User login
In the Eye of the Storm
A 37‐year‐old man presented to an ophthalmologist in July 2004 with a history of slowly decreasing vision in both eyes for several weeks. His vision on presentation was 20/400 in the right eye and 20/200 in the left eye. Slit‐lamp examination showed a bilateral anterior uveitis with 360 degrees of posterior synechiae (adhesions) and a dense vitritis (posterior uveitis) that obscured the view of the retina in both eyes. He was diagnosed with panuveitis and started on topical steroid and cycloplegic drops. He was referred to a uveitis specialist for investigation but missed his appointments.
One year later he presented to the emergency room with fever and severe pain in his left eye. On initial assessment he had no complaints of mouth or genital ulcers, recent or remote rashes, joint symptoms, or penile discharge. He denied any prior eye trauma or surgery. He reported that his last sexual encounter had been 8 months prior with a male and that his most recent HIV screen was negative 6 months ago. His family history was negative for autoimmune disorders.
On inspection, he appeared cachectic, lethargic, and very ill. He was febrile and tachycardic; the remainder of his vital signs were normal. There was no lymphadenopathy. His neck was supple with no meningismal signs. There were no heart murmurs, oral ulcers, swollen joints, mucosal eschar, or skin lesions. Respiratory and abdominal examinations were unremarkable.
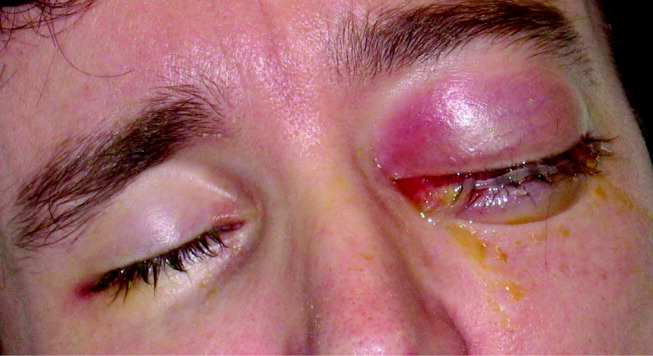
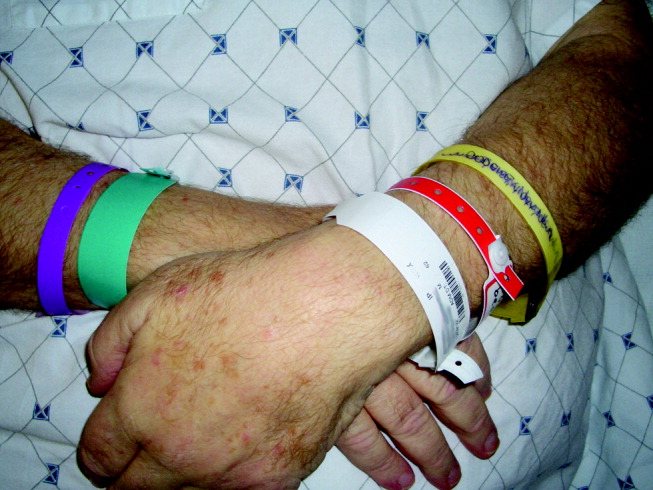
His visual acuity was light perception in right eye and no light perception in the left eye. There was significant eyelid edema, erythema and purulent discharge with mild proptosis of the left eye (Fig. 1). Pupils were 3 mm and fixed, with 360 of posterior synechiae. Intraocular pressure was elevated in the left eye (42 mm Hg, where normal is 20 mm Hg). There was moderate uveitis in both eyes, with a 1‐mm hypopyon in the left eye and forward bowing of the iris (iris bomb). A dense vitritis was present in both eyes, preventing visualization of the retina. B‐scan ultrasound examination showed bilateral retinal detachments, worse in the left eye.

Because of the high intraocular pressure in the left eye, the patient was given topical Cosopt (dorzolamide hydrochloride‐timolol maleate), bromonidine 0.15%, and oral acetazolamide to lower intraocular pressures. He was started on a preliminary treatment of hourly topical prednisolone acetate 1%, atropine 1% 4 times daily, and topical moxifloxacin 0.5%. He was admitted to hospital to investigate the source of his panophthalmitis (suppurative infection of the eye and sclera, extending to involve the orbit).
Blood and urine cultures, HIV, rapid plasma reagin test (RPR), HLA B27, toxoplasmosis serology, and ANA rheumatoid factor were sent. Overnight, he developed classic Janeway lesions on his palms and soles, and both blood and urine cultures grew gram‐positive cocci in clusters. Repeat blood cultures were taken. He was started on IV vancomycin empirically. Ultimately, all 3 blood cultures grew Staphylococcus aureus.
A transesophageal echocardiogram diagnosed endocarditis with a pedunculated mobile mass identified on the posterior mitral valve leaflet. Mild mitral regurgitation was noted. The aortic valve was normal, as were ventricular size and function. Antibiotics were modified to cloxacillin and gentamicin IV 2 days later, once sensitivities were reported.
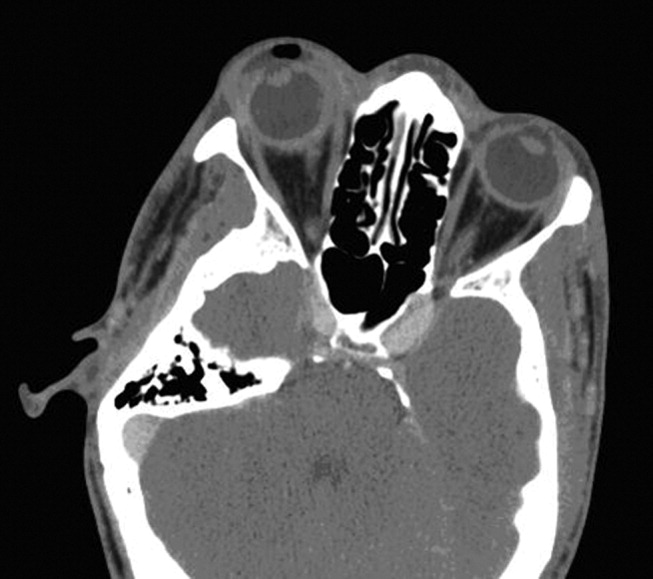
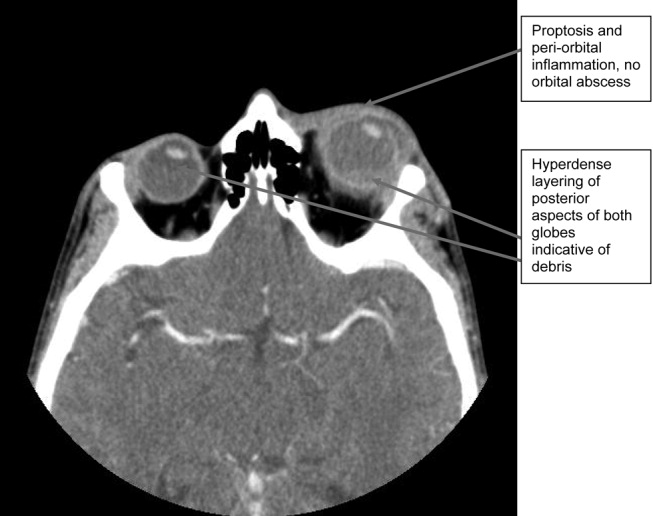
A CT scan of the orbits revealed diffuse orbital inflammation with no evidence of an orbital abscess (Fig. 2). The inflammation and proptosis of the left eye continued to worsen, and a vitreous paracentesis of the left eye was performed for 1.5 mL of dark brown fluid. The aspirated sample was sent for C&S, PCR (for HSV, CMV), acid‐fast stain, and fungal, viral, and mycobacterial cultures. Intravitreal injections of vancomycin and ceftazidime were given. Bacterial cultures showed a heavy intraocular growth of S. aureus, giving the diagnosis of endophthalmitis (bacterial or fungal infection of the vitreous or aqueous humor); all remaining stains and cultures were negative.
Over the next several days, the initial blood work returned with the following abnormal results: CD4 count was 70/L, and HIV serology was positive. The rapid plasma reagin test (RPR) was positive (titer 1:64). The enzyme immunoassay (EIA) and Treponema pallidum particle agglutination (TPPA) were also positive.
A lumbar puncture was performed, and CSF analysis indicated CSF fluid was clear, 2 erythrocytes and 2 leukocytes in the fourth tube, CSF glucose of 2.7 mmol/L (serum glucose 8.2 mmol/L), and CSF total protein of 1100 mg/L. There were no bacteria seen on the gram stain, and a rapid agglutination test for cryptococcal antigen was negative. The CSF RPR titer was 1:2, and the Treponema pallidum particle agglutination assay (TP‐PA) was reactive. The MRI of the brain indicated diffuse white matter disease but no meningeal enhancement. In combination, these results were indicative of neurosyphilis, and penicillin G IV therapy was initiated. He received a total of 14 days of IV therapy, followed by 3 weekly IM doses of benzathine penicillin. He also received a total of 28 days of IV cloxacillin therapy with 5 days of concomitant IV gentamicin for endocarditis treatment.
Over 8 weeks, the patient's panophthalmitis slowly improved. However, he maintained only light perception in the right eye and did not regain any vision in the left eye. He was discharged home to follow‐up with the infectious diseases and ophthalmology departments. The issue of initiating antiretroviral therapy, deferred during hospital admission because of his poor compliance history and the threat of immune reconstitution symptoms, was to be readdressed at this time. He missed both appointments and returned to the emergency room several months later with widespread Kaposi's sarcoma.
DISCUSSION
One of the key learning points from this case underlines that panuveitis carries a broad differential including inflammatory and infectious conditions, as well as lymphoma. Systemic infections include tuberculosis, syphilis, and in cases of severe immunosuppression, toxoplasmosis. Cytomegalovirus and candidiasis are less likely as they are not associated with intraocular inflammation. HIV is also on the differential, although it rarely causes severe panuveitis on its own. Inflammatory disorders such as Behcet syndrome, sarcoidosis, and, rarely, lens‐associated uveitis (if presented with a history of lens trauma or surgery) are also included on the differential. A systematic approach to the history and physical examination must be undertaken to narrow the search. A syphilis screen should always be included in the differential when investigating uveitis,1 especially given the resurgence of syphilis since 2000.2
Our patient presents an interesting study as he was coinfected with both syphilis and HIV. The progression of syphilis is far more aggressive in this scenario,3 as there is a higher frequency of initial presentation as secondary syphilis4 and with multiple persisting chancres.5 Secondary‐stage skin lesions are also more aggressive in coinfected patients (nodular or ulcerative lesions with necrotic centers), although the same dermatological presentations can be seen in HIV‐negative patients.6 It has not been definitively established whether HIV‐positive patients develop neurological complications of syphilis more frequently or earlier in disease, but most patients present with early neurosyphilis at the time of diagnosis.7 In keeping with these findings, our patient's initial presentation included both ocular and neurosyphilis as diagnostic features.
An atypical link highlighted by our case is that of endogenous, bacterial endophthalmitis secondary to endocarditis. Although traumatic or surgical complications are the most common causes of endophthalmitis, seeding from an endogenous infective source, although rare, is possible.810 Staphylococcus aureus endocarditis is one of the most common causes of endogenous spread.9 In our patient, his chronic uveitis and decompensated blood‐ocular barrier may have contributed to S. aureus seeding of his eye. As is the case with many patients diagnosed with S. aureus endocarditis, the source of infection was unknown, although several risk factors for S. aureus bacteremia have been documented. These risk factors include hospitalization, dialysis, transplantation, HIV‐positive status, heart disease, cancer, diabetes, and intravenous drug use. In a population‐based surveillance study from 1999 to 2000, 550 invasive isolates of S. aureus were obtained; the relative risk in HIV‐positive patients was 23.7.11 In a similar study, the source of the S. aureus bacteremia/endocarditis was not identified in 26% of patients with underlying medical conditions such as HIV infection.12
This case has demonstrated several intertwined disease presentations in a patient coinfected with multiple organisms. In an immunocompromised patient, Occam's razor does not necessarily hold true, and the possibility of multiple diagnoses must be entertained. Thus, clinicians must maintain a high index of suspicion for atypical presentations of typical diseases if their patients are to survive in the eye of the storm.
- ,.Ocular syphilis.Surv Ophthalmol.1992;37:203.
- ,,, et al.Primary and secondary syphilis—United States, 2003‐2004.MMWR.2006;55:269–273.
- ,,.Update on syphilis—resurgence of an old problem.JAMA.2003;290:1510.
- ,,,,.Altered clinical presentation of early syphilis in patients with human immunodeficiency virus infection.Ann Intern Med.1994;121:94–100.
- ,,, et al.A randomized trial of enhanced therapy for early syphilis in patients with and without human immunodeficiency virus infection. The Syphilis and HIV Study Group.N Engl J Med.1997;337:307–314.
- ,.Prominent osseous and unusual dermatologic manifestations of early syphilis in two patients with discordant serological statuses for human immunodeficiency virus infection.Clin Infect Dis.1996;23:462–467.
- ,,,,,.Neurosyphilis during the AIDS epidemic, San Francisco, 1985‐1992.J Infect Dis.1998;177:931–940.
- ,,, et al.Nosocomial endophthalmitis survey: Current incidence of infection after intraocular surgery.Ophthalmology.1991;98:227.
- ,,,.Endophthalmitis following open‐globe injuries.Curr Opin Ophthalmol.1998;9:59.
- ,,, et al.Endogenous bacterial endophthalmitis: Report of a ten‐year retrospective study.Ophthalmology.1994;101:832.
- ,,, et al.Population‐based study of the epidemiology of and the risk factors for invasive Staphylococcus aureus infections.J Infect Dis.2003;187:1452–1459.
- ,.Population‐based incidence and characteristics of community‐onset Staphylococcus aureus infections with bacteremia in 4 metropolitan Connecticut areas, 1998.J Infect Dis.2001;184:1029–1034.
A 37‐year‐old man presented to an ophthalmologist in July 2004 with a history of slowly decreasing vision in both eyes for several weeks. His vision on presentation was 20/400 in the right eye and 20/200 in the left eye. Slit‐lamp examination showed a bilateral anterior uveitis with 360 degrees of posterior synechiae (adhesions) and a dense vitritis (posterior uveitis) that obscured the view of the retina in both eyes. He was diagnosed with panuveitis and started on topical steroid and cycloplegic drops. He was referred to a uveitis specialist for investigation but missed his appointments.
One year later he presented to the emergency room with fever and severe pain in his left eye. On initial assessment he had no complaints of mouth or genital ulcers, recent or remote rashes, joint symptoms, or penile discharge. He denied any prior eye trauma or surgery. He reported that his last sexual encounter had been 8 months prior with a male and that his most recent HIV screen was negative 6 months ago. His family history was negative for autoimmune disorders.
On inspection, he appeared cachectic, lethargic, and very ill. He was febrile and tachycardic; the remainder of his vital signs were normal. There was no lymphadenopathy. His neck was supple with no meningismal signs. There were no heart murmurs, oral ulcers, swollen joints, mucosal eschar, or skin lesions. Respiratory and abdominal examinations were unremarkable.
His visual acuity was light perception in right eye and no light perception in the left eye. There was significant eyelid edema, erythema and purulent discharge with mild proptosis of the left eye (Fig. 1). Pupils were 3 mm and fixed, with 360 of posterior synechiae. Intraocular pressure was elevated in the left eye (42 mm Hg, where normal is 20 mm Hg). There was moderate uveitis in both eyes, with a 1‐mm hypopyon in the left eye and forward bowing of the iris (iris bomb). A dense vitritis was present in both eyes, preventing visualization of the retina. B‐scan ultrasound examination showed bilateral retinal detachments, worse in the left eye.

Because of the high intraocular pressure in the left eye, the patient was given topical Cosopt (dorzolamide hydrochloride‐timolol maleate), bromonidine 0.15%, and oral acetazolamide to lower intraocular pressures. He was started on a preliminary treatment of hourly topical prednisolone acetate 1%, atropine 1% 4 times daily, and topical moxifloxacin 0.5%. He was admitted to hospital to investigate the source of his panophthalmitis (suppurative infection of the eye and sclera, extending to involve the orbit).
Blood and urine cultures, HIV, rapid plasma reagin test (RPR), HLA B27, toxoplasmosis serology, and ANA rheumatoid factor were sent. Overnight, he developed classic Janeway lesions on his palms and soles, and both blood and urine cultures grew gram‐positive cocci in clusters. Repeat blood cultures were taken. He was started on IV vancomycin empirically. Ultimately, all 3 blood cultures grew Staphylococcus aureus.
A transesophageal echocardiogram diagnosed endocarditis with a pedunculated mobile mass identified on the posterior mitral valve leaflet. Mild mitral regurgitation was noted. The aortic valve was normal, as were ventricular size and function. Antibiotics were modified to cloxacillin and gentamicin IV 2 days later, once sensitivities were reported.
A CT scan of the orbits revealed diffuse orbital inflammation with no evidence of an orbital abscess (Fig. 2). The inflammation and proptosis of the left eye continued to worsen, and a vitreous paracentesis of the left eye was performed for 1.5 mL of dark brown fluid. The aspirated sample was sent for C&S, PCR (for HSV, CMV), acid‐fast stain, and fungal, viral, and mycobacterial cultures. Intravitreal injections of vancomycin and ceftazidime were given. Bacterial cultures showed a heavy intraocular growth of S. aureus, giving the diagnosis of endophthalmitis (bacterial or fungal infection of the vitreous or aqueous humor); all remaining stains and cultures were negative.

Over the next several days, the initial blood work returned with the following abnormal results: CD4 count was 70/L, and HIV serology was positive. The rapid plasma reagin test (RPR) was positive (titer 1:64). The enzyme immunoassay (EIA) and Treponema pallidum particle agglutination (TPPA) were also positive.
A lumbar puncture was performed, and CSF analysis indicated CSF fluid was clear, 2 erythrocytes and 2 leukocytes in the fourth tube, CSF glucose of 2.7 mmol/L (serum glucose 8.2 mmol/L), and CSF total protein of 1100 mg/L. There were no bacteria seen on the gram stain, and a rapid agglutination test for cryptococcal antigen was negative. The CSF RPR titer was 1:2, and the Treponema pallidum particle agglutination assay (TP‐PA) was reactive. The MRI of the brain indicated diffuse white matter disease but no meningeal enhancement. In combination, these results were indicative of neurosyphilis, and penicillin G IV therapy was initiated. He received a total of 14 days of IV therapy, followed by 3 weekly IM doses of benzathine penicillin. He also received a total of 28 days of IV cloxacillin therapy with 5 days of concomitant IV gentamicin for endocarditis treatment.
Over 8 weeks, the patient's panophthalmitis slowly improved. However, he maintained only light perception in the right eye and did not regain any vision in the left eye. He was discharged home to follow‐up with the infectious diseases and ophthalmology departments. The issue of initiating antiretroviral therapy, deferred during hospital admission because of his poor compliance history and the threat of immune reconstitution symptoms, was to be readdressed at this time. He missed both appointments and returned to the emergency room several months later with widespread Kaposi's sarcoma.
DISCUSSION
One of the key learning points from this case underlines that panuveitis carries a broad differential including inflammatory and infectious conditions, as well as lymphoma. Systemic infections include tuberculosis, syphilis, and in cases of severe immunosuppression, toxoplasmosis. Cytomegalovirus and candidiasis are less likely as they are not associated with intraocular inflammation. HIV is also on the differential, although it rarely causes severe panuveitis on its own. Inflammatory disorders such as Behcet syndrome, sarcoidosis, and, rarely, lens‐associated uveitis (if presented with a history of lens trauma or surgery) are also included on the differential. A systematic approach to the history and physical examination must be undertaken to narrow the search. A syphilis screen should always be included in the differential when investigating uveitis,1 especially given the resurgence of syphilis since 2000.2
Our patient presents an interesting study as he was coinfected with both syphilis and HIV. The progression of syphilis is far more aggressive in this scenario,3 as there is a higher frequency of initial presentation as secondary syphilis4 and with multiple persisting chancres.5 Secondary‐stage skin lesions are also more aggressive in coinfected patients (nodular or ulcerative lesions with necrotic centers), although the same dermatological presentations can be seen in HIV‐negative patients.6 It has not been definitively established whether HIV‐positive patients develop neurological complications of syphilis more frequently or earlier in disease, but most patients present with early neurosyphilis at the time of diagnosis.7 In keeping with these findings, our patient's initial presentation included both ocular and neurosyphilis as diagnostic features.
An atypical link highlighted by our case is that of endogenous, bacterial endophthalmitis secondary to endocarditis. Although traumatic or surgical complications are the most common causes of endophthalmitis, seeding from an endogenous infective source, although rare, is possible.810 Staphylococcus aureus endocarditis is one of the most common causes of endogenous spread.9 In our patient, his chronic uveitis and decompensated blood‐ocular barrier may have contributed to S. aureus seeding of his eye. As is the case with many patients diagnosed with S. aureus endocarditis, the source of infection was unknown, although several risk factors for S. aureus bacteremia have been documented. These risk factors include hospitalization, dialysis, transplantation, HIV‐positive status, heart disease, cancer, diabetes, and intravenous drug use. In a population‐based surveillance study from 1999 to 2000, 550 invasive isolates of S. aureus were obtained; the relative risk in HIV‐positive patients was 23.7.11 In a similar study, the source of the S. aureus bacteremia/endocarditis was not identified in 26% of patients with underlying medical conditions such as HIV infection.12
This case has demonstrated several intertwined disease presentations in a patient coinfected with multiple organisms. In an immunocompromised patient, Occam's razor does not necessarily hold true, and the possibility of multiple diagnoses must be entertained. Thus, clinicians must maintain a high index of suspicion for atypical presentations of typical diseases if their patients are to survive in the eye of the storm.
A 37‐year‐old man presented to an ophthalmologist in July 2004 with a history of slowly decreasing vision in both eyes for several weeks. His vision on presentation was 20/400 in the right eye and 20/200 in the left eye. Slit‐lamp examination showed a bilateral anterior uveitis with 360 degrees of posterior synechiae (adhesions) and a dense vitritis (posterior uveitis) that obscured the view of the retina in both eyes. He was diagnosed with panuveitis and started on topical steroid and cycloplegic drops. He was referred to a uveitis specialist for investigation but missed his appointments.
One year later he presented to the emergency room with fever and severe pain in his left eye. On initial assessment he had no complaints of mouth or genital ulcers, recent or remote rashes, joint symptoms, or penile discharge. He denied any prior eye trauma or surgery. He reported that his last sexual encounter had been 8 months prior with a male and that his most recent HIV screen was negative 6 months ago. His family history was negative for autoimmune disorders.
On inspection, he appeared cachectic, lethargic, and very ill. He was febrile and tachycardic; the remainder of his vital signs were normal. There was no lymphadenopathy. His neck was supple with no meningismal signs. There were no heart murmurs, oral ulcers, swollen joints, mucosal eschar, or skin lesions. Respiratory and abdominal examinations were unremarkable.
His visual acuity was light perception in right eye and no light perception in the left eye. There was significant eyelid edema, erythema and purulent discharge with mild proptosis of the left eye (Fig. 1). Pupils were 3 mm and fixed, with 360 of posterior synechiae. Intraocular pressure was elevated in the left eye (42 mm Hg, where normal is 20 mm Hg). There was moderate uveitis in both eyes, with a 1‐mm hypopyon in the left eye and forward bowing of the iris (iris bomb). A dense vitritis was present in both eyes, preventing visualization of the retina. B‐scan ultrasound examination showed bilateral retinal detachments, worse in the left eye.

Because of the high intraocular pressure in the left eye, the patient was given topical Cosopt (dorzolamide hydrochloride‐timolol maleate), bromonidine 0.15%, and oral acetazolamide to lower intraocular pressures. He was started on a preliminary treatment of hourly topical prednisolone acetate 1%, atropine 1% 4 times daily, and topical moxifloxacin 0.5%. He was admitted to hospital to investigate the source of his panophthalmitis (suppurative infection of the eye and sclera, extending to involve the orbit).
Blood and urine cultures, HIV, rapid plasma reagin test (RPR), HLA B27, toxoplasmosis serology, and ANA rheumatoid factor were sent. Overnight, he developed classic Janeway lesions on his palms and soles, and both blood and urine cultures grew gram‐positive cocci in clusters. Repeat blood cultures were taken. He was started on IV vancomycin empirically. Ultimately, all 3 blood cultures grew Staphylococcus aureus.
A transesophageal echocardiogram diagnosed endocarditis with a pedunculated mobile mass identified on the posterior mitral valve leaflet. Mild mitral regurgitation was noted. The aortic valve was normal, as were ventricular size and function. Antibiotics were modified to cloxacillin and gentamicin IV 2 days later, once sensitivities were reported.
A CT scan of the orbits revealed diffuse orbital inflammation with no evidence of an orbital abscess (Fig. 2). The inflammation and proptosis of the left eye continued to worsen, and a vitreous paracentesis of the left eye was performed for 1.5 mL of dark brown fluid. The aspirated sample was sent for C&S, PCR (for HSV, CMV), acid‐fast stain, and fungal, viral, and mycobacterial cultures. Intravitreal injections of vancomycin and ceftazidime were given. Bacterial cultures showed a heavy intraocular growth of S. aureus, giving the diagnosis of endophthalmitis (bacterial or fungal infection of the vitreous or aqueous humor); all remaining stains and cultures were negative.

Over the next several days, the initial blood work returned with the following abnormal results: CD4 count was 70/L, and HIV serology was positive. The rapid plasma reagin test (RPR) was positive (titer 1:64). The enzyme immunoassay (EIA) and Treponema pallidum particle agglutination (TPPA) were also positive.
A lumbar puncture was performed, and CSF analysis indicated CSF fluid was clear, 2 erythrocytes and 2 leukocytes in the fourth tube, CSF glucose of 2.7 mmol/L (serum glucose 8.2 mmol/L), and CSF total protein of 1100 mg/L. There were no bacteria seen on the gram stain, and a rapid agglutination test for cryptococcal antigen was negative. The CSF RPR titer was 1:2, and the Treponema pallidum particle agglutination assay (TP‐PA) was reactive. The MRI of the brain indicated diffuse white matter disease but no meningeal enhancement. In combination, these results were indicative of neurosyphilis, and penicillin G IV therapy was initiated. He received a total of 14 days of IV therapy, followed by 3 weekly IM doses of benzathine penicillin. He also received a total of 28 days of IV cloxacillin therapy with 5 days of concomitant IV gentamicin for endocarditis treatment.
Over 8 weeks, the patient's panophthalmitis slowly improved. However, he maintained only light perception in the right eye and did not regain any vision in the left eye. He was discharged home to follow‐up with the infectious diseases and ophthalmology departments. The issue of initiating antiretroviral therapy, deferred during hospital admission because of his poor compliance history and the threat of immune reconstitution symptoms, was to be readdressed at this time. He missed both appointments and returned to the emergency room several months later with widespread Kaposi's sarcoma.
DISCUSSION
One of the key learning points from this case underlines that panuveitis carries a broad differential including inflammatory and infectious conditions, as well as lymphoma. Systemic infections include tuberculosis, syphilis, and in cases of severe immunosuppression, toxoplasmosis. Cytomegalovirus and candidiasis are less likely as they are not associated with intraocular inflammation. HIV is also on the differential, although it rarely causes severe panuveitis on its own. Inflammatory disorders such as Behcet syndrome, sarcoidosis, and, rarely, lens‐associated uveitis (if presented with a history of lens trauma or surgery) are also included on the differential. A systematic approach to the history and physical examination must be undertaken to narrow the search. A syphilis screen should always be included in the differential when investigating uveitis,1 especially given the resurgence of syphilis since 2000.2
Our patient presents an interesting study as he was coinfected with both syphilis and HIV. The progression of syphilis is far more aggressive in this scenario,3 as there is a higher frequency of initial presentation as secondary syphilis4 and with multiple persisting chancres.5 Secondary‐stage skin lesions are also more aggressive in coinfected patients (nodular or ulcerative lesions with necrotic centers), although the same dermatological presentations can be seen in HIV‐negative patients.6 It has not been definitively established whether HIV‐positive patients develop neurological complications of syphilis more frequently or earlier in disease, but most patients present with early neurosyphilis at the time of diagnosis.7 In keeping with these findings, our patient's initial presentation included both ocular and neurosyphilis as diagnostic features.
An atypical link highlighted by our case is that of endogenous, bacterial endophthalmitis secondary to endocarditis. Although traumatic or surgical complications are the most common causes of endophthalmitis, seeding from an endogenous infective source, although rare, is possible.810 Staphylococcus aureus endocarditis is one of the most common causes of endogenous spread.9 In our patient, his chronic uveitis and decompensated blood‐ocular barrier may have contributed to S. aureus seeding of his eye. As is the case with many patients diagnosed with S. aureus endocarditis, the source of infection was unknown, although several risk factors for S. aureus bacteremia have been documented. These risk factors include hospitalization, dialysis, transplantation, HIV‐positive status, heart disease, cancer, diabetes, and intravenous drug use. In a population‐based surveillance study from 1999 to 2000, 550 invasive isolates of S. aureus were obtained; the relative risk in HIV‐positive patients was 23.7.11 In a similar study, the source of the S. aureus bacteremia/endocarditis was not identified in 26% of patients with underlying medical conditions such as HIV infection.12
This case has demonstrated several intertwined disease presentations in a patient coinfected with multiple organisms. In an immunocompromised patient, Occam's razor does not necessarily hold true, and the possibility of multiple diagnoses must be entertained. Thus, clinicians must maintain a high index of suspicion for atypical presentations of typical diseases if their patients are to survive in the eye of the storm.
- ,.Ocular syphilis.Surv Ophthalmol.1992;37:203.
- ,,, et al.Primary and secondary syphilis—United States, 2003‐2004.MMWR.2006;55:269–273.
- ,,.Update on syphilis—resurgence of an old problem.JAMA.2003;290:1510.
- ,,,,.Altered clinical presentation of early syphilis in patients with human immunodeficiency virus infection.Ann Intern Med.1994;121:94–100.
- ,,, et al.A randomized trial of enhanced therapy for early syphilis in patients with and without human immunodeficiency virus infection. The Syphilis and HIV Study Group.N Engl J Med.1997;337:307–314.
- ,.Prominent osseous and unusual dermatologic manifestations of early syphilis in two patients with discordant serological statuses for human immunodeficiency virus infection.Clin Infect Dis.1996;23:462–467.
- ,,,,,.Neurosyphilis during the AIDS epidemic, San Francisco, 1985‐1992.J Infect Dis.1998;177:931–940.
- ,,, et al.Nosocomial endophthalmitis survey: Current incidence of infection after intraocular surgery.Ophthalmology.1991;98:227.
- ,,,.Endophthalmitis following open‐globe injuries.Curr Opin Ophthalmol.1998;9:59.
- ,,, et al.Endogenous bacterial endophthalmitis: Report of a ten‐year retrospective study.Ophthalmology.1994;101:832.
- ,,, et al.Population‐based study of the epidemiology of and the risk factors for invasive Staphylococcus aureus infections.J Infect Dis.2003;187:1452–1459.
- ,.Population‐based incidence and characteristics of community‐onset Staphylococcus aureus infections with bacteremia in 4 metropolitan Connecticut areas, 1998.J Infect Dis.2001;184:1029–1034.
- ,.Ocular syphilis.Surv Ophthalmol.1992;37:203.
- ,,, et al.Primary and secondary syphilis—United States, 2003‐2004.MMWR.2006;55:269–273.
- ,,.Update on syphilis—resurgence of an old problem.JAMA.2003;290:1510.
- ,,,,.Altered clinical presentation of early syphilis in patients with human immunodeficiency virus infection.Ann Intern Med.1994;121:94–100.
- ,,, et al.A randomized trial of enhanced therapy for early syphilis in patients with and without human immunodeficiency virus infection. The Syphilis and HIV Study Group.N Engl J Med.1997;337:307–314.
- ,.Prominent osseous and unusual dermatologic manifestations of early syphilis in two patients with discordant serological statuses for human immunodeficiency virus infection.Clin Infect Dis.1996;23:462–467.
- ,,,,,.Neurosyphilis during the AIDS epidemic, San Francisco, 1985‐1992.J Infect Dis.1998;177:931–940.
- ,,, et al.Nosocomial endophthalmitis survey: Current incidence of infection after intraocular surgery.Ophthalmology.1991;98:227.
- ,,,.Endophthalmitis following open‐globe injuries.Curr Opin Ophthalmol.1998;9:59.
- ,,, et al.Endogenous bacterial endophthalmitis: Report of a ten‐year retrospective study.Ophthalmology.1994;101:832.
- ,,, et al.Population‐based study of the epidemiology of and the risk factors for invasive Staphylococcus aureus infections.J Infect Dis.2003;187:1452–1459.
- ,.Population‐based incidence and characteristics of community‐onset Staphylococcus aureus infections with bacteremia in 4 metropolitan Connecticut areas, 1998.J Infect Dis.2001;184:1029–1034.
Liver Abscess and Metastatic Endophthalmitis
Klebsiella pneumoniae liver abscess is known to be associated with metastatic endophthalmitis,1 although most cases have been clustered in Taiwan, with few reports in the United States.2 The first reported case of Klebsiella liver abscess with endophthalmitis in the United States was in a 38‐year‐old man with a new diagnosis of diabetes, a known risk factor for hematogenous spread of Klebsiella to metastatic sites.3
CASE REPORT
A previously healthy 43‐year old Haitian man presented after experiencing 5 days of right eye pain with associated fever and swelling. The patient denied preceding trauma, manipulation of the eye, contact lens use, or illicit drug use and had no significant medical history. He had moved to the United States from Haiti more than 15 years ago and had not traveled out of the state of Florida since that time.
Physical exam showed tachycardia (rate = 110/min), tachypnea (rate = 20/min), and a temperature of 101.5F. The right eye had injected conjunctiva, a swollen lid, and decreased palpebral fissure, and visual acuity on the left was 20/60, whereas visual acuity on the right was recorded as the ability to count fingers at 3 feet. The remainder of his physical exam was within normal limits including the abdominal exam.
Laboratory data on admission included a white blood cell count of 37,500/L significant for 12% bands, total bilirubin of 2.8 mg/dL, AST of 141 U/L, ALT of 130 U/L, and alkaline phosphatase of 196 U/L. HIV testing was negative, and urine toxicology did not detect the presence of any illicit drugs. Vitreous cultures grew Klebsiella pneumoniae.
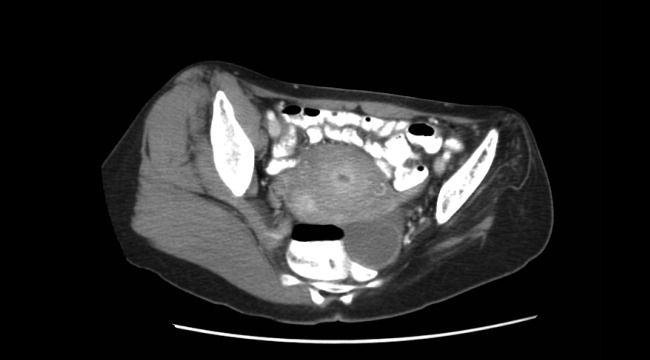
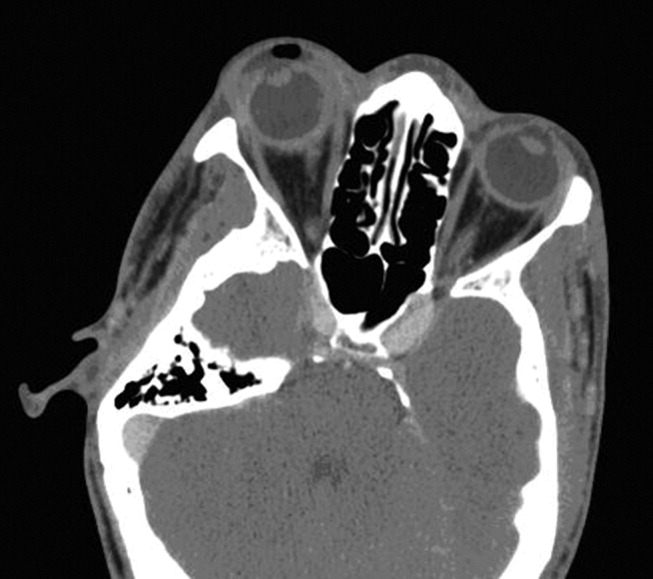
The initial CT scan of the orbit (Fig. 1) showed periorbital swelling and a preseptal collection anterior to the right globe consistent with an abscess. Because of the abnormal results of the liver panel in the presence of the ophthalmologic infection, an abdominal CT was obtained that showed an 11.5 by 8.0 cm lesion involving all segments of the right lobe of the liver with a 0.9‐mm cylindrical extension toward the right hepatic vein.
Percutaneous drainage of the liver abscess was performed, yielding positive cultures for K. pneumoniae. The patient was treated with oral gatifloxacin and intravenous ceftriaxone. Gatifloxacin therapy was chosen for its excellent penetrance into the vitreous.4 Despite antibiotic therapy, a repeat CT scan of the orbit showed further extension of the collection, and the decision was made to drain the abscess and perform right eye enucleation. The patient was discharged home on oral gatifloxacin. A follow‐up abdominal ultrasound 2 weeks after discharge showed complete resolution of the liver abscess.
DISCUSSION
Bacterial endophthalmitis is a rare infection involving the vitreous humor and other deep intraocular structures. It is most commonly exogenous in origin, caused by intraocular surgery, penetrating injury, a corneal ulcer, or periocular infection. Endogenous endophthalmitis occurs when organisms reach the eye hematogenously and accounts for fewer than 6% of all cases of endophthalmitis.5
Klebsiella liver abscesses have been increasing in incidence worldwide and since the mid‐1990s have become a common cause of liver abscess in the United States, along with Escherichia coli. The association with endophthalmitis was first reported in a series of 7 cases from Taiwan in 1986,1 and subsequent East Asian cases have been reported, usually in diabetic patients.6, 7 The association of Klebsiella liver abscesses with endogenous endophthalmitis has been rarely reported in the United States, with review of the literature from 1966 to 2003 revealing only 3 reported cases.2 One of these patients had diabetes, whereas another had beta‐thalassemia with previous splenectomy. Another study looking at only pyogenic liver abscess found biliary disease, hypertension, intraabdominal infection, and diabetes to be the most common underlying or concurrent conditions.8 Our patient did not appear to have any of these risk factors.
Our patient had no known risk factors to promote metastatic spread of the causative organisms. The patient was HIV negative, had no personal or family history of diabetes, and was not found to have elevated glucose levels at any point during admission. Although the ultimate etiology may never be determined, the possibility of undetected malignancy or cardiovascular or inflammatory disease cannot be excluded.
Physicians need to be aware of the global emergence of a hypervirulent strain of K. pneumonia causing liver abscesses and metastatic complications, especially endophthalmitis.9 Mucoviscosity associated gene A (magA) has been found in some liver isolates of K. pneumoniae.10 It has been suggested that as many as one‐third of patients infected with hyperviscous strains of K. pneumoniae will develop an invasive infection.11 Although it is unclear why metastatic endophthalmitis from Klebsiella liver abscess would be more common in East Asia, the magA gene may account for the observed difference. It was not possible to determine if the infectious organism that had infected our patient had the magA gene, although the clinical use of this information may not have changed management because the patient presented with metastatic infection. If this patient's particular organism had tested positive for the magA gene, it might explain why an apparently immunocompetent patient developed metastatic endophthalmitis not simply a liver abscess.
Patients with evidence of endogenous endophthalmitis without clear risk factors should be covered for K. pneumoniae, and extraocular sources should be sought, particularly the liver, even in the absence of diabetes. Early recognition and prompt initiation of antimicrobial therapy is essential if the patient's vision is to be preserved.
- ,,.Klebsiella pneumoniae liver abscess associated with septic endophthalmitis.Arch Intern Med.1986;146:1913–1916.
- ,.Pyogenic liver abscess with a focus on Klebsiella pneumoniae as a primary pathogen: an emerging disease with unique clinical characteristics.Am J Gastroenterol.2005;100:322–331.
- .Klebsiella pneumoniae liver abscess, endophthalmitis, and meningitis in a man with newly recognized diabetes mellitus.Clin Infect Dis.1999;29:1570–1571.
- ,,.Vitreous and aqueous penetration of orally administered gatifloxacin in humans.Arch Ophthalmol.2003;121:345–350.
- ,,,.Endogenous bacterial endophthalmitis: a 17‐year prospective series and review of 267 reported cases.Surv Ophthalmol.2003;48:403–423.
- ,,, et al.Primary liver abscess due to Klebsiella pneumoniae in Taiwan.Clin Infect Dis.1998;26:1434–1438.
- ,,,,.Septic metastatic lesions of pyogenic liver abscess. Their association with Klebsiella pneumoniae bacteremia in diabetic patients.Arch Intern Med.1991;151:1557–1559.
- ,,,.Pyogenic liver abscess: recent trends in etiology and mortality.Clin Infect Dis.2004;39:1654–1659.
- ,,, et al.A global emerging disease of Klebsiella pneumoniae liver abscess: is serotype K1 an important factor for complicated endophthalmitis?Gut.2002;50:420–424.
- ,,.Liver abscess caused by magA+ Klebsiella pneumoniae in North America.J Clin Microbiol.2005;43:991–992.
- ,,, et al.Clinical implications of hypermucoviscosity phenotype in Klebsiella pneumoniae isolates: association with invasive syndrome in patients with community‐acquired bacteraemia.J Intern Med.2006;259:606–614.
Klebsiella pneumoniae liver abscess is known to be associated with metastatic endophthalmitis,1 although most cases have been clustered in Taiwan, with few reports in the United States.2 The first reported case of Klebsiella liver abscess with endophthalmitis in the United States was in a 38‐year‐old man with a new diagnosis of diabetes, a known risk factor for hematogenous spread of Klebsiella to metastatic sites.3
CASE REPORT
A previously healthy 43‐year old Haitian man presented after experiencing 5 days of right eye pain with associated fever and swelling. The patient denied preceding trauma, manipulation of the eye, contact lens use, or illicit drug use and had no significant medical history. He had moved to the United States from Haiti more than 15 years ago and had not traveled out of the state of Florida since that time.
Physical exam showed tachycardia (rate = 110/min), tachypnea (rate = 20/min), and a temperature of 101.5F. The right eye had injected conjunctiva, a swollen lid, and decreased palpebral fissure, and visual acuity on the left was 20/60, whereas visual acuity on the right was recorded as the ability to count fingers at 3 feet. The remainder of his physical exam was within normal limits including the abdominal exam.
Laboratory data on admission included a white blood cell count of 37,500/L significant for 12% bands, total bilirubin of 2.8 mg/dL, AST of 141 U/L, ALT of 130 U/L, and alkaline phosphatase of 196 U/L. HIV testing was negative, and urine toxicology did not detect the presence of any illicit drugs. Vitreous cultures grew Klebsiella pneumoniae.
The initial CT scan of the orbit (Fig. 1) showed periorbital swelling and a preseptal collection anterior to the right globe consistent with an abscess. Because of the abnormal results of the liver panel in the presence of the ophthalmologic infection, an abdominal CT was obtained that showed an 11.5 by 8.0 cm lesion involving all segments of the right lobe of the liver with a 0.9‐mm cylindrical extension toward the right hepatic vein.

Percutaneous drainage of the liver abscess was performed, yielding positive cultures for K. pneumoniae. The patient was treated with oral gatifloxacin and intravenous ceftriaxone. Gatifloxacin therapy was chosen for its excellent penetrance into the vitreous.4 Despite antibiotic therapy, a repeat CT scan of the orbit showed further extension of the collection, and the decision was made to drain the abscess and perform right eye enucleation. The patient was discharged home on oral gatifloxacin. A follow‐up abdominal ultrasound 2 weeks after discharge showed complete resolution of the liver abscess.
DISCUSSION
Bacterial endophthalmitis is a rare infection involving the vitreous humor and other deep intraocular structures. It is most commonly exogenous in origin, caused by intraocular surgery, penetrating injury, a corneal ulcer, or periocular infection. Endogenous endophthalmitis occurs when organisms reach the eye hematogenously and accounts for fewer than 6% of all cases of endophthalmitis.5
Klebsiella liver abscesses have been increasing in incidence worldwide and since the mid‐1990s have become a common cause of liver abscess in the United States, along with Escherichia coli. The association with endophthalmitis was first reported in a series of 7 cases from Taiwan in 1986,1 and subsequent East Asian cases have been reported, usually in diabetic patients.6, 7 The association of Klebsiella liver abscesses with endogenous endophthalmitis has been rarely reported in the United States, with review of the literature from 1966 to 2003 revealing only 3 reported cases.2 One of these patients had diabetes, whereas another had beta‐thalassemia with previous splenectomy. Another study looking at only pyogenic liver abscess found biliary disease, hypertension, intraabdominal infection, and diabetes to be the most common underlying or concurrent conditions.8 Our patient did not appear to have any of these risk factors.
Our patient had no known risk factors to promote metastatic spread of the causative organisms. The patient was HIV negative, had no personal or family history of diabetes, and was not found to have elevated glucose levels at any point during admission. Although the ultimate etiology may never be determined, the possibility of undetected malignancy or cardiovascular or inflammatory disease cannot be excluded.
Physicians need to be aware of the global emergence of a hypervirulent strain of K. pneumonia causing liver abscesses and metastatic complications, especially endophthalmitis.9 Mucoviscosity associated gene A (magA) has been found in some liver isolates of K. pneumoniae.10 It has been suggested that as many as one‐third of patients infected with hyperviscous strains of K. pneumoniae will develop an invasive infection.11 Although it is unclear why metastatic endophthalmitis from Klebsiella liver abscess would be more common in East Asia, the magA gene may account for the observed difference. It was not possible to determine if the infectious organism that had infected our patient had the magA gene, although the clinical use of this information may not have changed management because the patient presented with metastatic infection. If this patient's particular organism had tested positive for the magA gene, it might explain why an apparently immunocompetent patient developed metastatic endophthalmitis not simply a liver abscess.
Patients with evidence of endogenous endophthalmitis without clear risk factors should be covered for K. pneumoniae, and extraocular sources should be sought, particularly the liver, even in the absence of diabetes. Early recognition and prompt initiation of antimicrobial therapy is essential if the patient's vision is to be preserved.
Klebsiella pneumoniae liver abscess is known to be associated with metastatic endophthalmitis,1 although most cases have been clustered in Taiwan, with few reports in the United States.2 The first reported case of Klebsiella liver abscess with endophthalmitis in the United States was in a 38‐year‐old man with a new diagnosis of diabetes, a known risk factor for hematogenous spread of Klebsiella to metastatic sites.3
CASE REPORT
A previously healthy 43‐year old Haitian man presented after experiencing 5 days of right eye pain with associated fever and swelling. The patient denied preceding trauma, manipulation of the eye, contact lens use, or illicit drug use and had no significant medical history. He had moved to the United States from Haiti more than 15 years ago and had not traveled out of the state of Florida since that time.
Physical exam showed tachycardia (rate = 110/min), tachypnea (rate = 20/min), and a temperature of 101.5F. The right eye had injected conjunctiva, a swollen lid, and decreased palpebral fissure, and visual acuity on the left was 20/60, whereas visual acuity on the right was recorded as the ability to count fingers at 3 feet. The remainder of his physical exam was within normal limits including the abdominal exam.
Laboratory data on admission included a white blood cell count of 37,500/L significant for 12% bands, total bilirubin of 2.8 mg/dL, AST of 141 U/L, ALT of 130 U/L, and alkaline phosphatase of 196 U/L. HIV testing was negative, and urine toxicology did not detect the presence of any illicit drugs. Vitreous cultures grew Klebsiella pneumoniae.
The initial CT scan of the orbit (Fig. 1) showed periorbital swelling and a preseptal collection anterior to the right globe consistent with an abscess. Because of the abnormal results of the liver panel in the presence of the ophthalmologic infection, an abdominal CT was obtained that showed an 11.5 by 8.0 cm lesion involving all segments of the right lobe of the liver with a 0.9‐mm cylindrical extension toward the right hepatic vein.

Percutaneous drainage of the liver abscess was performed, yielding positive cultures for K. pneumoniae. The patient was treated with oral gatifloxacin and intravenous ceftriaxone. Gatifloxacin therapy was chosen for its excellent penetrance into the vitreous.4 Despite antibiotic therapy, a repeat CT scan of the orbit showed further extension of the collection, and the decision was made to drain the abscess and perform right eye enucleation. The patient was discharged home on oral gatifloxacin. A follow‐up abdominal ultrasound 2 weeks after discharge showed complete resolution of the liver abscess.
DISCUSSION
Bacterial endophthalmitis is a rare infection involving the vitreous humor and other deep intraocular structures. It is most commonly exogenous in origin, caused by intraocular surgery, penetrating injury, a corneal ulcer, or periocular infection. Endogenous endophthalmitis occurs when organisms reach the eye hematogenously and accounts for fewer than 6% of all cases of endophthalmitis.5
Klebsiella liver abscesses have been increasing in incidence worldwide and since the mid‐1990s have become a common cause of liver abscess in the United States, along with Escherichia coli. The association with endophthalmitis was first reported in a series of 7 cases from Taiwan in 1986,1 and subsequent East Asian cases have been reported, usually in diabetic patients.6, 7 The association of Klebsiella liver abscesses with endogenous endophthalmitis has been rarely reported in the United States, with review of the literature from 1966 to 2003 revealing only 3 reported cases.2 One of these patients had diabetes, whereas another had beta‐thalassemia with previous splenectomy. Another study looking at only pyogenic liver abscess found biliary disease, hypertension, intraabdominal infection, and diabetes to be the most common underlying or concurrent conditions.8 Our patient did not appear to have any of these risk factors.
Our patient had no known risk factors to promote metastatic spread of the causative organisms. The patient was HIV negative, had no personal or family history of diabetes, and was not found to have elevated glucose levels at any point during admission. Although the ultimate etiology may never be determined, the possibility of undetected malignancy or cardiovascular or inflammatory disease cannot be excluded.
Physicians need to be aware of the global emergence of a hypervirulent strain of K. pneumonia causing liver abscesses and metastatic complications, especially endophthalmitis.9 Mucoviscosity associated gene A (magA) has been found in some liver isolates of K. pneumoniae.10 It has been suggested that as many as one‐third of patients infected with hyperviscous strains of K. pneumoniae will develop an invasive infection.11 Although it is unclear why metastatic endophthalmitis from Klebsiella liver abscess would be more common in East Asia, the magA gene may account for the observed difference. It was not possible to determine if the infectious organism that had infected our patient had the magA gene, although the clinical use of this information may not have changed management because the patient presented with metastatic infection. If this patient's particular organism had tested positive for the magA gene, it might explain why an apparently immunocompetent patient developed metastatic endophthalmitis not simply a liver abscess.
Patients with evidence of endogenous endophthalmitis without clear risk factors should be covered for K. pneumoniae, and extraocular sources should be sought, particularly the liver, even in the absence of diabetes. Early recognition and prompt initiation of antimicrobial therapy is essential if the patient's vision is to be preserved.
- ,,.Klebsiella pneumoniae liver abscess associated with septic endophthalmitis.Arch Intern Med.1986;146:1913–1916.
- ,.Pyogenic liver abscess with a focus on Klebsiella pneumoniae as a primary pathogen: an emerging disease with unique clinical characteristics.Am J Gastroenterol.2005;100:322–331.
- .Klebsiella pneumoniae liver abscess, endophthalmitis, and meningitis in a man with newly recognized diabetes mellitus.Clin Infect Dis.1999;29:1570–1571.
- ,,.Vitreous and aqueous penetration of orally administered gatifloxacin in humans.Arch Ophthalmol.2003;121:345–350.
- ,,,.Endogenous bacterial endophthalmitis: a 17‐year prospective series and review of 267 reported cases.Surv Ophthalmol.2003;48:403–423.
- ,,, et al.Primary liver abscess due to Klebsiella pneumoniae in Taiwan.Clin Infect Dis.1998;26:1434–1438.
- ,,,,.Septic metastatic lesions of pyogenic liver abscess. Their association with Klebsiella pneumoniae bacteremia in diabetic patients.Arch Intern Med.1991;151:1557–1559.
- ,,,.Pyogenic liver abscess: recent trends in etiology and mortality.Clin Infect Dis.2004;39:1654–1659.
- ,,, et al.A global emerging disease of Klebsiella pneumoniae liver abscess: is serotype K1 an important factor for complicated endophthalmitis?Gut.2002;50:420–424.
- ,,.Liver abscess caused by magA+ Klebsiella pneumoniae in North America.J Clin Microbiol.2005;43:991–992.
- ,,, et al.Clinical implications of hypermucoviscosity phenotype in Klebsiella pneumoniae isolates: association with invasive syndrome in patients with community‐acquired bacteraemia.J Intern Med.2006;259:606–614.
- ,,.Klebsiella pneumoniae liver abscess associated with septic endophthalmitis.Arch Intern Med.1986;146:1913–1916.
- ,.Pyogenic liver abscess with a focus on Klebsiella pneumoniae as a primary pathogen: an emerging disease with unique clinical characteristics.Am J Gastroenterol.2005;100:322–331.
- .Klebsiella pneumoniae liver abscess, endophthalmitis, and meningitis in a man with newly recognized diabetes mellitus.Clin Infect Dis.1999;29:1570–1571.
- ,,.Vitreous and aqueous penetration of orally administered gatifloxacin in humans.Arch Ophthalmol.2003;121:345–350.
- ,,,.Endogenous bacterial endophthalmitis: a 17‐year prospective series and review of 267 reported cases.Surv Ophthalmol.2003;48:403–423.
- ,,, et al.Primary liver abscess due to Klebsiella pneumoniae in Taiwan.Clin Infect Dis.1998;26:1434–1438.
- ,,,,.Septic metastatic lesions of pyogenic liver abscess. Their association with Klebsiella pneumoniae bacteremia in diabetic patients.Arch Intern Med.1991;151:1557–1559.
- ,,,.Pyogenic liver abscess: recent trends in etiology and mortality.Clin Infect Dis.2004;39:1654–1659.
- ,,, et al.A global emerging disease of Klebsiella pneumoniae liver abscess: is serotype K1 an important factor for complicated endophthalmitis?Gut.2002;50:420–424.
- ,,.Liver abscess caused by magA+ Klebsiella pneumoniae in North America.J Clin Microbiol.2005;43:991–992.
- ,,, et al.Clinical implications of hypermucoviscosity phenotype in Klebsiella pneumoniae isolates: association with invasive syndrome in patients with community‐acquired bacteraemia.J Intern Med.2006;259:606–614.
A Rash Decision
A 38‐year‐old HIV+ Ohio man with a recent CD4+ count of 534 cells/mL presented to his physician with 3 weeks of fever as high as 102F. He noted mild myalgias, pruritus, and an occasional cough but no headache, sore throat, dyspnea, rash, or gastrointestinal or genitourinary complaints. He had been seen elsewhere 2 weeks previously, when he had reported a single episode of receptive oral sex with a male partner several weeks earlier. He had been prescribed ciprofloxacin and azithromycin, but a throat swab came back negative for Chlamydia and Neisseria gonorrhoeae, and he reported no change in his symptoms after the course of antibiotics. He denied smoking or using street drugs. His only medications were citalopram and trazodone for depression.
This is a HIV+ man with a mild degree of immunosuppression with a fever of unknown origin (FUO). It is not yet known if the requisite basic infectious evaluation has been completed to meet this definition, but the duration certainly qualifies, and regardless of semantics, the FUO framework is a helpful starting point. The primary considerations in FUO are infections, neoplasms, and autoimmune illnesses. Autoimmune diseases are relatively less common in HIV patients. Although pruritis is quite common in HIV alone, it may also herald renal failure, cholestasis, or a malignancy (usually hematologic). Drugs must also be considered as a cause of unexplained fever; the pruritis might suggest an allergic reaction, although I do not think of citalopram or trazodone as having this effect. The failure to respond to broad‐spectrum antimicrobials (along with the duration) lowers my suspicion for common infections such as pneumonia, urinary tract infection, or cellulitis. Among sexually transmitted diseases, syphilis can be protean and merits consideration.
On examination he appeared well. His temperature was 102.4F, pulse 111 beats/min, blood pressure 138/78 mm Hg. The head, neck, cardiovascular system, and lungs appeared normal on examination. The abdomen was soft and nontender without organomegaly; skin, extremities, and neurological system were unremarkable. Rectal examination showed small anal condylomata. Hemoglobin was 14.3 g/dL, white blood cell count 6200/cm3, and platelet count 230,000/cm3. Serum electrolytes and lactate dehydrogenase were normal. The results of his liver function tests (LFTs) demonstrated a serum aspartate transaminase of 60 U/L (normal, 7‐40 U/L), alanine transaminase of 125 U/L (normal, 5‐50 U/L), alkaline phosphatase 218 U/L (normal, 40‐150 U/L), and total bilirubin 2.1 mg/dL (normal, 0.0‐1.5 mg/dL). Urinalysis demonstrated 2+ bilirubin and was otherwise normal. His erythrocyte sedimentation rate was 32 mm/hr (normal, 0‐15 mm/hr).
After 3 weeks of illness, his CBC demonstrates no signs of chronic illness (such as anemia of a chronic disease or a reactive leukocytosis or thrombocytosis). The results of his liver function tests showed moderate elevation, slightly more cholestatic than hepatocellular. This finding may reflect a disease process involving the liver, but such abnormal findings are often nonspecific in acute and chronic illnesses. With an unremitting fever, infectious complications in the liver merit early consideration. The time course rules out common biliary disorders such as cholangitis or cholecystitis. Pyogenic or amoebic liver abscesses are possible (homosexual men are at increased risk for the latter), but the absence of pain or abdominal tenderness is atypical. This biochemical profile can also be seen in chronic (but not acute) viral infections of the liver. Chronic hepatitis B and C predispose to hepatocellular carcinoma (HCC), which can be associated with fever. Cancers that infiltrate the liver, such as lymphoma or carcinoma, could also account for this picture. Indolent infections such as tuberculosis (TB) and syphilis are also possible, so associated signs of these systemic diseases should be sought. I do not believe either of his antibiotics is commonly associated with LFT abnormalities, and his CD4 count is too high for HIV cholangiopathy. In sum, a host of liver diseases are possible, but an extrahepatic systemic disease deserves equal attention.
His CD4+ count was 537 cells/mL, and his HIV RNA viral load was 44,300 copies/mL. Radiographs of the chest were normal. Two sets of blood cultures were negative. The rapid plasma reagin (RPR) was nonreactive. The results of serologies for acute hepatitis A, B, C, and E, chronic hepatitis B and C, and toxoplasmosis were negative. Testing for both Epstein‐Barr virus and cytomegalovirus showed evidence of remote infection. Results of serologies for bartonella species, human herpesviruses 6 and 7, and parvovirus B19 were negative.
The negative RPR makes disseminated (secondary) syphilis improbable, provided the prozone phenomenon has been excluded. An extensive serological workup is common in the evaluation of fever of unknown origin, although the threat of false‐positive results always looms when many studies are sent simultaneously. This must be considered in advance here, as his relatively preserved CD4 count affords him significant protection against many opportunistic infections. His HIV infection, however, regardless of CD4 count, increases his risk for TB and lymphoma, which remain high on my list. Both may be residing primarily in the liver. In FUO, the abdominal CT is frequently a high‐yield test (primarily by demonstrating unsuspected tumors and abscesses), even in the absence of symptoms, and would certainly be of interest here given the liver function test results. Imaging could diagnose febrile tumors such as lymphoma, HCC, or renal cell carcinoma. In the event that imaging is unrevealing, causes of granulomatous hepatitis should be entertained. The constellation of cough, LFT abnormalities, and fever is compatible with Q fever. As with any FUO case, I would also carefully revisit this patient's history to discern where he was born, where he has been, and what activities or exposures he is engaged in.
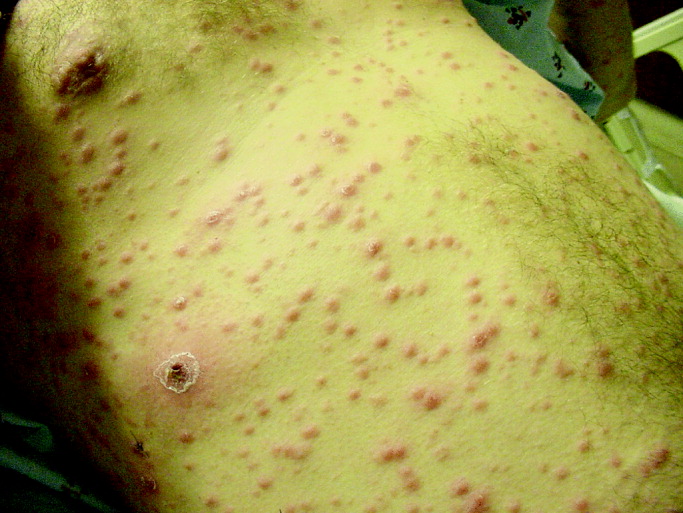
He was seen 2 days later with fever of 104F and new papules over his sternal area. Over the next week, he had intermittent fevers and severe fatigue. The rash progressed, predominantly involving his chest and back, but also his legs, arms, and face (see Fig. 1). The lesions spared his palms and soles. The exanthem was intensely pruritic and maculopapular, consisting of lesions with a diameter of 0.5 cm or less, with some scaling. There were no vesicles or pustular lesions. There were no other new findings on examination. His transaminase and bilirubin had normalized, and his CBC and electrolytes were unchanged. Repeat blood cultures held for extended incubation were negative. Computerized tomography of the chest, abdomen, and pelvis demonstrated mild lymphadenopathy at the porta hepatis with increased portocaval and periaortic lymphadenopathy.
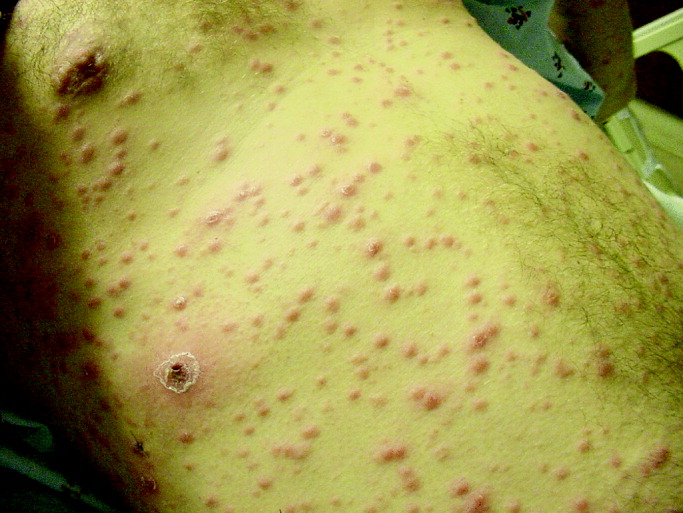
The only LFT abnormality that persists is the elevated alkaline phosphatase, which suggests (1) that liver involvement was not specific and that there is a disease process involving the bone, (2) that there is a persistent infiltrative disorder of the liver such as infection or malignancy or, less likely, amyloidosis or sarcoidosis, or (3) that the porta hepatis lymphadenopathy is causing biliary obstruction. The underlying diagnosis must explain the rash, intraabdominal lymphadenopathy, and fever. The time course does somewhat limit the extensive differential of fever and rash. After 3 weeks of illness, some of the most life‐threatening entities such as meningococcal disease, Rocky Mountain spotted fever, and toxic shock syndrome are unlikely. Concern remains for infections that are more indolent, such as mycobacteria, fungi, or spirochetes. The most striking elements of the rash are the extensive distribution, rapid progression, large number, and discreteness of the lesions, which collectively point more toward disseminated fungal (eg, histoplasmosis, as he lives in Ohio), spirochetal, rickettsial, or viral etiologies, rather than bacterial or mycobacterial entities. The absence of vesicles detracts from the diagnosis of a disseminated herpes virus such as herpes simplex or varicella. I believe that this rash is too disseminated to be caused by a common mycobacterial illness. This extent of cutaneous metastases would usually accompany a far more ill patient with an obvious primary cancer (none is seen on imaging, including the liver), and it appears too extensive to be caused by a paraneoplastic phenomenon such as Sweet's syndrome. A systemic vasculitis or another autoimmune disease remains possible, but there is minimal evidence of visceral organ involvement. All the aforementioned diseases could explain the intraabdominal lymphadenopathy, but my suspicion is highest for infection. I would biopsy and culture the skin lesions, repeat the RPR and/or send a treponemal‐specific test, place a PPD skin test, and send fungal studies (serum serologies and urine antigens) for evaluation. If the results of these noninvasive studies are unrevealing, I would consider a liver biopsy.
The patient's medications were discontinued, and a skin biopsy of the rash from his chest showed atypical lymphohistiocytic infiltrates without acute inflammatory cells and with negative Gomori methenamine silver (GMS), acid‐fast bacilli (AFB), and Fite (for Nocardia) stains. The infiltrates were predominantly T cells with a 1:1 CD4:CD8 ratio. This was read as suspicious for cytotoxic (CD8) mycosis fungoides.
I do not have reason to doubt the pathologist's impression of mycosis fungoides on histopathologic grounds, but from a clinical standpoint, I do not think mycosis fungoides is a disease that has a prolonged febrile prodrome or an explosive cutaneous onset. Rather, it is frequently preceded by nonspecific skin findings over a long period. Thinking broadly and pathophysiologically and noting that T cells are the predominant lymphocytes in skin, I wonder if they could represent a nonmalignant, immunological reaction in the skin. The stains, although not perfectly sensitive, make mycobacterial and fungal diseases less likely, although incubation of cultures is necessary.
Over the next 10 days (bringing the total duration of the patient's illness to 6 weeks), the skin lesions increased in number. In the physician's office at his next follow‐up, the patient had a temperature of 104.1F, was uncomfortable, shivering, and ill‐appearing. His blood pressure was 108/66 mm Hg, and his pulse 114 beats/min. He complained of severe shooting pains, predominantly in his pretibial regions and arms. Examination showed no other new findings, including no focal neurological findings. The results of the T‐cell rearrangement study from the skin biopsy showed evidence of a monoclonal T‐cell population. He was admitted to the hospital for further evaluation and treatment.
The extremity dysesthesias could represent a lesion of the spinal cord (including the CSF/meninges), a polyradiculopathy, or a polyneuropathy. Unfortunately, this does not add a tremendous amount of diagnostic resolution, as infection, malignancy, and autoimmune syndromes, such as vasculitis, may all involve the nervous system in these ways. In general, I associate monoclonal lymphocyte responses with hematological malignancies and polyclonal responses with the less specific inflammation that could accompany infection, autoimmunity, or solid malignancies. His age, fever, and rapid progression seem atypical for mycosis fungoides, but given the monoclonal T cells, this must now be considered. Adult T‐cell leukemia/lymphoma, with its prominent skin manifestations and its association with HLTV‐1, is an alternative T‐cell malignancy that could explain the fever, neurological symptoms, and possible visceral involvement (elevated alkaline phosphatase, which could reflect liver or bone). In cases that are diagnostic challenges, one of the highest‐yield maneuvers is to repeat the preceding evaluation, starting with the history, exam, and basic labs, and if necessary, to review or repeat the imaging or skin biopsy. Given the elevated alkaline phosphatase, disseminated rash, new neurological symptoms, and his HIV status, I remain particularly concerned about syphilis and would do further testing (accounting for the prozone phenomenon) before proceeding with the malignancy evaluation.
A lumbar puncture demonstrated clear cerebrospinal fluid, with 2 leukocytes and 195 erythrocytes/cm3, protein of 26 mg/dL, and glucose of 52 mg/dL. Bacterial and fungal cultures of the fluid were negative. The results of colonoscopy were normal. A bone marrow biopsy demonstrated ring granulomas. GMS, AFB, Fite, and Steiner (for spirochetes) stains were negative, cultures of the aspirate were negative for bacteria, and smears were negative for fungi and mycobacteria. Antibody tests for human T‐cell lymphotropic virus types I and II, Coxiella burnetii, and Bartonella henselae were negative. The dermatology consultant believed the absence of lymphadenopathy and the pruritic nature of the lesions was atypical for cytotoxic T‐cell lymphoma (CTCL). Before initiating therapy for CTCL, she suggested repeating the skin biopsy and RPR.
The repeat RPR was positive at 1:64 dilutions, and a confirmatory fluorescent treponemal antibody absorption test showed a positive result. He was prescribed intramuscular benzathine pencillin 2.4 million units weekly for 3 weeks, with almost immediate defervescence and slower resolution of his rash and shooting pains in his limbs. The repeat skin biopsy done during the hospitalization demonstrated lichenoid‐type dermatitis with interstitial and perivascular lymphohistiocytic infiltrates and granulomas. Steiner stains for spirochetes were positive. Immunohistochemical stains ruled out a lymphoproliferative process. One year later his RPR was nonreactive.
COMMENTARY
Fever of unknown origin (FUO) was first defined by Petersdorf and Beeson in 1961 as a temperature higher than 38.3C on several occasions lasting longer than 3 weeks and defying diagnosis despite 1 week of inpatient investigation.1 Dramatic changes in medical practice have rendered this definition outdated, with more recent proposals allowing thoughtful outpatient investigation to serve as a surrogate for hospitalization. Some have proposed that HIV‐associated FUO be considered a distinct entity, with the most complete North American series finding the etiology of the HIV‐associated FUO in 56 of 70 patients.2 The mean CD4+ count in this series was 58/cm3. Disseminated M. avium was the most frequently diagnosed cause, followed by P. jirovecii pneumonia, cytomegalovirus infection, disseminated histoplasmosis, and lymphoma. Of 14 patients with fever of no definable etiology, 12 eventually proved to have self‐limiting illness.
Despite numerous attempts to reduce the investigation of the patient with FUO to an algorithm, the approach must be individualized. A thorough history and careful, serial physical examinations are frequently and appropriately stressed as the foundation, followed by thoughtful selection of laboratory and imaging studies. Although FUO has a lengthy differential diagnosis, it often proves to be, as Mackowiak and Durack stress, an unusual manifestation of a common disease, rather than a typical presentation of a rare disease.3 A relatively uncommon disease in conjunction with an initially negative diagnostic test result, as was the case with this patient, may lead to a protracted diagnostic puzzle.
Syphilis is a rare cause of FUO. In 6 large studies of a total of 947 patients published over a 40‐year period, only 2 cases of syphilis (1 secondary and 1 neurosyphilis) were reported.1, 48 Syphilis as a cause of prolonged cryptic fever appears to have been seen with greater frequency in the preantibiotic era.9 In the first half of the 20th century, syphilis was known as the great imitator, with its unusual manifestations recognized and indeed expected. As a result of the dramatically lower incidence of syphilis in recent decades, these lessons have largely been forgotten, however, which may lead to diagnostic confusion when syphilis presents atypically. The manifestations of secondary syphilis are protean, including a variety of rashes, aphthous ulcers, arthralgias, pharyngitis, weight loss, fever, meningitis, ocular symptoms, cranial nerve palsies, glomerulonephritis, hepatitis, and periostitis (which afflicted this patient, who complained of severe shooting pains in his arms and shins).
After declining in the last decade of the 20th century, the rates of primary and secondary syphilis are rising in the United States.10 Oral sex is a clear risk factor for syphilis transmission, particularly for men who have sex with men.11 Because of the patient's exposure history and clinical picture, his outpatient physician considered the diagnosis of secondary syphilis early in the course of his illness. The diagnosis was not entertained further when an RPR test, highly sensitive at this stage of the disease, returned nonreactive. Likewise, when a rash subsequently appeared, the lack of palm and sole involvement dissuaded multiple clinicians from reconsidering the diagnosis of syphilis. A skin biopsy that appeared to lead in a distinctly different direction understandably confused the picture still further. Even at the time of the lumbar puncture, VDRL of the CSF was not ordered.
In retrospect, the chief confounder in the case was the false‐negative RPR test, as the discussant suspected early on. Although nontreponemal tests are generally accurate in individuals with HIV, delayed seropositivity and false‐negatives have been reported in this population.12 The false‐negative could have also been a result of the prozone phenomenon, an unusual event, occurring in fewer than 2% of cases of secondary syphilis and attributed to a mismatch between antibody and very high antigen level. The prozone reaction can be corrected for by requesting dilution of the serum prior to repeating the test. Simple lab error must be considered as well, but without access to this patient's serum from his original testing, the cause of his initial false‐negative test cannot be known with certainty.
An unusual presentation in conjunction with failure to recognize the causes of rare false‐negative testing for secondary syphilis led to a delayed diagnosis in this patient. Although syphilis and mycosis fungoides have previously been reported to mimic one another both clinically and histopathologically, the potential for secondary syphilis to be misdiagnosed in this fashion is not generally appreciated.1315 Recognition of the possibility of secondary syphilis occurred just in time to spare this patient the rash decision of treating him with cytotoxic therapy directed against CTCL.
Teaching Points
-
HIV‐associated FUO can be a diagnostic challenge, but an etiology can be found in most cases.
-
Syphilis continues to be an unusual cause of FUO and can have protean manifestations affecting nearly every organ system
-
The sensitivity of RPR is extremely high in secondary syphilis, but false‐negative tests can be seen in HIV because of both the prozone phenomenon and a delayed rise in antibodies.
- ,.Fever of unexplained origin: Report on 100 cases.Medicine.1961;40:1–30.
- ,,.Human immunodeficiency virus‐associated fever of unknown origin: A study of 70 patients in the United States and review.Clin Infect Dis.1999;28:341–345.
- ,.Fever of unknown origin. In:Mandell GL,Bennett JE,Dolin R, eds.Principles and Practice of Infectious Diseases.6th ed.Philadelphia:Elsevier Churchill Livingstone;2005:718–729.
- ,,.Fever of unknown origin: Diagnosis and follow‐up of 105 cases, 1970‐1980.Medicine.1982;61:269–292.
- ,,,.Fever of unknown origin in the 1980s: An update of the diagnostic spectrum.Arch Intern Med.1992;152:51–55.
- .Fever of unknown origin: Review of 86 patients treated in community hospitals.Clin Infect Dis.1992;15:968–973.
- ,,.Fever of unknown origin (FUO). I. A prospective multicenter study of 167 patients with FUO, using fixed epidemiologic entry criteria. The Netherlands FUO study group.Medicine.1997;76:392–400.
- ,,, et al.From prolonged febrile illness to fever of unknown origin: The challenge continues.Arch Intern Med.2003;163:1033–1041.
- ,.The diagnosis of obscure fever. II. The diagnosis of unexplained high fever.Bull Johns Hopkins Hosp.1936;58:307–331.
- Centers for Disease Control and Prevention.Primary and secondary syphilis—United States, 2003–2004.MMWR.2006;55:269–273.
- Transmission of primary and secondary syphilis by oral sex—Chicago, Illinois, 1998‐2202.MMWR.2004;53:966–968.
- ,,, et al.Seronegative secondary syphilis in 2 patients coinfected with human immunodeficiency virus.Arch Dermatol.2005;141:431–433.
- ,,,.Secondary syphilis mimicking mycosis fungoides.J Am Acad Dermatol.1980;3:92–94
- ,.A case of lues maligna in a patient with acquired immunodeficiency syndrome (AIDS).Scand J Infect Dis.2005;37:697–700.
- ,,,,.Unusual presentation of secondary syphilis in 2 HIV‐1 positive patients.Cutis.2000;66:383–389.
A 38‐year‐old HIV+ Ohio man with a recent CD4+ count of 534 cells/mL presented to his physician with 3 weeks of fever as high as 102F. He noted mild myalgias, pruritus, and an occasional cough but no headache, sore throat, dyspnea, rash, or gastrointestinal or genitourinary complaints. He had been seen elsewhere 2 weeks previously, when he had reported a single episode of receptive oral sex with a male partner several weeks earlier. He had been prescribed ciprofloxacin and azithromycin, but a throat swab came back negative for Chlamydia and Neisseria gonorrhoeae, and he reported no change in his symptoms after the course of antibiotics. He denied smoking or using street drugs. His only medications were citalopram and trazodone for depression.
This is a HIV+ man with a mild degree of immunosuppression with a fever of unknown origin (FUO). It is not yet known if the requisite basic infectious evaluation has been completed to meet this definition, but the duration certainly qualifies, and regardless of semantics, the FUO framework is a helpful starting point. The primary considerations in FUO are infections, neoplasms, and autoimmune illnesses. Autoimmune diseases are relatively less common in HIV patients. Although pruritis is quite common in HIV alone, it may also herald renal failure, cholestasis, or a malignancy (usually hematologic). Drugs must also be considered as a cause of unexplained fever; the pruritis might suggest an allergic reaction, although I do not think of citalopram or trazodone as having this effect. The failure to respond to broad‐spectrum antimicrobials (along with the duration) lowers my suspicion for common infections such as pneumonia, urinary tract infection, or cellulitis. Among sexually transmitted diseases, syphilis can be protean and merits consideration.
On examination he appeared well. His temperature was 102.4F, pulse 111 beats/min, blood pressure 138/78 mm Hg. The head, neck, cardiovascular system, and lungs appeared normal on examination. The abdomen was soft and nontender without organomegaly; skin, extremities, and neurological system were unremarkable. Rectal examination showed small anal condylomata. Hemoglobin was 14.3 g/dL, white blood cell count 6200/cm3, and platelet count 230,000/cm3. Serum electrolytes and lactate dehydrogenase were normal. The results of his liver function tests (LFTs) demonstrated a serum aspartate transaminase of 60 U/L (normal, 7‐40 U/L), alanine transaminase of 125 U/L (normal, 5‐50 U/L), alkaline phosphatase 218 U/L (normal, 40‐150 U/L), and total bilirubin 2.1 mg/dL (normal, 0.0‐1.5 mg/dL). Urinalysis demonstrated 2+ bilirubin and was otherwise normal. His erythrocyte sedimentation rate was 32 mm/hr (normal, 0‐15 mm/hr).
After 3 weeks of illness, his CBC demonstrates no signs of chronic illness (such as anemia of a chronic disease or a reactive leukocytosis or thrombocytosis). The results of his liver function tests showed moderate elevation, slightly more cholestatic than hepatocellular. This finding may reflect a disease process involving the liver, but such abnormal findings are often nonspecific in acute and chronic illnesses. With an unremitting fever, infectious complications in the liver merit early consideration. The time course rules out common biliary disorders such as cholangitis or cholecystitis. Pyogenic or amoebic liver abscesses are possible (homosexual men are at increased risk for the latter), but the absence of pain or abdominal tenderness is atypical. This biochemical profile can also be seen in chronic (but not acute) viral infections of the liver. Chronic hepatitis B and C predispose to hepatocellular carcinoma (HCC), which can be associated with fever. Cancers that infiltrate the liver, such as lymphoma or carcinoma, could also account for this picture. Indolent infections such as tuberculosis (TB) and syphilis are also possible, so associated signs of these systemic diseases should be sought. I do not believe either of his antibiotics is commonly associated with LFT abnormalities, and his CD4 count is too high for HIV cholangiopathy. In sum, a host of liver diseases are possible, but an extrahepatic systemic disease deserves equal attention.
His CD4+ count was 537 cells/mL, and his HIV RNA viral load was 44,300 copies/mL. Radiographs of the chest were normal. Two sets of blood cultures were negative. The rapid plasma reagin (RPR) was nonreactive. The results of serologies for acute hepatitis A, B, C, and E, chronic hepatitis B and C, and toxoplasmosis were negative. Testing for both Epstein‐Barr virus and cytomegalovirus showed evidence of remote infection. Results of serologies for bartonella species, human herpesviruses 6 and 7, and parvovirus B19 were negative.
The negative RPR makes disseminated (secondary) syphilis improbable, provided the prozone phenomenon has been excluded. An extensive serological workup is common in the evaluation of fever of unknown origin, although the threat of false‐positive results always looms when many studies are sent simultaneously. This must be considered in advance here, as his relatively preserved CD4 count affords him significant protection against many opportunistic infections. His HIV infection, however, regardless of CD4 count, increases his risk for TB and lymphoma, which remain high on my list. Both may be residing primarily in the liver. In FUO, the abdominal CT is frequently a high‐yield test (primarily by demonstrating unsuspected tumors and abscesses), even in the absence of symptoms, and would certainly be of interest here given the liver function test results. Imaging could diagnose febrile tumors such as lymphoma, HCC, or renal cell carcinoma. In the event that imaging is unrevealing, causes of granulomatous hepatitis should be entertained. The constellation of cough, LFT abnormalities, and fever is compatible with Q fever. As with any FUO case, I would also carefully revisit this patient's history to discern where he was born, where he has been, and what activities or exposures he is engaged in.
He was seen 2 days later with fever of 104F and new papules over his sternal area. Over the next week, he had intermittent fevers and severe fatigue. The rash progressed, predominantly involving his chest and back, but also his legs, arms, and face (see Fig. 1). The lesions spared his palms and soles. The exanthem was intensely pruritic and maculopapular, consisting of lesions with a diameter of 0.5 cm or less, with some scaling. There were no vesicles or pustular lesions. There were no other new findings on examination. His transaminase and bilirubin had normalized, and his CBC and electrolytes were unchanged. Repeat blood cultures held for extended incubation were negative. Computerized tomography of the chest, abdomen, and pelvis demonstrated mild lymphadenopathy at the porta hepatis with increased portocaval and periaortic lymphadenopathy.

The only LFT abnormality that persists is the elevated alkaline phosphatase, which suggests (1) that liver involvement was not specific and that there is a disease process involving the bone, (2) that there is a persistent infiltrative disorder of the liver such as infection or malignancy or, less likely, amyloidosis or sarcoidosis, or (3) that the porta hepatis lymphadenopathy is causing biliary obstruction. The underlying diagnosis must explain the rash, intraabdominal lymphadenopathy, and fever. The time course does somewhat limit the extensive differential of fever and rash. After 3 weeks of illness, some of the most life‐threatening entities such as meningococcal disease, Rocky Mountain spotted fever, and toxic shock syndrome are unlikely. Concern remains for infections that are more indolent, such as mycobacteria, fungi, or spirochetes. The most striking elements of the rash are the extensive distribution, rapid progression, large number, and discreteness of the lesions, which collectively point more toward disseminated fungal (eg, histoplasmosis, as he lives in Ohio), spirochetal, rickettsial, or viral etiologies, rather than bacterial or mycobacterial entities. The absence of vesicles detracts from the diagnosis of a disseminated herpes virus such as herpes simplex or varicella. I believe that this rash is too disseminated to be caused by a common mycobacterial illness. This extent of cutaneous metastases would usually accompany a far more ill patient with an obvious primary cancer (none is seen on imaging, including the liver), and it appears too extensive to be caused by a paraneoplastic phenomenon such as Sweet's syndrome. A systemic vasculitis or another autoimmune disease remains possible, but there is minimal evidence of visceral organ involvement. All the aforementioned diseases could explain the intraabdominal lymphadenopathy, but my suspicion is highest for infection. I would biopsy and culture the skin lesions, repeat the RPR and/or send a treponemal‐specific test, place a PPD skin test, and send fungal studies (serum serologies and urine antigens) for evaluation. If the results of these noninvasive studies are unrevealing, I would consider a liver biopsy.
The patient's medications were discontinued, and a skin biopsy of the rash from his chest showed atypical lymphohistiocytic infiltrates without acute inflammatory cells and with negative Gomori methenamine silver (GMS), acid‐fast bacilli (AFB), and Fite (for Nocardia) stains. The infiltrates were predominantly T cells with a 1:1 CD4:CD8 ratio. This was read as suspicious for cytotoxic (CD8) mycosis fungoides.
I do not have reason to doubt the pathologist's impression of mycosis fungoides on histopathologic grounds, but from a clinical standpoint, I do not think mycosis fungoides is a disease that has a prolonged febrile prodrome or an explosive cutaneous onset. Rather, it is frequently preceded by nonspecific skin findings over a long period. Thinking broadly and pathophysiologically and noting that T cells are the predominant lymphocytes in skin, I wonder if they could represent a nonmalignant, immunological reaction in the skin. The stains, although not perfectly sensitive, make mycobacterial and fungal diseases less likely, although incubation of cultures is necessary.
Over the next 10 days (bringing the total duration of the patient's illness to 6 weeks), the skin lesions increased in number. In the physician's office at his next follow‐up, the patient had a temperature of 104.1F, was uncomfortable, shivering, and ill‐appearing. His blood pressure was 108/66 mm Hg, and his pulse 114 beats/min. He complained of severe shooting pains, predominantly in his pretibial regions and arms. Examination showed no other new findings, including no focal neurological findings. The results of the T‐cell rearrangement study from the skin biopsy showed evidence of a monoclonal T‐cell population. He was admitted to the hospital for further evaluation and treatment.
The extremity dysesthesias could represent a lesion of the spinal cord (including the CSF/meninges), a polyradiculopathy, or a polyneuropathy. Unfortunately, this does not add a tremendous amount of diagnostic resolution, as infection, malignancy, and autoimmune syndromes, such as vasculitis, may all involve the nervous system in these ways. In general, I associate monoclonal lymphocyte responses with hematological malignancies and polyclonal responses with the less specific inflammation that could accompany infection, autoimmunity, or solid malignancies. His age, fever, and rapid progression seem atypical for mycosis fungoides, but given the monoclonal T cells, this must now be considered. Adult T‐cell leukemia/lymphoma, with its prominent skin manifestations and its association with HLTV‐1, is an alternative T‐cell malignancy that could explain the fever, neurological symptoms, and possible visceral involvement (elevated alkaline phosphatase, which could reflect liver or bone). In cases that are diagnostic challenges, one of the highest‐yield maneuvers is to repeat the preceding evaluation, starting with the history, exam, and basic labs, and if necessary, to review or repeat the imaging or skin biopsy. Given the elevated alkaline phosphatase, disseminated rash, new neurological symptoms, and his HIV status, I remain particularly concerned about syphilis and would do further testing (accounting for the prozone phenomenon) before proceeding with the malignancy evaluation.
A lumbar puncture demonstrated clear cerebrospinal fluid, with 2 leukocytes and 195 erythrocytes/cm3, protein of 26 mg/dL, and glucose of 52 mg/dL. Bacterial and fungal cultures of the fluid were negative. The results of colonoscopy were normal. A bone marrow biopsy demonstrated ring granulomas. GMS, AFB, Fite, and Steiner (for spirochetes) stains were negative, cultures of the aspirate were negative for bacteria, and smears were negative for fungi and mycobacteria. Antibody tests for human T‐cell lymphotropic virus types I and II, Coxiella burnetii, and Bartonella henselae were negative. The dermatology consultant believed the absence of lymphadenopathy and the pruritic nature of the lesions was atypical for cytotoxic T‐cell lymphoma (CTCL). Before initiating therapy for CTCL, she suggested repeating the skin biopsy and RPR.
The repeat RPR was positive at 1:64 dilutions, and a confirmatory fluorescent treponemal antibody absorption test showed a positive result. He was prescribed intramuscular benzathine pencillin 2.4 million units weekly for 3 weeks, with almost immediate defervescence and slower resolution of his rash and shooting pains in his limbs. The repeat skin biopsy done during the hospitalization demonstrated lichenoid‐type dermatitis with interstitial and perivascular lymphohistiocytic infiltrates and granulomas. Steiner stains for spirochetes were positive. Immunohistochemical stains ruled out a lymphoproliferative process. One year later his RPR was nonreactive.
COMMENTARY
Fever of unknown origin (FUO) was first defined by Petersdorf and Beeson in 1961 as a temperature higher than 38.3C on several occasions lasting longer than 3 weeks and defying diagnosis despite 1 week of inpatient investigation.1 Dramatic changes in medical practice have rendered this definition outdated, with more recent proposals allowing thoughtful outpatient investigation to serve as a surrogate for hospitalization. Some have proposed that HIV‐associated FUO be considered a distinct entity, with the most complete North American series finding the etiology of the HIV‐associated FUO in 56 of 70 patients.2 The mean CD4+ count in this series was 58/cm3. Disseminated M. avium was the most frequently diagnosed cause, followed by P. jirovecii pneumonia, cytomegalovirus infection, disseminated histoplasmosis, and lymphoma. Of 14 patients with fever of no definable etiology, 12 eventually proved to have self‐limiting illness.
Despite numerous attempts to reduce the investigation of the patient with FUO to an algorithm, the approach must be individualized. A thorough history and careful, serial physical examinations are frequently and appropriately stressed as the foundation, followed by thoughtful selection of laboratory and imaging studies. Although FUO has a lengthy differential diagnosis, it often proves to be, as Mackowiak and Durack stress, an unusual manifestation of a common disease, rather than a typical presentation of a rare disease.3 A relatively uncommon disease in conjunction with an initially negative diagnostic test result, as was the case with this patient, may lead to a protracted diagnostic puzzle.
Syphilis is a rare cause of FUO. In 6 large studies of a total of 947 patients published over a 40‐year period, only 2 cases of syphilis (1 secondary and 1 neurosyphilis) were reported.1, 48 Syphilis as a cause of prolonged cryptic fever appears to have been seen with greater frequency in the preantibiotic era.9 In the first half of the 20th century, syphilis was known as the great imitator, with its unusual manifestations recognized and indeed expected. As a result of the dramatically lower incidence of syphilis in recent decades, these lessons have largely been forgotten, however, which may lead to diagnostic confusion when syphilis presents atypically. The manifestations of secondary syphilis are protean, including a variety of rashes, aphthous ulcers, arthralgias, pharyngitis, weight loss, fever, meningitis, ocular symptoms, cranial nerve palsies, glomerulonephritis, hepatitis, and periostitis (which afflicted this patient, who complained of severe shooting pains in his arms and shins).
After declining in the last decade of the 20th century, the rates of primary and secondary syphilis are rising in the United States.10 Oral sex is a clear risk factor for syphilis transmission, particularly for men who have sex with men.11 Because of the patient's exposure history and clinical picture, his outpatient physician considered the diagnosis of secondary syphilis early in the course of his illness. The diagnosis was not entertained further when an RPR test, highly sensitive at this stage of the disease, returned nonreactive. Likewise, when a rash subsequently appeared, the lack of palm and sole involvement dissuaded multiple clinicians from reconsidering the diagnosis of syphilis. A skin biopsy that appeared to lead in a distinctly different direction understandably confused the picture still further. Even at the time of the lumbar puncture, VDRL of the CSF was not ordered.
In retrospect, the chief confounder in the case was the false‐negative RPR test, as the discussant suspected early on. Although nontreponemal tests are generally accurate in individuals with HIV, delayed seropositivity and false‐negatives have been reported in this population.12 The false‐negative could have also been a result of the prozone phenomenon, an unusual event, occurring in fewer than 2% of cases of secondary syphilis and attributed to a mismatch between antibody and very high antigen level. The prozone reaction can be corrected for by requesting dilution of the serum prior to repeating the test. Simple lab error must be considered as well, but without access to this patient's serum from his original testing, the cause of his initial false‐negative test cannot be known with certainty.
An unusual presentation in conjunction with failure to recognize the causes of rare false‐negative testing for secondary syphilis led to a delayed diagnosis in this patient. Although syphilis and mycosis fungoides have previously been reported to mimic one another both clinically and histopathologically, the potential for secondary syphilis to be misdiagnosed in this fashion is not generally appreciated.1315 Recognition of the possibility of secondary syphilis occurred just in time to spare this patient the rash decision of treating him with cytotoxic therapy directed against CTCL.
Teaching Points
-
HIV‐associated FUO can be a diagnostic challenge, but an etiology can be found in most cases.
-
Syphilis continues to be an unusual cause of FUO and can have protean manifestations affecting nearly every organ system
-
The sensitivity of RPR is extremely high in secondary syphilis, but false‐negative tests can be seen in HIV because of both the prozone phenomenon and a delayed rise in antibodies.
A 38‐year‐old HIV+ Ohio man with a recent CD4+ count of 534 cells/mL presented to his physician with 3 weeks of fever as high as 102F. He noted mild myalgias, pruritus, and an occasional cough but no headache, sore throat, dyspnea, rash, or gastrointestinal or genitourinary complaints. He had been seen elsewhere 2 weeks previously, when he had reported a single episode of receptive oral sex with a male partner several weeks earlier. He had been prescribed ciprofloxacin and azithromycin, but a throat swab came back negative for Chlamydia and Neisseria gonorrhoeae, and he reported no change in his symptoms after the course of antibiotics. He denied smoking or using street drugs. His only medications were citalopram and trazodone for depression.
This is a HIV+ man with a mild degree of immunosuppression with a fever of unknown origin (FUO). It is not yet known if the requisite basic infectious evaluation has been completed to meet this definition, but the duration certainly qualifies, and regardless of semantics, the FUO framework is a helpful starting point. The primary considerations in FUO are infections, neoplasms, and autoimmune illnesses. Autoimmune diseases are relatively less common in HIV patients. Although pruritis is quite common in HIV alone, it may also herald renal failure, cholestasis, or a malignancy (usually hematologic). Drugs must also be considered as a cause of unexplained fever; the pruritis might suggest an allergic reaction, although I do not think of citalopram or trazodone as having this effect. The failure to respond to broad‐spectrum antimicrobials (along with the duration) lowers my suspicion for common infections such as pneumonia, urinary tract infection, or cellulitis. Among sexually transmitted diseases, syphilis can be protean and merits consideration.
On examination he appeared well. His temperature was 102.4F, pulse 111 beats/min, blood pressure 138/78 mm Hg. The head, neck, cardiovascular system, and lungs appeared normal on examination. The abdomen was soft and nontender without organomegaly; skin, extremities, and neurological system were unremarkable. Rectal examination showed small anal condylomata. Hemoglobin was 14.3 g/dL, white blood cell count 6200/cm3, and platelet count 230,000/cm3. Serum electrolytes and lactate dehydrogenase were normal. The results of his liver function tests (LFTs) demonstrated a serum aspartate transaminase of 60 U/L (normal, 7‐40 U/L), alanine transaminase of 125 U/L (normal, 5‐50 U/L), alkaline phosphatase 218 U/L (normal, 40‐150 U/L), and total bilirubin 2.1 mg/dL (normal, 0.0‐1.5 mg/dL). Urinalysis demonstrated 2+ bilirubin and was otherwise normal. His erythrocyte sedimentation rate was 32 mm/hr (normal, 0‐15 mm/hr).
After 3 weeks of illness, his CBC demonstrates no signs of chronic illness (such as anemia of a chronic disease or a reactive leukocytosis or thrombocytosis). The results of his liver function tests showed moderate elevation, slightly more cholestatic than hepatocellular. This finding may reflect a disease process involving the liver, but such abnormal findings are often nonspecific in acute and chronic illnesses. With an unremitting fever, infectious complications in the liver merit early consideration. The time course rules out common biliary disorders such as cholangitis or cholecystitis. Pyogenic or amoebic liver abscesses are possible (homosexual men are at increased risk for the latter), but the absence of pain or abdominal tenderness is atypical. This biochemical profile can also be seen in chronic (but not acute) viral infections of the liver. Chronic hepatitis B and C predispose to hepatocellular carcinoma (HCC), which can be associated with fever. Cancers that infiltrate the liver, such as lymphoma or carcinoma, could also account for this picture. Indolent infections such as tuberculosis (TB) and syphilis are also possible, so associated signs of these systemic diseases should be sought. I do not believe either of his antibiotics is commonly associated with LFT abnormalities, and his CD4 count is too high for HIV cholangiopathy. In sum, a host of liver diseases are possible, but an extrahepatic systemic disease deserves equal attention.
His CD4+ count was 537 cells/mL, and his HIV RNA viral load was 44,300 copies/mL. Radiographs of the chest were normal. Two sets of blood cultures were negative. The rapid plasma reagin (RPR) was nonreactive. The results of serologies for acute hepatitis A, B, C, and E, chronic hepatitis B and C, and toxoplasmosis were negative. Testing for both Epstein‐Barr virus and cytomegalovirus showed evidence of remote infection. Results of serologies for bartonella species, human herpesviruses 6 and 7, and parvovirus B19 were negative.
The negative RPR makes disseminated (secondary) syphilis improbable, provided the prozone phenomenon has been excluded. An extensive serological workup is common in the evaluation of fever of unknown origin, although the threat of false‐positive results always looms when many studies are sent simultaneously. This must be considered in advance here, as his relatively preserved CD4 count affords him significant protection against many opportunistic infections. His HIV infection, however, regardless of CD4 count, increases his risk for TB and lymphoma, which remain high on my list. Both may be residing primarily in the liver. In FUO, the abdominal CT is frequently a high‐yield test (primarily by demonstrating unsuspected tumors and abscesses), even in the absence of symptoms, and would certainly be of interest here given the liver function test results. Imaging could diagnose febrile tumors such as lymphoma, HCC, or renal cell carcinoma. In the event that imaging is unrevealing, causes of granulomatous hepatitis should be entertained. The constellation of cough, LFT abnormalities, and fever is compatible with Q fever. As with any FUO case, I would also carefully revisit this patient's history to discern where he was born, where he has been, and what activities or exposures he is engaged in.
He was seen 2 days later with fever of 104F and new papules over his sternal area. Over the next week, he had intermittent fevers and severe fatigue. The rash progressed, predominantly involving his chest and back, but also his legs, arms, and face (see Fig. 1). The lesions spared his palms and soles. The exanthem was intensely pruritic and maculopapular, consisting of lesions with a diameter of 0.5 cm or less, with some scaling. There were no vesicles or pustular lesions. There were no other new findings on examination. His transaminase and bilirubin had normalized, and his CBC and electrolytes were unchanged. Repeat blood cultures held for extended incubation were negative. Computerized tomography of the chest, abdomen, and pelvis demonstrated mild lymphadenopathy at the porta hepatis with increased portocaval and periaortic lymphadenopathy.

The only LFT abnormality that persists is the elevated alkaline phosphatase, which suggests (1) that liver involvement was not specific and that there is a disease process involving the bone, (2) that there is a persistent infiltrative disorder of the liver such as infection or malignancy or, less likely, amyloidosis or sarcoidosis, or (3) that the porta hepatis lymphadenopathy is causing biliary obstruction. The underlying diagnosis must explain the rash, intraabdominal lymphadenopathy, and fever. The time course does somewhat limit the extensive differential of fever and rash. After 3 weeks of illness, some of the most life‐threatening entities such as meningococcal disease, Rocky Mountain spotted fever, and toxic shock syndrome are unlikely. Concern remains for infections that are more indolent, such as mycobacteria, fungi, or spirochetes. The most striking elements of the rash are the extensive distribution, rapid progression, large number, and discreteness of the lesions, which collectively point more toward disseminated fungal (eg, histoplasmosis, as he lives in Ohio), spirochetal, rickettsial, or viral etiologies, rather than bacterial or mycobacterial entities. The absence of vesicles detracts from the diagnosis of a disseminated herpes virus such as herpes simplex or varicella. I believe that this rash is too disseminated to be caused by a common mycobacterial illness. This extent of cutaneous metastases would usually accompany a far more ill patient with an obvious primary cancer (none is seen on imaging, including the liver), and it appears too extensive to be caused by a paraneoplastic phenomenon such as Sweet's syndrome. A systemic vasculitis or another autoimmune disease remains possible, but there is minimal evidence of visceral organ involvement. All the aforementioned diseases could explain the intraabdominal lymphadenopathy, but my suspicion is highest for infection. I would biopsy and culture the skin lesions, repeat the RPR and/or send a treponemal‐specific test, place a PPD skin test, and send fungal studies (serum serologies and urine antigens) for evaluation. If the results of these noninvasive studies are unrevealing, I would consider a liver biopsy.
The patient's medications were discontinued, and a skin biopsy of the rash from his chest showed atypical lymphohistiocytic infiltrates without acute inflammatory cells and with negative Gomori methenamine silver (GMS), acid‐fast bacilli (AFB), and Fite (for Nocardia) stains. The infiltrates were predominantly T cells with a 1:1 CD4:CD8 ratio. This was read as suspicious for cytotoxic (CD8) mycosis fungoides.
I do not have reason to doubt the pathologist's impression of mycosis fungoides on histopathologic grounds, but from a clinical standpoint, I do not think mycosis fungoides is a disease that has a prolonged febrile prodrome or an explosive cutaneous onset. Rather, it is frequently preceded by nonspecific skin findings over a long period. Thinking broadly and pathophysiologically and noting that T cells are the predominant lymphocytes in skin, I wonder if they could represent a nonmalignant, immunological reaction in the skin. The stains, although not perfectly sensitive, make mycobacterial and fungal diseases less likely, although incubation of cultures is necessary.
Over the next 10 days (bringing the total duration of the patient's illness to 6 weeks), the skin lesions increased in number. In the physician's office at his next follow‐up, the patient had a temperature of 104.1F, was uncomfortable, shivering, and ill‐appearing. His blood pressure was 108/66 mm Hg, and his pulse 114 beats/min. He complained of severe shooting pains, predominantly in his pretibial regions and arms. Examination showed no other new findings, including no focal neurological findings. The results of the T‐cell rearrangement study from the skin biopsy showed evidence of a monoclonal T‐cell population. He was admitted to the hospital for further evaluation and treatment.
The extremity dysesthesias could represent a lesion of the spinal cord (including the CSF/meninges), a polyradiculopathy, or a polyneuropathy. Unfortunately, this does not add a tremendous amount of diagnostic resolution, as infection, malignancy, and autoimmune syndromes, such as vasculitis, may all involve the nervous system in these ways. In general, I associate monoclonal lymphocyte responses with hematological malignancies and polyclonal responses with the less specific inflammation that could accompany infection, autoimmunity, or solid malignancies. His age, fever, and rapid progression seem atypical for mycosis fungoides, but given the monoclonal T cells, this must now be considered. Adult T‐cell leukemia/lymphoma, with its prominent skin manifestations and its association with HLTV‐1, is an alternative T‐cell malignancy that could explain the fever, neurological symptoms, and possible visceral involvement (elevated alkaline phosphatase, which could reflect liver or bone). In cases that are diagnostic challenges, one of the highest‐yield maneuvers is to repeat the preceding evaluation, starting with the history, exam, and basic labs, and if necessary, to review or repeat the imaging or skin biopsy. Given the elevated alkaline phosphatase, disseminated rash, new neurological symptoms, and his HIV status, I remain particularly concerned about syphilis and would do further testing (accounting for the prozone phenomenon) before proceeding with the malignancy evaluation.
A lumbar puncture demonstrated clear cerebrospinal fluid, with 2 leukocytes and 195 erythrocytes/cm3, protein of 26 mg/dL, and glucose of 52 mg/dL. Bacterial and fungal cultures of the fluid were negative. The results of colonoscopy were normal. A bone marrow biopsy demonstrated ring granulomas. GMS, AFB, Fite, and Steiner (for spirochetes) stains were negative, cultures of the aspirate were negative for bacteria, and smears were negative for fungi and mycobacteria. Antibody tests for human T‐cell lymphotropic virus types I and II, Coxiella burnetii, and Bartonella henselae were negative. The dermatology consultant believed the absence of lymphadenopathy and the pruritic nature of the lesions was atypical for cytotoxic T‐cell lymphoma (CTCL). Before initiating therapy for CTCL, she suggested repeating the skin biopsy and RPR.
The repeat RPR was positive at 1:64 dilutions, and a confirmatory fluorescent treponemal antibody absorption test showed a positive result. He was prescribed intramuscular benzathine pencillin 2.4 million units weekly for 3 weeks, with almost immediate defervescence and slower resolution of his rash and shooting pains in his limbs. The repeat skin biopsy done during the hospitalization demonstrated lichenoid‐type dermatitis with interstitial and perivascular lymphohistiocytic infiltrates and granulomas. Steiner stains for spirochetes were positive. Immunohistochemical stains ruled out a lymphoproliferative process. One year later his RPR was nonreactive.
COMMENTARY
Fever of unknown origin (FUO) was first defined by Petersdorf and Beeson in 1961 as a temperature higher than 38.3C on several occasions lasting longer than 3 weeks and defying diagnosis despite 1 week of inpatient investigation.1 Dramatic changes in medical practice have rendered this definition outdated, with more recent proposals allowing thoughtful outpatient investigation to serve as a surrogate for hospitalization. Some have proposed that HIV‐associated FUO be considered a distinct entity, with the most complete North American series finding the etiology of the HIV‐associated FUO in 56 of 70 patients.2 The mean CD4+ count in this series was 58/cm3. Disseminated M. avium was the most frequently diagnosed cause, followed by P. jirovecii pneumonia, cytomegalovirus infection, disseminated histoplasmosis, and lymphoma. Of 14 patients with fever of no definable etiology, 12 eventually proved to have self‐limiting illness.
Despite numerous attempts to reduce the investigation of the patient with FUO to an algorithm, the approach must be individualized. A thorough history and careful, serial physical examinations are frequently and appropriately stressed as the foundation, followed by thoughtful selection of laboratory and imaging studies. Although FUO has a lengthy differential diagnosis, it often proves to be, as Mackowiak and Durack stress, an unusual manifestation of a common disease, rather than a typical presentation of a rare disease.3 A relatively uncommon disease in conjunction with an initially negative diagnostic test result, as was the case with this patient, may lead to a protracted diagnostic puzzle.
Syphilis is a rare cause of FUO. In 6 large studies of a total of 947 patients published over a 40‐year period, only 2 cases of syphilis (1 secondary and 1 neurosyphilis) were reported.1, 48 Syphilis as a cause of prolonged cryptic fever appears to have been seen with greater frequency in the preantibiotic era.9 In the first half of the 20th century, syphilis was known as the great imitator, with its unusual manifestations recognized and indeed expected. As a result of the dramatically lower incidence of syphilis in recent decades, these lessons have largely been forgotten, however, which may lead to diagnostic confusion when syphilis presents atypically. The manifestations of secondary syphilis are protean, including a variety of rashes, aphthous ulcers, arthralgias, pharyngitis, weight loss, fever, meningitis, ocular symptoms, cranial nerve palsies, glomerulonephritis, hepatitis, and periostitis (which afflicted this patient, who complained of severe shooting pains in his arms and shins).
After declining in the last decade of the 20th century, the rates of primary and secondary syphilis are rising in the United States.10 Oral sex is a clear risk factor for syphilis transmission, particularly for men who have sex with men.11 Because of the patient's exposure history and clinical picture, his outpatient physician considered the diagnosis of secondary syphilis early in the course of his illness. The diagnosis was not entertained further when an RPR test, highly sensitive at this stage of the disease, returned nonreactive. Likewise, when a rash subsequently appeared, the lack of palm and sole involvement dissuaded multiple clinicians from reconsidering the diagnosis of syphilis. A skin biopsy that appeared to lead in a distinctly different direction understandably confused the picture still further. Even at the time of the lumbar puncture, VDRL of the CSF was not ordered.
In retrospect, the chief confounder in the case was the false‐negative RPR test, as the discussant suspected early on. Although nontreponemal tests are generally accurate in individuals with HIV, delayed seropositivity and false‐negatives have been reported in this population.12 The false‐negative could have also been a result of the prozone phenomenon, an unusual event, occurring in fewer than 2% of cases of secondary syphilis and attributed to a mismatch between antibody and very high antigen level. The prozone reaction can be corrected for by requesting dilution of the serum prior to repeating the test. Simple lab error must be considered as well, but without access to this patient's serum from his original testing, the cause of his initial false‐negative test cannot be known with certainty.
An unusual presentation in conjunction with failure to recognize the causes of rare false‐negative testing for secondary syphilis led to a delayed diagnosis in this patient. Although syphilis and mycosis fungoides have previously been reported to mimic one another both clinically and histopathologically, the potential for secondary syphilis to be misdiagnosed in this fashion is not generally appreciated.1315 Recognition of the possibility of secondary syphilis occurred just in time to spare this patient the rash decision of treating him with cytotoxic therapy directed against CTCL.
Teaching Points
-
HIV‐associated FUO can be a diagnostic challenge, but an etiology can be found in most cases.
-
Syphilis continues to be an unusual cause of FUO and can have protean manifestations affecting nearly every organ system
-
The sensitivity of RPR is extremely high in secondary syphilis, but false‐negative tests can be seen in HIV because of both the prozone phenomenon and a delayed rise in antibodies.
- ,.Fever of unexplained origin: Report on 100 cases.Medicine.1961;40:1–30.
- ,,.Human immunodeficiency virus‐associated fever of unknown origin: A study of 70 patients in the United States and review.Clin Infect Dis.1999;28:341–345.
- ,.Fever of unknown origin. In:Mandell GL,Bennett JE,Dolin R, eds.Principles and Practice of Infectious Diseases.6th ed.Philadelphia:Elsevier Churchill Livingstone;2005:718–729.
- ,,.Fever of unknown origin: Diagnosis and follow‐up of 105 cases, 1970‐1980.Medicine.1982;61:269–292.
- ,,,.Fever of unknown origin in the 1980s: An update of the diagnostic spectrum.Arch Intern Med.1992;152:51–55.
- .Fever of unknown origin: Review of 86 patients treated in community hospitals.Clin Infect Dis.1992;15:968–973.
- ,,.Fever of unknown origin (FUO). I. A prospective multicenter study of 167 patients with FUO, using fixed epidemiologic entry criteria. The Netherlands FUO study group.Medicine.1997;76:392–400.
- ,,, et al.From prolonged febrile illness to fever of unknown origin: The challenge continues.Arch Intern Med.2003;163:1033–1041.
- ,.The diagnosis of obscure fever. II. The diagnosis of unexplained high fever.Bull Johns Hopkins Hosp.1936;58:307–331.
- Centers for Disease Control and Prevention.Primary and secondary syphilis—United States, 2003–2004.MMWR.2006;55:269–273.
- Transmission of primary and secondary syphilis by oral sex—Chicago, Illinois, 1998‐2202.MMWR.2004;53:966–968.
- ,,, et al.Seronegative secondary syphilis in 2 patients coinfected with human immunodeficiency virus.Arch Dermatol.2005;141:431–433.
- ,,,.Secondary syphilis mimicking mycosis fungoides.J Am Acad Dermatol.1980;3:92–94
- ,.A case of lues maligna in a patient with acquired immunodeficiency syndrome (AIDS).Scand J Infect Dis.2005;37:697–700.
- ,,,,.Unusual presentation of secondary syphilis in 2 HIV‐1 positive patients.Cutis.2000;66:383–389.
- ,.Fever of unexplained origin: Report on 100 cases.Medicine.1961;40:1–30.
- ,,.Human immunodeficiency virus‐associated fever of unknown origin: A study of 70 patients in the United States and review.Clin Infect Dis.1999;28:341–345.
- ,.Fever of unknown origin. In:Mandell GL,Bennett JE,Dolin R, eds.Principles and Practice of Infectious Diseases.6th ed.Philadelphia:Elsevier Churchill Livingstone;2005:718–729.
- ,,.Fever of unknown origin: Diagnosis and follow‐up of 105 cases, 1970‐1980.Medicine.1982;61:269–292.
- ,,,.Fever of unknown origin in the 1980s: An update of the diagnostic spectrum.Arch Intern Med.1992;152:51–55.
- .Fever of unknown origin: Review of 86 patients treated in community hospitals.Clin Infect Dis.1992;15:968–973.
- ,,.Fever of unknown origin (FUO). I. A prospective multicenter study of 167 patients with FUO, using fixed epidemiologic entry criteria. The Netherlands FUO study group.Medicine.1997;76:392–400.
- ,,, et al.From prolonged febrile illness to fever of unknown origin: The challenge continues.Arch Intern Med.2003;163:1033–1041.
- ,.The diagnosis of obscure fever. II. The diagnosis of unexplained high fever.Bull Johns Hopkins Hosp.1936;58:307–331.
- Centers for Disease Control and Prevention.Primary and secondary syphilis—United States, 2003–2004.MMWR.2006;55:269–273.
- Transmission of primary and secondary syphilis by oral sex—Chicago, Illinois, 1998‐2202.MMWR.2004;53:966–968.
- ,,, et al.Seronegative secondary syphilis in 2 patients coinfected with human immunodeficiency virus.Arch Dermatol.2005;141:431–433.
- ,,,.Secondary syphilis mimicking mycosis fungoides.J Am Acad Dermatol.1980;3:92–94
- ,.A case of lues maligna in a patient with acquired immunodeficiency syndrome (AIDS).Scand J Infect Dis.2005;37:697–700.
- ,,,,.Unusual presentation of secondary syphilis in 2 HIV‐1 positive patients.Cutis.2000;66:383–389.
Asymmetric muscle atrophy from childhood polio
A 45‐year‐old Vietnamese woman presented to the emergency department reporting 4 days of vomiting, diarrhea, and epigastric pain. Other than poliomyelitis as a child, she had an unremarkable medical history. A slight left‐sided limp was noted on exam. Although she was diagnosed with gastroenteritis, laboratory studies revealed her blood urea nitrogen was low, at 4 mg/dL, and her creatinine was 0.2 mg/dL, raising concern for an intercurrent abdominal malignancy. A CT scan of the abdomen and pelvis was obtained.
The CT scan did not reveal any abdominal pathology but, incidentally, showed near‐complete atrophy of the left pelvic girdle and proximal femur. Specifically noted was near‐complete fatty atrophy and involution of the left iliacus, piriformis, and gluteus muscles (Fig. 1). Atrophy also involved muscles surrounding the left proximal femur (Fig. 2). The CT and laboratory findings were attributed to childhood poliomyelitis. The patient was discharged home in good condition.
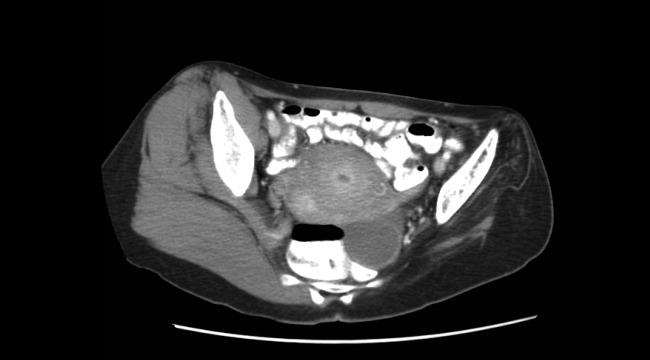
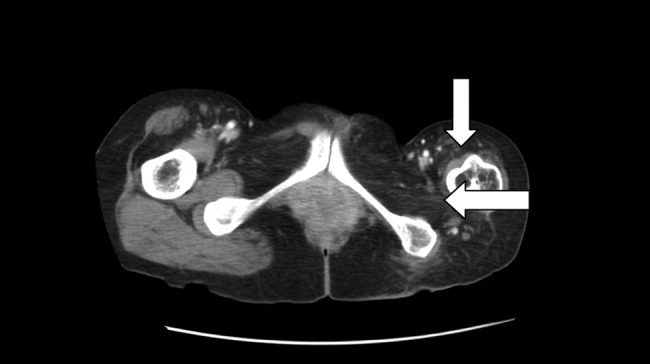
Poliovirus, a small RNA enterovirus, causes destruction of the motor neuron ganglia of the brain stem and anterior horn cells of the spine. Resultant Wallerian degeneration leads to muscle atrophy, as seen here.
A 45‐year‐old Vietnamese woman presented to the emergency department reporting 4 days of vomiting, diarrhea, and epigastric pain. Other than poliomyelitis as a child, she had an unremarkable medical history. A slight left‐sided limp was noted on exam. Although she was diagnosed with gastroenteritis, laboratory studies revealed her blood urea nitrogen was low, at 4 mg/dL, and her creatinine was 0.2 mg/dL, raising concern for an intercurrent abdominal malignancy. A CT scan of the abdomen and pelvis was obtained.
The CT scan did not reveal any abdominal pathology but, incidentally, showed near‐complete atrophy of the left pelvic girdle and proximal femur. Specifically noted was near‐complete fatty atrophy and involution of the left iliacus, piriformis, and gluteus muscles (Fig. 1). Atrophy also involved muscles surrounding the left proximal femur (Fig. 2). The CT and laboratory findings were attributed to childhood poliomyelitis. The patient was discharged home in good condition.


Poliovirus, a small RNA enterovirus, causes destruction of the motor neuron ganglia of the brain stem and anterior horn cells of the spine. Resultant Wallerian degeneration leads to muscle atrophy, as seen here.
A 45‐year‐old Vietnamese woman presented to the emergency department reporting 4 days of vomiting, diarrhea, and epigastric pain. Other than poliomyelitis as a child, she had an unremarkable medical history. A slight left‐sided limp was noted on exam. Although she was diagnosed with gastroenteritis, laboratory studies revealed her blood urea nitrogen was low, at 4 mg/dL, and her creatinine was 0.2 mg/dL, raising concern for an intercurrent abdominal malignancy. A CT scan of the abdomen and pelvis was obtained.
The CT scan did not reveal any abdominal pathology but, incidentally, showed near‐complete atrophy of the left pelvic girdle and proximal femur. Specifically noted was near‐complete fatty atrophy and involution of the left iliacus, piriformis, and gluteus muscles (Fig. 1). Atrophy also involved muscles surrounding the left proximal femur (Fig. 2). The CT and laboratory findings were attributed to childhood poliomyelitis. The patient was discharged home in good condition.


Poliovirus, a small RNA enterovirus, causes destruction of the motor neuron ganglia of the brain stem and anterior horn cells of the spine. Resultant Wallerian degeneration leads to muscle atrophy, as seen here.
Handoffs
Presurgery Discontinuation of Antiplatelet Therapy in Patients
There are currently limited data to guide perioperative management of antiplatelet therapy after drug‐eluting stent (DES) implantation. The clinician must balance the risk of excessive bleeding if antiplatelet agents are continued perioperatively with the risk of stent thrombosis if antiplatelet agents are discontinued for surgerya risk that may be amplified in the perioperative period because of the prothrombotic state that accompanies the stress of surgery.
Paclitaxel‐ and sirolimus‐eluting stents have supplanted bare‐metal stents as first‐line treatment for coronary stenosis because of their efficacy in preventing in‐stent restenosis by inhibiting neointimal proliferation. However, the antiproliferative effects of DESs may also delay endothelialization, rendering them vulnerable to stent thrombosis when antiplatelet therapy is prematurely discontinued.15 Some patients with DESs may be vulnerable to stent thrombosis when antiplatelet therapy is discontinued even after a year or more of treatment.6 Although stent thrombosis is uncommon, it is deadly, with a mortality rate approaching 50%.1 Generally, antiplatelet therapy is discontinued prior to surgery. This presents a clinical dilemma for patients with DES because guidelines recommend lifelong aspirin therapy and at least 36 months of clopidogrel for patients who have undergone DES placement.79
In the bare‐metal stent era, studies demonstrated an alarming risk of stent thrombosis in the setting of noncardiac surgery within 26 weeks of stent placement.10, 11 However, the appropriate interval before elective noncardiac surgery following DES placement has not been defined and may be longer. Case reports and case series have highlighted this risk12 and have even suggested that a DES may be susceptible to stent thrombosis as long as a year after its placement.6 More recently, pooled data from controlled trials have suggested that although the overall rate of DES thrombosis may not be consistently higher than that of bare‐metal stents, the risk appears to persist far longer (probably from delayed endothelialization of the target vessel) and may be more pronounced following discontinuation of antiplatelet agents.9, 1316 This has led to recent recommendations to continue dual antiplatelet therapy (with aspirin and clopidogrel) for at least a year following DES placement and possibly indefinitely, provided that the therapy is tolerated.9 Whether this risk is accentuated in the perioperative setting independent of discontinuation of antiplatelet therapy remains unknown. In 1 registry, the strongest predictor of DES thrombosis was premature discontinuation of antiplatelet therapy (hazard ratio 90, 95% confidence interval 30270, P < .001), and noncardiac surgery was the most frequent reason for discontinuation of antiplatelet therapy.1 However, the actual incidence of stent thrombosis in patients undergoing surgery was unavailable because the denominator was unknown (ie, number of patients with stents who underwent surgery). Although it is certainly plausible that the prothrombotic and proinflammatory postoperative state augments the risk of stent thrombosis independent of discontinuation of antiplatelet therapy alone, this remains unproven.
At the time of the present study, protocol‐based clinical practice at the Cleveland Clinic Foundation's Internal Medicine Preoperative Assessment Consultation and Treatment (IMPACT) Center included routine discontinuation of all antiplatelet agents (including aspirin and clopidogrel) at least 7 days prior to noncardiac surgery, including in patients with coronary stents. Exceptions to this policy were generally made only for very minor procedures. The purpose of this study was to systematically quantify the risk of adverse cardiovascular events in patients who had DES placement and subsequently underwent elective or semielective noncardiac surgery, most of whom had discontinued all antiplatelet agents at least 7 days before surgery.
Methods
We identified all patients who had DES placement at the Cleveland Clinic who subsequently underwent preoperative evaluation for noncardiac surgery at the IMPACT Center between July 2003 and July 2005. About half the patients undergoing surgery at the Cleveland Clinic were seen in the IMPACT Center prior to surgery during the study period. Preoperative evaluation at the IMPACT Center included a standardized assessment by a hospitalist with expertise in preoperative medicine. Clinical data for each patient were contemporaneously entered into an electronic medical record. Written preoperative medication instructions were provided to each patient and documented in the electronic record, indicating specific instructions to discontinue any antiplatelet agents 710 days preoperatively.
The IMPACT Center database was crosslinked to the Cleveland Clinic Foundation Heart Center Database, which contains records of all patients who have undergone coronary stenting at the Cleveland Clinic. Computerized and written medical records of all patients in both databases were reviewed using a standardized data collection instrument. All medical data generated up to 30 days postoperatively at the Cleveland Clinic were reviewed. Social Security numbers were linked with the Social Security Death Index to verify that no patients died within 30 days of surgery.
Predefined outcomes included catheterization‐confirmed DES thrombosis, any myocardial infarction, and major bleeding within 30 days of the surgical procedure. Myocardial infarction was defined as elevation of troponin T to more than twice the upper limit of normal (0.2 mg/mL) with or without associated electrocardiographic changes or symptoms. This biochemically based definition was used with the understanding that cardiac enzyme tests are consistently ordered for patients at the Cleveland Clinic with suspected coronary events and that postoperative myocardial infarction may be atypical in presentation (eg, delirium or hypotension without chest pain). Stent thrombosis was considered present if confirmed by catheterization or autopsy and considered possible if a patient suffered from a myocardial infarction but did not have a definitive diagnostic procedure performed. DES thrombosis was considered absent if a patient underwent postoperative catheterization and the DES appeared patent. Major bleeding was defined as any bleeding requiring unplanned reoperation or bleeding in a critical location (intracranial or retroperitoneal). Invasiveness of surgery was defined prospectively according to a Cleveland Clinic bleeding classification scheme based on that of Pasternak17, 18:
Category 1. Minimal risk to patient; little or no anticipated blood loss (eg, breast biopsy, cystoscopy).
Category 2. Mild risk to patient; minimal to moderately invasive procedure; estimated blood loss < 500 cc (eg, laparoscopy, arthroscopy, hernia repair).
Category 3. Moderate risk to patient and moderate to significantly invasive; blood loss potential 5001000 cc (eg, laminectomy, total hip or knee replacement).
Category 4. Major risk to patient; highly invasive procedure; anticipated blood loss > 1500 cc (eg, major spinal reconstruction, major reconstruction of GI tract, major vascular repair without intensive care unit stay).
Category 5. Critical risk to patient; highly invasive procedure; anticipated blood loss > 1500 cc with anticipated postoperative intensive care unit stay (eg, cardiac procedure, major vascular repair with anticipated intensive care unit stay).
Statistical analyses were descriptive. We determined the rate of adverse outcomes with 95% confidence intervals (CIs) in the entire patient cohort and among prespecified patient subsets, based on timing of discontinuation of antiplatelet therapy. Predefined subsets included those who had clopidogrel and aspirin discontinued less than 3 months and less than 6 months following DES implantation. The 2 test was used to test the hypothesis that discontinuation of antiplatelet therapy was a function of the type of surgery or timing of stent placement.
The study was approved by the Cleveland Clinic Foundation's institutional review board. The requirement for informed consent was waived.
RESULTS
In total, 114 patients were evaluated in the IMPACT Center following DES placement. Baseline patient characteristics are shown in Table 1. The median age was 71 years (interquartile range 6476 years), and 66% were male. Patients had a moderate degree of comorbidity: 41% had diabetes, 12% had an ejection fraction < 45%, 34% had undergone coronary bypass, 17% had atrial fibrillation or flutter, and 20% had chronic renal insufficiency (creatinine 2.0 or end‐stage renal disease). Most patients received ‐adrenergic blockers (97%), statins (95%), and either angiotensin‐converting enzyme (ACE) inhibitors or angiotensin receptor blockers (77%) preoperatively. Patients underwent a variety of surgeries (Table 1).
| Characteristic | n (%) unless otherwise noted |
|---|---|
| |
| Demographics | |
| Age (years), median (IQR) | 71 (6476) |
| Male | 75 (65.7%) |
| White | 88 (77.2%) |
| Comorbid illnesses | |
| Diabetes mellitus | 47 (41.2%) |
| History of prior myocardial infarction | 48 (42.1%) |
| Hypertension | 108 (94.7%) |
| History of stroke or transient cerebral ischemia | 15 (13.2%) |
| Dyslipidemia or treatment with lipid‐lowering drugs | 106 (93.0%) |
| Ejection fraction < 45% | 14 (12.3%) |
| History of coronary artery bypass | 39 (34.2%) |
| Atrial fibrillation or flutter | 19 (16.7%) |
| End‐stage renal disease on dialysis | 13 (11.4%) |
| Chronic renal impairment (creatinine 2.0) without dialysis | 10 (8.8%) |
| Other medical treatments | |
| Angiotensin converting enzyme inhibitor or angiotensin receptor blocker | 88 (77.2%) |
| ‐blocker | 111 (97.3%) |
| Statin | 108 (94.7%) |
| Invasiveness of surgery* | |
| Category 1 (lowest risk) | 37 (32.5%) |
| Category 2 | 22 (19.3%) |
| Category 3 | 48 (42.1%) |
| Category 4 | 7 (6.1%) |
| Category 5 (highest risk) | 0 (0%) |
| Outpatient or short‐stay surgery | 50 (47.2%) |
| Type of surgery | |
| Major orthopedic | 39 (34.2%) |
| Minor orthopedic | 5 (4.4%) |
| Ophthalmologic | 30 (26.3%) |
| General abdominal | 8 (7.0%) |
| Gynecological | 5 (4.4%) |
| Urological | 11 (9.6%) |
| Head and neck | 5 (4.4%) |
| Vascular | 1 (0.9%) |
| Other | 10 (8.8%) |
Patients had received both paclitaxel and sirolimus stents (28% and 73% of patients, respectively); 33% of patients had had more than 1 DES (Table 2). Most patients underwent surgery within 1 year of stent placement (77%), but only 40% had surgery within 180 days of stenting and only 13% within 90 days of stenting. Most patients (77%) had antiplatelet therapy completely discontinued a median of 10 days before surgery and remained off antiplatelet therapy for a median of 14 days total. Ten of the 15 patients (67%) who underwent surgery within 90 days of stenting had all antiplatelet agents discontinued preoperatively, 24 of the 30 patients (80%) who had surgery between 91 and 180 days after stenting had antiplatelet therapy completely discontinued, and 54 of the 69 patients (78%) who had surgery more than 180 days after stenting had antiplatelet therapy completely discontinued. There was no significant relationship between timing of stent placement relative to surgery (<90, 91180, or >180 days) and decision about whether to discontinue antiplatelet therapy (P = .59). However, invasiveness of the surgery was associated with antiplatelet management: 85% of those who continued antiplatelet therapy (aspirin or aspirin and clopidogrel) during the perioperative period were patients who underwent minimally invasive surgery (P < .0001).
| Characteristic | n (%) unless otherwise noted |
|---|---|
| |
| Timing of surgery and antiplatelet agent discontinuation relative to Percutaneous coronary intervention | |
| Duration of most recent intervention relative to surgery (days), median (IQR) | 236 (125354) |
| Surgery within 90 days of DES placement | 15 (13.2%) |
| Surgery within 180 days of DES placement | 45 (39.5%) |
| Surgery within 1 year of DES placement | 88 (77.2%) |
| Percutaneous Coronary Intervention History | |
| Number of drug‐eluting stents | |
| 1 | 76 (66.7%) |
| 2 | 26 (22.8%) |
| 3+ | 12 (10.5%) |
| Paclitaxel stent 1 | 32 (28.1%) |
| Sirolimus stent 1 | 83 (72.8%) |
| Bare‐metal stent 1 | 10 (8.8%) |
| Perioperative antiplatelet treatment | |
| Clopidogrel and aspirin continued through surgery | 24 (21.1%) |
| Aspirin alone continued through surgery | 2 (1.8%) |
| Clopidogrel alone continued through surgery | 0 (0%) |
| No antiplatelet treatment at time of surgery | 88 (77.2%) |
| Among the 15 patients who had surgery within 90 days of stenting | 10 (66.7%) |
| Among the 45 patients who had surgery within 180 days of stenting | 34 (75.6%) |
| Duration of discontinuance of aspirin | |
| Median number of days discontinued preoperatively (IQR) | 10 (812) |
| Median total duration of discontinuance [days, IQR) | 14 (1019) |
| Duration of discontinuance of clopidogrel | |
| Median number of days discontinued preoperatively [days, IQR] | 10 (813) |
| Median number of days discontinued in total (IQR) | 14 (1020) |
The outcome events are presented in Table 3. Two patients (1.8%, 95% CI 0.5%6.2%) suffered a non‐ST‐elevation myocardial infarction (NSTEMI) postoperatively, and another patient (0.9%, 95% CI 0.2%4.8%) developed major bleeding, a retroperitoneal hemorrhage following kidney transplantation. This patient had been taking both aspirin and clopidogrel until 7 days prior to surgery and began to hemorrhage the day after surgery; antiplatelet agents were resumed 12 days postoperatively. No patients died (0%, 95% CI 0%3.3%). One of the 2 patients who suffered an MI was a 72‐year‐old man who had had placement of a single sirolimus‐eluting stent in the posterior descending artery 284 days prior to elective hip arthroplasty. He had no history of myocardial infarction but had undergone coronary bypass surgery 4 years earlier. Echocardiography showed he had aortic stenosis, with a calculated valve area of 0.9 cm2. He had a baseline left ventricular ejection fraction of 45%. His preoperative cardiac medications included lovastatin, lisinopril, and atenolol; he discontinued both aspirin and clopidogrel 7 days before the surgery. His NSTEMI occurred on the day of his operation, presenting with hypotension and anterolateral ST depressions. His troponin T peaked at 0.48 mg/mL, with a peak creatinine kinase of 795 U/L (MB fraction 6%). His left ventricular ejection fraction was 45% on postoperative day 2 (unchanged from baseline). He was discharged on postoperative day 8 and returned for catheterization 3 weeks later, at which time he was found to have a 70% ostial lesion in a saphenous vein graft to an obtuse marginal, which was stented. The previously placed DES was widely patent. The other patient who suffered a postoperative NSTEMI was a 68‐year‐old man with a history of carotid artery stenting and renal artery stenosis who had undergone placement of 3 sirolimus‐eluting stents in the right coronary artery 50 days prior to cervical laminectomy. He had had elective placement of the stents following a positive pharmacologic stress test. He was taking 50 mg of atenolol daily and had been taking aspirin and clopidogrel until 17 days before surgery. On postoperative day 3 he developed dyspnea, and leads V4 and V5 showed ST depressions. His troponin T peaked at 1.24 mg/mL, with a peak creatinine kinase of 879 U/L (MB fraction 6%). The patient underwent left‐heart catheterization on hospital day 10. All 3 DESs were widely patent. His left ventricular ejection fraction was estimated at 65%. He was discharged on postoperative day 15. Because neither of the patients who had a postoperative NSTEMI showed evidence of stent thrombosis on catheterization, the overall rate of stent thrombosis was 0% (95% CI 0%3.3%).
| Outcome | Entire cohort (n = 114) [all antiplatelet therapy stopped in 88 patients (77%)] | Surgery < 90 days after DES (n = 15) [all antiplatelet therapy stopped in 10 patients (67%)] | Surgery < 180 days after DES (n = 45) [all antiplatelet therapy stopped in 34 patients (76%)] |
|---|---|---|---|
| |||
| Death | 0 (0%, 0%3.3%) | 0 (0%, 0%20.4%) | 0 (0%, 0%7.9%) |
| Any myocardial infarction | 2 (1.8%, 0.5%6.2%) | 1 (6.7%, 1.2%29.8%) | 1 (2.2%, 0.4%11.6%) |
| DES thrombosis | 0 (0%, 0%3.3%) | 0 (0%, 0%20.4%) | 0 (0%, 0%7.9%) |
| Major bleeding | 1 (0.9%, 0.2%4.8%) | 0 (0%, 0%20.4%) | 0 (0%, 0%7.9%) |
DISCUSSION
Although 2 patients in our study cohort suffered a postoperative myocardial infarction and underwent postoperative catheterization, neither was found to have stent thrombosis, and the MIs of both patients were NSTEMIs with modest cardiac enzyme elevations only. No patients died. A rate of myocardial infarction of less than 2% is well within that expected for patients with established coronary disease undergoing noncardiac surgery.19 That most of our patients discontinued both aspirin and clopidogrel and did not receive antiplatelet agents for a median of 14 days suggests that transient termination of antiplatelet agents in the perioperative setting is not associated with high morbidity or mortality in patients with DES, even when patients have had their stents implanted in the preceding 36 months.
Our study builds on the limited data on this topic. One small case series examined outcomes in 38 patients who had had DES placement and subsequently underwent noncardiac surgery a median of 297 days after stenting.20 None of the patients in this series suffered from stent thrombosis or myocardial infarction, but most underwent surgery without discontinuing aspirin, and 41% underwent surgery without discontinuing clopidogrel. Another recent study demonstrated a high rate of adverse cardiovascular events in patients with coronary stents who underwent noncardiac surgery up to a year after stenting, but the authors of this study did not differentiate between drug‐eluting and bare‐metal stents, and all patients were continued on antiplatelet agents and received parenteral antithrombotic treatment.21
The major strength of our study was its systematic approach. Using a computerized and comprehensive search strategy, we identified all patients who had undergone DES placement at the Cleveland Clinic who subsequently had a preoperative evaluation at the IMPACT Center. Therefore, we are confident that the number of patients in our cohort truly reflects a well‐defined at‐risk population, allowing for an accurate calculation of event rates. This approach contrasts sharply with prior case reports and case series, in which the number of patients at risk was unknown. Nevertheless, these previous reports demonstrate that DES thromboses do occur and can be devastating, so even a small risk of DES thrombosis should be taken seriously. The upper bound of the 95% confidence interval of our estimate of the rate of DES thrombosis was 3.3%, so it is entirely plausible that sampling error contributed to the low rate of thrombosis that we observed.
One major limitation of our study is its sample size. Although our cohort was more than 3 times larger than the only other published cohort of DES patients undergoing noncardiac surgery,20 we had only limited precision to quantify the risk of DES thrombosis. This limitation is particularly relevant for patients who have undergone stent implantation within 36 months of surgery, as they are the patients most likely to have incomplete reendothelialization of the stented artery. We believe that when possible it remains prudent to delay noncardiac surgery for at least 36 months and perhaps up to 12 months following DES implantation, in keeping with recent guidelines.7, 8 However, for patients with conditions such as cancer whose surgery is semielective or patients with nonsurgical bleeding problems (such as gastrointestinal bleeding), our study provides at least some reassurance that short‐term discontinuation of antiplatelet agents may not be as dangerous as some authors have suggested,1 even within 36 months of DES placement. Another important limitation of our study is potential referral bias. At the Cleveland Clinic, most patients undergoing vascular and thoracic procedures are not evaluated at the IMPACT Center. Similarly, some of the patients with severe cardiovascular disease may also have bypassed the IMPACT Center and gone to a cardiologist for preoperative evaluation. As such, we believe our findings should not be generalized to high‐risk cardiac patients or to those undergoing high‐risk procedures.
A noteworthy distinction between our cohort and the cohort reported by Compton and colleagues is that in the perioperative period, most of our patients underwent complete discontinuation of antiplatelet therapy and remained off both aspirin and clopidogrel for an average of 2 weeks, whereas most patients in the other cohort were continued on antiplatelet therapy.20 This highlights the continued controversy surrounding management of antiplatelet therapy in perioperative patients with established coronary disease, who are at substantial risk for both bleeding and myocardial infarction because of the surgery.22 Our data offer little guidance on the optimal management of antiplatelet agents perioperatively because the incidence of both bleeding and thrombosis was low and whether or not patients were continued on antiplatelet agents was not random. We advocate individualized management strategies of perioperative patients with DES. Patients undergoing procedures that carry a high risk of outcome‐affecting bleeding (such as brain surgery) should probably have their antiplatelet agents discontinued preoperatively, whereas those undergoing minor surgery may have their antiplatelet agents continued, provided the surgeon and the anesthesiologist are in agreement with this approach. The timing of DES placement should also be factored into this decision because recently placed stents carry a higher risk of thrombosis.
In summary, our findings clarify the risks of stent thrombosis and postoperative myocardial infarction in clinically stable patients with DES who undergo low‐ and intermediate‐risk noncardiac surgery. Because it is unlikely to ever be ethically appropriate or logistically feasible to conduct a randomized study of patients with DES having early versus delayed noncardiac surgery, observational cohorts will have to suffice. Additional similar studies will help to validate (or refute) our findings and to more precisely quantify the risk of adverse cardiac events when patients with DES undergo surgery, which is real, feared, and potentially catastrophic but may be overestimated.
- ,,, et al.Incidence, predictors, and outcome of thrombosis after successful implantation of drug‐eluting stents.JAMA.2005;293:2126–230.
- ,,, et al.Pathology of drug‐eluting stents in humans: delayed healing and late thrombotic risk.J Am Coll Cardiol.2006;48:193–202.
- ,,, et al.Correlates and long‐term outcomes of angiographically proven stent thrombosis with sirolimus‐ and paclitaxel‐eluting stents.Circulation.2006;113:1108–1113.
- ,,, et al.Prevalence, predictors, and outcomes of premature discontinuation of thienopyridine therapy after drug‐eluting stent placement: results from the PREMIER registry.Circulation.2006;113:2803–2809.
- .Trading restenosis for thrombosis? New questions about drug‐eluting stents.N Engl J Med.2006;355:1949–1952.
- ,,, et al.Late thrombosis in drug‐eluting coronary stents after discontinuation of antiplatelet therapy.Lancet2004;364:1519–1521.
- ,,, et al.ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update 2001 Guidelines for Percutaneous Coronary Intervention).Circulation.2006;113:e166–e286.
- ,,, et al.ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction).Circulation.2004;110:588–636.
- ,,, et al.Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents: a science advisory from the American Heart Association, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association, with representation from the American College of Physicians.Circulation.2007;115:813–818.
- ,,, et al.Clinical outcome of patients undergoing non‐cardiac surgery in the two months following coronary stenting.J Am Coll Cardiol.2003;42:234–240.
- ,,,,.Catastrophic outcomes of noncardiac surgery soon after coronary stenting.J Am Coll Cardiol.2000;35:1288–1294.
- ,.Thrombosis of sirolimus‐eluting coronary stent in the postanesthesia care unit.Anesth Analg.2005;101:971–973.
- ,,,,,.Late thrombosis of drug‐eluting stents: a meta‐analysis of randomized clinical trials.Am J Med.2006;119:1056–1061.
- ,,,,,.Long‐term outcomes with drug‐eluting stents versus bare‐metal stents in Sweden.N Engl J Med.2007;356:1009–1019.
- ,,, et al.Safety and efficacy of sirolimus‐ and paclitaxel‐eluting coronary stents.N Engl J Med.2007;356:998–1008.
- ,,,,.A pooled analysis of data comparing sirolimus‐eluting stents with bare‐metal stents.N Engl J Med.2007;356:989–997.
- .Preoperative assessment: guidelines and challenges.Acta Anaesthesiol ScandSuppl.1997;111:318–320.
- .Preoperative assessment of the ambulatory and same day admission patient.Wellcome Trends Anesthesiol.1991;9:3–11.
- ,,, et al.Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery.Circulation.1999;100:1043–1049.
- ,,,,.Risk of noncardiac surgery after coronary drug‐eluting stent implantation.Am J Cardiol.2006;98:1212–1213.
- ,,,,,.Coronary artery stenting and non‐cardiac surgery—a prospective outcome study.Br J Anaesth.2006;96:686–693.
- ,,.Double jeopardy: balance between bleeding and stent thrombosis with prolonged dual antiplatelet therapy after drug‐eluting stent implantation.Cardiovasc Revasc Med.2006;7:155–158.
There are currently limited data to guide perioperative management of antiplatelet therapy after drug‐eluting stent (DES) implantation. The clinician must balance the risk of excessive bleeding if antiplatelet agents are continued perioperatively with the risk of stent thrombosis if antiplatelet agents are discontinued for surgerya risk that may be amplified in the perioperative period because of the prothrombotic state that accompanies the stress of surgery.
Paclitaxel‐ and sirolimus‐eluting stents have supplanted bare‐metal stents as first‐line treatment for coronary stenosis because of their efficacy in preventing in‐stent restenosis by inhibiting neointimal proliferation. However, the antiproliferative effects of DESs may also delay endothelialization, rendering them vulnerable to stent thrombosis when antiplatelet therapy is prematurely discontinued.15 Some patients with DESs may be vulnerable to stent thrombosis when antiplatelet therapy is discontinued even after a year or more of treatment.6 Although stent thrombosis is uncommon, it is deadly, with a mortality rate approaching 50%.1 Generally, antiplatelet therapy is discontinued prior to surgery. This presents a clinical dilemma for patients with DES because guidelines recommend lifelong aspirin therapy and at least 36 months of clopidogrel for patients who have undergone DES placement.79
In the bare‐metal stent era, studies demonstrated an alarming risk of stent thrombosis in the setting of noncardiac surgery within 26 weeks of stent placement.10, 11 However, the appropriate interval before elective noncardiac surgery following DES placement has not been defined and may be longer. Case reports and case series have highlighted this risk12 and have even suggested that a DES may be susceptible to stent thrombosis as long as a year after its placement.6 More recently, pooled data from controlled trials have suggested that although the overall rate of DES thrombosis may not be consistently higher than that of bare‐metal stents, the risk appears to persist far longer (probably from delayed endothelialization of the target vessel) and may be more pronounced following discontinuation of antiplatelet agents.9, 1316 This has led to recent recommendations to continue dual antiplatelet therapy (with aspirin and clopidogrel) for at least a year following DES placement and possibly indefinitely, provided that the therapy is tolerated.9 Whether this risk is accentuated in the perioperative setting independent of discontinuation of antiplatelet therapy remains unknown. In 1 registry, the strongest predictor of DES thrombosis was premature discontinuation of antiplatelet therapy (hazard ratio 90, 95% confidence interval 30270, P < .001), and noncardiac surgery was the most frequent reason for discontinuation of antiplatelet therapy.1 However, the actual incidence of stent thrombosis in patients undergoing surgery was unavailable because the denominator was unknown (ie, number of patients with stents who underwent surgery). Although it is certainly plausible that the prothrombotic and proinflammatory postoperative state augments the risk of stent thrombosis independent of discontinuation of antiplatelet therapy alone, this remains unproven.
At the time of the present study, protocol‐based clinical practice at the Cleveland Clinic Foundation's Internal Medicine Preoperative Assessment Consultation and Treatment (IMPACT) Center included routine discontinuation of all antiplatelet agents (including aspirin and clopidogrel) at least 7 days prior to noncardiac surgery, including in patients with coronary stents. Exceptions to this policy were generally made only for very minor procedures. The purpose of this study was to systematically quantify the risk of adverse cardiovascular events in patients who had DES placement and subsequently underwent elective or semielective noncardiac surgery, most of whom had discontinued all antiplatelet agents at least 7 days before surgery.
Methods
We identified all patients who had DES placement at the Cleveland Clinic who subsequently underwent preoperative evaluation for noncardiac surgery at the IMPACT Center between July 2003 and July 2005. About half the patients undergoing surgery at the Cleveland Clinic were seen in the IMPACT Center prior to surgery during the study period. Preoperative evaluation at the IMPACT Center included a standardized assessment by a hospitalist with expertise in preoperative medicine. Clinical data for each patient were contemporaneously entered into an electronic medical record. Written preoperative medication instructions were provided to each patient and documented in the electronic record, indicating specific instructions to discontinue any antiplatelet agents 710 days preoperatively.
The IMPACT Center database was crosslinked to the Cleveland Clinic Foundation Heart Center Database, which contains records of all patients who have undergone coronary stenting at the Cleveland Clinic. Computerized and written medical records of all patients in both databases were reviewed using a standardized data collection instrument. All medical data generated up to 30 days postoperatively at the Cleveland Clinic were reviewed. Social Security numbers were linked with the Social Security Death Index to verify that no patients died within 30 days of surgery.
Predefined outcomes included catheterization‐confirmed DES thrombosis, any myocardial infarction, and major bleeding within 30 days of the surgical procedure. Myocardial infarction was defined as elevation of troponin T to more than twice the upper limit of normal (0.2 mg/mL) with or without associated electrocardiographic changes or symptoms. This biochemically based definition was used with the understanding that cardiac enzyme tests are consistently ordered for patients at the Cleveland Clinic with suspected coronary events and that postoperative myocardial infarction may be atypical in presentation (eg, delirium or hypotension without chest pain). Stent thrombosis was considered present if confirmed by catheterization or autopsy and considered possible if a patient suffered from a myocardial infarction but did not have a definitive diagnostic procedure performed. DES thrombosis was considered absent if a patient underwent postoperative catheterization and the DES appeared patent. Major bleeding was defined as any bleeding requiring unplanned reoperation or bleeding in a critical location (intracranial or retroperitoneal). Invasiveness of surgery was defined prospectively according to a Cleveland Clinic bleeding classification scheme based on that of Pasternak17, 18:
Category 1. Minimal risk to patient; little or no anticipated blood loss (eg, breast biopsy, cystoscopy).
Category 2. Mild risk to patient; minimal to moderately invasive procedure; estimated blood loss < 500 cc (eg, laparoscopy, arthroscopy, hernia repair).
Category 3. Moderate risk to patient and moderate to significantly invasive; blood loss potential 5001000 cc (eg, laminectomy, total hip or knee replacement).
Category 4. Major risk to patient; highly invasive procedure; anticipated blood loss > 1500 cc (eg, major spinal reconstruction, major reconstruction of GI tract, major vascular repair without intensive care unit stay).
Category 5. Critical risk to patient; highly invasive procedure; anticipated blood loss > 1500 cc with anticipated postoperative intensive care unit stay (eg, cardiac procedure, major vascular repair with anticipated intensive care unit stay).
Statistical analyses were descriptive. We determined the rate of adverse outcomes with 95% confidence intervals (CIs) in the entire patient cohort and among prespecified patient subsets, based on timing of discontinuation of antiplatelet therapy. Predefined subsets included those who had clopidogrel and aspirin discontinued less than 3 months and less than 6 months following DES implantation. The 2 test was used to test the hypothesis that discontinuation of antiplatelet therapy was a function of the type of surgery or timing of stent placement.
The study was approved by the Cleveland Clinic Foundation's institutional review board. The requirement for informed consent was waived.
RESULTS
In total, 114 patients were evaluated in the IMPACT Center following DES placement. Baseline patient characteristics are shown in Table 1. The median age was 71 years (interquartile range 6476 years), and 66% were male. Patients had a moderate degree of comorbidity: 41% had diabetes, 12% had an ejection fraction < 45%, 34% had undergone coronary bypass, 17% had atrial fibrillation or flutter, and 20% had chronic renal insufficiency (creatinine 2.0 or end‐stage renal disease). Most patients received ‐adrenergic blockers (97%), statins (95%), and either angiotensin‐converting enzyme (ACE) inhibitors or angiotensin receptor blockers (77%) preoperatively. Patients underwent a variety of surgeries (Table 1).
| Characteristic | n (%) unless otherwise noted |
|---|---|
| |
| Demographics | |
| Age (years), median (IQR) | 71 (6476) |
| Male | 75 (65.7%) |
| White | 88 (77.2%) |
| Comorbid illnesses | |
| Diabetes mellitus | 47 (41.2%) |
| History of prior myocardial infarction | 48 (42.1%) |
| Hypertension | 108 (94.7%) |
| History of stroke or transient cerebral ischemia | 15 (13.2%) |
| Dyslipidemia or treatment with lipid‐lowering drugs | 106 (93.0%) |
| Ejection fraction < 45% | 14 (12.3%) |
| History of coronary artery bypass | 39 (34.2%) |
| Atrial fibrillation or flutter | 19 (16.7%) |
| End‐stage renal disease on dialysis | 13 (11.4%) |
| Chronic renal impairment (creatinine 2.0) without dialysis | 10 (8.8%) |
| Other medical treatments | |
| Angiotensin converting enzyme inhibitor or angiotensin receptor blocker | 88 (77.2%) |
| ‐blocker | 111 (97.3%) |
| Statin | 108 (94.7%) |
| Invasiveness of surgery* | |
| Category 1 (lowest risk) | 37 (32.5%) |
| Category 2 | 22 (19.3%) |
| Category 3 | 48 (42.1%) |
| Category 4 | 7 (6.1%) |
| Category 5 (highest risk) | 0 (0%) |
| Outpatient or short‐stay surgery | 50 (47.2%) |
| Type of surgery | |
| Major orthopedic | 39 (34.2%) |
| Minor orthopedic | 5 (4.4%) |
| Ophthalmologic | 30 (26.3%) |
| General abdominal | 8 (7.0%) |
| Gynecological | 5 (4.4%) |
| Urological | 11 (9.6%) |
| Head and neck | 5 (4.4%) |
| Vascular | 1 (0.9%) |
| Other | 10 (8.8%) |
Patients had received both paclitaxel and sirolimus stents (28% and 73% of patients, respectively); 33% of patients had had more than 1 DES (Table 2). Most patients underwent surgery within 1 year of stent placement (77%), but only 40% had surgery within 180 days of stenting and only 13% within 90 days of stenting. Most patients (77%) had antiplatelet therapy completely discontinued a median of 10 days before surgery and remained off antiplatelet therapy for a median of 14 days total. Ten of the 15 patients (67%) who underwent surgery within 90 days of stenting had all antiplatelet agents discontinued preoperatively, 24 of the 30 patients (80%) who had surgery between 91 and 180 days after stenting had antiplatelet therapy completely discontinued, and 54 of the 69 patients (78%) who had surgery more than 180 days after stenting had antiplatelet therapy completely discontinued. There was no significant relationship between timing of stent placement relative to surgery (<90, 91180, or >180 days) and decision about whether to discontinue antiplatelet therapy (P = .59). However, invasiveness of the surgery was associated with antiplatelet management: 85% of those who continued antiplatelet therapy (aspirin or aspirin and clopidogrel) during the perioperative period were patients who underwent minimally invasive surgery (P < .0001).
| Characteristic | n (%) unless otherwise noted |
|---|---|
| |
| Timing of surgery and antiplatelet agent discontinuation relative to Percutaneous coronary intervention | |
| Duration of most recent intervention relative to surgery (days), median (IQR) | 236 (125354) |
| Surgery within 90 days of DES placement | 15 (13.2%) |
| Surgery within 180 days of DES placement | 45 (39.5%) |
| Surgery within 1 year of DES placement | 88 (77.2%) |
| Percutaneous Coronary Intervention History | |
| Number of drug‐eluting stents | |
| 1 | 76 (66.7%) |
| 2 | 26 (22.8%) |
| 3+ | 12 (10.5%) |
| Paclitaxel stent 1 | 32 (28.1%) |
| Sirolimus stent 1 | 83 (72.8%) |
| Bare‐metal stent 1 | 10 (8.8%) |
| Perioperative antiplatelet treatment | |
| Clopidogrel and aspirin continued through surgery | 24 (21.1%) |
| Aspirin alone continued through surgery | 2 (1.8%) |
| Clopidogrel alone continued through surgery | 0 (0%) |
| No antiplatelet treatment at time of surgery | 88 (77.2%) |
| Among the 15 patients who had surgery within 90 days of stenting | 10 (66.7%) |
| Among the 45 patients who had surgery within 180 days of stenting | 34 (75.6%) |
| Duration of discontinuance of aspirin | |
| Median number of days discontinued preoperatively (IQR) | 10 (812) |
| Median total duration of discontinuance [days, IQR) | 14 (1019) |
| Duration of discontinuance of clopidogrel | |
| Median number of days discontinued preoperatively [days, IQR] | 10 (813) |
| Median number of days discontinued in total (IQR) | 14 (1020) |
The outcome events are presented in Table 3. Two patients (1.8%, 95% CI 0.5%6.2%) suffered a non‐ST‐elevation myocardial infarction (NSTEMI) postoperatively, and another patient (0.9%, 95% CI 0.2%4.8%) developed major bleeding, a retroperitoneal hemorrhage following kidney transplantation. This patient had been taking both aspirin and clopidogrel until 7 days prior to surgery and began to hemorrhage the day after surgery; antiplatelet agents were resumed 12 days postoperatively. No patients died (0%, 95% CI 0%3.3%). One of the 2 patients who suffered an MI was a 72‐year‐old man who had had placement of a single sirolimus‐eluting stent in the posterior descending artery 284 days prior to elective hip arthroplasty. He had no history of myocardial infarction but had undergone coronary bypass surgery 4 years earlier. Echocardiography showed he had aortic stenosis, with a calculated valve area of 0.9 cm2. He had a baseline left ventricular ejection fraction of 45%. His preoperative cardiac medications included lovastatin, lisinopril, and atenolol; he discontinued both aspirin and clopidogrel 7 days before the surgery. His NSTEMI occurred on the day of his operation, presenting with hypotension and anterolateral ST depressions. His troponin T peaked at 0.48 mg/mL, with a peak creatinine kinase of 795 U/L (MB fraction 6%). His left ventricular ejection fraction was 45% on postoperative day 2 (unchanged from baseline). He was discharged on postoperative day 8 and returned for catheterization 3 weeks later, at which time he was found to have a 70% ostial lesion in a saphenous vein graft to an obtuse marginal, which was stented. The previously placed DES was widely patent. The other patient who suffered a postoperative NSTEMI was a 68‐year‐old man with a history of carotid artery stenting and renal artery stenosis who had undergone placement of 3 sirolimus‐eluting stents in the right coronary artery 50 days prior to cervical laminectomy. He had had elective placement of the stents following a positive pharmacologic stress test. He was taking 50 mg of atenolol daily and had been taking aspirin and clopidogrel until 17 days before surgery. On postoperative day 3 he developed dyspnea, and leads V4 and V5 showed ST depressions. His troponin T peaked at 1.24 mg/mL, with a peak creatinine kinase of 879 U/L (MB fraction 6%). The patient underwent left‐heart catheterization on hospital day 10. All 3 DESs were widely patent. His left ventricular ejection fraction was estimated at 65%. He was discharged on postoperative day 15. Because neither of the patients who had a postoperative NSTEMI showed evidence of stent thrombosis on catheterization, the overall rate of stent thrombosis was 0% (95% CI 0%3.3%).
| Outcome | Entire cohort (n = 114) [all antiplatelet therapy stopped in 88 patients (77%)] | Surgery < 90 days after DES (n = 15) [all antiplatelet therapy stopped in 10 patients (67%)] | Surgery < 180 days after DES (n = 45) [all antiplatelet therapy stopped in 34 patients (76%)] |
|---|---|---|---|
| |||
| Death | 0 (0%, 0%3.3%) | 0 (0%, 0%20.4%) | 0 (0%, 0%7.9%) |
| Any myocardial infarction | 2 (1.8%, 0.5%6.2%) | 1 (6.7%, 1.2%29.8%) | 1 (2.2%, 0.4%11.6%) |
| DES thrombosis | 0 (0%, 0%3.3%) | 0 (0%, 0%20.4%) | 0 (0%, 0%7.9%) |
| Major bleeding | 1 (0.9%, 0.2%4.8%) | 0 (0%, 0%20.4%) | 0 (0%, 0%7.9%) |
DISCUSSION
Although 2 patients in our study cohort suffered a postoperative myocardial infarction and underwent postoperative catheterization, neither was found to have stent thrombosis, and the MIs of both patients were NSTEMIs with modest cardiac enzyme elevations only. No patients died. A rate of myocardial infarction of less than 2% is well within that expected for patients with established coronary disease undergoing noncardiac surgery.19 That most of our patients discontinued both aspirin and clopidogrel and did not receive antiplatelet agents for a median of 14 days suggests that transient termination of antiplatelet agents in the perioperative setting is not associated with high morbidity or mortality in patients with DES, even when patients have had their stents implanted in the preceding 36 months.
Our study builds on the limited data on this topic. One small case series examined outcomes in 38 patients who had had DES placement and subsequently underwent noncardiac surgery a median of 297 days after stenting.20 None of the patients in this series suffered from stent thrombosis or myocardial infarction, but most underwent surgery without discontinuing aspirin, and 41% underwent surgery without discontinuing clopidogrel. Another recent study demonstrated a high rate of adverse cardiovascular events in patients with coronary stents who underwent noncardiac surgery up to a year after stenting, but the authors of this study did not differentiate between drug‐eluting and bare‐metal stents, and all patients were continued on antiplatelet agents and received parenteral antithrombotic treatment.21
The major strength of our study was its systematic approach. Using a computerized and comprehensive search strategy, we identified all patients who had undergone DES placement at the Cleveland Clinic who subsequently had a preoperative evaluation at the IMPACT Center. Therefore, we are confident that the number of patients in our cohort truly reflects a well‐defined at‐risk population, allowing for an accurate calculation of event rates. This approach contrasts sharply with prior case reports and case series, in which the number of patients at risk was unknown. Nevertheless, these previous reports demonstrate that DES thromboses do occur and can be devastating, so even a small risk of DES thrombosis should be taken seriously. The upper bound of the 95% confidence interval of our estimate of the rate of DES thrombosis was 3.3%, so it is entirely plausible that sampling error contributed to the low rate of thrombosis that we observed.
One major limitation of our study is its sample size. Although our cohort was more than 3 times larger than the only other published cohort of DES patients undergoing noncardiac surgery,20 we had only limited precision to quantify the risk of DES thrombosis. This limitation is particularly relevant for patients who have undergone stent implantation within 36 months of surgery, as they are the patients most likely to have incomplete reendothelialization of the stented artery. We believe that when possible it remains prudent to delay noncardiac surgery for at least 36 months and perhaps up to 12 months following DES implantation, in keeping with recent guidelines.7, 8 However, for patients with conditions such as cancer whose surgery is semielective or patients with nonsurgical bleeding problems (such as gastrointestinal bleeding), our study provides at least some reassurance that short‐term discontinuation of antiplatelet agents may not be as dangerous as some authors have suggested,1 even within 36 months of DES placement. Another important limitation of our study is potential referral bias. At the Cleveland Clinic, most patients undergoing vascular and thoracic procedures are not evaluated at the IMPACT Center. Similarly, some of the patients with severe cardiovascular disease may also have bypassed the IMPACT Center and gone to a cardiologist for preoperative evaluation. As such, we believe our findings should not be generalized to high‐risk cardiac patients or to those undergoing high‐risk procedures.
A noteworthy distinction between our cohort and the cohort reported by Compton and colleagues is that in the perioperative period, most of our patients underwent complete discontinuation of antiplatelet therapy and remained off both aspirin and clopidogrel for an average of 2 weeks, whereas most patients in the other cohort were continued on antiplatelet therapy.20 This highlights the continued controversy surrounding management of antiplatelet therapy in perioperative patients with established coronary disease, who are at substantial risk for both bleeding and myocardial infarction because of the surgery.22 Our data offer little guidance on the optimal management of antiplatelet agents perioperatively because the incidence of both bleeding and thrombosis was low and whether or not patients were continued on antiplatelet agents was not random. We advocate individualized management strategies of perioperative patients with DES. Patients undergoing procedures that carry a high risk of outcome‐affecting bleeding (such as brain surgery) should probably have their antiplatelet agents discontinued preoperatively, whereas those undergoing minor surgery may have their antiplatelet agents continued, provided the surgeon and the anesthesiologist are in agreement with this approach. The timing of DES placement should also be factored into this decision because recently placed stents carry a higher risk of thrombosis.
In summary, our findings clarify the risks of stent thrombosis and postoperative myocardial infarction in clinically stable patients with DES who undergo low‐ and intermediate‐risk noncardiac surgery. Because it is unlikely to ever be ethically appropriate or logistically feasible to conduct a randomized study of patients with DES having early versus delayed noncardiac surgery, observational cohorts will have to suffice. Additional similar studies will help to validate (or refute) our findings and to more precisely quantify the risk of adverse cardiac events when patients with DES undergo surgery, which is real, feared, and potentially catastrophic but may be overestimated.
There are currently limited data to guide perioperative management of antiplatelet therapy after drug‐eluting stent (DES) implantation. The clinician must balance the risk of excessive bleeding if antiplatelet agents are continued perioperatively with the risk of stent thrombosis if antiplatelet agents are discontinued for surgerya risk that may be amplified in the perioperative period because of the prothrombotic state that accompanies the stress of surgery.
Paclitaxel‐ and sirolimus‐eluting stents have supplanted bare‐metal stents as first‐line treatment for coronary stenosis because of their efficacy in preventing in‐stent restenosis by inhibiting neointimal proliferation. However, the antiproliferative effects of DESs may also delay endothelialization, rendering them vulnerable to stent thrombosis when antiplatelet therapy is prematurely discontinued.15 Some patients with DESs may be vulnerable to stent thrombosis when antiplatelet therapy is discontinued even after a year or more of treatment.6 Although stent thrombosis is uncommon, it is deadly, with a mortality rate approaching 50%.1 Generally, antiplatelet therapy is discontinued prior to surgery. This presents a clinical dilemma for patients with DES because guidelines recommend lifelong aspirin therapy and at least 36 months of clopidogrel for patients who have undergone DES placement.79
In the bare‐metal stent era, studies demonstrated an alarming risk of stent thrombosis in the setting of noncardiac surgery within 26 weeks of stent placement.10, 11 However, the appropriate interval before elective noncardiac surgery following DES placement has not been defined and may be longer. Case reports and case series have highlighted this risk12 and have even suggested that a DES may be susceptible to stent thrombosis as long as a year after its placement.6 More recently, pooled data from controlled trials have suggested that although the overall rate of DES thrombosis may not be consistently higher than that of bare‐metal stents, the risk appears to persist far longer (probably from delayed endothelialization of the target vessel) and may be more pronounced following discontinuation of antiplatelet agents.9, 1316 This has led to recent recommendations to continue dual antiplatelet therapy (with aspirin and clopidogrel) for at least a year following DES placement and possibly indefinitely, provided that the therapy is tolerated.9 Whether this risk is accentuated in the perioperative setting independent of discontinuation of antiplatelet therapy remains unknown. In 1 registry, the strongest predictor of DES thrombosis was premature discontinuation of antiplatelet therapy (hazard ratio 90, 95% confidence interval 30270, P < .001), and noncardiac surgery was the most frequent reason for discontinuation of antiplatelet therapy.1 However, the actual incidence of stent thrombosis in patients undergoing surgery was unavailable because the denominator was unknown (ie, number of patients with stents who underwent surgery). Although it is certainly plausible that the prothrombotic and proinflammatory postoperative state augments the risk of stent thrombosis independent of discontinuation of antiplatelet therapy alone, this remains unproven.
At the time of the present study, protocol‐based clinical practice at the Cleveland Clinic Foundation's Internal Medicine Preoperative Assessment Consultation and Treatment (IMPACT) Center included routine discontinuation of all antiplatelet agents (including aspirin and clopidogrel) at least 7 days prior to noncardiac surgery, including in patients with coronary stents. Exceptions to this policy were generally made only for very minor procedures. The purpose of this study was to systematically quantify the risk of adverse cardiovascular events in patients who had DES placement and subsequently underwent elective or semielective noncardiac surgery, most of whom had discontinued all antiplatelet agents at least 7 days before surgery.
Methods
We identified all patients who had DES placement at the Cleveland Clinic who subsequently underwent preoperative evaluation for noncardiac surgery at the IMPACT Center between July 2003 and July 2005. About half the patients undergoing surgery at the Cleveland Clinic were seen in the IMPACT Center prior to surgery during the study period. Preoperative evaluation at the IMPACT Center included a standardized assessment by a hospitalist with expertise in preoperative medicine. Clinical data for each patient were contemporaneously entered into an electronic medical record. Written preoperative medication instructions were provided to each patient and documented in the electronic record, indicating specific instructions to discontinue any antiplatelet agents 710 days preoperatively.
The IMPACT Center database was crosslinked to the Cleveland Clinic Foundation Heart Center Database, which contains records of all patients who have undergone coronary stenting at the Cleveland Clinic. Computerized and written medical records of all patients in both databases were reviewed using a standardized data collection instrument. All medical data generated up to 30 days postoperatively at the Cleveland Clinic were reviewed. Social Security numbers were linked with the Social Security Death Index to verify that no patients died within 30 days of surgery.
Predefined outcomes included catheterization‐confirmed DES thrombosis, any myocardial infarction, and major bleeding within 30 days of the surgical procedure. Myocardial infarction was defined as elevation of troponin T to more than twice the upper limit of normal (0.2 mg/mL) with or without associated electrocardiographic changes or symptoms. This biochemically based definition was used with the understanding that cardiac enzyme tests are consistently ordered for patients at the Cleveland Clinic with suspected coronary events and that postoperative myocardial infarction may be atypical in presentation (eg, delirium or hypotension without chest pain). Stent thrombosis was considered present if confirmed by catheterization or autopsy and considered possible if a patient suffered from a myocardial infarction but did not have a definitive diagnostic procedure performed. DES thrombosis was considered absent if a patient underwent postoperative catheterization and the DES appeared patent. Major bleeding was defined as any bleeding requiring unplanned reoperation or bleeding in a critical location (intracranial or retroperitoneal). Invasiveness of surgery was defined prospectively according to a Cleveland Clinic bleeding classification scheme based on that of Pasternak17, 18:
Category 1. Minimal risk to patient; little or no anticipated blood loss (eg, breast biopsy, cystoscopy).
Category 2. Mild risk to patient; minimal to moderately invasive procedure; estimated blood loss < 500 cc (eg, laparoscopy, arthroscopy, hernia repair).
Category 3. Moderate risk to patient and moderate to significantly invasive; blood loss potential 5001000 cc (eg, laminectomy, total hip or knee replacement).
Category 4. Major risk to patient; highly invasive procedure; anticipated blood loss > 1500 cc (eg, major spinal reconstruction, major reconstruction of GI tract, major vascular repair without intensive care unit stay).
Category 5. Critical risk to patient; highly invasive procedure; anticipated blood loss > 1500 cc with anticipated postoperative intensive care unit stay (eg, cardiac procedure, major vascular repair with anticipated intensive care unit stay).
Statistical analyses were descriptive. We determined the rate of adverse outcomes with 95% confidence intervals (CIs) in the entire patient cohort and among prespecified patient subsets, based on timing of discontinuation of antiplatelet therapy. Predefined subsets included those who had clopidogrel and aspirin discontinued less than 3 months and less than 6 months following DES implantation. The 2 test was used to test the hypothesis that discontinuation of antiplatelet therapy was a function of the type of surgery or timing of stent placement.
The study was approved by the Cleveland Clinic Foundation's institutional review board. The requirement for informed consent was waived.
RESULTS
In total, 114 patients were evaluated in the IMPACT Center following DES placement. Baseline patient characteristics are shown in Table 1. The median age was 71 years (interquartile range 6476 years), and 66% were male. Patients had a moderate degree of comorbidity: 41% had diabetes, 12% had an ejection fraction < 45%, 34% had undergone coronary bypass, 17% had atrial fibrillation or flutter, and 20% had chronic renal insufficiency (creatinine 2.0 or end‐stage renal disease). Most patients received ‐adrenergic blockers (97%), statins (95%), and either angiotensin‐converting enzyme (ACE) inhibitors or angiotensin receptor blockers (77%) preoperatively. Patients underwent a variety of surgeries (Table 1).
| Characteristic | n (%) unless otherwise noted |
|---|---|
| |
| Demographics | |
| Age (years), median (IQR) | 71 (6476) |
| Male | 75 (65.7%) |
| White | 88 (77.2%) |
| Comorbid illnesses | |
| Diabetes mellitus | 47 (41.2%) |
| History of prior myocardial infarction | 48 (42.1%) |
| Hypertension | 108 (94.7%) |
| History of stroke or transient cerebral ischemia | 15 (13.2%) |
| Dyslipidemia or treatment with lipid‐lowering drugs | 106 (93.0%) |
| Ejection fraction < 45% | 14 (12.3%) |
| History of coronary artery bypass | 39 (34.2%) |
| Atrial fibrillation or flutter | 19 (16.7%) |
| End‐stage renal disease on dialysis | 13 (11.4%) |
| Chronic renal impairment (creatinine 2.0) without dialysis | 10 (8.8%) |
| Other medical treatments | |
| Angiotensin converting enzyme inhibitor or angiotensin receptor blocker | 88 (77.2%) |
| ‐blocker | 111 (97.3%) |
| Statin | 108 (94.7%) |
| Invasiveness of surgery* | |
| Category 1 (lowest risk) | 37 (32.5%) |
| Category 2 | 22 (19.3%) |
| Category 3 | 48 (42.1%) |
| Category 4 | 7 (6.1%) |
| Category 5 (highest risk) | 0 (0%) |
| Outpatient or short‐stay surgery | 50 (47.2%) |
| Type of surgery | |
| Major orthopedic | 39 (34.2%) |
| Minor orthopedic | 5 (4.4%) |
| Ophthalmologic | 30 (26.3%) |
| General abdominal | 8 (7.0%) |
| Gynecological | 5 (4.4%) |
| Urological | 11 (9.6%) |
| Head and neck | 5 (4.4%) |
| Vascular | 1 (0.9%) |
| Other | 10 (8.8%) |
Patients had received both paclitaxel and sirolimus stents (28% and 73% of patients, respectively); 33% of patients had had more than 1 DES (Table 2). Most patients underwent surgery within 1 year of stent placement (77%), but only 40% had surgery within 180 days of stenting and only 13% within 90 days of stenting. Most patients (77%) had antiplatelet therapy completely discontinued a median of 10 days before surgery and remained off antiplatelet therapy for a median of 14 days total. Ten of the 15 patients (67%) who underwent surgery within 90 days of stenting had all antiplatelet agents discontinued preoperatively, 24 of the 30 patients (80%) who had surgery between 91 and 180 days after stenting had antiplatelet therapy completely discontinued, and 54 of the 69 patients (78%) who had surgery more than 180 days after stenting had antiplatelet therapy completely discontinued. There was no significant relationship between timing of stent placement relative to surgery (<90, 91180, or >180 days) and decision about whether to discontinue antiplatelet therapy (P = .59). However, invasiveness of the surgery was associated with antiplatelet management: 85% of those who continued antiplatelet therapy (aspirin or aspirin and clopidogrel) during the perioperative period were patients who underwent minimally invasive surgery (P < .0001).
| Characteristic | n (%) unless otherwise noted |
|---|---|
| |
| Timing of surgery and antiplatelet agent discontinuation relative to Percutaneous coronary intervention | |
| Duration of most recent intervention relative to surgery (days), median (IQR) | 236 (125354) |
| Surgery within 90 days of DES placement | 15 (13.2%) |
| Surgery within 180 days of DES placement | 45 (39.5%) |
| Surgery within 1 year of DES placement | 88 (77.2%) |
| Percutaneous Coronary Intervention History | |
| Number of drug‐eluting stents | |
| 1 | 76 (66.7%) |
| 2 | 26 (22.8%) |
| 3+ | 12 (10.5%) |
| Paclitaxel stent 1 | 32 (28.1%) |
| Sirolimus stent 1 | 83 (72.8%) |
| Bare‐metal stent 1 | 10 (8.8%) |
| Perioperative antiplatelet treatment | |
| Clopidogrel and aspirin continued through surgery | 24 (21.1%) |
| Aspirin alone continued through surgery | 2 (1.8%) |
| Clopidogrel alone continued through surgery | 0 (0%) |
| No antiplatelet treatment at time of surgery | 88 (77.2%) |
| Among the 15 patients who had surgery within 90 days of stenting | 10 (66.7%) |
| Among the 45 patients who had surgery within 180 days of stenting | 34 (75.6%) |
| Duration of discontinuance of aspirin | |
| Median number of days discontinued preoperatively (IQR) | 10 (812) |
| Median total duration of discontinuance [days, IQR) | 14 (1019) |
| Duration of discontinuance of clopidogrel | |
| Median number of days discontinued preoperatively [days, IQR] | 10 (813) |
| Median number of days discontinued in total (IQR) | 14 (1020) |
The outcome events are presented in Table 3. Two patients (1.8%, 95% CI 0.5%6.2%) suffered a non‐ST‐elevation myocardial infarction (NSTEMI) postoperatively, and another patient (0.9%, 95% CI 0.2%4.8%) developed major bleeding, a retroperitoneal hemorrhage following kidney transplantation. This patient had been taking both aspirin and clopidogrel until 7 days prior to surgery and began to hemorrhage the day after surgery; antiplatelet agents were resumed 12 days postoperatively. No patients died (0%, 95% CI 0%3.3%). One of the 2 patients who suffered an MI was a 72‐year‐old man who had had placement of a single sirolimus‐eluting stent in the posterior descending artery 284 days prior to elective hip arthroplasty. He had no history of myocardial infarction but had undergone coronary bypass surgery 4 years earlier. Echocardiography showed he had aortic stenosis, with a calculated valve area of 0.9 cm2. He had a baseline left ventricular ejection fraction of 45%. His preoperative cardiac medications included lovastatin, lisinopril, and atenolol; he discontinued both aspirin and clopidogrel 7 days before the surgery. His NSTEMI occurred on the day of his operation, presenting with hypotension and anterolateral ST depressions. His troponin T peaked at 0.48 mg/mL, with a peak creatinine kinase of 795 U/L (MB fraction 6%). His left ventricular ejection fraction was 45% on postoperative day 2 (unchanged from baseline). He was discharged on postoperative day 8 and returned for catheterization 3 weeks later, at which time he was found to have a 70% ostial lesion in a saphenous vein graft to an obtuse marginal, which was stented. The previously placed DES was widely patent. The other patient who suffered a postoperative NSTEMI was a 68‐year‐old man with a history of carotid artery stenting and renal artery stenosis who had undergone placement of 3 sirolimus‐eluting stents in the right coronary artery 50 days prior to cervical laminectomy. He had had elective placement of the stents following a positive pharmacologic stress test. He was taking 50 mg of atenolol daily and had been taking aspirin and clopidogrel until 17 days before surgery. On postoperative day 3 he developed dyspnea, and leads V4 and V5 showed ST depressions. His troponin T peaked at 1.24 mg/mL, with a peak creatinine kinase of 879 U/L (MB fraction 6%). The patient underwent left‐heart catheterization on hospital day 10. All 3 DESs were widely patent. His left ventricular ejection fraction was estimated at 65%. He was discharged on postoperative day 15. Because neither of the patients who had a postoperative NSTEMI showed evidence of stent thrombosis on catheterization, the overall rate of stent thrombosis was 0% (95% CI 0%3.3%).
| Outcome | Entire cohort (n = 114) [all antiplatelet therapy stopped in 88 patients (77%)] | Surgery < 90 days after DES (n = 15) [all antiplatelet therapy stopped in 10 patients (67%)] | Surgery < 180 days after DES (n = 45) [all antiplatelet therapy stopped in 34 patients (76%)] |
|---|---|---|---|
| |||
| Death | 0 (0%, 0%3.3%) | 0 (0%, 0%20.4%) | 0 (0%, 0%7.9%) |
| Any myocardial infarction | 2 (1.8%, 0.5%6.2%) | 1 (6.7%, 1.2%29.8%) | 1 (2.2%, 0.4%11.6%) |
| DES thrombosis | 0 (0%, 0%3.3%) | 0 (0%, 0%20.4%) | 0 (0%, 0%7.9%) |
| Major bleeding | 1 (0.9%, 0.2%4.8%) | 0 (0%, 0%20.4%) | 0 (0%, 0%7.9%) |
DISCUSSION
Although 2 patients in our study cohort suffered a postoperative myocardial infarction and underwent postoperative catheterization, neither was found to have stent thrombosis, and the MIs of both patients were NSTEMIs with modest cardiac enzyme elevations only. No patients died. A rate of myocardial infarction of less than 2% is well within that expected for patients with established coronary disease undergoing noncardiac surgery.19 That most of our patients discontinued both aspirin and clopidogrel and did not receive antiplatelet agents for a median of 14 days suggests that transient termination of antiplatelet agents in the perioperative setting is not associated with high morbidity or mortality in patients with DES, even when patients have had their stents implanted in the preceding 36 months.
Our study builds on the limited data on this topic. One small case series examined outcomes in 38 patients who had had DES placement and subsequently underwent noncardiac surgery a median of 297 days after stenting.20 None of the patients in this series suffered from stent thrombosis or myocardial infarction, but most underwent surgery without discontinuing aspirin, and 41% underwent surgery without discontinuing clopidogrel. Another recent study demonstrated a high rate of adverse cardiovascular events in patients with coronary stents who underwent noncardiac surgery up to a year after stenting, but the authors of this study did not differentiate between drug‐eluting and bare‐metal stents, and all patients were continued on antiplatelet agents and received parenteral antithrombotic treatment.21
The major strength of our study was its systematic approach. Using a computerized and comprehensive search strategy, we identified all patients who had undergone DES placement at the Cleveland Clinic who subsequently had a preoperative evaluation at the IMPACT Center. Therefore, we are confident that the number of patients in our cohort truly reflects a well‐defined at‐risk population, allowing for an accurate calculation of event rates. This approach contrasts sharply with prior case reports and case series, in which the number of patients at risk was unknown. Nevertheless, these previous reports demonstrate that DES thromboses do occur and can be devastating, so even a small risk of DES thrombosis should be taken seriously. The upper bound of the 95% confidence interval of our estimate of the rate of DES thrombosis was 3.3%, so it is entirely plausible that sampling error contributed to the low rate of thrombosis that we observed.
One major limitation of our study is its sample size. Although our cohort was more than 3 times larger than the only other published cohort of DES patients undergoing noncardiac surgery,20 we had only limited precision to quantify the risk of DES thrombosis. This limitation is particularly relevant for patients who have undergone stent implantation within 36 months of surgery, as they are the patients most likely to have incomplete reendothelialization of the stented artery. We believe that when possible it remains prudent to delay noncardiac surgery for at least 36 months and perhaps up to 12 months following DES implantation, in keeping with recent guidelines.7, 8 However, for patients with conditions such as cancer whose surgery is semielective or patients with nonsurgical bleeding problems (such as gastrointestinal bleeding), our study provides at least some reassurance that short‐term discontinuation of antiplatelet agents may not be as dangerous as some authors have suggested,1 even within 36 months of DES placement. Another important limitation of our study is potential referral bias. At the Cleveland Clinic, most patients undergoing vascular and thoracic procedures are not evaluated at the IMPACT Center. Similarly, some of the patients with severe cardiovascular disease may also have bypassed the IMPACT Center and gone to a cardiologist for preoperative evaluation. As such, we believe our findings should not be generalized to high‐risk cardiac patients or to those undergoing high‐risk procedures.
A noteworthy distinction between our cohort and the cohort reported by Compton and colleagues is that in the perioperative period, most of our patients underwent complete discontinuation of antiplatelet therapy and remained off both aspirin and clopidogrel for an average of 2 weeks, whereas most patients in the other cohort were continued on antiplatelet therapy.20 This highlights the continued controversy surrounding management of antiplatelet therapy in perioperative patients with established coronary disease, who are at substantial risk for both bleeding and myocardial infarction because of the surgery.22 Our data offer little guidance on the optimal management of antiplatelet agents perioperatively because the incidence of both bleeding and thrombosis was low and whether or not patients were continued on antiplatelet agents was not random. We advocate individualized management strategies of perioperative patients with DES. Patients undergoing procedures that carry a high risk of outcome‐affecting bleeding (such as brain surgery) should probably have their antiplatelet agents discontinued preoperatively, whereas those undergoing minor surgery may have their antiplatelet agents continued, provided the surgeon and the anesthesiologist are in agreement with this approach. The timing of DES placement should also be factored into this decision because recently placed stents carry a higher risk of thrombosis.
In summary, our findings clarify the risks of stent thrombosis and postoperative myocardial infarction in clinically stable patients with DES who undergo low‐ and intermediate‐risk noncardiac surgery. Because it is unlikely to ever be ethically appropriate or logistically feasible to conduct a randomized study of patients with DES having early versus delayed noncardiac surgery, observational cohorts will have to suffice. Additional similar studies will help to validate (or refute) our findings and to more precisely quantify the risk of adverse cardiac events when patients with DES undergo surgery, which is real, feared, and potentially catastrophic but may be overestimated.
- ,,, et al.Incidence, predictors, and outcome of thrombosis after successful implantation of drug‐eluting stents.JAMA.2005;293:2126–230.
- ,,, et al.Pathology of drug‐eluting stents in humans: delayed healing and late thrombotic risk.J Am Coll Cardiol.2006;48:193–202.
- ,,, et al.Correlates and long‐term outcomes of angiographically proven stent thrombosis with sirolimus‐ and paclitaxel‐eluting stents.Circulation.2006;113:1108–1113.
- ,,, et al.Prevalence, predictors, and outcomes of premature discontinuation of thienopyridine therapy after drug‐eluting stent placement: results from the PREMIER registry.Circulation.2006;113:2803–2809.
- .Trading restenosis for thrombosis? New questions about drug‐eluting stents.N Engl J Med.2006;355:1949–1952.
- ,,, et al.Late thrombosis in drug‐eluting coronary stents after discontinuation of antiplatelet therapy.Lancet2004;364:1519–1521.
- ,,, et al.ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update 2001 Guidelines for Percutaneous Coronary Intervention).Circulation.2006;113:e166–e286.
- ,,, et al.ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction).Circulation.2004;110:588–636.
- ,,, et al.Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents: a science advisory from the American Heart Association, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association, with representation from the American College of Physicians.Circulation.2007;115:813–818.
- ,,, et al.Clinical outcome of patients undergoing non‐cardiac surgery in the two months following coronary stenting.J Am Coll Cardiol.2003;42:234–240.
- ,,,,.Catastrophic outcomes of noncardiac surgery soon after coronary stenting.J Am Coll Cardiol.2000;35:1288–1294.
- ,.Thrombosis of sirolimus‐eluting coronary stent in the postanesthesia care unit.Anesth Analg.2005;101:971–973.
- ,,,,,.Late thrombosis of drug‐eluting stents: a meta‐analysis of randomized clinical trials.Am J Med.2006;119:1056–1061.
- ,,,,,.Long‐term outcomes with drug‐eluting stents versus bare‐metal stents in Sweden.N Engl J Med.2007;356:1009–1019.
- ,,, et al.Safety and efficacy of sirolimus‐ and paclitaxel‐eluting coronary stents.N Engl J Med.2007;356:998–1008.
- ,,,,.A pooled analysis of data comparing sirolimus‐eluting stents with bare‐metal stents.N Engl J Med.2007;356:989–997.
- .Preoperative assessment: guidelines and challenges.Acta Anaesthesiol ScandSuppl.1997;111:318–320.
- .Preoperative assessment of the ambulatory and same day admission patient.Wellcome Trends Anesthesiol.1991;9:3–11.
- ,,, et al.Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery.Circulation.1999;100:1043–1049.
- ,,,,.Risk of noncardiac surgery after coronary drug‐eluting stent implantation.Am J Cardiol.2006;98:1212–1213.
- ,,,,,.Coronary artery stenting and non‐cardiac surgery—a prospective outcome study.Br J Anaesth.2006;96:686–693.
- ,,.Double jeopardy: balance between bleeding and stent thrombosis with prolonged dual antiplatelet therapy after drug‐eluting stent implantation.Cardiovasc Revasc Med.2006;7:155–158.
- ,,, et al.Incidence, predictors, and outcome of thrombosis after successful implantation of drug‐eluting stents.JAMA.2005;293:2126–230.
- ,,, et al.Pathology of drug‐eluting stents in humans: delayed healing and late thrombotic risk.J Am Coll Cardiol.2006;48:193–202.
- ,,, et al.Correlates and long‐term outcomes of angiographically proven stent thrombosis with sirolimus‐ and paclitaxel‐eluting stents.Circulation.2006;113:1108–1113.
- ,,, et al.Prevalence, predictors, and outcomes of premature discontinuation of thienopyridine therapy after drug‐eluting stent placement: results from the PREMIER registry.Circulation.2006;113:2803–2809.
- .Trading restenosis for thrombosis? New questions about drug‐eluting stents.N Engl J Med.2006;355:1949–1952.
- ,,, et al.Late thrombosis in drug‐eluting coronary stents after discontinuation of antiplatelet therapy.Lancet2004;364:1519–1521.
- ,,, et al.ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update 2001 Guidelines for Percutaneous Coronary Intervention).Circulation.2006;113:e166–e286.
- ,,, et al.ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction).Circulation.2004;110:588–636.
- ,,, et al.Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents: a science advisory from the American Heart Association, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association, with representation from the American College of Physicians.Circulation.2007;115:813–818.
- ,,, et al.Clinical outcome of patients undergoing non‐cardiac surgery in the two months following coronary stenting.J Am Coll Cardiol.2003;42:234–240.
- ,,,,.Catastrophic outcomes of noncardiac surgery soon after coronary stenting.J Am Coll Cardiol.2000;35:1288–1294.
- ,.Thrombosis of sirolimus‐eluting coronary stent in the postanesthesia care unit.Anesth Analg.2005;101:971–973.
- ,,,,,.Late thrombosis of drug‐eluting stents: a meta‐analysis of randomized clinical trials.Am J Med.2006;119:1056–1061.
- ,,,,,.Long‐term outcomes with drug‐eluting stents versus bare‐metal stents in Sweden.N Engl J Med.2007;356:1009–1019.
- ,,, et al.Safety and efficacy of sirolimus‐ and paclitaxel‐eluting coronary stents.N Engl J Med.2007;356:998–1008.
- ,,,,.A pooled analysis of data comparing sirolimus‐eluting stents with bare‐metal stents.N Engl J Med.2007;356:989–997.
- .Preoperative assessment: guidelines and challenges.Acta Anaesthesiol ScandSuppl.1997;111:318–320.
- .Preoperative assessment of the ambulatory and same day admission patient.Wellcome Trends Anesthesiol.1991;9:3–11.
- ,,, et al.Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery.Circulation.1999;100:1043–1049.
- ,,,,.Risk of noncardiac surgery after coronary drug‐eluting stent implantation.Am J Cardiol.2006;98:1212–1213.
- ,,,,,.Coronary artery stenting and non‐cardiac surgery—a prospective outcome study.Br J Anaesth.2006;96:686–693.
- ,,.Double jeopardy: balance between bleeding and stent thrombosis with prolonged dual antiplatelet therapy after drug‐eluting stent implantation.Cardiovasc Revasc Med.2006;7:155–158.
Copyright © 2007 Society of Hospital Medicine
Trial of Safety Nets in Hospitalized Patients
Physical restraints, such as bed rails, Posey vests, and 2‐point and 4‐point soft or hard restraints, are commonly used in acute care hospitals to protect agitated patients from harming themselves or others.1 Yet restraints are viewed by patient advocates and health care practitioners as inhumane and overly restrictive. Furthermore, currently used physical restraints have been linked to minor injuries such as sores and abrasions, intensification of agitation, and even death.2, 3 Hospitals and nursing homes are therefore required to try alternative and less severe means of alleviating agitation and delirium among patients before resorting to physical restraints. However, despite a general dislike of restraints and stricter federal guidelines governing their use, the application of restraints is often unavoidable for some patients. It is estimated that between 4% and 25% of in‐patients will have physical restraints applied at some point during their hospital stay.4
Given these numbers, it is surprising that newer and potentially safer restraint systems have not been explored. Safe enclosures may provide health care facilities with an alternative option. This type of restraint consists of a nylon net canopy that safely surrounds both the patient and the mattress. The potential for safe enclosures to provide a safe, humane, and acceptable method of restraint for both hospital staff and patients warranted investigation. In addition, because this system does not restrict a person's ability to move within the enclosure, the many potential hazards of immobility associated with standard restraints may be reduced or eliminated. However, to our knowledge, there have been no reports published of randomized trials comparing standard restraints to newer and possibly safer restraint systems.
We report a randomized controlled trial that compared the use of safe enclosures with standard restraints among agitated, hospitalized patients. Compared with patients in standard restraints, we hypothesized that safe enclosures would: (1) be perceived as more acceptable and humane by family members, physicians, and nurses; (2) lead to improved health outcomes such as decreased duration of restraint use, decreased agitation, shorter length of stay, decreased need to administer medication to treat agitation, and fewer injuries to the patient.
METHODS
Design and Setting
This was a prospective, single‐center, randomized, controlled trial conducted at a community hospital in Connecticut.
Subjects
Male and female hospitalized patients at least 18 years old in the general medicine in‐patient services at a community hospital in Connecticut were assessed for eligibility to participate in this study if they had been put in restraints by the health care team independent of the study for one of these acute conditions: (1) delirium from any cause, including drug or alcohol withdrawal, or other medical conditions resulting in acute delirium; (2) confusional state from any cause; (3) agitation and disruptive behavior requiring restraints; (4) psychosis, hallucinations, or delusions requiring acute intervention (such as medication, restraints, or sitter); or (5) suicidality. Once in restraints, patients were screened for eligibility to participate in this study.
Exclusion criteria included: (1) need for acute respiratory or hemodynamic support or cardiac or septic shock; (2) terminal illness; (3) documented history of claustrophobia; (4) refusal by the family to give consent; (5) hospital stay < 24 hours; and (6) need for intravenous vasopressors, intubation, or ventilatory support. We also excluded patients who had been in restraints for more than 48 hours prior to potential study enrollment. Because safe enclosure would be a redundant system of restraint for patients requiring more than 1 limb in restraints, those patients were also excluded from the study. Figure 1 shows participation flow.
A number of screened patients were excluded for not being appropriate candidates for the safe enclosure. Of these, 20% required more than 1 limb in restraints, 13% required restraint not for agitation but for IV or catheter protection only, 10% were in critical care or on ventilators, and 13% were not appropriate for various other reasons including claustrophobia. The remaining excluded patients may have been eligible but either were preparing for discharge on screening (26%), were in restraints for more than 48 hours on screening (11%), stayed in the hospital less than 24 hours (4%), or had previously been a study participant (3%).
A stratified permuted block randomization was used to control for age (65 vs. >65 years) and sex (male vs. female) to ensure equal representation in both study arms. The study was approved by the institutional review board of the study site, and written informed consent was obtained by the study coordinator from patients' families. Because eligible patients suffered from acute delirium or agitation, most were not sufficiently cognizant to participate in the consent process. As a result, consent was largely obtained from patients' family members. Although the intent of this trial was to recruit 60 patients over an 18‐month period, the study was closed at 49 after 2 years because of slow recruitment and a lack of remaining funds.
Intervention
The safe enclosure, also known as a net bed or safety net, is an alternative to standard restraints. It consists of: (1) a metal frame that sits on the floor completely enclosing a standard hospital bed and (2) a nylon net canopy that encloses the patient and the mattress. We used the SOMA Safe Enclosure (Vivax Medical Corporation, Torrington, CT,
Procedures
Patients were enrolled in this study from April 2003 to February 2005. Once a patient had been placed in restraints by a physician, the nurse in charge alerted the study investigators by beeper of a potential subject, who was then screened based on the above eligibility criteria. We also actively screened restraint log sheets maintained by the nursing staff on most weekdays to monitor new patients who may have been put on restraints. Subjects were randomized to remain in standard restraints or be transferred to the safe enclosure. The randomization scheme was generated using software available at
Modification of the use of restraints is common in hospital settings, as patient needs fluctuate. In the 2 study groups, restraint crossover did occur at the discretion of the attending physician or nurse. Four patients in the safe enclosure group required either additional restraints or had the safe enclosure removed and alternate restraints applied. Similarly, 4 patients in the standard restraint group were either given the safe enclosure or had additional standard restraints applied. Under the principle of intent to treat, patients remained in their original randomized group for the purpose of analyses. This approach provided the most conservative analyses by keeping the sickest patients in the intervention group, thus guarding against type I error.
Measurements
Baseline data obtained at enrollment included: (1) demographic information, (2) clinical information, and (3) restraint information (ie, time of restraint application, clinical indication for use, type of restraint used, ordering physician, and alternative treatment tried before decision to start restraints).
Primary outcomes included: (1) perception survey scores of family members, physicians, and nurses regarding patient comfort, acceptability, and safety of the restraint device; and (2) patient agitation scores. A preplanned subgroup analysis separated nurses into 2 categories (primary and secondary). The admitting nurse was designated the primary nurse; all other nurses were considered secondary nurses. This analysis was performed to examine any differences in perception resulting from a nurse's level of patient involvement.
Family and provider perceptions were assessed with a self‐administered survey containing 11 items each measured on a 10‐point scale (from 1 = viewed negatively to 10 = viewed positively; maximum score 110 points). Surveyed physicians, nurses, and relatives were asked to rate: (1) patient comfort, (2) accessibility to patient, (3) ease of communication with patient, (4) how calm patient was, (5) perceived safety of patient, (6) patient's feeding convenience, (7) ease of bedpan use, (8) impact on recovery time; (9) how humane and ethical the restraint was, (10) recommendation for use on other patients, and (11) how demanding or difficult caring for the patient was.
Agitation was measured using 2 distinct methods: the Alcohol Withdrawal Assessment Form (AWAF)5 and the Agitated Behavior Scale (ABS).6, 7 Both techniques have been widely used to assess delirium of hospitalized patients. The AWAF measures agitation by analyzing key physiologic indicators such as blood pressure and heart rate on a 0‐ to 22‐point scale. The ABS is a 14‐item scale that measures specific behaviors related to agitation (eg, distractibility, uncooperativeness, and restlessness). Each behavior is rated on a 4‐point Likert scale (0‐3); total score ranges from 0 to 42. Each scale was completed once per 8‐hour shift by the nurse on duty.
Secondary outcomes consisted of total length of stay, duration of restraint use, time from application of restraints until time of discharge, and time from admission until time of application of restraints. Length of stay was calculated as the number of days from the time the patient was admitted to the time the patient was discharged. Time from admission until time of restraint application, duration of restraint use, and time from restraint application until discharge were assessed in minutes. These measurements were based on written restraint order forms and nursing progress reports. Hospital protocol regarding restraint requires hospital staff members to document the application, removal, and adjustment of restraints.
Additional outcomes measured included total amount of medications used to treat agitation and number of injuries incurred. Total amount of medication administered was determined with an equivalence system for different drugs used to treat agitation or delirium.811 Medications were separated into 4 groups: antianxiety medications, antidepressants, antipsychotics, and opioid analgesics. Total amount included both regularly administered and as‐needed dosages of medication. We identified injuries through reports of a subject's primary nurse and by review of medical records.
Data Analyses
Sample size was calculated using a 2‐sample t test formula based on the primary outcome. The study was designed to detect an absolute difference in points of 10% (total absolute score difference of 11 per survey or a total difference of 33). The 2‐sided alpha was initially set at 0.05 and the power at 80%, with an estimated standard deviation of 20. The alpha level was Bonferroni‐adjusted for up to 6 additional comparisons, with each significance level of 0.0071 (z = 2.70).
To assess differences in patient characteristics between the standard restraints and safe enclosure groups, we used the Student t test for continuous variables and Fisher's 2‐sided exact test for categorical variables. Differences in family and staff perceptions of the restraint mechanisms were measured using the Student t test with Satterthwaite's method for calculating variance. However, to account for questions marked not applicable by the responder, weighted scores, defined as the total score divided by the percentage of questions answered, were calculated.
Differences in agitation scores (ABS and AWAF) were analyzed using 2 strategies. First, the ABS and AWAF scores 24 hours after study enrollment were compared across groups using the Student t test with Satterthwaite's method for calculating variance. Then, separate comparisons of the ABS and AWAF scores 48 and 72 hours after enrollment were conducted using the Student t test with a pooled variance. Next, 2 longitudinal analyses were performed using a mixed‐effects (fixed and random) model. These analyses modeled change in the ABS or AWAF scores over (1) the first 3 days and (2) the first 6 days of hospitalization as a function of being restrained with the safe enclosure or being restrained with the hospital's standard restraint systems. For these comparisons, the model included not only the main effects of type of restraint and time, but also the interaction between type of restraint and time and the covariates sex, age, and initial ABS or AWAF score. For these models, a backward elimination procedure was undertaken using a significance level of = 0.05 in order to determine the most parsimonious model.
To determine if the total length of subject stay in the hospital was different between groups, the Student t test was used with Satterthwaite's method for calculating variance. Differences in time from admission until time of restraint application, duration of restraint use, and time from application of restraints until time of discharge were analyzed with the Student t test with pooled variances.
To compare the amount of medication used, equivalent dosage conversions were used for each of the 4 medication categories (antianxiety medications, antidepressants, antipsychotics, and opioid analgesics). To determine if the amounts of these 4 categories of medications differed between groups, the Student t test was used. Last, to determine if there was a difference in the number of patient injuries between groups, Fisher's 2‐sided exact test was used.
RESULTS
Study Population
Of the 49 subjects enrolled in the study, 20 were randomized to the safe enclosure and 29 to standard restraints. This imbalance was likely a result of the premature termination of the study, which in turn was a result of slow recruitment. Table 1 shows selected baseline characteristics of the enrolled subjects. There were no significant differences between the 2 groups in sex, age, patient diagnoses, reason for restraint, or type of medication. However, the subjects randomized to the safe enclosure were less likely to have hypertension than those randomized to standard restraints (36.8% vs. 72.4%, P = .019).
| Variable | All (n = 49) | SOMA Safe Enclosure (n = 20) | Standard restraint system (n = 29) |
|---|---|---|---|
| |||
| Sex (male) | 26 (53.1%) | 11 (55.0%) | 15 (51.7%) |
| Age (years) | 81.3 (13.1%) | 77.2 (15.6%) | 84.2 (10.3%) |
| Alzheimer's disease | 23 (47.9%) | 11 (57.9%) | 12 (41.4%) |
| Dementia | 3 (6.3%) | 2 (10.5%) | 1 (3.5%) |
| Coronary artery disease | 19 (39.6%) | 10 (52.6%) | 9 (31.0%) |
| Hypertension* | 28 (58.3%) | 7 (36.8%) | 21 (72.4%) |
| Congestive heart failure | 6 (12.5%) | 3 (15.8%) | 3 (10.3%) |
| Atrial fibrillation | 7 (14.6%) | 1 (5.3%) | 6 (20.7%) |
| Transient ischemic attacks/cerebral vascular accidents | 7 (14.6%) | 2 (10.5%) | 5 (17.2%) |
| Chronic obstructive pulmonary disease | 3 (6.3%) | 1 (5.3%) | 2 (6.9%) |
| Diabetes mellitus | 11 (22.9%) | 5 (26.3%) | 6 (20.7%) |
| Alcohol abuse | 7 (14.6%) | 2 (10.5%) | 5 (17.2%) |
| Drug abuse | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Where admitted | |||
| General medicine floor | 41 (83.7%) | 17 (85.0%) | 24 (82.8%) |
| Telemetry | 7 (14.3%) | 2 (10.0%) | 5 (17.2%) |
| ICU | 1 (2.0%) | 1 (5.0%) | 0 (0.0%) |
Primary Outcomes
The rates of response to the perception survey were: relatives/next of kin, 90%; physicians, 90%; primary nurses, 100%; and secondary nurses, 78%. Family members and physicians viewed the safe enclosure significantly more positively than they viewed standard restraints (P < .0001 and P < .0001, respectively; Table 2). There was a trend toward more positive perceptions of the safe enclosure among nurses; however, this trend did not achieve statistical significance (P = .0836). The subgroup analysis of nurses (primary vs. secondary) revealed that secondary nurses viewed the safe enclosure more positively (P = .023). Primary nurses tended to view the safe enclosure more positively than the standard restraints, but the association was not significant (P = .1313).
| Variable | SOMA Safe Enclosure (n = 20) | Standard restraint system (n = 29) | P value (observed power)* |
|---|---|---|---|
| |||
| 1. Perception Survey | |||
| Relative or next of kin | 86.84 | 68.47 | < .0001 (96%) |
| Physician | 83.38 | 65.76 | < .0001 (96%) |
| All nurses | 75.20 | 69.45 | .086 (40%) |
| Primary nurse | 75.45 | 69.72 | .1313 (31%) |
| Secondary nurse | 80.35 | 69.82 | .0230 (58%) |
| 2. Alcohol Withdrawal Assessment Form | |||
| 24 hours | 3.06 | 3.25 | .7972 (6%) |
| 48 hours | 3.23 | 3.40 | .8516 (5%) |
| 72 hours | 3.44 | 2.67 | .6163 (7%) |
| 3. Agitated Behavior Scale score | |||
| 24 hours | 11.93 | 8.33 | .2312 (27%) |
| 48 hours | 6.00 | 8.75 | .3743 (13%) |
| 72 hours | 7.83 | 7.11 | .7762 (6%) |
There were no statistically significant differences between the 2 randomized groups in ABS or AWAF scores 24, 48, or 72 hours after restraint application (Table 2). In addition, there were no statistically significant differences during the study between the groups in the rates of change in ABS or AWAF score . This was the case when looking at the first 3 days of hospitalization as well as the first 6 days (data not shown). All results were also calculated after adjusting for length of stay; this covariate did not affect any of the results.
Table 3 details the results for each perception survey question. Perceived comfort, calmness, and safety of patients were rated higher in the safe enclosure group by physicians, relatives, and all nurses. With the exception of perceived accessibility to patients, relatives rated the safe enclosure higher than standard restraints on all other perception measures. Table 4 illustrates the differences in the responses of primary and secondary nurse to each perception survey question. Primary and secondary nurses viewed the safety of the safe enclosure significantly more positively than they did the standard restraints.
| Variable | Relative/next of kin (n = 16, 28) | Primary and secondary nurses (n = 29, 29) | Physician (n = 29, 29) |
|---|---|---|---|
| |||
| Comfort | (8.78, 7.29).0033 | (7.98, 6.78).0194 | (8.40, 6.77).0003 |
| Accessibility | (8.28, 8.07).6486 | (7.68, 8.29).2236 | (8.35, 7.58).1056 |
| Communication | (9.11, 8.19).0214 | (8.29, 8.12).7333 | (8.40, 8.31).8469 |
| Calmness | (8.72, 6.29).0005 | (7.68, 6.53).0382 | (7.70, 5.92).0062 |
| Safety | (9.11, 6.74) < 0.001 | (8.53, 6.76).0024 | (8.60, 5.96).0002 |
| Feeding convenience | (8.50, 7.04).0164 | (7.11, 7.74).2327 | (8.25, 6.28).0047 |
| Ease of bedpan use | (7.91, 6.06).0224 | (7.36, 6.90).3977 | (6.82, 6.25).5376 |
| Impact on recovery time | (7.53, 6.07).0244 | (6.29, 5.66).3864 | (6.95, 6.43).4254 |
| Humane/ethical | (7.94, 5.50).0026 | (6.88, 6.31).4049 | (7.95, 5.96).0052 |
| Recommend for other patients | (8.71, 5.50).0002 | (7.15, 6.12).1395 | (8.05, 6.04).0037 |
| Ease of caring for patient | (8.44, 5.70) < .001 | (7.55, 6.38).0749 | (8.05, 6.25).0028 |
| Variable | Primary Nurse (n = 20, 29) (SOMA safe enclosure, control)* P value | Secondary Nurse (n = 12, 26) (SOMA safe enclosure, control)* P value |
|---|---|---|
| ||
| Comfort | (7.85, 6.62).0270 | (8.33, 6.81).0346 |
| Accessibility | (7.55, 8.21).2656 | (8.17, 8.16).9902 |
| Communication | (8.21, 8.34).8093 | (8.83, 7.88).0705 |
| Calmness | (7.70, 6.28).0153 | (7.83, 6.85).2001 |
| Safety | (8.55, 6.34).0012 | (8.67, 6.76).0435 |
| Feeding convenience | (7.37, 7.93).3861 | (7.60, 7.42).8282 |
| Ease of bedpan use | (7.53, 6.95).3945 | (7.00, 6.84).8602 |
| Impact on recovery time | (6.16, 5.39).3251 | (7.50, 6.50).2340 |
| Humane/ethical | (6.45, 6.66).7871 | (7.73, 5.96).0571 |
| Recommend for other patients | (6.70, 6.11).4553 | (8.42, 5.92).0075 |
| Ease of caring for patient | (7.65, 6.41).0860 | (7.83, 6.38).0565 |
Secondary Outcomes
There was a trend toward shorter total length of stay, time from admission until restraint application, duration of restraint use, and time from restraint application until discharge among subjects restrained by the safe enclosure compared with those restrained with standard restraints. However, these unadjusted differences were not statistically significant. We examined secondary outcomes after adjusting for 2 covariates, age and sex. Age but not sex affected the results. We found that subjects in the intervention group younger than 80 years of age had a shorter length of stay for 2 of the 4 related outcomes: time of admittance to time of discharge (P = .0199) and time of restraint to time of discharge (P = .0274). Time of admission to time of restraint application and duration of restraint did not differ between groups. The former outcome was not expected to differ between groups.
Additional Outcomes
There were no differences between groups in the amounts of 3 of the 4 types of medications used to treat agitation or delirium (ie, antianxiety medications, antipsychotic medications, opioid analgesic medications). The proportion of patients on these medications did not differ by group (P = .59). Only 5% of patients in standard restraints were on antidepressants, and about 5% were on opioids in each group. There was only 1 minor patient injury recorded during the study. This minor abrasion was to a patient assigned to the standard restraint group. No injuries were reported in the safe enclosure group.
DISCUSSION
We have demonstrated that the SOMA Safe Enclosure may be a more acceptable alternative to the restraints currently in use. Our results show that the safe enclosure was rated as more acceptable by family members, physicians, and secondary nurses in our composite perception scores. The results from the primary nurses did not show a significant difference between the 2 groups. An analysis of the individual perception variables found that family members viewed the safe enclosure as more acceptable for 10 of the 11 variables examined. Furthermore, in this small‐scale study, safe enclosures appeared to be safe, as there were no injuries reported in the intervention group. As stated above, there was 1 minor injury reported in the standard restraint groups.
Restraints are commonly used to protect agitated hospitalized patients from harming themselves or others. Despite the significant reluctance of hospital staff members to use restraints, they continue to be necessary in certain situations. Factors such as a general nursing shortage and the expense required to allocate nursing or other ancillary health care workers as sitters contribute to the use of restraints. Therefore, it is reasonable to conclude that restraint use in some form or fashion will continue into the foreseeable future. There are no clear estimates of the prevalence of restraint use in acute care hospitals. A chart review study from Canada reported physical restraints in about 7.7% of in‐patients.12 Other studies have reported the use of restraints on patients in the range of 4%‐25%.2 Given the prevalence of restraint use in acute care hospitals, surprisingly little innovative research has been undertaken to develop more effective and humane systems of restraint. Furthermore, no research has examined how restraint use may affect important clinical outcomes such as length of stay. To our knowledge, this is the first clinical trial to compare currently used restraints to a newer method of restraint using the SOMA Safe Enclosure.
The idea that restraint use can lead to further agitation is not supported by our data. We observed a decrease in agitated behavior scale scores from 9.6 to 7.4 from the 24‐ and to the 72‐hour assessments; however, these results were not significant and appeared to be more dramatic for the safe enclosure group because of higher baseline levels. Our adjusted analyses of length of stayrelated outcomes indicated an association with age. Total length of stay and time from restraint application until discharge were significantly reduced for those subjects younger than 80 years of age in the safe enclosure group. The basis for this finding is not entirely clear. It may be a chance finding, or there may have been a complex combination of factors at work.
There was a reduction in overall length of stay by 1.5 days among those in the safe enclosure group when compared with the standard restraint group. Similarly, total duration of restraint use of the safe enclosure group was 551 minutes (9 hours) shorter. Although these findings were not significant, they warrant further investigation in a larger trial. If safe enclosure use truly reduces length of stay and duration of restraint use, it is an important finding, for it could translate into meaningful cost savings for acute care hospitals. It is possible, however, that any potential cost savings could be tempered by the additional time required to set up the enclosure. Ethically, if restraints are to be used, their use should be minimized, and in that sense, safe enclosures may help acute care hospitals achieve this goal more effectively.
Limitations of this trial include its small sample size and inadequate power to determine certain outcomes. Although we saw encouraging trends in several outcomes, they failed to reach statistical significance because of the limited power. For instance, the observed power for total length of stay difference was only 17%. It is conceivable that a larger trial powered specifically for length of stayrelated outcomes may show significant results. Because subjects in this study were patients in a single midsize community teaching hospital, the results may not be generalizable to patients in, for example, tertiary‐care centers or nursing homes. However, these results may apply to a large proportion of patients in the United States, as most are treated in community hospitals. We found that many patients required 2 wrist restraints in order to protect IV lines, and this resulted in exclusion of a large proportion of potential subjects. Therefore, safe enclosures may not be appropriate for all agitated patients. They may be an ideal method of restraining patients who are not at risk of pulling out their IV line or catheters but require restraints for other reasons. This could include patients in nursing homes or rehabilitation centers.
It is also important to discuss the issue of practitioner acceptability of a newer method of restraint in acute care hospitals. As expected, we found the nursing staff was originally reluctant to use the safe enclosure, even as part of a trial. This may have been because of fear of change and having a high level of comfort with the restraint systems already in use. The setup of safe enclosures can take 10‐15 minutes, whereas the use of 2‐point soft restraints or Posey vests can be accomplished in as little as a minute. However, we found that after initial use of the safe enclosure, resistance among nurses declined. In fact, in our hospital, nurses began using safe enclosures for confused and agitated patients not enrolled in the study in order to prevent wandering and falls at night. Another difficulty reported by the nursing staff was feeling somewhat limited in their access to patients by a safe enclosure. Nurses had to open a zipped flap to access the patient to administer medication or provide food. Health care providers must remember to close the flap to avoid potential falls.
In summary, safe enclosures seem to be a safe and more acceptable alternative to the restraints currently in use in acute care hospitals. These findings should be replicated in a larger trial.
- ,,,,.Prevalence and patterns of physical restraint use in the acute care setting.J Nurs Adm.1998;28(11):19–24.
- ,Strumpf. Myths about Elder Restraint.J Nurs Scholarsh.1990;22(2):124–128.
- , et al.Deadly restraint: a Hartford Courant investigative report.Hartford Courant1998; October 11‐15.
- ,,,,,, et al.Jt Comm J Qual Improv2001;27:605–618.
- ,,,,.Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale.Br J Addict.1989;84:1353–1357.
- ,.Development of an agitated behavior rating scale for discrete temporal observations.Nursing Meas1993;1:115–124.
- ,,,.Reliability of the Agitated Behavior Scale.J Head Trauma Rehabil.1999;14:91–96.
- University of Newcastle. The Ashton Manual. Available at: http://www.benzo.org.uk/manual/bzcha01.htm. Accessed November 15,2006.
- Postgraduate Medicine Online. Sedation and analgesia in intensive care. Available at: http://www.postgradmed.com/issues/2002/02_02/blanchard.htm. Accessed November 15,2006.
- Anti‐psychotic Comparison chart. Available at: http://meds.queensu.ca/∼clpsych/orientation/Antipsychotics%20Comparison%20Chart.pdf. Accessed November 15,2006.
- Anti‐depressant comparison chart. Available at: http://meds.queensu.ca/∼clpsych/orientation/Antidepressant%20comparison%20Chart.pdf. Accessed November 15,2006.
- ,.Use of physical and chemical restraints in medical teaching units.Can Med Assoc J.2000;162:339–340.
Physical restraints, such as bed rails, Posey vests, and 2‐point and 4‐point soft or hard restraints, are commonly used in acute care hospitals to protect agitated patients from harming themselves or others.1 Yet restraints are viewed by patient advocates and health care practitioners as inhumane and overly restrictive. Furthermore, currently used physical restraints have been linked to minor injuries such as sores and abrasions, intensification of agitation, and even death.2, 3 Hospitals and nursing homes are therefore required to try alternative and less severe means of alleviating agitation and delirium among patients before resorting to physical restraints. However, despite a general dislike of restraints and stricter federal guidelines governing their use, the application of restraints is often unavoidable for some patients. It is estimated that between 4% and 25% of in‐patients will have physical restraints applied at some point during their hospital stay.4
Given these numbers, it is surprising that newer and potentially safer restraint systems have not been explored. Safe enclosures may provide health care facilities with an alternative option. This type of restraint consists of a nylon net canopy that safely surrounds both the patient and the mattress. The potential for safe enclosures to provide a safe, humane, and acceptable method of restraint for both hospital staff and patients warranted investigation. In addition, because this system does not restrict a person's ability to move within the enclosure, the many potential hazards of immobility associated with standard restraints may be reduced or eliminated. However, to our knowledge, there have been no reports published of randomized trials comparing standard restraints to newer and possibly safer restraint systems.
We report a randomized controlled trial that compared the use of safe enclosures with standard restraints among agitated, hospitalized patients. Compared with patients in standard restraints, we hypothesized that safe enclosures would: (1) be perceived as more acceptable and humane by family members, physicians, and nurses; (2) lead to improved health outcomes such as decreased duration of restraint use, decreased agitation, shorter length of stay, decreased need to administer medication to treat agitation, and fewer injuries to the patient.
METHODS
Design and Setting
This was a prospective, single‐center, randomized, controlled trial conducted at a community hospital in Connecticut.
Subjects
Male and female hospitalized patients at least 18 years old in the general medicine in‐patient services at a community hospital in Connecticut were assessed for eligibility to participate in this study if they had been put in restraints by the health care team independent of the study for one of these acute conditions: (1) delirium from any cause, including drug or alcohol withdrawal, or other medical conditions resulting in acute delirium; (2) confusional state from any cause; (3) agitation and disruptive behavior requiring restraints; (4) psychosis, hallucinations, or delusions requiring acute intervention (such as medication, restraints, or sitter); or (5) suicidality. Once in restraints, patients were screened for eligibility to participate in this study.
Exclusion criteria included: (1) need for acute respiratory or hemodynamic support or cardiac or septic shock; (2) terminal illness; (3) documented history of claustrophobia; (4) refusal by the family to give consent; (5) hospital stay < 24 hours; and (6) need for intravenous vasopressors, intubation, or ventilatory support. We also excluded patients who had been in restraints for more than 48 hours prior to potential study enrollment. Because safe enclosure would be a redundant system of restraint for patients requiring more than 1 limb in restraints, those patients were also excluded from the study. Figure 1 shows participation flow.

A number of screened patients were excluded for not being appropriate candidates for the safe enclosure. Of these, 20% required more than 1 limb in restraints, 13% required restraint not for agitation but for IV or catheter protection only, 10% were in critical care or on ventilators, and 13% were not appropriate for various other reasons including claustrophobia. The remaining excluded patients may have been eligible but either were preparing for discharge on screening (26%), were in restraints for more than 48 hours on screening (11%), stayed in the hospital less than 24 hours (4%), or had previously been a study participant (3%).
A stratified permuted block randomization was used to control for age (65 vs. >65 years) and sex (male vs. female) to ensure equal representation in both study arms. The study was approved by the institutional review board of the study site, and written informed consent was obtained by the study coordinator from patients' families. Because eligible patients suffered from acute delirium or agitation, most were not sufficiently cognizant to participate in the consent process. As a result, consent was largely obtained from patients' family members. Although the intent of this trial was to recruit 60 patients over an 18‐month period, the study was closed at 49 after 2 years because of slow recruitment and a lack of remaining funds.
Intervention
The safe enclosure, also known as a net bed or safety net, is an alternative to standard restraints. It consists of: (1) a metal frame that sits on the floor completely enclosing a standard hospital bed and (2) a nylon net canopy that encloses the patient and the mattress. We used the SOMA Safe Enclosure (Vivax Medical Corporation, Torrington, CT,
Procedures
Patients were enrolled in this study from April 2003 to February 2005. Once a patient had been placed in restraints by a physician, the nurse in charge alerted the study investigators by beeper of a potential subject, who was then screened based on the above eligibility criteria. We also actively screened restraint log sheets maintained by the nursing staff on most weekdays to monitor new patients who may have been put on restraints. Subjects were randomized to remain in standard restraints or be transferred to the safe enclosure. The randomization scheme was generated using software available at
Modification of the use of restraints is common in hospital settings, as patient needs fluctuate. In the 2 study groups, restraint crossover did occur at the discretion of the attending physician or nurse. Four patients in the safe enclosure group required either additional restraints or had the safe enclosure removed and alternate restraints applied. Similarly, 4 patients in the standard restraint group were either given the safe enclosure or had additional standard restraints applied. Under the principle of intent to treat, patients remained in their original randomized group for the purpose of analyses. This approach provided the most conservative analyses by keeping the sickest patients in the intervention group, thus guarding against type I error.
Measurements
Baseline data obtained at enrollment included: (1) demographic information, (2) clinical information, and (3) restraint information (ie, time of restraint application, clinical indication for use, type of restraint used, ordering physician, and alternative treatment tried before decision to start restraints).
Primary outcomes included: (1) perception survey scores of family members, physicians, and nurses regarding patient comfort, acceptability, and safety of the restraint device; and (2) patient agitation scores. A preplanned subgroup analysis separated nurses into 2 categories (primary and secondary). The admitting nurse was designated the primary nurse; all other nurses were considered secondary nurses. This analysis was performed to examine any differences in perception resulting from a nurse's level of patient involvement.
Family and provider perceptions were assessed with a self‐administered survey containing 11 items each measured on a 10‐point scale (from 1 = viewed negatively to 10 = viewed positively; maximum score 110 points). Surveyed physicians, nurses, and relatives were asked to rate: (1) patient comfort, (2) accessibility to patient, (3) ease of communication with patient, (4) how calm patient was, (5) perceived safety of patient, (6) patient's feeding convenience, (7) ease of bedpan use, (8) impact on recovery time; (9) how humane and ethical the restraint was, (10) recommendation for use on other patients, and (11) how demanding or difficult caring for the patient was.
Agitation was measured using 2 distinct methods: the Alcohol Withdrawal Assessment Form (AWAF)5 and the Agitated Behavior Scale (ABS).6, 7 Both techniques have been widely used to assess delirium of hospitalized patients. The AWAF measures agitation by analyzing key physiologic indicators such as blood pressure and heart rate on a 0‐ to 22‐point scale. The ABS is a 14‐item scale that measures specific behaviors related to agitation (eg, distractibility, uncooperativeness, and restlessness). Each behavior is rated on a 4‐point Likert scale (0‐3); total score ranges from 0 to 42. Each scale was completed once per 8‐hour shift by the nurse on duty.
Secondary outcomes consisted of total length of stay, duration of restraint use, time from application of restraints until time of discharge, and time from admission until time of application of restraints. Length of stay was calculated as the number of days from the time the patient was admitted to the time the patient was discharged. Time from admission until time of restraint application, duration of restraint use, and time from restraint application until discharge were assessed in minutes. These measurements were based on written restraint order forms and nursing progress reports. Hospital protocol regarding restraint requires hospital staff members to document the application, removal, and adjustment of restraints.
Additional outcomes measured included total amount of medications used to treat agitation and number of injuries incurred. Total amount of medication administered was determined with an equivalence system for different drugs used to treat agitation or delirium.811 Medications were separated into 4 groups: antianxiety medications, antidepressants, antipsychotics, and opioid analgesics. Total amount included both regularly administered and as‐needed dosages of medication. We identified injuries through reports of a subject's primary nurse and by review of medical records.
Data Analyses
Sample size was calculated using a 2‐sample t test formula based on the primary outcome. The study was designed to detect an absolute difference in points of 10% (total absolute score difference of 11 per survey or a total difference of 33). The 2‐sided alpha was initially set at 0.05 and the power at 80%, with an estimated standard deviation of 20. The alpha level was Bonferroni‐adjusted for up to 6 additional comparisons, with each significance level of 0.0071 (z = 2.70).
To assess differences in patient characteristics between the standard restraints and safe enclosure groups, we used the Student t test for continuous variables and Fisher's 2‐sided exact test for categorical variables. Differences in family and staff perceptions of the restraint mechanisms were measured using the Student t test with Satterthwaite's method for calculating variance. However, to account for questions marked not applicable by the responder, weighted scores, defined as the total score divided by the percentage of questions answered, were calculated.
Differences in agitation scores (ABS and AWAF) were analyzed using 2 strategies. First, the ABS and AWAF scores 24 hours after study enrollment were compared across groups using the Student t test with Satterthwaite's method for calculating variance. Then, separate comparisons of the ABS and AWAF scores 48 and 72 hours after enrollment were conducted using the Student t test with a pooled variance. Next, 2 longitudinal analyses were performed using a mixed‐effects (fixed and random) model. These analyses modeled change in the ABS or AWAF scores over (1) the first 3 days and (2) the first 6 days of hospitalization as a function of being restrained with the safe enclosure or being restrained with the hospital's standard restraint systems. For these comparisons, the model included not only the main effects of type of restraint and time, but also the interaction between type of restraint and time and the covariates sex, age, and initial ABS or AWAF score. For these models, a backward elimination procedure was undertaken using a significance level of = 0.05 in order to determine the most parsimonious model.
To determine if the total length of subject stay in the hospital was different between groups, the Student t test was used with Satterthwaite's method for calculating variance. Differences in time from admission until time of restraint application, duration of restraint use, and time from application of restraints until time of discharge were analyzed with the Student t test with pooled variances.
To compare the amount of medication used, equivalent dosage conversions were used for each of the 4 medication categories (antianxiety medications, antidepressants, antipsychotics, and opioid analgesics). To determine if the amounts of these 4 categories of medications differed between groups, the Student t test was used. Last, to determine if there was a difference in the number of patient injuries between groups, Fisher's 2‐sided exact test was used.
RESULTS
Study Population
Of the 49 subjects enrolled in the study, 20 were randomized to the safe enclosure and 29 to standard restraints. This imbalance was likely a result of the premature termination of the study, which in turn was a result of slow recruitment. Table 1 shows selected baseline characteristics of the enrolled subjects. There were no significant differences between the 2 groups in sex, age, patient diagnoses, reason for restraint, or type of medication. However, the subjects randomized to the safe enclosure were less likely to have hypertension than those randomized to standard restraints (36.8% vs. 72.4%, P = .019).
| Variable | All (n = 49) | SOMA Safe Enclosure (n = 20) | Standard restraint system (n = 29) |
|---|---|---|---|
| |||
| Sex (male) | 26 (53.1%) | 11 (55.0%) | 15 (51.7%) |
| Age (years) | 81.3 (13.1%) | 77.2 (15.6%) | 84.2 (10.3%) |
| Alzheimer's disease | 23 (47.9%) | 11 (57.9%) | 12 (41.4%) |
| Dementia | 3 (6.3%) | 2 (10.5%) | 1 (3.5%) |
| Coronary artery disease | 19 (39.6%) | 10 (52.6%) | 9 (31.0%) |
| Hypertension* | 28 (58.3%) | 7 (36.8%) | 21 (72.4%) |
| Congestive heart failure | 6 (12.5%) | 3 (15.8%) | 3 (10.3%) |
| Atrial fibrillation | 7 (14.6%) | 1 (5.3%) | 6 (20.7%) |
| Transient ischemic attacks/cerebral vascular accidents | 7 (14.6%) | 2 (10.5%) | 5 (17.2%) |
| Chronic obstructive pulmonary disease | 3 (6.3%) | 1 (5.3%) | 2 (6.9%) |
| Diabetes mellitus | 11 (22.9%) | 5 (26.3%) | 6 (20.7%) |
| Alcohol abuse | 7 (14.6%) | 2 (10.5%) | 5 (17.2%) |
| Drug abuse | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Where admitted | |||
| General medicine floor | 41 (83.7%) | 17 (85.0%) | 24 (82.8%) |
| Telemetry | 7 (14.3%) | 2 (10.0%) | 5 (17.2%) |
| ICU | 1 (2.0%) | 1 (5.0%) | 0 (0.0%) |
Primary Outcomes
The rates of response to the perception survey were: relatives/next of kin, 90%; physicians, 90%; primary nurses, 100%; and secondary nurses, 78%. Family members and physicians viewed the safe enclosure significantly more positively than they viewed standard restraints (P < .0001 and P < .0001, respectively; Table 2). There was a trend toward more positive perceptions of the safe enclosure among nurses; however, this trend did not achieve statistical significance (P = .0836). The subgroup analysis of nurses (primary vs. secondary) revealed that secondary nurses viewed the safe enclosure more positively (P = .023). Primary nurses tended to view the safe enclosure more positively than the standard restraints, but the association was not significant (P = .1313).
| Variable | SOMA Safe Enclosure (n = 20) | Standard restraint system (n = 29) | P value (observed power)* |
|---|---|---|---|
| |||
| 1. Perception Survey | |||
| Relative or next of kin | 86.84 | 68.47 | < .0001 (96%) |
| Physician | 83.38 | 65.76 | < .0001 (96%) |
| All nurses | 75.20 | 69.45 | .086 (40%) |
| Primary nurse | 75.45 | 69.72 | .1313 (31%) |
| Secondary nurse | 80.35 | 69.82 | .0230 (58%) |
| 2. Alcohol Withdrawal Assessment Form | |||
| 24 hours | 3.06 | 3.25 | .7972 (6%) |
| 48 hours | 3.23 | 3.40 | .8516 (5%) |
| 72 hours | 3.44 | 2.67 | .6163 (7%) |
| 3. Agitated Behavior Scale score | |||
| 24 hours | 11.93 | 8.33 | .2312 (27%) |
| 48 hours | 6.00 | 8.75 | .3743 (13%) |
| 72 hours | 7.83 | 7.11 | .7762 (6%) |
There were no statistically significant differences between the 2 randomized groups in ABS or AWAF scores 24, 48, or 72 hours after restraint application (Table 2). In addition, there were no statistically significant differences during the study between the groups in the rates of change in ABS or AWAF score . This was the case when looking at the first 3 days of hospitalization as well as the first 6 days (data not shown). All results were also calculated after adjusting for length of stay; this covariate did not affect any of the results.
Table 3 details the results for each perception survey question. Perceived comfort, calmness, and safety of patients were rated higher in the safe enclosure group by physicians, relatives, and all nurses. With the exception of perceived accessibility to patients, relatives rated the safe enclosure higher than standard restraints on all other perception measures. Table 4 illustrates the differences in the responses of primary and secondary nurse to each perception survey question. Primary and secondary nurses viewed the safety of the safe enclosure significantly more positively than they did the standard restraints.
| Variable | Relative/next of kin (n = 16, 28) | Primary and secondary nurses (n = 29, 29) | Physician (n = 29, 29) |
|---|---|---|---|
| |||
| Comfort | (8.78, 7.29).0033 | (7.98, 6.78).0194 | (8.40, 6.77).0003 |
| Accessibility | (8.28, 8.07).6486 | (7.68, 8.29).2236 | (8.35, 7.58).1056 |
| Communication | (9.11, 8.19).0214 | (8.29, 8.12).7333 | (8.40, 8.31).8469 |
| Calmness | (8.72, 6.29).0005 | (7.68, 6.53).0382 | (7.70, 5.92).0062 |
| Safety | (9.11, 6.74) < 0.001 | (8.53, 6.76).0024 | (8.60, 5.96).0002 |
| Feeding convenience | (8.50, 7.04).0164 | (7.11, 7.74).2327 | (8.25, 6.28).0047 |
| Ease of bedpan use | (7.91, 6.06).0224 | (7.36, 6.90).3977 | (6.82, 6.25).5376 |
| Impact on recovery time | (7.53, 6.07).0244 | (6.29, 5.66).3864 | (6.95, 6.43).4254 |
| Humane/ethical | (7.94, 5.50).0026 | (6.88, 6.31).4049 | (7.95, 5.96).0052 |
| Recommend for other patients | (8.71, 5.50).0002 | (7.15, 6.12).1395 | (8.05, 6.04).0037 |
| Ease of caring for patient | (8.44, 5.70) < .001 | (7.55, 6.38).0749 | (8.05, 6.25).0028 |
| Variable | Primary Nurse (n = 20, 29) (SOMA safe enclosure, control)* P value | Secondary Nurse (n = 12, 26) (SOMA safe enclosure, control)* P value |
|---|---|---|
| ||
| Comfort | (7.85, 6.62).0270 | (8.33, 6.81).0346 |
| Accessibility | (7.55, 8.21).2656 | (8.17, 8.16).9902 |
| Communication | (8.21, 8.34).8093 | (8.83, 7.88).0705 |
| Calmness | (7.70, 6.28).0153 | (7.83, 6.85).2001 |
| Safety | (8.55, 6.34).0012 | (8.67, 6.76).0435 |
| Feeding convenience | (7.37, 7.93).3861 | (7.60, 7.42).8282 |
| Ease of bedpan use | (7.53, 6.95).3945 | (7.00, 6.84).8602 |
| Impact on recovery time | (6.16, 5.39).3251 | (7.50, 6.50).2340 |
| Humane/ethical | (6.45, 6.66).7871 | (7.73, 5.96).0571 |
| Recommend for other patients | (6.70, 6.11).4553 | (8.42, 5.92).0075 |
| Ease of caring for patient | (7.65, 6.41).0860 | (7.83, 6.38).0565 |
Secondary Outcomes
There was a trend toward shorter total length of stay, time from admission until restraint application, duration of restraint use, and time from restraint application until discharge among subjects restrained by the safe enclosure compared with those restrained with standard restraints. However, these unadjusted differences were not statistically significant. We examined secondary outcomes after adjusting for 2 covariates, age and sex. Age but not sex affected the results. We found that subjects in the intervention group younger than 80 years of age had a shorter length of stay for 2 of the 4 related outcomes: time of admittance to time of discharge (P = .0199) and time of restraint to time of discharge (P = .0274). Time of admission to time of restraint application and duration of restraint did not differ between groups. The former outcome was not expected to differ between groups.
Additional Outcomes
There were no differences between groups in the amounts of 3 of the 4 types of medications used to treat agitation or delirium (ie, antianxiety medications, antipsychotic medications, opioid analgesic medications). The proportion of patients on these medications did not differ by group (P = .59). Only 5% of patients in standard restraints were on antidepressants, and about 5% were on opioids in each group. There was only 1 minor patient injury recorded during the study. This minor abrasion was to a patient assigned to the standard restraint group. No injuries were reported in the safe enclosure group.
DISCUSSION
We have demonstrated that the SOMA Safe Enclosure may be a more acceptable alternative to the restraints currently in use. Our results show that the safe enclosure was rated as more acceptable by family members, physicians, and secondary nurses in our composite perception scores. The results from the primary nurses did not show a significant difference between the 2 groups. An analysis of the individual perception variables found that family members viewed the safe enclosure as more acceptable for 10 of the 11 variables examined. Furthermore, in this small‐scale study, safe enclosures appeared to be safe, as there were no injuries reported in the intervention group. As stated above, there was 1 minor injury reported in the standard restraint groups.
Restraints are commonly used to protect agitated hospitalized patients from harming themselves or others. Despite the significant reluctance of hospital staff members to use restraints, they continue to be necessary in certain situations. Factors such as a general nursing shortage and the expense required to allocate nursing or other ancillary health care workers as sitters contribute to the use of restraints. Therefore, it is reasonable to conclude that restraint use in some form or fashion will continue into the foreseeable future. There are no clear estimates of the prevalence of restraint use in acute care hospitals. A chart review study from Canada reported physical restraints in about 7.7% of in‐patients.12 Other studies have reported the use of restraints on patients in the range of 4%‐25%.2 Given the prevalence of restraint use in acute care hospitals, surprisingly little innovative research has been undertaken to develop more effective and humane systems of restraint. Furthermore, no research has examined how restraint use may affect important clinical outcomes such as length of stay. To our knowledge, this is the first clinical trial to compare currently used restraints to a newer method of restraint using the SOMA Safe Enclosure.
The idea that restraint use can lead to further agitation is not supported by our data. We observed a decrease in agitated behavior scale scores from 9.6 to 7.4 from the 24‐ and to the 72‐hour assessments; however, these results were not significant and appeared to be more dramatic for the safe enclosure group because of higher baseline levels. Our adjusted analyses of length of stayrelated outcomes indicated an association with age. Total length of stay and time from restraint application until discharge were significantly reduced for those subjects younger than 80 years of age in the safe enclosure group. The basis for this finding is not entirely clear. It may be a chance finding, or there may have been a complex combination of factors at work.
There was a reduction in overall length of stay by 1.5 days among those in the safe enclosure group when compared with the standard restraint group. Similarly, total duration of restraint use of the safe enclosure group was 551 minutes (9 hours) shorter. Although these findings were not significant, they warrant further investigation in a larger trial. If safe enclosure use truly reduces length of stay and duration of restraint use, it is an important finding, for it could translate into meaningful cost savings for acute care hospitals. It is possible, however, that any potential cost savings could be tempered by the additional time required to set up the enclosure. Ethically, if restraints are to be used, their use should be minimized, and in that sense, safe enclosures may help acute care hospitals achieve this goal more effectively.
Limitations of this trial include its small sample size and inadequate power to determine certain outcomes. Although we saw encouraging trends in several outcomes, they failed to reach statistical significance because of the limited power. For instance, the observed power for total length of stay difference was only 17%. It is conceivable that a larger trial powered specifically for length of stayrelated outcomes may show significant results. Because subjects in this study were patients in a single midsize community teaching hospital, the results may not be generalizable to patients in, for example, tertiary‐care centers or nursing homes. However, these results may apply to a large proportion of patients in the United States, as most are treated in community hospitals. We found that many patients required 2 wrist restraints in order to protect IV lines, and this resulted in exclusion of a large proportion of potential subjects. Therefore, safe enclosures may not be appropriate for all agitated patients. They may be an ideal method of restraining patients who are not at risk of pulling out their IV line or catheters but require restraints for other reasons. This could include patients in nursing homes or rehabilitation centers.
It is also important to discuss the issue of practitioner acceptability of a newer method of restraint in acute care hospitals. As expected, we found the nursing staff was originally reluctant to use the safe enclosure, even as part of a trial. This may have been because of fear of change and having a high level of comfort with the restraint systems already in use. The setup of safe enclosures can take 10‐15 minutes, whereas the use of 2‐point soft restraints or Posey vests can be accomplished in as little as a minute. However, we found that after initial use of the safe enclosure, resistance among nurses declined. In fact, in our hospital, nurses began using safe enclosures for confused and agitated patients not enrolled in the study in order to prevent wandering and falls at night. Another difficulty reported by the nursing staff was feeling somewhat limited in their access to patients by a safe enclosure. Nurses had to open a zipped flap to access the patient to administer medication or provide food. Health care providers must remember to close the flap to avoid potential falls.
In summary, safe enclosures seem to be a safe and more acceptable alternative to the restraints currently in use in acute care hospitals. These findings should be replicated in a larger trial.
Physical restraints, such as bed rails, Posey vests, and 2‐point and 4‐point soft or hard restraints, are commonly used in acute care hospitals to protect agitated patients from harming themselves or others.1 Yet restraints are viewed by patient advocates and health care practitioners as inhumane and overly restrictive. Furthermore, currently used physical restraints have been linked to minor injuries such as sores and abrasions, intensification of agitation, and even death.2, 3 Hospitals and nursing homes are therefore required to try alternative and less severe means of alleviating agitation and delirium among patients before resorting to physical restraints. However, despite a general dislike of restraints and stricter federal guidelines governing their use, the application of restraints is often unavoidable for some patients. It is estimated that between 4% and 25% of in‐patients will have physical restraints applied at some point during their hospital stay.4
Given these numbers, it is surprising that newer and potentially safer restraint systems have not been explored. Safe enclosures may provide health care facilities with an alternative option. This type of restraint consists of a nylon net canopy that safely surrounds both the patient and the mattress. The potential for safe enclosures to provide a safe, humane, and acceptable method of restraint for both hospital staff and patients warranted investigation. In addition, because this system does not restrict a person's ability to move within the enclosure, the many potential hazards of immobility associated with standard restraints may be reduced or eliminated. However, to our knowledge, there have been no reports published of randomized trials comparing standard restraints to newer and possibly safer restraint systems.
We report a randomized controlled trial that compared the use of safe enclosures with standard restraints among agitated, hospitalized patients. Compared with patients in standard restraints, we hypothesized that safe enclosures would: (1) be perceived as more acceptable and humane by family members, physicians, and nurses; (2) lead to improved health outcomes such as decreased duration of restraint use, decreased agitation, shorter length of stay, decreased need to administer medication to treat agitation, and fewer injuries to the patient.
METHODS
Design and Setting
This was a prospective, single‐center, randomized, controlled trial conducted at a community hospital in Connecticut.
Subjects
Male and female hospitalized patients at least 18 years old in the general medicine in‐patient services at a community hospital in Connecticut were assessed for eligibility to participate in this study if they had been put in restraints by the health care team independent of the study for one of these acute conditions: (1) delirium from any cause, including drug or alcohol withdrawal, or other medical conditions resulting in acute delirium; (2) confusional state from any cause; (3) agitation and disruptive behavior requiring restraints; (4) psychosis, hallucinations, or delusions requiring acute intervention (such as medication, restraints, or sitter); or (5) suicidality. Once in restraints, patients were screened for eligibility to participate in this study.
Exclusion criteria included: (1) need for acute respiratory or hemodynamic support or cardiac or septic shock; (2) terminal illness; (3) documented history of claustrophobia; (4) refusal by the family to give consent; (5) hospital stay < 24 hours; and (6) need for intravenous vasopressors, intubation, or ventilatory support. We also excluded patients who had been in restraints for more than 48 hours prior to potential study enrollment. Because safe enclosure would be a redundant system of restraint for patients requiring more than 1 limb in restraints, those patients were also excluded from the study. Figure 1 shows participation flow.

A number of screened patients were excluded for not being appropriate candidates for the safe enclosure. Of these, 20% required more than 1 limb in restraints, 13% required restraint not for agitation but for IV or catheter protection only, 10% were in critical care or on ventilators, and 13% were not appropriate for various other reasons including claustrophobia. The remaining excluded patients may have been eligible but either were preparing for discharge on screening (26%), were in restraints for more than 48 hours on screening (11%), stayed in the hospital less than 24 hours (4%), or had previously been a study participant (3%).
A stratified permuted block randomization was used to control for age (65 vs. >65 years) and sex (male vs. female) to ensure equal representation in both study arms. The study was approved by the institutional review board of the study site, and written informed consent was obtained by the study coordinator from patients' families. Because eligible patients suffered from acute delirium or agitation, most were not sufficiently cognizant to participate in the consent process. As a result, consent was largely obtained from patients' family members. Although the intent of this trial was to recruit 60 patients over an 18‐month period, the study was closed at 49 after 2 years because of slow recruitment and a lack of remaining funds.
Intervention
The safe enclosure, also known as a net bed or safety net, is an alternative to standard restraints. It consists of: (1) a metal frame that sits on the floor completely enclosing a standard hospital bed and (2) a nylon net canopy that encloses the patient and the mattress. We used the SOMA Safe Enclosure (Vivax Medical Corporation, Torrington, CT,
Procedures
Patients were enrolled in this study from April 2003 to February 2005. Once a patient had been placed in restraints by a physician, the nurse in charge alerted the study investigators by beeper of a potential subject, who was then screened based on the above eligibility criteria. We also actively screened restraint log sheets maintained by the nursing staff on most weekdays to monitor new patients who may have been put on restraints. Subjects were randomized to remain in standard restraints or be transferred to the safe enclosure. The randomization scheme was generated using software available at
Modification of the use of restraints is common in hospital settings, as patient needs fluctuate. In the 2 study groups, restraint crossover did occur at the discretion of the attending physician or nurse. Four patients in the safe enclosure group required either additional restraints or had the safe enclosure removed and alternate restraints applied. Similarly, 4 patients in the standard restraint group were either given the safe enclosure or had additional standard restraints applied. Under the principle of intent to treat, patients remained in their original randomized group for the purpose of analyses. This approach provided the most conservative analyses by keeping the sickest patients in the intervention group, thus guarding against type I error.
Measurements
Baseline data obtained at enrollment included: (1) demographic information, (2) clinical information, and (3) restraint information (ie, time of restraint application, clinical indication for use, type of restraint used, ordering physician, and alternative treatment tried before decision to start restraints).
Primary outcomes included: (1) perception survey scores of family members, physicians, and nurses regarding patient comfort, acceptability, and safety of the restraint device; and (2) patient agitation scores. A preplanned subgroup analysis separated nurses into 2 categories (primary and secondary). The admitting nurse was designated the primary nurse; all other nurses were considered secondary nurses. This analysis was performed to examine any differences in perception resulting from a nurse's level of patient involvement.
Family and provider perceptions were assessed with a self‐administered survey containing 11 items each measured on a 10‐point scale (from 1 = viewed negatively to 10 = viewed positively; maximum score 110 points). Surveyed physicians, nurses, and relatives were asked to rate: (1) patient comfort, (2) accessibility to patient, (3) ease of communication with patient, (4) how calm patient was, (5) perceived safety of patient, (6) patient's feeding convenience, (7) ease of bedpan use, (8) impact on recovery time; (9) how humane and ethical the restraint was, (10) recommendation for use on other patients, and (11) how demanding or difficult caring for the patient was.
Agitation was measured using 2 distinct methods: the Alcohol Withdrawal Assessment Form (AWAF)5 and the Agitated Behavior Scale (ABS).6, 7 Both techniques have been widely used to assess delirium of hospitalized patients. The AWAF measures agitation by analyzing key physiologic indicators such as blood pressure and heart rate on a 0‐ to 22‐point scale. The ABS is a 14‐item scale that measures specific behaviors related to agitation (eg, distractibility, uncooperativeness, and restlessness). Each behavior is rated on a 4‐point Likert scale (0‐3); total score ranges from 0 to 42. Each scale was completed once per 8‐hour shift by the nurse on duty.
Secondary outcomes consisted of total length of stay, duration of restraint use, time from application of restraints until time of discharge, and time from admission until time of application of restraints. Length of stay was calculated as the number of days from the time the patient was admitted to the time the patient was discharged. Time from admission until time of restraint application, duration of restraint use, and time from restraint application until discharge were assessed in minutes. These measurements were based on written restraint order forms and nursing progress reports. Hospital protocol regarding restraint requires hospital staff members to document the application, removal, and adjustment of restraints.
Additional outcomes measured included total amount of medications used to treat agitation and number of injuries incurred. Total amount of medication administered was determined with an equivalence system for different drugs used to treat agitation or delirium.811 Medications were separated into 4 groups: antianxiety medications, antidepressants, antipsychotics, and opioid analgesics. Total amount included both regularly administered and as‐needed dosages of medication. We identified injuries through reports of a subject's primary nurse and by review of medical records.
Data Analyses
Sample size was calculated using a 2‐sample t test formula based on the primary outcome. The study was designed to detect an absolute difference in points of 10% (total absolute score difference of 11 per survey or a total difference of 33). The 2‐sided alpha was initially set at 0.05 and the power at 80%, with an estimated standard deviation of 20. The alpha level was Bonferroni‐adjusted for up to 6 additional comparisons, with each significance level of 0.0071 (z = 2.70).
To assess differences in patient characteristics between the standard restraints and safe enclosure groups, we used the Student t test for continuous variables and Fisher's 2‐sided exact test for categorical variables. Differences in family and staff perceptions of the restraint mechanisms were measured using the Student t test with Satterthwaite's method for calculating variance. However, to account for questions marked not applicable by the responder, weighted scores, defined as the total score divided by the percentage of questions answered, were calculated.
Differences in agitation scores (ABS and AWAF) were analyzed using 2 strategies. First, the ABS and AWAF scores 24 hours after study enrollment were compared across groups using the Student t test with Satterthwaite's method for calculating variance. Then, separate comparisons of the ABS and AWAF scores 48 and 72 hours after enrollment were conducted using the Student t test with a pooled variance. Next, 2 longitudinal analyses were performed using a mixed‐effects (fixed and random) model. These analyses modeled change in the ABS or AWAF scores over (1) the first 3 days and (2) the first 6 days of hospitalization as a function of being restrained with the safe enclosure or being restrained with the hospital's standard restraint systems. For these comparisons, the model included not only the main effects of type of restraint and time, but also the interaction between type of restraint and time and the covariates sex, age, and initial ABS or AWAF score. For these models, a backward elimination procedure was undertaken using a significance level of = 0.05 in order to determine the most parsimonious model.
To determine if the total length of subject stay in the hospital was different between groups, the Student t test was used with Satterthwaite's method for calculating variance. Differences in time from admission until time of restraint application, duration of restraint use, and time from application of restraints until time of discharge were analyzed with the Student t test with pooled variances.
To compare the amount of medication used, equivalent dosage conversions were used for each of the 4 medication categories (antianxiety medications, antidepressants, antipsychotics, and opioid analgesics). To determine if the amounts of these 4 categories of medications differed between groups, the Student t test was used. Last, to determine if there was a difference in the number of patient injuries between groups, Fisher's 2‐sided exact test was used.
RESULTS
Study Population
Of the 49 subjects enrolled in the study, 20 were randomized to the safe enclosure and 29 to standard restraints. This imbalance was likely a result of the premature termination of the study, which in turn was a result of slow recruitment. Table 1 shows selected baseline characteristics of the enrolled subjects. There were no significant differences between the 2 groups in sex, age, patient diagnoses, reason for restraint, or type of medication. However, the subjects randomized to the safe enclosure were less likely to have hypertension than those randomized to standard restraints (36.8% vs. 72.4%, P = .019).
| Variable | All (n = 49) | SOMA Safe Enclosure (n = 20) | Standard restraint system (n = 29) |
|---|---|---|---|
| |||
| Sex (male) | 26 (53.1%) | 11 (55.0%) | 15 (51.7%) |
| Age (years) | 81.3 (13.1%) | 77.2 (15.6%) | 84.2 (10.3%) |
| Alzheimer's disease | 23 (47.9%) | 11 (57.9%) | 12 (41.4%) |
| Dementia | 3 (6.3%) | 2 (10.5%) | 1 (3.5%) |
| Coronary artery disease | 19 (39.6%) | 10 (52.6%) | 9 (31.0%) |
| Hypertension* | 28 (58.3%) | 7 (36.8%) | 21 (72.4%) |
| Congestive heart failure | 6 (12.5%) | 3 (15.8%) | 3 (10.3%) |
| Atrial fibrillation | 7 (14.6%) | 1 (5.3%) | 6 (20.7%) |
| Transient ischemic attacks/cerebral vascular accidents | 7 (14.6%) | 2 (10.5%) | 5 (17.2%) |
| Chronic obstructive pulmonary disease | 3 (6.3%) | 1 (5.3%) | 2 (6.9%) |
| Diabetes mellitus | 11 (22.9%) | 5 (26.3%) | 6 (20.7%) |
| Alcohol abuse | 7 (14.6%) | 2 (10.5%) | 5 (17.2%) |
| Drug abuse | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Where admitted | |||
| General medicine floor | 41 (83.7%) | 17 (85.0%) | 24 (82.8%) |
| Telemetry | 7 (14.3%) | 2 (10.0%) | 5 (17.2%) |
| ICU | 1 (2.0%) | 1 (5.0%) | 0 (0.0%) |
Primary Outcomes
The rates of response to the perception survey were: relatives/next of kin, 90%; physicians, 90%; primary nurses, 100%; and secondary nurses, 78%. Family members and physicians viewed the safe enclosure significantly more positively than they viewed standard restraints (P < .0001 and P < .0001, respectively; Table 2). There was a trend toward more positive perceptions of the safe enclosure among nurses; however, this trend did not achieve statistical significance (P = .0836). The subgroup analysis of nurses (primary vs. secondary) revealed that secondary nurses viewed the safe enclosure more positively (P = .023). Primary nurses tended to view the safe enclosure more positively than the standard restraints, but the association was not significant (P = .1313).
| Variable | SOMA Safe Enclosure (n = 20) | Standard restraint system (n = 29) | P value (observed power)* |
|---|---|---|---|
| |||
| 1. Perception Survey | |||
| Relative or next of kin | 86.84 | 68.47 | < .0001 (96%) |
| Physician | 83.38 | 65.76 | < .0001 (96%) |
| All nurses | 75.20 | 69.45 | .086 (40%) |
| Primary nurse | 75.45 | 69.72 | .1313 (31%) |
| Secondary nurse | 80.35 | 69.82 | .0230 (58%) |
| 2. Alcohol Withdrawal Assessment Form | |||
| 24 hours | 3.06 | 3.25 | .7972 (6%) |
| 48 hours | 3.23 | 3.40 | .8516 (5%) |
| 72 hours | 3.44 | 2.67 | .6163 (7%) |
| 3. Agitated Behavior Scale score | |||
| 24 hours | 11.93 | 8.33 | .2312 (27%) |
| 48 hours | 6.00 | 8.75 | .3743 (13%) |
| 72 hours | 7.83 | 7.11 | .7762 (6%) |
There were no statistically significant differences between the 2 randomized groups in ABS or AWAF scores 24, 48, or 72 hours after restraint application (Table 2). In addition, there were no statistically significant differences during the study between the groups in the rates of change in ABS or AWAF score . This was the case when looking at the first 3 days of hospitalization as well as the first 6 days (data not shown). All results were also calculated after adjusting for length of stay; this covariate did not affect any of the results.
Table 3 details the results for each perception survey question. Perceived comfort, calmness, and safety of patients were rated higher in the safe enclosure group by physicians, relatives, and all nurses. With the exception of perceived accessibility to patients, relatives rated the safe enclosure higher than standard restraints on all other perception measures. Table 4 illustrates the differences in the responses of primary and secondary nurse to each perception survey question. Primary and secondary nurses viewed the safety of the safe enclosure significantly more positively than they did the standard restraints.
| Variable | Relative/next of kin (n = 16, 28) | Primary and secondary nurses (n = 29, 29) | Physician (n = 29, 29) |
|---|---|---|---|
| |||
| Comfort | (8.78, 7.29).0033 | (7.98, 6.78).0194 | (8.40, 6.77).0003 |
| Accessibility | (8.28, 8.07).6486 | (7.68, 8.29).2236 | (8.35, 7.58).1056 |
| Communication | (9.11, 8.19).0214 | (8.29, 8.12).7333 | (8.40, 8.31).8469 |
| Calmness | (8.72, 6.29).0005 | (7.68, 6.53).0382 | (7.70, 5.92).0062 |
| Safety | (9.11, 6.74) < 0.001 | (8.53, 6.76).0024 | (8.60, 5.96).0002 |
| Feeding convenience | (8.50, 7.04).0164 | (7.11, 7.74).2327 | (8.25, 6.28).0047 |
| Ease of bedpan use | (7.91, 6.06).0224 | (7.36, 6.90).3977 | (6.82, 6.25).5376 |
| Impact on recovery time | (7.53, 6.07).0244 | (6.29, 5.66).3864 | (6.95, 6.43).4254 |
| Humane/ethical | (7.94, 5.50).0026 | (6.88, 6.31).4049 | (7.95, 5.96).0052 |
| Recommend for other patients | (8.71, 5.50).0002 | (7.15, 6.12).1395 | (8.05, 6.04).0037 |
| Ease of caring for patient | (8.44, 5.70) < .001 | (7.55, 6.38).0749 | (8.05, 6.25).0028 |
| Variable | Primary Nurse (n = 20, 29) (SOMA safe enclosure, control)* P value | Secondary Nurse (n = 12, 26) (SOMA safe enclosure, control)* P value |
|---|---|---|
| ||
| Comfort | (7.85, 6.62).0270 | (8.33, 6.81).0346 |
| Accessibility | (7.55, 8.21).2656 | (8.17, 8.16).9902 |
| Communication | (8.21, 8.34).8093 | (8.83, 7.88).0705 |
| Calmness | (7.70, 6.28).0153 | (7.83, 6.85).2001 |
| Safety | (8.55, 6.34).0012 | (8.67, 6.76).0435 |
| Feeding convenience | (7.37, 7.93).3861 | (7.60, 7.42).8282 |
| Ease of bedpan use | (7.53, 6.95).3945 | (7.00, 6.84).8602 |
| Impact on recovery time | (6.16, 5.39).3251 | (7.50, 6.50).2340 |
| Humane/ethical | (6.45, 6.66).7871 | (7.73, 5.96).0571 |
| Recommend for other patients | (6.70, 6.11).4553 | (8.42, 5.92).0075 |
| Ease of caring for patient | (7.65, 6.41).0860 | (7.83, 6.38).0565 |
Secondary Outcomes
There was a trend toward shorter total length of stay, time from admission until restraint application, duration of restraint use, and time from restraint application until discharge among subjects restrained by the safe enclosure compared with those restrained with standard restraints. However, these unadjusted differences were not statistically significant. We examined secondary outcomes after adjusting for 2 covariates, age and sex. Age but not sex affected the results. We found that subjects in the intervention group younger than 80 years of age had a shorter length of stay for 2 of the 4 related outcomes: time of admittance to time of discharge (P = .0199) and time of restraint to time of discharge (P = .0274). Time of admission to time of restraint application and duration of restraint did not differ between groups. The former outcome was not expected to differ between groups.
Additional Outcomes
There were no differences between groups in the amounts of 3 of the 4 types of medications used to treat agitation or delirium (ie, antianxiety medications, antipsychotic medications, opioid analgesic medications). The proportion of patients on these medications did not differ by group (P = .59). Only 5% of patients in standard restraints were on antidepressants, and about 5% were on opioids in each group. There was only 1 minor patient injury recorded during the study. This minor abrasion was to a patient assigned to the standard restraint group. No injuries were reported in the safe enclosure group.
DISCUSSION
We have demonstrated that the SOMA Safe Enclosure may be a more acceptable alternative to the restraints currently in use. Our results show that the safe enclosure was rated as more acceptable by family members, physicians, and secondary nurses in our composite perception scores. The results from the primary nurses did not show a significant difference between the 2 groups. An analysis of the individual perception variables found that family members viewed the safe enclosure as more acceptable for 10 of the 11 variables examined. Furthermore, in this small‐scale study, safe enclosures appeared to be safe, as there were no injuries reported in the intervention group. As stated above, there was 1 minor injury reported in the standard restraint groups.
Restraints are commonly used to protect agitated hospitalized patients from harming themselves or others. Despite the significant reluctance of hospital staff members to use restraints, they continue to be necessary in certain situations. Factors such as a general nursing shortage and the expense required to allocate nursing or other ancillary health care workers as sitters contribute to the use of restraints. Therefore, it is reasonable to conclude that restraint use in some form or fashion will continue into the foreseeable future. There are no clear estimates of the prevalence of restraint use in acute care hospitals. A chart review study from Canada reported physical restraints in about 7.7% of in‐patients.12 Other studies have reported the use of restraints on patients in the range of 4%‐25%.2 Given the prevalence of restraint use in acute care hospitals, surprisingly little innovative research has been undertaken to develop more effective and humane systems of restraint. Furthermore, no research has examined how restraint use may affect important clinical outcomes such as length of stay. To our knowledge, this is the first clinical trial to compare currently used restraints to a newer method of restraint using the SOMA Safe Enclosure.
The idea that restraint use can lead to further agitation is not supported by our data. We observed a decrease in agitated behavior scale scores from 9.6 to 7.4 from the 24‐ and to the 72‐hour assessments; however, these results were not significant and appeared to be more dramatic for the safe enclosure group because of higher baseline levels. Our adjusted analyses of length of stayrelated outcomes indicated an association with age. Total length of stay and time from restraint application until discharge were significantly reduced for those subjects younger than 80 years of age in the safe enclosure group. The basis for this finding is not entirely clear. It may be a chance finding, or there may have been a complex combination of factors at work.
There was a reduction in overall length of stay by 1.5 days among those in the safe enclosure group when compared with the standard restraint group. Similarly, total duration of restraint use of the safe enclosure group was 551 minutes (9 hours) shorter. Although these findings were not significant, they warrant further investigation in a larger trial. If safe enclosure use truly reduces length of stay and duration of restraint use, it is an important finding, for it could translate into meaningful cost savings for acute care hospitals. It is possible, however, that any potential cost savings could be tempered by the additional time required to set up the enclosure. Ethically, if restraints are to be used, their use should be minimized, and in that sense, safe enclosures may help acute care hospitals achieve this goal more effectively.
Limitations of this trial include its small sample size and inadequate power to determine certain outcomes. Although we saw encouraging trends in several outcomes, they failed to reach statistical significance because of the limited power. For instance, the observed power for total length of stay difference was only 17%. It is conceivable that a larger trial powered specifically for length of stayrelated outcomes may show significant results. Because subjects in this study were patients in a single midsize community teaching hospital, the results may not be generalizable to patients in, for example, tertiary‐care centers or nursing homes. However, these results may apply to a large proportion of patients in the United States, as most are treated in community hospitals. We found that many patients required 2 wrist restraints in order to protect IV lines, and this resulted in exclusion of a large proportion of potential subjects. Therefore, safe enclosures may not be appropriate for all agitated patients. They may be an ideal method of restraining patients who are not at risk of pulling out their IV line or catheters but require restraints for other reasons. This could include patients in nursing homes or rehabilitation centers.
It is also important to discuss the issue of practitioner acceptability of a newer method of restraint in acute care hospitals. As expected, we found the nursing staff was originally reluctant to use the safe enclosure, even as part of a trial. This may have been because of fear of change and having a high level of comfort with the restraint systems already in use. The setup of safe enclosures can take 10‐15 minutes, whereas the use of 2‐point soft restraints or Posey vests can be accomplished in as little as a minute. However, we found that after initial use of the safe enclosure, resistance among nurses declined. In fact, in our hospital, nurses began using safe enclosures for confused and agitated patients not enrolled in the study in order to prevent wandering and falls at night. Another difficulty reported by the nursing staff was feeling somewhat limited in their access to patients by a safe enclosure. Nurses had to open a zipped flap to access the patient to administer medication or provide food. Health care providers must remember to close the flap to avoid potential falls.
In summary, safe enclosures seem to be a safe and more acceptable alternative to the restraints currently in use in acute care hospitals. These findings should be replicated in a larger trial.
- ,,,,.Prevalence and patterns of physical restraint use in the acute care setting.J Nurs Adm.1998;28(11):19–24.
- ,Strumpf. Myths about Elder Restraint.J Nurs Scholarsh.1990;22(2):124–128.
- , et al.Deadly restraint: a Hartford Courant investigative report.Hartford Courant1998; October 11‐15.
- ,,,,,, et al.Jt Comm J Qual Improv2001;27:605–618.
- ,,,,.Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale.Br J Addict.1989;84:1353–1357.
- ,.Development of an agitated behavior rating scale for discrete temporal observations.Nursing Meas1993;1:115–124.
- ,,,.Reliability of the Agitated Behavior Scale.J Head Trauma Rehabil.1999;14:91–96.
- University of Newcastle. The Ashton Manual. Available at: http://www.benzo.org.uk/manual/bzcha01.htm. Accessed November 15,2006.
- Postgraduate Medicine Online. Sedation and analgesia in intensive care. Available at: http://www.postgradmed.com/issues/2002/02_02/blanchard.htm. Accessed November 15,2006.
- Anti‐psychotic Comparison chart. Available at: http://meds.queensu.ca/∼clpsych/orientation/Antipsychotics%20Comparison%20Chart.pdf. Accessed November 15,2006.
- Anti‐depressant comparison chart. Available at: http://meds.queensu.ca/∼clpsych/orientation/Antidepressant%20comparison%20Chart.pdf. Accessed November 15,2006.
- ,.Use of physical and chemical restraints in medical teaching units.Can Med Assoc J.2000;162:339–340.
- ,,,,.Prevalence and patterns of physical restraint use in the acute care setting.J Nurs Adm.1998;28(11):19–24.
- ,Strumpf. Myths about Elder Restraint.J Nurs Scholarsh.1990;22(2):124–128.
- , et al.Deadly restraint: a Hartford Courant investigative report.Hartford Courant1998; October 11‐15.
- ,,,,,, et al.Jt Comm J Qual Improv2001;27:605–618.
- ,,,,.Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale.Br J Addict.1989;84:1353–1357.
- ,.Development of an agitated behavior rating scale for discrete temporal observations.Nursing Meas1993;1:115–124.
- ,,,.Reliability of the Agitated Behavior Scale.J Head Trauma Rehabil.1999;14:91–96.
- University of Newcastle. The Ashton Manual. Available at: http://www.benzo.org.uk/manual/bzcha01.htm. Accessed November 15,2006.
- Postgraduate Medicine Online. Sedation and analgesia in intensive care. Available at: http://www.postgradmed.com/issues/2002/02_02/blanchard.htm. Accessed November 15,2006.
- Anti‐psychotic Comparison chart. Available at: http://meds.queensu.ca/∼clpsych/orientation/Antipsychotics%20Comparison%20Chart.pdf. Accessed November 15,2006.
- Anti‐depressant comparison chart. Available at: http://meds.queensu.ca/∼clpsych/orientation/Antidepressant%20comparison%20Chart.pdf. Accessed November 15,2006.
- ,.Use of physical and chemical restraints in medical teaching units.Can Med Assoc J.2000;162:339–340.
Copyright © 2007 Society of Hospital Medicine
Color‐coded wristbands: Promoting safety or confusion?
A 62‐year‐old man was transferred from an outside hospital for evaluation of a complicated spinal infection. Like many patients, he had color‐coded wristbands to help identify potential safety hazards (see Fig. 1). The patient, an educated and alert individual, could describe the indications for only 3 of the 5 wristbands, and the transferring hospital supplied no legend. As it turned out, the green band represented a fall risk, the red one a drug allergy alert, and the purple one a tape allergy, whereas the white one was for patient identification. We're still not certain what the yellow one represented, but it was not a Lance Armstrong Livestrong bracelet; such wristbands have been reported to cause confusion in hospitals that have adopted yellow for their do not resuscitate wristbands.1 Although attempts at ensuring patient safety by using color‐coded wristbands are a common practice, the lack of standardization may pose an unknown hazard. Elsewhere in this journal, we present findings from a survey reinforcing the need for standardization around this issue.
- .Wristbands called patient safety risk.St. Petersburg Times 10 Dec2004. p1A.
A 62‐year‐old man was transferred from an outside hospital for evaluation of a complicated spinal infection. Like many patients, he had color‐coded wristbands to help identify potential safety hazards (see Fig. 1). The patient, an educated and alert individual, could describe the indications for only 3 of the 5 wristbands, and the transferring hospital supplied no legend. As it turned out, the green band represented a fall risk, the red one a drug allergy alert, and the purple one a tape allergy, whereas the white one was for patient identification. We're still not certain what the yellow one represented, but it was not a Lance Armstrong Livestrong bracelet; such wristbands have been reported to cause confusion in hospitals that have adopted yellow for their do not resuscitate wristbands.1 Although attempts at ensuring patient safety by using color‐coded wristbands are a common practice, the lack of standardization may pose an unknown hazard. Elsewhere in this journal, we present findings from a survey reinforcing the need for standardization around this issue.

A 62‐year‐old man was transferred from an outside hospital for evaluation of a complicated spinal infection. Like many patients, he had color‐coded wristbands to help identify potential safety hazards (see Fig. 1). The patient, an educated and alert individual, could describe the indications for only 3 of the 5 wristbands, and the transferring hospital supplied no legend. As it turned out, the green band represented a fall risk, the red one a drug allergy alert, and the purple one a tape allergy, whereas the white one was for patient identification. We're still not certain what the yellow one represented, but it was not a Lance Armstrong Livestrong bracelet; such wristbands have been reported to cause confusion in hospitals that have adopted yellow for their do not resuscitate wristbands.1 Although attempts at ensuring patient safety by using color‐coded wristbands are a common practice, the lack of standardization may pose an unknown hazard. Elsewhere in this journal, we present findings from a survey reinforcing the need for standardization around this issue.

- .Wristbands called patient safety risk.St. Petersburg Times 10 Dec2004. p1A.
- .Wristbands called patient safety risk.St. Petersburg Times 10 Dec2004. p1A.
Breaking Bad News
Broadly defined, bad news is any information that negatively alters a person's expectation about the present and the future.1 Importantly, news is defined as bad based on the patient's perspective about the information. Providers must remember that it will not always be obvious what patients will interpret as bad news. Although all would agree that the diagnosis of a new cancer would qualify as bad news, to some patients discovering hypertension would be deeply disturbing. Delivering bad news is difficult and stressful to all involved. Substantial data are now available describing patient preferences in these interactions, the impact on physicians who participate in these conversations, and specific recommendations for the delivery of bad news.
Hospitalists face additional challenges: lacking long‐standing relationships with patients and dealing with discontinuity in patient care and patient handoffs on a regular basis. Using an actual case as an example, this article examines the patient/family perspective and the provider perspective and reviews practical advice, actual phrases, useful mnemonics, and communication techniques to make these conversations more successful and less stressful. Opportunities to increase training in this area of palliative care are discussed. Adequate preparation, effective communication skills, empathy, and planned follow‐up are essential steps to assure that the goals for these difficult interactions are met.2, 3
CASE
The following scenario is based on an actual patient. The details and initials have been changed to maintain anonymity.
A 52‐year‐old Latino man, JR, was admitted with new‐onset ascites. He had a known diagnosis of end‐stage liver disease from prior alcohol use. Paracentesis revealed spontaneous bacterial peritonitis, and appropriate antibiotics were started. The fluid was sent for cytological analysis; the final diagnosis reported adenocarcinoma. A subsequent workup including PSA and CT of the abdomen/pelvis did not reveal the primary site of this malignancy.
JR had a supportive family and an established primary care physician. His spouse was no longer involved in his life, but his 2 daughters provided strong social support. His primary language was Spanish.
During the first 3 days of JR's hospital stay, he developed increasing abdominal pain, requiring escalating doses of narcotics. On the fourth day, the team received the cytology results, and the medical resident discussed the new diagnosis of cancer with JR. This conversation was not supervised by an attending, no interpreter was present, and no family members were in the room.
On entering the room, the resident said to the patient, I have bad news for you, JR.
The patient turned and said, Yes.
The resident continued, JR, you have cancer, and we don't know where it originated from.
The patient was silent and without expression. Unclear about how to proceed, the resident went on to say, The oncologist will be coming by later to discuss options with you. As there was no response, verbal or otherwise, the resident exited the room. The resident reported that the patient was unexpectedly calm after the news.
Provider Perspective
The responsibility of breaking bad news to patients weighs heavily on clinicians. As in this case, most providers' first experience with breaking bad news occurs with patients they have known for only a few hours or days. Even for the more experienced, this part of the job is rated as at least moderately stressful. Notably, most also feel that this stress lasts beyond the encounter, despite their perceived ability to manage their own stress during these situations.4 Additional training on clinicians' own coping skills may alleviate some of the emotional burden.
Provider's awareness and management of distress may enhance ability to provide comfort to patients or to specifically address their needs. Medical providers may try to suppress personal thoughts and feelings in these situations, but they bring emotional attachments to almost all encounters with patients.5 Emotional preparation by the provider is an important step prior to delivering bad news. Self‐reflection helps to identify personal emotions of sadness, anger, fear, or guilt and will help the provider not to disengage from the delivery of bad news.6 It is normal to have strong feelings, especially in difficult situations. Encouraging and validating these emotions personally will lead to a more therapeutic presence during a patient's time of need.7
Clinicians' perceptions of their interactions with patients when discussing bad news are probably more strongly influenced by the content of the discussion rather than the process itself. When asked to analyze their own videotaped consultations, doctors thought performance was worse when discussing palliative therapy than when discussing curative therapy.8
Traditionally, greatest emphasis has been placed on the acquisition and assessment of medical knowledge in medical training, and thus the focus on content is understandable. But more recently, efforts have been made at all levels of medical education to shift this focus toward encompassing many other competencies including professionalism and communication skills, which should translate into equal emphasis on the quality of these interactions.
As many hospitalists work closely with trainees, they are in the ideal position to serve as mentors and role models for communication. The case discussed in this article provides an example of a missed teaching opportunity. Ideally, the attending would have gone through the steps of preparation with the resident prior to the meeting, reviewed one or several of the suggested approaches discussed below, and observed the conversation and provided immediate feedback and a forum for processing afterward. It is especially helpful when first developing this skill to be familiar with helpful phrases to open the conversation, clarify patient preferences for communication, and convey empathy. It is also helpful to be aware of phrases that should be avoided (Table 1).
| Phrases to consider |
|---|
| To start a conversation |
| I am sorry to have to tell you this. |
| I know this is not good news. |
| I wish I had better news. |
| To elicit patient preferences |
| Would you like your family here when we talk about this? |
| Would you rather I speak with you about this or your daughter? |
| Some people want to be very involved in making decisions about their medical care, and some people want their doctor to just give them a recommendationhow do you feel about that? |
| To facilitate empathy |
| I can see how upsetting this is. |
| Is it okay if I hold your hand? |
| Phrases to avoid |
| There is nothing more we can do for you. |
| I know what this must be like. |
| I understand what you are going through. |
CASE
The following morning the attending physician, medical resident, and oncology fellow met with the patient and his daughter for a more extensive discussion. The goals of this discussion were to review the diagnosis and discuss the prognosis and future approaches to care. The entire discussion was conducted via a professional Spanish interpreter.
The attending physician began the conversation by asking, What do you remember about what the resident doctor told you, JR? pointing to the resident.
JR replied, I don't remember, and then went on to say, Please talk to my daughter, who was sitting across the room.
Family/Patient Perspective
As patient preferences for receiving bad news vary widely, it is these preferences that should determine the approach to the delivery, content, and context in which the news will be received. Some patients want information, and some do not; this needs to be clarified before beginning the discussion. The amount of detail should be negotiated in advance as well. As suggested by Back et al., soliciting patient preference prior to a discussion is important.9 These authors recommended using an approach called asktellask. This approach emphasizes the importance of asking questions to assess a patient's needs, telling the patient the information that meets those needs, and asking again to assess the patient's understanding.
Patients will rarely raise the issue of bad news with providers. In general, the provider must initiate the discussion.10 Surveys of patient preferences for delivery of bad news lend insight into this process and help guide providers during this challenging time. Patients report poor delivery is often characterized by bluntness, a lack of hope, and initiation of this serious conversation at an inappropriate time or place.11 Patients prefer providers to speak in clear, simple terms, being careful not to use technical jargon.12 Clinicians often use euphemisms to soften the blow of bad news, but this can lead to ambiguity. In addition to the clarity of the message, privacy, the attitude of the doctor, and the ability to answer questions are most important to patients and families receiving bad news.13 Although most would encourage touching in these difficult situations, it has been reported that up to a third of patients surveyed do not want physical contact.
Contrary to what providers may believe, diagnostic disclosure is not the most important part of a bad‐news discussion. Many patients believe it is most important to receive information on prognosis and treatment options. Often, patients want to discuss life expectancy. However, physicians are hesitant to address this issue. One study revealed that despite these requests, 22% of physicians would not provide any estimates at all, and when they did, 36% offered an overestimate.14 The authors hypothesized that how confident physicians are in this prognostic estimate and how much and what type of practice experience they have may influence their willingness to communicate a frank survival estimate.
The traditional dilemma of balancing hope with realism is reframed by Hagerty et al., who found that 98% of the patients they surveyed preferred a realistic and individualized approach.15 Use of euphemism and apparent unease of the provider actually decreases hope. Clayton et al. added that nurturing hope can also be facilitated by emphasizing what can be done, such as symptomatic management, emotional support, and practical support, particularly in terms of day‐to‐day living.16
In the case discussed in this article, preparation should have included asking the patient (1) whom he wanted present during the meeting, (2) how much information he and his family wanted to know, and (3) how involved he wanted his family to be. The informational needs of patients and their families will evolve over time as they process and accept the news. Thus, the asktellask approach remains a key concept to keep in mind as the dialogue continues beyond the initial encounter.
CASE
The attending physician attempted to continue the discussion by addressing both patient and daughter. He restated, JR, you have cancer in the fluid in your belly, and it is likely widespread throughout your body.
At that moment, the daughter became very tearful and emotional. There were several minutes of silence. The patient began to sob as well. The oncology fellow broke the silence by adding, Unfortunately, there is nothing that can be done when cancer is so widespread. The daughter broke into audible sobs; the patient looked away from the team and gazed through the window into the distance. The team fell silent and quietly left the room.
Importance of Empathy
The team failed in its inability to respond to emotion in this case. The emotional turmoil was apparent, but the team members made no attempt to acknowledge this emotion or to arrange additional emotional support. This could, at least in part, be a result of the providers' inability to process and manage their own emotions. A preparatory meeting beforehand and a debriefing session afterward for all the team members may have helped. Awareness of patients' coping strategies and various effective responses to these coping strategies may have better prepared the team to react and validate this patient's emotions. The role that psychology and emotion play cannot be overemphasized and clearly are important considerations. A useful mnemonic highlighting the components of the empathetic response is NURSE: naming, understanding, respect, supporting, and exploring.9 Using this technique, the provider starts by naming the emotion (anger, fear, disbelief); confirms a clear understanding of the patient's feelings; expresses respect verbally or nonverbally, letting the patient know the emotion is important; uses supporting statements that may express concern, reiterate understanding, or indicate a willingness to help; and closes by exploring additional concerns.
Certain phrases such as the one uttered by the fellowThere is nothing that can be doneshould be avoided.
Cultural Issues
Cultural diversity is increasingly common in contemporary medical practice in the United States. Some have suggested the Western value of autonomy is not embraced by all cultures. It has been suggested that non‐English‐speaking patients may receive less optimal end‐of‐life care than their English‐speaking counterparts.17 Beyond the language barrier, this observation may be a reflection of associated cultural barriers as well. Effective strategies for key issues of truth telling, language, family involvement, and decision making may help effective cross‐cultural communication and understanding and thus be effective patient‐centered care.18
A study of Korean patients and family members revealed a marked discordance between family and patients in the desire for disclosure.19 Almost all patients wanted to be informed that they had terminal illness, whereas a quarter of family members did not want physicians to relay this bad news to the patient. Interestingly, this study found patients would prefer to be told by the physician, whereas their family members would prefer to be the ones to deliver the news.
In some cultures it is believed that disclosure of bad news may cause patients to lose hope and hasten death. Physicians in these cultures may be more likely to honor family wishes. Language barriers may make a difficult situation even more complex. It is important to ascertain early on in what language patients and their families want to hold discussions. A medical interpreter should always be utilized for discussions with patients and their families. Dependence on a family member to interpret is not advised because the objective point of view may be lost in the interpretation. In addition, this places an enormous burden on the family member to be the bearer of bad news, which could have a lasting emotional impact. Although in the case discussed in this article, the patient's daughter was bilingual and could have translated, an interpreter should have been present for all discussions with this patient. Again, the importance of soliciting the patient's preference is critical.
Prior knowledge of the language requirement and of the patient's need for his daughter's involvement would also aid in the planning process. Because the patient's primary care physician (PCP) shared JR's Hispanic heritage, consultation with this PCP might have provided important insight, resulting in better preparation and planning.
How to Deliver Bad News
A number of guidelines are available to help physicians structure their conversations.2022 Baile and Buckman outline a 6‐step approach (Table 2). Rabow and Mcphee recommend the ABCDE mnemonic to help providers remember techniques for delivering bad news (Table 3). These recommendations are largely based on the literature to date. Both these approaches first emphasize preparations and planning. A private and quiet space, the presence of significant others if desired, arrangement to minimize interruptions, and provider emotional preparations are all prerequisites for the success of this type of encounter before the actual dialogue begins. As the encounter begins, it is key to assess a patient's needs if not already done before conveying information. Unidirectional transfer of information most likely will fail to satisfy the patient. The resident's initial conversation suffered in this key aspect.
| Setting up interview | Maintain privacy, involve significant others, sit down, make a connection, minimize interruptions. |
| Assessing patient perceptions | What have you been told about your illness? |
| Obtaining patient's invitation | How would you like me to give the information about your test results? |
| Giving knowledge and information to patient | Begin with warning statement, avoid jargon, avoid excessive bluntness. |
| Addressing patient's emotions | Listen, observe, acknowledge the emotion. |
| Providing strategy and summary | Give prognosis and treatment options and address symptoms. |
| Advance preparation |
|---|
| Ask what the patient already knows and understands. |
| Arrange for the presence of a support person and appropriate family. |
| Arrange a time and place that will be undisturbed (hand off beeper). |
| Prepare emotionally. |
| Decide which words and phrases to use (write down a script). |
| Practice delivering the news. |
| Build a therapeutic environment/relationship |
| Arrange a private, quiet place without interruptions. |
| Provide adequate seating for all. |
| Sit close enough to touch if appropriate. |
| Reassure about pain, suffering, abandonment. |
| Communicate well |
| Be direct (I am sorry, I have bad news). |
| Do not use euphemisms, jargon, or acronyms. |
| Do say cancer or death. |
| Allow for silence. |
| Use touch appropriately. |
| Ask patient to repeat his or her understanding of the news. |
| Arrange additional meetings. |
| Use repetition and written explanations of reminders. |
| Deal with patient and family reactions |
| Assess patient reaction |
| Physiologic responses: flight/fight, conservation/withdrawal; |
| Cognitive coping strategies: denial, blame, intellectualization, disbelief, acceptance; |
| Affective responses: anger/rage, fear/terror, anxiety, helplessness, hopelessness, shame, relief, guilt, sadness, anticipatory grief; |
| Listen actively, explore feelings, express empathy. |
| Encourage and validate emotions |
| Correct distortions. |
| Offer to tell others on behalf of the patient. |
| Evaluate the effects of the news. |
| Explore what the news means to the patient. |
| Address further needs, determine the patient's immediate and near‐term plans, assess suicidality. |
| Make appropriate referrals for more support, provide written materials, and arrange follow up. |
| Process your own feelings. |
Armed with knowledge of a patient's individual preferences, it is then possible to effectively convey information in a clear manner without jargon, using a direct but not blunt style. Both the SPIKES and ABCDE approaches similarly emphasize the asktellask approach. The attending physician was fairly effective in applying this communication approach in the subsequent encounter. However, the team left the room without providing a summary and follow‐up plan. Even though the patient and his daughter were quite emotional, acknowledging their reactions and appropriately ending the meeting with a summary and plans for the next steps would have been helpful in this continuing dialogue.
Hospitalist‐Specific Issues
Hospitalists may face special challenges when delivering bad news to patients. Without the benefit of preexisting longitudinal relationships with their patients, they lack prior understanding of a patient's values, family support system, and other cultural, spiritual, and social issues. Thus, preparation for these conversations is more difficult, and establishing rapport is more time‐consuming. There are no data available to describe the impact that not having a previous relationship with a patient has on these encounters. It is possible that the newness of the hospitalistpatient relationship may allow more candid, transparent communication than would be possible with established providers, who may themselves be struggling with the news and how it reflects on their care or the emotional impact of the impending loss.
Handoffs are a frequent part of the care the hospitalist provides, but communicating bad news is often a longitudinal process. One hospitalist may have the initial conversation regarding the patient's disease and prognosis, but the follow‐up often falls to a different hospitalist. Continuity of communication and awareness about what has been said previously are critical. It is important to explicitly document these conversations and their content in the medical record. In additional, summaries of pivotal conversations should be included in sign‐out. At discharge, whether patients are transitioning to postacute care or back to the outpatient arena, the hospitalist should carefully and vigilantly communicate critical conversations and predictions about patients' emotional needs.
Hospitalists do have some advantages when it comes to communication with patients. Unlike in outpatient practice, where clinicians are under pressure to keep up with a heavily loaded patient schedule, the hospitalist often has the flexibility and ability to allot time to each patient according to that patient's need. In addition, by definition, a hospitalist is in a hospital; this availability allows for more timely meetings, minimal delay in delivery of news, and accommodating the schedules of other people the patient may want included in any conversations.
CASE
A subsequent meeting occurred between the patient, his daughter, and the team, this time including a social worker and a hospice nurse.
The social worker began this discussion by stating, I understand you were quite upset last time and understandably so, and then inquired, What questions can we help you answer?
The patient and his daughter appropriately asked about alternatives to the usual aggressive treatment, and he made clear his desire to eventually spend his last days at home with family.
Through the translator, the hospice nurse succinctly explained the concept of palliative care with emphasis on symptomatic management as an alternative to aggressive curative therapy. JR and his daughter chose this palliative approach to care. This decision to focus on palliation was conveyed to JR's PCP. JR was eventually discharged to a short‐term postacute facility for rehabilitation and palliative care.
The health care team was finally able to acknowledge and validate JR's emotions with the help of the additional expertise of a social worker and a hospice nurse. This multidisciplinary approach allows team members to complement each other's strengths and weaknesses. Further, the patient had time to process his feelings and articulate his questions, values, and desires. Time often is required for this type of news to be more fully understood and eventually accepted. Breaking bad news is not a single event but a continuing dialogue and ultimately a relationship. Thus, proper delivery of bad news not only requires planning, effective communication, and empathy, but also deliberate follow‐up.
Training
What can be done to improve the effectiveness and satisfaction of these interactions for patients, providers, and families? Awareness of guidelines and effective strategies is a start but is unlikely to really change behavior or improve skills. Communication skills must be practiced, implemented, and observed with opportunity for feedback.23 Graduate and postgraduate training is probably the best time to develop these skills, and formal training in this area should be incorporated in a curriculum. Workshops on communicating bad news are offered frequently to oncologists and oncology fellows at various regional and national meetings. Ideally, these workshops would be offered at CME meetings specifically designed for hospitalists already in practice. Comprehensive palliative care training and materials, including specific modules and live workshops for delivering bad news, are available via the Education in Palliative and End‐of‐Life Care Project (EPEC) and the End‐of‐Life/Palliative Education Resource Center (EPERC) at the University of Wisconsin.
Hospitalists and trainees fortunate enough to practice in an institution with a palliative care service have the opportunity to learn from a multidisciplinary team, often including social workers, nursing staff, physicians and spiritual leaders. This interdisciplinary model is likely a more effective way to address the diverse physical, emotional, social, and spiritual needs of patients receiving difficult news and provides an ideal framework for this training.
CONCLUSIONS
Hospitalists are frequently called on to deliver bad news. A specific skills set is needed to be an effective communicator, especially in these stressful situations. Familiarity with an evidence‐based approach to this process and incorporation of the key steps into each of these encounters will likely improve patient and provider satisfaction as well as patient care during these critical times. Patient and family preferences for communication vary; so communication should be adjusted for each patient using the asktellask approach. Providers must remember to respond empathetically to emotion expressed by the patient and family and should keep the NURSE mnemonic in mind to guide the discussion. Providers should seek hands‐on training opportunities, which include supervision and feedback. Medical educators should incorporate training on the communication of bad news into curricula for students and trainees. Hospitalists may take a leadership role in teaching these skills at their institutions.
- .Breaking bad news: why is it still so difficult?BMJ.1984;288:1597–1599.
- ,.Bad news: delivery, dialogue, and dilemmas.Arch Intern Med.1991;151:463–468.
- ,.Beyond breaking bad news: how to help patients who suffer.WJM.1999;171:260–263.
- ,,,.Breaking bad news to patients: physicians' perception of the process.Support Care Cancer.1999;7:113–120.
- ..Giving bad news to cancer patients: matching process and content.J Clin Oncol.2001;19:2575–2577.
- ,.Beyond breaking bad news:how to help patients who suffer.West J Med.1999;171:260–263.
- .Breaking bad news.Am Fam Physician.2001;64:1975–1978
- ,,.Truth may hurt but deceit hurts more: communication in palliative care.Palliat Med.2002;16:297–303.
- ,,, et al.Approaching difficult communication tasks in oncology.CA Cancer J Clin.2005;55:164–177.
- ,,,.Enhancing physician‐patient communication.Hematology Am Soc Hematol Educ Program.2002;464–483.
- ,,,,,.Communicating with dying patients within the spectrum of care from terminal diagnosis to death.Arch Intern Med.2001;161:868–874.
- ,,,.Communicating prognosis in early breast cancer: do women understand the language used?Med J Aust.1999;171:290–294.
- ,,,.Giving bad news: the family perspective.J Trauma.2000;48:865–870
- ,.Prognostic disclosure to patients with cancer near the end of life.Ann Intern Med.2001;134:1096–1105.
- ,,, et al.Communicating with realism and hope: incurable cancer patients' views on the disclosure of prognosis.J Clin Oncol.2005;23:1278–1288.
- ,,, et al.Fostering coping and nurturing hope when discussing the future with terminally ill cancer patients and their caregivers.Cancer.2005;103:1965–1975.
- ,.Comparison of palliative care needs of English and non‐English‐speaking patients.J Palliat Care.1999;15(1):26–30
- ,.Negotiating cross‐cultural issues at the end of life.JAMA.2001;286:2993–3001
- ,,,,,.The attitudes of cancer patients and their families towards disclosure of terminal illness.J Clin Oncol.2004;22:307–314.
- ,.Breaking bad news:consensus guidelines for medical practitioners.J Clin Oncol.1995;13:2449–2456
- ,,, et al.SPIKES—a six step protocol for delivering bad news: application to the patient with cancer.Oncologist.2000;5:302–311
- ,.Beyond breaking bad news:how to help patients who suffer.West J Med.1999;171:260–263
- ,,.Communication skills training for health professionals working with cancer patients, their families, and/or careers.Cochrane Database Syst Rev.2003;2:CD003751.
Broadly defined, bad news is any information that negatively alters a person's expectation about the present and the future.1 Importantly, news is defined as bad based on the patient's perspective about the information. Providers must remember that it will not always be obvious what patients will interpret as bad news. Although all would agree that the diagnosis of a new cancer would qualify as bad news, to some patients discovering hypertension would be deeply disturbing. Delivering bad news is difficult and stressful to all involved. Substantial data are now available describing patient preferences in these interactions, the impact on physicians who participate in these conversations, and specific recommendations for the delivery of bad news.
Hospitalists face additional challenges: lacking long‐standing relationships with patients and dealing with discontinuity in patient care and patient handoffs on a regular basis. Using an actual case as an example, this article examines the patient/family perspective and the provider perspective and reviews practical advice, actual phrases, useful mnemonics, and communication techniques to make these conversations more successful and less stressful. Opportunities to increase training in this area of palliative care are discussed. Adequate preparation, effective communication skills, empathy, and planned follow‐up are essential steps to assure that the goals for these difficult interactions are met.2, 3
CASE
The following scenario is based on an actual patient. The details and initials have been changed to maintain anonymity.
A 52‐year‐old Latino man, JR, was admitted with new‐onset ascites. He had a known diagnosis of end‐stage liver disease from prior alcohol use. Paracentesis revealed spontaneous bacterial peritonitis, and appropriate antibiotics were started. The fluid was sent for cytological analysis; the final diagnosis reported adenocarcinoma. A subsequent workup including PSA and CT of the abdomen/pelvis did not reveal the primary site of this malignancy.
JR had a supportive family and an established primary care physician. His spouse was no longer involved in his life, but his 2 daughters provided strong social support. His primary language was Spanish.
During the first 3 days of JR's hospital stay, he developed increasing abdominal pain, requiring escalating doses of narcotics. On the fourth day, the team received the cytology results, and the medical resident discussed the new diagnosis of cancer with JR. This conversation was not supervised by an attending, no interpreter was present, and no family members were in the room.
On entering the room, the resident said to the patient, I have bad news for you, JR.
The patient turned and said, Yes.
The resident continued, JR, you have cancer, and we don't know where it originated from.
The patient was silent and without expression. Unclear about how to proceed, the resident went on to say, The oncologist will be coming by later to discuss options with you. As there was no response, verbal or otherwise, the resident exited the room. The resident reported that the patient was unexpectedly calm after the news.
Provider Perspective
The responsibility of breaking bad news to patients weighs heavily on clinicians. As in this case, most providers' first experience with breaking bad news occurs with patients they have known for only a few hours or days. Even for the more experienced, this part of the job is rated as at least moderately stressful. Notably, most also feel that this stress lasts beyond the encounter, despite their perceived ability to manage their own stress during these situations.4 Additional training on clinicians' own coping skills may alleviate some of the emotional burden.
Provider's awareness and management of distress may enhance ability to provide comfort to patients or to specifically address their needs. Medical providers may try to suppress personal thoughts and feelings in these situations, but they bring emotional attachments to almost all encounters with patients.5 Emotional preparation by the provider is an important step prior to delivering bad news. Self‐reflection helps to identify personal emotions of sadness, anger, fear, or guilt and will help the provider not to disengage from the delivery of bad news.6 It is normal to have strong feelings, especially in difficult situations. Encouraging and validating these emotions personally will lead to a more therapeutic presence during a patient's time of need.7
Clinicians' perceptions of their interactions with patients when discussing bad news are probably more strongly influenced by the content of the discussion rather than the process itself. When asked to analyze their own videotaped consultations, doctors thought performance was worse when discussing palliative therapy than when discussing curative therapy.8
Traditionally, greatest emphasis has been placed on the acquisition and assessment of medical knowledge in medical training, and thus the focus on content is understandable. But more recently, efforts have been made at all levels of medical education to shift this focus toward encompassing many other competencies including professionalism and communication skills, which should translate into equal emphasis on the quality of these interactions.
As many hospitalists work closely with trainees, they are in the ideal position to serve as mentors and role models for communication. The case discussed in this article provides an example of a missed teaching opportunity. Ideally, the attending would have gone through the steps of preparation with the resident prior to the meeting, reviewed one or several of the suggested approaches discussed below, and observed the conversation and provided immediate feedback and a forum for processing afterward. It is especially helpful when first developing this skill to be familiar with helpful phrases to open the conversation, clarify patient preferences for communication, and convey empathy. It is also helpful to be aware of phrases that should be avoided (Table 1).
| Phrases to consider |
|---|
| To start a conversation |
| I am sorry to have to tell you this. |
| I know this is not good news. |
| I wish I had better news. |
| To elicit patient preferences |
| Would you like your family here when we talk about this? |
| Would you rather I speak with you about this or your daughter? |
| Some people want to be very involved in making decisions about their medical care, and some people want their doctor to just give them a recommendationhow do you feel about that? |
| To facilitate empathy |
| I can see how upsetting this is. |
| Is it okay if I hold your hand? |
| Phrases to avoid |
| There is nothing more we can do for you. |
| I know what this must be like. |
| I understand what you are going through. |
CASE
The following morning the attending physician, medical resident, and oncology fellow met with the patient and his daughter for a more extensive discussion. The goals of this discussion were to review the diagnosis and discuss the prognosis and future approaches to care. The entire discussion was conducted via a professional Spanish interpreter.
The attending physician began the conversation by asking, What do you remember about what the resident doctor told you, JR? pointing to the resident.
JR replied, I don't remember, and then went on to say, Please talk to my daughter, who was sitting across the room.
Family/Patient Perspective
As patient preferences for receiving bad news vary widely, it is these preferences that should determine the approach to the delivery, content, and context in which the news will be received. Some patients want information, and some do not; this needs to be clarified before beginning the discussion. The amount of detail should be negotiated in advance as well. As suggested by Back et al., soliciting patient preference prior to a discussion is important.9 These authors recommended using an approach called asktellask. This approach emphasizes the importance of asking questions to assess a patient's needs, telling the patient the information that meets those needs, and asking again to assess the patient's understanding.
Patients will rarely raise the issue of bad news with providers. In general, the provider must initiate the discussion.10 Surveys of patient preferences for delivery of bad news lend insight into this process and help guide providers during this challenging time. Patients report poor delivery is often characterized by bluntness, a lack of hope, and initiation of this serious conversation at an inappropriate time or place.11 Patients prefer providers to speak in clear, simple terms, being careful not to use technical jargon.12 Clinicians often use euphemisms to soften the blow of bad news, but this can lead to ambiguity. In addition to the clarity of the message, privacy, the attitude of the doctor, and the ability to answer questions are most important to patients and families receiving bad news.13 Although most would encourage touching in these difficult situations, it has been reported that up to a third of patients surveyed do not want physical contact.
Contrary to what providers may believe, diagnostic disclosure is not the most important part of a bad‐news discussion. Many patients believe it is most important to receive information on prognosis and treatment options. Often, patients want to discuss life expectancy. However, physicians are hesitant to address this issue. One study revealed that despite these requests, 22% of physicians would not provide any estimates at all, and when they did, 36% offered an overestimate.14 The authors hypothesized that how confident physicians are in this prognostic estimate and how much and what type of practice experience they have may influence their willingness to communicate a frank survival estimate.
The traditional dilemma of balancing hope with realism is reframed by Hagerty et al., who found that 98% of the patients they surveyed preferred a realistic and individualized approach.15 Use of euphemism and apparent unease of the provider actually decreases hope. Clayton et al. added that nurturing hope can also be facilitated by emphasizing what can be done, such as symptomatic management, emotional support, and practical support, particularly in terms of day‐to‐day living.16
In the case discussed in this article, preparation should have included asking the patient (1) whom he wanted present during the meeting, (2) how much information he and his family wanted to know, and (3) how involved he wanted his family to be. The informational needs of patients and their families will evolve over time as they process and accept the news. Thus, the asktellask approach remains a key concept to keep in mind as the dialogue continues beyond the initial encounter.
CASE
The attending physician attempted to continue the discussion by addressing both patient and daughter. He restated, JR, you have cancer in the fluid in your belly, and it is likely widespread throughout your body.
At that moment, the daughter became very tearful and emotional. There were several minutes of silence. The patient began to sob as well. The oncology fellow broke the silence by adding, Unfortunately, there is nothing that can be done when cancer is so widespread. The daughter broke into audible sobs; the patient looked away from the team and gazed through the window into the distance. The team fell silent and quietly left the room.
Importance of Empathy
The team failed in its inability to respond to emotion in this case. The emotional turmoil was apparent, but the team members made no attempt to acknowledge this emotion or to arrange additional emotional support. This could, at least in part, be a result of the providers' inability to process and manage their own emotions. A preparatory meeting beforehand and a debriefing session afterward for all the team members may have helped. Awareness of patients' coping strategies and various effective responses to these coping strategies may have better prepared the team to react and validate this patient's emotions. The role that psychology and emotion play cannot be overemphasized and clearly are important considerations. A useful mnemonic highlighting the components of the empathetic response is NURSE: naming, understanding, respect, supporting, and exploring.9 Using this technique, the provider starts by naming the emotion (anger, fear, disbelief); confirms a clear understanding of the patient's feelings; expresses respect verbally or nonverbally, letting the patient know the emotion is important; uses supporting statements that may express concern, reiterate understanding, or indicate a willingness to help; and closes by exploring additional concerns.
Certain phrases such as the one uttered by the fellowThere is nothing that can be doneshould be avoided.
Cultural Issues
Cultural diversity is increasingly common in contemporary medical practice in the United States. Some have suggested the Western value of autonomy is not embraced by all cultures. It has been suggested that non‐English‐speaking patients may receive less optimal end‐of‐life care than their English‐speaking counterparts.17 Beyond the language barrier, this observation may be a reflection of associated cultural barriers as well. Effective strategies for key issues of truth telling, language, family involvement, and decision making may help effective cross‐cultural communication and understanding and thus be effective patient‐centered care.18
A study of Korean patients and family members revealed a marked discordance between family and patients in the desire for disclosure.19 Almost all patients wanted to be informed that they had terminal illness, whereas a quarter of family members did not want physicians to relay this bad news to the patient. Interestingly, this study found patients would prefer to be told by the physician, whereas their family members would prefer to be the ones to deliver the news.
In some cultures it is believed that disclosure of bad news may cause patients to lose hope and hasten death. Physicians in these cultures may be more likely to honor family wishes. Language barriers may make a difficult situation even more complex. It is important to ascertain early on in what language patients and their families want to hold discussions. A medical interpreter should always be utilized for discussions with patients and their families. Dependence on a family member to interpret is not advised because the objective point of view may be lost in the interpretation. In addition, this places an enormous burden on the family member to be the bearer of bad news, which could have a lasting emotional impact. Although in the case discussed in this article, the patient's daughter was bilingual and could have translated, an interpreter should have been present for all discussions with this patient. Again, the importance of soliciting the patient's preference is critical.
Prior knowledge of the language requirement and of the patient's need for his daughter's involvement would also aid in the planning process. Because the patient's primary care physician (PCP) shared JR's Hispanic heritage, consultation with this PCP might have provided important insight, resulting in better preparation and planning.
How to Deliver Bad News
A number of guidelines are available to help physicians structure their conversations.2022 Baile and Buckman outline a 6‐step approach (Table 2). Rabow and Mcphee recommend the ABCDE mnemonic to help providers remember techniques for delivering bad news (Table 3). These recommendations are largely based on the literature to date. Both these approaches first emphasize preparations and planning. A private and quiet space, the presence of significant others if desired, arrangement to minimize interruptions, and provider emotional preparations are all prerequisites for the success of this type of encounter before the actual dialogue begins. As the encounter begins, it is key to assess a patient's needs if not already done before conveying information. Unidirectional transfer of information most likely will fail to satisfy the patient. The resident's initial conversation suffered in this key aspect.
| Setting up interview | Maintain privacy, involve significant others, sit down, make a connection, minimize interruptions. |
| Assessing patient perceptions | What have you been told about your illness? |
| Obtaining patient's invitation | How would you like me to give the information about your test results? |
| Giving knowledge and information to patient | Begin with warning statement, avoid jargon, avoid excessive bluntness. |
| Addressing patient's emotions | Listen, observe, acknowledge the emotion. |
| Providing strategy and summary | Give prognosis and treatment options and address symptoms. |
| Advance preparation |
|---|
| Ask what the patient already knows and understands. |
| Arrange for the presence of a support person and appropriate family. |
| Arrange a time and place that will be undisturbed (hand off beeper). |
| Prepare emotionally. |
| Decide which words and phrases to use (write down a script). |
| Practice delivering the news. |
| Build a therapeutic environment/relationship |
| Arrange a private, quiet place without interruptions. |
| Provide adequate seating for all. |
| Sit close enough to touch if appropriate. |
| Reassure about pain, suffering, abandonment. |
| Communicate well |
| Be direct (I am sorry, I have bad news). |
| Do not use euphemisms, jargon, or acronyms. |
| Do say cancer or death. |
| Allow for silence. |
| Use touch appropriately. |
| Ask patient to repeat his or her understanding of the news. |
| Arrange additional meetings. |
| Use repetition and written explanations of reminders. |
| Deal with patient and family reactions |
| Assess patient reaction |
| Physiologic responses: flight/fight, conservation/withdrawal; |
| Cognitive coping strategies: denial, blame, intellectualization, disbelief, acceptance; |
| Affective responses: anger/rage, fear/terror, anxiety, helplessness, hopelessness, shame, relief, guilt, sadness, anticipatory grief; |
| Listen actively, explore feelings, express empathy. |
| Encourage and validate emotions |
| Correct distortions. |
| Offer to tell others on behalf of the patient. |
| Evaluate the effects of the news. |
| Explore what the news means to the patient. |
| Address further needs, determine the patient's immediate and near‐term plans, assess suicidality. |
| Make appropriate referrals for more support, provide written materials, and arrange follow up. |
| Process your own feelings. |
Armed with knowledge of a patient's individual preferences, it is then possible to effectively convey information in a clear manner without jargon, using a direct but not blunt style. Both the SPIKES and ABCDE approaches similarly emphasize the asktellask approach. The attending physician was fairly effective in applying this communication approach in the subsequent encounter. However, the team left the room without providing a summary and follow‐up plan. Even though the patient and his daughter were quite emotional, acknowledging their reactions and appropriately ending the meeting with a summary and plans for the next steps would have been helpful in this continuing dialogue.
Hospitalist‐Specific Issues
Hospitalists may face special challenges when delivering bad news to patients. Without the benefit of preexisting longitudinal relationships with their patients, they lack prior understanding of a patient's values, family support system, and other cultural, spiritual, and social issues. Thus, preparation for these conversations is more difficult, and establishing rapport is more time‐consuming. There are no data available to describe the impact that not having a previous relationship with a patient has on these encounters. It is possible that the newness of the hospitalistpatient relationship may allow more candid, transparent communication than would be possible with established providers, who may themselves be struggling with the news and how it reflects on their care or the emotional impact of the impending loss.
Handoffs are a frequent part of the care the hospitalist provides, but communicating bad news is often a longitudinal process. One hospitalist may have the initial conversation regarding the patient's disease and prognosis, but the follow‐up often falls to a different hospitalist. Continuity of communication and awareness about what has been said previously are critical. It is important to explicitly document these conversations and their content in the medical record. In additional, summaries of pivotal conversations should be included in sign‐out. At discharge, whether patients are transitioning to postacute care or back to the outpatient arena, the hospitalist should carefully and vigilantly communicate critical conversations and predictions about patients' emotional needs.
Hospitalists do have some advantages when it comes to communication with patients. Unlike in outpatient practice, where clinicians are under pressure to keep up with a heavily loaded patient schedule, the hospitalist often has the flexibility and ability to allot time to each patient according to that patient's need. In addition, by definition, a hospitalist is in a hospital; this availability allows for more timely meetings, minimal delay in delivery of news, and accommodating the schedules of other people the patient may want included in any conversations.
CASE
A subsequent meeting occurred between the patient, his daughter, and the team, this time including a social worker and a hospice nurse.
The social worker began this discussion by stating, I understand you were quite upset last time and understandably so, and then inquired, What questions can we help you answer?
The patient and his daughter appropriately asked about alternatives to the usual aggressive treatment, and he made clear his desire to eventually spend his last days at home with family.
Through the translator, the hospice nurse succinctly explained the concept of palliative care with emphasis on symptomatic management as an alternative to aggressive curative therapy. JR and his daughter chose this palliative approach to care. This decision to focus on palliation was conveyed to JR's PCP. JR was eventually discharged to a short‐term postacute facility for rehabilitation and palliative care.
The health care team was finally able to acknowledge and validate JR's emotions with the help of the additional expertise of a social worker and a hospice nurse. This multidisciplinary approach allows team members to complement each other's strengths and weaknesses. Further, the patient had time to process his feelings and articulate his questions, values, and desires. Time often is required for this type of news to be more fully understood and eventually accepted. Breaking bad news is not a single event but a continuing dialogue and ultimately a relationship. Thus, proper delivery of bad news not only requires planning, effective communication, and empathy, but also deliberate follow‐up.
Training
What can be done to improve the effectiveness and satisfaction of these interactions for patients, providers, and families? Awareness of guidelines and effective strategies is a start but is unlikely to really change behavior or improve skills. Communication skills must be practiced, implemented, and observed with opportunity for feedback.23 Graduate and postgraduate training is probably the best time to develop these skills, and formal training in this area should be incorporated in a curriculum. Workshops on communicating bad news are offered frequently to oncologists and oncology fellows at various regional and national meetings. Ideally, these workshops would be offered at CME meetings specifically designed for hospitalists already in practice. Comprehensive palliative care training and materials, including specific modules and live workshops for delivering bad news, are available via the Education in Palliative and End‐of‐Life Care Project (EPEC) and the End‐of‐Life/Palliative Education Resource Center (EPERC) at the University of Wisconsin.
Hospitalists and trainees fortunate enough to practice in an institution with a palliative care service have the opportunity to learn from a multidisciplinary team, often including social workers, nursing staff, physicians and spiritual leaders. This interdisciplinary model is likely a more effective way to address the diverse physical, emotional, social, and spiritual needs of patients receiving difficult news and provides an ideal framework for this training.
CONCLUSIONS
Hospitalists are frequently called on to deliver bad news. A specific skills set is needed to be an effective communicator, especially in these stressful situations. Familiarity with an evidence‐based approach to this process and incorporation of the key steps into each of these encounters will likely improve patient and provider satisfaction as well as patient care during these critical times. Patient and family preferences for communication vary; so communication should be adjusted for each patient using the asktellask approach. Providers must remember to respond empathetically to emotion expressed by the patient and family and should keep the NURSE mnemonic in mind to guide the discussion. Providers should seek hands‐on training opportunities, which include supervision and feedback. Medical educators should incorporate training on the communication of bad news into curricula for students and trainees. Hospitalists may take a leadership role in teaching these skills at their institutions.
Broadly defined, bad news is any information that negatively alters a person's expectation about the present and the future.1 Importantly, news is defined as bad based on the patient's perspective about the information. Providers must remember that it will not always be obvious what patients will interpret as bad news. Although all would agree that the diagnosis of a new cancer would qualify as bad news, to some patients discovering hypertension would be deeply disturbing. Delivering bad news is difficult and stressful to all involved. Substantial data are now available describing patient preferences in these interactions, the impact on physicians who participate in these conversations, and specific recommendations for the delivery of bad news.
Hospitalists face additional challenges: lacking long‐standing relationships with patients and dealing with discontinuity in patient care and patient handoffs on a regular basis. Using an actual case as an example, this article examines the patient/family perspective and the provider perspective and reviews practical advice, actual phrases, useful mnemonics, and communication techniques to make these conversations more successful and less stressful. Opportunities to increase training in this area of palliative care are discussed. Adequate preparation, effective communication skills, empathy, and planned follow‐up are essential steps to assure that the goals for these difficult interactions are met.2, 3
CASE
The following scenario is based on an actual patient. The details and initials have been changed to maintain anonymity.
A 52‐year‐old Latino man, JR, was admitted with new‐onset ascites. He had a known diagnosis of end‐stage liver disease from prior alcohol use. Paracentesis revealed spontaneous bacterial peritonitis, and appropriate antibiotics were started. The fluid was sent for cytological analysis; the final diagnosis reported adenocarcinoma. A subsequent workup including PSA and CT of the abdomen/pelvis did not reveal the primary site of this malignancy.
JR had a supportive family and an established primary care physician. His spouse was no longer involved in his life, but his 2 daughters provided strong social support. His primary language was Spanish.
During the first 3 days of JR's hospital stay, he developed increasing abdominal pain, requiring escalating doses of narcotics. On the fourth day, the team received the cytology results, and the medical resident discussed the new diagnosis of cancer with JR. This conversation was not supervised by an attending, no interpreter was present, and no family members were in the room.
On entering the room, the resident said to the patient, I have bad news for you, JR.
The patient turned and said, Yes.
The resident continued, JR, you have cancer, and we don't know where it originated from.
The patient was silent and without expression. Unclear about how to proceed, the resident went on to say, The oncologist will be coming by later to discuss options with you. As there was no response, verbal or otherwise, the resident exited the room. The resident reported that the patient was unexpectedly calm after the news.
Provider Perspective
The responsibility of breaking bad news to patients weighs heavily on clinicians. As in this case, most providers' first experience with breaking bad news occurs with patients they have known for only a few hours or days. Even for the more experienced, this part of the job is rated as at least moderately stressful. Notably, most also feel that this stress lasts beyond the encounter, despite their perceived ability to manage their own stress during these situations.4 Additional training on clinicians' own coping skills may alleviate some of the emotional burden.
Provider's awareness and management of distress may enhance ability to provide comfort to patients or to specifically address their needs. Medical providers may try to suppress personal thoughts and feelings in these situations, but they bring emotional attachments to almost all encounters with patients.5 Emotional preparation by the provider is an important step prior to delivering bad news. Self‐reflection helps to identify personal emotions of sadness, anger, fear, or guilt and will help the provider not to disengage from the delivery of bad news.6 It is normal to have strong feelings, especially in difficult situations. Encouraging and validating these emotions personally will lead to a more therapeutic presence during a patient's time of need.7
Clinicians' perceptions of their interactions with patients when discussing bad news are probably more strongly influenced by the content of the discussion rather than the process itself. When asked to analyze their own videotaped consultations, doctors thought performance was worse when discussing palliative therapy than when discussing curative therapy.8
Traditionally, greatest emphasis has been placed on the acquisition and assessment of medical knowledge in medical training, and thus the focus on content is understandable. But more recently, efforts have been made at all levels of medical education to shift this focus toward encompassing many other competencies including professionalism and communication skills, which should translate into equal emphasis on the quality of these interactions.
As many hospitalists work closely with trainees, they are in the ideal position to serve as mentors and role models for communication. The case discussed in this article provides an example of a missed teaching opportunity. Ideally, the attending would have gone through the steps of preparation with the resident prior to the meeting, reviewed one or several of the suggested approaches discussed below, and observed the conversation and provided immediate feedback and a forum for processing afterward. It is especially helpful when first developing this skill to be familiar with helpful phrases to open the conversation, clarify patient preferences for communication, and convey empathy. It is also helpful to be aware of phrases that should be avoided (Table 1).
| Phrases to consider |
|---|
| To start a conversation |
| I am sorry to have to tell you this. |
| I know this is not good news. |
| I wish I had better news. |
| To elicit patient preferences |
| Would you like your family here when we talk about this? |
| Would you rather I speak with you about this or your daughter? |
| Some people want to be very involved in making decisions about their medical care, and some people want their doctor to just give them a recommendationhow do you feel about that? |
| To facilitate empathy |
| I can see how upsetting this is. |
| Is it okay if I hold your hand? |
| Phrases to avoid |
| There is nothing more we can do for you. |
| I know what this must be like. |
| I understand what you are going through. |
CASE
The following morning the attending physician, medical resident, and oncology fellow met with the patient and his daughter for a more extensive discussion. The goals of this discussion were to review the diagnosis and discuss the prognosis and future approaches to care. The entire discussion was conducted via a professional Spanish interpreter.
The attending physician began the conversation by asking, What do you remember about what the resident doctor told you, JR? pointing to the resident.
JR replied, I don't remember, and then went on to say, Please talk to my daughter, who was sitting across the room.
Family/Patient Perspective
As patient preferences for receiving bad news vary widely, it is these preferences that should determine the approach to the delivery, content, and context in which the news will be received. Some patients want information, and some do not; this needs to be clarified before beginning the discussion. The amount of detail should be negotiated in advance as well. As suggested by Back et al., soliciting patient preference prior to a discussion is important.9 These authors recommended using an approach called asktellask. This approach emphasizes the importance of asking questions to assess a patient's needs, telling the patient the information that meets those needs, and asking again to assess the patient's understanding.
Patients will rarely raise the issue of bad news with providers. In general, the provider must initiate the discussion.10 Surveys of patient preferences for delivery of bad news lend insight into this process and help guide providers during this challenging time. Patients report poor delivery is often characterized by bluntness, a lack of hope, and initiation of this serious conversation at an inappropriate time or place.11 Patients prefer providers to speak in clear, simple terms, being careful not to use technical jargon.12 Clinicians often use euphemisms to soften the blow of bad news, but this can lead to ambiguity. In addition to the clarity of the message, privacy, the attitude of the doctor, and the ability to answer questions are most important to patients and families receiving bad news.13 Although most would encourage touching in these difficult situations, it has been reported that up to a third of patients surveyed do not want physical contact.
Contrary to what providers may believe, diagnostic disclosure is not the most important part of a bad‐news discussion. Many patients believe it is most important to receive information on prognosis and treatment options. Often, patients want to discuss life expectancy. However, physicians are hesitant to address this issue. One study revealed that despite these requests, 22% of physicians would not provide any estimates at all, and when they did, 36% offered an overestimate.14 The authors hypothesized that how confident physicians are in this prognostic estimate and how much and what type of practice experience they have may influence their willingness to communicate a frank survival estimate.
The traditional dilemma of balancing hope with realism is reframed by Hagerty et al., who found that 98% of the patients they surveyed preferred a realistic and individualized approach.15 Use of euphemism and apparent unease of the provider actually decreases hope. Clayton et al. added that nurturing hope can also be facilitated by emphasizing what can be done, such as symptomatic management, emotional support, and practical support, particularly in terms of day‐to‐day living.16
In the case discussed in this article, preparation should have included asking the patient (1) whom he wanted present during the meeting, (2) how much information he and his family wanted to know, and (3) how involved he wanted his family to be. The informational needs of patients and their families will evolve over time as they process and accept the news. Thus, the asktellask approach remains a key concept to keep in mind as the dialogue continues beyond the initial encounter.
CASE
The attending physician attempted to continue the discussion by addressing both patient and daughter. He restated, JR, you have cancer in the fluid in your belly, and it is likely widespread throughout your body.
At that moment, the daughter became very tearful and emotional. There were several minutes of silence. The patient began to sob as well. The oncology fellow broke the silence by adding, Unfortunately, there is nothing that can be done when cancer is so widespread. The daughter broke into audible sobs; the patient looked away from the team and gazed through the window into the distance. The team fell silent and quietly left the room.
Importance of Empathy
The team failed in its inability to respond to emotion in this case. The emotional turmoil was apparent, but the team members made no attempt to acknowledge this emotion or to arrange additional emotional support. This could, at least in part, be a result of the providers' inability to process and manage their own emotions. A preparatory meeting beforehand and a debriefing session afterward for all the team members may have helped. Awareness of patients' coping strategies and various effective responses to these coping strategies may have better prepared the team to react and validate this patient's emotions. The role that psychology and emotion play cannot be overemphasized and clearly are important considerations. A useful mnemonic highlighting the components of the empathetic response is NURSE: naming, understanding, respect, supporting, and exploring.9 Using this technique, the provider starts by naming the emotion (anger, fear, disbelief); confirms a clear understanding of the patient's feelings; expresses respect verbally or nonverbally, letting the patient know the emotion is important; uses supporting statements that may express concern, reiterate understanding, or indicate a willingness to help; and closes by exploring additional concerns.
Certain phrases such as the one uttered by the fellowThere is nothing that can be doneshould be avoided.
Cultural Issues
Cultural diversity is increasingly common in contemporary medical practice in the United States. Some have suggested the Western value of autonomy is not embraced by all cultures. It has been suggested that non‐English‐speaking patients may receive less optimal end‐of‐life care than their English‐speaking counterparts.17 Beyond the language barrier, this observation may be a reflection of associated cultural barriers as well. Effective strategies for key issues of truth telling, language, family involvement, and decision making may help effective cross‐cultural communication and understanding and thus be effective patient‐centered care.18
A study of Korean patients and family members revealed a marked discordance between family and patients in the desire for disclosure.19 Almost all patients wanted to be informed that they had terminal illness, whereas a quarter of family members did not want physicians to relay this bad news to the patient. Interestingly, this study found patients would prefer to be told by the physician, whereas their family members would prefer to be the ones to deliver the news.
In some cultures it is believed that disclosure of bad news may cause patients to lose hope and hasten death. Physicians in these cultures may be more likely to honor family wishes. Language barriers may make a difficult situation even more complex. It is important to ascertain early on in what language patients and their families want to hold discussions. A medical interpreter should always be utilized for discussions with patients and their families. Dependence on a family member to interpret is not advised because the objective point of view may be lost in the interpretation. In addition, this places an enormous burden on the family member to be the bearer of bad news, which could have a lasting emotional impact. Although in the case discussed in this article, the patient's daughter was bilingual and could have translated, an interpreter should have been present for all discussions with this patient. Again, the importance of soliciting the patient's preference is critical.
Prior knowledge of the language requirement and of the patient's need for his daughter's involvement would also aid in the planning process. Because the patient's primary care physician (PCP) shared JR's Hispanic heritage, consultation with this PCP might have provided important insight, resulting in better preparation and planning.
How to Deliver Bad News
A number of guidelines are available to help physicians structure their conversations.2022 Baile and Buckman outline a 6‐step approach (Table 2). Rabow and Mcphee recommend the ABCDE mnemonic to help providers remember techniques for delivering bad news (Table 3). These recommendations are largely based on the literature to date. Both these approaches first emphasize preparations and planning. A private and quiet space, the presence of significant others if desired, arrangement to minimize interruptions, and provider emotional preparations are all prerequisites for the success of this type of encounter before the actual dialogue begins. As the encounter begins, it is key to assess a patient's needs if not already done before conveying information. Unidirectional transfer of information most likely will fail to satisfy the patient. The resident's initial conversation suffered in this key aspect.
| Setting up interview | Maintain privacy, involve significant others, sit down, make a connection, minimize interruptions. |
| Assessing patient perceptions | What have you been told about your illness? |
| Obtaining patient's invitation | How would you like me to give the information about your test results? |
| Giving knowledge and information to patient | Begin with warning statement, avoid jargon, avoid excessive bluntness. |
| Addressing patient's emotions | Listen, observe, acknowledge the emotion. |
| Providing strategy and summary | Give prognosis and treatment options and address symptoms. |
| Advance preparation |
|---|
| Ask what the patient already knows and understands. |
| Arrange for the presence of a support person and appropriate family. |
| Arrange a time and place that will be undisturbed (hand off beeper). |
| Prepare emotionally. |
| Decide which words and phrases to use (write down a script). |
| Practice delivering the news. |
| Build a therapeutic environment/relationship |
| Arrange a private, quiet place without interruptions. |
| Provide adequate seating for all. |
| Sit close enough to touch if appropriate. |
| Reassure about pain, suffering, abandonment. |
| Communicate well |
| Be direct (I am sorry, I have bad news). |
| Do not use euphemisms, jargon, or acronyms. |
| Do say cancer or death. |
| Allow for silence. |
| Use touch appropriately. |
| Ask patient to repeat his or her understanding of the news. |
| Arrange additional meetings. |
| Use repetition and written explanations of reminders. |
| Deal with patient and family reactions |
| Assess patient reaction |
| Physiologic responses: flight/fight, conservation/withdrawal; |
| Cognitive coping strategies: denial, blame, intellectualization, disbelief, acceptance; |
| Affective responses: anger/rage, fear/terror, anxiety, helplessness, hopelessness, shame, relief, guilt, sadness, anticipatory grief; |
| Listen actively, explore feelings, express empathy. |
| Encourage and validate emotions |
| Correct distortions. |
| Offer to tell others on behalf of the patient. |
| Evaluate the effects of the news. |
| Explore what the news means to the patient. |
| Address further needs, determine the patient's immediate and near‐term plans, assess suicidality. |
| Make appropriate referrals for more support, provide written materials, and arrange follow up. |
| Process your own feelings. |
Armed with knowledge of a patient's individual preferences, it is then possible to effectively convey information in a clear manner without jargon, using a direct but not blunt style. Both the SPIKES and ABCDE approaches similarly emphasize the asktellask approach. The attending physician was fairly effective in applying this communication approach in the subsequent encounter. However, the team left the room without providing a summary and follow‐up plan. Even though the patient and his daughter were quite emotional, acknowledging their reactions and appropriately ending the meeting with a summary and plans for the next steps would have been helpful in this continuing dialogue.
Hospitalist‐Specific Issues
Hospitalists may face special challenges when delivering bad news to patients. Without the benefit of preexisting longitudinal relationships with their patients, they lack prior understanding of a patient's values, family support system, and other cultural, spiritual, and social issues. Thus, preparation for these conversations is more difficult, and establishing rapport is more time‐consuming. There are no data available to describe the impact that not having a previous relationship with a patient has on these encounters. It is possible that the newness of the hospitalistpatient relationship may allow more candid, transparent communication than would be possible with established providers, who may themselves be struggling with the news and how it reflects on their care or the emotional impact of the impending loss.
Handoffs are a frequent part of the care the hospitalist provides, but communicating bad news is often a longitudinal process. One hospitalist may have the initial conversation regarding the patient's disease and prognosis, but the follow‐up often falls to a different hospitalist. Continuity of communication and awareness about what has been said previously are critical. It is important to explicitly document these conversations and their content in the medical record. In additional, summaries of pivotal conversations should be included in sign‐out. At discharge, whether patients are transitioning to postacute care or back to the outpatient arena, the hospitalist should carefully and vigilantly communicate critical conversations and predictions about patients' emotional needs.
Hospitalists do have some advantages when it comes to communication with patients. Unlike in outpatient practice, where clinicians are under pressure to keep up with a heavily loaded patient schedule, the hospitalist often has the flexibility and ability to allot time to each patient according to that patient's need. In addition, by definition, a hospitalist is in a hospital; this availability allows for more timely meetings, minimal delay in delivery of news, and accommodating the schedules of other people the patient may want included in any conversations.
CASE
A subsequent meeting occurred between the patient, his daughter, and the team, this time including a social worker and a hospice nurse.
The social worker began this discussion by stating, I understand you were quite upset last time and understandably so, and then inquired, What questions can we help you answer?
The patient and his daughter appropriately asked about alternatives to the usual aggressive treatment, and he made clear his desire to eventually spend his last days at home with family.
Through the translator, the hospice nurse succinctly explained the concept of palliative care with emphasis on symptomatic management as an alternative to aggressive curative therapy. JR and his daughter chose this palliative approach to care. This decision to focus on palliation was conveyed to JR's PCP. JR was eventually discharged to a short‐term postacute facility for rehabilitation and palliative care.
The health care team was finally able to acknowledge and validate JR's emotions with the help of the additional expertise of a social worker and a hospice nurse. This multidisciplinary approach allows team members to complement each other's strengths and weaknesses. Further, the patient had time to process his feelings and articulate his questions, values, and desires. Time often is required for this type of news to be more fully understood and eventually accepted. Breaking bad news is not a single event but a continuing dialogue and ultimately a relationship. Thus, proper delivery of bad news not only requires planning, effective communication, and empathy, but also deliberate follow‐up.
Training
What can be done to improve the effectiveness and satisfaction of these interactions for patients, providers, and families? Awareness of guidelines and effective strategies is a start but is unlikely to really change behavior or improve skills. Communication skills must be practiced, implemented, and observed with opportunity for feedback.23 Graduate and postgraduate training is probably the best time to develop these skills, and formal training in this area should be incorporated in a curriculum. Workshops on communicating bad news are offered frequently to oncologists and oncology fellows at various regional and national meetings. Ideally, these workshops would be offered at CME meetings specifically designed for hospitalists already in practice. Comprehensive palliative care training and materials, including specific modules and live workshops for delivering bad news, are available via the Education in Palliative and End‐of‐Life Care Project (EPEC) and the End‐of‐Life/Palliative Education Resource Center (EPERC) at the University of Wisconsin.
Hospitalists and trainees fortunate enough to practice in an institution with a palliative care service have the opportunity to learn from a multidisciplinary team, often including social workers, nursing staff, physicians and spiritual leaders. This interdisciplinary model is likely a more effective way to address the diverse physical, emotional, social, and spiritual needs of patients receiving difficult news and provides an ideal framework for this training.
CONCLUSIONS
Hospitalists are frequently called on to deliver bad news. A specific skills set is needed to be an effective communicator, especially in these stressful situations. Familiarity with an evidence‐based approach to this process and incorporation of the key steps into each of these encounters will likely improve patient and provider satisfaction as well as patient care during these critical times. Patient and family preferences for communication vary; so communication should be adjusted for each patient using the asktellask approach. Providers must remember to respond empathetically to emotion expressed by the patient and family and should keep the NURSE mnemonic in mind to guide the discussion. Providers should seek hands‐on training opportunities, which include supervision and feedback. Medical educators should incorporate training on the communication of bad news into curricula for students and trainees. Hospitalists may take a leadership role in teaching these skills at their institutions.
- .Breaking bad news: why is it still so difficult?BMJ.1984;288:1597–1599.
- ,.Bad news: delivery, dialogue, and dilemmas.Arch Intern Med.1991;151:463–468.
- ,.Beyond breaking bad news: how to help patients who suffer.WJM.1999;171:260–263.
- ,,,.Breaking bad news to patients: physicians' perception of the process.Support Care Cancer.1999;7:113–120.
- ..Giving bad news to cancer patients: matching process and content.J Clin Oncol.2001;19:2575–2577.
- ,.Beyond breaking bad news:how to help patients who suffer.West J Med.1999;171:260–263.
- .Breaking bad news.Am Fam Physician.2001;64:1975–1978
- ,,.Truth may hurt but deceit hurts more: communication in palliative care.Palliat Med.2002;16:297–303.
- ,,, et al.Approaching difficult communication tasks in oncology.CA Cancer J Clin.2005;55:164–177.
- ,,,.Enhancing physician‐patient communication.Hematology Am Soc Hematol Educ Program.2002;464–483.
- ,,,,,.Communicating with dying patients within the spectrum of care from terminal diagnosis to death.Arch Intern Med.2001;161:868–874.
- ,,,.Communicating prognosis in early breast cancer: do women understand the language used?Med J Aust.1999;171:290–294.
- ,,,.Giving bad news: the family perspective.J Trauma.2000;48:865–870
- ,.Prognostic disclosure to patients with cancer near the end of life.Ann Intern Med.2001;134:1096–1105.
- ,,, et al.Communicating with realism and hope: incurable cancer patients' views on the disclosure of prognosis.J Clin Oncol.2005;23:1278–1288.
- ,,, et al.Fostering coping and nurturing hope when discussing the future with terminally ill cancer patients and their caregivers.Cancer.2005;103:1965–1975.
- ,.Comparison of palliative care needs of English and non‐English‐speaking patients.J Palliat Care.1999;15(1):26–30
- ,.Negotiating cross‐cultural issues at the end of life.JAMA.2001;286:2993–3001
- ,,,,,.The attitudes of cancer patients and their families towards disclosure of terminal illness.J Clin Oncol.2004;22:307–314.
- ,.Breaking bad news:consensus guidelines for medical practitioners.J Clin Oncol.1995;13:2449–2456
- ,,, et al.SPIKES—a six step protocol for delivering bad news: application to the patient with cancer.Oncologist.2000;5:302–311
- ,.Beyond breaking bad news:how to help patients who suffer.West J Med.1999;171:260–263
- ,,.Communication skills training for health professionals working with cancer patients, their families, and/or careers.Cochrane Database Syst Rev.2003;2:CD003751.
- .Breaking bad news: why is it still so difficult?BMJ.1984;288:1597–1599.
- ,.Bad news: delivery, dialogue, and dilemmas.Arch Intern Med.1991;151:463–468.
- ,.Beyond breaking bad news: how to help patients who suffer.WJM.1999;171:260–263.
- ,,,.Breaking bad news to patients: physicians' perception of the process.Support Care Cancer.1999;7:113–120.
- ..Giving bad news to cancer patients: matching process and content.J Clin Oncol.2001;19:2575–2577.
- ,.Beyond breaking bad news:how to help patients who suffer.West J Med.1999;171:260–263.
- .Breaking bad news.Am Fam Physician.2001;64:1975–1978
- ,,.Truth may hurt but deceit hurts more: communication in palliative care.Palliat Med.2002;16:297–303.
- ,,, et al.Approaching difficult communication tasks in oncology.CA Cancer J Clin.2005;55:164–177.
- ,,,.Enhancing physician‐patient communication.Hematology Am Soc Hematol Educ Program.2002;464–483.
- ,,,,,.Communicating with dying patients within the spectrum of care from terminal diagnosis to death.Arch Intern Med.2001;161:868–874.
- ,,,.Communicating prognosis in early breast cancer: do women understand the language used?Med J Aust.1999;171:290–294.
- ,,,.Giving bad news: the family perspective.J Trauma.2000;48:865–870
- ,.Prognostic disclosure to patients with cancer near the end of life.Ann Intern Med.2001;134:1096–1105.
- ,,, et al.Communicating with realism and hope: incurable cancer patients' views on the disclosure of prognosis.J Clin Oncol.2005;23:1278–1288.
- ,,, et al.Fostering coping and nurturing hope when discussing the future with terminally ill cancer patients and their caregivers.Cancer.2005;103:1965–1975.
- ,.Comparison of palliative care needs of English and non‐English‐speaking patients.J Palliat Care.1999;15(1):26–30
- ,.Negotiating cross‐cultural issues at the end of life.JAMA.2001;286:2993–3001
- ,,,,,.The attitudes of cancer patients and their families towards disclosure of terminal illness.J Clin Oncol.2004;22:307–314.
- ,.Breaking bad news:consensus guidelines for medical practitioners.J Clin Oncol.1995;13:2449–2456
- ,,, et al.SPIKES—a six step protocol for delivering bad news: application to the patient with cancer.Oncologist.2000;5:302–311
- ,.Beyond breaking bad news:how to help patients who suffer.West J Med.1999;171:260–263
- ,,.Communication skills training for health professionals working with cancer patients, their families, and/or careers.Cochrane Database Syst Rev.2003;2:CD003751.
Evaluation of Clerkship Structure
The third‐year pediatric clerkship at the University of Utah School of Medicine has a relatively unique inpatient service, the Glasgow Service, which consists of an academic attending, a third‐year pediatric resident, and 4 third‐year medical students, but no interns. (This service was named in honor of Lowell Glasgow, chair of pediatrics, 1972‐82.) This structure was introduced in 1992 by the chair of pediatrics, Michael Simmons, the residency program director, Richard Molteni, and the clerkship director, Karen Hansen. These individuals desired to improve students' inpatient experience by providing greater responsibility for patient care. An additional motive was to increase the total number of patients followed by house staff without increasing the size of the residency program.
This inpatient service is a part of a 6‐week pediatric clerkship. All students perform the 3‐week inpatient portion of their clerkship at Primary Children's Medical Center, a tertiary‐care, freestanding children's hospital. (The students also spend 1 week each in a newborn nursery, an outpatient clinic, and a subspecialty setting). The academic attendings include generalists, hospitalists, and specialists who concurrently have other clinical responsibilities. The students take in‐house call every fourth night, supervised by senior residents who are not necessarily members of their service. All students share the same formal teaching activities, including morning report, a noon conference, and a student conference.
Patients are assigned to the ward services by a senior admitting resident. The admitting resident distributes patients among the services based on the complexity and acuity of the patients' conditions as well as the census on the various services. The senior resident supervising a particular service then assigns patients among the members of that service. Each third‐year medical student is expected to care for 2 or 3 patients at a time.
In addition to the intervention service, students also rotate on 2 similar traditional services. These services are traditional in the sense that they are composed of an academic attending, a community attending, a third‐year pediatric resident, 4 interns, and up to 2 fourth‐year and 2 third‐year medical students. Faculty preferences regarding service assignments were accommodated when possible. Therefore, some faculty attended only on one type of service, intervention or traditional, and others attended on both types. Because they have more members and because interns are capable of caring for more patients than are medical students, the traditional services cared for more patients than the intervention service. Although identical in composition, the 2 traditional services differ with each other in several ways. One service typically admits children 3 years old and younger, whereas the other admits children who are between 3 and 12 years old. The service that admits older children also admits most of the hematology‐oncology patients.
Although other authors have described similar inpatient clerkship structures, to our knowledge, none have evaluated them through a prospective randomized controlled trial.1, 2 The recent literature on ambulatory experiences during third‐year clerkships provided a methodological framework for this study. Collectively, such studies have evaluated outcomes with a variety of measures, including patient logs,35 evaluations,3, 4, 6, 7 examinations,37 surveys,3, 5, 7, 8 and career choices.4, 68 Additional outcomes, such as the effect of educational interventions on patient care, have been emphasized.9
In the light of this research, we conducted a prospective, randomized controlled trial to compare outcomes on the intervention service with those on the traditional services. We hypothesized that, compared with the traditional services, the intervention service would show:
improved process measures in terms of increased number of patients admitted, number of key diagnoses encountered in the patients cared for, and range of ages of the patients admitted;
similar or improved student performance, as measured by faculty and resident evaluations and a National Board of Medical Examiners (NBME) subject examination;
increased student satisfaction, as assessed by an end‐of‐rotation questionnaire;
increased interest in pediatric and, more broadly, primary care careers, as measured by subinternship and internship selections; and
comparable or improved resource utilization in terms of length of stay and total charges.
METHODS
All students enrolled in the third‐year pediatric rotation during the 2001‐2003 academic years were individually randomized by the clerkship assistant to the intervention service or 1 of the 2 traditional services without respect to career preference. A 5:3 student randomization ratio was used to fulfill the requirement that 4 students be assigned to the intervention service during every 3‐week block. This permitted the service to have call every fourth night.
To evaluate the adequacy of the randomization process, we obtained baseline student characteristics on age, sex, and United States Medical Licensing Examination (USMLE) Step 1 score from the Dean of Student Affairs. The dean also reported the discipline each student enrolled in for the required fourth‐year subinternship(s) and matched in for internship. These data were reported anonymously and linked to the service to which the student was assigned. In this study, pediatrics, internal medicine, and family practice were all considered primary care, but preliminary or transitional internships were not.
Process Measures
Students were required to submit logs at the end of their rotations, recording patients' names, ages, diagnoses, and admission dates. The accuracy and completeness of these logs were not independently verified.
As there was no authoritative list of key diagnoses third‐year medical students should encounter in the patients they care for during their inpatient rotations, we relied on expert opinion at our institution. The Council on Medical Student Education in Pediatrics' curriculum was not used because it did not differentiate between inpatient and ambulatory contexts. A preliminary list of 93 diagnoses was developed from the table of contents of Pediatric Hospital Medicine.10 This list was distributed to the 26 clinical faculty members in the Divisions of Pediatric Inpatient Medicine and General Pediatrics who were asked to select the 10 most important diagnoses. Surveys were numerically coded to permit 1 reminder.
The survey had a response rate of 92.3% (24 of 26 surveys). One survey was excluded because the respondent significantly deviated from the instructions. The 10 key diagnoses and the percentages of respondents who selected each individual diagnosis are: asthma (100%), febrile infant (95.6%), diarrhea and dehydration (91.3%), bronchiolitis (78.2%), diabetes mellitus and diabetic ketoacidemia (60.9%), failure to thrive (56.5%), urinary tract infections (52.1%), pneumonia (47.8%), upper airway infections such as croup (43.5%), and seizures and status epilepticus (43.5%).
Two of the authors independently coded the diagnoses on the students' patient logs in terms of these 93 diagnoses. The authors were blinded to the students' service assignment. As many students reported more than 1 diagnosis, the authors prioritized primary, secondary, and tertiary diagnoses to simplify the evaluation. The most likely cause of admission was listed as the primary diagnosis. If the authors could not reconcile divergent views, a third party was consulted.
Student Performance
Students were evaluated by both the attending physician(s) and senior resident(s) using a standardized evaluation form available from the corresponding author. The evaluation contained 18 items in 7 categories: data gathering, data recording/reporting, knowledge, data interpretation, clinical performance, professional attitudes, and professional demeanor. The student was rated exceptional, above expectations, meets expectations, below expectations, unacceptable, or not observed on each item. A short narrative description illustrated each rating. The ratings were converted to a 5‐point scale, with exceptional being 5. If the evaluator marked the line between 2 ratings, it was recorded as half. When multiple attendings or residents evaluated a student, the scores for a given item were collapsed into an average score.
Students also completed a NBME pediatric subject examination on the last day of their rotation.
Additionally, students were requested to complete a questionnaire during the final week of the clerkship. The items on the questionnaire were meant to access students' perceptions of the quality of their attendings' and residents' teaching, a potentially confounding variable. The survey was piloted on a group of similar subjects. Informed consent was obtained for survey completion. The survey was anonymous and required approximately 7 minutes to complete.
Resource Utilization
Last, resource utilization data, length of stay and total charges, for the 4 most common primary diagnoses were compared between the intervention and the traditional services. The 4 most common primary diagnoses and the percentage of total diagnoses (n = 2047) that each represents were bronchiolitis, 13%; febrile infant, 8.6%; pneumonia, 7.1%; and asthma, 6.5% (the diagnosis other accounted for 12% of the total diagnoses). Unique patient identifiers were used to obtain length of stay and total charges from the hospital's database. All‐Patient‐Refined Diagnosis‐Related Groups Severity of Illness (APR‐DRG‐SOI) were also obtained and used to construct multivariate models. Patients who were admitted to the pediatric intensive care unit (PICU) were excluded from the analysis.
Statistical Analysis
Statistical analyses were conducted and frequencies and percentages were calculated using Stata SE version 8.0 (College Station, TX). For all interval and ratio‐scaled variables, distributions were tested for normality using the Shapiro‐Wilks test to determine whether to use parametric or nonparametric statistical tests. For distributions meeting the normality assumption, the unpaired t test was used to compare the intervention service with traditional services. Where the normality assumption was not met, the Mann‐Whitney test was used. Categorically scaled data were compared using Pearson's chi‐square test. The standardized mean differences, reported as d values, were calculated to determine the effect size. Small, medium, and large effect sizes were defined as d values of 0.20, 0.50, and 0.80, respectively.11 Teaching quality, an effect modifier, was entered as a covariate into a linear regression model. Analyses of length of stay and total charges were conducted using multivariate linear regression controlling for patient age and severity of illness.
This study was approved by the University of Utah and Primary Children's Medical Center's Institutional Review Board.
RESULTS
Two hundred and three students enrolled in the third‐year pediatric clerkship during the study period, and all students completed the clerkship on their assigned services. One hundred and twenty‐eight were randomized to the intervention service and 75 to the traditional services. There were no statistically significant differences in median age, percentage of male students, or mean USMLE Step 1 score between the students randomized to the intervention service and those randomized to the traditional services (Table 1).
| Intervention service | Traditional services | P value | |
|---|---|---|---|
| |||
| Age (median) | 28 | 28 | .76* |
| Sex (% male) | 58.6 | 62.7 | .57 |
| USMLE Step 1 score | 217 | 217 | .94 |
Process Measures
Overall, 96.6% of students (196 of 203) submitted patient logs; 97.7% of students (125 of 128) on the intervention service and 94.7% of students (71 of 75) on the traditional services. The students on the intervention service admitted a median of 10 patients, whereas the students on the traditional services admitted a median of 11 patients (d = 0.45, P < .01). Age data were recorded on 137 patient logs (69.9% of submitted logs, 72.0% of students on the intervention service vs. 66.2% of students on the traditional services). The percentage of students who saw at least 1 newborn (birth‐23 months), child (2‐12 years), and adolescent (12‐18 years) was 34.8% on the intervention service and 33.3% on the traditional services (P = .87) (Table 2).
| Intervention service | Traditional services | d | P value | |
|---|---|---|---|---|
| ||||
| Median number of patients | 10 | 11 | 0.45 | < .01* |
| Percent of students who saw 1 newborn, child, and adolescent | 34.8% | 33.3% | 0.03 | .87 |
| Top 10 diagnoses cared for (n) | 4.4 | 3.6 | 0.48 | < .01 |
| Percent of patients cared for whose diagnoses were in top 10 | 59.3% | 46.8% | 0.62# | < .01 |
| Percent of unique diagnoses (median) | 80.0% | 80.0% | 0.02 | .62 |
Students on the intervention service encountered, on average, a larger number of the 10 key diagnoses (4.4 vs. 3.6, d = 0.48, P < .01) and a higher percentage of their patients had clinical conditions among the key diagnoses (59.3 vs. 46.8, d = 0.62, P < .01). To determine if this higher percentage was the result of admitting multiple patients with the same diagnosis, we examined the percentage of unique primary diagnosesthe number of different primary diagnoses divided by the total number of patientsand found no differences (Table 2).
Student Performance
The faculty and resident evaluations of the students showed statistically significant differences between those in the intervention service and those in the traditional services in only 2 of the 18 items. These items were analysis in the data interpretation category (3.81 vs. 3.64, d = 0.35, P = .02) and patient interaction in the professional demeanor category (3.89 vs. 3.76, d = 0.31, P < .05). Both differences favored the intervention service. There were no statistical differences by service in student performance on the NBME subject examination (73.2 vs. 72.3, P = .39).
Student Satisfaction
Overall, 87.2% of students (177 of 203) completed the survey; 87.5% of students (112 of 124) on the intervention service and 86.7% of students (65 of 75) on the traditional services. The students on the intervention service both had a more positive overall attitude about their rotation and were more likely to find it a satisfying educational experience. Students on the intervention service also reported greater participation in patient care. Effect sizes ranged from small to medium (Table 3). The internal consistency of answers about participation in patient care was high (Pearson correlation coefficient r = 0.80).
| Intervention service | Traditional services | d | P value | |
|---|---|---|---|---|
| ||||
| My overall attitude toward this rotation is: 1. highly negative to 5. highly positive | 4.48 | 4.26 | 0.26 | .02* |
| I found this rotation a satisfying educational experience: 1. strongly disagree to 5. strongly agree | 4.49 | 4.22 | 0.35 | < .01* |
| My role on this rotation was that of an: 1. observer, 3. participant, 5. director | 3.77 | 3.33 | 0.60# | < .01 |
| My supervising interns/residents were _____ teachers: 1. poor, 3. good, 5. exemplary | 3.91 | 3.75 | 0.17 | .26* |
| My input into patient care decisions was: 1. strongly discouraged to 5. strongly encouraged | 4.45 | 3.98 | 0.66# | < .01* |
| I was able to make a significant contribution to patient care: 1. strongly disagree to 5. strongly agree | 4.19 | 3.92 | 0.34 | .02* |
| I had direct responsibility for patient care: 1. strongly disagree to 5. strongly agree | 4.33 | 3.95 | 0.46 | .01* |
| My attendings were _____ teachers: 1. poor, 3. good, 5. exemplary | 4.09 | 3.75 | 0.40 | < .01* |
| I found the feedback I received during this rotation to be: 1. insufficient, 3. appropriate, 5. excessive | 2.84 | 2.65 | 0.22 | .17* |
| The following best describes the quality of my supervision during this rotation: 1. I was expected to do things beyond my competence unsupervised 3. The degree of supervision was appropriate for my level of training 5. I was excessively supervised on skills I had already demonstrated | 2.95 | 3.06 | 0.18 | .19 |
| During this rotation: 1. I was expected to see too many patients 3. I was expected to see an appropriate number of patients 5. I expected to see more patients | 3.46 | 3.31 | 0.18 | .33* |
| Before this rotation I _____ pediatrics as a career choice: 1. had rejected, 3. was considering, 5. had decided on | 2.37 | 2.14 | 0.22 | .11* |
| This rotation increased my interest in pursuing pediatrics as a career: 1. strongly disagree to 5. strongly agree | 3.74 | 3.60 | 0.14 | .32* |
Students on the intervention service rated the teaching of their attendings, but not of their residents, higher than did students on the traditional services. Controlling for the perceived quality of the attending, 3 of 6 satisfaction outcomes remained statistically significant: role on rotation (P < .01), input into patient care decisions (P < .01), and direct responsibility for patient care (P = .04). Students on both services believed they were appropriately supervised (P = .19). Despite the students on the traditional services on average admitting more patients, there was no significant difference by service in the students' rating of patient load (P = .33).
Career Choice
The odds ratio and 95% confidence interval for students enrolling in a pediatric subinternship was 1.94 (0.83‐4.49) and matching in a pediatric residency was 2.52 (0.99‐6.37). There were no statistically significant differences by service in the percentage of students enrolling in primary care (pediatric, internal medicine, and family practice) subinternships or residencies (Table 4).
| Intervention service | Traditional services | Odds ratio (95% CI) | |
|---|---|---|---|
| Pediatric subinternship | 19.5% | 11.1% | 1.94 (0.83‐4.49) |
| Primary care subinternship | 68.3% | 70.8% | 0.89 (0.47‐1.67) |
| Pediatric residency | 18.6% | 8.3% | 2.52 (0.99‐6.37) |
| Primary care residency | 40.7% | 31.9% | 1.46 (0.79‐2.70) |
Resource Utilization
One hundred and thirty‐five patients were excluded from the resource utilization analysis (n = 594) because their unique identifiers could not be found or they had been admitted to the PICU. Univariate analysis demonstrated statistically significant differences for patients with asthma, but not patients with bronchiolitis, febrile infants, or patients with pneumonia, favoring the intervention service. Patients with asthma admitted to the intervention service had a shorter length of stay (49.9 vs. 70.1 hours, P = .02) and lower total charges ($3600 vs. $4600, P = .02), as shown in Table 5. Of 4 multivariate models controlling for age and severity of illness, each with length of stay and total charges as the dependant variables, length of stay was significantly less for patients with asthma admitted to the intervention service only. Such patients were discharged an average of 23.3 hours earlier than patients with asthma admitted to the traditional services (P = .02).
| Diagnosis (n) | n | Length of stay (hours) | P value | Total charges | ||||
|---|---|---|---|---|---|---|---|---|
| Intervention service | Traditional services | Intervention service | Traditional services | Intervention service | Traditional services | P value | ||
| ||||||||
| Bronchiolitis (210) | 159 | 51 | 63.7 | 70.5 | .20* | $4300 | $4800 | .20* |
| Febrile infant (152) | 105 | 47 | 58.8 | 58.9 | .50* | $4800 | $4900 | .28* |
| Pneumonia (123) | 82 | 41 | 84.3 | 116.8 | .71* | $6300 | $9200 | .63* |
| Asthma (109) | 80 | 29 | 49.9 | 70.1 | .02* | $3600 | $4600 | .02* |
DISCUSSION
This study's objective was to evaluate a third‐year pediatric clerkship structure that focuses on students, using multiple outcome parameters. Utilizing a robust design, the results of this study have demonstrated that the intervention service is more successful than the traditional services in several outcomes. Students assigned to the intervention service were more satisfied and more likely to select pediatrics as a career. These improvements were accomplished while maintaining similar process measures, student performance, and resource utilization compared with those of the traditional services.
Methods
The methods used in this study compare favorably with other evaluations of educational interventions. The present study incorporated a randomized controlled design.12 Although several studies of ambulatory clerkships used a randomized design, few randomized all eligible students.7, 8 The others used some form of selection prior to randomization. For example, in the Pangaro et al. study, students selected their clerkship site by lottery, with students selecting a certain site then offered the opportunity to participate in the intervention.6 The present study manifested several additional strengths. Multiple outcomes, including effects on patient care, were evaluated. Moreover, this study had a relatively large intervention group and total sample size compared with those in other medical education studies. Finally, because the intervention service had been in place for several years prior to its evaluation, the confounding influence of difficulties working out its implementation was minimized.
Results
Few studies of ambulatory experiences demonstrated statistically significant, let alone clinically significant, results. Most studies showed no statistically significant differences in student evaluations or examination scores. An exception is Grum et al., who showed improvements on 3 of 5 examinations.4 A few studies have found improved student satisfaction.3 None of the randomized controlled trials demonstrate increases in students matching in internal medicine or primary care residencies.4, 68 In contrast, this study produced statistically or programmatically significant results in process measures, evaluations, satisfaction, and career choices.
Several of our specific findings deserve additional comment. Although the admitting residents were instructed to assign patients to the intervention service based on their acuity and complexity, it is important to examine these residents' actual behavior. Several of our hypotheses were not validated. The students on the intervention service admitted fewer patients and were no more likely to see at least 1 patient in each age category. The admitting resident may have limited the number of patients admitted to the intervention service based on the workload of the supervising resident not that of the student. The supervising resident on the intervention service must round on all the patients, whereas the oversight of patients seen by students on the traditional services is shared with the interns. Having the attending on the intervention service share this supervising responsibility might improve this outcome.
Students on the intervention service had more positive attitudes toward the rotation. In addition, potentially negative attitudes were not manifest. For example, it might be argued that third‐year medical students are not prepared to bear this increased responsibility. However, there was not a significant difference in students' perception of the quality of supervision or the workload.
Although the goal of medical education is the production of competent physicians, it is important that the process not place undo burdens on patients and the health care system. Univariate analysis showed similar resource utilization. It might be contended that the admitting resident assigned the intervention service patients who were less acutely ill. Therefore, we performed multivariate analysis using APR‐DRG‐SOI to control for severity of illness. Of 8 comparisons, the only statistically significant difference, length of stay of patients with asthma, favored the intervention service.
Limitations
Although this study had numerous strengths, it also had several limitations. The primary limitations were lack of generalizability, difficulty in obtaining authentic assessments, the potential difference between statistical and educational significance, and inability to identify which components of the intervention service were responsible for the outcomes. This study's findings may not be generalizable to other institutions. For example, institutions without age or organ systembased teams may not observe increases in the number of key diagnoses encountered in the patients cared for. Regarding the assessments, there may be better measures of clinical competence, such as an objective structured clinical examination (OSCE),13 than those used in this study. However, there were not sufficient resources to implement an OSCE at the end of the rotation.
Some might question whether the statistically significant differences have educational significance. Although that is an important concern, this study should be compared with other educational interventions that found few statistically significant, let alone educationally significant, differences. To address this concern, we calculated effect sizes. The differences in student satisfaction were small to moderate. Although the lower limit of the 95% confidence interval of the odds ratio for matching in a pediatric residency was 0.99, the magnitude was programmatically important.
Finally, this study was an evaluation of an existing program. The authors were unable to control some potential confounders including patient allocation, average daily census, and quality of teaching. For example, Griffith and colleagues have shown that working with the best teachers improves student performance.14 We were not able to randomly assign the faculty among the services, and unequal distribution of better teachers could have biased this study's outcomes. The students on the intervention service rated their attendings, but not their residents, higher than did the students on the other services. However, the linear regression model showed that the perceived quality of the attending did not account for all the differences in student satisfaction. It was not possible to control for this factor in comparing student performance or subinternship or residency selection because the survey, which included the faculty evaluations, was anonymous and therefore could not be linked to the other data sets.
The perceived differences in the quality of teaching may not have been the result of differences in the attendings but instead of differences in the structure of the services. Accessibility is one of the characteristics of excellent clinical teachers.15 The intervention structure may permit faculty to spend more time with students, and this may increase the perceived quality of the teaching. However, it is not possible to resolve this issue with the available data.
CONCLUSIONS
The intervention service is a structure for the pediatric inpatient rotation of third‐year medical students that, instead of dividing the faculty and supervising resident's attention between interns and students, focuses their attention on the students. Although it has been difficult to demonstrate improvements as a result of the educational interventions, we have shown several improvements in the evaluations of the students. Moreover, the pattern of increased student satisfaction and a tendency toward more student selecting careers in pediatrics are remarkable. This was accomplished with similar resource utilization. Therefore, this program merits being continued at our institution and possibly adopted at other medical schools. Further research is needed to determine which aspects of the intervention are responsible for its effects. Some components, such as focused time with students, may be applicable to traditional services.
Acknowledgements
The authors thank Ronald Bloom for encouraging us to conduct this study; Kathy Bailey, Alice Dowling, and Margie Thompson for their assistance in the data collection; and Elizabeth Allen, Ronald Bloom, Flory Nkoy, Louis Pangaro, Stephanie Richardson, and Rajendu Srivastava for manuscript review.
- ,,.The role of the student ward in the medical clerkships.J Med Educ.1985;60:524–529.
- .Changing the fourth‐year medicine clerkship structure: A successful model for a teaching service without housestaff.J Gen Intern Med.1993;8:31–32.
- ,.A randomized, controlled pilot study of placing third‐year medical clerks in a continuity clinic.Acad Med.1993;68:845–847.
- ,,.Consequences of shifting medical‐student education to the outpatient setting: effects on performance and experiences.Acad Med.1996;71(suppl 1):S99–S101.
- ,.Learning outcomes of an ambulatory care rotation in internal medicine for junior medical students.J Gen Intern Med.1993;8:189–192.
- ,,,,.A prospective, randomized trial of a six‐week ambulatory medicine rotation.Acad Med.1995;70:537–541.
- ,,,,.Ambulatory versus inpatient rotations in teaching third‐year students internal medicine.J Gen Intern Med.1998;13:327–330.
- ,,,,.The effect of an ambulatory internal medicine rotation on students' career choices.Acad Med.1997;72:147–149.
- .Theme issue on medical education: Call for papers.JAMA.2005;293:742.
- Perkin RM,Swift JD,Newton DA, eds.Pediatric Hospital Medicine: Textbook of Inpatient Management.Philadelphia:Lippincott Williams 2003.
- .Call for greater emphasis on effect‐size measures in published articles in Teaching and Learning in Medicine.Teach Learn Med.2002;14:206–210.
- .Educational research and randomised trials.Med Educ.2002;36:1002–1003.
- ,.The objective structured clinical examination.Arch Pediatr Adolesc Med.2000;154:736–741.
- ,,,.Relationship of how well attending physicians teach to their student's performances and residency choices.Acad Med.1997;72(suppl 1):S118–S120.
- ,.Assessing quality and costs of education in the ambulatory setting: A review of the literature.Acad Med.2002;77:621–680.
The third‐year pediatric clerkship at the University of Utah School of Medicine has a relatively unique inpatient service, the Glasgow Service, which consists of an academic attending, a third‐year pediatric resident, and 4 third‐year medical students, but no interns. (This service was named in honor of Lowell Glasgow, chair of pediatrics, 1972‐82.) This structure was introduced in 1992 by the chair of pediatrics, Michael Simmons, the residency program director, Richard Molteni, and the clerkship director, Karen Hansen. These individuals desired to improve students' inpatient experience by providing greater responsibility for patient care. An additional motive was to increase the total number of patients followed by house staff without increasing the size of the residency program.
This inpatient service is a part of a 6‐week pediatric clerkship. All students perform the 3‐week inpatient portion of their clerkship at Primary Children's Medical Center, a tertiary‐care, freestanding children's hospital. (The students also spend 1 week each in a newborn nursery, an outpatient clinic, and a subspecialty setting). The academic attendings include generalists, hospitalists, and specialists who concurrently have other clinical responsibilities. The students take in‐house call every fourth night, supervised by senior residents who are not necessarily members of their service. All students share the same formal teaching activities, including morning report, a noon conference, and a student conference.
Patients are assigned to the ward services by a senior admitting resident. The admitting resident distributes patients among the services based on the complexity and acuity of the patients' conditions as well as the census on the various services. The senior resident supervising a particular service then assigns patients among the members of that service. Each third‐year medical student is expected to care for 2 or 3 patients at a time.
In addition to the intervention service, students also rotate on 2 similar traditional services. These services are traditional in the sense that they are composed of an academic attending, a community attending, a third‐year pediatric resident, 4 interns, and up to 2 fourth‐year and 2 third‐year medical students. Faculty preferences regarding service assignments were accommodated when possible. Therefore, some faculty attended only on one type of service, intervention or traditional, and others attended on both types. Because they have more members and because interns are capable of caring for more patients than are medical students, the traditional services cared for more patients than the intervention service. Although identical in composition, the 2 traditional services differ with each other in several ways. One service typically admits children 3 years old and younger, whereas the other admits children who are between 3 and 12 years old. The service that admits older children also admits most of the hematology‐oncology patients.
Although other authors have described similar inpatient clerkship structures, to our knowledge, none have evaluated them through a prospective randomized controlled trial.1, 2 The recent literature on ambulatory experiences during third‐year clerkships provided a methodological framework for this study. Collectively, such studies have evaluated outcomes with a variety of measures, including patient logs,35 evaluations,3, 4, 6, 7 examinations,37 surveys,3, 5, 7, 8 and career choices.4, 68 Additional outcomes, such as the effect of educational interventions on patient care, have been emphasized.9
In the light of this research, we conducted a prospective, randomized controlled trial to compare outcomes on the intervention service with those on the traditional services. We hypothesized that, compared with the traditional services, the intervention service would show:
improved process measures in terms of increased number of patients admitted, number of key diagnoses encountered in the patients cared for, and range of ages of the patients admitted;
similar or improved student performance, as measured by faculty and resident evaluations and a National Board of Medical Examiners (NBME) subject examination;
increased student satisfaction, as assessed by an end‐of‐rotation questionnaire;
increased interest in pediatric and, more broadly, primary care careers, as measured by subinternship and internship selections; and
comparable or improved resource utilization in terms of length of stay and total charges.
METHODS
All students enrolled in the third‐year pediatric rotation during the 2001‐2003 academic years were individually randomized by the clerkship assistant to the intervention service or 1 of the 2 traditional services without respect to career preference. A 5:3 student randomization ratio was used to fulfill the requirement that 4 students be assigned to the intervention service during every 3‐week block. This permitted the service to have call every fourth night.
To evaluate the adequacy of the randomization process, we obtained baseline student characteristics on age, sex, and United States Medical Licensing Examination (USMLE) Step 1 score from the Dean of Student Affairs. The dean also reported the discipline each student enrolled in for the required fourth‐year subinternship(s) and matched in for internship. These data were reported anonymously and linked to the service to which the student was assigned. In this study, pediatrics, internal medicine, and family practice were all considered primary care, but preliminary or transitional internships were not.
Process Measures
Students were required to submit logs at the end of their rotations, recording patients' names, ages, diagnoses, and admission dates. The accuracy and completeness of these logs were not independently verified.
As there was no authoritative list of key diagnoses third‐year medical students should encounter in the patients they care for during their inpatient rotations, we relied on expert opinion at our institution. The Council on Medical Student Education in Pediatrics' curriculum was not used because it did not differentiate between inpatient and ambulatory contexts. A preliminary list of 93 diagnoses was developed from the table of contents of Pediatric Hospital Medicine.10 This list was distributed to the 26 clinical faculty members in the Divisions of Pediatric Inpatient Medicine and General Pediatrics who were asked to select the 10 most important diagnoses. Surveys were numerically coded to permit 1 reminder.
The survey had a response rate of 92.3% (24 of 26 surveys). One survey was excluded because the respondent significantly deviated from the instructions. The 10 key diagnoses and the percentages of respondents who selected each individual diagnosis are: asthma (100%), febrile infant (95.6%), diarrhea and dehydration (91.3%), bronchiolitis (78.2%), diabetes mellitus and diabetic ketoacidemia (60.9%), failure to thrive (56.5%), urinary tract infections (52.1%), pneumonia (47.8%), upper airway infections such as croup (43.5%), and seizures and status epilepticus (43.5%).
Two of the authors independently coded the diagnoses on the students' patient logs in terms of these 93 diagnoses. The authors were blinded to the students' service assignment. As many students reported more than 1 diagnosis, the authors prioritized primary, secondary, and tertiary diagnoses to simplify the evaluation. The most likely cause of admission was listed as the primary diagnosis. If the authors could not reconcile divergent views, a third party was consulted.
Student Performance
Students were evaluated by both the attending physician(s) and senior resident(s) using a standardized evaluation form available from the corresponding author. The evaluation contained 18 items in 7 categories: data gathering, data recording/reporting, knowledge, data interpretation, clinical performance, professional attitudes, and professional demeanor. The student was rated exceptional, above expectations, meets expectations, below expectations, unacceptable, or not observed on each item. A short narrative description illustrated each rating. The ratings were converted to a 5‐point scale, with exceptional being 5. If the evaluator marked the line between 2 ratings, it was recorded as half. When multiple attendings or residents evaluated a student, the scores for a given item were collapsed into an average score.
Students also completed a NBME pediatric subject examination on the last day of their rotation.
Additionally, students were requested to complete a questionnaire during the final week of the clerkship. The items on the questionnaire were meant to access students' perceptions of the quality of their attendings' and residents' teaching, a potentially confounding variable. The survey was piloted on a group of similar subjects. Informed consent was obtained for survey completion. The survey was anonymous and required approximately 7 minutes to complete.
Resource Utilization
Last, resource utilization data, length of stay and total charges, for the 4 most common primary diagnoses were compared between the intervention and the traditional services. The 4 most common primary diagnoses and the percentage of total diagnoses (n = 2047) that each represents were bronchiolitis, 13%; febrile infant, 8.6%; pneumonia, 7.1%; and asthma, 6.5% (the diagnosis other accounted for 12% of the total diagnoses). Unique patient identifiers were used to obtain length of stay and total charges from the hospital's database. All‐Patient‐Refined Diagnosis‐Related Groups Severity of Illness (APR‐DRG‐SOI) were also obtained and used to construct multivariate models. Patients who were admitted to the pediatric intensive care unit (PICU) were excluded from the analysis.
Statistical Analysis
Statistical analyses were conducted and frequencies and percentages were calculated using Stata SE version 8.0 (College Station, TX). For all interval and ratio‐scaled variables, distributions were tested for normality using the Shapiro‐Wilks test to determine whether to use parametric or nonparametric statistical tests. For distributions meeting the normality assumption, the unpaired t test was used to compare the intervention service with traditional services. Where the normality assumption was not met, the Mann‐Whitney test was used. Categorically scaled data were compared using Pearson's chi‐square test. The standardized mean differences, reported as d values, were calculated to determine the effect size. Small, medium, and large effect sizes were defined as d values of 0.20, 0.50, and 0.80, respectively.11 Teaching quality, an effect modifier, was entered as a covariate into a linear regression model. Analyses of length of stay and total charges were conducted using multivariate linear regression controlling for patient age and severity of illness.
This study was approved by the University of Utah and Primary Children's Medical Center's Institutional Review Board.
RESULTS
Two hundred and three students enrolled in the third‐year pediatric clerkship during the study period, and all students completed the clerkship on their assigned services. One hundred and twenty‐eight were randomized to the intervention service and 75 to the traditional services. There were no statistically significant differences in median age, percentage of male students, or mean USMLE Step 1 score between the students randomized to the intervention service and those randomized to the traditional services (Table 1).
| Intervention service | Traditional services | P value | |
|---|---|---|---|
| |||
| Age (median) | 28 | 28 | .76* |
| Sex (% male) | 58.6 | 62.7 | .57 |
| USMLE Step 1 score | 217 | 217 | .94 |
Process Measures
Overall, 96.6% of students (196 of 203) submitted patient logs; 97.7% of students (125 of 128) on the intervention service and 94.7% of students (71 of 75) on the traditional services. The students on the intervention service admitted a median of 10 patients, whereas the students on the traditional services admitted a median of 11 patients (d = 0.45, P < .01). Age data were recorded on 137 patient logs (69.9% of submitted logs, 72.0% of students on the intervention service vs. 66.2% of students on the traditional services). The percentage of students who saw at least 1 newborn (birth‐23 months), child (2‐12 years), and adolescent (12‐18 years) was 34.8% on the intervention service and 33.3% on the traditional services (P = .87) (Table 2).
| Intervention service | Traditional services | d | P value | |
|---|---|---|---|---|
| ||||
| Median number of patients | 10 | 11 | 0.45 | < .01* |
| Percent of students who saw 1 newborn, child, and adolescent | 34.8% | 33.3% | 0.03 | .87 |
| Top 10 diagnoses cared for (n) | 4.4 | 3.6 | 0.48 | < .01 |
| Percent of patients cared for whose diagnoses were in top 10 | 59.3% | 46.8% | 0.62# | < .01 |
| Percent of unique diagnoses (median) | 80.0% | 80.0% | 0.02 | .62 |
Students on the intervention service encountered, on average, a larger number of the 10 key diagnoses (4.4 vs. 3.6, d = 0.48, P < .01) and a higher percentage of their patients had clinical conditions among the key diagnoses (59.3 vs. 46.8, d = 0.62, P < .01). To determine if this higher percentage was the result of admitting multiple patients with the same diagnosis, we examined the percentage of unique primary diagnosesthe number of different primary diagnoses divided by the total number of patientsand found no differences (Table 2).
Student Performance
The faculty and resident evaluations of the students showed statistically significant differences between those in the intervention service and those in the traditional services in only 2 of the 18 items. These items were analysis in the data interpretation category (3.81 vs. 3.64, d = 0.35, P = .02) and patient interaction in the professional demeanor category (3.89 vs. 3.76, d = 0.31, P < .05). Both differences favored the intervention service. There were no statistical differences by service in student performance on the NBME subject examination (73.2 vs. 72.3, P = .39).
Student Satisfaction
Overall, 87.2% of students (177 of 203) completed the survey; 87.5% of students (112 of 124) on the intervention service and 86.7% of students (65 of 75) on the traditional services. The students on the intervention service both had a more positive overall attitude about their rotation and were more likely to find it a satisfying educational experience. Students on the intervention service also reported greater participation in patient care. Effect sizes ranged from small to medium (Table 3). The internal consistency of answers about participation in patient care was high (Pearson correlation coefficient r = 0.80).
| Intervention service | Traditional services | d | P value | |
|---|---|---|---|---|
| ||||
| My overall attitude toward this rotation is: 1. highly negative to 5. highly positive | 4.48 | 4.26 | 0.26 | .02* |
| I found this rotation a satisfying educational experience: 1. strongly disagree to 5. strongly agree | 4.49 | 4.22 | 0.35 | < .01* |
| My role on this rotation was that of an: 1. observer, 3. participant, 5. director | 3.77 | 3.33 | 0.60# | < .01 |
| My supervising interns/residents were _____ teachers: 1. poor, 3. good, 5. exemplary | 3.91 | 3.75 | 0.17 | .26* |
| My input into patient care decisions was: 1. strongly discouraged to 5. strongly encouraged | 4.45 | 3.98 | 0.66# | < .01* |
| I was able to make a significant contribution to patient care: 1. strongly disagree to 5. strongly agree | 4.19 | 3.92 | 0.34 | .02* |
| I had direct responsibility for patient care: 1. strongly disagree to 5. strongly agree | 4.33 | 3.95 | 0.46 | .01* |
| My attendings were _____ teachers: 1. poor, 3. good, 5. exemplary | 4.09 | 3.75 | 0.40 | < .01* |
| I found the feedback I received during this rotation to be: 1. insufficient, 3. appropriate, 5. excessive | 2.84 | 2.65 | 0.22 | .17* |
| The following best describes the quality of my supervision during this rotation: 1. I was expected to do things beyond my competence unsupervised 3. The degree of supervision was appropriate for my level of training 5. I was excessively supervised on skills I had already demonstrated | 2.95 | 3.06 | 0.18 | .19 |
| During this rotation: 1. I was expected to see too many patients 3. I was expected to see an appropriate number of patients 5. I expected to see more patients | 3.46 | 3.31 | 0.18 | .33* |
| Before this rotation I _____ pediatrics as a career choice: 1. had rejected, 3. was considering, 5. had decided on | 2.37 | 2.14 | 0.22 | .11* |
| This rotation increased my interest in pursuing pediatrics as a career: 1. strongly disagree to 5. strongly agree | 3.74 | 3.60 | 0.14 | .32* |
Students on the intervention service rated the teaching of their attendings, but not of their residents, higher than did students on the traditional services. Controlling for the perceived quality of the attending, 3 of 6 satisfaction outcomes remained statistically significant: role on rotation (P < .01), input into patient care decisions (P < .01), and direct responsibility for patient care (P = .04). Students on both services believed they were appropriately supervised (P = .19). Despite the students on the traditional services on average admitting more patients, there was no significant difference by service in the students' rating of patient load (P = .33).
Career Choice
The odds ratio and 95% confidence interval for students enrolling in a pediatric subinternship was 1.94 (0.83‐4.49) and matching in a pediatric residency was 2.52 (0.99‐6.37). There were no statistically significant differences by service in the percentage of students enrolling in primary care (pediatric, internal medicine, and family practice) subinternships or residencies (Table 4).
| Intervention service | Traditional services | Odds ratio (95% CI) | |
|---|---|---|---|
| Pediatric subinternship | 19.5% | 11.1% | 1.94 (0.83‐4.49) |
| Primary care subinternship | 68.3% | 70.8% | 0.89 (0.47‐1.67) |
| Pediatric residency | 18.6% | 8.3% | 2.52 (0.99‐6.37) |
| Primary care residency | 40.7% | 31.9% | 1.46 (0.79‐2.70) |
Resource Utilization
One hundred and thirty‐five patients were excluded from the resource utilization analysis (n = 594) because their unique identifiers could not be found or they had been admitted to the PICU. Univariate analysis demonstrated statistically significant differences for patients with asthma, but not patients with bronchiolitis, febrile infants, or patients with pneumonia, favoring the intervention service. Patients with asthma admitted to the intervention service had a shorter length of stay (49.9 vs. 70.1 hours, P = .02) and lower total charges ($3600 vs. $4600, P = .02), as shown in Table 5. Of 4 multivariate models controlling for age and severity of illness, each with length of stay and total charges as the dependant variables, length of stay was significantly less for patients with asthma admitted to the intervention service only. Such patients were discharged an average of 23.3 hours earlier than patients with asthma admitted to the traditional services (P = .02).
| Diagnosis (n) | n | Length of stay (hours) | P value | Total charges | ||||
|---|---|---|---|---|---|---|---|---|
| Intervention service | Traditional services | Intervention service | Traditional services | Intervention service | Traditional services | P value | ||
| ||||||||
| Bronchiolitis (210) | 159 | 51 | 63.7 | 70.5 | .20* | $4300 | $4800 | .20* |
| Febrile infant (152) | 105 | 47 | 58.8 | 58.9 | .50* | $4800 | $4900 | .28* |
| Pneumonia (123) | 82 | 41 | 84.3 | 116.8 | .71* | $6300 | $9200 | .63* |
| Asthma (109) | 80 | 29 | 49.9 | 70.1 | .02* | $3600 | $4600 | .02* |
DISCUSSION
This study's objective was to evaluate a third‐year pediatric clerkship structure that focuses on students, using multiple outcome parameters. Utilizing a robust design, the results of this study have demonstrated that the intervention service is more successful than the traditional services in several outcomes. Students assigned to the intervention service were more satisfied and more likely to select pediatrics as a career. These improvements were accomplished while maintaining similar process measures, student performance, and resource utilization compared with those of the traditional services.
Methods
The methods used in this study compare favorably with other evaluations of educational interventions. The present study incorporated a randomized controlled design.12 Although several studies of ambulatory clerkships used a randomized design, few randomized all eligible students.7, 8 The others used some form of selection prior to randomization. For example, in the Pangaro et al. study, students selected their clerkship site by lottery, with students selecting a certain site then offered the opportunity to participate in the intervention.6 The present study manifested several additional strengths. Multiple outcomes, including effects on patient care, were evaluated. Moreover, this study had a relatively large intervention group and total sample size compared with those in other medical education studies. Finally, because the intervention service had been in place for several years prior to its evaluation, the confounding influence of difficulties working out its implementation was minimized.
Results
Few studies of ambulatory experiences demonstrated statistically significant, let alone clinically significant, results. Most studies showed no statistically significant differences in student evaluations or examination scores. An exception is Grum et al., who showed improvements on 3 of 5 examinations.4 A few studies have found improved student satisfaction.3 None of the randomized controlled trials demonstrate increases in students matching in internal medicine or primary care residencies.4, 68 In contrast, this study produced statistically or programmatically significant results in process measures, evaluations, satisfaction, and career choices.
Several of our specific findings deserve additional comment. Although the admitting residents were instructed to assign patients to the intervention service based on their acuity and complexity, it is important to examine these residents' actual behavior. Several of our hypotheses were not validated. The students on the intervention service admitted fewer patients and were no more likely to see at least 1 patient in each age category. The admitting resident may have limited the number of patients admitted to the intervention service based on the workload of the supervising resident not that of the student. The supervising resident on the intervention service must round on all the patients, whereas the oversight of patients seen by students on the traditional services is shared with the interns. Having the attending on the intervention service share this supervising responsibility might improve this outcome.
Students on the intervention service had more positive attitudes toward the rotation. In addition, potentially negative attitudes were not manifest. For example, it might be argued that third‐year medical students are not prepared to bear this increased responsibility. However, there was not a significant difference in students' perception of the quality of supervision or the workload.
Although the goal of medical education is the production of competent physicians, it is important that the process not place undo burdens on patients and the health care system. Univariate analysis showed similar resource utilization. It might be contended that the admitting resident assigned the intervention service patients who were less acutely ill. Therefore, we performed multivariate analysis using APR‐DRG‐SOI to control for severity of illness. Of 8 comparisons, the only statistically significant difference, length of stay of patients with asthma, favored the intervention service.
Limitations
Although this study had numerous strengths, it also had several limitations. The primary limitations were lack of generalizability, difficulty in obtaining authentic assessments, the potential difference between statistical and educational significance, and inability to identify which components of the intervention service were responsible for the outcomes. This study's findings may not be generalizable to other institutions. For example, institutions without age or organ systembased teams may not observe increases in the number of key diagnoses encountered in the patients cared for. Regarding the assessments, there may be better measures of clinical competence, such as an objective structured clinical examination (OSCE),13 than those used in this study. However, there were not sufficient resources to implement an OSCE at the end of the rotation.
Some might question whether the statistically significant differences have educational significance. Although that is an important concern, this study should be compared with other educational interventions that found few statistically significant, let alone educationally significant, differences. To address this concern, we calculated effect sizes. The differences in student satisfaction were small to moderate. Although the lower limit of the 95% confidence interval of the odds ratio for matching in a pediatric residency was 0.99, the magnitude was programmatically important.
Finally, this study was an evaluation of an existing program. The authors were unable to control some potential confounders including patient allocation, average daily census, and quality of teaching. For example, Griffith and colleagues have shown that working with the best teachers improves student performance.14 We were not able to randomly assign the faculty among the services, and unequal distribution of better teachers could have biased this study's outcomes. The students on the intervention service rated their attendings, but not their residents, higher than did the students on the other services. However, the linear regression model showed that the perceived quality of the attending did not account for all the differences in student satisfaction. It was not possible to control for this factor in comparing student performance or subinternship or residency selection because the survey, which included the faculty evaluations, was anonymous and therefore could not be linked to the other data sets.
The perceived differences in the quality of teaching may not have been the result of differences in the attendings but instead of differences in the structure of the services. Accessibility is one of the characteristics of excellent clinical teachers.15 The intervention structure may permit faculty to spend more time with students, and this may increase the perceived quality of the teaching. However, it is not possible to resolve this issue with the available data.
CONCLUSIONS
The intervention service is a structure for the pediatric inpatient rotation of third‐year medical students that, instead of dividing the faculty and supervising resident's attention between interns and students, focuses their attention on the students. Although it has been difficult to demonstrate improvements as a result of the educational interventions, we have shown several improvements in the evaluations of the students. Moreover, the pattern of increased student satisfaction and a tendency toward more student selecting careers in pediatrics are remarkable. This was accomplished with similar resource utilization. Therefore, this program merits being continued at our institution and possibly adopted at other medical schools. Further research is needed to determine which aspects of the intervention are responsible for its effects. Some components, such as focused time with students, may be applicable to traditional services.
Acknowledgements
The authors thank Ronald Bloom for encouraging us to conduct this study; Kathy Bailey, Alice Dowling, and Margie Thompson for their assistance in the data collection; and Elizabeth Allen, Ronald Bloom, Flory Nkoy, Louis Pangaro, Stephanie Richardson, and Rajendu Srivastava for manuscript review.
The third‐year pediatric clerkship at the University of Utah School of Medicine has a relatively unique inpatient service, the Glasgow Service, which consists of an academic attending, a third‐year pediatric resident, and 4 third‐year medical students, but no interns. (This service was named in honor of Lowell Glasgow, chair of pediatrics, 1972‐82.) This structure was introduced in 1992 by the chair of pediatrics, Michael Simmons, the residency program director, Richard Molteni, and the clerkship director, Karen Hansen. These individuals desired to improve students' inpatient experience by providing greater responsibility for patient care. An additional motive was to increase the total number of patients followed by house staff without increasing the size of the residency program.
This inpatient service is a part of a 6‐week pediatric clerkship. All students perform the 3‐week inpatient portion of their clerkship at Primary Children's Medical Center, a tertiary‐care, freestanding children's hospital. (The students also spend 1 week each in a newborn nursery, an outpatient clinic, and a subspecialty setting). The academic attendings include generalists, hospitalists, and specialists who concurrently have other clinical responsibilities. The students take in‐house call every fourth night, supervised by senior residents who are not necessarily members of their service. All students share the same formal teaching activities, including morning report, a noon conference, and a student conference.
Patients are assigned to the ward services by a senior admitting resident. The admitting resident distributes patients among the services based on the complexity and acuity of the patients' conditions as well as the census on the various services. The senior resident supervising a particular service then assigns patients among the members of that service. Each third‐year medical student is expected to care for 2 or 3 patients at a time.
In addition to the intervention service, students also rotate on 2 similar traditional services. These services are traditional in the sense that they are composed of an academic attending, a community attending, a third‐year pediatric resident, 4 interns, and up to 2 fourth‐year and 2 third‐year medical students. Faculty preferences regarding service assignments were accommodated when possible. Therefore, some faculty attended only on one type of service, intervention or traditional, and others attended on both types. Because they have more members and because interns are capable of caring for more patients than are medical students, the traditional services cared for more patients than the intervention service. Although identical in composition, the 2 traditional services differ with each other in several ways. One service typically admits children 3 years old and younger, whereas the other admits children who are between 3 and 12 years old. The service that admits older children also admits most of the hematology‐oncology patients.
Although other authors have described similar inpatient clerkship structures, to our knowledge, none have evaluated them through a prospective randomized controlled trial.1, 2 The recent literature on ambulatory experiences during third‐year clerkships provided a methodological framework for this study. Collectively, such studies have evaluated outcomes with a variety of measures, including patient logs,35 evaluations,3, 4, 6, 7 examinations,37 surveys,3, 5, 7, 8 and career choices.4, 68 Additional outcomes, such as the effect of educational interventions on patient care, have been emphasized.9
In the light of this research, we conducted a prospective, randomized controlled trial to compare outcomes on the intervention service with those on the traditional services. We hypothesized that, compared with the traditional services, the intervention service would show:
improved process measures in terms of increased number of patients admitted, number of key diagnoses encountered in the patients cared for, and range of ages of the patients admitted;
similar or improved student performance, as measured by faculty and resident evaluations and a National Board of Medical Examiners (NBME) subject examination;
increased student satisfaction, as assessed by an end‐of‐rotation questionnaire;
increased interest in pediatric and, more broadly, primary care careers, as measured by subinternship and internship selections; and
comparable or improved resource utilization in terms of length of stay and total charges.
METHODS
All students enrolled in the third‐year pediatric rotation during the 2001‐2003 academic years were individually randomized by the clerkship assistant to the intervention service or 1 of the 2 traditional services without respect to career preference. A 5:3 student randomization ratio was used to fulfill the requirement that 4 students be assigned to the intervention service during every 3‐week block. This permitted the service to have call every fourth night.
To evaluate the adequacy of the randomization process, we obtained baseline student characteristics on age, sex, and United States Medical Licensing Examination (USMLE) Step 1 score from the Dean of Student Affairs. The dean also reported the discipline each student enrolled in for the required fourth‐year subinternship(s) and matched in for internship. These data were reported anonymously and linked to the service to which the student was assigned. In this study, pediatrics, internal medicine, and family practice were all considered primary care, but preliminary or transitional internships were not.
Process Measures
Students were required to submit logs at the end of their rotations, recording patients' names, ages, diagnoses, and admission dates. The accuracy and completeness of these logs were not independently verified.
As there was no authoritative list of key diagnoses third‐year medical students should encounter in the patients they care for during their inpatient rotations, we relied on expert opinion at our institution. The Council on Medical Student Education in Pediatrics' curriculum was not used because it did not differentiate between inpatient and ambulatory contexts. A preliminary list of 93 diagnoses was developed from the table of contents of Pediatric Hospital Medicine.10 This list was distributed to the 26 clinical faculty members in the Divisions of Pediatric Inpatient Medicine and General Pediatrics who were asked to select the 10 most important diagnoses. Surveys were numerically coded to permit 1 reminder.
The survey had a response rate of 92.3% (24 of 26 surveys). One survey was excluded because the respondent significantly deviated from the instructions. The 10 key diagnoses and the percentages of respondents who selected each individual diagnosis are: asthma (100%), febrile infant (95.6%), diarrhea and dehydration (91.3%), bronchiolitis (78.2%), diabetes mellitus and diabetic ketoacidemia (60.9%), failure to thrive (56.5%), urinary tract infections (52.1%), pneumonia (47.8%), upper airway infections such as croup (43.5%), and seizures and status epilepticus (43.5%).
Two of the authors independently coded the diagnoses on the students' patient logs in terms of these 93 diagnoses. The authors were blinded to the students' service assignment. As many students reported more than 1 diagnosis, the authors prioritized primary, secondary, and tertiary diagnoses to simplify the evaluation. The most likely cause of admission was listed as the primary diagnosis. If the authors could not reconcile divergent views, a third party was consulted.
Student Performance
Students were evaluated by both the attending physician(s) and senior resident(s) using a standardized evaluation form available from the corresponding author. The evaluation contained 18 items in 7 categories: data gathering, data recording/reporting, knowledge, data interpretation, clinical performance, professional attitudes, and professional demeanor. The student was rated exceptional, above expectations, meets expectations, below expectations, unacceptable, or not observed on each item. A short narrative description illustrated each rating. The ratings were converted to a 5‐point scale, with exceptional being 5. If the evaluator marked the line between 2 ratings, it was recorded as half. When multiple attendings or residents evaluated a student, the scores for a given item were collapsed into an average score.
Students also completed a NBME pediatric subject examination on the last day of their rotation.
Additionally, students were requested to complete a questionnaire during the final week of the clerkship. The items on the questionnaire were meant to access students' perceptions of the quality of their attendings' and residents' teaching, a potentially confounding variable. The survey was piloted on a group of similar subjects. Informed consent was obtained for survey completion. The survey was anonymous and required approximately 7 minutes to complete.
Resource Utilization
Last, resource utilization data, length of stay and total charges, for the 4 most common primary diagnoses were compared between the intervention and the traditional services. The 4 most common primary diagnoses and the percentage of total diagnoses (n = 2047) that each represents were bronchiolitis, 13%; febrile infant, 8.6%; pneumonia, 7.1%; and asthma, 6.5% (the diagnosis other accounted for 12% of the total diagnoses). Unique patient identifiers were used to obtain length of stay and total charges from the hospital's database. All‐Patient‐Refined Diagnosis‐Related Groups Severity of Illness (APR‐DRG‐SOI) were also obtained and used to construct multivariate models. Patients who were admitted to the pediatric intensive care unit (PICU) were excluded from the analysis.
Statistical Analysis
Statistical analyses were conducted and frequencies and percentages were calculated using Stata SE version 8.0 (College Station, TX). For all interval and ratio‐scaled variables, distributions were tested for normality using the Shapiro‐Wilks test to determine whether to use parametric or nonparametric statistical tests. For distributions meeting the normality assumption, the unpaired t test was used to compare the intervention service with traditional services. Where the normality assumption was not met, the Mann‐Whitney test was used. Categorically scaled data were compared using Pearson's chi‐square test. The standardized mean differences, reported as d values, were calculated to determine the effect size. Small, medium, and large effect sizes were defined as d values of 0.20, 0.50, and 0.80, respectively.11 Teaching quality, an effect modifier, was entered as a covariate into a linear regression model. Analyses of length of stay and total charges were conducted using multivariate linear regression controlling for patient age and severity of illness.
This study was approved by the University of Utah and Primary Children's Medical Center's Institutional Review Board.
RESULTS
Two hundred and three students enrolled in the third‐year pediatric clerkship during the study period, and all students completed the clerkship on their assigned services. One hundred and twenty‐eight were randomized to the intervention service and 75 to the traditional services. There were no statistically significant differences in median age, percentage of male students, or mean USMLE Step 1 score between the students randomized to the intervention service and those randomized to the traditional services (Table 1).
| Intervention service | Traditional services | P value | |
|---|---|---|---|
| |||
| Age (median) | 28 | 28 | .76* |
| Sex (% male) | 58.6 | 62.7 | .57 |
| USMLE Step 1 score | 217 | 217 | .94 |
Process Measures
Overall, 96.6% of students (196 of 203) submitted patient logs; 97.7% of students (125 of 128) on the intervention service and 94.7% of students (71 of 75) on the traditional services. The students on the intervention service admitted a median of 10 patients, whereas the students on the traditional services admitted a median of 11 patients (d = 0.45, P < .01). Age data were recorded on 137 patient logs (69.9% of submitted logs, 72.0% of students on the intervention service vs. 66.2% of students on the traditional services). The percentage of students who saw at least 1 newborn (birth‐23 months), child (2‐12 years), and adolescent (12‐18 years) was 34.8% on the intervention service and 33.3% on the traditional services (P = .87) (Table 2).
| Intervention service | Traditional services | d | P value | |
|---|---|---|---|---|
| ||||
| Median number of patients | 10 | 11 | 0.45 | < .01* |
| Percent of students who saw 1 newborn, child, and adolescent | 34.8% | 33.3% | 0.03 | .87 |
| Top 10 diagnoses cared for (n) | 4.4 | 3.6 | 0.48 | < .01 |
| Percent of patients cared for whose diagnoses were in top 10 | 59.3% | 46.8% | 0.62# | < .01 |
| Percent of unique diagnoses (median) | 80.0% | 80.0% | 0.02 | .62 |
Students on the intervention service encountered, on average, a larger number of the 10 key diagnoses (4.4 vs. 3.6, d = 0.48, P < .01) and a higher percentage of their patients had clinical conditions among the key diagnoses (59.3 vs. 46.8, d = 0.62, P < .01). To determine if this higher percentage was the result of admitting multiple patients with the same diagnosis, we examined the percentage of unique primary diagnosesthe number of different primary diagnoses divided by the total number of patientsand found no differences (Table 2).
Student Performance
The faculty and resident evaluations of the students showed statistically significant differences between those in the intervention service and those in the traditional services in only 2 of the 18 items. These items were analysis in the data interpretation category (3.81 vs. 3.64, d = 0.35, P = .02) and patient interaction in the professional demeanor category (3.89 vs. 3.76, d = 0.31, P < .05). Both differences favored the intervention service. There were no statistical differences by service in student performance on the NBME subject examination (73.2 vs. 72.3, P = .39).
Student Satisfaction
Overall, 87.2% of students (177 of 203) completed the survey; 87.5% of students (112 of 124) on the intervention service and 86.7% of students (65 of 75) on the traditional services. The students on the intervention service both had a more positive overall attitude about their rotation and were more likely to find it a satisfying educational experience. Students on the intervention service also reported greater participation in patient care. Effect sizes ranged from small to medium (Table 3). The internal consistency of answers about participation in patient care was high (Pearson correlation coefficient r = 0.80).
| Intervention service | Traditional services | d | P value | |
|---|---|---|---|---|
| ||||
| My overall attitude toward this rotation is: 1. highly negative to 5. highly positive | 4.48 | 4.26 | 0.26 | .02* |
| I found this rotation a satisfying educational experience: 1. strongly disagree to 5. strongly agree | 4.49 | 4.22 | 0.35 | < .01* |
| My role on this rotation was that of an: 1. observer, 3. participant, 5. director | 3.77 | 3.33 | 0.60# | < .01 |
| My supervising interns/residents were _____ teachers: 1. poor, 3. good, 5. exemplary | 3.91 | 3.75 | 0.17 | .26* |
| My input into patient care decisions was: 1. strongly discouraged to 5. strongly encouraged | 4.45 | 3.98 | 0.66# | < .01* |
| I was able to make a significant contribution to patient care: 1. strongly disagree to 5. strongly agree | 4.19 | 3.92 | 0.34 | .02* |
| I had direct responsibility for patient care: 1. strongly disagree to 5. strongly agree | 4.33 | 3.95 | 0.46 | .01* |
| My attendings were _____ teachers: 1. poor, 3. good, 5. exemplary | 4.09 | 3.75 | 0.40 | < .01* |
| I found the feedback I received during this rotation to be: 1. insufficient, 3. appropriate, 5. excessive | 2.84 | 2.65 | 0.22 | .17* |
| The following best describes the quality of my supervision during this rotation: 1. I was expected to do things beyond my competence unsupervised 3. The degree of supervision was appropriate for my level of training 5. I was excessively supervised on skills I had already demonstrated | 2.95 | 3.06 | 0.18 | .19 |
| During this rotation: 1. I was expected to see too many patients 3. I was expected to see an appropriate number of patients 5. I expected to see more patients | 3.46 | 3.31 | 0.18 | .33* |
| Before this rotation I _____ pediatrics as a career choice: 1. had rejected, 3. was considering, 5. had decided on | 2.37 | 2.14 | 0.22 | .11* |
| This rotation increased my interest in pursuing pediatrics as a career: 1. strongly disagree to 5. strongly agree | 3.74 | 3.60 | 0.14 | .32* |
Students on the intervention service rated the teaching of their attendings, but not of their residents, higher than did students on the traditional services. Controlling for the perceived quality of the attending, 3 of 6 satisfaction outcomes remained statistically significant: role on rotation (P < .01), input into patient care decisions (P < .01), and direct responsibility for patient care (P = .04). Students on both services believed they were appropriately supervised (P = .19). Despite the students on the traditional services on average admitting more patients, there was no significant difference by service in the students' rating of patient load (P = .33).
Career Choice
The odds ratio and 95% confidence interval for students enrolling in a pediatric subinternship was 1.94 (0.83‐4.49) and matching in a pediatric residency was 2.52 (0.99‐6.37). There were no statistically significant differences by service in the percentage of students enrolling in primary care (pediatric, internal medicine, and family practice) subinternships or residencies (Table 4).
| Intervention service | Traditional services | Odds ratio (95% CI) | |
|---|---|---|---|
| Pediatric subinternship | 19.5% | 11.1% | 1.94 (0.83‐4.49) |
| Primary care subinternship | 68.3% | 70.8% | 0.89 (0.47‐1.67) |
| Pediatric residency | 18.6% | 8.3% | 2.52 (0.99‐6.37) |
| Primary care residency | 40.7% | 31.9% | 1.46 (0.79‐2.70) |
Resource Utilization
One hundred and thirty‐five patients were excluded from the resource utilization analysis (n = 594) because their unique identifiers could not be found or they had been admitted to the PICU. Univariate analysis demonstrated statistically significant differences for patients with asthma, but not patients with bronchiolitis, febrile infants, or patients with pneumonia, favoring the intervention service. Patients with asthma admitted to the intervention service had a shorter length of stay (49.9 vs. 70.1 hours, P = .02) and lower total charges ($3600 vs. $4600, P = .02), as shown in Table 5. Of 4 multivariate models controlling for age and severity of illness, each with length of stay and total charges as the dependant variables, length of stay was significantly less for patients with asthma admitted to the intervention service only. Such patients were discharged an average of 23.3 hours earlier than patients with asthma admitted to the traditional services (P = .02).
| Diagnosis (n) | n | Length of stay (hours) | P value | Total charges | ||||
|---|---|---|---|---|---|---|---|---|
| Intervention service | Traditional services | Intervention service | Traditional services | Intervention service | Traditional services | P value | ||
| ||||||||
| Bronchiolitis (210) | 159 | 51 | 63.7 | 70.5 | .20* | $4300 | $4800 | .20* |
| Febrile infant (152) | 105 | 47 | 58.8 | 58.9 | .50* | $4800 | $4900 | .28* |
| Pneumonia (123) | 82 | 41 | 84.3 | 116.8 | .71* | $6300 | $9200 | .63* |
| Asthma (109) | 80 | 29 | 49.9 | 70.1 | .02* | $3600 | $4600 | .02* |
DISCUSSION
This study's objective was to evaluate a third‐year pediatric clerkship structure that focuses on students, using multiple outcome parameters. Utilizing a robust design, the results of this study have demonstrated that the intervention service is more successful than the traditional services in several outcomes. Students assigned to the intervention service were more satisfied and more likely to select pediatrics as a career. These improvements were accomplished while maintaining similar process measures, student performance, and resource utilization compared with those of the traditional services.
Methods
The methods used in this study compare favorably with other evaluations of educational interventions. The present study incorporated a randomized controlled design.12 Although several studies of ambulatory clerkships used a randomized design, few randomized all eligible students.7, 8 The others used some form of selection prior to randomization. For example, in the Pangaro et al. study, students selected their clerkship site by lottery, with students selecting a certain site then offered the opportunity to participate in the intervention.6 The present study manifested several additional strengths. Multiple outcomes, including effects on patient care, were evaluated. Moreover, this study had a relatively large intervention group and total sample size compared with those in other medical education studies. Finally, because the intervention service had been in place for several years prior to its evaluation, the confounding influence of difficulties working out its implementation was minimized.
Results
Few studies of ambulatory experiences demonstrated statistically significant, let alone clinically significant, results. Most studies showed no statistically significant differences in student evaluations or examination scores. An exception is Grum et al., who showed improvements on 3 of 5 examinations.4 A few studies have found improved student satisfaction.3 None of the randomized controlled trials demonstrate increases in students matching in internal medicine or primary care residencies.4, 68 In contrast, this study produced statistically or programmatically significant results in process measures, evaluations, satisfaction, and career choices.
Several of our specific findings deserve additional comment. Although the admitting residents were instructed to assign patients to the intervention service based on their acuity and complexity, it is important to examine these residents' actual behavior. Several of our hypotheses were not validated. The students on the intervention service admitted fewer patients and were no more likely to see at least 1 patient in each age category. The admitting resident may have limited the number of patients admitted to the intervention service based on the workload of the supervising resident not that of the student. The supervising resident on the intervention service must round on all the patients, whereas the oversight of patients seen by students on the traditional services is shared with the interns. Having the attending on the intervention service share this supervising responsibility might improve this outcome.
Students on the intervention service had more positive attitudes toward the rotation. In addition, potentially negative attitudes were not manifest. For example, it might be argued that third‐year medical students are not prepared to bear this increased responsibility. However, there was not a significant difference in students' perception of the quality of supervision or the workload.
Although the goal of medical education is the production of competent physicians, it is important that the process not place undo burdens on patients and the health care system. Univariate analysis showed similar resource utilization. It might be contended that the admitting resident assigned the intervention service patients who were less acutely ill. Therefore, we performed multivariate analysis using APR‐DRG‐SOI to control for severity of illness. Of 8 comparisons, the only statistically significant difference, length of stay of patients with asthma, favored the intervention service.
Limitations
Although this study had numerous strengths, it also had several limitations. The primary limitations were lack of generalizability, difficulty in obtaining authentic assessments, the potential difference between statistical and educational significance, and inability to identify which components of the intervention service were responsible for the outcomes. This study's findings may not be generalizable to other institutions. For example, institutions without age or organ systembased teams may not observe increases in the number of key diagnoses encountered in the patients cared for. Regarding the assessments, there may be better measures of clinical competence, such as an objective structured clinical examination (OSCE),13 than those used in this study. However, there were not sufficient resources to implement an OSCE at the end of the rotation.
Some might question whether the statistically significant differences have educational significance. Although that is an important concern, this study should be compared with other educational interventions that found few statistically significant, let alone educationally significant, differences. To address this concern, we calculated effect sizes. The differences in student satisfaction were small to moderate. Although the lower limit of the 95% confidence interval of the odds ratio for matching in a pediatric residency was 0.99, the magnitude was programmatically important.
Finally, this study was an evaluation of an existing program. The authors were unable to control some potential confounders including patient allocation, average daily census, and quality of teaching. For example, Griffith and colleagues have shown that working with the best teachers improves student performance.14 We were not able to randomly assign the faculty among the services, and unequal distribution of better teachers could have biased this study's outcomes. The students on the intervention service rated their attendings, but not their residents, higher than did the students on the other services. However, the linear regression model showed that the perceived quality of the attending did not account for all the differences in student satisfaction. It was not possible to control for this factor in comparing student performance or subinternship or residency selection because the survey, which included the faculty evaluations, was anonymous and therefore could not be linked to the other data sets.
The perceived differences in the quality of teaching may not have been the result of differences in the attendings but instead of differences in the structure of the services. Accessibility is one of the characteristics of excellent clinical teachers.15 The intervention structure may permit faculty to spend more time with students, and this may increase the perceived quality of the teaching. However, it is not possible to resolve this issue with the available data.
CONCLUSIONS
The intervention service is a structure for the pediatric inpatient rotation of third‐year medical students that, instead of dividing the faculty and supervising resident's attention between interns and students, focuses their attention on the students. Although it has been difficult to demonstrate improvements as a result of the educational interventions, we have shown several improvements in the evaluations of the students. Moreover, the pattern of increased student satisfaction and a tendency toward more student selecting careers in pediatrics are remarkable. This was accomplished with similar resource utilization. Therefore, this program merits being continued at our institution and possibly adopted at other medical schools. Further research is needed to determine which aspects of the intervention are responsible for its effects. Some components, such as focused time with students, may be applicable to traditional services.
Acknowledgements
The authors thank Ronald Bloom for encouraging us to conduct this study; Kathy Bailey, Alice Dowling, and Margie Thompson for their assistance in the data collection; and Elizabeth Allen, Ronald Bloom, Flory Nkoy, Louis Pangaro, Stephanie Richardson, and Rajendu Srivastava for manuscript review.
- ,,.The role of the student ward in the medical clerkships.J Med Educ.1985;60:524–529.
- .Changing the fourth‐year medicine clerkship structure: A successful model for a teaching service without housestaff.J Gen Intern Med.1993;8:31–32.
- ,.A randomized, controlled pilot study of placing third‐year medical clerks in a continuity clinic.Acad Med.1993;68:845–847.
- ,,.Consequences of shifting medical‐student education to the outpatient setting: effects on performance and experiences.Acad Med.1996;71(suppl 1):S99–S101.
- ,.Learning outcomes of an ambulatory care rotation in internal medicine for junior medical students.J Gen Intern Med.1993;8:189–192.
- ,,,,.A prospective, randomized trial of a six‐week ambulatory medicine rotation.Acad Med.1995;70:537–541.
- ,,,,.Ambulatory versus inpatient rotations in teaching third‐year students internal medicine.J Gen Intern Med.1998;13:327–330.
- ,,,,.The effect of an ambulatory internal medicine rotation on students' career choices.Acad Med.1997;72:147–149.
- .Theme issue on medical education: Call for papers.JAMA.2005;293:742.
- Perkin RM,Swift JD,Newton DA, eds.Pediatric Hospital Medicine: Textbook of Inpatient Management.Philadelphia:Lippincott Williams 2003.
- .Call for greater emphasis on effect‐size measures in published articles in Teaching and Learning in Medicine.Teach Learn Med.2002;14:206–210.
- .Educational research and randomised trials.Med Educ.2002;36:1002–1003.
- ,.The objective structured clinical examination.Arch Pediatr Adolesc Med.2000;154:736–741.
- ,,,.Relationship of how well attending physicians teach to their student's performances and residency choices.Acad Med.1997;72(suppl 1):S118–S120.
- ,.Assessing quality and costs of education in the ambulatory setting: A review of the literature.Acad Med.2002;77:621–680.
- ,,.The role of the student ward in the medical clerkships.J Med Educ.1985;60:524–529.
- .Changing the fourth‐year medicine clerkship structure: A successful model for a teaching service without housestaff.J Gen Intern Med.1993;8:31–32.
- ,.A randomized, controlled pilot study of placing third‐year medical clerks in a continuity clinic.Acad Med.1993;68:845–847.
- ,,.Consequences of shifting medical‐student education to the outpatient setting: effects on performance and experiences.Acad Med.1996;71(suppl 1):S99–S101.
- ,.Learning outcomes of an ambulatory care rotation in internal medicine for junior medical students.J Gen Intern Med.1993;8:189–192.
- ,,,,.A prospective, randomized trial of a six‐week ambulatory medicine rotation.Acad Med.1995;70:537–541.
- ,,,,.Ambulatory versus inpatient rotations in teaching third‐year students internal medicine.J Gen Intern Med.1998;13:327–330.
- ,,,,.The effect of an ambulatory internal medicine rotation on students' career choices.Acad Med.1997;72:147–149.
- .Theme issue on medical education: Call for papers.JAMA.2005;293:742.
- Perkin RM,Swift JD,Newton DA, eds.Pediatric Hospital Medicine: Textbook of Inpatient Management.Philadelphia:Lippincott Williams 2003.
- .Call for greater emphasis on effect‐size measures in published articles in Teaching and Learning in Medicine.Teach Learn Med.2002;14:206–210.
- .Educational research and randomised trials.Med Educ.2002;36:1002–1003.
- ,.The objective structured clinical examination.Arch Pediatr Adolesc Med.2000;154:736–741.
- ,,,.Relationship of how well attending physicians teach to their student's performances and residency choices.Acad Med.1997;72(suppl 1):S118–S120.
- ,.Assessing quality and costs of education in the ambulatory setting: A review of the literature.Acad Med.2002;77:621–680.
Copyright © 2007 Society of Hospital Medicine