User login
Defending access to reproductive health care
The 1973 Supreme Court of the United States (SCOTUS) decision in Roe v Wade was a landmark ruling,1 establishing that the United States Constitution provides a fundamental “right to privacy,” protecting pregnant people’s freedom to access all available reproductive health care options. Recognizing that the right to abortion was not absolute, the majority of justices supported a trimester system. In the first trimester, decisions about abortion care are fully controlled by patients and clinicians, and no government could place restrictions on access to abortion. In the second trimester, SCOTUS ruled that states may choose to regulate abortion to protect maternal health. (As an example of such state restrictions, in Massachusetts, for many years, but no longer, the state required that abortions occur in a hospital when the patient was between 18 and 24 weeks’ gestation in order to facilitate comprehensive emergency care for complications.) Beginning in the third trimester, a point at which a fetus could be viable, the Court ruled that a government could prohibit abortion except when an abortion was necessary to protect the life or health of the pregnant person. In 1992, the SCOTUS decision in Planned Parenthood v Casey2 rejected the trimester system, reaffirming the right to an abortion before fetal viability, and adopting a new standard that states may not create an undue burden on a person seeking an abortion b
If, as anticipated, the 2022 SCOTUS decision in Dobbs v Jackson Women’s Health Organization3 overturns the precedents set in Roe v Wade and Planned Parenthood v Casey, decisions on abortion law will be relegated to elected legislators and state courts.4 It is expected that at least 26 state legislatures and governors will enact stringent new restrictions on access to abortion. This cataclysmic reversal of judicial opinion creates a historic challenge to obstetrician-gynecologists and their patients and could threaten access to other vital reproductive services beyond abortion, like contraception. We will be fighting, state by state, for people’s right to access all available reproductive health procedures. This will also significantly affect the ability for providers in women’s reproductive health to obtain appropriate and necessary education and training in a critical skills. If access to safe abortion is restricted, we fear patients may be forced to consider unsafe abortion, raising the specter of a return to the 1960s, when an epidemic of unsafe abortion caused countless injuries and deaths.5,6
How do we best prepare for these challenges?
- We will need to be flexible and continually evolve our clinical practices to be adherent with state and local legislation and regulation.
- To reduce unintended pregnancies, we need to strengthen our efforts to ensure that every patient has ready access to all available contraceptive options with no out-of-pocket cost.
- When a contraceptive is desired, we will focus on educating people about effectiveness, and offering them highly reliable contraception, such as the implant or intrauterine devices.
- We need to ensure timely access to abortion if state-based laws permit abortion before 6 or 7 weeks’ gestation. Providing medication abortion without an in-person visit using a telehealth option would be one option to expand rapid access to early first trimester abortion.
- Clinicians in states with access to abortion services will need to collaborate with colleagues in states with restrictions on abortion services to improve patient access across state borders.
On a national level, advancing our effective advocacy in Congress may lead to national legislation passed and signed by the President. This could supersede most state laws prohibiting access to comprehensive women’s reproductive health and create a unified, national approach to abortion care, allowing for the appropriate training of all obstetrician-gynecologists. We will also need to develop teams in every state capable of advocating for laws that ensure access to all reproductive health care options. The American College of Obstetricians and Gynecologists has leaders trained and tasked with legislative advocacy in every state.7 This network will be a foundation upon which to build additional advocacy efforts.
As women’s health care professionals, our responsibility to our patients, is to work to ensure universal access to safe and effective comprehensive reproductive options, and to ensure that our workforce is prepared to meet the needs of our patients by defending the patient-clinician relationship. Abortion care saves lives of pregnant patients and reduces maternal morbidity.8 Access to safe abortion care as part of comprehensive reproductive services is an important component of health care. ●
- Roe v Wade, 410 U.S. 113 (1973).
- Planned Parenthood v Casey, 505 U.S. 833 (1992).
- Dobbs v Jackson Women’s Health Organization, 19-1392. https://www.supremecourt.gov/search .aspx?filename=/docket/docketfiles/html /public/19-1392.html. Accessed May 18, 2022.
- Gerstein J, Ward A. Supreme Court has voted to overturn abortion rights, draft opinion shows. Politico. May 5, 2022. Updated May 3, 2022.
- Gold RB. Lessons from before Roe: will past be prologue? Guttmacher Institute. March 1, 2003. https://www.guttmacher.org/gpr/2003/03 /lessons-roe-will-past-be-prologue. Accessed May 18, 2022.
- Edelin KC. Broken Justice: A True Story of Race, Sex and Revenge in a Boston Courtroom. Pond View Press; 2007.
- The American College of Obstetricians and Gynecologists. Get involved in your state. ACOG web site. https://www.acog.org/advocacy /get-involved/get-involved-in-your-state. Accessed May 18, 2022.
- Institute of Medicine (US) Committee on Improving Birth Outcomes. Bale JR, Stoll BJ, Lucas AO, eds. Reducing maternal mortality and morbidity. In: Improving Birth Outcomes: Meeting the Challenge in the Developing World. Washington, DC: National Academies Press (US); 2003.
The 1973 Supreme Court of the United States (SCOTUS) decision in Roe v Wade was a landmark ruling,1 establishing that the United States Constitution provides a fundamental “right to privacy,” protecting pregnant people’s freedom to access all available reproductive health care options. Recognizing that the right to abortion was not absolute, the majority of justices supported a trimester system. In the first trimester, decisions about abortion care are fully controlled by patients and clinicians, and no government could place restrictions on access to abortion. In the second trimester, SCOTUS ruled that states may choose to regulate abortion to protect maternal health. (As an example of such state restrictions, in Massachusetts, for many years, but no longer, the state required that abortions occur in a hospital when the patient was between 18 and 24 weeks’ gestation in order to facilitate comprehensive emergency care for complications.) Beginning in the third trimester, a point at which a fetus could be viable, the Court ruled that a government could prohibit abortion except when an abortion was necessary to protect the life or health of the pregnant person. In 1992, the SCOTUS decision in Planned Parenthood v Casey2 rejected the trimester system, reaffirming the right to an abortion before fetal viability, and adopting a new standard that states may not create an undue burden on a person seeking an abortion b
If, as anticipated, the 2022 SCOTUS decision in Dobbs v Jackson Women’s Health Organization3 overturns the precedents set in Roe v Wade and Planned Parenthood v Casey, decisions on abortion law will be relegated to elected legislators and state courts.4 It is expected that at least 26 state legislatures and governors will enact stringent new restrictions on access to abortion. This cataclysmic reversal of judicial opinion creates a historic challenge to obstetrician-gynecologists and their patients and could threaten access to other vital reproductive services beyond abortion, like contraception. We will be fighting, state by state, for people’s right to access all available reproductive health procedures. This will also significantly affect the ability for providers in women’s reproductive health to obtain appropriate and necessary education and training in a critical skills. If access to safe abortion is restricted, we fear patients may be forced to consider unsafe abortion, raising the specter of a return to the 1960s, when an epidemic of unsafe abortion caused countless injuries and deaths.5,6
How do we best prepare for these challenges?
- We will need to be flexible and continually evolve our clinical practices to be adherent with state and local legislation and regulation.
- To reduce unintended pregnancies, we need to strengthen our efforts to ensure that every patient has ready access to all available contraceptive options with no out-of-pocket cost.
- When a contraceptive is desired, we will focus on educating people about effectiveness, and offering them highly reliable contraception, such as the implant or intrauterine devices.
- We need to ensure timely access to abortion if state-based laws permit abortion before 6 or 7 weeks’ gestation. Providing medication abortion without an in-person visit using a telehealth option would be one option to expand rapid access to early first trimester abortion.
- Clinicians in states with access to abortion services will need to collaborate with colleagues in states with restrictions on abortion services to improve patient access across state borders.
On a national level, advancing our effective advocacy in Congress may lead to national legislation passed and signed by the President. This could supersede most state laws prohibiting access to comprehensive women’s reproductive health and create a unified, national approach to abortion care, allowing for the appropriate training of all obstetrician-gynecologists. We will also need to develop teams in every state capable of advocating for laws that ensure access to all reproductive health care options. The American College of Obstetricians and Gynecologists has leaders trained and tasked with legislative advocacy in every state.7 This network will be a foundation upon which to build additional advocacy efforts.
As women’s health care professionals, our responsibility to our patients, is to work to ensure universal access to safe and effective comprehensive reproductive options, and to ensure that our workforce is prepared to meet the needs of our patients by defending the patient-clinician relationship. Abortion care saves lives of pregnant patients and reduces maternal morbidity.8 Access to safe abortion care as part of comprehensive reproductive services is an important component of health care. ●
The 1973 Supreme Court of the United States (SCOTUS) decision in Roe v Wade was a landmark ruling,1 establishing that the United States Constitution provides a fundamental “right to privacy,” protecting pregnant people’s freedom to access all available reproductive health care options. Recognizing that the right to abortion was not absolute, the majority of justices supported a trimester system. In the first trimester, decisions about abortion care are fully controlled by patients and clinicians, and no government could place restrictions on access to abortion. In the second trimester, SCOTUS ruled that states may choose to regulate abortion to protect maternal health. (As an example of such state restrictions, in Massachusetts, for many years, but no longer, the state required that abortions occur in a hospital when the patient was between 18 and 24 weeks’ gestation in order to facilitate comprehensive emergency care for complications.) Beginning in the third trimester, a point at which a fetus could be viable, the Court ruled that a government could prohibit abortion except when an abortion was necessary to protect the life or health of the pregnant person. In 1992, the SCOTUS decision in Planned Parenthood v Casey2 rejected the trimester system, reaffirming the right to an abortion before fetal viability, and adopting a new standard that states may not create an undue burden on a person seeking an abortion b
If, as anticipated, the 2022 SCOTUS decision in Dobbs v Jackson Women’s Health Organization3 overturns the precedents set in Roe v Wade and Planned Parenthood v Casey, decisions on abortion law will be relegated to elected legislators and state courts.4 It is expected that at least 26 state legislatures and governors will enact stringent new restrictions on access to abortion. This cataclysmic reversal of judicial opinion creates a historic challenge to obstetrician-gynecologists and their patients and could threaten access to other vital reproductive services beyond abortion, like contraception. We will be fighting, state by state, for people’s right to access all available reproductive health procedures. This will also significantly affect the ability for providers in women’s reproductive health to obtain appropriate and necessary education and training in a critical skills. If access to safe abortion is restricted, we fear patients may be forced to consider unsafe abortion, raising the specter of a return to the 1960s, when an epidemic of unsafe abortion caused countless injuries and deaths.5,6
How do we best prepare for these challenges?
- We will need to be flexible and continually evolve our clinical practices to be adherent with state and local legislation and regulation.
- To reduce unintended pregnancies, we need to strengthen our efforts to ensure that every patient has ready access to all available contraceptive options with no out-of-pocket cost.
- When a contraceptive is desired, we will focus on educating people about effectiveness, and offering them highly reliable contraception, such as the implant or intrauterine devices.
- We need to ensure timely access to abortion if state-based laws permit abortion before 6 or 7 weeks’ gestation. Providing medication abortion without an in-person visit using a telehealth option would be one option to expand rapid access to early first trimester abortion.
- Clinicians in states with access to abortion services will need to collaborate with colleagues in states with restrictions on abortion services to improve patient access across state borders.
On a national level, advancing our effective advocacy in Congress may lead to national legislation passed and signed by the President. This could supersede most state laws prohibiting access to comprehensive women’s reproductive health and create a unified, national approach to abortion care, allowing for the appropriate training of all obstetrician-gynecologists. We will also need to develop teams in every state capable of advocating for laws that ensure access to all reproductive health care options. The American College of Obstetricians and Gynecologists has leaders trained and tasked with legislative advocacy in every state.7 This network will be a foundation upon which to build additional advocacy efforts.
As women’s health care professionals, our responsibility to our patients, is to work to ensure universal access to safe and effective comprehensive reproductive options, and to ensure that our workforce is prepared to meet the needs of our patients by defending the patient-clinician relationship. Abortion care saves lives of pregnant patients and reduces maternal morbidity.8 Access to safe abortion care as part of comprehensive reproductive services is an important component of health care. ●
- Roe v Wade, 410 U.S. 113 (1973).
- Planned Parenthood v Casey, 505 U.S. 833 (1992).
- Dobbs v Jackson Women’s Health Organization, 19-1392. https://www.supremecourt.gov/search .aspx?filename=/docket/docketfiles/html /public/19-1392.html. Accessed May 18, 2022.
- Gerstein J, Ward A. Supreme Court has voted to overturn abortion rights, draft opinion shows. Politico. May 5, 2022. Updated May 3, 2022.
- Gold RB. Lessons from before Roe: will past be prologue? Guttmacher Institute. March 1, 2003. https://www.guttmacher.org/gpr/2003/03 /lessons-roe-will-past-be-prologue. Accessed May 18, 2022.
- Edelin KC. Broken Justice: A True Story of Race, Sex and Revenge in a Boston Courtroom. Pond View Press; 2007.
- The American College of Obstetricians and Gynecologists. Get involved in your state. ACOG web site. https://www.acog.org/advocacy /get-involved/get-involved-in-your-state. Accessed May 18, 2022.
- Institute of Medicine (US) Committee on Improving Birth Outcomes. Bale JR, Stoll BJ, Lucas AO, eds. Reducing maternal mortality and morbidity. In: Improving Birth Outcomes: Meeting the Challenge in the Developing World. Washington, DC: National Academies Press (US); 2003.
- Roe v Wade, 410 U.S. 113 (1973).
- Planned Parenthood v Casey, 505 U.S. 833 (1992).
- Dobbs v Jackson Women’s Health Organization, 19-1392. https://www.supremecourt.gov/search .aspx?filename=/docket/docketfiles/html /public/19-1392.html. Accessed May 18, 2022.
- Gerstein J, Ward A. Supreme Court has voted to overturn abortion rights, draft opinion shows. Politico. May 5, 2022. Updated May 3, 2022.
- Gold RB. Lessons from before Roe: will past be prologue? Guttmacher Institute. March 1, 2003. https://www.guttmacher.org/gpr/2003/03 /lessons-roe-will-past-be-prologue. Accessed May 18, 2022.
- Edelin KC. Broken Justice: A True Story of Race, Sex and Revenge in a Boston Courtroom. Pond View Press; 2007.
- The American College of Obstetricians and Gynecologists. Get involved in your state. ACOG web site. https://www.acog.org/advocacy /get-involved/get-involved-in-your-state. Accessed May 18, 2022.
- Institute of Medicine (US) Committee on Improving Birth Outcomes. Bale JR, Stoll BJ, Lucas AO, eds. Reducing maternal mortality and morbidity. In: Improving Birth Outcomes: Meeting the Challenge in the Developing World. Washington, DC: National Academies Press (US); 2003.
FDA denies petition to disqualify researchers over controversial ketamine studies
The U.S. Food and Drug Administration has declined to take further action against a group of investigators at Hennepin County Medical Center/Hennepin Healthcare (HCMC) who conducted controversial studies involving ketamine and other sedatives on agitated persons without their consent.
A citizen petition filed by Public Citizen, a consumer advocacy group, had asked the FDA to initiate clinical-investigator disqualification proceedings against Jon Cole, MD, and Lauren Klein, MD, along with other researchers who participated in the studies, for “repeatedly and deliberately initiating and conducting clinical investigations of investigational drug products” without having submitted or having in effect the investigational new drug applications (INDs) required by the FDA.
In certain situations, wherein the FDA alleges that a clinical investigator has violated applicable regulations, the agency may initiate clinical investigator disqualification proceedings. The names of the disqualified researchers are then added to a federal database.
The petition, which was filed in November 2021, also requested that the FDA initiate disqualification proceedings against the institutional review board (IRB) at HCMC for repeatedly failing to comply with federal regulations that adversely affected the rights and welfare of the individuals who were enrolled in the study without their consent.
Of note, Public Citizen stated that the FDA should have required the hospital to contact the more than 1,700 patients who “were unwittingly enrolled in unethical experiments” and inform them that their rights had been violated and their health potentially endangered by the research team.
Michael A. Carome, MD, director of Public Citizen’s Health Research Group, told this news organization that it is uncommon for the FDA to disqualify researchers. “It should be more common than it is,” he said. “I think that FDA is just reluctant to take more action.”
The actions of the Hennepin investigators were “repetitive and appeared to be in deliberate violation of regulations,” he added. “The case for the FDA disqualifying the HCMC researchers is overwhelming. The FDA’s slap-on-the-wrist approach to such appalling regulatory and ethical violations risks emboldening other researchers to disregard the rights and welfare of human subjects.”
Carl Elliott, MD, PhD, a bioethicist at the University of Minnesota, Minneapolis, agrees that the researcher from HCMC should be disqualified. “They didn’t just conduct risky, exploitative studies – they conducted them after the FDA had warned them not to proceed,” he said. “The message sent by this slap on the wrist is that investigators can do whatever they want to nonconsenting subjects, and the FDA will look the other way.”
Initial complaint
Public Citizen initially filed a complaint with the FDA in 2018, after learning that researchers affiliated with HCMC were conducting high-risk clinical trials involving ketamine to control agitation outside of the hospital setting. The complaint was cosigned by 64 doctors, bioethicists, and academic researchers and was also submitted to the Office for Human Research Protections.
The FDA typically allows investigational drugs to be used in emergency situation without obtaining informed consent if the therapies are known to carry a minimal risk. The IRB at HCMC had determined that this was the case with ketamine and approved the trials.
But according to Public Citizen’s complaint, prior research had suggested that ketamine could cause more complications and severe adverse events, compared with other sedatives.
The trials were conducted between 2014 and 2018, and in its letter, Public Citizen alleged that the investigators and the IRB had allowed these trials to proceed without obtaining informed consent from patients. The goal was to evaluate how well ketamine worked, compared with other drugs in calming agitated individuals: “The patients were given either ketamine or haloperidol for agitation by paramedics who responded to medical emergencies, and the goal was to see which drug worked faster,” said Dr. Carome. “Patients were only notified afterwards that they had received a sedative. Informed consent had been waived by IRB.”
In the first clinical trial conducted by HCMC, published in 2016, the researchers had hypothesized that 5 mg/kg of intramuscular ketamine would be superior to 10 mg of intramuscular haloperidol for severe prehospital agitation. Time to adequate sedation was the primary outcome measure. The study included 146 people; 64 received ketamine and 82 received haloperidol. They found that ketamine worked far more quickly than haloperidol (5 minutes vs. 17 minutes) but that the risk for complications was much higher. Complications occurred in 49% of patients receiving ketamine, compared with 5%.
“There was a 10-fold risk of adverse events,” said Dr. Carome. “And 39% of patients given ketamine had respiratory problems requiring intubation, compared to 4% who received haloperidol.”
A second study was launched in 2017, wherein ketamine was compared with midazolam in agitated patients. During the first 6-month period of the study, individuals would receive a ketamine-based protocol for prehospital agitation, and during the second 6 months, that would switch to midazolam. However, the study was halted in June 2018 after the local newspaper, the Star Tribune, reported that the city police had encouraged medical personnel to sedate agitated patients. This included individuals who had already been physically restrained.
The report stated that “in many cases, the individual being detained or arrested was not only handcuffed but strapped down on a stretcher in an ambulance before receiving ketamine,” and that it raised a “concerning question” over why these people were given the drug before they were transported to the hospital, “given the immediate effects on breathing and heart function that the drug induces.”
Along with halting the trial, HCMC asked for a review of cases involving its paramedics; an independent investigation led by former U.S. Deputy Attorney General Sally Yates was initiated to assess whether the Minneapolis police had crossed a line and urged paramedics to use ketamine.
“The decision to use ketamine was based on the study’s timeline and not on clinical judgment,” said Dr. Carome.
The FDA acknowledged receipt of the complaint and inspected the IRB records and the clinical trial data. Preliminary reports received by Public Citizen confirmed their allegations. “There were not appropriate protections for vulnerable subjects,” he said. “In 2019, the FDA did further investigations, and those reports had similar findings.”
FDA letters
The FDA had sent warning letters to Dr. Cole and Dr. Klein, citing them for ignoring federal safety laws in experimental research on the public. In their investigations, the FDA cited “objectionable conditions” for the studies led by Dr. Cole and Dr. Klein, according to the letters. Both researchers seemingly ignored FDA regulations and used practices that subjected patients to “significantly increased risk,” and the hospital defended its research with “factually incorrect” statements.
In a letter to Dr. Cole, the FDA noted that he never filed INDs for the trials with the FDA, as required by law, and that he also failed to write appropriate protocols to ensure that children and pregnant women were not enrolled in the research. Individuals under the influence of intoxicants also were not excluded, though the use of ketamine is cautioned in this population.
“Administration of the investigational drugs to these subjects placed them at significantly increased risk of the adverse events associated with the investigational products and decreased the acceptability of those risks,” the FDA said in its letter. “Your failure to exclude, and the lack of any precautions for, subjects under the influence of various intoxicants significantly increased the risks and/or decreased the acceptability of the risks associated with the investigational drugs.”
However, Dr. Cole conducted both studies in the prehospital setting and failed to initiate any specific measures to protect study participants, according to the FDA.
Petition denied
Dr. Carome noted that the researchers had committed repetitive egregious regulatory violations over a 4-year period, which were documented by the FDA in their warning letters to Dr. Cole and Dr. Klein. “We felt that they were so egregious that we need to send a signal to the community that this sort of behavior will not be tolerated,” he said. “The FDA denied our petition, and we think that sends the wrong signal to the research community.”
In their response, the FDA noted that as with judicial enforcement, “the Agency makes decisions regarding whether to pursue administrative enforcement action, including disqualification proceedings, on a case-by-case basis, considering all relevant facts and circumstances.” They added that at this time, they would not be taking further action against Dr. Cole and Dr. Klein.
“However, we intend to continue to consider all the options available to the Agency as we determine whether to pursue additional compliance actions related to this matter,” the FDA concluded.
The FDA declined to comment further on their decision.
Dr. Cole also declined to comment, but Hennepin Healthcare told this news organization that the “decision by the FDA to deny the petition validates the changes we made to strengthen and improve the clinical research program across the institution since the closing of the studies in 2018. We look forward to continuing to work with the FDA to ensure full compliance with the standards in place to protect research subjects.”
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has declined to take further action against a group of investigators at Hennepin County Medical Center/Hennepin Healthcare (HCMC) who conducted controversial studies involving ketamine and other sedatives on agitated persons without their consent.
A citizen petition filed by Public Citizen, a consumer advocacy group, had asked the FDA to initiate clinical-investigator disqualification proceedings against Jon Cole, MD, and Lauren Klein, MD, along with other researchers who participated in the studies, for “repeatedly and deliberately initiating and conducting clinical investigations of investigational drug products” without having submitted or having in effect the investigational new drug applications (INDs) required by the FDA.
In certain situations, wherein the FDA alleges that a clinical investigator has violated applicable regulations, the agency may initiate clinical investigator disqualification proceedings. The names of the disqualified researchers are then added to a federal database.
The petition, which was filed in November 2021, also requested that the FDA initiate disqualification proceedings against the institutional review board (IRB) at HCMC for repeatedly failing to comply with federal regulations that adversely affected the rights and welfare of the individuals who were enrolled in the study without their consent.
Of note, Public Citizen stated that the FDA should have required the hospital to contact the more than 1,700 patients who “were unwittingly enrolled in unethical experiments” and inform them that their rights had been violated and their health potentially endangered by the research team.
Michael A. Carome, MD, director of Public Citizen’s Health Research Group, told this news organization that it is uncommon for the FDA to disqualify researchers. “It should be more common than it is,” he said. “I think that FDA is just reluctant to take more action.”
The actions of the Hennepin investigators were “repetitive and appeared to be in deliberate violation of regulations,” he added. “The case for the FDA disqualifying the HCMC researchers is overwhelming. The FDA’s slap-on-the-wrist approach to such appalling regulatory and ethical violations risks emboldening other researchers to disregard the rights and welfare of human subjects.”
Carl Elliott, MD, PhD, a bioethicist at the University of Minnesota, Minneapolis, agrees that the researcher from HCMC should be disqualified. “They didn’t just conduct risky, exploitative studies – they conducted them after the FDA had warned them not to proceed,” he said. “The message sent by this slap on the wrist is that investigators can do whatever they want to nonconsenting subjects, and the FDA will look the other way.”
Initial complaint
Public Citizen initially filed a complaint with the FDA in 2018, after learning that researchers affiliated with HCMC were conducting high-risk clinical trials involving ketamine to control agitation outside of the hospital setting. The complaint was cosigned by 64 doctors, bioethicists, and academic researchers and was also submitted to the Office for Human Research Protections.
The FDA typically allows investigational drugs to be used in emergency situation without obtaining informed consent if the therapies are known to carry a minimal risk. The IRB at HCMC had determined that this was the case with ketamine and approved the trials.
But according to Public Citizen’s complaint, prior research had suggested that ketamine could cause more complications and severe adverse events, compared with other sedatives.
The trials were conducted between 2014 and 2018, and in its letter, Public Citizen alleged that the investigators and the IRB had allowed these trials to proceed without obtaining informed consent from patients. The goal was to evaluate how well ketamine worked, compared with other drugs in calming agitated individuals: “The patients were given either ketamine or haloperidol for agitation by paramedics who responded to medical emergencies, and the goal was to see which drug worked faster,” said Dr. Carome. “Patients were only notified afterwards that they had received a sedative. Informed consent had been waived by IRB.”
In the first clinical trial conducted by HCMC, published in 2016, the researchers had hypothesized that 5 mg/kg of intramuscular ketamine would be superior to 10 mg of intramuscular haloperidol for severe prehospital agitation. Time to adequate sedation was the primary outcome measure. The study included 146 people; 64 received ketamine and 82 received haloperidol. They found that ketamine worked far more quickly than haloperidol (5 minutes vs. 17 minutes) but that the risk for complications was much higher. Complications occurred in 49% of patients receiving ketamine, compared with 5%.
“There was a 10-fold risk of adverse events,” said Dr. Carome. “And 39% of patients given ketamine had respiratory problems requiring intubation, compared to 4% who received haloperidol.”
A second study was launched in 2017, wherein ketamine was compared with midazolam in agitated patients. During the first 6-month period of the study, individuals would receive a ketamine-based protocol for prehospital agitation, and during the second 6 months, that would switch to midazolam. However, the study was halted in June 2018 after the local newspaper, the Star Tribune, reported that the city police had encouraged medical personnel to sedate agitated patients. This included individuals who had already been physically restrained.
The report stated that “in many cases, the individual being detained or arrested was not only handcuffed but strapped down on a stretcher in an ambulance before receiving ketamine,” and that it raised a “concerning question” over why these people were given the drug before they were transported to the hospital, “given the immediate effects on breathing and heart function that the drug induces.”
Along with halting the trial, HCMC asked for a review of cases involving its paramedics; an independent investigation led by former U.S. Deputy Attorney General Sally Yates was initiated to assess whether the Minneapolis police had crossed a line and urged paramedics to use ketamine.
“The decision to use ketamine was based on the study’s timeline and not on clinical judgment,” said Dr. Carome.
The FDA acknowledged receipt of the complaint and inspected the IRB records and the clinical trial data. Preliminary reports received by Public Citizen confirmed their allegations. “There were not appropriate protections for vulnerable subjects,” he said. “In 2019, the FDA did further investigations, and those reports had similar findings.”
FDA letters
The FDA had sent warning letters to Dr. Cole and Dr. Klein, citing them for ignoring federal safety laws in experimental research on the public. In their investigations, the FDA cited “objectionable conditions” for the studies led by Dr. Cole and Dr. Klein, according to the letters. Both researchers seemingly ignored FDA regulations and used practices that subjected patients to “significantly increased risk,” and the hospital defended its research with “factually incorrect” statements.
In a letter to Dr. Cole, the FDA noted that he never filed INDs for the trials with the FDA, as required by law, and that he also failed to write appropriate protocols to ensure that children and pregnant women were not enrolled in the research. Individuals under the influence of intoxicants also were not excluded, though the use of ketamine is cautioned in this population.
“Administration of the investigational drugs to these subjects placed them at significantly increased risk of the adverse events associated with the investigational products and decreased the acceptability of those risks,” the FDA said in its letter. “Your failure to exclude, and the lack of any precautions for, subjects under the influence of various intoxicants significantly increased the risks and/or decreased the acceptability of the risks associated with the investigational drugs.”
However, Dr. Cole conducted both studies in the prehospital setting and failed to initiate any specific measures to protect study participants, according to the FDA.
Petition denied
Dr. Carome noted that the researchers had committed repetitive egregious regulatory violations over a 4-year period, which were documented by the FDA in their warning letters to Dr. Cole and Dr. Klein. “We felt that they were so egregious that we need to send a signal to the community that this sort of behavior will not be tolerated,” he said. “The FDA denied our petition, and we think that sends the wrong signal to the research community.”
In their response, the FDA noted that as with judicial enforcement, “the Agency makes decisions regarding whether to pursue administrative enforcement action, including disqualification proceedings, on a case-by-case basis, considering all relevant facts and circumstances.” They added that at this time, they would not be taking further action against Dr. Cole and Dr. Klein.
“However, we intend to continue to consider all the options available to the Agency as we determine whether to pursue additional compliance actions related to this matter,” the FDA concluded.
The FDA declined to comment further on their decision.
Dr. Cole also declined to comment, but Hennepin Healthcare told this news organization that the “decision by the FDA to deny the petition validates the changes we made to strengthen and improve the clinical research program across the institution since the closing of the studies in 2018. We look forward to continuing to work with the FDA to ensure full compliance with the standards in place to protect research subjects.”
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has declined to take further action against a group of investigators at Hennepin County Medical Center/Hennepin Healthcare (HCMC) who conducted controversial studies involving ketamine and other sedatives on agitated persons without their consent.
A citizen petition filed by Public Citizen, a consumer advocacy group, had asked the FDA to initiate clinical-investigator disqualification proceedings against Jon Cole, MD, and Lauren Klein, MD, along with other researchers who participated in the studies, for “repeatedly and deliberately initiating and conducting clinical investigations of investigational drug products” without having submitted or having in effect the investigational new drug applications (INDs) required by the FDA.
In certain situations, wherein the FDA alleges that a clinical investigator has violated applicable regulations, the agency may initiate clinical investigator disqualification proceedings. The names of the disqualified researchers are then added to a federal database.
The petition, which was filed in November 2021, also requested that the FDA initiate disqualification proceedings against the institutional review board (IRB) at HCMC for repeatedly failing to comply with federal regulations that adversely affected the rights and welfare of the individuals who were enrolled in the study without their consent.
Of note, Public Citizen stated that the FDA should have required the hospital to contact the more than 1,700 patients who “were unwittingly enrolled in unethical experiments” and inform them that their rights had been violated and their health potentially endangered by the research team.
Michael A. Carome, MD, director of Public Citizen’s Health Research Group, told this news organization that it is uncommon for the FDA to disqualify researchers. “It should be more common than it is,” he said. “I think that FDA is just reluctant to take more action.”
The actions of the Hennepin investigators were “repetitive and appeared to be in deliberate violation of regulations,” he added. “The case for the FDA disqualifying the HCMC researchers is overwhelming. The FDA’s slap-on-the-wrist approach to such appalling regulatory and ethical violations risks emboldening other researchers to disregard the rights and welfare of human subjects.”
Carl Elliott, MD, PhD, a bioethicist at the University of Minnesota, Minneapolis, agrees that the researcher from HCMC should be disqualified. “They didn’t just conduct risky, exploitative studies – they conducted them after the FDA had warned them not to proceed,” he said. “The message sent by this slap on the wrist is that investigators can do whatever they want to nonconsenting subjects, and the FDA will look the other way.”
Initial complaint
Public Citizen initially filed a complaint with the FDA in 2018, after learning that researchers affiliated with HCMC were conducting high-risk clinical trials involving ketamine to control agitation outside of the hospital setting. The complaint was cosigned by 64 doctors, bioethicists, and academic researchers and was also submitted to the Office for Human Research Protections.
The FDA typically allows investigational drugs to be used in emergency situation without obtaining informed consent if the therapies are known to carry a minimal risk. The IRB at HCMC had determined that this was the case with ketamine and approved the trials.
But according to Public Citizen’s complaint, prior research had suggested that ketamine could cause more complications and severe adverse events, compared with other sedatives.
The trials were conducted between 2014 and 2018, and in its letter, Public Citizen alleged that the investigators and the IRB had allowed these trials to proceed without obtaining informed consent from patients. The goal was to evaluate how well ketamine worked, compared with other drugs in calming agitated individuals: “The patients were given either ketamine or haloperidol for agitation by paramedics who responded to medical emergencies, and the goal was to see which drug worked faster,” said Dr. Carome. “Patients were only notified afterwards that they had received a sedative. Informed consent had been waived by IRB.”
In the first clinical trial conducted by HCMC, published in 2016, the researchers had hypothesized that 5 mg/kg of intramuscular ketamine would be superior to 10 mg of intramuscular haloperidol for severe prehospital agitation. Time to adequate sedation was the primary outcome measure. The study included 146 people; 64 received ketamine and 82 received haloperidol. They found that ketamine worked far more quickly than haloperidol (5 minutes vs. 17 minutes) but that the risk for complications was much higher. Complications occurred in 49% of patients receiving ketamine, compared with 5%.
“There was a 10-fold risk of adverse events,” said Dr. Carome. “And 39% of patients given ketamine had respiratory problems requiring intubation, compared to 4% who received haloperidol.”
A second study was launched in 2017, wherein ketamine was compared with midazolam in agitated patients. During the first 6-month period of the study, individuals would receive a ketamine-based protocol for prehospital agitation, and during the second 6 months, that would switch to midazolam. However, the study was halted in June 2018 after the local newspaper, the Star Tribune, reported that the city police had encouraged medical personnel to sedate agitated patients. This included individuals who had already been physically restrained.
The report stated that “in many cases, the individual being detained or arrested was not only handcuffed but strapped down on a stretcher in an ambulance before receiving ketamine,” and that it raised a “concerning question” over why these people were given the drug before they were transported to the hospital, “given the immediate effects on breathing and heart function that the drug induces.”
Along with halting the trial, HCMC asked for a review of cases involving its paramedics; an independent investigation led by former U.S. Deputy Attorney General Sally Yates was initiated to assess whether the Minneapolis police had crossed a line and urged paramedics to use ketamine.
“The decision to use ketamine was based on the study’s timeline and not on clinical judgment,” said Dr. Carome.
The FDA acknowledged receipt of the complaint and inspected the IRB records and the clinical trial data. Preliminary reports received by Public Citizen confirmed their allegations. “There were not appropriate protections for vulnerable subjects,” he said. “In 2019, the FDA did further investigations, and those reports had similar findings.”
FDA letters
The FDA had sent warning letters to Dr. Cole and Dr. Klein, citing them for ignoring federal safety laws in experimental research on the public. In their investigations, the FDA cited “objectionable conditions” for the studies led by Dr. Cole and Dr. Klein, according to the letters. Both researchers seemingly ignored FDA regulations and used practices that subjected patients to “significantly increased risk,” and the hospital defended its research with “factually incorrect” statements.
In a letter to Dr. Cole, the FDA noted that he never filed INDs for the trials with the FDA, as required by law, and that he also failed to write appropriate protocols to ensure that children and pregnant women were not enrolled in the research. Individuals under the influence of intoxicants also were not excluded, though the use of ketamine is cautioned in this population.
“Administration of the investigational drugs to these subjects placed them at significantly increased risk of the adverse events associated with the investigational products and decreased the acceptability of those risks,” the FDA said in its letter. “Your failure to exclude, and the lack of any precautions for, subjects under the influence of various intoxicants significantly increased the risks and/or decreased the acceptability of the risks associated with the investigational drugs.”
However, Dr. Cole conducted both studies in the prehospital setting and failed to initiate any specific measures to protect study participants, according to the FDA.
Petition denied
Dr. Carome noted that the researchers had committed repetitive egregious regulatory violations over a 4-year period, which were documented by the FDA in their warning letters to Dr. Cole and Dr. Klein. “We felt that they were so egregious that we need to send a signal to the community that this sort of behavior will not be tolerated,” he said. “The FDA denied our petition, and we think that sends the wrong signal to the research community.”
In their response, the FDA noted that as with judicial enforcement, “the Agency makes decisions regarding whether to pursue administrative enforcement action, including disqualification proceedings, on a case-by-case basis, considering all relevant facts and circumstances.” They added that at this time, they would not be taking further action against Dr. Cole and Dr. Klein.
“However, we intend to continue to consider all the options available to the Agency as we determine whether to pursue additional compliance actions related to this matter,” the FDA concluded.
The FDA declined to comment further on their decision.
Dr. Cole also declined to comment, but Hennepin Healthcare told this news organization that the “decision by the FDA to deny the petition validates the changes we made to strengthen and improve the clinical research program across the institution since the closing of the studies in 2018. We look forward to continuing to work with the FDA to ensure full compliance with the standards in place to protect research subjects.”
A version of this article first appeared on Medscape.com.
Where Does the Hospital Belong? Perspectives on Hospital at Home in the 21st Century
From Medically Home Group, Boston, MA.
Brick-and-mortar hospitals in the United States have historically been considered the dominant setting for providing care to patients. The coordination and delivery of care has previously been bound to physical hospitals largely because multidisciplinary services were only accessible in an individual location. While the fundamental make-up of these services remains unchanged, these services are now available in alternate settings. Some of these services include access to a patient care team, supplies, diagnostics, pharmacy, and advanced therapeutic interventions. Presently, the physical environment is becoming increasingly irrelevant as the core of what makes the traditional hospital—the professional staff, collaborative work processes, and the dynamics of the space—have all been translated into a modern digitally integrated environment. The elements necessary to providing safe, effective care in a physical hospital setting are now available in a patient’s home.
Impetus for the Model
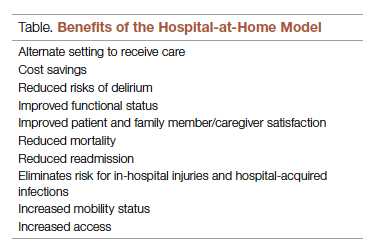
As hospitals reconsider how and where they deliver patient care because of limited resources, the hospital-at-home model has gained significant momentum and interest. This model transforms a home into a hospital. The inpatient acute care episode is entirely substituted with an intensive at-home hospital admission enabled by technology, multidisciplinary teams, and ancillary services. Furthermore, patients requiring post-acute support can be transitioned to their next phase of care seamlessly. Given the nationwide nursing shortage, aging population, challenges uncovered by the COVID-19 pandemic, rising hospital costs, nurse/provider burnout related to challenging work environments, and capacity constraints, a shift toward the combination of virtual and in-home care is imperative. The hospital-at-home model has been associated with superior patient outcomes, including reduced risks of delirium, improved functional status, improved patient and family member satisfaction, reduced mortality, reduced readmissions, and significantly lower costs.1 COVID-19 alone has unmasked major facility-based deficiencies and limitations of our health care system. While the pandemic is not the impetus for the hospital-at-home model, the extended stress of this event has created a unique opportunity to reimagine and transform our health care delivery system so that it is less fragmented and more flexible.
Nursing in the Model
Nursing is central to the hospital-at-home model. Virtual nurses provide meticulous care plan oversight, assessment, and documentation across in-home service providers, to ensure holistic, safe, transparent, and continuous progression toward care plan milestones. The virtual nurse monitors patients using in-home technology that is set up at the time of admission. Connecting with patients to verify social and medical needs, the virtual nurse advocates for their patients and uses these technologies to care and deploy on-demand hands-on services to the patient. Service providers such as paramedics, infusion nurses, or home health nurses may be deployed to provide services in the patient’s home. By bringing in supplies, therapeutics, and interdisciplinary team members, the capabilities of a brick-and-mortar hospital are replicated in the home. All actions that occur wherever the patient is receiving care are overseen by professional nursing staff; in short, virtual nurses are the equivalent of bedside nurses in the brick-and-mortar health care facilities.
Potential Benefits
There are many benefits to the hospital-at-home model (Table). This health care model can be particularly helpful for patients who require frequent admission to acute care facilities, and is well suited for patients with a range of conditions, including those with COVID-19, pneumonia, cellulitis, or congestive heart failure. This care model helps eliminate some of the stressors for patients who have chronic illnesses or other conditions that require frequent hospital admissions. Patients can independently recover at home and can also be surrounded by their loved ones and pets while recovering. This care approach additionally eliminates the risk of hospital-acquired infections and injuries. The hospital-at-home model allows for increased mobility,2 as patients are familiar with their surroundings, resulting in reduced onset of delirium. Additionally, patients with improved mobility performance are less likely to experience negative health outcomes.3 There is less chance of sleep disruption as the patient is sleeping in their own bed—no unfamiliar roommate, no call bells or health care personnel frequently coming into the room. The in-home technology set up for remote patient monitoring is designed with the user in mind. Ease of use empowers the patient to collaborate with their care team on their own terms and center the priorities of themselves and their families.
Positive Outcomes
The hospital-at-home model is associated with positive outcomes. The authors of a systematic review identified 10 randomized controlled trials of hospital-at-home programs (with a total of 1372 patients), but were able to obtain data for only 5 of these trials (with a total of 844 patients).4 They found a 38% reduction in 6-month mortality for patients who received hospital care at home, as well as significantly higher patient satisfaction across a range of medical conditions, including patients with cellulitis and community-acquired pneumonia, as well as elderly patients with multiple medical conditions. The authors concluded that hospital care at home was less expensive than admission to an acute care hospital.4 Similarly, a meta-analysis done by Caplan et al5 that included 61 randomized controlled trials concluded that hospital at home is associated with reductions in mortality, readmission rates, and cost, and increases in patient and caregiver satisfaction. Levine et al2 found reduced costs and utilization with home hospitalization compared to in-hospital care, as well as improved patient mobility status.
The home is the ideal place to empower patients and caregivers to engage in self-management.2 Receiving hospital care at home eliminates the need for dealing with transportation arrangements, traffic, road tolls, and time/scheduling constraints, or finding care for a dependent family member, some of the many stressors that may be experienced by patients who require frequent trips to the hospital. For patients who may not be clinically suitable candidates for hospital at home, such as those requiring critical care intervention and support, the brick-and-mortar hospital is still the appropriate site of care. The hospital-at-home model helps prevent bed shortages in brick-and-mortar hospital settings by allowing hospital care at home for patients who meet preset criteria. These patients can be hospitalized in alternative locations such as their own homes or the residence of a friend. This helps increase health system capacity as well as resiliency.
In addition to expanding safe and appropriate treatment spaces, the hospital-at-home model helps increase access to care for patients during nonstandard hours, including weekends, holidays, or when the waiting time in the emergency room is painfully long. Furthermore, providing care in the home gives the clinical team valuable insight into the patient’s daily life and routine. Performing medication reconciliation with the medicine cabinet in sight and dietary education in a patient’s kitchen are powerful touch points.2 For example, a patient with congestive heart failure who must undergo diuresis is much more likely to meet their care goals when their home diet is aligned with the treatment goal. By being able to see exactly what is in a patient’s pantry and fridge, the care team can create a much more tailored approach to sodium intake and fluid management. Providers can create and execute true patient-centric care as they gain direct insight into the patient’s lifestyle, which is clearly valuable when creating care plans for complex chronic health issues.
Challenges to Implementation and Scaling
Although there are clear benefits to hospital at home, how to best implement and scale this model presents a challenge. In addition to educating patients and families about this model of care, health care systems must expand their hospital-at-home programs and provide education about this model to clinical staff and trainees, and insurers must create reimbursement paradigms. Patients meeting eligibility criteria to enroll in hospital at home is the easiest hurdle, as hospital-at-home programs function best when they enroll and service as many patients as possible, including underserved populations.
Upfront Costs and Cost Savings
While there are upfront costs to set up technology and coordinate services, hospital at home also provides significant total cost savings when compared to coordination associated with brick-and-mortar admission. Hospital care accounts for about one-third of total medical expenditures and is a leading cause of debt.2 Eliminating fixed hospital costs such as facility, overhead, and equipment costs through adoption of the hospital-at-home model can lead to a reduction in expenditures. It has been found that fewer laboratory and diagnostic tests are ordered for hospital-at-home patients when compared to similar patients in brick-and-mortar hospital settings, with comparable or better clinical patient outcomes.6 Furthermore, it is estimated that there are cost savings of 19% to 30% when compared to traditional inpatient care.6 Without legislative action, upon the end of the current COVID-19 public health emergency, the Centers for Medicare & Medicaid Service’s Acute Hospital Care at Home waiver will terminate. This could slow down scaling of the model.However, over the past 2 years there has been enough buy-in from major health systems and patients to continue the momentum of the model’s growth. When setting up a hospital-at-home program, it would be wise to consider a few factors: where in the hospital or health system entity structure the hospital-at-home program will reside, which existing resources can be leveraged within the hospital or health system, and what are the state or federal regulatory requirements for such a program. This type of program continues to fill gaps within the US health care system, meeting the needs of widely overlooked populations and increasing access to essential ancillary services.
Conclusion
It is time to consider our bias toward hospital-first options when managing the care needs of our patients. Health care providers have the option to advocate for holistic care, better experience, and better outcomes. Home-based options are safe, equitable, and patient-centric. Increased costs, consumerism, and technology have pushed us to think about alternative approaches to patient care delivery, and the pandemic created a unique opportunity to see just how far the health care system could stretch itself with capacity constraints, insufficient resources, and staff shortages. In light of new possibilities, it is time to reimagine and transform our health care delivery system so that it is unified, seamless, cohesive, and flexible.
Corresponding author: Payal Sharma, DNP, MSN, RN, FNP-BC, CBN; psharma@medicallyhome.com.
Disclosures: None reported.
1. Cai S, Laurel PA, Makineni R, Marks ML. Evaluation of a hospital-in-home program implemented among veterans. Am J Manag Care. 2017;23(8):482-487.
2. Levine DM, Ouchi K, Blanchfield B, et al. Hospital-level care at home for acutely ill adults: a pilot randomized controlled trial. J Gen Intern Med. 2018;33(5):729-736. doi:10.1007/s11606-018-4307-z
3. Shuman V, Coyle PC, Perera S,et al. Association between improved mobility and distal health outcomes. J Gerontol A Biol Sci Med Sci. 2020;75(12):2412-2417. doi:10.1093/gerona/glaa086
4. Shepperd S, Doll H, Angus RM, et al. Avoiding hospital admission through provision of hospital care at home: a systematic review and meta-analysis of individual patient data. CMAJ. 2009;180(2):175-182. doi:10.1503/cmaj.081491
5. Caplan GA, Sulaiman NS, Mangin DA, et al. A meta-analysis of “hospital in the home”. Med J Aust. 2012;197(9):512-519. doi:10.5694/mja12.10480
6. Hospital at Home. Johns Hopkins Medicine. Healthcare Solutions. Accessed May 20, 2022. https://www.johnshopkinssolutions.com/solution/hospital-at-home/
From Medically Home Group, Boston, MA.
Brick-and-mortar hospitals in the United States have historically been considered the dominant setting for providing care to patients. The coordination and delivery of care has previously been bound to physical hospitals largely because multidisciplinary services were only accessible in an individual location. While the fundamental make-up of these services remains unchanged, these services are now available in alternate settings. Some of these services include access to a patient care team, supplies, diagnostics, pharmacy, and advanced therapeutic interventions. Presently, the physical environment is becoming increasingly irrelevant as the core of what makes the traditional hospital—the professional staff, collaborative work processes, and the dynamics of the space—have all been translated into a modern digitally integrated environment. The elements necessary to providing safe, effective care in a physical hospital setting are now available in a patient’s home.
Impetus for the Model
As hospitals reconsider how and where they deliver patient care because of limited resources, the hospital-at-home model has gained significant momentum and interest. This model transforms a home into a hospital. The inpatient acute care episode is entirely substituted with an intensive at-home hospital admission enabled by technology, multidisciplinary teams, and ancillary services. Furthermore, patients requiring post-acute support can be transitioned to their next phase of care seamlessly. Given the nationwide nursing shortage, aging population, challenges uncovered by the COVID-19 pandemic, rising hospital costs, nurse/provider burnout related to challenging work environments, and capacity constraints, a shift toward the combination of virtual and in-home care is imperative. The hospital-at-home model has been associated with superior patient outcomes, including reduced risks of delirium, improved functional status, improved patient and family member satisfaction, reduced mortality, reduced readmissions, and significantly lower costs.1 COVID-19 alone has unmasked major facility-based deficiencies and limitations of our health care system. While the pandemic is not the impetus for the hospital-at-home model, the extended stress of this event has created a unique opportunity to reimagine and transform our health care delivery system so that it is less fragmented and more flexible.
Nursing in the Model
Nursing is central to the hospital-at-home model. Virtual nurses provide meticulous care plan oversight, assessment, and documentation across in-home service providers, to ensure holistic, safe, transparent, and continuous progression toward care plan milestones. The virtual nurse monitors patients using in-home technology that is set up at the time of admission. Connecting with patients to verify social and medical needs, the virtual nurse advocates for their patients and uses these technologies to care and deploy on-demand hands-on services to the patient. Service providers such as paramedics, infusion nurses, or home health nurses may be deployed to provide services in the patient’s home. By bringing in supplies, therapeutics, and interdisciplinary team members, the capabilities of a brick-and-mortar hospital are replicated in the home. All actions that occur wherever the patient is receiving care are overseen by professional nursing staff; in short, virtual nurses are the equivalent of bedside nurses in the brick-and-mortar health care facilities.
Potential Benefits
There are many benefits to the hospital-at-home model (Table). This health care model can be particularly helpful for patients who require frequent admission to acute care facilities, and is well suited for patients with a range of conditions, including those with COVID-19, pneumonia, cellulitis, or congestive heart failure. This care model helps eliminate some of the stressors for patients who have chronic illnesses or other conditions that require frequent hospital admissions. Patients can independently recover at home and can also be surrounded by their loved ones and pets while recovering. This care approach additionally eliminates the risk of hospital-acquired infections and injuries. The hospital-at-home model allows for increased mobility,2 as patients are familiar with their surroundings, resulting in reduced onset of delirium. Additionally, patients with improved mobility performance are less likely to experience negative health outcomes.3 There is less chance of sleep disruption as the patient is sleeping in their own bed—no unfamiliar roommate, no call bells or health care personnel frequently coming into the room. The in-home technology set up for remote patient monitoring is designed with the user in mind. Ease of use empowers the patient to collaborate with their care team on their own terms and center the priorities of themselves and their families.
Positive Outcomes
The hospital-at-home model is associated with positive outcomes. The authors of a systematic review identified 10 randomized controlled trials of hospital-at-home programs (with a total of 1372 patients), but were able to obtain data for only 5 of these trials (with a total of 844 patients).4 They found a 38% reduction in 6-month mortality for patients who received hospital care at home, as well as significantly higher patient satisfaction across a range of medical conditions, including patients with cellulitis and community-acquired pneumonia, as well as elderly patients with multiple medical conditions. The authors concluded that hospital care at home was less expensive than admission to an acute care hospital.4 Similarly, a meta-analysis done by Caplan et al5 that included 61 randomized controlled trials concluded that hospital at home is associated with reductions in mortality, readmission rates, and cost, and increases in patient and caregiver satisfaction. Levine et al2 found reduced costs and utilization with home hospitalization compared to in-hospital care, as well as improved patient mobility status.
The home is the ideal place to empower patients and caregivers to engage in self-management.2 Receiving hospital care at home eliminates the need for dealing with transportation arrangements, traffic, road tolls, and time/scheduling constraints, or finding care for a dependent family member, some of the many stressors that may be experienced by patients who require frequent trips to the hospital. For patients who may not be clinically suitable candidates for hospital at home, such as those requiring critical care intervention and support, the brick-and-mortar hospital is still the appropriate site of care. The hospital-at-home model helps prevent bed shortages in brick-and-mortar hospital settings by allowing hospital care at home for patients who meet preset criteria. These patients can be hospitalized in alternative locations such as their own homes or the residence of a friend. This helps increase health system capacity as well as resiliency.
In addition to expanding safe and appropriate treatment spaces, the hospital-at-home model helps increase access to care for patients during nonstandard hours, including weekends, holidays, or when the waiting time in the emergency room is painfully long. Furthermore, providing care in the home gives the clinical team valuable insight into the patient’s daily life and routine. Performing medication reconciliation with the medicine cabinet in sight and dietary education in a patient’s kitchen are powerful touch points.2 For example, a patient with congestive heart failure who must undergo diuresis is much more likely to meet their care goals when their home diet is aligned with the treatment goal. By being able to see exactly what is in a patient’s pantry and fridge, the care team can create a much more tailored approach to sodium intake and fluid management. Providers can create and execute true patient-centric care as they gain direct insight into the patient’s lifestyle, which is clearly valuable when creating care plans for complex chronic health issues.
Challenges to Implementation and Scaling
Although there are clear benefits to hospital at home, how to best implement and scale this model presents a challenge. In addition to educating patients and families about this model of care, health care systems must expand their hospital-at-home programs and provide education about this model to clinical staff and trainees, and insurers must create reimbursement paradigms. Patients meeting eligibility criteria to enroll in hospital at home is the easiest hurdle, as hospital-at-home programs function best when they enroll and service as many patients as possible, including underserved populations.
Upfront Costs and Cost Savings
While there are upfront costs to set up technology and coordinate services, hospital at home also provides significant total cost savings when compared to coordination associated with brick-and-mortar admission. Hospital care accounts for about one-third of total medical expenditures and is a leading cause of debt.2 Eliminating fixed hospital costs such as facility, overhead, and equipment costs through adoption of the hospital-at-home model can lead to a reduction in expenditures. It has been found that fewer laboratory and diagnostic tests are ordered for hospital-at-home patients when compared to similar patients in brick-and-mortar hospital settings, with comparable or better clinical patient outcomes.6 Furthermore, it is estimated that there are cost savings of 19% to 30% when compared to traditional inpatient care.6 Without legislative action, upon the end of the current COVID-19 public health emergency, the Centers for Medicare & Medicaid Service’s Acute Hospital Care at Home waiver will terminate. This could slow down scaling of the model.However, over the past 2 years there has been enough buy-in from major health systems and patients to continue the momentum of the model’s growth. When setting up a hospital-at-home program, it would be wise to consider a few factors: where in the hospital or health system entity structure the hospital-at-home program will reside, which existing resources can be leveraged within the hospital or health system, and what are the state or federal regulatory requirements for such a program. This type of program continues to fill gaps within the US health care system, meeting the needs of widely overlooked populations and increasing access to essential ancillary services.
Conclusion
It is time to consider our bias toward hospital-first options when managing the care needs of our patients. Health care providers have the option to advocate for holistic care, better experience, and better outcomes. Home-based options are safe, equitable, and patient-centric. Increased costs, consumerism, and technology have pushed us to think about alternative approaches to patient care delivery, and the pandemic created a unique opportunity to see just how far the health care system could stretch itself with capacity constraints, insufficient resources, and staff shortages. In light of new possibilities, it is time to reimagine and transform our health care delivery system so that it is unified, seamless, cohesive, and flexible.
Corresponding author: Payal Sharma, DNP, MSN, RN, FNP-BC, CBN; psharma@medicallyhome.com.
Disclosures: None reported.
From Medically Home Group, Boston, MA.
Brick-and-mortar hospitals in the United States have historically been considered the dominant setting for providing care to patients. The coordination and delivery of care has previously been bound to physical hospitals largely because multidisciplinary services were only accessible in an individual location. While the fundamental make-up of these services remains unchanged, these services are now available in alternate settings. Some of these services include access to a patient care team, supplies, diagnostics, pharmacy, and advanced therapeutic interventions. Presently, the physical environment is becoming increasingly irrelevant as the core of what makes the traditional hospital—the professional staff, collaborative work processes, and the dynamics of the space—have all been translated into a modern digitally integrated environment. The elements necessary to providing safe, effective care in a physical hospital setting are now available in a patient’s home.
Impetus for the Model
As hospitals reconsider how and where they deliver patient care because of limited resources, the hospital-at-home model has gained significant momentum and interest. This model transforms a home into a hospital. The inpatient acute care episode is entirely substituted with an intensive at-home hospital admission enabled by technology, multidisciplinary teams, and ancillary services. Furthermore, patients requiring post-acute support can be transitioned to their next phase of care seamlessly. Given the nationwide nursing shortage, aging population, challenges uncovered by the COVID-19 pandemic, rising hospital costs, nurse/provider burnout related to challenging work environments, and capacity constraints, a shift toward the combination of virtual and in-home care is imperative. The hospital-at-home model has been associated with superior patient outcomes, including reduced risks of delirium, improved functional status, improved patient and family member satisfaction, reduced mortality, reduced readmissions, and significantly lower costs.1 COVID-19 alone has unmasked major facility-based deficiencies and limitations of our health care system. While the pandemic is not the impetus for the hospital-at-home model, the extended stress of this event has created a unique opportunity to reimagine and transform our health care delivery system so that it is less fragmented and more flexible.
Nursing in the Model
Nursing is central to the hospital-at-home model. Virtual nurses provide meticulous care plan oversight, assessment, and documentation across in-home service providers, to ensure holistic, safe, transparent, and continuous progression toward care plan milestones. The virtual nurse monitors patients using in-home technology that is set up at the time of admission. Connecting with patients to verify social and medical needs, the virtual nurse advocates for their patients and uses these technologies to care and deploy on-demand hands-on services to the patient. Service providers such as paramedics, infusion nurses, or home health nurses may be deployed to provide services in the patient’s home. By bringing in supplies, therapeutics, and interdisciplinary team members, the capabilities of a brick-and-mortar hospital are replicated in the home. All actions that occur wherever the patient is receiving care are overseen by professional nursing staff; in short, virtual nurses are the equivalent of bedside nurses in the brick-and-mortar health care facilities.
Potential Benefits
There are many benefits to the hospital-at-home model (Table). This health care model can be particularly helpful for patients who require frequent admission to acute care facilities, and is well suited for patients with a range of conditions, including those with COVID-19, pneumonia, cellulitis, or congestive heart failure. This care model helps eliminate some of the stressors for patients who have chronic illnesses or other conditions that require frequent hospital admissions. Patients can independently recover at home and can also be surrounded by their loved ones and pets while recovering. This care approach additionally eliminates the risk of hospital-acquired infections and injuries. The hospital-at-home model allows for increased mobility,2 as patients are familiar with their surroundings, resulting in reduced onset of delirium. Additionally, patients with improved mobility performance are less likely to experience negative health outcomes.3 There is less chance of sleep disruption as the patient is sleeping in their own bed—no unfamiliar roommate, no call bells or health care personnel frequently coming into the room. The in-home technology set up for remote patient monitoring is designed with the user in mind. Ease of use empowers the patient to collaborate with their care team on their own terms and center the priorities of themselves and their families.
Positive Outcomes
The hospital-at-home model is associated with positive outcomes. The authors of a systematic review identified 10 randomized controlled trials of hospital-at-home programs (with a total of 1372 patients), but were able to obtain data for only 5 of these trials (with a total of 844 patients).4 They found a 38% reduction in 6-month mortality for patients who received hospital care at home, as well as significantly higher patient satisfaction across a range of medical conditions, including patients with cellulitis and community-acquired pneumonia, as well as elderly patients with multiple medical conditions. The authors concluded that hospital care at home was less expensive than admission to an acute care hospital.4 Similarly, a meta-analysis done by Caplan et al5 that included 61 randomized controlled trials concluded that hospital at home is associated with reductions in mortality, readmission rates, and cost, and increases in patient and caregiver satisfaction. Levine et al2 found reduced costs and utilization with home hospitalization compared to in-hospital care, as well as improved patient mobility status.
The home is the ideal place to empower patients and caregivers to engage in self-management.2 Receiving hospital care at home eliminates the need for dealing with transportation arrangements, traffic, road tolls, and time/scheduling constraints, or finding care for a dependent family member, some of the many stressors that may be experienced by patients who require frequent trips to the hospital. For patients who may not be clinically suitable candidates for hospital at home, such as those requiring critical care intervention and support, the brick-and-mortar hospital is still the appropriate site of care. The hospital-at-home model helps prevent bed shortages in brick-and-mortar hospital settings by allowing hospital care at home for patients who meet preset criteria. These patients can be hospitalized in alternative locations such as their own homes or the residence of a friend. This helps increase health system capacity as well as resiliency.
In addition to expanding safe and appropriate treatment spaces, the hospital-at-home model helps increase access to care for patients during nonstandard hours, including weekends, holidays, or when the waiting time in the emergency room is painfully long. Furthermore, providing care in the home gives the clinical team valuable insight into the patient’s daily life and routine. Performing medication reconciliation with the medicine cabinet in sight and dietary education in a patient’s kitchen are powerful touch points.2 For example, a patient with congestive heart failure who must undergo diuresis is much more likely to meet their care goals when their home diet is aligned with the treatment goal. By being able to see exactly what is in a patient’s pantry and fridge, the care team can create a much more tailored approach to sodium intake and fluid management. Providers can create and execute true patient-centric care as they gain direct insight into the patient’s lifestyle, which is clearly valuable when creating care plans for complex chronic health issues.
Challenges to Implementation and Scaling
Although there are clear benefits to hospital at home, how to best implement and scale this model presents a challenge. In addition to educating patients and families about this model of care, health care systems must expand their hospital-at-home programs and provide education about this model to clinical staff and trainees, and insurers must create reimbursement paradigms. Patients meeting eligibility criteria to enroll in hospital at home is the easiest hurdle, as hospital-at-home programs function best when they enroll and service as many patients as possible, including underserved populations.
Upfront Costs and Cost Savings
While there are upfront costs to set up technology and coordinate services, hospital at home also provides significant total cost savings when compared to coordination associated with brick-and-mortar admission. Hospital care accounts for about one-third of total medical expenditures and is a leading cause of debt.2 Eliminating fixed hospital costs such as facility, overhead, and equipment costs through adoption of the hospital-at-home model can lead to a reduction in expenditures. It has been found that fewer laboratory and diagnostic tests are ordered for hospital-at-home patients when compared to similar patients in brick-and-mortar hospital settings, with comparable or better clinical patient outcomes.6 Furthermore, it is estimated that there are cost savings of 19% to 30% when compared to traditional inpatient care.6 Without legislative action, upon the end of the current COVID-19 public health emergency, the Centers for Medicare & Medicaid Service’s Acute Hospital Care at Home waiver will terminate. This could slow down scaling of the model.However, over the past 2 years there has been enough buy-in from major health systems and patients to continue the momentum of the model’s growth. When setting up a hospital-at-home program, it would be wise to consider a few factors: where in the hospital or health system entity structure the hospital-at-home program will reside, which existing resources can be leveraged within the hospital or health system, and what are the state or federal regulatory requirements for such a program. This type of program continues to fill gaps within the US health care system, meeting the needs of widely overlooked populations and increasing access to essential ancillary services.
Conclusion
It is time to consider our bias toward hospital-first options when managing the care needs of our patients. Health care providers have the option to advocate for holistic care, better experience, and better outcomes. Home-based options are safe, equitable, and patient-centric. Increased costs, consumerism, and technology have pushed us to think about alternative approaches to patient care delivery, and the pandemic created a unique opportunity to see just how far the health care system could stretch itself with capacity constraints, insufficient resources, and staff shortages. In light of new possibilities, it is time to reimagine and transform our health care delivery system so that it is unified, seamless, cohesive, and flexible.
Corresponding author: Payal Sharma, DNP, MSN, RN, FNP-BC, CBN; psharma@medicallyhome.com.
Disclosures: None reported.
1. Cai S, Laurel PA, Makineni R, Marks ML. Evaluation of a hospital-in-home program implemented among veterans. Am J Manag Care. 2017;23(8):482-487.
2. Levine DM, Ouchi K, Blanchfield B, et al. Hospital-level care at home for acutely ill adults: a pilot randomized controlled trial. J Gen Intern Med. 2018;33(5):729-736. doi:10.1007/s11606-018-4307-z
3. Shuman V, Coyle PC, Perera S,et al. Association between improved mobility and distal health outcomes. J Gerontol A Biol Sci Med Sci. 2020;75(12):2412-2417. doi:10.1093/gerona/glaa086
4. Shepperd S, Doll H, Angus RM, et al. Avoiding hospital admission through provision of hospital care at home: a systematic review and meta-analysis of individual patient data. CMAJ. 2009;180(2):175-182. doi:10.1503/cmaj.081491
5. Caplan GA, Sulaiman NS, Mangin DA, et al. A meta-analysis of “hospital in the home”. Med J Aust. 2012;197(9):512-519. doi:10.5694/mja12.10480
6. Hospital at Home. Johns Hopkins Medicine. Healthcare Solutions. Accessed May 20, 2022. https://www.johnshopkinssolutions.com/solution/hospital-at-home/
1. Cai S, Laurel PA, Makineni R, Marks ML. Evaluation of a hospital-in-home program implemented among veterans. Am J Manag Care. 2017;23(8):482-487.
2. Levine DM, Ouchi K, Blanchfield B, et al. Hospital-level care at home for acutely ill adults: a pilot randomized controlled trial. J Gen Intern Med. 2018;33(5):729-736. doi:10.1007/s11606-018-4307-z
3. Shuman V, Coyle PC, Perera S,et al. Association between improved mobility and distal health outcomes. J Gerontol A Biol Sci Med Sci. 2020;75(12):2412-2417. doi:10.1093/gerona/glaa086
4. Shepperd S, Doll H, Angus RM, et al. Avoiding hospital admission through provision of hospital care at home: a systematic review and meta-analysis of individual patient data. CMAJ. 2009;180(2):175-182. doi:10.1503/cmaj.081491
5. Caplan GA, Sulaiman NS, Mangin DA, et al. A meta-analysis of “hospital in the home”. Med J Aust. 2012;197(9):512-519. doi:10.5694/mja12.10480
6. Hospital at Home. Johns Hopkins Medicine. Healthcare Solutions. Accessed May 20, 2022. https://www.johnshopkinssolutions.com/solution/hospital-at-home/
The Intersection of Clinical Quality Improvement Research and Implementation Science
The Institute of Medicine brought much-needed attention to the need for process improvement in medicine with its seminal report To Err Is Human: Building a Safer Health System, which was issued in 1999, leading to the quality movement’s call to close health care performance gaps in Crossing the Quality Chasm: A New Health System for the 21st Century.1,2 Quality improvement science in medicine has evolved over the past 2 decades to include a broad spectrum of approaches, from agile improvement to continuous learning and improvement. Current efforts focus on Lean-based process improvement along with a reduction in variation in clinical practice to align practice with the principles of evidence-based medicine in a patient-centered approach.3 Further, the definition of quality improvement under the Affordable Care Act was framed as an equitable, timely, value-based, patient-centered approach to achieving population-level health goals.4 Thus, the science of quality improvement drives the core principles of care delivery improvement, and the rigorous evidence needed to expand innovation is embedded within the same framework.5,6 In clinical practice, quality improvement projects aim to define gaps and then specific steps are undertaken to improve the evidence-based practice of a specific process. The overarching goal is to enhance the efficacy of the practice by reducing waste within a particular domain. Thus, quality improvement and implementation research eventually unify how clinical practice is advanced concurrently to bridge identified gaps.7
System redesign through a patient-centered framework forms the core of an overarching strategy to support system-level processes. Both require a deep understanding of the fields of quality improvement science and implementation science.8 Furthermore, aligning clinical research needs, system aims, patients’ values, and clinical care give the new design a clear path forward. Patient-centered improvement includes the essential elements of system redesign around human factors, including communication, physical resources, and updated information during episodes of care. The patient-centered improvement design is juxtaposed with care planning and establishing continuum of care processes.9 It is essential to note that safety is rooted within the quality domain as a top priority in medicine.10 The best implementation methods and approaches are discussed and debated, and the improvement progress continues on multiple fronts.11 Patient safety systems are implemented simultaneously during the redesign phase. Moreover, identifying and testing the health care delivery methods in the era of competing strategic priorities to achieve the desirable clinical outcomes highlights the importance of implementation, while contemplating the methods of dissemination, scalability, and sustainability of the best evidence-based clinical practice.
The cycle of quality improvement research completes the system implementation efforts. The conceptual framework of quality improvement includes multiple areas of care and transition, along with applying the best clinical practices in a culture that emphasizes continuous improvement and learning. At the same time, the operating principles should include continuous improvement in a simple and continuous system of learning as a core concept. Our proposed implementation approach involves taking simple and practical steps while separating the process from the outcomes measures, extracting effectiveness throughout the process. It is essential to keep in mind that building a proactive and systematic improvement environment requires a framework for safety, reliability, and effective care, as well as the alignment of the physical system, communication, and professional environment and culture (Figure).
In summary, system design for quality improvement research should incorporate the principles and conceptual framework that embody effective implementation strategies, with a focus on operational and practical steps. Continuous improvement will be reached through the multidimensional development of current health care system metrics and the incorporation of implementation science methods.
Corresponding author: Ebrahim Barkoudah, MD, MPH, Department of Medicine, Brigham and Women’s Hospital, Boston, MA; ebarkoudah@bwh.harvard.edu
Disclosures: None reported.
1. Institute of Medicine (US) Committee on Quality of Health Care in America. To Err is Human: Building a Safer Health System. Kohn LT, Corrigan JM, Donaldson MS, editors. Washington (DC): National Academies Press (US); 2000.
2. Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington (DC): National Academies Press (US); 2001.
3. Berwick DM. The science of improvement. JAMA. 2008;299(10):1182-1184. doi:10.1001/jama.299.10.1182
4. Mazurenko O, Balio CP, Agarwal R, Carroll AE, Menachemi N. The effects of Medicaid expansion under the ACA: a systematic review. Health Affairs. 2018;37(6):944-950. doi: 10.1377/hlthaff.2017.1491
5. Fan E, Needham DM. The science of quality improvement. JAMA. 2008;300(4):390-391. doi:10.1001/jama.300.4.390-b
6. Alexander JA, Hearld LR. The science of quality improvement implementation: developing capacity to make a difference. Med Care. 2011:S6-20. doi:10.1097/MLR.0b013e3181e1709c
7. Rohweder C, Wangen M, Black M, et al. Understanding quality improvement collaboratives through an implementation science lens. Prev Med. 2019;129:105859. doi: 10.1016/j.ypmed.2019.105859
8. Bergeson SC, Dean JD. A systems approach to patient-centered care. JAMA. 2006;296(23):2848-2851. doi:10.1001/jama.296.23.2848
9. Leonard M, Graham S, Bonacum D. The human factor: the critical importance of effective teamwork and communication in providing safe care. Qual Saf Health Care. 2004;13 Suppl 1(Suppl 1):i85-90. doi:10.1136/qhc.13.suppl_1.i85
10. Leape LL, Berwick DM, Bates DW. What practices will most improve safety? Evidence-based medicine meets patient safety. JAMA. 2002;288(4):501-507. doi:10.1001/jama.288.4.501
11. Auerbach AD, Landefeld CS, Shojania KG. The tension between needing to improve care and knowing how to do it. N Engl J Med. 2007;357(6):608-613. doi:10.1056/NEJMsb070738
The Institute of Medicine brought much-needed attention to the need for process improvement in medicine with its seminal report To Err Is Human: Building a Safer Health System, which was issued in 1999, leading to the quality movement’s call to close health care performance gaps in Crossing the Quality Chasm: A New Health System for the 21st Century.1,2 Quality improvement science in medicine has evolved over the past 2 decades to include a broad spectrum of approaches, from agile improvement to continuous learning and improvement. Current efforts focus on Lean-based process improvement along with a reduction in variation in clinical practice to align practice with the principles of evidence-based medicine in a patient-centered approach.3 Further, the definition of quality improvement under the Affordable Care Act was framed as an equitable, timely, value-based, patient-centered approach to achieving population-level health goals.4 Thus, the science of quality improvement drives the core principles of care delivery improvement, and the rigorous evidence needed to expand innovation is embedded within the same framework.5,6 In clinical practice, quality improvement projects aim to define gaps and then specific steps are undertaken to improve the evidence-based practice of a specific process. The overarching goal is to enhance the efficacy of the practice by reducing waste within a particular domain. Thus, quality improvement and implementation research eventually unify how clinical practice is advanced concurrently to bridge identified gaps.7
System redesign through a patient-centered framework forms the core of an overarching strategy to support system-level processes. Both require a deep understanding of the fields of quality improvement science and implementation science.8 Furthermore, aligning clinical research needs, system aims, patients’ values, and clinical care give the new design a clear path forward. Patient-centered improvement includes the essential elements of system redesign around human factors, including communication, physical resources, and updated information during episodes of care. The patient-centered improvement design is juxtaposed with care planning and establishing continuum of care processes.9 It is essential to note that safety is rooted within the quality domain as a top priority in medicine.10 The best implementation methods and approaches are discussed and debated, and the improvement progress continues on multiple fronts.11 Patient safety systems are implemented simultaneously during the redesign phase. Moreover, identifying and testing the health care delivery methods in the era of competing strategic priorities to achieve the desirable clinical outcomes highlights the importance of implementation, while contemplating the methods of dissemination, scalability, and sustainability of the best evidence-based clinical practice.
The cycle of quality improvement research completes the system implementation efforts. The conceptual framework of quality improvement includes multiple areas of care and transition, along with applying the best clinical practices in a culture that emphasizes continuous improvement and learning. At the same time, the operating principles should include continuous improvement in a simple and continuous system of learning as a core concept. Our proposed implementation approach involves taking simple and practical steps while separating the process from the outcomes measures, extracting effectiveness throughout the process. It is essential to keep in mind that building a proactive and systematic improvement environment requires a framework for safety, reliability, and effective care, as well as the alignment of the physical system, communication, and professional environment and culture (Figure).
In summary, system design for quality improvement research should incorporate the principles and conceptual framework that embody effective implementation strategies, with a focus on operational and practical steps. Continuous improvement will be reached through the multidimensional development of current health care system metrics and the incorporation of implementation science methods.
Corresponding author: Ebrahim Barkoudah, MD, MPH, Department of Medicine, Brigham and Women’s Hospital, Boston, MA; ebarkoudah@bwh.harvard.edu
Disclosures: None reported.
The Institute of Medicine brought much-needed attention to the need for process improvement in medicine with its seminal report To Err Is Human: Building a Safer Health System, which was issued in 1999, leading to the quality movement’s call to close health care performance gaps in Crossing the Quality Chasm: A New Health System for the 21st Century.1,2 Quality improvement science in medicine has evolved over the past 2 decades to include a broad spectrum of approaches, from agile improvement to continuous learning and improvement. Current efforts focus on Lean-based process improvement along with a reduction in variation in clinical practice to align practice with the principles of evidence-based medicine in a patient-centered approach.3 Further, the definition of quality improvement under the Affordable Care Act was framed as an equitable, timely, value-based, patient-centered approach to achieving population-level health goals.4 Thus, the science of quality improvement drives the core principles of care delivery improvement, and the rigorous evidence needed to expand innovation is embedded within the same framework.5,6 In clinical practice, quality improvement projects aim to define gaps and then specific steps are undertaken to improve the evidence-based practice of a specific process. The overarching goal is to enhance the efficacy of the practice by reducing waste within a particular domain. Thus, quality improvement and implementation research eventually unify how clinical practice is advanced concurrently to bridge identified gaps.7
System redesign through a patient-centered framework forms the core of an overarching strategy to support system-level processes. Both require a deep understanding of the fields of quality improvement science and implementation science.8 Furthermore, aligning clinical research needs, system aims, patients’ values, and clinical care give the new design a clear path forward. Patient-centered improvement includes the essential elements of system redesign around human factors, including communication, physical resources, and updated information during episodes of care. The patient-centered improvement design is juxtaposed with care planning and establishing continuum of care processes.9 It is essential to note that safety is rooted within the quality domain as a top priority in medicine.10 The best implementation methods and approaches are discussed and debated, and the improvement progress continues on multiple fronts.11 Patient safety systems are implemented simultaneously during the redesign phase. Moreover, identifying and testing the health care delivery methods in the era of competing strategic priorities to achieve the desirable clinical outcomes highlights the importance of implementation, while contemplating the methods of dissemination, scalability, and sustainability of the best evidence-based clinical practice.
The cycle of quality improvement research completes the system implementation efforts. The conceptual framework of quality improvement includes multiple areas of care and transition, along with applying the best clinical practices in a culture that emphasizes continuous improvement and learning. At the same time, the operating principles should include continuous improvement in a simple and continuous system of learning as a core concept. Our proposed implementation approach involves taking simple and practical steps while separating the process from the outcomes measures, extracting effectiveness throughout the process. It is essential to keep in mind that building a proactive and systematic improvement environment requires a framework for safety, reliability, and effective care, as well as the alignment of the physical system, communication, and professional environment and culture (Figure).
In summary, system design for quality improvement research should incorporate the principles and conceptual framework that embody effective implementation strategies, with a focus on operational and practical steps. Continuous improvement will be reached through the multidimensional development of current health care system metrics and the incorporation of implementation science methods.
Corresponding author: Ebrahim Barkoudah, MD, MPH, Department of Medicine, Brigham and Women’s Hospital, Boston, MA; ebarkoudah@bwh.harvard.edu
Disclosures: None reported.
1. Institute of Medicine (US) Committee on Quality of Health Care in America. To Err is Human: Building a Safer Health System. Kohn LT, Corrigan JM, Donaldson MS, editors. Washington (DC): National Academies Press (US); 2000.
2. Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington (DC): National Academies Press (US); 2001.
3. Berwick DM. The science of improvement. JAMA. 2008;299(10):1182-1184. doi:10.1001/jama.299.10.1182
4. Mazurenko O, Balio CP, Agarwal R, Carroll AE, Menachemi N. The effects of Medicaid expansion under the ACA: a systematic review. Health Affairs. 2018;37(6):944-950. doi: 10.1377/hlthaff.2017.1491
5. Fan E, Needham DM. The science of quality improvement. JAMA. 2008;300(4):390-391. doi:10.1001/jama.300.4.390-b
6. Alexander JA, Hearld LR. The science of quality improvement implementation: developing capacity to make a difference. Med Care. 2011:S6-20. doi:10.1097/MLR.0b013e3181e1709c
7. Rohweder C, Wangen M, Black M, et al. Understanding quality improvement collaboratives through an implementation science lens. Prev Med. 2019;129:105859. doi: 10.1016/j.ypmed.2019.105859
8. Bergeson SC, Dean JD. A systems approach to patient-centered care. JAMA. 2006;296(23):2848-2851. doi:10.1001/jama.296.23.2848
9. Leonard M, Graham S, Bonacum D. The human factor: the critical importance of effective teamwork and communication in providing safe care. Qual Saf Health Care. 2004;13 Suppl 1(Suppl 1):i85-90. doi:10.1136/qhc.13.suppl_1.i85
10. Leape LL, Berwick DM, Bates DW. What practices will most improve safety? Evidence-based medicine meets patient safety. JAMA. 2002;288(4):501-507. doi:10.1001/jama.288.4.501
11. Auerbach AD, Landefeld CS, Shojania KG. The tension between needing to improve care and knowing how to do it. N Engl J Med. 2007;357(6):608-613. doi:10.1056/NEJMsb070738
1. Institute of Medicine (US) Committee on Quality of Health Care in America. To Err is Human: Building a Safer Health System. Kohn LT, Corrigan JM, Donaldson MS, editors. Washington (DC): National Academies Press (US); 2000.
2. Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington (DC): National Academies Press (US); 2001.
3. Berwick DM. The science of improvement. JAMA. 2008;299(10):1182-1184. doi:10.1001/jama.299.10.1182
4. Mazurenko O, Balio CP, Agarwal R, Carroll AE, Menachemi N. The effects of Medicaid expansion under the ACA: a systematic review. Health Affairs. 2018;37(6):944-950. doi: 10.1377/hlthaff.2017.1491
5. Fan E, Needham DM. The science of quality improvement. JAMA. 2008;300(4):390-391. doi:10.1001/jama.300.4.390-b
6. Alexander JA, Hearld LR. The science of quality improvement implementation: developing capacity to make a difference. Med Care. 2011:S6-20. doi:10.1097/MLR.0b013e3181e1709c
7. Rohweder C, Wangen M, Black M, et al. Understanding quality improvement collaboratives through an implementation science lens. Prev Med. 2019;129:105859. doi: 10.1016/j.ypmed.2019.105859
8. Bergeson SC, Dean JD. A systems approach to patient-centered care. JAMA. 2006;296(23):2848-2851. doi:10.1001/jama.296.23.2848
9. Leonard M, Graham S, Bonacum D. The human factor: the critical importance of effective teamwork and communication in providing safe care. Qual Saf Health Care. 2004;13 Suppl 1(Suppl 1):i85-90. doi:10.1136/qhc.13.suppl_1.i85
10. Leape LL, Berwick DM, Bates DW. What practices will most improve safety? Evidence-based medicine meets patient safety. JAMA. 2002;288(4):501-507. doi:10.1001/jama.288.4.501
11. Auerbach AD, Landefeld CS, Shojania KG. The tension between needing to improve care and knowing how to do it. N Engl J Med. 2007;357(6):608-613. doi:10.1056/NEJMsb070738
Fall Injury Among Community-Dwelling Older Adults: Effect of a Multifactorial Intervention and a Home Hazard Removal Program
Study 1 Overview (Bhasin et al)
Objective: To examine the effect of a multifactorial intervention for fall prevention on fall injury in community-dwelling older adults.
Design: This was a pragmatic, cluster randomized trial conducted in 86 primary care practices across 10 health care systems.
Setting and participants: The primary care sites were selected based on the prespecified criteria of size, ability to implement the intervention, proximity to other practices, accessibility to electronic health records, and access to community-based exercise programs. The primary care practices were randomly assigned to intervention or control.
Eligibility criteria for participants at those practices included age 70 years or older, dwelling in the community, and having an increased risk of falls, as determined by a history of fall-related injury in the past year, 2 or more falls in the past year, or being afraid of falling because of problems with balance or walking. Exclusion criteria were inability to provide consent or lack of proxy consent for participants who were determined to have cognitive impairment based on screening, and inability to speak English or Spanish. A total of 2802 participants were enrolled in the intervention group, and 2649 participants were enrolled in the control group.
Intervention: The intervention contained 5 components: a standardized assessment of 7 modifiable risk factors for fall injuries; standardized protocol-driven recommendations for management of risk factors; an individualized care plan focused on 1 to 3 risk factors; implementation of care plans, including referrals to community-based programs; and follow-up care conducted by telephone or in person. The modifiable risk factors included impairment of strength, gait, or balance; use of medications related to falls; postural hypotension; problems with feet or footwear; visual impairment; osteoporosis or vitamin D deficiency; and home safety hazards. The intervention was delivered by nurses who had completed online training modules and face-to-face training sessions focused on the intervention and motivational interviewing along with continuing education, in partnership with participants and their primary care providers. In the control group, participants received enhanced usual care, including an informational pamphlet, and were encouraged to discuss fall prevention with their primary care provider, including the results of their screening evaluation.
Main outcome measures: The primary outcome of the study was the first serious fall injury in a time-to-event analysis, defined as a fall resulting in a fracture (other than thoracic or lumbar vertebral fracture), joint dislocation, cut requiring closure, head injury requiring hospitalization, sprain or strain, bruising or swelling, or other serious injury. The secondary outcome was first patient-reported fall injury, also in a time-to-event analysis, ascertained by telephone interviews conducted every 4 months. Other outcomes included hospital admissions, emergency department visits, and other health care utilization. Adjudication of fall events and injuries was conducted by a team blinded to treatment assignment and verified using administrative claims data, encounter data, or electronic health record review.
Main results: The intervention and control groups were similar in terms of sex and age: 62.5% vs 61.5% of participants were women, and mean (SD) age was 79.9 (5.7) years and 79.5 (5.8) years, respectively. Other demographic characteristics were similar between groups. For the primary outcome, the rate of first serious injury was 4.9 per 100 person-years in the intervention group and 5.3 per 100 person-years in the control group, with a hazard ratio of 0.92 (95% CI, 0.80-1.06; P = .25). For the secondary outcome of patient-reported fall injury, there were 25.6 events per 100 person-years in the intervention group and 28.6 in the control group, with a hazard ratio of 0.90 (95% CI, 0.83-0.99; P =0.004). Rates of hospitalization and other secondary outcomes were similar between groups.
Conclusion: The multifactorial STRIDE intervention did not reduce the rate of serious fall injury when compared to enhanced usual care. The intervention did result in lower rates of fall injury by patient report, but no other significant outcomes were seen.
Study 2 Overview (Stark et al)
Objective: To examine the effect of a behavioral home hazard removal intervention for fall prevention on risk of fall in community-dwelling older adults.
Design: This randomized clinical trial was conducted at a single site in St. Louis, Missouri. Participants were community-dwelling older adults who received services from the Area Agency on Aging (AAA). Inclusion criteria included age 65 years and older, having 1 or more falls in the previous 12 months or being worried about falling by self report, and currently receiving services from an AAA. Exclusion criteria included living in an institution or being severely cognitively impaired and unable to follow directions or report falls. Participants who met the criteria were contacted by phone and invited to participate. A total of 310 participants were enrolled in the study, with an equal number of participants assigned to the intervention and control groups.
Intervention: The intervention included hazard identification and removal after a comprehensive assessment of participants, their behaviors, and the environment; this assessment took place during the first visit, which lasted approximately 80 minutes. A home hazard removal plan was developed, and in the second session, which lasted approximately 40 minutes, remediation of hazards was carried out. A third session for home modification that lasted approximately 30 minutes was conducted, if needed. At 6 months after the intervention, a booster session to identify and remediate any new home hazards and address issues was conducted. Specific interventions, as identified by the assessment, included minor home repair such as grab bars, adaptive equipment, task modification, and education. Shared decision making that enabled older adults to control changes in their homes, self-management strategies to improve awareness, and motivational enhancement strategies to improve acceptance were employed. Scripted algorithms and checklists were used to deliver the intervention. For usual care, an annual assessment and referrals to community services, if needed, were conducted in the AAA.
Main outcome measures: The primary outcome of the study was the number of days to first fall in 12 months. Falls were defined as unintentional movements to the floor, ground, or object below knee level, and falls were recorded through a daily journal for 12 months. Participants were contacted by phone if they did not return the journal or reported a fall. Participants were interviewed to verify falls and determine whether a fall was injurious. Secondary outcomes included rate of falls per person per 12 months; daily activity performance measured using the Older Americans Resources and Services Activities of Daily Living scale; falls self-efficacy, which measures confidence performing daily activities without falling; and quality of life using the SF-36 at 12 months.
Main results: Most of the study participants were women (74%), and mean (SD) age was 75 (7.4) years. Study retention was similar between the intervention and control groups, with 82% completing the study in the intervention group compared with 81% in the control group. Fidelity to the intervention, as measured by a checklist by the interventionist, was 99%, and adherence to home modification, as measured by number of home modifications in use by self report, was high at 92% at 6 months and 91% at 12 months. For the primary outcome, fall hazard was not different between the intervention and control groups (hazard ratio, 0.9; 95% CI, 0.66-1.27). For the secondary outcomes, the rate of falling was lower in the intervention group compared with the control group, with a relative risk of 0.62 (95% CI, 0.40-0.95). There was no difference in other secondary outcomes of daily activity performance, falls self-efficacy, or quality of life.
Conclusion: Despite high adherence to home modifications and fidelity to the intervention, this home hazard removal program did not reduce the risk of falling when compared to usual care. It did reduce the rate of falls, although no other effects were observed.
Commentary
Observational studies have identified factors that contribute to falls,1 and over the past 30 years a number of intervention trials designed to reduce the risk of falling have been conducted. A recent Cochrane review, published prior to the Bhasin et al and Stark et al trials, looked at the effect of multifactorial interventions for fall prevention across 62 trials that included 19,935 older adults living in the community. The review concluded that multifactorial interventions may reduce the rate of falls, but this conclusion was based on low-quality evidence and there was significant heterogeneity across the studies.2
The STRIDE randomized trial represents the latest effort to address the evidence gap around fall prevention, with the STRIDE investigators hoping this would be the definitive trial that leads to practice change in fall prevention. Smaller trials that have demonstrated effectiveness were brought to scale in this large randomized trial that included 86 practices and more than 5000 participants. The investigators used risk of injurious falls as the primary outcome, as this outcome is considered the most clinically meaningful for the study population. The results, however, were disappointing: the multifactorial intervention in STRIDE did not result in a reduction of risk of injurious falls. Challenges in the implementation of this large trial may have contributed to its results; falls care managers, key to this multifactorial intervention, reported difficulties in navigating complex relationships with patients, families, study staff, and primary care practices during the study. Barriers reported included clinical space limitations, variable buy-in from providers, and turnover of practice staff and providers.3 Such implementation factors may have resulted in the divergent results between smaller clinical trials and this large-scale trial conducted across multiple settings.
The second study, by Stark et al, examined a home modification program and its effect on risk of falls. A prior Cochrane review examining the effect of home safety assessment and modification indicates that these strategies are effective in reducing the rate of falls as well as the risk of falling.4 The results of the current trial showed a reduction in the rate of falls but not in the risk of falling; however, this study did not examine outcomes of serious injurious falls, which may be more clinically meaningful. The Stark et al study adds to the existing literature showing that home modification may have an impact on fall rates. One noteworthy aspect of the Stark et al trial is the high adherence rate to home modification in a community-based approach; perhaps the investigators’ approach can be translated to real-world use.
Applications for Clinical Practice and System Implementation
The role of exercise programs in reducing fall rates is well established,5 but neither of these studies focused on exercise interventions. STRIDE offered community-based exercise program referral, but there is variability in such programs and study staff reported challenges in matching participants with appropriate exercise programs.3 Further studies that examine combinations of multifactorial falls risk reduction, exercise, and home safety, with careful consideration of implementation challenges to assure fidelity and adherence to the intervention, are needed to ascertain the best strategy for fall prevention for older adults at risk.
Given the results of these trials, it is difficult to recommend one falls prevention intervention over another. Clinicians should continue to identify falls risk factors using standardized assessments and determine which factors are modifiable.
Practice Points
- Incorporating assessments of falls risk in primary care is feasible, and such assessments can identify important risk factors.
- Clinicians and health systems should identify avenues, such as developing programmatic approaches, to providing home safety assessment and intervention, exercise options, medication review, and modification of other risk factors.
- Ensuring delivery of these elements reliably through programmatic approaches with adequate follow-up is key to preventing falls in this population.
—William W. Hung, MD, MPH
1. Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988; 319:1701-1707. doi:10.1056/NEJM198812293192604
2. Hopewell S, Adedire O, Copsey BJ, et al. Multifactorial and multiple component interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2018;7(7):CD012221. doi:0.1002/14651858.CD012221.pub2
3. Reckrey JM, Gazarian P, Reuben DB, et al. Barriers to implementation of STRIDE, a national study to prevent fall-related injuries. J Am Geriatr Soc. 2021;69(5):1334-1342. doi:10.1111/jgs.17056
4. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012;2012(9):CD007146. doi:10.1002/14651858.CD007146.pub3
5. Sherrington C, Fairhall NJ, Wallbank GK, et al. Exercise for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2019;1(1):CD012424. doi:10.1002/14651858.CD012424.pub2
Study 1 Overview (Bhasin et al)
Objective: To examine the effect of a multifactorial intervention for fall prevention on fall injury in community-dwelling older adults.
Design: This was a pragmatic, cluster randomized trial conducted in 86 primary care practices across 10 health care systems.
Setting and participants: The primary care sites were selected based on the prespecified criteria of size, ability to implement the intervention, proximity to other practices, accessibility to electronic health records, and access to community-based exercise programs. The primary care practices were randomly assigned to intervention or control.
Eligibility criteria for participants at those practices included age 70 years or older, dwelling in the community, and having an increased risk of falls, as determined by a history of fall-related injury in the past year, 2 or more falls in the past year, or being afraid of falling because of problems with balance or walking. Exclusion criteria were inability to provide consent or lack of proxy consent for participants who were determined to have cognitive impairment based on screening, and inability to speak English or Spanish. A total of 2802 participants were enrolled in the intervention group, and 2649 participants were enrolled in the control group.
Intervention: The intervention contained 5 components: a standardized assessment of 7 modifiable risk factors for fall injuries; standardized protocol-driven recommendations for management of risk factors; an individualized care plan focused on 1 to 3 risk factors; implementation of care plans, including referrals to community-based programs; and follow-up care conducted by telephone or in person. The modifiable risk factors included impairment of strength, gait, or balance; use of medications related to falls; postural hypotension; problems with feet or footwear; visual impairment; osteoporosis or vitamin D deficiency; and home safety hazards. The intervention was delivered by nurses who had completed online training modules and face-to-face training sessions focused on the intervention and motivational interviewing along with continuing education, in partnership with participants and their primary care providers. In the control group, participants received enhanced usual care, including an informational pamphlet, and were encouraged to discuss fall prevention with their primary care provider, including the results of their screening evaluation.
Main outcome measures: The primary outcome of the study was the first serious fall injury in a time-to-event analysis, defined as a fall resulting in a fracture (other than thoracic or lumbar vertebral fracture), joint dislocation, cut requiring closure, head injury requiring hospitalization, sprain or strain, bruising or swelling, or other serious injury. The secondary outcome was first patient-reported fall injury, also in a time-to-event analysis, ascertained by telephone interviews conducted every 4 months. Other outcomes included hospital admissions, emergency department visits, and other health care utilization. Adjudication of fall events and injuries was conducted by a team blinded to treatment assignment and verified using administrative claims data, encounter data, or electronic health record review.
Main results: The intervention and control groups were similar in terms of sex and age: 62.5% vs 61.5% of participants were women, and mean (SD) age was 79.9 (5.7) years and 79.5 (5.8) years, respectively. Other demographic characteristics were similar between groups. For the primary outcome, the rate of first serious injury was 4.9 per 100 person-years in the intervention group and 5.3 per 100 person-years in the control group, with a hazard ratio of 0.92 (95% CI, 0.80-1.06; P = .25). For the secondary outcome of patient-reported fall injury, there were 25.6 events per 100 person-years in the intervention group and 28.6 in the control group, with a hazard ratio of 0.90 (95% CI, 0.83-0.99; P =0.004). Rates of hospitalization and other secondary outcomes were similar between groups.
Conclusion: The multifactorial STRIDE intervention did not reduce the rate of serious fall injury when compared to enhanced usual care. The intervention did result in lower rates of fall injury by patient report, but no other significant outcomes were seen.
Study 2 Overview (Stark et al)
Objective: To examine the effect of a behavioral home hazard removal intervention for fall prevention on risk of fall in community-dwelling older adults.
Design: This randomized clinical trial was conducted at a single site in St. Louis, Missouri. Participants were community-dwelling older adults who received services from the Area Agency on Aging (AAA). Inclusion criteria included age 65 years and older, having 1 or more falls in the previous 12 months or being worried about falling by self report, and currently receiving services from an AAA. Exclusion criteria included living in an institution or being severely cognitively impaired and unable to follow directions or report falls. Participants who met the criteria were contacted by phone and invited to participate. A total of 310 participants were enrolled in the study, with an equal number of participants assigned to the intervention and control groups.
Intervention: The intervention included hazard identification and removal after a comprehensive assessment of participants, their behaviors, and the environment; this assessment took place during the first visit, which lasted approximately 80 minutes. A home hazard removal plan was developed, and in the second session, which lasted approximately 40 minutes, remediation of hazards was carried out. A third session for home modification that lasted approximately 30 minutes was conducted, if needed. At 6 months after the intervention, a booster session to identify and remediate any new home hazards and address issues was conducted. Specific interventions, as identified by the assessment, included minor home repair such as grab bars, adaptive equipment, task modification, and education. Shared decision making that enabled older adults to control changes in their homes, self-management strategies to improve awareness, and motivational enhancement strategies to improve acceptance were employed. Scripted algorithms and checklists were used to deliver the intervention. For usual care, an annual assessment and referrals to community services, if needed, were conducted in the AAA.
Main outcome measures: The primary outcome of the study was the number of days to first fall in 12 months. Falls were defined as unintentional movements to the floor, ground, or object below knee level, and falls were recorded through a daily journal for 12 months. Participants were contacted by phone if they did not return the journal or reported a fall. Participants were interviewed to verify falls and determine whether a fall was injurious. Secondary outcomes included rate of falls per person per 12 months; daily activity performance measured using the Older Americans Resources and Services Activities of Daily Living scale; falls self-efficacy, which measures confidence performing daily activities without falling; and quality of life using the SF-36 at 12 months.
Main results: Most of the study participants were women (74%), and mean (SD) age was 75 (7.4) years. Study retention was similar between the intervention and control groups, with 82% completing the study in the intervention group compared with 81% in the control group. Fidelity to the intervention, as measured by a checklist by the interventionist, was 99%, and adherence to home modification, as measured by number of home modifications in use by self report, was high at 92% at 6 months and 91% at 12 months. For the primary outcome, fall hazard was not different between the intervention and control groups (hazard ratio, 0.9; 95% CI, 0.66-1.27). For the secondary outcomes, the rate of falling was lower in the intervention group compared with the control group, with a relative risk of 0.62 (95% CI, 0.40-0.95). There was no difference in other secondary outcomes of daily activity performance, falls self-efficacy, or quality of life.
Conclusion: Despite high adherence to home modifications and fidelity to the intervention, this home hazard removal program did not reduce the risk of falling when compared to usual care. It did reduce the rate of falls, although no other effects were observed.
Commentary
Observational studies have identified factors that contribute to falls,1 and over the past 30 years a number of intervention trials designed to reduce the risk of falling have been conducted. A recent Cochrane review, published prior to the Bhasin et al and Stark et al trials, looked at the effect of multifactorial interventions for fall prevention across 62 trials that included 19,935 older adults living in the community. The review concluded that multifactorial interventions may reduce the rate of falls, but this conclusion was based on low-quality evidence and there was significant heterogeneity across the studies.2
The STRIDE randomized trial represents the latest effort to address the evidence gap around fall prevention, with the STRIDE investigators hoping this would be the definitive trial that leads to practice change in fall prevention. Smaller trials that have demonstrated effectiveness were brought to scale in this large randomized trial that included 86 practices and more than 5000 participants. The investigators used risk of injurious falls as the primary outcome, as this outcome is considered the most clinically meaningful for the study population. The results, however, were disappointing: the multifactorial intervention in STRIDE did not result in a reduction of risk of injurious falls. Challenges in the implementation of this large trial may have contributed to its results; falls care managers, key to this multifactorial intervention, reported difficulties in navigating complex relationships with patients, families, study staff, and primary care practices during the study. Barriers reported included clinical space limitations, variable buy-in from providers, and turnover of practice staff and providers.3 Such implementation factors may have resulted in the divergent results between smaller clinical trials and this large-scale trial conducted across multiple settings.
The second study, by Stark et al, examined a home modification program and its effect on risk of falls. A prior Cochrane review examining the effect of home safety assessment and modification indicates that these strategies are effective in reducing the rate of falls as well as the risk of falling.4 The results of the current trial showed a reduction in the rate of falls but not in the risk of falling; however, this study did not examine outcomes of serious injurious falls, which may be more clinically meaningful. The Stark et al study adds to the existing literature showing that home modification may have an impact on fall rates. One noteworthy aspect of the Stark et al trial is the high adherence rate to home modification in a community-based approach; perhaps the investigators’ approach can be translated to real-world use.
Applications for Clinical Practice and System Implementation
The role of exercise programs in reducing fall rates is well established,5 but neither of these studies focused on exercise interventions. STRIDE offered community-based exercise program referral, but there is variability in such programs and study staff reported challenges in matching participants with appropriate exercise programs.3 Further studies that examine combinations of multifactorial falls risk reduction, exercise, and home safety, with careful consideration of implementation challenges to assure fidelity and adherence to the intervention, are needed to ascertain the best strategy for fall prevention for older adults at risk.
Given the results of these trials, it is difficult to recommend one falls prevention intervention over another. Clinicians should continue to identify falls risk factors using standardized assessments and determine which factors are modifiable.
Practice Points
- Incorporating assessments of falls risk in primary care is feasible, and such assessments can identify important risk factors.
- Clinicians and health systems should identify avenues, such as developing programmatic approaches, to providing home safety assessment and intervention, exercise options, medication review, and modification of other risk factors.
- Ensuring delivery of these elements reliably through programmatic approaches with adequate follow-up is key to preventing falls in this population.
—William W. Hung, MD, MPH
Study 1 Overview (Bhasin et al)
Objective: To examine the effect of a multifactorial intervention for fall prevention on fall injury in community-dwelling older adults.
Design: This was a pragmatic, cluster randomized trial conducted in 86 primary care practices across 10 health care systems.
Setting and participants: The primary care sites were selected based on the prespecified criteria of size, ability to implement the intervention, proximity to other practices, accessibility to electronic health records, and access to community-based exercise programs. The primary care practices were randomly assigned to intervention or control.
Eligibility criteria for participants at those practices included age 70 years or older, dwelling in the community, and having an increased risk of falls, as determined by a history of fall-related injury in the past year, 2 or more falls in the past year, or being afraid of falling because of problems with balance or walking. Exclusion criteria were inability to provide consent or lack of proxy consent for participants who were determined to have cognitive impairment based on screening, and inability to speak English or Spanish. A total of 2802 participants were enrolled in the intervention group, and 2649 participants were enrolled in the control group.
Intervention: The intervention contained 5 components: a standardized assessment of 7 modifiable risk factors for fall injuries; standardized protocol-driven recommendations for management of risk factors; an individualized care plan focused on 1 to 3 risk factors; implementation of care plans, including referrals to community-based programs; and follow-up care conducted by telephone or in person. The modifiable risk factors included impairment of strength, gait, or balance; use of medications related to falls; postural hypotension; problems with feet or footwear; visual impairment; osteoporosis or vitamin D deficiency; and home safety hazards. The intervention was delivered by nurses who had completed online training modules and face-to-face training sessions focused on the intervention and motivational interviewing along with continuing education, in partnership with participants and their primary care providers. In the control group, participants received enhanced usual care, including an informational pamphlet, and were encouraged to discuss fall prevention with their primary care provider, including the results of their screening evaluation.
Main outcome measures: The primary outcome of the study was the first serious fall injury in a time-to-event analysis, defined as a fall resulting in a fracture (other than thoracic or lumbar vertebral fracture), joint dislocation, cut requiring closure, head injury requiring hospitalization, sprain or strain, bruising or swelling, or other serious injury. The secondary outcome was first patient-reported fall injury, also in a time-to-event analysis, ascertained by telephone interviews conducted every 4 months. Other outcomes included hospital admissions, emergency department visits, and other health care utilization. Adjudication of fall events and injuries was conducted by a team blinded to treatment assignment and verified using administrative claims data, encounter data, or electronic health record review.
Main results: The intervention and control groups were similar in terms of sex and age: 62.5% vs 61.5% of participants were women, and mean (SD) age was 79.9 (5.7) years and 79.5 (5.8) years, respectively. Other demographic characteristics were similar between groups. For the primary outcome, the rate of first serious injury was 4.9 per 100 person-years in the intervention group and 5.3 per 100 person-years in the control group, with a hazard ratio of 0.92 (95% CI, 0.80-1.06; P = .25). For the secondary outcome of patient-reported fall injury, there were 25.6 events per 100 person-years in the intervention group and 28.6 in the control group, with a hazard ratio of 0.90 (95% CI, 0.83-0.99; P =0.004). Rates of hospitalization and other secondary outcomes were similar between groups.
Conclusion: The multifactorial STRIDE intervention did not reduce the rate of serious fall injury when compared to enhanced usual care. The intervention did result in lower rates of fall injury by patient report, but no other significant outcomes were seen.
Study 2 Overview (Stark et al)
Objective: To examine the effect of a behavioral home hazard removal intervention for fall prevention on risk of fall in community-dwelling older adults.
Design: This randomized clinical trial was conducted at a single site in St. Louis, Missouri. Participants were community-dwelling older adults who received services from the Area Agency on Aging (AAA). Inclusion criteria included age 65 years and older, having 1 or more falls in the previous 12 months or being worried about falling by self report, and currently receiving services from an AAA. Exclusion criteria included living in an institution or being severely cognitively impaired and unable to follow directions or report falls. Participants who met the criteria were contacted by phone and invited to participate. A total of 310 participants were enrolled in the study, with an equal number of participants assigned to the intervention and control groups.
Intervention: The intervention included hazard identification and removal after a comprehensive assessment of participants, their behaviors, and the environment; this assessment took place during the first visit, which lasted approximately 80 minutes. A home hazard removal plan was developed, and in the second session, which lasted approximately 40 minutes, remediation of hazards was carried out. A third session for home modification that lasted approximately 30 minutes was conducted, if needed. At 6 months after the intervention, a booster session to identify and remediate any new home hazards and address issues was conducted. Specific interventions, as identified by the assessment, included minor home repair such as grab bars, adaptive equipment, task modification, and education. Shared decision making that enabled older adults to control changes in their homes, self-management strategies to improve awareness, and motivational enhancement strategies to improve acceptance were employed. Scripted algorithms and checklists were used to deliver the intervention. For usual care, an annual assessment and referrals to community services, if needed, were conducted in the AAA.
Main outcome measures: The primary outcome of the study was the number of days to first fall in 12 months. Falls were defined as unintentional movements to the floor, ground, or object below knee level, and falls were recorded through a daily journal for 12 months. Participants were contacted by phone if they did not return the journal or reported a fall. Participants were interviewed to verify falls and determine whether a fall was injurious. Secondary outcomes included rate of falls per person per 12 months; daily activity performance measured using the Older Americans Resources and Services Activities of Daily Living scale; falls self-efficacy, which measures confidence performing daily activities without falling; and quality of life using the SF-36 at 12 months.
Main results: Most of the study participants were women (74%), and mean (SD) age was 75 (7.4) years. Study retention was similar between the intervention and control groups, with 82% completing the study in the intervention group compared with 81% in the control group. Fidelity to the intervention, as measured by a checklist by the interventionist, was 99%, and adherence to home modification, as measured by number of home modifications in use by self report, was high at 92% at 6 months and 91% at 12 months. For the primary outcome, fall hazard was not different between the intervention and control groups (hazard ratio, 0.9; 95% CI, 0.66-1.27). For the secondary outcomes, the rate of falling was lower in the intervention group compared with the control group, with a relative risk of 0.62 (95% CI, 0.40-0.95). There was no difference in other secondary outcomes of daily activity performance, falls self-efficacy, or quality of life.
Conclusion: Despite high adherence to home modifications and fidelity to the intervention, this home hazard removal program did not reduce the risk of falling when compared to usual care. It did reduce the rate of falls, although no other effects were observed.
Commentary
Observational studies have identified factors that contribute to falls,1 and over the past 30 years a number of intervention trials designed to reduce the risk of falling have been conducted. A recent Cochrane review, published prior to the Bhasin et al and Stark et al trials, looked at the effect of multifactorial interventions for fall prevention across 62 trials that included 19,935 older adults living in the community. The review concluded that multifactorial interventions may reduce the rate of falls, but this conclusion was based on low-quality evidence and there was significant heterogeneity across the studies.2
The STRIDE randomized trial represents the latest effort to address the evidence gap around fall prevention, with the STRIDE investigators hoping this would be the definitive trial that leads to practice change in fall prevention. Smaller trials that have demonstrated effectiveness were brought to scale in this large randomized trial that included 86 practices and more than 5000 participants. The investigators used risk of injurious falls as the primary outcome, as this outcome is considered the most clinically meaningful for the study population. The results, however, were disappointing: the multifactorial intervention in STRIDE did not result in a reduction of risk of injurious falls. Challenges in the implementation of this large trial may have contributed to its results; falls care managers, key to this multifactorial intervention, reported difficulties in navigating complex relationships with patients, families, study staff, and primary care practices during the study. Barriers reported included clinical space limitations, variable buy-in from providers, and turnover of practice staff and providers.3 Such implementation factors may have resulted in the divergent results between smaller clinical trials and this large-scale trial conducted across multiple settings.
The second study, by Stark et al, examined a home modification program and its effect on risk of falls. A prior Cochrane review examining the effect of home safety assessment and modification indicates that these strategies are effective in reducing the rate of falls as well as the risk of falling.4 The results of the current trial showed a reduction in the rate of falls but not in the risk of falling; however, this study did not examine outcomes of serious injurious falls, which may be more clinically meaningful. The Stark et al study adds to the existing literature showing that home modification may have an impact on fall rates. One noteworthy aspect of the Stark et al trial is the high adherence rate to home modification in a community-based approach; perhaps the investigators’ approach can be translated to real-world use.
Applications for Clinical Practice and System Implementation
The role of exercise programs in reducing fall rates is well established,5 but neither of these studies focused on exercise interventions. STRIDE offered community-based exercise program referral, but there is variability in such programs and study staff reported challenges in matching participants with appropriate exercise programs.3 Further studies that examine combinations of multifactorial falls risk reduction, exercise, and home safety, with careful consideration of implementation challenges to assure fidelity and adherence to the intervention, are needed to ascertain the best strategy for fall prevention for older adults at risk.
Given the results of these trials, it is difficult to recommend one falls prevention intervention over another. Clinicians should continue to identify falls risk factors using standardized assessments and determine which factors are modifiable.
Practice Points
- Incorporating assessments of falls risk in primary care is feasible, and such assessments can identify important risk factors.
- Clinicians and health systems should identify avenues, such as developing programmatic approaches, to providing home safety assessment and intervention, exercise options, medication review, and modification of other risk factors.
- Ensuring delivery of these elements reliably through programmatic approaches with adequate follow-up is key to preventing falls in this population.
—William W. Hung, MD, MPH
1. Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988; 319:1701-1707. doi:10.1056/NEJM198812293192604
2. Hopewell S, Adedire O, Copsey BJ, et al. Multifactorial and multiple component interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2018;7(7):CD012221. doi:0.1002/14651858.CD012221.pub2
3. Reckrey JM, Gazarian P, Reuben DB, et al. Barriers to implementation of STRIDE, a national study to prevent fall-related injuries. J Am Geriatr Soc. 2021;69(5):1334-1342. doi:10.1111/jgs.17056
4. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012;2012(9):CD007146. doi:10.1002/14651858.CD007146.pub3
5. Sherrington C, Fairhall NJ, Wallbank GK, et al. Exercise for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2019;1(1):CD012424. doi:10.1002/14651858.CD012424.pub2
1. Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988; 319:1701-1707. doi:10.1056/NEJM198812293192604
2. Hopewell S, Adedire O, Copsey BJ, et al. Multifactorial and multiple component interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2018;7(7):CD012221. doi:0.1002/14651858.CD012221.pub2
3. Reckrey JM, Gazarian P, Reuben DB, et al. Barriers to implementation of STRIDE, a national study to prevent fall-related injuries. J Am Geriatr Soc. 2021;69(5):1334-1342. doi:10.1111/jgs.17056
4. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012;2012(9):CD007146. doi:10.1002/14651858.CD007146.pub3
5. Sherrington C, Fairhall NJ, Wallbank GK, et al. Exercise for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2019;1(1):CD012424. doi:10.1002/14651858.CD012424.pub2
FDA allows import of 2 million cans of baby formula from U.K.
The U.S. Food and Drug Administration is easing rules to allow infant formula imports from the United Kingdom, which would bring about 2 million cans to the U.S. in coming weeks.
Kendal Nutricare will be able to offer certain infant formula products under the Kendamil brand to ease the nationwide formula shortage.
“Importantly, we anticipate additional infant formula products may be safely and quickly imported in the U.S. in the near-term, based on ongoing discussions with manufacturers and suppliers worldwide,” Robert Califf, MD, the FDA commissioner, said in a statement.
Kendal Nutricare has more than 40,000 cans in stock for immediate dispatch, the FDA said, and the U.S. Department of Health and Human Services is talking to the company about the best ways to get the products to the U.S. as quickly as possible.
Kendamil has set up a website for consumers to receive updates and find products once they arrive in the U.S.
After an evaluation, the FDA said it had no safety or nutrition concerns about the products. The evaluation reviewed the company’s microbiological testing, labeling, and information about facility production and inspection history.
On May 24, the FDA announced that Abbott Nutrition will release about 300,000 cans of its EleCare specialty amino acid-based formula to families that need urgent, life-sustaining supplies. The products had more tests for microbes before release.
Although some EleCare products were included in Abbott’s infant formula recall earlier this year, the cans that will be released were in different lots, have never been released, and have been maintained in storage, the FDA said.
“These EleCare product lots were not part of the recall but have been on hold due to concerns that they were produced under unsanitary conditions observed at Abbott Nutrition’s Sturgis, Michigan, facility,” the FDA wrote.
The FDA encourages parents and caregivers to talk with their health care providers to weigh the potential risk of bacterial infection with the critical need for the product, based on its special dietary formulation for infants with severe food allergies or gut disorders.
The FDA also said that Abbott confirmed the EleCare products will be the first formula produced at the Sturgis facility when it restarts production soon. Other specialty metabolic formulas will follow.
Abbott plans to restart production at the Sturgis facility on June 4, the company said in a statement, noting that the early batches of EleCare would be available to consumers around June 20.
The products being released now are EleCare (for infants under 1 year) and EleCare Jr. (for ages 1 and older). Those who want to request products should contact their health care providers or call Abbott directly at 800-881-0876.
A version of this article first appeared on WebMD.com.
The U.S. Food and Drug Administration is easing rules to allow infant formula imports from the United Kingdom, which would bring about 2 million cans to the U.S. in coming weeks.
Kendal Nutricare will be able to offer certain infant formula products under the Kendamil brand to ease the nationwide formula shortage.
“Importantly, we anticipate additional infant formula products may be safely and quickly imported in the U.S. in the near-term, based on ongoing discussions with manufacturers and suppliers worldwide,” Robert Califf, MD, the FDA commissioner, said in a statement.
Kendal Nutricare has more than 40,000 cans in stock for immediate dispatch, the FDA said, and the U.S. Department of Health and Human Services is talking to the company about the best ways to get the products to the U.S. as quickly as possible.
Kendamil has set up a website for consumers to receive updates and find products once they arrive in the U.S.
After an evaluation, the FDA said it had no safety or nutrition concerns about the products. The evaluation reviewed the company’s microbiological testing, labeling, and information about facility production and inspection history.
On May 24, the FDA announced that Abbott Nutrition will release about 300,000 cans of its EleCare specialty amino acid-based formula to families that need urgent, life-sustaining supplies. The products had more tests for microbes before release.
Although some EleCare products were included in Abbott’s infant formula recall earlier this year, the cans that will be released were in different lots, have never been released, and have been maintained in storage, the FDA said.
“These EleCare product lots were not part of the recall but have been on hold due to concerns that they were produced under unsanitary conditions observed at Abbott Nutrition’s Sturgis, Michigan, facility,” the FDA wrote.
The FDA encourages parents and caregivers to talk with their health care providers to weigh the potential risk of bacterial infection with the critical need for the product, based on its special dietary formulation for infants with severe food allergies or gut disorders.
The FDA also said that Abbott confirmed the EleCare products will be the first formula produced at the Sturgis facility when it restarts production soon. Other specialty metabolic formulas will follow.
Abbott plans to restart production at the Sturgis facility on June 4, the company said in a statement, noting that the early batches of EleCare would be available to consumers around June 20.
The products being released now are EleCare (for infants under 1 year) and EleCare Jr. (for ages 1 and older). Those who want to request products should contact their health care providers or call Abbott directly at 800-881-0876.
A version of this article first appeared on WebMD.com.
The U.S. Food and Drug Administration is easing rules to allow infant formula imports from the United Kingdom, which would bring about 2 million cans to the U.S. in coming weeks.
Kendal Nutricare will be able to offer certain infant formula products under the Kendamil brand to ease the nationwide formula shortage.
“Importantly, we anticipate additional infant formula products may be safely and quickly imported in the U.S. in the near-term, based on ongoing discussions with manufacturers and suppliers worldwide,” Robert Califf, MD, the FDA commissioner, said in a statement.
Kendal Nutricare has more than 40,000 cans in stock for immediate dispatch, the FDA said, and the U.S. Department of Health and Human Services is talking to the company about the best ways to get the products to the U.S. as quickly as possible.
Kendamil has set up a website for consumers to receive updates and find products once they arrive in the U.S.
After an evaluation, the FDA said it had no safety or nutrition concerns about the products. The evaluation reviewed the company’s microbiological testing, labeling, and information about facility production and inspection history.
On May 24, the FDA announced that Abbott Nutrition will release about 300,000 cans of its EleCare specialty amino acid-based formula to families that need urgent, life-sustaining supplies. The products had more tests for microbes before release.
Although some EleCare products were included in Abbott’s infant formula recall earlier this year, the cans that will be released were in different lots, have never been released, and have been maintained in storage, the FDA said.
“These EleCare product lots were not part of the recall but have been on hold due to concerns that they were produced under unsanitary conditions observed at Abbott Nutrition’s Sturgis, Michigan, facility,” the FDA wrote.
The FDA encourages parents and caregivers to talk with their health care providers to weigh the potential risk of bacterial infection with the critical need for the product, based on its special dietary formulation for infants with severe food allergies or gut disorders.
The FDA also said that Abbott confirmed the EleCare products will be the first formula produced at the Sturgis facility when it restarts production soon. Other specialty metabolic formulas will follow.
Abbott plans to restart production at the Sturgis facility on June 4, the company said in a statement, noting that the early batches of EleCare would be available to consumers around June 20.
The products being released now are EleCare (for infants under 1 year) and EleCare Jr. (for ages 1 and older). Those who want to request products should contact their health care providers or call Abbott directly at 800-881-0876.
A version of this article first appeared on WebMD.com.
CDC signs off on COVID boosters in children ages 5-11
Centers for Disease Control and Prevention Director Rochelle Walensky, MD, signed off May 19 on an advisory panel’s recommendation that children ages 5 to 11 years should receive a Pfizer-BioNTech COVID-19 vaccine booster dose at least 5 months after completion of the primary series.
The CDC’s Advisory Committee on Immunization Practices (ACIP) voted 11:1, with one abstention, on a question about whether it recommended these additional shots in this age group.
The U.S. Food and Drug Administration on May 17 amended the emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 vaccine to cover a single booster dose for administration to individuals 5 through 11 years of age.
At the request of CDC staff, ACIP members considered whether there should be softer wording for this recommendation, stating that children in this age group “may” receive a booster. This kind of phrasing would better reflect uncertainty about the course of COVID in the months ahead and allow flexibility for a stronger recommendation in the fall.
ACIP panelists and members of key groups argued strongly for a “should” recommendation, despite the uncertainties.
They also called for stronger efforts to make sure eligible children received their initial COVID-19 shots. Data gathered between November and April show only 14.4% of children ages 5 to 11 in rural areas have received at least one dose of COVID-19 vaccination, with top rates of 39.8% in large urban communities and 36% in larger suburban regions, CDC staff said.
CDC staff also said nearly 40% of parents in rural areas reported that their children’s pediatricians did not recommend COVID-19 vaccinations, compared with only 8% of parents in urban communities. These figures concerned ACIP members and liaisons from medical associations who take part in the panel’s deliberations but not in its votes.
“People will hear the word ‘m-a-y’ as ‘m-e-h’,” said Patricia Stinchfield, RN, MS, who served as the liaison for National Association of Pediatric Nurse Practitioners to ACIP. “I think we need to add urgency” to efforts to increase use of COVID vaccinations, she said.
Voting no on Thursday was Helen Keipp Talbot, MD, of Vanderbilt University. She explained after the vote that she is in favor of having young children vaccinated, but she’s concerned about the low rates of initial uptake of the COVID-19 shots.
“Boosters are great once we’ve gotten everyone their first round,” she said. “That needs to be our priority in this.”
Sandra Fryhofer, MD, the American Medical Association’s liaison to ACIP, stressed the add-on benefits from more widespread vaccination of children against COVID. Dr. Fryhofer said she serves adults in her practice as an internal medicine physician, with many of her patients being at high risk for complications from COVID.
Too many people are assuming the spread of infections in the community has lessened the risk of the virus, Dr. Fryhofer said.
“Not everyone’s had COVID yet, and my patients will be likely to get COVID if their grandchildren get it. We’re going through pandemic fatigue in this country,” she said. “Unfortunately, masks are now more off than on. Winter’s coming. They’re more variants” of the virus likely to emerge.
The data emerging so far suggests COVID vaccines will become a three-dose medicine, as is already accepted for other shots like hepatitis B vaccine, Dr. Fryhofer said.
Data gathered to date show the vaccine decreases risk of hospitalization for COVID and for complications such as multisystem inflammatory syndrome in children (MIS-C), she said.
“The bottom line is children in this age group are getting COVID,” Dr. Fryhofer said of the 5- to 11-year-olds. “Some do fine. Some are getting real sick. Some are hospitalized, some have died.”
At the meeting, CDC staff cited data from a paper published in the New England Journal of Medicine in March showing that vaccination had reduced the risk of hospitalization for COVID-19 among children 5 to 11 years of age by two-thirds during the Omicron period; most children with critical COVID-19 were unvaccinated.
COVID-19 led to 66 deaths among children ages 5 to 11 in the October 2020 to October 2021 timeframe, said ACIP member Matthew F. Daley, MD, of Kaiser Permanente Colorado during a presentation to his fellow panel members.
Parents may underestimate children’s risk from COVID and thus hold off on vaccinations, stressed AMA President Gerald E. Harmon, MD, in a statement issued after the meeting.
“It is concerning that only 1 in 3 children between the ages of 5 and 11 in the United States have received two doses of the vaccine, in part because parents believe them to be at lower risk for severe disease than adults,” Dr. Harmon said. “But the Omicron variant brought about change that should alter that calculus.”
Responding to early data
As Dr. Fryhofer put it, the medical community has been learning in “real time” about how COVID vaccines work and how to use them.
The EUA granted on May 17 for booster shots for children ages 5 to 11 was based on an analysis of immune response data in a subset of children from an ongoing randomized placebo-controlled trial, the FDA said.
Antibody responses were evaluated in 67 study participants who received a booster dose 7 to 9 months after completing a two-dose primary series of the Pfizer-BioNTech COVID-19 Vaccine. The EUA for the booster shot was intended to respond to emerging data that suggest that vaccine effectiveness against COVID-19 wanes after the second dose of the vaccine, the FDA said.
CDC seeks help tracking vaccine complications
At the ACIP meeting, a top CDC vaccine-safety official, Tom Shimabukuro, MD, MPH, MBA, asked physicians to make sure their patients know about the agency’s V-Safe program for gathering reports from the public about their experiences with COVID vaccines. This is intended to help the CDC monitor for side effects of these medications.
“We need your help,” he said during a presentation about adverse events reported to date in children ages 5 to 11 who took the Pfizer vaccine.
About 18.1 million doses of Pfizer-BioNTech vaccine have been administered to children ages 5 to 11 years in the United States so far. Most of the reports of adverse events following vaccination were not serious, he said. But there were 20 reports of myocarditis verified to meet CDC case definition among children ages 5 to 11 years.
One case involved a death with histopathologic evidence of myocarditis on autopsy. The CDC continues to assist with case review, he said.
A version of this article first appeared on Medscape.com.
Centers for Disease Control and Prevention Director Rochelle Walensky, MD, signed off May 19 on an advisory panel’s recommendation that children ages 5 to 11 years should receive a Pfizer-BioNTech COVID-19 vaccine booster dose at least 5 months after completion of the primary series.
The CDC’s Advisory Committee on Immunization Practices (ACIP) voted 11:1, with one abstention, on a question about whether it recommended these additional shots in this age group.
The U.S. Food and Drug Administration on May 17 amended the emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 vaccine to cover a single booster dose for administration to individuals 5 through 11 years of age.
At the request of CDC staff, ACIP members considered whether there should be softer wording for this recommendation, stating that children in this age group “may” receive a booster. This kind of phrasing would better reflect uncertainty about the course of COVID in the months ahead and allow flexibility for a stronger recommendation in the fall.
ACIP panelists and members of key groups argued strongly for a “should” recommendation, despite the uncertainties.
They also called for stronger efforts to make sure eligible children received their initial COVID-19 shots. Data gathered between November and April show only 14.4% of children ages 5 to 11 in rural areas have received at least one dose of COVID-19 vaccination, with top rates of 39.8% in large urban communities and 36% in larger suburban regions, CDC staff said.
CDC staff also said nearly 40% of parents in rural areas reported that their children’s pediatricians did not recommend COVID-19 vaccinations, compared with only 8% of parents in urban communities. These figures concerned ACIP members and liaisons from medical associations who take part in the panel’s deliberations but not in its votes.
“People will hear the word ‘m-a-y’ as ‘m-e-h’,” said Patricia Stinchfield, RN, MS, who served as the liaison for National Association of Pediatric Nurse Practitioners to ACIP. “I think we need to add urgency” to efforts to increase use of COVID vaccinations, she said.
Voting no on Thursday was Helen Keipp Talbot, MD, of Vanderbilt University. She explained after the vote that she is in favor of having young children vaccinated, but she’s concerned about the low rates of initial uptake of the COVID-19 shots.
“Boosters are great once we’ve gotten everyone their first round,” she said. “That needs to be our priority in this.”
Sandra Fryhofer, MD, the American Medical Association’s liaison to ACIP, stressed the add-on benefits from more widespread vaccination of children against COVID. Dr. Fryhofer said she serves adults in her practice as an internal medicine physician, with many of her patients being at high risk for complications from COVID.
Too many people are assuming the spread of infections in the community has lessened the risk of the virus, Dr. Fryhofer said.
“Not everyone’s had COVID yet, and my patients will be likely to get COVID if their grandchildren get it. We’re going through pandemic fatigue in this country,” she said. “Unfortunately, masks are now more off than on. Winter’s coming. They’re more variants” of the virus likely to emerge.
The data emerging so far suggests COVID vaccines will become a three-dose medicine, as is already accepted for other shots like hepatitis B vaccine, Dr. Fryhofer said.
Data gathered to date show the vaccine decreases risk of hospitalization for COVID and for complications such as multisystem inflammatory syndrome in children (MIS-C), she said.
“The bottom line is children in this age group are getting COVID,” Dr. Fryhofer said of the 5- to 11-year-olds. “Some do fine. Some are getting real sick. Some are hospitalized, some have died.”
At the meeting, CDC staff cited data from a paper published in the New England Journal of Medicine in March showing that vaccination had reduced the risk of hospitalization for COVID-19 among children 5 to 11 years of age by two-thirds during the Omicron period; most children with critical COVID-19 were unvaccinated.
COVID-19 led to 66 deaths among children ages 5 to 11 in the October 2020 to October 2021 timeframe, said ACIP member Matthew F. Daley, MD, of Kaiser Permanente Colorado during a presentation to his fellow panel members.
Parents may underestimate children’s risk from COVID and thus hold off on vaccinations, stressed AMA President Gerald E. Harmon, MD, in a statement issued after the meeting.
“It is concerning that only 1 in 3 children between the ages of 5 and 11 in the United States have received two doses of the vaccine, in part because parents believe them to be at lower risk for severe disease than adults,” Dr. Harmon said. “But the Omicron variant brought about change that should alter that calculus.”
Responding to early data
As Dr. Fryhofer put it, the medical community has been learning in “real time” about how COVID vaccines work and how to use them.
The EUA granted on May 17 for booster shots for children ages 5 to 11 was based on an analysis of immune response data in a subset of children from an ongoing randomized placebo-controlled trial, the FDA said.
Antibody responses were evaluated in 67 study participants who received a booster dose 7 to 9 months after completing a two-dose primary series of the Pfizer-BioNTech COVID-19 Vaccine. The EUA for the booster shot was intended to respond to emerging data that suggest that vaccine effectiveness against COVID-19 wanes after the second dose of the vaccine, the FDA said.
CDC seeks help tracking vaccine complications
At the ACIP meeting, a top CDC vaccine-safety official, Tom Shimabukuro, MD, MPH, MBA, asked physicians to make sure their patients know about the agency’s V-Safe program for gathering reports from the public about their experiences with COVID vaccines. This is intended to help the CDC monitor for side effects of these medications.
“We need your help,” he said during a presentation about adverse events reported to date in children ages 5 to 11 who took the Pfizer vaccine.
About 18.1 million doses of Pfizer-BioNTech vaccine have been administered to children ages 5 to 11 years in the United States so far. Most of the reports of adverse events following vaccination were not serious, he said. But there were 20 reports of myocarditis verified to meet CDC case definition among children ages 5 to 11 years.
One case involved a death with histopathologic evidence of myocarditis on autopsy. The CDC continues to assist with case review, he said.
A version of this article first appeared on Medscape.com.
Centers for Disease Control and Prevention Director Rochelle Walensky, MD, signed off May 19 on an advisory panel’s recommendation that children ages 5 to 11 years should receive a Pfizer-BioNTech COVID-19 vaccine booster dose at least 5 months after completion of the primary series.
The CDC’s Advisory Committee on Immunization Practices (ACIP) voted 11:1, with one abstention, on a question about whether it recommended these additional shots in this age group.
The U.S. Food and Drug Administration on May 17 amended the emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 vaccine to cover a single booster dose for administration to individuals 5 through 11 years of age.
At the request of CDC staff, ACIP members considered whether there should be softer wording for this recommendation, stating that children in this age group “may” receive a booster. This kind of phrasing would better reflect uncertainty about the course of COVID in the months ahead and allow flexibility for a stronger recommendation in the fall.
ACIP panelists and members of key groups argued strongly for a “should” recommendation, despite the uncertainties.
They also called for stronger efforts to make sure eligible children received their initial COVID-19 shots. Data gathered between November and April show only 14.4% of children ages 5 to 11 in rural areas have received at least one dose of COVID-19 vaccination, with top rates of 39.8% in large urban communities and 36% in larger suburban regions, CDC staff said.
CDC staff also said nearly 40% of parents in rural areas reported that their children’s pediatricians did not recommend COVID-19 vaccinations, compared with only 8% of parents in urban communities. These figures concerned ACIP members and liaisons from medical associations who take part in the panel’s deliberations but not in its votes.
“People will hear the word ‘m-a-y’ as ‘m-e-h’,” said Patricia Stinchfield, RN, MS, who served as the liaison for National Association of Pediatric Nurse Practitioners to ACIP. “I think we need to add urgency” to efforts to increase use of COVID vaccinations, she said.
Voting no on Thursday was Helen Keipp Talbot, MD, of Vanderbilt University. She explained after the vote that she is in favor of having young children vaccinated, but she’s concerned about the low rates of initial uptake of the COVID-19 shots.
“Boosters are great once we’ve gotten everyone their first round,” she said. “That needs to be our priority in this.”
Sandra Fryhofer, MD, the American Medical Association’s liaison to ACIP, stressed the add-on benefits from more widespread vaccination of children against COVID. Dr. Fryhofer said she serves adults in her practice as an internal medicine physician, with many of her patients being at high risk for complications from COVID.
Too many people are assuming the spread of infections in the community has lessened the risk of the virus, Dr. Fryhofer said.
“Not everyone’s had COVID yet, and my patients will be likely to get COVID if their grandchildren get it. We’re going through pandemic fatigue in this country,” she said. “Unfortunately, masks are now more off than on. Winter’s coming. They’re more variants” of the virus likely to emerge.
The data emerging so far suggests COVID vaccines will become a three-dose medicine, as is already accepted for other shots like hepatitis B vaccine, Dr. Fryhofer said.
Data gathered to date show the vaccine decreases risk of hospitalization for COVID and for complications such as multisystem inflammatory syndrome in children (MIS-C), she said.
“The bottom line is children in this age group are getting COVID,” Dr. Fryhofer said of the 5- to 11-year-olds. “Some do fine. Some are getting real sick. Some are hospitalized, some have died.”
At the meeting, CDC staff cited data from a paper published in the New England Journal of Medicine in March showing that vaccination had reduced the risk of hospitalization for COVID-19 among children 5 to 11 years of age by two-thirds during the Omicron period; most children with critical COVID-19 were unvaccinated.
COVID-19 led to 66 deaths among children ages 5 to 11 in the October 2020 to October 2021 timeframe, said ACIP member Matthew F. Daley, MD, of Kaiser Permanente Colorado during a presentation to his fellow panel members.
Parents may underestimate children’s risk from COVID and thus hold off on vaccinations, stressed AMA President Gerald E. Harmon, MD, in a statement issued after the meeting.
“It is concerning that only 1 in 3 children between the ages of 5 and 11 in the United States have received two doses of the vaccine, in part because parents believe them to be at lower risk for severe disease than adults,” Dr. Harmon said. “But the Omicron variant brought about change that should alter that calculus.”
Responding to early data
As Dr. Fryhofer put it, the medical community has been learning in “real time” about how COVID vaccines work and how to use them.
The EUA granted on May 17 for booster shots for children ages 5 to 11 was based on an analysis of immune response data in a subset of children from an ongoing randomized placebo-controlled trial, the FDA said.
Antibody responses were evaluated in 67 study participants who received a booster dose 7 to 9 months after completing a two-dose primary series of the Pfizer-BioNTech COVID-19 Vaccine. The EUA for the booster shot was intended to respond to emerging data that suggest that vaccine effectiveness against COVID-19 wanes after the second dose of the vaccine, the FDA said.
CDC seeks help tracking vaccine complications
At the ACIP meeting, a top CDC vaccine-safety official, Tom Shimabukuro, MD, MPH, MBA, asked physicians to make sure their patients know about the agency’s V-Safe program for gathering reports from the public about their experiences with COVID vaccines. This is intended to help the CDC monitor for side effects of these medications.
“We need your help,” he said during a presentation about adverse events reported to date in children ages 5 to 11 who took the Pfizer vaccine.
About 18.1 million doses of Pfizer-BioNTech vaccine have been administered to children ages 5 to 11 years in the United States so far. Most of the reports of adverse events following vaccination were not serious, he said. But there were 20 reports of myocarditis verified to meet CDC case definition among children ages 5 to 11 years.
One case involved a death with histopathologic evidence of myocarditis on autopsy. The CDC continues to assist with case review, he said.
A version of this article first appeared on Medscape.com.
Improved cancer survival in states with ACA Medicaid expansion
compared with patients in states that did not adopt the expansion.
The finding comes from an American Cancer Society study of more than 2 million patients with newly diagnosed cancer, published online in the Journal of the National Cancer Institute.
The analysis also showed that the evidence was strongest for malignancies with poor prognosis such as lung, pancreatic, and liver cancer, and also for colorectal cancer.
Importantly, improvements in survival were larger in non-Hispanic Black patients and individuals residing in rural areas, suggesting there was a narrowing of disparities in cancer survival by race and rurality.
“Our findings provide further evidence of the importance of expanding Medicaid eligibility in all states, particularly considering the economic crisis and health care disruptions caused by the COVID-19 pandemic,” said lead author Xuesong Han, PhD, scientific director of health services research at the American Cancer Society, in a statement. “What’s encouraging is the American Rescue Plan Act of 2021 provides new incentives for Medicaid expansion in states that have yet to increase eligibility.”
The ACA provided states with incentives to expand Medicaid eligibility to all low-income adults under 138% federal poverty level, regardless of parental status.
As of last month, just 12 states have not yet opted for Medicaid expansion, even though the American Rescue Plan Act of 2021 provides new incentives for those remaining jurisdictions. But to date, none of the remaining states have taken advantage of these new incentives.
An interactive map showing the status of Medicare expansion by state is available here. The 12 states that have not adopted Medicare expansion (as of April) are Alabama, Florida, Georgia, Kansas, Mississippi, North Carolina, South Carolina, South Dakota, Tennessee, Texas, Wisconsin, and Wyoming.
The benefit of Medicaid expansion on cancer outcomes has already been observed in other studies. The first study to show a survival benefit was presented at the 2020 American Society of Clinical Oncology annual meeting. That analysis showed that cancer mortality declined by 29% in states that expanded Medicaid and by 25% in those that did not. The authors also noted that the greatest mortality benefit was observed in Hispanic patients.
Improved survival with expansion
In the current paper, Dr. Han and colleagues used population-based cancer registries from 42 states and compared data on patients aged 18-62 years who were diagnosed with cancer in a period of 2 years before (2010-2012) and after (2014-2016) ACA Medicaid expansion. They were followed through Sept. 30, 2013, and Dec. 31, 2017, respectively.
The analysis involved a total of 2.5 million patients, of whom 1.52 million lived in states that adopted Medicaid expansion and compared with 1 million patients were in states that did not.
Patients with grouped by sex, race and ethnicity, census tract-level poverty, and rurality. The authors note that non-Hispanic Black patients and those from high poverty areas and nonmetropolitan areas were disproportionately represented in nonexpansion states.
During the 2-year follow-up period, a total of 453,487 deaths occurred (257,950 in expansion states and 195,537 in nonexpansion states).
Overall, patients in expansion states generally had better survival versus those in nonexpansion states, the authors comment. However, for most cancer types, overall survival improved after the ACA for both groups of states.
The 2-year overall survival increased from 80.6% before the ACA to 82.2% post ACA in expansion states and from 78.7% to 80% in nonexpansion states.
This extrapolated to net increase of 0.44 percentage points in expansion states after adjusting for sociodemographic factors. By cancer site, the net increase was greater for colorectal cancer, lung cancer, non-Hodgkin’s lymphoma, pancreatic cancer, and liver cancer.
For Hispanic patients, 2-year survival also increased but was similar in expansion and nonexpansion states, and little net change was associated with Medicaid expansion.
“Our study shows that the increase was largely driven by improvements in survival for cancer types with poor prognosis, suggesting improved access to timely and effective treatments,” said Dr. Han. “It adds to accumulating evidence of the multiple benefits of Medicaid expansion.”
A version of this article first appeared on Medscape.com.
compared with patients in states that did not adopt the expansion.
The finding comes from an American Cancer Society study of more than 2 million patients with newly diagnosed cancer, published online in the Journal of the National Cancer Institute.
The analysis also showed that the evidence was strongest for malignancies with poor prognosis such as lung, pancreatic, and liver cancer, and also for colorectal cancer.
Importantly, improvements in survival were larger in non-Hispanic Black patients and individuals residing in rural areas, suggesting there was a narrowing of disparities in cancer survival by race and rurality.
“Our findings provide further evidence of the importance of expanding Medicaid eligibility in all states, particularly considering the economic crisis and health care disruptions caused by the COVID-19 pandemic,” said lead author Xuesong Han, PhD, scientific director of health services research at the American Cancer Society, in a statement. “What’s encouraging is the American Rescue Plan Act of 2021 provides new incentives for Medicaid expansion in states that have yet to increase eligibility.”
The ACA provided states with incentives to expand Medicaid eligibility to all low-income adults under 138% federal poverty level, regardless of parental status.
As of last month, just 12 states have not yet opted for Medicaid expansion, even though the American Rescue Plan Act of 2021 provides new incentives for those remaining jurisdictions. But to date, none of the remaining states have taken advantage of these new incentives.
An interactive map showing the status of Medicare expansion by state is available here. The 12 states that have not adopted Medicare expansion (as of April) are Alabama, Florida, Georgia, Kansas, Mississippi, North Carolina, South Carolina, South Dakota, Tennessee, Texas, Wisconsin, and Wyoming.
The benefit of Medicaid expansion on cancer outcomes has already been observed in other studies. The first study to show a survival benefit was presented at the 2020 American Society of Clinical Oncology annual meeting. That analysis showed that cancer mortality declined by 29% in states that expanded Medicaid and by 25% in those that did not. The authors also noted that the greatest mortality benefit was observed in Hispanic patients.
Improved survival with expansion
In the current paper, Dr. Han and colleagues used population-based cancer registries from 42 states and compared data on patients aged 18-62 years who were diagnosed with cancer in a period of 2 years before (2010-2012) and after (2014-2016) ACA Medicaid expansion. They were followed through Sept. 30, 2013, and Dec. 31, 2017, respectively.
The analysis involved a total of 2.5 million patients, of whom 1.52 million lived in states that adopted Medicaid expansion and compared with 1 million patients were in states that did not.
Patients with grouped by sex, race and ethnicity, census tract-level poverty, and rurality. The authors note that non-Hispanic Black patients and those from high poverty areas and nonmetropolitan areas were disproportionately represented in nonexpansion states.
During the 2-year follow-up period, a total of 453,487 deaths occurred (257,950 in expansion states and 195,537 in nonexpansion states).
Overall, patients in expansion states generally had better survival versus those in nonexpansion states, the authors comment. However, for most cancer types, overall survival improved after the ACA for both groups of states.
The 2-year overall survival increased from 80.6% before the ACA to 82.2% post ACA in expansion states and from 78.7% to 80% in nonexpansion states.
This extrapolated to net increase of 0.44 percentage points in expansion states after adjusting for sociodemographic factors. By cancer site, the net increase was greater for colorectal cancer, lung cancer, non-Hodgkin’s lymphoma, pancreatic cancer, and liver cancer.
For Hispanic patients, 2-year survival also increased but was similar in expansion and nonexpansion states, and little net change was associated with Medicaid expansion.
“Our study shows that the increase was largely driven by improvements in survival for cancer types with poor prognosis, suggesting improved access to timely and effective treatments,” said Dr. Han. “It adds to accumulating evidence of the multiple benefits of Medicaid expansion.”
A version of this article first appeared on Medscape.com.
compared with patients in states that did not adopt the expansion.
The finding comes from an American Cancer Society study of more than 2 million patients with newly diagnosed cancer, published online in the Journal of the National Cancer Institute.
The analysis also showed that the evidence was strongest for malignancies with poor prognosis such as lung, pancreatic, and liver cancer, and also for colorectal cancer.
Importantly, improvements in survival were larger in non-Hispanic Black patients and individuals residing in rural areas, suggesting there was a narrowing of disparities in cancer survival by race and rurality.
“Our findings provide further evidence of the importance of expanding Medicaid eligibility in all states, particularly considering the economic crisis and health care disruptions caused by the COVID-19 pandemic,” said lead author Xuesong Han, PhD, scientific director of health services research at the American Cancer Society, in a statement. “What’s encouraging is the American Rescue Plan Act of 2021 provides new incentives for Medicaid expansion in states that have yet to increase eligibility.”
The ACA provided states with incentives to expand Medicaid eligibility to all low-income adults under 138% federal poverty level, regardless of parental status.
As of last month, just 12 states have not yet opted for Medicaid expansion, even though the American Rescue Plan Act of 2021 provides new incentives for those remaining jurisdictions. But to date, none of the remaining states have taken advantage of these new incentives.
An interactive map showing the status of Medicare expansion by state is available here. The 12 states that have not adopted Medicare expansion (as of April) are Alabama, Florida, Georgia, Kansas, Mississippi, North Carolina, South Carolina, South Dakota, Tennessee, Texas, Wisconsin, and Wyoming.
The benefit of Medicaid expansion on cancer outcomes has already been observed in other studies. The first study to show a survival benefit was presented at the 2020 American Society of Clinical Oncology annual meeting. That analysis showed that cancer mortality declined by 29% in states that expanded Medicaid and by 25% in those that did not. The authors also noted that the greatest mortality benefit was observed in Hispanic patients.
Improved survival with expansion
In the current paper, Dr. Han and colleagues used population-based cancer registries from 42 states and compared data on patients aged 18-62 years who were diagnosed with cancer in a period of 2 years before (2010-2012) and after (2014-2016) ACA Medicaid expansion. They were followed through Sept. 30, 2013, and Dec. 31, 2017, respectively.
The analysis involved a total of 2.5 million patients, of whom 1.52 million lived in states that adopted Medicaid expansion and compared with 1 million patients were in states that did not.
Patients with grouped by sex, race and ethnicity, census tract-level poverty, and rurality. The authors note that non-Hispanic Black patients and those from high poverty areas and nonmetropolitan areas were disproportionately represented in nonexpansion states.
During the 2-year follow-up period, a total of 453,487 deaths occurred (257,950 in expansion states and 195,537 in nonexpansion states).
Overall, patients in expansion states generally had better survival versus those in nonexpansion states, the authors comment. However, for most cancer types, overall survival improved after the ACA for both groups of states.
The 2-year overall survival increased from 80.6% before the ACA to 82.2% post ACA in expansion states and from 78.7% to 80% in nonexpansion states.
This extrapolated to net increase of 0.44 percentage points in expansion states after adjusting for sociodemographic factors. By cancer site, the net increase was greater for colorectal cancer, lung cancer, non-Hodgkin’s lymphoma, pancreatic cancer, and liver cancer.
For Hispanic patients, 2-year survival also increased but was similar in expansion and nonexpansion states, and little net change was associated with Medicaid expansion.
“Our study shows that the increase was largely driven by improvements in survival for cancer types with poor prognosis, suggesting improved access to timely and effective treatments,” said Dr. Han. “It adds to accumulating evidence of the multiple benefits of Medicaid expansion.”
A version of this article first appeared on Medscape.com.
FDA authorizes Pfizer’s COVID booster for kids ages 5 to 11
emergency use authorization (EUA), allowing the Pfizer-BioNTech COVID-19 booster shot for children ages 5 to 11 who are at least 5 months out from their first vaccine series.
According to the most recent data from the Centers for Disease Control and Prevention, 28.6% of children in this age group have received both initial doses of Pfizer’s COVID-19 vaccine, and 35.3% have received their first dose.
Pfizer’s vaccine trial involving 4,500 children showed few side effects among children younger than 12 who received a booster, or third dose, according to a company statement.
Pfizer asked the FDA for an amended authorization in April, after submitting data showing that a third dose in children between 5 and 11 raised antibodies targeting the Omicron variant by 36 times.
“While it has largely been the case that COVID-19 tends to be less severe in children than adults, the omicron wave has seen more kids getting sick with the disease and being hospitalized, and children may also experience longer-term effects, even following initially mild disease,” FDA Commissioner Robert M. Califf, MD, said in a news release.
A study done by the New York State Department of Health showed the effectiveness of Pfizer’s two-dose vaccine series fell from 68% to 12% 4-5 months after the second dose was given to children 5 to 11 during the Omicron surge. A CDC study published in March also showed that the Pfizer shot reduced the risk of Omicron by 31% in children 5 to 11, a significantly lower rate than for kids 12 to 15, who had a 59% risk reduction after receiving two doses.
To some experts, this data suggest an even greater need for children under 12 to be eligible for a third dose.
“Since authorizing the vaccine for children down to 5 years of age in October 2021, emerging data suggest that vaccine effectiveness against COVID-19 wanes after the second dose of the vaccine in all authorized populations,” says Peter Marks, MD, PhD, the director of the FDA’s Center for Biologics Evaluation and Research.
The CDC still needs to sign off on the shots before they can be allowed. The agency’s Advisory Committee on Immunization Practices is set to meet on May 19 to discuss boosters in this age group.
FDA advisory panels plan to meet next month to discuss allowing Pfizer’s and Moderna’s COVID-19 vaccines for children under 6 years old.
A version of this article first appeared on WebMD.com.
emergency use authorization (EUA), allowing the Pfizer-BioNTech COVID-19 booster shot for children ages 5 to 11 who are at least 5 months out from their first vaccine series.
According to the most recent data from the Centers for Disease Control and Prevention, 28.6% of children in this age group have received both initial doses of Pfizer’s COVID-19 vaccine, and 35.3% have received their first dose.
Pfizer’s vaccine trial involving 4,500 children showed few side effects among children younger than 12 who received a booster, or third dose, according to a company statement.
Pfizer asked the FDA for an amended authorization in April, after submitting data showing that a third dose in children between 5 and 11 raised antibodies targeting the Omicron variant by 36 times.
“While it has largely been the case that COVID-19 tends to be less severe in children than adults, the omicron wave has seen more kids getting sick with the disease and being hospitalized, and children may also experience longer-term effects, even following initially mild disease,” FDA Commissioner Robert M. Califf, MD, said in a news release.
A study done by the New York State Department of Health showed the effectiveness of Pfizer’s two-dose vaccine series fell from 68% to 12% 4-5 months after the second dose was given to children 5 to 11 during the Omicron surge. A CDC study published in March also showed that the Pfizer shot reduced the risk of Omicron by 31% in children 5 to 11, a significantly lower rate than for kids 12 to 15, who had a 59% risk reduction after receiving two doses.
To some experts, this data suggest an even greater need for children under 12 to be eligible for a third dose.
“Since authorizing the vaccine for children down to 5 years of age in October 2021, emerging data suggest that vaccine effectiveness against COVID-19 wanes after the second dose of the vaccine in all authorized populations,” says Peter Marks, MD, PhD, the director of the FDA’s Center for Biologics Evaluation and Research.
The CDC still needs to sign off on the shots before they can be allowed. The agency’s Advisory Committee on Immunization Practices is set to meet on May 19 to discuss boosters in this age group.
FDA advisory panels plan to meet next month to discuss allowing Pfizer’s and Moderna’s COVID-19 vaccines for children under 6 years old.
A version of this article first appeared on WebMD.com.
emergency use authorization (EUA), allowing the Pfizer-BioNTech COVID-19 booster shot for children ages 5 to 11 who are at least 5 months out from their first vaccine series.
According to the most recent data from the Centers for Disease Control and Prevention, 28.6% of children in this age group have received both initial doses of Pfizer’s COVID-19 vaccine, and 35.3% have received their first dose.
Pfizer’s vaccine trial involving 4,500 children showed few side effects among children younger than 12 who received a booster, or third dose, according to a company statement.
Pfizer asked the FDA for an amended authorization in April, after submitting data showing that a third dose in children between 5 and 11 raised antibodies targeting the Omicron variant by 36 times.
“While it has largely been the case that COVID-19 tends to be less severe in children than adults, the omicron wave has seen more kids getting sick with the disease and being hospitalized, and children may also experience longer-term effects, even following initially mild disease,” FDA Commissioner Robert M. Califf, MD, said in a news release.
A study done by the New York State Department of Health showed the effectiveness of Pfizer’s two-dose vaccine series fell from 68% to 12% 4-5 months after the second dose was given to children 5 to 11 during the Omicron surge. A CDC study published in March also showed that the Pfizer shot reduced the risk of Omicron by 31% in children 5 to 11, a significantly lower rate than for kids 12 to 15, who had a 59% risk reduction after receiving two doses.
To some experts, this data suggest an even greater need for children under 12 to be eligible for a third dose.
“Since authorizing the vaccine for children down to 5 years of age in October 2021, emerging data suggest that vaccine effectiveness against COVID-19 wanes after the second dose of the vaccine in all authorized populations,” says Peter Marks, MD, PhD, the director of the FDA’s Center for Biologics Evaluation and Research.
The CDC still needs to sign off on the shots before they can be allowed. The agency’s Advisory Committee on Immunization Practices is set to meet on May 19 to discuss boosters in this age group.
FDA advisory panels plan to meet next month to discuss allowing Pfizer’s and Moderna’s COVID-19 vaccines for children under 6 years old.
A version of this article first appeared on WebMD.com.
FDA working to improve U.S. baby formula supply
The Food and Drug Administration announced on May 10 that it is taking several steps to improve the supply of baby formula in the United States.
The nationwide formula shortage has grown worse in recent weeks due to supply chain issues and a recall of certain Abbott Nutrition products, including major labels such as Similac, Alimentum, and EleCare.
“We recognize that many consumers have been unable to access infant formula and critical medical foods they are accustomed to using and are frustrated by their inability to do so,” FDA Commissioner Robert Califf, MD, said in a statement.
“We are doing everything in our power to ensure there is adequate product available where and when they need it,” he said.
About three-quarters of babies are fed formula for the first 6 months of their lives as a substitute for human milk, Axios reported.
In mid-February, the FDA warned consumers not to use certain powdered infant formula products from Abbott’s facility in Sturgis, Mich. Since then, the FDA has been working with Abbott and other manufacturers to increase the supply in the U.S. market.
“In fact, other infant formula manufacturers are meeting or exceeding capacity levels to meet current demand,” the FDA said in the statement. “Notably, more infant formula was purchased in the month of April than in the month prior to the recall.”
The FDA released a list of steps the agency is taking to increase supply, such as meeting with major infant formula makers to increase output and prioritize product lines in high demand, particularly specialty formulas for infants with allergies or specific diet needs.
But other manufacturers have struggled to quickly increase production because their operations tend to focus on a steady level of supply, according to The New York Times.
“Some industries are very good at ramping up and ramping down,” Rudi Leuschner, PhD, an associate professor of supply chain management at Rutgers Business School, Newark, N.J., told the newspaper.
“You flip a switch and they can produce 10 times as much,” he said. “Baby formula is not that type of a product.”
The FDA is also keeping an eye on the infant formula shortage by using the agency’s 21 Forward food supply chain continuity system. The system was developed during the pandemic to provide a full understanding of how COVID-19 is impacting food supply chains, the FDA said.
The FDA is compiling data on trends for in-stock rates at national and regional levels to understand where infant formula is available and where it should go.
Products are also being brought in from other countries, the FDA said. The agency is trying to speed up the process to get more formula into the U.S. and move it more quickly around the country.
For babies on a special diet, the FDA has decided to release some Abbott products that have been on hold at the Sturgis facility to those who need an urgent supply of metabolic formulas, on a case-by-case basis.
“In these circumstances, the benefit of allowing caregivers, in consultation with their health care providers, to access these products may outweigh the potential risk of bacterial infection,” the FDA said in the statement.
The FDA continues to advise against making homemade infant formulas and recommends talking to the child’s health care provider for recommendations on changing feeding practices or switching to other formulas, if necessary.
A version of this article first appeared on WebMd.com.
The Food and Drug Administration announced on May 10 that it is taking several steps to improve the supply of baby formula in the United States.
The nationwide formula shortage has grown worse in recent weeks due to supply chain issues and a recall of certain Abbott Nutrition products, including major labels such as Similac, Alimentum, and EleCare.
“We recognize that many consumers have been unable to access infant formula and critical medical foods they are accustomed to using and are frustrated by their inability to do so,” FDA Commissioner Robert Califf, MD, said in a statement.
“We are doing everything in our power to ensure there is adequate product available where and when they need it,” he said.
About three-quarters of babies are fed formula for the first 6 months of their lives as a substitute for human milk, Axios reported.
In mid-February, the FDA warned consumers not to use certain powdered infant formula products from Abbott’s facility in Sturgis, Mich. Since then, the FDA has been working with Abbott and other manufacturers to increase the supply in the U.S. market.
“In fact, other infant formula manufacturers are meeting or exceeding capacity levels to meet current demand,” the FDA said in the statement. “Notably, more infant formula was purchased in the month of April than in the month prior to the recall.”
The FDA released a list of steps the agency is taking to increase supply, such as meeting with major infant formula makers to increase output and prioritize product lines in high demand, particularly specialty formulas for infants with allergies or specific diet needs.
But other manufacturers have struggled to quickly increase production because their operations tend to focus on a steady level of supply, according to The New York Times.
“Some industries are very good at ramping up and ramping down,” Rudi Leuschner, PhD, an associate professor of supply chain management at Rutgers Business School, Newark, N.J., told the newspaper.
“You flip a switch and they can produce 10 times as much,” he said. “Baby formula is not that type of a product.”
The FDA is also keeping an eye on the infant formula shortage by using the agency’s 21 Forward food supply chain continuity system. The system was developed during the pandemic to provide a full understanding of how COVID-19 is impacting food supply chains, the FDA said.
The FDA is compiling data on trends for in-stock rates at national and regional levels to understand where infant formula is available and where it should go.
Products are also being brought in from other countries, the FDA said. The agency is trying to speed up the process to get more formula into the U.S. and move it more quickly around the country.
For babies on a special diet, the FDA has decided to release some Abbott products that have been on hold at the Sturgis facility to those who need an urgent supply of metabolic formulas, on a case-by-case basis.
“In these circumstances, the benefit of allowing caregivers, in consultation with their health care providers, to access these products may outweigh the potential risk of bacterial infection,” the FDA said in the statement.
The FDA continues to advise against making homemade infant formulas and recommends talking to the child’s health care provider for recommendations on changing feeding practices or switching to other formulas, if necessary.
A version of this article first appeared on WebMd.com.
The Food and Drug Administration announced on May 10 that it is taking several steps to improve the supply of baby formula in the United States.
The nationwide formula shortage has grown worse in recent weeks due to supply chain issues and a recall of certain Abbott Nutrition products, including major labels such as Similac, Alimentum, and EleCare.
“We recognize that many consumers have been unable to access infant formula and critical medical foods they are accustomed to using and are frustrated by their inability to do so,” FDA Commissioner Robert Califf, MD, said in a statement.
“We are doing everything in our power to ensure there is adequate product available where and when they need it,” he said.
About three-quarters of babies are fed formula for the first 6 months of their lives as a substitute for human milk, Axios reported.
In mid-February, the FDA warned consumers not to use certain powdered infant formula products from Abbott’s facility in Sturgis, Mich. Since then, the FDA has been working with Abbott and other manufacturers to increase the supply in the U.S. market.
“In fact, other infant formula manufacturers are meeting or exceeding capacity levels to meet current demand,” the FDA said in the statement. “Notably, more infant formula was purchased in the month of April than in the month prior to the recall.”
The FDA released a list of steps the agency is taking to increase supply, such as meeting with major infant formula makers to increase output and prioritize product lines in high demand, particularly specialty formulas for infants with allergies or specific diet needs.
But other manufacturers have struggled to quickly increase production because their operations tend to focus on a steady level of supply, according to The New York Times.
“Some industries are very good at ramping up and ramping down,” Rudi Leuschner, PhD, an associate professor of supply chain management at Rutgers Business School, Newark, N.J., told the newspaper.
“You flip a switch and they can produce 10 times as much,” he said. “Baby formula is not that type of a product.”
The FDA is also keeping an eye on the infant formula shortage by using the agency’s 21 Forward food supply chain continuity system. The system was developed during the pandemic to provide a full understanding of how COVID-19 is impacting food supply chains, the FDA said.
The FDA is compiling data on trends for in-stock rates at national and regional levels to understand where infant formula is available and where it should go.
Products are also being brought in from other countries, the FDA said. The agency is trying to speed up the process to get more formula into the U.S. and move it more quickly around the country.
For babies on a special diet, the FDA has decided to release some Abbott products that have been on hold at the Sturgis facility to those who need an urgent supply of metabolic formulas, on a case-by-case basis.
“In these circumstances, the benefit of allowing caregivers, in consultation with their health care providers, to access these products may outweigh the potential risk of bacterial infection,” the FDA said in the statement.
The FDA continues to advise against making homemade infant formulas and recommends talking to the child’s health care provider for recommendations on changing feeding practices or switching to other formulas, if necessary.
A version of this article first appeared on WebMd.com.