User login
Assessing the Effectiveness of Knowledge-Based Interventions in Increasing Skin Cancer Awareness, Knowledge, and Protective Behaviors in Skin of Color Populations
Malignant melanoma, basal cell carcinoma, and squamous cell carcinoma account for approximately 40% of all neoplasms among the white population in the United States. Skin cancer is the most common malignancy in the United States.1 However, despite this occurrence, there are limited data regarding skin cancer in individuals with skin of color (SOC). The 5-year survival rates for melanoma are 58.2% for black individuals, 69.7% for Hispanics, and 70.9% for Asians compared to 79.8% for white individuals in the United States.2 Even though SOC populations have lower incidences of skin cancer—melanoma, basal cell carcinoma, and squamous cell carcinoma—they exhibit higher death rates.3-7 Nonetheless, no specific guidelines exist to address sun exposure and safety habits in SOC populations.6,8 Furthermore, current demographics suggest that by the year 2050, approximately half of the US population will be nonwhite.4 Paradoxically, despite having increased sun protection from greater amounts of melanin in their skin, black individuals are more likely to present with advanced-stage melanoma (eg, stage III/IV) compared to white individuals.8-12 Furthermore, those of nonwhite populations are more likely to present with more advanced stages of acral lentiginous melanomas than white individuals.13,14 Hispanics also face an increasing incidence of more invasive acral lentiginous melanomas.15 Overall, SOC patients have the poorest skin cancer prognosis, and the data suggest that the reason for this paradox is delayed diagnosis.1
Although skin cancer is largely a preventable condition, the literature suggests that lack of awareness of melanoma among ethnic minorities is one of the main reasons for their poor skin cancer prognosis.16 This lack of awareness decreases the likelihood that an SOC patient would be alert to early detection of cancerous changes.17 Because educating at-risk SOC populations is key to decreasing skin cancer risk, this study focused on determining the efficacy of major knowledge-based interventions conducted to date.1 Overall, we sought to answer the question, do knowledge-based interventions increase skin cancer awareness, knowledge, and protective behavior among people of color?
Methods
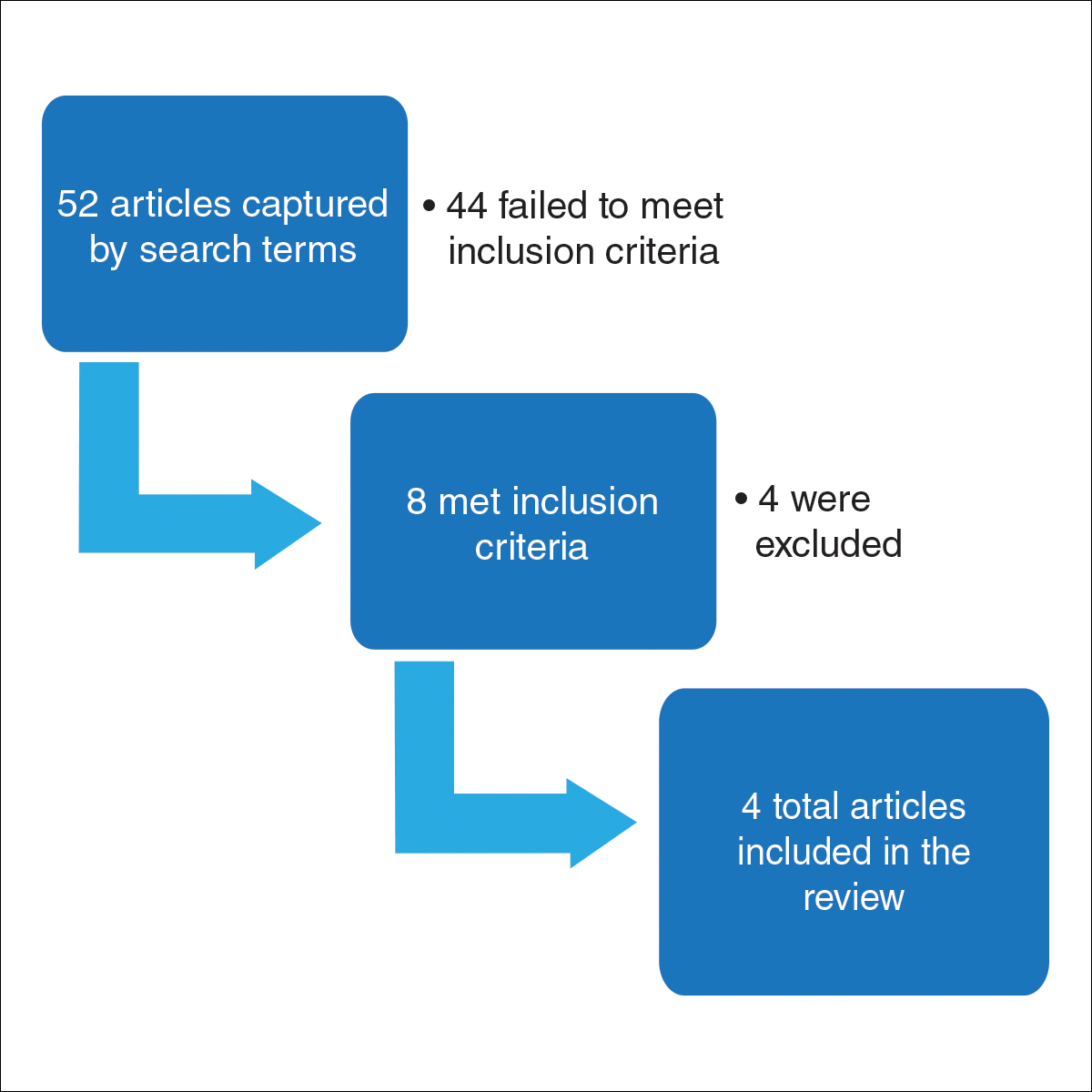
For this review, the Cochrane method of analysis was used to conduct a thorough search of PubMed articles indexed for MEDLINE (1994-2016), as well as a search of CINAHL (1997-2016), PsycINFO (1999-2016), and Web of Science (1965-2016), using a combination of more than 100 search terms including but not limited to skin cancer, skin of color, intervention, and ethnic skin. The search yielded a total of 52 articles (Figure). Following review, only 8 articles met inclusion criteria, which were as follows: (1) study was related to skin cancer in SOC patients, which included an intervention to increase skin cancer awareness and knowledge; (2) study included adult participants or adolescents aged 12 to 18 years; (3) study was written in English; and (4) study was published in a peer-reviewed journal. Of the remaining 8 articles, 4 were excluded due to the following criteria: (1) study failed to provide both preintervention and postintervention data, (2) study failed to provide quantitative data, and (3) study included participants who worked as health care professionals or ancillary staff. As a result, a total of 4 articles were analyzed and discussed in this review (Table).
Results
Robinson et al18 conducted 12 focus groups with 120 total participants (40 black, 40 Asian, and 40 Hispanic patients). Participants engaged in a 2-hour tape-recorded focus group with a moderator guide on melanoma and skin cancer. Furthermore, they also were asked to assess skin cancer risk in 5 celebrities with different skin tones. The statistically significant preintervention results of the study (χ2=4.6, P<.001) were as follows: only 2%, 4%, and 14% correctly reported that celebrities with a very fair skin type, a fair skin type, and very dark skin type, respectively, could get sunburn, compared to 75%, 76%, and 62% post-intervention. Additionally, prior to intervention, 14% of the study population believed that dark brown skin type could get sunburn compared to 62% of the same group postintervention. This study demonstrated that the intervention helped SOC patients better identify their ability to get sunburn and identify their skin cancer risk.18
Hernandez et al19 used a video-based intervention in a Hispanic community, which was in contrast to the multiracial focus group intervention conducted by Robinson et al.18 Eighty Hispanic individuals were recruited from beauty salons to participate in the study. Participants watched two 3-minute videos in Spanish and completed a preintervention and postintervention survey. The first video emphasized the photoaging benefits of sun protection, while the second focused on skin cancer prevention. Preintervention surveys indicated that only 54 (68%) participants believed that fair-skinned Hispanics were at risk for skin cancer, which improved to 72 (90%) participants postintervention. Furthermore, initially only 44 (55%) participants thought those with darker skin types could develop skin cancer, but this number increased to 69 (86%) postintervention. For both questions regarding fair and dark skin, the agreement proportion was significantly different between the preeducation and posteducation videos (P<.0002 for the fair skin question and P<.0001 for the dark skin question). This study greatly increased awareness of skin cancer risk among Hispanics,19 similar to the Robinson et al18 study.
In contrast to 2-hour focus groups or 3-minute video–based interventions, a study by Kundu et al17 employed a 20-minute educational class-based intervention with both verbal and visual instruction. This study assessed the efficacy of an educational tutorial on improving awareness and early detection of melanoma in SOC individuals. Photographs were used to help participants recognize the ABCDEs of melanoma and to show examples of acral lentiginous melanomas in white individuals. A total of 71 participants completed a preintervention questionnaire, participated in a 20-minute class, and completed a postintervention questionnaire immediately after and 3 months following the class. The study population included 44 black, 15 Asian, 10 Hispanic, and 2 multiethnic participants. Knowledge that melanoma is a skin cancer increased from 83.9% to 100% immediately postintervention (P=.0001) and 97.2% at 3 months postintervention (P=.0075). Additionally, knowledge that people of color are at risk for melanoma increased from 48.4% preintervention to 82.8% immediately postintervention (P<.0001). However, only 40.8% of participants retained this knowledge at 3 months postintervention. Because only 1 participant reported a family history of skin cancer, the authors hypothesized that the reason for this loss of knowledge was that most participants were not personally affected by friends or family members with melanoma. A future study with an appropriate control group would be needed to support this claim. This study shed light on the potential of class-based interventions to increase both awareness and knowledge of skin cancer in SOC populations.17
A study by Chapman et al20 examined the effects of a sun protection educational program on increasing awareness of skin cancer in Hispanic and black middle school students in southern Los Angeles, California. It was the only study we reviewed that focused primarily on adolescents. Furthermore, it included the largest sample size (N=148) analyzed here. Students were given a preintervention questionnaire to evaluate their awareness of skin cancer and current sun-protection practices. Based on these results, the investigators devised a set of learning goals and incorporated them into an educational pamphlet. The intervention, called “Skin Teaching Day,” was a 1-day program discussing skin cancer and the importance of sun protection. Prior to the intervention, 68% of participants reported that they used sunscreen. Three months after completing the program, 80% of participants reported sunscreen use, an increase of 12% prior to the intervention. The results of this study demonstrated the unique effectiveness and potential of pamphlets in increasing sunscreen use.20
Comment
Overall, various methods of interventions such as focus groups, videos, pamphlets, and lectures improved knowledge of skin cancer risk and sun-protection behaviors in SOC populations. Furthermore, the unique differences of each study provided important insights into the successful design of an intervention.
An important characteristic of the Robinson et al18 study was the addition of photographs, which allowed participants not only to visualize different skin tones but also provided them with the opportunity to relate themselves to the photographs; by doing so, participants could effectively pick out the skin tone that best suited them. Written SOC scales are limited to mere descriptions and thus make it more difficult for participants to accurately identify the tone that best fits them. Kundu et al17 used photographs to teach skin self-examination and ABCDEs for detection of melanoma. Additionally, both studies used photographs to demonstrate examples of skin cancer.17,18 Recent evidence suggests the use of visuals can be efficacious for improving skin cancer knowledge and awareness; a study in 16 SOC kidney transplant recipients found that the addition of photographs of squamous cell carcinoma in various skin tones to a sun-protection educational pamphlet was more effective than the original pamphlet without photographs.21
In contrast to the Robinson et al18 study and Hernandez et al19 study, the Kundu et al17 study showed photographs of acral lentiginous melanomas in white patients rather than SOC patients. However, SOC populations may be less likely to relate to or identify skin changes in skin types that are different from their own. This technique was still beneficial, as acral lentiginous melanoma is the most common type of melanoma in SOC populations. Another benefit of the study was that it was the only study reviewed that included a follow-up postintervention questionnaire. Such data is useful, as it demonstrates how muchinformation is retained by participants and may be more likely to predict compliance with skin cancer protective behaviors.17
The Hernandez et al19 study is unique in that it was the only one to include an educational intervention entirely in Spanish, which is important to consider, as language may be a hindrance to participants’ understanding in the other studies, particularly Hispanics, possibly leading to a lack of information retention regarding sun-protective behaviors. Furthermore, it also was the only study to utilize videos as a method for interventions. The 3-minute videos demonstrated that interventions could be efficient as compared to the 2-hour in-class intervention used by Robinson et al18 and the 20-minute intervention used by Kundu et al.17 Additionally, videos also could be more cost-effective, as incentives for large focus groups would no longer be needed. Furthermore, in the Hernandez et al19 study, there was minimal to no disruption in the participants’ daily routine, as the participants were getting cosmetic services while watching the videos, perhaps allowing them to be more attentive. In contrast, both the Robinson et al18 and Kundu et al17 studies required time out from the participants’ daily schedules. In addition, these studies were notably longer than the Hernandez et al19 study. The 8-hour intervention in the Chapman et al20 study also may not be feasible for the general population because of its excessive length. However, the intervention was successful among the adolescent participants, which suggested that shorter durations are effective in the adult population and longer interventions may be more appropriate for adolescents because they benefit from peer activity.
Despite the success of the educational interventions as outlined in the 4 studies described here, a major epidemiologic flaw is that these interventions included only a small percentage of the target population. The largest total number of adults surveyed and undergoing an intervention in any of the populations was only 120.17 By failing to reach a substantial proportion of the population at risk, the number of preventable deaths likely will not decrease. The authors believe a larger-scale intervention would provide meaningful change. Australia’s SunSmart campaign to increase skin cancer awareness in the Australian population is an example of one such large-scale national intervention. The campaign focused on massive television advertisements in the summer to educate participants about the dangers of skin cancer and the importance of protective behaviors. Telephone surveys conducted from 1987 to 2011 demonstrated that more exposure to the advertisements in the SunSmart campaign meant that individuals were more likely to use sunscreen and avoid sun exposure.22 In the United States, a similar intervention would be of great benefit in educating SOC populations regarding skin cancer risk. Additionally, dermatology residents need to be adequately trained to educate patients of color about the risk for skin cancer, as survey data indicated more than 80% of Australian dermatologists desired more SOC teaching during their training and 50% indicated that they would have time to learn it during their training if offered.23 Furthermore, one study suggested that future interventions must include primary-, secondary-, and tertiary-prevention methods to effectively reduce skin cancer risk among patients of color.24 Primary prevention involves sun avoidance, secondary prevention involves detecting cancerous lesions, and tertiary prevention involves undergoing treatment of skin malignancies. However, increased knowledge does not necessarily mean increased preventative action will be employed (eg, sunscreen use, wearing sun-protective clothing and sunglasses, avoiding tanning beds and excessive sun exposure). Additional studies that demonstrate a notable increase in sun-protective behaviors related to increased knowledge are needed.
Because retention of skin cancer knowledge decreased in several postintervention surveys, there also is a dire need for continuing skin cancer education in patients of color, which may be accomplished through a combination effort of television advertisement campaigns, pamphlets, social media, community health departments, or even community members. For example, a pilot program found that Hispanic lay health workers who are educated about skin cancer may serve as a bridge between medical providers and the Hispanic community by encouraging individuals in this population to get regular skin examinations from a physician.25 Overall, there are currently gaps in the understanding and treatment of skin cancer in people of color.26 Identifying the advantages and disadvantages of all relevant skin cancer interventions conducted in the SOC population will hopefully guide future studies to help close these gaps by allowing others to design the best possible intervention. By doing so, researchers can generate an intervention that is precise, well-informed, and effective in decreasing mortality rates from skin cancer among SOC populations.
Conclusion
All of the studies reviewed demonstrated that instructional and educational interventions are promising methods for improving either knowledge, awareness, or safe skin practices and sun-protective behaviors in SOC populations to differing degrees (Table). Although each of the 4 interventions employed their own methods, they all increased 1 or more of the 3 aforementioned concepts—knowledge, awareness, or safe skin practices and sun-protective behaviors—when comparing postsurvey to presurvey data. However, the critically important message derived from this research is that there is a tremendous need for a substantial large-scale educational intervention to increase knowledge regarding skin cancer in SOC populations.
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762.
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914.
- Gloster HM Jr, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-760.
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991.
- Byrd KM, Wilson DC, Hoyler SS, et al. Advanced presentation of melanoma in African Americans. J Am Acad Dermatol. 2004;50:21-24.
- Hu S, Parmet Y, Allen G, et al. Disparity in melanoma: a trend analysis of melanoma incidence and stage at diagnosis among whites, Hispanics, and blacks in Florida. Arch Dermatol. 2009;145:1369-1374.
- Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5, suppl 1):S26-S37.
- Byrd-Miles K, Toombs EL, Peck GL. Skin cancer in individuals of African, Asian, Latin-American, and American-Indian descent: differences in incidence, clinical presentation, and survival compared to Caucasians. J Drugs Dermatol. 2007;6:10-16.
- Hu S, Soza-Vento RM, Parker DF, et al. Comparison of stage at diagnosis of melanoma among Hispanic, black, and white patients in Miami-Dade County, Florida. Arch Dermatol. 2006;142:704-708.
- Hu S, Parker DF, Thomas AG, et al. Advanced presentation of melanoma in African Americans: the Miami-Dade County experience. J Am Acad Dermatol. 2004;5:1031-1032.
- Bellows CF, Belafsky P, Fortgang IS, et al. Melanoma in African-Americans: trends in biological behavior and clinical characteristics over two decades. J Surg Oncol. 2001;78:10-16.
- Pritchett EN, Doyle A, Shaver CM, et al. Nonmelanoma skin cancer in nonwhite organ transplant recipients. JAMA Dermatol. 2016;152:1348-1353.
- Shin S, Palis BE, Phillips JL, et al. Cutaneous melanoma in Asian-Americans. J Surg Oncol. 2009;99:114-118.
- Stubblefield J, Kelly B. Melanoma in non-caucasian populations. Surg Clin North Am. 2014;94:1115-1126.
- Bradford PT, Goldstein AM, McMaster ML, et al. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Arch Dermatol. 2009;145:427-434.
- Pichon LC, Corral I, Landrine H, et al. Perceived skin cancer risk and sunscreen use among African American adults. J Health Psychol. 2010;15:1181-1189.
- Kundu RV, Kamaria M, Ortiz S, et al. Effectiveness of a knowledge-based intervention for melanoma among those with ethnic skin. J Am Acad Dermatol. 2010;62:777-784.
- Robinson JK, Joshi KM, Ortiz S, et al. Melanoma knowledge, perception, and awareness in ethnic minorities in Chicago: recommendations regarding education. Psychooncology. 2010;20:313-320.
- Hernandez C, Wang S, Abraham I, et al. Evaluation of educational videos to increase skin cancer risk awareness and sun safe behaviors among adult Hispanics. J Cancer Educ. 2014;29:563-569.
- Chapman LW, Ochoa A, Tenconi F, et al. Dermatologic health literacy in underserved communities: a case report of south Los Angeles middle schools. Dermatol Online J. 2015;21. pii:13030/qt8671p40n.
- Yanina G, Gaber R, Clayman ML, et al. Sun protection education for diverse audiences: need for skin cancer pictures. J Cancer Educ. 2015;30:187-189.
- Dobbinson SJ, Volkov A, Wakefield MA. Continued impact of sunsmart advertising on youth and adults’ behaviors. Am J Prev Med. 2015;49:20-28.
- Rodrigues MA, Ross AL, Gilmore S, et al. Australian dermatologists’ perspective on skin of colour: results of a national survey [published online December 9, 2016]. Australas J Dermatol. doi:10.1111/ajd.12556.
- Jacobsen A, Galvan A, Lachapelle CC, et al. Defining the need for skin cancer prevention education in uninsured, minority, and immigrant communities. JAMA Dermatol. 2016;152:1342-1347.
- Hernandez C, Kim H, Mauleon G, et al. A pilot program in collaboration with community centers to increase awareness and participation in skin cancer screening among Latinos in Chicago. J Cancer Educ. 2013;28:342-345.
- Kailas A, Solomon JA, Mostow EN, et al. Gaps in the understanding and treatment of skin cancer in people of color. J Am Acad Dermatol. 2016;74:144-149.
Malignant melanoma, basal cell carcinoma, and squamous cell carcinoma account for approximately 40% of all neoplasms among the white population in the United States. Skin cancer is the most common malignancy in the United States.1 However, despite this occurrence, there are limited data regarding skin cancer in individuals with skin of color (SOC). The 5-year survival rates for melanoma are 58.2% for black individuals, 69.7% for Hispanics, and 70.9% for Asians compared to 79.8% for white individuals in the United States.2 Even though SOC populations have lower incidences of skin cancer—melanoma, basal cell carcinoma, and squamous cell carcinoma—they exhibit higher death rates.3-7 Nonetheless, no specific guidelines exist to address sun exposure and safety habits in SOC populations.6,8 Furthermore, current demographics suggest that by the year 2050, approximately half of the US population will be nonwhite.4 Paradoxically, despite having increased sun protection from greater amounts of melanin in their skin, black individuals are more likely to present with advanced-stage melanoma (eg, stage III/IV) compared to white individuals.8-12 Furthermore, those of nonwhite populations are more likely to present with more advanced stages of acral lentiginous melanomas than white individuals.13,14 Hispanics also face an increasing incidence of more invasive acral lentiginous melanomas.15 Overall, SOC patients have the poorest skin cancer prognosis, and the data suggest that the reason for this paradox is delayed diagnosis.1
Although skin cancer is largely a preventable condition, the literature suggests that lack of awareness of melanoma among ethnic minorities is one of the main reasons for their poor skin cancer prognosis.16 This lack of awareness decreases the likelihood that an SOC patient would be alert to early detection of cancerous changes.17 Because educating at-risk SOC populations is key to decreasing skin cancer risk, this study focused on determining the efficacy of major knowledge-based interventions conducted to date.1 Overall, we sought to answer the question, do knowledge-based interventions increase skin cancer awareness, knowledge, and protective behavior among people of color?
Methods
For this review, the Cochrane method of analysis was used to conduct a thorough search of PubMed articles indexed for MEDLINE (1994-2016), as well as a search of CINAHL (1997-2016), PsycINFO (1999-2016), and Web of Science (1965-2016), using a combination of more than 100 search terms including but not limited to skin cancer, skin of color, intervention, and ethnic skin. The search yielded a total of 52 articles (Figure). Following review, only 8 articles met inclusion criteria, which were as follows: (1) study was related to skin cancer in SOC patients, which included an intervention to increase skin cancer awareness and knowledge; (2) study included adult participants or adolescents aged 12 to 18 years; (3) study was written in English; and (4) study was published in a peer-reviewed journal. Of the remaining 8 articles, 4 were excluded due to the following criteria: (1) study failed to provide both preintervention and postintervention data, (2) study failed to provide quantitative data, and (3) study included participants who worked as health care professionals or ancillary staff. As a result, a total of 4 articles were analyzed and discussed in this review (Table).

Results
Robinson et al18 conducted 12 focus groups with 120 total participants (40 black, 40 Asian, and 40 Hispanic patients). Participants engaged in a 2-hour tape-recorded focus group with a moderator guide on melanoma and skin cancer. Furthermore, they also were asked to assess skin cancer risk in 5 celebrities with different skin tones. The statistically significant preintervention results of the study (χ2=4.6, P<.001) were as follows: only 2%, 4%, and 14% correctly reported that celebrities with a very fair skin type, a fair skin type, and very dark skin type, respectively, could get sunburn, compared to 75%, 76%, and 62% post-intervention. Additionally, prior to intervention, 14% of the study population believed that dark brown skin type could get sunburn compared to 62% of the same group postintervention. This study demonstrated that the intervention helped SOC patients better identify their ability to get sunburn and identify their skin cancer risk.18
Hernandez et al19 used a video-based intervention in a Hispanic community, which was in contrast to the multiracial focus group intervention conducted by Robinson et al.18 Eighty Hispanic individuals were recruited from beauty salons to participate in the study. Participants watched two 3-minute videos in Spanish and completed a preintervention and postintervention survey. The first video emphasized the photoaging benefits of sun protection, while the second focused on skin cancer prevention. Preintervention surveys indicated that only 54 (68%) participants believed that fair-skinned Hispanics were at risk for skin cancer, which improved to 72 (90%) participants postintervention. Furthermore, initially only 44 (55%) participants thought those with darker skin types could develop skin cancer, but this number increased to 69 (86%) postintervention. For both questions regarding fair and dark skin, the agreement proportion was significantly different between the preeducation and posteducation videos (P<.0002 for the fair skin question and P<.0001 for the dark skin question). This study greatly increased awareness of skin cancer risk among Hispanics,19 similar to the Robinson et al18 study.
In contrast to 2-hour focus groups or 3-minute video–based interventions, a study by Kundu et al17 employed a 20-minute educational class-based intervention with both verbal and visual instruction. This study assessed the efficacy of an educational tutorial on improving awareness and early detection of melanoma in SOC individuals. Photographs were used to help participants recognize the ABCDEs of melanoma and to show examples of acral lentiginous melanomas in white individuals. A total of 71 participants completed a preintervention questionnaire, participated in a 20-minute class, and completed a postintervention questionnaire immediately after and 3 months following the class. The study population included 44 black, 15 Asian, 10 Hispanic, and 2 multiethnic participants. Knowledge that melanoma is a skin cancer increased from 83.9% to 100% immediately postintervention (P=.0001) and 97.2% at 3 months postintervention (P=.0075). Additionally, knowledge that people of color are at risk for melanoma increased from 48.4% preintervention to 82.8% immediately postintervention (P<.0001). However, only 40.8% of participants retained this knowledge at 3 months postintervention. Because only 1 participant reported a family history of skin cancer, the authors hypothesized that the reason for this loss of knowledge was that most participants were not personally affected by friends or family members with melanoma. A future study with an appropriate control group would be needed to support this claim. This study shed light on the potential of class-based interventions to increase both awareness and knowledge of skin cancer in SOC populations.17
A study by Chapman et al20 examined the effects of a sun protection educational program on increasing awareness of skin cancer in Hispanic and black middle school students in southern Los Angeles, California. It was the only study we reviewed that focused primarily on adolescents. Furthermore, it included the largest sample size (N=148) analyzed here. Students were given a preintervention questionnaire to evaluate their awareness of skin cancer and current sun-protection practices. Based on these results, the investigators devised a set of learning goals and incorporated them into an educational pamphlet. The intervention, called “Skin Teaching Day,” was a 1-day program discussing skin cancer and the importance of sun protection. Prior to the intervention, 68% of participants reported that they used sunscreen. Three months after completing the program, 80% of participants reported sunscreen use, an increase of 12% prior to the intervention. The results of this study demonstrated the unique effectiveness and potential of pamphlets in increasing sunscreen use.20
Comment
Overall, various methods of interventions such as focus groups, videos, pamphlets, and lectures improved knowledge of skin cancer risk and sun-protection behaviors in SOC populations. Furthermore, the unique differences of each study provided important insights into the successful design of an intervention.
An important characteristic of the Robinson et al18 study was the addition of photographs, which allowed participants not only to visualize different skin tones but also provided them with the opportunity to relate themselves to the photographs; by doing so, participants could effectively pick out the skin tone that best suited them. Written SOC scales are limited to mere descriptions and thus make it more difficult for participants to accurately identify the tone that best fits them. Kundu et al17 used photographs to teach skin self-examination and ABCDEs for detection of melanoma. Additionally, both studies used photographs to demonstrate examples of skin cancer.17,18 Recent evidence suggests the use of visuals can be efficacious for improving skin cancer knowledge and awareness; a study in 16 SOC kidney transplant recipients found that the addition of photographs of squamous cell carcinoma in various skin tones to a sun-protection educational pamphlet was more effective than the original pamphlet without photographs.21
In contrast to the Robinson et al18 study and Hernandez et al19 study, the Kundu et al17 study showed photographs of acral lentiginous melanomas in white patients rather than SOC patients. However, SOC populations may be less likely to relate to or identify skin changes in skin types that are different from their own. This technique was still beneficial, as acral lentiginous melanoma is the most common type of melanoma in SOC populations. Another benefit of the study was that it was the only study reviewed that included a follow-up postintervention questionnaire. Such data is useful, as it demonstrates how muchinformation is retained by participants and may be more likely to predict compliance with skin cancer protective behaviors.17
The Hernandez et al19 study is unique in that it was the only one to include an educational intervention entirely in Spanish, which is important to consider, as language may be a hindrance to participants’ understanding in the other studies, particularly Hispanics, possibly leading to a lack of information retention regarding sun-protective behaviors. Furthermore, it also was the only study to utilize videos as a method for interventions. The 3-minute videos demonstrated that interventions could be efficient as compared to the 2-hour in-class intervention used by Robinson et al18 and the 20-minute intervention used by Kundu et al.17 Additionally, videos also could be more cost-effective, as incentives for large focus groups would no longer be needed. Furthermore, in the Hernandez et al19 study, there was minimal to no disruption in the participants’ daily routine, as the participants were getting cosmetic services while watching the videos, perhaps allowing them to be more attentive. In contrast, both the Robinson et al18 and Kundu et al17 studies required time out from the participants’ daily schedules. In addition, these studies were notably longer than the Hernandez et al19 study. The 8-hour intervention in the Chapman et al20 study also may not be feasible for the general population because of its excessive length. However, the intervention was successful among the adolescent participants, which suggested that shorter durations are effective in the adult population and longer interventions may be more appropriate for adolescents because they benefit from peer activity.
Despite the success of the educational interventions as outlined in the 4 studies described here, a major epidemiologic flaw is that these interventions included only a small percentage of the target population. The largest total number of adults surveyed and undergoing an intervention in any of the populations was only 120.17 By failing to reach a substantial proportion of the population at risk, the number of preventable deaths likely will not decrease. The authors believe a larger-scale intervention would provide meaningful change. Australia’s SunSmart campaign to increase skin cancer awareness in the Australian population is an example of one such large-scale national intervention. The campaign focused on massive television advertisements in the summer to educate participants about the dangers of skin cancer and the importance of protective behaviors. Telephone surveys conducted from 1987 to 2011 demonstrated that more exposure to the advertisements in the SunSmart campaign meant that individuals were more likely to use sunscreen and avoid sun exposure.22 In the United States, a similar intervention would be of great benefit in educating SOC populations regarding skin cancer risk. Additionally, dermatology residents need to be adequately trained to educate patients of color about the risk for skin cancer, as survey data indicated more than 80% of Australian dermatologists desired more SOC teaching during their training and 50% indicated that they would have time to learn it during their training if offered.23 Furthermore, one study suggested that future interventions must include primary-, secondary-, and tertiary-prevention methods to effectively reduce skin cancer risk among patients of color.24 Primary prevention involves sun avoidance, secondary prevention involves detecting cancerous lesions, and tertiary prevention involves undergoing treatment of skin malignancies. However, increased knowledge does not necessarily mean increased preventative action will be employed (eg, sunscreen use, wearing sun-protective clothing and sunglasses, avoiding tanning beds and excessive sun exposure). Additional studies that demonstrate a notable increase in sun-protective behaviors related to increased knowledge are needed.
Because retention of skin cancer knowledge decreased in several postintervention surveys, there also is a dire need for continuing skin cancer education in patients of color, which may be accomplished through a combination effort of television advertisement campaigns, pamphlets, social media, community health departments, or even community members. For example, a pilot program found that Hispanic lay health workers who are educated about skin cancer may serve as a bridge between medical providers and the Hispanic community by encouraging individuals in this population to get regular skin examinations from a physician.25 Overall, there are currently gaps in the understanding and treatment of skin cancer in people of color.26 Identifying the advantages and disadvantages of all relevant skin cancer interventions conducted in the SOC population will hopefully guide future studies to help close these gaps by allowing others to design the best possible intervention. By doing so, researchers can generate an intervention that is precise, well-informed, and effective in decreasing mortality rates from skin cancer among SOC populations.
Conclusion
All of the studies reviewed demonstrated that instructional and educational interventions are promising methods for improving either knowledge, awareness, or safe skin practices and sun-protective behaviors in SOC populations to differing degrees (Table). Although each of the 4 interventions employed their own methods, they all increased 1 or more of the 3 aforementioned concepts—knowledge, awareness, or safe skin practices and sun-protective behaviors—when comparing postsurvey to presurvey data. However, the critically important message derived from this research is that there is a tremendous need for a substantial large-scale educational intervention to increase knowledge regarding skin cancer in SOC populations.
Malignant melanoma, basal cell carcinoma, and squamous cell carcinoma account for approximately 40% of all neoplasms among the white population in the United States. Skin cancer is the most common malignancy in the United States.1 However, despite this occurrence, there are limited data regarding skin cancer in individuals with skin of color (SOC). The 5-year survival rates for melanoma are 58.2% for black individuals, 69.7% for Hispanics, and 70.9% for Asians compared to 79.8% for white individuals in the United States.2 Even though SOC populations have lower incidences of skin cancer—melanoma, basal cell carcinoma, and squamous cell carcinoma—they exhibit higher death rates.3-7 Nonetheless, no specific guidelines exist to address sun exposure and safety habits in SOC populations.6,8 Furthermore, current demographics suggest that by the year 2050, approximately half of the US population will be nonwhite.4 Paradoxically, despite having increased sun protection from greater amounts of melanin in their skin, black individuals are more likely to present with advanced-stage melanoma (eg, stage III/IV) compared to white individuals.8-12 Furthermore, those of nonwhite populations are more likely to present with more advanced stages of acral lentiginous melanomas than white individuals.13,14 Hispanics also face an increasing incidence of more invasive acral lentiginous melanomas.15 Overall, SOC patients have the poorest skin cancer prognosis, and the data suggest that the reason for this paradox is delayed diagnosis.1
Although skin cancer is largely a preventable condition, the literature suggests that lack of awareness of melanoma among ethnic minorities is one of the main reasons for their poor skin cancer prognosis.16 This lack of awareness decreases the likelihood that an SOC patient would be alert to early detection of cancerous changes.17 Because educating at-risk SOC populations is key to decreasing skin cancer risk, this study focused on determining the efficacy of major knowledge-based interventions conducted to date.1 Overall, we sought to answer the question, do knowledge-based interventions increase skin cancer awareness, knowledge, and protective behavior among people of color?
Methods
For this review, the Cochrane method of analysis was used to conduct a thorough search of PubMed articles indexed for MEDLINE (1994-2016), as well as a search of CINAHL (1997-2016), PsycINFO (1999-2016), and Web of Science (1965-2016), using a combination of more than 100 search terms including but not limited to skin cancer, skin of color, intervention, and ethnic skin. The search yielded a total of 52 articles (Figure). Following review, only 8 articles met inclusion criteria, which were as follows: (1) study was related to skin cancer in SOC patients, which included an intervention to increase skin cancer awareness and knowledge; (2) study included adult participants or adolescents aged 12 to 18 years; (3) study was written in English; and (4) study was published in a peer-reviewed journal. Of the remaining 8 articles, 4 were excluded due to the following criteria: (1) study failed to provide both preintervention and postintervention data, (2) study failed to provide quantitative data, and (3) study included participants who worked as health care professionals or ancillary staff. As a result, a total of 4 articles were analyzed and discussed in this review (Table).

Results
Robinson et al18 conducted 12 focus groups with 120 total participants (40 black, 40 Asian, and 40 Hispanic patients). Participants engaged in a 2-hour tape-recorded focus group with a moderator guide on melanoma and skin cancer. Furthermore, they also were asked to assess skin cancer risk in 5 celebrities with different skin tones. The statistically significant preintervention results of the study (χ2=4.6, P<.001) were as follows: only 2%, 4%, and 14% correctly reported that celebrities with a very fair skin type, a fair skin type, and very dark skin type, respectively, could get sunburn, compared to 75%, 76%, and 62% post-intervention. Additionally, prior to intervention, 14% of the study population believed that dark brown skin type could get sunburn compared to 62% of the same group postintervention. This study demonstrated that the intervention helped SOC patients better identify their ability to get sunburn and identify their skin cancer risk.18
Hernandez et al19 used a video-based intervention in a Hispanic community, which was in contrast to the multiracial focus group intervention conducted by Robinson et al.18 Eighty Hispanic individuals were recruited from beauty salons to participate in the study. Participants watched two 3-minute videos in Spanish and completed a preintervention and postintervention survey. The first video emphasized the photoaging benefits of sun protection, while the second focused on skin cancer prevention. Preintervention surveys indicated that only 54 (68%) participants believed that fair-skinned Hispanics were at risk for skin cancer, which improved to 72 (90%) participants postintervention. Furthermore, initially only 44 (55%) participants thought those with darker skin types could develop skin cancer, but this number increased to 69 (86%) postintervention. For both questions regarding fair and dark skin, the agreement proportion was significantly different between the preeducation and posteducation videos (P<.0002 for the fair skin question and P<.0001 for the dark skin question). This study greatly increased awareness of skin cancer risk among Hispanics,19 similar to the Robinson et al18 study.
In contrast to 2-hour focus groups or 3-minute video–based interventions, a study by Kundu et al17 employed a 20-minute educational class-based intervention with both verbal and visual instruction. This study assessed the efficacy of an educational tutorial on improving awareness and early detection of melanoma in SOC individuals. Photographs were used to help participants recognize the ABCDEs of melanoma and to show examples of acral lentiginous melanomas in white individuals. A total of 71 participants completed a preintervention questionnaire, participated in a 20-minute class, and completed a postintervention questionnaire immediately after and 3 months following the class. The study population included 44 black, 15 Asian, 10 Hispanic, and 2 multiethnic participants. Knowledge that melanoma is a skin cancer increased from 83.9% to 100% immediately postintervention (P=.0001) and 97.2% at 3 months postintervention (P=.0075). Additionally, knowledge that people of color are at risk for melanoma increased from 48.4% preintervention to 82.8% immediately postintervention (P<.0001). However, only 40.8% of participants retained this knowledge at 3 months postintervention. Because only 1 participant reported a family history of skin cancer, the authors hypothesized that the reason for this loss of knowledge was that most participants were not personally affected by friends or family members with melanoma. A future study with an appropriate control group would be needed to support this claim. This study shed light on the potential of class-based interventions to increase both awareness and knowledge of skin cancer in SOC populations.17
A study by Chapman et al20 examined the effects of a sun protection educational program on increasing awareness of skin cancer in Hispanic and black middle school students in southern Los Angeles, California. It was the only study we reviewed that focused primarily on adolescents. Furthermore, it included the largest sample size (N=148) analyzed here. Students were given a preintervention questionnaire to evaluate their awareness of skin cancer and current sun-protection practices. Based on these results, the investigators devised a set of learning goals and incorporated them into an educational pamphlet. The intervention, called “Skin Teaching Day,” was a 1-day program discussing skin cancer and the importance of sun protection. Prior to the intervention, 68% of participants reported that they used sunscreen. Three months after completing the program, 80% of participants reported sunscreen use, an increase of 12% prior to the intervention. The results of this study demonstrated the unique effectiveness and potential of pamphlets in increasing sunscreen use.20
Comment
Overall, various methods of interventions such as focus groups, videos, pamphlets, and lectures improved knowledge of skin cancer risk and sun-protection behaviors in SOC populations. Furthermore, the unique differences of each study provided important insights into the successful design of an intervention.
An important characteristic of the Robinson et al18 study was the addition of photographs, which allowed participants not only to visualize different skin tones but also provided them with the opportunity to relate themselves to the photographs; by doing so, participants could effectively pick out the skin tone that best suited them. Written SOC scales are limited to mere descriptions and thus make it more difficult for participants to accurately identify the tone that best fits them. Kundu et al17 used photographs to teach skin self-examination and ABCDEs for detection of melanoma. Additionally, both studies used photographs to demonstrate examples of skin cancer.17,18 Recent evidence suggests the use of visuals can be efficacious for improving skin cancer knowledge and awareness; a study in 16 SOC kidney transplant recipients found that the addition of photographs of squamous cell carcinoma in various skin tones to a sun-protection educational pamphlet was more effective than the original pamphlet without photographs.21
In contrast to the Robinson et al18 study and Hernandez et al19 study, the Kundu et al17 study showed photographs of acral lentiginous melanomas in white patients rather than SOC patients. However, SOC populations may be less likely to relate to or identify skin changes in skin types that are different from their own. This technique was still beneficial, as acral lentiginous melanoma is the most common type of melanoma in SOC populations. Another benefit of the study was that it was the only study reviewed that included a follow-up postintervention questionnaire. Such data is useful, as it demonstrates how muchinformation is retained by participants and may be more likely to predict compliance with skin cancer protective behaviors.17
The Hernandez et al19 study is unique in that it was the only one to include an educational intervention entirely in Spanish, which is important to consider, as language may be a hindrance to participants’ understanding in the other studies, particularly Hispanics, possibly leading to a lack of information retention regarding sun-protective behaviors. Furthermore, it also was the only study to utilize videos as a method for interventions. The 3-minute videos demonstrated that interventions could be efficient as compared to the 2-hour in-class intervention used by Robinson et al18 and the 20-minute intervention used by Kundu et al.17 Additionally, videos also could be more cost-effective, as incentives for large focus groups would no longer be needed. Furthermore, in the Hernandez et al19 study, there was minimal to no disruption in the participants’ daily routine, as the participants were getting cosmetic services while watching the videos, perhaps allowing them to be more attentive. In contrast, both the Robinson et al18 and Kundu et al17 studies required time out from the participants’ daily schedules. In addition, these studies were notably longer than the Hernandez et al19 study. The 8-hour intervention in the Chapman et al20 study also may not be feasible for the general population because of its excessive length. However, the intervention was successful among the adolescent participants, which suggested that shorter durations are effective in the adult population and longer interventions may be more appropriate for adolescents because they benefit from peer activity.
Despite the success of the educational interventions as outlined in the 4 studies described here, a major epidemiologic flaw is that these interventions included only a small percentage of the target population. The largest total number of adults surveyed and undergoing an intervention in any of the populations was only 120.17 By failing to reach a substantial proportion of the population at risk, the number of preventable deaths likely will not decrease. The authors believe a larger-scale intervention would provide meaningful change. Australia’s SunSmart campaign to increase skin cancer awareness in the Australian population is an example of one such large-scale national intervention. The campaign focused on massive television advertisements in the summer to educate participants about the dangers of skin cancer and the importance of protective behaviors. Telephone surveys conducted from 1987 to 2011 demonstrated that more exposure to the advertisements in the SunSmart campaign meant that individuals were more likely to use sunscreen and avoid sun exposure.22 In the United States, a similar intervention would be of great benefit in educating SOC populations regarding skin cancer risk. Additionally, dermatology residents need to be adequately trained to educate patients of color about the risk for skin cancer, as survey data indicated more than 80% of Australian dermatologists desired more SOC teaching during their training and 50% indicated that they would have time to learn it during their training if offered.23 Furthermore, one study suggested that future interventions must include primary-, secondary-, and tertiary-prevention methods to effectively reduce skin cancer risk among patients of color.24 Primary prevention involves sun avoidance, secondary prevention involves detecting cancerous lesions, and tertiary prevention involves undergoing treatment of skin malignancies. However, increased knowledge does not necessarily mean increased preventative action will be employed (eg, sunscreen use, wearing sun-protective clothing and sunglasses, avoiding tanning beds and excessive sun exposure). Additional studies that demonstrate a notable increase in sun-protective behaviors related to increased knowledge are needed.
Because retention of skin cancer knowledge decreased in several postintervention surveys, there also is a dire need for continuing skin cancer education in patients of color, which may be accomplished through a combination effort of television advertisement campaigns, pamphlets, social media, community health departments, or even community members. For example, a pilot program found that Hispanic lay health workers who are educated about skin cancer may serve as a bridge between medical providers and the Hispanic community by encouraging individuals in this population to get regular skin examinations from a physician.25 Overall, there are currently gaps in the understanding and treatment of skin cancer in people of color.26 Identifying the advantages and disadvantages of all relevant skin cancer interventions conducted in the SOC population will hopefully guide future studies to help close these gaps by allowing others to design the best possible intervention. By doing so, researchers can generate an intervention that is precise, well-informed, and effective in decreasing mortality rates from skin cancer among SOC populations.
Conclusion
All of the studies reviewed demonstrated that instructional and educational interventions are promising methods for improving either knowledge, awareness, or safe skin practices and sun-protective behaviors in SOC populations to differing degrees (Table). Although each of the 4 interventions employed their own methods, they all increased 1 or more of the 3 aforementioned concepts—knowledge, awareness, or safe skin practices and sun-protective behaviors—when comparing postsurvey to presurvey data. However, the critically important message derived from this research is that there is a tremendous need for a substantial large-scale educational intervention to increase knowledge regarding skin cancer in SOC populations.
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762.
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914.
- Gloster HM Jr, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-760.
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991.
- Byrd KM, Wilson DC, Hoyler SS, et al. Advanced presentation of melanoma in African Americans. J Am Acad Dermatol. 2004;50:21-24.
- Hu S, Parmet Y, Allen G, et al. Disparity in melanoma: a trend analysis of melanoma incidence and stage at diagnosis among whites, Hispanics, and blacks in Florida. Arch Dermatol. 2009;145:1369-1374.
- Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5, suppl 1):S26-S37.
- Byrd-Miles K, Toombs EL, Peck GL. Skin cancer in individuals of African, Asian, Latin-American, and American-Indian descent: differences in incidence, clinical presentation, and survival compared to Caucasians. J Drugs Dermatol. 2007;6:10-16.
- Hu S, Soza-Vento RM, Parker DF, et al. Comparison of stage at diagnosis of melanoma among Hispanic, black, and white patients in Miami-Dade County, Florida. Arch Dermatol. 2006;142:704-708.
- Hu S, Parker DF, Thomas AG, et al. Advanced presentation of melanoma in African Americans: the Miami-Dade County experience. J Am Acad Dermatol. 2004;5:1031-1032.
- Bellows CF, Belafsky P, Fortgang IS, et al. Melanoma in African-Americans: trends in biological behavior and clinical characteristics over two decades. J Surg Oncol. 2001;78:10-16.
- Pritchett EN, Doyle A, Shaver CM, et al. Nonmelanoma skin cancer in nonwhite organ transplant recipients. JAMA Dermatol. 2016;152:1348-1353.
- Shin S, Palis BE, Phillips JL, et al. Cutaneous melanoma in Asian-Americans. J Surg Oncol. 2009;99:114-118.
- Stubblefield J, Kelly B. Melanoma in non-caucasian populations. Surg Clin North Am. 2014;94:1115-1126.
- Bradford PT, Goldstein AM, McMaster ML, et al. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Arch Dermatol. 2009;145:427-434.
- Pichon LC, Corral I, Landrine H, et al. Perceived skin cancer risk and sunscreen use among African American adults. J Health Psychol. 2010;15:1181-1189.
- Kundu RV, Kamaria M, Ortiz S, et al. Effectiveness of a knowledge-based intervention for melanoma among those with ethnic skin. J Am Acad Dermatol. 2010;62:777-784.
- Robinson JK, Joshi KM, Ortiz S, et al. Melanoma knowledge, perception, and awareness in ethnic minorities in Chicago: recommendations regarding education. Psychooncology. 2010;20:313-320.
- Hernandez C, Wang S, Abraham I, et al. Evaluation of educational videos to increase skin cancer risk awareness and sun safe behaviors among adult Hispanics. J Cancer Educ. 2014;29:563-569.
- Chapman LW, Ochoa A, Tenconi F, et al. Dermatologic health literacy in underserved communities: a case report of south Los Angeles middle schools. Dermatol Online J. 2015;21. pii:13030/qt8671p40n.
- Yanina G, Gaber R, Clayman ML, et al. Sun protection education for diverse audiences: need for skin cancer pictures. J Cancer Educ. 2015;30:187-189.
- Dobbinson SJ, Volkov A, Wakefield MA. Continued impact of sunsmart advertising on youth and adults’ behaviors. Am J Prev Med. 2015;49:20-28.
- Rodrigues MA, Ross AL, Gilmore S, et al. Australian dermatologists’ perspective on skin of colour: results of a national survey [published online December 9, 2016]. Australas J Dermatol. doi:10.1111/ajd.12556.
- Jacobsen A, Galvan A, Lachapelle CC, et al. Defining the need for skin cancer prevention education in uninsured, minority, and immigrant communities. JAMA Dermatol. 2016;152:1342-1347.
- Hernandez C, Kim H, Mauleon G, et al. A pilot program in collaboration with community centers to increase awareness and participation in skin cancer screening among Latinos in Chicago. J Cancer Educ. 2013;28:342-345.
- Kailas A, Solomon JA, Mostow EN, et al. Gaps in the understanding and treatment of skin cancer in people of color. J Am Acad Dermatol. 2016;74:144-149.
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762.
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914.
- Gloster HM Jr, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-760.
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991.
- Byrd KM, Wilson DC, Hoyler SS, et al. Advanced presentation of melanoma in African Americans. J Am Acad Dermatol. 2004;50:21-24.
- Hu S, Parmet Y, Allen G, et al. Disparity in melanoma: a trend analysis of melanoma incidence and stage at diagnosis among whites, Hispanics, and blacks in Florida. Arch Dermatol. 2009;145:1369-1374.
- Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5, suppl 1):S26-S37.
- Byrd-Miles K, Toombs EL, Peck GL. Skin cancer in individuals of African, Asian, Latin-American, and American-Indian descent: differences in incidence, clinical presentation, and survival compared to Caucasians. J Drugs Dermatol. 2007;6:10-16.
- Hu S, Soza-Vento RM, Parker DF, et al. Comparison of stage at diagnosis of melanoma among Hispanic, black, and white patients in Miami-Dade County, Florida. Arch Dermatol. 2006;142:704-708.
- Hu S, Parker DF, Thomas AG, et al. Advanced presentation of melanoma in African Americans: the Miami-Dade County experience. J Am Acad Dermatol. 2004;5:1031-1032.
- Bellows CF, Belafsky P, Fortgang IS, et al. Melanoma in African-Americans: trends in biological behavior and clinical characteristics over two decades. J Surg Oncol. 2001;78:10-16.
- Pritchett EN, Doyle A, Shaver CM, et al. Nonmelanoma skin cancer in nonwhite organ transplant recipients. JAMA Dermatol. 2016;152:1348-1353.
- Shin S, Palis BE, Phillips JL, et al. Cutaneous melanoma in Asian-Americans. J Surg Oncol. 2009;99:114-118.
- Stubblefield J, Kelly B. Melanoma in non-caucasian populations. Surg Clin North Am. 2014;94:1115-1126.
- Bradford PT, Goldstein AM, McMaster ML, et al. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Arch Dermatol. 2009;145:427-434.
- Pichon LC, Corral I, Landrine H, et al. Perceived skin cancer risk and sunscreen use among African American adults. J Health Psychol. 2010;15:1181-1189.
- Kundu RV, Kamaria M, Ortiz S, et al. Effectiveness of a knowledge-based intervention for melanoma among those with ethnic skin. J Am Acad Dermatol. 2010;62:777-784.
- Robinson JK, Joshi KM, Ortiz S, et al. Melanoma knowledge, perception, and awareness in ethnic minorities in Chicago: recommendations regarding education. Psychooncology. 2010;20:313-320.
- Hernandez C, Wang S, Abraham I, et al. Evaluation of educational videos to increase skin cancer risk awareness and sun safe behaviors among adult Hispanics. J Cancer Educ. 2014;29:563-569.
- Chapman LW, Ochoa A, Tenconi F, et al. Dermatologic health literacy in underserved communities: a case report of south Los Angeles middle schools. Dermatol Online J. 2015;21. pii:13030/qt8671p40n.
- Yanina G, Gaber R, Clayman ML, et al. Sun protection education for diverse audiences: need for skin cancer pictures. J Cancer Educ. 2015;30:187-189.
- Dobbinson SJ, Volkov A, Wakefield MA. Continued impact of sunsmart advertising on youth and adults’ behaviors. Am J Prev Med. 2015;49:20-28.
- Rodrigues MA, Ross AL, Gilmore S, et al. Australian dermatologists’ perspective on skin of colour: results of a national survey [published online December 9, 2016]. Australas J Dermatol. doi:10.1111/ajd.12556.
- Jacobsen A, Galvan A, Lachapelle CC, et al. Defining the need for skin cancer prevention education in uninsured, minority, and immigrant communities. JAMA Dermatol. 2016;152:1342-1347.
- Hernandez C, Kim H, Mauleon G, et al. A pilot program in collaboration with community centers to increase awareness and participation in skin cancer screening among Latinos in Chicago. J Cancer Educ. 2013;28:342-345.
- Kailas A, Solomon JA, Mostow EN, et al. Gaps in the understanding and treatment of skin cancer in people of color. J Am Acad Dermatol. 2016;74:144-149.
Practice Points
- Patients of color should be informed that they are at risk for skin cancer including melanoma.
- Patients of color should be taught to identify suspicious skin lesions including the ABCDEs of melanoma.
- Patients of color should be instructed to perform self-body skin examinations, especially of the palms and soles, for any evolving skin lesions. Patients should be instructed on the importance of visiting a physician for an evolving or suspicious mole or lesion.
Do Infants Fed Rice and Rice Products Have an Increased Risk for Skin Cancer?
To the Editor:
Rice and rice products, such as rice cereal and rice snacks, contain inorganic arsenic. Exposure to arsenicin utero and during early life may be associated with adverse fetal growth, adverse infant and child immune response, and adverse neurodevelopmental outcomes. Therefore, the World Health Organization, the Food and Agriculture Organization of the United Nations, the European Union, and the US Food and Drug Administration have suggested maximum arsenic ingestion recommendations for infants: 100 ng/g for inorganic arsenic in products geared toward infants. However, infants consuming only a few servings of rice products may exceed the weekly tolerable intake of arsenic.
Karagas et al1 obtained dietary data on 759 infants who were enrolled in the New Hampshire Birth Cohort Study from 2011 to 2014. They noted that 80% of the infants had been introduced to rice cereal during the first year. Additional data on diet and total urinary arsenic at 12 months was available for 129 infants: 32.6% of these infants were fed rice snacks. In addition, the total urinary arsenic concentration was higher among infants who ate rice cereal or rice snacks as compared to infants who did not eat rice or rice products.
Chronic arsenic exposure can result in patchy dark brown hyperpigmentation with scattered pale spots referred to as “raindrops on a dusty road.” The axilla, eyelids, groin, neck, nipples, and temples often are affected. However, the hyperpigmentation can extend across the chest, abdomen, and back in severe cases.
Horizontal white lines across the nails (Mees lines) may develop. Keratoses, often on the palms (arsenic keratoses), may appear; they persist and may progress to skin cancers. In addition, patients with arsenic exposure are more susceptible to developing nonmelanoma skin cancers.2
It is unknown if exposure to inorganic arsenic in infancy predisposes these individuals to skin cancer when they become adults. Long-term longitudinal follow-up of the participants in this study may provide additional insight. Perhaps infants should not receive rice cereals and rice snacks or their parents should more carefully monitor the amount of rice and rice products that they ingest.
- Karagas MR, Punshon T, Sayarath V, et al. Association of rice and rice-product consumption with arsenic exposure early in life. JAMA Pediatr. 2016;170:609-616.
- Mayer JE, Goldman RH. Arsenic and skin cancer in the USA: the current evidence regarding arsenic-contaminated drinking water. Int J Dermatol. 2016;55;e585-e591.
To the Editor:
Rice and rice products, such as rice cereal and rice snacks, contain inorganic arsenic. Exposure to arsenicin utero and during early life may be associated with adverse fetal growth, adverse infant and child immune response, and adverse neurodevelopmental outcomes. Therefore, the World Health Organization, the Food and Agriculture Organization of the United Nations, the European Union, and the US Food and Drug Administration have suggested maximum arsenic ingestion recommendations for infants: 100 ng/g for inorganic arsenic in products geared toward infants. However, infants consuming only a few servings of rice products may exceed the weekly tolerable intake of arsenic.
Karagas et al1 obtained dietary data on 759 infants who were enrolled in the New Hampshire Birth Cohort Study from 2011 to 2014. They noted that 80% of the infants had been introduced to rice cereal during the first year. Additional data on diet and total urinary arsenic at 12 months was available for 129 infants: 32.6% of these infants were fed rice snacks. In addition, the total urinary arsenic concentration was higher among infants who ate rice cereal or rice snacks as compared to infants who did not eat rice or rice products.
Chronic arsenic exposure can result in patchy dark brown hyperpigmentation with scattered pale spots referred to as “raindrops on a dusty road.” The axilla, eyelids, groin, neck, nipples, and temples often are affected. However, the hyperpigmentation can extend across the chest, abdomen, and back in severe cases.
Horizontal white lines across the nails (Mees lines) may develop. Keratoses, often on the palms (arsenic keratoses), may appear; they persist and may progress to skin cancers. In addition, patients with arsenic exposure are more susceptible to developing nonmelanoma skin cancers.2
It is unknown if exposure to inorganic arsenic in infancy predisposes these individuals to skin cancer when they become adults. Long-term longitudinal follow-up of the participants in this study may provide additional insight. Perhaps infants should not receive rice cereals and rice snacks or their parents should more carefully monitor the amount of rice and rice products that they ingest.
To the Editor:
Rice and rice products, such as rice cereal and rice snacks, contain inorganic arsenic. Exposure to arsenicin utero and during early life may be associated with adverse fetal growth, adverse infant and child immune response, and adverse neurodevelopmental outcomes. Therefore, the World Health Organization, the Food and Agriculture Organization of the United Nations, the European Union, and the US Food and Drug Administration have suggested maximum arsenic ingestion recommendations for infants: 100 ng/g for inorganic arsenic in products geared toward infants. However, infants consuming only a few servings of rice products may exceed the weekly tolerable intake of arsenic.
Karagas et al1 obtained dietary data on 759 infants who were enrolled in the New Hampshire Birth Cohort Study from 2011 to 2014. They noted that 80% of the infants had been introduced to rice cereal during the first year. Additional data on diet and total urinary arsenic at 12 months was available for 129 infants: 32.6% of these infants were fed rice snacks. In addition, the total urinary arsenic concentration was higher among infants who ate rice cereal or rice snacks as compared to infants who did not eat rice or rice products.
Chronic arsenic exposure can result in patchy dark brown hyperpigmentation with scattered pale spots referred to as “raindrops on a dusty road.” The axilla, eyelids, groin, neck, nipples, and temples often are affected. However, the hyperpigmentation can extend across the chest, abdomen, and back in severe cases.
Horizontal white lines across the nails (Mees lines) may develop. Keratoses, often on the palms (arsenic keratoses), may appear; they persist and may progress to skin cancers. In addition, patients with arsenic exposure are more susceptible to developing nonmelanoma skin cancers.2
It is unknown if exposure to inorganic arsenic in infancy predisposes these individuals to skin cancer when they become adults. Long-term longitudinal follow-up of the participants in this study may provide additional insight. Perhaps infants should not receive rice cereals and rice snacks or their parents should more carefully monitor the amount of rice and rice products that they ingest.
- Karagas MR, Punshon T, Sayarath V, et al. Association of rice and rice-product consumption with arsenic exposure early in life. JAMA Pediatr. 2016;170:609-616.
- Mayer JE, Goldman RH. Arsenic and skin cancer in the USA: the current evidence regarding arsenic-contaminated drinking water. Int J Dermatol. 2016;55;e585-e591.
- Karagas MR, Punshon T, Sayarath V, et al. Association of rice and rice-product consumption with arsenic exposure early in life. JAMA Pediatr. 2016;170:609-616.
- Mayer JE, Goldman RH. Arsenic and skin cancer in the USA: the current evidence regarding arsenic-contaminated drinking water. Int J Dermatol. 2016;55;e585-e591.
Must-Have Dermatology App for Skin Cancer Detection: Report From the Mount Sinai Fall Symposium
Imaging Overview: Report From the Mount Sinai Fall Symposium
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Skin Cancer in Military Pilots: A Special Population With Special Risk Factors
Military dermatologists are charged with caring for a diverse population of active-duty members, civilian dependents, and military retirees. Although certain risk factors for cutaneous malignancies are common in all of these groups, the active-duty population experiences unique exposures to be considered when determining their risk for skin cancer. One subset that may be at a higher risk is military pilots who fly at high altitudes on irregular schedules in austere environments. Through the unparalleled comradeship inherent in many military units, pilots “hear” from their fellow pilots that they are at increased risk for skin cancer. Do their occupational exposures translate into increased risk for cutaneous malignancy? This article will survey the literature pertaining to pilots and skin cancer so that all dermatologists may better care for this unique population.
Epidemiology
Anecdotally, we have observed basal cell carcinoma in pilots in their 20s and early 30s, earlier than would be expected in an otherwise healthy prescreened military population.1 Woolley and Hughes2 published a case report of skin cancer in a young military aviator. The patient was a 32-year-old male helicopter pilot with Fitzpatrick skin type II and no personal or family history of skin cancer who was diagnosed with a periocular nodular basal cell carcinoma. He deployed to locations with high UV radiation (UVR) indices, and his vacation time also was spent in such areas.2 UV radiation exposure and Fitzpatrick skin type are known risk factors across occupations, but are there special exposures that come with military aviation service?
To better understand the risk for malignancy in this special population, the US Air Force examined the rates of all cancer types among a cohort of flying versus nonflying officers.3 Aviation personnel showed increased incidence of testicular, bladder, and all-site cancers combined. Noticeably absent was a statistically significant increased risk for malignant melanoma (MM) and nonmelanoma skin cancer (NMSC). Other epidemiological studies examined the incidence rates of MM in the US Armed Forces compared with age- and race-matched civilian populations and showed mixed results: 2 studies showed increased risk,4,5 while a third showed decreased risk.6 Despite finding opposite results of MM rates in military members versus the civilian population, 2 of these studies showed US Air Force members to have higher rates of MM than those in the US Army or Navy.4,6 Interestingly, the air force has the highest number of pilots among all the services, with 4000 more pilots than the army and navy.7 Further studies are needed to determine if the higher air force MM rates occur in pilots.
Although there are mixed and limited data pertaining to military flight crews, there is more robust literature concerning civilian flight personnel. One meta-analysis pooled studies related to cancer risk in cabin crews and civil and military pilots.8 In military pilots, they found a standardized incidence ratio (SIR) of 1.43 (95% confidence interval [CI], 1.09-1.87) for MM and 1.80 (95% CI, 1.25-2.80) for NMSC. The SIRs were higher for male cabin attendants (3.42 and 7.46, respectively) and civil pilots (2.18 and 1.88, respectively). They also found the most common cause of mortality in civilian cabin crews was AIDS, possibly explaining the higher SIRs for all types of malignancy in that population.8 In the United States, many civilian pilots previously were military pilots9 who likely served in the military for at least 10 years.10 A 2015 meta-analysis of 19 studies of more than 266,000 civil pilots and aircrew members found an SIR for MM of 2.22 (95% CI, 1.67-2.93) for civil pilots and 2.09 (95% CI, 1.67-2.62) for aircrews, stating the risk for MM is at least twice that of the general population.11
Risk Factors
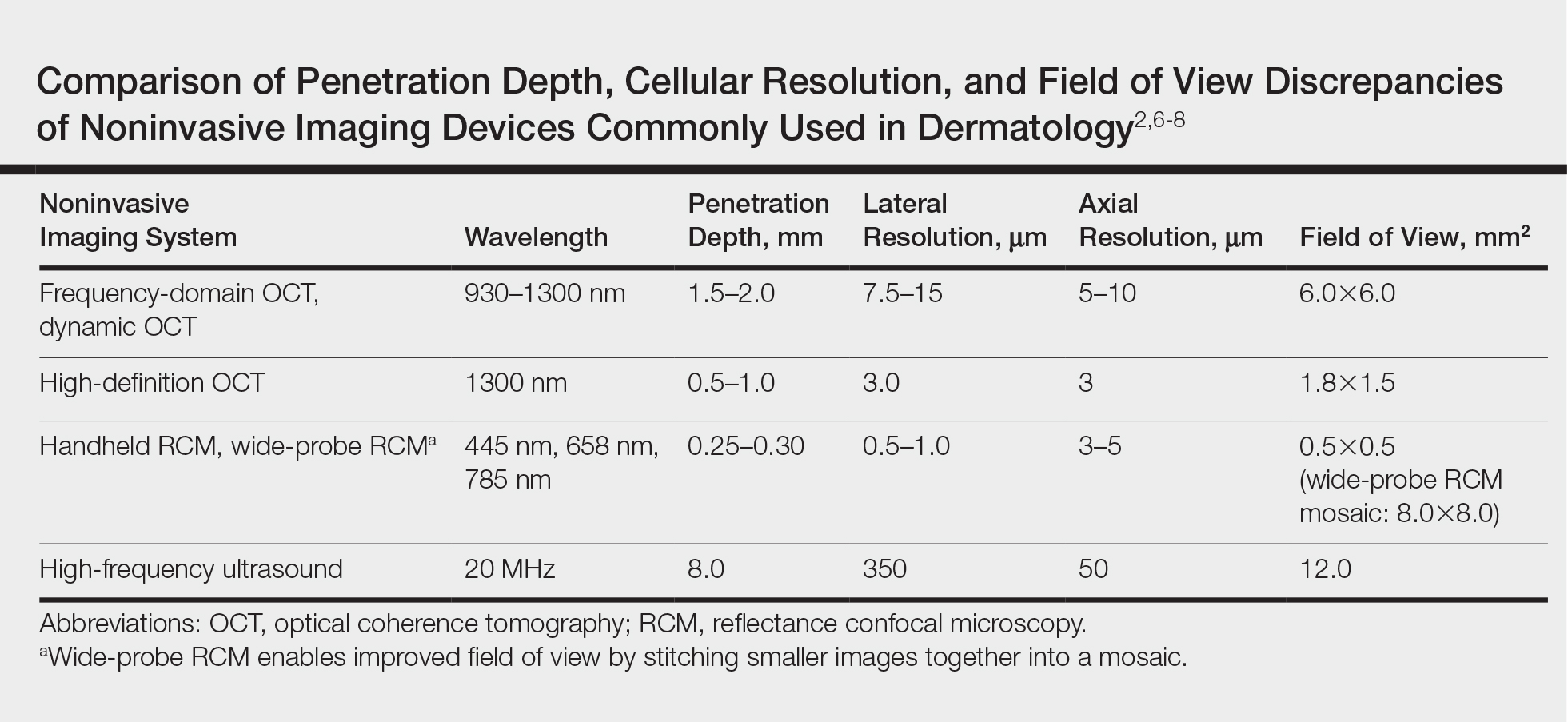
UV Radiation
These studies suggest flight duties increase the risk for cutaneous malignancy. UV radiation is a known risk factor for skin cancer.12 The main body of the aircraft may protect the cabin’s crew and passengers from UVR, but pilots are exposed to more UVR, especially in aircraft with larger windshields. A government study in 2007 examined the transmittance of UVR through windscreens of 8 aircraft: 3 commercial jets, 2 commercial propeller planes, 1 private jet, and 2 small propeller planes.13 UVB was attenuated by all the windscreens (<1% transmittance), but 43% to 54% of UVA was transmitted, with plastic windshields attenuating more than glass. Sanlorenzo et al14 measured UVA irradiance at the pilot’s seat of a turboprop aircraft at 30,000-ft altitude. They compared this exposure to a UVA tanning bed and estimated that 57 minutes of flight at 30,000-ft altitude was equivalent to 20 minutes inside a UVA tanning booth, a startling finding.14
Cosmic Radiation
Cosmic radiation consists of neutrons and gamma rays that originate outside Earth’s atmosphere. Pilots are exposed to higher doses of cosmic radiation than nonpilots, but the health effects are difficult to study. Boice et al15 described how factors such as altitude, latitude, and flight time determine pilots’ cumulative exposure. With longer flight times at higher altitudes, a pilot’s exposure to cosmic radiation is increasing over the years.15 A 2012 review found that aircrews have low-level cosmic radiation exposure. Despite increases in MM and NMSC in pilots and increased rates of breast cancer in female aircrew, overall cancer-related mortality was lower in flying versus nonflying controls.16 Thus, cosmic radiation may not be as onerous of an occupational hazard for pilots as has been postulated.
Altered Circadian Rhythms
Aviation duties, especially in the military, require irregular work schedules that repeatedly interfere with normal sleep-wake cycles, disrupt circadian rhythms, and lead to reduced melatonin levels.8 Evidence suggests that low levels of melatonin could increase the risk for breast and prostate cancer—both cancers that occur more frequently in female aircrew and male pilots, respectively—by reducing melatonin’s natural protective role in such malignancies.17,18 A World Health Organization working group categorized shift work as “probably carcinogenic” and cited alterations of melatonin levels, changes in other circadian rhythm–related gene pathways, and relative immunosuppression as likely causative factors.19 In a 2011 study, exposing mice to UVR during times when nucleotide excision repair mechanisms were at their lowest activity caused an increased rate of skin cancers.20 A 2014 review discussed how epidemiological studies of shift workers such as nurses, firefighters, pilots, and flight crews found contradictory data, but molecular studies show that circadian rhythm–linked repair and tumorigenesis mechanisms are altered by aberrations in the normal sleep-wake cycle.21
Cockpit Instrumentation
Electromagnetic energy from the flight instruments in the cockpit also could influence malignancy risk. Nicholas et al22 found magnetic field measurements within the cockpit to be 2 to 10 times that experienced within the home or office. However, no studies examining the health effects of cockpit flight instruments and magnetic fields were found.
Final Thoughts
It is important to counsel pilots on the generally recognized, nonaviation-specific risk factors of family history, skin type, and UVR exposure in the development of skin cancer. Additionally, it is important to explain the possible role of exposure to UVR at higher altitudes, cosmic radiation, and electromagnetic energy from cockpit instruments, as well as altered sleep-wake cycles. A pilot’s risk for MM may be twice that of matched controls, and the risk for NMSC could be higher.8,11 Although the literature lacks specific recommendations for pilots, it is reasonable to screen pilots once per year to better assess their individual risk and encourage diligent use of sunscreen and sun-protective measures when flying. It also may be important to advocate for the development of engineering controls that decrease UVR transmittance through windscreens, particularly for aircraft flying at higher altitudes for longer flights. More research is needed to determine if changes in circadian rhythm and decreases in melatonin increase skin cancer risk, which could impact how pilots’ schedules are managed. Together, we can ensure adequate surveillance, diagnosis, and treatment in this at-risk population.
- Roewert‐Huber J, Lange-Asschenfeldt B, Stockfleth E, et al. Epidemiology and aetiology of basal cell carcinoma. Br J Dermatol. 2007;157(suppl 2):47-51.
- Woolley SD, Hughes C. A young military pilot presents with a periocular basal cell carcinoma: a case report. Travel Med Infect Dis. 2013;11:435-437.
- Grayson JK, Lyons TJ. Cancer incidence in United States Air Force aircrew, 1975-89. Aviat Space Environ Med. 1996;67:101-104.
- Lea CS, Efird JT, Toland AE, et al. Melanoma incidence rates in active duty military personnel compared with a population-based registry in the United States, 2000-2007. Mil Med. 2014;179:247-253.
- Garland FC, White MR, Garland CF, et al. Occupational sunlight exposure and melanoma in the US Navy. Arc Environ Health. 1990;45:261-267.
- Zhou J, Enewold L, Zahm SH, et al. Melanoma incidence rates among whites in the US military. Cancer Epidemiol Biomarkers Prev. 2011;20:318-323.
- Active Duty Master Personnel File: Active Duty Tactical Operations Officers. Seaside, CA: Defense Manpower Data Center; August 31, 2017. Accessed September 22, 2017.
- Buja A, Lange JH, Perissinotto E, et al. Cancer incidence among male military and civil pilots and flight attendants: an analysis on published data. Toxicol Ind Health. 2005;21:273-282.
- Jansen HS, Oster CV, eds. Taking Flight: Education and Training for Aviation Careers. Washington, DC: National Academy Press; 1997.
- About AFROTC Service Commitment. US Air Force ROTC website. https://www.afrotc.com/about/service. Accessed September 20, 2017.
- Sanlorenzo M, Wehner MR, Linos E, et al. The risk of melanoma in airline pilots and cabin crew: a meta-analysis. JAMA Dermatol. 2015;151:51-58.
- Ananthaswamy HN, Pierceall WE. Molecular mechanisms of ultraviolet radiation carcinogenesis. Photochem Photobiol. 1990;52:1119-1136.
- Nakagawara VB, Montgomery RW, Marshall WJ. Optical Radiation Transmittance of Aircraft Windscreens and Pilot Vision. Oklahoma City, OK: Federal Aviation Administration; 2007.
- Sanlorenzo M, Vujic I, Posch C, et al. The risk of melanoma in pilots and cabin crew: UV measurements in flying airplanes. JAMA Dermatol. 2015;151:450-452.
- Boice JD, Blettner M, Auvinen A. Epidemiologic studies of pilots and aircrew. Health Phys. 2000;79:576-584.
- Zeeb H, Hammer GP, Blettner M. Epidemiological investigations of aircrew: an occupational group with low-level cosmic radiation exposure [published online March 6, 2012]. J Radiol Prot. 2012;32:N15-N19.
- Stevens RG. Circadian disruption and breast cancer: from melatonin to clock genes. Epidemiology. 2005;16:254-258.
- Siu SW, Lau KW, Tam PC, et al. Melatonin and prostate cancer cell proliferation: interplay with castration, epidermal growth factor, and androgen sensitivity. Prostate. 2002;52:106-122.
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Painting, Firefighting, and Shiftwork. Lyon, France: World Health Organization International Agency for Research on Cancer; 2010.
- Gaddameedhi S, Selby CP, Kaufmann WK, et al. Control of skin cancer by the circadian rhythm. Proc Natl Acad Sci. 2011;108:18790-18795.
- Markova-Car EP, Jurišic´ D, Ilic´ N, et al. Running for time: circadian rhythms and melanoma. Tumour Biol. 2014;35:8359-8368.
- Nicholas JS, Lackland DT, Butler GC, et al. Cosmic radiation and magnetic field exposure to airline flight crews. Am J Ind Med. 1998;34:574-580.
Military dermatologists are charged with caring for a diverse population of active-duty members, civilian dependents, and military retirees. Although certain risk factors for cutaneous malignancies are common in all of these groups, the active-duty population experiences unique exposures to be considered when determining their risk for skin cancer. One subset that may be at a higher risk is military pilots who fly at high altitudes on irregular schedules in austere environments. Through the unparalleled comradeship inherent in many military units, pilots “hear” from their fellow pilots that they are at increased risk for skin cancer. Do their occupational exposures translate into increased risk for cutaneous malignancy? This article will survey the literature pertaining to pilots and skin cancer so that all dermatologists may better care for this unique population.
Epidemiology
Anecdotally, we have observed basal cell carcinoma in pilots in their 20s and early 30s, earlier than would be expected in an otherwise healthy prescreened military population.1 Woolley and Hughes2 published a case report of skin cancer in a young military aviator. The patient was a 32-year-old male helicopter pilot with Fitzpatrick skin type II and no personal or family history of skin cancer who was diagnosed with a periocular nodular basal cell carcinoma. He deployed to locations with high UV radiation (UVR) indices, and his vacation time also was spent in such areas.2 UV radiation exposure and Fitzpatrick skin type are known risk factors across occupations, but are there special exposures that come with military aviation service?
To better understand the risk for malignancy in this special population, the US Air Force examined the rates of all cancer types among a cohort of flying versus nonflying officers.3 Aviation personnel showed increased incidence of testicular, bladder, and all-site cancers combined. Noticeably absent was a statistically significant increased risk for malignant melanoma (MM) and nonmelanoma skin cancer (NMSC). Other epidemiological studies examined the incidence rates of MM in the US Armed Forces compared with age- and race-matched civilian populations and showed mixed results: 2 studies showed increased risk,4,5 while a third showed decreased risk.6 Despite finding opposite results of MM rates in military members versus the civilian population, 2 of these studies showed US Air Force members to have higher rates of MM than those in the US Army or Navy.4,6 Interestingly, the air force has the highest number of pilots among all the services, with 4000 more pilots than the army and navy.7 Further studies are needed to determine if the higher air force MM rates occur in pilots.
Although there are mixed and limited data pertaining to military flight crews, there is more robust literature concerning civilian flight personnel. One meta-analysis pooled studies related to cancer risk in cabin crews and civil and military pilots.8 In military pilots, they found a standardized incidence ratio (SIR) of 1.43 (95% confidence interval [CI], 1.09-1.87) for MM and 1.80 (95% CI, 1.25-2.80) for NMSC. The SIRs were higher for male cabin attendants (3.42 and 7.46, respectively) and civil pilots (2.18 and 1.88, respectively). They also found the most common cause of mortality in civilian cabin crews was AIDS, possibly explaining the higher SIRs for all types of malignancy in that population.8 In the United States, many civilian pilots previously were military pilots9 who likely served in the military for at least 10 years.10 A 2015 meta-analysis of 19 studies of more than 266,000 civil pilots and aircrew members found an SIR for MM of 2.22 (95% CI, 1.67-2.93) for civil pilots and 2.09 (95% CI, 1.67-2.62) for aircrews, stating the risk for MM is at least twice that of the general population.11
Risk Factors
UV Radiation
These studies suggest flight duties increase the risk for cutaneous malignancy. UV radiation is a known risk factor for skin cancer.12 The main body of the aircraft may protect the cabin’s crew and passengers from UVR, but pilots are exposed to more UVR, especially in aircraft with larger windshields. A government study in 2007 examined the transmittance of UVR through windscreens of 8 aircraft: 3 commercial jets, 2 commercial propeller planes, 1 private jet, and 2 small propeller planes.13 UVB was attenuated by all the windscreens (<1% transmittance), but 43% to 54% of UVA was transmitted, with plastic windshields attenuating more than glass. Sanlorenzo et al14 measured UVA irradiance at the pilot’s seat of a turboprop aircraft at 30,000-ft altitude. They compared this exposure to a UVA tanning bed and estimated that 57 minutes of flight at 30,000-ft altitude was equivalent to 20 minutes inside a UVA tanning booth, a startling finding.14
Cosmic Radiation
Cosmic radiation consists of neutrons and gamma rays that originate outside Earth’s atmosphere. Pilots are exposed to higher doses of cosmic radiation than nonpilots, but the health effects are difficult to study. Boice et al15 described how factors such as altitude, latitude, and flight time determine pilots’ cumulative exposure. With longer flight times at higher altitudes, a pilot’s exposure to cosmic radiation is increasing over the years.15 A 2012 review found that aircrews have low-level cosmic radiation exposure. Despite increases in MM and NMSC in pilots and increased rates of breast cancer in female aircrew, overall cancer-related mortality was lower in flying versus nonflying controls.16 Thus, cosmic radiation may not be as onerous of an occupational hazard for pilots as has been postulated.
Altered Circadian Rhythms
Aviation duties, especially in the military, require irregular work schedules that repeatedly interfere with normal sleep-wake cycles, disrupt circadian rhythms, and lead to reduced melatonin levels.8 Evidence suggests that low levels of melatonin could increase the risk for breast and prostate cancer—both cancers that occur more frequently in female aircrew and male pilots, respectively—by reducing melatonin’s natural protective role in such malignancies.17,18 A World Health Organization working group categorized shift work as “probably carcinogenic” and cited alterations of melatonin levels, changes in other circadian rhythm–related gene pathways, and relative immunosuppression as likely causative factors.19 In a 2011 study, exposing mice to UVR during times when nucleotide excision repair mechanisms were at their lowest activity caused an increased rate of skin cancers.20 A 2014 review discussed how epidemiological studies of shift workers such as nurses, firefighters, pilots, and flight crews found contradictory data, but molecular studies show that circadian rhythm–linked repair and tumorigenesis mechanisms are altered by aberrations in the normal sleep-wake cycle.21
Cockpit Instrumentation
Electromagnetic energy from the flight instruments in the cockpit also could influence malignancy risk. Nicholas et al22 found magnetic field measurements within the cockpit to be 2 to 10 times that experienced within the home or office. However, no studies examining the health effects of cockpit flight instruments and magnetic fields were found.
Final Thoughts
It is important to counsel pilots on the generally recognized, nonaviation-specific risk factors of family history, skin type, and UVR exposure in the development of skin cancer. Additionally, it is important to explain the possible role of exposure to UVR at higher altitudes, cosmic radiation, and electromagnetic energy from cockpit instruments, as well as altered sleep-wake cycles. A pilot’s risk for MM may be twice that of matched controls, and the risk for NMSC could be higher.8,11 Although the literature lacks specific recommendations for pilots, it is reasonable to screen pilots once per year to better assess their individual risk and encourage diligent use of sunscreen and sun-protective measures when flying. It also may be important to advocate for the development of engineering controls that decrease UVR transmittance through windscreens, particularly for aircraft flying at higher altitudes for longer flights. More research is needed to determine if changes in circadian rhythm and decreases in melatonin increase skin cancer risk, which could impact how pilots’ schedules are managed. Together, we can ensure adequate surveillance, diagnosis, and treatment in this at-risk population.
Military dermatologists are charged with caring for a diverse population of active-duty members, civilian dependents, and military retirees. Although certain risk factors for cutaneous malignancies are common in all of these groups, the active-duty population experiences unique exposures to be considered when determining their risk for skin cancer. One subset that may be at a higher risk is military pilots who fly at high altitudes on irregular schedules in austere environments. Through the unparalleled comradeship inherent in many military units, pilots “hear” from their fellow pilots that they are at increased risk for skin cancer. Do their occupational exposures translate into increased risk for cutaneous malignancy? This article will survey the literature pertaining to pilots and skin cancer so that all dermatologists may better care for this unique population.
Epidemiology
Anecdotally, we have observed basal cell carcinoma in pilots in their 20s and early 30s, earlier than would be expected in an otherwise healthy prescreened military population.1 Woolley and Hughes2 published a case report of skin cancer in a young military aviator. The patient was a 32-year-old male helicopter pilot with Fitzpatrick skin type II and no personal or family history of skin cancer who was diagnosed with a periocular nodular basal cell carcinoma. He deployed to locations with high UV radiation (UVR) indices, and his vacation time also was spent in such areas.2 UV radiation exposure and Fitzpatrick skin type are known risk factors across occupations, but are there special exposures that come with military aviation service?
To better understand the risk for malignancy in this special population, the US Air Force examined the rates of all cancer types among a cohort of flying versus nonflying officers.3 Aviation personnel showed increased incidence of testicular, bladder, and all-site cancers combined. Noticeably absent was a statistically significant increased risk for malignant melanoma (MM) and nonmelanoma skin cancer (NMSC). Other epidemiological studies examined the incidence rates of MM in the US Armed Forces compared with age- and race-matched civilian populations and showed mixed results: 2 studies showed increased risk,4,5 while a third showed decreased risk.6 Despite finding opposite results of MM rates in military members versus the civilian population, 2 of these studies showed US Air Force members to have higher rates of MM than those in the US Army or Navy.4,6 Interestingly, the air force has the highest number of pilots among all the services, with 4000 more pilots than the army and navy.7 Further studies are needed to determine if the higher air force MM rates occur in pilots.
Although there are mixed and limited data pertaining to military flight crews, there is more robust literature concerning civilian flight personnel. One meta-analysis pooled studies related to cancer risk in cabin crews and civil and military pilots.8 In military pilots, they found a standardized incidence ratio (SIR) of 1.43 (95% confidence interval [CI], 1.09-1.87) for MM and 1.80 (95% CI, 1.25-2.80) for NMSC. The SIRs were higher for male cabin attendants (3.42 and 7.46, respectively) and civil pilots (2.18 and 1.88, respectively). They also found the most common cause of mortality in civilian cabin crews was AIDS, possibly explaining the higher SIRs for all types of malignancy in that population.8 In the United States, many civilian pilots previously were military pilots9 who likely served in the military for at least 10 years.10 A 2015 meta-analysis of 19 studies of more than 266,000 civil pilots and aircrew members found an SIR for MM of 2.22 (95% CI, 1.67-2.93) for civil pilots and 2.09 (95% CI, 1.67-2.62) for aircrews, stating the risk for MM is at least twice that of the general population.11
Risk Factors
UV Radiation
These studies suggest flight duties increase the risk for cutaneous malignancy. UV radiation is a known risk factor for skin cancer.12 The main body of the aircraft may protect the cabin’s crew and passengers from UVR, but pilots are exposed to more UVR, especially in aircraft with larger windshields. A government study in 2007 examined the transmittance of UVR through windscreens of 8 aircraft: 3 commercial jets, 2 commercial propeller planes, 1 private jet, and 2 small propeller planes.13 UVB was attenuated by all the windscreens (<1% transmittance), but 43% to 54% of UVA was transmitted, with plastic windshields attenuating more than glass. Sanlorenzo et al14 measured UVA irradiance at the pilot’s seat of a turboprop aircraft at 30,000-ft altitude. They compared this exposure to a UVA tanning bed and estimated that 57 minutes of flight at 30,000-ft altitude was equivalent to 20 minutes inside a UVA tanning booth, a startling finding.14
Cosmic Radiation
Cosmic radiation consists of neutrons and gamma rays that originate outside Earth’s atmosphere. Pilots are exposed to higher doses of cosmic radiation than nonpilots, but the health effects are difficult to study. Boice et al15 described how factors such as altitude, latitude, and flight time determine pilots’ cumulative exposure. With longer flight times at higher altitudes, a pilot’s exposure to cosmic radiation is increasing over the years.15 A 2012 review found that aircrews have low-level cosmic radiation exposure. Despite increases in MM and NMSC in pilots and increased rates of breast cancer in female aircrew, overall cancer-related mortality was lower in flying versus nonflying controls.16 Thus, cosmic radiation may not be as onerous of an occupational hazard for pilots as has been postulated.
Altered Circadian Rhythms
Aviation duties, especially in the military, require irregular work schedules that repeatedly interfere with normal sleep-wake cycles, disrupt circadian rhythms, and lead to reduced melatonin levels.8 Evidence suggests that low levels of melatonin could increase the risk for breast and prostate cancer—both cancers that occur more frequently in female aircrew and male pilots, respectively—by reducing melatonin’s natural protective role in such malignancies.17,18 A World Health Organization working group categorized shift work as “probably carcinogenic” and cited alterations of melatonin levels, changes in other circadian rhythm–related gene pathways, and relative immunosuppression as likely causative factors.19 In a 2011 study, exposing mice to UVR during times when nucleotide excision repair mechanisms were at their lowest activity caused an increased rate of skin cancers.20 A 2014 review discussed how epidemiological studies of shift workers such as nurses, firefighters, pilots, and flight crews found contradictory data, but molecular studies show that circadian rhythm–linked repair and tumorigenesis mechanisms are altered by aberrations in the normal sleep-wake cycle.21
Cockpit Instrumentation
Electromagnetic energy from the flight instruments in the cockpit also could influence malignancy risk. Nicholas et al22 found magnetic field measurements within the cockpit to be 2 to 10 times that experienced within the home or office. However, no studies examining the health effects of cockpit flight instruments and magnetic fields were found.
Final Thoughts
It is important to counsel pilots on the generally recognized, nonaviation-specific risk factors of family history, skin type, and UVR exposure in the development of skin cancer. Additionally, it is important to explain the possible role of exposure to UVR at higher altitudes, cosmic radiation, and electromagnetic energy from cockpit instruments, as well as altered sleep-wake cycles. A pilot’s risk for MM may be twice that of matched controls, and the risk for NMSC could be higher.8,11 Although the literature lacks specific recommendations for pilots, it is reasonable to screen pilots once per year to better assess their individual risk and encourage diligent use of sunscreen and sun-protective measures when flying. It also may be important to advocate for the development of engineering controls that decrease UVR transmittance through windscreens, particularly for aircraft flying at higher altitudes for longer flights. More research is needed to determine if changes in circadian rhythm and decreases in melatonin increase skin cancer risk, which could impact how pilots’ schedules are managed. Together, we can ensure adequate surveillance, diagnosis, and treatment in this at-risk population.
- Roewert‐Huber J, Lange-Asschenfeldt B, Stockfleth E, et al. Epidemiology and aetiology of basal cell carcinoma. Br J Dermatol. 2007;157(suppl 2):47-51.
- Woolley SD, Hughes C. A young military pilot presents with a periocular basal cell carcinoma: a case report. Travel Med Infect Dis. 2013;11:435-437.
- Grayson JK, Lyons TJ. Cancer incidence in United States Air Force aircrew, 1975-89. Aviat Space Environ Med. 1996;67:101-104.
- Lea CS, Efird JT, Toland AE, et al. Melanoma incidence rates in active duty military personnel compared with a population-based registry in the United States, 2000-2007. Mil Med. 2014;179:247-253.
- Garland FC, White MR, Garland CF, et al. Occupational sunlight exposure and melanoma in the US Navy. Arc Environ Health. 1990;45:261-267.
- Zhou J, Enewold L, Zahm SH, et al. Melanoma incidence rates among whites in the US military. Cancer Epidemiol Biomarkers Prev. 2011;20:318-323.
- Active Duty Master Personnel File: Active Duty Tactical Operations Officers. Seaside, CA: Defense Manpower Data Center; August 31, 2017. Accessed September 22, 2017.
- Buja A, Lange JH, Perissinotto E, et al. Cancer incidence among male military and civil pilots and flight attendants: an analysis on published data. Toxicol Ind Health. 2005;21:273-282.
- Jansen HS, Oster CV, eds. Taking Flight: Education and Training for Aviation Careers. Washington, DC: National Academy Press; 1997.
- About AFROTC Service Commitment. US Air Force ROTC website. https://www.afrotc.com/about/service. Accessed September 20, 2017.
- Sanlorenzo M, Wehner MR, Linos E, et al. The risk of melanoma in airline pilots and cabin crew: a meta-analysis. JAMA Dermatol. 2015;151:51-58.
- Ananthaswamy HN, Pierceall WE. Molecular mechanisms of ultraviolet radiation carcinogenesis. Photochem Photobiol. 1990;52:1119-1136.
- Nakagawara VB, Montgomery RW, Marshall WJ. Optical Radiation Transmittance of Aircraft Windscreens and Pilot Vision. Oklahoma City, OK: Federal Aviation Administration; 2007.
- Sanlorenzo M, Vujic I, Posch C, et al. The risk of melanoma in pilots and cabin crew: UV measurements in flying airplanes. JAMA Dermatol. 2015;151:450-452.
- Boice JD, Blettner M, Auvinen A. Epidemiologic studies of pilots and aircrew. Health Phys. 2000;79:576-584.
- Zeeb H, Hammer GP, Blettner M. Epidemiological investigations of aircrew: an occupational group with low-level cosmic radiation exposure [published online March 6, 2012]. J Radiol Prot. 2012;32:N15-N19.
- Stevens RG. Circadian disruption and breast cancer: from melatonin to clock genes. Epidemiology. 2005;16:254-258.
- Siu SW, Lau KW, Tam PC, et al. Melatonin and prostate cancer cell proliferation: interplay with castration, epidermal growth factor, and androgen sensitivity. Prostate. 2002;52:106-122.
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Painting, Firefighting, and Shiftwork. Lyon, France: World Health Organization International Agency for Research on Cancer; 2010.
- Gaddameedhi S, Selby CP, Kaufmann WK, et al. Control of skin cancer by the circadian rhythm. Proc Natl Acad Sci. 2011;108:18790-18795.
- Markova-Car EP, Jurišic´ D, Ilic´ N, et al. Running for time: circadian rhythms and melanoma. Tumour Biol. 2014;35:8359-8368.
- Nicholas JS, Lackland DT, Butler GC, et al. Cosmic radiation and magnetic field exposure to airline flight crews. Am J Ind Med. 1998;34:574-580.
- Roewert‐Huber J, Lange-Asschenfeldt B, Stockfleth E, et al. Epidemiology and aetiology of basal cell carcinoma. Br J Dermatol. 2007;157(suppl 2):47-51.
- Woolley SD, Hughes C. A young military pilot presents with a periocular basal cell carcinoma: a case report. Travel Med Infect Dis. 2013;11:435-437.
- Grayson JK, Lyons TJ. Cancer incidence in United States Air Force aircrew, 1975-89. Aviat Space Environ Med. 1996;67:101-104.
- Lea CS, Efird JT, Toland AE, et al. Melanoma incidence rates in active duty military personnel compared with a population-based registry in the United States, 2000-2007. Mil Med. 2014;179:247-253.
- Garland FC, White MR, Garland CF, et al. Occupational sunlight exposure and melanoma in the US Navy. Arc Environ Health. 1990;45:261-267.
- Zhou J, Enewold L, Zahm SH, et al. Melanoma incidence rates among whites in the US military. Cancer Epidemiol Biomarkers Prev. 2011;20:318-323.
- Active Duty Master Personnel File: Active Duty Tactical Operations Officers. Seaside, CA: Defense Manpower Data Center; August 31, 2017. Accessed September 22, 2017.
- Buja A, Lange JH, Perissinotto E, et al. Cancer incidence among male military and civil pilots and flight attendants: an analysis on published data. Toxicol Ind Health. 2005;21:273-282.
- Jansen HS, Oster CV, eds. Taking Flight: Education and Training for Aviation Careers. Washington, DC: National Academy Press; 1997.
- About AFROTC Service Commitment. US Air Force ROTC website. https://www.afrotc.com/about/service. Accessed September 20, 2017.
- Sanlorenzo M, Wehner MR, Linos E, et al. The risk of melanoma in airline pilots and cabin crew: a meta-analysis. JAMA Dermatol. 2015;151:51-58.
- Ananthaswamy HN, Pierceall WE. Molecular mechanisms of ultraviolet radiation carcinogenesis. Photochem Photobiol. 1990;52:1119-1136.
- Nakagawara VB, Montgomery RW, Marshall WJ. Optical Radiation Transmittance of Aircraft Windscreens and Pilot Vision. Oklahoma City, OK: Federal Aviation Administration; 2007.
- Sanlorenzo M, Vujic I, Posch C, et al. The risk of melanoma in pilots and cabin crew: UV measurements in flying airplanes. JAMA Dermatol. 2015;151:450-452.
- Boice JD, Blettner M, Auvinen A. Epidemiologic studies of pilots and aircrew. Health Phys. 2000;79:576-584.
- Zeeb H, Hammer GP, Blettner M. Epidemiological investigations of aircrew: an occupational group with low-level cosmic radiation exposure [published online March 6, 2012]. J Radiol Prot. 2012;32:N15-N19.
- Stevens RG. Circadian disruption and breast cancer: from melatonin to clock genes. Epidemiology. 2005;16:254-258.
- Siu SW, Lau KW, Tam PC, et al. Melatonin and prostate cancer cell proliferation: interplay with castration, epidermal growth factor, and androgen sensitivity. Prostate. 2002;52:106-122.
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Painting, Firefighting, and Shiftwork. Lyon, France: World Health Organization International Agency for Research on Cancer; 2010.
- Gaddameedhi S, Selby CP, Kaufmann WK, et al. Control of skin cancer by the circadian rhythm. Proc Natl Acad Sci. 2011;108:18790-18795.
- Markova-Car EP, Jurišic´ D, Ilic´ N, et al. Running for time: circadian rhythms and melanoma. Tumour Biol. 2014;35:8359-8368.
- Nicholas JS, Lackland DT, Butler GC, et al. Cosmic radiation and magnetic field exposure to airline flight crews. Am J Ind Med. 1998;34:574-580.
Practice Points
- Military and civilian pilots have an increased risk for melanoma and nonmelanoma skin cancer, likely due to unique occupational exposures.
- We recommend annual skin cancer screening for all pilots to help assess their individual risk.
- Pilots should be educated on their increased risk for skin cancer and encouraged to use sun-protective measures during their flying duties and leisure activities.
Noninvasive Imaging: Report From the Mount Sinai Fall Symposium
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Atypical Fibroxanthoma Arising Within Erosive Pustular Dermatosis of the Scalp
Atypical fibroxanthoma (AFX) is a low-grade dermal malignancy comprised of atypical spindle cells.1 Classified as a superficial fibrohistiocytic tumor with intermediate malignant potential, AFX has an incidence of approximately 0.24% worldwide.2 The tumor appears mainly on the head and neck in sun-exposed areas but can occur less frequently on the trunk and limbs in non–sun-exposed areas. There is a 70% to 80% predominance in men aged 69 to 77 years, with lesions primarily occurring in sun-exposed areas of the head and neck.3 A median period of 4 months between time of onset and time of diagnosis has been previously established.4
When AFX does occur in non–sun-exposed areas, it tends to be in a younger patient population. Clinically, it presents as a rather nondescript, firm, erythematous papule or nodule less than 2 cm in diameter. Atypical fibroxanthoma most often presents asymptomatically, but the tumor may ulcerate and bleed, though pain and pruritus are uncommon.5 Findings are nonspecific, and the diagnosis must be confirmed with biopsy, as it can resemble other common dermatological lesions. The pathogenesis of AFX has been controversial. Two different studies looked at AFX using electron microscopy and concluded that the tumor most closely resembled a myofibroblast,6,7 which is consistent with current thinking today.
Atypical fibroxanthoma is believed to be associated with p53 mutation and is closely linked with exposure to UV radiation due to its predominance in sun-exposed areas. Other predisposing factors may include prior exposure to UV radiation, history of organ transplantation, immunosuppression, advanced age in men, and xeroderma pigmentosum. The differential diagnosis for AFX encompasses basal cell carcinoma, squamous cell carcinoma, Merkel cell carcinoma, adnexal tumor, and pyogenic granuloma.
Case Report
On physical examination, the lesions appeared erosive with crusting and granulation tissue (Figure 1A). The presentation was consistent with erosive pustular dermatosis of the scalp. Biopsy revealed granulation tissue. The patient underwent PDT and prednisone treatment with improvement. Additional biopsies revealed AKs. His condition improved with 2 PDT sessions but never fully cleared. During the PDT sessions, the patient reported intense unilateral headaches without visual changes. The headaches were intermittent and not apparently related to the treatments. He was referred for a temporal artery biopsy and rebiopsy of the remaining lesion on the scalp. The temporal artery biopsy was negative. The lesion that remained was a large nodule on the vertex scalp, and biopsy revealed AFX.
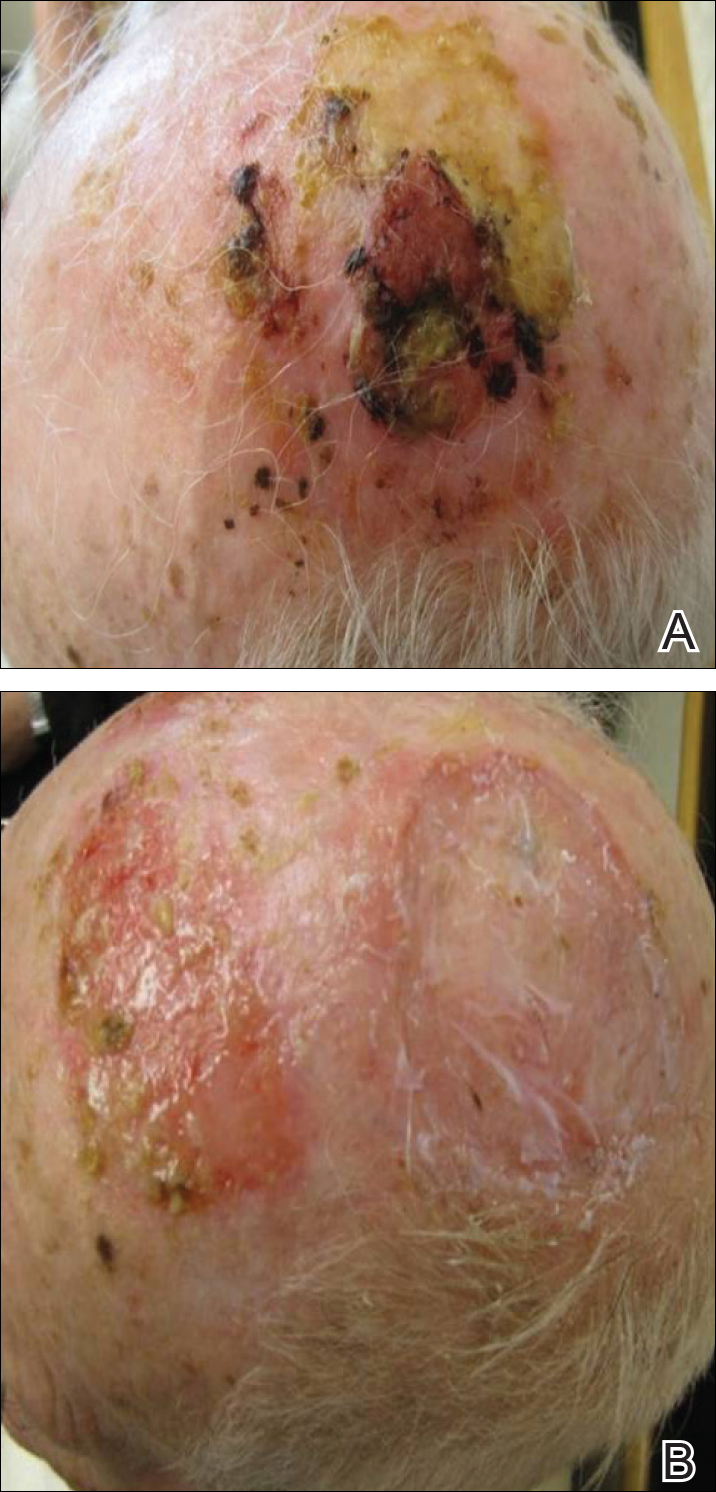
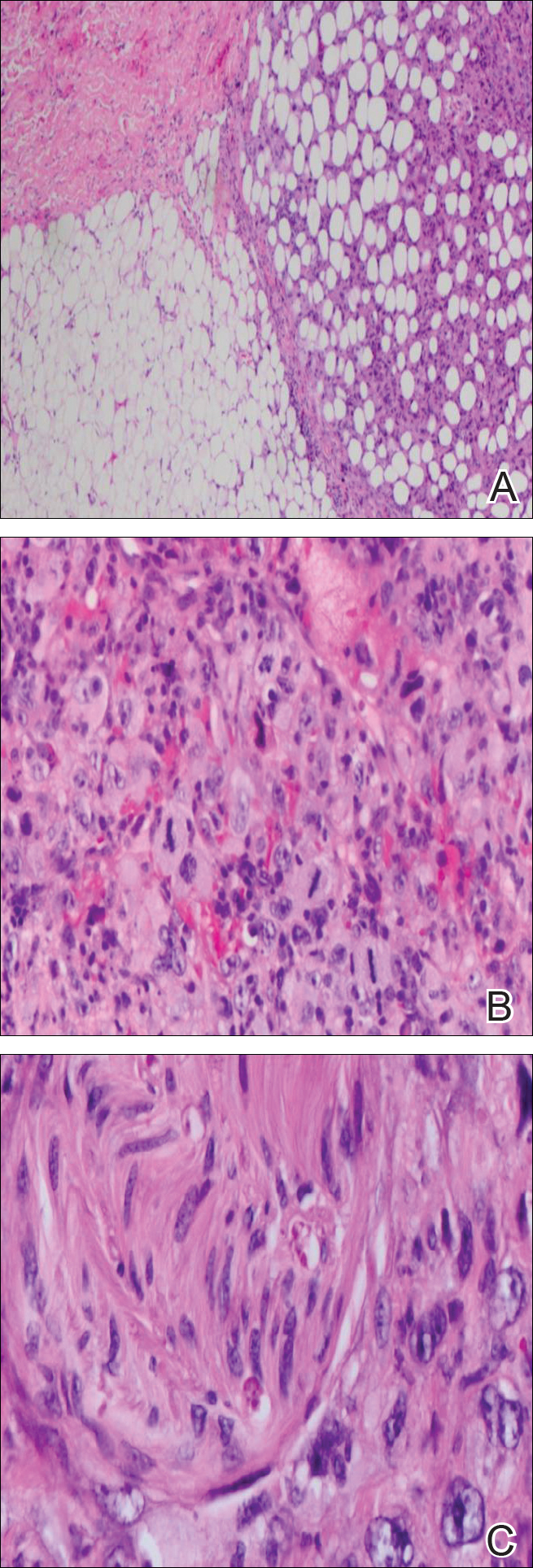
Immunohistochemical marker studies for S-100 and cytokeratin were negative. Invasion into subcutaneous fat was encountered (Figure 2A). Highly atypical spindle cells and mitoses were present (Figure 2B). Neoplastic cells were noted adjacent to nerve (Figure 2C). Excision of the lesion was curative, and his symptoms of pain and erosive pustular dermatosis resolved weeks thereafter (Figure 1B). The area of erosive pustular dermatosis was not excised, but symptoms resolved weeks following excision of the AFX.
Comment
Our case of AFX is unique due to the patient’s atypical presentation of severe pain. Because AFX usually presents asymptomatically, pain is an uncommon symptom. Based on the histologic findings in our case, we suspected that neural involvement of the tumor most likely explained the intense pain that our patient experienced.
The presence of erosive pustular dermatosis of the scalp also is interesting in our case. This elderly man had an extensive history of actinic damage and had reported pustules, scaling, itching, and scabbing of the scalp. It is possible that erosive pustular dermatosis was superimposed over the tumor and could have been the reason that multiple biopsies were needed to eventually arrive at a diagnosis. The coexistence of the 2 entities suggests that the chronic actinic damage played a role in the etiology of both.
Classification
There is a question regarding nomenclature when discussing AFX. Atypical fibroxanthoma has been referred to as a variant of undifferentiated pleomorphic sarcoma, which is a type of soft tissue sarcoma. Atypical fibroxanthoma can be referred to as undifferentiated pleomorphic sarcoma if it is more than 2 cm in diameter, if it involves the fascia or subcutaneous tissue, or if there is evidence of necrosis.3 Atypical fibroxanthoma generally is confined to the head and neck region and usually is less than 2 cm in diameter. In this patient, the presentation was consistent with AFX, as there was evidence of necrosis and invasion into the subcutaneous fat. The fact that the lesion also appeared on the scalp further supported the diagnosis of AFX.
Pathology
Biopsy of AFX typically reveals a spindle cell proliferation that usually arises in the setting of profound actinic damage. The epidermis may or may not be ulcerated, and in most cases, it is seen in close proximity to the overlying epidermis but not arising from it.8 Classic AFX is composed of highly atypical histiocytelike (epithelioid) cells admixed with pleomorphic spindle cells and giant cells, all showing frequent mitoses including atypical ones.9 Several histologic subtypes of AFX have been described, including clear cell, granular cell, pigmented cell, chondroid, osteoid, osteoclastic, and the most common spindle cell subtype.9 Features that indicate potential aggressive behavior include infiltration into the subcutaneous tissue, vascular invasion, and presence of necrosis. A diagnosis of AFX is made by exclusion of other malignant neoplasms with similar morphology, namely spindle cell squamous cell carcinoma, spindle cell melanoma, and leiomyoscarcoma.9 As such, immunohistochemistry plays a critical role in distinguishing these lesions, as they arise as part of the differential diagnosis. A panel of immunohistochemical stains is helpful for diagnosis and commonly includes but is not limited to S-100, Melan-A, smooth muscle actin, desmin, and cytokeratin.
Sampling error is an inherent flaw in any biopsy specimen. The eventual diagnosis of AFX in our case supports the argument for multiple biopsies of an unknown lesion, seeing as the affected area was interpreted as both granulation tissue and AK prior to the eventual diagnosis. Repeat biopsies, especially if a lesion is nonhealing, often can help clinicians arrive at a definitive diagnosis.
Treatment
Different treatment options have been used to manage AFX. Mohs micrographic surgery is most often used because of its tissue-sparing potential, often giving the most cosmetically appealing result. Wide local excision is another surgical technique utilized, generally with fixed margins of at least 1 cm.10 Radiation at the tumor site is used as a treatment method but most often during cases of reoccurrence. Cryotherapy as well as electrodesiccation and curettage are possible treatment options but are not the standard of care.
- Helwig EB. Atypical fibroxanthoma, in tumor seminar. proceedings of 18th Annual Seminar of San Antonio Society of Pathologists, 1961. Tex State J Med. 1963;59:664-667.
- Anderson HL, Joseph AK. A pilot feasibility study of a rare skin tumor database. Dermatol Surg. 2007;33:693-696.
- Iorizzo LJ 3rd, Brown MD. Atypical fibroxanthoma: a review of the literature. Dermatol Surg. 2011;37:146-157.
- Fretzin DF, Helwig EB. Atypical fibroxanthoma of the skin. a clinicopathologic study of 140 cases. Cancer. 1973;31:1541-1552.
- Vandergriff TW, Reed JA, Orengo IF. An unusual presentation of atypical fibroxanthoma. Dermatol Online J. 2008;14:6.
- Weedon D, Kerr JF. Atypical fibroxanthoma of skin: an electron microscope study. Pathology. 1975;7:173-177.
- Woyke S, Domagala W, Olszewski W, et al. Pseudosarcoma of the skin. an electron microscopic study and comparison with the fine structure of spindle-cell variant of squamous carcinoma. Cancer. 1974;33:970-980.
- Edward S, Yung A. Essential Dermatopathology. Philadelphia, PA: Lippincott Williams & Wilkins; 2012.
- Luzar B, Calonje E. Morphologic and immunohistochemical characteristics of atypical fibroxanthoma with a special emphasis on potential diagnostic pitfalls: a review. J Cutan Pathol. 2010;37:301-309.
- González-García R, Nam-Cha SH, Muñoz-Guerra MF, et al. Atypical fibroxanthoma of the head and neck: report of 5 cases. J Oral Maxillofac Surg. 2007;65:526-531.
Atypical fibroxanthoma (AFX) is a low-grade dermal malignancy comprised of atypical spindle cells.1 Classified as a superficial fibrohistiocytic tumor with intermediate malignant potential, AFX has an incidence of approximately 0.24% worldwide.2 The tumor appears mainly on the head and neck in sun-exposed areas but can occur less frequently on the trunk and limbs in non–sun-exposed areas. There is a 70% to 80% predominance in men aged 69 to 77 years, with lesions primarily occurring in sun-exposed areas of the head and neck.3 A median period of 4 months between time of onset and time of diagnosis has been previously established.4
When AFX does occur in non–sun-exposed areas, it tends to be in a younger patient population. Clinically, it presents as a rather nondescript, firm, erythematous papule or nodule less than 2 cm in diameter. Atypical fibroxanthoma most often presents asymptomatically, but the tumor may ulcerate and bleed, though pain and pruritus are uncommon.5 Findings are nonspecific, and the diagnosis must be confirmed with biopsy, as it can resemble other common dermatological lesions. The pathogenesis of AFX has been controversial. Two different studies looked at AFX using electron microscopy and concluded that the tumor most closely resembled a myofibroblast,6,7 which is consistent with current thinking today.
Atypical fibroxanthoma is believed to be associated with p53 mutation and is closely linked with exposure to UV radiation due to its predominance in sun-exposed areas. Other predisposing factors may include prior exposure to UV radiation, history of organ transplantation, immunosuppression, advanced age in men, and xeroderma pigmentosum. The differential diagnosis for AFX encompasses basal cell carcinoma, squamous cell carcinoma, Merkel cell carcinoma, adnexal tumor, and pyogenic granuloma.
Case Report
On physical examination, the lesions appeared erosive with crusting and granulation tissue (Figure 1A). The presentation was consistent with erosive pustular dermatosis of the scalp. Biopsy revealed granulation tissue. The patient underwent PDT and prednisone treatment with improvement. Additional biopsies revealed AKs. His condition improved with 2 PDT sessions but never fully cleared. During the PDT sessions, the patient reported intense unilateral headaches without visual changes. The headaches were intermittent and not apparently related to the treatments. He was referred for a temporal artery biopsy and rebiopsy of the remaining lesion on the scalp. The temporal artery biopsy was negative. The lesion that remained was a large nodule on the vertex scalp, and biopsy revealed AFX.


Immunohistochemical marker studies for S-100 and cytokeratin were negative. Invasion into subcutaneous fat was encountered (Figure 2A). Highly atypical spindle cells and mitoses were present (Figure 2B). Neoplastic cells were noted adjacent to nerve (Figure 2C). Excision of the lesion was curative, and his symptoms of pain and erosive pustular dermatosis resolved weeks thereafter (Figure 1B). The area of erosive pustular dermatosis was not excised, but symptoms resolved weeks following excision of the AFX.
Comment
Our case of AFX is unique due to the patient’s atypical presentation of severe pain. Because AFX usually presents asymptomatically, pain is an uncommon symptom. Based on the histologic findings in our case, we suspected that neural involvement of the tumor most likely explained the intense pain that our patient experienced.
The presence of erosive pustular dermatosis of the scalp also is interesting in our case. This elderly man had an extensive history of actinic damage and had reported pustules, scaling, itching, and scabbing of the scalp. It is possible that erosive pustular dermatosis was superimposed over the tumor and could have been the reason that multiple biopsies were needed to eventually arrive at a diagnosis. The coexistence of the 2 entities suggests that the chronic actinic damage played a role in the etiology of both.
Classification
There is a question regarding nomenclature when discussing AFX. Atypical fibroxanthoma has been referred to as a variant of undifferentiated pleomorphic sarcoma, which is a type of soft tissue sarcoma. Atypical fibroxanthoma can be referred to as undifferentiated pleomorphic sarcoma if it is more than 2 cm in diameter, if it involves the fascia or subcutaneous tissue, or if there is evidence of necrosis.3 Atypical fibroxanthoma generally is confined to the head and neck region and usually is less than 2 cm in diameter. In this patient, the presentation was consistent with AFX, as there was evidence of necrosis and invasion into the subcutaneous fat. The fact that the lesion also appeared on the scalp further supported the diagnosis of AFX.
Pathology
Biopsy of AFX typically reveals a spindle cell proliferation that usually arises in the setting of profound actinic damage. The epidermis may or may not be ulcerated, and in most cases, it is seen in close proximity to the overlying epidermis but not arising from it.8 Classic AFX is composed of highly atypical histiocytelike (epithelioid) cells admixed with pleomorphic spindle cells and giant cells, all showing frequent mitoses including atypical ones.9 Several histologic subtypes of AFX have been described, including clear cell, granular cell, pigmented cell, chondroid, osteoid, osteoclastic, and the most common spindle cell subtype.9 Features that indicate potential aggressive behavior include infiltration into the subcutaneous tissue, vascular invasion, and presence of necrosis. A diagnosis of AFX is made by exclusion of other malignant neoplasms with similar morphology, namely spindle cell squamous cell carcinoma, spindle cell melanoma, and leiomyoscarcoma.9 As such, immunohistochemistry plays a critical role in distinguishing these lesions, as they arise as part of the differential diagnosis. A panel of immunohistochemical stains is helpful for diagnosis and commonly includes but is not limited to S-100, Melan-A, smooth muscle actin, desmin, and cytokeratin.
Sampling error is an inherent flaw in any biopsy specimen. The eventual diagnosis of AFX in our case supports the argument for multiple biopsies of an unknown lesion, seeing as the affected area was interpreted as both granulation tissue and AK prior to the eventual diagnosis. Repeat biopsies, especially if a lesion is nonhealing, often can help clinicians arrive at a definitive diagnosis.
Treatment
Different treatment options have been used to manage AFX. Mohs micrographic surgery is most often used because of its tissue-sparing potential, often giving the most cosmetically appealing result. Wide local excision is another surgical technique utilized, generally with fixed margins of at least 1 cm.10 Radiation at the tumor site is used as a treatment method but most often during cases of reoccurrence. Cryotherapy as well as electrodesiccation and curettage are possible treatment options but are not the standard of care.
Atypical fibroxanthoma (AFX) is a low-grade dermal malignancy comprised of atypical spindle cells.1 Classified as a superficial fibrohistiocytic tumor with intermediate malignant potential, AFX has an incidence of approximately 0.24% worldwide.2 The tumor appears mainly on the head and neck in sun-exposed areas but can occur less frequently on the trunk and limbs in non–sun-exposed areas. There is a 70% to 80% predominance in men aged 69 to 77 years, with lesions primarily occurring in sun-exposed areas of the head and neck.3 A median period of 4 months between time of onset and time of diagnosis has been previously established.4
When AFX does occur in non–sun-exposed areas, it tends to be in a younger patient population. Clinically, it presents as a rather nondescript, firm, erythematous papule or nodule less than 2 cm in diameter. Atypical fibroxanthoma most often presents asymptomatically, but the tumor may ulcerate and bleed, though pain and pruritus are uncommon.5 Findings are nonspecific, and the diagnosis must be confirmed with biopsy, as it can resemble other common dermatological lesions. The pathogenesis of AFX has been controversial. Two different studies looked at AFX using electron microscopy and concluded that the tumor most closely resembled a myofibroblast,6,7 which is consistent with current thinking today.
Atypical fibroxanthoma is believed to be associated with p53 mutation and is closely linked with exposure to UV radiation due to its predominance in sun-exposed areas. Other predisposing factors may include prior exposure to UV radiation, history of organ transplantation, immunosuppression, advanced age in men, and xeroderma pigmentosum. The differential diagnosis for AFX encompasses basal cell carcinoma, squamous cell carcinoma, Merkel cell carcinoma, adnexal tumor, and pyogenic granuloma.
Case Report
On physical examination, the lesions appeared erosive with crusting and granulation tissue (Figure 1A). The presentation was consistent with erosive pustular dermatosis of the scalp. Biopsy revealed granulation tissue. The patient underwent PDT and prednisone treatment with improvement. Additional biopsies revealed AKs. His condition improved with 2 PDT sessions but never fully cleared. During the PDT sessions, the patient reported intense unilateral headaches without visual changes. The headaches were intermittent and not apparently related to the treatments. He was referred for a temporal artery biopsy and rebiopsy of the remaining lesion on the scalp. The temporal artery biopsy was negative. The lesion that remained was a large nodule on the vertex scalp, and biopsy revealed AFX.


Immunohistochemical marker studies for S-100 and cytokeratin were negative. Invasion into subcutaneous fat was encountered (Figure 2A). Highly atypical spindle cells and mitoses were present (Figure 2B). Neoplastic cells were noted adjacent to nerve (Figure 2C). Excision of the lesion was curative, and his symptoms of pain and erosive pustular dermatosis resolved weeks thereafter (Figure 1B). The area of erosive pustular dermatosis was not excised, but symptoms resolved weeks following excision of the AFX.
Comment
Our case of AFX is unique due to the patient’s atypical presentation of severe pain. Because AFX usually presents asymptomatically, pain is an uncommon symptom. Based on the histologic findings in our case, we suspected that neural involvement of the tumor most likely explained the intense pain that our patient experienced.
The presence of erosive pustular dermatosis of the scalp also is interesting in our case. This elderly man had an extensive history of actinic damage and had reported pustules, scaling, itching, and scabbing of the scalp. It is possible that erosive pustular dermatosis was superimposed over the tumor and could have been the reason that multiple biopsies were needed to eventually arrive at a diagnosis. The coexistence of the 2 entities suggests that the chronic actinic damage played a role in the etiology of both.
Classification
There is a question regarding nomenclature when discussing AFX. Atypical fibroxanthoma has been referred to as a variant of undifferentiated pleomorphic sarcoma, which is a type of soft tissue sarcoma. Atypical fibroxanthoma can be referred to as undifferentiated pleomorphic sarcoma if it is more than 2 cm in diameter, if it involves the fascia or subcutaneous tissue, or if there is evidence of necrosis.3 Atypical fibroxanthoma generally is confined to the head and neck region and usually is less than 2 cm in diameter. In this patient, the presentation was consistent with AFX, as there was evidence of necrosis and invasion into the subcutaneous fat. The fact that the lesion also appeared on the scalp further supported the diagnosis of AFX.
Pathology
Biopsy of AFX typically reveals a spindle cell proliferation that usually arises in the setting of profound actinic damage. The epidermis may or may not be ulcerated, and in most cases, it is seen in close proximity to the overlying epidermis but not arising from it.8 Classic AFX is composed of highly atypical histiocytelike (epithelioid) cells admixed with pleomorphic spindle cells and giant cells, all showing frequent mitoses including atypical ones.9 Several histologic subtypes of AFX have been described, including clear cell, granular cell, pigmented cell, chondroid, osteoid, osteoclastic, and the most common spindle cell subtype.9 Features that indicate potential aggressive behavior include infiltration into the subcutaneous tissue, vascular invasion, and presence of necrosis. A diagnosis of AFX is made by exclusion of other malignant neoplasms with similar morphology, namely spindle cell squamous cell carcinoma, spindle cell melanoma, and leiomyoscarcoma.9 As such, immunohistochemistry plays a critical role in distinguishing these lesions, as they arise as part of the differential diagnosis. A panel of immunohistochemical stains is helpful for diagnosis and commonly includes but is not limited to S-100, Melan-A, smooth muscle actin, desmin, and cytokeratin.
Sampling error is an inherent flaw in any biopsy specimen. The eventual diagnosis of AFX in our case supports the argument for multiple biopsies of an unknown lesion, seeing as the affected area was interpreted as both granulation tissue and AK prior to the eventual diagnosis. Repeat biopsies, especially if a lesion is nonhealing, often can help clinicians arrive at a definitive diagnosis.
Treatment
Different treatment options have been used to manage AFX. Mohs micrographic surgery is most often used because of its tissue-sparing potential, often giving the most cosmetically appealing result. Wide local excision is another surgical technique utilized, generally with fixed margins of at least 1 cm.10 Radiation at the tumor site is used as a treatment method but most often during cases of reoccurrence. Cryotherapy as well as electrodesiccation and curettage are possible treatment options but are not the standard of care.
- Helwig EB. Atypical fibroxanthoma, in tumor seminar. proceedings of 18th Annual Seminar of San Antonio Society of Pathologists, 1961. Tex State J Med. 1963;59:664-667.
- Anderson HL, Joseph AK. A pilot feasibility study of a rare skin tumor database. Dermatol Surg. 2007;33:693-696.
- Iorizzo LJ 3rd, Brown MD. Atypical fibroxanthoma: a review of the literature. Dermatol Surg. 2011;37:146-157.
- Fretzin DF, Helwig EB. Atypical fibroxanthoma of the skin. a clinicopathologic study of 140 cases. Cancer. 1973;31:1541-1552.
- Vandergriff TW, Reed JA, Orengo IF. An unusual presentation of atypical fibroxanthoma. Dermatol Online J. 2008;14:6.
- Weedon D, Kerr JF. Atypical fibroxanthoma of skin: an electron microscope study. Pathology. 1975;7:173-177.
- Woyke S, Domagala W, Olszewski W, et al. Pseudosarcoma of the skin. an electron microscopic study and comparison with the fine structure of spindle-cell variant of squamous carcinoma. Cancer. 1974;33:970-980.
- Edward S, Yung A. Essential Dermatopathology. Philadelphia, PA: Lippincott Williams & Wilkins; 2012.
- Luzar B, Calonje E. Morphologic and immunohistochemical characteristics of atypical fibroxanthoma with a special emphasis on potential diagnostic pitfalls: a review. J Cutan Pathol. 2010;37:301-309.
- González-García R, Nam-Cha SH, Muñoz-Guerra MF, et al. Atypical fibroxanthoma of the head and neck: report of 5 cases. J Oral Maxillofac Surg. 2007;65:526-531.
- Helwig EB. Atypical fibroxanthoma, in tumor seminar. proceedings of 18th Annual Seminar of San Antonio Society of Pathologists, 1961. Tex State J Med. 1963;59:664-667.
- Anderson HL, Joseph AK. A pilot feasibility study of a rare skin tumor database. Dermatol Surg. 2007;33:693-696.
- Iorizzo LJ 3rd, Brown MD. Atypical fibroxanthoma: a review of the literature. Dermatol Surg. 2011;37:146-157.
- Fretzin DF, Helwig EB. Atypical fibroxanthoma of the skin. a clinicopathologic study of 140 cases. Cancer. 1973;31:1541-1552.
- Vandergriff TW, Reed JA, Orengo IF. An unusual presentation of atypical fibroxanthoma. Dermatol Online J. 2008;14:6.
- Weedon D, Kerr JF. Atypical fibroxanthoma of skin: an electron microscope study. Pathology. 1975;7:173-177.
- Woyke S, Domagala W, Olszewski W, et al. Pseudosarcoma of the skin. an electron microscopic study and comparison with the fine structure of spindle-cell variant of squamous carcinoma. Cancer. 1974;33:970-980.
- Edward S, Yung A. Essential Dermatopathology. Philadelphia, PA: Lippincott Williams & Wilkins; 2012.
- Luzar B, Calonje E. Morphologic and immunohistochemical characteristics of atypical fibroxanthoma with a special emphasis on potential diagnostic pitfalls: a review. J Cutan Pathol. 2010;37:301-309.
- González-García R, Nam-Cha SH, Muñoz-Guerra MF, et al. Atypical fibroxanthoma of the head and neck: report of 5 cases. J Oral Maxillofac Surg. 2007;65:526-531.
Practice Points
- Atypical fibroxanthoma predominantly occurs in older men on the head and neck.
- Erosive pustular dermatosis may be a benign entity, but if it does not resolve, continue to rebiopsy, as rare tumors may mimic this condition.
Diagnosing high-risk keratinocyte carcinomas in the dermatology clinic
SAN FRANCISCO – Patients with high-risk keratinocyte carcinomas sometimes present with neurologic symptoms mimicking Bell’s palsy or trigeminal neuralgia, making the diagnosis of these perineural tumors challenging, Siegrid Yu, MD, said at the annual meeting of the Pacific Dermatologic Association.
Eventually, skin manifestations can land them in a dermatologist’s office. “There is a high incidence of delayed diagnosis and misdiagnosis, which affects the outcome of these patients,” said Dr. Yu of the department of dermatology, University of California, San Francisco.
She presented several cases illustrating the central role that dermatologists can play in the diagnosis and management of high-risk keratinocyte carcinomas. “All of these patients were seen by various doctors, sometimes multiple times, without a diagnosis,” she said.
Perineural invasion occurs in 2.6%-6% of squamous cell carcinoma (SCC) cases and 2% of basal cell carcinoma (BCC) cases. “Perineural invasion presenting with neurologic symptoms is not that common, which is part of why I think it’s easy to misdiagnose these patients,” said Dr. Yu, director of the Mohs Micrographic Surgery and Cutaneous Oncology Fellowship at the UCSF Dermatologic Surgery and Laser Center. In many cases, patients were diagnosed as having Bell’s palsy or trigeminal neuralgia for years before being diagnosed with skin cancer.
Common features of perineural invasion cases include midface location of the tumor, male gender, tumor size larger than 2 cm, recurrence, and poor histologic differentiation. Symptoms often include formication, pain, numbness, and facial weakness. Diagnosis is often delayed by 6 months to 2 years.
One case she described involved a 57-year-old immunosuppressed man who had previously undergone Mohs micrographic surgery for a primary SCC of the nasal sidewall. He experienced delayed numbness and pain of the upper lip and cheek near the surgical site 1 year later. There was no sign of cutaneous recurrence, and MRIs of the head and neck were normal. Examinations by dermatologists, neurologists, and otorhinolaryngologists yielded no diagnosis.
Two years after his initial surgery, the patient developed thickening of the scar from the Mohs surgery, without any overlying skin change. A punch biopsy showed only scar tissue, but a deeper incisional biopsy revealed a recurrence of the SCC. A second head/neck MRI, using a perineural protocol, showed abnormal enhancement at the V2 branch of the trigeminal nerve leading to the foramen rotundum. The patient underwent intensity-modulated radiation, which relies on computer-modeling to deliver doses to the precise location of the tumor. An MRI 2 months later showed a reduction in tumor size and radiographic resolution of trigeminal nerve involvement.
Another case involved a 75-year-old man with progressive right facial droop, who had experienced neurologic symptoms on the right side of his face, including numbness, tingling, oculomotor dysfunction, and radiating pain. He had been diagnosed with shingles on the right side of his face more than 20 years previously, but there was no history of postherpetic neuralgia. He also had hypertension and hypothyroidism, and had been prescribed levothyroxine, amlodipine, losartan, and gabapentin.
He had been evaluated by primary care, dermatology, and ophthalmology with no diagnosis. He then sequentially sought the opinion of four neurologists, and underwent lumbar puncture, serologic evaluation, head CT, and MRI with no findings that correlated with his symptoms. The patient’s neurological symptoms improved transiently with prednisone, and his pain improved slightly with gabapentin.
Finally, a skin biopsy of an ill-defined firmness in the right temple revealed infiltrative SCC. A repeat MRI, this time with perineural protocol, showed perineural spread along the trigeminal nerve, with involvement of the V2 and V3 branches, and possibly the V1 branch.
In another case, complete hemifacial palsy due to perineural spread of SCC was overlooked as having been related to the patient’s history of stroke. However, upon further questioning, the facial palsy involved all branches of the facial nerve, while the patient’s residual stroke symptoms of expressive aphasia and dysphagia were improving. “If you think about head and neck anatomy, an upper motor neuron lesion would not lead to complete facial nerve palsy. It could lead to palsy of the lower two-thirds of the face, sparing the temporal nerve due to cross innervation of the forehead. Only a lower motor neuron can result in progressive palsy of all branches of the facial nerve,” Dr. Yu said. In this case, the facial palsy was due to a large SCC of the external auditory canal.
Dr. Yu highlighted several considerations to keep in mind when examining these patients, including vigilance around prior skin cancer surgeries in cases with neurologic symptoms, the potential need for repeated imaging along with communication with the radiologist regarding suspicion of perineural spread, consideration of anatomy during the clinical exam, and correlation of clinical exam, histopathology, and radiographic findings.
When it comes to imaging, MRI is the most sensitive technique, she noted. It can show increase in nerve diameter, destruction of the nerve-blood barrier, obliteration of the fat below a foramen, nerve enhancement, and denervation atrophy.
Dr. Yu reported having no financial disclosures.
SAN FRANCISCO – Patients with high-risk keratinocyte carcinomas sometimes present with neurologic symptoms mimicking Bell’s palsy or trigeminal neuralgia, making the diagnosis of these perineural tumors challenging, Siegrid Yu, MD, said at the annual meeting of the Pacific Dermatologic Association.
Eventually, skin manifestations can land them in a dermatologist’s office. “There is a high incidence of delayed diagnosis and misdiagnosis, which affects the outcome of these patients,” said Dr. Yu of the department of dermatology, University of California, San Francisco.
She presented several cases illustrating the central role that dermatologists can play in the diagnosis and management of high-risk keratinocyte carcinomas. “All of these patients were seen by various doctors, sometimes multiple times, without a diagnosis,” she said.
Perineural invasion occurs in 2.6%-6% of squamous cell carcinoma (SCC) cases and 2% of basal cell carcinoma (BCC) cases. “Perineural invasion presenting with neurologic symptoms is not that common, which is part of why I think it’s easy to misdiagnose these patients,” said Dr. Yu, director of the Mohs Micrographic Surgery and Cutaneous Oncology Fellowship at the UCSF Dermatologic Surgery and Laser Center. In many cases, patients were diagnosed as having Bell’s palsy or trigeminal neuralgia for years before being diagnosed with skin cancer.
Common features of perineural invasion cases include midface location of the tumor, male gender, tumor size larger than 2 cm, recurrence, and poor histologic differentiation. Symptoms often include formication, pain, numbness, and facial weakness. Diagnosis is often delayed by 6 months to 2 years.
One case she described involved a 57-year-old immunosuppressed man who had previously undergone Mohs micrographic surgery for a primary SCC of the nasal sidewall. He experienced delayed numbness and pain of the upper lip and cheek near the surgical site 1 year later. There was no sign of cutaneous recurrence, and MRIs of the head and neck were normal. Examinations by dermatologists, neurologists, and otorhinolaryngologists yielded no diagnosis.
Two years after his initial surgery, the patient developed thickening of the scar from the Mohs surgery, without any overlying skin change. A punch biopsy showed only scar tissue, but a deeper incisional biopsy revealed a recurrence of the SCC. A second head/neck MRI, using a perineural protocol, showed abnormal enhancement at the V2 branch of the trigeminal nerve leading to the foramen rotundum. The patient underwent intensity-modulated radiation, which relies on computer-modeling to deliver doses to the precise location of the tumor. An MRI 2 months later showed a reduction in tumor size and radiographic resolution of trigeminal nerve involvement.
Another case involved a 75-year-old man with progressive right facial droop, who had experienced neurologic symptoms on the right side of his face, including numbness, tingling, oculomotor dysfunction, and radiating pain. He had been diagnosed with shingles on the right side of his face more than 20 years previously, but there was no history of postherpetic neuralgia. He also had hypertension and hypothyroidism, and had been prescribed levothyroxine, amlodipine, losartan, and gabapentin.
He had been evaluated by primary care, dermatology, and ophthalmology with no diagnosis. He then sequentially sought the opinion of four neurologists, and underwent lumbar puncture, serologic evaluation, head CT, and MRI with no findings that correlated with his symptoms. The patient’s neurological symptoms improved transiently with prednisone, and his pain improved slightly with gabapentin.
Finally, a skin biopsy of an ill-defined firmness in the right temple revealed infiltrative SCC. A repeat MRI, this time with perineural protocol, showed perineural spread along the trigeminal nerve, with involvement of the V2 and V3 branches, and possibly the V1 branch.
In another case, complete hemifacial palsy due to perineural spread of SCC was overlooked as having been related to the patient’s history of stroke. However, upon further questioning, the facial palsy involved all branches of the facial nerve, while the patient’s residual stroke symptoms of expressive aphasia and dysphagia were improving. “If you think about head and neck anatomy, an upper motor neuron lesion would not lead to complete facial nerve palsy. It could lead to palsy of the lower two-thirds of the face, sparing the temporal nerve due to cross innervation of the forehead. Only a lower motor neuron can result in progressive palsy of all branches of the facial nerve,” Dr. Yu said. In this case, the facial palsy was due to a large SCC of the external auditory canal.
Dr. Yu highlighted several considerations to keep in mind when examining these patients, including vigilance around prior skin cancer surgeries in cases with neurologic symptoms, the potential need for repeated imaging along with communication with the radiologist regarding suspicion of perineural spread, consideration of anatomy during the clinical exam, and correlation of clinical exam, histopathology, and radiographic findings.
When it comes to imaging, MRI is the most sensitive technique, she noted. It can show increase in nerve diameter, destruction of the nerve-blood barrier, obliteration of the fat below a foramen, nerve enhancement, and denervation atrophy.
Dr. Yu reported having no financial disclosures.
SAN FRANCISCO – Patients with high-risk keratinocyte carcinomas sometimes present with neurologic symptoms mimicking Bell’s palsy or trigeminal neuralgia, making the diagnosis of these perineural tumors challenging, Siegrid Yu, MD, said at the annual meeting of the Pacific Dermatologic Association.
Eventually, skin manifestations can land them in a dermatologist’s office. “There is a high incidence of delayed diagnosis and misdiagnosis, which affects the outcome of these patients,” said Dr. Yu of the department of dermatology, University of California, San Francisco.
She presented several cases illustrating the central role that dermatologists can play in the diagnosis and management of high-risk keratinocyte carcinomas. “All of these patients were seen by various doctors, sometimes multiple times, without a diagnosis,” she said.
Perineural invasion occurs in 2.6%-6% of squamous cell carcinoma (SCC) cases and 2% of basal cell carcinoma (BCC) cases. “Perineural invasion presenting with neurologic symptoms is not that common, which is part of why I think it’s easy to misdiagnose these patients,” said Dr. Yu, director of the Mohs Micrographic Surgery and Cutaneous Oncology Fellowship at the UCSF Dermatologic Surgery and Laser Center. In many cases, patients were diagnosed as having Bell’s palsy or trigeminal neuralgia for years before being diagnosed with skin cancer.
Common features of perineural invasion cases include midface location of the tumor, male gender, tumor size larger than 2 cm, recurrence, and poor histologic differentiation. Symptoms often include formication, pain, numbness, and facial weakness. Diagnosis is often delayed by 6 months to 2 years.
One case she described involved a 57-year-old immunosuppressed man who had previously undergone Mohs micrographic surgery for a primary SCC of the nasal sidewall. He experienced delayed numbness and pain of the upper lip and cheek near the surgical site 1 year later. There was no sign of cutaneous recurrence, and MRIs of the head and neck were normal. Examinations by dermatologists, neurologists, and otorhinolaryngologists yielded no diagnosis.
Two years after his initial surgery, the patient developed thickening of the scar from the Mohs surgery, without any overlying skin change. A punch biopsy showed only scar tissue, but a deeper incisional biopsy revealed a recurrence of the SCC. A second head/neck MRI, using a perineural protocol, showed abnormal enhancement at the V2 branch of the trigeminal nerve leading to the foramen rotundum. The patient underwent intensity-modulated radiation, which relies on computer-modeling to deliver doses to the precise location of the tumor. An MRI 2 months later showed a reduction in tumor size and radiographic resolution of trigeminal nerve involvement.
Another case involved a 75-year-old man with progressive right facial droop, who had experienced neurologic symptoms on the right side of his face, including numbness, tingling, oculomotor dysfunction, and radiating pain. He had been diagnosed with shingles on the right side of his face more than 20 years previously, but there was no history of postherpetic neuralgia. He also had hypertension and hypothyroidism, and had been prescribed levothyroxine, amlodipine, losartan, and gabapentin.
He had been evaluated by primary care, dermatology, and ophthalmology with no diagnosis. He then sequentially sought the opinion of four neurologists, and underwent lumbar puncture, serologic evaluation, head CT, and MRI with no findings that correlated with his symptoms. The patient’s neurological symptoms improved transiently with prednisone, and his pain improved slightly with gabapentin.
Finally, a skin biopsy of an ill-defined firmness in the right temple revealed infiltrative SCC. A repeat MRI, this time with perineural protocol, showed perineural spread along the trigeminal nerve, with involvement of the V2 and V3 branches, and possibly the V1 branch.
In another case, complete hemifacial palsy due to perineural spread of SCC was overlooked as having been related to the patient’s history of stroke. However, upon further questioning, the facial palsy involved all branches of the facial nerve, while the patient’s residual stroke symptoms of expressive aphasia and dysphagia were improving. “If you think about head and neck anatomy, an upper motor neuron lesion would not lead to complete facial nerve palsy. It could lead to palsy of the lower two-thirds of the face, sparing the temporal nerve due to cross innervation of the forehead. Only a lower motor neuron can result in progressive palsy of all branches of the facial nerve,” Dr. Yu said. In this case, the facial palsy was due to a large SCC of the external auditory canal.
Dr. Yu highlighted several considerations to keep in mind when examining these patients, including vigilance around prior skin cancer surgeries in cases with neurologic symptoms, the potential need for repeated imaging along with communication with the radiologist regarding suspicion of perineural spread, consideration of anatomy during the clinical exam, and correlation of clinical exam, histopathology, and radiographic findings.
When it comes to imaging, MRI is the most sensitive technique, she noted. It can show increase in nerve diameter, destruction of the nerve-blood barrier, obliteration of the fat below a foramen, nerve enhancement, and denervation atrophy.
Dr. Yu reported having no financial disclosures.
AT PDA 2017
Optical Coherence Tomography in Dermatology
Optical coherence tomography (OCT) is a noninvasive imaging technique that is cleared by the US Food and Drug Administration as a 510(k) class II regulatory device to visualize biological tissues in vivo and in real time.1-3 In July 2017, OCT received 2 category III Current Procedural Terminology (CPT) codes from the American Medical Association—0470T and 0471T—enabling physicians to report and track the usage of this emerging imaging method.4 Category III CPT codes remain investigational and therefore are not easily reimbursed by insurance.5 The goal of OCT manufacturers and providers within the next 5 years is to upgrade to category I coding before the present codes are archived. Although documented advantages of OCT include its unique ability to effectively differentiate and monitor skin lesions throughout nonsurgical treatment as well as to efficiently delineate presurgical margins, additional research reporting its efficacy may facilitate the coding conversion and encourage greater usage of OCT technology. We present a brief review of OCT imaging in dermatology, including its indications and limitations.
RELATED VIDEO: Imaging Overview: Report From the Mount Sinai Fall Symposium
Types of OCT
Optical coherence tomography, based on the principle of low-coherence interferometry, uses infrared light to extract fine details from within highly scattering turbid media to visualize the subsurface of the skin.2 Since its introduction for use in dermatology, OCT has been used to study skin in both the research and clinical settings.2,3 Current OCT devices on the market are mobile and easy to use in a busy dermatology practice. The Table reviews the most commonly used noninvasive imaging tools for the skin, depicting the inverse relationship between penetration depth and cellular resolution as well as field of view discrepancies.2,6-8 Optical coherence tomography technology collects cross-sectional (vertical) images similar to histology and en face (horizontal) images similar to reflective confocal microscopy (RCM) of skin areas with adequate cellular resolution and without compromising penetration depth as well as a field of view comparable to the probe aperture contacting the skin.
RELATED VIDEO: Noninvasive Imaging: Report From the Mount Sinai Fall Symposium
Conventional OCT
Due to multiple simultaneous beams, conventional frequency-domain OCT (FD-OCT) provides enhanced lateral resolution of 7.5 to 15 µm and axial resolution of 5 to 10 µm with a field of view of 6.0×6.0 mm2 and depth of 1.5 to 2.0 mm.2,6,8 Conventional FD-OCT detects architectural details within tissue with better cellular clarity than high-frequency ultrasound and better depth than RCM, yet FD-OCT is not sufficient to distinguish individual cells.
Dynamic OCT
The recent development of dynamic OCT (D-OCT) software based on speckle-variance has the added ability to visualize the skin microvasculature and therefore detect blood vessels and their distribution within specific lesions. This angiographic variant of FD-OCT detects motion corresponding to blood flow in the images and may enhance diagnostic accuracy, particularly in the differentiation of nevi and malignant melanomas.8-11
High-Definition OCT
High-definition OCT (HD-OCT), a hybrid of RCM and FD-OCT, provides improved optical resolution of 3 μm for both lateral and axial imaging approaching a resolution similar to RCM making it possible to visualize individual cells, though at the expense of lower penetration depth of 0.5 to 1.0 mm and reduced field of view of 1.8×1.5 mm2 to FD-OCT. High-definition OCT combines 2 different views to produce a 3-dimensional image for additional data interpretation (Table).7,8,12
Current CPT Guidelines
Two category III CPT codes—0470T and 0471T—allow the medical community to collect and track the usage of the emerging OCT technology. Code 0470T is used for microstructural and morphological skin imaging, specifically acquisition, interpretation, and reading of the images. Code 0471T is used for each additional skin lesion imaged.4
Current Procedural Terminology category III codes remain investigational in contrast to the permanent category I codes. Reimbursement for CPT III codes is difficult because it is not generally an accepted service covered by insurance.5 The goal within the next 5 years is to convert to category I CPT codes, meanwhile the CPT III codes should encourage increased utilization of OCT technology.
Indications for OCT
Depiction of Healthy Versus Diseased Skin
Optical coherence tomography is a valuable tool in visualizing normal skin morphology including principal skin layers, namely the dermis, epidermis, and dermoepidermal junction, as well as structures such as hair follicles, blood vessels, and glands.2,13 The OCT images show architectural changes of the skin layers and can be used to differentiate abnormal from normal tissue in vivo.2
Diagnosis and Treatment Monitoring of Skin Cancers
Optical coherence tomography is well established for use in the diagnosis and management of nonmelanoma skin cancers and to determine clinical end points of nonsurgical treatment without the need for skin biopsy. Promising diagnostic criteria have been developed for nonmelanoma skin cancers including basal cell carcinoma (BCC) and squamous cell carcinoma, as well as premalignant actinic keratoses using FD-OCT and the newer D-OCT and HD-OCT devices.9-17 For example, FD-OCT offers improved diagnosis of lesions suspicious for BCC, the most common type of skin cancer, showing improved sensitivity (79%–96%) and specificity (75%–96%) when compared with clinical assessment and dermoscopy alone.12,14 Typical OCT features differentiating BCC from other lesions include hyporeflective ovoid nests with a dark rim and an alteration of the dermoepidermal junction. In addition to providing a good diagnostic overview of skin, OCT devices show promise in monitoring the effects of treatment on primary and recurrent lesions.14-16
In Vivo Excision Planning
Additionally, OCT is a helpful tool in delineating tumor margins prior to surgical resection to achieve optimal cosmesis. By detecting subclinical tumor extension, this preoperative technique has been shown to reduce the number of surgical stages. Pomerantz et al17 showed that mapping BCC tumor margins with OCT prior to Mohs micrographic surgery closely approximated the final surgical defects. Alawi et al18 showed that the OCT-defined lateral margins correctly indicated complete removal of tumors. These studies illustrate the ability of OCT to minimize the amount of skin excised without compromising the integrity of tumor-free borders. The use of ex vivo OCT to detect residual tumors is not recommended based on current studies.6,17,18
Diagnosis and Treatment Monitoring of Other Diseases
Further applications of OCT include diagnosis of noncancerous lesions such as nail conditions, scleroderma, psoriatic arthritis, blistering diseases, and vascular lesions, as well as assessment of skin moisture and hydration, burn depth, wound healing, skin atrophy, and UV damage.2 For example, Aldahan et al19 demonstrated the utility of D-OCT to identify structural and vascular features specific to nail psoriasis useful in the diagnosis and treatment monitoring of the condition.
Limitations of OCT
Resolution
Frequency-domain OCT enables the detection of architectural details within tissue, but its image resolution is not sufficient to distinguish individual cells, therefore restricting its use in evaluating pigmented benign and malignant lesions such as dysplastic nevi and melanomas. Higher-resolution RCM is superior for imaging these lesions, as its device can better evaluate microscopic structures. With the advent of D-OCT and HD-OCT, research is being conducted to assess their use in differentiating pigmented lesions.8,20 Schuh et al9 and Gambichler et al20 reported preliminary results indicating the utility of D-OCT and HD-OCT to differentiate dysplastic nevi from melanomas and melanoma in situ, respectively.
Depth Measurement
Another limitation is associated with measuring lesion depth for advanced tumors. Although the typical imaging depth of OCT is significantly deeper than most other noninvasive imaging modalities used on skin, imaging deep tumor margins and invasion is restricted.
Image Interpretation
Diagnostic imaging requires image interpretation leading to potential interobserver and intraobserver variation. Experienced observers in OCT more accurately differentiated normal from lesional skin compared to novices, which suggests that training could improve agreement.21,22
Reimbursement and Device Cost
Other practical limitations to widespread OCT utilization at this time include its initial laser device cost and lack of reimbursement. As such, large academic and research centers remain the primary sites to utilize these devices.
Future Directions
Optical coherence tomography complements other established noninvasive imaging tools allowing for real-time visualization of the skin without interfering with the tissue and offering images with a good balance of depth, resolution, and field of view. Although a single histology cut has superior cellular resolution to any imaging modality, OCT provides additional information that is not provided by a physical biopsy, given the multiple vertical sections of data. Optical coherence tomography is a useful diagnostic technique enabling patients to avoid unnecessary biopsies while increasing early lesion diagnosis. Furthermore, OCT helps to decrease repetitive biopsies throughout nonsurgical treatments. With the availability of newer technology such as D-OCT and HD-OCT, OCT will play an increasing role in patient management. Clinicians and researchers should work to convert from category III to category I CPT codes and obtain reimbursement for imaging, with the ultimate goal of increasing its use in clinical practice and improving patient care.
- Michelson Diagnostics secures CPT codes for optical coherence tomography imaging of skin [press release]. Maidstone, Kent, United Kingdom: Michelson Diagnostics; July 14, 2017. https://vivosight.com/wp-content/uploads/2017/07/Press-Release-CPT-code-announcement-12-July-2017.pdf. Accessed August 17, 2017.
- Schmitz L, Reinhold U, Bierhoff E, et al. Optical coherence tomography: its role in daily dermatological practice. J Dtsch Dermatol Ges. 2013;11:499-507.
- Hibler BP, Qi Q, Rossi AM. Current state of imaging in dermatology. Semin Cutan Med Surg. 2016;35:2-8.
- Current Procedural Terminology 2018, Professional Edition. Chicago IL: American Medical Association; 2017.
- Current Procedural Terminology 2017, Professional Edition. Chicago IL: American Medical Association; 2016.
- Cheng HM, Guitera P. Systemic review of optical coherence tomography usage in the diagnosis and management of basal cell carcinoma. Br J Dermatol. 2015;173:1371-1380.
- Cao T, Tey HL. High-definition optical coherence tomography—an aid to clinical practice and research in dermatology. J Dtsch Dermatol Ges. 2015;13:886-890.
- Schwartz M, Siegel DM, Markowitz O. Commentary on the diagnostic utility of non-invasive imaging devices for field cancerization. Exp Dermatol. 2016;25:855-856.
- Schuh S, Holmes J, Ulrich M, et al. Imaging blood vessel morphology in skin: dynamic optical coherence tomography as a novel potential diagnostic tool in dermatology. Dermatol Ther. 2017;7:187-202.
- Themstrup L, Pellacani G, Welzel J, et al. In vivo microvascular imaging of cutaneous actinic keratosis, Bowen’s disease and squamous cell carcinoma using dynamic optical coherence tomography [published online May 14, 2017]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.14335.
- Markowitz O, Schwartz M, Minhas S, et al. DM. Speckle-variance optical coherence tomography: a novel approach to skin cancer characterization using vascular patterns. Dermatol Online J. 2016;18:22. pii:13030/qt7w10290r.
- Ulrich M, von Braunmuehl T, Kurzen H, et al. The sensitivity and specificity of optical coherence tomography for the assisted diagnosis of nonpigmented basal cell carcinoma: an observational study. Br J Dermatol. 2015;173:428-435.
- Hussain AA, Themstrup L, Jemec GB. Optical coherence tomography in the diagnosis of basal cell carcinoma. Arch Dermatol Res. 2015;307:1-10.
- Markowitz O, Schwartz M, Feldman E, et al. Evaluation of optical coherence tomography as a means of identifying earlier stage basal carcinomas while reducing the use of diagnostic biopsy. J Clin Aesthet Dermatol. 2015;8:14-20.
- Banzhaf CA, Themstrup L, Ring HC, et al. Optical coherence tomography imaging of non-melanoma skin cancer undergoing imiquimod therapy. Skin Res Technol. 2014;20:170-176.
- Themstrup L, Banzhaf CA, Mogensen M, et al. Optical coherence tomography imaging of non-melanoma skin cancer undergoing photodynamic therapy reveals subclinical residual lesions. Photodiagnosis Photodyn Ther. 2014;11:7-12.
- Pomerantz R, Zell D, McKenzie G, et al. Optical coherence tomography used as a modality to delineate basal cell carcinoma prior to Mohs micrographic surgery. Case Rep Dermatol. 2011;3:212-218.
- Alawi SA, Kuck M, Wahrlich C, et al. Optical coherence tomography for presurgical margin assessment of non-melanoma skin cancer—a practical approach. Exp Dermatol. 2013;22:547-551.
- Aldahan AS, Chen LL, Fertig RM, et al. Vascular features of nail psoriasis using dynamic optical coherence tomography. Skin Appendage Disord. 2017;2:102-108.
- Gambichler T, Plura I, Schmid-Wendtner M, et al. High-definition optical coherence tomography of melanocytic skin lesions. J Biophotonics. 2015;8:681-686.
- Mogensen M, Joergensen TM, Nurnberg BM, et al. Assessment of optical coherence tomography imaging in the diagnosis of non-melanoma skin cancer and benign lesions versus normal skin: observer-blinded evaluation by dermatologists. Dermatol Surg. 2009;35:965-972.
- Olsen J, Themstrup L, De Carbalho N, et al. Diagnostic accuracy of optical coherence tomography in actinic keratosis and basal cell carcinoma. Photodiagnosis Photodyn Ther. 2016;16:44-49.
Optical coherence tomography (OCT) is a noninvasive imaging technique that is cleared by the US Food and Drug Administration as a 510(k) class II regulatory device to visualize biological tissues in vivo and in real time.1-3 In July 2017, OCT received 2 category III Current Procedural Terminology (CPT) codes from the American Medical Association—0470T and 0471T—enabling physicians to report and track the usage of this emerging imaging method.4 Category III CPT codes remain investigational and therefore are not easily reimbursed by insurance.5 The goal of OCT manufacturers and providers within the next 5 years is to upgrade to category I coding before the present codes are archived. Although documented advantages of OCT include its unique ability to effectively differentiate and monitor skin lesions throughout nonsurgical treatment as well as to efficiently delineate presurgical margins, additional research reporting its efficacy may facilitate the coding conversion and encourage greater usage of OCT technology. We present a brief review of OCT imaging in dermatology, including its indications and limitations.
RELATED VIDEO: Imaging Overview: Report From the Mount Sinai Fall Symposium
Types of OCT
Optical coherence tomography, based on the principle of low-coherence interferometry, uses infrared light to extract fine details from within highly scattering turbid media to visualize the subsurface of the skin.2 Since its introduction for use in dermatology, OCT has been used to study skin in both the research and clinical settings.2,3 Current OCT devices on the market are mobile and easy to use in a busy dermatology practice. The Table reviews the most commonly used noninvasive imaging tools for the skin, depicting the inverse relationship between penetration depth and cellular resolution as well as field of view discrepancies.2,6-8 Optical coherence tomography technology collects cross-sectional (vertical) images similar to histology and en face (horizontal) images similar to reflective confocal microscopy (RCM) of skin areas with adequate cellular resolution and without compromising penetration depth as well as a field of view comparable to the probe aperture contacting the skin.
RELATED VIDEO: Noninvasive Imaging: Report From the Mount Sinai Fall Symposium
Conventional OCT
Due to multiple simultaneous beams, conventional frequency-domain OCT (FD-OCT) provides enhanced lateral resolution of 7.5 to 15 µm and axial resolution of 5 to 10 µm with a field of view of 6.0×6.0 mm2 and depth of 1.5 to 2.0 mm.2,6,8 Conventional FD-OCT detects architectural details within tissue with better cellular clarity than high-frequency ultrasound and better depth than RCM, yet FD-OCT is not sufficient to distinguish individual cells.
Dynamic OCT
The recent development of dynamic OCT (D-OCT) software based on speckle-variance has the added ability to visualize the skin microvasculature and therefore detect blood vessels and their distribution within specific lesions. This angiographic variant of FD-OCT detects motion corresponding to blood flow in the images and may enhance diagnostic accuracy, particularly in the differentiation of nevi and malignant melanomas.8-11
High-Definition OCT
High-definition OCT (HD-OCT), a hybrid of RCM and FD-OCT, provides improved optical resolution of 3 μm for both lateral and axial imaging approaching a resolution similar to RCM making it possible to visualize individual cells, though at the expense of lower penetration depth of 0.5 to 1.0 mm and reduced field of view of 1.8×1.5 mm2 to FD-OCT. High-definition OCT combines 2 different views to produce a 3-dimensional image for additional data interpretation (Table).7,8,12
Current CPT Guidelines
Two category III CPT codes—0470T and 0471T—allow the medical community to collect and track the usage of the emerging OCT technology. Code 0470T is used for microstructural and morphological skin imaging, specifically acquisition, interpretation, and reading of the images. Code 0471T is used for each additional skin lesion imaged.4
Current Procedural Terminology category III codes remain investigational in contrast to the permanent category I codes. Reimbursement for CPT III codes is difficult because it is not generally an accepted service covered by insurance.5 The goal within the next 5 years is to convert to category I CPT codes, meanwhile the CPT III codes should encourage increased utilization of OCT technology.
Indications for OCT
Depiction of Healthy Versus Diseased Skin
Optical coherence tomography is a valuable tool in visualizing normal skin morphology including principal skin layers, namely the dermis, epidermis, and dermoepidermal junction, as well as structures such as hair follicles, blood vessels, and glands.2,13 The OCT images show architectural changes of the skin layers and can be used to differentiate abnormal from normal tissue in vivo.2
Diagnosis and Treatment Monitoring of Skin Cancers
Optical coherence tomography is well established for use in the diagnosis and management of nonmelanoma skin cancers and to determine clinical end points of nonsurgical treatment without the need for skin biopsy. Promising diagnostic criteria have been developed for nonmelanoma skin cancers including basal cell carcinoma (BCC) and squamous cell carcinoma, as well as premalignant actinic keratoses using FD-OCT and the newer D-OCT and HD-OCT devices.9-17 For example, FD-OCT offers improved diagnosis of lesions suspicious for BCC, the most common type of skin cancer, showing improved sensitivity (79%–96%) and specificity (75%–96%) when compared with clinical assessment and dermoscopy alone.12,14 Typical OCT features differentiating BCC from other lesions include hyporeflective ovoid nests with a dark rim and an alteration of the dermoepidermal junction. In addition to providing a good diagnostic overview of skin, OCT devices show promise in monitoring the effects of treatment on primary and recurrent lesions.14-16
In Vivo Excision Planning
Additionally, OCT is a helpful tool in delineating tumor margins prior to surgical resection to achieve optimal cosmesis. By detecting subclinical tumor extension, this preoperative technique has been shown to reduce the number of surgical stages. Pomerantz et al17 showed that mapping BCC tumor margins with OCT prior to Mohs micrographic surgery closely approximated the final surgical defects. Alawi et al18 showed that the OCT-defined lateral margins correctly indicated complete removal of tumors. These studies illustrate the ability of OCT to minimize the amount of skin excised without compromising the integrity of tumor-free borders. The use of ex vivo OCT to detect residual tumors is not recommended based on current studies.6,17,18
Diagnosis and Treatment Monitoring of Other Diseases
Further applications of OCT include diagnosis of noncancerous lesions such as nail conditions, scleroderma, psoriatic arthritis, blistering diseases, and vascular lesions, as well as assessment of skin moisture and hydration, burn depth, wound healing, skin atrophy, and UV damage.2 For example, Aldahan et al19 demonstrated the utility of D-OCT to identify structural and vascular features specific to nail psoriasis useful in the diagnosis and treatment monitoring of the condition.
Limitations of OCT
Resolution
Frequency-domain OCT enables the detection of architectural details within tissue, but its image resolution is not sufficient to distinguish individual cells, therefore restricting its use in evaluating pigmented benign and malignant lesions such as dysplastic nevi and melanomas. Higher-resolution RCM is superior for imaging these lesions, as its device can better evaluate microscopic structures. With the advent of D-OCT and HD-OCT, research is being conducted to assess their use in differentiating pigmented lesions.8,20 Schuh et al9 and Gambichler et al20 reported preliminary results indicating the utility of D-OCT and HD-OCT to differentiate dysplastic nevi from melanomas and melanoma in situ, respectively.
Depth Measurement
Another limitation is associated with measuring lesion depth for advanced tumors. Although the typical imaging depth of OCT is significantly deeper than most other noninvasive imaging modalities used on skin, imaging deep tumor margins and invasion is restricted.
Image Interpretation
Diagnostic imaging requires image interpretation leading to potential interobserver and intraobserver variation. Experienced observers in OCT more accurately differentiated normal from lesional skin compared to novices, which suggests that training could improve agreement.21,22
Reimbursement and Device Cost
Other practical limitations to widespread OCT utilization at this time include its initial laser device cost and lack of reimbursement. As such, large academic and research centers remain the primary sites to utilize these devices.
Future Directions
Optical coherence tomography complements other established noninvasive imaging tools allowing for real-time visualization of the skin without interfering with the tissue and offering images with a good balance of depth, resolution, and field of view. Although a single histology cut has superior cellular resolution to any imaging modality, OCT provides additional information that is not provided by a physical biopsy, given the multiple vertical sections of data. Optical coherence tomography is a useful diagnostic technique enabling patients to avoid unnecessary biopsies while increasing early lesion diagnosis. Furthermore, OCT helps to decrease repetitive biopsies throughout nonsurgical treatments. With the availability of newer technology such as D-OCT and HD-OCT, OCT will play an increasing role in patient management. Clinicians and researchers should work to convert from category III to category I CPT codes and obtain reimbursement for imaging, with the ultimate goal of increasing its use in clinical practice and improving patient care.
Optical coherence tomography (OCT) is a noninvasive imaging technique that is cleared by the US Food and Drug Administration as a 510(k) class II regulatory device to visualize biological tissues in vivo and in real time.1-3 In July 2017, OCT received 2 category III Current Procedural Terminology (CPT) codes from the American Medical Association—0470T and 0471T—enabling physicians to report and track the usage of this emerging imaging method.4 Category III CPT codes remain investigational and therefore are not easily reimbursed by insurance.5 The goal of OCT manufacturers and providers within the next 5 years is to upgrade to category I coding before the present codes are archived. Although documented advantages of OCT include its unique ability to effectively differentiate and monitor skin lesions throughout nonsurgical treatment as well as to efficiently delineate presurgical margins, additional research reporting its efficacy may facilitate the coding conversion and encourage greater usage of OCT technology. We present a brief review of OCT imaging in dermatology, including its indications and limitations.
RELATED VIDEO: Imaging Overview: Report From the Mount Sinai Fall Symposium
Types of OCT
Optical coherence tomography, based on the principle of low-coherence interferometry, uses infrared light to extract fine details from within highly scattering turbid media to visualize the subsurface of the skin.2 Since its introduction for use in dermatology, OCT has been used to study skin in both the research and clinical settings.2,3 Current OCT devices on the market are mobile and easy to use in a busy dermatology practice. The Table reviews the most commonly used noninvasive imaging tools for the skin, depicting the inverse relationship between penetration depth and cellular resolution as well as field of view discrepancies.2,6-8 Optical coherence tomography technology collects cross-sectional (vertical) images similar to histology and en face (horizontal) images similar to reflective confocal microscopy (RCM) of skin areas with adequate cellular resolution and without compromising penetration depth as well as a field of view comparable to the probe aperture contacting the skin.
RELATED VIDEO: Noninvasive Imaging: Report From the Mount Sinai Fall Symposium
Conventional OCT
Due to multiple simultaneous beams, conventional frequency-domain OCT (FD-OCT) provides enhanced lateral resolution of 7.5 to 15 µm and axial resolution of 5 to 10 µm with a field of view of 6.0×6.0 mm2 and depth of 1.5 to 2.0 mm.2,6,8 Conventional FD-OCT detects architectural details within tissue with better cellular clarity than high-frequency ultrasound and better depth than RCM, yet FD-OCT is not sufficient to distinguish individual cells.
Dynamic OCT
The recent development of dynamic OCT (D-OCT) software based on speckle-variance has the added ability to visualize the skin microvasculature and therefore detect blood vessels and their distribution within specific lesions. This angiographic variant of FD-OCT detects motion corresponding to blood flow in the images and may enhance diagnostic accuracy, particularly in the differentiation of nevi and malignant melanomas.8-11
High-Definition OCT
High-definition OCT (HD-OCT), a hybrid of RCM and FD-OCT, provides improved optical resolution of 3 μm for both lateral and axial imaging approaching a resolution similar to RCM making it possible to visualize individual cells, though at the expense of lower penetration depth of 0.5 to 1.0 mm and reduced field of view of 1.8×1.5 mm2 to FD-OCT. High-definition OCT combines 2 different views to produce a 3-dimensional image for additional data interpretation (Table).7,8,12
Current CPT Guidelines
Two category III CPT codes—0470T and 0471T—allow the medical community to collect and track the usage of the emerging OCT technology. Code 0470T is used for microstructural and morphological skin imaging, specifically acquisition, interpretation, and reading of the images. Code 0471T is used for each additional skin lesion imaged.4
Current Procedural Terminology category III codes remain investigational in contrast to the permanent category I codes. Reimbursement for CPT III codes is difficult because it is not generally an accepted service covered by insurance.5 The goal within the next 5 years is to convert to category I CPT codes, meanwhile the CPT III codes should encourage increased utilization of OCT technology.
Indications for OCT
Depiction of Healthy Versus Diseased Skin
Optical coherence tomography is a valuable tool in visualizing normal skin morphology including principal skin layers, namely the dermis, epidermis, and dermoepidermal junction, as well as structures such as hair follicles, blood vessels, and glands.2,13 The OCT images show architectural changes of the skin layers and can be used to differentiate abnormal from normal tissue in vivo.2
Diagnosis and Treatment Monitoring of Skin Cancers
Optical coherence tomography is well established for use in the diagnosis and management of nonmelanoma skin cancers and to determine clinical end points of nonsurgical treatment without the need for skin biopsy. Promising diagnostic criteria have been developed for nonmelanoma skin cancers including basal cell carcinoma (BCC) and squamous cell carcinoma, as well as premalignant actinic keratoses using FD-OCT and the newer D-OCT and HD-OCT devices.9-17 For example, FD-OCT offers improved diagnosis of lesions suspicious for BCC, the most common type of skin cancer, showing improved sensitivity (79%–96%) and specificity (75%–96%) when compared with clinical assessment and dermoscopy alone.12,14 Typical OCT features differentiating BCC from other lesions include hyporeflective ovoid nests with a dark rim and an alteration of the dermoepidermal junction. In addition to providing a good diagnostic overview of skin, OCT devices show promise in monitoring the effects of treatment on primary and recurrent lesions.14-16
In Vivo Excision Planning
Additionally, OCT is a helpful tool in delineating tumor margins prior to surgical resection to achieve optimal cosmesis. By detecting subclinical tumor extension, this preoperative technique has been shown to reduce the number of surgical stages. Pomerantz et al17 showed that mapping BCC tumor margins with OCT prior to Mohs micrographic surgery closely approximated the final surgical defects. Alawi et al18 showed that the OCT-defined lateral margins correctly indicated complete removal of tumors. These studies illustrate the ability of OCT to minimize the amount of skin excised without compromising the integrity of tumor-free borders. The use of ex vivo OCT to detect residual tumors is not recommended based on current studies.6,17,18
Diagnosis and Treatment Monitoring of Other Diseases
Further applications of OCT include diagnosis of noncancerous lesions such as nail conditions, scleroderma, psoriatic arthritis, blistering diseases, and vascular lesions, as well as assessment of skin moisture and hydration, burn depth, wound healing, skin atrophy, and UV damage.2 For example, Aldahan et al19 demonstrated the utility of D-OCT to identify structural and vascular features specific to nail psoriasis useful in the diagnosis and treatment monitoring of the condition.
Limitations of OCT
Resolution
Frequency-domain OCT enables the detection of architectural details within tissue, but its image resolution is not sufficient to distinguish individual cells, therefore restricting its use in evaluating pigmented benign and malignant lesions such as dysplastic nevi and melanomas. Higher-resolution RCM is superior for imaging these lesions, as its device can better evaluate microscopic structures. With the advent of D-OCT and HD-OCT, research is being conducted to assess their use in differentiating pigmented lesions.8,20 Schuh et al9 and Gambichler et al20 reported preliminary results indicating the utility of D-OCT and HD-OCT to differentiate dysplastic nevi from melanomas and melanoma in situ, respectively.
Depth Measurement
Another limitation is associated with measuring lesion depth for advanced tumors. Although the typical imaging depth of OCT is significantly deeper than most other noninvasive imaging modalities used on skin, imaging deep tumor margins and invasion is restricted.
Image Interpretation
Diagnostic imaging requires image interpretation leading to potential interobserver and intraobserver variation. Experienced observers in OCT more accurately differentiated normal from lesional skin compared to novices, which suggests that training could improve agreement.21,22
Reimbursement and Device Cost
Other practical limitations to widespread OCT utilization at this time include its initial laser device cost and lack of reimbursement. As such, large academic and research centers remain the primary sites to utilize these devices.
Future Directions
Optical coherence tomography complements other established noninvasive imaging tools allowing for real-time visualization of the skin without interfering with the tissue and offering images with a good balance of depth, resolution, and field of view. Although a single histology cut has superior cellular resolution to any imaging modality, OCT provides additional information that is not provided by a physical biopsy, given the multiple vertical sections of data. Optical coherence tomography is a useful diagnostic technique enabling patients to avoid unnecessary biopsies while increasing early lesion diagnosis. Furthermore, OCT helps to decrease repetitive biopsies throughout nonsurgical treatments. With the availability of newer technology such as D-OCT and HD-OCT, OCT will play an increasing role in patient management. Clinicians and researchers should work to convert from category III to category I CPT codes and obtain reimbursement for imaging, with the ultimate goal of increasing its use in clinical practice and improving patient care.
- Michelson Diagnostics secures CPT codes for optical coherence tomography imaging of skin [press release]. Maidstone, Kent, United Kingdom: Michelson Diagnostics; July 14, 2017. https://vivosight.com/wp-content/uploads/2017/07/Press-Release-CPT-code-announcement-12-July-2017.pdf. Accessed August 17, 2017.
- Schmitz L, Reinhold U, Bierhoff E, et al. Optical coherence tomography: its role in daily dermatological practice. J Dtsch Dermatol Ges. 2013;11:499-507.
- Hibler BP, Qi Q, Rossi AM. Current state of imaging in dermatology. Semin Cutan Med Surg. 2016;35:2-8.
- Current Procedural Terminology 2018, Professional Edition. Chicago IL: American Medical Association; 2017.
- Current Procedural Terminology 2017, Professional Edition. Chicago IL: American Medical Association; 2016.
- Cheng HM, Guitera P. Systemic review of optical coherence tomography usage in the diagnosis and management of basal cell carcinoma. Br J Dermatol. 2015;173:1371-1380.
- Cao T, Tey HL. High-definition optical coherence tomography—an aid to clinical practice and research in dermatology. J Dtsch Dermatol Ges. 2015;13:886-890.
- Schwartz M, Siegel DM, Markowitz O. Commentary on the diagnostic utility of non-invasive imaging devices for field cancerization. Exp Dermatol. 2016;25:855-856.
- Schuh S, Holmes J, Ulrich M, et al. Imaging blood vessel morphology in skin: dynamic optical coherence tomography as a novel potential diagnostic tool in dermatology. Dermatol Ther. 2017;7:187-202.
- Themstrup L, Pellacani G, Welzel J, et al. In vivo microvascular imaging of cutaneous actinic keratosis, Bowen’s disease and squamous cell carcinoma using dynamic optical coherence tomography [published online May 14, 2017]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.14335.
- Markowitz O, Schwartz M, Minhas S, et al. DM. Speckle-variance optical coherence tomography: a novel approach to skin cancer characterization using vascular patterns. Dermatol Online J. 2016;18:22. pii:13030/qt7w10290r.
- Ulrich M, von Braunmuehl T, Kurzen H, et al. The sensitivity and specificity of optical coherence tomography for the assisted diagnosis of nonpigmented basal cell carcinoma: an observational study. Br J Dermatol. 2015;173:428-435.
- Hussain AA, Themstrup L, Jemec GB. Optical coherence tomography in the diagnosis of basal cell carcinoma. Arch Dermatol Res. 2015;307:1-10.
- Markowitz O, Schwartz M, Feldman E, et al. Evaluation of optical coherence tomography as a means of identifying earlier stage basal carcinomas while reducing the use of diagnostic biopsy. J Clin Aesthet Dermatol. 2015;8:14-20.
- Banzhaf CA, Themstrup L, Ring HC, et al. Optical coherence tomography imaging of non-melanoma skin cancer undergoing imiquimod therapy. Skin Res Technol. 2014;20:170-176.
- Themstrup L, Banzhaf CA, Mogensen M, et al. Optical coherence tomography imaging of non-melanoma skin cancer undergoing photodynamic therapy reveals subclinical residual lesions. Photodiagnosis Photodyn Ther. 2014;11:7-12.
- Pomerantz R, Zell D, McKenzie G, et al. Optical coherence tomography used as a modality to delineate basal cell carcinoma prior to Mohs micrographic surgery. Case Rep Dermatol. 2011;3:212-218.
- Alawi SA, Kuck M, Wahrlich C, et al. Optical coherence tomography for presurgical margin assessment of non-melanoma skin cancer—a practical approach. Exp Dermatol. 2013;22:547-551.
- Aldahan AS, Chen LL, Fertig RM, et al. Vascular features of nail psoriasis using dynamic optical coherence tomography. Skin Appendage Disord. 2017;2:102-108.
- Gambichler T, Plura I, Schmid-Wendtner M, et al. High-definition optical coherence tomography of melanocytic skin lesions. J Biophotonics. 2015;8:681-686.
- Mogensen M, Joergensen TM, Nurnberg BM, et al. Assessment of optical coherence tomography imaging in the diagnosis of non-melanoma skin cancer and benign lesions versus normal skin: observer-blinded evaluation by dermatologists. Dermatol Surg. 2009;35:965-972.
- Olsen J, Themstrup L, De Carbalho N, et al. Diagnostic accuracy of optical coherence tomography in actinic keratosis and basal cell carcinoma. Photodiagnosis Photodyn Ther. 2016;16:44-49.
- Michelson Diagnostics secures CPT codes for optical coherence tomography imaging of skin [press release]. Maidstone, Kent, United Kingdom: Michelson Diagnostics; July 14, 2017. https://vivosight.com/wp-content/uploads/2017/07/Press-Release-CPT-code-announcement-12-July-2017.pdf. Accessed August 17, 2017.
- Schmitz L, Reinhold U, Bierhoff E, et al. Optical coherence tomography: its role in daily dermatological practice. J Dtsch Dermatol Ges. 2013;11:499-507.
- Hibler BP, Qi Q, Rossi AM. Current state of imaging in dermatology. Semin Cutan Med Surg. 2016;35:2-8.
- Current Procedural Terminology 2018, Professional Edition. Chicago IL: American Medical Association; 2017.
- Current Procedural Terminology 2017, Professional Edition. Chicago IL: American Medical Association; 2016.
- Cheng HM, Guitera P. Systemic review of optical coherence tomography usage in the diagnosis and management of basal cell carcinoma. Br J Dermatol. 2015;173:1371-1380.
- Cao T, Tey HL. High-definition optical coherence tomography—an aid to clinical practice and research in dermatology. J Dtsch Dermatol Ges. 2015;13:886-890.
- Schwartz M, Siegel DM, Markowitz O. Commentary on the diagnostic utility of non-invasive imaging devices for field cancerization. Exp Dermatol. 2016;25:855-856.
- Schuh S, Holmes J, Ulrich M, et al. Imaging blood vessel morphology in skin: dynamic optical coherence tomography as a novel potential diagnostic tool in dermatology. Dermatol Ther. 2017;7:187-202.
- Themstrup L, Pellacani G, Welzel J, et al. In vivo microvascular imaging of cutaneous actinic keratosis, Bowen’s disease and squamous cell carcinoma using dynamic optical coherence tomography [published online May 14, 2017]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.14335.
- Markowitz O, Schwartz M, Minhas S, et al. DM. Speckle-variance optical coherence tomography: a novel approach to skin cancer characterization using vascular patterns. Dermatol Online J. 2016;18:22. pii:13030/qt7w10290r.
- Ulrich M, von Braunmuehl T, Kurzen H, et al. The sensitivity and specificity of optical coherence tomography for the assisted diagnosis of nonpigmented basal cell carcinoma: an observational study. Br J Dermatol. 2015;173:428-435.
- Hussain AA, Themstrup L, Jemec GB. Optical coherence tomography in the diagnosis of basal cell carcinoma. Arch Dermatol Res. 2015;307:1-10.
- Markowitz O, Schwartz M, Feldman E, et al. Evaluation of optical coherence tomography as a means of identifying earlier stage basal carcinomas while reducing the use of diagnostic biopsy. J Clin Aesthet Dermatol. 2015;8:14-20.
- Banzhaf CA, Themstrup L, Ring HC, et al. Optical coherence tomography imaging of non-melanoma skin cancer undergoing imiquimod therapy. Skin Res Technol. 2014;20:170-176.
- Themstrup L, Banzhaf CA, Mogensen M, et al. Optical coherence tomography imaging of non-melanoma skin cancer undergoing photodynamic therapy reveals subclinical residual lesions. Photodiagnosis Photodyn Ther. 2014;11:7-12.
- Pomerantz R, Zell D, McKenzie G, et al. Optical coherence tomography used as a modality to delineate basal cell carcinoma prior to Mohs micrographic surgery. Case Rep Dermatol. 2011;3:212-218.
- Alawi SA, Kuck M, Wahrlich C, et al. Optical coherence tomography for presurgical margin assessment of non-melanoma skin cancer—a practical approach. Exp Dermatol. 2013;22:547-551.
- Aldahan AS, Chen LL, Fertig RM, et al. Vascular features of nail psoriasis using dynamic optical coherence tomography. Skin Appendage Disord. 2017;2:102-108.
- Gambichler T, Plura I, Schmid-Wendtner M, et al. High-definition optical coherence tomography of melanocytic skin lesions. J Biophotonics. 2015;8:681-686.
- Mogensen M, Joergensen TM, Nurnberg BM, et al. Assessment of optical coherence tomography imaging in the diagnosis of non-melanoma skin cancer and benign lesions versus normal skin: observer-blinded evaluation by dermatologists. Dermatol Surg. 2009;35:965-972.
- Olsen J, Themstrup L, De Carbalho N, et al. Diagnostic accuracy of optical coherence tomography in actinic keratosis and basal cell carcinoma. Photodiagnosis Photodyn Ther. 2016;16:44-49.
Practice Points
- Optical coherence tomography (OCT) technology has considerable utility in research and clinical settings given its high resolution, wide field of view, moderate penetration depth, straightforward image acquisition, and accessibility to anatomically challenging areas.
- Potential benefits of OCT include its ability to noninvasively diagnose and monitor nonmelanoma skin cancers as well as to delineate presurgical margins and elucidate the course and mechanism of action of skin conditions at the bedside.
- Limitations of OCT include device cost, lack of reimbursement, and training, as well as restricted ability to image advanced deep tumors and differentiate melanocytic lesions.
- Optical coherence tomography recently received 2 category III Current Procedural Terminology (CPT) codes to track its utilization in clinical practice and will hopefully receive category I CPT codes within the next 5 years.
Videodermoscopy as a Novel Tool for Dermatologic Education
Dermoscopy, or the noninvasive in vivo examination of the epidermis and superficial dermis using magnification, facilitates the diagnosis of pigmented and nonpigmented skin lesions.1 Despite the benefit of dermoscopy in making early and accurate diagnoses of potentially life-limiting skin cancers, only 48% of dermatologists in the United States use dermoscopy in their practices.2 The most commonly cited reason for not using dermoscopy is lack of training.
Although the use of dermoscopy is associated with younger age and more recent graduation from residency compared to nonusers, dermatology resident physicians continue to receive limited training in dermoscopy.2 In a survey of 139 dermatology chief residents, 48% were not satisfied with the dermoscopy training that they had received during residency. Residents who received bedside instruction in dermoscopy reported greater satisfaction with their dermoscopy training compared to those who did not receive bedside instruction.3 This article provides a brief comparison of standard dermoscopy versus videodermoscopy for the instruction of trainees on common dermatologic diagnoses.
Bedside Dermoscopy
Standard optical dermatoscopes used for patient care and educational purposes typically incorporate 10-fold magnification and permit examination by a single viewer through a lens. With standard dermatoscopes, bedside dermoscopy instruction consists of the independent sequential viewing of skin lesions by instructors and trainees. Trainees must independently search for dermoscopic features noted by the instructor, which may be difficult for novice users. Simultaneous viewing of lesions would allow instructors to clearly indicate in real time pertinent dermoscopic features to their trainees.
Videodermatoscopes facilitate the simultaneous examination of cutaneous lesions by projecting the dermoscopic image onto a digital screen. Furthermore, these devices can incorporate magnifications of up to 200-fold or greater. In recent years, research pertaining to videodermoscopy has focused on the high magnification capabilities of these devices, specifically dermoscopic features that are visualized at magnifications greater than 10-fold, including the light brown nests of basal cell carcinomas that are seen at 50- to 70-fold magnification, twisted red capillary loops seen in active scalp psoriasis at 50-fold magnification, and longitudinal white indentations seen on nail plates affected by onychomycosis at 20-fold magnification.4-6 The potential value of videodermoscopy in medical education lies not only in the high magnification potential, which may make subtle dermoscopic findings more apparent to novice dermoscopists, but also in the ability to facilitate simultaneous dermoscopic examinations by instructors and trainees.
Educational Applications for Videodermoscopy
To illustrate the educational potential of videodermoscopy, images taken with a standard dermatoscope at 10-fold magnification are presented with videodermoscopic images taken at magnifications ranging from 60- to 185-fold (Figures 1–3). These examples demonstrate the potential for videodermoscopy to facilitate the visualization of subtle dermoscopic features by novice dermoscopists, relating to both the enhanced magnification potential and the potential for simultaneous rather than sequential examination.
Final Thoughts
High-magnification videodermoscopy may be a useful tool to further dermoscopic education. Videodermatoscopes vary in functionality and cost but are available at price points comparable to those of standard optical dermatoscopes. Owners of standard dermatoscopes can approximate some of the benefits of a digital videodermatoscope by using the standard dermatoscope in conjunction with a camera, including those integrated into mobile phones and tablets. By attaching the standard dermatoscope to a camera with a digital display, the digital zoom of the camera can be used to magnify the standard dermoscopic image, enhancing the ability of novice dermoscopists to visualize subtle findings. By presenting this magnified image on a digital display, dermoscopy instructors and trainees would be able to simultaneously view dermoscopic images of lesions, sometimes with magnifications comparable to videodermatoscopes.
In the setting of a dermatology residency program, videodermoscopy can be incorporated into bedside teaching with experienced dermoscopists and for the live presentation of dermoscopic features at departmental grand rounds. By facilitating the simultaneous, high-magnification and live viewing of skin lesions by dermoscopy instructors and trainees, digital videodermoscopy has the potential to address an area of weakness in dermatologic training.
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676.
- Engasser HC, Warshaw EM. Dermatoscopy use by US dermatologists: a cross-sectional survey [published online July 8, 2010]. J Am Acad Dermatol. 2010;63:412-419, 419.e1-419.e2.
- Wu TP, Newlove T, Smith L, et al. The importance of dedicated dermoscopy training during residency: a survey of US dermatology chief residents. J Am Acad Dermatol. 2013;68:1000-1005.
- Seidenari S, Bellucci C, Bassoli S, et al. High magnification digital dermoscopy of basal cell carcinoma: a single-centre study on 400 cases. Acta Derm Venereol. 2014;94:677-682.
- Ross EK, Vincenzi C, Tosti A. Videodermoscopy in the evaluation of hair and scalp disorders. J Am Acad Dermatol. 2006;55:799-806.
- Piraccini BM, Balestri R, Starace M, et al. Nail digital dermoscopy (onychoscopy) in the diagnosis of onychomycosis. J Eur Acad Dermatol Venereol. 2013;27:509-513.
Dermoscopy, or the noninvasive in vivo examination of the epidermis and superficial dermis using magnification, facilitates the diagnosis of pigmented and nonpigmented skin lesions.1 Despite the benefit of dermoscopy in making early and accurate diagnoses of potentially life-limiting skin cancers, only 48% of dermatologists in the United States use dermoscopy in their practices.2 The most commonly cited reason for not using dermoscopy is lack of training.
Although the use of dermoscopy is associated with younger age and more recent graduation from residency compared to nonusers, dermatology resident physicians continue to receive limited training in dermoscopy.2 In a survey of 139 dermatology chief residents, 48% were not satisfied with the dermoscopy training that they had received during residency. Residents who received bedside instruction in dermoscopy reported greater satisfaction with their dermoscopy training compared to those who did not receive bedside instruction.3 This article provides a brief comparison of standard dermoscopy versus videodermoscopy for the instruction of trainees on common dermatologic diagnoses.
Bedside Dermoscopy
Standard optical dermatoscopes used for patient care and educational purposes typically incorporate 10-fold magnification and permit examination by a single viewer through a lens. With standard dermatoscopes, bedside dermoscopy instruction consists of the independent sequential viewing of skin lesions by instructors and trainees. Trainees must independently search for dermoscopic features noted by the instructor, which may be difficult for novice users. Simultaneous viewing of lesions would allow instructors to clearly indicate in real time pertinent dermoscopic features to their trainees.
Videodermatoscopes facilitate the simultaneous examination of cutaneous lesions by projecting the dermoscopic image onto a digital screen. Furthermore, these devices can incorporate magnifications of up to 200-fold or greater. In recent years, research pertaining to videodermoscopy has focused on the high magnification capabilities of these devices, specifically dermoscopic features that are visualized at magnifications greater than 10-fold, including the light brown nests of basal cell carcinomas that are seen at 50- to 70-fold magnification, twisted red capillary loops seen in active scalp psoriasis at 50-fold magnification, and longitudinal white indentations seen on nail plates affected by onychomycosis at 20-fold magnification.4-6 The potential value of videodermoscopy in medical education lies not only in the high magnification potential, which may make subtle dermoscopic findings more apparent to novice dermoscopists, but also in the ability to facilitate simultaneous dermoscopic examinations by instructors and trainees.
Educational Applications for Videodermoscopy
To illustrate the educational potential of videodermoscopy, images taken with a standard dermatoscope at 10-fold magnification are presented with videodermoscopic images taken at magnifications ranging from 60- to 185-fold (Figures 1–3). These examples demonstrate the potential for videodermoscopy to facilitate the visualization of subtle dermoscopic features by novice dermoscopists, relating to both the enhanced magnification potential and the potential for simultaneous rather than sequential examination.



Final Thoughts
High-magnification videodermoscopy may be a useful tool to further dermoscopic education. Videodermatoscopes vary in functionality and cost but are available at price points comparable to those of standard optical dermatoscopes. Owners of standard dermatoscopes can approximate some of the benefits of a digital videodermatoscope by using the standard dermatoscope in conjunction with a camera, including those integrated into mobile phones and tablets. By attaching the standard dermatoscope to a camera with a digital display, the digital zoom of the camera can be used to magnify the standard dermoscopic image, enhancing the ability of novice dermoscopists to visualize subtle findings. By presenting this magnified image on a digital display, dermoscopy instructors and trainees would be able to simultaneously view dermoscopic images of lesions, sometimes with magnifications comparable to videodermatoscopes.
In the setting of a dermatology residency program, videodermoscopy can be incorporated into bedside teaching with experienced dermoscopists and for the live presentation of dermoscopic features at departmental grand rounds. By facilitating the simultaneous, high-magnification and live viewing of skin lesions by dermoscopy instructors and trainees, digital videodermoscopy has the potential to address an area of weakness in dermatologic training.
Dermoscopy, or the noninvasive in vivo examination of the epidermis and superficial dermis using magnification, facilitates the diagnosis of pigmented and nonpigmented skin lesions.1 Despite the benefit of dermoscopy in making early and accurate diagnoses of potentially life-limiting skin cancers, only 48% of dermatologists in the United States use dermoscopy in their practices.2 The most commonly cited reason for not using dermoscopy is lack of training.
Although the use of dermoscopy is associated with younger age and more recent graduation from residency compared to nonusers, dermatology resident physicians continue to receive limited training in dermoscopy.2 In a survey of 139 dermatology chief residents, 48% were not satisfied with the dermoscopy training that they had received during residency. Residents who received bedside instruction in dermoscopy reported greater satisfaction with their dermoscopy training compared to those who did not receive bedside instruction.3 This article provides a brief comparison of standard dermoscopy versus videodermoscopy for the instruction of trainees on common dermatologic diagnoses.
Bedside Dermoscopy
Standard optical dermatoscopes used for patient care and educational purposes typically incorporate 10-fold magnification and permit examination by a single viewer through a lens. With standard dermatoscopes, bedside dermoscopy instruction consists of the independent sequential viewing of skin lesions by instructors and trainees. Trainees must independently search for dermoscopic features noted by the instructor, which may be difficult for novice users. Simultaneous viewing of lesions would allow instructors to clearly indicate in real time pertinent dermoscopic features to their trainees.
Videodermatoscopes facilitate the simultaneous examination of cutaneous lesions by projecting the dermoscopic image onto a digital screen. Furthermore, these devices can incorporate magnifications of up to 200-fold or greater. In recent years, research pertaining to videodermoscopy has focused on the high magnification capabilities of these devices, specifically dermoscopic features that are visualized at magnifications greater than 10-fold, including the light brown nests of basal cell carcinomas that are seen at 50- to 70-fold magnification, twisted red capillary loops seen in active scalp psoriasis at 50-fold magnification, and longitudinal white indentations seen on nail plates affected by onychomycosis at 20-fold magnification.4-6 The potential value of videodermoscopy in medical education lies not only in the high magnification potential, which may make subtle dermoscopic findings more apparent to novice dermoscopists, but also in the ability to facilitate simultaneous dermoscopic examinations by instructors and trainees.
Educational Applications for Videodermoscopy
To illustrate the educational potential of videodermoscopy, images taken with a standard dermatoscope at 10-fold magnification are presented with videodermoscopic images taken at magnifications ranging from 60- to 185-fold (Figures 1–3). These examples demonstrate the potential for videodermoscopy to facilitate the visualization of subtle dermoscopic features by novice dermoscopists, relating to both the enhanced magnification potential and the potential for simultaneous rather than sequential examination.



Final Thoughts
High-magnification videodermoscopy may be a useful tool to further dermoscopic education. Videodermatoscopes vary in functionality and cost but are available at price points comparable to those of standard optical dermatoscopes. Owners of standard dermatoscopes can approximate some of the benefits of a digital videodermatoscope by using the standard dermatoscope in conjunction with a camera, including those integrated into mobile phones and tablets. By attaching the standard dermatoscope to a camera with a digital display, the digital zoom of the camera can be used to magnify the standard dermoscopic image, enhancing the ability of novice dermoscopists to visualize subtle findings. By presenting this magnified image on a digital display, dermoscopy instructors and trainees would be able to simultaneously view dermoscopic images of lesions, sometimes with magnifications comparable to videodermatoscopes.
In the setting of a dermatology residency program, videodermoscopy can be incorporated into bedside teaching with experienced dermoscopists and for the live presentation of dermoscopic features at departmental grand rounds. By facilitating the simultaneous, high-magnification and live viewing of skin lesions by dermoscopy instructors and trainees, digital videodermoscopy has the potential to address an area of weakness in dermatologic training.
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676.
- Engasser HC, Warshaw EM. Dermatoscopy use by US dermatologists: a cross-sectional survey [published online July 8, 2010]. J Am Acad Dermatol. 2010;63:412-419, 419.e1-419.e2.
- Wu TP, Newlove T, Smith L, et al. The importance of dedicated dermoscopy training during residency: a survey of US dermatology chief residents. J Am Acad Dermatol. 2013;68:1000-1005.
- Seidenari S, Bellucci C, Bassoli S, et al. High magnification digital dermoscopy of basal cell carcinoma: a single-centre study on 400 cases. Acta Derm Venereol. 2014;94:677-682.
- Ross EK, Vincenzi C, Tosti A. Videodermoscopy in the evaluation of hair and scalp disorders. J Am Acad Dermatol. 2006;55:799-806.
- Piraccini BM, Balestri R, Starace M, et al. Nail digital dermoscopy (onychoscopy) in the diagnosis of onychomycosis. J Eur Acad Dermatol Venereol. 2013;27:509-513.
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676.
- Engasser HC, Warshaw EM. Dermatoscopy use by US dermatologists: a cross-sectional survey [published online July 8, 2010]. J Am Acad Dermatol. 2010;63:412-419, 419.e1-419.e2.
- Wu TP, Newlove T, Smith L, et al. The importance of dedicated dermoscopy training during residency: a survey of US dermatology chief residents. J Am Acad Dermatol. 2013;68:1000-1005.
- Seidenari S, Bellucci C, Bassoli S, et al. High magnification digital dermoscopy of basal cell carcinoma: a single-centre study on 400 cases. Acta Derm Venereol. 2014;94:677-682.
- Ross EK, Vincenzi C, Tosti A. Videodermoscopy in the evaluation of hair and scalp disorders. J Am Acad Dermatol. 2006;55:799-806.
- Piraccini BM, Balestri R, Starace M, et al. Nail digital dermoscopy (onychoscopy) in the diagnosis of onychomycosis. J Eur Acad Dermatol Venereol. 2013;27:509-513.
Resident Pearl
- Bedside dermoscopy training can be enhanced through the use of videodermoscopy, which permits simultaneous, high-magnification viewing.