User login
Intraoral lesion • history of cirrhosis and smoking • Dx?
THE CASE
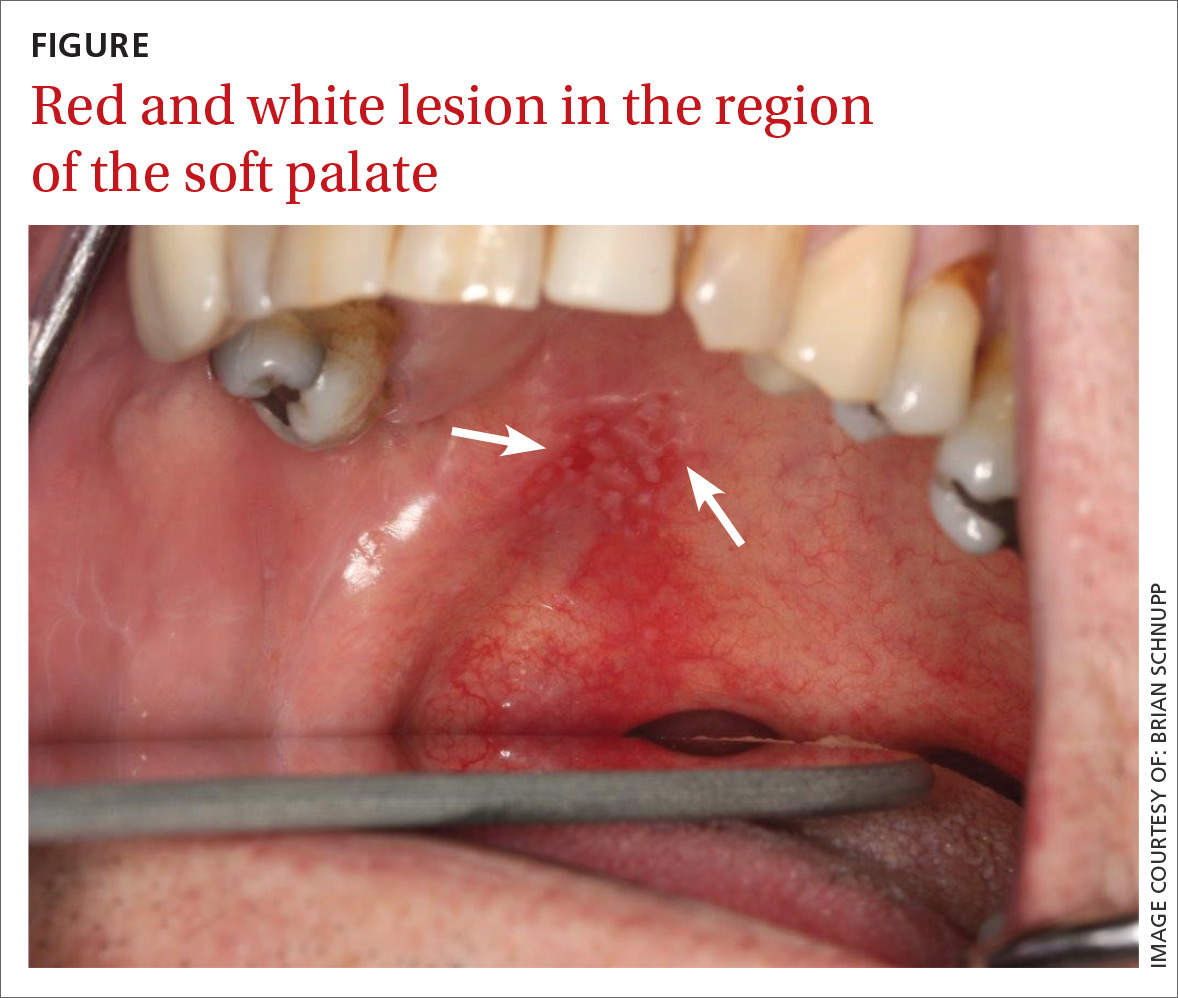
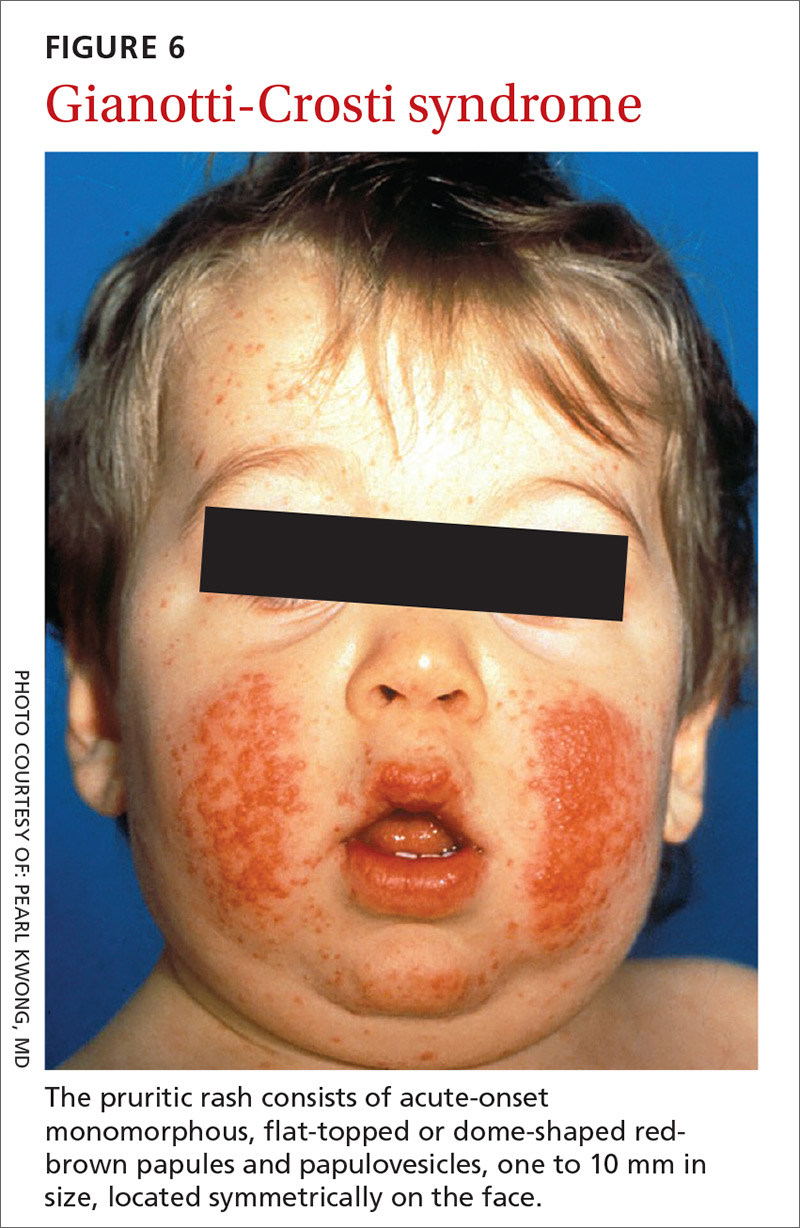
A 56-year-old white man presented at our dental clinic for routine care. The intraoral examination revealed an asymptomatic red lesion with white vesicle-like areas on the right side of the soft palate (FIGURE). The extraoral examination was normal, and regional lymph nodes were nonpalpable. The patient’s medical history included liver cirrhosis and pancreatitis. He also had a 30-year history of alcohol misuse (1-5 drinks per day) and a 30-pack-year smoking history. (The patient had stopped drinking at the time of presentation, and had quit smoking 2 years earlier.) We instructed him to gargle with warm salt water at home and return in 2 weeks. At follow-up, the lesion was unresolved, so a biopsy was performed.
THE DIAGNOSIS
The clinical diagnosis was erythroplakia. Trauma from food burn and inflammation of the salivary gland were both considered, but ultimately ruled out due to lack of symptoms and persistence of the lesion after 14 days. The pathology report confirmed a diagnosis of squamous cell carcinoma (SCC) in situ. Based on the pathology report, we referred the patient to an oral surgeon for wide surgical excision with evaluation of the margins.
Because of its location and subtle presentation, the lesion could have been easily overlooked, underscoring the importance of routinely going beyond dentition to examine the soft tissues of the mouth.
DISCUSSION
SCC is the most common cancer found in the oral cavity, accounting for 90% of all oral malignancies.1,2 Other malignancies include lymphomas, sarcomas, melanomas, salivary gland neoplasms, and metastasis from other sites.3,4 Predisposing factors include tobacco use (namely inhaled methods and chewing tobacco), alcohol misuse, human papillomavirus infection, and chewing betel nut.1,5 (Betel nuts grow on a species of palm tree mainly found in India, Pakistan, and Bangladesh. They are commonly chewed for their caffeine-like effect and are known to be carcinogenic.)
Presentation. SCC of the oral cavity can have various presentations. The lesion can appear as white, red, a mix of white and red, as a mass, or as a nonhealing ulcer. While some patients may be asymptomatic (as was ours), some may have signs and symptoms such as pain, bleeding, difficulty swallowing, difficulty wearing dentures, or a neck mass.6 A history of smoking and alcohol misuse, which was present in this case, should heighten suspicion and prompt further investigation of oral lesions.
Location. The most common intraoral site for oral cancer is the tongue (on the posterolateral border) followed by the floor of the mouth. Other common sites in descending order are the soft palate, gingiva, buccal mucosa, labial mucosa, and hard palate.1 (Our patient’s lesion was on the border of the hard and soft palate).
Treatment of oral cancer is surgical. In some cases, depending on the stage and size of the tumor, radiation and chemotherapy may be considered.3,5 Approximately two-thirds of oral cancers are detected in the later stages.7 The 5-year survival rate for people with oral SCC found at stages III or IV ranges from 32% to 45%, while the rate for those with SCC detected at stages I or II is 58% to 72%.1 Patients with a history of oral cancer have a 20-fold increased risk of a recurrence in the oral cavity or of developing cancer in the surrounding areas, such as the larynx, esophagus, and lungs, underscoring the necessity of adequate follow-up in these patients.2,3,5
Who is at risk?
In 2015, there were an estimated 45,780 new cases of oral cavity and pharyngeal cancer and 8650 deaths from these causes.8 Although oral cancer accounts for only 3% of all cancers in the United States, it is the eighth most common cancer in males and the 15th most common in females.1 Prevalence differs tremendously by location, however. In India, for example, oral cancer accounts for 30% of all cancers.9 Regardless of location, incidence increases with age; 62 is the average age at diagnosis.2 Oral cancers are also more common among African Americans than among Caucasians.1,3,5
Smokers are 2 to 3 times more likely to develop oral cancer than nonsmokers.1 This risk increases with amount and duration of smoking.1 The combination of smoking and alcohol use has a synergistic effect, increasing the likelihood of developing oral cancer 15 fold.1,3
Alcohol use. Among male patients with oral cancer, one-third are heavy alcohol users.1 In fact, one study found that 20% of these patients have cirrhosis of the liver.1 Thus, it makes good clinical sense to routinely examine the soft tissue of the oral cavity for abnormalities in patients with alcohol-induced cirrhosis of the liver.
Our patient. We placed our patient on a 3-month recall and stressed the importance of not smoking. The patient had surgery and a good outcome was documented. The patient indicated at follow-up that he’d started drinking again and was referred for counseling.
TAKEAWAY
It’s important to pay attention to color differences in the oral cavity on routine visits, particularly in patients with known risk factors for SCC. Patients with a lesion in the oral cavity should be seen again within 2 weeks. If the lesion is unresolved, the patient should be referred for further examination and/or biopsy. The possibility of recurrent oral cancer or cancer in the surrounding areas makes these patients good candidates for frequent follow-up examinations.
We strongly suggest that primary care physicians encourage their patients with the known predisposing risk factors of tobacco use and chronic alcohol misuse to quit these habits, visit their dentists for annual oral cancer screenings, and report any oral symptoms promptly to their medical and/or dental care providers. The asymptomatic nature of many of these lesions underscores the importance of following this advice. As is the case with most other cancers, survival rate is dependent on the stage of the disease at diagnosis.
1. Neville BW, Damm DD, Allen CM, et al. Oral and Maxillofacial Pathology. 4th ed. Philadelphia, PA: Elsevier, Inc; 2016:374-388.
2. American Cancer Society. What are the key statistics about oral cavity and oropharyngeal cancer? Available at: https://www.cancer.org/cancer/oral-cavity-and-oropharyngeal-cancer/about/key-statistics.html. Accessed August 28, 2017.
3. The Oral Cancer Foundation. Oral cancer facts. Available at: http://oralcancerfoundation.org/facts/. Accessed August 28, 2017.
4. Zini A, Czerninski R, Sqan-Cohen HD. Oral cancer over four decades: epidemiology, trends, histology, and survival by anatomical sites. J Oral Pathol Med. 2010;39:299-305.
5. National Institute of Health. National Cancer Institute. Oral Cavity and Oropharyngeal Cancer Screening (PDQ®)–Patient Version. Available at: https://www.cancer.gov/types/head-and-neck/patient/oral-screening-pdq. Accessed August 28, 2017.
6. Groome PA, Rohland SL, Hall SF, et al. A population-based study of factors associated with early versus late stage oral cavity cancer diagnoses. Oral Oncol. 2011;47:642-647.
7. Dodd VJ, Schenck DP, Chaney EH, et al. Assessing oral cancer awareness among rural Latino migrant workers. J Immigr Minor Health. 2016;18:552-560.
8. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29.
9. Coelho KR. Challenges of the oral cancer burden in India. J Cancer Epidemiol. 2012;2012:701932.
THE CASE
A 56-year-old white man presented at our dental clinic for routine care. The intraoral examination revealed an asymptomatic red lesion with white vesicle-like areas on the right side of the soft palate (FIGURE). The extraoral examination was normal, and regional lymph nodes were nonpalpable. The patient’s medical history included liver cirrhosis and pancreatitis. He also had a 30-year history of alcohol misuse (1-5 drinks per day) and a 30-pack-year smoking history. (The patient had stopped drinking at the time of presentation, and had quit smoking 2 years earlier.) We instructed him to gargle with warm salt water at home and return in 2 weeks. At follow-up, the lesion was unresolved, so a biopsy was performed.
THE DIAGNOSIS
The clinical diagnosis was erythroplakia. Trauma from food burn and inflammation of the salivary gland were both considered, but ultimately ruled out due to lack of symptoms and persistence of the lesion after 14 days. The pathology report confirmed a diagnosis of squamous cell carcinoma (SCC) in situ. Based on the pathology report, we referred the patient to an oral surgeon for wide surgical excision with evaluation of the margins.
Because of its location and subtle presentation, the lesion could have been easily overlooked, underscoring the importance of routinely going beyond dentition to examine the soft tissues of the mouth.
DISCUSSION
SCC is the most common cancer found in the oral cavity, accounting for 90% of all oral malignancies.1,2 Other malignancies include lymphomas, sarcomas, melanomas, salivary gland neoplasms, and metastasis from other sites.3,4 Predisposing factors include tobacco use (namely inhaled methods and chewing tobacco), alcohol misuse, human papillomavirus infection, and chewing betel nut.1,5 (Betel nuts grow on a species of palm tree mainly found in India, Pakistan, and Bangladesh. They are commonly chewed for their caffeine-like effect and are known to be carcinogenic.)
Presentation. SCC of the oral cavity can have various presentations. The lesion can appear as white, red, a mix of white and red, as a mass, or as a nonhealing ulcer. While some patients may be asymptomatic (as was ours), some may have signs and symptoms such as pain, bleeding, difficulty swallowing, difficulty wearing dentures, or a neck mass.6 A history of smoking and alcohol misuse, which was present in this case, should heighten suspicion and prompt further investigation of oral lesions.
Location. The most common intraoral site for oral cancer is the tongue (on the posterolateral border) followed by the floor of the mouth. Other common sites in descending order are the soft palate, gingiva, buccal mucosa, labial mucosa, and hard palate.1 (Our patient’s lesion was on the border of the hard and soft palate).
Treatment of oral cancer is surgical. In some cases, depending on the stage and size of the tumor, radiation and chemotherapy may be considered.3,5 Approximately two-thirds of oral cancers are detected in the later stages.7 The 5-year survival rate for people with oral SCC found at stages III or IV ranges from 32% to 45%, while the rate for those with SCC detected at stages I or II is 58% to 72%.1 Patients with a history of oral cancer have a 20-fold increased risk of a recurrence in the oral cavity or of developing cancer in the surrounding areas, such as the larynx, esophagus, and lungs, underscoring the necessity of adequate follow-up in these patients.2,3,5
Who is at risk?
In 2015, there were an estimated 45,780 new cases of oral cavity and pharyngeal cancer and 8650 deaths from these causes.8 Although oral cancer accounts for only 3% of all cancers in the United States, it is the eighth most common cancer in males and the 15th most common in females.1 Prevalence differs tremendously by location, however. In India, for example, oral cancer accounts for 30% of all cancers.9 Regardless of location, incidence increases with age; 62 is the average age at diagnosis.2 Oral cancers are also more common among African Americans than among Caucasians.1,3,5
Smokers are 2 to 3 times more likely to develop oral cancer than nonsmokers.1 This risk increases with amount and duration of smoking.1 The combination of smoking and alcohol use has a synergistic effect, increasing the likelihood of developing oral cancer 15 fold.1,3
Alcohol use. Among male patients with oral cancer, one-third are heavy alcohol users.1 In fact, one study found that 20% of these patients have cirrhosis of the liver.1 Thus, it makes good clinical sense to routinely examine the soft tissue of the oral cavity for abnormalities in patients with alcohol-induced cirrhosis of the liver.
Our patient. We placed our patient on a 3-month recall and stressed the importance of not smoking. The patient had surgery and a good outcome was documented. The patient indicated at follow-up that he’d started drinking again and was referred for counseling.
TAKEAWAY
It’s important to pay attention to color differences in the oral cavity on routine visits, particularly in patients with known risk factors for SCC. Patients with a lesion in the oral cavity should be seen again within 2 weeks. If the lesion is unresolved, the patient should be referred for further examination and/or biopsy. The possibility of recurrent oral cancer or cancer in the surrounding areas makes these patients good candidates for frequent follow-up examinations.
We strongly suggest that primary care physicians encourage their patients with the known predisposing risk factors of tobacco use and chronic alcohol misuse to quit these habits, visit their dentists for annual oral cancer screenings, and report any oral symptoms promptly to their medical and/or dental care providers. The asymptomatic nature of many of these lesions underscores the importance of following this advice. As is the case with most other cancers, survival rate is dependent on the stage of the disease at diagnosis.
THE CASE
A 56-year-old white man presented at our dental clinic for routine care. The intraoral examination revealed an asymptomatic red lesion with white vesicle-like areas on the right side of the soft palate (FIGURE). The extraoral examination was normal, and regional lymph nodes were nonpalpable. The patient’s medical history included liver cirrhosis and pancreatitis. He also had a 30-year history of alcohol misuse (1-5 drinks per day) and a 30-pack-year smoking history. (The patient had stopped drinking at the time of presentation, and had quit smoking 2 years earlier.) We instructed him to gargle with warm salt water at home and return in 2 weeks. At follow-up, the lesion was unresolved, so a biopsy was performed.
THE DIAGNOSIS
The clinical diagnosis was erythroplakia. Trauma from food burn and inflammation of the salivary gland were both considered, but ultimately ruled out due to lack of symptoms and persistence of the lesion after 14 days. The pathology report confirmed a diagnosis of squamous cell carcinoma (SCC) in situ. Based on the pathology report, we referred the patient to an oral surgeon for wide surgical excision with evaluation of the margins.
Because of its location and subtle presentation, the lesion could have been easily overlooked, underscoring the importance of routinely going beyond dentition to examine the soft tissues of the mouth.
DISCUSSION
SCC is the most common cancer found in the oral cavity, accounting for 90% of all oral malignancies.1,2 Other malignancies include lymphomas, sarcomas, melanomas, salivary gland neoplasms, and metastasis from other sites.3,4 Predisposing factors include tobacco use (namely inhaled methods and chewing tobacco), alcohol misuse, human papillomavirus infection, and chewing betel nut.1,5 (Betel nuts grow on a species of palm tree mainly found in India, Pakistan, and Bangladesh. They are commonly chewed for their caffeine-like effect and are known to be carcinogenic.)
Presentation. SCC of the oral cavity can have various presentations. The lesion can appear as white, red, a mix of white and red, as a mass, or as a nonhealing ulcer. While some patients may be asymptomatic (as was ours), some may have signs and symptoms such as pain, bleeding, difficulty swallowing, difficulty wearing dentures, or a neck mass.6 A history of smoking and alcohol misuse, which was present in this case, should heighten suspicion and prompt further investigation of oral lesions.
Location. The most common intraoral site for oral cancer is the tongue (on the posterolateral border) followed by the floor of the mouth. Other common sites in descending order are the soft palate, gingiva, buccal mucosa, labial mucosa, and hard palate.1 (Our patient’s lesion was on the border of the hard and soft palate).
Treatment of oral cancer is surgical. In some cases, depending on the stage and size of the tumor, radiation and chemotherapy may be considered.3,5 Approximately two-thirds of oral cancers are detected in the later stages.7 The 5-year survival rate for people with oral SCC found at stages III or IV ranges from 32% to 45%, while the rate for those with SCC detected at stages I or II is 58% to 72%.1 Patients with a history of oral cancer have a 20-fold increased risk of a recurrence in the oral cavity or of developing cancer in the surrounding areas, such as the larynx, esophagus, and lungs, underscoring the necessity of adequate follow-up in these patients.2,3,5
Who is at risk?
In 2015, there were an estimated 45,780 new cases of oral cavity and pharyngeal cancer and 8650 deaths from these causes.8 Although oral cancer accounts for only 3% of all cancers in the United States, it is the eighth most common cancer in males and the 15th most common in females.1 Prevalence differs tremendously by location, however. In India, for example, oral cancer accounts for 30% of all cancers.9 Regardless of location, incidence increases with age; 62 is the average age at diagnosis.2 Oral cancers are also more common among African Americans than among Caucasians.1,3,5
Smokers are 2 to 3 times more likely to develop oral cancer than nonsmokers.1 This risk increases with amount and duration of smoking.1 The combination of smoking and alcohol use has a synergistic effect, increasing the likelihood of developing oral cancer 15 fold.1,3
Alcohol use. Among male patients with oral cancer, one-third are heavy alcohol users.1 In fact, one study found that 20% of these patients have cirrhosis of the liver.1 Thus, it makes good clinical sense to routinely examine the soft tissue of the oral cavity for abnormalities in patients with alcohol-induced cirrhosis of the liver.
Our patient. We placed our patient on a 3-month recall and stressed the importance of not smoking. The patient had surgery and a good outcome was documented. The patient indicated at follow-up that he’d started drinking again and was referred for counseling.
TAKEAWAY
It’s important to pay attention to color differences in the oral cavity on routine visits, particularly in patients with known risk factors for SCC. Patients with a lesion in the oral cavity should be seen again within 2 weeks. If the lesion is unresolved, the patient should be referred for further examination and/or biopsy. The possibility of recurrent oral cancer or cancer in the surrounding areas makes these patients good candidates for frequent follow-up examinations.
We strongly suggest that primary care physicians encourage their patients with the known predisposing risk factors of tobacco use and chronic alcohol misuse to quit these habits, visit their dentists for annual oral cancer screenings, and report any oral symptoms promptly to their medical and/or dental care providers. The asymptomatic nature of many of these lesions underscores the importance of following this advice. As is the case with most other cancers, survival rate is dependent on the stage of the disease at diagnosis.
1. Neville BW, Damm DD, Allen CM, et al. Oral and Maxillofacial Pathology. 4th ed. Philadelphia, PA: Elsevier, Inc; 2016:374-388.
2. American Cancer Society. What are the key statistics about oral cavity and oropharyngeal cancer? Available at: https://www.cancer.org/cancer/oral-cavity-and-oropharyngeal-cancer/about/key-statistics.html. Accessed August 28, 2017.
3. The Oral Cancer Foundation. Oral cancer facts. Available at: http://oralcancerfoundation.org/facts/. Accessed August 28, 2017.
4. Zini A, Czerninski R, Sqan-Cohen HD. Oral cancer over four decades: epidemiology, trends, histology, and survival by anatomical sites. J Oral Pathol Med. 2010;39:299-305.
5. National Institute of Health. National Cancer Institute. Oral Cavity and Oropharyngeal Cancer Screening (PDQ®)–Patient Version. Available at: https://www.cancer.gov/types/head-and-neck/patient/oral-screening-pdq. Accessed August 28, 2017.
6. Groome PA, Rohland SL, Hall SF, et al. A population-based study of factors associated with early versus late stage oral cavity cancer diagnoses. Oral Oncol. 2011;47:642-647.
7. Dodd VJ, Schenck DP, Chaney EH, et al. Assessing oral cancer awareness among rural Latino migrant workers. J Immigr Minor Health. 2016;18:552-560.
8. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29.
9. Coelho KR. Challenges of the oral cancer burden in India. J Cancer Epidemiol. 2012;2012:701932.
1. Neville BW, Damm DD, Allen CM, et al. Oral and Maxillofacial Pathology. 4th ed. Philadelphia, PA: Elsevier, Inc; 2016:374-388.
2. American Cancer Society. What are the key statistics about oral cavity and oropharyngeal cancer? Available at: https://www.cancer.org/cancer/oral-cavity-and-oropharyngeal-cancer/about/key-statistics.html. Accessed August 28, 2017.
3. The Oral Cancer Foundation. Oral cancer facts. Available at: http://oralcancerfoundation.org/facts/. Accessed August 28, 2017.
4. Zini A, Czerninski R, Sqan-Cohen HD. Oral cancer over four decades: epidemiology, trends, histology, and survival by anatomical sites. J Oral Pathol Med. 2010;39:299-305.
5. National Institute of Health. National Cancer Institute. Oral Cavity and Oropharyngeal Cancer Screening (PDQ®)–Patient Version. Available at: https://www.cancer.gov/types/head-and-neck/patient/oral-screening-pdq. Accessed August 28, 2017.
6. Groome PA, Rohland SL, Hall SF, et al. A population-based study of factors associated with early versus late stage oral cavity cancer diagnoses. Oral Oncol. 2011;47:642-647.
7. Dodd VJ, Schenck DP, Chaney EH, et al. Assessing oral cancer awareness among rural Latino migrant workers. J Immigr Minor Health. 2016;18:552-560.
8. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29.
9. Coelho KR. Challenges of the oral cancer burden in India. J Cancer Epidemiol. 2012;2012:701932.
Is obesity a disease?
It depends on whom you ask. But if you ask me, obesity should not be labeled a disease.
I understand the rationale for calling obesity a disease—it helps legitimize the time we spend treating obesity and aids in getting paid for that time. Some people have distinct diseases, such as Prader-Willi syndrome, hypothyroidism, and Cushing’s syndrome that can cause obesity, and perhaps massive obesity is best categorized and treated as a disease. But the “garden variety” obesity that affects nearly 40% of the US adult population1 behaves more like a risk factor than a disease. Think of other continuous variables like blood pressure and cholesterol—the higher the measurement, the higher the risk of a plethora of medical problems.
Obesity is a global public health problem that is due largely—at least in this country—to the widespread availability of inexpensive, calorie-packed foods, as well as a desire by a screen-addicted society to stay home and “play” online rather than outdoors. Obesity is a health risk factor produced by our current social milieu and modified by genetics and personal health habits.
So what can we do? We need to recognize our limited, but important, role and remain nonjudgmental with our overweight and obese patients when they are unsuccessful at losing weight. It is easy to play the blame game, even in subtle ways. Recognizing that obesity is more of a social issue than a personal behavioral issue is a great place to start. Asking patients what they want to do and helping them set goals and find the resources to reach their goals can be helpful. Celebrating even small decreases in weight or increases in physical activity is always good medicine. Remember that a 5% to 10% weight loss has medically beneficial effects, especially for patients with diabetes.2
In addition to recommendations (and referrals) to help patients reduce calories and increase exercise, we have other weight-loss tools to draw upon. Gastric bypass surgery is certainly effective—especially for obese patients with diabetes. And
So whether you consider obesity a disease, or not, we now have even more ways with which to combat it.
1. Ogden CL, Carroll MD, Fryar CD, et al. Prevalence of obesity among adults and youth: United States, 2011-2014. NCHS Data Brief. 2015;219:1-8.
2. Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481-1486.
It depends on whom you ask. But if you ask me, obesity should not be labeled a disease.
I understand the rationale for calling obesity a disease—it helps legitimize the time we spend treating obesity and aids in getting paid for that time. Some people have distinct diseases, such as Prader-Willi syndrome, hypothyroidism, and Cushing’s syndrome that can cause obesity, and perhaps massive obesity is best categorized and treated as a disease. But the “garden variety” obesity that affects nearly 40% of the US adult population1 behaves more like a risk factor than a disease. Think of other continuous variables like blood pressure and cholesterol—the higher the measurement, the higher the risk of a plethora of medical problems.
Obesity is a global public health problem that is due largely—at least in this country—to the widespread availability of inexpensive, calorie-packed foods, as well as a desire by a screen-addicted society to stay home and “play” online rather than outdoors. Obesity is a health risk factor produced by our current social milieu and modified by genetics and personal health habits.
So what can we do? We need to recognize our limited, but important, role and remain nonjudgmental with our overweight and obese patients when they are unsuccessful at losing weight. It is easy to play the blame game, even in subtle ways. Recognizing that obesity is more of a social issue than a personal behavioral issue is a great place to start. Asking patients what they want to do and helping them set goals and find the resources to reach their goals can be helpful. Celebrating even small decreases in weight or increases in physical activity is always good medicine. Remember that a 5% to 10% weight loss has medically beneficial effects, especially for patients with diabetes.2
In addition to recommendations (and referrals) to help patients reduce calories and increase exercise, we have other weight-loss tools to draw upon. Gastric bypass surgery is certainly effective—especially for obese patients with diabetes. And
So whether you consider obesity a disease, or not, we now have even more ways with which to combat it.
It depends on whom you ask. But if you ask me, obesity should not be labeled a disease.
I understand the rationale for calling obesity a disease—it helps legitimize the time we spend treating obesity and aids in getting paid for that time. Some people have distinct diseases, such as Prader-Willi syndrome, hypothyroidism, and Cushing’s syndrome that can cause obesity, and perhaps massive obesity is best categorized and treated as a disease. But the “garden variety” obesity that affects nearly 40% of the US adult population1 behaves more like a risk factor than a disease. Think of other continuous variables like blood pressure and cholesterol—the higher the measurement, the higher the risk of a plethora of medical problems.
Obesity is a global public health problem that is due largely—at least in this country—to the widespread availability of inexpensive, calorie-packed foods, as well as a desire by a screen-addicted society to stay home and “play” online rather than outdoors. Obesity is a health risk factor produced by our current social milieu and modified by genetics and personal health habits.
So what can we do? We need to recognize our limited, but important, role and remain nonjudgmental with our overweight and obese patients when they are unsuccessful at losing weight. It is easy to play the blame game, even in subtle ways. Recognizing that obesity is more of a social issue than a personal behavioral issue is a great place to start. Asking patients what they want to do and helping them set goals and find the resources to reach their goals can be helpful. Celebrating even small decreases in weight or increases in physical activity is always good medicine. Remember that a 5% to 10% weight loss has medically beneficial effects, especially for patients with diabetes.2
In addition to recommendations (and referrals) to help patients reduce calories and increase exercise, we have other weight-loss tools to draw upon. Gastric bypass surgery is certainly effective—especially for obese patients with diabetes. And
So whether you consider obesity a disease, or not, we now have even more ways with which to combat it.
1. Ogden CL, Carroll MD, Fryar CD, et al. Prevalence of obesity among adults and youth: United States, 2011-2014. NCHS Data Brief. 2015;219:1-8.
2. Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481-1486.
1. Ogden CL, Carroll MD, Fryar CD, et al. Prevalence of obesity among adults and youth: United States, 2011-2014. NCHS Data Brief. 2015;219:1-8.
2. Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481-1486.
Posttraumatic stress disorder: Often missed in primary care
THE CASE
DeSean W,* a 47-year-old man, returned to his primary care clinic with a new complaint of epigastric burning that had been bothering him for the past 4 months. He had tried several over-the-counter remedies, which provided no relief. He also remained concerned—despite assurances to the contrary at previous clinic visits—that he had contracted a sexually-transmitted disease (STD) after going to a bar one night 4 to 5 months ago. At 2 other clinic visits since that time, STD test results were negative. At this current visit, symptoms and details of sexual history were unchanged since the last visit, with the exception of the epigastric pain.
When asked if he thought he had contracted an STD through a sexual encounter the night he went to the bar, he emphatically said he would not cheat on his wife. Surprisingly, given his concern, he avoided further discussion on modes of contracting an STD.
The physician prescribed ranitidine 150 mg bid for the epigastric burning and explained, once more, the significance of the STD test results. However, he also decided to further examine Mr. W’s concern about STDs and the night he may have contracted one.
HOW WOULD YOU PROCEED WITH THIS PATIENT?
*The patient’s name has been changed to protect his privacy.
SCOPE OF THE PROBLEM
Despite being as common as asthma, posttraumatic stress disorder (PTSD) often remains undiagnosed and untreated in primary care.1 In brief, the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) defines PTSD as persistent and long-term changes in thoughts or mood following actual or threatened exposure to death, serious injury, or sexual assault that leads to re-experiencing, functional impairment, physiologic stress reactions, and avoidance of thoughts or situations associated with the original trauma.2 More than one in 10 women and one in 20 men experience PTSD in their lifetime.2,3 Population-based studies have not yet determined the prevalence among children.3 Almost 40% of US adults report having experienced a trauma before age 13, and about one-third of these go on to develop PTSD.4
Individuals with PTSD have higher rates of somatic complaints, overall medical utilization, prescription use, physical and social disability, attempted suicide, and all-cause mortality.3,5-7 PTSD is associated with increased risk for cardiac, gastrointestinal, metabolic, and immunologic illnesses, other psychiatric illnesses, risky health behaviors, and decreased medical adherence.4,6 Additionally, prevention and treatment efforts for STDs and obesity are less effective among those with trauma histories.4 Thus, detection and treatment of PTSD improves the likelihood of successfully treating other health concerns.
THE ESSENTIALS OF A PTSD DIAGNOSIS
DSM-5 diagnosis of PTSD requires the experience of a trauma and resultant symptoms from each of 4 symptom-clusters:2
- one or more re-experiencing symptoms (eg, intrusive memories or recurrent distressing dreams, psychological distress or physiologic reactions to reminders of the trauma)
- one or more avoidance symptoms (eg, avoidance of trauma memories or of people and places that trigger a reminder of the trauma)
- two or more changes in thoughts or mood (eg, negative beliefs about self or others, social detachment, anhedonia)
- two or more changes in arousal activity (eg, sleep problems, hypervigilance, inability to concentrate).
Since many people experiencing traumas do not develop PTSD,5,8 symptoms must last at least one month to meet the criteria for diagnosis.2 Sexual trauma, experiencing multiple traumas, and lack of social support increase the risk that an individual will develop PTSD.9-11 Notably, symptom onset will be delayed 6 months or more in some individuals,2,8 making it more difficult for those patients and clinicians to connect symptoms to the trauma.12
Differential diagnosis
PTSD must be differentiated from other mental health conditions with overlapping symptoms (TABLE 12,8,13), but it may also be comorbid with one or more of these other conditions. When patients with PTSD do report mental health symptoms, providers often focus on the depressive symptoms that overlap with PTSD, and on substance use, which often accompanies PTSD, leaving PTSD undetected.9
Given that depressed/irritable mood, decreased participation in pleasurable activities, negative views of the world, attention difficulties, sleep difficulties, feelings of guilt, and agitation/restlessness are symptoms of both depression and PTSD,2 it is particularly important to screen patients with depressive symptoms for trauma history.
Why PTSD is often missed
Due to the impact of PTSD on overall health, the rates of PTSD in primary care clinics may be higher than in the general population.14 Thus, primary care clinicians are likely seeing PTSD more often than they realize. In fact, a systematic review showed that clinicians detected 0% to 52% of their patients with PTSD, missing at least half of all PTSD diagnoses.9
Detecting PTSD can be challenging for several reasons. Symptoms can span the emotional, social, physical, and behavioral aspects of an individual’s life, so patients and clinicians alike may regard symptoms as unrelated to PTSD.8 Primary symptom presentation may vary, with some people reporting anxiety symptoms, others mostly depressive symptoms, and others arousal, dissociative, or—as in our patient’s case—somatic symptoms.2 In affected children, parents may report emotional or behavioral problems without mentioning the trauma.2 Additionally, for traumas that were not a single event, such as long-term child abuse, patients may have difficulty identifying symptom onset.2
CASE
The physician screened Mr. W for trauma exposure as part of the differential. Mr. W revealed that he had blacked out at the bar, despite drinking only moderately, and that he awoke with anal pain. He believed he had been drugged and sexually assaulted. Further screening for PTSD symptoms related to this event confirmed multiple associated symptoms. He acknowledged that his epigastric pain had started soon after the trauma and, after further discussion, began to link his stomach pain and other new symptoms revealed by the PTSD screen (hypervigilance, avoidance, change in mood) to the trauma.
As happened in this case, most PTSD patients present with somatic complaints rather than reporting a traumatic experience and having associated effects. This in turn usually leads clinicians to consider only non-PTSD diagnoses.6,9,15 Core avoidance symptoms are a major reason for such a presentation in PTSD patients.14 Patients avoid thoughts, feelings, and conversations that remind them of the trauma.13 As a result, they are less likely to voluntarily report trauma. They avoid thinking about how their current symptoms may be associated with their trauma and are reluctant to talk about their trauma with clinicians.5,9,8,12
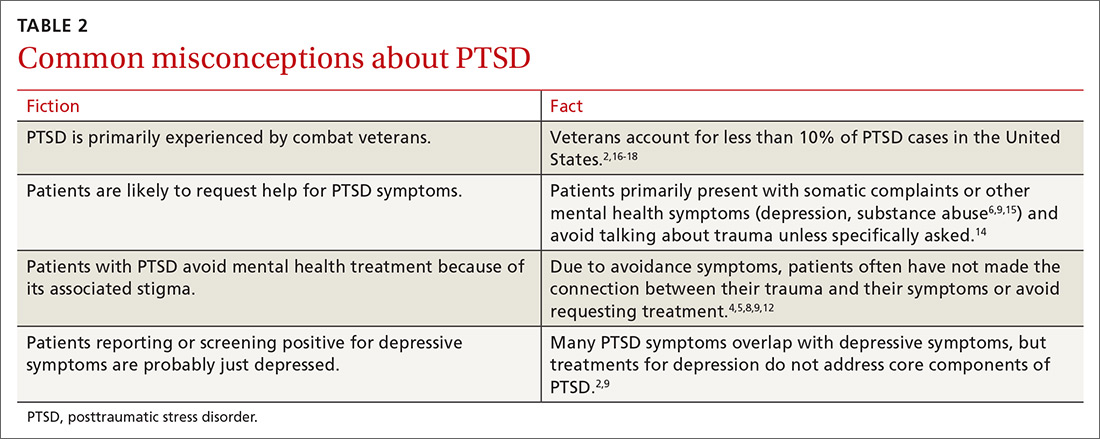
Another barrier to diagnosis is a belief that PTSD is primarily experienced by combat veterans1 (TABLE 22,4-6,8,9,12,14-18). While PTSD is detected more often among veterans due to regular screening through the Department of Veterans Affairs,14 the vast majority of PTSD cases are related to civilian traumas such as sexual assault, child abuse, and car accidents.5,9 In fact, the estimated
SCREENING: WHAT TO LOOK FOR
Since individuals with PTSD mainly seek treatment for associated physical symptoms,14 primary care is particularly important for identification of PTSD and treatment access. The US Preventative Services Task Force does not yet have any recommendations for screening for PTSD. The American Psychiatric Association recommends that a trauma history be included in all initial psychiatric evaluations of adults.19 Screens can target high-risk populations and can be repeated across the lifespan,9 as traumas can occur at any age and symptoms may not emerge until years after the trauma.2,4 Factors in a patient’s history associated with high risk of PTSD include the following:
- known trauma exposure (eg, treatment at the emergency department following motor vehicle collision, natural disaster, assault),6
- frequent medical visits or unexplained physical symptoms,5,8
- family members who are trauma victims,8
- involvement in juvenile justice system,4,12
- sensitive or invasive exams (eg, pelvic exams) that trigger symptoms or contribute to retraumatization,12,20 and
- any medical condition (eg, hypertension, chronic pain, sleep disorder), self-destructive behavior (eg, drug or alcohol abuse, low impulse control), or social/occupational issues (eg, unemployment, social isolation, fighting) with a known link to PTSD.2,4,6,8
The first step in screening. Given a patient’s reported symptoms, assess for trauma exposure to determine whether PTSD should be included in the differential diagnosis. Overlooking PTSD as a possible source of symptoms can result in misattributing them to other causes.4,8 Listing common traumas, or using a standardized list such as the Life Events Checklist, can help identify patients with trauma exposure.8,21 However, do not make the patient provide details of the traumatic event(s), as that can exacerbate symptoms if PTSD is present.6 It is sufficient to know the category of the trauma (eg, sexual assault) without making the patient describe who was involved and what happened.6
The second step in screening. If a patient reveals trauma exposure, consider using an instrument such as the Primary Care PTSD Screen (PC-PTSD) or the PTSD Checklist, both available online, to screen for PTSD symptoms related to the identified trauma.6,9,21-23 Since these measures screen for symptoms but do not ask about trauma exposure, false positives can occur if a trauma is not first identified (such as through the Life Events Checklist) due to symptom overlap with other conditions (TABLE 12,8,13).21
Treatment is effective, even decades after a traumatic event
Provide anyone who has been traumatized with information about common after effects, symptoms of PTSD, and available treatments.8 Keep in mind that initial symptom severity after trauma exposure does not correlate with long-term symptoms,8 and about half of adults will recover without treatment within 3 months.1,2,5 The first month of symptoms may be addressed with patient education and watchful waiting. But if symptoms don’t subside after a month, consider offering treatment1 with the understanding that, for some individuals, symptoms may yet resolve on their own.
Detecting and treating PTSD early can decrease its deleterious effects on health and cut down on years of functional impairment.1 Even decades after an initial traumatic event, PTSD treatments can be effective.8 Children may experience functional impairment without meeting full criteria for PTSD, and can also benefit from treatment.7
INTEGRATING EXPOSURE AND COGNITIVE THERAPIES IS KEY
Offer any patient who meets criteria for PTSD a referral for exposure therapy and trauma-focused cognitive behavioral therapy (TF-CBT), the first-line treatments for PTSD.1,4,8,24,25
Exposure therapies for PTSD are supported by strong evidence and help patients to become desensitized to distressful memories through gradual, repeated exposures in a relaxed or safe space.8,26
Cognitive methods, such as cognitive processing therapy, cognitive behavioral therapy, and cognitive reprocessing have moderate strength of evidence, and may be combined with exposure therapy.26 Cognitive therapies help patients change thoughts, beliefs, and behaviors that contribute to PTSD symptoms.8,26
Exposure and TF-CBT have the most empirical evidence for child, adolescent, and adult PTSD, and are effective for the range of PTSD symptoms,4,8,25 including avoidance—a fundamental component of PTSD that drives other PTSD symptoms27—comorbid depression, and other emotions associated with trauma (eg, shame, guilt, and anger).8,25 Family involvement is recommended for children and adolescents.4
For patients with comorbid substance abuse, offer integrated PTSD/substance abuse treatment, which is more effective than isolated treatment of each.4 Relaxation training can be helpful as an adjunct to TF-CBT, but is not sufficient as a stand-alone treatment.13 Other psychotherapies, such as supportive, psychodynamic, systemic, and hypnotherapy, have not proved effective.14
Eye Movement Desensitization and Reprocessing (EMDR), a much publicized but controversial treatment, integrates components of exposure and cognitive therapies with therapist-directed eye movements.28-30 Patients imagine their trauma while the therapist directs their eye movements, which is thought to provide exposure to trauma images and memories, thereby reducing symptoms. EMDR has been found to reduce PTSD symptoms with a low to moderate strength-of-evidence rating.26 However, it has not proved more effective than other exposure and cognitive therapies, and its unique component (eg, eye movements) has not added any effect to outcomes.28-31
Other newer therapies, such as Acceptance and Commitment Therapy7,24,27 and online and computer-assisted treatments, are being evaluated.14
Medications take on an adjunct role to therapy
Drug treatment of PTSD has not been effective in children or adolescents.4,8 In adults, medications have helped relieve some symptoms of PTSD. However, given their low effect sizes, medications are not recommended as first-line treatments over exposure and TF-CBT. Their usefulness lies chiefly in an adjunct role to exposure and cognitive therapies or for patients who refuse psychotherapy.4,8,25
Selective serotonin reuptake inhibitors such as fluoxetine, paroxetine, and sertraline, have been effective for such PTSD symptoms as intrusive thoughts, negative or irritable mood, anxiety, restlessness, attention difficulties, and hyperarousal.1,8
While benzodiazepines have been used to control anxiety, hyperarousal, and insomnia, they have not been effective for most other PTSD symptoms, including avoidance, re-experiencing, and cognitive symptoms. Furthermore, they are not recommended given their augmentative effect on other related symptoms and associated conditions (eg, dissociation, disinhibition, substance abuse) and possible interference with desensitization that occurs in exposure therapy.1,5
If a patient has significant insomnia and PTSD-related nightmares, consider starting prazosin at 1 mg/d and titrating up to an effective dose, which typically ranges from 5 to 20 mg per day.1,5 Additionally, trazodone or antihistamines may be used to enhance sleep.1
COORDINATION OF CARE
Upon identifying PTSD and offering treatment, introduce the patient to a mental health provider as part of the referral process, which strongly encourages patient engagement in treatment.14 Collaborate with the psychotherapist throughout treatment to facilitate a biopsychosocial approach to the patient’s care, and coordinate the monitoring and treatment of any comorbid physical conditions.
The Substance Abuse and Mental Health Services Administration has proposed a framework for multisystem Trauma-Informed Care (TIC), in which the primary care physician has many roles, including:12,20
- recording or communicating sensitive private information to other providers through the electronic medical record in a manner that does not interfere with a patient’s development of trust or lead to exposure and retraumatization,
- performing invasive physical exams in a sensitive and patient-centered manner, and
- using support and shared decision-making in clinical encounters.
Physicians can also connect patients with PTSD to programs or groups that aid in developing resilience, such as physical exercise classes, social support networks, and community involvement opportunities.4
CASE
The physician referred Mr. W to an onsite psychologist. At a subsequent clinic visit in which he was seen by a different primary care physician, Mr. W expressed new concerns about shoulder pain and changes in a mole. During this visit, Mr. W was asked whether he had followed up on the earlier referral for counseling. He replied that he had attended an intake appointment with the psychologist, but that he had not wanted to talk about what had happened to him and therefore avoided future appointments.*
He remained concerned that he might have an STD, but declined medication for PTSD because he felt he was “moving on” with his life.
*Author’s note: Getting patients to open up about their trauma exposure can be difficult. If the patient isn’t ready, simply bringing up the experience can trigger avoidance. It’s often helpful to encourage patients to first develop a relationship with their therapist, then later discuss the details of their trauma when they are ready. This encourages patients to engage in the counseling process.
CORRESPONDENCE
Adrienne A. Williams, PhD, Department of Family Medicine, University of Illinois at Chicago College of Medicine, 1919 W Taylor Street, MC663, Chicago, IL 60612; awms@uic.edu.
1. Bobo WV, Warner CH, Warner CM. The management of post traumatic stress disorder (PTSD) in the primary care setting. South Med J. 2007;100:797-802.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Publishing; 2013.
3. Gradus JL. Epidemiology of PTSD. National Center for PTSD. Available at: http://www.ptsd.va.gov/professional/PTSD-overview/epidemiological-facts-ptsd.asp. Updated 2017. Accessed August 16, 2017.
4. Gerson R, Rappaport N. Traumatic stress and posttraumatic stress disorder in youth: recent research findings on clinical impact, assessment, and treatment. J Adolesc Health. 2013;52:137-143.
5. Zohar J, Juven-Wetzler A, Myers V, et al. Post-traumatic stress disorder: facts and fiction. Curr Opin Psychiatry. 2008;21:74-77.
6. Spoont MR, Williams JW Jr, Kehle-Forbes S, et al. Does this patient have posttraumatic stress disorder? Rational clinical examination systematic review. JAMA. 2015;314:501-510.
7. Woidneck MR, Morrison KL, Twohig MP. Acceptance and commitment therapy for the treatment of posttraumatic stress among adolescents. Behav Modif. 2014;38:451-476.
8. National Collaborating Centre for Mental Health (UK). Post-traumatic stress disorder: the management of PTSD in adults and children in primary and secondary care. Available at: https://www.ncbi.nlm.nih.gov/books/NBK56494. Accessed August 16, 2017.
9. Greene T, Neria Y, Gross R. Prevalence, detection and correlates of PTSD in the primary care setting: a systematic review. J Clin Psychol Med Settings. 2016;23:160-180.
10. Gavranidou M, Rosner R. The weaker sex? Gender and post-traumatic stress disorder. Depress Anxiety. 2003;17:130-139.
11. Brewin CR, Andrews B, Valentine JD. Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. J Consult Clin Psychol. 2000;68:748-766.
12. SAMHSA’s Trauma and Justice Strategic Initiative. SAMHSA’s concept of trauma and guidance for a trauma-informed approach. Available at: http://store.samhsa.gov/shin/content/SMA14-4884/SMA14-4884.pdf. Accessed September 13, 2017.
13. Mulick PS, Landes SJ, Kanter JW. Contextual behavior therapies in the treatment of PTSD: a review. Int J Behav Consult Ther. 2005;1:223-238.
14. Possemato K. The current state of intervention research for posttraumatic stress disorder within the primary care setting. J Clin Psychol Med Settings. 2011;18:268-280.
15. Forneris CA, Gartlehner G, Brownley KA, et al. Interventions to prevent post-traumatic stress disorder: a systematic review. Am J Prev Med. 2013;44:635-650.
16. Trivedi RB, Post EP, Sun H, et al. Prevalence, comorbidity, and prognosis of mental health among US veterans. Am J Public Health. 2015;105:2564-2569.
17. United States Census Bureau. Facts for features: Veteran’s day 2016: Nov. 11. Available at: https://www.census.gov/newsroom/facts-for-features/2016/cb16-ff21.html. Accessed August 16, 2017.
18. United States Census Bureau. U.S. and World Population Clock. Available at: https://www.census.gov/popclock/. Accessed August 16, 2017.
19. American Psychiatric Association. Guidelines and implementation. In: Practice Guidelines for the Psychiatric Evaluation of Adults. 3rd ed. Arlington, Va: American Psychiatric Association; 2015:9-45.
20. Williams AA, Williams M. A guide to performing pelvic speculum exams: a patient-centered approach to reducing iatrogenic effects. Teach Learn Med. 2013;25:383-391.
21. U.S. Department of Veterans Affairs. Life events checklist for DSM-5 (LEC-5). Available at: http://www.ptsd.va.gov/professional/assessment/te-measures/life_events_checklist.asp. Accessed September 13, 2017.
22. U.S. Department of Veterans Affairs. Primary care PTSD screen for DSM-5 (PC-PTSD). Available at: http://www.ptsd.va.gov/professional/assessment/screens/pc-ptsd.asp. Accessed September 13, 2017.
23. Spoont M, Arbisi P, Fu S, et al. Screening for Post-Traumatic Stress Disorder (PTSD) in Primary Care: A Systematic Review. Available at: https://www.ncbi.nlm.nih.gov/books/NBK126691/. Accessed Sept 13, 2017
24. Gallagher MW, Thompson-Hollands J, Bourgeois ML, et al. Cognitive behavioral treatments for adult posttraumatic stress disorder: current status and future directions. J Contemp Psychother. 2015;45:235-243.
25. Kar N. Cognitive behavioral therapy for the treatment of post-traumatic stress disorder: a review. Neuropsychiatr Dis Treat. 2011;7:167-181.
26. Cusack K, Jonas DE, Forneris CA, et al. Psychological treatments for adults with posttraumatic stress disorder: a systematic review and meta-analysis. Clin Psychol Rev. 2016;43:128-141.
27. Thompson BL, Luoma JB, LeJeune JT. Using acceptance and commitment therapy to guide exposure-based interventions for posttraumatic stress disorder. J Contemp Psychother. 2013;43:133-140.
28. Lohr JM, Hooke W, Gist R, et al. Novel and controversial treatments for trauma-related stress disorders. In: Lilienfeld SO, Lynn SJ, Lohr JM, eds. Science and Pseudoscience in Clinical Psychology. New York, NY: Guilford Press; 2003:243-272.
29. Sikes C, Sikes V. EMDR: Why the controversy? Traumatol. 2003;9:169-182.
30. Davidson PR, Parker KCH. Eye movement desensitization and reprocessing (EMDR): a meta-analysis. J Consult Clin Psychol. 2001;69:305-316.
31. Devilly GJ. Power therapies and possible threats to the science of psychology and psychiatry. Aust N Z J Psychiatry. 2005;39:437-445.
THE CASE
DeSean W,* a 47-year-old man, returned to his primary care clinic with a new complaint of epigastric burning that had been bothering him for the past 4 months. He had tried several over-the-counter remedies, which provided no relief. He also remained concerned—despite assurances to the contrary at previous clinic visits—that he had contracted a sexually-transmitted disease (STD) after going to a bar one night 4 to 5 months ago. At 2 other clinic visits since that time, STD test results were negative. At this current visit, symptoms and details of sexual history were unchanged since the last visit, with the exception of the epigastric pain.
When asked if he thought he had contracted an STD through a sexual encounter the night he went to the bar, he emphatically said he would not cheat on his wife. Surprisingly, given his concern, he avoided further discussion on modes of contracting an STD.
The physician prescribed ranitidine 150 mg bid for the epigastric burning and explained, once more, the significance of the STD test results. However, he also decided to further examine Mr. W’s concern about STDs and the night he may have contracted one.
HOW WOULD YOU PROCEED WITH THIS PATIENT?
*The patient’s name has been changed to protect his privacy.
SCOPE OF THE PROBLEM
Despite being as common as asthma, posttraumatic stress disorder (PTSD) often remains undiagnosed and untreated in primary care.1 In brief, the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) defines PTSD as persistent and long-term changes in thoughts or mood following actual or threatened exposure to death, serious injury, or sexual assault that leads to re-experiencing, functional impairment, physiologic stress reactions, and avoidance of thoughts or situations associated with the original trauma.2 More than one in 10 women and one in 20 men experience PTSD in their lifetime.2,3 Population-based studies have not yet determined the prevalence among children.3 Almost 40% of US adults report having experienced a trauma before age 13, and about one-third of these go on to develop PTSD.4
Individuals with PTSD have higher rates of somatic complaints, overall medical utilization, prescription use, physical and social disability, attempted suicide, and all-cause mortality.3,5-7 PTSD is associated with increased risk for cardiac, gastrointestinal, metabolic, and immunologic illnesses, other psychiatric illnesses, risky health behaviors, and decreased medical adherence.4,6 Additionally, prevention and treatment efforts for STDs and obesity are less effective among those with trauma histories.4 Thus, detection and treatment of PTSD improves the likelihood of successfully treating other health concerns.
THE ESSENTIALS OF A PTSD DIAGNOSIS
DSM-5 diagnosis of PTSD requires the experience of a trauma and resultant symptoms from each of 4 symptom-clusters:2
- one or more re-experiencing symptoms (eg, intrusive memories or recurrent distressing dreams, psychological distress or physiologic reactions to reminders of the trauma)
- one or more avoidance symptoms (eg, avoidance of trauma memories or of people and places that trigger a reminder of the trauma)
- two or more changes in thoughts or mood (eg, negative beliefs about self or others, social detachment, anhedonia)
- two or more changes in arousal activity (eg, sleep problems, hypervigilance, inability to concentrate).
Since many people experiencing traumas do not develop PTSD,5,8 symptoms must last at least one month to meet the criteria for diagnosis.2 Sexual trauma, experiencing multiple traumas, and lack of social support increase the risk that an individual will develop PTSD.9-11 Notably, symptom onset will be delayed 6 months or more in some individuals,2,8 making it more difficult for those patients and clinicians to connect symptoms to the trauma.12
Differential diagnosis
PTSD must be differentiated from other mental health conditions with overlapping symptoms (TABLE 12,8,13), but it may also be comorbid with one or more of these other conditions. When patients with PTSD do report mental health symptoms, providers often focus on the depressive symptoms that overlap with PTSD, and on substance use, which often accompanies PTSD, leaving PTSD undetected.9
Given that depressed/irritable mood, decreased participation in pleasurable activities, negative views of the world, attention difficulties, sleep difficulties, feelings of guilt, and agitation/restlessness are symptoms of both depression and PTSD,2 it is particularly important to screen patients with depressive symptoms for trauma history.
Why PTSD is often missed
Due to the impact of PTSD on overall health, the rates of PTSD in primary care clinics may be higher than in the general population.14 Thus, primary care clinicians are likely seeing PTSD more often than they realize. In fact, a systematic review showed that clinicians detected 0% to 52% of their patients with PTSD, missing at least half of all PTSD diagnoses.9
Detecting PTSD can be challenging for several reasons. Symptoms can span the emotional, social, physical, and behavioral aspects of an individual’s life, so patients and clinicians alike may regard symptoms as unrelated to PTSD.8 Primary symptom presentation may vary, with some people reporting anxiety symptoms, others mostly depressive symptoms, and others arousal, dissociative, or—as in our patient’s case—somatic symptoms.2 In affected children, parents may report emotional or behavioral problems without mentioning the trauma.2 Additionally, for traumas that were not a single event, such as long-term child abuse, patients may have difficulty identifying symptom onset.2
CASE
The physician screened Mr. W for trauma exposure as part of the differential. Mr. W revealed that he had blacked out at the bar, despite drinking only moderately, and that he awoke with anal pain. He believed he had been drugged and sexually assaulted. Further screening for PTSD symptoms related to this event confirmed multiple associated symptoms. He acknowledged that his epigastric pain had started soon after the trauma and, after further discussion, began to link his stomach pain and other new symptoms revealed by the PTSD screen (hypervigilance, avoidance, change in mood) to the trauma.
As happened in this case, most PTSD patients present with somatic complaints rather than reporting a traumatic experience and having associated effects. This in turn usually leads clinicians to consider only non-PTSD diagnoses.6,9,15 Core avoidance symptoms are a major reason for such a presentation in PTSD patients.14 Patients avoid thoughts, feelings, and conversations that remind them of the trauma.13 As a result, they are less likely to voluntarily report trauma. They avoid thinking about how their current symptoms may be associated with their trauma and are reluctant to talk about their trauma with clinicians.5,9,8,12
Another barrier to diagnosis is a belief that PTSD is primarily experienced by combat veterans1 (TABLE 22,4-6,8,9,12,14-18). While PTSD is detected more often among veterans due to regular screening through the Department of Veterans Affairs,14 the vast majority of PTSD cases are related to civilian traumas such as sexual assault, child abuse, and car accidents.5,9 In fact, the estimated
SCREENING: WHAT TO LOOK FOR
Since individuals with PTSD mainly seek treatment for associated physical symptoms,14 primary care is particularly important for identification of PTSD and treatment access. The US Preventative Services Task Force does not yet have any recommendations for screening for PTSD. The American Psychiatric Association recommends that a trauma history be included in all initial psychiatric evaluations of adults.19 Screens can target high-risk populations and can be repeated across the lifespan,9 as traumas can occur at any age and symptoms may not emerge until years after the trauma.2,4 Factors in a patient’s history associated with high risk of PTSD include the following:
- known trauma exposure (eg, treatment at the emergency department following motor vehicle collision, natural disaster, assault),6
- frequent medical visits or unexplained physical symptoms,5,8
- family members who are trauma victims,8
- involvement in juvenile justice system,4,12
- sensitive or invasive exams (eg, pelvic exams) that trigger symptoms or contribute to retraumatization,12,20 and
- any medical condition (eg, hypertension, chronic pain, sleep disorder), self-destructive behavior (eg, drug or alcohol abuse, low impulse control), or social/occupational issues (eg, unemployment, social isolation, fighting) with a known link to PTSD.2,4,6,8
The first step in screening. Given a patient’s reported symptoms, assess for trauma exposure to determine whether PTSD should be included in the differential diagnosis. Overlooking PTSD as a possible source of symptoms can result in misattributing them to other causes.4,8 Listing common traumas, or using a standardized list such as the Life Events Checklist, can help identify patients with trauma exposure.8,21 However, do not make the patient provide details of the traumatic event(s), as that can exacerbate symptoms if PTSD is present.6 It is sufficient to know the category of the trauma (eg, sexual assault) without making the patient describe who was involved and what happened.6
The second step in screening. If a patient reveals trauma exposure, consider using an instrument such as the Primary Care PTSD Screen (PC-PTSD) or the PTSD Checklist, both available online, to screen for PTSD symptoms related to the identified trauma.6,9,21-23 Since these measures screen for symptoms but do not ask about trauma exposure, false positives can occur if a trauma is not first identified (such as through the Life Events Checklist) due to symptom overlap with other conditions (TABLE 12,8,13).21
Treatment is effective, even decades after a traumatic event
Provide anyone who has been traumatized with information about common after effects, symptoms of PTSD, and available treatments.8 Keep in mind that initial symptom severity after trauma exposure does not correlate with long-term symptoms,8 and about half of adults will recover without treatment within 3 months.1,2,5 The first month of symptoms may be addressed with patient education and watchful waiting. But if symptoms don’t subside after a month, consider offering treatment1 with the understanding that, for some individuals, symptoms may yet resolve on their own.
Detecting and treating PTSD early can decrease its deleterious effects on health and cut down on years of functional impairment.1 Even decades after an initial traumatic event, PTSD treatments can be effective.8 Children may experience functional impairment without meeting full criteria for PTSD, and can also benefit from treatment.7
INTEGRATING EXPOSURE AND COGNITIVE THERAPIES IS KEY
Offer any patient who meets criteria for PTSD a referral for exposure therapy and trauma-focused cognitive behavioral therapy (TF-CBT), the first-line treatments for PTSD.1,4,8,24,25
Exposure therapies for PTSD are supported by strong evidence and help patients to become desensitized to distressful memories through gradual, repeated exposures in a relaxed or safe space.8,26
Cognitive methods, such as cognitive processing therapy, cognitive behavioral therapy, and cognitive reprocessing have moderate strength of evidence, and may be combined with exposure therapy.26 Cognitive therapies help patients change thoughts, beliefs, and behaviors that contribute to PTSD symptoms.8,26
Exposure and TF-CBT have the most empirical evidence for child, adolescent, and adult PTSD, and are effective for the range of PTSD symptoms,4,8,25 including avoidance—a fundamental component of PTSD that drives other PTSD symptoms27—comorbid depression, and other emotions associated with trauma (eg, shame, guilt, and anger).8,25 Family involvement is recommended for children and adolescents.4
For patients with comorbid substance abuse, offer integrated PTSD/substance abuse treatment, which is more effective than isolated treatment of each.4 Relaxation training can be helpful as an adjunct to TF-CBT, but is not sufficient as a stand-alone treatment.13 Other psychotherapies, such as supportive, psychodynamic, systemic, and hypnotherapy, have not proved effective.14
Eye Movement Desensitization and Reprocessing (EMDR), a much publicized but controversial treatment, integrates components of exposure and cognitive therapies with therapist-directed eye movements.28-30 Patients imagine their trauma while the therapist directs their eye movements, which is thought to provide exposure to trauma images and memories, thereby reducing symptoms. EMDR has been found to reduce PTSD symptoms with a low to moderate strength-of-evidence rating.26 However, it has not proved more effective than other exposure and cognitive therapies, and its unique component (eg, eye movements) has not added any effect to outcomes.28-31
Other newer therapies, such as Acceptance and Commitment Therapy7,24,27 and online and computer-assisted treatments, are being evaluated.14
Medications take on an adjunct role to therapy
Drug treatment of PTSD has not been effective in children or adolescents.4,8 In adults, medications have helped relieve some symptoms of PTSD. However, given their low effect sizes, medications are not recommended as first-line treatments over exposure and TF-CBT. Their usefulness lies chiefly in an adjunct role to exposure and cognitive therapies or for patients who refuse psychotherapy.4,8,25
Selective serotonin reuptake inhibitors such as fluoxetine, paroxetine, and sertraline, have been effective for such PTSD symptoms as intrusive thoughts, negative or irritable mood, anxiety, restlessness, attention difficulties, and hyperarousal.1,8
While benzodiazepines have been used to control anxiety, hyperarousal, and insomnia, they have not been effective for most other PTSD symptoms, including avoidance, re-experiencing, and cognitive symptoms. Furthermore, they are not recommended given their augmentative effect on other related symptoms and associated conditions (eg, dissociation, disinhibition, substance abuse) and possible interference with desensitization that occurs in exposure therapy.1,5
If a patient has significant insomnia and PTSD-related nightmares, consider starting prazosin at 1 mg/d and titrating up to an effective dose, which typically ranges from 5 to 20 mg per day.1,5 Additionally, trazodone or antihistamines may be used to enhance sleep.1
COORDINATION OF CARE
Upon identifying PTSD and offering treatment, introduce the patient to a mental health provider as part of the referral process, which strongly encourages patient engagement in treatment.14 Collaborate with the psychotherapist throughout treatment to facilitate a biopsychosocial approach to the patient’s care, and coordinate the monitoring and treatment of any comorbid physical conditions.
The Substance Abuse and Mental Health Services Administration has proposed a framework for multisystem Trauma-Informed Care (TIC), in which the primary care physician has many roles, including:12,20
- recording or communicating sensitive private information to other providers through the electronic medical record in a manner that does not interfere with a patient’s development of trust or lead to exposure and retraumatization,
- performing invasive physical exams in a sensitive and patient-centered manner, and
- using support and shared decision-making in clinical encounters.
Physicians can also connect patients with PTSD to programs or groups that aid in developing resilience, such as physical exercise classes, social support networks, and community involvement opportunities.4
CASE
The physician referred Mr. W to an onsite psychologist. At a subsequent clinic visit in which he was seen by a different primary care physician, Mr. W expressed new concerns about shoulder pain and changes in a mole. During this visit, Mr. W was asked whether he had followed up on the earlier referral for counseling. He replied that he had attended an intake appointment with the psychologist, but that he had not wanted to talk about what had happened to him and therefore avoided future appointments.*
He remained concerned that he might have an STD, but declined medication for PTSD because he felt he was “moving on” with his life.
*Author’s note: Getting patients to open up about their trauma exposure can be difficult. If the patient isn’t ready, simply bringing up the experience can trigger avoidance. It’s often helpful to encourage patients to first develop a relationship with their therapist, then later discuss the details of their trauma when they are ready. This encourages patients to engage in the counseling process.
CORRESPONDENCE
Adrienne A. Williams, PhD, Department of Family Medicine, University of Illinois at Chicago College of Medicine, 1919 W Taylor Street, MC663, Chicago, IL 60612; awms@uic.edu.
THE CASE
DeSean W,* a 47-year-old man, returned to his primary care clinic with a new complaint of epigastric burning that had been bothering him for the past 4 months. He had tried several over-the-counter remedies, which provided no relief. He also remained concerned—despite assurances to the contrary at previous clinic visits—that he had contracted a sexually-transmitted disease (STD) after going to a bar one night 4 to 5 months ago. At 2 other clinic visits since that time, STD test results were negative. At this current visit, symptoms and details of sexual history were unchanged since the last visit, with the exception of the epigastric pain.
When asked if he thought he had contracted an STD through a sexual encounter the night he went to the bar, he emphatically said he would not cheat on his wife. Surprisingly, given his concern, he avoided further discussion on modes of contracting an STD.
The physician prescribed ranitidine 150 mg bid for the epigastric burning and explained, once more, the significance of the STD test results. However, he also decided to further examine Mr. W’s concern about STDs and the night he may have contracted one.
HOW WOULD YOU PROCEED WITH THIS PATIENT?
*The patient’s name has been changed to protect his privacy.
SCOPE OF THE PROBLEM
Despite being as common as asthma, posttraumatic stress disorder (PTSD) often remains undiagnosed and untreated in primary care.1 In brief, the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) defines PTSD as persistent and long-term changes in thoughts or mood following actual or threatened exposure to death, serious injury, or sexual assault that leads to re-experiencing, functional impairment, physiologic stress reactions, and avoidance of thoughts or situations associated with the original trauma.2 More than one in 10 women and one in 20 men experience PTSD in their lifetime.2,3 Population-based studies have not yet determined the prevalence among children.3 Almost 40% of US adults report having experienced a trauma before age 13, and about one-third of these go on to develop PTSD.4
Individuals with PTSD have higher rates of somatic complaints, overall medical utilization, prescription use, physical and social disability, attempted suicide, and all-cause mortality.3,5-7 PTSD is associated with increased risk for cardiac, gastrointestinal, metabolic, and immunologic illnesses, other psychiatric illnesses, risky health behaviors, and decreased medical adherence.4,6 Additionally, prevention and treatment efforts for STDs and obesity are less effective among those with trauma histories.4 Thus, detection and treatment of PTSD improves the likelihood of successfully treating other health concerns.
THE ESSENTIALS OF A PTSD DIAGNOSIS
DSM-5 diagnosis of PTSD requires the experience of a trauma and resultant symptoms from each of 4 symptom-clusters:2
- one or more re-experiencing symptoms (eg, intrusive memories or recurrent distressing dreams, psychological distress or physiologic reactions to reminders of the trauma)
- one or more avoidance symptoms (eg, avoidance of trauma memories or of people and places that trigger a reminder of the trauma)
- two or more changes in thoughts or mood (eg, negative beliefs about self or others, social detachment, anhedonia)
- two or more changes in arousal activity (eg, sleep problems, hypervigilance, inability to concentrate).
Since many people experiencing traumas do not develop PTSD,5,8 symptoms must last at least one month to meet the criteria for diagnosis.2 Sexual trauma, experiencing multiple traumas, and lack of social support increase the risk that an individual will develop PTSD.9-11 Notably, symptom onset will be delayed 6 months or more in some individuals,2,8 making it more difficult for those patients and clinicians to connect symptoms to the trauma.12
Differential diagnosis
PTSD must be differentiated from other mental health conditions with overlapping symptoms (TABLE 12,8,13), but it may also be comorbid with one or more of these other conditions. When patients with PTSD do report mental health symptoms, providers often focus on the depressive symptoms that overlap with PTSD, and on substance use, which often accompanies PTSD, leaving PTSD undetected.9
Given that depressed/irritable mood, decreased participation in pleasurable activities, negative views of the world, attention difficulties, sleep difficulties, feelings of guilt, and agitation/restlessness are symptoms of both depression and PTSD,2 it is particularly important to screen patients with depressive symptoms for trauma history.
Why PTSD is often missed
Due to the impact of PTSD on overall health, the rates of PTSD in primary care clinics may be higher than in the general population.14 Thus, primary care clinicians are likely seeing PTSD more often than they realize. In fact, a systematic review showed that clinicians detected 0% to 52% of their patients with PTSD, missing at least half of all PTSD diagnoses.9
Detecting PTSD can be challenging for several reasons. Symptoms can span the emotional, social, physical, and behavioral aspects of an individual’s life, so patients and clinicians alike may regard symptoms as unrelated to PTSD.8 Primary symptom presentation may vary, with some people reporting anxiety symptoms, others mostly depressive symptoms, and others arousal, dissociative, or—as in our patient’s case—somatic symptoms.2 In affected children, parents may report emotional or behavioral problems without mentioning the trauma.2 Additionally, for traumas that were not a single event, such as long-term child abuse, patients may have difficulty identifying symptom onset.2
CASE
The physician screened Mr. W for trauma exposure as part of the differential. Mr. W revealed that he had blacked out at the bar, despite drinking only moderately, and that he awoke with anal pain. He believed he had been drugged and sexually assaulted. Further screening for PTSD symptoms related to this event confirmed multiple associated symptoms. He acknowledged that his epigastric pain had started soon after the trauma and, after further discussion, began to link his stomach pain and other new symptoms revealed by the PTSD screen (hypervigilance, avoidance, change in mood) to the trauma.
As happened in this case, most PTSD patients present with somatic complaints rather than reporting a traumatic experience and having associated effects. This in turn usually leads clinicians to consider only non-PTSD diagnoses.6,9,15 Core avoidance symptoms are a major reason for such a presentation in PTSD patients.14 Patients avoid thoughts, feelings, and conversations that remind them of the trauma.13 As a result, they are less likely to voluntarily report trauma. They avoid thinking about how their current symptoms may be associated with their trauma and are reluctant to talk about their trauma with clinicians.5,9,8,12
Another barrier to diagnosis is a belief that PTSD is primarily experienced by combat veterans1 (TABLE 22,4-6,8,9,12,14-18). While PTSD is detected more often among veterans due to regular screening through the Department of Veterans Affairs,14 the vast majority of PTSD cases are related to civilian traumas such as sexual assault, child abuse, and car accidents.5,9 In fact, the estimated
SCREENING: WHAT TO LOOK FOR
Since individuals with PTSD mainly seek treatment for associated physical symptoms,14 primary care is particularly important for identification of PTSD and treatment access. The US Preventative Services Task Force does not yet have any recommendations for screening for PTSD. The American Psychiatric Association recommends that a trauma history be included in all initial psychiatric evaluations of adults.19 Screens can target high-risk populations and can be repeated across the lifespan,9 as traumas can occur at any age and symptoms may not emerge until years after the trauma.2,4 Factors in a patient’s history associated with high risk of PTSD include the following:
- known trauma exposure (eg, treatment at the emergency department following motor vehicle collision, natural disaster, assault),6
- frequent medical visits or unexplained physical symptoms,5,8
- family members who are trauma victims,8
- involvement in juvenile justice system,4,12
- sensitive or invasive exams (eg, pelvic exams) that trigger symptoms or contribute to retraumatization,12,20 and
- any medical condition (eg, hypertension, chronic pain, sleep disorder), self-destructive behavior (eg, drug or alcohol abuse, low impulse control), or social/occupational issues (eg, unemployment, social isolation, fighting) with a known link to PTSD.2,4,6,8
The first step in screening. Given a patient’s reported symptoms, assess for trauma exposure to determine whether PTSD should be included in the differential diagnosis. Overlooking PTSD as a possible source of symptoms can result in misattributing them to other causes.4,8 Listing common traumas, or using a standardized list such as the Life Events Checklist, can help identify patients with trauma exposure.8,21 However, do not make the patient provide details of the traumatic event(s), as that can exacerbate symptoms if PTSD is present.6 It is sufficient to know the category of the trauma (eg, sexual assault) without making the patient describe who was involved and what happened.6
The second step in screening. If a patient reveals trauma exposure, consider using an instrument such as the Primary Care PTSD Screen (PC-PTSD) or the PTSD Checklist, both available online, to screen for PTSD symptoms related to the identified trauma.6,9,21-23 Since these measures screen for symptoms but do not ask about trauma exposure, false positives can occur if a trauma is not first identified (such as through the Life Events Checklist) due to symptom overlap with other conditions (TABLE 12,8,13).21
Treatment is effective, even decades after a traumatic event
Provide anyone who has been traumatized with information about common after effects, symptoms of PTSD, and available treatments.8 Keep in mind that initial symptom severity after trauma exposure does not correlate with long-term symptoms,8 and about half of adults will recover without treatment within 3 months.1,2,5 The first month of symptoms may be addressed with patient education and watchful waiting. But if symptoms don’t subside after a month, consider offering treatment1 with the understanding that, for some individuals, symptoms may yet resolve on their own.
Detecting and treating PTSD early can decrease its deleterious effects on health and cut down on years of functional impairment.1 Even decades after an initial traumatic event, PTSD treatments can be effective.8 Children may experience functional impairment without meeting full criteria for PTSD, and can also benefit from treatment.7
INTEGRATING EXPOSURE AND COGNITIVE THERAPIES IS KEY
Offer any patient who meets criteria for PTSD a referral for exposure therapy and trauma-focused cognitive behavioral therapy (TF-CBT), the first-line treatments for PTSD.1,4,8,24,25
Exposure therapies for PTSD are supported by strong evidence and help patients to become desensitized to distressful memories through gradual, repeated exposures in a relaxed or safe space.8,26
Cognitive methods, such as cognitive processing therapy, cognitive behavioral therapy, and cognitive reprocessing have moderate strength of evidence, and may be combined with exposure therapy.26 Cognitive therapies help patients change thoughts, beliefs, and behaviors that contribute to PTSD symptoms.8,26
Exposure and TF-CBT have the most empirical evidence for child, adolescent, and adult PTSD, and are effective for the range of PTSD symptoms,4,8,25 including avoidance—a fundamental component of PTSD that drives other PTSD symptoms27—comorbid depression, and other emotions associated with trauma (eg, shame, guilt, and anger).8,25 Family involvement is recommended for children and adolescents.4
For patients with comorbid substance abuse, offer integrated PTSD/substance abuse treatment, which is more effective than isolated treatment of each.4 Relaxation training can be helpful as an adjunct to TF-CBT, but is not sufficient as a stand-alone treatment.13 Other psychotherapies, such as supportive, psychodynamic, systemic, and hypnotherapy, have not proved effective.14
Eye Movement Desensitization and Reprocessing (EMDR), a much publicized but controversial treatment, integrates components of exposure and cognitive therapies with therapist-directed eye movements.28-30 Patients imagine their trauma while the therapist directs their eye movements, which is thought to provide exposure to trauma images and memories, thereby reducing symptoms. EMDR has been found to reduce PTSD symptoms with a low to moderate strength-of-evidence rating.26 However, it has not proved more effective than other exposure and cognitive therapies, and its unique component (eg, eye movements) has not added any effect to outcomes.28-31
Other newer therapies, such as Acceptance and Commitment Therapy7,24,27 and online and computer-assisted treatments, are being evaluated.14
Medications take on an adjunct role to therapy
Drug treatment of PTSD has not been effective in children or adolescents.4,8 In adults, medications have helped relieve some symptoms of PTSD. However, given their low effect sizes, medications are not recommended as first-line treatments over exposure and TF-CBT. Their usefulness lies chiefly in an adjunct role to exposure and cognitive therapies or for patients who refuse psychotherapy.4,8,25
Selective serotonin reuptake inhibitors such as fluoxetine, paroxetine, and sertraline, have been effective for such PTSD symptoms as intrusive thoughts, negative or irritable mood, anxiety, restlessness, attention difficulties, and hyperarousal.1,8
While benzodiazepines have been used to control anxiety, hyperarousal, and insomnia, they have not been effective for most other PTSD symptoms, including avoidance, re-experiencing, and cognitive symptoms. Furthermore, they are not recommended given their augmentative effect on other related symptoms and associated conditions (eg, dissociation, disinhibition, substance abuse) and possible interference with desensitization that occurs in exposure therapy.1,5
If a patient has significant insomnia and PTSD-related nightmares, consider starting prazosin at 1 mg/d and titrating up to an effective dose, which typically ranges from 5 to 20 mg per day.1,5 Additionally, trazodone or antihistamines may be used to enhance sleep.1
COORDINATION OF CARE
Upon identifying PTSD and offering treatment, introduce the patient to a mental health provider as part of the referral process, which strongly encourages patient engagement in treatment.14 Collaborate with the psychotherapist throughout treatment to facilitate a biopsychosocial approach to the patient’s care, and coordinate the monitoring and treatment of any comorbid physical conditions.
The Substance Abuse and Mental Health Services Administration has proposed a framework for multisystem Trauma-Informed Care (TIC), in which the primary care physician has many roles, including:12,20
- recording or communicating sensitive private information to other providers through the electronic medical record in a manner that does not interfere with a patient’s development of trust or lead to exposure and retraumatization,
- performing invasive physical exams in a sensitive and patient-centered manner, and
- using support and shared decision-making in clinical encounters.
Physicians can also connect patients with PTSD to programs or groups that aid in developing resilience, such as physical exercise classes, social support networks, and community involvement opportunities.4
CASE
The physician referred Mr. W to an onsite psychologist. At a subsequent clinic visit in which he was seen by a different primary care physician, Mr. W expressed new concerns about shoulder pain and changes in a mole. During this visit, Mr. W was asked whether he had followed up on the earlier referral for counseling. He replied that he had attended an intake appointment with the psychologist, but that he had not wanted to talk about what had happened to him and therefore avoided future appointments.*
He remained concerned that he might have an STD, but declined medication for PTSD because he felt he was “moving on” with his life.
*Author’s note: Getting patients to open up about their trauma exposure can be difficult. If the patient isn’t ready, simply bringing up the experience can trigger avoidance. It’s often helpful to encourage patients to first develop a relationship with their therapist, then later discuss the details of their trauma when they are ready. This encourages patients to engage in the counseling process.
CORRESPONDENCE
Adrienne A. Williams, PhD, Department of Family Medicine, University of Illinois at Chicago College of Medicine, 1919 W Taylor Street, MC663, Chicago, IL 60612; awms@uic.edu.
1. Bobo WV, Warner CH, Warner CM. The management of post traumatic stress disorder (PTSD) in the primary care setting. South Med J. 2007;100:797-802.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Publishing; 2013.
3. Gradus JL. Epidemiology of PTSD. National Center for PTSD. Available at: http://www.ptsd.va.gov/professional/PTSD-overview/epidemiological-facts-ptsd.asp. Updated 2017. Accessed August 16, 2017.
4. Gerson R, Rappaport N. Traumatic stress and posttraumatic stress disorder in youth: recent research findings on clinical impact, assessment, and treatment. J Adolesc Health. 2013;52:137-143.
5. Zohar J, Juven-Wetzler A, Myers V, et al. Post-traumatic stress disorder: facts and fiction. Curr Opin Psychiatry. 2008;21:74-77.
6. Spoont MR, Williams JW Jr, Kehle-Forbes S, et al. Does this patient have posttraumatic stress disorder? Rational clinical examination systematic review. JAMA. 2015;314:501-510.
7. Woidneck MR, Morrison KL, Twohig MP. Acceptance and commitment therapy for the treatment of posttraumatic stress among adolescents. Behav Modif. 2014;38:451-476.
8. National Collaborating Centre for Mental Health (UK). Post-traumatic stress disorder: the management of PTSD in adults and children in primary and secondary care. Available at: https://www.ncbi.nlm.nih.gov/books/NBK56494. Accessed August 16, 2017.
9. Greene T, Neria Y, Gross R. Prevalence, detection and correlates of PTSD in the primary care setting: a systematic review. J Clin Psychol Med Settings. 2016;23:160-180.
10. Gavranidou M, Rosner R. The weaker sex? Gender and post-traumatic stress disorder. Depress Anxiety. 2003;17:130-139.
11. Brewin CR, Andrews B, Valentine JD. Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. J Consult Clin Psychol. 2000;68:748-766.
12. SAMHSA’s Trauma and Justice Strategic Initiative. SAMHSA’s concept of trauma and guidance for a trauma-informed approach. Available at: http://store.samhsa.gov/shin/content/SMA14-4884/SMA14-4884.pdf. Accessed September 13, 2017.
13. Mulick PS, Landes SJ, Kanter JW. Contextual behavior therapies in the treatment of PTSD: a review. Int J Behav Consult Ther. 2005;1:223-238.
14. Possemato K. The current state of intervention research for posttraumatic stress disorder within the primary care setting. J Clin Psychol Med Settings. 2011;18:268-280.
15. Forneris CA, Gartlehner G, Brownley KA, et al. Interventions to prevent post-traumatic stress disorder: a systematic review. Am J Prev Med. 2013;44:635-650.
16. Trivedi RB, Post EP, Sun H, et al. Prevalence, comorbidity, and prognosis of mental health among US veterans. Am J Public Health. 2015;105:2564-2569.
17. United States Census Bureau. Facts for features: Veteran’s day 2016: Nov. 11. Available at: https://www.census.gov/newsroom/facts-for-features/2016/cb16-ff21.html. Accessed August 16, 2017.
18. United States Census Bureau. U.S. and World Population Clock. Available at: https://www.census.gov/popclock/. Accessed August 16, 2017.
19. American Psychiatric Association. Guidelines and implementation. In: Practice Guidelines for the Psychiatric Evaluation of Adults. 3rd ed. Arlington, Va: American Psychiatric Association; 2015:9-45.
20. Williams AA, Williams M. A guide to performing pelvic speculum exams: a patient-centered approach to reducing iatrogenic effects. Teach Learn Med. 2013;25:383-391.
21. U.S. Department of Veterans Affairs. Life events checklist for DSM-5 (LEC-5). Available at: http://www.ptsd.va.gov/professional/assessment/te-measures/life_events_checklist.asp. Accessed September 13, 2017.
22. U.S. Department of Veterans Affairs. Primary care PTSD screen for DSM-5 (PC-PTSD). Available at: http://www.ptsd.va.gov/professional/assessment/screens/pc-ptsd.asp. Accessed September 13, 2017.
23. Spoont M, Arbisi P, Fu S, et al. Screening for Post-Traumatic Stress Disorder (PTSD) in Primary Care: A Systematic Review. Available at: https://www.ncbi.nlm.nih.gov/books/NBK126691/. Accessed Sept 13, 2017
24. Gallagher MW, Thompson-Hollands J, Bourgeois ML, et al. Cognitive behavioral treatments for adult posttraumatic stress disorder: current status and future directions. J Contemp Psychother. 2015;45:235-243.
25. Kar N. Cognitive behavioral therapy for the treatment of post-traumatic stress disorder: a review. Neuropsychiatr Dis Treat. 2011;7:167-181.
26. Cusack K, Jonas DE, Forneris CA, et al. Psychological treatments for adults with posttraumatic stress disorder: a systematic review and meta-analysis. Clin Psychol Rev. 2016;43:128-141.
27. Thompson BL, Luoma JB, LeJeune JT. Using acceptance and commitment therapy to guide exposure-based interventions for posttraumatic stress disorder. J Contemp Psychother. 2013;43:133-140.
28. Lohr JM, Hooke W, Gist R, et al. Novel and controversial treatments for trauma-related stress disorders. In: Lilienfeld SO, Lynn SJ, Lohr JM, eds. Science and Pseudoscience in Clinical Psychology. New York, NY: Guilford Press; 2003:243-272.
29. Sikes C, Sikes V. EMDR: Why the controversy? Traumatol. 2003;9:169-182.
30. Davidson PR, Parker KCH. Eye movement desensitization and reprocessing (EMDR): a meta-analysis. J Consult Clin Psychol. 2001;69:305-316.
31. Devilly GJ. Power therapies and possible threats to the science of psychology and psychiatry. Aust N Z J Psychiatry. 2005;39:437-445.
1. Bobo WV, Warner CH, Warner CM. The management of post traumatic stress disorder (PTSD) in the primary care setting. South Med J. 2007;100:797-802.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Publishing; 2013.
3. Gradus JL. Epidemiology of PTSD. National Center for PTSD. Available at: http://www.ptsd.va.gov/professional/PTSD-overview/epidemiological-facts-ptsd.asp. Updated 2017. Accessed August 16, 2017.
4. Gerson R, Rappaport N. Traumatic stress and posttraumatic stress disorder in youth: recent research findings on clinical impact, assessment, and treatment. J Adolesc Health. 2013;52:137-143.
5. Zohar J, Juven-Wetzler A, Myers V, et al. Post-traumatic stress disorder: facts and fiction. Curr Opin Psychiatry. 2008;21:74-77.
6. Spoont MR, Williams JW Jr, Kehle-Forbes S, et al. Does this patient have posttraumatic stress disorder? Rational clinical examination systematic review. JAMA. 2015;314:501-510.
7. Woidneck MR, Morrison KL, Twohig MP. Acceptance and commitment therapy for the treatment of posttraumatic stress among adolescents. Behav Modif. 2014;38:451-476.
8. National Collaborating Centre for Mental Health (UK). Post-traumatic stress disorder: the management of PTSD in adults and children in primary and secondary care. Available at: https://www.ncbi.nlm.nih.gov/books/NBK56494. Accessed August 16, 2017.
9. Greene T, Neria Y, Gross R. Prevalence, detection and correlates of PTSD in the primary care setting: a systematic review. J Clin Psychol Med Settings. 2016;23:160-180.
10. Gavranidou M, Rosner R. The weaker sex? Gender and post-traumatic stress disorder. Depress Anxiety. 2003;17:130-139.
11. Brewin CR, Andrews B, Valentine JD. Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. J Consult Clin Psychol. 2000;68:748-766.
12. SAMHSA’s Trauma and Justice Strategic Initiative. SAMHSA’s concept of trauma and guidance for a trauma-informed approach. Available at: http://store.samhsa.gov/shin/content/SMA14-4884/SMA14-4884.pdf. Accessed September 13, 2017.
13. Mulick PS, Landes SJ, Kanter JW. Contextual behavior therapies in the treatment of PTSD: a review. Int J Behav Consult Ther. 2005;1:223-238.
14. Possemato K. The current state of intervention research for posttraumatic stress disorder within the primary care setting. J Clin Psychol Med Settings. 2011;18:268-280.
15. Forneris CA, Gartlehner G, Brownley KA, et al. Interventions to prevent post-traumatic stress disorder: a systematic review. Am J Prev Med. 2013;44:635-650.
16. Trivedi RB, Post EP, Sun H, et al. Prevalence, comorbidity, and prognosis of mental health among US veterans. Am J Public Health. 2015;105:2564-2569.
17. United States Census Bureau. Facts for features: Veteran’s day 2016: Nov. 11. Available at: https://www.census.gov/newsroom/facts-for-features/2016/cb16-ff21.html. Accessed August 16, 2017.
18. United States Census Bureau. U.S. and World Population Clock. Available at: https://www.census.gov/popclock/. Accessed August 16, 2017.
19. American Psychiatric Association. Guidelines and implementation. In: Practice Guidelines for the Psychiatric Evaluation of Adults. 3rd ed. Arlington, Va: American Psychiatric Association; 2015:9-45.
20. Williams AA, Williams M. A guide to performing pelvic speculum exams: a patient-centered approach to reducing iatrogenic effects. Teach Learn Med. 2013;25:383-391.
21. U.S. Department of Veterans Affairs. Life events checklist for DSM-5 (LEC-5). Available at: http://www.ptsd.va.gov/professional/assessment/te-measures/life_events_checklist.asp. Accessed September 13, 2017.
22. U.S. Department of Veterans Affairs. Primary care PTSD screen for DSM-5 (PC-PTSD). Available at: http://www.ptsd.va.gov/professional/assessment/screens/pc-ptsd.asp. Accessed September 13, 2017.
23. Spoont M, Arbisi P, Fu S, et al. Screening for Post-Traumatic Stress Disorder (PTSD) in Primary Care: A Systematic Review. Available at: https://www.ncbi.nlm.nih.gov/books/NBK126691/. Accessed Sept 13, 2017
24. Gallagher MW, Thompson-Hollands J, Bourgeois ML, et al. Cognitive behavioral treatments for adult posttraumatic stress disorder: current status and future directions. J Contemp Psychother. 2015;45:235-243.
25. Kar N. Cognitive behavioral therapy for the treatment of post-traumatic stress disorder: a review. Neuropsychiatr Dis Treat. 2011;7:167-181.
26. Cusack K, Jonas DE, Forneris CA, et al. Psychological treatments for adults with posttraumatic stress disorder: a systematic review and meta-analysis. Clin Psychol Rev. 2016;43:128-141.
27. Thompson BL, Luoma JB, LeJeune JT. Using acceptance and commitment therapy to guide exposure-based interventions for posttraumatic stress disorder. J Contemp Psychother. 2013;43:133-140.
28. Lohr JM, Hooke W, Gist R, et al. Novel and controversial treatments for trauma-related stress disorders. In: Lilienfeld SO, Lynn SJ, Lohr JM, eds. Science and Pseudoscience in Clinical Psychology. New York, NY: Guilford Press; 2003:243-272.
29. Sikes C, Sikes V. EMDR: Why the controversy? Traumatol. 2003;9:169-182.
30. Davidson PR, Parker KCH. Eye movement desensitization and reprocessing (EMDR): a meta-analysis. J Consult Clin Psychol. 2001;69:305-316.
31. Devilly GJ. Power therapies and possible threats to the science of psychology and psychiatry. Aust N Z J Psychiatry. 2005;39:437-445.
Effectively engaging patients in everyday health-care decisions
The discipline of family medicine is committed to providing patient-centered care through recommendations that are grounded both in evidence and in patients’ personal values.1,2 The current health care environment, however, often demands heavy reliance on outcome-based performance metrics that can be insensitive to patient preferences.3 This tension necessitates models of decision-making that maximize reliance on measured performance, yet fulfill the clinician’s fiduciary responsibility to prioritize patients’ interests. The philosophy and practice of shared decision-making (SDM) can facilitate these aims.
The 3 elements of shared decision-making
SDM provides a framework for offering everyday medical advice and facilitating informed consent.4 Its 3 elements are:
- discussing with patients relevant information about their health conditions, possible treatments, and likely outcomes,
- clarifying and understanding a patient’s unique values and priorities and how they relate to the treatment options, and
- enabling a patient to select a care plan that is in keeping with his or her personal goals.5
This model is significant not only from a theoretical perspective, but also from a practical one. Studies have shown that both health outcomes and patient satisfaction improve when patients participate more actively in health care decision-making.6,7
Unfortunately, there is evidence that some decision-making practices in primary care settings remain inadequate. For example, unlike the standard disclosure of procedure risks in surgical settings, the burdens of cancer screening are frequently omitted from primary care discussions.8 Moreover, agreement about what should be disclosed, as well as how to disclose it, is still not sufficient. The following 3 recommendations, one for each element of SDM, aim to help clinicians effectively engage patients in everyday decision-making.
1. Provide patients with relevant information
The first element of SDM requires discussing the health-related information that is relevant to the patient’s decision-making process. The literature about informed consent supports explaining the risks that are common, as well as those that are particularly dangerous, and the likely benefits of recommended treatment, nontreatment, and alternative treatments.9 Moreover, adequate informed consent requires identifying what a reasonable person in a particular patient’s position would want to know.10
Accounting for “a patient’s position” is significant because it signals that personal factors (eg, the individual’s beliefs, goals, and familial responsibilities) are as important as the patient’s external clinical situation and what can be known by reviewing medical evidence. Incorporating a patient’s particular circumstances distinguishes patient-centered care from the mechanical application of generic best practices. This is the standard for what information should be provided.
When evidence is lacking. Clinicians facilitating decisions for which data is lacking should convey the best available evidence, including the inherent uncertainties. Like evidence-based medicine (EBM), the principles of SDM should be at work in most clinical encounters. The extent to which one engages in SDM depends upon the seriousness of the proposed interventions, the degree to which the decision is preference-sensitive, and the availability of evidence.
Statistics: Explain absolute and baseline risk
It is generally better to provide absolute risk rather than relative risk, because people perceive absolute risk reductions more accurately.11 Presentation of risks, in terms of relative risk or relative risk reduction, typically exaggerates the benefits of treatment, especially when the risk is small. This exaggeration makes it more likely that patients will accept interventions they might otherwise have rejected after reviewing the data more fully. Furthermore, relative risk statistics can impact clinicians’ perceptions, leading them to recommend an intervention more often than they might when absolute risk statistics are discussed.12
But even absolute risk, if presented on its own, can be misleading. A reasonable person trying to determine the value of an intervention needs to know his or her baseline risk of an event, in addition to his or her absolute risk with the intervention. For example, a hypothetical absolute risk reduction associated with a breast cancer treatment of 10% has different meanings, depending upon whether the baseline risk is 10% or 80%. A reduction from 10% to 0% would be a miraculous cure, while going from 80% to 70% may be viewed as only a slight improvement. Try as we might to present a single neat statistic, presenting both baseline risk and absolute risk with intervention is often necessary.13
How to effectively communicate medical information
Many patients struggle with processing information that is expressed as a probability.14 Patients process frequencies (eg, 10 in 100) better than probabilities (eg, 10%), and there is evidence that they understand best when decision aids are used.15 Decision aids, such as pictographs (FIGURE16), are supplementary, evidence-based tools for effectively communicating with patients and their families in a way that facilitates comparison between available options. Such aids are readily available online for many conditions or can be created using various software tools.16,17
Pictographs reveal that there is a values-sensitive decision to be made and visually demonstrate the outcomes associated with each option. Both pictographs and bar graphs have been shown to improve patient understanding and satisfaction.11 The benefit of pictographs is their ability to effectively, and simultaneously, convey both the numerator and the denominator in frequency statistics.12,18
There is high-quality evidence demonstrating that decision aids enhance an individual’s knowledge about the treatment and screening options available to them. A 2014 Cochrane review of the effects of decision aids found that they increased average knowledge scores when compared to usual care.15 Decision aids also improved accurate perception of risk.15 It is our belief that one of the reasons pictographs work so well is that they combine the salience of absolute risks with and without intervention.12,13
Beyond increased understanding, the Cochrane review also found high-quality evidence indicating that people who make decisions using decision aids feel less decisional conflict when compared to usual care.15 Moreover, in the context of SDM, decisional conflict may contribute to patients passing the decision-making responsibility to their clinician.19 And finally, there is moderate-quality evidence that patients are more likely to participate in decision-making when given tools such as pictographs.15
A potential barrier to putting pictographs into practice concerns perceptions that decision aids increase the length of office visits. Indeed, previous studies have identified perceived time constraints as one of the major barriers to enacting SDM in clinical settings.20 On this topic, the Cochrane review offers variable yet potentially promising results: Studies of the effects on appointment length ranged from a decrease of 8 minutes to an increase of 23 minutes.15 These results suggest that, under the right circumstances, pictographs can be used to facilitate SDM within the constraints of current clinical practice. More research is needed to determine the optimal circumstances that promote efficient SDM.
2. Elicit the patient’s unique values and priorities
Formalized approaches to building rapport with patients have been popular for more than 2 decades,21,22 and they are now routinely part of medical training. Nevertheless, there is always room for improvement when it comes to aligning treatment and screening recommendations with patient values. Some decision aids are designed to offer the added benefit of clarifying individual values and, thus, increase the likelihood that patients will make decisions that are more in line with their goals.15
When decision aids are not available to elicit patient values, clinicians can integrate preference-clarifying questions as part of the standard patient encounter.23 These questions are aimed at surfacing the values underlying what the patient wants, what the patient does not want, and most importantly, why.
“Why” matters because it ultimately helps the clinician understand the patient’s mindset, enabling the clinician to help the patient make choices that serve his or her values.24 Eliciting values not only promotes patient well-being and self-determination, but also facilitates the development of empathic patient-clinician partnerships.
Categorizing decisions. Regardless of the particular method chosen to elicit patient values, the underlying questions faced by many patients often fit into one of 2 categories: 1) Do I prefer quality of life over length of life? or 2) Am I willing to be inconvenienced now to prevent more severe illness later? Clarifying the category into which a decision falls may open the conversation and help to explore patients’ values and priorities. Alternatively, asking questions such as, “Thinking about this decision, what is the most important aspect for you to consider?”25 may facilitate the conversation.
Much of the research on techniques geared to elicit values comes from the palliative care and oncology literature.26 Although this research generally focuses on decisions about serious illness or end-of-life preferences, preference-sensitive decisions in primary care settings create a need for clinicians who are effective in eliciting patient values.
The more serious and preference-sensitive the decision, the deeper the clinician needs to explore the patient’s personal goals. Despite scant literature about seemingly innocuous decisions, we recommend that clinicians elicit from their patients a brief, but overt, acknowledgement of the values guiding their choice for most preference-sensitive decisions.
3. Offer a professional recommendation
Once clinicians have a sense of an individual’s values and priorities, they are positioned to make a professional recommendation that aligns with these values and priorities, and leaves room for the patient to reach a decision. Historically, one of the clinician’s major roles was to provide advice and recommendations to patients. For a long time, this was done without the patient’s involvement in the decision-making.27
With an increasing emphasis on patient self-determination over the last 50 years, there has been some concern that the pendulum is swinging too far in the opposite direction, with clinicians shying away from providing specific recommendations.28 Although this line of thinking acknowledges the power of the clinician to influence patients, it falls short of distinguishing between a personal recommendation and a professional one. While personal recommendations have no place in medical decision-making, clinicians should offer patients a professional recommendation, along with their rationale.
How do personal and professional recommendations differ?
Personal recommendations arise from clinicians considering what they themselves might decide if they were in the patient’s position. Such recommendations are inappropriate because every person has unique values and priorities.
In contrast, professional recommendations stem from the clinician’s knowledge of the best available evidence, his or her understanding of the patient’s values, and his or her weaving of these pieces together in the context of the patient’s specific clinical presentation. Experienced clinicians bring all 3 elements of SDM to bear in making professional recommendations, even if these recommendations are at odds with what they might choose for themselves.
EBM and SDM: Not so different after all?
Another way to understand the legitimacy of a professional recommendation is to view the parallels between SDM and EBM. From the outset, EBM positioned itself as arising from the best available evidence, the patient’s values, and clinical expertise29—elements that are strikingly similar to the components of SDM.
Although commonly overlooked, the concept of EBM recognizes that established evidence alone is not sufficient for decision-making.30 Additionally, EBM allows for making a recommendation that may not appear to be guideline-based, because guidelines typically do not take into account individual patient preferences.30-32 What’s more, both EBM and SDM highlight the essential contribution of the clinician’s judgment about his or her patient’s unique presentation.
Thus, both EBM and SDM are dependent on the professional communicating a recommendation to the patient. This communication involves not only making clear what one recommends, but also why one recommends it. For example, a clinician might say the following to a patient with worsening asthma symptoms:
“The asthma guidelines give us 2 treatment options. We can either double the dose of your inhaled corticosteroid, or start a 5-day course of corticosteroid pills. Given your concerns about the adverse effects of the pills, and the moderate severity of this exacerbation, I recommend doubling the dose of your inhaled corticosteroid. We can reconsider the pills if your symptoms worsen or if you don’t improve within the next week. How does that sound to you?”
An informed choice. Explaining the evidence, articulating the patient’s values, and summarizing the clinical elements that went into the clinician’s recommendation clarifies and signals to the patient that this is a professional recommendation. Ultimately, the process of SDM concludes with the patient considering the clinician’s recommendation and making an informed choice from the available options.
CORRESPONDENCE
David J. Satin, MD, 2020 E 28th St., Minneapolis, MN 55407; sati0003@umn.edu.
1. Medalie JH. Family Medicine: Principles and Applications. Baltimore, MD: Williams and Wilkins; 1978.
2. Philips RL Jr, Brundgardt S, Lesko SE, et al. The future role of the family physician in the United States: a rigorous exercise in definition. Ann Fam Med. 2014;12:250-255.
3. Berwick DM. Era 3 for medicine and health care. JAMA. 2016;315:1329-1330.
4. Edwards A, Elwyn G. Shared decision making in health care: achieving evidence-based patient choice. 2nd ed. Oxford, United Kingdom: Oxford University Press; 2009.
5. American Medical Association. Shared decision making H-373.997. Available at: https://policysearch.ama-assn.org/policyfinder/detail/H-373.997%20?uri=%2FAMADoc%2FHOD.xml-0-3162.xml. Accessed September 6, 2017.
6. Hibbard JH, Greene J. What the evidence shows about patient activation: better health outcomes and care experiences; fewer data on costs. Health Aff (Millwood). 2013;32:207-214.
7. Parchman ML, Zeber JE, Palmer RF. Participatory decision making, patient activation, medication adherence, and intermediate clinical outcomes in type 2 diabetes: a STARNet study. Ann Fam Med. 2010;8:410-417.
8. Fowler FJ Jr, Gerstein BS, Barry MJ. How patient centered are medical decisions? Results of a national survey. JAMA Intern Med. 2013;173:1215-1221.
9. Spatz ES, Krumhoiz HM, Moulton BW. The new era of informed consent: getting to a reasonable-patient standard through shared decision making. JAMA. 2016;315:2063-2064.
10. Faden RR, Becker C, Lewis C, et al. Disclosure of information to patients in medical care. Med Care. 1981;19:718-733.
11. Zipkin DA, Umscheid CA, Keating NL, et al. Evidence-based risk communication: a systematic review. Ann Intern Med. 2014;161:270-280.
12. Fagerlin A, Zikmund-Fisher BJ, Ubel PA. Helping patients decide: ten steps to better risk communication. J Natl Cancer Inst. 2011;103:1436-1443.
13. Stovitz SD, Shrier I. Medical decision making and the importance of baseline risk. Br J Gen Pract. 2013;63:e795-e797.
14. Lipkus IM, Samsa G, Rimer BK. General performance on a numeracy scale among highly educated samples. Med Decis Making. 2001;21:37-44.
15. Stacey D, Bennett CL, Barry MJ, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2011:CD001431.
16. Mayo Clinic. Statin choice decision aid. Available at: https://statindecisionaid.mayoclinic.org/index.php/statin/index. Accessed September 6, 2017.
17. Risk Science Center and Center for Bioethics and Social Sciences in Medicine, University of Michigan. Icon Array. Available at: http://www.iconarray.com/. Accessed September 6, 2017.
18. Price M, Cameron R, Butow P. Communicating risk information: the influence of graphical display format on quantitative information perception–accuracy, comprehension and preferences. Patient Educ Couns. 2007;69:121-128.
19. Kon AA. The shared decision making continuum. JAMA. 2010;304:903-904.
20. Légaré F1, Ratté S, Gravel K, et al. Barriers and facilitators to implementing shared decision making in clinical practice: update of a systematic review of health professionals’ perceptions. Patient Educ Couns. 2008;73:526-535.
21. Haidet P, Paterniti DA. “Building” a history rather than “taking” one: a perspective on information sharing during the medical interview. Arch Intern Med. 2003;163:1134-1140.
22. Frankel RM, Stein R. Getting the most out of the clinical encounter: the four habits model. J Med Pract Manage. 2001;16:184-194.
23. Delbanco TL. Enriching the doctor-patient relationship by inviting the patient’s perspective. Ann Intern Med. 1992;116:414-418.
24. Doukas DJ, McCullough LB. The values history: the evaluation of the patient’s values and advance directives. J Fam Pract. 1991;32:145-153.
25. Stiggelbout AM, Van der Weijden T, De Wit MP, et al. Shared decision making: really putting patients at the centre of healthcare. BMJ. 2012;344:e256.
26. Bernacki RE, Block SD, American College of Physicians High Value Care Task Force. Communication about serious illness care goals: a review and synthesis of best practices. JAMA Intern Med. 2014;174:1994-2003.
27. Katz J. The Silent World of Doctor and Patient. Baltimore, MD, and London, England: The Johns Hopkins University Press; 1984.
28. Baylis F, Downie J. Professional recommendations: disclosing facts and values. J Med Ethics. 2001;27:20-24.
29. Sackett DL, Rosenburg WM, Gray JA, et al. Evidence based medicine: what it is and what it isn’t. BMJ. 1996;312:71-72.
30. Montori VM, Brito JP, Murad MH. The optimal practice of evidence-based medicine: incorporating patient preferences in practice guidelines. JAMA. 2013;310:2053-2054.
31. Mora S, Ames JM, Manson JE. Low-dose aspirin in the primary prevention of cardiovascular disease: shared decision making in clinical practice. JAMA. 2016;316:709-710.
32. Stovitz SD, Satin D, Shrier I. Shared decision making regarding aspirin in primary prevention of cardiovascular disease. JAMA. 2016;316:2276.
The discipline of family medicine is committed to providing patient-centered care through recommendations that are grounded both in evidence and in patients’ personal values.1,2 The current health care environment, however, often demands heavy reliance on outcome-based performance metrics that can be insensitive to patient preferences.3 This tension necessitates models of decision-making that maximize reliance on measured performance, yet fulfill the clinician’s fiduciary responsibility to prioritize patients’ interests. The philosophy and practice of shared decision-making (SDM) can facilitate these aims.
The 3 elements of shared decision-making
SDM provides a framework for offering everyday medical advice and facilitating informed consent.4 Its 3 elements are:
- discussing with patients relevant information about their health conditions, possible treatments, and likely outcomes,
- clarifying and understanding a patient’s unique values and priorities and how they relate to the treatment options, and
- enabling a patient to select a care plan that is in keeping with his or her personal goals.5
This model is significant not only from a theoretical perspective, but also from a practical one. Studies have shown that both health outcomes and patient satisfaction improve when patients participate more actively in health care decision-making.6,7
Unfortunately, there is evidence that some decision-making practices in primary care settings remain inadequate. For example, unlike the standard disclosure of procedure risks in surgical settings, the burdens of cancer screening are frequently omitted from primary care discussions.8 Moreover, agreement about what should be disclosed, as well as how to disclose it, is still not sufficient. The following 3 recommendations, one for each element of SDM, aim to help clinicians effectively engage patients in everyday decision-making.
1. Provide patients with relevant information
The first element of SDM requires discussing the health-related information that is relevant to the patient’s decision-making process. The literature about informed consent supports explaining the risks that are common, as well as those that are particularly dangerous, and the likely benefits of recommended treatment, nontreatment, and alternative treatments.9 Moreover, adequate informed consent requires identifying what a reasonable person in a particular patient’s position would want to know.10
Accounting for “a patient’s position” is significant because it signals that personal factors (eg, the individual’s beliefs, goals, and familial responsibilities) are as important as the patient’s external clinical situation and what can be known by reviewing medical evidence. Incorporating a patient’s particular circumstances distinguishes patient-centered care from the mechanical application of generic best practices. This is the standard for what information should be provided.
When evidence is lacking. Clinicians facilitating decisions for which data is lacking should convey the best available evidence, including the inherent uncertainties. Like evidence-based medicine (EBM), the principles of SDM should be at work in most clinical encounters. The extent to which one engages in SDM depends upon the seriousness of the proposed interventions, the degree to which the decision is preference-sensitive, and the availability of evidence.
Statistics: Explain absolute and baseline risk
It is generally better to provide absolute risk rather than relative risk, because people perceive absolute risk reductions more accurately.11 Presentation of risks, in terms of relative risk or relative risk reduction, typically exaggerates the benefits of treatment, especially when the risk is small. This exaggeration makes it more likely that patients will accept interventions they might otherwise have rejected after reviewing the data more fully. Furthermore, relative risk statistics can impact clinicians’ perceptions, leading them to recommend an intervention more often than they might when absolute risk statistics are discussed.12
But even absolute risk, if presented on its own, can be misleading. A reasonable person trying to determine the value of an intervention needs to know his or her baseline risk of an event, in addition to his or her absolute risk with the intervention. For example, a hypothetical absolute risk reduction associated with a breast cancer treatment of 10% has different meanings, depending upon whether the baseline risk is 10% or 80%. A reduction from 10% to 0% would be a miraculous cure, while going from 80% to 70% may be viewed as only a slight improvement. Try as we might to present a single neat statistic, presenting both baseline risk and absolute risk with intervention is often necessary.13
How to effectively communicate medical information
Many patients struggle with processing information that is expressed as a probability.14 Patients process frequencies (eg, 10 in 100) better than probabilities (eg, 10%), and there is evidence that they understand best when decision aids are used.15 Decision aids, such as pictographs (FIGURE16), are supplementary, evidence-based tools for effectively communicating with patients and their families in a way that facilitates comparison between available options. Such aids are readily available online for many conditions or can be created using various software tools.16,17
Pictographs reveal that there is a values-sensitive decision to be made and visually demonstrate the outcomes associated with each option. Both pictographs and bar graphs have been shown to improve patient understanding and satisfaction.11 The benefit of pictographs is their ability to effectively, and simultaneously, convey both the numerator and the denominator in frequency statistics.12,18
There is high-quality evidence demonstrating that decision aids enhance an individual’s knowledge about the treatment and screening options available to them. A 2014 Cochrane review of the effects of decision aids found that they increased average knowledge scores when compared to usual care.15 Decision aids also improved accurate perception of risk.15 It is our belief that one of the reasons pictographs work so well is that they combine the salience of absolute risks with and without intervention.12,13
Beyond increased understanding, the Cochrane review also found high-quality evidence indicating that people who make decisions using decision aids feel less decisional conflict when compared to usual care.15 Moreover, in the context of SDM, decisional conflict may contribute to patients passing the decision-making responsibility to their clinician.19 And finally, there is moderate-quality evidence that patients are more likely to participate in decision-making when given tools such as pictographs.15
A potential barrier to putting pictographs into practice concerns perceptions that decision aids increase the length of office visits. Indeed, previous studies have identified perceived time constraints as one of the major barriers to enacting SDM in clinical settings.20 On this topic, the Cochrane review offers variable yet potentially promising results: Studies of the effects on appointment length ranged from a decrease of 8 minutes to an increase of 23 minutes.15 These results suggest that, under the right circumstances, pictographs can be used to facilitate SDM within the constraints of current clinical practice. More research is needed to determine the optimal circumstances that promote efficient SDM.
2. Elicit the patient’s unique values and priorities
Formalized approaches to building rapport with patients have been popular for more than 2 decades,21,22 and they are now routinely part of medical training. Nevertheless, there is always room for improvement when it comes to aligning treatment and screening recommendations with patient values. Some decision aids are designed to offer the added benefit of clarifying individual values and, thus, increase the likelihood that patients will make decisions that are more in line with their goals.15
When decision aids are not available to elicit patient values, clinicians can integrate preference-clarifying questions as part of the standard patient encounter.23 These questions are aimed at surfacing the values underlying what the patient wants, what the patient does not want, and most importantly, why.
“Why” matters because it ultimately helps the clinician understand the patient’s mindset, enabling the clinician to help the patient make choices that serve his or her values.24 Eliciting values not only promotes patient well-being and self-determination, but also facilitates the development of empathic patient-clinician partnerships.
Categorizing decisions. Regardless of the particular method chosen to elicit patient values, the underlying questions faced by many patients often fit into one of 2 categories: 1) Do I prefer quality of life over length of life? or 2) Am I willing to be inconvenienced now to prevent more severe illness later? Clarifying the category into which a decision falls may open the conversation and help to explore patients’ values and priorities. Alternatively, asking questions such as, “Thinking about this decision, what is the most important aspect for you to consider?”25 may facilitate the conversation.
Much of the research on techniques geared to elicit values comes from the palliative care and oncology literature.26 Although this research generally focuses on decisions about serious illness or end-of-life preferences, preference-sensitive decisions in primary care settings create a need for clinicians who are effective in eliciting patient values.
The more serious and preference-sensitive the decision, the deeper the clinician needs to explore the patient’s personal goals. Despite scant literature about seemingly innocuous decisions, we recommend that clinicians elicit from their patients a brief, but overt, acknowledgement of the values guiding their choice for most preference-sensitive decisions.
3. Offer a professional recommendation
Once clinicians have a sense of an individual’s values and priorities, they are positioned to make a professional recommendation that aligns with these values and priorities, and leaves room for the patient to reach a decision. Historically, one of the clinician’s major roles was to provide advice and recommendations to patients. For a long time, this was done without the patient’s involvement in the decision-making.27
With an increasing emphasis on patient self-determination over the last 50 years, there has been some concern that the pendulum is swinging too far in the opposite direction, with clinicians shying away from providing specific recommendations.28 Although this line of thinking acknowledges the power of the clinician to influence patients, it falls short of distinguishing between a personal recommendation and a professional one. While personal recommendations have no place in medical decision-making, clinicians should offer patients a professional recommendation, along with their rationale.
How do personal and professional recommendations differ?
Personal recommendations arise from clinicians considering what they themselves might decide if they were in the patient’s position. Such recommendations are inappropriate because every person has unique values and priorities.
In contrast, professional recommendations stem from the clinician’s knowledge of the best available evidence, his or her understanding of the patient’s values, and his or her weaving of these pieces together in the context of the patient’s specific clinical presentation. Experienced clinicians bring all 3 elements of SDM to bear in making professional recommendations, even if these recommendations are at odds with what they might choose for themselves.
EBM and SDM: Not so different after all?
Another way to understand the legitimacy of a professional recommendation is to view the parallels between SDM and EBM. From the outset, EBM positioned itself as arising from the best available evidence, the patient’s values, and clinical expertise29—elements that are strikingly similar to the components of SDM.
Although commonly overlooked, the concept of EBM recognizes that established evidence alone is not sufficient for decision-making.30 Additionally, EBM allows for making a recommendation that may not appear to be guideline-based, because guidelines typically do not take into account individual patient preferences.30-32 What’s more, both EBM and SDM highlight the essential contribution of the clinician’s judgment about his or her patient’s unique presentation.
Thus, both EBM and SDM are dependent on the professional communicating a recommendation to the patient. This communication involves not only making clear what one recommends, but also why one recommends it. For example, a clinician might say the following to a patient with worsening asthma symptoms:
“The asthma guidelines give us 2 treatment options. We can either double the dose of your inhaled corticosteroid, or start a 5-day course of corticosteroid pills. Given your concerns about the adverse effects of the pills, and the moderate severity of this exacerbation, I recommend doubling the dose of your inhaled corticosteroid. We can reconsider the pills if your symptoms worsen or if you don’t improve within the next week. How does that sound to you?”
An informed choice. Explaining the evidence, articulating the patient’s values, and summarizing the clinical elements that went into the clinician’s recommendation clarifies and signals to the patient that this is a professional recommendation. Ultimately, the process of SDM concludes with the patient considering the clinician’s recommendation and making an informed choice from the available options.
CORRESPONDENCE
David J. Satin, MD, 2020 E 28th St., Minneapolis, MN 55407; sati0003@umn.edu.
The discipline of family medicine is committed to providing patient-centered care through recommendations that are grounded both in evidence and in patients’ personal values.1,2 The current health care environment, however, often demands heavy reliance on outcome-based performance metrics that can be insensitive to patient preferences.3 This tension necessitates models of decision-making that maximize reliance on measured performance, yet fulfill the clinician’s fiduciary responsibility to prioritize patients’ interests. The philosophy and practice of shared decision-making (SDM) can facilitate these aims.
The 3 elements of shared decision-making
SDM provides a framework for offering everyday medical advice and facilitating informed consent.4 Its 3 elements are:
- discussing with patients relevant information about their health conditions, possible treatments, and likely outcomes,
- clarifying and understanding a patient’s unique values and priorities and how they relate to the treatment options, and
- enabling a patient to select a care plan that is in keeping with his or her personal goals.5
This model is significant not only from a theoretical perspective, but also from a practical one. Studies have shown that both health outcomes and patient satisfaction improve when patients participate more actively in health care decision-making.6,7
Unfortunately, there is evidence that some decision-making practices in primary care settings remain inadequate. For example, unlike the standard disclosure of procedure risks in surgical settings, the burdens of cancer screening are frequently omitted from primary care discussions.8 Moreover, agreement about what should be disclosed, as well as how to disclose it, is still not sufficient. The following 3 recommendations, one for each element of SDM, aim to help clinicians effectively engage patients in everyday decision-making.
1. Provide patients with relevant information
The first element of SDM requires discussing the health-related information that is relevant to the patient’s decision-making process. The literature about informed consent supports explaining the risks that are common, as well as those that are particularly dangerous, and the likely benefits of recommended treatment, nontreatment, and alternative treatments.9 Moreover, adequate informed consent requires identifying what a reasonable person in a particular patient’s position would want to know.10
Accounting for “a patient’s position” is significant because it signals that personal factors (eg, the individual’s beliefs, goals, and familial responsibilities) are as important as the patient’s external clinical situation and what can be known by reviewing medical evidence. Incorporating a patient’s particular circumstances distinguishes patient-centered care from the mechanical application of generic best practices. This is the standard for what information should be provided.
When evidence is lacking. Clinicians facilitating decisions for which data is lacking should convey the best available evidence, including the inherent uncertainties. Like evidence-based medicine (EBM), the principles of SDM should be at work in most clinical encounters. The extent to which one engages in SDM depends upon the seriousness of the proposed interventions, the degree to which the decision is preference-sensitive, and the availability of evidence.
Statistics: Explain absolute and baseline risk
It is generally better to provide absolute risk rather than relative risk, because people perceive absolute risk reductions more accurately.11 Presentation of risks, in terms of relative risk or relative risk reduction, typically exaggerates the benefits of treatment, especially when the risk is small. This exaggeration makes it more likely that patients will accept interventions they might otherwise have rejected after reviewing the data more fully. Furthermore, relative risk statistics can impact clinicians’ perceptions, leading them to recommend an intervention more often than they might when absolute risk statistics are discussed.12
But even absolute risk, if presented on its own, can be misleading. A reasonable person trying to determine the value of an intervention needs to know his or her baseline risk of an event, in addition to his or her absolute risk with the intervention. For example, a hypothetical absolute risk reduction associated with a breast cancer treatment of 10% has different meanings, depending upon whether the baseline risk is 10% or 80%. A reduction from 10% to 0% would be a miraculous cure, while going from 80% to 70% may be viewed as only a slight improvement. Try as we might to present a single neat statistic, presenting both baseline risk and absolute risk with intervention is often necessary.13
How to effectively communicate medical information
Many patients struggle with processing information that is expressed as a probability.14 Patients process frequencies (eg, 10 in 100) better than probabilities (eg, 10%), and there is evidence that they understand best when decision aids are used.15 Decision aids, such as pictographs (FIGURE16), are supplementary, evidence-based tools for effectively communicating with patients and their families in a way that facilitates comparison between available options. Such aids are readily available online for many conditions or can be created using various software tools.16,17
Pictographs reveal that there is a values-sensitive decision to be made and visually demonstrate the outcomes associated with each option. Both pictographs and bar graphs have been shown to improve patient understanding and satisfaction.11 The benefit of pictographs is their ability to effectively, and simultaneously, convey both the numerator and the denominator in frequency statistics.12,18
There is high-quality evidence demonstrating that decision aids enhance an individual’s knowledge about the treatment and screening options available to them. A 2014 Cochrane review of the effects of decision aids found that they increased average knowledge scores when compared to usual care.15 Decision aids also improved accurate perception of risk.15 It is our belief that one of the reasons pictographs work so well is that they combine the salience of absolute risks with and without intervention.12,13
Beyond increased understanding, the Cochrane review also found high-quality evidence indicating that people who make decisions using decision aids feel less decisional conflict when compared to usual care.15 Moreover, in the context of SDM, decisional conflict may contribute to patients passing the decision-making responsibility to their clinician.19 And finally, there is moderate-quality evidence that patients are more likely to participate in decision-making when given tools such as pictographs.15
A potential barrier to putting pictographs into practice concerns perceptions that decision aids increase the length of office visits. Indeed, previous studies have identified perceived time constraints as one of the major barriers to enacting SDM in clinical settings.20 On this topic, the Cochrane review offers variable yet potentially promising results: Studies of the effects on appointment length ranged from a decrease of 8 minutes to an increase of 23 minutes.15 These results suggest that, under the right circumstances, pictographs can be used to facilitate SDM within the constraints of current clinical practice. More research is needed to determine the optimal circumstances that promote efficient SDM.
2. Elicit the patient’s unique values and priorities
Formalized approaches to building rapport with patients have been popular for more than 2 decades,21,22 and they are now routinely part of medical training. Nevertheless, there is always room for improvement when it comes to aligning treatment and screening recommendations with patient values. Some decision aids are designed to offer the added benefit of clarifying individual values and, thus, increase the likelihood that patients will make decisions that are more in line with their goals.15
When decision aids are not available to elicit patient values, clinicians can integrate preference-clarifying questions as part of the standard patient encounter.23 These questions are aimed at surfacing the values underlying what the patient wants, what the patient does not want, and most importantly, why.
“Why” matters because it ultimately helps the clinician understand the patient’s mindset, enabling the clinician to help the patient make choices that serve his or her values.24 Eliciting values not only promotes patient well-being and self-determination, but also facilitates the development of empathic patient-clinician partnerships.
Categorizing decisions. Regardless of the particular method chosen to elicit patient values, the underlying questions faced by many patients often fit into one of 2 categories: 1) Do I prefer quality of life over length of life? or 2) Am I willing to be inconvenienced now to prevent more severe illness later? Clarifying the category into which a decision falls may open the conversation and help to explore patients’ values and priorities. Alternatively, asking questions such as, “Thinking about this decision, what is the most important aspect for you to consider?”25 may facilitate the conversation.
Much of the research on techniques geared to elicit values comes from the palliative care and oncology literature.26 Although this research generally focuses on decisions about serious illness or end-of-life preferences, preference-sensitive decisions in primary care settings create a need for clinicians who are effective in eliciting patient values.
The more serious and preference-sensitive the decision, the deeper the clinician needs to explore the patient’s personal goals. Despite scant literature about seemingly innocuous decisions, we recommend that clinicians elicit from their patients a brief, but overt, acknowledgement of the values guiding their choice for most preference-sensitive decisions.
3. Offer a professional recommendation
Once clinicians have a sense of an individual’s values and priorities, they are positioned to make a professional recommendation that aligns with these values and priorities, and leaves room for the patient to reach a decision. Historically, one of the clinician’s major roles was to provide advice and recommendations to patients. For a long time, this was done without the patient’s involvement in the decision-making.27
With an increasing emphasis on patient self-determination over the last 50 years, there has been some concern that the pendulum is swinging too far in the opposite direction, with clinicians shying away from providing specific recommendations.28 Although this line of thinking acknowledges the power of the clinician to influence patients, it falls short of distinguishing between a personal recommendation and a professional one. While personal recommendations have no place in medical decision-making, clinicians should offer patients a professional recommendation, along with their rationale.
How do personal and professional recommendations differ?
Personal recommendations arise from clinicians considering what they themselves might decide if they were in the patient’s position. Such recommendations are inappropriate because every person has unique values and priorities.
In contrast, professional recommendations stem from the clinician’s knowledge of the best available evidence, his or her understanding of the patient’s values, and his or her weaving of these pieces together in the context of the patient’s specific clinical presentation. Experienced clinicians bring all 3 elements of SDM to bear in making professional recommendations, even if these recommendations are at odds with what they might choose for themselves.
EBM and SDM: Not so different after all?
Another way to understand the legitimacy of a professional recommendation is to view the parallels between SDM and EBM. From the outset, EBM positioned itself as arising from the best available evidence, the patient’s values, and clinical expertise29—elements that are strikingly similar to the components of SDM.
Although commonly overlooked, the concept of EBM recognizes that established evidence alone is not sufficient for decision-making.30 Additionally, EBM allows for making a recommendation that may not appear to be guideline-based, because guidelines typically do not take into account individual patient preferences.30-32 What’s more, both EBM and SDM highlight the essential contribution of the clinician’s judgment about his or her patient’s unique presentation.
Thus, both EBM and SDM are dependent on the professional communicating a recommendation to the patient. This communication involves not only making clear what one recommends, but also why one recommends it. For example, a clinician might say the following to a patient with worsening asthma symptoms:
“The asthma guidelines give us 2 treatment options. We can either double the dose of your inhaled corticosteroid, or start a 5-day course of corticosteroid pills. Given your concerns about the adverse effects of the pills, and the moderate severity of this exacerbation, I recommend doubling the dose of your inhaled corticosteroid. We can reconsider the pills if your symptoms worsen or if you don’t improve within the next week. How does that sound to you?”
An informed choice. Explaining the evidence, articulating the patient’s values, and summarizing the clinical elements that went into the clinician’s recommendation clarifies and signals to the patient that this is a professional recommendation. Ultimately, the process of SDM concludes with the patient considering the clinician’s recommendation and making an informed choice from the available options.
CORRESPONDENCE
David J. Satin, MD, 2020 E 28th St., Minneapolis, MN 55407; sati0003@umn.edu.
1. Medalie JH. Family Medicine: Principles and Applications. Baltimore, MD: Williams and Wilkins; 1978.
2. Philips RL Jr, Brundgardt S, Lesko SE, et al. The future role of the family physician in the United States: a rigorous exercise in definition. Ann Fam Med. 2014;12:250-255.
3. Berwick DM. Era 3 for medicine and health care. JAMA. 2016;315:1329-1330.
4. Edwards A, Elwyn G. Shared decision making in health care: achieving evidence-based patient choice. 2nd ed. Oxford, United Kingdom: Oxford University Press; 2009.
5. American Medical Association. Shared decision making H-373.997. Available at: https://policysearch.ama-assn.org/policyfinder/detail/H-373.997%20?uri=%2FAMADoc%2FHOD.xml-0-3162.xml. Accessed September 6, 2017.
6. Hibbard JH, Greene J. What the evidence shows about patient activation: better health outcomes and care experiences; fewer data on costs. Health Aff (Millwood). 2013;32:207-214.
7. Parchman ML, Zeber JE, Palmer RF. Participatory decision making, patient activation, medication adherence, and intermediate clinical outcomes in type 2 diabetes: a STARNet study. Ann Fam Med. 2010;8:410-417.
8. Fowler FJ Jr, Gerstein BS, Barry MJ. How patient centered are medical decisions? Results of a national survey. JAMA Intern Med. 2013;173:1215-1221.
9. Spatz ES, Krumhoiz HM, Moulton BW. The new era of informed consent: getting to a reasonable-patient standard through shared decision making. JAMA. 2016;315:2063-2064.
10. Faden RR, Becker C, Lewis C, et al. Disclosure of information to patients in medical care. Med Care. 1981;19:718-733.
11. Zipkin DA, Umscheid CA, Keating NL, et al. Evidence-based risk communication: a systematic review. Ann Intern Med. 2014;161:270-280.
12. Fagerlin A, Zikmund-Fisher BJ, Ubel PA. Helping patients decide: ten steps to better risk communication. J Natl Cancer Inst. 2011;103:1436-1443.
13. Stovitz SD, Shrier I. Medical decision making and the importance of baseline risk. Br J Gen Pract. 2013;63:e795-e797.
14. Lipkus IM, Samsa G, Rimer BK. General performance on a numeracy scale among highly educated samples. Med Decis Making. 2001;21:37-44.
15. Stacey D, Bennett CL, Barry MJ, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2011:CD001431.
16. Mayo Clinic. Statin choice decision aid. Available at: https://statindecisionaid.mayoclinic.org/index.php/statin/index. Accessed September 6, 2017.
17. Risk Science Center and Center for Bioethics and Social Sciences in Medicine, University of Michigan. Icon Array. Available at: http://www.iconarray.com/. Accessed September 6, 2017.
18. Price M, Cameron R, Butow P. Communicating risk information: the influence of graphical display format on quantitative information perception–accuracy, comprehension and preferences. Patient Educ Couns. 2007;69:121-128.
19. Kon AA. The shared decision making continuum. JAMA. 2010;304:903-904.
20. Légaré F1, Ratté S, Gravel K, et al. Barriers and facilitators to implementing shared decision making in clinical practice: update of a systematic review of health professionals’ perceptions. Patient Educ Couns. 2008;73:526-535.
21. Haidet P, Paterniti DA. “Building” a history rather than “taking” one: a perspective on information sharing during the medical interview. Arch Intern Med. 2003;163:1134-1140.
22. Frankel RM, Stein R. Getting the most out of the clinical encounter: the four habits model. J Med Pract Manage. 2001;16:184-194.
23. Delbanco TL. Enriching the doctor-patient relationship by inviting the patient’s perspective. Ann Intern Med. 1992;116:414-418.
24. Doukas DJ, McCullough LB. The values history: the evaluation of the patient’s values and advance directives. J Fam Pract. 1991;32:145-153.
25. Stiggelbout AM, Van der Weijden T, De Wit MP, et al. Shared decision making: really putting patients at the centre of healthcare. BMJ. 2012;344:e256.
26. Bernacki RE, Block SD, American College of Physicians High Value Care Task Force. Communication about serious illness care goals: a review and synthesis of best practices. JAMA Intern Med. 2014;174:1994-2003.
27. Katz J. The Silent World of Doctor and Patient. Baltimore, MD, and London, England: The Johns Hopkins University Press; 1984.
28. Baylis F, Downie J. Professional recommendations: disclosing facts and values. J Med Ethics. 2001;27:20-24.
29. Sackett DL, Rosenburg WM, Gray JA, et al. Evidence based medicine: what it is and what it isn’t. BMJ. 1996;312:71-72.
30. Montori VM, Brito JP, Murad MH. The optimal practice of evidence-based medicine: incorporating patient preferences in practice guidelines. JAMA. 2013;310:2053-2054.
31. Mora S, Ames JM, Manson JE. Low-dose aspirin in the primary prevention of cardiovascular disease: shared decision making in clinical practice. JAMA. 2016;316:709-710.
32. Stovitz SD, Satin D, Shrier I. Shared decision making regarding aspirin in primary prevention of cardiovascular disease. JAMA. 2016;316:2276.
1. Medalie JH. Family Medicine: Principles and Applications. Baltimore, MD: Williams and Wilkins; 1978.
2. Philips RL Jr, Brundgardt S, Lesko SE, et al. The future role of the family physician in the United States: a rigorous exercise in definition. Ann Fam Med. 2014;12:250-255.
3. Berwick DM. Era 3 for medicine and health care. JAMA. 2016;315:1329-1330.
4. Edwards A, Elwyn G. Shared decision making in health care: achieving evidence-based patient choice. 2nd ed. Oxford, United Kingdom: Oxford University Press; 2009.
5. American Medical Association. Shared decision making H-373.997. Available at: https://policysearch.ama-assn.org/policyfinder/detail/H-373.997%20?uri=%2FAMADoc%2FHOD.xml-0-3162.xml. Accessed September 6, 2017.
6. Hibbard JH, Greene J. What the evidence shows about patient activation: better health outcomes and care experiences; fewer data on costs. Health Aff (Millwood). 2013;32:207-214.
7. Parchman ML, Zeber JE, Palmer RF. Participatory decision making, patient activation, medication adherence, and intermediate clinical outcomes in type 2 diabetes: a STARNet study. Ann Fam Med. 2010;8:410-417.
8. Fowler FJ Jr, Gerstein BS, Barry MJ. How patient centered are medical decisions? Results of a national survey. JAMA Intern Med. 2013;173:1215-1221.
9. Spatz ES, Krumhoiz HM, Moulton BW. The new era of informed consent: getting to a reasonable-patient standard through shared decision making. JAMA. 2016;315:2063-2064.
10. Faden RR, Becker C, Lewis C, et al. Disclosure of information to patients in medical care. Med Care. 1981;19:718-733.
11. Zipkin DA, Umscheid CA, Keating NL, et al. Evidence-based risk communication: a systematic review. Ann Intern Med. 2014;161:270-280.
12. Fagerlin A, Zikmund-Fisher BJ, Ubel PA. Helping patients decide: ten steps to better risk communication. J Natl Cancer Inst. 2011;103:1436-1443.
13. Stovitz SD, Shrier I. Medical decision making and the importance of baseline risk. Br J Gen Pract. 2013;63:e795-e797.
14. Lipkus IM, Samsa G, Rimer BK. General performance on a numeracy scale among highly educated samples. Med Decis Making. 2001;21:37-44.
15. Stacey D, Bennett CL, Barry MJ, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2011:CD001431.
16. Mayo Clinic. Statin choice decision aid. Available at: https://statindecisionaid.mayoclinic.org/index.php/statin/index. Accessed September 6, 2017.
17. Risk Science Center and Center for Bioethics and Social Sciences in Medicine, University of Michigan. Icon Array. Available at: http://www.iconarray.com/. Accessed September 6, 2017.
18. Price M, Cameron R, Butow P. Communicating risk information: the influence of graphical display format on quantitative information perception–accuracy, comprehension and preferences. Patient Educ Couns. 2007;69:121-128.
19. Kon AA. The shared decision making continuum. JAMA. 2010;304:903-904.
20. Légaré F1, Ratté S, Gravel K, et al. Barriers and facilitators to implementing shared decision making in clinical practice: update of a systematic review of health professionals’ perceptions. Patient Educ Couns. 2008;73:526-535.
21. Haidet P, Paterniti DA. “Building” a history rather than “taking” one: a perspective on information sharing during the medical interview. Arch Intern Med. 2003;163:1134-1140.
22. Frankel RM, Stein R. Getting the most out of the clinical encounter: the four habits model. J Med Pract Manage. 2001;16:184-194.
23. Delbanco TL. Enriching the doctor-patient relationship by inviting the patient’s perspective. Ann Intern Med. 1992;116:414-418.
24. Doukas DJ, McCullough LB. The values history: the evaluation of the patient’s values and advance directives. J Fam Pract. 1991;32:145-153.
25. Stiggelbout AM, Van der Weijden T, De Wit MP, et al. Shared decision making: really putting patients at the centre of healthcare. BMJ. 2012;344:e256.
26. Bernacki RE, Block SD, American College of Physicians High Value Care Task Force. Communication about serious illness care goals: a review and synthesis of best practices. JAMA Intern Med. 2014;174:1994-2003.
27. Katz J. The Silent World of Doctor and Patient. Baltimore, MD, and London, England: The Johns Hopkins University Press; 1984.
28. Baylis F, Downie J. Professional recommendations: disclosing facts and values. J Med Ethics. 2001;27:20-24.
29. Sackett DL, Rosenburg WM, Gray JA, et al. Evidence based medicine: what it is and what it isn’t. BMJ. 1996;312:71-72.
30. Montori VM, Brito JP, Murad MH. The optimal practice of evidence-based medicine: incorporating patient preferences in practice guidelines. JAMA. 2013;310:2053-2054.
31. Mora S, Ames JM, Manson JE. Low-dose aspirin in the primary prevention of cardiovascular disease: shared decision making in clinical practice. JAMA. 2016;316:709-710.
32. Stovitz SD, Satin D, Shrier I. Shared decision making regarding aspirin in primary prevention of cardiovascular disease. JAMA. 2016;316:2276.
PRACTICE RECOMMENDATIONS
› Provide patients with information in terms of absolute and baseline risks, ideally using pictograph decision aids. A
› Elicit the patient’s values and priorities by categorizing decisions and asking broad open-ended questions. C
› Offer patients a professional (not a personal) recommendation, including your rationale. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
8 viral exanthems of childhood
Family physicians encounter skin rashes on a daily basis. First steps in making the diagnosis include identifying the characteristics of the rash and determining whether the eruption is accompanied by fever or any other symptoms. In the article that follows, we review 8 viral exanthems of childhood that range from the common (chickenpox) to the not-so-common (Gianotti-Crosti syndrome).
Varicella-zoster virus
Varicella-zoster virus (VZV) is a human neurotropic alphaherpesvirus that causes a primary infection commonly known as chickenpox (varicella).1 This disease is usually mild and resolves spontaneously.
This highly contagious virus is transmitted by directly touching the blisters, saliva, or mucus of an infected person. It is also transmitted through the air by coughing and sneezing. VZV initiates primary infection by inoculating the respiratory mucosa. It then establishes a lifelong presence in the sensory ganglionic neurons and, thus, can reactivate later in life causing herpes zoster (shingles), which can affect cranial, thoracic, and lumbosacral dermatomes. Acute or chronic neurologic disorders, including cranial nerve palsies, zoster paresis, vasculopathy, meningoencephalitis, and multiple ocular disorders, have been reported after VZV reactivation resulting in herpes-zoster.1
Presentation. With varicella, an extremely pruritic rash follows a brief prodromal stage consisting of a low-grade fever, upper respiratory tract symptoms, tiredness, and fatigue. This exanthem develops rapidly, often beginning on the chest, trunk, or scalp and then spreading to the arms and legs (centrifugally) (FIGURE 1). Varicella also affects mucosal areas of the body, such as the conjunctiva, mouth, rectum, and vagina.
The lesions are papules that rapidly become vesicular with clear fluid inside. Subsequently, the lesions begin to crust. Scabbing occurs within 10 to 14 days. A sure sign of chickenpox is the presence of papules, vesicles, and crusting lesions in close proximity.
Complications. The most common complications of chickenpox—especially in children—are invasive streptococcal and staphylococcal infections.2 The most serious complication occurs when the virus invades the spinal cord, causing myelitis or affecting the cerebral arteries, leading to vasculopathy. Diagnosis of VZV in the central nervous system is based on isolation of the virus in cerebral spinal fluid by polymerase chain reaction (PCR). Early diagnosis is important to minimize morbidity and mortality.
Reactivation is sometimes associated with post-herpetic neuralgia (PHN), a severe neuropathic pain syndrome isolated to the dermatomes affected by VZV. PHN can cause pain and suffering for years after shingles resolves, and sometimes is refractory to treatment. PHN may reflect a chronic varicella virus ganglionitis.
A number of treatment choices exist for shingles, but not so much for varicella
Oral treatment. Oral medications such as acyclovir and its prodrug valacyclovir are the current gold standards for the treatment of VZV.3
Famciclovir, the prodrug of penciclovir, is more effective than valacyclovir at resolving acute herpes zoster rash and shortening the duration of PHN.4 Gabapentinoids (eg, pregabalin) are the only oral medications approved by the US Food and Drug Administration (FDA) to treat PHN.5
Topical medications can also be used. Lidocaine 5% is favored as first-line therapy for the amelioration of pain due to shingles, as it provides modest pain relief with a better safety and tolerability profile than capsaicin 8% patch, which is a second-line choice. The latter must be applied multiple times daily, has minimal analgesic efficacy, and frequently causes initial pain upon application.
Gabapentinoids and topical analgesics can be used in combination due to the low propensity for drug interactions.6,7 The treatment of choice for focal vasculopathy is intravenous acyclovir, usually for 14 days, although immunocompromised patients should be treated for a longer period of time. Also consider 5 days of steroid therapy for patients with VZV vasculopathy.8
Non-FDA approved treatments include tricyclic antidepressants (TCA), such as amitriptyline, nortriptyline, and desipramine, which are sometimes used as first-line therapy for shingles. TCAs may not work well in patients with burning pain, and can have significant adverse effects, including possible cardiotoxicity.9
Opioids, including oxycodone, morphine, methadone, and tramadol, are sometimes used in pain management, but concern exists for abuse. Because patients may develop physical dependence, use opioids with considerable caution.10
Prevention. The United States became the first country to institute a routine varicella immunization program after a varicella vaccine (Varivax) was licensed in 1995.11 The vaccine has reduced the number of varicella infection cases dramatically.11 Vaccine effectiveness is high, and protective herd immunity is obtained after 2 doses.11-13 The vaccine is administered to children after one year of age with a booster dose administered after the fourth birthday.
A live, attenuated VZV vaccine (Zostavax) is given to individuals ≥60 years of age to prevent or attenuate herpes zoster infection. This vaccine is used to boost VZV-specific cell-mediated immunity in adults, thereby decreasing the burden of herpes zoster and the pain associated with PHN.14
Roseola
Roseola infantum, also known as exanthema subitum and sixth disease, is a common mild acute febrile illness of childhood caused by infection with human herpesvirus (HHV) 6 (the primary agent causing roseola) or 7 (a secondary causal agent for roseola).15 HHV-6 has 2 variants (HHV-6a and HHV-6b). Roseola infantum is mostly associated with the HHV-6b variant, which predominantly affects children 6 to 36 months of age.16
The virus replicates in the salivary glands and is shed through saliva, which is the route of transmission. After a 10- to 15-day incubation period, it remains latent in lymphocytes and monocytes, thus persisting in cells and tissues. It may reactivate late in life, particularly in immunosuppressed individuals. Reactivated infection in immunocompromised patients may be associated with serious illness such as encephalitis/encephalopathy. In patients who have received a bone marrow transplant, it can induce graft vs host disease.17
Presentation. The virus causes a 5- to 6-day illness characterized by high fever (temperature as high as 105°-106° F), miringitis (inflammation of tympanic membranes), and irritability. Once defervescence occurs, an erythematous morbilliform exanthem appears.The rash, which has a discrete macular/papular soft-pink appearance, starts on the trunk and spreads centrifugally to the extremities, neck, and face (FIGURE 2). It usually resolves within one to 2 days.
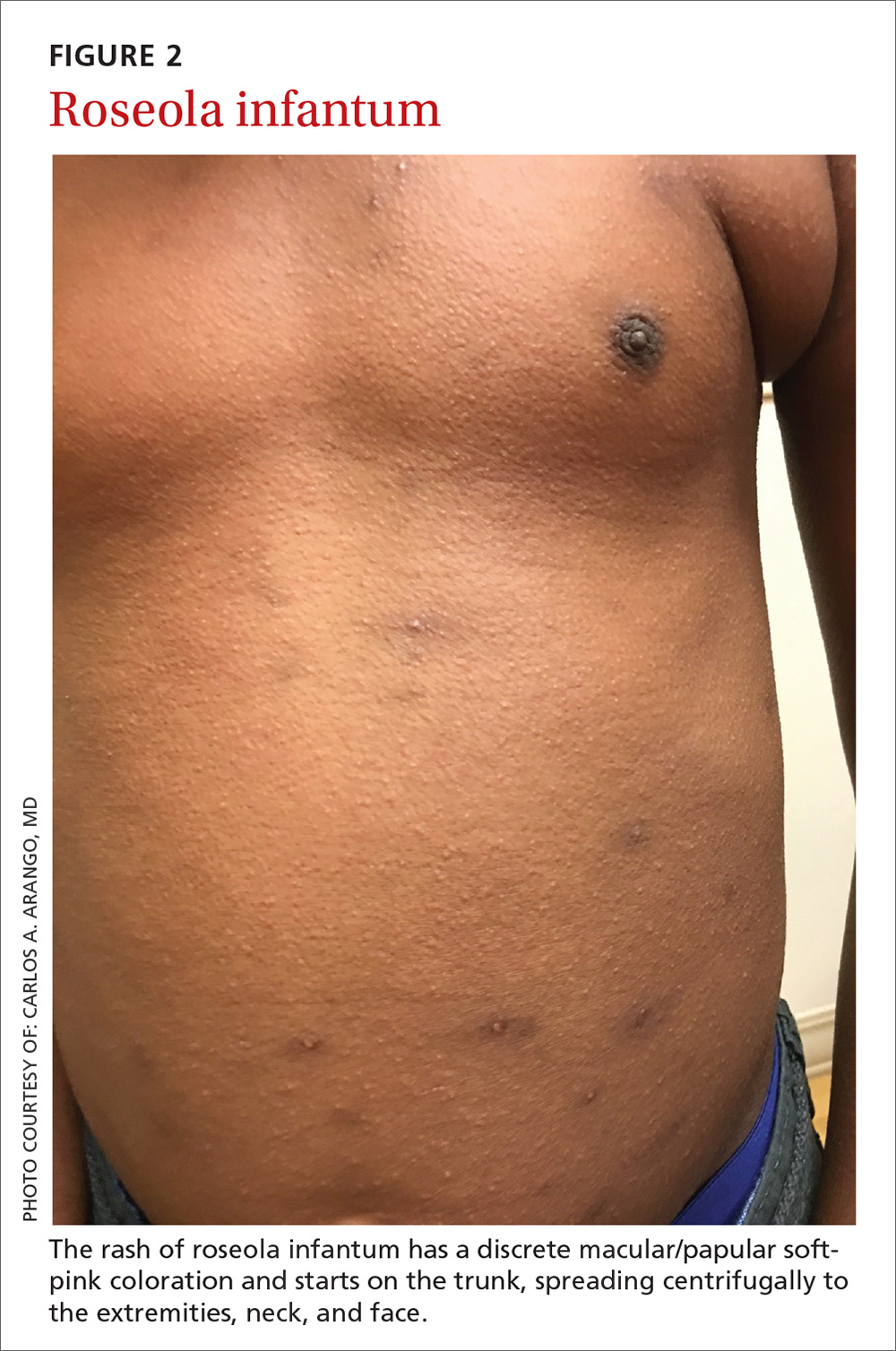
Complications. The most common complication of roseola is febrile seizures.17 Less common ones include encephalitis, encephalopathy, fatal hemophagocytic syndrome,18 or fulminant hepatitis.19
Treatment and prevention. Treatment depends on symptoms and may include antipyretics for fever management and liquids to maintain hydration. Recovery is usually complete with no significant sequelae. If a child develops a seizure, no antiepileptic drugs are recommended. No vaccine exists.
Fifth disease
Human parvovirus B19, a minute ssDNA virus, was first associated with human disease in 1981, when it was linked to an aplastic crisis in a patient with sickle cell disease.20 Since then researchers have determined that it is also the cause of erythema infectiosum or fifth disease of childhood. The B19 virus can also cause anemia in the fetus as well as hydrops fetalis. It has been linked to arthralgia and arthritis (especially in adults). There is an association with autoimmune diseases with characteristics similar to rheumatoid arthritis.20
The B19 virus is transmitted via aerosolized respiratory secretions, contaminated blood, or the placenta. The virus replicates in erythroid cells in bone marrow and peripheral blood, thus inhibiting erythropoiesis.21 Once the rash appears, the virus is no longer infectious.22 Seasonal peaks occur in the late winter and spring, with sporadic infections throughout the year.23 More than 70% of the adult population is seropositive for this virus.20
Presentation. Erythema infectiosum is a mild illness in childhood with an incubation period of 6 to 18 days. It presents with a characteristic malar rash on the face that gives patients a slapped cheek appearance (FIGURE 3A). A softer pink-colored “lacy” reticulated rash that blanches when touched may appear on the trunk, arms, and legs (FIGURE 3B).
Another presentation, which involves the hands and feet (glove and sock syndrome) (FIGURES 3C and 3D), consists of a purpuric eruption with painful edema and numerous small confluent petechiae.22,24 A majority of patients present with inflammatory symptoms that tend to resolve without sequelae within 3 weeks of infection.23
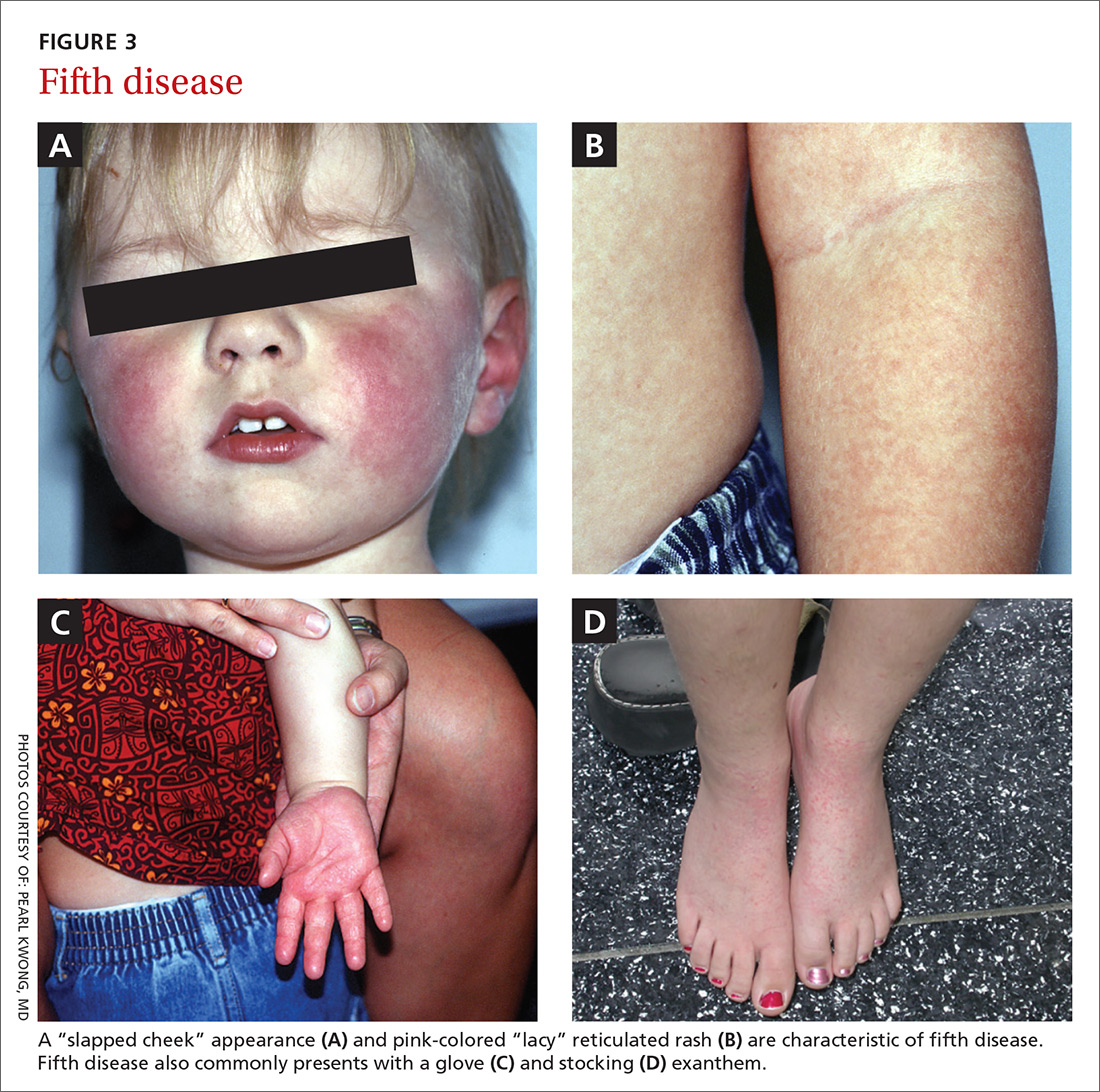
A rash is not as prevalent in adults as in children. Adults often present with more systemic systems, such as a debilitating influenza-like illness, arthropathy, transient aplastic anemia in sickle cell-affected individuals, and persistent viral suppression of erythrocyte production in immunocompromised patients and organ-transplant recipients.
Complications. The B19 virus can cause spontaneous abortion in pregnant women and anemia and hydrops fetalis in the fetus.22 Arthritis can occur in children, but is more common in adults, especially in women. The arthritis tends to be symmetrical and affects small joints such as the hands, wrists, and knees.
In one study of parvovirus B19 involving 633 children with sickle cell disease, 68 children developed transient red cell aplasia, 19% of them developed splenic sequestration, and 12% developed acute chest syndrome, a lung-related complication of sickle cell disease that can lower the level of oxygen in the blood and can be fatal.25
Treatment and prevention. Treatment of B19 infection is symptomatic; for example, nonsteroidal anti-inflammatory drugs (NSAIDs) are used if joint pain develops. No vaccine exists.
Hand, foot, and mouth disease
Hand, foot, and mouth disease (HFMD) is caused by the picornavirus family, including the Coxsackievirus, Enterovirus, and Echovirus. Infections commonly occur in the spring, summer, and fall. The virus primarily affects infants and children <10 years of age with the infection typically lasting 7 to 10 days.26
Presentation. The disease usually presents with a febrile episode, progressing to nasal congestion and malaise. One to 2 days later, the classic rash appears. Patients with HFMD usually present with papular or vesicular lesions on the hands and feet and painful oral lesions (FIGURES 4A, 4B, and 4C). The rash may also affect other parts of the body including the legs and buttocks. Desquamation of nails may occur up to one month after the HFM infection.27 Most cases are diagnosed by clinical presentation, but infection can be confirmed by PCR of vesicular lesion fluid.
Complications. In addition to being caused by Coxsackievirus, HFMD may be caused by human Enterovirus A serotype 71 (HEVA-71), which is associated with a high prevalence of acute neurologic disease including aseptic meningitis, poliomyelitis-like paralysis, and encephalitis.26 Of 764 HFMD patients enrolled in a prospective study, 214 cases were associated with Coxsackievirus A 16 (CVA16) infection and 173 cases were associated with HEVA-71 infection. Rare cases of HFMD have led to encephalitis, meningitis, flaccid paralysis, and death.26
Treatment and prevention. HFMD is usually self-limited, and treatment is supportive. There has been interest in developing an HFMD vaccine, but no products are as yet commercially available.
Rubella
Rubella, also known as the German measles or the 3-day measles, is caused by the rubella virus, which is transmitted via respiratory droplets. Up to half of rubella infections are asymptomatic.28-30
Presentation. Rubella typically has an incubation period of 12 to 24 days, with a 5-day prodromal period characterized by fever, headache, and other symptoms typical of an upper respiratory infection, including sore throat, arthralgia, and tender lymphadenopathy.28
The rash often starts as erythematous or as rose-pink macules on the face that progress down the body. The rash can cover the trunk and extremities within 24 hours. (For photos, see https://www.cdc.gov/rubella/about/photos.html.)
Patients are infectious from 7 days prior to the appearance of the rash to 7 days after resolution of the rash. Given the potentially prolonged infectious period, patients hospitalized for rubella infection should be placed on droplet precautions, and children should be kept from day care and school for 7 days after the appearance of the rash.28
Rubella is typically a mild disease in immunocompetent patients; however, immunocompromised patients may develop pneumonia, coagulopathies, or neurologic sequelae including encephalitis.
Complications. Rubella infection, especially during the first trimester, can lead to spontaneous abortion, stillbirth, or congenital rubella syndrome (CRS), a condition characterized by congenital cataracts and “blueberry muffin” skin lesions.31 Infants affected by CRS can also have heart defects, intellectual disabilities, deafness, and low birth weight. Diagnosis of primary maternal infection should be made with serologic tests. Fetal infection can be determined by detection of fetal serum IgM after 22 to 24 weeks of gestation or with viral culture of amniotic fluid.31
Treatment and prevention. Currently, no antiviral treatments are available; however, vaccines are highly effective at preventing infection. Rubella vaccine is usually given as part of the measles, mumps, rubella (MMR) vaccine, which is administered at age 12 to 15 months and again between 4 and 6 years of age.
Measles
Measles is a highly contagious disease caused by a virus that belongs to the Morbillivirus genus of the family Paramyxoviridae. Infection occurs through inhalation of, or direct contact with, respiratory droplets from infected people.32
Presentation. People with measles often present with what starts as a macular rash on the face that then spreads downward to the neck, trunk, and extremities (for photos, see http://www.cdc.gov/measles/hcp/index.html). As the disease progresses, papules can develop and the lesions may coalesce.
The rash is often preceded by 3 to 4 days of malaise and fever (temperature often greater than 104° F), along with the classic symptom triad of cough, coryza, and conjunctivitis. Koplik spots—clustered white lesions on the buccal mucosa—are often present prior to the measles rash and are pathognomonic for measles infection.33
Because the symptoms of measles are easily confused with other viral infections, suspected cases of measles should be confirmed via IgG and IgM antibody tests, by reverse transcription-PCR, or both.34,35 For limited and unusual cases, the Centers for Disease Control and Prevention can perform a plaque reduction neutralization assay.35
Complications. Measles infection is self-limited in immunocompetent patients. The most common complications are diarrhea and ear infections, but more serious complications, such as pneumonia, hearing loss, and encephalitis, can occur. Children <2 years of age, particularly boys, are at an increased risk of developing subacute sclerosing panencephalitis, a fatal neurologic disorder that can develop years after the initial measles infection.33,36
Treatment and prevention. Treatment is supportive and usually consists of acetaminophen or NSAIDs and fluids.
A live attenuated version of the measles vaccine is highly effective against the measles virus and has greatly reduced the number of measles cases globally.37 The measles vaccine is usually given in 2 doses—the first one after one year of age, and the second one before entering kindergarten. The most common adverse reactions to the vaccine are pain at the injection site and fever. Despite the fact that the MMR vaccine is effective and relatively benign, measles outbreaks continue to occur, as some parents forego routine childhood immunizations because of religious or other personal beliefs or safety concerns.38
Molluscum contagiosum
Molluscum contagiosum (MC) is caused by the MC virus, a member of the poxvirus family. The virus is transmitted by direct contact with the skin lesions. This skin condition is seen mainly in children, although it can occur in adults.
A study conducted in England and Wales that obtained information from the Royal College of General Practitioners reported an incidence of 15.0-17.2/1000 population over a 10-year period (1994-2003) with no variation between sexes.39 There is an association between atopic dermatitis and MC; 24% of children with atopic dermatitis develop MC.40 There might also be an association between recent swimming in a public pool and development of MC lesions.41
Presentation. Lesions caused by MC are small, discrete, waxy dome-shaped papules with central umbilication that are usually 3 to 5 mm in diameter (FIGURE 5).42,43 In immunocompetent patients, there are usually fewer than 20 lesions, which resolve within a year. However, in immunocompromised patients, the number of lesions is usually higher, and the diameter of each may be greater than 1 cm.42
Complications. The lesions are usually self-limited, but on occasion can become secondarily infected, usually with gram-positive organisms such as Staphylococcus aureus. Very rarely, abscesses develop requiring topical and/or systemic antimicrobials and perhaps incision and drainage.44
Treatment and prevention. Because the infection is often self-limited and benign, the preferred therapeutic modality is watchful waiting. Other treatments for MC include curettage, chemical agents, immune modulators, and antiviral drugs. A 2009 Cochran review of 11 studies involving 495 patients found “no single intervention to be convincingly effective in the treatment of molluscum contagiosum.”45 And no vaccine exists.
Gianotti-Crosti syndrome
Gianotti-Crosti syndrome (GCS), also known as papular acrodermatitis of childhood, is a relatively rare, self-limited exanthema that usually affects infants and children 6 months to 12 years of age (peak occurrence is in one- to 6-year-olds). Although there have been reports of adults with this syndrome, it is unusual in this age group.
Pathogenesis is still unknown. Although GCS itself isn’t contagious, the viruses that can cause it may be. Initially, researchers believed that GCS was linked to acute hepatitis B virus infection, but more recently other viral and bacterial infections have been associated with the condition.46
The most commonly associated virus in the United States is Epstein-Barr virus; other viruses include hepatitis A virus, cytomegalovirus, coxsackievirus, respiratory syncytial virus, parainfluenza virus, rotavirus, the mumps virus, parvovirus, and molluscum contagiosum.
Bacterial infections, such as those caused by Bartonella henselae, Mycoplasma pneumoniae, and group A streptococci may trigger GCS.47-49
Vaccines that have been implicated in GCS include those for polio, diphtheria/pertussis/tetanus, MMR, hepatitis A and B, as well as the influenza vaccine.48-51
Presentation. While GCS is relatively rare, its presentation is classic, making it easy to diagnose once it’s included in the differential. The pruritic rash usually consists of acute-onset monomorphous, flat-topped or dome-shaped red-brown papules and papulovesicles, one to 10 mm in size, located symmetrically on the face (FIGURE 6), the extensor surfaces of the arms and legs, and, less commonly, the buttocks. It rarely affects other parts of the body.48
The diagnosis is usually based on the characteristic rash and the benign nature of the condition; other than the rash, patients are typically asymptomatic and healthy. Sometimes a biopsy is performed and it reveals a dense lichenoid lymphohistiocytic infiltrate with a strong cytoplasmic immunopositivity for beta-defensin-4 in the stratum corneum, granulosum, and spinosum.52
The lesions spontaneously resolve within 8 to 12 weeks. GCS usually presents during spring and early summer and affects both sexes equally.46
Treatment and prevention. Treatment is usually symptomatic, with the use of oral antihistamines if the lesions become pruritic. Topical steroids may be used, and, in a few cases, oral corticosteroids may be considered. No vaccine exists.
CORRESPONDENCE
Carlos A. Arango, MD, 8399 Bayberry Road, Jacksonville, FL 32256; carlos.arango@jax.ufl.edu.
1. Kennedy PG, Rovnak J, Badani H, et al. A comparison of herpes simplex virus type 1 and varicella-zoster virus latency and reactivation. J Gen Virol. 2015;96(Pt 7):1581-1602.
2. Blumental S, Sabbe M, Lepage P, the Belgian Group for Varicella. Varicella paediatric hospitalisations in Belgium: a 1-year national survey. Arch Dis Child. 2016;101:16-22.
3. Sampathkumar P, Drage LA, Martin DP. Herpes zoster (shingles) and postherpetic neuralgia. Mayo Clinic Proc. 2009;84:274-280.
4. Ono F, Yasumoto S, Furumura M, et al. Comparison between famciclovir and valacyclovir for acute pain in adult Japanese immunocompetent patients with herpes zoster. J Dermatol. 2012;39:902-908.
5. Massengill JS, Kittredge JL. Practical considerations in the pharmacological treatment of post-herpetic neuralgia for the primary care provider. J Pain Res. 2014;7:125-132.
6. Nalamachu S, Morley-Forster P. Diagnosing and managing postherpetic neuralgia. Drugs & Aging. 2012;29:863-869.
7. Hempenstall K, Nurmikko TJ, Johnson RW, et al. Analgesic therapy in postherpetic neuralgia: a quantitative systematic review. PLoS Med. 2005;2:e164.
8. Gilden D, Cohrs RJ, Mahalingam R, et al. Varicella zoster virus vasculopathies: diverse clinical manifestations, laboratory features, pathogenesis, and treatment. Lancet Neurol. 2009;9:731-740.
9. Stankus SJ, Dlugopolski M, Packer D. Management of herpes zoster (shingles) and post herpetic neuralgia. Am Fam Physician. 2000;61:2437-2444.
10. Dworkin RH, O’Connor AB, Audette J, et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc. 2010;85(3 Suppl):S3-S14.
11. Thomas CA, Shwe T, Bixler D, et al. Two-dose varicella vaccine effectiveness and rash severity in outbreaks of varicella among public school students. Pediatr Infect Dis J. 2014;33:1164-1168.
12. Helmuth IG, Poulsen A, Suppli CH, et al. Varicella in Europe-a review of the epidemiology and experience with vaccination. Vaccine. 2015;33:2406-2413.
13. Marin M, Marti M, Kambhampati A, et al. Global varicella vaccine effectiveness: a metanalysis. Pediatrics. 2016;137:e20153741.
14. Centers for Disease Control and Prevention. What everyone should know about shingles vaccine. Available at: www.cdc.gov/vaccines/vpd/shingles/public/index.html. Accessed September 12, 2017.
15. Tanaka K, Kondo T, Torigoe S, et al. Human herpesvirus 7: another causal agent for roseola (exanthem subitum). J Pediatr. 1994;125:1-5.
16. Caserta MT, Mock DJ, Dewhurst S. Human herpesvirus 6. Clin Infect Dis. 2001;33:829-833.
17. Koch WC. Fifth (human parvovirus) and sixth (herpesvirus 6) diseases. Curr Opin Infect Dis. 2001;14:343-356.
18. Marabelle A, Bergeron C, Billaud G, et al. Hemophagocytic syndrome revealing primary HHV-6 infection. J Pediatr. 2010;157:511.
19. Charnot-Katsikasa A, Baewer D, Cook L, et al. Fulminant hepatic failure attributed to infection with human herpesvirus 6 (HHV-6) in an immunocompetent woman: a case report and review of the literature. J Clin Virol. 2016;75:27-32.
20. Corcoran A, Doyle S. Advances in the biology, diagnosis and host-pathogen interactions of parvovirus B19. J Med Microbiol. 2004;53(Pt 6):459-475.
21. Dolin R. Parvovirus erythema infectiousum, Aplastic anemia. In: Mandell, Douglas, Bennett’s Principles and Practice of Infectious Diseases. 3rd ed. New York, NY: Churchill Livingstone Inc; 1990:1231-1232.
22. Servey JT, Reamy BV, Hodge J. Clinical presentations of parvovirus B19 infection. Am Fam Physician. 2007;75:373-376.
23. Martin DR, Schlott DW, Flynn JA. Clinical problem-solving. No respecter of age. N Engl J Med. 2007;357:1856-1859.
24. Ozaydin V, Eceviz A, Sari Dogan F, et al. An adult patient who presented to emergency service with a papular purpuric gloves and socks syndrome: a case report. Turk J Emerg Med. 2014;14:179-181.
25. Smith-Whitley K, Zhao H, Hodinka RL, et al. Epidemiology of human parvovirus B19 in children with sickle cell disease. Blood. 2004;103:422-427.
26. Tu PV, Thao NT, Perera D, et al. Epidemiologic and virologic investigation of hand, foot, and mouth disease, Southern Vietnam, 2005. Emerg Infect Dis. 2007;13:1733-1741.
27. Ferrari B, Taliercio V, Hornos L, et al. Onychomadesis associated with mouth, hand and foot disease. Arch Argent Pediatr. 2013;111:e148-e151.
28. Alter SJ, Bennett JS, Koranyi K, et al. Common childhood viral infections. Curr Probl Pediatr Adolesc Health Care. 2015;45:21-53.
29. Lambert N, Strebel P, Orenstein W, et al. Rubella. Lancet. 2015;385:2297-2307.
30. Silasi M, Cardenas I, Kwon JY, et al. Viral infections during pregnancy. Am J Reprod Immunol. 2015;73:199-213.
31. Tang JW, Aarons E, Hesketh LM, et al. Prenatal diagnosis of congenital rubella infection in the second trimester of pregnancy Prenat Diagn. 2003;23:509-512.
32. Naim HY. Measles virus. Hum Vaccin Immunother. 2015;11:21-26.
33. Centers for Disease Control and Prevention. Measles (Rubeola). Available at: http://www.cdc.gov/measles/hcp/index.html. Accessed April 28, 2016.
34. Takao S, Shigemoto N, Shimazu Y, et al. Detection of exanthematic viruses using a TaqMan real-time PCR assay panel in patients with clinically diagnosed or suspected measles. Jpn J Infect Dis. 2012;65:444-448.
35. Centers for Disease Control and Prevention. Measles (Rubeola). Available at: https://www.cdc.gov/measles/lab-tools/rt-pcr.html. Accessed April 28, 2016.
36. Griffin DE, Lin WH, Pan CH. Measles virus, immune control, and persistence. FEMS Microbiol Rev. 2012;36:649-662.
37. Centers for Disease Control and Prevention. Measles, vaccination. Available at: https://www.cdc.gov/measles/vaccination.html. Accessed April 28, 2016.
38. Campos-Outcalt D. Measles: Why it’s still a threat. J Fam Pract. 2017;66:446-449.
39. Olsen JR, Gallacher J, Piguet V, et al. Epidemiology of molluscum contagiosum in children: a systematic review. Fam Pract. 2014;31:130-136.
40. Dohil MA, Lin P, Lee J, et al. The epidemiology of molluscum contagiosum in children. J Am Acad Dermatol. 2006;54:47-54.
41. Choong KY, Roberts LJ. Molluscum contagiosum, swimming and bathing: a clinical analysis. Australas J Dermatol. 1999;40:89-92.
42. Martin P. Interventions for molluscum contagiosum in people infected with human immunodeficiency virus: a systematic review. Int J Dermatol. 2016;55:956-966.
43. Chen X, Anstey AV, Bugert JJ. Molluscum contagiosum virus infection. Lancet Infect Dis. 2013;13:877-888.
44. Lacour M, Posfay-Barbe KM, La Scala GC. Staphylococcus lugdunensis abscesses complicating molluscum contagiosum in two children. Pediatr Dermatol. 2015;32:289-291.
45. van der Wouden JC, van der Sande R, van Suijlekom-Smit LW, et al. Interventions for cutaneous molluscum contagiosum. Cochrane Database Syst Rev. 2009;CD004767.
46. Tagawa C, Speakman M. Photo quiz. Papular rash in a child after a fever. Gianotti-Crosti syndrome. Am Fam Physician. 2013;87:59-60.
47. Brandt O, Abeck D, Gianotti R, et al. Gianotti-Crosti syndrome. J Am Acad Dermatol. 2006;54:136-145.
48. Retrouvey M, Koch LH, Williams JV. Gianotti-Crosti syndrome following childhood vaccinations. Pediatr Dermatol. 2013;30:137-138.
49. Velangi SS, Tidman MJ. Gianotti-Crosti syndrome after measles, mumps, and rubella vaccination. Br J Dermatol. 1998;139:1122-1123.
50. Lacour M, Harms M. Gianotti-Crosti syndrome as a result of vaccination and Epstein-Barr virus infection. Eur J Pediatr. 1995;154:688-689.
51. Kroeskop A, Lewis AB, Barril FA, et al. Gianotti-Crosti syndrome after H1N1-influenza vaccine. Pediatr Dermatol. 2011;28:595-596.
52. Caltabiano R, Vecchio GM, De Pasquale R, et al. Human beta-defensin 4 expression in Gianotti-Crosti. Acta Dermatovenerol Croat. 2013;21:43-47.
Family physicians encounter skin rashes on a daily basis. First steps in making the diagnosis include identifying the characteristics of the rash and determining whether the eruption is accompanied by fever or any other symptoms. In the article that follows, we review 8 viral exanthems of childhood that range from the common (chickenpox) to the not-so-common (Gianotti-Crosti syndrome).
Varicella-zoster virus
Varicella-zoster virus (VZV) is a human neurotropic alphaherpesvirus that causes a primary infection commonly known as chickenpox (varicella).1 This disease is usually mild and resolves spontaneously.
This highly contagious virus is transmitted by directly touching the blisters, saliva, or mucus of an infected person. It is also transmitted through the air by coughing and sneezing. VZV initiates primary infection by inoculating the respiratory mucosa. It then establishes a lifelong presence in the sensory ganglionic neurons and, thus, can reactivate later in life causing herpes zoster (shingles), which can affect cranial, thoracic, and lumbosacral dermatomes. Acute or chronic neurologic disorders, including cranial nerve palsies, zoster paresis, vasculopathy, meningoencephalitis, and multiple ocular disorders, have been reported after VZV reactivation resulting in herpes-zoster.1
Presentation. With varicella, an extremely pruritic rash follows a brief prodromal stage consisting of a low-grade fever, upper respiratory tract symptoms, tiredness, and fatigue. This exanthem develops rapidly, often beginning on the chest, trunk, or scalp and then spreading to the arms and legs (centrifugally) (FIGURE 1). Varicella also affects mucosal areas of the body, such as the conjunctiva, mouth, rectum, and vagina.
The lesions are papules that rapidly become vesicular with clear fluid inside. Subsequently, the lesions begin to crust. Scabbing occurs within 10 to 14 days. A sure sign of chickenpox is the presence of papules, vesicles, and crusting lesions in close proximity.
Complications. The most common complications of chickenpox—especially in children—are invasive streptococcal and staphylococcal infections.2 The most serious complication occurs when the virus invades the spinal cord, causing myelitis or affecting the cerebral arteries, leading to vasculopathy. Diagnosis of VZV in the central nervous system is based on isolation of the virus in cerebral spinal fluid by polymerase chain reaction (PCR). Early diagnosis is important to minimize morbidity and mortality.
Reactivation is sometimes associated with post-herpetic neuralgia (PHN), a severe neuropathic pain syndrome isolated to the dermatomes affected by VZV. PHN can cause pain and suffering for years after shingles resolves, and sometimes is refractory to treatment. PHN may reflect a chronic varicella virus ganglionitis.
A number of treatment choices exist for shingles, but not so much for varicella
Oral treatment. Oral medications such as acyclovir and its prodrug valacyclovir are the current gold standards for the treatment of VZV.3
Famciclovir, the prodrug of penciclovir, is more effective than valacyclovir at resolving acute herpes zoster rash and shortening the duration of PHN.4 Gabapentinoids (eg, pregabalin) are the only oral medications approved by the US Food and Drug Administration (FDA) to treat PHN.5
Topical medications can also be used. Lidocaine 5% is favored as first-line therapy for the amelioration of pain due to shingles, as it provides modest pain relief with a better safety and tolerability profile than capsaicin 8% patch, which is a second-line choice. The latter must be applied multiple times daily, has minimal analgesic efficacy, and frequently causes initial pain upon application.
Gabapentinoids and topical analgesics can be used in combination due to the low propensity for drug interactions.6,7 The treatment of choice for focal vasculopathy is intravenous acyclovir, usually for 14 days, although immunocompromised patients should be treated for a longer period of time. Also consider 5 days of steroid therapy for patients with VZV vasculopathy.8
Non-FDA approved treatments include tricyclic antidepressants (TCA), such as amitriptyline, nortriptyline, and desipramine, which are sometimes used as first-line therapy for shingles. TCAs may not work well in patients with burning pain, and can have significant adverse effects, including possible cardiotoxicity.9
Opioids, including oxycodone, morphine, methadone, and tramadol, are sometimes used in pain management, but concern exists for abuse. Because patients may develop physical dependence, use opioids with considerable caution.10
Prevention. The United States became the first country to institute a routine varicella immunization program after a varicella vaccine (Varivax) was licensed in 1995.11 The vaccine has reduced the number of varicella infection cases dramatically.11 Vaccine effectiveness is high, and protective herd immunity is obtained after 2 doses.11-13 The vaccine is administered to children after one year of age with a booster dose administered after the fourth birthday.
A live, attenuated VZV vaccine (Zostavax) is given to individuals ≥60 years of age to prevent or attenuate herpes zoster infection. This vaccine is used to boost VZV-specific cell-mediated immunity in adults, thereby decreasing the burden of herpes zoster and the pain associated with PHN.14
Roseola
Roseola infantum, also known as exanthema subitum and sixth disease, is a common mild acute febrile illness of childhood caused by infection with human herpesvirus (HHV) 6 (the primary agent causing roseola) or 7 (a secondary causal agent for roseola).15 HHV-6 has 2 variants (HHV-6a and HHV-6b). Roseola infantum is mostly associated with the HHV-6b variant, which predominantly affects children 6 to 36 months of age.16
The virus replicates in the salivary glands and is shed through saliva, which is the route of transmission. After a 10- to 15-day incubation period, it remains latent in lymphocytes and monocytes, thus persisting in cells and tissues. It may reactivate late in life, particularly in immunosuppressed individuals. Reactivated infection in immunocompromised patients may be associated with serious illness such as encephalitis/encephalopathy. In patients who have received a bone marrow transplant, it can induce graft vs host disease.17
Presentation. The virus causes a 5- to 6-day illness characterized by high fever (temperature as high as 105°-106° F), miringitis (inflammation of tympanic membranes), and irritability. Once defervescence occurs, an erythematous morbilliform exanthem appears.The rash, which has a discrete macular/papular soft-pink appearance, starts on the trunk and spreads centrifugally to the extremities, neck, and face (FIGURE 2). It usually resolves within one to 2 days.

Complications. The most common complication of roseola is febrile seizures.17 Less common ones include encephalitis, encephalopathy, fatal hemophagocytic syndrome,18 or fulminant hepatitis.19
Treatment and prevention. Treatment depends on symptoms and may include antipyretics for fever management and liquids to maintain hydration. Recovery is usually complete with no significant sequelae. If a child develops a seizure, no antiepileptic drugs are recommended. No vaccine exists.
Fifth disease
Human parvovirus B19, a minute ssDNA virus, was first associated with human disease in 1981, when it was linked to an aplastic crisis in a patient with sickle cell disease.20 Since then researchers have determined that it is also the cause of erythema infectiosum or fifth disease of childhood. The B19 virus can also cause anemia in the fetus as well as hydrops fetalis. It has been linked to arthralgia and arthritis (especially in adults). There is an association with autoimmune diseases with characteristics similar to rheumatoid arthritis.20
The B19 virus is transmitted via aerosolized respiratory secretions, contaminated blood, or the placenta. The virus replicates in erythroid cells in bone marrow and peripheral blood, thus inhibiting erythropoiesis.21 Once the rash appears, the virus is no longer infectious.22 Seasonal peaks occur in the late winter and spring, with sporadic infections throughout the year.23 More than 70% of the adult population is seropositive for this virus.20
Presentation. Erythema infectiosum is a mild illness in childhood with an incubation period of 6 to 18 days. It presents with a characteristic malar rash on the face that gives patients a slapped cheek appearance (FIGURE 3A). A softer pink-colored “lacy” reticulated rash that blanches when touched may appear on the trunk, arms, and legs (FIGURE 3B).
Another presentation, which involves the hands and feet (glove and sock syndrome) (FIGURES 3C and 3D), consists of a purpuric eruption with painful edema and numerous small confluent petechiae.22,24 A majority of patients present with inflammatory symptoms that tend to resolve without sequelae within 3 weeks of infection.23

A rash is not as prevalent in adults as in children. Adults often present with more systemic systems, such as a debilitating influenza-like illness, arthropathy, transient aplastic anemia in sickle cell-affected individuals, and persistent viral suppression of erythrocyte production in immunocompromised patients and organ-transplant recipients.
Complications. The B19 virus can cause spontaneous abortion in pregnant women and anemia and hydrops fetalis in the fetus.22 Arthritis can occur in children, but is more common in adults, especially in women. The arthritis tends to be symmetrical and affects small joints such as the hands, wrists, and knees.
In one study of parvovirus B19 involving 633 children with sickle cell disease, 68 children developed transient red cell aplasia, 19% of them developed splenic sequestration, and 12% developed acute chest syndrome, a lung-related complication of sickle cell disease that can lower the level of oxygen in the blood and can be fatal.25
Treatment and prevention. Treatment of B19 infection is symptomatic; for example, nonsteroidal anti-inflammatory drugs (NSAIDs) are used if joint pain develops. No vaccine exists.
Hand, foot, and mouth disease
Hand, foot, and mouth disease (HFMD) is caused by the picornavirus family, including the Coxsackievirus, Enterovirus, and Echovirus. Infections commonly occur in the spring, summer, and fall. The virus primarily affects infants and children <10 years of age with the infection typically lasting 7 to 10 days.26
Presentation. The disease usually presents with a febrile episode, progressing to nasal congestion and malaise. One to 2 days later, the classic rash appears. Patients with HFMD usually present with papular or vesicular lesions on the hands and feet and painful oral lesions (FIGURES 4A, 4B, and 4C). The rash may also affect other parts of the body including the legs and buttocks. Desquamation of nails may occur up to one month after the HFM infection.27 Most cases are diagnosed by clinical presentation, but infection can be confirmed by PCR of vesicular lesion fluid.
Complications. In addition to being caused by Coxsackievirus, HFMD may be caused by human Enterovirus A serotype 71 (HEVA-71), which is associated with a high prevalence of acute neurologic disease including aseptic meningitis, poliomyelitis-like paralysis, and encephalitis.26 Of 764 HFMD patients enrolled in a prospective study, 214 cases were associated with Coxsackievirus A 16 (CVA16) infection and 173 cases were associated with HEVA-71 infection. Rare cases of HFMD have led to encephalitis, meningitis, flaccid paralysis, and death.26
Treatment and prevention. HFMD is usually self-limited, and treatment is supportive. There has been interest in developing an HFMD vaccine, but no products are as yet commercially available.
Rubella
Rubella, also known as the German measles or the 3-day measles, is caused by the rubella virus, which is transmitted via respiratory droplets. Up to half of rubella infections are asymptomatic.28-30
Presentation. Rubella typically has an incubation period of 12 to 24 days, with a 5-day prodromal period characterized by fever, headache, and other symptoms typical of an upper respiratory infection, including sore throat, arthralgia, and tender lymphadenopathy.28
The rash often starts as erythematous or as rose-pink macules on the face that progress down the body. The rash can cover the trunk and extremities within 24 hours. (For photos, see https://www.cdc.gov/rubella/about/photos.html.)
Patients are infectious from 7 days prior to the appearance of the rash to 7 days after resolution of the rash. Given the potentially prolonged infectious period, patients hospitalized for rubella infection should be placed on droplet precautions, and children should be kept from day care and school for 7 days after the appearance of the rash.28
Rubella is typically a mild disease in immunocompetent patients; however, immunocompromised patients may develop pneumonia, coagulopathies, or neurologic sequelae including encephalitis.
Complications. Rubella infection, especially during the first trimester, can lead to spontaneous abortion, stillbirth, or congenital rubella syndrome (CRS), a condition characterized by congenital cataracts and “blueberry muffin” skin lesions.31 Infants affected by CRS can also have heart defects, intellectual disabilities, deafness, and low birth weight. Diagnosis of primary maternal infection should be made with serologic tests. Fetal infection can be determined by detection of fetal serum IgM after 22 to 24 weeks of gestation or with viral culture of amniotic fluid.31
Treatment and prevention. Currently, no antiviral treatments are available; however, vaccines are highly effective at preventing infection. Rubella vaccine is usually given as part of the measles, mumps, rubella (MMR) vaccine, which is administered at age 12 to 15 months and again between 4 and 6 years of age.
Measles
Measles is a highly contagious disease caused by a virus that belongs to the Morbillivirus genus of the family Paramyxoviridae. Infection occurs through inhalation of, or direct contact with, respiratory droplets from infected people.32
Presentation. People with measles often present with what starts as a macular rash on the face that then spreads downward to the neck, trunk, and extremities (for photos, see http://www.cdc.gov/measles/hcp/index.html). As the disease progresses, papules can develop and the lesions may coalesce.
The rash is often preceded by 3 to 4 days of malaise and fever (temperature often greater than 104° F), along with the classic symptom triad of cough, coryza, and conjunctivitis. Koplik spots—clustered white lesions on the buccal mucosa—are often present prior to the measles rash and are pathognomonic for measles infection.33
Because the symptoms of measles are easily confused with other viral infections, suspected cases of measles should be confirmed via IgG and IgM antibody tests, by reverse transcription-PCR, or both.34,35 For limited and unusual cases, the Centers for Disease Control and Prevention can perform a plaque reduction neutralization assay.35
Complications. Measles infection is self-limited in immunocompetent patients. The most common complications are diarrhea and ear infections, but more serious complications, such as pneumonia, hearing loss, and encephalitis, can occur. Children <2 years of age, particularly boys, are at an increased risk of developing subacute sclerosing panencephalitis, a fatal neurologic disorder that can develop years after the initial measles infection.33,36
Treatment and prevention. Treatment is supportive and usually consists of acetaminophen or NSAIDs and fluids.
A live attenuated version of the measles vaccine is highly effective against the measles virus and has greatly reduced the number of measles cases globally.37 The measles vaccine is usually given in 2 doses—the first one after one year of age, and the second one before entering kindergarten. The most common adverse reactions to the vaccine are pain at the injection site and fever. Despite the fact that the MMR vaccine is effective and relatively benign, measles outbreaks continue to occur, as some parents forego routine childhood immunizations because of religious or other personal beliefs or safety concerns.38
Molluscum contagiosum
Molluscum contagiosum (MC) is caused by the MC virus, a member of the poxvirus family. The virus is transmitted by direct contact with the skin lesions. This skin condition is seen mainly in children, although it can occur in adults.
A study conducted in England and Wales that obtained information from the Royal College of General Practitioners reported an incidence of 15.0-17.2/1000 population over a 10-year period (1994-2003) with no variation between sexes.39 There is an association between atopic dermatitis and MC; 24% of children with atopic dermatitis develop MC.40 There might also be an association between recent swimming in a public pool and development of MC lesions.41
Presentation. Lesions caused by MC are small, discrete, waxy dome-shaped papules with central umbilication that are usually 3 to 5 mm in diameter (FIGURE 5).42,43 In immunocompetent patients, there are usually fewer than 20 lesions, which resolve within a year. However, in immunocompromised patients, the number of lesions is usually higher, and the diameter of each may be greater than 1 cm.42
Complications. The lesions are usually self-limited, but on occasion can become secondarily infected, usually with gram-positive organisms such as Staphylococcus aureus. Very rarely, abscesses develop requiring topical and/or systemic antimicrobials and perhaps incision and drainage.44
Treatment and prevention. Because the infection is often self-limited and benign, the preferred therapeutic modality is watchful waiting. Other treatments for MC include curettage, chemical agents, immune modulators, and antiviral drugs. A 2009 Cochran review of 11 studies involving 495 patients found “no single intervention to be convincingly effective in the treatment of molluscum contagiosum.”45 And no vaccine exists.
Gianotti-Crosti syndrome
Gianotti-Crosti syndrome (GCS), also known as papular acrodermatitis of childhood, is a relatively rare, self-limited exanthema that usually affects infants and children 6 months to 12 years of age (peak occurrence is in one- to 6-year-olds). Although there have been reports of adults with this syndrome, it is unusual in this age group.
Pathogenesis is still unknown. Although GCS itself isn’t contagious, the viruses that can cause it may be. Initially, researchers believed that GCS was linked to acute hepatitis B virus infection, but more recently other viral and bacterial infections have been associated with the condition.46
The most commonly associated virus in the United States is Epstein-Barr virus; other viruses include hepatitis A virus, cytomegalovirus, coxsackievirus, respiratory syncytial virus, parainfluenza virus, rotavirus, the mumps virus, parvovirus, and molluscum contagiosum.
Bacterial infections, such as those caused by Bartonella henselae, Mycoplasma pneumoniae, and group A streptococci may trigger GCS.47-49
Vaccines that have been implicated in GCS include those for polio, diphtheria/pertussis/tetanus, MMR, hepatitis A and B, as well as the influenza vaccine.48-51
Presentation. While GCS is relatively rare, its presentation is classic, making it easy to diagnose once it’s included in the differential. The pruritic rash usually consists of acute-onset monomorphous, flat-topped or dome-shaped red-brown papules and papulovesicles, one to 10 mm in size, located symmetrically on the face (FIGURE 6), the extensor surfaces of the arms and legs, and, less commonly, the buttocks. It rarely affects other parts of the body.48
The diagnosis is usually based on the characteristic rash and the benign nature of the condition; other than the rash, patients are typically asymptomatic and healthy. Sometimes a biopsy is performed and it reveals a dense lichenoid lymphohistiocytic infiltrate with a strong cytoplasmic immunopositivity for beta-defensin-4 in the stratum corneum, granulosum, and spinosum.52
The lesions spontaneously resolve within 8 to 12 weeks. GCS usually presents during spring and early summer and affects both sexes equally.46
Treatment and prevention. Treatment is usually symptomatic, with the use of oral antihistamines if the lesions become pruritic. Topical steroids may be used, and, in a few cases, oral corticosteroids may be considered. No vaccine exists.
CORRESPONDENCE
Carlos A. Arango, MD, 8399 Bayberry Road, Jacksonville, FL 32256; carlos.arango@jax.ufl.edu.
Family physicians encounter skin rashes on a daily basis. First steps in making the diagnosis include identifying the characteristics of the rash and determining whether the eruption is accompanied by fever or any other symptoms. In the article that follows, we review 8 viral exanthems of childhood that range from the common (chickenpox) to the not-so-common (Gianotti-Crosti syndrome).
Varicella-zoster virus
Varicella-zoster virus (VZV) is a human neurotropic alphaherpesvirus that causes a primary infection commonly known as chickenpox (varicella).1 This disease is usually mild and resolves spontaneously.
This highly contagious virus is transmitted by directly touching the blisters, saliva, or mucus of an infected person. It is also transmitted through the air by coughing and sneezing. VZV initiates primary infection by inoculating the respiratory mucosa. It then establishes a lifelong presence in the sensory ganglionic neurons and, thus, can reactivate later in life causing herpes zoster (shingles), which can affect cranial, thoracic, and lumbosacral dermatomes. Acute or chronic neurologic disorders, including cranial nerve palsies, zoster paresis, vasculopathy, meningoencephalitis, and multiple ocular disorders, have been reported after VZV reactivation resulting in herpes-zoster.1
Presentation. With varicella, an extremely pruritic rash follows a brief prodromal stage consisting of a low-grade fever, upper respiratory tract symptoms, tiredness, and fatigue. This exanthem develops rapidly, often beginning on the chest, trunk, or scalp and then spreading to the arms and legs (centrifugally) (FIGURE 1). Varicella also affects mucosal areas of the body, such as the conjunctiva, mouth, rectum, and vagina.
The lesions are papules that rapidly become vesicular with clear fluid inside. Subsequently, the lesions begin to crust. Scabbing occurs within 10 to 14 days. A sure sign of chickenpox is the presence of papules, vesicles, and crusting lesions in close proximity.
Complications. The most common complications of chickenpox—especially in children—are invasive streptococcal and staphylococcal infections.2 The most serious complication occurs when the virus invades the spinal cord, causing myelitis or affecting the cerebral arteries, leading to vasculopathy. Diagnosis of VZV in the central nervous system is based on isolation of the virus in cerebral spinal fluid by polymerase chain reaction (PCR). Early diagnosis is important to minimize morbidity and mortality.
Reactivation is sometimes associated with post-herpetic neuralgia (PHN), a severe neuropathic pain syndrome isolated to the dermatomes affected by VZV. PHN can cause pain and suffering for years after shingles resolves, and sometimes is refractory to treatment. PHN may reflect a chronic varicella virus ganglionitis.
A number of treatment choices exist for shingles, but not so much for varicella
Oral treatment. Oral medications such as acyclovir and its prodrug valacyclovir are the current gold standards for the treatment of VZV.3
Famciclovir, the prodrug of penciclovir, is more effective than valacyclovir at resolving acute herpes zoster rash and shortening the duration of PHN.4 Gabapentinoids (eg, pregabalin) are the only oral medications approved by the US Food and Drug Administration (FDA) to treat PHN.5
Topical medications can also be used. Lidocaine 5% is favored as first-line therapy for the amelioration of pain due to shingles, as it provides modest pain relief with a better safety and tolerability profile than capsaicin 8% patch, which is a second-line choice. The latter must be applied multiple times daily, has minimal analgesic efficacy, and frequently causes initial pain upon application.
Gabapentinoids and topical analgesics can be used in combination due to the low propensity for drug interactions.6,7 The treatment of choice for focal vasculopathy is intravenous acyclovir, usually for 14 days, although immunocompromised patients should be treated for a longer period of time. Also consider 5 days of steroid therapy for patients with VZV vasculopathy.8
Non-FDA approved treatments include tricyclic antidepressants (TCA), such as amitriptyline, nortriptyline, and desipramine, which are sometimes used as first-line therapy for shingles. TCAs may not work well in patients with burning pain, and can have significant adverse effects, including possible cardiotoxicity.9
Opioids, including oxycodone, morphine, methadone, and tramadol, are sometimes used in pain management, but concern exists for abuse. Because patients may develop physical dependence, use opioids with considerable caution.10
Prevention. The United States became the first country to institute a routine varicella immunization program after a varicella vaccine (Varivax) was licensed in 1995.11 The vaccine has reduced the number of varicella infection cases dramatically.11 Vaccine effectiveness is high, and protective herd immunity is obtained after 2 doses.11-13 The vaccine is administered to children after one year of age with a booster dose administered after the fourth birthday.
A live, attenuated VZV vaccine (Zostavax) is given to individuals ≥60 years of age to prevent or attenuate herpes zoster infection. This vaccine is used to boost VZV-specific cell-mediated immunity in adults, thereby decreasing the burden of herpes zoster and the pain associated with PHN.14
Roseola
Roseola infantum, also known as exanthema subitum and sixth disease, is a common mild acute febrile illness of childhood caused by infection with human herpesvirus (HHV) 6 (the primary agent causing roseola) or 7 (a secondary causal agent for roseola).15 HHV-6 has 2 variants (HHV-6a and HHV-6b). Roseola infantum is mostly associated with the HHV-6b variant, which predominantly affects children 6 to 36 months of age.16
The virus replicates in the salivary glands and is shed through saliva, which is the route of transmission. After a 10- to 15-day incubation period, it remains latent in lymphocytes and monocytes, thus persisting in cells and tissues. It may reactivate late in life, particularly in immunosuppressed individuals. Reactivated infection in immunocompromised patients may be associated with serious illness such as encephalitis/encephalopathy. In patients who have received a bone marrow transplant, it can induce graft vs host disease.17
Presentation. The virus causes a 5- to 6-day illness characterized by high fever (temperature as high as 105°-106° F), miringitis (inflammation of tympanic membranes), and irritability. Once defervescence occurs, an erythematous morbilliform exanthem appears.The rash, which has a discrete macular/papular soft-pink appearance, starts on the trunk and spreads centrifugally to the extremities, neck, and face (FIGURE 2). It usually resolves within one to 2 days.

Complications. The most common complication of roseola is febrile seizures.17 Less common ones include encephalitis, encephalopathy, fatal hemophagocytic syndrome,18 or fulminant hepatitis.19
Treatment and prevention. Treatment depends on symptoms and may include antipyretics for fever management and liquids to maintain hydration. Recovery is usually complete with no significant sequelae. If a child develops a seizure, no antiepileptic drugs are recommended. No vaccine exists.
Fifth disease
Human parvovirus B19, a minute ssDNA virus, was first associated with human disease in 1981, when it was linked to an aplastic crisis in a patient with sickle cell disease.20 Since then researchers have determined that it is also the cause of erythema infectiosum or fifth disease of childhood. The B19 virus can also cause anemia in the fetus as well as hydrops fetalis. It has been linked to arthralgia and arthritis (especially in adults). There is an association with autoimmune diseases with characteristics similar to rheumatoid arthritis.20
The B19 virus is transmitted via aerosolized respiratory secretions, contaminated blood, or the placenta. The virus replicates in erythroid cells in bone marrow and peripheral blood, thus inhibiting erythropoiesis.21 Once the rash appears, the virus is no longer infectious.22 Seasonal peaks occur in the late winter and spring, with sporadic infections throughout the year.23 More than 70% of the adult population is seropositive for this virus.20
Presentation. Erythema infectiosum is a mild illness in childhood with an incubation period of 6 to 18 days. It presents with a characteristic malar rash on the face that gives patients a slapped cheek appearance (FIGURE 3A). A softer pink-colored “lacy” reticulated rash that blanches when touched may appear on the trunk, arms, and legs (FIGURE 3B).
Another presentation, which involves the hands and feet (glove and sock syndrome) (FIGURES 3C and 3D), consists of a purpuric eruption with painful edema and numerous small confluent petechiae.22,24 A majority of patients present with inflammatory symptoms that tend to resolve without sequelae within 3 weeks of infection.23

A rash is not as prevalent in adults as in children. Adults often present with more systemic systems, such as a debilitating influenza-like illness, arthropathy, transient aplastic anemia in sickle cell-affected individuals, and persistent viral suppression of erythrocyte production in immunocompromised patients and organ-transplant recipients.
Complications. The B19 virus can cause spontaneous abortion in pregnant women and anemia and hydrops fetalis in the fetus.22 Arthritis can occur in children, but is more common in adults, especially in women. The arthritis tends to be symmetrical and affects small joints such as the hands, wrists, and knees.
In one study of parvovirus B19 involving 633 children with sickle cell disease, 68 children developed transient red cell aplasia, 19% of them developed splenic sequestration, and 12% developed acute chest syndrome, a lung-related complication of sickle cell disease that can lower the level of oxygen in the blood and can be fatal.25
Treatment and prevention. Treatment of B19 infection is symptomatic; for example, nonsteroidal anti-inflammatory drugs (NSAIDs) are used if joint pain develops. No vaccine exists.
Hand, foot, and mouth disease
Hand, foot, and mouth disease (HFMD) is caused by the picornavirus family, including the Coxsackievirus, Enterovirus, and Echovirus. Infections commonly occur in the spring, summer, and fall. The virus primarily affects infants and children <10 years of age with the infection typically lasting 7 to 10 days.26
Presentation. The disease usually presents with a febrile episode, progressing to nasal congestion and malaise. One to 2 days later, the classic rash appears. Patients with HFMD usually present with papular or vesicular lesions on the hands and feet and painful oral lesions (FIGURES 4A, 4B, and 4C). The rash may also affect other parts of the body including the legs and buttocks. Desquamation of nails may occur up to one month after the HFM infection.27 Most cases are diagnosed by clinical presentation, but infection can be confirmed by PCR of vesicular lesion fluid.
Complications. In addition to being caused by Coxsackievirus, HFMD may be caused by human Enterovirus A serotype 71 (HEVA-71), which is associated with a high prevalence of acute neurologic disease including aseptic meningitis, poliomyelitis-like paralysis, and encephalitis.26 Of 764 HFMD patients enrolled in a prospective study, 214 cases were associated with Coxsackievirus A 16 (CVA16) infection and 173 cases were associated with HEVA-71 infection. Rare cases of HFMD have led to encephalitis, meningitis, flaccid paralysis, and death.26
Treatment and prevention. HFMD is usually self-limited, and treatment is supportive. There has been interest in developing an HFMD vaccine, but no products are as yet commercially available.
Rubella
Rubella, also known as the German measles or the 3-day measles, is caused by the rubella virus, which is transmitted via respiratory droplets. Up to half of rubella infections are asymptomatic.28-30
Presentation. Rubella typically has an incubation period of 12 to 24 days, with a 5-day prodromal period characterized by fever, headache, and other symptoms typical of an upper respiratory infection, including sore throat, arthralgia, and tender lymphadenopathy.28
The rash often starts as erythematous or as rose-pink macules on the face that progress down the body. The rash can cover the trunk and extremities within 24 hours. (For photos, see https://www.cdc.gov/rubella/about/photos.html.)
Patients are infectious from 7 days prior to the appearance of the rash to 7 days after resolution of the rash. Given the potentially prolonged infectious period, patients hospitalized for rubella infection should be placed on droplet precautions, and children should be kept from day care and school for 7 days after the appearance of the rash.28
Rubella is typically a mild disease in immunocompetent patients; however, immunocompromised patients may develop pneumonia, coagulopathies, or neurologic sequelae including encephalitis.
Complications. Rubella infection, especially during the first trimester, can lead to spontaneous abortion, stillbirth, or congenital rubella syndrome (CRS), a condition characterized by congenital cataracts and “blueberry muffin” skin lesions.31 Infants affected by CRS can also have heart defects, intellectual disabilities, deafness, and low birth weight. Diagnosis of primary maternal infection should be made with serologic tests. Fetal infection can be determined by detection of fetal serum IgM after 22 to 24 weeks of gestation or with viral culture of amniotic fluid.31
Treatment and prevention. Currently, no antiviral treatments are available; however, vaccines are highly effective at preventing infection. Rubella vaccine is usually given as part of the measles, mumps, rubella (MMR) vaccine, which is administered at age 12 to 15 months and again between 4 and 6 years of age.
Measles
Measles is a highly contagious disease caused by a virus that belongs to the Morbillivirus genus of the family Paramyxoviridae. Infection occurs through inhalation of, or direct contact with, respiratory droplets from infected people.32
Presentation. People with measles often present with what starts as a macular rash on the face that then spreads downward to the neck, trunk, and extremities (for photos, see http://www.cdc.gov/measles/hcp/index.html). As the disease progresses, papules can develop and the lesions may coalesce.
The rash is often preceded by 3 to 4 days of malaise and fever (temperature often greater than 104° F), along with the classic symptom triad of cough, coryza, and conjunctivitis. Koplik spots—clustered white lesions on the buccal mucosa—are often present prior to the measles rash and are pathognomonic for measles infection.33
Because the symptoms of measles are easily confused with other viral infections, suspected cases of measles should be confirmed via IgG and IgM antibody tests, by reverse transcription-PCR, or both.34,35 For limited and unusual cases, the Centers for Disease Control and Prevention can perform a plaque reduction neutralization assay.35
Complications. Measles infection is self-limited in immunocompetent patients. The most common complications are diarrhea and ear infections, but more serious complications, such as pneumonia, hearing loss, and encephalitis, can occur. Children <2 years of age, particularly boys, are at an increased risk of developing subacute sclerosing panencephalitis, a fatal neurologic disorder that can develop years after the initial measles infection.33,36
Treatment and prevention. Treatment is supportive and usually consists of acetaminophen or NSAIDs and fluids.
A live attenuated version of the measles vaccine is highly effective against the measles virus and has greatly reduced the number of measles cases globally.37 The measles vaccine is usually given in 2 doses—the first one after one year of age, and the second one before entering kindergarten. The most common adverse reactions to the vaccine are pain at the injection site and fever. Despite the fact that the MMR vaccine is effective and relatively benign, measles outbreaks continue to occur, as some parents forego routine childhood immunizations because of religious or other personal beliefs or safety concerns.38
Molluscum contagiosum
Molluscum contagiosum (MC) is caused by the MC virus, a member of the poxvirus family. The virus is transmitted by direct contact with the skin lesions. This skin condition is seen mainly in children, although it can occur in adults.
A study conducted in England and Wales that obtained information from the Royal College of General Practitioners reported an incidence of 15.0-17.2/1000 population over a 10-year period (1994-2003) with no variation between sexes.39 There is an association between atopic dermatitis and MC; 24% of children with atopic dermatitis develop MC.40 There might also be an association between recent swimming in a public pool and development of MC lesions.41
Presentation. Lesions caused by MC are small, discrete, waxy dome-shaped papules with central umbilication that are usually 3 to 5 mm in diameter (FIGURE 5).42,43 In immunocompetent patients, there are usually fewer than 20 lesions, which resolve within a year. However, in immunocompromised patients, the number of lesions is usually higher, and the diameter of each may be greater than 1 cm.42
Complications. The lesions are usually self-limited, but on occasion can become secondarily infected, usually with gram-positive organisms such as Staphylococcus aureus. Very rarely, abscesses develop requiring topical and/or systemic antimicrobials and perhaps incision and drainage.44
Treatment and prevention. Because the infection is often self-limited and benign, the preferred therapeutic modality is watchful waiting. Other treatments for MC include curettage, chemical agents, immune modulators, and antiviral drugs. A 2009 Cochran review of 11 studies involving 495 patients found “no single intervention to be convincingly effective in the treatment of molluscum contagiosum.”45 And no vaccine exists.
Gianotti-Crosti syndrome
Gianotti-Crosti syndrome (GCS), also known as papular acrodermatitis of childhood, is a relatively rare, self-limited exanthema that usually affects infants and children 6 months to 12 years of age (peak occurrence is in one- to 6-year-olds). Although there have been reports of adults with this syndrome, it is unusual in this age group.
Pathogenesis is still unknown. Although GCS itself isn’t contagious, the viruses that can cause it may be. Initially, researchers believed that GCS was linked to acute hepatitis B virus infection, but more recently other viral and bacterial infections have been associated with the condition.46
The most commonly associated virus in the United States is Epstein-Barr virus; other viruses include hepatitis A virus, cytomegalovirus, coxsackievirus, respiratory syncytial virus, parainfluenza virus, rotavirus, the mumps virus, parvovirus, and molluscum contagiosum.
Bacterial infections, such as those caused by Bartonella henselae, Mycoplasma pneumoniae, and group A streptococci may trigger GCS.47-49
Vaccines that have been implicated in GCS include those for polio, diphtheria/pertussis/tetanus, MMR, hepatitis A and B, as well as the influenza vaccine.48-51
Presentation. While GCS is relatively rare, its presentation is classic, making it easy to diagnose once it’s included in the differential. The pruritic rash usually consists of acute-onset monomorphous, flat-topped or dome-shaped red-brown papules and papulovesicles, one to 10 mm in size, located symmetrically on the face (FIGURE 6), the extensor surfaces of the arms and legs, and, less commonly, the buttocks. It rarely affects other parts of the body.48
The diagnosis is usually based on the characteristic rash and the benign nature of the condition; other than the rash, patients are typically asymptomatic and healthy. Sometimes a biopsy is performed and it reveals a dense lichenoid lymphohistiocytic infiltrate with a strong cytoplasmic immunopositivity for beta-defensin-4 in the stratum corneum, granulosum, and spinosum.52
The lesions spontaneously resolve within 8 to 12 weeks. GCS usually presents during spring and early summer and affects both sexes equally.46
Treatment and prevention. Treatment is usually symptomatic, with the use of oral antihistamines if the lesions become pruritic. Topical steroids may be used, and, in a few cases, oral corticosteroids may be considered. No vaccine exists.
CORRESPONDENCE
Carlos A. Arango, MD, 8399 Bayberry Road, Jacksonville, FL 32256; carlos.arango@jax.ufl.edu.
1. Kennedy PG, Rovnak J, Badani H, et al. A comparison of herpes simplex virus type 1 and varicella-zoster virus latency and reactivation. J Gen Virol. 2015;96(Pt 7):1581-1602.
2. Blumental S, Sabbe M, Lepage P, the Belgian Group for Varicella. Varicella paediatric hospitalisations in Belgium: a 1-year national survey. Arch Dis Child. 2016;101:16-22.
3. Sampathkumar P, Drage LA, Martin DP. Herpes zoster (shingles) and postherpetic neuralgia. Mayo Clinic Proc. 2009;84:274-280.
4. Ono F, Yasumoto S, Furumura M, et al. Comparison between famciclovir and valacyclovir for acute pain in adult Japanese immunocompetent patients with herpes zoster. J Dermatol. 2012;39:902-908.
5. Massengill JS, Kittredge JL. Practical considerations in the pharmacological treatment of post-herpetic neuralgia for the primary care provider. J Pain Res. 2014;7:125-132.
6. Nalamachu S, Morley-Forster P. Diagnosing and managing postherpetic neuralgia. Drugs & Aging. 2012;29:863-869.
7. Hempenstall K, Nurmikko TJ, Johnson RW, et al. Analgesic therapy in postherpetic neuralgia: a quantitative systematic review. PLoS Med. 2005;2:e164.
8. Gilden D, Cohrs RJ, Mahalingam R, et al. Varicella zoster virus vasculopathies: diverse clinical manifestations, laboratory features, pathogenesis, and treatment. Lancet Neurol. 2009;9:731-740.
9. Stankus SJ, Dlugopolski M, Packer D. Management of herpes zoster (shingles) and post herpetic neuralgia. Am Fam Physician. 2000;61:2437-2444.
10. Dworkin RH, O’Connor AB, Audette J, et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc. 2010;85(3 Suppl):S3-S14.
11. Thomas CA, Shwe T, Bixler D, et al. Two-dose varicella vaccine effectiveness and rash severity in outbreaks of varicella among public school students. Pediatr Infect Dis J. 2014;33:1164-1168.
12. Helmuth IG, Poulsen A, Suppli CH, et al. Varicella in Europe-a review of the epidemiology and experience with vaccination. Vaccine. 2015;33:2406-2413.
13. Marin M, Marti M, Kambhampati A, et al. Global varicella vaccine effectiveness: a metanalysis. Pediatrics. 2016;137:e20153741.
14. Centers for Disease Control and Prevention. What everyone should know about shingles vaccine. Available at: www.cdc.gov/vaccines/vpd/shingles/public/index.html. Accessed September 12, 2017.
15. Tanaka K, Kondo T, Torigoe S, et al. Human herpesvirus 7: another causal agent for roseola (exanthem subitum). J Pediatr. 1994;125:1-5.
16. Caserta MT, Mock DJ, Dewhurst S. Human herpesvirus 6. Clin Infect Dis. 2001;33:829-833.
17. Koch WC. Fifth (human parvovirus) and sixth (herpesvirus 6) diseases. Curr Opin Infect Dis. 2001;14:343-356.
18. Marabelle A, Bergeron C, Billaud G, et al. Hemophagocytic syndrome revealing primary HHV-6 infection. J Pediatr. 2010;157:511.
19. Charnot-Katsikasa A, Baewer D, Cook L, et al. Fulminant hepatic failure attributed to infection with human herpesvirus 6 (HHV-6) in an immunocompetent woman: a case report and review of the literature. J Clin Virol. 2016;75:27-32.
20. Corcoran A, Doyle S. Advances in the biology, diagnosis and host-pathogen interactions of parvovirus B19. J Med Microbiol. 2004;53(Pt 6):459-475.
21. Dolin R. Parvovirus erythema infectiousum, Aplastic anemia. In: Mandell, Douglas, Bennett’s Principles and Practice of Infectious Diseases. 3rd ed. New York, NY: Churchill Livingstone Inc; 1990:1231-1232.
22. Servey JT, Reamy BV, Hodge J. Clinical presentations of parvovirus B19 infection. Am Fam Physician. 2007;75:373-376.
23. Martin DR, Schlott DW, Flynn JA. Clinical problem-solving. No respecter of age. N Engl J Med. 2007;357:1856-1859.
24. Ozaydin V, Eceviz A, Sari Dogan F, et al. An adult patient who presented to emergency service with a papular purpuric gloves and socks syndrome: a case report. Turk J Emerg Med. 2014;14:179-181.
25. Smith-Whitley K, Zhao H, Hodinka RL, et al. Epidemiology of human parvovirus B19 in children with sickle cell disease. Blood. 2004;103:422-427.
26. Tu PV, Thao NT, Perera D, et al. Epidemiologic and virologic investigation of hand, foot, and mouth disease, Southern Vietnam, 2005. Emerg Infect Dis. 2007;13:1733-1741.
27. Ferrari B, Taliercio V, Hornos L, et al. Onychomadesis associated with mouth, hand and foot disease. Arch Argent Pediatr. 2013;111:e148-e151.
28. Alter SJ, Bennett JS, Koranyi K, et al. Common childhood viral infections. Curr Probl Pediatr Adolesc Health Care. 2015;45:21-53.
29. Lambert N, Strebel P, Orenstein W, et al. Rubella. Lancet. 2015;385:2297-2307.
30. Silasi M, Cardenas I, Kwon JY, et al. Viral infections during pregnancy. Am J Reprod Immunol. 2015;73:199-213.
31. Tang JW, Aarons E, Hesketh LM, et al. Prenatal diagnosis of congenital rubella infection in the second trimester of pregnancy Prenat Diagn. 2003;23:509-512.
32. Naim HY. Measles virus. Hum Vaccin Immunother. 2015;11:21-26.
33. Centers for Disease Control and Prevention. Measles (Rubeola). Available at: http://www.cdc.gov/measles/hcp/index.html. Accessed April 28, 2016.
34. Takao S, Shigemoto N, Shimazu Y, et al. Detection of exanthematic viruses using a TaqMan real-time PCR assay panel in patients with clinically diagnosed or suspected measles. Jpn J Infect Dis. 2012;65:444-448.
35. Centers for Disease Control and Prevention. Measles (Rubeola). Available at: https://www.cdc.gov/measles/lab-tools/rt-pcr.html. Accessed April 28, 2016.
36. Griffin DE, Lin WH, Pan CH. Measles virus, immune control, and persistence. FEMS Microbiol Rev. 2012;36:649-662.
37. Centers for Disease Control and Prevention. Measles, vaccination. Available at: https://www.cdc.gov/measles/vaccination.html. Accessed April 28, 2016.
38. Campos-Outcalt D. Measles: Why it’s still a threat. J Fam Pract. 2017;66:446-449.
39. Olsen JR, Gallacher J, Piguet V, et al. Epidemiology of molluscum contagiosum in children: a systematic review. Fam Pract. 2014;31:130-136.
40. Dohil MA, Lin P, Lee J, et al. The epidemiology of molluscum contagiosum in children. J Am Acad Dermatol. 2006;54:47-54.
41. Choong KY, Roberts LJ. Molluscum contagiosum, swimming and bathing: a clinical analysis. Australas J Dermatol. 1999;40:89-92.
42. Martin P. Interventions for molluscum contagiosum in people infected with human immunodeficiency virus: a systematic review. Int J Dermatol. 2016;55:956-966.
43. Chen X, Anstey AV, Bugert JJ. Molluscum contagiosum virus infection. Lancet Infect Dis. 2013;13:877-888.
44. Lacour M, Posfay-Barbe KM, La Scala GC. Staphylococcus lugdunensis abscesses complicating molluscum contagiosum in two children. Pediatr Dermatol. 2015;32:289-291.
45. van der Wouden JC, van der Sande R, van Suijlekom-Smit LW, et al. Interventions for cutaneous molluscum contagiosum. Cochrane Database Syst Rev. 2009;CD004767.
46. Tagawa C, Speakman M. Photo quiz. Papular rash in a child after a fever. Gianotti-Crosti syndrome. Am Fam Physician. 2013;87:59-60.
47. Brandt O, Abeck D, Gianotti R, et al. Gianotti-Crosti syndrome. J Am Acad Dermatol. 2006;54:136-145.
48. Retrouvey M, Koch LH, Williams JV. Gianotti-Crosti syndrome following childhood vaccinations. Pediatr Dermatol. 2013;30:137-138.
49. Velangi SS, Tidman MJ. Gianotti-Crosti syndrome after measles, mumps, and rubella vaccination. Br J Dermatol. 1998;139:1122-1123.
50. Lacour M, Harms M. Gianotti-Crosti syndrome as a result of vaccination and Epstein-Barr virus infection. Eur J Pediatr. 1995;154:688-689.
51. Kroeskop A, Lewis AB, Barril FA, et al. Gianotti-Crosti syndrome after H1N1-influenza vaccine. Pediatr Dermatol. 2011;28:595-596.
52. Caltabiano R, Vecchio GM, De Pasquale R, et al. Human beta-defensin 4 expression in Gianotti-Crosti. Acta Dermatovenerol Croat. 2013;21:43-47.
1. Kennedy PG, Rovnak J, Badani H, et al. A comparison of herpes simplex virus type 1 and varicella-zoster virus latency and reactivation. J Gen Virol. 2015;96(Pt 7):1581-1602.
2. Blumental S, Sabbe M, Lepage P, the Belgian Group for Varicella. Varicella paediatric hospitalisations in Belgium: a 1-year national survey. Arch Dis Child. 2016;101:16-22.
3. Sampathkumar P, Drage LA, Martin DP. Herpes zoster (shingles) and postherpetic neuralgia. Mayo Clinic Proc. 2009;84:274-280.
4. Ono F, Yasumoto S, Furumura M, et al. Comparison between famciclovir and valacyclovir for acute pain in adult Japanese immunocompetent patients with herpes zoster. J Dermatol. 2012;39:902-908.
5. Massengill JS, Kittredge JL. Practical considerations in the pharmacological treatment of post-herpetic neuralgia for the primary care provider. J Pain Res. 2014;7:125-132.
6. Nalamachu S, Morley-Forster P. Diagnosing and managing postherpetic neuralgia. Drugs & Aging. 2012;29:863-869.
7. Hempenstall K, Nurmikko TJ, Johnson RW, et al. Analgesic therapy in postherpetic neuralgia: a quantitative systematic review. PLoS Med. 2005;2:e164.
8. Gilden D, Cohrs RJ, Mahalingam R, et al. Varicella zoster virus vasculopathies: diverse clinical manifestations, laboratory features, pathogenesis, and treatment. Lancet Neurol. 2009;9:731-740.
9. Stankus SJ, Dlugopolski M, Packer D. Management of herpes zoster (shingles) and post herpetic neuralgia. Am Fam Physician. 2000;61:2437-2444.
10. Dworkin RH, O’Connor AB, Audette J, et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc. 2010;85(3 Suppl):S3-S14.
11. Thomas CA, Shwe T, Bixler D, et al. Two-dose varicella vaccine effectiveness and rash severity in outbreaks of varicella among public school students. Pediatr Infect Dis J. 2014;33:1164-1168.
12. Helmuth IG, Poulsen A, Suppli CH, et al. Varicella in Europe-a review of the epidemiology and experience with vaccination. Vaccine. 2015;33:2406-2413.
13. Marin M, Marti M, Kambhampati A, et al. Global varicella vaccine effectiveness: a metanalysis. Pediatrics. 2016;137:e20153741.
14. Centers for Disease Control and Prevention. What everyone should know about shingles vaccine. Available at: www.cdc.gov/vaccines/vpd/shingles/public/index.html. Accessed September 12, 2017.
15. Tanaka K, Kondo T, Torigoe S, et al. Human herpesvirus 7: another causal agent for roseola (exanthem subitum). J Pediatr. 1994;125:1-5.
16. Caserta MT, Mock DJ, Dewhurst S. Human herpesvirus 6. Clin Infect Dis. 2001;33:829-833.
17. Koch WC. Fifth (human parvovirus) and sixth (herpesvirus 6) diseases. Curr Opin Infect Dis. 2001;14:343-356.
18. Marabelle A, Bergeron C, Billaud G, et al. Hemophagocytic syndrome revealing primary HHV-6 infection. J Pediatr. 2010;157:511.
19. Charnot-Katsikasa A, Baewer D, Cook L, et al. Fulminant hepatic failure attributed to infection with human herpesvirus 6 (HHV-6) in an immunocompetent woman: a case report and review of the literature. J Clin Virol. 2016;75:27-32.
20. Corcoran A, Doyle S. Advances in the biology, diagnosis and host-pathogen interactions of parvovirus B19. J Med Microbiol. 2004;53(Pt 6):459-475.
21. Dolin R. Parvovirus erythema infectiousum, Aplastic anemia. In: Mandell, Douglas, Bennett’s Principles and Practice of Infectious Diseases. 3rd ed. New York, NY: Churchill Livingstone Inc; 1990:1231-1232.
22. Servey JT, Reamy BV, Hodge J. Clinical presentations of parvovirus B19 infection. Am Fam Physician. 2007;75:373-376.
23. Martin DR, Schlott DW, Flynn JA. Clinical problem-solving. No respecter of age. N Engl J Med. 2007;357:1856-1859.
24. Ozaydin V, Eceviz A, Sari Dogan F, et al. An adult patient who presented to emergency service with a papular purpuric gloves and socks syndrome: a case report. Turk J Emerg Med. 2014;14:179-181.
25. Smith-Whitley K, Zhao H, Hodinka RL, et al. Epidemiology of human parvovirus B19 in children with sickle cell disease. Blood. 2004;103:422-427.
26. Tu PV, Thao NT, Perera D, et al. Epidemiologic and virologic investigation of hand, foot, and mouth disease, Southern Vietnam, 2005. Emerg Infect Dis. 2007;13:1733-1741.
27. Ferrari B, Taliercio V, Hornos L, et al. Onychomadesis associated with mouth, hand and foot disease. Arch Argent Pediatr. 2013;111:e148-e151.
28. Alter SJ, Bennett JS, Koranyi K, et al. Common childhood viral infections. Curr Probl Pediatr Adolesc Health Care. 2015;45:21-53.
29. Lambert N, Strebel P, Orenstein W, et al. Rubella. Lancet. 2015;385:2297-2307.
30. Silasi M, Cardenas I, Kwon JY, et al. Viral infections during pregnancy. Am J Reprod Immunol. 2015;73:199-213.
31. Tang JW, Aarons E, Hesketh LM, et al. Prenatal diagnosis of congenital rubella infection in the second trimester of pregnancy Prenat Diagn. 2003;23:509-512.
32. Naim HY. Measles virus. Hum Vaccin Immunother. 2015;11:21-26.
33. Centers for Disease Control and Prevention. Measles (Rubeola). Available at: http://www.cdc.gov/measles/hcp/index.html. Accessed April 28, 2016.
34. Takao S, Shigemoto N, Shimazu Y, et al. Detection of exanthematic viruses using a TaqMan real-time PCR assay panel in patients with clinically diagnosed or suspected measles. Jpn J Infect Dis. 2012;65:444-448.
35. Centers for Disease Control and Prevention. Measles (Rubeola). Available at: https://www.cdc.gov/measles/lab-tools/rt-pcr.html. Accessed April 28, 2016.
36. Griffin DE, Lin WH, Pan CH. Measles virus, immune control, and persistence. FEMS Microbiol Rev. 2012;36:649-662.
37. Centers for Disease Control and Prevention. Measles, vaccination. Available at: https://www.cdc.gov/measles/vaccination.html. Accessed April 28, 2016.
38. Campos-Outcalt D. Measles: Why it’s still a threat. J Fam Pract. 2017;66:446-449.
39. Olsen JR, Gallacher J, Piguet V, et al. Epidemiology of molluscum contagiosum in children: a systematic review. Fam Pract. 2014;31:130-136.
40. Dohil MA, Lin P, Lee J, et al. The epidemiology of molluscum contagiosum in children. J Am Acad Dermatol. 2006;54:47-54.
41. Choong KY, Roberts LJ. Molluscum contagiosum, swimming and bathing: a clinical analysis. Australas J Dermatol. 1999;40:89-92.
42. Martin P. Interventions for molluscum contagiosum in people infected with human immunodeficiency virus: a systematic review. Int J Dermatol. 2016;55:956-966.
43. Chen X, Anstey AV, Bugert JJ. Molluscum contagiosum virus infection. Lancet Infect Dis. 2013;13:877-888.
44. Lacour M, Posfay-Barbe KM, La Scala GC. Staphylococcus lugdunensis abscesses complicating molluscum contagiosum in two children. Pediatr Dermatol. 2015;32:289-291.
45. van der Wouden JC, van der Sande R, van Suijlekom-Smit LW, et al. Interventions for cutaneous molluscum contagiosum. Cochrane Database Syst Rev. 2009;CD004767.
46. Tagawa C, Speakman M. Photo quiz. Papular rash in a child after a fever. Gianotti-Crosti syndrome. Am Fam Physician. 2013;87:59-60.
47. Brandt O, Abeck D, Gianotti R, et al. Gianotti-Crosti syndrome. J Am Acad Dermatol. 2006;54:136-145.
48. Retrouvey M, Koch LH, Williams JV. Gianotti-Crosti syndrome following childhood vaccinations. Pediatr Dermatol. 2013;30:137-138.
49. Velangi SS, Tidman MJ. Gianotti-Crosti syndrome after measles, mumps, and rubella vaccination. Br J Dermatol. 1998;139:1122-1123.
50. Lacour M, Harms M. Gianotti-Crosti syndrome as a result of vaccination and Epstein-Barr virus infection. Eur J Pediatr. 1995;154:688-689.
51. Kroeskop A, Lewis AB, Barril FA, et al. Gianotti-Crosti syndrome after H1N1-influenza vaccine. Pediatr Dermatol. 2011;28:595-596.
52. Caltabiano R, Vecchio GM, De Pasquale R, et al. Human beta-defensin 4 expression in Gianotti-Crosti. Acta Dermatovenerol Croat. 2013;21:43-47.
From The Journal of Family Practice | 2017;66(10):598-606.
PRACTICE RECOMMENDATIONS
› Administer the varicella-zoster vaccine to all adults ≥60 years of age to prevent or attenuate herpes zoster infection. A
› Avoid congenital rubella syndrome by vaccinating all at-risk pregnant women. A
› Administer 2 doses of the measles vaccine (one at 12-15 months of age and one at 4-6 years of age) to all children to avoid a resurgence. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
How to protect your patient—and yourself—when prescribing opioids
Statins for primary prevention of CVD: To start or not to start?
Resources
- Ioannidis JPA. Inconsistent guideline recommendations for cardiovascular prevention and the debate about zeroing in on and zeroing LDL-C levels with PCSK9 inhibitors. JAMA. 2017;318:419-420. Available at: https://jamanetwork.com/journals/jama/fullarticle/2645741.
- Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. J Am Coll Cardiol. 2014;63:2889-2934. Available at: http://www.sciencedirect.com/science/article/pii/S0735109713060282?via%3Dihub.
- Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:1997-2007. Available at: https://jamanetwork.com/journals/jama/fullarticle/2584058.
- Catapano AL, Graham I, De Backer G, et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016;37:2999-3058. Available at: https://academic.oup.com/eurheartj/article-lookup/doi/10.1093/eurheartj/ehw272.
- Anderson TJ, Grégoire J, Pearson GJ, et al. 2016 Canadian cardiovascular society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2016;32:1263-1282. Available at: http://www.onlinecjc.ca/article/S0828-282X(16)30732-2/fulltext.
- Pagidipati NJ, Navar AM, Mulder H, et al. Comparison of recommended eligibility for primary statin therapy based on the US Preventive Services Task Force recommendations vs the ACC/AHA guidelines. JAMA. 2017;317:1563-1567. Available at: https://jamanetwork.com/journals/jama/fullarticle/2618621.
Resources
- Ioannidis JPA. Inconsistent guideline recommendations for cardiovascular prevention and the debate about zeroing in on and zeroing LDL-C levels with PCSK9 inhibitors. JAMA. 2017;318:419-420. Available at: https://jamanetwork.com/journals/jama/fullarticle/2645741.
- Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. J Am Coll Cardiol. 2014;63:2889-2934. Available at: http://www.sciencedirect.com/science/article/pii/S0735109713060282?via%3Dihub.
- Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:1997-2007. Available at: https://jamanetwork.com/journals/jama/fullarticle/2584058.
- Catapano AL, Graham I, De Backer G, et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016;37:2999-3058. Available at: https://academic.oup.com/eurheartj/article-lookup/doi/10.1093/eurheartj/ehw272.
- Anderson TJ, Grégoire J, Pearson GJ, et al. 2016 Canadian cardiovascular society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2016;32:1263-1282. Available at: http://www.onlinecjc.ca/article/S0828-282X(16)30732-2/fulltext.
- Pagidipati NJ, Navar AM, Mulder H, et al. Comparison of recommended eligibility for primary statin therapy based on the US Preventive Services Task Force recommendations vs the ACC/AHA guidelines. JAMA. 2017;317:1563-1567. Available at: https://jamanetwork.com/journals/jama/fullarticle/2618621.
Resources
- Ioannidis JPA. Inconsistent guideline recommendations for cardiovascular prevention and the debate about zeroing in on and zeroing LDL-C levels with PCSK9 inhibitors. JAMA. 2017;318:419-420. Available at: https://jamanetwork.com/journals/jama/fullarticle/2645741.
- Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. J Am Coll Cardiol. 2014;63:2889-2934. Available at: http://www.sciencedirect.com/science/article/pii/S0735109713060282?via%3Dihub.
- Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:1997-2007. Available at: https://jamanetwork.com/journals/jama/fullarticle/2584058.
- Catapano AL, Graham I, De Backer G, et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016;37:2999-3058. Available at: https://academic.oup.com/eurheartj/article-lookup/doi/10.1093/eurheartj/ehw272.
- Anderson TJ, Grégoire J, Pearson GJ, et al. 2016 Canadian cardiovascular society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2016;32:1263-1282. Available at: http://www.onlinecjc.ca/article/S0828-282X(16)30732-2/fulltext.
- Pagidipati NJ, Navar AM, Mulder H, et al. Comparison of recommended eligibility for primary statin therapy based on the US Preventive Services Task Force recommendations vs the ACC/AHA guidelines. JAMA. 2017;317:1563-1567. Available at: https://jamanetwork.com/journals/jama/fullarticle/2618621.
Haste Makes Waste
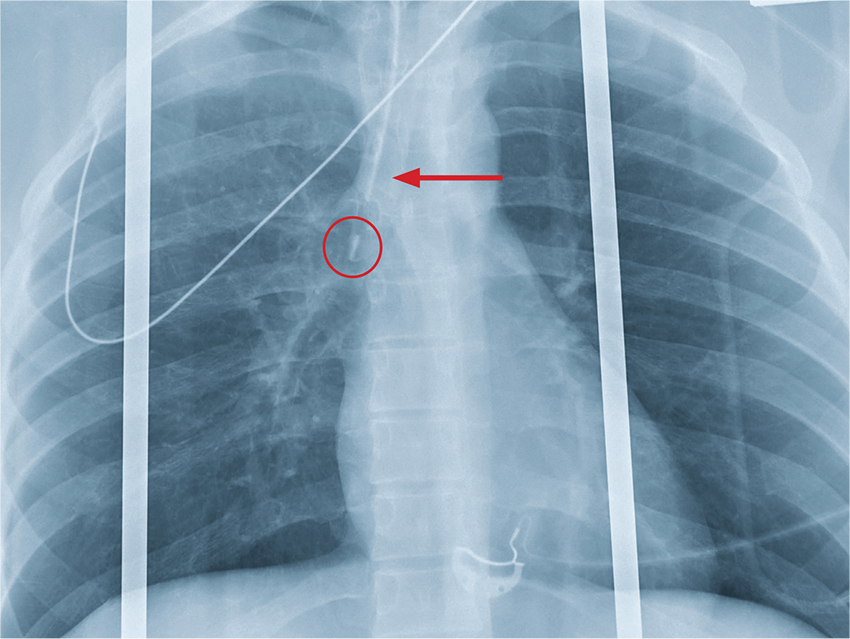
ANSWER
The chest radiograph shows an endotracheal tube within the right main stem bronchus. There is no evidence of any other acute pathology (eg, fracture, contusion, pneumothorax).
The tube needs to be withdrawn so that it sits just above the carina (see arrow). If not promptly addressed, incorrect placement of an endotracheal tube can lead to complications, including hypoxemia, pneumothorax, atelectasis, or complete collapse of the left lung.

ANSWER
The chest radiograph shows an endotracheal tube within the right main stem bronchus. There is no evidence of any other acute pathology (eg, fracture, contusion, pneumothorax).
The tube needs to be withdrawn so that it sits just above the carina (see arrow). If not promptly addressed, incorrect placement of an endotracheal tube can lead to complications, including hypoxemia, pneumothorax, atelectasis, or complete collapse of the left lung.

ANSWER
The chest radiograph shows an endotracheal tube within the right main stem bronchus. There is no evidence of any other acute pathology (eg, fracture, contusion, pneumothorax).
The tube needs to be withdrawn so that it sits just above the carina (see arrow). If not promptly addressed, incorrect placement of an endotracheal tube can lead to complications, including hypoxemia, pneumothorax, atelectasis, or complete collapse of the left lung.
A woman who looks to be 30 years old is brought to your facility as a trauma code following a car accident. She was a restrained driver, traveling at a high speed when she lost control of her vehicle and hit a retaining wall.
When first responders arrived, the patient had extricated herself but demonstrated a decreased level of consciousness, severe respiratory distress, and a Glasgow Coma Scale score of 7. She was intubated at the scene by emergency medical personnel.
On evaluation, you note a young, intubated, unresponsive female. Her blood pressure is 90/50 mm Hg; heart rate, 90 beats/min; and O2 saturation, 100%. Rapid primary survey shows
A portable chest radiograph is obtained (shown). What is your impression?
New ADA hypertension and diabetes treatment guide features visual aid
Clinicians can consult a diagram to plan treatment of hypertension in diabetes patients as part of the new American Diabetes Association guidelines.
“Diabetes and Hypertension: A Position Statement by the American Diabetes Association” was published in the September 2017 issue of Diabetes Care, and online on Aug. 22. The statement updates the ADA’s previous statement on hypertension and diabetes published in 2003.
“Numerous studies have shown that antihypertensive therapy reduces ASCVD [atherosclerotic cardiovascular disease] events, heart failure, and microvascular complications in people with diabetes,” wrote Ian H. de Boer, MD, of the University of Washington, Seattle, and his colleagues.
The statement is a collaboration between nine diabetes experts from the United States, Europe, and Australia whose specialties include endocrinology, nephrology, cardiology, and internal medicine (Diabetes Care. 2017 Sep.;40:1273-84).
The statement recommends that diabetes patients have their blood pressure checked at every routine clinical visit and that those with an elevated blood pressure on a clinical visit (defined as office-based measurements of 140/90 mm Hg and higher) have multiple measurements, including on a separate day to confirm the diagnosis.
In addition, during the initial evaluation, and then periodically, diabetes patients should be assessed for orthostatic hypotension “to individualize blood pressure goals, select the most appropriate antihypertensive agents, and minimize adverse effects of antihypertensive therapy,” according to the recommendations.
For most patients with diabetes and hypertension, the goal should be a blood pressure below 140/90 mm Hg, and even lower targets may be appropriate for patients at high cardiovascular disease risk, the researchers said.
The guidelines include recommendations for managing hypertension and diabetes through lifestyle modifications such as increasing physical activity, achieving and maintaining a healthy weight, and following a healthy diet with minimal sodium intake and an emphasis on fruits, vegetables, and low-fat dairy products.
The guidelines also emphasize the need for caution when treating older adults who are taking multiple medications. “Systolic blood pressure should be the main target of treatment,” for adults aged 65 years and older with diabetes and hypertension, the authors said.
In addition, the guidelines provide direction for clinicians treating pregnant women. “During pregnancy, treatment with ACE inhibitors, ARBs [angiotensin receptor blockers], or spironolactone is contraindicated, as [these medications] may cause fetal damage,” the authors wrote. Pregnant women with preexisting hypertension or with mild gestational hypertension with systolic blood pressure below 160 mm Hg, a diastolic blood pressure below 105 mm Hg, and no sign of end-organ damage need not take antihypertensive medications, they said. For pregnant women who require antihypertensive treatment, the aim should be a systolic blood pressure between 120 mm Hg and 160 mm Hg and a diastolic blood pressure between 80 mm Hg and 105 mm Hg.
The authors concluded that there currently is insufficient evidence to support blood pressure medication for diabetes patients without hypertension.
The recommendations reference several key clinical trials that compared intensive and standard hypertension treatment strategies: the ACCORD BP (Action to Control Cardiovascular Risk in Diabetes – Blood Pressure) trial, the ADVANCE BP (Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation – Blood Pressure) trial, the HOT (Hypertension Optimal Treatment) trial, and SPRINT (the Systolic Blood Pressure Intervention Trial).
Lead author Dr. de Boer reported serving as a consultant for Boehringer Ingelheim and Ironwood Pharmaceuticals, and his institution has received research equipment and supplies from Medtronic and Abbott. Study coauthors disclosed relationships with multiple companies including Merck, Abbott, Pfizer, and AstraZeneca.
Clinicians can consult a diagram to plan treatment of hypertension in diabetes patients as part of the new American Diabetes Association guidelines.
“Diabetes and Hypertension: A Position Statement by the American Diabetes Association” was published in the September 2017 issue of Diabetes Care, and online on Aug. 22. The statement updates the ADA’s previous statement on hypertension and diabetes published in 2003.
“Numerous studies have shown that antihypertensive therapy reduces ASCVD [atherosclerotic cardiovascular disease] events, heart failure, and microvascular complications in people with diabetes,” wrote Ian H. de Boer, MD, of the University of Washington, Seattle, and his colleagues.
The statement is a collaboration between nine diabetes experts from the United States, Europe, and Australia whose specialties include endocrinology, nephrology, cardiology, and internal medicine (Diabetes Care. 2017 Sep.;40:1273-84).
The statement recommends that diabetes patients have their blood pressure checked at every routine clinical visit and that those with an elevated blood pressure on a clinical visit (defined as office-based measurements of 140/90 mm Hg and higher) have multiple measurements, including on a separate day to confirm the diagnosis.
In addition, during the initial evaluation, and then periodically, diabetes patients should be assessed for orthostatic hypotension “to individualize blood pressure goals, select the most appropriate antihypertensive agents, and minimize adverse effects of antihypertensive therapy,” according to the recommendations.
For most patients with diabetes and hypertension, the goal should be a blood pressure below 140/90 mm Hg, and even lower targets may be appropriate for patients at high cardiovascular disease risk, the researchers said.
The guidelines include recommendations for managing hypertension and diabetes through lifestyle modifications such as increasing physical activity, achieving and maintaining a healthy weight, and following a healthy diet with minimal sodium intake and an emphasis on fruits, vegetables, and low-fat dairy products.
The guidelines also emphasize the need for caution when treating older adults who are taking multiple medications. “Systolic blood pressure should be the main target of treatment,” for adults aged 65 years and older with diabetes and hypertension, the authors said.
In addition, the guidelines provide direction for clinicians treating pregnant women. “During pregnancy, treatment with ACE inhibitors, ARBs [angiotensin receptor blockers], or spironolactone is contraindicated, as [these medications] may cause fetal damage,” the authors wrote. Pregnant women with preexisting hypertension or with mild gestational hypertension with systolic blood pressure below 160 mm Hg, a diastolic blood pressure below 105 mm Hg, and no sign of end-organ damage need not take antihypertensive medications, they said. For pregnant women who require antihypertensive treatment, the aim should be a systolic blood pressure between 120 mm Hg and 160 mm Hg and a diastolic blood pressure between 80 mm Hg and 105 mm Hg.
The authors concluded that there currently is insufficient evidence to support blood pressure medication for diabetes patients without hypertension.
The recommendations reference several key clinical trials that compared intensive and standard hypertension treatment strategies: the ACCORD BP (Action to Control Cardiovascular Risk in Diabetes – Blood Pressure) trial, the ADVANCE BP (Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation – Blood Pressure) trial, the HOT (Hypertension Optimal Treatment) trial, and SPRINT (the Systolic Blood Pressure Intervention Trial).
Lead author Dr. de Boer reported serving as a consultant for Boehringer Ingelheim and Ironwood Pharmaceuticals, and his institution has received research equipment and supplies from Medtronic and Abbott. Study coauthors disclosed relationships with multiple companies including Merck, Abbott, Pfizer, and AstraZeneca.
Clinicians can consult a diagram to plan treatment of hypertension in diabetes patients as part of the new American Diabetes Association guidelines.
“Diabetes and Hypertension: A Position Statement by the American Diabetes Association” was published in the September 2017 issue of Diabetes Care, and online on Aug. 22. The statement updates the ADA’s previous statement on hypertension and diabetes published in 2003.
“Numerous studies have shown that antihypertensive therapy reduces ASCVD [atherosclerotic cardiovascular disease] events, heart failure, and microvascular complications in people with diabetes,” wrote Ian H. de Boer, MD, of the University of Washington, Seattle, and his colleagues.
The statement is a collaboration between nine diabetes experts from the United States, Europe, and Australia whose specialties include endocrinology, nephrology, cardiology, and internal medicine (Diabetes Care. 2017 Sep.;40:1273-84).
The statement recommends that diabetes patients have their blood pressure checked at every routine clinical visit and that those with an elevated blood pressure on a clinical visit (defined as office-based measurements of 140/90 mm Hg and higher) have multiple measurements, including on a separate day to confirm the diagnosis.
In addition, during the initial evaluation, and then periodically, diabetes patients should be assessed for orthostatic hypotension “to individualize blood pressure goals, select the most appropriate antihypertensive agents, and minimize adverse effects of antihypertensive therapy,” according to the recommendations.
For most patients with diabetes and hypertension, the goal should be a blood pressure below 140/90 mm Hg, and even lower targets may be appropriate for patients at high cardiovascular disease risk, the researchers said.
The guidelines include recommendations for managing hypertension and diabetes through lifestyle modifications such as increasing physical activity, achieving and maintaining a healthy weight, and following a healthy diet with minimal sodium intake and an emphasis on fruits, vegetables, and low-fat dairy products.
The guidelines also emphasize the need for caution when treating older adults who are taking multiple medications. “Systolic blood pressure should be the main target of treatment,” for adults aged 65 years and older with diabetes and hypertension, the authors said.
In addition, the guidelines provide direction for clinicians treating pregnant women. “During pregnancy, treatment with ACE inhibitors, ARBs [angiotensin receptor blockers], or spironolactone is contraindicated, as [these medications] may cause fetal damage,” the authors wrote. Pregnant women with preexisting hypertension or with mild gestational hypertension with systolic blood pressure below 160 mm Hg, a diastolic blood pressure below 105 mm Hg, and no sign of end-organ damage need not take antihypertensive medications, they said. For pregnant women who require antihypertensive treatment, the aim should be a systolic blood pressure between 120 mm Hg and 160 mm Hg and a diastolic blood pressure between 80 mm Hg and 105 mm Hg.
The authors concluded that there currently is insufficient evidence to support blood pressure medication for diabetes patients without hypertension.
The recommendations reference several key clinical trials that compared intensive and standard hypertension treatment strategies: the ACCORD BP (Action to Control Cardiovascular Risk in Diabetes – Blood Pressure) trial, the ADVANCE BP (Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation – Blood Pressure) trial, the HOT (Hypertension Optimal Treatment) trial, and SPRINT (the Systolic Blood Pressure Intervention Trial).
Lead author Dr. de Boer reported serving as a consultant for Boehringer Ingelheim and Ironwood Pharmaceuticals, and his institution has received research equipment and supplies from Medtronic and Abbott. Study coauthors disclosed relationships with multiple companies including Merck, Abbott, Pfizer, and AstraZeneca.
FROM DIABETES CARE
Nocturia linked to hypertension, diuretic use in community-based study of black men
SAN FRANCISCO – Nocturia is a sign of uncontrolled hypertension and also is associated with diuretic use in middle-aged black men, according to a study conducted at 53 barbershops in and around Los Angeles.
In the study, investigators averaged the last three of five automated blood pressure readings in 1,748 black men aged 35-49 years old and asked them about symptoms of nocturia – defined in the study as getting up two or more times per night to urinate – and about what blood pressure medications they were taking, if any.
Men with untreated hypertension – defined as at or above 135/85 mm Hg – were 34% more likely to report nocturia than were men who were normotensive.
However, “what really grabbed our attention was the treated group,” lead investigator Ronald Victor, MD said at the joint scientific sessions of the AHA Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
The highest risk of nocturia, more than three times the risk of normotensive men, was among men treated with a diuretic who still had elevated blood pressure readings in the barbershop. Their risk was about 2.5 times greater in men who were treated but uncontrolled and were not on a diuretic, said Dr. Victor, director of the hypertension center at Cedars-Sinai Medical Center, Los Angeles.
Among men treated and controlled down to a systolic blood pressure of almost 120 mm Hg, those with a diuretic included in their regimen had about twice the risk of nocturia as did normotensive men; those controlled without a diuretic had no elevated risk of nocturia. The results were statistically significant and were adjusted for nocturia confounders, including diabetes, body mass index, sleep apnea, and an enlarged prostate.
“Nocturia is far more likely when hypertension is inadequately treated,” especially with a diuretic, than when untreated, Dr. Victor said. “The data suggest that nocturia may be a side effect of blood pressure drugs unless strict blood pressure control is achieved. Appropriate treatment of blood pressure without a diuretic may be really beneficial in terms of reducing nocturia.”
Not all the data on the specific medications the men in the study were taking were available, but the most common antihypertensive prescribed for them was short-acting, low-dose hydrochlorothiazide, he noted.
It has been shown that hydrochlorothiazide wears off in the evening when dosed in the morning, so blood pressure might appear to be well-controlled in the daytime, but patients become hypertensive at night, leading to pressure natriuresis and nocturia. “This might [help] explain our data,” Dr. Victor said, noting that longer-acting, more potent diuretics, such as chlorthalidone, might reduce the risk.
The team plans further work to see if tighter nighttime blood pressure control reduces nocturia and improves sleep. Maybe, he noted, “if you take your blood pressure meds correctly, you sleep better. That would be a fantastic public health message; we’ll see.”
Middle-aged black men are underrepresented in hypertension research, in part because of a mistrust of doctors and medical institutions. Enrolling black men in barbershops seemed a good way to address the problem; barbers are trusted and respected members of the community, and the shops themselves are warm and relaxed, which is why hypertension was defined in the study a bit lower than the usual 140/90 mm Hg, Dr. Victor commented.
A total of 45% of the men were hypertensive; only 16% were controlled on medication. Nocturia prevalence was higher than expected in the general population, at about 29% overall, and ranged from 24% in normotensive men to 50% in men whose hypertension was treated but uncontrolled.
Average blood pressures were 120/71 mm Hg in the normotensive men; 143/87 mm Hg in untreated hypertensive men; and 148/91 mm Hg in treated but uncontrolled men. The mean age in the study was 43 years. “We capped it at 49 because after that, nocturia is so prevalent, and dominated by prostate disease,” Dr. Victor said.
The investigators had no disclosures. The National Institutes of Health funded the work.
SAN FRANCISCO – Nocturia is a sign of uncontrolled hypertension and also is associated with diuretic use in middle-aged black men, according to a study conducted at 53 barbershops in and around Los Angeles.
In the study, investigators averaged the last three of five automated blood pressure readings in 1,748 black men aged 35-49 years old and asked them about symptoms of nocturia – defined in the study as getting up two or more times per night to urinate – and about what blood pressure medications they were taking, if any.
Men with untreated hypertension – defined as at or above 135/85 mm Hg – were 34% more likely to report nocturia than were men who were normotensive.
However, “what really grabbed our attention was the treated group,” lead investigator Ronald Victor, MD said at the joint scientific sessions of the AHA Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
The highest risk of nocturia, more than three times the risk of normotensive men, was among men treated with a diuretic who still had elevated blood pressure readings in the barbershop. Their risk was about 2.5 times greater in men who were treated but uncontrolled and were not on a diuretic, said Dr. Victor, director of the hypertension center at Cedars-Sinai Medical Center, Los Angeles.
Among men treated and controlled down to a systolic blood pressure of almost 120 mm Hg, those with a diuretic included in their regimen had about twice the risk of nocturia as did normotensive men; those controlled without a diuretic had no elevated risk of nocturia. The results were statistically significant and were adjusted for nocturia confounders, including diabetes, body mass index, sleep apnea, and an enlarged prostate.
“Nocturia is far more likely when hypertension is inadequately treated,” especially with a diuretic, than when untreated, Dr. Victor said. “The data suggest that nocturia may be a side effect of blood pressure drugs unless strict blood pressure control is achieved. Appropriate treatment of blood pressure without a diuretic may be really beneficial in terms of reducing nocturia.”
Not all the data on the specific medications the men in the study were taking were available, but the most common antihypertensive prescribed for them was short-acting, low-dose hydrochlorothiazide, he noted.
It has been shown that hydrochlorothiazide wears off in the evening when dosed in the morning, so blood pressure might appear to be well-controlled in the daytime, but patients become hypertensive at night, leading to pressure natriuresis and nocturia. “This might [help] explain our data,” Dr. Victor said, noting that longer-acting, more potent diuretics, such as chlorthalidone, might reduce the risk.
The team plans further work to see if tighter nighttime blood pressure control reduces nocturia and improves sleep. Maybe, he noted, “if you take your blood pressure meds correctly, you sleep better. That would be a fantastic public health message; we’ll see.”
Middle-aged black men are underrepresented in hypertension research, in part because of a mistrust of doctors and medical institutions. Enrolling black men in barbershops seemed a good way to address the problem; barbers are trusted and respected members of the community, and the shops themselves are warm and relaxed, which is why hypertension was defined in the study a bit lower than the usual 140/90 mm Hg, Dr. Victor commented.
A total of 45% of the men were hypertensive; only 16% were controlled on medication. Nocturia prevalence was higher than expected in the general population, at about 29% overall, and ranged from 24% in normotensive men to 50% in men whose hypertension was treated but uncontrolled.
Average blood pressures were 120/71 mm Hg in the normotensive men; 143/87 mm Hg in untreated hypertensive men; and 148/91 mm Hg in treated but uncontrolled men. The mean age in the study was 43 years. “We capped it at 49 because after that, nocturia is so prevalent, and dominated by prostate disease,” Dr. Victor said.
The investigators had no disclosures. The National Institutes of Health funded the work.
SAN FRANCISCO – Nocturia is a sign of uncontrolled hypertension and also is associated with diuretic use in middle-aged black men, according to a study conducted at 53 barbershops in and around Los Angeles.
In the study, investigators averaged the last three of five automated blood pressure readings in 1,748 black men aged 35-49 years old and asked them about symptoms of nocturia – defined in the study as getting up two or more times per night to urinate – and about what blood pressure medications they were taking, if any.
Men with untreated hypertension – defined as at or above 135/85 mm Hg – were 34% more likely to report nocturia than were men who were normotensive.
However, “what really grabbed our attention was the treated group,” lead investigator Ronald Victor, MD said at the joint scientific sessions of the AHA Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
The highest risk of nocturia, more than three times the risk of normotensive men, was among men treated with a diuretic who still had elevated blood pressure readings in the barbershop. Their risk was about 2.5 times greater in men who were treated but uncontrolled and were not on a diuretic, said Dr. Victor, director of the hypertension center at Cedars-Sinai Medical Center, Los Angeles.
Among men treated and controlled down to a systolic blood pressure of almost 120 mm Hg, those with a diuretic included in their regimen had about twice the risk of nocturia as did normotensive men; those controlled without a diuretic had no elevated risk of nocturia. The results were statistically significant and were adjusted for nocturia confounders, including diabetes, body mass index, sleep apnea, and an enlarged prostate.
“Nocturia is far more likely when hypertension is inadequately treated,” especially with a diuretic, than when untreated, Dr. Victor said. “The data suggest that nocturia may be a side effect of blood pressure drugs unless strict blood pressure control is achieved. Appropriate treatment of blood pressure without a diuretic may be really beneficial in terms of reducing nocturia.”
Not all the data on the specific medications the men in the study were taking were available, but the most common antihypertensive prescribed for them was short-acting, low-dose hydrochlorothiazide, he noted.
It has been shown that hydrochlorothiazide wears off in the evening when dosed in the morning, so blood pressure might appear to be well-controlled in the daytime, but patients become hypertensive at night, leading to pressure natriuresis and nocturia. “This might [help] explain our data,” Dr. Victor said, noting that longer-acting, more potent diuretics, such as chlorthalidone, might reduce the risk.
The team plans further work to see if tighter nighttime blood pressure control reduces nocturia and improves sleep. Maybe, he noted, “if you take your blood pressure meds correctly, you sleep better. That would be a fantastic public health message; we’ll see.”
Middle-aged black men are underrepresented in hypertension research, in part because of a mistrust of doctors and medical institutions. Enrolling black men in barbershops seemed a good way to address the problem; barbers are trusted and respected members of the community, and the shops themselves are warm and relaxed, which is why hypertension was defined in the study a bit lower than the usual 140/90 mm Hg, Dr. Victor commented.
A total of 45% of the men were hypertensive; only 16% were controlled on medication. Nocturia prevalence was higher than expected in the general population, at about 29% overall, and ranged from 24% in normotensive men to 50% in men whose hypertension was treated but uncontrolled.
Average blood pressures were 120/71 mm Hg in the normotensive men; 143/87 mm Hg in untreated hypertensive men; and 148/91 mm Hg in treated but uncontrolled men. The mean age in the study was 43 years. “We capped it at 49 because after that, nocturia is so prevalent, and dominated by prostate disease,” Dr. Victor said.
The investigators had no disclosures. The National Institutes of Health funded the work.
AT JOINT HYPERTENSION 2017
Key clinical point:
Major finding: The highest risk of nocturia, more than 3 times the risk of normotensive men, was among men treated with a diuretic who still had elevated blood pressure.
Data source: A community-based, cross-sectional study of 1,748 black men aged 35-49 years with blood pressures measured in 53 barbershops.
Disclosures: The investigators had no disclosures. The National Institutes of Health funded the work.