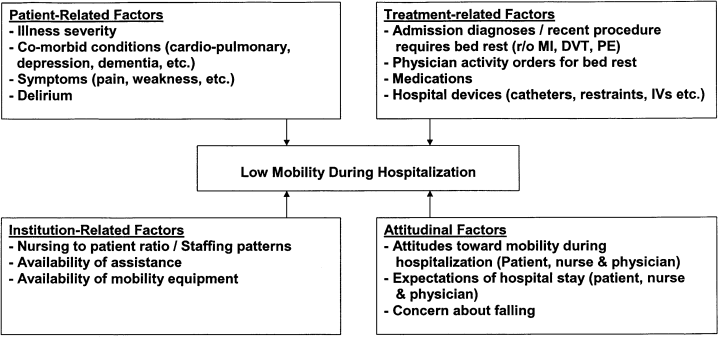
User login
Does marijuana use play a role in the recreational use of sildenafil?
- There is a strong association between men who use cannabis and men who obtain sildenafil from sources other than a prescribing physician.
Purpose This study examined the ways by which patients obtain nonprescription sildenafil and the patient predictors associated with nonprescribed use.
Methods We conducted this descriptive study via questionnaire-guided interviews with 231 male sildenafil users (ages 18 to 80) between December 1, 2002 and April 30, 2003 at outpatient Family Medicine and Urology Clinics at The Brooklyn Hospital Center, Brooklyn, NY. Patients were divided into 2 groups: those with erectile dysfunction (reported by the patients as defined by their physician) and those without.
Results The prevalence of erectile dysfunction in our total study population of sildenafil users (n=231) was 40.3% (n=93); 59.7% (n=138) did not have erectile dysfunction. Of those without erectile dysfunction, 76.1% (n=105) admitted to cannabis use, compared with 7.5% (n=7) of the subjects with erectile dysfunction. Patients without erectile dysfunction and history of cannabis abuse reported obtaining sildenafil from friends and street vendors significantly more often than non-cannabis users with erectile dysfunction (54.3%, n=57 vs 9.3%, n=8; P<.0001).
Conclusion Illicit use of cannabis is a strong predictor of recreational sildenafil use among patients without erectile dysfunction.
Published reports of improved sexual performance have prompted men without erectile dysfunction to use sildenafil inappropriately.1-4 Sildenafil has also been used to counteract the impotence-inducing effects of “club drugs” such as ecstasy.5
Cannabis, another widely abused street drug, is a known inhibitor of the cytochrome P450 3A4 isoenzyme pathway, the same pathway in which sildenafil is metabolized.6 Cannabis can thus potentiate the effect of sildenafil. A case report from 2002 has indicated that a young man using sildenafil and cannabis concomitantly suffered a myocardial infarction.7
In this study, we sought to answer the following questions:
- What methods did the men use to obtain sildenafil without a prescription?
- Why were the men taking sildenafil?
- Did these men increase the dose without physician supervision?
Methods
Setting, participants, and design
Two senior resident physicians from the Department of Family Practice at The Brooklyn Hospital Center asked male patients between the ages of 18 and 80 if they would be interested in participating in this descriptive study. Two hundred and thirty-one patients agreed to be interviewed during their outpatient clinic appointments in the Departments of Family Practice and Urology. We conducted these interviews between December 1, 2002 and April 30, 2003.
Patients with and without physician-diagnosed erectile dysfunction who were using sildenafil were included in this study. Patients were excluded if they were taking nitrates, had cognitive disabilities, were female, or if they could not read English.
The 1-page, 35-item questionnaire was read to the subjects by a resident, who provided additional explanations if needed. The researchers noted their responses to questions on demographics, medical history, social history, treatment duration of erectile dysfunction, method of procurement of sildenafil, and knowledge about the indications of sildenafil. The questionnaire was pretested on a small sample for comprehension prior to distribution.
We developed descriptive statistics and performed cross-tabulations using SPSS version 11.0 (SPSS, Inc, Chicago, Ill). We used a chi-square test to determine statistical significance between cannabis abuse and illicit sildenafil use. We established statistical significance at P<.05. The Institutional Review Board at the Brooklyn Hospital Center approved our research protocol, and we obtained consent from all the study participants.
Results
Strong link between cannabis use and recreational sildenafil
TABLE 1 shows the demographic information of the entire study population (n=231). Our study found that patient predictors for recreational sildenafil use are a younger unmarried male who smokes cannabis. Of the men in the study, 138 (59.7%) reported erectile function prior to the use of sildenafil.
We decided to examine data from this subgroup of our study population. As shown in TABLE 2, patients with erectile function but with a history of cannabis abuse reported obtaining sildenafil from friends, street vendors, and the Internet significantly more often than those with erectile dysfunction who did not use cannabis.
Discussion: Is there a danger?
Our study showed a strong association between individuals who obtained sildenafil from sources other than a prescribing physician and those who used cannabis. These men purchased this prescription medication from street vendors, friends, family, or via the Internet. Illicit sildenafil users took the medication mainly to improve performance and increase desire–and they often increased the dose of the medication at will. The differences between patients with erectile dysfunction compared with those without were so great that much of our data proved to be statistically significant (TABLE 1).
Data from our subgroup of patients who smoked cannabis supported our suspicion that patients who were able to maintain an erection prior to their use of sildenafil used the medication to improve sexual performance and counteract alterations in libido caused by cannabis.8
TABLE 1
Our study group: Who they were, why they were taking sildenafil
| ALL SUBJECTS (N=231) | WITH ED (N=93) | WITHOUTED (N=138) | P-VALUE | |
|---|---|---|---|---|
| DEMOGRAPHICS | ||||
| Age (years) | ||||
| 18–30 | 17 (7.4%) | 2 (2.2%) | 15 (10.9%) | .03 |
| 31–40 | 56 (24.2%) | 1 (1.1%) | 55 (39.9%) | <.0001 |
| 41–50 | 68 (29.4%) | 19 (20.4%) | 49 (35.5%) | .02 |
| 51–60 | 38 (16.5%) | 25 (26.9%) | 13 (9.4%) | .001 |
| 61–70 | 35 (15.2%) | 29 (31.2%) | 6 (4.3%) | <.0001 |
| 71–80 | 17 (7.4%) | 17 (18.3%) | 0 (0.0%) | <.0001 |
| Health insurance | ||||
| Self-pay | 23 (10%) | 4 (4.3%) | 19 (13.8%) | .03 |
| Medicaid | 73 (31.6%) | 30 (32.3%) | 43 (31.2%) | .97 |
| Medicare | 17 (7.4%) | 15 (16.1%) | 2 (1.4%) | <.0001 |
| Private carriers | 118 (51.1%) | 44 (47.3%) | 74 (53.6%) | <.42 |
| Marital status | ||||
| Married | 107 (46.3%) | 56 (60.2%) | 51 (37.0%) | .001 |
| Unmarried | 92 (39.8%) | 14 (15.1%) | 78 (56.5%) | <.0001 |
| Divorced | 32 (13.9%) | 23 (24.7%) | 9 (6.5%) | <.0001 |
| Drug use | ||||
| Yes | 121 (52.4%) | 9 (9.7%) | 112 (81.2%) | <.0001 |
| No | 110 (47.6%) | 84 (90.3%) | 26 (18.8%) | |
| Marijuana use | ||||
| Yes | 112 (48.5%) | 7 (7.5%) | 105 (76.1%) | <.0001 |
| No | 119 (51.5%) | 86 (92.5%) | 33 (23.9%) | <.0001 |
| USE OF SILDENAFIL | ||||
| Purchased from a friend/street vendor | ||||
| Yes | 149 (64.5%) | 26 (28.0%) | 123 (89.1%) | <.0001 |
| No | 82 (35.5%) | 67 (72.0%) | 15 (10.9%) | |
| Sold to a friend | ||||
| Yes | 72 (31.2%) | 7 (7.5%) | 65 (47.1%) | <.0001 |
| No | 159 (68.8%) | 86 (92.5%) | 73 (52.9%) | |
| Increased dose without physician authorization? | ||||
| Yes | 150 (64.9%) | 40 (43.0%) | 110 (79.7%) | <.0001 |
| No | 81 (35.1%) | 53 (57.0%) | 28 (20.3%) | |
| SEXUAL PROBLEM | ||||
| Lack of desire/interest | 14 (6.1%) | 2 (2.2%) | 12 (8.7%) | .08 |
| Lack of erection/difficulty in achieving erection | 82 (35.5%) | 69 (74.2%) | 13 (9.4%) | <.0001 |
| Difficulty in performance/endurance | 59 (25.5%) | 4 (4.3%) | 55 (39.9%) | <.0001 |
| Difficulty in orgasm/ejaculation | 14 (6.1%) | 3 (3.2%) | 11 (8.0%) | .22 |
| Lack of desire/lack of erection | 29 (12.6%) | 13 (14.0%) | 16 (11.6%) | .74 |
| Lack of desire+difficulty with performance | 33 (14.3%) | 2 (2.2%) | 31 (22.5%) | <.0001 |
| ED, erectile dysfunction | ||||
TABLE 2
Where did 2 subsets of subjects obtain sildenafil?
| SOURCE | NO ED/CANNABIS USERS (N=105) | ED/NON-CANNABIS USERS (N=86) | P-VALUE |
|---|---|---|---|
| PCP/specialist | 12 (11.4%) | 75 (87.2%) | <.0001 |
| Over-the-counter* | 8 (7.6%) | 2 (2.3%) | .19 |
| Friends/street vendors | 57 (54.3%) | 8 (9.3%) | <.0001 |
| Internet | 28 (26.7%) | 1 (1.2%) | <.0001 |
| * Purchased without a prescription from a privately owned business (such as a convenience store). | |||
Limitations of this study
The main limitation of this study was that the data obtained were self-reported. A chart review could have provided objective data on the patients’ ED diagnosis and medications.
Conclusion
The illicit use of sildenafil raises many issues. Patients with cardiovascular disease, even without the use of nitrates, may be at risk of myocardial infarction. Be aware that younger, male patient with an admitted history of drug abuse may be taking sildenafil without your knowledge, even without a diagnosis of erectile dysfunction.
Funding
Material support was provided by the Department of Family Medicine at The Brooklyn Hospital, Brooklyn, NY.
Acknowledgments
The contents of this manuscript were presented at the New York State Academy of Family Practice and The Albany County Chapter Regional Family Medicine Conference at Lake Placid, NY on September 6, 2003.
Correspondence
Marie L. Eloi-Stiven, MD, Director of Research, The Brooklyn Hospital Center, Department of Family Medicine, 121 Dekalb Ave, Brooklyn, NY 11201; dad9022@nyp.org
1. Crosby R, Diclemente RJ. Use of recreational sildenafil citrate among men having sex with men. Sex Transm Infect 2004;80:466-468.
2. Chu Pl, McFarland W, Gibson S, et al. Sildenafil citrate use in a community-recruited sample of men who have sex with men, San Francisco. J Acquir Immune Defic Syndr 2003;33:191-193.
3. Modaini N, Ponchietti R, Muir GH. Sildenafil citrate does not improve sexual function in men without erectile dysfunction but does reduce the postorgasmic refractory time. Int J Impot Res 2003;15:225-228.
4. Romanelli F, Smith KM. Recreational use of sildenafil citrate by HIV-positive and negative homosexual/bisexual males. Ann Pharmacother 2004;38:1024-1030.Epub 2004 Apr 27.
5. Breslau K. The “sextasy” craze. Clubland’s dangerous party mix: Viagra and ecstasy. Newsweek. 2002 Jun 3;139(22):30.-
6. McLeod AL, McKenna CJ, Northridge DB. Myocardial Infaraction following the combined recreational use of viagra and cannabis. Clin Cardiol 2002;25:133-134.
7. El-Galley R, Rutland H, Talic R, Keane T, Clark H. Long-term efficacy of sildenafil and tachyphylaxis effect. J Urol 2001;166:927-931.
8. Hubbard JR, Franco SE, Onaivi ES. Marijuana: medical implications. Am Fam Physician 1999;60:2583-2599.
- There is a strong association between men who use cannabis and men who obtain sildenafil from sources other than a prescribing physician.
Purpose This study examined the ways by which patients obtain nonprescription sildenafil and the patient predictors associated with nonprescribed use.
Methods We conducted this descriptive study via questionnaire-guided interviews with 231 male sildenafil users (ages 18 to 80) between December 1, 2002 and April 30, 2003 at outpatient Family Medicine and Urology Clinics at The Brooklyn Hospital Center, Brooklyn, NY. Patients were divided into 2 groups: those with erectile dysfunction (reported by the patients as defined by their physician) and those without.
Results The prevalence of erectile dysfunction in our total study population of sildenafil users (n=231) was 40.3% (n=93); 59.7% (n=138) did not have erectile dysfunction. Of those without erectile dysfunction, 76.1% (n=105) admitted to cannabis use, compared with 7.5% (n=7) of the subjects with erectile dysfunction. Patients without erectile dysfunction and history of cannabis abuse reported obtaining sildenafil from friends and street vendors significantly more often than non-cannabis users with erectile dysfunction (54.3%, n=57 vs 9.3%, n=8; P<.0001).
Conclusion Illicit use of cannabis is a strong predictor of recreational sildenafil use among patients without erectile dysfunction.
Published reports of improved sexual performance have prompted men without erectile dysfunction to use sildenafil inappropriately.1-4 Sildenafil has also been used to counteract the impotence-inducing effects of “club drugs” such as ecstasy.5
Cannabis, another widely abused street drug, is a known inhibitor of the cytochrome P450 3A4 isoenzyme pathway, the same pathway in which sildenafil is metabolized.6 Cannabis can thus potentiate the effect of sildenafil. A case report from 2002 has indicated that a young man using sildenafil and cannabis concomitantly suffered a myocardial infarction.7
In this study, we sought to answer the following questions:
- What methods did the men use to obtain sildenafil without a prescription?
- Why were the men taking sildenafil?
- Did these men increase the dose without physician supervision?
Methods
Setting, participants, and design
Two senior resident physicians from the Department of Family Practice at The Brooklyn Hospital Center asked male patients between the ages of 18 and 80 if they would be interested in participating in this descriptive study. Two hundred and thirty-one patients agreed to be interviewed during their outpatient clinic appointments in the Departments of Family Practice and Urology. We conducted these interviews between December 1, 2002 and April 30, 2003.
Patients with and without physician-diagnosed erectile dysfunction who were using sildenafil were included in this study. Patients were excluded if they were taking nitrates, had cognitive disabilities, were female, or if they could not read English.
The 1-page, 35-item questionnaire was read to the subjects by a resident, who provided additional explanations if needed. The researchers noted their responses to questions on demographics, medical history, social history, treatment duration of erectile dysfunction, method of procurement of sildenafil, and knowledge about the indications of sildenafil. The questionnaire was pretested on a small sample for comprehension prior to distribution.
We developed descriptive statistics and performed cross-tabulations using SPSS version 11.0 (SPSS, Inc, Chicago, Ill). We used a chi-square test to determine statistical significance between cannabis abuse and illicit sildenafil use. We established statistical significance at P<.05. The Institutional Review Board at the Brooklyn Hospital Center approved our research protocol, and we obtained consent from all the study participants.
Results
Strong link between cannabis use and recreational sildenafil
TABLE 1 shows the demographic information of the entire study population (n=231). Our study found that patient predictors for recreational sildenafil use are a younger unmarried male who smokes cannabis. Of the men in the study, 138 (59.7%) reported erectile function prior to the use of sildenafil.
We decided to examine data from this subgroup of our study population. As shown in TABLE 2, patients with erectile function but with a history of cannabis abuse reported obtaining sildenafil from friends, street vendors, and the Internet significantly more often than those with erectile dysfunction who did not use cannabis.
Discussion: Is there a danger?
Our study showed a strong association between individuals who obtained sildenafil from sources other than a prescribing physician and those who used cannabis. These men purchased this prescription medication from street vendors, friends, family, or via the Internet. Illicit sildenafil users took the medication mainly to improve performance and increase desire–and they often increased the dose of the medication at will. The differences between patients with erectile dysfunction compared with those without were so great that much of our data proved to be statistically significant (TABLE 1).
Data from our subgroup of patients who smoked cannabis supported our suspicion that patients who were able to maintain an erection prior to their use of sildenafil used the medication to improve sexual performance and counteract alterations in libido caused by cannabis.8
TABLE 1
Our study group: Who they were, why they were taking sildenafil
| ALL SUBJECTS (N=231) | WITH ED (N=93) | WITHOUTED (N=138) | P-VALUE | |
|---|---|---|---|---|
| DEMOGRAPHICS | ||||
| Age (years) | ||||
| 18–30 | 17 (7.4%) | 2 (2.2%) | 15 (10.9%) | .03 |
| 31–40 | 56 (24.2%) | 1 (1.1%) | 55 (39.9%) | <.0001 |
| 41–50 | 68 (29.4%) | 19 (20.4%) | 49 (35.5%) | .02 |
| 51–60 | 38 (16.5%) | 25 (26.9%) | 13 (9.4%) | .001 |
| 61–70 | 35 (15.2%) | 29 (31.2%) | 6 (4.3%) | <.0001 |
| 71–80 | 17 (7.4%) | 17 (18.3%) | 0 (0.0%) | <.0001 |
| Health insurance | ||||
| Self-pay | 23 (10%) | 4 (4.3%) | 19 (13.8%) | .03 |
| Medicaid | 73 (31.6%) | 30 (32.3%) | 43 (31.2%) | .97 |
| Medicare | 17 (7.4%) | 15 (16.1%) | 2 (1.4%) | <.0001 |
| Private carriers | 118 (51.1%) | 44 (47.3%) | 74 (53.6%) | <.42 |
| Marital status | ||||
| Married | 107 (46.3%) | 56 (60.2%) | 51 (37.0%) | .001 |
| Unmarried | 92 (39.8%) | 14 (15.1%) | 78 (56.5%) | <.0001 |
| Divorced | 32 (13.9%) | 23 (24.7%) | 9 (6.5%) | <.0001 |
| Drug use | ||||
| Yes | 121 (52.4%) | 9 (9.7%) | 112 (81.2%) | <.0001 |
| No | 110 (47.6%) | 84 (90.3%) | 26 (18.8%) | |
| Marijuana use | ||||
| Yes | 112 (48.5%) | 7 (7.5%) | 105 (76.1%) | <.0001 |
| No | 119 (51.5%) | 86 (92.5%) | 33 (23.9%) | <.0001 |
| USE OF SILDENAFIL | ||||
| Purchased from a friend/street vendor | ||||
| Yes | 149 (64.5%) | 26 (28.0%) | 123 (89.1%) | <.0001 |
| No | 82 (35.5%) | 67 (72.0%) | 15 (10.9%) | |
| Sold to a friend | ||||
| Yes | 72 (31.2%) | 7 (7.5%) | 65 (47.1%) | <.0001 |
| No | 159 (68.8%) | 86 (92.5%) | 73 (52.9%) | |
| Increased dose without physician authorization? | ||||
| Yes | 150 (64.9%) | 40 (43.0%) | 110 (79.7%) | <.0001 |
| No | 81 (35.1%) | 53 (57.0%) | 28 (20.3%) | |
| SEXUAL PROBLEM | ||||
| Lack of desire/interest | 14 (6.1%) | 2 (2.2%) | 12 (8.7%) | .08 |
| Lack of erection/difficulty in achieving erection | 82 (35.5%) | 69 (74.2%) | 13 (9.4%) | <.0001 |
| Difficulty in performance/endurance | 59 (25.5%) | 4 (4.3%) | 55 (39.9%) | <.0001 |
| Difficulty in orgasm/ejaculation | 14 (6.1%) | 3 (3.2%) | 11 (8.0%) | .22 |
| Lack of desire/lack of erection | 29 (12.6%) | 13 (14.0%) | 16 (11.6%) | .74 |
| Lack of desire+difficulty with performance | 33 (14.3%) | 2 (2.2%) | 31 (22.5%) | <.0001 |
| ED, erectile dysfunction | ||||
TABLE 2
Where did 2 subsets of subjects obtain sildenafil?
| SOURCE | NO ED/CANNABIS USERS (N=105) | ED/NON-CANNABIS USERS (N=86) | P-VALUE |
|---|---|---|---|
| PCP/specialist | 12 (11.4%) | 75 (87.2%) | <.0001 |
| Over-the-counter* | 8 (7.6%) | 2 (2.3%) | .19 |
| Friends/street vendors | 57 (54.3%) | 8 (9.3%) | <.0001 |
| Internet | 28 (26.7%) | 1 (1.2%) | <.0001 |
| * Purchased without a prescription from a privately owned business (such as a convenience store). | |||
Limitations of this study
The main limitation of this study was that the data obtained were self-reported. A chart review could have provided objective data on the patients’ ED diagnosis and medications.
Conclusion
The illicit use of sildenafil raises many issues. Patients with cardiovascular disease, even without the use of nitrates, may be at risk of myocardial infarction. Be aware that younger, male patient with an admitted history of drug abuse may be taking sildenafil without your knowledge, even without a diagnosis of erectile dysfunction.
Funding
Material support was provided by the Department of Family Medicine at The Brooklyn Hospital, Brooklyn, NY.
Acknowledgments
The contents of this manuscript were presented at the New York State Academy of Family Practice and The Albany County Chapter Regional Family Medicine Conference at Lake Placid, NY on September 6, 2003.
Correspondence
Marie L. Eloi-Stiven, MD, Director of Research, The Brooklyn Hospital Center, Department of Family Medicine, 121 Dekalb Ave, Brooklyn, NY 11201; dad9022@nyp.org
- There is a strong association between men who use cannabis and men who obtain sildenafil from sources other than a prescribing physician.
Purpose This study examined the ways by which patients obtain nonprescription sildenafil and the patient predictors associated with nonprescribed use.
Methods We conducted this descriptive study via questionnaire-guided interviews with 231 male sildenafil users (ages 18 to 80) between December 1, 2002 and April 30, 2003 at outpatient Family Medicine and Urology Clinics at The Brooklyn Hospital Center, Brooklyn, NY. Patients were divided into 2 groups: those with erectile dysfunction (reported by the patients as defined by their physician) and those without.
Results The prevalence of erectile dysfunction in our total study population of sildenafil users (n=231) was 40.3% (n=93); 59.7% (n=138) did not have erectile dysfunction. Of those without erectile dysfunction, 76.1% (n=105) admitted to cannabis use, compared with 7.5% (n=7) of the subjects with erectile dysfunction. Patients without erectile dysfunction and history of cannabis abuse reported obtaining sildenafil from friends and street vendors significantly more often than non-cannabis users with erectile dysfunction (54.3%, n=57 vs 9.3%, n=8; P<.0001).
Conclusion Illicit use of cannabis is a strong predictor of recreational sildenafil use among patients without erectile dysfunction.
Published reports of improved sexual performance have prompted men without erectile dysfunction to use sildenafil inappropriately.1-4 Sildenafil has also been used to counteract the impotence-inducing effects of “club drugs” such as ecstasy.5
Cannabis, another widely abused street drug, is a known inhibitor of the cytochrome P450 3A4 isoenzyme pathway, the same pathway in which sildenafil is metabolized.6 Cannabis can thus potentiate the effect of sildenafil. A case report from 2002 has indicated that a young man using sildenafil and cannabis concomitantly suffered a myocardial infarction.7
In this study, we sought to answer the following questions:
- What methods did the men use to obtain sildenafil without a prescription?
- Why were the men taking sildenafil?
- Did these men increase the dose without physician supervision?
Methods
Setting, participants, and design
Two senior resident physicians from the Department of Family Practice at The Brooklyn Hospital Center asked male patients between the ages of 18 and 80 if they would be interested in participating in this descriptive study. Two hundred and thirty-one patients agreed to be interviewed during their outpatient clinic appointments in the Departments of Family Practice and Urology. We conducted these interviews between December 1, 2002 and April 30, 2003.
Patients with and without physician-diagnosed erectile dysfunction who were using sildenafil were included in this study. Patients were excluded if they were taking nitrates, had cognitive disabilities, were female, or if they could not read English.
The 1-page, 35-item questionnaire was read to the subjects by a resident, who provided additional explanations if needed. The researchers noted their responses to questions on demographics, medical history, social history, treatment duration of erectile dysfunction, method of procurement of sildenafil, and knowledge about the indications of sildenafil. The questionnaire was pretested on a small sample for comprehension prior to distribution.
We developed descriptive statistics and performed cross-tabulations using SPSS version 11.0 (SPSS, Inc, Chicago, Ill). We used a chi-square test to determine statistical significance between cannabis abuse and illicit sildenafil use. We established statistical significance at P<.05. The Institutional Review Board at the Brooklyn Hospital Center approved our research protocol, and we obtained consent from all the study participants.
Results
Strong link between cannabis use and recreational sildenafil
TABLE 1 shows the demographic information of the entire study population (n=231). Our study found that patient predictors for recreational sildenafil use are a younger unmarried male who smokes cannabis. Of the men in the study, 138 (59.7%) reported erectile function prior to the use of sildenafil.
We decided to examine data from this subgroup of our study population. As shown in TABLE 2, patients with erectile function but with a history of cannabis abuse reported obtaining sildenafil from friends, street vendors, and the Internet significantly more often than those with erectile dysfunction who did not use cannabis.
Discussion: Is there a danger?
Our study showed a strong association between individuals who obtained sildenafil from sources other than a prescribing physician and those who used cannabis. These men purchased this prescription medication from street vendors, friends, family, or via the Internet. Illicit sildenafil users took the medication mainly to improve performance and increase desire–and they often increased the dose of the medication at will. The differences between patients with erectile dysfunction compared with those without were so great that much of our data proved to be statistically significant (TABLE 1).
Data from our subgroup of patients who smoked cannabis supported our suspicion that patients who were able to maintain an erection prior to their use of sildenafil used the medication to improve sexual performance and counteract alterations in libido caused by cannabis.8
TABLE 1
Our study group: Who they were, why they were taking sildenafil
| ALL SUBJECTS (N=231) | WITH ED (N=93) | WITHOUTED (N=138) | P-VALUE | |
|---|---|---|---|---|
| DEMOGRAPHICS | ||||
| Age (years) | ||||
| 18–30 | 17 (7.4%) | 2 (2.2%) | 15 (10.9%) | .03 |
| 31–40 | 56 (24.2%) | 1 (1.1%) | 55 (39.9%) | <.0001 |
| 41–50 | 68 (29.4%) | 19 (20.4%) | 49 (35.5%) | .02 |
| 51–60 | 38 (16.5%) | 25 (26.9%) | 13 (9.4%) | .001 |
| 61–70 | 35 (15.2%) | 29 (31.2%) | 6 (4.3%) | <.0001 |
| 71–80 | 17 (7.4%) | 17 (18.3%) | 0 (0.0%) | <.0001 |
| Health insurance | ||||
| Self-pay | 23 (10%) | 4 (4.3%) | 19 (13.8%) | .03 |
| Medicaid | 73 (31.6%) | 30 (32.3%) | 43 (31.2%) | .97 |
| Medicare | 17 (7.4%) | 15 (16.1%) | 2 (1.4%) | <.0001 |
| Private carriers | 118 (51.1%) | 44 (47.3%) | 74 (53.6%) | <.42 |
| Marital status | ||||
| Married | 107 (46.3%) | 56 (60.2%) | 51 (37.0%) | .001 |
| Unmarried | 92 (39.8%) | 14 (15.1%) | 78 (56.5%) | <.0001 |
| Divorced | 32 (13.9%) | 23 (24.7%) | 9 (6.5%) | <.0001 |
| Drug use | ||||
| Yes | 121 (52.4%) | 9 (9.7%) | 112 (81.2%) | <.0001 |
| No | 110 (47.6%) | 84 (90.3%) | 26 (18.8%) | |
| Marijuana use | ||||
| Yes | 112 (48.5%) | 7 (7.5%) | 105 (76.1%) | <.0001 |
| No | 119 (51.5%) | 86 (92.5%) | 33 (23.9%) | <.0001 |
| USE OF SILDENAFIL | ||||
| Purchased from a friend/street vendor | ||||
| Yes | 149 (64.5%) | 26 (28.0%) | 123 (89.1%) | <.0001 |
| No | 82 (35.5%) | 67 (72.0%) | 15 (10.9%) | |
| Sold to a friend | ||||
| Yes | 72 (31.2%) | 7 (7.5%) | 65 (47.1%) | <.0001 |
| No | 159 (68.8%) | 86 (92.5%) | 73 (52.9%) | |
| Increased dose without physician authorization? | ||||
| Yes | 150 (64.9%) | 40 (43.0%) | 110 (79.7%) | <.0001 |
| No | 81 (35.1%) | 53 (57.0%) | 28 (20.3%) | |
| SEXUAL PROBLEM | ||||
| Lack of desire/interest | 14 (6.1%) | 2 (2.2%) | 12 (8.7%) | .08 |
| Lack of erection/difficulty in achieving erection | 82 (35.5%) | 69 (74.2%) | 13 (9.4%) | <.0001 |
| Difficulty in performance/endurance | 59 (25.5%) | 4 (4.3%) | 55 (39.9%) | <.0001 |
| Difficulty in orgasm/ejaculation | 14 (6.1%) | 3 (3.2%) | 11 (8.0%) | .22 |
| Lack of desire/lack of erection | 29 (12.6%) | 13 (14.0%) | 16 (11.6%) | .74 |
| Lack of desire+difficulty with performance | 33 (14.3%) | 2 (2.2%) | 31 (22.5%) | <.0001 |
| ED, erectile dysfunction | ||||
TABLE 2
Where did 2 subsets of subjects obtain sildenafil?
| SOURCE | NO ED/CANNABIS USERS (N=105) | ED/NON-CANNABIS USERS (N=86) | P-VALUE |
|---|---|---|---|
| PCP/specialist | 12 (11.4%) | 75 (87.2%) | <.0001 |
| Over-the-counter* | 8 (7.6%) | 2 (2.3%) | .19 |
| Friends/street vendors | 57 (54.3%) | 8 (9.3%) | <.0001 |
| Internet | 28 (26.7%) | 1 (1.2%) | <.0001 |
| * Purchased without a prescription from a privately owned business (such as a convenience store). | |||
Limitations of this study
The main limitation of this study was that the data obtained were self-reported. A chart review could have provided objective data on the patients’ ED diagnosis and medications.
Conclusion
The illicit use of sildenafil raises many issues. Patients with cardiovascular disease, even without the use of nitrates, may be at risk of myocardial infarction. Be aware that younger, male patient with an admitted history of drug abuse may be taking sildenafil without your knowledge, even without a diagnosis of erectile dysfunction.
Funding
Material support was provided by the Department of Family Medicine at The Brooklyn Hospital, Brooklyn, NY.
Acknowledgments
The contents of this manuscript were presented at the New York State Academy of Family Practice and The Albany County Chapter Regional Family Medicine Conference at Lake Placid, NY on September 6, 2003.
Correspondence
Marie L. Eloi-Stiven, MD, Director of Research, The Brooklyn Hospital Center, Department of Family Medicine, 121 Dekalb Ave, Brooklyn, NY 11201; dad9022@nyp.org
1. Crosby R, Diclemente RJ. Use of recreational sildenafil citrate among men having sex with men. Sex Transm Infect 2004;80:466-468.
2. Chu Pl, McFarland W, Gibson S, et al. Sildenafil citrate use in a community-recruited sample of men who have sex with men, San Francisco. J Acquir Immune Defic Syndr 2003;33:191-193.
3. Modaini N, Ponchietti R, Muir GH. Sildenafil citrate does not improve sexual function in men without erectile dysfunction but does reduce the postorgasmic refractory time. Int J Impot Res 2003;15:225-228.
4. Romanelli F, Smith KM. Recreational use of sildenafil citrate by HIV-positive and negative homosexual/bisexual males. Ann Pharmacother 2004;38:1024-1030.Epub 2004 Apr 27.
5. Breslau K. The “sextasy” craze. Clubland’s dangerous party mix: Viagra and ecstasy. Newsweek. 2002 Jun 3;139(22):30.-
6. McLeod AL, McKenna CJ, Northridge DB. Myocardial Infaraction following the combined recreational use of viagra and cannabis. Clin Cardiol 2002;25:133-134.
7. El-Galley R, Rutland H, Talic R, Keane T, Clark H. Long-term efficacy of sildenafil and tachyphylaxis effect. J Urol 2001;166:927-931.
8. Hubbard JR, Franco SE, Onaivi ES. Marijuana: medical implications. Am Fam Physician 1999;60:2583-2599.
1. Crosby R, Diclemente RJ. Use of recreational sildenafil citrate among men having sex with men. Sex Transm Infect 2004;80:466-468.
2. Chu Pl, McFarland W, Gibson S, et al. Sildenafil citrate use in a community-recruited sample of men who have sex with men, San Francisco. J Acquir Immune Defic Syndr 2003;33:191-193.
3. Modaini N, Ponchietti R, Muir GH. Sildenafil citrate does not improve sexual function in men without erectile dysfunction but does reduce the postorgasmic refractory time. Int J Impot Res 2003;15:225-228.
4. Romanelli F, Smith KM. Recreational use of sildenafil citrate by HIV-positive and negative homosexual/bisexual males. Ann Pharmacother 2004;38:1024-1030.Epub 2004 Apr 27.
5. Breslau K. The “sextasy” craze. Clubland’s dangerous party mix: Viagra and ecstasy. Newsweek. 2002 Jun 3;139(22):30.-
6. McLeod AL, McKenna CJ, Northridge DB. Myocardial Infaraction following the combined recreational use of viagra and cannabis. Clin Cardiol 2002;25:133-134.
7. El-Galley R, Rutland H, Talic R, Keane T, Clark H. Long-term efficacy of sildenafil and tachyphylaxis effect. J Urol 2001;166:927-931.
8. Hubbard JR, Franco SE, Onaivi ES. Marijuana: medical implications. Am Fam Physician 1999;60:2583-2599.
Stroke prevention: Age alone does not rule out warfarin
Warfarin is as safe as aspirin and more effective for stroke prevention in elders with atrial fibrillation
Strength of recommendation (SOR)
A: Well-designed randomized controlled trial of elderly patients in the primary care setting, consistent with findings from prior RCTs
Mant et al. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study [BAFTA]): a randomised controlled trial. Lancet 2007;370:493–503.1
Illustrative Case
An 85-year-old woman with hypertension and chronic atrial fibrillation has transferred her care to you. She takes an aspirin a day for cardiovascular prevention. You know that warfarin is better than aspirin for preventing stroke but worry about the increased risk of bleeding with warfarin.
Should you recommend that she stay on aspirin or switch to warfarin?
Background: BAFTA: A realistic study
We have been reluctant to use warfarin in elders with atrial fibrillation for good reason: risk of hemorrhage. Since there are few trials looking at use of warfarin among elders in primary care settings, we are uncertain about the balance of benefits and harms.
The BAFTA study1 is the first trial to compare outcomes of warfarin vs aspirin in elders specifically, in the less-than-ideal conditions of real life.
Guidelines mirror uncertainties
This uncertainty is reflected even in guidelines for anticoagulation in elderly patients with atrial fibrillation.
- The 2004 American College of Chest Physicians Seventh Conference on Antithrombotic and Thrombolytic Therapy recommends treating all patients with atrial fibrillation and high risk of stroke with warfarin. Their definition of high-risk includes any patient with 1 or more of the following risk factors: age >75 years, prior ischemic stroke, transient ischemic attack or systemic embolism, congestive heart failure, impaired left ventricular systolic function, hypertension, or diabetes mellitus.2
- In contrast, the 2006 guidelines for the management of patients with atrial fibrillation from the American College of Cardiology, American Heart Association, and European Society of Cardiology, are more conservative. They recommend that patients with more than 1 risk factor take warfarin, and patients with only 1 risk factor (for example, a patient older than 75 years of age with no other risk factors) take either warfarin or aspirin.3
Clinical context: Reasonable concerns
Fewer than half of the 10% to 12% of people older than 75 with atrial fibrillation are taking warfarin for stroke prevention. In one study, only 35% of patients 85 years and older with no known contraindication to anticoagulation received warfarin.4 Possible reasons for this low rate include:
- cost of monitoring warfarin
- concerns about compliance
- increased risk of hemorrhage
- prior studies focused on younger patients, in closely monitored settings.
These factors lead us to speculate that many physicians believe that the risks of warfarin in elderly patients in primary care settings outweigh any potential benefit.
We think this study demonstrates that we should seriously discuss and consider warfarin therapy for most of our elderly patients with atrial fibrillation.
The key finding from the BAFTA study is that advanced age alone is not a contraindication to the use of warfarin for stroke prevention in elderly patients with atrial fibrillation
Study summary: Primary care setting, elders only
This prospective randomized open-label trial was designed to test the effectiveness and safety of warfarin vs aspirin in the elderly, in a realistic primary care setting. The study compared the frequency of stroke, intracranial hemorrhage, and other significant arterial embolism in patients taking either warfarin or aspirin.
Inclusion criteria. Patients were at least 75 years old (average 81.5 years) with an ECG within the previous 2 years showing atrial fibrillation or atrial flutter. Seventy percent of the patients had been previously diagnosed with atrial fibrillation and 30% were identified because they had an irregular pulse on exam.
Exclusion criteria included rheumatic heart disease, major nontraumatic hemorrhage in the past 5 years, intracranial hemorrhage, endoscopically proven peptic ulcer disease in the past year, esophageal varices, allergy to either study drug, terminal illness, surgery in past 3 months, blood pressure greater than 180/110 mm Hg, or if the primary physician judged that a patient should either be on warfarin or not, based on risk factors.
Patient characteristics. The patients were recruited from 260 general practices in England and Wales. At baseline, 39% to 40% of the patients were already taking warfarin, 12% to 13% had had a prior stroke, 53% to 55% had hypertension, 13% to 14% had diabetes, 19% to 20% had heart failure, and 10% to 12% had a history of myocardial infarction. Patients were followed for an average of 2.7 years.
Aspirin and warfarin regimens. Patients were assigned to either aspirin at a dose of 75 mg/day or warfarin with a target international normalized ratio (INR) of 2.5 and an acceptable range of 2 to 3. Because the study aimed to reflect a realistic primary care setting, the frequency and method of INR testing was left to the discretion of participating physicians.
Patients who had been taking aspirin or warfarin prior to the study discontinued that medicine if they were assigned to the other treatment. Sixty-seven percent of the patients assigned to warfarin continued this treatment throughout the study, and 78% of those who either stopped taking warfarin or never started it were put on either aspirin or clopidogrel. Seventy-six percent of the patients assigned to aspirin took the medicine for the entire study period, while 70% of those who stopped taking aspirin or never started it were either switched to or stayed on warfarin.
INR values. Patients on warfarin had INR values between 2.0 and 3.0 for 67% of the time, below range for 19%, of the time, and above range for 14% of the time. Twenty-two percent of practices had all components of INR monitoring done at the hospital (phlebotomy, INR analysis, and warfarin dosing), 19% of the practices completed all 3 components on site, and the remaining practices had various combinations of onsite and hospital monitoring.
The primary outcomes included disabling stroke (ischemic or hemorrhagic) or clinically significant arterial embolism. There were 24 primary events (1.8% per year) in patients assigned to warfarin compared with 48 primary events (3.8% per year) in those assigned to aspirin, with a relative risk of 0.48 (95% confidence interval [CI], 0.28–0.80 (TABLE). The number needed to treat for 1 year to prevent 1 primary event was 50, when warfarin was compared to aspirin. Warfarin was superior to aspirin in all subgroup analyses, including patients over 85 years old.
Secondary outcomes. There were no significant differences between the warfarin and aspirin groups in the secondary outcomes: hospital admission or death as a result of a non-stroke vascular event (6.1% risk per year with warfarin vs 6.3% risk per year with aspirin), all-cause mortality (8.0% vs 8.4%), and major extracranial hemorrhage (1.4% vs 1.6%). Patients assigned to warfarin, including the subgroup of patients older than 85, did not have an increased risk of a major hemorrhage when compared with those assigned to aspirin (1.9% risk per year with warfarin vs 2.0% risk per year with aspirin; relative risk=0.96; 95% CI, 0.53–1.75).1
TABLE
BAFTA study: Warfarin was as safe as aspirin and more effective in preventing stroke in the elderly
| WARFARIN (488 patients) | ASPIRIN (485 patients) | ||||
|---|---|---|---|---|---|
| PRIMARY EVENTS | Total events | Risk per year | Total events | Risk per year | WARFARIN VA ASPIRIN |
| Stroke | 21 | 1.6% | 44 | 3.4% | RR=0.46 (95% CI, 0.26–0.79) P=.003 |
| Stroke, other intracranial hemorrhage, or systemic embolism | 24 | 1.8% | 48 | 3.8% | RR=0.48 (95% CI, 0.28–0.80) P=.003 |
| RR, relative risk; CI, confidence interval. | |||||
| Source: Mant J, Hobbs FD, Fletcher K et al. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet 2007;370:493-503. | |||||
What’s new?: Age alone does not preclude warfarin
The key finding from the BAFTA study is that advanced age alone is not a contraindication to the use of warfarin for stroke prevention in elderly patients with atrial fibrillation.
This is the first randomized controlled trial of warfarin for atrial fibrillation that included only patients ages 75 and older, conducted in a primary care setting.5
Limitations of earlier studies. The most recent meta-analysis of antithrombotic therapy for stroke prevention in patients with atrial fibrillation included 29 trials with 28,044 patients. This analysis concluded that although both warfarin and aspirin are effective in reducing the risk of stroke in patients with atrial fibrillation (warfarin by 60% and aspirin by 20%), warfarin was more effective than aspirin (relative risk reduction of 39%), with very small (≤0.3% per year) absolute increases in major extracranial hemorrhage.
The average age of patients in those trials, however, was 71. The authors identified the lack of data on older patients (who are at higher risk for serious bleeding events) as a limitation of the meta-analysis. Many of these trials took place in settings with closer monitoring of INR and warfarin dosing than is customary in a primary care setting.5
Caveats: Consider the evidence on benefits and risks
Major bleeding from warfarin is a concern, especially in the elderly. A recent cohort study6 (summarized as a POEM in this journal7) reported high rates of major bleeding (13.1 per hundred person-years or 13.1%) in patients ≥80 years of age during their first year of warfarin therapy. Despite the high risk of bleeding events in this cohort study, there was considerable benefit from warfarin therapy.
None of the patients who remained on warfarin had a thrombotic stroke (personal communication with Dr Hylek by the author). The expected rate of thrombotic stroke is in the range of 5% to 6% per year in this high-risk group.
Furthermore, most of the bleeding events were gastrointestinal and did not lead to catastrophic outcomes.
Do not add warfarin to aspirin in patients >75 years
Dr Hylek also noted that 40% of the patients in their cohort study were taking both warfarin and aspirin, and, although her study did not have sufficient power to detect a difference, prior studies noted increased risk of bleeding with this combination compared to warfarin alone.8,9 For this reason we think the combination of warfarin and aspirin should be avoided in patients over 75.
Target INR <3
Our caveat is the same as the POEM author’s conclusion:7 Patients over 80 should be carefully monitored to keep the INR below 3.0 or for signs of bleeding, especially in the first 90 days of therapy when bleeding is more likely to occur.
A final point that the BAFTA authors make, which is worth repeating here, is that the prior studies showing an increased risk of bleeding complications had INR target rates of 4 to 5, whereas the target in this study was 2 to 3. Two previous studies that also compared aspirin to warfarin with an INR goal of 2 to 3 similarly showed no difference in major bleeding between the 2 groups.10,11
Challenges to Implementation: Meticulous monitoring, patient education
- Managing warfarin therapy requires meticulous care to avoid complications and optimize treatment effect.
- Patients may be reluctant to take warfarin because they may fear bleeding.
- Patients who do agree to take warfarin need education about possible medication interactions, the need for regular INR monitoring, dosage changes, and dietary issues (eg, maintaining a consistent intake of foods containing vitamin K).
Contraindications
Contraindications to the use of warfarin include hypersensitivity to warfarin, severe hepatic disease, alcoholism, recent trauma or surgery, history of falling or significant risk of falls, and active gastrointestinal, respiratory, or genitourinary bleeding.
INR testing systems
Several randomized trials support the use of monitoring systems such as a pharmacist managed anticoagulation service or decision support software, both of which can improve the percentage of patients with therapeutic INR values.12,13
Using point-of-care INR tests in the office provides immediate results which allow for more timely adjustments of warfarin dose.14
PURLs methodology
This study was selected and evaluated using the Family Physician Inquiries Network’s Priority Updates from the Research Literature Surveillance System (PURLs) methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed here.
1. Mant J, Hobbs FD, Fletcher K, et al. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet 2007;370:493-503.
2. Singer DE, Albers GW, Dalen JE, et al. Antithrombotic therapy in atrial fibrillation: The seventh ACCP (American College of Chest Physicians) conference on antithrombotic and thrombolytic therapy. Chest 2004;126:429S-456S.
3. Fuster V, Rydén LE, Cannom DS, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation). J Am Coll Cardiol 2006;48:854-906.
4. Go AS, Hylek EM, Borowsky LH, et al. Warfarin use among ambulatory patients with non-valvular atrial fibrillation: The Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study. Ann Intern Med 1999;131:927.-
5. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: Antithrombotic therapy to prevent stroke in patients who have non-valvular atrial fibrillation. Ann Intern Med 2007;146:857-867.
6. Hylek EM, Evans-Molina C, Shea C, et al. Major hemorrhage and tolerability of warfarin in the first year of therapy among elderly patients with atrial fibrillation. Circulation 2007;115:2689-2696.
7. POEM: Bleeding risk with warfarin is high among the elderly. J Fam Pract 2007;6:709.-
8. Garcia D, Hylek E. Stroke prevention in elderly patients with atrial fibrillation. Lancet 2007;370:460-461.
9. Perez-Gomez F, Alegria E, Bejon J, et al. Comparative effects of antiplatelet, anticoagulant, or combined therapy in patients with valvular and non-valvular atrial fibrillation: a randomized multicenter study. J Am Coll Cardiol 2004;44:1557-1556.
10. Rash A, Downes T, Portner R, et al. A randomized controlled trial of warfarin versus aspirin for stroke preventions in octogenarians with atrial fibrillation (WASPO). Age Ageing 2007;36:151-156.
11. Gullov AL, Koeford BG, Petersen P, et al. Fixed minidose warfarin and aspirin alone and in combination vs adjusted-dose warfarin for stroke prevention in atrial fibrillation. Arch Intern Med 1998;158:1513-1521.
12. Witt DM, Sadler MA, Shanahan RL, et al. Effect of a centralized clinical pharmacy anticoagulation service on outcomes of anticoagulation therapy. Chest 2005;127:1515-1522.
13. Wurster M, Doran T. Anticoagulation management: a new approach. Disease Management 2006;9:201-209.
14. Dorfman DM, Goonan EM, Boutilier MK, et al. Point-of-care (POC) versus central laboratory instrumentation for monitoring oral anticoagulation. Vasc Med 2005;10:23-27.
Warfarin is as safe as aspirin and more effective for stroke prevention in elders with atrial fibrillation
Strength of recommendation (SOR)
A: Well-designed randomized controlled trial of elderly patients in the primary care setting, consistent with findings from prior RCTs
Mant et al. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study [BAFTA]): a randomised controlled trial. Lancet 2007;370:493–503.1
Illustrative Case
An 85-year-old woman with hypertension and chronic atrial fibrillation has transferred her care to you. She takes an aspirin a day for cardiovascular prevention. You know that warfarin is better than aspirin for preventing stroke but worry about the increased risk of bleeding with warfarin.
Should you recommend that she stay on aspirin or switch to warfarin?
Background: BAFTA: A realistic study
We have been reluctant to use warfarin in elders with atrial fibrillation for good reason: risk of hemorrhage. Since there are few trials looking at use of warfarin among elders in primary care settings, we are uncertain about the balance of benefits and harms.
The BAFTA study1 is the first trial to compare outcomes of warfarin vs aspirin in elders specifically, in the less-than-ideal conditions of real life.
Guidelines mirror uncertainties
This uncertainty is reflected even in guidelines for anticoagulation in elderly patients with atrial fibrillation.
- The 2004 American College of Chest Physicians Seventh Conference on Antithrombotic and Thrombolytic Therapy recommends treating all patients with atrial fibrillation and high risk of stroke with warfarin. Their definition of high-risk includes any patient with 1 or more of the following risk factors: age >75 years, prior ischemic stroke, transient ischemic attack or systemic embolism, congestive heart failure, impaired left ventricular systolic function, hypertension, or diabetes mellitus.2
- In contrast, the 2006 guidelines for the management of patients with atrial fibrillation from the American College of Cardiology, American Heart Association, and European Society of Cardiology, are more conservative. They recommend that patients with more than 1 risk factor take warfarin, and patients with only 1 risk factor (for example, a patient older than 75 years of age with no other risk factors) take either warfarin or aspirin.3
Clinical context: Reasonable concerns
Fewer than half of the 10% to 12% of people older than 75 with atrial fibrillation are taking warfarin for stroke prevention. In one study, only 35% of patients 85 years and older with no known contraindication to anticoagulation received warfarin.4 Possible reasons for this low rate include:
- cost of monitoring warfarin
- concerns about compliance
- increased risk of hemorrhage
- prior studies focused on younger patients, in closely monitored settings.
These factors lead us to speculate that many physicians believe that the risks of warfarin in elderly patients in primary care settings outweigh any potential benefit.
We think this study demonstrates that we should seriously discuss and consider warfarin therapy for most of our elderly patients with atrial fibrillation.
The key finding from the BAFTA study is that advanced age alone is not a contraindication to the use of warfarin for stroke prevention in elderly patients with atrial fibrillation
Study summary: Primary care setting, elders only
This prospective randomized open-label trial was designed to test the effectiveness and safety of warfarin vs aspirin in the elderly, in a realistic primary care setting. The study compared the frequency of stroke, intracranial hemorrhage, and other significant arterial embolism in patients taking either warfarin or aspirin.
Inclusion criteria. Patients were at least 75 years old (average 81.5 years) with an ECG within the previous 2 years showing atrial fibrillation or atrial flutter. Seventy percent of the patients had been previously diagnosed with atrial fibrillation and 30% were identified because they had an irregular pulse on exam.
Exclusion criteria included rheumatic heart disease, major nontraumatic hemorrhage in the past 5 years, intracranial hemorrhage, endoscopically proven peptic ulcer disease in the past year, esophageal varices, allergy to either study drug, terminal illness, surgery in past 3 months, blood pressure greater than 180/110 mm Hg, or if the primary physician judged that a patient should either be on warfarin or not, based on risk factors.
Patient characteristics. The patients were recruited from 260 general practices in England and Wales. At baseline, 39% to 40% of the patients were already taking warfarin, 12% to 13% had had a prior stroke, 53% to 55% had hypertension, 13% to 14% had diabetes, 19% to 20% had heart failure, and 10% to 12% had a history of myocardial infarction. Patients were followed for an average of 2.7 years.
Aspirin and warfarin regimens. Patients were assigned to either aspirin at a dose of 75 mg/day or warfarin with a target international normalized ratio (INR) of 2.5 and an acceptable range of 2 to 3. Because the study aimed to reflect a realistic primary care setting, the frequency and method of INR testing was left to the discretion of participating physicians.
Patients who had been taking aspirin or warfarin prior to the study discontinued that medicine if they were assigned to the other treatment. Sixty-seven percent of the patients assigned to warfarin continued this treatment throughout the study, and 78% of those who either stopped taking warfarin or never started it were put on either aspirin or clopidogrel. Seventy-six percent of the patients assigned to aspirin took the medicine for the entire study period, while 70% of those who stopped taking aspirin or never started it were either switched to or stayed on warfarin.
INR values. Patients on warfarin had INR values between 2.0 and 3.0 for 67% of the time, below range for 19%, of the time, and above range for 14% of the time. Twenty-two percent of practices had all components of INR monitoring done at the hospital (phlebotomy, INR analysis, and warfarin dosing), 19% of the practices completed all 3 components on site, and the remaining practices had various combinations of onsite and hospital monitoring.
The primary outcomes included disabling stroke (ischemic or hemorrhagic) or clinically significant arterial embolism. There were 24 primary events (1.8% per year) in patients assigned to warfarin compared with 48 primary events (3.8% per year) in those assigned to aspirin, with a relative risk of 0.48 (95% confidence interval [CI], 0.28–0.80 (TABLE). The number needed to treat for 1 year to prevent 1 primary event was 50, when warfarin was compared to aspirin. Warfarin was superior to aspirin in all subgroup analyses, including patients over 85 years old.
Secondary outcomes. There were no significant differences between the warfarin and aspirin groups in the secondary outcomes: hospital admission or death as a result of a non-stroke vascular event (6.1% risk per year with warfarin vs 6.3% risk per year with aspirin), all-cause mortality (8.0% vs 8.4%), and major extracranial hemorrhage (1.4% vs 1.6%). Patients assigned to warfarin, including the subgroup of patients older than 85, did not have an increased risk of a major hemorrhage when compared with those assigned to aspirin (1.9% risk per year with warfarin vs 2.0% risk per year with aspirin; relative risk=0.96; 95% CI, 0.53–1.75).1
TABLE
BAFTA study: Warfarin was as safe as aspirin and more effective in preventing stroke in the elderly
| WARFARIN (488 patients) | ASPIRIN (485 patients) | ||||
|---|---|---|---|---|---|
| PRIMARY EVENTS | Total events | Risk per year | Total events | Risk per year | WARFARIN VA ASPIRIN |
| Stroke | 21 | 1.6% | 44 | 3.4% | RR=0.46 (95% CI, 0.26–0.79) P=.003 |
| Stroke, other intracranial hemorrhage, or systemic embolism | 24 | 1.8% | 48 | 3.8% | RR=0.48 (95% CI, 0.28–0.80) P=.003 |
| RR, relative risk; CI, confidence interval. | |||||
| Source: Mant J, Hobbs FD, Fletcher K et al. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet 2007;370:493-503. | |||||
What’s new?: Age alone does not preclude warfarin
The key finding from the BAFTA study is that advanced age alone is not a contraindication to the use of warfarin for stroke prevention in elderly patients with atrial fibrillation.
This is the first randomized controlled trial of warfarin for atrial fibrillation that included only patients ages 75 and older, conducted in a primary care setting.5
Limitations of earlier studies. The most recent meta-analysis of antithrombotic therapy for stroke prevention in patients with atrial fibrillation included 29 trials with 28,044 patients. This analysis concluded that although both warfarin and aspirin are effective in reducing the risk of stroke in patients with atrial fibrillation (warfarin by 60% and aspirin by 20%), warfarin was more effective than aspirin (relative risk reduction of 39%), with very small (≤0.3% per year) absolute increases in major extracranial hemorrhage.
The average age of patients in those trials, however, was 71. The authors identified the lack of data on older patients (who are at higher risk for serious bleeding events) as a limitation of the meta-analysis. Many of these trials took place in settings with closer monitoring of INR and warfarin dosing than is customary in a primary care setting.5
Caveats: Consider the evidence on benefits and risks
Major bleeding from warfarin is a concern, especially in the elderly. A recent cohort study6 (summarized as a POEM in this journal7) reported high rates of major bleeding (13.1 per hundred person-years or 13.1%) in patients ≥80 years of age during their first year of warfarin therapy. Despite the high risk of bleeding events in this cohort study, there was considerable benefit from warfarin therapy.
None of the patients who remained on warfarin had a thrombotic stroke (personal communication with Dr Hylek by the author). The expected rate of thrombotic stroke is in the range of 5% to 6% per year in this high-risk group.
Furthermore, most of the bleeding events were gastrointestinal and did not lead to catastrophic outcomes.
Do not add warfarin to aspirin in patients >75 years
Dr Hylek also noted that 40% of the patients in their cohort study were taking both warfarin and aspirin, and, although her study did not have sufficient power to detect a difference, prior studies noted increased risk of bleeding with this combination compared to warfarin alone.8,9 For this reason we think the combination of warfarin and aspirin should be avoided in patients over 75.
Target INR <3
Our caveat is the same as the POEM author’s conclusion:7 Patients over 80 should be carefully monitored to keep the INR below 3.0 or for signs of bleeding, especially in the first 90 days of therapy when bleeding is more likely to occur.
A final point that the BAFTA authors make, which is worth repeating here, is that the prior studies showing an increased risk of bleeding complications had INR target rates of 4 to 5, whereas the target in this study was 2 to 3. Two previous studies that also compared aspirin to warfarin with an INR goal of 2 to 3 similarly showed no difference in major bleeding between the 2 groups.10,11
Challenges to Implementation: Meticulous monitoring, patient education
- Managing warfarin therapy requires meticulous care to avoid complications and optimize treatment effect.
- Patients may be reluctant to take warfarin because they may fear bleeding.
- Patients who do agree to take warfarin need education about possible medication interactions, the need for regular INR monitoring, dosage changes, and dietary issues (eg, maintaining a consistent intake of foods containing vitamin K).
Contraindications
Contraindications to the use of warfarin include hypersensitivity to warfarin, severe hepatic disease, alcoholism, recent trauma or surgery, history of falling or significant risk of falls, and active gastrointestinal, respiratory, or genitourinary bleeding.
INR testing systems
Several randomized trials support the use of monitoring systems such as a pharmacist managed anticoagulation service or decision support software, both of which can improve the percentage of patients with therapeutic INR values.12,13
Using point-of-care INR tests in the office provides immediate results which allow for more timely adjustments of warfarin dose.14
PURLs methodology
This study was selected and evaluated using the Family Physician Inquiries Network’s Priority Updates from the Research Literature Surveillance System (PURLs) methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed here.
Warfarin is as safe as aspirin and more effective for stroke prevention in elders with atrial fibrillation
Strength of recommendation (SOR)
A: Well-designed randomized controlled trial of elderly patients in the primary care setting, consistent with findings from prior RCTs
Mant et al. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study [BAFTA]): a randomised controlled trial. Lancet 2007;370:493–503.1
Illustrative Case
An 85-year-old woman with hypertension and chronic atrial fibrillation has transferred her care to you. She takes an aspirin a day for cardiovascular prevention. You know that warfarin is better than aspirin for preventing stroke but worry about the increased risk of bleeding with warfarin.
Should you recommend that she stay on aspirin or switch to warfarin?
Background: BAFTA: A realistic study
We have been reluctant to use warfarin in elders with atrial fibrillation for good reason: risk of hemorrhage. Since there are few trials looking at use of warfarin among elders in primary care settings, we are uncertain about the balance of benefits and harms.
The BAFTA study1 is the first trial to compare outcomes of warfarin vs aspirin in elders specifically, in the less-than-ideal conditions of real life.
Guidelines mirror uncertainties
This uncertainty is reflected even in guidelines for anticoagulation in elderly patients with atrial fibrillation.
- The 2004 American College of Chest Physicians Seventh Conference on Antithrombotic and Thrombolytic Therapy recommends treating all patients with atrial fibrillation and high risk of stroke with warfarin. Their definition of high-risk includes any patient with 1 or more of the following risk factors: age >75 years, prior ischemic stroke, transient ischemic attack or systemic embolism, congestive heart failure, impaired left ventricular systolic function, hypertension, or diabetes mellitus.2
- In contrast, the 2006 guidelines for the management of patients with atrial fibrillation from the American College of Cardiology, American Heart Association, and European Society of Cardiology, are more conservative. They recommend that patients with more than 1 risk factor take warfarin, and patients with only 1 risk factor (for example, a patient older than 75 years of age with no other risk factors) take either warfarin or aspirin.3
Clinical context: Reasonable concerns
Fewer than half of the 10% to 12% of people older than 75 with atrial fibrillation are taking warfarin for stroke prevention. In one study, only 35% of patients 85 years and older with no known contraindication to anticoagulation received warfarin.4 Possible reasons for this low rate include:
- cost of monitoring warfarin
- concerns about compliance
- increased risk of hemorrhage
- prior studies focused on younger patients, in closely monitored settings.
These factors lead us to speculate that many physicians believe that the risks of warfarin in elderly patients in primary care settings outweigh any potential benefit.
We think this study demonstrates that we should seriously discuss and consider warfarin therapy for most of our elderly patients with atrial fibrillation.
The key finding from the BAFTA study is that advanced age alone is not a contraindication to the use of warfarin for stroke prevention in elderly patients with atrial fibrillation
Study summary: Primary care setting, elders only
This prospective randomized open-label trial was designed to test the effectiveness and safety of warfarin vs aspirin in the elderly, in a realistic primary care setting. The study compared the frequency of stroke, intracranial hemorrhage, and other significant arterial embolism in patients taking either warfarin or aspirin.
Inclusion criteria. Patients were at least 75 years old (average 81.5 years) with an ECG within the previous 2 years showing atrial fibrillation or atrial flutter. Seventy percent of the patients had been previously diagnosed with atrial fibrillation and 30% were identified because they had an irregular pulse on exam.
Exclusion criteria included rheumatic heart disease, major nontraumatic hemorrhage in the past 5 years, intracranial hemorrhage, endoscopically proven peptic ulcer disease in the past year, esophageal varices, allergy to either study drug, terminal illness, surgery in past 3 months, blood pressure greater than 180/110 mm Hg, or if the primary physician judged that a patient should either be on warfarin or not, based on risk factors.
Patient characteristics. The patients were recruited from 260 general practices in England and Wales. At baseline, 39% to 40% of the patients were already taking warfarin, 12% to 13% had had a prior stroke, 53% to 55% had hypertension, 13% to 14% had diabetes, 19% to 20% had heart failure, and 10% to 12% had a history of myocardial infarction. Patients were followed for an average of 2.7 years.
Aspirin and warfarin regimens. Patients were assigned to either aspirin at a dose of 75 mg/day or warfarin with a target international normalized ratio (INR) of 2.5 and an acceptable range of 2 to 3. Because the study aimed to reflect a realistic primary care setting, the frequency and method of INR testing was left to the discretion of participating physicians.
Patients who had been taking aspirin or warfarin prior to the study discontinued that medicine if they were assigned to the other treatment. Sixty-seven percent of the patients assigned to warfarin continued this treatment throughout the study, and 78% of those who either stopped taking warfarin or never started it were put on either aspirin or clopidogrel. Seventy-six percent of the patients assigned to aspirin took the medicine for the entire study period, while 70% of those who stopped taking aspirin or never started it were either switched to or stayed on warfarin.
INR values. Patients on warfarin had INR values between 2.0 and 3.0 for 67% of the time, below range for 19%, of the time, and above range for 14% of the time. Twenty-two percent of practices had all components of INR monitoring done at the hospital (phlebotomy, INR analysis, and warfarin dosing), 19% of the practices completed all 3 components on site, and the remaining practices had various combinations of onsite and hospital monitoring.
The primary outcomes included disabling stroke (ischemic or hemorrhagic) or clinically significant arterial embolism. There were 24 primary events (1.8% per year) in patients assigned to warfarin compared with 48 primary events (3.8% per year) in those assigned to aspirin, with a relative risk of 0.48 (95% confidence interval [CI], 0.28–0.80 (TABLE). The number needed to treat for 1 year to prevent 1 primary event was 50, when warfarin was compared to aspirin. Warfarin was superior to aspirin in all subgroup analyses, including patients over 85 years old.
Secondary outcomes. There were no significant differences between the warfarin and aspirin groups in the secondary outcomes: hospital admission or death as a result of a non-stroke vascular event (6.1% risk per year with warfarin vs 6.3% risk per year with aspirin), all-cause mortality (8.0% vs 8.4%), and major extracranial hemorrhage (1.4% vs 1.6%). Patients assigned to warfarin, including the subgroup of patients older than 85, did not have an increased risk of a major hemorrhage when compared with those assigned to aspirin (1.9% risk per year with warfarin vs 2.0% risk per year with aspirin; relative risk=0.96; 95% CI, 0.53–1.75).1
TABLE
BAFTA study: Warfarin was as safe as aspirin and more effective in preventing stroke in the elderly
| WARFARIN (488 patients) | ASPIRIN (485 patients) | ||||
|---|---|---|---|---|---|
| PRIMARY EVENTS | Total events | Risk per year | Total events | Risk per year | WARFARIN VA ASPIRIN |
| Stroke | 21 | 1.6% | 44 | 3.4% | RR=0.46 (95% CI, 0.26–0.79) P=.003 |
| Stroke, other intracranial hemorrhage, or systemic embolism | 24 | 1.8% | 48 | 3.8% | RR=0.48 (95% CI, 0.28–0.80) P=.003 |
| RR, relative risk; CI, confidence interval. | |||||
| Source: Mant J, Hobbs FD, Fletcher K et al. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet 2007;370:493-503. | |||||
What’s new?: Age alone does not preclude warfarin
The key finding from the BAFTA study is that advanced age alone is not a contraindication to the use of warfarin for stroke prevention in elderly patients with atrial fibrillation.
This is the first randomized controlled trial of warfarin for atrial fibrillation that included only patients ages 75 and older, conducted in a primary care setting.5
Limitations of earlier studies. The most recent meta-analysis of antithrombotic therapy for stroke prevention in patients with atrial fibrillation included 29 trials with 28,044 patients. This analysis concluded that although both warfarin and aspirin are effective in reducing the risk of stroke in patients with atrial fibrillation (warfarin by 60% and aspirin by 20%), warfarin was more effective than aspirin (relative risk reduction of 39%), with very small (≤0.3% per year) absolute increases in major extracranial hemorrhage.
The average age of patients in those trials, however, was 71. The authors identified the lack of data on older patients (who are at higher risk for serious bleeding events) as a limitation of the meta-analysis. Many of these trials took place in settings with closer monitoring of INR and warfarin dosing than is customary in a primary care setting.5
Caveats: Consider the evidence on benefits and risks
Major bleeding from warfarin is a concern, especially in the elderly. A recent cohort study6 (summarized as a POEM in this journal7) reported high rates of major bleeding (13.1 per hundred person-years or 13.1%) in patients ≥80 years of age during their first year of warfarin therapy. Despite the high risk of bleeding events in this cohort study, there was considerable benefit from warfarin therapy.
None of the patients who remained on warfarin had a thrombotic stroke (personal communication with Dr Hylek by the author). The expected rate of thrombotic stroke is in the range of 5% to 6% per year in this high-risk group.
Furthermore, most of the bleeding events were gastrointestinal and did not lead to catastrophic outcomes.
Do not add warfarin to aspirin in patients >75 years
Dr Hylek also noted that 40% of the patients in their cohort study were taking both warfarin and aspirin, and, although her study did not have sufficient power to detect a difference, prior studies noted increased risk of bleeding with this combination compared to warfarin alone.8,9 For this reason we think the combination of warfarin and aspirin should be avoided in patients over 75.
Target INR <3
Our caveat is the same as the POEM author’s conclusion:7 Patients over 80 should be carefully monitored to keep the INR below 3.0 or for signs of bleeding, especially in the first 90 days of therapy when bleeding is more likely to occur.
A final point that the BAFTA authors make, which is worth repeating here, is that the prior studies showing an increased risk of bleeding complications had INR target rates of 4 to 5, whereas the target in this study was 2 to 3. Two previous studies that also compared aspirin to warfarin with an INR goal of 2 to 3 similarly showed no difference in major bleeding between the 2 groups.10,11
Challenges to Implementation: Meticulous monitoring, patient education
- Managing warfarin therapy requires meticulous care to avoid complications and optimize treatment effect.
- Patients may be reluctant to take warfarin because they may fear bleeding.
- Patients who do agree to take warfarin need education about possible medication interactions, the need for regular INR monitoring, dosage changes, and dietary issues (eg, maintaining a consistent intake of foods containing vitamin K).
Contraindications
Contraindications to the use of warfarin include hypersensitivity to warfarin, severe hepatic disease, alcoholism, recent trauma or surgery, history of falling or significant risk of falls, and active gastrointestinal, respiratory, or genitourinary bleeding.
INR testing systems
Several randomized trials support the use of monitoring systems such as a pharmacist managed anticoagulation service or decision support software, both of which can improve the percentage of patients with therapeutic INR values.12,13
Using point-of-care INR tests in the office provides immediate results which allow for more timely adjustments of warfarin dose.14
PURLs methodology
This study was selected and evaluated using the Family Physician Inquiries Network’s Priority Updates from the Research Literature Surveillance System (PURLs) methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed here.
1. Mant J, Hobbs FD, Fletcher K, et al. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet 2007;370:493-503.
2. Singer DE, Albers GW, Dalen JE, et al. Antithrombotic therapy in atrial fibrillation: The seventh ACCP (American College of Chest Physicians) conference on antithrombotic and thrombolytic therapy. Chest 2004;126:429S-456S.
3. Fuster V, Rydén LE, Cannom DS, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation). J Am Coll Cardiol 2006;48:854-906.
4. Go AS, Hylek EM, Borowsky LH, et al. Warfarin use among ambulatory patients with non-valvular atrial fibrillation: The Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study. Ann Intern Med 1999;131:927.-
5. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: Antithrombotic therapy to prevent stroke in patients who have non-valvular atrial fibrillation. Ann Intern Med 2007;146:857-867.
6. Hylek EM, Evans-Molina C, Shea C, et al. Major hemorrhage and tolerability of warfarin in the first year of therapy among elderly patients with atrial fibrillation. Circulation 2007;115:2689-2696.
7. POEM: Bleeding risk with warfarin is high among the elderly. J Fam Pract 2007;6:709.-
8. Garcia D, Hylek E. Stroke prevention in elderly patients with atrial fibrillation. Lancet 2007;370:460-461.
9. Perez-Gomez F, Alegria E, Bejon J, et al. Comparative effects of antiplatelet, anticoagulant, or combined therapy in patients with valvular and non-valvular atrial fibrillation: a randomized multicenter study. J Am Coll Cardiol 2004;44:1557-1556.
10. Rash A, Downes T, Portner R, et al. A randomized controlled trial of warfarin versus aspirin for stroke preventions in octogenarians with atrial fibrillation (WASPO). Age Ageing 2007;36:151-156.
11. Gullov AL, Koeford BG, Petersen P, et al. Fixed minidose warfarin and aspirin alone and in combination vs adjusted-dose warfarin for stroke prevention in atrial fibrillation. Arch Intern Med 1998;158:1513-1521.
12. Witt DM, Sadler MA, Shanahan RL, et al. Effect of a centralized clinical pharmacy anticoagulation service on outcomes of anticoagulation therapy. Chest 2005;127:1515-1522.
13. Wurster M, Doran T. Anticoagulation management: a new approach. Disease Management 2006;9:201-209.
14. Dorfman DM, Goonan EM, Boutilier MK, et al. Point-of-care (POC) versus central laboratory instrumentation for monitoring oral anticoagulation. Vasc Med 2005;10:23-27.
1. Mant J, Hobbs FD, Fletcher K, et al. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet 2007;370:493-503.
2. Singer DE, Albers GW, Dalen JE, et al. Antithrombotic therapy in atrial fibrillation: The seventh ACCP (American College of Chest Physicians) conference on antithrombotic and thrombolytic therapy. Chest 2004;126:429S-456S.
3. Fuster V, Rydén LE, Cannom DS, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation). J Am Coll Cardiol 2006;48:854-906.
4. Go AS, Hylek EM, Borowsky LH, et al. Warfarin use among ambulatory patients with non-valvular atrial fibrillation: The Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study. Ann Intern Med 1999;131:927.-
5. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: Antithrombotic therapy to prevent stroke in patients who have non-valvular atrial fibrillation. Ann Intern Med 2007;146:857-867.
6. Hylek EM, Evans-Molina C, Shea C, et al. Major hemorrhage and tolerability of warfarin in the first year of therapy among elderly patients with atrial fibrillation. Circulation 2007;115:2689-2696.
7. POEM: Bleeding risk with warfarin is high among the elderly. J Fam Pract 2007;6:709.-
8. Garcia D, Hylek E. Stroke prevention in elderly patients with atrial fibrillation. Lancet 2007;370:460-461.
9. Perez-Gomez F, Alegria E, Bejon J, et al. Comparative effects of antiplatelet, anticoagulant, or combined therapy in patients with valvular and non-valvular atrial fibrillation: a randomized multicenter study. J Am Coll Cardiol 2004;44:1557-1556.
10. Rash A, Downes T, Portner R, et al. A randomized controlled trial of warfarin versus aspirin for stroke preventions in octogenarians with atrial fibrillation (WASPO). Age Ageing 2007;36:151-156.
11. Gullov AL, Koeford BG, Petersen P, et al. Fixed minidose warfarin and aspirin alone and in combination vs adjusted-dose warfarin for stroke prevention in atrial fibrillation. Arch Intern Med 1998;158:1513-1521.
12. Witt DM, Sadler MA, Shanahan RL, et al. Effect of a centralized clinical pharmacy anticoagulation service on outcomes of anticoagulation therapy. Chest 2005;127:1515-1522.
13. Wurster M, Doran T. Anticoagulation management: a new approach. Disease Management 2006;9:201-209.
14. Dorfman DM, Goonan EM, Boutilier MK, et al. Point-of-care (POC) versus central laboratory instrumentation for monitoring oral anticoagulation. Vasc Med 2005;10:23-27.
Copyright © 2007 The Family Physicians Inquiries Network.
All rights reserved.
How can you improve vaccination rates among older African Americans?
- Recommend the flu shot and make it convenient to get vaccinated.
- Tell your patients that the flu is a serious illness and that they may be susceptible, even if they haven’t had it before. reinforce the idea that they should practice good hygiene, such as washing their hands regularly.
- Tell patients that the flu shot is safe; it will not give them the flu or interact with any of their medications.
- Give patients printed materials. excellent resources are available through the CDC (www.cdc.gov/flu) or the National Institute of Allergy and Infectious Disease (www3.niaid.nih.gov).
Purpose Adults 65 and older are at greatest risk for complications and death from influenza, yet one third of those at risk do not receive the influenza vaccine; African American vaccination rates are even lower. This study explored older African Americans’ concerns about getting the flu vaccine and vaccine providers’ level of awareness of these concerns.
Methods Focus groups and in-depth interviews were conducted among African Americans who were 50 years of age and older, and vaccine providers.
Results Older African Americans’ fear of getting the flu from vaccination was widespread, as were concerns about vaccine interaction with medications and allergic reactions. older African Americans also doubted the vaccine’s effectiveness, and distrusted both the vaccine and the healthcare system. For their part, providers understood patients’ concerns and recognized that fear of illness caused by the shot was a major issue. They did not, however, recognize the importance of asking about, and discussing, patients’ fears of allergies and medication interactions when administering the vaccine.
Conclusions In order to improve vaccination rates among older African Americans, health care providers would be wise to take the time to discuss the vaccine and address vaccine efficacy, safety, side effects, and drug interactions.
Why are older African Americans less likely than whites to get a flu vaccination? Despite the existence of an effective flu vaccine, usage rates still remain low: 66% on average,1 and are even lower among minority groups.2 One survey found only 47% of older African Americans were vaccinated against the flu.3
These disparities are not easily explained. Even when controlling for increased risk, age distribution, perceived health status, family size and marital status, poverty level, education, and access to medical care and health insurance, African Americans are still less likely than whites to get vaccinated.3,4
In light of this disparity, we explored barriers to flu vaccination among this population, including concerns over safety and adverse events, and the role that health care providers can play in overcoming these issues.
What are the barriers?
Structural factors, such as having access to a location that provides vaccinations,5 and social factors, such as believing that others support vaccination,6 increase vaccination rates. Vaccination increases with age5,7 and is more likely in those who have previously been vaccinated.8
People who believe they aren’t susceptible to a disease are less likely to get vaccinated.9,10 Fear of side effects is also a significant barrier.2,6,7,11,12 Fear of illness from the vaccine is often cited by both white and African American Medicare patients, older adults, and most notably by older African Americans.2,5,12 African Americans and other minority groups are less likely to accept the vaccine as safe.13
A physician’s recommendation to get the flu vaccine appears to motivate patients in risk groups to get vaccinated,6,7,12 and is a significant determinant of vaccine acceptance in surveys of older Americans, high-risk patients, and older African Americans in particular.6,12
Methods
Focus groups and interviews
The study team conducted qualitative formative research with 2 pertinent audiences: African American adults (hereafter, “public participants”) and clinicians who administer the flu vaccine (hereafter, “providers”). The Saint Louis University Institutional Review Board approved the research.
We recruited older African American public participants through local community contacts, and screened to identify those ambivalent about getting a flu shot. A trained moderator then conducted focus groups and interviews with those who were ambivalent about the flu shot to assess their knowledge, beliefs, norms, and intentions related to vaccination.
We also recruited vaccine providers by calling local hospitals, doctors’ offices, health departments, and clinics that serve African Americans. To identify providers’ perceptions of patients’ concerns, we carried out interviews at the clinicians’ place of business or in a university conference room; a focus group was conducted at a community clinic.
All focus groups and interviews were audiotaped and transcribed. In pairs, research team members (all authors except for HJ) coded each of the transcripts independently, reviewed and discussed their codes, and then came to agreement on the final codes. Coded transcripts were entered into Atlas.ti (Atlas.ti GmbH, Berlin, Germany), a qualitative data analysis software program, and were analyzed with summary reports drafted for each focus group and interview. Findings were then synthesized across groups and interviews, and across segments.
Results
Description of participants
Four focus groups (N=35) and 8 in-depth interviews were conducted with the public participants—African American adults ages 50 and over. As shown in the TABLE, most participants were female, had children, had less than a college degree, and earned less than $30,000 a year. Widows and widowers made up the largest percentage of participants.
One focus group (N=9) and 5 interviews were conducted with vaccine providers—professionals working in clinics that offered the flu vaccine and served African Americans—for a total sample of 14. As the providers were sampled according to their professional affiliation, demographics were not systematically recorded. These informants were nurses, physicians’ assistants, and vaccination program administrators.
TABLE
4 focus groups and 8 interviews included 43 public participants*
| VARIABLE | FOCUS GROUPS (N=35) | INTERVIEWS (N=8) |
|---|---|---|
| Age | ||
| 50–55 | 9 (26%) | 3 (38%) |
| 56–60 | 5 (14%) | 1 (12%) |
| 61–65 | 4 (11%) | — |
| 66–70 | 4 (11%) | — |
| 71–75 | 3 (9%) | 1 (12%) |
| 76–80 | — | — |
| 81–85 | 1 (3%) | 2 (25%) |
| 86 and older | 1 (3%) | 1 (12%) |
| Gender | ||
| Male | 6 (17%) | 2 (25%) |
| Female | 29 (83%) | 6 (75%) |
| Education | ||
| Less than high school | 4 (11%) | 1 (12%) |
| Some high school | 7 (20%) | 1 (12%) |
| High school diploma/GED | 7 (20%) | 1 (12%) |
| Some college | 9 (26%) | 4 (50%) |
| College degree | 5 (14%) | 1 (12%) |
| Graduate degree | 2 (6%) | — |
| Marital status | ||
| Single | 9 (26%) | 4 (50%) |
| Married/living with partner | 4 (11%) | 1 (12%) |
| Divorced or separated | 4 (11%) | — |
| Widowed | 17 (49%) | 3 (38%) |
| Children | ||
| Yes | 33 (94%) | 6 (75%) |
| No | 1 (3%) | 2 (25%) |
| Family income | ||
| Less than $10,000 | 13 (37%) | 3 (37%) |
| $10,000–$19,999 | 8 (23%) | 3 (37%) |
| $20,000–$29,999 | 3 (9%) | 1 (12%) |
| $30,000–$39,999 | — — | |
| $40,000–$49,999 | 3 (9%) | — |
| $50,000–$59,999 | 1 (3%) | — |
| $60,000–$69,999 | — | — |
| $70,000–$79,999 | 1 (3%) | — |
| * Not all participants answered all questions | ||
How patients and clinicians see things differently
Susceptibility. Most public participants were familiar with only a few of the high-risk groups recommended for vaccination. Many participants said that children needed the flu shot, but did not state that those in their 50s were also recommended to get the flu shot. Those never stricken with the flu didn’t consider themselves susceptible. “Because I have never gotten it before…. I get a lot of other things but I don’t get colds and things like that.”
The health care providers identified senior citizens as a high-risk group for influenza because of the high occurrence of comorbid conditions. Providers were especially concerned that seniors who don’t go out much may incorrectly believe they don’t need to get vaccinated.
Severity of the flu. While some of the public participants didn’t see influenza as a deadly disease, others did.
Providers recognized that some members of the public regarded the flu as more of a nuisance then a serious illness and emphasized the need to raise awareness.
Vaccine efficacy. The opinions that the public participants had about the effectiveness of the influenza vaccine varied. Some said it was effective; others believed the vaccine was not effective because they knew they could still get the flu even after being vaccinated. “I know people who got the flu shot and they still got the flu.” Many believed that home remedies, cleanliness, and staying away from others were more effective means of prevention.
Providers believed the vaccine is effective if it matches the correct strain of flu virus. They noted that even if a patient is vaccinated against the wrong strain, flu symptoms will be milder. Providers recognized patient concerns about vaccine efficacy, but none mentioned that it was important to encourage vaccination along with cleanliness and avoidance measures.
Safety and side effects. The most common concern about vaccine safety that the public participants discussed was that the influenza vaccine causes flu illness. Several respondents substantiated this concern with their own experiences, or those of others, where shortly after vaccination a flulike illness resulted. “It gave them the flu. And they were sick for 2 or 3 weeks.”
Another common safety concern was that the vaccine would interact with prescription medications for chronic illnesses. Many public participants also noted that health care providers neglected to discuss the matter. “[M]y reason for not taking the flu shot is because I’m on other medicine and I do have some concerns.… What are you putting in my body?” Some were also concerned about the safety of vaccine components and receiving a tainted vaccine. These participants also mentioned their fear of an allergic reaction to the flu vaccine.
Overall, providers did not have concerns about vaccine safety; however, they understood patients were afraid the influenza vaccine would give them the flu. They felt they should inform patients that full protection from the influenza vaccine takes up to 2 weeks. Providers were also aware that many African Americans who are 50 years of age and older distrust the medical system.
Main reason for vaccination: Doctor’s advice
All of the African American adults agreed that physicians and other health care professionals were important sources of vaccine information. Though initially ambivalent, a majority also reported receiving the vaccine primarily because of recommendations from their doctor. Some noted that providers can do more to encourage vaccination when patients express concerns. “I would have taken it if he had said, ‘I think you should take it.’” The public participants also got vaccine information from family, friends, broadcast media, and print material. They noted that they wanted to see the following in flu vaccination information: the pros and cons of the vaccine, efficacy of the vaccine, how the vaccine reduces flu severity, vaccine safety, and history and background of the vaccine.
Providers noted the importance of: promoting informed decision making, one-on-one communication, using a matriarchal figure to promote vaccination and using the media to promote vaccination. “You would have to have a mother, or a grandmother, or aunt figure because that is usually who is responsible, who takes care of the family.” Providers also pointed out that patients often voice concerns about not having enough information to make informed decisions, and that patients rely on convenience and doctors’ recommendations when deciding about vaccination.
Patients may distrust the system, but they trust their doctor
Our study’s findings from both providers and older African Americans suggest that physicians are the most influential source of information when patients are deciding about flu vaccination. This is true despite the fact that the public makes no secret of its distrust of the medical system and the safety of the vaccine. The African American participants also suggested that physicians do not adequately address patient concerns through discussion and the information they provide.
Providers were concerned that many people do not believe the flu is a severe illness or that they are susceptible. Although the African Americans in the study recognized some high-risk groups, they tended not to consider themselves part of any of those groups.
Doctors didn’t realize that patients fear drug interactions
One of the interesting findings of this study was that armed with the knowledge that the flu shot does not guarantee flu prevention, African American adults were willing to forego the vaccine. This absence of a guarantee also reinforced their beliefs that other prevention methods, such as handwashing and avoidance, are more effective.
Not surprisingly, of course, was the finding that patients continue to avoid the flu shot for fear of getting the flu.2,6 What was a bit surprising was that providers did not recognize that fear of medication reactions (drug interactions and allergic reactions) was also a barrier to flu vaccination. Providers also missed out on an educational opportunity, since many of the African American participants wanted to discuss the possibility of interactions with them.
Limitations of the study
External validity is limited because the findings cannot be generalized to every African American population in the US. The participants made up a non-random convenience sample of older African Americans in a Midwestern city, although the community-based recruitment strategy succeeded in reaching members of a lower income urban population. This study included only those who were ambivalent about the vaccine and who were open to both the pros and cons of vaccination. Project staff minimized possible interviewer bias by using experienced moderators, ensuring the consistent use of moderator guides, and using consensus coding procedures.
Funding
This research was funded by grant #6465 from the National Immunization Program at the Centers for Disease Control and Prevention, via Special Interest Project 11, to the Prevention research Center at the Saint louis University School of Public Health.
Acknowledgments
We thank Katie Duggan at the School of Public Health and edith Gary and Pascale Wortley at the CDC for their support. This article is dedicated to the memory of Joe D. Wray, MD, who suggested it.
Correspondence
Ricardo J. Wray, PhD, Community Health, Saint Louis University School of Public Health, 3545 Lafayette Avenue, St. Louis, MO 63104; wray@slu.edu
US Department of Health and Human Services. Immunization and infectious disease. Progress Report. Healthy People 2010. Available at: www.healthypeople.gov/Document/HTML/Volume1/14Immunization.htm. Accessed on September 28, 2007.
2. Centers for Disease Control and Prevention. Reasons reported by Medicare beneficiaries for not receiving influenza and pneumococcal vaccinations—United States, 1996. MMWR Morb Mortal Wkly Rpt 1999;48:889-890.
3. Marin M, Johanson W, Salas-lopez D. Influenza vaccination among minority populations in the United States. Prev Med 2002;34:236-241.
4. Fiscella K, Franks P, Doescher M, Saver B. Disparities in health care by race, ethnicity, and language among the insured: Findings from a national sample. Med Care 2002;40:52-59.
5. Ludwig-Beymer P, Gerc S. An influenza prevention campaign: The employee perspective. J Nurs Care Qual 2002;16(3):1-12.
6. Zimmerman R, Santibanez T, Janosky J, et al. What affects influenza vaccination rates among older patients? An analysis from inner-city, suburban, rural, and veterans Affairs practice. Am J Med 2003;114:31-38.
7. Nexoe J, Oltarzewska A, Sawicka-Powierza J, Kragstrup J, Kristiansen I. Perception of risk information: Similarities and differences between Danish and Polish general practitioners. Scand J Prim Health Care 2002;20:183-187.
8. Nichol K, Lofgren R, Gapinski J. Influenza vaccination: Knowledge, attitudes, and behavior among high-risk outpatients. Arch Intern Med 1992;152:106-110.
9. Santibanez T, Nowalk M, Zimmerman R, et al. Knowledge and beliefs about influenza, pneumococcal disease, and immunizations among older people. J Am Geriatr Soc 2002;51:1711-1716.
10. Demicheli V, Jefferson T, Rivetti D, Deeks J. Prevention and early treatment of influenza in healthy adults. Vaccine 2000;18:957-1030.
11. Telford R, Rogers A. What influences elderly peoples’ decisions about whether to accept the influenza vaccination? A qualitative study. Health Educ Res 2003;18:743-753.
12. Nicoleau A, Nicoleau C, Balzora J, Oboh A, Siddiqui N, Rosenberg C. Elderly African-Americans and the influenza vaccine: The impact of the primary care physician. J Am Med Dir Assoc 2001;2:56-59.
13. Riddiough M, Willems J, Sanders C, Kemp K. Factors affecting the use of vaccines: Considerations for immunization program planners. Public Health Rep 1981;96:528-535.
14. Steuart G. Social and behavioral change strategies. In: Phillips H, Gaylord S, eds. Aging and Public Health New York, NY: Springer;1985.
- Recommend the flu shot and make it convenient to get vaccinated.
- Tell your patients that the flu is a serious illness and that they may be susceptible, even if they haven’t had it before. reinforce the idea that they should practice good hygiene, such as washing their hands regularly.
- Tell patients that the flu shot is safe; it will not give them the flu or interact with any of their medications.
- Give patients printed materials. excellent resources are available through the CDC (www.cdc.gov/flu) or the National Institute of Allergy and Infectious Disease (www3.niaid.nih.gov).
Purpose Adults 65 and older are at greatest risk for complications and death from influenza, yet one third of those at risk do not receive the influenza vaccine; African American vaccination rates are even lower. This study explored older African Americans’ concerns about getting the flu vaccine and vaccine providers’ level of awareness of these concerns.
Methods Focus groups and in-depth interviews were conducted among African Americans who were 50 years of age and older, and vaccine providers.
Results Older African Americans’ fear of getting the flu from vaccination was widespread, as were concerns about vaccine interaction with medications and allergic reactions. older African Americans also doubted the vaccine’s effectiveness, and distrusted both the vaccine and the healthcare system. For their part, providers understood patients’ concerns and recognized that fear of illness caused by the shot was a major issue. They did not, however, recognize the importance of asking about, and discussing, patients’ fears of allergies and medication interactions when administering the vaccine.
Conclusions In order to improve vaccination rates among older African Americans, health care providers would be wise to take the time to discuss the vaccine and address vaccine efficacy, safety, side effects, and drug interactions.
Why are older African Americans less likely than whites to get a flu vaccination? Despite the existence of an effective flu vaccine, usage rates still remain low: 66% on average,1 and are even lower among minority groups.2 One survey found only 47% of older African Americans were vaccinated against the flu.3
These disparities are not easily explained. Even when controlling for increased risk, age distribution, perceived health status, family size and marital status, poverty level, education, and access to medical care and health insurance, African Americans are still less likely than whites to get vaccinated.3,4
In light of this disparity, we explored barriers to flu vaccination among this population, including concerns over safety and adverse events, and the role that health care providers can play in overcoming these issues.
What are the barriers?
Structural factors, such as having access to a location that provides vaccinations,5 and social factors, such as believing that others support vaccination,6 increase vaccination rates. Vaccination increases with age5,7 and is more likely in those who have previously been vaccinated.8
People who believe they aren’t susceptible to a disease are less likely to get vaccinated.9,10 Fear of side effects is also a significant barrier.2,6,7,11,12 Fear of illness from the vaccine is often cited by both white and African American Medicare patients, older adults, and most notably by older African Americans.2,5,12 African Americans and other minority groups are less likely to accept the vaccine as safe.13
A physician’s recommendation to get the flu vaccine appears to motivate patients in risk groups to get vaccinated,6,7,12 and is a significant determinant of vaccine acceptance in surveys of older Americans, high-risk patients, and older African Americans in particular.6,12
Methods
Focus groups and interviews
The study team conducted qualitative formative research with 2 pertinent audiences: African American adults (hereafter, “public participants”) and clinicians who administer the flu vaccine (hereafter, “providers”). The Saint Louis University Institutional Review Board approved the research.
We recruited older African American public participants through local community contacts, and screened to identify those ambivalent about getting a flu shot. A trained moderator then conducted focus groups and interviews with those who were ambivalent about the flu shot to assess their knowledge, beliefs, norms, and intentions related to vaccination.
We also recruited vaccine providers by calling local hospitals, doctors’ offices, health departments, and clinics that serve African Americans. To identify providers’ perceptions of patients’ concerns, we carried out interviews at the clinicians’ place of business or in a university conference room; a focus group was conducted at a community clinic.
All focus groups and interviews were audiotaped and transcribed. In pairs, research team members (all authors except for HJ) coded each of the transcripts independently, reviewed and discussed their codes, and then came to agreement on the final codes. Coded transcripts were entered into Atlas.ti (Atlas.ti GmbH, Berlin, Germany), a qualitative data analysis software program, and were analyzed with summary reports drafted for each focus group and interview. Findings were then synthesized across groups and interviews, and across segments.
Results
Description of participants
Four focus groups (N=35) and 8 in-depth interviews were conducted with the public participants—African American adults ages 50 and over. As shown in the TABLE, most participants were female, had children, had less than a college degree, and earned less than $30,000 a year. Widows and widowers made up the largest percentage of participants.
One focus group (N=9) and 5 interviews were conducted with vaccine providers—professionals working in clinics that offered the flu vaccine and served African Americans—for a total sample of 14. As the providers were sampled according to their professional affiliation, demographics were not systematically recorded. These informants were nurses, physicians’ assistants, and vaccination program administrators.
TABLE
4 focus groups and 8 interviews included 43 public participants*
| VARIABLE | FOCUS GROUPS (N=35) | INTERVIEWS (N=8) |
|---|---|---|
| Age | ||
| 50–55 | 9 (26%) | 3 (38%) |
| 56–60 | 5 (14%) | 1 (12%) |
| 61–65 | 4 (11%) | — |
| 66–70 | 4 (11%) | — |
| 71–75 | 3 (9%) | 1 (12%) |
| 76–80 | — | — |
| 81–85 | 1 (3%) | 2 (25%) |
| 86 and older | 1 (3%) | 1 (12%) |
| Gender | ||
| Male | 6 (17%) | 2 (25%) |
| Female | 29 (83%) | 6 (75%) |
| Education | ||
| Less than high school | 4 (11%) | 1 (12%) |
| Some high school | 7 (20%) | 1 (12%) |
| High school diploma/GED | 7 (20%) | 1 (12%) |
| Some college | 9 (26%) | 4 (50%) |
| College degree | 5 (14%) | 1 (12%) |
| Graduate degree | 2 (6%) | — |
| Marital status | ||
| Single | 9 (26%) | 4 (50%) |
| Married/living with partner | 4 (11%) | 1 (12%) |
| Divorced or separated | 4 (11%) | — |
| Widowed | 17 (49%) | 3 (38%) |
| Children | ||
| Yes | 33 (94%) | 6 (75%) |
| No | 1 (3%) | 2 (25%) |
| Family income | ||
| Less than $10,000 | 13 (37%) | 3 (37%) |
| $10,000–$19,999 | 8 (23%) | 3 (37%) |
| $20,000–$29,999 | 3 (9%) | 1 (12%) |
| $30,000–$39,999 | — — | |
| $40,000–$49,999 | 3 (9%) | — |
| $50,000–$59,999 | 1 (3%) | — |
| $60,000–$69,999 | — | — |
| $70,000–$79,999 | 1 (3%) | — |
| * Not all participants answered all questions | ||
How patients and clinicians see things differently
Susceptibility. Most public participants were familiar with only a few of the high-risk groups recommended for vaccination. Many participants said that children needed the flu shot, but did not state that those in their 50s were also recommended to get the flu shot. Those never stricken with the flu didn’t consider themselves susceptible. “Because I have never gotten it before…. I get a lot of other things but I don’t get colds and things like that.”
The health care providers identified senior citizens as a high-risk group for influenza because of the high occurrence of comorbid conditions. Providers were especially concerned that seniors who don’t go out much may incorrectly believe they don’t need to get vaccinated.
Severity of the flu. While some of the public participants didn’t see influenza as a deadly disease, others did.
Providers recognized that some members of the public regarded the flu as more of a nuisance then a serious illness and emphasized the need to raise awareness.
Vaccine efficacy. The opinions that the public participants had about the effectiveness of the influenza vaccine varied. Some said it was effective; others believed the vaccine was not effective because they knew they could still get the flu even after being vaccinated. “I know people who got the flu shot and they still got the flu.” Many believed that home remedies, cleanliness, and staying away from others were more effective means of prevention.
Providers believed the vaccine is effective if it matches the correct strain of flu virus. They noted that even if a patient is vaccinated against the wrong strain, flu symptoms will be milder. Providers recognized patient concerns about vaccine efficacy, but none mentioned that it was important to encourage vaccination along with cleanliness and avoidance measures.
Safety and side effects. The most common concern about vaccine safety that the public participants discussed was that the influenza vaccine causes flu illness. Several respondents substantiated this concern with their own experiences, or those of others, where shortly after vaccination a flulike illness resulted. “It gave them the flu. And they were sick for 2 or 3 weeks.”
Another common safety concern was that the vaccine would interact with prescription medications for chronic illnesses. Many public participants also noted that health care providers neglected to discuss the matter. “[M]y reason for not taking the flu shot is because I’m on other medicine and I do have some concerns.… What are you putting in my body?” Some were also concerned about the safety of vaccine components and receiving a tainted vaccine. These participants also mentioned their fear of an allergic reaction to the flu vaccine.
Overall, providers did not have concerns about vaccine safety; however, they understood patients were afraid the influenza vaccine would give them the flu. They felt they should inform patients that full protection from the influenza vaccine takes up to 2 weeks. Providers were also aware that many African Americans who are 50 years of age and older distrust the medical system.
Main reason for vaccination: Doctor’s advice
All of the African American adults agreed that physicians and other health care professionals were important sources of vaccine information. Though initially ambivalent, a majority also reported receiving the vaccine primarily because of recommendations from their doctor. Some noted that providers can do more to encourage vaccination when patients express concerns. “I would have taken it if he had said, ‘I think you should take it.’” The public participants also got vaccine information from family, friends, broadcast media, and print material. They noted that they wanted to see the following in flu vaccination information: the pros and cons of the vaccine, efficacy of the vaccine, how the vaccine reduces flu severity, vaccine safety, and history and background of the vaccine.
Providers noted the importance of: promoting informed decision making, one-on-one communication, using a matriarchal figure to promote vaccination and using the media to promote vaccination. “You would have to have a mother, or a grandmother, or aunt figure because that is usually who is responsible, who takes care of the family.” Providers also pointed out that patients often voice concerns about not having enough information to make informed decisions, and that patients rely on convenience and doctors’ recommendations when deciding about vaccination.
Patients may distrust the system, but they trust their doctor
Our study’s findings from both providers and older African Americans suggest that physicians are the most influential source of information when patients are deciding about flu vaccination. This is true despite the fact that the public makes no secret of its distrust of the medical system and the safety of the vaccine. The African American participants also suggested that physicians do not adequately address patient concerns through discussion and the information they provide.
Providers were concerned that many people do not believe the flu is a severe illness or that they are susceptible. Although the African Americans in the study recognized some high-risk groups, they tended not to consider themselves part of any of those groups.
Doctors didn’t realize that patients fear drug interactions
One of the interesting findings of this study was that armed with the knowledge that the flu shot does not guarantee flu prevention, African American adults were willing to forego the vaccine. This absence of a guarantee also reinforced their beliefs that other prevention methods, such as handwashing and avoidance, are more effective.
Not surprisingly, of course, was the finding that patients continue to avoid the flu shot for fear of getting the flu.2,6 What was a bit surprising was that providers did not recognize that fear of medication reactions (drug interactions and allergic reactions) was also a barrier to flu vaccination. Providers also missed out on an educational opportunity, since many of the African American participants wanted to discuss the possibility of interactions with them.
Limitations of the study
External validity is limited because the findings cannot be generalized to every African American population in the US. The participants made up a non-random convenience sample of older African Americans in a Midwestern city, although the community-based recruitment strategy succeeded in reaching members of a lower income urban population. This study included only those who were ambivalent about the vaccine and who were open to both the pros and cons of vaccination. Project staff minimized possible interviewer bias by using experienced moderators, ensuring the consistent use of moderator guides, and using consensus coding procedures.
Funding
This research was funded by grant #6465 from the National Immunization Program at the Centers for Disease Control and Prevention, via Special Interest Project 11, to the Prevention research Center at the Saint louis University School of Public Health.
Acknowledgments
We thank Katie Duggan at the School of Public Health and edith Gary and Pascale Wortley at the CDC for their support. This article is dedicated to the memory of Joe D. Wray, MD, who suggested it.
Correspondence
Ricardo J. Wray, PhD, Community Health, Saint Louis University School of Public Health, 3545 Lafayette Avenue, St. Louis, MO 63104; wray@slu.edu
- Recommend the flu shot and make it convenient to get vaccinated.
- Tell your patients that the flu is a serious illness and that they may be susceptible, even if they haven’t had it before. reinforce the idea that they should practice good hygiene, such as washing their hands regularly.
- Tell patients that the flu shot is safe; it will not give them the flu or interact with any of their medications.
- Give patients printed materials. excellent resources are available through the CDC (www.cdc.gov/flu) or the National Institute of Allergy and Infectious Disease (www3.niaid.nih.gov).
Purpose Adults 65 and older are at greatest risk for complications and death from influenza, yet one third of those at risk do not receive the influenza vaccine; African American vaccination rates are even lower. This study explored older African Americans’ concerns about getting the flu vaccine and vaccine providers’ level of awareness of these concerns.
Methods Focus groups and in-depth interviews were conducted among African Americans who were 50 years of age and older, and vaccine providers.
Results Older African Americans’ fear of getting the flu from vaccination was widespread, as were concerns about vaccine interaction with medications and allergic reactions. older African Americans also doubted the vaccine’s effectiveness, and distrusted both the vaccine and the healthcare system. For their part, providers understood patients’ concerns and recognized that fear of illness caused by the shot was a major issue. They did not, however, recognize the importance of asking about, and discussing, patients’ fears of allergies and medication interactions when administering the vaccine.
Conclusions In order to improve vaccination rates among older African Americans, health care providers would be wise to take the time to discuss the vaccine and address vaccine efficacy, safety, side effects, and drug interactions.
Why are older African Americans less likely than whites to get a flu vaccination? Despite the existence of an effective flu vaccine, usage rates still remain low: 66% on average,1 and are even lower among minority groups.2 One survey found only 47% of older African Americans were vaccinated against the flu.3
These disparities are not easily explained. Even when controlling for increased risk, age distribution, perceived health status, family size and marital status, poverty level, education, and access to medical care and health insurance, African Americans are still less likely than whites to get vaccinated.3,4
In light of this disparity, we explored barriers to flu vaccination among this population, including concerns over safety and adverse events, and the role that health care providers can play in overcoming these issues.
What are the barriers?
Structural factors, such as having access to a location that provides vaccinations,5 and social factors, such as believing that others support vaccination,6 increase vaccination rates. Vaccination increases with age5,7 and is more likely in those who have previously been vaccinated.8
People who believe they aren’t susceptible to a disease are less likely to get vaccinated.9,10 Fear of side effects is also a significant barrier.2,6,7,11,12 Fear of illness from the vaccine is often cited by both white and African American Medicare patients, older adults, and most notably by older African Americans.2,5,12 African Americans and other minority groups are less likely to accept the vaccine as safe.13
A physician’s recommendation to get the flu vaccine appears to motivate patients in risk groups to get vaccinated,6,7,12 and is a significant determinant of vaccine acceptance in surveys of older Americans, high-risk patients, and older African Americans in particular.6,12
Methods
Focus groups and interviews
The study team conducted qualitative formative research with 2 pertinent audiences: African American adults (hereafter, “public participants”) and clinicians who administer the flu vaccine (hereafter, “providers”). The Saint Louis University Institutional Review Board approved the research.
We recruited older African American public participants through local community contacts, and screened to identify those ambivalent about getting a flu shot. A trained moderator then conducted focus groups and interviews with those who were ambivalent about the flu shot to assess their knowledge, beliefs, norms, and intentions related to vaccination.
We also recruited vaccine providers by calling local hospitals, doctors’ offices, health departments, and clinics that serve African Americans. To identify providers’ perceptions of patients’ concerns, we carried out interviews at the clinicians’ place of business or in a university conference room; a focus group was conducted at a community clinic.
All focus groups and interviews were audiotaped and transcribed. In pairs, research team members (all authors except for HJ) coded each of the transcripts independently, reviewed and discussed their codes, and then came to agreement on the final codes. Coded transcripts were entered into Atlas.ti (Atlas.ti GmbH, Berlin, Germany), a qualitative data analysis software program, and were analyzed with summary reports drafted for each focus group and interview. Findings were then synthesized across groups and interviews, and across segments.
Results
Description of participants
Four focus groups (N=35) and 8 in-depth interviews were conducted with the public participants—African American adults ages 50 and over. As shown in the TABLE, most participants were female, had children, had less than a college degree, and earned less than $30,000 a year. Widows and widowers made up the largest percentage of participants.
One focus group (N=9) and 5 interviews were conducted with vaccine providers—professionals working in clinics that offered the flu vaccine and served African Americans—for a total sample of 14. As the providers were sampled according to their professional affiliation, demographics were not systematically recorded. These informants were nurses, physicians’ assistants, and vaccination program administrators.
TABLE
4 focus groups and 8 interviews included 43 public participants*
| VARIABLE | FOCUS GROUPS (N=35) | INTERVIEWS (N=8) |
|---|---|---|
| Age | ||
| 50–55 | 9 (26%) | 3 (38%) |
| 56–60 | 5 (14%) | 1 (12%) |
| 61–65 | 4 (11%) | — |
| 66–70 | 4 (11%) | — |
| 71–75 | 3 (9%) | 1 (12%) |
| 76–80 | — | — |
| 81–85 | 1 (3%) | 2 (25%) |
| 86 and older | 1 (3%) | 1 (12%) |
| Gender | ||
| Male | 6 (17%) | 2 (25%) |
| Female | 29 (83%) | 6 (75%) |
| Education | ||
| Less than high school | 4 (11%) | 1 (12%) |
| Some high school | 7 (20%) | 1 (12%) |
| High school diploma/GED | 7 (20%) | 1 (12%) |
| Some college | 9 (26%) | 4 (50%) |
| College degree | 5 (14%) | 1 (12%) |
| Graduate degree | 2 (6%) | — |
| Marital status | ||
| Single | 9 (26%) | 4 (50%) |
| Married/living with partner | 4 (11%) | 1 (12%) |
| Divorced or separated | 4 (11%) | — |
| Widowed | 17 (49%) | 3 (38%) |
| Children | ||
| Yes | 33 (94%) | 6 (75%) |
| No | 1 (3%) | 2 (25%) |
| Family income | ||
| Less than $10,000 | 13 (37%) | 3 (37%) |
| $10,000–$19,999 | 8 (23%) | 3 (37%) |
| $20,000–$29,999 | 3 (9%) | 1 (12%) |
| $30,000–$39,999 | — — | |
| $40,000–$49,999 | 3 (9%) | — |
| $50,000–$59,999 | 1 (3%) | — |
| $60,000–$69,999 | — | — |
| $70,000–$79,999 | 1 (3%) | — |
| * Not all participants answered all questions | ||
How patients and clinicians see things differently
Susceptibility. Most public participants were familiar with only a few of the high-risk groups recommended for vaccination. Many participants said that children needed the flu shot, but did not state that those in their 50s were also recommended to get the flu shot. Those never stricken with the flu didn’t consider themselves susceptible. “Because I have never gotten it before…. I get a lot of other things but I don’t get colds and things like that.”
The health care providers identified senior citizens as a high-risk group for influenza because of the high occurrence of comorbid conditions. Providers were especially concerned that seniors who don’t go out much may incorrectly believe they don’t need to get vaccinated.
Severity of the flu. While some of the public participants didn’t see influenza as a deadly disease, others did.
Providers recognized that some members of the public regarded the flu as more of a nuisance then a serious illness and emphasized the need to raise awareness.
Vaccine efficacy. The opinions that the public participants had about the effectiveness of the influenza vaccine varied. Some said it was effective; others believed the vaccine was not effective because they knew they could still get the flu even after being vaccinated. “I know people who got the flu shot and they still got the flu.” Many believed that home remedies, cleanliness, and staying away from others were more effective means of prevention.
Providers believed the vaccine is effective if it matches the correct strain of flu virus. They noted that even if a patient is vaccinated against the wrong strain, flu symptoms will be milder. Providers recognized patient concerns about vaccine efficacy, but none mentioned that it was important to encourage vaccination along with cleanliness and avoidance measures.
Safety and side effects. The most common concern about vaccine safety that the public participants discussed was that the influenza vaccine causes flu illness. Several respondents substantiated this concern with their own experiences, or those of others, where shortly after vaccination a flulike illness resulted. “It gave them the flu. And they were sick for 2 or 3 weeks.”
Another common safety concern was that the vaccine would interact with prescription medications for chronic illnesses. Many public participants also noted that health care providers neglected to discuss the matter. “[M]y reason for not taking the flu shot is because I’m on other medicine and I do have some concerns.… What are you putting in my body?” Some were also concerned about the safety of vaccine components and receiving a tainted vaccine. These participants also mentioned their fear of an allergic reaction to the flu vaccine.
Overall, providers did not have concerns about vaccine safety; however, they understood patients were afraid the influenza vaccine would give them the flu. They felt they should inform patients that full protection from the influenza vaccine takes up to 2 weeks. Providers were also aware that many African Americans who are 50 years of age and older distrust the medical system.
Main reason for vaccination: Doctor’s advice
All of the African American adults agreed that physicians and other health care professionals were important sources of vaccine information. Though initially ambivalent, a majority also reported receiving the vaccine primarily because of recommendations from their doctor. Some noted that providers can do more to encourage vaccination when patients express concerns. “I would have taken it if he had said, ‘I think you should take it.’” The public participants also got vaccine information from family, friends, broadcast media, and print material. They noted that they wanted to see the following in flu vaccination information: the pros and cons of the vaccine, efficacy of the vaccine, how the vaccine reduces flu severity, vaccine safety, and history and background of the vaccine.
Providers noted the importance of: promoting informed decision making, one-on-one communication, using a matriarchal figure to promote vaccination and using the media to promote vaccination. “You would have to have a mother, or a grandmother, or aunt figure because that is usually who is responsible, who takes care of the family.” Providers also pointed out that patients often voice concerns about not having enough information to make informed decisions, and that patients rely on convenience and doctors’ recommendations when deciding about vaccination.
Patients may distrust the system, but they trust their doctor
Our study’s findings from both providers and older African Americans suggest that physicians are the most influential source of information when patients are deciding about flu vaccination. This is true despite the fact that the public makes no secret of its distrust of the medical system and the safety of the vaccine. The African American participants also suggested that physicians do not adequately address patient concerns through discussion and the information they provide.
Providers were concerned that many people do not believe the flu is a severe illness or that they are susceptible. Although the African Americans in the study recognized some high-risk groups, they tended not to consider themselves part of any of those groups.
Doctors didn’t realize that patients fear drug interactions
One of the interesting findings of this study was that armed with the knowledge that the flu shot does not guarantee flu prevention, African American adults were willing to forego the vaccine. This absence of a guarantee also reinforced their beliefs that other prevention methods, such as handwashing and avoidance, are more effective.
Not surprisingly, of course, was the finding that patients continue to avoid the flu shot for fear of getting the flu.2,6 What was a bit surprising was that providers did not recognize that fear of medication reactions (drug interactions and allergic reactions) was also a barrier to flu vaccination. Providers also missed out on an educational opportunity, since many of the African American participants wanted to discuss the possibility of interactions with them.
Limitations of the study
External validity is limited because the findings cannot be generalized to every African American population in the US. The participants made up a non-random convenience sample of older African Americans in a Midwestern city, although the community-based recruitment strategy succeeded in reaching members of a lower income urban population. This study included only those who were ambivalent about the vaccine and who were open to both the pros and cons of vaccination. Project staff minimized possible interviewer bias by using experienced moderators, ensuring the consistent use of moderator guides, and using consensus coding procedures.
Funding
This research was funded by grant #6465 from the National Immunization Program at the Centers for Disease Control and Prevention, via Special Interest Project 11, to the Prevention research Center at the Saint louis University School of Public Health.
Acknowledgments
We thank Katie Duggan at the School of Public Health and edith Gary and Pascale Wortley at the CDC for their support. This article is dedicated to the memory of Joe D. Wray, MD, who suggested it.
Correspondence
Ricardo J. Wray, PhD, Community Health, Saint Louis University School of Public Health, 3545 Lafayette Avenue, St. Louis, MO 63104; wray@slu.edu
US Department of Health and Human Services. Immunization and infectious disease. Progress Report. Healthy People 2010. Available at: www.healthypeople.gov/Document/HTML/Volume1/14Immunization.htm. Accessed on September 28, 2007.
2. Centers for Disease Control and Prevention. Reasons reported by Medicare beneficiaries for not receiving influenza and pneumococcal vaccinations—United States, 1996. MMWR Morb Mortal Wkly Rpt 1999;48:889-890.
3. Marin M, Johanson W, Salas-lopez D. Influenza vaccination among minority populations in the United States. Prev Med 2002;34:236-241.
4. Fiscella K, Franks P, Doescher M, Saver B. Disparities in health care by race, ethnicity, and language among the insured: Findings from a national sample. Med Care 2002;40:52-59.
5. Ludwig-Beymer P, Gerc S. An influenza prevention campaign: The employee perspective. J Nurs Care Qual 2002;16(3):1-12.
6. Zimmerman R, Santibanez T, Janosky J, et al. What affects influenza vaccination rates among older patients? An analysis from inner-city, suburban, rural, and veterans Affairs practice. Am J Med 2003;114:31-38.
7. Nexoe J, Oltarzewska A, Sawicka-Powierza J, Kragstrup J, Kristiansen I. Perception of risk information: Similarities and differences between Danish and Polish general practitioners. Scand J Prim Health Care 2002;20:183-187.
8. Nichol K, Lofgren R, Gapinski J. Influenza vaccination: Knowledge, attitudes, and behavior among high-risk outpatients. Arch Intern Med 1992;152:106-110.
9. Santibanez T, Nowalk M, Zimmerman R, et al. Knowledge and beliefs about influenza, pneumococcal disease, and immunizations among older people. J Am Geriatr Soc 2002;51:1711-1716.
10. Demicheli V, Jefferson T, Rivetti D, Deeks J. Prevention and early treatment of influenza in healthy adults. Vaccine 2000;18:957-1030.
11. Telford R, Rogers A. What influences elderly peoples’ decisions about whether to accept the influenza vaccination? A qualitative study. Health Educ Res 2003;18:743-753.
12. Nicoleau A, Nicoleau C, Balzora J, Oboh A, Siddiqui N, Rosenberg C. Elderly African-Americans and the influenza vaccine: The impact of the primary care physician. J Am Med Dir Assoc 2001;2:56-59.
13. Riddiough M, Willems J, Sanders C, Kemp K. Factors affecting the use of vaccines: Considerations for immunization program planners. Public Health Rep 1981;96:528-535.
14. Steuart G. Social and behavioral change strategies. In: Phillips H, Gaylord S, eds. Aging and Public Health New York, NY: Springer;1985.
US Department of Health and Human Services. Immunization and infectious disease. Progress Report. Healthy People 2010. Available at: www.healthypeople.gov/Document/HTML/Volume1/14Immunization.htm. Accessed on September 28, 2007.
2. Centers for Disease Control and Prevention. Reasons reported by Medicare beneficiaries for not receiving influenza and pneumococcal vaccinations—United States, 1996. MMWR Morb Mortal Wkly Rpt 1999;48:889-890.
3. Marin M, Johanson W, Salas-lopez D. Influenza vaccination among minority populations in the United States. Prev Med 2002;34:236-241.
4. Fiscella K, Franks P, Doescher M, Saver B. Disparities in health care by race, ethnicity, and language among the insured: Findings from a national sample. Med Care 2002;40:52-59.
5. Ludwig-Beymer P, Gerc S. An influenza prevention campaign: The employee perspective. J Nurs Care Qual 2002;16(3):1-12.
6. Zimmerman R, Santibanez T, Janosky J, et al. What affects influenza vaccination rates among older patients? An analysis from inner-city, suburban, rural, and veterans Affairs practice. Am J Med 2003;114:31-38.
7. Nexoe J, Oltarzewska A, Sawicka-Powierza J, Kragstrup J, Kristiansen I. Perception of risk information: Similarities and differences between Danish and Polish general practitioners. Scand J Prim Health Care 2002;20:183-187.
8. Nichol K, Lofgren R, Gapinski J. Influenza vaccination: Knowledge, attitudes, and behavior among high-risk outpatients. Arch Intern Med 1992;152:106-110.
9. Santibanez T, Nowalk M, Zimmerman R, et al. Knowledge and beliefs about influenza, pneumococcal disease, and immunizations among older people. J Am Geriatr Soc 2002;51:1711-1716.
10. Demicheli V, Jefferson T, Rivetti D, Deeks J. Prevention and early treatment of influenza in healthy adults. Vaccine 2000;18:957-1030.
11. Telford R, Rogers A. What influences elderly peoples’ decisions about whether to accept the influenza vaccination? A qualitative study. Health Educ Res 2003;18:743-753.
12. Nicoleau A, Nicoleau C, Balzora J, Oboh A, Siddiqui N, Rosenberg C. Elderly African-Americans and the influenza vaccine: The impact of the primary care physician. J Am Med Dir Assoc 2001;2:56-59.
13. Riddiough M, Willems J, Sanders C, Kemp K. Factors affecting the use of vaccines: Considerations for immunization program planners. Public Health Rep 1981;96:528-535.
14. Steuart G. Social and behavioral change strategies. In: Phillips H, Gaylord S, eds. Aging and Public Health New York, NY: Springer;1985.
Book Review

Stolen Kisses (by Emily Osborn)
Poetry, without question, is a tricky thing. For many Americans it is an unapproachable art form that resides in a fortress guarded by elite intellectuals. For the minority of Americans who read it, it is a personal thingtough to define what works for some readers and tougher to understand for most. For the occasional reader of poetry, the favorite poem is usually something that sparks a familiar memory and puts it in perspectivea first love, the sight of the moon rising over a ridge in the mountains in the winter, or the memory of a summer night in youth. For those of us who don't read much poetry, it is the commonality of experience buried in the words speaking to something deep down inside our common existence as humans that tends to attract us to a poemthe I've been there or I've felt that experience.
Although the language in many of the poems in Body Language is striking, what draws in the physician‐reader more than anything else is the commonality of experiences inherent in these works. There are many remarkable landscapes in these poems, from the struggle to understand the intricate detail of the human body in anatomy class to the indelible memories of the patients who are manic or hopelessly depressed during the psychiatry core clerkship. It is mostly all here, the things we have experienced, in the form of poetry, evoking those moments that most of us painfully internalized or stepped around or ignored for lack of time to pay any attention to. For some of us, both the subtle and more profound experiences have become shadows or scars and for many, things we just never understood very well to begin with and try not to think about any more. These are our stories almost as much as they are those of the physician and medical student poets who wrote them. This book brings our experiences back, whether sadly, bluntly, humorously, or subtly, in a way that reminds us of all the things we've been blessed and cursed to see and be part of.
Body Language was the brain child of Neeta Jain, when she was still a medical student at the University of Rochester. She collaborated with another medical student from Yale University, Dagan Coppock, with the support of her University of Rochester faculty adviser, Stephanie Brown Clark. During the waning months of medical school, they solicited submissions from students, residents, and attending physicians from throughout the United States. Out of hundreds of submissions, they chose approximately 90 poems to create this anthology.
Perhaps I am cynical or perhaps I just don't really believe that given the frantic nature of modern medicine, there are many doctors who can devote the time to polishing their poetry in the tradition of William Carlos Williams, a New Jersey general practitioner who practiced before the era of information overload. Williams wrote on a typewriter between patients, during the time we reserve for looking up a question on Up‐to‐Date or answering a 1‐week old e‐mail.
But I was wrong. I came home from work exhausted one evening and picked up the book to discover another world, however familiar that world was. In that world are poems that occasionally jump off the page. Many of these poems were written by serious poets, poets published long before this book came along, and some are written by relative novices. But what unites these poems is the powerthe raw emotionof so many of the experiences described. We're reminded of overwhelming fatigue so harsh one envies the dead or the mundane call to pronounce a patient's death before fading back into the halls of the hospital. It is all here, our experiences in training and in the practice of medicine.
The anthology is divided into 6 sections: medical student, first year; medical student, second year; medical student, clinical years; intern; resident; and attending. It is almost impossible not to find a situation or emotion in a poem in each section that all physicians have experienced at some point in our lives. For example, life that occasionally interjects itself into the mind‐numbing lecture hall of our preclinical years of medical school (Richard M. Berlin):
Medical School Lovers
And for residents, the soft admit in the night (Mindy Shah):
MAO
After reading this book cover to cover, I was not surprised to learn that Garrison Keillor had asked permission to read some of its contents on his radio show. It is great stuff that speaks about many of the things we've been through that we're too tired or too busy or too afraid to stop and ponder over our years of practicing medicine. This anthology is easily worth the $15 it costs, if only because it repays us as a guide through the remarkable landscapes we have known.

Stolen Kisses (by Emily Osborn)
Poetry, without question, is a tricky thing. For many Americans it is an unapproachable art form that resides in a fortress guarded by elite intellectuals. For the minority of Americans who read it, it is a personal thingtough to define what works for some readers and tougher to understand for most. For the occasional reader of poetry, the favorite poem is usually something that sparks a familiar memory and puts it in perspectivea first love, the sight of the moon rising over a ridge in the mountains in the winter, or the memory of a summer night in youth. For those of us who don't read much poetry, it is the commonality of experience buried in the words speaking to something deep down inside our common existence as humans that tends to attract us to a poemthe I've been there or I've felt that experience.
Although the language in many of the poems in Body Language is striking, what draws in the physician‐reader more than anything else is the commonality of experiences inherent in these works. There are many remarkable landscapes in these poems, from the struggle to understand the intricate detail of the human body in anatomy class to the indelible memories of the patients who are manic or hopelessly depressed during the psychiatry core clerkship. It is mostly all here, the things we have experienced, in the form of poetry, evoking those moments that most of us painfully internalized or stepped around or ignored for lack of time to pay any attention to. For some of us, both the subtle and more profound experiences have become shadows or scars and for many, things we just never understood very well to begin with and try not to think about any more. These are our stories almost as much as they are those of the physician and medical student poets who wrote them. This book brings our experiences back, whether sadly, bluntly, humorously, or subtly, in a way that reminds us of all the things we've been blessed and cursed to see and be part of.
Body Language was the brain child of Neeta Jain, when she was still a medical student at the University of Rochester. She collaborated with another medical student from Yale University, Dagan Coppock, with the support of her University of Rochester faculty adviser, Stephanie Brown Clark. During the waning months of medical school, they solicited submissions from students, residents, and attending physicians from throughout the United States. Out of hundreds of submissions, they chose approximately 90 poems to create this anthology.
Perhaps I am cynical or perhaps I just don't really believe that given the frantic nature of modern medicine, there are many doctors who can devote the time to polishing their poetry in the tradition of William Carlos Williams, a New Jersey general practitioner who practiced before the era of information overload. Williams wrote on a typewriter between patients, during the time we reserve for looking up a question on Up‐to‐Date or answering a 1‐week old e‐mail.
But I was wrong. I came home from work exhausted one evening and picked up the book to discover another world, however familiar that world was. In that world are poems that occasionally jump off the page. Many of these poems were written by serious poets, poets published long before this book came along, and some are written by relative novices. But what unites these poems is the powerthe raw emotionof so many of the experiences described. We're reminded of overwhelming fatigue so harsh one envies the dead or the mundane call to pronounce a patient's death before fading back into the halls of the hospital. It is all here, our experiences in training and in the practice of medicine.
The anthology is divided into 6 sections: medical student, first year; medical student, second year; medical student, clinical years; intern; resident; and attending. It is almost impossible not to find a situation or emotion in a poem in each section that all physicians have experienced at some point in our lives. For example, life that occasionally interjects itself into the mind‐numbing lecture hall of our preclinical years of medical school (Richard M. Berlin):
Medical School Lovers
And for residents, the soft admit in the night (Mindy Shah):
MAO
After reading this book cover to cover, I was not surprised to learn that Garrison Keillor had asked permission to read some of its contents on his radio show. It is great stuff that speaks about many of the things we've been through that we're too tired or too busy or too afraid to stop and ponder over our years of practicing medicine. This anthology is easily worth the $15 it costs, if only because it repays us as a guide through the remarkable landscapes we have known.

Stolen Kisses (by Emily Osborn)
Poetry, without question, is a tricky thing. For many Americans it is an unapproachable art form that resides in a fortress guarded by elite intellectuals. For the minority of Americans who read it, it is a personal thingtough to define what works for some readers and tougher to understand for most. For the occasional reader of poetry, the favorite poem is usually something that sparks a familiar memory and puts it in perspectivea first love, the sight of the moon rising over a ridge in the mountains in the winter, or the memory of a summer night in youth. For those of us who don't read much poetry, it is the commonality of experience buried in the words speaking to something deep down inside our common existence as humans that tends to attract us to a poemthe I've been there or I've felt that experience.
Although the language in many of the poems in Body Language is striking, what draws in the physician‐reader more than anything else is the commonality of experiences inherent in these works. There are many remarkable landscapes in these poems, from the struggle to understand the intricate detail of the human body in anatomy class to the indelible memories of the patients who are manic or hopelessly depressed during the psychiatry core clerkship. It is mostly all here, the things we have experienced, in the form of poetry, evoking those moments that most of us painfully internalized or stepped around or ignored for lack of time to pay any attention to. For some of us, both the subtle and more profound experiences have become shadows or scars and for many, things we just never understood very well to begin with and try not to think about any more. These are our stories almost as much as they are those of the physician and medical student poets who wrote them. This book brings our experiences back, whether sadly, bluntly, humorously, or subtly, in a way that reminds us of all the things we've been blessed and cursed to see and be part of.
Body Language was the brain child of Neeta Jain, when she was still a medical student at the University of Rochester. She collaborated with another medical student from Yale University, Dagan Coppock, with the support of her University of Rochester faculty adviser, Stephanie Brown Clark. During the waning months of medical school, they solicited submissions from students, residents, and attending physicians from throughout the United States. Out of hundreds of submissions, they chose approximately 90 poems to create this anthology.
Perhaps I am cynical or perhaps I just don't really believe that given the frantic nature of modern medicine, there are many doctors who can devote the time to polishing their poetry in the tradition of William Carlos Williams, a New Jersey general practitioner who practiced before the era of information overload. Williams wrote on a typewriter between patients, during the time we reserve for looking up a question on Up‐to‐Date or answering a 1‐week old e‐mail.
But I was wrong. I came home from work exhausted one evening and picked up the book to discover another world, however familiar that world was. In that world are poems that occasionally jump off the page. Many of these poems were written by serious poets, poets published long before this book came along, and some are written by relative novices. But what unites these poems is the powerthe raw emotionof so many of the experiences described. We're reminded of overwhelming fatigue so harsh one envies the dead or the mundane call to pronounce a patient's death before fading back into the halls of the hospital. It is all here, our experiences in training and in the practice of medicine.
The anthology is divided into 6 sections: medical student, first year; medical student, second year; medical student, clinical years; intern; resident; and attending. It is almost impossible not to find a situation or emotion in a poem in each section that all physicians have experienced at some point in our lives. For example, life that occasionally interjects itself into the mind‐numbing lecture hall of our preclinical years of medical school (Richard M. Berlin):
Medical School Lovers
And for residents, the soft admit in the night (Mindy Shah):
MAO
After reading this book cover to cover, I was not surprised to learn that Garrison Keillor had asked permission to read some of its contents on his radio show. It is great stuff that speaks about many of the things we've been through that we're too tired or too busy or too afraid to stop and ponder over our years of practicing medicine. This anthology is easily worth the $15 it costs, if only because it repays us as a guide through the remarkable landscapes we have known.
Hospital Discharge Information and Older Patients
Transitions from the acute hospital to other sites of care are critical and potentially dangerous times for patients. Improving coordination of care among health care settings is a major area of emphasis in the Institute of Medicine publication, Crossing the Quality Chasm: A New Health System for the 21st Century.1 System factors such as poor information transmission processes, inadequate training of discharging staff, and inadequate time for discharge teaching can prevent patients from having the information they need when being discharged home. Patient factors such as nervousness, home distractions, and poor health literacy further limit the implementation of discharge plans. Misalignment of system and patient factors can result in a bewildered patient with a failed discharge process that subverts the intentions of even the best posthospital plan. Regardless of whether system and/or patient factors underlie the problem, the perception of that bewildered patient is that of receiving inadequate instruction for self‐care after discharge from the hospital. As self‐care and medication compliance play an important role in health outcomes and whether patients are readmitted, such a perceived (or actual) lack of instruction can have important implications for future health, physical function, and quality of life. Patients cared for in settings where health literacy is generally low and socioeconomic conditions poor may be at especially high risk of problems with communication of the discharge plan.
The objectives of this study were to (1) describe patient recall regarding pre‐discharge communication between hospital staff and patients regarding discharge instructions, and (2) to demonstrate that a post‐hospitalization survey was both feasible and revealing in an urban, public hospital setting.
METHODS
This cross‐sectional survey of older inpatients discharged from Grady Memorial Hospital, an academically affiliated, 953‐bed public teaching hospital in downtown Atlanta, Georgia, was conducted by telephone.
Subjects
All discharges of inpatients age 70 years and older (n = 714) were identified through a computer search performed on weekdays over the study period by the Grady Information Service Office. According to the computerized discharge information, 114 patients had either died in the hospital or were discharged to a nursing home. No attempt was made to contact the nursing home patients. When attempts were made to contact the remaining 600 potential subjects, it was determined that 331 either had died, had been admitted to a nursing home, or had unusable contact information. The remaining 269 patients and their families were interviewed for this study. Nobody refused to participate. Proxies answered survey questions instead of patients when the surveyor was informed by the contacted individual that the patient would not be able to answer the questions. This study was approved by the Internal Review Board of Emory University School of Medicine.
Survey
Telephone interviews were conducted from September 7, 2004, to January 19, 2005. The survey was developed by the investigators. The interview was constructed to include important information to be communicated to patients being discharged home from the hospital. The content was based on a literature review and clinical experience. The survey was pilot‐tested for feasibility and clarity, then revised once prior to data collection.
The survey instrument (see Appendix) has 37 questions regarding 5 main components: (1) demographic information, (2) care instruction at discharge, (3) patient self‐rating of care during hospitalization, (4) needs and functioning once discharged home, and (5) patient opinion about the public hospital health system in general. Only data on the first 3 areas are presented in this article. Each interview took approximately 2030 minutes to complete. All interviews were performed by a single trained interviewer with a Master of Social Work degree employed by the Grady Health System Social Services Department. Most interviews were conducted by the third day after discharge (range of 110 days). For subjects admitted multiple times data were only collected for the first discharge during the study period.
Measurements
Descriptive data analysis was used to analyze open‐ended questions. Responses of Don't recall and Unsure and questions with no response were all classified as a single category, No answer. Several questions in the survey were only asked to some respondents contingent on their previous answers. For example, Do you remember who spoke with you? was only asked to those who had answered yes to the previous question, Did anyone talk with you about how to care for yourself after this hospitalization? The number of admissions to the study facility over the study period was determined by hospital administrative data.
Two investigators (W.P. and A.S.) independently analyzed the content of open‐ended questions and then compared their analyses in order to establish interrater reliability on themes. Chi‐square analysis was used to determine the relationship between patients recollecting instructions and the outcome of interest (understanding instructions, medication compliance, calling for problems).
RESULTS
We found the survey to be feasible and easily administered. Over the study period the mean length of stay of the respondents was 5.6 days (range, 056 days), and the mean number of times admitted was 1.6 (range, 17). Interviews were conducted an average of 3 days after discharge (range, 110 days). Other demographic information on the respondents is summarized in Table 1.
| Characteristic | Mean | Range |
|---|---|---|
| Age (years) | 78.7 | (70100) |
| Length of stay (days) | 5.6 | (056) |
| Number of admissions | 1.5 | (17) |
| Days postdischarge | 3 | (010) |
| N | % | |
| Sex | ||
| Male | 84 | 31 |
| Female | 185 | 69 |
| Marital status | ||
| Widowed | 164 | 61 |
| Married | 60 | 22 |
| Divorced | 15 | 6 |
| Separated | 14 | 5 |
| Single | 10 | 4 |
| No answer | 6 | 2 |
| Survey respondent | ||
| Patient | 187 | 70 |
| Child | 35 | 13 |
| Other | 33 | 12 |
| Spouse | 14 | 5 |
Most of those surveyed (81.8%, or 242 respondents) were able to answer the question Can you tell me what was explained to you [about why you were hospitalized]? The results of the content analysis of the answers to this open‐ended question are summarized in Table 2. The self‐reported problem area most frequently mentioned was the heart. Responses to questions about instructions and education received while in the hospital are reported in Table 3.
| Reason | n |
|---|---|
| Heart problem | 46 |
| Nonspecific (I am sick/I have chronic disease/all kinds of problems) | 29 |
| Don't know/no answer | 27 |
| Blood problem/bleeding/blood clot | 17 |
| Blood pressure problem (high or low) | 14 |
| Kidney problem | 14 |
| Surgery | 13 |
| Breathing problem | 13 |
| Stroke | 11 |
| Cold | 10 |
| Infection | 9 |
| Arm/leg/hand/feet/knee/bone | 8 |
| Fall | 8 |
| Stomach problem | 8 |
| Cancer | 7 |
| Diabetes | 7 |
| Dehydration | 6 |
| Lung problem | 6 |
| Bladder problem | 5 |
| Mental problem | 4 |
| Seizure | 4 |
| Automobile accident | 1 |
| Need medication | 1 |
| Prostate problem | 1 |
| Question | N | Yes (%) | No (%) | No Answer (%) |
|---|---|---|---|---|
| ||||
| Did someone explain to you why you were hospitalized ? | 269 | 224 (85%) | 35 (13%) | 6 (2.%) |
| Did the doctor explain your medical problems to you? | 269 | 205 (76%) | 49 (18%) | 15 (6%) |
| Did anyone talk with you about how to care for yourself after this hospitalization? | 269 | 115 (43%) | 141 (52%) | 13 (5%) |
| Do you remember who spoke with you?* | 115 | 91 (79%) | 22 (19%) | 2 (2%) |
| Doctor?* | 91 | 74 (81%) | 12 (13%) | 5 (6%) |
| Nurse?* | 91 | 44 (48%) | 43 (47%) | 4 (4%) |
| Other professional?* | 91 | 14 (15%) | 73 (80%) | 4 (4%) |
| If you had questions were they answered?* | 115 | 99 (86%) | 10 (9%) | 6 (5%) |
| Were you given a telephone number or name of a person to call if you needed help after you returned home? | 269 | 72 (27%) | 127 (47%) | 70 (26%) |
| Were you told what to do if you experienced problems at home? | 269 | 89 (33%) | 111 (41%) | 69 (26%) |
| Have you had to call about any problems since you arrived home? | 269 | 36 (13%) | 212 (79%) | 19 (8%) |
| Were your medications changed during this hospitalization? | 269 | 114 (42%) | 138 (52%) | 17 (6%) |
| Did someone explain how to take them?* | 114 | 90 (79%) | 16 (14%) | 8 (7%) |
| Doctor?* | 90 | 47 (52%) | 42 (47%) | 1 (1%) |
| Nurse?* | 90 | 39 (43%) | 51 (57%) | 0 (0%) |
| Pharmacist?* | 90 | 57 (63%) | 33 (37%) | 0 (0%) |
| Did you get your medication?* | 114 | 61 (53%) | 9 (8%) | 44 (39%) |
| Are you taking them the way they were explained to you?* | 114 | 95 (84%) | 13 (11%) | 6 (5%) |
| Since you have been home from the hospital do you feel you are receiving enough help? | 269 | 216 (80%) | 47 (18%) | 6 (2%) |
| If no, have you asked for more help?** | 47 | 26 (55%) | 12 (26%) | 9 (19%) |
A correlation was found between providing information in written and verbal fashion and self‐reported understanding of the instructions. For example, of the 103 respondents who answered affirmatively to Did anyone talk with you about how to care for yourself at home after this hospitalization? and also recalled the source of that information, 66.0% (n = 68) reported receiving instructions verbally, 10.7% (n = 11) reported receiving them in writing, and 23.3% (n = 24) reported receiving both. Patients who received both verbal and written instructions were more likely to report that they understood the care instructions very well versus somewhat or very little (2 = 29.612, df = 4, P = .000).
The association between the perceived provision of information to patients and effective use of that information was explored. For example, perceived medication compliance and instruction on medication use had a positive association. Among those who recalled receiving instruction on how to take their medications (n = 88), 76 (86.4%) stated that they were taking them correctly, 8 (9.1%) that they were not taking them correctly, and 4 (4.5%) were unsure. Among those who said that they did not receive instruction or did not recall being instructed on how to take their medications (n = 26), 16 (61.5%) believed that they were taking their mediations correctly, 4 (15.4%) that they were not taking them correctly, and 5 (19.2%) were unsure. Respondents who recalled receiving medication instruction were more likely to comply with taking medication. (2 = 7.321, P = .026)
There was also a positive association between being told what to do if problems were experienced at home and calling about problems after arriving home. Among those who believed they were instructed on what to do if problems were experienced at home (N = 86), 23 (26.7%) reported they called about a problem. Among those who did not recall being instructed about what to do if problems were experienced at home (N = 183), only 13 (7.1%) reported calling about a problem. Respondents who believed they had been instructed on what to do at home were significantly more likely to call from home about problems (2 = 16.740, df = 2, P = .000).
DISCUSSION
According to Bull and Roberts,2 there are 3 types of communication gaps in discharge planning: (1) gaps between health care providers in the hospital and those involved in the hospital‐community interface, (2) gaps between providers and patients, and (3) gaps between health care providers and family caregivers for elders. The present study focuses on the latter 2 types of communication gaps. In discharge planning it is critical not only to transmit information, but also to make sure that patients understand that information in the way health care providers intended. Systemic, cultural, emotional, and cognitive barriers may interact to limit the effectiveness of this communication.
In the present study, a large number of patients discharged from an academically affiliated public hospital were unaware of important discharge information, even though according to hospital protocol, all patients are given a discharge information sheet. Approximately 15% did not know why they were hospitalized. About 20% of those who reported their medications were changed in the hospital could not recall anyone explaining how to take these medications. More than half of respondents did not recall anyone speaking with them about how to take care of themselves following hospitalization. More than 60% of respondents did not recall getting information on what to do if they had a problem after being discharge home.
That patients were unaware of information that had been provided to them has significant implications for successful implementation of the spirit of the Joint Commission on Accreditation of Healthcare Organizations standards. These disease‐specific standards as well as medication standards are generally written as process measures. Although requiring that routine and clear standards of information be provided to patients is a significant step forward in patient safety, surveys such as the present one, done over time, should be an important part of any ongoing quality improvement process. As evident by the high response rate in our study and others,3 patients and their proxies are very willing to participate in such surveys. The results of the Consumer Assessment of Healthcare Providers and Systems (CHAMPS) survey,4 which will be published, should provide an important impetus for this ongoing quality improvement process. Interestingly, 2 of the CHAMPS questionsDuring this hospital stay, did doctors, nurses, or other hospital staff talk with you about whether you would have the help you needed when you left the hospital? and During this hospital stay, did you get information in writing about what symptoms or health problems to look out for after you left the hospital?are very similar to questions used in the present study. In our health system, site of the present study, these data have prompted a complete revision of the discharge instruction sheet, creation of a care transitions task force, and initiation of a pilot care transitions project. Follow‐up surveys will be performed to evaluate whether these changes have been effective in improving discharge information transfer and, more importantly, in patient outcomes.
The present study had several limitations. First, although the results are inconsistent with the findings of other studies, the present study took place at a single urban institution most of whose patients socioeconomically disadvantaged. Second, patient responses were combined with caregiver responses (elicited when a patient was unable to respond to the survey questions). Although it is not proven that these 2 groups are equivalent, from a practical point of view this was justified because the clinical issue is whether the person taking care of the patient (be it patient or caregiver) has the critical information needed after discharge from the hospital. Third, only those who could be reached by phone were included. This is likely to bias the results in a more favorable direction, given that the socioeconomic implications of not having a phone and/or the cognitive implications of not being able to use a phone would likely be reflected in even greater impediments to communication. Fourth, no attempt was made to evaluate the cognitive status or health literacy of either the subjects or their proxies. Fifth, generalizability of the survey to other research groups is unclear, as no additional attempt was made to define the interrater reliability of the survey. Finally, although it seems reasonable to presume that simpler discharge plans would be more effectively communicated, this study did not define the complexity of each discharge plan. Strengths of the study include a single trained interviewer, relatively rapid follow‐up of patients, and large sample size.
Effective communication during the care transition is important for improving patient outcomes and satisfaction. One study of 40 patient‐caregiver dyads showed that patients had a lower rate of medical problems postdischarge when they and their caregivers received verbal and/or printed information about activity and complications that could occur at home.5 Indeed, a study of 134 elder/family caregiver dyads interviewed 2 weeks after hospitalization found that receipt of information about the patient's condition, medications, and activities was an important contributor to both patient and family caregiver satisfaction with discharge care.6
At first glance, these findings may seem surprising, given that all patients discharged from the hospital should receive (by protocol) a discharge information sheet with postdischarge instructions. This study did not define what exactly transpired between hospital staff and patients, review discharge sheets, or validate the extent to which these instruction sheets are completely filled out and adequately reviewed with patients. The results of previous studies suggest that even when conversations are verified to have occurred, transmission is often inadequate. One study of 54 adult patients discharged after being hospitalized for pneumonia or acute myocardial infarction found that physicians believed that 88.9% of patients understood potential side effects of postdischarge medications, but only 57.4% of patients reported that they did understand instructions about side effects (P < .001).7 Another study of 47 patients discharged from a municipal teaching hospital in New York City showed that only 42% were able to state their diagnoses and only 28% were able to list all their medicines.8
The solutions to these problems may be as unapparent as they are difficult, and many of the challenges as well as some potential solutions have been recently reviewed.9 Arguing that physicians and/or hospital staff should spend more time with all their patients oversimplifies the problem and is not likely to occur. The present study confirmed earlier findings that providing verbal and written health information on hospital discharge significantly increases the knowledge of patients and caregivers.10 Risk stratificationtargeting those most at risk of medication noncompliance to receive augmented medication compliance instruction, such as the scheme suggested by Rosenow11has significant merit and warrants extension to discharge instructions in general and to prospective testing.
Discharge teaching videos have been shown to have some effectiveness in the emergency room setting12 and along with an audio‐only or CD option could be developed to supplement the written discharge information provided to patients. Calling patients after discharge to make sure they understand their prescribed medical regimen, have their prescriptions and home health equipment, and have a follow‐up visit scheduled with their doctors has been identified as a key characteristic of high‐achieving hospitalist programs. One care site found that 80% of patients have questions about their follow‐up care that could jeopardize their recovery.13 This strategy could just as well be implemented by a case manager or health educator. Follow‐up phone contact combined with Telecare has been shown to be effective in reducing hospital readmissions, emergency visits, and cost of care for patients with heart failure.14 Formal care transition instruments and interventions show promise for enabling patients and caregivers to take a more active role during care transition processes and improve outcomes. Whether this approach will be widely generalizable awaits demonstration.15
CONCLUSIONS
In summary, we found that a posthospitalization survey was both feasible and revealing and had a high acceptance rate in this urban public hospital population. Furthermore, subject recall about predischarge communication from hospital staff regarding discharge instructions demonstrated significant gaps about transfer of information. If these findings prove to be applicable to the large numbers of older as well as younger patients discharged from the hospital each year, the implications for patients health, safety, and satisfaction are enormous. It should not be assumed that this is a problem limited to older patients. As health care systems build bridges across gaps in the quality chasm, developing and testing more effective communication strategies for patients is imperative.
- Institute of Medicine.Crossing the Quality Chasm: A New Health System of the 21st Century. Committee on Quality of Health Care in America, editor.Washington, DC:National Academy Press;2001.
- ,.Components of a proper hospital discharge for elders.J Adv Nurs.2001;35:571–581.
- ,,.Assessing the quality of preparation for posthospital care from the patient's perspective—the care transitions measure.Med Care.2005;43:246–255.
- Available at http://www.hcahpsonline.org/default.aspx. Accessed September 23,2006.
- .Managing post‐discharge care at home: an analysis of patients' and their carers' perceptions of information received during their stay in hospital.J Advan Nurs.2000;31:1165–1173.
- ...Predictors of elder and family caregiver satisfaction with discharge planning.J Cardiovasc Nurs.2000;14:76–87.
- ,,,,,, et al.Patient‐physician communication at hospital discharge and patients' understanding of the postdischarge treatment plan.Arch Intern Med.1997;157:1026–1030.
- ,.Patient understanding of their treatment plans and diagnosis at discharge.Mayo Clin Proc.2005;80:991–994.
- ,.Lost in transition: challenges and opportunities for improving the quality of transitional care.Ann Intern Med.2004;140:533–536.
- ,,.Written and verbal information versus verbal information only for patients being discharged from acute hospital settings to home.Cochrane Database Syst Rev.2003;4:CD003716.
- .Patients' understanding of and compliance with medications: the sixth vital sign?Mayo Clin Proc.2005;80:983–987.
- ,,,,,.Development of an ED teaching program aimed at reducing prehospital delays for patients with chest pain.J Emerg Nurs.1998;24:316–319.
- .Remedy for high health care costs: hospitalist programs. January 5,2004.Phoenix Bus J. Available at: http://www.cogenthealthcare.com/Remedyopedfinal.pdf. Accessed August 22,year="2005"2005.
- ,,.Reducing the cost of frequent hospital admissions for congestive heart failure: a randomized trial of a home telecare intervention.Med Care,2001;39:1234–1245.
- ,,,,,.Preparing patients and caregivers to participate in care delivered across settings: the care transitions intervention.J Am Geriatr Soc.2004;52:1817–1825.
Transitions from the acute hospital to other sites of care are critical and potentially dangerous times for patients. Improving coordination of care among health care settings is a major area of emphasis in the Institute of Medicine publication, Crossing the Quality Chasm: A New Health System for the 21st Century.1 System factors such as poor information transmission processes, inadequate training of discharging staff, and inadequate time for discharge teaching can prevent patients from having the information they need when being discharged home. Patient factors such as nervousness, home distractions, and poor health literacy further limit the implementation of discharge plans. Misalignment of system and patient factors can result in a bewildered patient with a failed discharge process that subverts the intentions of even the best posthospital plan. Regardless of whether system and/or patient factors underlie the problem, the perception of that bewildered patient is that of receiving inadequate instruction for self‐care after discharge from the hospital. As self‐care and medication compliance play an important role in health outcomes and whether patients are readmitted, such a perceived (or actual) lack of instruction can have important implications for future health, physical function, and quality of life. Patients cared for in settings where health literacy is generally low and socioeconomic conditions poor may be at especially high risk of problems with communication of the discharge plan.
The objectives of this study were to (1) describe patient recall regarding pre‐discharge communication between hospital staff and patients regarding discharge instructions, and (2) to demonstrate that a post‐hospitalization survey was both feasible and revealing in an urban, public hospital setting.
METHODS
This cross‐sectional survey of older inpatients discharged from Grady Memorial Hospital, an academically affiliated, 953‐bed public teaching hospital in downtown Atlanta, Georgia, was conducted by telephone.
Subjects
All discharges of inpatients age 70 years and older (n = 714) were identified through a computer search performed on weekdays over the study period by the Grady Information Service Office. According to the computerized discharge information, 114 patients had either died in the hospital or were discharged to a nursing home. No attempt was made to contact the nursing home patients. When attempts were made to contact the remaining 600 potential subjects, it was determined that 331 either had died, had been admitted to a nursing home, or had unusable contact information. The remaining 269 patients and their families were interviewed for this study. Nobody refused to participate. Proxies answered survey questions instead of patients when the surveyor was informed by the contacted individual that the patient would not be able to answer the questions. This study was approved by the Internal Review Board of Emory University School of Medicine.
Survey
Telephone interviews were conducted from September 7, 2004, to January 19, 2005. The survey was developed by the investigators. The interview was constructed to include important information to be communicated to patients being discharged home from the hospital. The content was based on a literature review and clinical experience. The survey was pilot‐tested for feasibility and clarity, then revised once prior to data collection.
The survey instrument (see Appendix) has 37 questions regarding 5 main components: (1) demographic information, (2) care instruction at discharge, (3) patient self‐rating of care during hospitalization, (4) needs and functioning once discharged home, and (5) patient opinion about the public hospital health system in general. Only data on the first 3 areas are presented in this article. Each interview took approximately 2030 minutes to complete. All interviews were performed by a single trained interviewer with a Master of Social Work degree employed by the Grady Health System Social Services Department. Most interviews were conducted by the third day after discharge (range of 110 days). For subjects admitted multiple times data were only collected for the first discharge during the study period.
Measurements
Descriptive data analysis was used to analyze open‐ended questions. Responses of Don't recall and Unsure and questions with no response were all classified as a single category, No answer. Several questions in the survey were only asked to some respondents contingent on their previous answers. For example, Do you remember who spoke with you? was only asked to those who had answered yes to the previous question, Did anyone talk with you about how to care for yourself after this hospitalization? The number of admissions to the study facility over the study period was determined by hospital administrative data.
Two investigators (W.P. and A.S.) independently analyzed the content of open‐ended questions and then compared their analyses in order to establish interrater reliability on themes. Chi‐square analysis was used to determine the relationship between patients recollecting instructions and the outcome of interest (understanding instructions, medication compliance, calling for problems).
RESULTS
We found the survey to be feasible and easily administered. Over the study period the mean length of stay of the respondents was 5.6 days (range, 056 days), and the mean number of times admitted was 1.6 (range, 17). Interviews were conducted an average of 3 days after discharge (range, 110 days). Other demographic information on the respondents is summarized in Table 1.
| Characteristic | Mean | Range |
|---|---|---|
| Age (years) | 78.7 | (70100) |
| Length of stay (days) | 5.6 | (056) |
| Number of admissions | 1.5 | (17) |
| Days postdischarge | 3 | (010) |
| N | % | |
| Sex | ||
| Male | 84 | 31 |
| Female | 185 | 69 |
| Marital status | ||
| Widowed | 164 | 61 |
| Married | 60 | 22 |
| Divorced | 15 | 6 |
| Separated | 14 | 5 |
| Single | 10 | 4 |
| No answer | 6 | 2 |
| Survey respondent | ||
| Patient | 187 | 70 |
| Child | 35 | 13 |
| Other | 33 | 12 |
| Spouse | 14 | 5 |
Most of those surveyed (81.8%, or 242 respondents) were able to answer the question Can you tell me what was explained to you [about why you were hospitalized]? The results of the content analysis of the answers to this open‐ended question are summarized in Table 2. The self‐reported problem area most frequently mentioned was the heart. Responses to questions about instructions and education received while in the hospital are reported in Table 3.
| Reason | n |
|---|---|
| Heart problem | 46 |
| Nonspecific (I am sick/I have chronic disease/all kinds of problems) | 29 |
| Don't know/no answer | 27 |
| Blood problem/bleeding/blood clot | 17 |
| Blood pressure problem (high or low) | 14 |
| Kidney problem | 14 |
| Surgery | 13 |
| Breathing problem | 13 |
| Stroke | 11 |
| Cold | 10 |
| Infection | 9 |
| Arm/leg/hand/feet/knee/bone | 8 |
| Fall | 8 |
| Stomach problem | 8 |
| Cancer | 7 |
| Diabetes | 7 |
| Dehydration | 6 |
| Lung problem | 6 |
| Bladder problem | 5 |
| Mental problem | 4 |
| Seizure | 4 |
| Automobile accident | 1 |
| Need medication | 1 |
| Prostate problem | 1 |
| Question | N | Yes (%) | No (%) | No Answer (%) |
|---|---|---|---|---|
| ||||
| Did someone explain to you why you were hospitalized ? | 269 | 224 (85%) | 35 (13%) | 6 (2.%) |
| Did the doctor explain your medical problems to you? | 269 | 205 (76%) | 49 (18%) | 15 (6%) |
| Did anyone talk with you about how to care for yourself after this hospitalization? | 269 | 115 (43%) | 141 (52%) | 13 (5%) |
| Do you remember who spoke with you?* | 115 | 91 (79%) | 22 (19%) | 2 (2%) |
| Doctor?* | 91 | 74 (81%) | 12 (13%) | 5 (6%) |
| Nurse?* | 91 | 44 (48%) | 43 (47%) | 4 (4%) |
| Other professional?* | 91 | 14 (15%) | 73 (80%) | 4 (4%) |
| If you had questions were they answered?* | 115 | 99 (86%) | 10 (9%) | 6 (5%) |
| Were you given a telephone number or name of a person to call if you needed help after you returned home? | 269 | 72 (27%) | 127 (47%) | 70 (26%) |
| Were you told what to do if you experienced problems at home? | 269 | 89 (33%) | 111 (41%) | 69 (26%) |
| Have you had to call about any problems since you arrived home? | 269 | 36 (13%) | 212 (79%) | 19 (8%) |
| Were your medications changed during this hospitalization? | 269 | 114 (42%) | 138 (52%) | 17 (6%) |
| Did someone explain how to take them?* | 114 | 90 (79%) | 16 (14%) | 8 (7%) |
| Doctor?* | 90 | 47 (52%) | 42 (47%) | 1 (1%) |
| Nurse?* | 90 | 39 (43%) | 51 (57%) | 0 (0%) |
| Pharmacist?* | 90 | 57 (63%) | 33 (37%) | 0 (0%) |
| Did you get your medication?* | 114 | 61 (53%) | 9 (8%) | 44 (39%) |
| Are you taking them the way they were explained to you?* | 114 | 95 (84%) | 13 (11%) | 6 (5%) |
| Since you have been home from the hospital do you feel you are receiving enough help? | 269 | 216 (80%) | 47 (18%) | 6 (2%) |
| If no, have you asked for more help?** | 47 | 26 (55%) | 12 (26%) | 9 (19%) |
A correlation was found between providing information in written and verbal fashion and self‐reported understanding of the instructions. For example, of the 103 respondents who answered affirmatively to Did anyone talk with you about how to care for yourself at home after this hospitalization? and also recalled the source of that information, 66.0% (n = 68) reported receiving instructions verbally, 10.7% (n = 11) reported receiving them in writing, and 23.3% (n = 24) reported receiving both. Patients who received both verbal and written instructions were more likely to report that they understood the care instructions very well versus somewhat or very little (2 = 29.612, df = 4, P = .000).
The association between the perceived provision of information to patients and effective use of that information was explored. For example, perceived medication compliance and instruction on medication use had a positive association. Among those who recalled receiving instruction on how to take their medications (n = 88), 76 (86.4%) stated that they were taking them correctly, 8 (9.1%) that they were not taking them correctly, and 4 (4.5%) were unsure. Among those who said that they did not receive instruction or did not recall being instructed on how to take their medications (n = 26), 16 (61.5%) believed that they were taking their mediations correctly, 4 (15.4%) that they were not taking them correctly, and 5 (19.2%) were unsure. Respondents who recalled receiving medication instruction were more likely to comply with taking medication. (2 = 7.321, P = .026)
There was also a positive association between being told what to do if problems were experienced at home and calling about problems after arriving home. Among those who believed they were instructed on what to do if problems were experienced at home (N = 86), 23 (26.7%) reported they called about a problem. Among those who did not recall being instructed about what to do if problems were experienced at home (N = 183), only 13 (7.1%) reported calling about a problem. Respondents who believed they had been instructed on what to do at home were significantly more likely to call from home about problems (2 = 16.740, df = 2, P = .000).
DISCUSSION
According to Bull and Roberts,2 there are 3 types of communication gaps in discharge planning: (1) gaps between health care providers in the hospital and those involved in the hospital‐community interface, (2) gaps between providers and patients, and (3) gaps between health care providers and family caregivers for elders. The present study focuses on the latter 2 types of communication gaps. In discharge planning it is critical not only to transmit information, but also to make sure that patients understand that information in the way health care providers intended. Systemic, cultural, emotional, and cognitive barriers may interact to limit the effectiveness of this communication.
In the present study, a large number of patients discharged from an academically affiliated public hospital were unaware of important discharge information, even though according to hospital protocol, all patients are given a discharge information sheet. Approximately 15% did not know why they were hospitalized. About 20% of those who reported their medications were changed in the hospital could not recall anyone explaining how to take these medications. More than half of respondents did not recall anyone speaking with them about how to take care of themselves following hospitalization. More than 60% of respondents did not recall getting information on what to do if they had a problem after being discharge home.
That patients were unaware of information that had been provided to them has significant implications for successful implementation of the spirit of the Joint Commission on Accreditation of Healthcare Organizations standards. These disease‐specific standards as well as medication standards are generally written as process measures. Although requiring that routine and clear standards of information be provided to patients is a significant step forward in patient safety, surveys such as the present one, done over time, should be an important part of any ongoing quality improvement process. As evident by the high response rate in our study and others,3 patients and their proxies are very willing to participate in such surveys. The results of the Consumer Assessment of Healthcare Providers and Systems (CHAMPS) survey,4 which will be published, should provide an important impetus for this ongoing quality improvement process. Interestingly, 2 of the CHAMPS questionsDuring this hospital stay, did doctors, nurses, or other hospital staff talk with you about whether you would have the help you needed when you left the hospital? and During this hospital stay, did you get information in writing about what symptoms or health problems to look out for after you left the hospital?are very similar to questions used in the present study. In our health system, site of the present study, these data have prompted a complete revision of the discharge instruction sheet, creation of a care transitions task force, and initiation of a pilot care transitions project. Follow‐up surveys will be performed to evaluate whether these changes have been effective in improving discharge information transfer and, more importantly, in patient outcomes.
The present study had several limitations. First, although the results are inconsistent with the findings of other studies, the present study took place at a single urban institution most of whose patients socioeconomically disadvantaged. Second, patient responses were combined with caregiver responses (elicited when a patient was unable to respond to the survey questions). Although it is not proven that these 2 groups are equivalent, from a practical point of view this was justified because the clinical issue is whether the person taking care of the patient (be it patient or caregiver) has the critical information needed after discharge from the hospital. Third, only those who could be reached by phone were included. This is likely to bias the results in a more favorable direction, given that the socioeconomic implications of not having a phone and/or the cognitive implications of not being able to use a phone would likely be reflected in even greater impediments to communication. Fourth, no attempt was made to evaluate the cognitive status or health literacy of either the subjects or their proxies. Fifth, generalizability of the survey to other research groups is unclear, as no additional attempt was made to define the interrater reliability of the survey. Finally, although it seems reasonable to presume that simpler discharge plans would be more effectively communicated, this study did not define the complexity of each discharge plan. Strengths of the study include a single trained interviewer, relatively rapid follow‐up of patients, and large sample size.
Effective communication during the care transition is important for improving patient outcomes and satisfaction. One study of 40 patient‐caregiver dyads showed that patients had a lower rate of medical problems postdischarge when they and their caregivers received verbal and/or printed information about activity and complications that could occur at home.5 Indeed, a study of 134 elder/family caregiver dyads interviewed 2 weeks after hospitalization found that receipt of information about the patient's condition, medications, and activities was an important contributor to both patient and family caregiver satisfaction with discharge care.6
At first glance, these findings may seem surprising, given that all patients discharged from the hospital should receive (by protocol) a discharge information sheet with postdischarge instructions. This study did not define what exactly transpired between hospital staff and patients, review discharge sheets, or validate the extent to which these instruction sheets are completely filled out and adequately reviewed with patients. The results of previous studies suggest that even when conversations are verified to have occurred, transmission is often inadequate. One study of 54 adult patients discharged after being hospitalized for pneumonia or acute myocardial infarction found that physicians believed that 88.9% of patients understood potential side effects of postdischarge medications, but only 57.4% of patients reported that they did understand instructions about side effects (P < .001).7 Another study of 47 patients discharged from a municipal teaching hospital in New York City showed that only 42% were able to state their diagnoses and only 28% were able to list all their medicines.8
The solutions to these problems may be as unapparent as they are difficult, and many of the challenges as well as some potential solutions have been recently reviewed.9 Arguing that physicians and/or hospital staff should spend more time with all their patients oversimplifies the problem and is not likely to occur. The present study confirmed earlier findings that providing verbal and written health information on hospital discharge significantly increases the knowledge of patients and caregivers.10 Risk stratificationtargeting those most at risk of medication noncompliance to receive augmented medication compliance instruction, such as the scheme suggested by Rosenow11has significant merit and warrants extension to discharge instructions in general and to prospective testing.
Discharge teaching videos have been shown to have some effectiveness in the emergency room setting12 and along with an audio‐only or CD option could be developed to supplement the written discharge information provided to patients. Calling patients after discharge to make sure they understand their prescribed medical regimen, have their prescriptions and home health equipment, and have a follow‐up visit scheduled with their doctors has been identified as a key characteristic of high‐achieving hospitalist programs. One care site found that 80% of patients have questions about their follow‐up care that could jeopardize their recovery.13 This strategy could just as well be implemented by a case manager or health educator. Follow‐up phone contact combined with Telecare has been shown to be effective in reducing hospital readmissions, emergency visits, and cost of care for patients with heart failure.14 Formal care transition instruments and interventions show promise for enabling patients and caregivers to take a more active role during care transition processes and improve outcomes. Whether this approach will be widely generalizable awaits demonstration.15
CONCLUSIONS
In summary, we found that a posthospitalization survey was both feasible and revealing and had a high acceptance rate in this urban public hospital population. Furthermore, subject recall about predischarge communication from hospital staff regarding discharge instructions demonstrated significant gaps about transfer of information. If these findings prove to be applicable to the large numbers of older as well as younger patients discharged from the hospital each year, the implications for patients health, safety, and satisfaction are enormous. It should not be assumed that this is a problem limited to older patients. As health care systems build bridges across gaps in the quality chasm, developing and testing more effective communication strategies for patients is imperative.
Transitions from the acute hospital to other sites of care are critical and potentially dangerous times for patients. Improving coordination of care among health care settings is a major area of emphasis in the Institute of Medicine publication, Crossing the Quality Chasm: A New Health System for the 21st Century.1 System factors such as poor information transmission processes, inadequate training of discharging staff, and inadequate time for discharge teaching can prevent patients from having the information they need when being discharged home. Patient factors such as nervousness, home distractions, and poor health literacy further limit the implementation of discharge plans. Misalignment of system and patient factors can result in a bewildered patient with a failed discharge process that subverts the intentions of even the best posthospital plan. Regardless of whether system and/or patient factors underlie the problem, the perception of that bewildered patient is that of receiving inadequate instruction for self‐care after discharge from the hospital. As self‐care and medication compliance play an important role in health outcomes and whether patients are readmitted, such a perceived (or actual) lack of instruction can have important implications for future health, physical function, and quality of life. Patients cared for in settings where health literacy is generally low and socioeconomic conditions poor may be at especially high risk of problems with communication of the discharge plan.
The objectives of this study were to (1) describe patient recall regarding pre‐discharge communication between hospital staff and patients regarding discharge instructions, and (2) to demonstrate that a post‐hospitalization survey was both feasible and revealing in an urban, public hospital setting.
METHODS
This cross‐sectional survey of older inpatients discharged from Grady Memorial Hospital, an academically affiliated, 953‐bed public teaching hospital in downtown Atlanta, Georgia, was conducted by telephone.
Subjects
All discharges of inpatients age 70 years and older (n = 714) were identified through a computer search performed on weekdays over the study period by the Grady Information Service Office. According to the computerized discharge information, 114 patients had either died in the hospital or were discharged to a nursing home. No attempt was made to contact the nursing home patients. When attempts were made to contact the remaining 600 potential subjects, it was determined that 331 either had died, had been admitted to a nursing home, or had unusable contact information. The remaining 269 patients and their families were interviewed for this study. Nobody refused to participate. Proxies answered survey questions instead of patients when the surveyor was informed by the contacted individual that the patient would not be able to answer the questions. This study was approved by the Internal Review Board of Emory University School of Medicine.
Survey
Telephone interviews were conducted from September 7, 2004, to January 19, 2005. The survey was developed by the investigators. The interview was constructed to include important information to be communicated to patients being discharged home from the hospital. The content was based on a literature review and clinical experience. The survey was pilot‐tested for feasibility and clarity, then revised once prior to data collection.
The survey instrument (see Appendix) has 37 questions regarding 5 main components: (1) demographic information, (2) care instruction at discharge, (3) patient self‐rating of care during hospitalization, (4) needs and functioning once discharged home, and (5) patient opinion about the public hospital health system in general. Only data on the first 3 areas are presented in this article. Each interview took approximately 2030 minutes to complete. All interviews were performed by a single trained interviewer with a Master of Social Work degree employed by the Grady Health System Social Services Department. Most interviews were conducted by the third day after discharge (range of 110 days). For subjects admitted multiple times data were only collected for the first discharge during the study period.
Measurements
Descriptive data analysis was used to analyze open‐ended questions. Responses of Don't recall and Unsure and questions with no response were all classified as a single category, No answer. Several questions in the survey were only asked to some respondents contingent on their previous answers. For example, Do you remember who spoke with you? was only asked to those who had answered yes to the previous question, Did anyone talk with you about how to care for yourself after this hospitalization? The number of admissions to the study facility over the study period was determined by hospital administrative data.
Two investigators (W.P. and A.S.) independently analyzed the content of open‐ended questions and then compared their analyses in order to establish interrater reliability on themes. Chi‐square analysis was used to determine the relationship between patients recollecting instructions and the outcome of interest (understanding instructions, medication compliance, calling for problems).
RESULTS
We found the survey to be feasible and easily administered. Over the study period the mean length of stay of the respondents was 5.6 days (range, 056 days), and the mean number of times admitted was 1.6 (range, 17). Interviews were conducted an average of 3 days after discharge (range, 110 days). Other demographic information on the respondents is summarized in Table 1.
| Characteristic | Mean | Range |
|---|---|---|
| Age (years) | 78.7 | (70100) |
| Length of stay (days) | 5.6 | (056) |
| Number of admissions | 1.5 | (17) |
| Days postdischarge | 3 | (010) |
| N | % | |
| Sex | ||
| Male | 84 | 31 |
| Female | 185 | 69 |
| Marital status | ||
| Widowed | 164 | 61 |
| Married | 60 | 22 |
| Divorced | 15 | 6 |
| Separated | 14 | 5 |
| Single | 10 | 4 |
| No answer | 6 | 2 |
| Survey respondent | ||
| Patient | 187 | 70 |
| Child | 35 | 13 |
| Other | 33 | 12 |
| Spouse | 14 | 5 |
Most of those surveyed (81.8%, or 242 respondents) were able to answer the question Can you tell me what was explained to you [about why you were hospitalized]? The results of the content analysis of the answers to this open‐ended question are summarized in Table 2. The self‐reported problem area most frequently mentioned was the heart. Responses to questions about instructions and education received while in the hospital are reported in Table 3.
| Reason | n |
|---|---|
| Heart problem | 46 |
| Nonspecific (I am sick/I have chronic disease/all kinds of problems) | 29 |
| Don't know/no answer | 27 |
| Blood problem/bleeding/blood clot | 17 |
| Blood pressure problem (high or low) | 14 |
| Kidney problem | 14 |
| Surgery | 13 |
| Breathing problem | 13 |
| Stroke | 11 |
| Cold | 10 |
| Infection | 9 |
| Arm/leg/hand/feet/knee/bone | 8 |
| Fall | 8 |
| Stomach problem | 8 |
| Cancer | 7 |
| Diabetes | 7 |
| Dehydration | 6 |
| Lung problem | 6 |
| Bladder problem | 5 |
| Mental problem | 4 |
| Seizure | 4 |
| Automobile accident | 1 |
| Need medication | 1 |
| Prostate problem | 1 |
| Question | N | Yes (%) | No (%) | No Answer (%) |
|---|---|---|---|---|
| ||||
| Did someone explain to you why you were hospitalized ? | 269 | 224 (85%) | 35 (13%) | 6 (2.%) |
| Did the doctor explain your medical problems to you? | 269 | 205 (76%) | 49 (18%) | 15 (6%) |
| Did anyone talk with you about how to care for yourself after this hospitalization? | 269 | 115 (43%) | 141 (52%) | 13 (5%) |
| Do you remember who spoke with you?* | 115 | 91 (79%) | 22 (19%) | 2 (2%) |
| Doctor?* | 91 | 74 (81%) | 12 (13%) | 5 (6%) |
| Nurse?* | 91 | 44 (48%) | 43 (47%) | 4 (4%) |
| Other professional?* | 91 | 14 (15%) | 73 (80%) | 4 (4%) |
| If you had questions were they answered?* | 115 | 99 (86%) | 10 (9%) | 6 (5%) |
| Were you given a telephone number or name of a person to call if you needed help after you returned home? | 269 | 72 (27%) | 127 (47%) | 70 (26%) |
| Were you told what to do if you experienced problems at home? | 269 | 89 (33%) | 111 (41%) | 69 (26%) |
| Have you had to call about any problems since you arrived home? | 269 | 36 (13%) | 212 (79%) | 19 (8%) |
| Were your medications changed during this hospitalization? | 269 | 114 (42%) | 138 (52%) | 17 (6%) |
| Did someone explain how to take them?* | 114 | 90 (79%) | 16 (14%) | 8 (7%) |
| Doctor?* | 90 | 47 (52%) | 42 (47%) | 1 (1%) |
| Nurse?* | 90 | 39 (43%) | 51 (57%) | 0 (0%) |
| Pharmacist?* | 90 | 57 (63%) | 33 (37%) | 0 (0%) |
| Did you get your medication?* | 114 | 61 (53%) | 9 (8%) | 44 (39%) |
| Are you taking them the way they were explained to you?* | 114 | 95 (84%) | 13 (11%) | 6 (5%) |
| Since you have been home from the hospital do you feel you are receiving enough help? | 269 | 216 (80%) | 47 (18%) | 6 (2%) |
| If no, have you asked for more help?** | 47 | 26 (55%) | 12 (26%) | 9 (19%) |
A correlation was found between providing information in written and verbal fashion and self‐reported understanding of the instructions. For example, of the 103 respondents who answered affirmatively to Did anyone talk with you about how to care for yourself at home after this hospitalization? and also recalled the source of that information, 66.0% (n = 68) reported receiving instructions verbally, 10.7% (n = 11) reported receiving them in writing, and 23.3% (n = 24) reported receiving both. Patients who received both verbal and written instructions were more likely to report that they understood the care instructions very well versus somewhat or very little (2 = 29.612, df = 4, P = .000).
The association between the perceived provision of information to patients and effective use of that information was explored. For example, perceived medication compliance and instruction on medication use had a positive association. Among those who recalled receiving instruction on how to take their medications (n = 88), 76 (86.4%) stated that they were taking them correctly, 8 (9.1%) that they were not taking them correctly, and 4 (4.5%) were unsure. Among those who said that they did not receive instruction or did not recall being instructed on how to take their medications (n = 26), 16 (61.5%) believed that they were taking their mediations correctly, 4 (15.4%) that they were not taking them correctly, and 5 (19.2%) were unsure. Respondents who recalled receiving medication instruction were more likely to comply with taking medication. (2 = 7.321, P = .026)
There was also a positive association between being told what to do if problems were experienced at home and calling about problems after arriving home. Among those who believed they were instructed on what to do if problems were experienced at home (N = 86), 23 (26.7%) reported they called about a problem. Among those who did not recall being instructed about what to do if problems were experienced at home (N = 183), only 13 (7.1%) reported calling about a problem. Respondents who believed they had been instructed on what to do at home were significantly more likely to call from home about problems (2 = 16.740, df = 2, P = .000).
DISCUSSION
According to Bull and Roberts,2 there are 3 types of communication gaps in discharge planning: (1) gaps between health care providers in the hospital and those involved in the hospital‐community interface, (2) gaps between providers and patients, and (3) gaps between health care providers and family caregivers for elders. The present study focuses on the latter 2 types of communication gaps. In discharge planning it is critical not only to transmit information, but also to make sure that patients understand that information in the way health care providers intended. Systemic, cultural, emotional, and cognitive barriers may interact to limit the effectiveness of this communication.
In the present study, a large number of patients discharged from an academically affiliated public hospital were unaware of important discharge information, even though according to hospital protocol, all patients are given a discharge information sheet. Approximately 15% did not know why they were hospitalized. About 20% of those who reported their medications were changed in the hospital could not recall anyone explaining how to take these medications. More than half of respondents did not recall anyone speaking with them about how to take care of themselves following hospitalization. More than 60% of respondents did not recall getting information on what to do if they had a problem after being discharge home.
That patients were unaware of information that had been provided to them has significant implications for successful implementation of the spirit of the Joint Commission on Accreditation of Healthcare Organizations standards. These disease‐specific standards as well as medication standards are generally written as process measures. Although requiring that routine and clear standards of information be provided to patients is a significant step forward in patient safety, surveys such as the present one, done over time, should be an important part of any ongoing quality improvement process. As evident by the high response rate in our study and others,3 patients and their proxies are very willing to participate in such surveys. The results of the Consumer Assessment of Healthcare Providers and Systems (CHAMPS) survey,4 which will be published, should provide an important impetus for this ongoing quality improvement process. Interestingly, 2 of the CHAMPS questionsDuring this hospital stay, did doctors, nurses, or other hospital staff talk with you about whether you would have the help you needed when you left the hospital? and During this hospital stay, did you get information in writing about what symptoms or health problems to look out for after you left the hospital?are very similar to questions used in the present study. In our health system, site of the present study, these data have prompted a complete revision of the discharge instruction sheet, creation of a care transitions task force, and initiation of a pilot care transitions project. Follow‐up surveys will be performed to evaluate whether these changes have been effective in improving discharge information transfer and, more importantly, in patient outcomes.
The present study had several limitations. First, although the results are inconsistent with the findings of other studies, the present study took place at a single urban institution most of whose patients socioeconomically disadvantaged. Second, patient responses were combined with caregiver responses (elicited when a patient was unable to respond to the survey questions). Although it is not proven that these 2 groups are equivalent, from a practical point of view this was justified because the clinical issue is whether the person taking care of the patient (be it patient or caregiver) has the critical information needed after discharge from the hospital. Third, only those who could be reached by phone were included. This is likely to bias the results in a more favorable direction, given that the socioeconomic implications of not having a phone and/or the cognitive implications of not being able to use a phone would likely be reflected in even greater impediments to communication. Fourth, no attempt was made to evaluate the cognitive status or health literacy of either the subjects or their proxies. Fifth, generalizability of the survey to other research groups is unclear, as no additional attempt was made to define the interrater reliability of the survey. Finally, although it seems reasonable to presume that simpler discharge plans would be more effectively communicated, this study did not define the complexity of each discharge plan. Strengths of the study include a single trained interviewer, relatively rapid follow‐up of patients, and large sample size.
Effective communication during the care transition is important for improving patient outcomes and satisfaction. One study of 40 patient‐caregiver dyads showed that patients had a lower rate of medical problems postdischarge when they and their caregivers received verbal and/or printed information about activity and complications that could occur at home.5 Indeed, a study of 134 elder/family caregiver dyads interviewed 2 weeks after hospitalization found that receipt of information about the patient's condition, medications, and activities was an important contributor to both patient and family caregiver satisfaction with discharge care.6
At first glance, these findings may seem surprising, given that all patients discharged from the hospital should receive (by protocol) a discharge information sheet with postdischarge instructions. This study did not define what exactly transpired between hospital staff and patients, review discharge sheets, or validate the extent to which these instruction sheets are completely filled out and adequately reviewed with patients. The results of previous studies suggest that even when conversations are verified to have occurred, transmission is often inadequate. One study of 54 adult patients discharged after being hospitalized for pneumonia or acute myocardial infarction found that physicians believed that 88.9% of patients understood potential side effects of postdischarge medications, but only 57.4% of patients reported that they did understand instructions about side effects (P < .001).7 Another study of 47 patients discharged from a municipal teaching hospital in New York City showed that only 42% were able to state their diagnoses and only 28% were able to list all their medicines.8
The solutions to these problems may be as unapparent as they are difficult, and many of the challenges as well as some potential solutions have been recently reviewed.9 Arguing that physicians and/or hospital staff should spend more time with all their patients oversimplifies the problem and is not likely to occur. The present study confirmed earlier findings that providing verbal and written health information on hospital discharge significantly increases the knowledge of patients and caregivers.10 Risk stratificationtargeting those most at risk of medication noncompliance to receive augmented medication compliance instruction, such as the scheme suggested by Rosenow11has significant merit and warrants extension to discharge instructions in general and to prospective testing.
Discharge teaching videos have been shown to have some effectiveness in the emergency room setting12 and along with an audio‐only or CD option could be developed to supplement the written discharge information provided to patients. Calling patients after discharge to make sure they understand their prescribed medical regimen, have their prescriptions and home health equipment, and have a follow‐up visit scheduled with their doctors has been identified as a key characteristic of high‐achieving hospitalist programs. One care site found that 80% of patients have questions about their follow‐up care that could jeopardize their recovery.13 This strategy could just as well be implemented by a case manager or health educator. Follow‐up phone contact combined with Telecare has been shown to be effective in reducing hospital readmissions, emergency visits, and cost of care for patients with heart failure.14 Formal care transition instruments and interventions show promise for enabling patients and caregivers to take a more active role during care transition processes and improve outcomes. Whether this approach will be widely generalizable awaits demonstration.15
CONCLUSIONS
In summary, we found that a posthospitalization survey was both feasible and revealing and had a high acceptance rate in this urban public hospital population. Furthermore, subject recall about predischarge communication from hospital staff regarding discharge instructions demonstrated significant gaps about transfer of information. If these findings prove to be applicable to the large numbers of older as well as younger patients discharged from the hospital each year, the implications for patients health, safety, and satisfaction are enormous. It should not be assumed that this is a problem limited to older patients. As health care systems build bridges across gaps in the quality chasm, developing and testing more effective communication strategies for patients is imperative.
- Institute of Medicine.Crossing the Quality Chasm: A New Health System of the 21st Century. Committee on Quality of Health Care in America, editor.Washington, DC:National Academy Press;2001.
- ,.Components of a proper hospital discharge for elders.J Adv Nurs.2001;35:571–581.
- ,,.Assessing the quality of preparation for posthospital care from the patient's perspective—the care transitions measure.Med Care.2005;43:246–255.
- Available at http://www.hcahpsonline.org/default.aspx. Accessed September 23,2006.
- .Managing post‐discharge care at home: an analysis of patients' and their carers' perceptions of information received during their stay in hospital.J Advan Nurs.2000;31:1165–1173.
- ...Predictors of elder and family caregiver satisfaction with discharge planning.J Cardiovasc Nurs.2000;14:76–87.
- ,,,,,, et al.Patient‐physician communication at hospital discharge and patients' understanding of the postdischarge treatment plan.Arch Intern Med.1997;157:1026–1030.
- ,.Patient understanding of their treatment plans and diagnosis at discharge.Mayo Clin Proc.2005;80:991–994.
- ,.Lost in transition: challenges and opportunities for improving the quality of transitional care.Ann Intern Med.2004;140:533–536.
- ,,.Written and verbal information versus verbal information only for patients being discharged from acute hospital settings to home.Cochrane Database Syst Rev.2003;4:CD003716.
- .Patients' understanding of and compliance with medications: the sixth vital sign?Mayo Clin Proc.2005;80:983–987.
- ,,,,,.Development of an ED teaching program aimed at reducing prehospital delays for patients with chest pain.J Emerg Nurs.1998;24:316–319.
- .Remedy for high health care costs: hospitalist programs. January 5,2004.Phoenix Bus J. Available at: http://www.cogenthealthcare.com/Remedyopedfinal.pdf. Accessed August 22,year="2005"2005.
- ,,.Reducing the cost of frequent hospital admissions for congestive heart failure: a randomized trial of a home telecare intervention.Med Care,2001;39:1234–1245.
- ,,,,,.Preparing patients and caregivers to participate in care delivered across settings: the care transitions intervention.J Am Geriatr Soc.2004;52:1817–1825.
- Institute of Medicine.Crossing the Quality Chasm: A New Health System of the 21st Century. Committee on Quality of Health Care in America, editor.Washington, DC:National Academy Press;2001.
- ,.Components of a proper hospital discharge for elders.J Adv Nurs.2001;35:571–581.
- ,,.Assessing the quality of preparation for posthospital care from the patient's perspective—the care transitions measure.Med Care.2005;43:246–255.
- Available at http://www.hcahpsonline.org/default.aspx. Accessed September 23,2006.
- .Managing post‐discharge care at home: an analysis of patients' and their carers' perceptions of information received during their stay in hospital.J Advan Nurs.2000;31:1165–1173.
- ...Predictors of elder and family caregiver satisfaction with discharge planning.J Cardiovasc Nurs.2000;14:76–87.
- ,,,,,, et al.Patient‐physician communication at hospital discharge and patients' understanding of the postdischarge treatment plan.Arch Intern Med.1997;157:1026–1030.
- ,.Patient understanding of their treatment plans and diagnosis at discharge.Mayo Clin Proc.2005;80:991–994.
- ,.Lost in transition: challenges and opportunities for improving the quality of transitional care.Ann Intern Med.2004;140:533–536.
- ,,.Written and verbal information versus verbal information only for patients being discharged from acute hospital settings to home.Cochrane Database Syst Rev.2003;4:CD003716.
- .Patients' understanding of and compliance with medications: the sixth vital sign?Mayo Clin Proc.2005;80:983–987.
- ,,,,,.Development of an ED teaching program aimed at reducing prehospital delays for patients with chest pain.J Emerg Nurs.1998;24:316–319.
- .Remedy for high health care costs: hospitalist programs. January 5,2004.Phoenix Bus J. Available at: http://www.cogenthealthcare.com/Remedyopedfinal.pdf. Accessed August 22,year="2005"2005.
- ,,.Reducing the cost of frequent hospital admissions for congestive heart failure: a randomized trial of a home telecare intervention.Med Care,2001;39:1234–1245.
- ,,,,,.Preparing patients and caregivers to participate in care delivered across settings: the care transitions intervention.J Am Geriatr Soc.2004;52:1817–1825.
Copyright © 2007 Society of Hospital Medicine
Tongue necrosis from temporal arteritis
A 77‐year‐old woman with hypothyroidism presented with a 2‐week history of head, neck, jaw, and tongue pain. She had also developed slurred speech and difficulty chewing. On examination she had a temperature of 38.0C. She was without neurological deficits. However, she did have difficulty protruding her tongue, which had a cyanotic appearance and was painful. Laboratory findings showed an erythrocyte sedimentation rate of 68 mm/hr. Temporal arteritis was suspected, and the patient was started on corticosteroids. A subsequent temporal artery biopsy revealed inflammation and thrombus formation consistent with temporal arteritis. On hospital day 3, she developed unilateral ischemia in her tongue, which eventually became necrotic (Fig. 1). Although tongue necrosis is rare, temporal arteritis is the most frequent cause. It is usually unilateral and caused by compromised blood supply as a result of vasculitis in one of the lingual arteries. Other causes of tongue necrosis such as embolus, abscess, syphilis, tongue carcinoma, and Hodgkin's disease should be excluded.1, 2 Although necrotic tongue tissue must sometimes be extensively debrided or resected, our patient required minimal debridement. At follow‐up 1 month later, she was recovering at home with ongoing speech therapy and a corticosteroid taper.
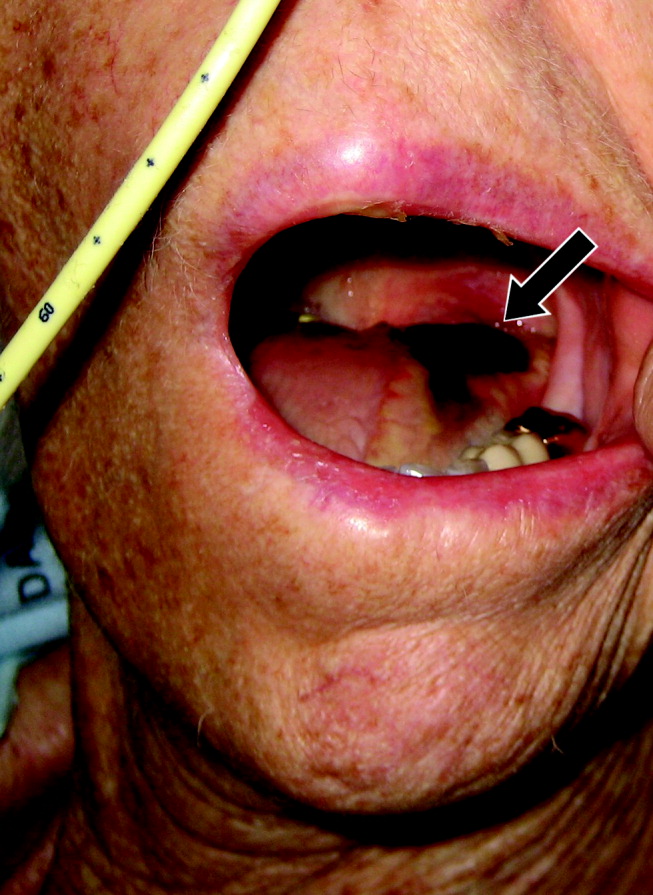
- ,,.Lingual infarction: a review of the literature.Ann Vasc Surg.1992;6:450–452.
- ,.The ESR in the diagnosis and management of the polymyalgia rheumatica/giant cell arteritis syndrome.Ann Rheum Dis.1983;42:168–170.
A 77‐year‐old woman with hypothyroidism presented with a 2‐week history of head, neck, jaw, and tongue pain. She had also developed slurred speech and difficulty chewing. On examination she had a temperature of 38.0C. She was without neurological deficits. However, she did have difficulty protruding her tongue, which had a cyanotic appearance and was painful. Laboratory findings showed an erythrocyte sedimentation rate of 68 mm/hr. Temporal arteritis was suspected, and the patient was started on corticosteroids. A subsequent temporal artery biopsy revealed inflammation and thrombus formation consistent with temporal arteritis. On hospital day 3, she developed unilateral ischemia in her tongue, which eventually became necrotic (Fig. 1). Although tongue necrosis is rare, temporal arteritis is the most frequent cause. It is usually unilateral and caused by compromised blood supply as a result of vasculitis in one of the lingual arteries. Other causes of tongue necrosis such as embolus, abscess, syphilis, tongue carcinoma, and Hodgkin's disease should be excluded.1, 2 Although necrotic tongue tissue must sometimes be extensively debrided or resected, our patient required minimal debridement. At follow‐up 1 month later, she was recovering at home with ongoing speech therapy and a corticosteroid taper.

A 77‐year‐old woman with hypothyroidism presented with a 2‐week history of head, neck, jaw, and tongue pain. She had also developed slurred speech and difficulty chewing. On examination she had a temperature of 38.0C. She was without neurological deficits. However, she did have difficulty protruding her tongue, which had a cyanotic appearance and was painful. Laboratory findings showed an erythrocyte sedimentation rate of 68 mm/hr. Temporal arteritis was suspected, and the patient was started on corticosteroids. A subsequent temporal artery biopsy revealed inflammation and thrombus formation consistent with temporal arteritis. On hospital day 3, she developed unilateral ischemia in her tongue, which eventually became necrotic (Fig. 1). Although tongue necrosis is rare, temporal arteritis is the most frequent cause. It is usually unilateral and caused by compromised blood supply as a result of vasculitis in one of the lingual arteries. Other causes of tongue necrosis such as embolus, abscess, syphilis, tongue carcinoma, and Hodgkin's disease should be excluded.1, 2 Although necrotic tongue tissue must sometimes be extensively debrided or resected, our patient required minimal debridement. At follow‐up 1 month later, she was recovering at home with ongoing speech therapy and a corticosteroid taper.

- ,,.Lingual infarction: a review of the literature.Ann Vasc Surg.1992;6:450–452.
- ,.The ESR in the diagnosis and management of the polymyalgia rheumatica/giant cell arteritis syndrome.Ann Rheum Dis.1983;42:168–170.
- ,,.Lingual infarction: a review of the literature.Ann Vasc Surg.1992;6:450–452.
- ,.The ESR in the diagnosis and management of the polymyalgia rheumatica/giant cell arteritis syndrome.Ann Rheum Dis.1983;42:168–170.
Linezolid‐ and vancomycin‐resistant Enterococcus faecium endocarditis: Successful treatment with tigecycline and daptomycin
Enterococci are a leading cause of endocarditis and nosocomial infections. Vancomycin‐resistant enterococci (VRE) emerged in the 1980s and now represent most nosocomial isolates in the United States. The first case of VRE endocarditis was reported in 1996.1 Although increasing enterococcal antibiotic resistance has prompted increasing reliance on newer antibiotics,2 a recent review of VRE endocarditis noted that survival rates were similar to those for vancomycin‐sensitive enterococcal endocarditis.1 Cure was achieved in several patients with bacteriostatic agents in the absence of valve replacement, but no patients were infected with truly linezolid‐resistant organisms. This case of linezolid‐resistant VRE endocarditis represents the first reported cure of infective endocarditis with a tigecycline‐containing regimen.
CASE REPORT
A 62‐year‐old man presented with hypoglycemia and delirium. His medical history included diabetes mellitus, coronary and peripheral arterial disease, and end‐stage renal disease. He had had endocarditis of an unknown type 12 years prior to admission. He had recently developed septic shock because of a Candida parapsilosis, Enterobacter cloacae, and Staphylococcus epidermidis infection of a peripherally inserted central catheter (PICC) and received 14 days of vancomycin, meropenem, and fluconazole administered through a new PICC. This catheter was not removed, and 39 days after completion of the antibiotic therapy, he developed hypoglycemia, which was attributed to weight loss without adjustment of his insulin regimen. He was afebrile; examination revealed a new 3/6 holosystolic murmur radiating to the axilla. There were no other stigmata of infective endocarditis, and his PICC and arteriovenous fistula sites appeared normal. Delirium resolved after administration of intravenous glucose.
E. faecium grew from all 6 initial blood cultures. A transesophageal echocardiogram revealed a new 3‐mm mitral valve vegetation with perforation and severe regurgitation. He had definite endocarditis on the basis of 2 major criteria.3 He was given vancomycin (1 g IV, then administered by levels), then switched to linezolid (600 mg orally every 12 hours), and finally tigecycline (100 mg IV followed by 50 mg IV every 12 hours) plus daptomycin (6 mg/kg IV every 48 hours) as further sensitivity data became available.
The organism was resistant to ampicillin, chloramphenicol, and linezolid (MIC > 20 g/mL), as well as vancomycin (MIC > 50 g/mL), quinupristin/dalfopristin (MIC 2.5 g/mL), and gentamicin (MIC > 200 g/mL), and demonstrated high‐level streptomycin resistance (>2000 g/mL). It was intermediate to doxycycline (MIC 5 g/mL). It was susceptible to daptomycin (MIC 4 g/mL) and tigecycline (MIC 0.06 g/mL).
Blood cultures done on hospital days 1, 4, 6, and 7 (day 1 of tigecycline) were positive, and multiple cultures were negative from day 10 on. Because of the lack of experience with tigecycline in infective endocarditis, unrevascularized left‐main coronary artery disease, and severe mitral regurgitation, the patient was advised to undergo valve replacement and coronary artery bypass surgery after antibiotic therapy. Because he feared surgical complications, he refused and received 70 days of tigecycline plus daptomycin therapy, which was complicated only by nausea. He remained clinically well and had negative blood cultures 16 weeks after completion of therapy.
DISCUSSION
Tigecycline, the first available glycylcycline, is a minocycline‐derived antibiotic that remains active in the presence of the ribosomal modifications and efflux pumps that mediate tetracycline resistance. Thus, it possesses broad‐spectrum bacteriostatic activity, including activity against VRE. A PubMed search revealed no published data about the use of tigecycline for endocarditis in humans. However, tetracyclines have been used to treat endocarditis due to such organisms as Bartonella, Coxiella burnetti, or methicillin‐resistant Staphylococcus aureus (MRSA), frequently for prolonged courses. Tetracyclines were combined with other antibiotics in 5 published cases of VRE endocarditis. All patients survived; 3 were cured with the tetracycline regimen and 2 with other antimicrobials.1 In animal models of endocarditis, tigecycline stabilized vegetation counts of E. faecalis and reduced vegetation counts of MRSA and 1 strain of E. faecium.4
Daptomycin, the first available cyclic lipopeptide, kills by nonlytic depolarization of the bacterial cell membrane. In a recent study, daptomycin was non‐inferior to vancomycin or antistaphylococcal penicillins for S. aureus bacteremia or endocarditis. Although a few patients had left‐sided endocarditis, only 1 of them experienced a successful outcome with daptomycin therapy, and daptomycin displayed a trend toward higher rates of persistent or relapsing infection.5 Less evidence supports the use of daptomycin for serious enterococcal infections.2 One report noted the deaths of 6 of 10 patients treated with daptomycin for VRE bacteremia, including both patients with endocarditis.6 Daptomycin was used successfully in a case of VRE endocarditis in combination with gentamicin and rifampin for 11 weeks1 and at least 6 other reported cases of VRE bacteremia.7, 8
In summary, despite tigecycline's lack of bactericidal activity or proven efficacy in endocarditis, daptomycin's prior performance in VRE bacteremia, and the isolate's borderline daptomycin susceptibility, prolonged combination therapy resulted in a cure of VRE endocarditis. This success extends the experience with using both agents in the treatment of resistant infections. As linezolid‐resistant VRE and other resistant pathogens become more common, the need for research on treatment options becomes more urgent, and familiarity with novel and lesser‐used antibiotics becomes more crucial for hospitalists.
- ,.Endocarditis due to vancomycin‐resistant enterococci: case report and review of the literature.Clin Infect Dis.2005;41:1134–1142.
- ,.Approaches to vancomycin resistant enterococci.Curr Opin Infect Dis.2004;17:541–547.
- ,,, et al.Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis.Clin Infect Dis.2000;4:633–638.
- ,,, et al.Activity and diffusion of tigecycline (GAR‐936) in experimental enterococcal endocarditis.Antimicrob Agents Chemother.2003;47:216–222.
- ,,, et al.Daptomycin versus standard therapy for bacteremia and endocarditis caused by staphylococcus aureus.New Engl J Med.2006;355:653–665.
- ,,.Daptomycin for the treatment of gram‐positive bacteremia and infective endocarditis: a retrospective case series of 31 patients.Pharmacotherapy.2006;26:347–352.
- ,,,,.Daptomycin in the treatment of vancomycin‐resistant Enterococcus faecium bacteremia in neutropenic patients.J Infect.2007;54:567–571.
- ,,,,.Daptomycin for the treatment of vancomycin resistant Enterococcus faecium bacteremia.Scand J Infect Dis.2006;38:290–292.
Enterococci are a leading cause of endocarditis and nosocomial infections. Vancomycin‐resistant enterococci (VRE) emerged in the 1980s and now represent most nosocomial isolates in the United States. The first case of VRE endocarditis was reported in 1996.1 Although increasing enterococcal antibiotic resistance has prompted increasing reliance on newer antibiotics,2 a recent review of VRE endocarditis noted that survival rates were similar to those for vancomycin‐sensitive enterococcal endocarditis.1 Cure was achieved in several patients with bacteriostatic agents in the absence of valve replacement, but no patients were infected with truly linezolid‐resistant organisms. This case of linezolid‐resistant VRE endocarditis represents the first reported cure of infective endocarditis with a tigecycline‐containing regimen.
CASE REPORT
A 62‐year‐old man presented with hypoglycemia and delirium. His medical history included diabetes mellitus, coronary and peripheral arterial disease, and end‐stage renal disease. He had had endocarditis of an unknown type 12 years prior to admission. He had recently developed septic shock because of a Candida parapsilosis, Enterobacter cloacae, and Staphylococcus epidermidis infection of a peripherally inserted central catheter (PICC) and received 14 days of vancomycin, meropenem, and fluconazole administered through a new PICC. This catheter was not removed, and 39 days after completion of the antibiotic therapy, he developed hypoglycemia, which was attributed to weight loss without adjustment of his insulin regimen. He was afebrile; examination revealed a new 3/6 holosystolic murmur radiating to the axilla. There were no other stigmata of infective endocarditis, and his PICC and arteriovenous fistula sites appeared normal. Delirium resolved after administration of intravenous glucose.
E. faecium grew from all 6 initial blood cultures. A transesophageal echocardiogram revealed a new 3‐mm mitral valve vegetation with perforation and severe regurgitation. He had definite endocarditis on the basis of 2 major criteria.3 He was given vancomycin (1 g IV, then administered by levels), then switched to linezolid (600 mg orally every 12 hours), and finally tigecycline (100 mg IV followed by 50 mg IV every 12 hours) plus daptomycin (6 mg/kg IV every 48 hours) as further sensitivity data became available.
The organism was resistant to ampicillin, chloramphenicol, and linezolid (MIC > 20 g/mL), as well as vancomycin (MIC > 50 g/mL), quinupristin/dalfopristin (MIC 2.5 g/mL), and gentamicin (MIC > 200 g/mL), and demonstrated high‐level streptomycin resistance (>2000 g/mL). It was intermediate to doxycycline (MIC 5 g/mL). It was susceptible to daptomycin (MIC 4 g/mL) and tigecycline (MIC 0.06 g/mL).
Blood cultures done on hospital days 1, 4, 6, and 7 (day 1 of tigecycline) were positive, and multiple cultures were negative from day 10 on. Because of the lack of experience with tigecycline in infective endocarditis, unrevascularized left‐main coronary artery disease, and severe mitral regurgitation, the patient was advised to undergo valve replacement and coronary artery bypass surgery after antibiotic therapy. Because he feared surgical complications, he refused and received 70 days of tigecycline plus daptomycin therapy, which was complicated only by nausea. He remained clinically well and had negative blood cultures 16 weeks after completion of therapy.
DISCUSSION
Tigecycline, the first available glycylcycline, is a minocycline‐derived antibiotic that remains active in the presence of the ribosomal modifications and efflux pumps that mediate tetracycline resistance. Thus, it possesses broad‐spectrum bacteriostatic activity, including activity against VRE. A PubMed search revealed no published data about the use of tigecycline for endocarditis in humans. However, tetracyclines have been used to treat endocarditis due to such organisms as Bartonella, Coxiella burnetti, or methicillin‐resistant Staphylococcus aureus (MRSA), frequently for prolonged courses. Tetracyclines were combined with other antibiotics in 5 published cases of VRE endocarditis. All patients survived; 3 were cured with the tetracycline regimen and 2 with other antimicrobials.1 In animal models of endocarditis, tigecycline stabilized vegetation counts of E. faecalis and reduced vegetation counts of MRSA and 1 strain of E. faecium.4
Daptomycin, the first available cyclic lipopeptide, kills by nonlytic depolarization of the bacterial cell membrane. In a recent study, daptomycin was non‐inferior to vancomycin or antistaphylococcal penicillins for S. aureus bacteremia or endocarditis. Although a few patients had left‐sided endocarditis, only 1 of them experienced a successful outcome with daptomycin therapy, and daptomycin displayed a trend toward higher rates of persistent or relapsing infection.5 Less evidence supports the use of daptomycin for serious enterococcal infections.2 One report noted the deaths of 6 of 10 patients treated with daptomycin for VRE bacteremia, including both patients with endocarditis.6 Daptomycin was used successfully in a case of VRE endocarditis in combination with gentamicin and rifampin for 11 weeks1 and at least 6 other reported cases of VRE bacteremia.7, 8
In summary, despite tigecycline's lack of bactericidal activity or proven efficacy in endocarditis, daptomycin's prior performance in VRE bacteremia, and the isolate's borderline daptomycin susceptibility, prolonged combination therapy resulted in a cure of VRE endocarditis. This success extends the experience with using both agents in the treatment of resistant infections. As linezolid‐resistant VRE and other resistant pathogens become more common, the need for research on treatment options becomes more urgent, and familiarity with novel and lesser‐used antibiotics becomes more crucial for hospitalists.
Enterococci are a leading cause of endocarditis and nosocomial infections. Vancomycin‐resistant enterococci (VRE) emerged in the 1980s and now represent most nosocomial isolates in the United States. The first case of VRE endocarditis was reported in 1996.1 Although increasing enterococcal antibiotic resistance has prompted increasing reliance on newer antibiotics,2 a recent review of VRE endocarditis noted that survival rates were similar to those for vancomycin‐sensitive enterococcal endocarditis.1 Cure was achieved in several patients with bacteriostatic agents in the absence of valve replacement, but no patients were infected with truly linezolid‐resistant organisms. This case of linezolid‐resistant VRE endocarditis represents the first reported cure of infective endocarditis with a tigecycline‐containing regimen.
CASE REPORT
A 62‐year‐old man presented with hypoglycemia and delirium. His medical history included diabetes mellitus, coronary and peripheral arterial disease, and end‐stage renal disease. He had had endocarditis of an unknown type 12 years prior to admission. He had recently developed septic shock because of a Candida parapsilosis, Enterobacter cloacae, and Staphylococcus epidermidis infection of a peripherally inserted central catheter (PICC) and received 14 days of vancomycin, meropenem, and fluconazole administered through a new PICC. This catheter was not removed, and 39 days after completion of the antibiotic therapy, he developed hypoglycemia, which was attributed to weight loss without adjustment of his insulin regimen. He was afebrile; examination revealed a new 3/6 holosystolic murmur radiating to the axilla. There were no other stigmata of infective endocarditis, and his PICC and arteriovenous fistula sites appeared normal. Delirium resolved after administration of intravenous glucose.
E. faecium grew from all 6 initial blood cultures. A transesophageal echocardiogram revealed a new 3‐mm mitral valve vegetation with perforation and severe regurgitation. He had definite endocarditis on the basis of 2 major criteria.3 He was given vancomycin (1 g IV, then administered by levels), then switched to linezolid (600 mg orally every 12 hours), and finally tigecycline (100 mg IV followed by 50 mg IV every 12 hours) plus daptomycin (6 mg/kg IV every 48 hours) as further sensitivity data became available.
The organism was resistant to ampicillin, chloramphenicol, and linezolid (MIC > 20 g/mL), as well as vancomycin (MIC > 50 g/mL), quinupristin/dalfopristin (MIC 2.5 g/mL), and gentamicin (MIC > 200 g/mL), and demonstrated high‐level streptomycin resistance (>2000 g/mL). It was intermediate to doxycycline (MIC 5 g/mL). It was susceptible to daptomycin (MIC 4 g/mL) and tigecycline (MIC 0.06 g/mL).
Blood cultures done on hospital days 1, 4, 6, and 7 (day 1 of tigecycline) were positive, and multiple cultures were negative from day 10 on. Because of the lack of experience with tigecycline in infective endocarditis, unrevascularized left‐main coronary artery disease, and severe mitral regurgitation, the patient was advised to undergo valve replacement and coronary artery bypass surgery after antibiotic therapy. Because he feared surgical complications, he refused and received 70 days of tigecycline plus daptomycin therapy, which was complicated only by nausea. He remained clinically well and had negative blood cultures 16 weeks after completion of therapy.
DISCUSSION
Tigecycline, the first available glycylcycline, is a minocycline‐derived antibiotic that remains active in the presence of the ribosomal modifications and efflux pumps that mediate tetracycline resistance. Thus, it possesses broad‐spectrum bacteriostatic activity, including activity against VRE. A PubMed search revealed no published data about the use of tigecycline for endocarditis in humans. However, tetracyclines have been used to treat endocarditis due to such organisms as Bartonella, Coxiella burnetti, or methicillin‐resistant Staphylococcus aureus (MRSA), frequently for prolonged courses. Tetracyclines were combined with other antibiotics in 5 published cases of VRE endocarditis. All patients survived; 3 were cured with the tetracycline regimen and 2 with other antimicrobials.1 In animal models of endocarditis, tigecycline stabilized vegetation counts of E. faecalis and reduced vegetation counts of MRSA and 1 strain of E. faecium.4
Daptomycin, the first available cyclic lipopeptide, kills by nonlytic depolarization of the bacterial cell membrane. In a recent study, daptomycin was non‐inferior to vancomycin or antistaphylococcal penicillins for S. aureus bacteremia or endocarditis. Although a few patients had left‐sided endocarditis, only 1 of them experienced a successful outcome with daptomycin therapy, and daptomycin displayed a trend toward higher rates of persistent or relapsing infection.5 Less evidence supports the use of daptomycin for serious enterococcal infections.2 One report noted the deaths of 6 of 10 patients treated with daptomycin for VRE bacteremia, including both patients with endocarditis.6 Daptomycin was used successfully in a case of VRE endocarditis in combination with gentamicin and rifampin for 11 weeks1 and at least 6 other reported cases of VRE bacteremia.7, 8
In summary, despite tigecycline's lack of bactericidal activity or proven efficacy in endocarditis, daptomycin's prior performance in VRE bacteremia, and the isolate's borderline daptomycin susceptibility, prolonged combination therapy resulted in a cure of VRE endocarditis. This success extends the experience with using both agents in the treatment of resistant infections. As linezolid‐resistant VRE and other resistant pathogens become more common, the need for research on treatment options becomes more urgent, and familiarity with novel and lesser‐used antibiotics becomes more crucial for hospitalists.
- ,.Endocarditis due to vancomycin‐resistant enterococci: case report and review of the literature.Clin Infect Dis.2005;41:1134–1142.
- ,.Approaches to vancomycin resistant enterococci.Curr Opin Infect Dis.2004;17:541–547.
- ,,, et al.Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis.Clin Infect Dis.2000;4:633–638.
- ,,, et al.Activity and diffusion of tigecycline (GAR‐936) in experimental enterococcal endocarditis.Antimicrob Agents Chemother.2003;47:216–222.
- ,,, et al.Daptomycin versus standard therapy for bacteremia and endocarditis caused by staphylococcus aureus.New Engl J Med.2006;355:653–665.
- ,,.Daptomycin for the treatment of gram‐positive bacteremia and infective endocarditis: a retrospective case series of 31 patients.Pharmacotherapy.2006;26:347–352.
- ,,,,.Daptomycin in the treatment of vancomycin‐resistant Enterococcus faecium bacteremia in neutropenic patients.J Infect.2007;54:567–571.
- ,,,,.Daptomycin for the treatment of vancomycin resistant Enterococcus faecium bacteremia.Scand J Infect Dis.2006;38:290–292.
- ,.Endocarditis due to vancomycin‐resistant enterococci: case report and review of the literature.Clin Infect Dis.2005;41:1134–1142.
- ,.Approaches to vancomycin resistant enterococci.Curr Opin Infect Dis.2004;17:541–547.
- ,,, et al.Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis.Clin Infect Dis.2000;4:633–638.
- ,,, et al.Activity and diffusion of tigecycline (GAR‐936) in experimental enterococcal endocarditis.Antimicrob Agents Chemother.2003;47:216–222.
- ,,, et al.Daptomycin versus standard therapy for bacteremia and endocarditis caused by staphylococcus aureus.New Engl J Med.2006;355:653–665.
- ,,.Daptomycin for the treatment of gram‐positive bacteremia and infective endocarditis: a retrospective case series of 31 patients.Pharmacotherapy.2006;26:347–352.
- ,,,,.Daptomycin in the treatment of vancomycin‐resistant Enterococcus faecium bacteremia in neutropenic patients.J Infect.2007;54:567–571.
- ,,,,.Daptomycin for the treatment of vancomycin resistant Enterococcus faecium bacteremia.Scand J Infect Dis.2006;38:290–292.
Fishing for a Diagnosis
A 54‐year‐old man with hypertension and type 2 diabetes mellitus entered the Chest Pain Evaluation Unit of a teaching hospital after 12 hours of intermittent thoracic discomfort. The pain began during dinner and was sharp, bandlike, and located beneath the sternum and across the entire chest. He had dyspnea but no diaphoresis or nausea. A recumbent position relieved the pain after dinner, but it recurred during the night and again the following morning. He did not smoke and had no family history of coronary artery disease.
A useful approach in evaluating acute chest pain is to employ a hierarchical differential diagnosis that emphasizes life‐threatening disorders requiring prompt recognition and intervention. Most prominent are cardiac ischemia, pericardial tamponade, pneumothorax, pulmonary embolus, esophageal rupture, and aortic dissection. The concurrent dyspnea and retrosternal location and intermittent nature of the pain that this patient has are consistent with myocardial ischemia, but the sharp quality of the pain and the relief gained by being recumbent are atypical. Pain with pericarditis is characteristically pleuritic and often worse when lying down. The pain of pneumothorax is typically unilateral, not intermittent, and unlikely to improve with recumbency. Although pain during eating suggests the possibility of an esophageal source, spontaneous rupture usually follows vomiting. The pain is typically continuous and severe. The pain of pulmonary embolism may be unilateral and pleuritic but often is more diffuse. Relief by recumbency is unusual, but the intermittent nature could suggest recurrent emboli. The patient has a history of hypertension, which predisposes him to aortic dissection, in which the pain is typically sharp, continuous, and severe but occasionally intermittent. Among numerous less urgent diagnoses are esophagitis and thoracic diabetic radiculopathy.
Important features to look for during this patient's examination include: disappearance of the radial pulse during inhalation, a simple screening test that is insensitive but very specific for pericardial tamponade; elevated neck veins, which can occur with tension pneumothorax, massive pulmonary embolism, and pericardial tamponade; pericardial and pleural friction rubs; discrepant blood pressures in the 2 arms, sometimes a sign of aortic dissection; local thoracic tenderness from chest wall disorders; and sensory examination of the chest surface, which is often abnormal in diabetic thoracic radiculopathy. Given this patient's age and history of diabetes, I am most concerned about myocardial ischemia. The most appropriate diagnostic tests include an electrocardiogram and a chest radiograph.
The patient appeared apprehensive but reported no pain. He had a temperature of 36.0 C, heart rate of 95 beats/minute, blood pressure of 138/77 mm Hg, respiratory rate of 16/minute, and oxygen saturation of 99% while breathing ambient air. Blood pressures were equal in both arms. Jugular venous distention was absent, and the lung and cardiac examinations had normal results. His pain did not increase on chest wall palpation. Examination of the abdomen, extremities, and the neurologic system showed normal results.
The results of laboratory tests showed a leukocyte count of 12,700/cm3, with 85% neutrophils, 8% lymphocytes, 6% monocytes, and 1% eosinophils. The hematocrit was 45%, and the platelet count was 172,000/cm3. The results of his chemistry panel were remarkable only for a glucose of 225 mg/dL. An electrocardiogram (ECG) showed a normal sinus rhythm and left anterior fascicular block without acute ST‐ or T‐wave changes. Prior ECGs were unavailable. An anteroposterior radiograph disclosed low lung volumes and bibasilar opacities. No pleural effusion was noted. Serial serum troponin and creatine kinase levels were normal. A Tc‐99m tetrofosmin cardiac nuclear perfusion test performed at rest demonstrated a moderate area of mildly decreased uptake along the inferior wall extending to the apex. An exercise treadmill test, terminated after 1 minute, 45 seconds because of chest pain, provoked no ECG changes diagnostic of ischemic disease.
The absence of elevated jugular venous pressure virtually eliminates pericardial tamponade as a diagnosis, and the chest film excludes pneumothorax. The intermittent nature of the chest pain, the absence on the chest radiograph of such findings as mediastinal gas, left pneumothorax, or hydropneumothorax, and the lack of a predisposing cause make esophageal rupture unlikely. Pulmonary emboli remain a consideration despite the normal oxygen saturation because there is no hypoxemia in a substantial minority of such cases. The normal cardiac enzyme levels and the lack of significant changes on the ECG exclude that a myocardial infarction has recently occurred, but cardiac ischemia remains a possibility, especially because the patient had chest pain on exercise and the nuclear scan indicated diminished blood flow to the inferior left ventricle. Aortic dissection still lurks as a possibility. The inferior wall abnormalities seen on the scan could result from dissection into the right coronary artery, which is more frequently involved than the left, or compression of it by an enlarged aorta, but they also may be artifacts. The leukocytosis may be a nonspecific response to stress but could indicate, although unlikely, infections such as mediastinitis from esophageal rupture or bacterial aortitis.
A conscientious clinician would repeat the history, reexamine the patient, and scrutinize the chest film to determine what the bilateral opacities represent. Given the story so far, however, I might consider a thoracic computed tomography (CT) angiogram because I am most concerned about pulmonary emboli and aortic dissection.
On hospital day 3, the patient had worsening dyspnea and persistent chest pain. His temperature was 39.3C, and his oxygen saturation decreased to 89% while breathing room air. Repeat chest radiography showed new bilateral pleural effusions and increased bibasilar opacification (Fig. 1). His leukocyte count was 19,000/cm3, with 88% neutrophils, 6% lymphocytes, 5% monocytes, and 1% eosinophils. Care was transferred from the chest pain team to an inpatient general medicine ward team. A pulmonary CT angiogram showed no large central clots but suggested emboli in the right superior subsegmental artery and a right upper lobe subsegmental artery. Bilateral pleural effusions were observed, as were bilateral pleural‐based atelectasis or infiltrates in the lower lungs. A hiatal hernia was noted, but no aortic dissection. The patient received supplemental oxygen, intravenous levofloxacin, and unfractionated heparin by continuous infusion.
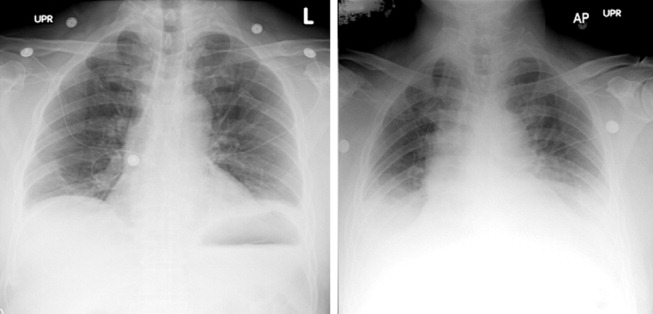
Without other information, I will assume that the fever is part of the patient's original disease and not a nosocomial infection or drug fever. At this point, a crucial part of the evaluation is examining the CT scan with experienced radiologists to determine whether the abnormalities noted are genuinely convincing for pulmonary emboli. If the findings are equivocal, the next step might be a pulmonary angiogram or the indirect approach of evaluating the leg veins with ultrasound, reasoning that the presence of proximal leg vein thromboses would require anticoagulation in any event.
The patient's worsening chest pain and hypoxemia are consistent with multiple pulmonary emboli. Bilateral pleural effusions and leukocytosis can occur but are uncommon. Because of the fever, another possibility is septic pulmonary emboli, but he has no evidence of suppurative thrombophlebitis of the peripheral veins, apparent infection elsewhere, or previous intravenous drug abuse causing right‐sided endocarditis. An alternative diagnosis is infection of an initially bland pulmonary infarct.
An important consideration is a thoracentesis, depending on how persuasive the CT diagnosis of pulmonary embolism is and the size of the pleural effusions. It should be done before instituting antimicrobial therapy, which may decrease the yield of the cultures, and before starting heparin, which increases the risk of bleeding and occasionally causes a substantial, even fatal hemothorax.
The patient's oxygenation and dyspnea did not improve. Over the next day, he repeatedly mentioned that swallowing, particularly solid foods, worsened his chest pain. He had a temperature of 39.9C, a heart rate of 121 beats/minute, blood pressure of 149/94 mm Hg, a respiratory rate of 28/minute, and oxygen saturation of 93% while breathing 40% oxygen. He had inspiratory splinting, percussive dullness at both lung bases, and distant heart sounds. A contrast esophagogram showed distal narrowing that prevented solid contrast from passing, but no hiatal hernia. Blood and urine cultures obtained before antibiotic therapy were sterile. Duplex ultrasonography of bilateral lower extremities showed no evidence of deep venous thrombosis. A pulmonary angiogram revealed no emboli, and heparin was discontinued. Bilateral thoracentesis yielded grossly bloody fluid. Repeat chest CT (Fig. 2) demonstrated large bilateral effusions, a new large pericardial effusion, and a prominence at the gastroesophageal junction more concerning for a soft‐tissue mass than for a hiatal hernia, although the quality of the study was suboptimal because of an absence of oral contrast.
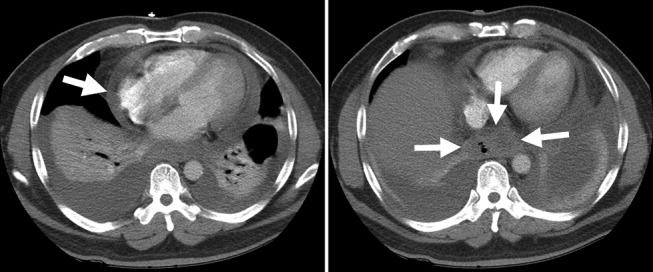
The CT scan suggests a paraesophageal abscess from an esophageal rupture. As mentioned earlier, if rupture occurs spontaneously, it typically follows retching or vomiting and is called Boerhaave's syndrome. Another consideration is a rupture secondary to an external insult, such as trauma or ingestion of a caustic substance. In evaluating these possibilities, the patient should have been asked 4 questions at the initial interview that I neglected to explicitly highlight earlier. First, what was he eating when he developed the chest pain? Second, did the pain begin during swallowing? Third, did he have previous symptoms suggesting an esophageal disorder such as dysphagia, odynophagia, or heartburn? These might indicate a cancer that could perforate or another problem such as a stricture or disordered esophageal motility that might have caused a swallowed item to lodge in the esophagus. Finally, did he have retching or vomiting? Though not routinely part of the review of systems, the former 2 questions are an appropriate history‐prompted line of questioning of a patient with onset of chest pain while eating.
At this point, a reasonable approach would be an esophagoscopy to delineate any intraluminal problems, such as a cancer or a foreign body. The apparent obstruction seen on barium swallow may be from extrinsic pressure from a paraesophageal abscess. The patient should receive broad‐spectrum antimicrobial therapy effective against oral anaerobes. Although occasionally patients recover with antibiotics alone, surgery is usually required. I am surprised that the original CT scan did not show evidence of an esophageal perforation. Possibly, the hiatal hernia was a paraesophageal abscess poorly characterized because of the lack of oral contrast.
The team, concerned about esophageal perforation, began the patient on intravenous clindamycin. The patient underwent video‐assisted thoracoscopic drainage, which yielded a moderate amount of turbid, bloody fluid from each hemithorax. The pericardium contained approximately 500 cm3 of turbid fluid. Gram stain and culture of these fluids were negative. No esophageal or mediastinal mass was noted during surgery. Intraoperative esophagogastroduodenoscopy with endoscopic ultrasound showed a healing linear mucosal tear in the distal esophagus (Fig. 3) as well as air/fluid collection in the esophageal soft tissue (not shown).
On further questioning postoperatively, the patient reported eating bony fish during the dinner when he first experienced chest pain. The patient received a 21‐day course of oral clindamycin and completely recovered. Five weeks later, a chest CT showed decreased distal esophageal thickening and no mediastinal air.
COMMENTARY
Esophageal perforation is an uncommon but life‐threatening cause of chest pain. In most series iatrogenic injury accounts for more than 70% of cases, whereas most of the other cases have spontaneous (5%20%) or traumatic (4%10%) causes (Table 1).14 Perforation as a complication of ingesting fish bones, although rare, is well described and continues to be reported.57
| Etiology | Percent |
|---|---|
| |
| Iatrogenic | 45%77% |
| Rigid or flexible endoscopy, balloon dilation, Blakemore tube, sclerotherapy, operative injury | |
| Increased intraesophageal pressure (Boerhaave's syndrome) | 5%20% |
| Vomiting or retching, weightlifting, childbirth | |
| Traumatic | 4%10% |
| Penetrating or blunt injury to neck or chest | |
| Ingestion | 0%12% |
| Foreign body, toxic or caustic substance | |
| Miscellaneous | 0%5% |
| Malignancy, Barrett's esophagus, infection, aortic dissection | |
Diagnosis of esophageal perforation secondary to a foreign body may be difficult because of the considerable overlap of symptoms with other causes of chest pain and failure to consider this infrequent condition in the absence of a classic history of retching. To diagnose such a disease, physicians must gather data from various sourcesespecially the history, physical examination, and medical recordformulate hypotheses, integrate results from diagnostic tests, and then assess the importance of the available information in the context of a differential diagnosis. Incorrectly evaluating or failing to obtain essential data can lead to incorrect or delayed diagnoses.
In This Patient's Evaluation, What Prevented Prompt Recognition of Esophageal Perforation?
The critical misstep was an incomplete history, both on arrival and when the patient was transferred to a second team. The presence of risk factors for coronary artery disease led the providers to first consider myocardial ischemia. They failed to ask crucial questions about the onset of the painwhen it occurred during the meal and what he was eatingeven when the patient later complained of odynophagia. As a result of the incomplete history, the providers, puzzled by the patient's ongoing and evolving symptoms, ordered numerous unnecessary diagnostic tests that gave false‐positive results, leading to potentially harmful treatment including anticoagulation. The discussant mentions that the preferred response to a puzzling clinical situation is to return to the bedside and repeat the history, reexamine the patient, and reevaluate available informationsimple steps that can often resolve diagnostic dilemmas.
There is ongoing concern that the history‐taking and physical examination skills of clinicians are in decline.814 Many speculate this is in part due to reliance on increasingly sophisticated diagnostic tests. Providers may overly rely on modern diagnostic tests because of their familiarity with the sensitivity and specificity of such tests, fear of malpractice litigation, diminishing opportunity to elucidate the complete history and physical exam, or lack of confidence in their history‐taking and examination skills.814 Although the rapid development and implementation of advanced diagnostic technologies have had a significant impact on diagnostic accuracy, the estimated rate of disease misdiagnosis remains elevated at 24%.1518 In contrast to technology‐based testing, the history and physical provide an inexpensive, safe, and effective means of at arriving at a correct diagnosis. In outpatient medical visits the history and physical, when completely elicited, result in a correct diagnosis of up to 70%90% of patients.8, 19, 20 Even for illnesses whose diagnosis requires confirmation by a diagnostic test, the definitive test can only be selected after a sufficient history and exam provide an assessment of the pretest probability of disease.
In evaluating chest pain there is an additional potential factor that diminishes reliance on bedside assessment. Modern quality assurance measures and chest pain units encourage clinicians to evaluate patients with chest pain quickly because any delay diminishes the benefits of therapies for acute coronary syndromes. In the emergency room, these patients find themselves on a rapidly moving diagnostic conveyor belt, an approach that is efficient and appropriate given the high prevalence of coronary disease but that also contributes to inattentiveness and error for patients with unusual diagnoses.
How Could Clinicians in Our Case Use Bedside Evidence to Help Differentiate Our Patient?
For most patients with chest pain there is no finding that would change diagnostic probabilities enough to take them off the diagnostic conveyor belt. Nevertheless, several bedside findings can help providers to rank‐order a differential diagnosis, thereby improving the sequence in which diagnostic testing is done. For patients with chest pain the ECG has the highest predictive ability of all studied history, physical exam, and ECG findings (Table 2).21 A history of sharp and positional pain descriptors diminishes the probability of myocardial ischemia.21 Unfortunately, no history, exam, or ECG feature is sensitive enough, either alone or in combination, to effectively rule out myocardial ischemia.
| Finding | Positive LR* |
|---|---|
| Myocardial ischemia | |
| ST segment elevation or Q wave | 22 |
| S3 gallop, blood pressure < 100 mm Hg, or ST segment depression | 3.0 |
| Sharp or positional pain | 0.3 |
| Pulmonary embolism | |
| Low clinical probability | 0.2 |
| Medium clinical probability | 1.8 |
| High clinical probability | 17.1 |
| Aortic dissection | |
| Tearing or ripping pain | 10.8 |
| Focal neurologic deficits | 6.633 |
| Ipsilateral versus contralateral pulse deficit | 5.7 |
| Cardiac tamponade | |
| Pulsus paradoxus > 12 mm Hg | 5.9 |
| Esophageal perforation | |
| Dysphagia, odynophagia, retching, vomiting, or subcutaneous emphysema | ? |
The history and exam can also facilitate differentiation of noncoronary causes of life‐threatening chest pain. The dismal performance of individual bedside findings for pulmonary embolism is what led to development of quantitative D‐dimer assays and objective methods based on bedside evaluation, including the widely used Wells Score.22 This score can be used to classify patients as having low, medium, and high risk of pulmonary embolism, facilitating management decisions after diagnostic imaging is obtained.23 Fewer than half of all patients with thoracic aortic dissection have classic exam findings; however, when present, they can appropriately raise the probability of dissection higher on the differential diagnosis.24 Importantly, no history or exam finding argues against dissection.24 Most patients with cardiac tamponade will have elevated jugular venous pressure (76%100%); however, poor interobserver agreement about this finding may decrease its detection.11, 25, 26 As the discussant notes, total paradox, defined as the palpable pulse disappearing with inspiration, is an insensitive test for tamponade, present in only 23% of patients with the disorder. In contrast, an inspiratory drop in systolic blood pressure of more than 12 mm Hg should prompt consideration for tamponade.11, 26 Commonly taught features of esophageal perforation, including chest pain, dysphagia, odynophagia, prior retching or vomiting, subcutaneous emphysema, dyspnea, and pleural effusions, vary in their reported sensitivity, but their specificity is virtually never reported.27
Like most patients with chest pain, our patient lacked all these symptoms and signs, arguing for myocardial ischemia, although he had a few signs that argued against it (sharp and positional chest pain). After the initial CXR and ECG, further testing with cardiac biomarkers was appropriate, but a fundamental error was made in not returning to the patient's bedside to repeat the interview and examination after the cardiac biomarkers were found to be normal. Had this been done, several cluesdysphagia, onset of pain with eating bony fish, and feverwould have pushed esophageal perforation to the top of the differential diagnosis. Subsequent testing would have led to the correct diagnosis and avoided a potentially harmful diagnostic fishing expedition.
Take‐Home Points
-
Esophageal perforation is an uncommon but life‐threatening cause of chest pain that is difficult to diagnose because of its nonspecific symptoms.
-
An accurate and complete history and exam can reveal signs and symptoms that influence the likelihood of each life‐threatening cause of chest pain. Evaluating patients for these features is vital to the rank ordering of a differential diagnosis and the selection of appropriate diagnostic tests.
-
There is no substitute for repeating the history, reexamining the patient, and reevaluating available information when confronted with a confusing constellation of symptoms.
Acknowledgements
The authors thank Steve McGee for his thoughtful review and comments on the manuscript.
- ,.Esophageal perforations: a 15 year experience.Am J Surg.1982;143:495–503.
- ,,.Esophageal perforation: emphasis on management.Ann Thorac Surg.1996;61:1447–1451; discussion1451–1452.
- ,,,,,.Evolving options in the management of esophageal perforation.Ann Thorac Surg.2004;77:1475–1783.
- ,.Personal management of 57 consecutive patients with esophageal perforation.Am J Surg.2004;187:58–63.
- ,,,,.Perforation of the oesophagus and aorta after eating fish: an unusual cause of chest pain.Emerg Med J.2003;20:385–386.
- ,,,.Esophageal perforation and mediastinitis from fish bone ingestion.South Med J.2003;96:516–520.
- ,,,.A 73‐year‐old man with chest pain 4 days after a fish dinner.Chest.2004;126:294–297.
- ,.The science of the art of the clinical examination.JAMA.1992;267:2650–2652.
- .Clinical skills in the 21st century.Arch Intern Med.1994;154:22–24.
- ,.Pulmonary auscultatory skills during training in internal medicine and family practice.Am J Respir Crit Care Med.1999;159:1119–1124.
- .Evidence‐Based Physical Diagnosis.Philadelphia, PA:Saunders;2001.
- .Simple is beautiful: the neglected power of simple tests.Arch Intern Med.2004;164:2198–2200.
- ,.Pearls and pitfalls in patient care: need to revive traditional clinical values.Am J Med Sci.2004;327:79–85.
- ,. Physical diagnosis: a lost art? Agency for Health Research and Quality. WebM75:29–40.
- .Low‐tech autopsies in the era of high‐tech medicine: continued value for quality assurance and patient safety.JAMA.1998;280:1273–1274.
- ,,,.Has misdiagnosis of appendicitis decreased over time? A population‐based analysis.JAMA.2001;286:1748–1753.
- ,,,.Changes in rates of autopsy‐detected diagnostic errors over time: a systematic review.JAMA.2003;289:2849–2856.
- .Diagnostic Process.J Coll Gen Pract.1963;54:579–589.
- .The importance of the history in the medical clinic and the cost of unnecessary tests.Am Heart J.1980;100:928–931.
- ,.Bedside diagnosis of coronary artery disease: a systematic review.Am J Med.2004;117:334–343.
- ,,, et al.Use of a clinical model for safe management of patients with suspected pulmonary embolism.Ann Intern Med.1998;129:997–1005.
- ,,, et al.Diagnostic pathways in acute pulmonary embolism: recommendations of the PIOPED II investigators.Am J Med.2006;119:1048–1055.
- .Does this patient have an acute thoracic aortic dissection?JAMA.2002;287:2262–2272.
- ,.The rational clinical examination. Does this patient have abnormal central venous pressure?JAMA.1996;275:630–634.
- ,,,.Does this patient with a pericardial effusion have cardiac tamponade?JAMA.2007;297:1810–1818.
- ,.Spontaneous esophageal rupture: a frequently missed diagnosis.Am Surg.1999;65:449–452.
A 54‐year‐old man with hypertension and type 2 diabetes mellitus entered the Chest Pain Evaluation Unit of a teaching hospital after 12 hours of intermittent thoracic discomfort. The pain began during dinner and was sharp, bandlike, and located beneath the sternum and across the entire chest. He had dyspnea but no diaphoresis or nausea. A recumbent position relieved the pain after dinner, but it recurred during the night and again the following morning. He did not smoke and had no family history of coronary artery disease.
A useful approach in evaluating acute chest pain is to employ a hierarchical differential diagnosis that emphasizes life‐threatening disorders requiring prompt recognition and intervention. Most prominent are cardiac ischemia, pericardial tamponade, pneumothorax, pulmonary embolus, esophageal rupture, and aortic dissection. The concurrent dyspnea and retrosternal location and intermittent nature of the pain that this patient has are consistent with myocardial ischemia, but the sharp quality of the pain and the relief gained by being recumbent are atypical. Pain with pericarditis is characteristically pleuritic and often worse when lying down. The pain of pneumothorax is typically unilateral, not intermittent, and unlikely to improve with recumbency. Although pain during eating suggests the possibility of an esophageal source, spontaneous rupture usually follows vomiting. The pain is typically continuous and severe. The pain of pulmonary embolism may be unilateral and pleuritic but often is more diffuse. Relief by recumbency is unusual, but the intermittent nature could suggest recurrent emboli. The patient has a history of hypertension, which predisposes him to aortic dissection, in which the pain is typically sharp, continuous, and severe but occasionally intermittent. Among numerous less urgent diagnoses are esophagitis and thoracic diabetic radiculopathy.
Important features to look for during this patient's examination include: disappearance of the radial pulse during inhalation, a simple screening test that is insensitive but very specific for pericardial tamponade; elevated neck veins, which can occur with tension pneumothorax, massive pulmonary embolism, and pericardial tamponade; pericardial and pleural friction rubs; discrepant blood pressures in the 2 arms, sometimes a sign of aortic dissection; local thoracic tenderness from chest wall disorders; and sensory examination of the chest surface, which is often abnormal in diabetic thoracic radiculopathy. Given this patient's age and history of diabetes, I am most concerned about myocardial ischemia. The most appropriate diagnostic tests include an electrocardiogram and a chest radiograph.
The patient appeared apprehensive but reported no pain. He had a temperature of 36.0 C, heart rate of 95 beats/minute, blood pressure of 138/77 mm Hg, respiratory rate of 16/minute, and oxygen saturation of 99% while breathing ambient air. Blood pressures were equal in both arms. Jugular venous distention was absent, and the lung and cardiac examinations had normal results. His pain did not increase on chest wall palpation. Examination of the abdomen, extremities, and the neurologic system showed normal results.
The results of laboratory tests showed a leukocyte count of 12,700/cm3, with 85% neutrophils, 8% lymphocytes, 6% monocytes, and 1% eosinophils. The hematocrit was 45%, and the platelet count was 172,000/cm3. The results of his chemistry panel were remarkable only for a glucose of 225 mg/dL. An electrocardiogram (ECG) showed a normal sinus rhythm and left anterior fascicular block without acute ST‐ or T‐wave changes. Prior ECGs were unavailable. An anteroposterior radiograph disclosed low lung volumes and bibasilar opacities. No pleural effusion was noted. Serial serum troponin and creatine kinase levels were normal. A Tc‐99m tetrofosmin cardiac nuclear perfusion test performed at rest demonstrated a moderate area of mildly decreased uptake along the inferior wall extending to the apex. An exercise treadmill test, terminated after 1 minute, 45 seconds because of chest pain, provoked no ECG changes diagnostic of ischemic disease.
The absence of elevated jugular venous pressure virtually eliminates pericardial tamponade as a diagnosis, and the chest film excludes pneumothorax. The intermittent nature of the chest pain, the absence on the chest radiograph of such findings as mediastinal gas, left pneumothorax, or hydropneumothorax, and the lack of a predisposing cause make esophageal rupture unlikely. Pulmonary emboli remain a consideration despite the normal oxygen saturation because there is no hypoxemia in a substantial minority of such cases. The normal cardiac enzyme levels and the lack of significant changes on the ECG exclude that a myocardial infarction has recently occurred, but cardiac ischemia remains a possibility, especially because the patient had chest pain on exercise and the nuclear scan indicated diminished blood flow to the inferior left ventricle. Aortic dissection still lurks as a possibility. The inferior wall abnormalities seen on the scan could result from dissection into the right coronary artery, which is more frequently involved than the left, or compression of it by an enlarged aorta, but they also may be artifacts. The leukocytosis may be a nonspecific response to stress but could indicate, although unlikely, infections such as mediastinitis from esophageal rupture or bacterial aortitis.
A conscientious clinician would repeat the history, reexamine the patient, and scrutinize the chest film to determine what the bilateral opacities represent. Given the story so far, however, I might consider a thoracic computed tomography (CT) angiogram because I am most concerned about pulmonary emboli and aortic dissection.
On hospital day 3, the patient had worsening dyspnea and persistent chest pain. His temperature was 39.3C, and his oxygen saturation decreased to 89% while breathing room air. Repeat chest radiography showed new bilateral pleural effusions and increased bibasilar opacification (Fig. 1). His leukocyte count was 19,000/cm3, with 88% neutrophils, 6% lymphocytes, 5% monocytes, and 1% eosinophils. Care was transferred from the chest pain team to an inpatient general medicine ward team. A pulmonary CT angiogram showed no large central clots but suggested emboli in the right superior subsegmental artery and a right upper lobe subsegmental artery. Bilateral pleural effusions were observed, as were bilateral pleural‐based atelectasis or infiltrates in the lower lungs. A hiatal hernia was noted, but no aortic dissection. The patient received supplemental oxygen, intravenous levofloxacin, and unfractionated heparin by continuous infusion.

Without other information, I will assume that the fever is part of the patient's original disease and not a nosocomial infection or drug fever. At this point, a crucial part of the evaluation is examining the CT scan with experienced radiologists to determine whether the abnormalities noted are genuinely convincing for pulmonary emboli. If the findings are equivocal, the next step might be a pulmonary angiogram or the indirect approach of evaluating the leg veins with ultrasound, reasoning that the presence of proximal leg vein thromboses would require anticoagulation in any event.
The patient's worsening chest pain and hypoxemia are consistent with multiple pulmonary emboli. Bilateral pleural effusions and leukocytosis can occur but are uncommon. Because of the fever, another possibility is septic pulmonary emboli, but he has no evidence of suppurative thrombophlebitis of the peripheral veins, apparent infection elsewhere, or previous intravenous drug abuse causing right‐sided endocarditis. An alternative diagnosis is infection of an initially bland pulmonary infarct.
An important consideration is a thoracentesis, depending on how persuasive the CT diagnosis of pulmonary embolism is and the size of the pleural effusions. It should be done before instituting antimicrobial therapy, which may decrease the yield of the cultures, and before starting heparin, which increases the risk of bleeding and occasionally causes a substantial, even fatal hemothorax.
The patient's oxygenation and dyspnea did not improve. Over the next day, he repeatedly mentioned that swallowing, particularly solid foods, worsened his chest pain. He had a temperature of 39.9C, a heart rate of 121 beats/minute, blood pressure of 149/94 mm Hg, a respiratory rate of 28/minute, and oxygen saturation of 93% while breathing 40% oxygen. He had inspiratory splinting, percussive dullness at both lung bases, and distant heart sounds. A contrast esophagogram showed distal narrowing that prevented solid contrast from passing, but no hiatal hernia. Blood and urine cultures obtained before antibiotic therapy were sterile. Duplex ultrasonography of bilateral lower extremities showed no evidence of deep venous thrombosis. A pulmonary angiogram revealed no emboli, and heparin was discontinued. Bilateral thoracentesis yielded grossly bloody fluid. Repeat chest CT (Fig. 2) demonstrated large bilateral effusions, a new large pericardial effusion, and a prominence at the gastroesophageal junction more concerning for a soft‐tissue mass than for a hiatal hernia, although the quality of the study was suboptimal because of an absence of oral contrast.

The CT scan suggests a paraesophageal abscess from an esophageal rupture. As mentioned earlier, if rupture occurs spontaneously, it typically follows retching or vomiting and is called Boerhaave's syndrome. Another consideration is a rupture secondary to an external insult, such as trauma or ingestion of a caustic substance. In evaluating these possibilities, the patient should have been asked 4 questions at the initial interview that I neglected to explicitly highlight earlier. First, what was he eating when he developed the chest pain? Second, did the pain begin during swallowing? Third, did he have previous symptoms suggesting an esophageal disorder such as dysphagia, odynophagia, or heartburn? These might indicate a cancer that could perforate or another problem such as a stricture or disordered esophageal motility that might have caused a swallowed item to lodge in the esophagus. Finally, did he have retching or vomiting? Though not routinely part of the review of systems, the former 2 questions are an appropriate history‐prompted line of questioning of a patient with onset of chest pain while eating.
At this point, a reasonable approach would be an esophagoscopy to delineate any intraluminal problems, such as a cancer or a foreign body. The apparent obstruction seen on barium swallow may be from extrinsic pressure from a paraesophageal abscess. The patient should receive broad‐spectrum antimicrobial therapy effective against oral anaerobes. Although occasionally patients recover with antibiotics alone, surgery is usually required. I am surprised that the original CT scan did not show evidence of an esophageal perforation. Possibly, the hiatal hernia was a paraesophageal abscess poorly characterized because of the lack of oral contrast.
The team, concerned about esophageal perforation, began the patient on intravenous clindamycin. The patient underwent video‐assisted thoracoscopic drainage, which yielded a moderate amount of turbid, bloody fluid from each hemithorax. The pericardium contained approximately 500 cm3 of turbid fluid. Gram stain and culture of these fluids were negative. No esophageal or mediastinal mass was noted during surgery. Intraoperative esophagogastroduodenoscopy with endoscopic ultrasound showed a healing linear mucosal tear in the distal esophagus (Fig. 3) as well as air/fluid collection in the esophageal soft tissue (not shown).

On further questioning postoperatively, the patient reported eating bony fish during the dinner when he first experienced chest pain. The patient received a 21‐day course of oral clindamycin and completely recovered. Five weeks later, a chest CT showed decreased distal esophageal thickening and no mediastinal air.
COMMENTARY
Esophageal perforation is an uncommon but life‐threatening cause of chest pain. In most series iatrogenic injury accounts for more than 70% of cases, whereas most of the other cases have spontaneous (5%20%) or traumatic (4%10%) causes (Table 1).14 Perforation as a complication of ingesting fish bones, although rare, is well described and continues to be reported.57
| Etiology | Percent |
|---|---|
| |
| Iatrogenic | 45%77% |
| Rigid or flexible endoscopy, balloon dilation, Blakemore tube, sclerotherapy, operative injury | |
| Increased intraesophageal pressure (Boerhaave's syndrome) | 5%20% |
| Vomiting or retching, weightlifting, childbirth | |
| Traumatic | 4%10% |
| Penetrating or blunt injury to neck or chest | |
| Ingestion | 0%12% |
| Foreign body, toxic or caustic substance | |
| Miscellaneous | 0%5% |
| Malignancy, Barrett's esophagus, infection, aortic dissection | |
Diagnosis of esophageal perforation secondary to a foreign body may be difficult because of the considerable overlap of symptoms with other causes of chest pain and failure to consider this infrequent condition in the absence of a classic history of retching. To diagnose such a disease, physicians must gather data from various sourcesespecially the history, physical examination, and medical recordformulate hypotheses, integrate results from diagnostic tests, and then assess the importance of the available information in the context of a differential diagnosis. Incorrectly evaluating or failing to obtain essential data can lead to incorrect or delayed diagnoses.
In This Patient's Evaluation, What Prevented Prompt Recognition of Esophageal Perforation?
The critical misstep was an incomplete history, both on arrival and when the patient was transferred to a second team. The presence of risk factors for coronary artery disease led the providers to first consider myocardial ischemia. They failed to ask crucial questions about the onset of the painwhen it occurred during the meal and what he was eatingeven when the patient later complained of odynophagia. As a result of the incomplete history, the providers, puzzled by the patient's ongoing and evolving symptoms, ordered numerous unnecessary diagnostic tests that gave false‐positive results, leading to potentially harmful treatment including anticoagulation. The discussant mentions that the preferred response to a puzzling clinical situation is to return to the bedside and repeat the history, reexamine the patient, and reevaluate available informationsimple steps that can often resolve diagnostic dilemmas.
There is ongoing concern that the history‐taking and physical examination skills of clinicians are in decline.814 Many speculate this is in part due to reliance on increasingly sophisticated diagnostic tests. Providers may overly rely on modern diagnostic tests because of their familiarity with the sensitivity and specificity of such tests, fear of malpractice litigation, diminishing opportunity to elucidate the complete history and physical exam, or lack of confidence in their history‐taking and examination skills.814 Although the rapid development and implementation of advanced diagnostic technologies have had a significant impact on diagnostic accuracy, the estimated rate of disease misdiagnosis remains elevated at 24%.1518 In contrast to technology‐based testing, the history and physical provide an inexpensive, safe, and effective means of at arriving at a correct diagnosis. In outpatient medical visits the history and physical, when completely elicited, result in a correct diagnosis of up to 70%90% of patients.8, 19, 20 Even for illnesses whose diagnosis requires confirmation by a diagnostic test, the definitive test can only be selected after a sufficient history and exam provide an assessment of the pretest probability of disease.
In evaluating chest pain there is an additional potential factor that diminishes reliance on bedside assessment. Modern quality assurance measures and chest pain units encourage clinicians to evaluate patients with chest pain quickly because any delay diminishes the benefits of therapies for acute coronary syndromes. In the emergency room, these patients find themselves on a rapidly moving diagnostic conveyor belt, an approach that is efficient and appropriate given the high prevalence of coronary disease but that also contributes to inattentiveness and error for patients with unusual diagnoses.
How Could Clinicians in Our Case Use Bedside Evidence to Help Differentiate Our Patient?
For most patients with chest pain there is no finding that would change diagnostic probabilities enough to take them off the diagnostic conveyor belt. Nevertheless, several bedside findings can help providers to rank‐order a differential diagnosis, thereby improving the sequence in which diagnostic testing is done. For patients with chest pain the ECG has the highest predictive ability of all studied history, physical exam, and ECG findings (Table 2).21 A history of sharp and positional pain descriptors diminishes the probability of myocardial ischemia.21 Unfortunately, no history, exam, or ECG feature is sensitive enough, either alone or in combination, to effectively rule out myocardial ischemia.
| Finding | Positive LR* |
|---|---|
| Myocardial ischemia | |
| ST segment elevation or Q wave | 22 |
| S3 gallop, blood pressure < 100 mm Hg, or ST segment depression | 3.0 |
| Sharp or positional pain | 0.3 |
| Pulmonary embolism | |
| Low clinical probability | 0.2 |
| Medium clinical probability | 1.8 |
| High clinical probability | 17.1 |
| Aortic dissection | |
| Tearing or ripping pain | 10.8 |
| Focal neurologic deficits | 6.633 |
| Ipsilateral versus contralateral pulse deficit | 5.7 |
| Cardiac tamponade | |
| Pulsus paradoxus > 12 mm Hg | 5.9 |
| Esophageal perforation | |
| Dysphagia, odynophagia, retching, vomiting, or subcutaneous emphysema | ? |
The history and exam can also facilitate differentiation of noncoronary causes of life‐threatening chest pain. The dismal performance of individual bedside findings for pulmonary embolism is what led to development of quantitative D‐dimer assays and objective methods based on bedside evaluation, including the widely used Wells Score.22 This score can be used to classify patients as having low, medium, and high risk of pulmonary embolism, facilitating management decisions after diagnostic imaging is obtained.23 Fewer than half of all patients with thoracic aortic dissection have classic exam findings; however, when present, they can appropriately raise the probability of dissection higher on the differential diagnosis.24 Importantly, no history or exam finding argues against dissection.24 Most patients with cardiac tamponade will have elevated jugular venous pressure (76%100%); however, poor interobserver agreement about this finding may decrease its detection.11, 25, 26 As the discussant notes, total paradox, defined as the palpable pulse disappearing with inspiration, is an insensitive test for tamponade, present in only 23% of patients with the disorder. In contrast, an inspiratory drop in systolic blood pressure of more than 12 mm Hg should prompt consideration for tamponade.11, 26 Commonly taught features of esophageal perforation, including chest pain, dysphagia, odynophagia, prior retching or vomiting, subcutaneous emphysema, dyspnea, and pleural effusions, vary in their reported sensitivity, but their specificity is virtually never reported.27
Like most patients with chest pain, our patient lacked all these symptoms and signs, arguing for myocardial ischemia, although he had a few signs that argued against it (sharp and positional chest pain). After the initial CXR and ECG, further testing with cardiac biomarkers was appropriate, but a fundamental error was made in not returning to the patient's bedside to repeat the interview and examination after the cardiac biomarkers were found to be normal. Had this been done, several cluesdysphagia, onset of pain with eating bony fish, and feverwould have pushed esophageal perforation to the top of the differential diagnosis. Subsequent testing would have led to the correct diagnosis and avoided a potentially harmful diagnostic fishing expedition.
Take‐Home Points
-
Esophageal perforation is an uncommon but life‐threatening cause of chest pain that is difficult to diagnose because of its nonspecific symptoms.
-
An accurate and complete history and exam can reveal signs and symptoms that influence the likelihood of each life‐threatening cause of chest pain. Evaluating patients for these features is vital to the rank ordering of a differential diagnosis and the selection of appropriate diagnostic tests.
-
There is no substitute for repeating the history, reexamining the patient, and reevaluating available information when confronted with a confusing constellation of symptoms.
Acknowledgements
The authors thank Steve McGee for his thoughtful review and comments on the manuscript.
A 54‐year‐old man with hypertension and type 2 diabetes mellitus entered the Chest Pain Evaluation Unit of a teaching hospital after 12 hours of intermittent thoracic discomfort. The pain began during dinner and was sharp, bandlike, and located beneath the sternum and across the entire chest. He had dyspnea but no diaphoresis or nausea. A recumbent position relieved the pain after dinner, but it recurred during the night and again the following morning. He did not smoke and had no family history of coronary artery disease.
A useful approach in evaluating acute chest pain is to employ a hierarchical differential diagnosis that emphasizes life‐threatening disorders requiring prompt recognition and intervention. Most prominent are cardiac ischemia, pericardial tamponade, pneumothorax, pulmonary embolus, esophageal rupture, and aortic dissection. The concurrent dyspnea and retrosternal location and intermittent nature of the pain that this patient has are consistent with myocardial ischemia, but the sharp quality of the pain and the relief gained by being recumbent are atypical. Pain with pericarditis is characteristically pleuritic and often worse when lying down. The pain of pneumothorax is typically unilateral, not intermittent, and unlikely to improve with recumbency. Although pain during eating suggests the possibility of an esophageal source, spontaneous rupture usually follows vomiting. The pain is typically continuous and severe. The pain of pulmonary embolism may be unilateral and pleuritic but often is more diffuse. Relief by recumbency is unusual, but the intermittent nature could suggest recurrent emboli. The patient has a history of hypertension, which predisposes him to aortic dissection, in which the pain is typically sharp, continuous, and severe but occasionally intermittent. Among numerous less urgent diagnoses are esophagitis and thoracic diabetic radiculopathy.
Important features to look for during this patient's examination include: disappearance of the radial pulse during inhalation, a simple screening test that is insensitive but very specific for pericardial tamponade; elevated neck veins, which can occur with tension pneumothorax, massive pulmonary embolism, and pericardial tamponade; pericardial and pleural friction rubs; discrepant blood pressures in the 2 arms, sometimes a sign of aortic dissection; local thoracic tenderness from chest wall disorders; and sensory examination of the chest surface, which is often abnormal in diabetic thoracic radiculopathy. Given this patient's age and history of diabetes, I am most concerned about myocardial ischemia. The most appropriate diagnostic tests include an electrocardiogram and a chest radiograph.
The patient appeared apprehensive but reported no pain. He had a temperature of 36.0 C, heart rate of 95 beats/minute, blood pressure of 138/77 mm Hg, respiratory rate of 16/minute, and oxygen saturation of 99% while breathing ambient air. Blood pressures were equal in both arms. Jugular venous distention was absent, and the lung and cardiac examinations had normal results. His pain did not increase on chest wall palpation. Examination of the abdomen, extremities, and the neurologic system showed normal results.
The results of laboratory tests showed a leukocyte count of 12,700/cm3, with 85% neutrophils, 8% lymphocytes, 6% monocytes, and 1% eosinophils. The hematocrit was 45%, and the platelet count was 172,000/cm3. The results of his chemistry panel were remarkable only for a glucose of 225 mg/dL. An electrocardiogram (ECG) showed a normal sinus rhythm and left anterior fascicular block without acute ST‐ or T‐wave changes. Prior ECGs were unavailable. An anteroposterior radiograph disclosed low lung volumes and bibasilar opacities. No pleural effusion was noted. Serial serum troponin and creatine kinase levels were normal. A Tc‐99m tetrofosmin cardiac nuclear perfusion test performed at rest demonstrated a moderate area of mildly decreased uptake along the inferior wall extending to the apex. An exercise treadmill test, terminated after 1 minute, 45 seconds because of chest pain, provoked no ECG changes diagnostic of ischemic disease.
The absence of elevated jugular venous pressure virtually eliminates pericardial tamponade as a diagnosis, and the chest film excludes pneumothorax. The intermittent nature of the chest pain, the absence on the chest radiograph of such findings as mediastinal gas, left pneumothorax, or hydropneumothorax, and the lack of a predisposing cause make esophageal rupture unlikely. Pulmonary emboli remain a consideration despite the normal oxygen saturation because there is no hypoxemia in a substantial minority of such cases. The normal cardiac enzyme levels and the lack of significant changes on the ECG exclude that a myocardial infarction has recently occurred, but cardiac ischemia remains a possibility, especially because the patient had chest pain on exercise and the nuclear scan indicated diminished blood flow to the inferior left ventricle. Aortic dissection still lurks as a possibility. The inferior wall abnormalities seen on the scan could result from dissection into the right coronary artery, which is more frequently involved than the left, or compression of it by an enlarged aorta, but they also may be artifacts. The leukocytosis may be a nonspecific response to stress but could indicate, although unlikely, infections such as mediastinitis from esophageal rupture or bacterial aortitis.
A conscientious clinician would repeat the history, reexamine the patient, and scrutinize the chest film to determine what the bilateral opacities represent. Given the story so far, however, I might consider a thoracic computed tomography (CT) angiogram because I am most concerned about pulmonary emboli and aortic dissection.
On hospital day 3, the patient had worsening dyspnea and persistent chest pain. His temperature was 39.3C, and his oxygen saturation decreased to 89% while breathing room air. Repeat chest radiography showed new bilateral pleural effusions and increased bibasilar opacification (Fig. 1). His leukocyte count was 19,000/cm3, with 88% neutrophils, 6% lymphocytes, 5% monocytes, and 1% eosinophils. Care was transferred from the chest pain team to an inpatient general medicine ward team. A pulmonary CT angiogram showed no large central clots but suggested emboli in the right superior subsegmental artery and a right upper lobe subsegmental artery. Bilateral pleural effusions were observed, as were bilateral pleural‐based atelectasis or infiltrates in the lower lungs. A hiatal hernia was noted, but no aortic dissection. The patient received supplemental oxygen, intravenous levofloxacin, and unfractionated heparin by continuous infusion.

Without other information, I will assume that the fever is part of the patient's original disease and not a nosocomial infection or drug fever. At this point, a crucial part of the evaluation is examining the CT scan with experienced radiologists to determine whether the abnormalities noted are genuinely convincing for pulmonary emboli. If the findings are equivocal, the next step might be a pulmonary angiogram or the indirect approach of evaluating the leg veins with ultrasound, reasoning that the presence of proximal leg vein thromboses would require anticoagulation in any event.
The patient's worsening chest pain and hypoxemia are consistent with multiple pulmonary emboli. Bilateral pleural effusions and leukocytosis can occur but are uncommon. Because of the fever, another possibility is septic pulmonary emboli, but he has no evidence of suppurative thrombophlebitis of the peripheral veins, apparent infection elsewhere, or previous intravenous drug abuse causing right‐sided endocarditis. An alternative diagnosis is infection of an initially bland pulmonary infarct.
An important consideration is a thoracentesis, depending on how persuasive the CT diagnosis of pulmonary embolism is and the size of the pleural effusions. It should be done before instituting antimicrobial therapy, which may decrease the yield of the cultures, and before starting heparin, which increases the risk of bleeding and occasionally causes a substantial, even fatal hemothorax.
The patient's oxygenation and dyspnea did not improve. Over the next day, he repeatedly mentioned that swallowing, particularly solid foods, worsened his chest pain. He had a temperature of 39.9C, a heart rate of 121 beats/minute, blood pressure of 149/94 mm Hg, a respiratory rate of 28/minute, and oxygen saturation of 93% while breathing 40% oxygen. He had inspiratory splinting, percussive dullness at both lung bases, and distant heart sounds. A contrast esophagogram showed distal narrowing that prevented solid contrast from passing, but no hiatal hernia. Blood and urine cultures obtained before antibiotic therapy were sterile. Duplex ultrasonography of bilateral lower extremities showed no evidence of deep venous thrombosis. A pulmonary angiogram revealed no emboli, and heparin was discontinued. Bilateral thoracentesis yielded grossly bloody fluid. Repeat chest CT (Fig. 2) demonstrated large bilateral effusions, a new large pericardial effusion, and a prominence at the gastroesophageal junction more concerning for a soft‐tissue mass than for a hiatal hernia, although the quality of the study was suboptimal because of an absence of oral contrast.

The CT scan suggests a paraesophageal abscess from an esophageal rupture. As mentioned earlier, if rupture occurs spontaneously, it typically follows retching or vomiting and is called Boerhaave's syndrome. Another consideration is a rupture secondary to an external insult, such as trauma or ingestion of a caustic substance. In evaluating these possibilities, the patient should have been asked 4 questions at the initial interview that I neglected to explicitly highlight earlier. First, what was he eating when he developed the chest pain? Second, did the pain begin during swallowing? Third, did he have previous symptoms suggesting an esophageal disorder such as dysphagia, odynophagia, or heartburn? These might indicate a cancer that could perforate or another problem such as a stricture or disordered esophageal motility that might have caused a swallowed item to lodge in the esophagus. Finally, did he have retching or vomiting? Though not routinely part of the review of systems, the former 2 questions are an appropriate history‐prompted line of questioning of a patient with onset of chest pain while eating.
At this point, a reasonable approach would be an esophagoscopy to delineate any intraluminal problems, such as a cancer or a foreign body. The apparent obstruction seen on barium swallow may be from extrinsic pressure from a paraesophageal abscess. The patient should receive broad‐spectrum antimicrobial therapy effective against oral anaerobes. Although occasionally patients recover with antibiotics alone, surgery is usually required. I am surprised that the original CT scan did not show evidence of an esophageal perforation. Possibly, the hiatal hernia was a paraesophageal abscess poorly characterized because of the lack of oral contrast.
The team, concerned about esophageal perforation, began the patient on intravenous clindamycin. The patient underwent video‐assisted thoracoscopic drainage, which yielded a moderate amount of turbid, bloody fluid from each hemithorax. The pericardium contained approximately 500 cm3 of turbid fluid. Gram stain and culture of these fluids were negative. No esophageal or mediastinal mass was noted during surgery. Intraoperative esophagogastroduodenoscopy with endoscopic ultrasound showed a healing linear mucosal tear in the distal esophagus (Fig. 3) as well as air/fluid collection in the esophageal soft tissue (not shown).

On further questioning postoperatively, the patient reported eating bony fish during the dinner when he first experienced chest pain. The patient received a 21‐day course of oral clindamycin and completely recovered. Five weeks later, a chest CT showed decreased distal esophageal thickening and no mediastinal air.
COMMENTARY
Esophageal perforation is an uncommon but life‐threatening cause of chest pain. In most series iatrogenic injury accounts for more than 70% of cases, whereas most of the other cases have spontaneous (5%20%) or traumatic (4%10%) causes (Table 1).14 Perforation as a complication of ingesting fish bones, although rare, is well described and continues to be reported.57
| Etiology | Percent |
|---|---|
| |
| Iatrogenic | 45%77% |
| Rigid or flexible endoscopy, balloon dilation, Blakemore tube, sclerotherapy, operative injury | |
| Increased intraesophageal pressure (Boerhaave's syndrome) | 5%20% |
| Vomiting or retching, weightlifting, childbirth | |
| Traumatic | 4%10% |
| Penetrating or blunt injury to neck or chest | |
| Ingestion | 0%12% |
| Foreign body, toxic or caustic substance | |
| Miscellaneous | 0%5% |
| Malignancy, Barrett's esophagus, infection, aortic dissection | |
Diagnosis of esophageal perforation secondary to a foreign body may be difficult because of the considerable overlap of symptoms with other causes of chest pain and failure to consider this infrequent condition in the absence of a classic history of retching. To diagnose such a disease, physicians must gather data from various sourcesespecially the history, physical examination, and medical recordformulate hypotheses, integrate results from diagnostic tests, and then assess the importance of the available information in the context of a differential diagnosis. Incorrectly evaluating or failing to obtain essential data can lead to incorrect or delayed diagnoses.
In This Patient's Evaluation, What Prevented Prompt Recognition of Esophageal Perforation?
The critical misstep was an incomplete history, both on arrival and when the patient was transferred to a second team. The presence of risk factors for coronary artery disease led the providers to first consider myocardial ischemia. They failed to ask crucial questions about the onset of the painwhen it occurred during the meal and what he was eatingeven when the patient later complained of odynophagia. As a result of the incomplete history, the providers, puzzled by the patient's ongoing and evolving symptoms, ordered numerous unnecessary diagnostic tests that gave false‐positive results, leading to potentially harmful treatment including anticoagulation. The discussant mentions that the preferred response to a puzzling clinical situation is to return to the bedside and repeat the history, reexamine the patient, and reevaluate available informationsimple steps that can often resolve diagnostic dilemmas.
There is ongoing concern that the history‐taking and physical examination skills of clinicians are in decline.814 Many speculate this is in part due to reliance on increasingly sophisticated diagnostic tests. Providers may overly rely on modern diagnostic tests because of their familiarity with the sensitivity and specificity of such tests, fear of malpractice litigation, diminishing opportunity to elucidate the complete history and physical exam, or lack of confidence in their history‐taking and examination skills.814 Although the rapid development and implementation of advanced diagnostic technologies have had a significant impact on diagnostic accuracy, the estimated rate of disease misdiagnosis remains elevated at 24%.1518 In contrast to technology‐based testing, the history and physical provide an inexpensive, safe, and effective means of at arriving at a correct diagnosis. In outpatient medical visits the history and physical, when completely elicited, result in a correct diagnosis of up to 70%90% of patients.8, 19, 20 Even for illnesses whose diagnosis requires confirmation by a diagnostic test, the definitive test can only be selected after a sufficient history and exam provide an assessment of the pretest probability of disease.
In evaluating chest pain there is an additional potential factor that diminishes reliance on bedside assessment. Modern quality assurance measures and chest pain units encourage clinicians to evaluate patients with chest pain quickly because any delay diminishes the benefits of therapies for acute coronary syndromes. In the emergency room, these patients find themselves on a rapidly moving diagnostic conveyor belt, an approach that is efficient and appropriate given the high prevalence of coronary disease but that also contributes to inattentiveness and error for patients with unusual diagnoses.
How Could Clinicians in Our Case Use Bedside Evidence to Help Differentiate Our Patient?
For most patients with chest pain there is no finding that would change diagnostic probabilities enough to take them off the diagnostic conveyor belt. Nevertheless, several bedside findings can help providers to rank‐order a differential diagnosis, thereby improving the sequence in which diagnostic testing is done. For patients with chest pain the ECG has the highest predictive ability of all studied history, physical exam, and ECG findings (Table 2).21 A history of sharp and positional pain descriptors diminishes the probability of myocardial ischemia.21 Unfortunately, no history, exam, or ECG feature is sensitive enough, either alone or in combination, to effectively rule out myocardial ischemia.
| Finding | Positive LR* |
|---|---|
| Myocardial ischemia | |
| ST segment elevation or Q wave | 22 |
| S3 gallop, blood pressure < 100 mm Hg, or ST segment depression | 3.0 |
| Sharp or positional pain | 0.3 |
| Pulmonary embolism | |
| Low clinical probability | 0.2 |
| Medium clinical probability | 1.8 |
| High clinical probability | 17.1 |
| Aortic dissection | |
| Tearing or ripping pain | 10.8 |
| Focal neurologic deficits | 6.633 |
| Ipsilateral versus contralateral pulse deficit | 5.7 |
| Cardiac tamponade | |
| Pulsus paradoxus > 12 mm Hg | 5.9 |
| Esophageal perforation | |
| Dysphagia, odynophagia, retching, vomiting, or subcutaneous emphysema | ? |
The history and exam can also facilitate differentiation of noncoronary causes of life‐threatening chest pain. The dismal performance of individual bedside findings for pulmonary embolism is what led to development of quantitative D‐dimer assays and objective methods based on bedside evaluation, including the widely used Wells Score.22 This score can be used to classify patients as having low, medium, and high risk of pulmonary embolism, facilitating management decisions after diagnostic imaging is obtained.23 Fewer than half of all patients with thoracic aortic dissection have classic exam findings; however, when present, they can appropriately raise the probability of dissection higher on the differential diagnosis.24 Importantly, no history or exam finding argues against dissection.24 Most patients with cardiac tamponade will have elevated jugular venous pressure (76%100%); however, poor interobserver agreement about this finding may decrease its detection.11, 25, 26 As the discussant notes, total paradox, defined as the palpable pulse disappearing with inspiration, is an insensitive test for tamponade, present in only 23% of patients with the disorder. In contrast, an inspiratory drop in systolic blood pressure of more than 12 mm Hg should prompt consideration for tamponade.11, 26 Commonly taught features of esophageal perforation, including chest pain, dysphagia, odynophagia, prior retching or vomiting, subcutaneous emphysema, dyspnea, and pleural effusions, vary in their reported sensitivity, but their specificity is virtually never reported.27
Like most patients with chest pain, our patient lacked all these symptoms and signs, arguing for myocardial ischemia, although he had a few signs that argued against it (sharp and positional chest pain). After the initial CXR and ECG, further testing with cardiac biomarkers was appropriate, but a fundamental error was made in not returning to the patient's bedside to repeat the interview and examination after the cardiac biomarkers were found to be normal. Had this been done, several cluesdysphagia, onset of pain with eating bony fish, and feverwould have pushed esophageal perforation to the top of the differential diagnosis. Subsequent testing would have led to the correct diagnosis and avoided a potentially harmful diagnostic fishing expedition.
Take‐Home Points
-
Esophageal perforation is an uncommon but life‐threatening cause of chest pain that is difficult to diagnose because of its nonspecific symptoms.
-
An accurate and complete history and exam can reveal signs and symptoms that influence the likelihood of each life‐threatening cause of chest pain. Evaluating patients for these features is vital to the rank ordering of a differential diagnosis and the selection of appropriate diagnostic tests.
-
There is no substitute for repeating the history, reexamining the patient, and reevaluating available information when confronted with a confusing constellation of symptoms.
Acknowledgements
The authors thank Steve McGee for his thoughtful review and comments on the manuscript.
- ,.Esophageal perforations: a 15 year experience.Am J Surg.1982;143:495–503.
- ,,.Esophageal perforation: emphasis on management.Ann Thorac Surg.1996;61:1447–1451; discussion1451–1452.
- ,,,,,.Evolving options in the management of esophageal perforation.Ann Thorac Surg.2004;77:1475–1783.
- ,.Personal management of 57 consecutive patients with esophageal perforation.Am J Surg.2004;187:58–63.
- ,,,,.Perforation of the oesophagus and aorta after eating fish: an unusual cause of chest pain.Emerg Med J.2003;20:385–386.
- ,,,.Esophageal perforation and mediastinitis from fish bone ingestion.South Med J.2003;96:516–520.
- ,,,.A 73‐year‐old man with chest pain 4 days after a fish dinner.Chest.2004;126:294–297.
- ,.The science of the art of the clinical examination.JAMA.1992;267:2650–2652.
- .Clinical skills in the 21st century.Arch Intern Med.1994;154:22–24.
- ,.Pulmonary auscultatory skills during training in internal medicine and family practice.Am J Respir Crit Care Med.1999;159:1119–1124.
- .Evidence‐Based Physical Diagnosis.Philadelphia, PA:Saunders;2001.
- .Simple is beautiful: the neglected power of simple tests.Arch Intern Med.2004;164:2198–2200.
- ,.Pearls and pitfalls in patient care: need to revive traditional clinical values.Am J Med Sci.2004;327:79–85.
- ,. Physical diagnosis: a lost art? Agency for Health Research and Quality. WebM75:29–40.
- .Low‐tech autopsies in the era of high‐tech medicine: continued value for quality assurance and patient safety.JAMA.1998;280:1273–1274.
- ,,,.Has misdiagnosis of appendicitis decreased over time? A population‐based analysis.JAMA.2001;286:1748–1753.
- ,,,.Changes in rates of autopsy‐detected diagnostic errors over time: a systematic review.JAMA.2003;289:2849–2856.
- .Diagnostic Process.J Coll Gen Pract.1963;54:579–589.
- .The importance of the history in the medical clinic and the cost of unnecessary tests.Am Heart J.1980;100:928–931.
- ,.Bedside diagnosis of coronary artery disease: a systematic review.Am J Med.2004;117:334–343.
- ,,, et al.Use of a clinical model for safe management of patients with suspected pulmonary embolism.Ann Intern Med.1998;129:997–1005.
- ,,, et al.Diagnostic pathways in acute pulmonary embolism: recommendations of the PIOPED II investigators.Am J Med.2006;119:1048–1055.
- .Does this patient have an acute thoracic aortic dissection?JAMA.2002;287:2262–2272.
- ,.The rational clinical examination. Does this patient have abnormal central venous pressure?JAMA.1996;275:630–634.
- ,,,.Does this patient with a pericardial effusion have cardiac tamponade?JAMA.2007;297:1810–1818.
- ,.Spontaneous esophageal rupture: a frequently missed diagnosis.Am Surg.1999;65:449–452.
- ,.Esophageal perforations: a 15 year experience.Am J Surg.1982;143:495–503.
- ,,.Esophageal perforation: emphasis on management.Ann Thorac Surg.1996;61:1447–1451; discussion1451–1452.
- ,,,,,.Evolving options in the management of esophageal perforation.Ann Thorac Surg.2004;77:1475–1783.
- ,.Personal management of 57 consecutive patients with esophageal perforation.Am J Surg.2004;187:58–63.
- ,,,,.Perforation of the oesophagus and aorta after eating fish: an unusual cause of chest pain.Emerg Med J.2003;20:385–386.
- ,,,.Esophageal perforation and mediastinitis from fish bone ingestion.South Med J.2003;96:516–520.
- ,,,.A 73‐year‐old man with chest pain 4 days after a fish dinner.Chest.2004;126:294–297.
- ,.The science of the art of the clinical examination.JAMA.1992;267:2650–2652.
- .Clinical skills in the 21st century.Arch Intern Med.1994;154:22–24.
- ,.Pulmonary auscultatory skills during training in internal medicine and family practice.Am J Respir Crit Care Med.1999;159:1119–1124.
- .Evidence‐Based Physical Diagnosis.Philadelphia, PA:Saunders;2001.
- .Simple is beautiful: the neglected power of simple tests.Arch Intern Med.2004;164:2198–2200.
- ,.Pearls and pitfalls in patient care: need to revive traditional clinical values.Am J Med Sci.2004;327:79–85.
- ,. Physical diagnosis: a lost art? Agency for Health Research and Quality. WebM75:29–40.
- .Low‐tech autopsies in the era of high‐tech medicine: continued value for quality assurance and patient safety.JAMA.1998;280:1273–1274.
- ,,,.Has misdiagnosis of appendicitis decreased over time? A population‐based analysis.JAMA.2001;286:1748–1753.
- ,,,.Changes in rates of autopsy‐detected diagnostic errors over time: a systematic review.JAMA.2003;289:2849–2856.
- .Diagnostic Process.J Coll Gen Pract.1963;54:579–589.
- .The importance of the history in the medical clinic and the cost of unnecessary tests.Am Heart J.1980;100:928–931.
- ,.Bedside diagnosis of coronary artery disease: a systematic review.Am J Med.2004;117:334–343.
- ,,, et al.Use of a clinical model for safe management of patients with suspected pulmonary embolism.Ann Intern Med.1998;129:997–1005.
- ,,, et al.Diagnostic pathways in acute pulmonary embolism: recommendations of the PIOPED II investigators.Am J Med.2006;119:1048–1055.
- .Does this patient have an acute thoracic aortic dissection?JAMA.2002;287:2262–2272.
- ,.The rational clinical examination. Does this patient have abnormal central venous pressure?JAMA.1996;275:630–634.
- ,,,.Does this patient with a pericardial effusion have cardiac tamponade?JAMA.2007;297:1810–1818.
- ,.Spontaneous esophageal rupture: a frequently missed diagnosis.Am Surg.1999;65:449–452.
“Non‐Heart‐Beating” Organ Donation
In April 2003 the Health Resources and Services Administration of the U.S. Department of Health and Human Services (DHHS) announced the formation of the Organ Donation Breakthrough Collaborative (ODBC).1 The Organ Donation Breakthrough Collaborative created 58 national donation service areas (DSAs) to organize the transplant community across the United States. Each of the 58 organ procurement organizations (OPOs) is joined to a regional transplant center or centers and donor hospitals to form a DSA. The ODBC's goal is to achieve a cadaveric organ donation rate of 75% or higher from hospitals within each DSA.2
A requirement for organ donation from patients facing imminent or cardiac death has been introduced to increase the supply of transplantable organs and shorten the waiting time for transplantation candidates.35 This type of organ donation represents a significant source of organs required for future expansion of transplantation practice in the United States. The requirement for donation in imminent or cardiac death is implemented through the collaboration of the Advisory Committee on Organ Transplantation of the DHHS (Table 1), the Centers for Medicare & Medicaid Services (CMS), and the Joint Commission on Accreditation of Healthcare Organizations (JCAHO).3, 57 The organ donor pool of those facing imminent or cardiac death has also been expanded to include neurologically intact patients who may not fulfill brain death criteria before withdrawal of life support.4, 8, 9
| State law (year)* | |
|---|---|
| |
| Uniform Anatomical Gift Act (1968) | Any 18‐year‐old with a sound mind may donate his or her body after death to be used for medical research or as a source of transplantable tissues and organs and barring others from overriding a donor's decision to make an anatomical gift. |
| Amendment (1987) | Minors who can apply for a driver's license are empowered to make anatomical gifts, but either parent can revoke the gift if the minor dies before the age of 18. |
| Revision (2006) | Declaration of a gift does not require any witnesses. |
| Amendment (2007) | Document of a gift or donor registry is sufficient for the removal of organs, which means an OPO does not need consent of the spouse or the family. |
| Enables an OPO to gain access to documents of gifts in donor registries, medical records, and records of a state motor vehicle department. | |
| Facilitates donations by expanding the list of those who may make an anatomical gift for another individual who has not declared a preference for or against donation. | |
| Permits removal of organs by medical personnel without explicit consent from a potential donor or from a relative of the donor, so long as the appropriate medical personnel or authorities have made a reasonable effort to discover any objection by the donor or the donor's family. | |
| Require hospitals to notify an OPO or third party designated by the OPO of an individual whose death is imminent or who has died in the hospital in order to increase donation opportunity. | |
| Gives an OPO the right to inspect a patient's medical records. | |
| Measures necessary to ensure the medical suitability of a part not be withdrawn while an examination is being made to determine whether an individual who has been referred to OPO has a part that could be the subject of an anatomical gift. If, following such an examination, it is determined by the OPO that the individual has a part that could be the subject of an anatomical gift, the individual is a prospective donor under this act unless the individual had signed a refusal. | |
| Forbids the buying and selling of organs. | |
| Measures necessary to ensure the medical suitability of an organ for transplantation or therapy may not be withheld or withdrawn from a prospective donor who has an advance health‐care directive or declaration unless the directive or declaration expressly provides to the contrary. The section presumes that for prospective donors the desire to save lives by making an anatomical gift trumps the desire to have life support systems withheld or withdrawn. Individuals who desire to overcome this presumption can do so by express language in their advance health‐care directive or declaration. | |
| Uniform Determination of Death Act (1981) | An individual who has sustained either (1) irreversible cessation of circulatory and respiratory functions, or (2) irreversible cessation of all functions of the entire brain including the brain stem is dead. A determination of death must be made in accordance with accepted medical standards. |
| Federal laws | |
| National Organ Transplant Act (1984) | Calls for a unified transplant network to be operated by a private, nonprofit organization under federal contract and the establishment of a Task Force in Organ Procurement and Transplantation and an Organ Procurement and Transplantation Registry. |
| Public Health and Welfare Act Title 42 USC (1999) | |
| Section 273 | Establishes guidelines to be a qualified OPO that can receive federal grants. |
| Section 274 | Establishes the OPTN & Scientific Registry for Transplantation. |
| Federal regulations | |
| Title Code 42 CFR Part 121 (1999) | Lists regulations of the OPTN final rule. |
| Explains the OPTN structure. | |
| Lists the policy that the OPTN board of directors is responsible for developing. | |
| Explains rules that an OPTN member must obey when including a person on the organ waiting list. | |
| Describes the requirements and tests for determining the suitability of donated organs. | |
| Explains how the OPTN identifies an organ recipient, allocates the organ, and transports it to the recipient. | |
| Describes how the board of directors should develop allocation policies to guarantee they are both efficient and just and allocation performance goals to ensure the best possible use and most equitable allocation of organs. | |
| Lists designated transplant program requirements. | |
| Describes how the HHS conducts reviews and evaluations and enforces rules. | |
| Describes the recording and reporting requirements of the various groups involved in the transplantation process. | |
| Establishes the Advisory Committee on Organ Transplantation (ACOT), which advises the HHS secretary on organ donation, procurement, allocation, and transplantation The HHS secretary may ask for ACOT's opinion of proposed OPTN policies. | |
| Title Code 42 CFR Parts 413, 441, 486 and 498 (2006) | Lists regulations of the OPO final rule. |
| Establishes new conditions for coverage for OPOs that include multiple new outcome and process performance measures based on organ donor potential and other related factors in each donation service area of qualified OPOs. | |
The President's Council on Bioethics independently evaluated the issues surrounding deceased organ donation and procurement.10 The President's Council on Bioethics has expressed major concerns about several issues pertinent to cardiac or imminent death organ donation that have not been addressed explicitly by the bodies that have made recommendations for reforming or expanding that type of organ donation in the United States. The debate on organ procurement in imminent or cardiac death has come to the forefront because of doubts about its ethical appropriateness and acceptance within the medical profession and the community at large. This review focuses on the serious issues related to organ procurement from patients facing imminent or cardiac death.
Organ Procurement and the Dead Donor Rule
Organ procurement is only permitted when the donor is already dead (ie, the dead donor rule), and the act of organ recovery cannot have been the immediate act to cause that death.10, 11 There are 2 criteria for death as defined in the Uniform Determination of Death Act (UDDA; Table 1): an individual who has sustained irreversible cessation of either (1) circulatory and respiratory functions or (2) all functions of the entire brain, including the brain stem, is considered dead and this determination of death must have been made in accordance with accepted medical standards.12 When organs are procured from an individual in whom all brain function has ceased but normal cardiac pump activity is continuing, it is referred to as heart‐beating organ donation. Organ procurement after cessation of cardiac pump activity and cardiorespiratory functions is referred to as non‐heart‐beating organ donation (NHBOD). Organ procurement from an individual in imminent or cardiac death is considered NHBOD.
Non‐heart‐beating organ donors can be neurologically intact and do not fulfill the brain death criterion prior to cessation of cardiac pump activity. In response to this dilemma, the University of Pittsburgh Medical Centre developed a protocol for donation of organs that permitted their procurement from patients who were pulseless and apneic for 2 minutes and did not fulfill brain criteria and who had previously given consent for organ donation.13 Because it is uncertain if cessation of cardiorespiratory function is irreversible after only a short time, the Institute of Medicine (IOM) extended the time required for pulselessness and apnea from 2 to 5 minutes before permitting organ procurement.14 Waiting longer than 5 minutes for the determination of death would compromise the quality of procured organs because of warm ischemia time and would influence the functioning of grafts in transplant recipients.
One of the pivotal assumptions for NHBOD acceptance is that 5 minutes of pulselessness and apnea eliminates the possibility that the procurement process itself could be the cause of death and fulfills the dead donor rule.14, 15 The cardiorespiratory death criteria were derived from observations that did not evaluate delayed autoresuscitation (spontaneous return of circulation) or simultaneous cessation of brain electrical activity (as recorded in brain death).1618 The death criteria applied for organ procurement must also comply with the irreversibility requirement of the UDDA.11, 12
The true incidence, temporal characteristics, and predictors of autoresuscitation in humans remain unknown because of underreporting in the literature. However, there have been case reports of autoresuscitation with return of neurologic function (also called the Lazarus phenomenon) after 10 minutes of cardiac asystole.19, 20 Maleck et al. and Adhiyaman et al. described autoresuscitation 5 minutes or longer after cardiorespiratory arrest in 44% and 50% of the published case reports, respectively.19, 20 Although cardiac asystole leads to the loss of arterial pulse pressure, circulatory arterial mean pressure is maintained in diastole by arteriolar vasomotor tone. The relaxation (diastole) phase systemic arterial to venous pressure gradient provides the perfusion pressure for vital organs and the spontaneous return of circulation after circulatory arrest.21 It is likely that autoresuscitation occurs because of the persistence of circulatory vasomotor tone after cessation of cardiac function. The time course of systemic vascular tone after circulatory arrest has not been well characterized in humans. However, the IOM criteria did not account for the incidence of delayed autoresuscitation in humans even though the Maastricht protocol (developed by the University of Zurich, Zurich, Switzerland) acknowledged this phenomenon and required at least 10 minutes to elapse after cardiorespiratory arrest before starting organ procurement.22 The 10‐minute waiting time did not compromise the quality of the organs procured.
The cardiorespiratory death criteria for organ procurement also ignore cardiac electrical activity (such as pulseless electrical activity or ventricular fibrillation) on an electrocardiogram. Research with cardiac ultrasonography and indwelling arterial catheters confirms that pulseless cardiac electrical activity can be associated with cardiac mechanical contractions, although these contractions are too weak to be detected by blood pressure monitoring.23 The presence of cardiac electrical activity on an electrocardiogram can also increase the likelihood that delayed autoresuscitation will occur.19, 20 Furthermore, whether there is brain electrical activity or neurologic function when cardiac electrical activity is still observed on an electrocardiogram remains unknown.24 It can be argued that donors who have already suffered severe neurologic injury cannot have meaningful neurologic function at the time of cardiorespiratory death. However, the presence of brain activity becomes relevant for organ donors with intact neurologic and brain function prior to cardiorespiratory arrest when only cardiorespiratory criteria for organ procurement are being used.4, 8, 9
In situ circulatory support with extracorporeal perfusion in organ donors has also refuted that cardiorespiratory arrest for 5 minutes fulfills the UDDA requirement because reversibility can occur during the procurement process. In situ extracorporeal perfusion is initiated 5 minutes after cardiorespiratory arrest of donors in order to preserve organs for procurement.25 Coronary and cerebral reperfusion can lead to the return of cardiac and neurologic functions (also called reanimation) of donors during the procurement process. Mechanical occlusion of the aortic arch and pharmacological agents are required to suppress donor reanimation during organ procurement.26 Martin et al. documented that in situ extracorporeal perfusion returned full neurologic and cardiac function 25 minute after cardiorespiratory arrest that occurred outside the hospital.27 Similar observations of full neurologic recovery and survival to hospital discharge were reported after in situ extracorporeal perfusion for in‐hospital cardiorespiratory arrest.28 These observations confirm that the time required for irreversible loss of neurologic function after cessation of circulation is much longer than the 25 minutes of cardiorespiratory arrest required to begin the process of organ procurement in NHBOD.
The incidence of donor reanimation during procurement is unknown because its reporting violates the dead donor rule and can create legal concerns.11 It can be argued that reanimation of organ donors is irrelevant because it does not mean survival. However, the occurrence of reanimation invalidates the premise that the cardiorespiratory criteria for organ procurement comply with the uniform determination of death. Others have accepted the inaccuracy of these criteria for determining death for procurement of organs from deceased donors and proposed abandoning the dead donor rule in order to permit recovery of transplantable organs before death.29
In the face of the uncertainty in determining death and in response to a media and marketing campaign by organ procurement organizations (OPOs) to promote public enrollment in deceased organ donation, the transplant community renamed NHBOD cardiac death organ donation.30, 31 The use of the term cardiac death is scientifically inaccurate and perhaps misleading. This term is used to denote the cessation of circulation and cardiac pump activity. The term cardiac death can be misinterpreted as meaning the heart has irreversibly ceased at the time of procurement, thus contradicting the scientific evidence for spontaneous resumption of cardiac function and autoresuscitation.19, 20 Alternatively, the use of this term can falsely imply that neurologic activity or brain stem function has ceased irreversibly after loss of cardiac activity, when scientific evidence suggests that brain stem function can remain after cardiac arrest.32
Consent for Organ Donation
Several state laws and federal regulations have been enacted to ensure that organ donation and transplantation practice comply with the ethical and legal standards of society (Table 1). The current regulations require hospitals across the United States to provde regional OPOs with early notification of all patients whose deaths are imminent before life support has been withdrawn so that discussion of organ donation with surrogate decision makers can be initiated independently and consent obtained.3, 5, 9 The Organ Donation Breakthrough Collaborative has set a goal of each OPO accomplishing a target organ donation rate of 75% or higher at local hospitals within an assigned donation service area (DSA).1, 2, 5 The financial and administrative incentives for the OPO to achieve that target organ donation rate have introduced undisclosed conflict within the donation consent process.33 Self‐serving bias of OPOs can influence whether pertinent information necessary for surrogate decision makers to provide informed consent is disclosed.34 As an example of this bias, alternative options for care and palliation may be discussed with surrogate decision makers with less enthusiasm than are the benefits and altruistic notion of organ donation. In obtaining donation consent, OPOs often avoid disclosing details of perimortem interventions performed on donors that are required for successful procurement of transplantable organs.10, 34, 35 After receiving consent for organ donation, OPO staffs also assume the responsibility of planning donor medical care and treatment pathways essential for maintaining organ viability and of preparing for subsequent procurement.5, 36 In essence, the care of the dying patient is guided by a team whose primary interest is the preservation of organs until procurement has been accomplished.
The Uniform Anatomical Gift Act (UAGA) assigns explicit priority to the donor's expressed intent so that consent for organ donation becomes irrevocable and does not require the consent or concurrence of any person after the donor's death (Table 1).9, 37 The donor's authorization to donate, recorded on an organ donor card, the individual's driver's license, or a donor registry, becomes a legally binding advance directive. The UAGA amendment enables OPOs to procure organs without family consent and in certain instances after family refusal to donate.37
Other consent options for organ donation from deceased donors have been proposed to maximize OPO recovery of transplantable organs in the United States (Table 2).8 The IOM has considered presumed consent for organ donation as a favorable option.8 Presumed consent means the default option is consent to donation, that if an individual has not expressly rejected donation, that individual is considered to have consented to organ donation. Legislative enactment of presumed consent enables OPOs to avoid the potential for surrogates to deny consent for donation, thus increasing the pool of future organ donors. The revised UAGA replaces nondonation with the intent to donate organs as the default option. In the default option, all measures necessary to ensure the medical suitability of an organ for transplantation can not be withheld or withdrawn until the OPO has determined medical suitability of the individual as a prospective donor (Table 1). The default option overrides the expression of intent in a declaration or advance health‐care directives not to have life prolonged by withholding or withdrawing life support system unless the individual has expressed refusal of donation (Tables 1 and 2). The revised UAGA presumes that for prospective donors the desire to save lives by making an anatomical gift trumps the desire to have life support systems withheld or withdrawn. Mandated consent (or conscription) has also been proposed for recovery of cadaveric organs (Table 2).38 Under mandated consent, consent for organ donation is automatic from all deceased individuals; therefore, OPOs would not require or request consent because removal of all needed transplantable cadaveric organs would be compulsory. OPO staffs would no longer have to discuss organ procurement from potential donors with family members or other surrogates. An alternative form of donation consent is mandated choice, which requires each individual to decide in advance either to agree to organ donation or to refuse it. Mandated choice is the IOM's least favorite option because it would require extensive public informational programs on organ donation to facilitate individual choices and decision making (Table 2).8
| Type | Description |
|---|---|
| Requested (expressed) consent | An individual is asked to voluntarily agree to organ donation. |
| Presumed (implied) consent | Unless an individual has expressly refused to donate organs, the default option is agreement to donate organs. |
| Mandated consent (conscription) | An individual is not required to decide on organ donation before death, and there is an automatic right to procure organs from any and all deceased individuals. |
| Mandated choice | An individual must choose between 2 options before death: agreement or refusal to donate organs. |
End‐of‐Life Care
Quality of end‐of‐life (EOL) care for an organ donor, as for any individual whose treatment is being withdrawn, is considered the highest priority of care and must not be compromised by the donation process. Yet no studies have investigated the impact of organ donation on the quality of EOL care in NHBOD.35 Previous reports have criticized the quality of EOL care offered to dying patients in intensive care units (ICUs).39, 40 Many of these patients are undergoing withdrawal of life support in anticipation of death and are considered candidates for NHBOD. The Robert Wood Johnson Foundation (RWJF) Critical Care End‐Of‐Life Peer Workgroup developed 53 EOL quality indicators to standardize and measure the quality of EOL care.41 These quality indicators, organized in 7 domains, focus on delivering patient‐ and family‐centered care and facilitating a good death experience in the ICU (Table 3).
| Domains of comprehensive quality indicators of EOLC (n = 53)41 | Abbreviated quality indicators of EOLC (n = 18)46 |
|---|---|
| |
| Patient and family‐centered decision making (n = 13) | |
| Recognize the patient and family as the unit of care | |
| Assess the patient's and family's decision‐making style and preferences | |
| Address conflicts in decision making within the family | |
| Assess, together with appropriate clinical consultants, the patient's capacity to participate in decision making about treatment and document assessment | Assessment of the patient's decisional capacity |
| Initiate advance care planning with the patient and family | |
| Clarify and document the status of the patient's advance directive | Documentation of the presence and, if present, contents of advance directives |
| Identify the healthcare proxy or surrogate decision maker | Documentation of a surrogate decision maker within 24 hours of admission |
| Clarify and document resuscitation orders | |
| Assure patients and families that decision making by the health care team will incorporate their preferences | |
| Follow ethical and legal guidelines for patients who lack both capacity to make decisions and a surrogate decision maker | |
| Establish and document clear, realistic, and appropriate goals of care in consultation with the patient and family | Documentation of the goals of care |
| Help the patient and family assess the benefits and burdens of alternative treatment choices as the patient's condition changes | |
| Forgo life‐sustaining treatments in a way that ensures patient and family preferences are elicited and respected | |
| Communication within the team and with patients and families (n = 10) | |
| Meet as interdisciplinary team to discuss the patient's condition, clarify goals of treatment, and identify the patient's and family's needs and preferences | Documentation of a timely interdisciplinary clinicianfamily conference |
| Address conflicts among the clinical team before meeting with the patient and/or family | |
| Utilize expert clinical, ethical, and spiritual consultants when appropriate | |
| Recognize the adaptations in communication strategy required for patients and families according to the chronic versus acute nature of the illness, cultural and spiritual differences, and other influences | |
| Meet with the patient and/or family on a regular basis to review patient's status and to answer questions | Documentation of timely physician communication with the family |
| Communicate all information to the patient and family, including distressing news, in a clear, sensitive, unhurried manner and in an appropriate setting | |
| Clarify the patient's and family's understanding of the patient's condition and goals of care at the beginning and end of each meeting | |
| Designate primary clinical liaison(s) who will communicate with the family daily | |
| Identify a family member who will serve as the contact person for the family | |
| Prepare the patient and family for the dying process | |
| Continuity of care (n = 3) | |
| Maximize continuity of care across clinicians, consultants, and settings | |
| Orient new clinicians to the status of the patient and family | Transmission of key information with transfer of the patient out of the ICU |
| Policy for continuity of nursing services | |
| Prepare the patient and/or family for a change of clinician(s) and introduce new clinicians | |
| Emotional and practical support for patients and families (n = 8) | |
| Elicit and attend to the needs of the dying person and his/her family | |
| Distribute written material (booklet) for families that includes orientation to the ICU environment and open visitation guidelines, logistical information (nearby hotels, banks, restaurants, directions), listings of financial consultation services, and bereavement programs and resources | Open visitation policy for family members |
| Facilitate strengthening of patientfamily relationships and communication | |
| Maximize privacy of the patient and family | |
| Value and support the patient's and family's cultural traditions | |
| Arrange for social support for patients who do not have family or friends | Documentation that psychosocial support has been offered |
| Distribute written material (booklet) containing essential logistical information and listings of financial consultation services and bereavement support programs/resources | |
| Support family members through the patient's death and their bereavement | |
| Symptom management and comfort care (n = 10) | |
| Emphasize the comprehensive comfort care that will be provided to the patient rather than the removal of life‐sustaining treatments | |
| Institute and use uniform quantitative symptom assessment scales appropriate for communicative and noncommunicative patients on a routine basis | Documentation of pain assessment |
| Documentation of respiratory distress assessment | |
| Standardize and follow best clinical practices for symptom management | Protocol for analgesia/sedation in terminal withdrawal of mechanical ventilation |
| Appropriate medications available during withdrawal of mechanical ventilation | |
| Use nonpharmacologic as well as pharmacologic measures to maximize comfort as appropriate and desired by the patient and family | |
| Reassess and document symptoms following interventions | Documentation of pain management |
| Documentation of respiratory distress management | |
| Know and follow best clinical practices for withdrawing life‐sustaining treatments to avoid patient and family distress | |
| Eliminate unnecessary tests and procedures (laboratory work, weights, routine vital signs) and only maintain intravenous catheters for symptom management when life support is being withdrawn | |
| Minimize noxious stimuli (monitors, strong lights) | |
| Attend to patient's appearance and hygiene | |
| Ensure family's and/or clinician's presence so the patient is not dying alone | |
| Spiritual support for patients and families (n = 3) | |
| Assess and document spiritual needs of the patient and family on an ongoing basis | Documentation that spiritual support was offered |
| Encourage access to spiritual resources | |
| Elicit and facilitate spiritual and cultural practices that the patient and family find comforting | |
| Emotional and organizational support for ICU clinicians (n = 6) | |
| Support health care team colleagues caring for dying patients | Opportunity to review experience of caring for dying patients by ICU clinicians |
| Adjust nursing staff and medical rotation schedules to maximize continuity of care providers for dying patients | |
| Communicate regularly with interdisciplinary team about goals of care | |
| Establish a staff support group, based on the input and needs of ICU staff and experienced group facilitators, and integrate meeting times into the routine of the ICU | |
| Enlist palliative care experts, pastoral care representatives, and other consultants to teach and model aspects of EOLC | |
| Facilitate rituals for the staff to mark the death of patients | |
| Strategies to improve EOLC and provide a good death42, 43 | |
| EOL care quality monitoring | |
| Making environmental changes to promote dying with dignity | |
| Managing patients' pain and discomfort | |
| Knowing and following patients' wishes for end‐of‐life care | |
| Bereavement program or service | |
| Regular meetings of senior ICU physician and nurse with patients' families | |
| Training of ICU clinicians in end‐of‐life communication skills | |
| Role modeling and supervision of trainees by clinicians experienced in end‐of‐life care | |
| Formal mechanism for emotional support of staff caring for dying patients | |
| Access to palliative care consultants | |
| Training of ICU clinicians in symptom management | |
| Scheduling staff to promote continuity of care for dying patients | |
| Formal system for scaled assessment and charting of patients' symptoms | |
| Method to help resolve differences about appropriate care goals | |
| Resources to accommodate diversity among patients/families at the end of life | |
| Access to clinical ethics consultants | |
| Regular pastoral care visits to the ICU | |
In a subsequent U.S. survey, the Critical Care Peer Workgroup of the Promoting Excellence in End‐of‐Life Care Project reported that more than 75% of ICUs were still not monitoring the quality of EOL care.42 The survey also identified multiple barriers to optimal EOL care found in most ICUs. The study group proposed several strategies to overcome these barriers and improve the quality of EOL care (Table 3).42, 43 It can also be inferred from the survey findings that most ICUs are unprepared and lack the necessary tools to appropriately inform patients and families of the trade‐off in EOL care for NHBOD. The President's Council for Bioethics has also warned that NHBOD can transform EOL care from a peaceful dignified death into a profanely high‐tech death experience for donors' families.10
Several aspects of medical care that are neither palliative nor beneficial are performed for donor management for NHBOD and can explain the feared transformation of the death experience. The revised UAGA reaffirms that all measures necessary to ensure the medical suitability of an organ for transplantation cannot be withheld or withdrawn from the prospective donor and overrides the expression of intent by a prospective donor in either a declaration or advance health‐care directive not to have life prolonged by use of life support systems (Table 1).9 The 2007 amendment to revised UAGA section 21 recognizes the conflict between all measures necessary to ensure organs viability for transplantation and appropriate EOL care and requires the attending physician and OPO to resolve the conflict with the prospective donor or surrogate decision‐maker.9
OPOs apply donor management critical pathways to potential organ donors in order to maintain organ viability for successful execution of organ procurement.36 The University of Wisconsin developed a protocol and an evaluation tool to determine the eligibility of potential candidates for NHBOD.44 The protocol entails temporary discontinuation of mechanical ventilation for a trial of spontaneous respiration lasting up to 10 minutes to determine the likelihood of cardiorespiratory death within 60‐90 minutes of the withdrawal of life support. Those patients predicted by the University of Wisconsin evaluation tool to survive a longer time are not candidates for NHBOD and are transferred to palliative care. Those patients who meet the necessary criteria of the University of Wisconsin evaluation tool become candidates for NHBOD and undergo additional antemortem testing, invasive vascular instrumentation, and infusion of medications essential for organ preservation.36 The instrumentation and medications used for organ preservation can also expedite death on withdrawal of life support.45 Other interventions (such as circulatory support with invasive and noninvasive devices, extracorporeal perfusion and oxygenation, endotracheal reintubation, mechanical ventilation, and bronchoscopy) are performed when cardiorespiratory death is pronounced in order to maintain organ viability and can inadvertently reanimate the donor during the procurement process.26
The process of obtaining donation consent and subsequent donor management protocols for NHBOD deviate from more than 60% of the RWJF quality indicators recommended for optimal EOL care.35, 36, 41 Therefore, NHBOD can have a profound impact on the quality of EOL care. There has been a recent proposal to abbreviate the original RWJF quality indicators to include 14 of the 53 (26%) original quality indicators described for optimal EOL care in the ICU (Table 3).46 Many of the quality indicators expected to be negatively affected by NHBOD are not included in the proposal for an abbreviated list. There has been a concern that the application of an abbreviated rather than a comprehensive metrics for EOL care can portray an incomplete assessment and perhaps misinform donors and their families about the potential trade‐off in EOL care. The President's Council on Bioethics has emphasized that comprehensive evaluation of the quality of EOL care is an ethical imperative so that families can decide if the trade‐off is acceptable for organ donation.10 Deciding to donate organs at the end of life can be stressful for many families, and therefore they must be fully informed of the possible consequences. Posttraumatic stress disorders, anxiety, depression, and decreased quality of life have been reported in the deceased's family members who shared in stressful EOL decisions.47 Posttraumatic stress disorders have been reported in family members of deceased organ donors.48 Organ‐focused behavior by professionals requesting consent for organ donation and ambivalent decision making by family members appeared to increase the risk of relatives of deceased donors subsequently developing traumatic memories and stress disorders. The processes required for successful accomplishment of donation consent and subsequent organ recovery can interfere with many of the interventions that lessen the burden of bereavement of relatives of ICU decedents.49
The variability in decision making by health care providers about medical futility and EOL care has been given as a reason for concern about the implementation of NHBOD.50 The variability of EOL practice raises the possibility of conflicted decision making on medical futility within institutions that have transplant programs.50 Ethical conflicts and moral distress have been reported among health care providers who were directly involved in organ procurement in NHBOD.51 The pressure to recover transplantable organs from NHBOD candidates has been associated with health care professionals' perception of euthanasia and premature determination of medical futility and withdrawal of life support. The long‐term psychological impact of NHBOD practice on caregivers, health care providers, and professionals remains unknown.
CONCLUSIONS
In conclusion, NHBOD influences medical care at critical time points to maximize the procurement of transplantable organs and minimize their warm ischemia time with negative consequences on the EOL care for the prospective donors and their families (see Figure 1).
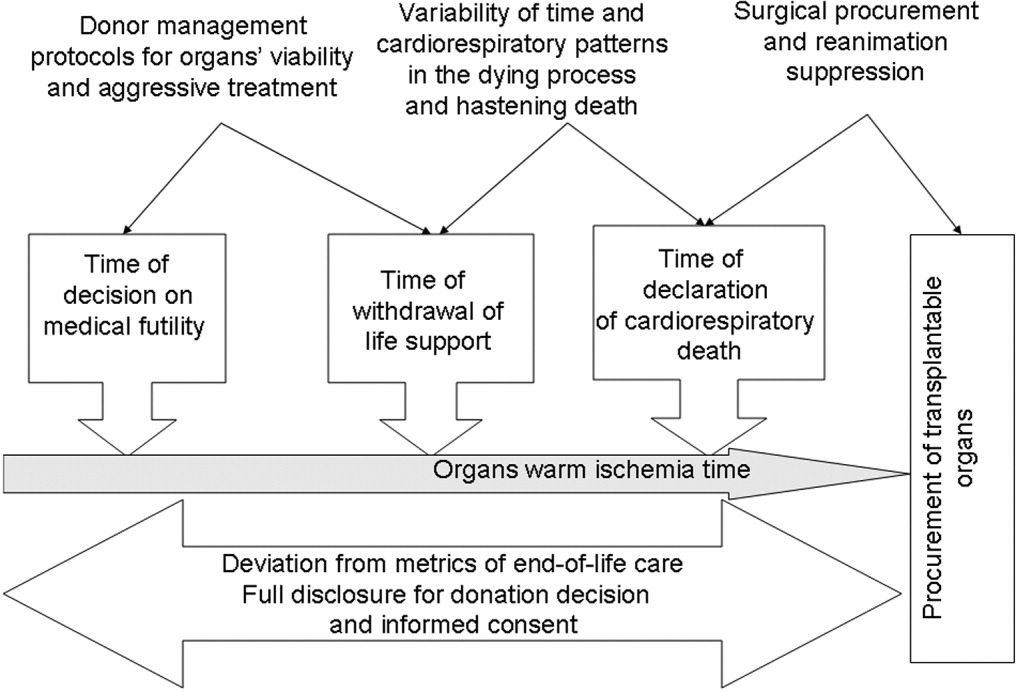
Mandatory implementation of NHBOD in the face of difficulties surrounding the quality of EOL care for donors raises concern across the medical profession and community. There is a need for better scientific validation of the timing of organ procurement to ensure that organ recovery is not the irreversible event defining death in NHBOD. The desire of OPOs or their affiliates to maximize recovery of transplantable organs introduces self‐serving bias into obtaining consent for organ donation and violates the basic tenet of true informed consent. The use of comprehensive quality indicators of EOL care will help to determine the impact of NHBOD on donors, families, caregivers, and health care providers.
- ,,, et al.Organ donation and utilization, 1995‐2004: Entering the collaborative era.Am J Transplant.2006;6:1101–1110.
- U.S. Department of Health and Human Servcies—Organ Donation and Transplant Breakthrough Collaborative. Measurement strategy: Organ Donation and Transplant Breakthrough Collaborative. Available at: http://www.organdonationnow.org/. Accessed December 15,2006.
- U.S. Department of Health and Human Services Advisory Committee on Organ Transplantation. Consensus Recommendations to the HHS Secretary. Available at: http://www.organdonor.gov/research/acot.htm. Accessed January 30,2007.
- ,,, et al.Report of a National Conference on Donation after Cardiac Death.Am J Transplant.2006;6:281–291.
- Centers for Medicare and Medicaid Services, Department of Health and Human Services.Medicare and Medicaid Programs. Conditions for coverage for organ procurement organizations (OPOs); final rule.Federal Register.2006;71:30981–31054.
- [JCAHO] Joint Commission on Accreditation of Healthcare Organizations. Health care at the crossroads: strategies for narrowing the organ donation gap and protecting patients. Available at: http://www.jointcommission.org/PublicPolicy/organ_donation.htm. Accessed January 30,2007.
- [JCAHO] Joint Commission on Accreditation of Healthcare Organizations.Revisions to Standard LD.3.110.Jt Comm Perspect.2006;26:7.
- Committee on Increasing Rates of Organ Donation‐Board on Health Sciences Policy‐Institute of Medicine.Organ Donation: Opportunities for Action.Washington, DC:National Academies Press;2006.
- National Conference of Commissioners on Uniform State Laws. Revised Uniform Anatomical Gift Act (2006) and Amendment to Section 21 (2007). http://www.law.upenn.edu/bll/ulc/uaga/2006final.htm and http://www.anatomicalgiftact.org/DesktopDefault.aspx?tabindex=0
In April 2003 the Health Resources and Services Administration of the U.S. Department of Health and Human Services (DHHS) announced the formation of the Organ Donation Breakthrough Collaborative (ODBC).1 The Organ Donation Breakthrough Collaborative created 58 national donation service areas (DSAs) to organize the transplant community across the United States. Each of the 58 organ procurement organizations (OPOs) is joined to a regional transplant center or centers and donor hospitals to form a DSA. The ODBC's goal is to achieve a cadaveric organ donation rate of 75% or higher from hospitals within each DSA.2
A requirement for organ donation from patients facing imminent or cardiac death has been introduced to increase the supply of transplantable organs and shorten the waiting time for transplantation candidates.35 This type of organ donation represents a significant source of organs required for future expansion of transplantation practice in the United States. The requirement for donation in imminent or cardiac death is implemented through the collaboration of the Advisory Committee on Organ Transplantation of the DHHS (Table 1), the Centers for Medicare & Medicaid Services (CMS), and the Joint Commission on Accreditation of Healthcare Organizations (JCAHO).3, 57 The organ donor pool of those facing imminent or cardiac death has also been expanded to include neurologically intact patients who may not fulfill brain death criteria before withdrawal of life support.4, 8, 9
| State law (year)* | |
|---|---|
| |
| Uniform Anatomical Gift Act (1968) | Any 18‐year‐old with a sound mind may donate his or her body after death to be used for medical research or as a source of transplantable tissues and organs and barring others from overriding a donor's decision to make an anatomical gift. |
| Amendment (1987) | Minors who can apply for a driver's license are empowered to make anatomical gifts, but either parent can revoke the gift if the minor dies before the age of 18. |
| Revision (2006) | Declaration of a gift does not require any witnesses. |
| Amendment (2007) | Document of a gift or donor registry is sufficient for the removal of organs, which means an OPO does not need consent of the spouse or the family. |
| Enables an OPO to gain access to documents of gifts in donor registries, medical records, and records of a state motor vehicle department. | |
| Facilitates donations by expanding the list of those who may make an anatomical gift for another individual who has not declared a preference for or against donation. | |
| Permits removal of organs by medical personnel without explicit consent from a potential donor or from a relative of the donor, so long as the appropriate medical personnel or authorities have made a reasonable effort to discover any objection by the donor or the donor's family. | |
| Require hospitals to notify an OPO or third party designated by the OPO of an individual whose death is imminent or who has died in the hospital in order to increase donation opportunity. | |
| Gives an OPO the right to inspect a patient's medical records. | |
| Measures necessary to ensure the medical suitability of a part not be withdrawn while an examination is being made to determine whether an individual who has been referred to OPO has a part that could be the subject of an anatomical gift. If, following such an examination, it is determined by the OPO that the individual has a part that could be the subject of an anatomical gift, the individual is a prospective donor under this act unless the individual had signed a refusal. | |
| Forbids the buying and selling of organs. | |
| Measures necessary to ensure the medical suitability of an organ for transplantation or therapy may not be withheld or withdrawn from a prospective donor who has an advance health‐care directive or declaration unless the directive or declaration expressly provides to the contrary. The section presumes that for prospective donors the desire to save lives by making an anatomical gift trumps the desire to have life support systems withheld or withdrawn. Individuals who desire to overcome this presumption can do so by express language in their advance health‐care directive or declaration. | |
| Uniform Determination of Death Act (1981) | An individual who has sustained either (1) irreversible cessation of circulatory and respiratory functions, or (2) irreversible cessation of all functions of the entire brain including the brain stem is dead. A determination of death must be made in accordance with accepted medical standards. |
| Federal laws | |
| National Organ Transplant Act (1984) | Calls for a unified transplant network to be operated by a private, nonprofit organization under federal contract and the establishment of a Task Force in Organ Procurement and Transplantation and an Organ Procurement and Transplantation Registry. |
| Public Health and Welfare Act Title 42 USC (1999) | |
| Section 273 | Establishes guidelines to be a qualified OPO that can receive federal grants. |
| Section 274 | Establishes the OPTN & Scientific Registry for Transplantation. |
| Federal regulations | |
| Title Code 42 CFR Part 121 (1999) | Lists regulations of the OPTN final rule. |
| Explains the OPTN structure. | |
| Lists the policy that the OPTN board of directors is responsible for developing. | |
| Explains rules that an OPTN member must obey when including a person on the organ waiting list. | |
| Describes the requirements and tests for determining the suitability of donated organs. | |
| Explains how the OPTN identifies an organ recipient, allocates the organ, and transports it to the recipient. | |
| Describes how the board of directors should develop allocation policies to guarantee they are both efficient and just and allocation performance goals to ensure the best possible use and most equitable allocation of organs. | |
| Lists designated transplant program requirements. | |
| Describes how the HHS conducts reviews and evaluations and enforces rules. | |
| Describes the recording and reporting requirements of the various groups involved in the transplantation process. | |
| Establishes the Advisory Committee on Organ Transplantation (ACOT), which advises the HHS secretary on organ donation, procurement, allocation, and transplantation The HHS secretary may ask for ACOT's opinion of proposed OPTN policies. | |
| Title Code 42 CFR Parts 413, 441, 486 and 498 (2006) | Lists regulations of the OPO final rule. |
| Establishes new conditions for coverage for OPOs that include multiple new outcome and process performance measures based on organ donor potential and other related factors in each donation service area of qualified OPOs. | |
The President's Council on Bioethics independently evaluated the issues surrounding deceased organ donation and procurement.10 The President's Council on Bioethics has expressed major concerns about several issues pertinent to cardiac or imminent death organ donation that have not been addressed explicitly by the bodies that have made recommendations for reforming or expanding that type of organ donation in the United States. The debate on organ procurement in imminent or cardiac death has come to the forefront because of doubts about its ethical appropriateness and acceptance within the medical profession and the community at large. This review focuses on the serious issues related to organ procurement from patients facing imminent or cardiac death.
Organ Procurement and the Dead Donor Rule
Organ procurement is only permitted when the donor is already dead (ie, the dead donor rule), and the act of organ recovery cannot have been the immediate act to cause that death.10, 11 There are 2 criteria for death as defined in the Uniform Determination of Death Act (UDDA; Table 1): an individual who has sustained irreversible cessation of either (1) circulatory and respiratory functions or (2) all functions of the entire brain, including the brain stem, is considered dead and this determination of death must have been made in accordance with accepted medical standards.12 When organs are procured from an individual in whom all brain function has ceased but normal cardiac pump activity is continuing, it is referred to as heart‐beating organ donation. Organ procurement after cessation of cardiac pump activity and cardiorespiratory functions is referred to as non‐heart‐beating organ donation (NHBOD). Organ procurement from an individual in imminent or cardiac death is considered NHBOD.
Non‐heart‐beating organ donors can be neurologically intact and do not fulfill the brain death criterion prior to cessation of cardiac pump activity. In response to this dilemma, the University of Pittsburgh Medical Centre developed a protocol for donation of organs that permitted their procurement from patients who were pulseless and apneic for 2 minutes and did not fulfill brain criteria and who had previously given consent for organ donation.13 Because it is uncertain if cessation of cardiorespiratory function is irreversible after only a short time, the Institute of Medicine (IOM) extended the time required for pulselessness and apnea from 2 to 5 minutes before permitting organ procurement.14 Waiting longer than 5 minutes for the determination of death would compromise the quality of procured organs because of warm ischemia time and would influence the functioning of grafts in transplant recipients.
One of the pivotal assumptions for NHBOD acceptance is that 5 minutes of pulselessness and apnea eliminates the possibility that the procurement process itself could be the cause of death and fulfills the dead donor rule.14, 15 The cardiorespiratory death criteria were derived from observations that did not evaluate delayed autoresuscitation (spontaneous return of circulation) or simultaneous cessation of brain electrical activity (as recorded in brain death).1618 The death criteria applied for organ procurement must also comply with the irreversibility requirement of the UDDA.11, 12
The true incidence, temporal characteristics, and predictors of autoresuscitation in humans remain unknown because of underreporting in the literature. However, there have been case reports of autoresuscitation with return of neurologic function (also called the Lazarus phenomenon) after 10 minutes of cardiac asystole.19, 20 Maleck et al. and Adhiyaman et al. described autoresuscitation 5 minutes or longer after cardiorespiratory arrest in 44% and 50% of the published case reports, respectively.19, 20 Although cardiac asystole leads to the loss of arterial pulse pressure, circulatory arterial mean pressure is maintained in diastole by arteriolar vasomotor tone. The relaxation (diastole) phase systemic arterial to venous pressure gradient provides the perfusion pressure for vital organs and the spontaneous return of circulation after circulatory arrest.21 It is likely that autoresuscitation occurs because of the persistence of circulatory vasomotor tone after cessation of cardiac function. The time course of systemic vascular tone after circulatory arrest has not been well characterized in humans. However, the IOM criteria did not account for the incidence of delayed autoresuscitation in humans even though the Maastricht protocol (developed by the University of Zurich, Zurich, Switzerland) acknowledged this phenomenon and required at least 10 minutes to elapse after cardiorespiratory arrest before starting organ procurement.22 The 10‐minute waiting time did not compromise the quality of the organs procured.
The cardiorespiratory death criteria for organ procurement also ignore cardiac electrical activity (such as pulseless electrical activity or ventricular fibrillation) on an electrocardiogram. Research with cardiac ultrasonography and indwelling arterial catheters confirms that pulseless cardiac electrical activity can be associated with cardiac mechanical contractions, although these contractions are too weak to be detected by blood pressure monitoring.23 The presence of cardiac electrical activity on an electrocardiogram can also increase the likelihood that delayed autoresuscitation will occur.19, 20 Furthermore, whether there is brain electrical activity or neurologic function when cardiac electrical activity is still observed on an electrocardiogram remains unknown.24 It can be argued that donors who have already suffered severe neurologic injury cannot have meaningful neurologic function at the time of cardiorespiratory death. However, the presence of brain activity becomes relevant for organ donors with intact neurologic and brain function prior to cardiorespiratory arrest when only cardiorespiratory criteria for organ procurement are being used.4, 8, 9
In situ circulatory support with extracorporeal perfusion in organ donors has also refuted that cardiorespiratory arrest for 5 minutes fulfills the UDDA requirement because reversibility can occur during the procurement process. In situ extracorporeal perfusion is initiated 5 minutes after cardiorespiratory arrest of donors in order to preserve organs for procurement.25 Coronary and cerebral reperfusion can lead to the return of cardiac and neurologic functions (also called reanimation) of donors during the procurement process. Mechanical occlusion of the aortic arch and pharmacological agents are required to suppress donor reanimation during organ procurement.26 Martin et al. documented that in situ extracorporeal perfusion returned full neurologic and cardiac function 25 minute after cardiorespiratory arrest that occurred outside the hospital.27 Similar observations of full neurologic recovery and survival to hospital discharge were reported after in situ extracorporeal perfusion for in‐hospital cardiorespiratory arrest.28 These observations confirm that the time required for irreversible loss of neurologic function after cessation of circulation is much longer than the 25 minutes of cardiorespiratory arrest required to begin the process of organ procurement in NHBOD.
The incidence of donor reanimation during procurement is unknown because its reporting violates the dead donor rule and can create legal concerns.11 It can be argued that reanimation of organ donors is irrelevant because it does not mean survival. However, the occurrence of reanimation invalidates the premise that the cardiorespiratory criteria for organ procurement comply with the uniform determination of death. Others have accepted the inaccuracy of these criteria for determining death for procurement of organs from deceased donors and proposed abandoning the dead donor rule in order to permit recovery of transplantable organs before death.29
In the face of the uncertainty in determining death and in response to a media and marketing campaign by organ procurement organizations (OPOs) to promote public enrollment in deceased organ donation, the transplant community renamed NHBOD cardiac death organ donation.30, 31 The use of the term cardiac death is scientifically inaccurate and perhaps misleading. This term is used to denote the cessation of circulation and cardiac pump activity. The term cardiac death can be misinterpreted as meaning the heart has irreversibly ceased at the time of procurement, thus contradicting the scientific evidence for spontaneous resumption of cardiac function and autoresuscitation.19, 20 Alternatively, the use of this term can falsely imply that neurologic activity or brain stem function has ceased irreversibly after loss of cardiac activity, when scientific evidence suggests that brain stem function can remain after cardiac arrest.32
Consent for Organ Donation
Several state laws and federal regulations have been enacted to ensure that organ donation and transplantation practice comply with the ethical and legal standards of society (Table 1). The current regulations require hospitals across the United States to provde regional OPOs with early notification of all patients whose deaths are imminent before life support has been withdrawn so that discussion of organ donation with surrogate decision makers can be initiated independently and consent obtained.3, 5, 9 The Organ Donation Breakthrough Collaborative has set a goal of each OPO accomplishing a target organ donation rate of 75% or higher at local hospitals within an assigned donation service area (DSA).1, 2, 5 The financial and administrative incentives for the OPO to achieve that target organ donation rate have introduced undisclosed conflict within the donation consent process.33 Self‐serving bias of OPOs can influence whether pertinent information necessary for surrogate decision makers to provide informed consent is disclosed.34 As an example of this bias, alternative options for care and palliation may be discussed with surrogate decision makers with less enthusiasm than are the benefits and altruistic notion of organ donation. In obtaining donation consent, OPOs often avoid disclosing details of perimortem interventions performed on donors that are required for successful procurement of transplantable organs.10, 34, 35 After receiving consent for organ donation, OPO staffs also assume the responsibility of planning donor medical care and treatment pathways essential for maintaining organ viability and of preparing for subsequent procurement.5, 36 In essence, the care of the dying patient is guided by a team whose primary interest is the preservation of organs until procurement has been accomplished.
The Uniform Anatomical Gift Act (UAGA) assigns explicit priority to the donor's expressed intent so that consent for organ donation becomes irrevocable and does not require the consent or concurrence of any person after the donor's death (Table 1).9, 37 The donor's authorization to donate, recorded on an organ donor card, the individual's driver's license, or a donor registry, becomes a legally binding advance directive. The UAGA amendment enables OPOs to procure organs without family consent and in certain instances after family refusal to donate.37
Other consent options for organ donation from deceased donors have been proposed to maximize OPO recovery of transplantable organs in the United States (Table 2).8 The IOM has considered presumed consent for organ donation as a favorable option.8 Presumed consent means the default option is consent to donation, that if an individual has not expressly rejected donation, that individual is considered to have consented to organ donation. Legislative enactment of presumed consent enables OPOs to avoid the potential for surrogates to deny consent for donation, thus increasing the pool of future organ donors. The revised UAGA replaces nondonation with the intent to donate organs as the default option. In the default option, all measures necessary to ensure the medical suitability of an organ for transplantation can not be withheld or withdrawn until the OPO has determined medical suitability of the individual as a prospective donor (Table 1). The default option overrides the expression of intent in a declaration or advance health‐care directives not to have life prolonged by withholding or withdrawing life support system unless the individual has expressed refusal of donation (Tables 1 and 2). The revised UAGA presumes that for prospective donors the desire to save lives by making an anatomical gift trumps the desire to have life support systems withheld or withdrawn. Mandated consent (or conscription) has also been proposed for recovery of cadaveric organs (Table 2).38 Under mandated consent, consent for organ donation is automatic from all deceased individuals; therefore, OPOs would not require or request consent because removal of all needed transplantable cadaveric organs would be compulsory. OPO staffs would no longer have to discuss organ procurement from potential donors with family members or other surrogates. An alternative form of donation consent is mandated choice, which requires each individual to decide in advance either to agree to organ donation or to refuse it. Mandated choice is the IOM's least favorite option because it would require extensive public informational programs on organ donation to facilitate individual choices and decision making (Table 2).8
| Type | Description |
|---|---|
| Requested (expressed) consent | An individual is asked to voluntarily agree to organ donation. |
| Presumed (implied) consent | Unless an individual has expressly refused to donate organs, the default option is agreement to donate organs. |
| Mandated consent (conscription) | An individual is not required to decide on organ donation before death, and there is an automatic right to procure organs from any and all deceased individuals. |
| Mandated choice | An individual must choose between 2 options before death: agreement or refusal to donate organs. |
End‐of‐Life Care
Quality of end‐of‐life (EOL) care for an organ donor, as for any individual whose treatment is being withdrawn, is considered the highest priority of care and must not be compromised by the donation process. Yet no studies have investigated the impact of organ donation on the quality of EOL care in NHBOD.35 Previous reports have criticized the quality of EOL care offered to dying patients in intensive care units (ICUs).39, 40 Many of these patients are undergoing withdrawal of life support in anticipation of death and are considered candidates for NHBOD. The Robert Wood Johnson Foundation (RWJF) Critical Care End‐Of‐Life Peer Workgroup developed 53 EOL quality indicators to standardize and measure the quality of EOL care.41 These quality indicators, organized in 7 domains, focus on delivering patient‐ and family‐centered care and facilitating a good death experience in the ICU (Table 3).
| Domains of comprehensive quality indicators of EOLC (n = 53)41 | Abbreviated quality indicators of EOLC (n = 18)46 |
|---|---|
| |
| Patient and family‐centered decision making (n = 13) | |
| Recognize the patient and family as the unit of care | |
| Assess the patient's and family's decision‐making style and preferences | |
| Address conflicts in decision making within the family | |
| Assess, together with appropriate clinical consultants, the patient's capacity to participate in decision making about treatment and document assessment | Assessment of the patient's decisional capacity |
| Initiate advance care planning with the patient and family | |
| Clarify and document the status of the patient's advance directive | Documentation of the presence and, if present, contents of advance directives |
| Identify the healthcare proxy or surrogate decision maker | Documentation of a surrogate decision maker within 24 hours of admission |
| Clarify and document resuscitation orders | |
| Assure patients and families that decision making by the health care team will incorporate their preferences | |
| Follow ethical and legal guidelines for patients who lack both capacity to make decisions and a surrogate decision maker | |
| Establish and document clear, realistic, and appropriate goals of care in consultation with the patient and family | Documentation of the goals of care |
| Help the patient and family assess the benefits and burdens of alternative treatment choices as the patient's condition changes | |
| Forgo life‐sustaining treatments in a way that ensures patient and family preferences are elicited and respected | |
| Communication within the team and with patients and families (n = 10) | |
| Meet as interdisciplinary team to discuss the patient's condition, clarify goals of treatment, and identify the patient's and family's needs and preferences | Documentation of a timely interdisciplinary clinicianfamily conference |
| Address conflicts among the clinical team before meeting with the patient and/or family | |
| Utilize expert clinical, ethical, and spiritual consultants when appropriate | |
| Recognize the adaptations in communication strategy required for patients and families according to the chronic versus acute nature of the illness, cultural and spiritual differences, and other influences | |
| Meet with the patient and/or family on a regular basis to review patient's status and to answer questions | Documentation of timely physician communication with the family |
| Communicate all information to the patient and family, including distressing news, in a clear, sensitive, unhurried manner and in an appropriate setting | |
| Clarify the patient's and family's understanding of the patient's condition and goals of care at the beginning and end of each meeting | |
| Designate primary clinical liaison(s) who will communicate with the family daily | |
| Identify a family member who will serve as the contact person for the family | |
| Prepare the patient and family for the dying process | |
| Continuity of care (n = 3) | |
| Maximize continuity of care across clinicians, consultants, and settings | |
| Orient new clinicians to the status of the patient and family | Transmission of key information with transfer of the patient out of the ICU |
| Policy for continuity of nursing services | |
| Prepare the patient and/or family for a change of clinician(s) and introduce new clinicians | |
| Emotional and practical support for patients and families (n = 8) | |
| Elicit and attend to the needs of the dying person and his/her family | |
| Distribute written material (booklet) for families that includes orientation to the ICU environment and open visitation guidelines, logistical information (nearby hotels, banks, restaurants, directions), listings of financial consultation services, and bereavement programs and resources | Open visitation policy for family members |
| Facilitate strengthening of patientfamily relationships and communication | |
| Maximize privacy of the patient and family | |
| Value and support the patient's and family's cultural traditions | |
| Arrange for social support for patients who do not have family or friends | Documentation that psychosocial support has been offered |
| Distribute written material (booklet) containing essential logistical information and listings of financial consultation services and bereavement support programs/resources | |
| Support family members through the patient's death and their bereavement | |
| Symptom management and comfort care (n = 10) | |
| Emphasize the comprehensive comfort care that will be provided to the patient rather than the removal of life‐sustaining treatments | |
| Institute and use uniform quantitative symptom assessment scales appropriate for communicative and noncommunicative patients on a routine basis | Documentation of pain assessment |
| Documentation of respiratory distress assessment | |
| Standardize and follow best clinical practices for symptom management | Protocol for analgesia/sedation in terminal withdrawal of mechanical ventilation |
| Appropriate medications available during withdrawal of mechanical ventilation | |
| Use nonpharmacologic as well as pharmacologic measures to maximize comfort as appropriate and desired by the patient and family | |
| Reassess and document symptoms following interventions | Documentation of pain management |
| Documentation of respiratory distress management | |
| Know and follow best clinical practices for withdrawing life‐sustaining treatments to avoid patient and family distress | |
| Eliminate unnecessary tests and procedures (laboratory work, weights, routine vital signs) and only maintain intravenous catheters for symptom management when life support is being withdrawn | |
| Minimize noxious stimuli (monitors, strong lights) | |
| Attend to patient's appearance and hygiene | |
| Ensure family's and/or clinician's presence so the patient is not dying alone | |
| Spiritual support for patients and families (n = 3) | |
| Assess and document spiritual needs of the patient and family on an ongoing basis | Documentation that spiritual support was offered |
| Encourage access to spiritual resources | |
| Elicit and facilitate spiritual and cultural practices that the patient and family find comforting | |
| Emotional and organizational support for ICU clinicians (n = 6) | |
| Support health care team colleagues caring for dying patients | Opportunity to review experience of caring for dying patients by ICU clinicians |
| Adjust nursing staff and medical rotation schedules to maximize continuity of care providers for dying patients | |
| Communicate regularly with interdisciplinary team about goals of care | |
| Establish a staff support group, based on the input and needs of ICU staff and experienced group facilitators, and integrate meeting times into the routine of the ICU | |
| Enlist palliative care experts, pastoral care representatives, and other consultants to teach and model aspects of EOLC | |
| Facilitate rituals for the staff to mark the death of patients | |
| Strategies to improve EOLC and provide a good death42, 43 | |
| EOL care quality monitoring | |
| Making environmental changes to promote dying with dignity | |
| Managing patients' pain and discomfort | |
| Knowing and following patients' wishes for end‐of‐life care | |
| Bereavement program or service | |
| Regular meetings of senior ICU physician and nurse with patients' families | |
| Training of ICU clinicians in end‐of‐life communication skills | |
| Role modeling and supervision of trainees by clinicians experienced in end‐of‐life care | |
| Formal mechanism for emotional support of staff caring for dying patients | |
| Access to palliative care consultants | |
| Training of ICU clinicians in symptom management | |
| Scheduling staff to promote continuity of care for dying patients | |
| Formal system for scaled assessment and charting of patients' symptoms | |
| Method to help resolve differences about appropriate care goals | |
| Resources to accommodate diversity among patients/families at the end of life | |
| Access to clinical ethics consultants | |
| Regular pastoral care visits to the ICU | |
In a subsequent U.S. survey, the Critical Care Peer Workgroup of the Promoting Excellence in End‐of‐Life Care Project reported that more than 75% of ICUs were still not monitoring the quality of EOL care.42 The survey also identified multiple barriers to optimal EOL care found in most ICUs. The study group proposed several strategies to overcome these barriers and improve the quality of EOL care (Table 3).42, 43 It can also be inferred from the survey findings that most ICUs are unprepared and lack the necessary tools to appropriately inform patients and families of the trade‐off in EOL care for NHBOD. The President's Council for Bioethics has also warned that NHBOD can transform EOL care from a peaceful dignified death into a profanely high‐tech death experience for donors' families.10
Several aspects of medical care that are neither palliative nor beneficial are performed for donor management for NHBOD and can explain the feared transformation of the death experience. The revised UAGA reaffirms that all measures necessary to ensure the medical suitability of an organ for transplantation cannot be withheld or withdrawn from the prospective donor and overrides the expression of intent by a prospective donor in either a declaration or advance health‐care directive not to have life prolonged by use of life support systems (Table 1).9 The 2007 amendment to revised UAGA section 21 recognizes the conflict between all measures necessary to ensure organs viability for transplantation and appropriate EOL care and requires the attending physician and OPO to resolve the conflict with the prospective donor or surrogate decision‐maker.9
OPOs apply donor management critical pathways to potential organ donors in order to maintain organ viability for successful execution of organ procurement.36 The University of Wisconsin developed a protocol and an evaluation tool to determine the eligibility of potential candidates for NHBOD.44 The protocol entails temporary discontinuation of mechanical ventilation for a trial of spontaneous respiration lasting up to 10 minutes to determine the likelihood of cardiorespiratory death within 60‐90 minutes of the withdrawal of life support. Those patients predicted by the University of Wisconsin evaluation tool to survive a longer time are not candidates for NHBOD and are transferred to palliative care. Those patients who meet the necessary criteria of the University of Wisconsin evaluation tool become candidates for NHBOD and undergo additional antemortem testing, invasive vascular instrumentation, and infusion of medications essential for organ preservation.36 The instrumentation and medications used for organ preservation can also expedite death on withdrawal of life support.45 Other interventions (such as circulatory support with invasive and noninvasive devices, extracorporeal perfusion and oxygenation, endotracheal reintubation, mechanical ventilation, and bronchoscopy) are performed when cardiorespiratory death is pronounced in order to maintain organ viability and can inadvertently reanimate the donor during the procurement process.26
The process of obtaining donation consent and subsequent donor management protocols for NHBOD deviate from more than 60% of the RWJF quality indicators recommended for optimal EOL care.35, 36, 41 Therefore, NHBOD can have a profound impact on the quality of EOL care. There has been a recent proposal to abbreviate the original RWJF quality indicators to include 14 of the 53 (26%) original quality indicators described for optimal EOL care in the ICU (Table 3).46 Many of the quality indicators expected to be negatively affected by NHBOD are not included in the proposal for an abbreviated list. There has been a concern that the application of an abbreviated rather than a comprehensive metrics for EOL care can portray an incomplete assessment and perhaps misinform donors and their families about the potential trade‐off in EOL care. The President's Council on Bioethics has emphasized that comprehensive evaluation of the quality of EOL care is an ethical imperative so that families can decide if the trade‐off is acceptable for organ donation.10 Deciding to donate organs at the end of life can be stressful for many families, and therefore they must be fully informed of the possible consequences. Posttraumatic stress disorders, anxiety, depression, and decreased quality of life have been reported in the deceased's family members who shared in stressful EOL decisions.47 Posttraumatic stress disorders have been reported in family members of deceased organ donors.48 Organ‐focused behavior by professionals requesting consent for organ donation and ambivalent decision making by family members appeared to increase the risk of relatives of deceased donors subsequently developing traumatic memories and stress disorders. The processes required for successful accomplishment of donation consent and subsequent organ recovery can interfere with many of the interventions that lessen the burden of bereavement of relatives of ICU decedents.49
The variability in decision making by health care providers about medical futility and EOL care has been given as a reason for concern about the implementation of NHBOD.50 The variability of EOL practice raises the possibility of conflicted decision making on medical futility within institutions that have transplant programs.50 Ethical conflicts and moral distress have been reported among health care providers who were directly involved in organ procurement in NHBOD.51 The pressure to recover transplantable organs from NHBOD candidates has been associated with health care professionals' perception of euthanasia and premature determination of medical futility and withdrawal of life support. The long‐term psychological impact of NHBOD practice on caregivers, health care providers, and professionals remains unknown.
CONCLUSIONS
In conclusion, NHBOD influences medical care at critical time points to maximize the procurement of transplantable organs and minimize their warm ischemia time with negative consequences on the EOL care for the prospective donors and their families (see Figure 1).

Mandatory implementation of NHBOD in the face of difficulties surrounding the quality of EOL care for donors raises concern across the medical profession and community. There is a need for better scientific validation of the timing of organ procurement to ensure that organ recovery is not the irreversible event defining death in NHBOD. The desire of OPOs or their affiliates to maximize recovery of transplantable organs introduces self‐serving bias into obtaining consent for organ donation and violates the basic tenet of true informed consent. The use of comprehensive quality indicators of EOL care will help to determine the impact of NHBOD on donors, families, caregivers, and health care providers.
In April 2003 the Health Resources and Services Administration of the U.S. Department of Health and Human Services (DHHS) announced the formation of the Organ Donation Breakthrough Collaborative (ODBC).1 The Organ Donation Breakthrough Collaborative created 58 national donation service areas (DSAs) to organize the transplant community across the United States. Each of the 58 organ procurement organizations (OPOs) is joined to a regional transplant center or centers and donor hospitals to form a DSA. The ODBC's goal is to achieve a cadaveric organ donation rate of 75% or higher from hospitals within each DSA.2
A requirement for organ donation from patients facing imminent or cardiac death has been introduced to increase the supply of transplantable organs and shorten the waiting time for transplantation candidates.35 This type of organ donation represents a significant source of organs required for future expansion of transplantation practice in the United States. The requirement for donation in imminent or cardiac death is implemented through the collaboration of the Advisory Committee on Organ Transplantation of the DHHS (Table 1), the Centers for Medicare & Medicaid Services (CMS), and the Joint Commission on Accreditation of Healthcare Organizations (JCAHO).3, 57 The organ donor pool of those facing imminent or cardiac death has also been expanded to include neurologically intact patients who may not fulfill brain death criteria before withdrawal of life support.4, 8, 9
| State law (year)* | |
|---|---|
| |
| Uniform Anatomical Gift Act (1968) | Any 18‐year‐old with a sound mind may donate his or her body after death to be used for medical research or as a source of transplantable tissues and organs and barring others from overriding a donor's decision to make an anatomical gift. |
| Amendment (1987) | Minors who can apply for a driver's license are empowered to make anatomical gifts, but either parent can revoke the gift if the minor dies before the age of 18. |
| Revision (2006) | Declaration of a gift does not require any witnesses. |
| Amendment (2007) | Document of a gift or donor registry is sufficient for the removal of organs, which means an OPO does not need consent of the spouse or the family. |
| Enables an OPO to gain access to documents of gifts in donor registries, medical records, and records of a state motor vehicle department. | |
| Facilitates donations by expanding the list of those who may make an anatomical gift for another individual who has not declared a preference for or against donation. | |
| Permits removal of organs by medical personnel without explicit consent from a potential donor or from a relative of the donor, so long as the appropriate medical personnel or authorities have made a reasonable effort to discover any objection by the donor or the donor's family. | |
| Require hospitals to notify an OPO or third party designated by the OPO of an individual whose death is imminent or who has died in the hospital in order to increase donation opportunity. | |
| Gives an OPO the right to inspect a patient's medical records. | |
| Measures necessary to ensure the medical suitability of a part not be withdrawn while an examination is being made to determine whether an individual who has been referred to OPO has a part that could be the subject of an anatomical gift. If, following such an examination, it is determined by the OPO that the individual has a part that could be the subject of an anatomical gift, the individual is a prospective donor under this act unless the individual had signed a refusal. | |
| Forbids the buying and selling of organs. | |
| Measures necessary to ensure the medical suitability of an organ for transplantation or therapy may not be withheld or withdrawn from a prospective donor who has an advance health‐care directive or declaration unless the directive or declaration expressly provides to the contrary. The section presumes that for prospective donors the desire to save lives by making an anatomical gift trumps the desire to have life support systems withheld or withdrawn. Individuals who desire to overcome this presumption can do so by express language in their advance health‐care directive or declaration. | |
| Uniform Determination of Death Act (1981) | An individual who has sustained either (1) irreversible cessation of circulatory and respiratory functions, or (2) irreversible cessation of all functions of the entire brain including the brain stem is dead. A determination of death must be made in accordance with accepted medical standards. |
| Federal laws | |
| National Organ Transplant Act (1984) | Calls for a unified transplant network to be operated by a private, nonprofit organization under federal contract and the establishment of a Task Force in Organ Procurement and Transplantation and an Organ Procurement and Transplantation Registry. |
| Public Health and Welfare Act Title 42 USC (1999) | |
| Section 273 | Establishes guidelines to be a qualified OPO that can receive federal grants. |
| Section 274 | Establishes the OPTN & Scientific Registry for Transplantation. |
| Federal regulations | |
| Title Code 42 CFR Part 121 (1999) | Lists regulations of the OPTN final rule. |
| Explains the OPTN structure. | |
| Lists the policy that the OPTN board of directors is responsible for developing. | |
| Explains rules that an OPTN member must obey when including a person on the organ waiting list. | |
| Describes the requirements and tests for determining the suitability of donated organs. | |
| Explains how the OPTN identifies an organ recipient, allocates the organ, and transports it to the recipient. | |
| Describes how the board of directors should develop allocation policies to guarantee they are both efficient and just and allocation performance goals to ensure the best possible use and most equitable allocation of organs. | |
| Lists designated transplant program requirements. | |
| Describes how the HHS conducts reviews and evaluations and enforces rules. | |
| Describes the recording and reporting requirements of the various groups involved in the transplantation process. | |
| Establishes the Advisory Committee on Organ Transplantation (ACOT), which advises the HHS secretary on organ donation, procurement, allocation, and transplantation The HHS secretary may ask for ACOT's opinion of proposed OPTN policies. | |
| Title Code 42 CFR Parts 413, 441, 486 and 498 (2006) | Lists regulations of the OPO final rule. |
| Establishes new conditions for coverage for OPOs that include multiple new outcome and process performance measures based on organ donor potential and other related factors in each donation service area of qualified OPOs. | |
The President's Council on Bioethics independently evaluated the issues surrounding deceased organ donation and procurement.10 The President's Council on Bioethics has expressed major concerns about several issues pertinent to cardiac or imminent death organ donation that have not been addressed explicitly by the bodies that have made recommendations for reforming or expanding that type of organ donation in the United States. The debate on organ procurement in imminent or cardiac death has come to the forefront because of doubts about its ethical appropriateness and acceptance within the medical profession and the community at large. This review focuses on the serious issues related to organ procurement from patients facing imminent or cardiac death.
Organ Procurement and the Dead Donor Rule
Organ procurement is only permitted when the donor is already dead (ie, the dead donor rule), and the act of organ recovery cannot have been the immediate act to cause that death.10, 11 There are 2 criteria for death as defined in the Uniform Determination of Death Act (UDDA; Table 1): an individual who has sustained irreversible cessation of either (1) circulatory and respiratory functions or (2) all functions of the entire brain, including the brain stem, is considered dead and this determination of death must have been made in accordance with accepted medical standards.12 When organs are procured from an individual in whom all brain function has ceased but normal cardiac pump activity is continuing, it is referred to as heart‐beating organ donation. Organ procurement after cessation of cardiac pump activity and cardiorespiratory functions is referred to as non‐heart‐beating organ donation (NHBOD). Organ procurement from an individual in imminent or cardiac death is considered NHBOD.
Non‐heart‐beating organ donors can be neurologically intact and do not fulfill the brain death criterion prior to cessation of cardiac pump activity. In response to this dilemma, the University of Pittsburgh Medical Centre developed a protocol for donation of organs that permitted their procurement from patients who were pulseless and apneic for 2 minutes and did not fulfill brain criteria and who had previously given consent for organ donation.13 Because it is uncertain if cessation of cardiorespiratory function is irreversible after only a short time, the Institute of Medicine (IOM) extended the time required for pulselessness and apnea from 2 to 5 minutes before permitting organ procurement.14 Waiting longer than 5 minutes for the determination of death would compromise the quality of procured organs because of warm ischemia time and would influence the functioning of grafts in transplant recipients.
One of the pivotal assumptions for NHBOD acceptance is that 5 minutes of pulselessness and apnea eliminates the possibility that the procurement process itself could be the cause of death and fulfills the dead donor rule.14, 15 The cardiorespiratory death criteria were derived from observations that did not evaluate delayed autoresuscitation (spontaneous return of circulation) or simultaneous cessation of brain electrical activity (as recorded in brain death).1618 The death criteria applied for organ procurement must also comply with the irreversibility requirement of the UDDA.11, 12
The true incidence, temporal characteristics, and predictors of autoresuscitation in humans remain unknown because of underreporting in the literature. However, there have been case reports of autoresuscitation with return of neurologic function (also called the Lazarus phenomenon) after 10 minutes of cardiac asystole.19, 20 Maleck et al. and Adhiyaman et al. described autoresuscitation 5 minutes or longer after cardiorespiratory arrest in 44% and 50% of the published case reports, respectively.19, 20 Although cardiac asystole leads to the loss of arterial pulse pressure, circulatory arterial mean pressure is maintained in diastole by arteriolar vasomotor tone. The relaxation (diastole) phase systemic arterial to venous pressure gradient provides the perfusion pressure for vital organs and the spontaneous return of circulation after circulatory arrest.21 It is likely that autoresuscitation occurs because of the persistence of circulatory vasomotor tone after cessation of cardiac function. The time course of systemic vascular tone after circulatory arrest has not been well characterized in humans. However, the IOM criteria did not account for the incidence of delayed autoresuscitation in humans even though the Maastricht protocol (developed by the University of Zurich, Zurich, Switzerland) acknowledged this phenomenon and required at least 10 minutes to elapse after cardiorespiratory arrest before starting organ procurement.22 The 10‐minute waiting time did not compromise the quality of the organs procured.
The cardiorespiratory death criteria for organ procurement also ignore cardiac electrical activity (such as pulseless electrical activity or ventricular fibrillation) on an electrocardiogram. Research with cardiac ultrasonography and indwelling arterial catheters confirms that pulseless cardiac electrical activity can be associated with cardiac mechanical contractions, although these contractions are too weak to be detected by blood pressure monitoring.23 The presence of cardiac electrical activity on an electrocardiogram can also increase the likelihood that delayed autoresuscitation will occur.19, 20 Furthermore, whether there is brain electrical activity or neurologic function when cardiac electrical activity is still observed on an electrocardiogram remains unknown.24 It can be argued that donors who have already suffered severe neurologic injury cannot have meaningful neurologic function at the time of cardiorespiratory death. However, the presence of brain activity becomes relevant for organ donors with intact neurologic and brain function prior to cardiorespiratory arrest when only cardiorespiratory criteria for organ procurement are being used.4, 8, 9
In situ circulatory support with extracorporeal perfusion in organ donors has also refuted that cardiorespiratory arrest for 5 minutes fulfills the UDDA requirement because reversibility can occur during the procurement process. In situ extracorporeal perfusion is initiated 5 minutes after cardiorespiratory arrest of donors in order to preserve organs for procurement.25 Coronary and cerebral reperfusion can lead to the return of cardiac and neurologic functions (also called reanimation) of donors during the procurement process. Mechanical occlusion of the aortic arch and pharmacological agents are required to suppress donor reanimation during organ procurement.26 Martin et al. documented that in situ extracorporeal perfusion returned full neurologic and cardiac function 25 minute after cardiorespiratory arrest that occurred outside the hospital.27 Similar observations of full neurologic recovery and survival to hospital discharge were reported after in situ extracorporeal perfusion for in‐hospital cardiorespiratory arrest.28 These observations confirm that the time required for irreversible loss of neurologic function after cessation of circulation is much longer than the 25 minutes of cardiorespiratory arrest required to begin the process of organ procurement in NHBOD.
The incidence of donor reanimation during procurement is unknown because its reporting violates the dead donor rule and can create legal concerns.11 It can be argued that reanimation of organ donors is irrelevant because it does not mean survival. However, the occurrence of reanimation invalidates the premise that the cardiorespiratory criteria for organ procurement comply with the uniform determination of death. Others have accepted the inaccuracy of these criteria for determining death for procurement of organs from deceased donors and proposed abandoning the dead donor rule in order to permit recovery of transplantable organs before death.29
In the face of the uncertainty in determining death and in response to a media and marketing campaign by organ procurement organizations (OPOs) to promote public enrollment in deceased organ donation, the transplant community renamed NHBOD cardiac death organ donation.30, 31 The use of the term cardiac death is scientifically inaccurate and perhaps misleading. This term is used to denote the cessation of circulation and cardiac pump activity. The term cardiac death can be misinterpreted as meaning the heart has irreversibly ceased at the time of procurement, thus contradicting the scientific evidence for spontaneous resumption of cardiac function and autoresuscitation.19, 20 Alternatively, the use of this term can falsely imply that neurologic activity or brain stem function has ceased irreversibly after loss of cardiac activity, when scientific evidence suggests that brain stem function can remain after cardiac arrest.32
Consent for Organ Donation
Several state laws and federal regulations have been enacted to ensure that organ donation and transplantation practice comply with the ethical and legal standards of society (Table 1). The current regulations require hospitals across the United States to provde regional OPOs with early notification of all patients whose deaths are imminent before life support has been withdrawn so that discussion of organ donation with surrogate decision makers can be initiated independently and consent obtained.3, 5, 9 The Organ Donation Breakthrough Collaborative has set a goal of each OPO accomplishing a target organ donation rate of 75% or higher at local hospitals within an assigned donation service area (DSA).1, 2, 5 The financial and administrative incentives for the OPO to achieve that target organ donation rate have introduced undisclosed conflict within the donation consent process.33 Self‐serving bias of OPOs can influence whether pertinent information necessary for surrogate decision makers to provide informed consent is disclosed.34 As an example of this bias, alternative options for care and palliation may be discussed with surrogate decision makers with less enthusiasm than are the benefits and altruistic notion of organ donation. In obtaining donation consent, OPOs often avoid disclosing details of perimortem interventions performed on donors that are required for successful procurement of transplantable organs.10, 34, 35 After receiving consent for organ donation, OPO staffs also assume the responsibility of planning donor medical care and treatment pathways essential for maintaining organ viability and of preparing for subsequent procurement.5, 36 In essence, the care of the dying patient is guided by a team whose primary interest is the preservation of organs until procurement has been accomplished.
The Uniform Anatomical Gift Act (UAGA) assigns explicit priority to the donor's expressed intent so that consent for organ donation becomes irrevocable and does not require the consent or concurrence of any person after the donor's death (Table 1).9, 37 The donor's authorization to donate, recorded on an organ donor card, the individual's driver's license, or a donor registry, becomes a legally binding advance directive. The UAGA amendment enables OPOs to procure organs without family consent and in certain instances after family refusal to donate.37
Other consent options for organ donation from deceased donors have been proposed to maximize OPO recovery of transplantable organs in the United States (Table 2).8 The IOM has considered presumed consent for organ donation as a favorable option.8 Presumed consent means the default option is consent to donation, that if an individual has not expressly rejected donation, that individual is considered to have consented to organ donation. Legislative enactment of presumed consent enables OPOs to avoid the potential for surrogates to deny consent for donation, thus increasing the pool of future organ donors. The revised UAGA replaces nondonation with the intent to donate organs as the default option. In the default option, all measures necessary to ensure the medical suitability of an organ for transplantation can not be withheld or withdrawn until the OPO has determined medical suitability of the individual as a prospective donor (Table 1). The default option overrides the expression of intent in a declaration or advance health‐care directives not to have life prolonged by withholding or withdrawing life support system unless the individual has expressed refusal of donation (Tables 1 and 2). The revised UAGA presumes that for prospective donors the desire to save lives by making an anatomical gift trumps the desire to have life support systems withheld or withdrawn. Mandated consent (or conscription) has also been proposed for recovery of cadaveric organs (Table 2).38 Under mandated consent, consent for organ donation is automatic from all deceased individuals; therefore, OPOs would not require or request consent because removal of all needed transplantable cadaveric organs would be compulsory. OPO staffs would no longer have to discuss organ procurement from potential donors with family members or other surrogates. An alternative form of donation consent is mandated choice, which requires each individual to decide in advance either to agree to organ donation or to refuse it. Mandated choice is the IOM's least favorite option because it would require extensive public informational programs on organ donation to facilitate individual choices and decision making (Table 2).8
| Type | Description |
|---|---|
| Requested (expressed) consent | An individual is asked to voluntarily agree to organ donation. |
| Presumed (implied) consent | Unless an individual has expressly refused to donate organs, the default option is agreement to donate organs. |
| Mandated consent (conscription) | An individual is not required to decide on organ donation before death, and there is an automatic right to procure organs from any and all deceased individuals. |
| Mandated choice | An individual must choose between 2 options before death: agreement or refusal to donate organs. |
End‐of‐Life Care
Quality of end‐of‐life (EOL) care for an organ donor, as for any individual whose treatment is being withdrawn, is considered the highest priority of care and must not be compromised by the donation process. Yet no studies have investigated the impact of organ donation on the quality of EOL care in NHBOD.35 Previous reports have criticized the quality of EOL care offered to dying patients in intensive care units (ICUs).39, 40 Many of these patients are undergoing withdrawal of life support in anticipation of death and are considered candidates for NHBOD. The Robert Wood Johnson Foundation (RWJF) Critical Care End‐Of‐Life Peer Workgroup developed 53 EOL quality indicators to standardize and measure the quality of EOL care.41 These quality indicators, organized in 7 domains, focus on delivering patient‐ and family‐centered care and facilitating a good death experience in the ICU (Table 3).
| Domains of comprehensive quality indicators of EOLC (n = 53)41 | Abbreviated quality indicators of EOLC (n = 18)46 |
|---|---|
| |
| Patient and family‐centered decision making (n = 13) | |
| Recognize the patient and family as the unit of care | |
| Assess the patient's and family's decision‐making style and preferences | |
| Address conflicts in decision making within the family | |
| Assess, together with appropriate clinical consultants, the patient's capacity to participate in decision making about treatment and document assessment | Assessment of the patient's decisional capacity |
| Initiate advance care planning with the patient and family | |
| Clarify and document the status of the patient's advance directive | Documentation of the presence and, if present, contents of advance directives |
| Identify the healthcare proxy or surrogate decision maker | Documentation of a surrogate decision maker within 24 hours of admission |
| Clarify and document resuscitation orders | |
| Assure patients and families that decision making by the health care team will incorporate their preferences | |
| Follow ethical and legal guidelines for patients who lack both capacity to make decisions and a surrogate decision maker | |
| Establish and document clear, realistic, and appropriate goals of care in consultation with the patient and family | Documentation of the goals of care |
| Help the patient and family assess the benefits and burdens of alternative treatment choices as the patient's condition changes | |
| Forgo life‐sustaining treatments in a way that ensures patient and family preferences are elicited and respected | |
| Communication within the team and with patients and families (n = 10) | |
| Meet as interdisciplinary team to discuss the patient's condition, clarify goals of treatment, and identify the patient's and family's needs and preferences | Documentation of a timely interdisciplinary clinicianfamily conference |
| Address conflicts among the clinical team before meeting with the patient and/or family | |
| Utilize expert clinical, ethical, and spiritual consultants when appropriate | |
| Recognize the adaptations in communication strategy required for patients and families according to the chronic versus acute nature of the illness, cultural and spiritual differences, and other influences | |
| Meet with the patient and/or family on a regular basis to review patient's status and to answer questions | Documentation of timely physician communication with the family |
| Communicate all information to the patient and family, including distressing news, in a clear, sensitive, unhurried manner and in an appropriate setting | |
| Clarify the patient's and family's understanding of the patient's condition and goals of care at the beginning and end of each meeting | |
| Designate primary clinical liaison(s) who will communicate with the family daily | |
| Identify a family member who will serve as the contact person for the family | |
| Prepare the patient and family for the dying process | |
| Continuity of care (n = 3) | |
| Maximize continuity of care across clinicians, consultants, and settings | |
| Orient new clinicians to the status of the patient and family | Transmission of key information with transfer of the patient out of the ICU |
| Policy for continuity of nursing services | |
| Prepare the patient and/or family for a change of clinician(s) and introduce new clinicians | |
| Emotional and practical support for patients and families (n = 8) | |
| Elicit and attend to the needs of the dying person and his/her family | |
| Distribute written material (booklet) for families that includes orientation to the ICU environment and open visitation guidelines, logistical information (nearby hotels, banks, restaurants, directions), listings of financial consultation services, and bereavement programs and resources | Open visitation policy for family members |
| Facilitate strengthening of patientfamily relationships and communication | |
| Maximize privacy of the patient and family | |
| Value and support the patient's and family's cultural traditions | |
| Arrange for social support for patients who do not have family or friends | Documentation that psychosocial support has been offered |
| Distribute written material (booklet) containing essential logistical information and listings of financial consultation services and bereavement support programs/resources | |
| Support family members through the patient's death and their bereavement | |
| Symptom management and comfort care (n = 10) | |
| Emphasize the comprehensive comfort care that will be provided to the patient rather than the removal of life‐sustaining treatments | |
| Institute and use uniform quantitative symptom assessment scales appropriate for communicative and noncommunicative patients on a routine basis | Documentation of pain assessment |
| Documentation of respiratory distress assessment | |
| Standardize and follow best clinical practices for symptom management | Protocol for analgesia/sedation in terminal withdrawal of mechanical ventilation |
| Appropriate medications available during withdrawal of mechanical ventilation | |
| Use nonpharmacologic as well as pharmacologic measures to maximize comfort as appropriate and desired by the patient and family | |
| Reassess and document symptoms following interventions | Documentation of pain management |
| Documentation of respiratory distress management | |
| Know and follow best clinical practices for withdrawing life‐sustaining treatments to avoid patient and family distress | |
| Eliminate unnecessary tests and procedures (laboratory work, weights, routine vital signs) and only maintain intravenous catheters for symptom management when life support is being withdrawn | |
| Minimize noxious stimuli (monitors, strong lights) | |
| Attend to patient's appearance and hygiene | |
| Ensure family's and/or clinician's presence so the patient is not dying alone | |
| Spiritual support for patients and families (n = 3) | |
| Assess and document spiritual needs of the patient and family on an ongoing basis | Documentation that spiritual support was offered |
| Encourage access to spiritual resources | |
| Elicit and facilitate spiritual and cultural practices that the patient and family find comforting | |
| Emotional and organizational support for ICU clinicians (n = 6) | |
| Support health care team colleagues caring for dying patients | Opportunity to review experience of caring for dying patients by ICU clinicians |
| Adjust nursing staff and medical rotation schedules to maximize continuity of care providers for dying patients | |
| Communicate regularly with interdisciplinary team about goals of care | |
| Establish a staff support group, based on the input and needs of ICU staff and experienced group facilitators, and integrate meeting times into the routine of the ICU | |
| Enlist palliative care experts, pastoral care representatives, and other consultants to teach and model aspects of EOLC | |
| Facilitate rituals for the staff to mark the death of patients | |
| Strategies to improve EOLC and provide a good death42, 43 | |
| EOL care quality monitoring | |
| Making environmental changes to promote dying with dignity | |
| Managing patients' pain and discomfort | |
| Knowing and following patients' wishes for end‐of‐life care | |
| Bereavement program or service | |
| Regular meetings of senior ICU physician and nurse with patients' families | |
| Training of ICU clinicians in end‐of‐life communication skills | |
| Role modeling and supervision of trainees by clinicians experienced in end‐of‐life care | |
| Formal mechanism for emotional support of staff caring for dying patients | |
| Access to palliative care consultants | |
| Training of ICU clinicians in symptom management | |
| Scheduling staff to promote continuity of care for dying patients | |
| Formal system for scaled assessment and charting of patients' symptoms | |
| Method to help resolve differences about appropriate care goals | |
| Resources to accommodate diversity among patients/families at the end of life | |
| Access to clinical ethics consultants | |
| Regular pastoral care visits to the ICU | |
In a subsequent U.S. survey, the Critical Care Peer Workgroup of the Promoting Excellence in End‐of‐Life Care Project reported that more than 75% of ICUs were still not monitoring the quality of EOL care.42 The survey also identified multiple barriers to optimal EOL care found in most ICUs. The study group proposed several strategies to overcome these barriers and improve the quality of EOL care (Table 3).42, 43 It can also be inferred from the survey findings that most ICUs are unprepared and lack the necessary tools to appropriately inform patients and families of the trade‐off in EOL care for NHBOD. The President's Council for Bioethics has also warned that NHBOD can transform EOL care from a peaceful dignified death into a profanely high‐tech death experience for donors' families.10
Several aspects of medical care that are neither palliative nor beneficial are performed for donor management for NHBOD and can explain the feared transformation of the death experience. The revised UAGA reaffirms that all measures necessary to ensure the medical suitability of an organ for transplantation cannot be withheld or withdrawn from the prospective donor and overrides the expression of intent by a prospective donor in either a declaration or advance health‐care directive not to have life prolonged by use of life support systems (Table 1).9 The 2007 amendment to revised UAGA section 21 recognizes the conflict between all measures necessary to ensure organs viability for transplantation and appropriate EOL care and requires the attending physician and OPO to resolve the conflict with the prospective donor or surrogate decision‐maker.9
OPOs apply donor management critical pathways to potential organ donors in order to maintain organ viability for successful execution of organ procurement.36 The University of Wisconsin developed a protocol and an evaluation tool to determine the eligibility of potential candidates for NHBOD.44 The protocol entails temporary discontinuation of mechanical ventilation for a trial of spontaneous respiration lasting up to 10 minutes to determine the likelihood of cardiorespiratory death within 60‐90 minutes of the withdrawal of life support. Those patients predicted by the University of Wisconsin evaluation tool to survive a longer time are not candidates for NHBOD and are transferred to palliative care. Those patients who meet the necessary criteria of the University of Wisconsin evaluation tool become candidates for NHBOD and undergo additional antemortem testing, invasive vascular instrumentation, and infusion of medications essential for organ preservation.36 The instrumentation and medications used for organ preservation can also expedite death on withdrawal of life support.45 Other interventions (such as circulatory support with invasive and noninvasive devices, extracorporeal perfusion and oxygenation, endotracheal reintubation, mechanical ventilation, and bronchoscopy) are performed when cardiorespiratory death is pronounced in order to maintain organ viability and can inadvertently reanimate the donor during the procurement process.26
The process of obtaining donation consent and subsequent donor management protocols for NHBOD deviate from more than 60% of the RWJF quality indicators recommended for optimal EOL care.35, 36, 41 Therefore, NHBOD can have a profound impact on the quality of EOL care. There has been a recent proposal to abbreviate the original RWJF quality indicators to include 14 of the 53 (26%) original quality indicators described for optimal EOL care in the ICU (Table 3).46 Many of the quality indicators expected to be negatively affected by NHBOD are not included in the proposal for an abbreviated list. There has been a concern that the application of an abbreviated rather than a comprehensive metrics for EOL care can portray an incomplete assessment and perhaps misinform donors and their families about the potential trade‐off in EOL care. The President's Council on Bioethics has emphasized that comprehensive evaluation of the quality of EOL care is an ethical imperative so that families can decide if the trade‐off is acceptable for organ donation.10 Deciding to donate organs at the end of life can be stressful for many families, and therefore they must be fully informed of the possible consequences. Posttraumatic stress disorders, anxiety, depression, and decreased quality of life have been reported in the deceased's family members who shared in stressful EOL decisions.47 Posttraumatic stress disorders have been reported in family members of deceased organ donors.48 Organ‐focused behavior by professionals requesting consent for organ donation and ambivalent decision making by family members appeared to increase the risk of relatives of deceased donors subsequently developing traumatic memories and stress disorders. The processes required for successful accomplishment of donation consent and subsequent organ recovery can interfere with many of the interventions that lessen the burden of bereavement of relatives of ICU decedents.49
The variability in decision making by health care providers about medical futility and EOL care has been given as a reason for concern about the implementation of NHBOD.50 The variability of EOL practice raises the possibility of conflicted decision making on medical futility within institutions that have transplant programs.50 Ethical conflicts and moral distress have been reported among health care providers who were directly involved in organ procurement in NHBOD.51 The pressure to recover transplantable organs from NHBOD candidates has been associated with health care professionals' perception of euthanasia and premature determination of medical futility and withdrawal of life support. The long‐term psychological impact of NHBOD practice on caregivers, health care providers, and professionals remains unknown.
CONCLUSIONS
In conclusion, NHBOD influences medical care at critical time points to maximize the procurement of transplantable organs and minimize their warm ischemia time with negative consequences on the EOL care for the prospective donors and their families (see Figure 1).

Mandatory implementation of NHBOD in the face of difficulties surrounding the quality of EOL care for donors raises concern across the medical profession and community. There is a need for better scientific validation of the timing of organ procurement to ensure that organ recovery is not the irreversible event defining death in NHBOD. The desire of OPOs or their affiliates to maximize recovery of transplantable organs introduces self‐serving bias into obtaining consent for organ donation and violates the basic tenet of true informed consent. The use of comprehensive quality indicators of EOL care will help to determine the impact of NHBOD on donors, families, caregivers, and health care providers.
- ,,, et al.Organ donation and utilization, 1995‐2004: Entering the collaborative era.Am J Transplant.2006;6:1101–1110.
- U.S. Department of Health and Human Servcies—Organ Donation and Transplant Breakthrough Collaborative. Measurement strategy: Organ Donation and Transplant Breakthrough Collaborative. Available at: http://www.organdonationnow.org/. Accessed December 15,2006.
- U.S. Department of Health and Human Services Advisory Committee on Organ Transplantation. Consensus Recommendations to the HHS Secretary. Available at: http://www.organdonor.gov/research/acot.htm. Accessed January 30,2007.
- ,,, et al.Report of a National Conference on Donation after Cardiac Death.Am J Transplant.2006;6:281–291.
- Centers for Medicare and Medicaid Services, Department of Health and Human Services.Medicare and Medicaid Programs. Conditions for coverage for organ procurement organizations (OPOs); final rule.Federal Register.2006;71:30981–31054.
- [JCAHO] Joint Commission on Accreditation of Healthcare Organizations. Health care at the crossroads: strategies for narrowing the organ donation gap and protecting patients. Available at: http://www.jointcommission.org/PublicPolicy/organ_donation.htm. Accessed January 30,2007.
- [JCAHO] Joint Commission on Accreditation of Healthcare Organizations.Revisions to Standard LD.3.110.Jt Comm Perspect.2006;26:7.
- Committee on Increasing Rates of Organ Donation‐Board on Health Sciences Policy‐Institute of Medicine.Organ Donation: Opportunities for Action.Washington, DC:National Academies Press;2006.
- National Conference of Commissioners on Uniform State Laws. Revised Uniform Anatomical Gift Act (2006) and Amendment to Section 21 (2007). http://www.law.upenn.edu/bll/ulc/uaga/2006final.htm and http://www.anatomicalgiftact.org/DesktopDefault.aspx?tabindex=0
- ,,, et al.Organ donation and utilization, 1995‐2004: Entering the collaborative era.Am J Transplant.2006;6:1101–1110.
- U.S. Department of Health and Human Servcies—Organ Donation and Transplant Breakthrough Collaborative. Measurement strategy: Organ Donation and Transplant Breakthrough Collaborative. Available at: http://www.organdonationnow.org/. Accessed December 15,2006.
- U.S. Department of Health and Human Services Advisory Committee on Organ Transplantation. Consensus Recommendations to the HHS Secretary. Available at: http://www.organdonor.gov/research/acot.htm. Accessed January 30,2007.
- ,,, et al.Report of a National Conference on Donation after Cardiac Death.Am J Transplant.2006;6:281–291.
- Centers for Medicare and Medicaid Services, Department of Health and Human Services.Medicare and Medicaid Programs. Conditions for coverage for organ procurement organizations (OPOs); final rule.Federal Register.2006;71:30981–31054.
- [JCAHO] Joint Commission on Accreditation of Healthcare Organizations. Health care at the crossroads: strategies for narrowing the organ donation gap and protecting patients. Available at: http://www.jointcommission.org/PublicPolicy/organ_donation.htm. Accessed January 30,2007.
- [JCAHO] Joint Commission on Accreditation of Healthcare Organizations.Revisions to Standard LD.3.110.Jt Comm Perspect.2006;26:7.
- Committee on Increasing Rates of Organ Donation‐Board on Health Sciences Policy‐Institute of Medicine.Organ Donation: Opportunities for Action.Washington, DC:National Academies Press;2006.
- National Conference of Commissioners on Uniform State Laws. Revised Uniform Anatomical Gift Act (2006) and Amendment to Section 21 (2007). http://www.law.upenn.edu/bll/ulc/uaga/2006final.htm and http://www.anatomicalgiftact.org/DesktopDefault.aspx?tabindex=0
Barriers to Mobility During Hospitalization
The adverse outcomes associated with hospitalization of older patients, such as functional decline and increased nursing home placement, have been well documented.19 Low mobility, defined as being limited to a bed or chair, has also been associated with these adverse outcomes, even after controlling for severity of illness.10 Early ambulation has been a common practice for years following many types of orthopedic operations, including hip fracture repair and total joint replacement.11, 12 A recent study demonstrated that time to ambulation after surgery was an independent predictor of the development of postoperative complications such as pneumonia and delirium.13
In the early 1980s, early ambulation became the cornerstone of cardiac rehabilitation after acute myocardial infarction.14, 15 Until recently, with the exception of postmyocardial infarction, the use of early ambulation for patients admitted with medical illnesses has not been studied. In the last few years, researchers have begun to explore the use of early ambulation for patients after cardiac catheterization and for those admitted with deep‐vein thrombosis and pneumonia.1620 Although many of these studies have been small, they have found early ambulation not to be associated with worse outcomes. Indeed, a study of early ambulation for patients with community‐acquired pneumonia demonstrated decreased hospital costs and increased functional ability prior to discharge.20
Although the literature documents the adverse consequences associated with bed rest2123 and the beneficial effects of early ambulation, patients continue to spend a significant amount of their hospital stay limited to a bed or a chair. The prevalence of low mobility in older patients ranges from 23% to 33% during hospitalization for medical illness.9, 10 Despite the high prevalence and the associated adverse outcomes of low mobility among hospitalized older adults, the factors associated with low mobility in the hospital setting have not been systematically explored. Identification of such factors is the first step toward recognizing potentially modifiable factors and developing targeted interventions to improve hospital care.
We conceptualized a variety of factors or barriers that could potentially affect the level of mobility achieved by older hospitalized patients. Using professional experience and expert opinion, a conceptual model of potential barriers to mobility was developed (Fig. 1). As Figure 1 illustrates, the model has 4 major categories: patient‐related factors, including illness severity or comorbid conditions; treatment‐related factors such as catheters and intravenous lines; institution‐related factors such as nursing‐to‐patient ratio; and attitudinal factors related to perspectives on mobility and concerns about falling. This model was reviewed and feedback provided by a multidisciplinary group of colleagues including physicians, nurses, physical therapists, medical educators, and medical sociologists.
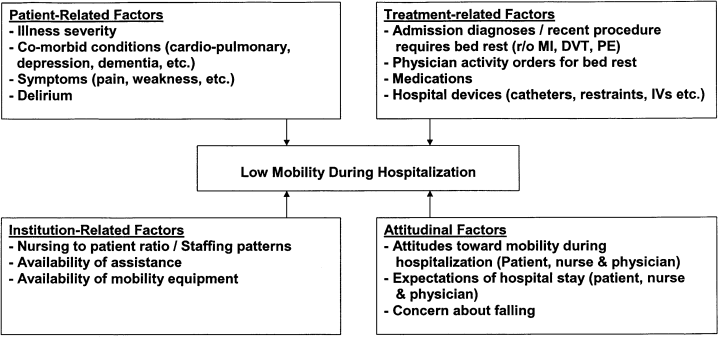
The objectives of this study were to employ qualitative methodology to identify and contextualize perceived barriers to mobility during hospitalization from the perspectives of older patients, their primary nurses, and their resident physicians; to compare and contrast the perceived barriers among these 3 groups; and to compare perceived barriers to mobility with our conceptual model.
METHODS
Setting and Patients
Patients aged 75 years admitted to the medical wards of the University Hospital either directly or through the Emergency Department were recruited for this study. In addition, the primary nurses and resident physicians of enrolled patients were also recruited because of their roles in providing hands‐on care to these patients in the university hospital setting. This project was supported in part by a VA Research Career Development Award and a training support grant from the Hartford Foundationfunded Southeast Center of Excellence in Geriatric Medicine. Written informed consent for participation was obtained from the patients, their nurses, and their resident physicians according to procedures approved by the Institutional Review Board of the University of Alabama at Birmingham. Recruitment was continued until no new barriers to hospital mobility were identified and data saturation was achieved. In all, 29 persons were enrolled: 10 patients, 10 nurses, and 9 physicians.
Patient exclusion criteria included factors that made it difficult for an individual to participate in the interview and to self‐report on mobility: (1) inability to be interviewed (ie, obtunded, aphasic), (2) a significant language barrier, requiring a translator, (3) Mini Mental State Examination Score24 < 16, (4) delirium at the time of the interview as documented by the Confusion Assessment Method (CAM)25, and (5) self‐reported inability to ambulate or transfer 2 weeks prior to hospital admission; Additional exclusion criteria were (6) previous enrollment in the study by the patient, the primary nurse, or the physician; and (7) refusal by patient, family, or physician to participate.
Questionnaire Development
A semistructured interview guide approach was used to encourage participants to discuss their perception of barriers to mobility during hospitalization. Pilot testing at a local retirement center was used to revise the initial patient questionnaire. Domains of inquiry were identified through the pilot testing and based on the conceptualized model (Fig. 1). These domains included attitudes toward mobility, expectations of care regarding walking/mobility, patient‐related factors that influence mobility, situational factors that influence mobility, and specific perceived barriers to mobility. Similar but not identical questions were used for patients and health care providers so answers could be compared between participant subgroups. For example, patients were asked, What might make it easier for you to get out of bed and walk more frequently or for longer periods than you are now? and health care providers were asked, What would make it easier for the patient to get out of bed and walk more frequently or for longer periods of time? Responses were categorized as being spontaneous or prompted depending on how the responses were elicited. Spontaneous responses about mobility barriers were elicited during general questioning about the hospital stay; for example: Tell me what you think about getting out of bed and moving around during this hospitalization? Prompted responses were elicited by asking a specific question about a potential barrier: Do you have any concerns about falling during your hospital stay?
Data Analysis
Interviews were conducted by the principal investigator (PI) between September 2004 and January 2005. Using an iterative approach, analysis began after the first interview with emerging themes being explored in subsequent interviews. All interviews were audiotaped and transcribed verbatim by a medical transcriptionist. Each participant was assigned a unique identifier. Interviews were reviewed by the PI to verify content. Participants were also given an opportunity to review the content of their interviews, with 76% choosing to do this. Using the grounded theory approach, an analytic technique that systematically analyzes raw interview data to generate hypotheses and develop theory; the data were analyzed by generating categories and themes.26
Interviews were independently reviewed and coded by the research assistant and the PI, with a third reviewer available to resolve disputes throughout the analyses. Initially, reviewers coded any item they believed to fit the category of barriers. Discrepancies between reviewers led to review of the original data to determine if the item could be considered a barrier. This process continued until the reviewers were in agreement about what to include as a barrier. Next, the themes generated by the coders were compared. Any discrepancies again led to review of the original data and revision of themes as indicated. This process continued until agreement was achieved for categories and themes between the coders for each of the 29 interviews. Independent themes were identified and counted and are presented as percentages for the purpose of comparing and contrasting between groups.
RESULTS
Fifty‐seven patients age 75 years were admitted to the medical service during the study period. Of those, 18 were excluded because they were too ill according to their physician (n = 6), had a nonmedical illness (n = 5), were discharged before being interviewed (n = 3), or were being cared for by a previously enrolled nurse or physician (n = 4). Of the 39 who were eligible for the study, 7 declined participation (6 patients, 1 physician), and data were lost for 3 as a result of technical difficulties with the tape recorder. A total of 29 participants had an interview transcribed: 10 patients, 10 nurses, and 9 physicians. As in other qualitative inquiries, data saturation was achieved after about 10 interviews,27 but we continued recruitment to permit comparison of themes identified by patients, nurses, and physicians.
Table 1 presents baseline characteristics of the patients. The mean age of the nurses was 34.9 9.9 years, all were female, and 60% were black. The mean age of the resident physicians was 29.1 2.2 years; 44% were female; and 78% were white, 22% were Latino, and none were black.
| Characteristic | Mean (SD) or N (%) |
|---|---|
| Age (years) | 84 (6.0) |
| Sex Male | 3 (30) |
| Female | 7 (70) |
| Race Black | 3 (30) |
| White | 7 (70) |
| Mini Mental State Examination score | 24 (3.3) |
| Number of comorbidities | 6 (2.3) |
| Number of medications at admission | 9 (2.3) |
Figure 2 presents barriers most frequently noted by participants. These barriers were similar among the 3 groups for symptoms and hospital‐related factors like catheters and lack of staff. Lack of patient motivation, lack of ambulatory devices, and medical reasons necessitating bed rest were reported more frequently by health care providers than by patients. On admission, 40% of patients had bed rest ordered. By hospital day 3, all patients had out‐of‐bed orders, and 70% had had physical therapy ordered.
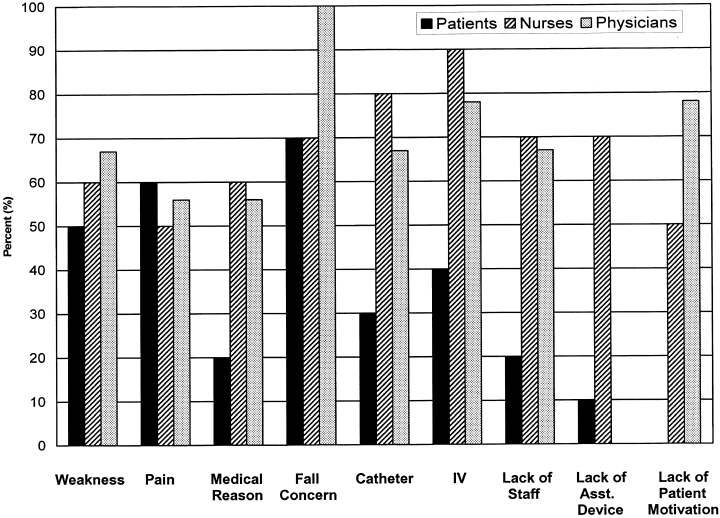
Symptoms
Symptoms were spontaneously mentioned by 97% of participants (28 of 29) as a cause of limited mobility, with weakness cited most frequently: I know it is going to be painful (to walk). My head swims. This side of my all up and down to my toes is weak. When that comes, you kind of be scared. Pain and fatigue were also mentioned by more than 35% of all participants. Although 42% of health care providers (8 of 19) identified dyspnea or shortness of breath as a barrier, no patients reported this symptom as a barrier.
Medical Devices
When asked directly about the impact of urinary catheters and intravenous lines, most patients expressed the belief that the device could either be disconnected or carried and therefore was not a barrier: They can take that off [the catheter] and hook it on the wall. Only 30% of the patients described their mobility as hampered by the medical devices, and only 1 patient (10%) spontaneously indicated the catheter was a barrier: I have had that catheter hooked up to me until today. That was a relief to get that outI couldn't hardly do nothing with that.
In contrast, most health care providers (89%) believed medical devices adversely affected mobility. However, only 32% spontaneously noted the IV or catheter was a barrier, with the other 67% requiring prompting to consider the medical devices as barriers. The rationale for this belief varied widely. Some providers focused on the impact of the patient having to push the IV pole: They get tangled, they hurt when they are mobilized. You have to push the little cart, so patients actually need some assistance. Other providers mentioned the impact of a patient's medical devices on the nurse's ability to assist patients out of bed: I hate to say it, but I think on some days, it does [affect mobility]. You have patients who have TPN and blood and Foleys and chest tubes, you are probably less likely to [get people out of bed]. One physician commented on a less obvious impact of catheters: Some people might be embarrassed to walk around with that Foley catheter.
Need for Assistance and Lack of Staff
Patients, nurses, and physicians alike spontaneously identified the patient's need for assistance with ambulation as a barrier. This observation was frequently followed by comments about staff shortages and time constraints that limited the availability of staff to assist patients with ambulation. The impact of a perceived lack of staff was expressed differently by patients and health care providers. Patients frequently talked about not wanting to bother the nurses: I know it would be good for me, but I just don't want to impose upon them; I try not to worry the girls to take me walking because they have their own patients. Health care providers focused on the variety of other nursing duties that tended to take precedence over helping patients with mobility: I just don't think the nurses have time. They are too busy doing other things to walk their patients up and down the hallway. I think if you really want your patient up walking down the hall, you need to have a relative help them or physical therapy. Among staff, the most frequently mentioned solution was to refer patients to physical therapy, a strategy endorsed equally by nurses and physicians: That is why we try to encourage the doctors to order physical therapy, because we don't have time to ambulate patients in the hallway like the doctor expects.
Lack of Ambulatory Devices
The nurses frequently mentioned the absence of ambulatory devices to assist patients with walking. Nurses also expressed concern about the ability of patients to walk safely without such devices: Sometimes if a patient requires a lot of help, then I think you really need to involve physical therapy to ensure the safety of the person getting up. Because a lot of times you are limited with equipment that you need and they [physical therapists] have that equipment to make sure they don't fall. No physicians and only 1 patient cited lack of ambulatory devices as a barrier to mobility.
Fear of a Patient Falling
The potential for a fall during a hospital stay was of concern for more than 75% of the participants, with physicians spontaneously expressing concern for falls as a perceived barrier more frequently than did patients or nurses: I probably don't encourage her to move as much as I should. And when I do, I tell her to be sure to have someone with her when she is getting up. I am probably a little more protective because I am afraid she might fall. Another physician stated, I think nurses in general would prefer the patient to stay in bed. I believe they perceive it as a risk for falls and a risk for pulling out their IVs or any other medical device, and it is probably not viewed as an important factor in someone who is recovering from an illness. Another physician noted fall prevention was a secondary reason for limited mobility but also expressed the idea that bed rest was easier than mobility: While they are in bed they are not giving trouble to anybody. It is less work and, second, because of liability issues in terms of patients falling and hurting themselves while they are in the hospital. I think everybody is very concerned with that, but I think mainly because it is less work. Although 68% of health care providers spontaneously noted falls as a barrier to mobility, only 1 patient spontaneously verbalized this concern. However, with prompting to consider falls a barrier, 60% of patients stated falls were a potential barrier: As old as I am, your legs don't last long and they give away. It would be dangerous because I haven't got the strength. Now, since I've fallen, yes, I have to be careful.
Lack of Patient Motivation
Lack of patient motivation was mentioned by 50% of the nurses and 78% of the physicians and was often linked to a patient's age: I just think he is older now, and he is not as motivated as younger people are, and he has been through so much. I just don't think he wants to do it. I don't know if it is because it is the elderly or because they just seem more stubborn. They are sometimes more content to stay in the bed, and you have to really stress to them to get up. However, none of the patients indicated a lack of motivation was the reason for not getting out of bed. Indeed, they commented on the staff's apparent lack of interest or their perception that the staff did not consider increased mobility important: I don't believe they are going to get me out of bed while I am here. If I said I really needed to get out of bed, they try to do what you want them to do. But evidently they don't think it is that important.
Hospital Environment
Although not frequently mentioned, issues of an environment not conducive to mobility did emerge as a theme. Several patients expressed this environmental barrier as a difficulty with the hospital gowns that are required garb during a hospital stay. One patient wrote the following additional thoughts after reviewing her transcript: Gowns lead to embarrassing moments, are designed for benefit of staff, not patients. This sentiment was echoed by a physician who, when queried about the impact of gowns, responded, I think the gown exposes the patient a lot and they might feel embarrassed to go around. And outside the hospital room, nobody wants to be perceived as sick and draw attention. Finally, 2 physicians commented on the lack of chairs in the room and the physical setup of the room not encouraging mobility: I think that patients, when they are in the hospital, they feel they are supposed to be in bed. And they are more comfortable there and a lot of times they can see the TV better.
DISCUSSION
Many of the barriers described in the original conceptual model (Fig. 1) were cited by participants from all 3 groups: patients, nurses and physicians. These included patient‐related factors like symptoms and need for assistance, concern about falls, and lack of staff to assist with ambulation. Although attitudes toward mobility were cited in the model and by participants, there was significant disagreement between the 3 groups about the cause of the attitudinal barrier. Health care providers cited lack of patient motivation, whereas patients perceived health care providers as not being interested in mobility or viewing it as important. Health care providers frequently employed stereotypes to describe the potential reasons for the perceived attitudes toward mobility, often linking lack of motivation or interest in getting out of bed to patients being old. Patients linked the lack of importance attached to mobility to the numerous duties of staff members and believed that assistance with mobility was less important than other duties. Physicians and nurses were both more likely than patients to mention factors like urinary catheters, intravenous lines, and other medical reasons that necessitated bed rest. Although more than half the nurses commented on the lack of ambulatory devices for ambulation, no physicians and only 1 patient perceived this lack to be a barrier.
The model presented appears to have face validity, with participants citing many of the factors originally identified as barriers to mobility. The original model did not include consideration of environmental factors such as the number of chairs in the room or the location of the television. Such environmental factors can be conceptualized as institution‐related factors.
In addition, the impact of physician activity orders for bed rest was not specifically discussed by participants, although 45% of participants did comment on the need for bed rest because of a medical condition. A review of the medical records for activity orders indicated 40% of the patients initially had orders for bed rest. Another recent study demonstrated 33% of older patients were on bed rest at some point during their hospital stay10 and should be retained in the model as a treatment‐related consideration.
Several barriers noted in the original model and by participants may not be modifiable, such as comorbid conditions and illness severity. But other perceived barriers may be, and recognition of these factors present potential targets for a future multicomponent intervention to enhance hospital mobility. This multicomponent approach has been highly successful for geriatric syndromes like falls28 and delirium.29
During hospitalization, a focus on early removal of catheters and intravenous lines may encourage mobility. In a recent study absence of a urinary catheter was a predictor of patients with activity limitations regaining ambulatory ability while hospitalized.30 Availability of ambulatory devices may allow nurses to ambulate patients without consulting physical therapy. Another potential solution would be a hospitalwide walking program. The feasibility of a walking program was demonstrated in a small pilot study at a community‐based hospital using specially trained transporters to walk ward patients during slow periods. These periods included nights and weekends, when patients were more likely to be available and when physical therapy was often not present. On average, participants spent 2.4 days in the program, with an average of 5.6 walks per patient. However, additional research is needed, as the study was too small to demonstrate the effects of the walking program on length of stay or functional decline.31
Other barriers, while potentially modifiable, may be more difficult to address and may involve changing the culture of the hospital. For instance, concern about falls was a common theme, echoed by all 3 groups. Physicians were the most likely to spontaneously mention falls as a concern. The nurses shared this concern, frequently citing the use of bed rails as a part of their fall protocol, despite available literature demonstrating that this approach was not efficacious.3234 Nurses also consistently reported asking patients to call for help to ambulate, yet both patients and nurses noted a lack of nursing time to assist with ambulation. The default solution providers reported using was utilizing physical therapists, who were available to walk with patients only once or twice a day. Research on the best methods to prevent falls during hospitalization is limited. Given the current medicolegal environment and the emphasis on fall prevention by the Joint Commission on Accreditation of Healthcare Organizations and other government entities, it is not surprising that bed rest and mobility limitation are being used as a method of minimizing falls. Until data about successful fall‐prevention strategies are available, minimization of mobility may remain the default solution.
Strengths of the present study include the use of qualitative methods to explore potential barriers to mobility, a method that allows participants to describe in their own words their attitudes, beliefs, and expectations about mobility during hospitalization. Face‐to‐face interviewing of the 3 major groups involved in the hospital experience (patients, nurses, and physicians) facilitated the collection of detailed contextualized information on factors expected to affect a patient's level of mobility during hospitalization. This enabled hypothesis generation and sensitization to issues that need further quantitative investigation with larger groups.
The study also had several limitations. First, only resident physicians were included, and their answers may not reflect the opinions of other, more experienced physicians. However, resident physicians were chosen for this study because they play important roles in delivering hands‐on care in teaching hospitals and would need to be involved in any future interventions designed to enhanced patient mobility. Second, a sample size of 29 participants, approximately 10 persons in each group, may not reflect the thinking of those throughout the hospital. However, sampling was continued until no new themes or barriers emerged, and major themes emerged consistently throughout the interviews with all 3 groups. Interviews were conducted at a large, urban university hospital, and so results may not be generalizable to smaller community hospitals. Last, although the 3 major groupspatients, nurses, and physicianswere included in the interviews, the opinions of other stakeholders who may have had perspectives on mobility such as family members were not solicited and may need to be incorporated into the model.
This study presents the perceived barriers to mobility from the perspectives of patients their nurses and physicians. The modifiable and nonmodifiable factors that might affect mobility during hospitalization that made up the original theoretical model were consistent with the barriers cited by the participants. Importantly, this research has led to the identification of other barriers such as environmental factors that may also influence the mobility of older patients. This research has provided insights into potentially modifiable factors of the well‐documented phenomenon of low mobility of hospitalized older persons10 and identified several targets for a multicomponent intervention to minimize low mobility. Possible interventions include a progressive walking program initiated early in the hospital stay, provision of assistive devices to patients who need them, and early removal of catheters and intravenous lines. Further research is needed to explore other factors associated with low mobility, such as specific medical conditions for which bed rest may be ordered, and to evaluate the impact that specific interventions may have on the mobility of older persons during hospitalization.
Acknowledgements
The authors are indebted to the patients, nurses, and resident physicians at University Hospital who participated in the study; to Stephanie Stone, MBA, for her invaluable assistance with study execution; and to Robert H. Brown, MDiv, for his critical review of the manuscript.
APPENDIX
Questionnaire Guide for Patients
Attitudes toward Mobility
Tell me how much walking do you do at home when you are not sick.
Do you leave your bedroom when you are at home? Do you need help to do this?
Do you go out of the house when you are at home? Do you need help to do this?
Do you go out of your neighborhood when you are at home? Do you need help to do this?
Tell me what you think about getting out of bed and moving around during this hospitalization? Do you think it is a good idea or a bad one?
How important do you believe it is for you to rest while you are in the hospital? Why did you choose that answer?
How important do you believe it is for you to walk while you are in the hospital? Why did you choose that answer?
Do you believe it to be dangerous for you to get up out of bed and walk? Why or why not?
Do you think your doctor wants you to get out of bed and walk? Why or why not?
Do you think your nurse wants you to get out of bed and walk? Why or why not?
Expectations of Care about Walking/Mobility
Can you tell me what you believe the nurse is supposed to do for you while you are in the hospital?
When you leave the hospital, do you believe you will be able to walk on your own, or will you need help?
Needs help: Why do you believe you will need help? What type of help will you need?
No help needed: Why do you believe you will not need help?
When you leave the hospital, do you believe you will be able to care for yourself, or will you need help? Needs help: Why do you believe you will need help? What type of help will you need?
No help needed: Why do you believe you will not need help?
If you needed help, who would be available to help you when you go home?
Person Factors That Influence Mobility
Tell me about the illness that brought you into the hospital.
How serious do you believe your illness to be? Why do you believe that?
How easy will it be for you to get better from this illness? Why do you believe that?
Situational Factors That Influence Mobility
Do you have any thoughts about what might make it easier for you to get out of bed and walk more frequently or for longer periods than you are now?
If you decided to go to the bathroom, would you call for help?
Why did you choose that answer?
Perceived Barriers
Tell me what, if anything, would prevent you from getting out of bed and walking during your hospital stay?
Do you have any concerns about falling during your hospital stay?
Are there other factors that influence if you will walk during your hospitalization that I haven't asked about?
New Questions
As this is a qualitative study, if participants bring up new topics that have not been previously explored with the questionnaire guide, these questions will be added to the questionnaire. Future participants will be asked the new questions.
- ,,.Predictors of functional decline in hospitalized elderly patients: a systematic review.J Gerontol Med Sci.2002;57A:M569–M577.
- ,,, et al.Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age.J Am Geriatr Soc.2003;51:451–458.
- ,,, et al.Functional outcomes of acute medical illness and hospitalization in older persons.Arch Intern Med.1996;156:645–652.
- ,,,,.Effect of a geriatric consultation team on functional status of elderly hospitalized patients.Ann Intern Med.1989;110:79–84.
- ,,, et al.Functional disability in the hospitalized elderly.JAMA.1982;248:847–850.
- ,,, et al.Predictors of immediate and 6‐month outcomes in hospitalized elderly patients.J Am Geriatr Soc.1988;36:775–783.
- ,,.Adverse consequences of hospitalization in the elderly.Soc Sci Med.1982;16:1033–1038.
- ,.Complications of illness in geriatric patients in hospital.J Chronic Dis.1966;19:307–313.
- ,,,,.The provision of physical activity to hospitalized elderly patients.Arch Intern Med.1991;151:2452–2456.
- ,,.Prevalence and outcomes of low mobility in hospitalized older patients.J Am Geriatr Soc.2004;52:1263–1270.
- ,,.Cost effectiveness of accelerated rehabilitation after proximal femoral fracture.J Clin Epidemiol.1994;47:1307–1313.
- ,,,,.Early inpatient rehabilitation after elective hip and knee arthroplasty.JAMA.1998;279:847–852.
- ,,,,.Time to ambulation after hip fracture surgery: relation to hospitalization outcomes.J Gerontol.2003;58A:1042–1045.
- ,,, et al.Controlled trial of early mobilization and discharge from hospital in uncomplicated myocardial infarction.Lancet.1971;2:1359–1360.
- ,,,,.Value of early ambulation in patients with and without complications after acute myocardial infarction.N Engl J Med.1975;292:719–722.
- ,,.Bed rest: a potentially harmful treatment needing more careful evaluation.Lancet.1999;354:1229–1233.
- ,,, et al.Early ambulation after 5 French diagnostic cardiac catheterization: results of a multicenter trial.J Am Coll Cardiol.1990;15:1475–1483.
- ,,, et al.Position and mobilisation post‐angiography study (PAMPAS): a comparison of 4.5 hours and 2.5 hours bed rest.Hear.2003;89:447–448.
- ,,, et al.Bed rest or ambulation in the initial treatment of patients with acute deep vein thrombosis or pulmonary embolism: findings from the RIETE Registry.Chest.2005;127:1631–1636.
- ,,,,.Early mobilization of patients hospitalized with community‐acquired pneumonia.Chest.2003;124:883–889.
- .Hazards of hospitalization of the elderly.Ann Intern Med.1993;118:219–223.
- ,.Physiology and complications of bedrest.J Am Geriatr Soc.1988;36:1047–1054.
- ,.Hospital‐associated deconditioning and dysfunction.J Am Geriatr Soc.1991;39:220–222.
- ,,.“Mini‐mental state.” A practical method for grading the cognitive status of patients for the clinician.J Psychiatr Rev.1975;12:189–198.
- ,,,,,.Clarifying confusion: the confusion assessment method. A new method for detection of delirium.Ann Intern Med.1990;113:941–948.
- ,.The Discovery of Grounded Theory. Strategies for Qualitative Research.Chicago, IL:Aldine;1967.
- ,,.How many interviews are enough?Field Methods.2006;18:59–82.
- ,,, et al.A multifactorial intervention to reduce the risk of falling among elderly people living in the community.N Engl J Med.1994;331:821–827.
- ,,, et al.A multicomponent intervention to prevent delirium in hospitalized older patients.N Engl J Med.1999;340:669–676.
- ,,,.Predictors of regaining ambulatory ability during hospitalization.J Hosp Med.2006;1:277–284.
- ,,.Walking for Wellness: a collaborative program to maintain mobility in hospitalized older adults.Geriatr Nurs.2004;25:242–245.
- ,,.Use of restraints and bed rails in a British hospital.J Am Geriatr Soc.1996;44:1086–1088.
- ,,.An analysis of falls in the hospital: can we do without bedrails?J Am Geriatr Soc.1999;47:529–31.
- ,,,.Side rail use and bed‐related outcomes among nursing home residents.J Am Geriatr Soc.2002;50:90–96.
The adverse outcomes associated with hospitalization of older patients, such as functional decline and increased nursing home placement, have been well documented.19 Low mobility, defined as being limited to a bed or chair, has also been associated with these adverse outcomes, even after controlling for severity of illness.10 Early ambulation has been a common practice for years following many types of orthopedic operations, including hip fracture repair and total joint replacement.11, 12 A recent study demonstrated that time to ambulation after surgery was an independent predictor of the development of postoperative complications such as pneumonia and delirium.13
In the early 1980s, early ambulation became the cornerstone of cardiac rehabilitation after acute myocardial infarction.14, 15 Until recently, with the exception of postmyocardial infarction, the use of early ambulation for patients admitted with medical illnesses has not been studied. In the last few years, researchers have begun to explore the use of early ambulation for patients after cardiac catheterization and for those admitted with deep‐vein thrombosis and pneumonia.1620 Although many of these studies have been small, they have found early ambulation not to be associated with worse outcomes. Indeed, a study of early ambulation for patients with community‐acquired pneumonia demonstrated decreased hospital costs and increased functional ability prior to discharge.20
Although the literature documents the adverse consequences associated with bed rest2123 and the beneficial effects of early ambulation, patients continue to spend a significant amount of their hospital stay limited to a bed or a chair. The prevalence of low mobility in older patients ranges from 23% to 33% during hospitalization for medical illness.9, 10 Despite the high prevalence and the associated adverse outcomes of low mobility among hospitalized older adults, the factors associated with low mobility in the hospital setting have not been systematically explored. Identification of such factors is the first step toward recognizing potentially modifiable factors and developing targeted interventions to improve hospital care.
We conceptualized a variety of factors or barriers that could potentially affect the level of mobility achieved by older hospitalized patients. Using professional experience and expert opinion, a conceptual model of potential barriers to mobility was developed (Fig. 1). As Figure 1 illustrates, the model has 4 major categories: patient‐related factors, including illness severity or comorbid conditions; treatment‐related factors such as catheters and intravenous lines; institution‐related factors such as nursing‐to‐patient ratio; and attitudinal factors related to perspectives on mobility and concerns about falling. This model was reviewed and feedback provided by a multidisciplinary group of colleagues including physicians, nurses, physical therapists, medical educators, and medical sociologists.

The objectives of this study were to employ qualitative methodology to identify and contextualize perceived barriers to mobility during hospitalization from the perspectives of older patients, their primary nurses, and their resident physicians; to compare and contrast the perceived barriers among these 3 groups; and to compare perceived barriers to mobility with our conceptual model.
METHODS
Setting and Patients
Patients aged 75 years admitted to the medical wards of the University Hospital either directly or through the Emergency Department were recruited for this study. In addition, the primary nurses and resident physicians of enrolled patients were also recruited because of their roles in providing hands‐on care to these patients in the university hospital setting. This project was supported in part by a VA Research Career Development Award and a training support grant from the Hartford Foundationfunded Southeast Center of Excellence in Geriatric Medicine. Written informed consent for participation was obtained from the patients, their nurses, and their resident physicians according to procedures approved by the Institutional Review Board of the University of Alabama at Birmingham. Recruitment was continued until no new barriers to hospital mobility were identified and data saturation was achieved. In all, 29 persons were enrolled: 10 patients, 10 nurses, and 9 physicians.
Patient exclusion criteria included factors that made it difficult for an individual to participate in the interview and to self‐report on mobility: (1) inability to be interviewed (ie, obtunded, aphasic), (2) a significant language barrier, requiring a translator, (3) Mini Mental State Examination Score24 < 16, (4) delirium at the time of the interview as documented by the Confusion Assessment Method (CAM)25, and (5) self‐reported inability to ambulate or transfer 2 weeks prior to hospital admission; Additional exclusion criteria were (6) previous enrollment in the study by the patient, the primary nurse, or the physician; and (7) refusal by patient, family, or physician to participate.
Questionnaire Development
A semistructured interview guide approach was used to encourage participants to discuss their perception of barriers to mobility during hospitalization. Pilot testing at a local retirement center was used to revise the initial patient questionnaire. Domains of inquiry were identified through the pilot testing and based on the conceptualized model (Fig. 1). These domains included attitudes toward mobility, expectations of care regarding walking/mobility, patient‐related factors that influence mobility, situational factors that influence mobility, and specific perceived barriers to mobility. Similar but not identical questions were used for patients and health care providers so answers could be compared between participant subgroups. For example, patients were asked, What might make it easier for you to get out of bed and walk more frequently or for longer periods than you are now? and health care providers were asked, What would make it easier for the patient to get out of bed and walk more frequently or for longer periods of time? Responses were categorized as being spontaneous or prompted depending on how the responses were elicited. Spontaneous responses about mobility barriers were elicited during general questioning about the hospital stay; for example: Tell me what you think about getting out of bed and moving around during this hospitalization? Prompted responses were elicited by asking a specific question about a potential barrier: Do you have any concerns about falling during your hospital stay?
Data Analysis
Interviews were conducted by the principal investigator (PI) between September 2004 and January 2005. Using an iterative approach, analysis began after the first interview with emerging themes being explored in subsequent interviews. All interviews were audiotaped and transcribed verbatim by a medical transcriptionist. Each participant was assigned a unique identifier. Interviews were reviewed by the PI to verify content. Participants were also given an opportunity to review the content of their interviews, with 76% choosing to do this. Using the grounded theory approach, an analytic technique that systematically analyzes raw interview data to generate hypotheses and develop theory; the data were analyzed by generating categories and themes.26
Interviews were independently reviewed and coded by the research assistant and the PI, with a third reviewer available to resolve disputes throughout the analyses. Initially, reviewers coded any item they believed to fit the category of barriers. Discrepancies between reviewers led to review of the original data to determine if the item could be considered a barrier. This process continued until the reviewers were in agreement about what to include as a barrier. Next, the themes generated by the coders were compared. Any discrepancies again led to review of the original data and revision of themes as indicated. This process continued until agreement was achieved for categories and themes between the coders for each of the 29 interviews. Independent themes were identified and counted and are presented as percentages for the purpose of comparing and contrasting between groups.
RESULTS
Fifty‐seven patients age 75 years were admitted to the medical service during the study period. Of those, 18 were excluded because they were too ill according to their physician (n = 6), had a nonmedical illness (n = 5), were discharged before being interviewed (n = 3), or were being cared for by a previously enrolled nurse or physician (n = 4). Of the 39 who were eligible for the study, 7 declined participation (6 patients, 1 physician), and data were lost for 3 as a result of technical difficulties with the tape recorder. A total of 29 participants had an interview transcribed: 10 patients, 10 nurses, and 9 physicians. As in other qualitative inquiries, data saturation was achieved after about 10 interviews,27 but we continued recruitment to permit comparison of themes identified by patients, nurses, and physicians.
Table 1 presents baseline characteristics of the patients. The mean age of the nurses was 34.9 9.9 years, all were female, and 60% were black. The mean age of the resident physicians was 29.1 2.2 years; 44% were female; and 78% were white, 22% were Latino, and none were black.
| Characteristic | Mean (SD) or N (%) |
|---|---|
| Age (years) | 84 (6.0) |
| Sex Male | 3 (30) |
| Female | 7 (70) |
| Race Black | 3 (30) |
| White | 7 (70) |
| Mini Mental State Examination score | 24 (3.3) |
| Number of comorbidities | 6 (2.3) |
| Number of medications at admission | 9 (2.3) |
Figure 2 presents barriers most frequently noted by participants. These barriers were similar among the 3 groups for symptoms and hospital‐related factors like catheters and lack of staff. Lack of patient motivation, lack of ambulatory devices, and medical reasons necessitating bed rest were reported more frequently by health care providers than by patients. On admission, 40% of patients had bed rest ordered. By hospital day 3, all patients had out‐of‐bed orders, and 70% had had physical therapy ordered.

Symptoms
Symptoms were spontaneously mentioned by 97% of participants (28 of 29) as a cause of limited mobility, with weakness cited most frequently: I know it is going to be painful (to walk). My head swims. This side of my all up and down to my toes is weak. When that comes, you kind of be scared. Pain and fatigue were also mentioned by more than 35% of all participants. Although 42% of health care providers (8 of 19) identified dyspnea or shortness of breath as a barrier, no patients reported this symptom as a barrier.
Medical Devices
When asked directly about the impact of urinary catheters and intravenous lines, most patients expressed the belief that the device could either be disconnected or carried and therefore was not a barrier: They can take that off [the catheter] and hook it on the wall. Only 30% of the patients described their mobility as hampered by the medical devices, and only 1 patient (10%) spontaneously indicated the catheter was a barrier: I have had that catheter hooked up to me until today. That was a relief to get that outI couldn't hardly do nothing with that.
In contrast, most health care providers (89%) believed medical devices adversely affected mobility. However, only 32% spontaneously noted the IV or catheter was a barrier, with the other 67% requiring prompting to consider the medical devices as barriers. The rationale for this belief varied widely. Some providers focused on the impact of the patient having to push the IV pole: They get tangled, they hurt when they are mobilized. You have to push the little cart, so patients actually need some assistance. Other providers mentioned the impact of a patient's medical devices on the nurse's ability to assist patients out of bed: I hate to say it, but I think on some days, it does [affect mobility]. You have patients who have TPN and blood and Foleys and chest tubes, you are probably less likely to [get people out of bed]. One physician commented on a less obvious impact of catheters: Some people might be embarrassed to walk around with that Foley catheter.
Need for Assistance and Lack of Staff
Patients, nurses, and physicians alike spontaneously identified the patient's need for assistance with ambulation as a barrier. This observation was frequently followed by comments about staff shortages and time constraints that limited the availability of staff to assist patients with ambulation. The impact of a perceived lack of staff was expressed differently by patients and health care providers. Patients frequently talked about not wanting to bother the nurses: I know it would be good for me, but I just don't want to impose upon them; I try not to worry the girls to take me walking because they have their own patients. Health care providers focused on the variety of other nursing duties that tended to take precedence over helping patients with mobility: I just don't think the nurses have time. They are too busy doing other things to walk their patients up and down the hallway. I think if you really want your patient up walking down the hall, you need to have a relative help them or physical therapy. Among staff, the most frequently mentioned solution was to refer patients to physical therapy, a strategy endorsed equally by nurses and physicians: That is why we try to encourage the doctors to order physical therapy, because we don't have time to ambulate patients in the hallway like the doctor expects.
Lack of Ambulatory Devices
The nurses frequently mentioned the absence of ambulatory devices to assist patients with walking. Nurses also expressed concern about the ability of patients to walk safely without such devices: Sometimes if a patient requires a lot of help, then I think you really need to involve physical therapy to ensure the safety of the person getting up. Because a lot of times you are limited with equipment that you need and they [physical therapists] have that equipment to make sure they don't fall. No physicians and only 1 patient cited lack of ambulatory devices as a barrier to mobility.
Fear of a Patient Falling
The potential for a fall during a hospital stay was of concern for more than 75% of the participants, with physicians spontaneously expressing concern for falls as a perceived barrier more frequently than did patients or nurses: I probably don't encourage her to move as much as I should. And when I do, I tell her to be sure to have someone with her when she is getting up. I am probably a little more protective because I am afraid she might fall. Another physician stated, I think nurses in general would prefer the patient to stay in bed. I believe they perceive it as a risk for falls and a risk for pulling out their IVs or any other medical device, and it is probably not viewed as an important factor in someone who is recovering from an illness. Another physician noted fall prevention was a secondary reason for limited mobility but also expressed the idea that bed rest was easier than mobility: While they are in bed they are not giving trouble to anybody. It is less work and, second, because of liability issues in terms of patients falling and hurting themselves while they are in the hospital. I think everybody is very concerned with that, but I think mainly because it is less work. Although 68% of health care providers spontaneously noted falls as a barrier to mobility, only 1 patient spontaneously verbalized this concern. However, with prompting to consider falls a barrier, 60% of patients stated falls were a potential barrier: As old as I am, your legs don't last long and they give away. It would be dangerous because I haven't got the strength. Now, since I've fallen, yes, I have to be careful.
Lack of Patient Motivation
Lack of patient motivation was mentioned by 50% of the nurses and 78% of the physicians and was often linked to a patient's age: I just think he is older now, and he is not as motivated as younger people are, and he has been through so much. I just don't think he wants to do it. I don't know if it is because it is the elderly or because they just seem more stubborn. They are sometimes more content to stay in the bed, and you have to really stress to them to get up. However, none of the patients indicated a lack of motivation was the reason for not getting out of bed. Indeed, they commented on the staff's apparent lack of interest or their perception that the staff did not consider increased mobility important: I don't believe they are going to get me out of bed while I am here. If I said I really needed to get out of bed, they try to do what you want them to do. But evidently they don't think it is that important.
Hospital Environment
Although not frequently mentioned, issues of an environment not conducive to mobility did emerge as a theme. Several patients expressed this environmental barrier as a difficulty with the hospital gowns that are required garb during a hospital stay. One patient wrote the following additional thoughts after reviewing her transcript: Gowns lead to embarrassing moments, are designed for benefit of staff, not patients. This sentiment was echoed by a physician who, when queried about the impact of gowns, responded, I think the gown exposes the patient a lot and they might feel embarrassed to go around. And outside the hospital room, nobody wants to be perceived as sick and draw attention. Finally, 2 physicians commented on the lack of chairs in the room and the physical setup of the room not encouraging mobility: I think that patients, when they are in the hospital, they feel they are supposed to be in bed. And they are more comfortable there and a lot of times they can see the TV better.
DISCUSSION
Many of the barriers described in the original conceptual model (Fig. 1) were cited by participants from all 3 groups: patients, nurses and physicians. These included patient‐related factors like symptoms and need for assistance, concern about falls, and lack of staff to assist with ambulation. Although attitudes toward mobility were cited in the model and by participants, there was significant disagreement between the 3 groups about the cause of the attitudinal barrier. Health care providers cited lack of patient motivation, whereas patients perceived health care providers as not being interested in mobility or viewing it as important. Health care providers frequently employed stereotypes to describe the potential reasons for the perceived attitudes toward mobility, often linking lack of motivation or interest in getting out of bed to patients being old. Patients linked the lack of importance attached to mobility to the numerous duties of staff members and believed that assistance with mobility was less important than other duties. Physicians and nurses were both more likely than patients to mention factors like urinary catheters, intravenous lines, and other medical reasons that necessitated bed rest. Although more than half the nurses commented on the lack of ambulatory devices for ambulation, no physicians and only 1 patient perceived this lack to be a barrier.
The model presented appears to have face validity, with participants citing many of the factors originally identified as barriers to mobility. The original model did not include consideration of environmental factors such as the number of chairs in the room or the location of the television. Such environmental factors can be conceptualized as institution‐related factors.
In addition, the impact of physician activity orders for bed rest was not specifically discussed by participants, although 45% of participants did comment on the need for bed rest because of a medical condition. A review of the medical records for activity orders indicated 40% of the patients initially had orders for bed rest. Another recent study demonstrated 33% of older patients were on bed rest at some point during their hospital stay10 and should be retained in the model as a treatment‐related consideration.
Several barriers noted in the original model and by participants may not be modifiable, such as comorbid conditions and illness severity. But other perceived barriers may be, and recognition of these factors present potential targets for a future multicomponent intervention to enhance hospital mobility. This multicomponent approach has been highly successful for geriatric syndromes like falls28 and delirium.29
During hospitalization, a focus on early removal of catheters and intravenous lines may encourage mobility. In a recent study absence of a urinary catheter was a predictor of patients with activity limitations regaining ambulatory ability while hospitalized.30 Availability of ambulatory devices may allow nurses to ambulate patients without consulting physical therapy. Another potential solution would be a hospitalwide walking program. The feasibility of a walking program was demonstrated in a small pilot study at a community‐based hospital using specially trained transporters to walk ward patients during slow periods. These periods included nights and weekends, when patients were more likely to be available and when physical therapy was often not present. On average, participants spent 2.4 days in the program, with an average of 5.6 walks per patient. However, additional research is needed, as the study was too small to demonstrate the effects of the walking program on length of stay or functional decline.31
Other barriers, while potentially modifiable, may be more difficult to address and may involve changing the culture of the hospital. For instance, concern about falls was a common theme, echoed by all 3 groups. Physicians were the most likely to spontaneously mention falls as a concern. The nurses shared this concern, frequently citing the use of bed rails as a part of their fall protocol, despite available literature demonstrating that this approach was not efficacious.3234 Nurses also consistently reported asking patients to call for help to ambulate, yet both patients and nurses noted a lack of nursing time to assist with ambulation. The default solution providers reported using was utilizing physical therapists, who were available to walk with patients only once or twice a day. Research on the best methods to prevent falls during hospitalization is limited. Given the current medicolegal environment and the emphasis on fall prevention by the Joint Commission on Accreditation of Healthcare Organizations and other government entities, it is not surprising that bed rest and mobility limitation are being used as a method of minimizing falls. Until data about successful fall‐prevention strategies are available, minimization of mobility may remain the default solution.
Strengths of the present study include the use of qualitative methods to explore potential barriers to mobility, a method that allows participants to describe in their own words their attitudes, beliefs, and expectations about mobility during hospitalization. Face‐to‐face interviewing of the 3 major groups involved in the hospital experience (patients, nurses, and physicians) facilitated the collection of detailed contextualized information on factors expected to affect a patient's level of mobility during hospitalization. This enabled hypothesis generation and sensitization to issues that need further quantitative investigation with larger groups.
The study also had several limitations. First, only resident physicians were included, and their answers may not reflect the opinions of other, more experienced physicians. However, resident physicians were chosen for this study because they play important roles in delivering hands‐on care in teaching hospitals and would need to be involved in any future interventions designed to enhanced patient mobility. Second, a sample size of 29 participants, approximately 10 persons in each group, may not reflect the thinking of those throughout the hospital. However, sampling was continued until no new themes or barriers emerged, and major themes emerged consistently throughout the interviews with all 3 groups. Interviews were conducted at a large, urban university hospital, and so results may not be generalizable to smaller community hospitals. Last, although the 3 major groupspatients, nurses, and physicianswere included in the interviews, the opinions of other stakeholders who may have had perspectives on mobility such as family members were not solicited and may need to be incorporated into the model.
This study presents the perceived barriers to mobility from the perspectives of patients their nurses and physicians. The modifiable and nonmodifiable factors that might affect mobility during hospitalization that made up the original theoretical model were consistent with the barriers cited by the participants. Importantly, this research has led to the identification of other barriers such as environmental factors that may also influence the mobility of older patients. This research has provided insights into potentially modifiable factors of the well‐documented phenomenon of low mobility of hospitalized older persons10 and identified several targets for a multicomponent intervention to minimize low mobility. Possible interventions include a progressive walking program initiated early in the hospital stay, provision of assistive devices to patients who need them, and early removal of catheters and intravenous lines. Further research is needed to explore other factors associated with low mobility, such as specific medical conditions for which bed rest may be ordered, and to evaluate the impact that specific interventions may have on the mobility of older persons during hospitalization.
Acknowledgements
The authors are indebted to the patients, nurses, and resident physicians at University Hospital who participated in the study; to Stephanie Stone, MBA, for her invaluable assistance with study execution; and to Robert H. Brown, MDiv, for his critical review of the manuscript.
APPENDIX
Questionnaire Guide for Patients
Attitudes toward Mobility
Tell me how much walking do you do at home when you are not sick.
Do you leave your bedroom when you are at home? Do you need help to do this?
Do you go out of the house when you are at home? Do you need help to do this?
Do you go out of your neighborhood when you are at home? Do you need help to do this?
Tell me what you think about getting out of bed and moving around during this hospitalization? Do you think it is a good idea or a bad one?
How important do you believe it is for you to rest while you are in the hospital? Why did you choose that answer?
How important do you believe it is for you to walk while you are in the hospital? Why did you choose that answer?
Do you believe it to be dangerous for you to get up out of bed and walk? Why or why not?
Do you think your doctor wants you to get out of bed and walk? Why or why not?
Do you think your nurse wants you to get out of bed and walk? Why or why not?
Expectations of Care about Walking/Mobility
Can you tell me what you believe the nurse is supposed to do for you while you are in the hospital?
When you leave the hospital, do you believe you will be able to walk on your own, or will you need help?
Needs help: Why do you believe you will need help? What type of help will you need?
No help needed: Why do you believe you will not need help?
When you leave the hospital, do you believe you will be able to care for yourself, or will you need help? Needs help: Why do you believe you will need help? What type of help will you need?
No help needed: Why do you believe you will not need help?
If you needed help, who would be available to help you when you go home?
Person Factors That Influence Mobility
Tell me about the illness that brought you into the hospital.
How serious do you believe your illness to be? Why do you believe that?
How easy will it be for you to get better from this illness? Why do you believe that?
Situational Factors That Influence Mobility
Do you have any thoughts about what might make it easier for you to get out of bed and walk more frequently or for longer periods than you are now?
If you decided to go to the bathroom, would you call for help?
Why did you choose that answer?
Perceived Barriers
Tell me what, if anything, would prevent you from getting out of bed and walking during your hospital stay?
Do you have any concerns about falling during your hospital stay?
Are there other factors that influence if you will walk during your hospitalization that I haven't asked about?
New Questions
As this is a qualitative study, if participants bring up new topics that have not been previously explored with the questionnaire guide, these questions will be added to the questionnaire. Future participants will be asked the new questions.
The adverse outcomes associated with hospitalization of older patients, such as functional decline and increased nursing home placement, have been well documented.19 Low mobility, defined as being limited to a bed or chair, has also been associated with these adverse outcomes, even after controlling for severity of illness.10 Early ambulation has been a common practice for years following many types of orthopedic operations, including hip fracture repair and total joint replacement.11, 12 A recent study demonstrated that time to ambulation after surgery was an independent predictor of the development of postoperative complications such as pneumonia and delirium.13
In the early 1980s, early ambulation became the cornerstone of cardiac rehabilitation after acute myocardial infarction.14, 15 Until recently, with the exception of postmyocardial infarction, the use of early ambulation for patients admitted with medical illnesses has not been studied. In the last few years, researchers have begun to explore the use of early ambulation for patients after cardiac catheterization and for those admitted with deep‐vein thrombosis and pneumonia.1620 Although many of these studies have been small, they have found early ambulation not to be associated with worse outcomes. Indeed, a study of early ambulation for patients with community‐acquired pneumonia demonstrated decreased hospital costs and increased functional ability prior to discharge.20
Although the literature documents the adverse consequences associated with bed rest2123 and the beneficial effects of early ambulation, patients continue to spend a significant amount of their hospital stay limited to a bed or a chair. The prevalence of low mobility in older patients ranges from 23% to 33% during hospitalization for medical illness.9, 10 Despite the high prevalence and the associated adverse outcomes of low mobility among hospitalized older adults, the factors associated with low mobility in the hospital setting have not been systematically explored. Identification of such factors is the first step toward recognizing potentially modifiable factors and developing targeted interventions to improve hospital care.
We conceptualized a variety of factors or barriers that could potentially affect the level of mobility achieved by older hospitalized patients. Using professional experience and expert opinion, a conceptual model of potential barriers to mobility was developed (Fig. 1). As Figure 1 illustrates, the model has 4 major categories: patient‐related factors, including illness severity or comorbid conditions; treatment‐related factors such as catheters and intravenous lines; institution‐related factors such as nursing‐to‐patient ratio; and attitudinal factors related to perspectives on mobility and concerns about falling. This model was reviewed and feedback provided by a multidisciplinary group of colleagues including physicians, nurses, physical therapists, medical educators, and medical sociologists.

The objectives of this study were to employ qualitative methodology to identify and contextualize perceived barriers to mobility during hospitalization from the perspectives of older patients, their primary nurses, and their resident physicians; to compare and contrast the perceived barriers among these 3 groups; and to compare perceived barriers to mobility with our conceptual model.
METHODS
Setting and Patients
Patients aged 75 years admitted to the medical wards of the University Hospital either directly or through the Emergency Department were recruited for this study. In addition, the primary nurses and resident physicians of enrolled patients were also recruited because of their roles in providing hands‐on care to these patients in the university hospital setting. This project was supported in part by a VA Research Career Development Award and a training support grant from the Hartford Foundationfunded Southeast Center of Excellence in Geriatric Medicine. Written informed consent for participation was obtained from the patients, their nurses, and their resident physicians according to procedures approved by the Institutional Review Board of the University of Alabama at Birmingham. Recruitment was continued until no new barriers to hospital mobility were identified and data saturation was achieved. In all, 29 persons were enrolled: 10 patients, 10 nurses, and 9 physicians.
Patient exclusion criteria included factors that made it difficult for an individual to participate in the interview and to self‐report on mobility: (1) inability to be interviewed (ie, obtunded, aphasic), (2) a significant language barrier, requiring a translator, (3) Mini Mental State Examination Score24 < 16, (4) delirium at the time of the interview as documented by the Confusion Assessment Method (CAM)25, and (5) self‐reported inability to ambulate or transfer 2 weeks prior to hospital admission; Additional exclusion criteria were (6) previous enrollment in the study by the patient, the primary nurse, or the physician; and (7) refusal by patient, family, or physician to participate.
Questionnaire Development
A semistructured interview guide approach was used to encourage participants to discuss their perception of barriers to mobility during hospitalization. Pilot testing at a local retirement center was used to revise the initial patient questionnaire. Domains of inquiry were identified through the pilot testing and based on the conceptualized model (Fig. 1). These domains included attitudes toward mobility, expectations of care regarding walking/mobility, patient‐related factors that influence mobility, situational factors that influence mobility, and specific perceived barriers to mobility. Similar but not identical questions were used for patients and health care providers so answers could be compared between participant subgroups. For example, patients were asked, What might make it easier for you to get out of bed and walk more frequently or for longer periods than you are now? and health care providers were asked, What would make it easier for the patient to get out of bed and walk more frequently or for longer periods of time? Responses were categorized as being spontaneous or prompted depending on how the responses were elicited. Spontaneous responses about mobility barriers were elicited during general questioning about the hospital stay; for example: Tell me what you think about getting out of bed and moving around during this hospitalization? Prompted responses were elicited by asking a specific question about a potential barrier: Do you have any concerns about falling during your hospital stay?
Data Analysis
Interviews were conducted by the principal investigator (PI) between September 2004 and January 2005. Using an iterative approach, analysis began after the first interview with emerging themes being explored in subsequent interviews. All interviews were audiotaped and transcribed verbatim by a medical transcriptionist. Each participant was assigned a unique identifier. Interviews were reviewed by the PI to verify content. Participants were also given an opportunity to review the content of their interviews, with 76% choosing to do this. Using the grounded theory approach, an analytic technique that systematically analyzes raw interview data to generate hypotheses and develop theory; the data were analyzed by generating categories and themes.26
Interviews were independently reviewed and coded by the research assistant and the PI, with a third reviewer available to resolve disputes throughout the analyses. Initially, reviewers coded any item they believed to fit the category of barriers. Discrepancies between reviewers led to review of the original data to determine if the item could be considered a barrier. This process continued until the reviewers were in agreement about what to include as a barrier. Next, the themes generated by the coders were compared. Any discrepancies again led to review of the original data and revision of themes as indicated. This process continued until agreement was achieved for categories and themes between the coders for each of the 29 interviews. Independent themes were identified and counted and are presented as percentages for the purpose of comparing and contrasting between groups.
RESULTS
Fifty‐seven patients age 75 years were admitted to the medical service during the study period. Of those, 18 were excluded because they were too ill according to their physician (n = 6), had a nonmedical illness (n = 5), were discharged before being interviewed (n = 3), or were being cared for by a previously enrolled nurse or physician (n = 4). Of the 39 who were eligible for the study, 7 declined participation (6 patients, 1 physician), and data were lost for 3 as a result of technical difficulties with the tape recorder. A total of 29 participants had an interview transcribed: 10 patients, 10 nurses, and 9 physicians. As in other qualitative inquiries, data saturation was achieved after about 10 interviews,27 but we continued recruitment to permit comparison of themes identified by patients, nurses, and physicians.
Table 1 presents baseline characteristics of the patients. The mean age of the nurses was 34.9 9.9 years, all were female, and 60% were black. The mean age of the resident physicians was 29.1 2.2 years; 44% were female; and 78% were white, 22% were Latino, and none were black.
| Characteristic | Mean (SD) or N (%) |
|---|---|
| Age (years) | 84 (6.0) |
| Sex Male | 3 (30) |
| Female | 7 (70) |
| Race Black | 3 (30) |
| White | 7 (70) |
| Mini Mental State Examination score | 24 (3.3) |
| Number of comorbidities | 6 (2.3) |
| Number of medications at admission | 9 (2.3) |
Figure 2 presents barriers most frequently noted by participants. These barriers were similar among the 3 groups for symptoms and hospital‐related factors like catheters and lack of staff. Lack of patient motivation, lack of ambulatory devices, and medical reasons necessitating bed rest were reported more frequently by health care providers than by patients. On admission, 40% of patients had bed rest ordered. By hospital day 3, all patients had out‐of‐bed orders, and 70% had had physical therapy ordered.

Symptoms
Symptoms were spontaneously mentioned by 97% of participants (28 of 29) as a cause of limited mobility, with weakness cited most frequently: I know it is going to be painful (to walk). My head swims. This side of my all up and down to my toes is weak. When that comes, you kind of be scared. Pain and fatigue were also mentioned by more than 35% of all participants. Although 42% of health care providers (8 of 19) identified dyspnea or shortness of breath as a barrier, no patients reported this symptom as a barrier.
Medical Devices
When asked directly about the impact of urinary catheters and intravenous lines, most patients expressed the belief that the device could either be disconnected or carried and therefore was not a barrier: They can take that off [the catheter] and hook it on the wall. Only 30% of the patients described their mobility as hampered by the medical devices, and only 1 patient (10%) spontaneously indicated the catheter was a barrier: I have had that catheter hooked up to me until today. That was a relief to get that outI couldn't hardly do nothing with that.
In contrast, most health care providers (89%) believed medical devices adversely affected mobility. However, only 32% spontaneously noted the IV or catheter was a barrier, with the other 67% requiring prompting to consider the medical devices as barriers. The rationale for this belief varied widely. Some providers focused on the impact of the patient having to push the IV pole: They get tangled, they hurt when they are mobilized. You have to push the little cart, so patients actually need some assistance. Other providers mentioned the impact of a patient's medical devices on the nurse's ability to assist patients out of bed: I hate to say it, but I think on some days, it does [affect mobility]. You have patients who have TPN and blood and Foleys and chest tubes, you are probably less likely to [get people out of bed]. One physician commented on a less obvious impact of catheters: Some people might be embarrassed to walk around with that Foley catheter.
Need for Assistance and Lack of Staff
Patients, nurses, and physicians alike spontaneously identified the patient's need for assistance with ambulation as a barrier. This observation was frequently followed by comments about staff shortages and time constraints that limited the availability of staff to assist patients with ambulation. The impact of a perceived lack of staff was expressed differently by patients and health care providers. Patients frequently talked about not wanting to bother the nurses: I know it would be good for me, but I just don't want to impose upon them; I try not to worry the girls to take me walking because they have their own patients. Health care providers focused on the variety of other nursing duties that tended to take precedence over helping patients with mobility: I just don't think the nurses have time. They are too busy doing other things to walk their patients up and down the hallway. I think if you really want your patient up walking down the hall, you need to have a relative help them or physical therapy. Among staff, the most frequently mentioned solution was to refer patients to physical therapy, a strategy endorsed equally by nurses and physicians: That is why we try to encourage the doctors to order physical therapy, because we don't have time to ambulate patients in the hallway like the doctor expects.
Lack of Ambulatory Devices
The nurses frequently mentioned the absence of ambulatory devices to assist patients with walking. Nurses also expressed concern about the ability of patients to walk safely without such devices: Sometimes if a patient requires a lot of help, then I think you really need to involve physical therapy to ensure the safety of the person getting up. Because a lot of times you are limited with equipment that you need and they [physical therapists] have that equipment to make sure they don't fall. No physicians and only 1 patient cited lack of ambulatory devices as a barrier to mobility.
Fear of a Patient Falling
The potential for a fall during a hospital stay was of concern for more than 75% of the participants, with physicians spontaneously expressing concern for falls as a perceived barrier more frequently than did patients or nurses: I probably don't encourage her to move as much as I should. And when I do, I tell her to be sure to have someone with her when she is getting up. I am probably a little more protective because I am afraid she might fall. Another physician stated, I think nurses in general would prefer the patient to stay in bed. I believe they perceive it as a risk for falls and a risk for pulling out their IVs or any other medical device, and it is probably not viewed as an important factor in someone who is recovering from an illness. Another physician noted fall prevention was a secondary reason for limited mobility but also expressed the idea that bed rest was easier than mobility: While they are in bed they are not giving trouble to anybody. It is less work and, second, because of liability issues in terms of patients falling and hurting themselves while they are in the hospital. I think everybody is very concerned with that, but I think mainly because it is less work. Although 68% of health care providers spontaneously noted falls as a barrier to mobility, only 1 patient spontaneously verbalized this concern. However, with prompting to consider falls a barrier, 60% of patients stated falls were a potential barrier: As old as I am, your legs don't last long and they give away. It would be dangerous because I haven't got the strength. Now, since I've fallen, yes, I have to be careful.
Lack of Patient Motivation
Lack of patient motivation was mentioned by 50% of the nurses and 78% of the physicians and was often linked to a patient's age: I just think he is older now, and he is not as motivated as younger people are, and he has been through so much. I just don't think he wants to do it. I don't know if it is because it is the elderly or because they just seem more stubborn. They are sometimes more content to stay in the bed, and you have to really stress to them to get up. However, none of the patients indicated a lack of motivation was the reason for not getting out of bed. Indeed, they commented on the staff's apparent lack of interest or their perception that the staff did not consider increased mobility important: I don't believe they are going to get me out of bed while I am here. If I said I really needed to get out of bed, they try to do what you want them to do. But evidently they don't think it is that important.
Hospital Environment
Although not frequently mentioned, issues of an environment not conducive to mobility did emerge as a theme. Several patients expressed this environmental barrier as a difficulty with the hospital gowns that are required garb during a hospital stay. One patient wrote the following additional thoughts after reviewing her transcript: Gowns lead to embarrassing moments, are designed for benefit of staff, not patients. This sentiment was echoed by a physician who, when queried about the impact of gowns, responded, I think the gown exposes the patient a lot and they might feel embarrassed to go around. And outside the hospital room, nobody wants to be perceived as sick and draw attention. Finally, 2 physicians commented on the lack of chairs in the room and the physical setup of the room not encouraging mobility: I think that patients, when they are in the hospital, they feel they are supposed to be in bed. And they are more comfortable there and a lot of times they can see the TV better.
DISCUSSION
Many of the barriers described in the original conceptual model (Fig. 1) were cited by participants from all 3 groups: patients, nurses and physicians. These included patient‐related factors like symptoms and need for assistance, concern about falls, and lack of staff to assist with ambulation. Although attitudes toward mobility were cited in the model and by participants, there was significant disagreement between the 3 groups about the cause of the attitudinal barrier. Health care providers cited lack of patient motivation, whereas patients perceived health care providers as not being interested in mobility or viewing it as important. Health care providers frequently employed stereotypes to describe the potential reasons for the perceived attitudes toward mobility, often linking lack of motivation or interest in getting out of bed to patients being old. Patients linked the lack of importance attached to mobility to the numerous duties of staff members and believed that assistance with mobility was less important than other duties. Physicians and nurses were both more likely than patients to mention factors like urinary catheters, intravenous lines, and other medical reasons that necessitated bed rest. Although more than half the nurses commented on the lack of ambulatory devices for ambulation, no physicians and only 1 patient perceived this lack to be a barrier.
The model presented appears to have face validity, with participants citing many of the factors originally identified as barriers to mobility. The original model did not include consideration of environmental factors such as the number of chairs in the room or the location of the television. Such environmental factors can be conceptualized as institution‐related factors.
In addition, the impact of physician activity orders for bed rest was not specifically discussed by participants, although 45% of participants did comment on the need for bed rest because of a medical condition. A review of the medical records for activity orders indicated 40% of the patients initially had orders for bed rest. Another recent study demonstrated 33% of older patients were on bed rest at some point during their hospital stay10 and should be retained in the model as a treatment‐related consideration.
Several barriers noted in the original model and by participants may not be modifiable, such as comorbid conditions and illness severity. But other perceived barriers may be, and recognition of these factors present potential targets for a future multicomponent intervention to enhance hospital mobility. This multicomponent approach has been highly successful for geriatric syndromes like falls28 and delirium.29
During hospitalization, a focus on early removal of catheters and intravenous lines may encourage mobility. In a recent study absence of a urinary catheter was a predictor of patients with activity limitations regaining ambulatory ability while hospitalized.30 Availability of ambulatory devices may allow nurses to ambulate patients without consulting physical therapy. Another potential solution would be a hospitalwide walking program. The feasibility of a walking program was demonstrated in a small pilot study at a community‐based hospital using specially trained transporters to walk ward patients during slow periods. These periods included nights and weekends, when patients were more likely to be available and when physical therapy was often not present. On average, participants spent 2.4 days in the program, with an average of 5.6 walks per patient. However, additional research is needed, as the study was too small to demonstrate the effects of the walking program on length of stay or functional decline.31
Other barriers, while potentially modifiable, may be more difficult to address and may involve changing the culture of the hospital. For instance, concern about falls was a common theme, echoed by all 3 groups. Physicians were the most likely to spontaneously mention falls as a concern. The nurses shared this concern, frequently citing the use of bed rails as a part of their fall protocol, despite available literature demonstrating that this approach was not efficacious.3234 Nurses also consistently reported asking patients to call for help to ambulate, yet both patients and nurses noted a lack of nursing time to assist with ambulation. The default solution providers reported using was utilizing physical therapists, who were available to walk with patients only once or twice a day. Research on the best methods to prevent falls during hospitalization is limited. Given the current medicolegal environment and the emphasis on fall prevention by the Joint Commission on Accreditation of Healthcare Organizations and other government entities, it is not surprising that bed rest and mobility limitation are being used as a method of minimizing falls. Until data about successful fall‐prevention strategies are available, minimization of mobility may remain the default solution.
Strengths of the present study include the use of qualitative methods to explore potential barriers to mobility, a method that allows participants to describe in their own words their attitudes, beliefs, and expectations about mobility during hospitalization. Face‐to‐face interviewing of the 3 major groups involved in the hospital experience (patients, nurses, and physicians) facilitated the collection of detailed contextualized information on factors expected to affect a patient's level of mobility during hospitalization. This enabled hypothesis generation and sensitization to issues that need further quantitative investigation with larger groups.
The study also had several limitations. First, only resident physicians were included, and their answers may not reflect the opinions of other, more experienced physicians. However, resident physicians were chosen for this study because they play important roles in delivering hands‐on care in teaching hospitals and would need to be involved in any future interventions designed to enhanced patient mobility. Second, a sample size of 29 participants, approximately 10 persons in each group, may not reflect the thinking of those throughout the hospital. However, sampling was continued until no new themes or barriers emerged, and major themes emerged consistently throughout the interviews with all 3 groups. Interviews were conducted at a large, urban university hospital, and so results may not be generalizable to smaller community hospitals. Last, although the 3 major groupspatients, nurses, and physicianswere included in the interviews, the opinions of other stakeholders who may have had perspectives on mobility such as family members were not solicited and may need to be incorporated into the model.
This study presents the perceived barriers to mobility from the perspectives of patients their nurses and physicians. The modifiable and nonmodifiable factors that might affect mobility during hospitalization that made up the original theoretical model were consistent with the barriers cited by the participants. Importantly, this research has led to the identification of other barriers such as environmental factors that may also influence the mobility of older patients. This research has provided insights into potentially modifiable factors of the well‐documented phenomenon of low mobility of hospitalized older persons10 and identified several targets for a multicomponent intervention to minimize low mobility. Possible interventions include a progressive walking program initiated early in the hospital stay, provision of assistive devices to patients who need them, and early removal of catheters and intravenous lines. Further research is needed to explore other factors associated with low mobility, such as specific medical conditions for which bed rest may be ordered, and to evaluate the impact that specific interventions may have on the mobility of older persons during hospitalization.
Acknowledgements
The authors are indebted to the patients, nurses, and resident physicians at University Hospital who participated in the study; to Stephanie Stone, MBA, for her invaluable assistance with study execution; and to Robert H. Brown, MDiv, for his critical review of the manuscript.
APPENDIX
Questionnaire Guide for Patients
Attitudes toward Mobility
Tell me how much walking do you do at home when you are not sick.
Do you leave your bedroom when you are at home? Do you need help to do this?
Do you go out of the house when you are at home? Do you need help to do this?
Do you go out of your neighborhood when you are at home? Do you need help to do this?
Tell me what you think about getting out of bed and moving around during this hospitalization? Do you think it is a good idea or a bad one?
How important do you believe it is for you to rest while you are in the hospital? Why did you choose that answer?
How important do you believe it is for you to walk while you are in the hospital? Why did you choose that answer?
Do you believe it to be dangerous for you to get up out of bed and walk? Why or why not?
Do you think your doctor wants you to get out of bed and walk? Why or why not?
Do you think your nurse wants you to get out of bed and walk? Why or why not?
Expectations of Care about Walking/Mobility
Can you tell me what you believe the nurse is supposed to do for you while you are in the hospital?
When you leave the hospital, do you believe you will be able to walk on your own, or will you need help?
Needs help: Why do you believe you will need help? What type of help will you need?
No help needed: Why do you believe you will not need help?
When you leave the hospital, do you believe you will be able to care for yourself, or will you need help? Needs help: Why do you believe you will need help? What type of help will you need?
No help needed: Why do you believe you will not need help?
If you needed help, who would be available to help you when you go home?
Person Factors That Influence Mobility
Tell me about the illness that brought you into the hospital.
How serious do you believe your illness to be? Why do you believe that?
How easy will it be for you to get better from this illness? Why do you believe that?
Situational Factors That Influence Mobility
Do you have any thoughts about what might make it easier for you to get out of bed and walk more frequently or for longer periods than you are now?
If you decided to go to the bathroom, would you call for help?
Why did you choose that answer?
Perceived Barriers
Tell me what, if anything, would prevent you from getting out of bed and walking during your hospital stay?
Do you have any concerns about falling during your hospital stay?
Are there other factors that influence if you will walk during your hospitalization that I haven't asked about?
New Questions
As this is a qualitative study, if participants bring up new topics that have not been previously explored with the questionnaire guide, these questions will be added to the questionnaire. Future participants will be asked the new questions.
- ,,.Predictors of functional decline in hospitalized elderly patients: a systematic review.J Gerontol Med Sci.2002;57A:M569–M577.
- ,,, et al.Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age.J Am Geriatr Soc.2003;51:451–458.
- ,,, et al.Functional outcomes of acute medical illness and hospitalization in older persons.Arch Intern Med.1996;156:645–652.
- ,,,,.Effect of a geriatric consultation team on functional status of elderly hospitalized patients.Ann Intern Med.1989;110:79–84.
- ,,, et al.Functional disability in the hospitalized elderly.JAMA.1982;248:847–850.
- ,,, et al.Predictors of immediate and 6‐month outcomes in hospitalized elderly patients.J Am Geriatr Soc.1988;36:775–783.
- ,,.Adverse consequences of hospitalization in the elderly.Soc Sci Med.1982;16:1033–1038.
- ,.Complications of illness in geriatric patients in hospital.J Chronic Dis.1966;19:307–313.
- ,,,,.The provision of physical activity to hospitalized elderly patients.Arch Intern Med.1991;151:2452–2456.
- ,,.Prevalence and outcomes of low mobility in hospitalized older patients.J Am Geriatr Soc.2004;52:1263–1270.
- ,,.Cost effectiveness of accelerated rehabilitation after proximal femoral fracture.J Clin Epidemiol.1994;47:1307–1313.
- ,,,,.Early inpatient rehabilitation after elective hip and knee arthroplasty.JAMA.1998;279:847–852.
- ,,,,.Time to ambulation after hip fracture surgery: relation to hospitalization outcomes.J Gerontol.2003;58A:1042–1045.
- ,,, et al.Controlled trial of early mobilization and discharge from hospital in uncomplicated myocardial infarction.Lancet.1971;2:1359–1360.
- ,,,,.Value of early ambulation in patients with and without complications after acute myocardial infarction.N Engl J Med.1975;292:719–722.
- ,,.Bed rest: a potentially harmful treatment needing more careful evaluation.Lancet.1999;354:1229–1233.
- ,,, et al.Early ambulation after 5 French diagnostic cardiac catheterization: results of a multicenter trial.J Am Coll Cardiol.1990;15:1475–1483.
- ,,, et al.Position and mobilisation post‐angiography study (PAMPAS): a comparison of 4.5 hours and 2.5 hours bed rest.Hear.2003;89:447–448.
- ,,, et al.Bed rest or ambulation in the initial treatment of patients with acute deep vein thrombosis or pulmonary embolism: findings from the RIETE Registry.Chest.2005;127:1631–1636.
- ,,,,.Early mobilization of patients hospitalized with community‐acquired pneumonia.Chest.2003;124:883–889.
- .Hazards of hospitalization of the elderly.Ann Intern Med.1993;118:219–223.
- ,.Physiology and complications of bedrest.J Am Geriatr Soc.1988;36:1047–1054.
- ,.Hospital‐associated deconditioning and dysfunction.J Am Geriatr Soc.1991;39:220–222.
- ,,.“Mini‐mental state.” A practical method for grading the cognitive status of patients for the clinician.J Psychiatr Rev.1975;12:189–198.
- ,,,,,.Clarifying confusion: the confusion assessment method. A new method for detection of delirium.Ann Intern Med.1990;113:941–948.
- ,.The Discovery of Grounded Theory. Strategies for Qualitative Research.Chicago, IL:Aldine;1967.
- ,,.How many interviews are enough?Field Methods.2006;18:59–82.
- ,,, et al.A multifactorial intervention to reduce the risk of falling among elderly people living in the community.N Engl J Med.1994;331:821–827.
- ,,, et al.A multicomponent intervention to prevent delirium in hospitalized older patients.N Engl J Med.1999;340:669–676.
- ,,,.Predictors of regaining ambulatory ability during hospitalization.J Hosp Med.2006;1:277–284.
- ,,.Walking for Wellness: a collaborative program to maintain mobility in hospitalized older adults.Geriatr Nurs.2004;25:242–245.
- ,,.Use of restraints and bed rails in a British hospital.J Am Geriatr Soc.1996;44:1086–1088.
- ,,.An analysis of falls in the hospital: can we do without bedrails?J Am Geriatr Soc.1999;47:529–31.
- ,,,.Side rail use and bed‐related outcomes among nursing home residents.J Am Geriatr Soc.2002;50:90–96.
- ,,.Predictors of functional decline in hospitalized elderly patients: a systematic review.J Gerontol Med Sci.2002;57A:M569–M577.
- ,,, et al.Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age.J Am Geriatr Soc.2003;51:451–458.
- ,,, et al.Functional outcomes of acute medical illness and hospitalization in older persons.Arch Intern Med.1996;156:645–652.
- ,,,,.Effect of a geriatric consultation team on functional status of elderly hospitalized patients.Ann Intern Med.1989;110:79–84.
- ,,, et al.Functional disability in the hospitalized elderly.JAMA.1982;248:847–850.
- ,,, et al.Predictors of immediate and 6‐month outcomes in hospitalized elderly patients.J Am Geriatr Soc.1988;36:775–783.
- ,,.Adverse consequences of hospitalization in the elderly.Soc Sci Med.1982;16:1033–1038.
- ,.Complications of illness in geriatric patients in hospital.J Chronic Dis.1966;19:307–313.
- ,,,,.The provision of physical activity to hospitalized elderly patients.Arch Intern Med.1991;151:2452–2456.
- ,,.Prevalence and outcomes of low mobility in hospitalized older patients.J Am Geriatr Soc.2004;52:1263–1270.
- ,,.Cost effectiveness of accelerated rehabilitation after proximal femoral fracture.J Clin Epidemiol.1994;47:1307–1313.
- ,,,,.Early inpatient rehabilitation after elective hip and knee arthroplasty.JAMA.1998;279:847–852.
- ,,,,.Time to ambulation after hip fracture surgery: relation to hospitalization outcomes.J Gerontol.2003;58A:1042–1045.
- ,,, et al.Controlled trial of early mobilization and discharge from hospital in uncomplicated myocardial infarction.Lancet.1971;2:1359–1360.
- ,,,,.Value of early ambulation in patients with and without complications after acute myocardial infarction.N Engl J Med.1975;292:719–722.
- ,,.Bed rest: a potentially harmful treatment needing more careful evaluation.Lancet.1999;354:1229–1233.
- ,,, et al.Early ambulation after 5 French diagnostic cardiac catheterization: results of a multicenter trial.J Am Coll Cardiol.1990;15:1475–1483.
- ,,, et al.Position and mobilisation post‐angiography study (PAMPAS): a comparison of 4.5 hours and 2.5 hours bed rest.Hear.2003;89:447–448.
- ,,, et al.Bed rest or ambulation in the initial treatment of patients with acute deep vein thrombosis or pulmonary embolism: findings from the RIETE Registry.Chest.2005;127:1631–1636.
- ,,,,.Early mobilization of patients hospitalized with community‐acquired pneumonia.Chest.2003;124:883–889.
- .Hazards of hospitalization of the elderly.Ann Intern Med.1993;118:219–223.
- ,.Physiology and complications of bedrest.J Am Geriatr Soc.1988;36:1047–1054.
- ,.Hospital‐associated deconditioning and dysfunction.J Am Geriatr Soc.1991;39:220–222.
- ,,.“Mini‐mental state.” A practical method for grading the cognitive status of patients for the clinician.J Psychiatr Rev.1975;12:189–198.
- ,,,,,.Clarifying confusion: the confusion assessment method. A new method for detection of delirium.Ann Intern Med.1990;113:941–948.
- ,.The Discovery of Grounded Theory. Strategies for Qualitative Research.Chicago, IL:Aldine;1967.
- ,,.How many interviews are enough?Field Methods.2006;18:59–82.
- ,,, et al.A multifactorial intervention to reduce the risk of falling among elderly people living in the community.N Engl J Med.1994;331:821–827.
- ,,, et al.A multicomponent intervention to prevent delirium in hospitalized older patients.N Engl J Med.1999;340:669–676.
- ,,,.Predictors of regaining ambulatory ability during hospitalization.J Hosp Med.2006;1:277–284.
- ,,.Walking for Wellness: a collaborative program to maintain mobility in hospitalized older adults.Geriatr Nurs.2004;25:242–245.
- ,,.Use of restraints and bed rails in a British hospital.J Am Geriatr Soc.1996;44:1086–1088.
- ,,.An analysis of falls in the hospital: can we do without bedrails?J Am Geriatr Soc.1999;47:529–31.
- ,,,.Side rail use and bed‐related outcomes among nursing home residents.J Am Geriatr Soc.2002;50:90–96.
Copyright © 2007 Society of Hospital Medicine