User login
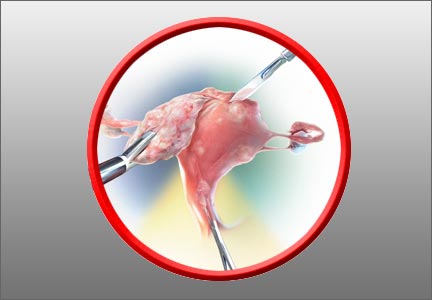
In the absence of a definitive FDA decision on the future of power morcellation in minimally invasive gynecologic surgery, many surgeons have stopped offering the option, often in response to constraints placed by their institutions, or have greatly expanded the informed consent discussion.
In Part 1 of this two-part roundtable discussion, which appeared in the September 2014 issue of OBG Management, our expert panelists discussed their current approach to tissue extraction during hysterectomy and myomectomy, as well as their preferred approach to both procedures amid this changing surgical environment. Here, in Part 2, they discuss patient counseling and the likely effects of FDA action.
How has your counseling changed?
OBG Management: Given recent concerns about the use of power morcellation, how has your counseling of the patient changed?
Kimberly Kho, MD, MPH: Though I look forward to the development of instruments and techniques that will make contained power morcellation safer, I am not using it currently and have been able to find minimally invasive alternatives such as minilaparotomy and vaginal removal of masses for the cases I would have considered for power morcellation.
Certainly, with power morcellation or any type of morcellation, it’s important to discuss the risks and benefits, as well as alternatives. Discussion should include the potential for:
- iatrogenic injury and tissue seeding of both benign and malignant tissue
- exacerbation of any occult malignancy and possible worsening of prognosis
- missing or mischaracterizing an occult malignancy.
Although there is no surefire way to avoid cellular dissemination with any type of surgery, I think it’s equally important to explain that, often, the only way to completely avoid fragmenting a large mass is to remove it en bloc, which would mean a large laparotomy for many patients. Women should understand the risks of laparotomy as well, including more frequent wound complications, longer hospitalization, and slower recovery.
Arnold P. Advincula, MD: If a clinician anticipates or plans the use of power morcellation, he or she certainly needs to go through an informed consent process with the patient. This process may include a separate form specific to power morcellation as well as detailed documentation during the preoperative visit.
OBG Management: What elements of the preoperative visit do you believe are important to document?
Dr. Advincula: It is important to clearly document the indications and alternatives for the surgery, as well as the decision-making process that led to the selection of a particular procedure and route of access. If any type of morcellation (power-driven or not) is anticipated, then the risks associated with it must be thoroughly discussed and documented in addition to the standard risks associated with any type of abdominal-pelvic surgery. No surgical procedure is without risks. Therefore, the process of informed consent cannot be taken lightly and is a critical part of the process that allows a patient to decide upon a particular intervention.
Jason D. Wright, MD: I believe the current role of power morcellation is limited. Patients considering the procedure should be counseled about the risks of cancer as well as other adverse pathologic abnormalities, including smooth muscle tumors of uncertain malignant potential, disseminated leiomyomatosis, and endometrial hyperplasia that may be associated with an occult cancer.
OBG Management: Do you recommend a separate consent form for power morcellation, as Dr. Advincula suggested?
Dr. Wright: Given the risk of adverse pathology, I think the role of electric power morcellation is limited. Patients should be carefully counseled about alternative surgical approaches that avoid tissue disruption and understand that the sensitivity of preoperative testing and intraoperative evaluation of smooth muscle neoplasms is limited. Further, patients considering contained morcellation also should be informed that the data examining the efficacy of these techniques are sparse.
Linda D. Bradley, MD: As I mentioned in Part 1 of our discussion, I’m giving patients new information about our concerns regarding occult malignancy, quoting the risk estimates given by the FDA this year.1 And the fact that we no longer use power morcellation at the Cleveland Clinic means that I no longer discuss it as an option, although one or two patients have asked for it in recent months.
I think many patients have read about it in the news or, once hysterectomy or myomectomy was planned, found discussion of the controversy surrounding it during their research. I’ve even had patients who underwent hysteroscopic myomectomy 2 or more years ago contacting me to find out whether power morcellation was used, and I have had to explain that hysteroscopic morcellation is different from the laparoscopic variant.
Patients are critical readers and are much more knowledgeable as a result of social media, so I do find myself spending more time discussing their procedure with them.
For myomectomy in particular, we send for a frozen section intraoperatively. Although that approach still is not 100% sensitive, it does guide what we do during surgery. If a sarcoma is found, for example, we call in the oncologists. I discuss that possibility with the patient as well. So I am spending more time with patients, but I don’t go into power morcellation because that is no longer an option for me.
OBG Management: Dr. Iglesia, has your counseling of patients changed in any way?
Cheryl Iglesia, MD: I do not routinely use power morcellation. However, the findings from the FDA and Dr. Wright about the higher risk of occult malignancy in fibroids is information I share with patients preoperatively.1,2
For women with fibroids who want uterine conservation procedures or who desire medical management, such as focused ultrasound or uterine fibroid embolization, MRI is routine. However, we make patients aware that this imaging modality is not 100% sensitive in detecting occult cancer—and neither are random biopsies of fibroids. Patients also need to be made aware that treatment with fibroid embolization or other medical options also could delay the detection of cancer and sarcoma. Any morcellation technique (power, hand, vaginal) does have the risk of potential cancer spread and upstaging, so morcellation should not be used in any women with suspected or known malignancy.
Effects of likely FDA actions
OBG Management: If the FDA decides to ban power morcellation outright, in some ways the approach to patient counseling will be simpler, as one option will have been permanently eliminated. But if the FDA allows power morcellation to continue, with stricter labeling, would that affect how you counsel patients? And would you reconsider power morcellation in that light?
Dr. Kho: I think the current discussion has highlighted again how important the informed consent process is as an opportunity for information sharing. It’s an ongoing discussion of risks, benefits, and alternatives. It also offers us an opportunity to understand the patient’s values and perspectives throughout the process of surgical planning. So, no, I don’t think the FDA’s actions will change how I counsel patients. Regardless of the FDA’s decisions, I think open power morcellation as we currently know it may be obviated as new instruments for contained morcellation—as well as other techniques we’ve discussed—become more popular. But it’s critical that we meaningfully monitor these techniques for long-term safety. In order to make evidence-based decisions, we will need good data.
Dr. Iglesia: I cannot comment on a final FDA decision. However, my feeling is that any information that patients can use to become educated about treatment alternatives—including the risks and benefits of each option—will help inform and improve the shared decision-making process.
Dr. Advincula: Regardless of the verdict rendered by the FDA, the way we approach tissue extraction in minimally invasive surgery has been changed forever. It is always important to take a critical look at the way things are done, but not at the expense of throwing the proverbial baby out with the bath water. If power morcellation were to remain a viable option, my counseling would remain as is, as it already has been modified and quite detailed in the wake of this whole controversy. I still believe there is a role for power morcellation, albeit modified from its current iteration, when applied by the right physician in a properly evaluated patient with the right indication.
Summing up
OBG Management: Do you have any additional comments about this issue?
Dr. Advincula: The ability to accurately and reliably detect an occult uterine malignancy—specifically, leiomyosarcoma—is lacking at present. Whether or not power morcellation remains a viable option in the future, the bottom line is that patients will still present with occult uterine malignancy. Minimizing the mishandling of this unfortunate diagnosis will depend on sound clinical judgment as well as improvements in diagnosis. It always will be important to avoid blaming the lack of sound clinical practice on surgical devices that, when used appropriately, have the potential to benefit the majority of women.
Dr. Kho: The current attention on power morcellators presents an opportunity to improve upon our current practices and find solutions to the issues we are encountering. I think this is an exciting time for examining preoperative risk stratification, the innovation of new techniques, repopularization and improvement of older ones such as vaginal tissue extraction, and, overall, to improve our system of safety monitoring and surgical device surveillance.
Dr. Iglesia: Intraperitoneal power morcellation should not be used in cases of malignancy or suspected malignancy or in postmenopausal patients with bleeding or growing fibroids. The availability of power morcellators may be limited as manufacturers
cease distribution, hospitals ban use, or insurers refuse payment for use.
Alternative minimally invasive approaches—especially the transvaginal approach—should be considered, since there are fewer complications associated with vaginal surgery, especially compared with open and laparoscopic surgery.
Dr. Wright: Although electric power morcellation may allow some women to undergo a minimally invasive procedure, the data currently available clearly suggest that adverse pathology is more common in women who undergo morcellation than was previously thought.
Although the debate around morcellation has focused on leiomyosarcoma, epithelial endometrial tumors and other preinvasive abnormalities are also common. These unexpected pathologic findings in women who underwent electric power morcellation highlight the importance of performing more rigorous evaluation of new methods of tissue extraction.
Quick Poll:
If you are using, plan to use, or anticipate the possibility of using power morcellation during minimally invasive gynecologic surgery, does your consent process include a separate form specific to power morcellation?
Please provide your answer to this question in the Quick Poll on the OBG Management home page, and then see how your peers have voted. Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication.http://www.fda.gov/medicaldevices/safety/alertsandnotices/ucm393576.htm. Published April 17, 2014. Accessed September 18, 2014.
2. Wright JD, Tergas AI, Burke WM, et al. Uterine pathology in women undergoing minimally invasive hysterectomy with morcellation [published online ahead of print July 22, 2014]. JAMA. doi:10.1001/jama.2014.9005.
In the absence of a definitive FDA decision on the future of power morcellation in minimally invasive gynecologic surgery, many surgeons have stopped offering the option, often in response to constraints placed by their institutions, or have greatly expanded the informed consent discussion.
In Part 1 of this two-part roundtable discussion, which appeared in the September 2014 issue of OBG Management, our expert panelists discussed their current approach to tissue extraction during hysterectomy and myomectomy, as well as their preferred approach to both procedures amid this changing surgical environment. Here, in Part 2, they discuss patient counseling and the likely effects of FDA action.
How has your counseling changed?
OBG Management: Given recent concerns about the use of power morcellation, how has your counseling of the patient changed?
Kimberly Kho, MD, MPH: Though I look forward to the development of instruments and techniques that will make contained power morcellation safer, I am not using it currently and have been able to find minimally invasive alternatives such as minilaparotomy and vaginal removal of masses for the cases I would have considered for power morcellation.
Certainly, with power morcellation or any type of morcellation, it’s important to discuss the risks and benefits, as well as alternatives. Discussion should include the potential for:
- iatrogenic injury and tissue seeding of both benign and malignant tissue
- exacerbation of any occult malignancy and possible worsening of prognosis
- missing or mischaracterizing an occult malignancy.
Although there is no surefire way to avoid cellular dissemination with any type of surgery, I think it’s equally important to explain that, often, the only way to completely avoid fragmenting a large mass is to remove it en bloc, which would mean a large laparotomy for many patients. Women should understand the risks of laparotomy as well, including more frequent wound complications, longer hospitalization, and slower recovery.
Arnold P. Advincula, MD: If a clinician anticipates or plans the use of power morcellation, he or she certainly needs to go through an informed consent process with the patient. This process may include a separate form specific to power morcellation as well as detailed documentation during the preoperative visit.
OBG Management: What elements of the preoperative visit do you believe are important to document?
Dr. Advincula: It is important to clearly document the indications and alternatives for the surgery, as well as the decision-making process that led to the selection of a particular procedure and route of access. If any type of morcellation (power-driven or not) is anticipated, then the risks associated with it must be thoroughly discussed and documented in addition to the standard risks associated with any type of abdominal-pelvic surgery. No surgical procedure is without risks. Therefore, the process of informed consent cannot be taken lightly and is a critical part of the process that allows a patient to decide upon a particular intervention.
Jason D. Wright, MD: I believe the current role of power morcellation is limited. Patients considering the procedure should be counseled about the risks of cancer as well as other adverse pathologic abnormalities, including smooth muscle tumors of uncertain malignant potential, disseminated leiomyomatosis, and endometrial hyperplasia that may be associated with an occult cancer.
OBG Management: Do you recommend a separate consent form for power morcellation, as Dr. Advincula suggested?
Dr. Wright: Given the risk of adverse pathology, I think the role of electric power morcellation is limited. Patients should be carefully counseled about alternative surgical approaches that avoid tissue disruption and understand that the sensitivity of preoperative testing and intraoperative evaluation of smooth muscle neoplasms is limited. Further, patients considering contained morcellation also should be informed that the data examining the efficacy of these techniques are sparse.
Linda D. Bradley, MD: As I mentioned in Part 1 of our discussion, I’m giving patients new information about our concerns regarding occult malignancy, quoting the risk estimates given by the FDA this year.1 And the fact that we no longer use power morcellation at the Cleveland Clinic means that I no longer discuss it as an option, although one or two patients have asked for it in recent months.
I think many patients have read about it in the news or, once hysterectomy or myomectomy was planned, found discussion of the controversy surrounding it during their research. I’ve even had patients who underwent hysteroscopic myomectomy 2 or more years ago contacting me to find out whether power morcellation was used, and I have had to explain that hysteroscopic morcellation is different from the laparoscopic variant.
Patients are critical readers and are much more knowledgeable as a result of social media, so I do find myself spending more time discussing their procedure with them.
For myomectomy in particular, we send for a frozen section intraoperatively. Although that approach still is not 100% sensitive, it does guide what we do during surgery. If a sarcoma is found, for example, we call in the oncologists. I discuss that possibility with the patient as well. So I am spending more time with patients, but I don’t go into power morcellation because that is no longer an option for me.
OBG Management: Dr. Iglesia, has your counseling of patients changed in any way?
Cheryl Iglesia, MD: I do not routinely use power morcellation. However, the findings from the FDA and Dr. Wright about the higher risk of occult malignancy in fibroids is information I share with patients preoperatively.1,2
For women with fibroids who want uterine conservation procedures or who desire medical management, such as focused ultrasound or uterine fibroid embolization, MRI is routine. However, we make patients aware that this imaging modality is not 100% sensitive in detecting occult cancer—and neither are random biopsies of fibroids. Patients also need to be made aware that treatment with fibroid embolization or other medical options also could delay the detection of cancer and sarcoma. Any morcellation technique (power, hand, vaginal) does have the risk of potential cancer spread and upstaging, so morcellation should not be used in any women with suspected or known malignancy.
Effects of likely FDA actions
OBG Management: If the FDA decides to ban power morcellation outright, in some ways the approach to patient counseling will be simpler, as one option will have been permanently eliminated. But if the FDA allows power morcellation to continue, with stricter labeling, would that affect how you counsel patients? And would you reconsider power morcellation in that light?
Dr. Kho: I think the current discussion has highlighted again how important the informed consent process is as an opportunity for information sharing. It’s an ongoing discussion of risks, benefits, and alternatives. It also offers us an opportunity to understand the patient’s values and perspectives throughout the process of surgical planning. So, no, I don’t think the FDA’s actions will change how I counsel patients. Regardless of the FDA’s decisions, I think open power morcellation as we currently know it may be obviated as new instruments for contained morcellation—as well as other techniques we’ve discussed—become more popular. But it’s critical that we meaningfully monitor these techniques for long-term safety. In order to make evidence-based decisions, we will need good data.
Dr. Iglesia: I cannot comment on a final FDA decision. However, my feeling is that any information that patients can use to become educated about treatment alternatives—including the risks and benefits of each option—will help inform and improve the shared decision-making process.
Dr. Advincula: Regardless of the verdict rendered by the FDA, the way we approach tissue extraction in minimally invasive surgery has been changed forever. It is always important to take a critical look at the way things are done, but not at the expense of throwing the proverbial baby out with the bath water. If power morcellation were to remain a viable option, my counseling would remain as is, as it already has been modified and quite detailed in the wake of this whole controversy. I still believe there is a role for power morcellation, albeit modified from its current iteration, when applied by the right physician in a properly evaluated patient with the right indication.
Summing up
OBG Management: Do you have any additional comments about this issue?
Dr. Advincula: The ability to accurately and reliably detect an occult uterine malignancy—specifically, leiomyosarcoma—is lacking at present. Whether or not power morcellation remains a viable option in the future, the bottom line is that patients will still present with occult uterine malignancy. Minimizing the mishandling of this unfortunate diagnosis will depend on sound clinical judgment as well as improvements in diagnosis. It always will be important to avoid blaming the lack of sound clinical practice on surgical devices that, when used appropriately, have the potential to benefit the majority of women.
Dr. Kho: The current attention on power morcellators presents an opportunity to improve upon our current practices and find solutions to the issues we are encountering. I think this is an exciting time for examining preoperative risk stratification, the innovation of new techniques, repopularization and improvement of older ones such as vaginal tissue extraction, and, overall, to improve our system of safety monitoring and surgical device surveillance.
Dr. Iglesia: Intraperitoneal power morcellation should not be used in cases of malignancy or suspected malignancy or in postmenopausal patients with bleeding or growing fibroids. The availability of power morcellators may be limited as manufacturers
cease distribution, hospitals ban use, or insurers refuse payment for use.
Alternative minimally invasive approaches—especially the transvaginal approach—should be considered, since there are fewer complications associated with vaginal surgery, especially compared with open and laparoscopic surgery.
Dr. Wright: Although electric power morcellation may allow some women to undergo a minimally invasive procedure, the data currently available clearly suggest that adverse pathology is more common in women who undergo morcellation than was previously thought.
Although the debate around morcellation has focused on leiomyosarcoma, epithelial endometrial tumors and other preinvasive abnormalities are also common. These unexpected pathologic findings in women who underwent electric power morcellation highlight the importance of performing more rigorous evaluation of new methods of tissue extraction.
Quick Poll:
If you are using, plan to use, or anticipate the possibility of using power morcellation during minimally invasive gynecologic surgery, does your consent process include a separate form specific to power morcellation?
Please provide your answer to this question in the Quick Poll on the OBG Management home page, and then see how your peers have voted. Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
In the absence of a definitive FDA decision on the future of power morcellation in minimally invasive gynecologic surgery, many surgeons have stopped offering the option, often in response to constraints placed by their institutions, or have greatly expanded the informed consent discussion.
In Part 1 of this two-part roundtable discussion, which appeared in the September 2014 issue of OBG Management, our expert panelists discussed their current approach to tissue extraction during hysterectomy and myomectomy, as well as their preferred approach to both procedures amid this changing surgical environment. Here, in Part 2, they discuss patient counseling and the likely effects of FDA action.
How has your counseling changed?
OBG Management: Given recent concerns about the use of power morcellation, how has your counseling of the patient changed?
Kimberly Kho, MD, MPH: Though I look forward to the development of instruments and techniques that will make contained power morcellation safer, I am not using it currently and have been able to find minimally invasive alternatives such as minilaparotomy and vaginal removal of masses for the cases I would have considered for power morcellation.
Certainly, with power morcellation or any type of morcellation, it’s important to discuss the risks and benefits, as well as alternatives. Discussion should include the potential for:
- iatrogenic injury and tissue seeding of both benign and malignant tissue
- exacerbation of any occult malignancy and possible worsening of prognosis
- missing or mischaracterizing an occult malignancy.
Although there is no surefire way to avoid cellular dissemination with any type of surgery, I think it’s equally important to explain that, often, the only way to completely avoid fragmenting a large mass is to remove it en bloc, which would mean a large laparotomy for many patients. Women should understand the risks of laparotomy as well, including more frequent wound complications, longer hospitalization, and slower recovery.
Arnold P. Advincula, MD: If a clinician anticipates or plans the use of power morcellation, he or she certainly needs to go through an informed consent process with the patient. This process may include a separate form specific to power morcellation as well as detailed documentation during the preoperative visit.
OBG Management: What elements of the preoperative visit do you believe are important to document?
Dr. Advincula: It is important to clearly document the indications and alternatives for the surgery, as well as the decision-making process that led to the selection of a particular procedure and route of access. If any type of morcellation (power-driven or not) is anticipated, then the risks associated with it must be thoroughly discussed and documented in addition to the standard risks associated with any type of abdominal-pelvic surgery. No surgical procedure is without risks. Therefore, the process of informed consent cannot be taken lightly and is a critical part of the process that allows a patient to decide upon a particular intervention.
Jason D. Wright, MD: I believe the current role of power morcellation is limited. Patients considering the procedure should be counseled about the risks of cancer as well as other adverse pathologic abnormalities, including smooth muscle tumors of uncertain malignant potential, disseminated leiomyomatosis, and endometrial hyperplasia that may be associated with an occult cancer.
OBG Management: Do you recommend a separate consent form for power morcellation, as Dr. Advincula suggested?
Dr. Wright: Given the risk of adverse pathology, I think the role of electric power morcellation is limited. Patients should be carefully counseled about alternative surgical approaches that avoid tissue disruption and understand that the sensitivity of preoperative testing and intraoperative evaluation of smooth muscle neoplasms is limited. Further, patients considering contained morcellation also should be informed that the data examining the efficacy of these techniques are sparse.
Linda D. Bradley, MD: As I mentioned in Part 1 of our discussion, I’m giving patients new information about our concerns regarding occult malignancy, quoting the risk estimates given by the FDA this year.1 And the fact that we no longer use power morcellation at the Cleveland Clinic means that I no longer discuss it as an option, although one or two patients have asked for it in recent months.
I think many patients have read about it in the news or, once hysterectomy or myomectomy was planned, found discussion of the controversy surrounding it during their research. I’ve even had patients who underwent hysteroscopic myomectomy 2 or more years ago contacting me to find out whether power morcellation was used, and I have had to explain that hysteroscopic morcellation is different from the laparoscopic variant.
Patients are critical readers and are much more knowledgeable as a result of social media, so I do find myself spending more time discussing their procedure with them.
For myomectomy in particular, we send for a frozen section intraoperatively. Although that approach still is not 100% sensitive, it does guide what we do during surgery. If a sarcoma is found, for example, we call in the oncologists. I discuss that possibility with the patient as well. So I am spending more time with patients, but I don’t go into power morcellation because that is no longer an option for me.
OBG Management: Dr. Iglesia, has your counseling of patients changed in any way?
Cheryl Iglesia, MD: I do not routinely use power morcellation. However, the findings from the FDA and Dr. Wright about the higher risk of occult malignancy in fibroids is information I share with patients preoperatively.1,2
For women with fibroids who want uterine conservation procedures or who desire medical management, such as focused ultrasound or uterine fibroid embolization, MRI is routine. However, we make patients aware that this imaging modality is not 100% sensitive in detecting occult cancer—and neither are random biopsies of fibroids. Patients also need to be made aware that treatment with fibroid embolization or other medical options also could delay the detection of cancer and sarcoma. Any morcellation technique (power, hand, vaginal) does have the risk of potential cancer spread and upstaging, so morcellation should not be used in any women with suspected or known malignancy.
Effects of likely FDA actions
OBG Management: If the FDA decides to ban power morcellation outright, in some ways the approach to patient counseling will be simpler, as one option will have been permanently eliminated. But if the FDA allows power morcellation to continue, with stricter labeling, would that affect how you counsel patients? And would you reconsider power morcellation in that light?
Dr. Kho: I think the current discussion has highlighted again how important the informed consent process is as an opportunity for information sharing. It’s an ongoing discussion of risks, benefits, and alternatives. It also offers us an opportunity to understand the patient’s values and perspectives throughout the process of surgical planning. So, no, I don’t think the FDA’s actions will change how I counsel patients. Regardless of the FDA’s decisions, I think open power morcellation as we currently know it may be obviated as new instruments for contained morcellation—as well as other techniques we’ve discussed—become more popular. But it’s critical that we meaningfully monitor these techniques for long-term safety. In order to make evidence-based decisions, we will need good data.
Dr. Iglesia: I cannot comment on a final FDA decision. However, my feeling is that any information that patients can use to become educated about treatment alternatives—including the risks and benefits of each option—will help inform and improve the shared decision-making process.
Dr. Advincula: Regardless of the verdict rendered by the FDA, the way we approach tissue extraction in minimally invasive surgery has been changed forever. It is always important to take a critical look at the way things are done, but not at the expense of throwing the proverbial baby out with the bath water. If power morcellation were to remain a viable option, my counseling would remain as is, as it already has been modified and quite detailed in the wake of this whole controversy. I still believe there is a role for power morcellation, albeit modified from its current iteration, when applied by the right physician in a properly evaluated patient with the right indication.
Summing up
OBG Management: Do you have any additional comments about this issue?
Dr. Advincula: The ability to accurately and reliably detect an occult uterine malignancy—specifically, leiomyosarcoma—is lacking at present. Whether or not power morcellation remains a viable option in the future, the bottom line is that patients will still present with occult uterine malignancy. Minimizing the mishandling of this unfortunate diagnosis will depend on sound clinical judgment as well as improvements in diagnosis. It always will be important to avoid blaming the lack of sound clinical practice on surgical devices that, when used appropriately, have the potential to benefit the majority of women.
Dr. Kho: The current attention on power morcellators presents an opportunity to improve upon our current practices and find solutions to the issues we are encountering. I think this is an exciting time for examining preoperative risk stratification, the innovation of new techniques, repopularization and improvement of older ones such as vaginal tissue extraction, and, overall, to improve our system of safety monitoring and surgical device surveillance.
Dr. Iglesia: Intraperitoneal power morcellation should not be used in cases of malignancy or suspected malignancy or in postmenopausal patients with bleeding or growing fibroids. The availability of power morcellators may be limited as manufacturers
cease distribution, hospitals ban use, or insurers refuse payment for use.
Alternative minimally invasive approaches—especially the transvaginal approach—should be considered, since there are fewer complications associated with vaginal surgery, especially compared with open and laparoscopic surgery.
Dr. Wright: Although electric power morcellation may allow some women to undergo a minimally invasive procedure, the data currently available clearly suggest that adverse pathology is more common in women who undergo morcellation than was previously thought.
Although the debate around morcellation has focused on leiomyosarcoma, epithelial endometrial tumors and other preinvasive abnormalities are also common. These unexpected pathologic findings in women who underwent electric power morcellation highlight the importance of performing more rigorous evaluation of new methods of tissue extraction.
Quick Poll:
If you are using, plan to use, or anticipate the possibility of using power morcellation during minimally invasive gynecologic surgery, does your consent process include a separate form specific to power morcellation?
Please provide your answer to this question in the Quick Poll on the OBG Management home page, and then see how your peers have voted. Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication.http://www.fda.gov/medicaldevices/safety/alertsandnotices/ucm393576.htm. Published April 17, 2014. Accessed September 18, 2014.
2. Wright JD, Tergas AI, Burke WM, et al. Uterine pathology in women undergoing minimally invasive hysterectomy with morcellation [published online ahead of print July 22, 2014]. JAMA. doi:10.1001/jama.2014.9005.
1. US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication.http://www.fda.gov/medicaldevices/safety/alertsandnotices/ucm393576.htm. Published April 17, 2014. Accessed September 18, 2014.
2. Wright JD, Tergas AI, Burke WM, et al. Uterine pathology in women undergoing minimally invasive hysterectomy with morcellation [published online ahead of print July 22, 2014]. JAMA. doi:10.1001/jama.2014.9005.