User login
FDA Okays Osimertinib After CRT in Locally Advanced, Unresectable NSCLC
Specifically, the third-generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) was approved for patients whose disease has not progressed during or after concurrent or sequential platinum-based chemoradiation therapy and whose tumors have EGFR exon 19 deletions or exon 21 L858R mutations. Such EGFR mutations can be detected by an FDA-approved test.
The FDA approved osimertinib in combination with platinum-based chemotherapy as first-line treatment for patients with locally advanced or metastatic NSCLC with the same mutations in February. The EGFR-TKI also carries other indications, including as first-line monotherapy for locally advanced or metastatic EGFR-mutated NSCLC.
Trial Findings Supporting Latest Approval
AstraZeneca announced in June that osimertinib had been granted Priority Review and Breakthrough Therapy Designation for its newest indication.
The September 25 approval was based on findings from the randomized, placebo-controlled LAURA trial of 216 patients, which demonstrated improved median progression-free survival with osimertinib vs placebo (39.1 vs 5.6 months; hazard ratio, 0.16). Overall survival results were immature at the most recent analysis, but “no trend towards a detriment was observed,” with 36% of prespecified deaths for the final analysis reported, according to an FDA press release.
Adverse Events
Study participants were randomized 2:1 to receive the osimertinib recommended dose of 80 mg given orally once daily or placebo until disease progression or unacceptable toxicity. The most common adverse reactions, occurring in at least 20% of patients, were lymphopenia, leukopenia, interstitial lung disease/pneumonitis, thrombocytopenia, neutropenia, rash, diarrhea, nail toxicity, musculoskeletal pain, cough, and COVID-19 infection.
A version of this article first appeared on Medscape.com.
Specifically, the third-generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) was approved for patients whose disease has not progressed during or after concurrent or sequential platinum-based chemoradiation therapy and whose tumors have EGFR exon 19 deletions or exon 21 L858R mutations. Such EGFR mutations can be detected by an FDA-approved test.
The FDA approved osimertinib in combination with platinum-based chemotherapy as first-line treatment for patients with locally advanced or metastatic NSCLC with the same mutations in February. The EGFR-TKI also carries other indications, including as first-line monotherapy for locally advanced or metastatic EGFR-mutated NSCLC.
Trial Findings Supporting Latest Approval
AstraZeneca announced in June that osimertinib had been granted Priority Review and Breakthrough Therapy Designation for its newest indication.
The September 25 approval was based on findings from the randomized, placebo-controlled LAURA trial of 216 patients, which demonstrated improved median progression-free survival with osimertinib vs placebo (39.1 vs 5.6 months; hazard ratio, 0.16). Overall survival results were immature at the most recent analysis, but “no trend towards a detriment was observed,” with 36% of prespecified deaths for the final analysis reported, according to an FDA press release.
Adverse Events
Study participants were randomized 2:1 to receive the osimertinib recommended dose of 80 mg given orally once daily or placebo until disease progression or unacceptable toxicity. The most common adverse reactions, occurring in at least 20% of patients, were lymphopenia, leukopenia, interstitial lung disease/pneumonitis, thrombocytopenia, neutropenia, rash, diarrhea, nail toxicity, musculoskeletal pain, cough, and COVID-19 infection.
A version of this article first appeared on Medscape.com.
Specifically, the third-generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) was approved for patients whose disease has not progressed during or after concurrent or sequential platinum-based chemoradiation therapy and whose tumors have EGFR exon 19 deletions or exon 21 L858R mutations. Such EGFR mutations can be detected by an FDA-approved test.
The FDA approved osimertinib in combination with platinum-based chemotherapy as first-line treatment for patients with locally advanced or metastatic NSCLC with the same mutations in February. The EGFR-TKI also carries other indications, including as first-line monotherapy for locally advanced or metastatic EGFR-mutated NSCLC.
Trial Findings Supporting Latest Approval
AstraZeneca announced in June that osimertinib had been granted Priority Review and Breakthrough Therapy Designation for its newest indication.
The September 25 approval was based on findings from the randomized, placebo-controlled LAURA trial of 216 patients, which demonstrated improved median progression-free survival with osimertinib vs placebo (39.1 vs 5.6 months; hazard ratio, 0.16). Overall survival results were immature at the most recent analysis, but “no trend towards a detriment was observed,” with 36% of prespecified deaths for the final analysis reported, according to an FDA press release.
Adverse Events
Study participants were randomized 2:1 to receive the osimertinib recommended dose of 80 mg given orally once daily or placebo until disease progression or unacceptable toxicity. The most common adverse reactions, occurring in at least 20% of patients, were lymphopenia, leukopenia, interstitial lung disease/pneumonitis, thrombocytopenia, neutropenia, rash, diarrhea, nail toxicity, musculoskeletal pain, cough, and COVID-19 infection.
A version of this article first appeared on Medscape.com.
Pertussis Rates Up Compared With Recent Years
, according to data from the Centers for Disease Control and Prevention (CDC). Reports from several states illustrate this trend, thought to be due to reduced immunity across the country.
The Alaska Department of Health issued a statement on its website about the significant increase in pertussis cases in the state during the summer, with 90 cases in July and 61 in August, compared with 24 in June and a total of 26 cases in 2023.
Similarly, the Florida Department of Health reported a pertussis increase in July 2024 that was higher than the June 2024 case count and also above the previous 5-year average.
Experts in these and other states suggest that several factors are driving the nationwide increase, including the fact that fewer people are consistently wearing masks. The mass masking during the COVID-19 pandemic caused a significant drop in pertussis, but the latest data suggest a return to prepandemic levels, and waning immunity likely plays a role as well.
Pertussis, also known as whooping cough, typically begins with symptoms similar to those of the common cold, including runny nose, sneezing, mild fever, and cough, according to the CDC. However, babies with whooping cough may experience trouble breathing rather than a cough. The coughing fits often associated with pertussis may not start until 2 weeks after the onset of other symptoms, according to the CDC.
Those who have been vaccinated against pertussis can still become infected, but the risk is lower, and the illness, if it occurs, is likely to be milder. Complications such as apnea, pneumonia, and convulsions can occur in babies younger than 1 year, especially if they have not been vaccinated, according to the CDC.
Beyond Easing Pandemic Precautions
Many respiratory-based infections dipped during the COVID-19 pandemic, almost certainly from the multifactorial interventions of masking, distancing, and the general lack of comingling, said David J. Cennimo, MD, associate professor of medicine & pediatrics in the Division of Infectious Diseases at Rutgers New Jersey Medical School, Newark, New Jersey, in an interview.
The number of cases of many of these diseases returned to previous levels after COVID-19 restrictions were lifted, he said.
“However, we know pertussis immunity wanes over time. Children get DTaP at 2, 4, 6, and 15 months, and a Tdap booster at 11-12 years old gets them to adulthood,” Dr. Cennimo said. Adults should be getting a Tdap every 10 years, he added.
The latest available CDC data indicate that Tdap vaccine coverage in adults is approximately 40%, which means that there may be a large number of susceptible people who can become infected and propagate to others, said Dr. Cennimo.
Not Just the Young Ones
A recent pertussis outbreak among college students in Virginia highlighted the fact that the infection can affect all ages, and that the effectiveness of childhood vaccines may decrease over time. The majority of the recently diagnosed cases occurred in individuals who had been previously vaccinated, according to a press release from the Virginia Department of Health.
Clinical Clues
The initial stage of pertussis infection looks like a common cold with symptoms of upper respiratory infection, Dr. Cennimo told this news organization. “Unless there is reason to suspect pertussis exposure, it would almost certainly be missed,” he noted.
The characteristic barking/seal-like cough is mostly seen in children, said Dr. Cennimo. Adults and children can experience coughing fits that can lead to shortness of breath and/or vomiting, which would raise suspicion for pertussis, but is not universally present, he said. The convalescent stage of pertussis can be prolonged and is characterized by chronic coughing. “In the past, pertussis had been called the 100-day cough,” and at that point, treatment is ineffective, Dr. Cennimo said.
In clinical practice, “I advise everyone to get the Tdap vaccine every 10 years,” and remember that the “Td” is the every 10-year tetanus shot as well, Dr. Cennimo told this news organization. Reassure patients that the Tdap can be given with other vaccines, he said, and remind patients that, as with any of the respiratory illnesses, they should stay home if sick, cover a cough, consider wearing a mask in public, and wash hands frequently, he said.
Dr. Cennimo had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
, according to data from the Centers for Disease Control and Prevention (CDC). Reports from several states illustrate this trend, thought to be due to reduced immunity across the country.
The Alaska Department of Health issued a statement on its website about the significant increase in pertussis cases in the state during the summer, with 90 cases in July and 61 in August, compared with 24 in June and a total of 26 cases in 2023.
Similarly, the Florida Department of Health reported a pertussis increase in July 2024 that was higher than the June 2024 case count and also above the previous 5-year average.
Experts in these and other states suggest that several factors are driving the nationwide increase, including the fact that fewer people are consistently wearing masks. The mass masking during the COVID-19 pandemic caused a significant drop in pertussis, but the latest data suggest a return to prepandemic levels, and waning immunity likely plays a role as well.
Pertussis, also known as whooping cough, typically begins with symptoms similar to those of the common cold, including runny nose, sneezing, mild fever, and cough, according to the CDC. However, babies with whooping cough may experience trouble breathing rather than a cough. The coughing fits often associated with pertussis may not start until 2 weeks after the onset of other symptoms, according to the CDC.
Those who have been vaccinated against pertussis can still become infected, but the risk is lower, and the illness, if it occurs, is likely to be milder. Complications such as apnea, pneumonia, and convulsions can occur in babies younger than 1 year, especially if they have not been vaccinated, according to the CDC.
Beyond Easing Pandemic Precautions
Many respiratory-based infections dipped during the COVID-19 pandemic, almost certainly from the multifactorial interventions of masking, distancing, and the general lack of comingling, said David J. Cennimo, MD, associate professor of medicine & pediatrics in the Division of Infectious Diseases at Rutgers New Jersey Medical School, Newark, New Jersey, in an interview.
The number of cases of many of these diseases returned to previous levels after COVID-19 restrictions were lifted, he said.
“However, we know pertussis immunity wanes over time. Children get DTaP at 2, 4, 6, and 15 months, and a Tdap booster at 11-12 years old gets them to adulthood,” Dr. Cennimo said. Adults should be getting a Tdap every 10 years, he added.
The latest available CDC data indicate that Tdap vaccine coverage in adults is approximately 40%, which means that there may be a large number of susceptible people who can become infected and propagate to others, said Dr. Cennimo.
Not Just the Young Ones
A recent pertussis outbreak among college students in Virginia highlighted the fact that the infection can affect all ages, and that the effectiveness of childhood vaccines may decrease over time. The majority of the recently diagnosed cases occurred in individuals who had been previously vaccinated, according to a press release from the Virginia Department of Health.
Clinical Clues
The initial stage of pertussis infection looks like a common cold with symptoms of upper respiratory infection, Dr. Cennimo told this news organization. “Unless there is reason to suspect pertussis exposure, it would almost certainly be missed,” he noted.
The characteristic barking/seal-like cough is mostly seen in children, said Dr. Cennimo. Adults and children can experience coughing fits that can lead to shortness of breath and/or vomiting, which would raise suspicion for pertussis, but is not universally present, he said. The convalescent stage of pertussis can be prolonged and is characterized by chronic coughing. “In the past, pertussis had been called the 100-day cough,” and at that point, treatment is ineffective, Dr. Cennimo said.
In clinical practice, “I advise everyone to get the Tdap vaccine every 10 years,” and remember that the “Td” is the every 10-year tetanus shot as well, Dr. Cennimo told this news organization. Reassure patients that the Tdap can be given with other vaccines, he said, and remind patients that, as with any of the respiratory illnesses, they should stay home if sick, cover a cough, consider wearing a mask in public, and wash hands frequently, he said.
Dr. Cennimo had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
, according to data from the Centers for Disease Control and Prevention (CDC). Reports from several states illustrate this trend, thought to be due to reduced immunity across the country.
The Alaska Department of Health issued a statement on its website about the significant increase in pertussis cases in the state during the summer, with 90 cases in July and 61 in August, compared with 24 in June and a total of 26 cases in 2023.
Similarly, the Florida Department of Health reported a pertussis increase in July 2024 that was higher than the June 2024 case count and also above the previous 5-year average.
Experts in these and other states suggest that several factors are driving the nationwide increase, including the fact that fewer people are consistently wearing masks. The mass masking during the COVID-19 pandemic caused a significant drop in pertussis, but the latest data suggest a return to prepandemic levels, and waning immunity likely plays a role as well.
Pertussis, also known as whooping cough, typically begins with symptoms similar to those of the common cold, including runny nose, sneezing, mild fever, and cough, according to the CDC. However, babies with whooping cough may experience trouble breathing rather than a cough. The coughing fits often associated with pertussis may not start until 2 weeks after the onset of other symptoms, according to the CDC.
Those who have been vaccinated against pertussis can still become infected, but the risk is lower, and the illness, if it occurs, is likely to be milder. Complications such as apnea, pneumonia, and convulsions can occur in babies younger than 1 year, especially if they have not been vaccinated, according to the CDC.
Beyond Easing Pandemic Precautions
Many respiratory-based infections dipped during the COVID-19 pandemic, almost certainly from the multifactorial interventions of masking, distancing, and the general lack of comingling, said David J. Cennimo, MD, associate professor of medicine & pediatrics in the Division of Infectious Diseases at Rutgers New Jersey Medical School, Newark, New Jersey, in an interview.
The number of cases of many of these diseases returned to previous levels after COVID-19 restrictions were lifted, he said.
“However, we know pertussis immunity wanes over time. Children get DTaP at 2, 4, 6, and 15 months, and a Tdap booster at 11-12 years old gets them to adulthood,” Dr. Cennimo said. Adults should be getting a Tdap every 10 years, he added.
The latest available CDC data indicate that Tdap vaccine coverage in adults is approximately 40%, which means that there may be a large number of susceptible people who can become infected and propagate to others, said Dr. Cennimo.
Not Just the Young Ones
A recent pertussis outbreak among college students in Virginia highlighted the fact that the infection can affect all ages, and that the effectiveness of childhood vaccines may decrease over time. The majority of the recently diagnosed cases occurred in individuals who had been previously vaccinated, according to a press release from the Virginia Department of Health.
Clinical Clues
The initial stage of pertussis infection looks like a common cold with symptoms of upper respiratory infection, Dr. Cennimo told this news organization. “Unless there is reason to suspect pertussis exposure, it would almost certainly be missed,” he noted.
The characteristic barking/seal-like cough is mostly seen in children, said Dr. Cennimo. Adults and children can experience coughing fits that can lead to shortness of breath and/or vomiting, which would raise suspicion for pertussis, but is not universally present, he said. The convalescent stage of pertussis can be prolonged and is characterized by chronic coughing. “In the past, pertussis had been called the 100-day cough,” and at that point, treatment is ineffective, Dr. Cennimo said.
In clinical practice, “I advise everyone to get the Tdap vaccine every 10 years,” and remember that the “Td” is the every 10-year tetanus shot as well, Dr. Cennimo told this news organization. Reassure patients that the Tdap can be given with other vaccines, he said, and remind patients that, as with any of the respiratory illnesses, they should stay home if sick, cover a cough, consider wearing a mask in public, and wash hands frequently, he said.
Dr. Cennimo had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
A Rare Case of a Splenic Abscess as the Origin of Illness in Exudative Pleural Effusion
Splenic abscesses are a rare occurrence that represent a marginal proportion of intra-abdominal infections. One study found splenic abscesses in only 0.14% to 0.70% of autopsies and none of the 540 abdominal abscesses they examined originated in the spleen.1 Patients with splenic abscesses tend to present with nonspecific symptoms such as fevers, chills, and abdominal pain.2 Imaging modalities such as abdominal ultrasound and computed tomography (CT) are vital to the workup and diagnosis identification.2 Splenic abscesses are generally associated with another underlying process, as seen in patients who are affected by endocarditis, trauma, metastatic infection, splenic infarction, or neoplasia.2
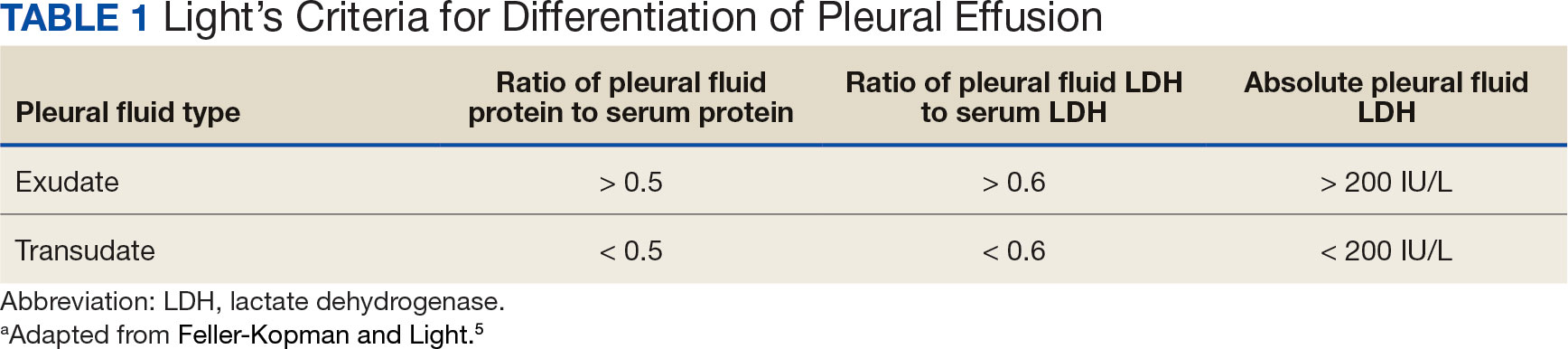
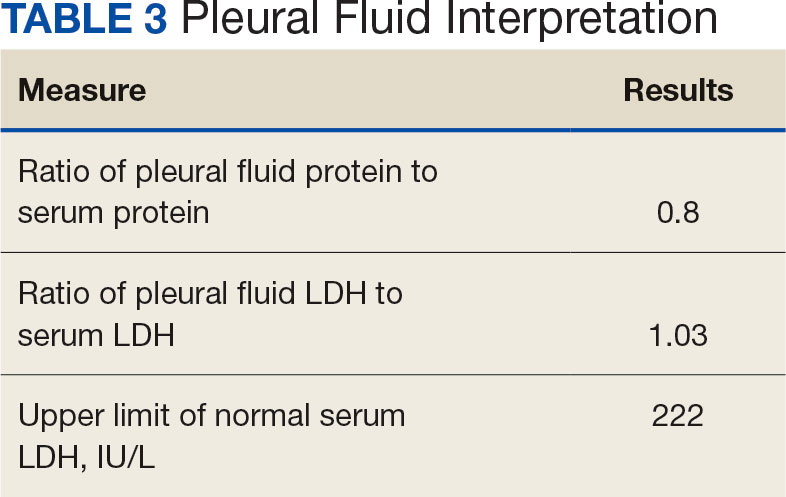
Pleural effusions, or the buildup of fluid within the pleural space, is a common condition typically secondary to another disease.3 Clinical identification of the primary condition may be challenging.3 In the absence of a clear etiology, such as obvious signs of congestive heart failure, further differentiation relies upon pleural fluid analysis, beginning with the distinction between exudate (inflammatory) and transudate (noninflammatory). 3,4 This distinction can be made using Light’s criteria, which relies on protein and lactate dehydrogenase (LDH) ratios between the pleural fluid and serum (Table 1).5 Though rare, half of splenic abscesses are associated with pleural effusion.6 As an inflammatory condition, splenic abscesses have been classically described as a cause of exudative pleural effusions.5,6
A myelodysplastic syndrome is a group of diseases that arise from malignant hematopoietic stem cells, leading to the proliferation of the malignant cells and faulty production of other bone marrow products.7 These disorders can range from single to multilineage dysplasia. Cells are often left in an immature blast form, unable to function appropriately, and vulnerable to destruction. Patients with myeloproliferative disorders frequently suffer from leukopenia and infections attributable to known quantitative and qualitative defects of neutrophils.8
CASE PRESENTATION
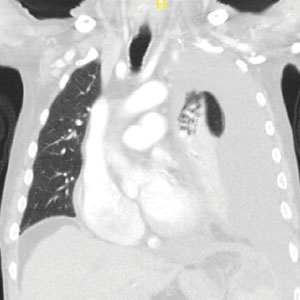
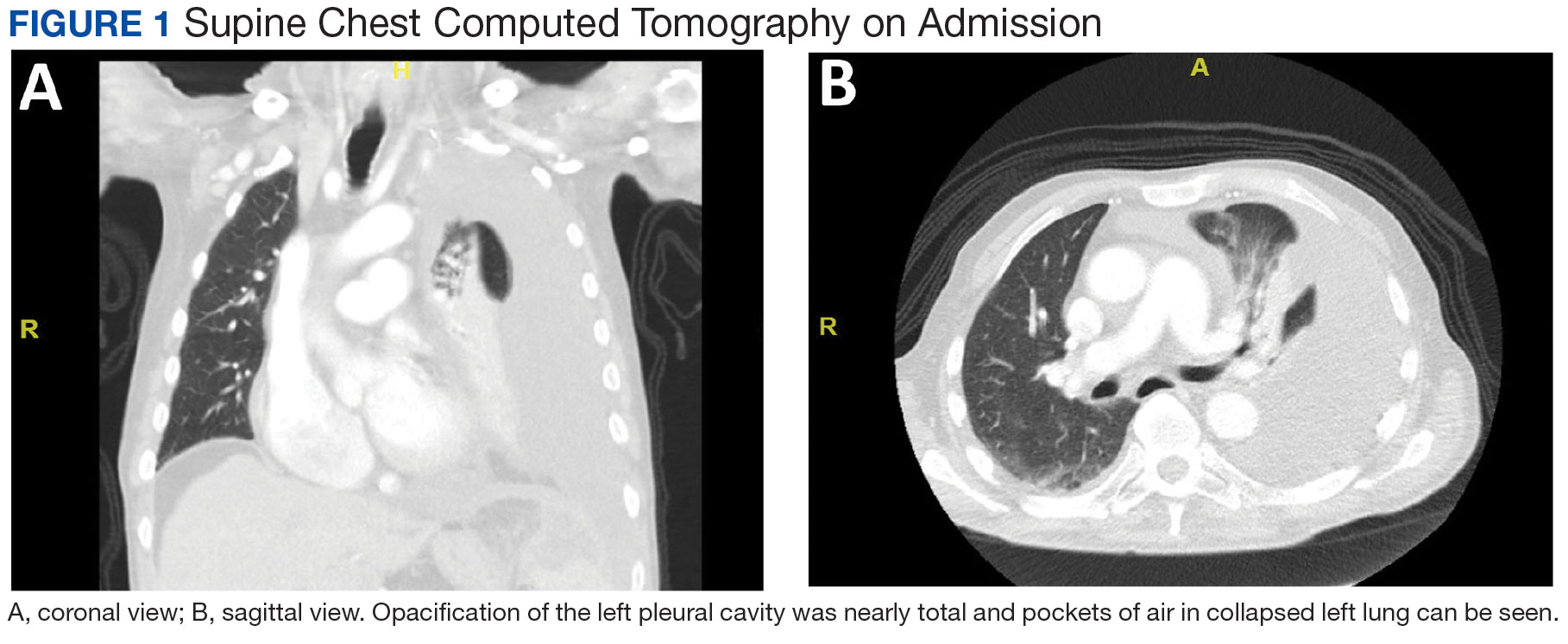
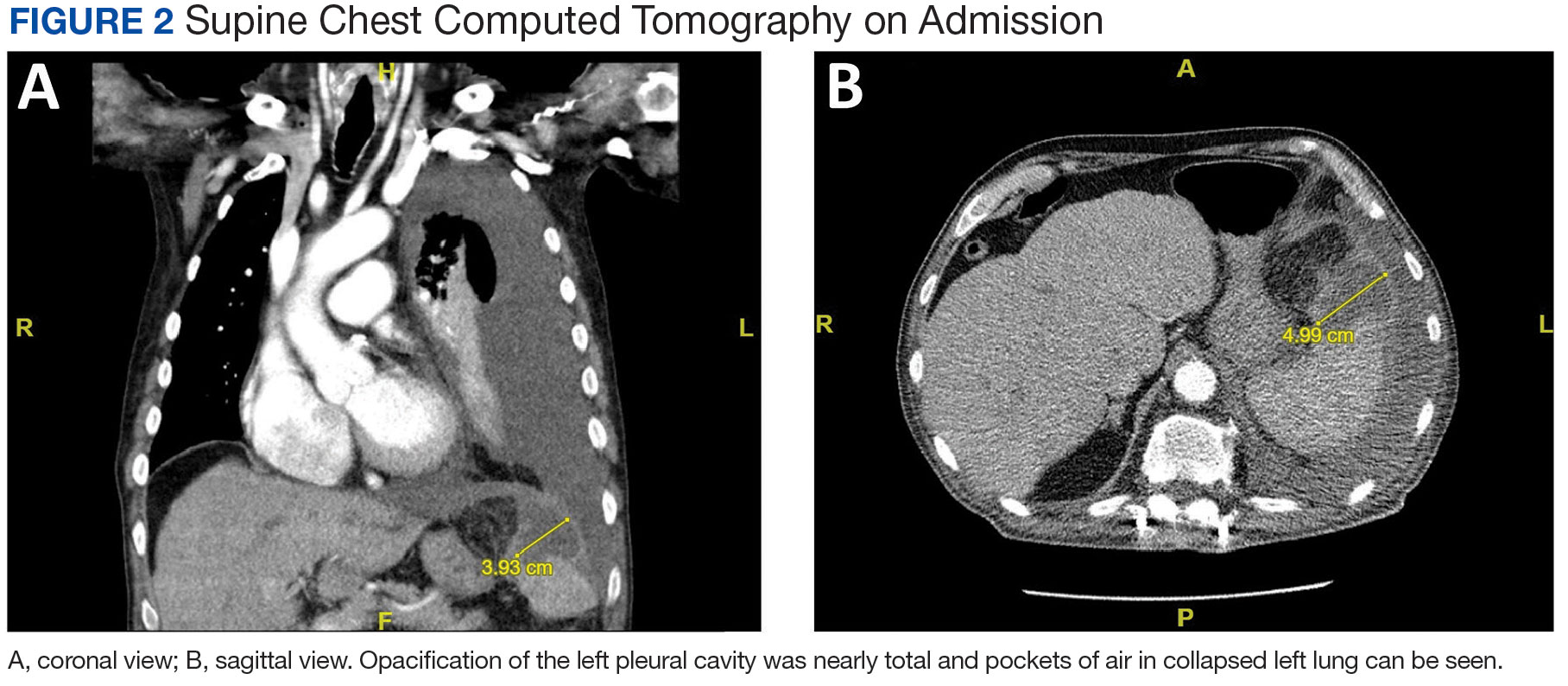
A male aged 80 years presented to the Central Texas Veterans Affairs Hospital (CTVAH) with shortness of breath, weight loss, and fever. On admission, his medical history was notable for atrial fibrillation, myelodysplastic syndrome, hypertension, hyperlipidemia, stable ascending aortic aneurysm, and Vitamin B12 deficiency. A chest CT showed a large left pleural effusion (Figure 1). Additionally, the radiology report noted a nonspecific 4- to 5-cm lobulated subdiaphragmatic mass within the anterior dome of the spleen with surrounding soft tissue swelling and splenomegaly (Figure 2).
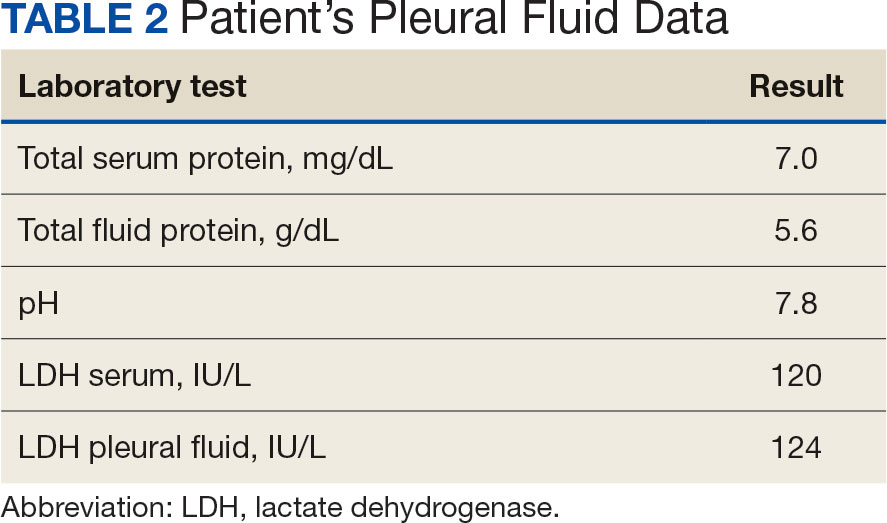
Initial thoracentesis was performed with 1500 mL of straw-colored fluid negative for bacteria, fungi, malignancy, and acid-fast organisms (Tables 2 and 3). The pleural effusion persisted, requiring a second thoracentesis 2 days later that was positive for Escherichia coli (E coli). Given the exudative nature and positive culture, a chest tube was placed, and the pleural effusion was therefore felt to be an empyema, arousing suspicion that the splenic mass seen on CT was an abscess. The site was accessed by interventional radiology, purulent fluid aspirated, and a drain was placed. Cultures grew E coli sensitive to ceftriaxone. Despite receiving intravenous ceftriaxone 2 g daily, the pleural effusion became further complicated due to chest tube obstruction and persistent drainage.
The patient was discharged to Baylor Scott & White Medical Center in Temple, Texas where he underwent decortication with cardiothoracic surgery with several pleural adhesions noted. Following surgery the patient was readmitted to CTVAH and continued ceftriaxone therapy following the infectious disease specialist's recommendation. He was discharged with plans to return to CTVAH for continued care. The patient was readmitted and transitioned to oral levofloxacin 500 mg daily and received physical and occupational therapy. He showed dramatic improvement on this regimen, with a 3-week follow-up CT that indicated only a small left pleural effusion and a 28 mm × 11 mm × 10 mm lesion in the anterior superior spleen. The patient had not returned for further evaluation by thoracic surgery; however, he has continued to see CTVAH primary care without reported recurrence of symptoms.
DISCUSSION
Splenic abscesses are a rare condition typically characterized by hematogenous spread of bacteria from another source, most commonly the endocardium.2 Other differential diagnoses include bacteremia or spread from an intra-abdominal site.2 Staphylococcus aureus and E coli are the most common bacteria seen in splenic abscesses. 2 Treatment includes antibiotics, percutaneous drainage, and, as a last resort, splenectomy.2
Our patient was found to have grown E coli, but no source indicative of spread was identified. He had negative blood cultures, negative findings for intra-abdominal pathologies on CT scans, and a negative echocardiogram for endocarditis. A bronchoscopy showed no evidence of a source from the lungs, and specimens taken from the pleural adhesions were negative for malignancy and bacteria.
This patient had risk factors for the illness, namely his history of being immunocompromised secondary to myelodysplastic syndrome.7 Accordingly, the patient showed persistent leukopenia with neutropenia and lymphocytopenia, which would not be expected for most patients with such an extensive infection. 8 While being immunocompromised undoubtedly contributed to the severity of the patient’s presentation and slow recovery, it does not explain the etiology or origin of his infection. This patient differs from current literature in that his splenic abscess was truly idiopathic rather than resulting from an alternative source.
Complications of splenic abscesses include pleural effusions, as seen with this patient, as well as pneumonia, pneumothorax, hemorrhage, subphrenic abscess, and intraabdominal perforation, among others.2 We determined conclusively that the patient’s pleural effusion was secondary to the splenic abscess, and excluded other bacterial foci strongly suggests that the spleen was the origin of the illness.
CONCLUSIONS
This case suggests splenic abscesses should be considered when evaluating pleural effusion. It further demonstrates that the spleen may be the central source of infection in the absence of iatrogenic inoculation or bacteremia. We hope our findings may lead to earlier identification in similar scenarios and improved patient outcomes in a multidisciplinary approach.
- Lee WS, Choi ST, Kim KK. Splenic abscess: a single institution study and review of the literature. Yonsei Med J. 2011;52(2):288-292. doi:10.3349/ymj.2011.52.2.288
- Lotfollahzadeh S, Mathew G, Zemaitis MR. Splenic Abscess. In: StatPearls. StatPearls Publishing; June 3, 2023.
- Jany B, Welte T. Pleural effusion in adults-etiology, diagnosis, and treatment. Dtsch Arztebl Int. 2019;116(21):377- 386. doi:10.3238/arztebl.2019.0377
- Light RW. Pleural effusions. Med Clin North Am. 2011;95(6):1055-1070. doi:10.1016/j.mcna.2011.08.005
- Feller-Kopman D, Light R. Pleural Disease. N Engl J Med. 2018;378(18):1754. doi:10.1056/NEJMc1803858
- Ferreiro L, Casal A, Toubes ME, et al. Pleural effusion due to nonmalignant gastrointestinal disease. ERJ Open Res. 2023;9(3):00290-2022. doi:10.1183/23120541.00290-2022
- Hasserjian RP. Myelodysplastic syndrome updated. Pathobiology. 2019;86(1):7-13. doi:10.1159/000489702
- Toma A, Fenaux P, Dreyfus F, Cordonnier C. Infections in myelodysplastic syndromes. Haematologica. 2012;97(10):1459- 1470. doi:10.3324/haematol2012.063420
Splenic abscesses are a rare occurrence that represent a marginal proportion of intra-abdominal infections. One study found splenic abscesses in only 0.14% to 0.70% of autopsies and none of the 540 abdominal abscesses they examined originated in the spleen.1 Patients with splenic abscesses tend to present with nonspecific symptoms such as fevers, chills, and abdominal pain.2 Imaging modalities such as abdominal ultrasound and computed tomography (CT) are vital to the workup and diagnosis identification.2 Splenic abscesses are generally associated with another underlying process, as seen in patients who are affected by endocarditis, trauma, metastatic infection, splenic infarction, or neoplasia.2
Pleural effusions, or the buildup of fluid within the pleural space, is a common condition typically secondary to another disease.3 Clinical identification of the primary condition may be challenging.3 In the absence of a clear etiology, such as obvious signs of congestive heart failure, further differentiation relies upon pleural fluid analysis, beginning with the distinction between exudate (inflammatory) and transudate (noninflammatory). 3,4 This distinction can be made using Light’s criteria, which relies on protein and lactate dehydrogenase (LDH) ratios between the pleural fluid and serum (Table 1).5 Though rare, half of splenic abscesses are associated with pleural effusion.6 As an inflammatory condition, splenic abscesses have been classically described as a cause of exudative pleural effusions.5,6

A myelodysplastic syndrome is a group of diseases that arise from malignant hematopoietic stem cells, leading to the proliferation of the malignant cells and faulty production of other bone marrow products.7 These disorders can range from single to multilineage dysplasia. Cells are often left in an immature blast form, unable to function appropriately, and vulnerable to destruction. Patients with myeloproliferative disorders frequently suffer from leukopenia and infections attributable to known quantitative and qualitative defects of neutrophils.8
CASE PRESENTATION
A male aged 80 years presented to the Central Texas Veterans Affairs Hospital (CTVAH) with shortness of breath, weight loss, and fever. On admission, his medical history was notable for atrial fibrillation, myelodysplastic syndrome, hypertension, hyperlipidemia, stable ascending aortic aneurysm, and Vitamin B12 deficiency. A chest CT showed a large left pleural effusion (Figure 1). Additionally, the radiology report noted a nonspecific 4- to 5-cm lobulated subdiaphragmatic mass within the anterior dome of the spleen with surrounding soft tissue swelling and splenomegaly (Figure 2).


Initial thoracentesis was performed with 1500 mL of straw-colored fluid negative for bacteria, fungi, malignancy, and acid-fast organisms (Tables 2 and 3). The pleural effusion persisted, requiring a second thoracentesis 2 days later that was positive for Escherichia coli (E coli). Given the exudative nature and positive culture, a chest tube was placed, and the pleural effusion was therefore felt to be an empyema, arousing suspicion that the splenic mass seen on CT was an abscess. The site was accessed by interventional radiology, purulent fluid aspirated, and a drain was placed. Cultures grew E coli sensitive to ceftriaxone. Despite receiving intravenous ceftriaxone 2 g daily, the pleural effusion became further complicated due to chest tube obstruction and persistent drainage.


The patient was discharged to Baylor Scott & White Medical Center in Temple, Texas where he underwent decortication with cardiothoracic surgery with several pleural adhesions noted. Following surgery the patient was readmitted to CTVAH and continued ceftriaxone therapy following the infectious disease specialist's recommendation. He was discharged with plans to return to CTVAH for continued care. The patient was readmitted and transitioned to oral levofloxacin 500 mg daily and received physical and occupational therapy. He showed dramatic improvement on this regimen, with a 3-week follow-up CT that indicated only a small left pleural effusion and a 28 mm × 11 mm × 10 mm lesion in the anterior superior spleen. The patient had not returned for further evaluation by thoracic surgery; however, he has continued to see CTVAH primary care without reported recurrence of symptoms.
DISCUSSION
Splenic abscesses are a rare condition typically characterized by hematogenous spread of bacteria from another source, most commonly the endocardium.2 Other differential diagnoses include bacteremia or spread from an intra-abdominal site.2 Staphylococcus aureus and E coli are the most common bacteria seen in splenic abscesses. 2 Treatment includes antibiotics, percutaneous drainage, and, as a last resort, splenectomy.2
Our patient was found to have grown E coli, but no source indicative of spread was identified. He had negative blood cultures, negative findings for intra-abdominal pathologies on CT scans, and a negative echocardiogram for endocarditis. A bronchoscopy showed no evidence of a source from the lungs, and specimens taken from the pleural adhesions were negative for malignancy and bacteria.
This patient had risk factors for the illness, namely his history of being immunocompromised secondary to myelodysplastic syndrome.7 Accordingly, the patient showed persistent leukopenia with neutropenia and lymphocytopenia, which would not be expected for most patients with such an extensive infection. 8 While being immunocompromised undoubtedly contributed to the severity of the patient’s presentation and slow recovery, it does not explain the etiology or origin of his infection. This patient differs from current literature in that his splenic abscess was truly idiopathic rather than resulting from an alternative source.
Complications of splenic abscesses include pleural effusions, as seen with this patient, as well as pneumonia, pneumothorax, hemorrhage, subphrenic abscess, and intraabdominal perforation, among others.2 We determined conclusively that the patient’s pleural effusion was secondary to the splenic abscess, and excluded other bacterial foci strongly suggests that the spleen was the origin of the illness.
CONCLUSIONS
This case suggests splenic abscesses should be considered when evaluating pleural effusion. It further demonstrates that the spleen may be the central source of infection in the absence of iatrogenic inoculation or bacteremia. We hope our findings may lead to earlier identification in similar scenarios and improved patient outcomes in a multidisciplinary approach.
Splenic abscesses are a rare occurrence that represent a marginal proportion of intra-abdominal infections. One study found splenic abscesses in only 0.14% to 0.70% of autopsies and none of the 540 abdominal abscesses they examined originated in the spleen.1 Patients with splenic abscesses tend to present with nonspecific symptoms such as fevers, chills, and abdominal pain.2 Imaging modalities such as abdominal ultrasound and computed tomography (CT) are vital to the workup and diagnosis identification.2 Splenic abscesses are generally associated with another underlying process, as seen in patients who are affected by endocarditis, trauma, metastatic infection, splenic infarction, or neoplasia.2
Pleural effusions, or the buildup of fluid within the pleural space, is a common condition typically secondary to another disease.3 Clinical identification of the primary condition may be challenging.3 In the absence of a clear etiology, such as obvious signs of congestive heart failure, further differentiation relies upon pleural fluid analysis, beginning with the distinction between exudate (inflammatory) and transudate (noninflammatory). 3,4 This distinction can be made using Light’s criteria, which relies on protein and lactate dehydrogenase (LDH) ratios between the pleural fluid and serum (Table 1).5 Though rare, half of splenic abscesses are associated with pleural effusion.6 As an inflammatory condition, splenic abscesses have been classically described as a cause of exudative pleural effusions.5,6

A myelodysplastic syndrome is a group of diseases that arise from malignant hematopoietic stem cells, leading to the proliferation of the malignant cells and faulty production of other bone marrow products.7 These disorders can range from single to multilineage dysplasia. Cells are often left in an immature blast form, unable to function appropriately, and vulnerable to destruction. Patients with myeloproliferative disorders frequently suffer from leukopenia and infections attributable to known quantitative and qualitative defects of neutrophils.8
CASE PRESENTATION
A male aged 80 years presented to the Central Texas Veterans Affairs Hospital (CTVAH) with shortness of breath, weight loss, and fever. On admission, his medical history was notable for atrial fibrillation, myelodysplastic syndrome, hypertension, hyperlipidemia, stable ascending aortic aneurysm, and Vitamin B12 deficiency. A chest CT showed a large left pleural effusion (Figure 1). Additionally, the radiology report noted a nonspecific 4- to 5-cm lobulated subdiaphragmatic mass within the anterior dome of the spleen with surrounding soft tissue swelling and splenomegaly (Figure 2).


Initial thoracentesis was performed with 1500 mL of straw-colored fluid negative for bacteria, fungi, malignancy, and acid-fast organisms (Tables 2 and 3). The pleural effusion persisted, requiring a second thoracentesis 2 days later that was positive for Escherichia coli (E coli). Given the exudative nature and positive culture, a chest tube was placed, and the pleural effusion was therefore felt to be an empyema, arousing suspicion that the splenic mass seen on CT was an abscess. The site was accessed by interventional radiology, purulent fluid aspirated, and a drain was placed. Cultures grew E coli sensitive to ceftriaxone. Despite receiving intravenous ceftriaxone 2 g daily, the pleural effusion became further complicated due to chest tube obstruction and persistent drainage.


The patient was discharged to Baylor Scott & White Medical Center in Temple, Texas where he underwent decortication with cardiothoracic surgery with several pleural adhesions noted. Following surgery the patient was readmitted to CTVAH and continued ceftriaxone therapy following the infectious disease specialist's recommendation. He was discharged with plans to return to CTVAH for continued care. The patient was readmitted and transitioned to oral levofloxacin 500 mg daily and received physical and occupational therapy. He showed dramatic improvement on this regimen, with a 3-week follow-up CT that indicated only a small left pleural effusion and a 28 mm × 11 mm × 10 mm lesion in the anterior superior spleen. The patient had not returned for further evaluation by thoracic surgery; however, he has continued to see CTVAH primary care without reported recurrence of symptoms.
DISCUSSION
Splenic abscesses are a rare condition typically characterized by hematogenous spread of bacteria from another source, most commonly the endocardium.2 Other differential diagnoses include bacteremia or spread from an intra-abdominal site.2 Staphylococcus aureus and E coli are the most common bacteria seen in splenic abscesses. 2 Treatment includes antibiotics, percutaneous drainage, and, as a last resort, splenectomy.2
Our patient was found to have grown E coli, but no source indicative of spread was identified. He had negative blood cultures, negative findings for intra-abdominal pathologies on CT scans, and a negative echocardiogram for endocarditis. A bronchoscopy showed no evidence of a source from the lungs, and specimens taken from the pleural adhesions were negative for malignancy and bacteria.
This patient had risk factors for the illness, namely his history of being immunocompromised secondary to myelodysplastic syndrome.7 Accordingly, the patient showed persistent leukopenia with neutropenia and lymphocytopenia, which would not be expected for most patients with such an extensive infection. 8 While being immunocompromised undoubtedly contributed to the severity of the patient’s presentation and slow recovery, it does not explain the etiology or origin of his infection. This patient differs from current literature in that his splenic abscess was truly idiopathic rather than resulting from an alternative source.
Complications of splenic abscesses include pleural effusions, as seen with this patient, as well as pneumonia, pneumothorax, hemorrhage, subphrenic abscess, and intraabdominal perforation, among others.2 We determined conclusively that the patient’s pleural effusion was secondary to the splenic abscess, and excluded other bacterial foci strongly suggests that the spleen was the origin of the illness.
CONCLUSIONS
This case suggests splenic abscesses should be considered when evaluating pleural effusion. It further demonstrates that the spleen may be the central source of infection in the absence of iatrogenic inoculation or bacteremia. We hope our findings may lead to earlier identification in similar scenarios and improved patient outcomes in a multidisciplinary approach.
- Lee WS, Choi ST, Kim KK. Splenic abscess: a single institution study and review of the literature. Yonsei Med J. 2011;52(2):288-292. doi:10.3349/ymj.2011.52.2.288
- Lotfollahzadeh S, Mathew G, Zemaitis MR. Splenic Abscess. In: StatPearls. StatPearls Publishing; June 3, 2023.
- Jany B, Welte T. Pleural effusion in adults-etiology, diagnosis, and treatment. Dtsch Arztebl Int. 2019;116(21):377- 386. doi:10.3238/arztebl.2019.0377
- Light RW. Pleural effusions. Med Clin North Am. 2011;95(6):1055-1070. doi:10.1016/j.mcna.2011.08.005
- Feller-Kopman D, Light R. Pleural Disease. N Engl J Med. 2018;378(18):1754. doi:10.1056/NEJMc1803858
- Ferreiro L, Casal A, Toubes ME, et al. Pleural effusion due to nonmalignant gastrointestinal disease. ERJ Open Res. 2023;9(3):00290-2022. doi:10.1183/23120541.00290-2022
- Hasserjian RP. Myelodysplastic syndrome updated. Pathobiology. 2019;86(1):7-13. doi:10.1159/000489702
- Toma A, Fenaux P, Dreyfus F, Cordonnier C. Infections in myelodysplastic syndromes. Haematologica. 2012;97(10):1459- 1470. doi:10.3324/haematol2012.063420
- Lee WS, Choi ST, Kim KK. Splenic abscess: a single institution study and review of the literature. Yonsei Med J. 2011;52(2):288-292. doi:10.3349/ymj.2011.52.2.288
- Lotfollahzadeh S, Mathew G, Zemaitis MR. Splenic Abscess. In: StatPearls. StatPearls Publishing; June 3, 2023.
- Jany B, Welte T. Pleural effusion in adults-etiology, diagnosis, and treatment. Dtsch Arztebl Int. 2019;116(21):377- 386. doi:10.3238/arztebl.2019.0377
- Light RW. Pleural effusions. Med Clin North Am. 2011;95(6):1055-1070. doi:10.1016/j.mcna.2011.08.005
- Feller-Kopman D, Light R. Pleural Disease. N Engl J Med. 2018;378(18):1754. doi:10.1056/NEJMc1803858
- Ferreiro L, Casal A, Toubes ME, et al. Pleural effusion due to nonmalignant gastrointestinal disease. ERJ Open Res. 2023;9(3):00290-2022. doi:10.1183/23120541.00290-2022
- Hasserjian RP. Myelodysplastic syndrome updated. Pathobiology. 2019;86(1):7-13. doi:10.1159/000489702
- Toma A, Fenaux P, Dreyfus F, Cordonnier C. Infections in myelodysplastic syndromes. Haematologica. 2012;97(10):1459- 1470. doi:10.3324/haematol2012.063420
FDA Expands Indication for Amivantamab in Lung Cancer
Amivantamab with carboplatin and pemetrexed is now indicated for adults with locally advanced or metastatic NSCLC with EGFR exon 19 deletions or exon 21 L858R substitution mutations whose disease has progressed on or after treatment with an EGFR tyrosine kinase inhibitor (TKI).
The FDA has already approved first-line use of amivantamab in combination with carboplatin and pemetrexed in patients with locally advanced or metastatic NSCLC with EGFR exon 20 insertion mutations, as reported by Medscape Medical News.
The second-line approval for amivantamab plus chemotherapy “may address the most common mechanisms of treatment resistance to third-generation EGFR TKIs, such as osimertinib, in the first line,” Martin Dietrich, MD, PhD, oncologist, Cancer Care Centers of Brevard in Florida, said in a company news release.
“This multitargeted combination extended progression-free survival (PFS) and improved overall response compared to chemotherapy alone, offering an important and effective new second-line option for patients,” Dr. Dietrich added.
The second-line indication is supported by the phase 3 MARIPOSA-2 study, which included 657 patients with locally advanced or metastatic NSCLC with EGFR exon 19 deletions or exon 21 L858R substitution mutations and disease progression on or after receiving osimertinib.
The study demonstrated a 52% reduced risk of disease progression or death when amivantamab was added to carboplatin and pemetrexed (hazard ratio, 0.48).
Median PFS was 6.3 months with amivantamab vs 4.2 months with chemotherapy alone. The confirmed objective response rate was 53% in the amivantamab plus chemotherapy group vs 29% in the chemotherapy only group.
The most common adverse reactions, occurring in at least 20% of patients, were rash, infusion-related reactions, fatigue, nail toxicity, nausea, constipation, edema, stomatitis, decreased appetite, musculoskeletal pain, vomiting, and COVID-19 infection.
The company noted that amivantamab in combination with chemotherapy is the only category 1 treatment option in National Comprehensive Cancer Network clinical practice guidelines for patients with EGFR-mutated NSCLC who have progressed on osimertinib and who are symptomatic with multiple lesions.
A version of this article appeared on Medscape.com.
Amivantamab with carboplatin and pemetrexed is now indicated for adults with locally advanced or metastatic NSCLC with EGFR exon 19 deletions or exon 21 L858R substitution mutations whose disease has progressed on or after treatment with an EGFR tyrosine kinase inhibitor (TKI).
The FDA has already approved first-line use of amivantamab in combination with carboplatin and pemetrexed in patients with locally advanced or metastatic NSCLC with EGFR exon 20 insertion mutations, as reported by Medscape Medical News.
The second-line approval for amivantamab plus chemotherapy “may address the most common mechanisms of treatment resistance to third-generation EGFR TKIs, such as osimertinib, in the first line,” Martin Dietrich, MD, PhD, oncologist, Cancer Care Centers of Brevard in Florida, said in a company news release.
“This multitargeted combination extended progression-free survival (PFS) and improved overall response compared to chemotherapy alone, offering an important and effective new second-line option for patients,” Dr. Dietrich added.
The second-line indication is supported by the phase 3 MARIPOSA-2 study, which included 657 patients with locally advanced or metastatic NSCLC with EGFR exon 19 deletions or exon 21 L858R substitution mutations and disease progression on or after receiving osimertinib.
The study demonstrated a 52% reduced risk of disease progression or death when amivantamab was added to carboplatin and pemetrexed (hazard ratio, 0.48).
Median PFS was 6.3 months with amivantamab vs 4.2 months with chemotherapy alone. The confirmed objective response rate was 53% in the amivantamab plus chemotherapy group vs 29% in the chemotherapy only group.
The most common adverse reactions, occurring in at least 20% of patients, were rash, infusion-related reactions, fatigue, nail toxicity, nausea, constipation, edema, stomatitis, decreased appetite, musculoskeletal pain, vomiting, and COVID-19 infection.
The company noted that amivantamab in combination with chemotherapy is the only category 1 treatment option in National Comprehensive Cancer Network clinical practice guidelines for patients with EGFR-mutated NSCLC who have progressed on osimertinib and who are symptomatic with multiple lesions.
A version of this article appeared on Medscape.com.
Amivantamab with carboplatin and pemetrexed is now indicated for adults with locally advanced or metastatic NSCLC with EGFR exon 19 deletions or exon 21 L858R substitution mutations whose disease has progressed on or after treatment with an EGFR tyrosine kinase inhibitor (TKI).
The FDA has already approved first-line use of amivantamab in combination with carboplatin and pemetrexed in patients with locally advanced or metastatic NSCLC with EGFR exon 20 insertion mutations, as reported by Medscape Medical News.
The second-line approval for amivantamab plus chemotherapy “may address the most common mechanisms of treatment resistance to third-generation EGFR TKIs, such as osimertinib, in the first line,” Martin Dietrich, MD, PhD, oncologist, Cancer Care Centers of Brevard in Florida, said in a company news release.
“This multitargeted combination extended progression-free survival (PFS) and improved overall response compared to chemotherapy alone, offering an important and effective new second-line option for patients,” Dr. Dietrich added.
The second-line indication is supported by the phase 3 MARIPOSA-2 study, which included 657 patients with locally advanced or metastatic NSCLC with EGFR exon 19 deletions or exon 21 L858R substitution mutations and disease progression on or after receiving osimertinib.
The study demonstrated a 52% reduced risk of disease progression or death when amivantamab was added to carboplatin and pemetrexed (hazard ratio, 0.48).
Median PFS was 6.3 months with amivantamab vs 4.2 months with chemotherapy alone. The confirmed objective response rate was 53% in the amivantamab plus chemotherapy group vs 29% in the chemotherapy only group.
The most common adverse reactions, occurring in at least 20% of patients, were rash, infusion-related reactions, fatigue, nail toxicity, nausea, constipation, edema, stomatitis, decreased appetite, musculoskeletal pain, vomiting, and COVID-19 infection.
The company noted that amivantamab in combination with chemotherapy is the only category 1 treatment option in National Comprehensive Cancer Network clinical practice guidelines for patients with EGFR-mutated NSCLC who have progressed on osimertinib and who are symptomatic with multiple lesions.
A version of this article appeared on Medscape.com.
Cancer Risk: Are Pesticides the New Smoking?
Pesticides have transformed modern agriculture by boosting production yields and helping alleviate food insecurity amid rapid global population growth. However, from a public health perspective, exposure to pesticides has been linked to numerous harmful effects, including neurologic disorders like Parkinson’s disease, weakened immune function, and an increased risk for cancer.
thus offering a limited perspective.
A comprehensive assessment of how pesticide use affects cancer risk across a broader population has yet to be conducted.
A recent population-level study aimed to address this gap by evaluating cancer risks in the US population using a model that accounts for pesticide use and adjusts for various factors. The goal was to identify regional disparities in exposure and contribute to the development of public health policies that protect populations from potential harm.
Calculating Cancer Risk
Researchers developed a model using several data sources to estimate the additional cancer risk from agricultural pesticide use. Key data included:
- Pesticide use data from the US Geological Survey in 2019, which covered 69 agricultural pesticides across 3143 counties
- Cancer incidence rates per 100,000 people, which were collected between 2015 and 2019 by the National Institutes of Health and the Centers for Disease Control and Prevention; these data covered various cancers, including bladder, colorectal, leukemia, lung, non-Hodgkin lymphoma, and pancreatic cancers
- Covariates, including smoking prevalence, the Social Vulnerability Index, agricultural land use, and total US population in 2019
Pesticide use profile patterns were developed using latent class analysis, a statistical method used to identify homogeneous subgroups within a heterogeneous population. A generalized linear model then estimated how these pesticide use patterns and the covariates affected cancer incidence.
The model highlighted regions with the highest and lowest “additional” cancer risks linked to pesticide exposure, calculating the estimated increase in cancer cases per year that resulted from variations in agricultural pesticide use.
Midwest Most Affected
While this model doesn’t establish causality or assess individual risk, it reveals regional trends in the association between pesticide use patterns and cancer incidence from a population-based perspective.
The Midwest, known for its high corn production, emerged as the region most affected by pesticide use. Compared with regions with the lowest risk, the Midwest faced an additional 154,541 cancer cases annually across all types. For colorectal and pancreatic cancers, the yearly increases were 20,927 and 3835 cases, respectively. Similar trends were observed for leukemia and non-Hodgkin lymphoma.
Pesticides vs Smoking
The researchers also estimated the additional cancer risk related to smoking, using the same model. They found that pesticides contributed to a higher risk for cancer than smoking in several cases.
The most significant difference was observed with non-Hodgkin lymphoma, where pesticides were linked to 154.1% more cases than smoking. For all cancers combined, as well as bladder cancer and leukemia, the increases were moderate: 18.7%, 19.3%, and 21.0%, respectively.
This result highlights the importance of considering pesticide exposure alongside smoking when studying cancer risks.
Expanding Scope of Research
Some limitations of this study should be noted. Certain counties lacked complete data, and there was heterogeneity in the size and population of the counties studied. The research also did not account for seasonal and migrant workers, who are likely to be heavily exposed. In addition, the data used in the study were not independently validated, and they could not be used to assess individual risk.
The effect of pesticides on human health is a vast and critical field of research, often focusing on a limited range of pesticides or specific cancers. This study stands out by taking a broader, more holistic approach, aiming to highlight regional inequalities and identify less-studied pesticides that could be future research priorities.
Given the significant public health impact, the authors encouraged the authorities to share these findings with the most vulnerable communities to raise awareness.
This story was translated from JIM using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Pesticides have transformed modern agriculture by boosting production yields and helping alleviate food insecurity amid rapid global population growth. However, from a public health perspective, exposure to pesticides has been linked to numerous harmful effects, including neurologic disorders like Parkinson’s disease, weakened immune function, and an increased risk for cancer.
thus offering a limited perspective.
A comprehensive assessment of how pesticide use affects cancer risk across a broader population has yet to be conducted.
A recent population-level study aimed to address this gap by evaluating cancer risks in the US population using a model that accounts for pesticide use and adjusts for various factors. The goal was to identify regional disparities in exposure and contribute to the development of public health policies that protect populations from potential harm.
Calculating Cancer Risk
Researchers developed a model using several data sources to estimate the additional cancer risk from agricultural pesticide use. Key data included:
- Pesticide use data from the US Geological Survey in 2019, which covered 69 agricultural pesticides across 3143 counties
- Cancer incidence rates per 100,000 people, which were collected between 2015 and 2019 by the National Institutes of Health and the Centers for Disease Control and Prevention; these data covered various cancers, including bladder, colorectal, leukemia, lung, non-Hodgkin lymphoma, and pancreatic cancers
- Covariates, including smoking prevalence, the Social Vulnerability Index, agricultural land use, and total US population in 2019
Pesticide use profile patterns were developed using latent class analysis, a statistical method used to identify homogeneous subgroups within a heterogeneous population. A generalized linear model then estimated how these pesticide use patterns and the covariates affected cancer incidence.
The model highlighted regions with the highest and lowest “additional” cancer risks linked to pesticide exposure, calculating the estimated increase in cancer cases per year that resulted from variations in agricultural pesticide use.
Midwest Most Affected
While this model doesn’t establish causality or assess individual risk, it reveals regional trends in the association between pesticide use patterns and cancer incidence from a population-based perspective.
The Midwest, known for its high corn production, emerged as the region most affected by pesticide use. Compared with regions with the lowest risk, the Midwest faced an additional 154,541 cancer cases annually across all types. For colorectal and pancreatic cancers, the yearly increases were 20,927 and 3835 cases, respectively. Similar trends were observed for leukemia and non-Hodgkin lymphoma.
Pesticides vs Smoking
The researchers also estimated the additional cancer risk related to smoking, using the same model. They found that pesticides contributed to a higher risk for cancer than smoking in several cases.
The most significant difference was observed with non-Hodgkin lymphoma, where pesticides were linked to 154.1% more cases than smoking. For all cancers combined, as well as bladder cancer and leukemia, the increases were moderate: 18.7%, 19.3%, and 21.0%, respectively.
This result highlights the importance of considering pesticide exposure alongside smoking when studying cancer risks.
Expanding Scope of Research
Some limitations of this study should be noted. Certain counties lacked complete data, and there was heterogeneity in the size and population of the counties studied. The research also did not account for seasonal and migrant workers, who are likely to be heavily exposed. In addition, the data used in the study were not independently validated, and they could not be used to assess individual risk.
The effect of pesticides on human health is a vast and critical field of research, often focusing on a limited range of pesticides or specific cancers. This study stands out by taking a broader, more holistic approach, aiming to highlight regional inequalities and identify less-studied pesticides that could be future research priorities.
Given the significant public health impact, the authors encouraged the authorities to share these findings with the most vulnerable communities to raise awareness.
This story was translated from JIM using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Pesticides have transformed modern agriculture by boosting production yields and helping alleviate food insecurity amid rapid global population growth. However, from a public health perspective, exposure to pesticides has been linked to numerous harmful effects, including neurologic disorders like Parkinson’s disease, weakened immune function, and an increased risk for cancer.
thus offering a limited perspective.
A comprehensive assessment of how pesticide use affects cancer risk across a broader population has yet to be conducted.
A recent population-level study aimed to address this gap by evaluating cancer risks in the US population using a model that accounts for pesticide use and adjusts for various factors. The goal was to identify regional disparities in exposure and contribute to the development of public health policies that protect populations from potential harm.
Calculating Cancer Risk
Researchers developed a model using several data sources to estimate the additional cancer risk from agricultural pesticide use. Key data included:
- Pesticide use data from the US Geological Survey in 2019, which covered 69 agricultural pesticides across 3143 counties
- Cancer incidence rates per 100,000 people, which were collected between 2015 and 2019 by the National Institutes of Health and the Centers for Disease Control and Prevention; these data covered various cancers, including bladder, colorectal, leukemia, lung, non-Hodgkin lymphoma, and pancreatic cancers
- Covariates, including smoking prevalence, the Social Vulnerability Index, agricultural land use, and total US population in 2019
Pesticide use profile patterns were developed using latent class analysis, a statistical method used to identify homogeneous subgroups within a heterogeneous population. A generalized linear model then estimated how these pesticide use patterns and the covariates affected cancer incidence.
The model highlighted regions with the highest and lowest “additional” cancer risks linked to pesticide exposure, calculating the estimated increase in cancer cases per year that resulted from variations in agricultural pesticide use.
Midwest Most Affected
While this model doesn’t establish causality or assess individual risk, it reveals regional trends in the association between pesticide use patterns and cancer incidence from a population-based perspective.
The Midwest, known for its high corn production, emerged as the region most affected by pesticide use. Compared with regions with the lowest risk, the Midwest faced an additional 154,541 cancer cases annually across all types. For colorectal and pancreatic cancers, the yearly increases were 20,927 and 3835 cases, respectively. Similar trends were observed for leukemia and non-Hodgkin lymphoma.
Pesticides vs Smoking
The researchers also estimated the additional cancer risk related to smoking, using the same model. They found that pesticides contributed to a higher risk for cancer than smoking in several cases.
The most significant difference was observed with non-Hodgkin lymphoma, where pesticides were linked to 154.1% more cases than smoking. For all cancers combined, as well as bladder cancer and leukemia, the increases were moderate: 18.7%, 19.3%, and 21.0%, respectively.
This result highlights the importance of considering pesticide exposure alongside smoking when studying cancer risks.
Expanding Scope of Research
Some limitations of this study should be noted. Certain counties lacked complete data, and there was heterogeneity in the size and population of the counties studied. The research also did not account for seasonal and migrant workers, who are likely to be heavily exposed. In addition, the data used in the study were not independently validated, and they could not be used to assess individual risk.
The effect of pesticides on human health is a vast and critical field of research, often focusing on a limited range of pesticides or specific cancers. This study stands out by taking a broader, more holistic approach, aiming to highlight regional inequalities and identify less-studied pesticides that could be future research priorities.
Given the significant public health impact, the authors encouraged the authorities to share these findings with the most vulnerable communities to raise awareness.
This story was translated from JIM using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Benralizumab Now FDA Approved to Treat EGPA Vasculitis
The Food and Drug Administration (FDA) has approved benralizumab (Fasenra) for the treatment of adults with eosinophilic granulomatosis with polyangiitis (EGPA), formerly known as Churg-Strauss syndrome.
The drug is the second approved biologic for the treatment of EGPA. The first, mepolizumab (Nucala), was approved in 2017.
“This disease has a devastating impact on patients and the quality of their life, and they need more treatment options. The approval of another treatment in EGPA is welcome news to the approximately 15,000 patients living in the US with this difficult-to-treat rare disease,” said Joyce Kullman, executive director of the Vasculitis Foundation, in a press release on September 18.
Benralizumab, developed by AstraZeneca, is a monoclonal antibody against the interleukin-5 alpha receptor expressed on eosinophils. The drug was first approved in 2017 as an add-on treatment for patients 12 years and older with severe eosinophilic asthma, and is now approved for use in children aged 6 years and older.
The new indication was based on positive results from a noninferiority trial comparing benralizumab and mepolizumab. For the trial, published in the New England Journal of Medicine earlier in 2024, 140 adults with relapsing or refractory EGPA were randomized to a 30-mg subcutaneous injection of benralizumab or three separate 100-mg mepolizumab injections every 4 weeks for 1 year. At weeks 36 and 48, 59% of patients in the benralizumab group and 56% of patients in the mepolizumab group achieved remission (95% CI, –13 to 18; P = .73 for superiority). From week 42 to 52, 41% of patients who received benralizumab completely stopped taking oral glucocorticoids, compared with 26% of those who received mepolizumab.
“Patients often rely on long-term oral corticosteroids, which can cause serious and lasting side effects. Benralizumab is a much-needed treatment option, with data showing that not only is remission an achievable goal for EGPA patients, but benralizumab can also help patients taper off steroid therapy,” Michael Wechsler, MD, director of The Asthma Institute at National Jewish Health in Denver, Colorado, and the international coordinating investigator for the clinical trial, said in the press release.
Benralizumab is administered via subcutaneous injection. In adults with EGPA, the recommended dosage is 30 mg every 4 weeks for the first three doses, then once every 8 weeks.
The most common adverse reactions include headache and pharyngitis, according to the prescribing information.
Benralizumab is also in development for the treatment of chronic obstructive pulmonary disease, chronic rhinosinusitis with nasal polyps, and hypereosinophilic syndrome.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration (FDA) has approved benralizumab (Fasenra) for the treatment of adults with eosinophilic granulomatosis with polyangiitis (EGPA), formerly known as Churg-Strauss syndrome.
The drug is the second approved biologic for the treatment of EGPA. The first, mepolizumab (Nucala), was approved in 2017.
“This disease has a devastating impact on patients and the quality of their life, and they need more treatment options. The approval of another treatment in EGPA is welcome news to the approximately 15,000 patients living in the US with this difficult-to-treat rare disease,” said Joyce Kullman, executive director of the Vasculitis Foundation, in a press release on September 18.
Benralizumab, developed by AstraZeneca, is a monoclonal antibody against the interleukin-5 alpha receptor expressed on eosinophils. The drug was first approved in 2017 as an add-on treatment for patients 12 years and older with severe eosinophilic asthma, and is now approved for use in children aged 6 years and older.
The new indication was based on positive results from a noninferiority trial comparing benralizumab and mepolizumab. For the trial, published in the New England Journal of Medicine earlier in 2024, 140 adults with relapsing or refractory EGPA were randomized to a 30-mg subcutaneous injection of benralizumab or three separate 100-mg mepolizumab injections every 4 weeks for 1 year. At weeks 36 and 48, 59% of patients in the benralizumab group and 56% of patients in the mepolizumab group achieved remission (95% CI, –13 to 18; P = .73 for superiority). From week 42 to 52, 41% of patients who received benralizumab completely stopped taking oral glucocorticoids, compared with 26% of those who received mepolizumab.
“Patients often rely on long-term oral corticosteroids, which can cause serious and lasting side effects. Benralizumab is a much-needed treatment option, with data showing that not only is remission an achievable goal for EGPA patients, but benralizumab can also help patients taper off steroid therapy,” Michael Wechsler, MD, director of The Asthma Institute at National Jewish Health in Denver, Colorado, and the international coordinating investigator for the clinical trial, said in the press release.
Benralizumab is administered via subcutaneous injection. In adults with EGPA, the recommended dosage is 30 mg every 4 weeks for the first three doses, then once every 8 weeks.
The most common adverse reactions include headache and pharyngitis, according to the prescribing information.
Benralizumab is also in development for the treatment of chronic obstructive pulmonary disease, chronic rhinosinusitis with nasal polyps, and hypereosinophilic syndrome.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration (FDA) has approved benralizumab (Fasenra) for the treatment of adults with eosinophilic granulomatosis with polyangiitis (EGPA), formerly known as Churg-Strauss syndrome.
The drug is the second approved biologic for the treatment of EGPA. The first, mepolizumab (Nucala), was approved in 2017.
“This disease has a devastating impact on patients and the quality of their life, and they need more treatment options. The approval of another treatment in EGPA is welcome news to the approximately 15,000 patients living in the US with this difficult-to-treat rare disease,” said Joyce Kullman, executive director of the Vasculitis Foundation, in a press release on September 18.
Benralizumab, developed by AstraZeneca, is a monoclonal antibody against the interleukin-5 alpha receptor expressed on eosinophils. The drug was first approved in 2017 as an add-on treatment for patients 12 years and older with severe eosinophilic asthma, and is now approved for use in children aged 6 years and older.
The new indication was based on positive results from a noninferiority trial comparing benralizumab and mepolizumab. For the trial, published in the New England Journal of Medicine earlier in 2024, 140 adults with relapsing or refractory EGPA were randomized to a 30-mg subcutaneous injection of benralizumab or three separate 100-mg mepolizumab injections every 4 weeks for 1 year. At weeks 36 and 48, 59% of patients in the benralizumab group and 56% of patients in the mepolizumab group achieved remission (95% CI, –13 to 18; P = .73 for superiority). From week 42 to 52, 41% of patients who received benralizumab completely stopped taking oral glucocorticoids, compared with 26% of those who received mepolizumab.
“Patients often rely on long-term oral corticosteroids, which can cause serious and lasting side effects. Benralizumab is a much-needed treatment option, with data showing that not only is remission an achievable goal for EGPA patients, but benralizumab can also help patients taper off steroid therapy,” Michael Wechsler, MD, director of The Asthma Institute at National Jewish Health in Denver, Colorado, and the international coordinating investigator for the clinical trial, said in the press release.
Benralizumab is administered via subcutaneous injection. In adults with EGPA, the recommended dosage is 30 mg every 4 weeks for the first three doses, then once every 8 weeks.
The most common adverse reactions include headache and pharyngitis, according to the prescribing information.
Benralizumab is also in development for the treatment of chronic obstructive pulmonary disease, chronic rhinosinusitis with nasal polyps, and hypereosinophilic syndrome.
A version of this article first appeared on Medscape.com.
Rheumatology Clinic Interventions for Smoking, Blood Pressure ‘Make a Big Difference’
Two relatively simple interventions — addressing high blood pressure (BP) and smoking cessation — could make a huge difference for patients with rheumatic disease. Patients with autoimmune disease are up to three times more likely to develop cardiovascular disease (CVD) than the general population. In addition to compounding CVD, smoking is tied to the development of certain autoimmune conditions, as well as worse outcomes. Christie Bartels, MD, chief of the Division of Rheumatology at the University of Wisconsin School of Medicine and Public Health, Madison, has focused her research on improving cardiac health in inflammatory diseases. This news organization spoke with Bartels about two short interventions she developed that tackle hypertension and smoking cessation during regular visits, each taking less than 3 minutes.
How Do These Programs Address Cardiac Disease Prevention?
The BP and Quit Connect programs help clinics systematically address the two most modifiable risk factors for CVD: high BP and smoking. There’s also evidence that addressing these two risk factors improves outcomes in rheumatic diseases. Hypertension predicts an increase in lupus damage. Particularly in lupus nephritis, hypertension will increase the risk for CVD and kidney failure. People who use tobacco have worse outcomes in diseases like rheumatoid arthritis, psoriatic arthritis, and lupus, as well as more CVD, and antirheumatic drugs may not work as well.
In 90 seconds to 3 minutes, staff can do protocol-based care, which we’ve done across 20,000-plus visits. We showed we can improve population level rates of high BP and BP control, as well as increase smoking quitting rates across different patient settings.
What Is the Quit Connect Program?
The Quit Connect program is a 10- to 90-second point of care intervention. During rooming, staff (medical assistants and nurses) ask patients: “A) Do you smoke? and B) Have you thought about cutting back or quitting in the next 30 days?”
It turns out, when you ask the question that way, between a third and a half of people say that they’ve thought about cutting back or quitting. Then, we can get patients connected directly to Quitline, a free public service across all 50 states that smokers can use to get cessation support.
If patients are ready, we ask if we can arrange for them to receive a call from a Quitline coach about setting a quit date or receiving free nicotine replacement therapy. The beautiful thing is when that all happens, A) it’s free to the patient, and B) the results from the Quitline can be recorded right back to the electronic health record.
In our most recent publication in Arthritis Care & Research, we documented bringing Quit Connect to Grady Hospital in downtown Atlanta. It’s a safety net hospital, where 80% patients are Black and 70%-80% patients are on public insurance or uninsured. Using this protocol, we improved Quitline referrals 20-fold.
What Is the BP Connect Program?
At least half of the encounters in United States happen in specialty clinics. Unfortunately, when patients get their BP measured in a specialty clinic that’s not a cardiology or a vascular clinic, often, even if the pressure is high, the clinic doesn’t give patients feedback on that. The problem is because we haven’t said anything, that gives people the false reassurance that their BP is okay.
We’ve developed a 3-minute protocol to ask, advise, and connect. The idea is that if we measure a high BP, then we remeasure and confirm that it’s high. Then, we advise why it matters in rheumatic disease: Patients with rheumatic diseases are already at an increased risk for heart disease, and controlling BP can make a big difference. Then, we connect patients with high BP back to primary care.
Specifically, a SmartSet — an electronic medical record feature — prompts different actions based on confirmed high BP readings:
- If systolic BP ≥ 140-159, the SmartSet directs scheduling a visit to a nurse or primary care provider.
- If systolic BP ≥ 160-179, the next primary care visit anticipates the need to see a prescriber.
- If systolic BP ≥ 180, then the medical assistant or nurse at the visit is instructed to notify the provider who can arrange a provider-to-provider handoff for safety to exclude a hypertensive emergency.
That order goes to the scheduler to call primary care to coordinate follow-up. BP Connect doubled the likelihood of a guideline-recommended follow-up in primary care within 30 days. All patients benefited, and disparities decreased. BP Connect has had 1100 downloads, and both BP and Quit Connect programs are endorsed by the Centers for Disease Control and Prevention and Million Hearts.
How Do These Programs Affect Clinical Practice?
We developed these interventions with a health system engineer, and we time stamped everything. Part of the sustainability of this model is that it fits within a regular workflow. As a practicing rheumatologist, I understand that time is a precious commodity.
The interventions are in partnership with frontline staff. We’ve received feedback that they feel pride participating in these initiatives. They can say, because of me, 30 patients followed up last month for high BP, or 10 patients took a referral to the Quitline last year. We celebrate these accomplishments with the staff.
What Are the Next Steps for These Programs?
Public-facing toolkits for both BP and Quit Connect programs are available online. We have implemented [these programs] in a rural setting, in an urban setting, in Milwaukee and in Atlanta, and we are looking in the future to do a larger, multistate implementation study. If folks are interested, we’d love to partner with them to look at disseminating this further.
A version of this article appeared on Medscape.com.
Two relatively simple interventions — addressing high blood pressure (BP) and smoking cessation — could make a huge difference for patients with rheumatic disease. Patients with autoimmune disease are up to three times more likely to develop cardiovascular disease (CVD) than the general population. In addition to compounding CVD, smoking is tied to the development of certain autoimmune conditions, as well as worse outcomes. Christie Bartels, MD, chief of the Division of Rheumatology at the University of Wisconsin School of Medicine and Public Health, Madison, has focused her research on improving cardiac health in inflammatory diseases. This news organization spoke with Bartels about two short interventions she developed that tackle hypertension and smoking cessation during regular visits, each taking less than 3 minutes.
How Do These Programs Address Cardiac Disease Prevention?
The BP and Quit Connect programs help clinics systematically address the two most modifiable risk factors for CVD: high BP and smoking. There’s also evidence that addressing these two risk factors improves outcomes in rheumatic diseases. Hypertension predicts an increase in lupus damage. Particularly in lupus nephritis, hypertension will increase the risk for CVD and kidney failure. People who use tobacco have worse outcomes in diseases like rheumatoid arthritis, psoriatic arthritis, and lupus, as well as more CVD, and antirheumatic drugs may not work as well.
In 90 seconds to 3 minutes, staff can do protocol-based care, which we’ve done across 20,000-plus visits. We showed we can improve population level rates of high BP and BP control, as well as increase smoking quitting rates across different patient settings.
What Is the Quit Connect Program?
The Quit Connect program is a 10- to 90-second point of care intervention. During rooming, staff (medical assistants and nurses) ask patients: “A) Do you smoke? and B) Have you thought about cutting back or quitting in the next 30 days?”
It turns out, when you ask the question that way, between a third and a half of people say that they’ve thought about cutting back or quitting. Then, we can get patients connected directly to Quitline, a free public service across all 50 states that smokers can use to get cessation support.
If patients are ready, we ask if we can arrange for them to receive a call from a Quitline coach about setting a quit date or receiving free nicotine replacement therapy. The beautiful thing is when that all happens, A) it’s free to the patient, and B) the results from the Quitline can be recorded right back to the electronic health record.
In our most recent publication in Arthritis Care & Research, we documented bringing Quit Connect to Grady Hospital in downtown Atlanta. It’s a safety net hospital, where 80% patients are Black and 70%-80% patients are on public insurance or uninsured. Using this protocol, we improved Quitline referrals 20-fold.
What Is the BP Connect Program?
At least half of the encounters in United States happen in specialty clinics. Unfortunately, when patients get their BP measured in a specialty clinic that’s not a cardiology or a vascular clinic, often, even if the pressure is high, the clinic doesn’t give patients feedback on that. The problem is because we haven’t said anything, that gives people the false reassurance that their BP is okay.
We’ve developed a 3-minute protocol to ask, advise, and connect. The idea is that if we measure a high BP, then we remeasure and confirm that it’s high. Then, we advise why it matters in rheumatic disease: Patients with rheumatic diseases are already at an increased risk for heart disease, and controlling BP can make a big difference. Then, we connect patients with high BP back to primary care.
Specifically, a SmartSet — an electronic medical record feature — prompts different actions based on confirmed high BP readings:
- If systolic BP ≥ 140-159, the SmartSet directs scheduling a visit to a nurse or primary care provider.
- If systolic BP ≥ 160-179, the next primary care visit anticipates the need to see a prescriber.
- If systolic BP ≥ 180, then the medical assistant or nurse at the visit is instructed to notify the provider who can arrange a provider-to-provider handoff for safety to exclude a hypertensive emergency.
That order goes to the scheduler to call primary care to coordinate follow-up. BP Connect doubled the likelihood of a guideline-recommended follow-up in primary care within 30 days. All patients benefited, and disparities decreased. BP Connect has had 1100 downloads, and both BP and Quit Connect programs are endorsed by the Centers for Disease Control and Prevention and Million Hearts.
How Do These Programs Affect Clinical Practice?
We developed these interventions with a health system engineer, and we time stamped everything. Part of the sustainability of this model is that it fits within a regular workflow. As a practicing rheumatologist, I understand that time is a precious commodity.
The interventions are in partnership with frontline staff. We’ve received feedback that they feel pride participating in these initiatives. They can say, because of me, 30 patients followed up last month for high BP, or 10 patients took a referral to the Quitline last year. We celebrate these accomplishments with the staff.
What Are the Next Steps for These Programs?
Public-facing toolkits for both BP and Quit Connect programs are available online. We have implemented [these programs] in a rural setting, in an urban setting, in Milwaukee and in Atlanta, and we are looking in the future to do a larger, multistate implementation study. If folks are interested, we’d love to partner with them to look at disseminating this further.
A version of this article appeared on Medscape.com.
Two relatively simple interventions — addressing high blood pressure (BP) and smoking cessation — could make a huge difference for patients with rheumatic disease. Patients with autoimmune disease are up to three times more likely to develop cardiovascular disease (CVD) than the general population. In addition to compounding CVD, smoking is tied to the development of certain autoimmune conditions, as well as worse outcomes. Christie Bartels, MD, chief of the Division of Rheumatology at the University of Wisconsin School of Medicine and Public Health, Madison, has focused her research on improving cardiac health in inflammatory diseases. This news organization spoke with Bartels about two short interventions she developed that tackle hypertension and smoking cessation during regular visits, each taking less than 3 minutes.
How Do These Programs Address Cardiac Disease Prevention?
The BP and Quit Connect programs help clinics systematically address the two most modifiable risk factors for CVD: high BP and smoking. There’s also evidence that addressing these two risk factors improves outcomes in rheumatic diseases. Hypertension predicts an increase in lupus damage. Particularly in lupus nephritis, hypertension will increase the risk for CVD and kidney failure. People who use tobacco have worse outcomes in diseases like rheumatoid arthritis, psoriatic arthritis, and lupus, as well as more CVD, and antirheumatic drugs may not work as well.
In 90 seconds to 3 minutes, staff can do protocol-based care, which we’ve done across 20,000-plus visits. We showed we can improve population level rates of high BP and BP control, as well as increase smoking quitting rates across different patient settings.
What Is the Quit Connect Program?
The Quit Connect program is a 10- to 90-second point of care intervention. During rooming, staff (medical assistants and nurses) ask patients: “A) Do you smoke? and B) Have you thought about cutting back or quitting in the next 30 days?”
It turns out, when you ask the question that way, between a third and a half of people say that they’ve thought about cutting back or quitting. Then, we can get patients connected directly to Quitline, a free public service across all 50 states that smokers can use to get cessation support.
If patients are ready, we ask if we can arrange for them to receive a call from a Quitline coach about setting a quit date or receiving free nicotine replacement therapy. The beautiful thing is when that all happens, A) it’s free to the patient, and B) the results from the Quitline can be recorded right back to the electronic health record.
In our most recent publication in Arthritis Care & Research, we documented bringing Quit Connect to Grady Hospital in downtown Atlanta. It’s a safety net hospital, where 80% patients are Black and 70%-80% patients are on public insurance or uninsured. Using this protocol, we improved Quitline referrals 20-fold.
What Is the BP Connect Program?
At least half of the encounters in United States happen in specialty clinics. Unfortunately, when patients get their BP measured in a specialty clinic that’s not a cardiology or a vascular clinic, often, even if the pressure is high, the clinic doesn’t give patients feedback on that. The problem is because we haven’t said anything, that gives people the false reassurance that their BP is okay.
We’ve developed a 3-minute protocol to ask, advise, and connect. The idea is that if we measure a high BP, then we remeasure and confirm that it’s high. Then, we advise why it matters in rheumatic disease: Patients with rheumatic diseases are already at an increased risk for heart disease, and controlling BP can make a big difference. Then, we connect patients with high BP back to primary care.
Specifically, a SmartSet — an electronic medical record feature — prompts different actions based on confirmed high BP readings:
- If systolic BP ≥ 140-159, the SmartSet directs scheduling a visit to a nurse or primary care provider.
- If systolic BP ≥ 160-179, the next primary care visit anticipates the need to see a prescriber.
- If systolic BP ≥ 180, then the medical assistant or nurse at the visit is instructed to notify the provider who can arrange a provider-to-provider handoff for safety to exclude a hypertensive emergency.
That order goes to the scheduler to call primary care to coordinate follow-up. BP Connect doubled the likelihood of a guideline-recommended follow-up in primary care within 30 days. All patients benefited, and disparities decreased. BP Connect has had 1100 downloads, and both BP and Quit Connect programs are endorsed by the Centers for Disease Control and Prevention and Million Hearts.
How Do These Programs Affect Clinical Practice?
We developed these interventions with a health system engineer, and we time stamped everything. Part of the sustainability of this model is that it fits within a regular workflow. As a practicing rheumatologist, I understand that time is a precious commodity.
The interventions are in partnership with frontline staff. We’ve received feedback that they feel pride participating in these initiatives. They can say, because of me, 30 patients followed up last month for high BP, or 10 patients took a referral to the Quitline last year. We celebrate these accomplishments with the staff.
What Are the Next Steps for These Programs?
Public-facing toolkits for both BP and Quit Connect programs are available online. We have implemented [these programs] in a rural setting, in an urban setting, in Milwaukee and in Atlanta, and we are looking in the future to do a larger, multistate implementation study. If folks are interested, we’d love to partner with them to look at disseminating this further.
A version of this article appeared on Medscape.com.
FDA OKs Subcutaneous Atezolizumab Formulation for Multiple Cancer Indications
Approved indications include non–small cell lung cancer (NSCLC), SCLC, hepatocellular carcinoma, melanoma, and alveolar soft part sarcoma. Specific indications are available with the full prescribing information at Drugs@FDA.
This is the first programmed death–ligand 1 inhibitor to gain approval for subcutaneous administration.
“This approval represents a significant option to improve the patient experience,” Ann Fish-Steagall, RN, Senior Vice President of Patient Services at the LUNGevity Foundation stated in a Genentech press release.
Subcutaneous atezolizumab and hyaluronidase-tqjs was evaluated in the open-label, randomized IMscin001 trial of 371 adult patients with locally advanced or metastatic NSCLC who were not previously exposed to cancer immunotherapy and who had disease progression following treatment with platinum-based chemotherapy. Patients were randomized 2:1 to receive subcutaneous or IV administration until disease progression or unacceptable toxicity.
Atezolizumab exposure, the primary outcome measure of the study, met the lower limit of geometric mean ratio above the prespecified threshold of 0.8 (cycle 1C trough, 1.05; area under the curve for days 0-21, 0.87).
No notable differences were observed in overall response rate, progression-free survival, or overall survival between the two formulations, according to the FDA approval notice.
The confirmed overall response rate was 9% in the subcutaneous arm and 8% intravenous arm.
Adverse events of any grade occurring in at least 10% of patients were fatigue, musculoskeletal pain, cough, dyspnea, and decreased appetite.
The recommended dose for subcutaneous injection is one 15 mL injection, which contains 1875 mg of atezolizumab and 30,000 units of hyaluronidase.
Injections should be administered in the thigh over approximately 7 minutes every 3 weeks. By contrast, IV administration generally takes 30-60 minutes.
A version of this article first appeared on Medscape.com.
Approved indications include non–small cell lung cancer (NSCLC), SCLC, hepatocellular carcinoma, melanoma, and alveolar soft part sarcoma. Specific indications are available with the full prescribing information at Drugs@FDA.
This is the first programmed death–ligand 1 inhibitor to gain approval for subcutaneous administration.
“This approval represents a significant option to improve the patient experience,” Ann Fish-Steagall, RN, Senior Vice President of Patient Services at the LUNGevity Foundation stated in a Genentech press release.
Subcutaneous atezolizumab and hyaluronidase-tqjs was evaluated in the open-label, randomized IMscin001 trial of 371 adult patients with locally advanced or metastatic NSCLC who were not previously exposed to cancer immunotherapy and who had disease progression following treatment with platinum-based chemotherapy. Patients were randomized 2:1 to receive subcutaneous or IV administration until disease progression or unacceptable toxicity.
Atezolizumab exposure, the primary outcome measure of the study, met the lower limit of geometric mean ratio above the prespecified threshold of 0.8 (cycle 1C trough, 1.05; area under the curve for days 0-21, 0.87).
No notable differences were observed in overall response rate, progression-free survival, or overall survival between the two formulations, according to the FDA approval notice.
The confirmed overall response rate was 9% in the subcutaneous arm and 8% intravenous arm.
Adverse events of any grade occurring in at least 10% of patients were fatigue, musculoskeletal pain, cough, dyspnea, and decreased appetite.
The recommended dose for subcutaneous injection is one 15 mL injection, which contains 1875 mg of atezolizumab and 30,000 units of hyaluronidase.
Injections should be administered in the thigh over approximately 7 minutes every 3 weeks. By contrast, IV administration generally takes 30-60 minutes.
A version of this article first appeared on Medscape.com.
Approved indications include non–small cell lung cancer (NSCLC), SCLC, hepatocellular carcinoma, melanoma, and alveolar soft part sarcoma. Specific indications are available with the full prescribing information at Drugs@FDA.
This is the first programmed death–ligand 1 inhibitor to gain approval for subcutaneous administration.
“This approval represents a significant option to improve the patient experience,” Ann Fish-Steagall, RN, Senior Vice President of Patient Services at the LUNGevity Foundation stated in a Genentech press release.
Subcutaneous atezolizumab and hyaluronidase-tqjs was evaluated in the open-label, randomized IMscin001 trial of 371 adult patients with locally advanced or metastatic NSCLC who were not previously exposed to cancer immunotherapy and who had disease progression following treatment with platinum-based chemotherapy. Patients were randomized 2:1 to receive subcutaneous or IV administration until disease progression or unacceptable toxicity.
Atezolizumab exposure, the primary outcome measure of the study, met the lower limit of geometric mean ratio above the prespecified threshold of 0.8 (cycle 1C trough, 1.05; area under the curve for days 0-21, 0.87).
No notable differences were observed in overall response rate, progression-free survival, or overall survival between the two formulations, according to the FDA approval notice.
The confirmed overall response rate was 9% in the subcutaneous arm and 8% intravenous arm.
Adverse events of any grade occurring in at least 10% of patients were fatigue, musculoskeletal pain, cough, dyspnea, and decreased appetite.
The recommended dose for subcutaneous injection is one 15 mL injection, which contains 1875 mg of atezolizumab and 30,000 units of hyaluronidase.
Injections should be administered in the thigh over approximately 7 minutes every 3 weeks. By contrast, IV administration generally takes 30-60 minutes.
A version of this article first appeared on Medscape.com.
Osimertinib/Savolitinib Combo Shows Promise in NSCLC
according to results of the phase 2 FLOWERS study.
Compared with EGFR inhibitor osimertinib alone, the combination demonstrated a clinically meaningful improvement in the objective response rate — the study’s primary endpoint — with a positive trend in progression-free survival and a manageable safety profile.
About 30% patients with EGFR-mutated NSCLC fail to respond well to EGFR–tyrosine kinase inhibitors (TKIs), explained Jin-Ji Yang, MD, with Guangdong Lung Cancer Institute, Guangzhou, China, who reported the study results at the annual meeting of the World Conference on Lung Cancer.
Data suggested that de novo MET amplification occurs in up to 5% patients with treatment-naive, EGFR-mutated advanced NSCLC, and MET overexpression occurs in up to 15% these patients.
Coexistence of EGFR mutation and MET amplification/overexpression reduces sensitivity to EGFR-TKI therapy “and is likely the mechanism for mediating primary resistance to first-line EGFR-TKI monotherapy,” Dr. Yang explained in her presentation.
Osimertinib is a third-generation EGFR-TKI recommended as the first-line treatment for EGFR-mutant advanced NSCLC. Savolitinib is a highly selective MET-TKI which has demonstrated antitumor activity in various cancers with MET alterations.
The FLOWERS study is the first to test whether combining the two agents could improve efficacy and overcome MET-driven primary resistance in these patients.
The phase 2 study enrolled 44 treatment-naive patients with de novo MET-aberrant, EGFR-mutant, stage IIIB-IV NSCLC; 23 were randomly allocated to receive oral osimertinib (80 mg once daily) alone and 21 to receive oral osimertinib (80 mg once daily) plus savolitinib (300 mg twice daily).
At a median follow-up of 8.2 months, the objective response rate was 60.9% with osimertinib monotherapy vs 90.5% with combination therapy. The disease control rate was also better with the combination therapy than with monotherapy (95.2% vs 87%).
Median duration of response (not yet mature) was 8.4 months with monotherapy vs 18.6 months with combination therapy.
Preliminary progression-free survival data also showed a trend in favor of combination therapy over monotherapy (a median of 19.3 vs 9.3 months; hazard ratio, 0.59).
Most treatment-related adverse events were grade 1 or 2, and there were no fatal adverse events.
Treatment-related adverse events of grade 3 or higher were more common with combination therapy (57.1% vs 8.7%). The most common events with monotherapy were diarrhea (56.5%), rash (52.2%), and pruritus (43.5%) and with dual therapy were rash (52.4%), thrombocytopenia (52.4%), and peripheral edema (42.9%).
The results showed that the combination therapy has the potential to become a first-line treatment option for patients who do not respond well to EGFR-TKIs alone, Dr. Yang said in a press release.
Discussant for the study Paul Paik, MD, thoracic oncologist at Memorial Sloan Kettering Cancer Center in New York City, said this study “adds to data suggesting high MET expression might be a poor prognostic or predictive marker, the outcomes of which are improved with MET inhibition.”
He cautioned, however, that there appears to be “quality of life, side-effect trade-offs with dual MET plus EGFR TKI upfront.”
Dr. Paik said he looks forward to results from FLOWERS on serial circulating tumor DNA and formal androgen receptor testing, which “might aid in further assessing clonality and characterizing MET as a co-driver in this setting.”
The study was funded by AstraZeneca China. Dr. Yang had no disclosures. Dr. Paik disclosed relationships with EMD Serono, Bicara, Novartis, and Summit.
A version of this article first appeared on Medscape.com.
according to results of the phase 2 FLOWERS study.
Compared with EGFR inhibitor osimertinib alone, the combination demonstrated a clinically meaningful improvement in the objective response rate — the study’s primary endpoint — with a positive trend in progression-free survival and a manageable safety profile.
About 30% patients with EGFR-mutated NSCLC fail to respond well to EGFR–tyrosine kinase inhibitors (TKIs), explained Jin-Ji Yang, MD, with Guangdong Lung Cancer Institute, Guangzhou, China, who reported the study results at the annual meeting of the World Conference on Lung Cancer.
Data suggested that de novo MET amplification occurs in up to 5% patients with treatment-naive, EGFR-mutated advanced NSCLC, and MET overexpression occurs in up to 15% these patients.
Coexistence of EGFR mutation and MET amplification/overexpression reduces sensitivity to EGFR-TKI therapy “and is likely the mechanism for mediating primary resistance to first-line EGFR-TKI monotherapy,” Dr. Yang explained in her presentation.
Osimertinib is a third-generation EGFR-TKI recommended as the first-line treatment for EGFR-mutant advanced NSCLC. Savolitinib is a highly selective MET-TKI which has demonstrated antitumor activity in various cancers with MET alterations.
The FLOWERS study is the first to test whether combining the two agents could improve efficacy and overcome MET-driven primary resistance in these patients.
The phase 2 study enrolled 44 treatment-naive patients with de novo MET-aberrant, EGFR-mutant, stage IIIB-IV NSCLC; 23 were randomly allocated to receive oral osimertinib (80 mg once daily) alone and 21 to receive oral osimertinib (80 mg once daily) plus savolitinib (300 mg twice daily).
At a median follow-up of 8.2 months, the objective response rate was 60.9% with osimertinib monotherapy vs 90.5% with combination therapy. The disease control rate was also better with the combination therapy than with monotherapy (95.2% vs 87%).
Median duration of response (not yet mature) was 8.4 months with monotherapy vs 18.6 months with combination therapy.
Preliminary progression-free survival data also showed a trend in favor of combination therapy over monotherapy (a median of 19.3 vs 9.3 months; hazard ratio, 0.59).
Most treatment-related adverse events were grade 1 or 2, and there were no fatal adverse events.
Treatment-related adverse events of grade 3 or higher were more common with combination therapy (57.1% vs 8.7%). The most common events with monotherapy were diarrhea (56.5%), rash (52.2%), and pruritus (43.5%) and with dual therapy were rash (52.4%), thrombocytopenia (52.4%), and peripheral edema (42.9%).
The results showed that the combination therapy has the potential to become a first-line treatment option for patients who do not respond well to EGFR-TKIs alone, Dr. Yang said in a press release.
Discussant for the study Paul Paik, MD, thoracic oncologist at Memorial Sloan Kettering Cancer Center in New York City, said this study “adds to data suggesting high MET expression might be a poor prognostic or predictive marker, the outcomes of which are improved with MET inhibition.”
He cautioned, however, that there appears to be “quality of life, side-effect trade-offs with dual MET plus EGFR TKI upfront.”
Dr. Paik said he looks forward to results from FLOWERS on serial circulating tumor DNA and formal androgen receptor testing, which “might aid in further assessing clonality and characterizing MET as a co-driver in this setting.”
The study was funded by AstraZeneca China. Dr. Yang had no disclosures. Dr. Paik disclosed relationships with EMD Serono, Bicara, Novartis, and Summit.
A version of this article first appeared on Medscape.com.
according to results of the phase 2 FLOWERS study.
Compared with EGFR inhibitor osimertinib alone, the combination demonstrated a clinically meaningful improvement in the objective response rate — the study’s primary endpoint — with a positive trend in progression-free survival and a manageable safety profile.
About 30% patients with EGFR-mutated NSCLC fail to respond well to EGFR–tyrosine kinase inhibitors (TKIs), explained Jin-Ji Yang, MD, with Guangdong Lung Cancer Institute, Guangzhou, China, who reported the study results at the annual meeting of the World Conference on Lung Cancer.
Data suggested that de novo MET amplification occurs in up to 5% patients with treatment-naive, EGFR-mutated advanced NSCLC, and MET overexpression occurs in up to 15% these patients.
Coexistence of EGFR mutation and MET amplification/overexpression reduces sensitivity to EGFR-TKI therapy “and is likely the mechanism for mediating primary resistance to first-line EGFR-TKI monotherapy,” Dr. Yang explained in her presentation.
Osimertinib is a third-generation EGFR-TKI recommended as the first-line treatment for EGFR-mutant advanced NSCLC. Savolitinib is a highly selective MET-TKI which has demonstrated antitumor activity in various cancers with MET alterations.
The FLOWERS study is the first to test whether combining the two agents could improve efficacy and overcome MET-driven primary resistance in these patients.
The phase 2 study enrolled 44 treatment-naive patients with de novo MET-aberrant, EGFR-mutant, stage IIIB-IV NSCLC; 23 were randomly allocated to receive oral osimertinib (80 mg once daily) alone and 21 to receive oral osimertinib (80 mg once daily) plus savolitinib (300 mg twice daily).
At a median follow-up of 8.2 months, the objective response rate was 60.9% with osimertinib monotherapy vs 90.5% with combination therapy. The disease control rate was also better with the combination therapy than with monotherapy (95.2% vs 87%).
Median duration of response (not yet mature) was 8.4 months with monotherapy vs 18.6 months with combination therapy.
Preliminary progression-free survival data also showed a trend in favor of combination therapy over monotherapy (a median of 19.3 vs 9.3 months; hazard ratio, 0.59).
Most treatment-related adverse events were grade 1 or 2, and there were no fatal adverse events.
Treatment-related adverse events of grade 3 or higher were more common with combination therapy (57.1% vs 8.7%). The most common events with monotherapy were diarrhea (56.5%), rash (52.2%), and pruritus (43.5%) and with dual therapy were rash (52.4%), thrombocytopenia (52.4%), and peripheral edema (42.9%).
The results showed that the combination therapy has the potential to become a first-line treatment option for patients who do not respond well to EGFR-TKIs alone, Dr. Yang said in a press release.
Discussant for the study Paul Paik, MD, thoracic oncologist at Memorial Sloan Kettering Cancer Center in New York City, said this study “adds to data suggesting high MET expression might be a poor prognostic or predictive marker, the outcomes of which are improved with MET inhibition.”
He cautioned, however, that there appears to be “quality of life, side-effect trade-offs with dual MET plus EGFR TKI upfront.”
Dr. Paik said he looks forward to results from FLOWERS on serial circulating tumor DNA and formal androgen receptor testing, which “might aid in further assessing clonality and characterizing MET as a co-driver in this setting.”
The study was funded by AstraZeneca China. Dr. Yang had no disclosures. Dr. Paik disclosed relationships with EMD Serono, Bicara, Novartis, and Summit.
A version of this article first appeared on Medscape.com.
FROM WCLC 2024
Majority of Hospitalized Patients With COPD Misuse Inhalers
Approximately two thirds of hospitalized adults with chronic obstructive pulmonary disease (COPD) received suboptimal treatment with inhalers, mainly resulting from errors, based on data from 96 individuals.
“Numerous studies have highlighted the significant issue of improper inhaler use in outpatient settings, but the extent of this problem within hospital settings remains poorly documented,” said lead author Gaël Grandmaison, MD, of the University of Fribourg in Switzerland, in an interview.
“This gap in knowledge is concerning, especially considering that several factors associated with suboptimal inhaler use, such as improper inhalation techniques, insufficient inspiratory flow, or the use of inhalers that are not suited to the patient’s specific characteristics, are associated with poorer disease control, more frequent exacerbations, and increased costs,” Dr. Grandmaison said.
To better characterize the prevalence of and factors associated with inhaler misuse in hospitalized patients with COPD, the researchers reviewed data from consecutive patients with COPD who were hospitalized in the general internal medicine department of a single institution between August 2022 and April 2023. Patients were assessed for peak inspiratory flow (PIF) and inhaler technique.
The primary outcome was the proportion of misused inhalers, which was defined as any inhaler used with either insufficient PIF and/or a critical error. The mean age of the patients was 71.6 years, 63% were men, and 67% were hospitalized for COPD exacerbations. Patients used 3.0 inhalers on average.
The study included 96 patients and 160 inhalers that were assessed at hospital admission. Overall, 111 were misused. Of those misused, 105 were associated with a critical error in the inhalation technique, and 22 were used with an insufficient PIF. After an episode of misuse, patients received targeted teaching on correct use that was repeated until they performed the technique without errors.
The percentage of inhaler misuse decreased over the course of the teaching sessions. The proportion of inhaler misuse decreased to 20.6%, 9.4%, and 5.6% after one, two, and three sessions, respectively.
“The inhalation technique was classified as ‘non-teachable’ if the patient continued to exhibit critical errors despite receiving three repetitions of the instructions,” the researchers wrote. Factors associated with inhaler misuse included cognitive disorders, fine motor disorders, poor coordination between inhaler activation and aspiration, and the inability to hold one’s breath.
Overall, the proportion of misused inhalers did not vary by age or gender. In an analysis at the patient level, 79 patients used at least one misused inhaler, 78 used at least one inhaler with a critical error, and 21 used inhalers with insufficient PIF.
“This study is particularly timely because reasons for hospitalization, such as COPD exacerbations or confusional states, could exacerbate the problem, leading to a potentially higher prevalence of suboptimal inhaler use compared to outpatient settings,” Dr. Grandmaison said.
The researchers also examined secondary outcomes including the prevalence of inhalers that were not suited to them and the number of patients using at least one misused inhaler.
The study findings confirm that suboptimal inhaler use is a significant problem in the hospital setting and provide new insights into the specific reasons behind this suboptimal usage, Dr. Grandmaison said.
“In the majority of cases, poor inhalation technique is the primary cause, which can generally be corrected through targeted therapeutic education,” she said. However, the study also revealed that 20% of patients are unable to use at least one of their inhalers correctly because of insufficient inspiratory force. Another 10% struggle despite receiving proper instruction, often because of cognitive impairments or difficulty with fine motor skills.
The results underscore the need for a comprehensive approach to inhaler use in hospitalized patients that combines continuous therapeutic education with personalized assessment in order to improve technique and subsequently enhance patient outcomes, she said.
Changing Clinical Practice
“As hospital physicians, these findings have led us to systematically evaluate the inhalers used by COPD patients, regardless of their reason for hospitalization,” Dr. Grandmaison said. Consequently, the hospital has implemented an assessment of inhaler use among patients that includes a review of techniques, an evaluation of the appropriateness of the inhaler prescribed, and an algorithm to help clinicians choose the most appropriate inhaler. Since its inception, the targeted intervention has significantly reduced improper inhaler use at discharge.
Limitations and Next Steps
The findings were limited by several factors including the possible underreporting of misuse caused by inadequate PIF, a lack of consensus on what constitutes a critical error, and the small sample of patients from a single center.
Despite these limitations, the study adds to the understanding of improper inhaler use in the hospital setting, Dr. Grandmaison said. “Our subsequent research demonstrated that a systematic evaluation of inhalers, combined with therapeutic education and an algorithm to select an inhaler suited to the patient’s characteristics, significantly reduces the number of improperly used inhalers at hospital discharge.”
However, several areas require further investigation, said Dr. Grandmaison. The most effective methods and frequency for teaching inhalation techniques must be defined, and more research is needed to understand the factors influencing PIF and its progression over the course of disease. The next steps for the current research are to evaluate the impact of the intervention on long-term symptom control and disease progression.
“Moreover, adapting the strategy developed in our institution for use in outpatient care is a priority, and multicenter studies would be valuable in validating these findings across different hospital settings,” she added.
In-Hospital Inhaler Education Falls Short
“Poor inhaler technique can lead to ineffective inhaler use and suboptimal treatment of COPD,” said Arianne K. Baldomero, MD, a pulmonologist and assistant professor of medicine at the University of Minnesota, Minneapolis, in an interview.
“The results from this study are consistent with prior studies showing a high prevalence of suboptimal inhaler use,” said Dr. Baldomero, who was not involved in the current study.
“The investigators also found that therapeutic education led to a significant reduction in the number of critical errors,” she said.
“What is surprising is that it can take up to three lessons to reduce this critical error down to 3.8%,” Dr. Baldomero said. “In most real-world clinic settings, many patients are not taught how to properly use inhalers, and many patients who receive inhaler technique education only receive instructions once.”
Dr. Baldomero’s takeaway from the study is that teaching patients to properly use their inhalers is critical, but that this education may need to be repeated multiple times. The findings also remind clinicians that some types of inhaler delivery are not suited for patients who cannot generate adequate respiratory flow.
Looking ahead, a larger sample size is needed to better identify which patients need additional teaching, Dr. Baldomero said. Also, the current study is limited by the focus on hospitalized patients. “I am interested in learning about the characteristics of patients in the outpatient settings who would benefit from additional inhaler teaching,” she noted.
The study was supported by a grant from the Hospital of Fribourg in Switzerland. The researchers had no financial conflicts to disclose. Dr. Baldomero had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
Approximately two thirds of hospitalized adults with chronic obstructive pulmonary disease (COPD) received suboptimal treatment with inhalers, mainly resulting from errors, based on data from 96 individuals.
“Numerous studies have highlighted the significant issue of improper inhaler use in outpatient settings, but the extent of this problem within hospital settings remains poorly documented,” said lead author Gaël Grandmaison, MD, of the University of Fribourg in Switzerland, in an interview.
“This gap in knowledge is concerning, especially considering that several factors associated with suboptimal inhaler use, such as improper inhalation techniques, insufficient inspiratory flow, or the use of inhalers that are not suited to the patient’s specific characteristics, are associated with poorer disease control, more frequent exacerbations, and increased costs,” Dr. Grandmaison said.
To better characterize the prevalence of and factors associated with inhaler misuse in hospitalized patients with COPD, the researchers reviewed data from consecutive patients with COPD who were hospitalized in the general internal medicine department of a single institution between August 2022 and April 2023. Patients were assessed for peak inspiratory flow (PIF) and inhaler technique.
The primary outcome was the proportion of misused inhalers, which was defined as any inhaler used with either insufficient PIF and/or a critical error. The mean age of the patients was 71.6 years, 63% were men, and 67% were hospitalized for COPD exacerbations. Patients used 3.0 inhalers on average.
The study included 96 patients and 160 inhalers that were assessed at hospital admission. Overall, 111 were misused. Of those misused, 105 were associated with a critical error in the inhalation technique, and 22 were used with an insufficient PIF. After an episode of misuse, patients received targeted teaching on correct use that was repeated until they performed the technique without errors.
The percentage of inhaler misuse decreased over the course of the teaching sessions. The proportion of inhaler misuse decreased to 20.6%, 9.4%, and 5.6% after one, two, and three sessions, respectively.
“The inhalation technique was classified as ‘non-teachable’ if the patient continued to exhibit critical errors despite receiving three repetitions of the instructions,” the researchers wrote. Factors associated with inhaler misuse included cognitive disorders, fine motor disorders, poor coordination between inhaler activation and aspiration, and the inability to hold one’s breath.
Overall, the proportion of misused inhalers did not vary by age or gender. In an analysis at the patient level, 79 patients used at least one misused inhaler, 78 used at least one inhaler with a critical error, and 21 used inhalers with insufficient PIF.
“This study is particularly timely because reasons for hospitalization, such as COPD exacerbations or confusional states, could exacerbate the problem, leading to a potentially higher prevalence of suboptimal inhaler use compared to outpatient settings,” Dr. Grandmaison said.
The researchers also examined secondary outcomes including the prevalence of inhalers that were not suited to them and the number of patients using at least one misused inhaler.
The study findings confirm that suboptimal inhaler use is a significant problem in the hospital setting and provide new insights into the specific reasons behind this suboptimal usage, Dr. Grandmaison said.
“In the majority of cases, poor inhalation technique is the primary cause, which can generally be corrected through targeted therapeutic education,” she said. However, the study also revealed that 20% of patients are unable to use at least one of their inhalers correctly because of insufficient inspiratory force. Another 10% struggle despite receiving proper instruction, often because of cognitive impairments or difficulty with fine motor skills.
The results underscore the need for a comprehensive approach to inhaler use in hospitalized patients that combines continuous therapeutic education with personalized assessment in order to improve technique and subsequently enhance patient outcomes, she said.
Changing Clinical Practice
“As hospital physicians, these findings have led us to systematically evaluate the inhalers used by COPD patients, regardless of their reason for hospitalization,” Dr. Grandmaison said. Consequently, the hospital has implemented an assessment of inhaler use among patients that includes a review of techniques, an evaluation of the appropriateness of the inhaler prescribed, and an algorithm to help clinicians choose the most appropriate inhaler. Since its inception, the targeted intervention has significantly reduced improper inhaler use at discharge.
Limitations and Next Steps
The findings were limited by several factors including the possible underreporting of misuse caused by inadequate PIF, a lack of consensus on what constitutes a critical error, and the small sample of patients from a single center.
Despite these limitations, the study adds to the understanding of improper inhaler use in the hospital setting, Dr. Grandmaison said. “Our subsequent research demonstrated that a systematic evaluation of inhalers, combined with therapeutic education and an algorithm to select an inhaler suited to the patient’s characteristics, significantly reduces the number of improperly used inhalers at hospital discharge.”
However, several areas require further investigation, said Dr. Grandmaison. The most effective methods and frequency for teaching inhalation techniques must be defined, and more research is needed to understand the factors influencing PIF and its progression over the course of disease. The next steps for the current research are to evaluate the impact of the intervention on long-term symptom control and disease progression.
“Moreover, adapting the strategy developed in our institution for use in outpatient care is a priority, and multicenter studies would be valuable in validating these findings across different hospital settings,” she added.
In-Hospital Inhaler Education Falls Short
“Poor inhaler technique can lead to ineffective inhaler use and suboptimal treatment of COPD,” said Arianne K. Baldomero, MD, a pulmonologist and assistant professor of medicine at the University of Minnesota, Minneapolis, in an interview.
“The results from this study are consistent with prior studies showing a high prevalence of suboptimal inhaler use,” said Dr. Baldomero, who was not involved in the current study.
“The investigators also found that therapeutic education led to a significant reduction in the number of critical errors,” she said.
“What is surprising is that it can take up to three lessons to reduce this critical error down to 3.8%,” Dr. Baldomero said. “In most real-world clinic settings, many patients are not taught how to properly use inhalers, and many patients who receive inhaler technique education only receive instructions once.”
Dr. Baldomero’s takeaway from the study is that teaching patients to properly use their inhalers is critical, but that this education may need to be repeated multiple times. The findings also remind clinicians that some types of inhaler delivery are not suited for patients who cannot generate adequate respiratory flow.
Looking ahead, a larger sample size is needed to better identify which patients need additional teaching, Dr. Baldomero said. Also, the current study is limited by the focus on hospitalized patients. “I am interested in learning about the characteristics of patients in the outpatient settings who would benefit from additional inhaler teaching,” she noted.
The study was supported by a grant from the Hospital of Fribourg in Switzerland. The researchers had no financial conflicts to disclose. Dr. Baldomero had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
Approximately two thirds of hospitalized adults with chronic obstructive pulmonary disease (COPD) received suboptimal treatment with inhalers, mainly resulting from errors, based on data from 96 individuals.
“Numerous studies have highlighted the significant issue of improper inhaler use in outpatient settings, but the extent of this problem within hospital settings remains poorly documented,” said lead author Gaël Grandmaison, MD, of the University of Fribourg in Switzerland, in an interview.
“This gap in knowledge is concerning, especially considering that several factors associated with suboptimal inhaler use, such as improper inhalation techniques, insufficient inspiratory flow, or the use of inhalers that are not suited to the patient’s specific characteristics, are associated with poorer disease control, more frequent exacerbations, and increased costs,” Dr. Grandmaison said.
To better characterize the prevalence of and factors associated with inhaler misuse in hospitalized patients with COPD, the researchers reviewed data from consecutive patients with COPD who were hospitalized in the general internal medicine department of a single institution between August 2022 and April 2023. Patients were assessed for peak inspiratory flow (PIF) and inhaler technique.
The primary outcome was the proportion of misused inhalers, which was defined as any inhaler used with either insufficient PIF and/or a critical error. The mean age of the patients was 71.6 years, 63% were men, and 67% were hospitalized for COPD exacerbations. Patients used 3.0 inhalers on average.
The study included 96 patients and 160 inhalers that were assessed at hospital admission. Overall, 111 were misused. Of those misused, 105 were associated with a critical error in the inhalation technique, and 22 were used with an insufficient PIF. After an episode of misuse, patients received targeted teaching on correct use that was repeated until they performed the technique without errors.
The percentage of inhaler misuse decreased over the course of the teaching sessions. The proportion of inhaler misuse decreased to 20.6%, 9.4%, and 5.6% after one, two, and three sessions, respectively.
“The inhalation technique was classified as ‘non-teachable’ if the patient continued to exhibit critical errors despite receiving three repetitions of the instructions,” the researchers wrote. Factors associated with inhaler misuse included cognitive disorders, fine motor disorders, poor coordination between inhaler activation and aspiration, and the inability to hold one’s breath.
Overall, the proportion of misused inhalers did not vary by age or gender. In an analysis at the patient level, 79 patients used at least one misused inhaler, 78 used at least one inhaler with a critical error, and 21 used inhalers with insufficient PIF.
“This study is particularly timely because reasons for hospitalization, such as COPD exacerbations or confusional states, could exacerbate the problem, leading to a potentially higher prevalence of suboptimal inhaler use compared to outpatient settings,” Dr. Grandmaison said.
The researchers also examined secondary outcomes including the prevalence of inhalers that were not suited to them and the number of patients using at least one misused inhaler.
The study findings confirm that suboptimal inhaler use is a significant problem in the hospital setting and provide new insights into the specific reasons behind this suboptimal usage, Dr. Grandmaison said.
“In the majority of cases, poor inhalation technique is the primary cause, which can generally be corrected through targeted therapeutic education,” she said. However, the study also revealed that 20% of patients are unable to use at least one of their inhalers correctly because of insufficient inspiratory force. Another 10% struggle despite receiving proper instruction, often because of cognitive impairments or difficulty with fine motor skills.
The results underscore the need for a comprehensive approach to inhaler use in hospitalized patients that combines continuous therapeutic education with personalized assessment in order to improve technique and subsequently enhance patient outcomes, she said.
Changing Clinical Practice
“As hospital physicians, these findings have led us to systematically evaluate the inhalers used by COPD patients, regardless of their reason for hospitalization,” Dr. Grandmaison said. Consequently, the hospital has implemented an assessment of inhaler use among patients that includes a review of techniques, an evaluation of the appropriateness of the inhaler prescribed, and an algorithm to help clinicians choose the most appropriate inhaler. Since its inception, the targeted intervention has significantly reduced improper inhaler use at discharge.
Limitations and Next Steps
The findings were limited by several factors including the possible underreporting of misuse caused by inadequate PIF, a lack of consensus on what constitutes a critical error, and the small sample of patients from a single center.
Despite these limitations, the study adds to the understanding of improper inhaler use in the hospital setting, Dr. Grandmaison said. “Our subsequent research demonstrated that a systematic evaluation of inhalers, combined with therapeutic education and an algorithm to select an inhaler suited to the patient’s characteristics, significantly reduces the number of improperly used inhalers at hospital discharge.”
However, several areas require further investigation, said Dr. Grandmaison. The most effective methods and frequency for teaching inhalation techniques must be defined, and more research is needed to understand the factors influencing PIF and its progression over the course of disease. The next steps for the current research are to evaluate the impact of the intervention on long-term symptom control and disease progression.
“Moreover, adapting the strategy developed in our institution for use in outpatient care is a priority, and multicenter studies would be valuable in validating these findings across different hospital settings,” she added.
In-Hospital Inhaler Education Falls Short
“Poor inhaler technique can lead to ineffective inhaler use and suboptimal treatment of COPD,” said Arianne K. Baldomero, MD, a pulmonologist and assistant professor of medicine at the University of Minnesota, Minneapolis, in an interview.
“The results from this study are consistent with prior studies showing a high prevalence of suboptimal inhaler use,” said Dr. Baldomero, who was not involved in the current study.
“The investigators also found that therapeutic education led to a significant reduction in the number of critical errors,” she said.
“What is surprising is that it can take up to three lessons to reduce this critical error down to 3.8%,” Dr. Baldomero said. “In most real-world clinic settings, many patients are not taught how to properly use inhalers, and many patients who receive inhaler technique education only receive instructions once.”
Dr. Baldomero’s takeaway from the study is that teaching patients to properly use their inhalers is critical, but that this education may need to be repeated multiple times. The findings also remind clinicians that some types of inhaler delivery are not suited for patients who cannot generate adequate respiratory flow.
Looking ahead, a larger sample size is needed to better identify which patients need additional teaching, Dr. Baldomero said. Also, the current study is limited by the focus on hospitalized patients. “I am interested in learning about the characteristics of patients in the outpatient settings who would benefit from additional inhaler teaching,” she noted.
The study was supported by a grant from the Hospital of Fribourg in Switzerland. The researchers had no financial conflicts to disclose. Dr. Baldomero had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
FROM CHRONIC OBSTRUCTIVE PULMONARY DISEASES