User login
Plan for Discharge
Discharge planning typically begins at the time of admission. Physicians and hospital staff manage the patient’s acute issues throughout the stay while simultaneously trying to anticipate the patient’s discharge needs. Physicians capture these associated efforts by reporting discharge day management codes 99238 or 99239.
Code Use
Use of discharge day management codes 99238-99239 is reserved for the admitting physician/group, unless a formal transfer of care occurs (e.g., patient is transferred from the intensive care unit by the critical care physician to the medical-surgical floor on the hospitalist’s service).
Report one discharge code per hospitalization, but only when the service occurs after the initial date of admission. Codes 99238 or 99239 are not permitted for use when the patient is admitted and discharged on the same calendar date. When this occurs, the physician selects from 99221-99223 (initial inpatient care) or 99234-99236 (admission and discharge on the same day). Choose 99234-99238 when the patient stay is eight or more hours on the same calendar day and the insurer accepts these codes.
Documentation must also reflect two components of service: the corresponding elements of both the admission and discharge. Alternately, if the patient stays less than eight hours, or the insurer does not recognize 99234-99236 (admission and discharge on the same day), report 9922x (initial inpatient care) as appropriate.
Don’t mistakenly report discharge services for merely dictating the discharge summary. Discharge day management, as with most payable evaluation and management (E/M) services, requires a face-to-face visit between the physician and the patient on discharge day.
The entire visit need not take place at the bedside and may include other discharge-related elements performed on the patient’s unit/floor such as discussions with other healthcare professionals, patient/caregiver instruction and coordination of follow-up care. The discharge code description indicates that a final examination of the patient is included, but only “as appropriate.” In other words, an exam may not occur, or may not be documented, yet this does not preclude the physician from reporting 99238-99239. However, inclusion of the exam in the discharge day documentation is the best way to justify that a face-to-face service occurred on discharge day. This may be included in the discharge summary or a separate progress note in the medical record.
Time-Based Service
Discharge day management codes reflect the time accumulated on a calendar date, ending when the patient physically leaves the hospital. Services performed in a location other than the patient’s unit/floor (e.g., dictating the discharge summary from the outpatient office), do not count toward the cumulative time. Additionally, discharge-related services performed by residents, students or ancillary staff (i.e., registered nurses), such as reviewing instructions with the patient, do not count toward the discharge service time.
To support the discharge day management claim, documentation should reference the discharge status and other clinically relevant information. Time is not required when documenting 99238 because this service code constitutes any amount of time up to and including 30 minutes. When reporting 99239, documentation must include the physician’s cumulative service time (more than 30 minutes).
Medicare currently initiates a prepayment review (i.e., request for documentation to review the service prior to any payment consideration) for claims involving 99239. Failure to respond to the prepayment request or failure to include the time component in the documentation often results in claim denial. Payment can be recovered only through the appeal process or claim correction, when applicable.
Rules For Surgery
Surgeons are prohibited from separately reporting inpatient postoperative services related to the surgery, including discharge day management (99238-99239). Additionally, when the surgeon admits a patient to the hospital and discharge services are performed postoperatively by the hospitalist, discharge day management is included in the surgical package.
The reasons are two-fold: If the surgeon transfers the remaining inpatient care to the hospitalist, these discharge services are considered part of the global surgical package.
If no transfer occurs (as the surgeon is typically responsible and paid for all care up to 90 days following surgery), only the admitting physician/group (i.e., the surgeon) may report discharge day management codes 99238-99239.
In the latter scenario, the hospitalist reports subsequent hospital care (99231-99233) for all medically necessary services involving the patient’s medical management, even if provided on the day of discharge.
Pronouncement of Death
One of the most underreported services involves pronouncement of death. A physician who performs this service may qualify to report discharge day management code 99238-99239. To pronounce death, the physician must examine the patient, thus satisfying the face-to-face visit requirement.
Additionally, the physician may have to coordinate the necessary services, speak with family members or other healthcare providers, and fill out the necessary documentation.
If performed on the patient’s unit/floor, these services count toward the cumulative discharge service time. Documentation must include the time (if reporting 99239) as well as the patient’s discharge status and clinically relevant information. Completion of the death certificate alone is not sufficient for billing. TH
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center, Philadelphia. She is also on the faculty of SHM’s inpatient coding course.
Discharge planning typically begins at the time of admission. Physicians and hospital staff manage the patient’s acute issues throughout the stay while simultaneously trying to anticipate the patient’s discharge needs. Physicians capture these associated efforts by reporting discharge day management codes 99238 or 99239.
Code Use
Use of discharge day management codes 99238-99239 is reserved for the admitting physician/group, unless a formal transfer of care occurs (e.g., patient is transferred from the intensive care unit by the critical care physician to the medical-surgical floor on the hospitalist’s service).
Report one discharge code per hospitalization, but only when the service occurs after the initial date of admission. Codes 99238 or 99239 are not permitted for use when the patient is admitted and discharged on the same calendar date. When this occurs, the physician selects from 99221-99223 (initial inpatient care) or 99234-99236 (admission and discharge on the same day). Choose 99234-99238 when the patient stay is eight or more hours on the same calendar day and the insurer accepts these codes.
Documentation must also reflect two components of service: the corresponding elements of both the admission and discharge. Alternately, if the patient stays less than eight hours, or the insurer does not recognize 99234-99236 (admission and discharge on the same day), report 9922x (initial inpatient care) as appropriate.
Don’t mistakenly report discharge services for merely dictating the discharge summary. Discharge day management, as with most payable evaluation and management (E/M) services, requires a face-to-face visit between the physician and the patient on discharge day.
The entire visit need not take place at the bedside and may include other discharge-related elements performed on the patient’s unit/floor such as discussions with other healthcare professionals, patient/caregiver instruction and coordination of follow-up care. The discharge code description indicates that a final examination of the patient is included, but only “as appropriate.” In other words, an exam may not occur, or may not be documented, yet this does not preclude the physician from reporting 99238-99239. However, inclusion of the exam in the discharge day documentation is the best way to justify that a face-to-face service occurred on discharge day. This may be included in the discharge summary or a separate progress note in the medical record.
Time-Based Service
Discharge day management codes reflect the time accumulated on a calendar date, ending when the patient physically leaves the hospital. Services performed in a location other than the patient’s unit/floor (e.g., dictating the discharge summary from the outpatient office), do not count toward the cumulative time. Additionally, discharge-related services performed by residents, students or ancillary staff (i.e., registered nurses), such as reviewing instructions with the patient, do not count toward the discharge service time.
To support the discharge day management claim, documentation should reference the discharge status and other clinically relevant information. Time is not required when documenting 99238 because this service code constitutes any amount of time up to and including 30 minutes. When reporting 99239, documentation must include the physician’s cumulative service time (more than 30 minutes).
Medicare currently initiates a prepayment review (i.e., request for documentation to review the service prior to any payment consideration) for claims involving 99239. Failure to respond to the prepayment request or failure to include the time component in the documentation often results in claim denial. Payment can be recovered only through the appeal process or claim correction, when applicable.
Rules For Surgery
Surgeons are prohibited from separately reporting inpatient postoperative services related to the surgery, including discharge day management (99238-99239). Additionally, when the surgeon admits a patient to the hospital and discharge services are performed postoperatively by the hospitalist, discharge day management is included in the surgical package.
The reasons are two-fold: If the surgeon transfers the remaining inpatient care to the hospitalist, these discharge services are considered part of the global surgical package.
If no transfer occurs (as the surgeon is typically responsible and paid for all care up to 90 days following surgery), only the admitting physician/group (i.e., the surgeon) may report discharge day management codes 99238-99239.
In the latter scenario, the hospitalist reports subsequent hospital care (99231-99233) for all medically necessary services involving the patient’s medical management, even if provided on the day of discharge.
Pronouncement of Death
One of the most underreported services involves pronouncement of death. A physician who performs this service may qualify to report discharge day management code 99238-99239. To pronounce death, the physician must examine the patient, thus satisfying the face-to-face visit requirement.
Additionally, the physician may have to coordinate the necessary services, speak with family members or other healthcare providers, and fill out the necessary documentation.
If performed on the patient’s unit/floor, these services count toward the cumulative discharge service time. Documentation must include the time (if reporting 99239) as well as the patient’s discharge status and clinically relevant information. Completion of the death certificate alone is not sufficient for billing. TH
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center, Philadelphia. She is also on the faculty of SHM’s inpatient coding course.
Discharge planning typically begins at the time of admission. Physicians and hospital staff manage the patient’s acute issues throughout the stay while simultaneously trying to anticipate the patient’s discharge needs. Physicians capture these associated efforts by reporting discharge day management codes 99238 or 99239.
Code Use
Use of discharge day management codes 99238-99239 is reserved for the admitting physician/group, unless a formal transfer of care occurs (e.g., patient is transferred from the intensive care unit by the critical care physician to the medical-surgical floor on the hospitalist’s service).
Report one discharge code per hospitalization, but only when the service occurs after the initial date of admission. Codes 99238 or 99239 are not permitted for use when the patient is admitted and discharged on the same calendar date. When this occurs, the physician selects from 99221-99223 (initial inpatient care) or 99234-99236 (admission and discharge on the same day). Choose 99234-99238 when the patient stay is eight or more hours on the same calendar day and the insurer accepts these codes.
Documentation must also reflect two components of service: the corresponding elements of both the admission and discharge. Alternately, if the patient stays less than eight hours, or the insurer does not recognize 99234-99236 (admission and discharge on the same day), report 9922x (initial inpatient care) as appropriate.
Don’t mistakenly report discharge services for merely dictating the discharge summary. Discharge day management, as with most payable evaluation and management (E/M) services, requires a face-to-face visit between the physician and the patient on discharge day.
The entire visit need not take place at the bedside and may include other discharge-related elements performed on the patient’s unit/floor such as discussions with other healthcare professionals, patient/caregiver instruction and coordination of follow-up care. The discharge code description indicates that a final examination of the patient is included, but only “as appropriate.” In other words, an exam may not occur, or may not be documented, yet this does not preclude the physician from reporting 99238-99239. However, inclusion of the exam in the discharge day documentation is the best way to justify that a face-to-face service occurred on discharge day. This may be included in the discharge summary or a separate progress note in the medical record.
Time-Based Service
Discharge day management codes reflect the time accumulated on a calendar date, ending when the patient physically leaves the hospital. Services performed in a location other than the patient’s unit/floor (e.g., dictating the discharge summary from the outpatient office), do not count toward the cumulative time. Additionally, discharge-related services performed by residents, students or ancillary staff (i.e., registered nurses), such as reviewing instructions with the patient, do not count toward the discharge service time.
To support the discharge day management claim, documentation should reference the discharge status and other clinically relevant information. Time is not required when documenting 99238 because this service code constitutes any amount of time up to and including 30 minutes. When reporting 99239, documentation must include the physician’s cumulative service time (more than 30 minutes).
Medicare currently initiates a prepayment review (i.e., request for documentation to review the service prior to any payment consideration) for claims involving 99239. Failure to respond to the prepayment request or failure to include the time component in the documentation often results in claim denial. Payment can be recovered only through the appeal process or claim correction, when applicable.
Rules For Surgery
Surgeons are prohibited from separately reporting inpatient postoperative services related to the surgery, including discharge day management (99238-99239). Additionally, when the surgeon admits a patient to the hospital and discharge services are performed postoperatively by the hospitalist, discharge day management is included in the surgical package.
The reasons are two-fold: If the surgeon transfers the remaining inpatient care to the hospitalist, these discharge services are considered part of the global surgical package.
If no transfer occurs (as the surgeon is typically responsible and paid for all care up to 90 days following surgery), only the admitting physician/group (i.e., the surgeon) may report discharge day management codes 99238-99239.
In the latter scenario, the hospitalist reports subsequent hospital care (99231-99233) for all medically necessary services involving the patient’s medical management, even if provided on the day of discharge.
Pronouncement of Death
One of the most underreported services involves pronouncement of death. A physician who performs this service may qualify to report discharge day management code 99238-99239. To pronounce death, the physician must examine the patient, thus satisfying the face-to-face visit requirement.
Additionally, the physician may have to coordinate the necessary services, speak with family members or other healthcare providers, and fill out the necessary documentation.
If performed on the patient’s unit/floor, these services count toward the cumulative discharge service time. Documentation must include the time (if reporting 99239) as well as the patient’s discharge status and clinically relevant information. Completion of the death certificate alone is not sufficient for billing. TH
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center, Philadelphia. She is also on the faculty of SHM’s inpatient coding course.
AHRQ in the Lead
What exactly is the Agency for Healthcare Research and Quality (AHRQ), and why are hospitalists urged to increase its portion of the federal budget pie each year?
According to its mission statement, the AHRQ is “the lead federal agency charged with improving the quality, safety, efficiency, and effectiveness of healthcare for all Americans.” This includes supporting high-quality, impartial research that specifically improves healthcare quality, reduces costs, advances patient safety, decreases medical errors, eliminates healthcare disparities, and broadens access to essential services.
“Supporting AHRQ is supporting an unbiased government organization that’s clearly on the side of patient safety, and that gets important information out fast,” says Andrew Fishmann, MD, FCCP, FACP, a member of AHRQ’s National Advisory Council and director of intensive care at Good Samaritan Hospital in Los Angeles. “Where’s the argument?”
Fight over Funding
The argument is over money, plain and simple. Each year, medical associations like SHM push for increased federal funding for AHRQ so the agency’s research can be expanded. And each year, Congress refuses those increases. Lawmakers have granted a slight boost in funding: Since 2002, AHRQ’s budget has increased by $2 million, or 6.7%.
Proponents of AHRQ believe precarious funding levels threaten the agency’s ability to achieve its essential mission. Last year, SHM lobbied for an increase in federal funding for AHRQ to $350 million in fiscal year 2008—$31 million more than the agency’s fiscal 2007 budget. By late 2007, Congress was weighing an increase of $329 million, plus $5 million targeted for comparative-effectiveness research.
“Think of AHRQ compared to the $28 billion that NIH gets,” says Dr. Fishmann. “[AHRQ’s] is a small budget relative to what they do.”
How much does AHRQ need to provide adequate research information? The answer is, apparently, as much as they can get. There are countless areas in healthcare the agency could address.
“If they got $500 million, could they spend it?” asks Dr. Fishmann. “Yes. They could look at the top 20 diseases instead of the top 10.”
What AHRQ Does
Regardless of the final budget amount they receive, AHRQ spends roughly 80% on grants and contracts focused on improving healthcare.
“AHRQ doesn’t do its own research or create its own data,” explains Dr. Fishmann. Rather, AHRQ conducts and supports health services research in leading academic institutions, hospitals, and other settings. In 2005, two hospitalists received separate grants for projects that have already had an effect on hospital medicine. Greg Maynard, MD, MS, division chief of hospital medicine at University of California San Diego School of Medicine, used AHRQ funds for an intervention project to prevent hospital-acquired venous thromboembolism (VTE). Dr. Maynard’s project continued to grow since that grant and has yielded key findings such as a risk-assessment model for VTE. Data and lessons learned are available in the VTE Resource Room on SHM’s Web site at www.hospitalmedicine.org/ResourceRoomRedesign/RR_LandingPage.cfm.
Asked why he went after AHRQ funding, Dr. Maynard explains: “AHRQ is one of the few [funding] agencies that focuses on the realm of implementation—that impact the patient immediately. It was a perfect marriage of what we wanted to do.” The other AHRQ-funded hospital medicine project was conducted by Mark V. Williams, MD, FACP, professor and chief, division of hospital medicine at Northwestern University Feinberg School of Medicine in Chicago, and editor of the Journal of Hospital Medicine. Working for Emory University’s hospital medicine program in Atlanta at the time, Dr. Williams used the grant to create a “discharge bundle” of patient safety interventions such as medication reconciliation and patient-centered education to improve patient safety transitions out of the hospital setting.
“We would not have been able to conduct the study without the support of AHRQ,” says Dr. Williams. “We certainly need more research funds such as this. AHRQ is the primary federal agency funding health services research—however, they receive less than 5% of the funding that goes to NIH and fund more basic science-oriented research. As few as one in 10 grants submitted to AHRQ are actually funded.”
Like Dr. Maynard’s work on VTE prevention, the injection of AHRQ funds also allowed Dr. Williams’ project to continue and grow. “With support from the Society of Hospital Medicine, we have been quite fortunate to utilize the momentum from the AHRQ Patient Safe-[Discharge] grant to obtain a $1.4 million grant from the John A. Hartford Grant to develop a discharge toolkit and facilitate implementation of it at hundreds of hospital,” he explains. “The BOOST [Better Outcomes for Older adults through Safe Transitions] project aims to improve care delivery to older adults at hospitals across America as they transition from the hospital to home.”
Additional research is developed in AHRQ’s Centers for Education and Research in Therapeutics (CERTS). Each of the 11 CERTS has a specific charge and gathers data on the benefits, risks, and cost-effectiveness of therapeutic products such as drugs, medical devices, and biological products.
AHRQ disseminates current healthcare data quickly and more effectively than private channels. “They look at healthcare as a whole,” explains Dr. Fishmann. “For five years, they’ve published the annual National Quality Report and the National Disparity Report. They try to zero in on information to share with the public and with physicians, including all issues related to patient safety. They allow anyone access to the information: One market is hospitalists.”
AHRQ and Hospitalists
Of course, the research and information that AHRQ provides is vital to all physicians. But Dr. Fishmann believes hospitalists find the agency particularly valuable.
“SHM perceives AHRQ as their champion,” he says. “It’s a great partnership: AHRQ documents the value of having hospitalists. SHM provides an efficient way to disseminate new information relevant to hospitals.”
Many essential data and resources for physicians can be found on AHRQ’s Web site at www.ahrq.gov.
“The average hospitalist already uses this site, but I don’t think the average resident does,” says Dr. Fishmann. “I hope everyone knows about it.” TH
Jane Jerrard has written for The Hospitalist since 2005.
What exactly is the Agency for Healthcare Research and Quality (AHRQ), and why are hospitalists urged to increase its portion of the federal budget pie each year?
According to its mission statement, the AHRQ is “the lead federal agency charged with improving the quality, safety, efficiency, and effectiveness of healthcare for all Americans.” This includes supporting high-quality, impartial research that specifically improves healthcare quality, reduces costs, advances patient safety, decreases medical errors, eliminates healthcare disparities, and broadens access to essential services.
“Supporting AHRQ is supporting an unbiased government organization that’s clearly on the side of patient safety, and that gets important information out fast,” says Andrew Fishmann, MD, FCCP, FACP, a member of AHRQ’s National Advisory Council and director of intensive care at Good Samaritan Hospital in Los Angeles. “Where’s the argument?”
Fight over Funding
The argument is over money, plain and simple. Each year, medical associations like SHM push for increased federal funding for AHRQ so the agency’s research can be expanded. And each year, Congress refuses those increases. Lawmakers have granted a slight boost in funding: Since 2002, AHRQ’s budget has increased by $2 million, or 6.7%.
Proponents of AHRQ believe precarious funding levels threaten the agency’s ability to achieve its essential mission. Last year, SHM lobbied for an increase in federal funding for AHRQ to $350 million in fiscal year 2008—$31 million more than the agency’s fiscal 2007 budget. By late 2007, Congress was weighing an increase of $329 million, plus $5 million targeted for comparative-effectiveness research.
“Think of AHRQ compared to the $28 billion that NIH gets,” says Dr. Fishmann. “[AHRQ’s] is a small budget relative to what they do.”
How much does AHRQ need to provide adequate research information? The answer is, apparently, as much as they can get. There are countless areas in healthcare the agency could address.
“If they got $500 million, could they spend it?” asks Dr. Fishmann. “Yes. They could look at the top 20 diseases instead of the top 10.”
What AHRQ Does
Regardless of the final budget amount they receive, AHRQ spends roughly 80% on grants and contracts focused on improving healthcare.
“AHRQ doesn’t do its own research or create its own data,” explains Dr. Fishmann. Rather, AHRQ conducts and supports health services research in leading academic institutions, hospitals, and other settings. In 2005, two hospitalists received separate grants for projects that have already had an effect on hospital medicine. Greg Maynard, MD, MS, division chief of hospital medicine at University of California San Diego School of Medicine, used AHRQ funds for an intervention project to prevent hospital-acquired venous thromboembolism (VTE). Dr. Maynard’s project continued to grow since that grant and has yielded key findings such as a risk-assessment model for VTE. Data and lessons learned are available in the VTE Resource Room on SHM’s Web site at www.hospitalmedicine.org/ResourceRoomRedesign/RR_LandingPage.cfm.
Asked why he went after AHRQ funding, Dr. Maynard explains: “AHRQ is one of the few [funding] agencies that focuses on the realm of implementation—that impact the patient immediately. It was a perfect marriage of what we wanted to do.” The other AHRQ-funded hospital medicine project was conducted by Mark V. Williams, MD, FACP, professor and chief, division of hospital medicine at Northwestern University Feinberg School of Medicine in Chicago, and editor of the Journal of Hospital Medicine. Working for Emory University’s hospital medicine program in Atlanta at the time, Dr. Williams used the grant to create a “discharge bundle” of patient safety interventions such as medication reconciliation and patient-centered education to improve patient safety transitions out of the hospital setting.
“We would not have been able to conduct the study without the support of AHRQ,” says Dr. Williams. “We certainly need more research funds such as this. AHRQ is the primary federal agency funding health services research—however, they receive less than 5% of the funding that goes to NIH and fund more basic science-oriented research. As few as one in 10 grants submitted to AHRQ are actually funded.”
Like Dr. Maynard’s work on VTE prevention, the injection of AHRQ funds also allowed Dr. Williams’ project to continue and grow. “With support from the Society of Hospital Medicine, we have been quite fortunate to utilize the momentum from the AHRQ Patient Safe-[Discharge] grant to obtain a $1.4 million grant from the John A. Hartford Grant to develop a discharge toolkit and facilitate implementation of it at hundreds of hospital,” he explains. “The BOOST [Better Outcomes for Older adults through Safe Transitions] project aims to improve care delivery to older adults at hospitals across America as they transition from the hospital to home.”
Additional research is developed in AHRQ’s Centers for Education and Research in Therapeutics (CERTS). Each of the 11 CERTS has a specific charge and gathers data on the benefits, risks, and cost-effectiveness of therapeutic products such as drugs, medical devices, and biological products.
AHRQ disseminates current healthcare data quickly and more effectively than private channels. “They look at healthcare as a whole,” explains Dr. Fishmann. “For five years, they’ve published the annual National Quality Report and the National Disparity Report. They try to zero in on information to share with the public and with physicians, including all issues related to patient safety. They allow anyone access to the information: One market is hospitalists.”
AHRQ and Hospitalists
Of course, the research and information that AHRQ provides is vital to all physicians. But Dr. Fishmann believes hospitalists find the agency particularly valuable.
“SHM perceives AHRQ as their champion,” he says. “It’s a great partnership: AHRQ documents the value of having hospitalists. SHM provides an efficient way to disseminate new information relevant to hospitals.”
Many essential data and resources for physicians can be found on AHRQ’s Web site at www.ahrq.gov.
“The average hospitalist already uses this site, but I don’t think the average resident does,” says Dr. Fishmann. “I hope everyone knows about it.” TH
Jane Jerrard has written for The Hospitalist since 2005.
What exactly is the Agency for Healthcare Research and Quality (AHRQ), and why are hospitalists urged to increase its portion of the federal budget pie each year?
According to its mission statement, the AHRQ is “the lead federal agency charged with improving the quality, safety, efficiency, and effectiveness of healthcare for all Americans.” This includes supporting high-quality, impartial research that specifically improves healthcare quality, reduces costs, advances patient safety, decreases medical errors, eliminates healthcare disparities, and broadens access to essential services.
“Supporting AHRQ is supporting an unbiased government organization that’s clearly on the side of patient safety, and that gets important information out fast,” says Andrew Fishmann, MD, FCCP, FACP, a member of AHRQ’s National Advisory Council and director of intensive care at Good Samaritan Hospital in Los Angeles. “Where’s the argument?”
Fight over Funding
The argument is over money, plain and simple. Each year, medical associations like SHM push for increased federal funding for AHRQ so the agency’s research can be expanded. And each year, Congress refuses those increases. Lawmakers have granted a slight boost in funding: Since 2002, AHRQ’s budget has increased by $2 million, or 6.7%.
Proponents of AHRQ believe precarious funding levels threaten the agency’s ability to achieve its essential mission. Last year, SHM lobbied for an increase in federal funding for AHRQ to $350 million in fiscal year 2008—$31 million more than the agency’s fiscal 2007 budget. By late 2007, Congress was weighing an increase of $329 million, plus $5 million targeted for comparative-effectiveness research.
“Think of AHRQ compared to the $28 billion that NIH gets,” says Dr. Fishmann. “[AHRQ’s] is a small budget relative to what they do.”
How much does AHRQ need to provide adequate research information? The answer is, apparently, as much as they can get. There are countless areas in healthcare the agency could address.
“If they got $500 million, could they spend it?” asks Dr. Fishmann. “Yes. They could look at the top 20 diseases instead of the top 10.”
What AHRQ Does
Regardless of the final budget amount they receive, AHRQ spends roughly 80% on grants and contracts focused on improving healthcare.
“AHRQ doesn’t do its own research or create its own data,” explains Dr. Fishmann. Rather, AHRQ conducts and supports health services research in leading academic institutions, hospitals, and other settings. In 2005, two hospitalists received separate grants for projects that have already had an effect on hospital medicine. Greg Maynard, MD, MS, division chief of hospital medicine at University of California San Diego School of Medicine, used AHRQ funds for an intervention project to prevent hospital-acquired venous thromboembolism (VTE). Dr. Maynard’s project continued to grow since that grant and has yielded key findings such as a risk-assessment model for VTE. Data and lessons learned are available in the VTE Resource Room on SHM’s Web site at www.hospitalmedicine.org/ResourceRoomRedesign/RR_LandingPage.cfm.
Asked why he went after AHRQ funding, Dr. Maynard explains: “AHRQ is one of the few [funding] agencies that focuses on the realm of implementation—that impact the patient immediately. It was a perfect marriage of what we wanted to do.” The other AHRQ-funded hospital medicine project was conducted by Mark V. Williams, MD, FACP, professor and chief, division of hospital medicine at Northwestern University Feinberg School of Medicine in Chicago, and editor of the Journal of Hospital Medicine. Working for Emory University’s hospital medicine program in Atlanta at the time, Dr. Williams used the grant to create a “discharge bundle” of patient safety interventions such as medication reconciliation and patient-centered education to improve patient safety transitions out of the hospital setting.
“We would not have been able to conduct the study without the support of AHRQ,” says Dr. Williams. “We certainly need more research funds such as this. AHRQ is the primary federal agency funding health services research—however, they receive less than 5% of the funding that goes to NIH and fund more basic science-oriented research. As few as one in 10 grants submitted to AHRQ are actually funded.”
Like Dr. Maynard’s work on VTE prevention, the injection of AHRQ funds also allowed Dr. Williams’ project to continue and grow. “With support from the Society of Hospital Medicine, we have been quite fortunate to utilize the momentum from the AHRQ Patient Safe-[Discharge] grant to obtain a $1.4 million grant from the John A. Hartford Grant to develop a discharge toolkit and facilitate implementation of it at hundreds of hospital,” he explains. “The BOOST [Better Outcomes for Older adults through Safe Transitions] project aims to improve care delivery to older adults at hospitals across America as they transition from the hospital to home.”
Additional research is developed in AHRQ’s Centers for Education and Research in Therapeutics (CERTS). Each of the 11 CERTS has a specific charge and gathers data on the benefits, risks, and cost-effectiveness of therapeutic products such as drugs, medical devices, and biological products.
AHRQ disseminates current healthcare data quickly and more effectively than private channels. “They look at healthcare as a whole,” explains Dr. Fishmann. “For five years, they’ve published the annual National Quality Report and the National Disparity Report. They try to zero in on information to share with the public and with physicians, including all issues related to patient safety. They allow anyone access to the information: One market is hospitalists.”
AHRQ and Hospitalists
Of course, the research and information that AHRQ provides is vital to all physicians. But Dr. Fishmann believes hospitalists find the agency particularly valuable.
“SHM perceives AHRQ as their champion,” he says. “It’s a great partnership: AHRQ documents the value of having hospitalists. SHM provides an efficient way to disseminate new information relevant to hospitals.”
Many essential data and resources for physicians can be found on AHRQ’s Web site at www.ahrq.gov.
“The average hospitalist already uses this site, but I don’t think the average resident does,” says Dr. Fishmann. “I hope everyone knows about it.” TH
Jane Jerrard has written for The Hospitalist since 2005.
Medical Mediation
How many conflicts do you witness during your average shift? How many are you embroiled in? Are any of them resolved amicably? How many can you resolve?
“Everyone does conflict resolution on some level,” says Leonard J. Marcus, PhD, director of the Program for Health Care Negotiation and Conflict Resolution, Harvard School of Public Health in Cambridge, Mass. “If you’re in a relationship, if you have kids—we all do it. The difference is that hospitalists do it as part of their professional work, and that requires a different level of complexity.” Dr. Marcus teaches conflict resolution skills in SHM’s Leadership Academy.
Everyone’s Best Interest
Dr. Marcus’ colleague and associate director of his program is Barry C. Dorn, MD, MHCM. Dr. Dorn clarifies: “Conflict is not bad. But unresolved conflict can be costly.”
A good leader can—and should—resolve problems on his team for the sake of the team, the project or work, and the hospital. Understanding that is easy—it’s how to end the problem that can be tricky.
“There are two poles to conflict resolution,” explains Dr. Marcus. “Positional bargaining is the adversarial win-lose approach to problem solving, and many people believe that’s the only way to resolve a conflict. However, interest-based negotiation focuses on what different people want to accomplish. It’s what we call a collaborative, cooperative approach—it’s gain-gain negotiating.”
How Hard Can It Be?
As Dr. Marcus says, everyone resolves conflicts. So is training in how to go about it really necessary?
“Absolutely,” asserts Dr. Dorn. “[Hospitalist leaders] need some sort of training, though a lot of it can be self-taught. I think this training is the most important thing a physician can do. The stresses and rapid changes in healthcare today make people crazy. It’s not just hospitalists—all physicians have conflicts with other groups. That conflict takes its toll; it’s a tremendous waste of time and energy. And it’s very costly to an institution to have people constantly at odds.”
Dr. Marcus agrees. “Given the role of the hospitalist, having specific skills training in conflict resolution is a huge plus,” he says. “They regularly face challenges in engaging other departments and other physicians, which can lead to turf wars and territoriality. They have to go beyond the simple ability to resolve conflicts and get to the core of the issue quickly. That’s where the training comes in.”
Conflicts among Peers
To further complicate the conflicts they face, hospitalists often find themselves managing a group of peers as committee chairs or lead researchers. They don’t have the title or authority to tell fellow hospitalists, other physicians, hospital staff, and administrators what to do. This can lead to some delicate conflict resolution.
“They’re dealing with people who lead individual silos within the healthcare system,” says Dr. Dorn. “And when someone else wants to step into their silo, it makes you and them uncomfortable. Leaders have to make others feel comfortable and learn to speak their language. Hospitalists have to lead across silos as well as within their own silo [of hospital medicine]; then they have to lead up, because hospital administrators have a lot of control. There are many nuances to leadership.”
As group leaders, hospitalists may face a wide range of conflicts, says Dr. Marcus, “from differences of opinion to resistance to downright draw-the-line-in-the-sand and get out of my way. The other piece is that some issues are clinical, whether between physicians, between physician and patient or family member, and some are administrative or managerial. Hospitalists are at the hub of all those issues; they serve as the fulcrum.”
According to Dr. Dorn, physician-physician conflicts can be disagreements of opinion, of training, of personality, and of reimbursement issues. “Physicians are very concerned with reimbursement—they want to know what it is going to cost them in time and money,” he explains.
For a hospitalist serving as a committee chair, says Dr. Dorn, “The critical thing is that when they assume these positions without authority, the only way to make it work is to increase their level of influence. The level of influence over authority is the indication of a good leader.”
You can acquire the necessary influence by learning solid conflict resolution skills.
Resolve to Study
There are a number of resources available for hospitalists interested in studying conflict resolution. Drs. Dorn and Marcus have co-written a book on the subject, Renegotiating Health Care: Resolving Conflict to Build Collaboration. “The conflicts we deal with [in the book] are right at the core of what’s going on in healthcare right now,” Dr. Marcus says.
Dr. Dorn also recommends some general books on resolving conflict. “Most of conflict resolution is interest-based negotiation, and the father of interest-based negotiation is Roger Fisher,” he says. “With Bill Ury, he wrote Getting to Yes. I think a better book for physicians is Getting Past No. It’s very simple and concise. These are basic books on conflict resolution.”
For a more detailed textbook, Dr. Dorn suggests The Mediation Process: Practical Strategies for Resolving Conflicts by Chris Moore. “This is the definitive text,” he says. “I also like Difficult Conversations by Stone, Patton, and Heen.”
Whether you’re in a leadership role or a hospitalist doing straight clinical work, successfully resolving conflicts on the job can be a much-appreciated skill. “[Conflict resolution training] will make your life so much easier, so much more pleasant,” promises Dr. Dorn. TH
Jane Jerrard writes “Public Policy” for The Hospitalist.
How many conflicts do you witness during your average shift? How many are you embroiled in? Are any of them resolved amicably? How many can you resolve?
“Everyone does conflict resolution on some level,” says Leonard J. Marcus, PhD, director of the Program for Health Care Negotiation and Conflict Resolution, Harvard School of Public Health in Cambridge, Mass. “If you’re in a relationship, if you have kids—we all do it. The difference is that hospitalists do it as part of their professional work, and that requires a different level of complexity.” Dr. Marcus teaches conflict resolution skills in SHM’s Leadership Academy.
Everyone’s Best Interest
Dr. Marcus’ colleague and associate director of his program is Barry C. Dorn, MD, MHCM. Dr. Dorn clarifies: “Conflict is not bad. But unresolved conflict can be costly.”
A good leader can—and should—resolve problems on his team for the sake of the team, the project or work, and the hospital. Understanding that is easy—it’s how to end the problem that can be tricky.
“There are two poles to conflict resolution,” explains Dr. Marcus. “Positional bargaining is the adversarial win-lose approach to problem solving, and many people believe that’s the only way to resolve a conflict. However, interest-based negotiation focuses on what different people want to accomplish. It’s what we call a collaborative, cooperative approach—it’s gain-gain negotiating.”
How Hard Can It Be?
As Dr. Marcus says, everyone resolves conflicts. So is training in how to go about it really necessary?
“Absolutely,” asserts Dr. Dorn. “[Hospitalist leaders] need some sort of training, though a lot of it can be self-taught. I think this training is the most important thing a physician can do. The stresses and rapid changes in healthcare today make people crazy. It’s not just hospitalists—all physicians have conflicts with other groups. That conflict takes its toll; it’s a tremendous waste of time and energy. And it’s very costly to an institution to have people constantly at odds.”
Dr. Marcus agrees. “Given the role of the hospitalist, having specific skills training in conflict resolution is a huge plus,” he says. “They regularly face challenges in engaging other departments and other physicians, which can lead to turf wars and territoriality. They have to go beyond the simple ability to resolve conflicts and get to the core of the issue quickly. That’s where the training comes in.”
Conflicts among Peers
To further complicate the conflicts they face, hospitalists often find themselves managing a group of peers as committee chairs or lead researchers. They don’t have the title or authority to tell fellow hospitalists, other physicians, hospital staff, and administrators what to do. This can lead to some delicate conflict resolution.
“They’re dealing with people who lead individual silos within the healthcare system,” says Dr. Dorn. “And when someone else wants to step into their silo, it makes you and them uncomfortable. Leaders have to make others feel comfortable and learn to speak their language. Hospitalists have to lead across silos as well as within their own silo [of hospital medicine]; then they have to lead up, because hospital administrators have a lot of control. There are many nuances to leadership.”
As group leaders, hospitalists may face a wide range of conflicts, says Dr. Marcus, “from differences of opinion to resistance to downright draw-the-line-in-the-sand and get out of my way. The other piece is that some issues are clinical, whether between physicians, between physician and patient or family member, and some are administrative or managerial. Hospitalists are at the hub of all those issues; they serve as the fulcrum.”
According to Dr. Dorn, physician-physician conflicts can be disagreements of opinion, of training, of personality, and of reimbursement issues. “Physicians are very concerned with reimbursement—they want to know what it is going to cost them in time and money,” he explains.
For a hospitalist serving as a committee chair, says Dr. Dorn, “The critical thing is that when they assume these positions without authority, the only way to make it work is to increase their level of influence. The level of influence over authority is the indication of a good leader.”
You can acquire the necessary influence by learning solid conflict resolution skills.
Resolve to Study
There are a number of resources available for hospitalists interested in studying conflict resolution. Drs. Dorn and Marcus have co-written a book on the subject, Renegotiating Health Care: Resolving Conflict to Build Collaboration. “The conflicts we deal with [in the book] are right at the core of what’s going on in healthcare right now,” Dr. Marcus says.
Dr. Dorn also recommends some general books on resolving conflict. “Most of conflict resolution is interest-based negotiation, and the father of interest-based negotiation is Roger Fisher,” he says. “With Bill Ury, he wrote Getting to Yes. I think a better book for physicians is Getting Past No. It’s very simple and concise. These are basic books on conflict resolution.”
For a more detailed textbook, Dr. Dorn suggests The Mediation Process: Practical Strategies for Resolving Conflicts by Chris Moore. “This is the definitive text,” he says. “I also like Difficult Conversations by Stone, Patton, and Heen.”
Whether you’re in a leadership role or a hospitalist doing straight clinical work, successfully resolving conflicts on the job can be a much-appreciated skill. “[Conflict resolution training] will make your life so much easier, so much more pleasant,” promises Dr. Dorn. TH
Jane Jerrard writes “Public Policy” for The Hospitalist.
How many conflicts do you witness during your average shift? How many are you embroiled in? Are any of them resolved amicably? How many can you resolve?
“Everyone does conflict resolution on some level,” says Leonard J. Marcus, PhD, director of the Program for Health Care Negotiation and Conflict Resolution, Harvard School of Public Health in Cambridge, Mass. “If you’re in a relationship, if you have kids—we all do it. The difference is that hospitalists do it as part of their professional work, and that requires a different level of complexity.” Dr. Marcus teaches conflict resolution skills in SHM’s Leadership Academy.
Everyone’s Best Interest
Dr. Marcus’ colleague and associate director of his program is Barry C. Dorn, MD, MHCM. Dr. Dorn clarifies: “Conflict is not bad. But unresolved conflict can be costly.”
A good leader can—and should—resolve problems on his team for the sake of the team, the project or work, and the hospital. Understanding that is easy—it’s how to end the problem that can be tricky.
“There are two poles to conflict resolution,” explains Dr. Marcus. “Positional bargaining is the adversarial win-lose approach to problem solving, and many people believe that’s the only way to resolve a conflict. However, interest-based negotiation focuses on what different people want to accomplish. It’s what we call a collaborative, cooperative approach—it’s gain-gain negotiating.”
How Hard Can It Be?
As Dr. Marcus says, everyone resolves conflicts. So is training in how to go about it really necessary?
“Absolutely,” asserts Dr. Dorn. “[Hospitalist leaders] need some sort of training, though a lot of it can be self-taught. I think this training is the most important thing a physician can do. The stresses and rapid changes in healthcare today make people crazy. It’s not just hospitalists—all physicians have conflicts with other groups. That conflict takes its toll; it’s a tremendous waste of time and energy. And it’s very costly to an institution to have people constantly at odds.”
Dr. Marcus agrees. “Given the role of the hospitalist, having specific skills training in conflict resolution is a huge plus,” he says. “They regularly face challenges in engaging other departments and other physicians, which can lead to turf wars and territoriality. They have to go beyond the simple ability to resolve conflicts and get to the core of the issue quickly. That’s where the training comes in.”
Conflicts among Peers
To further complicate the conflicts they face, hospitalists often find themselves managing a group of peers as committee chairs or lead researchers. They don’t have the title or authority to tell fellow hospitalists, other physicians, hospital staff, and administrators what to do. This can lead to some delicate conflict resolution.
“They’re dealing with people who lead individual silos within the healthcare system,” says Dr. Dorn. “And when someone else wants to step into their silo, it makes you and them uncomfortable. Leaders have to make others feel comfortable and learn to speak their language. Hospitalists have to lead across silos as well as within their own silo [of hospital medicine]; then they have to lead up, because hospital administrators have a lot of control. There are many nuances to leadership.”
As group leaders, hospitalists may face a wide range of conflicts, says Dr. Marcus, “from differences of opinion to resistance to downright draw-the-line-in-the-sand and get out of my way. The other piece is that some issues are clinical, whether between physicians, between physician and patient or family member, and some are administrative or managerial. Hospitalists are at the hub of all those issues; they serve as the fulcrum.”
According to Dr. Dorn, physician-physician conflicts can be disagreements of opinion, of training, of personality, and of reimbursement issues. “Physicians are very concerned with reimbursement—they want to know what it is going to cost them in time and money,” he explains.
For a hospitalist serving as a committee chair, says Dr. Dorn, “The critical thing is that when they assume these positions without authority, the only way to make it work is to increase their level of influence. The level of influence over authority is the indication of a good leader.”
You can acquire the necessary influence by learning solid conflict resolution skills.
Resolve to Study
There are a number of resources available for hospitalists interested in studying conflict resolution. Drs. Dorn and Marcus have co-written a book on the subject, Renegotiating Health Care: Resolving Conflict to Build Collaboration. “The conflicts we deal with [in the book] are right at the core of what’s going on in healthcare right now,” Dr. Marcus says.
Dr. Dorn also recommends some general books on resolving conflict. “Most of conflict resolution is interest-based negotiation, and the father of interest-based negotiation is Roger Fisher,” he says. “With Bill Ury, he wrote Getting to Yes. I think a better book for physicians is Getting Past No. It’s very simple and concise. These are basic books on conflict resolution.”
For a more detailed textbook, Dr. Dorn suggests The Mediation Process: Practical Strategies for Resolving Conflicts by Chris Moore. “This is the definitive text,” he says. “I also like Difficult Conversations by Stone, Patton, and Heen.”
Whether you’re in a leadership role or a hospitalist doing straight clinical work, successfully resolving conflicts on the job can be a much-appreciated skill. “[Conflict resolution training] will make your life so much easier, so much more pleasant,” promises Dr. Dorn. TH
Jane Jerrard writes “Public Policy” for The Hospitalist.
Hail Fellows
Because hospital medicine is still a new specialty, the finer points of hospitalist education and training are being developed. According to two papers in this month’s Journal of Hospital Medicine, students, hospitalists, employers—even patients—are eager for programs that allow hospitalists to hone their skills.
In one study, investigators at the University of California, San Francisco (UCSF), designed a program for third-year medical students and pharmacy graduate students emphasizing the issues involved in making the transition from inpatient to outpatient care.
Patients often are overwhelmed by the change, and the situation is ripe for miscommunication and error. Students accustomed to seeing these individuals only in the hospital often underestimate the challenges patients confront when they leave the hospital with a bewildering array of instructions and medications.
Medical students also receive little exposure to the roles of caregivers from other fields, yet good transitional care involves professionals from several disciplines. The authors, led by Cindy Lai, MD, assistant clinical professor of medicine at UCSF, reason that “training students in interdisciplinary collaboration may improve their ability to provide quality care.”
They designed an inpatient medicine clerkship curriculum in which teams of medical and pharmacy students paid a home visit to a patient they had cared for in the hospital. After the visits, the students wrote summary letters to each patient’s primary care physician.
The home visits lasted one to two hours and in general consisted of an introduction to the patient’s living quarters, a review of symptoms and medication, a brief physical examination, and a home tour to check for relevant issues such as safety hazards or the patient’s ability to function independently. Students quickly discovered the visits consisted of much more than that.
“Across the board, the response that came back was the ability to view the patient as a person,” says Heather Nye, MD, PhD, assistant clinical professor of internal medicine and one of the authors of the study. Students found it inspiring to see patients as people in control of their surroundings and also were surprised how well or poorly some people did away from the hospital.
They learned how to maximize their interaction with the pharmacy students and how to anticipate problems patients might encounter at home, such as taking medicines appropriately or scheduling and keeping follow-up appointments.
Apparently, the lessons went both ways, with some patients inviting students to stay for dinner or dessert. “That human aspect was one of the most profound features of the visits,” Dr. Nye says.
She acknowledges that scheduling home visits regularly would require a commitment of time and money that is simply not feasible in today’s environment, especially after medical school. But she urged that instruction in transitional and interdisciplinary care be incorporated into the curriculum whenever possible. “We all understand that safe discharges require multiple disciplines,” she said. “It’s never too early to start teaching about transitional care.”
Medical school training is especially important because students and residents who specialize in hospital medicine will find fellowships in short supply. The few that exist function more to train educators rather than practicing physicians.
—Philip Goodman, MD, professor of internal medicine and biomedical engineering, University of Nevada, Reno, School of Medicine
This despite the fact hospital medicine has grown at a near-exponential pace, from 2000 practitioners in 1998 to 15,000 in 2005, with 30,000 projected by 2010.
Practicing hospitalists and residents Philip Goodman, MD, MS, and Andrius Januska, BS, of the University of Nevada, Reno, School of Medicine set about to gauge the value of and interest in a practical fellowship in hospital medicine to employers. They sent questionnaires to employers and practicing hospitalists. Of 103 employers, two-thirds indicated they would offer fellowship graduates a signing bonus or salary premium ranging from $10,000 to more than $20,000.
Of 101 practicing hospitalists, 58% felt a clinical fellowship probably or strongly would be a good career move. Further, 91% said it would at least possibly be a good move. And 57% of the residents thinking of a career in hospital medicine said they would consider a one-year clinical fellowship if one were available.
“I was surprised at how strongly practicing hospitalists, most of whom are not academics, supported the value of an intense year of clinical hospital medicine fellowship training,” says Dr. Goodman, professor of internal medicine and biomedical engineering. “Most felt that graduating internal medicine residents ‘probably’ or ‘strongly’ should consider such fellowship training. I had expected a more neutral response, reflecting a balanced response bias of those with strong feelings at either extreme.”
Such training can offer new physicians a chance to develop expertise and leadership capabilities that might otherwise require years of on-the-job experience, he explains. Fellowship training also might elevate hospitalists to a level of prestige equaling that of other subspecialties, he says.
Ironically, the specialty’s rapid growth is probably slowing the establishment of fellowship programs, because residents can command annual salaries of $160,000 to $200,000 upon graduation with no special fellowship training. But a few months into it, they often realize a fellowship would have helped them master some of the unique aspects of hospital medicine, such as process of care, communication, productivity and medicolegal insight, and quality improvement, Dr. Goodman notes.
The University of Nevada will start training its first six hospitalist fellows next year. “I wouldn’t be surprised if most applicants were those who had recently taken hospitalist positions but realized the professional impact a year of polishing school can provide,” he says. TH
Norra MacReady is a medical writer based in California.
Because hospital medicine is still a new specialty, the finer points of hospitalist education and training are being developed. According to two papers in this month’s Journal of Hospital Medicine, students, hospitalists, employers—even patients—are eager for programs that allow hospitalists to hone their skills.
In one study, investigators at the University of California, San Francisco (UCSF), designed a program for third-year medical students and pharmacy graduate students emphasizing the issues involved in making the transition from inpatient to outpatient care.
Patients often are overwhelmed by the change, and the situation is ripe for miscommunication and error. Students accustomed to seeing these individuals only in the hospital often underestimate the challenges patients confront when they leave the hospital with a bewildering array of instructions and medications.
Medical students also receive little exposure to the roles of caregivers from other fields, yet good transitional care involves professionals from several disciplines. The authors, led by Cindy Lai, MD, assistant clinical professor of medicine at UCSF, reason that “training students in interdisciplinary collaboration may improve their ability to provide quality care.”
They designed an inpatient medicine clerkship curriculum in which teams of medical and pharmacy students paid a home visit to a patient they had cared for in the hospital. After the visits, the students wrote summary letters to each patient’s primary care physician.
The home visits lasted one to two hours and in general consisted of an introduction to the patient’s living quarters, a review of symptoms and medication, a brief physical examination, and a home tour to check for relevant issues such as safety hazards or the patient’s ability to function independently. Students quickly discovered the visits consisted of much more than that.
“Across the board, the response that came back was the ability to view the patient as a person,” says Heather Nye, MD, PhD, assistant clinical professor of internal medicine and one of the authors of the study. Students found it inspiring to see patients as people in control of their surroundings and also were surprised how well or poorly some people did away from the hospital.
They learned how to maximize their interaction with the pharmacy students and how to anticipate problems patients might encounter at home, such as taking medicines appropriately or scheduling and keeping follow-up appointments.
Apparently, the lessons went both ways, with some patients inviting students to stay for dinner or dessert. “That human aspect was one of the most profound features of the visits,” Dr. Nye says.
She acknowledges that scheduling home visits regularly would require a commitment of time and money that is simply not feasible in today’s environment, especially after medical school. But she urged that instruction in transitional and interdisciplinary care be incorporated into the curriculum whenever possible. “We all understand that safe discharges require multiple disciplines,” she said. “It’s never too early to start teaching about transitional care.”
Medical school training is especially important because students and residents who specialize in hospital medicine will find fellowships in short supply. The few that exist function more to train educators rather than practicing physicians.
—Philip Goodman, MD, professor of internal medicine and biomedical engineering, University of Nevada, Reno, School of Medicine
This despite the fact hospital medicine has grown at a near-exponential pace, from 2000 practitioners in 1998 to 15,000 in 2005, with 30,000 projected by 2010.
Practicing hospitalists and residents Philip Goodman, MD, MS, and Andrius Januska, BS, of the University of Nevada, Reno, School of Medicine set about to gauge the value of and interest in a practical fellowship in hospital medicine to employers. They sent questionnaires to employers and practicing hospitalists. Of 103 employers, two-thirds indicated they would offer fellowship graduates a signing bonus or salary premium ranging from $10,000 to more than $20,000.
Of 101 practicing hospitalists, 58% felt a clinical fellowship probably or strongly would be a good career move. Further, 91% said it would at least possibly be a good move. And 57% of the residents thinking of a career in hospital medicine said they would consider a one-year clinical fellowship if one were available.
“I was surprised at how strongly practicing hospitalists, most of whom are not academics, supported the value of an intense year of clinical hospital medicine fellowship training,” says Dr. Goodman, professor of internal medicine and biomedical engineering. “Most felt that graduating internal medicine residents ‘probably’ or ‘strongly’ should consider such fellowship training. I had expected a more neutral response, reflecting a balanced response bias of those with strong feelings at either extreme.”
Such training can offer new physicians a chance to develop expertise and leadership capabilities that might otherwise require years of on-the-job experience, he explains. Fellowship training also might elevate hospitalists to a level of prestige equaling that of other subspecialties, he says.
Ironically, the specialty’s rapid growth is probably slowing the establishment of fellowship programs, because residents can command annual salaries of $160,000 to $200,000 upon graduation with no special fellowship training. But a few months into it, they often realize a fellowship would have helped them master some of the unique aspects of hospital medicine, such as process of care, communication, productivity and medicolegal insight, and quality improvement, Dr. Goodman notes.
The University of Nevada will start training its first six hospitalist fellows next year. “I wouldn’t be surprised if most applicants were those who had recently taken hospitalist positions but realized the professional impact a year of polishing school can provide,” he says. TH
Norra MacReady is a medical writer based in California.
Because hospital medicine is still a new specialty, the finer points of hospitalist education and training are being developed. According to two papers in this month’s Journal of Hospital Medicine, students, hospitalists, employers—even patients—are eager for programs that allow hospitalists to hone their skills.
In one study, investigators at the University of California, San Francisco (UCSF), designed a program for third-year medical students and pharmacy graduate students emphasizing the issues involved in making the transition from inpatient to outpatient care.
Patients often are overwhelmed by the change, and the situation is ripe for miscommunication and error. Students accustomed to seeing these individuals only in the hospital often underestimate the challenges patients confront when they leave the hospital with a bewildering array of instructions and medications.
Medical students also receive little exposure to the roles of caregivers from other fields, yet good transitional care involves professionals from several disciplines. The authors, led by Cindy Lai, MD, assistant clinical professor of medicine at UCSF, reason that “training students in interdisciplinary collaboration may improve their ability to provide quality care.”
They designed an inpatient medicine clerkship curriculum in which teams of medical and pharmacy students paid a home visit to a patient they had cared for in the hospital. After the visits, the students wrote summary letters to each patient’s primary care physician.
The home visits lasted one to two hours and in general consisted of an introduction to the patient’s living quarters, a review of symptoms and medication, a brief physical examination, and a home tour to check for relevant issues such as safety hazards or the patient’s ability to function independently. Students quickly discovered the visits consisted of much more than that.
“Across the board, the response that came back was the ability to view the patient as a person,” says Heather Nye, MD, PhD, assistant clinical professor of internal medicine and one of the authors of the study. Students found it inspiring to see patients as people in control of their surroundings and also were surprised how well or poorly some people did away from the hospital.
They learned how to maximize their interaction with the pharmacy students and how to anticipate problems patients might encounter at home, such as taking medicines appropriately or scheduling and keeping follow-up appointments.
Apparently, the lessons went both ways, with some patients inviting students to stay for dinner or dessert. “That human aspect was one of the most profound features of the visits,” Dr. Nye says.
She acknowledges that scheduling home visits regularly would require a commitment of time and money that is simply not feasible in today’s environment, especially after medical school. But she urged that instruction in transitional and interdisciplinary care be incorporated into the curriculum whenever possible. “We all understand that safe discharges require multiple disciplines,” she said. “It’s never too early to start teaching about transitional care.”
Medical school training is especially important because students and residents who specialize in hospital medicine will find fellowships in short supply. The few that exist function more to train educators rather than practicing physicians.
—Philip Goodman, MD, professor of internal medicine and biomedical engineering, University of Nevada, Reno, School of Medicine
This despite the fact hospital medicine has grown at a near-exponential pace, from 2000 practitioners in 1998 to 15,000 in 2005, with 30,000 projected by 2010.
Practicing hospitalists and residents Philip Goodman, MD, MS, and Andrius Januska, BS, of the University of Nevada, Reno, School of Medicine set about to gauge the value of and interest in a practical fellowship in hospital medicine to employers. They sent questionnaires to employers and practicing hospitalists. Of 103 employers, two-thirds indicated they would offer fellowship graduates a signing bonus or salary premium ranging from $10,000 to more than $20,000.
Of 101 practicing hospitalists, 58% felt a clinical fellowship probably or strongly would be a good career move. Further, 91% said it would at least possibly be a good move. And 57% of the residents thinking of a career in hospital medicine said they would consider a one-year clinical fellowship if one were available.
“I was surprised at how strongly practicing hospitalists, most of whom are not academics, supported the value of an intense year of clinical hospital medicine fellowship training,” says Dr. Goodman, professor of internal medicine and biomedical engineering. “Most felt that graduating internal medicine residents ‘probably’ or ‘strongly’ should consider such fellowship training. I had expected a more neutral response, reflecting a balanced response bias of those with strong feelings at either extreme.”
Such training can offer new physicians a chance to develop expertise and leadership capabilities that might otherwise require years of on-the-job experience, he explains. Fellowship training also might elevate hospitalists to a level of prestige equaling that of other subspecialties, he says.
Ironically, the specialty’s rapid growth is probably slowing the establishment of fellowship programs, because residents can command annual salaries of $160,000 to $200,000 upon graduation with no special fellowship training. But a few months into it, they often realize a fellowship would have helped them master some of the unique aspects of hospital medicine, such as process of care, communication, productivity and medicolegal insight, and quality improvement, Dr. Goodman notes.
The University of Nevada will start training its first six hospitalist fellows next year. “I wouldn’t be surprised if most applicants were those who had recently taken hospitalist positions but realized the professional impact a year of polishing school can provide,” he says. TH
Norra MacReady is a medical writer based in California.
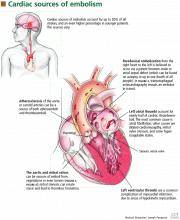
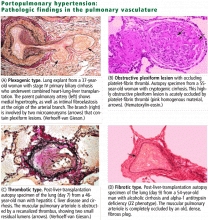
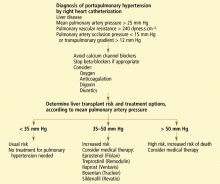
Liver Risks Abound
Drug-induced liver injury (DILI) or hepatotoxicity accounts for more than 50% of all cases of acute liver failure. DILI, often life-threatening, is the leading cause of patient referral for liver transplantation.1,2
DILI is an important diagnostic challenge for the treating clinician because of its presentation, which is often a diagnosis of exclusion. Determination of all potential causes of hepatic injury need to be assessed through onset of symptoms and a careful drug history (including prescription and over-the-counter medication, dietary supplements, and complementary and alternative therapies).3
DILI has brought an increase in Food and Drug Administration (FDA) “black box” warnings. Among the drugs affected are ketoconazole, pemoline, tolcapone, valproate sodium, and zalcitabine.4
A number of drugs have been withdrawn from the U.S. market after DILI or interactions with those that are hepatically metabolized, such as:
- Astemizole (cardiotoxicity);
- Bromfenac sodium, cisapride (cardiotoxicity);
- Felbamate, mibefradil (cardiotoxicity);
- Temafloxacin (abnormal liver function tests, as well as renal failure and other serious adverse events);
- Terfenadine (cardiotoxicity);
- Troglitazone; and
- Trovafloxacin mesylate.
One of the most common causes of DILI is intentional or unintentional overdose with acetaminophen.
DILI has been classified into two major types: cholestatic and hepatocellular, or cytolytic injury. In cholestatic liver injury, the serum alkaline phosphatase (ALP) is elevated; total bilirubin level (TBL) and the alanine aminotransferase (ALT) may also be elevated. In hepatocellular injury, initial elevation is noted in the ALT.
There may also be overlap in the pattern of injury (mixed-pattern injury) whereby ALP and ALT are elevated. These patterns of injury may be defined further by the degree of enzyme elevation, such as an ALT level of three or more times the upper limit of normal (ULN), an ALP level two or more times ULN, and TBL two or more times ULN if associated with an elevation of ALP or ALT.
Hepatotoxicity is often predictable—but not always. When predictable, the reaction is usually dose-dependent, such as in the case of acetaminophen. These reactions usually occur shortly after a threshold for toxicity has been reached. Unpredictable reactions can occur days or months after exposure, usually without warning. Hypersensitivity reactions are often delayed and occur upon repeated exposure to the agent. Symptoms of immunologic injury may include rash, fever, or eosinophilia. More severe forms include Stevens-Johnson syndrome, toxic epidermal necrolysis, or cytopenias. Reactions are more severe upon repeat exposure or rechallenge of the offending agent.
Symptomatology
Patients usually present with vague symptoms that may include nausea, anorexia, fatigue, right upper-quadrant discomfort, jaundice, or dark urine. Patients with cholestatic liver disease may also present with pruritus.
Any of these along with laboratory evidence of liver injury should indicate further investigation into possible DILI. Impaired hepatic function such as increased prothrombin time and encephalopathy (signs of acute liver failure) indicate severe hepatic injury.
The Agents
Common causes of hepatocellular injury include: acetaminophen, fluoxetine, highly active antiretroviral therapies, kava kava (other herbal products), non-steroidal anti-inflammatory drugs, paroxetine, rifampin, risperidone, statins, trazodone, and troglitazone.
Common causes of cholestatic injury include: ampicillin-clavulanic acid, anabolic steroids, chlorpromazine, clopidogrel, estrogens, mirtazapine, terbinafine, and tricyclics.
Common causes of mixed-pattern injury include: amitriptyline, azathioprine, captopril, carbamazepine, enalapril, erythromycins, flutamide, phenytoin, sulfonamides, trazodone, and trimethoprim-sulfamethoxazole.
Most cases of non-fulminant hepatitis will improve upon cessation of offending or potentially offending agent(s). Assess hepatic injury immediately via continuously obtained biochemical tests.
Consult a hepatologist or gastroenterologist immediately if jaundice, impaired hepatic function or clinical signs of acute hepatic failure (e.g., encephalopathy) are evident.
Report all cases of potential DILI to the FDA’s adverse events reporting program, Medwatch, at www.fda.gov/ medwatch or by calling (800) 332-1088. For patients receiving potentially hepatotoxic agents, liver function test monitoring is recommended following a baseline assessment. Some agents require monthly rather than periodic monitoring. For a list of some agents that require hepatic monitoring and the recommended frequency, visit www.factsandcomparisons.com/assets/hospitalpharm/feb2002_HepSp.pdf.5 TH
Michele B. Kaufman is a freelance medical writer based in New York City.
References
- Nathwani RA, Kaplowitz N. Drug hepatotoxicity. Clin Liver Dis. 2006;10:207-217.
- Navarro VJ, Senior JR. Drug-related hepatotoxicity. N Engl J Med. 2006;354(7):731-739.
- Hepatic Toxicity Possibly Associated with Kava-Containing Products—United States, Germany, and Switzerland, 1999-2002. MMWR Weekly, Nov. 29, 2002/51(47):1065-1067. Available at: www.cdc.gov/MMWR/previews/mmwrhtml/mm5147a1.htm. Last accessed Nov. 5, 2007.
- Lasser KE, Allen PD, Woolhandler SJ, et al. Timing of new black box warnings and withdrawals for prescription medications. JAMA. 2002;287:17:2215-2220.
- Tice SA, Parr D. Medications that require hepatic monitoring. Hospital Pharmacy 2004;39(6):595-606.
Drug-induced liver injury (DILI) or hepatotoxicity accounts for more than 50% of all cases of acute liver failure. DILI, often life-threatening, is the leading cause of patient referral for liver transplantation.1,2
DILI is an important diagnostic challenge for the treating clinician because of its presentation, which is often a diagnosis of exclusion. Determination of all potential causes of hepatic injury need to be assessed through onset of symptoms and a careful drug history (including prescription and over-the-counter medication, dietary supplements, and complementary and alternative therapies).3
DILI has brought an increase in Food and Drug Administration (FDA) “black box” warnings. Among the drugs affected are ketoconazole, pemoline, tolcapone, valproate sodium, and zalcitabine.4
A number of drugs have been withdrawn from the U.S. market after DILI or interactions with those that are hepatically metabolized, such as:
- Astemizole (cardiotoxicity);
- Bromfenac sodium, cisapride (cardiotoxicity);
- Felbamate, mibefradil (cardiotoxicity);
- Temafloxacin (abnormal liver function tests, as well as renal failure and other serious adverse events);
- Terfenadine (cardiotoxicity);
- Troglitazone; and
- Trovafloxacin mesylate.
One of the most common causes of DILI is intentional or unintentional overdose with acetaminophen.
DILI has been classified into two major types: cholestatic and hepatocellular, or cytolytic injury. In cholestatic liver injury, the serum alkaline phosphatase (ALP) is elevated; total bilirubin level (TBL) and the alanine aminotransferase (ALT) may also be elevated. In hepatocellular injury, initial elevation is noted in the ALT.
There may also be overlap in the pattern of injury (mixed-pattern injury) whereby ALP and ALT are elevated. These patterns of injury may be defined further by the degree of enzyme elevation, such as an ALT level of three or more times the upper limit of normal (ULN), an ALP level two or more times ULN, and TBL two or more times ULN if associated with an elevation of ALP or ALT.
Hepatotoxicity is often predictable—but not always. When predictable, the reaction is usually dose-dependent, such as in the case of acetaminophen. These reactions usually occur shortly after a threshold for toxicity has been reached. Unpredictable reactions can occur days or months after exposure, usually without warning. Hypersensitivity reactions are often delayed and occur upon repeated exposure to the agent. Symptoms of immunologic injury may include rash, fever, or eosinophilia. More severe forms include Stevens-Johnson syndrome, toxic epidermal necrolysis, or cytopenias. Reactions are more severe upon repeat exposure or rechallenge of the offending agent.
Symptomatology
Patients usually present with vague symptoms that may include nausea, anorexia, fatigue, right upper-quadrant discomfort, jaundice, or dark urine. Patients with cholestatic liver disease may also present with pruritus.
Any of these along with laboratory evidence of liver injury should indicate further investigation into possible DILI. Impaired hepatic function such as increased prothrombin time and encephalopathy (signs of acute liver failure) indicate severe hepatic injury.
The Agents
Common causes of hepatocellular injury include: acetaminophen, fluoxetine, highly active antiretroviral therapies, kava kava (other herbal products), non-steroidal anti-inflammatory drugs, paroxetine, rifampin, risperidone, statins, trazodone, and troglitazone.
Common causes of cholestatic injury include: ampicillin-clavulanic acid, anabolic steroids, chlorpromazine, clopidogrel, estrogens, mirtazapine, terbinafine, and tricyclics.
Common causes of mixed-pattern injury include: amitriptyline, azathioprine, captopril, carbamazepine, enalapril, erythromycins, flutamide, phenytoin, sulfonamides, trazodone, and trimethoprim-sulfamethoxazole.
Most cases of non-fulminant hepatitis will improve upon cessation of offending or potentially offending agent(s). Assess hepatic injury immediately via continuously obtained biochemical tests.
Consult a hepatologist or gastroenterologist immediately if jaundice, impaired hepatic function or clinical signs of acute hepatic failure (e.g., encephalopathy) are evident.
Report all cases of potential DILI to the FDA’s adverse events reporting program, Medwatch, at www.fda.gov/ medwatch or by calling (800) 332-1088. For patients receiving potentially hepatotoxic agents, liver function test monitoring is recommended following a baseline assessment. Some agents require monthly rather than periodic monitoring. For a list of some agents that require hepatic monitoring and the recommended frequency, visit www.factsandcomparisons.com/assets/hospitalpharm/feb2002_HepSp.pdf.5 TH
Michele B. Kaufman is a freelance medical writer based in New York City.
References
- Nathwani RA, Kaplowitz N. Drug hepatotoxicity. Clin Liver Dis. 2006;10:207-217.
- Navarro VJ, Senior JR. Drug-related hepatotoxicity. N Engl J Med. 2006;354(7):731-739.
- Hepatic Toxicity Possibly Associated with Kava-Containing Products—United States, Germany, and Switzerland, 1999-2002. MMWR Weekly, Nov. 29, 2002/51(47):1065-1067. Available at: www.cdc.gov/MMWR/previews/mmwrhtml/mm5147a1.htm. Last accessed Nov. 5, 2007.
- Lasser KE, Allen PD, Woolhandler SJ, et al. Timing of new black box warnings and withdrawals for prescription medications. JAMA. 2002;287:17:2215-2220.
- Tice SA, Parr D. Medications that require hepatic monitoring. Hospital Pharmacy 2004;39(6):595-606.
Drug-induced liver injury (DILI) or hepatotoxicity accounts for more than 50% of all cases of acute liver failure. DILI, often life-threatening, is the leading cause of patient referral for liver transplantation.1,2
DILI is an important diagnostic challenge for the treating clinician because of its presentation, which is often a diagnosis of exclusion. Determination of all potential causes of hepatic injury need to be assessed through onset of symptoms and a careful drug history (including prescription and over-the-counter medication, dietary supplements, and complementary and alternative therapies).3
DILI has brought an increase in Food and Drug Administration (FDA) “black box” warnings. Among the drugs affected are ketoconazole, pemoline, tolcapone, valproate sodium, and zalcitabine.4
A number of drugs have been withdrawn from the U.S. market after DILI or interactions with those that are hepatically metabolized, such as:
- Astemizole (cardiotoxicity);
- Bromfenac sodium, cisapride (cardiotoxicity);
- Felbamate, mibefradil (cardiotoxicity);
- Temafloxacin (abnormal liver function tests, as well as renal failure and other serious adverse events);
- Terfenadine (cardiotoxicity);
- Troglitazone; and
- Trovafloxacin mesylate.
One of the most common causes of DILI is intentional or unintentional overdose with acetaminophen.
DILI has been classified into two major types: cholestatic and hepatocellular, or cytolytic injury. In cholestatic liver injury, the serum alkaline phosphatase (ALP) is elevated; total bilirubin level (TBL) and the alanine aminotransferase (ALT) may also be elevated. In hepatocellular injury, initial elevation is noted in the ALT.
There may also be overlap in the pattern of injury (mixed-pattern injury) whereby ALP and ALT are elevated. These patterns of injury may be defined further by the degree of enzyme elevation, such as an ALT level of three or more times the upper limit of normal (ULN), an ALP level two or more times ULN, and TBL two or more times ULN if associated with an elevation of ALP or ALT.
Hepatotoxicity is often predictable—but not always. When predictable, the reaction is usually dose-dependent, such as in the case of acetaminophen. These reactions usually occur shortly after a threshold for toxicity has been reached. Unpredictable reactions can occur days or months after exposure, usually without warning. Hypersensitivity reactions are often delayed and occur upon repeated exposure to the agent. Symptoms of immunologic injury may include rash, fever, or eosinophilia. More severe forms include Stevens-Johnson syndrome, toxic epidermal necrolysis, or cytopenias. Reactions are more severe upon repeat exposure or rechallenge of the offending agent.
Symptomatology
Patients usually present with vague symptoms that may include nausea, anorexia, fatigue, right upper-quadrant discomfort, jaundice, or dark urine. Patients with cholestatic liver disease may also present with pruritus.
Any of these along with laboratory evidence of liver injury should indicate further investigation into possible DILI. Impaired hepatic function such as increased prothrombin time and encephalopathy (signs of acute liver failure) indicate severe hepatic injury.
The Agents
Common causes of hepatocellular injury include: acetaminophen, fluoxetine, highly active antiretroviral therapies, kava kava (other herbal products), non-steroidal anti-inflammatory drugs, paroxetine, rifampin, risperidone, statins, trazodone, and troglitazone.
Common causes of cholestatic injury include: ampicillin-clavulanic acid, anabolic steroids, chlorpromazine, clopidogrel, estrogens, mirtazapine, terbinafine, and tricyclics.
Common causes of mixed-pattern injury include: amitriptyline, azathioprine, captopril, carbamazepine, enalapril, erythromycins, flutamide, phenytoin, sulfonamides, trazodone, and trimethoprim-sulfamethoxazole.
Most cases of non-fulminant hepatitis will improve upon cessation of offending or potentially offending agent(s). Assess hepatic injury immediately via continuously obtained biochemical tests.
Consult a hepatologist or gastroenterologist immediately if jaundice, impaired hepatic function or clinical signs of acute hepatic failure (e.g., encephalopathy) are evident.
Report all cases of potential DILI to the FDA’s adverse events reporting program, Medwatch, at www.fda.gov/ medwatch or by calling (800) 332-1088. For patients receiving potentially hepatotoxic agents, liver function test monitoring is recommended following a baseline assessment. Some agents require monthly rather than periodic monitoring. For a list of some agents that require hepatic monitoring and the recommended frequency, visit www.factsandcomparisons.com/assets/hospitalpharm/feb2002_HepSp.pdf.5 TH
Michele B. Kaufman is a freelance medical writer based in New York City.
References
- Nathwani RA, Kaplowitz N. Drug hepatotoxicity. Clin Liver Dis. 2006;10:207-217.
- Navarro VJ, Senior JR. Drug-related hepatotoxicity. N Engl J Med. 2006;354(7):731-739.
- Hepatic Toxicity Possibly Associated with Kava-Containing Products—United States, Germany, and Switzerland, 1999-2002. MMWR Weekly, Nov. 29, 2002/51(47):1065-1067. Available at: www.cdc.gov/MMWR/previews/mmwrhtml/mm5147a1.htm. Last accessed Nov. 5, 2007.
- Lasser KE, Allen PD, Woolhandler SJ, et al. Timing of new black box warnings and withdrawals for prescription medications. JAMA. 2002;287:17:2215-2220.
- Tice SA, Parr D. Medications that require hepatic monitoring. Hospital Pharmacy 2004;39(6):595-606.
Preventing and managing diabetic complications in elderly patients
In elderly patients, as in all patients, diabetes is much more than the blood glucose level. However, in elderly patients the disease accelerates other common conditions of that population and markedly complicates their management.
Hypertension, coronary artery disease, and cerebrovascular attacks are more common in patients with diabetes.1 Longitudinal studies of elderly and middle-aged people with diabetes show increased rates of cognitive decline and dementia.2–4 Depression, urinary incontinence, and falls are also more common in elderly patients with diabetes. Physical disability is also increased: women with diabetes are half as likely to be able to manage ordinary physical tasks such as walking, climbing stairs, and doing housework as women without diabetes.5
In an earlier paper in this journal,6 we reviewed the management of diabetes per se in elderly patients. In the pages that follow, we review the management of its associated conditions.
HEART RISK TRUMPS BLOOD SUGAR
Coronary artery disease is by far the leading cause of death in elderly people with diabetes: 40% to 50% of patients with type 2 diabetes die of cardiac disease.7–9 The conventional risk factors—hypertension, hyperlipidemia, smoking, and diabetes—remain risk factors throughout old age. Risk reduction should focus on treating hypertension and dyslipidemia, smoking cessation, aspirin therapy, and exercise. While glycemic control reduces the risk of microvascular complications (eg, diabetic retinopathy and nephropathy) after about 8 years of treatment, benefits from control of elevated blood pressure and cholesterol occur after only 2 to 3 years.
Tight control of hypertension confers significant benefit
The United Kingdom Prospective Diabetes Study (UKPDS)10 found that patients who had tight control of blood pressure (mean treated blood pressure 144/82 mm Hg) had 24% fewer diabetes-related end points, 32% fewer diabetes-related deaths, 44% fewer strokes, a 34% reduced risk of deterioration of retinopathy, and a 47% reduced risk of visual deterioration than patients who had usual control (mean treated blood pressure 157/87 mm Hg). The benefit of treating hypertension outweighed the benefits of tight glycemic control.
A strong focus on blood pressure control should be a major focus of any treatment program. The American Geriatrics Society goal for blood pressure is less than 140/80 mm Hg if tolerated. Others have proposed more stringent targets.
Lipid control
Lipid control is integral to managing elderly patients with diabetes. In the Cholesterol and Recurrent Events trial11 and the Heart Protection Study,12 the cardiovascular benefits of reducing serum low-density lipoprotein cholesterol (LDL-C) levels were similar in elderly and younger patients with diabetes. In a meta-analysis of secondary prevention trials, absolute risk reduction was greatest in subjects older than 65 years with either diabetes or diastolic hypertension.
The American Diabetes Association,13 the American Geriatrics Society,14 and the Department of Veterans Affairs15,16 have all set a goal for serum LDL-C of less than 100 mg/dL. In addition, the American Diabetes Association has set goal levels for triglycerides (< 150 mg/dL) and high-density lipoprotein cholesterol (> 40 mg/dL).
Glycemic control
The importance of tight glycemic control in preventing coronary heart disease in the elderly is somewhat controversial. Treatment guidelines for elderly patients with diabetes are mainly extrapolated from the UKPDS, in which patients were a mean of 54 years old at the start of the study. After 10 years, the mean hemoglobin A1c levels were 7.9% in patients receiving conventional control and 7.0% in patients with intensive therapy. Every 1% reduction in hemoglobin A1c was associated with a 37% decline in microvascular complications of diabetes, a 14% decline in myocardial infarctions, and a 21% decline in any diabetes-related outcome.17
In the original trial,18 the rate of myocardial infarction was 17.4% in the conventional treatment group vs 14.7% in the intensive group (P = .052), and the risk of stroke did not differ. No thresholds for realizing benefits from reducing fasting glucose or hemoglobin A1c levels were detected.
A recent cohort study involving about 10,000 participants aged 45 to 79 years found that the risk of cardiovascular disease and death from any cause increased continuously with increasing hemoglobin A1c levels in people with or without diabetes.19 However, the impact of treatment remains to be clarified. The Action to Control Cardiovascular Risk in Diabetes trial will address this question (and others), but results will not be available for several years.
RETINOPATHY IS A MAJOR CAUSE OF BLINDNESS
Diabetic retinopathy, a leading cause of blindness in the United States, is perhaps the most threatening of the chronic microvascular complications of diabetes for elderly patients. The strongest predictor of retinopathy is the duration of diabetes.20–22 Retinopathy is classified as being nonproliferative, preproliferative, or proliferative.
Ischemia is believed to be the major cause of diabetic retinopathy, and glucose control has been shown to be of major benefit. A study of young adults with type 1 diabetes found that intensive therapy reduced the risk of developing retinopathy by 76% and slowed the progression of retinopathy by 54%. Comparable data for patients with type 2 diabetes are lacking.
Of some concern is a study in which retinopathy progressed more rapidly during the first year of aggressive insulin therapy in elderly patients with diabetes and baseline retinopathy.23 Further research is needed to identify which subgroups would benefit most from aggressive glycemic control.
In addition to specific ophthalmologic treatment, managing cardiovascular risk factors may reduce the progression of retinopathy: each cardiovascular risk factor has been found to also be a risk factor for retinopathy. Hypertension is an independent risk factor for any retinopathy, and its tight control reduces progression.20,24 Aspirin therapy has not been found to confer either risk or benefit.25,26
Although guidelines typically call for yearly ophthalmic examinations to screen for retinopathy, whether this is cost-effective has been questioned.27,28 But people older than 65 years with diabetes also have twice the risk of developing cataracts and three times the risk of developing glaucoma than those without diabetes. Considering the effects of visual loss on quality of life as well as the subsequent higher risk of accidents, eye examinations by an ophthalmologist at the time of diagnosis and annually thereafter are recommended. Tight glycemic and blood pressure control remains the cornerstone in the primary prevention of diabetic retinopathy. Panretinal and focal retinal laser photocoagulation reduces the risk of visual loss in patients with severe retinopathy and macular edema, respectively.29
NEUROPATHY PRESENTS IN MANY FORMS
Neuropathy is a particularly distressing complication and can lead to loss of sleep, limitation of activity, and depression.26,30,31 Diabetic neuropathies include focal neuropathies (entrapment syndromes and mono-neuropathies), polyneuropathy, and autonomic neuropathy.
Distal symmetric polyneuropathy (“glove and stocking” sensory symptoms) is the most common neuropathy of elderly people with diabetes. Pain, which can interrupt sleep and limit activity, can be treated with the anticonvulsants gabapentin (Gabarone, Neurontin), phenytoin (Dilantin, Phenytek) and carbamazepine (Carbatrol, Epitol, Equetro, Tegretol), and with tricyclic antidepressants. However, the anticholinergic effects of tricyclic antidepressants limit their use in older patients. Newer agents, such as duloxetine (Cymbalta) and pregabalin (Lyrica) show promise.30,31 Dysesthesia of a burning quality is sometimes treated with topical capsaicin or with oral mexiletine (Mexitil), although their role in treating older patients is not well established.
Patients with distal sensory polyneuropathy are predisposed to develop Charcot joints, which may mimic gout or degenerative joint disease. Plain radiography of the foot can help differentiate these diseases. Distal sensory polyneuropathy also predisposes patients to neuropathic foot ulcer, the leading cause of foot amputation in the United States.32
Feet should be inspected at each office visit. Testing sensation with a monofilament detects sensory neuropathy. Patients should be encouraged to examine their feet daily. Therapeutic shoes, prescribed by a podiatrist and individually designed to prevent blisters, calluses, and ulcers, are covered by Medicare for peripheral neuropathy if any of the following are also present: callus formation, poor circulation, foot deformity, or a history of foot callus, ulcer, or amputation (partial or complete). Medicare will pay for one pair of shoes plus three pairs of inserts per year.
Proximal motor neuropathy (diabetic amyotrophy) primarily affects elderly patients. It begins with unilateral thigh pain, which becomes bilateral and progresses to proximal muscle weakness and wasting. Distal symmetric polyneuropathy may also be present. Treatment includes glycemic control (usually with insulin) and physical therapy. Some forms of amyotrophy respond to immunotherapy.
Autonomic neuropathy, although not painful, can be the most life-threatening form of diabetic neuropathy.33 Tachycardia increases the risk of sudden death, while postural hypotension increases the risk of syncope, falling, and injury. Other forms of autonomic neuropathy include neurogenic bladder, sexual dysfunction, gastropathy (which is particularly sensitive to glycemic control), enteropathy, and gustatory sweating. Patients with autonomic neuropathy are more likely to have hypoglycemic unawareness.
NEPHROPATHY CAN PROGRESS RAPIDLY
Elderly patients with diabetes are especially at risk of developing nephropathy, which progresses from microalbuminuria to overt proteinuria to renal insufficiency and end-stage renal disease. Nephropathy may develop over a shorter time than the typical 10 to 20 years in younger patients. Independent risk factors for proteinuria and renal insufficiency include poor glycemic control over many years, hypertension, longer duration of diabetes, male sex, high serum total cholesterol levels, and smoking. Elderly patients are also at risk of renal insults such as receiving intravenous iodinated contrast agents in the course of radiologic procedures, nephrotoxic drugs, and comorbid illness such as congestive heart failure.
The diagnosis of diabetic nephropathy is usually made clinically and not by renal biopsy. Diabetic nephropathy can be diagnosed with almost 100% specificity in type 1 diabetes and more than 85% specificity in type 2 diabetes by a urinary albumin excretion of more than 300 mg per day and an appropriate time course in the absence of other obvious causes of renal disease. The urinary albumin-to-creatinine ratio can be used to screen for microalbuminuria (the precursor of frank proteinuria and renal insufficiency). A value of more than 30 mg of albumin per gram of creatinine suggests that albumin excretion exceeds 30 mg and that microalbuminuria is present.
Prevention is a cornerstone of management. Good glycemic control reduces the risk of microalbuminuria, the progression of albuminuria, and the development of renal insufficiency. Lowering blood pressure reduces the decline in glomerular filtration rate and albuminuria. Angiotensin-converting enzyme (ACE) inhibitors reduce the rate of progression of proteinuria and reduce the rate of end-stage renal disease, although the data are stronger in patients with type 1 diabetes.34 When side effects such as cough limit the use of ACE inhibitors, angiotensin receptor blockers can be used as an alternative. Blood pressure should be controlled to reduce stroke and cardiovascular complications, regardless of whether microalbuminuria is present.35
End-stage renal disease in elderly patients with diabetes is becoming increasingly frequent. Nephropathy in older patients is different from that in younger patients. In elderly patients, the pathologic findings may suggest ischemia and hypertension, and the classic Kimmelstiel-Wilson lesions may be absent. Patients may present with end-stage renal disease following an episode of acute renal failure that does not resolve, which may occur after a radiologic procedure involving an iodinated contrast agent.
NONKETOTIC HYPEROSMOLAR COMA
Nonketotic hyperosmolar coma occurs predominantly in elderly patients with type 2 diabetes. Predisposing factors include dementia, infection, stroke, and myocardial infarction. Coma results from osmotic diuresis due to hyperglycemia and consequent dehydration. A drop in the glomerular filtration rate promotes further hyperglycemia and dehydration in a vicious circle. Glucose levels commonly reach 600 mg/dL or more, and serum osmolality often exceeds 320 mOsm/L. A fluid deficit of 5 to 10 L is typical.
Fluid replacement is the mainstay of treatment. Because free water is typically lost in an osmotic diuresis, 0.9% (normal) saline is usually given if hemodynamic instability is present or 0.45% (half-normal) saline otherwise. Insulin is also required, as is specific treatment of the precipitating cause, eg, infection. Ketoacidosis may also occur in the elderly.
Recovery from coma or improvement in mental status may lag behind correction of the serum osmolality and may take several days. Mortality rates can be high: severe hyperosmolarity, advanced age, and nursing home residence are the major risk factors for death.
INFECTIONS: SEVERE AND UNUSUAL
Elderly patients with diabetes are at increased risk of developing severe and unusual infections, particularly malignant external otitis. Necrotizing Pseudomonas aeruginosa infection initially involves the external ear canal and progresses to the mastoid air cells, the skull base, or temporal bone. The clinical presentation consists of fever, otalgia, otorrhea, and less commonly, cranial nerve palsy. Treatment involves surgical debridement and antibiotics.
Other infections associated with diabetes include rhinocerebral mucormycosis, necrotizing fasciitis, emphysematous cholecystitis, and emphysematous pyelonephritis. An elderly patient with diabetes is also at increased risk of renal papillary necrosis, which presents as insidious renal failure.
COGNITIVE IMPAIRMENT
Elderly people with diabetes are at increased risk of cognitive impairment, which poses a barrier to taking medications appropriately and performing other tasks of self-management.
Because dementia may go undetected, particularly in the early stages, cognitive function should be assessed in elderly patients when they fail to take therapy correctly or have frequent episodes of hypoglycemia, or if glycemic control deteriorates without an obvious explanation. Caregivers play a critical role in detecting and reporting early cognitive impairment.
DEPRESSION IS OFTEN UNDETECTED
Elderly patients with diabetes have a higher rate of depression than do age-matched controls, but it is commonly underdetected and undertreated.5,36 Depression has been associated with poor glycemic control, and treatment of depression is associated with improved control. Routine screening for depression should be performed; a variety of diagnostic instruments are available. Particular attention should be given to medications that are associated with depression.
POLYPHARMACY
Many elderly patients take multiple medications. Polypharmacy increases the risk of drug side effects, interactions, and nonadherence to taking medications.37–39 This problem is increased in diabetes, in which several medications are necessary to manage hyper-glycemia, hyperlipidemia, hypertension, and other associated conditions.
Patients should keep accurate medication lists, including over-the-counter medications, herbs, and nutritional supplements. Physicians should carefully review each medication to check if it is appropriate and used correctly.
FALLS
Elderly patients with diabetes mellitus are at increased risk of injurious falls, which are associated with high rates of complications, death, and functional decline.40,41 Risk factors include frailty and functional disability, visual impairment, peripheral or autonomic neuropathy, hypoglycemia, and polypharmacy.
Elderly patients should be screened for their risk of falls, and appropriate measures should be instituted. The American Geriatrics Society has guidelines for preventing falls in the elderly.41
URINARY INCONTINENCE
Elderly women with diabetes are at increased risk of developing urinary incontinence. Risk factors include autonomic neuropathy (causing either neurogenic bladder or fecal impaction), polyuria due to hyperglycemia, and urinary tract and vaginal infections. Although evidence is lacking that urinary incontinence affects glycemic control, assessing and treating the condition improves quality of life.
SUMMARY
Diabetes is a common problem in the elderly, accounting for considerable morbidity and mortality. In a large longitudinal analysis (> 50,000 patients), elderly persons newly diagnosed as having diabetes experienced high rates of complications during 10-year follow-up, far in excess of elderly persons without diabetes.42 Diabetes is underdiagnosed in the elderly and is frequently undertreated. Management of the elderly with diabetes presents unique challenges because of associated comorbidities, but with attention to detail and individualized approaches, quality and duration of life can be optimized. The greatest attention should be given to reduction of overall cardiovascular risk. Glycemic goals and the treatment regimens to achieve those goals should be individualized and chosen to control hyperglycemic symptoms and achieve the maximal glycemic control possible while minimizing the risk of hypoglycemia. Diabetes will continue to be a challenge to the patient, the physician, the care team, and the health care system.
- Gregg EW, Engelgau MM, Narayan V. Complications of diabetes in elderly people. BMJ 2002; 325:916–917.
- Knopman D, Boland LL, Mosley T, et al. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology 2001; 56:42–48.
- Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology 1999; 53:1937–1942.
- Fontbonne A, Berr C, Ducimetiere P, Alperovitch A. Changes in cognitive abilities over a 4-year period are unfavorably affected in elderly diabetic subjects: results of the Epidemiology of Vascular Aging Study. Diabetes Care 2001; 24:366–370.
- Gregg EW, Mangione CM, Cauley JA, et al. Diabetes and incidence of functional disability in older women. Diabetes Care 2002; 25:61–67.
- Hornick T, Aron DC. Managing diabetes in the elderly: go easy, individualize. Cleve Clin J Med 2008; 75:70–78.
- Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339:229–234.
- Bertoni AG, Krop JS, Anderson GF, Brancati FL. Diabetes-related morbidity and mortality in a national sample of U.S. elders. Diabetes Care 2002; 25:471–475.
- Bertoni AG, Kirk JK, Goff DC, Wagenknecht LE. Excess mortality related to diabetes mellitus in elderly Medicare beneficiaries. Ann Epidemiol 2004; 14:362–367.
- UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998; 317:703–713. Erratum in: BMJ 1999; 318:29.
- Goldberg RB, Mellies MJ, Sacks FM, et al. Cardiovascular events and their reduction with pravastatin in diabetic and glucose-intolerant myocardial infarction survivors with average cholesterol levels: subgroup analyses in the Cholesterol and Recurrent Events (CARE) trial. The CARE Investigators. Circulation 1998; 98:2513–2519.
- Collins R, Armitage J, Parish S, Sleigh P, Peto R. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes. Lancet 2003; 361:2005–2016.
- American Diabetes Association. Standards of medical care in diabetes. Diabetes Care 2005; 28:S4–S36.
- Brown AF, Mangione CM, Saliba D, Sarkisian CA California Healthcare Foundation/American Geriatrics Society Panel on Improving Care for Elders with Diabetes. Guidelines for improving the care of the older person with diabetes mellitus. J Am Geriatr Soc 2003; 51:S265–S280.
- VA/DoD Clinical Practice Guideline for the Management of Diabetes Mellitus in the Primary Care Setting 2003. Accessed January 4, 2008. www.oqp.med.va.gov/cpg/dm/DM3_cpg/content/introduction.htm.
- Pogach LM, Brietzke SA, Cowan CL, Conlin P, Walder DJ, Sawin CT VA/DoD Diabetes Guideline Development Group. Development of evidence-based clinical practice guidelines for diabetes: the Department of Veterans Affairs/Department of Defense guidelines initiative. Diabetes Care 2004; 27:B82–B89.
- Stratton IM, Asler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000; 321:405–412.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853. Erratum in: Lancet 1999; 354:602.
- Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann Intern Med 2004; 141:413–420.
- Matthews DR, Stratton IM, Aldington SJ, Holman RR, Kohner EM UK Prospective Diabetes Study Group. Risks of progression of retinopathy and vision loss related to tight blood pressure control in type 2 diabetes mellitus: UKPDS 69. Arch Ophthalmol 2004; 122:1631–1640.
- Cahill M, Halley A, Codd M, et al. Prevalence of diabetic retinopathy in patients with diabetic mellitus diagnosed after the age of 70 years. Br J Opthalmol 1997; 81:218–222.
- Hirvela H, Laatikainen L. Diabetic retinopathy in people aged 70 years or older. The Oulu Eye Study. Br J Ophthalmol 1997; 81:214–217.
- Tovi J, Ingemansson SO, Engfeldt P. Insulin treatment of elderly type 2 diabetic patients: effects on retinopathy. Diabetes Metab 1998; 24:442–447.
- Schrier RW, Estacio RO, Esler A, Mehler P. Effects of aggressive blood pressure control in normotensive type 2 diabetic patients on albuminuria, retinopathy and strokes. Kidney Int 2002; 61:1086–1097.
- Kohner EM. Aspirin for diabetic retinopathy. BMJ 2003; 327:1060–1061.
- Greene DA, Stevens MJ, Feldman EL. Diabetic neuropathy: scope of the syndrome. Am J Med 1999; 107:2S–8S.
- Hutchinson A, McIntosh A, Peters J, et al. Effectiveness of screening and monitoring tests for diabetic retinopathy—a systematic review. Diabet Med 2000; 17:495–506.
- Vijan S, Hofer TP, Hayward RA. Cost-utility analysis of screening intervals for diabetic retinopathy in patients with type 2 diabetes mellitus. JAMA 2000; 283:889–896.
- Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review. JAMA 2007; 298:902–916.
- Argoff CE, Cole BE, Fishbain DA, Irving GA. Diabetic peripheral neuropathic pain: clinical and quality-of-life issues. Mayo Clin Proc 2006; 81:S3–S11.
- Wong MC, Chung JW, Wong TK. Effects of treatments for symptoms of painful diabetic neuropathy: systematic review. BMJ 2007; 335:87: epubl June 11, 2007.
- Bild DE, Selby JV, Sinnock P, Browner WS, Braveman P, Showstack JA. Lower-extremity amputation in people with diabetes. Epidemiology and prevention. Diabetes Care 1989; 12:24–31.
- Wheeler SG, Ahroni JH, Boyko EJ. Prospective study of autonomic neuropathy as a predictor of mortality in patients with diabetes. Diabetes Res Clin Pract 2002; 58:131–138.
- Brenner BM, Cooper ME, de Zeeuw D RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345:861–869.
- UK Prospective Diabetes Study Group. Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. BMJ 1998; 317:713–720.
- Sinclair AJ, Girling AJ, Bayer AJ. Cognitive dysfunction in older subjects with diabetes mellitus: impact on diabetes self-management and use of care services. All Wales Research into Elderly (AWARE) Study. Diabetes Res Clin Pract 2000; 50:203–212.
- Moisan J, Gaudet M, Gregoire JP, Bouchard R. Non-compliance with drug treatment and reading difficulties with regard to prescription labelling among seniors. Gerontology 2002; 48:44–51.
- Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA 2005; 294:716–724.
- Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA 2002; 288:462–467.
- Schwartz AV, Hillier TA, Sellmeyer DE, et al. Older women with diabetes have a higher risk of falls: a prospective study. Diabetes Care 2002; 25:1749–1754.
- American Geriatrics Society, British Geriatrics Society, and American Academy of Orthopaedic Surgeons Panel on Falls Prevention. Guideline for the prevention of falls in older persons. J Am Geriatr Soc 2001; 49:664–672.
- Bethel MA, Sloan FA, Belsky D, Feinglos MN. Longitudinal incidence and prevalence of adverse outcomes of diabetes mellitus in elderly patients. Arch Intern Med 2007; 167:921–927.
In elderly patients, as in all patients, diabetes is much more than the blood glucose level. However, in elderly patients the disease accelerates other common conditions of that population and markedly complicates their management.
Hypertension, coronary artery disease, and cerebrovascular attacks are more common in patients with diabetes.1 Longitudinal studies of elderly and middle-aged people with diabetes show increased rates of cognitive decline and dementia.2–4 Depression, urinary incontinence, and falls are also more common in elderly patients with diabetes. Physical disability is also increased: women with diabetes are half as likely to be able to manage ordinary physical tasks such as walking, climbing stairs, and doing housework as women without diabetes.5
In an earlier paper in this journal,6 we reviewed the management of diabetes per se in elderly patients. In the pages that follow, we review the management of its associated conditions.
HEART RISK TRUMPS BLOOD SUGAR
Coronary artery disease is by far the leading cause of death in elderly people with diabetes: 40% to 50% of patients with type 2 diabetes die of cardiac disease.7–9 The conventional risk factors—hypertension, hyperlipidemia, smoking, and diabetes—remain risk factors throughout old age. Risk reduction should focus on treating hypertension and dyslipidemia, smoking cessation, aspirin therapy, and exercise. While glycemic control reduces the risk of microvascular complications (eg, diabetic retinopathy and nephropathy) after about 8 years of treatment, benefits from control of elevated blood pressure and cholesterol occur after only 2 to 3 years.
Tight control of hypertension confers significant benefit
The United Kingdom Prospective Diabetes Study (UKPDS)10 found that patients who had tight control of blood pressure (mean treated blood pressure 144/82 mm Hg) had 24% fewer diabetes-related end points, 32% fewer diabetes-related deaths, 44% fewer strokes, a 34% reduced risk of deterioration of retinopathy, and a 47% reduced risk of visual deterioration than patients who had usual control (mean treated blood pressure 157/87 mm Hg). The benefit of treating hypertension outweighed the benefits of tight glycemic control.
A strong focus on blood pressure control should be a major focus of any treatment program. The American Geriatrics Society goal for blood pressure is less than 140/80 mm Hg if tolerated. Others have proposed more stringent targets.
Lipid control
Lipid control is integral to managing elderly patients with diabetes. In the Cholesterol and Recurrent Events trial11 and the Heart Protection Study,12 the cardiovascular benefits of reducing serum low-density lipoprotein cholesterol (LDL-C) levels were similar in elderly and younger patients with diabetes. In a meta-analysis of secondary prevention trials, absolute risk reduction was greatest in subjects older than 65 years with either diabetes or diastolic hypertension.
The American Diabetes Association,13 the American Geriatrics Society,14 and the Department of Veterans Affairs15,16 have all set a goal for serum LDL-C of less than 100 mg/dL. In addition, the American Diabetes Association has set goal levels for triglycerides (< 150 mg/dL) and high-density lipoprotein cholesterol (> 40 mg/dL).
Glycemic control
The importance of tight glycemic control in preventing coronary heart disease in the elderly is somewhat controversial. Treatment guidelines for elderly patients with diabetes are mainly extrapolated from the UKPDS, in which patients were a mean of 54 years old at the start of the study. After 10 years, the mean hemoglobin A1c levels were 7.9% in patients receiving conventional control and 7.0% in patients with intensive therapy. Every 1% reduction in hemoglobin A1c was associated with a 37% decline in microvascular complications of diabetes, a 14% decline in myocardial infarctions, and a 21% decline in any diabetes-related outcome.17
In the original trial,18 the rate of myocardial infarction was 17.4% in the conventional treatment group vs 14.7% in the intensive group (P = .052), and the risk of stroke did not differ. No thresholds for realizing benefits from reducing fasting glucose or hemoglobin A1c levels were detected.
A recent cohort study involving about 10,000 participants aged 45 to 79 years found that the risk of cardiovascular disease and death from any cause increased continuously with increasing hemoglobin A1c levels in people with or without diabetes.19 However, the impact of treatment remains to be clarified. The Action to Control Cardiovascular Risk in Diabetes trial will address this question (and others), but results will not be available for several years.
RETINOPATHY IS A MAJOR CAUSE OF BLINDNESS
Diabetic retinopathy, a leading cause of blindness in the United States, is perhaps the most threatening of the chronic microvascular complications of diabetes for elderly patients. The strongest predictor of retinopathy is the duration of diabetes.20–22 Retinopathy is classified as being nonproliferative, preproliferative, or proliferative.
Ischemia is believed to be the major cause of diabetic retinopathy, and glucose control has been shown to be of major benefit. A study of young adults with type 1 diabetes found that intensive therapy reduced the risk of developing retinopathy by 76% and slowed the progression of retinopathy by 54%. Comparable data for patients with type 2 diabetes are lacking.
Of some concern is a study in which retinopathy progressed more rapidly during the first year of aggressive insulin therapy in elderly patients with diabetes and baseline retinopathy.23 Further research is needed to identify which subgroups would benefit most from aggressive glycemic control.
In addition to specific ophthalmologic treatment, managing cardiovascular risk factors may reduce the progression of retinopathy: each cardiovascular risk factor has been found to also be a risk factor for retinopathy. Hypertension is an independent risk factor for any retinopathy, and its tight control reduces progression.20,24 Aspirin therapy has not been found to confer either risk or benefit.25,26
Although guidelines typically call for yearly ophthalmic examinations to screen for retinopathy, whether this is cost-effective has been questioned.27,28 But people older than 65 years with diabetes also have twice the risk of developing cataracts and three times the risk of developing glaucoma than those without diabetes. Considering the effects of visual loss on quality of life as well as the subsequent higher risk of accidents, eye examinations by an ophthalmologist at the time of diagnosis and annually thereafter are recommended. Tight glycemic and blood pressure control remains the cornerstone in the primary prevention of diabetic retinopathy. Panretinal and focal retinal laser photocoagulation reduces the risk of visual loss in patients with severe retinopathy and macular edema, respectively.29
NEUROPATHY PRESENTS IN MANY FORMS
Neuropathy is a particularly distressing complication and can lead to loss of sleep, limitation of activity, and depression.26,30,31 Diabetic neuropathies include focal neuropathies (entrapment syndromes and mono-neuropathies), polyneuropathy, and autonomic neuropathy.
Distal symmetric polyneuropathy (“glove and stocking” sensory symptoms) is the most common neuropathy of elderly people with diabetes. Pain, which can interrupt sleep and limit activity, can be treated with the anticonvulsants gabapentin (Gabarone, Neurontin), phenytoin (Dilantin, Phenytek) and carbamazepine (Carbatrol, Epitol, Equetro, Tegretol), and with tricyclic antidepressants. However, the anticholinergic effects of tricyclic antidepressants limit their use in older patients. Newer agents, such as duloxetine (Cymbalta) and pregabalin (Lyrica) show promise.30,31 Dysesthesia of a burning quality is sometimes treated with topical capsaicin or with oral mexiletine (Mexitil), although their role in treating older patients is not well established.
Patients with distal sensory polyneuropathy are predisposed to develop Charcot joints, which may mimic gout or degenerative joint disease. Plain radiography of the foot can help differentiate these diseases. Distal sensory polyneuropathy also predisposes patients to neuropathic foot ulcer, the leading cause of foot amputation in the United States.32
Feet should be inspected at each office visit. Testing sensation with a monofilament detects sensory neuropathy. Patients should be encouraged to examine their feet daily. Therapeutic shoes, prescribed by a podiatrist and individually designed to prevent blisters, calluses, and ulcers, are covered by Medicare for peripheral neuropathy if any of the following are also present: callus formation, poor circulation, foot deformity, or a history of foot callus, ulcer, or amputation (partial or complete). Medicare will pay for one pair of shoes plus three pairs of inserts per year.
Proximal motor neuropathy (diabetic amyotrophy) primarily affects elderly patients. It begins with unilateral thigh pain, which becomes bilateral and progresses to proximal muscle weakness and wasting. Distal symmetric polyneuropathy may also be present. Treatment includes glycemic control (usually with insulin) and physical therapy. Some forms of amyotrophy respond to immunotherapy.
Autonomic neuropathy, although not painful, can be the most life-threatening form of diabetic neuropathy.33 Tachycardia increases the risk of sudden death, while postural hypotension increases the risk of syncope, falling, and injury. Other forms of autonomic neuropathy include neurogenic bladder, sexual dysfunction, gastropathy (which is particularly sensitive to glycemic control), enteropathy, and gustatory sweating. Patients with autonomic neuropathy are more likely to have hypoglycemic unawareness.
NEPHROPATHY CAN PROGRESS RAPIDLY
Elderly patients with diabetes are especially at risk of developing nephropathy, which progresses from microalbuminuria to overt proteinuria to renal insufficiency and end-stage renal disease. Nephropathy may develop over a shorter time than the typical 10 to 20 years in younger patients. Independent risk factors for proteinuria and renal insufficiency include poor glycemic control over many years, hypertension, longer duration of diabetes, male sex, high serum total cholesterol levels, and smoking. Elderly patients are also at risk of renal insults such as receiving intravenous iodinated contrast agents in the course of radiologic procedures, nephrotoxic drugs, and comorbid illness such as congestive heart failure.
The diagnosis of diabetic nephropathy is usually made clinically and not by renal biopsy. Diabetic nephropathy can be diagnosed with almost 100% specificity in type 1 diabetes and more than 85% specificity in type 2 diabetes by a urinary albumin excretion of more than 300 mg per day and an appropriate time course in the absence of other obvious causes of renal disease. The urinary albumin-to-creatinine ratio can be used to screen for microalbuminuria (the precursor of frank proteinuria and renal insufficiency). A value of more than 30 mg of albumin per gram of creatinine suggests that albumin excretion exceeds 30 mg and that microalbuminuria is present.
Prevention is a cornerstone of management. Good glycemic control reduces the risk of microalbuminuria, the progression of albuminuria, and the development of renal insufficiency. Lowering blood pressure reduces the decline in glomerular filtration rate and albuminuria. Angiotensin-converting enzyme (ACE) inhibitors reduce the rate of progression of proteinuria and reduce the rate of end-stage renal disease, although the data are stronger in patients with type 1 diabetes.34 When side effects such as cough limit the use of ACE inhibitors, angiotensin receptor blockers can be used as an alternative. Blood pressure should be controlled to reduce stroke and cardiovascular complications, regardless of whether microalbuminuria is present.35
End-stage renal disease in elderly patients with diabetes is becoming increasingly frequent. Nephropathy in older patients is different from that in younger patients. In elderly patients, the pathologic findings may suggest ischemia and hypertension, and the classic Kimmelstiel-Wilson lesions may be absent. Patients may present with end-stage renal disease following an episode of acute renal failure that does not resolve, which may occur after a radiologic procedure involving an iodinated contrast agent.
NONKETOTIC HYPEROSMOLAR COMA
Nonketotic hyperosmolar coma occurs predominantly in elderly patients with type 2 diabetes. Predisposing factors include dementia, infection, stroke, and myocardial infarction. Coma results from osmotic diuresis due to hyperglycemia and consequent dehydration. A drop in the glomerular filtration rate promotes further hyperglycemia and dehydration in a vicious circle. Glucose levels commonly reach 600 mg/dL or more, and serum osmolality often exceeds 320 mOsm/L. A fluid deficit of 5 to 10 L is typical.
Fluid replacement is the mainstay of treatment. Because free water is typically lost in an osmotic diuresis, 0.9% (normal) saline is usually given if hemodynamic instability is present or 0.45% (half-normal) saline otherwise. Insulin is also required, as is specific treatment of the precipitating cause, eg, infection. Ketoacidosis may also occur in the elderly.
Recovery from coma or improvement in mental status may lag behind correction of the serum osmolality and may take several days. Mortality rates can be high: severe hyperosmolarity, advanced age, and nursing home residence are the major risk factors for death.
INFECTIONS: SEVERE AND UNUSUAL
Elderly patients with diabetes are at increased risk of developing severe and unusual infections, particularly malignant external otitis. Necrotizing Pseudomonas aeruginosa infection initially involves the external ear canal and progresses to the mastoid air cells, the skull base, or temporal bone. The clinical presentation consists of fever, otalgia, otorrhea, and less commonly, cranial nerve palsy. Treatment involves surgical debridement and antibiotics.
Other infections associated with diabetes include rhinocerebral mucormycosis, necrotizing fasciitis, emphysematous cholecystitis, and emphysematous pyelonephritis. An elderly patient with diabetes is also at increased risk of renal papillary necrosis, which presents as insidious renal failure.
COGNITIVE IMPAIRMENT
Elderly people with diabetes are at increased risk of cognitive impairment, which poses a barrier to taking medications appropriately and performing other tasks of self-management.
Because dementia may go undetected, particularly in the early stages, cognitive function should be assessed in elderly patients when they fail to take therapy correctly or have frequent episodes of hypoglycemia, or if glycemic control deteriorates without an obvious explanation. Caregivers play a critical role in detecting and reporting early cognitive impairment.
DEPRESSION IS OFTEN UNDETECTED
Elderly patients with diabetes have a higher rate of depression than do age-matched controls, but it is commonly underdetected and undertreated.5,36 Depression has been associated with poor glycemic control, and treatment of depression is associated with improved control. Routine screening for depression should be performed; a variety of diagnostic instruments are available. Particular attention should be given to medications that are associated with depression.
POLYPHARMACY
Many elderly patients take multiple medications. Polypharmacy increases the risk of drug side effects, interactions, and nonadherence to taking medications.37–39 This problem is increased in diabetes, in which several medications are necessary to manage hyper-glycemia, hyperlipidemia, hypertension, and other associated conditions.
Patients should keep accurate medication lists, including over-the-counter medications, herbs, and nutritional supplements. Physicians should carefully review each medication to check if it is appropriate and used correctly.
FALLS
Elderly patients with diabetes mellitus are at increased risk of injurious falls, which are associated with high rates of complications, death, and functional decline.40,41 Risk factors include frailty and functional disability, visual impairment, peripheral or autonomic neuropathy, hypoglycemia, and polypharmacy.
Elderly patients should be screened for their risk of falls, and appropriate measures should be instituted. The American Geriatrics Society has guidelines for preventing falls in the elderly.41
URINARY INCONTINENCE
Elderly women with diabetes are at increased risk of developing urinary incontinence. Risk factors include autonomic neuropathy (causing either neurogenic bladder or fecal impaction), polyuria due to hyperglycemia, and urinary tract and vaginal infections. Although evidence is lacking that urinary incontinence affects glycemic control, assessing and treating the condition improves quality of life.
SUMMARY
Diabetes is a common problem in the elderly, accounting for considerable morbidity and mortality. In a large longitudinal analysis (> 50,000 patients), elderly persons newly diagnosed as having diabetes experienced high rates of complications during 10-year follow-up, far in excess of elderly persons without diabetes.42 Diabetes is underdiagnosed in the elderly and is frequently undertreated. Management of the elderly with diabetes presents unique challenges because of associated comorbidities, but with attention to detail and individualized approaches, quality and duration of life can be optimized. The greatest attention should be given to reduction of overall cardiovascular risk. Glycemic goals and the treatment regimens to achieve those goals should be individualized and chosen to control hyperglycemic symptoms and achieve the maximal glycemic control possible while minimizing the risk of hypoglycemia. Diabetes will continue to be a challenge to the patient, the physician, the care team, and the health care system.
In elderly patients, as in all patients, diabetes is much more than the blood glucose level. However, in elderly patients the disease accelerates other common conditions of that population and markedly complicates their management.
Hypertension, coronary artery disease, and cerebrovascular attacks are more common in patients with diabetes.1 Longitudinal studies of elderly and middle-aged people with diabetes show increased rates of cognitive decline and dementia.2–4 Depression, urinary incontinence, and falls are also more common in elderly patients with diabetes. Physical disability is also increased: women with diabetes are half as likely to be able to manage ordinary physical tasks such as walking, climbing stairs, and doing housework as women without diabetes.5
In an earlier paper in this journal,6 we reviewed the management of diabetes per se in elderly patients. In the pages that follow, we review the management of its associated conditions.
HEART RISK TRUMPS BLOOD SUGAR
Coronary artery disease is by far the leading cause of death in elderly people with diabetes: 40% to 50% of patients with type 2 diabetes die of cardiac disease.7–9 The conventional risk factors—hypertension, hyperlipidemia, smoking, and diabetes—remain risk factors throughout old age. Risk reduction should focus on treating hypertension and dyslipidemia, smoking cessation, aspirin therapy, and exercise. While glycemic control reduces the risk of microvascular complications (eg, diabetic retinopathy and nephropathy) after about 8 years of treatment, benefits from control of elevated blood pressure and cholesterol occur after only 2 to 3 years.
Tight control of hypertension confers significant benefit
The United Kingdom Prospective Diabetes Study (UKPDS)10 found that patients who had tight control of blood pressure (mean treated blood pressure 144/82 mm Hg) had 24% fewer diabetes-related end points, 32% fewer diabetes-related deaths, 44% fewer strokes, a 34% reduced risk of deterioration of retinopathy, and a 47% reduced risk of visual deterioration than patients who had usual control (mean treated blood pressure 157/87 mm Hg). The benefit of treating hypertension outweighed the benefits of tight glycemic control.
A strong focus on blood pressure control should be a major focus of any treatment program. The American Geriatrics Society goal for blood pressure is less than 140/80 mm Hg if tolerated. Others have proposed more stringent targets.
Lipid control
Lipid control is integral to managing elderly patients with diabetes. In the Cholesterol and Recurrent Events trial11 and the Heart Protection Study,12 the cardiovascular benefits of reducing serum low-density lipoprotein cholesterol (LDL-C) levels were similar in elderly and younger patients with diabetes. In a meta-analysis of secondary prevention trials, absolute risk reduction was greatest in subjects older than 65 years with either diabetes or diastolic hypertension.
The American Diabetes Association,13 the American Geriatrics Society,14 and the Department of Veterans Affairs15,16 have all set a goal for serum LDL-C of less than 100 mg/dL. In addition, the American Diabetes Association has set goal levels for triglycerides (< 150 mg/dL) and high-density lipoprotein cholesterol (> 40 mg/dL).
Glycemic control
The importance of tight glycemic control in preventing coronary heart disease in the elderly is somewhat controversial. Treatment guidelines for elderly patients with diabetes are mainly extrapolated from the UKPDS, in which patients were a mean of 54 years old at the start of the study. After 10 years, the mean hemoglobin A1c levels were 7.9% in patients receiving conventional control and 7.0% in patients with intensive therapy. Every 1% reduction in hemoglobin A1c was associated with a 37% decline in microvascular complications of diabetes, a 14% decline in myocardial infarctions, and a 21% decline in any diabetes-related outcome.17
In the original trial,18 the rate of myocardial infarction was 17.4% in the conventional treatment group vs 14.7% in the intensive group (P = .052), and the risk of stroke did not differ. No thresholds for realizing benefits from reducing fasting glucose or hemoglobin A1c levels were detected.
A recent cohort study involving about 10,000 participants aged 45 to 79 years found that the risk of cardiovascular disease and death from any cause increased continuously with increasing hemoglobin A1c levels in people with or without diabetes.19 However, the impact of treatment remains to be clarified. The Action to Control Cardiovascular Risk in Diabetes trial will address this question (and others), but results will not be available for several years.
RETINOPATHY IS A MAJOR CAUSE OF BLINDNESS
Diabetic retinopathy, a leading cause of blindness in the United States, is perhaps the most threatening of the chronic microvascular complications of diabetes for elderly patients. The strongest predictor of retinopathy is the duration of diabetes.20–22 Retinopathy is classified as being nonproliferative, preproliferative, or proliferative.
Ischemia is believed to be the major cause of diabetic retinopathy, and glucose control has been shown to be of major benefit. A study of young adults with type 1 diabetes found that intensive therapy reduced the risk of developing retinopathy by 76% and slowed the progression of retinopathy by 54%. Comparable data for patients with type 2 diabetes are lacking.
Of some concern is a study in which retinopathy progressed more rapidly during the first year of aggressive insulin therapy in elderly patients with diabetes and baseline retinopathy.23 Further research is needed to identify which subgroups would benefit most from aggressive glycemic control.
In addition to specific ophthalmologic treatment, managing cardiovascular risk factors may reduce the progression of retinopathy: each cardiovascular risk factor has been found to also be a risk factor for retinopathy. Hypertension is an independent risk factor for any retinopathy, and its tight control reduces progression.20,24 Aspirin therapy has not been found to confer either risk or benefit.25,26
Although guidelines typically call for yearly ophthalmic examinations to screen for retinopathy, whether this is cost-effective has been questioned.27,28 But people older than 65 years with diabetes also have twice the risk of developing cataracts and three times the risk of developing glaucoma than those without diabetes. Considering the effects of visual loss on quality of life as well as the subsequent higher risk of accidents, eye examinations by an ophthalmologist at the time of diagnosis and annually thereafter are recommended. Tight glycemic and blood pressure control remains the cornerstone in the primary prevention of diabetic retinopathy. Panretinal and focal retinal laser photocoagulation reduces the risk of visual loss in patients with severe retinopathy and macular edema, respectively.29
NEUROPATHY PRESENTS IN MANY FORMS
Neuropathy is a particularly distressing complication and can lead to loss of sleep, limitation of activity, and depression.26,30,31 Diabetic neuropathies include focal neuropathies (entrapment syndromes and mono-neuropathies), polyneuropathy, and autonomic neuropathy.
Distal symmetric polyneuropathy (“glove and stocking” sensory symptoms) is the most common neuropathy of elderly people with diabetes. Pain, which can interrupt sleep and limit activity, can be treated with the anticonvulsants gabapentin (Gabarone, Neurontin), phenytoin (Dilantin, Phenytek) and carbamazepine (Carbatrol, Epitol, Equetro, Tegretol), and with tricyclic antidepressants. However, the anticholinergic effects of tricyclic antidepressants limit their use in older patients. Newer agents, such as duloxetine (Cymbalta) and pregabalin (Lyrica) show promise.30,31 Dysesthesia of a burning quality is sometimes treated with topical capsaicin or with oral mexiletine (Mexitil), although their role in treating older patients is not well established.
Patients with distal sensory polyneuropathy are predisposed to develop Charcot joints, which may mimic gout or degenerative joint disease. Plain radiography of the foot can help differentiate these diseases. Distal sensory polyneuropathy also predisposes patients to neuropathic foot ulcer, the leading cause of foot amputation in the United States.32
Feet should be inspected at each office visit. Testing sensation with a monofilament detects sensory neuropathy. Patients should be encouraged to examine their feet daily. Therapeutic shoes, prescribed by a podiatrist and individually designed to prevent blisters, calluses, and ulcers, are covered by Medicare for peripheral neuropathy if any of the following are also present: callus formation, poor circulation, foot deformity, or a history of foot callus, ulcer, or amputation (partial or complete). Medicare will pay for one pair of shoes plus three pairs of inserts per year.
Proximal motor neuropathy (diabetic amyotrophy) primarily affects elderly patients. It begins with unilateral thigh pain, which becomes bilateral and progresses to proximal muscle weakness and wasting. Distal symmetric polyneuropathy may also be present. Treatment includes glycemic control (usually with insulin) and physical therapy. Some forms of amyotrophy respond to immunotherapy.
Autonomic neuropathy, although not painful, can be the most life-threatening form of diabetic neuropathy.33 Tachycardia increases the risk of sudden death, while postural hypotension increases the risk of syncope, falling, and injury. Other forms of autonomic neuropathy include neurogenic bladder, sexual dysfunction, gastropathy (which is particularly sensitive to glycemic control), enteropathy, and gustatory sweating. Patients with autonomic neuropathy are more likely to have hypoglycemic unawareness.
NEPHROPATHY CAN PROGRESS RAPIDLY
Elderly patients with diabetes are especially at risk of developing nephropathy, which progresses from microalbuminuria to overt proteinuria to renal insufficiency and end-stage renal disease. Nephropathy may develop over a shorter time than the typical 10 to 20 years in younger patients. Independent risk factors for proteinuria and renal insufficiency include poor glycemic control over many years, hypertension, longer duration of diabetes, male sex, high serum total cholesterol levels, and smoking. Elderly patients are also at risk of renal insults such as receiving intravenous iodinated contrast agents in the course of radiologic procedures, nephrotoxic drugs, and comorbid illness such as congestive heart failure.
The diagnosis of diabetic nephropathy is usually made clinically and not by renal biopsy. Diabetic nephropathy can be diagnosed with almost 100% specificity in type 1 diabetes and more than 85% specificity in type 2 diabetes by a urinary albumin excretion of more than 300 mg per day and an appropriate time course in the absence of other obvious causes of renal disease. The urinary albumin-to-creatinine ratio can be used to screen for microalbuminuria (the precursor of frank proteinuria and renal insufficiency). A value of more than 30 mg of albumin per gram of creatinine suggests that albumin excretion exceeds 30 mg and that microalbuminuria is present.
Prevention is a cornerstone of management. Good glycemic control reduces the risk of microalbuminuria, the progression of albuminuria, and the development of renal insufficiency. Lowering blood pressure reduces the decline in glomerular filtration rate and albuminuria. Angiotensin-converting enzyme (ACE) inhibitors reduce the rate of progression of proteinuria and reduce the rate of end-stage renal disease, although the data are stronger in patients with type 1 diabetes.34 When side effects such as cough limit the use of ACE inhibitors, angiotensin receptor blockers can be used as an alternative. Blood pressure should be controlled to reduce stroke and cardiovascular complications, regardless of whether microalbuminuria is present.35
End-stage renal disease in elderly patients with diabetes is becoming increasingly frequent. Nephropathy in older patients is different from that in younger patients. In elderly patients, the pathologic findings may suggest ischemia and hypertension, and the classic Kimmelstiel-Wilson lesions may be absent. Patients may present with end-stage renal disease following an episode of acute renal failure that does not resolve, which may occur after a radiologic procedure involving an iodinated contrast agent.
NONKETOTIC HYPEROSMOLAR COMA
Nonketotic hyperosmolar coma occurs predominantly in elderly patients with type 2 diabetes. Predisposing factors include dementia, infection, stroke, and myocardial infarction. Coma results from osmotic diuresis due to hyperglycemia and consequent dehydration. A drop in the glomerular filtration rate promotes further hyperglycemia and dehydration in a vicious circle. Glucose levels commonly reach 600 mg/dL or more, and serum osmolality often exceeds 320 mOsm/L. A fluid deficit of 5 to 10 L is typical.
Fluid replacement is the mainstay of treatment. Because free water is typically lost in an osmotic diuresis, 0.9% (normal) saline is usually given if hemodynamic instability is present or 0.45% (half-normal) saline otherwise. Insulin is also required, as is specific treatment of the precipitating cause, eg, infection. Ketoacidosis may also occur in the elderly.
Recovery from coma or improvement in mental status may lag behind correction of the serum osmolality and may take several days. Mortality rates can be high: severe hyperosmolarity, advanced age, and nursing home residence are the major risk factors for death.
INFECTIONS: SEVERE AND UNUSUAL
Elderly patients with diabetes are at increased risk of developing severe and unusual infections, particularly malignant external otitis. Necrotizing Pseudomonas aeruginosa infection initially involves the external ear canal and progresses to the mastoid air cells, the skull base, or temporal bone. The clinical presentation consists of fever, otalgia, otorrhea, and less commonly, cranial nerve palsy. Treatment involves surgical debridement and antibiotics.
Other infections associated with diabetes include rhinocerebral mucormycosis, necrotizing fasciitis, emphysematous cholecystitis, and emphysematous pyelonephritis. An elderly patient with diabetes is also at increased risk of renal papillary necrosis, which presents as insidious renal failure.
COGNITIVE IMPAIRMENT
Elderly people with diabetes are at increased risk of cognitive impairment, which poses a barrier to taking medications appropriately and performing other tasks of self-management.
Because dementia may go undetected, particularly in the early stages, cognitive function should be assessed in elderly patients when they fail to take therapy correctly or have frequent episodes of hypoglycemia, or if glycemic control deteriorates without an obvious explanation. Caregivers play a critical role in detecting and reporting early cognitive impairment.
DEPRESSION IS OFTEN UNDETECTED
Elderly patients with diabetes have a higher rate of depression than do age-matched controls, but it is commonly underdetected and undertreated.5,36 Depression has been associated with poor glycemic control, and treatment of depression is associated with improved control. Routine screening for depression should be performed; a variety of diagnostic instruments are available. Particular attention should be given to medications that are associated with depression.
POLYPHARMACY
Many elderly patients take multiple medications. Polypharmacy increases the risk of drug side effects, interactions, and nonadherence to taking medications.37–39 This problem is increased in diabetes, in which several medications are necessary to manage hyper-glycemia, hyperlipidemia, hypertension, and other associated conditions.
Patients should keep accurate medication lists, including over-the-counter medications, herbs, and nutritional supplements. Physicians should carefully review each medication to check if it is appropriate and used correctly.
FALLS
Elderly patients with diabetes mellitus are at increased risk of injurious falls, which are associated with high rates of complications, death, and functional decline.40,41 Risk factors include frailty and functional disability, visual impairment, peripheral or autonomic neuropathy, hypoglycemia, and polypharmacy.
Elderly patients should be screened for their risk of falls, and appropriate measures should be instituted. The American Geriatrics Society has guidelines for preventing falls in the elderly.41
URINARY INCONTINENCE
Elderly women with diabetes are at increased risk of developing urinary incontinence. Risk factors include autonomic neuropathy (causing either neurogenic bladder or fecal impaction), polyuria due to hyperglycemia, and urinary tract and vaginal infections. Although evidence is lacking that urinary incontinence affects glycemic control, assessing and treating the condition improves quality of life.
SUMMARY
Diabetes is a common problem in the elderly, accounting for considerable morbidity and mortality. In a large longitudinal analysis (> 50,000 patients), elderly persons newly diagnosed as having diabetes experienced high rates of complications during 10-year follow-up, far in excess of elderly persons without diabetes.42 Diabetes is underdiagnosed in the elderly and is frequently undertreated. Management of the elderly with diabetes presents unique challenges because of associated comorbidities, but with attention to detail and individualized approaches, quality and duration of life can be optimized. The greatest attention should be given to reduction of overall cardiovascular risk. Glycemic goals and the treatment regimens to achieve those goals should be individualized and chosen to control hyperglycemic symptoms and achieve the maximal glycemic control possible while minimizing the risk of hypoglycemia. Diabetes will continue to be a challenge to the patient, the physician, the care team, and the health care system.
- Gregg EW, Engelgau MM, Narayan V. Complications of diabetes in elderly people. BMJ 2002; 325:916–917.
- Knopman D, Boland LL, Mosley T, et al. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology 2001; 56:42–48.
- Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology 1999; 53:1937–1942.
- Fontbonne A, Berr C, Ducimetiere P, Alperovitch A. Changes in cognitive abilities over a 4-year period are unfavorably affected in elderly diabetic subjects: results of the Epidemiology of Vascular Aging Study. Diabetes Care 2001; 24:366–370.
- Gregg EW, Mangione CM, Cauley JA, et al. Diabetes and incidence of functional disability in older women. Diabetes Care 2002; 25:61–67.
- Hornick T, Aron DC. Managing diabetes in the elderly: go easy, individualize. Cleve Clin J Med 2008; 75:70–78.
- Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339:229–234.
- Bertoni AG, Krop JS, Anderson GF, Brancati FL. Diabetes-related morbidity and mortality in a national sample of U.S. elders. Diabetes Care 2002; 25:471–475.
- Bertoni AG, Kirk JK, Goff DC, Wagenknecht LE. Excess mortality related to diabetes mellitus in elderly Medicare beneficiaries. Ann Epidemiol 2004; 14:362–367.
- UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998; 317:703–713. Erratum in: BMJ 1999; 318:29.
- Goldberg RB, Mellies MJ, Sacks FM, et al. Cardiovascular events and their reduction with pravastatin in diabetic and glucose-intolerant myocardial infarction survivors with average cholesterol levels: subgroup analyses in the Cholesterol and Recurrent Events (CARE) trial. The CARE Investigators. Circulation 1998; 98:2513–2519.
- Collins R, Armitage J, Parish S, Sleigh P, Peto R. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes. Lancet 2003; 361:2005–2016.
- American Diabetes Association. Standards of medical care in diabetes. Diabetes Care 2005; 28:S4–S36.
- Brown AF, Mangione CM, Saliba D, Sarkisian CA California Healthcare Foundation/American Geriatrics Society Panel on Improving Care for Elders with Diabetes. Guidelines for improving the care of the older person with diabetes mellitus. J Am Geriatr Soc 2003; 51:S265–S280.
- VA/DoD Clinical Practice Guideline for the Management of Diabetes Mellitus in the Primary Care Setting 2003. Accessed January 4, 2008. www.oqp.med.va.gov/cpg/dm/DM3_cpg/content/introduction.htm.
- Pogach LM, Brietzke SA, Cowan CL, Conlin P, Walder DJ, Sawin CT VA/DoD Diabetes Guideline Development Group. Development of evidence-based clinical practice guidelines for diabetes: the Department of Veterans Affairs/Department of Defense guidelines initiative. Diabetes Care 2004; 27:B82–B89.
- Stratton IM, Asler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000; 321:405–412.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853. Erratum in: Lancet 1999; 354:602.
- Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann Intern Med 2004; 141:413–420.
- Matthews DR, Stratton IM, Aldington SJ, Holman RR, Kohner EM UK Prospective Diabetes Study Group. Risks of progression of retinopathy and vision loss related to tight blood pressure control in type 2 diabetes mellitus: UKPDS 69. Arch Ophthalmol 2004; 122:1631–1640.
- Cahill M, Halley A, Codd M, et al. Prevalence of diabetic retinopathy in patients with diabetic mellitus diagnosed after the age of 70 years. Br J Opthalmol 1997; 81:218–222.
- Hirvela H, Laatikainen L. Diabetic retinopathy in people aged 70 years or older. The Oulu Eye Study. Br J Ophthalmol 1997; 81:214–217.
- Tovi J, Ingemansson SO, Engfeldt P. Insulin treatment of elderly type 2 diabetic patients: effects on retinopathy. Diabetes Metab 1998; 24:442–447.
- Schrier RW, Estacio RO, Esler A, Mehler P. Effects of aggressive blood pressure control in normotensive type 2 diabetic patients on albuminuria, retinopathy and strokes. Kidney Int 2002; 61:1086–1097.
- Kohner EM. Aspirin for diabetic retinopathy. BMJ 2003; 327:1060–1061.
- Greene DA, Stevens MJ, Feldman EL. Diabetic neuropathy: scope of the syndrome. Am J Med 1999; 107:2S–8S.
- Hutchinson A, McIntosh A, Peters J, et al. Effectiveness of screening and monitoring tests for diabetic retinopathy—a systematic review. Diabet Med 2000; 17:495–506.
- Vijan S, Hofer TP, Hayward RA. Cost-utility analysis of screening intervals for diabetic retinopathy in patients with type 2 diabetes mellitus. JAMA 2000; 283:889–896.
- Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review. JAMA 2007; 298:902–916.
- Argoff CE, Cole BE, Fishbain DA, Irving GA. Diabetic peripheral neuropathic pain: clinical and quality-of-life issues. Mayo Clin Proc 2006; 81:S3–S11.
- Wong MC, Chung JW, Wong TK. Effects of treatments for symptoms of painful diabetic neuropathy: systematic review. BMJ 2007; 335:87: epubl June 11, 2007.
- Bild DE, Selby JV, Sinnock P, Browner WS, Braveman P, Showstack JA. Lower-extremity amputation in people with diabetes. Epidemiology and prevention. Diabetes Care 1989; 12:24–31.
- Wheeler SG, Ahroni JH, Boyko EJ. Prospective study of autonomic neuropathy as a predictor of mortality in patients with diabetes. Diabetes Res Clin Pract 2002; 58:131–138.
- Brenner BM, Cooper ME, de Zeeuw D RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345:861–869.
- UK Prospective Diabetes Study Group. Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. BMJ 1998; 317:713–720.
- Sinclair AJ, Girling AJ, Bayer AJ. Cognitive dysfunction in older subjects with diabetes mellitus: impact on diabetes self-management and use of care services. All Wales Research into Elderly (AWARE) Study. Diabetes Res Clin Pract 2000; 50:203–212.
- Moisan J, Gaudet M, Gregoire JP, Bouchard R. Non-compliance with drug treatment and reading difficulties with regard to prescription labelling among seniors. Gerontology 2002; 48:44–51.
- Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA 2005; 294:716–724.
- Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA 2002; 288:462–467.
- Schwartz AV, Hillier TA, Sellmeyer DE, et al. Older women with diabetes have a higher risk of falls: a prospective study. Diabetes Care 2002; 25:1749–1754.
- American Geriatrics Society, British Geriatrics Society, and American Academy of Orthopaedic Surgeons Panel on Falls Prevention. Guideline for the prevention of falls in older persons. J Am Geriatr Soc 2001; 49:664–672.
- Bethel MA, Sloan FA, Belsky D, Feinglos MN. Longitudinal incidence and prevalence of adverse outcomes of diabetes mellitus in elderly patients. Arch Intern Med 2007; 167:921–927.
- Gregg EW, Engelgau MM, Narayan V. Complications of diabetes in elderly people. BMJ 2002; 325:916–917.
- Knopman D, Boland LL, Mosley T, et al. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology 2001; 56:42–48.
- Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology 1999; 53:1937–1942.
- Fontbonne A, Berr C, Ducimetiere P, Alperovitch A. Changes in cognitive abilities over a 4-year period are unfavorably affected in elderly diabetic subjects: results of the Epidemiology of Vascular Aging Study. Diabetes Care 2001; 24:366–370.
- Gregg EW, Mangione CM, Cauley JA, et al. Diabetes and incidence of functional disability in older women. Diabetes Care 2002; 25:61–67.
- Hornick T, Aron DC. Managing diabetes in the elderly: go easy, individualize. Cleve Clin J Med 2008; 75:70–78.
- Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339:229–234.
- Bertoni AG, Krop JS, Anderson GF, Brancati FL. Diabetes-related morbidity and mortality in a national sample of U.S. elders. Diabetes Care 2002; 25:471–475.
- Bertoni AG, Kirk JK, Goff DC, Wagenknecht LE. Excess mortality related to diabetes mellitus in elderly Medicare beneficiaries. Ann Epidemiol 2004; 14:362–367.
- UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998; 317:703–713. Erratum in: BMJ 1999; 318:29.
- Goldberg RB, Mellies MJ, Sacks FM, et al. Cardiovascular events and their reduction with pravastatin in diabetic and glucose-intolerant myocardial infarction survivors with average cholesterol levels: subgroup analyses in the Cholesterol and Recurrent Events (CARE) trial. The CARE Investigators. Circulation 1998; 98:2513–2519.
- Collins R, Armitage J, Parish S, Sleigh P, Peto R. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes. Lancet 2003; 361:2005–2016.
- American Diabetes Association. Standards of medical care in diabetes. Diabetes Care 2005; 28:S4–S36.
- Brown AF, Mangione CM, Saliba D, Sarkisian CA California Healthcare Foundation/American Geriatrics Society Panel on Improving Care for Elders with Diabetes. Guidelines for improving the care of the older person with diabetes mellitus. J Am Geriatr Soc 2003; 51:S265–S280.
- VA/DoD Clinical Practice Guideline for the Management of Diabetes Mellitus in the Primary Care Setting 2003. Accessed January 4, 2008. www.oqp.med.va.gov/cpg/dm/DM3_cpg/content/introduction.htm.
- Pogach LM, Brietzke SA, Cowan CL, Conlin P, Walder DJ, Sawin CT VA/DoD Diabetes Guideline Development Group. Development of evidence-based clinical practice guidelines for diabetes: the Department of Veterans Affairs/Department of Defense guidelines initiative. Diabetes Care 2004; 27:B82–B89.
- Stratton IM, Asler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000; 321:405–412.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853. Erratum in: Lancet 1999; 354:602.
- Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann Intern Med 2004; 141:413–420.
- Matthews DR, Stratton IM, Aldington SJ, Holman RR, Kohner EM UK Prospective Diabetes Study Group. Risks of progression of retinopathy and vision loss related to tight blood pressure control in type 2 diabetes mellitus: UKPDS 69. Arch Ophthalmol 2004; 122:1631–1640.
- Cahill M, Halley A, Codd M, et al. Prevalence of diabetic retinopathy in patients with diabetic mellitus diagnosed after the age of 70 years. Br J Opthalmol 1997; 81:218–222.
- Hirvela H, Laatikainen L. Diabetic retinopathy in people aged 70 years or older. The Oulu Eye Study. Br J Ophthalmol 1997; 81:214–217.
- Tovi J, Ingemansson SO, Engfeldt P. Insulin treatment of elderly type 2 diabetic patients: effects on retinopathy. Diabetes Metab 1998; 24:442–447.
- Schrier RW, Estacio RO, Esler A, Mehler P. Effects of aggressive blood pressure control in normotensive type 2 diabetic patients on albuminuria, retinopathy and strokes. Kidney Int 2002; 61:1086–1097.
- Kohner EM. Aspirin for diabetic retinopathy. BMJ 2003; 327:1060–1061.
- Greene DA, Stevens MJ, Feldman EL. Diabetic neuropathy: scope of the syndrome. Am J Med 1999; 107:2S–8S.
- Hutchinson A, McIntosh A, Peters J, et al. Effectiveness of screening and monitoring tests for diabetic retinopathy—a systematic review. Diabet Med 2000; 17:495–506.
- Vijan S, Hofer TP, Hayward RA. Cost-utility analysis of screening intervals for diabetic retinopathy in patients with type 2 diabetes mellitus. JAMA 2000; 283:889–896.
- Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review. JAMA 2007; 298:902–916.
- Argoff CE, Cole BE, Fishbain DA, Irving GA. Diabetic peripheral neuropathic pain: clinical and quality-of-life issues. Mayo Clin Proc 2006; 81:S3–S11.
- Wong MC, Chung JW, Wong TK. Effects of treatments for symptoms of painful diabetic neuropathy: systematic review. BMJ 2007; 335:87: epubl June 11, 2007.
- Bild DE, Selby JV, Sinnock P, Browner WS, Braveman P, Showstack JA. Lower-extremity amputation in people with diabetes. Epidemiology and prevention. Diabetes Care 1989; 12:24–31.
- Wheeler SG, Ahroni JH, Boyko EJ. Prospective study of autonomic neuropathy as a predictor of mortality in patients with diabetes. Diabetes Res Clin Pract 2002; 58:131–138.
- Brenner BM, Cooper ME, de Zeeuw D RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345:861–869.
- UK Prospective Diabetes Study Group. Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. BMJ 1998; 317:713–720.
- Sinclair AJ, Girling AJ, Bayer AJ. Cognitive dysfunction in older subjects with diabetes mellitus: impact on diabetes self-management and use of care services. All Wales Research into Elderly (AWARE) Study. Diabetes Res Clin Pract 2000; 50:203–212.
- Moisan J, Gaudet M, Gregoire JP, Bouchard R. Non-compliance with drug treatment and reading difficulties with regard to prescription labelling among seniors. Gerontology 2002; 48:44–51.
- Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA 2005; 294:716–724.
- Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA 2002; 288:462–467.
- Schwartz AV, Hillier TA, Sellmeyer DE, et al. Older women with diabetes have a higher risk of falls: a prospective study. Diabetes Care 2002; 25:1749–1754.
- American Geriatrics Society, British Geriatrics Society, and American Academy of Orthopaedic Surgeons Panel on Falls Prevention. Guideline for the prevention of falls in older persons. J Am Geriatr Soc 2001; 49:664–672.
- Bethel MA, Sloan FA, Belsky D, Feinglos MN. Longitudinal incidence and prevalence of adverse outcomes of diabetes mellitus in elderly patients. Arch Intern Med 2007; 167:921–927.
KEY POINTS
- Compared with strict glycemic control, treating cardiovascular risk factors offers more benefit in a shorter time and should be a higher priority.
- Diabetic retinopathy is a leading cause of blindness. Yearly eye examinations are recommended.
- Elderly patients with diabetes are prone to rapidly progressive nephropathy, especially after receiving iodinated contrast agents. Good glycemic control and control of blood pressure, especially with angiotensin-converting enzyme inhibitors, reduce the risk and the rate of progression.
- Elderly patients with diabetes are at higher risk of cognitive decline, depression, and polypharmacy, all of which impede good diabetes management.
A young man with acute weakness of his right arm
A 42-year-old man was working at his computer when he suddenly became disoriented and lightheaded, had difficulty concentrating, and could not move his right arm. He could walk without difficulty, but he had a tingling sensation in his right leg. He did not lose consciousness or have any associated palpitations, chest pain, shortness of breath, nausea, vomiting, headaches, or visual changes.
He called 911, and an ambulance arrived 15 minutes later. By that time his symptoms had started to resolve. Now, in the emergency department, his only residual symptom is mild numbness of his right arm and shoulder.
Until now he has been healthy except for a history of dyslipidemia. He takes no prescription or over-the-counter medications and has no drug allergies. He has smoked one pack of cigarettes daily for the past 28 years and also smokes marijuana several times each month. He drinks alcohol occasionally. His family has no history of stroke, premature coronary artery disease, or sudden cardiac death.
INITIAL EVALUATION
His heart rate is 88 beats per minute, blood pressure 142/82 mm Hg, and blood oxygen saturation 98% while breathing room air. He is alert and in no acute distress and answers questions appropriately.
His breathing sounds are normal, without crackles or wheezes. His heart has normal first and second sounds, a normal rate and rhythm, and no extra sounds or murmurs. His abdomen is normal. His extremities are warm and well perfused with normal peripheral pulses and no edema.
On neurologic examination, his cranial nerves and visual fields are normal, and his strength is normal in all muscle groups except for the right upper arm, which is slightly weaker than the left when tested against resistance. Reflexes and response to light touch and pinprick are normal.
His serum chemistry levels, renal function, and blood counts are normal. His total cholesterol level is 155 mg/dL, high-density lipoprotein cholesterol 38 mg/dL, low-density lipoprotein cholesterol 108 mg/dL, and triglycerides 1,286 mg/dL. Electrocardiography is normal with sinus rhythm at a rate of 74.
Magnetic resonance imaging (MRI) of the head and neck with magnetic resonance angiography (MRA) of the intracranial and extracranial vessels is performed. Diffusion-weighted images show a hyperintense lesion in the left insular cortex, consistent with an infarct in the distribution of a branch of the left middle cerebral artery. There is no intracranial hemorrhage. All intracranial and extracranial major vessels are patent, and no stenoses are seen.
DIFFERENTIAL DIAGNOSIS
1. Which is the most likely cause of this patient’s stroke?
- Vertebral or carotid atherosclerosis
- Cervical arterial dissection
- A hematologic disorder
- Cocaine abuse
- Cardiac embolism
Atherosclerosis
Although 85% of all strokes are ischemic, and most ischemic strokes are caused by occlusive atherosclerosis of large vessels, most ischemic strokes occur in patients older than 65 years. In patients younger than 55 years, only about 10% of strokes are caused by large-vessel atherosclerotic disease, thus lowering the initial probability that this is the cause of our patient’s stroke.1 Furthermore, our patient’s MRA study showed no carotid artery stenoses, which effectively eliminates this as the cause of his stroke, as the diagnostic sensitivity of MRA for detecting carotid stenosis is approximately 97%.
Cervical arterial dissection
Cervical arterial dissection causes up to 20% of strokes in patients younger than 45 years.2 Dissections usually involve the extracranial portion of the vessel, and involve the internal carotid arteries at least three times as often as the vertebral arteries. In many cases the dissection is preceded by mild neck trauma, which may be as minor as a vigorous cough or turning of the head.
Typical features of dissection include neck pain, headache, and Horner syndrome, followed minutes to hours later by symptoms of ocular or cerebral ischemia, usually a transient ischemic attack rather than a stroke. Neurologic symptoms are most commonly due to thrombosis at the dissection site with distal embolization. Inherited disorders that are associated with increased risk of cervical arterial dissection include Ehlers-Danlos syndrome type IV, Marfan syndrome, autosomal-dominant polycystic kidney disease, osteogenesis imperfecta type I, and fibromuscular dysplasia.3 MRA and computed tomographic angiography are the diagnostic tests of choice.
Our patient’s symptoms began suddenly, without a history of trauma or neck pain, making arterial dissection less likely as the cause of his stroke. No dissection was seen on MRA, which also minimizes its likelihood.4
Hematologic disorders
Many hematologic disorders are associated with ischemic stroke. The disorders most likely to cause ischemic stroke in patients younger than 45 years are antiphospholipid antibody syndrome, sickle cell anemia, and heparin-induced thrombocytopenia,5 which are associated with arterial thrombosis.
Most of the common hereditary hypercoagulable disorders, such as factor V Leiden/activated protein C resistance, the prothrombin gene mutation (G20210A), antithrombin III deficiency, protein C deficiency, and protein S deficiency, typically cause venous thrombosis much more often than they cause arterial thrombosis. Thus, the most typical presentations of stroke in these disorders are cerebral venous thrombosis or paradoxical embolic stroke due to a patent foramen ovale. Antithrombin III deficiency and protein C and protein S deficiency have been associated with arterial thrombosis, but so infrequently that their likelihood in this patient is extremely low.
Clues to the diagnosis of a hypercoagulable state include venous thrombosis in the past, recurrent fetal loss, thrombocytopenia, livedo reticularis, antiphospholipid antibody syndrome, and skin necrosis at the start of oral anticoagulant therapy.
Of importance: the relationship between hereditary hypercoagulable disorders and stroke is considerably weaker than their association with venous thrombosis. Several studies in clinical and general populations have failed to show an independent association between stroke and protein C deficiency, protein S deficiency, antithrombin III deficiency, factor V Leiden/activated protein C resistance, or the prothrombin G20210A mutation.6–8 Therefore, most experts do not recommend screening all stroke patients for a hypercoagulable state—only those with a personal or family history of thrombosis or young patients with unexplained stroke.
Our patient does not have historical or clinical features that would suggest a specific hypercoagulable disorder, either acquired (eg, heparin-induced thrombocytopenia) or inherited. A laboratory workup for a hypercoagulable disorder would likely be of little value in determining the cause of his stroke, and even if a hereditary disorder were identified it would be difficult to determine causation. However, if no other explanation for his stroke can be found during the workup, one could consider testing for proteins C and S, antithrombin III, activated protein C resistance (and factor V Leiden if screening for activated protein C resistance is positive), prothrombin G20210A, fibrinogen, homocysteine, D-dimers, and antiphospholipid antibodies.
Cocaine abuse
Another important cause of ischemic stroke is the use of sympathomimetic drugs such as cocaine or amphetamines. The strongest association is with cocaine, which has been seen in case series to cause cerebral vasoconstriction in a dose-dependent manner. Vasoconstriction is also related to a longer duration of cocaine use.9 Several case-control studies have found that the risk of stroke is 4.5 to 6.5 times higher in drug abusers than in controls, and that use of catecholamines or cocaine alone was associated with a significantly increased risk of stroke.10,11
It is certainly advisable to ask about the use of illicit drugs and to send serum and urine samples for appropriate drug screening in young stroke patients, particularly if another cause cannot be found or if drug use is suspected.12
Cardiac embolism
Cardiac embolism is the most likely cause of the stroke in this patient. Up to 20% of the 500,000 strokes that occur annually in the United States are of cardiac embolic origin,13 and the prevalence is even higher in younger patients. In a registry of 428 strokes in patients 15 to 44 years of age, a cardiac source of embolism was the cause in 31.8%.14
- Masses, which include atherosclerotic plaques, cardiac tumors, and infective and noninfective valvular vegetations
- Passageways for paradoxical embolism, such as a patent foramen ovale or atrial septal defect (Figure 2)
- Stasis in the left atrium or left ventricle, with a resulting propensity for thrombosis.
Atrial thrombus is most often seen in patients with atrial fibrillation, mitral stenosis, or dilated cardiomyopathy. Echocardiography of the left atrium in patients with these conditions often reveals spontaneous echo contrast that resembles swirling “smoke,” which is thought to be produced by red blood cell aggregation due to blood stasis. This sign is strongly associated with left atrial thrombi.
Left ventricular thrombosis is one of the most common complications of myocardial infarction and is caused by blood stasis in regions of the ventricle in which the myocardium is hypokinetic or akinetic.
We cannot assume, however, that a potential cardioembolic source seen on echocardiography is the cause of a given patient’s stroke. The evidence proving a causal relationship between most potential cardiac embolic sources and stroke is less than robust. Most of the published data are from nonrandomized studies or case series, and there are no large, prospective studies available to clearly prove that a given cardioembolic source is directly related to embolic stroke.16
This being said, most studies have found high prevalence rates of cardioembolic sources in patients with embolic stroke, which suggests that a causative relationship may exist. However, many of these findings also have a relatively high prevalence among the general population without stroke, raising the possibility that the finding could be incidental and unrelated. Examples are patent foramen ovale, which exists in 27% of adults,17 and aortic arch atheroma, which is common in the elderly.
In the end, when the only potential source of embolism that can be found is in the heart (as is often the case in younger patients), the probability is much greater that it is indeed the cause of the stroke. The lack of direct evidence linking many sources of cardioembolism to stroke emphasizes the need for a thorough investigation of all possible causes of stroke.
DIAGNOSTIC EVALUATION
2. Which is the best study to evaluate for a cardiac embolic source in this patient?
- Transthoracic echocardiography (TTE)
- Transesophageal echocardiography (TEE)
- Transcranial Doppler ultrasonography
- Electrocardiography
The study of choice in this patient is TEE. Overall, TEE is better than TTE in identifying a cardiac source of embolism,18,19 mainly because the images are obtained from a probe in the esophagus, which is in close proximity to the heart, so that there is little additional soft tissue and bone between the probe and cardiac structures. In addition, higher-frequency probes can be used. Both of these result in ultrasonographic images with much greater spatial resolution than can be obtained with a transthoracic study.15
In a case series,20 TEE identified a potential cardiac source of embolism in 45 (57%) of 79 patients with cryptogenic stroke, compared with only 12 (15%) with TTE.
The main limitation of TEE is that it does not show the left ventricular apex very well, making an accurate assessment of left ventricular function or identification of a left ventricular apical thrombus much less likely.
In patients who lack evidence of atherosclerotic cerebrovascular disease, specific findings on history or physical examination could increase the chances of identifying an embolic source, such as left ventricular thrombus, on TTE. These findings could include a history of a myocardial infarction, congestive heart failure, left ventricular dysfunction, endocarditis, rheumatic heart disease, a prosthetic valve, or atrial fibrillation or flutter. TTE by itself is considered sufficient for making the diagnosis of mitral stenosis, left ventricular aneurysm, dilated cardiomyopathy, left ventricular thrombus, and mitral valve prolapse with myxomatous degeneration of the leaflets.
However, in patients without signs or symptoms of cardiac disease, the diagnostic value of TTE is significantly less. Several studies have demonstrated that in patients without evidence of cardiac disease, TTE identifies the source of embolism less than 10% of the time.21 Some series even suggest that the yield may be less than 1%.22 TEE has the advantage of being able to diagnose the above disorders and of having a higher sensitivity for identifying potential sources that may be missed by TTE, such as left atrial or left atrial appendage thrombus, aortic arch atheroma, patent foramen ovale, atrial septal aneurysm, or spontaneous echo contrast. It should be remembered, however, that TEE is a semi-invasive procedure that carries the risks of both the procedure and the sedation, eg, bronchospasm, hypoxia, arrhythmias, upper gastrointestinal trauma, and bleeding.23
Further clouding the decision are recent advances in TTE technology, such as contrast TTE with second harmonic imaging, which enhances the ability of TTE to identify potential sources of stroke such as patent foramen ovale nearly to the level of TEE.24
Unfortunately, guidelines from professional societies do not offer assistance on the best diagnostic approach. Current guidelines from the American Heart Association, American College of Cardiology, and American Society of Echocardiography do give echocardiography a class I indication in younger patients (< 45 years old) with cerebrovascular events or older patients (> 45 years old) with stroke without evidence of cerebrovascular disease or other obvious causes. However, there is no official recommendation on whether to choose TTE, TEE, or both studies.16 Given the multiple causes of cardioembolism and the variety of clinical factors that could influence the decision to choose a certain echo study, this decision is appropriately left to the individual physician.
A reasonable, evidence-based diagnostic approach in young stroke patients is to proceed to TEE when routine TTE and electrocardiography are unrevealing.25 In reality, this is the practice followed in most centers, including ours. Although TTE has a lower diagnostic yield in patients without symptoms, it has the advantages of being readily available in most centers, being noninvasive, and providing complementary information to TEE even when TTE does not reveal a potential cause of stroke.
As for the other studies:
Electrocardiography is valuable in identifying potential cardioembolic causes of stroke such as atrial fibrillation, left ventricular aneurysm, or myocardial infarction, but it is insufficient by itself to assess for many other potential sources of cardioembolism.
Transcranial Doppler ultrasonography is very sensitive for detecting patent foramen ovale and other right-to-left shunts that could be sources of cardioembolism. In this test, microbubbles from agitated saline are injected into the venous circulation and are detected in the cerebral arteries after passing through the shunt. It has no utility in identifying the other possibilities discussed above, nor can it discriminate whether these shunts are intra-cardiac or extracardiac.
Case continued
The patient undergoes TTE, which shows normal left ventricular size, wall thickness, and systolic function. His right ventricular function is normal, as are his left and right atrial size. Valvular function is normal, and no right-to-left interatrial shunt is detected with the use of agitated saline contrast.
MANAGEMENT
3. Which is the most appropriate way to manage the lesion?
- Surgical resection
- Periodic echocardiographic follow-up
- Anticoagulation and periodic echocardiographic follow-up
Cardiac papillary fibroelastomas are rare benign primary tumors of the heart. The true incidence is unknown because, when small, they can be asymptomatic and easily overlooked on gross examination. In adults, they are the second most common primary cardiac tumors, next to atrial myxoma.26
The histogenesis is not known, but the mean age at which they are detected is approximately 60 years, and most of the patients are men, likely because most of these tumors are found incidentally during echocardiography, open heart surgery, or autopsy.28
Most patients with cardiac papillary fibroelastomas have no symptoms; however, those who do have symptoms usually experience valve obstruction or embolization of tumor fragments, leading to stroke, myocardial infarction, or sudden death. Further increasing the risk of embolism, thrombus has been reported on the surface of some tumors, supporting the use of anticoagulation in patients who have experienced embolic phenomena.29
A case review of 725 patients with these tumors27 found that tumor mobility and location on the aortic valve were univariate predictors of tumor-related death and of nonfatal embolism. The only independent predictor of tumor-related death or nonfatal embolization was tumor mobility.
Surgical resection of the tumor is curative, and no recurrences have been reported, although the longest follow-up period has been 11 years.
Although no data exist to support the practice, patients with nonmobile or nonaortic valve tumors could be managed with anticoagulation and periodic echocardiographic follow-up until the tumor becomes mobile or symptomatic, but such a conservative strategy would seem inappropriate for our patient. His tumor is both mobile and located on the aortic valve, putting him at risk of death, and he has already experienced an embolic complication. Therefore, his lesion should be surgically resected.
Case continued
The patient receives anticoagulation therapy with subcutaneous enoxaparin (Lovenox) and warfarin (Coumadin). He undergoes successful surgical resection of the tumor without complication and is discharged to home on hospital day 5.
TAKE-HOME POINTS
The potential causes of stroke in patients younger than age 45 differ significantly from those in older patients. Cardiac embolism is the most frequent cause of stroke in young patients and is most often from left atrial or ventricular thrombus or from aortic atheroma.
In young patients, TEE is superior to TTE in identifying a specific source of cardiac embolism, particularly when clues from the history or physical examination are lacking and the preliminary diagnostic workup fails to identify the cause of the stroke.
Our patient’s history, physical examination, MRI, MRA, electrocardiography, and TTE all failed to disclose a probable cause of his stroke. Appropriately, TEE was performed, which confirmed the diagnosis of cardiac papillary fibroelastoma, a rare and benign primary tumor of the heart with the potential for disastrous consequences.
- Bogousslavsky J, Van Melle G, Regli F. The Lausanne Stroke Registry: analysis of 1,000 consecutive patients with first stroke. Stroke 1988; 19:1083–1092.
- Bogousslavsky J, Pierre P. Ischemic stroke in patients under age 45. Neurol Clin 1992; 10:113–124.
- Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med 2001; 344:898–906.
- Thanvi B, Munshi SK, Dawson SL, Ribinson TG. Carotid and vertebral artery dissection syndromes. Postgrad Med J 2005; 81:383–388.
- Levine SR. Hypercoagulable states and stroke: a selective review. CNS Spectr 2005; 10:567–578.
- Juul K, Tybjaerg-Hansen A, Steffensen R, Kofoed S, Jensen G, Nordestgaard BG. Factor V Leiden: The Copenhagen City Heart Study and 2 meta-analyses. Blood 2002; 100:3–10.
- Ridker PM, Hennekens CH, Lindpaintner K, Stampfer MJ, Eisenberg PR, Miletich JP. Mutation in the gene coding for coagulation factor V and the risk of myocardial infarction, stroke, and venous thrombosis in apparently healthy men. N Engl J Med 1995; 332:912–917.
- Hankey GJ, Eikelboom JW, van Bockxmeer FM, Lofthouse E, Staples N, Baker RI. Inherited thrombophilia in ischemic stroke and its pathogenic subtypes. Stroke 2001; 32:1793–1799.
- Kaufman MJ, Levin JM, Ross MH, et al. Cocaine-induced cerebral vasoconstriction detected in humans with magnetic resonance angiography. JAMA 1998; 279:376–380.
- Kaku DA, Lowenstein DH. Emergence of recreational drug abuse as a major risk factor for stroke in young adults. Ann Intern Med 1990; 113:821–827.
- Petitti DB, Sidney S, Quesenberry C, Bernstein A. Stroke and cocaine or amphetamine use. Epidemiology 1998; 9:596–600.
- Bruno A. Cerebrovascular complications of alcohol and sympathomimetic drug abuse. Curr Neurol Neurosci Rep 2003; 3:40–45.
- Cardiogenic brain embolism. The second report of the Cerebral Embolism Task Force. Arch Neurol 1989; 46:727–743.
- Kittner SJ, Stern BJ, Wozniak M, et al. Cerebral infarction in young adults: the Baltimore-Washington Cooperative Young Stroke Study. Neurology 1998; 50:890–894.
- Manning WJ. Role of transesophageal echocardiography in the management of thromboembolic stroke. Am J Cardiol 1997; 80 4C:19D–28D.
- Cheitlin MD, Armstrong WF, Aurigemma GP, et al American College of Cardiology; American Heart Association; American Society of Echocardiography. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography). Circulation 2003; 108:1146–1162.
- Kizer JR, Devereux RB. Clinical practice. Patent foramen ovale in young adults with unexplained stroke. N Engl J Med 2005; 353:2361–2372.
- Pearson AC. Transthoracic echocardiography versus transesophageal echocardiography in detecting cardiac sources of embolism. Echocardiography 1993; 10:397–403.
- DeRook FA, Comess KA, Albers GW, Popp RL. Transesophageal echocardiography in the evaluation of stroke. Ann Intern Med 1992; 117:922–932.
- Pearson AC, Labovitz AJ, Tatineni S, Gomez CR. Superiority of transesophageal echocardiography in detecting cardiac source of embolism in patients with cerebral ischemia of uncertain etiology. J Am Coll Cardiol 1991; 17:66–72.
- Rahmatullah AF, Rahko PS, Stein JH. Transesophageal echocardiography for the evaluation and management of patients with cerebral ischemia. Clin Cardiol 1999; 22:391–396.
- Come PC, Riley MF, Bivas NK. Roles of echocardiography and arrhythmia monitoring in the evaluation of patients with suspected systemic embolism. Ann Neurol 1983; 13:527–531.
- Daniel WG, Erbel R, Kasper W, et al. Safety of transesophageal echocardiography. A multicenter survey of 10,419 examinations. Circulation 1991; 83:817–821.
- Souteyrand G, Motreff P, Lusson JR, et al. Comparison of transthoracic echocardiography using second harmonic imaging, transcranial Doppler and transesophageal echocardiography for the detection of patent foramen ovale in stroke patients. Eur J Echocardiogr 2006; 7:147–154.
- Harloff A, Handke M, Reinhard M, Geibel A, Hetzel A. Therapeutic strategies after examination by transesophageal echocardiography in 503 patients with ischemic stroke. Stroke 2006; 37:859–864.
- Burke A, Virami R. Tumors of the heart and great vessels. Atlas of Tumor Pathology, 1996, 3rd Series, Fascicle 16. Washington, DC: Armed Forces Institute of Pathology.
- Gowda RM, Khan IA, Nair CK, Mehta NJ, Vasavada BC, Sacchi TJ. Cardiac papillary fibroelastoma: a comprehensive analysis of 725 cases. Am Heart J 2003; 146:404–410.
- Edwards FH, Hale D, Cohen A, Thompson L, Pezzella AT, Virmani R. Primary cardiac valve tumors. Ann Thorac Surg 1991; 52:1127–1131.
- Joffe II, Jacobs LE, Owen AN, Ioli A, Kotler MN. Rapid development of a papillary fibroelastoma with associated thrombus: the role of transthoracic and transesophageal echocardiography. Echocardiography 1997; 14:287–292.
A 42-year-old man was working at his computer when he suddenly became disoriented and lightheaded, had difficulty concentrating, and could not move his right arm. He could walk without difficulty, but he had a tingling sensation in his right leg. He did not lose consciousness or have any associated palpitations, chest pain, shortness of breath, nausea, vomiting, headaches, or visual changes.
He called 911, and an ambulance arrived 15 minutes later. By that time his symptoms had started to resolve. Now, in the emergency department, his only residual symptom is mild numbness of his right arm and shoulder.
Until now he has been healthy except for a history of dyslipidemia. He takes no prescription or over-the-counter medications and has no drug allergies. He has smoked one pack of cigarettes daily for the past 28 years and also smokes marijuana several times each month. He drinks alcohol occasionally. His family has no history of stroke, premature coronary artery disease, or sudden cardiac death.
INITIAL EVALUATION
His heart rate is 88 beats per minute, blood pressure 142/82 mm Hg, and blood oxygen saturation 98% while breathing room air. He is alert and in no acute distress and answers questions appropriately.
His breathing sounds are normal, without crackles or wheezes. His heart has normal first and second sounds, a normal rate and rhythm, and no extra sounds or murmurs. His abdomen is normal. His extremities are warm and well perfused with normal peripheral pulses and no edema.
On neurologic examination, his cranial nerves and visual fields are normal, and his strength is normal in all muscle groups except for the right upper arm, which is slightly weaker than the left when tested against resistance. Reflexes and response to light touch and pinprick are normal.
His serum chemistry levels, renal function, and blood counts are normal. His total cholesterol level is 155 mg/dL, high-density lipoprotein cholesterol 38 mg/dL, low-density lipoprotein cholesterol 108 mg/dL, and triglycerides 1,286 mg/dL. Electrocardiography is normal with sinus rhythm at a rate of 74.
Magnetic resonance imaging (MRI) of the head and neck with magnetic resonance angiography (MRA) of the intracranial and extracranial vessels is performed. Diffusion-weighted images show a hyperintense lesion in the left insular cortex, consistent with an infarct in the distribution of a branch of the left middle cerebral artery. There is no intracranial hemorrhage. All intracranial and extracranial major vessels are patent, and no stenoses are seen.
DIFFERENTIAL DIAGNOSIS
1. Which is the most likely cause of this patient’s stroke?
- Vertebral or carotid atherosclerosis
- Cervical arterial dissection
- A hematologic disorder
- Cocaine abuse
- Cardiac embolism
Atherosclerosis
Although 85% of all strokes are ischemic, and most ischemic strokes are caused by occlusive atherosclerosis of large vessels, most ischemic strokes occur in patients older than 65 years. In patients younger than 55 years, only about 10% of strokes are caused by large-vessel atherosclerotic disease, thus lowering the initial probability that this is the cause of our patient’s stroke.1 Furthermore, our patient’s MRA study showed no carotid artery stenoses, which effectively eliminates this as the cause of his stroke, as the diagnostic sensitivity of MRA for detecting carotid stenosis is approximately 97%.
Cervical arterial dissection
Cervical arterial dissection causes up to 20% of strokes in patients younger than 45 years.2 Dissections usually involve the extracranial portion of the vessel, and involve the internal carotid arteries at least three times as often as the vertebral arteries. In many cases the dissection is preceded by mild neck trauma, which may be as minor as a vigorous cough or turning of the head.
Typical features of dissection include neck pain, headache, and Horner syndrome, followed minutes to hours later by symptoms of ocular or cerebral ischemia, usually a transient ischemic attack rather than a stroke. Neurologic symptoms are most commonly due to thrombosis at the dissection site with distal embolization. Inherited disorders that are associated with increased risk of cervical arterial dissection include Ehlers-Danlos syndrome type IV, Marfan syndrome, autosomal-dominant polycystic kidney disease, osteogenesis imperfecta type I, and fibromuscular dysplasia.3 MRA and computed tomographic angiography are the diagnostic tests of choice.
Our patient’s symptoms began suddenly, without a history of trauma or neck pain, making arterial dissection less likely as the cause of his stroke. No dissection was seen on MRA, which also minimizes its likelihood.4
Hematologic disorders
Many hematologic disorders are associated with ischemic stroke. The disorders most likely to cause ischemic stroke in patients younger than 45 years are antiphospholipid antibody syndrome, sickle cell anemia, and heparin-induced thrombocytopenia,5 which are associated with arterial thrombosis.
Most of the common hereditary hypercoagulable disorders, such as factor V Leiden/activated protein C resistance, the prothrombin gene mutation (G20210A), antithrombin III deficiency, protein C deficiency, and protein S deficiency, typically cause venous thrombosis much more often than they cause arterial thrombosis. Thus, the most typical presentations of stroke in these disorders are cerebral venous thrombosis or paradoxical embolic stroke due to a patent foramen ovale. Antithrombin III deficiency and protein C and protein S deficiency have been associated with arterial thrombosis, but so infrequently that their likelihood in this patient is extremely low.
Clues to the diagnosis of a hypercoagulable state include venous thrombosis in the past, recurrent fetal loss, thrombocytopenia, livedo reticularis, antiphospholipid antibody syndrome, and skin necrosis at the start of oral anticoagulant therapy.
Of importance: the relationship between hereditary hypercoagulable disorders and stroke is considerably weaker than their association with venous thrombosis. Several studies in clinical and general populations have failed to show an independent association between stroke and protein C deficiency, protein S deficiency, antithrombin III deficiency, factor V Leiden/activated protein C resistance, or the prothrombin G20210A mutation.6–8 Therefore, most experts do not recommend screening all stroke patients for a hypercoagulable state—only those with a personal or family history of thrombosis or young patients with unexplained stroke.
Our patient does not have historical or clinical features that would suggest a specific hypercoagulable disorder, either acquired (eg, heparin-induced thrombocytopenia) or inherited. A laboratory workup for a hypercoagulable disorder would likely be of little value in determining the cause of his stroke, and even if a hereditary disorder were identified it would be difficult to determine causation. However, if no other explanation for his stroke can be found during the workup, one could consider testing for proteins C and S, antithrombin III, activated protein C resistance (and factor V Leiden if screening for activated protein C resistance is positive), prothrombin G20210A, fibrinogen, homocysteine, D-dimers, and antiphospholipid antibodies.
Cocaine abuse
Another important cause of ischemic stroke is the use of sympathomimetic drugs such as cocaine or amphetamines. The strongest association is with cocaine, which has been seen in case series to cause cerebral vasoconstriction in a dose-dependent manner. Vasoconstriction is also related to a longer duration of cocaine use.9 Several case-control studies have found that the risk of stroke is 4.5 to 6.5 times higher in drug abusers than in controls, and that use of catecholamines or cocaine alone was associated with a significantly increased risk of stroke.10,11
It is certainly advisable to ask about the use of illicit drugs and to send serum and urine samples for appropriate drug screening in young stroke patients, particularly if another cause cannot be found or if drug use is suspected.12
Cardiac embolism
Cardiac embolism is the most likely cause of the stroke in this patient. Up to 20% of the 500,000 strokes that occur annually in the United States are of cardiac embolic origin,13 and the prevalence is even higher in younger patients. In a registry of 428 strokes in patients 15 to 44 years of age, a cardiac source of embolism was the cause in 31.8%.14
- Masses, which include atherosclerotic plaques, cardiac tumors, and infective and noninfective valvular vegetations
- Passageways for paradoxical embolism, such as a patent foramen ovale or atrial septal defect (Figure 2)
- Stasis in the left atrium or left ventricle, with a resulting propensity for thrombosis.
Atrial thrombus is most often seen in patients with atrial fibrillation, mitral stenosis, or dilated cardiomyopathy. Echocardiography of the left atrium in patients with these conditions often reveals spontaneous echo contrast that resembles swirling “smoke,” which is thought to be produced by red blood cell aggregation due to blood stasis. This sign is strongly associated with left atrial thrombi.
Left ventricular thrombosis is one of the most common complications of myocardial infarction and is caused by blood stasis in regions of the ventricle in which the myocardium is hypokinetic or akinetic.
We cannot assume, however, that a potential cardioembolic source seen on echocardiography is the cause of a given patient’s stroke. The evidence proving a causal relationship between most potential cardiac embolic sources and stroke is less than robust. Most of the published data are from nonrandomized studies or case series, and there are no large, prospective studies available to clearly prove that a given cardioembolic source is directly related to embolic stroke.16
This being said, most studies have found high prevalence rates of cardioembolic sources in patients with embolic stroke, which suggests that a causative relationship may exist. However, many of these findings also have a relatively high prevalence among the general population without stroke, raising the possibility that the finding could be incidental and unrelated. Examples are patent foramen ovale, which exists in 27% of adults,17 and aortic arch atheroma, which is common in the elderly.
In the end, when the only potential source of embolism that can be found is in the heart (as is often the case in younger patients), the probability is much greater that it is indeed the cause of the stroke. The lack of direct evidence linking many sources of cardioembolism to stroke emphasizes the need for a thorough investigation of all possible causes of stroke.
DIAGNOSTIC EVALUATION
2. Which is the best study to evaluate for a cardiac embolic source in this patient?
- Transthoracic echocardiography (TTE)
- Transesophageal echocardiography (TEE)
- Transcranial Doppler ultrasonography
- Electrocardiography
The study of choice in this patient is TEE. Overall, TEE is better than TTE in identifying a cardiac source of embolism,18,19 mainly because the images are obtained from a probe in the esophagus, which is in close proximity to the heart, so that there is little additional soft tissue and bone between the probe and cardiac structures. In addition, higher-frequency probes can be used. Both of these result in ultrasonographic images with much greater spatial resolution than can be obtained with a transthoracic study.15
In a case series,20 TEE identified a potential cardiac source of embolism in 45 (57%) of 79 patients with cryptogenic stroke, compared with only 12 (15%) with TTE.
The main limitation of TEE is that it does not show the left ventricular apex very well, making an accurate assessment of left ventricular function or identification of a left ventricular apical thrombus much less likely.
In patients who lack evidence of atherosclerotic cerebrovascular disease, specific findings on history or physical examination could increase the chances of identifying an embolic source, such as left ventricular thrombus, on TTE. These findings could include a history of a myocardial infarction, congestive heart failure, left ventricular dysfunction, endocarditis, rheumatic heart disease, a prosthetic valve, or atrial fibrillation or flutter. TTE by itself is considered sufficient for making the diagnosis of mitral stenosis, left ventricular aneurysm, dilated cardiomyopathy, left ventricular thrombus, and mitral valve prolapse with myxomatous degeneration of the leaflets.
However, in patients without signs or symptoms of cardiac disease, the diagnostic value of TTE is significantly less. Several studies have demonstrated that in patients without evidence of cardiac disease, TTE identifies the source of embolism less than 10% of the time.21 Some series even suggest that the yield may be less than 1%.22 TEE has the advantage of being able to diagnose the above disorders and of having a higher sensitivity for identifying potential sources that may be missed by TTE, such as left atrial or left atrial appendage thrombus, aortic arch atheroma, patent foramen ovale, atrial septal aneurysm, or spontaneous echo contrast. It should be remembered, however, that TEE is a semi-invasive procedure that carries the risks of both the procedure and the sedation, eg, bronchospasm, hypoxia, arrhythmias, upper gastrointestinal trauma, and bleeding.23
Further clouding the decision are recent advances in TTE technology, such as contrast TTE with second harmonic imaging, which enhances the ability of TTE to identify potential sources of stroke such as patent foramen ovale nearly to the level of TEE.24
Unfortunately, guidelines from professional societies do not offer assistance on the best diagnostic approach. Current guidelines from the American Heart Association, American College of Cardiology, and American Society of Echocardiography do give echocardiography a class I indication in younger patients (< 45 years old) with cerebrovascular events or older patients (> 45 years old) with stroke without evidence of cerebrovascular disease or other obvious causes. However, there is no official recommendation on whether to choose TTE, TEE, or both studies.16 Given the multiple causes of cardioembolism and the variety of clinical factors that could influence the decision to choose a certain echo study, this decision is appropriately left to the individual physician.
A reasonable, evidence-based diagnostic approach in young stroke patients is to proceed to TEE when routine TTE and electrocardiography are unrevealing.25 In reality, this is the practice followed in most centers, including ours. Although TTE has a lower diagnostic yield in patients without symptoms, it has the advantages of being readily available in most centers, being noninvasive, and providing complementary information to TEE even when TTE does not reveal a potential cause of stroke.
As for the other studies:
Electrocardiography is valuable in identifying potential cardioembolic causes of stroke such as atrial fibrillation, left ventricular aneurysm, or myocardial infarction, but it is insufficient by itself to assess for many other potential sources of cardioembolism.
Transcranial Doppler ultrasonography is very sensitive for detecting patent foramen ovale and other right-to-left shunts that could be sources of cardioembolism. In this test, microbubbles from agitated saline are injected into the venous circulation and are detected in the cerebral arteries after passing through the shunt. It has no utility in identifying the other possibilities discussed above, nor can it discriminate whether these shunts are intra-cardiac or extracardiac.
Case continued
The patient undergoes TTE, which shows normal left ventricular size, wall thickness, and systolic function. His right ventricular function is normal, as are his left and right atrial size. Valvular function is normal, and no right-to-left interatrial shunt is detected with the use of agitated saline contrast.
MANAGEMENT
3. Which is the most appropriate way to manage the lesion?
- Surgical resection
- Periodic echocardiographic follow-up
- Anticoagulation and periodic echocardiographic follow-up
Cardiac papillary fibroelastomas are rare benign primary tumors of the heart. The true incidence is unknown because, when small, they can be asymptomatic and easily overlooked on gross examination. In adults, they are the second most common primary cardiac tumors, next to atrial myxoma.26
The histogenesis is not known, but the mean age at which they are detected is approximately 60 years, and most of the patients are men, likely because most of these tumors are found incidentally during echocardiography, open heart surgery, or autopsy.28
Most patients with cardiac papillary fibroelastomas have no symptoms; however, those who do have symptoms usually experience valve obstruction or embolization of tumor fragments, leading to stroke, myocardial infarction, or sudden death. Further increasing the risk of embolism, thrombus has been reported on the surface of some tumors, supporting the use of anticoagulation in patients who have experienced embolic phenomena.29
A case review of 725 patients with these tumors27 found that tumor mobility and location on the aortic valve were univariate predictors of tumor-related death and of nonfatal embolism. The only independent predictor of tumor-related death or nonfatal embolization was tumor mobility.
Surgical resection of the tumor is curative, and no recurrences have been reported, although the longest follow-up period has been 11 years.
Although no data exist to support the practice, patients with nonmobile or nonaortic valve tumors could be managed with anticoagulation and periodic echocardiographic follow-up until the tumor becomes mobile or symptomatic, but such a conservative strategy would seem inappropriate for our patient. His tumor is both mobile and located on the aortic valve, putting him at risk of death, and he has already experienced an embolic complication. Therefore, his lesion should be surgically resected.
Case continued
The patient receives anticoagulation therapy with subcutaneous enoxaparin (Lovenox) and warfarin (Coumadin). He undergoes successful surgical resection of the tumor without complication and is discharged to home on hospital day 5.
TAKE-HOME POINTS
The potential causes of stroke in patients younger than age 45 differ significantly from those in older patients. Cardiac embolism is the most frequent cause of stroke in young patients and is most often from left atrial or ventricular thrombus or from aortic atheroma.
In young patients, TEE is superior to TTE in identifying a specific source of cardiac embolism, particularly when clues from the history or physical examination are lacking and the preliminary diagnostic workup fails to identify the cause of the stroke.
Our patient’s history, physical examination, MRI, MRA, electrocardiography, and TTE all failed to disclose a probable cause of his stroke. Appropriately, TEE was performed, which confirmed the diagnosis of cardiac papillary fibroelastoma, a rare and benign primary tumor of the heart with the potential for disastrous consequences.
A 42-year-old man was working at his computer when he suddenly became disoriented and lightheaded, had difficulty concentrating, and could not move his right arm. He could walk without difficulty, but he had a tingling sensation in his right leg. He did not lose consciousness or have any associated palpitations, chest pain, shortness of breath, nausea, vomiting, headaches, or visual changes.
He called 911, and an ambulance arrived 15 minutes later. By that time his symptoms had started to resolve. Now, in the emergency department, his only residual symptom is mild numbness of his right arm and shoulder.
Until now he has been healthy except for a history of dyslipidemia. He takes no prescription or over-the-counter medications and has no drug allergies. He has smoked one pack of cigarettes daily for the past 28 years and also smokes marijuana several times each month. He drinks alcohol occasionally. His family has no history of stroke, premature coronary artery disease, or sudden cardiac death.
INITIAL EVALUATION
His heart rate is 88 beats per minute, blood pressure 142/82 mm Hg, and blood oxygen saturation 98% while breathing room air. He is alert and in no acute distress and answers questions appropriately.
His breathing sounds are normal, without crackles or wheezes. His heart has normal first and second sounds, a normal rate and rhythm, and no extra sounds or murmurs. His abdomen is normal. His extremities are warm and well perfused with normal peripheral pulses and no edema.
On neurologic examination, his cranial nerves and visual fields are normal, and his strength is normal in all muscle groups except for the right upper arm, which is slightly weaker than the left when tested against resistance. Reflexes and response to light touch and pinprick are normal.
His serum chemistry levels, renal function, and blood counts are normal. His total cholesterol level is 155 mg/dL, high-density lipoprotein cholesterol 38 mg/dL, low-density lipoprotein cholesterol 108 mg/dL, and triglycerides 1,286 mg/dL. Electrocardiography is normal with sinus rhythm at a rate of 74.
Magnetic resonance imaging (MRI) of the head and neck with magnetic resonance angiography (MRA) of the intracranial and extracranial vessels is performed. Diffusion-weighted images show a hyperintense lesion in the left insular cortex, consistent with an infarct in the distribution of a branch of the left middle cerebral artery. There is no intracranial hemorrhage. All intracranial and extracranial major vessels are patent, and no stenoses are seen.
DIFFERENTIAL DIAGNOSIS
1. Which is the most likely cause of this patient’s stroke?
- Vertebral or carotid atherosclerosis
- Cervical arterial dissection
- A hematologic disorder
- Cocaine abuse
- Cardiac embolism
Atherosclerosis
Although 85% of all strokes are ischemic, and most ischemic strokes are caused by occlusive atherosclerosis of large vessels, most ischemic strokes occur in patients older than 65 years. In patients younger than 55 years, only about 10% of strokes are caused by large-vessel atherosclerotic disease, thus lowering the initial probability that this is the cause of our patient’s stroke.1 Furthermore, our patient’s MRA study showed no carotid artery stenoses, which effectively eliminates this as the cause of his stroke, as the diagnostic sensitivity of MRA for detecting carotid stenosis is approximately 97%.
Cervical arterial dissection
Cervical arterial dissection causes up to 20% of strokes in patients younger than 45 years.2 Dissections usually involve the extracranial portion of the vessel, and involve the internal carotid arteries at least three times as often as the vertebral arteries. In many cases the dissection is preceded by mild neck trauma, which may be as minor as a vigorous cough or turning of the head.
Typical features of dissection include neck pain, headache, and Horner syndrome, followed minutes to hours later by symptoms of ocular or cerebral ischemia, usually a transient ischemic attack rather than a stroke. Neurologic symptoms are most commonly due to thrombosis at the dissection site with distal embolization. Inherited disorders that are associated with increased risk of cervical arterial dissection include Ehlers-Danlos syndrome type IV, Marfan syndrome, autosomal-dominant polycystic kidney disease, osteogenesis imperfecta type I, and fibromuscular dysplasia.3 MRA and computed tomographic angiography are the diagnostic tests of choice.
Our patient’s symptoms began suddenly, without a history of trauma or neck pain, making arterial dissection less likely as the cause of his stroke. No dissection was seen on MRA, which also minimizes its likelihood.4
Hematologic disorders
Many hematologic disorders are associated with ischemic stroke. The disorders most likely to cause ischemic stroke in patients younger than 45 years are antiphospholipid antibody syndrome, sickle cell anemia, and heparin-induced thrombocytopenia,5 which are associated with arterial thrombosis.
Most of the common hereditary hypercoagulable disorders, such as factor V Leiden/activated protein C resistance, the prothrombin gene mutation (G20210A), antithrombin III deficiency, protein C deficiency, and protein S deficiency, typically cause venous thrombosis much more often than they cause arterial thrombosis. Thus, the most typical presentations of stroke in these disorders are cerebral venous thrombosis or paradoxical embolic stroke due to a patent foramen ovale. Antithrombin III deficiency and protein C and protein S deficiency have been associated with arterial thrombosis, but so infrequently that their likelihood in this patient is extremely low.
Clues to the diagnosis of a hypercoagulable state include venous thrombosis in the past, recurrent fetal loss, thrombocytopenia, livedo reticularis, antiphospholipid antibody syndrome, and skin necrosis at the start of oral anticoagulant therapy.
Of importance: the relationship between hereditary hypercoagulable disorders and stroke is considerably weaker than their association with venous thrombosis. Several studies in clinical and general populations have failed to show an independent association between stroke and protein C deficiency, protein S deficiency, antithrombin III deficiency, factor V Leiden/activated protein C resistance, or the prothrombin G20210A mutation.6–8 Therefore, most experts do not recommend screening all stroke patients for a hypercoagulable state—only those with a personal or family history of thrombosis or young patients with unexplained stroke.
Our patient does not have historical or clinical features that would suggest a specific hypercoagulable disorder, either acquired (eg, heparin-induced thrombocytopenia) or inherited. A laboratory workup for a hypercoagulable disorder would likely be of little value in determining the cause of his stroke, and even if a hereditary disorder were identified it would be difficult to determine causation. However, if no other explanation for his stroke can be found during the workup, one could consider testing for proteins C and S, antithrombin III, activated protein C resistance (and factor V Leiden if screening for activated protein C resistance is positive), prothrombin G20210A, fibrinogen, homocysteine, D-dimers, and antiphospholipid antibodies.
Cocaine abuse
Another important cause of ischemic stroke is the use of sympathomimetic drugs such as cocaine or amphetamines. The strongest association is with cocaine, which has been seen in case series to cause cerebral vasoconstriction in a dose-dependent manner. Vasoconstriction is also related to a longer duration of cocaine use.9 Several case-control studies have found that the risk of stroke is 4.5 to 6.5 times higher in drug abusers than in controls, and that use of catecholamines or cocaine alone was associated with a significantly increased risk of stroke.10,11
It is certainly advisable to ask about the use of illicit drugs and to send serum and urine samples for appropriate drug screening in young stroke patients, particularly if another cause cannot be found or if drug use is suspected.12
Cardiac embolism
Cardiac embolism is the most likely cause of the stroke in this patient. Up to 20% of the 500,000 strokes that occur annually in the United States are of cardiac embolic origin,13 and the prevalence is even higher in younger patients. In a registry of 428 strokes in patients 15 to 44 years of age, a cardiac source of embolism was the cause in 31.8%.14
- Masses, which include atherosclerotic plaques, cardiac tumors, and infective and noninfective valvular vegetations
- Passageways for paradoxical embolism, such as a patent foramen ovale or atrial septal defect (Figure 2)
- Stasis in the left atrium or left ventricle, with a resulting propensity for thrombosis.
Atrial thrombus is most often seen in patients with atrial fibrillation, mitral stenosis, or dilated cardiomyopathy. Echocardiography of the left atrium in patients with these conditions often reveals spontaneous echo contrast that resembles swirling “smoke,” which is thought to be produced by red blood cell aggregation due to blood stasis. This sign is strongly associated with left atrial thrombi.
Left ventricular thrombosis is one of the most common complications of myocardial infarction and is caused by blood stasis in regions of the ventricle in which the myocardium is hypokinetic or akinetic.
We cannot assume, however, that a potential cardioembolic source seen on echocardiography is the cause of a given patient’s stroke. The evidence proving a causal relationship between most potential cardiac embolic sources and stroke is less than robust. Most of the published data are from nonrandomized studies or case series, and there are no large, prospective studies available to clearly prove that a given cardioembolic source is directly related to embolic stroke.16
This being said, most studies have found high prevalence rates of cardioembolic sources in patients with embolic stroke, which suggests that a causative relationship may exist. However, many of these findings also have a relatively high prevalence among the general population without stroke, raising the possibility that the finding could be incidental and unrelated. Examples are patent foramen ovale, which exists in 27% of adults,17 and aortic arch atheroma, which is common in the elderly.
In the end, when the only potential source of embolism that can be found is in the heart (as is often the case in younger patients), the probability is much greater that it is indeed the cause of the stroke. The lack of direct evidence linking many sources of cardioembolism to stroke emphasizes the need for a thorough investigation of all possible causes of stroke.
DIAGNOSTIC EVALUATION
2. Which is the best study to evaluate for a cardiac embolic source in this patient?
- Transthoracic echocardiography (TTE)
- Transesophageal echocardiography (TEE)
- Transcranial Doppler ultrasonography
- Electrocardiography
The study of choice in this patient is TEE. Overall, TEE is better than TTE in identifying a cardiac source of embolism,18,19 mainly because the images are obtained from a probe in the esophagus, which is in close proximity to the heart, so that there is little additional soft tissue and bone between the probe and cardiac structures. In addition, higher-frequency probes can be used. Both of these result in ultrasonographic images with much greater spatial resolution than can be obtained with a transthoracic study.15
In a case series,20 TEE identified a potential cardiac source of embolism in 45 (57%) of 79 patients with cryptogenic stroke, compared with only 12 (15%) with TTE.
The main limitation of TEE is that it does not show the left ventricular apex very well, making an accurate assessment of left ventricular function or identification of a left ventricular apical thrombus much less likely.
In patients who lack evidence of atherosclerotic cerebrovascular disease, specific findings on history or physical examination could increase the chances of identifying an embolic source, such as left ventricular thrombus, on TTE. These findings could include a history of a myocardial infarction, congestive heart failure, left ventricular dysfunction, endocarditis, rheumatic heart disease, a prosthetic valve, or atrial fibrillation or flutter. TTE by itself is considered sufficient for making the diagnosis of mitral stenosis, left ventricular aneurysm, dilated cardiomyopathy, left ventricular thrombus, and mitral valve prolapse with myxomatous degeneration of the leaflets.
However, in patients without signs or symptoms of cardiac disease, the diagnostic value of TTE is significantly less. Several studies have demonstrated that in patients without evidence of cardiac disease, TTE identifies the source of embolism less than 10% of the time.21 Some series even suggest that the yield may be less than 1%.22 TEE has the advantage of being able to diagnose the above disorders and of having a higher sensitivity for identifying potential sources that may be missed by TTE, such as left atrial or left atrial appendage thrombus, aortic arch atheroma, patent foramen ovale, atrial septal aneurysm, or spontaneous echo contrast. It should be remembered, however, that TEE is a semi-invasive procedure that carries the risks of both the procedure and the sedation, eg, bronchospasm, hypoxia, arrhythmias, upper gastrointestinal trauma, and bleeding.23
Further clouding the decision are recent advances in TTE technology, such as contrast TTE with second harmonic imaging, which enhances the ability of TTE to identify potential sources of stroke such as patent foramen ovale nearly to the level of TEE.24
Unfortunately, guidelines from professional societies do not offer assistance on the best diagnostic approach. Current guidelines from the American Heart Association, American College of Cardiology, and American Society of Echocardiography do give echocardiography a class I indication in younger patients (< 45 years old) with cerebrovascular events or older patients (> 45 years old) with stroke without evidence of cerebrovascular disease or other obvious causes. However, there is no official recommendation on whether to choose TTE, TEE, or both studies.16 Given the multiple causes of cardioembolism and the variety of clinical factors that could influence the decision to choose a certain echo study, this decision is appropriately left to the individual physician.
A reasonable, evidence-based diagnostic approach in young stroke patients is to proceed to TEE when routine TTE and electrocardiography are unrevealing.25 In reality, this is the practice followed in most centers, including ours. Although TTE has a lower diagnostic yield in patients without symptoms, it has the advantages of being readily available in most centers, being noninvasive, and providing complementary information to TEE even when TTE does not reveal a potential cause of stroke.
As for the other studies:
Electrocardiography is valuable in identifying potential cardioembolic causes of stroke such as atrial fibrillation, left ventricular aneurysm, or myocardial infarction, but it is insufficient by itself to assess for many other potential sources of cardioembolism.
Transcranial Doppler ultrasonography is very sensitive for detecting patent foramen ovale and other right-to-left shunts that could be sources of cardioembolism. In this test, microbubbles from agitated saline are injected into the venous circulation and are detected in the cerebral arteries after passing through the shunt. It has no utility in identifying the other possibilities discussed above, nor can it discriminate whether these shunts are intra-cardiac or extracardiac.
Case continued
The patient undergoes TTE, which shows normal left ventricular size, wall thickness, and systolic function. His right ventricular function is normal, as are his left and right atrial size. Valvular function is normal, and no right-to-left interatrial shunt is detected with the use of agitated saline contrast.
MANAGEMENT
3. Which is the most appropriate way to manage the lesion?
- Surgical resection
- Periodic echocardiographic follow-up
- Anticoagulation and periodic echocardiographic follow-up
Cardiac papillary fibroelastomas are rare benign primary tumors of the heart. The true incidence is unknown because, when small, they can be asymptomatic and easily overlooked on gross examination. In adults, they are the second most common primary cardiac tumors, next to atrial myxoma.26
The histogenesis is not known, but the mean age at which they are detected is approximately 60 years, and most of the patients are men, likely because most of these tumors are found incidentally during echocardiography, open heart surgery, or autopsy.28
Most patients with cardiac papillary fibroelastomas have no symptoms; however, those who do have symptoms usually experience valve obstruction or embolization of tumor fragments, leading to stroke, myocardial infarction, or sudden death. Further increasing the risk of embolism, thrombus has been reported on the surface of some tumors, supporting the use of anticoagulation in patients who have experienced embolic phenomena.29
A case review of 725 patients with these tumors27 found that tumor mobility and location on the aortic valve were univariate predictors of tumor-related death and of nonfatal embolism. The only independent predictor of tumor-related death or nonfatal embolization was tumor mobility.
Surgical resection of the tumor is curative, and no recurrences have been reported, although the longest follow-up period has been 11 years.
Although no data exist to support the practice, patients with nonmobile or nonaortic valve tumors could be managed with anticoagulation and periodic echocardiographic follow-up until the tumor becomes mobile or symptomatic, but such a conservative strategy would seem inappropriate for our patient. His tumor is both mobile and located on the aortic valve, putting him at risk of death, and he has already experienced an embolic complication. Therefore, his lesion should be surgically resected.
Case continued
The patient receives anticoagulation therapy with subcutaneous enoxaparin (Lovenox) and warfarin (Coumadin). He undergoes successful surgical resection of the tumor without complication and is discharged to home on hospital day 5.
TAKE-HOME POINTS
The potential causes of stroke in patients younger than age 45 differ significantly from those in older patients. Cardiac embolism is the most frequent cause of stroke in young patients and is most often from left atrial or ventricular thrombus or from aortic atheroma.
In young patients, TEE is superior to TTE in identifying a specific source of cardiac embolism, particularly when clues from the history or physical examination are lacking and the preliminary diagnostic workup fails to identify the cause of the stroke.
Our patient’s history, physical examination, MRI, MRA, electrocardiography, and TTE all failed to disclose a probable cause of his stroke. Appropriately, TEE was performed, which confirmed the diagnosis of cardiac papillary fibroelastoma, a rare and benign primary tumor of the heart with the potential for disastrous consequences.
- Bogousslavsky J, Van Melle G, Regli F. The Lausanne Stroke Registry: analysis of 1,000 consecutive patients with first stroke. Stroke 1988; 19:1083–1092.
- Bogousslavsky J, Pierre P. Ischemic stroke in patients under age 45. Neurol Clin 1992; 10:113–124.
- Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med 2001; 344:898–906.
- Thanvi B, Munshi SK, Dawson SL, Ribinson TG. Carotid and vertebral artery dissection syndromes. Postgrad Med J 2005; 81:383–388.
- Levine SR. Hypercoagulable states and stroke: a selective review. CNS Spectr 2005; 10:567–578.
- Juul K, Tybjaerg-Hansen A, Steffensen R, Kofoed S, Jensen G, Nordestgaard BG. Factor V Leiden: The Copenhagen City Heart Study and 2 meta-analyses. Blood 2002; 100:3–10.
- Ridker PM, Hennekens CH, Lindpaintner K, Stampfer MJ, Eisenberg PR, Miletich JP. Mutation in the gene coding for coagulation factor V and the risk of myocardial infarction, stroke, and venous thrombosis in apparently healthy men. N Engl J Med 1995; 332:912–917.
- Hankey GJ, Eikelboom JW, van Bockxmeer FM, Lofthouse E, Staples N, Baker RI. Inherited thrombophilia in ischemic stroke and its pathogenic subtypes. Stroke 2001; 32:1793–1799.
- Kaufman MJ, Levin JM, Ross MH, et al. Cocaine-induced cerebral vasoconstriction detected in humans with magnetic resonance angiography. JAMA 1998; 279:376–380.
- Kaku DA, Lowenstein DH. Emergence of recreational drug abuse as a major risk factor for stroke in young adults. Ann Intern Med 1990; 113:821–827.
- Petitti DB, Sidney S, Quesenberry C, Bernstein A. Stroke and cocaine or amphetamine use. Epidemiology 1998; 9:596–600.
- Bruno A. Cerebrovascular complications of alcohol and sympathomimetic drug abuse. Curr Neurol Neurosci Rep 2003; 3:40–45.
- Cardiogenic brain embolism. The second report of the Cerebral Embolism Task Force. Arch Neurol 1989; 46:727–743.
- Kittner SJ, Stern BJ, Wozniak M, et al. Cerebral infarction in young adults: the Baltimore-Washington Cooperative Young Stroke Study. Neurology 1998; 50:890–894.
- Manning WJ. Role of transesophageal echocardiography in the management of thromboembolic stroke. Am J Cardiol 1997; 80 4C:19D–28D.
- Cheitlin MD, Armstrong WF, Aurigemma GP, et al American College of Cardiology; American Heart Association; American Society of Echocardiography. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography). Circulation 2003; 108:1146–1162.
- Kizer JR, Devereux RB. Clinical practice. Patent foramen ovale in young adults with unexplained stroke. N Engl J Med 2005; 353:2361–2372.
- Pearson AC. Transthoracic echocardiography versus transesophageal echocardiography in detecting cardiac sources of embolism. Echocardiography 1993; 10:397–403.
- DeRook FA, Comess KA, Albers GW, Popp RL. Transesophageal echocardiography in the evaluation of stroke. Ann Intern Med 1992; 117:922–932.
- Pearson AC, Labovitz AJ, Tatineni S, Gomez CR. Superiority of transesophageal echocardiography in detecting cardiac source of embolism in patients with cerebral ischemia of uncertain etiology. J Am Coll Cardiol 1991; 17:66–72.
- Rahmatullah AF, Rahko PS, Stein JH. Transesophageal echocardiography for the evaluation and management of patients with cerebral ischemia. Clin Cardiol 1999; 22:391–396.
- Come PC, Riley MF, Bivas NK. Roles of echocardiography and arrhythmia monitoring in the evaluation of patients with suspected systemic embolism. Ann Neurol 1983; 13:527–531.
- Daniel WG, Erbel R, Kasper W, et al. Safety of transesophageal echocardiography. A multicenter survey of 10,419 examinations. Circulation 1991; 83:817–821.
- Souteyrand G, Motreff P, Lusson JR, et al. Comparison of transthoracic echocardiography using second harmonic imaging, transcranial Doppler and transesophageal echocardiography for the detection of patent foramen ovale in stroke patients. Eur J Echocardiogr 2006; 7:147–154.
- Harloff A, Handke M, Reinhard M, Geibel A, Hetzel A. Therapeutic strategies after examination by transesophageal echocardiography in 503 patients with ischemic stroke. Stroke 2006; 37:859–864.
- Burke A, Virami R. Tumors of the heart and great vessels. Atlas of Tumor Pathology, 1996, 3rd Series, Fascicle 16. Washington, DC: Armed Forces Institute of Pathology.
- Gowda RM, Khan IA, Nair CK, Mehta NJ, Vasavada BC, Sacchi TJ. Cardiac papillary fibroelastoma: a comprehensive analysis of 725 cases. Am Heart J 2003; 146:404–410.
- Edwards FH, Hale D, Cohen A, Thompson L, Pezzella AT, Virmani R. Primary cardiac valve tumors. Ann Thorac Surg 1991; 52:1127–1131.
- Joffe II, Jacobs LE, Owen AN, Ioli A, Kotler MN. Rapid development of a papillary fibroelastoma with associated thrombus: the role of transthoracic and transesophageal echocardiography. Echocardiography 1997; 14:287–292.
- Bogousslavsky J, Van Melle G, Regli F. The Lausanne Stroke Registry: analysis of 1,000 consecutive patients with first stroke. Stroke 1988; 19:1083–1092.
- Bogousslavsky J, Pierre P. Ischemic stroke in patients under age 45. Neurol Clin 1992; 10:113–124.
- Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med 2001; 344:898–906.
- Thanvi B, Munshi SK, Dawson SL, Ribinson TG. Carotid and vertebral artery dissection syndromes. Postgrad Med J 2005; 81:383–388.
- Levine SR. Hypercoagulable states and stroke: a selective review. CNS Spectr 2005; 10:567–578.
- Juul K, Tybjaerg-Hansen A, Steffensen R, Kofoed S, Jensen G, Nordestgaard BG. Factor V Leiden: The Copenhagen City Heart Study and 2 meta-analyses. Blood 2002; 100:3–10.
- Ridker PM, Hennekens CH, Lindpaintner K, Stampfer MJ, Eisenberg PR, Miletich JP. Mutation in the gene coding for coagulation factor V and the risk of myocardial infarction, stroke, and venous thrombosis in apparently healthy men. N Engl J Med 1995; 332:912–917.
- Hankey GJ, Eikelboom JW, van Bockxmeer FM, Lofthouse E, Staples N, Baker RI. Inherited thrombophilia in ischemic stroke and its pathogenic subtypes. Stroke 2001; 32:1793–1799.
- Kaufman MJ, Levin JM, Ross MH, et al. Cocaine-induced cerebral vasoconstriction detected in humans with magnetic resonance angiography. JAMA 1998; 279:376–380.
- Kaku DA, Lowenstein DH. Emergence of recreational drug abuse as a major risk factor for stroke in young adults. Ann Intern Med 1990; 113:821–827.
- Petitti DB, Sidney S, Quesenberry C, Bernstein A. Stroke and cocaine or amphetamine use. Epidemiology 1998; 9:596–600.
- Bruno A. Cerebrovascular complications of alcohol and sympathomimetic drug abuse. Curr Neurol Neurosci Rep 2003; 3:40–45.
- Cardiogenic brain embolism. The second report of the Cerebral Embolism Task Force. Arch Neurol 1989; 46:727–743.
- Kittner SJ, Stern BJ, Wozniak M, et al. Cerebral infarction in young adults: the Baltimore-Washington Cooperative Young Stroke Study. Neurology 1998; 50:890–894.
- Manning WJ. Role of transesophageal echocardiography in the management of thromboembolic stroke. Am J Cardiol 1997; 80 4C:19D–28D.
- Cheitlin MD, Armstrong WF, Aurigemma GP, et al American College of Cardiology; American Heart Association; American Society of Echocardiography. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography). Circulation 2003; 108:1146–1162.
- Kizer JR, Devereux RB. Clinical practice. Patent foramen ovale in young adults with unexplained stroke. N Engl J Med 2005; 353:2361–2372.
- Pearson AC. Transthoracic echocardiography versus transesophageal echocardiography in detecting cardiac sources of embolism. Echocardiography 1993; 10:397–403.
- DeRook FA, Comess KA, Albers GW, Popp RL. Transesophageal echocardiography in the evaluation of stroke. Ann Intern Med 1992; 117:922–932.
- Pearson AC, Labovitz AJ, Tatineni S, Gomez CR. Superiority of transesophageal echocardiography in detecting cardiac source of embolism in patients with cerebral ischemia of uncertain etiology. J Am Coll Cardiol 1991; 17:66–72.
- Rahmatullah AF, Rahko PS, Stein JH. Transesophageal echocardiography for the evaluation and management of patients with cerebral ischemia. Clin Cardiol 1999; 22:391–396.
- Come PC, Riley MF, Bivas NK. Roles of echocardiography and arrhythmia monitoring in the evaluation of patients with suspected systemic embolism. Ann Neurol 1983; 13:527–531.
- Daniel WG, Erbel R, Kasper W, et al. Safety of transesophageal echocardiography. A multicenter survey of 10,419 examinations. Circulation 1991; 83:817–821.
- Souteyrand G, Motreff P, Lusson JR, et al. Comparison of transthoracic echocardiography using second harmonic imaging, transcranial Doppler and transesophageal echocardiography for the detection of patent foramen ovale in stroke patients. Eur J Echocardiogr 2006; 7:147–154.
- Harloff A, Handke M, Reinhard M, Geibel A, Hetzel A. Therapeutic strategies after examination by transesophageal echocardiography in 503 patients with ischemic stroke. Stroke 2006; 37:859–864.
- Burke A, Virami R. Tumors of the heart and great vessels. Atlas of Tumor Pathology, 1996, 3rd Series, Fascicle 16. Washington, DC: Armed Forces Institute of Pathology.
- Gowda RM, Khan IA, Nair CK, Mehta NJ, Vasavada BC, Sacchi TJ. Cardiac papillary fibroelastoma: a comprehensive analysis of 725 cases. Am Heart J 2003; 146:404–410.
- Edwards FH, Hale D, Cohen A, Thompson L, Pezzella AT, Virmani R. Primary cardiac valve tumors. Ann Thorac Surg 1991; 52:1127–1131.
- Joffe II, Jacobs LE, Owen AN, Ioli A, Kotler MN. Rapid development of a papillary fibroelastoma with associated thrombus: the role of transthoracic and transesophageal echocardiography. Echocardiography 1997; 14:287–292.
Screen for portopulmonary hypertension, especially in liver transplant candidates
Portopulmonary hypertension poses difficulties for patients with liver disease. The elevated pulmonary artery pressure in this disorder makes liver transplantation more dangerous and in fact may rule out the procedure, although in a selected few patients, medical treatment may enable transplantation to proceed. In any event, portopulmonary hypertension should be looked for in patients with liver disease, especially if liver transplantation is being considered.
In this article we discuss the definition, pathophysiology, clinical features, diagnosis, and management of portopulmonary hypertension.
DEFINED BY HEMODYNAMIC CRITERIA
Portopulmonary hypertension—elevated pulmonary artery pressure due to increased resistance to blood flow in patients with portal hypertension—is one of several pulmonary complications of liver disease. A few others to be aware of are pleural effusions (hepatic hydrothorax), dilatation of the pulmonary vasculature with shunting and hypoxemia (hepatopulmonary syndrome), and elevation in pulmonary pressures due to the high cardiac output usually seen in liver disease (flow phenomenon).
The definition of portopulmonary hypertension has evolved as the various hemodynamic profiles that occur in liver disease and their consequences have been described. Currently, it is defined by the following criteria (obtained by right heart catheterization) in a patient with portal hypertension1:
- Elevated mean pulmonary artery pressure (> 25 mm Hg at rest, > 30 mm Hg with exercise);
- Increased pulmonary vascular resistance (> 240 dynes.s.cm−5; pulmonary vascular resistance = [(mean pulmonary artery pressure minus pulmonary artery occlusion pressure) /cardiac output] times 80); and
- Normal pulmonary artery occlusion pressure (< 15 mm Hg) or an elevated transpulmonary gradient (the mean pulmonary artery pressure minus the pulmonary artery occlusion pressure; abnormal is > 12 mm Hg).
The transpulmonary gradient sometimes helps in further assessing the resistance to blood flow in cases that do not meet the other criteria.2 For example, how should we classify a patient whose mean pulmonary artery pressure is 45 mm Hg but whose pulmonary vascular resistance is only 432 dynes.s.cm−5 and whose pulmonary artery occlusion pressure is slightly high at 18 mm Hg? Although this patient does not meet the hemodynamic criteria for portopulmonary hypertension listed above, intuitively, we should not exclude the diagnosis, as the transpulmonary gradient is high at 27 mm Hg.
FLOW PHENOMENON VS TRUE PORTOPULMONARY HYPERTENSION
The cardiopulmonary hemodynamic profile is different in patients with liver disease than in those without liver disease. Understanding the “normal” hemodynamics in liver disease is paramount in understanding the abnormal hemodynamics that occur in portopulmonary hypertension. In general, patients with liver disease have a high cardiac output at baseline (high flow). They may also have an increased blood volume due to fluid shifts (elevated pulmonary artery occlusion pressure).
Right heart catheterization is necessary to make the diagnosis of portopulmonary hypertension, as pulmonary artery pressures may be increased simply from increases in cardiac output and blood volume without an increase in pulmonary vascular resistance.
Consider, for example, a patient whose mean pulmonary artery pressure is 38 mm Hg, pulmonary artery occlusion pressure 14 mm Hg, and cardiac output 8.8 L/minute. In this case, the pulmonary vascular resistance is 218 dynes.s.cm−5. About 30% to 50% of patients with cirrhosis have this type of hyperdynamic pattern, with high cardiac output, low systemic vascular resistance, and low pulmonary vascular resistance.1,3,4 These patients typically have a much better prognosis than those with portopulmonary hypertension and do well with liver transplantation.
Right heart catheterization is also helpful in assessing whether elevated pulmonary pressures are due to increased volume (increased pulmonary artery occlusion pressure), in which case the patient might benefit from more aggressive diuresis.
In true portopulmonary hypertension, the pulmonary vascular resistance is increased due to obstruction of arterial blood flow. Cardiac output may be elevated initially and then decline as pulmonary hypertension becomes more severe. These hemodynamic patterns have different treatment implications and are important when liver transplantation is being considered.5
HOW COMMON IS PORTOPULMONARY HYPERTENSION?
The incidence and prevalence of portopulmonary hypertension is difficult to assess, as many of the estimates are in patients with severe liver disease undergoing evaluation for liver transplantation. Its prevalence in patients with cirrhosis and refractory ascites has been documented at 16.1%,6 while its prevalence in patients with cirrhosis without refractory ascites has been in the range of 0.25% to 4%.7–9
Overall, about 8% of candidates for liver transplantation have portopulmonary hypertension and are at risk of its complications.10 In view of this figure, screening for it should be performed before proceeding with liver transplantation.
VASOCONSTRICTION, REMODELING, THROMBOSIS
The pathogenesis of portopulmonary hypertension is not completely understood but likely involves a complex interaction of several mechanisms, including an imbalance of vascular mediators favoring vasoconstriction,11–13 endothelial damage with vascular remodeling due to excessive pulmonary blood flow,14,15 smooth muscle proliferation, and microvascular thrombosis.16,17
The pulmonary endothelium is a complex, dynamic organ capable of influencing a variety of vascular mediators and adapting to changes in pulmonary volume as necessary. Endothelial dysfunction may initiate the vascular changes seen in portopulmonary hypertension.
Endothelin-1 (ET-1) is a potent vasoconstrictor that has been implicated in the pathogenesis of idiopathic pulmonary artery hypertension. ET-1 levels are also increased in cirrhotic patients with refractory ascites.6
Other mediators favoring vasoconstriction include serotonin, angiotensin II, and norepinephrine. Whether these mediators influence the development of portopulmonary hypertension is not clear.
At the same time, production of vasodilatory mediators such as nitric oxide and prostacyclin may be decreased in portopulmonary hypertension, facilitating vascular remodeling and a proliferative vascular response. Prostacyclin is a potent vasodilator normally found in high concentrations in the lungs. Prostacyclin synthase is the precursor enzyme for the production of prostacyclin and is decreased in the lungs of patients with portopulmonary hypertension.18
Another way that portal hypertension may influence lung vascular tone is that endotoxin, cytokines, or both, released from the splanchnic circulation, may bypass the liver and get into the lungs.19 Evidence in support of this is that patients with portosystemic shunting can develop similar pathologic changes in the pulmonary vascular bed that normalize when the shunt is reversed. To date, however, no substance has been definitively identified.
Yet another proposed mechanism is shear stress on the pulmonary endothelium from the hyperdynamic cardiac output, with resultant vascular remodeling; however, other mechanisms must be involved, as not everyone with liver disease develops portopulmonary hypertension (see below).
These changes are identical to those in idiopathic and familial pulmonary arterial hypertension,21 and indeed, the World Health Organization now classifies portopulmonary hypertension in the same category as these primary forms of pulmonary hypertension rather than in the secondary forms.3
Why doesn’t everyone with liver disease develop portopulmonary hypertension?
The severity of liver disease or degree of portal hypertension does not appear to correlate with the severity of pulmonary hypertension,4 and portopulmonary hypertension does not develop in all patients with portal hypertension. Therefore, it is likely that some patients have a genetic or environmental susceptibility or suffer a “second hit” that triggers dysregulated pulmonary vascular proliferation and contributes to the development of pulmonary hypertension.
Whether genetic mutations play a role in portopulmonary hypertension remains unknown. Such a mutation could be similar to the one identified in the bone morphogenetic protein receptor type 2 gene (BMPR2) in familial pulmonary artery hypertension or the mutation in the activin-like kinase gene (ALK1) seen in pulmonary hypertension in patients with hereditary hemorrhagic telangiectasia.22
Current studies are investigating the role that bone-marrow-derived progenitor cells might play in the pathogenesis of portopulmonary hypertension.
CLINICAL FEATURES MAY NOT BE OBVIOUS AT FIRST
In the early stages of portopulmonary hypertension, patients may have no symptoms or only symptoms of liver disease, so it is important to have a high index of suspicion and screen for pulmonary hypertension. As its severity increases, symptoms may include fatigue, dyspnea, abdominal bloating, palpitations, chest pain or pressure, and syncope. The most common presenting symptom is dyspnea on exertion.
Similarly, the findings on physical examination also depend on the severity of pulmonary hypertension. Patients with mild portopulmonary hypertension may have only signs suggesting liver disease, such as spider telangiectases, jaundice, mild lower extremity edema, and ascites. As the severity of portopulmonary hypertension increases, however, findings of right heart pressure-and-volume overload become more obvious. These include peripheral edema, elevation of the jugular venous pressure, a right ventricular lift, a loud pulmonic valve closure, increased split of the second heart sound, a pulsatile liver, or a right-sided third or fourth heart sound.
SCREEN LIVER TRANSPLANT CANDIDATES
Screening for portopulmonary hypertension should be mandatory in patients undergoing evaluation for liver transplantation. This condition increases the risk of perioperative death, so it is not acceptable to make the diagnosis in the operating room!5
Electrocardiographic abnormalities that may raise the suspicion of portopulmonary hypertension include right atrial or ventricular enlargement and a right bundle branch pattern.
Chest radiographic signs are enlarged central pulmonary arteries and cardiomegaly. These electrocardiographic and radiographic signs tend to reflect advanced pulmonary hypertension.
Pulmonary function testing is not generally helpful, but the diffusing capacity may be decreased.
B-type natriuretic peptide (BNP) measurement may be helpful. BNP is released from the ventricles when the ventricles become dilated (due to pressure or volume overload), as in left or right heart failure. BNP testing is clinically useful in monitoring the severity of disease and the efficacy of treatment in patients with pulmonary hypertension; its role in portopulmonary hypertension requires prospective study.23
Transthoracic Doppler echocardiography is an excellent screening test and should be performed in patients undergoing evaluation for liver transplantation to exclude pulmonary hypertension.1 Findings on echocardiography that suggest portopulmonary hypertension include elevation of right ventricular systolic pressure (RVSP), which is calculated from the peak tricuspid regurgitant velocity (TRV) using the modified Bernoulli equation and an estimate of right atrial pressure (RAP):
RVSP = 4(TRV)2 + RAP.
Right atrial pressure is estimated from the filling characteristics of the inferior vena cava.
Transthoracic Doppler echocardiography has a sensitivity of 97% and a specificity of 77% in diagnosing moderate to severe pulmonary hypertension in patients undergoing evaluation for liver transplantation.24 Using an RVSP cutoff of 40 mm Hg, the sensitivity of Doppler echocardiography is about 80%, specificity 96%, positive predictive value 60%, and negative predictive value 98%.25
At Mayo Clinic, patients with an estimated RVSP greater than 50 mm Hg undergo right heart catheterization (see below). Such patients should also have repeat echocardiography at 1-year intervals to monitor for increasing pulmonary artery pressures5; for those on the waiting list for liver transplantation, the interval should probably be every 6 to 12 months.
RIGHT HEART CATHETERIZATION CONFIRMS THE DIAGNOSIS
The diagnosis of portopulmonary hypertension is confirmed with right heart catheterization to accurately measure pulmonary artery pressures, pulmonary artery occlusion pressure (to exclude volume overload), cardiac output (to exclude high-output pulmonary hypertension), and pulmonary vascular resistance. One study in patients with decompensated cirrhosis and refractory ascites found that a right atrial pressure of 14 mm Hg or greater had a positive predictive value of 83% for pulmonary hypertension.6
Other, potentially treatable causes of pulmonary hypertension must be excluded before diagnosing portopulmonary hypertension. These include thromboembolic disease, interstitial lung disease, connective tissue disease, untreated obstructive sleep apnea, and elevated pulmonary artery pressures due to increased cardiac output.
Vasodilator studies are being done less frequently in patients with portopulmonary hypertension, as they generally cannot tolerate calcium channel blocker therapy. Calcium channel blocker therapy is usually started in patients with idiopathic pulmonary artery hypertension who exhibit a positive vasodilator response. A positive vasodilator response also does not predict survival with or without liver transplantation. Unlike those with idiopathic pulmonary artery hypertension, many patients with portopulmonary hypertension cannot tolerate calcium channel blockers, as some of these drugs can exacerbate edema and portal hypertension.
GENERAL MANAGEMENT
Treatment of mild portopulmonary hypertension (mean pulmonary artery pressure < 35 mm Hg) is debatable. In these cases many patients do not have any symptoms attributable to portopulmonary hypertension, but only symptoms of liver disease, and they have a good functional status. As a group, such patients have not been formally studied to date.
Anticoagulation is often contraindicated in portopulmonary hypertension because of gastroesophageal varices, thrombocytopenia, or other coagulation abnormalities related to liver disease. If contraindications to anticoagulation do not exist, it should be considered.
Diuretics are a mainstay in the treatment of portopulmonary hypertension, both for the pulmonary hypertension and for the liver disease, especially if ascites or peripheral edema is present.
Oxygen should be given to patients with hypoxemia to keep the saturation greater than 90%.
Beta-blockers: A dilemma
Beta-blockers are used in many patients with liver disease as both primary and secondary prophylaxis of variceal bleeding.
However, one study has shown that in patients with moderate to severe portopulmonary hypertension, beta-blockers are associated with significant worsening of exercise capacity and pulmonary hemodynamic measurements.26 After beta-blockers were withdrawn, the 6-minute walking distance increased in 9 of 10 patients, and cardiac output increased with no change in mean pulmonary artery pressure, resulting in a 19% decrease in pulmonary vascular resistance. The increases in cardiac output were related to a 25% increase in heart rate. Long-term follow-up was not reported, and it remains unclear whether rates of gastrointestinal bleeding may increase when beta-blockers are withdrawn.
Beta-blocker therapy in portopulmonary hypertension needs to be carefully considered and if at all possible should be avoided.
VASODILATOR THERAPY
Several vasodilating or vasomodulating drugs are available. However, much of the information about them comes from studies in patients with idiopathic pulmonary artery hypertension or pulmonary hypertension due to connective tissue disease, and no randomized controlled trials in portopulmonary hypertension have been performed.
Prostanoids
Prostanoids have been used successfully to lower pulmonary pressures in portopulmonary hypertension.
Epoprostenol (Flolan) is a pulmonary and systemic vasodilator as well as an inhibitor of platelet aggregation. It is given as a continuous intravenous infusion via an indwelling central venous catheter and a portable infusion pump. It has a very short half-life, requires mixing, and must be kept cold with ice packs, making it somewhat cumbersome to administer.
This medication has been shown to improve cardiopulmonary hemodynamics and exercise capacity in portopulmonary hypertension, although a survival advantage has not been documented to date.27 In several case series, some patients with portopulmonary hypertension treated with intravenous epoprostenol responded with a reduction in pulmonary pressures and successfully underwent liver transplantation.28–31
Complications of intravenous epoprostenol therapy include central venous catheter thrombosis, infection, and infusion pump failure; a backup pump must be available at all times. Patients with portopulmonary hypertension may also develop progressive splenomegaly and thrombocytopenia that may be due to increased blood flow in the splanchnic circulation.32
Treprostinil (Remodulin) has a longer half-life and does not have to be kept cold. It is given as a 24-hour intravenous or subcutaneous infusion, using an infusion pump that is smaller than that used with epoprostenol.
Although treprostinil is easier for patients to use, larger doses are necessary to achieve the same effect as with epoprostenol. With subcutaneous administration, the biggest drawback is site pain. Prostacyclin-related side effects include flushing, diarrhea, jaw discomfort, and lower extremity pain.
Iloprost (Ventavis) has the advantage of being given by inhalation. It is very short-acting, however, and requires six to nine inhalations per day.
Endothelin receptor blockers
Bosentan (Tracleer) is an oral agent that has been approved by the US Food and Drug Administration (FDA) for the treatment of pulmonary hypertension, including in patients with portopulmonary hypertension who have mild hepatic derangement. This medication is a dual endothelin receptor antagonist, nonselectively blocking the endothelin A and B receptors on the endothelial and vascular smooth muscle cells so that ET-1 cannot bind and cause vasoconstriction.
In approximately 10% of patients, bosentan can cause elevations in aminotransferase, alkaline phosphatase, and bilirubin levels, which therefore must be checked monthly.33 Irreversible hepatic toxicity is uncommon; in most cases, liver function abnormalities return to baseline levels when the medication is stopped. The presumed mechanism is impairment of bile-salt transporters, leading to bile-salt accumulation in the liver.34 Bosentan’s use in patients with liver disease has not been well studied, although several case reports have described its use in patients with portopulmonary hypertension.35–38
Ambrisentan (Letairis) is a selective endothelin receptor-A blocker that has just received FDA approval for the treatment of pulmonary artery hypertension. It has not yet been studied in portopulmonary hypertension. Elevations in liver enzymes and bilirubin may also occur, and monthly monitoring is indicated.
Sildenafil
Another oral agent that might be effective in portopulmonary hypertension is sildenafil (Revatio). A phosphodiesterase-5 inhibitor, it selectively inhibits the cyclic guanosine monophosphatase-specific phosphodiesterase type 5 enzyme that is found in large concentrations in pulmonary artery smooth muscle cells.
In other forms of pulmonary hypertension, sildenafil has been shown to increase cardiac output and decrease pulmonary artery pressures and pulmonary vascular resistance without serious adverse events.39–41
In one reported case, treatment with sildenafil in a patient with portopulmonary hypertension decreased the mean pulmonary artery pressure from 56 mm Hg to 28 to 31 mm Hg, and the patient underwent successful liver transplantation.42 A recent case series of 14 patients with portopulmonary hypertension treated with sildenafil documents some improvement in 6-minute walking distance, suggesting that sildenafil as monotherapy or in combination therapy might be effective in portopulmonary hypertension.43 However, in 3 of these patients, the cardiac index decreased and pulmonary vascular resistance increased.44
We must emphasize that controlled studies in portopulmonary hypertension need to be done to find the optimal therapy.
LIVER TRANSPLANTATION MAY BENEFIT A FEW PATIENTS
Liver transplantation may be beneficial in highly selected patients with portopulmonary hypertension. However, this condition increases the risk of intraoperative and immediate postoperative complications of liver transplantation, so patients should be carefully evaluated5,45 at a liver transplantation center experienced in its management, including medical treatment with well-defined protocols regarding timing of liver transplantation.
Patients with mean pulmonary artery pressures greater than 50 mm Hg should not undergo liver transplantation. Those with mean pulmonary artery pressure between 35 and 50 mm Hg also have an increased mortality rate and may benefit from prolonged treatment for pulmonary hypertension.5,46
One successful case of living-related liver transplantation in a patient with portopulmonary hypertension has been published.47 (Most other successful transplants were from unrelated cadaver donors.)
Some patients who initially cannot undergo liver transplantation owing to severe pulmonary hypertension may eventually be able to do so if they receive medical therapy that improves their pulmonary hemodynamic profile, decreasing their mean pulmonary artery pressure and pulmonary vascular resistance. This would apply to a small subset of patients with portopulmonary hypertension.
When patients without pulmonary hypertension undergo liver transplantation, right ventricular function is preserved throughout all phases of the surgery.48 Patients with portopulmonary hypertension, however, may develop hemodynamic instability during liver transplantation. The most critical times are the induction of anesthesia, during and after graft reperfusion, and the immediate postoperative period.49,50
During the surgery, patients may require vasodilators if they have worsening pulmonary hypertension, or inotropic medications if they have right ventricular dysfunction and heart failure. In one study,51 eight patients with portopulmonary hypertension diagnosed at anesthesia induction for liver transplantation all required intraoperative vasodilator therapy after graft reperfusion because of marked increases in pulmonary artery pressures and pulmonary vascular resistance.
The increase in blood flow following reperfusion or necessary fluid challenges may exacerbate pulmonary hypertension, resulting in worsening right heart function and backup into the transplanted liver. Infusion of 1 liter of crystalloid over 10 minutes has been shown to increase mean pulmonary artery pressure and pulmonary artery occlusion pressure in liver transplantation candidates without pulmonary hypertension52; this response may be exaggerated in portopulmonary hypertension.
PROGNOSIS VARIES WITH SEVERITY OF DISEASE
The natural history of untreated portopulmonary hypertension varies with the degree of liver disease and the severity of pulmonary hypertension. Transplant-free survival was 85% at 1 year and 38% at 3 years in one study.45 The cardiac index appears to be the most significant prognostic variable.20
In a retrospective study of 78 patients with portopulmonary hypertension treated conservatively (before prostanoids were available) the median survival was 6 months (range 0–84 months) from the time of diagnosis.53 Causes of death included right heart failure, sudden death, gastrointestinal bleeding, and small bowel perforation.
Most of the data on outcomes of drug treatment and liver transplantation in patients with portopulmonary hypertension come from case series and retrospective reviews; prospective trials have been lacking.
If right ventricular function is normal and pulmonary hypertension is mild (mean pulmonary artery pressure < 35 mm Hg), patients tend to do well with liver transplantation.9
Outcomes are worse if pulmonary hypertension is more severe. In a database54 from 10 liver transplant centers from 1996 to 2001, 13 (36%) of 36 patients undergoing liver transplantation died in the hospital, emphasizing the importance of accurately assessing the severity of pulmonary hypertension before attempting liver transplantation.46 The rate was even higher—92%—in those with a mean pulmonary artery pressure greater than 35 mm Hg. The cause of death in severe pulmonary hypertension was failure of the right ventricle.
However, some patients with moderate to severe portopulmonary hypertension have been bridged with medications to lower pulmonary artery pressures and pulmonary vascular resistance so that liver transplantation can be safely done, and some have even been able to discontinue medications because their pulmonary hypertension resolved.29,31,41,42,47
Unlike in hepatopulmonary syndrome, liver transplantation is not the treatment of choice for portopulmonary hypertension, and pulmonary hypertension does not always resolve after liver transplantation. Many patients continue therapy for pulmonary hypertension after liver transplantation. Pulmonary hypertension may resolve, persist, or even develop de novo after liver transplantation.1 If pulmonary hypertension resolves, it does so over a prolonged time—months to years—favoring a vascular remodeling hypothesis as opposed to simply reversing vasoconstriction.
- Rodriguez-Roisin R, Krowka MJ, Hervé P, Fallon MB; ERS Task Force Pulmonary-Hepatic Vascular Disorders (PHD) Scientific Committee. Pulmonary-hepatic vascular disorders (PHD). Eur Respir J 2004; 24:861–880.
- Krowka MJ, Swanson KL, Frantz RP, et al. Portopulmonary hypertension: results from a 10-year screening algorithm. Hepatology 2006; 44:1502–1510.
- Simonneau G, Galie N, Rubin LJ, et al. Clinical classification of pulmonary hypertension. J Am Coll Cardiol 2004; 43:5S–12S.
- Hadengue A, Benhayoun MK, Lebrec D, et al. Pulmonary hypertension complicating portal hypertension: prevalence and relation to splanchnic hemodynamics. Gastroenterology 1991; 100:520–528.
- Krowka MJ, Plevak DJ, Findlay JY, et al. Pulmonary hemodynamics and perioperative cardiopulmonary-related mortality in patients with portopulmonary hypertension undergoing liver transplantation. Liver Transplant 2000; 6:443–450.
- Benjaminov FS, Prentice M, Sniderman KW, et al. Portopulmonary hypertension in decompensated cirrhosis with refractory ascites. Gut 2003; 52:1355–1362.
- McDonnell PJ, Toye PA, Hutchins GM. Primary pulmonary hypertension and cirrhosis: are they related? Am Rev Respir Dis 1983; 127:437–441.
- Cheng EY, Woehlck H. Pulmonary artery hypertension complicating anesthesia for liver transplantation. Anesthesiology 1992; 77:375–378.
- Castro M, Krowka MJ, Schroeder DR, et al. Frequency and clinical implications of increased pulmonary artery pressures in liver transplantation. Mayo Clin Proc 1996; 71:543–551.
- Ramsay MA, Simpson BR, Nguyen AT, et al. Severe pulmonary hypertension in liver transplant candidates. Liver Transplant Surg 1997; 3:494–500.
- Kiely DG, Cargill RI, Struthers AD, et al. Cardiopulmonary effects of endothelin-1 in man. Cardiovasc Res 1997; 33:378–386.
- Panos RJ, Baker SK. Mediators, cytokines, and growth factors in liver-lung interactions. Clin Chest Med 1996; 17:151–169.
- Higgenbottam T. Pathophysiology of pulmonary hypertension. Chest 1994; 105:7S–12S.
- Krowka MJ. Hepatopulmonary syndrome and portopulmonary hypertension: distinction and dilemmas. Hepatology 1997; 25:1282–1284.
- Hongqun L, Lee SS. Cardiopulmonary dysfunction in cirrhosis. Hepatology 2000; 14:600–608.
- Lebrec D, Brenot F, Simonneau G, et al. Pulmonary arterial hypertension in portal hypertension. Eur Respir J 1998; 11:1153–1166.
- Herve P, Lebrec D, Brenot F, et al. Pulmonary vascular disorders in portal hypertension. Eur Respir J 1998; 11:1153–1166.
- Tuder RM, Cool CD, Geraci MW, et al. Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. Am J Respir Crit Care Med 1999; 159:1925–1932.
- Hoeper MM, Krowka MJ, Strassburg CP. Portopulmonary hypertension and hepatopulmonary syndrome. Lancet 2004; 363:1461–1468.
- Edwards B, Weir K, Edwards WD, et al. Coexistent pulmonary and portal hypertension: morphologic and clinical features. J Am Coll Cardiol 1987; 10:1233–1238.
- Ramsay MAE, Simpson BR, Nguyen AT, Ramsay KJ, East C, Klintmalm GB. Severe pulmonary hypertension in liver transplant candidates. Liver Transplant Surg 1997; 3:494–500.
- Trembath RC. Clinical and molecular genetic features of pulmonary hypertension in patients with hereditary hemorrhagic telangiectasia. N Engl J Med 2001; 345:325–334.
- Leuchte HH, Holzapfel M, Baumgartner RA, et al. Clinical significance of brain natriuretic peptide in primary pulmonary hypertension. J Am Coll Cardiol 2004; 43:764–770.
- Kim WR, Krowka MJ, Plevak DJ, et al. Accuracy of Doppler echocardiography in the assessment of pulmonary hypertension in liver transplant candidates. Liver Transplant 2000; 6:453–458.
- Colle IO, Moreau R, Godinho E, et al. Diagnosis of portopulmonary hypertension in candidates for liver transplantation: a prospective study. Hepatology 2003; 37:401–409.
- Provencher S, Herve P, Jais X, et al. Deleterious effects of beta-blockers on exercise capacity and hemodynamics in patients with portopulmonary hypertension. Gastroenterology 2006; 130:120–126.
- Swanson KL, McGoon MD, Krowka MJ. Survival in patients with portopulmonary hypertension [abstract]. Am J Respir Crit Care Med 2003; 167:A693.
- Kuo PC, Johnson LB, Plotkin JS, et al. Continuous intravenous infusion of epoprostenol for the treatment of portopulmonary hypertension. Transplantation 1997; 63:604–616.
- Krowka MJ, Frantz RP, McGoon MD, et al. Improvement in pulmonary hemodynamics during intravenous epoprostenol (prostacyclin): A study of 15 patients with moderate to severe portopulmonary hypertension. Hepatology 1999; 30:641–648.
- Kähler CM, Graziadei I, Wiedermann CJ, Kneussl MP, Vogel W. Successful use of continuous intravenous prostacyclin in a patient with severe portopulmonary hypertension. Wien Klin Wochenschr 2000; 112:637–640.
- Sussman N, Kaza V, Barshes N, et al. Successful liver transplantation following medical management of portopulmonary hypertension: a single-center series. Am J Transplant 2006; 6:2177–2182.
- Findlay JY, Plevak DJ, Krowka MJ, et al. Progressive splenomegaly after epoprostenol therapy in portopulmonary hypertension. Liver Transplant Surg 1999; 5:381–387.
- Rubin LJ, Roux S. Bosentan: a dual endothelin receptor antagonist. Expert Opin Invest Drugs 2002; 11:991–1002.
- Fattinger K, Funk C, Pantze M, et al. The endothelin antagonist bosentan inhibits the canalicular bile salt export pump: a potential mechanism for hepatic adverse reactions. Clin Pharmacol Ther 2001; 69:223–231.
- Hinterhuber L, Graziadei IW, Kahler CM, et al. Endothelin-receptor anatgonist treatment of portopulmonary hypertension. Clin Gastroenterol Hepatol 2004; 2:1039–1042.
- Clift PF, Townend JN, Bramhall S, et al. Successful treatment of severe portopulmonary hypertension after liver transplantation by bosentan. Transplantation 2004; 77:1774–1775.
- Halank M, Miehlke S, Hoeffken G, et al. Use of oral endothelin-receptor antagonist bosentan in the treatment of portopulmonary hypertension. Transplantation 2004; 77:1775–1776.
- Kuntzen C, Gulberg V, Gerbes AL. Use of a mixed endothelin receptor antagonist in portopulmonary hypertension: a safe and effective therapy? Gastroenterology 2005; 128:164–168.
- Watanabe H, Ohashi K, Takeuchi K, et al. Sildenafil for primary and secondary pulmonary hypertension. Clin Pharmacol Ther 2002; 71:398–402.
- Michelakis E, Tymchak W, Lien D, et al. Oral sildenafil is an effective and specific pulmonary vasodilator in patients with pulmonary arterial hypertension: comparison with inhaled nitric oxide. Circulation 2002; 105:2398–2403.
- Ghofrani HA, Wiedemann R, Rose F, et al. Sildenafil for treatment of lung fibrosis and pulmonary hypertension: a randomised controlled trial. Lancet 2002; 360:895–900.
- Makisalo H, Koivusalo A, Vakkuri A, et al. Sildenafil for portopulmonary hypertension in a patient undergoing liver transplantation. Liver Transplant 2004; 10:945–950.
- Reichengerger F, Voswinckel R, Steveling E, et al. Sildenafil treatment for portopulmonary hypertension. Eur Respir J 2006; 28:563–567.
- Krowka MJ, Swanson KL. How should we treat portopulmonary hypertension? Eur Respir J 2006; 28:466–467.
- Kawut SM, Taichman DB, Ahya VN, et al. Hemodynamics and survival of patients with portopulmonary hypertension. Liver Transplant 2005; 11:1107–1111.
- Krowka MJ, Mandell MS, Ramsay MA, et al. Hepatopulmonary syndrome and portopulmonary hypertension: a report of the multicenter liver transplant database. Liver Transplant 2004; 10:174–182.
- Sulica R, Emre S, Poon M. Medical management of portopulmonary hypertension and right heart failure prior to living-related liver transplantation. Congest Heart Fail 2004; 10:192–194.
- De Wolf AM, Begliomini B, Gasior TA, et al. Right ventricular function during orthotopic liver transplantation. Anesthes Analges 1993; 76:562–568.
- Csete M. Intraoperative management of liver transplant patients with pulmonary hypertension. Liver Transplant Surg 1997; 3:454–455.
- Acosta F, Sansano T, Palenciano CG, et al. Portopulmonary hypertension and liver transplantation: hemodynamic consequences at reperfusion. Transplant Proc 2005; 37:3865–3866.
- Taura P, Garcia-Valdecasas JC, Beltran J, et al. Moderate primary pulmonary hypertension in patients undergoing liver transplantation. Anesthes Analges 1996; 83:675–680.
- Kuo PC, Schroeder RA, Vagelos RH, et al. Volume-mediated pulmonary responses in liver transplant candidates. Clin Transplant 1996; 10:521–527.
- Robalino BD, Moodie DS. Association between primary pulmonary hypertension and portal hypertension: analysis of its pathophysiology and clinical, laboratory and hemodynamic manifestations. J Am Coll Cardiol 1991; 17:492–498.
- Mandell MS, Krowka MJ. Formation of a national database on pulmonary hypertension and hepatopulmonary syndrome in chronic liver disease. Anesthesiology 1997; 87:450–451.
Portopulmonary hypertension poses difficulties for patients with liver disease. The elevated pulmonary artery pressure in this disorder makes liver transplantation more dangerous and in fact may rule out the procedure, although in a selected few patients, medical treatment may enable transplantation to proceed. In any event, portopulmonary hypertension should be looked for in patients with liver disease, especially if liver transplantation is being considered.
In this article we discuss the definition, pathophysiology, clinical features, diagnosis, and management of portopulmonary hypertension.
DEFINED BY HEMODYNAMIC CRITERIA
Portopulmonary hypertension—elevated pulmonary artery pressure due to increased resistance to blood flow in patients with portal hypertension—is one of several pulmonary complications of liver disease. A few others to be aware of are pleural effusions (hepatic hydrothorax), dilatation of the pulmonary vasculature with shunting and hypoxemia (hepatopulmonary syndrome), and elevation in pulmonary pressures due to the high cardiac output usually seen in liver disease (flow phenomenon).
The definition of portopulmonary hypertension has evolved as the various hemodynamic profiles that occur in liver disease and their consequences have been described. Currently, it is defined by the following criteria (obtained by right heart catheterization) in a patient with portal hypertension1:
- Elevated mean pulmonary artery pressure (> 25 mm Hg at rest, > 30 mm Hg with exercise);
- Increased pulmonary vascular resistance (> 240 dynes.s.cm−5; pulmonary vascular resistance = [(mean pulmonary artery pressure minus pulmonary artery occlusion pressure) /cardiac output] times 80); and
- Normal pulmonary artery occlusion pressure (< 15 mm Hg) or an elevated transpulmonary gradient (the mean pulmonary artery pressure minus the pulmonary artery occlusion pressure; abnormal is > 12 mm Hg).
The transpulmonary gradient sometimes helps in further assessing the resistance to blood flow in cases that do not meet the other criteria.2 For example, how should we classify a patient whose mean pulmonary artery pressure is 45 mm Hg but whose pulmonary vascular resistance is only 432 dynes.s.cm−5 and whose pulmonary artery occlusion pressure is slightly high at 18 mm Hg? Although this patient does not meet the hemodynamic criteria for portopulmonary hypertension listed above, intuitively, we should not exclude the diagnosis, as the transpulmonary gradient is high at 27 mm Hg.
FLOW PHENOMENON VS TRUE PORTOPULMONARY HYPERTENSION
The cardiopulmonary hemodynamic profile is different in patients with liver disease than in those without liver disease. Understanding the “normal” hemodynamics in liver disease is paramount in understanding the abnormal hemodynamics that occur in portopulmonary hypertension. In general, patients with liver disease have a high cardiac output at baseline (high flow). They may also have an increased blood volume due to fluid shifts (elevated pulmonary artery occlusion pressure).
Right heart catheterization is necessary to make the diagnosis of portopulmonary hypertension, as pulmonary artery pressures may be increased simply from increases in cardiac output and blood volume without an increase in pulmonary vascular resistance.
Consider, for example, a patient whose mean pulmonary artery pressure is 38 mm Hg, pulmonary artery occlusion pressure 14 mm Hg, and cardiac output 8.8 L/minute. In this case, the pulmonary vascular resistance is 218 dynes.s.cm−5. About 30% to 50% of patients with cirrhosis have this type of hyperdynamic pattern, with high cardiac output, low systemic vascular resistance, and low pulmonary vascular resistance.1,3,4 These patients typically have a much better prognosis than those with portopulmonary hypertension and do well with liver transplantation.
Right heart catheterization is also helpful in assessing whether elevated pulmonary pressures are due to increased volume (increased pulmonary artery occlusion pressure), in which case the patient might benefit from more aggressive diuresis.
In true portopulmonary hypertension, the pulmonary vascular resistance is increased due to obstruction of arterial blood flow. Cardiac output may be elevated initially and then decline as pulmonary hypertension becomes more severe. These hemodynamic patterns have different treatment implications and are important when liver transplantation is being considered.5
HOW COMMON IS PORTOPULMONARY HYPERTENSION?
The incidence and prevalence of portopulmonary hypertension is difficult to assess, as many of the estimates are in patients with severe liver disease undergoing evaluation for liver transplantation. Its prevalence in patients with cirrhosis and refractory ascites has been documented at 16.1%,6 while its prevalence in patients with cirrhosis without refractory ascites has been in the range of 0.25% to 4%.7–9
Overall, about 8% of candidates for liver transplantation have portopulmonary hypertension and are at risk of its complications.10 In view of this figure, screening for it should be performed before proceeding with liver transplantation.
VASOCONSTRICTION, REMODELING, THROMBOSIS
The pathogenesis of portopulmonary hypertension is not completely understood but likely involves a complex interaction of several mechanisms, including an imbalance of vascular mediators favoring vasoconstriction,11–13 endothelial damage with vascular remodeling due to excessive pulmonary blood flow,14,15 smooth muscle proliferation, and microvascular thrombosis.16,17
The pulmonary endothelium is a complex, dynamic organ capable of influencing a variety of vascular mediators and adapting to changes in pulmonary volume as necessary. Endothelial dysfunction may initiate the vascular changes seen in portopulmonary hypertension.
Endothelin-1 (ET-1) is a potent vasoconstrictor that has been implicated in the pathogenesis of idiopathic pulmonary artery hypertension. ET-1 levels are also increased in cirrhotic patients with refractory ascites.6
Other mediators favoring vasoconstriction include serotonin, angiotensin II, and norepinephrine. Whether these mediators influence the development of portopulmonary hypertension is not clear.
At the same time, production of vasodilatory mediators such as nitric oxide and prostacyclin may be decreased in portopulmonary hypertension, facilitating vascular remodeling and a proliferative vascular response. Prostacyclin is a potent vasodilator normally found in high concentrations in the lungs. Prostacyclin synthase is the precursor enzyme for the production of prostacyclin and is decreased in the lungs of patients with portopulmonary hypertension.18
Another way that portal hypertension may influence lung vascular tone is that endotoxin, cytokines, or both, released from the splanchnic circulation, may bypass the liver and get into the lungs.19 Evidence in support of this is that patients with portosystemic shunting can develop similar pathologic changes in the pulmonary vascular bed that normalize when the shunt is reversed. To date, however, no substance has been definitively identified.
Yet another proposed mechanism is shear stress on the pulmonary endothelium from the hyperdynamic cardiac output, with resultant vascular remodeling; however, other mechanisms must be involved, as not everyone with liver disease develops portopulmonary hypertension (see below).
These changes are identical to those in idiopathic and familial pulmonary arterial hypertension,21 and indeed, the World Health Organization now classifies portopulmonary hypertension in the same category as these primary forms of pulmonary hypertension rather than in the secondary forms.3
Why doesn’t everyone with liver disease develop portopulmonary hypertension?
The severity of liver disease or degree of portal hypertension does not appear to correlate with the severity of pulmonary hypertension,4 and portopulmonary hypertension does not develop in all patients with portal hypertension. Therefore, it is likely that some patients have a genetic or environmental susceptibility or suffer a “second hit” that triggers dysregulated pulmonary vascular proliferation and contributes to the development of pulmonary hypertension.
Whether genetic mutations play a role in portopulmonary hypertension remains unknown. Such a mutation could be similar to the one identified in the bone morphogenetic protein receptor type 2 gene (BMPR2) in familial pulmonary artery hypertension or the mutation in the activin-like kinase gene (ALK1) seen in pulmonary hypertension in patients with hereditary hemorrhagic telangiectasia.22
Current studies are investigating the role that bone-marrow-derived progenitor cells might play in the pathogenesis of portopulmonary hypertension.
CLINICAL FEATURES MAY NOT BE OBVIOUS AT FIRST
In the early stages of portopulmonary hypertension, patients may have no symptoms or only symptoms of liver disease, so it is important to have a high index of suspicion and screen for pulmonary hypertension. As its severity increases, symptoms may include fatigue, dyspnea, abdominal bloating, palpitations, chest pain or pressure, and syncope. The most common presenting symptom is dyspnea on exertion.
Similarly, the findings on physical examination also depend on the severity of pulmonary hypertension. Patients with mild portopulmonary hypertension may have only signs suggesting liver disease, such as spider telangiectases, jaundice, mild lower extremity edema, and ascites. As the severity of portopulmonary hypertension increases, however, findings of right heart pressure-and-volume overload become more obvious. These include peripheral edema, elevation of the jugular venous pressure, a right ventricular lift, a loud pulmonic valve closure, increased split of the second heart sound, a pulsatile liver, or a right-sided third or fourth heart sound.
SCREEN LIVER TRANSPLANT CANDIDATES
Screening for portopulmonary hypertension should be mandatory in patients undergoing evaluation for liver transplantation. This condition increases the risk of perioperative death, so it is not acceptable to make the diagnosis in the operating room!5
Electrocardiographic abnormalities that may raise the suspicion of portopulmonary hypertension include right atrial or ventricular enlargement and a right bundle branch pattern.
Chest radiographic signs are enlarged central pulmonary arteries and cardiomegaly. These electrocardiographic and radiographic signs tend to reflect advanced pulmonary hypertension.
Pulmonary function testing is not generally helpful, but the diffusing capacity may be decreased.
B-type natriuretic peptide (BNP) measurement may be helpful. BNP is released from the ventricles when the ventricles become dilated (due to pressure or volume overload), as in left or right heart failure. BNP testing is clinically useful in monitoring the severity of disease and the efficacy of treatment in patients with pulmonary hypertension; its role in portopulmonary hypertension requires prospective study.23
Transthoracic Doppler echocardiography is an excellent screening test and should be performed in patients undergoing evaluation for liver transplantation to exclude pulmonary hypertension.1 Findings on echocardiography that suggest portopulmonary hypertension include elevation of right ventricular systolic pressure (RVSP), which is calculated from the peak tricuspid regurgitant velocity (TRV) using the modified Bernoulli equation and an estimate of right atrial pressure (RAP):
RVSP = 4(TRV)2 + RAP.
Right atrial pressure is estimated from the filling characteristics of the inferior vena cava.
Transthoracic Doppler echocardiography has a sensitivity of 97% and a specificity of 77% in diagnosing moderate to severe pulmonary hypertension in patients undergoing evaluation for liver transplantation.24 Using an RVSP cutoff of 40 mm Hg, the sensitivity of Doppler echocardiography is about 80%, specificity 96%, positive predictive value 60%, and negative predictive value 98%.25
At Mayo Clinic, patients with an estimated RVSP greater than 50 mm Hg undergo right heart catheterization (see below). Such patients should also have repeat echocardiography at 1-year intervals to monitor for increasing pulmonary artery pressures5; for those on the waiting list for liver transplantation, the interval should probably be every 6 to 12 months.
RIGHT HEART CATHETERIZATION CONFIRMS THE DIAGNOSIS
The diagnosis of portopulmonary hypertension is confirmed with right heart catheterization to accurately measure pulmonary artery pressures, pulmonary artery occlusion pressure (to exclude volume overload), cardiac output (to exclude high-output pulmonary hypertension), and pulmonary vascular resistance. One study in patients with decompensated cirrhosis and refractory ascites found that a right atrial pressure of 14 mm Hg or greater had a positive predictive value of 83% for pulmonary hypertension.6
Other, potentially treatable causes of pulmonary hypertension must be excluded before diagnosing portopulmonary hypertension. These include thromboembolic disease, interstitial lung disease, connective tissue disease, untreated obstructive sleep apnea, and elevated pulmonary artery pressures due to increased cardiac output.
Vasodilator studies are being done less frequently in patients with portopulmonary hypertension, as they generally cannot tolerate calcium channel blocker therapy. Calcium channel blocker therapy is usually started in patients with idiopathic pulmonary artery hypertension who exhibit a positive vasodilator response. A positive vasodilator response also does not predict survival with or without liver transplantation. Unlike those with idiopathic pulmonary artery hypertension, many patients with portopulmonary hypertension cannot tolerate calcium channel blockers, as some of these drugs can exacerbate edema and portal hypertension.
GENERAL MANAGEMENT
Treatment of mild portopulmonary hypertension (mean pulmonary artery pressure < 35 mm Hg) is debatable. In these cases many patients do not have any symptoms attributable to portopulmonary hypertension, but only symptoms of liver disease, and they have a good functional status. As a group, such patients have not been formally studied to date.
Anticoagulation is often contraindicated in portopulmonary hypertension because of gastroesophageal varices, thrombocytopenia, or other coagulation abnormalities related to liver disease. If contraindications to anticoagulation do not exist, it should be considered.
Diuretics are a mainstay in the treatment of portopulmonary hypertension, both for the pulmonary hypertension and for the liver disease, especially if ascites or peripheral edema is present.
Oxygen should be given to patients with hypoxemia to keep the saturation greater than 90%.
Beta-blockers: A dilemma
Beta-blockers are used in many patients with liver disease as both primary and secondary prophylaxis of variceal bleeding.
However, one study has shown that in patients with moderate to severe portopulmonary hypertension, beta-blockers are associated with significant worsening of exercise capacity and pulmonary hemodynamic measurements.26 After beta-blockers were withdrawn, the 6-minute walking distance increased in 9 of 10 patients, and cardiac output increased with no change in mean pulmonary artery pressure, resulting in a 19% decrease in pulmonary vascular resistance. The increases in cardiac output were related to a 25% increase in heart rate. Long-term follow-up was not reported, and it remains unclear whether rates of gastrointestinal bleeding may increase when beta-blockers are withdrawn.
Beta-blocker therapy in portopulmonary hypertension needs to be carefully considered and if at all possible should be avoided.
VASODILATOR THERAPY
Several vasodilating or vasomodulating drugs are available. However, much of the information about them comes from studies in patients with idiopathic pulmonary artery hypertension or pulmonary hypertension due to connective tissue disease, and no randomized controlled trials in portopulmonary hypertension have been performed.
Prostanoids
Prostanoids have been used successfully to lower pulmonary pressures in portopulmonary hypertension.
Epoprostenol (Flolan) is a pulmonary and systemic vasodilator as well as an inhibitor of platelet aggregation. It is given as a continuous intravenous infusion via an indwelling central venous catheter and a portable infusion pump. It has a very short half-life, requires mixing, and must be kept cold with ice packs, making it somewhat cumbersome to administer.
This medication has been shown to improve cardiopulmonary hemodynamics and exercise capacity in portopulmonary hypertension, although a survival advantage has not been documented to date.27 In several case series, some patients with portopulmonary hypertension treated with intravenous epoprostenol responded with a reduction in pulmonary pressures and successfully underwent liver transplantation.28–31
Complications of intravenous epoprostenol therapy include central venous catheter thrombosis, infection, and infusion pump failure; a backup pump must be available at all times. Patients with portopulmonary hypertension may also develop progressive splenomegaly and thrombocytopenia that may be due to increased blood flow in the splanchnic circulation.32
Treprostinil (Remodulin) has a longer half-life and does not have to be kept cold. It is given as a 24-hour intravenous or subcutaneous infusion, using an infusion pump that is smaller than that used with epoprostenol.
Although treprostinil is easier for patients to use, larger doses are necessary to achieve the same effect as with epoprostenol. With subcutaneous administration, the biggest drawback is site pain. Prostacyclin-related side effects include flushing, diarrhea, jaw discomfort, and lower extremity pain.
Iloprost (Ventavis) has the advantage of being given by inhalation. It is very short-acting, however, and requires six to nine inhalations per day.
Endothelin receptor blockers
Bosentan (Tracleer) is an oral agent that has been approved by the US Food and Drug Administration (FDA) for the treatment of pulmonary hypertension, including in patients with portopulmonary hypertension who have mild hepatic derangement. This medication is a dual endothelin receptor antagonist, nonselectively blocking the endothelin A and B receptors on the endothelial and vascular smooth muscle cells so that ET-1 cannot bind and cause vasoconstriction.
In approximately 10% of patients, bosentan can cause elevations in aminotransferase, alkaline phosphatase, and bilirubin levels, which therefore must be checked monthly.33 Irreversible hepatic toxicity is uncommon; in most cases, liver function abnormalities return to baseline levels when the medication is stopped. The presumed mechanism is impairment of bile-salt transporters, leading to bile-salt accumulation in the liver.34 Bosentan’s use in patients with liver disease has not been well studied, although several case reports have described its use in patients with portopulmonary hypertension.35–38
Ambrisentan (Letairis) is a selective endothelin receptor-A blocker that has just received FDA approval for the treatment of pulmonary artery hypertension. It has not yet been studied in portopulmonary hypertension. Elevations in liver enzymes and bilirubin may also occur, and monthly monitoring is indicated.
Sildenafil
Another oral agent that might be effective in portopulmonary hypertension is sildenafil (Revatio). A phosphodiesterase-5 inhibitor, it selectively inhibits the cyclic guanosine monophosphatase-specific phosphodiesterase type 5 enzyme that is found in large concentrations in pulmonary artery smooth muscle cells.
In other forms of pulmonary hypertension, sildenafil has been shown to increase cardiac output and decrease pulmonary artery pressures and pulmonary vascular resistance without serious adverse events.39–41
In one reported case, treatment with sildenafil in a patient with portopulmonary hypertension decreased the mean pulmonary artery pressure from 56 mm Hg to 28 to 31 mm Hg, and the patient underwent successful liver transplantation.42 A recent case series of 14 patients with portopulmonary hypertension treated with sildenafil documents some improvement in 6-minute walking distance, suggesting that sildenafil as monotherapy or in combination therapy might be effective in portopulmonary hypertension.43 However, in 3 of these patients, the cardiac index decreased and pulmonary vascular resistance increased.44
We must emphasize that controlled studies in portopulmonary hypertension need to be done to find the optimal therapy.
LIVER TRANSPLANTATION MAY BENEFIT A FEW PATIENTS
Liver transplantation may be beneficial in highly selected patients with portopulmonary hypertension. However, this condition increases the risk of intraoperative and immediate postoperative complications of liver transplantation, so patients should be carefully evaluated5,45 at a liver transplantation center experienced in its management, including medical treatment with well-defined protocols regarding timing of liver transplantation.
Patients with mean pulmonary artery pressures greater than 50 mm Hg should not undergo liver transplantation. Those with mean pulmonary artery pressure between 35 and 50 mm Hg also have an increased mortality rate and may benefit from prolonged treatment for pulmonary hypertension.5,46
One successful case of living-related liver transplantation in a patient with portopulmonary hypertension has been published.47 (Most other successful transplants were from unrelated cadaver donors.)
Some patients who initially cannot undergo liver transplantation owing to severe pulmonary hypertension may eventually be able to do so if they receive medical therapy that improves their pulmonary hemodynamic profile, decreasing their mean pulmonary artery pressure and pulmonary vascular resistance. This would apply to a small subset of patients with portopulmonary hypertension.
When patients without pulmonary hypertension undergo liver transplantation, right ventricular function is preserved throughout all phases of the surgery.48 Patients with portopulmonary hypertension, however, may develop hemodynamic instability during liver transplantation. The most critical times are the induction of anesthesia, during and after graft reperfusion, and the immediate postoperative period.49,50
During the surgery, patients may require vasodilators if they have worsening pulmonary hypertension, or inotropic medications if they have right ventricular dysfunction and heart failure. In one study,51 eight patients with portopulmonary hypertension diagnosed at anesthesia induction for liver transplantation all required intraoperative vasodilator therapy after graft reperfusion because of marked increases in pulmonary artery pressures and pulmonary vascular resistance.
The increase in blood flow following reperfusion or necessary fluid challenges may exacerbate pulmonary hypertension, resulting in worsening right heart function and backup into the transplanted liver. Infusion of 1 liter of crystalloid over 10 minutes has been shown to increase mean pulmonary artery pressure and pulmonary artery occlusion pressure in liver transplantation candidates without pulmonary hypertension52; this response may be exaggerated in portopulmonary hypertension.
PROGNOSIS VARIES WITH SEVERITY OF DISEASE
The natural history of untreated portopulmonary hypertension varies with the degree of liver disease and the severity of pulmonary hypertension. Transplant-free survival was 85% at 1 year and 38% at 3 years in one study.45 The cardiac index appears to be the most significant prognostic variable.20
In a retrospective study of 78 patients with portopulmonary hypertension treated conservatively (before prostanoids were available) the median survival was 6 months (range 0–84 months) from the time of diagnosis.53 Causes of death included right heart failure, sudden death, gastrointestinal bleeding, and small bowel perforation.
Most of the data on outcomes of drug treatment and liver transplantation in patients with portopulmonary hypertension come from case series and retrospective reviews; prospective trials have been lacking.
If right ventricular function is normal and pulmonary hypertension is mild (mean pulmonary artery pressure < 35 mm Hg), patients tend to do well with liver transplantation.9
Outcomes are worse if pulmonary hypertension is more severe. In a database54 from 10 liver transplant centers from 1996 to 2001, 13 (36%) of 36 patients undergoing liver transplantation died in the hospital, emphasizing the importance of accurately assessing the severity of pulmonary hypertension before attempting liver transplantation.46 The rate was even higher—92%—in those with a mean pulmonary artery pressure greater than 35 mm Hg. The cause of death in severe pulmonary hypertension was failure of the right ventricle.
However, some patients with moderate to severe portopulmonary hypertension have been bridged with medications to lower pulmonary artery pressures and pulmonary vascular resistance so that liver transplantation can be safely done, and some have even been able to discontinue medications because their pulmonary hypertension resolved.29,31,41,42,47
Unlike in hepatopulmonary syndrome, liver transplantation is not the treatment of choice for portopulmonary hypertension, and pulmonary hypertension does not always resolve after liver transplantation. Many patients continue therapy for pulmonary hypertension after liver transplantation. Pulmonary hypertension may resolve, persist, or even develop de novo after liver transplantation.1 If pulmonary hypertension resolves, it does so over a prolonged time—months to years—favoring a vascular remodeling hypothesis as opposed to simply reversing vasoconstriction.
Portopulmonary hypertension poses difficulties for patients with liver disease. The elevated pulmonary artery pressure in this disorder makes liver transplantation more dangerous and in fact may rule out the procedure, although in a selected few patients, medical treatment may enable transplantation to proceed. In any event, portopulmonary hypertension should be looked for in patients with liver disease, especially if liver transplantation is being considered.
In this article we discuss the definition, pathophysiology, clinical features, diagnosis, and management of portopulmonary hypertension.
DEFINED BY HEMODYNAMIC CRITERIA
Portopulmonary hypertension—elevated pulmonary artery pressure due to increased resistance to blood flow in patients with portal hypertension—is one of several pulmonary complications of liver disease. A few others to be aware of are pleural effusions (hepatic hydrothorax), dilatation of the pulmonary vasculature with shunting and hypoxemia (hepatopulmonary syndrome), and elevation in pulmonary pressures due to the high cardiac output usually seen in liver disease (flow phenomenon).
The definition of portopulmonary hypertension has evolved as the various hemodynamic profiles that occur in liver disease and their consequences have been described. Currently, it is defined by the following criteria (obtained by right heart catheterization) in a patient with portal hypertension1:
- Elevated mean pulmonary artery pressure (> 25 mm Hg at rest, > 30 mm Hg with exercise);
- Increased pulmonary vascular resistance (> 240 dynes.s.cm−5; pulmonary vascular resistance = [(mean pulmonary artery pressure minus pulmonary artery occlusion pressure) /cardiac output] times 80); and
- Normal pulmonary artery occlusion pressure (< 15 mm Hg) or an elevated transpulmonary gradient (the mean pulmonary artery pressure minus the pulmonary artery occlusion pressure; abnormal is > 12 mm Hg).
The transpulmonary gradient sometimes helps in further assessing the resistance to blood flow in cases that do not meet the other criteria.2 For example, how should we classify a patient whose mean pulmonary artery pressure is 45 mm Hg but whose pulmonary vascular resistance is only 432 dynes.s.cm−5 and whose pulmonary artery occlusion pressure is slightly high at 18 mm Hg? Although this patient does not meet the hemodynamic criteria for portopulmonary hypertension listed above, intuitively, we should not exclude the diagnosis, as the transpulmonary gradient is high at 27 mm Hg.
FLOW PHENOMENON VS TRUE PORTOPULMONARY HYPERTENSION
The cardiopulmonary hemodynamic profile is different in patients with liver disease than in those without liver disease. Understanding the “normal” hemodynamics in liver disease is paramount in understanding the abnormal hemodynamics that occur in portopulmonary hypertension. In general, patients with liver disease have a high cardiac output at baseline (high flow). They may also have an increased blood volume due to fluid shifts (elevated pulmonary artery occlusion pressure).
Right heart catheterization is necessary to make the diagnosis of portopulmonary hypertension, as pulmonary artery pressures may be increased simply from increases in cardiac output and blood volume without an increase in pulmonary vascular resistance.
Consider, for example, a patient whose mean pulmonary artery pressure is 38 mm Hg, pulmonary artery occlusion pressure 14 mm Hg, and cardiac output 8.8 L/minute. In this case, the pulmonary vascular resistance is 218 dynes.s.cm−5. About 30% to 50% of patients with cirrhosis have this type of hyperdynamic pattern, with high cardiac output, low systemic vascular resistance, and low pulmonary vascular resistance.1,3,4 These patients typically have a much better prognosis than those with portopulmonary hypertension and do well with liver transplantation.
Right heart catheterization is also helpful in assessing whether elevated pulmonary pressures are due to increased volume (increased pulmonary artery occlusion pressure), in which case the patient might benefit from more aggressive diuresis.
In true portopulmonary hypertension, the pulmonary vascular resistance is increased due to obstruction of arterial blood flow. Cardiac output may be elevated initially and then decline as pulmonary hypertension becomes more severe. These hemodynamic patterns have different treatment implications and are important when liver transplantation is being considered.5
HOW COMMON IS PORTOPULMONARY HYPERTENSION?
The incidence and prevalence of portopulmonary hypertension is difficult to assess, as many of the estimates are in patients with severe liver disease undergoing evaluation for liver transplantation. Its prevalence in patients with cirrhosis and refractory ascites has been documented at 16.1%,6 while its prevalence in patients with cirrhosis without refractory ascites has been in the range of 0.25% to 4%.7–9
Overall, about 8% of candidates for liver transplantation have portopulmonary hypertension and are at risk of its complications.10 In view of this figure, screening for it should be performed before proceeding with liver transplantation.
VASOCONSTRICTION, REMODELING, THROMBOSIS
The pathogenesis of portopulmonary hypertension is not completely understood but likely involves a complex interaction of several mechanisms, including an imbalance of vascular mediators favoring vasoconstriction,11–13 endothelial damage with vascular remodeling due to excessive pulmonary blood flow,14,15 smooth muscle proliferation, and microvascular thrombosis.16,17
The pulmonary endothelium is a complex, dynamic organ capable of influencing a variety of vascular mediators and adapting to changes in pulmonary volume as necessary. Endothelial dysfunction may initiate the vascular changes seen in portopulmonary hypertension.
Endothelin-1 (ET-1) is a potent vasoconstrictor that has been implicated in the pathogenesis of idiopathic pulmonary artery hypertension. ET-1 levels are also increased in cirrhotic patients with refractory ascites.6
Other mediators favoring vasoconstriction include serotonin, angiotensin II, and norepinephrine. Whether these mediators influence the development of portopulmonary hypertension is not clear.
At the same time, production of vasodilatory mediators such as nitric oxide and prostacyclin may be decreased in portopulmonary hypertension, facilitating vascular remodeling and a proliferative vascular response. Prostacyclin is a potent vasodilator normally found in high concentrations in the lungs. Prostacyclin synthase is the precursor enzyme for the production of prostacyclin and is decreased in the lungs of patients with portopulmonary hypertension.18
Another way that portal hypertension may influence lung vascular tone is that endotoxin, cytokines, or both, released from the splanchnic circulation, may bypass the liver and get into the lungs.19 Evidence in support of this is that patients with portosystemic shunting can develop similar pathologic changes in the pulmonary vascular bed that normalize when the shunt is reversed. To date, however, no substance has been definitively identified.
Yet another proposed mechanism is shear stress on the pulmonary endothelium from the hyperdynamic cardiac output, with resultant vascular remodeling; however, other mechanisms must be involved, as not everyone with liver disease develops portopulmonary hypertension (see below).
These changes are identical to those in idiopathic and familial pulmonary arterial hypertension,21 and indeed, the World Health Organization now classifies portopulmonary hypertension in the same category as these primary forms of pulmonary hypertension rather than in the secondary forms.3
Why doesn’t everyone with liver disease develop portopulmonary hypertension?
The severity of liver disease or degree of portal hypertension does not appear to correlate with the severity of pulmonary hypertension,4 and portopulmonary hypertension does not develop in all patients with portal hypertension. Therefore, it is likely that some patients have a genetic or environmental susceptibility or suffer a “second hit” that triggers dysregulated pulmonary vascular proliferation and contributes to the development of pulmonary hypertension.
Whether genetic mutations play a role in portopulmonary hypertension remains unknown. Such a mutation could be similar to the one identified in the bone morphogenetic protein receptor type 2 gene (BMPR2) in familial pulmonary artery hypertension or the mutation in the activin-like kinase gene (ALK1) seen in pulmonary hypertension in patients with hereditary hemorrhagic telangiectasia.22
Current studies are investigating the role that bone-marrow-derived progenitor cells might play in the pathogenesis of portopulmonary hypertension.
CLINICAL FEATURES MAY NOT BE OBVIOUS AT FIRST
In the early stages of portopulmonary hypertension, patients may have no symptoms or only symptoms of liver disease, so it is important to have a high index of suspicion and screen for pulmonary hypertension. As its severity increases, symptoms may include fatigue, dyspnea, abdominal bloating, palpitations, chest pain or pressure, and syncope. The most common presenting symptom is dyspnea on exertion.
Similarly, the findings on physical examination also depend on the severity of pulmonary hypertension. Patients with mild portopulmonary hypertension may have only signs suggesting liver disease, such as spider telangiectases, jaundice, mild lower extremity edema, and ascites. As the severity of portopulmonary hypertension increases, however, findings of right heart pressure-and-volume overload become more obvious. These include peripheral edema, elevation of the jugular venous pressure, a right ventricular lift, a loud pulmonic valve closure, increased split of the second heart sound, a pulsatile liver, or a right-sided third or fourth heart sound.
SCREEN LIVER TRANSPLANT CANDIDATES
Screening for portopulmonary hypertension should be mandatory in patients undergoing evaluation for liver transplantation. This condition increases the risk of perioperative death, so it is not acceptable to make the diagnosis in the operating room!5
Electrocardiographic abnormalities that may raise the suspicion of portopulmonary hypertension include right atrial or ventricular enlargement and a right bundle branch pattern.
Chest radiographic signs are enlarged central pulmonary arteries and cardiomegaly. These electrocardiographic and radiographic signs tend to reflect advanced pulmonary hypertension.
Pulmonary function testing is not generally helpful, but the diffusing capacity may be decreased.
B-type natriuretic peptide (BNP) measurement may be helpful. BNP is released from the ventricles when the ventricles become dilated (due to pressure or volume overload), as in left or right heart failure. BNP testing is clinically useful in monitoring the severity of disease and the efficacy of treatment in patients with pulmonary hypertension; its role in portopulmonary hypertension requires prospective study.23
Transthoracic Doppler echocardiography is an excellent screening test and should be performed in patients undergoing evaluation for liver transplantation to exclude pulmonary hypertension.1 Findings on echocardiography that suggest portopulmonary hypertension include elevation of right ventricular systolic pressure (RVSP), which is calculated from the peak tricuspid regurgitant velocity (TRV) using the modified Bernoulli equation and an estimate of right atrial pressure (RAP):
RVSP = 4(TRV)2 + RAP.
Right atrial pressure is estimated from the filling characteristics of the inferior vena cava.
Transthoracic Doppler echocardiography has a sensitivity of 97% and a specificity of 77% in diagnosing moderate to severe pulmonary hypertension in patients undergoing evaluation for liver transplantation.24 Using an RVSP cutoff of 40 mm Hg, the sensitivity of Doppler echocardiography is about 80%, specificity 96%, positive predictive value 60%, and negative predictive value 98%.25
At Mayo Clinic, patients with an estimated RVSP greater than 50 mm Hg undergo right heart catheterization (see below). Such patients should also have repeat echocardiography at 1-year intervals to monitor for increasing pulmonary artery pressures5; for those on the waiting list for liver transplantation, the interval should probably be every 6 to 12 months.
RIGHT HEART CATHETERIZATION CONFIRMS THE DIAGNOSIS
The diagnosis of portopulmonary hypertension is confirmed with right heart catheterization to accurately measure pulmonary artery pressures, pulmonary artery occlusion pressure (to exclude volume overload), cardiac output (to exclude high-output pulmonary hypertension), and pulmonary vascular resistance. One study in patients with decompensated cirrhosis and refractory ascites found that a right atrial pressure of 14 mm Hg or greater had a positive predictive value of 83% for pulmonary hypertension.6
Other, potentially treatable causes of pulmonary hypertension must be excluded before diagnosing portopulmonary hypertension. These include thromboembolic disease, interstitial lung disease, connective tissue disease, untreated obstructive sleep apnea, and elevated pulmonary artery pressures due to increased cardiac output.
Vasodilator studies are being done less frequently in patients with portopulmonary hypertension, as they generally cannot tolerate calcium channel blocker therapy. Calcium channel blocker therapy is usually started in patients with idiopathic pulmonary artery hypertension who exhibit a positive vasodilator response. A positive vasodilator response also does not predict survival with or without liver transplantation. Unlike those with idiopathic pulmonary artery hypertension, many patients with portopulmonary hypertension cannot tolerate calcium channel blockers, as some of these drugs can exacerbate edema and portal hypertension.
GENERAL MANAGEMENT
Treatment of mild portopulmonary hypertension (mean pulmonary artery pressure < 35 mm Hg) is debatable. In these cases many patients do not have any symptoms attributable to portopulmonary hypertension, but only symptoms of liver disease, and they have a good functional status. As a group, such patients have not been formally studied to date.
Anticoagulation is often contraindicated in portopulmonary hypertension because of gastroesophageal varices, thrombocytopenia, or other coagulation abnormalities related to liver disease. If contraindications to anticoagulation do not exist, it should be considered.
Diuretics are a mainstay in the treatment of portopulmonary hypertension, both for the pulmonary hypertension and for the liver disease, especially if ascites or peripheral edema is present.
Oxygen should be given to patients with hypoxemia to keep the saturation greater than 90%.
Beta-blockers: A dilemma
Beta-blockers are used in many patients with liver disease as both primary and secondary prophylaxis of variceal bleeding.
However, one study has shown that in patients with moderate to severe portopulmonary hypertension, beta-blockers are associated with significant worsening of exercise capacity and pulmonary hemodynamic measurements.26 After beta-blockers were withdrawn, the 6-minute walking distance increased in 9 of 10 patients, and cardiac output increased with no change in mean pulmonary artery pressure, resulting in a 19% decrease in pulmonary vascular resistance. The increases in cardiac output were related to a 25% increase in heart rate. Long-term follow-up was not reported, and it remains unclear whether rates of gastrointestinal bleeding may increase when beta-blockers are withdrawn.
Beta-blocker therapy in portopulmonary hypertension needs to be carefully considered and if at all possible should be avoided.
VASODILATOR THERAPY
Several vasodilating or vasomodulating drugs are available. However, much of the information about them comes from studies in patients with idiopathic pulmonary artery hypertension or pulmonary hypertension due to connective tissue disease, and no randomized controlled trials in portopulmonary hypertension have been performed.
Prostanoids
Prostanoids have been used successfully to lower pulmonary pressures in portopulmonary hypertension.
Epoprostenol (Flolan) is a pulmonary and systemic vasodilator as well as an inhibitor of platelet aggregation. It is given as a continuous intravenous infusion via an indwelling central venous catheter and a portable infusion pump. It has a very short half-life, requires mixing, and must be kept cold with ice packs, making it somewhat cumbersome to administer.
This medication has been shown to improve cardiopulmonary hemodynamics and exercise capacity in portopulmonary hypertension, although a survival advantage has not been documented to date.27 In several case series, some patients with portopulmonary hypertension treated with intravenous epoprostenol responded with a reduction in pulmonary pressures and successfully underwent liver transplantation.28–31
Complications of intravenous epoprostenol therapy include central venous catheter thrombosis, infection, and infusion pump failure; a backup pump must be available at all times. Patients with portopulmonary hypertension may also develop progressive splenomegaly and thrombocytopenia that may be due to increased blood flow in the splanchnic circulation.32
Treprostinil (Remodulin) has a longer half-life and does not have to be kept cold. It is given as a 24-hour intravenous or subcutaneous infusion, using an infusion pump that is smaller than that used with epoprostenol.
Although treprostinil is easier for patients to use, larger doses are necessary to achieve the same effect as with epoprostenol. With subcutaneous administration, the biggest drawback is site pain. Prostacyclin-related side effects include flushing, diarrhea, jaw discomfort, and lower extremity pain.
Iloprost (Ventavis) has the advantage of being given by inhalation. It is very short-acting, however, and requires six to nine inhalations per day.
Endothelin receptor blockers
Bosentan (Tracleer) is an oral agent that has been approved by the US Food and Drug Administration (FDA) for the treatment of pulmonary hypertension, including in patients with portopulmonary hypertension who have mild hepatic derangement. This medication is a dual endothelin receptor antagonist, nonselectively blocking the endothelin A and B receptors on the endothelial and vascular smooth muscle cells so that ET-1 cannot bind and cause vasoconstriction.
In approximately 10% of patients, bosentan can cause elevations in aminotransferase, alkaline phosphatase, and bilirubin levels, which therefore must be checked monthly.33 Irreversible hepatic toxicity is uncommon; in most cases, liver function abnormalities return to baseline levels when the medication is stopped. The presumed mechanism is impairment of bile-salt transporters, leading to bile-salt accumulation in the liver.34 Bosentan’s use in patients with liver disease has not been well studied, although several case reports have described its use in patients with portopulmonary hypertension.35–38
Ambrisentan (Letairis) is a selective endothelin receptor-A blocker that has just received FDA approval for the treatment of pulmonary artery hypertension. It has not yet been studied in portopulmonary hypertension. Elevations in liver enzymes and bilirubin may also occur, and monthly monitoring is indicated.
Sildenafil
Another oral agent that might be effective in portopulmonary hypertension is sildenafil (Revatio). A phosphodiesterase-5 inhibitor, it selectively inhibits the cyclic guanosine monophosphatase-specific phosphodiesterase type 5 enzyme that is found in large concentrations in pulmonary artery smooth muscle cells.
In other forms of pulmonary hypertension, sildenafil has been shown to increase cardiac output and decrease pulmonary artery pressures and pulmonary vascular resistance without serious adverse events.39–41
In one reported case, treatment with sildenafil in a patient with portopulmonary hypertension decreased the mean pulmonary artery pressure from 56 mm Hg to 28 to 31 mm Hg, and the patient underwent successful liver transplantation.42 A recent case series of 14 patients with portopulmonary hypertension treated with sildenafil documents some improvement in 6-minute walking distance, suggesting that sildenafil as monotherapy or in combination therapy might be effective in portopulmonary hypertension.43 However, in 3 of these patients, the cardiac index decreased and pulmonary vascular resistance increased.44
We must emphasize that controlled studies in portopulmonary hypertension need to be done to find the optimal therapy.
LIVER TRANSPLANTATION MAY BENEFIT A FEW PATIENTS
Liver transplantation may be beneficial in highly selected patients with portopulmonary hypertension. However, this condition increases the risk of intraoperative and immediate postoperative complications of liver transplantation, so patients should be carefully evaluated5,45 at a liver transplantation center experienced in its management, including medical treatment with well-defined protocols regarding timing of liver transplantation.
Patients with mean pulmonary artery pressures greater than 50 mm Hg should not undergo liver transplantation. Those with mean pulmonary artery pressure between 35 and 50 mm Hg also have an increased mortality rate and may benefit from prolonged treatment for pulmonary hypertension.5,46
One successful case of living-related liver transplantation in a patient with portopulmonary hypertension has been published.47 (Most other successful transplants were from unrelated cadaver donors.)
Some patients who initially cannot undergo liver transplantation owing to severe pulmonary hypertension may eventually be able to do so if they receive medical therapy that improves their pulmonary hemodynamic profile, decreasing their mean pulmonary artery pressure and pulmonary vascular resistance. This would apply to a small subset of patients with portopulmonary hypertension.
When patients without pulmonary hypertension undergo liver transplantation, right ventricular function is preserved throughout all phases of the surgery.48 Patients with portopulmonary hypertension, however, may develop hemodynamic instability during liver transplantation. The most critical times are the induction of anesthesia, during and after graft reperfusion, and the immediate postoperative period.49,50
During the surgery, patients may require vasodilators if they have worsening pulmonary hypertension, or inotropic medications if they have right ventricular dysfunction and heart failure. In one study,51 eight patients with portopulmonary hypertension diagnosed at anesthesia induction for liver transplantation all required intraoperative vasodilator therapy after graft reperfusion because of marked increases in pulmonary artery pressures and pulmonary vascular resistance.
The increase in blood flow following reperfusion or necessary fluid challenges may exacerbate pulmonary hypertension, resulting in worsening right heart function and backup into the transplanted liver. Infusion of 1 liter of crystalloid over 10 minutes has been shown to increase mean pulmonary artery pressure and pulmonary artery occlusion pressure in liver transplantation candidates without pulmonary hypertension52; this response may be exaggerated in portopulmonary hypertension.
PROGNOSIS VARIES WITH SEVERITY OF DISEASE
The natural history of untreated portopulmonary hypertension varies with the degree of liver disease and the severity of pulmonary hypertension. Transplant-free survival was 85% at 1 year and 38% at 3 years in one study.45 The cardiac index appears to be the most significant prognostic variable.20
In a retrospective study of 78 patients with portopulmonary hypertension treated conservatively (before prostanoids were available) the median survival was 6 months (range 0–84 months) from the time of diagnosis.53 Causes of death included right heart failure, sudden death, gastrointestinal bleeding, and small bowel perforation.
Most of the data on outcomes of drug treatment and liver transplantation in patients with portopulmonary hypertension come from case series and retrospective reviews; prospective trials have been lacking.
If right ventricular function is normal and pulmonary hypertension is mild (mean pulmonary artery pressure < 35 mm Hg), patients tend to do well with liver transplantation.9
Outcomes are worse if pulmonary hypertension is more severe. In a database54 from 10 liver transplant centers from 1996 to 2001, 13 (36%) of 36 patients undergoing liver transplantation died in the hospital, emphasizing the importance of accurately assessing the severity of pulmonary hypertension before attempting liver transplantation.46 The rate was even higher—92%—in those with a mean pulmonary artery pressure greater than 35 mm Hg. The cause of death in severe pulmonary hypertension was failure of the right ventricle.
However, some patients with moderate to severe portopulmonary hypertension have been bridged with medications to lower pulmonary artery pressures and pulmonary vascular resistance so that liver transplantation can be safely done, and some have even been able to discontinue medications because their pulmonary hypertension resolved.29,31,41,42,47
Unlike in hepatopulmonary syndrome, liver transplantation is not the treatment of choice for portopulmonary hypertension, and pulmonary hypertension does not always resolve after liver transplantation. Many patients continue therapy for pulmonary hypertension after liver transplantation. Pulmonary hypertension may resolve, persist, or even develop de novo after liver transplantation.1 If pulmonary hypertension resolves, it does so over a prolonged time—months to years—favoring a vascular remodeling hypothesis as opposed to simply reversing vasoconstriction.
- Rodriguez-Roisin R, Krowka MJ, Hervé P, Fallon MB; ERS Task Force Pulmonary-Hepatic Vascular Disorders (PHD) Scientific Committee. Pulmonary-hepatic vascular disorders (PHD). Eur Respir J 2004; 24:861–880.
- Krowka MJ, Swanson KL, Frantz RP, et al. Portopulmonary hypertension: results from a 10-year screening algorithm. Hepatology 2006; 44:1502–1510.
- Simonneau G, Galie N, Rubin LJ, et al. Clinical classification of pulmonary hypertension. J Am Coll Cardiol 2004; 43:5S–12S.
- Hadengue A, Benhayoun MK, Lebrec D, et al. Pulmonary hypertension complicating portal hypertension: prevalence and relation to splanchnic hemodynamics. Gastroenterology 1991; 100:520–528.
- Krowka MJ, Plevak DJ, Findlay JY, et al. Pulmonary hemodynamics and perioperative cardiopulmonary-related mortality in patients with portopulmonary hypertension undergoing liver transplantation. Liver Transplant 2000; 6:443–450.
- Benjaminov FS, Prentice M, Sniderman KW, et al. Portopulmonary hypertension in decompensated cirrhosis with refractory ascites. Gut 2003; 52:1355–1362.
- McDonnell PJ, Toye PA, Hutchins GM. Primary pulmonary hypertension and cirrhosis: are they related? Am Rev Respir Dis 1983; 127:437–441.
- Cheng EY, Woehlck H. Pulmonary artery hypertension complicating anesthesia for liver transplantation. Anesthesiology 1992; 77:375–378.
- Castro M, Krowka MJ, Schroeder DR, et al. Frequency and clinical implications of increased pulmonary artery pressures in liver transplantation. Mayo Clin Proc 1996; 71:543–551.
- Ramsay MA, Simpson BR, Nguyen AT, et al. Severe pulmonary hypertension in liver transplant candidates. Liver Transplant Surg 1997; 3:494–500.
- Kiely DG, Cargill RI, Struthers AD, et al. Cardiopulmonary effects of endothelin-1 in man. Cardiovasc Res 1997; 33:378–386.
- Panos RJ, Baker SK. Mediators, cytokines, and growth factors in liver-lung interactions. Clin Chest Med 1996; 17:151–169.
- Higgenbottam T. Pathophysiology of pulmonary hypertension. Chest 1994; 105:7S–12S.
- Krowka MJ. Hepatopulmonary syndrome and portopulmonary hypertension: distinction and dilemmas. Hepatology 1997; 25:1282–1284.
- Hongqun L, Lee SS. Cardiopulmonary dysfunction in cirrhosis. Hepatology 2000; 14:600–608.
- Lebrec D, Brenot F, Simonneau G, et al. Pulmonary arterial hypertension in portal hypertension. Eur Respir J 1998; 11:1153–1166.
- Herve P, Lebrec D, Brenot F, et al. Pulmonary vascular disorders in portal hypertension. Eur Respir J 1998; 11:1153–1166.
- Tuder RM, Cool CD, Geraci MW, et al. Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. Am J Respir Crit Care Med 1999; 159:1925–1932.
- Hoeper MM, Krowka MJ, Strassburg CP. Portopulmonary hypertension and hepatopulmonary syndrome. Lancet 2004; 363:1461–1468.
- Edwards B, Weir K, Edwards WD, et al. Coexistent pulmonary and portal hypertension: morphologic and clinical features. J Am Coll Cardiol 1987; 10:1233–1238.
- Ramsay MAE, Simpson BR, Nguyen AT, Ramsay KJ, East C, Klintmalm GB. Severe pulmonary hypertension in liver transplant candidates. Liver Transplant Surg 1997; 3:494–500.
- Trembath RC. Clinical and molecular genetic features of pulmonary hypertension in patients with hereditary hemorrhagic telangiectasia. N Engl J Med 2001; 345:325–334.
- Leuchte HH, Holzapfel M, Baumgartner RA, et al. Clinical significance of brain natriuretic peptide in primary pulmonary hypertension. J Am Coll Cardiol 2004; 43:764–770.
- Kim WR, Krowka MJ, Plevak DJ, et al. Accuracy of Doppler echocardiography in the assessment of pulmonary hypertension in liver transplant candidates. Liver Transplant 2000; 6:453–458.
- Colle IO, Moreau R, Godinho E, et al. Diagnosis of portopulmonary hypertension in candidates for liver transplantation: a prospective study. Hepatology 2003; 37:401–409.
- Provencher S, Herve P, Jais X, et al. Deleterious effects of beta-blockers on exercise capacity and hemodynamics in patients with portopulmonary hypertension. Gastroenterology 2006; 130:120–126.
- Swanson KL, McGoon MD, Krowka MJ. Survival in patients with portopulmonary hypertension [abstract]. Am J Respir Crit Care Med 2003; 167:A693.
- Kuo PC, Johnson LB, Plotkin JS, et al. Continuous intravenous infusion of epoprostenol for the treatment of portopulmonary hypertension. Transplantation 1997; 63:604–616.
- Krowka MJ, Frantz RP, McGoon MD, et al. Improvement in pulmonary hemodynamics during intravenous epoprostenol (prostacyclin): A study of 15 patients with moderate to severe portopulmonary hypertension. Hepatology 1999; 30:641–648.
- Kähler CM, Graziadei I, Wiedermann CJ, Kneussl MP, Vogel W. Successful use of continuous intravenous prostacyclin in a patient with severe portopulmonary hypertension. Wien Klin Wochenschr 2000; 112:637–640.
- Sussman N, Kaza V, Barshes N, et al. Successful liver transplantation following medical management of portopulmonary hypertension: a single-center series. Am J Transplant 2006; 6:2177–2182.
- Findlay JY, Plevak DJ, Krowka MJ, et al. Progressive splenomegaly after epoprostenol therapy in portopulmonary hypertension. Liver Transplant Surg 1999; 5:381–387.
- Rubin LJ, Roux S. Bosentan: a dual endothelin receptor antagonist. Expert Opin Invest Drugs 2002; 11:991–1002.
- Fattinger K, Funk C, Pantze M, et al. The endothelin antagonist bosentan inhibits the canalicular bile salt export pump: a potential mechanism for hepatic adverse reactions. Clin Pharmacol Ther 2001; 69:223–231.
- Hinterhuber L, Graziadei IW, Kahler CM, et al. Endothelin-receptor anatgonist treatment of portopulmonary hypertension. Clin Gastroenterol Hepatol 2004; 2:1039–1042.
- Clift PF, Townend JN, Bramhall S, et al. Successful treatment of severe portopulmonary hypertension after liver transplantation by bosentan. Transplantation 2004; 77:1774–1775.
- Halank M, Miehlke S, Hoeffken G, et al. Use of oral endothelin-receptor antagonist bosentan in the treatment of portopulmonary hypertension. Transplantation 2004; 77:1775–1776.
- Kuntzen C, Gulberg V, Gerbes AL. Use of a mixed endothelin receptor antagonist in portopulmonary hypertension: a safe and effective therapy? Gastroenterology 2005; 128:164–168.
- Watanabe H, Ohashi K, Takeuchi K, et al. Sildenafil for primary and secondary pulmonary hypertension. Clin Pharmacol Ther 2002; 71:398–402.
- Michelakis E, Tymchak W, Lien D, et al. Oral sildenafil is an effective and specific pulmonary vasodilator in patients with pulmonary arterial hypertension: comparison with inhaled nitric oxide. Circulation 2002; 105:2398–2403.
- Ghofrani HA, Wiedemann R, Rose F, et al. Sildenafil for treatment of lung fibrosis and pulmonary hypertension: a randomised controlled trial. Lancet 2002; 360:895–900.
- Makisalo H, Koivusalo A, Vakkuri A, et al. Sildenafil for portopulmonary hypertension in a patient undergoing liver transplantation. Liver Transplant 2004; 10:945–950.
- Reichengerger F, Voswinckel R, Steveling E, et al. Sildenafil treatment for portopulmonary hypertension. Eur Respir J 2006; 28:563–567.
- Krowka MJ, Swanson KL. How should we treat portopulmonary hypertension? Eur Respir J 2006; 28:466–467.
- Kawut SM, Taichman DB, Ahya VN, et al. Hemodynamics and survival of patients with portopulmonary hypertension. Liver Transplant 2005; 11:1107–1111.
- Krowka MJ, Mandell MS, Ramsay MA, et al. Hepatopulmonary syndrome and portopulmonary hypertension: a report of the multicenter liver transplant database. Liver Transplant 2004; 10:174–182.
- Sulica R, Emre S, Poon M. Medical management of portopulmonary hypertension and right heart failure prior to living-related liver transplantation. Congest Heart Fail 2004; 10:192–194.
- De Wolf AM, Begliomini B, Gasior TA, et al. Right ventricular function during orthotopic liver transplantation. Anesthes Analges 1993; 76:562–568.
- Csete M. Intraoperative management of liver transplant patients with pulmonary hypertension. Liver Transplant Surg 1997; 3:454–455.
- Acosta F, Sansano T, Palenciano CG, et al. Portopulmonary hypertension and liver transplantation: hemodynamic consequences at reperfusion. Transplant Proc 2005; 37:3865–3866.
- Taura P, Garcia-Valdecasas JC, Beltran J, et al. Moderate primary pulmonary hypertension in patients undergoing liver transplantation. Anesthes Analges 1996; 83:675–680.
- Kuo PC, Schroeder RA, Vagelos RH, et al. Volume-mediated pulmonary responses in liver transplant candidates. Clin Transplant 1996; 10:521–527.
- Robalino BD, Moodie DS. Association between primary pulmonary hypertension and portal hypertension: analysis of its pathophysiology and clinical, laboratory and hemodynamic manifestations. J Am Coll Cardiol 1991; 17:492–498.
- Mandell MS, Krowka MJ. Formation of a national database on pulmonary hypertension and hepatopulmonary syndrome in chronic liver disease. Anesthesiology 1997; 87:450–451.
- Rodriguez-Roisin R, Krowka MJ, Hervé P, Fallon MB; ERS Task Force Pulmonary-Hepatic Vascular Disorders (PHD) Scientific Committee. Pulmonary-hepatic vascular disorders (PHD). Eur Respir J 2004; 24:861–880.
- Krowka MJ, Swanson KL, Frantz RP, et al. Portopulmonary hypertension: results from a 10-year screening algorithm. Hepatology 2006; 44:1502–1510.
- Simonneau G, Galie N, Rubin LJ, et al. Clinical classification of pulmonary hypertension. J Am Coll Cardiol 2004; 43:5S–12S.
- Hadengue A, Benhayoun MK, Lebrec D, et al. Pulmonary hypertension complicating portal hypertension: prevalence and relation to splanchnic hemodynamics. Gastroenterology 1991; 100:520–528.
- Krowka MJ, Plevak DJ, Findlay JY, et al. Pulmonary hemodynamics and perioperative cardiopulmonary-related mortality in patients with portopulmonary hypertension undergoing liver transplantation. Liver Transplant 2000; 6:443–450.
- Benjaminov FS, Prentice M, Sniderman KW, et al. Portopulmonary hypertension in decompensated cirrhosis with refractory ascites. Gut 2003; 52:1355–1362.
- McDonnell PJ, Toye PA, Hutchins GM. Primary pulmonary hypertension and cirrhosis: are they related? Am Rev Respir Dis 1983; 127:437–441.
- Cheng EY, Woehlck H. Pulmonary artery hypertension complicating anesthesia for liver transplantation. Anesthesiology 1992; 77:375–378.
- Castro M, Krowka MJ, Schroeder DR, et al. Frequency and clinical implications of increased pulmonary artery pressures in liver transplantation. Mayo Clin Proc 1996; 71:543–551.
- Ramsay MA, Simpson BR, Nguyen AT, et al. Severe pulmonary hypertension in liver transplant candidates. Liver Transplant Surg 1997; 3:494–500.
- Kiely DG, Cargill RI, Struthers AD, et al. Cardiopulmonary effects of endothelin-1 in man. Cardiovasc Res 1997; 33:378–386.
- Panos RJ, Baker SK. Mediators, cytokines, and growth factors in liver-lung interactions. Clin Chest Med 1996; 17:151–169.
- Higgenbottam T. Pathophysiology of pulmonary hypertension. Chest 1994; 105:7S–12S.
- Krowka MJ. Hepatopulmonary syndrome and portopulmonary hypertension: distinction and dilemmas. Hepatology 1997; 25:1282–1284.
- Hongqun L, Lee SS. Cardiopulmonary dysfunction in cirrhosis. Hepatology 2000; 14:600–608.
- Lebrec D, Brenot F, Simonneau G, et al. Pulmonary arterial hypertension in portal hypertension. Eur Respir J 1998; 11:1153–1166.
- Herve P, Lebrec D, Brenot F, et al. Pulmonary vascular disorders in portal hypertension. Eur Respir J 1998; 11:1153–1166.
- Tuder RM, Cool CD, Geraci MW, et al. Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. Am J Respir Crit Care Med 1999; 159:1925–1932.
- Hoeper MM, Krowka MJ, Strassburg CP. Portopulmonary hypertension and hepatopulmonary syndrome. Lancet 2004; 363:1461–1468.
- Edwards B, Weir K, Edwards WD, et al. Coexistent pulmonary and portal hypertension: morphologic and clinical features. J Am Coll Cardiol 1987; 10:1233–1238.
- Ramsay MAE, Simpson BR, Nguyen AT, Ramsay KJ, East C, Klintmalm GB. Severe pulmonary hypertension in liver transplant candidates. Liver Transplant Surg 1997; 3:494–500.
- Trembath RC. Clinical and molecular genetic features of pulmonary hypertension in patients with hereditary hemorrhagic telangiectasia. N Engl J Med 2001; 345:325–334.
- Leuchte HH, Holzapfel M, Baumgartner RA, et al. Clinical significance of brain natriuretic peptide in primary pulmonary hypertension. J Am Coll Cardiol 2004; 43:764–770.
- Kim WR, Krowka MJ, Plevak DJ, et al. Accuracy of Doppler echocardiography in the assessment of pulmonary hypertension in liver transplant candidates. Liver Transplant 2000; 6:453–458.
- Colle IO, Moreau R, Godinho E, et al. Diagnosis of portopulmonary hypertension in candidates for liver transplantation: a prospective study. Hepatology 2003; 37:401–409.
- Provencher S, Herve P, Jais X, et al. Deleterious effects of beta-blockers on exercise capacity and hemodynamics in patients with portopulmonary hypertension. Gastroenterology 2006; 130:120–126.
- Swanson KL, McGoon MD, Krowka MJ. Survival in patients with portopulmonary hypertension [abstract]. Am J Respir Crit Care Med 2003; 167:A693.
- Kuo PC, Johnson LB, Plotkin JS, et al. Continuous intravenous infusion of epoprostenol for the treatment of portopulmonary hypertension. Transplantation 1997; 63:604–616.
- Krowka MJ, Frantz RP, McGoon MD, et al. Improvement in pulmonary hemodynamics during intravenous epoprostenol (prostacyclin): A study of 15 patients with moderate to severe portopulmonary hypertension. Hepatology 1999; 30:641–648.
- Kähler CM, Graziadei I, Wiedermann CJ, Kneussl MP, Vogel W. Successful use of continuous intravenous prostacyclin in a patient with severe portopulmonary hypertension. Wien Klin Wochenschr 2000; 112:637–640.
- Sussman N, Kaza V, Barshes N, et al. Successful liver transplantation following medical management of portopulmonary hypertension: a single-center series. Am J Transplant 2006; 6:2177–2182.
- Findlay JY, Plevak DJ, Krowka MJ, et al. Progressive splenomegaly after epoprostenol therapy in portopulmonary hypertension. Liver Transplant Surg 1999; 5:381–387.
- Rubin LJ, Roux S. Bosentan: a dual endothelin receptor antagonist. Expert Opin Invest Drugs 2002; 11:991–1002.
- Fattinger K, Funk C, Pantze M, et al. The endothelin antagonist bosentan inhibits the canalicular bile salt export pump: a potential mechanism for hepatic adverse reactions. Clin Pharmacol Ther 2001; 69:223–231.
- Hinterhuber L, Graziadei IW, Kahler CM, et al. Endothelin-receptor anatgonist treatment of portopulmonary hypertension. Clin Gastroenterol Hepatol 2004; 2:1039–1042.
- Clift PF, Townend JN, Bramhall S, et al. Successful treatment of severe portopulmonary hypertension after liver transplantation by bosentan. Transplantation 2004; 77:1774–1775.
- Halank M, Miehlke S, Hoeffken G, et al. Use of oral endothelin-receptor antagonist bosentan in the treatment of portopulmonary hypertension. Transplantation 2004; 77:1775–1776.
- Kuntzen C, Gulberg V, Gerbes AL. Use of a mixed endothelin receptor antagonist in portopulmonary hypertension: a safe and effective therapy? Gastroenterology 2005; 128:164–168.
- Watanabe H, Ohashi K, Takeuchi K, et al. Sildenafil for primary and secondary pulmonary hypertension. Clin Pharmacol Ther 2002; 71:398–402.
- Michelakis E, Tymchak W, Lien D, et al. Oral sildenafil is an effective and specific pulmonary vasodilator in patients with pulmonary arterial hypertension: comparison with inhaled nitric oxide. Circulation 2002; 105:2398–2403.
- Ghofrani HA, Wiedemann R, Rose F, et al. Sildenafil for treatment of lung fibrosis and pulmonary hypertension: a randomised controlled trial. Lancet 2002; 360:895–900.
- Makisalo H, Koivusalo A, Vakkuri A, et al. Sildenafil for portopulmonary hypertension in a patient undergoing liver transplantation. Liver Transplant 2004; 10:945–950.
- Reichengerger F, Voswinckel R, Steveling E, et al. Sildenafil treatment for portopulmonary hypertension. Eur Respir J 2006; 28:563–567.
- Krowka MJ, Swanson KL. How should we treat portopulmonary hypertension? Eur Respir J 2006; 28:466–467.
- Kawut SM, Taichman DB, Ahya VN, et al. Hemodynamics and survival of patients with portopulmonary hypertension. Liver Transplant 2005; 11:1107–1111.
- Krowka MJ, Mandell MS, Ramsay MA, et al. Hepatopulmonary syndrome and portopulmonary hypertension: a report of the multicenter liver transplant database. Liver Transplant 2004; 10:174–182.
- Sulica R, Emre S, Poon M. Medical management of portopulmonary hypertension and right heart failure prior to living-related liver transplantation. Congest Heart Fail 2004; 10:192–194.
- De Wolf AM, Begliomini B, Gasior TA, et al. Right ventricular function during orthotopic liver transplantation. Anesthes Analges 1993; 76:562–568.
- Csete M. Intraoperative management of liver transplant patients with pulmonary hypertension. Liver Transplant Surg 1997; 3:454–455.
- Acosta F, Sansano T, Palenciano CG, et al. Portopulmonary hypertension and liver transplantation: hemodynamic consequences at reperfusion. Transplant Proc 2005; 37:3865–3866.
- Taura P, Garcia-Valdecasas JC, Beltran J, et al. Moderate primary pulmonary hypertension in patients undergoing liver transplantation. Anesthes Analges 1996; 83:675–680.
- Kuo PC, Schroeder RA, Vagelos RH, et al. Volume-mediated pulmonary responses in liver transplant candidates. Clin Transplant 1996; 10:521–527.
- Robalino BD, Moodie DS. Association between primary pulmonary hypertension and portal hypertension: analysis of its pathophysiology and clinical, laboratory and hemodynamic manifestations. J Am Coll Cardiol 1991; 17:492–498.
- Mandell MS, Krowka MJ. Formation of a national database on pulmonary hypertension and hepatopulmonary syndrome in chronic liver disease. Anesthesiology 1997; 87:450–451.
KEY POINTS
- In portopulmonary hypertension, the pulmonary artery pressures, pulmonary vascular resistance, and portal venous pressure are all elevated.
- All candidates for liver transplantation should undergo echocardiography to screen for portopulmonary hypertension. If the echocardiogram shows elevated pulmonary pressures, right heart catheterization must be performed to confirm the diagnosis.
- The ideal medical regimen remains to be determined. Although drug treatment may lower pulmonary artery pressures in selected patients so that liver transplantation can be safely done, morbidity and mortality rates remain higher in patients with moderate to severe portopulmonary hypertension.
- Liver transplantation is not the treatment of choice for portopulmonary hypertension.
Should all patients with chronic kidney disease take a statin?
We think some patients with chronic kidney disease should take a statin, particularly those in stages 1 through 4 (ie, not yet on dialysis1)* who have low-density lipoprotein cholesterol (LDL-C) levels higher than 100 mg/dL. However, few studies have addressed this question.
*Stages of chronic kidney disease1:
Stage 1—kidney damage with normal or high glomerular filtration rate (GFR ≥ 90 mL/min/1.73 m2)
Stage 2—kidney damage with mildly decreased GFR (60–89 mL/min/1.73 m2)
Stage 3—moderately decreased GFR (30–59 mL/min/1.73 m2)
Stage 4—severely decreased GFR (15–29 mL/min/1.73 m2)
Stage 5—kidney failure (GFR < 15 mL/min/1.73 m2 or dialysis)
The answer is murkier in patients on dialysis. Only one study has been done in this population, and it found no benefit from statin therapy. However, we would prescribe a statin for a dialysis patient who had known coronary artery disease and an LDL-C level higher than 100 mg/dL.
RATIONALE FOR STATIN USE: KIDNEY PATIENTS ARE AT RISK
Cardiovascular disease is common among patients with chronic kidney disease. While the risks of cardiovascular disease and death are highest among those requiring dialysis, earlier stages of chronic kidney disease also are associated with cardiovascular disease.2–4
The prevalence of traditional risk factors, particularly diabetes and hypertension, is high in all stages of kidney disease, and dyslipidemia is extremely common. Patients with chronic kidney disease who are not on dialysis tend to have lower levels of high-density lipoprotein cholesterol and higher levels of triglycerides, lipoprotein remnants, lipoprotein(a), and LDL-C. The lipid profile of dialysis patients is more complex, as malnutrition and inflammation in this population may lead to low cholesterol levels.
Since statins are effective for primary and secondary prevention of cardiovascular events in those in the general population with high LDL-C,5 we could expect that the same holds true for patients with chronic kidney disease. Furthermore, if kidney disease were considered a coronary heart disease equivalent, more than 85% of those with stage 3, 4, or 5 disease would qualify for lipid-lowering therapy by LDL-C criteria.6
However, compared with the large body of evidence in those without kidney disease, we have few data on the effect of statins on cardiovascular outcomes in those with kidney disease. Five of seven major trials of statins excluded patients with chronic kidney disease by using a creatinine cutoff or by excluding patients with known kidney disease.7
Renoprotective effects
Besides their cardiovascular effects, statins may slow the progression of kidney disease.
A subgroup analysis of the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) trial8 showed a 12% increase in creatinine clearance in the group receiving atorvastatin (Lipitor) (P = .0001). In comparison, creatinine clearance decreased by 4% in the placebo group.
A subgroup analysis of the Cholesterol and Recurrent Events (CARE) trial, a secondary prevention trial of pravastatin (Pravachol) vs placebo, showed a similar effect for patients with a glomerular filtration rate (GFR) less than 60 mL/min/1.73 m2 at baseline.9
A meta-analysis of 27 randomized trials (39,704 participants) concluded that, compared with no treatment, statins slowed the loss of GFR by a mean of 1.22 mL/min/year (95% confidence interval 0.44–2.0).10
Statins may confer this benefit independently of lipid-lowering. These drugs seem to decrease proteinuria, possibly by improving endothelial function or decreasing inflammation.11 A meta-analysis (1,384 patients) noted that 13 of 15 published studies found an antiproteinuric effect, with a greater effect in those with greater baseline proteinuria.12
The Prospective Evaluation of Proteinuria and Renal Function in Diabetic Patients With Progressive Renal Disease Trial (PLANET) will enroll 345 diabetic patients with protein-uria and hypercholesterolemia and examine the effects of rosuvastatin (Crestor) and atorvastatin on proteinuria and GFR.13
Cardioprotective effects in stages 1–4
Since patients with chronic kidney disease were excluded from most of the major statin trials, the best evidence in those with non-dialysis-dependent disease comes from post hoc analysis of data from the CARE study.14 While this trial excluded patients with more than 2+ proteinuria on dipstick analysis and those with creatinine values greater than 1.5 times the upper limit of normal, 1,711 of the initial 4,159 patients had a creatinine clearance of less than 75 mL/min; the mean creatinine clearance in this subgroup was 61. In this subgroup, pravastatin therapy was associated with a significantly lower risk of cardiovascular death or recurrent nonfatal myocardial infarction (MI) (hazard ratio 0.72, P < 0.05).
Similarly, in the 4,491 patients with chronic kidney disease (mean GFR 55 mL/min/1.73 m2) in the Pravastatin Pooling Project, the hazard of new MI, cardiovascular death, or cardiac intervention was nearly 25% lower in the pravastatin group.15
The ongoing Study of Heart and Renal Protection (SHARP),16 a randomized trial of ezetimibe/simvastatin (Vytorin) that enrolled 6,000 people with stages 3 to 4 kidney disease and 3,000 dialysis patients, will help in determining whether statin therapy prevents new vascular events. The study was launched in 2003 and has now completed enrollment. The primary outcome measure will be the time to first vascular event; secondary analyses will address whether statins decrease proteinuria or slow the progression of kidney disease.
Cardioprotective effects in dialysis patients
The only major randomized trial of statins ever conducted in dialysis patients with diabetes, the German Diabetes and Dialysis Study (4D), did not find atorvastatin 20 mg to have any benefit compared with placebo in reducing a composite end point of death from cardiac causes, stroke, and nonfatal MI over a median of 4 years of follow-up, despite a decrease in LDL-C of over 40% in the treatment group.17 Adverse events were similar in the two groups. The lack of a detectable benefit may be due to differences in the cardiovascular milieu in dialysis patients, who may have more advanced disease, with preexisting cardiac remodeling and congestive heart failure, which may not be modified to the same extent by statin therapy. Alternatively, the dose of atorvastatin may have been too low, or 4 years of treatment may not be sufficient to detect a benefit in these patients.
An ongoing prospective, randomized, placebo-controlled trial in 3,000 hemodialysis patients, called A Study to Evaluate the Use of Rosuvastatin in Subjects on Regular Haemodialysis: an Assessment of Survival and Cardiovascular Events (AURORA),18 will help to clarify the role of statins in this population.
CONCLUSION
The National Kidney Foundation guidelines1,19 note that people with chronic kidney disease are at high risk of cardiovascular disease and therefore should be treated according to guidelines for treating traditional risk factors in high-risk groups. We believe that those with dyslipidemia who are in stages 1 through 4, particularly those with other risk factors for coronary heart disease, should receive a statin, with an LDL-C target of less than 100 mg/dL, even though we have few data from large trials focused on this population and even though LDL-C may not be the only reason to consider statin use. The pleiotropic effects of statins on proteinuria and progression of kidney function loss may be of benefit in this population as well, although we would not recommend starting a statin solely for these effects until more data are available.
Despite the negative results of the 4D trial, given the relative safety of statins and the lack of any trial data suggesting harm in patients with chronic kidney disease, in our practice we treat dialysis patients with known cardiovascular disease with a statin, with a target LDL-C level less than 100 mg/dL. In dialysis patients without known cardiovascular disease, the use of a statin is even more controversial, and decisions should be made on an individual basis.
Results from the SHARP, AURORA, and PLANET trials, each of which is focused on patients with chronic kidney disease, will help determine whether statins benefit patients at this stage of disease.
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39 suppl 1:S1–S266.
- Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med 2005; 352:2049–2060.
- Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 1998; 32 suppl 3:S112–S119.
- Manjunath G, Tighiouart H, Coresh J, et al. Level of kidney function as a risk factor for cardiovascular outcomes in the elderly. Kidney Int 2003; 63:1121–1129.
- Baigent C, Keech A, Kearney PM, et al Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005; 366:1267–1278.
- Hyre AD, Fox CS, Astor BC, Cohen AJ, Muntner P. The impact of reclassifying moderate CKD as a coronary heart disease risk equivalent on the number of US adults recommended lipid-lowering treatment. Am J Kidney Dis 2007; 49:37–45.
- Coca SG, Krumholz HM, Garg AX, Parikh CR. Underrepresentation of renal disease in randomized controlled trials of cardiovascular disease. JAMA 2006; 296:1377–1384.
- Athyros VG, Mikhailidis DP, Papageorgiou AA, et al. The effect of statins versus untreated dyslipidaemia on renal function in patients with coronary heart disease. A subgroup analysis of the Greek atorvastatin and coronary heart disease evaluation (GREACE) study. J Clin Pathol 2004; 57:728–734.
- Tonelli M, Moye L, Sacks FM, Cole T, Curhan GC Cholesterol and Recurrent Events Trial Investigators. Effect of pravastatin on loss of renal function in people with moderate chronic renal insufficiency and cardiovascular disease. J Am Soc Nephrol 2003; 14:1605–1613.
- Sandhu S, Wiebe N, Fried LF, Tonelli M. Statins for improving renal outcomes: a meta-analysis. J Am Soc Nephrol 2006; 17:2006–2016.
- Balk EM, Lau J, Goudas LC, et al. Effects of statins on nonlipid serum markers associated with cardiovascular disease: a systematic review. Ann Intern Med 2003; 139:670–682.
- Douglas K, O’Malley PG, Jackson JL. Meta-analysis: the effect of statins on albuminuria. Ann Intern Med 2006; 145:117–124.
- US National Institutes of Health. Prospective Evaluation of Proteinuria and Renal Function in Diabetic Patients with Progressive Renal Disease (PLANET 1). Accessed December 6, 2007. www.clinicaltrials.gov/ct/show/NCT00296374?order=1.
- Tonelli M, Moye L, Sacks FM, Kiberd B, Curhan G Cholesterol and Recurrent Events (CARE) Trial Investigators. Pravastatin for secondary prevention of cardiovascular events in persons with mild chronic renal insufficiency. Ann Intern Med 2003; 138:98–104.
- Tonelli M, Isles C, Curhan GC, et al. Effect of pravastatin on cardiovascular events in people with chronic kidney disease. Circulation 2004; 110:1557–1563.
- Baigent C, Landry M. Study of Heart and Renal Protection (SHARP). Kidney Int Suppl 2003; 84:S207–S210.
- Wanner C, Krane V, Marz W, et al German Diabetes and Dialysis Study Investigators. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med 2005; 353:238–248.
- Fellstrom BC, Holdaas H, Jardine AG. Why do we need a statin trial in hemodialysis patients? Kidney Int 2003; 84 suppl:S204–S206.
- KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. Am J Kidney Dis 2007; 49 suppl 2:S12–154.
We think some patients with chronic kidney disease should take a statin, particularly those in stages 1 through 4 (ie, not yet on dialysis1)* who have low-density lipoprotein cholesterol (LDL-C) levels higher than 100 mg/dL. However, few studies have addressed this question.
*Stages of chronic kidney disease1:
Stage 1—kidney damage with normal or high glomerular filtration rate (GFR ≥ 90 mL/min/1.73 m2)
Stage 2—kidney damage with mildly decreased GFR (60–89 mL/min/1.73 m2)
Stage 3—moderately decreased GFR (30–59 mL/min/1.73 m2)
Stage 4—severely decreased GFR (15–29 mL/min/1.73 m2)
Stage 5—kidney failure (GFR < 15 mL/min/1.73 m2 or dialysis)
The answer is murkier in patients on dialysis. Only one study has been done in this population, and it found no benefit from statin therapy. However, we would prescribe a statin for a dialysis patient who had known coronary artery disease and an LDL-C level higher than 100 mg/dL.
RATIONALE FOR STATIN USE: KIDNEY PATIENTS ARE AT RISK
Cardiovascular disease is common among patients with chronic kidney disease. While the risks of cardiovascular disease and death are highest among those requiring dialysis, earlier stages of chronic kidney disease also are associated with cardiovascular disease.2–4
The prevalence of traditional risk factors, particularly diabetes and hypertension, is high in all stages of kidney disease, and dyslipidemia is extremely common. Patients with chronic kidney disease who are not on dialysis tend to have lower levels of high-density lipoprotein cholesterol and higher levels of triglycerides, lipoprotein remnants, lipoprotein(a), and LDL-C. The lipid profile of dialysis patients is more complex, as malnutrition and inflammation in this population may lead to low cholesterol levels.
Since statins are effective for primary and secondary prevention of cardiovascular events in those in the general population with high LDL-C,5 we could expect that the same holds true for patients with chronic kidney disease. Furthermore, if kidney disease were considered a coronary heart disease equivalent, more than 85% of those with stage 3, 4, or 5 disease would qualify for lipid-lowering therapy by LDL-C criteria.6
However, compared with the large body of evidence in those without kidney disease, we have few data on the effect of statins on cardiovascular outcomes in those with kidney disease. Five of seven major trials of statins excluded patients with chronic kidney disease by using a creatinine cutoff or by excluding patients with known kidney disease.7
Renoprotective effects
Besides their cardiovascular effects, statins may slow the progression of kidney disease.
A subgroup analysis of the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) trial8 showed a 12% increase in creatinine clearance in the group receiving atorvastatin (Lipitor) (P = .0001). In comparison, creatinine clearance decreased by 4% in the placebo group.
A subgroup analysis of the Cholesterol and Recurrent Events (CARE) trial, a secondary prevention trial of pravastatin (Pravachol) vs placebo, showed a similar effect for patients with a glomerular filtration rate (GFR) less than 60 mL/min/1.73 m2 at baseline.9
A meta-analysis of 27 randomized trials (39,704 participants) concluded that, compared with no treatment, statins slowed the loss of GFR by a mean of 1.22 mL/min/year (95% confidence interval 0.44–2.0).10
Statins may confer this benefit independently of lipid-lowering. These drugs seem to decrease proteinuria, possibly by improving endothelial function or decreasing inflammation.11 A meta-analysis (1,384 patients) noted that 13 of 15 published studies found an antiproteinuric effect, with a greater effect in those with greater baseline proteinuria.12
The Prospective Evaluation of Proteinuria and Renal Function in Diabetic Patients With Progressive Renal Disease Trial (PLANET) will enroll 345 diabetic patients with protein-uria and hypercholesterolemia and examine the effects of rosuvastatin (Crestor) and atorvastatin on proteinuria and GFR.13
Cardioprotective effects in stages 1–4
Since patients with chronic kidney disease were excluded from most of the major statin trials, the best evidence in those with non-dialysis-dependent disease comes from post hoc analysis of data from the CARE study.14 While this trial excluded patients with more than 2+ proteinuria on dipstick analysis and those with creatinine values greater than 1.5 times the upper limit of normal, 1,711 of the initial 4,159 patients had a creatinine clearance of less than 75 mL/min; the mean creatinine clearance in this subgroup was 61. In this subgroup, pravastatin therapy was associated with a significantly lower risk of cardiovascular death or recurrent nonfatal myocardial infarction (MI) (hazard ratio 0.72, P < 0.05).
Similarly, in the 4,491 patients with chronic kidney disease (mean GFR 55 mL/min/1.73 m2) in the Pravastatin Pooling Project, the hazard of new MI, cardiovascular death, or cardiac intervention was nearly 25% lower in the pravastatin group.15
The ongoing Study of Heart and Renal Protection (SHARP),16 a randomized trial of ezetimibe/simvastatin (Vytorin) that enrolled 6,000 people with stages 3 to 4 kidney disease and 3,000 dialysis patients, will help in determining whether statin therapy prevents new vascular events. The study was launched in 2003 and has now completed enrollment. The primary outcome measure will be the time to first vascular event; secondary analyses will address whether statins decrease proteinuria or slow the progression of kidney disease.
Cardioprotective effects in dialysis patients
The only major randomized trial of statins ever conducted in dialysis patients with diabetes, the German Diabetes and Dialysis Study (4D), did not find atorvastatin 20 mg to have any benefit compared with placebo in reducing a composite end point of death from cardiac causes, stroke, and nonfatal MI over a median of 4 years of follow-up, despite a decrease in LDL-C of over 40% in the treatment group.17 Adverse events were similar in the two groups. The lack of a detectable benefit may be due to differences in the cardiovascular milieu in dialysis patients, who may have more advanced disease, with preexisting cardiac remodeling and congestive heart failure, which may not be modified to the same extent by statin therapy. Alternatively, the dose of atorvastatin may have been too low, or 4 years of treatment may not be sufficient to detect a benefit in these patients.
An ongoing prospective, randomized, placebo-controlled trial in 3,000 hemodialysis patients, called A Study to Evaluate the Use of Rosuvastatin in Subjects on Regular Haemodialysis: an Assessment of Survival and Cardiovascular Events (AURORA),18 will help to clarify the role of statins in this population.
CONCLUSION
The National Kidney Foundation guidelines1,19 note that people with chronic kidney disease are at high risk of cardiovascular disease and therefore should be treated according to guidelines for treating traditional risk factors in high-risk groups. We believe that those with dyslipidemia who are in stages 1 through 4, particularly those with other risk factors for coronary heart disease, should receive a statin, with an LDL-C target of less than 100 mg/dL, even though we have few data from large trials focused on this population and even though LDL-C may not be the only reason to consider statin use. The pleiotropic effects of statins on proteinuria and progression of kidney function loss may be of benefit in this population as well, although we would not recommend starting a statin solely for these effects until more data are available.
Despite the negative results of the 4D trial, given the relative safety of statins and the lack of any trial data suggesting harm in patients with chronic kidney disease, in our practice we treat dialysis patients with known cardiovascular disease with a statin, with a target LDL-C level less than 100 mg/dL. In dialysis patients without known cardiovascular disease, the use of a statin is even more controversial, and decisions should be made on an individual basis.
Results from the SHARP, AURORA, and PLANET trials, each of which is focused on patients with chronic kidney disease, will help determine whether statins benefit patients at this stage of disease.
We think some patients with chronic kidney disease should take a statin, particularly those in stages 1 through 4 (ie, not yet on dialysis1)* who have low-density lipoprotein cholesterol (LDL-C) levels higher than 100 mg/dL. However, few studies have addressed this question.
*Stages of chronic kidney disease1:
Stage 1—kidney damage with normal or high glomerular filtration rate (GFR ≥ 90 mL/min/1.73 m2)
Stage 2—kidney damage with mildly decreased GFR (60–89 mL/min/1.73 m2)
Stage 3—moderately decreased GFR (30–59 mL/min/1.73 m2)
Stage 4—severely decreased GFR (15–29 mL/min/1.73 m2)
Stage 5—kidney failure (GFR < 15 mL/min/1.73 m2 or dialysis)
The answer is murkier in patients on dialysis. Only one study has been done in this population, and it found no benefit from statin therapy. However, we would prescribe a statin for a dialysis patient who had known coronary artery disease and an LDL-C level higher than 100 mg/dL.
RATIONALE FOR STATIN USE: KIDNEY PATIENTS ARE AT RISK
Cardiovascular disease is common among patients with chronic kidney disease. While the risks of cardiovascular disease and death are highest among those requiring dialysis, earlier stages of chronic kidney disease also are associated with cardiovascular disease.2–4
The prevalence of traditional risk factors, particularly diabetes and hypertension, is high in all stages of kidney disease, and dyslipidemia is extremely common. Patients with chronic kidney disease who are not on dialysis tend to have lower levels of high-density lipoprotein cholesterol and higher levels of triglycerides, lipoprotein remnants, lipoprotein(a), and LDL-C. The lipid profile of dialysis patients is more complex, as malnutrition and inflammation in this population may lead to low cholesterol levels.
Since statins are effective for primary and secondary prevention of cardiovascular events in those in the general population with high LDL-C,5 we could expect that the same holds true for patients with chronic kidney disease. Furthermore, if kidney disease were considered a coronary heart disease equivalent, more than 85% of those with stage 3, 4, or 5 disease would qualify for lipid-lowering therapy by LDL-C criteria.6
However, compared with the large body of evidence in those without kidney disease, we have few data on the effect of statins on cardiovascular outcomes in those with kidney disease. Five of seven major trials of statins excluded patients with chronic kidney disease by using a creatinine cutoff or by excluding patients with known kidney disease.7
Renoprotective effects
Besides their cardiovascular effects, statins may slow the progression of kidney disease.
A subgroup analysis of the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) trial8 showed a 12% increase in creatinine clearance in the group receiving atorvastatin (Lipitor) (P = .0001). In comparison, creatinine clearance decreased by 4% in the placebo group.
A subgroup analysis of the Cholesterol and Recurrent Events (CARE) trial, a secondary prevention trial of pravastatin (Pravachol) vs placebo, showed a similar effect for patients with a glomerular filtration rate (GFR) less than 60 mL/min/1.73 m2 at baseline.9
A meta-analysis of 27 randomized trials (39,704 participants) concluded that, compared with no treatment, statins slowed the loss of GFR by a mean of 1.22 mL/min/year (95% confidence interval 0.44–2.0).10
Statins may confer this benefit independently of lipid-lowering. These drugs seem to decrease proteinuria, possibly by improving endothelial function or decreasing inflammation.11 A meta-analysis (1,384 patients) noted that 13 of 15 published studies found an antiproteinuric effect, with a greater effect in those with greater baseline proteinuria.12
The Prospective Evaluation of Proteinuria and Renal Function in Diabetic Patients With Progressive Renal Disease Trial (PLANET) will enroll 345 diabetic patients with protein-uria and hypercholesterolemia and examine the effects of rosuvastatin (Crestor) and atorvastatin on proteinuria and GFR.13
Cardioprotective effects in stages 1–4
Since patients with chronic kidney disease were excluded from most of the major statin trials, the best evidence in those with non-dialysis-dependent disease comes from post hoc analysis of data from the CARE study.14 While this trial excluded patients with more than 2+ proteinuria on dipstick analysis and those with creatinine values greater than 1.5 times the upper limit of normal, 1,711 of the initial 4,159 patients had a creatinine clearance of less than 75 mL/min; the mean creatinine clearance in this subgroup was 61. In this subgroup, pravastatin therapy was associated with a significantly lower risk of cardiovascular death or recurrent nonfatal myocardial infarction (MI) (hazard ratio 0.72, P < 0.05).
Similarly, in the 4,491 patients with chronic kidney disease (mean GFR 55 mL/min/1.73 m2) in the Pravastatin Pooling Project, the hazard of new MI, cardiovascular death, or cardiac intervention was nearly 25% lower in the pravastatin group.15
The ongoing Study of Heart and Renal Protection (SHARP),16 a randomized trial of ezetimibe/simvastatin (Vytorin) that enrolled 6,000 people with stages 3 to 4 kidney disease and 3,000 dialysis patients, will help in determining whether statin therapy prevents new vascular events. The study was launched in 2003 and has now completed enrollment. The primary outcome measure will be the time to first vascular event; secondary analyses will address whether statins decrease proteinuria or slow the progression of kidney disease.
Cardioprotective effects in dialysis patients
The only major randomized trial of statins ever conducted in dialysis patients with diabetes, the German Diabetes and Dialysis Study (4D), did not find atorvastatin 20 mg to have any benefit compared with placebo in reducing a composite end point of death from cardiac causes, stroke, and nonfatal MI over a median of 4 years of follow-up, despite a decrease in LDL-C of over 40% in the treatment group.17 Adverse events were similar in the two groups. The lack of a detectable benefit may be due to differences in the cardiovascular milieu in dialysis patients, who may have more advanced disease, with preexisting cardiac remodeling and congestive heart failure, which may not be modified to the same extent by statin therapy. Alternatively, the dose of atorvastatin may have been too low, or 4 years of treatment may not be sufficient to detect a benefit in these patients.
An ongoing prospective, randomized, placebo-controlled trial in 3,000 hemodialysis patients, called A Study to Evaluate the Use of Rosuvastatin in Subjects on Regular Haemodialysis: an Assessment of Survival and Cardiovascular Events (AURORA),18 will help to clarify the role of statins in this population.
CONCLUSION
The National Kidney Foundation guidelines1,19 note that people with chronic kidney disease are at high risk of cardiovascular disease and therefore should be treated according to guidelines for treating traditional risk factors in high-risk groups. We believe that those with dyslipidemia who are in stages 1 through 4, particularly those with other risk factors for coronary heart disease, should receive a statin, with an LDL-C target of less than 100 mg/dL, even though we have few data from large trials focused on this population and even though LDL-C may not be the only reason to consider statin use. The pleiotropic effects of statins on proteinuria and progression of kidney function loss may be of benefit in this population as well, although we would not recommend starting a statin solely for these effects until more data are available.
Despite the negative results of the 4D trial, given the relative safety of statins and the lack of any trial data suggesting harm in patients with chronic kidney disease, in our practice we treat dialysis patients with known cardiovascular disease with a statin, with a target LDL-C level less than 100 mg/dL. In dialysis patients without known cardiovascular disease, the use of a statin is even more controversial, and decisions should be made on an individual basis.
Results from the SHARP, AURORA, and PLANET trials, each of which is focused on patients with chronic kidney disease, will help determine whether statins benefit patients at this stage of disease.
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39 suppl 1:S1–S266.
- Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med 2005; 352:2049–2060.
- Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 1998; 32 suppl 3:S112–S119.
- Manjunath G, Tighiouart H, Coresh J, et al. Level of kidney function as a risk factor for cardiovascular outcomes in the elderly. Kidney Int 2003; 63:1121–1129.
- Baigent C, Keech A, Kearney PM, et al Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005; 366:1267–1278.
- Hyre AD, Fox CS, Astor BC, Cohen AJ, Muntner P. The impact of reclassifying moderate CKD as a coronary heart disease risk equivalent on the number of US adults recommended lipid-lowering treatment. Am J Kidney Dis 2007; 49:37–45.
- Coca SG, Krumholz HM, Garg AX, Parikh CR. Underrepresentation of renal disease in randomized controlled trials of cardiovascular disease. JAMA 2006; 296:1377–1384.
- Athyros VG, Mikhailidis DP, Papageorgiou AA, et al. The effect of statins versus untreated dyslipidaemia on renal function in patients with coronary heart disease. A subgroup analysis of the Greek atorvastatin and coronary heart disease evaluation (GREACE) study. J Clin Pathol 2004; 57:728–734.
- Tonelli M, Moye L, Sacks FM, Cole T, Curhan GC Cholesterol and Recurrent Events Trial Investigators. Effect of pravastatin on loss of renal function in people with moderate chronic renal insufficiency and cardiovascular disease. J Am Soc Nephrol 2003; 14:1605–1613.
- Sandhu S, Wiebe N, Fried LF, Tonelli M. Statins for improving renal outcomes: a meta-analysis. J Am Soc Nephrol 2006; 17:2006–2016.
- Balk EM, Lau J, Goudas LC, et al. Effects of statins on nonlipid serum markers associated with cardiovascular disease: a systematic review. Ann Intern Med 2003; 139:670–682.
- Douglas K, O’Malley PG, Jackson JL. Meta-analysis: the effect of statins on albuminuria. Ann Intern Med 2006; 145:117–124.
- US National Institutes of Health. Prospective Evaluation of Proteinuria and Renal Function in Diabetic Patients with Progressive Renal Disease (PLANET 1). Accessed December 6, 2007. www.clinicaltrials.gov/ct/show/NCT00296374?order=1.
- Tonelli M, Moye L, Sacks FM, Kiberd B, Curhan G Cholesterol and Recurrent Events (CARE) Trial Investigators. Pravastatin for secondary prevention of cardiovascular events in persons with mild chronic renal insufficiency. Ann Intern Med 2003; 138:98–104.
- Tonelli M, Isles C, Curhan GC, et al. Effect of pravastatin on cardiovascular events in people with chronic kidney disease. Circulation 2004; 110:1557–1563.
- Baigent C, Landry M. Study of Heart and Renal Protection (SHARP). Kidney Int Suppl 2003; 84:S207–S210.
- Wanner C, Krane V, Marz W, et al German Diabetes and Dialysis Study Investigators. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med 2005; 353:238–248.
- Fellstrom BC, Holdaas H, Jardine AG. Why do we need a statin trial in hemodialysis patients? Kidney Int 2003; 84 suppl:S204–S206.
- KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. Am J Kidney Dis 2007; 49 suppl 2:S12–154.
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39 suppl 1:S1–S266.
- Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med 2005; 352:2049–2060.
- Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 1998; 32 suppl 3:S112–S119.
- Manjunath G, Tighiouart H, Coresh J, et al. Level of kidney function as a risk factor for cardiovascular outcomes in the elderly. Kidney Int 2003; 63:1121–1129.
- Baigent C, Keech A, Kearney PM, et al Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005; 366:1267–1278.
- Hyre AD, Fox CS, Astor BC, Cohen AJ, Muntner P. The impact of reclassifying moderate CKD as a coronary heart disease risk equivalent on the number of US adults recommended lipid-lowering treatment. Am J Kidney Dis 2007; 49:37–45.
- Coca SG, Krumholz HM, Garg AX, Parikh CR. Underrepresentation of renal disease in randomized controlled trials of cardiovascular disease. JAMA 2006; 296:1377–1384.
- Athyros VG, Mikhailidis DP, Papageorgiou AA, et al. The effect of statins versus untreated dyslipidaemia on renal function in patients with coronary heart disease. A subgroup analysis of the Greek atorvastatin and coronary heart disease evaluation (GREACE) study. J Clin Pathol 2004; 57:728–734.
- Tonelli M, Moye L, Sacks FM, Cole T, Curhan GC Cholesterol and Recurrent Events Trial Investigators. Effect of pravastatin on loss of renal function in people with moderate chronic renal insufficiency and cardiovascular disease. J Am Soc Nephrol 2003; 14:1605–1613.
- Sandhu S, Wiebe N, Fried LF, Tonelli M. Statins for improving renal outcomes: a meta-analysis. J Am Soc Nephrol 2006; 17:2006–2016.
- Balk EM, Lau J, Goudas LC, et al. Effects of statins on nonlipid serum markers associated with cardiovascular disease: a systematic review. Ann Intern Med 2003; 139:670–682.
- Douglas K, O’Malley PG, Jackson JL. Meta-analysis: the effect of statins on albuminuria. Ann Intern Med 2006; 145:117–124.
- US National Institutes of Health. Prospective Evaluation of Proteinuria and Renal Function in Diabetic Patients with Progressive Renal Disease (PLANET 1). Accessed December 6, 2007. www.clinicaltrials.gov/ct/show/NCT00296374?order=1.
- Tonelli M, Moye L, Sacks FM, Kiberd B, Curhan G Cholesterol and Recurrent Events (CARE) Trial Investigators. Pravastatin for secondary prevention of cardiovascular events in persons with mild chronic renal insufficiency. Ann Intern Med 2003; 138:98–104.
- Tonelli M, Isles C, Curhan GC, et al. Effect of pravastatin on cardiovascular events in people with chronic kidney disease. Circulation 2004; 110:1557–1563.
- Baigent C, Landry M. Study of Heart and Renal Protection (SHARP). Kidney Int Suppl 2003; 84:S207–S210.
- Wanner C, Krane V, Marz W, et al German Diabetes and Dialysis Study Investigators. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med 2005; 353:238–248.
- Fellstrom BC, Holdaas H, Jardine AG. Why do we need a statin trial in hemodialysis patients? Kidney Int 2003; 84 suppl:S204–S206.
- KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. Am J Kidney Dis 2007; 49 suppl 2:S12–154.