User login
Use Incentives, Moonlighters to Staff the Holidays
Holidays can pose a challenge for hospitalists who have to balance proper patient care with appropriate staffing. Good communication and chart documentation can make all the difference.
Bradley A. Sharpe, MD, assistant chief of medical service at the University of California, San Francisco, department of medicine, says patients admitted on holidays should be able to know treatment they receive is as good as on any other day of the year.
“All groups should probably act under the premise that a patient admitted on Thanksgiving day should get exactly the same care as one who gets admitted the following Tuesday,” he says. “Because there are fewer admissions, fewer tests, [and] fewer procedures, groups can probably dial down their staffing a bit. But it should not be a skeleton crew that could put patients at risk.”
Certainly, maintaining a high level of patient care depends on the attitudes of the caretakers.
Ken Simone, DO, president and founder of Hospitalist and Practice Solutions in Veazie, Maine, suggests making holiday work worthwhile for your staff.
“Hospitalist programs can make holidays more attractive to the hospitalist staff by offering a pay differential or by rewarding holiday work with additional days off,” he says.
Dr. Sharpe also recommends getting staff involved in the scheduling process early so they feel empowered.
—Ken Simone, DO, president and founder, Hospitalist and Practice Solutions, Veazie, Maine
“There is plenty of evidence that a lack of control contributes to unhappiness and burnout,” Dr. Sharpe says. “If the staff feels like they have been part of deciding how to do this, they will be less likely to complain.”
He also discourages using rank or seniority in dealing with holiday schedules.
“For our group, regardless of rank or seniority, everyone is expected to do the same number of major holidays over a three-year period, and then the cycle starts over,” he says.
Brigham and Women’s Hospital in Boston uses blocks of time—usually two weeks—rather than typical shifts; residents are available to cover off-hours, says Sylvia C. W. McKean, MD, FACP, medical director of the hospital and Faulkner Hospitalist Service.
“If there are difficulties with the availability of the existing staff, consider hiring moonlighters such as established physicians in the community, internal medicine or family practice residents, or utilizing locum tenens,” Dr. Simone says. “Non-physician clinicians can also be a resource.”
He notes that staffing needs should be estimated by the hospitalist inpatient census and average number of admissions and discharges per day.
Once that is determined, he recommends “a defined checkout process” between providers who will be off and the providers who work on holidays, similar to checkout protocol on weekends.
“Provider-to-provider checkout is a key component to ensuring patient continuity and safety,” he says. “Appropriate (e.g., clear and detailed) documentation in the patient’s medical record is also important.”
Dr. Sharpe agrees, adding that moonlighters especially should be made fully aware of the process.
“Groups should be sure they have a robust signout and sign-in system to make sure nothing is lost in the shuffle,” he cautions.
The bottom line is that working holiday shifts should not compromise patient care, Dr. Simone says. “Excellent communication, excellent chart documentation, and appropriate staffing are keys to maintaining continuity of care, quality patient care, and patient safety,” he says. TH
Molly Okeon is journalist based in California.
Holidays can pose a challenge for hospitalists who have to balance proper patient care with appropriate staffing. Good communication and chart documentation can make all the difference.
Bradley A. Sharpe, MD, assistant chief of medical service at the University of California, San Francisco, department of medicine, says patients admitted on holidays should be able to know treatment they receive is as good as on any other day of the year.
“All groups should probably act under the premise that a patient admitted on Thanksgiving day should get exactly the same care as one who gets admitted the following Tuesday,” he says. “Because there are fewer admissions, fewer tests, [and] fewer procedures, groups can probably dial down their staffing a bit. But it should not be a skeleton crew that could put patients at risk.”
Certainly, maintaining a high level of patient care depends on the attitudes of the caretakers.
Ken Simone, DO, president and founder of Hospitalist and Practice Solutions in Veazie, Maine, suggests making holiday work worthwhile for your staff.
“Hospitalist programs can make holidays more attractive to the hospitalist staff by offering a pay differential or by rewarding holiday work with additional days off,” he says.
Dr. Sharpe also recommends getting staff involved in the scheduling process early so they feel empowered.
—Ken Simone, DO, president and founder, Hospitalist and Practice Solutions, Veazie, Maine
“There is plenty of evidence that a lack of control contributes to unhappiness and burnout,” Dr. Sharpe says. “If the staff feels like they have been part of deciding how to do this, they will be less likely to complain.”
He also discourages using rank or seniority in dealing with holiday schedules.
“For our group, regardless of rank or seniority, everyone is expected to do the same number of major holidays over a three-year period, and then the cycle starts over,” he says.
Brigham and Women’s Hospital in Boston uses blocks of time—usually two weeks—rather than typical shifts; residents are available to cover off-hours, says Sylvia C. W. McKean, MD, FACP, medical director of the hospital and Faulkner Hospitalist Service.
“If there are difficulties with the availability of the existing staff, consider hiring moonlighters such as established physicians in the community, internal medicine or family practice residents, or utilizing locum tenens,” Dr. Simone says. “Non-physician clinicians can also be a resource.”
He notes that staffing needs should be estimated by the hospitalist inpatient census and average number of admissions and discharges per day.
Once that is determined, he recommends “a defined checkout process” between providers who will be off and the providers who work on holidays, similar to checkout protocol on weekends.
“Provider-to-provider checkout is a key component to ensuring patient continuity and safety,” he says. “Appropriate (e.g., clear and detailed) documentation in the patient’s medical record is also important.”
Dr. Sharpe agrees, adding that moonlighters especially should be made fully aware of the process.
“Groups should be sure they have a robust signout and sign-in system to make sure nothing is lost in the shuffle,” he cautions.
The bottom line is that working holiday shifts should not compromise patient care, Dr. Simone says. “Excellent communication, excellent chart documentation, and appropriate staffing are keys to maintaining continuity of care, quality patient care, and patient safety,” he says. TH
Molly Okeon is journalist based in California.
Holidays can pose a challenge for hospitalists who have to balance proper patient care with appropriate staffing. Good communication and chart documentation can make all the difference.
Bradley A. Sharpe, MD, assistant chief of medical service at the University of California, San Francisco, department of medicine, says patients admitted on holidays should be able to know treatment they receive is as good as on any other day of the year.
“All groups should probably act under the premise that a patient admitted on Thanksgiving day should get exactly the same care as one who gets admitted the following Tuesday,” he says. “Because there are fewer admissions, fewer tests, [and] fewer procedures, groups can probably dial down their staffing a bit. But it should not be a skeleton crew that could put patients at risk.”
Certainly, maintaining a high level of patient care depends on the attitudes of the caretakers.
Ken Simone, DO, president and founder of Hospitalist and Practice Solutions in Veazie, Maine, suggests making holiday work worthwhile for your staff.
“Hospitalist programs can make holidays more attractive to the hospitalist staff by offering a pay differential or by rewarding holiday work with additional days off,” he says.
Dr. Sharpe also recommends getting staff involved in the scheduling process early so they feel empowered.
—Ken Simone, DO, president and founder, Hospitalist and Practice Solutions, Veazie, Maine
“There is plenty of evidence that a lack of control contributes to unhappiness and burnout,” Dr. Sharpe says. “If the staff feels like they have been part of deciding how to do this, they will be less likely to complain.”
He also discourages using rank or seniority in dealing with holiday schedules.
“For our group, regardless of rank or seniority, everyone is expected to do the same number of major holidays over a three-year period, and then the cycle starts over,” he says.
Brigham and Women’s Hospital in Boston uses blocks of time—usually two weeks—rather than typical shifts; residents are available to cover off-hours, says Sylvia C. W. McKean, MD, FACP, medical director of the hospital and Faulkner Hospitalist Service.
“If there are difficulties with the availability of the existing staff, consider hiring moonlighters such as established physicians in the community, internal medicine or family practice residents, or utilizing locum tenens,” Dr. Simone says. “Non-physician clinicians can also be a resource.”
He notes that staffing needs should be estimated by the hospitalist inpatient census and average number of admissions and discharges per day.
Once that is determined, he recommends “a defined checkout process” between providers who will be off and the providers who work on holidays, similar to checkout protocol on weekends.
“Provider-to-provider checkout is a key component to ensuring patient continuity and safety,” he says. “Appropriate (e.g., clear and detailed) documentation in the patient’s medical record is also important.”
Dr. Sharpe agrees, adding that moonlighters especially should be made fully aware of the process.
“Groups should be sure they have a robust signout and sign-in system to make sure nothing is lost in the shuffle,” he cautions.
The bottom line is that working holiday shifts should not compromise patient care, Dr. Simone says. “Excellent communication, excellent chart documentation, and appropriate staffing are keys to maintaining continuity of care, quality patient care, and patient safety,” he says. TH
Molly Okeon is journalist based in California.
How Am I Doing?
How hospitalists assess their performance and hone their skills is critical to patient care. Continuing medical education (CME), relicensure, specialty recertification, and lifelong learning are all linked to hospitalists’ abilities to assess and meet their learning needs.
But the preponderance of evidence suggests physicians have limited ability to accurately assess their performance, according to a physician self-assessment literature review published in September 2006 in JAMA.1
“Self-assessment should be guided by tools designed by experts, based on standards, and aimed at filling gaps in knowledge, skills, and competencies—not simply the internally based self-rating of individual practitioners,” says C. Michael Fordis, MD, senior associate dean for con-
tinuing medical education at the Baylor College of Medicine in Houston, and one of the authors of the study.
“Hospitalists and other physicians are not doing themselves a service to rely on their own internal self-rated judgments of knowledge and performance,” Dr. Fordis says. “There’s too much to know, too much that’s changing, and too much that affects the implementation into practice of the knowledge that you have for any one person to be able to take care of patients and at the same time have some sense of whether there are gaps along that implementation pathway.”
“Guided” self-assessment represents the thinking of many experts who ask questions, consider guidelines, and suggest tools that can help physicians pursue the best ways of identifying those gaps that reflect differences in what they think they are doing and their actual performance.
Regular, consistent self-assessment is imperative for a self-regulating profession such as medicine. How well are hospitalists doing—and what mechanisms or tools do they use?
Group Assessment
Hospital medicine groups are increasingly able to measure their clinical competence against other hospitals’ and hospitalist groups. SHM’s Benchmarks Committee has been working on performance assessment at a program level.
“When the JCAHO [Joint Commission on Accreditation of Healthcare Organizations] Core Measures were coming out a few years back, as a whole most docs when reflecting on their practice would say they do a fine job within these measures,” says Burke T. Kealey, MD, chairman of the Benchmarks Committee from 2006-07. “For instance, [they might say] ‘I always send people out on ACE inhibitors and beta-blockers,’ or, ‘We always start people on aspirin when they come into the ER,’ but when you looked at the data, you found that their self-assessment was not as accurate as we hoped it would be.”
A lot of hard work went into discovering why their self-assessment was inaccurate. “We found there were documentation problems that they didn’t really incorporate a lot of the contraindications when giving their answer about self-assessment,” says Dr. Kealey, who leads the hospital medicine program at Regions Hospital and HealthPartners Medical Group in St. Paul, Minn.
If patients had kidney dysfunction or kidney failure, they were not discharged on ACE inhibitors.
“But we as doctors didn’t do a great job of explaining why we weren’t doing that,” Dr. Kealey says. “We were not transparent in our reasoning, but the core measures caused us to become more transparent, to explain what we were thinking and what we were doing in a way that the public could see.”
At SHM’s annual meeting in May, the Benchmarks Committee released the white paper “Measuring Hospitalist Performance: Metrics, Reports, and Dashboards” with the intent of assisting hospitals and hospital medicine programs develop or improve their performance monitoring and reporting.
“Hospitalists in general could do a better job of assessing themselves,” says Arpana Vidyarthi, MD, an assistant professor in the division of hospital medicine at the University of California, San Francisco (UCSF). “Self-assessment for those of us in cognitive specialties, like internists, is more complicated than in procedural specialties like surgery, partly because these procedural specialties have very specific outcomes that are linked to the procedure and that level of skill. With the new drivers of quality improvement and patient safety, and the dramatic increase of quality indicators for hospitals overall, this is now trickling down to thinking about how we truly assess the doctors themselves.”
The quality indicators that hospitalist groups are benchmarking may not be linked to the individual, she says. Dr. Vidyarthi, also director of quality for the Inpatient General Medicine Service at UCSF Medical Center, provides an example. “Pneumovax as a quality indicator is part of the Joint Commission core measures,” says Dr. Vidyarthi. “You can go online where it is publicly reported and choose this or other indicators to compare one hospital to another. That is the sort of benchmarking that some hospitalists groups are doing.”
But using that kind of evaluation for individual assessment misses the mark.
“Does the fact that the patient does not get Pneumovax reflect upon me and my abilities as a hospitalist? Not at all,” she says, “because my institution and those institutions who have done well with this specific indicator have taken it out of the hands of the doctors. It’s an automated sort of thing. At our hospital, the pharmacists do it.”
Although the American Board of Internal Medicine asks that the individual physician assess his or her own care as part of recredentialing, it’s more difficult for a hospitalist than for an outpatient internist. Hospitalists don’t have a panel of diabetic patients, for instance, for which the outcomes data can be easily analyzed.
Hospitalists as a group also haven’t had a tradition of self-assessment or peer assessment. Further, hospitalist groups differ as to how they handle assessments of individual physicians.
“In general if you ask our [UCSF] hospitalists, the way that we assess competency is generally through hospital privileging,” Dr. Vidyarthi says. Because the hospital as a whole reviews the competency of all the doctors that work there, the process known as “privileging” has consisted of asking a couple of colleagues to write letters of recommendation. “The division is changing this, but that is just on the cusp.
“We’ve built a new system for our quality committee in which one layer is peer assessment, looking at just the individual cases that bubble up from an incident report or a root-cause analysis or other sources. We’re looking at and identifying both systems issues and individual issues and trying to build a way to feed back those assessments.”
But that’s just half the equation, she says, the flip side being continual self-assessment for what a hospitalist is doing well.
To Dr. Kealey, self-assessment plays a significant role in helping physicians with their career goals and ensuring that their careers are on track and on target.
At HealthPartners, physicians fill out a self-evaluation form on which they list all activities they’ve been involved in over the previous year. Then they are asked what they got out of these activities, what their career goals are, and whether they are meeting them. They’re also asked how the group can help them reach those goals.
“We ask them to pause and reflect on where they’re headed with their career and their life, and put it down in writing so that in that moment they take the time to ask, ‘What is it that I’m ultimately after?’ ” says Dr. Kealey.
Day to day, they are immersed in patient care and focused on doing a good job. “But in the trajectory of where they are headed—the committees, projects, and educational activities they are involved in—are they all aligned and pointing in the same direction and the right direction?” Dr Kealey asks.
The process, which HealthPartners hospitalists have been using for about 10 years, was modified from the American College of Physician Executives course “Managing Physician Performance.”
“It is a tool to help hospitalists pause and reflect on their career and how to move it forward,” Dr. Kealey says.
Marc B. Westle, DO, FACP, president and managing partner of the Asheville Hospitalist Group, PA, in Asheville, N.C., relies on ongoing conversations. This group also uses Crimson’s Physician Management Software to track various group quality and cost indicators, looking at data from as many angles as possible.
“It’s an excellent tool to look at a group, it is a poor tool to look at an individual,” Dr. Westle says. “Although the insurance companies like to say you can apply it to the individual, in reality there is no good way to attribute that data down to the physician level.”
Within the group data, it may be possible to recognize underperformers, but still it is anecdotal, based on experience and interaction.
“Under, ‘How am I doing?’ there is an objective category in the software where there are hard end-points and measures you can look at,” says Dr. Westle
On the subjective side, Dr. Westle collects data on relative value units (RVUs), non-monetary, numeric values Medicare uses to represent the relative amount of physician time, resources, and expertise needed to provide various services to patients. They review total RVUs as well as individual-components that make up total RVUs.
“I’ll track how many simple, moderate, or complex follow-up visits were made, how many simple or moderate histories and physicals or consultations, how many procedures are they doing.” Dr. Westle says. “I’ll track every statistic that way for every individual and give them that feedback so they can see how they’re doing from a performance and a work standard, compared to their peers within the group, and nationally as published by Medicare.”
Dr. Westle uses charts and graphs to drive his points home.
“It just gives them an idea about where they are,’’ he says. “It doesn’t mean they’re doing a bad job. Our patients may be sicker than some other patients. And that is why we do it as a group, too, because their patients should be similar to the group’s patients and the group’s patients may be different than the average Medicare patient.”
They also look at hospitalists’ quality of life, their schedules, and the quantity of work the average physician is doing compared with those around the country. They discuss scheduling, income, disposable income, and the kind of work they’re doing in the hospital. “All this comes into a discussion of where they are in their lives and are they happy with what they’re doing,” Dr. Westle says. TH
Andrea Sattinger is a medical writer based in North Carolina.
Reference
- Davis DA, Mazmanian PE, Fordis M, et al. Accuracy of physician self-assessment compared with observed measures of competence: a systematic review. JAMA. 2006;296(9):1094-1102.
How hospitalists assess their performance and hone their skills is critical to patient care. Continuing medical education (CME), relicensure, specialty recertification, and lifelong learning are all linked to hospitalists’ abilities to assess and meet their learning needs.
But the preponderance of evidence suggests physicians have limited ability to accurately assess their performance, according to a physician self-assessment literature review published in September 2006 in JAMA.1
“Self-assessment should be guided by tools designed by experts, based on standards, and aimed at filling gaps in knowledge, skills, and competencies—not simply the internally based self-rating of individual practitioners,” says C. Michael Fordis, MD, senior associate dean for con-
tinuing medical education at the Baylor College of Medicine in Houston, and one of the authors of the study.
“Hospitalists and other physicians are not doing themselves a service to rely on their own internal self-rated judgments of knowledge and performance,” Dr. Fordis says. “There’s too much to know, too much that’s changing, and too much that affects the implementation into practice of the knowledge that you have for any one person to be able to take care of patients and at the same time have some sense of whether there are gaps along that implementation pathway.”
“Guided” self-assessment represents the thinking of many experts who ask questions, consider guidelines, and suggest tools that can help physicians pursue the best ways of identifying those gaps that reflect differences in what they think they are doing and their actual performance.
Regular, consistent self-assessment is imperative for a self-regulating profession such as medicine. How well are hospitalists doing—and what mechanisms or tools do they use?
Group Assessment
Hospital medicine groups are increasingly able to measure their clinical competence against other hospitals’ and hospitalist groups. SHM’s Benchmarks Committee has been working on performance assessment at a program level.
“When the JCAHO [Joint Commission on Accreditation of Healthcare Organizations] Core Measures were coming out a few years back, as a whole most docs when reflecting on their practice would say they do a fine job within these measures,” says Burke T. Kealey, MD, chairman of the Benchmarks Committee from 2006-07. “For instance, [they might say] ‘I always send people out on ACE inhibitors and beta-blockers,’ or, ‘We always start people on aspirin when they come into the ER,’ but when you looked at the data, you found that their self-assessment was not as accurate as we hoped it would be.”
A lot of hard work went into discovering why their self-assessment was inaccurate. “We found there were documentation problems that they didn’t really incorporate a lot of the contraindications when giving their answer about self-assessment,” says Dr. Kealey, who leads the hospital medicine program at Regions Hospital and HealthPartners Medical Group in St. Paul, Minn.
If patients had kidney dysfunction or kidney failure, they were not discharged on ACE inhibitors.
“But we as doctors didn’t do a great job of explaining why we weren’t doing that,” Dr. Kealey says. “We were not transparent in our reasoning, but the core measures caused us to become more transparent, to explain what we were thinking and what we were doing in a way that the public could see.”
At SHM’s annual meeting in May, the Benchmarks Committee released the white paper “Measuring Hospitalist Performance: Metrics, Reports, and Dashboards” with the intent of assisting hospitals and hospital medicine programs develop or improve their performance monitoring and reporting.
“Hospitalists in general could do a better job of assessing themselves,” says Arpana Vidyarthi, MD, an assistant professor in the division of hospital medicine at the University of California, San Francisco (UCSF). “Self-assessment for those of us in cognitive specialties, like internists, is more complicated than in procedural specialties like surgery, partly because these procedural specialties have very specific outcomes that are linked to the procedure and that level of skill. With the new drivers of quality improvement and patient safety, and the dramatic increase of quality indicators for hospitals overall, this is now trickling down to thinking about how we truly assess the doctors themselves.”
The quality indicators that hospitalist groups are benchmarking may not be linked to the individual, she says. Dr. Vidyarthi, also director of quality for the Inpatient General Medicine Service at UCSF Medical Center, provides an example. “Pneumovax as a quality indicator is part of the Joint Commission core measures,” says Dr. Vidyarthi. “You can go online where it is publicly reported and choose this or other indicators to compare one hospital to another. That is the sort of benchmarking that some hospitalists groups are doing.”
But using that kind of evaluation for individual assessment misses the mark.
“Does the fact that the patient does not get Pneumovax reflect upon me and my abilities as a hospitalist? Not at all,” she says, “because my institution and those institutions who have done well with this specific indicator have taken it out of the hands of the doctors. It’s an automated sort of thing. At our hospital, the pharmacists do it.”
Although the American Board of Internal Medicine asks that the individual physician assess his or her own care as part of recredentialing, it’s more difficult for a hospitalist than for an outpatient internist. Hospitalists don’t have a panel of diabetic patients, for instance, for which the outcomes data can be easily analyzed.
Hospitalists as a group also haven’t had a tradition of self-assessment or peer assessment. Further, hospitalist groups differ as to how they handle assessments of individual physicians.
“In general if you ask our [UCSF] hospitalists, the way that we assess competency is generally through hospital privileging,” Dr. Vidyarthi says. Because the hospital as a whole reviews the competency of all the doctors that work there, the process known as “privileging” has consisted of asking a couple of colleagues to write letters of recommendation. “The division is changing this, but that is just on the cusp.
“We’ve built a new system for our quality committee in which one layer is peer assessment, looking at just the individual cases that bubble up from an incident report or a root-cause analysis or other sources. We’re looking at and identifying both systems issues and individual issues and trying to build a way to feed back those assessments.”
But that’s just half the equation, she says, the flip side being continual self-assessment for what a hospitalist is doing well.
To Dr. Kealey, self-assessment plays a significant role in helping physicians with their career goals and ensuring that their careers are on track and on target.
At HealthPartners, physicians fill out a self-evaluation form on which they list all activities they’ve been involved in over the previous year. Then they are asked what they got out of these activities, what their career goals are, and whether they are meeting them. They’re also asked how the group can help them reach those goals.
“We ask them to pause and reflect on where they’re headed with their career and their life, and put it down in writing so that in that moment they take the time to ask, ‘What is it that I’m ultimately after?’ ” says Dr. Kealey.
Day to day, they are immersed in patient care and focused on doing a good job. “But in the trajectory of where they are headed—the committees, projects, and educational activities they are involved in—are they all aligned and pointing in the same direction and the right direction?” Dr Kealey asks.
The process, which HealthPartners hospitalists have been using for about 10 years, was modified from the American College of Physician Executives course “Managing Physician Performance.”
“It is a tool to help hospitalists pause and reflect on their career and how to move it forward,” Dr. Kealey says.
Marc B. Westle, DO, FACP, president and managing partner of the Asheville Hospitalist Group, PA, in Asheville, N.C., relies on ongoing conversations. This group also uses Crimson’s Physician Management Software to track various group quality and cost indicators, looking at data from as many angles as possible.
“It’s an excellent tool to look at a group, it is a poor tool to look at an individual,” Dr. Westle says. “Although the insurance companies like to say you can apply it to the individual, in reality there is no good way to attribute that data down to the physician level.”
Within the group data, it may be possible to recognize underperformers, but still it is anecdotal, based on experience and interaction.
“Under, ‘How am I doing?’ there is an objective category in the software where there are hard end-points and measures you can look at,” says Dr. Westle
On the subjective side, Dr. Westle collects data on relative value units (RVUs), non-monetary, numeric values Medicare uses to represent the relative amount of physician time, resources, and expertise needed to provide various services to patients. They review total RVUs as well as individual-components that make up total RVUs.
“I’ll track how many simple, moderate, or complex follow-up visits were made, how many simple or moderate histories and physicals or consultations, how many procedures are they doing.” Dr. Westle says. “I’ll track every statistic that way for every individual and give them that feedback so they can see how they’re doing from a performance and a work standard, compared to their peers within the group, and nationally as published by Medicare.”
Dr. Westle uses charts and graphs to drive his points home.
“It just gives them an idea about where they are,’’ he says. “It doesn’t mean they’re doing a bad job. Our patients may be sicker than some other patients. And that is why we do it as a group, too, because their patients should be similar to the group’s patients and the group’s patients may be different than the average Medicare patient.”
They also look at hospitalists’ quality of life, their schedules, and the quantity of work the average physician is doing compared with those around the country. They discuss scheduling, income, disposable income, and the kind of work they’re doing in the hospital. “All this comes into a discussion of where they are in their lives and are they happy with what they’re doing,” Dr. Westle says. TH
Andrea Sattinger is a medical writer based in North Carolina.
Reference
- Davis DA, Mazmanian PE, Fordis M, et al. Accuracy of physician self-assessment compared with observed measures of competence: a systematic review. JAMA. 2006;296(9):1094-1102.
How hospitalists assess their performance and hone their skills is critical to patient care. Continuing medical education (CME), relicensure, specialty recertification, and lifelong learning are all linked to hospitalists’ abilities to assess and meet their learning needs.
But the preponderance of evidence suggests physicians have limited ability to accurately assess their performance, according to a physician self-assessment literature review published in September 2006 in JAMA.1
“Self-assessment should be guided by tools designed by experts, based on standards, and aimed at filling gaps in knowledge, skills, and competencies—not simply the internally based self-rating of individual practitioners,” says C. Michael Fordis, MD, senior associate dean for con-
tinuing medical education at the Baylor College of Medicine in Houston, and one of the authors of the study.
“Hospitalists and other physicians are not doing themselves a service to rely on their own internal self-rated judgments of knowledge and performance,” Dr. Fordis says. “There’s too much to know, too much that’s changing, and too much that affects the implementation into practice of the knowledge that you have for any one person to be able to take care of patients and at the same time have some sense of whether there are gaps along that implementation pathway.”
“Guided” self-assessment represents the thinking of many experts who ask questions, consider guidelines, and suggest tools that can help physicians pursue the best ways of identifying those gaps that reflect differences in what they think they are doing and their actual performance.
Regular, consistent self-assessment is imperative for a self-regulating profession such as medicine. How well are hospitalists doing—and what mechanisms or tools do they use?
Group Assessment
Hospital medicine groups are increasingly able to measure their clinical competence against other hospitals’ and hospitalist groups. SHM’s Benchmarks Committee has been working on performance assessment at a program level.
“When the JCAHO [Joint Commission on Accreditation of Healthcare Organizations] Core Measures were coming out a few years back, as a whole most docs when reflecting on their practice would say they do a fine job within these measures,” says Burke T. Kealey, MD, chairman of the Benchmarks Committee from 2006-07. “For instance, [they might say] ‘I always send people out on ACE inhibitors and beta-blockers,’ or, ‘We always start people on aspirin when they come into the ER,’ but when you looked at the data, you found that their self-assessment was not as accurate as we hoped it would be.”
A lot of hard work went into discovering why their self-assessment was inaccurate. “We found there were documentation problems that they didn’t really incorporate a lot of the contraindications when giving their answer about self-assessment,” says Dr. Kealey, who leads the hospital medicine program at Regions Hospital and HealthPartners Medical Group in St. Paul, Minn.
If patients had kidney dysfunction or kidney failure, they were not discharged on ACE inhibitors.
“But we as doctors didn’t do a great job of explaining why we weren’t doing that,” Dr. Kealey says. “We were not transparent in our reasoning, but the core measures caused us to become more transparent, to explain what we were thinking and what we were doing in a way that the public could see.”
At SHM’s annual meeting in May, the Benchmarks Committee released the white paper “Measuring Hospitalist Performance: Metrics, Reports, and Dashboards” with the intent of assisting hospitals and hospital medicine programs develop or improve their performance monitoring and reporting.
“Hospitalists in general could do a better job of assessing themselves,” says Arpana Vidyarthi, MD, an assistant professor in the division of hospital medicine at the University of California, San Francisco (UCSF). “Self-assessment for those of us in cognitive specialties, like internists, is more complicated than in procedural specialties like surgery, partly because these procedural specialties have very specific outcomes that are linked to the procedure and that level of skill. With the new drivers of quality improvement and patient safety, and the dramatic increase of quality indicators for hospitals overall, this is now trickling down to thinking about how we truly assess the doctors themselves.”
The quality indicators that hospitalist groups are benchmarking may not be linked to the individual, she says. Dr. Vidyarthi, also director of quality for the Inpatient General Medicine Service at UCSF Medical Center, provides an example. “Pneumovax as a quality indicator is part of the Joint Commission core measures,” says Dr. Vidyarthi. “You can go online where it is publicly reported and choose this or other indicators to compare one hospital to another. That is the sort of benchmarking that some hospitalists groups are doing.”
But using that kind of evaluation for individual assessment misses the mark.
“Does the fact that the patient does not get Pneumovax reflect upon me and my abilities as a hospitalist? Not at all,” she says, “because my institution and those institutions who have done well with this specific indicator have taken it out of the hands of the doctors. It’s an automated sort of thing. At our hospital, the pharmacists do it.”
Although the American Board of Internal Medicine asks that the individual physician assess his or her own care as part of recredentialing, it’s more difficult for a hospitalist than for an outpatient internist. Hospitalists don’t have a panel of diabetic patients, for instance, for which the outcomes data can be easily analyzed.
Hospitalists as a group also haven’t had a tradition of self-assessment or peer assessment. Further, hospitalist groups differ as to how they handle assessments of individual physicians.
“In general if you ask our [UCSF] hospitalists, the way that we assess competency is generally through hospital privileging,” Dr. Vidyarthi says. Because the hospital as a whole reviews the competency of all the doctors that work there, the process known as “privileging” has consisted of asking a couple of colleagues to write letters of recommendation. “The division is changing this, but that is just on the cusp.
“We’ve built a new system for our quality committee in which one layer is peer assessment, looking at just the individual cases that bubble up from an incident report or a root-cause analysis or other sources. We’re looking at and identifying both systems issues and individual issues and trying to build a way to feed back those assessments.”
But that’s just half the equation, she says, the flip side being continual self-assessment for what a hospitalist is doing well.
To Dr. Kealey, self-assessment plays a significant role in helping physicians with their career goals and ensuring that their careers are on track and on target.
At HealthPartners, physicians fill out a self-evaluation form on which they list all activities they’ve been involved in over the previous year. Then they are asked what they got out of these activities, what their career goals are, and whether they are meeting them. They’re also asked how the group can help them reach those goals.
“We ask them to pause and reflect on where they’re headed with their career and their life, and put it down in writing so that in that moment they take the time to ask, ‘What is it that I’m ultimately after?’ ” says Dr. Kealey.
Day to day, they are immersed in patient care and focused on doing a good job. “But in the trajectory of where they are headed—the committees, projects, and educational activities they are involved in—are they all aligned and pointing in the same direction and the right direction?” Dr Kealey asks.
The process, which HealthPartners hospitalists have been using for about 10 years, was modified from the American College of Physician Executives course “Managing Physician Performance.”
“It is a tool to help hospitalists pause and reflect on their career and how to move it forward,” Dr. Kealey says.
Marc B. Westle, DO, FACP, president and managing partner of the Asheville Hospitalist Group, PA, in Asheville, N.C., relies on ongoing conversations. This group also uses Crimson’s Physician Management Software to track various group quality and cost indicators, looking at data from as many angles as possible.
“It’s an excellent tool to look at a group, it is a poor tool to look at an individual,” Dr. Westle says. “Although the insurance companies like to say you can apply it to the individual, in reality there is no good way to attribute that data down to the physician level.”
Within the group data, it may be possible to recognize underperformers, but still it is anecdotal, based on experience and interaction.
“Under, ‘How am I doing?’ there is an objective category in the software where there are hard end-points and measures you can look at,” says Dr. Westle
On the subjective side, Dr. Westle collects data on relative value units (RVUs), non-monetary, numeric values Medicare uses to represent the relative amount of physician time, resources, and expertise needed to provide various services to patients. They review total RVUs as well as individual-components that make up total RVUs.
“I’ll track how many simple, moderate, or complex follow-up visits were made, how many simple or moderate histories and physicals or consultations, how many procedures are they doing.” Dr. Westle says. “I’ll track every statistic that way for every individual and give them that feedback so they can see how they’re doing from a performance and a work standard, compared to their peers within the group, and nationally as published by Medicare.”
Dr. Westle uses charts and graphs to drive his points home.
“It just gives them an idea about where they are,’’ he says. “It doesn’t mean they’re doing a bad job. Our patients may be sicker than some other patients. And that is why we do it as a group, too, because their patients should be similar to the group’s patients and the group’s patients may be different than the average Medicare patient.”
They also look at hospitalists’ quality of life, their schedules, and the quantity of work the average physician is doing compared with those around the country. They discuss scheduling, income, disposable income, and the kind of work they’re doing in the hospital. “All this comes into a discussion of where they are in their lives and are they happy with what they’re doing,” Dr. Westle says. TH
Andrea Sattinger is a medical writer based in North Carolina.
Reference
- Davis DA, Mazmanian PE, Fordis M, et al. Accuracy of physician self-assessment compared with observed measures of competence: a systematic review. JAMA. 2006;296(9):1094-1102.
The Surgical Surge
New limits on resident work hours and the graying of the U.S. population are putting hospitalists in the forefront of helping surgeons manage their patients.
Because the Accreditation Council for Graduate Medical Education restricted resident duty hours, surgeons can no longer rely automatically on residents to medically manage their patients on the floors, says Amir K. Jaffer, MD, a hospitalist and an associate professor of medicine at the Cleveland Clinic Lerner College of Medicine of Case Western Reserve University in Ohio.
Meanwhile, the population over age 65 will double, increasing to 70 million over the next 10 to 15 years.1
“More patients living longer means an increase in surgeries along the way,” says Dr. Jaffer, who is also the medical director of the Internal Medicine Preoperative Assessment Consultation and Treatment program in the section of hospital medicine at the Cleveland Clinic. For him, the first place hospitalists need to co-manage is in the postoperative setting.
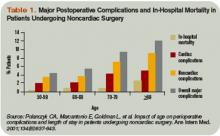
“Studies have suggested that as patients age there is an increase in cardiological complications, noncardiological complications, pulmonary complications, and overall mortality,” he continues. “In my opinion there is going to be a crisis in regard to managing medical issues and complications surrounding surgery.” (See Table 1, p. 24)
Medications issues are another major reason hospitalists are called for surgical consults, says Benny Gavi, MD, hospitalist at Stanford Hospitals and Clinics in Calif. “I got consulted for a patient with tachycardia in the inpatient setting,” says Dr. Gavi. “By the time we saw the patient, the orthopedic surgeon had already ordered an echocardiogram and added a beta-blocker. When I looked at the patient I realized he had a gout flare; the colchicine that he took daily for his gout was never started in the inpatient setting, which ultimately delayed his physical therapy and added three additional days to his hospital stay.”
Co-management makes sense for still other reasons, he says.
“The knowledge base of both surgery and medicine is growing rapidly; no one person can remain on top of what is needed for both fields,” says Dr. Gavi. “In the last 20 years there has been a dramatic rise in the number of medications and some are very complicated. Also, physicians and surgeons both are being approached to participate more in quality initiatives and increasing throughput. As a result, physicians have to work faster and do more.”
Opportunities
In the United States, approximately 100,000 surgeries are performed each day and 36 million surgeries are performed each year at a cost of $450 billion annually. More than 1 million serious surgical adverse events each year cost $45 billion. Within two decades, the surgeries will increase by 25%, the associated cost will increase 50%, and the cost of in-hospital and long-term complications will increase 100%.
Along with postoperative care, there are increasing opportunities in the preoperative setting.
“At our institution, which is a tertiary care center with a huge surgical hospital, we determined that there was a need for hospitalists to provide medical management of surgical patients 10 years ago,” Dr. Jaffer says. “Patients were often not adequately prepared when they went to surgery, and sometimes in the morning of surgery the anesthesiologists would cancel their cases.”
The traditional model of physicians calling in consultants when problems arise might need to change.
“We are increasingly looking for ways to identify patients who have a high likelihood of developing medical problems and proactively getting involved,” says Dr. Gavi.
To co-manage, hospitalists must take ownership of some medical issues under specific conditions (diabetes, anticoagulation, blood pressure), says Dr. Jaffer.
The Benefits
To Latha Sivaprasad, MD, hospitalist at Beth Israel Medical Center in New York City, there are three main advantages of hospitalists’ involvement in perioperative co-management:
- Hospitalists typically perform comprehensive, multisystemic patient evaluations;
- Hospitalists are extremely accessible; and
- Hospitalists are up to date on inpatient medicine.
How up to date?
“Periop isn’t routinely taught in residency,” says Ali Usmani, MD, a hospitalist at the Cleveland Clinic. “In fact, I had little information about perioperative care.”
When he joined the hospitalist group after a three-year residency at Cleveland Clinic, Dr. Usmani did preparatory reading. Later, the hospitalist group gave him a helpful collection of essays.
“I was very nervous because, of course, I had never done this before,” he says. “Surprisingly, I also had not done a general medicine consult service where we see postoperative patients. It was scary to some extent, but I found out that it is easier than I thought because there are guidelines you can follow from the AHA/ACC that are fairly straightforward. It also meant a nice schedule change from being on the floors.”
Although conducting preoperative evaluations with patients was technically outpatient work, it was not like he was seeing patients with such simple illnesses as a cold or a sore throat. Also, he says, there were no new surprises postoperatively because either he or a hospitalist colleague had seen the patient preoperatively.
Dr. Usmani, also a clinical assistant professor of medicine at Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, believes patients are happier when seen by hospitalists because they get a standardized, holistic preoperative assessment. And, helping to reduce the number of unnecessary tests ordered by primary care physicians or surgeons makes him feel as though he’s making a valuable contribution.
New Niche
Dr. Sivaprasad, who is also doing a one-year fellowship in quality improvement and patient safety at Beth Israel, has practiced hospital medicine in four hospitals ranging from 500 to 1,000 beds. “The primary reason we are consulted by surgeons is for perioperative cardiac risk assessment,” says Dr. Sivaprasad. “Other reasons include co-managing a patient with comorbidities such as a history of diabetes, hypertension, or renal failure.”
From 2003-2006, Dr. Sivaprasad was one of 14 hospitalists consulted often by surgeons at St. John’s Mercy Hospital in St Louis, a 1,000-bed Level I trauma center. “We were consulted for postoperative co-management, preoperative evaluation, or more urgent cases such as a patient experiencing hypotension, atrial fibrillation, shortness of breath, decreased urine output, or renal failure,” she says.
Dr. Sivaprasad recently attended the Johns Hopkins conference on Perioperative Management. The session made it easier for her to do a systems-based consult.
“All hospitalists differ to the degree of perioperative medicine they feel comfortable with,” she says. “Hospitalists understand perioperative medicine on different levels. They all can do an acceptable consult; but there is a spectrum of how detailed one can be and what service one can provide for the surgeon and the patient.”
Dr. Jaffer finds his work in perioperative care fulfilling and considers it another way hospitalists can increase their influence.
“Often when you manage medical patients in the hospital, it’s you, the medical patient, and the patient’s primary care physician,” Dr. Jaffer says. “But when you start to manage surgical patients, you are really being looked at by your surgical colleagues as an expert in managing medical problems, just as you view them as experts in managing surgical problems. What I realize from this is that I can be a perioperative medicine expert as well.”
Are there any downfalls to co-managing surgical patients?
“Sometimes the surgeons order unnecessary lab tests such as PTTs [partial thromboplastin time] because they are concerned about bleeding and complications,” Dr. Usmani says. “The next day if there is a deranged PTT, we need to figure out whether to suggest postponing the surgery or go ahead with the surgery based on the patients’ past medical/family history. We try to get our surgeons and our colleagues to work together with us in that regard because they don’t want to postpone surgery either.”
Drs. Usmani, Gavi, Jaffer, and Sivaprasad all say that when surgeons can observe firsthand their hospitalist partners exhibiting expertise in acute care it appears to improve surgeons’ attitudes about the role and value of hospitalists.
In fact, says Dr. Usmani, surgeons call him or one of his colleagues to thank them. “They say, ‘We really appreciate what you’ve done for this patient,’ ’’ he says. “Even if we suggest canceling surgery, they respect that we have seen a potential problem instead of letting it go ahead. They are happy to receive this advice.”
Another new relationship is between anesthesiologists and hospitalists. “I spend a lot of time calling anesthesiologists in regard to patient cases, and a good many of them are surprised to get a call from a hospitalist,” Dr. Gavi says. “We especially work closely together when we get complicated patients ready for surgery.”
A recent encounter proved to Dr. Gavi the complementary nature of the hospitalist-anesthesiologist relationship.2
“A patient came to the hospital two weeks ago to have an elective total knee replacement,” says Dr. Gavi. “She was an older woman with severe pulmonary disease. When the anesthesiologists saw her in the preoperative waiting area and realized how sick she is, they wanted to cancel the surgery. But the surgeon told the anesthesiologist that this patient had been seen in our own preoperative clinic and cleared by a hospitalist.”
Dr. Gavi had done what is customary for an internist. He took a more in-depth look at her pulmonology and cardiac records, called her cardiologist for further history, and reassured the anesthesiologist and surgeon. The patient had her surgery.
The Future
“Perioperative co-management is becoming more of a visible need,” says Dr. Sivaprasad. “It bridges the gap between surgeons and internists.”
To those of his hospitalist colleagues who have little information and are a bit afraid to begin perioperative care practice, Dr. Usmani recommends attending a perioperative summit conference.
The session should teach how to set up a perioperative center and what to do when managing patients with certain conditions.
“Although you meet with patients preoperatively in an office setting, you don’t feel like a primary care physician,” Dr. Usmani says. “You feel as if you are a specialist. You are respected, and you are contributing to postoperative outcomes to the benefit of the patient.”
Perioperative patient management is also financially rewarding because reimbursement is higher than customary hospital medicine duties.
Dr. Jaffer, soon to be chief of the division of hospital medicine at the University of Miami Medical Center in Florida, is proud of the work he and his colleagues have done to grow the Cleveland Clinic perioperative summit. This third summit, in September, was organized in collaboration with the Society of Perioperative Assessment and Quality Improvement.
“I think this is something that every hospitalist should try,” Dr. Usmani says. “It is definitely a niche.” TH
Andrea Sattinger is a frequent contributor to The Hospitalist.
References
- Mangano DT. Perioperative medicine: NHLBI working group deliberations and recommendations. J Cardiothorac Vasc Anesth. 2004;18(1):1-6.
- Adebola O, Adesanya AO, Joshi GP. Hospitalists and anesthesiologists as perioperative physicians: Are their roles complementary? Proc. (Bayl Univ Med Cent) 2007 April;20(2):140-142.
New limits on resident work hours and the graying of the U.S. population are putting hospitalists in the forefront of helping surgeons manage their patients.
Because the Accreditation Council for Graduate Medical Education restricted resident duty hours, surgeons can no longer rely automatically on residents to medically manage their patients on the floors, says Amir K. Jaffer, MD, a hospitalist and an associate professor of medicine at the Cleveland Clinic Lerner College of Medicine of Case Western Reserve University in Ohio.
Meanwhile, the population over age 65 will double, increasing to 70 million over the next 10 to 15 years.1
“More patients living longer means an increase in surgeries along the way,” says Dr. Jaffer, who is also the medical director of the Internal Medicine Preoperative Assessment Consultation and Treatment program in the section of hospital medicine at the Cleveland Clinic. For him, the first place hospitalists need to co-manage is in the postoperative setting.
“Studies have suggested that as patients age there is an increase in cardiological complications, noncardiological complications, pulmonary complications, and overall mortality,” he continues. “In my opinion there is going to be a crisis in regard to managing medical issues and complications surrounding surgery.” (See Table 1, p. 24)
Medications issues are another major reason hospitalists are called for surgical consults, says Benny Gavi, MD, hospitalist at Stanford Hospitals and Clinics in Calif. “I got consulted for a patient with tachycardia in the inpatient setting,” says Dr. Gavi. “By the time we saw the patient, the orthopedic surgeon had already ordered an echocardiogram and added a beta-blocker. When I looked at the patient I realized he had a gout flare; the colchicine that he took daily for his gout was never started in the inpatient setting, which ultimately delayed his physical therapy and added three additional days to his hospital stay.”
Co-management makes sense for still other reasons, he says.
“The knowledge base of both surgery and medicine is growing rapidly; no one person can remain on top of what is needed for both fields,” says Dr. Gavi. “In the last 20 years there has been a dramatic rise in the number of medications and some are very complicated. Also, physicians and surgeons both are being approached to participate more in quality initiatives and increasing throughput. As a result, physicians have to work faster and do more.”
Opportunities
In the United States, approximately 100,000 surgeries are performed each day and 36 million surgeries are performed each year at a cost of $450 billion annually. More than 1 million serious surgical adverse events each year cost $45 billion. Within two decades, the surgeries will increase by 25%, the associated cost will increase 50%, and the cost of in-hospital and long-term complications will increase 100%.
Along with postoperative care, there are increasing opportunities in the preoperative setting.
“At our institution, which is a tertiary care center with a huge surgical hospital, we determined that there was a need for hospitalists to provide medical management of surgical patients 10 years ago,” Dr. Jaffer says. “Patients were often not adequately prepared when they went to surgery, and sometimes in the morning of surgery the anesthesiologists would cancel their cases.”
The traditional model of physicians calling in consultants when problems arise might need to change.
“We are increasingly looking for ways to identify patients who have a high likelihood of developing medical problems and proactively getting involved,” says Dr. Gavi.
To co-manage, hospitalists must take ownership of some medical issues under specific conditions (diabetes, anticoagulation, blood pressure), says Dr. Jaffer.
The Benefits
To Latha Sivaprasad, MD, hospitalist at Beth Israel Medical Center in New York City, there are three main advantages of hospitalists’ involvement in perioperative co-management:
- Hospitalists typically perform comprehensive, multisystemic patient evaluations;
- Hospitalists are extremely accessible; and
- Hospitalists are up to date on inpatient medicine.
How up to date?
“Periop isn’t routinely taught in residency,” says Ali Usmani, MD, a hospitalist at the Cleveland Clinic. “In fact, I had little information about perioperative care.”
When he joined the hospitalist group after a three-year residency at Cleveland Clinic, Dr. Usmani did preparatory reading. Later, the hospitalist group gave him a helpful collection of essays.
“I was very nervous because, of course, I had never done this before,” he says. “Surprisingly, I also had not done a general medicine consult service where we see postoperative patients. It was scary to some extent, but I found out that it is easier than I thought because there are guidelines you can follow from the AHA/ACC that are fairly straightforward. It also meant a nice schedule change from being on the floors.”
Although conducting preoperative evaluations with patients was technically outpatient work, it was not like he was seeing patients with such simple illnesses as a cold or a sore throat. Also, he says, there were no new surprises postoperatively because either he or a hospitalist colleague had seen the patient preoperatively.
Dr. Usmani, also a clinical assistant professor of medicine at Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, believes patients are happier when seen by hospitalists because they get a standardized, holistic preoperative assessment. And, helping to reduce the number of unnecessary tests ordered by primary care physicians or surgeons makes him feel as though he’s making a valuable contribution.
New Niche
Dr. Sivaprasad, who is also doing a one-year fellowship in quality improvement and patient safety at Beth Israel, has practiced hospital medicine in four hospitals ranging from 500 to 1,000 beds. “The primary reason we are consulted by surgeons is for perioperative cardiac risk assessment,” says Dr. Sivaprasad. “Other reasons include co-managing a patient with comorbidities such as a history of diabetes, hypertension, or renal failure.”
From 2003-2006, Dr. Sivaprasad was one of 14 hospitalists consulted often by surgeons at St. John’s Mercy Hospital in St Louis, a 1,000-bed Level I trauma center. “We were consulted for postoperative co-management, preoperative evaluation, or more urgent cases such as a patient experiencing hypotension, atrial fibrillation, shortness of breath, decreased urine output, or renal failure,” she says.
Dr. Sivaprasad recently attended the Johns Hopkins conference on Perioperative Management. The session made it easier for her to do a systems-based consult.
“All hospitalists differ to the degree of perioperative medicine they feel comfortable with,” she says. “Hospitalists understand perioperative medicine on different levels. They all can do an acceptable consult; but there is a spectrum of how detailed one can be and what service one can provide for the surgeon and the patient.”
Dr. Jaffer finds his work in perioperative care fulfilling and considers it another way hospitalists can increase their influence.
“Often when you manage medical patients in the hospital, it’s you, the medical patient, and the patient’s primary care physician,” Dr. Jaffer says. “But when you start to manage surgical patients, you are really being looked at by your surgical colleagues as an expert in managing medical problems, just as you view them as experts in managing surgical problems. What I realize from this is that I can be a perioperative medicine expert as well.”
Are there any downfalls to co-managing surgical patients?
“Sometimes the surgeons order unnecessary lab tests such as PTTs [partial thromboplastin time] because they are concerned about bleeding and complications,” Dr. Usmani says. “The next day if there is a deranged PTT, we need to figure out whether to suggest postponing the surgery or go ahead with the surgery based on the patients’ past medical/family history. We try to get our surgeons and our colleagues to work together with us in that regard because they don’t want to postpone surgery either.”
Drs. Usmani, Gavi, Jaffer, and Sivaprasad all say that when surgeons can observe firsthand their hospitalist partners exhibiting expertise in acute care it appears to improve surgeons’ attitudes about the role and value of hospitalists.
In fact, says Dr. Usmani, surgeons call him or one of his colleagues to thank them. “They say, ‘We really appreciate what you’ve done for this patient,’ ’’ he says. “Even if we suggest canceling surgery, they respect that we have seen a potential problem instead of letting it go ahead. They are happy to receive this advice.”
Another new relationship is between anesthesiologists and hospitalists. “I spend a lot of time calling anesthesiologists in regard to patient cases, and a good many of them are surprised to get a call from a hospitalist,” Dr. Gavi says. “We especially work closely together when we get complicated patients ready for surgery.”
A recent encounter proved to Dr. Gavi the complementary nature of the hospitalist-anesthesiologist relationship.2
“A patient came to the hospital two weeks ago to have an elective total knee replacement,” says Dr. Gavi. “She was an older woman with severe pulmonary disease. When the anesthesiologists saw her in the preoperative waiting area and realized how sick she is, they wanted to cancel the surgery. But the surgeon told the anesthesiologist that this patient had been seen in our own preoperative clinic and cleared by a hospitalist.”
Dr. Gavi had done what is customary for an internist. He took a more in-depth look at her pulmonology and cardiac records, called her cardiologist for further history, and reassured the anesthesiologist and surgeon. The patient had her surgery.
The Future
“Perioperative co-management is becoming more of a visible need,” says Dr. Sivaprasad. “It bridges the gap between surgeons and internists.”
To those of his hospitalist colleagues who have little information and are a bit afraid to begin perioperative care practice, Dr. Usmani recommends attending a perioperative summit conference.
The session should teach how to set up a perioperative center and what to do when managing patients with certain conditions.
“Although you meet with patients preoperatively in an office setting, you don’t feel like a primary care physician,” Dr. Usmani says. “You feel as if you are a specialist. You are respected, and you are contributing to postoperative outcomes to the benefit of the patient.”
Perioperative patient management is also financially rewarding because reimbursement is higher than customary hospital medicine duties.
Dr. Jaffer, soon to be chief of the division of hospital medicine at the University of Miami Medical Center in Florida, is proud of the work he and his colleagues have done to grow the Cleveland Clinic perioperative summit. This third summit, in September, was organized in collaboration with the Society of Perioperative Assessment and Quality Improvement.
“I think this is something that every hospitalist should try,” Dr. Usmani says. “It is definitely a niche.” TH
Andrea Sattinger is a frequent contributor to The Hospitalist.
References
- Mangano DT. Perioperative medicine: NHLBI working group deliberations and recommendations. J Cardiothorac Vasc Anesth. 2004;18(1):1-6.
- Adebola O, Adesanya AO, Joshi GP. Hospitalists and anesthesiologists as perioperative physicians: Are their roles complementary? Proc. (Bayl Univ Med Cent) 2007 April;20(2):140-142.
New limits on resident work hours and the graying of the U.S. population are putting hospitalists in the forefront of helping surgeons manage their patients.
Because the Accreditation Council for Graduate Medical Education restricted resident duty hours, surgeons can no longer rely automatically on residents to medically manage their patients on the floors, says Amir K. Jaffer, MD, a hospitalist and an associate professor of medicine at the Cleveland Clinic Lerner College of Medicine of Case Western Reserve University in Ohio.
Meanwhile, the population over age 65 will double, increasing to 70 million over the next 10 to 15 years.1
“More patients living longer means an increase in surgeries along the way,” says Dr. Jaffer, who is also the medical director of the Internal Medicine Preoperative Assessment Consultation and Treatment program in the section of hospital medicine at the Cleveland Clinic. For him, the first place hospitalists need to co-manage is in the postoperative setting.
“Studies have suggested that as patients age there is an increase in cardiological complications, noncardiological complications, pulmonary complications, and overall mortality,” he continues. “In my opinion there is going to be a crisis in regard to managing medical issues and complications surrounding surgery.” (See Table 1, p. 24)
Medications issues are another major reason hospitalists are called for surgical consults, says Benny Gavi, MD, hospitalist at Stanford Hospitals and Clinics in Calif. “I got consulted for a patient with tachycardia in the inpatient setting,” says Dr. Gavi. “By the time we saw the patient, the orthopedic surgeon had already ordered an echocardiogram and added a beta-blocker. When I looked at the patient I realized he had a gout flare; the colchicine that he took daily for his gout was never started in the inpatient setting, which ultimately delayed his physical therapy and added three additional days to his hospital stay.”
Co-management makes sense for still other reasons, he says.
“The knowledge base of both surgery and medicine is growing rapidly; no one person can remain on top of what is needed for both fields,” says Dr. Gavi. “In the last 20 years there has been a dramatic rise in the number of medications and some are very complicated. Also, physicians and surgeons both are being approached to participate more in quality initiatives and increasing throughput. As a result, physicians have to work faster and do more.”
Opportunities
In the United States, approximately 100,000 surgeries are performed each day and 36 million surgeries are performed each year at a cost of $450 billion annually. More than 1 million serious surgical adverse events each year cost $45 billion. Within two decades, the surgeries will increase by 25%, the associated cost will increase 50%, and the cost of in-hospital and long-term complications will increase 100%.
Along with postoperative care, there are increasing opportunities in the preoperative setting.
“At our institution, which is a tertiary care center with a huge surgical hospital, we determined that there was a need for hospitalists to provide medical management of surgical patients 10 years ago,” Dr. Jaffer says. “Patients were often not adequately prepared when they went to surgery, and sometimes in the morning of surgery the anesthesiologists would cancel their cases.”
The traditional model of physicians calling in consultants when problems arise might need to change.
“We are increasingly looking for ways to identify patients who have a high likelihood of developing medical problems and proactively getting involved,” says Dr. Gavi.
To co-manage, hospitalists must take ownership of some medical issues under specific conditions (diabetes, anticoagulation, blood pressure), says Dr. Jaffer.
The Benefits
To Latha Sivaprasad, MD, hospitalist at Beth Israel Medical Center in New York City, there are three main advantages of hospitalists’ involvement in perioperative co-management:
- Hospitalists typically perform comprehensive, multisystemic patient evaluations;
- Hospitalists are extremely accessible; and
- Hospitalists are up to date on inpatient medicine.
How up to date?
“Periop isn’t routinely taught in residency,” says Ali Usmani, MD, a hospitalist at the Cleveland Clinic. “In fact, I had little information about perioperative care.”
When he joined the hospitalist group after a three-year residency at Cleveland Clinic, Dr. Usmani did preparatory reading. Later, the hospitalist group gave him a helpful collection of essays.
“I was very nervous because, of course, I had never done this before,” he says. “Surprisingly, I also had not done a general medicine consult service where we see postoperative patients. It was scary to some extent, but I found out that it is easier than I thought because there are guidelines you can follow from the AHA/ACC that are fairly straightforward. It also meant a nice schedule change from being on the floors.”
Although conducting preoperative evaluations with patients was technically outpatient work, it was not like he was seeing patients with such simple illnesses as a cold or a sore throat. Also, he says, there were no new surprises postoperatively because either he or a hospitalist colleague had seen the patient preoperatively.
Dr. Usmani, also a clinical assistant professor of medicine at Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, believes patients are happier when seen by hospitalists because they get a standardized, holistic preoperative assessment. And, helping to reduce the number of unnecessary tests ordered by primary care physicians or surgeons makes him feel as though he’s making a valuable contribution.
New Niche
Dr. Sivaprasad, who is also doing a one-year fellowship in quality improvement and patient safety at Beth Israel, has practiced hospital medicine in four hospitals ranging from 500 to 1,000 beds. “The primary reason we are consulted by surgeons is for perioperative cardiac risk assessment,” says Dr. Sivaprasad. “Other reasons include co-managing a patient with comorbidities such as a history of diabetes, hypertension, or renal failure.”
From 2003-2006, Dr. Sivaprasad was one of 14 hospitalists consulted often by surgeons at St. John’s Mercy Hospital in St Louis, a 1,000-bed Level I trauma center. “We were consulted for postoperative co-management, preoperative evaluation, or more urgent cases such as a patient experiencing hypotension, atrial fibrillation, shortness of breath, decreased urine output, or renal failure,” she says.
Dr. Sivaprasad recently attended the Johns Hopkins conference on Perioperative Management. The session made it easier for her to do a systems-based consult.
“All hospitalists differ to the degree of perioperative medicine they feel comfortable with,” she says. “Hospitalists understand perioperative medicine on different levels. They all can do an acceptable consult; but there is a spectrum of how detailed one can be and what service one can provide for the surgeon and the patient.”
Dr. Jaffer finds his work in perioperative care fulfilling and considers it another way hospitalists can increase their influence.
“Often when you manage medical patients in the hospital, it’s you, the medical patient, and the patient’s primary care physician,” Dr. Jaffer says. “But when you start to manage surgical patients, you are really being looked at by your surgical colleagues as an expert in managing medical problems, just as you view them as experts in managing surgical problems. What I realize from this is that I can be a perioperative medicine expert as well.”
Are there any downfalls to co-managing surgical patients?
“Sometimes the surgeons order unnecessary lab tests such as PTTs [partial thromboplastin time] because they are concerned about bleeding and complications,” Dr. Usmani says. “The next day if there is a deranged PTT, we need to figure out whether to suggest postponing the surgery or go ahead with the surgery based on the patients’ past medical/family history. We try to get our surgeons and our colleagues to work together with us in that regard because they don’t want to postpone surgery either.”
Drs. Usmani, Gavi, Jaffer, and Sivaprasad all say that when surgeons can observe firsthand their hospitalist partners exhibiting expertise in acute care it appears to improve surgeons’ attitudes about the role and value of hospitalists.
In fact, says Dr. Usmani, surgeons call him or one of his colleagues to thank them. “They say, ‘We really appreciate what you’ve done for this patient,’ ’’ he says. “Even if we suggest canceling surgery, they respect that we have seen a potential problem instead of letting it go ahead. They are happy to receive this advice.”
Another new relationship is between anesthesiologists and hospitalists. “I spend a lot of time calling anesthesiologists in regard to patient cases, and a good many of them are surprised to get a call from a hospitalist,” Dr. Gavi says. “We especially work closely together when we get complicated patients ready for surgery.”
A recent encounter proved to Dr. Gavi the complementary nature of the hospitalist-anesthesiologist relationship.2
“A patient came to the hospital two weeks ago to have an elective total knee replacement,” says Dr. Gavi. “She was an older woman with severe pulmonary disease. When the anesthesiologists saw her in the preoperative waiting area and realized how sick she is, they wanted to cancel the surgery. But the surgeon told the anesthesiologist that this patient had been seen in our own preoperative clinic and cleared by a hospitalist.”
Dr. Gavi had done what is customary for an internist. He took a more in-depth look at her pulmonology and cardiac records, called her cardiologist for further history, and reassured the anesthesiologist and surgeon. The patient had her surgery.
The Future
“Perioperative co-management is becoming more of a visible need,” says Dr. Sivaprasad. “It bridges the gap between surgeons and internists.”
To those of his hospitalist colleagues who have little information and are a bit afraid to begin perioperative care practice, Dr. Usmani recommends attending a perioperative summit conference.
The session should teach how to set up a perioperative center and what to do when managing patients with certain conditions.
“Although you meet with patients preoperatively in an office setting, you don’t feel like a primary care physician,” Dr. Usmani says. “You feel as if you are a specialist. You are respected, and you are contributing to postoperative outcomes to the benefit of the patient.”
Perioperative patient management is also financially rewarding because reimbursement is higher than customary hospital medicine duties.
Dr. Jaffer, soon to be chief of the division of hospital medicine at the University of Miami Medical Center in Florida, is proud of the work he and his colleagues have done to grow the Cleveland Clinic perioperative summit. This third summit, in September, was organized in collaboration with the Society of Perioperative Assessment and Quality Improvement.
“I think this is something that every hospitalist should try,” Dr. Usmani says. “It is definitely a niche.” TH
Andrea Sattinger is a frequent contributor to The Hospitalist.
References
- Mangano DT. Perioperative medicine: NHLBI working group deliberations and recommendations. J Cardiothorac Vasc Anesth. 2004;18(1):1-6.
- Adebola O, Adesanya AO, Joshi GP. Hospitalists and anesthesiologists as perioperative physicians: Are their roles complementary? Proc. (Bayl Univ Med Cent) 2007 April;20(2):140-142.
Masquerade: Medical causes of back pain
An elderly woman with asthma, eosinophilia, and septic shock
Obstructive sleep apnea and cardiovascular disease: Implications for clinical practice
What's Eating You? Pigeon Mite (Dermanyssus gallinae)
CPT codes diversify for hysterectomy and repair of paravaginal defects
There’s more: If you’ve been spending time on telephone or on-line counseling, codes that may get you paid for that service are about to make their debut.
Key additions and revisions to CPT for the new year are detailed in this article and in next issue’s Reimbursement Adviser.
Specify repair of paravaginal defect
57284 Paravaginal defect repair (including repair of cystocele, if performed); open abdominal approach
57285 Paravaginal defect repair (including repair of cystocele, if performed); vaginal approach
57423 Paravaginal defect repair (including repair of cystocele, if performed); laparoscopic approach
You’ll now have to carefully document your surgical approach to repairing a paravaginal defect, thanks to creation of two new codes and revision of the existing 57284.
Several bundles are still attached to the new codes, however. CPT did remove references to “stress urinary incontinence, and/or incomplete vaginal prolapse” from the revised and new codes, but repair of a cystocele, by any method, is still included.
CPT 2008 is, therefore, listing codes that cannot be reported additionally. In general, urethropexy codes 51840, 51841, 51990, 58152, and 58267 and cystocele repair codes 57240, 57260, and 57265 should not be reported when a paravaginal defect repair is performed.
Also, be alert for any National Correct Coding Initiatives (NCCI) bundles assigned by Medicare to these new codes if they are different from the ones that will be listed by CPT. In particular, 57288 [sling operation for stress incontinence (e.g., fascia or synthetic)] was permanently bundled into 57284. (If that bundle isn’t removed in 2008, I encourage you to contact ACOG and urge the College to discuss this inappropriate bundle with Medicare administrators.)
Total lap hysterectomy
58570 Laparoscopy, surgical, with total hysterectomy, for uterus 250 g or less
58571 Laparoscopy, surgical, with total hysterectomy, for uterus 250 g or less; with removal of tube(s) and/or ovary(ies)
58572 Laparoscopy, surgical, with total hysterectomy, for uterus greater than 250 g
58573 Laparoscopy, surgical, with total hysterectomy, for uterus greater than 250 g; with removal of tube(s) and/or ovary(ies)
For some time, surgeons have been able to perform a hysterectomy by completely detaching both the uterine cervix and the body of the uterus from their surrounding support structures laparoscopically, then closing the vaginal cuff via this approach as well. Before 2008, the only coding choices were laparoscopic-assisted hysterectomy codes (58550–58554) or the unlisted laparoscopic code 58578. The new codes—as with codes for any vaginal or laparoscopic approach—are selected based on 1) the documented weight of the uterus and 2) whether the fallopian tubes or ovaries have been removed.
Intraperitoneal tumors, coded by size
49203 Excision or destruction, open, intra-abdominal tumors, cysts or endometriomas, 1 or more peritoneal, mesenteric, or retroperitoneal primary or secondary tumors; largest tumor 5 cm diameter or less
49204 …largest tumor 5.1–10.0 cm diameter
49205 …largest tumor greater than 10.0 cm diameter
In 2007, documenting the removal of intraperitoneal or retroperitoneal tumors, cysts, and endometriomas via abdominal incision was fairly simple: There were two codes and you had only to decide if removal was extensive or not.
In 2008, codes 49200 and 49201 are deleted and replaced by three new codes—each of which requires you to document the size of the largest tumor or lesion removed.
The new codes will come in handy during surgery in which the originating organ has been removed but the patient is found to have additional tumors. For example: A patient had ovarian cancer and now there are additional tumors in the abdominal cavity, but an omentectomy is not being performed. Of course, the new codes can still be used for excision or destruction of cysts or endometriomas, as well. But CPT has also listed codes that cannot be billed with the new codes: Among them are 38770 [pelvic lymphadenectomy] and 58900–58960 [surgeries performed on the ovaries]. If the new codes don’t fit the surgery, the other option for tumor debulking after the organ has been removed is to report 58957 or 58958; note, however, that these codes include omentectomy and optional pelvic lymph node sampling.
Bladder aspiration is renumbered
51100 Aspiration of bladder; by needle
51101 …by trocar or intracatheter
51102 …with insertion of suprapubic catheter
If you have the old codes for bladder aspiration memorized, relearn them. Once again, CPT tinkered with placement of codes and decided that bladder aspiration codes are placed more appropriately under “Bladder, Removal” than “Bladder, Incision.” The uses of those codes are unchanged.
Giving flu, HPV vaccines
90661 Influenza virus vaccine, derived from cell cultures, subunit, preservative and antibiotic free, for intramuscular use
90662 Influenza virus vaccine, split virus, preservative free, enhanced immunogenicity via increased antigen content, for intramuscular use
90663 Influenza virus vaccine, pandemic formulation
90650 Human papillomavirus (HPV) vaccine, types 16 and 18, bivalent, 3-dose schedule, for intramuscular use
Four new codes for vaccines can be reported beginning January 1, but only those for the influenza vaccine appear in the CPT 2008 book. The code for the new bivalent HPV vaccine is a valid code for 2008 but will not appear in print until CPT 2009.
Changes made to “modifier -51” exemptions
CPT 2008 also reassessed codes that have been designated as “modifier - 51 exempt.” Typically, these are codes that do not involve significant preoperative or postoperative work. 36660 [catheterization, umbilical artery, newborn, for diagnosis or therapy] now requires a modifier when performed with other procedures, whereas 51797 [voiding pressure studies (VP); intra-abdominal voiding pressure (AP) (rectal, gastric, intraperitoneal)] becomes an add-on code that does not take a modifier -51. Beginning January 1, 51797 can be billed only if 51795 [voiding pressure studies (VP); bladder voiding pressure, any technique] has also been reported.
Fecal blood testing
If you bill 82272 [blood, occult, by peroxidase activity (e.g., guaiac), qualitative, feces, single specimen (e.g., from digital rectal exam)] for the annual fecal occult blood screening test, CPT has revised the code to make it clear that this code is not to be reported for a screening test.
The only two CPT codes that can be reported for the screening fecal occult blood test are 82270 [blood, occult, by peroxidase activity (e.g., guaiac), qualitative, feces, consecutive collected specimens with single determination, for colorectal neoplasm screening] (that is, the patient was provided three cards or a single triple card for consecutive collection) or 82274 [blood, occult, by fecal hemoglobin determination by immunoassay, qualitative, feces, 1–3 simultaneous determinations].
Note: The physician may collect the specimen for an immunoassay (except on a Medicare patient), but a guaiac test specimen must be collected by the patient.
Cervical biopsy
The descriptor for 57500 will now specifically refer to the cervix as the location for biopsy or excision of a lesion. Before this change, only the subheading title gave any indication of anatomic location.
Hysterectomy
If you perform a laparoscopic-assisted (58550–58554), total (58570–58573), or supracervical (58541–58544) hysterectomy, CPT has added a list of codes that you may not report as well. These include:
- 49320 [diagnostic laparoscopy]
- 57000 [colpotomy]
- 57180 [hemostatic vaginal packing]
- 57410 [EUA]
- 58140–58146, 58545–58546, 58561 [myomectomy]
- 58661 [removal of tubes and/or ovaries]
- 58670, 58671 [tubal ligations]
Vascular ultrasound
Last, CPT has clarified that, to bill 93975 or 93976 [duplex scan of arterial inflow and venous outflow of abdominal, pelvic, scrotal contents and/or retroperitoneal organs], the purpose of the exam must be to evaluate vascular structures. If color Doppler ultrasound is used to identify anatomic structures at the time of US scan, neither of those two codes may be billed additionally.
There’s more: If you’ve been spending time on telephone or on-line counseling, codes that may get you paid for that service are about to make their debut.
Key additions and revisions to CPT for the new year are detailed in this article and in next issue’s Reimbursement Adviser.
Specify repair of paravaginal defect
57284 Paravaginal defect repair (including repair of cystocele, if performed); open abdominal approach
57285 Paravaginal defect repair (including repair of cystocele, if performed); vaginal approach
57423 Paravaginal defect repair (including repair of cystocele, if performed); laparoscopic approach
You’ll now have to carefully document your surgical approach to repairing a paravaginal defect, thanks to creation of two new codes and revision of the existing 57284.
Several bundles are still attached to the new codes, however. CPT did remove references to “stress urinary incontinence, and/or incomplete vaginal prolapse” from the revised and new codes, but repair of a cystocele, by any method, is still included.
CPT 2008 is, therefore, listing codes that cannot be reported additionally. In general, urethropexy codes 51840, 51841, 51990, 58152, and 58267 and cystocele repair codes 57240, 57260, and 57265 should not be reported when a paravaginal defect repair is performed.
Also, be alert for any National Correct Coding Initiatives (NCCI) bundles assigned by Medicare to these new codes if they are different from the ones that will be listed by CPT. In particular, 57288 [sling operation for stress incontinence (e.g., fascia or synthetic)] was permanently bundled into 57284. (If that bundle isn’t removed in 2008, I encourage you to contact ACOG and urge the College to discuss this inappropriate bundle with Medicare administrators.)
Total lap hysterectomy
58570 Laparoscopy, surgical, with total hysterectomy, for uterus 250 g or less
58571 Laparoscopy, surgical, with total hysterectomy, for uterus 250 g or less; with removal of tube(s) and/or ovary(ies)
58572 Laparoscopy, surgical, with total hysterectomy, for uterus greater than 250 g
58573 Laparoscopy, surgical, with total hysterectomy, for uterus greater than 250 g; with removal of tube(s) and/or ovary(ies)
For some time, surgeons have been able to perform a hysterectomy by completely detaching both the uterine cervix and the body of the uterus from their surrounding support structures laparoscopically, then closing the vaginal cuff via this approach as well. Before 2008, the only coding choices were laparoscopic-assisted hysterectomy codes (58550–58554) or the unlisted laparoscopic code 58578. The new codes—as with codes for any vaginal or laparoscopic approach—are selected based on 1) the documented weight of the uterus and 2) whether the fallopian tubes or ovaries have been removed.
Intraperitoneal tumors, coded by size
49203 Excision or destruction, open, intra-abdominal tumors, cysts or endometriomas, 1 or more peritoneal, mesenteric, or retroperitoneal primary or secondary tumors; largest tumor 5 cm diameter or less
49204 …largest tumor 5.1–10.0 cm diameter
49205 …largest tumor greater than 10.0 cm diameter
In 2007, documenting the removal of intraperitoneal or retroperitoneal tumors, cysts, and endometriomas via abdominal incision was fairly simple: There were two codes and you had only to decide if removal was extensive or not.
In 2008, codes 49200 and 49201 are deleted and replaced by three new codes—each of which requires you to document the size of the largest tumor or lesion removed.
The new codes will come in handy during surgery in which the originating organ has been removed but the patient is found to have additional tumors. For example: A patient had ovarian cancer and now there are additional tumors in the abdominal cavity, but an omentectomy is not being performed. Of course, the new codes can still be used for excision or destruction of cysts or endometriomas, as well. But CPT has also listed codes that cannot be billed with the new codes: Among them are 38770 [pelvic lymphadenectomy] and 58900–58960 [surgeries performed on the ovaries]. If the new codes don’t fit the surgery, the other option for tumor debulking after the organ has been removed is to report 58957 or 58958; note, however, that these codes include omentectomy and optional pelvic lymph node sampling.
Bladder aspiration is renumbered
51100 Aspiration of bladder; by needle
51101 …by trocar or intracatheter
51102 …with insertion of suprapubic catheter
If you have the old codes for bladder aspiration memorized, relearn them. Once again, CPT tinkered with placement of codes and decided that bladder aspiration codes are placed more appropriately under “Bladder, Removal” than “Bladder, Incision.” The uses of those codes are unchanged.
Giving flu, HPV vaccines
90661 Influenza virus vaccine, derived from cell cultures, subunit, preservative and antibiotic free, for intramuscular use
90662 Influenza virus vaccine, split virus, preservative free, enhanced immunogenicity via increased antigen content, for intramuscular use
90663 Influenza virus vaccine, pandemic formulation
90650 Human papillomavirus (HPV) vaccine, types 16 and 18, bivalent, 3-dose schedule, for intramuscular use
Four new codes for vaccines can be reported beginning January 1, but only those for the influenza vaccine appear in the CPT 2008 book. The code for the new bivalent HPV vaccine is a valid code for 2008 but will not appear in print until CPT 2009.
Changes made to “modifier -51” exemptions
CPT 2008 also reassessed codes that have been designated as “modifier - 51 exempt.” Typically, these are codes that do not involve significant preoperative or postoperative work. 36660 [catheterization, umbilical artery, newborn, for diagnosis or therapy] now requires a modifier when performed with other procedures, whereas 51797 [voiding pressure studies (VP); intra-abdominal voiding pressure (AP) (rectal, gastric, intraperitoneal)] becomes an add-on code that does not take a modifier -51. Beginning January 1, 51797 can be billed only if 51795 [voiding pressure studies (VP); bladder voiding pressure, any technique] has also been reported.
Fecal blood testing
If you bill 82272 [blood, occult, by peroxidase activity (e.g., guaiac), qualitative, feces, single specimen (e.g., from digital rectal exam)] for the annual fecal occult blood screening test, CPT has revised the code to make it clear that this code is not to be reported for a screening test.
The only two CPT codes that can be reported for the screening fecal occult blood test are 82270 [blood, occult, by peroxidase activity (e.g., guaiac), qualitative, feces, consecutive collected specimens with single determination, for colorectal neoplasm screening] (that is, the patient was provided three cards or a single triple card for consecutive collection) or 82274 [blood, occult, by fecal hemoglobin determination by immunoassay, qualitative, feces, 1–3 simultaneous determinations].
Note: The physician may collect the specimen for an immunoassay (except on a Medicare patient), but a guaiac test specimen must be collected by the patient.
Cervical biopsy
The descriptor for 57500 will now specifically refer to the cervix as the location for biopsy or excision of a lesion. Before this change, only the subheading title gave any indication of anatomic location.
Hysterectomy
If you perform a laparoscopic-assisted (58550–58554), total (58570–58573), or supracervical (58541–58544) hysterectomy, CPT has added a list of codes that you may not report as well. These include:
- 49320 [diagnostic laparoscopy]
- 57000 [colpotomy]
- 57180 [hemostatic vaginal packing]
- 57410 [EUA]
- 58140–58146, 58545–58546, 58561 [myomectomy]
- 58661 [removal of tubes and/or ovaries]
- 58670, 58671 [tubal ligations]
Vascular ultrasound
Last, CPT has clarified that, to bill 93975 or 93976 [duplex scan of arterial inflow and venous outflow of abdominal, pelvic, scrotal contents and/or retroperitoneal organs], the purpose of the exam must be to evaluate vascular structures. If color Doppler ultrasound is used to identify anatomic structures at the time of US scan, neither of those two codes may be billed additionally.
There’s more: If you’ve been spending time on telephone or on-line counseling, codes that may get you paid for that service are about to make their debut.
Key additions and revisions to CPT for the new year are detailed in this article and in next issue’s Reimbursement Adviser.
Specify repair of paravaginal defect
57284 Paravaginal defect repair (including repair of cystocele, if performed); open abdominal approach
57285 Paravaginal defect repair (including repair of cystocele, if performed); vaginal approach
57423 Paravaginal defect repair (including repair of cystocele, if performed); laparoscopic approach
You’ll now have to carefully document your surgical approach to repairing a paravaginal defect, thanks to creation of two new codes and revision of the existing 57284.
Several bundles are still attached to the new codes, however. CPT did remove references to “stress urinary incontinence, and/or incomplete vaginal prolapse” from the revised and new codes, but repair of a cystocele, by any method, is still included.
CPT 2008 is, therefore, listing codes that cannot be reported additionally. In general, urethropexy codes 51840, 51841, 51990, 58152, and 58267 and cystocele repair codes 57240, 57260, and 57265 should not be reported when a paravaginal defect repair is performed.
Also, be alert for any National Correct Coding Initiatives (NCCI) bundles assigned by Medicare to these new codes if they are different from the ones that will be listed by CPT. In particular, 57288 [sling operation for stress incontinence (e.g., fascia or synthetic)] was permanently bundled into 57284. (If that bundle isn’t removed in 2008, I encourage you to contact ACOG and urge the College to discuss this inappropriate bundle with Medicare administrators.)
Total lap hysterectomy
58570 Laparoscopy, surgical, with total hysterectomy, for uterus 250 g or less
58571 Laparoscopy, surgical, with total hysterectomy, for uterus 250 g or less; with removal of tube(s) and/or ovary(ies)
58572 Laparoscopy, surgical, with total hysterectomy, for uterus greater than 250 g
58573 Laparoscopy, surgical, with total hysterectomy, for uterus greater than 250 g; with removal of tube(s) and/or ovary(ies)
For some time, surgeons have been able to perform a hysterectomy by completely detaching both the uterine cervix and the body of the uterus from their surrounding support structures laparoscopically, then closing the vaginal cuff via this approach as well. Before 2008, the only coding choices were laparoscopic-assisted hysterectomy codes (58550–58554) or the unlisted laparoscopic code 58578. The new codes—as with codes for any vaginal or laparoscopic approach—are selected based on 1) the documented weight of the uterus and 2) whether the fallopian tubes or ovaries have been removed.
Intraperitoneal tumors, coded by size
49203 Excision or destruction, open, intra-abdominal tumors, cysts or endometriomas, 1 or more peritoneal, mesenteric, or retroperitoneal primary or secondary tumors; largest tumor 5 cm diameter or less
49204 …largest tumor 5.1–10.0 cm diameter
49205 …largest tumor greater than 10.0 cm diameter
In 2007, documenting the removal of intraperitoneal or retroperitoneal tumors, cysts, and endometriomas via abdominal incision was fairly simple: There were two codes and you had only to decide if removal was extensive or not.
In 2008, codes 49200 and 49201 are deleted and replaced by three new codes—each of which requires you to document the size of the largest tumor or lesion removed.
The new codes will come in handy during surgery in which the originating organ has been removed but the patient is found to have additional tumors. For example: A patient had ovarian cancer and now there are additional tumors in the abdominal cavity, but an omentectomy is not being performed. Of course, the new codes can still be used for excision or destruction of cysts or endometriomas, as well. But CPT has also listed codes that cannot be billed with the new codes: Among them are 38770 [pelvic lymphadenectomy] and 58900–58960 [surgeries performed on the ovaries]. If the new codes don’t fit the surgery, the other option for tumor debulking after the organ has been removed is to report 58957 or 58958; note, however, that these codes include omentectomy and optional pelvic lymph node sampling.
Bladder aspiration is renumbered
51100 Aspiration of bladder; by needle
51101 …by trocar or intracatheter
51102 …with insertion of suprapubic catheter
If you have the old codes for bladder aspiration memorized, relearn them. Once again, CPT tinkered with placement of codes and decided that bladder aspiration codes are placed more appropriately under “Bladder, Removal” than “Bladder, Incision.” The uses of those codes are unchanged.
Giving flu, HPV vaccines
90661 Influenza virus vaccine, derived from cell cultures, subunit, preservative and antibiotic free, for intramuscular use
90662 Influenza virus vaccine, split virus, preservative free, enhanced immunogenicity via increased antigen content, for intramuscular use
90663 Influenza virus vaccine, pandemic formulation
90650 Human papillomavirus (HPV) vaccine, types 16 and 18, bivalent, 3-dose schedule, for intramuscular use
Four new codes for vaccines can be reported beginning January 1, but only those for the influenza vaccine appear in the CPT 2008 book. The code for the new bivalent HPV vaccine is a valid code for 2008 but will not appear in print until CPT 2009.
Changes made to “modifier -51” exemptions
CPT 2008 also reassessed codes that have been designated as “modifier - 51 exempt.” Typically, these are codes that do not involve significant preoperative or postoperative work. 36660 [catheterization, umbilical artery, newborn, for diagnosis or therapy] now requires a modifier when performed with other procedures, whereas 51797 [voiding pressure studies (VP); intra-abdominal voiding pressure (AP) (rectal, gastric, intraperitoneal)] becomes an add-on code that does not take a modifier -51. Beginning January 1, 51797 can be billed only if 51795 [voiding pressure studies (VP); bladder voiding pressure, any technique] has also been reported.
Fecal blood testing
If you bill 82272 [blood, occult, by peroxidase activity (e.g., guaiac), qualitative, feces, single specimen (e.g., from digital rectal exam)] for the annual fecal occult blood screening test, CPT has revised the code to make it clear that this code is not to be reported for a screening test.
The only two CPT codes that can be reported for the screening fecal occult blood test are 82270 [blood, occult, by peroxidase activity (e.g., guaiac), qualitative, feces, consecutive collected specimens with single determination, for colorectal neoplasm screening] (that is, the patient was provided three cards or a single triple card for consecutive collection) or 82274 [blood, occult, by fecal hemoglobin determination by immunoassay, qualitative, feces, 1–3 simultaneous determinations].
Note: The physician may collect the specimen for an immunoassay (except on a Medicare patient), but a guaiac test specimen must be collected by the patient.
Cervical biopsy
The descriptor for 57500 will now specifically refer to the cervix as the location for biopsy or excision of a lesion. Before this change, only the subheading title gave any indication of anatomic location.
Hysterectomy
If you perform a laparoscopic-assisted (58550–58554), total (58570–58573), or supracervical (58541–58544) hysterectomy, CPT has added a list of codes that you may not report as well. These include:
- 49320 [diagnostic laparoscopy]
- 57000 [colpotomy]
- 57180 [hemostatic vaginal packing]
- 57410 [EUA]
- 58140–58146, 58545–58546, 58561 [myomectomy]
- 58661 [removal of tubes and/or ovaries]
- 58670, 58671 [tubal ligations]
Vascular ultrasound
Last, CPT has clarified that, to bill 93975 or 93976 [duplex scan of arterial inflow and venous outflow of abdominal, pelvic, scrotal contents and/or retroperitoneal organs], the purpose of the exam must be to evaluate vascular structures. If color Doppler ultrasound is used to identify anatomic structures at the time of US scan, neither of those two codes may be billed additionally.
Empathy goes a long way in weight loss discussions
- A physician’s empathy, collaborative approach, and words of support can have a positive effect on overweight and obese women’s weight loss efforts.
Purpose This study explores how weight-related topics are discussed between physicians and their overweight and obese female patients.
Methods We surveyed and audio-recorded preventive health and chronic care visits with 25 overweight and obese female patients. We coded both for quantity (content and time) of weight-related discussions and quality (adherence to Motivational Interviewing [MI] techniques). We then tested correlations of these measures with patients’ reported attempts to lose weight, change diet, and change exercise patterns 1 month after the visit.
Results Weight was routinely addressed (19 of 25 encounters). Patients usually initiated the topic (67% of time). Physicians’ use of MI techniques resulted in patients attempting to lose weight and changing their exercise patterns.
Conclusion Physicians may benefit from MI training to help patients lose weight.
Research has shown that when physicians advise overweight patients to lose weight, improve their diet, or increase their physical activity, patients are more likely to report attempting to do so.1-3 In a study of 433 primary care patients, 46% reported trying to lose weight after their physician counseled them about nutrition, compared with 37% who were not counseled.1
The reality, though, is that physicians are not very likely to address weight loss. Data from the Behavioral Risk Factor Surveillance System indicate that patients report their providers address weight loss in fewer than 20% of their examinations.4 These low rates are concerning; when physicians do not advise patients to lose weight, patients may believe their weight is not a problem.5 Even more worrisome: Physicians are rarely trained on how to counsel patients about weight loss. So, when physicians do counsel patients, it may not be effective.
Using Motivational Interviewing
One effective style of counseling is Motivational Interviewing (MI). MI is a patient-centered, directive counseling style used to help patients explore and resolve their ambivalence related to a particular behavior change (see What is Motivational Interviewing?).6,7 Researchers have studied the use of MI by counselors and case managers (in handling smoking cessation),8-11 but not by physicians. Further, no one has examined whether physicians instinctively use MI techniques when discussing weight loss with their patients, or whether MI counseling results in patients trying to lose weight.
The primary aim of this study was to assess how overweight and obese female patients discuss weight loss with their physicians. We also wanted to explore the role that physicians’ way of discussing weight loss—and the use of MI in particular—might play in their patients’ motivation to lose weight.
Motivational Interviewing is a counseling style intended to create changes in behavior by helping patients to explore and resolve their ambivalence.7 In a patient-physician encounter guided by MI:
- The motivation to change comes from the patient.
- It is the patient’s job to articulate and resolve his or her ambivalence.
- Direct persuasion is not used; the physician is quiet and eliciting, but directive in helping the patient examine his or her ambivalence.
- Readiness for change is recognized not as a patient trait, but as a part of the interaction between physician and patient.
- The patient-physician relationship is regarded more like a partnership.
Methods
Setting and recruitment
All data were collected in a family practice clinic within Duke University Medical Center. We approached 9 physicians in the practice to participate, and all consented. Only 7 physicians had visits with overweight or obese patients and were included in this report. We reviewed their electronic patient appointment schedules twice a week to identify female patients meeting the following criteria: English-speaking, overweight or obese (body mass index [BMI] ≥25 kg/m2), 40 years of age or older, and with health maintenance or chronic care appointments scheduled at least 7 days later. We sent these patients a letter describing the study, and allowed them 7 days to call a toll-free number if they didn’t want to participate.
We took several steps to avoid priming physicians and patients about the purpose of the study. First, both physicians and patients were told the study was about how doctors and patients discuss preventive health topics—they were not told the study was about examining discussions of weight. Second, we surveyed physicians 1 month prior to audio-recording visits, and patients 1 week prior to their visit. Third, we included measures for other preventive health topics (eg, smoking and alcohol) to detract attention from weight.
Gathering data
1. Phone survey before patient visit. We telephoned those patients who did not refuse participation and conducted a baseline survey. We asked about date of birth, race, marital status, level of education, income, weight, height, history of weight loss attempts, and whether this was their first visit with that physician. We categorized women with a BMI ≥25 but <30 as overweight, and those with a BMI ≥30 as obese.12
We also assessed each patient’s
- self-efficacy—that is, confidence in their ability to lose weight. We asked: “How confident are you that you can lose weight?” (1=not at all confident, 5=extremely confident).
- readiness to lose weight. We asked: “Are you seriously considering trying to losing weight within the next 6 months?” and, if yes, “Are you planning to try to lose weight in the next 30 days?”13 Those not considering trying to lose weight were staged as precontemplation; those who were considering trying but not planning to try in the next 30 days were staged as contemplation; and those who were planning to try to lose weight in the next 30 days were staged as preparation.
2. Office visit. When patients came in for their appointments, the research assistant gave them consent forms to sign. The assistant then escorted the patient to the examination rooms and started the digital audio recorder. The exams typically took 27 minutes.
Immediately following the exam, the research assistant surveyed the patients. The assistant asked 2 questions we’d asked at baseline: “How confident are you that you can lose weight?” and “Are you seriously considering trying to losing weight within the next 6 months?” (If yes, “Are you planning to try to lose weight in the next 30 days?”) She also made an appointment to conduct a 1-month follow-up telephone survey.
3. One-month follow-up survey. During a follow-up phone survey, we asked patients whether they had attempted to lose weight by changing their diet, exercise patterns, or both. Subsequent to this call, we sent the study participants a $25 check.
Analyzing the patient-physician discussion
Content. Two authors coded 9 topics that physicians and patients discussed that were “weight-related.” Topics included: physical activity, diet, BMI, psychosocial issues, referral to a nutritionist, weight loss surgery, goal setting, weight loss medications, and health care avoidance. We also coded who first brought up the topic.
Time spent. We calculated time spent discussing weight-related topics and also the total time of the patient’s visit.
Motivational Interviewing. Two coders assessed MI. To assess fidelity to MI principles, we used sections of the Motivational Interviewing Treatment Integrity scale (MITI)14 to rate patient interactions on a scale of 1 (low) to 7 (high) in 2 categories: empathy and MI spirit.
- Empathy is when physicians convey understanding of patients’ perspective.
- MI spirit includes evocation, collaboration, and autonomy. Evocation is when physicians draw out patients’ own reasons for change. Collaboration is when physicians act as partners, supporting and exploring patients’ concerns. Autonomy is when physicians convey that decisions to change lie completely with patients. Inter-rater reliability for the Empathy and MI Spirit was adequate (ICC=.94 and .97, respectively).
- MI-adherent behaviors were those where the physician asked permission to do things, affirmed statements, offered words of support, and emphasized patient control. For instance, the physician might say, “It’s great that you have stopped drinking sweetened tea” or “Whether you lose weight is up to you.”
- MI-nonadherent behaviors were those where the physician advised without asking permission. For example, the physician might say, “Let me tell you what you need to do to make this work…” or “Well, if you want to continue on the way you are, you know your diabetes is only going to get worse.”
These were combined to create a ratio of percentage MI-adherent behaviors by dividing MI-adherent by MI-nonadherent. There was an excellent level of agreement between coders for MI-nonadherent (kappa=.80) and a moderate level of agreement for MI-adherent (kappa=.52) behaviors.
Data analysis
We used Spearman correlations to assess the relationship between our predictors, quantity (time spent and whether weight was addressed) and quality (MI techniques), and mediators of behavior change (readiness to lose weight and self-efficacy to lose weight) and behavior change (attempts to lose weight, change in diet, and change in exercise patterns). We used SAS 9.1 (SAS Institute, Inc, Cary, NC) for all analyses. The study was approved by the Duke University Medical Center Institutional Review Board.
Results
We identified 202 eligible female patients. Of those, 96 had appointments that passed before we could contact them; 11 called the 800 number to refuse. Of the remaining 95 women, we reached 94 by phone. Of those, 19 refused to participate, 46 were ineligible because we had reached the targeted number of women in their weight category, and 4 skipped their appointments. Thus, we audio-recorded 25 encounters (for 14 obese and 11 overweight patients). Of these 25 patients, 24 completed the 1-month follow-up.
Patient demographics. Patients had a mean age of 59 years (standard deviation [SD]=11). Half were white; 42% were college-educated. Forty-two percent reported being in poor to fair health (TABLE 1).
The typical participant was moderately confident and ready to lose weight both before and after their visit. One month after their visit, 63% reported attempting to lose weight. More than half attempted to change their diet (67%); slightly more than half changed their exercise patterns (58%) (TABLE 2).
Physician demographics. Physicians had a mean age of 43 years (SD=10). About half were white; about half were female. No physicians were overweight.
TABLE 1
Characteristics of patients and physicians
| CHARACTERISTIC | PATIENT (N=25) | PHYSICIAN (N=7) |
|---|---|---|
| Age (M, SD) | 59 (11) | 43 (10) |
| Race (%)* | ||
| White | 50 | 57 |
| Black | 50 | 29 |
| Indian | 14 | |
| Female (%) | 100 | 57 |
| Married (%) | 46 | — |
| Employed (%) | 54 | 100 |
| College graduate (%) | 42 | 100 |
| Health status, self-reported (%) | ||
| Poor to fair | 42 | — |
| Good | 37 | — |
| Very good to excellent | 21 | — |
| Times lost at least 10 lbs (mean, SD) | 5.8 (4.0) | — |
| New patient with physician (%) | 12 | — |
| Body mass index (mean, SD) | 37 (11) | 22 (3) |
| * One participant did not provide his/her race. | ||
TABLE 2
Feeling about weight loss before and after the visit
| BASELINE | POST-VISIT | 1 MONTH | |
|---|---|---|---|
| Mediators of behavior change | |||
| Confidence in losing weight (M, SD)* | 3.8 (1.4) | 3.8 (1.1) | – |
| Stage of readiness to lose weight (%) | |||
| Precontemplation | 25% | 28% | – |
| Contemplation | 8% | 8% | – |
| Preparation | 67% | 64% | – |
| Behavior change variables | |||
| Attempted to lose weight (%) | – | – | 63% |
| Attempted to change diet (%) | – | – | 67% |
| Changed exercise patterns (%) | – | – | 58% |
| * Scale ranged from 1=not at all confident to 5=extremely confident. | |||
Patients were more likely to raise the weight issue
Weight-related topics were addressed in 19 of the 25 encounters (11 out of 12 preventive health visits, 8 out of 13 chronic care visits). The mean time spent discussing weight-related topics was 6.9 minutes out of an mean total of 27.0 minutes, or 26% of the total patient-physician time. Weight was more likely to be addressed with obese patients (86%) than with overweight patients (63%).
Patients were more likely than physicians to initiate discussions on weight. Physicians raised weight-related topics 37% of the time. Obese patients were slightly more likely to raise weight-related topics (8 out of 12 times [67%]) than overweight patients (4 out of 7 times [57%]).
The weight-related topics addressed were, in order from most to least frequent: physical activity, diet, BMI, psychosocial issues (eg, motivation to lose weight, triggers for unhealthful eating [such as family cookouts], negative talk [such as telling oneself that losing weight is too hard]), referral to a nutritionist, weight loss surgery, goal setting, health care avoidance, and weight loss medication. When comparing those who attempted to lose weight (n=15) with those who did not (n=9), there was no significant difference in whether or how often a topic was addressed.
Physicians’ empathy scores are moderate
Physicians had a moderate score for Empathy (mean=3.8, standard deviation [SD]=1.5, on 7-point scale), a low score for MI Spirit (mean=2.4, standard deviation [SD]=1.4, on 7-point scale), and displayed fewer MI-adherent behaviors than MI-nonadherent behaviors (mean=0.4, SD=0.3). These means did not differ significantly based on the patients’ weight.
Weight loss conversations linked to patients’ readiness
The discussion of weight-related topics, and the time spent doing so, were related to patients’ readiness to lose weight after their initial examination, when patients’ baseline readiness to lose weight was controlled. The more ready patients were to lose weight after their visit, the more likely they had discussed weight (Spearman’s rank correlation coefficient [r]=.52, P=.01) and spent more time discussing weight (r=.42, P=.05). No other associations were statistically significant (TABLE W1).
Several of the Motivational Interviewing scores predicted patients’ outcomes. When physicians showed more empathy, patients were more likely to report changing their exercise patterns 1 month after the visit (r=.50, P=.02). When physicians displayed more of an MI Spirit, patients were more likely to be ready to lose weight (r=.63, P=.005) and change their exercise patterns (r=.47, P=.04). Further, when physicians used more MI-adherent techniques, patients were more likely to attempt to lose weight (r=.42, P=.08).
Discussion: Good quality discussions lead to change
While more discussion about weight loss led to a greater readiness to lose weight, it was the quality of the discussions that actually led to behavior changes. Most patients had virtually the same levels of readiness to lose weight before and after the visit. It is likely that patients who were ready to lose weight discussed their weight with their physicians—and spent more time discussing it than those patients who were not ready to lose weight.
How patients and physicians discussed weight influenced behavior change. When physicians were more empathic and used techniques consistent with Motivational Interviewing, patients were more likely to report changing their exercise routine and attempting to lose weight.
To date, no one has examined the effect of physicians’ MI techniques on weight-related behavior change in a large study. The low adherence to MI techniques suggests that physicians can improve their counseling skills.
Patients aren’t afraid to talk about their weight
Unexpectedly, patients were more likely than physicians to initiate weight-related discussions. Only one third of the time did physicians raise the topic. Patients appear to be “empowered” to initiate discussions about weight loss. We expected physicians and patients to both be somewhat apprehensive about raising this sensitive topic. However, these findings suggest that overweight and obese patients will initiate the discussion most of the time.
Limitations and strengths of this study
The small sample size limited the analyses. Nonetheless, we found strong correlations in this sample that suggest true relationships that were unlikely to have occurred by chance. Also, we were unable to conduct nested analyses to account for the clustering of patients seen by the same physicians. The results may not generalize to settings outside of academic medical centers and practices in which physicians have less time to spend with patients.
The physicians in this study were not overweight, which could limit the generalizability of the results. Patients may be less likely to raise the topic of weight with physicians who were themselves overweight. In addition, while we assessed single-item outcomes, more objective and extensive standard measures of diet, physical activity, and weight loss would have been optimal.
Some notable strengths of this study were that we used a comprehensive multimodal measurement in assessing both content and style of conversations in addition to patient self-report. We also examined Motivational Interviewing techniques among physicians with little or no MI training; most studies have examined MI among trained counselors only.
How to talk about weight loss: More study is needed
The most commonly addressed weight-related topics were diet and physical activity. However, when looking at the topics that were discussed, we found no patterns between those who attempted to lose weight and those who didn’t. This may mean that because weight loss is such a complex behavior, mention of any aspect of it—be it physical activity, diet, psychosocial issues, and so on—helps patients in their efforts. It also could be that the physician and patient discussed some other aspects in a previous visit; therefore, it was the cumulative effect of many conversations that influenced the patient to change.
These results need to be explored in a larger study to understand whether discussing certain topics is more influential than discussing others in promoting weight loss.
Acknowledgments
We thank Miranda West, Laura Fish, and Mary Sochaki for their work on this project. We are also grateful to the physicians and patients who agreed to have their encounters audio recorded.
Funding
This work was supported by National Cancer Institute grant 2P50 CA68438-06A2. The authors were supported in part by National Cancer Institute grants R01CA089053, R01CA100387, and National Institute of Diabetes and Digestive and Kidney Disorders grant R01DK64986.
CorrespondenceKathryn I. Pollak, PhD, Duke Comprehensive Cancer Center, Cancer Prevention, Detection and Control Research Program, 2424 Erwin Road, Room 6029, Hock Plaza I, Suite 602, Durham, NC 27705; kathryn.pollak@duke.edu.
1. Nawaz H, Adams ML, Katz DL. Physician-patient interactions regarding diet, exercise, and smoking. Prev Med 2000;31:652-657.
2. Sciamanna CN, Tate DF, Lang W, Wing RR. Who reports receiving advice to lose weight? Results from a multistate survey. Arch Intern Med 2000;160:2334-2339.
3. Mehrotra C, Naimi TS, Serdula M, Bolen J, Pearson K. Arthritis, body mass index, and professional advice to lose weight: implications for clinical medicine and public health. Am J Prev Med 2004;27:16-21.
4. National Center for Chronic Disease Prevention and Health Promotion. 2001 BRFSS Summary Prevalence Report. Bethesda, Md: CDC; 2001.
5. Wee CC, McCarthy EP, Davis RB, Phillips RS. Screening for cervical and breast cancer: is obesity an unrecognized barrier to preventive care? Ann Intern Med 2000;132:697-704.
6. Emmons KM, Rollnick S. Motivational interviewing in health care settings. Opportunities and limitations. Am J Prev Med 2001;20:68-74.
7. Miller WR, Rollnick S. Motivational Interviewing: Preparing People for Change. 2nd ed. New York, NY: Guilford Press; 2002.
8. Glasgow RE, Whitlock EP, Eakin EG, Lichtenstein E. A brief smoking cessation intervention for women in low-income planned parenthood clinics. Am J Pub Health 2000;90:786-789.
9. Valanis B, Lichtenstein E, Mullooly JP, et al. Maternal smoking cessation and relapse prevention during health care visits. Am J Prev Med 2001;20:1-8.
10. Stotts AL, Diclemente CC, Dolan-Mullen P. One-to-one: a motivational intervention for resistant pregnant smokers. Addict Behav 2002;27:275-292.
11. Stotts AL, DeLaune KA, Schmitz JM, Grabowski J. Impact of a motivational intervention on mechanisms of change in low-income pregnant smokers. Addict Behav 2004;29:1649-1657.
12. National Heart Lung and Blood Institute. Obesity Education Initiative Expert Panel. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. NIH publication no. 98-4083. Bethesda, MD: National Institutes of Health; 1998.
13. O’Connell D, Velicer WF. A decision balance measure and the stages of change model of weight loss. Int J Addict 1988;23:729-750.
14. Moyers TB, Martin T, Manuel JK, Hendrickson SM, Miller WR. Assessing competence in the use of motivational interviewing. J Substance Abuse Treat 2005;28:19-26.
- A physician’s empathy, collaborative approach, and words of support can have a positive effect on overweight and obese women’s weight loss efforts.
Purpose This study explores how weight-related topics are discussed between physicians and their overweight and obese female patients.
Methods We surveyed and audio-recorded preventive health and chronic care visits with 25 overweight and obese female patients. We coded both for quantity (content and time) of weight-related discussions and quality (adherence to Motivational Interviewing [MI] techniques). We then tested correlations of these measures with patients’ reported attempts to lose weight, change diet, and change exercise patterns 1 month after the visit.
Results Weight was routinely addressed (19 of 25 encounters). Patients usually initiated the topic (67% of time). Physicians’ use of MI techniques resulted in patients attempting to lose weight and changing their exercise patterns.
Conclusion Physicians may benefit from MI training to help patients lose weight.
Research has shown that when physicians advise overweight patients to lose weight, improve their diet, or increase their physical activity, patients are more likely to report attempting to do so.1-3 In a study of 433 primary care patients, 46% reported trying to lose weight after their physician counseled them about nutrition, compared with 37% who were not counseled.1
The reality, though, is that physicians are not very likely to address weight loss. Data from the Behavioral Risk Factor Surveillance System indicate that patients report their providers address weight loss in fewer than 20% of their examinations.4 These low rates are concerning; when physicians do not advise patients to lose weight, patients may believe their weight is not a problem.5 Even more worrisome: Physicians are rarely trained on how to counsel patients about weight loss. So, when physicians do counsel patients, it may not be effective.
Using Motivational Interviewing
One effective style of counseling is Motivational Interviewing (MI). MI is a patient-centered, directive counseling style used to help patients explore and resolve their ambivalence related to a particular behavior change (see What is Motivational Interviewing?).6,7 Researchers have studied the use of MI by counselors and case managers (in handling smoking cessation),8-11 but not by physicians. Further, no one has examined whether physicians instinctively use MI techniques when discussing weight loss with their patients, or whether MI counseling results in patients trying to lose weight.
The primary aim of this study was to assess how overweight and obese female patients discuss weight loss with their physicians. We also wanted to explore the role that physicians’ way of discussing weight loss—and the use of MI in particular—might play in their patients’ motivation to lose weight.
Motivational Interviewing is a counseling style intended to create changes in behavior by helping patients to explore and resolve their ambivalence.7 In a patient-physician encounter guided by MI:
- The motivation to change comes from the patient.
- It is the patient’s job to articulate and resolve his or her ambivalence.
- Direct persuasion is not used; the physician is quiet and eliciting, but directive in helping the patient examine his or her ambivalence.
- Readiness for change is recognized not as a patient trait, but as a part of the interaction between physician and patient.
- The patient-physician relationship is regarded more like a partnership.
Methods
Setting and recruitment
All data were collected in a family practice clinic within Duke University Medical Center. We approached 9 physicians in the practice to participate, and all consented. Only 7 physicians had visits with overweight or obese patients and were included in this report. We reviewed their electronic patient appointment schedules twice a week to identify female patients meeting the following criteria: English-speaking, overweight or obese (body mass index [BMI] ≥25 kg/m2), 40 years of age or older, and with health maintenance or chronic care appointments scheduled at least 7 days later. We sent these patients a letter describing the study, and allowed them 7 days to call a toll-free number if they didn’t want to participate.
We took several steps to avoid priming physicians and patients about the purpose of the study. First, both physicians and patients were told the study was about how doctors and patients discuss preventive health topics—they were not told the study was about examining discussions of weight. Second, we surveyed physicians 1 month prior to audio-recording visits, and patients 1 week prior to their visit. Third, we included measures for other preventive health topics (eg, smoking and alcohol) to detract attention from weight.
Gathering data
1. Phone survey before patient visit. We telephoned those patients who did not refuse participation and conducted a baseline survey. We asked about date of birth, race, marital status, level of education, income, weight, height, history of weight loss attempts, and whether this was their first visit with that physician. We categorized women with a BMI ≥25 but <30 as overweight, and those with a BMI ≥30 as obese.12
We also assessed each patient’s
- self-efficacy—that is, confidence in their ability to lose weight. We asked: “How confident are you that you can lose weight?” (1=not at all confident, 5=extremely confident).
- readiness to lose weight. We asked: “Are you seriously considering trying to losing weight within the next 6 months?” and, if yes, “Are you planning to try to lose weight in the next 30 days?”13 Those not considering trying to lose weight were staged as precontemplation; those who were considering trying but not planning to try in the next 30 days were staged as contemplation; and those who were planning to try to lose weight in the next 30 days were staged as preparation.
2. Office visit. When patients came in for their appointments, the research assistant gave them consent forms to sign. The assistant then escorted the patient to the examination rooms and started the digital audio recorder. The exams typically took 27 minutes.
Immediately following the exam, the research assistant surveyed the patients. The assistant asked 2 questions we’d asked at baseline: “How confident are you that you can lose weight?” and “Are you seriously considering trying to losing weight within the next 6 months?” (If yes, “Are you planning to try to lose weight in the next 30 days?”) She also made an appointment to conduct a 1-month follow-up telephone survey.
3. One-month follow-up survey. During a follow-up phone survey, we asked patients whether they had attempted to lose weight by changing their diet, exercise patterns, or both. Subsequent to this call, we sent the study participants a $25 check.
Analyzing the patient-physician discussion
Content. Two authors coded 9 topics that physicians and patients discussed that were “weight-related.” Topics included: physical activity, diet, BMI, psychosocial issues, referral to a nutritionist, weight loss surgery, goal setting, weight loss medications, and health care avoidance. We also coded who first brought up the topic.
Time spent. We calculated time spent discussing weight-related topics and also the total time of the patient’s visit.
Motivational Interviewing. Two coders assessed MI. To assess fidelity to MI principles, we used sections of the Motivational Interviewing Treatment Integrity scale (MITI)14 to rate patient interactions on a scale of 1 (low) to 7 (high) in 2 categories: empathy and MI spirit.
- Empathy is when physicians convey understanding of patients’ perspective.
- MI spirit includes evocation, collaboration, and autonomy. Evocation is when physicians draw out patients’ own reasons for change. Collaboration is when physicians act as partners, supporting and exploring patients’ concerns. Autonomy is when physicians convey that decisions to change lie completely with patients. Inter-rater reliability for the Empathy and MI Spirit was adequate (ICC=.94 and .97, respectively).
- MI-adherent behaviors were those where the physician asked permission to do things, affirmed statements, offered words of support, and emphasized patient control. For instance, the physician might say, “It’s great that you have stopped drinking sweetened tea” or “Whether you lose weight is up to you.”
- MI-nonadherent behaviors were those where the physician advised without asking permission. For example, the physician might say, “Let me tell you what you need to do to make this work…” or “Well, if you want to continue on the way you are, you know your diabetes is only going to get worse.”
These were combined to create a ratio of percentage MI-adherent behaviors by dividing MI-adherent by MI-nonadherent. There was an excellent level of agreement between coders for MI-nonadherent (kappa=.80) and a moderate level of agreement for MI-adherent (kappa=.52) behaviors.
Data analysis
We used Spearman correlations to assess the relationship between our predictors, quantity (time spent and whether weight was addressed) and quality (MI techniques), and mediators of behavior change (readiness to lose weight and self-efficacy to lose weight) and behavior change (attempts to lose weight, change in diet, and change in exercise patterns). We used SAS 9.1 (SAS Institute, Inc, Cary, NC) for all analyses. The study was approved by the Duke University Medical Center Institutional Review Board.
Results
We identified 202 eligible female patients. Of those, 96 had appointments that passed before we could contact them; 11 called the 800 number to refuse. Of the remaining 95 women, we reached 94 by phone. Of those, 19 refused to participate, 46 were ineligible because we had reached the targeted number of women in their weight category, and 4 skipped their appointments. Thus, we audio-recorded 25 encounters (for 14 obese and 11 overweight patients). Of these 25 patients, 24 completed the 1-month follow-up.
Patient demographics. Patients had a mean age of 59 years (standard deviation [SD]=11). Half were white; 42% were college-educated. Forty-two percent reported being in poor to fair health (TABLE 1).
The typical participant was moderately confident and ready to lose weight both before and after their visit. One month after their visit, 63% reported attempting to lose weight. More than half attempted to change their diet (67%); slightly more than half changed their exercise patterns (58%) (TABLE 2).
Physician demographics. Physicians had a mean age of 43 years (SD=10). About half were white; about half were female. No physicians were overweight.
TABLE 1
Characteristics of patients and physicians
| CHARACTERISTIC | PATIENT (N=25) | PHYSICIAN (N=7) |
|---|---|---|
| Age (M, SD) | 59 (11) | 43 (10) |
| Race (%)* | ||
| White | 50 | 57 |
| Black | 50 | 29 |
| Indian | 14 | |
| Female (%) | 100 | 57 |
| Married (%) | 46 | — |
| Employed (%) | 54 | 100 |
| College graduate (%) | 42 | 100 |
| Health status, self-reported (%) | ||
| Poor to fair | 42 | — |
| Good | 37 | — |
| Very good to excellent | 21 | — |
| Times lost at least 10 lbs (mean, SD) | 5.8 (4.0) | — |
| New patient with physician (%) | 12 | — |
| Body mass index (mean, SD) | 37 (11) | 22 (3) |
| * One participant did not provide his/her race. | ||
TABLE 2
Feeling about weight loss before and after the visit
| BASELINE | POST-VISIT | 1 MONTH | |
|---|---|---|---|
| Mediators of behavior change | |||
| Confidence in losing weight (M, SD)* | 3.8 (1.4) | 3.8 (1.1) | – |
| Stage of readiness to lose weight (%) | |||
| Precontemplation | 25% | 28% | – |
| Contemplation | 8% | 8% | – |
| Preparation | 67% | 64% | – |
| Behavior change variables | |||
| Attempted to lose weight (%) | – | – | 63% |
| Attempted to change diet (%) | – | – | 67% |
| Changed exercise patterns (%) | – | – | 58% |
| * Scale ranged from 1=not at all confident to 5=extremely confident. | |||
Patients were more likely to raise the weight issue
Weight-related topics were addressed in 19 of the 25 encounters (11 out of 12 preventive health visits, 8 out of 13 chronic care visits). The mean time spent discussing weight-related topics was 6.9 minutes out of an mean total of 27.0 minutes, or 26% of the total patient-physician time. Weight was more likely to be addressed with obese patients (86%) than with overweight patients (63%).
Patients were more likely than physicians to initiate discussions on weight. Physicians raised weight-related topics 37% of the time. Obese patients were slightly more likely to raise weight-related topics (8 out of 12 times [67%]) than overweight patients (4 out of 7 times [57%]).
The weight-related topics addressed were, in order from most to least frequent: physical activity, diet, BMI, psychosocial issues (eg, motivation to lose weight, triggers for unhealthful eating [such as family cookouts], negative talk [such as telling oneself that losing weight is too hard]), referral to a nutritionist, weight loss surgery, goal setting, health care avoidance, and weight loss medication. When comparing those who attempted to lose weight (n=15) with those who did not (n=9), there was no significant difference in whether or how often a topic was addressed.
Physicians’ empathy scores are moderate
Physicians had a moderate score for Empathy (mean=3.8, standard deviation [SD]=1.5, on 7-point scale), a low score for MI Spirit (mean=2.4, standard deviation [SD]=1.4, on 7-point scale), and displayed fewer MI-adherent behaviors than MI-nonadherent behaviors (mean=0.4, SD=0.3). These means did not differ significantly based on the patients’ weight.
Weight loss conversations linked to patients’ readiness
The discussion of weight-related topics, and the time spent doing so, were related to patients’ readiness to lose weight after their initial examination, when patients’ baseline readiness to lose weight was controlled. The more ready patients were to lose weight after their visit, the more likely they had discussed weight (Spearman’s rank correlation coefficient [r]=.52, P=.01) and spent more time discussing weight (r=.42, P=.05). No other associations were statistically significant (TABLE W1).
Several of the Motivational Interviewing scores predicted patients’ outcomes. When physicians showed more empathy, patients were more likely to report changing their exercise patterns 1 month after the visit (r=.50, P=.02). When physicians displayed more of an MI Spirit, patients were more likely to be ready to lose weight (r=.63, P=.005) and change their exercise patterns (r=.47, P=.04). Further, when physicians used more MI-adherent techniques, patients were more likely to attempt to lose weight (r=.42, P=.08).
Discussion: Good quality discussions lead to change
While more discussion about weight loss led to a greater readiness to lose weight, it was the quality of the discussions that actually led to behavior changes. Most patients had virtually the same levels of readiness to lose weight before and after the visit. It is likely that patients who were ready to lose weight discussed their weight with their physicians—and spent more time discussing it than those patients who were not ready to lose weight.
How patients and physicians discussed weight influenced behavior change. When physicians were more empathic and used techniques consistent with Motivational Interviewing, patients were more likely to report changing their exercise routine and attempting to lose weight.
To date, no one has examined the effect of physicians’ MI techniques on weight-related behavior change in a large study. The low adherence to MI techniques suggests that physicians can improve their counseling skills.
Patients aren’t afraid to talk about their weight
Unexpectedly, patients were more likely than physicians to initiate weight-related discussions. Only one third of the time did physicians raise the topic. Patients appear to be “empowered” to initiate discussions about weight loss. We expected physicians and patients to both be somewhat apprehensive about raising this sensitive topic. However, these findings suggest that overweight and obese patients will initiate the discussion most of the time.
Limitations and strengths of this study
The small sample size limited the analyses. Nonetheless, we found strong correlations in this sample that suggest true relationships that were unlikely to have occurred by chance. Also, we were unable to conduct nested analyses to account for the clustering of patients seen by the same physicians. The results may not generalize to settings outside of academic medical centers and practices in which physicians have less time to spend with patients.
The physicians in this study were not overweight, which could limit the generalizability of the results. Patients may be less likely to raise the topic of weight with physicians who were themselves overweight. In addition, while we assessed single-item outcomes, more objective and extensive standard measures of diet, physical activity, and weight loss would have been optimal.
Some notable strengths of this study were that we used a comprehensive multimodal measurement in assessing both content and style of conversations in addition to patient self-report. We also examined Motivational Interviewing techniques among physicians with little or no MI training; most studies have examined MI among trained counselors only.
How to talk about weight loss: More study is needed
The most commonly addressed weight-related topics were diet and physical activity. However, when looking at the topics that were discussed, we found no patterns between those who attempted to lose weight and those who didn’t. This may mean that because weight loss is such a complex behavior, mention of any aspect of it—be it physical activity, diet, psychosocial issues, and so on—helps patients in their efforts. It also could be that the physician and patient discussed some other aspects in a previous visit; therefore, it was the cumulative effect of many conversations that influenced the patient to change.
These results need to be explored in a larger study to understand whether discussing certain topics is more influential than discussing others in promoting weight loss.
Acknowledgments
We thank Miranda West, Laura Fish, and Mary Sochaki for their work on this project. We are also grateful to the physicians and patients who agreed to have their encounters audio recorded.
Funding
This work was supported by National Cancer Institute grant 2P50 CA68438-06A2. The authors were supported in part by National Cancer Institute grants R01CA089053, R01CA100387, and National Institute of Diabetes and Digestive and Kidney Disorders grant R01DK64986.
CorrespondenceKathryn I. Pollak, PhD, Duke Comprehensive Cancer Center, Cancer Prevention, Detection and Control Research Program, 2424 Erwin Road, Room 6029, Hock Plaza I, Suite 602, Durham, NC 27705; kathryn.pollak@duke.edu.
- A physician’s empathy, collaborative approach, and words of support can have a positive effect on overweight and obese women’s weight loss efforts.
Purpose This study explores how weight-related topics are discussed between physicians and their overweight and obese female patients.
Methods We surveyed and audio-recorded preventive health and chronic care visits with 25 overweight and obese female patients. We coded both for quantity (content and time) of weight-related discussions and quality (adherence to Motivational Interviewing [MI] techniques). We then tested correlations of these measures with patients’ reported attempts to lose weight, change diet, and change exercise patterns 1 month after the visit.
Results Weight was routinely addressed (19 of 25 encounters). Patients usually initiated the topic (67% of time). Physicians’ use of MI techniques resulted in patients attempting to lose weight and changing their exercise patterns.
Conclusion Physicians may benefit from MI training to help patients lose weight.
Research has shown that when physicians advise overweight patients to lose weight, improve their diet, or increase their physical activity, patients are more likely to report attempting to do so.1-3 In a study of 433 primary care patients, 46% reported trying to lose weight after their physician counseled them about nutrition, compared with 37% who were not counseled.1
The reality, though, is that physicians are not very likely to address weight loss. Data from the Behavioral Risk Factor Surveillance System indicate that patients report their providers address weight loss in fewer than 20% of their examinations.4 These low rates are concerning; when physicians do not advise patients to lose weight, patients may believe their weight is not a problem.5 Even more worrisome: Physicians are rarely trained on how to counsel patients about weight loss. So, when physicians do counsel patients, it may not be effective.
Using Motivational Interviewing
One effective style of counseling is Motivational Interviewing (MI). MI is a patient-centered, directive counseling style used to help patients explore and resolve their ambivalence related to a particular behavior change (see What is Motivational Interviewing?).6,7 Researchers have studied the use of MI by counselors and case managers (in handling smoking cessation),8-11 but not by physicians. Further, no one has examined whether physicians instinctively use MI techniques when discussing weight loss with their patients, or whether MI counseling results in patients trying to lose weight.
The primary aim of this study was to assess how overweight and obese female patients discuss weight loss with their physicians. We also wanted to explore the role that physicians’ way of discussing weight loss—and the use of MI in particular—might play in their patients’ motivation to lose weight.
Motivational Interviewing is a counseling style intended to create changes in behavior by helping patients to explore and resolve their ambivalence.7 In a patient-physician encounter guided by MI:
- The motivation to change comes from the patient.
- It is the patient’s job to articulate and resolve his or her ambivalence.
- Direct persuasion is not used; the physician is quiet and eliciting, but directive in helping the patient examine his or her ambivalence.
- Readiness for change is recognized not as a patient trait, but as a part of the interaction between physician and patient.
- The patient-physician relationship is regarded more like a partnership.
Methods
Setting and recruitment
All data were collected in a family practice clinic within Duke University Medical Center. We approached 9 physicians in the practice to participate, and all consented. Only 7 physicians had visits with overweight or obese patients and were included in this report. We reviewed their electronic patient appointment schedules twice a week to identify female patients meeting the following criteria: English-speaking, overweight or obese (body mass index [BMI] ≥25 kg/m2), 40 years of age or older, and with health maintenance or chronic care appointments scheduled at least 7 days later. We sent these patients a letter describing the study, and allowed them 7 days to call a toll-free number if they didn’t want to participate.
We took several steps to avoid priming physicians and patients about the purpose of the study. First, both physicians and patients were told the study was about how doctors and patients discuss preventive health topics—they were not told the study was about examining discussions of weight. Second, we surveyed physicians 1 month prior to audio-recording visits, and patients 1 week prior to their visit. Third, we included measures for other preventive health topics (eg, smoking and alcohol) to detract attention from weight.
Gathering data
1. Phone survey before patient visit. We telephoned those patients who did not refuse participation and conducted a baseline survey. We asked about date of birth, race, marital status, level of education, income, weight, height, history of weight loss attempts, and whether this was their first visit with that physician. We categorized women with a BMI ≥25 but <30 as overweight, and those with a BMI ≥30 as obese.12
We also assessed each patient’s
- self-efficacy—that is, confidence in their ability to lose weight. We asked: “How confident are you that you can lose weight?” (1=not at all confident, 5=extremely confident).
- readiness to lose weight. We asked: “Are you seriously considering trying to losing weight within the next 6 months?” and, if yes, “Are you planning to try to lose weight in the next 30 days?”13 Those not considering trying to lose weight were staged as precontemplation; those who were considering trying but not planning to try in the next 30 days were staged as contemplation; and those who were planning to try to lose weight in the next 30 days were staged as preparation.
2. Office visit. When patients came in for their appointments, the research assistant gave them consent forms to sign. The assistant then escorted the patient to the examination rooms and started the digital audio recorder. The exams typically took 27 minutes.
Immediately following the exam, the research assistant surveyed the patients. The assistant asked 2 questions we’d asked at baseline: “How confident are you that you can lose weight?” and “Are you seriously considering trying to losing weight within the next 6 months?” (If yes, “Are you planning to try to lose weight in the next 30 days?”) She also made an appointment to conduct a 1-month follow-up telephone survey.
3. One-month follow-up survey. During a follow-up phone survey, we asked patients whether they had attempted to lose weight by changing their diet, exercise patterns, or both. Subsequent to this call, we sent the study participants a $25 check.
Analyzing the patient-physician discussion
Content. Two authors coded 9 topics that physicians and patients discussed that were “weight-related.” Topics included: physical activity, diet, BMI, psychosocial issues, referral to a nutritionist, weight loss surgery, goal setting, weight loss medications, and health care avoidance. We also coded who first brought up the topic.
Time spent. We calculated time spent discussing weight-related topics and also the total time of the patient’s visit.
Motivational Interviewing. Two coders assessed MI. To assess fidelity to MI principles, we used sections of the Motivational Interviewing Treatment Integrity scale (MITI)14 to rate patient interactions on a scale of 1 (low) to 7 (high) in 2 categories: empathy and MI spirit.
- Empathy is when physicians convey understanding of patients’ perspective.
- MI spirit includes evocation, collaboration, and autonomy. Evocation is when physicians draw out patients’ own reasons for change. Collaboration is when physicians act as partners, supporting and exploring patients’ concerns. Autonomy is when physicians convey that decisions to change lie completely with patients. Inter-rater reliability for the Empathy and MI Spirit was adequate (ICC=.94 and .97, respectively).
- MI-adherent behaviors were those where the physician asked permission to do things, affirmed statements, offered words of support, and emphasized patient control. For instance, the physician might say, “It’s great that you have stopped drinking sweetened tea” or “Whether you lose weight is up to you.”
- MI-nonadherent behaviors were those where the physician advised without asking permission. For example, the physician might say, “Let me tell you what you need to do to make this work…” or “Well, if you want to continue on the way you are, you know your diabetes is only going to get worse.”
These were combined to create a ratio of percentage MI-adherent behaviors by dividing MI-adherent by MI-nonadherent. There was an excellent level of agreement between coders for MI-nonadherent (kappa=.80) and a moderate level of agreement for MI-adherent (kappa=.52) behaviors.
Data analysis
We used Spearman correlations to assess the relationship between our predictors, quantity (time spent and whether weight was addressed) and quality (MI techniques), and mediators of behavior change (readiness to lose weight and self-efficacy to lose weight) and behavior change (attempts to lose weight, change in diet, and change in exercise patterns). We used SAS 9.1 (SAS Institute, Inc, Cary, NC) for all analyses. The study was approved by the Duke University Medical Center Institutional Review Board.
Results
We identified 202 eligible female patients. Of those, 96 had appointments that passed before we could contact them; 11 called the 800 number to refuse. Of the remaining 95 women, we reached 94 by phone. Of those, 19 refused to participate, 46 were ineligible because we had reached the targeted number of women in their weight category, and 4 skipped their appointments. Thus, we audio-recorded 25 encounters (for 14 obese and 11 overweight patients). Of these 25 patients, 24 completed the 1-month follow-up.
Patient demographics. Patients had a mean age of 59 years (standard deviation [SD]=11). Half were white; 42% were college-educated. Forty-two percent reported being in poor to fair health (TABLE 1).
The typical participant was moderately confident and ready to lose weight both before and after their visit. One month after their visit, 63% reported attempting to lose weight. More than half attempted to change their diet (67%); slightly more than half changed their exercise patterns (58%) (TABLE 2).
Physician demographics. Physicians had a mean age of 43 years (SD=10). About half were white; about half were female. No physicians were overweight.
TABLE 1
Characteristics of patients and physicians
| CHARACTERISTIC | PATIENT (N=25) | PHYSICIAN (N=7) |
|---|---|---|
| Age (M, SD) | 59 (11) | 43 (10) |
| Race (%)* | ||
| White | 50 | 57 |
| Black | 50 | 29 |
| Indian | 14 | |
| Female (%) | 100 | 57 |
| Married (%) | 46 | — |
| Employed (%) | 54 | 100 |
| College graduate (%) | 42 | 100 |
| Health status, self-reported (%) | ||
| Poor to fair | 42 | — |
| Good | 37 | — |
| Very good to excellent | 21 | — |
| Times lost at least 10 lbs (mean, SD) | 5.8 (4.0) | — |
| New patient with physician (%) | 12 | — |
| Body mass index (mean, SD) | 37 (11) | 22 (3) |
| * One participant did not provide his/her race. | ||
TABLE 2
Feeling about weight loss before and after the visit
| BASELINE | POST-VISIT | 1 MONTH | |
|---|---|---|---|
| Mediators of behavior change | |||
| Confidence in losing weight (M, SD)* | 3.8 (1.4) | 3.8 (1.1) | – |
| Stage of readiness to lose weight (%) | |||
| Precontemplation | 25% | 28% | – |
| Contemplation | 8% | 8% | – |
| Preparation | 67% | 64% | – |
| Behavior change variables | |||
| Attempted to lose weight (%) | – | – | 63% |
| Attempted to change diet (%) | – | – | 67% |
| Changed exercise patterns (%) | – | – | 58% |
| * Scale ranged from 1=not at all confident to 5=extremely confident. | |||
Patients were more likely to raise the weight issue
Weight-related topics were addressed in 19 of the 25 encounters (11 out of 12 preventive health visits, 8 out of 13 chronic care visits). The mean time spent discussing weight-related topics was 6.9 minutes out of an mean total of 27.0 minutes, or 26% of the total patient-physician time. Weight was more likely to be addressed with obese patients (86%) than with overweight patients (63%).
Patients were more likely than physicians to initiate discussions on weight. Physicians raised weight-related topics 37% of the time. Obese patients were slightly more likely to raise weight-related topics (8 out of 12 times [67%]) than overweight patients (4 out of 7 times [57%]).
The weight-related topics addressed were, in order from most to least frequent: physical activity, diet, BMI, psychosocial issues (eg, motivation to lose weight, triggers for unhealthful eating [such as family cookouts], negative talk [such as telling oneself that losing weight is too hard]), referral to a nutritionist, weight loss surgery, goal setting, health care avoidance, and weight loss medication. When comparing those who attempted to lose weight (n=15) with those who did not (n=9), there was no significant difference in whether or how often a topic was addressed.
Physicians’ empathy scores are moderate
Physicians had a moderate score for Empathy (mean=3.8, standard deviation [SD]=1.5, on 7-point scale), a low score for MI Spirit (mean=2.4, standard deviation [SD]=1.4, on 7-point scale), and displayed fewer MI-adherent behaviors than MI-nonadherent behaviors (mean=0.4, SD=0.3). These means did not differ significantly based on the patients’ weight.
Weight loss conversations linked to patients’ readiness
The discussion of weight-related topics, and the time spent doing so, were related to patients’ readiness to lose weight after their initial examination, when patients’ baseline readiness to lose weight was controlled. The more ready patients were to lose weight after their visit, the more likely they had discussed weight (Spearman’s rank correlation coefficient [r]=.52, P=.01) and spent more time discussing weight (r=.42, P=.05). No other associations were statistically significant (TABLE W1).
Several of the Motivational Interviewing scores predicted patients’ outcomes. When physicians showed more empathy, patients were more likely to report changing their exercise patterns 1 month after the visit (r=.50, P=.02). When physicians displayed more of an MI Spirit, patients were more likely to be ready to lose weight (r=.63, P=.005) and change their exercise patterns (r=.47, P=.04). Further, when physicians used more MI-adherent techniques, patients were more likely to attempt to lose weight (r=.42, P=.08).
Discussion: Good quality discussions lead to change
While more discussion about weight loss led to a greater readiness to lose weight, it was the quality of the discussions that actually led to behavior changes. Most patients had virtually the same levels of readiness to lose weight before and after the visit. It is likely that patients who were ready to lose weight discussed their weight with their physicians—and spent more time discussing it than those patients who were not ready to lose weight.
How patients and physicians discussed weight influenced behavior change. When physicians were more empathic and used techniques consistent with Motivational Interviewing, patients were more likely to report changing their exercise routine and attempting to lose weight.
To date, no one has examined the effect of physicians’ MI techniques on weight-related behavior change in a large study. The low adherence to MI techniques suggests that physicians can improve their counseling skills.
Patients aren’t afraid to talk about their weight
Unexpectedly, patients were more likely than physicians to initiate weight-related discussions. Only one third of the time did physicians raise the topic. Patients appear to be “empowered” to initiate discussions about weight loss. We expected physicians and patients to both be somewhat apprehensive about raising this sensitive topic. However, these findings suggest that overweight and obese patients will initiate the discussion most of the time.
Limitations and strengths of this study
The small sample size limited the analyses. Nonetheless, we found strong correlations in this sample that suggest true relationships that were unlikely to have occurred by chance. Also, we were unable to conduct nested analyses to account for the clustering of patients seen by the same physicians. The results may not generalize to settings outside of academic medical centers and practices in which physicians have less time to spend with patients.
The physicians in this study were not overweight, which could limit the generalizability of the results. Patients may be less likely to raise the topic of weight with physicians who were themselves overweight. In addition, while we assessed single-item outcomes, more objective and extensive standard measures of diet, physical activity, and weight loss would have been optimal.
Some notable strengths of this study were that we used a comprehensive multimodal measurement in assessing both content and style of conversations in addition to patient self-report. We also examined Motivational Interviewing techniques among physicians with little or no MI training; most studies have examined MI among trained counselors only.
How to talk about weight loss: More study is needed
The most commonly addressed weight-related topics were diet and physical activity. However, when looking at the topics that were discussed, we found no patterns between those who attempted to lose weight and those who didn’t. This may mean that because weight loss is such a complex behavior, mention of any aspect of it—be it physical activity, diet, psychosocial issues, and so on—helps patients in their efforts. It also could be that the physician and patient discussed some other aspects in a previous visit; therefore, it was the cumulative effect of many conversations that influenced the patient to change.
These results need to be explored in a larger study to understand whether discussing certain topics is more influential than discussing others in promoting weight loss.
Acknowledgments
We thank Miranda West, Laura Fish, and Mary Sochaki for their work on this project. We are also grateful to the physicians and patients who agreed to have their encounters audio recorded.
Funding
This work was supported by National Cancer Institute grant 2P50 CA68438-06A2. The authors were supported in part by National Cancer Institute grants R01CA089053, R01CA100387, and National Institute of Diabetes and Digestive and Kidney Disorders grant R01DK64986.
CorrespondenceKathryn I. Pollak, PhD, Duke Comprehensive Cancer Center, Cancer Prevention, Detection and Control Research Program, 2424 Erwin Road, Room 6029, Hock Plaza I, Suite 602, Durham, NC 27705; kathryn.pollak@duke.edu.
1. Nawaz H, Adams ML, Katz DL. Physician-patient interactions regarding diet, exercise, and smoking. Prev Med 2000;31:652-657.
2. Sciamanna CN, Tate DF, Lang W, Wing RR. Who reports receiving advice to lose weight? Results from a multistate survey. Arch Intern Med 2000;160:2334-2339.
3. Mehrotra C, Naimi TS, Serdula M, Bolen J, Pearson K. Arthritis, body mass index, and professional advice to lose weight: implications for clinical medicine and public health. Am J Prev Med 2004;27:16-21.
4. National Center for Chronic Disease Prevention and Health Promotion. 2001 BRFSS Summary Prevalence Report. Bethesda, Md: CDC; 2001.
5. Wee CC, McCarthy EP, Davis RB, Phillips RS. Screening for cervical and breast cancer: is obesity an unrecognized barrier to preventive care? Ann Intern Med 2000;132:697-704.
6. Emmons KM, Rollnick S. Motivational interviewing in health care settings. Opportunities and limitations. Am J Prev Med 2001;20:68-74.
7. Miller WR, Rollnick S. Motivational Interviewing: Preparing People for Change. 2nd ed. New York, NY: Guilford Press; 2002.
8. Glasgow RE, Whitlock EP, Eakin EG, Lichtenstein E. A brief smoking cessation intervention for women in low-income planned parenthood clinics. Am J Pub Health 2000;90:786-789.
9. Valanis B, Lichtenstein E, Mullooly JP, et al. Maternal smoking cessation and relapse prevention during health care visits. Am J Prev Med 2001;20:1-8.
10. Stotts AL, Diclemente CC, Dolan-Mullen P. One-to-one: a motivational intervention for resistant pregnant smokers. Addict Behav 2002;27:275-292.
11. Stotts AL, DeLaune KA, Schmitz JM, Grabowski J. Impact of a motivational intervention on mechanisms of change in low-income pregnant smokers. Addict Behav 2004;29:1649-1657.
12. National Heart Lung and Blood Institute. Obesity Education Initiative Expert Panel. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. NIH publication no. 98-4083. Bethesda, MD: National Institutes of Health; 1998.
13. O’Connell D, Velicer WF. A decision balance measure and the stages of change model of weight loss. Int J Addict 1988;23:729-750.
14. Moyers TB, Martin T, Manuel JK, Hendrickson SM, Miller WR. Assessing competence in the use of motivational interviewing. J Substance Abuse Treat 2005;28:19-26.
1. Nawaz H, Adams ML, Katz DL. Physician-patient interactions regarding diet, exercise, and smoking. Prev Med 2000;31:652-657.
2. Sciamanna CN, Tate DF, Lang W, Wing RR. Who reports receiving advice to lose weight? Results from a multistate survey. Arch Intern Med 2000;160:2334-2339.
3. Mehrotra C, Naimi TS, Serdula M, Bolen J, Pearson K. Arthritis, body mass index, and professional advice to lose weight: implications for clinical medicine and public health. Am J Prev Med 2004;27:16-21.
4. National Center for Chronic Disease Prevention and Health Promotion. 2001 BRFSS Summary Prevalence Report. Bethesda, Md: CDC; 2001.
5. Wee CC, McCarthy EP, Davis RB, Phillips RS. Screening for cervical and breast cancer: is obesity an unrecognized barrier to preventive care? Ann Intern Med 2000;132:697-704.
6. Emmons KM, Rollnick S. Motivational interviewing in health care settings. Opportunities and limitations. Am J Prev Med 2001;20:68-74.
7. Miller WR, Rollnick S. Motivational Interviewing: Preparing People for Change. 2nd ed. New York, NY: Guilford Press; 2002.
8. Glasgow RE, Whitlock EP, Eakin EG, Lichtenstein E. A brief smoking cessation intervention for women in low-income planned parenthood clinics. Am J Pub Health 2000;90:786-789.
9. Valanis B, Lichtenstein E, Mullooly JP, et al. Maternal smoking cessation and relapse prevention during health care visits. Am J Prev Med 2001;20:1-8.
10. Stotts AL, Diclemente CC, Dolan-Mullen P. One-to-one: a motivational intervention for resistant pregnant smokers. Addict Behav 2002;27:275-292.
11. Stotts AL, DeLaune KA, Schmitz JM, Grabowski J. Impact of a motivational intervention on mechanisms of change in low-income pregnant smokers. Addict Behav 2004;29:1649-1657.
12. National Heart Lung and Blood Institute. Obesity Education Initiative Expert Panel. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. NIH publication no. 98-4083. Bethesda, MD: National Institutes of Health; 1998.
13. O’Connell D, Velicer WF. A decision balance measure and the stages of change model of weight loss. Int J Addict 1988;23:729-750.
14. Moyers TB, Martin T, Manuel JK, Hendrickson SM, Miller WR. Assessing competence in the use of motivational interviewing. J Substance Abuse Treat 2005;28:19-26.
Transdermal rivastigmine for dementia
The rivastigmine patch is the first transdermal treatment for symptoms of mild to moderate Alzheimer’s disease (AD) and mild to moderate Parkinson’s disease dementia (Table). Rivastigmine, a cholinesterase inhibitor, is the only therapy approved for both indications.
Table
Rivastigmine transdermal patch: Fast facts
| Brand name: Exelon Patch |
| Class: Cholinesterase inhibitor |
| Indication: Symptomatic treatment of mild to moderate Alzheimer’s-type dementia and mild to moderate dementia associated with Parkinson’s disease |
| Manufacturer: Novartis Pharmaceuticals, Inc. |
| Dosing forms: 4.6 and 9.5 mg/24 hours transdermal patches (5 cm2 and 10 cm2, respectively) |
| Recommended dosage: Start with 4.6 mg/24 hours patch for ≥4 weeks, followed by a one-step increase to the target dose 9.5 mg/24 hours patch* |
| *Unless the patient is taking oral rivastigmine (see ‘Transitioning to rivastigmine patch,’) |
Clinical implications
The rivastigmine patch offers continuous drug delivery through the skin into the bloodstream over 24 hours.1 This may reduce the incidence of side effects compared with oral rivastigmine,2 making optimal therapeutic doses easier to attain.3 The target dose 9.5 mg/24 hours patch provides efficacy similar to the highest recommended rivastigmine capsule dose (6 mg bid for a total of 12 mg/d).2
How it works
The rivastigmine patch uses matrix technology, which enables delivery of a large amount of drug from a small surface area.4 The patch is available in 2 dosage forms:
- a 5-cm2 size containing 9 mg of rivastigmine that delivers 4.6 mg/24 hours
- a 10-cm2 size containing 18 mg of rivastigmine that delivers 9.5 mg/24 hours.
Each patch consists of 4 layers: the backing layer, an acrylic drug matrix, a silicone adhesive matrix, and an overlapping release liner that is removed and discarded before the patch is applied.1
Cholinesterase inhibitors are believed to exert their effects by increasing available levels of the neurotransmitter acetylcholine in the brain. Two studies have demonstrated that cognitive improvements associated with rivastigmine treatment correlate significantly with cholinesterase inhibition.5,6 In 1 study, rivastigmine’s inhibitory effects on cholinesterase were sustained for 12 months.6
Pharmacokinetics
Rivastigmine is metabolized by its target cholinesterase enzymes to the decarbamylated metabolite NAP 226-90, which has minimal acetylcholinesterase inhibition and is excreted through the urine.1 As a result of its low accumulation potential and cytochrome P 450-independent metabolism, rivastigmine has low potential for pharmacokinetic drug–drug interactions. This lack of interaction has been confirmed for many drugs commonly taken by elderly patients, such as digoxin, nonsteroidal anti-inflammatory drugs, and estrogens.7
Rivastigmine has a half-life of 1 to 2 hours, so it is rapidly cleared.8 In the event of a serious reaction, significant clearance of rivastigmine from the body would occur within 3 hours of patch removal.
Centrally mediated cholinergic gastrointestinal (GI) side effects associated with oral rivastigmine are related to high maximum plasma concentrations (Cmax) and short time interval to Cmax (Tmax).9 In an open-label, parallel-group study of 51 AD patients that compared rivastigmine patches with rivastigmine capsules, transdermal administration was associated with slower increases to lower peak plasma concentrations (prolonged Tmax and reduced Cmax), and less fluctuation in plasma concentration.1 Despite these effects, the rivastigmine 9.5 mg/24 hours patch provided drug exposure comparable to the highest dose of capsules (6 mg bid for a total of 12 mg/d), with improved GI tolerability.3
Efficacy
Rivastigmine patch efficacy was evaluated in a single, 24-week, international, randomized, double-blind trial of 1,195 patients with AD.2 The study group represented typical patients with mild to moderate AD—age 50 to 85 years with Mini-Mental State Examination scores of 10 to 20 at baseline. Patients were randomly assigned to receive:
- 17.4 mg/24 hours rivastigmine patch (20-cm2 patch; n=303)
- 9.5 mg/24 hours rivastigmine patch (10-cm2 patch; n=293)
- 6 mg bid rivastigmine capsules (n=297)
- or placebo (n=302).
Data for the 17.4 mg/24 hours patch are not discussed here because this dose exceeds the FDA-approved maximum dosage (9.5 mg/24 hours) and is not available.
Patients in the 9.5 mg/24 hours patch group received a 4.6 mg/24 hours patch (5 cm2) for weeks 1 through 4, and then the 9.5 mg/24 hours patch for the remainder of the study. Patients in the capsule group started on 3 mg/d (1.5 mg bid) and were titrated every 4 weeks in steps of 3 mg/d to a maximum of 12 mg/d administered as 6 mg bid.
Primary outcomes were measured as mean change in score from baseline to endpoint on the Alzheimer’s Disease Assessment Scale–Cognitive Subscale (ADAS-Cog) and Alzheimer’s Disease Co-operative Study–Clinical Global Impression of Change (ADCS-CGIC). By study endpoint, the 9.5 mg/24 hours patch and capsules, 12 mg/d, showed comparable efficacy (Figure).2 Compared with those receiving placebo, patients in the 9.5 mg/24 hours patch and capsule groups showed significant improvements in dementia symptoms, including:
- cognition
- global performance
- attention
- activities of daily living.2
Based on my clinical experience, these improvements reflect small but clinically meaningful changes that are noted by patients and caregivers.
Figure
Efficacy of transdermal rivastigmine for Alzheimer’s symptoms
*P<0.05 vs placebo
ADAS-Cog: Alzheimer’s Disease Assessment Scale-Cognitive Subscale; ADCS-CGIC: Alzheimer’s Disease Cooperative Study-Clinical Global Impression of Change
Source: Adapted from reference 2
In a 24-week study, transdermal rivastigmine, 9.5 mg/24 hours, and the highest recommended dose of oral rivastigmine (6 mg bid) showed comparable efficacy as measured by mean change in score on scales commonly used in Alzheimer’s disease clinical trials. ADAS-Cog assesses orientation, memory, language, praxis, and visuospatial functions. ADCS-CGIC provides a single global rating of change from baseline based on interviews with the patient and caregiver.
Safety and tolerability
Adverse events associated with rivastigmine are predominantly cholinergic; GI side effects—nausea, vomiting, and diarrhea—are observed most frequently.2 These events occur less frequently with the patch than with capsules. In the efficacy trial, patients in the 9.5 mg/24 hours rivastigmine patch group had one-third as many reports of nausea (7.2% vs 23.1%) and vomiting (6.2% vs 17.0%) compared with the 6 mg bid capsule group.2
Diarrhea was reported by 6% of subjects receiving the 9.5 mg/24 hours patch, 5% of those taking 6-mg capsule bid, and 3% receiving placebo. Fewer subjects in the 9.5 mg/24 hours patch group (3%) experienced decreased weight compared with those in the capsule group (5%). The rate of decreased weight with placebo was 1%.
Dizziness affected 2% of those in the 9.5 mg/24 hours patch and placebo groups; incidence in the capsule group was significantly higher at 8%. Headache was similar with the 9.5 mg/24 hours patch (3%) and placebo (2%), with the capsule significantly higher at 6%.2
The proportion of patients who experienced no, slight, or mild skin irritation ranged from 90% to 98%.2 The most commonly reported moderate or severe skin irritations were erythema (8% rivastigmine patch vs 4% placebo) and pruritus (7% rivastigmine patch vs 3% placebo). Two percent of patients using active patch discontinued the trial because of skin irritation.
Rivastigmine appears not to produce adverse effects on cardiac function as assessed by ECG. In clinical trials of 2,791 patients, pooled 12-lead ECG data comparing oral rivastigmine and placebo groups did not differ significantly in heart rate or PR, QRS, and QTc intervals.10
Dosing
The rivastigmine patch is administered once daily, and the recommended maintenance dose is the 9.5 mg/24 hours patch. Start patients on a 4.6 mg/24 hours patch for at least 4 weeks and then increase to the 9.5 mg/24 hours target dose if the lower dose is well tolerated.
Dosage adjustment of rivastigmine is not necessary in patients with hepatic or renal disease because of minimal liver metabolism and the acetylcholinesterase-mediated hydrolysis of rivastigmine to the inactive decarbamylated metabolite NAP 226-90, which is excreted in the urine.11
Instruct patients or caregivers to apply the patch to clean, dry, hairless skin that is free of cuts, rashes, or irritation on the upper or lower back or upper arm or chest.1 The patch has shown good adhesive properties over 24 hours, remaining attached in a range of situations, including bathing and hot weather.2 In the 9.5 mg/24 hours group of the efficacy study, 96% of patches remained attached or had slight lifting of the edges (1,336 total patch evaluations).
Transitioning to rivastigmine patch
The efficacy study included an open-label extension, during which blinding was maintained. This provided information on patients beginning rivastigmine patch therapy directly from placebo2 or transitioning from rivastigmine capsules to the target dose 9.5 mg/24 hours patch.12 Based on these results, transition patients as follows:
- Patients taking oral rivastigmine, <6 mg/d: Switch to a 4.6 mg/24 hours patch for ≥4 weeks before increasing to a 9.5 mg/24 hours patch.
- Patients taking oral rivastigmine, 6 to 12 mg/d: Switch directly to a 9.5 mg/24 hours patch.
Apply the first patch the day after the last oral dose.
Related resource
- Rivastigmine transdermal system prescribing information. www.pharma.us.novartis.com/product/pi/pdf/exelonpatch.pdf.
Drug brand names
- Digoxin • Lanoxin
- Rivastigmine • Exelon
- Rivastigmine transdermal
- system • Exelon Patch
Disclosure
Dr. Sadowsky is a consultant to and speaker for Forest Pharmaceuticals and Novartis Pharmaceuticals.
Acknowledgment
The author thanks Christina Mackins, PhD, a medical writer for Alpha-Plus Medical Communications Ltd, for her editorial assistance with this article. Funding for her work was provided by Novartis Pharmaceuticals.
1. Lefèvre G, Sedek G, Jhee S, et al. Pharmacokinetics and pharmacodynamics of the novel daily rivastigmine transdermal patch compared with twice-daily capsules in Alzheimer’s disease patients. J Clin Pharmacol 2007;47:471-8.
2. Winblad B, Cummings J, Andreasen N, et al. A six-month, double-blind, randomized, placebo-controlled study of a transdermal patch in Alzheimer’s disease—rivastigmine patch versus capsule. Int J Geriatr Psychiatry 2007;22:456-67.
3. Oertel W, Ross JS, Eggert K, Adler G. Rationale for transdermal drug administration in Alzheimer disease. Neurology 2007;69(suppl 1):S4-S9.
4. Petersen TA. Transdermal drug formulations and process development. Pharmaceut Technol 2003;(suppl):18-21.
5. Giacobini E, Spiegel R, Enz A, et al. Inhibition of acetyl- and butyryl-cholinesterase in the cerebrospinal fluid of patients with Alzheimer’s disease by rivastigmine: correlation with cognitive benefit. J Neural Transm 2002;109:1053-65.
6. Darreh-Shori T, Almkvist O, Guan ZZ, et al. Sustained cholinesterase inhibition in AD patients receiving rivastigmine for 12 months. Neurology 2002;59:563-72.
7. Grossberg GT, Stahelin HB, Messina JC, et al. Lack of adverse pharmacodynamic drug interactions with rivastigmine and twenty-two classes of medications. Int J Geriatr Psychiatry 2000;15(3):242-7.
8. Polinsky RJ. Clinical pharmacology of rivastigmine: a new-generation acetylcholinesterase inhibitor for the treatment of Alzheimer’s disease. Clin Ther 1998;20:634-47.
9. Jann MW, Shirley KL, Small GW. Clinical pharmacokinetics and pharmacodynamics of cholinesterase inhibitors. Clin Pharmacokinet 2002;41:719-39.
10. Morganroth J, Graham S, Hartman R, et al. Electrocardiographic effects of rivastigmine. J Clin Pharmacol 2002;42:558-68.
11. Exelon patch [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2007.
12. Frölich L, Barone P, Förstl H, et al. IDEAL: A 28-week open-label extension of a 24-week double-blind study of the first transdermal patch in Alzheimer’s disease. Poster presented at: 11th Congress of the European Federation of Neurological Societies; August 25-28, 2007; Brussels, Belgium.
Dr. Sadowsky is associate clinical professor of neurology, Nova Southeastern University, Fort Lauderdale, FL, and director, Premier Research Institute, Palm Beach Neurology, West Palm Beach, FL.
The rivastigmine patch is the first transdermal treatment for symptoms of mild to moderate Alzheimer’s disease (AD) and mild to moderate Parkinson’s disease dementia (Table). Rivastigmine, a cholinesterase inhibitor, is the only therapy approved for both indications.
Table
Rivastigmine transdermal patch: Fast facts
| Brand name: Exelon Patch |
| Class: Cholinesterase inhibitor |
| Indication: Symptomatic treatment of mild to moderate Alzheimer’s-type dementia and mild to moderate dementia associated with Parkinson’s disease |
| Manufacturer: Novartis Pharmaceuticals, Inc. |
| Dosing forms: 4.6 and 9.5 mg/24 hours transdermal patches (5 cm2 and 10 cm2, respectively) |
| Recommended dosage: Start with 4.6 mg/24 hours patch for ≥4 weeks, followed by a one-step increase to the target dose 9.5 mg/24 hours patch* |
| *Unless the patient is taking oral rivastigmine (see ‘Transitioning to rivastigmine patch,’) |
Clinical implications
The rivastigmine patch offers continuous drug delivery through the skin into the bloodstream over 24 hours.1 This may reduce the incidence of side effects compared with oral rivastigmine,2 making optimal therapeutic doses easier to attain.3 The target dose 9.5 mg/24 hours patch provides efficacy similar to the highest recommended rivastigmine capsule dose (6 mg bid for a total of 12 mg/d).2
How it works
The rivastigmine patch uses matrix technology, which enables delivery of a large amount of drug from a small surface area.4 The patch is available in 2 dosage forms:
- a 5-cm2 size containing 9 mg of rivastigmine that delivers 4.6 mg/24 hours
- a 10-cm2 size containing 18 mg of rivastigmine that delivers 9.5 mg/24 hours.
Each patch consists of 4 layers: the backing layer, an acrylic drug matrix, a silicone adhesive matrix, and an overlapping release liner that is removed and discarded before the patch is applied.1
Cholinesterase inhibitors are believed to exert their effects by increasing available levels of the neurotransmitter acetylcholine in the brain. Two studies have demonstrated that cognitive improvements associated with rivastigmine treatment correlate significantly with cholinesterase inhibition.5,6 In 1 study, rivastigmine’s inhibitory effects on cholinesterase were sustained for 12 months.6
Pharmacokinetics
Rivastigmine is metabolized by its target cholinesterase enzymes to the decarbamylated metabolite NAP 226-90, which has minimal acetylcholinesterase inhibition and is excreted through the urine.1 As a result of its low accumulation potential and cytochrome P 450-independent metabolism, rivastigmine has low potential for pharmacokinetic drug–drug interactions. This lack of interaction has been confirmed for many drugs commonly taken by elderly patients, such as digoxin, nonsteroidal anti-inflammatory drugs, and estrogens.7
Rivastigmine has a half-life of 1 to 2 hours, so it is rapidly cleared.8 In the event of a serious reaction, significant clearance of rivastigmine from the body would occur within 3 hours of patch removal.
Centrally mediated cholinergic gastrointestinal (GI) side effects associated with oral rivastigmine are related to high maximum plasma concentrations (Cmax) and short time interval to Cmax (Tmax).9 In an open-label, parallel-group study of 51 AD patients that compared rivastigmine patches with rivastigmine capsules, transdermal administration was associated with slower increases to lower peak plasma concentrations (prolonged Tmax and reduced Cmax), and less fluctuation in plasma concentration.1 Despite these effects, the rivastigmine 9.5 mg/24 hours patch provided drug exposure comparable to the highest dose of capsules (6 mg bid for a total of 12 mg/d), with improved GI tolerability.3
Efficacy
Rivastigmine patch efficacy was evaluated in a single, 24-week, international, randomized, double-blind trial of 1,195 patients with AD.2 The study group represented typical patients with mild to moderate AD—age 50 to 85 years with Mini-Mental State Examination scores of 10 to 20 at baseline. Patients were randomly assigned to receive:
- 17.4 mg/24 hours rivastigmine patch (20-cm2 patch; n=303)
- 9.5 mg/24 hours rivastigmine patch (10-cm2 patch; n=293)
- 6 mg bid rivastigmine capsules (n=297)
- or placebo (n=302).
Data for the 17.4 mg/24 hours patch are not discussed here because this dose exceeds the FDA-approved maximum dosage (9.5 mg/24 hours) and is not available.
Patients in the 9.5 mg/24 hours patch group received a 4.6 mg/24 hours patch (5 cm2) for weeks 1 through 4, and then the 9.5 mg/24 hours patch for the remainder of the study. Patients in the capsule group started on 3 mg/d (1.5 mg bid) and were titrated every 4 weeks in steps of 3 mg/d to a maximum of 12 mg/d administered as 6 mg bid.
Primary outcomes were measured as mean change in score from baseline to endpoint on the Alzheimer’s Disease Assessment Scale–Cognitive Subscale (ADAS-Cog) and Alzheimer’s Disease Co-operative Study–Clinical Global Impression of Change (ADCS-CGIC). By study endpoint, the 9.5 mg/24 hours patch and capsules, 12 mg/d, showed comparable efficacy (Figure).2 Compared with those receiving placebo, patients in the 9.5 mg/24 hours patch and capsule groups showed significant improvements in dementia symptoms, including:
- cognition
- global performance
- attention
- activities of daily living.2
Based on my clinical experience, these improvements reflect small but clinically meaningful changes that are noted by patients and caregivers.
Figure
Efficacy of transdermal rivastigmine for Alzheimer’s symptoms

*P<0.05 vs placebo
ADAS-Cog: Alzheimer’s Disease Assessment Scale-Cognitive Subscale; ADCS-CGIC: Alzheimer’s Disease Cooperative Study-Clinical Global Impression of Change
Source: Adapted from reference 2
In a 24-week study, transdermal rivastigmine, 9.5 mg/24 hours, and the highest recommended dose of oral rivastigmine (6 mg bid) showed comparable efficacy as measured by mean change in score on scales commonly used in Alzheimer’s disease clinical trials. ADAS-Cog assesses orientation, memory, language, praxis, and visuospatial functions. ADCS-CGIC provides a single global rating of change from baseline based on interviews with the patient and caregiver.
Safety and tolerability
Adverse events associated with rivastigmine are predominantly cholinergic; GI side effects—nausea, vomiting, and diarrhea—are observed most frequently.2 These events occur less frequently with the patch than with capsules. In the efficacy trial, patients in the 9.5 mg/24 hours rivastigmine patch group had one-third as many reports of nausea (7.2% vs 23.1%) and vomiting (6.2% vs 17.0%) compared with the 6 mg bid capsule group.2
Diarrhea was reported by 6% of subjects receiving the 9.5 mg/24 hours patch, 5% of those taking 6-mg capsule bid, and 3% receiving placebo. Fewer subjects in the 9.5 mg/24 hours patch group (3%) experienced decreased weight compared with those in the capsule group (5%). The rate of decreased weight with placebo was 1%.
Dizziness affected 2% of those in the 9.5 mg/24 hours patch and placebo groups; incidence in the capsule group was significantly higher at 8%. Headache was similar with the 9.5 mg/24 hours patch (3%) and placebo (2%), with the capsule significantly higher at 6%.2
The proportion of patients who experienced no, slight, or mild skin irritation ranged from 90% to 98%.2 The most commonly reported moderate or severe skin irritations were erythema (8% rivastigmine patch vs 4% placebo) and pruritus (7% rivastigmine patch vs 3% placebo). Two percent of patients using active patch discontinued the trial because of skin irritation.
Rivastigmine appears not to produce adverse effects on cardiac function as assessed by ECG. In clinical trials of 2,791 patients, pooled 12-lead ECG data comparing oral rivastigmine and placebo groups did not differ significantly in heart rate or PR, QRS, and QTc intervals.10
Dosing
The rivastigmine patch is administered once daily, and the recommended maintenance dose is the 9.5 mg/24 hours patch. Start patients on a 4.6 mg/24 hours patch for at least 4 weeks and then increase to the 9.5 mg/24 hours target dose if the lower dose is well tolerated.
Dosage adjustment of rivastigmine is not necessary in patients with hepatic or renal disease because of minimal liver metabolism and the acetylcholinesterase-mediated hydrolysis of rivastigmine to the inactive decarbamylated metabolite NAP 226-90, which is excreted in the urine.11
Instruct patients or caregivers to apply the patch to clean, dry, hairless skin that is free of cuts, rashes, or irritation on the upper or lower back or upper arm or chest.1 The patch has shown good adhesive properties over 24 hours, remaining attached in a range of situations, including bathing and hot weather.2 In the 9.5 mg/24 hours group of the efficacy study, 96% of patches remained attached or had slight lifting of the edges (1,336 total patch evaluations).
Transitioning to rivastigmine patch
The efficacy study included an open-label extension, during which blinding was maintained. This provided information on patients beginning rivastigmine patch therapy directly from placebo2 or transitioning from rivastigmine capsules to the target dose 9.5 mg/24 hours patch.12 Based on these results, transition patients as follows:
- Patients taking oral rivastigmine, <6 mg/d: Switch to a 4.6 mg/24 hours patch for ≥4 weeks before increasing to a 9.5 mg/24 hours patch.
- Patients taking oral rivastigmine, 6 to 12 mg/d: Switch directly to a 9.5 mg/24 hours patch.
Apply the first patch the day after the last oral dose.
Related resource
- Rivastigmine transdermal system prescribing information. www.pharma.us.novartis.com/product/pi/pdf/exelonpatch.pdf.
Drug brand names
- Digoxin • Lanoxin
- Rivastigmine • Exelon
- Rivastigmine transdermal
- system • Exelon Patch
Disclosure
Dr. Sadowsky is a consultant to and speaker for Forest Pharmaceuticals and Novartis Pharmaceuticals.
Acknowledgment
The author thanks Christina Mackins, PhD, a medical writer for Alpha-Plus Medical Communications Ltd, for her editorial assistance with this article. Funding for her work was provided by Novartis Pharmaceuticals.
The rivastigmine patch is the first transdermal treatment for symptoms of mild to moderate Alzheimer’s disease (AD) and mild to moderate Parkinson’s disease dementia (Table). Rivastigmine, a cholinesterase inhibitor, is the only therapy approved for both indications.
Table
Rivastigmine transdermal patch: Fast facts
| Brand name: Exelon Patch |
| Class: Cholinesterase inhibitor |
| Indication: Symptomatic treatment of mild to moderate Alzheimer’s-type dementia and mild to moderate dementia associated with Parkinson’s disease |
| Manufacturer: Novartis Pharmaceuticals, Inc. |
| Dosing forms: 4.6 and 9.5 mg/24 hours transdermal patches (5 cm2 and 10 cm2, respectively) |
| Recommended dosage: Start with 4.6 mg/24 hours patch for ≥4 weeks, followed by a one-step increase to the target dose 9.5 mg/24 hours patch* |
| *Unless the patient is taking oral rivastigmine (see ‘Transitioning to rivastigmine patch,’) |
Clinical implications
The rivastigmine patch offers continuous drug delivery through the skin into the bloodstream over 24 hours.1 This may reduce the incidence of side effects compared with oral rivastigmine,2 making optimal therapeutic doses easier to attain.3 The target dose 9.5 mg/24 hours patch provides efficacy similar to the highest recommended rivastigmine capsule dose (6 mg bid for a total of 12 mg/d).2
How it works
The rivastigmine patch uses matrix technology, which enables delivery of a large amount of drug from a small surface area.4 The patch is available in 2 dosage forms:
- a 5-cm2 size containing 9 mg of rivastigmine that delivers 4.6 mg/24 hours
- a 10-cm2 size containing 18 mg of rivastigmine that delivers 9.5 mg/24 hours.
Each patch consists of 4 layers: the backing layer, an acrylic drug matrix, a silicone adhesive matrix, and an overlapping release liner that is removed and discarded before the patch is applied.1
Cholinesterase inhibitors are believed to exert their effects by increasing available levels of the neurotransmitter acetylcholine in the brain. Two studies have demonstrated that cognitive improvements associated with rivastigmine treatment correlate significantly with cholinesterase inhibition.5,6 In 1 study, rivastigmine’s inhibitory effects on cholinesterase were sustained for 12 months.6
Pharmacokinetics
Rivastigmine is metabolized by its target cholinesterase enzymes to the decarbamylated metabolite NAP 226-90, which has minimal acetylcholinesterase inhibition and is excreted through the urine.1 As a result of its low accumulation potential and cytochrome P 450-independent metabolism, rivastigmine has low potential for pharmacokinetic drug–drug interactions. This lack of interaction has been confirmed for many drugs commonly taken by elderly patients, such as digoxin, nonsteroidal anti-inflammatory drugs, and estrogens.7
Rivastigmine has a half-life of 1 to 2 hours, so it is rapidly cleared.8 In the event of a serious reaction, significant clearance of rivastigmine from the body would occur within 3 hours of patch removal.
Centrally mediated cholinergic gastrointestinal (GI) side effects associated with oral rivastigmine are related to high maximum plasma concentrations (Cmax) and short time interval to Cmax (Tmax).9 In an open-label, parallel-group study of 51 AD patients that compared rivastigmine patches with rivastigmine capsules, transdermal administration was associated with slower increases to lower peak plasma concentrations (prolonged Tmax and reduced Cmax), and less fluctuation in plasma concentration.1 Despite these effects, the rivastigmine 9.5 mg/24 hours patch provided drug exposure comparable to the highest dose of capsules (6 mg bid for a total of 12 mg/d), with improved GI tolerability.3
Efficacy
Rivastigmine patch efficacy was evaluated in a single, 24-week, international, randomized, double-blind trial of 1,195 patients with AD.2 The study group represented typical patients with mild to moderate AD—age 50 to 85 years with Mini-Mental State Examination scores of 10 to 20 at baseline. Patients were randomly assigned to receive:
- 17.4 mg/24 hours rivastigmine patch (20-cm2 patch; n=303)
- 9.5 mg/24 hours rivastigmine patch (10-cm2 patch; n=293)
- 6 mg bid rivastigmine capsules (n=297)
- or placebo (n=302).
Data for the 17.4 mg/24 hours patch are not discussed here because this dose exceeds the FDA-approved maximum dosage (9.5 mg/24 hours) and is not available.
Patients in the 9.5 mg/24 hours patch group received a 4.6 mg/24 hours patch (5 cm2) for weeks 1 through 4, and then the 9.5 mg/24 hours patch for the remainder of the study. Patients in the capsule group started on 3 mg/d (1.5 mg bid) and were titrated every 4 weeks in steps of 3 mg/d to a maximum of 12 mg/d administered as 6 mg bid.
Primary outcomes were measured as mean change in score from baseline to endpoint on the Alzheimer’s Disease Assessment Scale–Cognitive Subscale (ADAS-Cog) and Alzheimer’s Disease Co-operative Study–Clinical Global Impression of Change (ADCS-CGIC). By study endpoint, the 9.5 mg/24 hours patch and capsules, 12 mg/d, showed comparable efficacy (Figure).2 Compared with those receiving placebo, patients in the 9.5 mg/24 hours patch and capsule groups showed significant improvements in dementia symptoms, including:
- cognition
- global performance
- attention
- activities of daily living.2
Based on my clinical experience, these improvements reflect small but clinically meaningful changes that are noted by patients and caregivers.
Figure
Efficacy of transdermal rivastigmine for Alzheimer’s symptoms

*P<0.05 vs placebo
ADAS-Cog: Alzheimer’s Disease Assessment Scale-Cognitive Subscale; ADCS-CGIC: Alzheimer’s Disease Cooperative Study-Clinical Global Impression of Change
Source: Adapted from reference 2
In a 24-week study, transdermal rivastigmine, 9.5 mg/24 hours, and the highest recommended dose of oral rivastigmine (6 mg bid) showed comparable efficacy as measured by mean change in score on scales commonly used in Alzheimer’s disease clinical trials. ADAS-Cog assesses orientation, memory, language, praxis, and visuospatial functions. ADCS-CGIC provides a single global rating of change from baseline based on interviews with the patient and caregiver.
Safety and tolerability
Adverse events associated with rivastigmine are predominantly cholinergic; GI side effects—nausea, vomiting, and diarrhea—are observed most frequently.2 These events occur less frequently with the patch than with capsules. In the efficacy trial, patients in the 9.5 mg/24 hours rivastigmine patch group had one-third as many reports of nausea (7.2% vs 23.1%) and vomiting (6.2% vs 17.0%) compared with the 6 mg bid capsule group.2
Diarrhea was reported by 6% of subjects receiving the 9.5 mg/24 hours patch, 5% of those taking 6-mg capsule bid, and 3% receiving placebo. Fewer subjects in the 9.5 mg/24 hours patch group (3%) experienced decreased weight compared with those in the capsule group (5%). The rate of decreased weight with placebo was 1%.
Dizziness affected 2% of those in the 9.5 mg/24 hours patch and placebo groups; incidence in the capsule group was significantly higher at 8%. Headache was similar with the 9.5 mg/24 hours patch (3%) and placebo (2%), with the capsule significantly higher at 6%.2
The proportion of patients who experienced no, slight, or mild skin irritation ranged from 90% to 98%.2 The most commonly reported moderate or severe skin irritations were erythema (8% rivastigmine patch vs 4% placebo) and pruritus (7% rivastigmine patch vs 3% placebo). Two percent of patients using active patch discontinued the trial because of skin irritation.
Rivastigmine appears not to produce adverse effects on cardiac function as assessed by ECG. In clinical trials of 2,791 patients, pooled 12-lead ECG data comparing oral rivastigmine and placebo groups did not differ significantly in heart rate or PR, QRS, and QTc intervals.10
Dosing
The rivastigmine patch is administered once daily, and the recommended maintenance dose is the 9.5 mg/24 hours patch. Start patients on a 4.6 mg/24 hours patch for at least 4 weeks and then increase to the 9.5 mg/24 hours target dose if the lower dose is well tolerated.
Dosage adjustment of rivastigmine is not necessary in patients with hepatic or renal disease because of minimal liver metabolism and the acetylcholinesterase-mediated hydrolysis of rivastigmine to the inactive decarbamylated metabolite NAP 226-90, which is excreted in the urine.11
Instruct patients or caregivers to apply the patch to clean, dry, hairless skin that is free of cuts, rashes, or irritation on the upper or lower back or upper arm or chest.1 The patch has shown good adhesive properties over 24 hours, remaining attached in a range of situations, including bathing and hot weather.2 In the 9.5 mg/24 hours group of the efficacy study, 96% of patches remained attached or had slight lifting of the edges (1,336 total patch evaluations).
Transitioning to rivastigmine patch
The efficacy study included an open-label extension, during which blinding was maintained. This provided information on patients beginning rivastigmine patch therapy directly from placebo2 or transitioning from rivastigmine capsules to the target dose 9.5 mg/24 hours patch.12 Based on these results, transition patients as follows:
- Patients taking oral rivastigmine, <6 mg/d: Switch to a 4.6 mg/24 hours patch for ≥4 weeks before increasing to a 9.5 mg/24 hours patch.
- Patients taking oral rivastigmine, 6 to 12 mg/d: Switch directly to a 9.5 mg/24 hours patch.
Apply the first patch the day after the last oral dose.
Related resource
- Rivastigmine transdermal system prescribing information. www.pharma.us.novartis.com/product/pi/pdf/exelonpatch.pdf.
Drug brand names
- Digoxin • Lanoxin
- Rivastigmine • Exelon
- Rivastigmine transdermal
- system • Exelon Patch
Disclosure
Dr. Sadowsky is a consultant to and speaker for Forest Pharmaceuticals and Novartis Pharmaceuticals.
Acknowledgment
The author thanks Christina Mackins, PhD, a medical writer for Alpha-Plus Medical Communications Ltd, for her editorial assistance with this article. Funding for her work was provided by Novartis Pharmaceuticals.
1. Lefèvre G, Sedek G, Jhee S, et al. Pharmacokinetics and pharmacodynamics of the novel daily rivastigmine transdermal patch compared with twice-daily capsules in Alzheimer’s disease patients. J Clin Pharmacol 2007;47:471-8.
2. Winblad B, Cummings J, Andreasen N, et al. A six-month, double-blind, randomized, placebo-controlled study of a transdermal patch in Alzheimer’s disease—rivastigmine patch versus capsule. Int J Geriatr Psychiatry 2007;22:456-67.
3. Oertel W, Ross JS, Eggert K, Adler G. Rationale for transdermal drug administration in Alzheimer disease. Neurology 2007;69(suppl 1):S4-S9.
4. Petersen TA. Transdermal drug formulations and process development. Pharmaceut Technol 2003;(suppl):18-21.
5. Giacobini E, Spiegel R, Enz A, et al. Inhibition of acetyl- and butyryl-cholinesterase in the cerebrospinal fluid of patients with Alzheimer’s disease by rivastigmine: correlation with cognitive benefit. J Neural Transm 2002;109:1053-65.
6. Darreh-Shori T, Almkvist O, Guan ZZ, et al. Sustained cholinesterase inhibition in AD patients receiving rivastigmine for 12 months. Neurology 2002;59:563-72.
7. Grossberg GT, Stahelin HB, Messina JC, et al. Lack of adverse pharmacodynamic drug interactions with rivastigmine and twenty-two classes of medications. Int J Geriatr Psychiatry 2000;15(3):242-7.
8. Polinsky RJ. Clinical pharmacology of rivastigmine: a new-generation acetylcholinesterase inhibitor for the treatment of Alzheimer’s disease. Clin Ther 1998;20:634-47.
9. Jann MW, Shirley KL, Small GW. Clinical pharmacokinetics and pharmacodynamics of cholinesterase inhibitors. Clin Pharmacokinet 2002;41:719-39.
10. Morganroth J, Graham S, Hartman R, et al. Electrocardiographic effects of rivastigmine. J Clin Pharmacol 2002;42:558-68.
11. Exelon patch [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2007.
12. Frölich L, Barone P, Förstl H, et al. IDEAL: A 28-week open-label extension of a 24-week double-blind study of the first transdermal patch in Alzheimer’s disease. Poster presented at: 11th Congress of the European Federation of Neurological Societies; August 25-28, 2007; Brussels, Belgium.
Dr. Sadowsky is associate clinical professor of neurology, Nova Southeastern University, Fort Lauderdale, FL, and director, Premier Research Institute, Palm Beach Neurology, West Palm Beach, FL.
1. Lefèvre G, Sedek G, Jhee S, et al. Pharmacokinetics and pharmacodynamics of the novel daily rivastigmine transdermal patch compared with twice-daily capsules in Alzheimer’s disease patients. J Clin Pharmacol 2007;47:471-8.
2. Winblad B, Cummings J, Andreasen N, et al. A six-month, double-blind, randomized, placebo-controlled study of a transdermal patch in Alzheimer’s disease—rivastigmine patch versus capsule. Int J Geriatr Psychiatry 2007;22:456-67.
3. Oertel W, Ross JS, Eggert K, Adler G. Rationale for transdermal drug administration in Alzheimer disease. Neurology 2007;69(suppl 1):S4-S9.
4. Petersen TA. Transdermal drug formulations and process development. Pharmaceut Technol 2003;(suppl):18-21.
5. Giacobini E, Spiegel R, Enz A, et al. Inhibition of acetyl- and butyryl-cholinesterase in the cerebrospinal fluid of patients with Alzheimer’s disease by rivastigmine: correlation with cognitive benefit. J Neural Transm 2002;109:1053-65.
6. Darreh-Shori T, Almkvist O, Guan ZZ, et al. Sustained cholinesterase inhibition in AD patients receiving rivastigmine for 12 months. Neurology 2002;59:563-72.
7. Grossberg GT, Stahelin HB, Messina JC, et al. Lack of adverse pharmacodynamic drug interactions with rivastigmine and twenty-two classes of medications. Int J Geriatr Psychiatry 2000;15(3):242-7.
8. Polinsky RJ. Clinical pharmacology of rivastigmine: a new-generation acetylcholinesterase inhibitor for the treatment of Alzheimer’s disease. Clin Ther 1998;20:634-47.
9. Jann MW, Shirley KL, Small GW. Clinical pharmacokinetics and pharmacodynamics of cholinesterase inhibitors. Clin Pharmacokinet 2002;41:719-39.
10. Morganroth J, Graham S, Hartman R, et al. Electrocardiographic effects of rivastigmine. J Clin Pharmacol 2002;42:558-68.
11. Exelon patch [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2007.
12. Frölich L, Barone P, Förstl H, et al. IDEAL: A 28-week open-label extension of a 24-week double-blind study of the first transdermal patch in Alzheimer’s disease. Poster presented at: 11th Congress of the European Federation of Neurological Societies; August 25-28, 2007; Brussels, Belgium.
Dr. Sadowsky is associate clinical professor of neurology, Nova Southeastern University, Fort Lauderdale, FL, and director, Premier Research Institute, Palm Beach Neurology, West Palm Beach, FL.