User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Diagnose Pediatric Melanoma Using the CUP Criteria
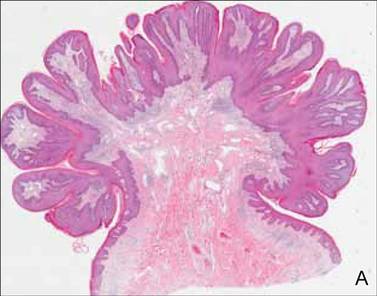
It is typically perceived that cutaneous malignancies in childhood and adolescence are uncommon, but the incidence of melanoma in this patient population is increasing. In 2014, the American Cancer Society estimated 310 cases of malignant melanoma in adolescents aged 15 to 19 years, making it the 8th most prevalent cancer in adolescents. Based on US data for pediatric patients aged 0 to 19 years (2006-2010), melanoma was reported to be more common in girls and non-Hispanic whites. For pediatric cases diagnosed in 2003-2009, the 5-year observed survival rate was 95%, an increase from 83% in 1975-1979. These findings serve as a reminder for practitioners to encourage young patients to practice good sun protection habits. Even if patients are careful, melanomas do occur in childhood and early diagnosis is key.
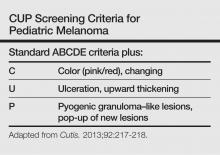
The ABCDE—asymmetry, border irregularity, color variegation, diameter, evolving—criteria have been used for melanoma screening in adults and may be helpful in detecting pediatric melanoma. However, Silverberg and McCuaig proposed a mnemonic specifically for screening children called the CUP criteria. Because melanomas of childhood do not necessarily arise in a preexisting nevus, the CUP criteria take into account melanomas that arise de novo and may present histologically as spitzoid neoplasms. The atypical presentation of melanomas in childhood includes color uniformity (pink/red), ulceration and upward thickening, and pyogenic granuloma–like lesions and pop-up of new lesions.
For more information on practice modifications from these screening criteria for pediatric melanoma, read Silverberg and McCuaig’s Cutis article “Melanoma in Childhood: Changing Our Mind-set.”
It is typically perceived that cutaneous malignancies in childhood and adolescence are uncommon, but the incidence of melanoma in this patient population is increasing. In 2014, the American Cancer Society estimated 310 cases of malignant melanoma in adolescents aged 15 to 19 years, making it the 8th most prevalent cancer in adolescents. Based on US data for pediatric patients aged 0 to 19 years (2006-2010), melanoma was reported to be more common in girls and non-Hispanic whites. For pediatric cases diagnosed in 2003-2009, the 5-year observed survival rate was 95%, an increase from 83% in 1975-1979. These findings serve as a reminder for practitioners to encourage young patients to practice good sun protection habits. Even if patients are careful, melanomas do occur in childhood and early diagnosis is key.
The ABCDE—asymmetry, border irregularity, color variegation, diameter, evolving—criteria have been used for melanoma screening in adults and may be helpful in detecting pediatric melanoma. However, Silverberg and McCuaig proposed a mnemonic specifically for screening children called the CUP criteria. Because melanomas of childhood do not necessarily arise in a preexisting nevus, the CUP criteria take into account melanomas that arise de novo and may present histologically as spitzoid neoplasms. The atypical presentation of melanomas in childhood includes color uniformity (pink/red), ulceration and upward thickening, and pyogenic granuloma–like lesions and pop-up of new lesions.
For more information on practice modifications from these screening criteria for pediatric melanoma, read Silverberg and McCuaig’s Cutis article “Melanoma in Childhood: Changing Our Mind-set.”
It is typically perceived that cutaneous malignancies in childhood and adolescence are uncommon, but the incidence of melanoma in this patient population is increasing. In 2014, the American Cancer Society estimated 310 cases of malignant melanoma in adolescents aged 15 to 19 years, making it the 8th most prevalent cancer in adolescents. Based on US data for pediatric patients aged 0 to 19 years (2006-2010), melanoma was reported to be more common in girls and non-Hispanic whites. For pediatric cases diagnosed in 2003-2009, the 5-year observed survival rate was 95%, an increase from 83% in 1975-1979. These findings serve as a reminder for practitioners to encourage young patients to practice good sun protection habits. Even if patients are careful, melanomas do occur in childhood and early diagnosis is key.
The ABCDE—asymmetry, border irregularity, color variegation, diameter, evolving—criteria have been used for melanoma screening in adults and may be helpful in detecting pediatric melanoma. However, Silverberg and McCuaig proposed a mnemonic specifically for screening children called the CUP criteria. Because melanomas of childhood do not necessarily arise in a preexisting nevus, the CUP criteria take into account melanomas that arise de novo and may present histologically as spitzoid neoplasms. The atypical presentation of melanomas in childhood includes color uniformity (pink/red), ulceration and upward thickening, and pyogenic granuloma–like lesions and pop-up of new lesions.
For more information on practice modifications from these screening criteria for pediatric melanoma, read Silverberg and McCuaig’s Cutis article “Melanoma in Childhood: Changing Our Mind-set.”
Product News: 02 2015
Bellafill
Suneva Medical, Inc, announces US Food and Drug Administration approval of the polymethylmethacrylate collagen filler Bellafill for the correction of moderate to severe, atrophic, distensible facial acne scars on the cheek in patients older than 21 years. Bellafill adds volume to the skin to lift and smooth out pitted acne scars to the level of the surrounding skin. Bellafill also is indicated for correction of nasolabial folds. For more information, visit www.bellafill.com.
Benzac
Galderma Laboratories, LP, launches Benzac Acne Solutions, an over-the-counter 3-step acne regimen consisting of a skin balancing foaming cleanser, intensive spot treatment, and blemish clearing hydrator. In addition to salicylic acid, Benzac also contains Kakadu plum (an antioxidant) to brighten the skin, lemon myrtle (an astringent) to reduce excess oil, and zinc to act as a barrier against skin moisture loss. Benzac contains pharmaceutical-grade East Indian sandalwood oil to calm and soothe the skin. For more information, visit www.benzac.com.
The Promius Promise App
Promius Pharma, LLC, marketers of Zenatane (isotretinoin capsules), launches The Promius Promise App designed to help educate and guide patients through the iPLEDGE program from the first visit to the last visit. The app is free for patients who have received a Zenatane prescription through The Promius Promise program. Patients can easily find important information, which may help with treatment compliance. The app will be useful for young adults and teenagers as well as their parents. For more information, visit www.zenatane.com/toolkit/Promius-Promise-Enrollment-Form.php.
Soolantra
Galderma Laboratories, LP, announces US Food and Drug Administration approval of Soolantra Cream 1% for the once-daily treatment of inflammatory lesions of rosacea. Ivermectin, the active ingredient in Soolantra, has both anti-inflammatory and antiparasitic activity. The basis for the cream formulation is Cetaphil Moisturizing Cream. Soolantra offers patients improvement as early as week 2 of treatment. For more information, visit www.soolantra.com/hcp.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at cutis@frontlinemedcom.com.
Bellafill
Suneva Medical, Inc, announces US Food and Drug Administration approval of the polymethylmethacrylate collagen filler Bellafill for the correction of moderate to severe, atrophic, distensible facial acne scars on the cheek in patients older than 21 years. Bellafill adds volume to the skin to lift and smooth out pitted acne scars to the level of the surrounding skin. Bellafill also is indicated for correction of nasolabial folds. For more information, visit www.bellafill.com.
Benzac
Galderma Laboratories, LP, launches Benzac Acne Solutions, an over-the-counter 3-step acne regimen consisting of a skin balancing foaming cleanser, intensive spot treatment, and blemish clearing hydrator. In addition to salicylic acid, Benzac also contains Kakadu plum (an antioxidant) to brighten the skin, lemon myrtle (an astringent) to reduce excess oil, and zinc to act as a barrier against skin moisture loss. Benzac contains pharmaceutical-grade East Indian sandalwood oil to calm and soothe the skin. For more information, visit www.benzac.com.
The Promius Promise App
Promius Pharma, LLC, marketers of Zenatane (isotretinoin capsules), launches The Promius Promise App designed to help educate and guide patients through the iPLEDGE program from the first visit to the last visit. The app is free for patients who have received a Zenatane prescription through The Promius Promise program. Patients can easily find important information, which may help with treatment compliance. The app will be useful for young adults and teenagers as well as their parents. For more information, visit www.zenatane.com/toolkit/Promius-Promise-Enrollment-Form.php.
Soolantra
Galderma Laboratories, LP, announces US Food and Drug Administration approval of Soolantra Cream 1% for the once-daily treatment of inflammatory lesions of rosacea. Ivermectin, the active ingredient in Soolantra, has both anti-inflammatory and antiparasitic activity. The basis for the cream formulation is Cetaphil Moisturizing Cream. Soolantra offers patients improvement as early as week 2 of treatment. For more information, visit www.soolantra.com/hcp.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at cutis@frontlinemedcom.com.
Bellafill
Suneva Medical, Inc, announces US Food and Drug Administration approval of the polymethylmethacrylate collagen filler Bellafill for the correction of moderate to severe, atrophic, distensible facial acne scars on the cheek in patients older than 21 years. Bellafill adds volume to the skin to lift and smooth out pitted acne scars to the level of the surrounding skin. Bellafill also is indicated for correction of nasolabial folds. For more information, visit www.bellafill.com.
Benzac
Galderma Laboratories, LP, launches Benzac Acne Solutions, an over-the-counter 3-step acne regimen consisting of a skin balancing foaming cleanser, intensive spot treatment, and blemish clearing hydrator. In addition to salicylic acid, Benzac also contains Kakadu plum (an antioxidant) to brighten the skin, lemon myrtle (an astringent) to reduce excess oil, and zinc to act as a barrier against skin moisture loss. Benzac contains pharmaceutical-grade East Indian sandalwood oil to calm and soothe the skin. For more information, visit www.benzac.com.
The Promius Promise App
Promius Pharma, LLC, marketers of Zenatane (isotretinoin capsules), launches The Promius Promise App designed to help educate and guide patients through the iPLEDGE program from the first visit to the last visit. The app is free for patients who have received a Zenatane prescription through The Promius Promise program. Patients can easily find important information, which may help with treatment compliance. The app will be useful for young adults and teenagers as well as their parents. For more information, visit www.zenatane.com/toolkit/Promius-Promise-Enrollment-Form.php.
Soolantra
Galderma Laboratories, LP, announces US Food and Drug Administration approval of Soolantra Cream 1% for the once-daily treatment of inflammatory lesions of rosacea. Ivermectin, the active ingredient in Soolantra, has both anti-inflammatory and antiparasitic activity. The basis for the cream formulation is Cetaphil Moisturizing Cream. Soolantra offers patients improvement as early as week 2 of treatment. For more information, visit www.soolantra.com/hcp.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at cutis@frontlinemedcom.com.
A Primer to Natural Hair Care Practices in Black Patients
The phenomenon of natural (nonchemically treated) hair in individuals of African and Afro-Caribbean descent is sweeping across the United States. The ideals of beauty among this patient population have shifted from a relaxed, straightened, noncurly look to a more natural curly and/or kinky appearance. The discussion on natural hair versus straight hair has been brought to the mainstream by films such as Good Hair (2009). Furthermore, major hair care companies have increased their marketing of natural hair products to address the needs of these patients.
Popular traumatic hair care practices such as chemical relaxation and thermal straightening may lead to hair damage. Although the role of hair care practices in various scalp and hair disorders is ambiguous, traumatic practices commonly are performed by patients who are diagnosed with dermatologic conditions such as scarring alopecia.1 Alopecia is the fourth most common dermatologic diagnosis in black patients.2 Central centrifugal cicatricial alopecia is the most common form of scarring alopecia in this patient population3 and has been associated with traumatic hair care practices. As a result, many patients have switched to natural hairstyles that are less traumatic and damaging, often due to recommendations by dermatologists.
As the US population continues to become more diverse, dermatologists will be faced with many questions regarding hair disease and natural hair care in patients with skin of color. A basic understanding of hair care practices among black individuals is important to aid in the diagnosis and treatment of hair shaft and scalp disorders.4 When patients switch to natural hairstyles, are dermatologists prepared to answer questions that may arise during this process? This article will familiarize dermatologists with basic hair care terminology and general recommendations they can make to black patients who are transitioning to natural hairstyles.
Characteristics of Hair in the Skin of Color Population
A basic understanding of the structural properties of hair is fundamental. Human hair is categorized into 3 groups: Asian, Caucasian, and African.5 African hair typically is curly and, depending on the degree of the curl, is more susceptible to damage due to increased mechanical fragility. It also has a tendency to form knots and fissures along the hair shaft, which causes additional fracturing with simple manipulation. African hair grows more slowly than Asian and Caucasian hair, which can be discouraging to patients. It also has a lower water concentration and does not become coated with sebum as naturally as straightened hair.5 A simplified explanation of these characteristics can help patients understand how to proceed in managing and styling their natural hair.
As physicians, it is important for us to treat any underlying conditions related to the hair and scalp in black patients. Common dermatologic conditions such as seborrheic dermatitis, lupus, folliculitis, and alopecia can affect patients’ hair health. In addition to traumatic hair care practices, inflammation secondary to bacterial infections can contribute to the onset of central centrifugal cicatricial alopecia.6 Therefore, a detailed history and physical examination are needed to evaluate the etiology of associated symptoms. Treatment of these associated symptoms will aid in the overall care of patients.
Transitioning to Natural Hairstyles
Following evaluation and treatment of any hair or scalp conditions, how can dermatologists help black patients transition to natural hairstyles? The term transition refers to the process of switching from a chemically relaxed or thermally straightened hairstyle to a natural hairstyle. Dermatologists must understand the common terminology used to describe natural hair practices in this patient population.
There are several methods patients can use to transition from chemically treated hairstyles to natural hairstyles. Patients may consider the option of the “big chop,” or cutting off all chemically treated hair. This option typically leaves women with very short hairstyles down to the new growth, or hair that has grown since the last chemical relaxer. Other commonly used methods during the transition phase include protective styling (eg, braids, weaves, extensions) or simply growing out the chemically treated hair.
Protective styling methods such as braids, weaves, and extensions allow hair to be easily styled while the chemically treated hair grows out over time.7 Typically, protective styles may be worn for weeks to months, allowing hair growth without hair breakage and shedding. Hair weaving is a practice that incorporates artificial (synthetic) or human hair into one’s natural scalp hair.8 There are various techniques to extend hair including clip-in extensions, hair bonding and fusion with adhesives, sewing hair into braided hair, or the application of single strands of hair into a cap made of nylon mesh known as a lace front. Braided styles, weaves, and hair extensions cannot be washed as often as natural hair, but it is important to remind patients to replenish moisture as often as possible. Moisturizing or greasing the exposed scalp and proximal hair shafts can assist with water retention. It is imperative to inform patients that overuse of tight braids and glues for weaves and extensions may further damage the hair and scalp. Some of the natural ingredients commonly used in moisturizers include olive oil, jojoba oil, coconut oil, castor oil, and glycerin. These products can commonly cause pomade acne, which should be recognized and treated by dermatologists. Furthermore, long weaves and extensions can put excess weight on natural hair causing breakage. To prevent breakage, wearing an updo (a hairstyle in which the hair is pulled upward) can reduce the heavy strain on the hair.
Dermatologists should remind patients who wish to grow out chemically treated hair to frequently moisturize the hair and scalp as well as to avoid trauma to prevent hair breakage. As the natural hair grows out, the patient will experience varying hair textures from the natural curly hair to the previously processed straightened hair; as a result, the hair may tangle and become damaged. Manual detangling and detangling conditioners can help prevent damage. Patients should be advised to detangle the hair in sections first with the fingers, then with a wide-tooth comb working retrograde from the hair end to the roots.
Frequent hair trimming, ranging from every 4 to 6 weeks to every 2 to 4 months, should be recommended to patients who are experiencing breakage or wish to prevent damage. Trimming damaged hair can relieve excess weight on the natural hair and remove split ends, which promotes hair growth. Braiding and other lengthening techniques can prevent the hair from curling upon itself or tangling, causing less kinking and thereby decreasing the need for trimming.7 Wearing bonnets, using satin pillowcases, and wearing protective hairstyles while sleeping also can decrease hair breakage and hair loss. A commonly used hairstyle to protect the hair while sleeping is called “pineappling,” which is used to preserve and protect curls. This technique is described as gathering the hair in a high but loose ponytail at the top of the head. For patients with straightened hair, wrapping the hair underneath a bonnet or satin scarf while sleeping can prevent damage.
Managing Natural Hairstyles
An important factor in the management of natural hairstyles is the retention of hair moisture, as there is less water content in African hair compared to other hair types.5 Overuse of heat and harsh shampoos can strip moisture from the hair. Similar to patients with atopic dermatitis who should restore and maintain the skin barrier to prevent transepidermal water loss, it is important to remind patients with natural hairstyles to avoid using products and styling practices that may further deplete water content in the hair. Moisture is crucial to healthy hair.
A common culprit in shampoos that leads to hair dryness is sodium lauryl sulfate/sodium laureth sulfate, a detergent/surfactant used as a foaming agent. Sodium lauryl sulfate is a potent degreaser that binds dirt and excess product on the hair and scalp. It also dissolves oil in the hair, causing additional dryness and breakage.
Patients with natural hairstyles commonly use sulfate-free shampoos to prevent stripping the hair of its moisture and natural oils. Another method used to prevent hair dryness is co-washing, or washing the hair with a conditioner. Co-washing can effectively cleanse the hair while maintaining moisture. The use of cationic ingredients in conditioners aids in sealing moisture within the hair shaft. Hair consists of the negatively charged protein keratin, which binds to cationic surfactants in conditioners.9 The hydrophobic ends of the surfactant prevent the substance from being rinsed out and act to restore the hair barrier.
Silicone is another important ingredient in hair care products. In patients with natural hair, there are varying views on the use of products containing silicone. Silicones are added to products designed to coat the hair, adding shine, retaining moisture, and providing thermal protection. Silicones are used to provide “slip.” Slip is a term that is commonly used among patients with natural hair to describe how slippery a product is and how easily the product will help comb or detangle the hair. There are 2 basic types of silicones: water insoluble and water soluble. Water-insoluble silicones traditionally build up on the hair and require surfactant-containing shampoos to becompletely removed. Residue buildup on the hair weighs the hair down and causes damage. In contrast, water-soluble silicones do not build up and typically do not cause damage.
Silicones with the prefixes PEG- or PPG- typically are water soluble and will not build up on the hair. Dimethicone copolyol and lauryl methicone copolyol are other water-soluble silicones. In general, water-soluble silicones provide moisturizing properties without leaving residue. Other silicones such as amodimethicone and cyclomethicone are not water soluble but have properties that prevent buildup.
It is common practice for patients with natural hairstyles to avoid using water-insoluble silicones. As dermatologists, we can recommend silicone-free conditioners or conditioners containing water-soluble silicones to prevent hair dehydration and subsequent breakage. It may be advantageous to have patients try various products to determine which ones work best for their hair.
More Resources for Patients
Dermatologists have extensive knowledge of the pathophysiology of skin, hair, and nail diseases; however, despite our vast knowledge, we also need to recognize our limits. In addition to increasing your own knowledge of natural hair care practices to help your patients, it is important to recommend that your patients search for additional resources to aid in their transition to natural hairstyles. Natural hairstylists can be great resources for patients to help with hair management. In the current digital age, there also are thousands of blogs and social media forums dedicated to the topic of natural hair care. Advising patients to consult natural hair care resources can be beneficial, but as hair specialists, it also is important for us to dispel any false information that our patients may receive. As physicians, it is essential not only to manage patients who present to our offices with conditions resulting from damaging hair practices but also to help prevent such conditions from occurring. Although there may not be an overwhelming amount of evidence-based medical research to guide our decisions, we also can learn from the thousands of patients who have articulated their stories and experiences. Through observing and listening to our patients, we can incorporate this new knowledge in the management of our patients.
1. Shah SK, Alexis AF. Central centrifugal cicatricial alopecia: retrospective chart review. J Cutan Med Surg. 2010;14:212-222.
2. Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
3. Uhlenhake EE, Mehregan DM. Prospective histologic examinations in patients who practice traumatic hairstyling [published online ahead of print March 3, 2013]. Int J Dermatol. 2013;52:1506-1512.
4. Roseborough IE, McMichael AJ. Hair care practices in African-American patients. Semin Cutan Med Surg. 2009;28:103-108.
5. Kelly AP, Taylor S, eds. Dermatology for Skin of Color. New York: McGraw-Hill; 2009.
6. Kyei A, Bergfeld WF, Piliang M, et al. Medical and environmental risk factors for the development of central centrifugal cicatricial alopecia: a population study [published online ahead of print April 11, 2011]. Arch Dermatol. 2011;147:909-914.
7. Walton N, Carter ET. Better Than Good Hair: The Curly Girl Guide to Healthy, Gorgeous Natural Hair! New York, NY: Amistad; 2013.
8. Quinn CR, Quinn TM, Kelly AP. Hair care practices in African American women. Cutis. 2003;72:280-282, 285-289.
9. Cruz CF, Fernandes MM, Gomes AC, et al. Keratins and lipids in ethnic hair [published online ahead of print January 24, 2013]. Int J Cosmet Sci. 2013;35:244-249.
The phenomenon of natural (nonchemically treated) hair in individuals of African and Afro-Caribbean descent is sweeping across the United States. The ideals of beauty among this patient population have shifted from a relaxed, straightened, noncurly look to a more natural curly and/or kinky appearance. The discussion on natural hair versus straight hair has been brought to the mainstream by films such as Good Hair (2009). Furthermore, major hair care companies have increased their marketing of natural hair products to address the needs of these patients.
Popular traumatic hair care practices such as chemical relaxation and thermal straightening may lead to hair damage. Although the role of hair care practices in various scalp and hair disorders is ambiguous, traumatic practices commonly are performed by patients who are diagnosed with dermatologic conditions such as scarring alopecia.1 Alopecia is the fourth most common dermatologic diagnosis in black patients.2 Central centrifugal cicatricial alopecia is the most common form of scarring alopecia in this patient population3 and has been associated with traumatic hair care practices. As a result, many patients have switched to natural hairstyles that are less traumatic and damaging, often due to recommendations by dermatologists.
As the US population continues to become more diverse, dermatologists will be faced with many questions regarding hair disease and natural hair care in patients with skin of color. A basic understanding of hair care practices among black individuals is important to aid in the diagnosis and treatment of hair shaft and scalp disorders.4 When patients switch to natural hairstyles, are dermatologists prepared to answer questions that may arise during this process? This article will familiarize dermatologists with basic hair care terminology and general recommendations they can make to black patients who are transitioning to natural hairstyles.
Characteristics of Hair in the Skin of Color Population
A basic understanding of the structural properties of hair is fundamental. Human hair is categorized into 3 groups: Asian, Caucasian, and African.5 African hair typically is curly and, depending on the degree of the curl, is more susceptible to damage due to increased mechanical fragility. It also has a tendency to form knots and fissures along the hair shaft, which causes additional fracturing with simple manipulation. African hair grows more slowly than Asian and Caucasian hair, which can be discouraging to patients. It also has a lower water concentration and does not become coated with sebum as naturally as straightened hair.5 A simplified explanation of these characteristics can help patients understand how to proceed in managing and styling their natural hair.
As physicians, it is important for us to treat any underlying conditions related to the hair and scalp in black patients. Common dermatologic conditions such as seborrheic dermatitis, lupus, folliculitis, and alopecia can affect patients’ hair health. In addition to traumatic hair care practices, inflammation secondary to bacterial infections can contribute to the onset of central centrifugal cicatricial alopecia.6 Therefore, a detailed history and physical examination are needed to evaluate the etiology of associated symptoms. Treatment of these associated symptoms will aid in the overall care of patients.
Transitioning to Natural Hairstyles
Following evaluation and treatment of any hair or scalp conditions, how can dermatologists help black patients transition to natural hairstyles? The term transition refers to the process of switching from a chemically relaxed or thermally straightened hairstyle to a natural hairstyle. Dermatologists must understand the common terminology used to describe natural hair practices in this patient population.
There are several methods patients can use to transition from chemically treated hairstyles to natural hairstyles. Patients may consider the option of the “big chop,” or cutting off all chemically treated hair. This option typically leaves women with very short hairstyles down to the new growth, or hair that has grown since the last chemical relaxer. Other commonly used methods during the transition phase include protective styling (eg, braids, weaves, extensions) or simply growing out the chemically treated hair.
Protective styling methods such as braids, weaves, and extensions allow hair to be easily styled while the chemically treated hair grows out over time.7 Typically, protective styles may be worn for weeks to months, allowing hair growth without hair breakage and shedding. Hair weaving is a practice that incorporates artificial (synthetic) or human hair into one’s natural scalp hair.8 There are various techniques to extend hair including clip-in extensions, hair bonding and fusion with adhesives, sewing hair into braided hair, or the application of single strands of hair into a cap made of nylon mesh known as a lace front. Braided styles, weaves, and hair extensions cannot be washed as often as natural hair, but it is important to remind patients to replenish moisture as often as possible. Moisturizing or greasing the exposed scalp and proximal hair shafts can assist with water retention. It is imperative to inform patients that overuse of tight braids and glues for weaves and extensions may further damage the hair and scalp. Some of the natural ingredients commonly used in moisturizers include olive oil, jojoba oil, coconut oil, castor oil, and glycerin. These products can commonly cause pomade acne, which should be recognized and treated by dermatologists. Furthermore, long weaves and extensions can put excess weight on natural hair causing breakage. To prevent breakage, wearing an updo (a hairstyle in which the hair is pulled upward) can reduce the heavy strain on the hair.
Dermatologists should remind patients who wish to grow out chemically treated hair to frequently moisturize the hair and scalp as well as to avoid trauma to prevent hair breakage. As the natural hair grows out, the patient will experience varying hair textures from the natural curly hair to the previously processed straightened hair; as a result, the hair may tangle and become damaged. Manual detangling and detangling conditioners can help prevent damage. Patients should be advised to detangle the hair in sections first with the fingers, then with a wide-tooth comb working retrograde from the hair end to the roots.
Frequent hair trimming, ranging from every 4 to 6 weeks to every 2 to 4 months, should be recommended to patients who are experiencing breakage or wish to prevent damage. Trimming damaged hair can relieve excess weight on the natural hair and remove split ends, which promotes hair growth. Braiding and other lengthening techniques can prevent the hair from curling upon itself or tangling, causing less kinking and thereby decreasing the need for trimming.7 Wearing bonnets, using satin pillowcases, and wearing protective hairstyles while sleeping also can decrease hair breakage and hair loss. A commonly used hairstyle to protect the hair while sleeping is called “pineappling,” which is used to preserve and protect curls. This technique is described as gathering the hair in a high but loose ponytail at the top of the head. For patients with straightened hair, wrapping the hair underneath a bonnet or satin scarf while sleeping can prevent damage.
Managing Natural Hairstyles
An important factor in the management of natural hairstyles is the retention of hair moisture, as there is less water content in African hair compared to other hair types.5 Overuse of heat and harsh shampoos can strip moisture from the hair. Similar to patients with atopic dermatitis who should restore and maintain the skin barrier to prevent transepidermal water loss, it is important to remind patients with natural hairstyles to avoid using products and styling practices that may further deplete water content in the hair. Moisture is crucial to healthy hair.
A common culprit in shampoos that leads to hair dryness is sodium lauryl sulfate/sodium laureth sulfate, a detergent/surfactant used as a foaming agent. Sodium lauryl sulfate is a potent degreaser that binds dirt and excess product on the hair and scalp. It also dissolves oil in the hair, causing additional dryness and breakage.
Patients with natural hairstyles commonly use sulfate-free shampoos to prevent stripping the hair of its moisture and natural oils. Another method used to prevent hair dryness is co-washing, or washing the hair with a conditioner. Co-washing can effectively cleanse the hair while maintaining moisture. The use of cationic ingredients in conditioners aids in sealing moisture within the hair shaft. Hair consists of the negatively charged protein keratin, which binds to cationic surfactants in conditioners.9 The hydrophobic ends of the surfactant prevent the substance from being rinsed out and act to restore the hair barrier.
Silicone is another important ingredient in hair care products. In patients with natural hair, there are varying views on the use of products containing silicone. Silicones are added to products designed to coat the hair, adding shine, retaining moisture, and providing thermal protection. Silicones are used to provide “slip.” Slip is a term that is commonly used among patients with natural hair to describe how slippery a product is and how easily the product will help comb or detangle the hair. There are 2 basic types of silicones: water insoluble and water soluble. Water-insoluble silicones traditionally build up on the hair and require surfactant-containing shampoos to becompletely removed. Residue buildup on the hair weighs the hair down and causes damage. In contrast, water-soluble silicones do not build up and typically do not cause damage.
Silicones with the prefixes PEG- or PPG- typically are water soluble and will not build up on the hair. Dimethicone copolyol and lauryl methicone copolyol are other water-soluble silicones. In general, water-soluble silicones provide moisturizing properties without leaving residue. Other silicones such as amodimethicone and cyclomethicone are not water soluble but have properties that prevent buildup.
It is common practice for patients with natural hairstyles to avoid using water-insoluble silicones. As dermatologists, we can recommend silicone-free conditioners or conditioners containing water-soluble silicones to prevent hair dehydration and subsequent breakage. It may be advantageous to have patients try various products to determine which ones work best for their hair.
More Resources for Patients
Dermatologists have extensive knowledge of the pathophysiology of skin, hair, and nail diseases; however, despite our vast knowledge, we also need to recognize our limits. In addition to increasing your own knowledge of natural hair care practices to help your patients, it is important to recommend that your patients search for additional resources to aid in their transition to natural hairstyles. Natural hairstylists can be great resources for patients to help with hair management. In the current digital age, there also are thousands of blogs and social media forums dedicated to the topic of natural hair care. Advising patients to consult natural hair care resources can be beneficial, but as hair specialists, it also is important for us to dispel any false information that our patients may receive. As physicians, it is essential not only to manage patients who present to our offices with conditions resulting from damaging hair practices but also to help prevent such conditions from occurring. Although there may not be an overwhelming amount of evidence-based medical research to guide our decisions, we also can learn from the thousands of patients who have articulated their stories and experiences. Through observing and listening to our patients, we can incorporate this new knowledge in the management of our patients.
The phenomenon of natural (nonchemically treated) hair in individuals of African and Afro-Caribbean descent is sweeping across the United States. The ideals of beauty among this patient population have shifted from a relaxed, straightened, noncurly look to a more natural curly and/or kinky appearance. The discussion on natural hair versus straight hair has been brought to the mainstream by films such as Good Hair (2009). Furthermore, major hair care companies have increased their marketing of natural hair products to address the needs of these patients.
Popular traumatic hair care practices such as chemical relaxation and thermal straightening may lead to hair damage. Although the role of hair care practices in various scalp and hair disorders is ambiguous, traumatic practices commonly are performed by patients who are diagnosed with dermatologic conditions such as scarring alopecia.1 Alopecia is the fourth most common dermatologic diagnosis in black patients.2 Central centrifugal cicatricial alopecia is the most common form of scarring alopecia in this patient population3 and has been associated with traumatic hair care practices. As a result, many patients have switched to natural hairstyles that are less traumatic and damaging, often due to recommendations by dermatologists.
As the US population continues to become more diverse, dermatologists will be faced with many questions regarding hair disease and natural hair care in patients with skin of color. A basic understanding of hair care practices among black individuals is important to aid in the diagnosis and treatment of hair shaft and scalp disorders.4 When patients switch to natural hairstyles, are dermatologists prepared to answer questions that may arise during this process? This article will familiarize dermatologists with basic hair care terminology and general recommendations they can make to black patients who are transitioning to natural hairstyles.
Characteristics of Hair in the Skin of Color Population
A basic understanding of the structural properties of hair is fundamental. Human hair is categorized into 3 groups: Asian, Caucasian, and African.5 African hair typically is curly and, depending on the degree of the curl, is more susceptible to damage due to increased mechanical fragility. It also has a tendency to form knots and fissures along the hair shaft, which causes additional fracturing with simple manipulation. African hair grows more slowly than Asian and Caucasian hair, which can be discouraging to patients. It also has a lower water concentration and does not become coated with sebum as naturally as straightened hair.5 A simplified explanation of these characteristics can help patients understand how to proceed in managing and styling their natural hair.
As physicians, it is important for us to treat any underlying conditions related to the hair and scalp in black patients. Common dermatologic conditions such as seborrheic dermatitis, lupus, folliculitis, and alopecia can affect patients’ hair health. In addition to traumatic hair care practices, inflammation secondary to bacterial infections can contribute to the onset of central centrifugal cicatricial alopecia.6 Therefore, a detailed history and physical examination are needed to evaluate the etiology of associated symptoms. Treatment of these associated symptoms will aid in the overall care of patients.
Transitioning to Natural Hairstyles
Following evaluation and treatment of any hair or scalp conditions, how can dermatologists help black patients transition to natural hairstyles? The term transition refers to the process of switching from a chemically relaxed or thermally straightened hairstyle to a natural hairstyle. Dermatologists must understand the common terminology used to describe natural hair practices in this patient population.
There are several methods patients can use to transition from chemically treated hairstyles to natural hairstyles. Patients may consider the option of the “big chop,” or cutting off all chemically treated hair. This option typically leaves women with very short hairstyles down to the new growth, or hair that has grown since the last chemical relaxer. Other commonly used methods during the transition phase include protective styling (eg, braids, weaves, extensions) or simply growing out the chemically treated hair.
Protective styling methods such as braids, weaves, and extensions allow hair to be easily styled while the chemically treated hair grows out over time.7 Typically, protective styles may be worn for weeks to months, allowing hair growth without hair breakage and shedding. Hair weaving is a practice that incorporates artificial (synthetic) or human hair into one’s natural scalp hair.8 There are various techniques to extend hair including clip-in extensions, hair bonding and fusion with adhesives, sewing hair into braided hair, or the application of single strands of hair into a cap made of nylon mesh known as a lace front. Braided styles, weaves, and hair extensions cannot be washed as often as natural hair, but it is important to remind patients to replenish moisture as often as possible. Moisturizing or greasing the exposed scalp and proximal hair shafts can assist with water retention. It is imperative to inform patients that overuse of tight braids and glues for weaves and extensions may further damage the hair and scalp. Some of the natural ingredients commonly used in moisturizers include olive oil, jojoba oil, coconut oil, castor oil, and glycerin. These products can commonly cause pomade acne, which should be recognized and treated by dermatologists. Furthermore, long weaves and extensions can put excess weight on natural hair causing breakage. To prevent breakage, wearing an updo (a hairstyle in which the hair is pulled upward) can reduce the heavy strain on the hair.
Dermatologists should remind patients who wish to grow out chemically treated hair to frequently moisturize the hair and scalp as well as to avoid trauma to prevent hair breakage. As the natural hair grows out, the patient will experience varying hair textures from the natural curly hair to the previously processed straightened hair; as a result, the hair may tangle and become damaged. Manual detangling and detangling conditioners can help prevent damage. Patients should be advised to detangle the hair in sections first with the fingers, then with a wide-tooth comb working retrograde from the hair end to the roots.
Frequent hair trimming, ranging from every 4 to 6 weeks to every 2 to 4 months, should be recommended to patients who are experiencing breakage or wish to prevent damage. Trimming damaged hair can relieve excess weight on the natural hair and remove split ends, which promotes hair growth. Braiding and other lengthening techniques can prevent the hair from curling upon itself or tangling, causing less kinking and thereby decreasing the need for trimming.7 Wearing bonnets, using satin pillowcases, and wearing protective hairstyles while sleeping also can decrease hair breakage and hair loss. A commonly used hairstyle to protect the hair while sleeping is called “pineappling,” which is used to preserve and protect curls. This technique is described as gathering the hair in a high but loose ponytail at the top of the head. For patients with straightened hair, wrapping the hair underneath a bonnet or satin scarf while sleeping can prevent damage.
Managing Natural Hairstyles
An important factor in the management of natural hairstyles is the retention of hair moisture, as there is less water content in African hair compared to other hair types.5 Overuse of heat and harsh shampoos can strip moisture from the hair. Similar to patients with atopic dermatitis who should restore and maintain the skin barrier to prevent transepidermal water loss, it is important to remind patients with natural hairstyles to avoid using products and styling practices that may further deplete water content in the hair. Moisture is crucial to healthy hair.
A common culprit in shampoos that leads to hair dryness is sodium lauryl sulfate/sodium laureth sulfate, a detergent/surfactant used as a foaming agent. Sodium lauryl sulfate is a potent degreaser that binds dirt and excess product on the hair and scalp. It also dissolves oil in the hair, causing additional dryness and breakage.
Patients with natural hairstyles commonly use sulfate-free shampoos to prevent stripping the hair of its moisture and natural oils. Another method used to prevent hair dryness is co-washing, or washing the hair with a conditioner. Co-washing can effectively cleanse the hair while maintaining moisture. The use of cationic ingredients in conditioners aids in sealing moisture within the hair shaft. Hair consists of the negatively charged protein keratin, which binds to cationic surfactants in conditioners.9 The hydrophobic ends of the surfactant prevent the substance from being rinsed out and act to restore the hair barrier.
Silicone is another important ingredient in hair care products. In patients with natural hair, there are varying views on the use of products containing silicone. Silicones are added to products designed to coat the hair, adding shine, retaining moisture, and providing thermal protection. Silicones are used to provide “slip.” Slip is a term that is commonly used among patients with natural hair to describe how slippery a product is and how easily the product will help comb or detangle the hair. There are 2 basic types of silicones: water insoluble and water soluble. Water-insoluble silicones traditionally build up on the hair and require surfactant-containing shampoos to becompletely removed. Residue buildup on the hair weighs the hair down and causes damage. In contrast, water-soluble silicones do not build up and typically do not cause damage.
Silicones with the prefixes PEG- or PPG- typically are water soluble and will not build up on the hair. Dimethicone copolyol and lauryl methicone copolyol are other water-soluble silicones. In general, water-soluble silicones provide moisturizing properties without leaving residue. Other silicones such as amodimethicone and cyclomethicone are not water soluble but have properties that prevent buildup.
It is common practice for patients with natural hairstyles to avoid using water-insoluble silicones. As dermatologists, we can recommend silicone-free conditioners or conditioners containing water-soluble silicones to prevent hair dehydration and subsequent breakage. It may be advantageous to have patients try various products to determine which ones work best for their hair.
More Resources for Patients
Dermatologists have extensive knowledge of the pathophysiology of skin, hair, and nail diseases; however, despite our vast knowledge, we also need to recognize our limits. In addition to increasing your own knowledge of natural hair care practices to help your patients, it is important to recommend that your patients search for additional resources to aid in their transition to natural hairstyles. Natural hairstylists can be great resources for patients to help with hair management. In the current digital age, there also are thousands of blogs and social media forums dedicated to the topic of natural hair care. Advising patients to consult natural hair care resources can be beneficial, but as hair specialists, it also is important for us to dispel any false information that our patients may receive. As physicians, it is essential not only to manage patients who present to our offices with conditions resulting from damaging hair practices but also to help prevent such conditions from occurring. Although there may not be an overwhelming amount of evidence-based medical research to guide our decisions, we also can learn from the thousands of patients who have articulated their stories and experiences. Through observing and listening to our patients, we can incorporate this new knowledge in the management of our patients.
1. Shah SK, Alexis AF. Central centrifugal cicatricial alopecia: retrospective chart review. J Cutan Med Surg. 2010;14:212-222.
2. Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
3. Uhlenhake EE, Mehregan DM. Prospective histologic examinations in patients who practice traumatic hairstyling [published online ahead of print March 3, 2013]. Int J Dermatol. 2013;52:1506-1512.
4. Roseborough IE, McMichael AJ. Hair care practices in African-American patients. Semin Cutan Med Surg. 2009;28:103-108.
5. Kelly AP, Taylor S, eds. Dermatology for Skin of Color. New York: McGraw-Hill; 2009.
6. Kyei A, Bergfeld WF, Piliang M, et al. Medical and environmental risk factors for the development of central centrifugal cicatricial alopecia: a population study [published online ahead of print April 11, 2011]. Arch Dermatol. 2011;147:909-914.
7. Walton N, Carter ET. Better Than Good Hair: The Curly Girl Guide to Healthy, Gorgeous Natural Hair! New York, NY: Amistad; 2013.
8. Quinn CR, Quinn TM, Kelly AP. Hair care practices in African American women. Cutis. 2003;72:280-282, 285-289.
9. Cruz CF, Fernandes MM, Gomes AC, et al. Keratins and lipids in ethnic hair [published online ahead of print January 24, 2013]. Int J Cosmet Sci. 2013;35:244-249.
1. Shah SK, Alexis AF. Central centrifugal cicatricial alopecia: retrospective chart review. J Cutan Med Surg. 2010;14:212-222.
2. Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
3. Uhlenhake EE, Mehregan DM. Prospective histologic examinations in patients who practice traumatic hairstyling [published online ahead of print March 3, 2013]. Int J Dermatol. 2013;52:1506-1512.
4. Roseborough IE, McMichael AJ. Hair care practices in African-American patients. Semin Cutan Med Surg. 2009;28:103-108.
5. Kelly AP, Taylor S, eds. Dermatology for Skin of Color. New York: McGraw-Hill; 2009.
6. Kyei A, Bergfeld WF, Piliang M, et al. Medical and environmental risk factors for the development of central centrifugal cicatricial alopecia: a population study [published online ahead of print April 11, 2011]. Arch Dermatol. 2011;147:909-914.
7. Walton N, Carter ET. Better Than Good Hair: The Curly Girl Guide to Healthy, Gorgeous Natural Hair! New York, NY: Amistad; 2013.
8. Quinn CR, Quinn TM, Kelly AP. Hair care practices in African American women. Cutis. 2003;72:280-282, 285-289.
9. Cruz CF, Fernandes MM, Gomes AC, et al. Keratins and lipids in ethnic hair [published online ahead of print January 24, 2013]. Int J Cosmet Sci. 2013;35:244-249.
Practice Points
- Many scalp and hair diseases in patients of African and Afro-Caribbean descent result from traumatic hairstyling practices and poor management. Proper care of these patients requires an understanding of hair variances and styling techniques across ethnicities.
- The use of protective hairstyles and adequate trimming can aid black patients in the transition to healthier natural hair.
- The use of natural oils for scalp health and the avoidance of products containing chemicals that remove moisture from the hair are helpful in maintaining healthy natural hair.
Multiple Papules on the Eyelid Margin
The Diagnosis: Molluscum Contagiosum
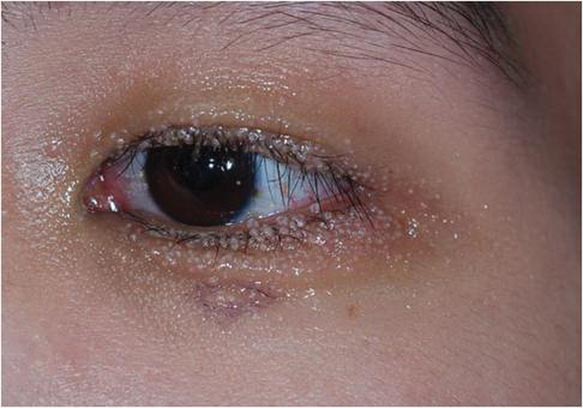
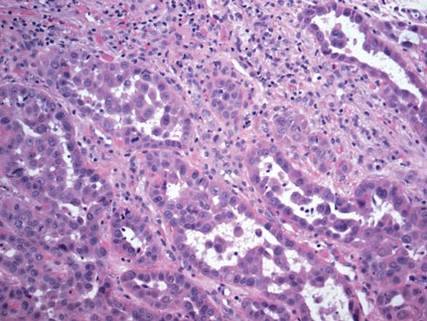
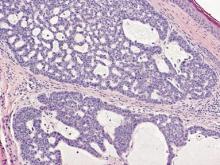
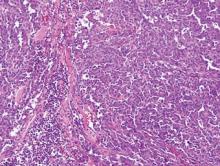
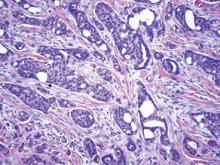
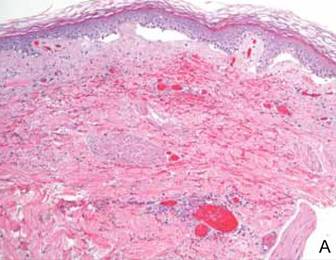
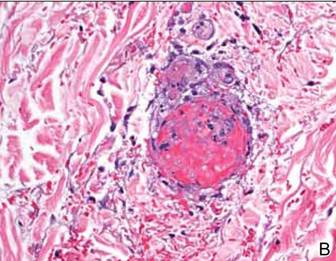
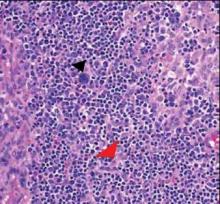
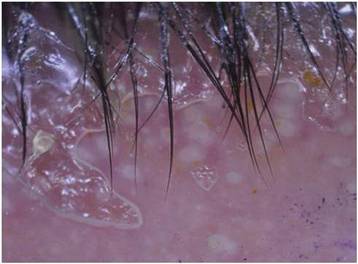
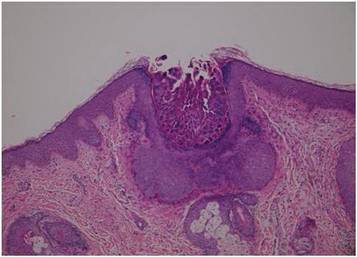
Dermoscopy showed multiple whitish amorphous structures with peripheral blood vessels (Figure 1). A skin biopsy specimen from the lower eyelid revealed loculated and endophytic epidermal hyperplasia. The keratinocytes contained large eosinophilic intracytoplasmic inclusion bodies, and the diagnosis of molluscum contagiosum (MC) was confirmed (Figure 2). Laboratory results were positive for human immunodeficiency virus (HIV) infection with a CD4 lymphocyte count of 18 cells/mm3 and viral load of 199,686 copies/mL. The patient was treated with CO2 laser therapy for eyelid lesions. A regimen of highly active antiretroviral therapy (HAART) was later started using a combination of lamivudine-zidovudine (150 mg and 300 mg) as well as lopinavir-ritonavir (400 mg and 100 mg), both twice daily. There was no recurrence at 3-month follow-up.
 |
 |
Molluscum contagiosum is a common cutaneous infection that is caused by a double-stranded DNA poxvirus. The clinical manifestations of MC are solitary or multiple, tiny, dome-shaped, pale, waxy or flesh-colored papules with central umbilication. The skin lesions can be located anywhere on the body. It occurs mostly in children, but adults also may be affected. In patients with atopic dermatitis or immunocompromised status such as AIDS, acute lymphoblastic leukemia, multiple myeloma, hyperimmunoglobulin E syndrome, or treatment with prednisone and methotrexate, the cutaneous lesions may be more extensive with an atypical presentation.1-6
The diagnosis of MC is mainly made by clinical inspection. However, Giemsa staining, Papanicolaou tests, and histopathology are useful for diagnosis of atypical MC.7 Dermoscopy is a noninvasive and fast diagnostic tool for MC.8,9 In dermoscopy, MC is characterized by multiple spherical, whitish, amorphous structures with a crown of blood vessels surrounding the periphery, termed red corona.8,9 The whitish amorphous structures and red corona correlate with inclusion bodies and dermal dilated blood vessels, respectively.
Approximately 13% of HIV patients have cutaneous MC, and the lesions tend to be more diffuse and refractory to treatment.10 A giant variant and abscess formation also have been described.11,12 Molluscum contagiosum of the eyelids often occurs in advanced HIV infection with a CD4 count less than 80 cells/mm3.1 These patients often have been diagnosed with HIV before developing eyelid MC. The severity of MC in immunocompromised patients may be related to the deficits of cell-mediated immunity, especially the T helper 1 (TH1) cytokine pathway. One case report also showed the clinical remission of MC after restoration of CD4 count with HAART.13 Our patient was not previously diagnosed with HIV and the MC of the eyelid margin was the early presentation of AIDS.
Molluscum contagiosum of the eyelids may cause chronic keratoconjunctivitis or even vascular infiltration and scarring of the peripheral cornea.14 These manifestations may be attributed to a hypersensitivity reaction to viral protein in tear film. Therefore, individuals with eyelid MC should accept thorough examination of the conjunctiva and cornea.
Treatment options include surgical excision, CO2 laser, curettage, and hyperfocal cryotherapy.15 Several reports also have demonstrated effectiveness of cidofovir for treatment of extensive MC lesions.16 Highly active antiretroviral therapy may play a role in the treatment of patients with AIDS by restoring the CD4 count.13 However, a few patients may develop immune reconstitution inflammatory syndrome, an intensive inflammatory reaction to pathogens after HAART, leading to paradoxical worsening of existing infection. Spontaneous corneal perforation due to immune reconstitution inflammatory syndrome in a case with eyelid and conjunctival MC has been reported.17 Therefore, physicians should perform MC therapy before HAART and mucocutaneous lesions should be followed regularly to prevent possible morbidity.
In summary, we report a case of AIDS with the initial presentation of MC on the eyelid margin. Physicians should test for HIV infection in patients with an atypical presentation of MC. The ocular mucosa also should be examined in patients with MC of the eyelid to prevent possible complications.
1. Pérez-Blázquez E, Villafruela I, Madero S. Eyelid molluscum contagiosum in patients with human immunodeficiency virus infection. Orbit. 1999;18:75-81.
2. Ozyürek E, Sentürk N, Kefeli M, et al. Ulcerating molluscum contagiosum in a boy with relapsed acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2011;33:e114-e116.
3. Moradi P, Bhogal M, Thaung C, et al. Epibulbar molluscum contagiosum lesions in multiple myeloma. Cornea. 2011;30:910-911.
4. Rosenberg EW, Yusk JW. Molluscum contagiosum. eruption following treatment with prednisone and methotrexate. Arch Dermatol. 1970;101:439-441.
5. Yang CH, Lee WI, Hsu TS. Disseminated white papules. Arch Dermatol. 2006;142:775-780.
6. Fotiadou C, Lazaridou E, Lekkas D, et al. Disseminated, eruptive molluscum contagiosum lesions in a psoriasis patient under treatment with methotrexate and cyclosporine. Eur J Dermatol. 2012;22:147-148.
7. Kumar N, Okiro P, Wasike R. Cytological diagnosis of molluscum contagiosum with an unusual clinical presentation at an unusual site. J Dermatol Case Rep. 2010;4:63-65.
8. Micali G, Lacarrubba F. Augmented diagnostic capability using videodermatoscopy on selected infectious and non-infectious penile growths. Int J Dermatol. 2011;50:1501-1505.
9. Micali G, Lacarrubba F, Massimino D, et al. Dermatoscopy: alternative uses in daily clinical practice. J Am Acad Dermatol. 2011;64:1135-1146.
10. Tzung TY, Yang CY, Chao SC, et al. Cutaneous manifestations of human immunodeficiency virus infection in Taiwan. Kaohsiung J Med Sci. 2004;20:216-224.
11. Chang W-Y, Chang C-P, Yang S-A, et al. Giant molluscum contagiosum with concurrence of molluscum dermatitis. Dermatol Sinica. 2005;23:81-85.
12. Bates CM, Carey PB, Dhar J, et al. Molluscum contagiosum—a novel presentation. Int J STD AIDS. 2001;12:614-615.
13. Schulz D, Sarra GM, Koerner UB, et al. Evolution of HIV-1-related conjunctival molluscum contagiosum under HAART: report of a bilaterally manifesting case and literature review. Graefes Arch Clin Exp Ophthalmol. 2004;242:951-955.
14. Redmond RM. Molluscum contagiosum is not always benign. BMJ. 2004;329:403.
15. Bardenstein DS, Elmets C. Hyperfocal cryotherapy of multiple molluscum contagiosum lesions in patients with the acquired immune deficiency syndrome. Ophthalmology. 1995;102:131-134.
16. Erickson C, Driscoll M, Gaspari A. Efficacy of intravenous cidofovir in the treatment of giant molluscum contagiosum in a patient with human immunodeficiency virus. Arch Dermatol. 2011;147:652-654.
17. Williamson W, Dorot N, Mortemousque B, et al. Spontaneous corneal perforation and conjunctival molluscum contagiosum in a AIDS patient [in French]. J Fr Ophtalmol. 1995;18:703-707.
The Diagnosis: Molluscum Contagiosum
Dermoscopy showed multiple whitish amorphous structures with peripheral blood vessels (Figure 1). A skin biopsy specimen from the lower eyelid revealed loculated and endophytic epidermal hyperplasia. The keratinocytes contained large eosinophilic intracytoplasmic inclusion bodies, and the diagnosis of molluscum contagiosum (MC) was confirmed (Figure 2). Laboratory results were positive for human immunodeficiency virus (HIV) infection with a CD4 lymphocyte count of 18 cells/mm3 and viral load of 199,686 copies/mL. The patient was treated with CO2 laser therapy for eyelid lesions. A regimen of highly active antiretroviral therapy (HAART) was later started using a combination of lamivudine-zidovudine (150 mg and 300 mg) as well as lopinavir-ritonavir (400 mg and 100 mg), both twice daily. There was no recurrence at 3-month follow-up.
 |
 |
Molluscum contagiosum is a common cutaneous infection that is caused by a double-stranded DNA poxvirus. The clinical manifestations of MC are solitary or multiple, tiny, dome-shaped, pale, waxy or flesh-colored papules with central umbilication. The skin lesions can be located anywhere on the body. It occurs mostly in children, but adults also may be affected. In patients with atopic dermatitis or immunocompromised status such as AIDS, acute lymphoblastic leukemia, multiple myeloma, hyperimmunoglobulin E syndrome, or treatment with prednisone and methotrexate, the cutaneous lesions may be more extensive with an atypical presentation.1-6
The diagnosis of MC is mainly made by clinical inspection. However, Giemsa staining, Papanicolaou tests, and histopathology are useful for diagnosis of atypical MC.7 Dermoscopy is a noninvasive and fast diagnostic tool for MC.8,9 In dermoscopy, MC is characterized by multiple spherical, whitish, amorphous structures with a crown of blood vessels surrounding the periphery, termed red corona.8,9 The whitish amorphous structures and red corona correlate with inclusion bodies and dermal dilated blood vessels, respectively.
Approximately 13% of HIV patients have cutaneous MC, and the lesions tend to be more diffuse and refractory to treatment.10 A giant variant and abscess formation also have been described.11,12 Molluscum contagiosum of the eyelids often occurs in advanced HIV infection with a CD4 count less than 80 cells/mm3.1 These patients often have been diagnosed with HIV before developing eyelid MC. The severity of MC in immunocompromised patients may be related to the deficits of cell-mediated immunity, especially the T helper 1 (TH1) cytokine pathway. One case report also showed the clinical remission of MC after restoration of CD4 count with HAART.13 Our patient was not previously diagnosed with HIV and the MC of the eyelid margin was the early presentation of AIDS.
Molluscum contagiosum of the eyelids may cause chronic keratoconjunctivitis or even vascular infiltration and scarring of the peripheral cornea.14 These manifestations may be attributed to a hypersensitivity reaction to viral protein in tear film. Therefore, individuals with eyelid MC should accept thorough examination of the conjunctiva and cornea.
Treatment options include surgical excision, CO2 laser, curettage, and hyperfocal cryotherapy.15 Several reports also have demonstrated effectiveness of cidofovir for treatment of extensive MC lesions.16 Highly active antiretroviral therapy may play a role in the treatment of patients with AIDS by restoring the CD4 count.13 However, a few patients may develop immune reconstitution inflammatory syndrome, an intensive inflammatory reaction to pathogens after HAART, leading to paradoxical worsening of existing infection. Spontaneous corneal perforation due to immune reconstitution inflammatory syndrome in a case with eyelid and conjunctival MC has been reported.17 Therefore, physicians should perform MC therapy before HAART and mucocutaneous lesions should be followed regularly to prevent possible morbidity.
In summary, we report a case of AIDS with the initial presentation of MC on the eyelid margin. Physicians should test for HIV infection in patients with an atypical presentation of MC. The ocular mucosa also should be examined in patients with MC of the eyelid to prevent possible complications.
The Diagnosis: Molluscum Contagiosum
Dermoscopy showed multiple whitish amorphous structures with peripheral blood vessels (Figure 1). A skin biopsy specimen from the lower eyelid revealed loculated and endophytic epidermal hyperplasia. The keratinocytes contained large eosinophilic intracytoplasmic inclusion bodies, and the diagnosis of molluscum contagiosum (MC) was confirmed (Figure 2). Laboratory results were positive for human immunodeficiency virus (HIV) infection with a CD4 lymphocyte count of 18 cells/mm3 and viral load of 199,686 copies/mL. The patient was treated with CO2 laser therapy for eyelid lesions. A regimen of highly active antiretroviral therapy (HAART) was later started using a combination of lamivudine-zidovudine (150 mg and 300 mg) as well as lopinavir-ritonavir (400 mg and 100 mg), both twice daily. There was no recurrence at 3-month follow-up.
 |
 |
Molluscum contagiosum is a common cutaneous infection that is caused by a double-stranded DNA poxvirus. The clinical manifestations of MC are solitary or multiple, tiny, dome-shaped, pale, waxy or flesh-colored papules with central umbilication. The skin lesions can be located anywhere on the body. It occurs mostly in children, but adults also may be affected. In patients with atopic dermatitis or immunocompromised status such as AIDS, acute lymphoblastic leukemia, multiple myeloma, hyperimmunoglobulin E syndrome, or treatment with prednisone and methotrexate, the cutaneous lesions may be more extensive with an atypical presentation.1-6
The diagnosis of MC is mainly made by clinical inspection. However, Giemsa staining, Papanicolaou tests, and histopathology are useful for diagnosis of atypical MC.7 Dermoscopy is a noninvasive and fast diagnostic tool for MC.8,9 In dermoscopy, MC is characterized by multiple spherical, whitish, amorphous structures with a crown of blood vessels surrounding the periphery, termed red corona.8,9 The whitish amorphous structures and red corona correlate with inclusion bodies and dermal dilated blood vessels, respectively.
Approximately 13% of HIV patients have cutaneous MC, and the lesions tend to be more diffuse and refractory to treatment.10 A giant variant and abscess formation also have been described.11,12 Molluscum contagiosum of the eyelids often occurs in advanced HIV infection with a CD4 count less than 80 cells/mm3.1 These patients often have been diagnosed with HIV before developing eyelid MC. The severity of MC in immunocompromised patients may be related to the deficits of cell-mediated immunity, especially the T helper 1 (TH1) cytokine pathway. One case report also showed the clinical remission of MC after restoration of CD4 count with HAART.13 Our patient was not previously diagnosed with HIV and the MC of the eyelid margin was the early presentation of AIDS.
Molluscum contagiosum of the eyelids may cause chronic keratoconjunctivitis or even vascular infiltration and scarring of the peripheral cornea.14 These manifestations may be attributed to a hypersensitivity reaction to viral protein in tear film. Therefore, individuals with eyelid MC should accept thorough examination of the conjunctiva and cornea.
Treatment options include surgical excision, CO2 laser, curettage, and hyperfocal cryotherapy.15 Several reports also have demonstrated effectiveness of cidofovir for treatment of extensive MC lesions.16 Highly active antiretroviral therapy may play a role in the treatment of patients with AIDS by restoring the CD4 count.13 However, a few patients may develop immune reconstitution inflammatory syndrome, an intensive inflammatory reaction to pathogens after HAART, leading to paradoxical worsening of existing infection. Spontaneous corneal perforation due to immune reconstitution inflammatory syndrome in a case with eyelid and conjunctival MC has been reported.17 Therefore, physicians should perform MC therapy before HAART and mucocutaneous lesions should be followed regularly to prevent possible morbidity.
In summary, we report a case of AIDS with the initial presentation of MC on the eyelid margin. Physicians should test for HIV infection in patients with an atypical presentation of MC. The ocular mucosa also should be examined in patients with MC of the eyelid to prevent possible complications.
1. Pérez-Blázquez E, Villafruela I, Madero S. Eyelid molluscum contagiosum in patients with human immunodeficiency virus infection. Orbit. 1999;18:75-81.
2. Ozyürek E, Sentürk N, Kefeli M, et al. Ulcerating molluscum contagiosum in a boy with relapsed acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2011;33:e114-e116.
3. Moradi P, Bhogal M, Thaung C, et al. Epibulbar molluscum contagiosum lesions in multiple myeloma. Cornea. 2011;30:910-911.
4. Rosenberg EW, Yusk JW. Molluscum contagiosum. eruption following treatment with prednisone and methotrexate. Arch Dermatol. 1970;101:439-441.
5. Yang CH, Lee WI, Hsu TS. Disseminated white papules. Arch Dermatol. 2006;142:775-780.
6. Fotiadou C, Lazaridou E, Lekkas D, et al. Disseminated, eruptive molluscum contagiosum lesions in a psoriasis patient under treatment with methotrexate and cyclosporine. Eur J Dermatol. 2012;22:147-148.
7. Kumar N, Okiro P, Wasike R. Cytological diagnosis of molluscum contagiosum with an unusual clinical presentation at an unusual site. J Dermatol Case Rep. 2010;4:63-65.
8. Micali G, Lacarrubba F. Augmented diagnostic capability using videodermatoscopy on selected infectious and non-infectious penile growths. Int J Dermatol. 2011;50:1501-1505.
9. Micali G, Lacarrubba F, Massimino D, et al. Dermatoscopy: alternative uses in daily clinical practice. J Am Acad Dermatol. 2011;64:1135-1146.
10. Tzung TY, Yang CY, Chao SC, et al. Cutaneous manifestations of human immunodeficiency virus infection in Taiwan. Kaohsiung J Med Sci. 2004;20:216-224.
11. Chang W-Y, Chang C-P, Yang S-A, et al. Giant molluscum contagiosum with concurrence of molluscum dermatitis. Dermatol Sinica. 2005;23:81-85.
12. Bates CM, Carey PB, Dhar J, et al. Molluscum contagiosum—a novel presentation. Int J STD AIDS. 2001;12:614-615.
13. Schulz D, Sarra GM, Koerner UB, et al. Evolution of HIV-1-related conjunctival molluscum contagiosum under HAART: report of a bilaterally manifesting case and literature review. Graefes Arch Clin Exp Ophthalmol. 2004;242:951-955.
14. Redmond RM. Molluscum contagiosum is not always benign. BMJ. 2004;329:403.
15. Bardenstein DS, Elmets C. Hyperfocal cryotherapy of multiple molluscum contagiosum lesions in patients with the acquired immune deficiency syndrome. Ophthalmology. 1995;102:131-134.
16. Erickson C, Driscoll M, Gaspari A. Efficacy of intravenous cidofovir in the treatment of giant molluscum contagiosum in a patient with human immunodeficiency virus. Arch Dermatol. 2011;147:652-654.
17. Williamson W, Dorot N, Mortemousque B, et al. Spontaneous corneal perforation and conjunctival molluscum contagiosum in a AIDS patient [in French]. J Fr Ophtalmol. 1995;18:703-707.
1. Pérez-Blázquez E, Villafruela I, Madero S. Eyelid molluscum contagiosum in patients with human immunodeficiency virus infection. Orbit. 1999;18:75-81.
2. Ozyürek E, Sentürk N, Kefeli M, et al. Ulcerating molluscum contagiosum in a boy with relapsed acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2011;33:e114-e116.
3. Moradi P, Bhogal M, Thaung C, et al. Epibulbar molluscum contagiosum lesions in multiple myeloma. Cornea. 2011;30:910-911.
4. Rosenberg EW, Yusk JW. Molluscum contagiosum. eruption following treatment with prednisone and methotrexate. Arch Dermatol. 1970;101:439-441.
5. Yang CH, Lee WI, Hsu TS. Disseminated white papules. Arch Dermatol. 2006;142:775-780.
6. Fotiadou C, Lazaridou E, Lekkas D, et al. Disseminated, eruptive molluscum contagiosum lesions in a psoriasis patient under treatment with methotrexate and cyclosporine. Eur J Dermatol. 2012;22:147-148.
7. Kumar N, Okiro P, Wasike R. Cytological diagnosis of molluscum contagiosum with an unusual clinical presentation at an unusual site. J Dermatol Case Rep. 2010;4:63-65.
8. Micali G, Lacarrubba F. Augmented diagnostic capability using videodermatoscopy on selected infectious and non-infectious penile growths. Int J Dermatol. 2011;50:1501-1505.
9. Micali G, Lacarrubba F, Massimino D, et al. Dermatoscopy: alternative uses in daily clinical practice. J Am Acad Dermatol. 2011;64:1135-1146.
10. Tzung TY, Yang CY, Chao SC, et al. Cutaneous manifestations of human immunodeficiency virus infection in Taiwan. Kaohsiung J Med Sci. 2004;20:216-224.
11. Chang W-Y, Chang C-P, Yang S-A, et al. Giant molluscum contagiosum with concurrence of molluscum dermatitis. Dermatol Sinica. 2005;23:81-85.
12. Bates CM, Carey PB, Dhar J, et al. Molluscum contagiosum—a novel presentation. Int J STD AIDS. 2001;12:614-615.
13. Schulz D, Sarra GM, Koerner UB, et al. Evolution of HIV-1-related conjunctival molluscum contagiosum under HAART: report of a bilaterally manifesting case and literature review. Graefes Arch Clin Exp Ophthalmol. 2004;242:951-955.
14. Redmond RM. Molluscum contagiosum is not always benign. BMJ. 2004;329:403.
15. Bardenstein DS, Elmets C. Hyperfocal cryotherapy of multiple molluscum contagiosum lesions in patients with the acquired immune deficiency syndrome. Ophthalmology. 1995;102:131-134.
16. Erickson C, Driscoll M, Gaspari A. Efficacy of intravenous cidofovir in the treatment of giant molluscum contagiosum in a patient with human immunodeficiency virus. Arch Dermatol. 2011;147:652-654.
17. Williamson W, Dorot N, Mortemousque B, et al. Spontaneous corneal perforation and conjunctival molluscum contagiosum in a AIDS patient [in French]. J Fr Ophtalmol. 1995;18:703-707.
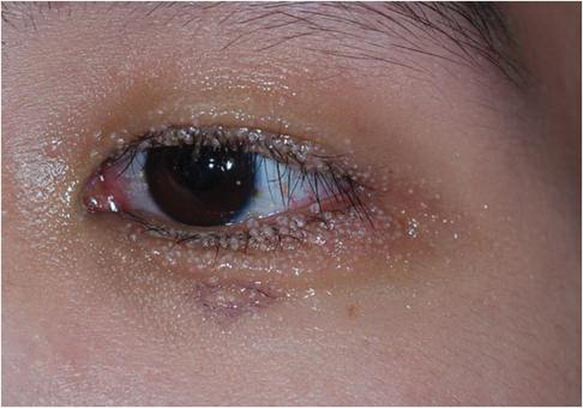
A 24-year-old man presented with multiple tiny papules over the left eyelid margin of 2 to 3 months’ duration. There were a couple of papules on the left upper eyelid initially, but they progressed to the upper and lower eyelid margin after scratching. On physical examination, multiple whitish to flesh-colored pearly papules measuring 1 to 2 mm were located on the left eyelid margin. Palpebral follicular conjunctivitis also was noted.
Modifier -25 Use in Dermatology
According to Current Procedural Terminology (CPT), modifier -25 is to be used to identify “significant, separately identifiable evaluation and management service by the same physician or other qualified health care professional on the same day of the procedure or other service.”1 Modifier -25 frequently is integral to the description of patient visits in dermatology. Dermatologists use modifier -25 more than physicians of any other specialty, and in recent years, more than 50% of dermatology evaluation and management (E/M) visits have been appended with this modifier.
When patients present for assessment and management of various skin findings, a dermatologist may deem it appropriate to proceed with a diagnostic or therapeutic procedure at the same visit after obtaining the patient’s medical history, completing a review of systems, and conducting a clinical examination. Most commonly, a skin biopsy or destruction of a benign or malignant lesion may be performed, but other simple procedures also may be appropriate. The ability to assess and intervene during the same visit is optimal for patients who subsequently may require fewer follow-up visits and experience more immediate relief from their symptoms.
When E/M Cannot Be Billed Separately
Regulatory guidance from the National Correct Coding Initiative (NCCI) dated January 2013 indicates that procedures with a global period of 90 days are major surgical procedures, and if an E/M service is performed on the same day as such a procedure to decide whether or not to perform that procedure, then the E/M service should be reported with modifier -57.2 On the other hand, CPT defines procedures with a 0- or 10-day global period as minor surgical procedures, and E/M services provided on the same day of service as these procedures are included in the procedure code and cannot be billed separately. For review, common dermatologic procedures with 0-day global periods include biopsies (CPT code 11000), shave removals (11300–11313), debridements (11000, 11011–11042), and Mohs micrographic surgery (17311–17315); procedures with 10-day global periods include destructions (17000–17286), excisions (11400–11646), and repairs (12001–13153). If an E/M service is performed on the same day as one of these procedures to decide whether to proceed with the minor surgical procedure, this E/M service cannot be reported separately. Additionally, the fact that the patient is new to the physician is not sufficient to allow reporting of an E/M with such a minor procedure.
When E/M Can Be Billed Separately
However, a “significant and separately identifiable E/M service unrelated to the decision to perform the minor procedure” is separately reportable with modifier -25. According to the NCCI, the minor procedure and the E/M do not require different diagnoses, but the E/M service must be above and beyond what is usually required for the minor surgical procedure.2 Because a certain amount of so-called preservice time is built into minor procedure codes, the implication is that substantially more E/M was needed than envisioned in this preservice time, necessitating inclusion of an E/M in addition to a minor procedure when there is a single diagnosis.
When there is a single diagnosis, the physician has to decide when such a significant and separately identifiable service exists. If the physician determines that it is appropriate to code for E/M in addition to the minor procedure, clear documentation of the additional E/M service provided will reduce the likelihood of this choice being questioned. Specifically, it may be helpful to describe the additional history, examination results, medical knowledge, professional skill, and work time above and beyond what is usually required for the minor surgical procedure.
When there are many diagnosis codes for a single visit and only a subset of them are associated with the minor procedure, as is common in dermatology, then the decision to include an E/M service is simpler. In this case, if E/M services were provided that pertained to a diagnosis or diagnoses other than the one(s) associated with the minor procedure(s), then these additional E/M services will clearly not be included in the preservice time for the procedure and an E/M can virtually always be coded separately. For instance, if a patient presents with a growing scaly bump (clinically apparent squamous cell carcinoma) on the leg that the dermatologist deems is appropriate for biopsy but concurrently notices nummular dermatitis of the legs, which the patient describes as itchy and uncomfortable, then the diagnosis and management of the dermatitis would clearly be a separate E/M service and would not be included in the workup for the biopsy. The E/M code that is applied should, of course, reflect the services provided exclusive of those integral to the minor procedure. To make it easier for regulators and auditors, it may be helpful to clearly itemize the additional diagnoses unrelated to the minor procedure and describe the specific E/M services provided for these diagnoses. Although it is certainly not necessary or required, it also may be helpful to physically separate the documentation for the minor procedure from the E/M services for the additional diagnoses within the medical chart.
Final Thoughts
It is clear that frequent use of modifier -25 is appropriate in routine, high-quality dermatologic practice. Simultaneous provision of E/M services and minor procedures often is in the patient’s best interest, as it minimizes unnecessary office visits and expedites treatment. When modifier -25 is appropriately appended, careful documentation by the dermatologist can help to clarify the precise basis for its use. Recent NCCI edits provide guidelines for use of this modifier that can be adapted by individual dermatologists for particular patient circumstances.2
1. CPT 2014 Professional Edition. Chicago, IL: American Medical Association; 2014.
2. National Correct Coding Initiative Policy Manual for Medicare Services. Carmel, IN: National Correct Coding Initiative; 2013.
According to Current Procedural Terminology (CPT), modifier -25 is to be used to identify “significant, separately identifiable evaluation and management service by the same physician or other qualified health care professional on the same day of the procedure or other service.”1 Modifier -25 frequently is integral to the description of patient visits in dermatology. Dermatologists use modifier -25 more than physicians of any other specialty, and in recent years, more than 50% of dermatology evaluation and management (E/M) visits have been appended with this modifier.
When patients present for assessment and management of various skin findings, a dermatologist may deem it appropriate to proceed with a diagnostic or therapeutic procedure at the same visit after obtaining the patient’s medical history, completing a review of systems, and conducting a clinical examination. Most commonly, a skin biopsy or destruction of a benign or malignant lesion may be performed, but other simple procedures also may be appropriate. The ability to assess and intervene during the same visit is optimal for patients who subsequently may require fewer follow-up visits and experience more immediate relief from their symptoms.
When E/M Cannot Be Billed Separately
Regulatory guidance from the National Correct Coding Initiative (NCCI) dated January 2013 indicates that procedures with a global period of 90 days are major surgical procedures, and if an E/M service is performed on the same day as such a procedure to decide whether or not to perform that procedure, then the E/M service should be reported with modifier -57.2 On the other hand, CPT defines procedures with a 0- or 10-day global period as minor surgical procedures, and E/M services provided on the same day of service as these procedures are included in the procedure code and cannot be billed separately. For review, common dermatologic procedures with 0-day global periods include biopsies (CPT code 11000), shave removals (11300–11313), debridements (11000, 11011–11042), and Mohs micrographic surgery (17311–17315); procedures with 10-day global periods include destructions (17000–17286), excisions (11400–11646), and repairs (12001–13153). If an E/M service is performed on the same day as one of these procedures to decide whether to proceed with the minor surgical procedure, this E/M service cannot be reported separately. Additionally, the fact that the patient is new to the physician is not sufficient to allow reporting of an E/M with such a minor procedure.
When E/M Can Be Billed Separately
However, a “significant and separately identifiable E/M service unrelated to the decision to perform the minor procedure” is separately reportable with modifier -25. According to the NCCI, the minor procedure and the E/M do not require different diagnoses, but the E/M service must be above and beyond what is usually required for the minor surgical procedure.2 Because a certain amount of so-called preservice time is built into minor procedure codes, the implication is that substantially more E/M was needed than envisioned in this preservice time, necessitating inclusion of an E/M in addition to a minor procedure when there is a single diagnosis.
When there is a single diagnosis, the physician has to decide when such a significant and separately identifiable service exists. If the physician determines that it is appropriate to code for E/M in addition to the minor procedure, clear documentation of the additional E/M service provided will reduce the likelihood of this choice being questioned. Specifically, it may be helpful to describe the additional history, examination results, medical knowledge, professional skill, and work time above and beyond what is usually required for the minor surgical procedure.
When there are many diagnosis codes for a single visit and only a subset of them are associated with the minor procedure, as is common in dermatology, then the decision to include an E/M service is simpler. In this case, if E/M services were provided that pertained to a diagnosis or diagnoses other than the one(s) associated with the minor procedure(s), then these additional E/M services will clearly not be included in the preservice time for the procedure and an E/M can virtually always be coded separately. For instance, if a patient presents with a growing scaly bump (clinically apparent squamous cell carcinoma) on the leg that the dermatologist deems is appropriate for biopsy but concurrently notices nummular dermatitis of the legs, which the patient describes as itchy and uncomfortable, then the diagnosis and management of the dermatitis would clearly be a separate E/M service and would not be included in the workup for the biopsy. The E/M code that is applied should, of course, reflect the services provided exclusive of those integral to the minor procedure. To make it easier for regulators and auditors, it may be helpful to clearly itemize the additional diagnoses unrelated to the minor procedure and describe the specific E/M services provided for these diagnoses. Although it is certainly not necessary or required, it also may be helpful to physically separate the documentation for the minor procedure from the E/M services for the additional diagnoses within the medical chart.
Final Thoughts
It is clear that frequent use of modifier -25 is appropriate in routine, high-quality dermatologic practice. Simultaneous provision of E/M services and minor procedures often is in the patient’s best interest, as it minimizes unnecessary office visits and expedites treatment. When modifier -25 is appropriately appended, careful documentation by the dermatologist can help to clarify the precise basis for its use. Recent NCCI edits provide guidelines for use of this modifier that can be adapted by individual dermatologists for particular patient circumstances.2
According to Current Procedural Terminology (CPT), modifier -25 is to be used to identify “significant, separately identifiable evaluation and management service by the same physician or other qualified health care professional on the same day of the procedure or other service.”1 Modifier -25 frequently is integral to the description of patient visits in dermatology. Dermatologists use modifier -25 more than physicians of any other specialty, and in recent years, more than 50% of dermatology evaluation and management (E/M) visits have been appended with this modifier.
When patients present for assessment and management of various skin findings, a dermatologist may deem it appropriate to proceed with a diagnostic or therapeutic procedure at the same visit after obtaining the patient’s medical history, completing a review of systems, and conducting a clinical examination. Most commonly, a skin biopsy or destruction of a benign or malignant lesion may be performed, but other simple procedures also may be appropriate. The ability to assess and intervene during the same visit is optimal for patients who subsequently may require fewer follow-up visits and experience more immediate relief from their symptoms.
When E/M Cannot Be Billed Separately
Regulatory guidance from the National Correct Coding Initiative (NCCI) dated January 2013 indicates that procedures with a global period of 90 days are major surgical procedures, and if an E/M service is performed on the same day as such a procedure to decide whether or not to perform that procedure, then the E/M service should be reported with modifier -57.2 On the other hand, CPT defines procedures with a 0- or 10-day global period as minor surgical procedures, and E/M services provided on the same day of service as these procedures are included in the procedure code and cannot be billed separately. For review, common dermatologic procedures with 0-day global periods include biopsies (CPT code 11000), shave removals (11300–11313), debridements (11000, 11011–11042), and Mohs micrographic surgery (17311–17315); procedures with 10-day global periods include destructions (17000–17286), excisions (11400–11646), and repairs (12001–13153). If an E/M service is performed on the same day as one of these procedures to decide whether to proceed with the minor surgical procedure, this E/M service cannot be reported separately. Additionally, the fact that the patient is new to the physician is not sufficient to allow reporting of an E/M with such a minor procedure.
When E/M Can Be Billed Separately
However, a “significant and separately identifiable E/M service unrelated to the decision to perform the minor procedure” is separately reportable with modifier -25. According to the NCCI, the minor procedure and the E/M do not require different diagnoses, but the E/M service must be above and beyond what is usually required for the minor surgical procedure.2 Because a certain amount of so-called preservice time is built into minor procedure codes, the implication is that substantially more E/M was needed than envisioned in this preservice time, necessitating inclusion of an E/M in addition to a minor procedure when there is a single diagnosis.
When there is a single diagnosis, the physician has to decide when such a significant and separately identifiable service exists. If the physician determines that it is appropriate to code for E/M in addition to the minor procedure, clear documentation of the additional E/M service provided will reduce the likelihood of this choice being questioned. Specifically, it may be helpful to describe the additional history, examination results, medical knowledge, professional skill, and work time above and beyond what is usually required for the minor surgical procedure.
When there are many diagnosis codes for a single visit and only a subset of them are associated with the minor procedure, as is common in dermatology, then the decision to include an E/M service is simpler. In this case, if E/M services were provided that pertained to a diagnosis or diagnoses other than the one(s) associated with the minor procedure(s), then these additional E/M services will clearly not be included in the preservice time for the procedure and an E/M can virtually always be coded separately. For instance, if a patient presents with a growing scaly bump (clinically apparent squamous cell carcinoma) on the leg that the dermatologist deems is appropriate for biopsy but concurrently notices nummular dermatitis of the legs, which the patient describes as itchy and uncomfortable, then the diagnosis and management of the dermatitis would clearly be a separate E/M service and would not be included in the workup for the biopsy. The E/M code that is applied should, of course, reflect the services provided exclusive of those integral to the minor procedure. To make it easier for regulators and auditors, it may be helpful to clearly itemize the additional diagnoses unrelated to the minor procedure and describe the specific E/M services provided for these diagnoses. Although it is certainly not necessary or required, it also may be helpful to physically separate the documentation for the minor procedure from the E/M services for the additional diagnoses within the medical chart.
Final Thoughts
It is clear that frequent use of modifier -25 is appropriate in routine, high-quality dermatologic practice. Simultaneous provision of E/M services and minor procedures often is in the patient’s best interest, as it minimizes unnecessary office visits and expedites treatment. When modifier -25 is appropriately appended, careful documentation by the dermatologist can help to clarify the precise basis for its use. Recent NCCI edits provide guidelines for use of this modifier that can be adapted by individual dermatologists for particular patient circumstances.2
1. CPT 2014 Professional Edition. Chicago, IL: American Medical Association; 2014.
2. National Correct Coding Initiative Policy Manual for Medicare Services. Carmel, IN: National Correct Coding Initiative; 2013.
1. CPT 2014 Professional Edition. Chicago, IL: American Medical Association; 2014.
2. National Correct Coding Initiative Policy Manual for Medicare Services. Carmel, IN: National Correct Coding Initiative; 2013.
Practice Points
- Frequent use of modifier -25 is appropriate in routine, high-quality dermatologic practice.
- The global period (0, 10, or 90 days) of a procedure dictates if evaluation and management services provided on the same day of service as the original procedure can be billed separately.
- Careful documentation by the dermatologist can help clarify the precise basis for the use of modifier -25.
Pseudoglandular Squamous Cell Carcinoma
Squamous cell carcinoma (SCC) is the second most common form of skin cancer. Pseudoglandular SCC, also known as adenoid SCC or acantholytic SCC, is an uncommon variant that was first described by Lever1 in 1947 as an adenoacanthoma of the sweat glands. Of the many variants of SCC, pseudoglandular SCC generally is considered to behave aggressively with intermediate (3%–10%) risk for metastasis.2 The metastatic potential of pseudoglandular SCC may be conferred in part by diminished expression of intercellular adhesion molecules, including desmoglein 3, epithelial cadherin, and syn-decan 1.3,4 Pseudoglandular SCC presents most often on sun-damaged skin of elderly patients, especially the face and ears, as a pink or red nodule with central ulceration and a raised indurated border. It may be mistaken clinically for basal cell carcinoma (BCC) or keratoacanthoma.
On microscopic examination, the lesion is predominantly located in the dermis and may extend to the subcutis. There usually is connection to the overlying epidermis, which often shows hyperkeratosis and parakeratosis. Epidermal squamous dysplasia may be present. The dermis typically contains nests of squamous cells with a variable degree of central acantholysis. The morphology on low-power magnification consists of tubules of irregular size and shape, which are present either focally or throughout the lesion (Figure 1). The tubules are typically admixed with foci of keratinization. One or more layers of cohesive cells line the tubules. Partial keratinization may be found in the lining of tubules with more than 1 cell layer. The tumor cells are polygonal with eosinophilic cytoplasm, ovoid hyperchromatic or vesicular nuclei, and prominent nucleoli. Mitoses are common. The tubular lumina are filled with acantholytic cells, either singly or in small clusters, which may demonstrate residual bridging to tubular lining cells (Figure 2). The acantholytic cells show some variability in size and may be large, multinucleated, or keratinized. The tubules may contain material that is amorphous, basophilic, periodic acid–Schiff positive, diastase sensitive, and mucicarmine negative.5 Eccrine ducts at the periphery of the tumor may show reactive dilatation and proliferation. Tumor cells show positive immunostaining for epithelial membrane antigen, 34βE12, CK5/6, and tumor protein p63.6-8 There is negative immunostaining for carcinoembryonic antigen, amylase, S-100 protein, and factor VIII.5
|
The differential diagnosis includes adenoid BCC, angiosarcoma, eccrine carcinoma, and metastatic adenocarcinoma of the skin. In adenoid BCC, excess stromal mucin imparts pseudoglandular architecture (Figure 3). However, features of conventional BCC, including peripheral nuclear palisading and retraction artifact often are present as well.
Angiosarcoma shows slitlike vascular spaces lined by hyperchromatic endothelial cells (Figure 4). Further, there is positive immunostaining for vascular markers CD31 and CD34.
In eccrine carcinoma, there are invasive ductal structures lined by either a single or double layer of cells that may contain luminal material that is periodic acid–Schiff positive and diastase resistant (Figure 5).9 The tumor cells show positive immunostaining for cytokeratins, epithelial membrane antigen, carcinoembryonic antigen, and S-100 protein.10
Pseudoglandular SCC is susceptible to misdiagnosis as adenocarcinoma by sampling error if biopsies do not capture areas with typical features of SCC, including dysplastic squamous epithelium and keratinization. Metastatic adenocarcinoma of the skin is more likely to present with multiple nodules in older individuals. Lack of epidermal connection of the tumor and minimal to no acantholytic dyskeratosis further support cutaneous metastasis (Figure 6). Review of the patient’s clinical history might be helpful if adenocarcinoma was previously diagnosed. Immunohistochemical evaluation may aid in the prediction of the primary site in patients with metastatic adenocarcinoma of unknown origin.11
1. Lever WF. Adenocanthoma of sweat glands; carcinoma of sweat glands with glandular and epidermal elements: report of four cases. Arch Derm Syphilol. 1947;56:157-171.
2. Bonerandi JJ, Beauvillain C, Caquant L, et al. Guidelines for the diagnosis and treatment of cutaneous squamous cell carcinoma and precursor lesions. J Eur Acad Dermatol Venereol. 2011;25(suppl 5):1-51.
3. Griffin JR, Wriston CC, Peters MS, et al. Decreased expression of intercellular adhesion molecules in acantholytic squamous cell carcinoma compared with invasive well-differentiated squamous cell carcinoma of the skin. Am J Clin Pathol. 2013;139:442-447.
4. Bayer-Garner IB, Smoller BR. The expression of syndecan-1 is preferentially reduced compared with that of E-cadherin in acantholytic squamous cell carcinoma. J Cutan Pathol. 2001;28:83-89.
5. Nappi O, Pettinato G, Wick MR. Adenoid (acantholytic) squamous cell carcinoma of the skin. J Cutan Pathol. 1989;16:114-121.
6. Sajin M, Hodorogea Prisăcaru A, Luchian MC, et al. Acantholytic squamous cell carcinoma: pathological study of nine cases with review of literature. Rom J Morphol Embryol. 2014;55:279-283.
7. Gray Y, Robidoux HJ, Farrell DS, et al. Squamous cell carcinoma detected by high-molecular-weight cytokeratin immunostaining mimicking atypical fibroxanthoma. Arch Pathol Lab Med. 2001;125:799-802.
8. Kanitakis J, Chouvet B. Expression of p63 in cutaneous metastases. Am J Clin Pathol. 2007;128:753-758.
9. Plaza JA, Prieto VG. Neoplastic Lesions of the Skin. New York, NY: Demos Medical Publishing; 2014.
10. Swanson PE, Cherwitz DL, Neumann MP, et al. Eccrine sweat gland carcinoma: an histologic and immunohistochemical study of 32 cases. J Cutan Pathol. 1987;14:65-86.
11. Dennis JL, Hvidsten TR, Wit EC, et al. Markers of adenocarcinoma characteristic of the site of origin: development of a diagnostic algorithm. Clin Cancer Res. 2005;11:3766-3772.
Squamous cell carcinoma (SCC) is the second most common form of skin cancer. Pseudoglandular SCC, also known as adenoid SCC or acantholytic SCC, is an uncommon variant that was first described by Lever1 in 1947 as an adenoacanthoma of the sweat glands. Of the many variants of SCC, pseudoglandular SCC generally is considered to behave aggressively with intermediate (3%–10%) risk for metastasis.2 The metastatic potential of pseudoglandular SCC may be conferred in part by diminished expression of intercellular adhesion molecules, including desmoglein 3, epithelial cadherin, and syn-decan 1.3,4 Pseudoglandular SCC presents most often on sun-damaged skin of elderly patients, especially the face and ears, as a pink or red nodule with central ulceration and a raised indurated border. It may be mistaken clinically for basal cell carcinoma (BCC) or keratoacanthoma.
On microscopic examination, the lesion is predominantly located in the dermis and may extend to the subcutis. There usually is connection to the overlying epidermis, which often shows hyperkeratosis and parakeratosis. Epidermal squamous dysplasia may be present. The dermis typically contains nests of squamous cells with a variable degree of central acantholysis. The morphology on low-power magnification consists of tubules of irregular size and shape, which are present either focally or throughout the lesion (Figure 1). The tubules are typically admixed with foci of keratinization. One or more layers of cohesive cells line the tubules. Partial keratinization may be found in the lining of tubules with more than 1 cell layer. The tumor cells are polygonal with eosinophilic cytoplasm, ovoid hyperchromatic or vesicular nuclei, and prominent nucleoli. Mitoses are common. The tubular lumina are filled with acantholytic cells, either singly or in small clusters, which may demonstrate residual bridging to tubular lining cells (Figure 2). The acantholytic cells show some variability in size and may be large, multinucleated, or keratinized. The tubules may contain material that is amorphous, basophilic, periodic acid–Schiff positive, diastase sensitive, and mucicarmine negative.5 Eccrine ducts at the periphery of the tumor may show reactive dilatation and proliferation. Tumor cells show positive immunostaining for epithelial membrane antigen, 34βE12, CK5/6, and tumor protein p63.6-8 There is negative immunostaining for carcinoembryonic antigen, amylase, S-100 protein, and factor VIII.5
|
The differential diagnosis includes adenoid BCC, angiosarcoma, eccrine carcinoma, and metastatic adenocarcinoma of the skin. In adenoid BCC, excess stromal mucin imparts pseudoglandular architecture (Figure 3). However, features of conventional BCC, including peripheral nuclear palisading and retraction artifact often are present as well.
Angiosarcoma shows slitlike vascular spaces lined by hyperchromatic endothelial cells (Figure 4). Further, there is positive immunostaining for vascular markers CD31 and CD34.
In eccrine carcinoma, there are invasive ductal structures lined by either a single or double layer of cells that may contain luminal material that is periodic acid–Schiff positive and diastase resistant (Figure 5).9 The tumor cells show positive immunostaining for cytokeratins, epithelial membrane antigen, carcinoembryonic antigen, and S-100 protein.10
Pseudoglandular SCC is susceptible to misdiagnosis as adenocarcinoma by sampling error if biopsies do not capture areas with typical features of SCC, including dysplastic squamous epithelium and keratinization. Metastatic adenocarcinoma of the skin is more likely to present with multiple nodules in older individuals. Lack of epidermal connection of the tumor and minimal to no acantholytic dyskeratosis further support cutaneous metastasis (Figure 6). Review of the patient’s clinical history might be helpful if adenocarcinoma was previously diagnosed. Immunohistochemical evaluation may aid in the prediction of the primary site in patients with metastatic adenocarcinoma of unknown origin.11
Squamous cell carcinoma (SCC) is the second most common form of skin cancer. Pseudoglandular SCC, also known as adenoid SCC or acantholytic SCC, is an uncommon variant that was first described by Lever1 in 1947 as an adenoacanthoma of the sweat glands. Of the many variants of SCC, pseudoglandular SCC generally is considered to behave aggressively with intermediate (3%–10%) risk for metastasis.2 The metastatic potential of pseudoglandular SCC may be conferred in part by diminished expression of intercellular adhesion molecules, including desmoglein 3, epithelial cadherin, and syn-decan 1.3,4 Pseudoglandular SCC presents most often on sun-damaged skin of elderly patients, especially the face and ears, as a pink or red nodule with central ulceration and a raised indurated border. It may be mistaken clinically for basal cell carcinoma (BCC) or keratoacanthoma.
On microscopic examination, the lesion is predominantly located in the dermis and may extend to the subcutis. There usually is connection to the overlying epidermis, which often shows hyperkeratosis and parakeratosis. Epidermal squamous dysplasia may be present. The dermis typically contains nests of squamous cells with a variable degree of central acantholysis. The morphology on low-power magnification consists of tubules of irregular size and shape, which are present either focally or throughout the lesion (Figure 1). The tubules are typically admixed with foci of keratinization. One or more layers of cohesive cells line the tubules. Partial keratinization may be found in the lining of tubules with more than 1 cell layer. The tumor cells are polygonal with eosinophilic cytoplasm, ovoid hyperchromatic or vesicular nuclei, and prominent nucleoli. Mitoses are common. The tubular lumina are filled with acantholytic cells, either singly or in small clusters, which may demonstrate residual bridging to tubular lining cells (Figure 2). The acantholytic cells show some variability in size and may be large, multinucleated, or keratinized. The tubules may contain material that is amorphous, basophilic, periodic acid–Schiff positive, diastase sensitive, and mucicarmine negative.5 Eccrine ducts at the periphery of the tumor may show reactive dilatation and proliferation. Tumor cells show positive immunostaining for epithelial membrane antigen, 34βE12, CK5/6, and tumor protein p63.6-8 There is negative immunostaining for carcinoembryonic antigen, amylase, S-100 protein, and factor VIII.5
|
The differential diagnosis includes adenoid BCC, angiosarcoma, eccrine carcinoma, and metastatic adenocarcinoma of the skin. In adenoid BCC, excess stromal mucin imparts pseudoglandular architecture (Figure 3). However, features of conventional BCC, including peripheral nuclear palisading and retraction artifact often are present as well.
Angiosarcoma shows slitlike vascular spaces lined by hyperchromatic endothelial cells (Figure 4). Further, there is positive immunostaining for vascular markers CD31 and CD34.
In eccrine carcinoma, there are invasive ductal structures lined by either a single or double layer of cells that may contain luminal material that is periodic acid–Schiff positive and diastase resistant (Figure 5).9 The tumor cells show positive immunostaining for cytokeratins, epithelial membrane antigen, carcinoembryonic antigen, and S-100 protein.10
Pseudoglandular SCC is susceptible to misdiagnosis as adenocarcinoma by sampling error if biopsies do not capture areas with typical features of SCC, including dysplastic squamous epithelium and keratinization. Metastatic adenocarcinoma of the skin is more likely to present with multiple nodules in older individuals. Lack of epidermal connection of the tumor and minimal to no acantholytic dyskeratosis further support cutaneous metastasis (Figure 6). Review of the patient’s clinical history might be helpful if adenocarcinoma was previously diagnosed. Immunohistochemical evaluation may aid in the prediction of the primary site in patients with metastatic adenocarcinoma of unknown origin.11
1. Lever WF. Adenocanthoma of sweat glands; carcinoma of sweat glands with glandular and epidermal elements: report of four cases. Arch Derm Syphilol. 1947;56:157-171.
2. Bonerandi JJ, Beauvillain C, Caquant L, et al. Guidelines for the diagnosis and treatment of cutaneous squamous cell carcinoma and precursor lesions. J Eur Acad Dermatol Venereol. 2011;25(suppl 5):1-51.
3. Griffin JR, Wriston CC, Peters MS, et al. Decreased expression of intercellular adhesion molecules in acantholytic squamous cell carcinoma compared with invasive well-differentiated squamous cell carcinoma of the skin. Am J Clin Pathol. 2013;139:442-447.
4. Bayer-Garner IB, Smoller BR. The expression of syndecan-1 is preferentially reduced compared with that of E-cadherin in acantholytic squamous cell carcinoma. J Cutan Pathol. 2001;28:83-89.
5. Nappi O, Pettinato G, Wick MR. Adenoid (acantholytic) squamous cell carcinoma of the skin. J Cutan Pathol. 1989;16:114-121.
6. Sajin M, Hodorogea Prisăcaru A, Luchian MC, et al. Acantholytic squamous cell carcinoma: pathological study of nine cases with review of literature. Rom J Morphol Embryol. 2014;55:279-283.
7. Gray Y, Robidoux HJ, Farrell DS, et al. Squamous cell carcinoma detected by high-molecular-weight cytokeratin immunostaining mimicking atypical fibroxanthoma. Arch Pathol Lab Med. 2001;125:799-802.
8. Kanitakis J, Chouvet B. Expression of p63 in cutaneous metastases. Am J Clin Pathol. 2007;128:753-758.
9. Plaza JA, Prieto VG. Neoplastic Lesions of the Skin. New York, NY: Demos Medical Publishing; 2014.
10. Swanson PE, Cherwitz DL, Neumann MP, et al. Eccrine sweat gland carcinoma: an histologic and immunohistochemical study of 32 cases. J Cutan Pathol. 1987;14:65-86.
11. Dennis JL, Hvidsten TR, Wit EC, et al. Markers of adenocarcinoma characteristic of the site of origin: development of a diagnostic algorithm. Clin Cancer Res. 2005;11:3766-3772.
1. Lever WF. Adenocanthoma of sweat glands; carcinoma of sweat glands with glandular and epidermal elements: report of four cases. Arch Derm Syphilol. 1947;56:157-171.
2. Bonerandi JJ, Beauvillain C, Caquant L, et al. Guidelines for the diagnosis and treatment of cutaneous squamous cell carcinoma and precursor lesions. J Eur Acad Dermatol Venereol. 2011;25(suppl 5):1-51.
3. Griffin JR, Wriston CC, Peters MS, et al. Decreased expression of intercellular adhesion molecules in acantholytic squamous cell carcinoma compared with invasive well-differentiated squamous cell carcinoma of the skin. Am J Clin Pathol. 2013;139:442-447.
4. Bayer-Garner IB, Smoller BR. The expression of syndecan-1 is preferentially reduced compared with that of E-cadherin in acantholytic squamous cell carcinoma. J Cutan Pathol. 2001;28:83-89.
5. Nappi O, Pettinato G, Wick MR. Adenoid (acantholytic) squamous cell carcinoma of the skin. J Cutan Pathol. 1989;16:114-121.
6. Sajin M, Hodorogea Prisăcaru A, Luchian MC, et al. Acantholytic squamous cell carcinoma: pathological study of nine cases with review of literature. Rom J Morphol Embryol. 2014;55:279-283.
7. Gray Y, Robidoux HJ, Farrell DS, et al. Squamous cell carcinoma detected by high-molecular-weight cytokeratin immunostaining mimicking atypical fibroxanthoma. Arch Pathol Lab Med. 2001;125:799-802.
8. Kanitakis J, Chouvet B. Expression of p63 in cutaneous metastases. Am J Clin Pathol. 2007;128:753-758.
9. Plaza JA, Prieto VG. Neoplastic Lesions of the Skin. New York, NY: Demos Medical Publishing; 2014.
10. Swanson PE, Cherwitz DL, Neumann MP, et al. Eccrine sweat gland carcinoma: an histologic and immunohistochemical study of 32 cases. J Cutan Pathol. 1987;14:65-86.
11. Dennis JL, Hvidsten TR, Wit EC, et al. Markers of adenocarcinoma characteristic of the site of origin: development of a diagnostic algorithm. Clin Cancer Res. 2005;11:3766-3772.
What Is Your Diagnosis? Acquired Lymphangiectasia
A 19-year-old woman presented with an umbilical mass of 5 months’ duration that had grown in size. Physical examination revealed a 1×1-cm brownish, pedunculated, cauliflower-shaped lesion on the umbilicus. There were no other signs or symptoms of disease. The patient’s personal and family disease history were unremarkable. An excisional biopsy was performed.
The Diagnosis: Acquired Lymphangiectasia
On histopathology numerous dilated channels lined by a single flat layer of endothelial cells were noted within the dermis. The overlying epidermis was papillomatous and acanthotic (Figure 1). The endothelial cells lining the dilated channels were D2-40 positive (Figure 2). Furthermore, the channels contained a pinkish amorphous material and a few red blood cells. The surrounding stroma showed scattered lymphocyte infiltration. These findings were consistent with lymphangiectasia. The lesion has not recurred 4 years following total excision.
|
Acquired lymphangiectasia is known by various names, including lymphangioma, acquired lymphangioma, and acquired lymphangioma circumscriptum, which has led to confusion.1 Acquired lymphangiectasia, which is characterized by dilated superficial lymphatics, develops following damage to previously normal lymphatic channels, leading to a buildup of lymph pressure and backflow.2 Acquired lymphangiectasia has been reported as clinically and histologically indistinguishable from lymphangioma circumscriptum2; however, unlike in lymphangiectasia, the suffix -oma denotes a tumor. Our case matched more closely with the typical concept of lymphangiectasia rather than lymphangioma.
Clinical findings of acquired lymphangiectasia usually include translucent, flat or slightly raised, 2- to 5-mm, flesh-colored papules and vesicles.3,4 Acquired lymphangiectasia has been described with lesions that have verrucous surfaces mimicking warts, condyloma acuminata, or molluscum contagiosum.5,6 Our case suggests that acquired lymphangiectasia also can present with a pedunculated cauliflowerlike appearance. In general, it develops secondary to certain conditions such as recovery from trauma or surgery, postsurgical fibrosis, and irradiation. Lymphangiectasia often is seen on the arms, axillae, chest wall, and genital area in women and the scrotum, penis, thighs, and pubic region in men, both who have undergone radical surgery and irradiation for treatment of breast and prostate cancer, respectively.3 Our patient did not report any history of trauma to the umbilicus.
On histopathology acquired lymphangiectasia typically shows edematous polypoid nodules with dilated lymphatics. The overlying epidermis usually shows a spectrum of proliferation ranging from mild acanthosis to florid pseudoepitheliomatous hyperplasia with marked hyperkeratosis and parakeratosis. The distinctive finding of lymphangiectasia is the presence of dilated lymphatic spaces within the dermis. The dilated channels are filled with lymphatic fluid and often red and white blood cells. The single layer of flattened endothelial cells generally exhibits immunoreactivity to D2-40 and CD31.1
Treatment of lymphangiectasia is focused on reducing the pressure within the lymph vessels and managing consequent lymphedema with compression dressings. Simple surgical excision of lesions on sites such as the vulva or legs often is effective.3 If surgical intervention is not an option, cryotherapy, sclerotherapy, cauterization, and treatment with CO2 lasers also have been utilized with good outcomes.7 In the current case, total surgical excision was performed, which provided good results.
1. Stewart CJ, Chan T, Platten M. Acquired lymphangiectasia (‘lymphangioma circumscriptum’) of the vulva: a report of eight cases. Pathology. 2009;41:448-453.
2. Celis AV, Gaughf CN, Sangueza OP, et al. Acquired lymphangiectasis. South Med J. 1999;92:69-72.
3. Verma SB. Lymphangiectasias of the skin: victims of confusing nomenclature. Clin Exp Dermatol. 2009;34:566-569.
4. Mortimer PS. Disorder of lymphatic vessels. In: Burns T, Breathnach S, Cox N, et al, eds. Rook’s Textbook of Dermatology. Vol 3. 8th ed. Hoboken, NJ: Wiley-Blackwell; 2010:48.28-48.29.
5. Sharma R, Tomar S, Chandra M. Acquired vulval lymphangiectases mimicking genital warts. Indian J Dermatol Venereol Leprol. 2002;68:166-167.
6. Horn LC, Kühndel K, Pawlowitsch T, et al. Acquired lymphangioma circumscriptum of the vulva mimicking genital warts. Eur J Obstet Gynecol Reprod Biol. 2005;123:118-120.
7. Patel GA, Schwartz RA. Cutaneous lymphangioma circumscriptum: frog spawn on the skin. Int J Dermatol. 2009;48:1290-1295.
A 19-year-old woman presented with an umbilical mass of 5 months’ duration that had grown in size. Physical examination revealed a 1×1-cm brownish, pedunculated, cauliflower-shaped lesion on the umbilicus. There were no other signs or symptoms of disease. The patient’s personal and family disease history were unremarkable. An excisional biopsy was performed.
The Diagnosis: Acquired Lymphangiectasia
On histopathology numerous dilated channels lined by a single flat layer of endothelial cells were noted within the dermis. The overlying epidermis was papillomatous and acanthotic (Figure 1). The endothelial cells lining the dilated channels were D2-40 positive (Figure 2). Furthermore, the channels contained a pinkish amorphous material and a few red blood cells. The surrounding stroma showed scattered lymphocyte infiltration. These findings were consistent with lymphangiectasia. The lesion has not recurred 4 years following total excision.
|
Acquired lymphangiectasia is known by various names, including lymphangioma, acquired lymphangioma, and acquired lymphangioma circumscriptum, which has led to confusion.1 Acquired lymphangiectasia, which is characterized by dilated superficial lymphatics, develops following damage to previously normal lymphatic channels, leading to a buildup of lymph pressure and backflow.2 Acquired lymphangiectasia has been reported as clinically and histologically indistinguishable from lymphangioma circumscriptum2; however, unlike in lymphangiectasia, the suffix -oma denotes a tumor. Our case matched more closely with the typical concept of lymphangiectasia rather than lymphangioma.
Clinical findings of acquired lymphangiectasia usually include translucent, flat or slightly raised, 2- to 5-mm, flesh-colored papules and vesicles.3,4 Acquired lymphangiectasia has been described with lesions that have verrucous surfaces mimicking warts, condyloma acuminata, or molluscum contagiosum.5,6 Our case suggests that acquired lymphangiectasia also can present with a pedunculated cauliflowerlike appearance. In general, it develops secondary to certain conditions such as recovery from trauma or surgery, postsurgical fibrosis, and irradiation. Lymphangiectasia often is seen on the arms, axillae, chest wall, and genital area in women and the scrotum, penis, thighs, and pubic region in men, both who have undergone radical surgery and irradiation for treatment of breast and prostate cancer, respectively.3 Our patient did not report any history of trauma to the umbilicus.
On histopathology acquired lymphangiectasia typically shows edematous polypoid nodules with dilated lymphatics. The overlying epidermis usually shows a spectrum of proliferation ranging from mild acanthosis to florid pseudoepitheliomatous hyperplasia with marked hyperkeratosis and parakeratosis. The distinctive finding of lymphangiectasia is the presence of dilated lymphatic spaces within the dermis. The dilated channels are filled with lymphatic fluid and often red and white blood cells. The single layer of flattened endothelial cells generally exhibits immunoreactivity to D2-40 and CD31.1
Treatment of lymphangiectasia is focused on reducing the pressure within the lymph vessels and managing consequent lymphedema with compression dressings. Simple surgical excision of lesions on sites such as the vulva or legs often is effective.3 If surgical intervention is not an option, cryotherapy, sclerotherapy, cauterization, and treatment with CO2 lasers also have been utilized with good outcomes.7 In the current case, total surgical excision was performed, which provided good results.
A 19-year-old woman presented with an umbilical mass of 5 months’ duration that had grown in size. Physical examination revealed a 1×1-cm brownish, pedunculated, cauliflower-shaped lesion on the umbilicus. There were no other signs or symptoms of disease. The patient’s personal and family disease history were unremarkable. An excisional biopsy was performed.
The Diagnosis: Acquired Lymphangiectasia
On histopathology numerous dilated channels lined by a single flat layer of endothelial cells were noted within the dermis. The overlying epidermis was papillomatous and acanthotic (Figure 1). The endothelial cells lining the dilated channels were D2-40 positive (Figure 2). Furthermore, the channels contained a pinkish amorphous material and a few red blood cells. The surrounding stroma showed scattered lymphocyte infiltration. These findings were consistent with lymphangiectasia. The lesion has not recurred 4 years following total excision.
|
Acquired lymphangiectasia is known by various names, including lymphangioma, acquired lymphangioma, and acquired lymphangioma circumscriptum, which has led to confusion.1 Acquired lymphangiectasia, which is characterized by dilated superficial lymphatics, develops following damage to previously normal lymphatic channels, leading to a buildup of lymph pressure and backflow.2 Acquired lymphangiectasia has been reported as clinically and histologically indistinguishable from lymphangioma circumscriptum2; however, unlike in lymphangiectasia, the suffix -oma denotes a tumor. Our case matched more closely with the typical concept of lymphangiectasia rather than lymphangioma.
Clinical findings of acquired lymphangiectasia usually include translucent, flat or slightly raised, 2- to 5-mm, flesh-colored papules and vesicles.3,4 Acquired lymphangiectasia has been described with lesions that have verrucous surfaces mimicking warts, condyloma acuminata, or molluscum contagiosum.5,6 Our case suggests that acquired lymphangiectasia also can present with a pedunculated cauliflowerlike appearance. In general, it develops secondary to certain conditions such as recovery from trauma or surgery, postsurgical fibrosis, and irradiation. Lymphangiectasia often is seen on the arms, axillae, chest wall, and genital area in women and the scrotum, penis, thighs, and pubic region in men, both who have undergone radical surgery and irradiation for treatment of breast and prostate cancer, respectively.3 Our patient did not report any history of trauma to the umbilicus.
On histopathology acquired lymphangiectasia typically shows edematous polypoid nodules with dilated lymphatics. The overlying epidermis usually shows a spectrum of proliferation ranging from mild acanthosis to florid pseudoepitheliomatous hyperplasia with marked hyperkeratosis and parakeratosis. The distinctive finding of lymphangiectasia is the presence of dilated lymphatic spaces within the dermis. The dilated channels are filled with lymphatic fluid and often red and white blood cells. The single layer of flattened endothelial cells generally exhibits immunoreactivity to D2-40 and CD31.1
Treatment of lymphangiectasia is focused on reducing the pressure within the lymph vessels and managing consequent lymphedema with compression dressings. Simple surgical excision of lesions on sites such as the vulva or legs often is effective.3 If surgical intervention is not an option, cryotherapy, sclerotherapy, cauterization, and treatment with CO2 lasers also have been utilized with good outcomes.7 In the current case, total surgical excision was performed, which provided good results.
1. Stewart CJ, Chan T, Platten M. Acquired lymphangiectasia (‘lymphangioma circumscriptum’) of the vulva: a report of eight cases. Pathology. 2009;41:448-453.
2. Celis AV, Gaughf CN, Sangueza OP, et al. Acquired lymphangiectasis. South Med J. 1999;92:69-72.
3. Verma SB. Lymphangiectasias of the skin: victims of confusing nomenclature. Clin Exp Dermatol. 2009;34:566-569.
4. Mortimer PS. Disorder of lymphatic vessels. In: Burns T, Breathnach S, Cox N, et al, eds. Rook’s Textbook of Dermatology. Vol 3. 8th ed. Hoboken, NJ: Wiley-Blackwell; 2010:48.28-48.29.
5. Sharma R, Tomar S, Chandra M. Acquired vulval lymphangiectases mimicking genital warts. Indian J Dermatol Venereol Leprol. 2002;68:166-167.
6. Horn LC, Kühndel K, Pawlowitsch T, et al. Acquired lymphangioma circumscriptum of the vulva mimicking genital warts. Eur J Obstet Gynecol Reprod Biol. 2005;123:118-120.
7. Patel GA, Schwartz RA. Cutaneous lymphangioma circumscriptum: frog spawn on the skin. Int J Dermatol. 2009;48:1290-1295.
1. Stewart CJ, Chan T, Platten M. Acquired lymphangiectasia (‘lymphangioma circumscriptum’) of the vulva: a report of eight cases. Pathology. 2009;41:448-453.
2. Celis AV, Gaughf CN, Sangueza OP, et al. Acquired lymphangiectasis. South Med J. 1999;92:69-72.
3. Verma SB. Lymphangiectasias of the skin: victims of confusing nomenclature. Clin Exp Dermatol. 2009;34:566-569.
4. Mortimer PS. Disorder of lymphatic vessels. In: Burns T, Breathnach S, Cox N, et al, eds. Rook’s Textbook of Dermatology. Vol 3. 8th ed. Hoboken, NJ: Wiley-Blackwell; 2010:48.28-48.29.
5. Sharma R, Tomar S, Chandra M. Acquired vulval lymphangiectases mimicking genital warts. Indian J Dermatol Venereol Leprol. 2002;68:166-167.
6. Horn LC, Kühndel K, Pawlowitsch T, et al. Acquired lymphangioma circumscriptum of the vulva mimicking genital warts. Eur J Obstet Gynecol Reprod Biol. 2005;123:118-120.
7. Patel GA, Schwartz RA. Cutaneous lymphangioma circumscriptum: frog spawn on the skin. Int J Dermatol. 2009;48:1290-1295.
Can Dermatologists Influence the Political Process?
Can dermatologists influence the political process? This age-old question raises its head when things are happening that we do not like or that we believe could be done better. Even though dermatologists have skills and expertise that allow us to be effective in our medical practice, most of us feel incompetent when it comes to dealing with the government. The political process is not the primary focus of our time and energy, not a topic in continuing medical education, and definitely not a place of comfort. At my medical school commencement ceremony, Leon Jaworski, a special prosecutor during Watergate, gave an address in which he scolded the medical community for not being more involved in the political process, insisting that society would benefit from our participation. This message was a lightning bolt out of the blue for me. It was the first time in all my medical school years that any respected senior figure had broached the concept that a physician’s role includes engagement in the workings of government. Jaworski was insistent, and he was right.
Influencing the political process (ie, advocating effectively) requires skills and expertise that can be acquired with attention to 3 primary principles: interfacing with legislators, establishing credibility, and joining forces within professional societies.
How to Interface With Legislators
Interfacing with legislators is exactly that: interFACEing. Based on 4 online surveys of congressional staff members (2010-2013) by the Congressional Management Foundation, the most influential way to communicate with a senator or representative is an in-person visit.1 Successful politicians regard keeping in touch with their constituents to be the most critical factor in their effectiveness.
Getting face time with a legislator can be difficult. In the short-term, the most successful strategy is for the request to come from a constituent who is affiliated with an association or corporation that represents the interests of many constituents. In the long-term, personal visits, letters (e-mail is preferred for security reasons), and telephone calls are most important when they come from constituents who are well known, highly regarded, and have gone out of their way to be helpful to the legislator’s office, which means we need to pay attention to building a relationship with our legislators. For example, I had a long-standing, one-sided correspondence with former representative Barney Frank (Democrat, Massachusetts), writing him regularly on issues that concerned me. I once chided him for not showing up for a vote I thought was important and in return I received a 3-page handwritten letter from him concerning his absence. YES! He knew my name, he knew what was important to me, and he had enough regard for my opinion to answer me personally.
Building a relationship with a staffer can be as important or sometimes even more important than with the legislators themselves. Staffers have direct access to legislators and understand the best way to facilitate moving your information and concerns forward. Meet with them, keep their contact information on hand, and direct your questions and comments to them. Staffers know how you can be helpful to their office, whether it is by supplying information, providing feedback on a position or comment, or hosting a neighborhood coffee gathering in your home so the legislator can meet other constituents. Attending events in your district or offering a simple fundraiser in your home for local and state legislators is an excellent way to be involved with candidates in a way that promotes a future relationship. When the Stark exemption was being discussed in relation to anatomic pathology at an in-office laboratory, I offered a tour of my Mohs unit to a staffer so she could see how integral and important it is for surgeons to perform histology examinations to ensure proper patient care. She did not take me up on the offer, but she subsequently called me with a question concerning a similar issue.
It is not optimal to develop relationships solely with legislators from the political party with which you identify. Dermatologists’ professional interests of concern will likely require bipartisan support for resolution.
Establish Your Credibility
Do some homework on your key issues; find the facts that support your position and also be sure to understand the opposition’s concerns. Be able to present your viewpoint in a focused and concise way with a clear sense of what you are asking the legislator to do.
Keep in mind that you want to develop a long-term, trusted relationship. It is important to be respectful and leave general political feelings out of the meeting. Schedule a meeting with the intention of presenting only 2 or 3 concrete requests. During the visit, it helps to set the context for the conversation if you reference a particular bill. Staffers often ask questions about the bill if it is not one they are currently following, which allows you to become a dependable source of good information. You can engage with staffers or legislators by asking them directly and politely for their views and position on the issue. It often is effective to leave them with a 1- to 2-page summary for review and to follow up later with a formal thank you for their time.
Participate in Professional Societies
Join professional societies to acquire the information you need, refine and consolidate messaging, and represent a larger constituency. Most of the societies that dermatologists belong to, including the American Academy of Dermatology Association, the American Society for Dermatologic Surgery Association, and the American College of Mohs Surgery, have identified legislative priorities and promote coordinated visits to Congress. The American Academy of Dermatology Association organizes its annual Legislative Conference in Washington, DC, each fall, which includes an in-depth program of speakers, discussions, and information concerning priority issues. Messages are refined and appointments are made for visits with legislators and/or their staffers. Your small group will include experienced colleagues so that you develop your skills with their mentorship. Many state societies also train their members to be effective at advocacy at the State House. Finally and most importantly, a sizeable war chest (yes, your dollars are needed too) for SkinPAC, the American Academy of Dermatology Association Political Action Committee, creates notice and respect for dermatology’s commitment to the issues of importance to the profession.
Dermatologists Have Impacted Legislation
Interaction by individual dermatologists with legislators has had a direct impact on health care issues. A Medicare Advantage Participant Bill of Rights recently was introduced and has garnered bipartisan support in the Senate due to meetings between dermatologists and key members of Congress and the Obama administration about concerns with narrow physician networks. There is a robust bipartisan Congressional Skin Cancer Caucus that representatives have joined because of discussions with their constituent dermatologists. The strong testimonies of 2 dermatologists in Maryland has picked up 3 more yes votes for passing a bill that prohibits use of tanning devices in minors younger than 18 years in that state. California dermatologists also put enough pressure on their state senators to defeat the elimination of the in-office exception to self-referral for anatomic pathology services.
Final Thoughts
Dermatologists do have a voice. We do have influence. We must “sit at the table.”2 Build a relationship with your legislators. Develop your message and become a trusted voice. Join forces with other dermatologists. “Let’s grab the front seat together.”2
1. Face-to-face with congress: before, during, and after meetings with legislators. Congressional Management Foundation Web site. http://www.congressfoundation.org/projects/communicating-with-congress/face-to-face. Accessed January 9, 2015.
2. Sandberg S. Why we have too few women leaders. http://www.ted.com/talks/sheryl_sandberg_why_we_have_too_few_women_leaders. Accessed January 6, 2015.
Can dermatologists influence the political process? This age-old question raises its head when things are happening that we do not like or that we believe could be done better. Even though dermatologists have skills and expertise that allow us to be effective in our medical practice, most of us feel incompetent when it comes to dealing with the government. The political process is not the primary focus of our time and energy, not a topic in continuing medical education, and definitely not a place of comfort. At my medical school commencement ceremony, Leon Jaworski, a special prosecutor during Watergate, gave an address in which he scolded the medical community for not being more involved in the political process, insisting that society would benefit from our participation. This message was a lightning bolt out of the blue for me. It was the first time in all my medical school years that any respected senior figure had broached the concept that a physician’s role includes engagement in the workings of government. Jaworski was insistent, and he was right.
Influencing the political process (ie, advocating effectively) requires skills and expertise that can be acquired with attention to 3 primary principles: interfacing with legislators, establishing credibility, and joining forces within professional societies.
How to Interface With Legislators
Interfacing with legislators is exactly that: interFACEing. Based on 4 online surveys of congressional staff members (2010-2013) by the Congressional Management Foundation, the most influential way to communicate with a senator or representative is an in-person visit.1 Successful politicians regard keeping in touch with their constituents to be the most critical factor in their effectiveness.
Getting face time with a legislator can be difficult. In the short-term, the most successful strategy is for the request to come from a constituent who is affiliated with an association or corporation that represents the interests of many constituents. In the long-term, personal visits, letters (e-mail is preferred for security reasons), and telephone calls are most important when they come from constituents who are well known, highly regarded, and have gone out of their way to be helpful to the legislator’s office, which means we need to pay attention to building a relationship with our legislators. For example, I had a long-standing, one-sided correspondence with former representative Barney Frank (Democrat, Massachusetts), writing him regularly on issues that concerned me. I once chided him for not showing up for a vote I thought was important and in return I received a 3-page handwritten letter from him concerning his absence. YES! He knew my name, he knew what was important to me, and he had enough regard for my opinion to answer me personally.
Building a relationship with a staffer can be as important or sometimes even more important than with the legislators themselves. Staffers have direct access to legislators and understand the best way to facilitate moving your information and concerns forward. Meet with them, keep their contact information on hand, and direct your questions and comments to them. Staffers know how you can be helpful to their office, whether it is by supplying information, providing feedback on a position or comment, or hosting a neighborhood coffee gathering in your home so the legislator can meet other constituents. Attending events in your district or offering a simple fundraiser in your home for local and state legislators is an excellent way to be involved with candidates in a way that promotes a future relationship. When the Stark exemption was being discussed in relation to anatomic pathology at an in-office laboratory, I offered a tour of my Mohs unit to a staffer so she could see how integral and important it is for surgeons to perform histology examinations to ensure proper patient care. She did not take me up on the offer, but she subsequently called me with a question concerning a similar issue.
It is not optimal to develop relationships solely with legislators from the political party with which you identify. Dermatologists’ professional interests of concern will likely require bipartisan support for resolution.
Establish Your Credibility
Do some homework on your key issues; find the facts that support your position and also be sure to understand the opposition’s concerns. Be able to present your viewpoint in a focused and concise way with a clear sense of what you are asking the legislator to do.
Keep in mind that you want to develop a long-term, trusted relationship. It is important to be respectful and leave general political feelings out of the meeting. Schedule a meeting with the intention of presenting only 2 or 3 concrete requests. During the visit, it helps to set the context for the conversation if you reference a particular bill. Staffers often ask questions about the bill if it is not one they are currently following, which allows you to become a dependable source of good information. You can engage with staffers or legislators by asking them directly and politely for their views and position on the issue. It often is effective to leave them with a 1- to 2-page summary for review and to follow up later with a formal thank you for their time.
Participate in Professional Societies
Join professional societies to acquire the information you need, refine and consolidate messaging, and represent a larger constituency. Most of the societies that dermatologists belong to, including the American Academy of Dermatology Association, the American Society for Dermatologic Surgery Association, and the American College of Mohs Surgery, have identified legislative priorities and promote coordinated visits to Congress. The American Academy of Dermatology Association organizes its annual Legislative Conference in Washington, DC, each fall, which includes an in-depth program of speakers, discussions, and information concerning priority issues. Messages are refined and appointments are made for visits with legislators and/or their staffers. Your small group will include experienced colleagues so that you develop your skills with their mentorship. Many state societies also train their members to be effective at advocacy at the State House. Finally and most importantly, a sizeable war chest (yes, your dollars are needed too) for SkinPAC, the American Academy of Dermatology Association Political Action Committee, creates notice and respect for dermatology’s commitment to the issues of importance to the profession.
Dermatologists Have Impacted Legislation
Interaction by individual dermatologists with legislators has had a direct impact on health care issues. A Medicare Advantage Participant Bill of Rights recently was introduced and has garnered bipartisan support in the Senate due to meetings between dermatologists and key members of Congress and the Obama administration about concerns with narrow physician networks. There is a robust bipartisan Congressional Skin Cancer Caucus that representatives have joined because of discussions with their constituent dermatologists. The strong testimonies of 2 dermatologists in Maryland has picked up 3 more yes votes for passing a bill that prohibits use of tanning devices in minors younger than 18 years in that state. California dermatologists also put enough pressure on their state senators to defeat the elimination of the in-office exception to self-referral for anatomic pathology services.
Final Thoughts
Dermatologists do have a voice. We do have influence. We must “sit at the table.”2 Build a relationship with your legislators. Develop your message and become a trusted voice. Join forces with other dermatologists. “Let’s grab the front seat together.”2
Can dermatologists influence the political process? This age-old question raises its head when things are happening that we do not like or that we believe could be done better. Even though dermatologists have skills and expertise that allow us to be effective in our medical practice, most of us feel incompetent when it comes to dealing with the government. The political process is not the primary focus of our time and energy, not a topic in continuing medical education, and definitely not a place of comfort. At my medical school commencement ceremony, Leon Jaworski, a special prosecutor during Watergate, gave an address in which he scolded the medical community for not being more involved in the political process, insisting that society would benefit from our participation. This message was a lightning bolt out of the blue for me. It was the first time in all my medical school years that any respected senior figure had broached the concept that a physician’s role includes engagement in the workings of government. Jaworski was insistent, and he was right.
Influencing the political process (ie, advocating effectively) requires skills and expertise that can be acquired with attention to 3 primary principles: interfacing with legislators, establishing credibility, and joining forces within professional societies.
How to Interface With Legislators
Interfacing with legislators is exactly that: interFACEing. Based on 4 online surveys of congressional staff members (2010-2013) by the Congressional Management Foundation, the most influential way to communicate with a senator or representative is an in-person visit.1 Successful politicians regard keeping in touch with their constituents to be the most critical factor in their effectiveness.
Getting face time with a legislator can be difficult. In the short-term, the most successful strategy is for the request to come from a constituent who is affiliated with an association or corporation that represents the interests of many constituents. In the long-term, personal visits, letters (e-mail is preferred for security reasons), and telephone calls are most important when they come from constituents who are well known, highly regarded, and have gone out of their way to be helpful to the legislator’s office, which means we need to pay attention to building a relationship with our legislators. For example, I had a long-standing, one-sided correspondence with former representative Barney Frank (Democrat, Massachusetts), writing him regularly on issues that concerned me. I once chided him for not showing up for a vote I thought was important and in return I received a 3-page handwritten letter from him concerning his absence. YES! He knew my name, he knew what was important to me, and he had enough regard for my opinion to answer me personally.
Building a relationship with a staffer can be as important or sometimes even more important than with the legislators themselves. Staffers have direct access to legislators and understand the best way to facilitate moving your information and concerns forward. Meet with them, keep their contact information on hand, and direct your questions and comments to them. Staffers know how you can be helpful to their office, whether it is by supplying information, providing feedback on a position or comment, or hosting a neighborhood coffee gathering in your home so the legislator can meet other constituents. Attending events in your district or offering a simple fundraiser in your home for local and state legislators is an excellent way to be involved with candidates in a way that promotes a future relationship. When the Stark exemption was being discussed in relation to anatomic pathology at an in-office laboratory, I offered a tour of my Mohs unit to a staffer so she could see how integral and important it is for surgeons to perform histology examinations to ensure proper patient care. She did not take me up on the offer, but she subsequently called me with a question concerning a similar issue.
It is not optimal to develop relationships solely with legislators from the political party with which you identify. Dermatologists’ professional interests of concern will likely require bipartisan support for resolution.
Establish Your Credibility
Do some homework on your key issues; find the facts that support your position and also be sure to understand the opposition’s concerns. Be able to present your viewpoint in a focused and concise way with a clear sense of what you are asking the legislator to do.
Keep in mind that you want to develop a long-term, trusted relationship. It is important to be respectful and leave general political feelings out of the meeting. Schedule a meeting with the intention of presenting only 2 or 3 concrete requests. During the visit, it helps to set the context for the conversation if you reference a particular bill. Staffers often ask questions about the bill if it is not one they are currently following, which allows you to become a dependable source of good information. You can engage with staffers or legislators by asking them directly and politely for their views and position on the issue. It often is effective to leave them with a 1- to 2-page summary for review and to follow up later with a formal thank you for their time.
Participate in Professional Societies
Join professional societies to acquire the information you need, refine and consolidate messaging, and represent a larger constituency. Most of the societies that dermatologists belong to, including the American Academy of Dermatology Association, the American Society for Dermatologic Surgery Association, and the American College of Mohs Surgery, have identified legislative priorities and promote coordinated visits to Congress. The American Academy of Dermatology Association organizes its annual Legislative Conference in Washington, DC, each fall, which includes an in-depth program of speakers, discussions, and information concerning priority issues. Messages are refined and appointments are made for visits with legislators and/or their staffers. Your small group will include experienced colleagues so that you develop your skills with their mentorship. Many state societies also train their members to be effective at advocacy at the State House. Finally and most importantly, a sizeable war chest (yes, your dollars are needed too) for SkinPAC, the American Academy of Dermatology Association Political Action Committee, creates notice and respect for dermatology’s commitment to the issues of importance to the profession.
Dermatologists Have Impacted Legislation
Interaction by individual dermatologists with legislators has had a direct impact on health care issues. A Medicare Advantage Participant Bill of Rights recently was introduced and has garnered bipartisan support in the Senate due to meetings between dermatologists and key members of Congress and the Obama administration about concerns with narrow physician networks. There is a robust bipartisan Congressional Skin Cancer Caucus that representatives have joined because of discussions with their constituent dermatologists. The strong testimonies of 2 dermatologists in Maryland has picked up 3 more yes votes for passing a bill that prohibits use of tanning devices in minors younger than 18 years in that state. California dermatologists also put enough pressure on their state senators to defeat the elimination of the in-office exception to self-referral for anatomic pathology services.
Final Thoughts
Dermatologists do have a voice. We do have influence. We must “sit at the table.”2 Build a relationship with your legislators. Develop your message and become a trusted voice. Join forces with other dermatologists. “Let’s grab the front seat together.”2
1. Face-to-face with congress: before, during, and after meetings with legislators. Congressional Management Foundation Web site. http://www.congressfoundation.org/projects/communicating-with-congress/face-to-face. Accessed January 9, 2015.
2. Sandberg S. Why we have too few women leaders. http://www.ted.com/talks/sheryl_sandberg_why_we_have_too_few_women_leaders. Accessed January 6, 2015.
1. Face-to-face with congress: before, during, and after meetings with legislators. Congressional Management Foundation Web site. http://www.congressfoundation.org/projects/communicating-with-congress/face-to-face. Accessed January 9, 2015.
2. Sandberg S. Why we have too few women leaders. http://www.ted.com/talks/sheryl_sandberg_why_we_have_too_few_women_leaders. Accessed January 6, 2015.
Angioimmunoblastic T-cell Lymphoma Presenting as Purpura Fulminans
Purpura fulminans is a hematologic emergency, with clinical skin necrosis and laboratory testing showing disseminated intravascular coagulation. The thrombotic occlusion usually affects small and medium-sized blood vessels and may involve any organ. Purpura fulminans has been implicated with sepsis, most commonly meningococcal infections; other infections such as Staphylococcus aureus, groups A and B β-hemolytic streptococci, Streptococcus pneumoniae, and Haemophilus influenzae; and as a sequela to benign childhood infections, such as varicella. Other associations with purpura fulminans include autoimmune disease and heritable or acquired deficiency of anticoagulant proteins, most commonly protein C. We present a rare case of purpura fulminans as the presenting sign of angioimmunoblastic T-cell lymphoma (AITL), an aggressive primary nodal peripheral T-cell lymphoma with a high mortality rate and nonspecific skin manifestations in roughly half of all patients involved.
Case Report
A 56-year-old woman presented with purpuric patches on the left foot (Figure 1A). Seven days after presentation the lesion progressed into ecchymotic geographic plaques and hemorrhagic bullae that spread upward and contralaterally, sparing the digits, trunk, head, neck, and mucous membranes. Ultimately, the involved skin became necrotic and involved 20% of the body surface area (Figure 1B). The lesions were painful with a burning sensation but were not pruritic. The patient also reported intermittent fevers, chills, myalgia, nausea, and shortness of breath. Enlarged lymph nodes were present in the right cervical chain. She denied new medications; stated she had been in good health prior to this episode; and had no history of spontaneous abortion, neurologic symptoms, or other serious illness.
  |
Computed tomography showed prominent diffuse mediastinal, mesenteric, retroperitoneal, and pelvic lymphadenopathy with involvement of the cervical and inguinal areas. Laboratory values showed thrombocytopenia and increased fibrin degradation products. Blood and tissue cultures were negative; the patient also had a negative viral serology, except for Epstein-Barr virus IgG titers (>1:2560). A skin biopsy of the left thigh demonstrated venules and capillaries in the mid and superficial dermis filled with fibrin thrombi without vasculitis (Figure 2). A lymph node biopsy was consistent with a diagnosis of AITL. The lymph node architecture was largely effaced by a polymorphous lymphoid infiltrate that predominantly expanded into paracortical areas and was associated with a prominent arborizing vascular proliferation. The infiltrate was composed of lymphocytes ranging in size from small to medium, with ample cytoplasm, coarsely clumped chromatin, and mildly irregular nuclear membranes. Large atypical lymphocytes with features of immunoblasts were easily identified. An associated inflammatory background composed of eosinophils, plasma cells, and histiocytes was present (Figure 3). The atypical lymphocytes stained positive for CD3and CD10 on immunohistochemistry. Additionally, a subset of large immunoblastlike lymphocytes was positive for Epstein-Barr–encoded small RNAs by in situ hybridization.
|
The patient was started on rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone. She received 2 cycles with positive response based on subsequent computed tomography and positron emission tomography scans that showed regression of her disease as well as the lack of formation of new skin lesions. She was transferred to a burn unit where she had continuing treatment and skin grafts. Despite 2 cycles of chemotherapy, broad-spectrum antibiotics, and daily wound care management, the patient died secondary to sepsis 6 months after presentation.
Comment
Angioimmunoblastic T-cell lymphoma is a primary nodal lymphoma with occasional cutaneous involvement. Cutaneous manifestations occur in roughly half of all patients with AITL1 and have mainly been described as erythematous macules and papules that can resemble a viral exanthem or a drug reaction.2 However, other skin manifestations include urticaria, papulovesicular lesions, nodules, erythroderma,3 and to a lesser degree purpura.4 The lesions have been noted to occur prior to, concurrent with, or anytime during the disease.3,5,6 This aggressive lymphoma has mortality rates ranging from 50% to 72%, and median survival ranges from 11 to 30 months.6
To arrive at the correct diagnosis of AITL, a nodal biopsy with immunochemistry is necessary. Classic findings on histopathology include effacement of normal architecture, marked vascular proliferation, and aggregates of atypical lymphoid cells. CD10 has been shown to be a good objective criterion for the diagnosis of AITL,4 with characteristic tumor cells expressing CD10. Nodal Epstein-Barr virus–positive lymphocytes often are present.2 Other T-cell lymphomas with primarily nodal presentation along with peripheral T-cell lymphoma include peripheral T-cell lymphoma unspecified type and anaplastic large cell lymphoma, according to the World Health Organization classification.7 Anaplastic large cell lymphoma is easily distinguished from AITL based on histopathology, immunostaining, and clinical presentation. Until recently, peripheral T-cell lymphoma unspecified type and reactive lymphoid hyperplasia presented a challenge to differentiate from AITL, especially in the early phases of the disease; however, the introduction of CD10 as a phenotypic marker has been instrumental in distinguishing AITL from other T-cell lymphomas with primary nodal involvement.1,4
The development of purpura fulminans and disseminated intravascular coagulation in a patient with AITL is rare. Although the exact mechanism for the thrombus formation in the skin has not been elucidated, purpura fulminans typically develops secondary to a severe infection. The exact incidence of purpura fulminans in the setting of AITL is unknown, but purpura as a cutaneous eruption has been associated as a clinical finding in AITL.6 Although our case may be a rare presentation of AITL, a prompt and accurate diagnosis can drastically change the prognosis of this aggressive disease.
1. Ferry JA. Angioimmunoblastic T-cell lymphoma. Adv Anat Pathol. 2002;9:273-279.
2. Brown HA, Macon WR, Kurtin PJ, et al. Cutaneous involvement by angioimmunoblastic T-cell lymphoma with remarkable heterogeneous Epstein-Barr virus expression. J Cutan Pathol. 2001;28:432-438.
3. Bernstein JE, Soltani K, Lorincz AL. Cutaneous manifestations of angioimmunoblastic lymphadenopathy. J Am Acad Dermatol. 1979;1:227-232.
4. Attygalle A, Al-Jehani R, Diss TC, et al. Neoplastic T cells in angioimmunoblastic T-cell lymphoma express CD10. Blood. 2002;99:627-633.
5. Jayaramna AG, Cassarino D, Advani R, et al. Cutaneous involvement by angioimmunoblastic T-cell lymphoma: a unique histologic presentation, mimicking an infectious etiology. J Cutan Pathol. 2006;33(suppl 2):6-11.
6. Martel P, Laroche L, Courville P, et al. Cutaneous involvement in patients with angioimmunoblastic lymphadenopathy with dysproteinemia: a clinical, immunohistological, and molecular analysis. Archives of Dermatology. 2000;136:881-886.
7. Jaffe ES, Harris NL, Stein H, et al, eds. Tumours of Haematopoietic and Lymphoid Tissues. 1st ed. Bethesda, MD: International Agency for Research on Cancer; 2001.
Purpura fulminans is a hematologic emergency, with clinical skin necrosis and laboratory testing showing disseminated intravascular coagulation. The thrombotic occlusion usually affects small and medium-sized blood vessels and may involve any organ. Purpura fulminans has been implicated with sepsis, most commonly meningococcal infections; other infections such as Staphylococcus aureus, groups A and B β-hemolytic streptococci, Streptococcus pneumoniae, and Haemophilus influenzae; and as a sequela to benign childhood infections, such as varicella. Other associations with purpura fulminans include autoimmune disease and heritable or acquired deficiency of anticoagulant proteins, most commonly protein C. We present a rare case of purpura fulminans as the presenting sign of angioimmunoblastic T-cell lymphoma (AITL), an aggressive primary nodal peripheral T-cell lymphoma with a high mortality rate and nonspecific skin manifestations in roughly half of all patients involved.
Case Report
A 56-year-old woman presented with purpuric patches on the left foot (Figure 1A). Seven days after presentation the lesion progressed into ecchymotic geographic plaques and hemorrhagic bullae that spread upward and contralaterally, sparing the digits, trunk, head, neck, and mucous membranes. Ultimately, the involved skin became necrotic and involved 20% of the body surface area (Figure 1B). The lesions were painful with a burning sensation but were not pruritic. The patient also reported intermittent fevers, chills, myalgia, nausea, and shortness of breath. Enlarged lymph nodes were present in the right cervical chain. She denied new medications; stated she had been in good health prior to this episode; and had no history of spontaneous abortion, neurologic symptoms, or other serious illness.
  |
Computed tomography showed prominent diffuse mediastinal, mesenteric, retroperitoneal, and pelvic lymphadenopathy with involvement of the cervical and inguinal areas. Laboratory values showed thrombocytopenia and increased fibrin degradation products. Blood and tissue cultures were negative; the patient also had a negative viral serology, except for Epstein-Barr virus IgG titers (>1:2560). A skin biopsy of the left thigh demonstrated venules and capillaries in the mid and superficial dermis filled with fibrin thrombi without vasculitis (Figure 2). A lymph node biopsy was consistent with a diagnosis of AITL. The lymph node architecture was largely effaced by a polymorphous lymphoid infiltrate that predominantly expanded into paracortical areas and was associated with a prominent arborizing vascular proliferation. The infiltrate was composed of lymphocytes ranging in size from small to medium, with ample cytoplasm, coarsely clumped chromatin, and mildly irregular nuclear membranes. Large atypical lymphocytes with features of immunoblasts were easily identified. An associated inflammatory background composed of eosinophils, plasma cells, and histiocytes was present (Figure 3). The atypical lymphocytes stained positive for CD3and CD10 on immunohistochemistry. Additionally, a subset of large immunoblastlike lymphocytes was positive for Epstein-Barr–encoded small RNAs by in situ hybridization.
|
The patient was started on rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone. She received 2 cycles with positive response based on subsequent computed tomography and positron emission tomography scans that showed regression of her disease as well as the lack of formation of new skin lesions. She was transferred to a burn unit where she had continuing treatment and skin grafts. Despite 2 cycles of chemotherapy, broad-spectrum antibiotics, and daily wound care management, the patient died secondary to sepsis 6 months after presentation.
Comment
Angioimmunoblastic T-cell lymphoma is a primary nodal lymphoma with occasional cutaneous involvement. Cutaneous manifestations occur in roughly half of all patients with AITL1 and have mainly been described as erythematous macules and papules that can resemble a viral exanthem or a drug reaction.2 However, other skin manifestations include urticaria, papulovesicular lesions, nodules, erythroderma,3 and to a lesser degree purpura.4 The lesions have been noted to occur prior to, concurrent with, or anytime during the disease.3,5,6 This aggressive lymphoma has mortality rates ranging from 50% to 72%, and median survival ranges from 11 to 30 months.6
To arrive at the correct diagnosis of AITL, a nodal biopsy with immunochemistry is necessary. Classic findings on histopathology include effacement of normal architecture, marked vascular proliferation, and aggregates of atypical lymphoid cells. CD10 has been shown to be a good objective criterion for the diagnosis of AITL,4 with characteristic tumor cells expressing CD10. Nodal Epstein-Barr virus–positive lymphocytes often are present.2 Other T-cell lymphomas with primarily nodal presentation along with peripheral T-cell lymphoma include peripheral T-cell lymphoma unspecified type and anaplastic large cell lymphoma, according to the World Health Organization classification.7 Anaplastic large cell lymphoma is easily distinguished from AITL based on histopathology, immunostaining, and clinical presentation. Until recently, peripheral T-cell lymphoma unspecified type and reactive lymphoid hyperplasia presented a challenge to differentiate from AITL, especially in the early phases of the disease; however, the introduction of CD10 as a phenotypic marker has been instrumental in distinguishing AITL from other T-cell lymphomas with primary nodal involvement.1,4
The development of purpura fulminans and disseminated intravascular coagulation in a patient with AITL is rare. Although the exact mechanism for the thrombus formation in the skin has not been elucidated, purpura fulminans typically develops secondary to a severe infection. The exact incidence of purpura fulminans in the setting of AITL is unknown, but purpura as a cutaneous eruption has been associated as a clinical finding in AITL.6 Although our case may be a rare presentation of AITL, a prompt and accurate diagnosis can drastically change the prognosis of this aggressive disease.
Purpura fulminans is a hematologic emergency, with clinical skin necrosis and laboratory testing showing disseminated intravascular coagulation. The thrombotic occlusion usually affects small and medium-sized blood vessels and may involve any organ. Purpura fulminans has been implicated with sepsis, most commonly meningococcal infections; other infections such as Staphylococcus aureus, groups A and B β-hemolytic streptococci, Streptococcus pneumoniae, and Haemophilus influenzae; and as a sequela to benign childhood infections, such as varicella. Other associations with purpura fulminans include autoimmune disease and heritable or acquired deficiency of anticoagulant proteins, most commonly protein C. We present a rare case of purpura fulminans as the presenting sign of angioimmunoblastic T-cell lymphoma (AITL), an aggressive primary nodal peripheral T-cell lymphoma with a high mortality rate and nonspecific skin manifestations in roughly half of all patients involved.
Case Report
A 56-year-old woman presented with purpuric patches on the left foot (Figure 1A). Seven days after presentation the lesion progressed into ecchymotic geographic plaques and hemorrhagic bullae that spread upward and contralaterally, sparing the digits, trunk, head, neck, and mucous membranes. Ultimately, the involved skin became necrotic and involved 20% of the body surface area (Figure 1B). The lesions were painful with a burning sensation but were not pruritic. The patient also reported intermittent fevers, chills, myalgia, nausea, and shortness of breath. Enlarged lymph nodes were present in the right cervical chain. She denied new medications; stated she had been in good health prior to this episode; and had no history of spontaneous abortion, neurologic symptoms, or other serious illness.
  |
Computed tomography showed prominent diffuse mediastinal, mesenteric, retroperitoneal, and pelvic lymphadenopathy with involvement of the cervical and inguinal areas. Laboratory values showed thrombocytopenia and increased fibrin degradation products. Blood and tissue cultures were negative; the patient also had a negative viral serology, except for Epstein-Barr virus IgG titers (>1:2560). A skin biopsy of the left thigh demonstrated venules and capillaries in the mid and superficial dermis filled with fibrin thrombi without vasculitis (Figure 2). A lymph node biopsy was consistent with a diagnosis of AITL. The lymph node architecture was largely effaced by a polymorphous lymphoid infiltrate that predominantly expanded into paracortical areas and was associated with a prominent arborizing vascular proliferation. The infiltrate was composed of lymphocytes ranging in size from small to medium, with ample cytoplasm, coarsely clumped chromatin, and mildly irregular nuclear membranes. Large atypical lymphocytes with features of immunoblasts were easily identified. An associated inflammatory background composed of eosinophils, plasma cells, and histiocytes was present (Figure 3). The atypical lymphocytes stained positive for CD3and CD10 on immunohistochemistry. Additionally, a subset of large immunoblastlike lymphocytes was positive for Epstein-Barr–encoded small RNAs by in situ hybridization.
|
The patient was started on rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone. She received 2 cycles with positive response based on subsequent computed tomography and positron emission tomography scans that showed regression of her disease as well as the lack of formation of new skin lesions. She was transferred to a burn unit where she had continuing treatment and skin grafts. Despite 2 cycles of chemotherapy, broad-spectrum antibiotics, and daily wound care management, the patient died secondary to sepsis 6 months after presentation.
Comment
Angioimmunoblastic T-cell lymphoma is a primary nodal lymphoma with occasional cutaneous involvement. Cutaneous manifestations occur in roughly half of all patients with AITL1 and have mainly been described as erythematous macules and papules that can resemble a viral exanthem or a drug reaction.2 However, other skin manifestations include urticaria, papulovesicular lesions, nodules, erythroderma,3 and to a lesser degree purpura.4 The lesions have been noted to occur prior to, concurrent with, or anytime during the disease.3,5,6 This aggressive lymphoma has mortality rates ranging from 50% to 72%, and median survival ranges from 11 to 30 months.6
To arrive at the correct diagnosis of AITL, a nodal biopsy with immunochemistry is necessary. Classic findings on histopathology include effacement of normal architecture, marked vascular proliferation, and aggregates of atypical lymphoid cells. CD10 has been shown to be a good objective criterion for the diagnosis of AITL,4 with characteristic tumor cells expressing CD10. Nodal Epstein-Barr virus–positive lymphocytes often are present.2 Other T-cell lymphomas with primarily nodal presentation along with peripheral T-cell lymphoma include peripheral T-cell lymphoma unspecified type and anaplastic large cell lymphoma, according to the World Health Organization classification.7 Anaplastic large cell lymphoma is easily distinguished from AITL based on histopathology, immunostaining, and clinical presentation. Until recently, peripheral T-cell lymphoma unspecified type and reactive lymphoid hyperplasia presented a challenge to differentiate from AITL, especially in the early phases of the disease; however, the introduction of CD10 as a phenotypic marker has been instrumental in distinguishing AITL from other T-cell lymphomas with primary nodal involvement.1,4
The development of purpura fulminans and disseminated intravascular coagulation in a patient with AITL is rare. Although the exact mechanism for the thrombus formation in the skin has not been elucidated, purpura fulminans typically develops secondary to a severe infection. The exact incidence of purpura fulminans in the setting of AITL is unknown, but purpura as a cutaneous eruption has been associated as a clinical finding in AITL.6 Although our case may be a rare presentation of AITL, a prompt and accurate diagnosis can drastically change the prognosis of this aggressive disease.
1. Ferry JA. Angioimmunoblastic T-cell lymphoma. Adv Anat Pathol. 2002;9:273-279.
2. Brown HA, Macon WR, Kurtin PJ, et al. Cutaneous involvement by angioimmunoblastic T-cell lymphoma with remarkable heterogeneous Epstein-Barr virus expression. J Cutan Pathol. 2001;28:432-438.
3. Bernstein JE, Soltani K, Lorincz AL. Cutaneous manifestations of angioimmunoblastic lymphadenopathy. J Am Acad Dermatol. 1979;1:227-232.
4. Attygalle A, Al-Jehani R, Diss TC, et al. Neoplastic T cells in angioimmunoblastic T-cell lymphoma express CD10. Blood. 2002;99:627-633.
5. Jayaramna AG, Cassarino D, Advani R, et al. Cutaneous involvement by angioimmunoblastic T-cell lymphoma: a unique histologic presentation, mimicking an infectious etiology. J Cutan Pathol. 2006;33(suppl 2):6-11.
6. Martel P, Laroche L, Courville P, et al. Cutaneous involvement in patients with angioimmunoblastic lymphadenopathy with dysproteinemia: a clinical, immunohistological, and molecular analysis. Archives of Dermatology. 2000;136:881-886.
7. Jaffe ES, Harris NL, Stein H, et al, eds. Tumours of Haematopoietic and Lymphoid Tissues. 1st ed. Bethesda, MD: International Agency for Research on Cancer; 2001.
1. Ferry JA. Angioimmunoblastic T-cell lymphoma. Adv Anat Pathol. 2002;9:273-279.
2. Brown HA, Macon WR, Kurtin PJ, et al. Cutaneous involvement by angioimmunoblastic T-cell lymphoma with remarkable heterogeneous Epstein-Barr virus expression. J Cutan Pathol. 2001;28:432-438.
3. Bernstein JE, Soltani K, Lorincz AL. Cutaneous manifestations of angioimmunoblastic lymphadenopathy. J Am Acad Dermatol. 1979;1:227-232.
4. Attygalle A, Al-Jehani R, Diss TC, et al. Neoplastic T cells in angioimmunoblastic T-cell lymphoma express CD10. Blood. 2002;99:627-633.
5. Jayaramna AG, Cassarino D, Advani R, et al. Cutaneous involvement by angioimmunoblastic T-cell lymphoma: a unique histologic presentation, mimicking an infectious etiology. J Cutan Pathol. 2006;33(suppl 2):6-11.
6. Martel P, Laroche L, Courville P, et al. Cutaneous involvement in patients with angioimmunoblastic lymphadenopathy with dysproteinemia: a clinical, immunohistological, and molecular analysis. Archives of Dermatology. 2000;136:881-886.
7. Jaffe ES, Harris NL, Stein H, et al, eds. Tumours of Haematopoietic and Lymphoid Tissues. 1st ed. Bethesda, MD: International Agency for Research on Cancer; 2001.
Practice Points
- Angioimmunoblastic T-cell lymphoma (AITL) is a primary nodal lymphoma with occasional nonspecific cutaneous involvement that may be morbilliform, maculopapular, erythrodermic, or rarely purpuric.
- To arrive at the correct diagnosis of AITL, a nodal biopsy with immunochemistry is necessary.
- CD10 positivity is a good objective criterion for the diagnosis of AITL, and Epstein-Barr virus–positive lymphocytes are nearly always present.
Dermatologic Emergencies
Dermatologic emergency may sound like an oxymoron, but there are many emergencies that dermatology residents may encounter in their careers. In some instances the skin is the primary organ that is affected, while in others cutaneous symptoms and life-threatening signs are important diagnostic clues for what may lie beneath the skin.
As residents who are occasionally on call or on consultation services, it is important for us to recognize dermatologic emergencies quickly because some of these conditions can acutely evolve and become lethal if a diagnosis is not made early in the disease course with the appropriate treatment administered. Dermatologic emergencies can range from severe drug reactions, infections, autoimmune exacerbations, and inflammatory conditions (eg, erythroderma) to environmental insults such as burns (Figure 1) and child abuse.1
Critical Infections
Some dermatologic emergencies are infectious in origin, and although these infections are most commonly bacterial (eg, necrotizing fasciitis), they also can range from viral to fungal (eg, mucormycosis) in nature. Some areas with large populations of immunocompromised patients (eg, human immunodeficiency virus–positive patients, organ transplant recipients) may warrant a high index of suspicion for possible zebras (rare conditions) and opportunistic infections that may quickly escalate to life-threatening situations.
Although few cutaneous manifestations in emergent infections are pathognomonic, they sometimes can be categorized according to the appearance of the primary lesion: erythrodermic (eg, staphylococcal scalded skin syndrome), maculopapular (eg, Lyme disease), purpuric/petechial (eg, Rocky Mountain spotted fever), pustular (eg, disseminated candidiasis), or vesicular (eg, neonatal herpes simplex virus)(Table). On consultations, dermatology residents frequently get called to evaluate hemorrhagic and ischemic lesions in inpatients (Figure 2). Aside from infectious causes, the differential diagnosis may include coagulation abnormalities (eg, concurrent anticoagulant therapies), vasculitides, poisoning, vascular disease, or Stevens-Johnson syndrome and toxic epidermal necrolysis, which can occasionally present with hemorrhagic lesions.1,2
Necrotizing Fasciitis
Dermatology residents may frequently encounter necrotizing fasciitis, either in clinic or on the wards (Figure 3). Recognition of the skin signs in this condition is essential to patient survival. As an intern, I once had an attending teach me that patients with necrotizing fasciitis only have a couple of hours to live. The rapid unfolding of this flesh-eating disease and its high morbidity and mortality has led to recent attention in the press and media.
Although necrotizing fasciitis may be caused by several different bacterial organisms (eg, gram positive, gram negative, polymicrobial), it usually is rapidly progressive, destroying muscle and subcutaneous tissues in a matter of hours.3 Bacteria usually enter through a traumatic or present wound and quickly move along fascial planes, destroying blood vessels and whatever subcutaneous tissues happen to be in the way. Within the first few hours, the involved area that was initially erythematous becomes indurated, woody, extremely painful, and dusky, indicating a lack of circulation to the area. Extensive debridement is required until reaching noninfected tissue that is no longer purulent, necrotic, or woody to the touch. If necrotizing fasciitis is not diagnosed and treated early, patients may lose one or several limbs and death may occur.
Key findings of necrotizing fasciitis include systemic toxicity, localized painful induration, well-defined dusky blue discoloration, and a lack of bleeding or purulent discharge on incision and squeezing of the affected tissue. Crepitation or a crackling sensation can occasionally be felt when palpating the area secondary to gas formation in the tissue, though it is not always present. Patients with necrotizing fasciitis often initially present to dermatology clinics because the first manifestation happens to be in the skin. The role of dermatologists in treating this critical condition may prompt recognition and collaboration with other specialists to reach a viable outcome for the patient.3
Drug Reactions
Cutaneous drug eruptions usually are relatively benign, consisting of a morbilliform eruption often without any other accompanying symptoms. However, sometimes these reactions can present as exfoliative dermatitis or red man syndrome in which patients can develop total body erythema with diffuse scaling and pruritus.4 Aside from drug reactions, other causes of exfoliative dermatitis such as psoriasis, atopic and seborrheic dermatitis, mycosis fungoides, and lymphoma should be ruled out. Other drug eruptions that can be classified as dermatologic emergencies include leukocytoclastic vasculitis, severe urticaria or angioedema, erythema multiforme, or Stevens-Johnson syndrome and toxic epidermal necrolysis.
Severe Acne
If not treated promptly, serious cases of acne can lead to severe scarring and psychologic problems. Acne fulminans is characterized by a rapid eruption of suppurative and large, highly inflamed nodules, plaques, and cysts that result in ragged ulcerations and cicatrization of the chest, back, and occasionally the face. Systemic symptoms of fever, arthralgia, leukocytosis, and myalgia suggest an upregulation of the immune system in affected patients.
Final Comment
In summary, dermatologic emergencies do exist and some may present with characteristic skin findings. In almost all cases, collaboration with other departments such as trauma, burn, internal medicine, rheumatology, and infectious diseases is extremely helpful in diagnosing and treating these medical emergencies. Collaboration can provide insight into how brainstorming through different approaches can lead to a better outcome whether it be solving the cause of a puzzling rash in a patient with multiple comorbidities or surgically removing a bullet from a trauma patient (Figure 4). Recognition of specific cutaneous manifestations and early diagnosis of dermatologic emergencies can be lifesaving.
1. McQueen A, Martin SA, Lio PA. Derm emergencies: detecting early signs of trouble. J Fam Pract. 2012;61:71-78.
2. Bennion S. Dermatologic emergencies. In: Fitzpatrick J, Morelli J, eds. Dermatology Secrets Plus. 4th ed. Philadelphia, PA: Mosby; 2011:442-452.
3. Sarani B, Strong M, Pascual J, et al. Necrotizing fasciitis: current concepts and review of the literature. J Am Coll Surg. 2009;208:279-288.
4. Wolf R, Orion E, Marcos B, et al. Life-threatening acute adverse cutaneous drug reactions. Clin Dermatol. 2005;23:171-181.
Dermatologic emergency may sound like an oxymoron, but there are many emergencies that dermatology residents may encounter in their careers. In some instances the skin is the primary organ that is affected, while in others cutaneous symptoms and life-threatening signs are important diagnostic clues for what may lie beneath the skin.
As residents who are occasionally on call or on consultation services, it is important for us to recognize dermatologic emergencies quickly because some of these conditions can acutely evolve and become lethal if a diagnosis is not made early in the disease course with the appropriate treatment administered. Dermatologic emergencies can range from severe drug reactions, infections, autoimmune exacerbations, and inflammatory conditions (eg, erythroderma) to environmental insults such as burns (Figure 1) and child abuse.1
Critical Infections
Some dermatologic emergencies are infectious in origin, and although these infections are most commonly bacterial (eg, necrotizing fasciitis), they also can range from viral to fungal (eg, mucormycosis) in nature. Some areas with large populations of immunocompromised patients (eg, human immunodeficiency virus–positive patients, organ transplant recipients) may warrant a high index of suspicion for possible zebras (rare conditions) and opportunistic infections that may quickly escalate to life-threatening situations.
Although few cutaneous manifestations in emergent infections are pathognomonic, they sometimes can be categorized according to the appearance of the primary lesion: erythrodermic (eg, staphylococcal scalded skin syndrome), maculopapular (eg, Lyme disease), purpuric/petechial (eg, Rocky Mountain spotted fever), pustular (eg, disseminated candidiasis), or vesicular (eg, neonatal herpes simplex virus)(Table). On consultations, dermatology residents frequently get called to evaluate hemorrhagic and ischemic lesions in inpatients (Figure 2). Aside from infectious causes, the differential diagnosis may include coagulation abnormalities (eg, concurrent anticoagulant therapies), vasculitides, poisoning, vascular disease, or Stevens-Johnson syndrome and toxic epidermal necrolysis, which can occasionally present with hemorrhagic lesions.1,2
Necrotizing Fasciitis
Dermatology residents may frequently encounter necrotizing fasciitis, either in clinic or on the wards (Figure 3). Recognition of the skin signs in this condition is essential to patient survival. As an intern, I once had an attending teach me that patients with necrotizing fasciitis only have a couple of hours to live. The rapid unfolding of this flesh-eating disease and its high morbidity and mortality has led to recent attention in the press and media.
Although necrotizing fasciitis may be caused by several different bacterial organisms (eg, gram positive, gram negative, polymicrobial), it usually is rapidly progressive, destroying muscle and subcutaneous tissues in a matter of hours.3 Bacteria usually enter through a traumatic or present wound and quickly move along fascial planes, destroying blood vessels and whatever subcutaneous tissues happen to be in the way. Within the first few hours, the involved area that was initially erythematous becomes indurated, woody, extremely painful, and dusky, indicating a lack of circulation to the area. Extensive debridement is required until reaching noninfected tissue that is no longer purulent, necrotic, or woody to the touch. If necrotizing fasciitis is not diagnosed and treated early, patients may lose one or several limbs and death may occur.
Key findings of necrotizing fasciitis include systemic toxicity, localized painful induration, well-defined dusky blue discoloration, and a lack of bleeding or purulent discharge on incision and squeezing of the affected tissue. Crepitation or a crackling sensation can occasionally be felt when palpating the area secondary to gas formation in the tissue, though it is not always present. Patients with necrotizing fasciitis often initially present to dermatology clinics because the first manifestation happens to be in the skin. The role of dermatologists in treating this critical condition may prompt recognition and collaboration with other specialists to reach a viable outcome for the patient.3
Drug Reactions
Cutaneous drug eruptions usually are relatively benign, consisting of a morbilliform eruption often without any other accompanying symptoms. However, sometimes these reactions can present as exfoliative dermatitis or red man syndrome in which patients can develop total body erythema with diffuse scaling and pruritus.4 Aside from drug reactions, other causes of exfoliative dermatitis such as psoriasis, atopic and seborrheic dermatitis, mycosis fungoides, and lymphoma should be ruled out. Other drug eruptions that can be classified as dermatologic emergencies include leukocytoclastic vasculitis, severe urticaria or angioedema, erythema multiforme, or Stevens-Johnson syndrome and toxic epidermal necrolysis.
Severe Acne
If not treated promptly, serious cases of acne can lead to severe scarring and psychologic problems. Acne fulminans is characterized by a rapid eruption of suppurative and large, highly inflamed nodules, plaques, and cysts that result in ragged ulcerations and cicatrization of the chest, back, and occasionally the face. Systemic symptoms of fever, arthralgia, leukocytosis, and myalgia suggest an upregulation of the immune system in affected patients.
Final Comment
In summary, dermatologic emergencies do exist and some may present with characteristic skin findings. In almost all cases, collaboration with other departments such as trauma, burn, internal medicine, rheumatology, and infectious diseases is extremely helpful in diagnosing and treating these medical emergencies. Collaboration can provide insight into how brainstorming through different approaches can lead to a better outcome whether it be solving the cause of a puzzling rash in a patient with multiple comorbidities or surgically removing a bullet from a trauma patient (Figure 4). Recognition of specific cutaneous manifestations and early diagnosis of dermatologic emergencies can be lifesaving.
Dermatologic emergency may sound like an oxymoron, but there are many emergencies that dermatology residents may encounter in their careers. In some instances the skin is the primary organ that is affected, while in others cutaneous symptoms and life-threatening signs are important diagnostic clues for what may lie beneath the skin.
As residents who are occasionally on call or on consultation services, it is important for us to recognize dermatologic emergencies quickly because some of these conditions can acutely evolve and become lethal if a diagnosis is not made early in the disease course with the appropriate treatment administered. Dermatologic emergencies can range from severe drug reactions, infections, autoimmune exacerbations, and inflammatory conditions (eg, erythroderma) to environmental insults such as burns (Figure 1) and child abuse.1
Critical Infections
Some dermatologic emergencies are infectious in origin, and although these infections are most commonly bacterial (eg, necrotizing fasciitis), they also can range from viral to fungal (eg, mucormycosis) in nature. Some areas with large populations of immunocompromised patients (eg, human immunodeficiency virus–positive patients, organ transplant recipients) may warrant a high index of suspicion for possible zebras (rare conditions) and opportunistic infections that may quickly escalate to life-threatening situations.
Although few cutaneous manifestations in emergent infections are pathognomonic, they sometimes can be categorized according to the appearance of the primary lesion: erythrodermic (eg, staphylococcal scalded skin syndrome), maculopapular (eg, Lyme disease), purpuric/petechial (eg, Rocky Mountain spotted fever), pustular (eg, disseminated candidiasis), or vesicular (eg, neonatal herpes simplex virus)(Table). On consultations, dermatology residents frequently get called to evaluate hemorrhagic and ischemic lesions in inpatients (Figure 2). Aside from infectious causes, the differential diagnosis may include coagulation abnormalities (eg, concurrent anticoagulant therapies), vasculitides, poisoning, vascular disease, or Stevens-Johnson syndrome and toxic epidermal necrolysis, which can occasionally present with hemorrhagic lesions.1,2
Necrotizing Fasciitis
Dermatology residents may frequently encounter necrotizing fasciitis, either in clinic or on the wards (Figure 3). Recognition of the skin signs in this condition is essential to patient survival. As an intern, I once had an attending teach me that patients with necrotizing fasciitis only have a couple of hours to live. The rapid unfolding of this flesh-eating disease and its high morbidity and mortality has led to recent attention in the press and media.
Although necrotizing fasciitis may be caused by several different bacterial organisms (eg, gram positive, gram negative, polymicrobial), it usually is rapidly progressive, destroying muscle and subcutaneous tissues in a matter of hours.3 Bacteria usually enter through a traumatic or present wound and quickly move along fascial planes, destroying blood vessels and whatever subcutaneous tissues happen to be in the way. Within the first few hours, the involved area that was initially erythematous becomes indurated, woody, extremely painful, and dusky, indicating a lack of circulation to the area. Extensive debridement is required until reaching noninfected tissue that is no longer purulent, necrotic, or woody to the touch. If necrotizing fasciitis is not diagnosed and treated early, patients may lose one or several limbs and death may occur.
Key findings of necrotizing fasciitis include systemic toxicity, localized painful induration, well-defined dusky blue discoloration, and a lack of bleeding or purulent discharge on incision and squeezing of the affected tissue. Crepitation or a crackling sensation can occasionally be felt when palpating the area secondary to gas formation in the tissue, though it is not always present. Patients with necrotizing fasciitis often initially present to dermatology clinics because the first manifestation happens to be in the skin. The role of dermatologists in treating this critical condition may prompt recognition and collaboration with other specialists to reach a viable outcome for the patient.3
Drug Reactions
Cutaneous drug eruptions usually are relatively benign, consisting of a morbilliform eruption often without any other accompanying symptoms. However, sometimes these reactions can present as exfoliative dermatitis or red man syndrome in which patients can develop total body erythema with diffuse scaling and pruritus.4 Aside from drug reactions, other causes of exfoliative dermatitis such as psoriasis, atopic and seborrheic dermatitis, mycosis fungoides, and lymphoma should be ruled out. Other drug eruptions that can be classified as dermatologic emergencies include leukocytoclastic vasculitis, severe urticaria or angioedema, erythema multiforme, or Stevens-Johnson syndrome and toxic epidermal necrolysis.
Severe Acne
If not treated promptly, serious cases of acne can lead to severe scarring and psychologic problems. Acne fulminans is characterized by a rapid eruption of suppurative and large, highly inflamed nodules, plaques, and cysts that result in ragged ulcerations and cicatrization of the chest, back, and occasionally the face. Systemic symptoms of fever, arthralgia, leukocytosis, and myalgia suggest an upregulation of the immune system in affected patients.
Final Comment
In summary, dermatologic emergencies do exist and some may present with characteristic skin findings. In almost all cases, collaboration with other departments such as trauma, burn, internal medicine, rheumatology, and infectious diseases is extremely helpful in diagnosing and treating these medical emergencies. Collaboration can provide insight into how brainstorming through different approaches can lead to a better outcome whether it be solving the cause of a puzzling rash in a patient with multiple comorbidities or surgically removing a bullet from a trauma patient (Figure 4). Recognition of specific cutaneous manifestations and early diagnosis of dermatologic emergencies can be lifesaving.
1. McQueen A, Martin SA, Lio PA. Derm emergencies: detecting early signs of trouble. J Fam Pract. 2012;61:71-78.
2. Bennion S. Dermatologic emergencies. In: Fitzpatrick J, Morelli J, eds. Dermatology Secrets Plus. 4th ed. Philadelphia, PA: Mosby; 2011:442-452.
3. Sarani B, Strong M, Pascual J, et al. Necrotizing fasciitis: current concepts and review of the literature. J Am Coll Surg. 2009;208:279-288.
4. Wolf R, Orion E, Marcos B, et al. Life-threatening acute adverse cutaneous drug reactions. Clin Dermatol. 2005;23:171-181.
1. McQueen A, Martin SA, Lio PA. Derm emergencies: detecting early signs of trouble. J Fam Pract. 2012;61:71-78.
2. Bennion S. Dermatologic emergencies. In: Fitzpatrick J, Morelli J, eds. Dermatology Secrets Plus. 4th ed. Philadelphia, PA: Mosby; 2011:442-452.
3. Sarani B, Strong M, Pascual J, et al. Necrotizing fasciitis: current concepts and review of the literature. J Am Coll Surg. 2009;208:279-288.
4. Wolf R, Orion E, Marcos B, et al. Life-threatening acute adverse cutaneous drug reactions. Clin Dermatol. 2005;23:171-181.