User login
The Effects of Sunscreen on Marine Environments
Coastal travel accounts for 80% of all tourism worldwide, a number that continues to grow. The number of travelers to the Mediterranean Sea alone is expected to rise to 350 million individuals per year within the next 20 years.1 As the number of tourists visiting the world’s oceans increases, the rate of sunscreen unintentionally washed into these marine environments also rises. One study estimated that approximately one-quarter of the sunscreen applied to the skin is washed off over a 20-minute period spent in the water.2 Four of the most common sunscreen agents—benzophenone-3 (BP-3),
Benzophenone-3
4-Methylbenzylidene Camphor
Environmental concerns have also been raised about another common chemical UV filter: 4-MBC, or enzacamene. In laboratory studies, 4-MBC has been shown to cause oxidative stress to Tetrahymena thermophila, an aquatic protozoan, which results in inhibited growth. At higher concentrations, damage to the cellular membrane was seen as soon as 4 hours after exposure.6 In embryonic zebrafish, elevated 4-MBC levels were correlated to improper nerve and muscular development, resulting in developmental defects.7 Another study demonstrated that 4-MBC was toxic to Mytilus galloprovincialis, known as the Mediterranean mussel, and Paracentrotus lividus, a species of sea urchin.8 Although these studies utilized highly controlled laboratory settings, further studies are needed to examine the effects of 4-MBC on these species at environmentally relevant concentrations.
Physical Sunscreens
Physical sunscreens, as compared to the chemical filters referenced above, use either zinc or titanium to protect the skin from the sun’s rays. Nanoparticles, in particular, are preferred because they do not leave a white film on the skin.9 Both titanium dioxide and zinc oxide nanoparticles have been found to inhibit the growth and photosynthesis of marine phytoplankton, the most abundant primary producers on Earth.10,11 These metal contaminants can be transferred to organisms of higher trophic levels, including zooplankton,12 and filter-feeding organisms, including marine abalone13 and the Mediterranean mussel.14 These nanoparticles have been shown to cause oxidative stress to these organisms, making them less fit to withstand environmental stressors. It is difficult to show their true impact, however, as it is challenging to accurately detect and quantify nanoparticle concentrations in vivo.15
Final Thoughts
- Marine problems: tourism & coastal development. World Wide Fund for Nature website. http://wwf.panda.org/about_our_earth/blue_planet/problems/tourism/. Published 2017. Accessed November 14, 2017.
- Danovaro R, Bongiorni L, Corinaldesi C, et al. Sunscreens cause coral bleaching by promoting viral infections. Environ Health Perspect. 2008;116:441-447.
- Downs C, Kramarsky-Winter E, Segal R, et al. Toxicopathological effects of the sunscreen UV filter, oxybenzone (benzophenone-3), on coral planulae and cultured primary cells and its environmental contamination in Hawaii and the US Virgin Islands. Arch Environ Contam Toxicol. 2016;70:265-288.
- Sánchez Rodríguez A, Rodrigo Sanz M, Betancort Rodríguez JR. Occurrence of eight UV filters in beaches of Gran Canaria (Canary Islands)[published online March 17, 2015]. Chemosphere. 2015;131:85-90.
- Bratkovics S, Sapozhnikova Y. Determination of seven commonly used organic UV filters in fresh and saline waters by liquid chromatography-tandem mass spectrometry. Analytical Methods. 2011;3:2943-2950.
- Gao L, Yuan T, Zhou C, et al. Effects of four commonly used UV filters on the growth, cell viability and oxidative stress responses of the Tetrahymena thermophila. Chemosphere. 2013;93:2507-2513.
- Li VW, Tsui MP, Chen X, et al. Effects of 4-methylbenzylidene camphor (4-MBC) on neuronal and muscular development in zebrafish (Danio rerio) embryos [published online February 18, 2016]. Environ Sci Pollut Res Int. 2016;23:8275-8285.
- Paredes E, Perez S, Rodil R, et al. Ecotoxicological evaluation of four UV filters using marine organisms from different trophic levels Isochrysis galbana, Mytilus galloprovincialis, Paracentrotus lividus, and Siriella armata. Chemosphere. 2014;104:44-50.
- Osterwalder U, Sohn M, Herzog B. Global state of sunscreens. Photodermatol Photoimmunol Photomed. 2014;30:62-80.
- Miller RJ, Bennett S, Keller AA, et al. TiO2 nanoparticles are phototoxic to marine phytoplankton. PloS One. 2012;7:E30321.
- Spisni E. Toxicity Assessment of Industrial- and Sunscreen-derived ZnO Nanoparticles [master’s thesis]. Coral Gables, FL: University of Miami Libraries Scholarly Repository; 2016. http://scholarlyrepository.miami.edu/cgi/viewcontent.cgi?article=1625&context=oa_theses. Accessed November 10, 2017.
- Jarvis TA, Miller RJ, Lenihan HS, et al. Toxicity of ZnO nanoparticles to the copepod Acartia tonsa, exposed through a phytoplankton diet [published online April 15, 2013]. Environ Toxicol Chem. 2013;32:1264-1269.
- Zhu X, Zhou J, Cai Z. The toxicity and oxidative stress of TiO2 nanoparticles in marine abalone (Haliotis diversicolor supertexta). Mar Pollut Bull. 2011;63:334-338.
- Barmo C, Ciacci C, Canonico B, et al. In vivo effects of n-TiO2 on digestive gland and immune function of the marine bivalve Mytilus galloprovincialis. Aquatic Toxicol. 2013;132:9-18.
- Sánchez-Quiles D, Tovar-Sánchez A. Are sunscreens a new environmental risk associated with coastal tourism? Environ Int. 2015;83:158-170.
- Xu S, Kwa M, Agarwal A, et al. Sunscreen product performance and other determinants of consumer preferences. JAMA Dermatol. 2016;152:920-927.
- Vesper I. Hawaii seeks to ban ‘reef-unfriendly’ sunscreen. Nature. February 3, 2017. https://www.nature.com/news/hawaii-seeks-to-ban-reef-unfriendly-sunscreen-1.21332. Accessed November 16, 2017.
Coastal travel accounts for 80% of all tourism worldwide, a number that continues to grow. The number of travelers to the Mediterranean Sea alone is expected to rise to 350 million individuals per year within the next 20 years.1 As the number of tourists visiting the world’s oceans increases, the rate of sunscreen unintentionally washed into these marine environments also rises. One study estimated that approximately one-quarter of the sunscreen applied to the skin is washed off over a 20-minute period spent in the water.2 Four of the most common sunscreen agents—benzophenone-3 (BP-3),
Benzophenone-3
4-Methylbenzylidene Camphor
Environmental concerns have also been raised about another common chemical UV filter: 4-MBC, or enzacamene. In laboratory studies, 4-MBC has been shown to cause oxidative stress to Tetrahymena thermophila, an aquatic protozoan, which results in inhibited growth. At higher concentrations, damage to the cellular membrane was seen as soon as 4 hours after exposure.6 In embryonic zebrafish, elevated 4-MBC levels were correlated to improper nerve and muscular development, resulting in developmental defects.7 Another study demonstrated that 4-MBC was toxic to Mytilus galloprovincialis, known as the Mediterranean mussel, and Paracentrotus lividus, a species of sea urchin.8 Although these studies utilized highly controlled laboratory settings, further studies are needed to examine the effects of 4-MBC on these species at environmentally relevant concentrations.
Physical Sunscreens
Physical sunscreens, as compared to the chemical filters referenced above, use either zinc or titanium to protect the skin from the sun’s rays. Nanoparticles, in particular, are preferred because they do not leave a white film on the skin.9 Both titanium dioxide and zinc oxide nanoparticles have been found to inhibit the growth and photosynthesis of marine phytoplankton, the most abundant primary producers on Earth.10,11 These metal contaminants can be transferred to organisms of higher trophic levels, including zooplankton,12 and filter-feeding organisms, including marine abalone13 and the Mediterranean mussel.14 These nanoparticles have been shown to cause oxidative stress to these organisms, making them less fit to withstand environmental stressors. It is difficult to show their true impact, however, as it is challenging to accurately detect and quantify nanoparticle concentrations in vivo.15
Final Thoughts
Coastal travel accounts for 80% of all tourism worldwide, a number that continues to grow. The number of travelers to the Mediterranean Sea alone is expected to rise to 350 million individuals per year within the next 20 years.1 As the number of tourists visiting the world’s oceans increases, the rate of sunscreen unintentionally washed into these marine environments also rises. One study estimated that approximately one-quarter of the sunscreen applied to the skin is washed off over a 20-minute period spent in the water.2 Four of the most common sunscreen agents—benzophenone-3 (BP-3),
Benzophenone-3
4-Methylbenzylidene Camphor
Environmental concerns have also been raised about another common chemical UV filter: 4-MBC, or enzacamene. In laboratory studies, 4-MBC has been shown to cause oxidative stress to Tetrahymena thermophila, an aquatic protozoan, which results in inhibited growth. At higher concentrations, damage to the cellular membrane was seen as soon as 4 hours after exposure.6 In embryonic zebrafish, elevated 4-MBC levels were correlated to improper nerve and muscular development, resulting in developmental defects.7 Another study demonstrated that 4-MBC was toxic to Mytilus galloprovincialis, known as the Mediterranean mussel, and Paracentrotus lividus, a species of sea urchin.8 Although these studies utilized highly controlled laboratory settings, further studies are needed to examine the effects of 4-MBC on these species at environmentally relevant concentrations.
Physical Sunscreens
Physical sunscreens, as compared to the chemical filters referenced above, use either zinc or titanium to protect the skin from the sun’s rays. Nanoparticles, in particular, are preferred because they do not leave a white film on the skin.9 Both titanium dioxide and zinc oxide nanoparticles have been found to inhibit the growth and photosynthesis of marine phytoplankton, the most abundant primary producers on Earth.10,11 These metal contaminants can be transferred to organisms of higher trophic levels, including zooplankton,12 and filter-feeding organisms, including marine abalone13 and the Mediterranean mussel.14 These nanoparticles have been shown to cause oxidative stress to these organisms, making them less fit to withstand environmental stressors. It is difficult to show their true impact, however, as it is challenging to accurately detect and quantify nanoparticle concentrations in vivo.15
Final Thoughts
- Marine problems: tourism & coastal development. World Wide Fund for Nature website. http://wwf.panda.org/about_our_earth/blue_planet/problems/tourism/. Published 2017. Accessed November 14, 2017.
- Danovaro R, Bongiorni L, Corinaldesi C, et al. Sunscreens cause coral bleaching by promoting viral infections. Environ Health Perspect. 2008;116:441-447.
- Downs C, Kramarsky-Winter E, Segal R, et al. Toxicopathological effects of the sunscreen UV filter, oxybenzone (benzophenone-3), on coral planulae and cultured primary cells and its environmental contamination in Hawaii and the US Virgin Islands. Arch Environ Contam Toxicol. 2016;70:265-288.
- Sánchez Rodríguez A, Rodrigo Sanz M, Betancort Rodríguez JR. Occurrence of eight UV filters in beaches of Gran Canaria (Canary Islands)[published online March 17, 2015]. Chemosphere. 2015;131:85-90.
- Bratkovics S, Sapozhnikova Y. Determination of seven commonly used organic UV filters in fresh and saline waters by liquid chromatography-tandem mass spectrometry. Analytical Methods. 2011;3:2943-2950.
- Gao L, Yuan T, Zhou C, et al. Effects of four commonly used UV filters on the growth, cell viability and oxidative stress responses of the Tetrahymena thermophila. Chemosphere. 2013;93:2507-2513.
- Li VW, Tsui MP, Chen X, et al. Effects of 4-methylbenzylidene camphor (4-MBC) on neuronal and muscular development in zebrafish (Danio rerio) embryos [published online February 18, 2016]. Environ Sci Pollut Res Int. 2016;23:8275-8285.
- Paredes E, Perez S, Rodil R, et al. Ecotoxicological evaluation of four UV filters using marine organisms from different trophic levels Isochrysis galbana, Mytilus galloprovincialis, Paracentrotus lividus, and Siriella armata. Chemosphere. 2014;104:44-50.
- Osterwalder U, Sohn M, Herzog B. Global state of sunscreens. Photodermatol Photoimmunol Photomed. 2014;30:62-80.
- Miller RJ, Bennett S, Keller AA, et al. TiO2 nanoparticles are phototoxic to marine phytoplankton. PloS One. 2012;7:E30321.
- Spisni E. Toxicity Assessment of Industrial- and Sunscreen-derived ZnO Nanoparticles [master’s thesis]. Coral Gables, FL: University of Miami Libraries Scholarly Repository; 2016. http://scholarlyrepository.miami.edu/cgi/viewcontent.cgi?article=1625&context=oa_theses. Accessed November 10, 2017.
- Jarvis TA, Miller RJ, Lenihan HS, et al. Toxicity of ZnO nanoparticles to the copepod Acartia tonsa, exposed through a phytoplankton diet [published online April 15, 2013]. Environ Toxicol Chem. 2013;32:1264-1269.
- Zhu X, Zhou J, Cai Z. The toxicity and oxidative stress of TiO2 nanoparticles in marine abalone (Haliotis diversicolor supertexta). Mar Pollut Bull. 2011;63:334-338.
- Barmo C, Ciacci C, Canonico B, et al. In vivo effects of n-TiO2 on digestive gland and immune function of the marine bivalve Mytilus galloprovincialis. Aquatic Toxicol. 2013;132:9-18.
- Sánchez-Quiles D, Tovar-Sánchez A. Are sunscreens a new environmental risk associated with coastal tourism? Environ Int. 2015;83:158-170.
- Xu S, Kwa M, Agarwal A, et al. Sunscreen product performance and other determinants of consumer preferences. JAMA Dermatol. 2016;152:920-927.
- Vesper I. Hawaii seeks to ban ‘reef-unfriendly’ sunscreen. Nature. February 3, 2017. https://www.nature.com/news/hawaii-seeks-to-ban-reef-unfriendly-sunscreen-1.21332. Accessed November 16, 2017.
- Marine problems: tourism & coastal development. World Wide Fund for Nature website. http://wwf.panda.org/about_our_earth/blue_planet/problems/tourism/. Published 2017. Accessed November 14, 2017.
- Danovaro R, Bongiorni L, Corinaldesi C, et al. Sunscreens cause coral bleaching by promoting viral infections. Environ Health Perspect. 2008;116:441-447.
- Downs C, Kramarsky-Winter E, Segal R, et al. Toxicopathological effects of the sunscreen UV filter, oxybenzone (benzophenone-3), on coral planulae and cultured primary cells and its environmental contamination in Hawaii and the US Virgin Islands. Arch Environ Contam Toxicol. 2016;70:265-288.
- Sánchez Rodríguez A, Rodrigo Sanz M, Betancort Rodríguez JR. Occurrence of eight UV filters in beaches of Gran Canaria (Canary Islands)[published online March 17, 2015]. Chemosphere. 2015;131:85-90.
- Bratkovics S, Sapozhnikova Y. Determination of seven commonly used organic UV filters in fresh and saline waters by liquid chromatography-tandem mass spectrometry. Analytical Methods. 2011;3:2943-2950.
- Gao L, Yuan T, Zhou C, et al. Effects of four commonly used UV filters on the growth, cell viability and oxidative stress responses of the Tetrahymena thermophila. Chemosphere. 2013;93:2507-2513.
- Li VW, Tsui MP, Chen X, et al. Effects of 4-methylbenzylidene camphor (4-MBC) on neuronal and muscular development in zebrafish (Danio rerio) embryos [published online February 18, 2016]. Environ Sci Pollut Res Int. 2016;23:8275-8285.
- Paredes E, Perez S, Rodil R, et al. Ecotoxicological evaluation of four UV filters using marine organisms from different trophic levels Isochrysis galbana, Mytilus galloprovincialis, Paracentrotus lividus, and Siriella armata. Chemosphere. 2014;104:44-50.
- Osterwalder U, Sohn M, Herzog B. Global state of sunscreens. Photodermatol Photoimmunol Photomed. 2014;30:62-80.
- Miller RJ, Bennett S, Keller AA, et al. TiO2 nanoparticles are phototoxic to marine phytoplankton. PloS One. 2012;7:E30321.
- Spisni E. Toxicity Assessment of Industrial- and Sunscreen-derived ZnO Nanoparticles [master’s thesis]. Coral Gables, FL: University of Miami Libraries Scholarly Repository; 2016. http://scholarlyrepository.miami.edu/cgi/viewcontent.cgi?article=1625&context=oa_theses. Accessed November 10, 2017.
- Jarvis TA, Miller RJ, Lenihan HS, et al. Toxicity of ZnO nanoparticles to the copepod Acartia tonsa, exposed through a phytoplankton diet [published online April 15, 2013]. Environ Toxicol Chem. 2013;32:1264-1269.
- Zhu X, Zhou J, Cai Z. The toxicity and oxidative stress of TiO2 nanoparticles in marine abalone (Haliotis diversicolor supertexta). Mar Pollut Bull. 2011;63:334-338.
- Barmo C, Ciacci C, Canonico B, et al. In vivo effects of n-TiO2 on digestive gland and immune function of the marine bivalve Mytilus galloprovincialis. Aquatic Toxicol. 2013;132:9-18.
- Sánchez-Quiles D, Tovar-Sánchez A. Are sunscreens a new environmental risk associated with coastal tourism? Environ Int. 2015;83:158-170.
- Xu S, Kwa M, Agarwal A, et al. Sunscreen product performance and other determinants of consumer preferences. JAMA Dermatol. 2016;152:920-927.
- Vesper I. Hawaii seeks to ban ‘reef-unfriendly’ sunscreen. Nature. February 3, 2017. https://www.nature.com/news/hawaii-seeks-to-ban-reef-unfriendly-sunscreen-1.21332. Accessed November 16, 2017.
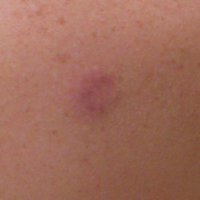
Asymptomatic Pink Plaque on the Scapula
The Diagnosis: Primary Cutaneous Follicle Center Lymphoma
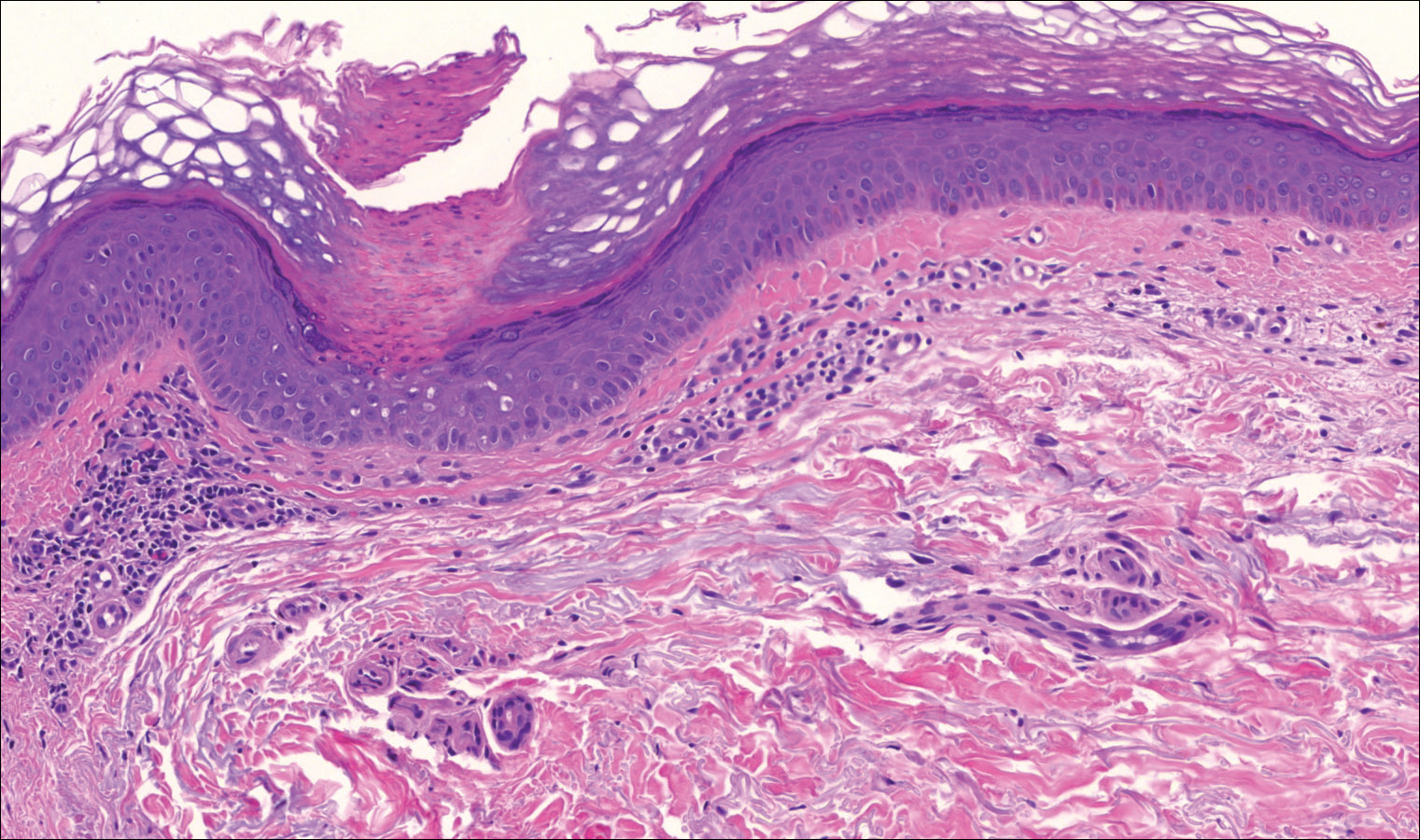
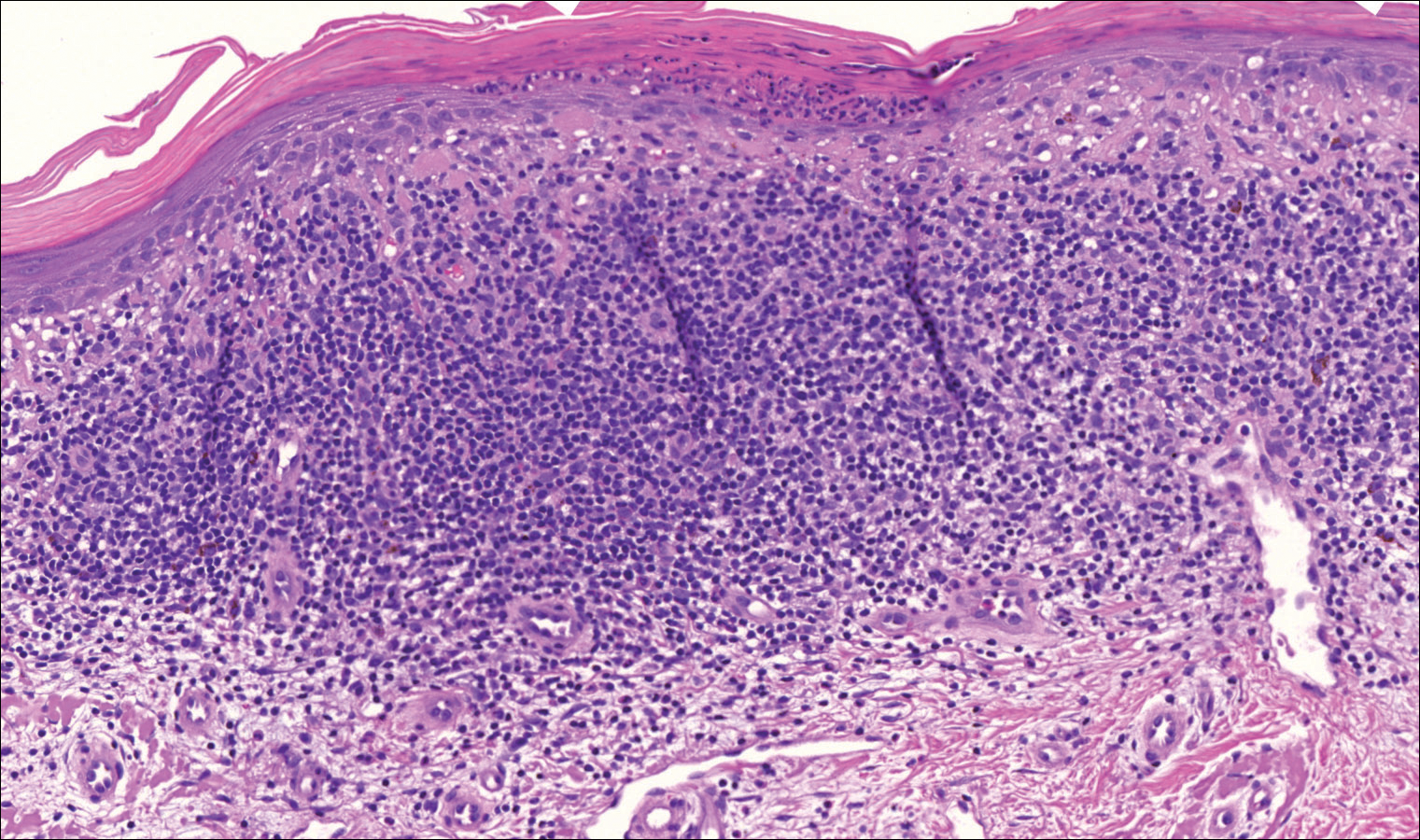
Immunohistochemistry revealed a nodular infiltrate consisting of small to large atypical lymphocytes forming an irregular germinal center with notably thinned mantle zones and lack of polarization (Figure, A). Atypical cells stained positively with Bcl-6, and CD20 was diffusely positive (Figure, B-D). Bcl-2 and CD3 colocalized to the reactive T-cell infiltrate, and CD10 was largely negative. Further workup with bone marrow biopsy and full-body positron emission tomography-computed tomography was unremarkable. Given these findings, a diagnosis of primary cutaneous follicle center lymphoma (FCL) was made. At 1 month following radiation therapy, complete clinical clearance of the lymphoma was achieved.
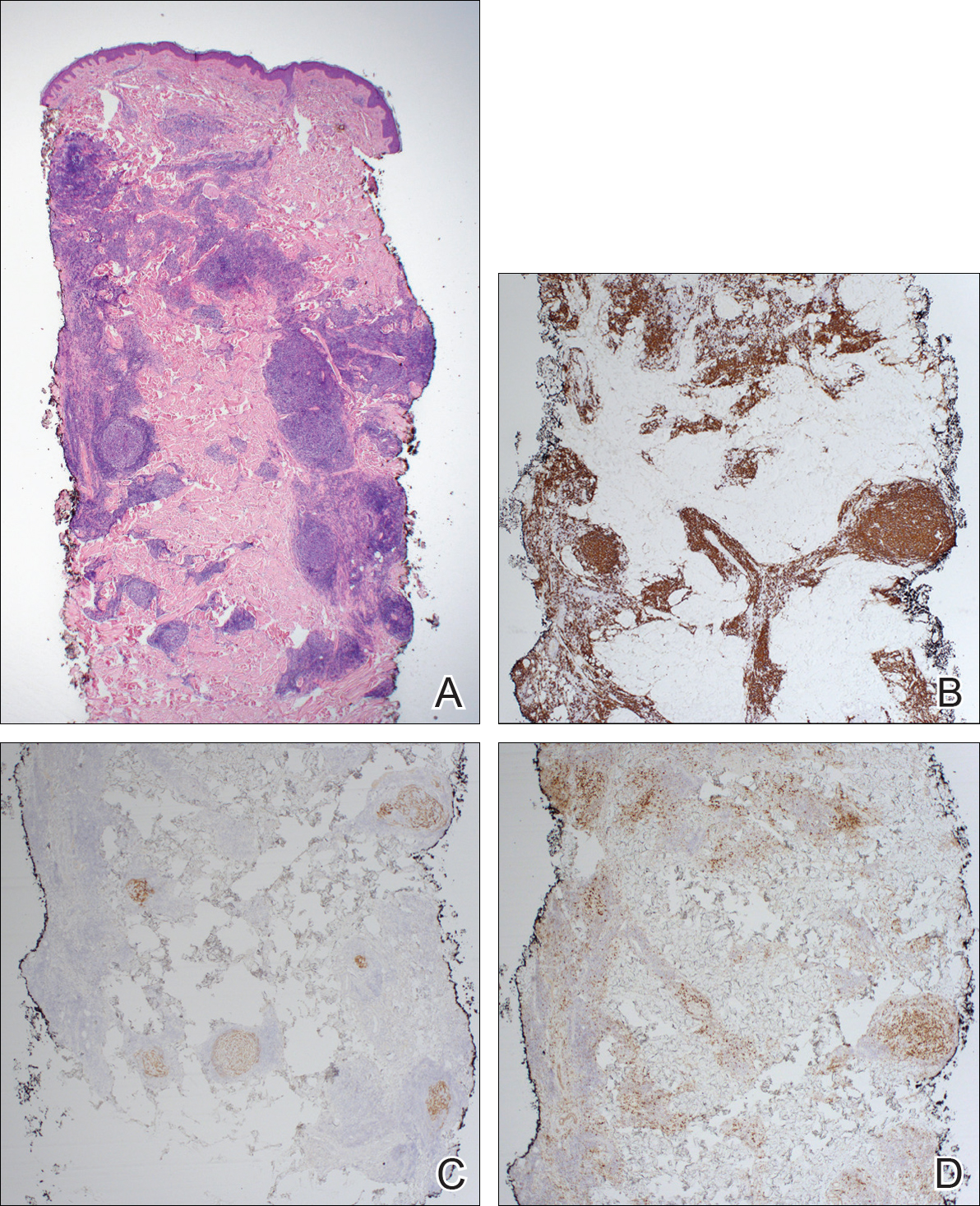
Follicle center lymphoma, also known as cutaneous follicular lymphoma, is the most common subtype of primary cutaneous B-cell lymphomas, representing approximately 57% of cases.1 Follicle center lymphoma typically affects older, non-Hispanic white adults with a median age of onset of 60 years. It has a predilection for the head, neck, and trunk.2 Lesions present as solitary erythematous to violaceous papules, plaques, or nodules, but they can more rarely be multifocal.3 Clinical diagnosis of FCL can be difficult, with papular lesions resembling acne, rosacea, folliculitis, or arthropod assault.4,5 As such, diagnosis of FCL typically relies on histopathologic analysis.
Histologically, FCL can present in several different patterns including follicular, nodular, diffuse, or a pleomorphic mix of these.2,6 The cells are comprised of germinal center B cells, staining positively for Bcl-6, CD20, and CD79a.7 Tumor cells do not exhibit the t(14;18) translocation seen in nodal follicular lymphomas.2,8 Unlike marginal zone lymphoma, FCL stains negatively for Bcl-2 and multiple myeloma 1/interferon regulatory factor 4 (MUM1/IRF-4).2,9 Forkhead box P1 (FOXP1) also is usually negative, but its presence can indicate a poorer prognosis.2 It is important to distinguish primary cutaneous B-cell lymphomas from systemic B-cell lymphoma with secondary cutaneous involvement, as they have a different clinical prognosis and management course. Further workup includes bone marrow biopsy, serum analysis for clonal involvement, and positron emission tomography-computed tomography imaging. Follicle center lymphoma generally has an indolent disease course with a favorable 5-year survival rate of approximately 95%.6,8
Untreated lesions may enlarge slowly or even spontaneously involute.10 The histologic growth pattern and number of lesions do not affect prognosis, but presence on the legs has a 5-year survival rate of 41%.2 Extracutaneous dissemination can occur in 5% to 10% of cases.2 Given the slow progression of FCL, conservative management with observation is an option. However, curative treatment can be reasonably attempted for solitary lesions by excision or radiation. Treatment of FCL often can be complicated by its predilection for the head and neck. Other treatment modalities include topical steroids, imiquimod, nitrogen mustard, and bexarotene.10 More generalized involvement may require systemic therapy with rituximab or chemotherapy. Recurrence after therapy is common, reported in 46.5% of patients, but does not affect prognosis.2
- Zinzani PL, Quaglino P, Pimpinelli N, et al. Prognostic factors in primary cutaneous B-cell lymphoma: The Italian Study Group for Cutaneous Lymphomas. J Clin Oncol. 2006;24:1376-1382.
- Suárez AL, Pulitzer M, Horwitz S, et al. Primary cutaneous B-cell lymphomas: part I. clinical features, diagnosis, and classification. J Am Acad Dermatol. 2013;69:1-13.
- Grange F, Bekkenk MW, Wechsler J, et al. Prognostic factors in primary cutaneous large B-cell lymphomas: a European multicenter study. J Clin Oncol. 2001;19:3602-3610.
- Soon CW, Pincus LB, Ai WZ, et al. Acneiform presentation of primary cutaneous follicle center lymphoma. J Am Acad Dermatol. 2011;65:887-889.
- Massone C, Fink-Puches R, Laimer M, et al. Miliary and agminated-type primary cutaneous follicle center lymphoma: a report of 18 cases. J Am Acad Dermatol. 2011;65:749-755.
- Wilcox RA. CME information: cutaneous B-cell lymphomas: 2015 update on diagnosis, risk-stratification, and management. Am J Hematol. 2015;90:73-76.
- Franco R, Fernandez-Vazquez A, Rodriguez-Peralto JL, et al. Cutaneous follicular B-cell lymphoma: description of a series of 18 cases. Am J Surg Pathol. 2001;25:875-883.
- Kempf W, Denisjuk N, Kerl K, et al. Primary cutaneous B-cell lymphomas. J Dtsch Dermatol Ges. 2012;10:12-22; quiz 23.
- de Leval L HN, Longtine J, Ferry JA, et al. Cutaneous B-cell lymphomas of follicular and marginal zone types: use of Bcl-6, CD10, Bcl-2, and CD21 in differential diagnosis and classification. Am J Surg Pathol. 2001;25:732-741.
- Suárez AL, Querfeld C, Horwitz S, et al. Primary cutaneous B-cell lymphomas: part II. therapy and future directions. J Am Acad Dermatol. 2013;69:1-11.
The Diagnosis: Primary Cutaneous Follicle Center Lymphoma
Immunohistochemistry revealed a nodular infiltrate consisting of small to large atypical lymphocytes forming an irregular germinal center with notably thinned mantle zones and lack of polarization (Figure, A). Atypical cells stained positively with Bcl-6, and CD20 was diffusely positive (Figure, B-D). Bcl-2 and CD3 colocalized to the reactive T-cell infiltrate, and CD10 was largely negative. Further workup with bone marrow biopsy and full-body positron emission tomography-computed tomography was unremarkable. Given these findings, a diagnosis of primary cutaneous follicle center lymphoma (FCL) was made. At 1 month following radiation therapy, complete clinical clearance of the lymphoma was achieved.

Follicle center lymphoma, also known as cutaneous follicular lymphoma, is the most common subtype of primary cutaneous B-cell lymphomas, representing approximately 57% of cases.1 Follicle center lymphoma typically affects older, non-Hispanic white adults with a median age of onset of 60 years. It has a predilection for the head, neck, and trunk.2 Lesions present as solitary erythematous to violaceous papules, plaques, or nodules, but they can more rarely be multifocal.3 Clinical diagnosis of FCL can be difficult, with papular lesions resembling acne, rosacea, folliculitis, or arthropod assault.4,5 As such, diagnosis of FCL typically relies on histopathologic analysis.
Histologically, FCL can present in several different patterns including follicular, nodular, diffuse, or a pleomorphic mix of these.2,6 The cells are comprised of germinal center B cells, staining positively for Bcl-6, CD20, and CD79a.7 Tumor cells do not exhibit the t(14;18) translocation seen in nodal follicular lymphomas.2,8 Unlike marginal zone lymphoma, FCL stains negatively for Bcl-2 and multiple myeloma 1/interferon regulatory factor 4 (MUM1/IRF-4).2,9 Forkhead box P1 (FOXP1) also is usually negative, but its presence can indicate a poorer prognosis.2 It is important to distinguish primary cutaneous B-cell lymphomas from systemic B-cell lymphoma with secondary cutaneous involvement, as they have a different clinical prognosis and management course. Further workup includes bone marrow biopsy, serum analysis for clonal involvement, and positron emission tomography-computed tomography imaging. Follicle center lymphoma generally has an indolent disease course with a favorable 5-year survival rate of approximately 95%.6,8
Untreated lesions may enlarge slowly or even spontaneously involute.10 The histologic growth pattern and number of lesions do not affect prognosis, but presence on the legs has a 5-year survival rate of 41%.2 Extracutaneous dissemination can occur in 5% to 10% of cases.2 Given the slow progression of FCL, conservative management with observation is an option. However, curative treatment can be reasonably attempted for solitary lesions by excision or radiation. Treatment of FCL often can be complicated by its predilection for the head and neck. Other treatment modalities include topical steroids, imiquimod, nitrogen mustard, and bexarotene.10 More generalized involvement may require systemic therapy with rituximab or chemotherapy. Recurrence after therapy is common, reported in 46.5% of patients, but does not affect prognosis.2
The Diagnosis: Primary Cutaneous Follicle Center Lymphoma
Immunohistochemistry revealed a nodular infiltrate consisting of small to large atypical lymphocytes forming an irregular germinal center with notably thinned mantle zones and lack of polarization (Figure, A). Atypical cells stained positively with Bcl-6, and CD20 was diffusely positive (Figure, B-D). Bcl-2 and CD3 colocalized to the reactive T-cell infiltrate, and CD10 was largely negative. Further workup with bone marrow biopsy and full-body positron emission tomography-computed tomography was unremarkable. Given these findings, a diagnosis of primary cutaneous follicle center lymphoma (FCL) was made. At 1 month following radiation therapy, complete clinical clearance of the lymphoma was achieved.

Follicle center lymphoma, also known as cutaneous follicular lymphoma, is the most common subtype of primary cutaneous B-cell lymphomas, representing approximately 57% of cases.1 Follicle center lymphoma typically affects older, non-Hispanic white adults with a median age of onset of 60 years. It has a predilection for the head, neck, and trunk.2 Lesions present as solitary erythematous to violaceous papules, plaques, or nodules, but they can more rarely be multifocal.3 Clinical diagnosis of FCL can be difficult, with papular lesions resembling acne, rosacea, folliculitis, or arthropod assault.4,5 As such, diagnosis of FCL typically relies on histopathologic analysis.
Histologically, FCL can present in several different patterns including follicular, nodular, diffuse, or a pleomorphic mix of these.2,6 The cells are comprised of germinal center B cells, staining positively for Bcl-6, CD20, and CD79a.7 Tumor cells do not exhibit the t(14;18) translocation seen in nodal follicular lymphomas.2,8 Unlike marginal zone lymphoma, FCL stains negatively for Bcl-2 and multiple myeloma 1/interferon regulatory factor 4 (MUM1/IRF-4).2,9 Forkhead box P1 (FOXP1) also is usually negative, but its presence can indicate a poorer prognosis.2 It is important to distinguish primary cutaneous B-cell lymphomas from systemic B-cell lymphoma with secondary cutaneous involvement, as they have a different clinical prognosis and management course. Further workup includes bone marrow biopsy, serum analysis for clonal involvement, and positron emission tomography-computed tomography imaging. Follicle center lymphoma generally has an indolent disease course with a favorable 5-year survival rate of approximately 95%.6,8
Untreated lesions may enlarge slowly or even spontaneously involute.10 The histologic growth pattern and number of lesions do not affect prognosis, but presence on the legs has a 5-year survival rate of 41%.2 Extracutaneous dissemination can occur in 5% to 10% of cases.2 Given the slow progression of FCL, conservative management with observation is an option. However, curative treatment can be reasonably attempted for solitary lesions by excision or radiation. Treatment of FCL often can be complicated by its predilection for the head and neck. Other treatment modalities include topical steroids, imiquimod, nitrogen mustard, and bexarotene.10 More generalized involvement may require systemic therapy with rituximab or chemotherapy. Recurrence after therapy is common, reported in 46.5% of patients, but does not affect prognosis.2
- Zinzani PL, Quaglino P, Pimpinelli N, et al. Prognostic factors in primary cutaneous B-cell lymphoma: The Italian Study Group for Cutaneous Lymphomas. J Clin Oncol. 2006;24:1376-1382.
- Suárez AL, Pulitzer M, Horwitz S, et al. Primary cutaneous B-cell lymphomas: part I. clinical features, diagnosis, and classification. J Am Acad Dermatol. 2013;69:1-13.
- Grange F, Bekkenk MW, Wechsler J, et al. Prognostic factors in primary cutaneous large B-cell lymphomas: a European multicenter study. J Clin Oncol. 2001;19:3602-3610.
- Soon CW, Pincus LB, Ai WZ, et al. Acneiform presentation of primary cutaneous follicle center lymphoma. J Am Acad Dermatol. 2011;65:887-889.
- Massone C, Fink-Puches R, Laimer M, et al. Miliary and agminated-type primary cutaneous follicle center lymphoma: a report of 18 cases. J Am Acad Dermatol. 2011;65:749-755.
- Wilcox RA. CME information: cutaneous B-cell lymphomas: 2015 update on diagnosis, risk-stratification, and management. Am J Hematol. 2015;90:73-76.
- Franco R, Fernandez-Vazquez A, Rodriguez-Peralto JL, et al. Cutaneous follicular B-cell lymphoma: description of a series of 18 cases. Am J Surg Pathol. 2001;25:875-883.
- Kempf W, Denisjuk N, Kerl K, et al. Primary cutaneous B-cell lymphomas. J Dtsch Dermatol Ges. 2012;10:12-22; quiz 23.
- de Leval L HN, Longtine J, Ferry JA, et al. Cutaneous B-cell lymphomas of follicular and marginal zone types: use of Bcl-6, CD10, Bcl-2, and CD21 in differential diagnosis and classification. Am J Surg Pathol. 2001;25:732-741.
- Suárez AL, Querfeld C, Horwitz S, et al. Primary cutaneous B-cell lymphomas: part II. therapy and future directions. J Am Acad Dermatol. 2013;69:1-11.
- Zinzani PL, Quaglino P, Pimpinelli N, et al. Prognostic factors in primary cutaneous B-cell lymphoma: The Italian Study Group for Cutaneous Lymphomas. J Clin Oncol. 2006;24:1376-1382.
- Suárez AL, Pulitzer M, Horwitz S, et al. Primary cutaneous B-cell lymphomas: part I. clinical features, diagnosis, and classification. J Am Acad Dermatol. 2013;69:1-13.
- Grange F, Bekkenk MW, Wechsler J, et al. Prognostic factors in primary cutaneous large B-cell lymphomas: a European multicenter study. J Clin Oncol. 2001;19:3602-3610.
- Soon CW, Pincus LB, Ai WZ, et al. Acneiform presentation of primary cutaneous follicle center lymphoma. J Am Acad Dermatol. 2011;65:887-889.
- Massone C, Fink-Puches R, Laimer M, et al. Miliary and agminated-type primary cutaneous follicle center lymphoma: a report of 18 cases. J Am Acad Dermatol. 2011;65:749-755.
- Wilcox RA. CME information: cutaneous B-cell lymphomas: 2015 update on diagnosis, risk-stratification, and management. Am J Hematol. 2015;90:73-76.
- Franco R, Fernandez-Vazquez A, Rodriguez-Peralto JL, et al. Cutaneous follicular B-cell lymphoma: description of a series of 18 cases. Am J Surg Pathol. 2001;25:875-883.
- Kempf W, Denisjuk N, Kerl K, et al. Primary cutaneous B-cell lymphomas. J Dtsch Dermatol Ges. 2012;10:12-22; quiz 23.
- de Leval L HN, Longtine J, Ferry JA, et al. Cutaneous B-cell lymphomas of follicular and marginal zone types: use of Bcl-6, CD10, Bcl-2, and CD21 in differential diagnosis and classification. Am J Surg Pathol. 2001;25:732-741.
- Suárez AL, Querfeld C, Horwitz S, et al. Primary cutaneous B-cell lymphomas: part II. therapy and future directions. J Am Acad Dermatol. 2013;69:1-11.
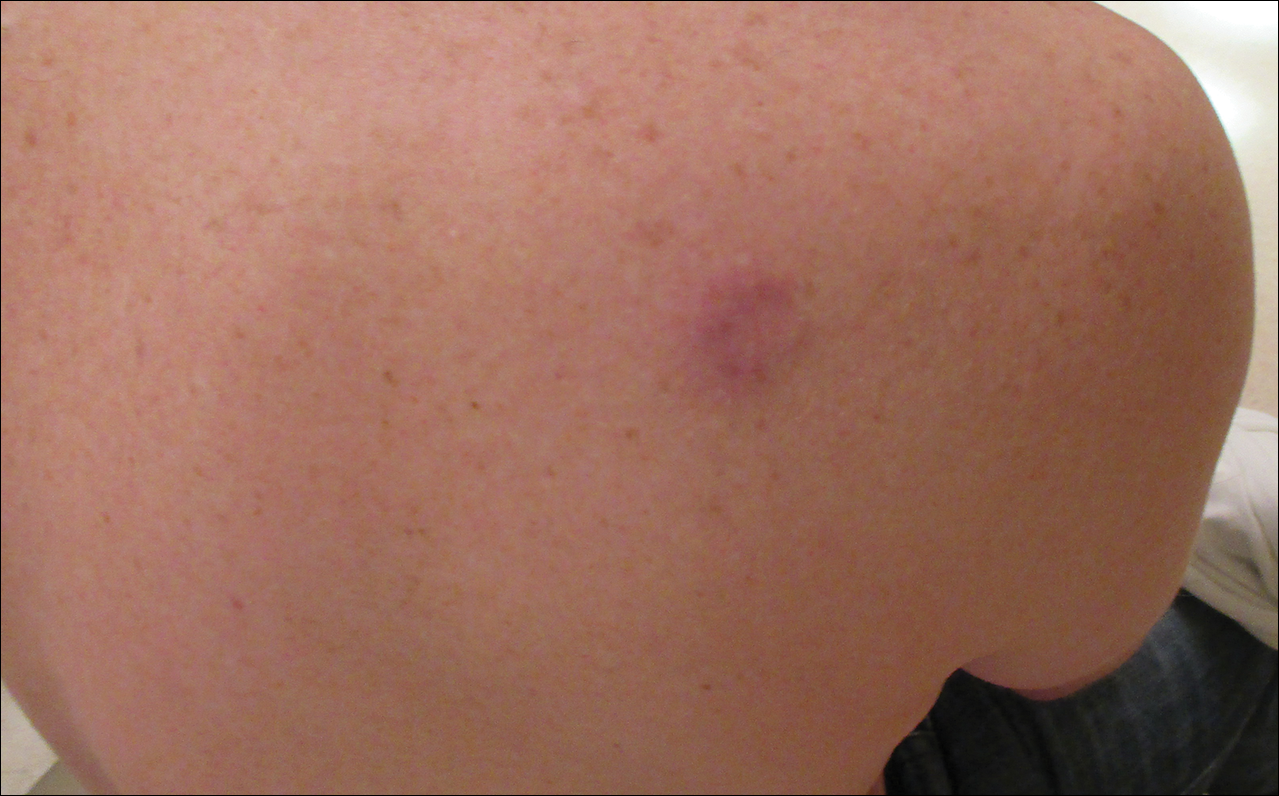
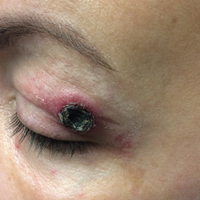
A 36-year-old man presented with a pink plaque on the right side of the scapula of 1 year's duration. The plaque had not grown and was completely asymptomatic. Physical examination revealed a violaceous, pink, 2-cm nodule with overlying telangiectasia. No other concerning lesions were identified on total-body skin examination. A punch biopsy was obtained.
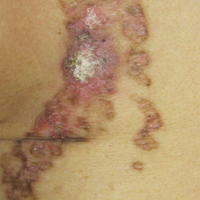
Linear Porokeratosis Associated With Multiple Squamous Cell Carcinomas
Lesions of porokeratosis are thought to arise from disordered keratinization, though the exact pathogenesis remains uncertain. At least 5 clinical subtypes of porokeratosis have been identified: porokeratosis of Mibelli, disseminated superficial porokeratosis and disseminated superficial actinic porokeratosis (DSAP), linear porokeratosis, punctuate porokeratosis, and porokeratosis palmaris et plantaris disseminata (PPPD).1,2 Linear porokeratosis is a rare subtype with a clinical differential diagnosis that includes lichen striatus, linear lichen planus, linear verrucous epidermal nevus, segmental Darier disease, and incontinentia pigmenti.3 Definitive diagnosis of linear porokeratosis is made by histopathologic examination demonstrating a cornoid lamella, defined as a column of parakeratotic cells that lies at 45°to the surface of the epidermis and contains pyknotic basophilic nuclei.4 Patients with linear porokeratosis typically develop lesions along the lines of Blaschko in infancy or childhood.5,6 Among the different subtypes of porokeratosis, linear porokeratosis demonstrates the highest rate of malignant transformation, therefore requiring close clinical observation.7
Case Report
An 83-year-old woman presented to the outpatient clinic with a large linear plaque on the right leg that had been present since birth. Ten years prior to presentation, a portion of the lesion started to bleed; biopsy of the area was performed by an outside provider demonstrating squamous cell carcinoma (SCC), which was treated with wide local excision. One year prior to presentation, a separate portion of the plaque was biopsied by an outside provider and another diagnosis of SCC was made.
On examination performed during the initial presentation to our clinic, there was a well-demarcated tan to violaceous linear plaque present at the lower buttock and extending along the posterior leg to the skin overlying the Achilles tendon and dorsal aspect of the right foot. Within the plaque, there were areas of atrophy and areas of inflammation, induration, and hyperkeratosis (Figures 1 and 2). Two punch biopsies were performed: one from the edge of the plaque and one from a hyperkeratotic region within the plaque. Histology from the edge of the plaque demonstrated a cornoid lamella, consistent with a porokeratosis (Figure 3), whereas the histology from the hyperkeratotic region demonstrated a lichenoid infiltrate (Figure 4).

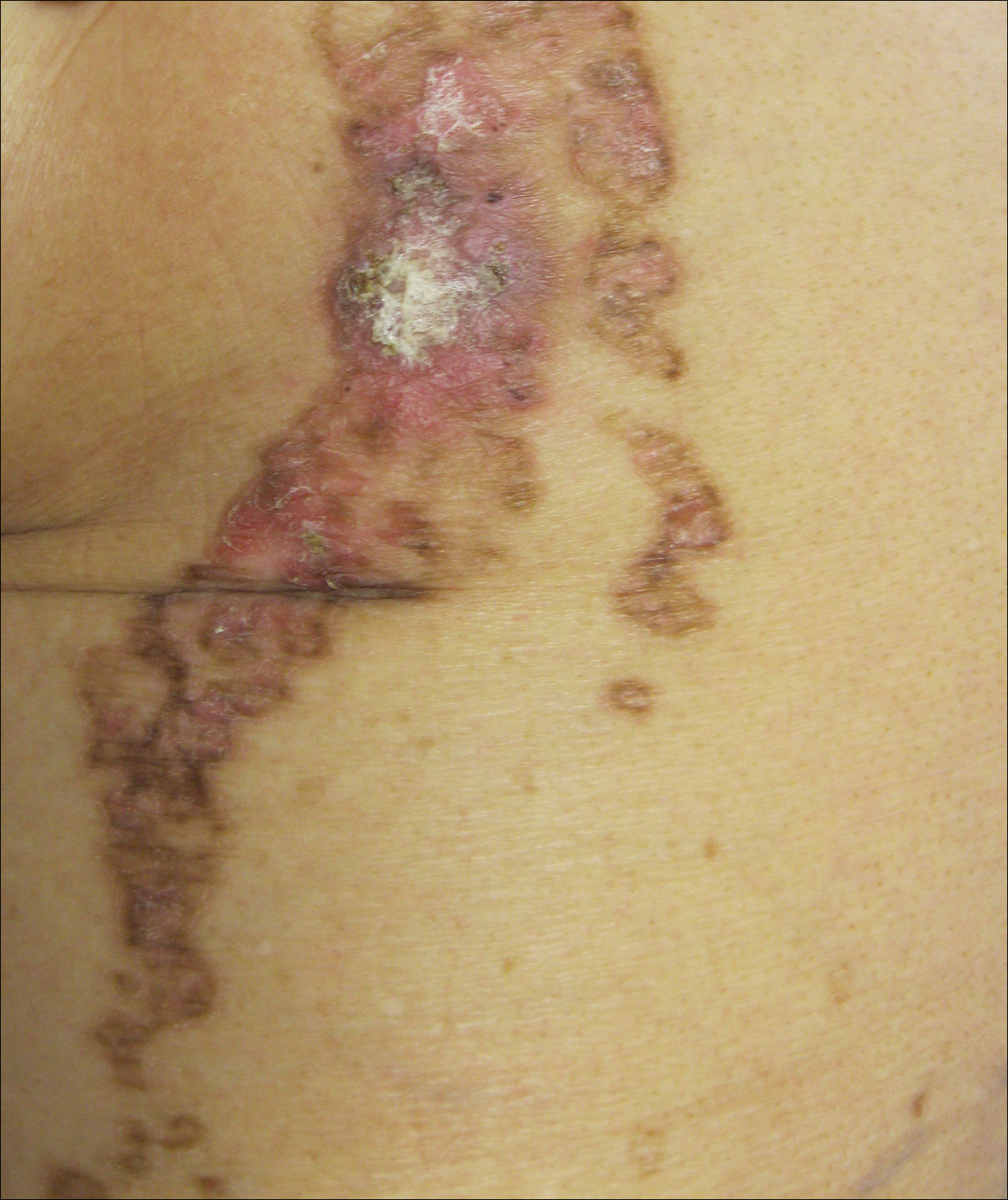
Several treatment options directed at the entire lesion were offered to the patient, but she declined these therapies and opted to address only those areas with clinical features of SCC, such as hyperkeratosis, bleeding, and rapid growth. Although biopsies performed by an outside provider were consistent with SCC, it had not been detected on biopsy performed during her initial visit to our clinic.
The patient was educated on the risk associated with her condition and instructed to follow up every 6 months to monitor for the development of SCC.
Comment
Porokeratosis is a disorder of keratinization with at least 5 clinical subtypes that share histologic similarities: porokeratosis of Mibelli, disseminated superficial porokeratosis and DSAP, linear porokeratosis, punctate porokeratosis, and PPPD.1,2 Other less common variants of porokeratosis include porokeratosis ptychotropica (a verrucous variant confined to the perianal area) and congenital unilateral linear porokeratosis.8,9
Linear porokeratosis appears in infancy or childhood with plaques that follow the lines of Blaschko.5,6 Most commonly, it presents unilaterally with annular plaques and linear hyperkeratotic papules that preferentially affect the extremities, though it also may present in a more generalized form or appear in a zosteriform pattern.10,11 Linear porokeratosis affects fewer than 20,000 individuals in the United States and accounts for fewer than 13% of all porokeratosis cases.12,13
Despite its relatively low prevalence, early identification of linear porokeratosis is important due to its high oncogenic potential, with malignant transformation to basal cell carcinoma or, more commonly, SCC reported in 19% of reported cases.1,5,7,14 The malignant transformation rate of linear porokeratosis is reported to be higher than rates seen in other porokeratosis subtypes (9.5%, 7.6%, and 3.4% for PPPD, porokeratosis of Mibelli, and DSAP, respectively).7 The risk of malignant transformation from porokeratosis increases with exposure to ionizing radiation, duration of the lesion, larger or coalescing lesions, and advanced age.7,15,16 Histologic studies have provided support for correlation between lesion size and oncogenic potential, with greater numbers of mitotic cells and more abnormal DNA ploidy seen in larger lesions.17
Histopathology
All subtypes of porokeratosis share certain histopathologic features that aid in the diagnosis of the disorder.18 Identification of the clinically observed hyperkeratotic ridged border or cornoid lamella is the primary means of definitively diagnosing porokeratosis; however, cornoid lamellae may be observed in other conditions, including verruca vulgaris and actinic keratosis.4,14
The cornoid lamella appears as a skewed column of densely packed parakeratotic cells with pyknotic basophilic nuclei extending through the stratum corneum from an epidermal invagination.4 Directly beneath the cornoid lamella, the granular layer is markedly diminished or absent, and cells of the stratum spinosum may demonstrate vacuolar changes or dyskeratosis.4,19 The superficial layer of the cornoid lamella may appear to be more centrifugally located and the cornoid lamella may be seen in several locations throughout the lesion.2,20 The degree of epidermal invagination, which is present under the cornoid lamella, varies by porokeratosis subtype; the central portion of the lesion may contain epidermis that ranges from hyperplastic to atrophic.2 Shumack et al21 noted that histologic changes under the cornoid lamella may include a lichenoid tissue reaction, papillary dermal lymphocytic infiltrate, vacuolar changes, dyskeratosis, and liquefaction degeneration of the basal layer. Because many of these histologic features also can be identified in lichen planus, a biopsy of the edge of lesions of porokeratosis is essential for making the correct diagnosis.
Heritability
Although linear porokeratosis has no identified pattern of inheritance and appears sporadic in onset, reports have described concomitant occurrence of linear porokeratosis and DSAP as well as linear porokeratosis arising in children of parents who have a diagnosis of DSAP.5,18,22,23 Based on these findings, it has been hypothesized that linear porokeratosis may represent a mosaic or segmental form of autosomal-dominant inherited subtypes of porokeratosis, such as DSAP.5 According to this hypothesis, loss of heterozygosity in patients with a DSAP mutation during early embryogenesis leads to proliferation of cells that are homozygous or hemizygous for the underlying mutation along lines of Blaschko.24 It has been suggested that the allelic loss implicated in the development of linear porokeratosis is the first step in a multistage process of carcinogenesis, which may help to explain the higher rates of malignant transformation that can be seen in linear porokeratosis.24
Management
Several treatment options exist for porokeratosis, including cryotherapy, topical 5-fluorouracil with or without adjunctive retinoid treatment, topical imiquimod, CO2 laser, shave and linear excision, curettage, dermabrasion, and oral acitretin for widespread lesions.1,25-29 One case report detailed successful treatment of adult-onset linear porokeratosis with tacrolimus ointment 0.1%.30 Treatments for porokeratosis demonstrate variable degrees of success, with the aim of eradicating the clonal population of mutant keratinocytes.2 Additionally, protection from UV radiation should be encouraged, especially in patients who have lesions that occur in areas of high actinic damage.1
Conclusion
We report of a case of linear porokeratosis with associated multiple SCCs that developed within the lesion. Definitive diagnosis of linear porokeratosis is important due to the higher rate of malignant transformation than the rate seen in other porokeratoses. In larger lesions, appropriate sampling and orientation of the pathology specimen is essential for identifying cornoid lamellae, thus allowing for appropriate follow-up and management. Several treatment options are available, though evidence for the effectiveness of any particular therapy is lacking. Research has shed light on possible genetic and molecular abnormalities in linear porokeratosis, but the exact pathogenesis of the disorder remains unclear.
- Curkova AK, Hegyi J, Kozub P, et al. A case of linear porokeratosis treated with photodynamic therapy with confocal microscopy surveillance. Dermatol Ther. 2014;27:144-147.
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Saunders; 2012.
- Behera B, Devi B, Nayak BB, et al. Giant inflammatory linear verrucous epidermal nevus: successfully treated with full thickness excision and skin grafting. Indian J Dermatol. 2013;58:461-463.
- Wade TR, Ackerman AB. Cornoid lamellation. a histologic reaction pattern. Am J Dermatopathol. 1980;2:5-15.
- Curnow P, Foley P, Baker C. Multiple squamous cell carcinomas complicating linear porokeratosis. Australas J Dermatol. 2003;44:136-139.
- Rahbari H, Cordero AA, Mehregan AH. Linear porokeratosis. a distinctive clinical variant of porokeratosis of Mibelli. Arch Dermatol. 1974;109:526-528.
- Sasson M, Krain AD. Porokeratosis and cutaneous malignancy. a review. Dermatol Surg. 1996;22:339-342.
- Yeo J, Winhoven S, Tallon B. Porokeratosis ptychotropica: a rare and evolving variant of porokeratosis. J Cutan Pathol. 2013;40:1042-1047.
- Scola N, Skrygan M, Wieland U, et al. Altered gene expression in squamous cell carcinoma arising from congenital unilateral linear porokeratosis. Clin Exp Dermatol. 2012;37:781-785.
- Sertznig P, von Felbert V, Megahed M. Porokeratosis: present concepts. J Eur Acad Dermatol Venereol. 2012;26:404-412.
- Goldner R. Zosteriform porokeratosis of Mibelli. Arch Dermatol. 1971;104:425-426.
- Malhotra SK, Puri KJ, Goyal T, et al. Linear porokeratosis. Dermatol Online J. 2007;13:15.
- Leow YH, Soon YH, Tham SN. A report of 31 cases of porokeratosis at the National Skin Centre. Ann Acad Med Singapore. 1996;25:837-841.
- Vivas AC, Maderal AD, Kirsner RS. Giant ulcerating squamous cell carcinoma arising from linear porokeratosis: a case study. Ostomy Wound Manage. 2012;58:18-20.
- Arranz-Salas I, Sanz-Trelles A, Ojeda DB. p53 alterations in porokeratosis. J Cutan Pathol. 2003;30:455-458.
- Otsuka F, Someya T, Ishibashi Y. Porokeratosis and malignant skin tumors. J Cancer Res Clin Oncol. 1991;117:55-60.
- Otsuka F, Umebayashi Y, Watanabe S, et al. Porokeratosis large skin lesions are susceptible to skin cancer development: histological and cytological explanation for the susceptibility. J Cancer Res Clin Oncol. 1993;119:395-400.
- Lohrer R, Neumann-Acikel A, Eming R, et al. A case of linear porokeratosis superimposed on disseminated superficial actinic porokeratosis. Case Rep Dermatol. 2010;2:130-134.
- Biswas A. Cornoid lamellation revisited: apropos of porokeratosis with emphasis on unusual clinicopathological variants. Am J Dermatopathol. 2015;37:145-155.
- Reed RJ, Leone P. Porokeratosis—a mutant clonal keratosis of the epidermis. I. histogenesis. Arch Dermatol. 1970;101:340-347.
- Shumack S, Commens C, Kossard S. Disseminated superficial actinic porokeratosis. a histological review of 61 cases with particular reference to lymphocytic inflammation. Am J Dermatopathol. 1991;13:26-31.
- Murase J, Gilliam AC. Disseminated superficial actinic porokeratosis co-existing with linear and verrucous porokeratosis in an elderly woman: update on the genetics and clinical expression of porokeratosis. J Am Acad Dermatol. 2010;63:886-891.
- Commens CA, Shumack SP. Linear porokeratosis in two families with disseminated superficial actinic porokeratosis. Pediatr Dermatol. 1987;4:209-214.
- Happle R. Cancer proneness of linear porokeratosis may be explained by allelic loss. Dermatology. 1997;195:20-25.
- Rabbin PE, Baldwin HE. Treatment of porokeratosis of Mibelli with CO2 laser vaporization versus surgical excision with split-thickness skin graft. a comparison. J Dermatol Surg Oncol. 1993;19:199-202.
- Spencer JM, Katz BE. Successful treatment of porokeratosis of Mibelli with diamond fraise dermabrasion. Arch Dermatol. 1992;128:1187-1188.
- Venkatarajan S, LeLeux TM, Yang D, et al. Porokeratosis of Mibelli: successful treatment with 5 percent topical imiquimod and topical 5 percent 5-fluorouracil. Dermatol Online J. 2010;16:10.
- McDonald SG, Peterka ES. Porokeratosis (Mibelli): treatment with topical 5-fluorouracil. J Am Acad Dermatol. 1983;8:107-110.
- Shumack SP, Commens CA. Disseminated superficial actinic porokeratosis: a clinical study. J Am Acad Dermatol. 1989;20:1015-1022.
- Parks AC, Conner KJ, Armstrong CA. Long-term clearance of linear porokeratosis with tacrolimus, 0.1%, ointment. JAMA Dermatol. 2014;150:194-196.
Lesions of porokeratosis are thought to arise from disordered keratinization, though the exact pathogenesis remains uncertain. At least 5 clinical subtypes of porokeratosis have been identified: porokeratosis of Mibelli, disseminated superficial porokeratosis and disseminated superficial actinic porokeratosis (DSAP), linear porokeratosis, punctuate porokeratosis, and porokeratosis palmaris et plantaris disseminata (PPPD).1,2 Linear porokeratosis is a rare subtype with a clinical differential diagnosis that includes lichen striatus, linear lichen planus, linear verrucous epidermal nevus, segmental Darier disease, and incontinentia pigmenti.3 Definitive diagnosis of linear porokeratosis is made by histopathologic examination demonstrating a cornoid lamella, defined as a column of parakeratotic cells that lies at 45°to the surface of the epidermis and contains pyknotic basophilic nuclei.4 Patients with linear porokeratosis typically develop lesions along the lines of Blaschko in infancy or childhood.5,6 Among the different subtypes of porokeratosis, linear porokeratosis demonstrates the highest rate of malignant transformation, therefore requiring close clinical observation.7
Case Report
An 83-year-old woman presented to the outpatient clinic with a large linear plaque on the right leg that had been present since birth. Ten years prior to presentation, a portion of the lesion started to bleed; biopsy of the area was performed by an outside provider demonstrating squamous cell carcinoma (SCC), which was treated with wide local excision. One year prior to presentation, a separate portion of the plaque was biopsied by an outside provider and another diagnosis of SCC was made.
On examination performed during the initial presentation to our clinic, there was a well-demarcated tan to violaceous linear plaque present at the lower buttock and extending along the posterior leg to the skin overlying the Achilles tendon and dorsal aspect of the right foot. Within the plaque, there were areas of atrophy and areas of inflammation, induration, and hyperkeratosis (Figures 1 and 2). Two punch biopsies were performed: one from the edge of the plaque and one from a hyperkeratotic region within the plaque. Histology from the edge of the plaque demonstrated a cornoid lamella, consistent with a porokeratosis (Figure 3), whereas the histology from the hyperkeratotic region demonstrated a lichenoid infiltrate (Figure 4).




Several treatment options directed at the entire lesion were offered to the patient, but she declined these therapies and opted to address only those areas with clinical features of SCC, such as hyperkeratosis, bleeding, and rapid growth. Although biopsies performed by an outside provider were consistent with SCC, it had not been detected on biopsy performed during her initial visit to our clinic.
The patient was educated on the risk associated with her condition and instructed to follow up every 6 months to monitor for the development of SCC.
Comment
Porokeratosis is a disorder of keratinization with at least 5 clinical subtypes that share histologic similarities: porokeratosis of Mibelli, disseminated superficial porokeratosis and DSAP, linear porokeratosis, punctate porokeratosis, and PPPD.1,2 Other less common variants of porokeratosis include porokeratosis ptychotropica (a verrucous variant confined to the perianal area) and congenital unilateral linear porokeratosis.8,9
Linear porokeratosis appears in infancy or childhood with plaques that follow the lines of Blaschko.5,6 Most commonly, it presents unilaterally with annular plaques and linear hyperkeratotic papules that preferentially affect the extremities, though it also may present in a more generalized form or appear in a zosteriform pattern.10,11 Linear porokeratosis affects fewer than 20,000 individuals in the United States and accounts for fewer than 13% of all porokeratosis cases.12,13
Despite its relatively low prevalence, early identification of linear porokeratosis is important due to its high oncogenic potential, with malignant transformation to basal cell carcinoma or, more commonly, SCC reported in 19% of reported cases.1,5,7,14 The malignant transformation rate of linear porokeratosis is reported to be higher than rates seen in other porokeratosis subtypes (9.5%, 7.6%, and 3.4% for PPPD, porokeratosis of Mibelli, and DSAP, respectively).7 The risk of malignant transformation from porokeratosis increases with exposure to ionizing radiation, duration of the lesion, larger or coalescing lesions, and advanced age.7,15,16 Histologic studies have provided support for correlation between lesion size and oncogenic potential, with greater numbers of mitotic cells and more abnormal DNA ploidy seen in larger lesions.17
Histopathology
All subtypes of porokeratosis share certain histopathologic features that aid in the diagnosis of the disorder.18 Identification of the clinically observed hyperkeratotic ridged border or cornoid lamella is the primary means of definitively diagnosing porokeratosis; however, cornoid lamellae may be observed in other conditions, including verruca vulgaris and actinic keratosis.4,14
The cornoid lamella appears as a skewed column of densely packed parakeratotic cells with pyknotic basophilic nuclei extending through the stratum corneum from an epidermal invagination.4 Directly beneath the cornoid lamella, the granular layer is markedly diminished or absent, and cells of the stratum spinosum may demonstrate vacuolar changes or dyskeratosis.4,19 The superficial layer of the cornoid lamella may appear to be more centrifugally located and the cornoid lamella may be seen in several locations throughout the lesion.2,20 The degree of epidermal invagination, which is present under the cornoid lamella, varies by porokeratosis subtype; the central portion of the lesion may contain epidermis that ranges from hyperplastic to atrophic.2 Shumack et al21 noted that histologic changes under the cornoid lamella may include a lichenoid tissue reaction, papillary dermal lymphocytic infiltrate, vacuolar changes, dyskeratosis, and liquefaction degeneration of the basal layer. Because many of these histologic features also can be identified in lichen planus, a biopsy of the edge of lesions of porokeratosis is essential for making the correct diagnosis.
Heritability
Although linear porokeratosis has no identified pattern of inheritance and appears sporadic in onset, reports have described concomitant occurrence of linear porokeratosis and DSAP as well as linear porokeratosis arising in children of parents who have a diagnosis of DSAP.5,18,22,23 Based on these findings, it has been hypothesized that linear porokeratosis may represent a mosaic or segmental form of autosomal-dominant inherited subtypes of porokeratosis, such as DSAP.5 According to this hypothesis, loss of heterozygosity in patients with a DSAP mutation during early embryogenesis leads to proliferation of cells that are homozygous or hemizygous for the underlying mutation along lines of Blaschko.24 It has been suggested that the allelic loss implicated in the development of linear porokeratosis is the first step in a multistage process of carcinogenesis, which may help to explain the higher rates of malignant transformation that can be seen in linear porokeratosis.24
Management
Several treatment options exist for porokeratosis, including cryotherapy, topical 5-fluorouracil with or without adjunctive retinoid treatment, topical imiquimod, CO2 laser, shave and linear excision, curettage, dermabrasion, and oral acitretin for widespread lesions.1,25-29 One case report detailed successful treatment of adult-onset linear porokeratosis with tacrolimus ointment 0.1%.30 Treatments for porokeratosis demonstrate variable degrees of success, with the aim of eradicating the clonal population of mutant keratinocytes.2 Additionally, protection from UV radiation should be encouraged, especially in patients who have lesions that occur in areas of high actinic damage.1
Conclusion
We report of a case of linear porokeratosis with associated multiple SCCs that developed within the lesion. Definitive diagnosis of linear porokeratosis is important due to the higher rate of malignant transformation than the rate seen in other porokeratoses. In larger lesions, appropriate sampling and orientation of the pathology specimen is essential for identifying cornoid lamellae, thus allowing for appropriate follow-up and management. Several treatment options are available, though evidence for the effectiveness of any particular therapy is lacking. Research has shed light on possible genetic and molecular abnormalities in linear porokeratosis, but the exact pathogenesis of the disorder remains unclear.
Lesions of porokeratosis are thought to arise from disordered keratinization, though the exact pathogenesis remains uncertain. At least 5 clinical subtypes of porokeratosis have been identified: porokeratosis of Mibelli, disseminated superficial porokeratosis and disseminated superficial actinic porokeratosis (DSAP), linear porokeratosis, punctuate porokeratosis, and porokeratosis palmaris et plantaris disseminata (PPPD).1,2 Linear porokeratosis is a rare subtype with a clinical differential diagnosis that includes lichen striatus, linear lichen planus, linear verrucous epidermal nevus, segmental Darier disease, and incontinentia pigmenti.3 Definitive diagnosis of linear porokeratosis is made by histopathologic examination demonstrating a cornoid lamella, defined as a column of parakeratotic cells that lies at 45°to the surface of the epidermis and contains pyknotic basophilic nuclei.4 Patients with linear porokeratosis typically develop lesions along the lines of Blaschko in infancy or childhood.5,6 Among the different subtypes of porokeratosis, linear porokeratosis demonstrates the highest rate of malignant transformation, therefore requiring close clinical observation.7
Case Report
An 83-year-old woman presented to the outpatient clinic with a large linear plaque on the right leg that had been present since birth. Ten years prior to presentation, a portion of the lesion started to bleed; biopsy of the area was performed by an outside provider demonstrating squamous cell carcinoma (SCC), which was treated with wide local excision. One year prior to presentation, a separate portion of the plaque was biopsied by an outside provider and another diagnosis of SCC was made.
On examination performed during the initial presentation to our clinic, there was a well-demarcated tan to violaceous linear plaque present at the lower buttock and extending along the posterior leg to the skin overlying the Achilles tendon and dorsal aspect of the right foot. Within the plaque, there were areas of atrophy and areas of inflammation, induration, and hyperkeratosis (Figures 1 and 2). Two punch biopsies were performed: one from the edge of the plaque and one from a hyperkeratotic region within the plaque. Histology from the edge of the plaque demonstrated a cornoid lamella, consistent with a porokeratosis (Figure 3), whereas the histology from the hyperkeratotic region demonstrated a lichenoid infiltrate (Figure 4).




Several treatment options directed at the entire lesion were offered to the patient, but she declined these therapies and opted to address only those areas with clinical features of SCC, such as hyperkeratosis, bleeding, and rapid growth. Although biopsies performed by an outside provider were consistent with SCC, it had not been detected on biopsy performed during her initial visit to our clinic.
The patient was educated on the risk associated with her condition and instructed to follow up every 6 months to monitor for the development of SCC.
Comment
Porokeratosis is a disorder of keratinization with at least 5 clinical subtypes that share histologic similarities: porokeratosis of Mibelli, disseminated superficial porokeratosis and DSAP, linear porokeratosis, punctate porokeratosis, and PPPD.1,2 Other less common variants of porokeratosis include porokeratosis ptychotropica (a verrucous variant confined to the perianal area) and congenital unilateral linear porokeratosis.8,9
Linear porokeratosis appears in infancy or childhood with plaques that follow the lines of Blaschko.5,6 Most commonly, it presents unilaterally with annular plaques and linear hyperkeratotic papules that preferentially affect the extremities, though it also may present in a more generalized form or appear in a zosteriform pattern.10,11 Linear porokeratosis affects fewer than 20,000 individuals in the United States and accounts for fewer than 13% of all porokeratosis cases.12,13
Despite its relatively low prevalence, early identification of linear porokeratosis is important due to its high oncogenic potential, with malignant transformation to basal cell carcinoma or, more commonly, SCC reported in 19% of reported cases.1,5,7,14 The malignant transformation rate of linear porokeratosis is reported to be higher than rates seen in other porokeratosis subtypes (9.5%, 7.6%, and 3.4% for PPPD, porokeratosis of Mibelli, and DSAP, respectively).7 The risk of malignant transformation from porokeratosis increases with exposure to ionizing radiation, duration of the lesion, larger or coalescing lesions, and advanced age.7,15,16 Histologic studies have provided support for correlation between lesion size and oncogenic potential, with greater numbers of mitotic cells and more abnormal DNA ploidy seen in larger lesions.17
Histopathology
All subtypes of porokeratosis share certain histopathologic features that aid in the diagnosis of the disorder.18 Identification of the clinically observed hyperkeratotic ridged border or cornoid lamella is the primary means of definitively diagnosing porokeratosis; however, cornoid lamellae may be observed in other conditions, including verruca vulgaris and actinic keratosis.4,14
The cornoid lamella appears as a skewed column of densely packed parakeratotic cells with pyknotic basophilic nuclei extending through the stratum corneum from an epidermal invagination.4 Directly beneath the cornoid lamella, the granular layer is markedly diminished or absent, and cells of the stratum spinosum may demonstrate vacuolar changes or dyskeratosis.4,19 The superficial layer of the cornoid lamella may appear to be more centrifugally located and the cornoid lamella may be seen in several locations throughout the lesion.2,20 The degree of epidermal invagination, which is present under the cornoid lamella, varies by porokeratosis subtype; the central portion of the lesion may contain epidermis that ranges from hyperplastic to atrophic.2 Shumack et al21 noted that histologic changes under the cornoid lamella may include a lichenoid tissue reaction, papillary dermal lymphocytic infiltrate, vacuolar changes, dyskeratosis, and liquefaction degeneration of the basal layer. Because many of these histologic features also can be identified in lichen planus, a biopsy of the edge of lesions of porokeratosis is essential for making the correct diagnosis.
Heritability
Although linear porokeratosis has no identified pattern of inheritance and appears sporadic in onset, reports have described concomitant occurrence of linear porokeratosis and DSAP as well as linear porokeratosis arising in children of parents who have a diagnosis of DSAP.5,18,22,23 Based on these findings, it has been hypothesized that linear porokeratosis may represent a mosaic or segmental form of autosomal-dominant inherited subtypes of porokeratosis, such as DSAP.5 According to this hypothesis, loss of heterozygosity in patients with a DSAP mutation during early embryogenesis leads to proliferation of cells that are homozygous or hemizygous for the underlying mutation along lines of Blaschko.24 It has been suggested that the allelic loss implicated in the development of linear porokeratosis is the first step in a multistage process of carcinogenesis, which may help to explain the higher rates of malignant transformation that can be seen in linear porokeratosis.24
Management
Several treatment options exist for porokeratosis, including cryotherapy, topical 5-fluorouracil with or without adjunctive retinoid treatment, topical imiquimod, CO2 laser, shave and linear excision, curettage, dermabrasion, and oral acitretin for widespread lesions.1,25-29 One case report detailed successful treatment of adult-onset linear porokeratosis with tacrolimus ointment 0.1%.30 Treatments for porokeratosis demonstrate variable degrees of success, with the aim of eradicating the clonal population of mutant keratinocytes.2 Additionally, protection from UV radiation should be encouraged, especially in patients who have lesions that occur in areas of high actinic damage.1
Conclusion
We report of a case of linear porokeratosis with associated multiple SCCs that developed within the lesion. Definitive diagnosis of linear porokeratosis is important due to the higher rate of malignant transformation than the rate seen in other porokeratoses. In larger lesions, appropriate sampling and orientation of the pathology specimen is essential for identifying cornoid lamellae, thus allowing for appropriate follow-up and management. Several treatment options are available, though evidence for the effectiveness of any particular therapy is lacking. Research has shed light on possible genetic and molecular abnormalities in linear porokeratosis, but the exact pathogenesis of the disorder remains unclear.
- Curkova AK, Hegyi J, Kozub P, et al. A case of linear porokeratosis treated with photodynamic therapy with confocal microscopy surveillance. Dermatol Ther. 2014;27:144-147.
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Saunders; 2012.
- Behera B, Devi B, Nayak BB, et al. Giant inflammatory linear verrucous epidermal nevus: successfully treated with full thickness excision and skin grafting. Indian J Dermatol. 2013;58:461-463.
- Wade TR, Ackerman AB. Cornoid lamellation. a histologic reaction pattern. Am J Dermatopathol. 1980;2:5-15.
- Curnow P, Foley P, Baker C. Multiple squamous cell carcinomas complicating linear porokeratosis. Australas J Dermatol. 2003;44:136-139.
- Rahbari H, Cordero AA, Mehregan AH. Linear porokeratosis. a distinctive clinical variant of porokeratosis of Mibelli. Arch Dermatol. 1974;109:526-528.
- Sasson M, Krain AD. Porokeratosis and cutaneous malignancy. a review. Dermatol Surg. 1996;22:339-342.
- Yeo J, Winhoven S, Tallon B. Porokeratosis ptychotropica: a rare and evolving variant of porokeratosis. J Cutan Pathol. 2013;40:1042-1047.
- Scola N, Skrygan M, Wieland U, et al. Altered gene expression in squamous cell carcinoma arising from congenital unilateral linear porokeratosis. Clin Exp Dermatol. 2012;37:781-785.
- Sertznig P, von Felbert V, Megahed M. Porokeratosis: present concepts. J Eur Acad Dermatol Venereol. 2012;26:404-412.
- Goldner R. Zosteriform porokeratosis of Mibelli. Arch Dermatol. 1971;104:425-426.
- Malhotra SK, Puri KJ, Goyal T, et al. Linear porokeratosis. Dermatol Online J. 2007;13:15.
- Leow YH, Soon YH, Tham SN. A report of 31 cases of porokeratosis at the National Skin Centre. Ann Acad Med Singapore. 1996;25:837-841.
- Vivas AC, Maderal AD, Kirsner RS. Giant ulcerating squamous cell carcinoma arising from linear porokeratosis: a case study. Ostomy Wound Manage. 2012;58:18-20.
- Arranz-Salas I, Sanz-Trelles A, Ojeda DB. p53 alterations in porokeratosis. J Cutan Pathol. 2003;30:455-458.
- Otsuka F, Someya T, Ishibashi Y. Porokeratosis and malignant skin tumors. J Cancer Res Clin Oncol. 1991;117:55-60.
- Otsuka F, Umebayashi Y, Watanabe S, et al. Porokeratosis large skin lesions are susceptible to skin cancer development: histological and cytological explanation for the susceptibility. J Cancer Res Clin Oncol. 1993;119:395-400.
- Lohrer R, Neumann-Acikel A, Eming R, et al. A case of linear porokeratosis superimposed on disseminated superficial actinic porokeratosis. Case Rep Dermatol. 2010;2:130-134.
- Biswas A. Cornoid lamellation revisited: apropos of porokeratosis with emphasis on unusual clinicopathological variants. Am J Dermatopathol. 2015;37:145-155.
- Reed RJ, Leone P. Porokeratosis—a mutant clonal keratosis of the epidermis. I. histogenesis. Arch Dermatol. 1970;101:340-347.
- Shumack S, Commens C, Kossard S. Disseminated superficial actinic porokeratosis. a histological review of 61 cases with particular reference to lymphocytic inflammation. Am J Dermatopathol. 1991;13:26-31.
- Murase J, Gilliam AC. Disseminated superficial actinic porokeratosis co-existing with linear and verrucous porokeratosis in an elderly woman: update on the genetics and clinical expression of porokeratosis. J Am Acad Dermatol. 2010;63:886-891.
- Commens CA, Shumack SP. Linear porokeratosis in two families with disseminated superficial actinic porokeratosis. Pediatr Dermatol. 1987;4:209-214.
- Happle R. Cancer proneness of linear porokeratosis may be explained by allelic loss. Dermatology. 1997;195:20-25.
- Rabbin PE, Baldwin HE. Treatment of porokeratosis of Mibelli with CO2 laser vaporization versus surgical excision with split-thickness skin graft. a comparison. J Dermatol Surg Oncol. 1993;19:199-202.
- Spencer JM, Katz BE. Successful treatment of porokeratosis of Mibelli with diamond fraise dermabrasion. Arch Dermatol. 1992;128:1187-1188.
- Venkatarajan S, LeLeux TM, Yang D, et al. Porokeratosis of Mibelli: successful treatment with 5 percent topical imiquimod and topical 5 percent 5-fluorouracil. Dermatol Online J. 2010;16:10.
- McDonald SG, Peterka ES. Porokeratosis (Mibelli): treatment with topical 5-fluorouracil. J Am Acad Dermatol. 1983;8:107-110.
- Shumack SP, Commens CA. Disseminated superficial actinic porokeratosis: a clinical study. J Am Acad Dermatol. 1989;20:1015-1022.
- Parks AC, Conner KJ, Armstrong CA. Long-term clearance of linear porokeratosis with tacrolimus, 0.1%, ointment. JAMA Dermatol. 2014;150:194-196.
- Curkova AK, Hegyi J, Kozub P, et al. A case of linear porokeratosis treated with photodynamic therapy with confocal microscopy surveillance. Dermatol Ther. 2014;27:144-147.
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Saunders; 2012.
- Behera B, Devi B, Nayak BB, et al. Giant inflammatory linear verrucous epidermal nevus: successfully treated with full thickness excision and skin grafting. Indian J Dermatol. 2013;58:461-463.
- Wade TR, Ackerman AB. Cornoid lamellation. a histologic reaction pattern. Am J Dermatopathol. 1980;2:5-15.
- Curnow P, Foley P, Baker C. Multiple squamous cell carcinomas complicating linear porokeratosis. Australas J Dermatol. 2003;44:136-139.
- Rahbari H, Cordero AA, Mehregan AH. Linear porokeratosis. a distinctive clinical variant of porokeratosis of Mibelli. Arch Dermatol. 1974;109:526-528.
- Sasson M, Krain AD. Porokeratosis and cutaneous malignancy. a review. Dermatol Surg. 1996;22:339-342.
- Yeo J, Winhoven S, Tallon B. Porokeratosis ptychotropica: a rare and evolving variant of porokeratosis. J Cutan Pathol. 2013;40:1042-1047.
- Scola N, Skrygan M, Wieland U, et al. Altered gene expression in squamous cell carcinoma arising from congenital unilateral linear porokeratosis. Clin Exp Dermatol. 2012;37:781-785.
- Sertznig P, von Felbert V, Megahed M. Porokeratosis: present concepts. J Eur Acad Dermatol Venereol. 2012;26:404-412.
- Goldner R. Zosteriform porokeratosis of Mibelli. Arch Dermatol. 1971;104:425-426.
- Malhotra SK, Puri KJ, Goyal T, et al. Linear porokeratosis. Dermatol Online J. 2007;13:15.
- Leow YH, Soon YH, Tham SN. A report of 31 cases of porokeratosis at the National Skin Centre. Ann Acad Med Singapore. 1996;25:837-841.
- Vivas AC, Maderal AD, Kirsner RS. Giant ulcerating squamous cell carcinoma arising from linear porokeratosis: a case study. Ostomy Wound Manage. 2012;58:18-20.
- Arranz-Salas I, Sanz-Trelles A, Ojeda DB. p53 alterations in porokeratosis. J Cutan Pathol. 2003;30:455-458.
- Otsuka F, Someya T, Ishibashi Y. Porokeratosis and malignant skin tumors. J Cancer Res Clin Oncol. 1991;117:55-60.
- Otsuka F, Umebayashi Y, Watanabe S, et al. Porokeratosis large skin lesions are susceptible to skin cancer development: histological and cytological explanation for the susceptibility. J Cancer Res Clin Oncol. 1993;119:395-400.
- Lohrer R, Neumann-Acikel A, Eming R, et al. A case of linear porokeratosis superimposed on disseminated superficial actinic porokeratosis. Case Rep Dermatol. 2010;2:130-134.
- Biswas A. Cornoid lamellation revisited: apropos of porokeratosis with emphasis on unusual clinicopathological variants. Am J Dermatopathol. 2015;37:145-155.
- Reed RJ, Leone P. Porokeratosis—a mutant clonal keratosis of the epidermis. I. histogenesis. Arch Dermatol. 1970;101:340-347.
- Shumack S, Commens C, Kossard S. Disseminated superficial actinic porokeratosis. a histological review of 61 cases with particular reference to lymphocytic inflammation. Am J Dermatopathol. 1991;13:26-31.
- Murase J, Gilliam AC. Disseminated superficial actinic porokeratosis co-existing with linear and verrucous porokeratosis in an elderly woman: update on the genetics and clinical expression of porokeratosis. J Am Acad Dermatol. 2010;63:886-891.
- Commens CA, Shumack SP. Linear porokeratosis in two families with disseminated superficial actinic porokeratosis. Pediatr Dermatol. 1987;4:209-214.
- Happle R. Cancer proneness of linear porokeratosis may be explained by allelic loss. Dermatology. 1997;195:20-25.
- Rabbin PE, Baldwin HE. Treatment of porokeratosis of Mibelli with CO2 laser vaporization versus surgical excision with split-thickness skin graft. a comparison. J Dermatol Surg Oncol. 1993;19:199-202.
- Spencer JM, Katz BE. Successful treatment of porokeratosis of Mibelli with diamond fraise dermabrasion. Arch Dermatol. 1992;128:1187-1188.
- Venkatarajan S, LeLeux TM, Yang D, et al. Porokeratosis of Mibelli: successful treatment with 5 percent topical imiquimod and topical 5 percent 5-fluorouracil. Dermatol Online J. 2010;16:10.
- McDonald SG, Peterka ES. Porokeratosis (Mibelli): treatment with topical 5-fluorouracil. J Am Acad Dermatol. 1983;8:107-110.
- Shumack SP, Commens CA. Disseminated superficial actinic porokeratosis: a clinical study. J Am Acad Dermatol. 1989;20:1015-1022.
- Parks AC, Conner KJ, Armstrong CA. Long-term clearance of linear porokeratosis with tacrolimus, 0.1%, ointment. JAMA Dermatol. 2014;150:194-196.
Practice Points
- Porokeratosis represents a heterogeneous group of skin disorders.
- Porokeratosis can be inherited in an autosomal-dominant pattern, though many patients lack a family history.
- The presence of a cornoid lamella is the characteristic finding of porokeratosis on histology.
- The rate of malignant transformation to squamous cell carcinoma is highest in linear porokeratosis, lowest in disseminated superficial actinic porokeratosis, and unreported in the punctate type.
Squamous cell carcinoma linked to 25% increase in all-cause mortality
FROM JAAD
Squamous cell carcinomas (SCC), but not basal cell carcinomas (BCC), were associated with a risk of death from any cause that was 25% higher than that seen in the general population, based on a systematic literature review and meta-analysis published in the Journal of American Academy of Dermatology (2017. doi: 10.1016/j.jaad.2017.11.026).
“Because these tumors often occur in the same patients and are both often caused by exposure to ultraviolet radiation, patients with BCC and SCC are often grouped together,” Mackenzie R. Wehner, MD, of the University of Pennsylvania, Philadelphia, and co-authors wrote. “Our data contributes to the argument that the carcinogenesis of these tumors and long-term outcomes for patients with these tumors may be distinct.”
Patients with SCC “may need additional education and age-appropriate screening to prevent deaths from major diseases,” the authors concluded.
Dr. Wehner and colleagues systematically searched the medical literature and found four studies encompassing a total of 175,849 patients with SCC and 464,230 patients with BCC.
Relative to the general population, mortality for those with an SCC was 1.25 (95% CI, 1.17-1.32). At 0.92 (95% CI 0.83-1.02), there was no significant difference in mortality for patients with a BCC.
Collectively and individually, the studies found a statistically significant increased relative mortality for having SCC.
There are clear distinctions between BCC and SCC with regard to histology, pathophysiology, survival, and other parameters, the study authors said. “While many patients get both BCC and SCC, future research should take into account that these cancers may have different long-term risks and outcomes.”
FROM JAAD
Squamous cell carcinomas (SCC), but not basal cell carcinomas (BCC), were associated with a risk of death from any cause that was 25% higher than that seen in the general population, based on a systematic literature review and meta-analysis published in the Journal of American Academy of Dermatology (2017. doi: 10.1016/j.jaad.2017.11.026).
“Because these tumors often occur in the same patients and are both often caused by exposure to ultraviolet radiation, patients with BCC and SCC are often grouped together,” Mackenzie R. Wehner, MD, of the University of Pennsylvania, Philadelphia, and co-authors wrote. “Our data contributes to the argument that the carcinogenesis of these tumors and long-term outcomes for patients with these tumors may be distinct.”
Patients with SCC “may need additional education and age-appropriate screening to prevent deaths from major diseases,” the authors concluded.
Dr. Wehner and colleagues systematically searched the medical literature and found four studies encompassing a total of 175,849 patients with SCC and 464,230 patients with BCC.
Relative to the general population, mortality for those with an SCC was 1.25 (95% CI, 1.17-1.32). At 0.92 (95% CI 0.83-1.02), there was no significant difference in mortality for patients with a BCC.
Collectively and individually, the studies found a statistically significant increased relative mortality for having SCC.
There are clear distinctions between BCC and SCC with regard to histology, pathophysiology, survival, and other parameters, the study authors said. “While many patients get both BCC and SCC, future research should take into account that these cancers may have different long-term risks and outcomes.”
FROM JAAD
Squamous cell carcinomas (SCC), but not basal cell carcinomas (BCC), were associated with a risk of death from any cause that was 25% higher than that seen in the general population, based on a systematic literature review and meta-analysis published in the Journal of American Academy of Dermatology (2017. doi: 10.1016/j.jaad.2017.11.026).
“Because these tumors often occur in the same patients and are both often caused by exposure to ultraviolet radiation, patients with BCC and SCC are often grouped together,” Mackenzie R. Wehner, MD, of the University of Pennsylvania, Philadelphia, and co-authors wrote. “Our data contributes to the argument that the carcinogenesis of these tumors and long-term outcomes for patients with these tumors may be distinct.”
Patients with SCC “may need additional education and age-appropriate screening to prevent deaths from major diseases,” the authors concluded.
Dr. Wehner and colleagues systematically searched the medical literature and found four studies encompassing a total of 175,849 patients with SCC and 464,230 patients with BCC.
Relative to the general population, mortality for those with an SCC was 1.25 (95% CI, 1.17-1.32). At 0.92 (95% CI 0.83-1.02), there was no significant difference in mortality for patients with a BCC.
Collectively and individually, the studies found a statistically significant increased relative mortality for having SCC.
There are clear distinctions between BCC and SCC with regard to histology, pathophysiology, survival, and other parameters, the study authors said. “While many patients get both BCC and SCC, future research should take into account that these cancers may have different long-term risks and outcomes.”
Primary Mucinous Carcinoma of the Eyelid Treated With Mohs Micrographic Surgery
To the Editor:
Primary mucinous carcinoma (PMC) is an exceedingly rare adnexal tumor with an incidence of 0.07 cases per million individuals.1,2 First described by Lennox et al3 in 1952, this entity often presents as slow-growing, solitary nodules that often are soft on palpation but may have an indurated quality and range in color from reddish blue to flesh colored to white.4 Primary mucinous carcinoma most commonly is found on the eyelid (38%) but may affect other sites on the face (20.3%), scalp (16%), and axilla (10%).5 Historically, it has been thought to be more common among men; however, a 2005 large case series by Kazakov et al5 found that women were twice as likely to be affected. Primary mucinous carcinoma most frequently is diagnosed in the fifth through seventh decades of life, with a median age at onset of 63 years.6,7 Because of its rarity, PMC is most frequently confused clinically with basal cell carcinoma, keratoacanthoma, apocrine hidrocystoma, epidermoid cyst, Kaposi sarcoma, neuroma, lacrimal sac tumor, squamous cell carcinoma, granulomatous tumors, and metastatic adenocarcinoma.1,8-10
Primary mucinous carcinoma is thought to be derived from sweat glands, and select features such as decapitation secretion are more suggestive of apocrine than eccrine differentiation.5,8 On histopathology, PMC classically is described as nests of epithelial cells floating in lakes of extracellular mucin, primarily in the dermis and subcutis. The nests are composed of basaloid cells in solid to cribriform arrangements, usually with a low mitotic count and little nuclear atypia. These nests are suspended within periodic acid–Schiff positive mucinous pools partitioned by delicate fibrous septa. The mucin produced by PMC is sialomucin, and as such it is hyaluronidase resistant and sialidase labile.6 At least 1 report has been made of the presence of psammoma bodies in PMC.11
The neoplasm is characterized by an indolent course with frequent recurrence but rare metastasis.5,12 Treatment is primarily surgical, with Mohs micrographic surgery (MMS) offering improved tissue conservation and reduced recurrence rates.12 The diagnostic challenge lies in distinguishing PMC from a variety of metastatic mucinous internal malignancies that portend a notably greater morbidity and mortality to the patient. We describe a case of PMC, discuss the differentiation of PMC from metastatic mucinous carcinoma, and review the literature regarding treatment of this rare neoplasm.
A 65-year-old white woman was referred to our tertiary-care dermatologic surgery clinic for treatment of an incompletely excised mucinous carcinoma of the right lateral canthus (Figure 1). The clinically evident scar measured 0.5×0.5 cm. Although difficult to appreciate in Figure 1, a slight textural change of the surrounding skin, including the upper and lower eyelid, was apparent. Prior to her arrival to our clinic, the referring physician had completed a thorough review of systems and physical examination, which did not suggest an underlying malignancy. Computed tomography of the head, neck, chest, abdomen, and pelvis revealed a mass in the thyroid that was removed and found to be benign. The patient’s cutaneous lesion was therefore considered to be a PMC of the skin.
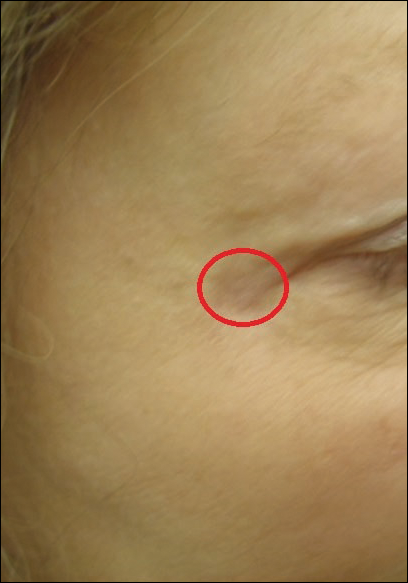
Given the prior incomplete excision of the lesion and its periocular location, we treated the patient with MMS. After 6 surgical stages, we continued to see evidence of the neoplasm as it tracked medially along the orbicularis oculi muscle (Figure 2). Due to the patient’s physical and emotional exhaustion at this point, we discontinued MMS and referred her to a colleague in plastic surgery for further excision of the remaining focus of positivity as well as repair. The final Mohs defect measured 4.2×4.0 cm (Figure 3). Approximately 2.3×1.0 cm of tissue in the area of remaining tumor was excised by plastic surgery, and the defect was repaired with a cervicofacial advancement flap closure of the right cheek and lower eyelid and full-thickness skin graft of the left upper eyelid. Histopathologic investigation found the additional tissue resected to be free of residual tumor.
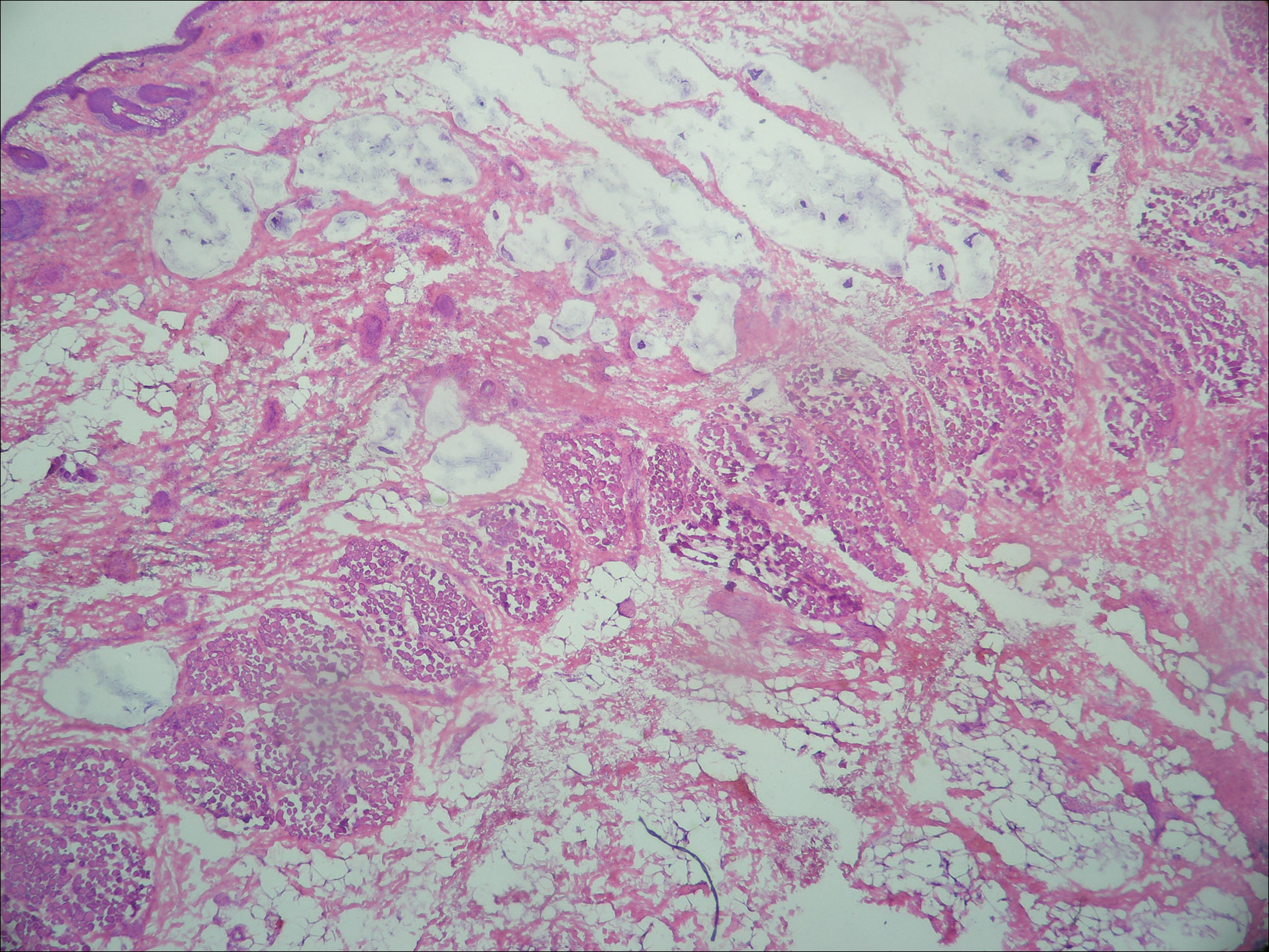
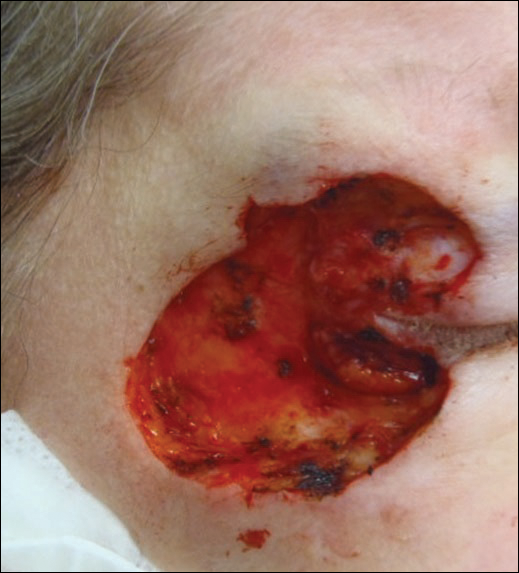
To diagnose a patient with PMC, one must first rule out cutaneous metastasis of various internal malignancies that may appear similar on histopathology. A full clinical investigation consisting of a thorough history, physical examination, and appropriate radiographic imaging is required. Cutaneous metastases most commonly arise from the breast or gastrointestinal tract (GIT) but also can originate from the prostate, lungs, ovaries, pancreas, and kidneys.5 Histologically, PMC may be identical to metastatic adenocarcinoma.13 Location on the body may be a clue to a lesion’s origin, as metastases from a mucinous adenocarcinoma of the breast typically occur on the chest, breast, or axilla,5 whereas PMC primarily is found on the head and neck.
Certain histopathologic features may be suggestive of either a primary or metastatic etiology. Lesions arising in the skin may reveal an in situ component representing ductal hyperplasia, atypical ductal hyperplasia, or ductal carcinoma in situ. Identification of an in situ component defines a cutaneous primary neoplasm, but its absence does not exclude PMC.5 Additionally, metastatic lesions from the GIT typically have greater pleomorphism and “dirty” necrosis defined as eosinophilic foci containing nuclear debris.5
The expression pattern of cytokeratins (CKs) also can be suggestive. Primary mucinous carcinoma and metastatic breast adenocarcinoma are both CK7+ and CK20−. By contrast, mucinous adenocarcinoma of the GIT stains CK20+ and CK7−.14 Another marker that stains PMC is CK5 and CK6, though infrequently present. Levy et al15 reported positive staining for CK5 and CK6 in only 1 of 5 PMC cases. Positive staining for CK5 and CK6 has not been reported in any metastatic mucinous carcinoma.
The role of p63 immunostaining in the setting of mucinous carcinoma is controversial.16-18 Some practi-tioners have reported using p63 immunostaining to assist in establishing the diagnosis of PMC but only after performing a clinical workup to search for any primary sites of mucinous carcinoma in other organs.11 Other studies, however, have found select metastatic lesions from the breast17,18 and GIT18 to stain positively with p63. It is important to remember that these clinical and pathologic features are only suggestive of the primary etiology and are not replacement for a full clinical investigation.
Primary mucinous carcinoma is considered an indolent tumor with the majority of patient morbidity attributable to local recurrence and regional metastasis. Although uncommon, regional and distant metastasis rates have been reported to be 11% and 3%, respectively.19 Direct lymphatic invasion has been reported and indicates a more aggressive tumor with shorter recurrence-free intervals and predicts nodal metastases. Paradela et al20 recommended the use of D2-40, a monoclonal antibody and specific marker for lymphatic endothelium, to detect lymphatic invasion, particularly in node-negative primary tumors.
In one case of PMC on the jaw of a 39-year-old Japanese man, no recurrence or metastases were discovered until the 11th year of follow-up. At that time, he was found to have lung and bone metastases and died after 3 years.21 Other investigators report death occurring 4 to 24 months following diagnosis of distant metastases.7,22 Direct extension of the tumor into skeletal muscle, periosteum, bone, and dura also has been documented.7
Treatment principally is surgical, with PMC known to be resistant to both chemotherapy and radiation therapy.19,22 The recommended margins for simple excision range from 1 to 2 cm, but this method of treatment yields recurrence rates upward of 30% to 40%, especially for lesions located on the eyelid.12,13 First utilized in PMC of the eyelid to conserve tissue, MMS is rapidly becoming the treatment of choice because of its notably improved recurrence rate. A case series of 4 PMCs of the eyelid treated via MMS or frozen section control found the recurrence rate to be 7%.23 Another report of 2 cases of PMC treated by MMS reported no recurrence after 42 and 26 months.13 Ortiz et al7 reported an additional case of a patient treated by MMS that was recurrence free for 30 months at the time of publication. Further investigation is required to definitively recommend MMS on the basis of improved recurrence rate but should now be considered standard of care in recurrent, sizeable, or eyelid PMC.
Despite its ascension as treatment of choice in many cases of PMC, MMS is not without its risk of metastasis and recurrence. Tam et al24 reported a case of PMC with multiple recurrences and metastases following 3 simple excisions and 2 excisions via MMS. Although the lesion’s previously recurrent nature increased the likelihood of failure of MMS, this case demonstrates that all patients should be followed periodically after the treatment of PMC.
We presented a case of PMC in which standard surgical margins would have been insufficient to clear the lesion. Mohs micrographic surgery was used to remove the majority of the tumor. As is common in PMC, the lesion was indolent and periocular in location. It also was incompletely excised due to notable subclinical extension, which is common for PMC. The distinction of PMC from metastatic mucinous carcinoma is paramount but sometimes difficult. Randomized controlled trials are lacking with regards to preferred method of treatment, but MMS has shown benefit and should be considered for recurrent lesions and lesions in cosmetically sensitive areas.
- Breiting L, Christensen L, Dahlstrom K, et al. Primary mucinous carcinoma of the skin: a population-based study. Int J Dermatol. 2008;47:242-245.
- Martinez SR, Young SE. Primary mucinous carcinoma of the skin: a review. Int J Oncol. 2005;2:432-437.
- Lennox B, Pearse AG, Richards HG. Mucin-secreting tumours of the skin with special reference to the so-called mixed-salivary tumour of the skin and its relation to hidradenoma. J Pathol Bacteriol. 1952;64:865-880.
- Marra DE, Schanbacher CF, Torres A. Mohs micrographic surgery of primary cutaneous mucinous carcinoma using immunohistochemistry for margin control. Dermatol Surg. 2004;30:799-802.
- Kazakov DV, Suster S, LeBoit PE, et al. Mucinous carcinoma of the skin, primary, and secondary: a clinicopathologic study of 63 cases with emphasis on the morphologic spectrum of primary cutaneous forms: homologies with mucinous lesions in the breast. Am J Surg Pathol. 2005;29:764-782.
- Mendoza S, Helwig EB. Mucinous (adenocystic) carcinoma of the skin. Arch Dermatol. 1971;103:68-78.
- Ortiz KJ, Gaughan MD, Bang RH, et al. A case of primary mucinous carcinoma of the scalp treated with Mohs surgery. Dermatol Surg. 2002;28:751-754.
- Bellezza G, Sidoni A, Bucciarelli E. Primary mucinous carcinoma of the skin. Am J Dermatopathol. 2000;22:166-170.
- Teng P, Muir J. Small primary cutaneous mucinous carcinoma mimicking an early basal cell carcinoma. Dermatol Online J. 2013;19:3.
- Terada T, Sato Y, Furukawa K, et al. Primary cutaneous mucinous carcinoma initially diagnosed as metastatic adenocarcinoma. Tohoku J Exp Med. 2004;203:345-348.
- Kalebi A, Hale M. Primary mucinous carcinoma of the skin: usefulness of p63 in excluding metastasis and first report of psammoma bodies. Am J Dermatopathol. 2008;30:510.
- Cabell CE, Helm KF, Sakol PJ, et al. Primary mucinous carcinoma in a 54-year-old man. J Am Acad Dermatol. 2003;49:941-943.
- Cecchi R, Rapicano V. Primary cutaneous mucinous carcinoma: report of two cases treated with Mohs’ micrographic surgery. Australas J Dermatol. 2006;47:192-194.
- Eckert F, Schmid U, Hardmeier T, et al. Cytokeratin expression in mucinous sweat gland carcinomas: an immunohistochemical analysis of four cases. Histopathology. 1992;21:161-165.
- Levy G, Finkelstein A, McNiff JM. Immunohistochemical techniques to compare primary vs. metastatic mucinous carcinoma of the skin. J Cutan Pathol. 2010;37:411-415.
- Ivan D, Hafeez Diwan A, Prieto VG. Expression of p63 in primary cutaneous adnexal neoplasms and adenocarcinoma metastatic to the skin. Mod Pathol. 2005;18:137-142.
- Kanitakis J, Chouvet B. Expression of p63 in cutaneous metastases. Am J Clin Pathol. 2007;128:753-758.
- Sariya D, Ruth K, Adams-McDonnell R, et al. Clinicopathologic correlation of cutaneous metastases: experience from a cancer center. Arch Dermatol. 2007;143:613-620.
- Snow SN, Reizner GT. Mucinous eccrine carcinoma of the eyelid. Cancer. 1992;70:2099-2104.
- Paradela S, Castiñeiras I, Cuevas J, et al. Mucinous carcinoma of the skin: evaluation of lymphatic invasion with D2-40. Am J Dermatopathol. 2008;30:504-508.
- Miyasaka M, Tanaka R, Hirabayashi K, et al. Primary mucinous carcinoma of the skin: a case of metastasis after 10 years of disease-free interval. Eur J Plast Surg. 2009;32:189-193.
- Yeung KY, Stinson JC. Mucinous (adenocystic) carcinoma of sweat glands with widespread metastasis. case report with ultrastructural study. Cancer. 1977;39:2556-2562.
- Papalas JA, Proia AD. Primary mucinous carcinoma of the eyelid: a clinicopathologic and immunohistochemical study of 4 cases and an update on recurrence rates. Arch Ophthalmol. 2010;128:1160-1165.
- Tam CC, Dare DM, DiGiovanni JJ, et al. Recurrent and metastatic primary cutaneous mucinous carcinoma after excision and Mohs micrographic surgery. Cutis. 2011;87:245-248.
To the Editor:
Primary mucinous carcinoma (PMC) is an exceedingly rare adnexal tumor with an incidence of 0.07 cases per million individuals.1,2 First described by Lennox et al3 in 1952, this entity often presents as slow-growing, solitary nodules that often are soft on palpation but may have an indurated quality and range in color from reddish blue to flesh colored to white.4 Primary mucinous carcinoma most commonly is found on the eyelid (38%) but may affect other sites on the face (20.3%), scalp (16%), and axilla (10%).5 Historically, it has been thought to be more common among men; however, a 2005 large case series by Kazakov et al5 found that women were twice as likely to be affected. Primary mucinous carcinoma most frequently is diagnosed in the fifth through seventh decades of life, with a median age at onset of 63 years.6,7 Because of its rarity, PMC is most frequently confused clinically with basal cell carcinoma, keratoacanthoma, apocrine hidrocystoma, epidermoid cyst, Kaposi sarcoma, neuroma, lacrimal sac tumor, squamous cell carcinoma, granulomatous tumors, and metastatic adenocarcinoma.1,8-10
Primary mucinous carcinoma is thought to be derived from sweat glands, and select features such as decapitation secretion are more suggestive of apocrine than eccrine differentiation.5,8 On histopathology, PMC classically is described as nests of epithelial cells floating in lakes of extracellular mucin, primarily in the dermis and subcutis. The nests are composed of basaloid cells in solid to cribriform arrangements, usually with a low mitotic count and little nuclear atypia. These nests are suspended within periodic acid–Schiff positive mucinous pools partitioned by delicate fibrous septa. The mucin produced by PMC is sialomucin, and as such it is hyaluronidase resistant and sialidase labile.6 At least 1 report has been made of the presence of psammoma bodies in PMC.11
The neoplasm is characterized by an indolent course with frequent recurrence but rare metastasis.5,12 Treatment is primarily surgical, with Mohs micrographic surgery (MMS) offering improved tissue conservation and reduced recurrence rates.12 The diagnostic challenge lies in distinguishing PMC from a variety of metastatic mucinous internal malignancies that portend a notably greater morbidity and mortality to the patient. We describe a case of PMC, discuss the differentiation of PMC from metastatic mucinous carcinoma, and review the literature regarding treatment of this rare neoplasm.
A 65-year-old white woman was referred to our tertiary-care dermatologic surgery clinic for treatment of an incompletely excised mucinous carcinoma of the right lateral canthus (Figure 1). The clinically evident scar measured 0.5×0.5 cm. Although difficult to appreciate in Figure 1, a slight textural change of the surrounding skin, including the upper and lower eyelid, was apparent. Prior to her arrival to our clinic, the referring physician had completed a thorough review of systems and physical examination, which did not suggest an underlying malignancy. Computed tomography of the head, neck, chest, abdomen, and pelvis revealed a mass in the thyroid that was removed and found to be benign. The patient’s cutaneous lesion was therefore considered to be a PMC of the skin.

Given the prior incomplete excision of the lesion and its periocular location, we treated the patient with MMS. After 6 surgical stages, we continued to see evidence of the neoplasm as it tracked medially along the orbicularis oculi muscle (Figure 2). Due to the patient’s physical and emotional exhaustion at this point, we discontinued MMS and referred her to a colleague in plastic surgery for further excision of the remaining focus of positivity as well as repair. The final Mohs defect measured 4.2×4.0 cm (Figure 3). Approximately 2.3×1.0 cm of tissue in the area of remaining tumor was excised by plastic surgery, and the defect was repaired with a cervicofacial advancement flap closure of the right cheek and lower eyelid and full-thickness skin graft of the left upper eyelid. Histopathologic investigation found the additional tissue resected to be free of residual tumor.


To diagnose a patient with PMC, one must first rule out cutaneous metastasis of various internal malignancies that may appear similar on histopathology. A full clinical investigation consisting of a thorough history, physical examination, and appropriate radiographic imaging is required. Cutaneous metastases most commonly arise from the breast or gastrointestinal tract (GIT) but also can originate from the prostate, lungs, ovaries, pancreas, and kidneys.5 Histologically, PMC may be identical to metastatic adenocarcinoma.13 Location on the body may be a clue to a lesion’s origin, as metastases from a mucinous adenocarcinoma of the breast typically occur on the chest, breast, or axilla,5 whereas PMC primarily is found on the head and neck.
Certain histopathologic features may be suggestive of either a primary or metastatic etiology. Lesions arising in the skin may reveal an in situ component representing ductal hyperplasia, atypical ductal hyperplasia, or ductal carcinoma in situ. Identification of an in situ component defines a cutaneous primary neoplasm, but its absence does not exclude PMC.5 Additionally, metastatic lesions from the GIT typically have greater pleomorphism and “dirty” necrosis defined as eosinophilic foci containing nuclear debris.5
The expression pattern of cytokeratins (CKs) also can be suggestive. Primary mucinous carcinoma and metastatic breast adenocarcinoma are both CK7+ and CK20−. By contrast, mucinous adenocarcinoma of the GIT stains CK20+ and CK7−.14 Another marker that stains PMC is CK5 and CK6, though infrequently present. Levy et al15 reported positive staining for CK5 and CK6 in only 1 of 5 PMC cases. Positive staining for CK5 and CK6 has not been reported in any metastatic mucinous carcinoma.
The role of p63 immunostaining in the setting of mucinous carcinoma is controversial.16-18 Some practi-tioners have reported using p63 immunostaining to assist in establishing the diagnosis of PMC but only after performing a clinical workup to search for any primary sites of mucinous carcinoma in other organs.11 Other studies, however, have found select metastatic lesions from the breast17,18 and GIT18 to stain positively with p63. It is important to remember that these clinical and pathologic features are only suggestive of the primary etiology and are not replacement for a full clinical investigation.
Primary mucinous carcinoma is considered an indolent tumor with the majority of patient morbidity attributable to local recurrence and regional metastasis. Although uncommon, regional and distant metastasis rates have been reported to be 11% and 3%, respectively.19 Direct lymphatic invasion has been reported and indicates a more aggressive tumor with shorter recurrence-free intervals and predicts nodal metastases. Paradela et al20 recommended the use of D2-40, a monoclonal antibody and specific marker for lymphatic endothelium, to detect lymphatic invasion, particularly in node-negative primary tumors.
In one case of PMC on the jaw of a 39-year-old Japanese man, no recurrence or metastases were discovered until the 11th year of follow-up. At that time, he was found to have lung and bone metastases and died after 3 years.21 Other investigators report death occurring 4 to 24 months following diagnosis of distant metastases.7,22 Direct extension of the tumor into skeletal muscle, periosteum, bone, and dura also has been documented.7
Treatment principally is surgical, with PMC known to be resistant to both chemotherapy and radiation therapy.19,22 The recommended margins for simple excision range from 1 to 2 cm, but this method of treatment yields recurrence rates upward of 30% to 40%, especially for lesions located on the eyelid.12,13 First utilized in PMC of the eyelid to conserve tissue, MMS is rapidly becoming the treatment of choice because of its notably improved recurrence rate. A case series of 4 PMCs of the eyelid treated via MMS or frozen section control found the recurrence rate to be 7%.23 Another report of 2 cases of PMC treated by MMS reported no recurrence after 42 and 26 months.13 Ortiz et al7 reported an additional case of a patient treated by MMS that was recurrence free for 30 months at the time of publication. Further investigation is required to definitively recommend MMS on the basis of improved recurrence rate but should now be considered standard of care in recurrent, sizeable, or eyelid PMC.
Despite its ascension as treatment of choice in many cases of PMC, MMS is not without its risk of metastasis and recurrence. Tam et al24 reported a case of PMC with multiple recurrences and metastases following 3 simple excisions and 2 excisions via MMS. Although the lesion’s previously recurrent nature increased the likelihood of failure of MMS, this case demonstrates that all patients should be followed periodically after the treatment of PMC.
We presented a case of PMC in which standard surgical margins would have been insufficient to clear the lesion. Mohs micrographic surgery was used to remove the majority of the tumor. As is common in PMC, the lesion was indolent and periocular in location. It also was incompletely excised due to notable subclinical extension, which is common for PMC. The distinction of PMC from metastatic mucinous carcinoma is paramount but sometimes difficult. Randomized controlled trials are lacking with regards to preferred method of treatment, but MMS has shown benefit and should be considered for recurrent lesions and lesions in cosmetically sensitive areas.
To the Editor:
Primary mucinous carcinoma (PMC) is an exceedingly rare adnexal tumor with an incidence of 0.07 cases per million individuals.1,2 First described by Lennox et al3 in 1952, this entity often presents as slow-growing, solitary nodules that often are soft on palpation but may have an indurated quality and range in color from reddish blue to flesh colored to white.4 Primary mucinous carcinoma most commonly is found on the eyelid (38%) but may affect other sites on the face (20.3%), scalp (16%), and axilla (10%).5 Historically, it has been thought to be more common among men; however, a 2005 large case series by Kazakov et al5 found that women were twice as likely to be affected. Primary mucinous carcinoma most frequently is diagnosed in the fifth through seventh decades of life, with a median age at onset of 63 years.6,7 Because of its rarity, PMC is most frequently confused clinically with basal cell carcinoma, keratoacanthoma, apocrine hidrocystoma, epidermoid cyst, Kaposi sarcoma, neuroma, lacrimal sac tumor, squamous cell carcinoma, granulomatous tumors, and metastatic adenocarcinoma.1,8-10
Primary mucinous carcinoma is thought to be derived from sweat glands, and select features such as decapitation secretion are more suggestive of apocrine than eccrine differentiation.5,8 On histopathology, PMC classically is described as nests of epithelial cells floating in lakes of extracellular mucin, primarily in the dermis and subcutis. The nests are composed of basaloid cells in solid to cribriform arrangements, usually with a low mitotic count and little nuclear atypia. These nests are suspended within periodic acid–Schiff positive mucinous pools partitioned by delicate fibrous septa. The mucin produced by PMC is sialomucin, and as such it is hyaluronidase resistant and sialidase labile.6 At least 1 report has been made of the presence of psammoma bodies in PMC.11
The neoplasm is characterized by an indolent course with frequent recurrence but rare metastasis.5,12 Treatment is primarily surgical, with Mohs micrographic surgery (MMS) offering improved tissue conservation and reduced recurrence rates.12 The diagnostic challenge lies in distinguishing PMC from a variety of metastatic mucinous internal malignancies that portend a notably greater morbidity and mortality to the patient. We describe a case of PMC, discuss the differentiation of PMC from metastatic mucinous carcinoma, and review the literature regarding treatment of this rare neoplasm.
A 65-year-old white woman was referred to our tertiary-care dermatologic surgery clinic for treatment of an incompletely excised mucinous carcinoma of the right lateral canthus (Figure 1). The clinically evident scar measured 0.5×0.5 cm. Although difficult to appreciate in Figure 1, a slight textural change of the surrounding skin, including the upper and lower eyelid, was apparent. Prior to her arrival to our clinic, the referring physician had completed a thorough review of systems and physical examination, which did not suggest an underlying malignancy. Computed tomography of the head, neck, chest, abdomen, and pelvis revealed a mass in the thyroid that was removed and found to be benign. The patient’s cutaneous lesion was therefore considered to be a PMC of the skin.

Given the prior incomplete excision of the lesion and its periocular location, we treated the patient with MMS. After 6 surgical stages, we continued to see evidence of the neoplasm as it tracked medially along the orbicularis oculi muscle (Figure 2). Due to the patient’s physical and emotional exhaustion at this point, we discontinued MMS and referred her to a colleague in plastic surgery for further excision of the remaining focus of positivity as well as repair. The final Mohs defect measured 4.2×4.0 cm (Figure 3). Approximately 2.3×1.0 cm of tissue in the area of remaining tumor was excised by plastic surgery, and the defect was repaired with a cervicofacial advancement flap closure of the right cheek and lower eyelid and full-thickness skin graft of the left upper eyelid. Histopathologic investigation found the additional tissue resected to be free of residual tumor.


To diagnose a patient with PMC, one must first rule out cutaneous metastasis of various internal malignancies that may appear similar on histopathology. A full clinical investigation consisting of a thorough history, physical examination, and appropriate radiographic imaging is required. Cutaneous metastases most commonly arise from the breast or gastrointestinal tract (GIT) but also can originate from the prostate, lungs, ovaries, pancreas, and kidneys.5 Histologically, PMC may be identical to metastatic adenocarcinoma.13 Location on the body may be a clue to a lesion’s origin, as metastases from a mucinous adenocarcinoma of the breast typically occur on the chest, breast, or axilla,5 whereas PMC primarily is found on the head and neck.
Certain histopathologic features may be suggestive of either a primary or metastatic etiology. Lesions arising in the skin may reveal an in situ component representing ductal hyperplasia, atypical ductal hyperplasia, or ductal carcinoma in situ. Identification of an in situ component defines a cutaneous primary neoplasm, but its absence does not exclude PMC.5 Additionally, metastatic lesions from the GIT typically have greater pleomorphism and “dirty” necrosis defined as eosinophilic foci containing nuclear debris.5
The expression pattern of cytokeratins (CKs) also can be suggestive. Primary mucinous carcinoma and metastatic breast adenocarcinoma are both CK7+ and CK20−. By contrast, mucinous adenocarcinoma of the GIT stains CK20+ and CK7−.14 Another marker that stains PMC is CK5 and CK6, though infrequently present. Levy et al15 reported positive staining for CK5 and CK6 in only 1 of 5 PMC cases. Positive staining for CK5 and CK6 has not been reported in any metastatic mucinous carcinoma.
The role of p63 immunostaining in the setting of mucinous carcinoma is controversial.16-18 Some practi-tioners have reported using p63 immunostaining to assist in establishing the diagnosis of PMC but only after performing a clinical workup to search for any primary sites of mucinous carcinoma in other organs.11 Other studies, however, have found select metastatic lesions from the breast17,18 and GIT18 to stain positively with p63. It is important to remember that these clinical and pathologic features are only suggestive of the primary etiology and are not replacement for a full clinical investigation.
Primary mucinous carcinoma is considered an indolent tumor with the majority of patient morbidity attributable to local recurrence and regional metastasis. Although uncommon, regional and distant metastasis rates have been reported to be 11% and 3%, respectively.19 Direct lymphatic invasion has been reported and indicates a more aggressive tumor with shorter recurrence-free intervals and predicts nodal metastases. Paradela et al20 recommended the use of D2-40, a monoclonal antibody and specific marker for lymphatic endothelium, to detect lymphatic invasion, particularly in node-negative primary tumors.
In one case of PMC on the jaw of a 39-year-old Japanese man, no recurrence or metastases were discovered until the 11th year of follow-up. At that time, he was found to have lung and bone metastases and died after 3 years.21 Other investigators report death occurring 4 to 24 months following diagnosis of distant metastases.7,22 Direct extension of the tumor into skeletal muscle, periosteum, bone, and dura also has been documented.7
Treatment principally is surgical, with PMC known to be resistant to both chemotherapy and radiation therapy.19,22 The recommended margins for simple excision range from 1 to 2 cm, but this method of treatment yields recurrence rates upward of 30% to 40%, especially for lesions located on the eyelid.12,13 First utilized in PMC of the eyelid to conserve tissue, MMS is rapidly becoming the treatment of choice because of its notably improved recurrence rate. A case series of 4 PMCs of the eyelid treated via MMS or frozen section control found the recurrence rate to be 7%.23 Another report of 2 cases of PMC treated by MMS reported no recurrence after 42 and 26 months.13 Ortiz et al7 reported an additional case of a patient treated by MMS that was recurrence free for 30 months at the time of publication. Further investigation is required to definitively recommend MMS on the basis of improved recurrence rate but should now be considered standard of care in recurrent, sizeable, or eyelid PMC.
Despite its ascension as treatment of choice in many cases of PMC, MMS is not without its risk of metastasis and recurrence. Tam et al24 reported a case of PMC with multiple recurrences and metastases following 3 simple excisions and 2 excisions via MMS. Although the lesion’s previously recurrent nature increased the likelihood of failure of MMS, this case demonstrates that all patients should be followed periodically after the treatment of PMC.
We presented a case of PMC in which standard surgical margins would have been insufficient to clear the lesion. Mohs micrographic surgery was used to remove the majority of the tumor. As is common in PMC, the lesion was indolent and periocular in location. It also was incompletely excised due to notable subclinical extension, which is common for PMC. The distinction of PMC from metastatic mucinous carcinoma is paramount but sometimes difficult. Randomized controlled trials are lacking with regards to preferred method of treatment, but MMS has shown benefit and should be considered for recurrent lesions and lesions in cosmetically sensitive areas.
- Breiting L, Christensen L, Dahlstrom K, et al. Primary mucinous carcinoma of the skin: a population-based study. Int J Dermatol. 2008;47:242-245.
- Martinez SR, Young SE. Primary mucinous carcinoma of the skin: a review. Int J Oncol. 2005;2:432-437.
- Lennox B, Pearse AG, Richards HG. Mucin-secreting tumours of the skin with special reference to the so-called mixed-salivary tumour of the skin and its relation to hidradenoma. J Pathol Bacteriol. 1952;64:865-880.
- Marra DE, Schanbacher CF, Torres A. Mohs micrographic surgery of primary cutaneous mucinous carcinoma using immunohistochemistry for margin control. Dermatol Surg. 2004;30:799-802.
- Kazakov DV, Suster S, LeBoit PE, et al. Mucinous carcinoma of the skin, primary, and secondary: a clinicopathologic study of 63 cases with emphasis on the morphologic spectrum of primary cutaneous forms: homologies with mucinous lesions in the breast. Am J Surg Pathol. 2005;29:764-782.
- Mendoza S, Helwig EB. Mucinous (adenocystic) carcinoma of the skin. Arch Dermatol. 1971;103:68-78.
- Ortiz KJ, Gaughan MD, Bang RH, et al. A case of primary mucinous carcinoma of the scalp treated with Mohs surgery. Dermatol Surg. 2002;28:751-754.
- Bellezza G, Sidoni A, Bucciarelli E. Primary mucinous carcinoma of the skin. Am J Dermatopathol. 2000;22:166-170.
- Teng P, Muir J. Small primary cutaneous mucinous carcinoma mimicking an early basal cell carcinoma. Dermatol Online J. 2013;19:3.
- Terada T, Sato Y, Furukawa K, et al. Primary cutaneous mucinous carcinoma initially diagnosed as metastatic adenocarcinoma. Tohoku J Exp Med. 2004;203:345-348.
- Kalebi A, Hale M. Primary mucinous carcinoma of the skin: usefulness of p63 in excluding metastasis and first report of psammoma bodies. Am J Dermatopathol. 2008;30:510.
- Cabell CE, Helm KF, Sakol PJ, et al. Primary mucinous carcinoma in a 54-year-old man. J Am Acad Dermatol. 2003;49:941-943.
- Cecchi R, Rapicano V. Primary cutaneous mucinous carcinoma: report of two cases treated with Mohs’ micrographic surgery. Australas J Dermatol. 2006;47:192-194.
- Eckert F, Schmid U, Hardmeier T, et al. Cytokeratin expression in mucinous sweat gland carcinomas: an immunohistochemical analysis of four cases. Histopathology. 1992;21:161-165.
- Levy G, Finkelstein A, McNiff JM. Immunohistochemical techniques to compare primary vs. metastatic mucinous carcinoma of the skin. J Cutan Pathol. 2010;37:411-415.
- Ivan D, Hafeez Diwan A, Prieto VG. Expression of p63 in primary cutaneous adnexal neoplasms and adenocarcinoma metastatic to the skin. Mod Pathol. 2005;18:137-142.
- Kanitakis J, Chouvet B. Expression of p63 in cutaneous metastases. Am J Clin Pathol. 2007;128:753-758.
- Sariya D, Ruth K, Adams-McDonnell R, et al. Clinicopathologic correlation of cutaneous metastases: experience from a cancer center. Arch Dermatol. 2007;143:613-620.
- Snow SN, Reizner GT. Mucinous eccrine carcinoma of the eyelid. Cancer. 1992;70:2099-2104.
- Paradela S, Castiñeiras I, Cuevas J, et al. Mucinous carcinoma of the skin: evaluation of lymphatic invasion with D2-40. Am J Dermatopathol. 2008;30:504-508.
- Miyasaka M, Tanaka R, Hirabayashi K, et al. Primary mucinous carcinoma of the skin: a case of metastasis after 10 years of disease-free interval. Eur J Plast Surg. 2009;32:189-193.
- Yeung KY, Stinson JC. Mucinous (adenocystic) carcinoma of sweat glands with widespread metastasis. case report with ultrastructural study. Cancer. 1977;39:2556-2562.
- Papalas JA, Proia AD. Primary mucinous carcinoma of the eyelid: a clinicopathologic and immunohistochemical study of 4 cases and an update on recurrence rates. Arch Ophthalmol. 2010;128:1160-1165.
- Tam CC, Dare DM, DiGiovanni JJ, et al. Recurrent and metastatic primary cutaneous mucinous carcinoma after excision and Mohs micrographic surgery. Cutis. 2011;87:245-248.
- Breiting L, Christensen L, Dahlstrom K, et al. Primary mucinous carcinoma of the skin: a population-based study. Int J Dermatol. 2008;47:242-245.
- Martinez SR, Young SE. Primary mucinous carcinoma of the skin: a review. Int J Oncol. 2005;2:432-437.
- Lennox B, Pearse AG, Richards HG. Mucin-secreting tumours of the skin with special reference to the so-called mixed-salivary tumour of the skin and its relation to hidradenoma. J Pathol Bacteriol. 1952;64:865-880.
- Marra DE, Schanbacher CF, Torres A. Mohs micrographic surgery of primary cutaneous mucinous carcinoma using immunohistochemistry for margin control. Dermatol Surg. 2004;30:799-802.
- Kazakov DV, Suster S, LeBoit PE, et al. Mucinous carcinoma of the skin, primary, and secondary: a clinicopathologic study of 63 cases with emphasis on the morphologic spectrum of primary cutaneous forms: homologies with mucinous lesions in the breast. Am J Surg Pathol. 2005;29:764-782.
- Mendoza S, Helwig EB. Mucinous (adenocystic) carcinoma of the skin. Arch Dermatol. 1971;103:68-78.
- Ortiz KJ, Gaughan MD, Bang RH, et al. A case of primary mucinous carcinoma of the scalp treated with Mohs surgery. Dermatol Surg. 2002;28:751-754.
- Bellezza G, Sidoni A, Bucciarelli E. Primary mucinous carcinoma of the skin. Am J Dermatopathol. 2000;22:166-170.
- Teng P, Muir J. Small primary cutaneous mucinous carcinoma mimicking an early basal cell carcinoma. Dermatol Online J. 2013;19:3.
- Terada T, Sato Y, Furukawa K, et al. Primary cutaneous mucinous carcinoma initially diagnosed as metastatic adenocarcinoma. Tohoku J Exp Med. 2004;203:345-348.
- Kalebi A, Hale M. Primary mucinous carcinoma of the skin: usefulness of p63 in excluding metastasis and first report of psammoma bodies. Am J Dermatopathol. 2008;30:510.
- Cabell CE, Helm KF, Sakol PJ, et al. Primary mucinous carcinoma in a 54-year-old man. J Am Acad Dermatol. 2003;49:941-943.
- Cecchi R, Rapicano V. Primary cutaneous mucinous carcinoma: report of two cases treated with Mohs’ micrographic surgery. Australas J Dermatol. 2006;47:192-194.
- Eckert F, Schmid U, Hardmeier T, et al. Cytokeratin expression in mucinous sweat gland carcinomas: an immunohistochemical analysis of four cases. Histopathology. 1992;21:161-165.
- Levy G, Finkelstein A, McNiff JM. Immunohistochemical techniques to compare primary vs. metastatic mucinous carcinoma of the skin. J Cutan Pathol. 2010;37:411-415.
- Ivan D, Hafeez Diwan A, Prieto VG. Expression of p63 in primary cutaneous adnexal neoplasms and adenocarcinoma metastatic to the skin. Mod Pathol. 2005;18:137-142.
- Kanitakis J, Chouvet B. Expression of p63 in cutaneous metastases. Am J Clin Pathol. 2007;128:753-758.
- Sariya D, Ruth K, Adams-McDonnell R, et al. Clinicopathologic correlation of cutaneous metastases: experience from a cancer center. Arch Dermatol. 2007;143:613-620.
- Snow SN, Reizner GT. Mucinous eccrine carcinoma of the eyelid. Cancer. 1992;70:2099-2104.
- Paradela S, Castiñeiras I, Cuevas J, et al. Mucinous carcinoma of the skin: evaluation of lymphatic invasion with D2-40. Am J Dermatopathol. 2008;30:504-508.
- Miyasaka M, Tanaka R, Hirabayashi K, et al. Primary mucinous carcinoma of the skin: a case of metastasis after 10 years of disease-free interval. Eur J Plast Surg. 2009;32:189-193.
- Yeung KY, Stinson JC. Mucinous (adenocystic) carcinoma of sweat glands with widespread metastasis. case report with ultrastructural study. Cancer. 1977;39:2556-2562.
- Papalas JA, Proia AD. Primary mucinous carcinoma of the eyelid: a clinicopathologic and immunohistochemical study of 4 cases and an update on recurrence rates. Arch Ophthalmol. 2010;128:1160-1165.
- Tam CC, Dare DM, DiGiovanni JJ, et al. Recurrent and metastatic primary cutaneous mucinous carcinoma after excision and Mohs micrographic surgery. Cutis. 2011;87:245-248.
Practice Points
- Primary mucinous carcinoma (PMC) of the skin is a rare adnexal tumor.
- Prior to treatment, the diagnostic importance lies in distinguishing PMC from metastatic mucinous malignancies, which portend a poorer prognosis.
- Treatment primarily is surgical, with Mohs micrographic surgery offering improved tissue conservation and reduced recurrence rates.
Ustekinumab may reduce risk of nonmelanoma skin cancer
GENEVA – Ustekinumab therapy appears to protect psoriasis patients against nonmelanoma skin cancer (NMSC), according to a new analysis from the PSOLAR registry.
Compared with psoriasis patients on methotrexate, the risk of developing on-treatment NMSC was lower among patients on the interleukin-12-/23 inhibitor ustekinumab (Stelara) and those on the three tumor necrosis factor (TNF) inhibitors included in the PSOLAR registry – infliximab (Remicade), etanercept (Enbrel), and adalimumab (Humira). The lower risk was statistically significant only for ustekinumab, although there was a favorable trend with the TNF inhibitors showing a 19% relative risk reduction, Bhaskar Srivastava, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
PSOLAR (Psoriasis Longitudinal Assessment and Registry) is an ongoing international prospective observational study evaluating long-term safety and clinical outcomes in psoriasis patients eligible for systemic therapies. The study is now fully enrolled, with 12,090 psoriasis patients and 48,870 patient-years of follow-up and climbing, noted Dr. Srivastava, an employee of Janssen Scientific Affairs, Spring House, Pa.
This analysis focused on 6,782 PSOLAR participants with a mean 18-year history of psoriasis and no history of NMSC at enrollment: 2,623 patients on ustekinumab with 7,900 patient-years of prospective follow-up, 3,727 on a TNF inhibitor with 10,580 patient-years of follow-up, and 432 controls on methotrexate with 781 patient-years of follow-up.
Patients on a biologic were significantly younger, with a mean age of 46.7 years, versus 53.6 years for those on methotrexate. Rates of past or current smoking were similar, in the 55%-60% range, regardless of which systemic agent patients were using.
The crude unadjusted incidence rate for NMSC among all patients on a biologic was 0.33 cancers/100 patient-years, compared with 1.41/100 patient-years for psoriasis patients on methotrexate.
Patients on ustekinumab had an NMSC incidence rate of 0.19/100 patient-years, with a basal cell carcinoma rate of 0.13/100 patient-years and a squamous cell carcinoma rate of 0.06/100 patient-years. Psoriasis patients on a TNF inhibitor had an NMSC incidence rate of 0.43/100 patient-years, with a basal cell carcinoma rate of 0.26/100 patient-years and a squamous cell carcinoma rate of 0.17/100 patient-years.
In a multivariate analysis adjusted for age, sex, race, location, duration of psoriasis, smoking, prior malignancy, skin type, and history of treatment with cyclosporine, methotrexate, other systemic agents, or phototherapy, patients taking ustekinumab had a statistically significant 65% reduction in the risk of NMSC compared with patients on methotrexate and a 74% relative risk reduction for basal cell carcinoma; however, the squamous cell carcinoma risk in the two patient groups was similar.
Dr. Srivastava said the PSOLAR data shouldn’t be taken as the final word regarding NMSC risk and the use of biologics. He noted that psoriasis itself is associated with an increased risk of NMSC. And methotrexate, which was used as the reference standard in this analysis, may alter the risk of NMSC.
“Overall, these results require further validation in psoriasis populations with larger numbers of exposed patients,” he said.
The PSOLAR registry is funded by Janssen, where Dr. Srivastava is employed.
GENEVA – Ustekinumab therapy appears to protect psoriasis patients against nonmelanoma skin cancer (NMSC), according to a new analysis from the PSOLAR registry.
Compared with psoriasis patients on methotrexate, the risk of developing on-treatment NMSC was lower among patients on the interleukin-12-/23 inhibitor ustekinumab (Stelara) and those on the three tumor necrosis factor (TNF) inhibitors included in the PSOLAR registry – infliximab (Remicade), etanercept (Enbrel), and adalimumab (Humira). The lower risk was statistically significant only for ustekinumab, although there was a favorable trend with the TNF inhibitors showing a 19% relative risk reduction, Bhaskar Srivastava, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
PSOLAR (Psoriasis Longitudinal Assessment and Registry) is an ongoing international prospective observational study evaluating long-term safety and clinical outcomes in psoriasis patients eligible for systemic therapies. The study is now fully enrolled, with 12,090 psoriasis patients and 48,870 patient-years of follow-up and climbing, noted Dr. Srivastava, an employee of Janssen Scientific Affairs, Spring House, Pa.
This analysis focused on 6,782 PSOLAR participants with a mean 18-year history of psoriasis and no history of NMSC at enrollment: 2,623 patients on ustekinumab with 7,900 patient-years of prospective follow-up, 3,727 on a TNF inhibitor with 10,580 patient-years of follow-up, and 432 controls on methotrexate with 781 patient-years of follow-up.
Patients on a biologic were significantly younger, with a mean age of 46.7 years, versus 53.6 years for those on methotrexate. Rates of past or current smoking were similar, in the 55%-60% range, regardless of which systemic agent patients were using.
The crude unadjusted incidence rate for NMSC among all patients on a biologic was 0.33 cancers/100 patient-years, compared with 1.41/100 patient-years for psoriasis patients on methotrexate.
Patients on ustekinumab had an NMSC incidence rate of 0.19/100 patient-years, with a basal cell carcinoma rate of 0.13/100 patient-years and a squamous cell carcinoma rate of 0.06/100 patient-years. Psoriasis patients on a TNF inhibitor had an NMSC incidence rate of 0.43/100 patient-years, with a basal cell carcinoma rate of 0.26/100 patient-years and a squamous cell carcinoma rate of 0.17/100 patient-years.
In a multivariate analysis adjusted for age, sex, race, location, duration of psoriasis, smoking, prior malignancy, skin type, and history of treatment with cyclosporine, methotrexate, other systemic agents, or phototherapy, patients taking ustekinumab had a statistically significant 65% reduction in the risk of NMSC compared with patients on methotrexate and a 74% relative risk reduction for basal cell carcinoma; however, the squamous cell carcinoma risk in the two patient groups was similar.
Dr. Srivastava said the PSOLAR data shouldn’t be taken as the final word regarding NMSC risk and the use of biologics. He noted that psoriasis itself is associated with an increased risk of NMSC. And methotrexate, which was used as the reference standard in this analysis, may alter the risk of NMSC.
“Overall, these results require further validation in psoriasis populations with larger numbers of exposed patients,” he said.
The PSOLAR registry is funded by Janssen, where Dr. Srivastava is employed.
GENEVA – Ustekinumab therapy appears to protect psoriasis patients against nonmelanoma skin cancer (NMSC), according to a new analysis from the PSOLAR registry.
Compared with psoriasis patients on methotrexate, the risk of developing on-treatment NMSC was lower among patients on the interleukin-12-/23 inhibitor ustekinumab (Stelara) and those on the three tumor necrosis factor (TNF) inhibitors included in the PSOLAR registry – infliximab (Remicade), etanercept (Enbrel), and adalimumab (Humira). The lower risk was statistically significant only for ustekinumab, although there was a favorable trend with the TNF inhibitors showing a 19% relative risk reduction, Bhaskar Srivastava, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
PSOLAR (Psoriasis Longitudinal Assessment and Registry) is an ongoing international prospective observational study evaluating long-term safety and clinical outcomes in psoriasis patients eligible for systemic therapies. The study is now fully enrolled, with 12,090 psoriasis patients and 48,870 patient-years of follow-up and climbing, noted Dr. Srivastava, an employee of Janssen Scientific Affairs, Spring House, Pa.
This analysis focused on 6,782 PSOLAR participants with a mean 18-year history of psoriasis and no history of NMSC at enrollment: 2,623 patients on ustekinumab with 7,900 patient-years of prospective follow-up, 3,727 on a TNF inhibitor with 10,580 patient-years of follow-up, and 432 controls on methotrexate with 781 patient-years of follow-up.
Patients on a biologic were significantly younger, with a mean age of 46.7 years, versus 53.6 years for those on methotrexate. Rates of past or current smoking were similar, in the 55%-60% range, regardless of which systemic agent patients were using.
The crude unadjusted incidence rate for NMSC among all patients on a biologic was 0.33 cancers/100 patient-years, compared with 1.41/100 patient-years for psoriasis patients on methotrexate.
Patients on ustekinumab had an NMSC incidence rate of 0.19/100 patient-years, with a basal cell carcinoma rate of 0.13/100 patient-years and a squamous cell carcinoma rate of 0.06/100 patient-years. Psoriasis patients on a TNF inhibitor had an NMSC incidence rate of 0.43/100 patient-years, with a basal cell carcinoma rate of 0.26/100 patient-years and a squamous cell carcinoma rate of 0.17/100 patient-years.
In a multivariate analysis adjusted for age, sex, race, location, duration of psoriasis, smoking, prior malignancy, skin type, and history of treatment with cyclosporine, methotrexate, other systemic agents, or phototherapy, patients taking ustekinumab had a statistically significant 65% reduction in the risk of NMSC compared with patients on methotrexate and a 74% relative risk reduction for basal cell carcinoma; however, the squamous cell carcinoma risk in the two patient groups was similar.
Dr. Srivastava said the PSOLAR data shouldn’t be taken as the final word regarding NMSC risk and the use of biologics. He noted that psoriasis itself is associated with an increased risk of NMSC. And methotrexate, which was used as the reference standard in this analysis, may alter the risk of NMSC.
“Overall, these results require further validation in psoriasis populations with larger numbers of exposed patients,” he said.
The PSOLAR registry is funded by Janssen, where Dr. Srivastava is employed.
AT THE EADV CONGRESS
Key clinical point:
Major finding: Psoriasis patients on ustekinumab had an adjusted 65% reduction in the risk of developing nonmelanoma skin cancer compared with patients on methotrexate.
Data source: An analysis of 6,782 psoriasis patients participating in an international prospective observational registry evaluating the long-term safety and clinical outcomes of systemic therapies.
Disclosures: The PSOLAR registry is funded by ustekinumab manufacturer Janssen; the study presenter is a company employee.
Black Eschars on the Face and Body
The Diagnosis: Lymphomatoid Papulosis
Histopathologic and immunohistochemical examination of the ulcer revealed a dense nodular and diffuse infiltrate in the papillary and reticular dermis comprised predominantly of atypical, CD30+, small T cells and large lymphoid cells admixed with neutrophils and eosinophils (Figures 1 and 2). Tissue cultures and infectious stains were negative. The complete blood cell count, metabolic panel, serum lactate dehydrogenase level, and peripheral blood flow cytometry were normal. Correlation of the lesions' self-healing nature with the histopathologic and immunohistochemical findings led to a diagnosis of lymphomatoid papulosis (LyP). In light of this diagnosis, a shave biopsy was obtained of one of the patient's poikilodermatous patches and was found to be consistent with poikilodermatous mycosis fungoides (MF).
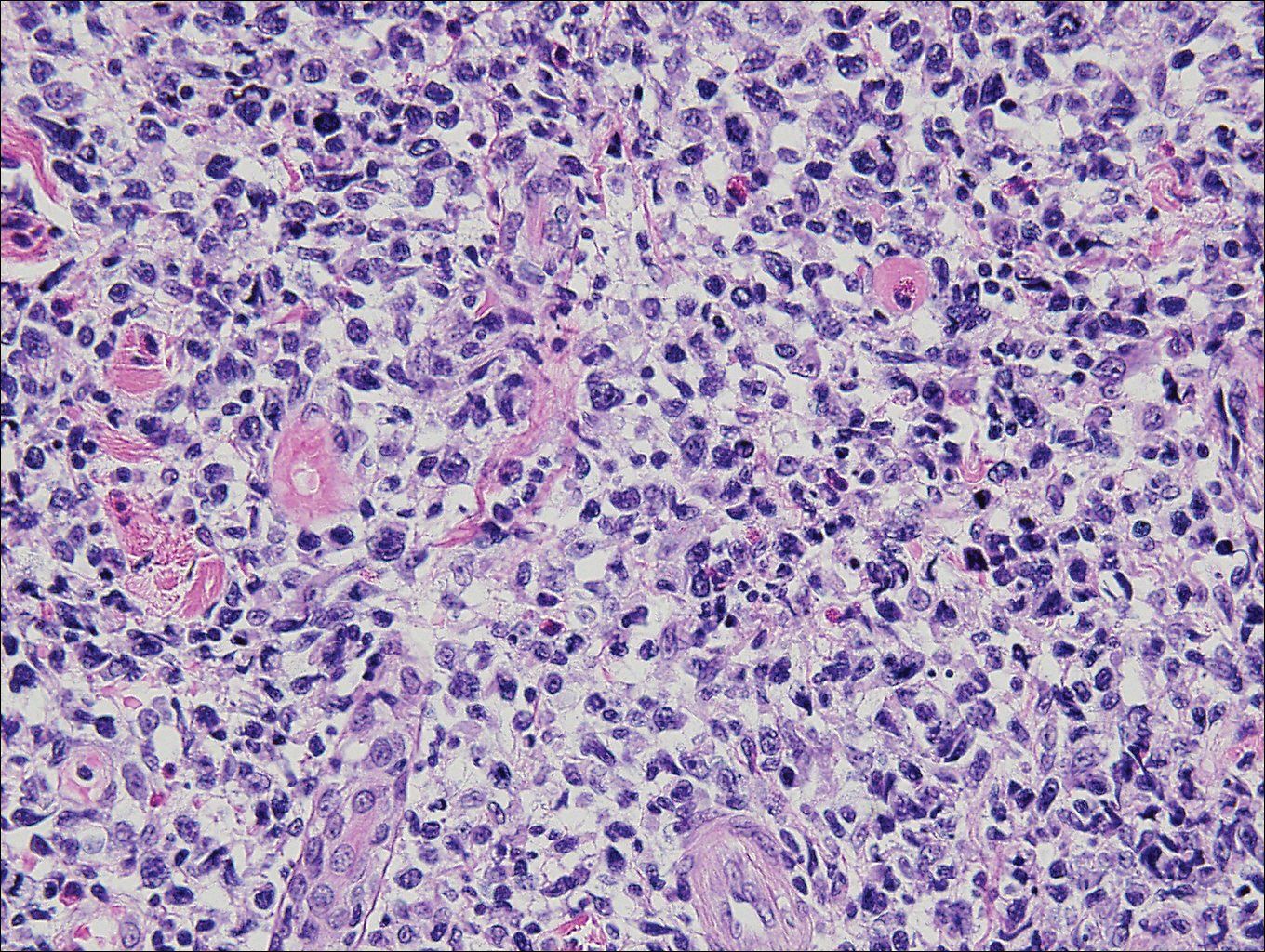
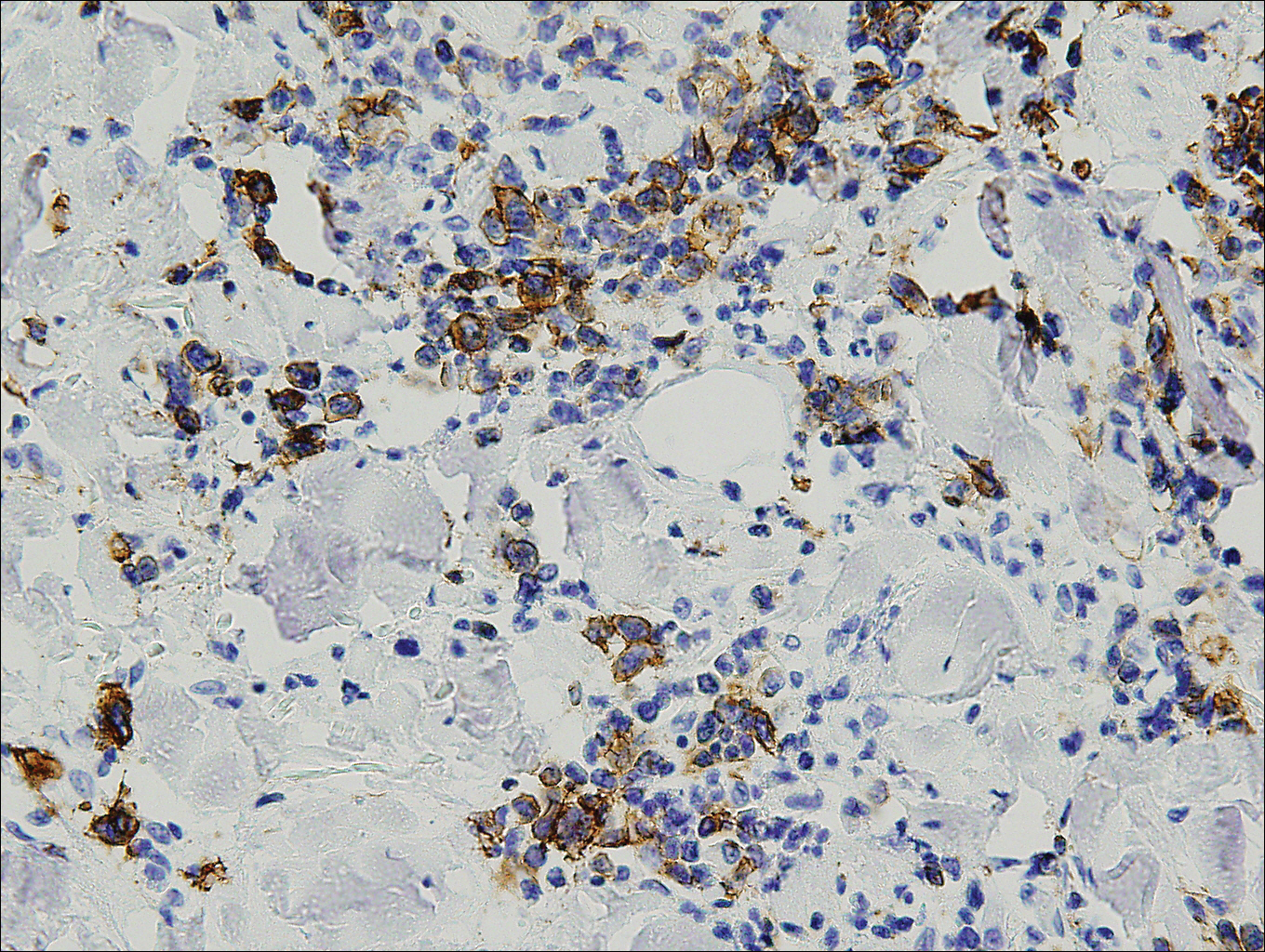
At 4-month follow-up, the patient reported that she continued to develop crops of 1 to 3 LyP lesions each month. She continued to deny systemic concerns, and the poikilodermatous MF appeared unchanged. As part of a hematologic workup, a positron emission tomography-computed tomography scan revealed glucose-avid lymph nodes in the axillary, supraclavicular, abdominal, and inguinal regions. These findings raised concern for possible lymphomatous involvement of the patient's MF. Systemic therapy may be required pending further surveillance.
Lymphomatoid papulosis is a chronic papulonecrotic disease characterized clinically by recurrent crops of self-healing papules. Histopathologically, LyP features a perivascular infiltrate with atypical dermal T cells. Macaulay1 first described LyP in 1968 in a 41-year-old woman with a several-year history of continuously self-resolving crops of necrotic papules, noting the paradox between the patient's benign clinical course and malignant-seeming histology featuring "an alarming infiltrate of anaplastic cells." Since this report, LyP has continued to spur debate regarding its malignant potential but is now recognized as an indolent cutaneous T-cell lymphoma with an excellent prognosis.2
There are several histopathologic subtypes of LyP, the most common of which are type A, resembling Hodgkin lymphoma; type B, resembling MF; type C, resembling primary cutaneous anaplastic large cell lymphoma (C-ALCL); and type D, resembling aggressive epidermotropic CD8+ cutaneous T-cell lymphoma.2
The multifocal ulcers and eschars of LyP may appropriately raise suspicion for an infectious process, as in the present case. Numerous reports show that LyP may be initially misdiagnosed as an infection, such as cellulitis,3 furunculosis,4 parapoxvirus Orf,5 and ecthyma.6 Furthermore, several cutaneous infections have histopathologic features indistinguishable from LyP.7 For example, herpes simplex virus infection, molluscum contagiosum, Milker nodule, syphilis, and leishmaniasis may contain an appreciable number of large CD30+ T cells, which is compatible with both LyP type C and C-ALCL.7 As in the present case, the final diagnosis rests on clinicopathologic correlation, with LyP often distinguished by its invariable self-resolution, unlike its numerous infectious mimickers. The self-regressing nature of LyP also helps differentiate LyP occurring in the setting of MF from MF that has underwent CD30+ large cell transformation. In addition, the diagnosis of MF-associated LyP is favored over transformed MF when, as in the present case, CD30+ lesions develop on skin distinct from MF-affected skin.
Although isolated LyP is benign, 18% (11/61) of patients will subsequently develop lymphoma. More commonly, lymphomas may precede or occur concomitantly with the onset of LyP. In a retrospective study of 84 LyP patients, for example, 40% (34/84) had prior or concomitant lymphoma.8 Owing to the well-established link between LyP and lymphoma, there is appropriate emphasis on close monitoring of these patients. In addition, a careful history and physical examination are necessary to evaluate for a preceding, previously undiagnosed lymphoma. In point of fact, our patient had undiagnosed poikilodermatous MF prior to developing LyP, which was proven by biopsy at the time of LyP diagnosis. A distinct clinical variant of MF, poikilodermatous MF is characterized by hyperpigmented and hypopigmented patches, atrophy, and telangiectasia. A study of 49 patients with poikilodermatous MF found that this variant had an earlier age of onset compared with other types of MF. The study also showed that 18% (9/49) of patients had coexistent LyP, suggesting that poikilodermatous MF and LyP may be more frequently associated than previously believed.9
Treatment of LyP is unnecessary beyond basic wound care to avoid bacterial superinfection.2,10 Therapy for poikilodermatous MF, similar to other types of MF, is based on disease stage. Topical therapy may be utilized for localized disease, while systemic therapies are reserved for recalcitrant cases and internal involvement.9
Acknowledgments
We thank David L. Ramsay, MD, for obtaining aspects of the patient's history, and Shane A. Meehan, MD, and Adnan Mir, MD, PhD, as well as Cynthia M. Magro, MD, (all from New York, New York) for performing the histopathologic and immunohistochemical analyses.
- Macaulay WL. Lymphomatoid papulosis. a continuing self-healing eruption, clinically benign--histologically malignant. Arch Dermatol. 1968;97:23-30.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Meena M, Martin PA, Abouseif C, et al. Lymphomatoid papulosis type C of the eyelid in a young girl: a case report and review of literature. Orbit. 2014;3:395-398.
- Dinotta F, Lacarrubba F, Micali G. Sixteen-year-old girl with papules and nodules on the face and upper limbs. Pediatr Dermatol. 2014;31:103-104.
- Eminger LA, Shinohara MM, Kim EJ, et al. Clinicopathologic challenge: acral lymphomatoid papulosis. Int J Dermatol. 2012;51:531-534.
- Harder D, Kuhn A, Mahrle G. Lymphomatoid papulosis resembling ecthyma. a case report. Z Hautkr. 1989;64:593-595.
- Werner B, Massone C, Kerl H, et al. Large CD30-positive cells in benign, atypical lymphoid infiltrates of the skin. J Cutan Pathol. 2008;35:1100-1107.
- Kunishige JH, McDonald H, Alvarez G, et al. Lymphomatoid papulosis and associated lymphomas: a retrospective case series of 84 patients. Clin Exp Dermatol. 2009;34:576-581.
- Abbott RA, Sahni D, Robson A, et al. Poikilodermatous mycosis fungoides: a study of its clinicopathological, immunophenotypic, and prognostic features. J Am Acad Dermatol. 2011;65:313-319.
- Kempf W, Pfaltz K, Vermeer MH, et al. EORTC, ISCL, and USCLC consensus recommendations for the treatment of primary cutaneous CD30-positive lymphoproliferative disorders: lymphomatoid papulosis and primary cutaneous anaplastic large-cell lymphoma. Blood. 2011;118:4024-4035.
The Diagnosis: Lymphomatoid Papulosis
Histopathologic and immunohistochemical examination of the ulcer revealed a dense nodular and diffuse infiltrate in the papillary and reticular dermis comprised predominantly of atypical, CD30+, small T cells and large lymphoid cells admixed with neutrophils and eosinophils (Figures 1 and 2). Tissue cultures and infectious stains were negative. The complete blood cell count, metabolic panel, serum lactate dehydrogenase level, and peripheral blood flow cytometry were normal. Correlation of the lesions' self-healing nature with the histopathologic and immunohistochemical findings led to a diagnosis of lymphomatoid papulosis (LyP). In light of this diagnosis, a shave biopsy was obtained of one of the patient's poikilodermatous patches and was found to be consistent with poikilodermatous mycosis fungoides (MF).


At 4-month follow-up, the patient reported that she continued to develop crops of 1 to 3 LyP lesions each month. She continued to deny systemic concerns, and the poikilodermatous MF appeared unchanged. As part of a hematologic workup, a positron emission tomography-computed tomography scan revealed glucose-avid lymph nodes in the axillary, supraclavicular, abdominal, and inguinal regions. These findings raised concern for possible lymphomatous involvement of the patient's MF. Systemic therapy may be required pending further surveillance.
Lymphomatoid papulosis is a chronic papulonecrotic disease characterized clinically by recurrent crops of self-healing papules. Histopathologically, LyP features a perivascular infiltrate with atypical dermal T cells. Macaulay1 first described LyP in 1968 in a 41-year-old woman with a several-year history of continuously self-resolving crops of necrotic papules, noting the paradox between the patient's benign clinical course and malignant-seeming histology featuring "an alarming infiltrate of anaplastic cells." Since this report, LyP has continued to spur debate regarding its malignant potential but is now recognized as an indolent cutaneous T-cell lymphoma with an excellent prognosis.2
There are several histopathologic subtypes of LyP, the most common of which are type A, resembling Hodgkin lymphoma; type B, resembling MF; type C, resembling primary cutaneous anaplastic large cell lymphoma (C-ALCL); and type D, resembling aggressive epidermotropic CD8+ cutaneous T-cell lymphoma.2
The multifocal ulcers and eschars of LyP may appropriately raise suspicion for an infectious process, as in the present case. Numerous reports show that LyP may be initially misdiagnosed as an infection, such as cellulitis,3 furunculosis,4 parapoxvirus Orf,5 and ecthyma.6 Furthermore, several cutaneous infections have histopathologic features indistinguishable from LyP.7 For example, herpes simplex virus infection, molluscum contagiosum, Milker nodule, syphilis, and leishmaniasis may contain an appreciable number of large CD30+ T cells, which is compatible with both LyP type C and C-ALCL.7 As in the present case, the final diagnosis rests on clinicopathologic correlation, with LyP often distinguished by its invariable self-resolution, unlike its numerous infectious mimickers. The self-regressing nature of LyP also helps differentiate LyP occurring in the setting of MF from MF that has underwent CD30+ large cell transformation. In addition, the diagnosis of MF-associated LyP is favored over transformed MF when, as in the present case, CD30+ lesions develop on skin distinct from MF-affected skin.
Although isolated LyP is benign, 18% (11/61) of patients will subsequently develop lymphoma. More commonly, lymphomas may precede or occur concomitantly with the onset of LyP. In a retrospective study of 84 LyP patients, for example, 40% (34/84) had prior or concomitant lymphoma.8 Owing to the well-established link between LyP and lymphoma, there is appropriate emphasis on close monitoring of these patients. In addition, a careful history and physical examination are necessary to evaluate for a preceding, previously undiagnosed lymphoma. In point of fact, our patient had undiagnosed poikilodermatous MF prior to developing LyP, which was proven by biopsy at the time of LyP diagnosis. A distinct clinical variant of MF, poikilodermatous MF is characterized by hyperpigmented and hypopigmented patches, atrophy, and telangiectasia. A study of 49 patients with poikilodermatous MF found that this variant had an earlier age of onset compared with other types of MF. The study also showed that 18% (9/49) of patients had coexistent LyP, suggesting that poikilodermatous MF and LyP may be more frequently associated than previously believed.9
Treatment of LyP is unnecessary beyond basic wound care to avoid bacterial superinfection.2,10 Therapy for poikilodermatous MF, similar to other types of MF, is based on disease stage. Topical therapy may be utilized for localized disease, while systemic therapies are reserved for recalcitrant cases and internal involvement.9
Acknowledgments
We thank David L. Ramsay, MD, for obtaining aspects of the patient's history, and Shane A. Meehan, MD, and Adnan Mir, MD, PhD, as well as Cynthia M. Magro, MD, (all from New York, New York) for performing the histopathologic and immunohistochemical analyses.
The Diagnosis: Lymphomatoid Papulosis
Histopathologic and immunohistochemical examination of the ulcer revealed a dense nodular and diffuse infiltrate in the papillary and reticular dermis comprised predominantly of atypical, CD30+, small T cells and large lymphoid cells admixed with neutrophils and eosinophils (Figures 1 and 2). Tissue cultures and infectious stains were negative. The complete blood cell count, metabolic panel, serum lactate dehydrogenase level, and peripheral blood flow cytometry were normal. Correlation of the lesions' self-healing nature with the histopathologic and immunohistochemical findings led to a diagnosis of lymphomatoid papulosis (LyP). In light of this diagnosis, a shave biopsy was obtained of one of the patient's poikilodermatous patches and was found to be consistent with poikilodermatous mycosis fungoides (MF).


At 4-month follow-up, the patient reported that she continued to develop crops of 1 to 3 LyP lesions each month. She continued to deny systemic concerns, and the poikilodermatous MF appeared unchanged. As part of a hematologic workup, a positron emission tomography-computed tomography scan revealed glucose-avid lymph nodes in the axillary, supraclavicular, abdominal, and inguinal regions. These findings raised concern for possible lymphomatous involvement of the patient's MF. Systemic therapy may be required pending further surveillance.
Lymphomatoid papulosis is a chronic papulonecrotic disease characterized clinically by recurrent crops of self-healing papules. Histopathologically, LyP features a perivascular infiltrate with atypical dermal T cells. Macaulay1 first described LyP in 1968 in a 41-year-old woman with a several-year history of continuously self-resolving crops of necrotic papules, noting the paradox between the patient's benign clinical course and malignant-seeming histology featuring "an alarming infiltrate of anaplastic cells." Since this report, LyP has continued to spur debate regarding its malignant potential but is now recognized as an indolent cutaneous T-cell lymphoma with an excellent prognosis.2
There are several histopathologic subtypes of LyP, the most common of which are type A, resembling Hodgkin lymphoma; type B, resembling MF; type C, resembling primary cutaneous anaplastic large cell lymphoma (C-ALCL); and type D, resembling aggressive epidermotropic CD8+ cutaneous T-cell lymphoma.2
The multifocal ulcers and eschars of LyP may appropriately raise suspicion for an infectious process, as in the present case. Numerous reports show that LyP may be initially misdiagnosed as an infection, such as cellulitis,3 furunculosis,4 parapoxvirus Orf,5 and ecthyma.6 Furthermore, several cutaneous infections have histopathologic features indistinguishable from LyP.7 For example, herpes simplex virus infection, molluscum contagiosum, Milker nodule, syphilis, and leishmaniasis may contain an appreciable number of large CD30+ T cells, which is compatible with both LyP type C and C-ALCL.7 As in the present case, the final diagnosis rests on clinicopathologic correlation, with LyP often distinguished by its invariable self-resolution, unlike its numerous infectious mimickers. The self-regressing nature of LyP also helps differentiate LyP occurring in the setting of MF from MF that has underwent CD30+ large cell transformation. In addition, the diagnosis of MF-associated LyP is favored over transformed MF when, as in the present case, CD30+ lesions develop on skin distinct from MF-affected skin.
Although isolated LyP is benign, 18% (11/61) of patients will subsequently develop lymphoma. More commonly, lymphomas may precede or occur concomitantly with the onset of LyP. In a retrospective study of 84 LyP patients, for example, 40% (34/84) had prior or concomitant lymphoma.8 Owing to the well-established link between LyP and lymphoma, there is appropriate emphasis on close monitoring of these patients. In addition, a careful history and physical examination are necessary to evaluate for a preceding, previously undiagnosed lymphoma. In point of fact, our patient had undiagnosed poikilodermatous MF prior to developing LyP, which was proven by biopsy at the time of LyP diagnosis. A distinct clinical variant of MF, poikilodermatous MF is characterized by hyperpigmented and hypopigmented patches, atrophy, and telangiectasia. A study of 49 patients with poikilodermatous MF found that this variant had an earlier age of onset compared with other types of MF. The study also showed that 18% (9/49) of patients had coexistent LyP, suggesting that poikilodermatous MF and LyP may be more frequently associated than previously believed.9
Treatment of LyP is unnecessary beyond basic wound care to avoid bacterial superinfection.2,10 Therapy for poikilodermatous MF, similar to other types of MF, is based on disease stage. Topical therapy may be utilized for localized disease, while systemic therapies are reserved for recalcitrant cases and internal involvement.9
Acknowledgments
We thank David L. Ramsay, MD, for obtaining aspects of the patient's history, and Shane A. Meehan, MD, and Adnan Mir, MD, PhD, as well as Cynthia M. Magro, MD, (all from New York, New York) for performing the histopathologic and immunohistochemical analyses.
- Macaulay WL. Lymphomatoid papulosis. a continuing self-healing eruption, clinically benign--histologically malignant. Arch Dermatol. 1968;97:23-30.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Meena M, Martin PA, Abouseif C, et al. Lymphomatoid papulosis type C of the eyelid in a young girl: a case report and review of literature. Orbit. 2014;3:395-398.
- Dinotta F, Lacarrubba F, Micali G. Sixteen-year-old girl with papules and nodules on the face and upper limbs. Pediatr Dermatol. 2014;31:103-104.
- Eminger LA, Shinohara MM, Kim EJ, et al. Clinicopathologic challenge: acral lymphomatoid papulosis. Int J Dermatol. 2012;51:531-534.
- Harder D, Kuhn A, Mahrle G. Lymphomatoid papulosis resembling ecthyma. a case report. Z Hautkr. 1989;64:593-595.
- Werner B, Massone C, Kerl H, et al. Large CD30-positive cells in benign, atypical lymphoid infiltrates of the skin. J Cutan Pathol. 2008;35:1100-1107.
- Kunishige JH, McDonald H, Alvarez G, et al. Lymphomatoid papulosis and associated lymphomas: a retrospective case series of 84 patients. Clin Exp Dermatol. 2009;34:576-581.
- Abbott RA, Sahni D, Robson A, et al. Poikilodermatous mycosis fungoides: a study of its clinicopathological, immunophenotypic, and prognostic features. J Am Acad Dermatol. 2011;65:313-319.
- Kempf W, Pfaltz K, Vermeer MH, et al. EORTC, ISCL, and USCLC consensus recommendations for the treatment of primary cutaneous CD30-positive lymphoproliferative disorders: lymphomatoid papulosis and primary cutaneous anaplastic large-cell lymphoma. Blood. 2011;118:4024-4035.
- Macaulay WL. Lymphomatoid papulosis. a continuing self-healing eruption, clinically benign--histologically malignant. Arch Dermatol. 1968;97:23-30.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Meena M, Martin PA, Abouseif C, et al. Lymphomatoid papulosis type C of the eyelid in a young girl: a case report and review of literature. Orbit. 2014;3:395-398.
- Dinotta F, Lacarrubba F, Micali G. Sixteen-year-old girl with papules and nodules on the face and upper limbs. Pediatr Dermatol. 2014;31:103-104.
- Eminger LA, Shinohara MM, Kim EJ, et al. Clinicopathologic challenge: acral lymphomatoid papulosis. Int J Dermatol. 2012;51:531-534.
- Harder D, Kuhn A, Mahrle G. Lymphomatoid papulosis resembling ecthyma. a case report. Z Hautkr. 1989;64:593-595.
- Werner B, Massone C, Kerl H, et al. Large CD30-positive cells in benign, atypical lymphoid infiltrates of the skin. J Cutan Pathol. 2008;35:1100-1107.
- Kunishige JH, McDonald H, Alvarez G, et al. Lymphomatoid papulosis and associated lymphomas: a retrospective case series of 84 patients. Clin Exp Dermatol. 2009;34:576-581.
- Abbott RA, Sahni D, Robson A, et al. Poikilodermatous mycosis fungoides: a study of its clinicopathological, immunophenotypic, and prognostic features. J Am Acad Dermatol. 2011;65:313-319.
- Kempf W, Pfaltz K, Vermeer MH, et al. EORTC, ISCL, and USCLC consensus recommendations for the treatment of primary cutaneous CD30-positive lymphoproliferative disorders: lymphomatoid papulosis and primary cutaneous anaplastic large-cell lymphoma. Blood. 2011;118:4024-4035.
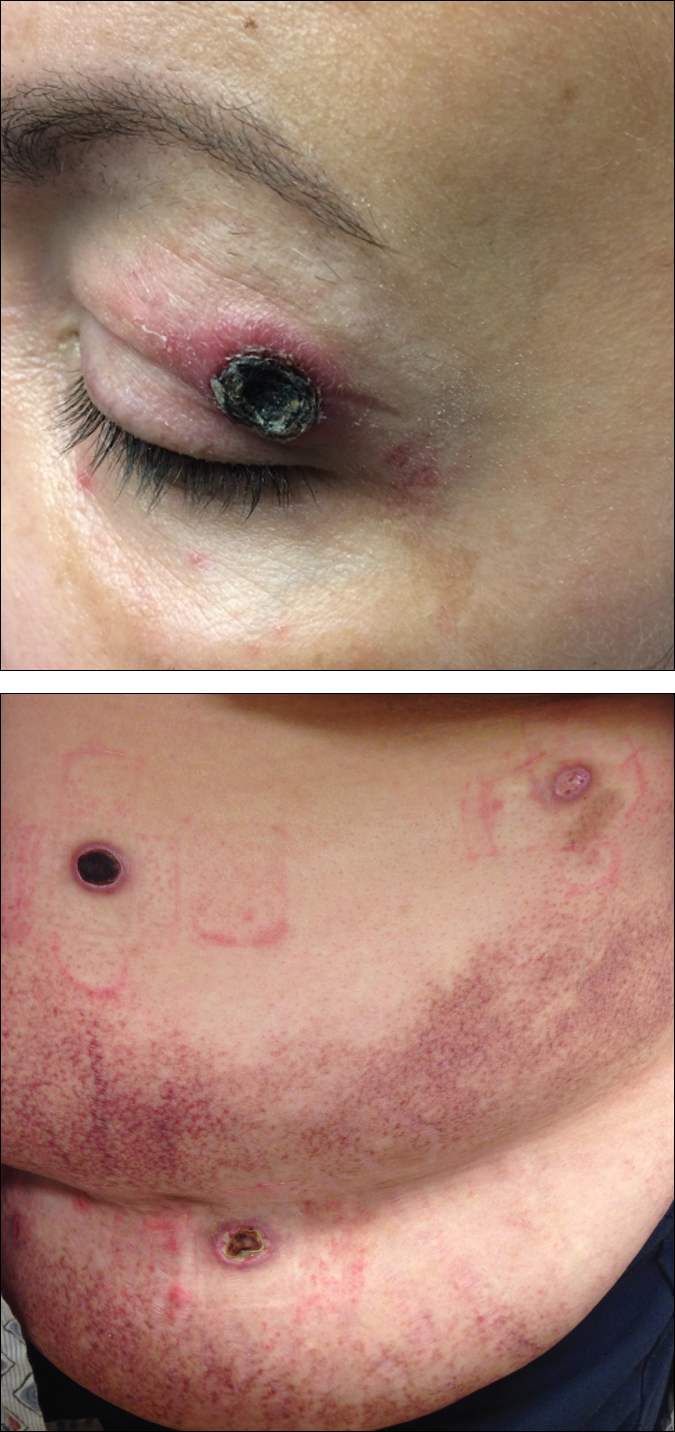
A 50-year-old woman presented for evaluation of black eschars on the face and body. Over the preceding 8 weeks she had developed several asymptomatic papules that gradually enlarged, ulcerated, and formed a black eschar, prior to gradually self-resolving over the course of several weeks. During this time, new lesions were forming. The resulting skin revealed dyspigmentation and scar formation. Prior to presentation, antimicrobial therapy had been initiated for a presumed infectious etiology; however, the eruption continued to progress. The patient denied sick contacts, livestock exposure, or recent travel. A complete review of systems, including fever, chills, or lymphadenopathy, was negative. Physical examination revealed 6 circular necrotic ulcers with an overlying black eschar on the face (top), trunk (bottom), hands, and thighs, all in various stages of healing. In addition, large, reticulated, poikilodermatous patches were incidentally noted in areas free of ulcers and eschars on the trunk (bottom) and bilateral arms and legs. Upon questioning, the patient said these patches had been present for more than 30 years. A punch biopsy from an ulcer on the chest was obtained and sent for histopathologic and immunohistochemical examination.
Debunking Actinic Keratosis Myths: Are Patients With Darker Skin At Risk for Actinic Keratoses?
Myth: Actinic keratoses are only seen in patients with lighter skin
Actinic keratoses (AKs) are precancerous lesions that may turn into squamous cell carcinoma if left untreated. UV rays cause AKs, either from outdoor sun exposure or tanning beds. According to the American Academy of Dermatology, AKs are more likely to develop in patients 40 years or older with fair skin; hair color that is naturally blonde or red; eye color that is naturally blue, green, or hazel; skin that freckles or burns when in the sun; a weakened immune system; and occupations involving substances that contain polycyclic aromatic hydrocarbons such as coal or tar.
A 2007 study compared the most common diagnoses among patients of different racial and ethnic groups in New York City. Alexis et al found that AK was in the top 10 diagnoses in white patients but not for black patients. They postulated that photoprotective factors in darkly pigmented skin such as larger and more numerous melanosomes that contain more melanin and are more dispersed throughout the epidermis result in a lower incidence of skin cancers in the skin of color (SOC) population.
RELATED ARTICLE: Common Dermatologic Disorders in Skin of Color: A Comparative Practice Survey
However, a recent skin cancer awareness study in Cutis reported that even though SOC populations have lower incidences of skin cancer such as melanoma, basal cell carcinoma, and squamous cell carcinoma, they exhibit higher death rates. Furthermore, black individuals are more likely to present with advanced-stage melanoma and acral lentiginous melanomas compared to white individuals. Kailas et al stated, “Overall, SOC patients have the poorest skin cancer prognosis, and the data suggest that the reason for this paradox is delayed diagnosis.” They evaluated several knowledge-based interventions for increasing skin cancer awareness, knowledge, and protective behaviors in SOC populations, including the use of visuals such as photographs to allow SOC patients to visualize different skin tones, educational interventions in another language, and pamphlets.
Dermatologists should be aware that education of SOC patients is important to eradicate the common misconception that these patients do not have to worry about AKs and other skin cancers. Remind these patients that they need to protect their skin from the sun, just as patients with fair skin do. Further research in the dermatology community should focus on educational interventions that will help increase knowledge regarding skin cancer in SOC populations.
Expert Commentary
Although more common in patients with lighter skin, actinic keratosis and skin cancer can be seen in patients of all skin types. Many patients are unaware of this risk and do not use sunscreen and other sun-protective measures. We, as a specialty, have to educate our patients and the public of the risk for actinic keratosis and skin cancer in all skin types.
—Gary Goldenberg, MD (New York, New York)
Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
American Academy of Dermatology. Actinic keratosis. https://www.aad.org/public/diseases/scaly-skin/actinic-keratosis. Accessed October 17, 2017.
Kailas A, Botwin AL, Pritchett EN, et al. Assessing the effectiveness of knowledge-based interventions in increasing skin cancer awareness, knowledge, and protective behaviors in skin of color populations. Cutis. 2017;100:235-240.
Myth: Actinic keratoses are only seen in patients with lighter skin
Actinic keratoses (AKs) are precancerous lesions that may turn into squamous cell carcinoma if left untreated. UV rays cause AKs, either from outdoor sun exposure or tanning beds. According to the American Academy of Dermatology, AKs are more likely to develop in patients 40 years or older with fair skin; hair color that is naturally blonde or red; eye color that is naturally blue, green, or hazel; skin that freckles or burns when in the sun; a weakened immune system; and occupations involving substances that contain polycyclic aromatic hydrocarbons such as coal or tar.
A 2007 study compared the most common diagnoses among patients of different racial and ethnic groups in New York City. Alexis et al found that AK was in the top 10 diagnoses in white patients but not for black patients. They postulated that photoprotective factors in darkly pigmented skin such as larger and more numerous melanosomes that contain more melanin and are more dispersed throughout the epidermis result in a lower incidence of skin cancers in the skin of color (SOC) population.
RELATED ARTICLE: Common Dermatologic Disorders in Skin of Color: A Comparative Practice Survey
However, a recent skin cancer awareness study in Cutis reported that even though SOC populations have lower incidences of skin cancer such as melanoma, basal cell carcinoma, and squamous cell carcinoma, they exhibit higher death rates. Furthermore, black individuals are more likely to present with advanced-stage melanoma and acral lentiginous melanomas compared to white individuals. Kailas et al stated, “Overall, SOC patients have the poorest skin cancer prognosis, and the data suggest that the reason for this paradox is delayed diagnosis.” They evaluated several knowledge-based interventions for increasing skin cancer awareness, knowledge, and protective behaviors in SOC populations, including the use of visuals such as photographs to allow SOC patients to visualize different skin tones, educational interventions in another language, and pamphlets.
Dermatologists should be aware that education of SOC patients is important to eradicate the common misconception that these patients do not have to worry about AKs and other skin cancers. Remind these patients that they need to protect their skin from the sun, just as patients with fair skin do. Further research in the dermatology community should focus on educational interventions that will help increase knowledge regarding skin cancer in SOC populations.
Expert Commentary
Although more common in patients with lighter skin, actinic keratosis and skin cancer can be seen in patients of all skin types. Many patients are unaware of this risk and do not use sunscreen and other sun-protective measures. We, as a specialty, have to educate our patients and the public of the risk for actinic keratosis and skin cancer in all skin types.
—Gary Goldenberg, MD (New York, New York)
Myth: Actinic keratoses are only seen in patients with lighter skin
Actinic keratoses (AKs) are precancerous lesions that may turn into squamous cell carcinoma if left untreated. UV rays cause AKs, either from outdoor sun exposure or tanning beds. According to the American Academy of Dermatology, AKs are more likely to develop in patients 40 years or older with fair skin; hair color that is naturally blonde or red; eye color that is naturally blue, green, or hazel; skin that freckles or burns when in the sun; a weakened immune system; and occupations involving substances that contain polycyclic aromatic hydrocarbons such as coal or tar.
A 2007 study compared the most common diagnoses among patients of different racial and ethnic groups in New York City. Alexis et al found that AK was in the top 10 diagnoses in white patients but not for black patients. They postulated that photoprotective factors in darkly pigmented skin such as larger and more numerous melanosomes that contain more melanin and are more dispersed throughout the epidermis result in a lower incidence of skin cancers in the skin of color (SOC) population.
RELATED ARTICLE: Common Dermatologic Disorders in Skin of Color: A Comparative Practice Survey
However, a recent skin cancer awareness study in Cutis reported that even though SOC populations have lower incidences of skin cancer such as melanoma, basal cell carcinoma, and squamous cell carcinoma, they exhibit higher death rates. Furthermore, black individuals are more likely to present with advanced-stage melanoma and acral lentiginous melanomas compared to white individuals. Kailas et al stated, “Overall, SOC patients have the poorest skin cancer prognosis, and the data suggest that the reason for this paradox is delayed diagnosis.” They evaluated several knowledge-based interventions for increasing skin cancer awareness, knowledge, and protective behaviors in SOC populations, including the use of visuals such as photographs to allow SOC patients to visualize different skin tones, educational interventions in another language, and pamphlets.
Dermatologists should be aware that education of SOC patients is important to eradicate the common misconception that these patients do not have to worry about AKs and other skin cancers. Remind these patients that they need to protect their skin from the sun, just as patients with fair skin do. Further research in the dermatology community should focus on educational interventions that will help increase knowledge regarding skin cancer in SOC populations.
Expert Commentary
Although more common in patients with lighter skin, actinic keratosis and skin cancer can be seen in patients of all skin types. Many patients are unaware of this risk and do not use sunscreen and other sun-protective measures. We, as a specialty, have to educate our patients and the public of the risk for actinic keratosis and skin cancer in all skin types.
—Gary Goldenberg, MD (New York, New York)
Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
American Academy of Dermatology. Actinic keratosis. https://www.aad.org/public/diseases/scaly-skin/actinic-keratosis. Accessed October 17, 2017.
Kailas A, Botwin AL, Pritchett EN, et al. Assessing the effectiveness of knowledge-based interventions in increasing skin cancer awareness, knowledge, and protective behaviors in skin of color populations. Cutis. 2017;100:235-240.
Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
American Academy of Dermatology. Actinic keratosis. https://www.aad.org/public/diseases/scaly-skin/actinic-keratosis. Accessed October 17, 2017.
Kailas A, Botwin AL, Pritchett EN, et al. Assessing the effectiveness of knowledge-based interventions in increasing skin cancer awareness, knowledge, and protective behaviors in skin of color populations. Cutis. 2017;100:235-240.
Painless Telangiectatic Lesion on the Wrist
The Diagnosis: Merkel Cell Carcinoma
A partial biopsy was performed during the dermatology examination. Histopathology demonstrated a dense dermal infiltrate of small, dark blue, pleomorphic cells (Figure 1). On high power, the individual cells were noted to have vesicular nuclei with finely granular and dusty chromatin (Figure 2). Numerous mitotic figures were present. Immunohistochemical stains were performed and revealed positive staining for cytokeratin 20 (with a perinuclear dot pattern), synaptophysin, and chromogranin.
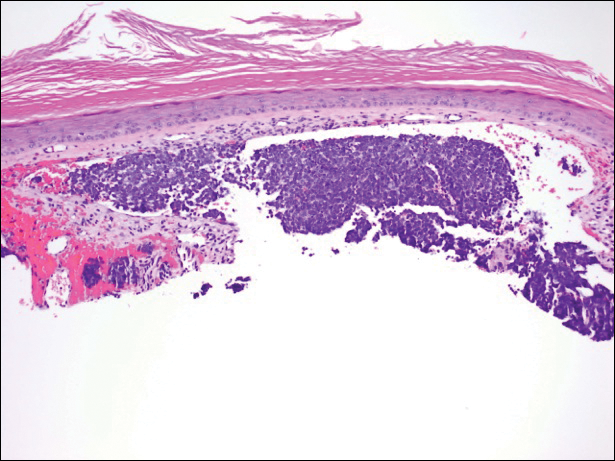
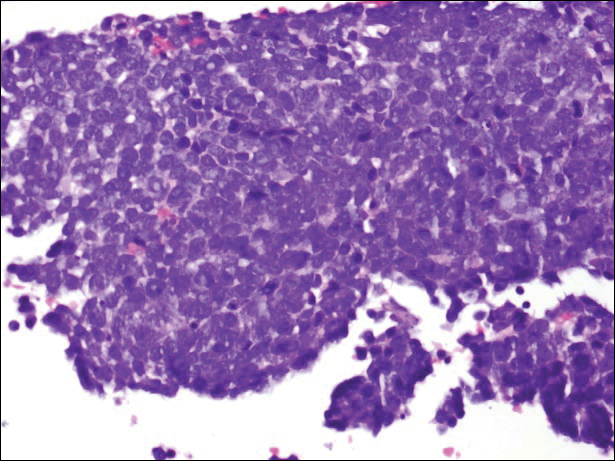
Merkel cell carcinoma (MCC) is an uncommon carcinoma of the epidermal neuroendocrine cells with approximately 1500 cases a year in the United States.1 Merkel cell carcinoma has a poor prognosis with approximately one-third of cases resulting in death within 5 years and with a survival rate strongly dependent on the stage of disease at presentation.2 A complete surgical excision with histologically verified clear margins is the main form of treatment of the primary cancer.3 Although the effectiveness of adjuvant therapy for MCC has been debated,4 retrospective analysis has shown that the high local recurrence rate of the primary tumor can be reduced by combining surgical excision with a form of radiation therapy.5
A systematic cohort study of 195 patients diagnosed with MCC summarized its most clinical factors with the acronym AEIOU: asymptomatic, expanding rapidly, immunosuppression, older than 50 years of age, and UV-exposed site on a fair-skinned individual.6 The role of immune function in MCC was highlighted by a 16-fold overrepresentation of immunosuppressed patients in the studied cohort as compared to the general US population. The immunosuppressed patients included individuals with human immunodeficiency virus, chronic lymphocytic leukemia, and iatrogenic suppression secondary to solid organ transplantation.6
In 2008, Merkel cell polyomavirus (MCPyV) was found in 80% (8/10) of MCC tumors tested.7 Since then, many different studies have suggested that MCPyV is an etiologic agent of MCC.8-10 A natural component of skin flora, MCPyV only becomes tumorigenic after integration into the host DNA and with mutations to the viral genome.11 Although there currently is no difference in treatment of MCPyV-positive and MCPyV-negative MCC,12 research is being done to determine how the discovery of the MCPyV could impact the treatment of MCC.
- Albores-Saavedra J, Batich K, Chable-Montero F, et al. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population based study. J Cutan Pathol. 2009;37:20-27.
- Allen PJ, Bowne WB, Jaques DP, et al. Merkel cell carcinoma: prognosis and treatment of patients from a single institution. J Clin Oncol. 2005;23:2300-2309.
- Eng TY, Boersma MG, Fuller CD, et al. A comprehensive review of the treatment of Merkel cell carcinoma. Am J Clin Oncol. 2007;30:624-636.
- Beenken SW, Urist MM. Treatment options for Merkel cell carcinoma. J Natl Compr Canc Netw. 2004;2:89-92.
- Decker RH, Wilson LD. Role of radiotherapy in the management of Merkel cell carcinoma of the skin. J Natl Compr Canc Netw. 2006;4:713-718.
- Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the "AEIOU" features. J Am Acad Dermatol. 2008;58:375-381.
- Feng H, Shuda M, Chang Y, et al. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096-1100.
- Duncavage EJ, Zehnbauer BA, Pfeifer JD. Prevalence of Merkel cell polyomavirus in Merkel cell carcinoma. Mod Pathol. 2009;22:516-521.
- Sastre-Garau X, Peter M, Avril MF, et al. Merkel cell carcinoma of the skin: pathological and molecular evidence for a causative role of MCV in oncogenesis. J Pathol. 2009;218:48-56.
- Varga E, Kiss M, Szabó K, et al. Detection of Merkel cell polyomavirus DNA in Merkel cell carcinomas. Br J Dermatol. 2009;161:930-932.
- Wendzicki JA, Moore PS, Chang Y. Large T and small T antigens of Merkel cell carcinoma. Curr Opin Virol. 2015;11:38-43.
- Duprat JP, Landman G, Salvajoli JV, et al. A review of the epidemiology and treatment of Merkel cell carcinoma. Clinics (Sao Paulo). 2011;66:1817-1823.
The Diagnosis: Merkel Cell Carcinoma
A partial biopsy was performed during the dermatology examination. Histopathology demonstrated a dense dermal infiltrate of small, dark blue, pleomorphic cells (Figure 1). On high power, the individual cells were noted to have vesicular nuclei with finely granular and dusty chromatin (Figure 2). Numerous mitotic figures were present. Immunohistochemical stains were performed and revealed positive staining for cytokeratin 20 (with a perinuclear dot pattern), synaptophysin, and chromogranin.


Merkel cell carcinoma (MCC) is an uncommon carcinoma of the epidermal neuroendocrine cells with approximately 1500 cases a year in the United States.1 Merkel cell carcinoma has a poor prognosis with approximately one-third of cases resulting in death within 5 years and with a survival rate strongly dependent on the stage of disease at presentation.2 A complete surgical excision with histologically verified clear margins is the main form of treatment of the primary cancer.3 Although the effectiveness of adjuvant therapy for MCC has been debated,4 retrospective analysis has shown that the high local recurrence rate of the primary tumor can be reduced by combining surgical excision with a form of radiation therapy.5
A systematic cohort study of 195 patients diagnosed with MCC summarized its most clinical factors with the acronym AEIOU: asymptomatic, expanding rapidly, immunosuppression, older than 50 years of age, and UV-exposed site on a fair-skinned individual.6 The role of immune function in MCC was highlighted by a 16-fold overrepresentation of immunosuppressed patients in the studied cohort as compared to the general US population. The immunosuppressed patients included individuals with human immunodeficiency virus, chronic lymphocytic leukemia, and iatrogenic suppression secondary to solid organ transplantation.6
In 2008, Merkel cell polyomavirus (MCPyV) was found in 80% (8/10) of MCC tumors tested.7 Since then, many different studies have suggested that MCPyV is an etiologic agent of MCC.8-10 A natural component of skin flora, MCPyV only becomes tumorigenic after integration into the host DNA and with mutations to the viral genome.11 Although there currently is no difference in treatment of MCPyV-positive and MCPyV-negative MCC,12 research is being done to determine how the discovery of the MCPyV could impact the treatment of MCC.
The Diagnosis: Merkel Cell Carcinoma
A partial biopsy was performed during the dermatology examination. Histopathology demonstrated a dense dermal infiltrate of small, dark blue, pleomorphic cells (Figure 1). On high power, the individual cells were noted to have vesicular nuclei with finely granular and dusty chromatin (Figure 2). Numerous mitotic figures were present. Immunohistochemical stains were performed and revealed positive staining for cytokeratin 20 (with a perinuclear dot pattern), synaptophysin, and chromogranin.


Merkel cell carcinoma (MCC) is an uncommon carcinoma of the epidermal neuroendocrine cells with approximately 1500 cases a year in the United States.1 Merkel cell carcinoma has a poor prognosis with approximately one-third of cases resulting in death within 5 years and with a survival rate strongly dependent on the stage of disease at presentation.2 A complete surgical excision with histologically verified clear margins is the main form of treatment of the primary cancer.3 Although the effectiveness of adjuvant therapy for MCC has been debated,4 retrospective analysis has shown that the high local recurrence rate of the primary tumor can be reduced by combining surgical excision with a form of radiation therapy.5
A systematic cohort study of 195 patients diagnosed with MCC summarized its most clinical factors with the acronym AEIOU: asymptomatic, expanding rapidly, immunosuppression, older than 50 years of age, and UV-exposed site on a fair-skinned individual.6 The role of immune function in MCC was highlighted by a 16-fold overrepresentation of immunosuppressed patients in the studied cohort as compared to the general US population. The immunosuppressed patients included individuals with human immunodeficiency virus, chronic lymphocytic leukemia, and iatrogenic suppression secondary to solid organ transplantation.6
In 2008, Merkel cell polyomavirus (MCPyV) was found in 80% (8/10) of MCC tumors tested.7 Since then, many different studies have suggested that MCPyV is an etiologic agent of MCC.8-10 A natural component of skin flora, MCPyV only becomes tumorigenic after integration into the host DNA and with mutations to the viral genome.11 Although there currently is no difference in treatment of MCPyV-positive and MCPyV-negative MCC,12 research is being done to determine how the discovery of the MCPyV could impact the treatment of MCC.
- Albores-Saavedra J, Batich K, Chable-Montero F, et al. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population based study. J Cutan Pathol. 2009;37:20-27.
- Allen PJ, Bowne WB, Jaques DP, et al. Merkel cell carcinoma: prognosis and treatment of patients from a single institution. J Clin Oncol. 2005;23:2300-2309.
- Eng TY, Boersma MG, Fuller CD, et al. A comprehensive review of the treatment of Merkel cell carcinoma. Am J Clin Oncol. 2007;30:624-636.
- Beenken SW, Urist MM. Treatment options for Merkel cell carcinoma. J Natl Compr Canc Netw. 2004;2:89-92.
- Decker RH, Wilson LD. Role of radiotherapy in the management of Merkel cell carcinoma of the skin. J Natl Compr Canc Netw. 2006;4:713-718.
- Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the "AEIOU" features. J Am Acad Dermatol. 2008;58:375-381.
- Feng H, Shuda M, Chang Y, et al. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096-1100.
- Duncavage EJ, Zehnbauer BA, Pfeifer JD. Prevalence of Merkel cell polyomavirus in Merkel cell carcinoma. Mod Pathol. 2009;22:516-521.
- Sastre-Garau X, Peter M, Avril MF, et al. Merkel cell carcinoma of the skin: pathological and molecular evidence for a causative role of MCV in oncogenesis. J Pathol. 2009;218:48-56.
- Varga E, Kiss M, Szabó K, et al. Detection of Merkel cell polyomavirus DNA in Merkel cell carcinomas. Br J Dermatol. 2009;161:930-932.
- Wendzicki JA, Moore PS, Chang Y. Large T and small T antigens of Merkel cell carcinoma. Curr Opin Virol. 2015;11:38-43.
- Duprat JP, Landman G, Salvajoli JV, et al. A review of the epidemiology and treatment of Merkel cell carcinoma. Clinics (Sao Paulo). 2011;66:1817-1823.
- Albores-Saavedra J, Batich K, Chable-Montero F, et al. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population based study. J Cutan Pathol. 2009;37:20-27.
- Allen PJ, Bowne WB, Jaques DP, et al. Merkel cell carcinoma: prognosis and treatment of patients from a single institution. J Clin Oncol. 2005;23:2300-2309.
- Eng TY, Boersma MG, Fuller CD, et al. A comprehensive review of the treatment of Merkel cell carcinoma. Am J Clin Oncol. 2007;30:624-636.
- Beenken SW, Urist MM. Treatment options for Merkel cell carcinoma. J Natl Compr Canc Netw. 2004;2:89-92.
- Decker RH, Wilson LD. Role of radiotherapy in the management of Merkel cell carcinoma of the skin. J Natl Compr Canc Netw. 2006;4:713-718.
- Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the "AEIOU" features. J Am Acad Dermatol. 2008;58:375-381.
- Feng H, Shuda M, Chang Y, et al. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096-1100.
- Duncavage EJ, Zehnbauer BA, Pfeifer JD. Prevalence of Merkel cell polyomavirus in Merkel cell carcinoma. Mod Pathol. 2009;22:516-521.
- Sastre-Garau X, Peter M, Avril MF, et al. Merkel cell carcinoma of the skin: pathological and molecular evidence for a causative role of MCV in oncogenesis. J Pathol. 2009;218:48-56.
- Varga E, Kiss M, Szabó K, et al. Detection of Merkel cell polyomavirus DNA in Merkel cell carcinomas. Br J Dermatol. 2009;161:930-932.
- Wendzicki JA, Moore PS, Chang Y. Large T and small T antigens of Merkel cell carcinoma. Curr Opin Virol. 2015;11:38-43.
- Duprat JP, Landman G, Salvajoli JV, et al. A review of the epidemiology and treatment of Merkel cell carcinoma. Clinics (Sao Paulo). 2011;66:1817-1823.
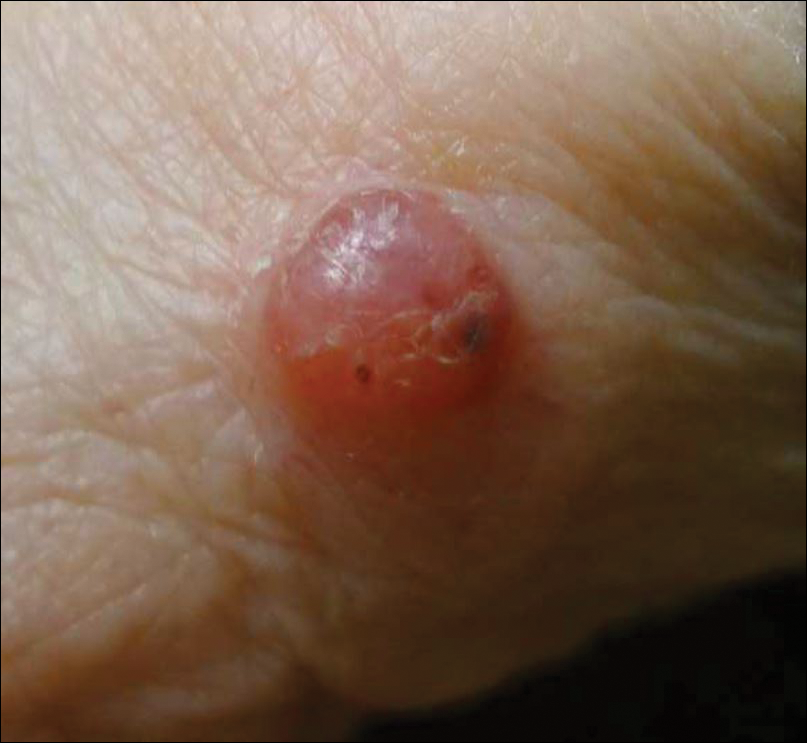
A 91-year-old white man with a history of atrial fibrillation, benign prostatic hyperplasia, dysphagia, gastroesophageal reflux disease, hypertension, hypothyroidism, osteoarthritis, and laryngeal cancer presented with an 8-mm firm, painless, pink lesion with telangiectasia on the left wrist. The lesion had been present for an unknown period of time and was asymptomatic at presentation.
Counsel fair-skinned patients on cancer prevention, says task force
The U.S. Preventive Services Task Force has recommended that clinicians counsel fair-skinned young adults, adolescents, children, and parents of young children about minimizing exposure to ultraviolet radiation to reduce their risk of skin cancer, in a draft recommendation statement that is available online.
The grade B recommendation applies to asymptomatic individuals who have fair skin, are aged 6 months to 24 years, and have no history of skin cancer; the recommendation is being issued because members of this population are at increased risk and are the subject of most of the existing research on skin cancer counseling, according to the USPSTF. The task force found a moderate net benefit when clinicians in a primary care setting offered behavioral counseling on skin cancer prevention to members of this population.
The draft recommendation is open for public comment until 8:00 p.m. Eastern Standard Time on Nov. 6, 2017.
The draft recommendation can be viewed and comments can be submitted online at the USPSTF site.
The U.S. Preventive Services Task Force has recommended that clinicians counsel fair-skinned young adults, adolescents, children, and parents of young children about minimizing exposure to ultraviolet radiation to reduce their risk of skin cancer, in a draft recommendation statement that is available online.
The grade B recommendation applies to asymptomatic individuals who have fair skin, are aged 6 months to 24 years, and have no history of skin cancer; the recommendation is being issued because members of this population are at increased risk and are the subject of most of the existing research on skin cancer counseling, according to the USPSTF. The task force found a moderate net benefit when clinicians in a primary care setting offered behavioral counseling on skin cancer prevention to members of this population.
The draft recommendation is open for public comment until 8:00 p.m. Eastern Standard Time on Nov. 6, 2017.
The draft recommendation can be viewed and comments can be submitted online at the USPSTF site.
The U.S. Preventive Services Task Force has recommended that clinicians counsel fair-skinned young adults, adolescents, children, and parents of young children about minimizing exposure to ultraviolet radiation to reduce their risk of skin cancer, in a draft recommendation statement that is available online.
The grade B recommendation applies to asymptomatic individuals who have fair skin, are aged 6 months to 24 years, and have no history of skin cancer; the recommendation is being issued because members of this population are at increased risk and are the subject of most of the existing research on skin cancer counseling, according to the USPSTF. The task force found a moderate net benefit when clinicians in a primary care setting offered behavioral counseling on skin cancer prevention to members of this population.
The draft recommendation is open for public comment until 8:00 p.m. Eastern Standard Time on Nov. 6, 2017.
The draft recommendation can be viewed and comments can be submitted online at the USPSTF site.
FROM USPSTF