User login
Defending access to reproductive health care
The 1973 Supreme Court of the United States (SCOTUS) decision in Roe v Wade was a landmark ruling,1 establishing that the United States Constitution provides a fundamental “right to privacy,” protecting pregnant people’s freedom to access all available reproductive health care options. Recognizing that the right to abortion was not absolute, the majority of justices supported a trimester system. In the first trimester, decisions about abortion care are fully controlled by patients and clinicians, and no government could place restrictions on access to abortion. In the second trimester, SCOTUS ruled that states may choose to regulate abortion to protect maternal health. (As an example of such state restrictions, in Massachusetts, for many years, but no longer, the state required that abortions occur in a hospital when the patient was between 18 and 24 weeks’ gestation in order to facilitate comprehensive emergency care for complications.) Beginning in the third trimester, a point at which a fetus could be viable, the Court ruled that a government could prohibit abortion except when an abortion was necessary to protect the life or health of the pregnant person. In 1992, the SCOTUS decision in Planned Parenthood v Casey2 rejected the trimester system, reaffirming the right to an abortion before fetal viability, and adopting a new standard that states may not create an undue burden on a person seeking an abortion b
If, as anticipated, the 2022 SCOTUS decision in Dobbs v Jackson Women’s Health Organization3 overturns the precedents set in Roe v Wade and Planned Parenthood v Casey, decisions on abortion law will be relegated to elected legislators and state courts.4 It is expected that at least 26 state legislatures and governors will enact stringent new restrictions on access to abortion. This cataclysmic reversal of judicial opinion creates a historic challenge to obstetrician-gynecologists and their patients and could threaten access to other vital reproductive services beyond abortion, like contraception. We will be fighting, state by state, for people’s right to access all available reproductive health procedures. This will also significantly affect the ability for providers in women’s reproductive health to obtain appropriate and necessary education and training in a critical skills. If access to safe abortion is restricted, we fear patients may be forced to consider unsafe abortion, raising the specter of a return to the 1960s, when an epidemic of unsafe abortion caused countless injuries and deaths.5,6
How do we best prepare for these challenges?
- We will need to be flexible and continually evolve our clinical practices to be adherent with state and local legislation and regulation.
- To reduce unintended pregnancies, we need to strengthen our efforts to ensure that every patient has ready access to all available contraceptive options with no out-of-pocket cost.
- When a contraceptive is desired, we will focus on educating people about effectiveness, and offering them highly reliable contraception, such as the implant or intrauterine devices.
- We need to ensure timely access to abortion if state-based laws permit abortion before 6 or 7 weeks’ gestation. Providing medication abortion without an in-person visit using a telehealth option would be one option to expand rapid access to early first trimester abortion.
- Clinicians in states with access to abortion services will need to collaborate with colleagues in states with restrictions on abortion services to improve patient access across state borders.
On a national level, advancing our effective advocacy in Congress may lead to national legislation passed and signed by the President. This could supersede most state laws prohibiting access to comprehensive women’s reproductive health and create a unified, national approach to abortion care, allowing for the appropriate training of all obstetrician-gynecologists. We will also need to develop teams in every state capable of advocating for laws that ensure access to all reproductive health care options. The American College of Obstetricians and Gynecologists has leaders trained and tasked with legislative advocacy in every state.7 This network will be a foundation upon which to build additional advocacy efforts.
As women’s health care professionals, our responsibility to our patients, is to work to ensure universal access to safe and effective comprehensive reproductive options, and to ensure that our workforce is prepared to meet the needs of our patients by defending the patient-clinician relationship. Abortion care saves lives of pregnant patients and reduces maternal morbidity.8 Access to safe abortion care as part of comprehensive reproductive services is an important component of health care. ●
- Roe v Wade, 410 U.S. 113 (1973).
- Planned Parenthood v Casey, 505 U.S. 833 (1992).
- Dobbs v Jackson Women’s Health Organization, 19-1392. https://www.supremecourt.gov/search .aspx?filename=/docket/docketfiles/html /public/19-1392.html. Accessed May 18, 2022.
- Gerstein J, Ward A. Supreme Court has voted to overturn abortion rights, draft opinion shows. Politico. May 5, 2022. Updated May 3, 2022.
- Gold RB. Lessons from before Roe: will past be prologue? Guttmacher Institute. March 1, 2003. https://www.guttmacher.org/gpr/2003/03 /lessons-roe-will-past-be-prologue. Accessed May 18, 2022.
- Edelin KC. Broken Justice: A True Story of Race, Sex and Revenge in a Boston Courtroom. Pond View Press; 2007.
- The American College of Obstetricians and Gynecologists. Get involved in your state. ACOG web site. https://www.acog.org/advocacy /get-involved/get-involved-in-your-state. Accessed May 18, 2022.
- Institute of Medicine (US) Committee on Improving Birth Outcomes. Bale JR, Stoll BJ, Lucas AO, eds. Reducing maternal mortality and morbidity. In: Improving Birth Outcomes: Meeting the Challenge in the Developing World. Washington, DC: National Academies Press (US); 2003.
The 1973 Supreme Court of the United States (SCOTUS) decision in Roe v Wade was a landmark ruling,1 establishing that the United States Constitution provides a fundamental “right to privacy,” protecting pregnant people’s freedom to access all available reproductive health care options. Recognizing that the right to abortion was not absolute, the majority of justices supported a trimester system. In the first trimester, decisions about abortion care are fully controlled by patients and clinicians, and no government could place restrictions on access to abortion. In the second trimester, SCOTUS ruled that states may choose to regulate abortion to protect maternal health. (As an example of such state restrictions, in Massachusetts, for many years, but no longer, the state required that abortions occur in a hospital when the patient was between 18 and 24 weeks’ gestation in order to facilitate comprehensive emergency care for complications.) Beginning in the third trimester, a point at which a fetus could be viable, the Court ruled that a government could prohibit abortion except when an abortion was necessary to protect the life or health of the pregnant person. In 1992, the SCOTUS decision in Planned Parenthood v Casey2 rejected the trimester system, reaffirming the right to an abortion before fetal viability, and adopting a new standard that states may not create an undue burden on a person seeking an abortion b
If, as anticipated, the 2022 SCOTUS decision in Dobbs v Jackson Women’s Health Organization3 overturns the precedents set in Roe v Wade and Planned Parenthood v Casey, decisions on abortion law will be relegated to elected legislators and state courts.4 It is expected that at least 26 state legislatures and governors will enact stringent new restrictions on access to abortion. This cataclysmic reversal of judicial opinion creates a historic challenge to obstetrician-gynecologists and their patients and could threaten access to other vital reproductive services beyond abortion, like contraception. We will be fighting, state by state, for people’s right to access all available reproductive health procedures. This will also significantly affect the ability for providers in women’s reproductive health to obtain appropriate and necessary education and training in a critical skills. If access to safe abortion is restricted, we fear patients may be forced to consider unsafe abortion, raising the specter of a return to the 1960s, when an epidemic of unsafe abortion caused countless injuries and deaths.5,6
How do we best prepare for these challenges?
- We will need to be flexible and continually evolve our clinical practices to be adherent with state and local legislation and regulation.
- To reduce unintended pregnancies, we need to strengthen our efforts to ensure that every patient has ready access to all available contraceptive options with no out-of-pocket cost.
- When a contraceptive is desired, we will focus on educating people about effectiveness, and offering them highly reliable contraception, such as the implant or intrauterine devices.
- We need to ensure timely access to abortion if state-based laws permit abortion before 6 or 7 weeks’ gestation. Providing medication abortion without an in-person visit using a telehealth option would be one option to expand rapid access to early first trimester abortion.
- Clinicians in states with access to abortion services will need to collaborate with colleagues in states with restrictions on abortion services to improve patient access across state borders.
On a national level, advancing our effective advocacy in Congress may lead to national legislation passed and signed by the President. This could supersede most state laws prohibiting access to comprehensive women’s reproductive health and create a unified, national approach to abortion care, allowing for the appropriate training of all obstetrician-gynecologists. We will also need to develop teams in every state capable of advocating for laws that ensure access to all reproductive health care options. The American College of Obstetricians and Gynecologists has leaders trained and tasked with legislative advocacy in every state.7 This network will be a foundation upon which to build additional advocacy efforts.
As women’s health care professionals, our responsibility to our patients, is to work to ensure universal access to safe and effective comprehensive reproductive options, and to ensure that our workforce is prepared to meet the needs of our patients by defending the patient-clinician relationship. Abortion care saves lives of pregnant patients and reduces maternal morbidity.8 Access to safe abortion care as part of comprehensive reproductive services is an important component of health care. ●
The 1973 Supreme Court of the United States (SCOTUS) decision in Roe v Wade was a landmark ruling,1 establishing that the United States Constitution provides a fundamental “right to privacy,” protecting pregnant people’s freedom to access all available reproductive health care options. Recognizing that the right to abortion was not absolute, the majority of justices supported a trimester system. In the first trimester, decisions about abortion care are fully controlled by patients and clinicians, and no government could place restrictions on access to abortion. In the second trimester, SCOTUS ruled that states may choose to regulate abortion to protect maternal health. (As an example of such state restrictions, in Massachusetts, for many years, but no longer, the state required that abortions occur in a hospital when the patient was between 18 and 24 weeks’ gestation in order to facilitate comprehensive emergency care for complications.) Beginning in the third trimester, a point at which a fetus could be viable, the Court ruled that a government could prohibit abortion except when an abortion was necessary to protect the life or health of the pregnant person. In 1992, the SCOTUS decision in Planned Parenthood v Casey2 rejected the trimester system, reaffirming the right to an abortion before fetal viability, and adopting a new standard that states may not create an undue burden on a person seeking an abortion b
If, as anticipated, the 2022 SCOTUS decision in Dobbs v Jackson Women’s Health Organization3 overturns the precedents set in Roe v Wade and Planned Parenthood v Casey, decisions on abortion law will be relegated to elected legislators and state courts.4 It is expected that at least 26 state legislatures and governors will enact stringent new restrictions on access to abortion. This cataclysmic reversal of judicial opinion creates a historic challenge to obstetrician-gynecologists and their patients and could threaten access to other vital reproductive services beyond abortion, like contraception. We will be fighting, state by state, for people’s right to access all available reproductive health procedures. This will also significantly affect the ability for providers in women’s reproductive health to obtain appropriate and necessary education and training in a critical skills. If access to safe abortion is restricted, we fear patients may be forced to consider unsafe abortion, raising the specter of a return to the 1960s, when an epidemic of unsafe abortion caused countless injuries and deaths.5,6
How do we best prepare for these challenges?
- We will need to be flexible and continually evolve our clinical practices to be adherent with state and local legislation and regulation.
- To reduce unintended pregnancies, we need to strengthen our efforts to ensure that every patient has ready access to all available contraceptive options with no out-of-pocket cost.
- When a contraceptive is desired, we will focus on educating people about effectiveness, and offering them highly reliable contraception, such as the implant or intrauterine devices.
- We need to ensure timely access to abortion if state-based laws permit abortion before 6 or 7 weeks’ gestation. Providing medication abortion without an in-person visit using a telehealth option would be one option to expand rapid access to early first trimester abortion.
- Clinicians in states with access to abortion services will need to collaborate with colleagues in states with restrictions on abortion services to improve patient access across state borders.
On a national level, advancing our effective advocacy in Congress may lead to national legislation passed and signed by the President. This could supersede most state laws prohibiting access to comprehensive women’s reproductive health and create a unified, national approach to abortion care, allowing for the appropriate training of all obstetrician-gynecologists. We will also need to develop teams in every state capable of advocating for laws that ensure access to all reproductive health care options. The American College of Obstetricians and Gynecologists has leaders trained and tasked with legislative advocacy in every state.7 This network will be a foundation upon which to build additional advocacy efforts.
As women’s health care professionals, our responsibility to our patients, is to work to ensure universal access to safe and effective comprehensive reproductive options, and to ensure that our workforce is prepared to meet the needs of our patients by defending the patient-clinician relationship. Abortion care saves lives of pregnant patients and reduces maternal morbidity.8 Access to safe abortion care as part of comprehensive reproductive services is an important component of health care. ●
- Roe v Wade, 410 U.S. 113 (1973).
- Planned Parenthood v Casey, 505 U.S. 833 (1992).
- Dobbs v Jackson Women’s Health Organization, 19-1392. https://www.supremecourt.gov/search .aspx?filename=/docket/docketfiles/html /public/19-1392.html. Accessed May 18, 2022.
- Gerstein J, Ward A. Supreme Court has voted to overturn abortion rights, draft opinion shows. Politico. May 5, 2022. Updated May 3, 2022.
- Gold RB. Lessons from before Roe: will past be prologue? Guttmacher Institute. March 1, 2003. https://www.guttmacher.org/gpr/2003/03 /lessons-roe-will-past-be-prologue. Accessed May 18, 2022.
- Edelin KC. Broken Justice: A True Story of Race, Sex and Revenge in a Boston Courtroom. Pond View Press; 2007.
- The American College of Obstetricians and Gynecologists. Get involved in your state. ACOG web site. https://www.acog.org/advocacy /get-involved/get-involved-in-your-state. Accessed May 18, 2022.
- Institute of Medicine (US) Committee on Improving Birth Outcomes. Bale JR, Stoll BJ, Lucas AO, eds. Reducing maternal mortality and morbidity. In: Improving Birth Outcomes: Meeting the Challenge in the Developing World. Washington, DC: National Academies Press (US); 2003.
- Roe v Wade, 410 U.S. 113 (1973).
- Planned Parenthood v Casey, 505 U.S. 833 (1992).
- Dobbs v Jackson Women’s Health Organization, 19-1392. https://www.supremecourt.gov/search .aspx?filename=/docket/docketfiles/html /public/19-1392.html. Accessed May 18, 2022.
- Gerstein J, Ward A. Supreme Court has voted to overturn abortion rights, draft opinion shows. Politico. May 5, 2022. Updated May 3, 2022.
- Gold RB. Lessons from before Roe: will past be prologue? Guttmacher Institute. March 1, 2003. https://www.guttmacher.org/gpr/2003/03 /lessons-roe-will-past-be-prologue. Accessed May 18, 2022.
- Edelin KC. Broken Justice: A True Story of Race, Sex and Revenge in a Boston Courtroom. Pond View Press; 2007.
- The American College of Obstetricians and Gynecologists. Get involved in your state. ACOG web site. https://www.acog.org/advocacy /get-involved/get-involved-in-your-state. Accessed May 18, 2022.
- Institute of Medicine (US) Committee on Improving Birth Outcomes. Bale JR, Stoll BJ, Lucas AO, eds. Reducing maternal mortality and morbidity. In: Improving Birth Outcomes: Meeting the Challenge in the Developing World. Washington, DC: National Academies Press (US); 2003.
2021 Update on pelvic floor disorders
With the increasing prevalence of pelvic floor disorders among our aging population, women’s health clinicians should be prepared to counsel patients on treatment options and posttreatment expectations. In this Update, we will review recent literature on surgical treatments for pelvic organ prolapse (POP) and stress urinary incontinence (SUI). We also include our review of an award-winning and practice-changing study on office-based pessary care. Lastly, we will finish with a summary of a recent Society of Gynecologic Surgeons collaborative systematic review on sexual function after surgery.
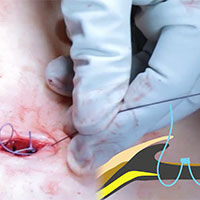
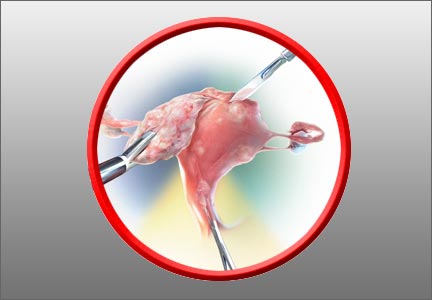
5-year RCT data on hysteropexy vs hysterectomy for POP
Nager CW, Visco AG, Richter HE, et al; National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Effect of sacrospinous hysteropexy with graft vs vaginal hysterectomy with uterosacral ligament suspension on treatment failure in women with uterovaginal prolapse: 5-year results of a randomized clinical trial. Am J Obstet Gynecol. 2021;225:153. e1-153.e31. doi: 10.1016/j.ajog.2021.03.012.
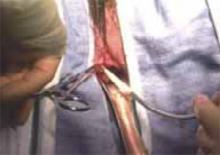
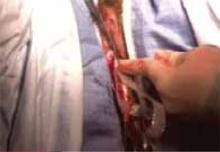
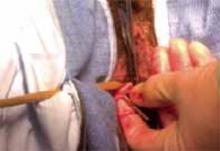
The Pelvic Floor Disorders Network conducted a multisite randomized superiority trial comparing sacrospinous hysteropexy with mesh graft to vaginal hysterectomy with uterosacral ligament suspension for POP.
Study details
Postmenopausal women who desired surgery for symptomatic uterovaginal prolapse were randomly assigned to sacrospinous hysteropexy with polypropylene mesh graft using the Uphold-LITE device (Boston Scientific) versus vaginal hysterectomy with uterosacral ligament suspension. Participants were masked to treatment allocation and completed study visits at 6-month intervals through 60 months. Quantitative prolapse POP-Q exams were performed and patients completed multiple validated questionnaires regarding the presence; severity; and impact of prolapse, urinary, bowel, and pelvic pain symptoms.
Results
A total of 183 postmenopausal women were randomized, and 156 (81 hysteropexy and 75 hysterectomy) patients completed 5-year follow up with no demographic differences between the 2 intervention groups. Operative time was statistically less in the hysteropexy group (111.5 min vs 156.7 min). There were fewer treatment failures (a composite including retreatment for prolapse, prolapse beyond the hymen, and/or bothersome bulge symptoms) in the hysteropexy than in the hysterectomy group (37% vs 54%, respectively) at 5 years of follow up. However, most patients with treatment failure were classified as an intermittent failure, with only 16% of hysteropexy patients and 22% of hysterectomy patients classified as persistent failures. There were no meaningful differences between patient-reported outcomes. Hysteropexy had an 8% mesh exposure risk, with none requiring surgical management.
This study represents the highest quality randomized trial design and boasts high patient retention rates and 5-year follow up. Findings support further investigation on the use of polypropylene mesh for POP. In April of 2019, the US Food and Drug Administration halted the selling and distribution of vaginal mesh products for prolapse repair given the lack of safety outcomes, concerns about mesh exposure rates, and possible increased rates of pelvic pain and adverse events. This study invites pelvic reconstructive surgeons to revisit the debate of hysteropexy versus hysterectomy and synthetic mesh versus native tissue repairs. The 8% mesh exposure rate represents a challenge for the future design and development of vaginal implant materials, weighing the balancing of improved long-term efficacy with the safety and complication concerns.
Continue to: Preliminary 12-month data for a single-incision sling for surgical management of SUI...
Preliminary 12-month data for a single-incision sling for surgical management of SUI
Erickson T, Roovers JP, Gheiler E, et al. A multicenter prospective study evaluating efficacy and safety of a single-incision sling procedure for stress urinary incontinence. J Minim Invasive Gynecol. 2021;28:93-99. doi: 10.1016/j.jmig.2020.04.014.
In this industry-sponsored study, researchers compared a novel single-incision sling to currently available midurethral slings for SUI with 12-month outcomes and adverse event details. However, results are primarily descriptive with no statistical testing.
Study details
Patients were eligible for inclusion in this prospective, nonrandomized cohort study if SUI was their primary incontinence symptom, with confirmatory office testing. Exclusion criteria included POP greater than stage 2, prior SUI surgery, plans for future pregnancy, elevated postvoid residuals, or concomitant surgical procedures. The single-incision Altis (Coloplast) sling was compared to all commercially available transobturator and retropubic midurethral slings. The primary outcome of this study was reduction in 24-hour pad weights, and secondary outcomes included negative cough-stress test and subjective patient-reported outcomes via validated questionnaires.
Results
A total of 184 women were enrolled in the Altis group and 171 in the comparator other sling group. Symptom severity was similar between groups, but more patients in the comparator group had mixed urinary incontinence, and more patients in the Altis group had intrinsic sphincter deficiency. The Altis group had a higher proportion of “dry patients,” but otherwise the outcomes were similar between the 2 groups, including negative cough-stress test and patientreported outcomes. Two patients in the Altis group and 7 patients in the comparator group underwent device revisions. Again, statistical analysis was not performed.
Single-incision slings may reduce the risk of groin pain associated with transobturator slings and may be a good option for patients who desire less mesh burden than the traditional retropubic slings or who are not good candidates. This trial suggests that the Altis single-incision sling may be similar in outcomes and adverse events to currently available midurethral slings, but further, more rigorous trials are underway to fully evaluate this—including a US-based multicenter randomized trial of Altis single-incision slings versus retropubic slings (ClinicalTrials.gov Identifier: NCT03520114).
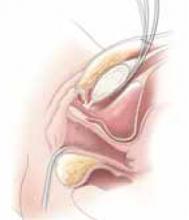
Office-based pessary care can be safely spaced out to 24 weeks without an increase in erosions
Propst K, Mellen C, O’Sullivan DM, et al. Timing of office-based pessary care: a randomized controlled trial. Obstet Gynecol. 2020;135:100-105. doi: 10.1097 /AOG.0000000000003580.
For women already using a pessary without issues, extending office visits to every 6 months does not increase rates of vaginal epithelial abnormalities, according to results of this randomized controlled trial.
Study details
Women already using a Gelhorn, ring, or incontinence dish pessary for POP, SUI, or both were randomized to continue routine care with office evaluation every 12 weeks versus the extended-care cohort (with office evaluation every 24 weeks). Women were excluded if they removed and replaced the pessary themselves or if there was a presence of vaginal epithelial abnormalities, such as erosion or granulation tissue.
Results
The rate of vaginal epithelium erosion was 7.4% in the routine arm and 1.7% in the extended-care arm, meeting criteria for noninferiority of extended care. The majority of patients with office visits every 24 weeks preferred the less frequent examinations, and there was no difference in degree of bother due to vaginal discharge. There was also no difference in the percentage of patients with unscheduled visits. The only factors associated with vaginal epithelium abnormalities were prior abnormalities and lifetime duration of pessary use.
As there are currently no evidenced-based guidelines for pessary care, this study contributes data to support extended office-based care up to 24 weeks, a common practice in the United Kingdom. During the COVID-19 pandemic, with reduced health care access, these findings should be reassuring to clinicians and patients.
Continue to: How can we counsel patients regarding changes in sexual activity and function after surgery for POP?...
How can we counsel patients regarding changes in sexual activity and function after surgery for POP?
Antosh DD, Dieter AA, Balk EM, et al. Sexual function after pelvic organ prolapse surgery: a systematic review comparing different approaches to pelvic floor repair. Am J Obstet Gynecol. 2021;2:S0002-9378(21)00610-4. doi: 10.1016/j.ajog.2021.05.042.
A secondary analysis of a recent systematic review found overall moderate- to high-quality evidence that were no differences in total dyspareunia, de novo dyspareunia, and scores on a validated sexual function questionnaire (PISQ12) when comparing postoperative sexual function outcomes of native tissue repair to sacrocolpopexy, transvaginal mesh, or biologic graft. Rates of postoperative dyspareunia were higher for transvaginal mesh than for sacrocolpopexy.
Study details
The Society of Gynecologic Surgeons Systematic Review Group identified 43 original prospective, comparative studies of reconstructive prolapse surgery that reported sexual function outcomes when comparing 2 different types of POP procedures. Thirty-seven of those studies were randomized controlled trials. Specifically, they looked at data comparing outcomes for native tissue versus sacrocolpopexy, native tissue versus transvaginal mesh, native tissue versus biologic graft, and transvaginal mesh versus sacrocolpopexy.
Results
Overall, the prevalence of postoperative dyspareunia was lower than preoperatively after all surgery types. The only statistical difference in this review demonstrated higher postoperative prevalence of dyspareunia after transvaginal mesh than sacrocolpopexy, based on 2 studies. When comparing native tissue prolapse repair to transvaginal mesh, sacrocolpopexy, or biologic grafts, there were no significant differences in sexual activity, baseline, or postoperative total dyspareunia, de-novo dyspareunia, or sexual function changes as measured by the PISQ12 validated questionnaire. ●
This systematic review further contributes to the growing evidence that, regardless of surgical approach to POP, sexual function generally improves and dyspareunia rates generally decrease postoperatively, with overall low rates of de novo dyspareunia. This will help patients and providers select the best-fit surgical approach without concern for worsened sexual function. It also underscores the need for inclusion of standardized sexual function terminology use and sexual health outcomes in future prolapse surgery research.

With the increasing prevalence of pelvic floor disorders among our aging population, women’s health clinicians should be prepared to counsel patients on treatment options and posttreatment expectations. In this Update, we will review recent literature on surgical treatments for pelvic organ prolapse (POP) and stress urinary incontinence (SUI). We also include our review of an award-winning and practice-changing study on office-based pessary care. Lastly, we will finish with a summary of a recent Society of Gynecologic Surgeons collaborative systematic review on sexual function after surgery.
5-year RCT data on hysteropexy vs hysterectomy for POP
Nager CW, Visco AG, Richter HE, et al; National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Effect of sacrospinous hysteropexy with graft vs vaginal hysterectomy with uterosacral ligament suspension on treatment failure in women with uterovaginal prolapse: 5-year results of a randomized clinical trial. Am J Obstet Gynecol. 2021;225:153. e1-153.e31. doi: 10.1016/j.ajog.2021.03.012.
The Pelvic Floor Disorders Network conducted a multisite randomized superiority trial comparing sacrospinous hysteropexy with mesh graft to vaginal hysterectomy with uterosacral ligament suspension for POP.
Study details
Postmenopausal women who desired surgery for symptomatic uterovaginal prolapse were randomly assigned to sacrospinous hysteropexy with polypropylene mesh graft using the Uphold-LITE device (Boston Scientific) versus vaginal hysterectomy with uterosacral ligament suspension. Participants were masked to treatment allocation and completed study visits at 6-month intervals through 60 months. Quantitative prolapse POP-Q exams were performed and patients completed multiple validated questionnaires regarding the presence; severity; and impact of prolapse, urinary, bowel, and pelvic pain symptoms.
Results
A total of 183 postmenopausal women were randomized, and 156 (81 hysteropexy and 75 hysterectomy) patients completed 5-year follow up with no demographic differences between the 2 intervention groups. Operative time was statistically less in the hysteropexy group (111.5 min vs 156.7 min). There were fewer treatment failures (a composite including retreatment for prolapse, prolapse beyond the hymen, and/or bothersome bulge symptoms) in the hysteropexy than in the hysterectomy group (37% vs 54%, respectively) at 5 years of follow up. However, most patients with treatment failure were classified as an intermittent failure, with only 16% of hysteropexy patients and 22% of hysterectomy patients classified as persistent failures. There were no meaningful differences between patient-reported outcomes. Hysteropexy had an 8% mesh exposure risk, with none requiring surgical management.
This study represents the highest quality randomized trial design and boasts high patient retention rates and 5-year follow up. Findings support further investigation on the use of polypropylene mesh for POP. In April of 2019, the US Food and Drug Administration halted the selling and distribution of vaginal mesh products for prolapse repair given the lack of safety outcomes, concerns about mesh exposure rates, and possible increased rates of pelvic pain and adverse events. This study invites pelvic reconstructive surgeons to revisit the debate of hysteropexy versus hysterectomy and synthetic mesh versus native tissue repairs. The 8% mesh exposure rate represents a challenge for the future design and development of vaginal implant materials, weighing the balancing of improved long-term efficacy with the safety and complication concerns.
Continue to: Preliminary 12-month data for a single-incision sling for surgical management of SUI...
Preliminary 12-month data for a single-incision sling for surgical management of SUI
Erickson T, Roovers JP, Gheiler E, et al. A multicenter prospective study evaluating efficacy and safety of a single-incision sling procedure for stress urinary incontinence. J Minim Invasive Gynecol. 2021;28:93-99. doi: 10.1016/j.jmig.2020.04.014.
In this industry-sponsored study, researchers compared a novel single-incision sling to currently available midurethral slings for SUI with 12-month outcomes and adverse event details. However, results are primarily descriptive with no statistical testing.
Study details
Patients were eligible for inclusion in this prospective, nonrandomized cohort study if SUI was their primary incontinence symptom, with confirmatory office testing. Exclusion criteria included POP greater than stage 2, prior SUI surgery, plans for future pregnancy, elevated postvoid residuals, or concomitant surgical procedures. The single-incision Altis (Coloplast) sling was compared to all commercially available transobturator and retropubic midurethral slings. The primary outcome of this study was reduction in 24-hour pad weights, and secondary outcomes included negative cough-stress test and subjective patient-reported outcomes via validated questionnaires.
Results
A total of 184 women were enrolled in the Altis group and 171 in the comparator other sling group. Symptom severity was similar between groups, but more patients in the comparator group had mixed urinary incontinence, and more patients in the Altis group had intrinsic sphincter deficiency. The Altis group had a higher proportion of “dry patients,” but otherwise the outcomes were similar between the 2 groups, including negative cough-stress test and patientreported outcomes. Two patients in the Altis group and 7 patients in the comparator group underwent device revisions. Again, statistical analysis was not performed.
Single-incision slings may reduce the risk of groin pain associated with transobturator slings and may be a good option for patients who desire less mesh burden than the traditional retropubic slings or who are not good candidates. This trial suggests that the Altis single-incision sling may be similar in outcomes and adverse events to currently available midurethral slings, but further, more rigorous trials are underway to fully evaluate this—including a US-based multicenter randomized trial of Altis single-incision slings versus retropubic slings (ClinicalTrials.gov Identifier: NCT03520114).
Office-based pessary care can be safely spaced out to 24 weeks without an increase in erosions
Propst K, Mellen C, O’Sullivan DM, et al. Timing of office-based pessary care: a randomized controlled trial. Obstet Gynecol. 2020;135:100-105. doi: 10.1097 /AOG.0000000000003580.
For women already using a pessary without issues, extending office visits to every 6 months does not increase rates of vaginal epithelial abnormalities, according to results of this randomized controlled trial.
Study details
Women already using a Gelhorn, ring, or incontinence dish pessary for POP, SUI, or both were randomized to continue routine care with office evaluation every 12 weeks versus the extended-care cohort (with office evaluation every 24 weeks). Women were excluded if they removed and replaced the pessary themselves or if there was a presence of vaginal epithelial abnormalities, such as erosion or granulation tissue.
Results
The rate of vaginal epithelium erosion was 7.4% in the routine arm and 1.7% in the extended-care arm, meeting criteria for noninferiority of extended care. The majority of patients with office visits every 24 weeks preferred the less frequent examinations, and there was no difference in degree of bother due to vaginal discharge. There was also no difference in the percentage of patients with unscheduled visits. The only factors associated with vaginal epithelium abnormalities were prior abnormalities and lifetime duration of pessary use.
As there are currently no evidenced-based guidelines for pessary care, this study contributes data to support extended office-based care up to 24 weeks, a common practice in the United Kingdom. During the COVID-19 pandemic, with reduced health care access, these findings should be reassuring to clinicians and patients.
Continue to: How can we counsel patients regarding changes in sexual activity and function after surgery for POP?...
How can we counsel patients regarding changes in sexual activity and function after surgery for POP?
Antosh DD, Dieter AA, Balk EM, et al. Sexual function after pelvic organ prolapse surgery: a systematic review comparing different approaches to pelvic floor repair. Am J Obstet Gynecol. 2021;2:S0002-9378(21)00610-4. doi: 10.1016/j.ajog.2021.05.042.
A secondary analysis of a recent systematic review found overall moderate- to high-quality evidence that were no differences in total dyspareunia, de novo dyspareunia, and scores on a validated sexual function questionnaire (PISQ12) when comparing postoperative sexual function outcomes of native tissue repair to sacrocolpopexy, transvaginal mesh, or biologic graft. Rates of postoperative dyspareunia were higher for transvaginal mesh than for sacrocolpopexy.
Study details
The Society of Gynecologic Surgeons Systematic Review Group identified 43 original prospective, comparative studies of reconstructive prolapse surgery that reported sexual function outcomes when comparing 2 different types of POP procedures. Thirty-seven of those studies were randomized controlled trials. Specifically, they looked at data comparing outcomes for native tissue versus sacrocolpopexy, native tissue versus transvaginal mesh, native tissue versus biologic graft, and transvaginal mesh versus sacrocolpopexy.
Results
Overall, the prevalence of postoperative dyspareunia was lower than preoperatively after all surgery types. The only statistical difference in this review demonstrated higher postoperative prevalence of dyspareunia after transvaginal mesh than sacrocolpopexy, based on 2 studies. When comparing native tissue prolapse repair to transvaginal mesh, sacrocolpopexy, or biologic grafts, there were no significant differences in sexual activity, baseline, or postoperative total dyspareunia, de-novo dyspareunia, or sexual function changes as measured by the PISQ12 validated questionnaire. ●
This systematic review further contributes to the growing evidence that, regardless of surgical approach to POP, sexual function generally improves and dyspareunia rates generally decrease postoperatively, with overall low rates of de novo dyspareunia. This will help patients and providers select the best-fit surgical approach without concern for worsened sexual function. It also underscores the need for inclusion of standardized sexual function terminology use and sexual health outcomes in future prolapse surgery research.

With the increasing prevalence of pelvic floor disorders among our aging population, women’s health clinicians should be prepared to counsel patients on treatment options and posttreatment expectations. In this Update, we will review recent literature on surgical treatments for pelvic organ prolapse (POP) and stress urinary incontinence (SUI). We also include our review of an award-winning and practice-changing study on office-based pessary care. Lastly, we will finish with a summary of a recent Society of Gynecologic Surgeons collaborative systematic review on sexual function after surgery.
5-year RCT data on hysteropexy vs hysterectomy for POP
Nager CW, Visco AG, Richter HE, et al; National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Effect of sacrospinous hysteropexy with graft vs vaginal hysterectomy with uterosacral ligament suspension on treatment failure in women with uterovaginal prolapse: 5-year results of a randomized clinical trial. Am J Obstet Gynecol. 2021;225:153. e1-153.e31. doi: 10.1016/j.ajog.2021.03.012.
The Pelvic Floor Disorders Network conducted a multisite randomized superiority trial comparing sacrospinous hysteropexy with mesh graft to vaginal hysterectomy with uterosacral ligament suspension for POP.
Study details
Postmenopausal women who desired surgery for symptomatic uterovaginal prolapse were randomly assigned to sacrospinous hysteropexy with polypropylene mesh graft using the Uphold-LITE device (Boston Scientific) versus vaginal hysterectomy with uterosacral ligament suspension. Participants were masked to treatment allocation and completed study visits at 6-month intervals through 60 months. Quantitative prolapse POP-Q exams were performed and patients completed multiple validated questionnaires regarding the presence; severity; and impact of prolapse, urinary, bowel, and pelvic pain symptoms.
Results
A total of 183 postmenopausal women were randomized, and 156 (81 hysteropexy and 75 hysterectomy) patients completed 5-year follow up with no demographic differences between the 2 intervention groups. Operative time was statistically less in the hysteropexy group (111.5 min vs 156.7 min). There were fewer treatment failures (a composite including retreatment for prolapse, prolapse beyond the hymen, and/or bothersome bulge symptoms) in the hysteropexy than in the hysterectomy group (37% vs 54%, respectively) at 5 years of follow up. However, most patients with treatment failure were classified as an intermittent failure, with only 16% of hysteropexy patients and 22% of hysterectomy patients classified as persistent failures. There were no meaningful differences between patient-reported outcomes. Hysteropexy had an 8% mesh exposure risk, with none requiring surgical management.
This study represents the highest quality randomized trial design and boasts high patient retention rates and 5-year follow up. Findings support further investigation on the use of polypropylene mesh for POP. In April of 2019, the US Food and Drug Administration halted the selling and distribution of vaginal mesh products for prolapse repair given the lack of safety outcomes, concerns about mesh exposure rates, and possible increased rates of pelvic pain and adverse events. This study invites pelvic reconstructive surgeons to revisit the debate of hysteropexy versus hysterectomy and synthetic mesh versus native tissue repairs. The 8% mesh exposure rate represents a challenge for the future design and development of vaginal implant materials, weighing the balancing of improved long-term efficacy with the safety and complication concerns.
Continue to: Preliminary 12-month data for a single-incision sling for surgical management of SUI...
Preliminary 12-month data for a single-incision sling for surgical management of SUI
Erickson T, Roovers JP, Gheiler E, et al. A multicenter prospective study evaluating efficacy and safety of a single-incision sling procedure for stress urinary incontinence. J Minim Invasive Gynecol. 2021;28:93-99. doi: 10.1016/j.jmig.2020.04.014.
In this industry-sponsored study, researchers compared a novel single-incision sling to currently available midurethral slings for SUI with 12-month outcomes and adverse event details. However, results are primarily descriptive with no statistical testing.
Study details
Patients were eligible for inclusion in this prospective, nonrandomized cohort study if SUI was their primary incontinence symptom, with confirmatory office testing. Exclusion criteria included POP greater than stage 2, prior SUI surgery, plans for future pregnancy, elevated postvoid residuals, or concomitant surgical procedures. The single-incision Altis (Coloplast) sling was compared to all commercially available transobturator and retropubic midurethral slings. The primary outcome of this study was reduction in 24-hour pad weights, and secondary outcomes included negative cough-stress test and subjective patient-reported outcomes via validated questionnaires.
Results
A total of 184 women were enrolled in the Altis group and 171 in the comparator other sling group. Symptom severity was similar between groups, but more patients in the comparator group had mixed urinary incontinence, and more patients in the Altis group had intrinsic sphincter deficiency. The Altis group had a higher proportion of “dry patients,” but otherwise the outcomes were similar between the 2 groups, including negative cough-stress test and patientreported outcomes. Two patients in the Altis group and 7 patients in the comparator group underwent device revisions. Again, statistical analysis was not performed.
Single-incision slings may reduce the risk of groin pain associated with transobturator slings and may be a good option for patients who desire less mesh burden than the traditional retropubic slings or who are not good candidates. This trial suggests that the Altis single-incision sling may be similar in outcomes and adverse events to currently available midurethral slings, but further, more rigorous trials are underway to fully evaluate this—including a US-based multicenter randomized trial of Altis single-incision slings versus retropubic slings (ClinicalTrials.gov Identifier: NCT03520114).
Office-based pessary care can be safely spaced out to 24 weeks without an increase in erosions
Propst K, Mellen C, O’Sullivan DM, et al. Timing of office-based pessary care: a randomized controlled trial. Obstet Gynecol. 2020;135:100-105. doi: 10.1097 /AOG.0000000000003580.
For women already using a pessary without issues, extending office visits to every 6 months does not increase rates of vaginal epithelial abnormalities, according to results of this randomized controlled trial.
Study details
Women already using a Gelhorn, ring, or incontinence dish pessary for POP, SUI, or both were randomized to continue routine care with office evaluation every 12 weeks versus the extended-care cohort (with office evaluation every 24 weeks). Women were excluded if they removed and replaced the pessary themselves or if there was a presence of vaginal epithelial abnormalities, such as erosion or granulation tissue.
Results
The rate of vaginal epithelium erosion was 7.4% in the routine arm and 1.7% in the extended-care arm, meeting criteria for noninferiority of extended care. The majority of patients with office visits every 24 weeks preferred the less frequent examinations, and there was no difference in degree of bother due to vaginal discharge. There was also no difference in the percentage of patients with unscheduled visits. The only factors associated with vaginal epithelium abnormalities were prior abnormalities and lifetime duration of pessary use.
As there are currently no evidenced-based guidelines for pessary care, this study contributes data to support extended office-based care up to 24 weeks, a common practice in the United Kingdom. During the COVID-19 pandemic, with reduced health care access, these findings should be reassuring to clinicians and patients.
Continue to: How can we counsel patients regarding changes in sexual activity and function after surgery for POP?...
How can we counsel patients regarding changes in sexual activity and function after surgery for POP?
Antosh DD, Dieter AA, Balk EM, et al. Sexual function after pelvic organ prolapse surgery: a systematic review comparing different approaches to pelvic floor repair. Am J Obstet Gynecol. 2021;2:S0002-9378(21)00610-4. doi: 10.1016/j.ajog.2021.05.042.
A secondary analysis of a recent systematic review found overall moderate- to high-quality evidence that were no differences in total dyspareunia, de novo dyspareunia, and scores on a validated sexual function questionnaire (PISQ12) when comparing postoperative sexual function outcomes of native tissue repair to sacrocolpopexy, transvaginal mesh, or biologic graft. Rates of postoperative dyspareunia were higher for transvaginal mesh than for sacrocolpopexy.
Study details
The Society of Gynecologic Surgeons Systematic Review Group identified 43 original prospective, comparative studies of reconstructive prolapse surgery that reported sexual function outcomes when comparing 2 different types of POP procedures. Thirty-seven of those studies were randomized controlled trials. Specifically, they looked at data comparing outcomes for native tissue versus sacrocolpopexy, native tissue versus transvaginal mesh, native tissue versus biologic graft, and transvaginal mesh versus sacrocolpopexy.
Results
Overall, the prevalence of postoperative dyspareunia was lower than preoperatively after all surgery types. The only statistical difference in this review demonstrated higher postoperative prevalence of dyspareunia after transvaginal mesh than sacrocolpopexy, based on 2 studies. When comparing native tissue prolapse repair to transvaginal mesh, sacrocolpopexy, or biologic grafts, there were no significant differences in sexual activity, baseline, or postoperative total dyspareunia, de-novo dyspareunia, or sexual function changes as measured by the PISQ12 validated questionnaire. ●
This systematic review further contributes to the growing evidence that, regardless of surgical approach to POP, sexual function generally improves and dyspareunia rates generally decrease postoperatively, with overall low rates of de novo dyspareunia. This will help patients and providers select the best-fit surgical approach without concern for worsened sexual function. It also underscores the need for inclusion of standardized sexual function terminology use and sexual health outcomes in future prolapse surgery research.
How to choose the right vaginal moisturizer or lubricant for your patient
Vaginal dryness, encompassed in the modern term genitourinary syndrome of menopause (GSM) affects up to 40% of menopausal women and up to 60% of postmenopausal breast cancer survivors.1,2 Premenopausal women also can have vulvovaginal dryness while breastfeeding (lactational amenorrhea) and while taking low-dose contraceptives.3 Vaginal moisturizers and lubricants are the first-line treatment options for vaginal dryness, dyspareunia, and GSM.4,5 In fact, approximately two-thirds of women have reported using a vaginal lubricant in their lifetime.6 Despite such ubiquitous use, many health care providers and patients have questions about the difference between vaginal moisturizers and lubricants and how to best choose a product.
Vaginal moisturizers
Vaginal moisturizers are designed to rehydrate the vaginal epithelium. Much like facial or skin moisturizers, they are intended to be applied regularly, every 2 to 3 days, but may be applied more often depending on the severity of symptoms. Vaginal moisturizers work by increasing the fluid content of the vaginal tissue and by lowering the vaginal pH to mimic that of natural vaginal secretions. Vaginal moisturizers are typically water based and use polymers to hydrate tissues.7 They change cell morphology but do not change vaginal maturation, indicating that they bring water to the tissue but do not shift the balance between superficial and basal cells and do not increase vaginal epithelial thickness as seen with vaginal estrogen.8 Vaginal moisturizers also have been found to be a safe alternative to vaginal estrogen therapy and may improve markers of vaginal health, including vaginal moisture, vaginal fluid volume, vaginal elasticity, and premenopausal pH.9 Commercially available vaginal moisturizers have been shown to be as effective as vaginal estrogens in reducing vaginal symptoms such as itching, irritation, and dyspareunia, but some caution should be taken when interpreting these results as neither vaginal moisturizer nor vaginal estrogen tablet were more effective than placebo in a recent randomized controlled trial.10,11 Small studies on hyaluronic acid have shown efficacy for the treatment of vaginal dryness.12,13 Hyaluronic acid is commercially available as a vaginal suppository ovule and as a liquid. It may also be obtained from a reliable compounding pharmacy. Vaginal suppository ovules may be a preferable formulation for women who find the liquids messy or cumbersome to apply.
Lubricants
Lubricants differ from vaginal moisturizers because they are specifically designed to be used during intercourse to provide short-term relief from vaginal dryness. They may be water-, silicone-, mineral oil-, or plant oil-based. The use of water- and silicone-based lubricants is associated with high satisfaction for intercourse as well as masturbation.14 These products may be particularly beneficial to women whose chief complaint is dyspareunia. In fact, women with dyspareunia report more lubricant use than women without dyspareunia, and the most common reason for lubricant use among these women was to reduce or alleviate pain.15 Overall, women both with and without dyspareunia have a positive perception regarding lubricant use and prefer sexual intercourse that feels more “wet,” and women in their forties have the most positive perception about lubricant use at the time of intercourse compared with other age groups.16 Furthermore, the World Health Organization (WHO) recommends that condom-compatible lubricants be used with condoms for menopausal and postmenopausal women.17 Both water-based and silicone-based lubricants may be used with latex condoms, while oil-based lubricants should be avoided as they can degrade the latex condom. While vaginal moisturizers and lubricants technically differ based on use, patients may use one product for both purposes, and some products are marketed as both a moisturizer and lubricant.
Continue to: Providing counsel to patients...
Providing counsel to patients
Patients often seek advice on how to choose vaginal moisturizers and lubricants. Understanding the compositions of these products and their scientific evidence is useful when helping patients make informed decisions regarding their pelvic health. Most commercially available lubricants are either water- or silicone- based. In one study comparing these two types of lubricants, water-based lubricants were associated with fewer genital symptoms than silicone-based products.14 Women may want to use a natural or organic product and may prefer plant-based oils such as coconut oil or olive oil. Patients should be counseled that latex condoms are not compatible with petroleum-, mineral oil- or plant oil-based lubricants.
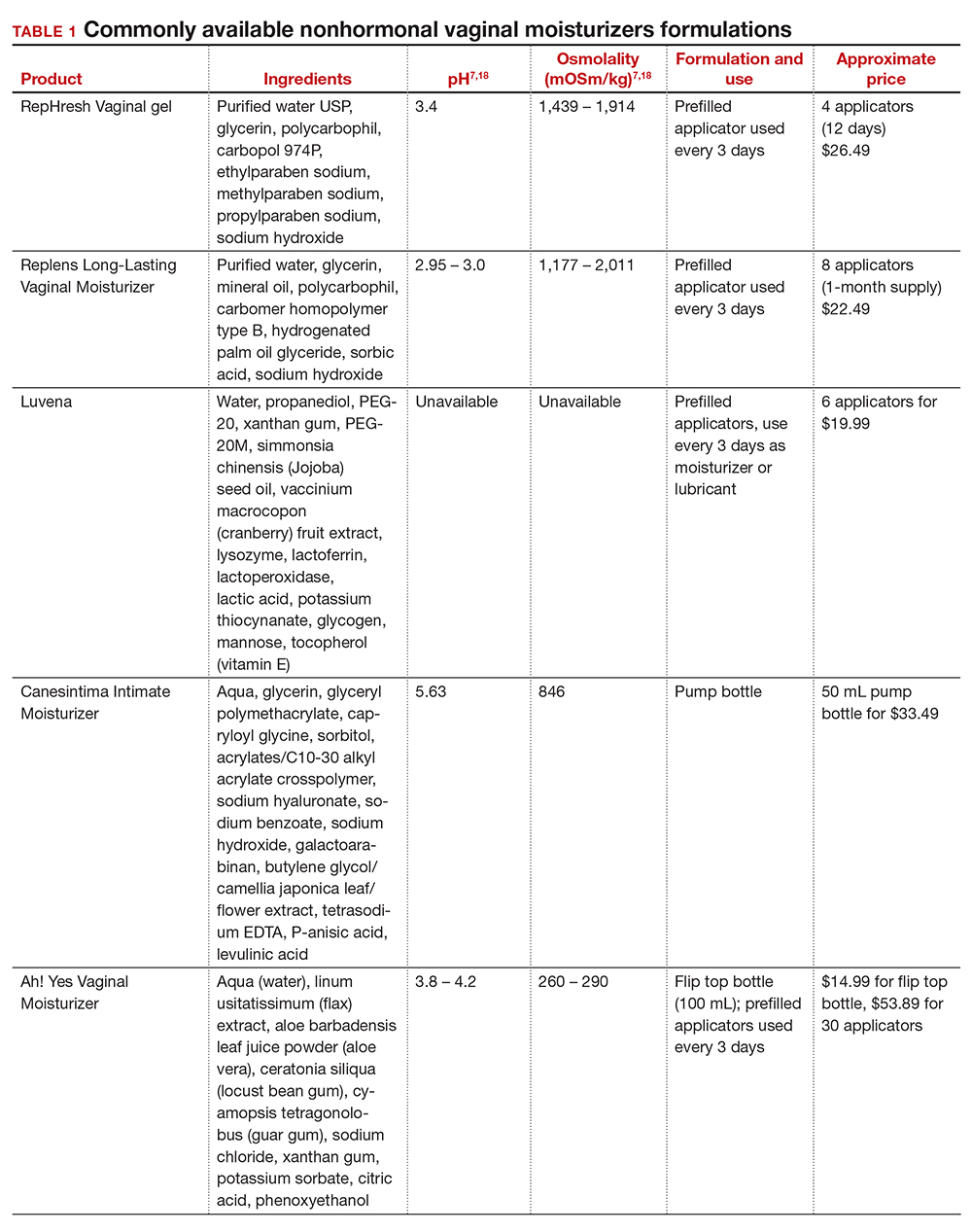
In our practice, we generally recommend silicone-based lubricants, as they are readily available and compatible with latex condoms and generally require a smaller amount than water-based lubricants. They tend to be more expensive than water-based lubricants. For vaginal moisturizers, we often recommend commercially available formulations that can be purchased at local pharmacies or drug stores. However, a patient may need to try different lubricants and moisturizers in order to find a preferred product. We have included in TABLES 1 and 27,17,18 a list of commercially available vaginal moisturizers and lubricants with ingredient list, pH, osmolality, common formulation, and cost when available, which has been compiled from WHO and published research data to help guide patient counseling.
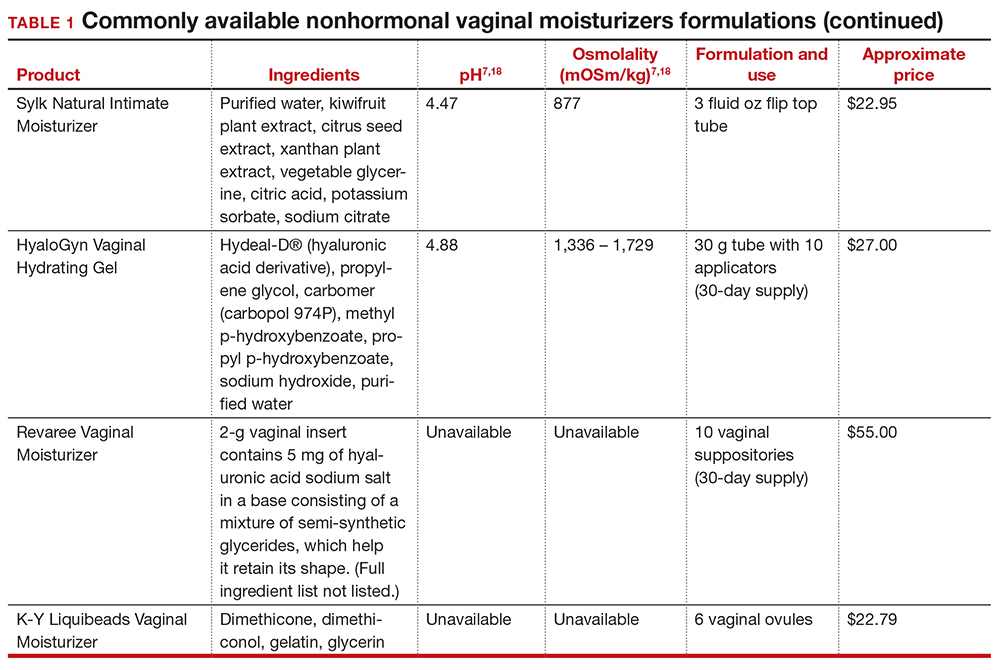
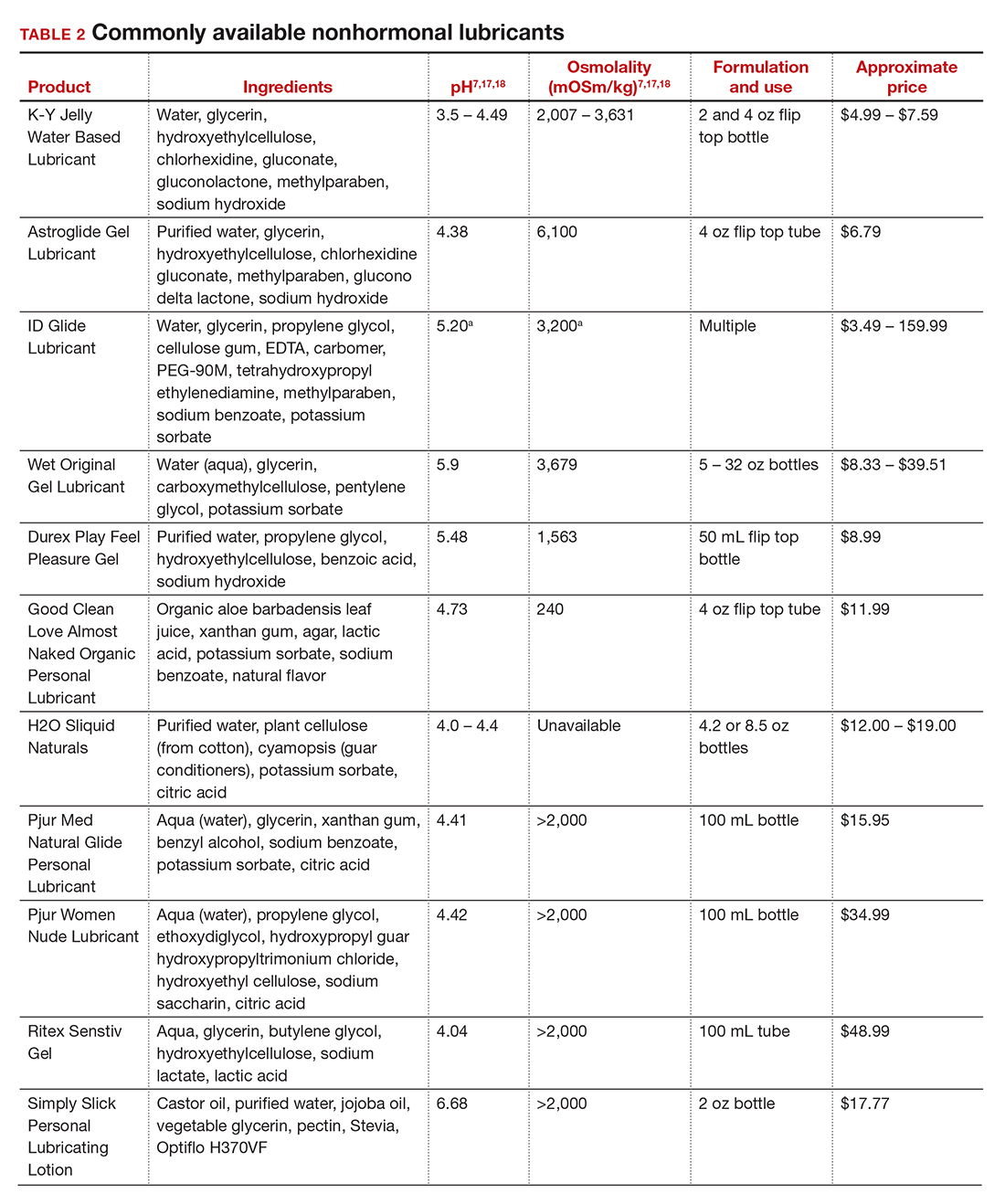
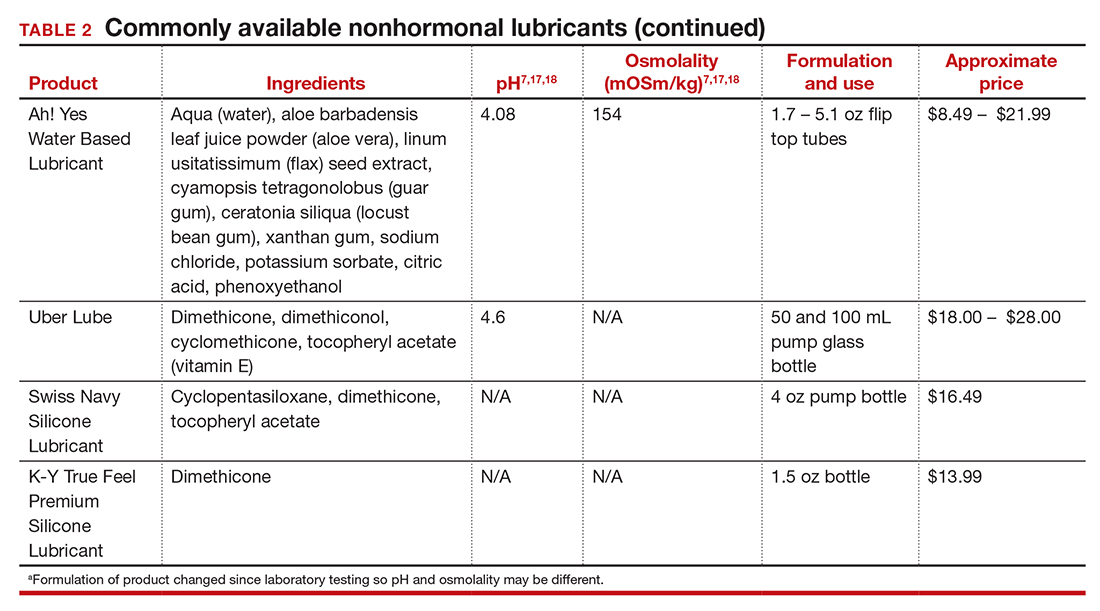
The effects of additives
Water-based moisturizers and lubricants may contain many ingredients, such as glycerols, fragrance, flavors, sweeteners, warming or cooling agents, buffering solutions, parabens and other preservatives, and numbing agents. These substances are added to water-based products to prolong water content, alter viscosity, alter pH, achieve certain sensations, and prevent bacterial contamination.7 The addition of these substances, however, will alter osmolality and pH balance of the product, which may be of clinical consequence. Silicone- or oil-based products do not contain water and therefore do not have a pH or an osmolality value.
Hyperosmolar formulations can theoretically injure epithelial tissue. In vitro studies have shown that hyperosmotic vaginal products can induce mild to moderate irritation, while very hyperosmolar formulations can induce severe irritation and tissue damage to vaginal epithelial and cervical cells.19,20 The WHO recommends that the osmolality of a vaginal product not exceed 380 mOsm/kg, but very few commercially available products meet these criteria so, clinically, the threshold is 1,200 mOsm/kg.17 It should be noted that most commercially available products exceed the 1,200 mOsm/kg threshold. Vaginal products may be a cause for vaginal irritation and should be considered in the differential diagnosis.
The normal vaginal pH is 3.8–4.5, and vaginal products should be pH balanced to this range. The exact role of pH in these products remains poorly understood. Nonetheless, products with a pH of 3 or lower are not recommended.18 Concerns about osmolality and pH remain theoretical, as a study of 12 commercially available lubricants of varying osmolality and pH found no cytotoxic effect in vivo.18
Vaginal moisturizers and lubricants contain many inactive ingredients, the most controversial of which are parabens. These substances are used in many cosmetic products as preservatives and are weakly estrogenic. These substances have been found in breast cancer tissue, but their possible role as a carcinogen remains uncertain.21,22 Nonetheless, the use of paraben-containing products is not recommended for women who have a history of hormonally-driven cancer or who are at high risk for developing cancer.7 Many lubricants contain glycerols (glycerol, glycerine, and propylene glycol) to alter viscosity or alter the water properties. The WHO recommends limits on the content of glycerols in these products.17 Glycerols have been associated with increased risk of bacterial vaginosis (adjusted odds ratio [aOR], 11.75; 95% confidence interval [CI], 1.96–70.27), and can serve as a food source for candida species, possibly increasing risk of yeast infections.7,23 Additionally, vaginal moisturizers and lubricants may contain preservatives such as chlorhexidine, which can disrupt normal vaginal flora and may cause tissue irritation.7
Continue to: Common concerns to be aware of...
Common concerns to be aware of
Women using vaginal products may be concerned about adverse effects, such as worsening vaginal irritation or infection. Vaginal moisturizers have not been shown to have increased risk of adverse effects compared with vaginal estrogens.9,10 In vitro studies have shown that vaginal moisturizers and lubricants inhibit the growth of Escherichia coli but may also inhibit Lactobacillus crispatus.24 Clinically, vaginal moisturizers have been shown to improve signs of bacterial vaginosis and have even been used to treat bacterial vaginosis.25,26 A study of commercially available vaginal lubricants inhibited the growth of L crispatus, which may predispose to irritation and infection.27 Nonetheless, the effect of the vaginal products on the vaginal microbiome and vaginal tissue remains poorly studied. Vaginal moisturizers and lubricants, while often helpful for patients, also can potentially cause irritation or predispose to infections. Providers should consider this when evaluating patients for new onset vaginal symptoms after starting vaginal products.
Bottom line
Vaginal products such as moisturizers and lubricants are often effective treatment options for women suffering from genitourinary syndrome of menopause and may be first-line treatment options, especially for women who may wish to avoid estrogen-containing products. Vaginal moisturizers can be recommended to any women experiencing vaginal irritation due to vaginal dryness while vaginal lubricants should be recommended to sexually active women who experience dyspareunia. Clinicians need to be aware of the formulations of these products and possible side effects in order to appropriately counsel patients. ●
- Castelo-Branco C, Cancelo MJ, Villero J, et al. Management of postmenopausal vaginal atrophy and atrophic vaginitis. Maturitas. 2005;52(suppl 1):S46-S52. doi: 10.1016/j.maturitas.2005.06.014.
- Crandall C, Peterson L, Ganz PA, et al. Association of breast cancer and its therapy with menopause-related symptoms. Menopause. 2004;11:519-530. doi: 10.1097/01.gme.0000117061.40493.ab.
- Bornstein J, Goldstein AT, Stockdale CK, et al. 2015 ISSVD, ISSWSH, and IPPS Consensus Terminology and Classification of Persistant Vulvar Pain and Vulvodynia. J Sex Med. 2016;13:607-612. doi: 10.1016/j.jsxm.2016.02.167.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216. doi: 10.1097/01.AOG.0000441353.20693.78.
- Faubion S, Larkin L, Stuenkel C, et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: consensus recommendation from The North American Menopause Society and the International Society for the Study for Women’s Sexual Health. Menopause. 2018;25:596-608. doi: 10.1097/GME.0000000000001121.
- Herbenick D, Reece M, Schick V, et al. Women’s use and perceptions of commercial lubricants: prevalence and characteristics in a nationally representative sample of American adults. J Sex Med. 2014;11:642-652. doi: 10.1111/jsm.12427.
- Edwards D, Panay N. Treating vulvovaginal atrophy/genitourinary syndrome of menopause: how important is vaginal lubricant and moisturizer composition? Climacteric. 2016;19:151-116. doi: 10.3109/13697137.2015.1124259.
- Van der Lakk JAWN, de Bie LMT, de Leeuw H, et al. The effect of Replens on vaginal cytology in the treatment of postmenopausal atrophy: cytomorphology versus computerized cytometry. J Clin Pathol. 2002;55:446-451. doi: 10.1136/jcp.55.6.446.
- Nachtigall LE. Comparitive study: Replens versus local estrogen in menopausal women. Fertil Steril. 1994;61:178-180. doi: 10.1016/s0015-0282(16)56474-7.
- Bygdeman M, Swahn ML. Replens versus dienoestrol cream in the symptomatic treatment of vaginal atrophy in postmenopausal women. Maturitas. 1996;23:259-263. doi: 10.1016/0378-5122(95)00955-8.
- Mitchell CM, Reed SD, Diem S, et al. Efficacy of vaginal estradiol or vaginal moisturizer vs placebo for treating postmenopausal vulvovaginal symptoms. JAMA Intern Med. 2018;178:681-690. doi: 10.1001/jamainternmed.2018.0116.
- Chen J, Geng L, Song X, et al. Evaluation of the efficacy and safety of hyaluronic acid vaginal gel to ease vaginal dryness: a multicenter, randomized, controlled, open-label, parallel-group, clinical trial. J Sex Med. 2013;10:1575-1584. doi: 10.1111/jsm.12125.
- Jokar A, Davari T, Asadi N, et al. Comparison of the hyaluronic acid vaginal cream and conjugated estrogen used in treatment of vaginal atrophy of menopause women: a randomized controlled clinical trial. IJCBNM. 2016;4:69-78.
- Herbenick D, Reece M, Hensel D, et al. Association of lubricant use with women’s sexual pleasure, sexual satisfaction, and genital symptoms: a prospective daily diary study. J Sex Med. 2011;8:202-212. doi: 10.1111/j.1743-6109.2010.02067.x.
- Sutton KS, Boyer SC, Goldfinger C, et al. To lube or not to lube: experiences and perceptions of lubricant use in women with and without dyspareunia. J Sex Med. 2012;9:240-250. doi: 10.1111/j.1743-6109.2011.02543.x.
- Jozkowski KN, Herbenick D, Schick V, et al. Women’s perceptions about lubricant use and vaginal wetness during sexual activity. J Sex Med. 2013;10:484-492. doi: 10.1111/jsm.12022.
- World Health Organization. Use and procurement of additional lubricants for male and female condoms: WHO /UNFPA/FHI360 advisory note. 2012. https://www.who. int/reproductivehealth/publications/rtis/rhr12_33/en/. Accessed February 13, 2021.
- Cunha AR, Machado RM, Palmeira de Oliveira A, et al. Characterization of commercially available vaginal lubricants: a safety perspective. Pharmaceuticals. 2014;6:530-542. doi: 10.3390/pharmaceutics6030530.
- Adriaens E, Remon JP. Mucosal irritation potential of personal lubricants relates to product osmolality as detected by the slug mucosal irritation assay. Sex Transm Dis. 2008;35:512-516. doi: 10.1097/OLQ.0b013e3181644669.
- Dezzuti CS, Brown ER, Moncla B, et al. Is wetter better? An evaluation of over-the-counter personal lubricants for safety and anti-HIV activity. PLoS One. 2012;7:e48328. doi: 10.1371/journal.pone.0048328.
- Harvey PW, Everett DJ. Significance of the detection of esters of p-hydroxybenzoic acid (parabens) in human breast tumours. J Appl Toxicol. 2004:24:1-4. doi: 10.1002/jat.957.
- Darbre PD, Alijarrah A, Miller WR, et al. Concentrations of parabens in human breast tumous. J Appl Toxicol. 2004;24:5-13. doi: 10.1002/jat.958.
- Brotman RM, Ravel J, Cone RA, et al. Rapid fluctuation of the vaginal microbiota measured by Gram stain analysis. Sex Transm Infect. 2010;86:297-302. doi: 10.1136/sti.2009.040592.
- Hung KJ, Hudson P, Bergerat A, et al. Effect of commercial vaginal products on the growth of uropathogenic and commensal vaginal bacteria. Sci Rep. 2020;10:7625.
- Wu JP, Fielding SL, Fiscell K. The effect of the polycarbophil gel (Replens) on bacterial vaginosis: a pilot study. Eur J Obstet Gynecol Reprod Biol. 2007;130:132-136. doi: 10.1016/j.ejogrb.2006.01.007.
- Fiorelli A, Molteni B, Milani M. Successful treatment of bacterial vaginosis with a polycarbophil-carbopol acidic vaginal gel: results from a randomized double-bling, placebo controlled trial. Eur J Obstet Gynecol Reprod Biol. 2005;120:202-205. doi: 10.1016/j.ejogrb.2004.10.011.
- Fashemi B, Delaney ML, Onderdonk AB, et al. Effects of feminine hygiene products on the vaginal mucosal biome. Microb Ecol Health Dis. 2013;24. doi: 10.3402/mehd.v24i0.19703.
Vaginal dryness, encompassed in the modern term genitourinary syndrome of menopause (GSM) affects up to 40% of menopausal women and up to 60% of postmenopausal breast cancer survivors.1,2 Premenopausal women also can have vulvovaginal dryness while breastfeeding (lactational amenorrhea) and while taking low-dose contraceptives.3 Vaginal moisturizers and lubricants are the first-line treatment options for vaginal dryness, dyspareunia, and GSM.4,5 In fact, approximately two-thirds of women have reported using a vaginal lubricant in their lifetime.6 Despite such ubiquitous use, many health care providers and patients have questions about the difference between vaginal moisturizers and lubricants and how to best choose a product.
Vaginal moisturizers
Vaginal moisturizers are designed to rehydrate the vaginal epithelium. Much like facial or skin moisturizers, they are intended to be applied regularly, every 2 to 3 days, but may be applied more often depending on the severity of symptoms. Vaginal moisturizers work by increasing the fluid content of the vaginal tissue and by lowering the vaginal pH to mimic that of natural vaginal secretions. Vaginal moisturizers are typically water based and use polymers to hydrate tissues.7 They change cell morphology but do not change vaginal maturation, indicating that they bring water to the tissue but do not shift the balance between superficial and basal cells and do not increase vaginal epithelial thickness as seen with vaginal estrogen.8 Vaginal moisturizers also have been found to be a safe alternative to vaginal estrogen therapy and may improve markers of vaginal health, including vaginal moisture, vaginal fluid volume, vaginal elasticity, and premenopausal pH.9 Commercially available vaginal moisturizers have been shown to be as effective as vaginal estrogens in reducing vaginal symptoms such as itching, irritation, and dyspareunia, but some caution should be taken when interpreting these results as neither vaginal moisturizer nor vaginal estrogen tablet were more effective than placebo in a recent randomized controlled trial.10,11 Small studies on hyaluronic acid have shown efficacy for the treatment of vaginal dryness.12,13 Hyaluronic acid is commercially available as a vaginal suppository ovule and as a liquid. It may also be obtained from a reliable compounding pharmacy. Vaginal suppository ovules may be a preferable formulation for women who find the liquids messy or cumbersome to apply.
Lubricants
Lubricants differ from vaginal moisturizers because they are specifically designed to be used during intercourse to provide short-term relief from vaginal dryness. They may be water-, silicone-, mineral oil-, or plant oil-based. The use of water- and silicone-based lubricants is associated with high satisfaction for intercourse as well as masturbation.14 These products may be particularly beneficial to women whose chief complaint is dyspareunia. In fact, women with dyspareunia report more lubricant use than women without dyspareunia, and the most common reason for lubricant use among these women was to reduce or alleviate pain.15 Overall, women both with and without dyspareunia have a positive perception regarding lubricant use and prefer sexual intercourse that feels more “wet,” and women in their forties have the most positive perception about lubricant use at the time of intercourse compared with other age groups.16 Furthermore, the World Health Organization (WHO) recommends that condom-compatible lubricants be used with condoms for menopausal and postmenopausal women.17 Both water-based and silicone-based lubricants may be used with latex condoms, while oil-based lubricants should be avoided as they can degrade the latex condom. While vaginal moisturizers and lubricants technically differ based on use, patients may use one product for both purposes, and some products are marketed as both a moisturizer and lubricant.
Continue to: Providing counsel to patients...
Providing counsel to patients
Patients often seek advice on how to choose vaginal moisturizers and lubricants. Understanding the compositions of these products and their scientific evidence is useful when helping patients make informed decisions regarding their pelvic health. Most commercially available lubricants are either water- or silicone- based. In one study comparing these two types of lubricants, water-based lubricants were associated with fewer genital symptoms than silicone-based products.14 Women may want to use a natural or organic product and may prefer plant-based oils such as coconut oil or olive oil. Patients should be counseled that latex condoms are not compatible with petroleum-, mineral oil- or plant oil-based lubricants.
In our practice, we generally recommend silicone-based lubricants, as they are readily available and compatible with latex condoms and generally require a smaller amount than water-based lubricants. They tend to be more expensive than water-based lubricants. For vaginal moisturizers, we often recommend commercially available formulations that can be purchased at local pharmacies or drug stores. However, a patient may need to try different lubricants and moisturizers in order to find a preferred product. We have included in TABLES 1 and 27,17,18 a list of commercially available vaginal moisturizers and lubricants with ingredient list, pH, osmolality, common formulation, and cost when available, which has been compiled from WHO and published research data to help guide patient counseling.




The effects of additives
Water-based moisturizers and lubricants may contain many ingredients, such as glycerols, fragrance, flavors, sweeteners, warming or cooling agents, buffering solutions, parabens and other preservatives, and numbing agents. These substances are added to water-based products to prolong water content, alter viscosity, alter pH, achieve certain sensations, and prevent bacterial contamination.7 The addition of these substances, however, will alter osmolality and pH balance of the product, which may be of clinical consequence. Silicone- or oil-based products do not contain water and therefore do not have a pH or an osmolality value.
Hyperosmolar formulations can theoretically injure epithelial tissue. In vitro studies have shown that hyperosmotic vaginal products can induce mild to moderate irritation, while very hyperosmolar formulations can induce severe irritation and tissue damage to vaginal epithelial and cervical cells.19,20 The WHO recommends that the osmolality of a vaginal product not exceed 380 mOsm/kg, but very few commercially available products meet these criteria so, clinically, the threshold is 1,200 mOsm/kg.17 It should be noted that most commercially available products exceed the 1,200 mOsm/kg threshold. Vaginal products may be a cause for vaginal irritation and should be considered in the differential diagnosis.
The normal vaginal pH is 3.8–4.5, and vaginal products should be pH balanced to this range. The exact role of pH in these products remains poorly understood. Nonetheless, products with a pH of 3 or lower are not recommended.18 Concerns about osmolality and pH remain theoretical, as a study of 12 commercially available lubricants of varying osmolality and pH found no cytotoxic effect in vivo.18
Vaginal moisturizers and lubricants contain many inactive ingredients, the most controversial of which are parabens. These substances are used in many cosmetic products as preservatives and are weakly estrogenic. These substances have been found in breast cancer tissue, but their possible role as a carcinogen remains uncertain.21,22 Nonetheless, the use of paraben-containing products is not recommended for women who have a history of hormonally-driven cancer or who are at high risk for developing cancer.7 Many lubricants contain glycerols (glycerol, glycerine, and propylene glycol) to alter viscosity or alter the water properties. The WHO recommends limits on the content of glycerols in these products.17 Glycerols have been associated with increased risk of bacterial vaginosis (adjusted odds ratio [aOR], 11.75; 95% confidence interval [CI], 1.96–70.27), and can serve as a food source for candida species, possibly increasing risk of yeast infections.7,23 Additionally, vaginal moisturizers and lubricants may contain preservatives such as chlorhexidine, which can disrupt normal vaginal flora and may cause tissue irritation.7
Continue to: Common concerns to be aware of...
Common concerns to be aware of
Women using vaginal products may be concerned about adverse effects, such as worsening vaginal irritation or infection. Vaginal moisturizers have not been shown to have increased risk of adverse effects compared with vaginal estrogens.9,10 In vitro studies have shown that vaginal moisturizers and lubricants inhibit the growth of Escherichia coli but may also inhibit Lactobacillus crispatus.24 Clinically, vaginal moisturizers have been shown to improve signs of bacterial vaginosis and have even been used to treat bacterial vaginosis.25,26 A study of commercially available vaginal lubricants inhibited the growth of L crispatus, which may predispose to irritation and infection.27 Nonetheless, the effect of the vaginal products on the vaginal microbiome and vaginal tissue remains poorly studied. Vaginal moisturizers and lubricants, while often helpful for patients, also can potentially cause irritation or predispose to infections. Providers should consider this when evaluating patients for new onset vaginal symptoms after starting vaginal products.
Bottom line
Vaginal products such as moisturizers and lubricants are often effective treatment options for women suffering from genitourinary syndrome of menopause and may be first-line treatment options, especially for women who may wish to avoid estrogen-containing products. Vaginal moisturizers can be recommended to any women experiencing vaginal irritation due to vaginal dryness while vaginal lubricants should be recommended to sexually active women who experience dyspareunia. Clinicians need to be aware of the formulations of these products and possible side effects in order to appropriately counsel patients. ●
Vaginal dryness, encompassed in the modern term genitourinary syndrome of menopause (GSM) affects up to 40% of menopausal women and up to 60% of postmenopausal breast cancer survivors.1,2 Premenopausal women also can have vulvovaginal dryness while breastfeeding (lactational amenorrhea) and while taking low-dose contraceptives.3 Vaginal moisturizers and lubricants are the first-line treatment options for vaginal dryness, dyspareunia, and GSM.4,5 In fact, approximately two-thirds of women have reported using a vaginal lubricant in their lifetime.6 Despite such ubiquitous use, many health care providers and patients have questions about the difference between vaginal moisturizers and lubricants and how to best choose a product.
Vaginal moisturizers
Vaginal moisturizers are designed to rehydrate the vaginal epithelium. Much like facial or skin moisturizers, they are intended to be applied regularly, every 2 to 3 days, but may be applied more often depending on the severity of symptoms. Vaginal moisturizers work by increasing the fluid content of the vaginal tissue and by lowering the vaginal pH to mimic that of natural vaginal secretions. Vaginal moisturizers are typically water based and use polymers to hydrate tissues.7 They change cell morphology but do not change vaginal maturation, indicating that they bring water to the tissue but do not shift the balance between superficial and basal cells and do not increase vaginal epithelial thickness as seen with vaginal estrogen.8 Vaginal moisturizers also have been found to be a safe alternative to vaginal estrogen therapy and may improve markers of vaginal health, including vaginal moisture, vaginal fluid volume, vaginal elasticity, and premenopausal pH.9 Commercially available vaginal moisturizers have been shown to be as effective as vaginal estrogens in reducing vaginal symptoms such as itching, irritation, and dyspareunia, but some caution should be taken when interpreting these results as neither vaginal moisturizer nor vaginal estrogen tablet were more effective than placebo in a recent randomized controlled trial.10,11 Small studies on hyaluronic acid have shown efficacy for the treatment of vaginal dryness.12,13 Hyaluronic acid is commercially available as a vaginal suppository ovule and as a liquid. It may also be obtained from a reliable compounding pharmacy. Vaginal suppository ovules may be a preferable formulation for women who find the liquids messy or cumbersome to apply.
Lubricants
Lubricants differ from vaginal moisturizers because they are specifically designed to be used during intercourse to provide short-term relief from vaginal dryness. They may be water-, silicone-, mineral oil-, or plant oil-based. The use of water- and silicone-based lubricants is associated with high satisfaction for intercourse as well as masturbation.14 These products may be particularly beneficial to women whose chief complaint is dyspareunia. In fact, women with dyspareunia report more lubricant use than women without dyspareunia, and the most common reason for lubricant use among these women was to reduce or alleviate pain.15 Overall, women both with and without dyspareunia have a positive perception regarding lubricant use and prefer sexual intercourse that feels more “wet,” and women in their forties have the most positive perception about lubricant use at the time of intercourse compared with other age groups.16 Furthermore, the World Health Organization (WHO) recommends that condom-compatible lubricants be used with condoms for menopausal and postmenopausal women.17 Both water-based and silicone-based lubricants may be used with latex condoms, while oil-based lubricants should be avoided as they can degrade the latex condom. While vaginal moisturizers and lubricants technically differ based on use, patients may use one product for both purposes, and some products are marketed as both a moisturizer and lubricant.
Continue to: Providing counsel to patients...
Providing counsel to patients
Patients often seek advice on how to choose vaginal moisturizers and lubricants. Understanding the compositions of these products and their scientific evidence is useful when helping patients make informed decisions regarding their pelvic health. Most commercially available lubricants are either water- or silicone- based. In one study comparing these two types of lubricants, water-based lubricants were associated with fewer genital symptoms than silicone-based products.14 Women may want to use a natural or organic product and may prefer plant-based oils such as coconut oil or olive oil. Patients should be counseled that latex condoms are not compatible with petroleum-, mineral oil- or plant oil-based lubricants.
In our practice, we generally recommend silicone-based lubricants, as they are readily available and compatible with latex condoms and generally require a smaller amount than water-based lubricants. They tend to be more expensive than water-based lubricants. For vaginal moisturizers, we often recommend commercially available formulations that can be purchased at local pharmacies or drug stores. However, a patient may need to try different lubricants and moisturizers in order to find a preferred product. We have included in TABLES 1 and 27,17,18 a list of commercially available vaginal moisturizers and lubricants with ingredient list, pH, osmolality, common formulation, and cost when available, which has been compiled from WHO and published research data to help guide patient counseling.




The effects of additives
Water-based moisturizers and lubricants may contain many ingredients, such as glycerols, fragrance, flavors, sweeteners, warming or cooling agents, buffering solutions, parabens and other preservatives, and numbing agents. These substances are added to water-based products to prolong water content, alter viscosity, alter pH, achieve certain sensations, and prevent bacterial contamination.7 The addition of these substances, however, will alter osmolality and pH balance of the product, which may be of clinical consequence. Silicone- or oil-based products do not contain water and therefore do not have a pH or an osmolality value.
Hyperosmolar formulations can theoretically injure epithelial tissue. In vitro studies have shown that hyperosmotic vaginal products can induce mild to moderate irritation, while very hyperosmolar formulations can induce severe irritation and tissue damage to vaginal epithelial and cervical cells.19,20 The WHO recommends that the osmolality of a vaginal product not exceed 380 mOsm/kg, but very few commercially available products meet these criteria so, clinically, the threshold is 1,200 mOsm/kg.17 It should be noted that most commercially available products exceed the 1,200 mOsm/kg threshold. Vaginal products may be a cause for vaginal irritation and should be considered in the differential diagnosis.
The normal vaginal pH is 3.8–4.5, and vaginal products should be pH balanced to this range. The exact role of pH in these products remains poorly understood. Nonetheless, products with a pH of 3 or lower are not recommended.18 Concerns about osmolality and pH remain theoretical, as a study of 12 commercially available lubricants of varying osmolality and pH found no cytotoxic effect in vivo.18
Vaginal moisturizers and lubricants contain many inactive ingredients, the most controversial of which are parabens. These substances are used in many cosmetic products as preservatives and are weakly estrogenic. These substances have been found in breast cancer tissue, but their possible role as a carcinogen remains uncertain.21,22 Nonetheless, the use of paraben-containing products is not recommended for women who have a history of hormonally-driven cancer or who are at high risk for developing cancer.7 Many lubricants contain glycerols (glycerol, glycerine, and propylene glycol) to alter viscosity or alter the water properties. The WHO recommends limits on the content of glycerols in these products.17 Glycerols have been associated with increased risk of bacterial vaginosis (adjusted odds ratio [aOR], 11.75; 95% confidence interval [CI], 1.96–70.27), and can serve as a food source for candida species, possibly increasing risk of yeast infections.7,23 Additionally, vaginal moisturizers and lubricants may contain preservatives such as chlorhexidine, which can disrupt normal vaginal flora and may cause tissue irritation.7
Continue to: Common concerns to be aware of...
Common concerns to be aware of
Women using vaginal products may be concerned about adverse effects, such as worsening vaginal irritation or infection. Vaginal moisturizers have not been shown to have increased risk of adverse effects compared with vaginal estrogens.9,10 In vitro studies have shown that vaginal moisturizers and lubricants inhibit the growth of Escherichia coli but may also inhibit Lactobacillus crispatus.24 Clinically, vaginal moisturizers have been shown to improve signs of bacterial vaginosis and have even been used to treat bacterial vaginosis.25,26 A study of commercially available vaginal lubricants inhibited the growth of L crispatus, which may predispose to irritation and infection.27 Nonetheless, the effect of the vaginal products on the vaginal microbiome and vaginal tissue remains poorly studied. Vaginal moisturizers and lubricants, while often helpful for patients, also can potentially cause irritation or predispose to infections. Providers should consider this when evaluating patients for new onset vaginal symptoms after starting vaginal products.
Bottom line
Vaginal products such as moisturizers and lubricants are often effective treatment options for women suffering from genitourinary syndrome of menopause and may be first-line treatment options, especially for women who may wish to avoid estrogen-containing products. Vaginal moisturizers can be recommended to any women experiencing vaginal irritation due to vaginal dryness while vaginal lubricants should be recommended to sexually active women who experience dyspareunia. Clinicians need to be aware of the formulations of these products and possible side effects in order to appropriately counsel patients. ●
- Castelo-Branco C, Cancelo MJ, Villero J, et al. Management of postmenopausal vaginal atrophy and atrophic vaginitis. Maturitas. 2005;52(suppl 1):S46-S52. doi: 10.1016/j.maturitas.2005.06.014.
- Crandall C, Peterson L, Ganz PA, et al. Association of breast cancer and its therapy with menopause-related symptoms. Menopause. 2004;11:519-530. doi: 10.1097/01.gme.0000117061.40493.ab.
- Bornstein J, Goldstein AT, Stockdale CK, et al. 2015 ISSVD, ISSWSH, and IPPS Consensus Terminology and Classification of Persistant Vulvar Pain and Vulvodynia. J Sex Med. 2016;13:607-612. doi: 10.1016/j.jsxm.2016.02.167.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216. doi: 10.1097/01.AOG.0000441353.20693.78.
- Faubion S, Larkin L, Stuenkel C, et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: consensus recommendation from The North American Menopause Society and the International Society for the Study for Women’s Sexual Health. Menopause. 2018;25:596-608. doi: 10.1097/GME.0000000000001121.
- Herbenick D, Reece M, Schick V, et al. Women’s use and perceptions of commercial lubricants: prevalence and characteristics in a nationally representative sample of American adults. J Sex Med. 2014;11:642-652. doi: 10.1111/jsm.12427.
- Edwards D, Panay N. Treating vulvovaginal atrophy/genitourinary syndrome of menopause: how important is vaginal lubricant and moisturizer composition? Climacteric. 2016;19:151-116. doi: 10.3109/13697137.2015.1124259.
- Van der Lakk JAWN, de Bie LMT, de Leeuw H, et al. The effect of Replens on vaginal cytology in the treatment of postmenopausal atrophy: cytomorphology versus computerized cytometry. J Clin Pathol. 2002;55:446-451. doi: 10.1136/jcp.55.6.446.
- Nachtigall LE. Comparitive study: Replens versus local estrogen in menopausal women. Fertil Steril. 1994;61:178-180. doi: 10.1016/s0015-0282(16)56474-7.
- Bygdeman M, Swahn ML. Replens versus dienoestrol cream in the symptomatic treatment of vaginal atrophy in postmenopausal women. Maturitas. 1996;23:259-263. doi: 10.1016/0378-5122(95)00955-8.
- Mitchell CM, Reed SD, Diem S, et al. Efficacy of vaginal estradiol or vaginal moisturizer vs placebo for treating postmenopausal vulvovaginal symptoms. JAMA Intern Med. 2018;178:681-690. doi: 10.1001/jamainternmed.2018.0116.
- Chen J, Geng L, Song X, et al. Evaluation of the efficacy and safety of hyaluronic acid vaginal gel to ease vaginal dryness: a multicenter, randomized, controlled, open-label, parallel-group, clinical trial. J Sex Med. 2013;10:1575-1584. doi: 10.1111/jsm.12125.
- Jokar A, Davari T, Asadi N, et al. Comparison of the hyaluronic acid vaginal cream and conjugated estrogen used in treatment of vaginal atrophy of menopause women: a randomized controlled clinical trial. IJCBNM. 2016;4:69-78.
- Herbenick D, Reece M, Hensel D, et al. Association of lubricant use with women’s sexual pleasure, sexual satisfaction, and genital symptoms: a prospective daily diary study. J Sex Med. 2011;8:202-212. doi: 10.1111/j.1743-6109.2010.02067.x.
- Sutton KS, Boyer SC, Goldfinger C, et al. To lube or not to lube: experiences and perceptions of lubricant use in women with and without dyspareunia. J Sex Med. 2012;9:240-250. doi: 10.1111/j.1743-6109.2011.02543.x.
- Jozkowski KN, Herbenick D, Schick V, et al. Women’s perceptions about lubricant use and vaginal wetness during sexual activity. J Sex Med. 2013;10:484-492. doi: 10.1111/jsm.12022.
- World Health Organization. Use and procurement of additional lubricants for male and female condoms: WHO /UNFPA/FHI360 advisory note. 2012. https://www.who. int/reproductivehealth/publications/rtis/rhr12_33/en/. Accessed February 13, 2021.
- Cunha AR, Machado RM, Palmeira de Oliveira A, et al. Characterization of commercially available vaginal lubricants: a safety perspective. Pharmaceuticals. 2014;6:530-542. doi: 10.3390/pharmaceutics6030530.
- Adriaens E, Remon JP. Mucosal irritation potential of personal lubricants relates to product osmolality as detected by the slug mucosal irritation assay. Sex Transm Dis. 2008;35:512-516. doi: 10.1097/OLQ.0b013e3181644669.
- Dezzuti CS, Brown ER, Moncla B, et al. Is wetter better? An evaluation of over-the-counter personal lubricants for safety and anti-HIV activity. PLoS One. 2012;7:e48328. doi: 10.1371/journal.pone.0048328.
- Harvey PW, Everett DJ. Significance of the detection of esters of p-hydroxybenzoic acid (parabens) in human breast tumours. J Appl Toxicol. 2004:24:1-4. doi: 10.1002/jat.957.
- Darbre PD, Alijarrah A, Miller WR, et al. Concentrations of parabens in human breast tumous. J Appl Toxicol. 2004;24:5-13. doi: 10.1002/jat.958.
- Brotman RM, Ravel J, Cone RA, et al. Rapid fluctuation of the vaginal microbiota measured by Gram stain analysis. Sex Transm Infect. 2010;86:297-302. doi: 10.1136/sti.2009.040592.
- Hung KJ, Hudson P, Bergerat A, et al. Effect of commercial vaginal products on the growth of uropathogenic and commensal vaginal bacteria. Sci Rep. 2020;10:7625.
- Wu JP, Fielding SL, Fiscell K. The effect of the polycarbophil gel (Replens) on bacterial vaginosis: a pilot study. Eur J Obstet Gynecol Reprod Biol. 2007;130:132-136. doi: 10.1016/j.ejogrb.2006.01.007.
- Fiorelli A, Molteni B, Milani M. Successful treatment of bacterial vaginosis with a polycarbophil-carbopol acidic vaginal gel: results from a randomized double-bling, placebo controlled trial. Eur J Obstet Gynecol Reprod Biol. 2005;120:202-205. doi: 10.1016/j.ejogrb.2004.10.011.
- Fashemi B, Delaney ML, Onderdonk AB, et al. Effects of feminine hygiene products on the vaginal mucosal biome. Microb Ecol Health Dis. 2013;24. doi: 10.3402/mehd.v24i0.19703.
- Castelo-Branco C, Cancelo MJ, Villero J, et al. Management of postmenopausal vaginal atrophy and atrophic vaginitis. Maturitas. 2005;52(suppl 1):S46-S52. doi: 10.1016/j.maturitas.2005.06.014.
- Crandall C, Peterson L, Ganz PA, et al. Association of breast cancer and its therapy with menopause-related symptoms. Menopause. 2004;11:519-530. doi: 10.1097/01.gme.0000117061.40493.ab.
- Bornstein J, Goldstein AT, Stockdale CK, et al. 2015 ISSVD, ISSWSH, and IPPS Consensus Terminology and Classification of Persistant Vulvar Pain and Vulvodynia. J Sex Med. 2016;13:607-612. doi: 10.1016/j.jsxm.2016.02.167.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216. doi: 10.1097/01.AOG.0000441353.20693.78.
- Faubion S, Larkin L, Stuenkel C, et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: consensus recommendation from The North American Menopause Society and the International Society for the Study for Women’s Sexual Health. Menopause. 2018;25:596-608. doi: 10.1097/GME.0000000000001121.
- Herbenick D, Reece M, Schick V, et al. Women’s use and perceptions of commercial lubricants: prevalence and characteristics in a nationally representative sample of American adults. J Sex Med. 2014;11:642-652. doi: 10.1111/jsm.12427.
- Edwards D, Panay N. Treating vulvovaginal atrophy/genitourinary syndrome of menopause: how important is vaginal lubricant and moisturizer composition? Climacteric. 2016;19:151-116. doi: 10.3109/13697137.2015.1124259.
- Van der Lakk JAWN, de Bie LMT, de Leeuw H, et al. The effect of Replens on vaginal cytology in the treatment of postmenopausal atrophy: cytomorphology versus computerized cytometry. J Clin Pathol. 2002;55:446-451. doi: 10.1136/jcp.55.6.446.
- Nachtigall LE. Comparitive study: Replens versus local estrogen in menopausal women. Fertil Steril. 1994;61:178-180. doi: 10.1016/s0015-0282(16)56474-7.
- Bygdeman M, Swahn ML. Replens versus dienoestrol cream in the symptomatic treatment of vaginal atrophy in postmenopausal women. Maturitas. 1996;23:259-263. doi: 10.1016/0378-5122(95)00955-8.
- Mitchell CM, Reed SD, Diem S, et al. Efficacy of vaginal estradiol or vaginal moisturizer vs placebo for treating postmenopausal vulvovaginal symptoms. JAMA Intern Med. 2018;178:681-690. doi: 10.1001/jamainternmed.2018.0116.
- Chen J, Geng L, Song X, et al. Evaluation of the efficacy and safety of hyaluronic acid vaginal gel to ease vaginal dryness: a multicenter, randomized, controlled, open-label, parallel-group, clinical trial. J Sex Med. 2013;10:1575-1584. doi: 10.1111/jsm.12125.
- Jokar A, Davari T, Asadi N, et al. Comparison of the hyaluronic acid vaginal cream and conjugated estrogen used in treatment of vaginal atrophy of menopause women: a randomized controlled clinical trial. IJCBNM. 2016;4:69-78.
- Herbenick D, Reece M, Hensel D, et al. Association of lubricant use with women’s sexual pleasure, sexual satisfaction, and genital symptoms: a prospective daily diary study. J Sex Med. 2011;8:202-212. doi: 10.1111/j.1743-6109.2010.02067.x.
- Sutton KS, Boyer SC, Goldfinger C, et al. To lube or not to lube: experiences and perceptions of lubricant use in women with and without dyspareunia. J Sex Med. 2012;9:240-250. doi: 10.1111/j.1743-6109.2011.02543.x.
- Jozkowski KN, Herbenick D, Schick V, et al. Women’s perceptions about lubricant use and vaginal wetness during sexual activity. J Sex Med. 2013;10:484-492. doi: 10.1111/jsm.12022.
- World Health Organization. Use and procurement of additional lubricants for male and female condoms: WHO /UNFPA/FHI360 advisory note. 2012. https://www.who. int/reproductivehealth/publications/rtis/rhr12_33/en/. Accessed February 13, 2021.
- Cunha AR, Machado RM, Palmeira de Oliveira A, et al. Characterization of commercially available vaginal lubricants: a safety perspective. Pharmaceuticals. 2014;6:530-542. doi: 10.3390/pharmaceutics6030530.
- Adriaens E, Remon JP. Mucosal irritation potential of personal lubricants relates to product osmolality as detected by the slug mucosal irritation assay. Sex Transm Dis. 2008;35:512-516. doi: 10.1097/OLQ.0b013e3181644669.
- Dezzuti CS, Brown ER, Moncla B, et al. Is wetter better? An evaluation of over-the-counter personal lubricants for safety and anti-HIV activity. PLoS One. 2012;7:e48328. doi: 10.1371/journal.pone.0048328.
- Harvey PW, Everett DJ. Significance of the detection of esters of p-hydroxybenzoic acid (parabens) in human breast tumours. J Appl Toxicol. 2004:24:1-4. doi: 10.1002/jat.957.
- Darbre PD, Alijarrah A, Miller WR, et al. Concentrations of parabens in human breast tumous. J Appl Toxicol. 2004;24:5-13. doi: 10.1002/jat.958.
- Brotman RM, Ravel J, Cone RA, et al. Rapid fluctuation of the vaginal microbiota measured by Gram stain analysis. Sex Transm Infect. 2010;86:297-302. doi: 10.1136/sti.2009.040592.
- Hung KJ, Hudson P, Bergerat A, et al. Effect of commercial vaginal products on the growth of uropathogenic and commensal vaginal bacteria. Sci Rep. 2020;10:7625.
- Wu JP, Fielding SL, Fiscell K. The effect of the polycarbophil gel (Replens) on bacterial vaginosis: a pilot study. Eur J Obstet Gynecol Reprod Biol. 2007;130:132-136. doi: 10.1016/j.ejogrb.2006.01.007.
- Fiorelli A, Molteni B, Milani M. Successful treatment of bacterial vaginosis with a polycarbophil-carbopol acidic vaginal gel: results from a randomized double-bling, placebo controlled trial. Eur J Obstet Gynecol Reprod Biol. 2005;120:202-205. doi: 10.1016/j.ejogrb.2004.10.011.
- Fashemi B, Delaney ML, Onderdonk AB, et al. Effects of feminine hygiene products on the vaginal mucosal biome. Microb Ecol Health Dis. 2013;24. doi: 10.3402/mehd.v24i0.19703.
A novel approach to complete transobturator sling mesh removal

Additional videos from SGS are available here, including these recent offerings:
- Embryologic development of the external genitalia as it relates to vaginoplasty for the transgender woman
- A stepwise approach to the difficult bladder flap to prevent urinary tract injury during laparoscopic hysterectomy
- Novel method to demarcate bladder dissection during posthysterectomy sacrocolpopexy

Additional videos from SGS are available here, including these recent offerings:
- Embryologic development of the external genitalia as it relates to vaginoplasty for the transgender woman
- A stepwise approach to the difficult bladder flap to prevent urinary tract injury during laparoscopic hysterectomy
- Novel method to demarcate bladder dissection during posthysterectomy sacrocolpopexy

Additional videos from SGS are available here, including these recent offerings:
- Embryologic development of the external genitalia as it relates to vaginoplasty for the transgender woman
- A stepwise approach to the difficult bladder flap to prevent urinary tract injury during laparoscopic hysterectomy
- Novel method to demarcate bladder dissection during posthysterectomy sacrocolpopexy
Tips and tricks for open laparoscopy

Visit the Society of Gynecologic Surgeons online: sgsonline.org
More videos from SGS:
- Complete colpectomy & colpocleisis: Model for simulation
- Natural orifice sacral colpopexy
- Alternative options for visualizing ureteral patency during intraoperative cystoscopy
- Use of suprapubic Carter-Thomason needle to assist in cystoscopic excision of an intravesical foreign object
- Uterine artery ligation: Advanced techniques and considerations for the difficult laparoscopic hysterectomy
- Cervical injection of methylene blue for identification of sentinel lymph nodes in cervical cancer
- Misplaced hysteroscopic sterilization micro-insert in the peritoneal cavity: A corpus alienum
- Laparoscopic cystectomy for large, bilateral ovarian dermoids
- Small bowel surgery for the benign gynecologist

Visit the Society of Gynecologic Surgeons online: sgsonline.org
More videos from SGS:
- Complete colpectomy & colpocleisis: Model for simulation
- Natural orifice sacral colpopexy
- Alternative options for visualizing ureteral patency during intraoperative cystoscopy
- Use of suprapubic Carter-Thomason needle to assist in cystoscopic excision of an intravesical foreign object
- Uterine artery ligation: Advanced techniques and considerations for the difficult laparoscopic hysterectomy
- Cervical injection of methylene blue for identification of sentinel lymph nodes in cervical cancer
- Misplaced hysteroscopic sterilization micro-insert in the peritoneal cavity: A corpus alienum
- Laparoscopic cystectomy for large, bilateral ovarian dermoids
- Small bowel surgery for the benign gynecologist

Visit the Society of Gynecologic Surgeons online: sgsonline.org
More videos from SGS:
- Complete colpectomy & colpocleisis: Model for simulation
- Natural orifice sacral colpopexy
- Alternative options for visualizing ureteral patency during intraoperative cystoscopy
- Use of suprapubic Carter-Thomason needle to assist in cystoscopic excision of an intravesical foreign object
- Uterine artery ligation: Advanced techniques and considerations for the difficult laparoscopic hysterectomy
- Cervical injection of methylene blue for identification of sentinel lymph nodes in cervical cancer
- Misplaced hysteroscopic sterilization micro-insert in the peritoneal cavity: A corpus alienum
- Laparoscopic cystectomy for large, bilateral ovarian dermoids
- Small bowel surgery for the benign gynecologist
This video is brought to you by
Tissue extraction during minimally invasive Gyn surgery. Second of 2 Parts: Counseling the patient
In the absence of a definitive FDA decision on the future of power morcellation in minimally invasive gynecologic surgery, many surgeons have stopped offering the option, often in response to constraints placed by their institutions, or have greatly expanded the informed consent discussion.
In Part 1 of this two-part roundtable discussion, which appeared in the September 2014 issue of OBG Management, our expert panelists discussed their current approach to tissue extraction during hysterectomy and myomectomy, as well as their preferred approach to both procedures amid this changing surgical environment. Here, in Part 2, they discuss patient counseling and the likely effects of FDA action.
How has your counseling changed?
OBG Management: Given recent concerns about the use of power morcellation, how has your counseling of the patient changed?
Kimberly Kho, MD, MPH: Though I look forward to the development of instruments and techniques that will make contained power morcellation safer, I am not using it currently and have been able to find minimally invasive alternatives such as minilaparotomy and vaginal removal of masses for the cases I would have considered for power morcellation.
Certainly, with power morcellation or any type of morcellation, it’s important to discuss the risks and benefits, as well as alternatives. Discussion should include the potential for:
- iatrogenic injury and tissue seeding of both benign and malignant tissue
- exacerbation of any occult malignancy and possible worsening of prognosis
- missing or mischaracterizing an occult malignancy.
Although there is no surefire way to avoid cellular dissemination with any type of surgery, I think it’s equally important to explain that, often, the only way to completely avoid fragmenting a large mass is to remove it en bloc, which would mean a large laparotomy for many patients. Women should understand the risks of laparotomy as well, including more frequent wound complications, longer hospitalization, and slower recovery.
Arnold P. Advincula, MD: If a clinician anticipates or plans the use of power morcellation, he or she certainly needs to go through an informed consent process with the patient. This process may include a separate form specific to power morcellation as well as detailed documentation during the preoperative visit.
OBG Management: What elements of the preoperative visit do you believe are important to document?
Dr. Advincula: It is important to clearly document the indications and alternatives for the surgery, as well as the decision-making process that led to the selection of a particular procedure and route of access. If any type of morcellation (power-driven or not) is anticipated, then the risks associated with it must be thoroughly discussed and documented in addition to the standard risks associated with any type of abdominal-pelvic surgery. No surgical procedure is without risks. Therefore, the process of informed consent cannot be taken lightly and is a critical part of the process that allows a patient to decide upon a particular intervention.
Jason D. Wright, MD: I believe the current role of power morcellation is limited. Patients considering the procedure should be counseled about the risks of cancer as well as other adverse pathologic abnormalities, including smooth muscle tumors of uncertain malignant potential, disseminated leiomyomatosis, and endometrial hyperplasia that may be associated with an occult cancer.
OBG Management: Do you recommend a separate consent form for power morcellation, as Dr. Advincula suggested?
Dr. Wright: Given the risk of adverse pathology, I think the role of electric power morcellation is limited. Patients should be carefully counseled about alternative surgical approaches that avoid tissue disruption and understand that the sensitivity of preoperative testing and intraoperative evaluation of smooth muscle neoplasms is limited. Further, patients considering contained morcellation also should be informed that the data examining the efficacy of these techniques are sparse.
Linda D. Bradley, MD: As I mentioned in Part 1 of our discussion, I’m giving patients new information about our concerns regarding occult malignancy, quoting the risk estimates given by the FDA this year.1 And the fact that we no longer use power morcellation at the Cleveland Clinic means that I no longer discuss it as an option, although one or two patients have asked for it in recent months.
I think many patients have read about it in the news or, once hysterectomy or myomectomy was planned, found discussion of the controversy surrounding it during their research. I’ve even had patients who underwent hysteroscopic myomectomy 2 or more years ago contacting me to find out whether power morcellation was used, and I have had to explain that hysteroscopic morcellation is different from the laparoscopic variant.
Patients are critical readers and are much more knowledgeable as a result of social media, so I do find myself spending more time discussing their procedure with them.
For myomectomy in particular, we send for a frozen section intraoperatively. Although that approach still is not 100% sensitive, it does guide what we do during surgery. If a sarcoma is found, for example, we call in the oncologists. I discuss that possibility with the patient as well. So I am spending more time with patients, but I don’t go into power morcellation because that is no longer an option for me.
OBG Management: Dr. Iglesia, has your counseling of patients changed in any way?
Cheryl Iglesia, MD: I do not routinely use power morcellation. However, the findings from the FDA and Dr. Wright about the higher risk of occult malignancy in fibroids is information I share with patients preoperatively.1,2
For women with fibroids who want uterine conservation procedures or who desire medical management, such as focused ultrasound or uterine fibroid embolization, MRI is routine. However, we make patients aware that this imaging modality is not 100% sensitive in detecting occult cancer—and neither are random biopsies of fibroids. Patients also need to be made aware that treatment with fibroid embolization or other medical options also could delay the detection of cancer and sarcoma. Any morcellation technique (power, hand, vaginal) does have the risk of potential cancer spread and upstaging, so morcellation should not be used in any women with suspected or known malignancy.
Effects of likely FDA actions
OBG Management: If the FDA decides to ban power morcellation outright, in some ways the approach to patient counseling will be simpler, as one option will have been permanently eliminated. But if the FDA allows power morcellation to continue, with stricter labeling, would that affect how you counsel patients? And would you reconsider power morcellation in that light?
Dr. Kho: I think the current discussion has highlighted again how important the informed consent process is as an opportunity for information sharing. It’s an ongoing discussion of risks, benefits, and alternatives. It also offers us an opportunity to understand the patient’s values and perspectives throughout the process of surgical planning. So, no, I don’t think the FDA’s actions will change how I counsel patients. Regardless of the FDA’s decisions, I think open power morcellation as we currently know it may be obviated as new instruments for contained morcellation—as well as other techniques we’ve discussed—become more popular. But it’s critical that we meaningfully monitor these techniques for long-term safety. In order to make evidence-based decisions, we will need good data.
Dr. Iglesia: I cannot comment on a final FDA decision. However, my feeling is that any information that patients can use to become educated about treatment alternatives—including the risks and benefits of each option—will help inform and improve the shared decision-making process.
Dr. Advincula: Regardless of the verdict rendered by the FDA, the way we approach tissue extraction in minimally invasive surgery has been changed forever. It is always important to take a critical look at the way things are done, but not at the expense of throwing the proverbial baby out with the bath water. If power morcellation were to remain a viable option, my counseling would remain as is, as it already has been modified and quite detailed in the wake of this whole controversy. I still believe there is a role for power morcellation, albeit modified from its current iteration, when applied by the right physician in a properly evaluated patient with the right indication.
Summing up
OBG Management: Do you have any additional comments about this issue?
Dr. Advincula: The ability to accurately and reliably detect an occult uterine malignancy—specifically, leiomyosarcoma—is lacking at present. Whether or not power morcellation remains a viable option in the future, the bottom line is that patients will still present with occult uterine malignancy. Minimizing the mishandling of this unfortunate diagnosis will depend on sound clinical judgment as well as improvements in diagnosis. It always will be important to avoid blaming the lack of sound clinical practice on surgical devices that, when used appropriately, have the potential to benefit the majority of women.
Dr. Kho: The current attention on power morcellators presents an opportunity to improve upon our current practices and find solutions to the issues we are encountering. I think this is an exciting time for examining preoperative risk stratification, the innovation of new techniques, repopularization and improvement of older ones such as vaginal tissue extraction, and, overall, to improve our system of safety monitoring and surgical device surveillance.
Dr. Iglesia: Intraperitoneal power morcellation should not be used in cases of malignancy or suspected malignancy or in postmenopausal patients with bleeding or growing fibroids. The availability of power morcellators may be limited as manufacturers
cease distribution, hospitals ban use, or insurers refuse payment for use.
Alternative minimally invasive approaches—especially the transvaginal approach—should be considered, since there are fewer complications associated with vaginal surgery, especially compared with open and laparoscopic surgery.
Dr. Wright: Although electric power morcellation may allow some women to undergo a minimally invasive procedure, the data currently available clearly suggest that adverse pathology is more common in women who undergo morcellation than was previously thought.
Although the debate around morcellation has focused on leiomyosarcoma, epithelial endometrial tumors and other preinvasive abnormalities are also common. These unexpected pathologic findings in women who underwent electric power morcellation highlight the importance of performing more rigorous evaluation of new methods of tissue extraction.
Quick Poll:
If you are using, plan to use, or anticipate the possibility of using power morcellation during minimally invasive gynecologic surgery, does your consent process include a separate form specific to power morcellation?
Please provide your answer to this question in the Quick Poll on the OBG Management home page, and then see how your peers have voted. Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication.http://www.fda.gov/medicaldevices/safety/alertsandnotices/ucm393576.htm. Published April 17, 2014. Accessed September 18, 2014.
2. Wright JD, Tergas AI, Burke WM, et al. Uterine pathology in women undergoing minimally invasive hysterectomy with morcellation [published online ahead of print July 22, 2014]. JAMA. doi:10.1001/jama.2014.9005.
In the absence of a definitive FDA decision on the future of power morcellation in minimally invasive gynecologic surgery, many surgeons have stopped offering the option, often in response to constraints placed by their institutions, or have greatly expanded the informed consent discussion.
In Part 1 of this two-part roundtable discussion, which appeared in the September 2014 issue of OBG Management, our expert panelists discussed their current approach to tissue extraction during hysterectomy and myomectomy, as well as their preferred approach to both procedures amid this changing surgical environment. Here, in Part 2, they discuss patient counseling and the likely effects of FDA action.
How has your counseling changed?
OBG Management: Given recent concerns about the use of power morcellation, how has your counseling of the patient changed?
Kimberly Kho, MD, MPH: Though I look forward to the development of instruments and techniques that will make contained power morcellation safer, I am not using it currently and have been able to find minimally invasive alternatives such as minilaparotomy and vaginal removal of masses for the cases I would have considered for power morcellation.
Certainly, with power morcellation or any type of morcellation, it’s important to discuss the risks and benefits, as well as alternatives. Discussion should include the potential for:
- iatrogenic injury and tissue seeding of both benign and malignant tissue
- exacerbation of any occult malignancy and possible worsening of prognosis
- missing or mischaracterizing an occult malignancy.
Although there is no surefire way to avoid cellular dissemination with any type of surgery, I think it’s equally important to explain that, often, the only way to completely avoid fragmenting a large mass is to remove it en bloc, which would mean a large laparotomy for many patients. Women should understand the risks of laparotomy as well, including more frequent wound complications, longer hospitalization, and slower recovery.
Arnold P. Advincula, MD: If a clinician anticipates or plans the use of power morcellation, he or she certainly needs to go through an informed consent process with the patient. This process may include a separate form specific to power morcellation as well as detailed documentation during the preoperative visit.
OBG Management: What elements of the preoperative visit do you believe are important to document?
Dr. Advincula: It is important to clearly document the indications and alternatives for the surgery, as well as the decision-making process that led to the selection of a particular procedure and route of access. If any type of morcellation (power-driven or not) is anticipated, then the risks associated with it must be thoroughly discussed and documented in addition to the standard risks associated with any type of abdominal-pelvic surgery. No surgical procedure is without risks. Therefore, the process of informed consent cannot be taken lightly and is a critical part of the process that allows a patient to decide upon a particular intervention.
Jason D. Wright, MD: I believe the current role of power morcellation is limited. Patients considering the procedure should be counseled about the risks of cancer as well as other adverse pathologic abnormalities, including smooth muscle tumors of uncertain malignant potential, disseminated leiomyomatosis, and endometrial hyperplasia that may be associated with an occult cancer.
OBG Management: Do you recommend a separate consent form for power morcellation, as Dr. Advincula suggested?
Dr. Wright: Given the risk of adverse pathology, I think the role of electric power morcellation is limited. Patients should be carefully counseled about alternative surgical approaches that avoid tissue disruption and understand that the sensitivity of preoperative testing and intraoperative evaluation of smooth muscle neoplasms is limited. Further, patients considering contained morcellation also should be informed that the data examining the efficacy of these techniques are sparse.
Linda D. Bradley, MD: As I mentioned in Part 1 of our discussion, I’m giving patients new information about our concerns regarding occult malignancy, quoting the risk estimates given by the FDA this year.1 And the fact that we no longer use power morcellation at the Cleveland Clinic means that I no longer discuss it as an option, although one or two patients have asked for it in recent months.
I think many patients have read about it in the news or, once hysterectomy or myomectomy was planned, found discussion of the controversy surrounding it during their research. I’ve even had patients who underwent hysteroscopic myomectomy 2 or more years ago contacting me to find out whether power morcellation was used, and I have had to explain that hysteroscopic morcellation is different from the laparoscopic variant.
Patients are critical readers and are much more knowledgeable as a result of social media, so I do find myself spending more time discussing their procedure with them.
For myomectomy in particular, we send for a frozen section intraoperatively. Although that approach still is not 100% sensitive, it does guide what we do during surgery. If a sarcoma is found, for example, we call in the oncologists. I discuss that possibility with the patient as well. So I am spending more time with patients, but I don’t go into power morcellation because that is no longer an option for me.
OBG Management: Dr. Iglesia, has your counseling of patients changed in any way?
Cheryl Iglesia, MD: I do not routinely use power morcellation. However, the findings from the FDA and Dr. Wright about the higher risk of occult malignancy in fibroids is information I share with patients preoperatively.1,2
For women with fibroids who want uterine conservation procedures or who desire medical management, such as focused ultrasound or uterine fibroid embolization, MRI is routine. However, we make patients aware that this imaging modality is not 100% sensitive in detecting occult cancer—and neither are random biopsies of fibroids. Patients also need to be made aware that treatment with fibroid embolization or other medical options also could delay the detection of cancer and sarcoma. Any morcellation technique (power, hand, vaginal) does have the risk of potential cancer spread and upstaging, so morcellation should not be used in any women with suspected or known malignancy.
Effects of likely FDA actions
OBG Management: If the FDA decides to ban power morcellation outright, in some ways the approach to patient counseling will be simpler, as one option will have been permanently eliminated. But if the FDA allows power morcellation to continue, with stricter labeling, would that affect how you counsel patients? And would you reconsider power morcellation in that light?
Dr. Kho: I think the current discussion has highlighted again how important the informed consent process is as an opportunity for information sharing. It’s an ongoing discussion of risks, benefits, and alternatives. It also offers us an opportunity to understand the patient’s values and perspectives throughout the process of surgical planning. So, no, I don’t think the FDA’s actions will change how I counsel patients. Regardless of the FDA’s decisions, I think open power morcellation as we currently know it may be obviated as new instruments for contained morcellation—as well as other techniques we’ve discussed—become more popular. But it’s critical that we meaningfully monitor these techniques for long-term safety. In order to make evidence-based decisions, we will need good data.
Dr. Iglesia: I cannot comment on a final FDA decision. However, my feeling is that any information that patients can use to become educated about treatment alternatives—including the risks and benefits of each option—will help inform and improve the shared decision-making process.
Dr. Advincula: Regardless of the verdict rendered by the FDA, the way we approach tissue extraction in minimally invasive surgery has been changed forever. It is always important to take a critical look at the way things are done, but not at the expense of throwing the proverbial baby out with the bath water. If power morcellation were to remain a viable option, my counseling would remain as is, as it already has been modified and quite detailed in the wake of this whole controversy. I still believe there is a role for power morcellation, albeit modified from its current iteration, when applied by the right physician in a properly evaluated patient with the right indication.
Summing up
OBG Management: Do you have any additional comments about this issue?
Dr. Advincula: The ability to accurately and reliably detect an occult uterine malignancy—specifically, leiomyosarcoma—is lacking at present. Whether or not power morcellation remains a viable option in the future, the bottom line is that patients will still present with occult uterine malignancy. Minimizing the mishandling of this unfortunate diagnosis will depend on sound clinical judgment as well as improvements in diagnosis. It always will be important to avoid blaming the lack of sound clinical practice on surgical devices that, when used appropriately, have the potential to benefit the majority of women.
Dr. Kho: The current attention on power morcellators presents an opportunity to improve upon our current practices and find solutions to the issues we are encountering. I think this is an exciting time for examining preoperative risk stratification, the innovation of new techniques, repopularization and improvement of older ones such as vaginal tissue extraction, and, overall, to improve our system of safety monitoring and surgical device surveillance.
Dr. Iglesia: Intraperitoneal power morcellation should not be used in cases of malignancy or suspected malignancy or in postmenopausal patients with bleeding or growing fibroids. The availability of power morcellators may be limited as manufacturers
cease distribution, hospitals ban use, or insurers refuse payment for use.
Alternative minimally invasive approaches—especially the transvaginal approach—should be considered, since there are fewer complications associated with vaginal surgery, especially compared with open and laparoscopic surgery.
Dr. Wright: Although electric power morcellation may allow some women to undergo a minimally invasive procedure, the data currently available clearly suggest that adverse pathology is more common in women who undergo morcellation than was previously thought.
Although the debate around morcellation has focused on leiomyosarcoma, epithelial endometrial tumors and other preinvasive abnormalities are also common. These unexpected pathologic findings in women who underwent electric power morcellation highlight the importance of performing more rigorous evaluation of new methods of tissue extraction.
Quick Poll:
If you are using, plan to use, or anticipate the possibility of using power morcellation during minimally invasive gynecologic surgery, does your consent process include a separate form specific to power morcellation?
Please provide your answer to this question in the Quick Poll on the OBG Management home page, and then see how your peers have voted. Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
In the absence of a definitive FDA decision on the future of power morcellation in minimally invasive gynecologic surgery, many surgeons have stopped offering the option, often in response to constraints placed by their institutions, or have greatly expanded the informed consent discussion.
In Part 1 of this two-part roundtable discussion, which appeared in the September 2014 issue of OBG Management, our expert panelists discussed their current approach to tissue extraction during hysterectomy and myomectomy, as well as their preferred approach to both procedures amid this changing surgical environment. Here, in Part 2, they discuss patient counseling and the likely effects of FDA action.
How has your counseling changed?
OBG Management: Given recent concerns about the use of power morcellation, how has your counseling of the patient changed?
Kimberly Kho, MD, MPH: Though I look forward to the development of instruments and techniques that will make contained power morcellation safer, I am not using it currently and have been able to find minimally invasive alternatives such as minilaparotomy and vaginal removal of masses for the cases I would have considered for power morcellation.
Certainly, with power morcellation or any type of morcellation, it’s important to discuss the risks and benefits, as well as alternatives. Discussion should include the potential for:
- iatrogenic injury and tissue seeding of both benign and malignant tissue
- exacerbation of any occult malignancy and possible worsening of prognosis
- missing or mischaracterizing an occult malignancy.
Although there is no surefire way to avoid cellular dissemination with any type of surgery, I think it’s equally important to explain that, often, the only way to completely avoid fragmenting a large mass is to remove it en bloc, which would mean a large laparotomy for many patients. Women should understand the risks of laparotomy as well, including more frequent wound complications, longer hospitalization, and slower recovery.
Arnold P. Advincula, MD: If a clinician anticipates or plans the use of power morcellation, he or she certainly needs to go through an informed consent process with the patient. This process may include a separate form specific to power morcellation as well as detailed documentation during the preoperative visit.
OBG Management: What elements of the preoperative visit do you believe are important to document?
Dr. Advincula: It is important to clearly document the indications and alternatives for the surgery, as well as the decision-making process that led to the selection of a particular procedure and route of access. If any type of morcellation (power-driven or not) is anticipated, then the risks associated with it must be thoroughly discussed and documented in addition to the standard risks associated with any type of abdominal-pelvic surgery. No surgical procedure is without risks. Therefore, the process of informed consent cannot be taken lightly and is a critical part of the process that allows a patient to decide upon a particular intervention.
Jason D. Wright, MD: I believe the current role of power morcellation is limited. Patients considering the procedure should be counseled about the risks of cancer as well as other adverse pathologic abnormalities, including smooth muscle tumors of uncertain malignant potential, disseminated leiomyomatosis, and endometrial hyperplasia that may be associated with an occult cancer.
OBG Management: Do you recommend a separate consent form for power morcellation, as Dr. Advincula suggested?
Dr. Wright: Given the risk of adverse pathology, I think the role of electric power morcellation is limited. Patients should be carefully counseled about alternative surgical approaches that avoid tissue disruption and understand that the sensitivity of preoperative testing and intraoperative evaluation of smooth muscle neoplasms is limited. Further, patients considering contained morcellation also should be informed that the data examining the efficacy of these techniques are sparse.
Linda D. Bradley, MD: As I mentioned in Part 1 of our discussion, I’m giving patients new information about our concerns regarding occult malignancy, quoting the risk estimates given by the FDA this year.1 And the fact that we no longer use power morcellation at the Cleveland Clinic means that I no longer discuss it as an option, although one or two patients have asked for it in recent months.
I think many patients have read about it in the news or, once hysterectomy or myomectomy was planned, found discussion of the controversy surrounding it during their research. I’ve even had patients who underwent hysteroscopic myomectomy 2 or more years ago contacting me to find out whether power morcellation was used, and I have had to explain that hysteroscopic morcellation is different from the laparoscopic variant.
Patients are critical readers and are much more knowledgeable as a result of social media, so I do find myself spending more time discussing their procedure with them.
For myomectomy in particular, we send for a frozen section intraoperatively. Although that approach still is not 100% sensitive, it does guide what we do during surgery. If a sarcoma is found, for example, we call in the oncologists. I discuss that possibility with the patient as well. So I am spending more time with patients, but I don’t go into power morcellation because that is no longer an option for me.
OBG Management: Dr. Iglesia, has your counseling of patients changed in any way?
Cheryl Iglesia, MD: I do not routinely use power morcellation. However, the findings from the FDA and Dr. Wright about the higher risk of occult malignancy in fibroids is information I share with patients preoperatively.1,2
For women with fibroids who want uterine conservation procedures or who desire medical management, such as focused ultrasound or uterine fibroid embolization, MRI is routine. However, we make patients aware that this imaging modality is not 100% sensitive in detecting occult cancer—and neither are random biopsies of fibroids. Patients also need to be made aware that treatment with fibroid embolization or other medical options also could delay the detection of cancer and sarcoma. Any morcellation technique (power, hand, vaginal) does have the risk of potential cancer spread and upstaging, so morcellation should not be used in any women with suspected or known malignancy.
Effects of likely FDA actions
OBG Management: If the FDA decides to ban power morcellation outright, in some ways the approach to patient counseling will be simpler, as one option will have been permanently eliminated. But if the FDA allows power morcellation to continue, with stricter labeling, would that affect how you counsel patients? And would you reconsider power morcellation in that light?
Dr. Kho: I think the current discussion has highlighted again how important the informed consent process is as an opportunity for information sharing. It’s an ongoing discussion of risks, benefits, and alternatives. It also offers us an opportunity to understand the patient’s values and perspectives throughout the process of surgical planning. So, no, I don’t think the FDA’s actions will change how I counsel patients. Regardless of the FDA’s decisions, I think open power morcellation as we currently know it may be obviated as new instruments for contained morcellation—as well as other techniques we’ve discussed—become more popular. But it’s critical that we meaningfully monitor these techniques for long-term safety. In order to make evidence-based decisions, we will need good data.
Dr. Iglesia: I cannot comment on a final FDA decision. However, my feeling is that any information that patients can use to become educated about treatment alternatives—including the risks and benefits of each option—will help inform and improve the shared decision-making process.
Dr. Advincula: Regardless of the verdict rendered by the FDA, the way we approach tissue extraction in minimally invasive surgery has been changed forever. It is always important to take a critical look at the way things are done, but not at the expense of throwing the proverbial baby out with the bath water. If power morcellation were to remain a viable option, my counseling would remain as is, as it already has been modified and quite detailed in the wake of this whole controversy. I still believe there is a role for power morcellation, albeit modified from its current iteration, when applied by the right physician in a properly evaluated patient with the right indication.
Summing up
OBG Management: Do you have any additional comments about this issue?
Dr. Advincula: The ability to accurately and reliably detect an occult uterine malignancy—specifically, leiomyosarcoma—is lacking at present. Whether or not power morcellation remains a viable option in the future, the bottom line is that patients will still present with occult uterine malignancy. Minimizing the mishandling of this unfortunate diagnosis will depend on sound clinical judgment as well as improvements in diagnosis. It always will be important to avoid blaming the lack of sound clinical practice on surgical devices that, when used appropriately, have the potential to benefit the majority of women.
Dr. Kho: The current attention on power morcellators presents an opportunity to improve upon our current practices and find solutions to the issues we are encountering. I think this is an exciting time for examining preoperative risk stratification, the innovation of new techniques, repopularization and improvement of older ones such as vaginal tissue extraction, and, overall, to improve our system of safety monitoring and surgical device surveillance.
Dr. Iglesia: Intraperitoneal power morcellation should not be used in cases of malignancy or suspected malignancy or in postmenopausal patients with bleeding or growing fibroids. The availability of power morcellators may be limited as manufacturers
cease distribution, hospitals ban use, or insurers refuse payment for use.
Alternative minimally invasive approaches—especially the transvaginal approach—should be considered, since there are fewer complications associated with vaginal surgery, especially compared with open and laparoscopic surgery.
Dr. Wright: Although electric power morcellation may allow some women to undergo a minimally invasive procedure, the data currently available clearly suggest that adverse pathology is more common in women who undergo morcellation than was previously thought.
Although the debate around morcellation has focused on leiomyosarcoma, epithelial endometrial tumors and other preinvasive abnormalities are also common. These unexpected pathologic findings in women who underwent electric power morcellation highlight the importance of performing more rigorous evaluation of new methods of tissue extraction.
Quick Poll:
If you are using, plan to use, or anticipate the possibility of using power morcellation during minimally invasive gynecologic surgery, does your consent process include a separate form specific to power morcellation?
Please provide your answer to this question in the Quick Poll on the OBG Management home page, and then see how your peers have voted. Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication.http://www.fda.gov/medicaldevices/safety/alertsandnotices/ucm393576.htm. Published April 17, 2014. Accessed September 18, 2014.
2. Wright JD, Tergas AI, Burke WM, et al. Uterine pathology in women undergoing minimally invasive hysterectomy with morcellation [published online ahead of print July 22, 2014]. JAMA. doi:10.1001/jama.2014.9005.
1. US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication.http://www.fda.gov/medicaldevices/safety/alertsandnotices/ucm393576.htm. Published April 17, 2014. Accessed September 18, 2014.
2. Wright JD, Tergas AI, Burke WM, et al. Uterine pathology in women undergoing minimally invasive hysterectomy with morcellation [published online ahead of print July 22, 2014]. JAMA. doi:10.1001/jama.2014.9005.
Why FDA hearing on morcellation safety could drive innovation
As a member of the Obstetrics & Gynecology Devices Panel FDA Advisory Committee, Dr. Iglesia digested the information presented at the hearing on July 10 and 11 and made her recommendations, along with her fellow panel members, for the fate of laparoscopic power morcellators to the FDA. Tune in to this special audiocast to hear Dr. Iglesia discuss the specific issues the panel weighed when making their final recommendations.
Dr. Iglesia is Director, Section of Female Pelvic Medicine and Reconstructive Surgery, MedStar Washington Hospital Center, and Associate Professor, Departments of ObGyn and Urology, Georgetown University School of Medicine, Washington, DC. She also serves on the OBG Management Board of Editors.
TRANSCRIPT
Janelle Yates: OBG Management Editorial Board Member Dr. Cheryl Iglesia attended the July 10th and 11th FDA hearing on microscopic power morcellation as a member of the Obstetrics and Gynecology Devices Panel Advisory Committee. In this audiocast, she describes the hearing and the panel’s recommendations as well as many of the fine points considered in weighing the risks and benefits of power morcellation. Dr. Iglesia is Director of the Section of Female Pelvic Medicine and Reconstructive Surgery at MedStar Washington Hospital Center, and Associate Professor in the Departments of Ob/Gyn and Neurology at Georgetown University’s School of Medicine in Washington, DC.
Dr. Iglesia, could you describe your role on the FDA’s Obstetrics and Gynecology Device Panel Advisory Committee?
Cheryl B. Iglesia, MD: I’m considered a special government employee and I have a 5-year term on the ObGyn Devices Panel. I was a member of the panel that reviewed vaginal mesh, and this is my second ObGyn devices panel as a consultant on power morcellation for laparoscopy. After the hearing, which was July 10th and 11th, we make recommendations as a panel but no final decisions are made until everything has been reviewed by officials at the FDA, and the FDA will come up with final decisions based in part on some of the recommendations that the panel has made. Therefore I can’t give you an official view, and what I’ll be talking about right now mostly represents my own opinion.
Ms. Yates: Just to review: What was the goal of the 2-day hearing, and whose points of view were represented to the panel?
Dr. Iglesia: The goal was to discuss the risk of disseminating unsuspected uterine malignancy with power morcellation. We talked on the panel about what the risk is of occult leiomyosarcoma in women with uterine fibroids. We talked about the preoperative screening evaluation process, talked about options for interoperative strategies to minimize or mitigate intraperitoneal fragmentation or dissemination of the tissue. They talked about various types of morcellators and, moving forward, if leiomyosarcoma was diagnosed, whether or not power morcellation upgraded an occult malignancy. And what the benchmarks should be for future devices, and whether or not future devices— not just for the power morcellators with containment, like containment bags—how they should be evaluated and tested moving forward. There was also some discussion about the role of registries.
Ms. Yates: What final recommendations did the panel make to the FDA?
Dr. Iglesia: Overall, there was a very long discussion about the risk of having an unsuspected sarcoma and the rates ranged from one in 350 to one in 7,450. What we as a panel realized is that while there are some indicators that could be suspicious for leiomyosarcoma, particularly on an MRI, that one cannot be 100% certain, particularly when you have a fibroid that’s degenerating, that it’s not just leiomyosarcoma but other occult malignancies.
The bottom line is the patients must be adequately worked up, particularly if there is abnormal bleeding. An evaluation would include normal cervical cytology, normal endometrial sampling, either sonograms or MRIs if indicated, and we talked about patient selection. In particular of being very worried about using morcellation in the postmenopausal woman who’s bleeding. We talked a lot about other options for morcellation. In general, if you can remove a uterus through the vagina or intact, that’s ideal because there’s a lot of data about the pros and cons from the vaginal approach to hysterectomy. But we’re not 100% certain that containment bags are going to be the “be-all and end-all.” In that, particularly if you’re doing subtotal hysterectomies, you still might be cutting through cancers and occult malignancies and the containment bags are very thin so that there’s a possibility that there could be leakage and/or breakage or unintended injury to other interperitoneal organs like bowels and vessels, etc. So we can’t be complacent about being 100% certain that things will go right even with the use of a bag.
Ms. Yates: Were there any final recommendations about informed consent?
Dr. Iglesia: A lot of discussion, particularly on the second day, was in the area of labeling special controls and it would be labeling for a patient and practitioners or physician surgeons who are using the morcellator. To the extent that—and there have been some precedents I think in silicon breast and other devices—where both the patient and the physician have to sign off that they’re aware that morcellators may be used, that there’s a potential for dissemination of an occult malignancy or even dissemination of benign disease like leiomyomatosis and incomplete removal. There are risks of using the morcellator in terms of injury, just a whole checklist. But it’s interesting for the labeling, both the patient and the physician in this: One of the recommendations for special controls would be included.
Ms. Yates: And would that involve a black box warning?
Dr. Iglesia: I think there were several discussions about the black box warning; I’m not sure what the final discussion is. Some people believe that with the black box on an administrative level, it sends a signal and a reminder to everyone about the labeling. But labeling can be done without a black box and it can be done with a black box. I’m not 100% certain how that will ultimately be decided upon by the FDA.
Ms. Yates: What were your reactions to the hearing, apart from your role on the panel? Did you feel that adequate testimony was heard from all the parties that have a stake in the immediate and long-term fate of laparoscopic power morcellation?
Dr. Iglesia: I think that the FDA did an excellent job in convening all the players, anybody who has interests in the stake I feel was represented--from industry and companies that make morcellators, companies that make containment bags, medical societies, ACOG, and AAGL gave testimony. AAGL’s testimony was very powerful, particularly by Dr. Jubilee Brown in mentioning that without the morcellator more women may be subjected to abdominal procedure, which in and of itself has some morbidity and mortality associated with that type of operation, and it was a nice study analysis. In terms of a decision tree what the potential harms would be without available morcellators to use, and I thought the MRI imaging that was done by the radiologist was also very interesting and discussed the limits of our ability to detect.
I also found some of the testimony to be extremely powerful from the patients, including that of Dr. Amy Reed and her family and the other women who presented. In some of the cases, we and several people on the panel, including myself, did wonder about the selection or the choice to use the morcellator in the first place in some cases, particularly in women who had uterine fibroids and they were postmenopausal. I think that that would be a particular case where you know go ahead and make an incision because there’s a potential higher index of suspicion for cancer in those kinds of cases.
Ms. Yates: Do you care to predict whether gynecologic surgeons will continue to use power morcellators after this controversy?
Dr. Iglesia: You know, and this was also discussed by Dr. Fisher and some of the officials from the FDA, that if anything this would be a call for innovation and improving products that could morcellate and contain at the same time. I know that we have some hysteroscopic morcellators that you can insert and there’s a vacuum and so things get kind of vacuumed up and whether or not we can develop something that has very little spill—obviously none at all would be key—and I do believe that at some point there will be some ingenuity and some improvements made to the current devices that will allow us to continue this is in our armamentarium.
What was interesting was that one of the questions that was addressed to the panel was, “When do you see that the benefits may outweigh the risks, in what population?” And leiomyosarcoma, which is just one of the occult malignancies—and there’s different types of sarcomas including endometrial stromal sarcomas and other endometrial cancer and malignancies. What’s interesting is that when you look at fibroids and even when you do a myomectomy it’s not necessarily just the power of morcellation, it’s just cutting through cancer or morcellating either vaginally or open. You’re doing an open myomectomy, just removal of the fibroid, and it turns out that that’s cancer. You know that is not a good prognosis to start with, but to spread it clearly is not good for the patient and makes a bad condition even worse.
But it really means that we need to do a better job in pretty accurately identifying patients. And while we’re there, we did mention on the panel that maybe we can develop risk calculators or ways to stratify based on the MRI imaging, patient age, patient race, and whether or not something has a higher index of suspicion for being cancerous. What I meant to say is the two benefits outweigh the risk.
One other case was the young 20-something-year-old fertility patient with the fibroids that clearly have an impact. I mean you don’t want to do a hysterectomy and you still want to remove the fibroid in as noninvasive a way as possible and morcellation may be the best in terms of creating less adhesions. The other case was something that I had mentioned in patients who have prolapse who you’re thinking about placing mesh. Some people do subtotal hysterectomies and then attach the mesh to the cervix as opposed to the vaginal cuff to decrease the risk of cuff erosion and that’s another technique where benefits might outweigh the risk, particularly in older postmenopausal women with not particularly enlarged uteri. So, more to come.
As a member of the Obstetrics & Gynecology Devices Panel FDA Advisory Committee, Dr. Iglesia digested the information presented at the hearing on July 10 and 11 and made her recommendations, along with her fellow panel members, for the fate of laparoscopic power morcellators to the FDA. Tune in to this special audiocast to hear Dr. Iglesia discuss the specific issues the panel weighed when making their final recommendations.
Dr. Iglesia is Director, Section of Female Pelvic Medicine and Reconstructive Surgery, MedStar Washington Hospital Center, and Associate Professor, Departments of ObGyn and Urology, Georgetown University School of Medicine, Washington, DC. She also serves on the OBG Management Board of Editors.
TRANSCRIPT
Janelle Yates: OBG Management Editorial Board Member Dr. Cheryl Iglesia attended the July 10th and 11th FDA hearing on microscopic power morcellation as a member of the Obstetrics and Gynecology Devices Panel Advisory Committee. In this audiocast, she describes the hearing and the panel’s recommendations as well as many of the fine points considered in weighing the risks and benefits of power morcellation. Dr. Iglesia is Director of the Section of Female Pelvic Medicine and Reconstructive Surgery at MedStar Washington Hospital Center, and Associate Professor in the Departments of Ob/Gyn and Neurology at Georgetown University’s School of Medicine in Washington, DC.
Dr. Iglesia, could you describe your role on the FDA’s Obstetrics and Gynecology Device Panel Advisory Committee?
Cheryl B. Iglesia, MD: I’m considered a special government employee and I have a 5-year term on the ObGyn Devices Panel. I was a member of the panel that reviewed vaginal mesh, and this is my second ObGyn devices panel as a consultant on power morcellation for laparoscopy. After the hearing, which was July 10th and 11th, we make recommendations as a panel but no final decisions are made until everything has been reviewed by officials at the FDA, and the FDA will come up with final decisions based in part on some of the recommendations that the panel has made. Therefore I can’t give you an official view, and what I’ll be talking about right now mostly represents my own opinion.
Ms. Yates: Just to review: What was the goal of the 2-day hearing, and whose points of view were represented to the panel?
Dr. Iglesia: The goal was to discuss the risk of disseminating unsuspected uterine malignancy with power morcellation. We talked on the panel about what the risk is of occult leiomyosarcoma in women with uterine fibroids. We talked about the preoperative screening evaluation process, talked about options for interoperative strategies to minimize or mitigate intraperitoneal fragmentation or dissemination of the tissue. They talked about various types of morcellators and, moving forward, if leiomyosarcoma was diagnosed, whether or not power morcellation upgraded an occult malignancy. And what the benchmarks should be for future devices, and whether or not future devices— not just for the power morcellators with containment, like containment bags—how they should be evaluated and tested moving forward. There was also some discussion about the role of registries.
Ms. Yates: What final recommendations did the panel make to the FDA?
Dr. Iglesia: Overall, there was a very long discussion about the risk of having an unsuspected sarcoma and the rates ranged from one in 350 to one in 7,450. What we as a panel realized is that while there are some indicators that could be suspicious for leiomyosarcoma, particularly on an MRI, that one cannot be 100% certain, particularly when you have a fibroid that’s degenerating, that it’s not just leiomyosarcoma but other occult malignancies.
The bottom line is the patients must be adequately worked up, particularly if there is abnormal bleeding. An evaluation would include normal cervical cytology, normal endometrial sampling, either sonograms or MRIs if indicated, and we talked about patient selection. In particular of being very worried about using morcellation in the postmenopausal woman who’s bleeding. We talked a lot about other options for morcellation. In general, if you can remove a uterus through the vagina or intact, that’s ideal because there’s a lot of data about the pros and cons from the vaginal approach to hysterectomy. But we’re not 100% certain that containment bags are going to be the “be-all and end-all.” In that, particularly if you’re doing subtotal hysterectomies, you still might be cutting through cancers and occult malignancies and the containment bags are very thin so that there’s a possibility that there could be leakage and/or breakage or unintended injury to other interperitoneal organs like bowels and vessels, etc. So we can’t be complacent about being 100% certain that things will go right even with the use of a bag.
Ms. Yates: Were there any final recommendations about informed consent?
Dr. Iglesia: A lot of discussion, particularly on the second day, was in the area of labeling special controls and it would be labeling for a patient and practitioners or physician surgeons who are using the morcellator. To the extent that—and there have been some precedents I think in silicon breast and other devices—where both the patient and the physician have to sign off that they’re aware that morcellators may be used, that there’s a potential for dissemination of an occult malignancy or even dissemination of benign disease like leiomyomatosis and incomplete removal. There are risks of using the morcellator in terms of injury, just a whole checklist. But it’s interesting for the labeling, both the patient and the physician in this: One of the recommendations for special controls would be included.
Ms. Yates: And would that involve a black box warning?
Dr. Iglesia: I think there were several discussions about the black box warning; I’m not sure what the final discussion is. Some people believe that with the black box on an administrative level, it sends a signal and a reminder to everyone about the labeling. But labeling can be done without a black box and it can be done with a black box. I’m not 100% certain how that will ultimately be decided upon by the FDA.
Ms. Yates: What were your reactions to the hearing, apart from your role on the panel? Did you feel that adequate testimony was heard from all the parties that have a stake in the immediate and long-term fate of laparoscopic power morcellation?
Dr. Iglesia: I think that the FDA did an excellent job in convening all the players, anybody who has interests in the stake I feel was represented--from industry and companies that make morcellators, companies that make containment bags, medical societies, ACOG, and AAGL gave testimony. AAGL’s testimony was very powerful, particularly by Dr. Jubilee Brown in mentioning that without the morcellator more women may be subjected to abdominal procedure, which in and of itself has some morbidity and mortality associated with that type of operation, and it was a nice study analysis. In terms of a decision tree what the potential harms would be without available morcellators to use, and I thought the MRI imaging that was done by the radiologist was also very interesting and discussed the limits of our ability to detect.
I also found some of the testimony to be extremely powerful from the patients, including that of Dr. Amy Reed and her family and the other women who presented. In some of the cases, we and several people on the panel, including myself, did wonder about the selection or the choice to use the morcellator in the first place in some cases, particularly in women who had uterine fibroids and they were postmenopausal. I think that that would be a particular case where you know go ahead and make an incision because there’s a potential higher index of suspicion for cancer in those kinds of cases.
Ms. Yates: Do you care to predict whether gynecologic surgeons will continue to use power morcellators after this controversy?
Dr. Iglesia: You know, and this was also discussed by Dr. Fisher and some of the officials from the FDA, that if anything this would be a call for innovation and improving products that could morcellate and contain at the same time. I know that we have some hysteroscopic morcellators that you can insert and there’s a vacuum and so things get kind of vacuumed up and whether or not we can develop something that has very little spill—obviously none at all would be key—and I do believe that at some point there will be some ingenuity and some improvements made to the current devices that will allow us to continue this is in our armamentarium.
What was interesting was that one of the questions that was addressed to the panel was, “When do you see that the benefits may outweigh the risks, in what population?” And leiomyosarcoma, which is just one of the occult malignancies—and there’s different types of sarcomas including endometrial stromal sarcomas and other endometrial cancer and malignancies. What’s interesting is that when you look at fibroids and even when you do a myomectomy it’s not necessarily just the power of morcellation, it’s just cutting through cancer or morcellating either vaginally or open. You’re doing an open myomectomy, just removal of the fibroid, and it turns out that that’s cancer. You know that is not a good prognosis to start with, but to spread it clearly is not good for the patient and makes a bad condition even worse.
But it really means that we need to do a better job in pretty accurately identifying patients. And while we’re there, we did mention on the panel that maybe we can develop risk calculators or ways to stratify based on the MRI imaging, patient age, patient race, and whether or not something has a higher index of suspicion for being cancerous. What I meant to say is the two benefits outweigh the risk.
One other case was the young 20-something-year-old fertility patient with the fibroids that clearly have an impact. I mean you don’t want to do a hysterectomy and you still want to remove the fibroid in as noninvasive a way as possible and morcellation may be the best in terms of creating less adhesions. The other case was something that I had mentioned in patients who have prolapse who you’re thinking about placing mesh. Some people do subtotal hysterectomies and then attach the mesh to the cervix as opposed to the vaginal cuff to decrease the risk of cuff erosion and that’s another technique where benefits might outweigh the risk, particularly in older postmenopausal women with not particularly enlarged uteri. So, more to come.
As a member of the Obstetrics & Gynecology Devices Panel FDA Advisory Committee, Dr. Iglesia digested the information presented at the hearing on July 10 and 11 and made her recommendations, along with her fellow panel members, for the fate of laparoscopic power morcellators to the FDA. Tune in to this special audiocast to hear Dr. Iglesia discuss the specific issues the panel weighed when making their final recommendations.
Dr. Iglesia is Director, Section of Female Pelvic Medicine and Reconstructive Surgery, MedStar Washington Hospital Center, and Associate Professor, Departments of ObGyn and Urology, Georgetown University School of Medicine, Washington, DC. She also serves on the OBG Management Board of Editors.
TRANSCRIPT
Janelle Yates: OBG Management Editorial Board Member Dr. Cheryl Iglesia attended the July 10th and 11th FDA hearing on microscopic power morcellation as a member of the Obstetrics and Gynecology Devices Panel Advisory Committee. In this audiocast, she describes the hearing and the panel’s recommendations as well as many of the fine points considered in weighing the risks and benefits of power morcellation. Dr. Iglesia is Director of the Section of Female Pelvic Medicine and Reconstructive Surgery at MedStar Washington Hospital Center, and Associate Professor in the Departments of Ob/Gyn and Neurology at Georgetown University’s School of Medicine in Washington, DC.
Dr. Iglesia, could you describe your role on the FDA’s Obstetrics and Gynecology Device Panel Advisory Committee?
Cheryl B. Iglesia, MD: I’m considered a special government employee and I have a 5-year term on the ObGyn Devices Panel. I was a member of the panel that reviewed vaginal mesh, and this is my second ObGyn devices panel as a consultant on power morcellation for laparoscopy. After the hearing, which was July 10th and 11th, we make recommendations as a panel but no final decisions are made until everything has been reviewed by officials at the FDA, and the FDA will come up with final decisions based in part on some of the recommendations that the panel has made. Therefore I can’t give you an official view, and what I’ll be talking about right now mostly represents my own opinion.
Ms. Yates: Just to review: What was the goal of the 2-day hearing, and whose points of view were represented to the panel?
Dr. Iglesia: The goal was to discuss the risk of disseminating unsuspected uterine malignancy with power morcellation. We talked on the panel about what the risk is of occult leiomyosarcoma in women with uterine fibroids. We talked about the preoperative screening evaluation process, talked about options for interoperative strategies to minimize or mitigate intraperitoneal fragmentation or dissemination of the tissue. They talked about various types of morcellators and, moving forward, if leiomyosarcoma was diagnosed, whether or not power morcellation upgraded an occult malignancy. And what the benchmarks should be for future devices, and whether or not future devices— not just for the power morcellators with containment, like containment bags—how they should be evaluated and tested moving forward. There was also some discussion about the role of registries.
Ms. Yates: What final recommendations did the panel make to the FDA?
Dr. Iglesia: Overall, there was a very long discussion about the risk of having an unsuspected sarcoma and the rates ranged from one in 350 to one in 7,450. What we as a panel realized is that while there are some indicators that could be suspicious for leiomyosarcoma, particularly on an MRI, that one cannot be 100% certain, particularly when you have a fibroid that’s degenerating, that it’s not just leiomyosarcoma but other occult malignancies.
The bottom line is the patients must be adequately worked up, particularly if there is abnormal bleeding. An evaluation would include normal cervical cytology, normal endometrial sampling, either sonograms or MRIs if indicated, and we talked about patient selection. In particular of being very worried about using morcellation in the postmenopausal woman who’s bleeding. We talked a lot about other options for morcellation. In general, if you can remove a uterus through the vagina or intact, that’s ideal because there’s a lot of data about the pros and cons from the vaginal approach to hysterectomy. But we’re not 100% certain that containment bags are going to be the “be-all and end-all.” In that, particularly if you’re doing subtotal hysterectomies, you still might be cutting through cancers and occult malignancies and the containment bags are very thin so that there’s a possibility that there could be leakage and/or breakage or unintended injury to other interperitoneal organs like bowels and vessels, etc. So we can’t be complacent about being 100% certain that things will go right even with the use of a bag.
Ms. Yates: Were there any final recommendations about informed consent?
Dr. Iglesia: A lot of discussion, particularly on the second day, was in the area of labeling special controls and it would be labeling for a patient and practitioners or physician surgeons who are using the morcellator. To the extent that—and there have been some precedents I think in silicon breast and other devices—where both the patient and the physician have to sign off that they’re aware that morcellators may be used, that there’s a potential for dissemination of an occult malignancy or even dissemination of benign disease like leiomyomatosis and incomplete removal. There are risks of using the morcellator in terms of injury, just a whole checklist. But it’s interesting for the labeling, both the patient and the physician in this: One of the recommendations for special controls would be included.
Ms. Yates: And would that involve a black box warning?
Dr. Iglesia: I think there were several discussions about the black box warning; I’m not sure what the final discussion is. Some people believe that with the black box on an administrative level, it sends a signal and a reminder to everyone about the labeling. But labeling can be done without a black box and it can be done with a black box. I’m not 100% certain how that will ultimately be decided upon by the FDA.
Ms. Yates: What were your reactions to the hearing, apart from your role on the panel? Did you feel that adequate testimony was heard from all the parties that have a stake in the immediate and long-term fate of laparoscopic power morcellation?
Dr. Iglesia: I think that the FDA did an excellent job in convening all the players, anybody who has interests in the stake I feel was represented--from industry and companies that make morcellators, companies that make containment bags, medical societies, ACOG, and AAGL gave testimony. AAGL’s testimony was very powerful, particularly by Dr. Jubilee Brown in mentioning that without the morcellator more women may be subjected to abdominal procedure, which in and of itself has some morbidity and mortality associated with that type of operation, and it was a nice study analysis. In terms of a decision tree what the potential harms would be without available morcellators to use, and I thought the MRI imaging that was done by the radiologist was also very interesting and discussed the limits of our ability to detect.
I also found some of the testimony to be extremely powerful from the patients, including that of Dr. Amy Reed and her family and the other women who presented. In some of the cases, we and several people on the panel, including myself, did wonder about the selection or the choice to use the morcellator in the first place in some cases, particularly in women who had uterine fibroids and they were postmenopausal. I think that that would be a particular case where you know go ahead and make an incision because there’s a potential higher index of suspicion for cancer in those kinds of cases.
Ms. Yates: Do you care to predict whether gynecologic surgeons will continue to use power morcellators after this controversy?
Dr. Iglesia: You know, and this was also discussed by Dr. Fisher and some of the officials from the FDA, that if anything this would be a call for innovation and improving products that could morcellate and contain at the same time. I know that we have some hysteroscopic morcellators that you can insert and there’s a vacuum and so things get kind of vacuumed up and whether or not we can develop something that has very little spill—obviously none at all would be key—and I do believe that at some point there will be some ingenuity and some improvements made to the current devices that will allow us to continue this is in our armamentarium.
What was interesting was that one of the questions that was addressed to the panel was, “When do you see that the benefits may outweigh the risks, in what population?” And leiomyosarcoma, which is just one of the occult malignancies—and there’s different types of sarcomas including endometrial stromal sarcomas and other endometrial cancer and malignancies. What’s interesting is that when you look at fibroids and even when you do a myomectomy it’s not necessarily just the power of morcellation, it’s just cutting through cancer or morcellating either vaginally or open. You’re doing an open myomectomy, just removal of the fibroid, and it turns out that that’s cancer. You know that is not a good prognosis to start with, but to spread it clearly is not good for the patient and makes a bad condition even worse.
But it really means that we need to do a better job in pretty accurately identifying patients. And while we’re there, we did mention on the panel that maybe we can develop risk calculators or ways to stratify based on the MRI imaging, patient age, patient race, and whether or not something has a higher index of suspicion for being cancerous. What I meant to say is the two benefits outweigh the risk.
One other case was the young 20-something-year-old fertility patient with the fibroids that clearly have an impact. I mean you don’t want to do a hysterectomy and you still want to remove the fibroid in as noninvasive a way as possible and morcellation may be the best in terms of creating less adhesions. The other case was something that I had mentioned in patients who have prolapse who you’re thinking about placing mesh. Some people do subtotal hysterectomies and then attach the mesh to the cervix as opposed to the vaginal cuff to decrease the risk of cuff erosion and that’s another technique where benefits might outweigh the risk, particularly in older postmenopausal women with not particularly enlarged uteri. So, more to come.
A guide to lotions and potions for treating vaginal atrophy
The authors would like to acknowledge Lauren Melcher, MD, an ObGyn resident at Washington Hospital Center, who contributed to this article.
CASE: New-onset dyspareunia in a menopausal patient
J. B., 53 years old, has been menopausal for 2 years. Several months after her annual examination, she schedules another appointment to discuss a worsening complaint: dyspareunia. She says she never had the problem until she reached menopause, and reports that it has become so severe that she has started avoiding sexual intercourse altogether. Even when she avoids intercourse, however, she is bothered by vaginal itching and burning.
What can you offer to her?
Various hormonal and nonhormonal products are available to relieve the frequent complaint, in menopausal women, of symptoms of vaginal atrophy: vaginal dryness, itching, burning, and dyspareunia.1-3 The array of products isn’t really surprising: As women advance through menopause, their complaints of vaginal dryness increase fivefold.4
Systemic and local estrogen therapies reverse some atrophic changes and alleviate symptoms.5 After menopause, local vaginal estrogen formulations are recommended as first-line treatment for women who experience moderate or severe symptoms of vaginal atrophy.3 Formulations such as the vaginal ring, vaginal tablet, and transdermal gels and sprays are increasingly popular.
In this article, we describe these and other products, including nonhormonal lubricants and moisturizers, to relieve:
- the range of symptoms of vaginal atrophy in menopausal women
- isolated vaginal dryness in premenopausal women.
Is hormonal therapy always necessary?
When a postmenopausal woman complains of chronic vaginal dryness, and the exam is consistent with vaginal atrophy, the recommended treatment is local vaginal estrogen. If she complains of vaginal dryness during sexual intercourse only, a vaginal lubricant is a suitable option.
When a premenopausal woman complains of vaginal dryness, a vaginal moisturizer is the best long-term treatment option. However, a vaginal lubricant is recommended for intermittent dryness during intercourse or dyspareunia.
Local estrogens avoid many risks of systemic therapy
Topical estrogen preparations are available as vaginal creams, tablets, and rings, and as transdermal lotions, gels, and patches (TABLE 1). Local preparations are preferred to systemic therapy for the treatment of atrophy because they bypass the gastrointestinal tract, undergo less conversion in the liver, and improve local tissue with minimal elevation of the serum estradiol level.1,3
The vaginal ring (Estring) delivers the lowest systemic estradiol level—approximately 5 to 10 μg of estradiol daily. Femring delivers more estradiol daily and requires the addition of progesterone in women who have an intact uterus.
Studies suggest that patients favor the estradiol-releasing vaginal ring because of its ease of use, comfort, and effectiveness, compared with vaginal estrogen cream.2,5
Local estrogen formulations were compared and reviewed in a systematic Cochrane meta-analysis of 19 trials that included 4,162 women.5 Vaginal cream, tablets, and rings were all equally effective in treating symptoms of atrophy. One trial found that cream (conjugated equine estrogen) increased the risk of uterine bleeding, breast pain, and perineal pain, compared with vaginal tablets.
Newer estrogen formulations include topical and transdermal patches, gels, lotions, and sprays (TABLE 1), all of which are systemic. They are effective in the treatment of vasomotor symptoms and vaginal atrophy.
TABLE 1
Topical estrogen formulations—a rundown of local and systemic options
| Product | Dosing | Administration | Source of active ingredient |
|---|---|---|---|
| Absorbed locally | |||
| VAGINAL TABLET | |||
| Vagifem | 25 μg of estradiol | One tablet intravaginally daily for 2 weeks; then, twice weekly | Synthesized from soy |
| VAGINAL CREAM | |||
| Premarin | 0.5 g (0.625 mg/g of conjugated estrogen) | Insert 0.5 g daily for 3 weeks; then, twice weekly (Note: Dosage can be increased to 2 g daily but this may require progesterone supplementation) | Urine of pregnant mares |
| Estrace | 0.1 mg of estradiol/g of cream | Insert 0.5 g daily for 1 or 2 weeks; then, twice weekly | Synthesized from soy and yams |
| VAGINAL RING | |||
| Estring | 2 mg (delivers 6–9 μg of estradiol daily) | Insert 1 ring intravaginally for 3 months | Synthesized from Mexican yams |
| Absorbed systemically | |||
| VAGINAL RING | |||
| Femring | Delivers 0.05 mg–0.1 mg of estradiol daily | Insert 1 ring intravaginally for 3 months | Synthesized from soy |
| ESTROGEN PATCH | |||
| Estraderm | Delivers 0.05 mg or 0.1 mg of estradiol daily | Apply patch twice weekly | Synthesized from Mexican yams |
| Estradiol (generic) | Delivers 0.05 mg or 0.1 mg of estradiol daily | ||
| Esclim | Delivers 0.025 mg, 0.0375 mg, 0.05 mg, 0.075 mg, or 0.1 mg of estradiol daily | ||
| Vivelle, Vivelle-Dot | Delivers 0.025 mg, 0.0375 mg, 0.05 mg, 0.075 mg, or 0.1 mg of estradiol daily | Synthesized from Mexican yams | |
| Climara | Delivers 0.025 mg, 0.0375 mg, 0.05 mg, 0.06 mg, 0.075 mg, or 0.1 mg of estradiol daily | Synthesized from soy | |
| Alora | Delivers 0.025 mg, 0.05 mg, 0.075 mg, or 0.1 mg of estradiol daily | ||
| Menostar | Delivers 0.014 mg of estradiol daily | Apply patch once weekly (Note: Indicated only for prevention of osteoporosis) | |
| CombiPatch | Delivers 0.05 mg or 0.14 mg daily of estradiol plus 0.05 mg or 0.25 mg daily of norethindrone | Apply patch twice weekly | Synthesized from soy (estradiol) and Mexican yams (norethrindrone) |
| ESTROGEN LOTION, GEL | |||
| Estrasorb (lotion) | Content of two pouches delivers 0.05 mg daily of estradiol | Apply one packet to each leg daily | Synthesized from soy |
| EstroGel (gel) | 1.25 g (0.75 mg of estradiol) | Apply one pump to arm once daily | |
| Divigel (gel) | 0.25 g, 0.5 g, or 1 g of 0.1% estradiol | Apply one packet to upper thigh daily | |
| Elestrin (gel) | 0.87 g (0.52 mg of estradiol) | Apply one pump to arm once daily | |
| ESTROGEN SPRAY | |||
| Evamist | 1.53 mg of estradiol in each spray | Apply 1-3 sprays to forearm daily | |
| Source: Cirigliano M. Bioidentical hormone therapy: a review of the evidence. J Womens Health (Larchmt). 2007;16:600–631. | |||
When to add a progestin
A progestin is recommended in addition to a systemic estrogen formulation in women who have a uterus. For low-dose, local vaginal estrogen formulations, a progestin is usually not needed.3 However, when the treatment is vaginal cream, consider progestin supplementation when the dosage exceeds 0.5 g twice weekly for an extended time (>1 year).
The serum estrogen level with local vaginal treatment is dose-dependent, and the long-term endometrial effects of vaginal estrogens are unknown. If vaginal bleeding develops, a workup is indicated and may necessitate imaging of the endometrial echo or endometrial sampling to rule out hyperplasia, neoplasia, and cancer.
Counsel the patient about any risks
If you prescribe transdermal or oral estrogen for a patient, be sure to counsel her about the risks of systemic therapy described in the Women’s Health Initiative.6
Consider the patient’s preference
Local estrogen treatment is recommended over systemic therapy for vaginal atrophy, but patient preference should also be considered. Some women may prefer the ring or tablet to minimize excess vaginal discharge, while others may prefer a cream because of its soothing effects. Always individualize management!
Lubricants and moisturizers
Insufficient lubrication during intercourse is a common complaint among both premenopausal and postmenopausal women: As many as 60% of women report intermittent episodes of insufficient lubrication.7
Many women and their partners use a vaginal lubricant to assist with sexual relations and to self-treat for pain. A wide variety of nonhormonal products are available—many of them advertised at pharmacies and in the media—despite little published scientific evaluation. Because gynecologists routinely counsel patients on sensitive matters, including sexual practices, you may find it valuable—with appropriate candidates—to open a line of questioning about difficulties with intercourse and resulting attempts to self-medicate using over-the-counter products.
What are the indications?
A vaginal lubricant is a solution used locally, and as a temporary measure, to moisten the vaginal epithelium to facilitate a medical examination or sexual intercourse.2 Because it has a short duration, it must be applied at the time of intercourse. Lubricants can be categorized as water-, silicone-, and oil-based. Each formulation may affect the local inflammatory response, viability of sperm, and condom integrity.
A vaginal moisturizer is a gel or cream used regularly to maintain hydration of the vaginal epithelium for long-term relief of vaginal dryness.2
Both lubricants and moisturizers have many indications for both medical and personal use. Personal lubricants can be used for assistance during sexual activity, such as intercourse, masturbation, or use of sex toys. These products reduce friction and are thought to enhance pleasure in women who suffer from vaginal dryness. However, we lack sufficient data to confirm that lubricants can improve sexual dysfunction and vaginal atrophy. In general, these products are affordable, readily available, and may be helpful in the treatment of sexual dysfunction and vaginal dryness.
See TABLE 2 for a list of personal lubricants and vaginal moisturizers.
TABLE 2
Vaginal moisturizers and lubricants are plentiful and diverse
| Product (Manufacturer) | Ingredients | Notes |
|---|---|---|
| MOISTURIZERS | ||
| Replens (Columbia Laboratories) | Water, carbomer, polycarbophil, paraffin, hydrogenated palm oil, glyceride, sorbic acid, and sodium hydroxide | Should be used 3 times weekly |
| Moist Again (Lake Consumer Products) | Water, carbomer, aloe, citric acid, chlorhexidine deglutinate, sodium benzoate, potassium sorbate, diazolidinyl urea, and sorbic acid | Safe to use with a latex condom; no data on effects on sperm motility |
| Vagisil Feminine Moisturizer (Combe) | Water, glycerin, propylene glycol, poloxamer 407, methylparaben, polyquaternium-32, propylparaben, chamomile, and aloe | |
| Feminease (Parnell Pharmaceuticals) | Water, mineral oil, glycerin, yerba santa, cetyl alcohol, and methyl paraben | Yerba santa (Eriodictyon spp), a plant native to the Pacific Northwest, is used as a moisturizer in place of aloe |
| K-Y Long Lasting Moisturizer (McNeil) | Purified water, glycerin, mineral oil, calcium/sodium PVM/MA copolymer, PVM/MA decadiene crosspolymer, hydrogenated palm glyceride, methylparaben, benzoic acid, tocopherol acetate, and sodium hydroxide | |
| K-Y Silk-E (McNeil) | Water, propylene glycol, sorbitol, polysorbate 60, hydroxyethylcellulose, benzoic acid, methylparaben, tocopherol, and aloe | |
| LUBRICANTS | ||
| Water-based | ||
| Slippery Stuff (Wallace-O’Farrell) | Water, polyoxyethylene, methylparaben, propylene glycol, isopropynol | |
| Astroglide (BioFilm) | Water, glycerin, methylparaben, propylparaben, polypropylene glycol, polyquaternium, hydroxyethylcellulose, and sodium benzoate | Also sold in a glycerin-free and paraben-free formulation |
| K-Y Jelly (McNeil) | Water, glycerin, hydroxyethylcellulose, parabens, and chlorhexidine | |
| Summer’s Eve Lubricant (C.B. Fleet) | Water, propylene glycol, methylcellulose, xanthan gum, sodium lactate, methylparaben, lactic acid, dextrose, sodium chloride, edatate disodium, pectin, and propylparaben | |
| FemGlide (WalMed) | Water, polyoxyethylene, methylparaben, and sodium carbomer | |
| Pre-Seed (INGfertility) | Water, hydroxyethylcellulose, arabinogalactan, paraben, and Pluronic copolymers | Promoted to women and their partners who are trying to conceive |
| Silicone-based | ||
| ID Millennium (Westridge Laboratories) | Cyclomethicone, dimethicone, and dimethiconol | Less drying than other lubricants |
| Pjur | Cyclopentasiloxane, dimethicone, and dimethiconol | Compatible with a condom |
| Pink | Dimethicone, vitamin E, aloe vera, dimethiconol, and cyclomethicone | |
| K-Y Liquibeads (McNeil) | Dimethicone, gelatin, glycerin, and dimethiconol | Active ingredients are contained in so-called ovules that release lubricant over several days |
| Oil-based | ||
| Élégance Women’s Lubricant | Natural oils | Does not contain alcohol, glycerin, or parabens; is incompatible with a condom; helpful for women who have vulvodynia or vestibulitis |
What to offer when estrogen is not an option
Some women may want to avoid hormonal treatment, or have a contraindication to it, such as estrogen-receptor–positive breast cancer.8 In premenopausal women, vaginal atrophy can occur with lactation or postpartum hormonal changes, or may result from the use of anti-estrogenic agents for breast cancer. Other candidates for nonhormonal therapy are women who have chronic vulvar pain syndromes. In these women, vaginal lubricants can be especially useful.
Although they are less effective than estrogen, vaginal moisturizers, such as Replens, have been shown to reverse symptoms of vaginal atrophy and decrease discomfort during intercourse.9
The therapeutic options for vaginal atrophy are likely to broaden in the near future. Ospemifene (Ophena), a selective estrogen receptor modulator (SERM) under development for the treatment of vaginal atrophy, has reached the end of Phase-3 clinical study, with positive efficacy results. A long-term safety study of the orally administered SERM has also been completed, reports QuatRx, the drug’s manufacturer. The company expects to file a New Drug Application with the US Food and Drug Administration early next year.
Phase-3 trials documented significant improvement in dryness, dyspareunia, and other endpoints
The first Phase-3 study of Ophena was announced by QuatRx in January 2008 and presented at the 90th annual meeting of the Endocrine Society. Women who were treated with 60 mg daily of Ophena experienced statistically significant improvement in vaginal dryness, dyspareunia, and the proportion of parabasal and superficial cells in the epithelium of vaginal walls. The vaginal pH level also declined. The drug did not cause hot flushes among users.
The second Phase-3 study was a randomized, double-blind, placebo-controlled study of 919 women who had vulvovaginal atrophy. It was conducted at 116 sites in the United States. Among the cohort of 605 women who identified dyspareunia as their most bothersome symptom, positive efficacy results were achieved in all four primary endpoints, including:
- a decrease in parabasal cells
- an increase in superficial cells
- a decrease in the vaginal pH level
- improvement in dyspareunia.
The trial demonstrated statistically significant improvement from baseline to week 12 in all four endpoints, compared with placebo (P.0001>
All women were supplied with a nonhormonal vaginal lubricant to be used as needed during the treatment period; the study found efficacy above and beyond usage of this lubricant, according to a press release from QuatRx.
Is the benefit worth the risk?
Some have questioned whether a systemic drug is overkill for a complaint like postmenopausal vaginal atrophy.14 Because Ophena is a SERM, it is likely to carry a cardiovascular risk profile similar to that of other drugs in its class. For example, in a large randomized trial, raloxifene (Evista) failed to reduce coronary artery disease and significantly increased the incidence of fatal stroke and venous thromboembolism.15 When local estrogen formulations that do not carry such risks are already available, some experts question the advisability of developing another systemic agent.
Another question: Is it realistic to expect the patient to take a drug every day when her chief complaint is postmenopausal dyspareunia and she is likely to have intercourse only once or twice a week?
These questions probably won’t be addressed until the drug enters the market—and physicians and their patients will be the ones providing the answers.
Specialty lubricants are unproven
In contrast to products designed to treat vaginal dryness and atrophy, some lubricants are marketed specifically for sexual enhancement. Warming lubricants cause a heating sensation on the skin and usually contain menthol, L-arginine, or capsaicin. Natural and artificial flavors are used to manufacture flavored lubricants.
None of these products have been scientifically proven to enhance sexual function.
Oil-based lubricants may impede condom integrity
It is estimated that 40% of couples who use condoms also use a lubricant to assist with intercourse.10 The integrity of latex condoms has been shown to deteriorate with the use of an oil-based lubricant or petrolatum. One study, in which the mean burst time of condoms was assessed during pressurized air inflation, showed a significant reduction in that time when vaginal lubricants that contained mineral or vegetable oil were used.11
Oil-based lubricants also have been shown to increase the slippage rate, with a trend toward increased breakage.10
Water-based lubricants may slightly increase slippage, but they reduce breakage.
Women should avoid oil-based lubricants when their partner uses a condom.
Some lubricants affect sperm quality
Choosing a vaginal lubricant can be of particular concern to a woman who is being treated for infertility. Lubricants may affect the integrity and function of sperm, even if they do not contain spermicide. Noncommercial products, such as glycerin, olive oil, vegetable oil, and, even, saliva have been associated with a loss of sperm function.12
A recent study found that Replens and Astroglide cause a dramatic decrease in sperm motility. FemGlide causes less of a decrease—but still a significant one.12 The nonphysiologic osmolality and pH of these products may be the cause of such sperm damage. Pre-Seed, which has a more physiologic pH level and isotonic quality, was found to cause minimal harm to sperm motility and chromatin quality.12
Avoid propylene glycol in women who have vulvodynia
Vaginal lubricants and moisturizers are also used in the treatment of vulvodynia or chronic vulvar pain syndromes. According to an ACOG Committee Opinion, topical application of preservative-free solutions, such as vegetable oil or plain petrolatum, is recommended to hold moisture within the tissues and provide a protective barrier.13 Adequate lubrication is also recommended during intercourse.
Products that contain propylene glycol, or alcohol, may act as an irritant in women who experience local pain and heightened vaginal sensitivity. For that reason, such products should be avoided in this population.
CASE RESOLVED
Careful examination reveals urogenital atrophy with absence of any fungal or bacterial infection of the vulva or vagina. The patient chooses to use a water-based lubricant during sexual relations and begins using intravaginal estradiol tablets (other options include the vaginal ring or cream). Symptoms of dyspareunia disappear almost immediately, and vaginal burning improves after 6 weeks.
1. Al-Baghdadi O, Ewies AA. Topical estrogen therapy in the management of postmenopausal vaginal atrophy: an up-to-date overview. Climacteric. 2009;12:91-105.
2. Willhite LA, O’Connell MB. Urogenital atrophy: prevention and treatment. Pharmacotherapy. 2001;21:464-480.
3. Archer DF. Efficacy and tolerability of local estrogen therapy for urogenital atrophy. Menopause. 2009;17:1-10.
4. Dennerstein L, Dudley EC, Hopper JL, Guthrie JR, Burger HG. A prospective population-based study of menopausal symptoms. Obstet Gynecol. 2000;96:351-358.
5. Suckling J, Lethaby A, Kennedy R. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2006;(4):CD001500.-
6. Anderson GL, Limacher M, Assaf AR, et al. Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701-1712.
7. Oberg K, Fugl-Meyer AR, Fugl-Meyer KS. On categorization and quantification of women’s sexual dysfunctions: an epidemiological approach. Int J Impot Res. 2004;16:261-269.
8. Loprinzi CL, Wolf SL, Barton DL, Laack NN. Symptom management in premenopausal patients with breast cancer. Lancet Oncol. 2008;9:993-1001.
9. Bygdeman M, Swahn ML. Replens versus dienoestrol cream in the symptomatic treatment of vaginal atrophy in postmenopausal women. Maturitas. 1996;23:259-263.
10. Steiner M, Piedrahita C, Glover L, Joanis C, Spruyt A, Foldesy R. The impact of lubricants on latex condoms during vaginal intercourse. Int J STD AIDS. 1994;5:29-36.
11. Rosen AD, Rosen T. Study of condom integrity after brief exposure to over-the-counter vaginal preparations. South Med J. 1999;92:305-307.
12. Agarwal A, Deepinder F, Cocuzza M, Short RA, Evenson DP. Effect of vaginal lubricants on sperm motility and chromatin integrity: a prospective comparative study. Fertil Steril. 2008;89:375-379.
13. ACOG Committee Opinion #345: Vulvodynia. October 2006. ACOG Committee on Gynecologic Practice. Obstet Gynecol. 2006;108:1049-1052.
14. Ophena is a me-too drug with an impractical mode of administration. Gerson Lehrman Group. Jan. 14, 2008. Available at: http://www.glgroup.com/News/Ophena-is-a-Me-Too-Drug-with-an-Impractical-Mode-of-Administration-20651.html. Accessed Nov. 11, 2009.
15. de Villiers TJ. Clinical issues regarding cardiovascular disease and selective estrogen receptor modulators in postmenopausal women. Climacteric. 2009;12 Suppl 1:108-111.
The authors would like to acknowledge Lauren Melcher, MD, an ObGyn resident at Washington Hospital Center, who contributed to this article.
CASE: New-onset dyspareunia in a menopausal patient
J. B., 53 years old, has been menopausal for 2 years. Several months after her annual examination, she schedules another appointment to discuss a worsening complaint: dyspareunia. She says she never had the problem until she reached menopause, and reports that it has become so severe that she has started avoiding sexual intercourse altogether. Even when she avoids intercourse, however, she is bothered by vaginal itching and burning.
What can you offer to her?
Various hormonal and nonhormonal products are available to relieve the frequent complaint, in menopausal women, of symptoms of vaginal atrophy: vaginal dryness, itching, burning, and dyspareunia.1-3 The array of products isn’t really surprising: As women advance through menopause, their complaints of vaginal dryness increase fivefold.4
Systemic and local estrogen therapies reverse some atrophic changes and alleviate symptoms.5 After menopause, local vaginal estrogen formulations are recommended as first-line treatment for women who experience moderate or severe symptoms of vaginal atrophy.3 Formulations such as the vaginal ring, vaginal tablet, and transdermal gels and sprays are increasingly popular.
In this article, we describe these and other products, including nonhormonal lubricants and moisturizers, to relieve:
- the range of symptoms of vaginal atrophy in menopausal women
- isolated vaginal dryness in premenopausal women.
Is hormonal therapy always necessary?
When a postmenopausal woman complains of chronic vaginal dryness, and the exam is consistent with vaginal atrophy, the recommended treatment is local vaginal estrogen. If she complains of vaginal dryness during sexual intercourse only, a vaginal lubricant is a suitable option.
When a premenopausal woman complains of vaginal dryness, a vaginal moisturizer is the best long-term treatment option. However, a vaginal lubricant is recommended for intermittent dryness during intercourse or dyspareunia.
Local estrogens avoid many risks of systemic therapy
Topical estrogen preparations are available as vaginal creams, tablets, and rings, and as transdermal lotions, gels, and patches (TABLE 1). Local preparations are preferred to systemic therapy for the treatment of atrophy because they bypass the gastrointestinal tract, undergo less conversion in the liver, and improve local tissue with minimal elevation of the serum estradiol level.1,3
The vaginal ring (Estring) delivers the lowest systemic estradiol level—approximately 5 to 10 μg of estradiol daily. Femring delivers more estradiol daily and requires the addition of progesterone in women who have an intact uterus.
Studies suggest that patients favor the estradiol-releasing vaginal ring because of its ease of use, comfort, and effectiveness, compared with vaginal estrogen cream.2,5
Local estrogen formulations were compared and reviewed in a systematic Cochrane meta-analysis of 19 trials that included 4,162 women.5 Vaginal cream, tablets, and rings were all equally effective in treating symptoms of atrophy. One trial found that cream (conjugated equine estrogen) increased the risk of uterine bleeding, breast pain, and perineal pain, compared with vaginal tablets.
Newer estrogen formulations include topical and transdermal patches, gels, lotions, and sprays (TABLE 1), all of which are systemic. They are effective in the treatment of vasomotor symptoms and vaginal atrophy.
TABLE 1
Topical estrogen formulations—a rundown of local and systemic options
| Product | Dosing | Administration | Source of active ingredient |
|---|---|---|---|
| Absorbed locally | |||
| VAGINAL TABLET | |||
| Vagifem | 25 μg of estradiol | One tablet intravaginally daily for 2 weeks; then, twice weekly | Synthesized from soy |
| VAGINAL CREAM | |||
| Premarin | 0.5 g (0.625 mg/g of conjugated estrogen) | Insert 0.5 g daily for 3 weeks; then, twice weekly (Note: Dosage can be increased to 2 g daily but this may require progesterone supplementation) | Urine of pregnant mares |
| Estrace | 0.1 mg of estradiol/g of cream | Insert 0.5 g daily for 1 or 2 weeks; then, twice weekly | Synthesized from soy and yams |
| VAGINAL RING | |||
| Estring | 2 mg (delivers 6–9 μg of estradiol daily) | Insert 1 ring intravaginally for 3 months | Synthesized from Mexican yams |
| Absorbed systemically | |||
| VAGINAL RING | |||
| Femring | Delivers 0.05 mg–0.1 mg of estradiol daily | Insert 1 ring intravaginally for 3 months | Synthesized from soy |
| ESTROGEN PATCH | |||
| Estraderm | Delivers 0.05 mg or 0.1 mg of estradiol daily | Apply patch twice weekly | Synthesized from Mexican yams |
| Estradiol (generic) | Delivers 0.05 mg or 0.1 mg of estradiol daily | ||
| Esclim | Delivers 0.025 mg, 0.0375 mg, 0.05 mg, 0.075 mg, or 0.1 mg of estradiol daily | ||
| Vivelle, Vivelle-Dot | Delivers 0.025 mg, 0.0375 mg, 0.05 mg, 0.075 mg, or 0.1 mg of estradiol daily | Synthesized from Mexican yams | |
| Climara | Delivers 0.025 mg, 0.0375 mg, 0.05 mg, 0.06 mg, 0.075 mg, or 0.1 mg of estradiol daily | Synthesized from soy | |
| Alora | Delivers 0.025 mg, 0.05 mg, 0.075 mg, or 0.1 mg of estradiol daily | ||
| Menostar | Delivers 0.014 mg of estradiol daily | Apply patch once weekly (Note: Indicated only for prevention of osteoporosis) | |
| CombiPatch | Delivers 0.05 mg or 0.14 mg daily of estradiol plus 0.05 mg or 0.25 mg daily of norethindrone | Apply patch twice weekly | Synthesized from soy (estradiol) and Mexican yams (norethrindrone) |
| ESTROGEN LOTION, GEL | |||
| Estrasorb (lotion) | Content of two pouches delivers 0.05 mg daily of estradiol | Apply one packet to each leg daily | Synthesized from soy |
| EstroGel (gel) | 1.25 g (0.75 mg of estradiol) | Apply one pump to arm once daily | |
| Divigel (gel) | 0.25 g, 0.5 g, or 1 g of 0.1% estradiol | Apply one packet to upper thigh daily | |
| Elestrin (gel) | 0.87 g (0.52 mg of estradiol) | Apply one pump to arm once daily | |
| ESTROGEN SPRAY | |||
| Evamist | 1.53 mg of estradiol in each spray | Apply 1-3 sprays to forearm daily | |
| Source: Cirigliano M. Bioidentical hormone therapy: a review of the evidence. J Womens Health (Larchmt). 2007;16:600–631. | |||
When to add a progestin
A progestin is recommended in addition to a systemic estrogen formulation in women who have a uterus. For low-dose, local vaginal estrogen formulations, a progestin is usually not needed.3 However, when the treatment is vaginal cream, consider progestin supplementation when the dosage exceeds 0.5 g twice weekly for an extended time (>1 year).
The serum estrogen level with local vaginal treatment is dose-dependent, and the long-term endometrial effects of vaginal estrogens are unknown. If vaginal bleeding develops, a workup is indicated and may necessitate imaging of the endometrial echo or endometrial sampling to rule out hyperplasia, neoplasia, and cancer.
Counsel the patient about any risks
If you prescribe transdermal or oral estrogen for a patient, be sure to counsel her about the risks of systemic therapy described in the Women’s Health Initiative.6
Consider the patient’s preference
Local estrogen treatment is recommended over systemic therapy for vaginal atrophy, but patient preference should also be considered. Some women may prefer the ring or tablet to minimize excess vaginal discharge, while others may prefer a cream because of its soothing effects. Always individualize management!
Lubricants and moisturizers
Insufficient lubrication during intercourse is a common complaint among both premenopausal and postmenopausal women: As many as 60% of women report intermittent episodes of insufficient lubrication.7
Many women and their partners use a vaginal lubricant to assist with sexual relations and to self-treat for pain. A wide variety of nonhormonal products are available—many of them advertised at pharmacies and in the media—despite little published scientific evaluation. Because gynecologists routinely counsel patients on sensitive matters, including sexual practices, you may find it valuable—with appropriate candidates—to open a line of questioning about difficulties with intercourse and resulting attempts to self-medicate using over-the-counter products.
What are the indications?
A vaginal lubricant is a solution used locally, and as a temporary measure, to moisten the vaginal epithelium to facilitate a medical examination or sexual intercourse.2 Because it has a short duration, it must be applied at the time of intercourse. Lubricants can be categorized as water-, silicone-, and oil-based. Each formulation may affect the local inflammatory response, viability of sperm, and condom integrity.
A vaginal moisturizer is a gel or cream used regularly to maintain hydration of the vaginal epithelium for long-term relief of vaginal dryness.2
Both lubricants and moisturizers have many indications for both medical and personal use. Personal lubricants can be used for assistance during sexual activity, such as intercourse, masturbation, or use of sex toys. These products reduce friction and are thought to enhance pleasure in women who suffer from vaginal dryness. However, we lack sufficient data to confirm that lubricants can improve sexual dysfunction and vaginal atrophy. In general, these products are affordable, readily available, and may be helpful in the treatment of sexual dysfunction and vaginal dryness.
See TABLE 2 for a list of personal lubricants and vaginal moisturizers.
TABLE 2
Vaginal moisturizers and lubricants are plentiful and diverse
| Product (Manufacturer) | Ingredients | Notes |
|---|---|---|
| MOISTURIZERS | ||
| Replens (Columbia Laboratories) | Water, carbomer, polycarbophil, paraffin, hydrogenated palm oil, glyceride, sorbic acid, and sodium hydroxide | Should be used 3 times weekly |
| Moist Again (Lake Consumer Products) | Water, carbomer, aloe, citric acid, chlorhexidine deglutinate, sodium benzoate, potassium sorbate, diazolidinyl urea, and sorbic acid | Safe to use with a latex condom; no data on effects on sperm motility |
| Vagisil Feminine Moisturizer (Combe) | Water, glycerin, propylene glycol, poloxamer 407, methylparaben, polyquaternium-32, propylparaben, chamomile, and aloe | |
| Feminease (Parnell Pharmaceuticals) | Water, mineral oil, glycerin, yerba santa, cetyl alcohol, and methyl paraben | Yerba santa (Eriodictyon spp), a plant native to the Pacific Northwest, is used as a moisturizer in place of aloe |
| K-Y Long Lasting Moisturizer (McNeil) | Purified water, glycerin, mineral oil, calcium/sodium PVM/MA copolymer, PVM/MA decadiene crosspolymer, hydrogenated palm glyceride, methylparaben, benzoic acid, tocopherol acetate, and sodium hydroxide | |
| K-Y Silk-E (McNeil) | Water, propylene glycol, sorbitol, polysorbate 60, hydroxyethylcellulose, benzoic acid, methylparaben, tocopherol, and aloe | |
| LUBRICANTS | ||
| Water-based | ||
| Slippery Stuff (Wallace-O’Farrell) | Water, polyoxyethylene, methylparaben, propylene glycol, isopropynol | |
| Astroglide (BioFilm) | Water, glycerin, methylparaben, propylparaben, polypropylene glycol, polyquaternium, hydroxyethylcellulose, and sodium benzoate | Also sold in a glycerin-free and paraben-free formulation |
| K-Y Jelly (McNeil) | Water, glycerin, hydroxyethylcellulose, parabens, and chlorhexidine | |
| Summer’s Eve Lubricant (C.B. Fleet) | Water, propylene glycol, methylcellulose, xanthan gum, sodium lactate, methylparaben, lactic acid, dextrose, sodium chloride, edatate disodium, pectin, and propylparaben | |
| FemGlide (WalMed) | Water, polyoxyethylene, methylparaben, and sodium carbomer | |
| Pre-Seed (INGfertility) | Water, hydroxyethylcellulose, arabinogalactan, paraben, and Pluronic copolymers | Promoted to women and their partners who are trying to conceive |
| Silicone-based | ||
| ID Millennium (Westridge Laboratories) | Cyclomethicone, dimethicone, and dimethiconol | Less drying than other lubricants |
| Pjur | Cyclopentasiloxane, dimethicone, and dimethiconol | Compatible with a condom |
| Pink | Dimethicone, vitamin E, aloe vera, dimethiconol, and cyclomethicone | |
| K-Y Liquibeads (McNeil) | Dimethicone, gelatin, glycerin, and dimethiconol | Active ingredients are contained in so-called ovules that release lubricant over several days |
| Oil-based | ||
| Élégance Women’s Lubricant | Natural oils | Does not contain alcohol, glycerin, or parabens; is incompatible with a condom; helpful for women who have vulvodynia or vestibulitis |
What to offer when estrogen is not an option
Some women may want to avoid hormonal treatment, or have a contraindication to it, such as estrogen-receptor–positive breast cancer.8 In premenopausal women, vaginal atrophy can occur with lactation or postpartum hormonal changes, or may result from the use of anti-estrogenic agents for breast cancer. Other candidates for nonhormonal therapy are women who have chronic vulvar pain syndromes. In these women, vaginal lubricants can be especially useful.
Although they are less effective than estrogen, vaginal moisturizers, such as Replens, have been shown to reverse symptoms of vaginal atrophy and decrease discomfort during intercourse.9
The therapeutic options for vaginal atrophy are likely to broaden in the near future. Ospemifene (Ophena), a selective estrogen receptor modulator (SERM) under development for the treatment of vaginal atrophy, has reached the end of Phase-3 clinical study, with positive efficacy results. A long-term safety study of the orally administered SERM has also been completed, reports QuatRx, the drug’s manufacturer. The company expects to file a New Drug Application with the US Food and Drug Administration early next year.
Phase-3 trials documented significant improvement in dryness, dyspareunia, and other endpoints
The first Phase-3 study of Ophena was announced by QuatRx in January 2008 and presented at the 90th annual meeting of the Endocrine Society. Women who were treated with 60 mg daily of Ophena experienced statistically significant improvement in vaginal dryness, dyspareunia, and the proportion of parabasal and superficial cells in the epithelium of vaginal walls. The vaginal pH level also declined. The drug did not cause hot flushes among users.
The second Phase-3 study was a randomized, double-blind, placebo-controlled study of 919 women who had vulvovaginal atrophy. It was conducted at 116 sites in the United States. Among the cohort of 605 women who identified dyspareunia as their most bothersome symptom, positive efficacy results were achieved in all four primary endpoints, including:
- a decrease in parabasal cells
- an increase in superficial cells
- a decrease in the vaginal pH level
- improvement in dyspareunia.
The trial demonstrated statistically significant improvement from baseline to week 12 in all four endpoints, compared with placebo (P.0001>
All women were supplied with a nonhormonal vaginal lubricant to be used as needed during the treatment period; the study found efficacy above and beyond usage of this lubricant, according to a press release from QuatRx.
Is the benefit worth the risk?
Some have questioned whether a systemic drug is overkill for a complaint like postmenopausal vaginal atrophy.14 Because Ophena is a SERM, it is likely to carry a cardiovascular risk profile similar to that of other drugs in its class. For example, in a large randomized trial, raloxifene (Evista) failed to reduce coronary artery disease and significantly increased the incidence of fatal stroke and venous thromboembolism.15 When local estrogen formulations that do not carry such risks are already available, some experts question the advisability of developing another systemic agent.
Another question: Is it realistic to expect the patient to take a drug every day when her chief complaint is postmenopausal dyspareunia and she is likely to have intercourse only once or twice a week?
These questions probably won’t be addressed until the drug enters the market—and physicians and their patients will be the ones providing the answers.
Specialty lubricants are unproven
In contrast to products designed to treat vaginal dryness and atrophy, some lubricants are marketed specifically for sexual enhancement. Warming lubricants cause a heating sensation on the skin and usually contain menthol, L-arginine, or capsaicin. Natural and artificial flavors are used to manufacture flavored lubricants.
None of these products have been scientifically proven to enhance sexual function.
Oil-based lubricants may impede condom integrity
It is estimated that 40% of couples who use condoms also use a lubricant to assist with intercourse.10 The integrity of latex condoms has been shown to deteriorate with the use of an oil-based lubricant or petrolatum. One study, in which the mean burst time of condoms was assessed during pressurized air inflation, showed a significant reduction in that time when vaginal lubricants that contained mineral or vegetable oil were used.11
Oil-based lubricants also have been shown to increase the slippage rate, with a trend toward increased breakage.10
Water-based lubricants may slightly increase slippage, but they reduce breakage.
Women should avoid oil-based lubricants when their partner uses a condom.
Some lubricants affect sperm quality
Choosing a vaginal lubricant can be of particular concern to a woman who is being treated for infertility. Lubricants may affect the integrity and function of sperm, even if they do not contain spermicide. Noncommercial products, such as glycerin, olive oil, vegetable oil, and, even, saliva have been associated with a loss of sperm function.12
A recent study found that Replens and Astroglide cause a dramatic decrease in sperm motility. FemGlide causes less of a decrease—but still a significant one.12 The nonphysiologic osmolality and pH of these products may be the cause of such sperm damage. Pre-Seed, which has a more physiologic pH level and isotonic quality, was found to cause minimal harm to sperm motility and chromatin quality.12
Avoid propylene glycol in women who have vulvodynia
Vaginal lubricants and moisturizers are also used in the treatment of vulvodynia or chronic vulvar pain syndromes. According to an ACOG Committee Opinion, topical application of preservative-free solutions, such as vegetable oil or plain petrolatum, is recommended to hold moisture within the tissues and provide a protective barrier.13 Adequate lubrication is also recommended during intercourse.
Products that contain propylene glycol, or alcohol, may act as an irritant in women who experience local pain and heightened vaginal sensitivity. For that reason, such products should be avoided in this population.
CASE RESOLVED
Careful examination reveals urogenital atrophy with absence of any fungal or bacterial infection of the vulva or vagina. The patient chooses to use a water-based lubricant during sexual relations and begins using intravaginal estradiol tablets (other options include the vaginal ring or cream). Symptoms of dyspareunia disappear almost immediately, and vaginal burning improves after 6 weeks.
The authors would like to acknowledge Lauren Melcher, MD, an ObGyn resident at Washington Hospital Center, who contributed to this article.
CASE: New-onset dyspareunia in a menopausal patient
J. B., 53 years old, has been menopausal for 2 years. Several months after her annual examination, she schedules another appointment to discuss a worsening complaint: dyspareunia. She says she never had the problem until she reached menopause, and reports that it has become so severe that she has started avoiding sexual intercourse altogether. Even when she avoids intercourse, however, she is bothered by vaginal itching and burning.
What can you offer to her?
Various hormonal and nonhormonal products are available to relieve the frequent complaint, in menopausal women, of symptoms of vaginal atrophy: vaginal dryness, itching, burning, and dyspareunia.1-3 The array of products isn’t really surprising: As women advance through menopause, their complaints of vaginal dryness increase fivefold.4
Systemic and local estrogen therapies reverse some atrophic changes and alleviate symptoms.5 After menopause, local vaginal estrogen formulations are recommended as first-line treatment for women who experience moderate or severe symptoms of vaginal atrophy.3 Formulations such as the vaginal ring, vaginal tablet, and transdermal gels and sprays are increasingly popular.
In this article, we describe these and other products, including nonhormonal lubricants and moisturizers, to relieve:
- the range of symptoms of vaginal atrophy in menopausal women
- isolated vaginal dryness in premenopausal women.
Is hormonal therapy always necessary?
When a postmenopausal woman complains of chronic vaginal dryness, and the exam is consistent with vaginal atrophy, the recommended treatment is local vaginal estrogen. If she complains of vaginal dryness during sexual intercourse only, a vaginal lubricant is a suitable option.
When a premenopausal woman complains of vaginal dryness, a vaginal moisturizer is the best long-term treatment option. However, a vaginal lubricant is recommended for intermittent dryness during intercourse or dyspareunia.
Local estrogens avoid many risks of systemic therapy
Topical estrogen preparations are available as vaginal creams, tablets, and rings, and as transdermal lotions, gels, and patches (TABLE 1). Local preparations are preferred to systemic therapy for the treatment of atrophy because they bypass the gastrointestinal tract, undergo less conversion in the liver, and improve local tissue with minimal elevation of the serum estradiol level.1,3
The vaginal ring (Estring) delivers the lowest systemic estradiol level—approximately 5 to 10 μg of estradiol daily. Femring delivers more estradiol daily and requires the addition of progesterone in women who have an intact uterus.
Studies suggest that patients favor the estradiol-releasing vaginal ring because of its ease of use, comfort, and effectiveness, compared with vaginal estrogen cream.2,5
Local estrogen formulations were compared and reviewed in a systematic Cochrane meta-analysis of 19 trials that included 4,162 women.5 Vaginal cream, tablets, and rings were all equally effective in treating symptoms of atrophy. One trial found that cream (conjugated equine estrogen) increased the risk of uterine bleeding, breast pain, and perineal pain, compared with vaginal tablets.
Newer estrogen formulations include topical and transdermal patches, gels, lotions, and sprays (TABLE 1), all of which are systemic. They are effective in the treatment of vasomotor symptoms and vaginal atrophy.
TABLE 1
Topical estrogen formulations—a rundown of local and systemic options
| Product | Dosing | Administration | Source of active ingredient |
|---|---|---|---|
| Absorbed locally | |||
| VAGINAL TABLET | |||
| Vagifem | 25 μg of estradiol | One tablet intravaginally daily for 2 weeks; then, twice weekly | Synthesized from soy |
| VAGINAL CREAM | |||
| Premarin | 0.5 g (0.625 mg/g of conjugated estrogen) | Insert 0.5 g daily for 3 weeks; then, twice weekly (Note: Dosage can be increased to 2 g daily but this may require progesterone supplementation) | Urine of pregnant mares |
| Estrace | 0.1 mg of estradiol/g of cream | Insert 0.5 g daily for 1 or 2 weeks; then, twice weekly | Synthesized from soy and yams |
| VAGINAL RING | |||
| Estring | 2 mg (delivers 6–9 μg of estradiol daily) | Insert 1 ring intravaginally for 3 months | Synthesized from Mexican yams |
| Absorbed systemically | |||
| VAGINAL RING | |||
| Femring | Delivers 0.05 mg–0.1 mg of estradiol daily | Insert 1 ring intravaginally for 3 months | Synthesized from soy |
| ESTROGEN PATCH | |||
| Estraderm | Delivers 0.05 mg or 0.1 mg of estradiol daily | Apply patch twice weekly | Synthesized from Mexican yams |
| Estradiol (generic) | Delivers 0.05 mg or 0.1 mg of estradiol daily | ||
| Esclim | Delivers 0.025 mg, 0.0375 mg, 0.05 mg, 0.075 mg, or 0.1 mg of estradiol daily | ||
| Vivelle, Vivelle-Dot | Delivers 0.025 mg, 0.0375 mg, 0.05 mg, 0.075 mg, or 0.1 mg of estradiol daily | Synthesized from Mexican yams | |
| Climara | Delivers 0.025 mg, 0.0375 mg, 0.05 mg, 0.06 mg, 0.075 mg, or 0.1 mg of estradiol daily | Synthesized from soy | |
| Alora | Delivers 0.025 mg, 0.05 mg, 0.075 mg, or 0.1 mg of estradiol daily | ||
| Menostar | Delivers 0.014 mg of estradiol daily | Apply patch once weekly (Note: Indicated only for prevention of osteoporosis) | |
| CombiPatch | Delivers 0.05 mg or 0.14 mg daily of estradiol plus 0.05 mg or 0.25 mg daily of norethindrone | Apply patch twice weekly | Synthesized from soy (estradiol) and Mexican yams (norethrindrone) |
| ESTROGEN LOTION, GEL | |||
| Estrasorb (lotion) | Content of two pouches delivers 0.05 mg daily of estradiol | Apply one packet to each leg daily | Synthesized from soy |
| EstroGel (gel) | 1.25 g (0.75 mg of estradiol) | Apply one pump to arm once daily | |
| Divigel (gel) | 0.25 g, 0.5 g, or 1 g of 0.1% estradiol | Apply one packet to upper thigh daily | |
| Elestrin (gel) | 0.87 g (0.52 mg of estradiol) | Apply one pump to arm once daily | |
| ESTROGEN SPRAY | |||
| Evamist | 1.53 mg of estradiol in each spray | Apply 1-3 sprays to forearm daily | |
| Source: Cirigliano M. Bioidentical hormone therapy: a review of the evidence. J Womens Health (Larchmt). 2007;16:600–631. | |||
When to add a progestin
A progestin is recommended in addition to a systemic estrogen formulation in women who have a uterus. For low-dose, local vaginal estrogen formulations, a progestin is usually not needed.3 However, when the treatment is vaginal cream, consider progestin supplementation when the dosage exceeds 0.5 g twice weekly for an extended time (>1 year).
The serum estrogen level with local vaginal treatment is dose-dependent, and the long-term endometrial effects of vaginal estrogens are unknown. If vaginal bleeding develops, a workup is indicated and may necessitate imaging of the endometrial echo or endometrial sampling to rule out hyperplasia, neoplasia, and cancer.
Counsel the patient about any risks
If you prescribe transdermal or oral estrogen for a patient, be sure to counsel her about the risks of systemic therapy described in the Women’s Health Initiative.6
Consider the patient’s preference
Local estrogen treatment is recommended over systemic therapy for vaginal atrophy, but patient preference should also be considered. Some women may prefer the ring or tablet to minimize excess vaginal discharge, while others may prefer a cream because of its soothing effects. Always individualize management!
Lubricants and moisturizers
Insufficient lubrication during intercourse is a common complaint among both premenopausal and postmenopausal women: As many as 60% of women report intermittent episodes of insufficient lubrication.7
Many women and their partners use a vaginal lubricant to assist with sexual relations and to self-treat for pain. A wide variety of nonhormonal products are available—many of them advertised at pharmacies and in the media—despite little published scientific evaluation. Because gynecologists routinely counsel patients on sensitive matters, including sexual practices, you may find it valuable—with appropriate candidates—to open a line of questioning about difficulties with intercourse and resulting attempts to self-medicate using over-the-counter products.
What are the indications?
A vaginal lubricant is a solution used locally, and as a temporary measure, to moisten the vaginal epithelium to facilitate a medical examination or sexual intercourse.2 Because it has a short duration, it must be applied at the time of intercourse. Lubricants can be categorized as water-, silicone-, and oil-based. Each formulation may affect the local inflammatory response, viability of sperm, and condom integrity.
A vaginal moisturizer is a gel or cream used regularly to maintain hydration of the vaginal epithelium for long-term relief of vaginal dryness.2
Both lubricants and moisturizers have many indications for both medical and personal use. Personal lubricants can be used for assistance during sexual activity, such as intercourse, masturbation, or use of sex toys. These products reduce friction and are thought to enhance pleasure in women who suffer from vaginal dryness. However, we lack sufficient data to confirm that lubricants can improve sexual dysfunction and vaginal atrophy. In general, these products are affordable, readily available, and may be helpful in the treatment of sexual dysfunction and vaginal dryness.
See TABLE 2 for a list of personal lubricants and vaginal moisturizers.
TABLE 2
Vaginal moisturizers and lubricants are plentiful and diverse
| Product (Manufacturer) | Ingredients | Notes |
|---|---|---|
| MOISTURIZERS | ||
| Replens (Columbia Laboratories) | Water, carbomer, polycarbophil, paraffin, hydrogenated palm oil, glyceride, sorbic acid, and sodium hydroxide | Should be used 3 times weekly |
| Moist Again (Lake Consumer Products) | Water, carbomer, aloe, citric acid, chlorhexidine deglutinate, sodium benzoate, potassium sorbate, diazolidinyl urea, and sorbic acid | Safe to use with a latex condom; no data on effects on sperm motility |
| Vagisil Feminine Moisturizer (Combe) | Water, glycerin, propylene glycol, poloxamer 407, methylparaben, polyquaternium-32, propylparaben, chamomile, and aloe | |
| Feminease (Parnell Pharmaceuticals) | Water, mineral oil, glycerin, yerba santa, cetyl alcohol, and methyl paraben | Yerba santa (Eriodictyon spp), a plant native to the Pacific Northwest, is used as a moisturizer in place of aloe |
| K-Y Long Lasting Moisturizer (McNeil) | Purified water, glycerin, mineral oil, calcium/sodium PVM/MA copolymer, PVM/MA decadiene crosspolymer, hydrogenated palm glyceride, methylparaben, benzoic acid, tocopherol acetate, and sodium hydroxide | |
| K-Y Silk-E (McNeil) | Water, propylene glycol, sorbitol, polysorbate 60, hydroxyethylcellulose, benzoic acid, methylparaben, tocopherol, and aloe | |
| LUBRICANTS | ||
| Water-based | ||
| Slippery Stuff (Wallace-O’Farrell) | Water, polyoxyethylene, methylparaben, propylene glycol, isopropynol | |
| Astroglide (BioFilm) | Water, glycerin, methylparaben, propylparaben, polypropylene glycol, polyquaternium, hydroxyethylcellulose, and sodium benzoate | Also sold in a glycerin-free and paraben-free formulation |
| K-Y Jelly (McNeil) | Water, glycerin, hydroxyethylcellulose, parabens, and chlorhexidine | |
| Summer’s Eve Lubricant (C.B. Fleet) | Water, propylene glycol, methylcellulose, xanthan gum, sodium lactate, methylparaben, lactic acid, dextrose, sodium chloride, edatate disodium, pectin, and propylparaben | |
| FemGlide (WalMed) | Water, polyoxyethylene, methylparaben, and sodium carbomer | |
| Pre-Seed (INGfertility) | Water, hydroxyethylcellulose, arabinogalactan, paraben, and Pluronic copolymers | Promoted to women and their partners who are trying to conceive |
| Silicone-based | ||
| ID Millennium (Westridge Laboratories) | Cyclomethicone, dimethicone, and dimethiconol | Less drying than other lubricants |
| Pjur | Cyclopentasiloxane, dimethicone, and dimethiconol | Compatible with a condom |
| Pink | Dimethicone, vitamin E, aloe vera, dimethiconol, and cyclomethicone | |
| K-Y Liquibeads (McNeil) | Dimethicone, gelatin, glycerin, and dimethiconol | Active ingredients are contained in so-called ovules that release lubricant over several days |
| Oil-based | ||
| Élégance Women’s Lubricant | Natural oils | Does not contain alcohol, glycerin, or parabens; is incompatible with a condom; helpful for women who have vulvodynia or vestibulitis |
What to offer when estrogen is not an option
Some women may want to avoid hormonal treatment, or have a contraindication to it, such as estrogen-receptor–positive breast cancer.8 In premenopausal women, vaginal atrophy can occur with lactation or postpartum hormonal changes, or may result from the use of anti-estrogenic agents for breast cancer. Other candidates for nonhormonal therapy are women who have chronic vulvar pain syndromes. In these women, vaginal lubricants can be especially useful.
Although they are less effective than estrogen, vaginal moisturizers, such as Replens, have been shown to reverse symptoms of vaginal atrophy and decrease discomfort during intercourse.9
The therapeutic options for vaginal atrophy are likely to broaden in the near future. Ospemifene (Ophena), a selective estrogen receptor modulator (SERM) under development for the treatment of vaginal atrophy, has reached the end of Phase-3 clinical study, with positive efficacy results. A long-term safety study of the orally administered SERM has also been completed, reports QuatRx, the drug’s manufacturer. The company expects to file a New Drug Application with the US Food and Drug Administration early next year.
Phase-3 trials documented significant improvement in dryness, dyspareunia, and other endpoints
The first Phase-3 study of Ophena was announced by QuatRx in January 2008 and presented at the 90th annual meeting of the Endocrine Society. Women who were treated with 60 mg daily of Ophena experienced statistically significant improvement in vaginal dryness, dyspareunia, and the proportion of parabasal and superficial cells in the epithelium of vaginal walls. The vaginal pH level also declined. The drug did not cause hot flushes among users.
The second Phase-3 study was a randomized, double-blind, placebo-controlled study of 919 women who had vulvovaginal atrophy. It was conducted at 116 sites in the United States. Among the cohort of 605 women who identified dyspareunia as their most bothersome symptom, positive efficacy results were achieved in all four primary endpoints, including:
- a decrease in parabasal cells
- an increase in superficial cells
- a decrease in the vaginal pH level
- improvement in dyspareunia.
The trial demonstrated statistically significant improvement from baseline to week 12 in all four endpoints, compared with placebo (P.0001>
All women were supplied with a nonhormonal vaginal lubricant to be used as needed during the treatment period; the study found efficacy above and beyond usage of this lubricant, according to a press release from QuatRx.
Is the benefit worth the risk?
Some have questioned whether a systemic drug is overkill for a complaint like postmenopausal vaginal atrophy.14 Because Ophena is a SERM, it is likely to carry a cardiovascular risk profile similar to that of other drugs in its class. For example, in a large randomized trial, raloxifene (Evista) failed to reduce coronary artery disease and significantly increased the incidence of fatal stroke and venous thromboembolism.15 When local estrogen formulations that do not carry such risks are already available, some experts question the advisability of developing another systemic agent.
Another question: Is it realistic to expect the patient to take a drug every day when her chief complaint is postmenopausal dyspareunia and she is likely to have intercourse only once or twice a week?
These questions probably won’t be addressed until the drug enters the market—and physicians and their patients will be the ones providing the answers.
Specialty lubricants are unproven
In contrast to products designed to treat vaginal dryness and atrophy, some lubricants are marketed specifically for sexual enhancement. Warming lubricants cause a heating sensation on the skin and usually contain menthol, L-arginine, or capsaicin. Natural and artificial flavors are used to manufacture flavored lubricants.
None of these products have been scientifically proven to enhance sexual function.
Oil-based lubricants may impede condom integrity
It is estimated that 40% of couples who use condoms also use a lubricant to assist with intercourse.10 The integrity of latex condoms has been shown to deteriorate with the use of an oil-based lubricant or petrolatum. One study, in which the mean burst time of condoms was assessed during pressurized air inflation, showed a significant reduction in that time when vaginal lubricants that contained mineral or vegetable oil were used.11
Oil-based lubricants also have been shown to increase the slippage rate, with a trend toward increased breakage.10
Water-based lubricants may slightly increase slippage, but they reduce breakage.
Women should avoid oil-based lubricants when their partner uses a condom.
Some lubricants affect sperm quality
Choosing a vaginal lubricant can be of particular concern to a woman who is being treated for infertility. Lubricants may affect the integrity and function of sperm, even if they do not contain spermicide. Noncommercial products, such as glycerin, olive oil, vegetable oil, and, even, saliva have been associated with a loss of sperm function.12
A recent study found that Replens and Astroglide cause a dramatic decrease in sperm motility. FemGlide causes less of a decrease—but still a significant one.12 The nonphysiologic osmolality and pH of these products may be the cause of such sperm damage. Pre-Seed, which has a more physiologic pH level and isotonic quality, was found to cause minimal harm to sperm motility and chromatin quality.12
Avoid propylene glycol in women who have vulvodynia
Vaginal lubricants and moisturizers are also used in the treatment of vulvodynia or chronic vulvar pain syndromes. According to an ACOG Committee Opinion, topical application of preservative-free solutions, such as vegetable oil or plain petrolatum, is recommended to hold moisture within the tissues and provide a protective barrier.13 Adequate lubrication is also recommended during intercourse.
Products that contain propylene glycol, or alcohol, may act as an irritant in women who experience local pain and heightened vaginal sensitivity. For that reason, such products should be avoided in this population.
CASE RESOLVED
Careful examination reveals urogenital atrophy with absence of any fungal or bacterial infection of the vulva or vagina. The patient chooses to use a water-based lubricant during sexual relations and begins using intravaginal estradiol tablets (other options include the vaginal ring or cream). Symptoms of dyspareunia disappear almost immediately, and vaginal burning improves after 6 weeks.
1. Al-Baghdadi O, Ewies AA. Topical estrogen therapy in the management of postmenopausal vaginal atrophy: an up-to-date overview. Climacteric. 2009;12:91-105.
2. Willhite LA, O’Connell MB. Urogenital atrophy: prevention and treatment. Pharmacotherapy. 2001;21:464-480.
3. Archer DF. Efficacy and tolerability of local estrogen therapy for urogenital atrophy. Menopause. 2009;17:1-10.
4. Dennerstein L, Dudley EC, Hopper JL, Guthrie JR, Burger HG. A prospective population-based study of menopausal symptoms. Obstet Gynecol. 2000;96:351-358.
5. Suckling J, Lethaby A, Kennedy R. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2006;(4):CD001500.-
6. Anderson GL, Limacher M, Assaf AR, et al. Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701-1712.
7. Oberg K, Fugl-Meyer AR, Fugl-Meyer KS. On categorization and quantification of women’s sexual dysfunctions: an epidemiological approach. Int J Impot Res. 2004;16:261-269.
8. Loprinzi CL, Wolf SL, Barton DL, Laack NN. Symptom management in premenopausal patients with breast cancer. Lancet Oncol. 2008;9:993-1001.
9. Bygdeman M, Swahn ML. Replens versus dienoestrol cream in the symptomatic treatment of vaginal atrophy in postmenopausal women. Maturitas. 1996;23:259-263.
10. Steiner M, Piedrahita C, Glover L, Joanis C, Spruyt A, Foldesy R. The impact of lubricants on latex condoms during vaginal intercourse. Int J STD AIDS. 1994;5:29-36.
11. Rosen AD, Rosen T. Study of condom integrity after brief exposure to over-the-counter vaginal preparations. South Med J. 1999;92:305-307.
12. Agarwal A, Deepinder F, Cocuzza M, Short RA, Evenson DP. Effect of vaginal lubricants on sperm motility and chromatin integrity: a prospective comparative study. Fertil Steril. 2008;89:375-379.
13. ACOG Committee Opinion #345: Vulvodynia. October 2006. ACOG Committee on Gynecologic Practice. Obstet Gynecol. 2006;108:1049-1052.
14. Ophena is a me-too drug with an impractical mode of administration. Gerson Lehrman Group. Jan. 14, 2008. Available at: http://www.glgroup.com/News/Ophena-is-a-Me-Too-Drug-with-an-Impractical-Mode-of-Administration-20651.html. Accessed Nov. 11, 2009.
15. de Villiers TJ. Clinical issues regarding cardiovascular disease and selective estrogen receptor modulators in postmenopausal women. Climacteric. 2009;12 Suppl 1:108-111.
1. Al-Baghdadi O, Ewies AA. Topical estrogen therapy in the management of postmenopausal vaginal atrophy: an up-to-date overview. Climacteric. 2009;12:91-105.
2. Willhite LA, O’Connell MB. Urogenital atrophy: prevention and treatment. Pharmacotherapy. 2001;21:464-480.
3. Archer DF. Efficacy and tolerability of local estrogen therapy for urogenital atrophy. Menopause. 2009;17:1-10.
4. Dennerstein L, Dudley EC, Hopper JL, Guthrie JR, Burger HG. A prospective population-based study of menopausal symptoms. Obstet Gynecol. 2000;96:351-358.
5. Suckling J, Lethaby A, Kennedy R. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2006;(4):CD001500.-
6. Anderson GL, Limacher M, Assaf AR, et al. Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701-1712.
7. Oberg K, Fugl-Meyer AR, Fugl-Meyer KS. On categorization and quantification of women’s sexual dysfunctions: an epidemiological approach. Int J Impot Res. 2004;16:261-269.
8. Loprinzi CL, Wolf SL, Barton DL, Laack NN. Symptom management in premenopausal patients with breast cancer. Lancet Oncol. 2008;9:993-1001.
9. Bygdeman M, Swahn ML. Replens versus dienoestrol cream in the symptomatic treatment of vaginal atrophy in postmenopausal women. Maturitas. 1996;23:259-263.
10. Steiner M, Piedrahita C, Glover L, Joanis C, Spruyt A, Foldesy R. The impact of lubricants on latex condoms during vaginal intercourse. Int J STD AIDS. 1994;5:29-36.
11. Rosen AD, Rosen T. Study of condom integrity after brief exposure to over-the-counter vaginal preparations. South Med J. 1999;92:305-307.
12. Agarwal A, Deepinder F, Cocuzza M, Short RA, Evenson DP. Effect of vaginal lubricants on sperm motility and chromatin integrity: a prospective comparative study. Fertil Steril. 2008;89:375-379.
13. ACOG Committee Opinion #345: Vulvodynia. October 2006. ACOG Committee on Gynecologic Practice. Obstet Gynecol. 2006;108:1049-1052.
14. Ophena is a me-too drug with an impractical mode of administration. Gerson Lehrman Group. Jan. 14, 2008. Available at: http://www.glgroup.com/News/Ophena-is-a-Me-Too-Drug-with-an-Impractical-Mode-of-Administration-20651.html. Accessed Nov. 11, 2009.
15. de Villiers TJ. Clinical issues regarding cardiovascular disease and selective estrogen receptor modulators in postmenopausal women. Climacteric. 2009;12 Suppl 1:108-111.
Should cystoscopy be routine at the time of hysterectomy?
The overall rate of ureteral injury at the time of hysterectomy and other gynecologic procedures for benign disease has been estimated at 8.8 injuries for every 1,000 procedures, with the highest rate (17.3/1,000) occurring during laparoscopic hysterectomy with bilateral salpingo-oophorectomy (BSO). The rate of bladder injury is estimated at 16.3 for every 1,000 gynecologic procedures and 29.2 for every 1,000 laparoscopic hysterectomies with BSO.1
Cystoscopy is a low-risk procedure that may be beneficial in surgeries associated with a high rate (1% to 2%) of lower urinary tract injury.2 Early detection of bladder or ureteral injury is preferable to avoid postoperative complications such as fistula formation, loss of renal function, and other complications requiring additional surgery and prolonged hospitalization. Early detection also reduces medicolegal risk.
Ibeanu and associates point out that many gynecologic surgeons do not perform cystoscopy routinely, because of either a lack of training or difficulty obtaining privileges to perform this urologic procedure. They also note that the benefits of cystoscopy clearly outweigh the risks.
Only 25.6% of injuries were detected by visual inspection
Roughly one in four injuries to the bladder and ureter were detected without the aid of cystoscopy; the rest were identified using cystoscopy.
Twenty-four cases of bladder injury (2.9%) and 15 cases of ureteral injury (1.8%) were identified at the time of rigid diagnostic cystoscopy after hysterectomy. The majority (544) of the hysterectomies were abdominal, followed by vaginal hysterectomy (227) and laparoscopically assisted vaginal hysterectomy (61) ( TABLE ).
Most ureteral injuries (80%) occurred at the level of the uterine artery. The ureter is difficult to visualize or palpate once it goes under the uterine artery and courses along the anterior vagina before entry into the urinary bladder.
Ureteral injury also was common at the level of the infundibulopelvic ligament. One patient developed vesicovaginal fistula postoperatively that was missed on initial cystoscopy.
TABLE
Injury rate, by hysterectomy procedure
| Type of procedure | Bladder injury | Ureteral injury |
|---|---|---|
| Total abdominal hysterectomy | 2.3% | 1.7% |
| Total vaginal hysterectomy (alone) | 1.8% | 0.9% |
| Total vaginal hysterectomy (with prolapse procedures) | 2.6% | 1.7% |
| Laparoscopically assisted vaginal hysterectomy | 3.3% | 0 |
Cystoscopy is imperfect, but effective, and its cost is justifiable
Cystoscopy should be performed routinely after any gynecologic procedure associated with a high risk of injury, such as difficult bladder or ureteral dissection. Findings that justify cystoscopy include de novo hematuria and air in the Foley bag during laparoscopy.
Although cystoscopy may not identify all injuries, its benefits likely outweigh any additional cost associated with the procedure when a high rate of injury is likely (greater than, say, 1.5%).3
This study was conducted over 8 years at three academic practices, so it may not be possible to generalize its findings broadly across practitioners.
Perform cystoscopy to verify integrity of the lower urinary tract at the time of hysterectomy for benign disease. Appropriate training to detect and repair injury is required to optimize surgical outcomes.—CHERYL IGLESIA, MD
1. Gilmour DT, Das S, Flowerdew G. Rates of urinary tract injury from gynecologic surgery and the role of intraoperative cystoscopy. Obstet Gynecol. 2006;107:1366-1372.
2. American College of Obstetricians and Gynecologists. ACOG Committee Opinion. Number 372. July 2007. The role of cystourethroscopy in the generalist obstetrician-gynecologist practice. Obstet Gynecol. 2007;110:221–224.
3. Visco AG, Taber KH, Weidner AC, Barber MD, Myers ER. Cost-effectiveness of universal cystoscopy to identify ureteral injury at hysterectomy. Obstet Gynecol. 2001;97:685-692.
The overall rate of ureteral injury at the time of hysterectomy and other gynecologic procedures for benign disease has been estimated at 8.8 injuries for every 1,000 procedures, with the highest rate (17.3/1,000) occurring during laparoscopic hysterectomy with bilateral salpingo-oophorectomy (BSO). The rate of bladder injury is estimated at 16.3 for every 1,000 gynecologic procedures and 29.2 for every 1,000 laparoscopic hysterectomies with BSO.1
Cystoscopy is a low-risk procedure that may be beneficial in surgeries associated with a high rate (1% to 2%) of lower urinary tract injury.2 Early detection of bladder or ureteral injury is preferable to avoid postoperative complications such as fistula formation, loss of renal function, and other complications requiring additional surgery and prolonged hospitalization. Early detection also reduces medicolegal risk.
Ibeanu and associates point out that many gynecologic surgeons do not perform cystoscopy routinely, because of either a lack of training or difficulty obtaining privileges to perform this urologic procedure. They also note that the benefits of cystoscopy clearly outweigh the risks.
Only 25.6% of injuries were detected by visual inspection
Roughly one in four injuries to the bladder and ureter were detected without the aid of cystoscopy; the rest were identified using cystoscopy.
Twenty-four cases of bladder injury (2.9%) and 15 cases of ureteral injury (1.8%) were identified at the time of rigid diagnostic cystoscopy after hysterectomy. The majority (544) of the hysterectomies were abdominal, followed by vaginal hysterectomy (227) and laparoscopically assisted vaginal hysterectomy (61) ( TABLE ).
Most ureteral injuries (80%) occurred at the level of the uterine artery. The ureter is difficult to visualize or palpate once it goes under the uterine artery and courses along the anterior vagina before entry into the urinary bladder.
Ureteral injury also was common at the level of the infundibulopelvic ligament. One patient developed vesicovaginal fistula postoperatively that was missed on initial cystoscopy.
TABLE
Injury rate, by hysterectomy procedure
| Type of procedure | Bladder injury | Ureteral injury |
|---|---|---|
| Total abdominal hysterectomy | 2.3% | 1.7% |
| Total vaginal hysterectomy (alone) | 1.8% | 0.9% |
| Total vaginal hysterectomy (with prolapse procedures) | 2.6% | 1.7% |
| Laparoscopically assisted vaginal hysterectomy | 3.3% | 0 |
Cystoscopy is imperfect, but effective, and its cost is justifiable
Cystoscopy should be performed routinely after any gynecologic procedure associated with a high risk of injury, such as difficult bladder or ureteral dissection. Findings that justify cystoscopy include de novo hematuria and air in the Foley bag during laparoscopy.
Although cystoscopy may not identify all injuries, its benefits likely outweigh any additional cost associated with the procedure when a high rate of injury is likely (greater than, say, 1.5%).3
This study was conducted over 8 years at three academic practices, so it may not be possible to generalize its findings broadly across practitioners.
Perform cystoscopy to verify integrity of the lower urinary tract at the time of hysterectomy for benign disease. Appropriate training to detect and repair injury is required to optimize surgical outcomes.—CHERYL IGLESIA, MD
The overall rate of ureteral injury at the time of hysterectomy and other gynecologic procedures for benign disease has been estimated at 8.8 injuries for every 1,000 procedures, with the highest rate (17.3/1,000) occurring during laparoscopic hysterectomy with bilateral salpingo-oophorectomy (BSO). The rate of bladder injury is estimated at 16.3 for every 1,000 gynecologic procedures and 29.2 for every 1,000 laparoscopic hysterectomies with BSO.1
Cystoscopy is a low-risk procedure that may be beneficial in surgeries associated with a high rate (1% to 2%) of lower urinary tract injury.2 Early detection of bladder or ureteral injury is preferable to avoid postoperative complications such as fistula formation, loss of renal function, and other complications requiring additional surgery and prolonged hospitalization. Early detection also reduces medicolegal risk.
Ibeanu and associates point out that many gynecologic surgeons do not perform cystoscopy routinely, because of either a lack of training or difficulty obtaining privileges to perform this urologic procedure. They also note that the benefits of cystoscopy clearly outweigh the risks.
Only 25.6% of injuries were detected by visual inspection
Roughly one in four injuries to the bladder and ureter were detected without the aid of cystoscopy; the rest were identified using cystoscopy.
Twenty-four cases of bladder injury (2.9%) and 15 cases of ureteral injury (1.8%) were identified at the time of rigid diagnostic cystoscopy after hysterectomy. The majority (544) of the hysterectomies were abdominal, followed by vaginal hysterectomy (227) and laparoscopically assisted vaginal hysterectomy (61) ( TABLE ).
Most ureteral injuries (80%) occurred at the level of the uterine artery. The ureter is difficult to visualize or palpate once it goes under the uterine artery and courses along the anterior vagina before entry into the urinary bladder.
Ureteral injury also was common at the level of the infundibulopelvic ligament. One patient developed vesicovaginal fistula postoperatively that was missed on initial cystoscopy.
TABLE
Injury rate, by hysterectomy procedure
| Type of procedure | Bladder injury | Ureteral injury |
|---|---|---|
| Total abdominal hysterectomy | 2.3% | 1.7% |
| Total vaginal hysterectomy (alone) | 1.8% | 0.9% |
| Total vaginal hysterectomy (with prolapse procedures) | 2.6% | 1.7% |
| Laparoscopically assisted vaginal hysterectomy | 3.3% | 0 |
Cystoscopy is imperfect, but effective, and its cost is justifiable
Cystoscopy should be performed routinely after any gynecologic procedure associated with a high risk of injury, such as difficult bladder or ureteral dissection. Findings that justify cystoscopy include de novo hematuria and air in the Foley bag during laparoscopy.
Although cystoscopy may not identify all injuries, its benefits likely outweigh any additional cost associated with the procedure when a high rate of injury is likely (greater than, say, 1.5%).3
This study was conducted over 8 years at three academic practices, so it may not be possible to generalize its findings broadly across practitioners.
Perform cystoscopy to verify integrity of the lower urinary tract at the time of hysterectomy for benign disease. Appropriate training to detect and repair injury is required to optimize surgical outcomes.—CHERYL IGLESIA, MD
1. Gilmour DT, Das S, Flowerdew G. Rates of urinary tract injury from gynecologic surgery and the role of intraoperative cystoscopy. Obstet Gynecol. 2006;107:1366-1372.
2. American College of Obstetricians and Gynecologists. ACOG Committee Opinion. Number 372. July 2007. The role of cystourethroscopy in the generalist obstetrician-gynecologist practice. Obstet Gynecol. 2007;110:221–224.
3. Visco AG, Taber KH, Weidner AC, Barber MD, Myers ER. Cost-effectiveness of universal cystoscopy to identify ureteral injury at hysterectomy. Obstet Gynecol. 2001;97:685-692.
1. Gilmour DT, Das S, Flowerdew G. Rates of urinary tract injury from gynecologic surgery and the role of intraoperative cystoscopy. Obstet Gynecol. 2006;107:1366-1372.
2. American College of Obstetricians and Gynecologists. ACOG Committee Opinion. Number 372. July 2007. The role of cystourethroscopy in the generalist obstetrician-gynecologist practice. Obstet Gynecol. 2007;110:221–224.
3. Visco AG, Taber KH, Weidner AC, Barber MD, Myers ER. Cost-effectiveness of universal cystoscopy to identify ureteral injury at hysterectomy. Obstet Gynecol. 2001;97:685-692.
Treating stress urinary incontinence with suburethral slings
- Suburethral sling procedures are effective in treating patients with urethral hypermobility, intrinsic sphincter deficiency, low-pressure urethras, and increased intra-abdominal pressure.
- Autologous slings may be a better choice in cases of severe urogenital atrophy, previous radiation, or extensive scarring from previous repairs.
- For both the tension-free vaginal tape and SPARC slings, mark the suprapubic region 1 cm above and 1 cm lateral to the pubic symphsis on the left and right sides and inject 20 cc of a 1:1 mixture of local anesthetic and normal saline into the marked regions.
- Once the trocars are in place, fill the bladder with 250 cc of water and perform a cough stress test to confirm continence.
When the suburethral sling was first described in 1907 by von Giordano, it entailed placing autologous tissue underneath the bladder neck and suspending it superiorly. Complications including urethral erosion, infection, bleeding, and fistula formation led many surgeons to use it sparingly.
Fast forward to the 21st century: Synthetic materials and new techniques were introduced, simplifying the sling procedures and raising the long-term success rates to 84%.1 As a result, slings now stand at the forefront of stress urinary incontinence (SUI) treatment. Among advances are the tension-free vaginal tape (TVT) sling (Gynecare, a division of Ethicon Inc., Somerville, NJ) and the SPARC sling (American Medical Systems, Inc., Minnetonka, Minn). The former, approved in the U.S. in 1998, calls for another look due to of the recent publication of a Cochrane review of outcomes studies, while the latter, approved by the FDA in August 2001, is the newest technique deserving examination. Clearly, with 83,010 incontinence procedures performed in the U.S. in 1999,2 a detailed look at the suburethral sling is warranted. Here, we review materials, indications, techniques, complications, and outcomes.
Materials
The choice of material—either organic or synthetic—depends on several factors: availability, cost, patient and surgeon preference, and clinical variables. (TABLE 1) outlines the advantages and disadvantages of each material type. Organic slings include autologous tissues (rectus fascia and fascia lata graft), and allografts or xenografts (cadaveric fascia lata graft, human dermal graft, or porcine small intestine and dermal graft). Synthetic slings are made of polyethylene terephthalate, expanded polytetrafluoroethylene, and polypropylene.
While sling procedures utilizing organic materials do have their benefits, synthetic slings, particularly the polypropylene mesh used in TVT and SPARC, have proven to be a stable material unlikely to deteriorate with time. Further, increased collagen metabolism around this synthetic sling promotes an ingrowth of tissue through the mesh.
TABLE 1
Slings: advantages and disadvantages of various materials
| SLING MATERIAL | ADVANTAGES | DISADVANTAGES |
|---|---|---|
| Autologous tissues (rectus fascia, fascia lata, or vaginal wall) |
|
|
| Allografts (cadaveric fascia lata or dermis) |
|
|
| Xenografts (porcine dermis or small intestine) | ||
| Synthetic mesh (polyethylene terephthalate, expanded polytetrafluoroethylene, or polypropylene) |
|
|
Indications
Suburethral sling procedures are typically used for the treatment of genuine stress urinary incontinence (GSUI), in which the urethra becomes either hypermobile and unstable or its intrinsic sphincter becomes incompetent. In fact, slings are technically easier to place in patients with anatomic urethrovesical junction hypermobility compared to those with fixed urethras. Several authors also have suggested the sling’s advantage in patients with low-pressure urethras.3
Use urodynamic criteria to diagnose intrinsic sphincter deficiency (ISD), which is defined as a Valsalva leak point pressure of less than 60 cm water or maximal urethral closure pressure of less than 20 cm water. (Bear in mind, however, that these cut-off criteria are controversial.4,5)
Also, consider slings in patients with recurrent GSUI, inherited collagen deficiency, and increased abdominal pressure (e.g., women with chronic obstructive pulmonary disease, obesity, or high-impact physical activity). The sling also can be used as an adjunct to other transvaginal surgeries (e.g., hysterectomy or prolapse repair).
Autologous slings may be a better choice than synthetic slings in cases of severe urogenital atrophy, previous radiation, or extensive scarring from previous repairs. In these instances, the patient may be at-risk for postoperative vaginal necrosis or erosion.6 Due to their biocompatibility, autologous slings are more likely to heal over a vaginal erosion and less likely to infect or erode into the urethra. In any event, urogenital atrophy should be treated with local estrogen preoperatively to prevent some of these complications.
Technique
Conventionally, suburethral slings were placed via a combined vaginal and abdominal approach into the retropubic space of Retzius. Alternatively, the procedure could be performed abdominally by creating a suburethral tunnel via pelvic incisions, but this is the most difficult route.
Most recently, technological advances have simplified the vaginal approach, which utilizes minimal suburethral dissection and small suprapubic incisions. This technique is subdivided into “bottom-up” and “top-down” approaches. In the bottom-up TVT, the sling is inserted into a vaginal incision and threaded up through the patient’s pelvis, exiting from a small suprapubic incision. The topdown SPARC entails a reverse approach, starting from a suprapubic incision and exiting from a vaginal incision. New modifications allow for an abdominal TVT approach, as well, which we describe in detail in a later section.
Surgeons who are familiar with traditional needle suspensions may be more comfortable with the top-down approach. The need for concomitant surgery (e.g., hysterectomy or prolapse repair) not only determines the type of incontinence procedure, but also dictates the approach.
Preparing the patient. Place the patient under regional or local anesthesia with sedation so that an intraoperative cough stress test can be performed. Then administer an intravenous dose of a broad-spectrum antibiotic. Insert a 16 to 18 French Foley catheter into the urethra. Mark the suprapubic region 1 cm above and 1 cm lateral to the pubic symphsis on the left and right sides of the patient. Inject approximately 20 cc of a 1:1 mixture of local anesthetic and normal saline into the marked areas. We typically use 60 cc of 0.25% bupivicaine with epinephrine, diluted 1:1 with 60 cc of normal saline. After administering the local anesthetic suprapubically, inject a similar solution into the anterior vaginal wall suburethrally in the midline and laterally toward the retropubic tunnels.
Making the incisions. Both the TVT and SPARC techniques utilize the same type and location of incisions. As such, make a 0.5-cm incision into the abdominal skin on each side of the midline, approximately 1 cm lateral to midline and 1 cm above the pubic symphsis. Next, make a 1.5- to 2-cm vertical incision in the vaginal mucosa, starting 1.5 cm from the urethral meatus (FIGURE 1). Use Metzenbaum scissors to dissect the vaginal mucosa from the pubocervical fascia sub- and para-urethrally on both sides (FIGURE 2). Insert a Foley catheter guide (similar to the Lowsley retractor) into the catheter and deviate it to the ipsilateral side, thereby retracting the bladder neck to the contralateral side. Proceed with the placement of either the TVT or SPARC sling.
Placing the TVT sling. Attach the TVT introducer to the curved needle trocar on 1 end of the polypropylene sling. Insert the trocar with the tape attached into the vaginal incision and push through the retropubic space, keeping the trocar in close contact with the posterior surface of the pubic bone (FIGURES 3 and 4). Continue pushing the trocar through the urogenital diaphragm until its tip comes through the suprapubic incision on the ipsilateral side (FIGURE 5). It is important to not deviate too laterally, medially, or cephalad during trocar insertion to prevent vessel, bladder, or bowel injury. Perform a cystoscopy to rule out cystotomy. Place the second trocar in a similar manner on the opposite side. After both trocars have been pulled through their respective incisions, perform a tension test.
Placing the SPARC/abdominal TVT sling. Guide the abdominal needles through the previously marked suprapubic incision and the patient’s retropubic cavity (keeping the needle behind the pubic bone), to a finger placed in the vaginal incision (FIGURE 6). Snap the abdominal needle guides with the attached polypropylene mesh to the sling connectors (FIGURE 7). Bring the abdominal needles through the suprapubic incisions. Perform a tension test. As with the TVT sling, perform a cystoscopy after each needle placement to rule out cystotomy. Then pass the sling through the tunnel.
Testing for continence. Once the sling is in place, fill the bladder with 250 cc of water and perform a cough stress test. Adjust sling tension by pulling up on both sling arms until only a few drops of leakage are noted. It is important not to secure the sling too tightly as this may lead to urinary retention, detrusor instability, or urethral erosion. We prefer placing a hemostat between the sling tape and the urethra to avoid over tightening.
Suspending the sling arms. Remove the plastic sheaths after tension adjustment and cut the sling flush with the skin (FIGURE 8). Compared to the conventional bone-anchored slings, the newer tension-free sling devices are not anchored but instead suspended through the retropubic space. At first, the sling is held in place by friction from the opposing tissues. Over time, collagen formation fixes the mesh more strongly within the suburethral and paravaginal tissues.
Finally, close the suprapubic and vaginal incisions with absorbable sutures.
Placing autologous or allogenic slings. Fashion the graft, typically 2 cm wide and 10 to 12 cm long, with permanent sutures at the edges. Make a 1-cm incision into the suprapubic rectus fascia. Use either a Stamey-type needle trocar or uterine packing forceps and guide the instrument “top down” from the retropubic incision to the vaginal tunnel. The tunnel is made directly into the retropubic space from the vaginal incision. Bring the sling arms up on each side. Attach the arms to the rectus fascia and tie them down once cystoscopy and the tension test are complete.
The sling also can be performed with bone anchors placed through the vaginal incision into the pubic bone. Placement requires vaginal dissection into the retropubic space with no suprapubic incision. Once anchored, the sutures are then passed through the chosen graft materials and tied down. Bear in mind that anchoring into the periosteum of the pubic bone may cause severe osteomyelitis or osteitis pubis, though the actual incidence is unknown.7
FIGURE 1 Surgical steps for tension-free vaginal tape (TVT)
Place a Sims speculum into the vagina and make a vertical incision 1.5 cm from the external urethra meatus.
FIGURE 2
Use Metzenbaum scissors to dissect the vaginal mucosa from the underlying fascia bilaterally. Insert a Foley catheter with guide.
FIGURE 3
Place TVT trocars through the vaginal incision. Place abdominal guides suprapubically and attach them to the TVT trocar tip.
FIGURE 4
Push the tape through the retropubic space, keeping the trocar in close contact with the posterior surface of the pubic bone.
FIGURE 5
Continue pushing the trocar through the urogenital diaphragm until its tip comes through the suprapubic incision on the ipsilateral side.
FIGURE 6 Surgical steps for SPARC
Guide the needle down the posterior side of the pubic bone, keeping the needle tip in contact with the pubic bone.
FIGURE 7
Snap the needle guide and sling onto the sling connectors and pull through the suprapubic incision.
FIGURE 8
Perform a tension test. Remove the plastic sheaths and cut the sling flush with the skin.
Complications
Intraoperative and immediate postoperative complications include bladder perforation, vaginal or retropubic bleeding, wound or urinary tract infection (UTI), and short-term urinary retention. Possible long-term problems include urethral or vaginal erosion, mesh infection, prolonged voiding dysfunction, fistula formation, or de novo urge incontinence (TABLE 2).
Specifically, the TVT sling, which has been placed in more than 50,000 women in the U.S. and 200,000 worldwide, carries the potential for significant vascular injury and bowel perforation. In addition, the Food and Drug Administration (FDA) reported 4 deaths (2 from unrecognized bowel injuries, 1 from retropubic bleeding in a patient with a bleeding disorder, and 1 from a heart attack more than 1 week after an incontinence repair procedure complicated by a vascular injury); 168 device malfunctions (mostly tape or sheath detachment from the trocar); and 128 other injuries, including bowel perforations and major vascular injuries to the obturator, external iliac, femoral, or inferior epigastrics (TABLE 3).8 In a review of 1,455 TVT sling cases at 38 hospitals, Kuuva and Nilsson9 found bladder perforation in 3.8% of the patients and retropubic hematoma in 1.9%, along with 1 case of vesicovaginal fistula, 1 obturator nerve injury, and 1 epigastric vessel injury.
According to the FDA, there have been 2 complications reported with the SPARC sling system. Both involved vaginal erosion subsequently repaired by oversewing the vaginal mucosa. One of these complications occurred in a woman undergoing her fourth vaginal procedure who was therefore deemed to have “poor tissue.”8
TABLE 2
Suburethral sling complications1
| COMPLICATION | 1,715 AUTOLOGOUS | 1,515 SYNTHETIC |
|---|---|---|
| Vaginal erosion | 1 (.0001%) | 10 (.007%) |
| Urethral erosion | 5 (.003%) | 27 (.02%) |
| Fistula | 6 (.003%) | 4 (.002%) |
| Wound sinus | 3 (.002%) | 11 (.007%) |
| Wound infection | 11 (.006%) | 15 (.009%) |
| Seroma | 6 (.003%) | 1 (.0007%) |
TABLE 3
TVT complications in 200,000 procedures worldwide
| COMPLICATION | U.S. | WORLD | TOTAL |
|---|---|---|---|
| Vascular injury | 3 | 25 | 28 |
| Vaginal mesh exposure | 15 | 2 | 17 |
| Urethral erosion | 8 | 0 | 8 |
| Bowel perforation | 4 | 6 | 10 |
| Nerve injury | 1 | 0 | 1 |
Outcomes studies
Unfortunately, most of the published clinical studies on the surgical management of stress urinary incontinence suffer from inadequate follow-up and sample size, unclear patient selection criteria, and poor postoperative documentation, especially with respect to quality of life. However, multiple studies to assess the effectiveness and safety of TVT slings have been published. The following is an outline of these preliminary yet important findings.
In 2002, the Cochrane Database evaluated 7 randomized and quasi-randomized trials of suburethral slings for the treatment of urinary incontinence.10 Of 682 women evaluated, 457 had some type of suburethral sling procedure. Four trials compared slings to retropubic urethropexies, 1 compared slings to Stamey needle suspensions, and 2 compared the use of different sling materials. The results indicated that the data were insufficient to suggest that slings were more effective than other incontinence procedures or that slings were associated with fewer postoperative complications. While TVT slings did provide similar cure rates as open retropubic urethropexy, research is still lacking with respect to other types of slings. More studies comparing TVT slings to traditional pubovaginal slings also are needed before the 2 can be deemed equivalent.
In Sweden and Finland, where the TVT procedure was developed,11 85 patients who had undergone the procedure were evaluated at 48 to 70 months. Of those, 84.7% were completely cured of stress incontinence, 10.6% had significantly improved symptoms, and 4.7% were regarded as failures.
A recent well-designed, multicenter, randomized, prospective trial in the U.K. and Ireland compared 146 open Burch colposuspensions to 170 TVTs. Similar cure rates (57% and 66%, respectively) were reported.12 Although these rates are low compared to the Nordic nonrandomized TVT studies mentioned, the U.K./Ireland outcome criteria were particularly stringent and included a negative cystometrogram for stress incontinence and negative pad test. These differences in reported success rates highlight the importance of clearly defining objective outcomes criteria from randomized trials.
Nonetheless, the U.K./Ireland study showed that TVT is less invasive than the Burch procedure and is associated with shorter recovery periods and greater cost savings. Follow-up on complications (bladder perforation and hematoma in TVTs and incisional hernia formation in Burch colposuspensions) will be the most crucial aspect of this study.13
Clearly, the question of whether a Burch retropubic urethropexy or a suburethral sling procedure is better for SUI needs to be further investigated. Weber and Walters sought to answer this question by developing a decision analytical model (without the aid of randomized, controlled trials) and discovered similar cure rates.14 However, there were higher rates of urinary retention and detrusor instability associated with the traditional pubovaginal sling. But, most importantly, sensitivity analyses proved that if the rate of permanent urinary retention after a sling procedure was less than 9%—as in most sling series—the overall effectiveness of slings was higher than that of the Burch.
Conclusion
The suburethral sling procedure has undergone many modifications since its first description nearly a century ago. As such, Ob/Gyns need to familiarize themselves with the current options. Typically, we perform up to 6 suburethral sling procedures per month. Of those, 50% are referrals from failed incontinence procedures. Recently, we have made the switch from using autologous slings to tension-free type slings due to ease and good outcomes. While more data from randomized, prospective, multicenter trials are needed to determine the best approach for individual patients, surgeons should become comfortable with the technique that works best for them.
The authors report no financial relationship with any companies whose products are mentioned in this article.
1. Leach GE, Dmochowski RR, Appell RA, et al. Female Stress Urinary Incontinence Clinical Guidelines Panel summary report on surgical management of female stress urinary incontinence. J Urol. 1997;875-880.
2. Nihira MA, Schaffer JI. Surgical procedures for stress urinary incontinence in 1999. Presented at: 28th Annual Scientific Meeting of the Society of Gynecologic Surgeons; March 6, 2002; Dallas, Texas.
3. Kobashi KC, Leach GE. Stress urinary incontinence. Curr Opin Urol. 1999;9:285-290.
4. Bowen LW, Sand PK, Ostergard DR, Franti CE. Unsuccessful Burch retropubic urethropexy: a case-controlled urodynamic study. Am J Obstet Gynecol. 1989;160:452-458.
5. Bump RC, Coates KW, Cundiff GW, Harris RL, Weidner AC. Diagnosing intrinsic sphincteric deficiency: Comparing urethral closure pressure, urethral axis, and Valsalva leak point pressures. Am J Obstet Gynecol. 1997;177:303-310.
6. Nichols DH, Randal CL. Operations for urinary stress incontinence. In: Vaginal Surgery. 4th ed. Baltimore, Md: Williams & Wilkins; 1996;402-415.
7. Rackley RR, Abdelmalak JB, Madjar S, Yanilmaz A, Appell RA, Tchetgen MB. Bone anchor infections in female pelvic reconstructive procedures: a literature review of series and case reports. J Urol. 2001;165:1975-1978.
8. Food and Drug Administration manufacturer and user facility device experience database. Available at: www.fda.gov/cdrh/maude.html. Accessed November 12, 2002.
9. Kuuva N, Nilsson CG. A nationwide analysis of complications associated with the tension-free vaginal tape (TVT) procedure. Neurourol Urodyn. 2000;19:394.-
10. Bezerra CA, Bruschini H. Suburethral sling operations for urinary incontinence in women. Cochrane Database Syst Rev. 2001;(3):CD001754.-
11. Nilsson CG, Kuuva N, Falconer C, Rezapour M, Ulmsten U. Long-term results of the tension-free vaginal tape procedure (TVT) for surgical treatment of female stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12(suppl 2):S5-S8.
12. Ward KL, Hilton P, Browning J. A randomized trial of colposuspension and tension-free vaginal tape (TVT) for primary genuine stress incontinence. Neurourol Urodyn. 2000;19:386.-
13. Ward K, Hilton P. Prospective multicenter randomized trial of tension-free vaginal tape and colposuspension as primary treatment for stress incontinence. BMJ. 2002;325:67.-
14. Weber A, Walters M. Burch procedure compared with sling for stress urinary incontinence: a decision analysis. Obstet Gynecol. 2000;96:867-873.
- Suburethral sling procedures are effective in treating patients with urethral hypermobility, intrinsic sphincter deficiency, low-pressure urethras, and increased intra-abdominal pressure.
- Autologous slings may be a better choice in cases of severe urogenital atrophy, previous radiation, or extensive scarring from previous repairs.
- For both the tension-free vaginal tape and SPARC slings, mark the suprapubic region 1 cm above and 1 cm lateral to the pubic symphsis on the left and right sides and inject 20 cc of a 1:1 mixture of local anesthetic and normal saline into the marked regions.
- Once the trocars are in place, fill the bladder with 250 cc of water and perform a cough stress test to confirm continence.
When the suburethral sling was first described in 1907 by von Giordano, it entailed placing autologous tissue underneath the bladder neck and suspending it superiorly. Complications including urethral erosion, infection, bleeding, and fistula formation led many surgeons to use it sparingly.
Fast forward to the 21st century: Synthetic materials and new techniques were introduced, simplifying the sling procedures and raising the long-term success rates to 84%.1 As a result, slings now stand at the forefront of stress urinary incontinence (SUI) treatment. Among advances are the tension-free vaginal tape (TVT) sling (Gynecare, a division of Ethicon Inc., Somerville, NJ) and the SPARC sling (American Medical Systems, Inc., Minnetonka, Minn). The former, approved in the U.S. in 1998, calls for another look due to of the recent publication of a Cochrane review of outcomes studies, while the latter, approved by the FDA in August 2001, is the newest technique deserving examination. Clearly, with 83,010 incontinence procedures performed in the U.S. in 1999,2 a detailed look at the suburethral sling is warranted. Here, we review materials, indications, techniques, complications, and outcomes.
Materials
The choice of material—either organic or synthetic—depends on several factors: availability, cost, patient and surgeon preference, and clinical variables. (TABLE 1) outlines the advantages and disadvantages of each material type. Organic slings include autologous tissues (rectus fascia and fascia lata graft), and allografts or xenografts (cadaveric fascia lata graft, human dermal graft, or porcine small intestine and dermal graft). Synthetic slings are made of polyethylene terephthalate, expanded polytetrafluoroethylene, and polypropylene.
While sling procedures utilizing organic materials do have their benefits, synthetic slings, particularly the polypropylene mesh used in TVT and SPARC, have proven to be a stable material unlikely to deteriorate with time. Further, increased collagen metabolism around this synthetic sling promotes an ingrowth of tissue through the mesh.
TABLE 1
Slings: advantages and disadvantages of various materials
| SLING MATERIAL | ADVANTAGES | DISADVANTAGES |
|---|---|---|
| Autologous tissues (rectus fascia, fascia lata, or vaginal wall) |
|
|
| Allografts (cadaveric fascia lata or dermis) |
|
|
| Xenografts (porcine dermis or small intestine) | ||
| Synthetic mesh (polyethylene terephthalate, expanded polytetrafluoroethylene, or polypropylene) |
|
|
Indications
Suburethral sling procedures are typically used for the treatment of genuine stress urinary incontinence (GSUI), in which the urethra becomes either hypermobile and unstable or its intrinsic sphincter becomes incompetent. In fact, slings are technically easier to place in patients with anatomic urethrovesical junction hypermobility compared to those with fixed urethras. Several authors also have suggested the sling’s advantage in patients with low-pressure urethras.3
Use urodynamic criteria to diagnose intrinsic sphincter deficiency (ISD), which is defined as a Valsalva leak point pressure of less than 60 cm water or maximal urethral closure pressure of less than 20 cm water. (Bear in mind, however, that these cut-off criteria are controversial.4,5)
Also, consider slings in patients with recurrent GSUI, inherited collagen deficiency, and increased abdominal pressure (e.g., women with chronic obstructive pulmonary disease, obesity, or high-impact physical activity). The sling also can be used as an adjunct to other transvaginal surgeries (e.g., hysterectomy or prolapse repair).
Autologous slings may be a better choice than synthetic slings in cases of severe urogenital atrophy, previous radiation, or extensive scarring from previous repairs. In these instances, the patient may be at-risk for postoperative vaginal necrosis or erosion.6 Due to their biocompatibility, autologous slings are more likely to heal over a vaginal erosion and less likely to infect or erode into the urethra. In any event, urogenital atrophy should be treated with local estrogen preoperatively to prevent some of these complications.
Technique
Conventionally, suburethral slings were placed via a combined vaginal and abdominal approach into the retropubic space of Retzius. Alternatively, the procedure could be performed abdominally by creating a suburethral tunnel via pelvic incisions, but this is the most difficult route.
Most recently, technological advances have simplified the vaginal approach, which utilizes minimal suburethral dissection and small suprapubic incisions. This technique is subdivided into “bottom-up” and “top-down” approaches. In the bottom-up TVT, the sling is inserted into a vaginal incision and threaded up through the patient’s pelvis, exiting from a small suprapubic incision. The topdown SPARC entails a reverse approach, starting from a suprapubic incision and exiting from a vaginal incision. New modifications allow for an abdominal TVT approach, as well, which we describe in detail in a later section.
Surgeons who are familiar with traditional needle suspensions may be more comfortable with the top-down approach. The need for concomitant surgery (e.g., hysterectomy or prolapse repair) not only determines the type of incontinence procedure, but also dictates the approach.
Preparing the patient. Place the patient under regional or local anesthesia with sedation so that an intraoperative cough stress test can be performed. Then administer an intravenous dose of a broad-spectrum antibiotic. Insert a 16 to 18 French Foley catheter into the urethra. Mark the suprapubic region 1 cm above and 1 cm lateral to the pubic symphsis on the left and right sides of the patient. Inject approximately 20 cc of a 1:1 mixture of local anesthetic and normal saline into the marked areas. We typically use 60 cc of 0.25% bupivicaine with epinephrine, diluted 1:1 with 60 cc of normal saline. After administering the local anesthetic suprapubically, inject a similar solution into the anterior vaginal wall suburethrally in the midline and laterally toward the retropubic tunnels.
Making the incisions. Both the TVT and SPARC techniques utilize the same type and location of incisions. As such, make a 0.5-cm incision into the abdominal skin on each side of the midline, approximately 1 cm lateral to midline and 1 cm above the pubic symphsis. Next, make a 1.5- to 2-cm vertical incision in the vaginal mucosa, starting 1.5 cm from the urethral meatus (FIGURE 1). Use Metzenbaum scissors to dissect the vaginal mucosa from the pubocervical fascia sub- and para-urethrally on both sides (FIGURE 2). Insert a Foley catheter guide (similar to the Lowsley retractor) into the catheter and deviate it to the ipsilateral side, thereby retracting the bladder neck to the contralateral side. Proceed with the placement of either the TVT or SPARC sling.
Placing the TVT sling. Attach the TVT introducer to the curved needle trocar on 1 end of the polypropylene sling. Insert the trocar with the tape attached into the vaginal incision and push through the retropubic space, keeping the trocar in close contact with the posterior surface of the pubic bone (FIGURES 3 and 4). Continue pushing the trocar through the urogenital diaphragm until its tip comes through the suprapubic incision on the ipsilateral side (FIGURE 5). It is important to not deviate too laterally, medially, or cephalad during trocar insertion to prevent vessel, bladder, or bowel injury. Perform a cystoscopy to rule out cystotomy. Place the second trocar in a similar manner on the opposite side. After both trocars have been pulled through their respective incisions, perform a tension test.
Placing the SPARC/abdominal TVT sling. Guide the abdominal needles through the previously marked suprapubic incision and the patient’s retropubic cavity (keeping the needle behind the pubic bone), to a finger placed in the vaginal incision (FIGURE 6). Snap the abdominal needle guides with the attached polypropylene mesh to the sling connectors (FIGURE 7). Bring the abdominal needles through the suprapubic incisions. Perform a tension test. As with the TVT sling, perform a cystoscopy after each needle placement to rule out cystotomy. Then pass the sling through the tunnel.
Testing for continence. Once the sling is in place, fill the bladder with 250 cc of water and perform a cough stress test. Adjust sling tension by pulling up on both sling arms until only a few drops of leakage are noted. It is important not to secure the sling too tightly as this may lead to urinary retention, detrusor instability, or urethral erosion. We prefer placing a hemostat between the sling tape and the urethra to avoid over tightening.
Suspending the sling arms. Remove the plastic sheaths after tension adjustment and cut the sling flush with the skin (FIGURE 8). Compared to the conventional bone-anchored slings, the newer tension-free sling devices are not anchored but instead suspended through the retropubic space. At first, the sling is held in place by friction from the opposing tissues. Over time, collagen formation fixes the mesh more strongly within the suburethral and paravaginal tissues.
Finally, close the suprapubic and vaginal incisions with absorbable sutures.
Placing autologous or allogenic slings. Fashion the graft, typically 2 cm wide and 10 to 12 cm long, with permanent sutures at the edges. Make a 1-cm incision into the suprapubic rectus fascia. Use either a Stamey-type needle trocar or uterine packing forceps and guide the instrument “top down” from the retropubic incision to the vaginal tunnel. The tunnel is made directly into the retropubic space from the vaginal incision. Bring the sling arms up on each side. Attach the arms to the rectus fascia and tie them down once cystoscopy and the tension test are complete.
The sling also can be performed with bone anchors placed through the vaginal incision into the pubic bone. Placement requires vaginal dissection into the retropubic space with no suprapubic incision. Once anchored, the sutures are then passed through the chosen graft materials and tied down. Bear in mind that anchoring into the periosteum of the pubic bone may cause severe osteomyelitis or osteitis pubis, though the actual incidence is unknown.7
FIGURE 1 Surgical steps for tension-free vaginal tape (TVT)
Place a Sims speculum into the vagina and make a vertical incision 1.5 cm from the external urethra meatus.
FIGURE 2
Use Metzenbaum scissors to dissect the vaginal mucosa from the underlying fascia bilaterally. Insert a Foley catheter with guide.
FIGURE 3
Place TVT trocars through the vaginal incision. Place abdominal guides suprapubically and attach them to the TVT trocar tip.
FIGURE 4
Push the tape through the retropubic space, keeping the trocar in close contact with the posterior surface of the pubic bone.
FIGURE 5
Continue pushing the trocar through the urogenital diaphragm until its tip comes through the suprapubic incision on the ipsilateral side.
FIGURE 6 Surgical steps for SPARC
Guide the needle down the posterior side of the pubic bone, keeping the needle tip in contact with the pubic bone.
FIGURE 7
Snap the needle guide and sling onto the sling connectors and pull through the suprapubic incision.
FIGURE 8
Perform a tension test. Remove the plastic sheaths and cut the sling flush with the skin.
Complications
Intraoperative and immediate postoperative complications include bladder perforation, vaginal or retropubic bleeding, wound or urinary tract infection (UTI), and short-term urinary retention. Possible long-term problems include urethral or vaginal erosion, mesh infection, prolonged voiding dysfunction, fistula formation, or de novo urge incontinence (TABLE 2).
Specifically, the TVT sling, which has been placed in more than 50,000 women in the U.S. and 200,000 worldwide, carries the potential for significant vascular injury and bowel perforation. In addition, the Food and Drug Administration (FDA) reported 4 deaths (2 from unrecognized bowel injuries, 1 from retropubic bleeding in a patient with a bleeding disorder, and 1 from a heart attack more than 1 week after an incontinence repair procedure complicated by a vascular injury); 168 device malfunctions (mostly tape or sheath detachment from the trocar); and 128 other injuries, including bowel perforations and major vascular injuries to the obturator, external iliac, femoral, or inferior epigastrics (TABLE 3).8 In a review of 1,455 TVT sling cases at 38 hospitals, Kuuva and Nilsson9 found bladder perforation in 3.8% of the patients and retropubic hematoma in 1.9%, along with 1 case of vesicovaginal fistula, 1 obturator nerve injury, and 1 epigastric vessel injury.
According to the FDA, there have been 2 complications reported with the SPARC sling system. Both involved vaginal erosion subsequently repaired by oversewing the vaginal mucosa. One of these complications occurred in a woman undergoing her fourth vaginal procedure who was therefore deemed to have “poor tissue.”8
TABLE 2
Suburethral sling complications1
| COMPLICATION | 1,715 AUTOLOGOUS | 1,515 SYNTHETIC |
|---|---|---|
| Vaginal erosion | 1 (.0001%) | 10 (.007%) |
| Urethral erosion | 5 (.003%) | 27 (.02%) |
| Fistula | 6 (.003%) | 4 (.002%) |
| Wound sinus | 3 (.002%) | 11 (.007%) |
| Wound infection | 11 (.006%) | 15 (.009%) |
| Seroma | 6 (.003%) | 1 (.0007%) |
TABLE 3
TVT complications in 200,000 procedures worldwide
| COMPLICATION | U.S. | WORLD | TOTAL |
|---|---|---|---|
| Vascular injury | 3 | 25 | 28 |
| Vaginal mesh exposure | 15 | 2 | 17 |
| Urethral erosion | 8 | 0 | 8 |
| Bowel perforation | 4 | 6 | 10 |
| Nerve injury | 1 | 0 | 1 |
Outcomes studies
Unfortunately, most of the published clinical studies on the surgical management of stress urinary incontinence suffer from inadequate follow-up and sample size, unclear patient selection criteria, and poor postoperative documentation, especially with respect to quality of life. However, multiple studies to assess the effectiveness and safety of TVT slings have been published. The following is an outline of these preliminary yet important findings.
In 2002, the Cochrane Database evaluated 7 randomized and quasi-randomized trials of suburethral slings for the treatment of urinary incontinence.10 Of 682 women evaluated, 457 had some type of suburethral sling procedure. Four trials compared slings to retropubic urethropexies, 1 compared slings to Stamey needle suspensions, and 2 compared the use of different sling materials. The results indicated that the data were insufficient to suggest that slings were more effective than other incontinence procedures or that slings were associated with fewer postoperative complications. While TVT slings did provide similar cure rates as open retropubic urethropexy, research is still lacking with respect to other types of slings. More studies comparing TVT slings to traditional pubovaginal slings also are needed before the 2 can be deemed equivalent.
In Sweden and Finland, where the TVT procedure was developed,11 85 patients who had undergone the procedure were evaluated at 48 to 70 months. Of those, 84.7% were completely cured of stress incontinence, 10.6% had significantly improved symptoms, and 4.7% were regarded as failures.
A recent well-designed, multicenter, randomized, prospective trial in the U.K. and Ireland compared 146 open Burch colposuspensions to 170 TVTs. Similar cure rates (57% and 66%, respectively) were reported.12 Although these rates are low compared to the Nordic nonrandomized TVT studies mentioned, the U.K./Ireland outcome criteria were particularly stringent and included a negative cystometrogram for stress incontinence and negative pad test. These differences in reported success rates highlight the importance of clearly defining objective outcomes criteria from randomized trials.
Nonetheless, the U.K./Ireland study showed that TVT is less invasive than the Burch procedure and is associated with shorter recovery periods and greater cost savings. Follow-up on complications (bladder perforation and hematoma in TVTs and incisional hernia formation in Burch colposuspensions) will be the most crucial aspect of this study.13
Clearly, the question of whether a Burch retropubic urethropexy or a suburethral sling procedure is better for SUI needs to be further investigated. Weber and Walters sought to answer this question by developing a decision analytical model (without the aid of randomized, controlled trials) and discovered similar cure rates.14 However, there were higher rates of urinary retention and detrusor instability associated with the traditional pubovaginal sling. But, most importantly, sensitivity analyses proved that if the rate of permanent urinary retention after a sling procedure was less than 9%—as in most sling series—the overall effectiveness of slings was higher than that of the Burch.
Conclusion
The suburethral sling procedure has undergone many modifications since its first description nearly a century ago. As such, Ob/Gyns need to familiarize themselves with the current options. Typically, we perform up to 6 suburethral sling procedures per month. Of those, 50% are referrals from failed incontinence procedures. Recently, we have made the switch from using autologous slings to tension-free type slings due to ease and good outcomes. While more data from randomized, prospective, multicenter trials are needed to determine the best approach for individual patients, surgeons should become comfortable with the technique that works best for them.
The authors report no financial relationship with any companies whose products are mentioned in this article.
- Suburethral sling procedures are effective in treating patients with urethral hypermobility, intrinsic sphincter deficiency, low-pressure urethras, and increased intra-abdominal pressure.
- Autologous slings may be a better choice in cases of severe urogenital atrophy, previous radiation, or extensive scarring from previous repairs.
- For both the tension-free vaginal tape and SPARC slings, mark the suprapubic region 1 cm above and 1 cm lateral to the pubic symphsis on the left and right sides and inject 20 cc of a 1:1 mixture of local anesthetic and normal saline into the marked regions.
- Once the trocars are in place, fill the bladder with 250 cc of water and perform a cough stress test to confirm continence.
When the suburethral sling was first described in 1907 by von Giordano, it entailed placing autologous tissue underneath the bladder neck and suspending it superiorly. Complications including urethral erosion, infection, bleeding, and fistula formation led many surgeons to use it sparingly.
Fast forward to the 21st century: Synthetic materials and new techniques were introduced, simplifying the sling procedures and raising the long-term success rates to 84%.1 As a result, slings now stand at the forefront of stress urinary incontinence (SUI) treatment. Among advances are the tension-free vaginal tape (TVT) sling (Gynecare, a division of Ethicon Inc., Somerville, NJ) and the SPARC sling (American Medical Systems, Inc., Minnetonka, Minn). The former, approved in the U.S. in 1998, calls for another look due to of the recent publication of a Cochrane review of outcomes studies, while the latter, approved by the FDA in August 2001, is the newest technique deserving examination. Clearly, with 83,010 incontinence procedures performed in the U.S. in 1999,2 a detailed look at the suburethral sling is warranted. Here, we review materials, indications, techniques, complications, and outcomes.
Materials
The choice of material—either organic or synthetic—depends on several factors: availability, cost, patient and surgeon preference, and clinical variables. (TABLE 1) outlines the advantages and disadvantages of each material type. Organic slings include autologous tissues (rectus fascia and fascia lata graft), and allografts or xenografts (cadaveric fascia lata graft, human dermal graft, or porcine small intestine and dermal graft). Synthetic slings are made of polyethylene terephthalate, expanded polytetrafluoroethylene, and polypropylene.
While sling procedures utilizing organic materials do have their benefits, synthetic slings, particularly the polypropylene mesh used in TVT and SPARC, have proven to be a stable material unlikely to deteriorate with time. Further, increased collagen metabolism around this synthetic sling promotes an ingrowth of tissue through the mesh.
TABLE 1
Slings: advantages and disadvantages of various materials
| SLING MATERIAL | ADVANTAGES | DISADVANTAGES |
|---|---|---|
| Autologous tissues (rectus fascia, fascia lata, or vaginal wall) |
|
|
| Allografts (cadaveric fascia lata or dermis) |
|
|
| Xenografts (porcine dermis or small intestine) | ||
| Synthetic mesh (polyethylene terephthalate, expanded polytetrafluoroethylene, or polypropylene) |
|
|
Indications
Suburethral sling procedures are typically used for the treatment of genuine stress urinary incontinence (GSUI), in which the urethra becomes either hypermobile and unstable or its intrinsic sphincter becomes incompetent. In fact, slings are technically easier to place in patients with anatomic urethrovesical junction hypermobility compared to those with fixed urethras. Several authors also have suggested the sling’s advantage in patients with low-pressure urethras.3
Use urodynamic criteria to diagnose intrinsic sphincter deficiency (ISD), which is defined as a Valsalva leak point pressure of less than 60 cm water or maximal urethral closure pressure of less than 20 cm water. (Bear in mind, however, that these cut-off criteria are controversial.4,5)
Also, consider slings in patients with recurrent GSUI, inherited collagen deficiency, and increased abdominal pressure (e.g., women with chronic obstructive pulmonary disease, obesity, or high-impact physical activity). The sling also can be used as an adjunct to other transvaginal surgeries (e.g., hysterectomy or prolapse repair).
Autologous slings may be a better choice than synthetic slings in cases of severe urogenital atrophy, previous radiation, or extensive scarring from previous repairs. In these instances, the patient may be at-risk for postoperative vaginal necrosis or erosion.6 Due to their biocompatibility, autologous slings are more likely to heal over a vaginal erosion and less likely to infect or erode into the urethra. In any event, urogenital atrophy should be treated with local estrogen preoperatively to prevent some of these complications.
Technique
Conventionally, suburethral slings were placed via a combined vaginal and abdominal approach into the retropubic space of Retzius. Alternatively, the procedure could be performed abdominally by creating a suburethral tunnel via pelvic incisions, but this is the most difficult route.
Most recently, technological advances have simplified the vaginal approach, which utilizes minimal suburethral dissection and small suprapubic incisions. This technique is subdivided into “bottom-up” and “top-down” approaches. In the bottom-up TVT, the sling is inserted into a vaginal incision and threaded up through the patient’s pelvis, exiting from a small suprapubic incision. The topdown SPARC entails a reverse approach, starting from a suprapubic incision and exiting from a vaginal incision. New modifications allow for an abdominal TVT approach, as well, which we describe in detail in a later section.
Surgeons who are familiar with traditional needle suspensions may be more comfortable with the top-down approach. The need for concomitant surgery (e.g., hysterectomy or prolapse repair) not only determines the type of incontinence procedure, but also dictates the approach.
Preparing the patient. Place the patient under regional or local anesthesia with sedation so that an intraoperative cough stress test can be performed. Then administer an intravenous dose of a broad-spectrum antibiotic. Insert a 16 to 18 French Foley catheter into the urethra. Mark the suprapubic region 1 cm above and 1 cm lateral to the pubic symphsis on the left and right sides of the patient. Inject approximately 20 cc of a 1:1 mixture of local anesthetic and normal saline into the marked areas. We typically use 60 cc of 0.25% bupivicaine with epinephrine, diluted 1:1 with 60 cc of normal saline. After administering the local anesthetic suprapubically, inject a similar solution into the anterior vaginal wall suburethrally in the midline and laterally toward the retropubic tunnels.
Making the incisions. Both the TVT and SPARC techniques utilize the same type and location of incisions. As such, make a 0.5-cm incision into the abdominal skin on each side of the midline, approximately 1 cm lateral to midline and 1 cm above the pubic symphsis. Next, make a 1.5- to 2-cm vertical incision in the vaginal mucosa, starting 1.5 cm from the urethral meatus (FIGURE 1). Use Metzenbaum scissors to dissect the vaginal mucosa from the pubocervical fascia sub- and para-urethrally on both sides (FIGURE 2). Insert a Foley catheter guide (similar to the Lowsley retractor) into the catheter and deviate it to the ipsilateral side, thereby retracting the bladder neck to the contralateral side. Proceed with the placement of either the TVT or SPARC sling.
Placing the TVT sling. Attach the TVT introducer to the curved needle trocar on 1 end of the polypropylene sling. Insert the trocar with the tape attached into the vaginal incision and push through the retropubic space, keeping the trocar in close contact with the posterior surface of the pubic bone (FIGURES 3 and 4). Continue pushing the trocar through the urogenital diaphragm until its tip comes through the suprapubic incision on the ipsilateral side (FIGURE 5). It is important to not deviate too laterally, medially, or cephalad during trocar insertion to prevent vessel, bladder, or bowel injury. Perform a cystoscopy to rule out cystotomy. Place the second trocar in a similar manner on the opposite side. After both trocars have been pulled through their respective incisions, perform a tension test.
Placing the SPARC/abdominal TVT sling. Guide the abdominal needles through the previously marked suprapubic incision and the patient’s retropubic cavity (keeping the needle behind the pubic bone), to a finger placed in the vaginal incision (FIGURE 6). Snap the abdominal needle guides with the attached polypropylene mesh to the sling connectors (FIGURE 7). Bring the abdominal needles through the suprapubic incisions. Perform a tension test. As with the TVT sling, perform a cystoscopy after each needle placement to rule out cystotomy. Then pass the sling through the tunnel.
Testing for continence. Once the sling is in place, fill the bladder with 250 cc of water and perform a cough stress test. Adjust sling tension by pulling up on both sling arms until only a few drops of leakage are noted. It is important not to secure the sling too tightly as this may lead to urinary retention, detrusor instability, or urethral erosion. We prefer placing a hemostat between the sling tape and the urethra to avoid over tightening.
Suspending the sling arms. Remove the plastic sheaths after tension adjustment and cut the sling flush with the skin (FIGURE 8). Compared to the conventional bone-anchored slings, the newer tension-free sling devices are not anchored but instead suspended through the retropubic space. At first, the sling is held in place by friction from the opposing tissues. Over time, collagen formation fixes the mesh more strongly within the suburethral and paravaginal tissues.
Finally, close the suprapubic and vaginal incisions with absorbable sutures.
Placing autologous or allogenic slings. Fashion the graft, typically 2 cm wide and 10 to 12 cm long, with permanent sutures at the edges. Make a 1-cm incision into the suprapubic rectus fascia. Use either a Stamey-type needle trocar or uterine packing forceps and guide the instrument “top down” from the retropubic incision to the vaginal tunnel. The tunnel is made directly into the retropubic space from the vaginal incision. Bring the sling arms up on each side. Attach the arms to the rectus fascia and tie them down once cystoscopy and the tension test are complete.
The sling also can be performed with bone anchors placed through the vaginal incision into the pubic bone. Placement requires vaginal dissection into the retropubic space with no suprapubic incision. Once anchored, the sutures are then passed through the chosen graft materials and tied down. Bear in mind that anchoring into the periosteum of the pubic bone may cause severe osteomyelitis or osteitis pubis, though the actual incidence is unknown.7
FIGURE 1 Surgical steps for tension-free vaginal tape (TVT)
Place a Sims speculum into the vagina and make a vertical incision 1.5 cm from the external urethra meatus.
FIGURE 2
Use Metzenbaum scissors to dissect the vaginal mucosa from the underlying fascia bilaterally. Insert a Foley catheter with guide.
FIGURE 3
Place TVT trocars through the vaginal incision. Place abdominal guides suprapubically and attach them to the TVT trocar tip.
FIGURE 4
Push the tape through the retropubic space, keeping the trocar in close contact with the posterior surface of the pubic bone.
FIGURE 5
Continue pushing the trocar through the urogenital diaphragm until its tip comes through the suprapubic incision on the ipsilateral side.
FIGURE 6 Surgical steps for SPARC
Guide the needle down the posterior side of the pubic bone, keeping the needle tip in contact with the pubic bone.
FIGURE 7
Snap the needle guide and sling onto the sling connectors and pull through the suprapubic incision.
FIGURE 8
Perform a tension test. Remove the plastic sheaths and cut the sling flush with the skin.
Complications
Intraoperative and immediate postoperative complications include bladder perforation, vaginal or retropubic bleeding, wound or urinary tract infection (UTI), and short-term urinary retention. Possible long-term problems include urethral or vaginal erosion, mesh infection, prolonged voiding dysfunction, fistula formation, or de novo urge incontinence (TABLE 2).
Specifically, the TVT sling, which has been placed in more than 50,000 women in the U.S. and 200,000 worldwide, carries the potential for significant vascular injury and bowel perforation. In addition, the Food and Drug Administration (FDA) reported 4 deaths (2 from unrecognized bowel injuries, 1 from retropubic bleeding in a patient with a bleeding disorder, and 1 from a heart attack more than 1 week after an incontinence repair procedure complicated by a vascular injury); 168 device malfunctions (mostly tape or sheath detachment from the trocar); and 128 other injuries, including bowel perforations and major vascular injuries to the obturator, external iliac, femoral, or inferior epigastrics (TABLE 3).8 In a review of 1,455 TVT sling cases at 38 hospitals, Kuuva and Nilsson9 found bladder perforation in 3.8% of the patients and retropubic hematoma in 1.9%, along with 1 case of vesicovaginal fistula, 1 obturator nerve injury, and 1 epigastric vessel injury.
According to the FDA, there have been 2 complications reported with the SPARC sling system. Both involved vaginal erosion subsequently repaired by oversewing the vaginal mucosa. One of these complications occurred in a woman undergoing her fourth vaginal procedure who was therefore deemed to have “poor tissue.”8
TABLE 2
Suburethral sling complications1
| COMPLICATION | 1,715 AUTOLOGOUS | 1,515 SYNTHETIC |
|---|---|---|
| Vaginal erosion | 1 (.0001%) | 10 (.007%) |
| Urethral erosion | 5 (.003%) | 27 (.02%) |
| Fistula | 6 (.003%) | 4 (.002%) |
| Wound sinus | 3 (.002%) | 11 (.007%) |
| Wound infection | 11 (.006%) | 15 (.009%) |
| Seroma | 6 (.003%) | 1 (.0007%) |
TABLE 3
TVT complications in 200,000 procedures worldwide
| COMPLICATION | U.S. | WORLD | TOTAL |
|---|---|---|---|
| Vascular injury | 3 | 25 | 28 |
| Vaginal mesh exposure | 15 | 2 | 17 |
| Urethral erosion | 8 | 0 | 8 |
| Bowel perforation | 4 | 6 | 10 |
| Nerve injury | 1 | 0 | 1 |
Outcomes studies
Unfortunately, most of the published clinical studies on the surgical management of stress urinary incontinence suffer from inadequate follow-up and sample size, unclear patient selection criteria, and poor postoperative documentation, especially with respect to quality of life. However, multiple studies to assess the effectiveness and safety of TVT slings have been published. The following is an outline of these preliminary yet important findings.
In 2002, the Cochrane Database evaluated 7 randomized and quasi-randomized trials of suburethral slings for the treatment of urinary incontinence.10 Of 682 women evaluated, 457 had some type of suburethral sling procedure. Four trials compared slings to retropubic urethropexies, 1 compared slings to Stamey needle suspensions, and 2 compared the use of different sling materials. The results indicated that the data were insufficient to suggest that slings were more effective than other incontinence procedures or that slings were associated with fewer postoperative complications. While TVT slings did provide similar cure rates as open retropubic urethropexy, research is still lacking with respect to other types of slings. More studies comparing TVT slings to traditional pubovaginal slings also are needed before the 2 can be deemed equivalent.
In Sweden and Finland, where the TVT procedure was developed,11 85 patients who had undergone the procedure were evaluated at 48 to 70 months. Of those, 84.7% were completely cured of stress incontinence, 10.6% had significantly improved symptoms, and 4.7% were regarded as failures.
A recent well-designed, multicenter, randomized, prospective trial in the U.K. and Ireland compared 146 open Burch colposuspensions to 170 TVTs. Similar cure rates (57% and 66%, respectively) were reported.12 Although these rates are low compared to the Nordic nonrandomized TVT studies mentioned, the U.K./Ireland outcome criteria were particularly stringent and included a negative cystometrogram for stress incontinence and negative pad test. These differences in reported success rates highlight the importance of clearly defining objective outcomes criteria from randomized trials.
Nonetheless, the U.K./Ireland study showed that TVT is less invasive than the Burch procedure and is associated with shorter recovery periods and greater cost savings. Follow-up on complications (bladder perforation and hematoma in TVTs and incisional hernia formation in Burch colposuspensions) will be the most crucial aspect of this study.13
Clearly, the question of whether a Burch retropubic urethropexy or a suburethral sling procedure is better for SUI needs to be further investigated. Weber and Walters sought to answer this question by developing a decision analytical model (without the aid of randomized, controlled trials) and discovered similar cure rates.14 However, there were higher rates of urinary retention and detrusor instability associated with the traditional pubovaginal sling. But, most importantly, sensitivity analyses proved that if the rate of permanent urinary retention after a sling procedure was less than 9%—as in most sling series—the overall effectiveness of slings was higher than that of the Burch.
Conclusion
The suburethral sling procedure has undergone many modifications since its first description nearly a century ago. As such, Ob/Gyns need to familiarize themselves with the current options. Typically, we perform up to 6 suburethral sling procedures per month. Of those, 50% are referrals from failed incontinence procedures. Recently, we have made the switch from using autologous slings to tension-free type slings due to ease and good outcomes. While more data from randomized, prospective, multicenter trials are needed to determine the best approach for individual patients, surgeons should become comfortable with the technique that works best for them.
The authors report no financial relationship with any companies whose products are mentioned in this article.
1. Leach GE, Dmochowski RR, Appell RA, et al. Female Stress Urinary Incontinence Clinical Guidelines Panel summary report on surgical management of female stress urinary incontinence. J Urol. 1997;875-880.
2. Nihira MA, Schaffer JI. Surgical procedures for stress urinary incontinence in 1999. Presented at: 28th Annual Scientific Meeting of the Society of Gynecologic Surgeons; March 6, 2002; Dallas, Texas.
3. Kobashi KC, Leach GE. Stress urinary incontinence. Curr Opin Urol. 1999;9:285-290.
4. Bowen LW, Sand PK, Ostergard DR, Franti CE. Unsuccessful Burch retropubic urethropexy: a case-controlled urodynamic study. Am J Obstet Gynecol. 1989;160:452-458.
5. Bump RC, Coates KW, Cundiff GW, Harris RL, Weidner AC. Diagnosing intrinsic sphincteric deficiency: Comparing urethral closure pressure, urethral axis, and Valsalva leak point pressures. Am J Obstet Gynecol. 1997;177:303-310.
6. Nichols DH, Randal CL. Operations for urinary stress incontinence. In: Vaginal Surgery. 4th ed. Baltimore, Md: Williams & Wilkins; 1996;402-415.
7. Rackley RR, Abdelmalak JB, Madjar S, Yanilmaz A, Appell RA, Tchetgen MB. Bone anchor infections in female pelvic reconstructive procedures: a literature review of series and case reports. J Urol. 2001;165:1975-1978.
8. Food and Drug Administration manufacturer and user facility device experience database. Available at: www.fda.gov/cdrh/maude.html. Accessed November 12, 2002.
9. Kuuva N, Nilsson CG. A nationwide analysis of complications associated with the tension-free vaginal tape (TVT) procedure. Neurourol Urodyn. 2000;19:394.-
10. Bezerra CA, Bruschini H. Suburethral sling operations for urinary incontinence in women. Cochrane Database Syst Rev. 2001;(3):CD001754.-
11. Nilsson CG, Kuuva N, Falconer C, Rezapour M, Ulmsten U. Long-term results of the tension-free vaginal tape procedure (TVT) for surgical treatment of female stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12(suppl 2):S5-S8.
12. Ward KL, Hilton P, Browning J. A randomized trial of colposuspension and tension-free vaginal tape (TVT) for primary genuine stress incontinence. Neurourol Urodyn. 2000;19:386.-
13. Ward K, Hilton P. Prospective multicenter randomized trial of tension-free vaginal tape and colposuspension as primary treatment for stress incontinence. BMJ. 2002;325:67.-
14. Weber A, Walters M. Burch procedure compared with sling for stress urinary incontinence: a decision analysis. Obstet Gynecol. 2000;96:867-873.
1. Leach GE, Dmochowski RR, Appell RA, et al. Female Stress Urinary Incontinence Clinical Guidelines Panel summary report on surgical management of female stress urinary incontinence. J Urol. 1997;875-880.
2. Nihira MA, Schaffer JI. Surgical procedures for stress urinary incontinence in 1999. Presented at: 28th Annual Scientific Meeting of the Society of Gynecologic Surgeons; March 6, 2002; Dallas, Texas.
3. Kobashi KC, Leach GE. Stress urinary incontinence. Curr Opin Urol. 1999;9:285-290.
4. Bowen LW, Sand PK, Ostergard DR, Franti CE. Unsuccessful Burch retropubic urethropexy: a case-controlled urodynamic study. Am J Obstet Gynecol. 1989;160:452-458.
5. Bump RC, Coates KW, Cundiff GW, Harris RL, Weidner AC. Diagnosing intrinsic sphincteric deficiency: Comparing urethral closure pressure, urethral axis, and Valsalva leak point pressures. Am J Obstet Gynecol. 1997;177:303-310.
6. Nichols DH, Randal CL. Operations for urinary stress incontinence. In: Vaginal Surgery. 4th ed. Baltimore, Md: Williams & Wilkins; 1996;402-415.
7. Rackley RR, Abdelmalak JB, Madjar S, Yanilmaz A, Appell RA, Tchetgen MB. Bone anchor infections in female pelvic reconstructive procedures: a literature review of series and case reports. J Urol. 2001;165:1975-1978.
8. Food and Drug Administration manufacturer and user facility device experience database. Available at: www.fda.gov/cdrh/maude.html. Accessed November 12, 2002.
9. Kuuva N, Nilsson CG. A nationwide analysis of complications associated with the tension-free vaginal tape (TVT) procedure. Neurourol Urodyn. 2000;19:394.-
10. Bezerra CA, Bruschini H. Suburethral sling operations for urinary incontinence in women. Cochrane Database Syst Rev. 2001;(3):CD001754.-
11. Nilsson CG, Kuuva N, Falconer C, Rezapour M, Ulmsten U. Long-term results of the tension-free vaginal tape procedure (TVT) for surgical treatment of female stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12(suppl 2):S5-S8.
12. Ward KL, Hilton P, Browning J. A randomized trial of colposuspension and tension-free vaginal tape (TVT) for primary genuine stress incontinence. Neurourol Urodyn. 2000;19:386.-
13. Ward K, Hilton P. Prospective multicenter randomized trial of tension-free vaginal tape and colposuspension as primary treatment for stress incontinence. BMJ. 2002;325:67.-
14. Weber A, Walters M. Burch procedure compared with sling for stress urinary incontinence: a decision analysis. Obstet Gynecol. 2000;96:867-873.