User login
Neurology Reviews covers innovative and emerging news in neurology and neuroscience every month, with a focus on practical approaches to treating Parkinson's disease, epilepsy, headache, stroke, multiple sclerosis, Alzheimer's disease, and other neurologic disorders.
PML
Progressive multifocal leukoencephalopathy
Rituxan
The leading independent newspaper covering neurology news and commentary.
Not always implemented or enforced: Harassment policies at work
Many companies, government agencies, and organizations have implemented policies and procedures to shield employees from sexual and other forms of harassment. The U.S. Department of Health & Human Services and the American Medical Association are just two examples.
Employers can tap a rich lode of guidance and resources to craft these antiharassment policies. The National Institutes of Health’s resource page is a good site for hospitals to check out.
But how effective have official policies proved in deterring harassment in medical workplaces? After all, in a study by the American Association of Medical Colleges, 34% of female faculty said they had experienced sexual harassment irrespective of such policies. And in a recent Medscape survey of more than 3,000 physicians, 27% reported that they had either witnessed or been subjected to sexual harassment or misconduct at work during the past 4 years.
When policies are absent or unenforced
She believes employer rules and policies generally are helpful in establishing who fields harassment complaints and in creating at least some accountability.
On the other hand, policies that don’t recognize anonymous complaints effectively discourage harassment victims from coming forward, Dr. Rohr-Kirchgraber argues. Even those policies that do allow anonymous complaints may have limitations.
For example, the NIH policy on reporting harassment acknowledges that “officials must follow up on all allegations of harassment and cannot guarantee that your identity will not become apparent during the process. Please note that if you remain anonymous, key details about the allegation or concern [may] be omitted. This will limit the NIH’s ability to conduct an inquiry and take corrective action as warranted.”
Risks in pressing a harassment case
A complainant whose name becomes public risks getting a reputation as a problem employee or suffering workplace retaliation, according to Dr. Rohr-Kirchgraber. She recalls a colleague who was on a clinical education track until she lodged a harassment complaint. Abruptly, she was told she was needed on a service with fewer teaching opportunities.
With such risks in mind, respondents to the Medscape survey advised employees in medical workplaces to familiarize themselves with policies and procedures before pressing a case.
“Document everything,” an ophthalmologist urged, including time, place, offender, and witnesses. Present that information to your supervisor, and if nothing is done, hire a lawyer, a gastroenterologist suggested.
But taking the situation to the Equal Employment Opportunity Commission can be complicated, Roberta Gebhard, DO, past AMWA president and founder of its Gender Equity Task Force, told this news organization.
“They talk to the employer and get the employer’s side of the story and eventually render a decision about whether you have a case you can put through and file a lawsuit,” she said. “I don’t know of any other situation in which you need ‘permission’ to file a lawsuit.”
Nevertheless, an attorney can be helpful with cases, and when someone is terminated, a lawyer can possibly have it overturned or converted to a resignation, Dr. Gebhard said.
“And always have a lawyer review your contract before you take the job,” she advised. The lawyer might adjust the contract’s verbiage in ways that can protect one down the road in the event of a potential termination. “It’s money very well spent.”
More education needed
Dr. Rohr-Kirchgraber said that protection against harassment goes beyond the employer’s policies and procedures. Building an overall consciousness of what harassment is should begin with employee onboarding, she said.
“The harasser may not even recognize that what they’re doing or saying is a form of harassment, so we need better education,” Dr. Rohr-Kirchgraber emphasized.
A version of this article originally appeared on Medscape.com.
Many companies, government agencies, and organizations have implemented policies and procedures to shield employees from sexual and other forms of harassment. The U.S. Department of Health & Human Services and the American Medical Association are just two examples.
Employers can tap a rich lode of guidance and resources to craft these antiharassment policies. The National Institutes of Health’s resource page is a good site for hospitals to check out.
But how effective have official policies proved in deterring harassment in medical workplaces? After all, in a study by the American Association of Medical Colleges, 34% of female faculty said they had experienced sexual harassment irrespective of such policies. And in a recent Medscape survey of more than 3,000 physicians, 27% reported that they had either witnessed or been subjected to sexual harassment or misconduct at work during the past 4 years.
When policies are absent or unenforced
She believes employer rules and policies generally are helpful in establishing who fields harassment complaints and in creating at least some accountability.
On the other hand, policies that don’t recognize anonymous complaints effectively discourage harassment victims from coming forward, Dr. Rohr-Kirchgraber argues. Even those policies that do allow anonymous complaints may have limitations.
For example, the NIH policy on reporting harassment acknowledges that “officials must follow up on all allegations of harassment and cannot guarantee that your identity will not become apparent during the process. Please note that if you remain anonymous, key details about the allegation or concern [may] be omitted. This will limit the NIH’s ability to conduct an inquiry and take corrective action as warranted.”
Risks in pressing a harassment case
A complainant whose name becomes public risks getting a reputation as a problem employee or suffering workplace retaliation, according to Dr. Rohr-Kirchgraber. She recalls a colleague who was on a clinical education track until she lodged a harassment complaint. Abruptly, she was told she was needed on a service with fewer teaching opportunities.
With such risks in mind, respondents to the Medscape survey advised employees in medical workplaces to familiarize themselves with policies and procedures before pressing a case.
“Document everything,” an ophthalmologist urged, including time, place, offender, and witnesses. Present that information to your supervisor, and if nothing is done, hire a lawyer, a gastroenterologist suggested.
But taking the situation to the Equal Employment Opportunity Commission can be complicated, Roberta Gebhard, DO, past AMWA president and founder of its Gender Equity Task Force, told this news organization.
“They talk to the employer and get the employer’s side of the story and eventually render a decision about whether you have a case you can put through and file a lawsuit,” she said. “I don’t know of any other situation in which you need ‘permission’ to file a lawsuit.”
Nevertheless, an attorney can be helpful with cases, and when someone is terminated, a lawyer can possibly have it overturned or converted to a resignation, Dr. Gebhard said.
“And always have a lawyer review your contract before you take the job,” she advised. The lawyer might adjust the contract’s verbiage in ways that can protect one down the road in the event of a potential termination. “It’s money very well spent.”
More education needed
Dr. Rohr-Kirchgraber said that protection against harassment goes beyond the employer’s policies and procedures. Building an overall consciousness of what harassment is should begin with employee onboarding, she said.
“The harasser may not even recognize that what they’re doing or saying is a form of harassment, so we need better education,” Dr. Rohr-Kirchgraber emphasized.
A version of this article originally appeared on Medscape.com.
Many companies, government agencies, and organizations have implemented policies and procedures to shield employees from sexual and other forms of harassment. The U.S. Department of Health & Human Services and the American Medical Association are just two examples.
Employers can tap a rich lode of guidance and resources to craft these antiharassment policies. The National Institutes of Health’s resource page is a good site for hospitals to check out.
But how effective have official policies proved in deterring harassment in medical workplaces? After all, in a study by the American Association of Medical Colleges, 34% of female faculty said they had experienced sexual harassment irrespective of such policies. And in a recent Medscape survey of more than 3,000 physicians, 27% reported that they had either witnessed or been subjected to sexual harassment or misconduct at work during the past 4 years.
When policies are absent or unenforced
She believes employer rules and policies generally are helpful in establishing who fields harassment complaints and in creating at least some accountability.
On the other hand, policies that don’t recognize anonymous complaints effectively discourage harassment victims from coming forward, Dr. Rohr-Kirchgraber argues. Even those policies that do allow anonymous complaints may have limitations.
For example, the NIH policy on reporting harassment acknowledges that “officials must follow up on all allegations of harassment and cannot guarantee that your identity will not become apparent during the process. Please note that if you remain anonymous, key details about the allegation or concern [may] be omitted. This will limit the NIH’s ability to conduct an inquiry and take corrective action as warranted.”
Risks in pressing a harassment case
A complainant whose name becomes public risks getting a reputation as a problem employee or suffering workplace retaliation, according to Dr. Rohr-Kirchgraber. She recalls a colleague who was on a clinical education track until she lodged a harassment complaint. Abruptly, she was told she was needed on a service with fewer teaching opportunities.
With such risks in mind, respondents to the Medscape survey advised employees in medical workplaces to familiarize themselves with policies and procedures before pressing a case.
“Document everything,” an ophthalmologist urged, including time, place, offender, and witnesses. Present that information to your supervisor, and if nothing is done, hire a lawyer, a gastroenterologist suggested.
But taking the situation to the Equal Employment Opportunity Commission can be complicated, Roberta Gebhard, DO, past AMWA president and founder of its Gender Equity Task Force, told this news organization.
“They talk to the employer and get the employer’s side of the story and eventually render a decision about whether you have a case you can put through and file a lawsuit,” she said. “I don’t know of any other situation in which you need ‘permission’ to file a lawsuit.”
Nevertheless, an attorney can be helpful with cases, and when someone is terminated, a lawyer can possibly have it overturned or converted to a resignation, Dr. Gebhard said.
“And always have a lawyer review your contract before you take the job,” she advised. The lawyer might adjust the contract’s verbiage in ways that can protect one down the road in the event of a potential termination. “It’s money very well spent.”
More education needed
Dr. Rohr-Kirchgraber said that protection against harassment goes beyond the employer’s policies and procedures. Building an overall consciousness of what harassment is should begin with employee onboarding, she said.
“The harasser may not even recognize that what they’re doing or saying is a form of harassment, so we need better education,” Dr. Rohr-Kirchgraber emphasized.
A version of this article originally appeared on Medscape.com.
Immunodeficiencies tied to psychiatric disorders in offspring
new research suggests.
Results from a cohort study of more than 4.2 million individuals showed that offspring of mothers with PIDs had a 17% increased risk for a psychiatric disorder and a 20% increased risk for suicidal behavior, compared with their peers with mothers who did not have PIDs.
The risk was more pronounced in offspring of mothers with both PIDs and autoimmune diseases. These risks remained after strictly controlling for different covariates, such as the parents’ psychiatric history, offspring PIDs, and offspring autoimmune diseases.
The investigators, led by Josef Isung, MD, PhD, Centre for Psychiatry Research, department of clinical neuroscience, Karolinska Institutet, Stockholm, noted that they could not “pinpoint a precise causal mechanism” underlying these findings.
Still, “the results add to the existing literature suggesting that the intrauterine immune environment may have implications for fetal neurodevelopment and that a compromised maternal immune system during pregnancy may be a risk factor for psychiatric disorders and suicidal behavior in their offspring in the long term,” they wrote.
The findings were published online in JAMA Psychiatry.
‘Natural experiment’
Maternal immune activation (MIA) is “an overarching term for aberrant and disrupted immune activity in the mother during gestation [and] has long been of interest in relation to adverse health outcomes in the offspring,” Dr. Isung noted.
“In relation to negative psychiatric outcomes, there is an abundance of preclinical evidence that has shown a negative impact on offspring secondary to MIA. And in humans, there are several observational studies supporting this link,” he said in an interview.
Dr. Isung added that PIDs are “rare conditions” known to be associated with repeated infections and high rates of autoimmune diseases, causing substantial disability.
“PIDs represent an interesting ‘natural experiment’ for researchers to understand more about the association between immune system dysfunctions and mental health,” he said.
Dr. Isung’s group previously showed that individuals with PIDs have increased odds of psychiatric disorders and suicidal behavior. The link was more pronounced in women with PIDs – and was even more pronounced in those with both PIDs and autoimmune diseases.
In the current study, “we wanted to see whether offspring of individuals were differentially at risk of psychiatric disorders and suicidal behavior, depending on being offspring of mothers or fathers with PIDs,” Dr. Isung said.
“Our hypothesis was that mothers with PIDs would have an increased risk of having offspring with neuropsychiatric outcomes, and that this risk could be due to MIA,” he added.
The researchers turned to Swedish nationwide health and administrative registers. They analyzed data on all individuals with diagnoses of PIDs identified between 1973 and 2013. Offspring born prior to 2003 were included, and parent-offspring pairs in which both parents had a history of PIDs were excluded.
The final study sample consisted of 4,294,169 offspring (51.4% boys). Of these participants, 7,270 (0.17%) had a parent with PIDs.
The researchers identified lifetime records of 10 psychiatric disorders: obsessive-compulsive disorder, ADHD, autism spectrum disorders, schizophrenia and other psychotic disorders, bipolar disorders, major depressive disorder and other mood disorders, anxiety and stress-related disorders, eating disorders, substance use disorders, and Tourette syndrome and chronic tic disorders.
The investigators included parental birth year, psychopathology, suicide attempts, suicide deaths, and autoimmune diseases as covariates, as well as offsprings’ birth year and gender.
Elucidation needed
Results showed that, of the 4,676 offspring of mothers with PID, 17.1% had a psychiatric disorder versus 12.7% of offspring of mothers without PIDs. This translated “into a 17% increased risk for offspring of mothers with PIDs in the fully adjusted model,” the investigators reported.
The risk was even higher for offspring of mothers who had not only PIDs but also one of six of the individual psychiatric disorders, with incident rate ratios ranging from 1.15 to 1.71.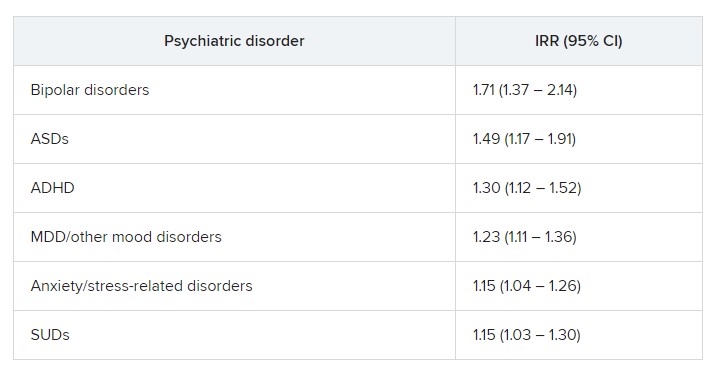
“In fully adjusted models, offspring of mothers with PIDs had an increased risk of any psychiatric disorder, while no such risks were observed in offspring of fathers with PIDs” (IRR, 1.17 vs. 1.03; P < .001), the researchers reported.
A higher risk for suicidal behavior was also observed among offspring of mothers with PIDS, in contrast to those of fathers with PIDs (IRR, 1.2 vs. 1.1; P = .01).
The greatest risk for any psychiatric disorder, as well as suicidal behavior, was found in offspring of mothers who had both PIDs and autoimmune diseases (IRRs, 1.24 and 1.44, respectively).
“The results could be seen as substantiating the hypothesis that immune disruption may be important in the pathophysiology of psychiatric disorders and suicidal behavior,” Dr. Isung said.
“Furthermore, the fact that only offspring of mothers and not offspring of fathers with PIDs had this association would align with our hypothesis that MIA is of importance,” he added.
However, he noted that “the specific mechanisms are most likely multifactorial and remain to be elucidated.”
Important piece of the puzzle?
In a comment, Michael Eriksen Benros, MD, PhD, professor of immunopsychiatry, department of immunology and microbiology, health, and medical sciences, University of Copenhagen, said this was a “high-quality study” that used a “rich data source.”
Dr. Benros, who is also head of research (biological and precision psychiatry) at the Copenhagen Research Centre for Mental Health, Copenhagen University Hospital, was not involved with the current study.
He noted that prior studies, including some conducted by his own group, have shown that maternal infections overall did not seem to be “specifically linked to mental disorders in the offspring.”
However, “specific maternal infections or specific brain-reactive antibodies during the pregnancy period have been shown to be associated with neurodevelopmental outcomes among the children,” such as intellectual disability, he said.
Regarding direct clinical implications of the study, “it is important to note that the increased risk of psychiatric disorders and suicidality in the offspring of mothers with PID were small,” Dr. Benros said.
“However, it adds an important part to the scientific puzzle regarding the role of maternal immune activation during pregnancy and the risk of mental disorders,” he added.
The study was funded by the Söderström König Foundation and the Fredrik and Ingrid Thuring Foundation. Neither Dr. Isung nor Dr. Benros reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests.
Results from a cohort study of more than 4.2 million individuals showed that offspring of mothers with PIDs had a 17% increased risk for a psychiatric disorder and a 20% increased risk for suicidal behavior, compared with their peers with mothers who did not have PIDs.
The risk was more pronounced in offspring of mothers with both PIDs and autoimmune diseases. These risks remained after strictly controlling for different covariates, such as the parents’ psychiatric history, offspring PIDs, and offspring autoimmune diseases.
The investigators, led by Josef Isung, MD, PhD, Centre for Psychiatry Research, department of clinical neuroscience, Karolinska Institutet, Stockholm, noted that they could not “pinpoint a precise causal mechanism” underlying these findings.
Still, “the results add to the existing literature suggesting that the intrauterine immune environment may have implications for fetal neurodevelopment and that a compromised maternal immune system during pregnancy may be a risk factor for psychiatric disorders and suicidal behavior in their offspring in the long term,” they wrote.
The findings were published online in JAMA Psychiatry.
‘Natural experiment’
Maternal immune activation (MIA) is “an overarching term for aberrant and disrupted immune activity in the mother during gestation [and] has long been of interest in relation to adverse health outcomes in the offspring,” Dr. Isung noted.
“In relation to negative psychiatric outcomes, there is an abundance of preclinical evidence that has shown a negative impact on offspring secondary to MIA. And in humans, there are several observational studies supporting this link,” he said in an interview.
Dr. Isung added that PIDs are “rare conditions” known to be associated with repeated infections and high rates of autoimmune diseases, causing substantial disability.
“PIDs represent an interesting ‘natural experiment’ for researchers to understand more about the association between immune system dysfunctions and mental health,” he said.
Dr. Isung’s group previously showed that individuals with PIDs have increased odds of psychiatric disorders and suicidal behavior. The link was more pronounced in women with PIDs – and was even more pronounced in those with both PIDs and autoimmune diseases.
In the current study, “we wanted to see whether offspring of individuals were differentially at risk of psychiatric disorders and suicidal behavior, depending on being offspring of mothers or fathers with PIDs,” Dr. Isung said.
“Our hypothesis was that mothers with PIDs would have an increased risk of having offspring with neuropsychiatric outcomes, and that this risk could be due to MIA,” he added.
The researchers turned to Swedish nationwide health and administrative registers. They analyzed data on all individuals with diagnoses of PIDs identified between 1973 and 2013. Offspring born prior to 2003 were included, and parent-offspring pairs in which both parents had a history of PIDs were excluded.
The final study sample consisted of 4,294,169 offspring (51.4% boys). Of these participants, 7,270 (0.17%) had a parent with PIDs.
The researchers identified lifetime records of 10 psychiatric disorders: obsessive-compulsive disorder, ADHD, autism spectrum disorders, schizophrenia and other psychotic disorders, bipolar disorders, major depressive disorder and other mood disorders, anxiety and stress-related disorders, eating disorders, substance use disorders, and Tourette syndrome and chronic tic disorders.
The investigators included parental birth year, psychopathology, suicide attempts, suicide deaths, and autoimmune diseases as covariates, as well as offsprings’ birth year and gender.
Elucidation needed
Results showed that, of the 4,676 offspring of mothers with PID, 17.1% had a psychiatric disorder versus 12.7% of offspring of mothers without PIDs. This translated “into a 17% increased risk for offspring of mothers with PIDs in the fully adjusted model,” the investigators reported.
The risk was even higher for offspring of mothers who had not only PIDs but also one of six of the individual psychiatric disorders, with incident rate ratios ranging from 1.15 to 1.71.
“In fully adjusted models, offspring of mothers with PIDs had an increased risk of any psychiatric disorder, while no such risks were observed in offspring of fathers with PIDs” (IRR, 1.17 vs. 1.03; P < .001), the researchers reported.
A higher risk for suicidal behavior was also observed among offspring of mothers with PIDS, in contrast to those of fathers with PIDs (IRR, 1.2 vs. 1.1; P = .01).
The greatest risk for any psychiatric disorder, as well as suicidal behavior, was found in offspring of mothers who had both PIDs and autoimmune diseases (IRRs, 1.24 and 1.44, respectively).
“The results could be seen as substantiating the hypothesis that immune disruption may be important in the pathophysiology of psychiatric disorders and suicidal behavior,” Dr. Isung said.
“Furthermore, the fact that only offspring of mothers and not offspring of fathers with PIDs had this association would align with our hypothesis that MIA is of importance,” he added.
However, he noted that “the specific mechanisms are most likely multifactorial and remain to be elucidated.”
Important piece of the puzzle?
In a comment, Michael Eriksen Benros, MD, PhD, professor of immunopsychiatry, department of immunology and microbiology, health, and medical sciences, University of Copenhagen, said this was a “high-quality study” that used a “rich data source.”
Dr. Benros, who is also head of research (biological and precision psychiatry) at the Copenhagen Research Centre for Mental Health, Copenhagen University Hospital, was not involved with the current study.
He noted that prior studies, including some conducted by his own group, have shown that maternal infections overall did not seem to be “specifically linked to mental disorders in the offspring.”
However, “specific maternal infections or specific brain-reactive antibodies during the pregnancy period have been shown to be associated with neurodevelopmental outcomes among the children,” such as intellectual disability, he said.
Regarding direct clinical implications of the study, “it is important to note that the increased risk of psychiatric disorders and suicidality in the offspring of mothers with PID were small,” Dr. Benros said.
“However, it adds an important part to the scientific puzzle regarding the role of maternal immune activation during pregnancy and the risk of mental disorders,” he added.
The study was funded by the Söderström König Foundation and the Fredrik and Ingrid Thuring Foundation. Neither Dr. Isung nor Dr. Benros reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests.
Results from a cohort study of more than 4.2 million individuals showed that offspring of mothers with PIDs had a 17% increased risk for a psychiatric disorder and a 20% increased risk for suicidal behavior, compared with their peers with mothers who did not have PIDs.
The risk was more pronounced in offspring of mothers with both PIDs and autoimmune diseases. These risks remained after strictly controlling for different covariates, such as the parents’ psychiatric history, offspring PIDs, and offspring autoimmune diseases.
The investigators, led by Josef Isung, MD, PhD, Centre for Psychiatry Research, department of clinical neuroscience, Karolinska Institutet, Stockholm, noted that they could not “pinpoint a precise causal mechanism” underlying these findings.
Still, “the results add to the existing literature suggesting that the intrauterine immune environment may have implications for fetal neurodevelopment and that a compromised maternal immune system during pregnancy may be a risk factor for psychiatric disorders and suicidal behavior in their offspring in the long term,” they wrote.
The findings were published online in JAMA Psychiatry.
‘Natural experiment’
Maternal immune activation (MIA) is “an overarching term for aberrant and disrupted immune activity in the mother during gestation [and] has long been of interest in relation to adverse health outcomes in the offspring,” Dr. Isung noted.
“In relation to negative psychiatric outcomes, there is an abundance of preclinical evidence that has shown a negative impact on offspring secondary to MIA. And in humans, there are several observational studies supporting this link,” he said in an interview.
Dr. Isung added that PIDs are “rare conditions” known to be associated with repeated infections and high rates of autoimmune diseases, causing substantial disability.
“PIDs represent an interesting ‘natural experiment’ for researchers to understand more about the association between immune system dysfunctions and mental health,” he said.
Dr. Isung’s group previously showed that individuals with PIDs have increased odds of psychiatric disorders and suicidal behavior. The link was more pronounced in women with PIDs – and was even more pronounced in those with both PIDs and autoimmune diseases.
In the current study, “we wanted to see whether offspring of individuals were differentially at risk of psychiatric disorders and suicidal behavior, depending on being offspring of mothers or fathers with PIDs,” Dr. Isung said.
“Our hypothesis was that mothers with PIDs would have an increased risk of having offspring with neuropsychiatric outcomes, and that this risk could be due to MIA,” he added.
The researchers turned to Swedish nationwide health and administrative registers. They analyzed data on all individuals with diagnoses of PIDs identified between 1973 and 2013. Offspring born prior to 2003 were included, and parent-offspring pairs in which both parents had a history of PIDs were excluded.
The final study sample consisted of 4,294,169 offspring (51.4% boys). Of these participants, 7,270 (0.17%) had a parent with PIDs.
The researchers identified lifetime records of 10 psychiatric disorders: obsessive-compulsive disorder, ADHD, autism spectrum disorders, schizophrenia and other psychotic disorders, bipolar disorders, major depressive disorder and other mood disorders, anxiety and stress-related disorders, eating disorders, substance use disorders, and Tourette syndrome and chronic tic disorders.
The investigators included parental birth year, psychopathology, suicide attempts, suicide deaths, and autoimmune diseases as covariates, as well as offsprings’ birth year and gender.
Elucidation needed
Results showed that, of the 4,676 offspring of mothers with PID, 17.1% had a psychiatric disorder versus 12.7% of offspring of mothers without PIDs. This translated “into a 17% increased risk for offspring of mothers with PIDs in the fully adjusted model,” the investigators reported.
The risk was even higher for offspring of mothers who had not only PIDs but also one of six of the individual psychiatric disorders, with incident rate ratios ranging from 1.15 to 1.71.
“In fully adjusted models, offspring of mothers with PIDs had an increased risk of any psychiatric disorder, while no such risks were observed in offspring of fathers with PIDs” (IRR, 1.17 vs. 1.03; P < .001), the researchers reported.
A higher risk for suicidal behavior was also observed among offspring of mothers with PIDS, in contrast to those of fathers with PIDs (IRR, 1.2 vs. 1.1; P = .01).
The greatest risk for any psychiatric disorder, as well as suicidal behavior, was found in offspring of mothers who had both PIDs and autoimmune diseases (IRRs, 1.24 and 1.44, respectively).
“The results could be seen as substantiating the hypothesis that immune disruption may be important in the pathophysiology of psychiatric disorders and suicidal behavior,” Dr. Isung said.
“Furthermore, the fact that only offspring of mothers and not offspring of fathers with PIDs had this association would align with our hypothesis that MIA is of importance,” he added.
However, he noted that “the specific mechanisms are most likely multifactorial and remain to be elucidated.”
Important piece of the puzzle?
In a comment, Michael Eriksen Benros, MD, PhD, professor of immunopsychiatry, department of immunology and microbiology, health, and medical sciences, University of Copenhagen, said this was a “high-quality study” that used a “rich data source.”
Dr. Benros, who is also head of research (biological and precision psychiatry) at the Copenhagen Research Centre for Mental Health, Copenhagen University Hospital, was not involved with the current study.
He noted that prior studies, including some conducted by his own group, have shown that maternal infections overall did not seem to be “specifically linked to mental disorders in the offspring.”
However, “specific maternal infections or specific brain-reactive antibodies during the pregnancy period have been shown to be associated with neurodevelopmental outcomes among the children,” such as intellectual disability, he said.
Regarding direct clinical implications of the study, “it is important to note that the increased risk of psychiatric disorders and suicidality in the offspring of mothers with PID were small,” Dr. Benros said.
“However, it adds an important part to the scientific puzzle regarding the role of maternal immune activation during pregnancy and the risk of mental disorders,” he added.
The study was funded by the Söderström König Foundation and the Fredrik and Ingrid Thuring Foundation. Neither Dr. Isung nor Dr. Benros reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM JAMA PSYCHIATRY
Slowing, not stopping, Alzheimer’s a better goal for clinical trials?
and may be a more realistic goal for clinical AD drug trials, a new report suggests.
The report is a yearlong undertaking by an expert work group convened by the Alzheimer’s Association and was prompted, in part, by the fallout from the U.S. Food and Drug Administration’s controversial decision to grant aducanumab (Aduhelm) accelerated approval, which came over the objection of an advisory panel that found the drug was ineffective.
The report’s authors call for a “reframing” of how researchers define “clinically meaningful” in randomized controlled trials (RCTs), noting that it’s time to adjust expectations of outcomes from relatively short clinical trials.
“Without lowering the bar, are we expecting too much from a clinical trial by expecting that unless the disease is halted in its tracks and there’s no progression, we failed at treatment?” the report’s lead author and group leader Ronald C. Petersen, MD, PhD, lead author, chair of the work group, and professor of neurology at the Mayo Clinic, Rochester, Minn., told this news organization.
Interpretations of clinical meaningfulness are used in the drug approval process and in decisions about whether an insurer will cover the cost of treatment, the authors note.
While the report doesn’t provide a consensus definition of clinically meaningful benefit, it does offer a starting point for a conversation about how the phrase should be defined in the context of RCTs for disease-modifying therapies (DMTs) in AD, Dr. Petersen said.
“What we tried to do was to put it into some kind of perspective and at least have people reflect on this: If you’re going to design the perfect drug trial in Alzheimer’s disease, what would it be? We wanted to get people to think about it without digging in their heels for or against,” he added.
The report was published online in Alzheimer’s and Dementia: The Journal of the Alzheimer’s Association.
A proactive measure
The expert group began its work in January 2022, less than a year after the FDA approved aducanumab. Since the panel began its work, the FDA has approved a second AD drug, lecanemab (Leqembi), and denied accelerated approval of a third medication, donanemab.
“At the time we started this group, we had one approved treatment, and we just knew that there were others on the way, and we needed to be prepared to have this conversation and be more proactive than reactive,” Christopher Weber, PhD, director of global science initiatives for the Alzheimer’s Association and co-author of the report, said in an interview.
The work group suggests that simply slowing disease progression might be a desired goal for drug trials, especially early on, before cognition and memory are affected.
They also note that a benefit identified during an 18-month clinical trial may ultimately lead to even more meaningful changes over coming years, well beyond the trial’s end.
In addition, the report authors call for the development of better research tools to more accurately assess meaningful change. The Clinical Dementia Rating (CDR) scale is currently the key instrument used as a primary outcome measure in RCTs. However, the report’s authors note that it may not be adequate to measure meaningful change in early-stage disease.
“Developing better tools certainly should be on the radar screen for all of us, because I think we can do better,” Dr. Petersen said. “The CDR, as good as it is and as long as it’s been used in the field, is a pretty blunt instrument, and it’s the result of subjective ratings.”
‘Quality of mind’
Jason Karlawish, MD, professor of medicine, medical ethics, health policy, and neurology at the University of Pennsylvania, Philadelphia, said measuring the actual impact of a drug on a patient’s disease and quality of life has been a hot topic in the AD field for some time, but settling on a definition of “clinically meaningful” that everyone agrees upon will be a challenge.
“I think the idea of ‘clinically meaningful’ is truly a socially constructed idea,” said Dr. Karlawish, co-director of Penn’s Memory Center, who did not work on the report.
“You can come up with objective measures of cognition, but a measure to call something ‘clinically meaningful’ ultimately requires some sort of negotiated social order among clinicians and patients and others who have immediate interest in the health and well-being of the patient.”
Dr. Karlawish added that he’s interested in the conversations the report might prompt and the challenges it could highlight, especially when it comes to how meaningful clinical benefit can be measured, regardless of how it’s defined.
“Hidden in this conversation about clinically meaningful treatments in Alzheimer’s disease is, frankly, not quality of life, but quality of mind,” said Dr. Karlawish. “No measure captures acceptably the very thing that everyone actually cares a lot about and why we view this disease as so dreadful, which is damage to our mind.”
More evidence needed
The development of such tools will take time. What does that mean for drugs already in the pipeline? Members of the work group argue that those trials must move forward at the same time new tools are being created.
“We need to continue to refine, develop better instruments, [and] develop tools that are going to assess the disease in its more subtle features early on, even in the so-called ‘pre-symptomatic’ stage of the disease,” said lead author Dr. Petersen. “We shouldn’t wait for the development of that before intervening if we have a drug that seems to work.”
However, not everyone who agrees with the premise of the report agrees with this position, including Joel S. Perlmutter, MD, professor of neurology, Washington University School of Medicine, St. Louis, who also commented on the report.
As reported by this news organization, Dr. Perlmutter was one of three physicians who resigned from the FDA advisory panel that voted against approving aducanumab after the agency moved forward anyway.
“We have to be careful not to recommend DMTs that we hope will help without strong evidence, especially when potential side effects are not trivial,” Dr. Perlmutter said. “We have to have evidence before making these recommendations so we don’t end up harming people more than helping them.”
The report received no specific funding. Dr. Petersen received consulting fees from Roche, Nestle, Merck, Biogen, Eisai, and Genentech. Full disclosures are included in the original article. Dr. Perlmutter and Dr. Karlawish report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
and may be a more realistic goal for clinical AD drug trials, a new report suggests.
The report is a yearlong undertaking by an expert work group convened by the Alzheimer’s Association and was prompted, in part, by the fallout from the U.S. Food and Drug Administration’s controversial decision to grant aducanumab (Aduhelm) accelerated approval, which came over the objection of an advisory panel that found the drug was ineffective.
The report’s authors call for a “reframing” of how researchers define “clinically meaningful” in randomized controlled trials (RCTs), noting that it’s time to adjust expectations of outcomes from relatively short clinical trials.
“Without lowering the bar, are we expecting too much from a clinical trial by expecting that unless the disease is halted in its tracks and there’s no progression, we failed at treatment?” the report’s lead author and group leader Ronald C. Petersen, MD, PhD, lead author, chair of the work group, and professor of neurology at the Mayo Clinic, Rochester, Minn., told this news organization.
Interpretations of clinical meaningfulness are used in the drug approval process and in decisions about whether an insurer will cover the cost of treatment, the authors note.
While the report doesn’t provide a consensus definition of clinically meaningful benefit, it does offer a starting point for a conversation about how the phrase should be defined in the context of RCTs for disease-modifying therapies (DMTs) in AD, Dr. Petersen said.
“What we tried to do was to put it into some kind of perspective and at least have people reflect on this: If you’re going to design the perfect drug trial in Alzheimer’s disease, what would it be? We wanted to get people to think about it without digging in their heels for or against,” he added.
The report was published online in Alzheimer’s and Dementia: The Journal of the Alzheimer’s Association.
A proactive measure
The expert group began its work in January 2022, less than a year after the FDA approved aducanumab. Since the panel began its work, the FDA has approved a second AD drug, lecanemab (Leqembi), and denied accelerated approval of a third medication, donanemab.
“At the time we started this group, we had one approved treatment, and we just knew that there were others on the way, and we needed to be prepared to have this conversation and be more proactive than reactive,” Christopher Weber, PhD, director of global science initiatives for the Alzheimer’s Association and co-author of the report, said in an interview.
The work group suggests that simply slowing disease progression might be a desired goal for drug trials, especially early on, before cognition and memory are affected.
They also note that a benefit identified during an 18-month clinical trial may ultimately lead to even more meaningful changes over coming years, well beyond the trial’s end.
In addition, the report authors call for the development of better research tools to more accurately assess meaningful change. The Clinical Dementia Rating (CDR) scale is currently the key instrument used as a primary outcome measure in RCTs. However, the report’s authors note that it may not be adequate to measure meaningful change in early-stage disease.
“Developing better tools certainly should be on the radar screen for all of us, because I think we can do better,” Dr. Petersen said. “The CDR, as good as it is and as long as it’s been used in the field, is a pretty blunt instrument, and it’s the result of subjective ratings.”
‘Quality of mind’
Jason Karlawish, MD, professor of medicine, medical ethics, health policy, and neurology at the University of Pennsylvania, Philadelphia, said measuring the actual impact of a drug on a patient’s disease and quality of life has been a hot topic in the AD field for some time, but settling on a definition of “clinically meaningful” that everyone agrees upon will be a challenge.
“I think the idea of ‘clinically meaningful’ is truly a socially constructed idea,” said Dr. Karlawish, co-director of Penn’s Memory Center, who did not work on the report.
“You can come up with objective measures of cognition, but a measure to call something ‘clinically meaningful’ ultimately requires some sort of negotiated social order among clinicians and patients and others who have immediate interest in the health and well-being of the patient.”
Dr. Karlawish added that he’s interested in the conversations the report might prompt and the challenges it could highlight, especially when it comes to how meaningful clinical benefit can be measured, regardless of how it’s defined.
“Hidden in this conversation about clinically meaningful treatments in Alzheimer’s disease is, frankly, not quality of life, but quality of mind,” said Dr. Karlawish. “No measure captures acceptably the very thing that everyone actually cares a lot about and why we view this disease as so dreadful, which is damage to our mind.”
More evidence needed
The development of such tools will take time. What does that mean for drugs already in the pipeline? Members of the work group argue that those trials must move forward at the same time new tools are being created.
“We need to continue to refine, develop better instruments, [and] develop tools that are going to assess the disease in its more subtle features early on, even in the so-called ‘pre-symptomatic’ stage of the disease,” said lead author Dr. Petersen. “We shouldn’t wait for the development of that before intervening if we have a drug that seems to work.”
However, not everyone who agrees with the premise of the report agrees with this position, including Joel S. Perlmutter, MD, professor of neurology, Washington University School of Medicine, St. Louis, who also commented on the report.
As reported by this news organization, Dr. Perlmutter was one of three physicians who resigned from the FDA advisory panel that voted against approving aducanumab after the agency moved forward anyway.
“We have to be careful not to recommend DMTs that we hope will help without strong evidence, especially when potential side effects are not trivial,” Dr. Perlmutter said. “We have to have evidence before making these recommendations so we don’t end up harming people more than helping them.”
The report received no specific funding. Dr. Petersen received consulting fees from Roche, Nestle, Merck, Biogen, Eisai, and Genentech. Full disclosures are included in the original article. Dr. Perlmutter and Dr. Karlawish report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
and may be a more realistic goal for clinical AD drug trials, a new report suggests.
The report is a yearlong undertaking by an expert work group convened by the Alzheimer’s Association and was prompted, in part, by the fallout from the U.S. Food and Drug Administration’s controversial decision to grant aducanumab (Aduhelm) accelerated approval, which came over the objection of an advisory panel that found the drug was ineffective.
The report’s authors call for a “reframing” of how researchers define “clinically meaningful” in randomized controlled trials (RCTs), noting that it’s time to adjust expectations of outcomes from relatively short clinical trials.
“Without lowering the bar, are we expecting too much from a clinical trial by expecting that unless the disease is halted in its tracks and there’s no progression, we failed at treatment?” the report’s lead author and group leader Ronald C. Petersen, MD, PhD, lead author, chair of the work group, and professor of neurology at the Mayo Clinic, Rochester, Minn., told this news organization.
Interpretations of clinical meaningfulness are used in the drug approval process and in decisions about whether an insurer will cover the cost of treatment, the authors note.
While the report doesn’t provide a consensus definition of clinically meaningful benefit, it does offer a starting point for a conversation about how the phrase should be defined in the context of RCTs for disease-modifying therapies (DMTs) in AD, Dr. Petersen said.
“What we tried to do was to put it into some kind of perspective and at least have people reflect on this: If you’re going to design the perfect drug trial in Alzheimer’s disease, what would it be? We wanted to get people to think about it without digging in their heels for or against,” he added.
The report was published online in Alzheimer’s and Dementia: The Journal of the Alzheimer’s Association.
A proactive measure
The expert group began its work in January 2022, less than a year after the FDA approved aducanumab. Since the panel began its work, the FDA has approved a second AD drug, lecanemab (Leqembi), and denied accelerated approval of a third medication, donanemab.
“At the time we started this group, we had one approved treatment, and we just knew that there were others on the way, and we needed to be prepared to have this conversation and be more proactive than reactive,” Christopher Weber, PhD, director of global science initiatives for the Alzheimer’s Association and co-author of the report, said in an interview.
The work group suggests that simply slowing disease progression might be a desired goal for drug trials, especially early on, before cognition and memory are affected.
They also note that a benefit identified during an 18-month clinical trial may ultimately lead to even more meaningful changes over coming years, well beyond the trial’s end.
In addition, the report authors call for the development of better research tools to more accurately assess meaningful change. The Clinical Dementia Rating (CDR) scale is currently the key instrument used as a primary outcome measure in RCTs. However, the report’s authors note that it may not be adequate to measure meaningful change in early-stage disease.
“Developing better tools certainly should be on the radar screen for all of us, because I think we can do better,” Dr. Petersen said. “The CDR, as good as it is and as long as it’s been used in the field, is a pretty blunt instrument, and it’s the result of subjective ratings.”
‘Quality of mind’
Jason Karlawish, MD, professor of medicine, medical ethics, health policy, and neurology at the University of Pennsylvania, Philadelphia, said measuring the actual impact of a drug on a patient’s disease and quality of life has been a hot topic in the AD field for some time, but settling on a definition of “clinically meaningful” that everyone agrees upon will be a challenge.
“I think the idea of ‘clinically meaningful’ is truly a socially constructed idea,” said Dr. Karlawish, co-director of Penn’s Memory Center, who did not work on the report.
“You can come up with objective measures of cognition, but a measure to call something ‘clinically meaningful’ ultimately requires some sort of negotiated social order among clinicians and patients and others who have immediate interest in the health and well-being of the patient.”
Dr. Karlawish added that he’s interested in the conversations the report might prompt and the challenges it could highlight, especially when it comes to how meaningful clinical benefit can be measured, regardless of how it’s defined.
“Hidden in this conversation about clinically meaningful treatments in Alzheimer’s disease is, frankly, not quality of life, but quality of mind,” said Dr. Karlawish. “No measure captures acceptably the very thing that everyone actually cares a lot about and why we view this disease as so dreadful, which is damage to our mind.”
More evidence needed
The development of such tools will take time. What does that mean for drugs already in the pipeline? Members of the work group argue that those trials must move forward at the same time new tools are being created.
“We need to continue to refine, develop better instruments, [and] develop tools that are going to assess the disease in its more subtle features early on, even in the so-called ‘pre-symptomatic’ stage of the disease,” said lead author Dr. Petersen. “We shouldn’t wait for the development of that before intervening if we have a drug that seems to work.”
However, not everyone who agrees with the premise of the report agrees with this position, including Joel S. Perlmutter, MD, professor of neurology, Washington University School of Medicine, St. Louis, who also commented on the report.
As reported by this news organization, Dr. Perlmutter was one of three physicians who resigned from the FDA advisory panel that voted against approving aducanumab after the agency moved forward anyway.
“We have to be careful not to recommend DMTs that we hope will help without strong evidence, especially when potential side effects are not trivial,” Dr. Perlmutter said. “We have to have evidence before making these recommendations so we don’t end up harming people more than helping them.”
The report received no specific funding. Dr. Petersen received consulting fees from Roche, Nestle, Merck, Biogen, Eisai, and Genentech. Full disclosures are included in the original article. Dr. Perlmutter and Dr. Karlawish report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM ALZHEIMER’S AND DEMENTIA
Novel celery seed–derived drug may improve stroke outcomes
a new report suggests.
Patients treated with butylphthalide had fewer severe neurologic symptoms and better function 90 days after the stroke, compared with those receiving placebo.
Butylphthalide is approved and available for use in China, where the study was conducted. However, the medication hasn’t been approved for use by the U.S. Food and Drug Administration.
“Patients who received butylphthalide had less severe neurological symptoms and a better living status at 90 days post stroke, compared to those who received the placebo,” said coauthor Baixue Jia, MD, an attending physician in interventional neuroradiology at the Beijing Tiantan Hospital of Capital Medical University and a faculty member at the China National Clinical Research Center for Neurological Diseases in Beijing. “If the results are confirmed in other trials, this may lead to more options to treat strokes caused by clots.”
The study was presented at the International Stroke Conference presented by the American Stroke Association, a division of the American Heart Association.
Studying stroke outcomes
The researchers described butylphthalide as a cerebroprotective drug that was originally extracted from seeds of Apium graveolens. In China, previous studies have shown that the drug has cerebroprotective effects in animal models of ischemia-reperfusion, they noted.
In this randomized, double-blind, placebo-controlled trial, Dr. Jia and colleagues evaluated whether treatment with butylphthalide could improve 90-day outcomes for adults with acute ischemic stroke who received intravenous recombinant tissue plasminogen activator (tPA), endovascular treatment, or both.
The participants were treated at one of 59 medical centers in China between July 2018 and February 2022. Those who had minimal stroke symptoms on their initial exam, defined as a score of 0-3 on the National Institutes of Health Stroke Scale, or had severe stroke symptoms, defined as having a score of 26 or higher on the NIHSS, were excluded from the study.
Along with an initial revascularization intervention chosen by their physician, participants were randomly selected to receive either butylphthalide or a placebo daily for 90 days. The drug was administered through daily intravenous injections for the first 14 days, after which patients received oral capsules for 76 days.
The research team defined the outcomes as “favorable” if a patient fell into one of the following categories 90 days after the stroke: an initially mild to moderate stroke (NIHSS, 4-7) and no symptoms after treatment, defined as a score of 0 on the Modified Rankin Scale (mRS), which measures disability and dependence; an initially moderate to serious stroke (NIHSS, 8-14) and no residual symptoms or mild symptoms that don’t impair the ability to perform routine activities of daily living without assistance (mRS, 0-1); or an initially serious to severe stroke (NIHSS, 15-25) and no remaining symptoms or a slight disability that impairs some activities but allows one to conduct daily living without assistance (mRS, 0-2).
Secondary outcomes included symptomatic intracranial hemorrhage, recurrent stroke, and mortality.
Among the 1,216 participants, 607 were assigned to the treatment group, and 609 were assigned to the placebo group. The average age was 66 years, and 68% were men.
Overall, participants in the butylphthalide group were 70% more likely to have a favorable 90-day outcome, compared with the placebo group. Favorable outcomes occurred in 344 patients (56.7%) in the butylphthalide group, compared with 268 patients (44%) in the placebo group (odds ratio, 1.70; 95% confidence interval, 1.35-2.14; P < .001).
In addition, butylphthalide improved function equally well for the patients who initially received tPA, those who received endovascular treatment, and those who received both tPA and endovascular treatment.
Secondary events, such as recurrent stroke and intracranial hemorrhage, weren’t significantly different between the butylphthalide and placebo groups.
Ongoing questions
Dr. Jia and colleagues noted the need to understand how butylphthalide works in the brain. Animal studies have suggested several possible mechanisms, but it remains unclear.
“The next step should be investigating the exact mechanisms of butylphthalide in humans,” Dr. Jia said.
Additional research should assess the medication in other populations, the authors noted, particularly because the study involved participants who received initial treatment with tPA, endovascular treatment, or both. The results may not be generalizable to stroke patients who receive other treatments or to populations outside of China.
“While these are interesting results, this is only one relatively small study on a fairly select population in China. Butylphthalide, a medication initially compounded from celery seed, is not ready for use in standard stroke treatment,” said Daniel Lackland, DrPH, professor of neurology and director of the division of translational neurosciences and population studies at the Medical University of South Carolina, Charleston.
Dr. Lackland, who wasn’t involved with the study, is a member of the American Stroke Association’s Stroke Council. Although butylphthalide was originally extracted from seeds, he noted, it’s not what patients would find commercially available.
“The medication used in this study is not the same as celery seed or celery seed extract supplements,” he said. “Stroke survivors should always consult with their neurologist or healthcare professional regarding diet after a stroke.”
The study was funded by the National Key Technology Research and Development Program of the Ministry of Science and Technology of the People’s Republic of China and Shijiazhuang Pharmaceutical Group dl-3-butylphthalide Pharmaceutical. Several authors are employed with Beijing Tiantan Hospital and the Beijing Institute of Brain Disorders. Dr. Lackland reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a new report suggests.
Patients treated with butylphthalide had fewer severe neurologic symptoms and better function 90 days after the stroke, compared with those receiving placebo.
Butylphthalide is approved and available for use in China, where the study was conducted. However, the medication hasn’t been approved for use by the U.S. Food and Drug Administration.
“Patients who received butylphthalide had less severe neurological symptoms and a better living status at 90 days post stroke, compared to those who received the placebo,” said coauthor Baixue Jia, MD, an attending physician in interventional neuroradiology at the Beijing Tiantan Hospital of Capital Medical University and a faculty member at the China National Clinical Research Center for Neurological Diseases in Beijing. “If the results are confirmed in other trials, this may lead to more options to treat strokes caused by clots.”
The study was presented at the International Stroke Conference presented by the American Stroke Association, a division of the American Heart Association.
Studying stroke outcomes
The researchers described butylphthalide as a cerebroprotective drug that was originally extracted from seeds of Apium graveolens. In China, previous studies have shown that the drug has cerebroprotective effects in animal models of ischemia-reperfusion, they noted.
In this randomized, double-blind, placebo-controlled trial, Dr. Jia and colleagues evaluated whether treatment with butylphthalide could improve 90-day outcomes for adults with acute ischemic stroke who received intravenous recombinant tissue plasminogen activator (tPA), endovascular treatment, or both.
The participants were treated at one of 59 medical centers in China between July 2018 and February 2022. Those who had minimal stroke symptoms on their initial exam, defined as a score of 0-3 on the National Institutes of Health Stroke Scale, or had severe stroke symptoms, defined as having a score of 26 or higher on the NIHSS, were excluded from the study.
Along with an initial revascularization intervention chosen by their physician, participants were randomly selected to receive either butylphthalide or a placebo daily for 90 days. The drug was administered through daily intravenous injections for the first 14 days, after which patients received oral capsules for 76 days.
The research team defined the outcomes as “favorable” if a patient fell into one of the following categories 90 days after the stroke: an initially mild to moderate stroke (NIHSS, 4-7) and no symptoms after treatment, defined as a score of 0 on the Modified Rankin Scale (mRS), which measures disability and dependence; an initially moderate to serious stroke (NIHSS, 8-14) and no residual symptoms or mild symptoms that don’t impair the ability to perform routine activities of daily living without assistance (mRS, 0-1); or an initially serious to severe stroke (NIHSS, 15-25) and no remaining symptoms or a slight disability that impairs some activities but allows one to conduct daily living without assistance (mRS, 0-2).
Secondary outcomes included symptomatic intracranial hemorrhage, recurrent stroke, and mortality.
Among the 1,216 participants, 607 were assigned to the treatment group, and 609 were assigned to the placebo group. The average age was 66 years, and 68% were men.
Overall, participants in the butylphthalide group were 70% more likely to have a favorable 90-day outcome, compared with the placebo group. Favorable outcomes occurred in 344 patients (56.7%) in the butylphthalide group, compared with 268 patients (44%) in the placebo group (odds ratio, 1.70; 95% confidence interval, 1.35-2.14; P < .001).
In addition, butylphthalide improved function equally well for the patients who initially received tPA, those who received endovascular treatment, and those who received both tPA and endovascular treatment.
Secondary events, such as recurrent stroke and intracranial hemorrhage, weren’t significantly different between the butylphthalide and placebo groups.
Ongoing questions
Dr. Jia and colleagues noted the need to understand how butylphthalide works in the brain. Animal studies have suggested several possible mechanisms, but it remains unclear.
“The next step should be investigating the exact mechanisms of butylphthalide in humans,” Dr. Jia said.
Additional research should assess the medication in other populations, the authors noted, particularly because the study involved participants who received initial treatment with tPA, endovascular treatment, or both. The results may not be generalizable to stroke patients who receive other treatments or to populations outside of China.
“While these are interesting results, this is only one relatively small study on a fairly select population in China. Butylphthalide, a medication initially compounded from celery seed, is not ready for use in standard stroke treatment,” said Daniel Lackland, DrPH, professor of neurology and director of the division of translational neurosciences and population studies at the Medical University of South Carolina, Charleston.
Dr. Lackland, who wasn’t involved with the study, is a member of the American Stroke Association’s Stroke Council. Although butylphthalide was originally extracted from seeds, he noted, it’s not what patients would find commercially available.
“The medication used in this study is not the same as celery seed or celery seed extract supplements,” he said. “Stroke survivors should always consult with their neurologist or healthcare professional regarding diet after a stroke.”
The study was funded by the National Key Technology Research and Development Program of the Ministry of Science and Technology of the People’s Republic of China and Shijiazhuang Pharmaceutical Group dl-3-butylphthalide Pharmaceutical. Several authors are employed with Beijing Tiantan Hospital and the Beijing Institute of Brain Disorders. Dr. Lackland reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a new report suggests.
Patients treated with butylphthalide had fewer severe neurologic symptoms and better function 90 days after the stroke, compared with those receiving placebo.
Butylphthalide is approved and available for use in China, where the study was conducted. However, the medication hasn’t been approved for use by the U.S. Food and Drug Administration.
“Patients who received butylphthalide had less severe neurological symptoms and a better living status at 90 days post stroke, compared to those who received the placebo,” said coauthor Baixue Jia, MD, an attending physician in interventional neuroradiology at the Beijing Tiantan Hospital of Capital Medical University and a faculty member at the China National Clinical Research Center for Neurological Diseases in Beijing. “If the results are confirmed in other trials, this may lead to more options to treat strokes caused by clots.”
The study was presented at the International Stroke Conference presented by the American Stroke Association, a division of the American Heart Association.
Studying stroke outcomes
The researchers described butylphthalide as a cerebroprotective drug that was originally extracted from seeds of Apium graveolens. In China, previous studies have shown that the drug has cerebroprotective effects in animal models of ischemia-reperfusion, they noted.
In this randomized, double-blind, placebo-controlled trial, Dr. Jia and colleagues evaluated whether treatment with butylphthalide could improve 90-day outcomes for adults with acute ischemic stroke who received intravenous recombinant tissue plasminogen activator (tPA), endovascular treatment, or both.
The participants were treated at one of 59 medical centers in China between July 2018 and February 2022. Those who had minimal stroke symptoms on their initial exam, defined as a score of 0-3 on the National Institutes of Health Stroke Scale, or had severe stroke symptoms, defined as having a score of 26 or higher on the NIHSS, were excluded from the study.
Along with an initial revascularization intervention chosen by their physician, participants were randomly selected to receive either butylphthalide or a placebo daily for 90 days. The drug was administered through daily intravenous injections for the first 14 days, after which patients received oral capsules for 76 days.
The research team defined the outcomes as “favorable” if a patient fell into one of the following categories 90 days after the stroke: an initially mild to moderate stroke (NIHSS, 4-7) and no symptoms after treatment, defined as a score of 0 on the Modified Rankin Scale (mRS), which measures disability and dependence; an initially moderate to serious stroke (NIHSS, 8-14) and no residual symptoms or mild symptoms that don’t impair the ability to perform routine activities of daily living without assistance (mRS, 0-1); or an initially serious to severe stroke (NIHSS, 15-25) and no remaining symptoms or a slight disability that impairs some activities but allows one to conduct daily living without assistance (mRS, 0-2).
Secondary outcomes included symptomatic intracranial hemorrhage, recurrent stroke, and mortality.
Among the 1,216 participants, 607 were assigned to the treatment group, and 609 were assigned to the placebo group. The average age was 66 years, and 68% were men.
Overall, participants in the butylphthalide group were 70% more likely to have a favorable 90-day outcome, compared with the placebo group. Favorable outcomes occurred in 344 patients (56.7%) in the butylphthalide group, compared with 268 patients (44%) in the placebo group (odds ratio, 1.70; 95% confidence interval, 1.35-2.14; P < .001).
In addition, butylphthalide improved function equally well for the patients who initially received tPA, those who received endovascular treatment, and those who received both tPA and endovascular treatment.
Secondary events, such as recurrent stroke and intracranial hemorrhage, weren’t significantly different between the butylphthalide and placebo groups.
Ongoing questions
Dr. Jia and colleagues noted the need to understand how butylphthalide works in the brain. Animal studies have suggested several possible mechanisms, but it remains unclear.
“The next step should be investigating the exact mechanisms of butylphthalide in humans,” Dr. Jia said.
Additional research should assess the medication in other populations, the authors noted, particularly because the study involved participants who received initial treatment with tPA, endovascular treatment, or both. The results may not be generalizable to stroke patients who receive other treatments or to populations outside of China.
“While these are interesting results, this is only one relatively small study on a fairly select population in China. Butylphthalide, a medication initially compounded from celery seed, is not ready for use in standard stroke treatment,” said Daniel Lackland, DrPH, professor of neurology and director of the division of translational neurosciences and population studies at the Medical University of South Carolina, Charleston.
Dr. Lackland, who wasn’t involved with the study, is a member of the American Stroke Association’s Stroke Council. Although butylphthalide was originally extracted from seeds, he noted, it’s not what patients would find commercially available.
“The medication used in this study is not the same as celery seed or celery seed extract supplements,” he said. “Stroke survivors should always consult with their neurologist or healthcare professional regarding diet after a stroke.”
The study was funded by the National Key Technology Research and Development Program of the Ministry of Science and Technology of the People’s Republic of China and Shijiazhuang Pharmaceutical Group dl-3-butylphthalide Pharmaceutical. Several authors are employed with Beijing Tiantan Hospital and the Beijing Institute of Brain Disorders. Dr. Lackland reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ISC 2023
What’s new in brain health?
This transcript has been edited for clarity.
Dear colleagues, I am Christoph Diener from the medical faculty of the University of Duisburg-Essen in Germany.
Treatment of tension-type headache
I would like to start with headache. You are all aware that we have several new studies regarding the prevention of migraine, but very few studies involving nondrug treatments for tension-type headache.
A working group in Göttingen, Germany, conducted a study in people with frequent episodic and chronic tension-type headache. The first of the four randomized groups received traditional Chinese acupuncture for 3 months. The second group received physical therapy and exercise for 1 hour per week for 12 weeks. The third group received a combination of acupuncture and exercise. The last was a control group that received only standard care.
The outcome parameters of tension-type headache were evaluated after 6 months and again after 12 months. Previously, these same researchers published that the intensity but not the frequency of tension-type headache was reduced by active therapy.
In Cephalalgia, they published the outcome for the endpoints of depression, anxiety, and quality of life. Acupuncture, exercise, and the combination of the two improved depression, anxiety, and quality of life. This shows that nonmedical treatment is effective in people with frequent episodic and chronic tension-type headache.
Headache after COVID-19
The next study was published in Headache and discusses headache after COVID-19. In this review of published studies, more than 50% of people with COVID-19 develop headache. It is more frequent in young patients and people with preexisting primary headaches, such as migraine and tension-type headache. Prognosis is usually good, but some patients develop new, daily persistent headache, which is a major problem because treatment is unclear. We desperately need studies investigating how to treat this new, daily persistent headache after COVID-19.
SSRIs during COVID-19 infection
The next study also focuses on COVID-19. We have conflicting results from several studies suggesting that selective serotonin reuptake inhibitors might be effective in people with mild COVID-19 infection. This hypothesis was tested in a study in Brazil and was published in JAMA, The study included 1,288 outpatients with mild COVID-19 who either received 50 mg of fluvoxamine twice daily for 10 days or placebo. There was no benefit of the treatment for any outcome.
Preventing dementia with antihypertensive treatment
The next study was published in the European Heart Journal and addresses the question of whether effective antihypertensive treatment in elderly persons can prevent dementia. This is a meta-analysis of five placebo-controlled trials with more than 28,000 patients. The meta-analysis clearly shows that treating hypertension in elderly patients does prevent dementia. The benefit is higher if the blood pressure is lowered by a larger amount which also stays true for elderly patients. There is no negative impact of lowering blood pressure in this population.
Antiplatelet therapy
The next study was published in Stroke and reexamines whether resumption of antiplatelet therapy should be early or late in people who had an intracerebral hemorrhage while on antiplatelet therapy. In the Taiwanese Health Registry, this was studied in 1,584 patients. The researchers divided participants into groups based on whether antiplatelet therapy was resumed within 30 days or after 30 days. In 1 year, the rate of recurrent intracerebral hemorrhage was 3.2%. There was no difference whether antiplatelet therapy was resumed early or late.
Regular exercise in Parkinson’s disease
The final study is a review of nonmedical therapy. This meta-analysis of 19 randomized trials looked at the benefit of regular exercise in patients with Parkinson’s disease and depression. The analysis clearly showed that rigorous and moderate exercise improved depression in patients with Parkinson’s disease. This is very important because exercise improves not only the symptoms of Parkinson’s disease but also comorbid depression while presenting no serious adverse events or side effects.
Dr. Diener is a professor in the department of neurology at Stroke Center–Headache Center, University Duisburg-Essen, Germany. He disclosed ties with Abbott, Addex Pharma, Alder, Allergan, Almirall, Amgen, Autonomic Technology, AstraZeneca, Bayer Vital, Berlin Chemie, Bristol-Myers Squibb, Boehringer Ingelheim, Chordate, CoAxia, Corimmun, Covidien, Coherex, CoLucid, Daiichi Sankyo, D-Pharm, Electrocore, Fresenius, GlaxoSmithKline, Grunenthal, Janssen-Cilag, Labrys Biologics Lilly, La Roche, Lundbeck, 3M Medica, MSD, Medtronic, Menarini, MindFrame, Minster, Neuroscore, Neurobiological Technologies, Novartis, Novo Nordisk, Johnson & Johnson, Knoll, Paion, Parke-Davis, Pierre Fabre, Pfizer Inc, Schaper and Brummer, Sanofi-Aventis, Schering-Plough, Servier, Solvay, St. Jude, Talecris, Thrombogenics, WebMD Global, Weber and Weber, Wyeth, and Yamanouchi. Dr. Diener has served as editor of Aktuelle Neurologie, Arzneimitteltherapie, Kopfschmerz News, Stroke News, and the Treatment Guidelines of the German Neurological Society; as co-editor of Cephalalgia; and on the editorial board of The Lancet Neurology, Stroke, European Neurology, and Cerebrovascular Disorders. The department of neurology in Essen is supported by the German Research Council, the German Ministry of Education and Research, European Union, National Institutes of Health, Bertelsmann Foundation, and Heinz Nixdorf Foundation. Dr. Diener has no ownership interest and does not own stocks in any pharmaceutical company. A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
Dear colleagues, I am Christoph Diener from the medical faculty of the University of Duisburg-Essen in Germany.
Treatment of tension-type headache
I would like to start with headache. You are all aware that we have several new studies regarding the prevention of migraine, but very few studies involving nondrug treatments for tension-type headache.
A working group in Göttingen, Germany, conducted a study in people with frequent episodic and chronic tension-type headache. The first of the four randomized groups received traditional Chinese acupuncture for 3 months. The second group received physical therapy and exercise for 1 hour per week for 12 weeks. The third group received a combination of acupuncture and exercise. The last was a control group that received only standard care.
The outcome parameters of tension-type headache were evaluated after 6 months and again after 12 months. Previously, these same researchers published that the intensity but not the frequency of tension-type headache was reduced by active therapy.
In Cephalalgia, they published the outcome for the endpoints of depression, anxiety, and quality of life. Acupuncture, exercise, and the combination of the two improved depression, anxiety, and quality of life. This shows that nonmedical treatment is effective in people with frequent episodic and chronic tension-type headache.
Headache after COVID-19
The next study was published in Headache and discusses headache after COVID-19. In this review of published studies, more than 50% of people with COVID-19 develop headache. It is more frequent in young patients and people with preexisting primary headaches, such as migraine and tension-type headache. Prognosis is usually good, but some patients develop new, daily persistent headache, which is a major problem because treatment is unclear. We desperately need studies investigating how to treat this new, daily persistent headache after COVID-19.
SSRIs during COVID-19 infection
The next study also focuses on COVID-19. We have conflicting results from several studies suggesting that selective serotonin reuptake inhibitors might be effective in people with mild COVID-19 infection. This hypothesis was tested in a study in Brazil and was published in JAMA, The study included 1,288 outpatients with mild COVID-19 who either received 50 mg of fluvoxamine twice daily for 10 days or placebo. There was no benefit of the treatment for any outcome.
Preventing dementia with antihypertensive treatment
The next study was published in the European Heart Journal and addresses the question of whether effective antihypertensive treatment in elderly persons can prevent dementia. This is a meta-analysis of five placebo-controlled trials with more than 28,000 patients. The meta-analysis clearly shows that treating hypertension in elderly patients does prevent dementia. The benefit is higher if the blood pressure is lowered by a larger amount which also stays true for elderly patients. There is no negative impact of lowering blood pressure in this population.
Antiplatelet therapy
The next study was published in Stroke and reexamines whether resumption of antiplatelet therapy should be early or late in people who had an intracerebral hemorrhage while on antiplatelet therapy. In the Taiwanese Health Registry, this was studied in 1,584 patients. The researchers divided participants into groups based on whether antiplatelet therapy was resumed within 30 days or after 30 days. In 1 year, the rate of recurrent intracerebral hemorrhage was 3.2%. There was no difference whether antiplatelet therapy was resumed early or late.
Regular exercise in Parkinson’s disease
The final study is a review of nonmedical therapy. This meta-analysis of 19 randomized trials looked at the benefit of regular exercise in patients with Parkinson’s disease and depression. The analysis clearly showed that rigorous and moderate exercise improved depression in patients with Parkinson’s disease. This is very important because exercise improves not only the symptoms of Parkinson’s disease but also comorbid depression while presenting no serious adverse events or side effects.
Dr. Diener is a professor in the department of neurology at Stroke Center–Headache Center, University Duisburg-Essen, Germany. He disclosed ties with Abbott, Addex Pharma, Alder, Allergan, Almirall, Amgen, Autonomic Technology, AstraZeneca, Bayer Vital, Berlin Chemie, Bristol-Myers Squibb, Boehringer Ingelheim, Chordate, CoAxia, Corimmun, Covidien, Coherex, CoLucid, Daiichi Sankyo, D-Pharm, Electrocore, Fresenius, GlaxoSmithKline, Grunenthal, Janssen-Cilag, Labrys Biologics Lilly, La Roche, Lundbeck, 3M Medica, MSD, Medtronic, Menarini, MindFrame, Minster, Neuroscore, Neurobiological Technologies, Novartis, Novo Nordisk, Johnson & Johnson, Knoll, Paion, Parke-Davis, Pierre Fabre, Pfizer Inc, Schaper and Brummer, Sanofi-Aventis, Schering-Plough, Servier, Solvay, St. Jude, Talecris, Thrombogenics, WebMD Global, Weber and Weber, Wyeth, and Yamanouchi. Dr. Diener has served as editor of Aktuelle Neurologie, Arzneimitteltherapie, Kopfschmerz News, Stroke News, and the Treatment Guidelines of the German Neurological Society; as co-editor of Cephalalgia; and on the editorial board of The Lancet Neurology, Stroke, European Neurology, and Cerebrovascular Disorders. The department of neurology in Essen is supported by the German Research Council, the German Ministry of Education and Research, European Union, National Institutes of Health, Bertelsmann Foundation, and Heinz Nixdorf Foundation. Dr. Diener has no ownership interest and does not own stocks in any pharmaceutical company. A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
Dear colleagues, I am Christoph Diener from the medical faculty of the University of Duisburg-Essen in Germany.
Treatment of tension-type headache
I would like to start with headache. You are all aware that we have several new studies regarding the prevention of migraine, but very few studies involving nondrug treatments for tension-type headache.
A working group in Göttingen, Germany, conducted a study in people with frequent episodic and chronic tension-type headache. The first of the four randomized groups received traditional Chinese acupuncture for 3 months. The second group received physical therapy and exercise for 1 hour per week for 12 weeks. The third group received a combination of acupuncture and exercise. The last was a control group that received only standard care.
The outcome parameters of tension-type headache were evaluated after 6 months and again after 12 months. Previously, these same researchers published that the intensity but not the frequency of tension-type headache was reduced by active therapy.
In Cephalalgia, they published the outcome for the endpoints of depression, anxiety, and quality of life. Acupuncture, exercise, and the combination of the two improved depression, anxiety, and quality of life. This shows that nonmedical treatment is effective in people with frequent episodic and chronic tension-type headache.
Headache after COVID-19
The next study was published in Headache and discusses headache after COVID-19. In this review of published studies, more than 50% of people with COVID-19 develop headache. It is more frequent in young patients and people with preexisting primary headaches, such as migraine and tension-type headache. Prognosis is usually good, but some patients develop new, daily persistent headache, which is a major problem because treatment is unclear. We desperately need studies investigating how to treat this new, daily persistent headache after COVID-19.
SSRIs during COVID-19 infection
The next study also focuses on COVID-19. We have conflicting results from several studies suggesting that selective serotonin reuptake inhibitors might be effective in people with mild COVID-19 infection. This hypothesis was tested in a study in Brazil and was published in JAMA, The study included 1,288 outpatients with mild COVID-19 who either received 50 mg of fluvoxamine twice daily for 10 days or placebo. There was no benefit of the treatment for any outcome.
Preventing dementia with antihypertensive treatment
The next study was published in the European Heart Journal and addresses the question of whether effective antihypertensive treatment in elderly persons can prevent dementia. This is a meta-analysis of five placebo-controlled trials with more than 28,000 patients. The meta-analysis clearly shows that treating hypertension in elderly patients does prevent dementia. The benefit is higher if the blood pressure is lowered by a larger amount which also stays true for elderly patients. There is no negative impact of lowering blood pressure in this population.
Antiplatelet therapy
The next study was published in Stroke and reexamines whether resumption of antiplatelet therapy should be early or late in people who had an intracerebral hemorrhage while on antiplatelet therapy. In the Taiwanese Health Registry, this was studied in 1,584 patients. The researchers divided participants into groups based on whether antiplatelet therapy was resumed within 30 days or after 30 days. In 1 year, the rate of recurrent intracerebral hemorrhage was 3.2%. There was no difference whether antiplatelet therapy was resumed early or late.
Regular exercise in Parkinson’s disease
The final study is a review of nonmedical therapy. This meta-analysis of 19 randomized trials looked at the benefit of regular exercise in patients with Parkinson’s disease and depression. The analysis clearly showed that rigorous and moderate exercise improved depression in patients with Parkinson’s disease. This is very important because exercise improves not only the symptoms of Parkinson’s disease but also comorbid depression while presenting no serious adverse events or side effects.
Dr. Diener is a professor in the department of neurology at Stroke Center–Headache Center, University Duisburg-Essen, Germany. He disclosed ties with Abbott, Addex Pharma, Alder, Allergan, Almirall, Amgen, Autonomic Technology, AstraZeneca, Bayer Vital, Berlin Chemie, Bristol-Myers Squibb, Boehringer Ingelheim, Chordate, CoAxia, Corimmun, Covidien, Coherex, CoLucid, Daiichi Sankyo, D-Pharm, Electrocore, Fresenius, GlaxoSmithKline, Grunenthal, Janssen-Cilag, Labrys Biologics Lilly, La Roche, Lundbeck, 3M Medica, MSD, Medtronic, Menarini, MindFrame, Minster, Neuroscore, Neurobiological Technologies, Novartis, Novo Nordisk, Johnson & Johnson, Knoll, Paion, Parke-Davis, Pierre Fabre, Pfizer Inc, Schaper and Brummer, Sanofi-Aventis, Schering-Plough, Servier, Solvay, St. Jude, Talecris, Thrombogenics, WebMD Global, Weber and Weber, Wyeth, and Yamanouchi. Dr. Diener has served as editor of Aktuelle Neurologie, Arzneimitteltherapie, Kopfschmerz News, Stroke News, and the Treatment Guidelines of the German Neurological Society; as co-editor of Cephalalgia; and on the editorial board of The Lancet Neurology, Stroke, European Neurology, and Cerebrovascular Disorders. The department of neurology in Essen is supported by the German Research Council, the German Ministry of Education and Research, European Union, National Institutes of Health, Bertelsmann Foundation, and Heinz Nixdorf Foundation. Dr. Diener has no ownership interest and does not own stocks in any pharmaceutical company. A version of this article originally appeared on Medscape.com.
Drug combo promising in vascular cognitive impairment: LACI-2 trial results
and is seen as a new therapeutic approach for patients with cerebral small-vessel disease. The drugs – isosorbide mononitrate and cilostazol – stabilize endothelial function, which is a new therapeutic target for patients with small-vessel disease stroke.
The phase 2 LACI-2 study, evaluating these drugs individually and in combination in patients with lacunar stroke, showed promising trends toward reductions in recurrent stroke, cognitive impairment, and dependency, some of which became significant when the drugs were given together. There was also some suggestion of positive impacts on mood and quality of life.
“Isosorbide mononitrate was associated with a reduction in recurrent stroke, a tendency toward a reduction in dependency and a reduction in cognitive impairment, and cilostazol also seemed to reduce dependency,” study investigator Joanna M. Wardlaw, MD, professor of applied neuroimaging at Edinburgh University, reported.
“When used together, they seemed to have more benefits than either drug on its own. So this is good preliminary evidence that the drugs are working together in a positive way,” she said. But she cautioned that these potential benefits will need to be confirmed in a larger phase 3 trial.
The LACI-2 study was presented at the International Stroke Conference by Dr. Wardlaw and coinvestigator Philip Bath, DSc, professor of medicine at the University of Nottingham (England).
They both highlighted the effect seen on cognitive impairment at the conference presented by the American Stroke Association, a division of the American Heart Association.
“We saw a significant reduction in the number of patients with cognitive impairment with the two drugs together in this phase 2 study,” Dr. Wardlaw said. “This is very encouraging since no study has previously found any medications that positively affect cognitive impairment in small-vessel disease strokes. We cautiously hope that these medications may have wider implications for other types of small-vessel disease as well.”
Dr. Bath added: “The results on cognitive impairment are particularly important. Many patients rate cognitive impairment as one of the most dreaded outcomes of a stroke even if they also have quite significant physical disability. People simply don’t want to lose their memory and thinking ability.”
“The results of LACI-2 also raise interesting questions about whether these drugs would be beneficial for other types of small-vessel disease which do not present as stroke, but maybe may manifest as headaches or memory impairment,” he noted.
‘Very intriguing results’
Outside experts were enthusiastic about these preliminary results. In an ISC highlights presentation, program chair Tudor Jovin, MD, Cooper Neurological Institute, Cherry Hill, N.J., said: “It is refreshing to finally see some positive signals in studies in small-vessel stroke. This is an area where we haven’t had answers for a long time.”
He described the reduction in cognitive impairment seen in the study as “very intriguing and very important.”
“I think we have underestimated the burden that cognitive impairment has in stroke, and the burden in general in society of vascular cognitive impairment. This is a very promising approach that definitely deserves to be investigated more thoroughly in a larger trial.”
Commenting on the study findings, Mitchell Elkind, MD, professor of neurology and epidemiology at Columbia University Irving Medical Center, New York, said this study “provides evidence that points us in at least two important directions.”
“First, it suggests that endothelial dysfunction, or problems with the lining of the blood vessels, may be an important contributor to small-vessel disease and the cognitive decline that often accompanies it. This is a new mechanism of action and different from blood clotting, blood pressure, and other conventional targets of treatment,” Dr. Elkind said.
“Second, and more generally, it suggests that stroke trials, particularly in the subtype of small-vessel disease, can and should explore not only the incidence of recurrent acute events but also the steady decline that occurs after stroke. Poststroke cognitive decline is a relatively new area of stroke research.”
Dr. Wardlaw noted that lacunar stroke is a common type of ischemic stroke, but it has been rather neglected in terms of research. It is assumed to be caused by atherosclerosis of the small vessel but there is now mounting evidence suggesting that it is a result of problems in the endothelium of the small vessels.
“We looked for potential available drugs that targeted endothelial dysfunction. Both the drugs we tested are already widely used – isosorbide mononitrate for the treatment of coronary artery disease and angina, and cilostazol, mainly in Asia, for stroke prevention,” she said.
LACI-2 was primarily a feasibility study looking at whether it was possible to recruit enough patients who had had a lacunar stroke and would take the drugs, individually or in combination, for up to a year. Outcomes were investigated on an exploratory basis. The study enrolled 363 patients who had experienced lacunar stroke from 26 stroke centers throughout the United Kingdom. They were randomly assigned to one of four treatment groups for 1 year:
- 40-60 mg/day of oral isosorbide mononitrate alone.
- 200 mg/day of oral cilostazol alone.
- Both medications.
- Neither medication.
Patients completed phone surveys at 6 and 12 months to assess health status, including recurrent stroke, myocardial infarction, cognitive tests, symptoms, quality of life surveys, and they also had brain imaging at 12 months.
Results showed 98% of patients were still taking their study medication at 1 year, and the drugs appeared to be safe on top of usual care with few deaths or hemorrhages in the study.
The composite outcome including recurrent stroke, MI, cognitive impairment, dependency (modified Rankin score > 2) and death was reduced by 20% in the isosorbide mononitrate–alone group (adjusted hazard ratio, 0.80; 95% confidence interval, 0.59-1.09).
The composite endpoint was reduced by 23% in the cilostazol group (aHR, 0.77; 95% CI, 0.57-1.05) and by 42% in the combination group (aHR, 0.58, 95% CI, 0.36-0.92) compared with those taking neither drug.
Isosorbide mononitrate alone showed trends toward a reduction in recurrent stroke, cognitive impairment, and dependency, whereas cilostazol alone reduced dependency with a trend toward a reduction in cognitive impairment. When used together, the drugs showed large reductions in cognitive impairment (aHR, 0.44; 95% CI, 0.19-0.99) and dependency (aHR ,0.14; 95% CI, 0.03-0.59).
During the highlights session, Dr. Jovin commented: “It is obvious that the investigators have put a lot of thought into the design of this trial. Presumably because of the composite score they were able to increase the power. We are used to trials which require thousands of patients, but here we are able to see significant results, although exploratory, with just a few hundred patients.”
Dr. Bath stressed that this was only a phase 2 study. “We now need to see if we can confirm these results in a larger phase 3 study.” That study, LACI-3, is planned to start later this year. He also suggested that it would be interesting to investigate whether these drugs would work in other types of ischemic stroke such as those caused by large-artery disease or cardioembolic strokes, as well as other forms of small-vessel disease such as patients with vascular cognitive impairment.
“There are many areas to investigate in future. It might be that in a few years’ time these drugs may be standard of care across many different forms of small-vessel disease,” he said.
Dr. Wardlaw noted that lacunar strokes are generally quite mild strokes, which could be one of the reasons why they have not been the target of much research to date. But Dr. Bath added: “While they may be labeled as a mild stroke on the NIHSS scale, patients can still be quite badly affected. About half of patients with a lacunar stroke develop cognitive impairment and eventually dementia – that is certainly not mild.”
The study was funded primarily by the British Heart Foundation, with support from the UK Alzheimer’s Society, the UK Dementia Research Institute, the Stroke Association, the Fondation Leducq, NHS Research Scotland, and the UK National Institutes of Health Research Clinical Research Networks. Dr. Bath is an adviser to CoMind, DiaMedica, Phagenesis, and Roche. Dr. Wardlaw reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
and is seen as a new therapeutic approach for patients with cerebral small-vessel disease. The drugs – isosorbide mononitrate and cilostazol – stabilize endothelial function, which is a new therapeutic target for patients with small-vessel disease stroke.
The phase 2 LACI-2 study, evaluating these drugs individually and in combination in patients with lacunar stroke, showed promising trends toward reductions in recurrent stroke, cognitive impairment, and dependency, some of which became significant when the drugs were given together. There was also some suggestion of positive impacts on mood and quality of life.
“Isosorbide mononitrate was associated with a reduction in recurrent stroke, a tendency toward a reduction in dependency and a reduction in cognitive impairment, and cilostazol also seemed to reduce dependency,” study investigator Joanna M. Wardlaw, MD, professor of applied neuroimaging at Edinburgh University, reported.
“When used together, they seemed to have more benefits than either drug on its own. So this is good preliminary evidence that the drugs are working together in a positive way,” she said. But she cautioned that these potential benefits will need to be confirmed in a larger phase 3 trial.
The LACI-2 study was presented at the International Stroke Conference by Dr. Wardlaw and coinvestigator Philip Bath, DSc, professor of medicine at the University of Nottingham (England).
They both highlighted the effect seen on cognitive impairment at the conference presented by the American Stroke Association, a division of the American Heart Association.
“We saw a significant reduction in the number of patients with cognitive impairment with the two drugs together in this phase 2 study,” Dr. Wardlaw said. “This is very encouraging since no study has previously found any medications that positively affect cognitive impairment in small-vessel disease strokes. We cautiously hope that these medications may have wider implications for other types of small-vessel disease as well.”
Dr. Bath added: “The results on cognitive impairment are particularly important. Many patients rate cognitive impairment as one of the most dreaded outcomes of a stroke even if they also have quite significant physical disability. People simply don’t want to lose their memory and thinking ability.”
“The results of LACI-2 also raise interesting questions about whether these drugs would be beneficial for other types of small-vessel disease which do not present as stroke, but maybe may manifest as headaches or memory impairment,” he noted.
‘Very intriguing results’
Outside experts were enthusiastic about these preliminary results. In an ISC highlights presentation, program chair Tudor Jovin, MD, Cooper Neurological Institute, Cherry Hill, N.J., said: “It is refreshing to finally see some positive signals in studies in small-vessel stroke. This is an area where we haven’t had answers for a long time.”
He described the reduction in cognitive impairment seen in the study as “very intriguing and very important.”
“I think we have underestimated the burden that cognitive impairment has in stroke, and the burden in general in society of vascular cognitive impairment. This is a very promising approach that definitely deserves to be investigated more thoroughly in a larger trial.”
Commenting on the study findings, Mitchell Elkind, MD, professor of neurology and epidemiology at Columbia University Irving Medical Center, New York, said this study “provides evidence that points us in at least two important directions.”
“First, it suggests that endothelial dysfunction, or problems with the lining of the blood vessels, may be an important contributor to small-vessel disease and the cognitive decline that often accompanies it. This is a new mechanism of action and different from blood clotting, blood pressure, and other conventional targets of treatment,” Dr. Elkind said.
“Second, and more generally, it suggests that stroke trials, particularly in the subtype of small-vessel disease, can and should explore not only the incidence of recurrent acute events but also the steady decline that occurs after stroke. Poststroke cognitive decline is a relatively new area of stroke research.”
Dr. Wardlaw noted that lacunar stroke is a common type of ischemic stroke, but it has been rather neglected in terms of research. It is assumed to be caused by atherosclerosis of the small vessel but there is now mounting evidence suggesting that it is a result of problems in the endothelium of the small vessels.
“We looked for potential available drugs that targeted endothelial dysfunction. Both the drugs we tested are already widely used – isosorbide mononitrate for the treatment of coronary artery disease and angina, and cilostazol, mainly in Asia, for stroke prevention,” she said.
LACI-2 was primarily a feasibility study looking at whether it was possible to recruit enough patients who had had a lacunar stroke and would take the drugs, individually or in combination, for up to a year. Outcomes were investigated on an exploratory basis. The study enrolled 363 patients who had experienced lacunar stroke from 26 stroke centers throughout the United Kingdom. They were randomly assigned to one of four treatment groups for 1 year:
- 40-60 mg/day of oral isosorbide mononitrate alone.
- 200 mg/day of oral cilostazol alone.
- Both medications.
- Neither medication.
Patients completed phone surveys at 6 and 12 months to assess health status, including recurrent stroke, myocardial infarction, cognitive tests, symptoms, quality of life surveys, and they also had brain imaging at 12 months.
Results showed 98% of patients were still taking their study medication at 1 year, and the drugs appeared to be safe on top of usual care with few deaths or hemorrhages in the study.
The composite outcome including recurrent stroke, MI, cognitive impairment, dependency (modified Rankin score > 2) and death was reduced by 20% in the isosorbide mononitrate–alone group (adjusted hazard ratio, 0.80; 95% confidence interval, 0.59-1.09).
The composite endpoint was reduced by 23% in the cilostazol group (aHR, 0.77; 95% CI, 0.57-1.05) and by 42% in the combination group (aHR, 0.58, 95% CI, 0.36-0.92) compared with those taking neither drug.
Isosorbide mononitrate alone showed trends toward a reduction in recurrent stroke, cognitive impairment, and dependency, whereas cilostazol alone reduced dependency with a trend toward a reduction in cognitive impairment. When used together, the drugs showed large reductions in cognitive impairment (aHR, 0.44; 95% CI, 0.19-0.99) and dependency (aHR ,0.14; 95% CI, 0.03-0.59).
During the highlights session, Dr. Jovin commented: “It is obvious that the investigators have put a lot of thought into the design of this trial. Presumably because of the composite score they were able to increase the power. We are used to trials which require thousands of patients, but here we are able to see significant results, although exploratory, with just a few hundred patients.”
Dr. Bath stressed that this was only a phase 2 study. “We now need to see if we can confirm these results in a larger phase 3 study.” That study, LACI-3, is planned to start later this year. He also suggested that it would be interesting to investigate whether these drugs would work in other types of ischemic stroke such as those caused by large-artery disease or cardioembolic strokes, as well as other forms of small-vessel disease such as patients with vascular cognitive impairment.
“There are many areas to investigate in future. It might be that in a few years’ time these drugs may be standard of care across many different forms of small-vessel disease,” he said.
Dr. Wardlaw noted that lacunar strokes are generally quite mild strokes, which could be one of the reasons why they have not been the target of much research to date. But Dr. Bath added: “While they may be labeled as a mild stroke on the NIHSS scale, patients can still be quite badly affected. About half of patients with a lacunar stroke develop cognitive impairment and eventually dementia – that is certainly not mild.”
The study was funded primarily by the British Heart Foundation, with support from the UK Alzheimer’s Society, the UK Dementia Research Institute, the Stroke Association, the Fondation Leducq, NHS Research Scotland, and the UK National Institutes of Health Research Clinical Research Networks. Dr. Bath is an adviser to CoMind, DiaMedica, Phagenesis, and Roche. Dr. Wardlaw reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
and is seen as a new therapeutic approach for patients with cerebral small-vessel disease. The drugs – isosorbide mononitrate and cilostazol – stabilize endothelial function, which is a new therapeutic target for patients with small-vessel disease stroke.
The phase 2 LACI-2 study, evaluating these drugs individually and in combination in patients with lacunar stroke, showed promising trends toward reductions in recurrent stroke, cognitive impairment, and dependency, some of which became significant when the drugs were given together. There was also some suggestion of positive impacts on mood and quality of life.
“Isosorbide mononitrate was associated with a reduction in recurrent stroke, a tendency toward a reduction in dependency and a reduction in cognitive impairment, and cilostazol also seemed to reduce dependency,” study investigator Joanna M. Wardlaw, MD, professor of applied neuroimaging at Edinburgh University, reported.
“When used together, they seemed to have more benefits than either drug on its own. So this is good preliminary evidence that the drugs are working together in a positive way,” she said. But she cautioned that these potential benefits will need to be confirmed in a larger phase 3 trial.
The LACI-2 study was presented at the International Stroke Conference by Dr. Wardlaw and coinvestigator Philip Bath, DSc, professor of medicine at the University of Nottingham (England).
They both highlighted the effect seen on cognitive impairment at the conference presented by the American Stroke Association, a division of the American Heart Association.
“We saw a significant reduction in the number of patients with cognitive impairment with the two drugs together in this phase 2 study,” Dr. Wardlaw said. “This is very encouraging since no study has previously found any medications that positively affect cognitive impairment in small-vessel disease strokes. We cautiously hope that these medications may have wider implications for other types of small-vessel disease as well.”
Dr. Bath added: “The results on cognitive impairment are particularly important. Many patients rate cognitive impairment as one of the most dreaded outcomes of a stroke even if they also have quite significant physical disability. People simply don’t want to lose their memory and thinking ability.”
“The results of LACI-2 also raise interesting questions about whether these drugs would be beneficial for other types of small-vessel disease which do not present as stroke, but maybe may manifest as headaches or memory impairment,” he noted.
‘Very intriguing results’
Outside experts were enthusiastic about these preliminary results. In an ISC highlights presentation, program chair Tudor Jovin, MD, Cooper Neurological Institute, Cherry Hill, N.J., said: “It is refreshing to finally see some positive signals in studies in small-vessel stroke. This is an area where we haven’t had answers for a long time.”
He described the reduction in cognitive impairment seen in the study as “very intriguing and very important.”
“I think we have underestimated the burden that cognitive impairment has in stroke, and the burden in general in society of vascular cognitive impairment. This is a very promising approach that definitely deserves to be investigated more thoroughly in a larger trial.”
Commenting on the study findings, Mitchell Elkind, MD, professor of neurology and epidemiology at Columbia University Irving Medical Center, New York, said this study “provides evidence that points us in at least two important directions.”
“First, it suggests that endothelial dysfunction, or problems with the lining of the blood vessels, may be an important contributor to small-vessel disease and the cognitive decline that often accompanies it. This is a new mechanism of action and different from blood clotting, blood pressure, and other conventional targets of treatment,” Dr. Elkind said.
“Second, and more generally, it suggests that stroke trials, particularly in the subtype of small-vessel disease, can and should explore not only the incidence of recurrent acute events but also the steady decline that occurs after stroke. Poststroke cognitive decline is a relatively new area of stroke research.”
Dr. Wardlaw noted that lacunar stroke is a common type of ischemic stroke, but it has been rather neglected in terms of research. It is assumed to be caused by atherosclerosis of the small vessel but there is now mounting evidence suggesting that it is a result of problems in the endothelium of the small vessels.
“We looked for potential available drugs that targeted endothelial dysfunction. Both the drugs we tested are already widely used – isosorbide mononitrate for the treatment of coronary artery disease and angina, and cilostazol, mainly in Asia, for stroke prevention,” she said.
LACI-2 was primarily a feasibility study looking at whether it was possible to recruit enough patients who had had a lacunar stroke and would take the drugs, individually or in combination, for up to a year. Outcomes were investigated on an exploratory basis. The study enrolled 363 patients who had experienced lacunar stroke from 26 stroke centers throughout the United Kingdom. They were randomly assigned to one of four treatment groups for 1 year:
- 40-60 mg/day of oral isosorbide mononitrate alone.
- 200 mg/day of oral cilostazol alone.
- Both medications.
- Neither medication.
Patients completed phone surveys at 6 and 12 months to assess health status, including recurrent stroke, myocardial infarction, cognitive tests, symptoms, quality of life surveys, and they also had brain imaging at 12 months.
Results showed 98% of patients were still taking their study medication at 1 year, and the drugs appeared to be safe on top of usual care with few deaths or hemorrhages in the study.
The composite outcome including recurrent stroke, MI, cognitive impairment, dependency (modified Rankin score > 2) and death was reduced by 20% in the isosorbide mononitrate–alone group (adjusted hazard ratio, 0.80; 95% confidence interval, 0.59-1.09).
The composite endpoint was reduced by 23% in the cilostazol group (aHR, 0.77; 95% CI, 0.57-1.05) and by 42% in the combination group (aHR, 0.58, 95% CI, 0.36-0.92) compared with those taking neither drug.
Isosorbide mononitrate alone showed trends toward a reduction in recurrent stroke, cognitive impairment, and dependency, whereas cilostazol alone reduced dependency with a trend toward a reduction in cognitive impairment. When used together, the drugs showed large reductions in cognitive impairment (aHR, 0.44; 95% CI, 0.19-0.99) and dependency (aHR ,0.14; 95% CI, 0.03-0.59).
During the highlights session, Dr. Jovin commented: “It is obvious that the investigators have put a lot of thought into the design of this trial. Presumably because of the composite score they were able to increase the power. We are used to trials which require thousands of patients, but here we are able to see significant results, although exploratory, with just a few hundred patients.”
Dr. Bath stressed that this was only a phase 2 study. “We now need to see if we can confirm these results in a larger phase 3 study.” That study, LACI-3, is planned to start later this year. He also suggested that it would be interesting to investigate whether these drugs would work in other types of ischemic stroke such as those caused by large-artery disease or cardioembolic strokes, as well as other forms of small-vessel disease such as patients with vascular cognitive impairment.
“There are many areas to investigate in future. It might be that in a few years’ time these drugs may be standard of care across many different forms of small-vessel disease,” he said.
Dr. Wardlaw noted that lacunar strokes are generally quite mild strokes, which could be one of the reasons why they have not been the target of much research to date. But Dr. Bath added: “While they may be labeled as a mild stroke on the NIHSS scale, patients can still be quite badly affected. About half of patients with a lacunar stroke develop cognitive impairment and eventually dementia – that is certainly not mild.”
The study was funded primarily by the British Heart Foundation, with support from the UK Alzheimer’s Society, the UK Dementia Research Institute, the Stroke Association, the Fondation Leducq, NHS Research Scotland, and the UK National Institutes of Health Research Clinical Research Networks. Dr. Bath is an adviser to CoMind, DiaMedica, Phagenesis, and Roche. Dr. Wardlaw reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ISC 2023
Tenecteplase noninferior to alteplase for ischemic stroke: TRACE-2
, a new study has found. “This was a pivotal trial in establishing the safety and efficacy of tenecteplase as an alternative to alteplase in the thrombolytic treatment of acute ischemic stroke within 4.5 hours in Asian patients,” said study author Shuya Li, MD, associate chief physician, department of neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing.
The findings in this all-Chinese population should have an impact on the use of tenecteplase going forward, said Dr. Li. “The results provide further evidence to support a worldwide switch to tenecteplase as the preferred thrombolytic for acute ischemic stroke.”
The findings were presented at the 2023 International Stroke Conference presented by the American Stroke Association, a division of the American Heart Association.
Single bolus
Use of alteplase (tissue plasminogen activator [tPA]) has for years been the main approach to thrombolytic reperfusion therapy for patients with acute stroke, but tenecteplase has recently emerged as a potential successor.
Tenecteplase is a tPA produced by recombinant DNA technology. It has a relatively long half-life and can be delivered in a single bolus instead of requiring an hour-long infusion, as is the case with alteplase.
The phase 3 noninferiority Tenecteplase Reperfusion Therapy in Acute ischemic Cerebrovascular Events (TRACE-2) trial – the first of its kind in an Asian population – included 1,430 adult ischemic stroke patients at 53 Chinese centers. Patients had to have a National Institutes of Health Stroke Scale (NIHSS) score of 5-25 and either not be eligible for or have refused endovascular therapy.
The mean age of study participants was about 66 years, and the percentage of women was about 31%. The mean baseline NIHSS score was 7 in both groups, and the symptom-onset-to-needle time was similar at 180 minutes for the tenecteplase group and 178.5 minutes for the alteplase group.
Researchers randomly assigned patients to receive tenecteplase or alteplase within 4.5 hours of symptom onset.
Those in the tenecteplase group received 0.25 mg/kg of the drug in a single IV bolus (maximum dose, 25 mg). Control group members who were treated with alteplase were given the drug as a 10% bolus, with the remainder given as a 1-hour infusion (0.9 mg/kg with a maximum dose of 90 mg).
Showed noninferiority
The primary efficacy outcome was a modified Rankins scale (mRS) score of 0-1 at 90 days, which is considered excellent function. About 62% of tenecteplase patients and 58% of alteplase patients attained this outcome (risk ratio, 1.09; 95% confidence interval, 1.00-1.18).
The P value was .001 for noninferiority and .06 for superiority, but Dr. Li explained that these values may change when considering the site effect.
There were no statistically significant differences between the two drugs on secondary outcomes of favorable function. For example, 73% of tenecteplase patients and 72% of alteplase patients had an mRS score of 0-2 at 3 months, and 50% in the tenecteplase and 49% in the alteplase group improved by 4 or more points on the NIHSS, or had a score of 1 or less, at 24 hours.
The groups also had comparable scores on the European quality-of-life visual analogue scale and on the Barthel index, which measures functional independence related to personal care and mobility.
Tenecteplase also turned out to be as safe at alteplase. About 2% in both groups had symptomatic intracranial hemorrhage within 36 hours, and both groups had that same percentage for such hemorrhages within 90 days. As well, the groups had a similar rate of any intracranial hemorrhage within 90 days (6% and 7%).
The mortality rate was 7% in the tenecteplase group, compared with 5% in the alteplase group.
Adverse events (AEs) occurred in 86% and 87%, and serious AEs in 16% and 15%, of the tenecteplase and alteplase groups, respectively, again with no statistically significant differences.
The research team aims to test the effectiveness of tenecteplase in other stroke patients, including those with minor strokes, those receiving thrombolysis in a later window, and those receiving endovascular therapy, said Dr. Li.
Strong evidence
Commenting on the study findings, Larry B. Goldstein, MD, professor and chair of neurology, University of Kentucky, Lexington, said it is important to determine the efficacy of tenecteplase among Asians, as they represent “an entirely different population” with unique concerns, such as bleeding complications from anticoagulants.
He noted an advantage of tenecteplase is ease of administration. “You don’t have to go through the loading dose and then the 1-hour infusion,” which poses an “additional hassle” when transferring patients between institutions, he said.
However, he noted that a possible “downside” to having both drugs available in the emergency department is “using the wrong drug at the wrong dose” because of their similar sounding names.
Also commenting on the study, Tudor G. Jovin, MD, professor and chair, department of neurology, Rowan University, Camden, N.J., said he welcomes another trial that confirms that these two drugs are biologically similar.
“I’m very glad this trial was done because it adds another very strong piece of evidence of equivalency.”
But the two drugs are not the same in some important respects, said Dr. Jovin, whose center switched to using tenecteplase almost 3 years ago. That switch has resulted in cutting 17 minutes from the door-to-needle time “which is quite significant,” he said.
“There’s no question that once we used tenecteplase in lieu of tPA, it’s been just so much easier to administer and affects the interhospital transfer protocols, because you’re not transferring the patient with a critical care IV. It’s a win-win situation for everyone.”
The study received funding from the National Science and Technology Major Project, the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences, the National Natural Science Foundation of China, and the China Shijiazhuang Pharmaceutical Company Recomgen Pharmaceutical (Guangzhou). Dr. Li, Dr. Goldstein, and Dr. Jovin report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a new study has found. “This was a pivotal trial in establishing the safety and efficacy of tenecteplase as an alternative to alteplase in the thrombolytic treatment of acute ischemic stroke within 4.5 hours in Asian patients,” said study author Shuya Li, MD, associate chief physician, department of neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing.
The findings in this all-Chinese population should have an impact on the use of tenecteplase going forward, said Dr. Li. “The results provide further evidence to support a worldwide switch to tenecteplase as the preferred thrombolytic for acute ischemic stroke.”
The findings were presented at the 2023 International Stroke Conference presented by the American Stroke Association, a division of the American Heart Association.
Single bolus
Use of alteplase (tissue plasminogen activator [tPA]) has for years been the main approach to thrombolytic reperfusion therapy for patients with acute stroke, but tenecteplase has recently emerged as a potential successor.
Tenecteplase is a tPA produced by recombinant DNA technology. It has a relatively long half-life and can be delivered in a single bolus instead of requiring an hour-long infusion, as is the case with alteplase.
The phase 3 noninferiority Tenecteplase Reperfusion Therapy in Acute ischemic Cerebrovascular Events (TRACE-2) trial – the first of its kind in an Asian population – included 1,430 adult ischemic stroke patients at 53 Chinese centers. Patients had to have a National Institutes of Health Stroke Scale (NIHSS) score of 5-25 and either not be eligible for or have refused endovascular therapy.
The mean age of study participants was about 66 years, and the percentage of women was about 31%. The mean baseline NIHSS score was 7 in both groups, and the symptom-onset-to-needle time was similar at 180 minutes for the tenecteplase group and 178.5 minutes for the alteplase group.
Researchers randomly assigned patients to receive tenecteplase or alteplase within 4.5 hours of symptom onset.
Those in the tenecteplase group received 0.25 mg/kg of the drug in a single IV bolus (maximum dose, 25 mg). Control group members who were treated with alteplase were given the drug as a 10% bolus, with the remainder given as a 1-hour infusion (0.9 mg/kg with a maximum dose of 90 mg).
Showed noninferiority
The primary efficacy outcome was a modified Rankins scale (mRS) score of 0-1 at 90 days, which is considered excellent function. About 62% of tenecteplase patients and 58% of alteplase patients attained this outcome (risk ratio, 1.09; 95% confidence interval, 1.00-1.18).
The P value was .001 for noninferiority and .06 for superiority, but Dr. Li explained that these values may change when considering the site effect.
There were no statistically significant differences between the two drugs on secondary outcomes of favorable function. For example, 73% of tenecteplase patients and 72% of alteplase patients had an mRS score of 0-2 at 3 months, and 50% in the tenecteplase and 49% in the alteplase group improved by 4 or more points on the NIHSS, or had a score of 1 or less, at 24 hours.
The groups also had comparable scores on the European quality-of-life visual analogue scale and on the Barthel index, which measures functional independence related to personal care and mobility.
Tenecteplase also turned out to be as safe at alteplase. About 2% in both groups had symptomatic intracranial hemorrhage within 36 hours, and both groups had that same percentage for such hemorrhages within 90 days. As well, the groups had a similar rate of any intracranial hemorrhage within 90 days (6% and 7%).
The mortality rate was 7% in the tenecteplase group, compared with 5% in the alteplase group.
Adverse events (AEs) occurred in 86% and 87%, and serious AEs in 16% and 15%, of the tenecteplase and alteplase groups, respectively, again with no statistically significant differences.
The research team aims to test the effectiveness of tenecteplase in other stroke patients, including those with minor strokes, those receiving thrombolysis in a later window, and those receiving endovascular therapy, said Dr. Li.
Strong evidence
Commenting on the study findings, Larry B. Goldstein, MD, professor and chair of neurology, University of Kentucky, Lexington, said it is important to determine the efficacy of tenecteplase among Asians, as they represent “an entirely different population” with unique concerns, such as bleeding complications from anticoagulants.
He noted an advantage of tenecteplase is ease of administration. “You don’t have to go through the loading dose and then the 1-hour infusion,” which poses an “additional hassle” when transferring patients between institutions, he said.
However, he noted that a possible “downside” to having both drugs available in the emergency department is “using the wrong drug at the wrong dose” because of their similar sounding names.
Also commenting on the study, Tudor G. Jovin, MD, professor and chair, department of neurology, Rowan University, Camden, N.J., said he welcomes another trial that confirms that these two drugs are biologically similar.
“I’m very glad this trial was done because it adds another very strong piece of evidence of equivalency.”
But the two drugs are not the same in some important respects, said Dr. Jovin, whose center switched to using tenecteplase almost 3 years ago. That switch has resulted in cutting 17 minutes from the door-to-needle time “which is quite significant,” he said.
“There’s no question that once we used tenecteplase in lieu of tPA, it’s been just so much easier to administer and affects the interhospital transfer protocols, because you’re not transferring the patient with a critical care IV. It’s a win-win situation for everyone.”
The study received funding from the National Science and Technology Major Project, the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences, the National Natural Science Foundation of China, and the China Shijiazhuang Pharmaceutical Company Recomgen Pharmaceutical (Guangzhou). Dr. Li, Dr. Goldstein, and Dr. Jovin report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a new study has found. “This was a pivotal trial in establishing the safety and efficacy of tenecteplase as an alternative to alteplase in the thrombolytic treatment of acute ischemic stroke within 4.5 hours in Asian patients,” said study author Shuya Li, MD, associate chief physician, department of neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing.
The findings in this all-Chinese population should have an impact on the use of tenecteplase going forward, said Dr. Li. “The results provide further evidence to support a worldwide switch to tenecteplase as the preferred thrombolytic for acute ischemic stroke.”
The findings were presented at the 2023 International Stroke Conference presented by the American Stroke Association, a division of the American Heart Association.
Single bolus
Use of alteplase (tissue plasminogen activator [tPA]) has for years been the main approach to thrombolytic reperfusion therapy for patients with acute stroke, but tenecteplase has recently emerged as a potential successor.
Tenecteplase is a tPA produced by recombinant DNA technology. It has a relatively long half-life and can be delivered in a single bolus instead of requiring an hour-long infusion, as is the case with alteplase.
The phase 3 noninferiority Tenecteplase Reperfusion Therapy in Acute ischemic Cerebrovascular Events (TRACE-2) trial – the first of its kind in an Asian population – included 1,430 adult ischemic stroke patients at 53 Chinese centers. Patients had to have a National Institutes of Health Stroke Scale (NIHSS) score of 5-25 and either not be eligible for or have refused endovascular therapy.
The mean age of study participants was about 66 years, and the percentage of women was about 31%. The mean baseline NIHSS score was 7 in both groups, and the symptom-onset-to-needle time was similar at 180 minutes for the tenecteplase group and 178.5 minutes for the alteplase group.
Researchers randomly assigned patients to receive tenecteplase or alteplase within 4.5 hours of symptom onset.
Those in the tenecteplase group received 0.25 mg/kg of the drug in a single IV bolus (maximum dose, 25 mg). Control group members who were treated with alteplase were given the drug as a 10% bolus, with the remainder given as a 1-hour infusion (0.9 mg/kg with a maximum dose of 90 mg).
Showed noninferiority
The primary efficacy outcome was a modified Rankins scale (mRS) score of 0-1 at 90 days, which is considered excellent function. About 62% of tenecteplase patients and 58% of alteplase patients attained this outcome (risk ratio, 1.09; 95% confidence interval, 1.00-1.18).
The P value was .001 for noninferiority and .06 for superiority, but Dr. Li explained that these values may change when considering the site effect.
There were no statistically significant differences between the two drugs on secondary outcomes of favorable function. For example, 73% of tenecteplase patients and 72% of alteplase patients had an mRS score of 0-2 at 3 months, and 50% in the tenecteplase and 49% in the alteplase group improved by 4 or more points on the NIHSS, or had a score of 1 or less, at 24 hours.
The groups also had comparable scores on the European quality-of-life visual analogue scale and on the Barthel index, which measures functional independence related to personal care and mobility.
Tenecteplase also turned out to be as safe at alteplase. About 2% in both groups had symptomatic intracranial hemorrhage within 36 hours, and both groups had that same percentage for such hemorrhages within 90 days. As well, the groups had a similar rate of any intracranial hemorrhage within 90 days (6% and 7%).
The mortality rate was 7% in the tenecteplase group, compared with 5% in the alteplase group.
Adverse events (AEs) occurred in 86% and 87%, and serious AEs in 16% and 15%, of the tenecteplase and alteplase groups, respectively, again with no statistically significant differences.
The research team aims to test the effectiveness of tenecteplase in other stroke patients, including those with minor strokes, those receiving thrombolysis in a later window, and those receiving endovascular therapy, said Dr. Li.
Strong evidence
Commenting on the study findings, Larry B. Goldstein, MD, professor and chair of neurology, University of Kentucky, Lexington, said it is important to determine the efficacy of tenecteplase among Asians, as they represent “an entirely different population” with unique concerns, such as bleeding complications from anticoagulants.
He noted an advantage of tenecteplase is ease of administration. “You don’t have to go through the loading dose and then the 1-hour infusion,” which poses an “additional hassle” when transferring patients between institutions, he said.
However, he noted that a possible “downside” to having both drugs available in the emergency department is “using the wrong drug at the wrong dose” because of their similar sounding names.
Also commenting on the study, Tudor G. Jovin, MD, professor and chair, department of neurology, Rowan University, Camden, N.J., said he welcomes another trial that confirms that these two drugs are biologically similar.
“I’m very glad this trial was done because it adds another very strong piece of evidence of equivalency.”
But the two drugs are not the same in some important respects, said Dr. Jovin, whose center switched to using tenecteplase almost 3 years ago. That switch has resulted in cutting 17 minutes from the door-to-needle time “which is quite significant,” he said.
“There’s no question that once we used tenecteplase in lieu of tPA, it’s been just so much easier to administer and affects the interhospital transfer protocols, because you’re not transferring the patient with a critical care IV. It’s a win-win situation for everyone.”
The study received funding from the National Science and Technology Major Project, the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences, the National Natural Science Foundation of China, and the China Shijiazhuang Pharmaceutical Company Recomgen Pharmaceutical (Guangzhou). Dr. Li, Dr. Goldstein, and Dr. Jovin report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ISC 2023
Medicare ‘offers’ cancer patient a choice: Less life or more debt
We’re gonna need a bigger meth lab
In case you’ve been living under a rock for the past 15 years, the TV show “Breaking Bad” details the spiraling rise and downfall of a high school chemistry teacher who, after developing a case of terminal lung cancer, starts producing methamphetamine to provide for his family in response to the steep cost of treatment for his cancer.
Meanwhile, here in 2023 in the real world, we have Paul Davis, a retired physician in Ohio, who’s being forced to choose between an expensive cancer treatment and bankrupting his family, since Medicare’s decided it doesn’t want to cover the cost. Hey, we’ve seen this one before!
A bit of backstory: In November 2019, Dr. Davis was diagnosed with uveal melanoma, a very rare type of cancer that affects eye tissue. The news got worse in 2022 when the cancer spread to his liver, a move which typically proves fatal within a year. However, in a stroke of great news, the Food and Drug Administration approved the drug Kimmtrak earlier that year, which could be used to treat his cancer. Not cure, of course, but it would give him more time.
His initial treatments with the drug went fine and were covered, but when he transferred his care from a hospital in Columbus to one closer to home, big problem. Medicare decided it didn’t like that hospital and abruptly cut off coverage, denying the local hospital’s claims. That leaves Dr. Davis on the hook for his cancer treatment, and it’s what you might call expensive. Expensive to the tune of $50,000.
A week.
Apparently the coding the local hospital submitted was wrong, indicating that Dr. Davis was receiving Kimmtrak for a type of cancer that the FDA hadn’t approved the drug for. So until the government bureaucracy works itself out, his treatment is on hold, leaving all his faith in Medicare working quickly to rectify its mistake. If it can rectify its mistake. We’re not hopeful.
And in case you were wondering, if Dr. Davis wanted to go full Walter White, the average street price of meth is about $20-$60 per gram, so to pay for his treatment, he’d need to make at least a kilogram of meth every week. That’s, uh, quite a lot of illegal drug, or what we here at the LOTME office would call a fun Saturday night.
When you give a mouse a movie
Researchers have been successfully testing Alzheimer drugs on mice for years, but none of the drugs has proved successful in humans. Recent work, however, might have found the missing link, and it’s a combination no one ever thought of before: mice and movies.
Turns out that Orson Welles’ 1958 film noir classic “Touch of Evil” tapped a part of the mouse brain that has been overlooked: the hippocampus, which is crucial for learning and memory. Previous researchers thought it was just used as a kind of GPS system, but that’s only partially true.
Not only did the mice choose to pay attention to the movie clip, but the hippocampus responded to the visual stimuli only when the rodents saw the scenes from the clip later in the order that they were presented and not in a scrambled order. These findings represent a “major paradigm shift” in studying mouse recall, Mayank Mehta, PhD, of the University of California, Los Angeles, said in a statement from the school.
This breakthrough could run parallel to Alzheimer’s patients struggling with similar defects. “Selective and episodic activation of the mouse hippocampus using a human movie opens up the possibility of directly testing human episodic memory disorders and therapies using mouse neurons, a major step forward,” said coauthor Chinmay Purandare, PhD, who is now at the University of California, San Francisco.
Who would have thought that a classic film would help advance Alzheimer research?
A less human way to study mosquitoes
We here at LOTME have a history with mosquitoes. We know they don’t like us, and they know that we don’t like them. Trust us, they know. So when humans gain a little ground in the war against the buzzy little bloodsuckers, we want to share the joy.
To know the enemy, scientists have to study the enemy, but there is a problem. “Many mosquito experiments still rely on human volunteers and animal subjects,” bioengineering graduate student Kevin Janson, said in a statement from Rice University. Most people don’t like being bitten by mosquitoes, so that kind of testing can be expensive.
Is there a way to automate the collection and processing of mosquito behavior data using inexpensive cameras and machine-learning software? We’re glad you asked, because Mr. Janson and the research team, which includes bioengineers from Rice and tropical medicine experts from Tulane University, have managed to eliminate the need for live volunteers by using patches of synthetic skin made with a 3D printer.
“Each patch of gelatin-like hydrogel comes complete with tiny passageways that can be filled with flowing blood” from a chicken, sheep, or cow, they explained, and proof-of-concept testing showed that mosquitoes would feed on hydrogels without any repellent and stay away from those treated with a repellent.
To conduct the feeding tests, the blood-infused hydrogels are placed in a clear plastic box that is surrounded by cameras.
A bunch of mosquitoes are then tossed in the box and the cameras record all their insect activities: how often they land at each location, how long they stay, whether or not they bite, how long they feed, etc. Humans don’t have to watch and don’t have to be food sources.
Humans don’t have to be food sources, and we just pictured the future of mosquito control. Imagine a dozen Arnold Schwarzenegger–style Terminators, covered in 3D-printed skin, walking through your neighborhood in the summer while wearing sweat-soaked, brightly colored clothing. The mosquitoes wouldn’t be able to stay away, but guess what? They’re feeding off robots with nonhuman skin and nonhuman blood, so we win. It’s good to have a cerebral cortex.
Getting medieval on brain surgery
Let’s get one thing clear: The so-called “Dark Ages” were not nearly as dark as they’re made out to be. For one thing, there’s a world beyond Western Europe. The Roman Empire didn’t collapse everywhere. But even in Western Europe, the centuries between the fall of Rome and the Renaissance were hardly lacking in cultural development.
That said, we wouldn’t want to be in the position of the seventh-century noblewoman whose remains were recently uncovered in a Byzantine fortress in central Italy with multiple cross-shaped incisions in her skull. Yes, this unfortunate woman underwent at least two brain surgeries.
Then again, maybe not. Nothing like it had been discovered at the site, and while the markings – signs of a procedure called trepanation – can be surgical in nature, there are other explanations. For example, the Avar people practiced ritual trepanation during the same time period, but they were hundreds of miles away in the Carpathian mountains, and there was no evidence to support that a different form of ritualistic trepanation ever took place in Byzantine-era Italy.
The investigators then moved on to a form of judicial punishment called decalvatio, which involves mutilation by scalping. Look, the Dark Ages weren’t dark, but no one said they were fun. Anyway, this was discarded, since decalvatio was only meted out to soldiers who deserted the battlefield.
That brings us back to surgery. While one of the trepanations was fully engraved into her skull, indicating that the woman died soon after the surgery, she also bore indications of a healed trepanation. A 50% success rate isn’t terrible for our medieval surgeon. Sure, the Incas managed 80%, but even during the Civil War brain surgery only had a 50% success rate. And that’s the end of the story, nothing more to say about our medieval Italian woman.
Nope. Nothing at all.
Fine. While a surgical procedure was deemed most likely, the study investigators found no direct evidence of a medical condition. No trauma, no tumor, nothing. Just a couple of suggestions of “a systemic pathological condition,” they said. Okay, we swear, it really wasn’t that bad in the Middle [Editor’s note: Approximately 5,000 more words on medieval culture not included. This is a medical column, thank you very much.]
We’re gonna need a bigger meth lab
In case you’ve been living under a rock for the past 15 years, the TV show “Breaking Bad” details the spiraling rise and downfall of a high school chemistry teacher who, after developing a case of terminal lung cancer, starts producing methamphetamine to provide for his family in response to the steep cost of treatment for his cancer.
Meanwhile, here in 2023 in the real world, we have Paul Davis, a retired physician in Ohio, who’s being forced to choose between an expensive cancer treatment and bankrupting his family, since Medicare’s decided it doesn’t want to cover the cost. Hey, we’ve seen this one before!
A bit of backstory: In November 2019, Dr. Davis was diagnosed with uveal melanoma, a very rare type of cancer that affects eye tissue. The news got worse in 2022 when the cancer spread to his liver, a move which typically proves fatal within a year. However, in a stroke of great news, the Food and Drug Administration approved the drug Kimmtrak earlier that year, which could be used to treat his cancer. Not cure, of course, but it would give him more time.
His initial treatments with the drug went fine and were covered, but when he transferred his care from a hospital in Columbus to one closer to home, big problem. Medicare decided it didn’t like that hospital and abruptly cut off coverage, denying the local hospital’s claims. That leaves Dr. Davis on the hook for his cancer treatment, and it’s what you might call expensive. Expensive to the tune of $50,000.
A week.
Apparently the coding the local hospital submitted was wrong, indicating that Dr. Davis was receiving Kimmtrak for a type of cancer that the FDA hadn’t approved the drug for. So until the government bureaucracy works itself out, his treatment is on hold, leaving all his faith in Medicare working quickly to rectify its mistake. If it can rectify its mistake. We’re not hopeful.
And in case you were wondering, if Dr. Davis wanted to go full Walter White, the average street price of meth is about $20-$60 per gram, so to pay for his treatment, he’d need to make at least a kilogram of meth every week. That’s, uh, quite a lot of illegal drug, or what we here at the LOTME office would call a fun Saturday night.
When you give a mouse a movie
Researchers have been successfully testing Alzheimer drugs on mice for years, but none of the drugs has proved successful in humans. Recent work, however, might have found the missing link, and it’s a combination no one ever thought of before: mice and movies.
Turns out that Orson Welles’ 1958 film noir classic “Touch of Evil” tapped a part of the mouse brain that has been overlooked: the hippocampus, which is crucial for learning and memory. Previous researchers thought it was just used as a kind of GPS system, but that’s only partially true.
Not only did the mice choose to pay attention to the movie clip, but the hippocampus responded to the visual stimuli only when the rodents saw the scenes from the clip later in the order that they were presented and not in a scrambled order. These findings represent a “major paradigm shift” in studying mouse recall, Mayank Mehta, PhD, of the University of California, Los Angeles, said in a statement from the school.
This breakthrough could run parallel to Alzheimer’s patients struggling with similar defects. “Selective and episodic activation of the mouse hippocampus using a human movie opens up the possibility of directly testing human episodic memory disorders and therapies using mouse neurons, a major step forward,” said coauthor Chinmay Purandare, PhD, who is now at the University of California, San Francisco.
Who would have thought that a classic film would help advance Alzheimer research?
A less human way to study mosquitoes
We here at LOTME have a history with mosquitoes. We know they don’t like us, and they know that we don’t like them. Trust us, they know. So when humans gain a little ground in the war against the buzzy little bloodsuckers, we want to share the joy.
To know the enemy, scientists have to study the enemy, but there is a problem. “Many mosquito experiments still rely on human volunteers and animal subjects,” bioengineering graduate student Kevin Janson, said in a statement from Rice University. Most people don’t like being bitten by mosquitoes, so that kind of testing can be expensive.
Is there a way to automate the collection and processing of mosquito behavior data using inexpensive cameras and machine-learning software? We’re glad you asked, because Mr. Janson and the research team, which includes bioengineers from Rice and tropical medicine experts from Tulane University, have managed to eliminate the need for live volunteers by using patches of synthetic skin made with a 3D printer.
“Each patch of gelatin-like hydrogel comes complete with tiny passageways that can be filled with flowing blood” from a chicken, sheep, or cow, they explained, and proof-of-concept testing showed that mosquitoes would feed on hydrogels without any repellent and stay away from those treated with a repellent.
To conduct the feeding tests, the blood-infused hydrogels are placed in a clear plastic box that is surrounded by cameras.
A bunch of mosquitoes are then tossed in the box and the cameras record all their insect activities: how often they land at each location, how long they stay, whether or not they bite, how long they feed, etc. Humans don’t have to watch and don’t have to be food sources.
Humans don’t have to be food sources, and we just pictured the future of mosquito control. Imagine a dozen Arnold Schwarzenegger–style Terminators, covered in 3D-printed skin, walking through your neighborhood in the summer while wearing sweat-soaked, brightly colored clothing. The mosquitoes wouldn’t be able to stay away, but guess what? They’re feeding off robots with nonhuman skin and nonhuman blood, so we win. It’s good to have a cerebral cortex.
Getting medieval on brain surgery
Let’s get one thing clear: The so-called “Dark Ages” were not nearly as dark as they’re made out to be. For one thing, there’s a world beyond Western Europe. The Roman Empire didn’t collapse everywhere. But even in Western Europe, the centuries between the fall of Rome and the Renaissance were hardly lacking in cultural development.
That said, we wouldn’t want to be in the position of the seventh-century noblewoman whose remains were recently uncovered in a Byzantine fortress in central Italy with multiple cross-shaped incisions in her skull. Yes, this unfortunate woman underwent at least two brain surgeries.
Then again, maybe not. Nothing like it had been discovered at the site, and while the markings – signs of a procedure called trepanation – can be surgical in nature, there are other explanations. For example, the Avar people practiced ritual trepanation during the same time period, but they were hundreds of miles away in the Carpathian mountains, and there was no evidence to support that a different form of ritualistic trepanation ever took place in Byzantine-era Italy.
The investigators then moved on to a form of judicial punishment called decalvatio, which involves mutilation by scalping. Look, the Dark Ages weren’t dark, but no one said they were fun. Anyway, this was discarded, since decalvatio was only meted out to soldiers who deserted the battlefield.
That brings us back to surgery. While one of the trepanations was fully engraved into her skull, indicating that the woman died soon after the surgery, she also bore indications of a healed trepanation. A 50% success rate isn’t terrible for our medieval surgeon. Sure, the Incas managed 80%, but even during the Civil War brain surgery only had a 50% success rate. And that’s the end of the story, nothing more to say about our medieval Italian woman.
Nope. Nothing at all.
Fine. While a surgical procedure was deemed most likely, the study investigators found no direct evidence of a medical condition. No trauma, no tumor, nothing. Just a couple of suggestions of “a systemic pathological condition,” they said. Okay, we swear, it really wasn’t that bad in the Middle [Editor’s note: Approximately 5,000 more words on medieval culture not included. This is a medical column, thank you very much.]
We’re gonna need a bigger meth lab
In case you’ve been living under a rock for the past 15 years, the TV show “Breaking Bad” details the spiraling rise and downfall of a high school chemistry teacher who, after developing a case of terminal lung cancer, starts producing methamphetamine to provide for his family in response to the steep cost of treatment for his cancer.
Meanwhile, here in 2023 in the real world, we have Paul Davis, a retired physician in Ohio, who’s being forced to choose between an expensive cancer treatment and bankrupting his family, since Medicare’s decided it doesn’t want to cover the cost. Hey, we’ve seen this one before!
A bit of backstory: In November 2019, Dr. Davis was diagnosed with uveal melanoma, a very rare type of cancer that affects eye tissue. The news got worse in 2022 when the cancer spread to his liver, a move which typically proves fatal within a year. However, in a stroke of great news, the Food and Drug Administration approved the drug Kimmtrak earlier that year, which could be used to treat his cancer. Not cure, of course, but it would give him more time.
His initial treatments with the drug went fine and were covered, but when he transferred his care from a hospital in Columbus to one closer to home, big problem. Medicare decided it didn’t like that hospital and abruptly cut off coverage, denying the local hospital’s claims. That leaves Dr. Davis on the hook for his cancer treatment, and it’s what you might call expensive. Expensive to the tune of $50,000.
A week.
Apparently the coding the local hospital submitted was wrong, indicating that Dr. Davis was receiving Kimmtrak for a type of cancer that the FDA hadn’t approved the drug for. So until the government bureaucracy works itself out, his treatment is on hold, leaving all his faith in Medicare working quickly to rectify its mistake. If it can rectify its mistake. We’re not hopeful.
And in case you were wondering, if Dr. Davis wanted to go full Walter White, the average street price of meth is about $20-$60 per gram, so to pay for his treatment, he’d need to make at least a kilogram of meth every week. That’s, uh, quite a lot of illegal drug, or what we here at the LOTME office would call a fun Saturday night.
When you give a mouse a movie
Researchers have been successfully testing Alzheimer drugs on mice for years, but none of the drugs has proved successful in humans. Recent work, however, might have found the missing link, and it’s a combination no one ever thought of before: mice and movies.
Turns out that Orson Welles’ 1958 film noir classic “Touch of Evil” tapped a part of the mouse brain that has been overlooked: the hippocampus, which is crucial for learning and memory. Previous researchers thought it was just used as a kind of GPS system, but that’s only partially true.
Not only did the mice choose to pay attention to the movie clip, but the hippocampus responded to the visual stimuli only when the rodents saw the scenes from the clip later in the order that they were presented and not in a scrambled order. These findings represent a “major paradigm shift” in studying mouse recall, Mayank Mehta, PhD, of the University of California, Los Angeles, said in a statement from the school.
This breakthrough could run parallel to Alzheimer’s patients struggling with similar defects. “Selective and episodic activation of the mouse hippocampus using a human movie opens up the possibility of directly testing human episodic memory disorders and therapies using mouse neurons, a major step forward,” said coauthor Chinmay Purandare, PhD, who is now at the University of California, San Francisco.
Who would have thought that a classic film would help advance Alzheimer research?
A less human way to study mosquitoes
We here at LOTME have a history with mosquitoes. We know they don’t like us, and they know that we don’t like them. Trust us, they know. So when humans gain a little ground in the war against the buzzy little bloodsuckers, we want to share the joy.
To know the enemy, scientists have to study the enemy, but there is a problem. “Many mosquito experiments still rely on human volunteers and animal subjects,” bioengineering graduate student Kevin Janson, said in a statement from Rice University. Most people don’t like being bitten by mosquitoes, so that kind of testing can be expensive.
Is there a way to automate the collection and processing of mosquito behavior data using inexpensive cameras and machine-learning software? We’re glad you asked, because Mr. Janson and the research team, which includes bioengineers from Rice and tropical medicine experts from Tulane University, have managed to eliminate the need for live volunteers by using patches of synthetic skin made with a 3D printer.
“Each patch of gelatin-like hydrogel comes complete with tiny passageways that can be filled with flowing blood” from a chicken, sheep, or cow, they explained, and proof-of-concept testing showed that mosquitoes would feed on hydrogels without any repellent and stay away from those treated with a repellent.
To conduct the feeding tests, the blood-infused hydrogels are placed in a clear plastic box that is surrounded by cameras.
A bunch of mosquitoes are then tossed in the box and the cameras record all their insect activities: how often they land at each location, how long they stay, whether or not they bite, how long they feed, etc. Humans don’t have to watch and don’t have to be food sources.
Humans don’t have to be food sources, and we just pictured the future of mosquito control. Imagine a dozen Arnold Schwarzenegger–style Terminators, covered in 3D-printed skin, walking through your neighborhood in the summer while wearing sweat-soaked, brightly colored clothing. The mosquitoes wouldn’t be able to stay away, but guess what? They’re feeding off robots with nonhuman skin and nonhuman blood, so we win. It’s good to have a cerebral cortex.
Getting medieval on brain surgery
Let’s get one thing clear: The so-called “Dark Ages” were not nearly as dark as they’re made out to be. For one thing, there’s a world beyond Western Europe. The Roman Empire didn’t collapse everywhere. But even in Western Europe, the centuries between the fall of Rome and the Renaissance were hardly lacking in cultural development.
That said, we wouldn’t want to be in the position of the seventh-century noblewoman whose remains were recently uncovered in a Byzantine fortress in central Italy with multiple cross-shaped incisions in her skull. Yes, this unfortunate woman underwent at least two brain surgeries.
Then again, maybe not. Nothing like it had been discovered at the site, and while the markings – signs of a procedure called trepanation – can be surgical in nature, there are other explanations. For example, the Avar people practiced ritual trepanation during the same time period, but they were hundreds of miles away in the Carpathian mountains, and there was no evidence to support that a different form of ritualistic trepanation ever took place in Byzantine-era Italy.
The investigators then moved on to a form of judicial punishment called decalvatio, which involves mutilation by scalping. Look, the Dark Ages weren’t dark, but no one said they were fun. Anyway, this was discarded, since decalvatio was only meted out to soldiers who deserted the battlefield.
That brings us back to surgery. While one of the trepanations was fully engraved into her skull, indicating that the woman died soon after the surgery, she also bore indications of a healed trepanation. A 50% success rate isn’t terrible for our medieval surgeon. Sure, the Incas managed 80%, but even during the Civil War brain surgery only had a 50% success rate. And that’s the end of the story, nothing more to say about our medieval Italian woman.
Nope. Nothing at all.
Fine. While a surgical procedure was deemed most likely, the study investigators found no direct evidence of a medical condition. No trauma, no tumor, nothing. Just a couple of suggestions of “a systemic pathological condition,” they said. Okay, we swear, it really wasn’t that bad in the Middle [Editor’s note: Approximately 5,000 more words on medieval culture not included. This is a medical column, thank you very much.]
Saying goodbye: How to transition teens to adult medical care
However, many clinicians feel insufficiently prepared to provide comprehensive transition services. This can result in the actual handoff or transfer into adult care being abrupt, incomplete, or outright unsuccessful. By following the recommended best practices of transitions, providers of pediatric care can ensure that this challenging goodbye prepares everyone for the next steps ahead.
Using a structured transition process
In 2011, a health care transition clinical report based on expert opinion and practice consensus and endorsed by the American Academy of Pediatrics, American Academy of Family Physicians, and American College of Physicians – Society of Internal Medicine was released. This report provided a decision-making algorithm for “practice-based implementation of transition for all youth beginning in early adolescence.”
The Got Transition organization, funded by the Maternal Child Health Bureau and Health Resources and Services Administration, provides web-based information and materials for health care providers and families to establish a smooth and successful transition. At the center of these recommendations are the Six Core Elements of Health Care Transition – the essential components of a structured transition process: 1) transition policy/guide; 2) tracking and monitoring; 3) readiness; 4) planning; 5) transfer of care, and 6) transition completion.
This transition process should start early in adolescence, preferably by age 12-14 years, to give adequate time to progress successfully through these elements and improve the likelihood of a smooth, final transfer into the care of an adult clinician.
Preparing your patients for transfer
Despite the availability of these recommendations, national surveys show that the overwhelming majority of adolescents with and without special health care needs report not receiving transition services. Lack of time, resources, interest, and patients being lost to care during adolescence all contribute to this deficit in care. Without transition preparation, the actual handoff or transfer to adult care can be difficult for adolescents, caregivers, and clinicians alike. Adolescents and caregivers may feel a sense of abandonment or have inadequate health knowledge/literacy, pediatric clinicians may fear that the patient is not ready for the expected independence, and adult clinicians face numerous challenges integrating these young patients into their practice.
A structured transition process can help the family and clinicians know what to expect during the transfer of care. Pediatric clinicians can gradually move from a pediatric model of care, in which the caregiver is the center of communication, to an adult model, putting the patient at the center. By encouraging the adolescent to be the direct communicator, the pediatric clinician can promote independence and assess health knowledge, allowing for education where gaps exist.
Assisting the patient in identifying and even meeting the adult clinician well ahead of the final transfer date can also make the process less daunting for the adolescent.
Adult clinicians should consider allowing more time for the first visit with a new young adult patient and welcome caregiver input early in the transfer process, particularly for patients with a chronic disease. By engaging patients and families in an intentional, gradual transition process with an expected outcome, all those involved will be more prepared for the final handoff.
Utilizing transition tools and engaging the adolescent
Numerous tools can assist in the preparation for transfer to adult care. These include transition summaries and emergency plans, which contain essential information such as current medical problems, allergies, medications, prior procedures and treatments, and sick day plans. Such tools can also be built into electronic medical records for easy modification and updating. They can be used as methods to engage and teach adolescents about their disease history and current regimen and can contain essential components for information handoff at the time of transfer to adult care. If the patient carries a rare diagnosis, or one that has historically been associated with lower survival to adulthood, these transfer documents can also include summary information about disease states and contact information for pediatric specialty clinicians.
Adolescent engagement in their health care during the time of transition can also be prompted through the use of patient portals within an electronic health record. Such portals put health information directly at the adolescent’s fingertips, provide them with an outlet for communication with their clinicians, and give reminders regarding health maintenance.
Completing the transfer: The final handoff
The best and most recommended means of relaying information at the time of transfer to adult care is a direct, verbal handoff between clinicians. This direct handoff has several goals:
(1) To ensure the patient has scheduled or attended the first appointment with the adult clinician
(2) To ensure record transfer has occurred successfully
(3) To answer any questions the receiving clinician may have about prior or ongoing care.
(4) To offer the adult clinician ongoing access to the pediatric clinician as an “expert” resource for additional questions.
By remaining available as a resource, the pediatric clinician can alleviate concerns for both the patient and caregiver as well as the receiving adult clinician.
As valuable as verbal handoffs can be, they are not always possible due to patients not having selected an adult clinician prior to leaving the pediatric clinician, an inability to reach the receiving clinician, and/or time limitations. Many of these barriers can be alleviated by early discussions of transitions of care as well as utilization of structured documentation tools as noted above.
It is also recommended that the pediatric clinician follows up with the patient and/or caregiver several months after the transfer is complete. This allows for the adolescent and/or the caregiver to reflect on the transition process and provide feedback to the pediatric clinicians and their practice for ongoing process improvement.
Reflection as a pediatrician
Ideally, all transition steps occur for the adolescent; in our opinion, a crucial component is to prepare the adolescent patient for the change from a pediatric to adult model of care, in which they are independent in their health communication and decision-making. By engaging adolescents to understand their health, how to maintain it, and when to seek care, we empower them to advocate for their own health as young adults. With appropriate health knowledge and literacy, adolescents are more likely to actively engage with their health care providers and make healthy lifestyle choices. So though saying goodbye may still be difficult, it can be done with the confidence that the patients will continue to get the care they need as they transition into adulthood.
Dr. Kim is assistant clinical professor, department of pediatrics, University of California, San Diego. Dr. Mennito is associate professor of pediatrics and internal medicine, Medical University of South Carolina, Charleston, S.C. Dr. Kim and Dr. Mennito have disclosed no relevant financial relationships. A version of this article originally appeared on Medscape.com.
However, many clinicians feel insufficiently prepared to provide comprehensive transition services. This can result in the actual handoff or transfer into adult care being abrupt, incomplete, or outright unsuccessful. By following the recommended best practices of transitions, providers of pediatric care can ensure that this challenging goodbye prepares everyone for the next steps ahead.
Using a structured transition process
In 2011, a health care transition clinical report based on expert opinion and practice consensus and endorsed by the American Academy of Pediatrics, American Academy of Family Physicians, and American College of Physicians – Society of Internal Medicine was released. This report provided a decision-making algorithm for “practice-based implementation of transition for all youth beginning in early adolescence.”
The Got Transition organization, funded by the Maternal Child Health Bureau and Health Resources and Services Administration, provides web-based information and materials for health care providers and families to establish a smooth and successful transition. At the center of these recommendations are the Six Core Elements of Health Care Transition – the essential components of a structured transition process: 1) transition policy/guide; 2) tracking and monitoring; 3) readiness; 4) planning; 5) transfer of care, and 6) transition completion.
This transition process should start early in adolescence, preferably by age 12-14 years, to give adequate time to progress successfully through these elements and improve the likelihood of a smooth, final transfer into the care of an adult clinician.
Preparing your patients for transfer
Despite the availability of these recommendations, national surveys show that the overwhelming majority of adolescents with and without special health care needs report not receiving transition services. Lack of time, resources, interest, and patients being lost to care during adolescence all contribute to this deficit in care. Without transition preparation, the actual handoff or transfer to adult care can be difficult for adolescents, caregivers, and clinicians alike. Adolescents and caregivers may feel a sense of abandonment or have inadequate health knowledge/literacy, pediatric clinicians may fear that the patient is not ready for the expected independence, and adult clinicians face numerous challenges integrating these young patients into their practice.
A structured transition process can help the family and clinicians know what to expect during the transfer of care. Pediatric clinicians can gradually move from a pediatric model of care, in which the caregiver is the center of communication, to an adult model, putting the patient at the center. By encouraging the adolescent to be the direct communicator, the pediatric clinician can promote independence and assess health knowledge, allowing for education where gaps exist.
Assisting the patient in identifying and even meeting the adult clinician well ahead of the final transfer date can also make the process less daunting for the adolescent.
Adult clinicians should consider allowing more time for the first visit with a new young adult patient and welcome caregiver input early in the transfer process, particularly for patients with a chronic disease. By engaging patients and families in an intentional, gradual transition process with an expected outcome, all those involved will be more prepared for the final handoff.
Utilizing transition tools and engaging the adolescent
Numerous tools can assist in the preparation for transfer to adult care. These include transition summaries and emergency plans, which contain essential information such as current medical problems, allergies, medications, prior procedures and treatments, and sick day plans. Such tools can also be built into electronic medical records for easy modification and updating. They can be used as methods to engage and teach adolescents about their disease history and current regimen and can contain essential components for information handoff at the time of transfer to adult care. If the patient carries a rare diagnosis, or one that has historically been associated with lower survival to adulthood, these transfer documents can also include summary information about disease states and contact information for pediatric specialty clinicians.
Adolescent engagement in their health care during the time of transition can also be prompted through the use of patient portals within an electronic health record. Such portals put health information directly at the adolescent’s fingertips, provide them with an outlet for communication with their clinicians, and give reminders regarding health maintenance.
Completing the transfer: The final handoff
The best and most recommended means of relaying information at the time of transfer to adult care is a direct, verbal handoff between clinicians. This direct handoff has several goals:
(1) To ensure the patient has scheduled or attended the first appointment with the adult clinician
(2) To ensure record transfer has occurred successfully
(3) To answer any questions the receiving clinician may have about prior or ongoing care.
(4) To offer the adult clinician ongoing access to the pediatric clinician as an “expert” resource for additional questions.
By remaining available as a resource, the pediatric clinician can alleviate concerns for both the patient and caregiver as well as the receiving adult clinician.
As valuable as verbal handoffs can be, they are not always possible due to patients not having selected an adult clinician prior to leaving the pediatric clinician, an inability to reach the receiving clinician, and/or time limitations. Many of these barriers can be alleviated by early discussions of transitions of care as well as utilization of structured documentation tools as noted above.
It is also recommended that the pediatric clinician follows up with the patient and/or caregiver several months after the transfer is complete. This allows for the adolescent and/or the caregiver to reflect on the transition process and provide feedback to the pediatric clinicians and their practice for ongoing process improvement.
Reflection as a pediatrician
Ideally, all transition steps occur for the adolescent; in our opinion, a crucial component is to prepare the adolescent patient for the change from a pediatric to adult model of care, in which they are independent in their health communication and decision-making. By engaging adolescents to understand their health, how to maintain it, and when to seek care, we empower them to advocate for their own health as young adults. With appropriate health knowledge and literacy, adolescents are more likely to actively engage with their health care providers and make healthy lifestyle choices. So though saying goodbye may still be difficult, it can be done with the confidence that the patients will continue to get the care they need as they transition into adulthood.
Dr. Kim is assistant clinical professor, department of pediatrics, University of California, San Diego. Dr. Mennito is associate professor of pediatrics and internal medicine, Medical University of South Carolina, Charleston, S.C. Dr. Kim and Dr. Mennito have disclosed no relevant financial relationships. A version of this article originally appeared on Medscape.com.
However, many clinicians feel insufficiently prepared to provide comprehensive transition services. This can result in the actual handoff or transfer into adult care being abrupt, incomplete, or outright unsuccessful. By following the recommended best practices of transitions, providers of pediatric care can ensure that this challenging goodbye prepares everyone for the next steps ahead.
Using a structured transition process
In 2011, a health care transition clinical report based on expert opinion and practice consensus and endorsed by the American Academy of Pediatrics, American Academy of Family Physicians, and American College of Physicians – Society of Internal Medicine was released. This report provided a decision-making algorithm for “practice-based implementation of transition for all youth beginning in early adolescence.”
The Got Transition organization, funded by the Maternal Child Health Bureau and Health Resources and Services Administration, provides web-based information and materials for health care providers and families to establish a smooth and successful transition. At the center of these recommendations are the Six Core Elements of Health Care Transition – the essential components of a structured transition process: 1) transition policy/guide; 2) tracking and monitoring; 3) readiness; 4) planning; 5) transfer of care, and 6) transition completion.
This transition process should start early in adolescence, preferably by age 12-14 years, to give adequate time to progress successfully through these elements and improve the likelihood of a smooth, final transfer into the care of an adult clinician.
Preparing your patients for transfer
Despite the availability of these recommendations, national surveys show that the overwhelming majority of adolescents with and without special health care needs report not receiving transition services. Lack of time, resources, interest, and patients being lost to care during adolescence all contribute to this deficit in care. Without transition preparation, the actual handoff or transfer to adult care can be difficult for adolescents, caregivers, and clinicians alike. Adolescents and caregivers may feel a sense of abandonment or have inadequate health knowledge/literacy, pediatric clinicians may fear that the patient is not ready for the expected independence, and adult clinicians face numerous challenges integrating these young patients into their practice.
A structured transition process can help the family and clinicians know what to expect during the transfer of care. Pediatric clinicians can gradually move from a pediatric model of care, in which the caregiver is the center of communication, to an adult model, putting the patient at the center. By encouraging the adolescent to be the direct communicator, the pediatric clinician can promote independence and assess health knowledge, allowing for education where gaps exist.
Assisting the patient in identifying and even meeting the adult clinician well ahead of the final transfer date can also make the process less daunting for the adolescent.
Adult clinicians should consider allowing more time for the first visit with a new young adult patient and welcome caregiver input early in the transfer process, particularly for patients with a chronic disease. By engaging patients and families in an intentional, gradual transition process with an expected outcome, all those involved will be more prepared for the final handoff.
Utilizing transition tools and engaging the adolescent
Numerous tools can assist in the preparation for transfer to adult care. These include transition summaries and emergency plans, which contain essential information such as current medical problems, allergies, medications, prior procedures and treatments, and sick day plans. Such tools can also be built into electronic medical records for easy modification and updating. They can be used as methods to engage and teach adolescents about their disease history and current regimen and can contain essential components for information handoff at the time of transfer to adult care. If the patient carries a rare diagnosis, or one that has historically been associated with lower survival to adulthood, these transfer documents can also include summary information about disease states and contact information for pediatric specialty clinicians.
Adolescent engagement in their health care during the time of transition can also be prompted through the use of patient portals within an electronic health record. Such portals put health information directly at the adolescent’s fingertips, provide them with an outlet for communication with their clinicians, and give reminders regarding health maintenance.
Completing the transfer: The final handoff
The best and most recommended means of relaying information at the time of transfer to adult care is a direct, verbal handoff between clinicians. This direct handoff has several goals:
(1) To ensure the patient has scheduled or attended the first appointment with the adult clinician
(2) To ensure record transfer has occurred successfully
(3) To answer any questions the receiving clinician may have about prior or ongoing care.
(4) To offer the adult clinician ongoing access to the pediatric clinician as an “expert” resource for additional questions.
By remaining available as a resource, the pediatric clinician can alleviate concerns for both the patient and caregiver as well as the receiving adult clinician.
As valuable as verbal handoffs can be, they are not always possible due to patients not having selected an adult clinician prior to leaving the pediatric clinician, an inability to reach the receiving clinician, and/or time limitations. Many of these barriers can be alleviated by early discussions of transitions of care as well as utilization of structured documentation tools as noted above.
It is also recommended that the pediatric clinician follows up with the patient and/or caregiver several months after the transfer is complete. This allows for the adolescent and/or the caregiver to reflect on the transition process and provide feedback to the pediatric clinicians and their practice for ongoing process improvement.
Reflection as a pediatrician
Ideally, all transition steps occur for the adolescent; in our opinion, a crucial component is to prepare the adolescent patient for the change from a pediatric to adult model of care, in which they are independent in their health communication and decision-making. By engaging adolescents to understand their health, how to maintain it, and when to seek care, we empower them to advocate for their own health as young adults. With appropriate health knowledge and literacy, adolescents are more likely to actively engage with their health care providers and make healthy lifestyle choices. So though saying goodbye may still be difficult, it can be done with the confidence that the patients will continue to get the care they need as they transition into adulthood.
Dr. Kim is assistant clinical professor, department of pediatrics, University of California, San Diego. Dr. Mennito is associate professor of pediatrics and internal medicine, Medical University of South Carolina, Charleston, S.C. Dr. Kim and Dr. Mennito have disclosed no relevant financial relationships. A version of this article originally appeared on Medscape.com.
Using devices to calm children can backfire long term
according to developmental behavioral pediatricians at University of Michigan Health C. S. Mott Children’s Hospital, Ann Arbor.
What to know
- Using a mobile device to distract children from how they are feeling may displace opportunities for them to develop independent, alternative methods to self-regulate, especially in early childhood.
- Signs of increased dysregulation could include rapid shifts between sadness and excitement, a sudden change in mood or feelings, and heightened impulsivity.
- The association between device-calming and emotional consequences may be particularly high among young boys and children who are already experiencing hyperactivity, impulsiveness, and a strong temperament that makes them more likely to react intensely to feelings such as anger, frustration, and sadness.
- While occasional use of media to occupy children is expected and understandable, it is important that it not become a primary or regular soothing tool, and children should be given clear expectations of when and where devices can be used.
- The preschool-to-kindergarten period is a developmental stage in which children may be more likely to exhibit difficult behaviors, such as tantrums, defiance, and intense emotions, but parents should resist using devices as a parenting strategy.
This is a summary of the article, “Longitudinal Association Between Use of Mobile Devices for Calming and Emotional Reactivity and Executive Functioning in Children Aged 3 to 5 Years,” published in JAMA Pediatrics on Dec. 20, 2022. The full article can be found on jamanetwork.com. A version of this article originally appeared on Medscape.com.
according to developmental behavioral pediatricians at University of Michigan Health C. S. Mott Children’s Hospital, Ann Arbor.
What to know
- Using a mobile device to distract children from how they are feeling may displace opportunities for them to develop independent, alternative methods to self-regulate, especially in early childhood.
- Signs of increased dysregulation could include rapid shifts between sadness and excitement, a sudden change in mood or feelings, and heightened impulsivity.
- The association between device-calming and emotional consequences may be particularly high among young boys and children who are already experiencing hyperactivity, impulsiveness, and a strong temperament that makes them more likely to react intensely to feelings such as anger, frustration, and sadness.
- While occasional use of media to occupy children is expected and understandable, it is important that it not become a primary or regular soothing tool, and children should be given clear expectations of when and where devices can be used.
- The preschool-to-kindergarten period is a developmental stage in which children may be more likely to exhibit difficult behaviors, such as tantrums, defiance, and intense emotions, but parents should resist using devices as a parenting strategy.
This is a summary of the article, “Longitudinal Association Between Use of Mobile Devices for Calming and Emotional Reactivity and Executive Functioning in Children Aged 3 to 5 Years,” published in JAMA Pediatrics on Dec. 20, 2022. The full article can be found on jamanetwork.com. A version of this article originally appeared on Medscape.com.
according to developmental behavioral pediatricians at University of Michigan Health C. S. Mott Children’s Hospital, Ann Arbor.
What to know
- Using a mobile device to distract children from how they are feeling may displace opportunities for them to develop independent, alternative methods to self-regulate, especially in early childhood.
- Signs of increased dysregulation could include rapid shifts between sadness and excitement, a sudden change in mood or feelings, and heightened impulsivity.
- The association between device-calming and emotional consequences may be particularly high among young boys and children who are already experiencing hyperactivity, impulsiveness, and a strong temperament that makes them more likely to react intensely to feelings such as anger, frustration, and sadness.
- While occasional use of media to occupy children is expected and understandable, it is important that it not become a primary or regular soothing tool, and children should be given clear expectations of when and where devices can be used.
- The preschool-to-kindergarten period is a developmental stage in which children may be more likely to exhibit difficult behaviors, such as tantrums, defiance, and intense emotions, but parents should resist using devices as a parenting strategy.
This is a summary of the article, “Longitudinal Association Between Use of Mobile Devices for Calming and Emotional Reactivity and Executive Functioning in Children Aged 3 to 5 Years,” published in JAMA Pediatrics on Dec. 20, 2022. The full article can be found on jamanetwork.com. A version of this article originally appeared on Medscape.com.




