User login
Multiple Primary Atypical Vascular Lesions Occurring in the Same Breast
Atypical vascular lesions (AVLs) of the breast are rare cutaneous vascular proliferations that present as erythematous, violaceous, or flesh-colored papules, patches, or plaques in women who have undergone radiation treatment for breast carcinoma.1,2 These lesions most commonly develop in the irradiated area within 3 to 6 years following radiation treatment.3
Various terms have been used to describe AVLs in the literature, including atypical hemangiomas, benign lymphangiomatous papules, benign lymphangioendotheliomas, lymphangioma circumscriptum, and acquired progressive lymphangiomas, suggesting benign behavior.4-10 However, their identity as benign lesions has been a source of controversy, with some investigators proposing that AVLs may be a precursor lesion to postirradiation angiosarcoma.2 Research has addressed if there are markers that can predict AVL types that are more likely to develop into angiosarcomas.1 Although most clinicians treat AVLs with complete excision, there currently are no specific guidelines to direct this practice.
We report the case of a patient with a history of 1 AVL that was excised who developed 3 additional AVLs in the same breast over the course of 15 months.
Case Report
A 55-year-old woman with a history of obesity, hypertension, and infiltrating ductal carcinoma in situ of the right breast (grade 2, estrogen receptor and progesterone receptor positive) underwent a right breast lumpectomy and sentinel lymph node dissection. Three months later, she underwent re-excision for positive margins and started adjuvant hormonal therapy with tamoxifen. One month later, she began external beam radiation therapy and received a total dose of 6040 cGy over the course of 9 weeks (34 total treatments).
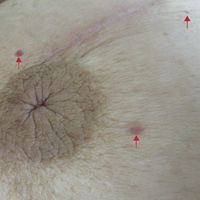
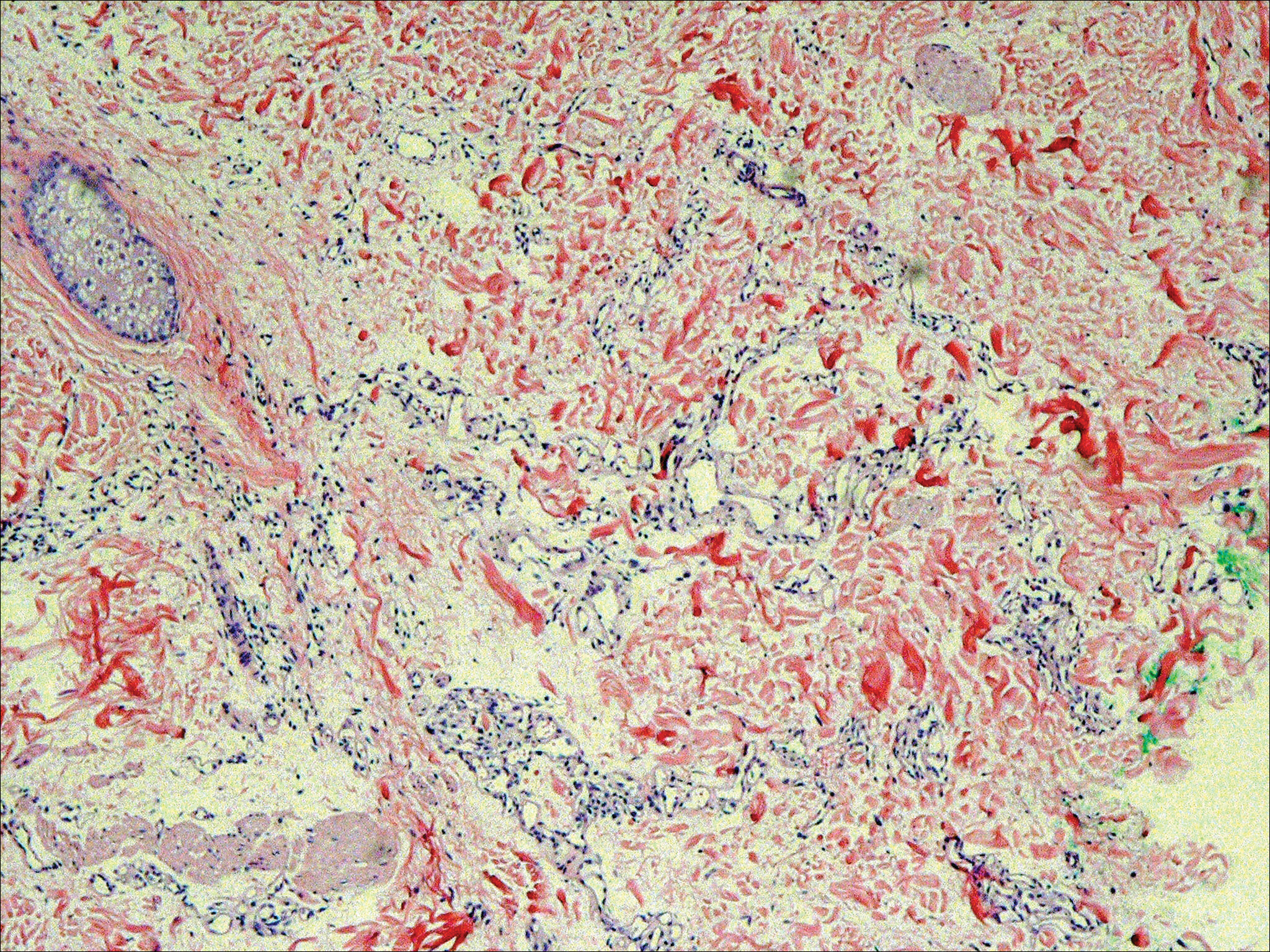
The patient presented to an outside dermatology clinic 2 years after completing external beam radiation therapy for evaluation of a new pink nodule on the right mid breast. The nodule was biopsied and discovered to be an AVL. Pathology showed an anastomosing proliferation of thin-walled vascular channels mainly located in the superficial dermis with notable endothelial nuclear atypia and hyperchromasia. There were several tiny foci with the beginnings of multilayering with prominent endothelial atypia (Figure 1). She underwent complete excision for this AVL with negative margins.
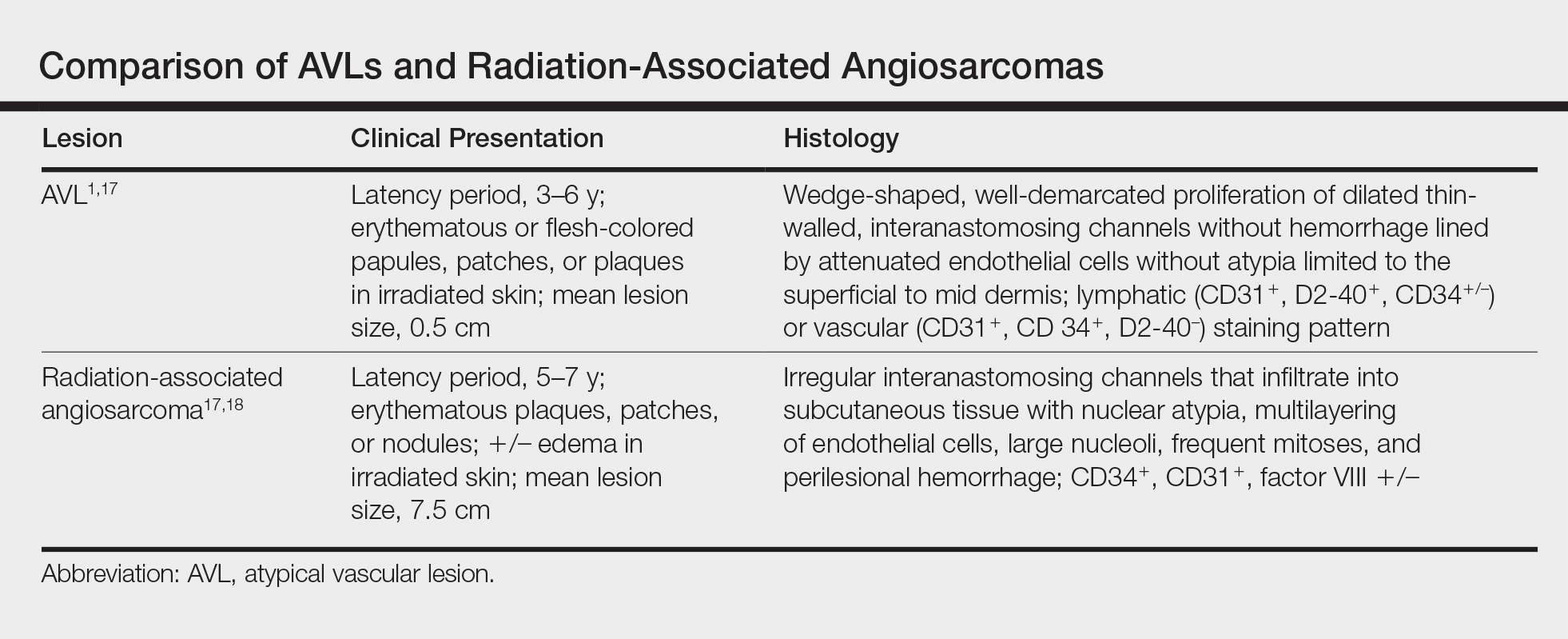
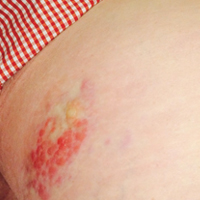
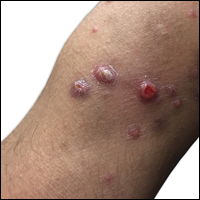
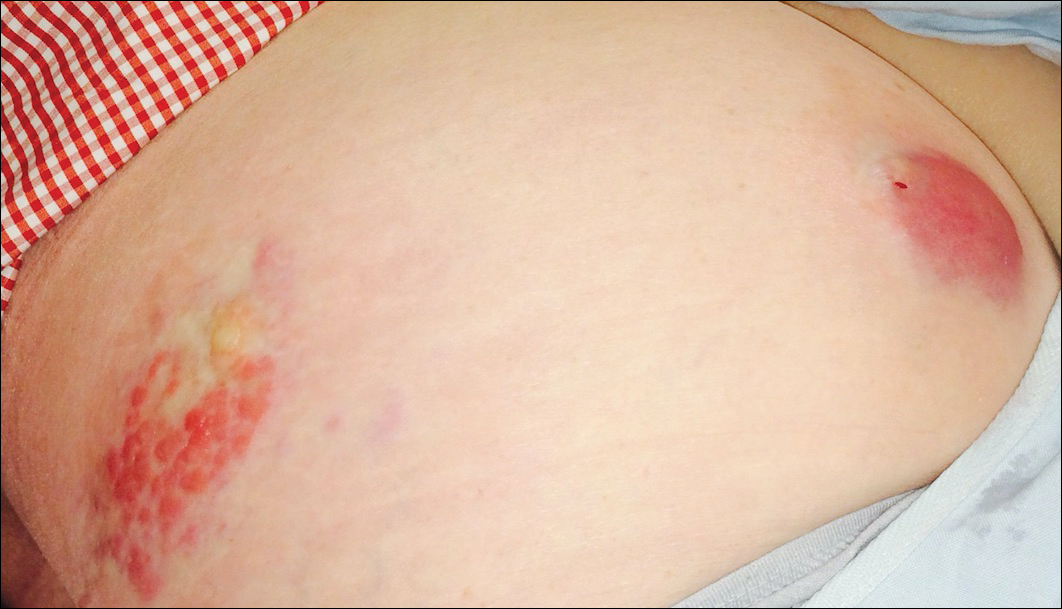
Six months after the initial AVL diagnosis, she presented to our dermatology clinic with another asymptomatic red bump on the right breast. On physical examination, a 4-mm firm, erythematous, well-circumscribed papule was noted on the medial aspect of the right breast along with a similar-appearing 4-mm papule on the right lateral aspect of the right breast (Figure 2). The patient was unsure of the duration of the second lesion but felt that it had been present at least as long as the other lesion. Both lesions clinically resembled typical capillary hemangiomas. A 6-mm punch biopsy of the right medial breast was performed and revealed enlarged vessels and capillaries in the upper dermis lined by endothelial cells with focal prominent nuclei without necrosis, overt atypia, mitosis, or tufting (Figure 3). Immunostaining was positive for CD34, factor VIII antigen, podoplanin (D2-40), and CD31, and negative for cytokeratin 7 and pankeratin. This staining was compatible with a lymphatic-type AVL.1 A diagnosis of AVL was made and complete excision with clear margins was performed. At the time of this excision, a biopsy of the right lateral breast was performed revealing thin-walled, dilated vascular channels in the superficial dermis with architecturally atypical angulated outlines, mild endothelial nuclear atypia, and hyperchromasia without endothelial multilayering. Clear margins were noted on the biopsy, but the patient subsequently declined re-excision of this third AVL.
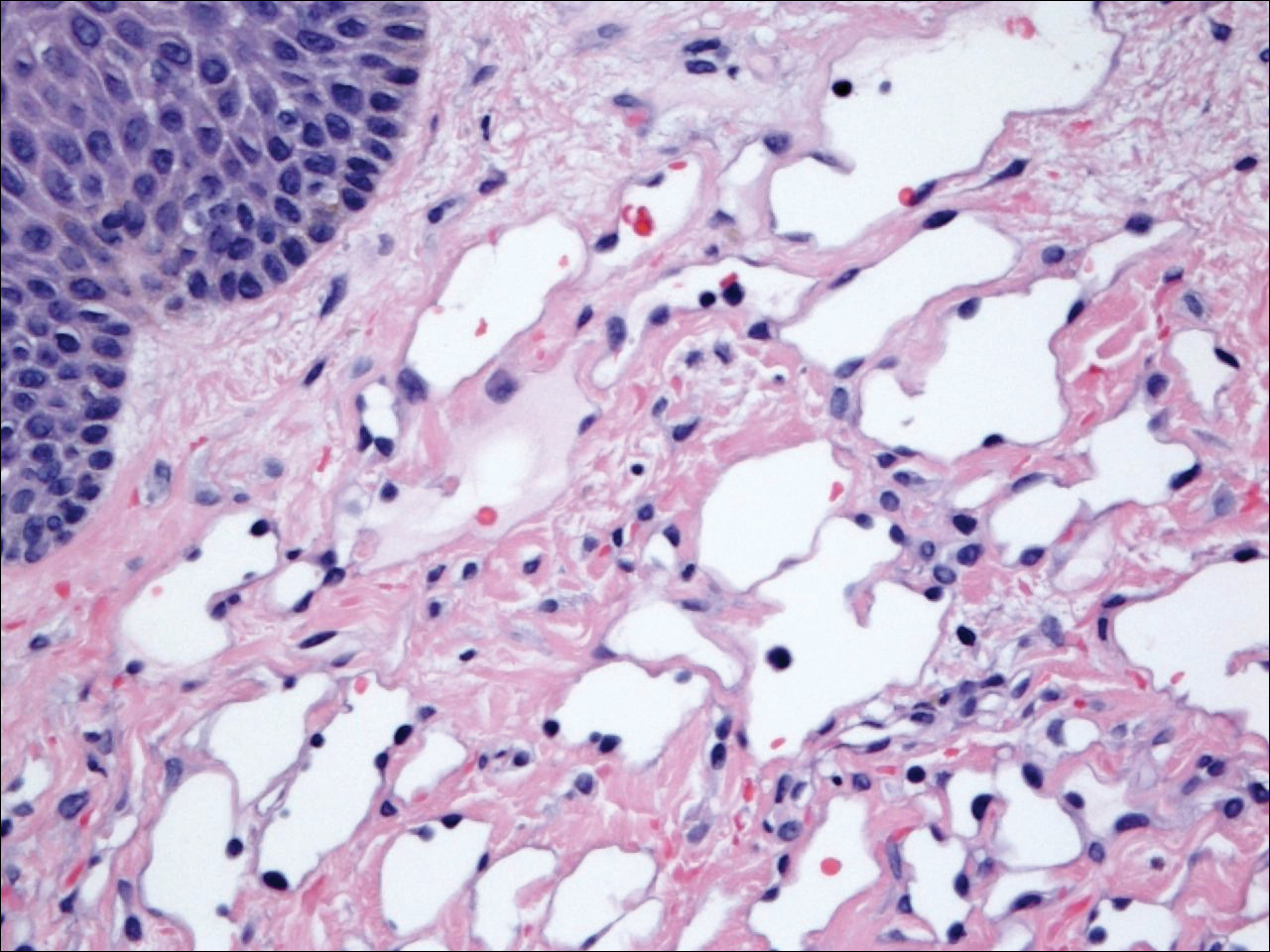
During a subsequent follow-up visit 9 months later, the patient was noted to have a 2-mm red, vascular-appearing papule on the right upper medial breast (Figure 2). A 6-mm biopsy was performed and revealed thin-walled vascular channels in the superficial dermis with endothelial nuclear atypia consistent with an AVL.
Comment
Fineberg and Rosen8 were the first to describe AVLs in their 1994 study of 4 women with cutaneous vascular proliferations that developed after radiation and chemotherapy for breast cancer. They concluded that these AVLs were benign lesions distinct from angiosarcomas.8 However, further research has challenged the benign nature of AVLs. In 2005, Brenn and Fletcher2 studied 42 women diagnosed with either angiosarcoma or atypical radiation-associated cutaneous vascular lesions. They suggested that AVLs resided on the same spectrum as angiosarcomas and that AVLs may be precursor lesions to angiosarcomas.2 Furthermore, Hildebrandt et al11 in 2001 and Di Tommaso and Fabbri12 in 2003 published case reports of individual patients who developed an angiosarcoma from a preexisting AVL.
The controversy continued when Patton et al1 published a study in 2008 in which 32 cases of AVLs were reviewed. In this study, 2 histologic types of AVLs were described: vascular type and lymphatic type. Vascular-type AVLs are characterized by irregularly dispersed, pericyte-invested, capillary-sized vessels within the papillary or reticular dermis that often are associated with extravasated erythrocytes or hemosiderin. On the other hand, lymphatic-type AVLs display thin-walled, variably anastomosing, lymphatic vessels lined by attenuated or slightly protuberant endothelial cells. These subtypes have been suggested based on the antigens known to be present in certain tissues, specifically vascular and lymphatic tissue. Despite these seemingly distinct histologies, 6 lesions classified as vascular type displayed some histologic overlap with the lymphatic-type AVLs. The authors concluded that the vascular type showed greater potential to develop into an angiosarcoma based on the degree of endothelial atypia.1
In 2011, Santi et al13 found that both AVLs and angiosarcomas share inactivation mutations in the tumor suppressor gene TP53, providing further evidence to suggest that AVLs may be precursors to angiosarcomas.
Although the malignant potential of AVLs remains questionable, research has shown that they do have a propensity to recur.3 In 2007, Gengler et al3 determined that 20% of patients with AVLs experienced recurrence after a biopsy or excision with varying margins; however, the group stated that these new vascular lesions might not be recurrences but rather entirely new lesions in the same irradiated field (field-effect phenomenon). Several other studies demonstrated that more than 30% of patients with 1 AVL developed more lesions within the same irradiated area.3,14-16 Despite the high rate of recurrence documented in the literature, only 5 of more than 100 diagnosed AVLs have progressed to angiosarcoma.1,3
Many differences can be noted when comparing the histology of AVLs versus angiosarcomas, though some are subtle (Table). Angiosarcomas display poorly circumscribed vascular infiltration into the subcutaneous tissue, multilayering of endothelial cells, prominent nucleoli, hemorrhage, mitoses, and notable aytpia. Atypical vascular lesions lack these features and tend to be wedge shaped and display chronic inflammation.8,15,17-19 Atypical vascular lesions show superficial localized growth without destruction of adjacent adnexa, display dilated vascular spaces, and exhibit large endothelial cells.5,6,8,14,15,19,20 However, there is overlap between AVLs and angiosarcomas that can make diagnosis difficult.2,14,16,17,19 Areas within or just outside of an angiosarcoma, especially in well-differentiated angiosarcomas, can appear histologically identical to AVLs, and multiple biopsies may be required for diagnosis.17,19,21
Conclusion
More research is needed in the arenas of classification, diagnosis, treatment, and follow-up recommendations for AVLs. In particular, more specific histologic markers may be needed to identify those AVLs that may progress to angiosarcomas. Although most AVLs are treated with excision, a consensus needs to be reached on adequate surgical margins. Lastly, due to the tendency of AVLs to recur coupled with their unknown malignant potential, recommendations are needed for consistent follow-up examinations.
- Patton KT, Deyrup AT, Weiss SW. Atypical vascular lesions after surgery and radiation of the breast: a clinicopathologic study of 32 cases analyzing histologic heterogeneity and association with angiosarcoma. Am J Surg Pathol. 2008;32:943-950.
- Brenn T, Fletcher CD. Radiation-associated cutaneous atypical vascular lesions and angiosarcoma: clinicopathologic analysis of 42 cases. Am J Surg Pathol. 2005;29:983-996.
- Gengler C, Coindre JM, Leroux A, et al. Vascular proliferations of the skin after radiation therapy for breast cancer: clinicopathologic analysis of a series in favor of a benign process; a study from the French sarcoma group. Cancer. 2007;109:1584-1598.
- Hoda SA, Cranor ML, Rosen PP. Hemangiomas of the breast with atypical histological features: further analysis of histological subtypes confirming their benign character. Am J Surg Pathol. 1992;16:553-560.
- Wagamon K, Ranchoff RE, Rosenberg AS, et al. Benign lymphangiomatous papules of the skin. J Am Acad Dermatol. 2005;52:912-913.
- Diaz-Cascajo C, Borghi S, Weyers W, et al. Benign lymphangiomatous papules of the skin following radiotherapy: a report of five new cases and review of the literature. Histopathology. 1999;35:319-327.
- Martín-González T, Sanz-Trelles A, Del Boz J, et al. Benign lymphangiomatous papules and plaques after radiotherapy [in Spanish]. Actas Dermosifiliogr. 2008;99:84-86.
- Fineberg S, Rosen PP. Cutaneous angiosarcoma and atypical vascular lesions of the skin and breast after radiation therapy for breast carcinoma. Am J Clin Pathol. 1994;102:757-763.
- Guillou L, Fletcher CD. Benign lymphangioendothelioma (acquired progressive lymphangioma): a lesion not to be confused with well-differentiated angiosarcoma and patch stage Kaposi’s sarcoma: clinicopathologic analysis of a series. Am J Surg Pathol. 2000;24:1047-1057.
- Rosso R, Gianelli U, Carnevali L. Acquired progressive lymphangioma of the skin following radiotherapy for breast carcinoma. J Cutan Pathol. 1995;22:164-167.
- Hildebrandt G, Mittag M, Gutz U, et al. Cutaneous breast angiosarcoma after conservative treatment of breast cancer. Eur J Dermatol. 2001;11:580-583.
- Di Tommaso L, Fabbri A. Cutaneous angiosarcoma arising after radiotherapy treatment of a breast carcinoma: description of a case and review of the literature [in Italian]. Pathologica. 2003;95:196-202.
- Santi R, Cetica V, Franchi A, et al. Tumour suppressor gene TP53 mutations in atypical vascular lesions of breast skin following radiotherapy. Histopathology. 2011;58:455-466.
- Requena L, Kutzner H, Mentzel T, et al. Benign vascular proliferations in irradiated skin. Am J Surg Pathol. 2002;26:328-337.
- Brodie C, Provenzano E. Vascular proliferations of the breast. Histopathology. 2008;52:30-44.
- Brenn T, Fletcher CD. Postradiation vascular proliferations: an increasing problem. Histopathology. 2006;48:106-114.
- Lucas DR. Angiosarcoma, radiation-associated angiosarcoma, and atypical vascular lesion. Arch Pathol Lab Med. 2009;133:1804-1809.
- Kardum-Skelin I, Jelić-Puskarić B, Pazur M, et al. A case report of breast angiosarcoma. Coll Antropol. 2010;34:645-648.
- Mattoch IW, Robbins JB, Kempson RL, et al. Post-radiotherapy vascular proliferations in mammary skin: a clinicopathologic study of 11 cases. J Am Acad Dermatol. 2007;57:126-133.
- Bodet D, Rodríguez-Cano L, Bartralot R, et al. Benign lymphangiomatous papules of the skin associated with ovarian fibroma. J Am Acad Dermatol. 2007;56(2 suppl):S41-S44.
- Losch A, Chilek KD, Zirwas MJ. Post-radiation atypical vascular proliferation mimicking angiosarcoma eight months following breast-conserving therapy for breast carcinoma. J Clin Aesthet Dermatol. 2011;4:47-48.
Atypical vascular lesions (AVLs) of the breast are rare cutaneous vascular proliferations that present as erythematous, violaceous, or flesh-colored papules, patches, or plaques in women who have undergone radiation treatment for breast carcinoma.1,2 These lesions most commonly develop in the irradiated area within 3 to 6 years following radiation treatment.3
Various terms have been used to describe AVLs in the literature, including atypical hemangiomas, benign lymphangiomatous papules, benign lymphangioendotheliomas, lymphangioma circumscriptum, and acquired progressive lymphangiomas, suggesting benign behavior.4-10 However, their identity as benign lesions has been a source of controversy, with some investigators proposing that AVLs may be a precursor lesion to postirradiation angiosarcoma.2 Research has addressed if there are markers that can predict AVL types that are more likely to develop into angiosarcomas.1 Although most clinicians treat AVLs with complete excision, there currently are no specific guidelines to direct this practice.
We report the case of a patient with a history of 1 AVL that was excised who developed 3 additional AVLs in the same breast over the course of 15 months.
Case Report
A 55-year-old woman with a history of obesity, hypertension, and infiltrating ductal carcinoma in situ of the right breast (grade 2, estrogen receptor and progesterone receptor positive) underwent a right breast lumpectomy and sentinel lymph node dissection. Three months later, she underwent re-excision for positive margins and started adjuvant hormonal therapy with tamoxifen. One month later, she began external beam radiation therapy and received a total dose of 6040 cGy over the course of 9 weeks (34 total treatments).
The patient presented to an outside dermatology clinic 2 years after completing external beam radiation therapy for evaluation of a new pink nodule on the right mid breast. The nodule was biopsied and discovered to be an AVL. Pathology showed an anastomosing proliferation of thin-walled vascular channels mainly located in the superficial dermis with notable endothelial nuclear atypia and hyperchromasia. There were several tiny foci with the beginnings of multilayering with prominent endothelial atypia (Figure 1). She underwent complete excision for this AVL with negative margins.

Six months after the initial AVL diagnosis, she presented to our dermatology clinic with another asymptomatic red bump on the right breast. On physical examination, a 4-mm firm, erythematous, well-circumscribed papule was noted on the medial aspect of the right breast along with a similar-appearing 4-mm papule on the right lateral aspect of the right breast (Figure 2). The patient was unsure of the duration of the second lesion but felt that it had been present at least as long as the other lesion. Both lesions clinically resembled typical capillary hemangiomas. A 6-mm punch biopsy of the right medial breast was performed and revealed enlarged vessels and capillaries in the upper dermis lined by endothelial cells with focal prominent nuclei without necrosis, overt atypia, mitosis, or tufting (Figure 3). Immunostaining was positive for CD34, factor VIII antigen, podoplanin (D2-40), and CD31, and negative for cytokeratin 7 and pankeratin. This staining was compatible with a lymphatic-type AVL.1 A diagnosis of AVL was made and complete excision with clear margins was performed. At the time of this excision, a biopsy of the right lateral breast was performed revealing thin-walled, dilated vascular channels in the superficial dermis with architecturally atypical angulated outlines, mild endothelial nuclear atypia, and hyperchromasia without endothelial multilayering. Clear margins were noted on the biopsy, but the patient subsequently declined re-excision of this third AVL.


During a subsequent follow-up visit 9 months later, the patient was noted to have a 2-mm red, vascular-appearing papule on the right upper medial breast (Figure 2). A 6-mm biopsy was performed and revealed thin-walled vascular channels in the superficial dermis with endothelial nuclear atypia consistent with an AVL.
Comment
Fineberg and Rosen8 were the first to describe AVLs in their 1994 study of 4 women with cutaneous vascular proliferations that developed after radiation and chemotherapy for breast cancer. They concluded that these AVLs were benign lesions distinct from angiosarcomas.8 However, further research has challenged the benign nature of AVLs. In 2005, Brenn and Fletcher2 studied 42 women diagnosed with either angiosarcoma or atypical radiation-associated cutaneous vascular lesions. They suggested that AVLs resided on the same spectrum as angiosarcomas and that AVLs may be precursor lesions to angiosarcomas.2 Furthermore, Hildebrandt et al11 in 2001 and Di Tommaso and Fabbri12 in 2003 published case reports of individual patients who developed an angiosarcoma from a preexisting AVL.
The controversy continued when Patton et al1 published a study in 2008 in which 32 cases of AVLs were reviewed. In this study, 2 histologic types of AVLs were described: vascular type and lymphatic type. Vascular-type AVLs are characterized by irregularly dispersed, pericyte-invested, capillary-sized vessels within the papillary or reticular dermis that often are associated with extravasated erythrocytes or hemosiderin. On the other hand, lymphatic-type AVLs display thin-walled, variably anastomosing, lymphatic vessels lined by attenuated or slightly protuberant endothelial cells. These subtypes have been suggested based on the antigens known to be present in certain tissues, specifically vascular and lymphatic tissue. Despite these seemingly distinct histologies, 6 lesions classified as vascular type displayed some histologic overlap with the lymphatic-type AVLs. The authors concluded that the vascular type showed greater potential to develop into an angiosarcoma based on the degree of endothelial atypia.1
In 2011, Santi et al13 found that both AVLs and angiosarcomas share inactivation mutations in the tumor suppressor gene TP53, providing further evidence to suggest that AVLs may be precursors to angiosarcomas.
Although the malignant potential of AVLs remains questionable, research has shown that they do have a propensity to recur.3 In 2007, Gengler et al3 determined that 20% of patients with AVLs experienced recurrence after a biopsy or excision with varying margins; however, the group stated that these new vascular lesions might not be recurrences but rather entirely new lesions in the same irradiated field (field-effect phenomenon). Several other studies demonstrated that more than 30% of patients with 1 AVL developed more lesions within the same irradiated area.3,14-16 Despite the high rate of recurrence documented in the literature, only 5 of more than 100 diagnosed AVLs have progressed to angiosarcoma.1,3
Many differences can be noted when comparing the histology of AVLs versus angiosarcomas, though some are subtle (Table). Angiosarcomas display poorly circumscribed vascular infiltration into the subcutaneous tissue, multilayering of endothelial cells, prominent nucleoli, hemorrhage, mitoses, and notable aytpia. Atypical vascular lesions lack these features and tend to be wedge shaped and display chronic inflammation.8,15,17-19 Atypical vascular lesions show superficial localized growth without destruction of adjacent adnexa, display dilated vascular spaces, and exhibit large endothelial cells.5,6,8,14,15,19,20 However, there is overlap between AVLs and angiosarcomas that can make diagnosis difficult.2,14,16,17,19 Areas within or just outside of an angiosarcoma, especially in well-differentiated angiosarcomas, can appear histologically identical to AVLs, and multiple biopsies may be required for diagnosis.17,19,21
Conclusion
More research is needed in the arenas of classification, diagnosis, treatment, and follow-up recommendations for AVLs. In particular, more specific histologic markers may be needed to identify those AVLs that may progress to angiosarcomas. Although most AVLs are treated with excision, a consensus needs to be reached on adequate surgical margins. Lastly, due to the tendency of AVLs to recur coupled with their unknown malignant potential, recommendations are needed for consistent follow-up examinations.
Atypical vascular lesions (AVLs) of the breast are rare cutaneous vascular proliferations that present as erythematous, violaceous, or flesh-colored papules, patches, or plaques in women who have undergone radiation treatment for breast carcinoma.1,2 These lesions most commonly develop in the irradiated area within 3 to 6 years following radiation treatment.3
Various terms have been used to describe AVLs in the literature, including atypical hemangiomas, benign lymphangiomatous papules, benign lymphangioendotheliomas, lymphangioma circumscriptum, and acquired progressive lymphangiomas, suggesting benign behavior.4-10 However, their identity as benign lesions has been a source of controversy, with some investigators proposing that AVLs may be a precursor lesion to postirradiation angiosarcoma.2 Research has addressed if there are markers that can predict AVL types that are more likely to develop into angiosarcomas.1 Although most clinicians treat AVLs with complete excision, there currently are no specific guidelines to direct this practice.
We report the case of a patient with a history of 1 AVL that was excised who developed 3 additional AVLs in the same breast over the course of 15 months.
Case Report
A 55-year-old woman with a history of obesity, hypertension, and infiltrating ductal carcinoma in situ of the right breast (grade 2, estrogen receptor and progesterone receptor positive) underwent a right breast lumpectomy and sentinel lymph node dissection. Three months later, she underwent re-excision for positive margins and started adjuvant hormonal therapy with tamoxifen. One month later, she began external beam radiation therapy and received a total dose of 6040 cGy over the course of 9 weeks (34 total treatments).
The patient presented to an outside dermatology clinic 2 years after completing external beam radiation therapy for evaluation of a new pink nodule on the right mid breast. The nodule was biopsied and discovered to be an AVL. Pathology showed an anastomosing proliferation of thin-walled vascular channels mainly located in the superficial dermis with notable endothelial nuclear atypia and hyperchromasia. There were several tiny foci with the beginnings of multilayering with prominent endothelial atypia (Figure 1). She underwent complete excision for this AVL with negative margins.

Six months after the initial AVL diagnosis, she presented to our dermatology clinic with another asymptomatic red bump on the right breast. On physical examination, a 4-mm firm, erythematous, well-circumscribed papule was noted on the medial aspect of the right breast along with a similar-appearing 4-mm papule on the right lateral aspect of the right breast (Figure 2). The patient was unsure of the duration of the second lesion but felt that it had been present at least as long as the other lesion. Both lesions clinically resembled typical capillary hemangiomas. A 6-mm punch biopsy of the right medial breast was performed and revealed enlarged vessels and capillaries in the upper dermis lined by endothelial cells with focal prominent nuclei without necrosis, overt atypia, mitosis, or tufting (Figure 3). Immunostaining was positive for CD34, factor VIII antigen, podoplanin (D2-40), and CD31, and negative for cytokeratin 7 and pankeratin. This staining was compatible with a lymphatic-type AVL.1 A diagnosis of AVL was made and complete excision with clear margins was performed. At the time of this excision, a biopsy of the right lateral breast was performed revealing thin-walled, dilated vascular channels in the superficial dermis with architecturally atypical angulated outlines, mild endothelial nuclear atypia, and hyperchromasia without endothelial multilayering. Clear margins were noted on the biopsy, but the patient subsequently declined re-excision of this third AVL.


During a subsequent follow-up visit 9 months later, the patient was noted to have a 2-mm red, vascular-appearing papule on the right upper medial breast (Figure 2). A 6-mm biopsy was performed and revealed thin-walled vascular channels in the superficial dermis with endothelial nuclear atypia consistent with an AVL.
Comment
Fineberg and Rosen8 were the first to describe AVLs in their 1994 study of 4 women with cutaneous vascular proliferations that developed after radiation and chemotherapy for breast cancer. They concluded that these AVLs were benign lesions distinct from angiosarcomas.8 However, further research has challenged the benign nature of AVLs. In 2005, Brenn and Fletcher2 studied 42 women diagnosed with either angiosarcoma or atypical radiation-associated cutaneous vascular lesions. They suggested that AVLs resided on the same spectrum as angiosarcomas and that AVLs may be precursor lesions to angiosarcomas.2 Furthermore, Hildebrandt et al11 in 2001 and Di Tommaso and Fabbri12 in 2003 published case reports of individual patients who developed an angiosarcoma from a preexisting AVL.
The controversy continued when Patton et al1 published a study in 2008 in which 32 cases of AVLs were reviewed. In this study, 2 histologic types of AVLs were described: vascular type and lymphatic type. Vascular-type AVLs are characterized by irregularly dispersed, pericyte-invested, capillary-sized vessels within the papillary or reticular dermis that often are associated with extravasated erythrocytes or hemosiderin. On the other hand, lymphatic-type AVLs display thin-walled, variably anastomosing, lymphatic vessels lined by attenuated or slightly protuberant endothelial cells. These subtypes have been suggested based on the antigens known to be present in certain tissues, specifically vascular and lymphatic tissue. Despite these seemingly distinct histologies, 6 lesions classified as vascular type displayed some histologic overlap with the lymphatic-type AVLs. The authors concluded that the vascular type showed greater potential to develop into an angiosarcoma based on the degree of endothelial atypia.1
In 2011, Santi et al13 found that both AVLs and angiosarcomas share inactivation mutations in the tumor suppressor gene TP53, providing further evidence to suggest that AVLs may be precursors to angiosarcomas.
Although the malignant potential of AVLs remains questionable, research has shown that they do have a propensity to recur.3 In 2007, Gengler et al3 determined that 20% of patients with AVLs experienced recurrence after a biopsy or excision with varying margins; however, the group stated that these new vascular lesions might not be recurrences but rather entirely new lesions in the same irradiated field (field-effect phenomenon). Several other studies demonstrated that more than 30% of patients with 1 AVL developed more lesions within the same irradiated area.3,14-16 Despite the high rate of recurrence documented in the literature, only 5 of more than 100 diagnosed AVLs have progressed to angiosarcoma.1,3
Many differences can be noted when comparing the histology of AVLs versus angiosarcomas, though some are subtle (Table). Angiosarcomas display poorly circumscribed vascular infiltration into the subcutaneous tissue, multilayering of endothelial cells, prominent nucleoli, hemorrhage, mitoses, and notable aytpia. Atypical vascular lesions lack these features and tend to be wedge shaped and display chronic inflammation.8,15,17-19 Atypical vascular lesions show superficial localized growth without destruction of adjacent adnexa, display dilated vascular spaces, and exhibit large endothelial cells.5,6,8,14,15,19,20 However, there is overlap between AVLs and angiosarcomas that can make diagnosis difficult.2,14,16,17,19 Areas within or just outside of an angiosarcoma, especially in well-differentiated angiosarcomas, can appear histologically identical to AVLs, and multiple biopsies may be required for diagnosis.17,19,21
Conclusion
More research is needed in the arenas of classification, diagnosis, treatment, and follow-up recommendations for AVLs. In particular, more specific histologic markers may be needed to identify those AVLs that may progress to angiosarcomas. Although most AVLs are treated with excision, a consensus needs to be reached on adequate surgical margins. Lastly, due to the tendency of AVLs to recur coupled with their unknown malignant potential, recommendations are needed for consistent follow-up examinations.
- Patton KT, Deyrup AT, Weiss SW. Atypical vascular lesions after surgery and radiation of the breast: a clinicopathologic study of 32 cases analyzing histologic heterogeneity and association with angiosarcoma. Am J Surg Pathol. 2008;32:943-950.
- Brenn T, Fletcher CD. Radiation-associated cutaneous atypical vascular lesions and angiosarcoma: clinicopathologic analysis of 42 cases. Am J Surg Pathol. 2005;29:983-996.
- Gengler C, Coindre JM, Leroux A, et al. Vascular proliferations of the skin after radiation therapy for breast cancer: clinicopathologic analysis of a series in favor of a benign process; a study from the French sarcoma group. Cancer. 2007;109:1584-1598.
- Hoda SA, Cranor ML, Rosen PP. Hemangiomas of the breast with atypical histological features: further analysis of histological subtypes confirming their benign character. Am J Surg Pathol. 1992;16:553-560.
- Wagamon K, Ranchoff RE, Rosenberg AS, et al. Benign lymphangiomatous papules of the skin. J Am Acad Dermatol. 2005;52:912-913.
- Diaz-Cascajo C, Borghi S, Weyers W, et al. Benign lymphangiomatous papules of the skin following radiotherapy: a report of five new cases and review of the literature. Histopathology. 1999;35:319-327.
- Martín-González T, Sanz-Trelles A, Del Boz J, et al. Benign lymphangiomatous papules and plaques after radiotherapy [in Spanish]. Actas Dermosifiliogr. 2008;99:84-86.
- Fineberg S, Rosen PP. Cutaneous angiosarcoma and atypical vascular lesions of the skin and breast after radiation therapy for breast carcinoma. Am J Clin Pathol. 1994;102:757-763.
- Guillou L, Fletcher CD. Benign lymphangioendothelioma (acquired progressive lymphangioma): a lesion not to be confused with well-differentiated angiosarcoma and patch stage Kaposi’s sarcoma: clinicopathologic analysis of a series. Am J Surg Pathol. 2000;24:1047-1057.
- Rosso R, Gianelli U, Carnevali L. Acquired progressive lymphangioma of the skin following radiotherapy for breast carcinoma. J Cutan Pathol. 1995;22:164-167.
- Hildebrandt G, Mittag M, Gutz U, et al. Cutaneous breast angiosarcoma after conservative treatment of breast cancer. Eur J Dermatol. 2001;11:580-583.
- Di Tommaso L, Fabbri A. Cutaneous angiosarcoma arising after radiotherapy treatment of a breast carcinoma: description of a case and review of the literature [in Italian]. Pathologica. 2003;95:196-202.
- Santi R, Cetica V, Franchi A, et al. Tumour suppressor gene TP53 mutations in atypical vascular lesions of breast skin following radiotherapy. Histopathology. 2011;58:455-466.
- Requena L, Kutzner H, Mentzel T, et al. Benign vascular proliferations in irradiated skin. Am J Surg Pathol. 2002;26:328-337.
- Brodie C, Provenzano E. Vascular proliferations of the breast. Histopathology. 2008;52:30-44.
- Brenn T, Fletcher CD. Postradiation vascular proliferations: an increasing problem. Histopathology. 2006;48:106-114.
- Lucas DR. Angiosarcoma, radiation-associated angiosarcoma, and atypical vascular lesion. Arch Pathol Lab Med. 2009;133:1804-1809.
- Kardum-Skelin I, Jelić-Puskarić B, Pazur M, et al. A case report of breast angiosarcoma. Coll Antropol. 2010;34:645-648.
- Mattoch IW, Robbins JB, Kempson RL, et al. Post-radiotherapy vascular proliferations in mammary skin: a clinicopathologic study of 11 cases. J Am Acad Dermatol. 2007;57:126-133.
- Bodet D, Rodríguez-Cano L, Bartralot R, et al. Benign lymphangiomatous papules of the skin associated with ovarian fibroma. J Am Acad Dermatol. 2007;56(2 suppl):S41-S44.
- Losch A, Chilek KD, Zirwas MJ. Post-radiation atypical vascular proliferation mimicking angiosarcoma eight months following breast-conserving therapy for breast carcinoma. J Clin Aesthet Dermatol. 2011;4:47-48.
- Patton KT, Deyrup AT, Weiss SW. Atypical vascular lesions after surgery and radiation of the breast: a clinicopathologic study of 32 cases analyzing histologic heterogeneity and association with angiosarcoma. Am J Surg Pathol. 2008;32:943-950.
- Brenn T, Fletcher CD. Radiation-associated cutaneous atypical vascular lesions and angiosarcoma: clinicopathologic analysis of 42 cases. Am J Surg Pathol. 2005;29:983-996.
- Gengler C, Coindre JM, Leroux A, et al. Vascular proliferations of the skin after radiation therapy for breast cancer: clinicopathologic analysis of a series in favor of a benign process; a study from the French sarcoma group. Cancer. 2007;109:1584-1598.
- Hoda SA, Cranor ML, Rosen PP. Hemangiomas of the breast with atypical histological features: further analysis of histological subtypes confirming their benign character. Am J Surg Pathol. 1992;16:553-560.
- Wagamon K, Ranchoff RE, Rosenberg AS, et al. Benign lymphangiomatous papules of the skin. J Am Acad Dermatol. 2005;52:912-913.
- Diaz-Cascajo C, Borghi S, Weyers W, et al. Benign lymphangiomatous papules of the skin following radiotherapy: a report of five new cases and review of the literature. Histopathology. 1999;35:319-327.
- Martín-González T, Sanz-Trelles A, Del Boz J, et al. Benign lymphangiomatous papules and plaques after radiotherapy [in Spanish]. Actas Dermosifiliogr. 2008;99:84-86.
- Fineberg S, Rosen PP. Cutaneous angiosarcoma and atypical vascular lesions of the skin and breast after radiation therapy for breast carcinoma. Am J Clin Pathol. 1994;102:757-763.
- Guillou L, Fletcher CD. Benign lymphangioendothelioma (acquired progressive lymphangioma): a lesion not to be confused with well-differentiated angiosarcoma and patch stage Kaposi’s sarcoma: clinicopathologic analysis of a series. Am J Surg Pathol. 2000;24:1047-1057.
- Rosso R, Gianelli U, Carnevali L. Acquired progressive lymphangioma of the skin following radiotherapy for breast carcinoma. J Cutan Pathol. 1995;22:164-167.
- Hildebrandt G, Mittag M, Gutz U, et al. Cutaneous breast angiosarcoma after conservative treatment of breast cancer. Eur J Dermatol. 2001;11:580-583.
- Di Tommaso L, Fabbri A. Cutaneous angiosarcoma arising after radiotherapy treatment of a breast carcinoma: description of a case and review of the literature [in Italian]. Pathologica. 2003;95:196-202.
- Santi R, Cetica V, Franchi A, et al. Tumour suppressor gene TP53 mutations in atypical vascular lesions of breast skin following radiotherapy. Histopathology. 2011;58:455-466.
- Requena L, Kutzner H, Mentzel T, et al. Benign vascular proliferations in irradiated skin. Am J Surg Pathol. 2002;26:328-337.
- Brodie C, Provenzano E. Vascular proliferations of the breast. Histopathology. 2008;52:30-44.
- Brenn T, Fletcher CD. Postradiation vascular proliferations: an increasing problem. Histopathology. 2006;48:106-114.
- Lucas DR. Angiosarcoma, radiation-associated angiosarcoma, and atypical vascular lesion. Arch Pathol Lab Med. 2009;133:1804-1809.
- Kardum-Skelin I, Jelić-Puskarić B, Pazur M, et al. A case report of breast angiosarcoma. Coll Antropol. 2010;34:645-648.
- Mattoch IW, Robbins JB, Kempson RL, et al. Post-radiotherapy vascular proliferations in mammary skin: a clinicopathologic study of 11 cases. J Am Acad Dermatol. 2007;57:126-133.
- Bodet D, Rodríguez-Cano L, Bartralot R, et al. Benign lymphangiomatous papules of the skin associated with ovarian fibroma. J Am Acad Dermatol. 2007;56(2 suppl):S41-S44.
- Losch A, Chilek KD, Zirwas MJ. Post-radiation atypical vascular proliferation mimicking angiosarcoma eight months following breast-conserving therapy for breast carcinoma. J Clin Aesthet Dermatol. 2011;4:47-48.
Practice Points
- Atypical vascular lesions (AVLs) of the breast can appear an average of 5 years following radiation therapy.
- Although the malignant potential of AVLs remains debatable, excision generally is recommended, as lesions tend to recur.
Paraneoplastic Acrokeratosis Bazex Syndrome: Unusual Association With In Situ Follicular Lymphoma and Response to Acitretin
To the Editor:
Paraneoplastic acrokeratosis (PA), also known as Bazex syndrome, is a rare paraneoplastic dermatosis first described in 1965 by Bazex et al.1 This entity is clinically characterized by dusky erythematous to violaceous keratoderma of the acral sites and commonly affects men older than 40 years. In most reported cases, there has been an underlying primary malignant neoplasm of the upper aerodigestive tract2; however, some other associated malignancies also have been reported. Skin changes tend to occur before the diagnosis of the associated tumor in 67% of cases. The cutaneous lesions usually resolve after successful treatment of the tumor and relapse in case of recurrence of the malignancy.3
A 53-year-old woman who was a smoker with no relevant medical background was referred to the dermatology department with an itching psoriasiform dermatitis on the palms and soles of 2 months' duration. There were no signs of systemic disease. Physical examination revealed well-demarcated, dusky red, thick, scaly plaques on the soles with sparing of the insteps (Figure, A). Scattered symmetric hyperkeratotic plaques were present on the palms (Figure, B). We also detected onychodystrophy on the hands. Other dermatologic findings were normal. Histologic examination of a biopsy specimen of the left sole showed hyperkeratosis, focal parakeratosis, acanthosis, hypergranulosis, and a predominantly perivascular dermal lymphocytic infiltrate.

With the diagnostic suspicion of PA, blood tests, chest radiograph, and colonoscopy were performed without revealing abnormalities. Positron emission tomography and computed tomography also was performed, showing cervical, mesenteric, retroperitoneal, and inguinal adenopathies. Histologic examination of both inguinal adenectomy and cervical lymph node biopsy revealed Bcl-2-positive in situ follicular lymphoma (ISFL). Examination of an iliac crest marrow aspirate showed minimal involvement of lymphoma (10%). Follow-up imaging performed 4 months after diagnosis showed no changes. The patient was diagnosed with a low-grade chronic lymphoproliferative disorder with histologic findings consistent with ISFL presenting with small disperse adenopathies and minimal bone marrow involvement. The hematology department opted for a wait-and-see approach with 6-month follow-up imaging.
The skin lesions were first treated with salicylic acid cream 10%, psoralen plus UVA therapy, and methotrexate 20 mg weekly for 2 months without remission. Replacing the other therapies, we initiated acitretin 25 mg daily, achieving sustained remission after 6 months of treatment, and then continued with a scaled dose reduction. The patient remained lesion free 1 year after starting the treatment, with a daily dose of 10 mg of acitretin.
Paraneoplastic acrokeratosis has been traditionally described as a paraneoplastic entity mainly associated with primary squamous cell carcinoma (SCC) of the upper aerodigestive tract or a metastatic SCC of the cervical lymph nodes with an unknown origin.4,5 However, uncommon associations such as adenocarcinoma of the prostate, lung, esophagus, stomach, and colon; transitional cell carcinoma of the bladder; small cell carcinoma of the lung; cutaneous SCC; breast cancer; metastatic thymic carcinoma; metastatic neuroendocrine tumor; bronchial carcinoid tumor; SCC of the vulvar region; simultaneous multiple genitourinary tumors; and liposarcoma also have been described.6 Regarding the association with lymphoma, PA has been reported with peripheral T-cell lymphoma7 and Hodgkin disease8; however, ISFL underlying PA is rare.
Follicular lymphoma is the second most common non-Hodgkin lymphoma in Western countries and comprises approximately 20% of all lymphomas.9 It is slightly more prevalent in females, and the majority of patients present with advanced-stage disease. Generally considered to be an incurable disease, a watchful-waiting approach of conservative management has been advocated in most cases, deferring treatment until symptoms appear.9
Histology of PA is nonspecific, as in our case. However, it facilitates a differential diagnosis of major dermatoses including psoriasis vulgaris, pityriasis rubra pilaris, and lupus erythematosus.
Paraneoplastic palmoplantar keratoderma also is characteristic of Howel-Evans syndrome, which is a rare inherited condition associated with esophageal cancer. In contrast to our case, palmoplantar keratoderma in these patients usually begins around 10 years of age, is caused by a mutation in the RHBDF2 gene, and is inherited in an autosomal pattern.10
The diagnosis in our case was supported by a typical clinical picture, nonspecific histology, and the concurrent finding of the underlying lymphoma. Treatment of PA must focus on the removal of the underlying malignancy, which implies the remission of the cutaneous lesions. Taking into account that a recurrence of the primary tumor leads to a relapse of skin manifestations while distant metastases do not cause a reappearance of PA, it could be suggested that pathogenetically relevant factors are produced by the primary tumor and by lymph node metastases but not by metastases elsewhere.
In this case, due to the wait-and-see approach, a specific treatment for the skin lesions was established. Although management of the skin itself generally is ineffective, there are isolated reports of response after corticosteroids, antibiotics, antimycotics, keratolytic measures, or psoralen plus UVA therapy.6 Wishart11 used etretinate to achieve an improvement of PA. We also achieved good response with acitretin. Retinoids are known to have antineoplastic activity, which may have been helpful in both the patient we presented and the one reported by Wishart.11 In summary, we propose adding ISFL to the expanding list of malignant neoplasms associated with PA, noting the response of skin lesions after acitretin.
- Bazex A, Salvador R, Dupré A, et al. Syndrome paranéoplasique à type d'hyperkératose des extremités. Guérison après le traitement de l'épithelioma laryngé. Bull Soc Fr Dermatol Syphiligr. 1965;72:182.
- Bazex A, Griffiths A. Acrokeratosis paraneoplasticae--a new cutaneous marker of malignancy. Br J Dermatol. 1980;103:301-306.
- Bolognia JL. Bazex syndrome: acrokeratosis paraneoplastica. Semin Dermatol. 1995;14:84-89.
- Witkowski JA, Parish LC. Bazex's syndrome. Paraneoplastic acrokeratosis. JAMA. 1982;248:2883-2884.
- Bolognia JL. Bazex's syndrome. Clin Dermatol. 1993;11:37-42.
- Sator PG, Breier F, Gschnait F. Acrokeratosis paraneoplastica (Bazex's syndrome): association with liposarcoma [published online August 28, 2006]. J Am Acad Dermatol. 2006;55:1103-1105.
- Lin YC, Chu CY, Chiu HC. Acrokeratosis paraneoplastica Bazex's syndrome: unusual association with a peripheral T-cell lymphoma. Acta Derm Venereol. 2001;81:440-441.
- Lucker GP, Steijlen PM. Acrokeratosis paraneoplastica (Bazex syndrome) occurring with acquired ichthyosis in Hodgkin's disease. Br J Dermatol. 1995;133:322-325.
- Jegalian AG, Eberle FC, Pack SD, et al. Follicular lymphoma in situ: clinical implications and comparisons with partial involvement by follicular lymphoma. Blood. 2011;118:2976-2984.
- Sroa N, Witman P. Howel-Evans syndrome: a variant of ectodermal dysplasia. Cutis. 2010;85:183-185.
- Wishart JM. Bazex paraneoplastic acrokeratosis: a case report and response to Tigason. Br J Dermatol. 1986;115:595-599.
To the Editor:
Paraneoplastic acrokeratosis (PA), also known as Bazex syndrome, is a rare paraneoplastic dermatosis first described in 1965 by Bazex et al.1 This entity is clinically characterized by dusky erythematous to violaceous keratoderma of the acral sites and commonly affects men older than 40 years. In most reported cases, there has been an underlying primary malignant neoplasm of the upper aerodigestive tract2; however, some other associated malignancies also have been reported. Skin changes tend to occur before the diagnosis of the associated tumor in 67% of cases. The cutaneous lesions usually resolve after successful treatment of the tumor and relapse in case of recurrence of the malignancy.3
A 53-year-old woman who was a smoker with no relevant medical background was referred to the dermatology department with an itching psoriasiform dermatitis on the palms and soles of 2 months' duration. There were no signs of systemic disease. Physical examination revealed well-demarcated, dusky red, thick, scaly plaques on the soles with sparing of the insteps (Figure, A). Scattered symmetric hyperkeratotic plaques were present on the palms (Figure, B). We also detected onychodystrophy on the hands. Other dermatologic findings were normal. Histologic examination of a biopsy specimen of the left sole showed hyperkeratosis, focal parakeratosis, acanthosis, hypergranulosis, and a predominantly perivascular dermal lymphocytic infiltrate.

With the diagnostic suspicion of PA, blood tests, chest radiograph, and colonoscopy were performed without revealing abnormalities. Positron emission tomography and computed tomography also was performed, showing cervical, mesenteric, retroperitoneal, and inguinal adenopathies. Histologic examination of both inguinal adenectomy and cervical lymph node biopsy revealed Bcl-2-positive in situ follicular lymphoma (ISFL). Examination of an iliac crest marrow aspirate showed minimal involvement of lymphoma (10%). Follow-up imaging performed 4 months after diagnosis showed no changes. The patient was diagnosed with a low-grade chronic lymphoproliferative disorder with histologic findings consistent with ISFL presenting with small disperse adenopathies and minimal bone marrow involvement. The hematology department opted for a wait-and-see approach with 6-month follow-up imaging.
The skin lesions were first treated with salicylic acid cream 10%, psoralen plus UVA therapy, and methotrexate 20 mg weekly for 2 months without remission. Replacing the other therapies, we initiated acitretin 25 mg daily, achieving sustained remission after 6 months of treatment, and then continued with a scaled dose reduction. The patient remained lesion free 1 year after starting the treatment, with a daily dose of 10 mg of acitretin.
Paraneoplastic acrokeratosis has been traditionally described as a paraneoplastic entity mainly associated with primary squamous cell carcinoma (SCC) of the upper aerodigestive tract or a metastatic SCC of the cervical lymph nodes with an unknown origin.4,5 However, uncommon associations such as adenocarcinoma of the prostate, lung, esophagus, stomach, and colon; transitional cell carcinoma of the bladder; small cell carcinoma of the lung; cutaneous SCC; breast cancer; metastatic thymic carcinoma; metastatic neuroendocrine tumor; bronchial carcinoid tumor; SCC of the vulvar region; simultaneous multiple genitourinary tumors; and liposarcoma also have been described.6 Regarding the association with lymphoma, PA has been reported with peripheral T-cell lymphoma7 and Hodgkin disease8; however, ISFL underlying PA is rare.
Follicular lymphoma is the second most common non-Hodgkin lymphoma in Western countries and comprises approximately 20% of all lymphomas.9 It is slightly more prevalent in females, and the majority of patients present with advanced-stage disease. Generally considered to be an incurable disease, a watchful-waiting approach of conservative management has been advocated in most cases, deferring treatment until symptoms appear.9
Histology of PA is nonspecific, as in our case. However, it facilitates a differential diagnosis of major dermatoses including psoriasis vulgaris, pityriasis rubra pilaris, and lupus erythematosus.
Paraneoplastic palmoplantar keratoderma also is characteristic of Howel-Evans syndrome, which is a rare inherited condition associated with esophageal cancer. In contrast to our case, palmoplantar keratoderma in these patients usually begins around 10 years of age, is caused by a mutation in the RHBDF2 gene, and is inherited in an autosomal pattern.10
The diagnosis in our case was supported by a typical clinical picture, nonspecific histology, and the concurrent finding of the underlying lymphoma. Treatment of PA must focus on the removal of the underlying malignancy, which implies the remission of the cutaneous lesions. Taking into account that a recurrence of the primary tumor leads to a relapse of skin manifestations while distant metastases do not cause a reappearance of PA, it could be suggested that pathogenetically relevant factors are produced by the primary tumor and by lymph node metastases but not by metastases elsewhere.
In this case, due to the wait-and-see approach, a specific treatment for the skin lesions was established. Although management of the skin itself generally is ineffective, there are isolated reports of response after corticosteroids, antibiotics, antimycotics, keratolytic measures, or psoralen plus UVA therapy.6 Wishart11 used etretinate to achieve an improvement of PA. We also achieved good response with acitretin. Retinoids are known to have antineoplastic activity, which may have been helpful in both the patient we presented and the one reported by Wishart.11 In summary, we propose adding ISFL to the expanding list of malignant neoplasms associated with PA, noting the response of skin lesions after acitretin.
To the Editor:
Paraneoplastic acrokeratosis (PA), also known as Bazex syndrome, is a rare paraneoplastic dermatosis first described in 1965 by Bazex et al.1 This entity is clinically characterized by dusky erythematous to violaceous keratoderma of the acral sites and commonly affects men older than 40 years. In most reported cases, there has been an underlying primary malignant neoplasm of the upper aerodigestive tract2; however, some other associated malignancies also have been reported. Skin changes tend to occur before the diagnosis of the associated tumor in 67% of cases. The cutaneous lesions usually resolve after successful treatment of the tumor and relapse in case of recurrence of the malignancy.3
A 53-year-old woman who was a smoker with no relevant medical background was referred to the dermatology department with an itching psoriasiform dermatitis on the palms and soles of 2 months' duration. There were no signs of systemic disease. Physical examination revealed well-demarcated, dusky red, thick, scaly plaques on the soles with sparing of the insteps (Figure, A). Scattered symmetric hyperkeratotic plaques were present on the palms (Figure, B). We also detected onychodystrophy on the hands. Other dermatologic findings were normal. Histologic examination of a biopsy specimen of the left sole showed hyperkeratosis, focal parakeratosis, acanthosis, hypergranulosis, and a predominantly perivascular dermal lymphocytic infiltrate.

With the diagnostic suspicion of PA, blood tests, chest radiograph, and colonoscopy were performed without revealing abnormalities. Positron emission tomography and computed tomography also was performed, showing cervical, mesenteric, retroperitoneal, and inguinal adenopathies. Histologic examination of both inguinal adenectomy and cervical lymph node biopsy revealed Bcl-2-positive in situ follicular lymphoma (ISFL). Examination of an iliac crest marrow aspirate showed minimal involvement of lymphoma (10%). Follow-up imaging performed 4 months after diagnosis showed no changes. The patient was diagnosed with a low-grade chronic lymphoproliferative disorder with histologic findings consistent with ISFL presenting with small disperse adenopathies and minimal bone marrow involvement. The hematology department opted for a wait-and-see approach with 6-month follow-up imaging.
The skin lesions were first treated with salicylic acid cream 10%, psoralen plus UVA therapy, and methotrexate 20 mg weekly for 2 months without remission. Replacing the other therapies, we initiated acitretin 25 mg daily, achieving sustained remission after 6 months of treatment, and then continued with a scaled dose reduction. The patient remained lesion free 1 year after starting the treatment, with a daily dose of 10 mg of acitretin.
Paraneoplastic acrokeratosis has been traditionally described as a paraneoplastic entity mainly associated with primary squamous cell carcinoma (SCC) of the upper aerodigestive tract or a metastatic SCC of the cervical lymph nodes with an unknown origin.4,5 However, uncommon associations such as adenocarcinoma of the prostate, lung, esophagus, stomach, and colon; transitional cell carcinoma of the bladder; small cell carcinoma of the lung; cutaneous SCC; breast cancer; metastatic thymic carcinoma; metastatic neuroendocrine tumor; bronchial carcinoid tumor; SCC of the vulvar region; simultaneous multiple genitourinary tumors; and liposarcoma also have been described.6 Regarding the association with lymphoma, PA has been reported with peripheral T-cell lymphoma7 and Hodgkin disease8; however, ISFL underlying PA is rare.
Follicular lymphoma is the second most common non-Hodgkin lymphoma in Western countries and comprises approximately 20% of all lymphomas.9 It is slightly more prevalent in females, and the majority of patients present with advanced-stage disease. Generally considered to be an incurable disease, a watchful-waiting approach of conservative management has been advocated in most cases, deferring treatment until symptoms appear.9
Histology of PA is nonspecific, as in our case. However, it facilitates a differential diagnosis of major dermatoses including psoriasis vulgaris, pityriasis rubra pilaris, and lupus erythematosus.
Paraneoplastic palmoplantar keratoderma also is characteristic of Howel-Evans syndrome, which is a rare inherited condition associated with esophageal cancer. In contrast to our case, palmoplantar keratoderma in these patients usually begins around 10 years of age, is caused by a mutation in the RHBDF2 gene, and is inherited in an autosomal pattern.10
The diagnosis in our case was supported by a typical clinical picture, nonspecific histology, and the concurrent finding of the underlying lymphoma. Treatment of PA must focus on the removal of the underlying malignancy, which implies the remission of the cutaneous lesions. Taking into account that a recurrence of the primary tumor leads to a relapse of skin manifestations while distant metastases do not cause a reappearance of PA, it could be suggested that pathogenetically relevant factors are produced by the primary tumor and by lymph node metastases but not by metastases elsewhere.
In this case, due to the wait-and-see approach, a specific treatment for the skin lesions was established. Although management of the skin itself generally is ineffective, there are isolated reports of response after corticosteroids, antibiotics, antimycotics, keratolytic measures, or psoralen plus UVA therapy.6 Wishart11 used etretinate to achieve an improvement of PA. We also achieved good response with acitretin. Retinoids are known to have antineoplastic activity, which may have been helpful in both the patient we presented and the one reported by Wishart.11 In summary, we propose adding ISFL to the expanding list of malignant neoplasms associated with PA, noting the response of skin lesions after acitretin.
- Bazex A, Salvador R, Dupré A, et al. Syndrome paranéoplasique à type d'hyperkératose des extremités. Guérison après le traitement de l'épithelioma laryngé. Bull Soc Fr Dermatol Syphiligr. 1965;72:182.
- Bazex A, Griffiths A. Acrokeratosis paraneoplasticae--a new cutaneous marker of malignancy. Br J Dermatol. 1980;103:301-306.
- Bolognia JL. Bazex syndrome: acrokeratosis paraneoplastica. Semin Dermatol. 1995;14:84-89.
- Witkowski JA, Parish LC. Bazex's syndrome. Paraneoplastic acrokeratosis. JAMA. 1982;248:2883-2884.
- Bolognia JL. Bazex's syndrome. Clin Dermatol. 1993;11:37-42.
- Sator PG, Breier F, Gschnait F. Acrokeratosis paraneoplastica (Bazex's syndrome): association with liposarcoma [published online August 28, 2006]. J Am Acad Dermatol. 2006;55:1103-1105.
- Lin YC, Chu CY, Chiu HC. Acrokeratosis paraneoplastica Bazex's syndrome: unusual association with a peripheral T-cell lymphoma. Acta Derm Venereol. 2001;81:440-441.
- Lucker GP, Steijlen PM. Acrokeratosis paraneoplastica (Bazex syndrome) occurring with acquired ichthyosis in Hodgkin's disease. Br J Dermatol. 1995;133:322-325.
- Jegalian AG, Eberle FC, Pack SD, et al. Follicular lymphoma in situ: clinical implications and comparisons with partial involvement by follicular lymphoma. Blood. 2011;118:2976-2984.
- Sroa N, Witman P. Howel-Evans syndrome: a variant of ectodermal dysplasia. Cutis. 2010;85:183-185.
- Wishart JM. Bazex paraneoplastic acrokeratosis: a case report and response to Tigason. Br J Dermatol. 1986;115:595-599.
- Bazex A, Salvador R, Dupré A, et al. Syndrome paranéoplasique à type d'hyperkératose des extremités. Guérison après le traitement de l'épithelioma laryngé. Bull Soc Fr Dermatol Syphiligr. 1965;72:182.
- Bazex A, Griffiths A. Acrokeratosis paraneoplasticae--a new cutaneous marker of malignancy. Br J Dermatol. 1980;103:301-306.
- Bolognia JL. Bazex syndrome: acrokeratosis paraneoplastica. Semin Dermatol. 1995;14:84-89.
- Witkowski JA, Parish LC. Bazex's syndrome. Paraneoplastic acrokeratosis. JAMA. 1982;248:2883-2884.
- Bolognia JL. Bazex's syndrome. Clin Dermatol. 1993;11:37-42.
- Sator PG, Breier F, Gschnait F. Acrokeratosis paraneoplastica (Bazex's syndrome): association with liposarcoma [published online August 28, 2006]. J Am Acad Dermatol. 2006;55:1103-1105.
- Lin YC, Chu CY, Chiu HC. Acrokeratosis paraneoplastica Bazex's syndrome: unusual association with a peripheral T-cell lymphoma. Acta Derm Venereol. 2001;81:440-441.
- Lucker GP, Steijlen PM. Acrokeratosis paraneoplastica (Bazex syndrome) occurring with acquired ichthyosis in Hodgkin's disease. Br J Dermatol. 1995;133:322-325.
- Jegalian AG, Eberle FC, Pack SD, et al. Follicular lymphoma in situ: clinical implications and comparisons with partial involvement by follicular lymphoma. Blood. 2011;118:2976-2984.
- Sroa N, Witman P. Howel-Evans syndrome: a variant of ectodermal dysplasia. Cutis. 2010;85:183-185.
- Wishart JM. Bazex paraneoplastic acrokeratosis: a case report and response to Tigason. Br J Dermatol. 1986;115:595-599.
Practice Points
- Paraneoplastic acrokeratosis may mimic palmo-plantar acrokeratosis in both clinical presentation and treatment.
- Uncommon associations of paraneoplastic acrokeratosis with different types of lymphoma have been described.
Aggressive Merkel Cell Carcinoma in a Liver Transplant Recipient
Merkel cell carcinoma (MCC) is a rare cutaneous neuroendocrine tumor derived from the nerve-associated Merkel cell touch receptors.1 It typically presents as a solitary, rapidly growing, red to violaceous, asymptomatic nodule, though ulcerated, acneform, and cystic lesions also have been described.2 Merkel cell carcinoma follows an aggressive clinical course with a tendency for rapid growth, local recurrence (26%–60% of cases), lymph node invasion, and distant metastases (18%–52% of cases).3
Several risk factors contribute to the development of MCC, including chronic immunosuppression, exposure to UV radiation, and infection with the Merkel cell polyomavirus. Immunosuppression has been shown to increase the risk for MCC and is associated with a worse prognosis independent of stage at diagnosis.4 Organ transplant recipients represent a subset of immunosuppressed patients who are at increased risk for the development of MCC. We report a case of metastatic MCC in a 67-year-old woman 6 years after liver transplantation.
Case Report
A 67-year-old woman presented to our clinic with 2 masses—1 on the left buttock and 1 on the left hip—of 4 months’ duration. The patient’s medical history was remarkable for autoimmune hepatitis requiring liver transplantation 6 years prior as well as hypertension and thyroid disorder. Her posttransplantation course was unremarkable, and she was maintained on chronic immunosuppression with tacrolimus and mycophenolate mofetil. Six years after transplantation, the patient was observed to have a 4-cm, red-violaceous, painless, dome-shaped tumor on the left buttock (Figure 1). She also was noted to have pink-red papulonodules forming a painless 8-cm plaque on the left hip that was present for 2 weeks prior to presentation (Figure 1). Both lesions were subsequently biopsied.
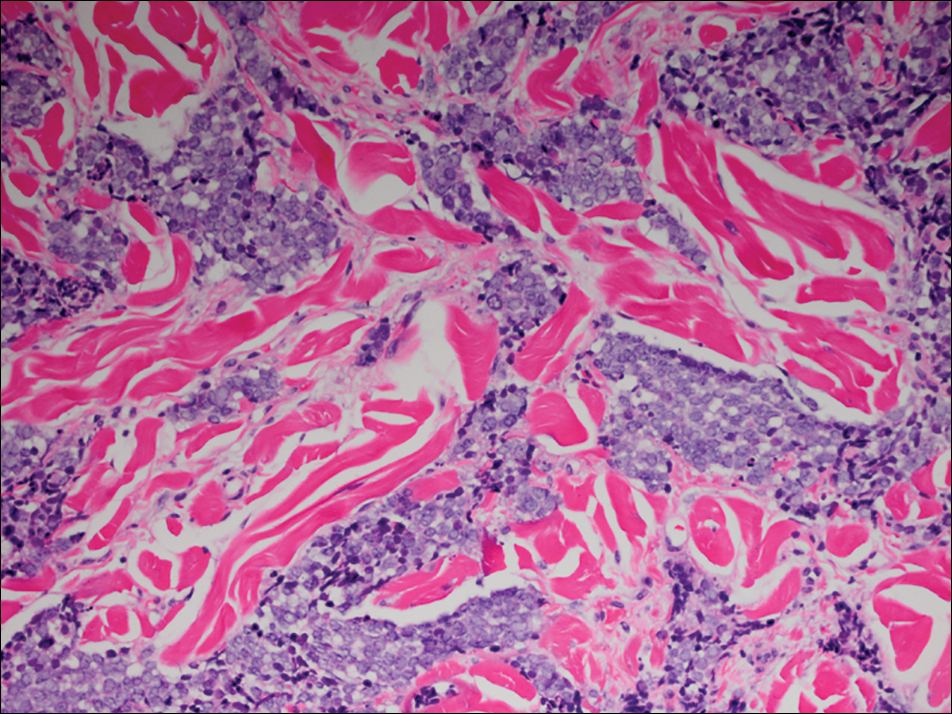
Microscopic examination of both lesions was consistent with the diagnosis of MCC. On histopathology, both samples exhibited a dense cellular dermis composed of atypical basophilic tumor cells with extension into superficial dilated lymphatic channels indicating lymphovascular invasion (Figure 2). Tumor cells were positive for the immunohistochemical markers pankeratin AE1/AE3, CAM 5.2, cytokeratin 20, synaptophysin, chromogranin A, and Merkel cell polyomavirus.
Total-body computed tomography and positron emission tomography revealed a hypermetabolic lobular density in the left gluteal region measuring 3.9×1.1 cm. The mass was associated with avid disease involving the left inguinal, bilateral iliac chain, and retroperitoneal lymph nodes. The patient was determined to have stage IV MCC based on the presence of distant lymph node metastases. The mass on the left hip was identified as an in-transit metastasis from the primary tumor on the left buttock.
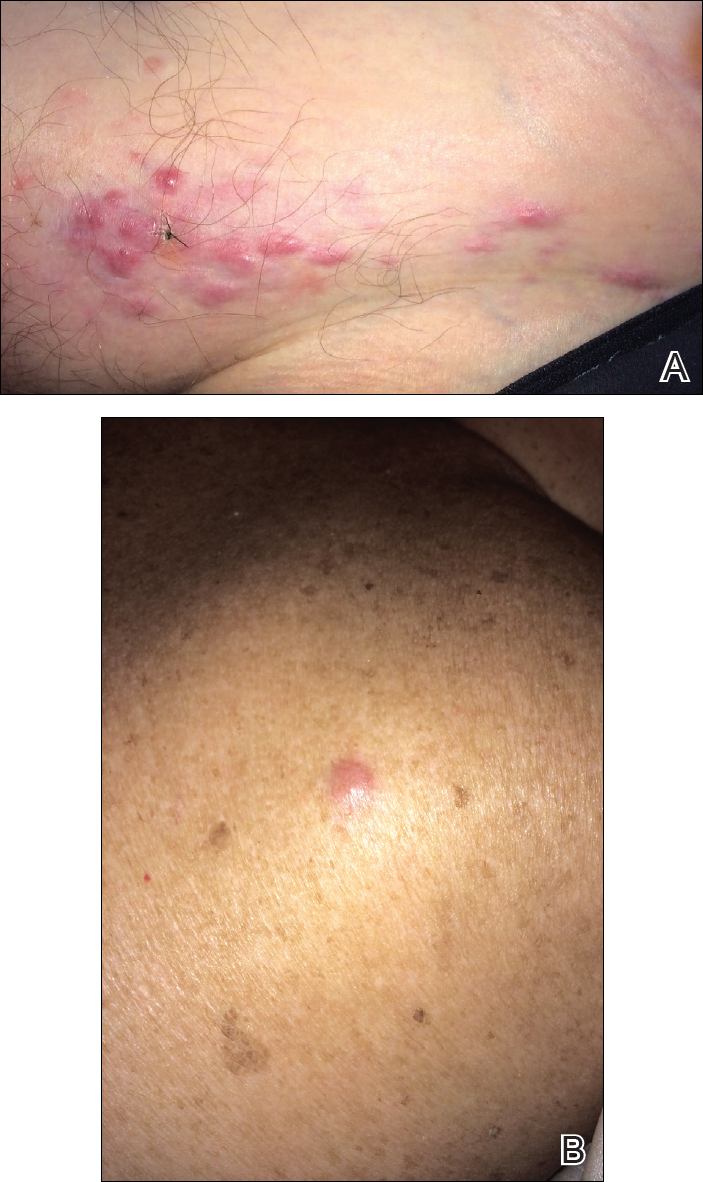
The patient was referred to surgical and medical oncology. The decision was made to start palliative chemotherapy without surgical intervention given the extent of metastases not amenable for resection. The patient was subsequently initiated on chemotherapy with etoposide and carboplatin. After one cycle of chemotherapy, both tumors initially decreased in size; however, 4 months later, despite multiple cycles of chemotherapy, the patient was noted to have growth of existing tumors and interval development of a new 7×5-cm erythematous plaque in the left groin (Figure 3A) and a 1.1×1.0-cm smooth nodule on the right upper back (Figure 3B), both also found to be consistent with distant skin metastases of MCC upon microscopic examination after biopsy. Despite chemotherapy, the patient’s tumor continued to spread and the patient died within 8 months of diagnosis.
Comment
Transplant recipients represent a well-described cohort of immunosuppressed patients prone to the development of MCC. Merkel cell carcinoma in organ transplant recipients has been most frequently documented to occur after kidney transplantation and less frequently after heart and liver transplantations.5,6 However, the role of organ type and immunosuppressive regimen is not well characterized in the literature. Clarke et al7 investigated the risk for MCC in a large cohort of solid organ transplant recipients based on specific immunosuppression medications. They found a higher risk for MCC in patients who were maintained on cyclosporine, azathioprine, and mTOR (mechanistic target of rapamycin) inhibitors rather than tacrolimus, mycophenolate mofetil, and corticosteroids. In comparison to combination tacrolimus–mycophenolate mofetil, cyclosporine-azathioprine was associated with an increased incidence of MCC; this risk rose remarkably in patients who resided in geographic locations with a higher average of UV exposure. The authors suggested that UV radiation and immunosuppression-induced DNA damage may be synergistic in the development of MCC.7
Merkel cell carcinoma most frequently occurs on sun-exposed sites, including the face, head, and neck (55%); upper and lower extremities (40%); and truncal regions (5%).8 However, case reports highlight MCC arising in atypical locations such as the buttocks and gluteal region in organ transplant recipients.7,9 In the general population, MCC predominantly arises in elderly patients (ie, >70 years), but it is more likely to present at an earlier age in transplant recipients.6,10 In a retrospective analysis of 41 solid organ transplant recipients, 12 were diagnosed before the age of 50 years.6 Data from the US Scientific Registry of Transplant Recipients showed a median age at diagnosis of 62 years, with the highest incidence occurring 10 or more years after transplantation.7
Merkel cell carcinoma behaves aggressively and is the most common cause of skin cancer death after melanoma.11 Organ transplant recipients with MCC have a worse prognosis than MCC patients who are not transplant recipients. In a retrospective registry analysis of 45 de novo cases, Buell at al5 found a 60% mortality rate in transplant recipients, almost double the 33% mortality rate of the general population. Furthermore, Arron et al10 revealed substantially increased rates of disease progression and decreased rates of disease-specific and overall survival in solid organ transplant recipients on immunosuppression compared to immunocompetent controls. The most important factor for poor prognosis is the presence of lymph node invasion, which lowers survival rate.12
Conclusion
Merkel cell carcinoma following liver transplantation is not well described in the literature. We highlight a case of an aggressive MCC arising in a sun-protected site with rapid metastasis 6 years after liver transplantation. This case emphasizes the importance of surveillance for cutaneous malignancy in solid organ transplant recipients.
- Gould VE, Moll R, Moll I, et al. Neuroendocrine (Merkel) cells of the skin: hyperplasias, dysplasias, and neoplasms. Lab Invest. 1985;52:334-353.
- Ratner D, Nelson BR, Brown MD, et al. Merkel cell carcinoma. J Am Acad Dermatol. 1993;29(2, pt 1):143-156.
- Pectasides D, Pectasides M, Economopoulos T. Merkel cell cancer of the skin. Ann Oncol. 2006;17:1489-1495.
- Paulson KG, Iyer JG, Blom A, et al. Systemic immune suppression predicts diminished Merkel cell carcinoma-specific survival independent of stage. J Invest Dermatol. 2013;133:642-646.
- Buell JF, Trofe J, Hanaway MJ, et al. Immunosuppression and Merkel cell cancer. Transplant Proc. 2002;34:1780-1781.
- Penn I, First MR. Merkel’s cell carcinoma in organ recipients: report of 41 cases. Transplantation. 1999;68:1717-1721.
- Clarke CA, Robbins HA, Tatalovich Z, et al. Risk of Merkel cell carcinoma after solid organ transplantation. J Natl Cancer Inst. 2015;107. pii:dju382. doi:10.1093/jnci/dju382.
- Rockville Merkel Cell Carcinoma Group. Merkel cell carcinoma: recent progress and current priorities on etiology, pathogenesis and clinical management [published online July 13, 2009]. J Clin Oncol. 2009;27:4021-4026.
- Krejčí K, Tichý T, Horák P, et al. Merkel cell carcinoma of the gluteal region with ipsilateral metastasis into the pancreatic graft of a patient after combined kidney-pancreas transplantation [published online September 20, 2010]. Onkologie. 2010;33:520-524.
- Arron ST, Canavan T, Yu SS. Organ transplant recipients with Merkel cell carcinoma have reduced progression-free, overall, and disease-specific survival independent of stage at presentation [published online July 1, 2014]. J Am Acad Dermatol. 2014;71:684-690.
- Albores-Saavedra J, Batich K, Chable-Montero F, et al. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population-based study [published online July 23, 2009]. J Cutan Pathol. 2010;37:20-27.
- Eng TY, Boersma MG, Fuller CD, et al. Treatment of Merkel cell carcinoma. Am J Clin Oncol. 2004;27:510-515.
Merkel cell carcinoma (MCC) is a rare cutaneous neuroendocrine tumor derived from the nerve-associated Merkel cell touch receptors.1 It typically presents as a solitary, rapidly growing, red to violaceous, asymptomatic nodule, though ulcerated, acneform, and cystic lesions also have been described.2 Merkel cell carcinoma follows an aggressive clinical course with a tendency for rapid growth, local recurrence (26%–60% of cases), lymph node invasion, and distant metastases (18%–52% of cases).3
Several risk factors contribute to the development of MCC, including chronic immunosuppression, exposure to UV radiation, and infection with the Merkel cell polyomavirus. Immunosuppression has been shown to increase the risk for MCC and is associated with a worse prognosis independent of stage at diagnosis.4 Organ transplant recipients represent a subset of immunosuppressed patients who are at increased risk for the development of MCC. We report a case of metastatic MCC in a 67-year-old woman 6 years after liver transplantation.
Case Report
A 67-year-old woman presented to our clinic with 2 masses—1 on the left buttock and 1 on the left hip—of 4 months’ duration. The patient’s medical history was remarkable for autoimmune hepatitis requiring liver transplantation 6 years prior as well as hypertension and thyroid disorder. Her posttransplantation course was unremarkable, and she was maintained on chronic immunosuppression with tacrolimus and mycophenolate mofetil. Six years after transplantation, the patient was observed to have a 4-cm, red-violaceous, painless, dome-shaped tumor on the left buttock (Figure 1). She also was noted to have pink-red papulonodules forming a painless 8-cm plaque on the left hip that was present for 2 weeks prior to presentation (Figure 1). Both lesions were subsequently biopsied.

Microscopic examination of both lesions was consistent with the diagnosis of MCC. On histopathology, both samples exhibited a dense cellular dermis composed of atypical basophilic tumor cells with extension into superficial dilated lymphatic channels indicating lymphovascular invasion (Figure 2). Tumor cells were positive for the immunohistochemical markers pankeratin AE1/AE3, CAM 5.2, cytokeratin 20, synaptophysin, chromogranin A, and Merkel cell polyomavirus.

Total-body computed tomography and positron emission tomography revealed a hypermetabolic lobular density in the left gluteal region measuring 3.9×1.1 cm. The mass was associated with avid disease involving the left inguinal, bilateral iliac chain, and retroperitoneal lymph nodes. The patient was determined to have stage IV MCC based on the presence of distant lymph node metastases. The mass on the left hip was identified as an in-transit metastasis from the primary tumor on the left buttock.
The patient was referred to surgical and medical oncology. The decision was made to start palliative chemotherapy without surgical intervention given the extent of metastases not amenable for resection. The patient was subsequently initiated on chemotherapy with etoposide and carboplatin. After one cycle of chemotherapy, both tumors initially decreased in size; however, 4 months later, despite multiple cycles of chemotherapy, the patient was noted to have growth of existing tumors and interval development of a new 7×5-cm erythematous plaque in the left groin (Figure 3A) and a 1.1×1.0-cm smooth nodule on the right upper back (Figure 3B), both also found to be consistent with distant skin metastases of MCC upon microscopic examination after biopsy. Despite chemotherapy, the patient’s tumor continued to spread and the patient died within 8 months of diagnosis.

Comment
Transplant recipients represent a well-described cohort of immunosuppressed patients prone to the development of MCC. Merkel cell carcinoma in organ transplant recipients has been most frequently documented to occur after kidney transplantation and less frequently after heart and liver transplantations.5,6 However, the role of organ type and immunosuppressive regimen is not well characterized in the literature. Clarke et al7 investigated the risk for MCC in a large cohort of solid organ transplant recipients based on specific immunosuppression medications. They found a higher risk for MCC in patients who were maintained on cyclosporine, azathioprine, and mTOR (mechanistic target of rapamycin) inhibitors rather than tacrolimus, mycophenolate mofetil, and corticosteroids. In comparison to combination tacrolimus–mycophenolate mofetil, cyclosporine-azathioprine was associated with an increased incidence of MCC; this risk rose remarkably in patients who resided in geographic locations with a higher average of UV exposure. The authors suggested that UV radiation and immunosuppression-induced DNA damage may be synergistic in the development of MCC.7
Merkel cell carcinoma most frequently occurs on sun-exposed sites, including the face, head, and neck (55%); upper and lower extremities (40%); and truncal regions (5%).8 However, case reports highlight MCC arising in atypical locations such as the buttocks and gluteal region in organ transplant recipients.7,9 In the general population, MCC predominantly arises in elderly patients (ie, >70 years), but it is more likely to present at an earlier age in transplant recipients.6,10 In a retrospective analysis of 41 solid organ transplant recipients, 12 were diagnosed before the age of 50 years.6 Data from the US Scientific Registry of Transplant Recipients showed a median age at diagnosis of 62 years, with the highest incidence occurring 10 or more years after transplantation.7
Merkel cell carcinoma behaves aggressively and is the most common cause of skin cancer death after melanoma.11 Organ transplant recipients with MCC have a worse prognosis than MCC patients who are not transplant recipients. In a retrospective registry analysis of 45 de novo cases, Buell at al5 found a 60% mortality rate in transplant recipients, almost double the 33% mortality rate of the general population. Furthermore, Arron et al10 revealed substantially increased rates of disease progression and decreased rates of disease-specific and overall survival in solid organ transplant recipients on immunosuppression compared to immunocompetent controls. The most important factor for poor prognosis is the presence of lymph node invasion, which lowers survival rate.12
Conclusion
Merkel cell carcinoma following liver transplantation is not well described in the literature. We highlight a case of an aggressive MCC arising in a sun-protected site with rapid metastasis 6 years after liver transplantation. This case emphasizes the importance of surveillance for cutaneous malignancy in solid organ transplant recipients.
Merkel cell carcinoma (MCC) is a rare cutaneous neuroendocrine tumor derived from the nerve-associated Merkel cell touch receptors.1 It typically presents as a solitary, rapidly growing, red to violaceous, asymptomatic nodule, though ulcerated, acneform, and cystic lesions also have been described.2 Merkel cell carcinoma follows an aggressive clinical course with a tendency for rapid growth, local recurrence (26%–60% of cases), lymph node invasion, and distant metastases (18%–52% of cases).3
Several risk factors contribute to the development of MCC, including chronic immunosuppression, exposure to UV radiation, and infection with the Merkel cell polyomavirus. Immunosuppression has been shown to increase the risk for MCC and is associated with a worse prognosis independent of stage at diagnosis.4 Organ transplant recipients represent a subset of immunosuppressed patients who are at increased risk for the development of MCC. We report a case of metastatic MCC in a 67-year-old woman 6 years after liver transplantation.
Case Report
A 67-year-old woman presented to our clinic with 2 masses—1 on the left buttock and 1 on the left hip—of 4 months’ duration. The patient’s medical history was remarkable for autoimmune hepatitis requiring liver transplantation 6 years prior as well as hypertension and thyroid disorder. Her posttransplantation course was unremarkable, and she was maintained on chronic immunosuppression with tacrolimus and mycophenolate mofetil. Six years after transplantation, the patient was observed to have a 4-cm, red-violaceous, painless, dome-shaped tumor on the left buttock (Figure 1). She also was noted to have pink-red papulonodules forming a painless 8-cm plaque on the left hip that was present for 2 weeks prior to presentation (Figure 1). Both lesions were subsequently biopsied.

Microscopic examination of both lesions was consistent with the diagnosis of MCC. On histopathology, both samples exhibited a dense cellular dermis composed of atypical basophilic tumor cells with extension into superficial dilated lymphatic channels indicating lymphovascular invasion (Figure 2). Tumor cells were positive for the immunohistochemical markers pankeratin AE1/AE3, CAM 5.2, cytokeratin 20, synaptophysin, chromogranin A, and Merkel cell polyomavirus.

Total-body computed tomography and positron emission tomography revealed a hypermetabolic lobular density in the left gluteal region measuring 3.9×1.1 cm. The mass was associated with avid disease involving the left inguinal, bilateral iliac chain, and retroperitoneal lymph nodes. The patient was determined to have stage IV MCC based on the presence of distant lymph node metastases. The mass on the left hip was identified as an in-transit metastasis from the primary tumor on the left buttock.
The patient was referred to surgical and medical oncology. The decision was made to start palliative chemotherapy without surgical intervention given the extent of metastases not amenable for resection. The patient was subsequently initiated on chemotherapy with etoposide and carboplatin. After one cycle of chemotherapy, both tumors initially decreased in size; however, 4 months later, despite multiple cycles of chemotherapy, the patient was noted to have growth of existing tumors and interval development of a new 7×5-cm erythematous plaque in the left groin (Figure 3A) and a 1.1×1.0-cm smooth nodule on the right upper back (Figure 3B), both also found to be consistent with distant skin metastases of MCC upon microscopic examination after biopsy. Despite chemotherapy, the patient’s tumor continued to spread and the patient died within 8 months of diagnosis.

Comment
Transplant recipients represent a well-described cohort of immunosuppressed patients prone to the development of MCC. Merkel cell carcinoma in organ transplant recipients has been most frequently documented to occur after kidney transplantation and less frequently after heart and liver transplantations.5,6 However, the role of organ type and immunosuppressive regimen is not well characterized in the literature. Clarke et al7 investigated the risk for MCC in a large cohort of solid organ transplant recipients based on specific immunosuppression medications. They found a higher risk for MCC in patients who were maintained on cyclosporine, azathioprine, and mTOR (mechanistic target of rapamycin) inhibitors rather than tacrolimus, mycophenolate mofetil, and corticosteroids. In comparison to combination tacrolimus–mycophenolate mofetil, cyclosporine-azathioprine was associated with an increased incidence of MCC; this risk rose remarkably in patients who resided in geographic locations with a higher average of UV exposure. The authors suggested that UV radiation and immunosuppression-induced DNA damage may be synergistic in the development of MCC.7
Merkel cell carcinoma most frequently occurs on sun-exposed sites, including the face, head, and neck (55%); upper and lower extremities (40%); and truncal regions (5%).8 However, case reports highlight MCC arising in atypical locations such as the buttocks and gluteal region in organ transplant recipients.7,9 In the general population, MCC predominantly arises in elderly patients (ie, >70 years), but it is more likely to present at an earlier age in transplant recipients.6,10 In a retrospective analysis of 41 solid organ transplant recipients, 12 were diagnosed before the age of 50 years.6 Data from the US Scientific Registry of Transplant Recipients showed a median age at diagnosis of 62 years, with the highest incidence occurring 10 or more years after transplantation.7
Merkel cell carcinoma behaves aggressively and is the most common cause of skin cancer death after melanoma.11 Organ transplant recipients with MCC have a worse prognosis than MCC patients who are not transplant recipients. In a retrospective registry analysis of 45 de novo cases, Buell at al5 found a 60% mortality rate in transplant recipients, almost double the 33% mortality rate of the general population. Furthermore, Arron et al10 revealed substantially increased rates of disease progression and decreased rates of disease-specific and overall survival in solid organ transplant recipients on immunosuppression compared to immunocompetent controls. The most important factor for poor prognosis is the presence of lymph node invasion, which lowers survival rate.12
Conclusion
Merkel cell carcinoma following liver transplantation is not well described in the literature. We highlight a case of an aggressive MCC arising in a sun-protected site with rapid metastasis 6 years after liver transplantation. This case emphasizes the importance of surveillance for cutaneous malignancy in solid organ transplant recipients.
- Gould VE, Moll R, Moll I, et al. Neuroendocrine (Merkel) cells of the skin: hyperplasias, dysplasias, and neoplasms. Lab Invest. 1985;52:334-353.
- Ratner D, Nelson BR, Brown MD, et al. Merkel cell carcinoma. J Am Acad Dermatol. 1993;29(2, pt 1):143-156.
- Pectasides D, Pectasides M, Economopoulos T. Merkel cell cancer of the skin. Ann Oncol. 2006;17:1489-1495.
- Paulson KG, Iyer JG, Blom A, et al. Systemic immune suppression predicts diminished Merkel cell carcinoma-specific survival independent of stage. J Invest Dermatol. 2013;133:642-646.
- Buell JF, Trofe J, Hanaway MJ, et al. Immunosuppression and Merkel cell cancer. Transplant Proc. 2002;34:1780-1781.
- Penn I, First MR. Merkel’s cell carcinoma in organ recipients: report of 41 cases. Transplantation. 1999;68:1717-1721.
- Clarke CA, Robbins HA, Tatalovich Z, et al. Risk of Merkel cell carcinoma after solid organ transplantation. J Natl Cancer Inst. 2015;107. pii:dju382. doi:10.1093/jnci/dju382.
- Rockville Merkel Cell Carcinoma Group. Merkel cell carcinoma: recent progress and current priorities on etiology, pathogenesis and clinical management [published online July 13, 2009]. J Clin Oncol. 2009;27:4021-4026.
- Krejčí K, Tichý T, Horák P, et al. Merkel cell carcinoma of the gluteal region with ipsilateral metastasis into the pancreatic graft of a patient after combined kidney-pancreas transplantation [published online September 20, 2010]. Onkologie. 2010;33:520-524.
- Arron ST, Canavan T, Yu SS. Organ transplant recipients with Merkel cell carcinoma have reduced progression-free, overall, and disease-specific survival independent of stage at presentation [published online July 1, 2014]. J Am Acad Dermatol. 2014;71:684-690.
- Albores-Saavedra J, Batich K, Chable-Montero F, et al. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population-based study [published online July 23, 2009]. J Cutan Pathol. 2010;37:20-27.
- Eng TY, Boersma MG, Fuller CD, et al. Treatment of Merkel cell carcinoma. Am J Clin Oncol. 2004;27:510-515.
- Gould VE, Moll R, Moll I, et al. Neuroendocrine (Merkel) cells of the skin: hyperplasias, dysplasias, and neoplasms. Lab Invest. 1985;52:334-353.
- Ratner D, Nelson BR, Brown MD, et al. Merkel cell carcinoma. J Am Acad Dermatol. 1993;29(2, pt 1):143-156.
- Pectasides D, Pectasides M, Economopoulos T. Merkel cell cancer of the skin. Ann Oncol. 2006;17:1489-1495.
- Paulson KG, Iyer JG, Blom A, et al. Systemic immune suppression predicts diminished Merkel cell carcinoma-specific survival independent of stage. J Invest Dermatol. 2013;133:642-646.
- Buell JF, Trofe J, Hanaway MJ, et al. Immunosuppression and Merkel cell cancer. Transplant Proc. 2002;34:1780-1781.
- Penn I, First MR. Merkel’s cell carcinoma in organ recipients: report of 41 cases. Transplantation. 1999;68:1717-1721.
- Clarke CA, Robbins HA, Tatalovich Z, et al. Risk of Merkel cell carcinoma after solid organ transplantation. J Natl Cancer Inst. 2015;107. pii:dju382. doi:10.1093/jnci/dju382.
- Rockville Merkel Cell Carcinoma Group. Merkel cell carcinoma: recent progress and current priorities on etiology, pathogenesis and clinical management [published online July 13, 2009]. J Clin Oncol. 2009;27:4021-4026.
- Krejčí K, Tichý T, Horák P, et al. Merkel cell carcinoma of the gluteal region with ipsilateral metastasis into the pancreatic graft of a patient after combined kidney-pancreas transplantation [published online September 20, 2010]. Onkologie. 2010;33:520-524.
- Arron ST, Canavan T, Yu SS. Organ transplant recipients with Merkel cell carcinoma have reduced progression-free, overall, and disease-specific survival independent of stage at presentation [published online July 1, 2014]. J Am Acad Dermatol. 2014;71:684-690.
- Albores-Saavedra J, Batich K, Chable-Montero F, et al. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population-based study [published online July 23, 2009]. J Cutan Pathol. 2010;37:20-27.
- Eng TY, Boersma MG, Fuller CD, et al. Treatment of Merkel cell carcinoma. Am J Clin Oncol. 2004;27:510-515.
Practice Points
- Organ transplant recipients are at an increased risk for Merkel cell carcinoma (MCC).
- Early recognition and diagnosis of MCC is important to improve morbidity and mortality.
Ex Vivo Confocal Microscopy: A Diagnostic Tool for Skin Malignancies
Skin cancer is diagnosed in approximately 5.4 million individuals annually in the United States, more than the total number of breast, lung, colon, and prostate cancers diagnosed per year.1 It is estimated that 1 in 5 Americans will develop skin cancer during their lifetime.2 The 2 most common forms of skin cancer are basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), accounting for 4 million and 1 million cases diagnosed each year, respectively.3 With the increasing incidence of these skin cancers, the use of noninvasive imaging tools for detection and diagnosis has grown.
Ex vivo confocal microscopy is a diagnostic imaging tool that can be used in real-time at the bedside to assess freshly excised tissue for malignancies. It images tissue samples with cellular resolution and within minutes of biopsy or excision. Ex vivo confocal microscopy is a versatile tool that can assist in the diagnosis and management of skin malignancies such as melanoma, BCC, and SCC.
Reflectance vs Fluorescence Mode
Excised lesions can be examined in reflectance or fluorescence mode in great detail but with slightly varying nuclear-to-dermis contrasts depending on the chromophore that is targeted. In reflectance mode (reflectance confocal microscopy [RCM]), melanin and keratin act as endogenous chromophores because of their high refractive index relative to water,4,5 which allows for the visualization of cellular structures of the skin at low power, as well as microscopic substructures such as melanosomes, cytoplasmic granules, and other cellular organelles at high power. Although an exogenous contrast agent is not required, acetic acid has the capability to highlight nuclei, enhancing the tumor cell-to-dermis contrast in RCM.6 Acetic acid is clinically used as a predictor for certain skin and mucosal membrane neoplasms that blanch when exposed to the solution. In the case of RCM, acetic acid increases the visibility of nuclei by inducing the compaction of chromatin. For the acetowhitening to be effective, the sample must be soaked in the solution for a specific amount of time, depending on the concentration.7 A concentration between 1% and 10% can be used, but the less concentrated the solution, the longer the time of soaking that is required to achieve sufficiently bright nuclei.6
The contrast with acetic acid, however, is quite weak when the tissue is imaged en face, or along the horizontal surface of the sample, due to the collagen in the dermal layer, which has a high reflectance index. This issue is rectified when using the confocal microscope in the fluorescence mode with an exogenous fluorescent dye as a nuclear stain. Fluorescence confocal microscopy (FCM), results in a stronger nuclear-to-dermal contrast because of the role of contrast agents.8 The 1000-fold increase in contrast between nuclei and dermis is the result of dye agents that preferentially bind to nuclear DNA, of which acridine orange is the most commonly used.5,8 Basal cell carcinoma and SCC tumor cells can be visualized with FCM because they appear hyperfluorescent when stained with acridine orange.9 The acridine orange–stained cells display bright nuclei, while the cytoplasm and collagen remains dark. A positive feature of acridine orange is that it does not alter the tissue sample during freezing or formalin fixation and thus has no effect on subsequent histopathology that may need to be performed on the sample.10
High-Resolution Images Aid in Diagnosis
After it is harvested, the tissue sample is soaked in a contrast agent or dye, if needed, depending on the confocal mode to be used. The confocal microscope is then used to take a series of high-resolution individual en face images that are then stitched together to create a final mosaic image that can be up to 12×12 mm.6,11 With a 200-µm depth visibility, confocal microscopy can capture the cellular structures in the epidermis, dermis, and (if compressed enough) subcutaneous fat in just under 3 minutes.12
The images produced through confocal microscopy have an excellent correlation to frozen histological sections and can aid in the diagnosis of many epidermal and dermal malignancies including melanoma, BCC, and SCC. New criteria have been established to aid in the interpretation of the confocal images and identify some of the more common skin cancers.5,12,13 Basal cell carcinoma samples imaged through fluorescence and reflectance in low-power mode display the distinct nodular patterns with well-demarcated edges, as seen on classical histopathology. In the case of FCM, the cells that make up the tumor display hyperfluorescent areas consistent with nucleated cells that are stained with acridine orange. The main features that identify BCC on FCM images include nuclear pleomorphism and crowding, peripheral palisading, clefting of the basaloid islands, increased nucleus-to-cytoplasm ratio, and the presence of a modified dermis surrounding the mass known as the tumoral stroma5,12 (Figure).
In addition to fluorescence and a well-defined tumor silhouette, SCC under FCM displays keratin pearls composed of keratinized squames, nuclear pleomorphism, and fluorescent scales in the stratum corneum that are a result of keratin formation.5,13 The extent of differentiation of the SCC lesion also can be determined by assessing if the silhouette is well defined. A well-defined tumor silhouette is consistent with the diagnosis of a well-differentiated SCC, and vice versa.13 Ex vivo RCM also has been shown to be useful in diagnosing malignant melanomas, with melanin acting as an endogenous chromophore. Some of the features seen on imaging include a disarranged epithelium, hyperreflective roundish and dendritic pagetoid cells, and large hyperreflective polymorphic cells in the superficial chorion.14
Comparison to Conventional Histopathology
Ex vivo confocal microscopy in both the reflectance and fluorescence mode has been shown to perform well compared to conventional histopathology in the diagnosis of biopsy specimens. Ex vivo FCM has been shown to have an overall sensitivity of 88% and specificity of 99% in detecting residual BCC at the margins of excised tissue samples and in the fraction of the time it takes to attain similar results with frozen histopathology.9 Ex vivo RCM has been shown to have a higher prognostic capability, with 100% sensitivity and specificity in identifying BCC when scanning the tissue samples en face.15
Qualitatively, the images produced by RCM and FCM are similar to histopathology in overall architecture. Both techniques enhance the contrast between the epithelium and stroma and create images that can be examined in low as well as high resolution. A substantial difference between confocal microscopy and conventional hematoxylin and eosin–stained histopathology is that the confocal microscope produces images in gray scale. One way to alter the black-and-white images to resemble hematoxylin and eosin–stained slides is through the use of digital staining,16 which could boost clinical acceptance by physicians who are accustomed to the classical pink-purple appearance of pathology slides and could potentially limit the learning curve needed to read the confocal images.
Application in Mohs Micrographic Surgery
An important clinical application of ex vivo FCM imaging that has emerged is the detection of malignant cells at the excision margins during Mohs micrographic surgery. The use of confocal microscopy has the potential to save time by eliminating the need for tissue fixation while still providing good diagnostic accuracy. Implementing FCM as an imaging tool to guide surgical excisions could provide rapid diagnosis of the tissue, expediting excisions and reconstruction or the Mohs procedure while eliminating patient wait time and the need for frozen histopathology. Ex vivo RCM also has been used to establish laser parameters for CO2 laser ablation of superficial and early nodular BCC lesions.17 Other potential uses for ex vivo RCM/FCM could include rapid evaluation of tissue during operating room procedures where rapid frozen sections are currently utilized.
Combining In Vivo and Ex Vivo Confocal Microscopy
Many of the diagnostic guidelines created with the use of ex vivo confocal microscopy have been applied to in vivo use, and therefore the use of both modalities is appealing. In vivo confocal microscopy is a noninvasive technique that has been used to map margins of skin tumors such as BCC and lentigo maligna at the bedside.5 It also has been shown to help plan both surgical and nonsurgical treatment modalities and reconstruction before the tumor is excised.18 This technique also can help the patient understand the extent of the excision and any subsequent reconstruction that may be needed.
Limitations
Ex vivo confocal microscopy used as a diagnostic tool does have some limitations. Its novelty may require surgeons and pathologists to be trained to interpret the images properly and correlate them to conventional diagnostic guidelines. The imaging also is limited to a depth of approximately 200 µm; however, the sample may be flipped so that the underside can be imaged as well, which increases the depth to approximately 400 µm. The tissue being imaged must be fixed flat, which may alter its shape. Complex tissue samples may be difficult to flatten out completely and therefore may be difficult to image. A special mount may be required for the sample to be fixed in a proper position for imaging.6
Final Thoughts
Despite some of these limitations, the need for rapid bedside tissue diagnosis makes ex vivo confocal microscopy an attractive device that can be used as an additional diagnostic tool to histopathology and also has been tested in other disciplines, such as breast cancer pathology. In the future, both in vivo and ex vivo confocal microscopy may be utilized to diagnose cutaneous malignancies, guide surgical excisions, and detect lesion progression, and it may become a basis for rapid diagnosis and detection.19
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016 [published online January 7, 2016]. CA Cancer J Clin. 2016;66:7-30.
- Robinson JK. Sun exposure, sun protection, and vitamin D. JAMA. 2005;294:1541-1543.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Welzel J, Kästle R, Sattler EC. Fluorescence (multiwave) confocal microscopy. Dermatol Clin. 2016;34:527-533.
- Longo C, Ragazzi M, Rajadhyaksha M, et al. In vivo and ex vivo confocal microscopy for dermatologic and Mohs surgeons. Dermatol Clin. 2016;34:497-504.
- Patel YG, Nehal KS, Aranda I, et al. Confocal reflectance mosaicing of basal cell carcinomas in Mohs surgical skin excisions. J Biomed Opt. 2007;12:034027.
- Rajadhyaksha M, Gonzalez S, Zavislan JM. Detectability of contrast agents for confocal reflectance imaging of skin and microcirculation. J Biomed Opt. 2004;9:323-331.
- Karen JK, Gareau DS, Dusza SW, et al. Detection of basal cell carcinomas in Mohs excisions with fluorescence confocal mosaicing microscopy. Br J Dermatol. 2009;160:1242-1250.
- Bennàssar A, Vilata A, Puig S, et al. Ex vivo fluorescence confocal microscopy for fast evaluation of tumour margins during Mohs surgery. Br J Dermatol. 2014;170:360-365.
- Gareau DS, Li Y, Huang B, et al. Confocal mosaicing microscopy in Mohs skin excisions: feasibility of rapid surgical pathology. J Biomed Opt. 2008;13:054001.
- Bini J, Spain J, Nehal K, et al. Confocal mosaicing microscopy of human skin ex vivo: spectral analysis for digital staining to simulate histology-like appearance. J Biomed Opt. 2011;16:076008.
- Bennàssar A, Carrera C, Puig S, et al. Fast evaluation of 69 basal cell carcinomas with ex vivo fluorescence confocal microscopy: criteria description, histopathological correlation, and interobserver agreement. JAMA Dermatol. 2013;149:839-847.
- Longo C, Ragazzi M, Gardini S, et al. Ex vivo fluorescence confocal microscopy in conjunction with Mohs micrographic surgery for cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2015;73:321-322.
- Cinotti E, Haouas M, Grivet D, et al. In vivo and ex vivo confocal microscopy for the management of a melanoma of the eyelid margin. Dermatol Surg. 2015;41:1437-1440.
- , , , ‘En face’ ex vivo reflectance confocal microscopy to help the surgery of basal cell carcinoma of the eyelid [published online December 19, 2016]. Clin Exp Ophthalmol. doi:10.1111/ceo.12904.
- Gareau DS, Jeon H, Nehal KS, et al. Rapid screening of cancer margins in tissue with multimodal confocal microscopy. J Surg Res. 2012;178:533-538.
- Sierra H, Damanpour S, Hibler B, et al. Confocal imaging of carbon dioxide laser-ablated basal cell carcinomas: an ex-vivo study on the uptake of contrast agent and ablation parameters [published online September 22, 2015]. Lasers Surg Med. 2016;48:133-139.
- Hibler BP, Yélamos O, Cordova M, et al. Handheld reflectance confocal microscopy to aid in the management of complex facial lentigo maligna. Cutis. 2017;99:346-352.
- Rajadhyaksha M, Marghoob A, Rossi A, et al. Reflectance confocal microscopy of skin in vivo: from bench to bedside. Lasers Surg Med. 2017;49:7-19.
Skin cancer is diagnosed in approximately 5.4 million individuals annually in the United States, more than the total number of breast, lung, colon, and prostate cancers diagnosed per year.1 It is estimated that 1 in 5 Americans will develop skin cancer during their lifetime.2 The 2 most common forms of skin cancer are basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), accounting for 4 million and 1 million cases diagnosed each year, respectively.3 With the increasing incidence of these skin cancers, the use of noninvasive imaging tools for detection and diagnosis has grown.
Ex vivo confocal microscopy is a diagnostic imaging tool that can be used in real-time at the bedside to assess freshly excised tissue for malignancies. It images tissue samples with cellular resolution and within minutes of biopsy or excision. Ex vivo confocal microscopy is a versatile tool that can assist in the diagnosis and management of skin malignancies such as melanoma, BCC, and SCC.
Reflectance vs Fluorescence Mode
Excised lesions can be examined in reflectance or fluorescence mode in great detail but with slightly varying nuclear-to-dermis contrasts depending on the chromophore that is targeted. In reflectance mode (reflectance confocal microscopy [RCM]), melanin and keratin act as endogenous chromophores because of their high refractive index relative to water,4,5 which allows for the visualization of cellular structures of the skin at low power, as well as microscopic substructures such as melanosomes, cytoplasmic granules, and other cellular organelles at high power. Although an exogenous contrast agent is not required, acetic acid has the capability to highlight nuclei, enhancing the tumor cell-to-dermis contrast in RCM.6 Acetic acid is clinically used as a predictor for certain skin and mucosal membrane neoplasms that blanch when exposed to the solution. In the case of RCM, acetic acid increases the visibility of nuclei by inducing the compaction of chromatin. For the acetowhitening to be effective, the sample must be soaked in the solution for a specific amount of time, depending on the concentration.7 A concentration between 1% and 10% can be used, but the less concentrated the solution, the longer the time of soaking that is required to achieve sufficiently bright nuclei.6
The contrast with acetic acid, however, is quite weak when the tissue is imaged en face, or along the horizontal surface of the sample, due to the collagen in the dermal layer, which has a high reflectance index. This issue is rectified when using the confocal microscope in the fluorescence mode with an exogenous fluorescent dye as a nuclear stain. Fluorescence confocal microscopy (FCM), results in a stronger nuclear-to-dermal contrast because of the role of contrast agents.8 The 1000-fold increase in contrast between nuclei and dermis is the result of dye agents that preferentially bind to nuclear DNA, of which acridine orange is the most commonly used.5,8 Basal cell carcinoma and SCC tumor cells can be visualized with FCM because they appear hyperfluorescent when stained with acridine orange.9 The acridine orange–stained cells display bright nuclei, while the cytoplasm and collagen remains dark. A positive feature of acridine orange is that it does not alter the tissue sample during freezing or formalin fixation and thus has no effect on subsequent histopathology that may need to be performed on the sample.10
High-Resolution Images Aid in Diagnosis
After it is harvested, the tissue sample is soaked in a contrast agent or dye, if needed, depending on the confocal mode to be used. The confocal microscope is then used to take a series of high-resolution individual en face images that are then stitched together to create a final mosaic image that can be up to 12×12 mm.6,11 With a 200-µm depth visibility, confocal microscopy can capture the cellular structures in the epidermis, dermis, and (if compressed enough) subcutaneous fat in just under 3 minutes.12
The images produced through confocal microscopy have an excellent correlation to frozen histological sections and can aid in the diagnosis of many epidermal and dermal malignancies including melanoma, BCC, and SCC. New criteria have been established to aid in the interpretation of the confocal images and identify some of the more common skin cancers.5,12,13 Basal cell carcinoma samples imaged through fluorescence and reflectance in low-power mode display the distinct nodular patterns with well-demarcated edges, as seen on classical histopathology. In the case of FCM, the cells that make up the tumor display hyperfluorescent areas consistent with nucleated cells that are stained with acridine orange. The main features that identify BCC on FCM images include nuclear pleomorphism and crowding, peripheral palisading, clefting of the basaloid islands, increased nucleus-to-cytoplasm ratio, and the presence of a modified dermis surrounding the mass known as the tumoral stroma5,12 (Figure).

In addition to fluorescence and a well-defined tumor silhouette, SCC under FCM displays keratin pearls composed of keratinized squames, nuclear pleomorphism, and fluorescent scales in the stratum corneum that are a result of keratin formation.5,13 The extent of differentiation of the SCC lesion also can be determined by assessing if the silhouette is well defined. A well-defined tumor silhouette is consistent with the diagnosis of a well-differentiated SCC, and vice versa.13 Ex vivo RCM also has been shown to be useful in diagnosing malignant melanomas, with melanin acting as an endogenous chromophore. Some of the features seen on imaging include a disarranged epithelium, hyperreflective roundish and dendritic pagetoid cells, and large hyperreflective polymorphic cells in the superficial chorion.14
Comparison to Conventional Histopathology
Ex vivo confocal microscopy in both the reflectance and fluorescence mode has been shown to perform well compared to conventional histopathology in the diagnosis of biopsy specimens. Ex vivo FCM has been shown to have an overall sensitivity of 88% and specificity of 99% in detecting residual BCC at the margins of excised tissue samples and in the fraction of the time it takes to attain similar results with frozen histopathology.9 Ex vivo RCM has been shown to have a higher prognostic capability, with 100% sensitivity and specificity in identifying BCC when scanning the tissue samples en face.15
Qualitatively, the images produced by RCM and FCM are similar to histopathology in overall architecture. Both techniques enhance the contrast between the epithelium and stroma and create images that can be examined in low as well as high resolution. A substantial difference between confocal microscopy and conventional hematoxylin and eosin–stained histopathology is that the confocal microscope produces images in gray scale. One way to alter the black-and-white images to resemble hematoxylin and eosin–stained slides is through the use of digital staining,16 which could boost clinical acceptance by physicians who are accustomed to the classical pink-purple appearance of pathology slides and could potentially limit the learning curve needed to read the confocal images.
Application in Mohs Micrographic Surgery
An important clinical application of ex vivo FCM imaging that has emerged is the detection of malignant cells at the excision margins during Mohs micrographic surgery. The use of confocal microscopy has the potential to save time by eliminating the need for tissue fixation while still providing good diagnostic accuracy. Implementing FCM as an imaging tool to guide surgical excisions could provide rapid diagnosis of the tissue, expediting excisions and reconstruction or the Mohs procedure while eliminating patient wait time and the need for frozen histopathology. Ex vivo RCM also has been used to establish laser parameters for CO2 laser ablation of superficial and early nodular BCC lesions.17 Other potential uses for ex vivo RCM/FCM could include rapid evaluation of tissue during operating room procedures where rapid frozen sections are currently utilized.
Combining In Vivo and Ex Vivo Confocal Microscopy
Many of the diagnostic guidelines created with the use of ex vivo confocal microscopy have been applied to in vivo use, and therefore the use of both modalities is appealing. In vivo confocal microscopy is a noninvasive technique that has been used to map margins of skin tumors such as BCC and lentigo maligna at the bedside.5 It also has been shown to help plan both surgical and nonsurgical treatment modalities and reconstruction before the tumor is excised.18 This technique also can help the patient understand the extent of the excision and any subsequent reconstruction that may be needed.
Limitations
Ex vivo confocal microscopy used as a diagnostic tool does have some limitations. Its novelty may require surgeons and pathologists to be trained to interpret the images properly and correlate them to conventional diagnostic guidelines. The imaging also is limited to a depth of approximately 200 µm; however, the sample may be flipped so that the underside can be imaged as well, which increases the depth to approximately 400 µm. The tissue being imaged must be fixed flat, which may alter its shape. Complex tissue samples may be difficult to flatten out completely and therefore may be difficult to image. A special mount may be required for the sample to be fixed in a proper position for imaging.6
Final Thoughts
Despite some of these limitations, the need for rapid bedside tissue diagnosis makes ex vivo confocal microscopy an attractive device that can be used as an additional diagnostic tool to histopathology and also has been tested in other disciplines, such as breast cancer pathology. In the future, both in vivo and ex vivo confocal microscopy may be utilized to diagnose cutaneous malignancies, guide surgical excisions, and detect lesion progression, and it may become a basis for rapid diagnosis and detection.19
Skin cancer is diagnosed in approximately 5.4 million individuals annually in the United States, more than the total number of breast, lung, colon, and prostate cancers diagnosed per year.1 It is estimated that 1 in 5 Americans will develop skin cancer during their lifetime.2 The 2 most common forms of skin cancer are basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), accounting for 4 million and 1 million cases diagnosed each year, respectively.3 With the increasing incidence of these skin cancers, the use of noninvasive imaging tools for detection and diagnosis has grown.
Ex vivo confocal microscopy is a diagnostic imaging tool that can be used in real-time at the bedside to assess freshly excised tissue for malignancies. It images tissue samples with cellular resolution and within minutes of biopsy or excision. Ex vivo confocal microscopy is a versatile tool that can assist in the diagnosis and management of skin malignancies such as melanoma, BCC, and SCC.
Reflectance vs Fluorescence Mode
Excised lesions can be examined in reflectance or fluorescence mode in great detail but with slightly varying nuclear-to-dermis contrasts depending on the chromophore that is targeted. In reflectance mode (reflectance confocal microscopy [RCM]), melanin and keratin act as endogenous chromophores because of their high refractive index relative to water,4,5 which allows for the visualization of cellular structures of the skin at low power, as well as microscopic substructures such as melanosomes, cytoplasmic granules, and other cellular organelles at high power. Although an exogenous contrast agent is not required, acetic acid has the capability to highlight nuclei, enhancing the tumor cell-to-dermis contrast in RCM.6 Acetic acid is clinically used as a predictor for certain skin and mucosal membrane neoplasms that blanch when exposed to the solution. In the case of RCM, acetic acid increases the visibility of nuclei by inducing the compaction of chromatin. For the acetowhitening to be effective, the sample must be soaked in the solution for a specific amount of time, depending on the concentration.7 A concentration between 1% and 10% can be used, but the less concentrated the solution, the longer the time of soaking that is required to achieve sufficiently bright nuclei.6
The contrast with acetic acid, however, is quite weak when the tissue is imaged en face, or along the horizontal surface of the sample, due to the collagen in the dermal layer, which has a high reflectance index. This issue is rectified when using the confocal microscope in the fluorescence mode with an exogenous fluorescent dye as a nuclear stain. Fluorescence confocal microscopy (FCM), results in a stronger nuclear-to-dermal contrast because of the role of contrast agents.8 The 1000-fold increase in contrast between nuclei and dermis is the result of dye agents that preferentially bind to nuclear DNA, of which acridine orange is the most commonly used.5,8 Basal cell carcinoma and SCC tumor cells can be visualized with FCM because they appear hyperfluorescent when stained with acridine orange.9 The acridine orange–stained cells display bright nuclei, while the cytoplasm and collagen remains dark. A positive feature of acridine orange is that it does not alter the tissue sample during freezing or formalin fixation and thus has no effect on subsequent histopathology that may need to be performed on the sample.10
High-Resolution Images Aid in Diagnosis
After it is harvested, the tissue sample is soaked in a contrast agent or dye, if needed, depending on the confocal mode to be used. The confocal microscope is then used to take a series of high-resolution individual en face images that are then stitched together to create a final mosaic image that can be up to 12×12 mm.6,11 With a 200-µm depth visibility, confocal microscopy can capture the cellular structures in the epidermis, dermis, and (if compressed enough) subcutaneous fat in just under 3 minutes.12
The images produced through confocal microscopy have an excellent correlation to frozen histological sections and can aid in the diagnosis of many epidermal and dermal malignancies including melanoma, BCC, and SCC. New criteria have been established to aid in the interpretation of the confocal images and identify some of the more common skin cancers.5,12,13 Basal cell carcinoma samples imaged through fluorescence and reflectance in low-power mode display the distinct nodular patterns with well-demarcated edges, as seen on classical histopathology. In the case of FCM, the cells that make up the tumor display hyperfluorescent areas consistent with nucleated cells that are stained with acridine orange. The main features that identify BCC on FCM images include nuclear pleomorphism and crowding, peripheral palisading, clefting of the basaloid islands, increased nucleus-to-cytoplasm ratio, and the presence of a modified dermis surrounding the mass known as the tumoral stroma5,12 (Figure).

In addition to fluorescence and a well-defined tumor silhouette, SCC under FCM displays keratin pearls composed of keratinized squames, nuclear pleomorphism, and fluorescent scales in the stratum corneum that are a result of keratin formation.5,13 The extent of differentiation of the SCC lesion also can be determined by assessing if the silhouette is well defined. A well-defined tumor silhouette is consistent with the diagnosis of a well-differentiated SCC, and vice versa.13 Ex vivo RCM also has been shown to be useful in diagnosing malignant melanomas, with melanin acting as an endogenous chromophore. Some of the features seen on imaging include a disarranged epithelium, hyperreflective roundish and dendritic pagetoid cells, and large hyperreflective polymorphic cells in the superficial chorion.14
Comparison to Conventional Histopathology
Ex vivo confocal microscopy in both the reflectance and fluorescence mode has been shown to perform well compared to conventional histopathology in the diagnosis of biopsy specimens. Ex vivo FCM has been shown to have an overall sensitivity of 88% and specificity of 99% in detecting residual BCC at the margins of excised tissue samples and in the fraction of the time it takes to attain similar results with frozen histopathology.9 Ex vivo RCM has been shown to have a higher prognostic capability, with 100% sensitivity and specificity in identifying BCC when scanning the tissue samples en face.15
Qualitatively, the images produced by RCM and FCM are similar to histopathology in overall architecture. Both techniques enhance the contrast between the epithelium and stroma and create images that can be examined in low as well as high resolution. A substantial difference between confocal microscopy and conventional hematoxylin and eosin–stained histopathology is that the confocal microscope produces images in gray scale. One way to alter the black-and-white images to resemble hematoxylin and eosin–stained slides is through the use of digital staining,16 which could boost clinical acceptance by physicians who are accustomed to the classical pink-purple appearance of pathology slides and could potentially limit the learning curve needed to read the confocal images.
Application in Mohs Micrographic Surgery
An important clinical application of ex vivo FCM imaging that has emerged is the detection of malignant cells at the excision margins during Mohs micrographic surgery. The use of confocal microscopy has the potential to save time by eliminating the need for tissue fixation while still providing good diagnostic accuracy. Implementing FCM as an imaging tool to guide surgical excisions could provide rapid diagnosis of the tissue, expediting excisions and reconstruction or the Mohs procedure while eliminating patient wait time and the need for frozen histopathology. Ex vivo RCM also has been used to establish laser parameters for CO2 laser ablation of superficial and early nodular BCC lesions.17 Other potential uses for ex vivo RCM/FCM could include rapid evaluation of tissue during operating room procedures where rapid frozen sections are currently utilized.
Combining In Vivo and Ex Vivo Confocal Microscopy
Many of the diagnostic guidelines created with the use of ex vivo confocal microscopy have been applied to in vivo use, and therefore the use of both modalities is appealing. In vivo confocal microscopy is a noninvasive technique that has been used to map margins of skin tumors such as BCC and lentigo maligna at the bedside.5 It also has been shown to help plan both surgical and nonsurgical treatment modalities and reconstruction before the tumor is excised.18 This technique also can help the patient understand the extent of the excision and any subsequent reconstruction that may be needed.
Limitations
Ex vivo confocal microscopy used as a diagnostic tool does have some limitations. Its novelty may require surgeons and pathologists to be trained to interpret the images properly and correlate them to conventional diagnostic guidelines. The imaging also is limited to a depth of approximately 200 µm; however, the sample may be flipped so that the underside can be imaged as well, which increases the depth to approximately 400 µm. The tissue being imaged must be fixed flat, which may alter its shape. Complex tissue samples may be difficult to flatten out completely and therefore may be difficult to image. A special mount may be required for the sample to be fixed in a proper position for imaging.6
Final Thoughts
Despite some of these limitations, the need for rapid bedside tissue diagnosis makes ex vivo confocal microscopy an attractive device that can be used as an additional diagnostic tool to histopathology and also has been tested in other disciplines, such as breast cancer pathology. In the future, both in vivo and ex vivo confocal microscopy may be utilized to diagnose cutaneous malignancies, guide surgical excisions, and detect lesion progression, and it may become a basis for rapid diagnosis and detection.19
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016 [published online January 7, 2016]. CA Cancer J Clin. 2016;66:7-30.
- Robinson JK. Sun exposure, sun protection, and vitamin D. JAMA. 2005;294:1541-1543.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Welzel J, Kästle R, Sattler EC. Fluorescence (multiwave) confocal microscopy. Dermatol Clin. 2016;34:527-533.
- Longo C, Ragazzi M, Rajadhyaksha M, et al. In vivo and ex vivo confocal microscopy for dermatologic and Mohs surgeons. Dermatol Clin. 2016;34:497-504.
- Patel YG, Nehal KS, Aranda I, et al. Confocal reflectance mosaicing of basal cell carcinomas in Mohs surgical skin excisions. J Biomed Opt. 2007;12:034027.
- Rajadhyaksha M, Gonzalez S, Zavislan JM. Detectability of contrast agents for confocal reflectance imaging of skin and microcirculation. J Biomed Opt. 2004;9:323-331.
- Karen JK, Gareau DS, Dusza SW, et al. Detection of basal cell carcinomas in Mohs excisions with fluorescence confocal mosaicing microscopy. Br J Dermatol. 2009;160:1242-1250.
- Bennàssar A, Vilata A, Puig S, et al. Ex vivo fluorescence confocal microscopy for fast evaluation of tumour margins during Mohs surgery. Br J Dermatol. 2014;170:360-365.
- Gareau DS, Li Y, Huang B, et al. Confocal mosaicing microscopy in Mohs skin excisions: feasibility of rapid surgical pathology. J Biomed Opt. 2008;13:054001.
- Bini J, Spain J, Nehal K, et al. Confocal mosaicing microscopy of human skin ex vivo: spectral analysis for digital staining to simulate histology-like appearance. J Biomed Opt. 2011;16:076008.
- Bennàssar A, Carrera C, Puig S, et al. Fast evaluation of 69 basal cell carcinomas with ex vivo fluorescence confocal microscopy: criteria description, histopathological correlation, and interobserver agreement. JAMA Dermatol. 2013;149:839-847.
- Longo C, Ragazzi M, Gardini S, et al. Ex vivo fluorescence confocal microscopy in conjunction with Mohs micrographic surgery for cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2015;73:321-322.
- Cinotti E, Haouas M, Grivet D, et al. In vivo and ex vivo confocal microscopy for the management of a melanoma of the eyelid margin. Dermatol Surg. 2015;41:1437-1440.
- , , , ‘En face’ ex vivo reflectance confocal microscopy to help the surgery of basal cell carcinoma of the eyelid [published online December 19, 2016]. Clin Exp Ophthalmol. doi:10.1111/ceo.12904.
- Gareau DS, Jeon H, Nehal KS, et al. Rapid screening of cancer margins in tissue with multimodal confocal microscopy. J Surg Res. 2012;178:533-538.
- Sierra H, Damanpour S, Hibler B, et al. Confocal imaging of carbon dioxide laser-ablated basal cell carcinomas: an ex-vivo study on the uptake of contrast agent and ablation parameters [published online September 22, 2015]. Lasers Surg Med. 2016;48:133-139.
- Hibler BP, Yélamos O, Cordova M, et al. Handheld reflectance confocal microscopy to aid in the management of complex facial lentigo maligna. Cutis. 2017;99:346-352.
- Rajadhyaksha M, Marghoob A, Rossi A, et al. Reflectance confocal microscopy of skin in vivo: from bench to bedside. Lasers Surg Med. 2017;49:7-19.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016 [published online January 7, 2016]. CA Cancer J Clin. 2016;66:7-30.
- Robinson JK. Sun exposure, sun protection, and vitamin D. JAMA. 2005;294:1541-1543.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Welzel J, Kästle R, Sattler EC. Fluorescence (multiwave) confocal microscopy. Dermatol Clin. 2016;34:527-533.
- Longo C, Ragazzi M, Rajadhyaksha M, et al. In vivo and ex vivo confocal microscopy for dermatologic and Mohs surgeons. Dermatol Clin. 2016;34:497-504.
- Patel YG, Nehal KS, Aranda I, et al. Confocal reflectance mosaicing of basal cell carcinomas in Mohs surgical skin excisions. J Biomed Opt. 2007;12:034027.
- Rajadhyaksha M, Gonzalez S, Zavislan JM. Detectability of contrast agents for confocal reflectance imaging of skin and microcirculation. J Biomed Opt. 2004;9:323-331.
- Karen JK, Gareau DS, Dusza SW, et al. Detection of basal cell carcinomas in Mohs excisions with fluorescence confocal mosaicing microscopy. Br J Dermatol. 2009;160:1242-1250.
- Bennàssar A, Vilata A, Puig S, et al. Ex vivo fluorescence confocal microscopy for fast evaluation of tumour margins during Mohs surgery. Br J Dermatol. 2014;170:360-365.
- Gareau DS, Li Y, Huang B, et al. Confocal mosaicing microscopy in Mohs skin excisions: feasibility of rapid surgical pathology. J Biomed Opt. 2008;13:054001.
- Bini J, Spain J, Nehal K, et al. Confocal mosaicing microscopy of human skin ex vivo: spectral analysis for digital staining to simulate histology-like appearance. J Biomed Opt. 2011;16:076008.
- Bennàssar A, Carrera C, Puig S, et al. Fast evaluation of 69 basal cell carcinomas with ex vivo fluorescence confocal microscopy: criteria description, histopathological correlation, and interobserver agreement. JAMA Dermatol. 2013;149:839-847.
- Longo C, Ragazzi M, Gardini S, et al. Ex vivo fluorescence confocal microscopy in conjunction with Mohs micrographic surgery for cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2015;73:321-322.
- Cinotti E, Haouas M, Grivet D, et al. In vivo and ex vivo confocal microscopy for the management of a melanoma of the eyelid margin. Dermatol Surg. 2015;41:1437-1440.
- , , , ‘En face’ ex vivo reflectance confocal microscopy to help the surgery of basal cell carcinoma of the eyelid [published online December 19, 2016]. Clin Exp Ophthalmol. doi:10.1111/ceo.12904.
- Gareau DS, Jeon H, Nehal KS, et al. Rapid screening of cancer margins in tissue with multimodal confocal microscopy. J Surg Res. 2012;178:533-538.
- Sierra H, Damanpour S, Hibler B, et al. Confocal imaging of carbon dioxide laser-ablated basal cell carcinomas: an ex-vivo study on the uptake of contrast agent and ablation parameters [published online September 22, 2015]. Lasers Surg Med. 2016;48:133-139.
- Hibler BP, Yélamos O, Cordova M, et al. Handheld reflectance confocal microscopy to aid in the management of complex facial lentigo maligna. Cutis. 2017;99:346-352.
- Rajadhyaksha M, Marghoob A, Rossi A, et al. Reflectance confocal microscopy of skin in vivo: from bench to bedside. Lasers Surg Med. 2017;49:7-19.
Practice Points
- Confocal microscopy is an imaging tool that can be used both in vivo and ex vivo to aid in the diagnosis and management of cutaneous neoplasms, including melanoma, basal cell carcinoma, and squamous cell carcinoma, as well as inflammatory dermatoses.
- Ex vivo confocal microscopy can be used in both reflectance and fluorescent modes to render diagnosis in excised tissue or check surgical margins.
- Both in vivo and ex vivo confocal microscopy produces images with cellular resolution with a main limitation being depth of imaging.
What’s on the dermatopathologist’s wish list
NEW YORK – If dermatopathologists had a wish list they could give their dermatologist colleagues, what might it include? High up on the list for many, said Robert Phelps, MD, might be to have them share the clinical picture, treat the specimen gently, and give the best landmarks possible.
Speaking at the summer meeting of the American Academy of Dermatology, Dr. Phelps, director of the dermatopathology service at Mount Sinai Medical Center in New York, led off the dermatopathologist-run session – appropriately titled “Help Me Help You” – by asking, “How can the clinician provide the optimal biopsy?”
It’s always helpful to have as much clinical information as possible, said Dr. Phelps, whose discussion focused on tips for neoplastic lesions. This might include prior history of malignancy, autoimmune disease, pathergy, or other relevant medical history, but clinical pictures can also be a big help, although there can be technical and patient privacy issues to overcome, he noted. If, for example, a larger lesion or rash is being biopsied rather than excised, it can be very helpful to see the larger field and full area of distribution of the lesion in question. Submitting multiple specimens for rashes and larger lesions is always a good idea too, he added.
Although curettage can be a great way to biopsy – and perhaps even definitively treat some lesions – problems can arise on the dermatopathologist’s side when melanocytic lesions are curetted for biopsy, according to Dr. Phelps, a practicing dermatologist and a dermatopathologist. “By virtue of the force of the biopsy, the specimen is often fragmented, and histology can be distorted,” he said. One element of that distortion can be that melanocytes can appear to be free floating, which is a problem. “Dyshesion of melanocytes is usually an indication of atypia … It is an important histologic clue as to the possibility of a malignancy supervening.”
These factors can make it tough for a dermatopathologist to make an accurate call. “If there are free-floating melanocytes from a curetted specimen, I can’t rule out invasive melanoma,” explained Dr. Phelps, since he can’t tell if he is seeing true atypia or disruption that’s an artifact of the collection technique.
In this instance, he said, a dermatopathologist would be “obligated to overcall, because one couldn’t really determine the pathology.” The bottom line? “Don’t curette biopsies of melanocytic lesions.”
Another technique that can interfere with the ability to read a tissue specimen accurately is electrodesiccation. Although it’s often performed in conjunction with curettage, electrodesiccation can cause changes in tissue consistent with thermal injury. “Essentially, the tissue has been burned,” Dr. Phelps pointed out. This can result in a characteristic streaming pattern of nuclei, and the dermis can acquire a “peculiar homogenized appearance,” he said.
Although electrodesiccation can be a useful technique to make sure margins are controlled, “when you do this, just be aware that the interpretation is difficult,” he noted. “It’s difficult to tell where the margins are and if they are the appropriate and correct margins,” he said.
When possible, try to avoid squeezing the tumor, Dr. Phelps advised. Excessive pressure on the specimen can distort cell architecture and make pathological diagnosis really challenging, particularly in lymphoid tumors, he said.
“Often, the tumor is not recognizable,” he added. Crush artifact can result in an appearance of small bluish clumps and smearing of collagen fibers. The effect, he said, can be particularly pronounced with small cell carcinoma and lymphoma, and with rapidly proliferating tumors.
Dr. Phelps said that during his training, he was taught not to use forceps to extract a stubborn punch biopsy specimen; rather, he was trained to use a needle to tease out the specimen. Fear of a self-inflicted needle stick with this technique may be a deterrent, he acknowledged. If forceps are used, he suggested being as gentle as possible and using the finest forceps available.
When pathologists receive an intact excised lesion – one not obtained using a Mohs technique, “delineation of the margin is essential,” Dr. Phelps said. Further, accurate mapping is critical to helping the examiner understand the anatomic orientation of the specimen, a key prerequisite that enables accurate communication from the dermatopathologist back to the clinician if there’s a question regarding the need for retreatment, he added.
For an elliptical excision, ideally, both poles of the ellipse would be suture-tagged, and at least one tag is essential, he said. Then superior and inferior borders can be inked with contrasting colors, and the epidermal borders of the lesion should be marked as well. When the specimen is submitted, it should be accompanied by an accurate map that clearly indicates the coding for medial, lateral, inferior, and superior aspects of the specimen. “Always prepare a specimen diagram for oriented specimens,” Dr. Phelps noted.
Don’t forget to make sure that the left-right orientation on the diagram corresponds to the specimen’s orientation on the patient, he added. Some facilities use a clock face system to indicate orientation and positioning, which may be the clearest method of all.
Sometimes, it’s difficult for the dermatopathologist to visualize whether the specimen is aligned in true medial-lateral fashion, or along skin tension lines, which tend to run diagonally, so “the more clinical information, the better,” he said. “With good mapping, precise retreatment can be optimal,” he said.
Dr. Phelps reported that he had no relevant conflicts of interest.
koakes@frontlinemedcom.com
On Twitter @karioakes
NEW YORK – If dermatopathologists had a wish list they could give their dermatologist colleagues, what might it include? High up on the list for many, said Robert Phelps, MD, might be to have them share the clinical picture, treat the specimen gently, and give the best landmarks possible.
Speaking at the summer meeting of the American Academy of Dermatology, Dr. Phelps, director of the dermatopathology service at Mount Sinai Medical Center in New York, led off the dermatopathologist-run session – appropriately titled “Help Me Help You” – by asking, “How can the clinician provide the optimal biopsy?”
It’s always helpful to have as much clinical information as possible, said Dr. Phelps, whose discussion focused on tips for neoplastic lesions. This might include prior history of malignancy, autoimmune disease, pathergy, or other relevant medical history, but clinical pictures can also be a big help, although there can be technical and patient privacy issues to overcome, he noted. If, for example, a larger lesion or rash is being biopsied rather than excised, it can be very helpful to see the larger field and full area of distribution of the lesion in question. Submitting multiple specimens for rashes and larger lesions is always a good idea too, he added.
Although curettage can be a great way to biopsy – and perhaps even definitively treat some lesions – problems can arise on the dermatopathologist’s side when melanocytic lesions are curetted for biopsy, according to Dr. Phelps, a practicing dermatologist and a dermatopathologist. “By virtue of the force of the biopsy, the specimen is often fragmented, and histology can be distorted,” he said. One element of that distortion can be that melanocytes can appear to be free floating, which is a problem. “Dyshesion of melanocytes is usually an indication of atypia … It is an important histologic clue as to the possibility of a malignancy supervening.”
These factors can make it tough for a dermatopathologist to make an accurate call. “If there are free-floating melanocytes from a curetted specimen, I can’t rule out invasive melanoma,” explained Dr. Phelps, since he can’t tell if he is seeing true atypia or disruption that’s an artifact of the collection technique.
In this instance, he said, a dermatopathologist would be “obligated to overcall, because one couldn’t really determine the pathology.” The bottom line? “Don’t curette biopsies of melanocytic lesions.”
Another technique that can interfere with the ability to read a tissue specimen accurately is electrodesiccation. Although it’s often performed in conjunction with curettage, electrodesiccation can cause changes in tissue consistent with thermal injury. “Essentially, the tissue has been burned,” Dr. Phelps pointed out. This can result in a characteristic streaming pattern of nuclei, and the dermis can acquire a “peculiar homogenized appearance,” he said.
Although electrodesiccation can be a useful technique to make sure margins are controlled, “when you do this, just be aware that the interpretation is difficult,” he noted. “It’s difficult to tell where the margins are and if they are the appropriate and correct margins,” he said.
When possible, try to avoid squeezing the tumor, Dr. Phelps advised. Excessive pressure on the specimen can distort cell architecture and make pathological diagnosis really challenging, particularly in lymphoid tumors, he said.
“Often, the tumor is not recognizable,” he added. Crush artifact can result in an appearance of small bluish clumps and smearing of collagen fibers. The effect, he said, can be particularly pronounced with small cell carcinoma and lymphoma, and with rapidly proliferating tumors.
Dr. Phelps said that during his training, he was taught not to use forceps to extract a stubborn punch biopsy specimen; rather, he was trained to use a needle to tease out the specimen. Fear of a self-inflicted needle stick with this technique may be a deterrent, he acknowledged. If forceps are used, he suggested being as gentle as possible and using the finest forceps available.
When pathologists receive an intact excised lesion – one not obtained using a Mohs technique, “delineation of the margin is essential,” Dr. Phelps said. Further, accurate mapping is critical to helping the examiner understand the anatomic orientation of the specimen, a key prerequisite that enables accurate communication from the dermatopathologist back to the clinician if there’s a question regarding the need for retreatment, he added.
For an elliptical excision, ideally, both poles of the ellipse would be suture-tagged, and at least one tag is essential, he said. Then superior and inferior borders can be inked with contrasting colors, and the epidermal borders of the lesion should be marked as well. When the specimen is submitted, it should be accompanied by an accurate map that clearly indicates the coding for medial, lateral, inferior, and superior aspects of the specimen. “Always prepare a specimen diagram for oriented specimens,” Dr. Phelps noted.
Don’t forget to make sure that the left-right orientation on the diagram corresponds to the specimen’s orientation on the patient, he added. Some facilities use a clock face system to indicate orientation and positioning, which may be the clearest method of all.
Sometimes, it’s difficult for the dermatopathologist to visualize whether the specimen is aligned in true medial-lateral fashion, or along skin tension lines, which tend to run diagonally, so “the more clinical information, the better,” he said. “With good mapping, precise retreatment can be optimal,” he said.
Dr. Phelps reported that he had no relevant conflicts of interest.
koakes@frontlinemedcom.com
On Twitter @karioakes
NEW YORK – If dermatopathologists had a wish list they could give their dermatologist colleagues, what might it include? High up on the list for many, said Robert Phelps, MD, might be to have them share the clinical picture, treat the specimen gently, and give the best landmarks possible.
Speaking at the summer meeting of the American Academy of Dermatology, Dr. Phelps, director of the dermatopathology service at Mount Sinai Medical Center in New York, led off the dermatopathologist-run session – appropriately titled “Help Me Help You” – by asking, “How can the clinician provide the optimal biopsy?”
It’s always helpful to have as much clinical information as possible, said Dr. Phelps, whose discussion focused on tips for neoplastic lesions. This might include prior history of malignancy, autoimmune disease, pathergy, or other relevant medical history, but clinical pictures can also be a big help, although there can be technical and patient privacy issues to overcome, he noted. If, for example, a larger lesion or rash is being biopsied rather than excised, it can be very helpful to see the larger field and full area of distribution of the lesion in question. Submitting multiple specimens for rashes and larger lesions is always a good idea too, he added.
Although curettage can be a great way to biopsy – and perhaps even definitively treat some lesions – problems can arise on the dermatopathologist’s side when melanocytic lesions are curetted for biopsy, according to Dr. Phelps, a practicing dermatologist and a dermatopathologist. “By virtue of the force of the biopsy, the specimen is often fragmented, and histology can be distorted,” he said. One element of that distortion can be that melanocytes can appear to be free floating, which is a problem. “Dyshesion of melanocytes is usually an indication of atypia … It is an important histologic clue as to the possibility of a malignancy supervening.”
These factors can make it tough for a dermatopathologist to make an accurate call. “If there are free-floating melanocytes from a curetted specimen, I can’t rule out invasive melanoma,” explained Dr. Phelps, since he can’t tell if he is seeing true atypia or disruption that’s an artifact of the collection technique.
In this instance, he said, a dermatopathologist would be “obligated to overcall, because one couldn’t really determine the pathology.” The bottom line? “Don’t curette biopsies of melanocytic lesions.”
Another technique that can interfere with the ability to read a tissue specimen accurately is electrodesiccation. Although it’s often performed in conjunction with curettage, electrodesiccation can cause changes in tissue consistent with thermal injury. “Essentially, the tissue has been burned,” Dr. Phelps pointed out. This can result in a characteristic streaming pattern of nuclei, and the dermis can acquire a “peculiar homogenized appearance,” he said.
Although electrodesiccation can be a useful technique to make sure margins are controlled, “when you do this, just be aware that the interpretation is difficult,” he noted. “It’s difficult to tell where the margins are and if they are the appropriate and correct margins,” he said.
When possible, try to avoid squeezing the tumor, Dr. Phelps advised. Excessive pressure on the specimen can distort cell architecture and make pathological diagnosis really challenging, particularly in lymphoid tumors, he said.
“Often, the tumor is not recognizable,” he added. Crush artifact can result in an appearance of small bluish clumps and smearing of collagen fibers. The effect, he said, can be particularly pronounced with small cell carcinoma and lymphoma, and with rapidly proliferating tumors.
Dr. Phelps said that during his training, he was taught not to use forceps to extract a stubborn punch biopsy specimen; rather, he was trained to use a needle to tease out the specimen. Fear of a self-inflicted needle stick with this technique may be a deterrent, he acknowledged. If forceps are used, he suggested being as gentle as possible and using the finest forceps available.
When pathologists receive an intact excised lesion – one not obtained using a Mohs technique, “delineation of the margin is essential,” Dr. Phelps said. Further, accurate mapping is critical to helping the examiner understand the anatomic orientation of the specimen, a key prerequisite that enables accurate communication from the dermatopathologist back to the clinician if there’s a question regarding the need for retreatment, he added.
For an elliptical excision, ideally, both poles of the ellipse would be suture-tagged, and at least one tag is essential, he said. Then superior and inferior borders can be inked with contrasting colors, and the epidermal borders of the lesion should be marked as well. When the specimen is submitted, it should be accompanied by an accurate map that clearly indicates the coding for medial, lateral, inferior, and superior aspects of the specimen. “Always prepare a specimen diagram for oriented specimens,” Dr. Phelps noted.
Don’t forget to make sure that the left-right orientation on the diagram corresponds to the specimen’s orientation on the patient, he added. Some facilities use a clock face system to indicate orientation and positioning, which may be the clearest method of all.
Sometimes, it’s difficult for the dermatopathologist to visualize whether the specimen is aligned in true medial-lateral fashion, or along skin tension lines, which tend to run diagonally, so “the more clinical information, the better,” he said. “With good mapping, precise retreatment can be optimal,” he said.
Dr. Phelps reported that he had no relevant conflicts of interest.
koakes@frontlinemedcom.com
On Twitter @karioakes
EXPERT ANALYSIS FROM THE 2017 AAD SUMMER MEETING
Topical Timolol May Improve Overall Scar Cosmesis in Acute Surgical Wounds
Timolol is a nonselective β-adrenergic receptor antagonist indicated for treating glaucoma, heart attacks, hypertension, and migraine headaches. It is made in both an oral and ophthalmic form. In dermatology, the beta-blocker propranolol is approved for the treatment of infantile hemangiomas (IHs). The exact mechanism of action of beta-blockers for the treatment of IHs is not yet completely understood, but it is postulated that they inhibit growth by at least 4 distinct mechanisms: (1) vasoconstriction, (2) inhibition of angiogenesis or vasculogenesis, (3) induction of apoptosis, and (4) recruitment of endothelial progenitor cells to the site of the hemangioma.1
Scar cosmesis can be calculated using the visual analog scale (VAS), which is a subjective scar assessment scored from poor to excellent. The multidimensional VAS is a photograph-based scale derived from evaluating standardized digital photographs in 4 dimensions—pigmentation, vascularity, acceptability, and observer comfort—plus contour. It uses the sum of the individual scores to obtain a single overall score ranging from excellent to poor.2 In this study, we sought to determine if the use of topical timolol after excision or Mohs micrographic surgery (MMS) treatment of nonmelanoma skin cancers improved the overall cosmesis of the scar.
Methods
The study protocol was approved by the institutional review board at Roger Williams Medical Center (Providence, Rhode Island). Eligibility criteria included patients who required excision or MMS for their nonmelanoma skin cancer located below the patella and those who agreed to allow their wounds to heal by secondary intention when given options for closure of their wounds. Patients were randomized to either the timolol (study medication) group or the saline (placebo) group. The initial defects were measured and photographed. Patients were educated on how to apply the study medication. All patients were prescribed 40 mm Hg compression stockings to wear following application of the study medication. Patients were asked to return at 1 and 5 weeks postsurgery and then every 1 to 2 weeks for wound assessment and measurement until their wounds had healed or at 13 weeks, depending on which came first. A healed wound was defined as having no exudate, exhibiting complete reepithelialization, and being stable for 1 week.
Healed wounds were assessed by a blinded outside dermatologist who examined photographs of the wounds and then completed the VAS for each participant’s scar.
Results
A total of 9 participants were enrolled in the study. Three participants were lost to follow-up; 6 completed the study (4 females, 2 males). The mean age was 70 years (age range, 46–89 years). The average wound size was 2×2 cm with a depth of 1 mm. Three participants were in the active medication group and 3 were in the control group.
A VAS was completed for each participant’s scar by an outside blinded dermatologist. Based on the VAS, wounds treated with timolol resulted in more cosmetically favorable scars (scored higher on the VAS) compared to control (mean [SD]: 6.5±0.9 vs 2.5±0.7; P<0.05). See Figures 1 and 2 for representative results.
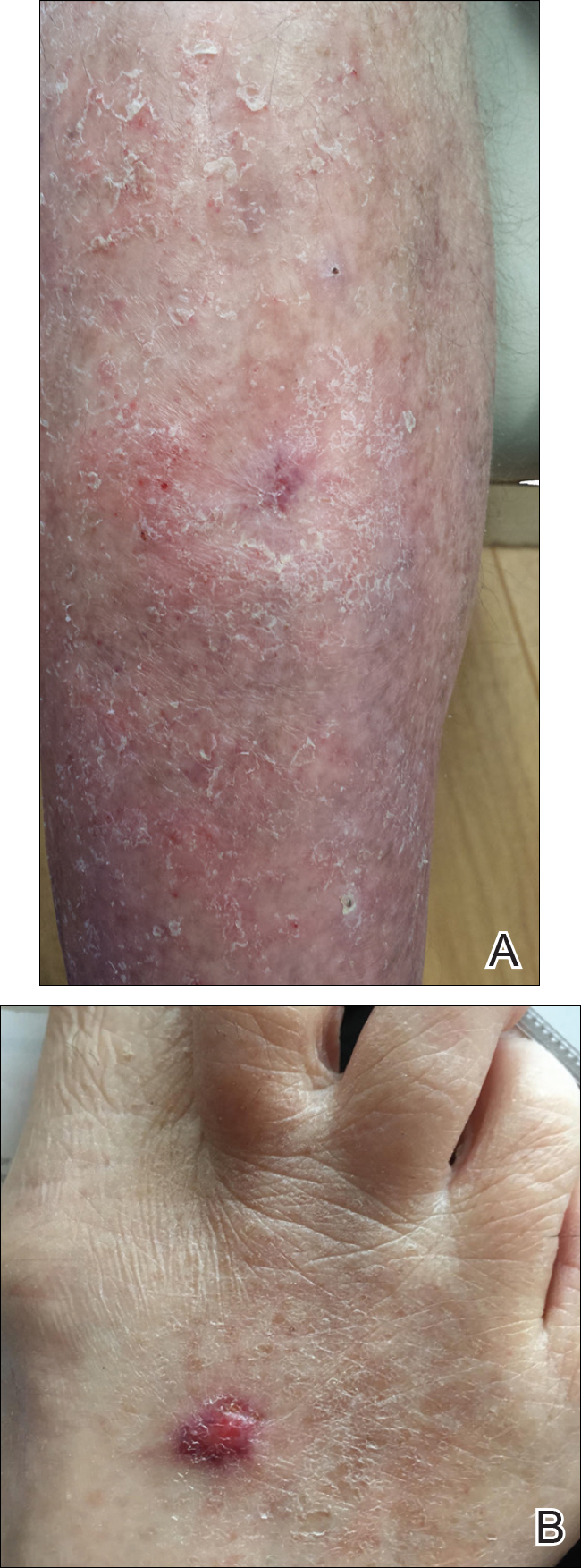
Comment
Dermatologists create acute wounds in patients on a daily basis. Ensuring that patients achieve the most desirable cosmetic outcome is a primary goal for dermatologists and an important component of patient satisfaction. A number of studies have examined patient satisfaction following MMS.3,4 Patient satisfaction is an especially important outcome measure in dermatology, as dermatologic diseases affect cosmetic appearance and are related to quality of life.3,4
Timolol is a nonselective β-adrenergic receptor antagonist that is used in dermatology to treat IHs. In this preliminary study, the authors sought to determine if topical timolol applied to acute wounds following surgical removal of nonmelanoma skin cancers could improve the overall cosmetic outcome of acute surgical scars. The results showed that compared to control, topical timolol resulted in a more cosmetically favorable scar. The results are preliminary, and it would be of future interest to further study the effects of topical timolol on acute surgical wounds from a wound-healing standpoint as well as to further test its effects on the cosmesis of these wounds.
- Chisholm KM, Chang KW, Truong MT, et al. β-Adrenergic receptor expression in vascular tumors [published online June 29, 2012]. Mod Pathol. 2012;25:1446-1451.
- Fearmonti R, Bond J, Erdmann D, et al. A review of scar scales and scar measuring devices. Eplasty. 2010;10:e43.
- Asgari MM, Warton EM, Neugebauer R, et al. Predictors of patient satisfaction with Mohs surgery: analysis of preoperative, intraoperative, and postoperative factors in a prospective cohort. Arch Dermatol. 2011;147:1387-1394.
- Asgari MM, Bertenthal D, Sen S, et al. Patient satisfaction after treatment of nonmelanoma skin cancer. Dermatol Surg. 2009;35:1041-1049.
Timolol is a nonselective β-adrenergic receptor antagonist indicated for treating glaucoma, heart attacks, hypertension, and migraine headaches. It is made in both an oral and ophthalmic form. In dermatology, the beta-blocker propranolol is approved for the treatment of infantile hemangiomas (IHs). The exact mechanism of action of beta-blockers for the treatment of IHs is not yet completely understood, but it is postulated that they inhibit growth by at least 4 distinct mechanisms: (1) vasoconstriction, (2) inhibition of angiogenesis or vasculogenesis, (3) induction of apoptosis, and (4) recruitment of endothelial progenitor cells to the site of the hemangioma.1
Scar cosmesis can be calculated using the visual analog scale (VAS), which is a subjective scar assessment scored from poor to excellent. The multidimensional VAS is a photograph-based scale derived from evaluating standardized digital photographs in 4 dimensions—pigmentation, vascularity, acceptability, and observer comfort—plus contour. It uses the sum of the individual scores to obtain a single overall score ranging from excellent to poor.2 In this study, we sought to determine if the use of topical timolol after excision or Mohs micrographic surgery (MMS) treatment of nonmelanoma skin cancers improved the overall cosmesis of the scar.
Methods
The study protocol was approved by the institutional review board at Roger Williams Medical Center (Providence, Rhode Island). Eligibility criteria included patients who required excision or MMS for their nonmelanoma skin cancer located below the patella and those who agreed to allow their wounds to heal by secondary intention when given options for closure of their wounds. Patients were randomized to either the timolol (study medication) group or the saline (placebo) group. The initial defects were measured and photographed. Patients were educated on how to apply the study medication. All patients were prescribed 40 mm Hg compression stockings to wear following application of the study medication. Patients were asked to return at 1 and 5 weeks postsurgery and then every 1 to 2 weeks for wound assessment and measurement until their wounds had healed or at 13 weeks, depending on which came first. A healed wound was defined as having no exudate, exhibiting complete reepithelialization, and being stable for 1 week.
Healed wounds were assessed by a blinded outside dermatologist who examined photographs of the wounds and then completed the VAS for each participant’s scar.
Results
A total of 9 participants were enrolled in the study. Three participants were lost to follow-up; 6 completed the study (4 females, 2 males). The mean age was 70 years (age range, 46–89 years). The average wound size was 2×2 cm with a depth of 1 mm. Three participants were in the active medication group and 3 were in the control group.
A VAS was completed for each participant’s scar by an outside blinded dermatologist. Based on the VAS, wounds treated with timolol resulted in more cosmetically favorable scars (scored higher on the VAS) compared to control (mean [SD]: 6.5±0.9 vs 2.5±0.7; P<0.05). See Figures 1 and 2 for representative results.


Comment
Dermatologists create acute wounds in patients on a daily basis. Ensuring that patients achieve the most desirable cosmetic outcome is a primary goal for dermatologists and an important component of patient satisfaction. A number of studies have examined patient satisfaction following MMS.3,4 Patient satisfaction is an especially important outcome measure in dermatology, as dermatologic diseases affect cosmetic appearance and are related to quality of life.3,4
Timolol is a nonselective β-adrenergic receptor antagonist that is used in dermatology to treat IHs. In this preliminary study, the authors sought to determine if topical timolol applied to acute wounds following surgical removal of nonmelanoma skin cancers could improve the overall cosmetic outcome of acute surgical scars. The results showed that compared to control, topical timolol resulted in a more cosmetically favorable scar. The results are preliminary, and it would be of future interest to further study the effects of topical timolol on acute surgical wounds from a wound-healing standpoint as well as to further test its effects on the cosmesis of these wounds.
Timolol is a nonselective β-adrenergic receptor antagonist indicated for treating glaucoma, heart attacks, hypertension, and migraine headaches. It is made in both an oral and ophthalmic form. In dermatology, the beta-blocker propranolol is approved for the treatment of infantile hemangiomas (IHs). The exact mechanism of action of beta-blockers for the treatment of IHs is not yet completely understood, but it is postulated that they inhibit growth by at least 4 distinct mechanisms: (1) vasoconstriction, (2) inhibition of angiogenesis or vasculogenesis, (3) induction of apoptosis, and (4) recruitment of endothelial progenitor cells to the site of the hemangioma.1
Scar cosmesis can be calculated using the visual analog scale (VAS), which is a subjective scar assessment scored from poor to excellent. The multidimensional VAS is a photograph-based scale derived from evaluating standardized digital photographs in 4 dimensions—pigmentation, vascularity, acceptability, and observer comfort—plus contour. It uses the sum of the individual scores to obtain a single overall score ranging from excellent to poor.2 In this study, we sought to determine if the use of topical timolol after excision or Mohs micrographic surgery (MMS) treatment of nonmelanoma skin cancers improved the overall cosmesis of the scar.
Methods
The study protocol was approved by the institutional review board at Roger Williams Medical Center (Providence, Rhode Island). Eligibility criteria included patients who required excision or MMS for their nonmelanoma skin cancer located below the patella and those who agreed to allow their wounds to heal by secondary intention when given options for closure of their wounds. Patients were randomized to either the timolol (study medication) group or the saline (placebo) group. The initial defects were measured and photographed. Patients were educated on how to apply the study medication. All patients were prescribed 40 mm Hg compression stockings to wear following application of the study medication. Patients were asked to return at 1 and 5 weeks postsurgery and then every 1 to 2 weeks for wound assessment and measurement until their wounds had healed or at 13 weeks, depending on which came first. A healed wound was defined as having no exudate, exhibiting complete reepithelialization, and being stable for 1 week.
Healed wounds were assessed by a blinded outside dermatologist who examined photographs of the wounds and then completed the VAS for each participant’s scar.
Results
A total of 9 participants were enrolled in the study. Three participants were lost to follow-up; 6 completed the study (4 females, 2 males). The mean age was 70 years (age range, 46–89 years). The average wound size was 2×2 cm with a depth of 1 mm. Three participants were in the active medication group and 3 were in the control group.
A VAS was completed for each participant’s scar by an outside blinded dermatologist. Based on the VAS, wounds treated with timolol resulted in more cosmetically favorable scars (scored higher on the VAS) compared to control (mean [SD]: 6.5±0.9 vs 2.5±0.7; P<0.05). See Figures 1 and 2 for representative results.


Comment
Dermatologists create acute wounds in patients on a daily basis. Ensuring that patients achieve the most desirable cosmetic outcome is a primary goal for dermatologists and an important component of patient satisfaction. A number of studies have examined patient satisfaction following MMS.3,4 Patient satisfaction is an especially important outcome measure in dermatology, as dermatologic diseases affect cosmetic appearance and are related to quality of life.3,4
Timolol is a nonselective β-adrenergic receptor antagonist that is used in dermatology to treat IHs. In this preliminary study, the authors sought to determine if topical timolol applied to acute wounds following surgical removal of nonmelanoma skin cancers could improve the overall cosmetic outcome of acute surgical scars. The results showed that compared to control, topical timolol resulted in a more cosmetically favorable scar. The results are preliminary, and it would be of future interest to further study the effects of topical timolol on acute surgical wounds from a wound-healing standpoint as well as to further test its effects on the cosmesis of these wounds.
- Chisholm KM, Chang KW, Truong MT, et al. β-Adrenergic receptor expression in vascular tumors [published online June 29, 2012]. Mod Pathol. 2012;25:1446-1451.
- Fearmonti R, Bond J, Erdmann D, et al. A review of scar scales and scar measuring devices. Eplasty. 2010;10:e43.
- Asgari MM, Warton EM, Neugebauer R, et al. Predictors of patient satisfaction with Mohs surgery: analysis of preoperative, intraoperative, and postoperative factors in a prospective cohort. Arch Dermatol. 2011;147:1387-1394.
- Asgari MM, Bertenthal D, Sen S, et al. Patient satisfaction after treatment of nonmelanoma skin cancer. Dermatol Surg. 2009;35:1041-1049.
- Chisholm KM, Chang KW, Truong MT, et al. β-Adrenergic receptor expression in vascular tumors [published online June 29, 2012]. Mod Pathol. 2012;25:1446-1451.
- Fearmonti R, Bond J, Erdmann D, et al. A review of scar scales and scar measuring devices. Eplasty. 2010;10:e43.
- Asgari MM, Warton EM, Neugebauer R, et al. Predictors of patient satisfaction with Mohs surgery: analysis of preoperative, intraoperative, and postoperative factors in a prospective cohort. Arch Dermatol. 2011;147:1387-1394.
- Asgari MM, Bertenthal D, Sen S, et al. Patient satisfaction after treatment of nonmelanoma skin cancer. Dermatol Surg. 2009;35:1041-1049.
Resident Pearl
- Dermatologists create acute surgical wounds on a daily basis. We should strive for excellent patient outcomes as well as the most desirable cosmetic result. This research article points to a possible new application of a longstanding medication to improve the cosmetic outcome in acute surgical wounds.
Chronic Diffuse Erythematous Papulonodules
The Diagnosis: Lymphomatoid Papulosis
A shave biopsy of an established lesion on the volar aspect of the left wrist was performed (Figure 1). The biopsy showed an ulcerated nodular lesion characterized by a dense mixed inflammatory cell infiltrate in the dermis composed of lymphocytes, histiocytes, scattered neutrophils, and numerous eosinophils (Figure 2). Notably there was a minor population of large atypical cells with immunoblastic and anaplastic morphology present individually and in small clusters most prominently within the upper dermis (Figures 3 and 4). Immunohistochemistry of the anaplastic cells revealed a CD30+, CD3−, CD4+, CD5−, CD8−, CD2−, CD7−, CD56−, ALK1− (anaplastic lymphoma kinase-1), PAX5− (paired box protein-5), CD20−, and CD15− phenotype. These morphologic and immunohistochemical features suggested a CD30+ cutaneous lymphoproliferative disorder. The clinical history of recurrent self-healing papulonodules in an otherwise-healthy patient established the diagnosis of lymphomatoid papulosis (LyP).

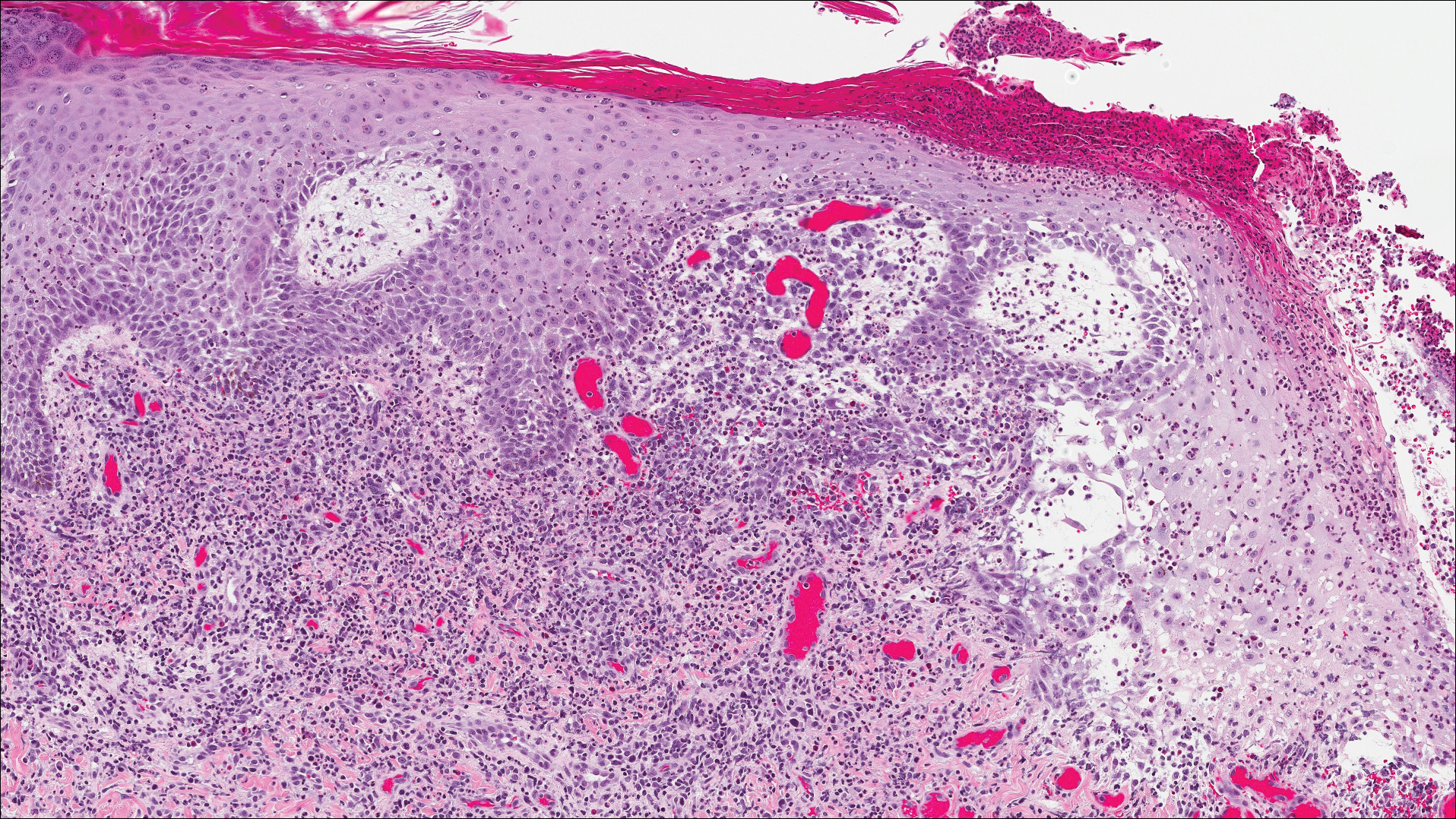
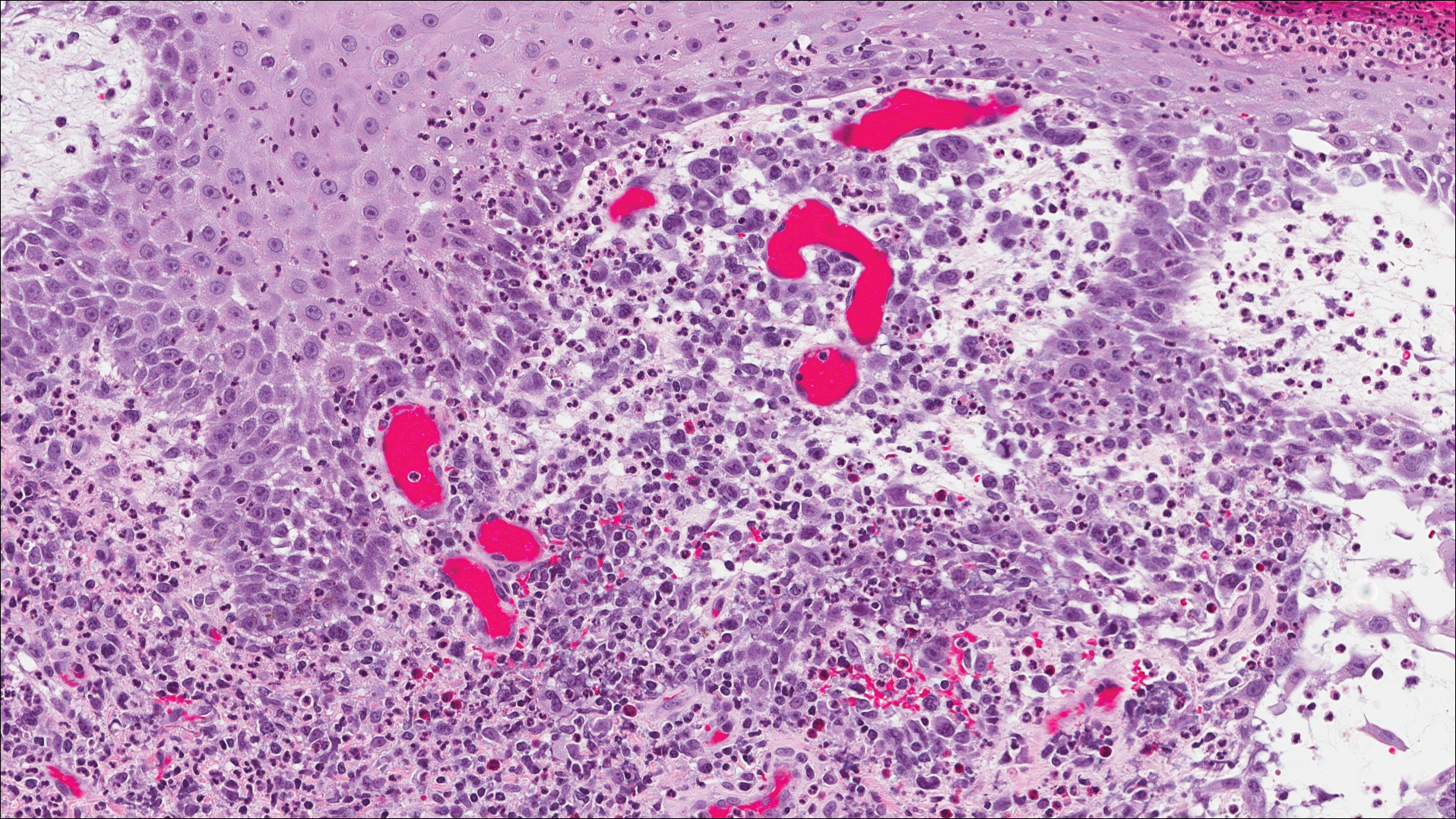
Lymphomatoid papulosis is a lymphoproliferative disorder characterized by recurrent crops of self-resolving eruptive papulonodular skin lesions that may show a variety of histologic features including a CD30+ malignant T-cell lymphoma.1 Lymphomatoid papulosis was first described in 19681 but debate continues whether the condition should be considered malignant or benign.2 Although the prognosis is excellent, LyP is characterized by a protracted course, often lasting many years. Additionally, these patients have a lifelong increased risk for development of a second cutaneous or systemic lymphoma such as mycosis fungoides (MF), cutaneous or nodal anaplastic large cell lymphoma (ALCL), or Hodgkin lymphoma, among others.
Lymphomatoid papulosis is a rare disease occurring in all ethnic groups and at any age, though most commonly presenting in the fifth decade of life. Finding large atypical T cells expressing CD30 in recurring skin lesions is highly suggestive of LyP; however, large CD30+ cells also can be seen in numerous benign reactive processes such as arthropod assault, drug eruption, viral skin infections, and other dermatoses, thus clinical correlation is always paramount. The cause of LyP is largely unknown; however, spontaneous regression may be explained by CD30-CD30 ligand interaction3 as well as an increased proapoptotic milieu.4 Specific translocations such as interferon regulatory factor-4 have been hypothesized as a risk factor for malignant progression.5-7 Additionally, an inactivating gene mutation resulting in loss of transforming growth factor β1 receptor expression and subsequent unresponsiveness to the growth inhibitory effect of transforming growth factor β may play a role in progression of LyP to ALCL.8
Clinically, LyP consists of red-brown papules and nodules generally smaller than 2 cm, often with central hemorrhage, necrosis, and crusting. Lesions are at different stages of eruption and resolution. They are often grouped but may be disseminated. Spontaneous regression typically occurs within 3 to 8 weeks. Pruritus or mild tenderness may occur as well as residual hyperpigmentation or scarring. Systemic symptoms are notably absent.
The histologic features of LyP vary according to the age of the lesion and subtype.2 Early lesions may only show a few inflammatory cells, but as lesions evolve, larger immunoblastlike CD30+ atypical cells accumulate that may resemble the Reed-Sternberg cells of Hodgkin lymphoma. Of the 5 subtypes, the most common is type A. It is characterized by a wedge-shaped infiltrate with a mixed population of scattered or clustered, large, atypical CD30+ cells, lymphocytes, neutrophils, eosinophils, and histiocytes.9 Frequent mitoses often are seen. Type B appears similar to MF due to a predominantly epidermotropic infiltrate of CD3+ and often CD30− atypical cells. Spontaneously regressing papules favor LyP, whereas persistent patches or plaques favor MF. Type C appears identical to ALCL with diffuse sheets of large atypical CD30+ cells and relatively few inflammatory cells, but spontaneously regressing lesions again favor LyP, whereas persistent tumors favor ALCL. Type D appears similar to primary cutaneous aggressive epidermotropic CD8+ cytotoxic T cell lymphoma due to a markedly epidermotropic infiltrate of small atypical CD8+ and CD30+ lymphocytes, often TIA-1+ (T-cell intracytoplasmic antigen-1) or granzyme B+, but CD30 positivity and self-resolving lesions favor LyP. Type E mimics extranodal natural killer/T cell lymphoma (nasal type) due to angioinvasive CD30+ and beta F1+ T lymphocytes, often CD8+ and/or TIA-1+, but self-resolving lesions again favor LyP, as well as absence of Epstein-Barr virus and CD56−.9
The most common therapeutic approaches to LyP include topical steroids, phototherapy, and low-dose methotrexate.10 However, treatment does not change overall disease course or reduce the future risk for developing an associated lymphoma. Accordingly, abstaining from active therapeutic intervention is reasonable, especially in patients with only a few asymptomatic lesions.
- Macaulay WL. Lymphomatoid papulosis: a continuing self-healing eruption, clinically benign--histologically malignant. Arch Dermatol. 1968;97:23-30.
- Slater DN. The new World Health Organization-European Organization for Research and Treatment of Cancer classification for cutaneous lymphomas: a practical marriage of two giants. Br J Dermatol. 2005;153:874-880.
- Mori M, Manuelli C, Pimpinelli N, et al. CD30-CD30 ligand interaction in primary cutaneous CD30(+) T-cell lymphomas: a clue to the pathophysiology of clinical regression. Blood. 1999;94:3077-3083.
- Greisser J, Doebbeling U, Roos M, et al. Apoptosis in CD30-positive lymphoproliferative disorders of the skin. Exp Dermatol. 2005;14:380-385.
- Kiran T, Demirkesen C, Eker C, et al. The significance of MUM1/IRF4 protein expression and IRF4 translocation of CD30(+) cutaneous T-cell lymphoproliferative disorders: a study of 53 cases. Leuk Res. 2013;37:396-400.
- Wada DA, Law ME, Hsi ED, et al. Specificity of IRF4 translocations for primary cutaneous anaplastic large cell lymphoma: a multicenter study of 204 skin biopsies. Mod Pathol. 2011;24:596-605.
- Pham-Ledard A, Prochazkova-Carlotti M, Laharanne E, et al. IRF4 gene rearrangements define a subgroup of CD30-positive cutaneous T-cell lymphoma: a study of 54 cases. J Invest Dermatol. 2010;130:816-825.
- Schiemann WP, Pfeifer WM, Levi E, et al. A deletion in the gene for transforming growth factor β type I receptor abolishes growth regulation by transforming growth factor β in a cutaneous T-cell lymphoma. Blood. 1999;94:2854-2861.
- Kempf W, Kazakov DV, Schärer L, et al. Angioinvasive lymphomatoid papulosis: a new variant simulating aggressive lymphomas. Am J Surg Pathol. 2013;37:1-13.
- Kempf W, Pfaltz K, Vermeer MH, et al. EORTC, ISCL, and USCLC consensus recommendations for the treatment of primary cutaneous CD30-positive lymphoproliferative disorders: lymphomatoid papulosis and primary cutaneous anaplastic large-cell lymphoma. Blood. 2011;118:4024-4035.
The Diagnosis: Lymphomatoid Papulosis
A shave biopsy of an established lesion on the volar aspect of the left wrist was performed (Figure 1). The biopsy showed an ulcerated nodular lesion characterized by a dense mixed inflammatory cell infiltrate in the dermis composed of lymphocytes, histiocytes, scattered neutrophils, and numerous eosinophils (Figure 2). Notably there was a minor population of large atypical cells with immunoblastic and anaplastic morphology present individually and in small clusters most prominently within the upper dermis (Figures 3 and 4). Immunohistochemistry of the anaplastic cells revealed a CD30+, CD3−, CD4+, CD5−, CD8−, CD2−, CD7−, CD56−, ALK1− (anaplastic lymphoma kinase-1), PAX5− (paired box protein-5), CD20−, and CD15− phenotype. These morphologic and immunohistochemical features suggested a CD30+ cutaneous lymphoproliferative disorder. The clinical history of recurrent self-healing papulonodules in an otherwise-healthy patient established the diagnosis of lymphomatoid papulosis (LyP).




Lymphomatoid papulosis is a lymphoproliferative disorder characterized by recurrent crops of self-resolving eruptive papulonodular skin lesions that may show a variety of histologic features including a CD30+ malignant T-cell lymphoma.1 Lymphomatoid papulosis was first described in 19681 but debate continues whether the condition should be considered malignant or benign.2 Although the prognosis is excellent, LyP is characterized by a protracted course, often lasting many years. Additionally, these patients have a lifelong increased risk for development of a second cutaneous or systemic lymphoma such as mycosis fungoides (MF), cutaneous or nodal anaplastic large cell lymphoma (ALCL), or Hodgkin lymphoma, among others.
Lymphomatoid papulosis is a rare disease occurring in all ethnic groups and at any age, though most commonly presenting in the fifth decade of life. Finding large atypical T cells expressing CD30 in recurring skin lesions is highly suggestive of LyP; however, large CD30+ cells also can be seen in numerous benign reactive processes such as arthropod assault, drug eruption, viral skin infections, and other dermatoses, thus clinical correlation is always paramount. The cause of LyP is largely unknown; however, spontaneous regression may be explained by CD30-CD30 ligand interaction3 as well as an increased proapoptotic milieu.4 Specific translocations such as interferon regulatory factor-4 have been hypothesized as a risk factor for malignant progression.5-7 Additionally, an inactivating gene mutation resulting in loss of transforming growth factor β1 receptor expression and subsequent unresponsiveness to the growth inhibitory effect of transforming growth factor β may play a role in progression of LyP to ALCL.8
Clinically, LyP consists of red-brown papules and nodules generally smaller than 2 cm, often with central hemorrhage, necrosis, and crusting. Lesions are at different stages of eruption and resolution. They are often grouped but may be disseminated. Spontaneous regression typically occurs within 3 to 8 weeks. Pruritus or mild tenderness may occur as well as residual hyperpigmentation or scarring. Systemic symptoms are notably absent.
The histologic features of LyP vary according to the age of the lesion and subtype.2 Early lesions may only show a few inflammatory cells, but as lesions evolve, larger immunoblastlike CD30+ atypical cells accumulate that may resemble the Reed-Sternberg cells of Hodgkin lymphoma. Of the 5 subtypes, the most common is type A. It is characterized by a wedge-shaped infiltrate with a mixed population of scattered or clustered, large, atypical CD30+ cells, lymphocytes, neutrophils, eosinophils, and histiocytes.9 Frequent mitoses often are seen. Type B appears similar to MF due to a predominantly epidermotropic infiltrate of CD3+ and often CD30− atypical cells. Spontaneously regressing papules favor LyP, whereas persistent patches or plaques favor MF. Type C appears identical to ALCL with diffuse sheets of large atypical CD30+ cells and relatively few inflammatory cells, but spontaneously regressing lesions again favor LyP, whereas persistent tumors favor ALCL. Type D appears similar to primary cutaneous aggressive epidermotropic CD8+ cytotoxic T cell lymphoma due to a markedly epidermotropic infiltrate of small atypical CD8+ and CD30+ lymphocytes, often TIA-1+ (T-cell intracytoplasmic antigen-1) or granzyme B+, but CD30 positivity and self-resolving lesions favor LyP. Type E mimics extranodal natural killer/T cell lymphoma (nasal type) due to angioinvasive CD30+ and beta F1+ T lymphocytes, often CD8+ and/or TIA-1+, but self-resolving lesions again favor LyP, as well as absence of Epstein-Barr virus and CD56−.9
The most common therapeutic approaches to LyP include topical steroids, phototherapy, and low-dose methotrexate.10 However, treatment does not change overall disease course or reduce the future risk for developing an associated lymphoma. Accordingly, abstaining from active therapeutic intervention is reasonable, especially in patients with only a few asymptomatic lesions.
The Diagnosis: Lymphomatoid Papulosis
A shave biopsy of an established lesion on the volar aspect of the left wrist was performed (Figure 1). The biopsy showed an ulcerated nodular lesion characterized by a dense mixed inflammatory cell infiltrate in the dermis composed of lymphocytes, histiocytes, scattered neutrophils, and numerous eosinophils (Figure 2). Notably there was a minor population of large atypical cells with immunoblastic and anaplastic morphology present individually and in small clusters most prominently within the upper dermis (Figures 3 and 4). Immunohistochemistry of the anaplastic cells revealed a CD30+, CD3−, CD4+, CD5−, CD8−, CD2−, CD7−, CD56−, ALK1− (anaplastic lymphoma kinase-1), PAX5− (paired box protein-5), CD20−, and CD15− phenotype. These morphologic and immunohistochemical features suggested a CD30+ cutaneous lymphoproliferative disorder. The clinical history of recurrent self-healing papulonodules in an otherwise-healthy patient established the diagnosis of lymphomatoid papulosis (LyP).




Lymphomatoid papulosis is a lymphoproliferative disorder characterized by recurrent crops of self-resolving eruptive papulonodular skin lesions that may show a variety of histologic features including a CD30+ malignant T-cell lymphoma.1 Lymphomatoid papulosis was first described in 19681 but debate continues whether the condition should be considered malignant or benign.2 Although the prognosis is excellent, LyP is characterized by a protracted course, often lasting many years. Additionally, these patients have a lifelong increased risk for development of a second cutaneous or systemic lymphoma such as mycosis fungoides (MF), cutaneous or nodal anaplastic large cell lymphoma (ALCL), or Hodgkin lymphoma, among others.
Lymphomatoid papulosis is a rare disease occurring in all ethnic groups and at any age, though most commonly presenting in the fifth decade of life. Finding large atypical T cells expressing CD30 in recurring skin lesions is highly suggestive of LyP; however, large CD30+ cells also can be seen in numerous benign reactive processes such as arthropod assault, drug eruption, viral skin infections, and other dermatoses, thus clinical correlation is always paramount. The cause of LyP is largely unknown; however, spontaneous regression may be explained by CD30-CD30 ligand interaction3 as well as an increased proapoptotic milieu.4 Specific translocations such as interferon regulatory factor-4 have been hypothesized as a risk factor for malignant progression.5-7 Additionally, an inactivating gene mutation resulting in loss of transforming growth factor β1 receptor expression and subsequent unresponsiveness to the growth inhibitory effect of transforming growth factor β may play a role in progression of LyP to ALCL.8
Clinically, LyP consists of red-brown papules and nodules generally smaller than 2 cm, often with central hemorrhage, necrosis, and crusting. Lesions are at different stages of eruption and resolution. They are often grouped but may be disseminated. Spontaneous regression typically occurs within 3 to 8 weeks. Pruritus or mild tenderness may occur as well as residual hyperpigmentation or scarring. Systemic symptoms are notably absent.
The histologic features of LyP vary according to the age of the lesion and subtype.2 Early lesions may only show a few inflammatory cells, but as lesions evolve, larger immunoblastlike CD30+ atypical cells accumulate that may resemble the Reed-Sternberg cells of Hodgkin lymphoma. Of the 5 subtypes, the most common is type A. It is characterized by a wedge-shaped infiltrate with a mixed population of scattered or clustered, large, atypical CD30+ cells, lymphocytes, neutrophils, eosinophils, and histiocytes.9 Frequent mitoses often are seen. Type B appears similar to MF due to a predominantly epidermotropic infiltrate of CD3+ and often CD30− atypical cells. Spontaneously regressing papules favor LyP, whereas persistent patches or plaques favor MF. Type C appears identical to ALCL with diffuse sheets of large atypical CD30+ cells and relatively few inflammatory cells, but spontaneously regressing lesions again favor LyP, whereas persistent tumors favor ALCL. Type D appears similar to primary cutaneous aggressive epidermotropic CD8+ cytotoxic T cell lymphoma due to a markedly epidermotropic infiltrate of small atypical CD8+ and CD30+ lymphocytes, often TIA-1+ (T-cell intracytoplasmic antigen-1) or granzyme B+, but CD30 positivity and self-resolving lesions favor LyP. Type E mimics extranodal natural killer/T cell lymphoma (nasal type) due to angioinvasive CD30+ and beta F1+ T lymphocytes, often CD8+ and/or TIA-1+, but self-resolving lesions again favor LyP, as well as absence of Epstein-Barr virus and CD56−.9
The most common therapeutic approaches to LyP include topical steroids, phototherapy, and low-dose methotrexate.10 However, treatment does not change overall disease course or reduce the future risk for developing an associated lymphoma. Accordingly, abstaining from active therapeutic intervention is reasonable, especially in patients with only a few asymptomatic lesions.
- Macaulay WL. Lymphomatoid papulosis: a continuing self-healing eruption, clinically benign--histologically malignant. Arch Dermatol. 1968;97:23-30.
- Slater DN. The new World Health Organization-European Organization for Research and Treatment of Cancer classification for cutaneous lymphomas: a practical marriage of two giants. Br J Dermatol. 2005;153:874-880.
- Mori M, Manuelli C, Pimpinelli N, et al. CD30-CD30 ligand interaction in primary cutaneous CD30(+) T-cell lymphomas: a clue to the pathophysiology of clinical regression. Blood. 1999;94:3077-3083.
- Greisser J, Doebbeling U, Roos M, et al. Apoptosis in CD30-positive lymphoproliferative disorders of the skin. Exp Dermatol. 2005;14:380-385.
- Kiran T, Demirkesen C, Eker C, et al. The significance of MUM1/IRF4 protein expression and IRF4 translocation of CD30(+) cutaneous T-cell lymphoproliferative disorders: a study of 53 cases. Leuk Res. 2013;37:396-400.
- Wada DA, Law ME, Hsi ED, et al. Specificity of IRF4 translocations for primary cutaneous anaplastic large cell lymphoma: a multicenter study of 204 skin biopsies. Mod Pathol. 2011;24:596-605.
- Pham-Ledard A, Prochazkova-Carlotti M, Laharanne E, et al. IRF4 gene rearrangements define a subgroup of CD30-positive cutaneous T-cell lymphoma: a study of 54 cases. J Invest Dermatol. 2010;130:816-825.
- Schiemann WP, Pfeifer WM, Levi E, et al. A deletion in the gene for transforming growth factor β type I receptor abolishes growth regulation by transforming growth factor β in a cutaneous T-cell lymphoma. Blood. 1999;94:2854-2861.
- Kempf W, Kazakov DV, Schärer L, et al. Angioinvasive lymphomatoid papulosis: a new variant simulating aggressive lymphomas. Am J Surg Pathol. 2013;37:1-13.
- Kempf W, Pfaltz K, Vermeer MH, et al. EORTC, ISCL, and USCLC consensus recommendations for the treatment of primary cutaneous CD30-positive lymphoproliferative disorders: lymphomatoid papulosis and primary cutaneous anaplastic large-cell lymphoma. Blood. 2011;118:4024-4035.
- Macaulay WL. Lymphomatoid papulosis: a continuing self-healing eruption, clinically benign--histologically malignant. Arch Dermatol. 1968;97:23-30.
- Slater DN. The new World Health Organization-European Organization for Research and Treatment of Cancer classification for cutaneous lymphomas: a practical marriage of two giants. Br J Dermatol. 2005;153:874-880.
- Mori M, Manuelli C, Pimpinelli N, et al. CD30-CD30 ligand interaction in primary cutaneous CD30(+) T-cell lymphomas: a clue to the pathophysiology of clinical regression. Blood. 1999;94:3077-3083.
- Greisser J, Doebbeling U, Roos M, et al. Apoptosis in CD30-positive lymphoproliferative disorders of the skin. Exp Dermatol. 2005;14:380-385.
- Kiran T, Demirkesen C, Eker C, et al. The significance of MUM1/IRF4 protein expression and IRF4 translocation of CD30(+) cutaneous T-cell lymphoproliferative disorders: a study of 53 cases. Leuk Res. 2013;37:396-400.
- Wada DA, Law ME, Hsi ED, et al. Specificity of IRF4 translocations for primary cutaneous anaplastic large cell lymphoma: a multicenter study of 204 skin biopsies. Mod Pathol. 2011;24:596-605.
- Pham-Ledard A, Prochazkova-Carlotti M, Laharanne E, et al. IRF4 gene rearrangements define a subgroup of CD30-positive cutaneous T-cell lymphoma: a study of 54 cases. J Invest Dermatol. 2010;130:816-825.
- Schiemann WP, Pfeifer WM, Levi E, et al. A deletion in the gene for transforming growth factor β type I receptor abolishes growth regulation by transforming growth factor β in a cutaneous T-cell lymphoma. Blood. 1999;94:2854-2861.
- Kempf W, Kazakov DV, Schärer L, et al. Angioinvasive lymphomatoid papulosis: a new variant simulating aggressive lymphomas. Am J Surg Pathol. 2013;37:1-13.
- Kempf W, Pfaltz K, Vermeer MH, et al. EORTC, ISCL, and USCLC consensus recommendations for the treatment of primary cutaneous CD30-positive lymphoproliferative disorders: lymphomatoid papulosis and primary cutaneous anaplastic large-cell lymphoma. Blood. 2011;118:4024-4035.
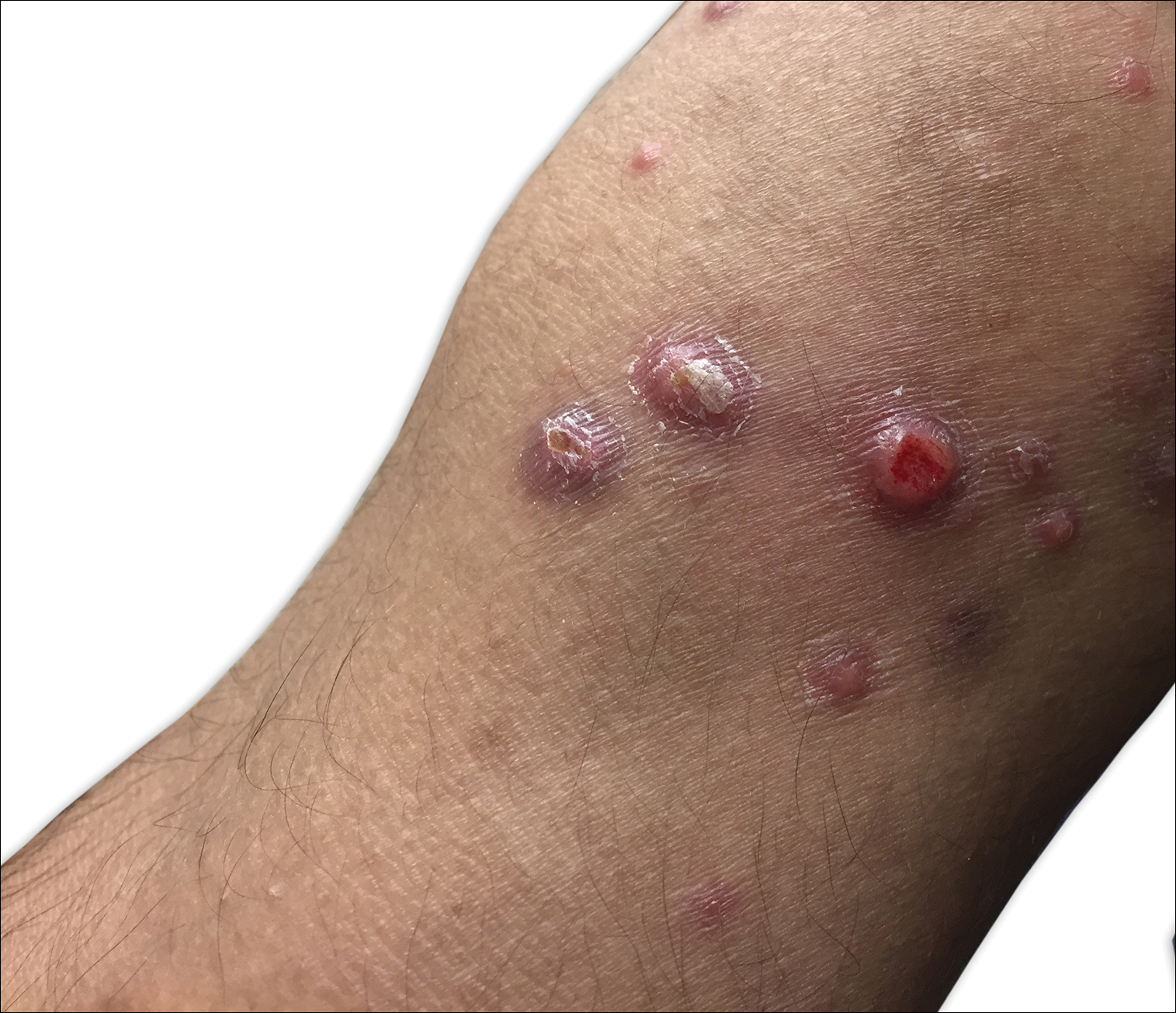
A 29-year-old man from Saudi Arabia presented with slightly tender skin lesions occurring in crops every few months over the last 7 years. The lesions typically would occur on the inguinal area, lower abdomen, buttocks, thighs, or arms, resolving within a few weeks despite no treatment. The patient denied having systemic symptoms such as fevers, chills, sweats, chest pain, shortness of breath, or unexpected weight loss. Physical examination revealed multiple erythematous papulonodules, some ulcerated with a superficial crust, grouped predominantly on the medial aspect of the right upper arm and left lower inguinal region. Isolated lesions also were present on the forearms, dorsal aspects of the hands, abdomen, and thighs. The grouped papulonodules were intermixed with faint hyperpigmented macules indicative of prior lesions. No oral lesions were noted, and there was no marked axillary or inguinal lymphadenopathy.
Ibrutinib and bleeding complications in Mohs surgery
Clinically significant bleeding events occurred in two elderly men who were taking ibrutinib and underwent Mohs micrographic surgery for squamous cell carcinomas, Cindy E. Parra and her colleagues reported in JAMA Dermatology.
On day 3 after his Mohs procedure, one 73-year-old man taking ibrutinib for Waldenstrom macroglobulinemia developed extensive bilateral periorbital ecchymosis that extended down to his upper chest. The other patient, an 88-year-old man taking ibrutinib for chronic lymphocytic leukemia, developed ecchymosis down to the chin. The first patient discontinued ibrutinib 3 days before his surgery; the second patient was taking ibrutinib at the time of his surgery.
“The increased incidence of nonmelanoma skin cancer and poorer outcomes in patients with non-Hodgkin lymphoma and CLL is well recognized, as is the importance of aggressive dermatologic management,” the researchers wrote (JAMA Dermatol. 2017 Jul 12. doi: 10.1001/jamadermatol.2017.1877). “It may be prudent to withhold ibrutinib treatment prior to dermatologic surgery to avoid potential bleeding complications.”
The findings argue for close collaboration between the dermatologic surgeon and the patient’s hematologist when scheduling extended-duration dermatologic procedures in patients taking ibrutinib.
Find the full summary here.
Clinically significant bleeding events occurred in two elderly men who were taking ibrutinib and underwent Mohs micrographic surgery for squamous cell carcinomas, Cindy E. Parra and her colleagues reported in JAMA Dermatology.
On day 3 after his Mohs procedure, one 73-year-old man taking ibrutinib for Waldenstrom macroglobulinemia developed extensive bilateral periorbital ecchymosis that extended down to his upper chest. The other patient, an 88-year-old man taking ibrutinib for chronic lymphocytic leukemia, developed ecchymosis down to the chin. The first patient discontinued ibrutinib 3 days before his surgery; the second patient was taking ibrutinib at the time of his surgery.
“The increased incidence of nonmelanoma skin cancer and poorer outcomes in patients with non-Hodgkin lymphoma and CLL is well recognized, as is the importance of aggressive dermatologic management,” the researchers wrote (JAMA Dermatol. 2017 Jul 12. doi: 10.1001/jamadermatol.2017.1877). “It may be prudent to withhold ibrutinib treatment prior to dermatologic surgery to avoid potential bleeding complications.”
The findings argue for close collaboration between the dermatologic surgeon and the patient’s hematologist when scheduling extended-duration dermatologic procedures in patients taking ibrutinib.
Find the full summary here.
Clinically significant bleeding events occurred in two elderly men who were taking ibrutinib and underwent Mohs micrographic surgery for squamous cell carcinomas, Cindy E. Parra and her colleagues reported in JAMA Dermatology.
On day 3 after his Mohs procedure, one 73-year-old man taking ibrutinib for Waldenstrom macroglobulinemia developed extensive bilateral periorbital ecchymosis that extended down to his upper chest. The other patient, an 88-year-old man taking ibrutinib for chronic lymphocytic leukemia, developed ecchymosis down to the chin. The first patient discontinued ibrutinib 3 days before his surgery; the second patient was taking ibrutinib at the time of his surgery.
“The increased incidence of nonmelanoma skin cancer and poorer outcomes in patients with non-Hodgkin lymphoma and CLL is well recognized, as is the importance of aggressive dermatologic management,” the researchers wrote (JAMA Dermatol. 2017 Jul 12. doi: 10.1001/jamadermatol.2017.1877). “It may be prudent to withhold ibrutinib treatment prior to dermatologic surgery to avoid potential bleeding complications.”
The findings argue for close collaboration between the dermatologic surgeon and the patient’s hematologist when scheduling extended-duration dermatologic procedures in patients taking ibrutinib.
Find the full summary here.
FROM JAMA DERMATOLOGY
Subsequent squamous cell carcinoma risk higher in HIV patients with low CD4 count
HIV-infected individuals who have experienced a nonmelanoma skin cancer may be at significantly greater risk of subsequent new squamous cell carcinoma if they have a lower CD4 cell count, a new study suggests.
In a study published online July 12 in JAMA Dermatology, researchers reported the results of a retrospective cohort study using medical record data from 455 HIV-infected and 1,952 HIV-uninfected patients who had previously been diagnosed with at least one nonmelanoma skin cancer.
Patients with CD4 cell counts below 200 cells/mcL had a 44% greater risk of a subsequent nonmelanoma skin cancer, compared with uninfected individuals (95% confidence interval, 1.10-1.88), while those with a viral load greater than 10,000 copies/mL had a 31% greater risk (95% CI, 1.00-1.72), after adjusting for age, sex, smoking status, and obesity.
The increase in nonmelanoma skin cancer risk was largely accounted for by an increased risk of squamous cell carcinoma (SCC). Among patients with lower CD4 cell counts and those with higher viral loads, the risk of SCC was more than twofold greater than among uninfected individuals. In contrast, while there was a trend toward a higher risk of basal cell carcinoma in those two groups, it did not reach significance (JAMA Dermatol. 2017 Jul 12. doi: 10.1001/jamadermatol.2017.1716).
Overall, HIV-infected individuals had a significant 15% increase in the risk of subsequent nonmelanoma skin cancer over an average follow-up period of 5 years, compared with uninfected individuals.
“This study addresses key questions regarding subsequent NMSC [nonmelanoma skin cancer] risk among a high-risk subgroup of HIV-infected population who, by virtue of having had a pathologically validated skin cancer, are at increased risk of subsequent NMSCs,” wrote Maryam M. Asgari, MD, of Massachusetts General Hospital, Boston, and her coauthors. “Specifically, it was previously not known precisely which NMSC subtype is increased in high-risk persons with HIV and whether biomarkers of HIV infections, such as degree of immune dysfunction, are associated with subsequent skin cancer risk.”
While the study wasn’t able to control for known skin cancer risk factors such as skin type, the patients were all non-Hispanic white, which the authors said selected for individuals with fair skin and some sun-exposure history.
The findings could have implications for skin cancer screening among individuals with HIV infection, with the authors suggesting more targeted monitoring for SCC among individuals with low CD4 counts or high viral loads.
“Our findings support immune dysfunction as a risk factor for SCCs and dovetail with SCC risk data from iatrogenic immunodeficiency states, such as organ transplantation and autoimmune diseases.”
The study was partly supported by Kaiser Permanente Northern California, and one author was supported by a grant from the National Cancer Institute. Two authors had previously served as investigators on studies funded by the pharmaceutical industry, one author declared research funding from the pharmaceutical industry, and one declared shares in two medical companies.
HIV-infected individuals who have experienced a nonmelanoma skin cancer may be at significantly greater risk of subsequent new squamous cell carcinoma if they have a lower CD4 cell count, a new study suggests.
In a study published online July 12 in JAMA Dermatology, researchers reported the results of a retrospective cohort study using medical record data from 455 HIV-infected and 1,952 HIV-uninfected patients who had previously been diagnosed with at least one nonmelanoma skin cancer.
Patients with CD4 cell counts below 200 cells/mcL had a 44% greater risk of a subsequent nonmelanoma skin cancer, compared with uninfected individuals (95% confidence interval, 1.10-1.88), while those with a viral load greater than 10,000 copies/mL had a 31% greater risk (95% CI, 1.00-1.72), after adjusting for age, sex, smoking status, and obesity.
The increase in nonmelanoma skin cancer risk was largely accounted for by an increased risk of squamous cell carcinoma (SCC). Among patients with lower CD4 cell counts and those with higher viral loads, the risk of SCC was more than twofold greater than among uninfected individuals. In contrast, while there was a trend toward a higher risk of basal cell carcinoma in those two groups, it did not reach significance (JAMA Dermatol. 2017 Jul 12. doi: 10.1001/jamadermatol.2017.1716).
Overall, HIV-infected individuals had a significant 15% increase in the risk of subsequent nonmelanoma skin cancer over an average follow-up period of 5 years, compared with uninfected individuals.
“This study addresses key questions regarding subsequent NMSC [nonmelanoma skin cancer] risk among a high-risk subgroup of HIV-infected population who, by virtue of having had a pathologically validated skin cancer, are at increased risk of subsequent NMSCs,” wrote Maryam M. Asgari, MD, of Massachusetts General Hospital, Boston, and her coauthors. “Specifically, it was previously not known precisely which NMSC subtype is increased in high-risk persons with HIV and whether biomarkers of HIV infections, such as degree of immune dysfunction, are associated with subsequent skin cancer risk.”
While the study wasn’t able to control for known skin cancer risk factors such as skin type, the patients were all non-Hispanic white, which the authors said selected for individuals with fair skin and some sun-exposure history.
The findings could have implications for skin cancer screening among individuals with HIV infection, with the authors suggesting more targeted monitoring for SCC among individuals with low CD4 counts or high viral loads.
“Our findings support immune dysfunction as a risk factor for SCCs and dovetail with SCC risk data from iatrogenic immunodeficiency states, such as organ transplantation and autoimmune diseases.”
The study was partly supported by Kaiser Permanente Northern California, and one author was supported by a grant from the National Cancer Institute. Two authors had previously served as investigators on studies funded by the pharmaceutical industry, one author declared research funding from the pharmaceutical industry, and one declared shares in two medical companies.
HIV-infected individuals who have experienced a nonmelanoma skin cancer may be at significantly greater risk of subsequent new squamous cell carcinoma if they have a lower CD4 cell count, a new study suggests.
In a study published online July 12 in JAMA Dermatology, researchers reported the results of a retrospective cohort study using medical record data from 455 HIV-infected and 1,952 HIV-uninfected patients who had previously been diagnosed with at least one nonmelanoma skin cancer.
Patients with CD4 cell counts below 200 cells/mcL had a 44% greater risk of a subsequent nonmelanoma skin cancer, compared with uninfected individuals (95% confidence interval, 1.10-1.88), while those with a viral load greater than 10,000 copies/mL had a 31% greater risk (95% CI, 1.00-1.72), after adjusting for age, sex, smoking status, and obesity.
The increase in nonmelanoma skin cancer risk was largely accounted for by an increased risk of squamous cell carcinoma (SCC). Among patients with lower CD4 cell counts and those with higher viral loads, the risk of SCC was more than twofold greater than among uninfected individuals. In contrast, while there was a trend toward a higher risk of basal cell carcinoma in those two groups, it did not reach significance (JAMA Dermatol. 2017 Jul 12. doi: 10.1001/jamadermatol.2017.1716).
Overall, HIV-infected individuals had a significant 15% increase in the risk of subsequent nonmelanoma skin cancer over an average follow-up period of 5 years, compared with uninfected individuals.
“This study addresses key questions regarding subsequent NMSC [nonmelanoma skin cancer] risk among a high-risk subgroup of HIV-infected population who, by virtue of having had a pathologically validated skin cancer, are at increased risk of subsequent NMSCs,” wrote Maryam M. Asgari, MD, of Massachusetts General Hospital, Boston, and her coauthors. “Specifically, it was previously not known precisely which NMSC subtype is increased in high-risk persons with HIV and whether biomarkers of HIV infections, such as degree of immune dysfunction, are associated with subsequent skin cancer risk.”
While the study wasn’t able to control for known skin cancer risk factors such as skin type, the patients were all non-Hispanic white, which the authors said selected for individuals with fair skin and some sun-exposure history.
The findings could have implications for skin cancer screening among individuals with HIV infection, with the authors suggesting more targeted monitoring for SCC among individuals with low CD4 counts or high viral loads.
“Our findings support immune dysfunction as a risk factor for SCCs and dovetail with SCC risk data from iatrogenic immunodeficiency states, such as organ transplantation and autoimmune diseases.”
The study was partly supported by Kaiser Permanente Northern California, and one author was supported by a grant from the National Cancer Institute. Two authors had previously served as investigators on studies funded by the pharmaceutical industry, one author declared research funding from the pharmaceutical industry, and one declared shares in two medical companies.
FROM JAMA DERMATOLOGY
Key clinical point: HIV-infected people who have had a previous nonmelanoma skin cancer are at significantly higher risk of subsequent SCC if they have a lower CD4 count or higher viral load.
Major finding: HIV-infected people with a low CD4 cell count or high viral load have a greater than twofold increased risk of subsequent SCC after a primary nonmelanoma skin cancer than do uninfected people who have had a previous nonmelanoma skin cancer.
Data source: A retrospective cohort study in 455 HIV-infected and 1,945 HIV-uninfected patients.
Disclosures: The study was partly supported by Kaiser Permanente, Northern California, and one author was supported by a grant from the National Cancer Institute. Two authors had previously served as investigators on studies funded by the pharmaceutical industry, one author declared research funding from the pharmaceutical industry, and one declared shares in two medical companies.
Dermatofibrosarcoma Protuberans
To the Editor:
A 41-year-old man presented with a slowly enlarging, tender, firm lesion on the left hallux of approximately 5 months' duration that initially appeared to be a blister. He reported no history of keloids or trauma to the left foot. On examination, a 3.5-cm, flesh-colored, pedunculated, firm nodule was present on the lateral aspect of the left great hallux (Figure 1). No lymphadenopathy was found. The lesion was diagnosed at that time as a keloid and treated with intralesional steroids without response. The patient was lost to follow-up, and after 5 months he presented again with pain and drainage from the lesion. Acute drainage resolved after antibiotic therapy. A shave biopsy was performed, which revealed findings consistent with a dermatofibrosarcoma protuberans (DFSP). A chest radiograph was unremarkable. Re-excision was performed with negative margins on frozen section but with positive peripheral and deep margins on permanent sections. The patient subsequently underwent amputation of the left great toe and was lost to follow-up after the initial postoperative period.
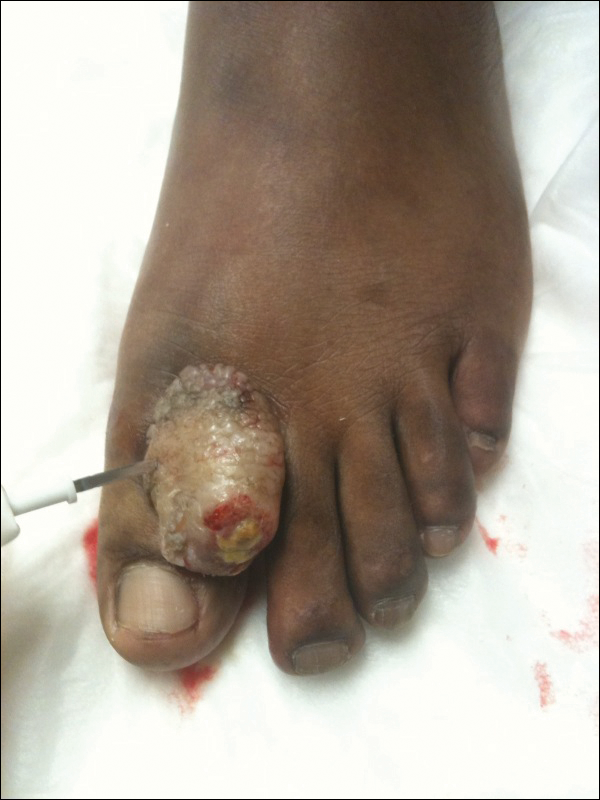
Histopathologic examination demonstrated a polypoid spindle cell tumor that filled the dermis and invaded into the subcutaneous adipose tissue (Figure 2). The spindle cells had tapered nuclei in a honeycomb arrangement with only mild nuclear pleomorphism arranged in fascicles with a herringbone formation. Areas showed a myxoid stroma with abundant mucin (Figure 3). Immunostaining demonstrated cells strongly positive for CD34 and negative for MART (melanoma-associated antigen recognized by T cells), S-100, and smooth muscle actin immunostains.
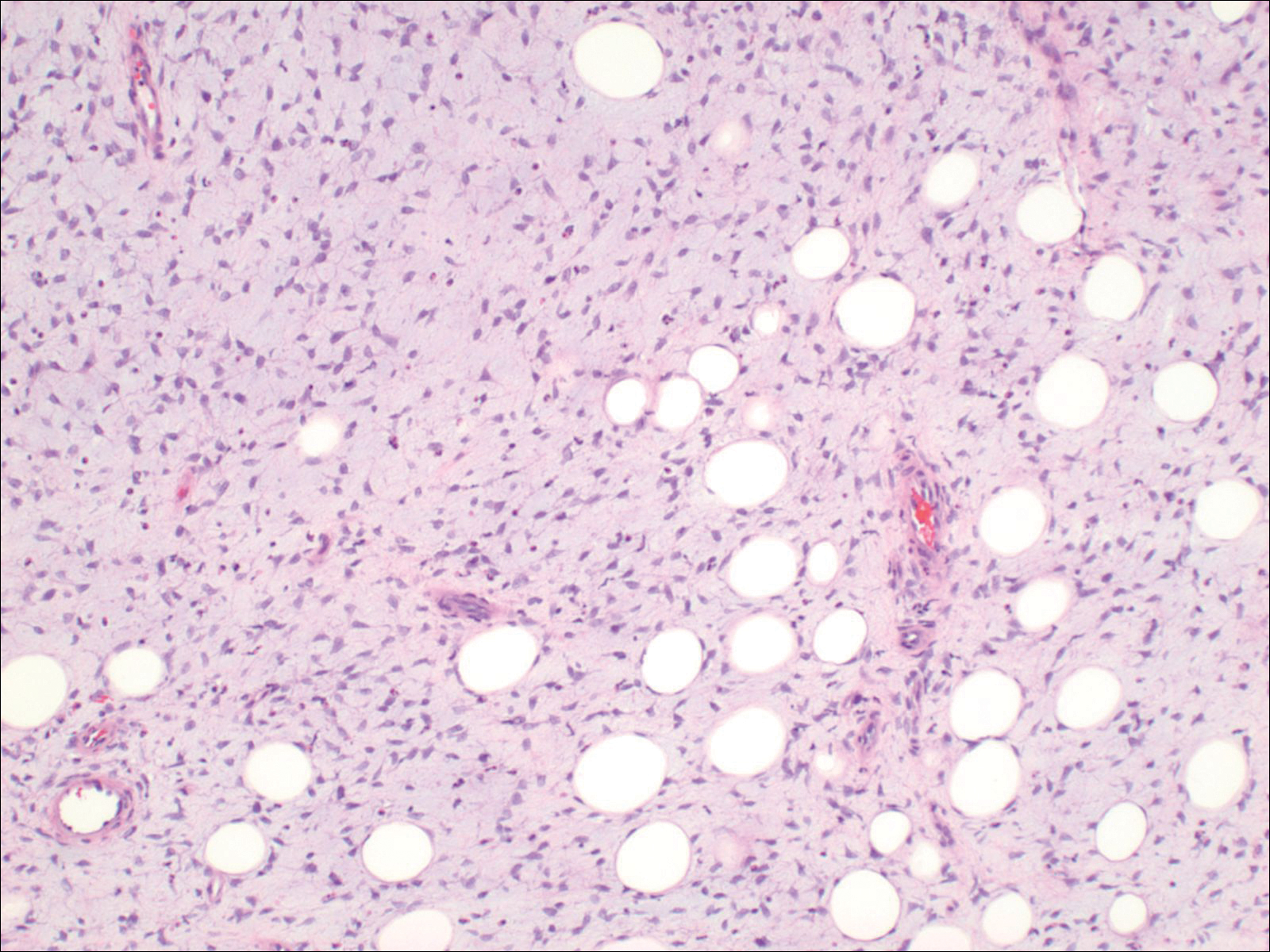
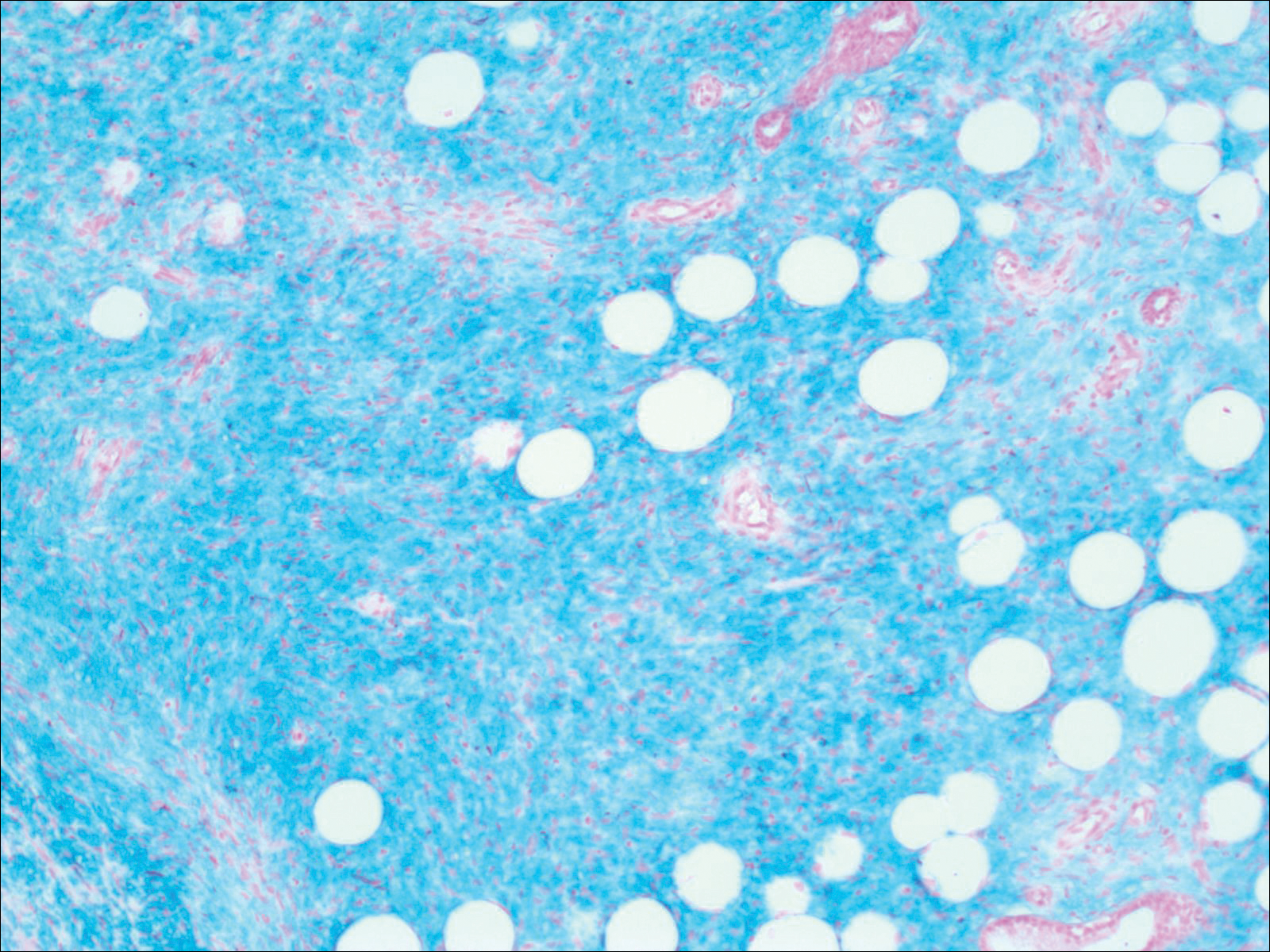
Dermatofibrosarcoma protuberans is a sarcoma that is locally aggressive and tends to recur after surgical excision, though rare cases of metastasis involving the lungs have been reported.12 Dermatofibrosarcoma protuberans usually affects young to middle-aged adults. Acral DFSP is rare in adults, with tumors most commonly occurring on the trunk (50%-60%), proximal extremities (20%-30%), or the head and neck (10%-15%).1,2 A higher rate of acral DFSP has been found in children, which may be due to the increased rate of extremity trauma. Dermatofibrosarcoma protuberans commonly presents as an asymptomatic, slowly growing, indurated plaque that may be flesh colored or hyperpigmented, followed by development of erythematous firm nodules of up to several centimeters.1,3 Dermatofibrosarcoma protuberans may be associated with a purulent exudate or ulceration, and pain may develop as the lesion grows.
Histopathologic evaluation shows an early plaque stage characterized by low cellularity, minimal nuclear atypia, and rare mitotic figures.4 In the nodular stage, the spindle cells are arranged as short fascicles in a storiform arrangement and infiltrate the subcutaneous tissue in a honeycomb pattern with hyperchromatic nuclei and mitotic figures. The nodules may develop myxomatous areas as well as less-differentiated foci with intersecting fascicles in a herringbone pattern. Anti-CD34 antibody immunostaining demonstrates strongly positive spindle cells, while DFSP is negative for stromelysin 3, factor XIIIa, and D2-40, which can help to differentiate DFSP from dermatofibroma.5 The myxoid subtype of DFSP does not differ clinically or prognostically from conventional DFSP, though its recognition can be of use in differentiating other myxoid tumors. Myxoid DFSP is nearly always positive for CD34 and negative for the neural marker S-100 protein.6
Some reports have demonstrated that Mohs micrographic surgery is superior to wide local excision in treatment of DFSP, as it results in fewer local recurrences and metastases.7,8 Because of cytogenic abnormalities such as a reciprocal chromosomal (17;22) translocation or supernumerary ring chromosome derived from t(17;22) that place the PDGFB gene under the control of COL1A1 promoter, imatinib mesylate has been tested in DFSP and resulted in dramatic responses in both adults and children.9,10 Suggested uses of imatinib include metastatic disease and locally invasive disease not suitable for surgical excision as well as a method to debulk tumors prior to resection.11
- Gloster HM Jr. Dermatofibrosarcoma protuberans. J Am Acad Dermatol. 1996;35(3, pt 1):355-374; quiz 375-376.
- Do AN, Goleno K, Geisse JK. Mohs micrographic surgery and partial amputation preserving function and aesthetics in digits: case reports of invasive melanoma and digital dermatofibrosarcoma protuberans. Dermatol Surg. 2006;32:1516-1521.
- Taylor HB, Helwig EB. Dermatofibrosarcoma protuberans: a study of 115 cases. Cancer. 1962;15:717-725.
- Kamino H, Reddy VB, Pui J. Dermatofibrosarcoma protuberans. In: Bolognia J, Jorizzo J, Rapini R, eds. Dermatology. 3rd ed. London, England: Elsevier; 2012:1961-1977.
- Cohen PR, Rapini RP, Farhood AI. Dermatofibroma and dermatofibrosarcoma protuberans: differential expression of CD34 and factor XIIIa. Am J Dermatopathol. 1994;16:573-574.
- Llombart B, Serra-Guillén C, Monteagudo C, et al. Dermatofibrosarcoma protuberans: a comprehensive review and update of diagnosis and management. Semin Diagn Pathol. 2013;30:13-28.
- Paradisi A, Abeni D, Rusciani A, et al. Dermatofibrosarcoma protuberans: wide local excision vs. Mohs micrographic surgery. Cancer Treat Rev. 2008;34:728-736.
- Foroozan M, Sei JF, Amini M, et al. Efficacy of Mohs micrographic surgery for the treatment of dermatofibrosarcoma protuberans: systematic review. Arch Dermatol. 2012;148:1055-1063.
- Patel KU, Szaebo SS, Hernandez VS, et al. Dermatofibrosarcoma protuberans COL1A1-PDGFB fusion is identified in virtually all dermatofibrosarcoma protuberans cases when investigated by newly developed multiplex reverse transcription polymerase chain reaction and fluorescence in situ hybridization assays. Hum Pathol. 2008;39:184-193.
- McArthur GA, Demetri GD, van Oosterom A, et al. Molecular and clinical analysis of locally advanced dermatofibrosarcoma protuberans treated with imatinib: Imatinib Target Exploration Consortium Study B2225. J Clin Oncol. 2005;23:866-873.
- Rutkowski P, Van Glabbeke M, Rankin CJ, et al; European Organisation for Research and Treatment of Cancer Soft Tissue/Bone Sarcoma Group, Southwest Oncology Group. Imatinib mesylate in advanced dermatofibrosarcoma protuberans: pooled analysis of two phase II clinical trials [published online March 1, 2010]. J Clin Oncol. 2010;28:1772-1779.
- Mentzel T, Beham A, Katenkamp D, et al. Fibrosarcomatous ("high-grade") dermatofibrosarcoma protuberans: clinicopathologic and immunohistochemical study of a series of 41 cases with emphasis on prognostic significance. Am J Surg Pathol. 1998;22:576-587.
To the Editor:
A 41-year-old man presented with a slowly enlarging, tender, firm lesion on the left hallux of approximately 5 months' duration that initially appeared to be a blister. He reported no history of keloids or trauma to the left foot. On examination, a 3.5-cm, flesh-colored, pedunculated, firm nodule was present on the lateral aspect of the left great hallux (Figure 1). No lymphadenopathy was found. The lesion was diagnosed at that time as a keloid and treated with intralesional steroids without response. The patient was lost to follow-up, and after 5 months he presented again with pain and drainage from the lesion. Acute drainage resolved after antibiotic therapy. A shave biopsy was performed, which revealed findings consistent with a dermatofibrosarcoma protuberans (DFSP). A chest radiograph was unremarkable. Re-excision was performed with negative margins on frozen section but with positive peripheral and deep margins on permanent sections. The patient subsequently underwent amputation of the left great toe and was lost to follow-up after the initial postoperative period.

Histopathologic examination demonstrated a polypoid spindle cell tumor that filled the dermis and invaded into the subcutaneous adipose tissue (Figure 2). The spindle cells had tapered nuclei in a honeycomb arrangement with only mild nuclear pleomorphism arranged in fascicles with a herringbone formation. Areas showed a myxoid stroma with abundant mucin (Figure 3). Immunostaining demonstrated cells strongly positive for CD34 and negative for MART (melanoma-associated antigen recognized by T cells), S-100, and smooth muscle actin immunostains.


Dermatofibrosarcoma protuberans is a sarcoma that is locally aggressive and tends to recur after surgical excision, though rare cases of metastasis involving the lungs have been reported.12 Dermatofibrosarcoma protuberans usually affects young to middle-aged adults. Acral DFSP is rare in adults, with tumors most commonly occurring on the trunk (50%-60%), proximal extremities (20%-30%), or the head and neck (10%-15%).1,2 A higher rate of acral DFSP has been found in children, which may be due to the increased rate of extremity trauma. Dermatofibrosarcoma protuberans commonly presents as an asymptomatic, slowly growing, indurated plaque that may be flesh colored or hyperpigmented, followed by development of erythematous firm nodules of up to several centimeters.1,3 Dermatofibrosarcoma protuberans may be associated with a purulent exudate or ulceration, and pain may develop as the lesion grows.
Histopathologic evaluation shows an early plaque stage characterized by low cellularity, minimal nuclear atypia, and rare mitotic figures.4 In the nodular stage, the spindle cells are arranged as short fascicles in a storiform arrangement and infiltrate the subcutaneous tissue in a honeycomb pattern with hyperchromatic nuclei and mitotic figures. The nodules may develop myxomatous areas as well as less-differentiated foci with intersecting fascicles in a herringbone pattern. Anti-CD34 antibody immunostaining demonstrates strongly positive spindle cells, while DFSP is negative for stromelysin 3, factor XIIIa, and D2-40, which can help to differentiate DFSP from dermatofibroma.5 The myxoid subtype of DFSP does not differ clinically or prognostically from conventional DFSP, though its recognition can be of use in differentiating other myxoid tumors. Myxoid DFSP is nearly always positive for CD34 and negative for the neural marker S-100 protein.6
Some reports have demonstrated that Mohs micrographic surgery is superior to wide local excision in treatment of DFSP, as it results in fewer local recurrences and metastases.7,8 Because of cytogenic abnormalities such as a reciprocal chromosomal (17;22) translocation or supernumerary ring chromosome derived from t(17;22) that place the PDGFB gene under the control of COL1A1 promoter, imatinib mesylate has been tested in DFSP and resulted in dramatic responses in both adults and children.9,10 Suggested uses of imatinib include metastatic disease and locally invasive disease not suitable for surgical excision as well as a method to debulk tumors prior to resection.11
To the Editor:
A 41-year-old man presented with a slowly enlarging, tender, firm lesion on the left hallux of approximately 5 months' duration that initially appeared to be a blister. He reported no history of keloids or trauma to the left foot. On examination, a 3.5-cm, flesh-colored, pedunculated, firm nodule was present on the lateral aspect of the left great hallux (Figure 1). No lymphadenopathy was found. The lesion was diagnosed at that time as a keloid and treated with intralesional steroids without response. The patient was lost to follow-up, and after 5 months he presented again with pain and drainage from the lesion. Acute drainage resolved after antibiotic therapy. A shave biopsy was performed, which revealed findings consistent with a dermatofibrosarcoma protuberans (DFSP). A chest radiograph was unremarkable. Re-excision was performed with negative margins on frozen section but with positive peripheral and deep margins on permanent sections. The patient subsequently underwent amputation of the left great toe and was lost to follow-up after the initial postoperative period.

Histopathologic examination demonstrated a polypoid spindle cell tumor that filled the dermis and invaded into the subcutaneous adipose tissue (Figure 2). The spindle cells had tapered nuclei in a honeycomb arrangement with only mild nuclear pleomorphism arranged in fascicles with a herringbone formation. Areas showed a myxoid stroma with abundant mucin (Figure 3). Immunostaining demonstrated cells strongly positive for CD34 and negative for MART (melanoma-associated antigen recognized by T cells), S-100, and smooth muscle actin immunostains.


Dermatofibrosarcoma protuberans is a sarcoma that is locally aggressive and tends to recur after surgical excision, though rare cases of metastasis involving the lungs have been reported.12 Dermatofibrosarcoma protuberans usually affects young to middle-aged adults. Acral DFSP is rare in adults, with tumors most commonly occurring on the trunk (50%-60%), proximal extremities (20%-30%), or the head and neck (10%-15%).1,2 A higher rate of acral DFSP has been found in children, which may be due to the increased rate of extremity trauma. Dermatofibrosarcoma protuberans commonly presents as an asymptomatic, slowly growing, indurated plaque that may be flesh colored or hyperpigmented, followed by development of erythematous firm nodules of up to several centimeters.1,3 Dermatofibrosarcoma protuberans may be associated with a purulent exudate or ulceration, and pain may develop as the lesion grows.
Histopathologic evaluation shows an early plaque stage characterized by low cellularity, minimal nuclear atypia, and rare mitotic figures.4 In the nodular stage, the spindle cells are arranged as short fascicles in a storiform arrangement and infiltrate the subcutaneous tissue in a honeycomb pattern with hyperchromatic nuclei and mitotic figures. The nodules may develop myxomatous areas as well as less-differentiated foci with intersecting fascicles in a herringbone pattern. Anti-CD34 antibody immunostaining demonstrates strongly positive spindle cells, while DFSP is negative for stromelysin 3, factor XIIIa, and D2-40, which can help to differentiate DFSP from dermatofibroma.5 The myxoid subtype of DFSP does not differ clinically or prognostically from conventional DFSP, though its recognition can be of use in differentiating other myxoid tumors. Myxoid DFSP is nearly always positive for CD34 and negative for the neural marker S-100 protein.6
Some reports have demonstrated that Mohs micrographic surgery is superior to wide local excision in treatment of DFSP, as it results in fewer local recurrences and metastases.7,8 Because of cytogenic abnormalities such as a reciprocal chromosomal (17;22) translocation or supernumerary ring chromosome derived from t(17;22) that place the PDGFB gene under the control of COL1A1 promoter, imatinib mesylate has been tested in DFSP and resulted in dramatic responses in both adults and children.9,10 Suggested uses of imatinib include metastatic disease and locally invasive disease not suitable for surgical excision as well as a method to debulk tumors prior to resection.11
- Gloster HM Jr. Dermatofibrosarcoma protuberans. J Am Acad Dermatol. 1996;35(3, pt 1):355-374; quiz 375-376.
- Do AN, Goleno K, Geisse JK. Mohs micrographic surgery and partial amputation preserving function and aesthetics in digits: case reports of invasive melanoma and digital dermatofibrosarcoma protuberans. Dermatol Surg. 2006;32:1516-1521.
- Taylor HB, Helwig EB. Dermatofibrosarcoma protuberans: a study of 115 cases. Cancer. 1962;15:717-725.
- Kamino H, Reddy VB, Pui J. Dermatofibrosarcoma protuberans. In: Bolognia J, Jorizzo J, Rapini R, eds. Dermatology. 3rd ed. London, England: Elsevier; 2012:1961-1977.
- Cohen PR, Rapini RP, Farhood AI. Dermatofibroma and dermatofibrosarcoma protuberans: differential expression of CD34 and factor XIIIa. Am J Dermatopathol. 1994;16:573-574.
- Llombart B, Serra-Guillén C, Monteagudo C, et al. Dermatofibrosarcoma protuberans: a comprehensive review and update of diagnosis and management. Semin Diagn Pathol. 2013;30:13-28.
- Paradisi A, Abeni D, Rusciani A, et al. Dermatofibrosarcoma protuberans: wide local excision vs. Mohs micrographic surgery. Cancer Treat Rev. 2008;34:728-736.
- Foroozan M, Sei JF, Amini M, et al. Efficacy of Mohs micrographic surgery for the treatment of dermatofibrosarcoma protuberans: systematic review. Arch Dermatol. 2012;148:1055-1063.
- Patel KU, Szaebo SS, Hernandez VS, et al. Dermatofibrosarcoma protuberans COL1A1-PDGFB fusion is identified in virtually all dermatofibrosarcoma protuberans cases when investigated by newly developed multiplex reverse transcription polymerase chain reaction and fluorescence in situ hybridization assays. Hum Pathol. 2008;39:184-193.
- McArthur GA, Demetri GD, van Oosterom A, et al. Molecular and clinical analysis of locally advanced dermatofibrosarcoma protuberans treated with imatinib: Imatinib Target Exploration Consortium Study B2225. J Clin Oncol. 2005;23:866-873.
- Rutkowski P, Van Glabbeke M, Rankin CJ, et al; European Organisation for Research and Treatment of Cancer Soft Tissue/Bone Sarcoma Group, Southwest Oncology Group. Imatinib mesylate in advanced dermatofibrosarcoma protuberans: pooled analysis of two phase II clinical trials [published online March 1, 2010]. J Clin Oncol. 2010;28:1772-1779.
- Mentzel T, Beham A, Katenkamp D, et al. Fibrosarcomatous ("high-grade") dermatofibrosarcoma protuberans: clinicopathologic and immunohistochemical study of a series of 41 cases with emphasis on prognostic significance. Am J Surg Pathol. 1998;22:576-587.
- Gloster HM Jr. Dermatofibrosarcoma protuberans. J Am Acad Dermatol. 1996;35(3, pt 1):355-374; quiz 375-376.
- Do AN, Goleno K, Geisse JK. Mohs micrographic surgery and partial amputation preserving function and aesthetics in digits: case reports of invasive melanoma and digital dermatofibrosarcoma protuberans. Dermatol Surg. 2006;32:1516-1521.
- Taylor HB, Helwig EB. Dermatofibrosarcoma protuberans: a study of 115 cases. Cancer. 1962;15:717-725.
- Kamino H, Reddy VB, Pui J. Dermatofibrosarcoma protuberans. In: Bolognia J, Jorizzo J, Rapini R, eds. Dermatology. 3rd ed. London, England: Elsevier; 2012:1961-1977.
- Cohen PR, Rapini RP, Farhood AI. Dermatofibroma and dermatofibrosarcoma protuberans: differential expression of CD34 and factor XIIIa. Am J Dermatopathol. 1994;16:573-574.
- Llombart B, Serra-Guillén C, Monteagudo C, et al. Dermatofibrosarcoma protuberans: a comprehensive review and update of diagnosis and management. Semin Diagn Pathol. 2013;30:13-28.
- Paradisi A, Abeni D, Rusciani A, et al. Dermatofibrosarcoma protuberans: wide local excision vs. Mohs micrographic surgery. Cancer Treat Rev. 2008;34:728-736.
- Foroozan M, Sei JF, Amini M, et al. Efficacy of Mohs micrographic surgery for the treatment of dermatofibrosarcoma protuberans: systematic review. Arch Dermatol. 2012;148:1055-1063.
- Patel KU, Szaebo SS, Hernandez VS, et al. Dermatofibrosarcoma protuberans COL1A1-PDGFB fusion is identified in virtually all dermatofibrosarcoma protuberans cases when investigated by newly developed multiplex reverse transcription polymerase chain reaction and fluorescence in situ hybridization assays. Hum Pathol. 2008;39:184-193.
- McArthur GA, Demetri GD, van Oosterom A, et al. Molecular and clinical analysis of locally advanced dermatofibrosarcoma protuberans treated with imatinib: Imatinib Target Exploration Consortium Study B2225. J Clin Oncol. 2005;23:866-873.
- Rutkowski P, Van Glabbeke M, Rankin CJ, et al; European Organisation for Research and Treatment of Cancer Soft Tissue/Bone Sarcoma Group, Southwest Oncology Group. Imatinib mesylate in advanced dermatofibrosarcoma protuberans: pooled analysis of two phase II clinical trials [published online March 1, 2010]. J Clin Oncol. 2010;28:1772-1779.
- Mentzel T, Beham A, Katenkamp D, et al. Fibrosarcomatous ("high-grade") dermatofibrosarcoma protuberans: clinicopathologic and immunohistochemical study of a series of 41 cases with emphasis on prognostic significance. Am J Surg Pathol. 1998;22:576-587.
Practice Points
- Consider dermatofibrosarcoma protuberans for a keloidlike enlarging lesion when there is no history of trauma or prior keloid formation.
- Treatments such as Mohs micrographic surgery or oral imatinib mesylate can provide lower recurrence rates in appropriate patients as stand-alone or adjuvant therapy.