User login
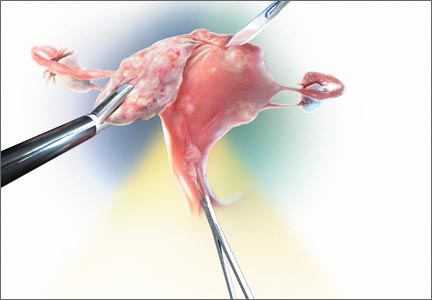
The world of minimally invasive gynecologic surgery has been transformed over the past 10 months—specifically in regard to the option of open power morcellation. From individual hospital bans of the procedure to an official warning from the US Food and Drug Administration (FDA)1 and the potential for further government action, the change has been swift and certain. Johnson & Johnson has recalled all power morcellators, many institutions now have bans in place, and one major insurer has announced its plan to discontinue coverage of power morcellation in three states.
What effect have these actions had on the availability of minimally invasive approaches to benign hysterectomy and myomectomy? And given new information on the risk of occult malignancy during these surgeries, how has patient selection and preoperative assessment changed? To address these and other questions, OBG Management convened a panel of experts in minimally invasive gynecology and asked them to share their perspective. In this case-based discussion, they offer their views on the morcellation controversy and their current approach to hysterectomy, myomectomy, and tissue extraction. Next month, in Part 2 of their discussion, they address patient counseling and FDA actions.
What is your preferred approach?
OBG Management: In light of the morcellation controversy, what is your preferred approach for benign hysterectomy?
Kimberly Kho, MD, MPH: Whenever possible and appropriate, vaginal hysterectomy is my preferred route. However, many surgical cases require evaluation of the abdominal cavity for pain, endometriosis, or a concerning adnexal mass. In such cases, and in cases involving a very large uterus, I prefer laparoscopic hysterectomy—either laparoscopic-assisted vaginal hysterectomy or total laparoscopic hysterectomy (TLH). I tend to perform TLH more frequently in these cases if the uterus lacks descent or the patient’s anatomy restricts vaginal access. Even in these cases, and with very large myomas and uteri, I have been successful removing the uterus vaginally, although this approach frequently involves vaginal morcellation with a scalpel.
Arnold P. Advincula, MD: My preferred approach for both benign hysterectomy and myomectomy is robot-assisted laparoscopy. I have used this approach over the past 13 years. In my hands, it is reproducible, safe, efficient, and cost-effective and affords me the ability to tackle a wide range of complex cases.
Cheryl Iglesia, MD: Like Dr. Kho, I prefer vaginal hysterectomy.
Jason D. Wright, MD: I also prefer the vaginal approach. In fact, I believe it should be the preferred approach for hysterectomy for benign gynecologic disease whenever it is feasible. And the laparoscopic and robot-assisted approaches carry less perioperative morbidity than abdominal hysterectomy.
Given the recent concerns about open power morcellation, I prefer to perform either vaginal hysterectomy or minimally invasive hysterectomy without morcellation. If neither approach is feasible, given anatomic considerations, I counsel the patient about the risks and benefits of abdominal hysterectomy, compared with minimally invasive hysterectomy with morcellation.
Linda D. Bradley, MD: For women who meet minimally invasive surgical criteria, I prefer the laparoscopic approach because of its many benefits, including a shorter hospital stay (which reduces the risk of hospital-acquired infection and iatrogenic complications of hospitalization), lower risk of incisional infection, lower requirement for pain medications, and faster return to work.
OBG Management: What about myomectomy? Would your approach be different?
Dr. Bradley: Many myomectomy cases can be done hysteroscopically. I would like to point out, however, that when we talk about hysteroscopy, the morcellation issue is moot. Although there are hysteroscopic surgical devices that have used the word “morcellator” in their names, hysteroscopic morcellation is performed within a closed system—the uterine cavity—and so carries none of the risks of laparoscopic morcellation.
I prefer to perform nonhysteroscopic cases using a laparoscopic approach, creating a small mini-laparotomy to remove the fibroid intact or using a knife to morcellate the tissue outside of the peritoneal cavity.
Dr. Kho: I use a similar laparoscopic approach for myomectomy, using laparoscopy to assess the uterus and fibroids, enucleate the fibroid and remove it from the uterus, and then creating a mini-laparotomy incision
3 cm to 4 cm in length to manually remove or morcellate the fibroid and reapproximate the myometrium.
Dr. Iglesia: I rarely perform myomectomy but would likely do it laparoscopically or robotically to achieve minimally invasive benefits such as fewer adhesions and less postoperative pain.
How do you manage tissue extraction?
OBG Management: What methods of tissue extraction do you currently use during hysterectomy and myomectomy?
Dr. Advincula: I currently utilize a contained, extracorporeal, transumbilical, manual scalpel-morcellation technique for all myomectomy cases, as well as hysterectomy cases not amenable to transvaginal extraction.
Dr. Iglesia: I rely on vaginal removal of tissue and vaginal morcellation.
Dr. Kho: I infrequently perform supracervical hysterectomy, so almost all the hysterectomies I do are total hysterectomies. I remove the uterus through the vagina. In addition, because the size of the specimen frequently is too large to remove through a colpotomy intact, I morcellate the uterus manually with a scalpel using coring, wedge resection, and myomectomy. I find this to be an efficient and controlled method for tissue removal, with minimal tissue scattering. I also have begun to perform the same type of vaginal morcellation with the specimen enclosed in a bag.
That being said, the spread of occult malignancy has been reported after all types of morcellation—not just with power morcellation but also with vaginal and abdominal morcellation. So we are increasingly performing tissue extraction in an enclosed fashion using manual morcellation in a containment bag through a mini-laparotomy or posterior colpotomy to minimize the risk of leaving tissue fragments behind.
Dr. Wright: Although different methods of tissue extraction, including morcellation within a bag, are commonly discussed, data documenting the safety of these methods are extremely limited and patients should be counseled accordingly.
Similarly, the risk of adverse pathology increases substantially with age, and morcellation should be considered with great caution—if at all—in older women.
Given the risks associated with power morcellation, I try to avoid uterine disruption at the time of hysterectomy and perform either vaginal or minimally invasive total hysterectomy. In older women, because of the higher risk of underlying pathology, I prefer laparotomy if anatomic considerations preclude a vaginal or minimally invasive total hysterectomy. Younger women can be counseled about the risks and benefits of various routes of extraction. Patients with any suspicious findings during preoperative evaluation or surgery itself should have their uterus removed without disruption or fragmentation.
In regard to myomectomy specifically, a significant portion of the data we have on the risks of power morcellation derives from studies of hysterectomy. There are minimal data describing the risk of occult pathology at the time of minimally invasive myomectomy. Although younger patients likely are at relatively low risk for occult malignancy, they should be counseled that population-based estimates of cancer at the time of myomectomy are lacking.
Dr. Bradley: Since the controversy over morcellation arose, the Cleveland Clinic not only has banned the procedure but also removed all morcellators from its shelves, and it is unclear whether the option will be revisited after the FDA renders its final verdict. So my approach to tissue extraction is either vaginal morcellation or using a mini-laparotomy to remove the whole specimen intact or put it in a bag and morcellate it with a knife.
Case 1: A premenopausal patient scheduled for myomectomy
OBG Management: Let’s move on to a specific case. Let’s say the patient is a 35-year-old woman with a large fibroid, to be removed by myomectomy. How would you quantify her risk of occult malignancy? And what would preoperative assessment entail?
Dr. Iglesia: This patient’s risk of occult malignancy is low. I would obtain pelvic ultrasonography and endometrial biopsy, with cervical cytology included. Preoperative magnetic resonance imaging (MRI) would be indicated if there is a possibility that power morcellation will be performed. If power morcellation were selected, I would perform it using a bag.
Dr. Bradley: At the Cleveland Clinic, we now utilize the FDA risk estimates for occult malignancy of 1 in 300 to 1 in 350 women,1 and I counsel patients using these figures. In the past several years, we have begun to use MRI with and without contrast to determine the size, number, and location of the fibroids, to determine our surgical approach, and to guide our discussion with the patient of what we will be able to do—for example, laparoscopy versus laparotomy.
OBG Management: Would the FDA figures you give be applicable to a young woman such as this 35-year-old?
Dr. Bradley: We’re using those figures with all of our premenopausal patients.
OBG Management: And does the MRI pick up sarcomas?
Dr. Bradley: No imaging is 100% sensitive in detecting sarcoma. We do MRI, and if the fibroid has any areas of necrosis, irregularity, or poor tissue planes that would arouse our suspicion of adenomyoma or sarcoma, we perform the myomectomy via laparotomy. But as I mentioned earlier, we don’t use power morcellation at all anymore—so this patient you describe would likely undergo laparoscopic removal using a bag and a knife to extract it to the skin level.
Although every patient is different, in general, if we have a patient with a single large fibroid 10 cm or less in size, we try to remove it laparoscopically or with robot assistance rather than via laparotomy. We also perform endometrial biopsy.
Dr. Advincula: First, it’s important to define prevalence and incidence when discussing risks. Prevalence would be the number of patients with a leiomyosarcoma per 100,000 women, whereas incidence is the number of patients given a diagnosis of leiomyosarcoma within a year per 100,000 women. In this case, a 35-year-old woman would have a prevalence of leiomyosarcoma, in the general population, of 3 to 7 per 100,000 women and an incidence of less than 1%.
My preoperative assessment would involve MRI of the pelvis with T2 weighted images to better characterize her uterus. Although there has been much discussion lately about the use of lactate dehydrogenase (LDH) isoenzyme panels in combination with MRI to detect occult leiomyosarcoma, the reliability and reproducibility of that combined approach are not fully vetted and, as yet, are not a standard part of my workup. Endometrial sampling would certainly be warranted with any associated history of abnormal uterine bleeding.
Dr. Wright: As I mentioned earlier, most data on power morcellation have been derived from studies of women undergoing hysterectomy. To date, accurate estimates to predict the risk of occult cancer in this patient planning to undergo myomectomy are largely lacking. For women undergoing hysterectomy using power morcellation, advanced age is the strongest risk factor for occult malignancy. Although this patient’s risk of cancer likely is relatively low, she should be counseled that precise estimates are lacking.
Preoperatively, she should undergo endometrial sampling if she has abnormal bleeding. However, the reliability of endometrial sampling, as well as imaging, is limited in the detection of uterine sarcomas.
Case 2: Perimenopausal patient undergoing hysterectomy
OBG Management: How would your approach to preoperative assessment change if this patient were a 47-year-old perimenopausal woman with a single large fibroid to be removed by hysterectomy?
Dr. Bradley: It would be the same preoperative assessment—an MRI and an endometrial biopsy.
Dr. Iglesia: The risk of occult malignancy would be greater than with the first patient. Again, I would use pelvic ultrasound, endometrial biopsy, and cervical cytology to assess her, and I would perform vaginal or TLH. MRI would be indicated if there is a possibility of performing intraperitoneal morcellation. I would prefer doing any morcellation in a bag or via laparotomy.
Dr. Wright: Based on age alone, this perimenopausal patient’s risk for an underlying cancer is 0.2%.2 If the patient has any abnormal bleeding, she should undergo endometrial sampling preoperatively. The diagnostic modalities currently available—which include endometrial sampling as well as imaging, even MRI—are unreliable in the diagnosis of uterine sarcomas, and the patient should be counseled accordingly if she is considering power morcellation.
If it is technically feasible, vaginal hysterectomy or a minimally invasive hysterectomy without power morcellation are preferred. If neither modality is feasible and the patient is considering power morcellation, she should be carefully counseled about the underlying risk not only of uterine cancer but also of other adverse pathologic abnormalities.
Case 3: Postmenopausal woman scheduled for hysterectomy
OBG Management: Let’s change the details of the case again. This time she’s 55 years old and postmenopausal. She, too, has a large fibroid to be removed via hysterectomy. What is her risk of occult cancer? How would you assess her preoperatively?
Dr. Bradley: My approach would be the same as in the first two cases. However, because this patient is menopausal, morcellation would be off the table. (And it already is off the table—for any patient—at the Cleveland Clinic.) I usually prefer open hysterectomy for these patients, which is very different from what we were doing 1 year ago.
I want to expand on Dr. Wright’s comments about other pathologic abnormalities. As a woman ages, her cancer risk becomes greatest for malignancy of the endometrium rather than cancer in a fibroid. If this were my 55-year-old patient, and I had been seeing her for 20 years, and her fibroids had remained the same size but she was now having bleeding, I’d be more concerned about an endometrial problem—hyperplasia, a polyp, or cancer.
If the patient were having bulk symptoms, new pain, and imaging that shows, over 10 years between perimenopause and postmenopause, that there has been growth of the fibroids, I would be concerned about a sarcoma.
Some women who present with postmenopausal bleeding have ovarian cancer, and some studies show that a significant percentage of women with ovarian cancer present with bleeding as a primary symptom.3 So in a postmenopausal patient, I really want to know about the health of the ovaries. Are they enlarged on imaging?
There is also a bimodal distribution of human papillomavirus (HPV) infection and cervical cancer, with peaks of infection at ages 26 to 30 years and again at 46 to 50 years in some populations. The second age peak is followed by an increase in cervical intraepithelial neoplasia (CIN) 2 and 3 and invasive cervical cancer 20 years later.4 So I also want to consider the possibility of cervical cancer in this population.
I tell my patients that pretty much everybody has fibroids. Because they are so common, I need to look at the whole picture.
Dr. Wright: For the perimenopausal and postmenopausal patients we are discussing, preoperative evaluation and counseling would be similar to that for the premenopausal woman. However, given recent data, it is important to note that the prevalence ratio for a uterine malignancy increases with increasing age.2 Clinicians need to be mindful of red flags in perimenopausal and postmenopausal women.
Dr. Kho: I agree that, for each of the cases we have discussed, we need to consider more than just the presence of the fibroid. Risk stratification based on clinical factors like age, menopausal status, BMI, the indication for surgery, and response to any other therapy is extremely important and should guide decision-making regarding the surgical approach.
Although we lack reliable methods to differentiate fibroids from leiomyosarcomas, there are other malignant conditions of the uterus, as Dr. Bradley pointed out. Premalignant conditions of the uterus and cervix and endometrial adenocarcinomas occur far more frequently in the population than sarcomas, and we may be increasing risks by morcellating unsuspected cancers of any type.
At this time, whenever I am considering morcellation in any patient, I obtain pelvic imaging, endometrial biopsy, and current cervical cancer screening. If any of these studies suggest a malignant or premalignant condition, I avoid morcellation. Similarly, if a patient’s clinical history raises suspicion of a potential underlying malignant process, such as new symptoms of an enlarging myoma in a postmenopausal woman, I will try to find an alternative to morcellation.
Why we need a national surgery registry
The controversy surrounding open power morcellation was precipitated by the reporting of a single case—that of a prominent physician who had an unsuspected cancer morcellated during the course of a hysterectomy and was later upgraded to Stage 4 leiomyosarcoma as a result. But it wasn’t a gynecologic surgeon who reported the case—it was the patient herself. And we all know she was by no means the first case of an inadvertently morcellated sarcoma.
I would wager that few physicians are well versed in how to contact the FDA’s Manufacturer and User Facility Device Experience (MAUDE) database. And not many are familiar with the other specialized registries in the United States, let alone know how to report to them. Another important but often unaddressed issue: Is it hassle-free to make a report? The answer: A resounding “No.”
Who do we inform about our experiences—successful or not—in the operating room? And who pays for data collection? At present, the system is piecemeal or scattershot at best.
Sorely needed is a system for reporting that is easy to use, broad, and deep. A nationally funded system would be best. Otherwise, who is going to maintain the database? Who will filter the data? Who will ensure that the information that is entered is correct so that outcomes can be followed accurately?
For those of us employed by a hospital or other institution, it tends to be the institution itself that gathers the data—when it is gathered. But what about surgeons in private practice? How do they monitor themselves? And what about privately owned outpatient surgery centers, where patients sojourn no longer than 23 hours? Is it reasonable to expect them to add the burden and expense of data collection without a national mandate?
I know firsthand some of the skewed information that results when reporting is piecemeal or manipulated. When I was a resident, for example, in some localities, it was not uncommon for sexually transmitted diseases to go unreported when the patient had private insurance. The result: Only those in the lower socioeconomic ranks appeared to experience this problem.
Clearly, we need national standards and a national protocol. And to achieve that we need leaders strong enough to argue that the expense represents dollars well spent.
- Linda D. Bradley, MD
Quick Poll:
If you are using, plan to use, or anticipate the possibility of using power morcellation during minimally invasive gynecologic surgery, does your consent process include a separate form specific to power morcellation?
Please provide your answer to this question in the Quick Poll on the OBG Management home page and then see how your peers have voted.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. April 17, 2014. http://www.fda.gov/medicaldevices/safety/alertsandnotices/ucm393576.htm. Accessed August 18, 2014.
2. Wright JD, Tergas AI, Burke WM, et al. Uterine pathology in women undergoing minimally invasive hysterectomy with morcellation [published online ahead of print July 22, 2014]. JAMA. doi: 10.1001/jama.2014.9005.
3. Lurie G, Thompson PJ, McDuffie KE, et al. Prediagnostic symptoms of ovarian carcinoma: a case-control study. Gynecol Oncol. 2009;114(2):231–236.
4. Chan PK, Chang AR, Yu MY, et al. Age distribution of human papillomavirus infection and cervical neoplasia reflects caveats of cervical screening policies. Int J Cancer. 2010;126(1):297–301.
The world of minimally invasive gynecologic surgery has been transformed over the past 10 months—specifically in regard to the option of open power morcellation. From individual hospital bans of the procedure to an official warning from the US Food and Drug Administration (FDA)1 and the potential for further government action, the change has been swift and certain. Johnson & Johnson has recalled all power morcellators, many institutions now have bans in place, and one major insurer has announced its plan to discontinue coverage of power morcellation in three states.
What effect have these actions had on the availability of minimally invasive approaches to benign hysterectomy and myomectomy? And given new information on the risk of occult malignancy during these surgeries, how has patient selection and preoperative assessment changed? To address these and other questions, OBG Management convened a panel of experts in minimally invasive gynecology and asked them to share their perspective. In this case-based discussion, they offer their views on the morcellation controversy and their current approach to hysterectomy, myomectomy, and tissue extraction. Next month, in Part 2 of their discussion, they address patient counseling and FDA actions.
What is your preferred approach?
OBG Management: In light of the morcellation controversy, what is your preferred approach for benign hysterectomy?
Kimberly Kho, MD, MPH: Whenever possible and appropriate, vaginal hysterectomy is my preferred route. However, many surgical cases require evaluation of the abdominal cavity for pain, endometriosis, or a concerning adnexal mass. In such cases, and in cases involving a very large uterus, I prefer laparoscopic hysterectomy—either laparoscopic-assisted vaginal hysterectomy or total laparoscopic hysterectomy (TLH). I tend to perform TLH more frequently in these cases if the uterus lacks descent or the patient’s anatomy restricts vaginal access. Even in these cases, and with very large myomas and uteri, I have been successful removing the uterus vaginally, although this approach frequently involves vaginal morcellation with a scalpel.
Arnold P. Advincula, MD: My preferred approach for both benign hysterectomy and myomectomy is robot-assisted laparoscopy. I have used this approach over the past 13 years. In my hands, it is reproducible, safe, efficient, and cost-effective and affords me the ability to tackle a wide range of complex cases.
Cheryl Iglesia, MD: Like Dr. Kho, I prefer vaginal hysterectomy.
Jason D. Wright, MD: I also prefer the vaginal approach. In fact, I believe it should be the preferred approach for hysterectomy for benign gynecologic disease whenever it is feasible. And the laparoscopic and robot-assisted approaches carry less perioperative morbidity than abdominal hysterectomy.
Given the recent concerns about open power morcellation, I prefer to perform either vaginal hysterectomy or minimally invasive hysterectomy without morcellation. If neither approach is feasible, given anatomic considerations, I counsel the patient about the risks and benefits of abdominal hysterectomy, compared with minimally invasive hysterectomy with morcellation.
Linda D. Bradley, MD: For women who meet minimally invasive surgical criteria, I prefer the laparoscopic approach because of its many benefits, including a shorter hospital stay (which reduces the risk of hospital-acquired infection and iatrogenic complications of hospitalization), lower risk of incisional infection, lower requirement for pain medications, and faster return to work.
OBG Management: What about myomectomy? Would your approach be different?
Dr. Bradley: Many myomectomy cases can be done hysteroscopically. I would like to point out, however, that when we talk about hysteroscopy, the morcellation issue is moot. Although there are hysteroscopic surgical devices that have used the word “morcellator” in their names, hysteroscopic morcellation is performed within a closed system—the uterine cavity—and so carries none of the risks of laparoscopic morcellation.
I prefer to perform nonhysteroscopic cases using a laparoscopic approach, creating a small mini-laparotomy to remove the fibroid intact or using a knife to morcellate the tissue outside of the peritoneal cavity.
Dr. Kho: I use a similar laparoscopic approach for myomectomy, using laparoscopy to assess the uterus and fibroids, enucleate the fibroid and remove it from the uterus, and then creating a mini-laparotomy incision
3 cm to 4 cm in length to manually remove or morcellate the fibroid and reapproximate the myometrium.
Dr. Iglesia: I rarely perform myomectomy but would likely do it laparoscopically or robotically to achieve minimally invasive benefits such as fewer adhesions and less postoperative pain.
How do you manage tissue extraction?
OBG Management: What methods of tissue extraction do you currently use during hysterectomy and myomectomy?
Dr. Advincula: I currently utilize a contained, extracorporeal, transumbilical, manual scalpel-morcellation technique for all myomectomy cases, as well as hysterectomy cases not amenable to transvaginal extraction.
Dr. Iglesia: I rely on vaginal removal of tissue and vaginal morcellation.
Dr. Kho: I infrequently perform supracervical hysterectomy, so almost all the hysterectomies I do are total hysterectomies. I remove the uterus through the vagina. In addition, because the size of the specimen frequently is too large to remove through a colpotomy intact, I morcellate the uterus manually with a scalpel using coring, wedge resection, and myomectomy. I find this to be an efficient and controlled method for tissue removal, with minimal tissue scattering. I also have begun to perform the same type of vaginal morcellation with the specimen enclosed in a bag.
That being said, the spread of occult malignancy has been reported after all types of morcellation—not just with power morcellation but also with vaginal and abdominal morcellation. So we are increasingly performing tissue extraction in an enclosed fashion using manual morcellation in a containment bag through a mini-laparotomy or posterior colpotomy to minimize the risk of leaving tissue fragments behind.
Dr. Wright: Although different methods of tissue extraction, including morcellation within a bag, are commonly discussed, data documenting the safety of these methods are extremely limited and patients should be counseled accordingly.
Similarly, the risk of adverse pathology increases substantially with age, and morcellation should be considered with great caution—if at all—in older women.
Given the risks associated with power morcellation, I try to avoid uterine disruption at the time of hysterectomy and perform either vaginal or minimally invasive total hysterectomy. In older women, because of the higher risk of underlying pathology, I prefer laparotomy if anatomic considerations preclude a vaginal or minimally invasive total hysterectomy. Younger women can be counseled about the risks and benefits of various routes of extraction. Patients with any suspicious findings during preoperative evaluation or surgery itself should have their uterus removed without disruption or fragmentation.
In regard to myomectomy specifically, a significant portion of the data we have on the risks of power morcellation derives from studies of hysterectomy. There are minimal data describing the risk of occult pathology at the time of minimally invasive myomectomy. Although younger patients likely are at relatively low risk for occult malignancy, they should be counseled that population-based estimates of cancer at the time of myomectomy are lacking.
Dr. Bradley: Since the controversy over morcellation arose, the Cleveland Clinic not only has banned the procedure but also removed all morcellators from its shelves, and it is unclear whether the option will be revisited after the FDA renders its final verdict. So my approach to tissue extraction is either vaginal morcellation or using a mini-laparotomy to remove the whole specimen intact or put it in a bag and morcellate it with a knife.
Case 1: A premenopausal patient scheduled for myomectomy
OBG Management: Let’s move on to a specific case. Let’s say the patient is a 35-year-old woman with a large fibroid, to be removed by myomectomy. How would you quantify her risk of occult malignancy? And what would preoperative assessment entail?
Dr. Iglesia: This patient’s risk of occult malignancy is low. I would obtain pelvic ultrasonography and endometrial biopsy, with cervical cytology included. Preoperative magnetic resonance imaging (MRI) would be indicated if there is a possibility that power morcellation will be performed. If power morcellation were selected, I would perform it using a bag.
Dr. Bradley: At the Cleveland Clinic, we now utilize the FDA risk estimates for occult malignancy of 1 in 300 to 1 in 350 women,1 and I counsel patients using these figures. In the past several years, we have begun to use MRI with and without contrast to determine the size, number, and location of the fibroids, to determine our surgical approach, and to guide our discussion with the patient of what we will be able to do—for example, laparoscopy versus laparotomy.
OBG Management: Would the FDA figures you give be applicable to a young woman such as this 35-year-old?
Dr. Bradley: We’re using those figures with all of our premenopausal patients.
OBG Management: And does the MRI pick up sarcomas?
Dr. Bradley: No imaging is 100% sensitive in detecting sarcoma. We do MRI, and if the fibroid has any areas of necrosis, irregularity, or poor tissue planes that would arouse our suspicion of adenomyoma or sarcoma, we perform the myomectomy via laparotomy. But as I mentioned earlier, we don’t use power morcellation at all anymore—so this patient you describe would likely undergo laparoscopic removal using a bag and a knife to extract it to the skin level.
Although every patient is different, in general, if we have a patient with a single large fibroid 10 cm or less in size, we try to remove it laparoscopically or with robot assistance rather than via laparotomy. We also perform endometrial biopsy.
Dr. Advincula: First, it’s important to define prevalence and incidence when discussing risks. Prevalence would be the number of patients with a leiomyosarcoma per 100,000 women, whereas incidence is the number of patients given a diagnosis of leiomyosarcoma within a year per 100,000 women. In this case, a 35-year-old woman would have a prevalence of leiomyosarcoma, in the general population, of 3 to 7 per 100,000 women and an incidence of less than 1%.
My preoperative assessment would involve MRI of the pelvis with T2 weighted images to better characterize her uterus. Although there has been much discussion lately about the use of lactate dehydrogenase (LDH) isoenzyme panels in combination with MRI to detect occult leiomyosarcoma, the reliability and reproducibility of that combined approach are not fully vetted and, as yet, are not a standard part of my workup. Endometrial sampling would certainly be warranted with any associated history of abnormal uterine bleeding.
Dr. Wright: As I mentioned earlier, most data on power morcellation have been derived from studies of women undergoing hysterectomy. To date, accurate estimates to predict the risk of occult cancer in this patient planning to undergo myomectomy are largely lacking. For women undergoing hysterectomy using power morcellation, advanced age is the strongest risk factor for occult malignancy. Although this patient’s risk of cancer likely is relatively low, she should be counseled that precise estimates are lacking.
Preoperatively, she should undergo endometrial sampling if she has abnormal bleeding. However, the reliability of endometrial sampling, as well as imaging, is limited in the detection of uterine sarcomas.
Case 2: Perimenopausal patient undergoing hysterectomy
OBG Management: How would your approach to preoperative assessment change if this patient were a 47-year-old perimenopausal woman with a single large fibroid to be removed by hysterectomy?
Dr. Bradley: It would be the same preoperative assessment—an MRI and an endometrial biopsy.
Dr. Iglesia: The risk of occult malignancy would be greater than with the first patient. Again, I would use pelvic ultrasound, endometrial biopsy, and cervical cytology to assess her, and I would perform vaginal or TLH. MRI would be indicated if there is a possibility of performing intraperitoneal morcellation. I would prefer doing any morcellation in a bag or via laparotomy.
Dr. Wright: Based on age alone, this perimenopausal patient’s risk for an underlying cancer is 0.2%.2 If the patient has any abnormal bleeding, she should undergo endometrial sampling preoperatively. The diagnostic modalities currently available—which include endometrial sampling as well as imaging, even MRI—are unreliable in the diagnosis of uterine sarcomas, and the patient should be counseled accordingly if she is considering power morcellation.
If it is technically feasible, vaginal hysterectomy or a minimally invasive hysterectomy without power morcellation are preferred. If neither modality is feasible and the patient is considering power morcellation, she should be carefully counseled about the underlying risk not only of uterine cancer but also of other adverse pathologic abnormalities.
Case 3: Postmenopausal woman scheduled for hysterectomy
OBG Management: Let’s change the details of the case again. This time she’s 55 years old and postmenopausal. She, too, has a large fibroid to be removed via hysterectomy. What is her risk of occult cancer? How would you assess her preoperatively?
Dr. Bradley: My approach would be the same as in the first two cases. However, because this patient is menopausal, morcellation would be off the table. (And it already is off the table—for any patient—at the Cleveland Clinic.) I usually prefer open hysterectomy for these patients, which is very different from what we were doing 1 year ago.
I want to expand on Dr. Wright’s comments about other pathologic abnormalities. As a woman ages, her cancer risk becomes greatest for malignancy of the endometrium rather than cancer in a fibroid. If this were my 55-year-old patient, and I had been seeing her for 20 years, and her fibroids had remained the same size but she was now having bleeding, I’d be more concerned about an endometrial problem—hyperplasia, a polyp, or cancer.
If the patient were having bulk symptoms, new pain, and imaging that shows, over 10 years between perimenopause and postmenopause, that there has been growth of the fibroids, I would be concerned about a sarcoma.
Some women who present with postmenopausal bleeding have ovarian cancer, and some studies show that a significant percentage of women with ovarian cancer present with bleeding as a primary symptom.3 So in a postmenopausal patient, I really want to know about the health of the ovaries. Are they enlarged on imaging?
There is also a bimodal distribution of human papillomavirus (HPV) infection and cervical cancer, with peaks of infection at ages 26 to 30 years and again at 46 to 50 years in some populations. The second age peak is followed by an increase in cervical intraepithelial neoplasia (CIN) 2 and 3 and invasive cervical cancer 20 years later.4 So I also want to consider the possibility of cervical cancer in this population.
I tell my patients that pretty much everybody has fibroids. Because they are so common, I need to look at the whole picture.
Dr. Wright: For the perimenopausal and postmenopausal patients we are discussing, preoperative evaluation and counseling would be similar to that for the premenopausal woman. However, given recent data, it is important to note that the prevalence ratio for a uterine malignancy increases with increasing age.2 Clinicians need to be mindful of red flags in perimenopausal and postmenopausal women.
Dr. Kho: I agree that, for each of the cases we have discussed, we need to consider more than just the presence of the fibroid. Risk stratification based on clinical factors like age, menopausal status, BMI, the indication for surgery, and response to any other therapy is extremely important and should guide decision-making regarding the surgical approach.
Although we lack reliable methods to differentiate fibroids from leiomyosarcomas, there are other malignant conditions of the uterus, as Dr. Bradley pointed out. Premalignant conditions of the uterus and cervix and endometrial adenocarcinomas occur far more frequently in the population than sarcomas, and we may be increasing risks by morcellating unsuspected cancers of any type.
At this time, whenever I am considering morcellation in any patient, I obtain pelvic imaging, endometrial biopsy, and current cervical cancer screening. If any of these studies suggest a malignant or premalignant condition, I avoid morcellation. Similarly, if a patient’s clinical history raises suspicion of a potential underlying malignant process, such as new symptoms of an enlarging myoma in a postmenopausal woman, I will try to find an alternative to morcellation.
Why we need a national surgery registry
The controversy surrounding open power morcellation was precipitated by the reporting of a single case—that of a prominent physician who had an unsuspected cancer morcellated during the course of a hysterectomy and was later upgraded to Stage 4 leiomyosarcoma as a result. But it wasn’t a gynecologic surgeon who reported the case—it was the patient herself. And we all know she was by no means the first case of an inadvertently morcellated sarcoma.
I would wager that few physicians are well versed in how to contact the FDA’s Manufacturer and User Facility Device Experience (MAUDE) database. And not many are familiar with the other specialized registries in the United States, let alone know how to report to them. Another important but often unaddressed issue: Is it hassle-free to make a report? The answer: A resounding “No.”
Who do we inform about our experiences—successful or not—in the operating room? And who pays for data collection? At present, the system is piecemeal or scattershot at best.
Sorely needed is a system for reporting that is easy to use, broad, and deep. A nationally funded system would be best. Otherwise, who is going to maintain the database? Who will filter the data? Who will ensure that the information that is entered is correct so that outcomes can be followed accurately?
For those of us employed by a hospital or other institution, it tends to be the institution itself that gathers the data—when it is gathered. But what about surgeons in private practice? How do they monitor themselves? And what about privately owned outpatient surgery centers, where patients sojourn no longer than 23 hours? Is it reasonable to expect them to add the burden and expense of data collection without a national mandate?
I know firsthand some of the skewed information that results when reporting is piecemeal or manipulated. When I was a resident, for example, in some localities, it was not uncommon for sexually transmitted diseases to go unreported when the patient had private insurance. The result: Only those in the lower socioeconomic ranks appeared to experience this problem.
Clearly, we need national standards and a national protocol. And to achieve that we need leaders strong enough to argue that the expense represents dollars well spent.
- Linda D. Bradley, MD
Quick Poll:
If you are using, plan to use, or anticipate the possibility of using power morcellation during minimally invasive gynecologic surgery, does your consent process include a separate form specific to power morcellation?
Please provide your answer to this question in the Quick Poll on the OBG Management home page and then see how your peers have voted.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
The world of minimally invasive gynecologic surgery has been transformed over the past 10 months—specifically in regard to the option of open power morcellation. From individual hospital bans of the procedure to an official warning from the US Food and Drug Administration (FDA)1 and the potential for further government action, the change has been swift and certain. Johnson & Johnson has recalled all power morcellators, many institutions now have bans in place, and one major insurer has announced its plan to discontinue coverage of power morcellation in three states.
What effect have these actions had on the availability of minimally invasive approaches to benign hysterectomy and myomectomy? And given new information on the risk of occult malignancy during these surgeries, how has patient selection and preoperative assessment changed? To address these and other questions, OBG Management convened a panel of experts in minimally invasive gynecology and asked them to share their perspective. In this case-based discussion, they offer their views on the morcellation controversy and their current approach to hysterectomy, myomectomy, and tissue extraction. Next month, in Part 2 of their discussion, they address patient counseling and FDA actions.
What is your preferred approach?
OBG Management: In light of the morcellation controversy, what is your preferred approach for benign hysterectomy?
Kimberly Kho, MD, MPH: Whenever possible and appropriate, vaginal hysterectomy is my preferred route. However, many surgical cases require evaluation of the abdominal cavity for pain, endometriosis, or a concerning adnexal mass. In such cases, and in cases involving a very large uterus, I prefer laparoscopic hysterectomy—either laparoscopic-assisted vaginal hysterectomy or total laparoscopic hysterectomy (TLH). I tend to perform TLH more frequently in these cases if the uterus lacks descent or the patient’s anatomy restricts vaginal access. Even in these cases, and with very large myomas and uteri, I have been successful removing the uterus vaginally, although this approach frequently involves vaginal morcellation with a scalpel.
Arnold P. Advincula, MD: My preferred approach for both benign hysterectomy and myomectomy is robot-assisted laparoscopy. I have used this approach over the past 13 years. In my hands, it is reproducible, safe, efficient, and cost-effective and affords me the ability to tackle a wide range of complex cases.
Cheryl Iglesia, MD: Like Dr. Kho, I prefer vaginal hysterectomy.
Jason D. Wright, MD: I also prefer the vaginal approach. In fact, I believe it should be the preferred approach for hysterectomy for benign gynecologic disease whenever it is feasible. And the laparoscopic and robot-assisted approaches carry less perioperative morbidity than abdominal hysterectomy.
Given the recent concerns about open power morcellation, I prefer to perform either vaginal hysterectomy or minimally invasive hysterectomy without morcellation. If neither approach is feasible, given anatomic considerations, I counsel the patient about the risks and benefits of abdominal hysterectomy, compared with minimally invasive hysterectomy with morcellation.
Linda D. Bradley, MD: For women who meet minimally invasive surgical criteria, I prefer the laparoscopic approach because of its many benefits, including a shorter hospital stay (which reduces the risk of hospital-acquired infection and iatrogenic complications of hospitalization), lower risk of incisional infection, lower requirement for pain medications, and faster return to work.
OBG Management: What about myomectomy? Would your approach be different?
Dr. Bradley: Many myomectomy cases can be done hysteroscopically. I would like to point out, however, that when we talk about hysteroscopy, the morcellation issue is moot. Although there are hysteroscopic surgical devices that have used the word “morcellator” in their names, hysteroscopic morcellation is performed within a closed system—the uterine cavity—and so carries none of the risks of laparoscopic morcellation.
I prefer to perform nonhysteroscopic cases using a laparoscopic approach, creating a small mini-laparotomy to remove the fibroid intact or using a knife to morcellate the tissue outside of the peritoneal cavity.
Dr. Kho: I use a similar laparoscopic approach for myomectomy, using laparoscopy to assess the uterus and fibroids, enucleate the fibroid and remove it from the uterus, and then creating a mini-laparotomy incision
3 cm to 4 cm in length to manually remove or morcellate the fibroid and reapproximate the myometrium.
Dr. Iglesia: I rarely perform myomectomy but would likely do it laparoscopically or robotically to achieve minimally invasive benefits such as fewer adhesions and less postoperative pain.
How do you manage tissue extraction?
OBG Management: What methods of tissue extraction do you currently use during hysterectomy and myomectomy?
Dr. Advincula: I currently utilize a contained, extracorporeal, transumbilical, manual scalpel-morcellation technique for all myomectomy cases, as well as hysterectomy cases not amenable to transvaginal extraction.
Dr. Iglesia: I rely on vaginal removal of tissue and vaginal morcellation.
Dr. Kho: I infrequently perform supracervical hysterectomy, so almost all the hysterectomies I do are total hysterectomies. I remove the uterus through the vagina. In addition, because the size of the specimen frequently is too large to remove through a colpotomy intact, I morcellate the uterus manually with a scalpel using coring, wedge resection, and myomectomy. I find this to be an efficient and controlled method for tissue removal, with minimal tissue scattering. I also have begun to perform the same type of vaginal morcellation with the specimen enclosed in a bag.
That being said, the spread of occult malignancy has been reported after all types of morcellation—not just with power morcellation but also with vaginal and abdominal morcellation. So we are increasingly performing tissue extraction in an enclosed fashion using manual morcellation in a containment bag through a mini-laparotomy or posterior colpotomy to minimize the risk of leaving tissue fragments behind.
Dr. Wright: Although different methods of tissue extraction, including morcellation within a bag, are commonly discussed, data documenting the safety of these methods are extremely limited and patients should be counseled accordingly.
Similarly, the risk of adverse pathology increases substantially with age, and morcellation should be considered with great caution—if at all—in older women.
Given the risks associated with power morcellation, I try to avoid uterine disruption at the time of hysterectomy and perform either vaginal or minimally invasive total hysterectomy. In older women, because of the higher risk of underlying pathology, I prefer laparotomy if anatomic considerations preclude a vaginal or minimally invasive total hysterectomy. Younger women can be counseled about the risks and benefits of various routes of extraction. Patients with any suspicious findings during preoperative evaluation or surgery itself should have their uterus removed without disruption or fragmentation.
In regard to myomectomy specifically, a significant portion of the data we have on the risks of power morcellation derives from studies of hysterectomy. There are minimal data describing the risk of occult pathology at the time of minimally invasive myomectomy. Although younger patients likely are at relatively low risk for occult malignancy, they should be counseled that population-based estimates of cancer at the time of myomectomy are lacking.
Dr. Bradley: Since the controversy over morcellation arose, the Cleveland Clinic not only has banned the procedure but also removed all morcellators from its shelves, and it is unclear whether the option will be revisited after the FDA renders its final verdict. So my approach to tissue extraction is either vaginal morcellation or using a mini-laparotomy to remove the whole specimen intact or put it in a bag and morcellate it with a knife.
Case 1: A premenopausal patient scheduled for myomectomy
OBG Management: Let’s move on to a specific case. Let’s say the patient is a 35-year-old woman with a large fibroid, to be removed by myomectomy. How would you quantify her risk of occult malignancy? And what would preoperative assessment entail?
Dr. Iglesia: This patient’s risk of occult malignancy is low. I would obtain pelvic ultrasonography and endometrial biopsy, with cervical cytology included. Preoperative magnetic resonance imaging (MRI) would be indicated if there is a possibility that power morcellation will be performed. If power morcellation were selected, I would perform it using a bag.
Dr. Bradley: At the Cleveland Clinic, we now utilize the FDA risk estimates for occult malignancy of 1 in 300 to 1 in 350 women,1 and I counsel patients using these figures. In the past several years, we have begun to use MRI with and without contrast to determine the size, number, and location of the fibroids, to determine our surgical approach, and to guide our discussion with the patient of what we will be able to do—for example, laparoscopy versus laparotomy.
OBG Management: Would the FDA figures you give be applicable to a young woman such as this 35-year-old?
Dr. Bradley: We’re using those figures with all of our premenopausal patients.
OBG Management: And does the MRI pick up sarcomas?
Dr. Bradley: No imaging is 100% sensitive in detecting sarcoma. We do MRI, and if the fibroid has any areas of necrosis, irregularity, or poor tissue planes that would arouse our suspicion of adenomyoma or sarcoma, we perform the myomectomy via laparotomy. But as I mentioned earlier, we don’t use power morcellation at all anymore—so this patient you describe would likely undergo laparoscopic removal using a bag and a knife to extract it to the skin level.
Although every patient is different, in general, if we have a patient with a single large fibroid 10 cm or less in size, we try to remove it laparoscopically or with robot assistance rather than via laparotomy. We also perform endometrial biopsy.
Dr. Advincula: First, it’s important to define prevalence and incidence when discussing risks. Prevalence would be the number of patients with a leiomyosarcoma per 100,000 women, whereas incidence is the number of patients given a diagnosis of leiomyosarcoma within a year per 100,000 women. In this case, a 35-year-old woman would have a prevalence of leiomyosarcoma, in the general population, of 3 to 7 per 100,000 women and an incidence of less than 1%.
My preoperative assessment would involve MRI of the pelvis with T2 weighted images to better characterize her uterus. Although there has been much discussion lately about the use of lactate dehydrogenase (LDH) isoenzyme panels in combination with MRI to detect occult leiomyosarcoma, the reliability and reproducibility of that combined approach are not fully vetted and, as yet, are not a standard part of my workup. Endometrial sampling would certainly be warranted with any associated history of abnormal uterine bleeding.
Dr. Wright: As I mentioned earlier, most data on power morcellation have been derived from studies of women undergoing hysterectomy. To date, accurate estimates to predict the risk of occult cancer in this patient planning to undergo myomectomy are largely lacking. For women undergoing hysterectomy using power morcellation, advanced age is the strongest risk factor for occult malignancy. Although this patient’s risk of cancer likely is relatively low, she should be counseled that precise estimates are lacking.
Preoperatively, she should undergo endometrial sampling if she has abnormal bleeding. However, the reliability of endometrial sampling, as well as imaging, is limited in the detection of uterine sarcomas.
Case 2: Perimenopausal patient undergoing hysterectomy
OBG Management: How would your approach to preoperative assessment change if this patient were a 47-year-old perimenopausal woman with a single large fibroid to be removed by hysterectomy?
Dr. Bradley: It would be the same preoperative assessment—an MRI and an endometrial biopsy.
Dr. Iglesia: The risk of occult malignancy would be greater than with the first patient. Again, I would use pelvic ultrasound, endometrial biopsy, and cervical cytology to assess her, and I would perform vaginal or TLH. MRI would be indicated if there is a possibility of performing intraperitoneal morcellation. I would prefer doing any morcellation in a bag or via laparotomy.
Dr. Wright: Based on age alone, this perimenopausal patient’s risk for an underlying cancer is 0.2%.2 If the patient has any abnormal bleeding, she should undergo endometrial sampling preoperatively. The diagnostic modalities currently available—which include endometrial sampling as well as imaging, even MRI—are unreliable in the diagnosis of uterine sarcomas, and the patient should be counseled accordingly if she is considering power morcellation.
If it is technically feasible, vaginal hysterectomy or a minimally invasive hysterectomy without power morcellation are preferred. If neither modality is feasible and the patient is considering power morcellation, she should be carefully counseled about the underlying risk not only of uterine cancer but also of other adverse pathologic abnormalities.
Case 3: Postmenopausal woman scheduled for hysterectomy
OBG Management: Let’s change the details of the case again. This time she’s 55 years old and postmenopausal. She, too, has a large fibroid to be removed via hysterectomy. What is her risk of occult cancer? How would you assess her preoperatively?
Dr. Bradley: My approach would be the same as in the first two cases. However, because this patient is menopausal, morcellation would be off the table. (And it already is off the table—for any patient—at the Cleveland Clinic.) I usually prefer open hysterectomy for these patients, which is very different from what we were doing 1 year ago.
I want to expand on Dr. Wright’s comments about other pathologic abnormalities. As a woman ages, her cancer risk becomes greatest for malignancy of the endometrium rather than cancer in a fibroid. If this were my 55-year-old patient, and I had been seeing her for 20 years, and her fibroids had remained the same size but she was now having bleeding, I’d be more concerned about an endometrial problem—hyperplasia, a polyp, or cancer.
If the patient were having bulk symptoms, new pain, and imaging that shows, over 10 years between perimenopause and postmenopause, that there has been growth of the fibroids, I would be concerned about a sarcoma.
Some women who present with postmenopausal bleeding have ovarian cancer, and some studies show that a significant percentage of women with ovarian cancer present with bleeding as a primary symptom.3 So in a postmenopausal patient, I really want to know about the health of the ovaries. Are they enlarged on imaging?
There is also a bimodal distribution of human papillomavirus (HPV) infection and cervical cancer, with peaks of infection at ages 26 to 30 years and again at 46 to 50 years in some populations. The second age peak is followed by an increase in cervical intraepithelial neoplasia (CIN) 2 and 3 and invasive cervical cancer 20 years later.4 So I also want to consider the possibility of cervical cancer in this population.
I tell my patients that pretty much everybody has fibroids. Because they are so common, I need to look at the whole picture.
Dr. Wright: For the perimenopausal and postmenopausal patients we are discussing, preoperative evaluation and counseling would be similar to that for the premenopausal woman. However, given recent data, it is important to note that the prevalence ratio for a uterine malignancy increases with increasing age.2 Clinicians need to be mindful of red flags in perimenopausal and postmenopausal women.
Dr. Kho: I agree that, for each of the cases we have discussed, we need to consider more than just the presence of the fibroid. Risk stratification based on clinical factors like age, menopausal status, BMI, the indication for surgery, and response to any other therapy is extremely important and should guide decision-making regarding the surgical approach.
Although we lack reliable methods to differentiate fibroids from leiomyosarcomas, there are other malignant conditions of the uterus, as Dr. Bradley pointed out. Premalignant conditions of the uterus and cervix and endometrial adenocarcinomas occur far more frequently in the population than sarcomas, and we may be increasing risks by morcellating unsuspected cancers of any type.
At this time, whenever I am considering morcellation in any patient, I obtain pelvic imaging, endometrial biopsy, and current cervical cancer screening. If any of these studies suggest a malignant or premalignant condition, I avoid morcellation. Similarly, if a patient’s clinical history raises suspicion of a potential underlying malignant process, such as new symptoms of an enlarging myoma in a postmenopausal woman, I will try to find an alternative to morcellation.
Why we need a national surgery registry
The controversy surrounding open power morcellation was precipitated by the reporting of a single case—that of a prominent physician who had an unsuspected cancer morcellated during the course of a hysterectomy and was later upgraded to Stage 4 leiomyosarcoma as a result. But it wasn’t a gynecologic surgeon who reported the case—it was the patient herself. And we all know she was by no means the first case of an inadvertently morcellated sarcoma.
I would wager that few physicians are well versed in how to contact the FDA’s Manufacturer and User Facility Device Experience (MAUDE) database. And not many are familiar with the other specialized registries in the United States, let alone know how to report to them. Another important but often unaddressed issue: Is it hassle-free to make a report? The answer: A resounding “No.”
Who do we inform about our experiences—successful or not—in the operating room? And who pays for data collection? At present, the system is piecemeal or scattershot at best.
Sorely needed is a system for reporting that is easy to use, broad, and deep. A nationally funded system would be best. Otherwise, who is going to maintain the database? Who will filter the data? Who will ensure that the information that is entered is correct so that outcomes can be followed accurately?
For those of us employed by a hospital or other institution, it tends to be the institution itself that gathers the data—when it is gathered. But what about surgeons in private practice? How do they monitor themselves? And what about privately owned outpatient surgery centers, where patients sojourn no longer than 23 hours? Is it reasonable to expect them to add the burden and expense of data collection without a national mandate?
I know firsthand some of the skewed information that results when reporting is piecemeal or manipulated. When I was a resident, for example, in some localities, it was not uncommon for sexually transmitted diseases to go unreported when the patient had private insurance. The result: Only those in the lower socioeconomic ranks appeared to experience this problem.
Clearly, we need national standards and a national protocol. And to achieve that we need leaders strong enough to argue that the expense represents dollars well spent.
- Linda D. Bradley, MD
Quick Poll:
If you are using, plan to use, or anticipate the possibility of using power morcellation during minimally invasive gynecologic surgery, does your consent process include a separate form specific to power morcellation?
Please provide your answer to this question in the Quick Poll on the OBG Management home page and then see how your peers have voted.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. April 17, 2014. http://www.fda.gov/medicaldevices/safety/alertsandnotices/ucm393576.htm. Accessed August 18, 2014.
2. Wright JD, Tergas AI, Burke WM, et al. Uterine pathology in women undergoing minimally invasive hysterectomy with morcellation [published online ahead of print July 22, 2014]. JAMA. doi: 10.1001/jama.2014.9005.
3. Lurie G, Thompson PJ, McDuffie KE, et al. Prediagnostic symptoms of ovarian carcinoma: a case-control study. Gynecol Oncol. 2009;114(2):231–236.
4. Chan PK, Chang AR, Yu MY, et al. Age distribution of human papillomavirus infection and cervical neoplasia reflects caveats of cervical screening policies. Int J Cancer. 2010;126(1):297–301.
1. US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. April 17, 2014. http://www.fda.gov/medicaldevices/safety/alertsandnotices/ucm393576.htm. Accessed August 18, 2014.
2. Wright JD, Tergas AI, Burke WM, et al. Uterine pathology in women undergoing minimally invasive hysterectomy with morcellation [published online ahead of print July 22, 2014]. JAMA. doi: 10.1001/jama.2014.9005.
3. Lurie G, Thompson PJ, McDuffie KE, et al. Prediagnostic symptoms of ovarian carcinoma: a case-control study. Gynecol Oncol. 2009;114(2):231–236.
4. Chan PK, Chang AR, Yu MY, et al. Age distribution of human papillomavirus infection and cervical neoplasia reflects caveats of cervical screening policies. Int J Cancer. 2010;126(1):297–301.