User login
Neurology Reviews covers innovative and emerging news in neurology and neuroscience every month, with a focus on practical approaches to treating Parkinson's disease, epilepsy, headache, stroke, multiple sclerosis, Alzheimer's disease, and other neurologic disorders.
PML
Progressive multifocal leukoencephalopathy
Rituxan
The leading independent newspaper covering neurology news and commentary.
Four PTSD blood biomarkers identified
“More accurate means of predicting or screening for PTSD could help to overcome the disorder by identifying individuals at high risk of developing PTSD and providing them with early intervention or prevention strategies,” said study investigator Stacy-Ann Miller, MS.
She also noted that the biomarkers could be used to monitor treatment for PTSD, identify subtypes of PTSD, and lead to a new understanding of the mechanisms underlying PTSD.
The findings were presented at Discover BMB, the annual meeting of the American Society for Biochemistry and Molecular Biology.
Toward better clinical assessment
The findings originated from research conducted by the Department of Defense–initiated PTSD Systems Biology Consortium. The consortium’s goals include developing a reproducible panel of blood-based biomarkers with high sensitivity and specificity for PTSD diagnosis and is made up of about 45 researchers, led by Marti Jett, PhD, Charles Marmar, MD, and Francis J. Doyle III, PhD.
The researchers analyzed blood samples from 1,000 active-duty Army personnel from the 101st Airborne at Fort Campbell, Ky. Participants were assessed before and after deployment to Afghanistan in February 2014 and are referred to as the Fort Campbell Cohort (FCC). Participants’ age ranged from 25 to 30 and approximately 6% were female.
Investigators collected blood samples from the service members and looked for four biomarkers: glycolytic ratio, arginine, serotonin, and glutamate. The team then divided the participants into four groups – those with PTSD (PTSD Checklist score above 30), those who were subthreshold for PTSD (PTSD Checklist score 15-30), those who had high resilience, and those who had low levels of resilience.
The resilience groups were determined based on answers to the Generalized Anxiety Disorder Questionnaire, Patient Health Questionnaire, Pittsburgh Sleep Quality Index, Intensive Combat Exposure (DRRI-D), the number of deployments, whether they had moderate or severe traumatic brain injury, and scores on the Alcohol Use Disorders Identification Test.
Those who scored in the high range at current or prior time points or who were PTSD/subthreshold at prior time points were placed in the low resilience group.
Ms. Miller noted that those in the PTSD group had more severe symptoms than those in the PTSD subthreshold group based on the longitudinal clinical assessment at 3-6 months, 5 years, and longer post deployment. The low resilience group had a much higher rate of PTSD post deployment than the high resilience group.
Investigators found participants with PTSD or subthreshold PTSD had significantly higher glycolic ratios and lower arginine than those with high resilience. They also found that those with PTSD had significantly lower serotonin and higher glutamate levels versus those with high resilience. These associations were independent of factors such as sex, age, body mass index, smoking, and caffeine consumption.
Ms. Miller said that the study results require further validation by the consortium’s labs and third-party labs.
“We are also interested in determining the most appropriate time to screen soldiers for PTSD, as it has been noted that the time period where we see the most psychological issues is around 2-3 months post return from deployment and when the soldier is preparing for their next assignment, perhaps a next deployment,” she said.
She added that previous studies have identified several promising biomarkers of PTSD. “However, like much of the research data, the study sample was comprised mainly of combat-exposed males. With more women serving on the front lines, the military faces new challenges in how combat affects females in the military,” including sex-specific biomarkers that will improve clinical assessment for female soldiers.
Eventually, the team would also like to be able to apply their research to the civilian population experiencing PTSD.
“Our research is anticipated to be useful in helping the medical provider select appropriate therapeutic interventions,” Ms. Miller said.
A version of this article first appeared on Medscape.com.
“More accurate means of predicting or screening for PTSD could help to overcome the disorder by identifying individuals at high risk of developing PTSD and providing them with early intervention or prevention strategies,” said study investigator Stacy-Ann Miller, MS.
She also noted that the biomarkers could be used to monitor treatment for PTSD, identify subtypes of PTSD, and lead to a new understanding of the mechanisms underlying PTSD.
The findings were presented at Discover BMB, the annual meeting of the American Society for Biochemistry and Molecular Biology.
Toward better clinical assessment
The findings originated from research conducted by the Department of Defense–initiated PTSD Systems Biology Consortium. The consortium’s goals include developing a reproducible panel of blood-based biomarkers with high sensitivity and specificity for PTSD diagnosis and is made up of about 45 researchers, led by Marti Jett, PhD, Charles Marmar, MD, and Francis J. Doyle III, PhD.
The researchers analyzed blood samples from 1,000 active-duty Army personnel from the 101st Airborne at Fort Campbell, Ky. Participants were assessed before and after deployment to Afghanistan in February 2014 and are referred to as the Fort Campbell Cohort (FCC). Participants’ age ranged from 25 to 30 and approximately 6% were female.
Investigators collected blood samples from the service members and looked for four biomarkers: glycolytic ratio, arginine, serotonin, and glutamate. The team then divided the participants into four groups – those with PTSD (PTSD Checklist score above 30), those who were subthreshold for PTSD (PTSD Checklist score 15-30), those who had high resilience, and those who had low levels of resilience.
The resilience groups were determined based on answers to the Generalized Anxiety Disorder Questionnaire, Patient Health Questionnaire, Pittsburgh Sleep Quality Index, Intensive Combat Exposure (DRRI-D), the number of deployments, whether they had moderate or severe traumatic brain injury, and scores on the Alcohol Use Disorders Identification Test.
Those who scored in the high range at current or prior time points or who were PTSD/subthreshold at prior time points were placed in the low resilience group.
Ms. Miller noted that those in the PTSD group had more severe symptoms than those in the PTSD subthreshold group based on the longitudinal clinical assessment at 3-6 months, 5 years, and longer post deployment. The low resilience group had a much higher rate of PTSD post deployment than the high resilience group.
Investigators found participants with PTSD or subthreshold PTSD had significantly higher glycolic ratios and lower arginine than those with high resilience. They also found that those with PTSD had significantly lower serotonin and higher glutamate levels versus those with high resilience. These associations were independent of factors such as sex, age, body mass index, smoking, and caffeine consumption.
Ms. Miller said that the study results require further validation by the consortium’s labs and third-party labs.
“We are also interested in determining the most appropriate time to screen soldiers for PTSD, as it has been noted that the time period where we see the most psychological issues is around 2-3 months post return from deployment and when the soldier is preparing for their next assignment, perhaps a next deployment,” she said.
She added that previous studies have identified several promising biomarkers of PTSD. “However, like much of the research data, the study sample was comprised mainly of combat-exposed males. With more women serving on the front lines, the military faces new challenges in how combat affects females in the military,” including sex-specific biomarkers that will improve clinical assessment for female soldiers.
Eventually, the team would also like to be able to apply their research to the civilian population experiencing PTSD.
“Our research is anticipated to be useful in helping the medical provider select appropriate therapeutic interventions,” Ms. Miller said.
A version of this article first appeared on Medscape.com.
“More accurate means of predicting or screening for PTSD could help to overcome the disorder by identifying individuals at high risk of developing PTSD and providing them with early intervention or prevention strategies,” said study investigator Stacy-Ann Miller, MS.
She also noted that the biomarkers could be used to monitor treatment for PTSD, identify subtypes of PTSD, and lead to a new understanding of the mechanisms underlying PTSD.
The findings were presented at Discover BMB, the annual meeting of the American Society for Biochemistry and Molecular Biology.
Toward better clinical assessment
The findings originated from research conducted by the Department of Defense–initiated PTSD Systems Biology Consortium. The consortium’s goals include developing a reproducible panel of blood-based biomarkers with high sensitivity and specificity for PTSD diagnosis and is made up of about 45 researchers, led by Marti Jett, PhD, Charles Marmar, MD, and Francis J. Doyle III, PhD.
The researchers analyzed blood samples from 1,000 active-duty Army personnel from the 101st Airborne at Fort Campbell, Ky. Participants were assessed before and after deployment to Afghanistan in February 2014 and are referred to as the Fort Campbell Cohort (FCC). Participants’ age ranged from 25 to 30 and approximately 6% were female.
Investigators collected blood samples from the service members and looked for four biomarkers: glycolytic ratio, arginine, serotonin, and glutamate. The team then divided the participants into four groups – those with PTSD (PTSD Checklist score above 30), those who were subthreshold for PTSD (PTSD Checklist score 15-30), those who had high resilience, and those who had low levels of resilience.
The resilience groups were determined based on answers to the Generalized Anxiety Disorder Questionnaire, Patient Health Questionnaire, Pittsburgh Sleep Quality Index, Intensive Combat Exposure (DRRI-D), the number of deployments, whether they had moderate or severe traumatic brain injury, and scores on the Alcohol Use Disorders Identification Test.
Those who scored in the high range at current or prior time points or who were PTSD/subthreshold at prior time points were placed in the low resilience group.
Ms. Miller noted that those in the PTSD group had more severe symptoms than those in the PTSD subthreshold group based on the longitudinal clinical assessment at 3-6 months, 5 years, and longer post deployment. The low resilience group had a much higher rate of PTSD post deployment than the high resilience group.
Investigators found participants with PTSD or subthreshold PTSD had significantly higher glycolic ratios and lower arginine than those with high resilience. They also found that those with PTSD had significantly lower serotonin and higher glutamate levels versus those with high resilience. These associations were independent of factors such as sex, age, body mass index, smoking, and caffeine consumption.
Ms. Miller said that the study results require further validation by the consortium’s labs and third-party labs.
“We are also interested in determining the most appropriate time to screen soldiers for PTSD, as it has been noted that the time period where we see the most psychological issues is around 2-3 months post return from deployment and when the soldier is preparing for their next assignment, perhaps a next deployment,” she said.
She added that previous studies have identified several promising biomarkers of PTSD. “However, like much of the research data, the study sample was comprised mainly of combat-exposed males. With more women serving on the front lines, the military faces new challenges in how combat affects females in the military,” including sex-specific biomarkers that will improve clinical assessment for female soldiers.
Eventually, the team would also like to be able to apply their research to the civilian population experiencing PTSD.
“Our research is anticipated to be useful in helping the medical provider select appropriate therapeutic interventions,” Ms. Miller said.
A version of this article first appeared on Medscape.com.
FROM DISCOVER BMB
Lack of food for thought: Starve a bacterium, feed an infection
A whole new, tiny level of hangry
Ever been so hungry that everything just got on your nerves? Maybe you feel a little snappy right now? Like you’ll just lash out unless you get something to eat? Been there. And so have bacteria.
New research shows that some bacteria go into a full-on Hulk smash if they’re not getting the nutrients they need by releasing toxins into the body. Sounds like a bacterial temper tantrum.
Even though two cells may be genetically identical, they don’t always behave the same in a bacterial community. Some do their job and stay in line, but some evil twins rage out and make people sick by releasing toxins into the environment, Adam Rosenthal, PhD, of the University of North Carolina and his colleagues discovered.
To figure out why some cells were all business as usual while others were not, the investigators looked at Clostridium perfringens, a bacterium found in the intestines of humans and other vertebrates. When the C. perfringens cells were fed a little acetate to munch on, the hangry cells calmed down faster than a kid with a bag of fruit snacks, reducing toxin levels. Some cells even disappeared, falling in line with their model-citizen counterparts.
So what does this really mean? More research, duh. Now that we know nutrients play a role in toxicity, it may open the door to finding a way to fight against antibiotic resistance in humans and reduce antibiotic use in the food industry.
So think to yourself. Are you bothered for no reason? Getting a little testy with your friends and coworkers? Maybe you just haven’t eaten in a while. You’re literally not alone. Even a single-cell organism can behave based on its hunger levels.
Now go have a snack. Your bacteria are getting restless.
The very hangry iguana?
Imagine yourself on a warm, sunny tropical beach. You are enjoying a piece of cake as you take in the slow beat of the waves lapping against the shore. Life is as good as it could be.
Then you feel a presence nearby. Hostility. Hunger. A set of feral, covetous eyes in the nearby jungle. A reptilian beast stalks you, and its all-encompassing sweet tooth desires your cake.
Wait, hold on, what?
As an unfortunate 3-year-old on vacation in Costa Rica found out, there’s at least one iguana in the world out there with a taste for sugar (better than a taste for blood, we suppose).
While out on the beach, the lizard darted out of nowhere, bit the girl on the back of the hand, and stole her cake. Still not the worst party guest ever. The child was taken to a local clinic, where the wound was cleaned and a 5-day antibiotic treatment (lizards carry salmonella) was provided. Things seemed fine, and the girl returned home without incident.
But of course, that’s not the end of the story. Five months later, the girl’s parents noticed a red bump at the wound site. Over the next 3 months, the surrounding skin grew red and painful. A trip to the hospital in California revealed that she had a ganglion cyst and a discharge of pus. Turns out our cake-obsessed lizard friend did give the little girl a gift: the first known human case of Mycobacterium marinum infection following an iguana bite on record.
M. marinum, which causes a disease similar to tuberculosis, typically infects fish but can infect humans if skin wounds are exposed to contaminated water. It’s also resistant to most antibiotics, which is why the first round didn’t clear up the infection. A second round of more-potent antibiotics seems to be working well.
So, to sum up, this poor child got bitten by a lizard, had her cake stolen, and contracted a rare illness in exchange. For a 3-year-old, that’s gotta be in the top-10 worst days ever. Unless, of course, we’re actually living in the Marvel universe (sorry, multiverse at this point). Then we’re totally going to see the emergence of the new superhero Iguana Girl in 15 years or so. Keep your eyes open.
No allergies? Let them give up cake
Allergy season is already here – starting earlier every year, it seems – and many people are not happy about it. So unhappy, actually, that there’s a list of things they would be willing to give up for a year to get rid of their of allergies, according to a survey conducted by OnePoll on behalf of Flonase.
Nearly 40% of 2,000 respondents with allergies would go a year without eating cake or chocolate or playing video games in exchange for allergy-free status, the survey results show. Almost as many would forgo coffee (38%) or pizza (37%) for a year, while 36% would stay off social media and 31% would take a pay cut or give up their smartphones, the Independent reported.
More than half of the allergic Americans – 54%, to be exact – who were polled this past winter – Feb. 24 to March 1, to be exact – consider allergy symptoms to be the most frustrating part of the spring. Annoying things that were less frustrating to the group included mosquitoes (41%), filing tax returns (38%), and daylight savings time (37%).
The Trump arraignment circus, of course, occurred too late to make the list, as did the big “We’re going back to the office! No wait, we’re closing the office forever!” email extravaganza and emotional roller coaster. That second one, however, did not get nearly as much media coverage.
A whole new, tiny level of hangry
Ever been so hungry that everything just got on your nerves? Maybe you feel a little snappy right now? Like you’ll just lash out unless you get something to eat? Been there. And so have bacteria.
New research shows that some bacteria go into a full-on Hulk smash if they’re not getting the nutrients they need by releasing toxins into the body. Sounds like a bacterial temper tantrum.
Even though two cells may be genetically identical, they don’t always behave the same in a bacterial community. Some do their job and stay in line, but some evil twins rage out and make people sick by releasing toxins into the environment, Adam Rosenthal, PhD, of the University of North Carolina and his colleagues discovered.
To figure out why some cells were all business as usual while others were not, the investigators looked at Clostridium perfringens, a bacterium found in the intestines of humans and other vertebrates. When the C. perfringens cells were fed a little acetate to munch on, the hangry cells calmed down faster than a kid with a bag of fruit snacks, reducing toxin levels. Some cells even disappeared, falling in line with their model-citizen counterparts.
So what does this really mean? More research, duh. Now that we know nutrients play a role in toxicity, it may open the door to finding a way to fight against antibiotic resistance in humans and reduce antibiotic use in the food industry.
So think to yourself. Are you bothered for no reason? Getting a little testy with your friends and coworkers? Maybe you just haven’t eaten in a while. You’re literally not alone. Even a single-cell organism can behave based on its hunger levels.
Now go have a snack. Your bacteria are getting restless.
The very hangry iguana?
Imagine yourself on a warm, sunny tropical beach. You are enjoying a piece of cake as you take in the slow beat of the waves lapping against the shore. Life is as good as it could be.
Then you feel a presence nearby. Hostility. Hunger. A set of feral, covetous eyes in the nearby jungle. A reptilian beast stalks you, and its all-encompassing sweet tooth desires your cake.
Wait, hold on, what?
As an unfortunate 3-year-old on vacation in Costa Rica found out, there’s at least one iguana in the world out there with a taste for sugar (better than a taste for blood, we suppose).
While out on the beach, the lizard darted out of nowhere, bit the girl on the back of the hand, and stole her cake. Still not the worst party guest ever. The child was taken to a local clinic, where the wound was cleaned and a 5-day antibiotic treatment (lizards carry salmonella) was provided. Things seemed fine, and the girl returned home without incident.
But of course, that’s not the end of the story. Five months later, the girl’s parents noticed a red bump at the wound site. Over the next 3 months, the surrounding skin grew red and painful. A trip to the hospital in California revealed that she had a ganglion cyst and a discharge of pus. Turns out our cake-obsessed lizard friend did give the little girl a gift: the first known human case of Mycobacterium marinum infection following an iguana bite on record.
M. marinum, which causes a disease similar to tuberculosis, typically infects fish but can infect humans if skin wounds are exposed to contaminated water. It’s also resistant to most antibiotics, which is why the first round didn’t clear up the infection. A second round of more-potent antibiotics seems to be working well.
So, to sum up, this poor child got bitten by a lizard, had her cake stolen, and contracted a rare illness in exchange. For a 3-year-old, that’s gotta be in the top-10 worst days ever. Unless, of course, we’re actually living in the Marvel universe (sorry, multiverse at this point). Then we’re totally going to see the emergence of the new superhero Iguana Girl in 15 years or so. Keep your eyes open.
No allergies? Let them give up cake
Allergy season is already here – starting earlier every year, it seems – and many people are not happy about it. So unhappy, actually, that there’s a list of things they would be willing to give up for a year to get rid of their of allergies, according to a survey conducted by OnePoll on behalf of Flonase.
Nearly 40% of 2,000 respondents with allergies would go a year without eating cake or chocolate or playing video games in exchange for allergy-free status, the survey results show. Almost as many would forgo coffee (38%) or pizza (37%) for a year, while 36% would stay off social media and 31% would take a pay cut or give up their smartphones, the Independent reported.
More than half of the allergic Americans – 54%, to be exact – who were polled this past winter – Feb. 24 to March 1, to be exact – consider allergy symptoms to be the most frustrating part of the spring. Annoying things that were less frustrating to the group included mosquitoes (41%), filing tax returns (38%), and daylight savings time (37%).
The Trump arraignment circus, of course, occurred too late to make the list, as did the big “We’re going back to the office! No wait, we’re closing the office forever!” email extravaganza and emotional roller coaster. That second one, however, did not get nearly as much media coverage.
A whole new, tiny level of hangry
Ever been so hungry that everything just got on your nerves? Maybe you feel a little snappy right now? Like you’ll just lash out unless you get something to eat? Been there. And so have bacteria.
New research shows that some bacteria go into a full-on Hulk smash if they’re not getting the nutrients they need by releasing toxins into the body. Sounds like a bacterial temper tantrum.
Even though two cells may be genetically identical, they don’t always behave the same in a bacterial community. Some do their job and stay in line, but some evil twins rage out and make people sick by releasing toxins into the environment, Adam Rosenthal, PhD, of the University of North Carolina and his colleagues discovered.
To figure out why some cells were all business as usual while others were not, the investigators looked at Clostridium perfringens, a bacterium found in the intestines of humans and other vertebrates. When the C. perfringens cells were fed a little acetate to munch on, the hangry cells calmed down faster than a kid with a bag of fruit snacks, reducing toxin levels. Some cells even disappeared, falling in line with their model-citizen counterparts.
So what does this really mean? More research, duh. Now that we know nutrients play a role in toxicity, it may open the door to finding a way to fight against antibiotic resistance in humans and reduce antibiotic use in the food industry.
So think to yourself. Are you bothered for no reason? Getting a little testy with your friends and coworkers? Maybe you just haven’t eaten in a while. You’re literally not alone. Even a single-cell organism can behave based on its hunger levels.
Now go have a snack. Your bacteria are getting restless.
The very hangry iguana?
Imagine yourself on a warm, sunny tropical beach. You are enjoying a piece of cake as you take in the slow beat of the waves lapping against the shore. Life is as good as it could be.
Then you feel a presence nearby. Hostility. Hunger. A set of feral, covetous eyes in the nearby jungle. A reptilian beast stalks you, and its all-encompassing sweet tooth desires your cake.
Wait, hold on, what?
As an unfortunate 3-year-old on vacation in Costa Rica found out, there’s at least one iguana in the world out there with a taste for sugar (better than a taste for blood, we suppose).
While out on the beach, the lizard darted out of nowhere, bit the girl on the back of the hand, and stole her cake. Still not the worst party guest ever. The child was taken to a local clinic, where the wound was cleaned and a 5-day antibiotic treatment (lizards carry salmonella) was provided. Things seemed fine, and the girl returned home without incident.
But of course, that’s not the end of the story. Five months later, the girl’s parents noticed a red bump at the wound site. Over the next 3 months, the surrounding skin grew red and painful. A trip to the hospital in California revealed that she had a ganglion cyst and a discharge of pus. Turns out our cake-obsessed lizard friend did give the little girl a gift: the first known human case of Mycobacterium marinum infection following an iguana bite on record.
M. marinum, which causes a disease similar to tuberculosis, typically infects fish but can infect humans if skin wounds are exposed to contaminated water. It’s also resistant to most antibiotics, which is why the first round didn’t clear up the infection. A second round of more-potent antibiotics seems to be working well.
So, to sum up, this poor child got bitten by a lizard, had her cake stolen, and contracted a rare illness in exchange. For a 3-year-old, that’s gotta be in the top-10 worst days ever. Unless, of course, we’re actually living in the Marvel universe (sorry, multiverse at this point). Then we’re totally going to see the emergence of the new superhero Iguana Girl in 15 years or so. Keep your eyes open.
No allergies? Let them give up cake
Allergy season is already here – starting earlier every year, it seems – and many people are not happy about it. So unhappy, actually, that there’s a list of things they would be willing to give up for a year to get rid of their of allergies, according to a survey conducted by OnePoll on behalf of Flonase.
Nearly 40% of 2,000 respondents with allergies would go a year without eating cake or chocolate or playing video games in exchange for allergy-free status, the survey results show. Almost as many would forgo coffee (38%) or pizza (37%) for a year, while 36% would stay off social media and 31% would take a pay cut or give up their smartphones, the Independent reported.
More than half of the allergic Americans – 54%, to be exact – who were polled this past winter – Feb. 24 to March 1, to be exact – consider allergy symptoms to be the most frustrating part of the spring. Annoying things that were less frustrating to the group included mosquitoes (41%), filing tax returns (38%), and daylight savings time (37%).
The Trump arraignment circus, of course, occurred too late to make the list, as did the big “We’re going back to the office! No wait, we’re closing the office forever!” email extravaganza and emotional roller coaster. That second one, however, did not get nearly as much media coverage.
SARS-CoV-2 crosses placenta and infects brains of two infants: ‘This is a first’
, according to a study published online today in Pediatrics .
One of the infants died at 13 months and the other remained in hospice care at time of manuscript submission.
Lead author Merline Benny, MD, with the division of neonatology, department of pediatrics at University of Miami, and colleagues briefed reporters today ahead of the release.
“This is a first,” said senior author Shahnaz Duara, MD, medical director of the Neonatal Intensive Care Unit at Holtz Children’s Hospital, Miami, explaining it is the first study to confirm cross-placental SARS-CoV-2 transmission leading to brain injury in a newborn.
Both infants negative for the virus at birth
The two infants were admitted in the early days of the pandemic in the Delta wave to the neonatal ICU at Holtz Children’s Hospital at University of Miami/Jackson Memorial Medical Center.
Both infants tested negative for the virus at birth, but had significantly elevated SARS-CoV-2 antibodies in their blood, indicating that either antibodies crossed the placenta, or the virus crossed and the immune response was the baby’s.
Dr. Benny explained that the researchers have seen, to this point, more than 700 mother/infant pairs in whom the mother tested positive for COVID in Jackson hospital.
Most who tested positive for COVID were asymptomatic and most of the mothers and infants left the hospital without complications.
“However, (these) two babies had a very unusual clinical picture,” Dr. Benny said.
Those infants were born to mothers who became COVID positive in the second trimester and delivered a few weeks later.
Seizures started on day 1 of life
The babies began to seize from the first day of life. They had profound low tone (hypotonia) in their clinical exam, Dr. Benny explained.
“We had absolutely no good explanation for the early seizures and the degree of brain injury we saw,” Dr. Duara said.
Dr. Benny said that as their bodies grew, they had very small head circumference. Unlike some babies born with the Zika virus, these babies were not microcephalic at birth. Brain imaging on the two babies indicated significant brain atrophy, and neurodevelopment exams showed significant delay.
Discussions began with the center’s multidisciplinary team including neurologists, pathologists, neuroradiologists, and obstetricians who cared for both the mothers and the babies.
The experts examined the placentas and found some characteristic COVID changes and presence of the COVID virus. This was accompanied by increased markers for inflammation and a severe reduction in a hormone critical for placental health and brain development.
Examining the infant’s autopsy findings further raised suspicions of maternal transmission, something that had not been documented before.
Coauthor Ali G. Saad, MD, pediatric and perinatal pathology director at Miami, said, “I have seen literally thousands of brains in autopsies over the last 14 years, and this was the most dramatic case of leukoencephalopathy or loss of white matter in a patient with no significant reason. That’s what triggered the investigation.”
Mothers had very different presentations
Coauthor Michael J. Paidas, MD, with the department of obstetrics, gynecology, and reproductive sciences at Miami, pointed out that the circumstances of the two mothers, who were in their 20s, were very different.
One mother delivered at 32 weeks and had a very severe COVID presentation and spent a month in the intensive care unit. The team decided to deliver the child to save the mother, Dr. Paidas said.
In contrast, the other mother had asymptomatic COVID infection in the second trimester and delivered at full term.
He said one of the early suspicions in the babies’ presentations was hypoxic ischemic encephalopathy. “But it wasn’t lack of blood flow to the placenta that caused this,” he said. “As best we can tell, it was the viral infection.”
Instances are rare
The researchers emphasized that these instances are rare and have not been seen before or since the period of this study to their knowledge.
Dr. Duara said, “This is something we want to alert the medical community to more than the general public. We do not want the lay public to be panicked. We’re trying to understand what made these two pregnancies different, so we can direct research towards protecting vulnerable babies.”
Previous data have indicated a relatively benign status in infants who test negative for the COVID virus after birth. Dr. Benny added that COVID vaccination has been found safe in pregnancy and both vaccination and breastfeeding can help passage of antibodies to the infant and help protect the baby. Because these cases happened in the early days of the pandemic, no vaccines were available.
Dr. Paidas received funding from BioIncept to study hypoxic-ischemic encephalopathy with Preimplantation Factor, is a scientific advisory board member, and has stock options. Dr. Paidas and coauthor Dr. Jayakumar are coinventors of SPIKENET, University of Miami, patent pending 2023. The other authors have no conflicts of interest to disclose.
, according to a study published online today in Pediatrics .
One of the infants died at 13 months and the other remained in hospice care at time of manuscript submission.
Lead author Merline Benny, MD, with the division of neonatology, department of pediatrics at University of Miami, and colleagues briefed reporters today ahead of the release.
“This is a first,” said senior author Shahnaz Duara, MD, medical director of the Neonatal Intensive Care Unit at Holtz Children’s Hospital, Miami, explaining it is the first study to confirm cross-placental SARS-CoV-2 transmission leading to brain injury in a newborn.
Both infants negative for the virus at birth
The two infants were admitted in the early days of the pandemic in the Delta wave to the neonatal ICU at Holtz Children’s Hospital at University of Miami/Jackson Memorial Medical Center.
Both infants tested negative for the virus at birth, but had significantly elevated SARS-CoV-2 antibodies in their blood, indicating that either antibodies crossed the placenta, or the virus crossed and the immune response was the baby’s.
Dr. Benny explained that the researchers have seen, to this point, more than 700 mother/infant pairs in whom the mother tested positive for COVID in Jackson hospital.
Most who tested positive for COVID were asymptomatic and most of the mothers and infants left the hospital without complications.
“However, (these) two babies had a very unusual clinical picture,” Dr. Benny said.
Those infants were born to mothers who became COVID positive in the second trimester and delivered a few weeks later.
Seizures started on day 1 of life
The babies began to seize from the first day of life. They had profound low tone (hypotonia) in their clinical exam, Dr. Benny explained.
“We had absolutely no good explanation for the early seizures and the degree of brain injury we saw,” Dr. Duara said.
Dr. Benny said that as their bodies grew, they had very small head circumference. Unlike some babies born with the Zika virus, these babies were not microcephalic at birth. Brain imaging on the two babies indicated significant brain atrophy, and neurodevelopment exams showed significant delay.
Discussions began with the center’s multidisciplinary team including neurologists, pathologists, neuroradiologists, and obstetricians who cared for both the mothers and the babies.
The experts examined the placentas and found some characteristic COVID changes and presence of the COVID virus. This was accompanied by increased markers for inflammation and a severe reduction in a hormone critical for placental health and brain development.
Examining the infant’s autopsy findings further raised suspicions of maternal transmission, something that had not been documented before.
Coauthor Ali G. Saad, MD, pediatric and perinatal pathology director at Miami, said, “I have seen literally thousands of brains in autopsies over the last 14 years, and this was the most dramatic case of leukoencephalopathy or loss of white matter in a patient with no significant reason. That’s what triggered the investigation.”
Mothers had very different presentations
Coauthor Michael J. Paidas, MD, with the department of obstetrics, gynecology, and reproductive sciences at Miami, pointed out that the circumstances of the two mothers, who were in their 20s, were very different.
One mother delivered at 32 weeks and had a very severe COVID presentation and spent a month in the intensive care unit. The team decided to deliver the child to save the mother, Dr. Paidas said.
In contrast, the other mother had asymptomatic COVID infection in the second trimester and delivered at full term.
He said one of the early suspicions in the babies’ presentations was hypoxic ischemic encephalopathy. “But it wasn’t lack of blood flow to the placenta that caused this,” he said. “As best we can tell, it was the viral infection.”
Instances are rare
The researchers emphasized that these instances are rare and have not been seen before or since the period of this study to their knowledge.
Dr. Duara said, “This is something we want to alert the medical community to more than the general public. We do not want the lay public to be panicked. We’re trying to understand what made these two pregnancies different, so we can direct research towards protecting vulnerable babies.”
Previous data have indicated a relatively benign status in infants who test negative for the COVID virus after birth. Dr. Benny added that COVID vaccination has been found safe in pregnancy and both vaccination and breastfeeding can help passage of antibodies to the infant and help protect the baby. Because these cases happened in the early days of the pandemic, no vaccines were available.
Dr. Paidas received funding from BioIncept to study hypoxic-ischemic encephalopathy with Preimplantation Factor, is a scientific advisory board member, and has stock options. Dr. Paidas and coauthor Dr. Jayakumar are coinventors of SPIKENET, University of Miami, patent pending 2023. The other authors have no conflicts of interest to disclose.
, according to a study published online today in Pediatrics .
One of the infants died at 13 months and the other remained in hospice care at time of manuscript submission.
Lead author Merline Benny, MD, with the division of neonatology, department of pediatrics at University of Miami, and colleagues briefed reporters today ahead of the release.
“This is a first,” said senior author Shahnaz Duara, MD, medical director of the Neonatal Intensive Care Unit at Holtz Children’s Hospital, Miami, explaining it is the first study to confirm cross-placental SARS-CoV-2 transmission leading to brain injury in a newborn.
Both infants negative for the virus at birth
The two infants were admitted in the early days of the pandemic in the Delta wave to the neonatal ICU at Holtz Children’s Hospital at University of Miami/Jackson Memorial Medical Center.
Both infants tested negative for the virus at birth, but had significantly elevated SARS-CoV-2 antibodies in their blood, indicating that either antibodies crossed the placenta, or the virus crossed and the immune response was the baby’s.
Dr. Benny explained that the researchers have seen, to this point, more than 700 mother/infant pairs in whom the mother tested positive for COVID in Jackson hospital.
Most who tested positive for COVID were asymptomatic and most of the mothers and infants left the hospital without complications.
“However, (these) two babies had a very unusual clinical picture,” Dr. Benny said.
Those infants were born to mothers who became COVID positive in the second trimester and delivered a few weeks later.
Seizures started on day 1 of life
The babies began to seize from the first day of life. They had profound low tone (hypotonia) in their clinical exam, Dr. Benny explained.
“We had absolutely no good explanation for the early seizures and the degree of brain injury we saw,” Dr. Duara said.
Dr. Benny said that as their bodies grew, they had very small head circumference. Unlike some babies born with the Zika virus, these babies were not microcephalic at birth. Brain imaging on the two babies indicated significant brain atrophy, and neurodevelopment exams showed significant delay.
Discussions began with the center’s multidisciplinary team including neurologists, pathologists, neuroradiologists, and obstetricians who cared for both the mothers and the babies.
The experts examined the placentas and found some characteristic COVID changes and presence of the COVID virus. This was accompanied by increased markers for inflammation and a severe reduction in a hormone critical for placental health and brain development.
Examining the infant’s autopsy findings further raised suspicions of maternal transmission, something that had not been documented before.
Coauthor Ali G. Saad, MD, pediatric and perinatal pathology director at Miami, said, “I have seen literally thousands of brains in autopsies over the last 14 years, and this was the most dramatic case of leukoencephalopathy or loss of white matter in a patient with no significant reason. That’s what triggered the investigation.”
Mothers had very different presentations
Coauthor Michael J. Paidas, MD, with the department of obstetrics, gynecology, and reproductive sciences at Miami, pointed out that the circumstances of the two mothers, who were in their 20s, were very different.
One mother delivered at 32 weeks and had a very severe COVID presentation and spent a month in the intensive care unit. The team decided to deliver the child to save the mother, Dr. Paidas said.
In contrast, the other mother had asymptomatic COVID infection in the second trimester and delivered at full term.
He said one of the early suspicions in the babies’ presentations was hypoxic ischemic encephalopathy. “But it wasn’t lack of blood flow to the placenta that caused this,” he said. “As best we can tell, it was the viral infection.”
Instances are rare
The researchers emphasized that these instances are rare and have not been seen before or since the period of this study to their knowledge.
Dr. Duara said, “This is something we want to alert the medical community to more than the general public. We do not want the lay public to be panicked. We’re trying to understand what made these two pregnancies different, so we can direct research towards protecting vulnerable babies.”
Previous data have indicated a relatively benign status in infants who test negative for the COVID virus after birth. Dr. Benny added that COVID vaccination has been found safe in pregnancy and both vaccination and breastfeeding can help passage of antibodies to the infant and help protect the baby. Because these cases happened in the early days of the pandemic, no vaccines were available.
Dr. Paidas received funding from BioIncept to study hypoxic-ischemic encephalopathy with Preimplantation Factor, is a scientific advisory board member, and has stock options. Dr. Paidas and coauthor Dr. Jayakumar are coinventors of SPIKENET, University of Miami, patent pending 2023. The other authors have no conflicts of interest to disclose.
FROM PEDIATRICS
New update on left atrial appendage closure recommendations
An updated consensus statement on transcatheter left atrial appendage closure (LAAC) has put a newfound focus on patient selection for the procedure, specifically recommending that the procedure is appropriate for patients with nonvalvular atrial fibrillation who have risk for thromboembolism, aren’t well suited for direct oral anticoagulants (DOACs) and have a good chance of living for at least another year.
The statement, published online in the Journal of the Society for Cardiovascular Angiography & Interventions, also makes recommendations for how much experience operators should have, how many procedures they should perform to keep their skills up, and when and how to use imaging and prescribe DOACs, among other suggestions.
The statement represents the first updated guidance for LAAC since 2015. “Since then this field has really expanded and evolved,” writing group chair Jacqueline Saw, MD, said in an interview. “For instance, the indications are more matured and specific, and the procedural technical steps have matured. Imaging has also advanced, there’s more understanding about postprocedural care and there are also new devices that have been approved.”
Dr. Saw, an interventional cardiologist at Vancouver General Hospital and St. Paul’s Hospital, and a professor at the University of British Columbia in Vancouver, called the statement “a piece that puts everything together.”
“This document really summarizes the whole practice for doing transcatheter procedures,” she added, “so it’s all-in-one document in terms of recommendation of who we do the procedure for, how we should do it, how we should image and guide the procedure, and what complications to look out for and how to manage patients post procedure, be it with antithrombotic therapy and/or device surveillance.”
13 recommendations
In all, the statement carries 13 recommendations for LAAC. The Society for Cardiovascular Angiography & Interventions and the Heart Rhythm Society commissioned the writing group. The American College of Cardiology and Society of Cardiovascular Computed Tomography have endorsed the statement. The following are among the recommendations:
- Transcatheter LAAC is appropriate for patients with nonvalvular atrial fibrillation with high thromboembolic risk but for whom long-term oral anticoagulation may be contraindicated and who have at least 1 year’s life expectancy.
- Operators should have performed at least 50 prior left-sided ablations or structural procedures and at least 25 transseptal punctures (TSPs). Interventional-imaging physicians should have experience in guiding 25 or more TSPs before supporting LAAC procedures independently.
- To maintain skills, operators should do 25 or more TSPs and at least 12 LAACs over each 2-year period.
- On-site cardiovascular surgery backup should be available for new programs and for operators early in their learning curve.
- Baseline imaging with transesophageal echocardiography (TEE) or cardiac computed tomography should be performed before LAAC.
- Intraprocedural imaging guidance with TEE or intracardiac echocardiography.
- Follow labeling of each specific LAAC device for technical aspects of the procedure.
- Familiarity with avoiding, recognizing, and managing LAAC complications.
- Predischarge 2-dimensional TEE to rule out pericardial effusion and device embolization.
- Anticoagulation for device-related thrombus.
- Make all efforts to minimize peridevice leaks during implantation because their clinical impact and management isn’t well understood.
- Antithrombotic therapy with warfarin, DOAC, or dual-antiplatelet therapy after LAAC based on the studied regimen and instructions for each specific device, tailored to the bleeding risks for each patient.
- TEE or cardiac computed tomography at 45-90 days after LAAC for device surveillance to assess for peridevice leak and device-related thrombus.
The statement also includes precautionary recommendations. It advises against using routine closure of LAAC-associated iatrogenic atrial septal defects and states that combined procedures with LAAC, such as structural interventions and pulmonary vein isolation, should be avoided because randomized controlled trial data are pending.
“These recommendations are based upon data from updated publications and randomized trial data as well as large registries, including the National Cardiovascular Data Registry, so I think this is a very practical statement that puts all these pieces together for any budding interventionalist doing this procedure and even experienced operations,” Dr. Saw said.
Authors of an accompanying editorial agreed that the “rigorous standards” set out in the statement will help maintain “a high level of procedural safety in the setting of rapid expansion.”
The editorialists, Faisal M. Merchant, MD, of Emory University, Atlanta, and Mohamad Alkhouli, MD, professor of medicine at Mayo Clinic School of Medicine, Rochester, Minn., point out that the incidence of pericardial effusion has decreased from more than 5% in the pivotal Watchman trials to less than 1.5% in the most recent report from the National Cardiovascular Data Registry, which shows that more than 100,000 procedures have been performed in the United States.
But most important as the field moves forward, they stress, is patient selection. The recommendation of limiting patients to those with a life expectancy of 1 year “is a tacit recognition of the fact that the benefits of LAAC take time to accrue, and many older and frail patients are unlikely to derive meaningful benefit.”
Dr. Merchant and Dr. Alkhouli also note that there remains a conundrum in patient selection that remains from the original LAAC trials, which enrolled patients who were eligible for anticoagulation. “Somewhat paradoxically, after its approval, LAAC is mostly prescribed to patients who are not felt to be good anticoagulation candidates.” This leaves physicians “in the precarious position of extrapolating data to patients who were excluded from the original clinical trials.”
Therefore, the consensus statement “is right to put patient selection front and center in its recommendations, but as the field of LAAC comes of age, better evidence to support patient selection will be the real sign of maturity.”
Dr. Saw said she envisions another update over the next 2 years or so as ongoing clinical trials comparing DOAC and LAAC, namely the CHAMPION-AF and OPTION trials, report results.
Dr. Saw and Dr. Merchant, reported no conflicts of interest. Dr. Alkhouli has financial ties to Boston Scientific, Abbott, and Philips.
An updated consensus statement on transcatheter left atrial appendage closure (LAAC) has put a newfound focus on patient selection for the procedure, specifically recommending that the procedure is appropriate for patients with nonvalvular atrial fibrillation who have risk for thromboembolism, aren’t well suited for direct oral anticoagulants (DOACs) and have a good chance of living for at least another year.
The statement, published online in the Journal of the Society for Cardiovascular Angiography & Interventions, also makes recommendations for how much experience operators should have, how many procedures they should perform to keep their skills up, and when and how to use imaging and prescribe DOACs, among other suggestions.
The statement represents the first updated guidance for LAAC since 2015. “Since then this field has really expanded and evolved,” writing group chair Jacqueline Saw, MD, said in an interview. “For instance, the indications are more matured and specific, and the procedural technical steps have matured. Imaging has also advanced, there’s more understanding about postprocedural care and there are also new devices that have been approved.”
Dr. Saw, an interventional cardiologist at Vancouver General Hospital and St. Paul’s Hospital, and a professor at the University of British Columbia in Vancouver, called the statement “a piece that puts everything together.”
“This document really summarizes the whole practice for doing transcatheter procedures,” she added, “so it’s all-in-one document in terms of recommendation of who we do the procedure for, how we should do it, how we should image and guide the procedure, and what complications to look out for and how to manage patients post procedure, be it with antithrombotic therapy and/or device surveillance.”
13 recommendations
In all, the statement carries 13 recommendations for LAAC. The Society for Cardiovascular Angiography & Interventions and the Heart Rhythm Society commissioned the writing group. The American College of Cardiology and Society of Cardiovascular Computed Tomography have endorsed the statement. The following are among the recommendations:
- Transcatheter LAAC is appropriate for patients with nonvalvular atrial fibrillation with high thromboembolic risk but for whom long-term oral anticoagulation may be contraindicated and who have at least 1 year’s life expectancy.
- Operators should have performed at least 50 prior left-sided ablations or structural procedures and at least 25 transseptal punctures (TSPs). Interventional-imaging physicians should have experience in guiding 25 or more TSPs before supporting LAAC procedures independently.
- To maintain skills, operators should do 25 or more TSPs and at least 12 LAACs over each 2-year period.
- On-site cardiovascular surgery backup should be available for new programs and for operators early in their learning curve.
- Baseline imaging with transesophageal echocardiography (TEE) or cardiac computed tomography should be performed before LAAC.
- Intraprocedural imaging guidance with TEE or intracardiac echocardiography.
- Follow labeling of each specific LAAC device for technical aspects of the procedure.
- Familiarity with avoiding, recognizing, and managing LAAC complications.
- Predischarge 2-dimensional TEE to rule out pericardial effusion and device embolization.
- Anticoagulation for device-related thrombus.
- Make all efforts to minimize peridevice leaks during implantation because their clinical impact and management isn’t well understood.
- Antithrombotic therapy with warfarin, DOAC, or dual-antiplatelet therapy after LAAC based on the studied regimen and instructions for each specific device, tailored to the bleeding risks for each patient.
- TEE or cardiac computed tomography at 45-90 days after LAAC for device surveillance to assess for peridevice leak and device-related thrombus.
The statement also includes precautionary recommendations. It advises against using routine closure of LAAC-associated iatrogenic atrial septal defects and states that combined procedures with LAAC, such as structural interventions and pulmonary vein isolation, should be avoided because randomized controlled trial data are pending.
“These recommendations are based upon data from updated publications and randomized trial data as well as large registries, including the National Cardiovascular Data Registry, so I think this is a very practical statement that puts all these pieces together for any budding interventionalist doing this procedure and even experienced operations,” Dr. Saw said.
Authors of an accompanying editorial agreed that the “rigorous standards” set out in the statement will help maintain “a high level of procedural safety in the setting of rapid expansion.”
The editorialists, Faisal M. Merchant, MD, of Emory University, Atlanta, and Mohamad Alkhouli, MD, professor of medicine at Mayo Clinic School of Medicine, Rochester, Minn., point out that the incidence of pericardial effusion has decreased from more than 5% in the pivotal Watchman trials to less than 1.5% in the most recent report from the National Cardiovascular Data Registry, which shows that more than 100,000 procedures have been performed in the United States.
But most important as the field moves forward, they stress, is patient selection. The recommendation of limiting patients to those with a life expectancy of 1 year “is a tacit recognition of the fact that the benefits of LAAC take time to accrue, and many older and frail patients are unlikely to derive meaningful benefit.”
Dr. Merchant and Dr. Alkhouli also note that there remains a conundrum in patient selection that remains from the original LAAC trials, which enrolled patients who were eligible for anticoagulation. “Somewhat paradoxically, after its approval, LAAC is mostly prescribed to patients who are not felt to be good anticoagulation candidates.” This leaves physicians “in the precarious position of extrapolating data to patients who were excluded from the original clinical trials.”
Therefore, the consensus statement “is right to put patient selection front and center in its recommendations, but as the field of LAAC comes of age, better evidence to support patient selection will be the real sign of maturity.”
Dr. Saw said she envisions another update over the next 2 years or so as ongoing clinical trials comparing DOAC and LAAC, namely the CHAMPION-AF and OPTION trials, report results.
Dr. Saw and Dr. Merchant, reported no conflicts of interest. Dr. Alkhouli has financial ties to Boston Scientific, Abbott, and Philips.
An updated consensus statement on transcatheter left atrial appendage closure (LAAC) has put a newfound focus on patient selection for the procedure, specifically recommending that the procedure is appropriate for patients with nonvalvular atrial fibrillation who have risk for thromboembolism, aren’t well suited for direct oral anticoagulants (DOACs) and have a good chance of living for at least another year.
The statement, published online in the Journal of the Society for Cardiovascular Angiography & Interventions, also makes recommendations for how much experience operators should have, how many procedures they should perform to keep their skills up, and when and how to use imaging and prescribe DOACs, among other suggestions.
The statement represents the first updated guidance for LAAC since 2015. “Since then this field has really expanded and evolved,” writing group chair Jacqueline Saw, MD, said in an interview. “For instance, the indications are more matured and specific, and the procedural technical steps have matured. Imaging has also advanced, there’s more understanding about postprocedural care and there are also new devices that have been approved.”
Dr. Saw, an interventional cardiologist at Vancouver General Hospital and St. Paul’s Hospital, and a professor at the University of British Columbia in Vancouver, called the statement “a piece that puts everything together.”
“This document really summarizes the whole practice for doing transcatheter procedures,” she added, “so it’s all-in-one document in terms of recommendation of who we do the procedure for, how we should do it, how we should image and guide the procedure, and what complications to look out for and how to manage patients post procedure, be it with antithrombotic therapy and/or device surveillance.”
13 recommendations
In all, the statement carries 13 recommendations for LAAC. The Society for Cardiovascular Angiography & Interventions and the Heart Rhythm Society commissioned the writing group. The American College of Cardiology and Society of Cardiovascular Computed Tomography have endorsed the statement. The following are among the recommendations:
- Transcatheter LAAC is appropriate for patients with nonvalvular atrial fibrillation with high thromboembolic risk but for whom long-term oral anticoagulation may be contraindicated and who have at least 1 year’s life expectancy.
- Operators should have performed at least 50 prior left-sided ablations or structural procedures and at least 25 transseptal punctures (TSPs). Interventional-imaging physicians should have experience in guiding 25 or more TSPs before supporting LAAC procedures independently.
- To maintain skills, operators should do 25 or more TSPs and at least 12 LAACs over each 2-year period.
- On-site cardiovascular surgery backup should be available for new programs and for operators early in their learning curve.
- Baseline imaging with transesophageal echocardiography (TEE) or cardiac computed tomography should be performed before LAAC.
- Intraprocedural imaging guidance with TEE or intracardiac echocardiography.
- Follow labeling of each specific LAAC device for technical aspects of the procedure.
- Familiarity with avoiding, recognizing, and managing LAAC complications.
- Predischarge 2-dimensional TEE to rule out pericardial effusion and device embolization.
- Anticoagulation for device-related thrombus.
- Make all efforts to minimize peridevice leaks during implantation because their clinical impact and management isn’t well understood.
- Antithrombotic therapy with warfarin, DOAC, or dual-antiplatelet therapy after LAAC based on the studied regimen and instructions for each specific device, tailored to the bleeding risks for each patient.
- TEE or cardiac computed tomography at 45-90 days after LAAC for device surveillance to assess for peridevice leak and device-related thrombus.
The statement also includes precautionary recommendations. It advises against using routine closure of LAAC-associated iatrogenic atrial septal defects and states that combined procedures with LAAC, such as structural interventions and pulmonary vein isolation, should be avoided because randomized controlled trial data are pending.
“These recommendations are based upon data from updated publications and randomized trial data as well as large registries, including the National Cardiovascular Data Registry, so I think this is a very practical statement that puts all these pieces together for any budding interventionalist doing this procedure and even experienced operations,” Dr. Saw said.
Authors of an accompanying editorial agreed that the “rigorous standards” set out in the statement will help maintain “a high level of procedural safety in the setting of rapid expansion.”
The editorialists, Faisal M. Merchant, MD, of Emory University, Atlanta, and Mohamad Alkhouli, MD, professor of medicine at Mayo Clinic School of Medicine, Rochester, Minn., point out that the incidence of pericardial effusion has decreased from more than 5% in the pivotal Watchman trials to less than 1.5% in the most recent report from the National Cardiovascular Data Registry, which shows that more than 100,000 procedures have been performed in the United States.
But most important as the field moves forward, they stress, is patient selection. The recommendation of limiting patients to those with a life expectancy of 1 year “is a tacit recognition of the fact that the benefits of LAAC take time to accrue, and many older and frail patients are unlikely to derive meaningful benefit.”
Dr. Merchant and Dr. Alkhouli also note that there remains a conundrum in patient selection that remains from the original LAAC trials, which enrolled patients who were eligible for anticoagulation. “Somewhat paradoxically, after its approval, LAAC is mostly prescribed to patients who are not felt to be good anticoagulation candidates.” This leaves physicians “in the precarious position of extrapolating data to patients who were excluded from the original clinical trials.”
Therefore, the consensus statement “is right to put patient selection front and center in its recommendations, but as the field of LAAC comes of age, better evidence to support patient selection will be the real sign of maturity.”
Dr. Saw said she envisions another update over the next 2 years or so as ongoing clinical trials comparing DOAC and LAAC, namely the CHAMPION-AF and OPTION trials, report results.
Dr. Saw and Dr. Merchant, reported no conflicts of interest. Dr. Alkhouli has financial ties to Boston Scientific, Abbott, and Philips.
FROM THE JOURNAL OF THE SOCIETY FOR CARDIOVASCULAR ANGIOGRAPHY & INTERVENTIONS
Kidney disease skews Alzheimer’s biomarker testing
New research provides more evidence that
In a cross-sectional study of adults with and those without cognitive impairment, chronic kidney disease was associated with increased plasma concentrations of phosphorylated tau (p-tau) 217 and 181.
However, there were no associations between chronic kidney disease and the ratio of p-tau217 to unphosphorylated tau 217 (pT217/T217), and the associations with p-tau181 to unphosphorylated tau 181 (pT181/T181) were attenuated in patients with cognitive impairment.
“These novel findings suggest that plasma measures of the phosphorylated to unphosphorylated tau ratios are more accurate than p-tau forms alone as they correlate less with individual difference in glomerular filtration rate or impaired kidney function,” reported an investigative team led by Oskar Hansson, MD, PhD, with Lund University in Sweden.
“Thus, to mitigate the effects of non-Alzheimer’s–related comorbidities like chronic kidney disease on the performance of plasma Alzheimer’s disease biomarkers, certain tau ratios, and specifically pT217/T217, should be considered for implementation in clinical practice and drug trials,” they added.
The study was published online in JAMA Neurology to coincide with a presentation at the International Conference on Alzheimer’s and Parkinson’s Diseases in Gothenburg, Sweden.
Skewed tau levels
Plasma biomarkers of amyloid-beta (Abeta) and tau pathologies, and in particular variants in p-tau217 and p-tau181, have shown promise for use in Alzheimer’s disease diagnosis and prognosis. However, previous reports have suggested that chronic kidney disease might influence plasma p-tau217 and p-tau181 concentrations, potentially decreasing their usefulness in the diagnostic workup of dementia.
Researchers investigated associations of chronic kidney disease with plasma ratios of p-tau217 and p-tau181 to the corresponding unphosphorylated peptides in Alzheimer’s disease.
The data came from 473 older adults, with and without cognitive impairment, from the Swedish BioFinder-2 study who had plasma tau assessments and chronic kidney disease status established within 6 months of plasma collection.
The researchers found that lower estimated glomerular filtration rate levels (indicative of kidney dysfunction) were associated with higher plasma concentrations of phosphorylated and unphosphorylated tau peptides measured simultaneously using the tau immunoprecipitation mass spectrometry assay.
However, the correlations with estimated glomerular filtration rate were nonsignificant for the pT217/T217 ratio in individuals with cognitive impairment and were significantly attenuated for pT217/T217 in cognitively unimpaired individuals and for pT181/T181 in both cognitively unimpaired and impaired individuals.
“Importantly, we demonstrate that there were no significant associations between chronic kidney disease and the pT217/T217 ratio and changes in plasma pT181/T181 associated with chronic kidney disease were small or nonsignificant,” the researchers noted.
“Our results indicate that by using p-tau/tau ratios we may be able to reduce the variability in plasma p-tau levels driven by impaired kidney function and consequently such ratios are more robust measures of brain p-tau pathology in individuals with both early- and later-stage Alzheimer’s disease,” they added.
The researchers believe this is likely true for the ratios of other related proteins – a view that is supported by findings of attenuated associations of chronic kidney disease with Abeta42/40, compared with Abeta42 and Abeta40 in the current study and in previous studies.
Important clinical implications
Reached for comment, Rebecca Edelmayer, PhD, Alzheimer’s Association senior director of scientific engagement, noted that research continues to indicate that blood biomarkers have “promise for improving, and possibly even redefining, the clinical workup for Alzheimer’s disease.
“Many of the Alzheimer’s blood tests in development today have focused on core blood biomarkers associated with amyloid accumulation and tau tangle formation in the brain,” Dr. Edelmayer said.
“Before these tests can be used more broadly in the clinic, we need to understand all of the variables that may impact the results of various blood biomarkers, including differences that may be driven by race, ethnicity, sex, and underlying health conditions, such as chronic kidney disease.
“This study corroborates other research suggesting that some Alzheimer’s-associated markers can be affected by chronic kidney disease, but by using ratios of amyloid or tau markers, we may be able to minimize these differences in results caused by underlying disease,” Dr. Edelmayer said.
Howard Fillit, MD, cofounder and chief science officer at the Alzheimer’s Drug Discovery Foundation, said, “Using these ratios makes a lot of sense because the ratios wouldn’t change with kidney function. I think this is an important advance in clinical utilization of these tests.”
Dr. Fillit noted that older people often have declining kidney function, which can be easily measured by glomerular filtration rate. Changes in blood levels of these markers with declining kidney function are “not unexpected, but [it’s] important that it’s been demonstrated.
“As we go forward, maybe the future utilization of these tests will be not only recording the ratios but also reporting the ratios in the context of somebody’s glomerular filtration rate. You could imagine a scenario where when the test is done, it’s automatically done alongside of glomerular filtration rate,” Dr. Fillit said in an interview.
The study was supported by Coins for Alzheimer’s Research Trust, the Tracy Family Stable Isotope Labeling Quantitation Center, and the National Institute of Neurological Disorders and Stroke. Dr. Hansson has received support to his institution from ADx, AVID Radiopharmaceuticals, Biogen, Eli Lilly, Eisai, Fujirebio, GE Healthcare, Pfizer, and Roche; consultancy/speaker fees from Amylyx, Alzpath, Biogen, Cerveau, Fujirebio, Genentech, Novartis, Roche, and Siemens; and personal fees from Eli Lilly, Eisai, Bioarctic, and Biogen outside the submitted work. Dr. Edelmayer and Dr. Fillit have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New research provides more evidence that
In a cross-sectional study of adults with and those without cognitive impairment, chronic kidney disease was associated with increased plasma concentrations of phosphorylated tau (p-tau) 217 and 181.
However, there were no associations between chronic kidney disease and the ratio of p-tau217 to unphosphorylated tau 217 (pT217/T217), and the associations with p-tau181 to unphosphorylated tau 181 (pT181/T181) were attenuated in patients with cognitive impairment.
“These novel findings suggest that plasma measures of the phosphorylated to unphosphorylated tau ratios are more accurate than p-tau forms alone as they correlate less with individual difference in glomerular filtration rate or impaired kidney function,” reported an investigative team led by Oskar Hansson, MD, PhD, with Lund University in Sweden.
“Thus, to mitigate the effects of non-Alzheimer’s–related comorbidities like chronic kidney disease on the performance of plasma Alzheimer’s disease biomarkers, certain tau ratios, and specifically pT217/T217, should be considered for implementation in clinical practice and drug trials,” they added.
The study was published online in JAMA Neurology to coincide with a presentation at the International Conference on Alzheimer’s and Parkinson’s Diseases in Gothenburg, Sweden.
Skewed tau levels
Plasma biomarkers of amyloid-beta (Abeta) and tau pathologies, and in particular variants in p-tau217 and p-tau181, have shown promise for use in Alzheimer’s disease diagnosis and prognosis. However, previous reports have suggested that chronic kidney disease might influence plasma p-tau217 and p-tau181 concentrations, potentially decreasing their usefulness in the diagnostic workup of dementia.
Researchers investigated associations of chronic kidney disease with plasma ratios of p-tau217 and p-tau181 to the corresponding unphosphorylated peptides in Alzheimer’s disease.
The data came from 473 older adults, with and without cognitive impairment, from the Swedish BioFinder-2 study who had plasma tau assessments and chronic kidney disease status established within 6 months of plasma collection.
The researchers found that lower estimated glomerular filtration rate levels (indicative of kidney dysfunction) were associated with higher plasma concentrations of phosphorylated and unphosphorylated tau peptides measured simultaneously using the tau immunoprecipitation mass spectrometry assay.
However, the correlations with estimated glomerular filtration rate were nonsignificant for the pT217/T217 ratio in individuals with cognitive impairment and were significantly attenuated for pT217/T217 in cognitively unimpaired individuals and for pT181/T181 in both cognitively unimpaired and impaired individuals.
“Importantly, we demonstrate that there were no significant associations between chronic kidney disease and the pT217/T217 ratio and changes in plasma pT181/T181 associated with chronic kidney disease were small or nonsignificant,” the researchers noted.
“Our results indicate that by using p-tau/tau ratios we may be able to reduce the variability in plasma p-tau levels driven by impaired kidney function and consequently such ratios are more robust measures of brain p-tau pathology in individuals with both early- and later-stage Alzheimer’s disease,” they added.
The researchers believe this is likely true for the ratios of other related proteins – a view that is supported by findings of attenuated associations of chronic kidney disease with Abeta42/40, compared with Abeta42 and Abeta40 in the current study and in previous studies.
Important clinical implications
Reached for comment, Rebecca Edelmayer, PhD, Alzheimer’s Association senior director of scientific engagement, noted that research continues to indicate that blood biomarkers have “promise for improving, and possibly even redefining, the clinical workup for Alzheimer’s disease.
“Many of the Alzheimer’s blood tests in development today have focused on core blood biomarkers associated with amyloid accumulation and tau tangle formation in the brain,” Dr. Edelmayer said.
“Before these tests can be used more broadly in the clinic, we need to understand all of the variables that may impact the results of various blood biomarkers, including differences that may be driven by race, ethnicity, sex, and underlying health conditions, such as chronic kidney disease.
“This study corroborates other research suggesting that some Alzheimer’s-associated markers can be affected by chronic kidney disease, but by using ratios of amyloid or tau markers, we may be able to minimize these differences in results caused by underlying disease,” Dr. Edelmayer said.
Howard Fillit, MD, cofounder and chief science officer at the Alzheimer’s Drug Discovery Foundation, said, “Using these ratios makes a lot of sense because the ratios wouldn’t change with kidney function. I think this is an important advance in clinical utilization of these tests.”
Dr. Fillit noted that older people often have declining kidney function, which can be easily measured by glomerular filtration rate. Changes in blood levels of these markers with declining kidney function are “not unexpected, but [it’s] important that it’s been demonstrated.
“As we go forward, maybe the future utilization of these tests will be not only recording the ratios but also reporting the ratios in the context of somebody’s glomerular filtration rate. You could imagine a scenario where when the test is done, it’s automatically done alongside of glomerular filtration rate,” Dr. Fillit said in an interview.
The study was supported by Coins for Alzheimer’s Research Trust, the Tracy Family Stable Isotope Labeling Quantitation Center, and the National Institute of Neurological Disorders and Stroke. Dr. Hansson has received support to his institution from ADx, AVID Radiopharmaceuticals, Biogen, Eli Lilly, Eisai, Fujirebio, GE Healthcare, Pfizer, and Roche; consultancy/speaker fees from Amylyx, Alzpath, Biogen, Cerveau, Fujirebio, Genentech, Novartis, Roche, and Siemens; and personal fees from Eli Lilly, Eisai, Bioarctic, and Biogen outside the submitted work. Dr. Edelmayer and Dr. Fillit have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New research provides more evidence that
In a cross-sectional study of adults with and those without cognitive impairment, chronic kidney disease was associated with increased plasma concentrations of phosphorylated tau (p-tau) 217 and 181.
However, there were no associations between chronic kidney disease and the ratio of p-tau217 to unphosphorylated tau 217 (pT217/T217), and the associations with p-tau181 to unphosphorylated tau 181 (pT181/T181) were attenuated in patients with cognitive impairment.
“These novel findings suggest that plasma measures of the phosphorylated to unphosphorylated tau ratios are more accurate than p-tau forms alone as they correlate less with individual difference in glomerular filtration rate or impaired kidney function,” reported an investigative team led by Oskar Hansson, MD, PhD, with Lund University in Sweden.
“Thus, to mitigate the effects of non-Alzheimer’s–related comorbidities like chronic kidney disease on the performance of plasma Alzheimer’s disease biomarkers, certain tau ratios, and specifically pT217/T217, should be considered for implementation in clinical practice and drug trials,” they added.
The study was published online in JAMA Neurology to coincide with a presentation at the International Conference on Alzheimer’s and Parkinson’s Diseases in Gothenburg, Sweden.
Skewed tau levels
Plasma biomarkers of amyloid-beta (Abeta) and tau pathologies, and in particular variants in p-tau217 and p-tau181, have shown promise for use in Alzheimer’s disease diagnosis and prognosis. However, previous reports have suggested that chronic kidney disease might influence plasma p-tau217 and p-tau181 concentrations, potentially decreasing their usefulness in the diagnostic workup of dementia.
Researchers investigated associations of chronic kidney disease with plasma ratios of p-tau217 and p-tau181 to the corresponding unphosphorylated peptides in Alzheimer’s disease.
The data came from 473 older adults, with and without cognitive impairment, from the Swedish BioFinder-2 study who had plasma tau assessments and chronic kidney disease status established within 6 months of plasma collection.
The researchers found that lower estimated glomerular filtration rate levels (indicative of kidney dysfunction) were associated with higher plasma concentrations of phosphorylated and unphosphorylated tau peptides measured simultaneously using the tau immunoprecipitation mass spectrometry assay.
However, the correlations with estimated glomerular filtration rate were nonsignificant for the pT217/T217 ratio in individuals with cognitive impairment and were significantly attenuated for pT217/T217 in cognitively unimpaired individuals and for pT181/T181 in both cognitively unimpaired and impaired individuals.
“Importantly, we demonstrate that there were no significant associations between chronic kidney disease and the pT217/T217 ratio and changes in plasma pT181/T181 associated with chronic kidney disease were small or nonsignificant,” the researchers noted.
“Our results indicate that by using p-tau/tau ratios we may be able to reduce the variability in plasma p-tau levels driven by impaired kidney function and consequently such ratios are more robust measures of brain p-tau pathology in individuals with both early- and later-stage Alzheimer’s disease,” they added.
The researchers believe this is likely true for the ratios of other related proteins – a view that is supported by findings of attenuated associations of chronic kidney disease with Abeta42/40, compared with Abeta42 and Abeta40 in the current study and in previous studies.
Important clinical implications
Reached for comment, Rebecca Edelmayer, PhD, Alzheimer’s Association senior director of scientific engagement, noted that research continues to indicate that blood biomarkers have “promise for improving, and possibly even redefining, the clinical workup for Alzheimer’s disease.
“Many of the Alzheimer’s blood tests in development today have focused on core blood biomarkers associated with amyloid accumulation and tau tangle formation in the brain,” Dr. Edelmayer said.
“Before these tests can be used more broadly in the clinic, we need to understand all of the variables that may impact the results of various blood biomarkers, including differences that may be driven by race, ethnicity, sex, and underlying health conditions, such as chronic kidney disease.
“This study corroborates other research suggesting that some Alzheimer’s-associated markers can be affected by chronic kidney disease, but by using ratios of amyloid or tau markers, we may be able to minimize these differences in results caused by underlying disease,” Dr. Edelmayer said.
Howard Fillit, MD, cofounder and chief science officer at the Alzheimer’s Drug Discovery Foundation, said, “Using these ratios makes a lot of sense because the ratios wouldn’t change with kidney function. I think this is an important advance in clinical utilization of these tests.”
Dr. Fillit noted that older people often have declining kidney function, which can be easily measured by glomerular filtration rate. Changes in blood levels of these markers with declining kidney function are “not unexpected, but [it’s] important that it’s been demonstrated.
“As we go forward, maybe the future utilization of these tests will be not only recording the ratios but also reporting the ratios in the context of somebody’s glomerular filtration rate. You could imagine a scenario where when the test is done, it’s automatically done alongside of glomerular filtration rate,” Dr. Fillit said in an interview.
The study was supported by Coins for Alzheimer’s Research Trust, the Tracy Family Stable Isotope Labeling Quantitation Center, and the National Institute of Neurological Disorders and Stroke. Dr. Hansson has received support to his institution from ADx, AVID Radiopharmaceuticals, Biogen, Eli Lilly, Eisai, Fujirebio, GE Healthcare, Pfizer, and Roche; consultancy/speaker fees from Amylyx, Alzpath, Biogen, Cerveau, Fujirebio, Genentech, Novartis, Roche, and Siemens; and personal fees from Eli Lilly, Eisai, Bioarctic, and Biogen outside the submitted work. Dr. Edelmayer and Dr. Fillit have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AD/PD 2023
Parkinson’s disease: What’s trauma got to do with it?
This transcript has been edited for clarity.
Kathrin LaFaver, MD: Hello. I’m happy to talk today to Dr. Indu Subramanian, clinical professor at University of California, Los Angeles, and director of the Parkinson’s Disease Research, Education and Clinical Center in Los Angeles. I am a neurologist in Saratoga Springs, New York, and we will be talking today about Indu’s new paper on childhood trauma and Parkinson’s disease. Welcome and thanks for taking the time.
Indu Subramanian, MD: Thank you so much for letting us highlight this important topic.
Dr. LaFaver: There are many papers published every month on Parkinson’s disease, but this topic stands out because it’s not a thing that has been commonly looked at. What gave you the idea to study this?
Neurology behind other specialties
Dr. Subramanian: Kathrin, you and I have been looking at things that can inform us about our patients – the person who’s standing in front of us when they come in and we’re giving them this diagnosis. I think that so much of what we’ve done [in the past] is a cookie cutter approach to giving everybody the standard treatment. [We’ve been assuming that] It doesn’t matter if they’re a man or woman. It doesn’t matter if they’re a veteran. It doesn’t matter if they may be from a minoritized population.
We’ve also been interested in approaches that are outside the box, right? We have this integrative medicine and lifestyle medicine background. I’ve been going to those meetings and really been struck by the mounting evidence on the importance of things like early adverse childhood events (ACEs), what zip code you live in, what your pollution index is, and how these things can affect people through their life and their health.
I think that it is high time neurologists pay attention to this. There’s been mounting evidence throughout many disease states, various types of cancers, and mental health. Cardiology is much more advanced, but we haven’t had much data in neurology. In fact, when we went to write this paper, there were just one or two papers that were looking at multiple sclerosis or general neurologic issues, but really nothing in Parkinson’s disease.
We know that Parkinson’s disease is not only a motor disease that affects mental health, but that it also affects nonmotor issues. Childhood adversity may affect how people progress or how quickly they may get a disease, and we were interested in how it may manifest in a disease like Parkinson’s disease.
That was the framework going to meetings. As we wrote this paper and were in various editing stages, there was a beautiful paper that came out by Nadine Burke Harris and team that really was a call to action for neurologists and caring about trauma.
Dr. LaFaver: I couldn’t agree more. It’s really an underrecognized issue. With my own background, being very interested in functional movement disorders, psychosomatic disorders, and so on, it becomes much more evident how common a trauma background is, not only for people we were traditionally asking about.
Why don’t you summarize your findings for us?
Adverse childhood events
Dr. Subramanian: This is a web-based survey, so obviously, these are patient self-reports of their disease. We have a large cohort of people that we’ve been following over 7 years. I’m looking at modifiable variables and what really impacts Parkinson’s disease. Some of our previous papers have looked at diet, exercise, and loneliness. This is the same cohort.
We ended up putting the ACEs questionnaire, which is 10 questions looking at whether you were exposed to certain things in your household below the age of 18. This is a relatively standard questionnaire that’s administered one time, and you get a score out of 10. This is something that has been pushed, at least in the state of California, as something that we should be checking more in all people coming in.
We introduced the survey, and we didn’t force everyone to take it. Unfortunately, there was 20% or so of our patients who chose not to answer these questions. One has to ask, who are those people that didn’t answer the questions? Are they the ones that may have had trauma and these questions were triggering? It was a gap. We didn’t add extra questions to explore why people didn’t answer those questions.
We have to also put this in context. We have a patient population that’s largely quite affluent, who are able to access web-based surveys through their computer, and largely Caucasian; there are not many minoritized populations in our cohort. We want to do better with that. We actually were able to gather a decent number of women. We represent women quite well in our survey. I think that’s because of this online approach and some of the things that we’re studying.
In our survey, we broke it down into people who had no ACEs, one to three ACEs, or four or more ACEs. This is a standard way to break down ACEs so that we’re able to categorize what to do with these patient populations.
What we saw – and it’s preliminary evidence – is that people who had higher ACE scores seemed to have more symptom severity when we controlled for things like years since diagnosis, age, and gender. They also seem to have a worse quality of life. There was some indication that there were more nonmotor issues in those populations, as you might expect, such as anxiety, depression, and things that presumably ACEs can affect separately.
There are some confounders, but I think we really want to use this as the first piece of evidence to hopefully pave the way for caring about trauma in Parkinson’s disease moving forward.
Dr. LaFaver: Thank you so much for that summary. You already mentioned the main methodology you used.
What is the next step for you? How do you see these findings informing our clinical care? Do you have suggestions for all of the neurologists listening in this regard?
PD not yet considered ACE-related
Dr. Subramanian: Dr. Burke Harris was the former surgeon general in California. She’s a woman of color and a brilliant speaker, and she had worked in inner cities, I think in San Francisco, with pediatric populations, seeing these effects of adversity in that time frame.
You see this population at risk, and then you’re following this cohort, which we knew from the Kaiser cohort determines earlier morbidity and mortality across a number of disease states. We’re seeing things like more heart attacks, more diabetes, and all kinds of things in these populations. This is not new news; we just have not been focusing on this.
In her paper, this call to action, they had talked about some ACE-related conditions that currently do not include Parkinson’s disease. There are three ACE-related neurologic conditions that people should be aware of. One is in the headache/pain universe. Another is in the stroke universe, and that’s understandable, given cardiovascular risk factors . Then the third is in this dementia risk category. I think Parkinson’s disease, as we know, can be associated with dementia. A large percentage of our patients get dementia, but we don’t have Parkinson’s disease called out in this framework.
What people are talking about is if you have no ACEs or are in this middle category of one to three ACEs and you don’t have an ACE-related diagnosis – which Parkinson’s disease is not currently – we just give some basic counseling about the importance of lifestyle. I think we would love to see that anyway. They’re talking about things like exercise, diet, sleep, social connection, getting out in nature, things like that, so just general counseling on the importance of that.
Then if you’re in this higher-risk category, and so with these ACE-related neurologic conditions, including dementia, headache, and stroke, if you had this middle range of one to three ACEs, they’re getting additional resources. Some of them may be referred for social work help or mental health support and things like that.
I’d really love to see that happening in Parkinson’s disease, because I think we have so many needs in our population. I’m always hoping to advocate for more mental health needs that are scarce and resources in the social support realm because I believe that social connection and social support is a huge buffer for this trauma.
ACEs are just one type of trauma. I take care of veterans in the Veterans [Affairs Department]. We have some information now coming out about posttraumatic stress disorder, predisposing to certain things in Parkinson’s disease, possibly head injury, and things like that. I think we have populations at risk that we can hopefully screen at intake, and I’m really pushing for that.
Maybe it’s not the neurologist that does this intake. It might be someone else on the team that can spend some time doing these questionnaires and understand if your patient has a high ACE score. Unless you ask, many patients don’t necessarily come forward to talk about this. I really am pushing for trying to screen and trying to advocate for more research in this area so that we can classify Parkinson’s disease as an ACE-related condition and thus give more resources from the mental health world, and also the social support world, to our patients.
Dr. LaFaver: Thank you. There are many important points, and I think it’s a very important thing to recognize that it may not be only trauma in childhood but also throughout life, as you said, and might really influence nonmotor symptoms of Parkinson’s disease in particular, including anxiety and pain, which are often difficult to treat.
I think there’s much more to do in research, advocacy, and education. We’re going to educate patients about this, and also educate other neurologists and providers. I think you mentioned that trauma-informed care is getting its spotlight in primary care and other specialties. I think we have catching up to do in neurology, and I think this is a really important work toward that goal.
Thank you so much for your work and for taking the time to share your thoughts. I hope to talk to you again soon.
Dr. Subramanian: Thank you so much, Kathrin.
Dr. LaFaver has disclosed no relevant financial relationships. Dr. Subramanian disclosed ties with Acorda Therapeutics.
A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
Kathrin LaFaver, MD: Hello. I’m happy to talk today to Dr. Indu Subramanian, clinical professor at University of California, Los Angeles, and director of the Parkinson’s Disease Research, Education and Clinical Center in Los Angeles. I am a neurologist in Saratoga Springs, New York, and we will be talking today about Indu’s new paper on childhood trauma and Parkinson’s disease. Welcome and thanks for taking the time.
Indu Subramanian, MD: Thank you so much for letting us highlight this important topic.
Dr. LaFaver: There are many papers published every month on Parkinson’s disease, but this topic stands out because it’s not a thing that has been commonly looked at. What gave you the idea to study this?
Neurology behind other specialties
Dr. Subramanian: Kathrin, you and I have been looking at things that can inform us about our patients – the person who’s standing in front of us when they come in and we’re giving them this diagnosis. I think that so much of what we’ve done [in the past] is a cookie cutter approach to giving everybody the standard treatment. [We’ve been assuming that] It doesn’t matter if they’re a man or woman. It doesn’t matter if they’re a veteran. It doesn’t matter if they may be from a minoritized population.
We’ve also been interested in approaches that are outside the box, right? We have this integrative medicine and lifestyle medicine background. I’ve been going to those meetings and really been struck by the mounting evidence on the importance of things like early adverse childhood events (ACEs), what zip code you live in, what your pollution index is, and how these things can affect people through their life and their health.
I think that it is high time neurologists pay attention to this. There’s been mounting evidence throughout many disease states, various types of cancers, and mental health. Cardiology is much more advanced, but we haven’t had much data in neurology. In fact, when we went to write this paper, there were just one or two papers that were looking at multiple sclerosis or general neurologic issues, but really nothing in Parkinson’s disease.
We know that Parkinson’s disease is not only a motor disease that affects mental health, but that it also affects nonmotor issues. Childhood adversity may affect how people progress or how quickly they may get a disease, and we were interested in how it may manifest in a disease like Parkinson’s disease.
That was the framework going to meetings. As we wrote this paper and were in various editing stages, there was a beautiful paper that came out by Nadine Burke Harris and team that really was a call to action for neurologists and caring about trauma.
Dr. LaFaver: I couldn’t agree more. It’s really an underrecognized issue. With my own background, being very interested in functional movement disorders, psychosomatic disorders, and so on, it becomes much more evident how common a trauma background is, not only for people we were traditionally asking about.
Why don’t you summarize your findings for us?
Adverse childhood events
Dr. Subramanian: This is a web-based survey, so obviously, these are patient self-reports of their disease. We have a large cohort of people that we’ve been following over 7 years. I’m looking at modifiable variables and what really impacts Parkinson’s disease. Some of our previous papers have looked at diet, exercise, and loneliness. This is the same cohort.
We ended up putting the ACEs questionnaire, which is 10 questions looking at whether you were exposed to certain things in your household below the age of 18. This is a relatively standard questionnaire that’s administered one time, and you get a score out of 10. This is something that has been pushed, at least in the state of California, as something that we should be checking more in all people coming in.
We introduced the survey, and we didn’t force everyone to take it. Unfortunately, there was 20% or so of our patients who chose not to answer these questions. One has to ask, who are those people that didn’t answer the questions? Are they the ones that may have had trauma and these questions were triggering? It was a gap. We didn’t add extra questions to explore why people didn’t answer those questions.
We have to also put this in context. We have a patient population that’s largely quite affluent, who are able to access web-based surveys through their computer, and largely Caucasian; there are not many minoritized populations in our cohort. We want to do better with that. We actually were able to gather a decent number of women. We represent women quite well in our survey. I think that’s because of this online approach and some of the things that we’re studying.
In our survey, we broke it down into people who had no ACEs, one to three ACEs, or four or more ACEs. This is a standard way to break down ACEs so that we’re able to categorize what to do with these patient populations.
What we saw – and it’s preliminary evidence – is that people who had higher ACE scores seemed to have more symptom severity when we controlled for things like years since diagnosis, age, and gender. They also seem to have a worse quality of life. There was some indication that there were more nonmotor issues in those populations, as you might expect, such as anxiety, depression, and things that presumably ACEs can affect separately.
There are some confounders, but I think we really want to use this as the first piece of evidence to hopefully pave the way for caring about trauma in Parkinson’s disease moving forward.
Dr. LaFaver: Thank you so much for that summary. You already mentioned the main methodology you used.
What is the next step for you? How do you see these findings informing our clinical care? Do you have suggestions for all of the neurologists listening in this regard?
PD not yet considered ACE-related
Dr. Subramanian: Dr. Burke Harris was the former surgeon general in California. She’s a woman of color and a brilliant speaker, and she had worked in inner cities, I think in San Francisco, with pediatric populations, seeing these effects of adversity in that time frame.
You see this population at risk, and then you’re following this cohort, which we knew from the Kaiser cohort determines earlier morbidity and mortality across a number of disease states. We’re seeing things like more heart attacks, more diabetes, and all kinds of things in these populations. This is not new news; we just have not been focusing on this.
In her paper, this call to action, they had talked about some ACE-related conditions that currently do not include Parkinson’s disease. There are three ACE-related neurologic conditions that people should be aware of. One is in the headache/pain universe. Another is in the stroke universe, and that’s understandable, given cardiovascular risk factors . Then the third is in this dementia risk category. I think Parkinson’s disease, as we know, can be associated with dementia. A large percentage of our patients get dementia, but we don’t have Parkinson’s disease called out in this framework.
What people are talking about is if you have no ACEs or are in this middle category of one to three ACEs and you don’t have an ACE-related diagnosis – which Parkinson’s disease is not currently – we just give some basic counseling about the importance of lifestyle. I think we would love to see that anyway. They’re talking about things like exercise, diet, sleep, social connection, getting out in nature, things like that, so just general counseling on the importance of that.
Then if you’re in this higher-risk category, and so with these ACE-related neurologic conditions, including dementia, headache, and stroke, if you had this middle range of one to three ACEs, they’re getting additional resources. Some of them may be referred for social work help or mental health support and things like that.
I’d really love to see that happening in Parkinson’s disease, because I think we have so many needs in our population. I’m always hoping to advocate for more mental health needs that are scarce and resources in the social support realm because I believe that social connection and social support is a huge buffer for this trauma.
ACEs are just one type of trauma. I take care of veterans in the Veterans [Affairs Department]. We have some information now coming out about posttraumatic stress disorder, predisposing to certain things in Parkinson’s disease, possibly head injury, and things like that. I think we have populations at risk that we can hopefully screen at intake, and I’m really pushing for that.
Maybe it’s not the neurologist that does this intake. It might be someone else on the team that can spend some time doing these questionnaires and understand if your patient has a high ACE score. Unless you ask, many patients don’t necessarily come forward to talk about this. I really am pushing for trying to screen and trying to advocate for more research in this area so that we can classify Parkinson’s disease as an ACE-related condition and thus give more resources from the mental health world, and also the social support world, to our patients.
Dr. LaFaver: Thank you. There are many important points, and I think it’s a very important thing to recognize that it may not be only trauma in childhood but also throughout life, as you said, and might really influence nonmotor symptoms of Parkinson’s disease in particular, including anxiety and pain, which are often difficult to treat.
I think there’s much more to do in research, advocacy, and education. We’re going to educate patients about this, and also educate other neurologists and providers. I think you mentioned that trauma-informed care is getting its spotlight in primary care and other specialties. I think we have catching up to do in neurology, and I think this is a really important work toward that goal.
Thank you so much for your work and for taking the time to share your thoughts. I hope to talk to you again soon.
Dr. Subramanian: Thank you so much, Kathrin.
Dr. LaFaver has disclosed no relevant financial relationships. Dr. Subramanian disclosed ties with Acorda Therapeutics.
A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
Kathrin LaFaver, MD: Hello. I’m happy to talk today to Dr. Indu Subramanian, clinical professor at University of California, Los Angeles, and director of the Parkinson’s Disease Research, Education and Clinical Center in Los Angeles. I am a neurologist in Saratoga Springs, New York, and we will be talking today about Indu’s new paper on childhood trauma and Parkinson’s disease. Welcome and thanks for taking the time.
Indu Subramanian, MD: Thank you so much for letting us highlight this important topic.
Dr. LaFaver: There are many papers published every month on Parkinson’s disease, but this topic stands out because it’s not a thing that has been commonly looked at. What gave you the idea to study this?
Neurology behind other specialties
Dr. Subramanian: Kathrin, you and I have been looking at things that can inform us about our patients – the person who’s standing in front of us when they come in and we’re giving them this diagnosis. I think that so much of what we’ve done [in the past] is a cookie cutter approach to giving everybody the standard treatment. [We’ve been assuming that] It doesn’t matter if they’re a man or woman. It doesn’t matter if they’re a veteran. It doesn’t matter if they may be from a minoritized population.
We’ve also been interested in approaches that are outside the box, right? We have this integrative medicine and lifestyle medicine background. I’ve been going to those meetings and really been struck by the mounting evidence on the importance of things like early adverse childhood events (ACEs), what zip code you live in, what your pollution index is, and how these things can affect people through their life and their health.
I think that it is high time neurologists pay attention to this. There’s been mounting evidence throughout many disease states, various types of cancers, and mental health. Cardiology is much more advanced, but we haven’t had much data in neurology. In fact, when we went to write this paper, there were just one or two papers that were looking at multiple sclerosis or general neurologic issues, but really nothing in Parkinson’s disease.
We know that Parkinson’s disease is not only a motor disease that affects mental health, but that it also affects nonmotor issues. Childhood adversity may affect how people progress or how quickly they may get a disease, and we were interested in how it may manifest in a disease like Parkinson’s disease.
That was the framework going to meetings. As we wrote this paper and were in various editing stages, there was a beautiful paper that came out by Nadine Burke Harris and team that really was a call to action for neurologists and caring about trauma.
Dr. LaFaver: I couldn’t agree more. It’s really an underrecognized issue. With my own background, being very interested in functional movement disorders, psychosomatic disorders, and so on, it becomes much more evident how common a trauma background is, not only for people we were traditionally asking about.
Why don’t you summarize your findings for us?
Adverse childhood events
Dr. Subramanian: This is a web-based survey, so obviously, these are patient self-reports of their disease. We have a large cohort of people that we’ve been following over 7 years. I’m looking at modifiable variables and what really impacts Parkinson’s disease. Some of our previous papers have looked at diet, exercise, and loneliness. This is the same cohort.
We ended up putting the ACEs questionnaire, which is 10 questions looking at whether you were exposed to certain things in your household below the age of 18. This is a relatively standard questionnaire that’s administered one time, and you get a score out of 10. This is something that has been pushed, at least in the state of California, as something that we should be checking more in all people coming in.
We introduced the survey, and we didn’t force everyone to take it. Unfortunately, there was 20% or so of our patients who chose not to answer these questions. One has to ask, who are those people that didn’t answer the questions? Are they the ones that may have had trauma and these questions were triggering? It was a gap. We didn’t add extra questions to explore why people didn’t answer those questions.
We have to also put this in context. We have a patient population that’s largely quite affluent, who are able to access web-based surveys through their computer, and largely Caucasian; there are not many minoritized populations in our cohort. We want to do better with that. We actually were able to gather a decent number of women. We represent women quite well in our survey. I think that’s because of this online approach and some of the things that we’re studying.
In our survey, we broke it down into people who had no ACEs, one to three ACEs, or four or more ACEs. This is a standard way to break down ACEs so that we’re able to categorize what to do with these patient populations.
What we saw – and it’s preliminary evidence – is that people who had higher ACE scores seemed to have more symptom severity when we controlled for things like years since diagnosis, age, and gender. They also seem to have a worse quality of life. There was some indication that there were more nonmotor issues in those populations, as you might expect, such as anxiety, depression, and things that presumably ACEs can affect separately.
There are some confounders, but I think we really want to use this as the first piece of evidence to hopefully pave the way for caring about trauma in Parkinson’s disease moving forward.
Dr. LaFaver: Thank you so much for that summary. You already mentioned the main methodology you used.
What is the next step for you? How do you see these findings informing our clinical care? Do you have suggestions for all of the neurologists listening in this regard?
PD not yet considered ACE-related
Dr. Subramanian: Dr. Burke Harris was the former surgeon general in California. She’s a woman of color and a brilliant speaker, and she had worked in inner cities, I think in San Francisco, with pediatric populations, seeing these effects of adversity in that time frame.
You see this population at risk, and then you’re following this cohort, which we knew from the Kaiser cohort determines earlier morbidity and mortality across a number of disease states. We’re seeing things like more heart attacks, more diabetes, and all kinds of things in these populations. This is not new news; we just have not been focusing on this.
In her paper, this call to action, they had talked about some ACE-related conditions that currently do not include Parkinson’s disease. There are three ACE-related neurologic conditions that people should be aware of. One is in the headache/pain universe. Another is in the stroke universe, and that’s understandable, given cardiovascular risk factors . Then the third is in this dementia risk category. I think Parkinson’s disease, as we know, can be associated with dementia. A large percentage of our patients get dementia, but we don’t have Parkinson’s disease called out in this framework.
What people are talking about is if you have no ACEs or are in this middle category of one to three ACEs and you don’t have an ACE-related diagnosis – which Parkinson’s disease is not currently – we just give some basic counseling about the importance of lifestyle. I think we would love to see that anyway. They’re talking about things like exercise, diet, sleep, social connection, getting out in nature, things like that, so just general counseling on the importance of that.
Then if you’re in this higher-risk category, and so with these ACE-related neurologic conditions, including dementia, headache, and stroke, if you had this middle range of one to three ACEs, they’re getting additional resources. Some of them may be referred for social work help or mental health support and things like that.
I’d really love to see that happening in Parkinson’s disease, because I think we have so many needs in our population. I’m always hoping to advocate for more mental health needs that are scarce and resources in the social support realm because I believe that social connection and social support is a huge buffer for this trauma.
ACEs are just one type of trauma. I take care of veterans in the Veterans [Affairs Department]. We have some information now coming out about posttraumatic stress disorder, predisposing to certain things in Parkinson’s disease, possibly head injury, and things like that. I think we have populations at risk that we can hopefully screen at intake, and I’m really pushing for that.
Maybe it’s not the neurologist that does this intake. It might be someone else on the team that can spend some time doing these questionnaires and understand if your patient has a high ACE score. Unless you ask, many patients don’t necessarily come forward to talk about this. I really am pushing for trying to screen and trying to advocate for more research in this area so that we can classify Parkinson’s disease as an ACE-related condition and thus give more resources from the mental health world, and also the social support world, to our patients.
Dr. LaFaver: Thank you. There are many important points, and I think it’s a very important thing to recognize that it may not be only trauma in childhood but also throughout life, as you said, and might really influence nonmotor symptoms of Parkinson’s disease in particular, including anxiety and pain, which are often difficult to treat.
I think there’s much more to do in research, advocacy, and education. We’re going to educate patients about this, and also educate other neurologists and providers. I think you mentioned that trauma-informed care is getting its spotlight in primary care and other specialties. I think we have catching up to do in neurology, and I think this is a really important work toward that goal.
Thank you so much for your work and for taking the time to share your thoughts. I hope to talk to you again soon.
Dr. Subramanian: Thank you so much, Kathrin.
Dr. LaFaver has disclosed no relevant financial relationships. Dr. Subramanian disclosed ties with Acorda Therapeutics.
A version of this article originally appeared on Medscape.com.
Magnesium-rich diet linked to lower dementia risk
Investigators studied more than 6,000 cognitively healthy individuals, aged 40-73, and found that those who consumed more than 550 mg of magnesium daily had a brain age approximately 1 year younger by age 55 years, compared with a person who consumed a normal magnesium intake (~360 mg per day).
“This research highlights the potential benefits of a diet high in magnesium and the role it plays in promoting good brain health,” lead author Khawlah Alateeq, a PhD candidate in neuroscience at Australian National University’s National Centre for Epidemiology and Population Health, said in an interview.
Clinicians “can use [the findings] to counsel patients on the benefits of increasing magnesium intake through a healthy diet and monitoring magnesium levels to prevent deficiencies,” she stated.
The study was published online in the European Journal of Nutrition.
Promising target
The researchers were motivated to conduct the study because of “the growing concern over the increasing prevalence of dementia,” Ms. Alateeq said.
“Since there is no cure for dementia, and the development of pharmacological treatment for dementia has been unsuccessful over the last 30 years, prevention has been suggested as an effective approach to address the issue,” she added.
Nutrition, Ms. Alateeq said, is a “modifiable risk factor that can influence brain health and is highly amenable to scalable and cost-effective interventions.” It represents “a promising target” for risk reduction at a population level.
Previous research shows individuals with lower magnesium levels are at higher risk for AD, while those with higher dietary magnesium intake may be at lower risk of progressing from normal aging to cognitive impairment.
Most previous studies, however, included participants older than age 60 years, and it’s “unclear when the neuroprotective effects of dietary magnesium become detectable,” the researchers note.
Moreover, dietary patterns change and fluctuate, potentially leading to changes in magnesium intake over time. These changes may have as much impact as absolute magnesium at any point in time.
In light of the “current lack of understanding of when and to what extent dietary magnesium exerts its protective effects on the brain,” the researchers examined the association between magnesium trajectories over time, brain matter, and white matter lesions.
They also examined the association between magnesium and several different blood pressure measures (mean arterial pressure, systolic blood pressure, diastolic blood pressure, and pulse pressure).
Since cardiovascular health, neurodegeneration, and brain shrinkage patterns differ between men and women, the researchers stratified their analyses by sex.
Brain volume differences
The researchers analyzed the dietary magnesium intake of 6,001 individuals (mean age, 55.3 years) selected from the UK Biobank – a prospective cohort study of participants aged 37-73 at baseline, who were assessed between 2005 and 2023.
For the current study, only participants with baseline DBP and SBP measurements and structural MRI scans were included. Participants were also required to be free of neurologic disorders and to have an available record of dietary magnesium intake.
Covariates included age, sex, education, health conditions, smoking status, body mass index, amount of physical activity, smoking status, and alcohol intake.
Over a 16-month period, participants completed an online questionnaire five times. Their responses were used to calculate daily magnesium intake. Foods of particular interest included leafy green vegetables, legumes, nuts, seeds, and whole grains, all of which are magnesium rich.
They used latent class analysis (LCA) to “identify mutually exclusive subgroup (classes) of magnesium intake trajectory separately for men and women.”
Men had a slightly higher prevalence of BP medication and diabetes, compared with women, and postmenopausal women had a higher prevalence of BP medication and diabetes, compared with premenopausal women.
Compared with lower baseline magnesium intake, higher baseline dietary intake of magnesium was associated with larger brain volumes in several regions in both men and women.
The latent class analysis identified three classes of magnesium intake:
In women in particular, the “high-decreasing” trajectory was significantly associated with larger brain volumes, compared with the “normal-stable” trajectory, while the “low-increasing” trajectory was associated with smaller brain volumes.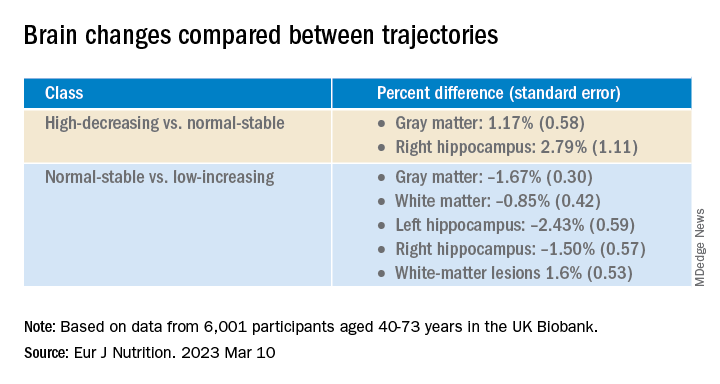
Even an increase of 1 mg of magnesium per day (above 350 mg/day) made a difference in brain volume, especially in women. The changes associated with every 1-mg increase are found in the table below: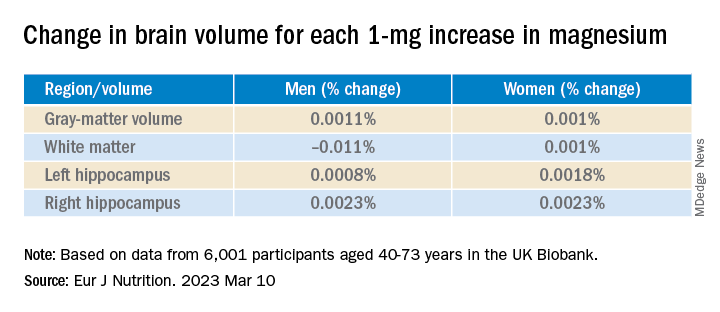
Associations between magnesium and BP measures were “mostly nonsignificant,” the researchers say, and the neuroprotective effect of higher magnesium intake in the high-decreasing trajectory was greater in postmenopausal versus premenopausal women.
“Our models indicate that compared to somebody with a normal magnesium intake (~350 mg per day), somebody in the top quartile of magnesium intake (≥ 550 mg per day) would be predicted to have a ~0.20% larger GM and ~0.46% larger RHC,” the authors summarize.
“In a population with an average age of 55 years, this effect corresponds to ~1 year of typical aging,” they note. “In other words, if this effect is generalizable to other populations, a 41% increase in magnesium intake may lead to significantly better brain health.”
Although the exact mechanisms underlying magnesium’s protective effects are “not yet clearly understood, there’s considerable evidence that magnesium levels are related to better cardiovascular health. Magnesium supplementation has been found to decrease blood pressure – and high blood pressure is a well-established risk factor for dementia,” said Ms. Alateeq.
Association, not causation
Yuko Hara, PhD, director of Aging and Prevention, Alzheimer’s Drug Discovery Foundation, noted that the study is observational and therefore shows an association, not causation.
“People eating a high-magnesium diet may also be eating a brain-healthy diet and getting high levels of nutrients/minerals other than magnesium alone,” suggested Dr. Hara, who was not involved with the study.
She noted that many foods are good sources of magnesium, including spinach, almonds, cashews, legumes, yogurt, brown rice, and avocados.
“Eating a brain-healthy diet (for example, the Mediterranean diet) is one of the Seven Steps to Protect Your Cognitive Vitality that ADDF’s Cognitive Vitality promotes,” she said.
Open Access funding was enabled and organized by the Council of Australian University Librarians and its Member Institutions. Ms. Alateeq, her co-authors, and Dr. Hara declare no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Investigators studied more than 6,000 cognitively healthy individuals, aged 40-73, and found that those who consumed more than 550 mg of magnesium daily had a brain age approximately 1 year younger by age 55 years, compared with a person who consumed a normal magnesium intake (~360 mg per day).
“This research highlights the potential benefits of a diet high in magnesium and the role it plays in promoting good brain health,” lead author Khawlah Alateeq, a PhD candidate in neuroscience at Australian National University’s National Centre for Epidemiology and Population Health, said in an interview.
Clinicians “can use [the findings] to counsel patients on the benefits of increasing magnesium intake through a healthy diet and monitoring magnesium levels to prevent deficiencies,” she stated.
The study was published online in the European Journal of Nutrition.
Promising target
The researchers were motivated to conduct the study because of “the growing concern over the increasing prevalence of dementia,” Ms. Alateeq said.
“Since there is no cure for dementia, and the development of pharmacological treatment for dementia has been unsuccessful over the last 30 years, prevention has been suggested as an effective approach to address the issue,” she added.
Nutrition, Ms. Alateeq said, is a “modifiable risk factor that can influence brain health and is highly amenable to scalable and cost-effective interventions.” It represents “a promising target” for risk reduction at a population level.
Previous research shows individuals with lower magnesium levels are at higher risk for AD, while those with higher dietary magnesium intake may be at lower risk of progressing from normal aging to cognitive impairment.
Most previous studies, however, included participants older than age 60 years, and it’s “unclear when the neuroprotective effects of dietary magnesium become detectable,” the researchers note.
Moreover, dietary patterns change and fluctuate, potentially leading to changes in magnesium intake over time. These changes may have as much impact as absolute magnesium at any point in time.
In light of the “current lack of understanding of when and to what extent dietary magnesium exerts its protective effects on the brain,” the researchers examined the association between magnesium trajectories over time, brain matter, and white matter lesions.
They also examined the association between magnesium and several different blood pressure measures (mean arterial pressure, systolic blood pressure, diastolic blood pressure, and pulse pressure).
Since cardiovascular health, neurodegeneration, and brain shrinkage patterns differ between men and women, the researchers stratified their analyses by sex.
Brain volume differences
The researchers analyzed the dietary magnesium intake of 6,001 individuals (mean age, 55.3 years) selected from the UK Biobank – a prospective cohort study of participants aged 37-73 at baseline, who were assessed between 2005 and 2023.
For the current study, only participants with baseline DBP and SBP measurements and structural MRI scans were included. Participants were also required to be free of neurologic disorders and to have an available record of dietary magnesium intake.
Covariates included age, sex, education, health conditions, smoking status, body mass index, amount of physical activity, smoking status, and alcohol intake.
Over a 16-month period, participants completed an online questionnaire five times. Their responses were used to calculate daily magnesium intake. Foods of particular interest included leafy green vegetables, legumes, nuts, seeds, and whole grains, all of which are magnesium rich.
They used latent class analysis (LCA) to “identify mutually exclusive subgroup (classes) of magnesium intake trajectory separately for men and women.”
Men had a slightly higher prevalence of BP medication and diabetes, compared with women, and postmenopausal women had a higher prevalence of BP medication and diabetes, compared with premenopausal women.
Compared with lower baseline magnesium intake, higher baseline dietary intake of magnesium was associated with larger brain volumes in several regions in both men and women.
The latent class analysis identified three classes of magnesium intake:
In women in particular, the “high-decreasing” trajectory was significantly associated with larger brain volumes, compared with the “normal-stable” trajectory, while the “low-increasing” trajectory was associated with smaller brain volumes.
Even an increase of 1 mg of magnesium per day (above 350 mg/day) made a difference in brain volume, especially in women. The changes associated with every 1-mg increase are found in the table below:
Associations between magnesium and BP measures were “mostly nonsignificant,” the researchers say, and the neuroprotective effect of higher magnesium intake in the high-decreasing trajectory was greater in postmenopausal versus premenopausal women.
“Our models indicate that compared to somebody with a normal magnesium intake (~350 mg per day), somebody in the top quartile of magnesium intake (≥ 550 mg per day) would be predicted to have a ~0.20% larger GM and ~0.46% larger RHC,” the authors summarize.
“In a population with an average age of 55 years, this effect corresponds to ~1 year of typical aging,” they note. “In other words, if this effect is generalizable to other populations, a 41% increase in magnesium intake may lead to significantly better brain health.”
Although the exact mechanisms underlying magnesium’s protective effects are “not yet clearly understood, there’s considerable evidence that magnesium levels are related to better cardiovascular health. Magnesium supplementation has been found to decrease blood pressure – and high blood pressure is a well-established risk factor for dementia,” said Ms. Alateeq.
Association, not causation
Yuko Hara, PhD, director of Aging and Prevention, Alzheimer’s Drug Discovery Foundation, noted that the study is observational and therefore shows an association, not causation.
“People eating a high-magnesium diet may also be eating a brain-healthy diet and getting high levels of nutrients/minerals other than magnesium alone,” suggested Dr. Hara, who was not involved with the study.
She noted that many foods are good sources of magnesium, including spinach, almonds, cashews, legumes, yogurt, brown rice, and avocados.
“Eating a brain-healthy diet (for example, the Mediterranean diet) is one of the Seven Steps to Protect Your Cognitive Vitality that ADDF’s Cognitive Vitality promotes,” she said.
Open Access funding was enabled and organized by the Council of Australian University Librarians and its Member Institutions. Ms. Alateeq, her co-authors, and Dr. Hara declare no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Investigators studied more than 6,000 cognitively healthy individuals, aged 40-73, and found that those who consumed more than 550 mg of magnesium daily had a brain age approximately 1 year younger by age 55 years, compared with a person who consumed a normal magnesium intake (~360 mg per day).
“This research highlights the potential benefits of a diet high in magnesium and the role it plays in promoting good brain health,” lead author Khawlah Alateeq, a PhD candidate in neuroscience at Australian National University’s National Centre for Epidemiology and Population Health, said in an interview.
Clinicians “can use [the findings] to counsel patients on the benefits of increasing magnesium intake through a healthy diet and monitoring magnesium levels to prevent deficiencies,” she stated.
The study was published online in the European Journal of Nutrition.
Promising target
The researchers were motivated to conduct the study because of “the growing concern over the increasing prevalence of dementia,” Ms. Alateeq said.
“Since there is no cure for dementia, and the development of pharmacological treatment for dementia has been unsuccessful over the last 30 years, prevention has been suggested as an effective approach to address the issue,” she added.
Nutrition, Ms. Alateeq said, is a “modifiable risk factor that can influence brain health and is highly amenable to scalable and cost-effective interventions.” It represents “a promising target” for risk reduction at a population level.
Previous research shows individuals with lower magnesium levels are at higher risk for AD, while those with higher dietary magnesium intake may be at lower risk of progressing from normal aging to cognitive impairment.
Most previous studies, however, included participants older than age 60 years, and it’s “unclear when the neuroprotective effects of dietary magnesium become detectable,” the researchers note.
Moreover, dietary patterns change and fluctuate, potentially leading to changes in magnesium intake over time. These changes may have as much impact as absolute magnesium at any point in time.
In light of the “current lack of understanding of when and to what extent dietary magnesium exerts its protective effects on the brain,” the researchers examined the association between magnesium trajectories over time, brain matter, and white matter lesions.
They also examined the association between magnesium and several different blood pressure measures (mean arterial pressure, systolic blood pressure, diastolic blood pressure, and pulse pressure).
Since cardiovascular health, neurodegeneration, and brain shrinkage patterns differ between men and women, the researchers stratified their analyses by sex.
Brain volume differences
The researchers analyzed the dietary magnesium intake of 6,001 individuals (mean age, 55.3 years) selected from the UK Biobank – a prospective cohort study of participants aged 37-73 at baseline, who were assessed between 2005 and 2023.
For the current study, only participants with baseline DBP and SBP measurements and structural MRI scans were included. Participants were also required to be free of neurologic disorders and to have an available record of dietary magnesium intake.
Covariates included age, sex, education, health conditions, smoking status, body mass index, amount of physical activity, smoking status, and alcohol intake.
Over a 16-month period, participants completed an online questionnaire five times. Their responses were used to calculate daily magnesium intake. Foods of particular interest included leafy green vegetables, legumes, nuts, seeds, and whole grains, all of which are magnesium rich.
They used latent class analysis (LCA) to “identify mutually exclusive subgroup (classes) of magnesium intake trajectory separately for men and women.”
Men had a slightly higher prevalence of BP medication and diabetes, compared with women, and postmenopausal women had a higher prevalence of BP medication and diabetes, compared with premenopausal women.
Compared with lower baseline magnesium intake, higher baseline dietary intake of magnesium was associated with larger brain volumes in several regions in both men and women.
The latent class analysis identified three classes of magnesium intake:
In women in particular, the “high-decreasing” trajectory was significantly associated with larger brain volumes, compared with the “normal-stable” trajectory, while the “low-increasing” trajectory was associated with smaller brain volumes.
Even an increase of 1 mg of magnesium per day (above 350 mg/day) made a difference in brain volume, especially in women. The changes associated with every 1-mg increase are found in the table below:
Associations between magnesium and BP measures were “mostly nonsignificant,” the researchers say, and the neuroprotective effect of higher magnesium intake in the high-decreasing trajectory was greater in postmenopausal versus premenopausal women.
“Our models indicate that compared to somebody with a normal magnesium intake (~350 mg per day), somebody in the top quartile of magnesium intake (≥ 550 mg per day) would be predicted to have a ~0.20% larger GM and ~0.46% larger RHC,” the authors summarize.
“In a population with an average age of 55 years, this effect corresponds to ~1 year of typical aging,” they note. “In other words, if this effect is generalizable to other populations, a 41% increase in magnesium intake may lead to significantly better brain health.”
Although the exact mechanisms underlying magnesium’s protective effects are “not yet clearly understood, there’s considerable evidence that magnesium levels are related to better cardiovascular health. Magnesium supplementation has been found to decrease blood pressure – and high blood pressure is a well-established risk factor for dementia,” said Ms. Alateeq.
Association, not causation
Yuko Hara, PhD, director of Aging and Prevention, Alzheimer’s Drug Discovery Foundation, noted that the study is observational and therefore shows an association, not causation.
“People eating a high-magnesium diet may also be eating a brain-healthy diet and getting high levels of nutrients/minerals other than magnesium alone,” suggested Dr. Hara, who was not involved with the study.
She noted that many foods are good sources of magnesium, including spinach, almonds, cashews, legumes, yogurt, brown rice, and avocados.
“Eating a brain-healthy diet (for example, the Mediterranean diet) is one of the Seven Steps to Protect Your Cognitive Vitality that ADDF’s Cognitive Vitality promotes,” she said.
Open Access funding was enabled and organized by the Council of Australian University Librarians and its Member Institutions. Ms. Alateeq, her co-authors, and Dr. Hara declare no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM EUROPEAN JOURNAL OF NUTRITION
Autism: Is it in the water?
This transcript has been edited for clarity.
Few diseases have stymied explanation like autism spectrum disorder (ASD). We know that the prevalence has been increasing dramatically, but we aren’t quite sure whether that is because of more screening and awareness or more fundamental changes. We know that much of the risk appears to be genetic, but there may be 1,000 genes involved in the syndrome. We know that certain environmental exposures, like pollution, might increase the risk – perhaps on a susceptible genetic background – but we’re not really sure which exposures are most harmful.
So, the search continues, across all domains of inquiry from cell culture to large epidemiologic analyses. And this week, a new player enters the field, and, as they say, it’s something in the water.
We’re talking about this paper, by Zeyan Liew and colleagues, appearing in JAMA Pediatrics.
Using the incredibly robust health data infrastructure in Denmark, the researchers were able to identify 8,842 children born between 2000 and 2013 with ASD and matched each one to five control kids of the same sex and age without autism.
They then mapped the location the mothers of these kids lived while they were pregnant – down to 5 meters resolution, actually – to groundwater lithium levels.
Once that was done, the analysis was straightforward. Would moms who were pregnant in areas with higher groundwater lithium levels be more likely to have kids with ASD?
The results show a rather steady and consistent association between higher lithium levels in groundwater and the prevalence of ASD in children.
We’re not talking huge numbers, but moms who lived in the areas of the highest quartile of lithium were about 46% more likely to have a child with ASD. That’s a relative risk, of course – this would be like an increase from 1 in 100 kids to 1.5 in 100 kids. But still, it’s intriguing.
But the case is far from closed here.
Groundwater concentration of lithium and the amount of lithium a pregnant mother ingests are not the same thing. It does turn out that virtually all drinking water in Denmark comes from groundwater sources – but not all lithium comes from drinking water. There are plenty of dietary sources of lithium as well. And, of course, there is medical lithium, but we’ll get to that in a second.
First, let’s talk about those lithium measurements. They were taken in 2013 – after all these kids were born. The authors acknowledge this limitation but show a high correlation between measured levels in 2013 and earlier measured levels from prior studies, suggesting that lithium levels in a given area are quite constant over time. That’s great – but if lithium levels are constant over time, this study does nothing to shed light on why autism diagnoses seem to be increasing.
Let’s put some numbers to the lithium concentrations the authors examined. The average was about 12 mcg/L.
As a reminder, a standard therapeutic dose of lithium used for bipolar disorder is like 600 mg. That means you’d need to drink more than 2,500 of those 5-gallon jugs that sit on your water cooler, per day, to approximate the dose you’d get from a lithium tablet. Of course, small doses can still cause toxicity – but I wanted to put this in perspective.
Also, we have some data on pregnant women who take medical lithium. An analysis of nine studies showed that first-trimester lithium use may be associated with congenital malformations – particularly some specific heart malformations – and some birth complications. But three of four separate studies looking at longer-term neurodevelopmental outcomes did not find any effect on development, attainment of milestones, or IQ. One study of 15 kids exposed to medical lithium in utero did note minor neurologic dysfunction in one child and a low verbal IQ in another – but that’s a very small study.
Of course, lithium levels vary around the world as well. The U.S. Geological Survey examined lithium content in groundwater in the United States, as you can see here.
Our numbers are pretty similar to Denmark’s – in the 0-60 range. But an area in the Argentine Andes has levels as high as 1,600 mcg/L. A study of 194 babies from that area found higher lithium exposure was associated with lower fetal size, but I haven’t seen follow-up on neurodevelopmental outcomes.
The point is that there is a lot of variability here. It would be really interesting to map groundwater lithium levels to autism rates around the world. As a teaser, I will point out that, if you look at worldwide autism rates, you may be able to convince yourself that they are higher in more arid climates, and arid climates tend to have more groundwater lithium. But I’m really reaching here. More work needs to be done.
And I hope it is done quickly. Lithium is in the midst of becoming a very important commodity thanks to the shift to electric vehicles. While we can hope that recycling will claim most of those batteries at the end of their life, some will escape reclamation and potentially put more lithium into the drinking water. I’d like to know how risky that is before it happens.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. He has disclosed no relevant financial relationships. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and his new book, “How Medicine Works and When It Doesn’t”, is available now.
A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
Few diseases have stymied explanation like autism spectrum disorder (ASD). We know that the prevalence has been increasing dramatically, but we aren’t quite sure whether that is because of more screening and awareness or more fundamental changes. We know that much of the risk appears to be genetic, but there may be 1,000 genes involved in the syndrome. We know that certain environmental exposures, like pollution, might increase the risk – perhaps on a susceptible genetic background – but we’re not really sure which exposures are most harmful.
So, the search continues, across all domains of inquiry from cell culture to large epidemiologic analyses. And this week, a new player enters the field, and, as they say, it’s something in the water.
We’re talking about this paper, by Zeyan Liew and colleagues, appearing in JAMA Pediatrics.
Using the incredibly robust health data infrastructure in Denmark, the researchers were able to identify 8,842 children born between 2000 and 2013 with ASD and matched each one to five control kids of the same sex and age without autism.
They then mapped the location the mothers of these kids lived while they were pregnant – down to 5 meters resolution, actually – to groundwater lithium levels.
Once that was done, the analysis was straightforward. Would moms who were pregnant in areas with higher groundwater lithium levels be more likely to have kids with ASD?
The results show a rather steady and consistent association between higher lithium levels in groundwater and the prevalence of ASD in children.
We’re not talking huge numbers, but moms who lived in the areas of the highest quartile of lithium were about 46% more likely to have a child with ASD. That’s a relative risk, of course – this would be like an increase from 1 in 100 kids to 1.5 in 100 kids. But still, it’s intriguing.
But the case is far from closed here.
Groundwater concentration of lithium and the amount of lithium a pregnant mother ingests are not the same thing. It does turn out that virtually all drinking water in Denmark comes from groundwater sources – but not all lithium comes from drinking water. There are plenty of dietary sources of lithium as well. And, of course, there is medical lithium, but we’ll get to that in a second.
First, let’s talk about those lithium measurements. They were taken in 2013 – after all these kids were born. The authors acknowledge this limitation but show a high correlation between measured levels in 2013 and earlier measured levels from prior studies, suggesting that lithium levels in a given area are quite constant over time. That’s great – but if lithium levels are constant over time, this study does nothing to shed light on why autism diagnoses seem to be increasing.
Let’s put some numbers to the lithium concentrations the authors examined. The average was about 12 mcg/L.
As a reminder, a standard therapeutic dose of lithium used for bipolar disorder is like 600 mg. That means you’d need to drink more than 2,500 of those 5-gallon jugs that sit on your water cooler, per day, to approximate the dose you’d get from a lithium tablet. Of course, small doses can still cause toxicity – but I wanted to put this in perspective.
Also, we have some data on pregnant women who take medical lithium. An analysis of nine studies showed that first-trimester lithium use may be associated with congenital malformations – particularly some specific heart malformations – and some birth complications. But three of four separate studies looking at longer-term neurodevelopmental outcomes did not find any effect on development, attainment of milestones, or IQ. One study of 15 kids exposed to medical lithium in utero did note minor neurologic dysfunction in one child and a low verbal IQ in another – but that’s a very small study.
Of course, lithium levels vary around the world as well. The U.S. Geological Survey examined lithium content in groundwater in the United States, as you can see here.
Our numbers are pretty similar to Denmark’s – in the 0-60 range. But an area in the Argentine Andes has levels as high as 1,600 mcg/L. A study of 194 babies from that area found higher lithium exposure was associated with lower fetal size, but I haven’t seen follow-up on neurodevelopmental outcomes.
The point is that there is a lot of variability here. It would be really interesting to map groundwater lithium levels to autism rates around the world. As a teaser, I will point out that, if you look at worldwide autism rates, you may be able to convince yourself that they are higher in more arid climates, and arid climates tend to have more groundwater lithium. But I’m really reaching here. More work needs to be done.
And I hope it is done quickly. Lithium is in the midst of becoming a very important commodity thanks to the shift to electric vehicles. While we can hope that recycling will claim most of those batteries at the end of their life, some will escape reclamation and potentially put more lithium into the drinking water. I’d like to know how risky that is before it happens.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. He has disclosed no relevant financial relationships. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and his new book, “How Medicine Works and When It Doesn’t”, is available now.
A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
Few diseases have stymied explanation like autism spectrum disorder (ASD). We know that the prevalence has been increasing dramatically, but we aren’t quite sure whether that is because of more screening and awareness or more fundamental changes. We know that much of the risk appears to be genetic, but there may be 1,000 genes involved in the syndrome. We know that certain environmental exposures, like pollution, might increase the risk – perhaps on a susceptible genetic background – but we’re not really sure which exposures are most harmful.
So, the search continues, across all domains of inquiry from cell culture to large epidemiologic analyses. And this week, a new player enters the field, and, as they say, it’s something in the water.
We’re talking about this paper, by Zeyan Liew and colleagues, appearing in JAMA Pediatrics.
Using the incredibly robust health data infrastructure in Denmark, the researchers were able to identify 8,842 children born between 2000 and 2013 with ASD and matched each one to five control kids of the same sex and age without autism.
They then mapped the location the mothers of these kids lived while they were pregnant – down to 5 meters resolution, actually – to groundwater lithium levels.
Once that was done, the analysis was straightforward. Would moms who were pregnant in areas with higher groundwater lithium levels be more likely to have kids with ASD?
The results show a rather steady and consistent association between higher lithium levels in groundwater and the prevalence of ASD in children.
We’re not talking huge numbers, but moms who lived in the areas of the highest quartile of lithium were about 46% more likely to have a child with ASD. That’s a relative risk, of course – this would be like an increase from 1 in 100 kids to 1.5 in 100 kids. But still, it’s intriguing.
But the case is far from closed here.
Groundwater concentration of lithium and the amount of lithium a pregnant mother ingests are not the same thing. It does turn out that virtually all drinking water in Denmark comes from groundwater sources – but not all lithium comes from drinking water. There are plenty of dietary sources of lithium as well. And, of course, there is medical lithium, but we’ll get to that in a second.
First, let’s talk about those lithium measurements. They were taken in 2013 – after all these kids were born. The authors acknowledge this limitation but show a high correlation between measured levels in 2013 and earlier measured levels from prior studies, suggesting that lithium levels in a given area are quite constant over time. That’s great – but if lithium levels are constant over time, this study does nothing to shed light on why autism diagnoses seem to be increasing.
Let’s put some numbers to the lithium concentrations the authors examined. The average was about 12 mcg/L.
As a reminder, a standard therapeutic dose of lithium used for bipolar disorder is like 600 mg. That means you’d need to drink more than 2,500 of those 5-gallon jugs that sit on your water cooler, per day, to approximate the dose you’d get from a lithium tablet. Of course, small doses can still cause toxicity – but I wanted to put this in perspective.
Also, we have some data on pregnant women who take medical lithium. An analysis of nine studies showed that first-trimester lithium use may be associated with congenital malformations – particularly some specific heart malformations – and some birth complications. But three of four separate studies looking at longer-term neurodevelopmental outcomes did not find any effect on development, attainment of milestones, or IQ. One study of 15 kids exposed to medical lithium in utero did note minor neurologic dysfunction in one child and a low verbal IQ in another – but that’s a very small study.
Of course, lithium levels vary around the world as well. The U.S. Geological Survey examined lithium content in groundwater in the United States, as you can see here.
Our numbers are pretty similar to Denmark’s – in the 0-60 range. But an area in the Argentine Andes has levels as high as 1,600 mcg/L. A study of 194 babies from that area found higher lithium exposure was associated with lower fetal size, but I haven’t seen follow-up on neurodevelopmental outcomes.
The point is that there is a lot of variability here. It would be really interesting to map groundwater lithium levels to autism rates around the world. As a teaser, I will point out that, if you look at worldwide autism rates, you may be able to convince yourself that they are higher in more arid climates, and arid climates tend to have more groundwater lithium. But I’m really reaching here. More work needs to be done.
And I hope it is done quickly. Lithium is in the midst of becoming a very important commodity thanks to the shift to electric vehicles. While we can hope that recycling will claim most of those batteries at the end of their life, some will escape reclamation and potentially put more lithium into the drinking water. I’d like to know how risky that is before it happens.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. He has disclosed no relevant financial relationships. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and his new book, “How Medicine Works and When It Doesn’t”, is available now.
A version of this article originally appeared on Medscape.com.
Specific brain damage links hypertension to cognitive impairment
Researchers have identified specific regions of the brain that appear to be damaged by high blood pressure. The finding may explain the link between hypertension and cognitive impairment.
They used genetic information from genome-wide association studies (GWASs) and MRI scans of the brain to study the relationship between hypertension, changes in brain structures, and cognitive impairment. Using Mendelian randomization techniques, they identified nine brain structures related to cognitive impairment that are affected by blood pressure.
“We knew before that raised blood pressure was related to changes in the brain, but our research has narrowed down the changes to those that appear to be potentially causally related to cognitive impairment,” senior author Tomasz Guzik, professor of cardiovascular medicine, at the University of Edinburgh and of the Jagiellonian University, Krakow, Poland, told this news organization.
“Our study confirms a potentially causal relationship between raised blood pressure and cognitive impairment, emphasizing the importance of preventing and treating hypertension,” Prof. Guzik noted.
“But it also identifies the brain culprits of this relationship,” he added.
In the future, it may be possible to assess these nine brain structures in people with high blood pressure to identify those at increased risk of developing cognitive impairment, he said. “These patients may need more intensive care for their blood pressure. We can also investigate these brain structures for potential signaling pathways and molecular changes to see if we can find new targets for treatment to prevent cognitive impairment.”
For this report, the investigators married together different research datasets to identify brain structures potentially responsible for the effects of blood pressure on cognitive function, using results from previous GWASs and observational data from 39,000 people in the UK Biobank registry for whom brain MRI data were available.
First, they mapped brain structures potentially influenced by blood pressure in midlife using MRI scans from people in the UK Biobank registry. Then they examined the relationship between blood pressure and cognitive function in the UK Biobank registry. Next, of the brain structures affected by blood pressure, they identified those that are causally linked to cognitive impairment.
This was possible thanks to genetic markers coding for increased blood pressure, brain structure imaging phenotypes, and those coding for cognitive impairment that could be used in Mendelian randomization studies.
“We looked at 3935 brain magnetic resonance imaging–derived phenotypes in the brain and cognitive function defined by fluid intelligence score to identify genetically predicted causal relationships,” Prof. Guzik said.
They identified 200 brain structures that were causally affected by systolic blood pressure. Of these, nine were also causally related to cognitive impairment. The results were validated in a second prospective cohort of patients with hypertension.
“Some of these structures, including putamen and the white matter regions spanning between the anterior corona radiata, anterior thalamic radiation, and anterior limb of the internal capsule, may represent the target brain regions at which systolic blood pressure acts on cognitive function,” the authors comment.
In an accompanying editorial, Ernesto Schiffrin, MD, and James Engert, PhD, McGill University, Montreal, say that further mechanistic studies of the effects of blood pressure on cognitive function are required to determine precise causal pathways and the roles of relevant brain regions.
“Eventually, biomarkers could be developed to inform antihypertensive trials. Whether clinical trials targeting the specific brain structures will be feasible or if specific antihypertensives could be found that target specific structures remains to be demonstrated,” they write.
“Thus, these new studies could lead to an understanding of the signaling pathways that explain how these structures relate vascular damage to cognitive impairment in hypertension, and contribute to the development of novel interventions to more successfully address the scourge of cognitive decline and dementia in the future,” the editorialists conclude.
The study was funded by the European Research Council, the British Heart Foundation, and the Italian Ministry of Health.
A version of this article first appeared on Medscape.com.
Researchers have identified specific regions of the brain that appear to be damaged by high blood pressure. The finding may explain the link between hypertension and cognitive impairment.
They used genetic information from genome-wide association studies (GWASs) and MRI scans of the brain to study the relationship between hypertension, changes in brain structures, and cognitive impairment. Using Mendelian randomization techniques, they identified nine brain structures related to cognitive impairment that are affected by blood pressure.
“We knew before that raised blood pressure was related to changes in the brain, but our research has narrowed down the changes to those that appear to be potentially causally related to cognitive impairment,” senior author Tomasz Guzik, professor of cardiovascular medicine, at the University of Edinburgh and of the Jagiellonian University, Krakow, Poland, told this news organization.
“Our study confirms a potentially causal relationship between raised blood pressure and cognitive impairment, emphasizing the importance of preventing and treating hypertension,” Prof. Guzik noted.
“But it also identifies the brain culprits of this relationship,” he added.
In the future, it may be possible to assess these nine brain structures in people with high blood pressure to identify those at increased risk of developing cognitive impairment, he said. “These patients may need more intensive care for their blood pressure. We can also investigate these brain structures for potential signaling pathways and molecular changes to see if we can find new targets for treatment to prevent cognitive impairment.”
For this report, the investigators married together different research datasets to identify brain structures potentially responsible for the effects of blood pressure on cognitive function, using results from previous GWASs and observational data from 39,000 people in the UK Biobank registry for whom brain MRI data were available.
First, they mapped brain structures potentially influenced by blood pressure in midlife using MRI scans from people in the UK Biobank registry. Then they examined the relationship between blood pressure and cognitive function in the UK Biobank registry. Next, of the brain structures affected by blood pressure, they identified those that are causally linked to cognitive impairment.
This was possible thanks to genetic markers coding for increased blood pressure, brain structure imaging phenotypes, and those coding for cognitive impairment that could be used in Mendelian randomization studies.
“We looked at 3935 brain magnetic resonance imaging–derived phenotypes in the brain and cognitive function defined by fluid intelligence score to identify genetically predicted causal relationships,” Prof. Guzik said.
They identified 200 brain structures that were causally affected by systolic blood pressure. Of these, nine were also causally related to cognitive impairment. The results were validated in a second prospective cohort of patients with hypertension.
“Some of these structures, including putamen and the white matter regions spanning between the anterior corona radiata, anterior thalamic radiation, and anterior limb of the internal capsule, may represent the target brain regions at which systolic blood pressure acts on cognitive function,” the authors comment.
In an accompanying editorial, Ernesto Schiffrin, MD, and James Engert, PhD, McGill University, Montreal, say that further mechanistic studies of the effects of blood pressure on cognitive function are required to determine precise causal pathways and the roles of relevant brain regions.
“Eventually, biomarkers could be developed to inform antihypertensive trials. Whether clinical trials targeting the specific brain structures will be feasible or if specific antihypertensives could be found that target specific structures remains to be demonstrated,” they write.
“Thus, these new studies could lead to an understanding of the signaling pathways that explain how these structures relate vascular damage to cognitive impairment in hypertension, and contribute to the development of novel interventions to more successfully address the scourge of cognitive decline and dementia in the future,” the editorialists conclude.
The study was funded by the European Research Council, the British Heart Foundation, and the Italian Ministry of Health.
A version of this article first appeared on Medscape.com.
Researchers have identified specific regions of the brain that appear to be damaged by high blood pressure. The finding may explain the link between hypertension and cognitive impairment.
They used genetic information from genome-wide association studies (GWASs) and MRI scans of the brain to study the relationship between hypertension, changes in brain structures, and cognitive impairment. Using Mendelian randomization techniques, they identified nine brain structures related to cognitive impairment that are affected by blood pressure.
“We knew before that raised blood pressure was related to changes in the brain, but our research has narrowed down the changes to those that appear to be potentially causally related to cognitive impairment,” senior author Tomasz Guzik, professor of cardiovascular medicine, at the University of Edinburgh and of the Jagiellonian University, Krakow, Poland, told this news organization.
“Our study confirms a potentially causal relationship between raised blood pressure and cognitive impairment, emphasizing the importance of preventing and treating hypertension,” Prof. Guzik noted.
“But it also identifies the brain culprits of this relationship,” he added.
In the future, it may be possible to assess these nine brain structures in people with high blood pressure to identify those at increased risk of developing cognitive impairment, he said. “These patients may need more intensive care for their blood pressure. We can also investigate these brain structures for potential signaling pathways and molecular changes to see if we can find new targets for treatment to prevent cognitive impairment.”
For this report, the investigators married together different research datasets to identify brain structures potentially responsible for the effects of blood pressure on cognitive function, using results from previous GWASs and observational data from 39,000 people in the UK Biobank registry for whom brain MRI data were available.
First, they mapped brain structures potentially influenced by blood pressure in midlife using MRI scans from people in the UK Biobank registry. Then they examined the relationship between blood pressure and cognitive function in the UK Biobank registry. Next, of the brain structures affected by blood pressure, they identified those that are causally linked to cognitive impairment.
This was possible thanks to genetic markers coding for increased blood pressure, brain structure imaging phenotypes, and those coding for cognitive impairment that could be used in Mendelian randomization studies.
“We looked at 3935 brain magnetic resonance imaging–derived phenotypes in the brain and cognitive function defined by fluid intelligence score to identify genetically predicted causal relationships,” Prof. Guzik said.
They identified 200 brain structures that were causally affected by systolic blood pressure. Of these, nine were also causally related to cognitive impairment. The results were validated in a second prospective cohort of patients with hypertension.
“Some of these structures, including putamen and the white matter regions spanning between the anterior corona radiata, anterior thalamic radiation, and anterior limb of the internal capsule, may represent the target brain regions at which systolic blood pressure acts on cognitive function,” the authors comment.
In an accompanying editorial, Ernesto Schiffrin, MD, and James Engert, PhD, McGill University, Montreal, say that further mechanistic studies of the effects of blood pressure on cognitive function are required to determine precise causal pathways and the roles of relevant brain regions.
“Eventually, biomarkers could be developed to inform antihypertensive trials. Whether clinical trials targeting the specific brain structures will be feasible or if specific antihypertensives could be found that target specific structures remains to be demonstrated,” they write.
“Thus, these new studies could lead to an understanding of the signaling pathways that explain how these structures relate vascular damage to cognitive impairment in hypertension, and contribute to the development of novel interventions to more successfully address the scourge of cognitive decline and dementia in the future,” the editorialists conclude.
The study was funded by the European Research Council, the British Heart Foundation, and the Italian Ministry of Health.
A version of this article first appeared on Medscape.com.
Cancer risk elevated after stroke in younger people
In young people, stroke might be the first manifestation of an underlying cancer, according to the investigators, led by Jamie Verhoeven, MD, PhD, with the department of neurology, Radboud University Medical Centre, Nijmegen, the Netherlands.
The new study can be viewed as a “stepping stone for future studies investigating the usefulness of screening for cancer after stroke,” the researchers say.
The study was published online in JAMA Network Open.
Currently, the diagnostic workup for young people with stroke includes searching for rare clotting disorders, although screening for cancer is not regularly performed.
Some research suggests that stroke and cancer are linked, but the literature is limited. In prior studies among people of all ages, cancer incidence after stroke has been variable – from 1% to 5% at 1 year and from 11% to 30% after 10 years.
To the team’s knowledge, only two studies have described the incidence of cancer after stroke among younger patients. One put the risk at 0.5% for people aged 18-50 years in the first year after stroke; the other described a cumulative risk of 17.3% in the 10 years after stroke for patients aged 18-55 years.
Using Dutch data, Dr. Verhoeven and colleagues identified 27,616 young stroke patients (age, 15-49 years; median age, 45 years) and 362,782 older stroke patients (median age, 76 years).
The cumulative incidence of any new cancer at 10 years was 3.7% among the younger stroke patients and 8.5% among the older stroke patients.
The incidence of a new cancer after stroke among younger patients was higher among women than men, while the opposite was true for older stroke patients.
Compared with the general population, younger stroke patients had a more than 2.5-fold greater likelihood of being diagnosed with a new cancer in the first year after ischemic stroke (standardized incidence ratio, 2.6). The risk was highest for lung cancer (SIR, 6.9), followed by hematologic cancers (SIR, 5.2).
Compared with the general population, younger stroke patients had nearly a 5.5-fold greater likelihood of being diagnosed with a new cancer in the first year after intracerebral hemorrhage (SIR, 5.4), and the risk was highest for hematologic cancers (SIR, 14.2).
In younger patients, the cumulative incidence of any cancer decreased over the years but remained significantly higher for 8 years following a stroke.
For patients aged 50 years or older, the 1-year risk for any new cancer after either ischemic stroke or intracerebral hemorrhage was 1.2 times higher, compared with the general population.
“We typically think of occult cancer as being a cause of stroke in an older population, given that the incidence of cancer increases over time [but] what this study shows is that we probably do need to consider occult cancer as an underlying cause of stroke even in a younger population,” said Laura Gioia, MD, stroke neurologist at the University of Montreal, who was not involved in the research.
Dr. Verhoeven and colleagues conclude that their finding supports the hypothesis of a causal link between cancer and stroke. Given the timing between stroke and cancer diagnosis, cancer may have been present when the stroke occurred and possibly played a role in causing it, the authors note. However, conclusions on causal mechanisms cannot be drawn from the current study.
The question of whether young stroke patients should be screened for cancer is a tough one, Dr. Gioia noted. “Cancer represents a small percentage of causes of stroke. That means you would have to screen a lot of people with a benefit that is still uncertain for the moment,” Dr. Gioia said in an interview.
“I think we need to keep cancer in mind as a cause of stroke in our young patients, and that should probably guide our history-taking with the patient and consider imaging when it’s appropriate and when we think that there could be an underlying occult cancer,” Dr. Gioia suggested.
The study was funded in part through unrestricted funding by Stryker, Medtronic, and Cerenovus. Dr. Verhoeven and Dr. Gioia have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In young people, stroke might be the first manifestation of an underlying cancer, according to the investigators, led by Jamie Verhoeven, MD, PhD, with the department of neurology, Radboud University Medical Centre, Nijmegen, the Netherlands.
The new study can be viewed as a “stepping stone for future studies investigating the usefulness of screening for cancer after stroke,” the researchers say.
The study was published online in JAMA Network Open.
Currently, the diagnostic workup for young people with stroke includes searching for rare clotting disorders, although screening for cancer is not regularly performed.
Some research suggests that stroke and cancer are linked, but the literature is limited. In prior studies among people of all ages, cancer incidence after stroke has been variable – from 1% to 5% at 1 year and from 11% to 30% after 10 years.
To the team’s knowledge, only two studies have described the incidence of cancer after stroke among younger patients. One put the risk at 0.5% for people aged 18-50 years in the first year after stroke; the other described a cumulative risk of 17.3% in the 10 years after stroke for patients aged 18-55 years.
Using Dutch data, Dr. Verhoeven and colleagues identified 27,616 young stroke patients (age, 15-49 years; median age, 45 years) and 362,782 older stroke patients (median age, 76 years).
The cumulative incidence of any new cancer at 10 years was 3.7% among the younger stroke patients and 8.5% among the older stroke patients.
The incidence of a new cancer after stroke among younger patients was higher among women than men, while the opposite was true for older stroke patients.
Compared with the general population, younger stroke patients had a more than 2.5-fold greater likelihood of being diagnosed with a new cancer in the first year after ischemic stroke (standardized incidence ratio, 2.6). The risk was highest for lung cancer (SIR, 6.9), followed by hematologic cancers (SIR, 5.2).
Compared with the general population, younger stroke patients had nearly a 5.5-fold greater likelihood of being diagnosed with a new cancer in the first year after intracerebral hemorrhage (SIR, 5.4), and the risk was highest for hematologic cancers (SIR, 14.2).
In younger patients, the cumulative incidence of any cancer decreased over the years but remained significantly higher for 8 years following a stroke.
For patients aged 50 years or older, the 1-year risk for any new cancer after either ischemic stroke or intracerebral hemorrhage was 1.2 times higher, compared with the general population.
“We typically think of occult cancer as being a cause of stroke in an older population, given that the incidence of cancer increases over time [but] what this study shows is that we probably do need to consider occult cancer as an underlying cause of stroke even in a younger population,” said Laura Gioia, MD, stroke neurologist at the University of Montreal, who was not involved in the research.
Dr. Verhoeven and colleagues conclude that their finding supports the hypothesis of a causal link between cancer and stroke. Given the timing between stroke and cancer diagnosis, cancer may have been present when the stroke occurred and possibly played a role in causing it, the authors note. However, conclusions on causal mechanisms cannot be drawn from the current study.
The question of whether young stroke patients should be screened for cancer is a tough one, Dr. Gioia noted. “Cancer represents a small percentage of causes of stroke. That means you would have to screen a lot of people with a benefit that is still uncertain for the moment,” Dr. Gioia said in an interview.
“I think we need to keep cancer in mind as a cause of stroke in our young patients, and that should probably guide our history-taking with the patient and consider imaging when it’s appropriate and when we think that there could be an underlying occult cancer,” Dr. Gioia suggested.
The study was funded in part through unrestricted funding by Stryker, Medtronic, and Cerenovus. Dr. Verhoeven and Dr. Gioia have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In young people, stroke might be the first manifestation of an underlying cancer, according to the investigators, led by Jamie Verhoeven, MD, PhD, with the department of neurology, Radboud University Medical Centre, Nijmegen, the Netherlands.
The new study can be viewed as a “stepping stone for future studies investigating the usefulness of screening for cancer after stroke,” the researchers say.
The study was published online in JAMA Network Open.
Currently, the diagnostic workup for young people with stroke includes searching for rare clotting disorders, although screening for cancer is not regularly performed.
Some research suggests that stroke and cancer are linked, but the literature is limited. In prior studies among people of all ages, cancer incidence after stroke has been variable – from 1% to 5% at 1 year and from 11% to 30% after 10 years.
To the team’s knowledge, only two studies have described the incidence of cancer after stroke among younger patients. One put the risk at 0.5% for people aged 18-50 years in the first year after stroke; the other described a cumulative risk of 17.3% in the 10 years after stroke for patients aged 18-55 years.
Using Dutch data, Dr. Verhoeven and colleagues identified 27,616 young stroke patients (age, 15-49 years; median age, 45 years) and 362,782 older stroke patients (median age, 76 years).
The cumulative incidence of any new cancer at 10 years was 3.7% among the younger stroke patients and 8.5% among the older stroke patients.
The incidence of a new cancer after stroke among younger patients was higher among women than men, while the opposite was true for older stroke patients.
Compared with the general population, younger stroke patients had a more than 2.5-fold greater likelihood of being diagnosed with a new cancer in the first year after ischemic stroke (standardized incidence ratio, 2.6). The risk was highest for lung cancer (SIR, 6.9), followed by hematologic cancers (SIR, 5.2).
Compared with the general population, younger stroke patients had nearly a 5.5-fold greater likelihood of being diagnosed with a new cancer in the first year after intracerebral hemorrhage (SIR, 5.4), and the risk was highest for hematologic cancers (SIR, 14.2).
In younger patients, the cumulative incidence of any cancer decreased over the years but remained significantly higher for 8 years following a stroke.
For patients aged 50 years or older, the 1-year risk for any new cancer after either ischemic stroke or intracerebral hemorrhage was 1.2 times higher, compared with the general population.
“We typically think of occult cancer as being a cause of stroke in an older population, given that the incidence of cancer increases over time [but] what this study shows is that we probably do need to consider occult cancer as an underlying cause of stroke even in a younger population,” said Laura Gioia, MD, stroke neurologist at the University of Montreal, who was not involved in the research.
Dr. Verhoeven and colleagues conclude that their finding supports the hypothesis of a causal link between cancer and stroke. Given the timing between stroke and cancer diagnosis, cancer may have been present when the stroke occurred and possibly played a role in causing it, the authors note. However, conclusions on causal mechanisms cannot be drawn from the current study.
The question of whether young stroke patients should be screened for cancer is a tough one, Dr. Gioia noted. “Cancer represents a small percentage of causes of stroke. That means you would have to screen a lot of people with a benefit that is still uncertain for the moment,” Dr. Gioia said in an interview.
“I think we need to keep cancer in mind as a cause of stroke in our young patients, and that should probably guide our history-taking with the patient and consider imaging when it’s appropriate and when we think that there could be an underlying occult cancer,” Dr. Gioia suggested.
The study was funded in part through unrestricted funding by Stryker, Medtronic, and Cerenovus. Dr. Verhoeven and Dr. Gioia have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN