User login
Persistent mood swings
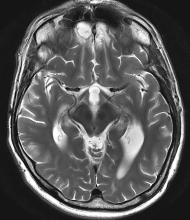
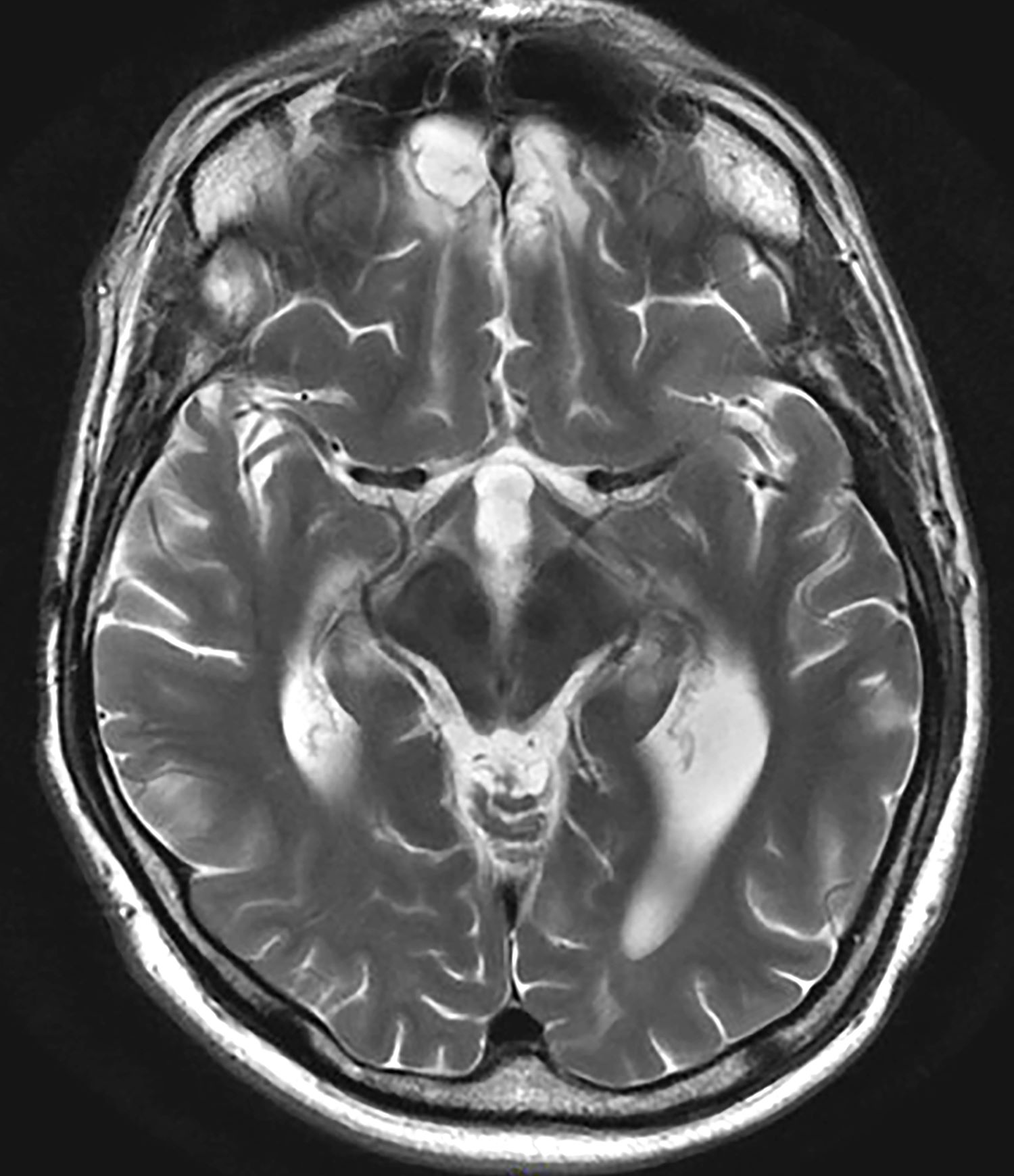
The most likely diagnosis for this patient is veteran posttraumatic stress disorder (PTSD), given his history of combat exposure and symptoms, such as severe headaches, difficulty concentrating, mood swings, nightmares, flashbacks, increased startle response, and hypervigilance. MRI findings showing significant changes in the limbic system and hippocampal regions support this diagnosis. Other potential diagnoses, like traumatic brain injury, chronic migraine, and major depressive disorder, are less likely because of their inability to account for the full range of his symptoms and specific MRI abnormalities.
PTSD, experienced by a subset of individuals after exposure to life-threatening events, has a lifetime prevalence of 4%-7% and a current prevalence of 1%-3%, with higher rates in older women, those with more trauma, and combat veterans. Nearly half of US veterans are aged 65 or older, many being Vietnam-era veterans at elevated risk for PTSD. Prevalence rates in older veterans range between 1% and 22%.
PTSD is characterized by intrusive thoughts, flashbacks, nightmares, avoidance of reminders, hypervigilance, and sleep difficulties, significantly disrupting interpersonal and occupational functioning. Screening tools like the primary care (PC) PTSD-5 and PCL-5, used in primary care settings, are effective for early detection, provisional diagnosis, and monitoring of symptom changes. The clinician-administered PTSD scale for Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition DSM-5 (CAPS-5) is the gold standard for diagnosis, particularly among veterans, with multimethod assessments combining self-report measures and semi-structured interviews recommended for accuracy. The DSM-5 criteria for PTSD diagnosis describe exposure to traumatic events, intrusion symptoms, avoidance behaviors, negative mood, and altered arousal, with symptoms persisting for over a month and causing significant distress or functional impairment.
Research has identified consistent anatomical and functional changes in PTSD patients, such as smaller hippocampi, decreased corpus callosum and prefrontal cortex, increased amygdala reactivity, and decreased prefrontal cortex activity. PTSD, linked to alterations in brain regions involved in fear learning and memory, shows diminished structural integrity in executive function areas, reduced cortical volumes in the cingulate brain cortex and frontal regions, and reduced white matter integrity in key brain pathways. Neuroimaging findings, however, are primarily used for research currently and have yet to be widely implemented in clinical guidelines.
International PTSD treatment guidelines consistently recognize trauma-focused cognitive-behavioral therapies (CBTs), such as cognitive processing therapy (CPT), prolonged exposure (PE), and eye movement desensitization and reprocessing (EMDR) as the gold standard. Recent guidelines have expanded the list of recommended treatments: The 2023 Department of Veterans Affairs and Department of Defense guidelines in the United States also endorse therapies like written narrative exposure and brief eclectic therapy. Internationally, guidelines do not perfectly coincide, as the 2018 update from the United Kingdom's National Institute for Health and Care Excellence (NICE) gives the highest recommendations to PE and CPT but rates EMDR slightly lower for military veterans because of limited evidence. Overall, guidelines consistently advocate for trauma-focused psychological interventions as the primary treatment for PTSD.
Guidelines from NICE and the World Health Organization do not recommend medications as the primary treatment; the American Psychiatric Association and the US Department of Veterans Affairs support selective serotonin reuptake inhibitors and prazosin but advise against benzodiazepines. Inpatient care may be necessary for individuals who pose a danger to themselves or others, or for those with severe PTSD from childhood abuse, to aid in emotional regulation and treatment.
Jasvinder Chawla, MD, Professor of Neurology, Loyola University Medical Center, Maywood; Director, Clinical Neurophysiology Lab, Department of Neurology, Hines VA Hospital, Hines, IL.
Jasvinder Chawla, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The most likely diagnosis for this patient is veteran posttraumatic stress disorder (PTSD), given his history of combat exposure and symptoms, such as severe headaches, difficulty concentrating, mood swings, nightmares, flashbacks, increased startle response, and hypervigilance. MRI findings showing significant changes in the limbic system and hippocampal regions support this diagnosis. Other potential diagnoses, like traumatic brain injury, chronic migraine, and major depressive disorder, are less likely because of their inability to account for the full range of his symptoms and specific MRI abnormalities.
PTSD, experienced by a subset of individuals after exposure to life-threatening events, has a lifetime prevalence of 4%-7% and a current prevalence of 1%-3%, with higher rates in older women, those with more trauma, and combat veterans. Nearly half of US veterans are aged 65 or older, many being Vietnam-era veterans at elevated risk for PTSD. Prevalence rates in older veterans range between 1% and 22%.
PTSD is characterized by intrusive thoughts, flashbacks, nightmares, avoidance of reminders, hypervigilance, and sleep difficulties, significantly disrupting interpersonal and occupational functioning. Screening tools like the primary care (PC) PTSD-5 and PCL-5, used in primary care settings, are effective for early detection, provisional diagnosis, and monitoring of symptom changes. The clinician-administered PTSD scale for Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition DSM-5 (CAPS-5) is the gold standard for diagnosis, particularly among veterans, with multimethod assessments combining self-report measures and semi-structured interviews recommended for accuracy. The DSM-5 criteria for PTSD diagnosis describe exposure to traumatic events, intrusion symptoms, avoidance behaviors, negative mood, and altered arousal, with symptoms persisting for over a month and causing significant distress or functional impairment.
Research has identified consistent anatomical and functional changes in PTSD patients, such as smaller hippocampi, decreased corpus callosum and prefrontal cortex, increased amygdala reactivity, and decreased prefrontal cortex activity. PTSD, linked to alterations in brain regions involved in fear learning and memory, shows diminished structural integrity in executive function areas, reduced cortical volumes in the cingulate brain cortex and frontal regions, and reduced white matter integrity in key brain pathways. Neuroimaging findings, however, are primarily used for research currently and have yet to be widely implemented in clinical guidelines.
International PTSD treatment guidelines consistently recognize trauma-focused cognitive-behavioral therapies (CBTs), such as cognitive processing therapy (CPT), prolonged exposure (PE), and eye movement desensitization and reprocessing (EMDR) as the gold standard. Recent guidelines have expanded the list of recommended treatments: The 2023 Department of Veterans Affairs and Department of Defense guidelines in the United States also endorse therapies like written narrative exposure and brief eclectic therapy. Internationally, guidelines do not perfectly coincide, as the 2018 update from the United Kingdom's National Institute for Health and Care Excellence (NICE) gives the highest recommendations to PE and CPT but rates EMDR slightly lower for military veterans because of limited evidence. Overall, guidelines consistently advocate for trauma-focused psychological interventions as the primary treatment for PTSD.
Guidelines from NICE and the World Health Organization do not recommend medications as the primary treatment; the American Psychiatric Association and the US Department of Veterans Affairs support selective serotonin reuptake inhibitors and prazosin but advise against benzodiazepines. Inpatient care may be necessary for individuals who pose a danger to themselves or others, or for those with severe PTSD from childhood abuse, to aid in emotional regulation and treatment.
Jasvinder Chawla, MD, Professor of Neurology, Loyola University Medical Center, Maywood; Director, Clinical Neurophysiology Lab, Department of Neurology, Hines VA Hospital, Hines, IL.
Jasvinder Chawla, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The most likely diagnosis for this patient is veteran posttraumatic stress disorder (PTSD), given his history of combat exposure and symptoms, such as severe headaches, difficulty concentrating, mood swings, nightmares, flashbacks, increased startle response, and hypervigilance. MRI findings showing significant changes in the limbic system and hippocampal regions support this diagnosis. Other potential diagnoses, like traumatic brain injury, chronic migraine, and major depressive disorder, are less likely because of their inability to account for the full range of his symptoms and specific MRI abnormalities.
PTSD, experienced by a subset of individuals after exposure to life-threatening events, has a lifetime prevalence of 4%-7% and a current prevalence of 1%-3%, with higher rates in older women, those with more trauma, and combat veterans. Nearly half of US veterans are aged 65 or older, many being Vietnam-era veterans at elevated risk for PTSD. Prevalence rates in older veterans range between 1% and 22%.
PTSD is characterized by intrusive thoughts, flashbacks, nightmares, avoidance of reminders, hypervigilance, and sleep difficulties, significantly disrupting interpersonal and occupational functioning. Screening tools like the primary care (PC) PTSD-5 and PCL-5, used in primary care settings, are effective for early detection, provisional diagnosis, and monitoring of symptom changes. The clinician-administered PTSD scale for Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition DSM-5 (CAPS-5) is the gold standard for diagnosis, particularly among veterans, with multimethod assessments combining self-report measures and semi-structured interviews recommended for accuracy. The DSM-5 criteria for PTSD diagnosis describe exposure to traumatic events, intrusion symptoms, avoidance behaviors, negative mood, and altered arousal, with symptoms persisting for over a month and causing significant distress or functional impairment.
Research has identified consistent anatomical and functional changes in PTSD patients, such as smaller hippocampi, decreased corpus callosum and prefrontal cortex, increased amygdala reactivity, and decreased prefrontal cortex activity. PTSD, linked to alterations in brain regions involved in fear learning and memory, shows diminished structural integrity in executive function areas, reduced cortical volumes in the cingulate brain cortex and frontal regions, and reduced white matter integrity in key brain pathways. Neuroimaging findings, however, are primarily used for research currently and have yet to be widely implemented in clinical guidelines.
International PTSD treatment guidelines consistently recognize trauma-focused cognitive-behavioral therapies (CBTs), such as cognitive processing therapy (CPT), prolonged exposure (PE), and eye movement desensitization and reprocessing (EMDR) as the gold standard. Recent guidelines have expanded the list of recommended treatments: The 2023 Department of Veterans Affairs and Department of Defense guidelines in the United States also endorse therapies like written narrative exposure and brief eclectic therapy. Internationally, guidelines do not perfectly coincide, as the 2018 update from the United Kingdom's National Institute for Health and Care Excellence (NICE) gives the highest recommendations to PE and CPT but rates EMDR slightly lower for military veterans because of limited evidence. Overall, guidelines consistently advocate for trauma-focused psychological interventions as the primary treatment for PTSD.
Guidelines from NICE and the World Health Organization do not recommend medications as the primary treatment; the American Psychiatric Association and the US Department of Veterans Affairs support selective serotonin reuptake inhibitors and prazosin but advise against benzodiazepines. Inpatient care may be necessary for individuals who pose a danger to themselves or others, or for those with severe PTSD from childhood abuse, to aid in emotional regulation and treatment.
Jasvinder Chawla, MD, Professor of Neurology, Loyola University Medical Center, Maywood; Director, Clinical Neurophysiology Lab, Department of Neurology, Hines VA Hospital, Hines, IL.
Jasvinder Chawla, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 35-year-old male veteran presents with a history of severe headaches, difficulty concentrating, and persistent mood swings. He served multiple tours in a combat zone, where he was exposed to several traumatic events, including the loss of close friends. His medical history reveals previous diagnoses of insomnia and anxiety, for which he has been prescribed various medications over the years with limited success. During his clinical evaluation, he describes frequent nightmares and flashbacks related to his time in service. He reports an increased startle response and hypervigilance, often feeling on edge and irritable. A recent MRI of the brain, as shown in the image here, reveals significant changes in the limbic system, with abnormalities in the hippocampal regions. Laboratory tests and physical exams are otherwise unremarkable, but his mental health assessment indicates severe distress, which is affecting his daily functioning and interpersonal relationships.
Hospital to home tracheostomy care
SLEEP MEDICINE NETWORK
Home-Based Mechanical Ventilation and Neuromuscular Section
Technological improvement has enhanced our ability to support these patients with complex conditions in their home settings. However, clinical practice guidelines are lacking, and current practice relies on a consensus of expert opinions.1-3
Once a patient who has had a tracheostomy begins transitioning care to home, identifying caregivers is vital.
Caregivers need to be educated on daily tracheostomy care, airway clearance, and ventilator management.
Protocols to standardize this transition, such as the “Trach Trail” protocol, help reduce ICU readmissions with new tracheostomies (P = .05), eliminate predischarge mortality (P = .05), and may decrease ICU length of stay (P = 0.72).4 Standardized protocols for aspects of tracheostomy care, such as the “Go-Bag” from Boston Children’s Hospital, ensure that a consistent approach keeps providers, families, and patients familiar with their equipment and safety procedures, improving outcomes and decreasing tracheostomy-related adverse events.4-6
Understanding the landscape surrounding which equipment companies have trained field respiratory therapists is crucial. Airway clearance is key to improving ventilation and oxygenation and maintaining tracheostomy patency. Knowing the types of airway clearance modalities used for each patient remains critical.
Trach care may look substantially different for some populations, like patients in the neonatal ICU. Trach changes may happen more frequently. Speaking valve times may be gradually increased while planning for possible decannulation. Skin care involving granulation tissue and stoma complications is particularly important for this population. Active infants need well-fitting trach ties to balance enough support to maintain their trach without causing skin breakdown or discomfort. Securing the trach to prevent pulling or dislodgement as infants become more active is crucial as developmental milestones are achieved.
We hope national societies prioritize standardizing care for this vulnerable population while promoting additional high-quality, patient-centered outcomes in research studies. Implementation strategies to promote interprofessional teams to enhance education, communication, and outcomes will reduce health care disparities.
References
1. Am J Respir Crit Care Med Vol 161. pp Sherman JM, Davis S, Albamonte-Petrick S, et al. Care of the child with a chronic tracheostomy. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 2000;161(1):297-308. doi: 10.1164/ajrccm.161.1.ats1-00 297-308, 2000
2. Mitchell RB, Hussey HM, Setzen G, et al. Clinical consensus statement: tracheostomy care. Otolaryngol Head Neck Surg. 2013;148(1):6-20. Preprint. Posted online September 18, 2012. PMID: 22990518. doi: 10.1177/0194599812460376
3. Sterni LM, Collaco JM, Baker CD, et al; ATS Pediatric Chronic Home Ventilation Workgroup. An official American Thoracic Society clinical practice guideline: pediatric chronic home invasive ventilation. Am J Respir Crit Care Med. 2016;193(8):e16-35. PMID: 27082538; PMCID: PMC5439679. doi: 10.1164/rccm.201602-0276ST
4. Cherney RL, Pandian V, Ninan A, et al. The Trach Trail: a systems-based pathway to improve quality of tracheostomy care and interdisciplinary collaboration. Otolaryngol Head Neck Surg. 2020;163(2):232-243. doi: 10.1177/0194599820917427
5. Brown J. Tracheostomy to noninvasive ventilation: from acute care to home. Sleep Med Clin. 2020;15(4):593-598. doi: 10.1016/j.jsmc.2020.08.003
6. Kohn J, McKeon M, Munhall D, Blanchette S, Wells S, Watters K. Standardization of pediatric tracheostomy care with “Go-bags.” Int J Pediatr Otorhinolaryngol. 2019;121:154-156. doi: 10.1016/j.ijporl.2019.03.022
SLEEP MEDICINE NETWORK
Home-Based Mechanical Ventilation and Neuromuscular Section
Technological improvement has enhanced our ability to support these patients with complex conditions in their home settings. However, clinical practice guidelines are lacking, and current practice relies on a consensus of expert opinions.1-3
Once a patient who has had a tracheostomy begins transitioning care to home, identifying caregivers is vital.
Caregivers need to be educated on daily tracheostomy care, airway clearance, and ventilator management.
Protocols to standardize this transition, such as the “Trach Trail” protocol, help reduce ICU readmissions with new tracheostomies (P = .05), eliminate predischarge mortality (P = .05), and may decrease ICU length of stay (P = 0.72).4 Standardized protocols for aspects of tracheostomy care, such as the “Go-Bag” from Boston Children’s Hospital, ensure that a consistent approach keeps providers, families, and patients familiar with their equipment and safety procedures, improving outcomes and decreasing tracheostomy-related adverse events.4-6
Understanding the landscape surrounding which equipment companies have trained field respiratory therapists is crucial. Airway clearance is key to improving ventilation and oxygenation and maintaining tracheostomy patency. Knowing the types of airway clearance modalities used for each patient remains critical.
Trach care may look substantially different for some populations, like patients in the neonatal ICU. Trach changes may happen more frequently. Speaking valve times may be gradually increased while planning for possible decannulation. Skin care involving granulation tissue and stoma complications is particularly important for this population. Active infants need well-fitting trach ties to balance enough support to maintain their trach without causing skin breakdown or discomfort. Securing the trach to prevent pulling or dislodgement as infants become more active is crucial as developmental milestones are achieved.
We hope national societies prioritize standardizing care for this vulnerable population while promoting additional high-quality, patient-centered outcomes in research studies. Implementation strategies to promote interprofessional teams to enhance education, communication, and outcomes will reduce health care disparities.
References
1. Am J Respir Crit Care Med Vol 161. pp Sherman JM, Davis S, Albamonte-Petrick S, et al. Care of the child with a chronic tracheostomy. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 2000;161(1):297-308. doi: 10.1164/ajrccm.161.1.ats1-00 297-308, 2000
2. Mitchell RB, Hussey HM, Setzen G, et al. Clinical consensus statement: tracheostomy care. Otolaryngol Head Neck Surg. 2013;148(1):6-20. Preprint. Posted online September 18, 2012. PMID: 22990518. doi: 10.1177/0194599812460376
3. Sterni LM, Collaco JM, Baker CD, et al; ATS Pediatric Chronic Home Ventilation Workgroup. An official American Thoracic Society clinical practice guideline: pediatric chronic home invasive ventilation. Am J Respir Crit Care Med. 2016;193(8):e16-35. PMID: 27082538; PMCID: PMC5439679. doi: 10.1164/rccm.201602-0276ST
4. Cherney RL, Pandian V, Ninan A, et al. The Trach Trail: a systems-based pathway to improve quality of tracheostomy care and interdisciplinary collaboration. Otolaryngol Head Neck Surg. 2020;163(2):232-243. doi: 10.1177/0194599820917427
5. Brown J. Tracheostomy to noninvasive ventilation: from acute care to home. Sleep Med Clin. 2020;15(4):593-598. doi: 10.1016/j.jsmc.2020.08.003
6. Kohn J, McKeon M, Munhall D, Blanchette S, Wells S, Watters K. Standardization of pediatric tracheostomy care with “Go-bags.” Int J Pediatr Otorhinolaryngol. 2019;121:154-156. doi: 10.1016/j.ijporl.2019.03.022
SLEEP MEDICINE NETWORK
Home-Based Mechanical Ventilation and Neuromuscular Section
Technological improvement has enhanced our ability to support these patients with complex conditions in their home settings. However, clinical practice guidelines are lacking, and current practice relies on a consensus of expert opinions.1-3
Once a patient who has had a tracheostomy begins transitioning care to home, identifying caregivers is vital.
Caregivers need to be educated on daily tracheostomy care, airway clearance, and ventilator management.
Protocols to standardize this transition, such as the “Trach Trail” protocol, help reduce ICU readmissions with new tracheostomies (P = .05), eliminate predischarge mortality (P = .05), and may decrease ICU length of stay (P = 0.72).4 Standardized protocols for aspects of tracheostomy care, such as the “Go-Bag” from Boston Children’s Hospital, ensure that a consistent approach keeps providers, families, and patients familiar with their equipment and safety procedures, improving outcomes and decreasing tracheostomy-related adverse events.4-6
Understanding the landscape surrounding which equipment companies have trained field respiratory therapists is crucial. Airway clearance is key to improving ventilation and oxygenation and maintaining tracheostomy patency. Knowing the types of airway clearance modalities used for each patient remains critical.
Trach care may look substantially different for some populations, like patients in the neonatal ICU. Trach changes may happen more frequently. Speaking valve times may be gradually increased while planning for possible decannulation. Skin care involving granulation tissue and stoma complications is particularly important for this population. Active infants need well-fitting trach ties to balance enough support to maintain their trach without causing skin breakdown or discomfort. Securing the trach to prevent pulling or dislodgement as infants become more active is crucial as developmental milestones are achieved.
We hope national societies prioritize standardizing care for this vulnerable population while promoting additional high-quality, patient-centered outcomes in research studies. Implementation strategies to promote interprofessional teams to enhance education, communication, and outcomes will reduce health care disparities.
References
1. Am J Respir Crit Care Med Vol 161. pp Sherman JM, Davis S, Albamonte-Petrick S, et al. Care of the child with a chronic tracheostomy. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 2000;161(1):297-308. doi: 10.1164/ajrccm.161.1.ats1-00 297-308, 2000
2. Mitchell RB, Hussey HM, Setzen G, et al. Clinical consensus statement: tracheostomy care. Otolaryngol Head Neck Surg. 2013;148(1):6-20. Preprint. Posted online September 18, 2012. PMID: 22990518. doi: 10.1177/0194599812460376
3. Sterni LM, Collaco JM, Baker CD, et al; ATS Pediatric Chronic Home Ventilation Workgroup. An official American Thoracic Society clinical practice guideline: pediatric chronic home invasive ventilation. Am J Respir Crit Care Med. 2016;193(8):e16-35. PMID: 27082538; PMCID: PMC5439679. doi: 10.1164/rccm.201602-0276ST
4. Cherney RL, Pandian V, Ninan A, et al. The Trach Trail: a systems-based pathway to improve quality of tracheostomy care and interdisciplinary collaboration. Otolaryngol Head Neck Surg. 2020;163(2):232-243. doi: 10.1177/0194599820917427
5. Brown J. Tracheostomy to noninvasive ventilation: from acute care to home. Sleep Med Clin. 2020;15(4):593-598. doi: 10.1016/j.jsmc.2020.08.003
6. Kohn J, McKeon M, Munhall D, Blanchette S, Wells S, Watters K. Standardization of pediatric tracheostomy care with “Go-bags.” Int J Pediatr Otorhinolaryngol. 2019;121:154-156. doi: 10.1016/j.ijporl.2019.03.022
HALT early recognition is key
DIFFUSE LUNG DISEASE AND LUNG TRANSPLANT NETWORK
Lung Transplant Section
Hyperammonemia after lung transplantation (HALT) is a rare but serious complication occurring in 1% to 4% of patients with high morbidity and mortality. Early recognition is crucial, as mortality rates can reach 75%.1
HALT arises from excess ammonia production or decreased clearance and is often linked to infections by urea-splitting organisms, including mycoplasma and ureaplasma. Prompt, aggressive treatment is essential and typically includes dietary protein restriction, renal replacement therapy (ideally intermittent hemodialysis), bowel decontamination (lactulose, rifaximin, metronidazole, or neomycin), amino acids (arginine and levocarnitine), nitrogen scavengers (sodium phenylbutyrate or glycerol phenylbutyrate), and empiric antimicrobial coverage for urea-splitting organisms.2 Given concerns for calcineurin inhibitor-induced hyperammonemia, transition to an alternative agent may be considered.
Given the severe risks associated with HALT, vigilance is vital, particularly in intubated and sedated patients where monitoring of neurologic status is more challenging. Protocols may involve routine serum ammonia monitoring, polymerase chain reaction testing for mycoplasma and ureaplasma at the time of transplant or with postoperative bronchoscopy, and empiric antimicrobial treatment. No definitive ammonia threshold exists, but altered sensorium with elevated levels warrants immediate and more aggressive treatment with levels >75 μmol/L. Early testing and symptom recognition can significantly improve survival rates in this potentially devastating condition.
References
1. Leger RF, Silverman MS, Hauck ES, Guvakova KD. Hyperammonemia post lung transplantation: a review. Clin Med Insights Circ Respir Pulm Med. 2020;14:1179548420966234. doi:10.1177/1179548420966234
2. Chen C, Bain KB, Luppa JA. Hyperammonemia syndrome after lung transplantation: a single center experience. Transplantation. 2016;100(3):678-684. doi:10.1097/TP.0000000000000868
DIFFUSE LUNG DISEASE AND LUNG TRANSPLANT NETWORK
Lung Transplant Section
Hyperammonemia after lung transplantation (HALT) is a rare but serious complication occurring in 1% to 4% of patients with high morbidity and mortality. Early recognition is crucial, as mortality rates can reach 75%.1
HALT arises from excess ammonia production or decreased clearance and is often linked to infections by urea-splitting organisms, including mycoplasma and ureaplasma. Prompt, aggressive treatment is essential and typically includes dietary protein restriction, renal replacement therapy (ideally intermittent hemodialysis), bowel decontamination (lactulose, rifaximin, metronidazole, or neomycin), amino acids (arginine and levocarnitine), nitrogen scavengers (sodium phenylbutyrate or glycerol phenylbutyrate), and empiric antimicrobial coverage for urea-splitting organisms.2 Given concerns for calcineurin inhibitor-induced hyperammonemia, transition to an alternative agent may be considered.
Given the severe risks associated with HALT, vigilance is vital, particularly in intubated and sedated patients where monitoring of neurologic status is more challenging. Protocols may involve routine serum ammonia monitoring, polymerase chain reaction testing for mycoplasma and ureaplasma at the time of transplant or with postoperative bronchoscopy, and empiric antimicrobial treatment. No definitive ammonia threshold exists, but altered sensorium with elevated levels warrants immediate and more aggressive treatment with levels >75 μmol/L. Early testing and symptom recognition can significantly improve survival rates in this potentially devastating condition.
References
1. Leger RF, Silverman MS, Hauck ES, Guvakova KD. Hyperammonemia post lung transplantation: a review. Clin Med Insights Circ Respir Pulm Med. 2020;14:1179548420966234. doi:10.1177/1179548420966234
2. Chen C, Bain KB, Luppa JA. Hyperammonemia syndrome after lung transplantation: a single center experience. Transplantation. 2016;100(3):678-684. doi:10.1097/TP.0000000000000868
DIFFUSE LUNG DISEASE AND LUNG TRANSPLANT NETWORK
Lung Transplant Section
Hyperammonemia after lung transplantation (HALT) is a rare but serious complication occurring in 1% to 4% of patients with high morbidity and mortality. Early recognition is crucial, as mortality rates can reach 75%.1
HALT arises from excess ammonia production or decreased clearance and is often linked to infections by urea-splitting organisms, including mycoplasma and ureaplasma. Prompt, aggressive treatment is essential and typically includes dietary protein restriction, renal replacement therapy (ideally intermittent hemodialysis), bowel decontamination (lactulose, rifaximin, metronidazole, or neomycin), amino acids (arginine and levocarnitine), nitrogen scavengers (sodium phenylbutyrate or glycerol phenylbutyrate), and empiric antimicrobial coverage for urea-splitting organisms.2 Given concerns for calcineurin inhibitor-induced hyperammonemia, transition to an alternative agent may be considered.
Given the severe risks associated with HALT, vigilance is vital, particularly in intubated and sedated patients where monitoring of neurologic status is more challenging. Protocols may involve routine serum ammonia monitoring, polymerase chain reaction testing for mycoplasma and ureaplasma at the time of transplant or with postoperative bronchoscopy, and empiric antimicrobial treatment. No definitive ammonia threshold exists, but altered sensorium with elevated levels warrants immediate and more aggressive treatment with levels >75 μmol/L. Early testing and symptom recognition can significantly improve survival rates in this potentially devastating condition.
References
1. Leger RF, Silverman MS, Hauck ES, Guvakova KD. Hyperammonemia post lung transplantation: a review. Clin Med Insights Circ Respir Pulm Med. 2020;14:1179548420966234. doi:10.1177/1179548420966234
2. Chen C, Bain KB, Luppa JA. Hyperammonemia syndrome after lung transplantation: a single center experience. Transplantation. 2016;100(3):678-684. doi:10.1097/TP.0000000000000868
SURMOUNT-OSA Results: ‘Impressive’ in Improving Sleep Apnea
This transcript has been edited for clarity.
Akshay B. Jain, MD: Welcome. I’m Dr. Akshay Jain, an endocrinologist in Vancouver, Canada, and with me is a very special guest. Today we have Dr. James Kim, a primary care physician working in Calgary, Canada. Both Dr. Kim and I were fortunate to attend the recently concluded American Diabetes Association annual conference in Orlando in June.
We thought we could share with you some of the key learnings that we found very insightful and clinically quite relevant. We were hoping to bring our own conclusion regarding what these findings were, both from a primary care perspective and an endocrinology perspective.
There were so many different studies that, frankly, it was difficult to pick them, but we handpicked a few studies we felt we could do a bit of a deeper dive on, and we’ll talk about each of these studies.
Welcome, Dr. Kim, and thanks for joining us.
James W. Kim, MBBCh, PgDip, MScCH: Thank you so much, Dr Jain. It’s a pleasure to be here.
Dr. Jain: Probably the best place to start would be with the SURMOUNT-OSA study. This was highlighted at the American Diabetes Association conference. Essentially, it looked at people who are living with obesity who also had obstructive sleep apnea.
This was a randomized controlled trial where individuals tested either got tirzepatide (trade name, Mounjaro) or placebo treatment. They looked at the change in their apnea-hypopnea index at the end of the study.
This included both people who were using CPAP machines and those who were not using CPAP machines at baseline. We do know that many individuals with sleep apnea may not use these machines.
That was a big reduction.
Dr. Kim, what’s the relevance of this study in primary care?
Dr. Kim: Oh, it’s massive. Obstructive sleep apnea is probably one of the most underdiagnosed yet huge cardiac risk factors that we tend to overlook in primary care. We sometimes say, oh, it’s just sleep apnea; what’s the big deal? We know it’s a big problem. We know that more than 50% of people with type 2 diabetes have obstructive sleep apnea, and some studies have even quoted that 90% of their population cohorts had sleep apnea. This is a big deal.
What do we know so far? We know that obstructive sleep apnea, which I’m just going to call OSA, increases the risk for hypertension, bad cholesterol, and worsening blood glucose in terms of A1c and fasting glucose, which eventually leads to myocardial infarction, arrhythmia, stroke, and eventually cardiovascular death.
We also know that people with type 2 diabetes have an increased risk for OSA. There seems to be a bidirectional relationship between diabetes and OSA. It seems like weight plays the biggest role in terms of developing OSA, and numerous studies have shown this.
Also, thankfully, some of the studies showed that weight loss improves not just OSA but also blood pressure, cholesterol, blood glucose, and insulin sensitivities. These have been fascinating. We see these patients every single day. If you think about it in your population, for 50%-90% of the patients to have OSA is a large number. If you haven’t seen a person with OSA this week, you probably missed them, very likely.
Therefore, the SURMOUNT-OSA trial was quite fascinating with, as you mentioned, 50%-60% reduction in the severity of OSA, which is very impressive. Even more impressive, I think, is that for about 50% of the patients on tirzepatide, the OSA improves so much that they may not even need to be on CPAP machines.
Those who were on CPAP may not need to be on CPAP any longer. These are huge data, especially for primary care, because as you mentioned, we see these people every single day.
Dr. Jain: Thanks for pointing that out. Clearly, it’s very clinically relevant. I think the most important takeaway for me from this study was the correlation between weight loss and AHI improvement.
Clearly, it showed that placebo had about a 6% drop in AHI, whereas there was a 60% drop in the tirzepatide group, so you can see that it’s significantly different. The placebo group did not have any significant degree of weight loss, whereas the tirzepatide group had nearly 20% weight loss. This again goes to show that there is a very close correlation between weight loss and improvement in OSA.
What’s very important to note is that we’ve seen this in the past as well. We had seen some of these data with other GLP-1 agents, but the extent of improvement that we have seen in the SURMOUNT-OSA trial is significantly more than what we’ve seen in previous studies. There is a ray of hope now where we have medical management to offer people who are living with obesity and obstructive sleep apnea.
Dr. Kim: I want to add that, from a primary care perspective, this study also showed the improvement of the sleep apnea–related symptoms as well. The biggest problem with sleep apnea — or at least what patients’ spouses complain of, is the person snoring too much; it’s a symptom.
It’s the next-day symptoms that really do disturb people, like chronic fatigue. I have numerous patients who say that, once they’ve been treated for sleep apnea, they feel like a brand-new person. They have sudden bursts of energy that they never felt before, and over 50% of these people have huge improvements in the symptoms as well.
This is a huge trial. The only thing that I wish this study included were people with mild obstructive sleep apnea who were symptomatic. I do understand that, with other studies in this population, the data have been conflicting, but it would have been really awesome if they had those patients included. However, it is still a significant study for primary care.
Dr. Jain: That’s a really good point. Fatigue improves and overall quality of life improves. That’s very important from a primary care perspective.
From an endocrinology perspective, we know that management of sleep apnea can often lead to improvement in male hypogonadism, polycystic ovary syndrome, and insulin resistance. The amount of insulin required, or the number of medications needed for managing diabetes, can improve. Hypertension can improve as well. There are multiple benefits that you can get from appropriate management of sleep apnea.
Thanks, Dr. Kim. We really appreciate your insights on SURMOUNT-OSA.
Dr. Jain is a clinical instructor, Department of Endocrinology, University of British Columbia, Vancouver. Dr. Kim is a clinical assistant professor, Department of Family Medicine, University of Calgary in Alberta. Both disclosed conflicts of interest with numerous pharmaceutical companies.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Akshay B. Jain, MD: Welcome. I’m Dr. Akshay Jain, an endocrinologist in Vancouver, Canada, and with me is a very special guest. Today we have Dr. James Kim, a primary care physician working in Calgary, Canada. Both Dr. Kim and I were fortunate to attend the recently concluded American Diabetes Association annual conference in Orlando in June.
We thought we could share with you some of the key learnings that we found very insightful and clinically quite relevant. We were hoping to bring our own conclusion regarding what these findings were, both from a primary care perspective and an endocrinology perspective.
There were so many different studies that, frankly, it was difficult to pick them, but we handpicked a few studies we felt we could do a bit of a deeper dive on, and we’ll talk about each of these studies.
Welcome, Dr. Kim, and thanks for joining us.
James W. Kim, MBBCh, PgDip, MScCH: Thank you so much, Dr Jain. It’s a pleasure to be here.
Dr. Jain: Probably the best place to start would be with the SURMOUNT-OSA study. This was highlighted at the American Diabetes Association conference. Essentially, it looked at people who are living with obesity who also had obstructive sleep apnea.
This was a randomized controlled trial where individuals tested either got tirzepatide (trade name, Mounjaro) or placebo treatment. They looked at the change in their apnea-hypopnea index at the end of the study.
This included both people who were using CPAP machines and those who were not using CPAP machines at baseline. We do know that many individuals with sleep apnea may not use these machines.
That was a big reduction.
Dr. Kim, what’s the relevance of this study in primary care?
Dr. Kim: Oh, it’s massive. Obstructive sleep apnea is probably one of the most underdiagnosed yet huge cardiac risk factors that we tend to overlook in primary care. We sometimes say, oh, it’s just sleep apnea; what’s the big deal? We know it’s a big problem. We know that more than 50% of people with type 2 diabetes have obstructive sleep apnea, and some studies have even quoted that 90% of their population cohorts had sleep apnea. This is a big deal.
What do we know so far? We know that obstructive sleep apnea, which I’m just going to call OSA, increases the risk for hypertension, bad cholesterol, and worsening blood glucose in terms of A1c and fasting glucose, which eventually leads to myocardial infarction, arrhythmia, stroke, and eventually cardiovascular death.
We also know that people with type 2 diabetes have an increased risk for OSA. There seems to be a bidirectional relationship between diabetes and OSA. It seems like weight plays the biggest role in terms of developing OSA, and numerous studies have shown this.
Also, thankfully, some of the studies showed that weight loss improves not just OSA but also blood pressure, cholesterol, blood glucose, and insulin sensitivities. These have been fascinating. We see these patients every single day. If you think about it in your population, for 50%-90% of the patients to have OSA is a large number. If you haven’t seen a person with OSA this week, you probably missed them, very likely.
Therefore, the SURMOUNT-OSA trial was quite fascinating with, as you mentioned, 50%-60% reduction in the severity of OSA, which is very impressive. Even more impressive, I think, is that for about 50% of the patients on tirzepatide, the OSA improves so much that they may not even need to be on CPAP machines.
Those who were on CPAP may not need to be on CPAP any longer. These are huge data, especially for primary care, because as you mentioned, we see these people every single day.
Dr. Jain: Thanks for pointing that out. Clearly, it’s very clinically relevant. I think the most important takeaway for me from this study was the correlation between weight loss and AHI improvement.
Clearly, it showed that placebo had about a 6% drop in AHI, whereas there was a 60% drop in the tirzepatide group, so you can see that it’s significantly different. The placebo group did not have any significant degree of weight loss, whereas the tirzepatide group had nearly 20% weight loss. This again goes to show that there is a very close correlation between weight loss and improvement in OSA.
What’s very important to note is that we’ve seen this in the past as well. We had seen some of these data with other GLP-1 agents, but the extent of improvement that we have seen in the SURMOUNT-OSA trial is significantly more than what we’ve seen in previous studies. There is a ray of hope now where we have medical management to offer people who are living with obesity and obstructive sleep apnea.
Dr. Kim: I want to add that, from a primary care perspective, this study also showed the improvement of the sleep apnea–related symptoms as well. The biggest problem with sleep apnea — or at least what patients’ spouses complain of, is the person snoring too much; it’s a symptom.
It’s the next-day symptoms that really do disturb people, like chronic fatigue. I have numerous patients who say that, once they’ve been treated for sleep apnea, they feel like a brand-new person. They have sudden bursts of energy that they never felt before, and over 50% of these people have huge improvements in the symptoms as well.
This is a huge trial. The only thing that I wish this study included were people with mild obstructive sleep apnea who were symptomatic. I do understand that, with other studies in this population, the data have been conflicting, but it would have been really awesome if they had those patients included. However, it is still a significant study for primary care.
Dr. Jain: That’s a really good point. Fatigue improves and overall quality of life improves. That’s very important from a primary care perspective.
From an endocrinology perspective, we know that management of sleep apnea can often lead to improvement in male hypogonadism, polycystic ovary syndrome, and insulin resistance. The amount of insulin required, or the number of medications needed for managing diabetes, can improve. Hypertension can improve as well. There are multiple benefits that you can get from appropriate management of sleep apnea.
Thanks, Dr. Kim. We really appreciate your insights on SURMOUNT-OSA.
Dr. Jain is a clinical instructor, Department of Endocrinology, University of British Columbia, Vancouver. Dr. Kim is a clinical assistant professor, Department of Family Medicine, University of Calgary in Alberta. Both disclosed conflicts of interest with numerous pharmaceutical companies.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Akshay B. Jain, MD: Welcome. I’m Dr. Akshay Jain, an endocrinologist in Vancouver, Canada, and with me is a very special guest. Today we have Dr. James Kim, a primary care physician working in Calgary, Canada. Both Dr. Kim and I were fortunate to attend the recently concluded American Diabetes Association annual conference in Orlando in June.
We thought we could share with you some of the key learnings that we found very insightful and clinically quite relevant. We were hoping to bring our own conclusion regarding what these findings were, both from a primary care perspective and an endocrinology perspective.
There were so many different studies that, frankly, it was difficult to pick them, but we handpicked a few studies we felt we could do a bit of a deeper dive on, and we’ll talk about each of these studies.
Welcome, Dr. Kim, and thanks for joining us.
James W. Kim, MBBCh, PgDip, MScCH: Thank you so much, Dr Jain. It’s a pleasure to be here.
Dr. Jain: Probably the best place to start would be with the SURMOUNT-OSA study. This was highlighted at the American Diabetes Association conference. Essentially, it looked at people who are living with obesity who also had obstructive sleep apnea.
This was a randomized controlled trial where individuals tested either got tirzepatide (trade name, Mounjaro) or placebo treatment. They looked at the change in their apnea-hypopnea index at the end of the study.
This included both people who were using CPAP machines and those who were not using CPAP machines at baseline. We do know that many individuals with sleep apnea may not use these machines.
That was a big reduction.
Dr. Kim, what’s the relevance of this study in primary care?
Dr. Kim: Oh, it’s massive. Obstructive sleep apnea is probably one of the most underdiagnosed yet huge cardiac risk factors that we tend to overlook in primary care. We sometimes say, oh, it’s just sleep apnea; what’s the big deal? We know it’s a big problem. We know that more than 50% of people with type 2 diabetes have obstructive sleep apnea, and some studies have even quoted that 90% of their population cohorts had sleep apnea. This is a big deal.
What do we know so far? We know that obstructive sleep apnea, which I’m just going to call OSA, increases the risk for hypertension, bad cholesterol, and worsening blood glucose in terms of A1c and fasting glucose, which eventually leads to myocardial infarction, arrhythmia, stroke, and eventually cardiovascular death.
We also know that people with type 2 diabetes have an increased risk for OSA. There seems to be a bidirectional relationship between diabetes and OSA. It seems like weight plays the biggest role in terms of developing OSA, and numerous studies have shown this.
Also, thankfully, some of the studies showed that weight loss improves not just OSA but also blood pressure, cholesterol, blood glucose, and insulin sensitivities. These have been fascinating. We see these patients every single day. If you think about it in your population, for 50%-90% of the patients to have OSA is a large number. If you haven’t seen a person with OSA this week, you probably missed them, very likely.
Therefore, the SURMOUNT-OSA trial was quite fascinating with, as you mentioned, 50%-60% reduction in the severity of OSA, which is very impressive. Even more impressive, I think, is that for about 50% of the patients on tirzepatide, the OSA improves so much that they may not even need to be on CPAP machines.
Those who were on CPAP may not need to be on CPAP any longer. These are huge data, especially for primary care, because as you mentioned, we see these people every single day.
Dr. Jain: Thanks for pointing that out. Clearly, it’s very clinically relevant. I think the most important takeaway for me from this study was the correlation between weight loss and AHI improvement.
Clearly, it showed that placebo had about a 6% drop in AHI, whereas there was a 60% drop in the tirzepatide group, so you can see that it’s significantly different. The placebo group did not have any significant degree of weight loss, whereas the tirzepatide group had nearly 20% weight loss. This again goes to show that there is a very close correlation between weight loss and improvement in OSA.
What’s very important to note is that we’ve seen this in the past as well. We had seen some of these data with other GLP-1 agents, but the extent of improvement that we have seen in the SURMOUNT-OSA trial is significantly more than what we’ve seen in previous studies. There is a ray of hope now where we have medical management to offer people who are living with obesity and obstructive sleep apnea.
Dr. Kim: I want to add that, from a primary care perspective, this study also showed the improvement of the sleep apnea–related symptoms as well. The biggest problem with sleep apnea — or at least what patients’ spouses complain of, is the person snoring too much; it’s a symptom.
It’s the next-day symptoms that really do disturb people, like chronic fatigue. I have numerous patients who say that, once they’ve been treated for sleep apnea, they feel like a brand-new person. They have sudden bursts of energy that they never felt before, and over 50% of these people have huge improvements in the symptoms as well.
This is a huge trial. The only thing that I wish this study included were people with mild obstructive sleep apnea who were symptomatic. I do understand that, with other studies in this population, the data have been conflicting, but it would have been really awesome if they had those patients included. However, it is still a significant study for primary care.
Dr. Jain: That’s a really good point. Fatigue improves and overall quality of life improves. That’s very important from a primary care perspective.
From an endocrinology perspective, we know that management of sleep apnea can often lead to improvement in male hypogonadism, polycystic ovary syndrome, and insulin resistance. The amount of insulin required, or the number of medications needed for managing diabetes, can improve. Hypertension can improve as well. There are multiple benefits that you can get from appropriate management of sleep apnea.
Thanks, Dr. Kim. We really appreciate your insights on SURMOUNT-OSA.
Dr. Jain is a clinical instructor, Department of Endocrinology, University of British Columbia, Vancouver. Dr. Kim is a clinical assistant professor, Department of Family Medicine, University of Calgary in Alberta. Both disclosed conflicts of interest with numerous pharmaceutical companies.
A version of this article appeared on Medscape.com.
Pulmonary Hypertension: Comorbidities and Novel Therapeutics
- Cullivan S, Gaine S, Sitbon O. New trends in pulmonary hypertension. Eur Respir Rev. 2023;32(167):220211. doi:10.1183/16000617.0211-2022
- Mocumbi A, Humbert M, Saxena A, et al. Pulmonary hypertension [published correction appears in Nat Rev Dis Primers. 2024;10(1):5]. Nat Rev Dis Primers. 2024;10(1):1. doi:10.1038/s41572-023-00486-7
- Lang IM, Palazzini M. The burden of comorbidities in pulmonary arterial hypertension. Eur Heart J Suppl. 2019;21(suppl K):K21-K28. doi:10.1093/ eurheartj/suz205
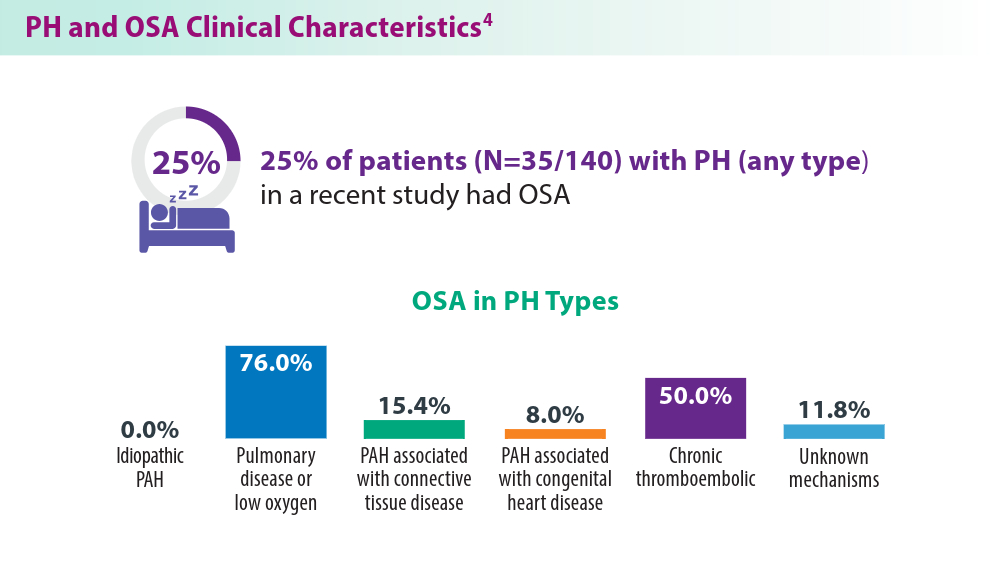
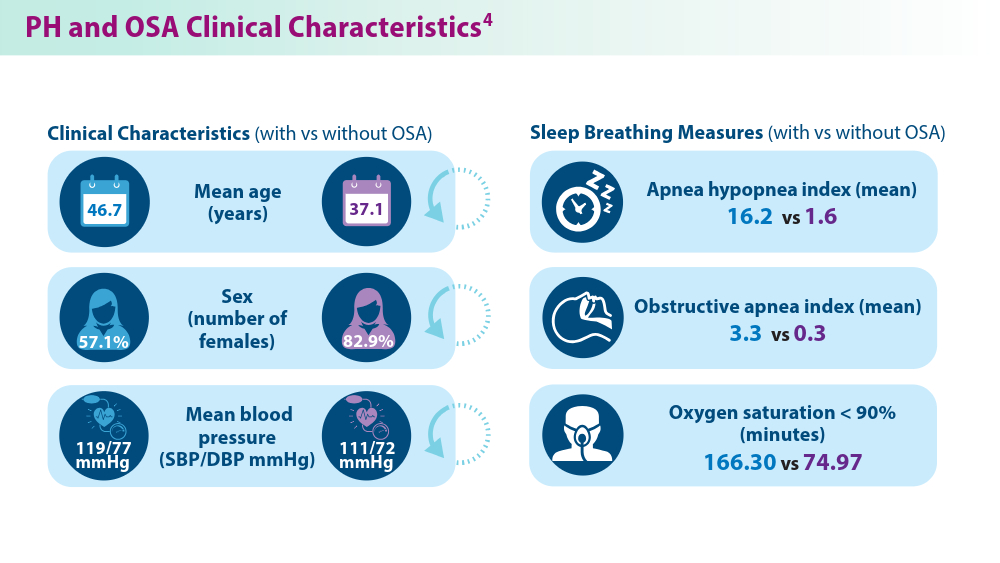
- Yan L, Zhao Z, Zhao Q, et al. The clinical characteristics of patients with pulmonary hypertension combined with obstructive sleep apnoea. BMC Pulm Med. 2021;21(1):378. doi:10.1186/s12890-021-01755-5
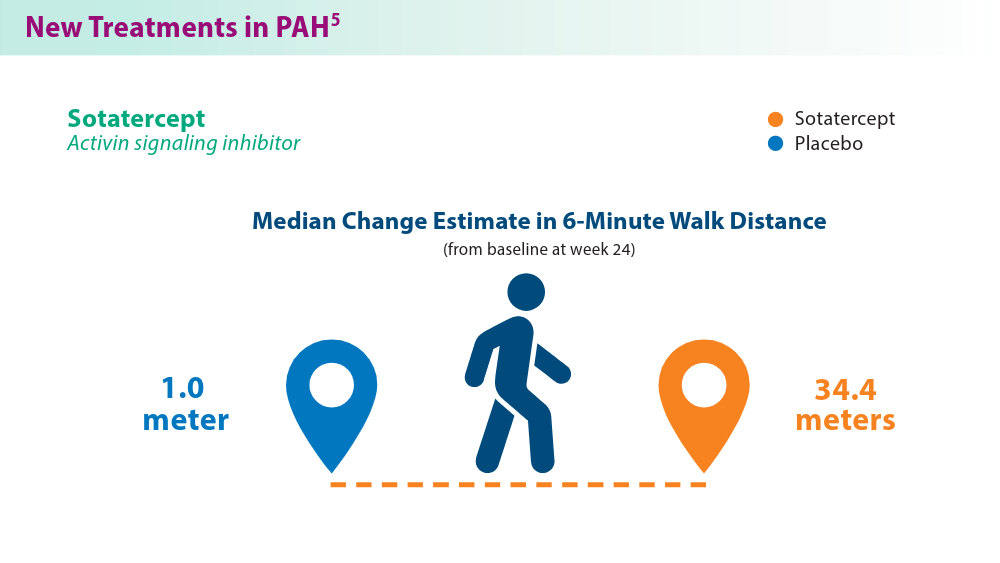
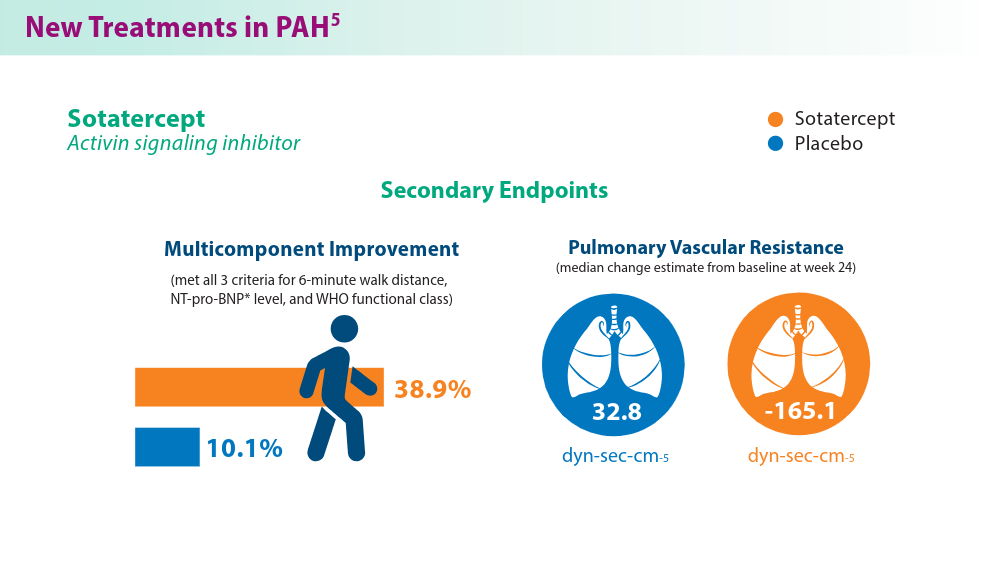
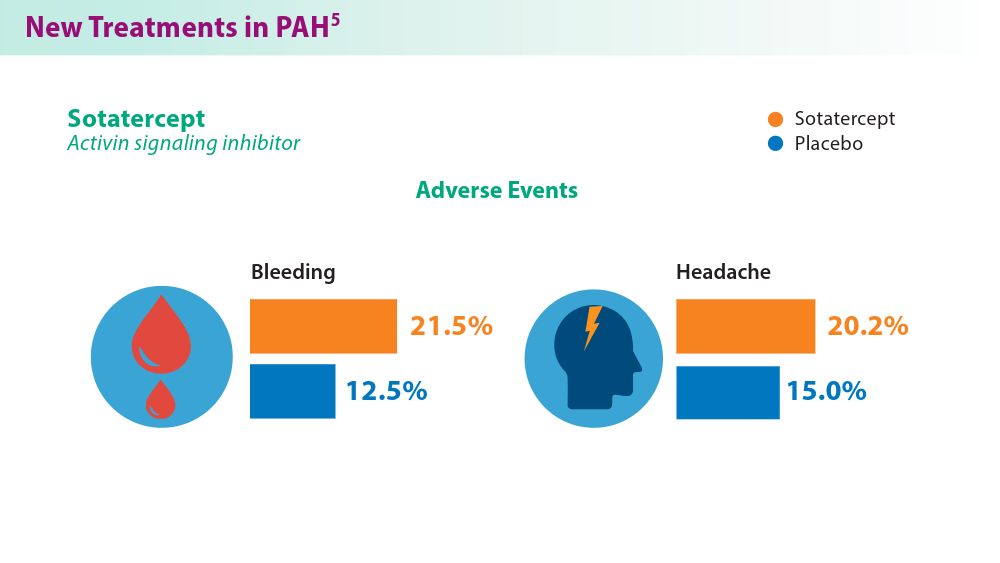
- Hoeper MM, Badesch DB, Ghofrani HA, et al; for the STELLAR Trial Investigators. Phase 3 trial of sotatercept for treatment of pulmonary arterial hypertension. N Engl J Med. 2023;388(16):1478-1490. doi:10.1056/NEJMoa2213558
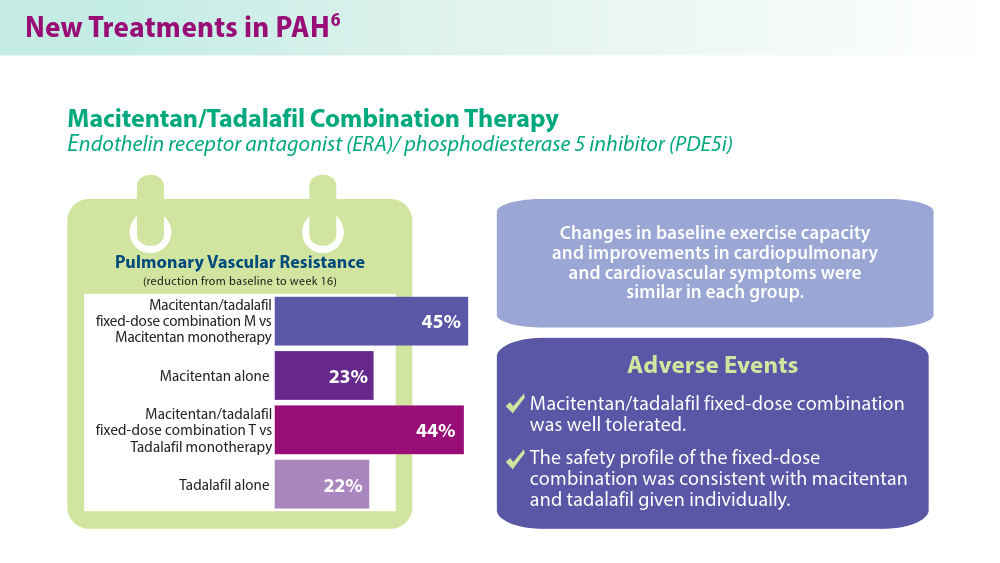
- Grünig E, Jansa P, Fan F, et al. Randomized trial of macitentan/tadalafil single-tablet combination therapy for pulmonary arterial hypertension. J Am Coll Cardiol. 2024;83(4):473-484. doi:10.1016/j.jacc.2023.10.045
- Higuchi S, Horinouchi H, Aoki T, et al. Balloon pulmonary angioplasty in the management of chronic thromboembolic pulmonary hypertension. Radiographics. 2022;42(6):1881-1896. doi:10.1148/rg.210102
- Cullivan S, Gaine S, Sitbon O. New trends in pulmonary hypertension. Eur Respir Rev. 2023;32(167):220211. doi:10.1183/16000617.0211-2022
- Mocumbi A, Humbert M, Saxena A, et al. Pulmonary hypertension [published correction appears in Nat Rev Dis Primers. 2024;10(1):5]. Nat Rev Dis Primers. 2024;10(1):1. doi:10.1038/s41572-023-00486-7
- Lang IM, Palazzini M. The burden of comorbidities in pulmonary arterial hypertension. Eur Heart J Suppl. 2019;21(suppl K):K21-K28. doi:10.1093/ eurheartj/suz205
- Yan L, Zhao Z, Zhao Q, et al. The clinical characteristics of patients with pulmonary hypertension combined with obstructive sleep apnoea. BMC Pulm Med. 2021;21(1):378. doi:10.1186/s12890-021-01755-5
- Hoeper MM, Badesch DB, Ghofrani HA, et al; for the STELLAR Trial Investigators. Phase 3 trial of sotatercept for treatment of pulmonary arterial hypertension. N Engl J Med. 2023;388(16):1478-1490. doi:10.1056/NEJMoa2213558
- Grünig E, Jansa P, Fan F, et al. Randomized trial of macitentan/tadalafil single-tablet combination therapy for pulmonary arterial hypertension. J Am Coll Cardiol. 2024;83(4):473-484. doi:10.1016/j.jacc.2023.10.045
- Higuchi S, Horinouchi H, Aoki T, et al. Balloon pulmonary angioplasty in the management of chronic thromboembolic pulmonary hypertension. Radiographics. 2022;42(6):1881-1896. doi:10.1148/rg.210102
- Cullivan S, Gaine S, Sitbon O. New trends in pulmonary hypertension. Eur Respir Rev. 2023;32(167):220211. doi:10.1183/16000617.0211-2022
- Mocumbi A, Humbert M, Saxena A, et al. Pulmonary hypertension [published correction appears in Nat Rev Dis Primers. 2024;10(1):5]. Nat Rev Dis Primers. 2024;10(1):1. doi:10.1038/s41572-023-00486-7
- Lang IM, Palazzini M. The burden of comorbidities in pulmonary arterial hypertension. Eur Heart J Suppl. 2019;21(suppl K):K21-K28. doi:10.1093/ eurheartj/suz205
- Yan L, Zhao Z, Zhao Q, et al. The clinical characteristics of patients with pulmonary hypertension combined with obstructive sleep apnoea. BMC Pulm Med. 2021;21(1):378. doi:10.1186/s12890-021-01755-5
- Hoeper MM, Badesch DB, Ghofrani HA, et al; for the STELLAR Trial Investigators. Phase 3 trial of sotatercept for treatment of pulmonary arterial hypertension. N Engl J Med. 2023;388(16):1478-1490. doi:10.1056/NEJMoa2213558
- Grünig E, Jansa P, Fan F, et al. Randomized trial of macitentan/tadalafil single-tablet combination therapy for pulmonary arterial hypertension. J Am Coll Cardiol. 2024;83(4):473-484. doi:10.1016/j.jacc.2023.10.045
- Higuchi S, Horinouchi H, Aoki T, et al. Balloon pulmonary angioplasty in the management of chronic thromboembolic pulmonary hypertension. Radiographics. 2022;42(6):1881-1896. doi:10.1148/rg.210102
Advancements in nutritional management for critically ill patients
CRITICAL CARE NETWORK
Nonrespiratory Critical Care Section
Nutrition plays an important role in the management and recovery of critically ill patients admitted to the ICU. Major guidelines recommend that critically ill patients should receive 1.2 to 2.0 g/kg/day of protein, with an emphasis on early (within 48 hours of ICU admission) enteral nutrition.1-3
In a randomized controlled trial involving 173 critically ill patients who stayed in the ICU in Zhejiang, China, Wang and colleagues studied the impact of early high protein intake (1.5 g/kg/day vs 0.8 g/kg/day).4 The primary outcome of 28-day mortality was lower among the high protein intake group (8.14% vs 19.54%). Still, this intention-to-treat analysis did not reach a statistical significance (P = .051). However, a time-to-event analysis using the Cox proportional hazard model showed that the high protein intake group had a significantly lower 28-day mortality rate, shorter ICU stays, and improved nutritional status, particularly in patients with sepsis (P = .045).
In a systematic review and meta-analysis involving 19 randomized controlled trials and 1,731 patients, there was no definitive evidence that higher protein intake significantly reduces mortality. However, it may improve specific clinical outcomes like muscle mass retention and shorter duration of mechanical ventilation.5 Similarly, a post hoc analysis on the EFFORT Protein Trial focusing on critically ill patients with acute kidney injury (AKI) showed that higher protein intake did not significantly impact the duration of kidney replacement therapy but was associated with higher serum urea levels and slower time-to-discharge-alive among patients with AKI.6
For critically ill patients, increasing early protein intake to 1.5 g/kg/day is safe and may be beneficial. We still need more data to guide the best approach to determining the protein intake.
References
1. Taylor BE, McClave SA, Martindale RG, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN). Crit Care Med. 2016;44(2):390-438. doi:10.1097/CCM.0000000000001525
2. Singer P, Blaser AR, Berger MM, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38(1):48-79. doi:10.1016/j.clnu.2018.08.037
3. McClave SA, Taylor BE, Martindale RG, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN). JJPEN J Parenter Enteral Nutr. 2016;40(2):159-211. doi:10.1177/0148607115621863
4. Wang Y, Ye Y, Xuan L, et al. Impact of early high protein intake in critically ill patients: a randomized controlled trial. Nutr Metab. 2024;21(1):39. doi.org/10.1186/s12986-024-00818-8
5. Lee ZY, Yap CSL, Hasan MS, et al. The effect of higher versus lower protein delivery in critically ill patients: a systematic review and meta-analysis of randomized controlled trials. Crit Care. 2021;25(1):260. doi.org/10.1186/s13054-021-03693-4
6. Stoppe C, Patel JJ, Zarbock A, et al. The impact of higher protein dosing on outcomes in critically ill patients with acute kidney injury: a post hoc analysis of the EFFORT protein trial. Crit Care. 2023;27(1):399. doi.org/10.1186/s13054-023-04663-8
CRITICAL CARE NETWORK
Nonrespiratory Critical Care Section
Nutrition plays an important role in the management and recovery of critically ill patients admitted to the ICU. Major guidelines recommend that critically ill patients should receive 1.2 to 2.0 g/kg/day of protein, with an emphasis on early (within 48 hours of ICU admission) enteral nutrition.1-3
In a randomized controlled trial involving 173 critically ill patients who stayed in the ICU in Zhejiang, China, Wang and colleagues studied the impact of early high protein intake (1.5 g/kg/day vs 0.8 g/kg/day).4 The primary outcome of 28-day mortality was lower among the high protein intake group (8.14% vs 19.54%). Still, this intention-to-treat analysis did not reach a statistical significance (P = .051). However, a time-to-event analysis using the Cox proportional hazard model showed that the high protein intake group had a significantly lower 28-day mortality rate, shorter ICU stays, and improved nutritional status, particularly in patients with sepsis (P = .045).
In a systematic review and meta-analysis involving 19 randomized controlled trials and 1,731 patients, there was no definitive evidence that higher protein intake significantly reduces mortality. However, it may improve specific clinical outcomes like muscle mass retention and shorter duration of mechanical ventilation.5 Similarly, a post hoc analysis on the EFFORT Protein Trial focusing on critically ill patients with acute kidney injury (AKI) showed that higher protein intake did not significantly impact the duration of kidney replacement therapy but was associated with higher serum urea levels and slower time-to-discharge-alive among patients with AKI.6
For critically ill patients, increasing early protein intake to 1.5 g/kg/day is safe and may be beneficial. We still need more data to guide the best approach to determining the protein intake.
References
1. Taylor BE, McClave SA, Martindale RG, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN). Crit Care Med. 2016;44(2):390-438. doi:10.1097/CCM.0000000000001525
2. Singer P, Blaser AR, Berger MM, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38(1):48-79. doi:10.1016/j.clnu.2018.08.037
3. McClave SA, Taylor BE, Martindale RG, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN). JJPEN J Parenter Enteral Nutr. 2016;40(2):159-211. doi:10.1177/0148607115621863
4. Wang Y, Ye Y, Xuan L, et al. Impact of early high protein intake in critically ill patients: a randomized controlled trial. Nutr Metab. 2024;21(1):39. doi.org/10.1186/s12986-024-00818-8
5. Lee ZY, Yap CSL, Hasan MS, et al. The effect of higher versus lower protein delivery in critically ill patients: a systematic review and meta-analysis of randomized controlled trials. Crit Care. 2021;25(1):260. doi.org/10.1186/s13054-021-03693-4
6. Stoppe C, Patel JJ, Zarbock A, et al. The impact of higher protein dosing on outcomes in critically ill patients with acute kidney injury: a post hoc analysis of the EFFORT protein trial. Crit Care. 2023;27(1):399. doi.org/10.1186/s13054-023-04663-8
CRITICAL CARE NETWORK
Nonrespiratory Critical Care Section
Nutrition plays an important role in the management and recovery of critically ill patients admitted to the ICU. Major guidelines recommend that critically ill patients should receive 1.2 to 2.0 g/kg/day of protein, with an emphasis on early (within 48 hours of ICU admission) enteral nutrition.1-3
In a randomized controlled trial involving 173 critically ill patients who stayed in the ICU in Zhejiang, China, Wang and colleagues studied the impact of early high protein intake (1.5 g/kg/day vs 0.8 g/kg/day).4 The primary outcome of 28-day mortality was lower among the high protein intake group (8.14% vs 19.54%). Still, this intention-to-treat analysis did not reach a statistical significance (P = .051). However, a time-to-event analysis using the Cox proportional hazard model showed that the high protein intake group had a significantly lower 28-day mortality rate, shorter ICU stays, and improved nutritional status, particularly in patients with sepsis (P = .045).
In a systematic review and meta-analysis involving 19 randomized controlled trials and 1,731 patients, there was no definitive evidence that higher protein intake significantly reduces mortality. However, it may improve specific clinical outcomes like muscle mass retention and shorter duration of mechanical ventilation.5 Similarly, a post hoc analysis on the EFFORT Protein Trial focusing on critically ill patients with acute kidney injury (AKI) showed that higher protein intake did not significantly impact the duration of kidney replacement therapy but was associated with higher serum urea levels and slower time-to-discharge-alive among patients with AKI.6
For critically ill patients, increasing early protein intake to 1.5 g/kg/day is safe and may be beneficial. We still need more data to guide the best approach to determining the protein intake.
References
1. Taylor BE, McClave SA, Martindale RG, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN). Crit Care Med. 2016;44(2):390-438. doi:10.1097/CCM.0000000000001525
2. Singer P, Blaser AR, Berger MM, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38(1):48-79. doi:10.1016/j.clnu.2018.08.037
3. McClave SA, Taylor BE, Martindale RG, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN). JJPEN J Parenter Enteral Nutr. 2016;40(2):159-211. doi:10.1177/0148607115621863
4. Wang Y, Ye Y, Xuan L, et al. Impact of early high protein intake in critically ill patients: a randomized controlled trial. Nutr Metab. 2024;21(1):39. doi.org/10.1186/s12986-024-00818-8
5. Lee ZY, Yap CSL, Hasan MS, et al. The effect of higher versus lower protein delivery in critically ill patients: a systematic review and meta-analysis of randomized controlled trials. Crit Care. 2021;25(1):260. doi.org/10.1186/s13054-021-03693-4
6. Stoppe C, Patel JJ, Zarbock A, et al. The impact of higher protein dosing on outcomes in critically ill patients with acute kidney injury: a post hoc analysis of the EFFORT protein trial. Crit Care. 2023;27(1):399. doi.org/10.1186/s13054-023-04663-8
PTSD Workup
Editor's Note: This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Editor's Note: This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Editor's Note: This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
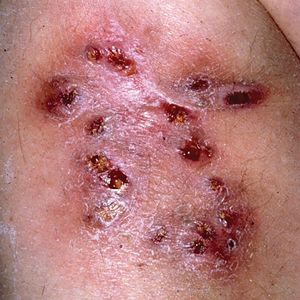
Multiple Draining Sinus Tracts on the Thigh
The Diagnosis: Mycobacterial Infection
An injury sustained in a wet environment that results in chronic indolent abscesses, nodules, or draining sinus tracts suggests a mycobacterial infection. In our patient, a culture revealed MycobacteriuM fortuitum, which is classified in the rapid grower nontuberculous mycobacteria (NTM) group, along with Mycobacterium chelonae and Mycobacterium abscessus.1 The patient’s history of skin injury while cutting wet grass and the common presence of M fortuitum in the environment suggested that the organism entered the wound. The patient healed completely following surgical excision and a 2-month course of clarithromycin 1 g daily and rifampin 600 mg daily.
MycobacteriuM fortuitum was first isolated from an amphibian source in 1905 and later identified in a human with cutaneous infection in 1938. It commonly is found in soil and water.2 Skin and soft-tissue infections with M fortuitum usually are acquired from direct entry of the organism through a damaged skin barrier from trauma, medical injection, surgery, or tattoo placement.2,3
Skin lesions caused by NTM often are nonspecific and can mimic a variety of other dermatologic conditions, making clinical diagnosis challenging. As such, cutaneous manifestations of M fortuitum infection can include recurrent cutaneous abscesses, nodular lesions, chronic discharging sinuses, cellulitis, and surgical site infections.4 Although cutaneous infection with M fortuitum classically manifests with a single subcutaneous nodule at the site of trauma or surgery,5 it also can manifest as multiple draining sinus tracts, as seen in our patient. Hence, the diagnosis and treatment of cutaneous NTM infection is challenging, especially when M fortuitum skin manifestations can take up to 4 to 6 weeks to develop after inoculation. Diagnosis often requires a detailed patient history, tissue cultures, and histopathology.5
In recent years, rapid detection with polymerase chain reaction (PCR) techniques has been employed more widely. Notably, a molecular system based on multiplex real-time PCR with high-resolution melting was shown to have a sensitivity of up to 54% for distinguishing M fortuitum from other NTM.6 More recently, a 2-step real-time PCR method has demonstrated diagnostic sensitivity and specificity for differentiating NTM from Mycobacterium tuberculosis infections and identifying the causative NTM agent.7
Compared to immunocompetent individuals, those who are immunocompromised are more susceptible to less pathogenic strains of NTM, which can cause dissemination and lead to tenosynovitis, myositis, osteomyelitis, and septic arthritis.8-12 Nonetheless, cases of infections with NTM—including M fortuitum—are becoming harder to treat. Several single nucleotide polymorphisms and point mutations have been demonstrated in the ribosomal RNA methylase gene erm(39) related to clarithromycin resistance and in the rrl gene related to linezolid resistance.13 Due to increasing inducible resistance to common classes of antibiotics, such as macrolides and linezolid, treatment of M fortuitum requires multidrug regimens.13,14 Drug susceptibility testing also may be required, as M fortuitum has shown low resistance to tigecycline, tetracycline, cefmetazole, imipenem, and aminoglycosides (eg, amikacin, tobramycin, neomycin, gentamycin). Surgery is an important adjunctive tool in treating M fortuitum infections; patients with a single lesion are more likely to undergo surgical treatment alone or in combination with antibiotic therapy.15 More recently, antimicrobial photodynamic therapy has been explored as an alternative to eliminate NTM, including M fortuitum.16
The differential diagnosis for skin lesions manifesting with draining fistulae and sinus tracts includes conditions with infectious (cellulitis and chromomycosis) and inflammatory (pyoderma gangrenosum [PG] and hidradenitis suppurativa [HS]) causes.
Cellulitis is a common infection of the skin and subcutaneous tissue that predominantly is caused by gram-positive organisms such as β-hemolytic streptococci.17 Clinical manifestations include acute skin erythema, swelling, tenderness, and warmth. The legs are the most common sites of infection, but any area of the skin can be involved.17 Cellulitis comprises 10% of all infectious disease hospitalizations and up to 11% of all dermatologic admissions.18,19 It frequently is misdiagnosed, perhaps due to the lack of a reliable confirmatory laboratory test or imaging study, in addition to the plethora of diseases that mimic cellulitis, such as stasis dermatitis, lipodermatosclerosis, contact dermatitis, lymphedema, eosinophilic cellulitis, and papular urticaria.20,21 The consequences of misdiagnosis include but are not limited to unnecessary hospitalizations, inappropriate antibiotic use, and delayed management of the disease; thus, there is an urgent need for a reliable standard test to confirm the diagnosis, especially among nonspecialist physicians. 20 Most patients with uncomplicated cellulitis can be treated with empiric oral antibiotics that target β-hemolytic streptococci (ie, penicillin V potassium, amoxicillin).17 Methicillin-resistant Staphylococcus aureus coverage generally is unnecessary for nonpurulent cellulitis, but clinicians can consider adding amoxicillin-clavulanate, dicloxacillin, and cephalexin to the regimen. For purulent cellulitis, incision and drainage should be performed. In severe cases that manifest with sepsis, altered mental status, or hemodynamic instability, inpatient management is required.17
Chromomycosis (also known as chromoblastomycosis) is a chronic, indolent, granulomatous, suppurative mycosis of the skin and subcutaneous tissue22 that is caused by traumatic inoculation of various fungi of the order Chaetothyriales and family Herpotrichiellaceae, which are present in soil, plants, and decomposing wood. Chromomycosis is prevalent in tropical and subtropical regions.23,24 Clinically, it manifests as oligosymptomatic or asymptomatic lesions around an infection site that can manifest as papules with centrifugal growth evolving into nodular, verrucous, plaque, tumoral, or atrophic forms.22 Diagnosis is made with direct microscopy using potassium hydroxide, which reveals muriform bodies. Fungal culture in Sabouraud agar also can be used to isolate the causative pathogen.22 Unfortunately, chromomycosis is difficult to treat, with low cure rates and high relapse rates. Antifungal agents combined with surgery, cryotherapy, or thermotherapy often are used, with cure rates ranging from 15% to 80%.22,25
Pyoderma gangrenosum is a reactive noninfectious inflammatory dermatosis associated with inflammatory bowel disease and rheumatoid arthritis. The exact etiology is not clearly understood, but it generally is considered an autoinflammatory disorder.26 The most common form—classical PG—occurs in approximately 85% of cases and manifests as a painful erythematous lesion that progresses to a blistered or necrotic ulcer. It primarily affects the lower legs but can occur in other body sites.27 The diagnosis is based on clinical symptoms after excluding other similar conditions; histopathology of biopsied wound tissues often are required for confirmation. Treatment of PG starts with fast-acting immunosuppressive drugs (corticosteroids and/or cyclosporine) followed by slowacting immunosuppressive drugs (biologics).26
Hidradenitis suppurativa is a chronic recurrent disease of the hair follicle unit that develops after puberty.28 Clinically, HS manifests with painful nodules, abscesses, chronically draining fistulas, and scarring in areas of the body rich in apocrine glands.29,30 Treatment of HS is challenging due to its diverse clinical manifestations and unclear etiology. Topical therapy, systemic treatments, biologic agents, surgery, and light therapy have shown variable results.28,31
- Franco-Paredes C, Marcos LA, Henao-Martínez AF, et al. Cutaneous mycobacterial infections. Clin Microbiol Rev. 2018;32: E00069-18. doi:10.1128/CMR.00069-18
- Brown TH. The rapidly growing mycobacteria—MycobacteriuM fortuitum and Mycobacterium chelonae. Infect Control. 1985;6:283-238. doi:10.1017/s0195941700061762
- Hooper J; Beltrami EJ; Santoro F; et al. Remember the fite: a case of cutaneous MycobacteriuM fortuitum infection. Am J Dermatopathol. 2023;45:214-215. doi:10.1097/DAD.0000000000002336
- Franco-Paredes C, Chastain DB, Allen L, et al. Overview of cutaneous mycobacterial infections. Curr Trop Med Rep. 2018;5:228-232. doi:10.1007/s40475-018-0161-7
- Gonzalez-Santiago TM, Drage LA. Nontuberculous mycobacteria: skin and soft tissue infections. Dermatol Clin. 2015;33:563-77. doi:10.1016/j.det.2015.03.017
- Peixoto ADS, Montenegro LML, Lima AS, et al. Identification of nontuberculous mycobacteria species by multiplex real-time PCR with high-resolution melting. Rev Soc Bras Med Trop. 2020;53:E20200211. doi:10.1590/0037-8682-0211-2020
- Park J, Kwak N, Chae JC, et al. A two-step real-time PCR method to identify Mycobacterium tuberculosis infections and six dominant nontuberculous mycobacterial infections from clinical specimens. Microbiol Spectr. 2023:E0160623. doi:10.1128/spectrum.01606-23
- Fowler J, Mahlen SD. Localized cutaneous infections in immunocompetent individuals due to rapidly growing mycobacteria. Arch Pathol Lab Med. 2014;138:1106-1109. doi:10.5858/arpa.2012-0203-RS
- Gardini G, Gregori N, Matteelli A, et al. Mycobacterial skin infection. Curr Opin Infect Dis. 2022;35:79-87. doi:10.1097/QCO.0000000000000820
- Wang SH, Pancholi P. Mycobacterial skin and soft tissue infection. Curr Infect Dis Rep. 2014;16:438. doi:10.1007/s11908-014-0438-5
- Griffith DE, Aksamit T, Brown-Elliott BA, et al; ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416. doi:10.1164/rccm.200604-571ST
- Mougari F, Guglielmetti L, Raskine L, et al. Infections caused by Mycobacterium abscessus: epidemiology, diagnostic tools and treatment. Expert Rev Anti Infect Ther. 2016;14:1139-1154. doi:10.1080/14787210.201 6.1238304
- Tu HZ, Lee HS, Chen YS, et al. High rates of antimicrobial resistance in rapidly growing mycobacterial infections in Taiwan. Pathogens. 2022;11:969. doi:10.3390/pathogens11090969
- Hashemzadeh M, Zadegan Dezfuli AA, Khosravi AD, et al. F requency of mutations in erm(39) related to clarithromycin resistance and in rrl related to linezolid resistance in clinical isolates of MycobacteriuM fortuitum in Iran. Acta Microbiol Immunol Hung. 2023;70:167-176. doi:10.1556/030.2023.02020
- Uslan DZ, Kowalski TJ, Wengenack NL, et al. Skin and soft tissue infections due to rapidly growing mycobacteria: comparison of clinical features, treatment, and susceptibility. Arch Dermatol. 2006;142:1287-1292. doi:10.1001/archderm.142.10.1287
- Miretti M, Juri L, Peralta A, et al. Photoinactivation of non-tuberculous mycobacteria using Zn-phthalocyanine loaded into liposomes. Tuberculosis (Edinb). 2022;136:102247. doi:10.1016/j.tube.2022.102247
- Bystritsky RJ. Cellulitis. Infect Dis Clin North Am. 2021;35:49-60. doi:10.1016/j.idc.2020.10.002
- Christensen K, Holman R, Steiner C, et al. Infectious disease hospitalizations in the United States. Clin Infect Dis. 2009;49:1025-1035. doi:10.1086/605562
- Yang JJ, Maloney NJ, Bach DQ, et al. Dermatology in the emergency department: prescriptions, rates of inpatient admission, and predictors of high utilization in the United States from 1996 to 2012. J Am Acad Dermatol. 2021;84:1480-1483. doi:10.1016/J.JAAD.2020.07.055
- Cutler TS, Jannat-Khah DP, Kam B, et al. Prevalence of misdiagnosis of cellulitis: a systematic review and meta-analysis. J Hosp Med. 2023;18:254-261. doi:10.1002/jhm.12977
- Keller EC, Tomecki KJ, Alraies MC. Distinguishing cellulitis from its mimics. Cleve Clin J Med. 2012;79:547-52. doi:10.3949/ccjm.79a.11121
- Brito AC, Bittencourt MJS. Chromoblastomycosis: an etiological, epidemiological, clinical, diagnostic, and treatment update. An Bras Dermatol. 2018;93:495-506. doi:10.1590/abd1806-4841.20187321
- McGinnis MR. Chromoblastomycosis and phaeohyphomycosis: new concepts, diagnosis, and mycology. J Am Acad Dermatol. 1983;8:1-16.
- Rubin HA, Bruce S, Rosen T, et al. Evidence for percutaneous inoculation as the mode of transmission for chromoblastomycosis. J Am Acad Dermatol. 1991;25:951-954.
- Bonifaz A, Paredes-Solís V, Saúl A. Treating chromoblastomycosis with systemic antifungals. Expert Opin Pharmacother. 2004;5:247-254.
- Maverakis E, Marzano AV, Le ST, et al. Pyoderma gangrenosum. Nat Rev Dis Primers. 2020;6:81. doi:10.1038/s41572-020-0213-x
- George C, Deroide F, Rustin M. Pyoderma gangrenosum—a guide to diagnosis and management. Clin Med (Lond). 2019;19:224-228. doi:10.7861/clinmedicine.19-3-224
- Narla S, Lyons AB, Hamzavi IH. The most recent advances in understanding and managing hidradenitis suppurativa. F1000Res. 2020;9:F1000 Faculty Rev-1049. doi:10.12688/f1000research.26083.1
- Garg A, Lavian J, Lin G, et al. Incidence of hidradenitis suppurativa in the United States: a sex- and age-adjusted population analysis. J Am Acad Dermatol. 2017;77:118-122. doi:10.1016/j.jaad.2017.02.005
- Daxhelet M, Suppa M, White J, et al. Proposed definitions of typical lesions in hidradenitis suppurativa. Dermatology. 2020;236:431-438. doi:10.1159/000507348
- Amat-Samaranch V, Agut-Busquet E, Vilarrasa E, et al. New perspectives on the treatment of hidradenitis suppurativa. Ther Adv Chronic Dis. 2021;12:20406223211055920. doi:10.1177/20406223211055920
The Diagnosis: Mycobacterial Infection
An injury sustained in a wet environment that results in chronic indolent abscesses, nodules, or draining sinus tracts suggests a mycobacterial infection. In our patient, a culture revealed MycobacteriuM fortuitum, which is classified in the rapid grower nontuberculous mycobacteria (NTM) group, along with Mycobacterium chelonae and Mycobacterium abscessus.1 The patient’s history of skin injury while cutting wet grass and the common presence of M fortuitum in the environment suggested that the organism entered the wound. The patient healed completely following surgical excision and a 2-month course of clarithromycin 1 g daily and rifampin 600 mg daily.
MycobacteriuM fortuitum was first isolated from an amphibian source in 1905 and later identified in a human with cutaneous infection in 1938. It commonly is found in soil and water.2 Skin and soft-tissue infections with M fortuitum usually are acquired from direct entry of the organism through a damaged skin barrier from trauma, medical injection, surgery, or tattoo placement.2,3
Skin lesions caused by NTM often are nonspecific and can mimic a variety of other dermatologic conditions, making clinical diagnosis challenging. As such, cutaneous manifestations of M fortuitum infection can include recurrent cutaneous abscesses, nodular lesions, chronic discharging sinuses, cellulitis, and surgical site infections.4 Although cutaneous infection with M fortuitum classically manifests with a single subcutaneous nodule at the site of trauma or surgery,5 it also can manifest as multiple draining sinus tracts, as seen in our patient. Hence, the diagnosis and treatment of cutaneous NTM infection is challenging, especially when M fortuitum skin manifestations can take up to 4 to 6 weeks to develop after inoculation. Diagnosis often requires a detailed patient history, tissue cultures, and histopathology.5
In recent years, rapid detection with polymerase chain reaction (PCR) techniques has been employed more widely. Notably, a molecular system based on multiplex real-time PCR with high-resolution melting was shown to have a sensitivity of up to 54% for distinguishing M fortuitum from other NTM.6 More recently, a 2-step real-time PCR method has demonstrated diagnostic sensitivity and specificity for differentiating NTM from Mycobacterium tuberculosis infections and identifying the causative NTM agent.7
Compared to immunocompetent individuals, those who are immunocompromised are more susceptible to less pathogenic strains of NTM, which can cause dissemination and lead to tenosynovitis, myositis, osteomyelitis, and septic arthritis.8-12 Nonetheless, cases of infections with NTM—including M fortuitum—are becoming harder to treat. Several single nucleotide polymorphisms and point mutations have been demonstrated in the ribosomal RNA methylase gene erm(39) related to clarithromycin resistance and in the rrl gene related to linezolid resistance.13 Due to increasing inducible resistance to common classes of antibiotics, such as macrolides and linezolid, treatment of M fortuitum requires multidrug regimens.13,14 Drug susceptibility testing also may be required, as M fortuitum has shown low resistance to tigecycline, tetracycline, cefmetazole, imipenem, and aminoglycosides (eg, amikacin, tobramycin, neomycin, gentamycin). Surgery is an important adjunctive tool in treating M fortuitum infections; patients with a single lesion are more likely to undergo surgical treatment alone or in combination with antibiotic therapy.15 More recently, antimicrobial photodynamic therapy has been explored as an alternative to eliminate NTM, including M fortuitum.16
The differential diagnosis for skin lesions manifesting with draining fistulae and sinus tracts includes conditions with infectious (cellulitis and chromomycosis) and inflammatory (pyoderma gangrenosum [PG] and hidradenitis suppurativa [HS]) causes.
Cellulitis is a common infection of the skin and subcutaneous tissue that predominantly is caused by gram-positive organisms such as β-hemolytic streptococci.17 Clinical manifestations include acute skin erythema, swelling, tenderness, and warmth. The legs are the most common sites of infection, but any area of the skin can be involved.17 Cellulitis comprises 10% of all infectious disease hospitalizations and up to 11% of all dermatologic admissions.18,19 It frequently is misdiagnosed, perhaps due to the lack of a reliable confirmatory laboratory test or imaging study, in addition to the plethora of diseases that mimic cellulitis, such as stasis dermatitis, lipodermatosclerosis, contact dermatitis, lymphedema, eosinophilic cellulitis, and papular urticaria.20,21 The consequences of misdiagnosis include but are not limited to unnecessary hospitalizations, inappropriate antibiotic use, and delayed management of the disease; thus, there is an urgent need for a reliable standard test to confirm the diagnosis, especially among nonspecialist physicians. 20 Most patients with uncomplicated cellulitis can be treated with empiric oral antibiotics that target β-hemolytic streptococci (ie, penicillin V potassium, amoxicillin).17 Methicillin-resistant Staphylococcus aureus coverage generally is unnecessary for nonpurulent cellulitis, but clinicians can consider adding amoxicillin-clavulanate, dicloxacillin, and cephalexin to the regimen. For purulent cellulitis, incision and drainage should be performed. In severe cases that manifest with sepsis, altered mental status, or hemodynamic instability, inpatient management is required.17
Chromomycosis (also known as chromoblastomycosis) is a chronic, indolent, granulomatous, suppurative mycosis of the skin and subcutaneous tissue22 that is caused by traumatic inoculation of various fungi of the order Chaetothyriales and family Herpotrichiellaceae, which are present in soil, plants, and decomposing wood. Chromomycosis is prevalent in tropical and subtropical regions.23,24 Clinically, it manifests as oligosymptomatic or asymptomatic lesions around an infection site that can manifest as papules with centrifugal growth evolving into nodular, verrucous, plaque, tumoral, or atrophic forms.22 Diagnosis is made with direct microscopy using potassium hydroxide, which reveals muriform bodies. Fungal culture in Sabouraud agar also can be used to isolate the causative pathogen.22 Unfortunately, chromomycosis is difficult to treat, with low cure rates and high relapse rates. Antifungal agents combined with surgery, cryotherapy, or thermotherapy often are used, with cure rates ranging from 15% to 80%.22,25
Pyoderma gangrenosum is a reactive noninfectious inflammatory dermatosis associated with inflammatory bowel disease and rheumatoid arthritis. The exact etiology is not clearly understood, but it generally is considered an autoinflammatory disorder.26 The most common form—classical PG—occurs in approximately 85% of cases and manifests as a painful erythematous lesion that progresses to a blistered or necrotic ulcer. It primarily affects the lower legs but can occur in other body sites.27 The diagnosis is based on clinical symptoms after excluding other similar conditions; histopathology of biopsied wound tissues often are required for confirmation. Treatment of PG starts with fast-acting immunosuppressive drugs (corticosteroids and/or cyclosporine) followed by slowacting immunosuppressive drugs (biologics).26
Hidradenitis suppurativa is a chronic recurrent disease of the hair follicle unit that develops after puberty.28 Clinically, HS manifests with painful nodules, abscesses, chronically draining fistulas, and scarring in areas of the body rich in apocrine glands.29,30 Treatment of HS is challenging due to its diverse clinical manifestations and unclear etiology. Topical therapy, systemic treatments, biologic agents, surgery, and light therapy have shown variable results.28,31
The Diagnosis: Mycobacterial Infection
An injury sustained in a wet environment that results in chronic indolent abscesses, nodules, or draining sinus tracts suggests a mycobacterial infection. In our patient, a culture revealed MycobacteriuM fortuitum, which is classified in the rapid grower nontuberculous mycobacteria (NTM) group, along with Mycobacterium chelonae and Mycobacterium abscessus.1 The patient’s history of skin injury while cutting wet grass and the common presence of M fortuitum in the environment suggested that the organism entered the wound. The patient healed completely following surgical excision and a 2-month course of clarithromycin 1 g daily and rifampin 600 mg daily.
MycobacteriuM fortuitum was first isolated from an amphibian source in 1905 and later identified in a human with cutaneous infection in 1938. It commonly is found in soil and water.2 Skin and soft-tissue infections with M fortuitum usually are acquired from direct entry of the organism through a damaged skin barrier from trauma, medical injection, surgery, or tattoo placement.2,3
Skin lesions caused by NTM often are nonspecific and can mimic a variety of other dermatologic conditions, making clinical diagnosis challenging. As such, cutaneous manifestations of M fortuitum infection can include recurrent cutaneous abscesses, nodular lesions, chronic discharging sinuses, cellulitis, and surgical site infections.4 Although cutaneous infection with M fortuitum classically manifests with a single subcutaneous nodule at the site of trauma or surgery,5 it also can manifest as multiple draining sinus tracts, as seen in our patient. Hence, the diagnosis and treatment of cutaneous NTM infection is challenging, especially when M fortuitum skin manifestations can take up to 4 to 6 weeks to develop after inoculation. Diagnosis often requires a detailed patient history, tissue cultures, and histopathology.5
In recent years, rapid detection with polymerase chain reaction (PCR) techniques has been employed more widely. Notably, a molecular system based on multiplex real-time PCR with high-resolution melting was shown to have a sensitivity of up to 54% for distinguishing M fortuitum from other NTM.6 More recently, a 2-step real-time PCR method has demonstrated diagnostic sensitivity and specificity for differentiating NTM from Mycobacterium tuberculosis infections and identifying the causative NTM agent.7
Compared to immunocompetent individuals, those who are immunocompromised are more susceptible to less pathogenic strains of NTM, which can cause dissemination and lead to tenosynovitis, myositis, osteomyelitis, and septic arthritis.8-12 Nonetheless, cases of infections with NTM—including M fortuitum—are becoming harder to treat. Several single nucleotide polymorphisms and point mutations have been demonstrated in the ribosomal RNA methylase gene erm(39) related to clarithromycin resistance and in the rrl gene related to linezolid resistance.13 Due to increasing inducible resistance to common classes of antibiotics, such as macrolides and linezolid, treatment of M fortuitum requires multidrug regimens.13,14 Drug susceptibility testing also may be required, as M fortuitum has shown low resistance to tigecycline, tetracycline, cefmetazole, imipenem, and aminoglycosides (eg, amikacin, tobramycin, neomycin, gentamycin). Surgery is an important adjunctive tool in treating M fortuitum infections; patients with a single lesion are more likely to undergo surgical treatment alone or in combination with antibiotic therapy.15 More recently, antimicrobial photodynamic therapy has been explored as an alternative to eliminate NTM, including M fortuitum.16
The differential diagnosis for skin lesions manifesting with draining fistulae and sinus tracts includes conditions with infectious (cellulitis and chromomycosis) and inflammatory (pyoderma gangrenosum [PG] and hidradenitis suppurativa [HS]) causes.
Cellulitis is a common infection of the skin and subcutaneous tissue that predominantly is caused by gram-positive organisms such as β-hemolytic streptococci.17 Clinical manifestations include acute skin erythema, swelling, tenderness, and warmth. The legs are the most common sites of infection, but any area of the skin can be involved.17 Cellulitis comprises 10% of all infectious disease hospitalizations and up to 11% of all dermatologic admissions.18,19 It frequently is misdiagnosed, perhaps due to the lack of a reliable confirmatory laboratory test or imaging study, in addition to the plethora of diseases that mimic cellulitis, such as stasis dermatitis, lipodermatosclerosis, contact dermatitis, lymphedema, eosinophilic cellulitis, and papular urticaria.20,21 The consequences of misdiagnosis include but are not limited to unnecessary hospitalizations, inappropriate antibiotic use, and delayed management of the disease; thus, there is an urgent need for a reliable standard test to confirm the diagnosis, especially among nonspecialist physicians. 20 Most patients with uncomplicated cellulitis can be treated with empiric oral antibiotics that target β-hemolytic streptococci (ie, penicillin V potassium, amoxicillin).17 Methicillin-resistant Staphylococcus aureus coverage generally is unnecessary for nonpurulent cellulitis, but clinicians can consider adding amoxicillin-clavulanate, dicloxacillin, and cephalexin to the regimen. For purulent cellulitis, incision and drainage should be performed. In severe cases that manifest with sepsis, altered mental status, or hemodynamic instability, inpatient management is required.17
Chromomycosis (also known as chromoblastomycosis) is a chronic, indolent, granulomatous, suppurative mycosis of the skin and subcutaneous tissue22 that is caused by traumatic inoculation of various fungi of the order Chaetothyriales and family Herpotrichiellaceae, which are present in soil, plants, and decomposing wood. Chromomycosis is prevalent in tropical and subtropical regions.23,24 Clinically, it manifests as oligosymptomatic or asymptomatic lesions around an infection site that can manifest as papules with centrifugal growth evolving into nodular, verrucous, plaque, tumoral, or atrophic forms.22 Diagnosis is made with direct microscopy using potassium hydroxide, which reveals muriform bodies. Fungal culture in Sabouraud agar also can be used to isolate the causative pathogen.22 Unfortunately, chromomycosis is difficult to treat, with low cure rates and high relapse rates. Antifungal agents combined with surgery, cryotherapy, or thermotherapy often are used, with cure rates ranging from 15% to 80%.22,25
Pyoderma gangrenosum is a reactive noninfectious inflammatory dermatosis associated with inflammatory bowel disease and rheumatoid arthritis. The exact etiology is not clearly understood, but it generally is considered an autoinflammatory disorder.26 The most common form—classical PG—occurs in approximately 85% of cases and manifests as a painful erythematous lesion that progresses to a blistered or necrotic ulcer. It primarily affects the lower legs but can occur in other body sites.27 The diagnosis is based on clinical symptoms after excluding other similar conditions; histopathology of biopsied wound tissues often are required for confirmation. Treatment of PG starts with fast-acting immunosuppressive drugs (corticosteroids and/or cyclosporine) followed by slowacting immunosuppressive drugs (biologics).26
Hidradenitis suppurativa is a chronic recurrent disease of the hair follicle unit that develops after puberty.28 Clinically, HS manifests with painful nodules, abscesses, chronically draining fistulas, and scarring in areas of the body rich in apocrine glands.29,30 Treatment of HS is challenging due to its diverse clinical manifestations and unclear etiology. Topical therapy, systemic treatments, biologic agents, surgery, and light therapy have shown variable results.28,31
- Franco-Paredes C, Marcos LA, Henao-Martínez AF, et al. Cutaneous mycobacterial infections. Clin Microbiol Rev. 2018;32: E00069-18. doi:10.1128/CMR.00069-18
- Brown TH. The rapidly growing mycobacteria—MycobacteriuM fortuitum and Mycobacterium chelonae. Infect Control. 1985;6:283-238. doi:10.1017/s0195941700061762
- Hooper J; Beltrami EJ; Santoro F; et al. Remember the fite: a case of cutaneous MycobacteriuM fortuitum infection. Am J Dermatopathol. 2023;45:214-215. doi:10.1097/DAD.0000000000002336
- Franco-Paredes C, Chastain DB, Allen L, et al. Overview of cutaneous mycobacterial infections. Curr Trop Med Rep. 2018;5:228-232. doi:10.1007/s40475-018-0161-7
- Gonzalez-Santiago TM, Drage LA. Nontuberculous mycobacteria: skin and soft tissue infections. Dermatol Clin. 2015;33:563-77. doi:10.1016/j.det.2015.03.017
- Peixoto ADS, Montenegro LML, Lima AS, et al. Identification of nontuberculous mycobacteria species by multiplex real-time PCR with high-resolution melting. Rev Soc Bras Med Trop. 2020;53:E20200211. doi:10.1590/0037-8682-0211-2020
- Park J, Kwak N, Chae JC, et al. A two-step real-time PCR method to identify Mycobacterium tuberculosis infections and six dominant nontuberculous mycobacterial infections from clinical specimens. Microbiol Spectr. 2023:E0160623. doi:10.1128/spectrum.01606-23
- Fowler J, Mahlen SD. Localized cutaneous infections in immunocompetent individuals due to rapidly growing mycobacteria. Arch Pathol Lab Med. 2014;138:1106-1109. doi:10.5858/arpa.2012-0203-RS
- Gardini G, Gregori N, Matteelli A, et al. Mycobacterial skin infection. Curr Opin Infect Dis. 2022;35:79-87. doi:10.1097/QCO.0000000000000820
- Wang SH, Pancholi P. Mycobacterial skin and soft tissue infection. Curr Infect Dis Rep. 2014;16:438. doi:10.1007/s11908-014-0438-5
- Griffith DE, Aksamit T, Brown-Elliott BA, et al; ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416. doi:10.1164/rccm.200604-571ST
- Mougari F, Guglielmetti L, Raskine L, et al. Infections caused by Mycobacterium abscessus: epidemiology, diagnostic tools and treatment. Expert Rev Anti Infect Ther. 2016;14:1139-1154. doi:10.1080/14787210.201 6.1238304
- Tu HZ, Lee HS, Chen YS, et al. High rates of antimicrobial resistance in rapidly growing mycobacterial infections in Taiwan. Pathogens. 2022;11:969. doi:10.3390/pathogens11090969
- Hashemzadeh M, Zadegan Dezfuli AA, Khosravi AD, et al. F requency of mutations in erm(39) related to clarithromycin resistance and in rrl related to linezolid resistance in clinical isolates of MycobacteriuM fortuitum in Iran. Acta Microbiol Immunol Hung. 2023;70:167-176. doi:10.1556/030.2023.02020
- Uslan DZ, Kowalski TJ, Wengenack NL, et al. Skin and soft tissue infections due to rapidly growing mycobacteria: comparison of clinical features, treatment, and susceptibility. Arch Dermatol. 2006;142:1287-1292. doi:10.1001/archderm.142.10.1287
- Miretti M, Juri L, Peralta A, et al. Photoinactivation of non-tuberculous mycobacteria using Zn-phthalocyanine loaded into liposomes. Tuberculosis (Edinb). 2022;136:102247. doi:10.1016/j.tube.2022.102247
- Bystritsky RJ. Cellulitis. Infect Dis Clin North Am. 2021;35:49-60. doi:10.1016/j.idc.2020.10.002
- Christensen K, Holman R, Steiner C, et al. Infectious disease hospitalizations in the United States. Clin Infect Dis. 2009;49:1025-1035. doi:10.1086/605562
- Yang JJ, Maloney NJ, Bach DQ, et al. Dermatology in the emergency department: prescriptions, rates of inpatient admission, and predictors of high utilization in the United States from 1996 to 2012. J Am Acad Dermatol. 2021;84:1480-1483. doi:10.1016/J.JAAD.2020.07.055
- Cutler TS, Jannat-Khah DP, Kam B, et al. Prevalence of misdiagnosis of cellulitis: a systematic review and meta-analysis. J Hosp Med. 2023;18:254-261. doi:10.1002/jhm.12977
- Keller EC, Tomecki KJ, Alraies MC. Distinguishing cellulitis from its mimics. Cleve Clin J Med. 2012;79:547-52. doi:10.3949/ccjm.79a.11121
- Brito AC, Bittencourt MJS. Chromoblastomycosis: an etiological, epidemiological, clinical, diagnostic, and treatment update. An Bras Dermatol. 2018;93:495-506. doi:10.1590/abd1806-4841.20187321
- McGinnis MR. Chromoblastomycosis and phaeohyphomycosis: new concepts, diagnosis, and mycology. J Am Acad Dermatol. 1983;8:1-16.
- Rubin HA, Bruce S, Rosen T, et al. Evidence for percutaneous inoculation as the mode of transmission for chromoblastomycosis. J Am Acad Dermatol. 1991;25:951-954.
- Bonifaz A, Paredes-Solís V, Saúl A. Treating chromoblastomycosis with systemic antifungals. Expert Opin Pharmacother. 2004;5:247-254.
- Maverakis E, Marzano AV, Le ST, et al. Pyoderma gangrenosum. Nat Rev Dis Primers. 2020;6:81. doi:10.1038/s41572-020-0213-x
- George C, Deroide F, Rustin M. Pyoderma gangrenosum—a guide to diagnosis and management. Clin Med (Lond). 2019;19:224-228. doi:10.7861/clinmedicine.19-3-224
- Narla S, Lyons AB, Hamzavi IH. The most recent advances in understanding and managing hidradenitis suppurativa. F1000Res. 2020;9:F1000 Faculty Rev-1049. doi:10.12688/f1000research.26083.1
- Garg A, Lavian J, Lin G, et al. Incidence of hidradenitis suppurativa in the United States: a sex- and age-adjusted population analysis. J Am Acad Dermatol. 2017;77:118-122. doi:10.1016/j.jaad.2017.02.005
- Daxhelet M, Suppa M, White J, et al. Proposed definitions of typical lesions in hidradenitis suppurativa. Dermatology. 2020;236:431-438. doi:10.1159/000507348
- Amat-Samaranch V, Agut-Busquet E, Vilarrasa E, et al. New perspectives on the treatment of hidradenitis suppurativa. Ther Adv Chronic Dis. 2021;12:20406223211055920. doi:10.1177/20406223211055920
- Franco-Paredes C, Marcos LA, Henao-Martínez AF, et al. Cutaneous mycobacterial infections. Clin Microbiol Rev. 2018;32: E00069-18. doi:10.1128/CMR.00069-18
- Brown TH. The rapidly growing mycobacteria—MycobacteriuM fortuitum and Mycobacterium chelonae. Infect Control. 1985;6:283-238. doi:10.1017/s0195941700061762
- Hooper J; Beltrami EJ; Santoro F; et al. Remember the fite: a case of cutaneous MycobacteriuM fortuitum infection. Am J Dermatopathol. 2023;45:214-215. doi:10.1097/DAD.0000000000002336
- Franco-Paredes C, Chastain DB, Allen L, et al. Overview of cutaneous mycobacterial infections. Curr Trop Med Rep. 2018;5:228-232. doi:10.1007/s40475-018-0161-7
- Gonzalez-Santiago TM, Drage LA. Nontuberculous mycobacteria: skin and soft tissue infections. Dermatol Clin. 2015;33:563-77. doi:10.1016/j.det.2015.03.017
- Peixoto ADS, Montenegro LML, Lima AS, et al. Identification of nontuberculous mycobacteria species by multiplex real-time PCR with high-resolution melting. Rev Soc Bras Med Trop. 2020;53:E20200211. doi:10.1590/0037-8682-0211-2020
- Park J, Kwak N, Chae JC, et al. A two-step real-time PCR method to identify Mycobacterium tuberculosis infections and six dominant nontuberculous mycobacterial infections from clinical specimens. Microbiol Spectr. 2023:E0160623. doi:10.1128/spectrum.01606-23
- Fowler J, Mahlen SD. Localized cutaneous infections in immunocompetent individuals due to rapidly growing mycobacteria. Arch Pathol Lab Med. 2014;138:1106-1109. doi:10.5858/arpa.2012-0203-RS
- Gardini G, Gregori N, Matteelli A, et al. Mycobacterial skin infection. Curr Opin Infect Dis. 2022;35:79-87. doi:10.1097/QCO.0000000000000820
- Wang SH, Pancholi P. Mycobacterial skin and soft tissue infection. Curr Infect Dis Rep. 2014;16:438. doi:10.1007/s11908-014-0438-5
- Griffith DE, Aksamit T, Brown-Elliott BA, et al; ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416. doi:10.1164/rccm.200604-571ST
- Mougari F, Guglielmetti L, Raskine L, et al. Infections caused by Mycobacterium abscessus: epidemiology, diagnostic tools and treatment. Expert Rev Anti Infect Ther. 2016;14:1139-1154. doi:10.1080/14787210.201 6.1238304
- Tu HZ, Lee HS, Chen YS, et al. High rates of antimicrobial resistance in rapidly growing mycobacterial infections in Taiwan. Pathogens. 2022;11:969. doi:10.3390/pathogens11090969
- Hashemzadeh M, Zadegan Dezfuli AA, Khosravi AD, et al. F requency of mutations in erm(39) related to clarithromycin resistance and in rrl related to linezolid resistance in clinical isolates of MycobacteriuM fortuitum in Iran. Acta Microbiol Immunol Hung. 2023;70:167-176. doi:10.1556/030.2023.02020
- Uslan DZ, Kowalski TJ, Wengenack NL, et al. Skin and soft tissue infections due to rapidly growing mycobacteria: comparison of clinical features, treatment, and susceptibility. Arch Dermatol. 2006;142:1287-1292. doi:10.1001/archderm.142.10.1287
- Miretti M, Juri L, Peralta A, et al. Photoinactivation of non-tuberculous mycobacteria using Zn-phthalocyanine loaded into liposomes. Tuberculosis (Edinb). 2022;136:102247. doi:10.1016/j.tube.2022.102247
- Bystritsky RJ. Cellulitis. Infect Dis Clin North Am. 2021;35:49-60. doi:10.1016/j.idc.2020.10.002
- Christensen K, Holman R, Steiner C, et al. Infectious disease hospitalizations in the United States. Clin Infect Dis. 2009;49:1025-1035. doi:10.1086/605562
- Yang JJ, Maloney NJ, Bach DQ, et al. Dermatology in the emergency department: prescriptions, rates of inpatient admission, and predictors of high utilization in the United States from 1996 to 2012. J Am Acad Dermatol. 2021;84:1480-1483. doi:10.1016/J.JAAD.2020.07.055
- Cutler TS, Jannat-Khah DP, Kam B, et al. Prevalence of misdiagnosis of cellulitis: a systematic review and meta-analysis. J Hosp Med. 2023;18:254-261. doi:10.1002/jhm.12977
- Keller EC, Tomecki KJ, Alraies MC. Distinguishing cellulitis from its mimics. Cleve Clin J Med. 2012;79:547-52. doi:10.3949/ccjm.79a.11121
- Brito AC, Bittencourt MJS. Chromoblastomycosis: an etiological, epidemiological, clinical, diagnostic, and treatment update. An Bras Dermatol. 2018;93:495-506. doi:10.1590/abd1806-4841.20187321
- McGinnis MR. Chromoblastomycosis and phaeohyphomycosis: new concepts, diagnosis, and mycology. J Am Acad Dermatol. 1983;8:1-16.
- Rubin HA, Bruce S, Rosen T, et al. Evidence for percutaneous inoculation as the mode of transmission for chromoblastomycosis. J Am Acad Dermatol. 1991;25:951-954.
- Bonifaz A, Paredes-Solís V, Saúl A. Treating chromoblastomycosis with systemic antifungals. Expert Opin Pharmacother. 2004;5:247-254.
- Maverakis E, Marzano AV, Le ST, et al. Pyoderma gangrenosum. Nat Rev Dis Primers. 2020;6:81. doi:10.1038/s41572-020-0213-x
- George C, Deroide F, Rustin M. Pyoderma gangrenosum—a guide to diagnosis and management. Clin Med (Lond). 2019;19:224-228. doi:10.7861/clinmedicine.19-3-224
- Narla S, Lyons AB, Hamzavi IH. The most recent advances in understanding and managing hidradenitis suppurativa. F1000Res. 2020;9:F1000 Faculty Rev-1049. doi:10.12688/f1000research.26083.1
- Garg A, Lavian J, Lin G, et al. Incidence of hidradenitis suppurativa in the United States: a sex- and age-adjusted population analysis. J Am Acad Dermatol. 2017;77:118-122. doi:10.1016/j.jaad.2017.02.005
- Daxhelet M, Suppa M, White J, et al. Proposed definitions of typical lesions in hidradenitis suppurativa. Dermatology. 2020;236:431-438. doi:10.1159/000507348
- Amat-Samaranch V, Agut-Busquet E, Vilarrasa E, et al. New perspectives on the treatment of hidradenitis suppurativa. Ther Adv Chronic Dis. 2021;12:20406223211055920. doi:10.1177/20406223211055920
A 40-year-old woman presented with multiple draining sinus tracts on the right thigh following an injury sustained weeks earlier while mowing wet grass.
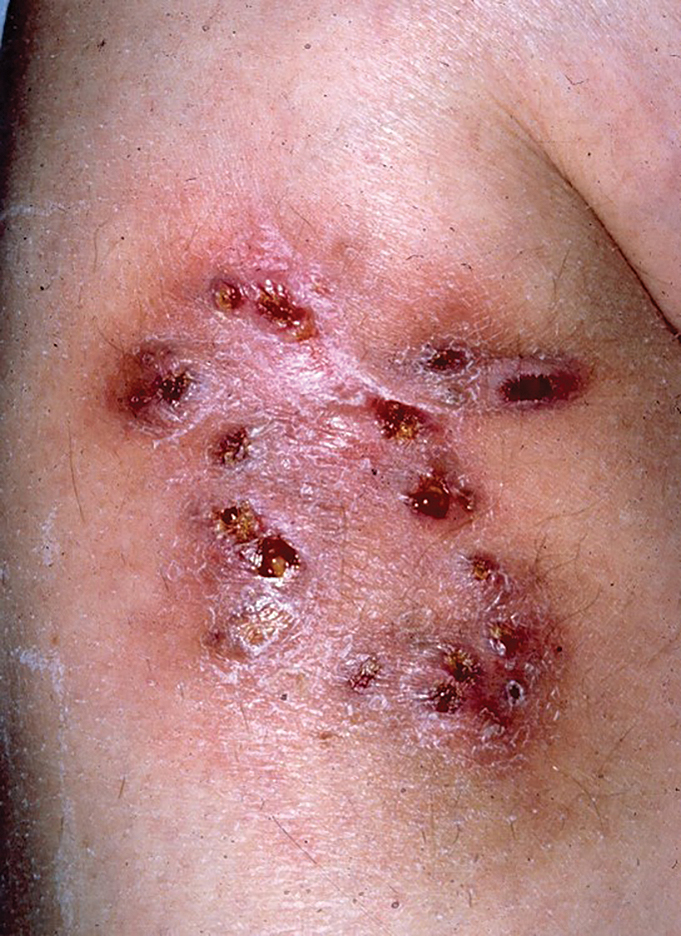
New developments on the forefront of intermediate-risk pulmonary embolism
PULMONARY VASCULAR AND CARDIOVASCULAR NETWORK
Cardiovascular Medicine and Surgery Section
Patients with intermediate-risk pulmonary embolism (IRPE), or those with right ventricular dysfunction without overt hemodynamic instability, represent a heterogenous population with short-term mortality ranging from 2% to 17%.1 While systemic anticoagulation is the mainstay therapy, select individuals may benefit from more immediate reperfusion. Unfortunately, only small, randomized trials exploring surrogate outcomes are available to guide modality and patient selection.2
To better define which patients with IRPE are best managed with which therapy, several large-scale randomized controlled trials are underway. PE-TRACT, a study funded by the National Institutes of Health, aims to randomize 500 patients with IRPE to anticoagulation alone vs one of several modalities of CBT with a focus on long-term functional outcomes, including peak oxygen consumption at 3 months and functional class at 1 year. Aspiration thrombectomy with the FlowTriever® device is being compared with anticoagulation alone in a study of 1,200 patients examining short-term composite end points.
While full-dose thrombolysis may decrease the composite outcome of death or hemodynamic deterioration in this population, the benefit is counterbalanced by the risk of significant bleeding. Whether reduced-dose thrombolysis is associated with improved outcomes has been questioned in several small studies. The PEITHO-3 trial plans to randomize 650 patients with IRPE to reduced-dose thrombolytics vs placebo, exploring several outcomes at 30 days. With multiple large trials ongoing, we anticipate important changes to the landscape of IRPE care over the coming years.
References
1. Fernández C, Bova C, Sanchez O, et al. Validation of a model for identification of patients at intermediate to high risk for complications associated with acute symptomatic pulmonary embolism. Chest. 2015;148(1):211-218. doi:10.1378/chest.14-2551
2. Yuriditsky E, Horowitz JM. The role of the PERT in the management and therapeutic decision-making in pulmonary embolism. Eur Heart J Acute Cardiovasc Care. 2022;11(9):693-694. doi:10.1093/ehjacc/zuac102
3. National Library of Medicine (US). A Randomized Trial of Ultrasound-facilitated, Catheter-directed, Thrombolysis Versus Anticoagulation for Acute Intermediate-high Risk Pulmonary Embolism: The Higher-risk Pulmonary Embolism Thrombolysis Study. Updated July 16, 2024. https://clinicaltrials.gov/study/NCT04790370
4. National Library of Medicine (US). PEERLESS II: RCT of FlowTriever vs. Anticoagulation Alone in Pulmonary Embolism. Updated July 17, 2024. https://clinicaltrials.gov/study/NCT06055920
5. National Library of Medicine (US). A Reduced Dose of Thrombolytic Treatment for Patients With Intermediate High-risk Acute Pulmonary Embolism: a Randomized Controled Trial. Updated July 17, 2024. https://clinicaltrials.gov/study/NCT04430569
PULMONARY VASCULAR AND CARDIOVASCULAR NETWORK
Cardiovascular Medicine and Surgery Section
Patients with intermediate-risk pulmonary embolism (IRPE), or those with right ventricular dysfunction without overt hemodynamic instability, represent a heterogenous population with short-term mortality ranging from 2% to 17%.1 While systemic anticoagulation is the mainstay therapy, select individuals may benefit from more immediate reperfusion. Unfortunately, only small, randomized trials exploring surrogate outcomes are available to guide modality and patient selection.2
To better define which patients with IRPE are best managed with which therapy, several large-scale randomized controlled trials are underway. PE-TRACT, a study funded by the National Institutes of Health, aims to randomize 500 patients with IRPE to anticoagulation alone vs one of several modalities of CBT with a focus on long-term functional outcomes, including peak oxygen consumption at 3 months and functional class at 1 year. Aspiration thrombectomy with the FlowTriever® device is being compared with anticoagulation alone in a study of 1,200 patients examining short-term composite end points.
While full-dose thrombolysis may decrease the composite outcome of death or hemodynamic deterioration in this population, the benefit is counterbalanced by the risk of significant bleeding. Whether reduced-dose thrombolysis is associated with improved outcomes has been questioned in several small studies. The PEITHO-3 trial plans to randomize 650 patients with IRPE to reduced-dose thrombolytics vs placebo, exploring several outcomes at 30 days. With multiple large trials ongoing, we anticipate important changes to the landscape of IRPE care over the coming years.
References
1. Fernández C, Bova C, Sanchez O, et al. Validation of a model for identification of patients at intermediate to high risk for complications associated with acute symptomatic pulmonary embolism. Chest. 2015;148(1):211-218. doi:10.1378/chest.14-2551
2. Yuriditsky E, Horowitz JM. The role of the PERT in the management and therapeutic decision-making in pulmonary embolism. Eur Heart J Acute Cardiovasc Care. 2022;11(9):693-694. doi:10.1093/ehjacc/zuac102
3. National Library of Medicine (US). A Randomized Trial of Ultrasound-facilitated, Catheter-directed, Thrombolysis Versus Anticoagulation for Acute Intermediate-high Risk Pulmonary Embolism: The Higher-risk Pulmonary Embolism Thrombolysis Study. Updated July 16, 2024. https://clinicaltrials.gov/study/NCT04790370
4. National Library of Medicine (US). PEERLESS II: RCT of FlowTriever vs. Anticoagulation Alone in Pulmonary Embolism. Updated July 17, 2024. https://clinicaltrials.gov/study/NCT06055920
5. National Library of Medicine (US). A Reduced Dose of Thrombolytic Treatment for Patients With Intermediate High-risk Acute Pulmonary Embolism: a Randomized Controled Trial. Updated July 17, 2024. https://clinicaltrials.gov/study/NCT04430569
PULMONARY VASCULAR AND CARDIOVASCULAR NETWORK
Cardiovascular Medicine and Surgery Section
Patients with intermediate-risk pulmonary embolism (IRPE), or those with right ventricular dysfunction without overt hemodynamic instability, represent a heterogenous population with short-term mortality ranging from 2% to 17%.1 While systemic anticoagulation is the mainstay therapy, select individuals may benefit from more immediate reperfusion. Unfortunately, only small, randomized trials exploring surrogate outcomes are available to guide modality and patient selection.2
To better define which patients with IRPE are best managed with which therapy, several large-scale randomized controlled trials are underway. PE-TRACT, a study funded by the National Institutes of Health, aims to randomize 500 patients with IRPE to anticoagulation alone vs one of several modalities of CBT with a focus on long-term functional outcomes, including peak oxygen consumption at 3 months and functional class at 1 year. Aspiration thrombectomy with the FlowTriever® device is being compared with anticoagulation alone in a study of 1,200 patients examining short-term composite end points.
While full-dose thrombolysis may decrease the composite outcome of death or hemodynamic deterioration in this population, the benefit is counterbalanced by the risk of significant bleeding. Whether reduced-dose thrombolysis is associated with improved outcomes has been questioned in several small studies. The PEITHO-3 trial plans to randomize 650 patients with IRPE to reduced-dose thrombolytics vs placebo, exploring several outcomes at 30 days. With multiple large trials ongoing, we anticipate important changes to the landscape of IRPE care over the coming years.
References
1. Fernández C, Bova C, Sanchez O, et al. Validation of a model for identification of patients at intermediate to high risk for complications associated with acute symptomatic pulmonary embolism. Chest. 2015;148(1):211-218. doi:10.1378/chest.14-2551
2. Yuriditsky E, Horowitz JM. The role of the PERT in the management and therapeutic decision-making in pulmonary embolism. Eur Heart J Acute Cardiovasc Care. 2022;11(9):693-694. doi:10.1093/ehjacc/zuac102
3. National Library of Medicine (US). A Randomized Trial of Ultrasound-facilitated, Catheter-directed, Thrombolysis Versus Anticoagulation for Acute Intermediate-high Risk Pulmonary Embolism: The Higher-risk Pulmonary Embolism Thrombolysis Study. Updated July 16, 2024. https://clinicaltrials.gov/study/NCT04790370
4. National Library of Medicine (US). PEERLESS II: RCT of FlowTriever vs. Anticoagulation Alone in Pulmonary Embolism. Updated July 17, 2024. https://clinicaltrials.gov/study/NCT06055920
5. National Library of Medicine (US). A Reduced Dose of Thrombolytic Treatment for Patients With Intermediate High-risk Acute Pulmonary Embolism: a Randomized Controled Trial. Updated July 17, 2024. https://clinicaltrials.gov/study/NCT04430569
Bronchiectasis: A call to action
AIRWAYS DISORDERS NETWORK
Bronchiectasis Section
For years, the noncystic fibrosis (CF) bronchiectasis community has been trying to organize to provide better care for more than half a million adults with bronchiectasis in the United States. Internationally, the Europeans created the European Bronchiectasis Registry, which has been a powerful tool including nearly 20,000 patients, to answer important epidemiologic and management questions. We must do more for the bronchiectasis community.
Clinicaltrials.gov indicates that there are 8 international phase 3 or 4 clinical trials that are currently enrolling; 3 of those have enrollment sites in the United States. One such study from University of North Carolina at Chapel Hill is looking at the use of nebulized hypertonic saline in patients with non-CF bronchiectasis to understand the effect it has on mucociliary clearance. Emory University is looking at the use of elexacaftor/tezacaftor/ivacaftor (Trikafta) in patients with non-CF bronchiectasis; these patients have only 1 targetable mutation and a phenotype that resembles CF. This 8-week, open-label, single-center study aims to measure both clinical and biomarker outcomes after treatment with Trikafta. Finally, a phase 3 trial out of Florida, the ICoN-1 study, is examining the efficacy and safety of inhaled clofazimine in the treatment of nontuberculous mycobacteria (NTM). This double-blind, randomized trial will look at culture conversion and quality of life measures. Additionally, the COPD Foundation has created the Bronchiectasis and NTM Research Registry, an American cohort containing more than 5,000 patients and data from 22 different sites, to answer some of the most important questions for clinicians and patients.
We have made significant progress in bronchiectasis research; however, there is still much to learn. Together, we must make a concerted effort to enroll patients in clinical trials. Doing so will allow us to define our epidemiologic profile more precisely and explore new treatments and airway clearance techniques.
AIRWAYS DISORDERS NETWORK
Bronchiectasis Section
For years, the noncystic fibrosis (CF) bronchiectasis community has been trying to organize to provide better care for more than half a million adults with bronchiectasis in the United States. Internationally, the Europeans created the European Bronchiectasis Registry, which has been a powerful tool including nearly 20,000 patients, to answer important epidemiologic and management questions. We must do more for the bronchiectasis community.
Clinicaltrials.gov indicates that there are 8 international phase 3 or 4 clinical trials that are currently enrolling; 3 of those have enrollment sites in the United States. One such study from University of North Carolina at Chapel Hill is looking at the use of nebulized hypertonic saline in patients with non-CF bronchiectasis to understand the effect it has on mucociliary clearance. Emory University is looking at the use of elexacaftor/tezacaftor/ivacaftor (Trikafta) in patients with non-CF bronchiectasis; these patients have only 1 targetable mutation and a phenotype that resembles CF. This 8-week, open-label, single-center study aims to measure both clinical and biomarker outcomes after treatment with Trikafta. Finally, a phase 3 trial out of Florida, the ICoN-1 study, is examining the efficacy and safety of inhaled clofazimine in the treatment of nontuberculous mycobacteria (NTM). This double-blind, randomized trial will look at culture conversion and quality of life measures. Additionally, the COPD Foundation has created the Bronchiectasis and NTM Research Registry, an American cohort containing more than 5,000 patients and data from 22 different sites, to answer some of the most important questions for clinicians and patients.
We have made significant progress in bronchiectasis research; however, there is still much to learn. Together, we must make a concerted effort to enroll patients in clinical trials. Doing so will allow us to define our epidemiologic profile more precisely and explore new treatments and airway clearance techniques.
AIRWAYS DISORDERS NETWORK
Bronchiectasis Section
For years, the noncystic fibrosis (CF) bronchiectasis community has been trying to organize to provide better care for more than half a million adults with bronchiectasis in the United States. Internationally, the Europeans created the European Bronchiectasis Registry, which has been a powerful tool including nearly 20,000 patients, to answer important epidemiologic and management questions. We must do more for the bronchiectasis community.
Clinicaltrials.gov indicates that there are 8 international phase 3 or 4 clinical trials that are currently enrolling; 3 of those have enrollment sites in the United States. One such study from University of North Carolina at Chapel Hill is looking at the use of nebulized hypertonic saline in patients with non-CF bronchiectasis to understand the effect it has on mucociliary clearance. Emory University is looking at the use of elexacaftor/tezacaftor/ivacaftor (Trikafta) in patients with non-CF bronchiectasis; these patients have only 1 targetable mutation and a phenotype that resembles CF. This 8-week, open-label, single-center study aims to measure both clinical and biomarker outcomes after treatment with Trikafta. Finally, a phase 3 trial out of Florida, the ICoN-1 study, is examining the efficacy and safety of inhaled clofazimine in the treatment of nontuberculous mycobacteria (NTM). This double-blind, randomized trial will look at culture conversion and quality of life measures. Additionally, the COPD Foundation has created the Bronchiectasis and NTM Research Registry, an American cohort containing more than 5,000 patients and data from 22 different sites, to answer some of the most important questions for clinicians and patients.
We have made significant progress in bronchiectasis research; however, there is still much to learn. Together, we must make a concerted effort to enroll patients in clinical trials. Doing so will allow us to define our epidemiologic profile more precisely and explore new treatments and airway clearance techniques.