User login
Is There a Relationship Between Facility Peer Review Findings and Quality in the Veterans Health Administration?
Hospital leaders report the most common aim of peer review (PR) is to improve quality and patient safety, thus it is a potentially powerful quality improvement (QI) driver.1 “When conducted systematically and credibly, peer review for quality management can result in both short-term and long-term improvements in patient care by revealing areas for improvement in the provision of care,” Veterans Health Administration (VHA) Directive 1190 states. “This ultimately contributes to organizational improvements.” At the same time, there are anecdotal concerns that PR may be used punitively and driven by case outcomes rather than by accepted best practices supporting QI.
Studies of the PR process suggest these concerns are valid. A key tenet of QI is standardization. PR is problematic in that regard; studies show poor interrater reliability for judgments on care, as well as hindsight bias—the fact that raters are strongly influenced by the outcome of care, not the process of care.2-5 There are concerns that case selection or review process when not standardized may be wielded as punitive too.6 In this study, we sought to identify the relationship between PR findings and subsequent institution quality metrics. If PR does lead to an improvement in quality, or if quality concerns are managed within the PR committee, it should be possible to identify a measurable relationship between the PR process and a facility’s subsequent quality measures.
A handful of studies describe the association between PR and quality of care. Itri and colleagues noted that random, not standardized PR in radiology does not achieve reductions in diagnostic error rate.7 However, adoption of just culture principles in PR resulted in a significant improvement in facility leaders’ self-reports of quality measures at surveyed institutions.8 The same author reported that increases in PR standardization and integration with performance improvement activities could explain up to 18% of objective quality measure variation.9
We sought to determine whether a specific aspect of the PR process, the PR committee judgment of quality of care by clinicians, was related to medical center quality in a cross-sectional study of 136 Veterans Health Administration (VHA) medical centers. The VHA is a good source of study because there are standardized PR processes and training for committee members and reviewers. Our hypothesis was that medical centers with a higher number of Level 2 (“most experienced and competent clinicians might have managed the case differently”) and Level 3 (“most experienced and competent providers would have managed the case differently”) PR findings would also have lower quality metric scores for processes and outcomes of care.
Methods
We used PR data from fiscal year 2018 and 2019. VHA PR data are available quarterly and are self-reported by each facility to the VHA Office of Clinical Risk Management. These data are broken down by facility. The following data, when available in both fiscal years 2018 and 2019, were used for this analysis: percent and number of PR that are ranked as level 1, 2, or 3; medical center group (MCG) acuity measure assigned by the VHA (1 is highest, 3 is lowest); and number of PR per 100,000 unique veteran encounters in 2019. Measures of facility quality are drawn from Strategic Analytics for Improvement and Learning (SAIL) data from 2019, which are available quarterly by facility and are rolling for 12 months. SAIL measures processes and outcomes of care. Table 1 indicates which measures are focused on outcomes vs quality processes.
SAS Version 9.2 was used to perform statistical analyses. We used Spearman correlation to estimate the PR and quality relationship.
Results
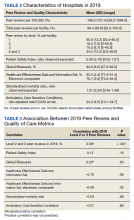
There were 136 facilities with 2 years of PR data available. The majority of these facilities (89) were highest complexity MCG 1 facilities; 19 were MCG 2, and 28 were MCG 3. Of 13,515 PRs, most of the 9555 PR findings were level 1 (70.7%). The between-facility range of level 2 and 3 findings was large, varying from 3.5% to nearly 70% in 2019 (Table 2). Findings were similar in 2018; facilities level 2 and 3 ratings ranged from 3.6% to 73.5% of all PR findings.
There was no correlation between most quality measures and facility PR findings (Table 3). The only exception was for Global Measures (GM90), an inpatient process of care measure. Unexpectedly, the correlation was positive—facilities with a higher percentage of level 2 and 3 PR findings had better inpatient processes of care SAIL score. The strongest correlation was between 2018 and 2019 PR findings.
Discussion
We hypothesized that a high percentage of level 2 and 3 PR findings would be negatively associated with objective facility measures of care processes in SAIL but we did not see this association. The only quality measure associated with PR findings was GM90, a score of inpatient care processes. However, the association was positive, with better performance associated with more level 2 and 3 PR findings.
The best predictor of the proportion of a facility’s PR findings is the previous year’s PR findings. With an R = 0.59, the previous year findings explain about 35% of the variability in level assignment. Our analysis may describe a new bias in PR, in which committees consistently assign either low or high proportions of level 2 and 3 findings. This correlation could be due to individual PR committee culture or composition, but it does not relate to objective quality measures.
Strengths
For this study we use objective measures of PR processes, the assignment of levels of care.
Limitations
Facilities self-report PR outcomes, so there could be errors in reporting. In addition, this study was cross sectional and not longitudinal and it is possible that change in quality measures over time are correlated with PR findings. Future studies using the VHA PR and SAIL data could evaluate whether changes over time, and perhaps in response to level 2 and 3 findings, would be a more sensitive indicator of the impact of the PR process on quality metrics. Future studies could incorporate the relationship between findings from the All Employee Survey, which is conducted annually, such as psychologic safety, as well as the distance the facility has gone on the high reliability organization journey, with PR findings and SAIL metrics. Finally, PR is focused on the practice of an individual clinician, while SAIL quality metrics reflect facility performance. Interventions possibly stay at the clinician level and do not drive subsequent QI processes.
What does this mean for PR? Since the early 1990s, there have been exhortations from experts to improve PR, by adopting a QI model, or for a deeper integration of PR and QI.1,2,10 Just culture tools, which include QI, are promoted as a means to improve PR.8,11,12 Other studies show PR remains problematic in terms of standardization, incorporation of best practices, redesigning systems of care, or demonstrable improvements to facility safety and care quality.1,4,6,8 Several publications have described interventions to improve PR. Deyo-Svedson discussed a program with standardized training and triggers, much like VHA.13 Itri and colleagues standardized PR in radiology to target areas of known diagnostic error, as well as use the issues assessed in PR to perform QI and education. One example of a successful QI effort involved changing the radiology reporting template to make sure areas that are prone to diagnostic error are addressed.7
Conclusions
Since 35% of PR level variance is correlated with prior year’s results, PR committees should look at increased standardization in reviews and findings. We endorse a strong focus on standardization, application of just culture tools to case reviews, and tighter linkage between process and outcome metrics measured by SAIL and PR case finding. Studies should be performed to pilot interventions to improve the linkage between PR and quality, so that greater and faster gains can be made in quality processes and, leading from this, outcomes. Additionally, future research should investigate why some facilities consistently choose higher or lower PR ratings.
Acknowledgments
We acknowledge Dr. George “Web” Ross for his helpful edits.
1. Edwards MT. In pursuit of quality and safety: an 8-year study of clinical peer review best practices in US hospitals. Int J Qual Health Care. 2018;30(8):602-607. doi:10.1093/intqhc/mzy069
2. Dans PE. Clinical peer Review: burnishing a tarnished icon. Ann Intern Med. 1993;118(7):566-568. doi:10.7326/0003-4819-118-7-199304010-00014
3. Goldman RL. The reliability of peer assessments of quality of care. JAMA. 1992;267(7):958-960. doi:10.1001/jama.1992.03480070074034
4. Swaroop R. Disrupting physician clinical practice peer review. Perm J. 2019;23:18-207. doi:10.7812/TPP/18-207
5. Caplan RA, Posner KL, Cheney FW. Effect of outcome on physician judgments of appropriateness of care. JAMA. 1991;265(15):1957–1960. doi:10.1001/jama.1991.03460150061024
6. Vyas D, Hozain AE. Clinical peer review in the United States: history, legal development and subsequent abuse. World J Gastroenterol. 2014;20(21):6357-6363. doi:10.3748/wjg.v20.i21.6357
7. Itri JN, Donithan A, Patel SH. Random versus nonrandom peer review: a case for more meaningful peer review. J Am Coll Radiol. 2018;15(7):1045-1052. doi:10.1016/j.jacr.2018.03.054
8. Edwards MT. An assessment of the impact of just culture on quality and safety in US hospitals. Am J Med Qual. 2018; 33(5):502-508. doi:10.1177/1062860618768057
9. Edwards MT. The objective impact of clinical peer review on hospital quality and safety. Am J Med Qual. 2011;26(2);110-119. doi:10.1177/1062860610380732
10. Berwick DM. Peer review and quality management: are they compatible?. QRB Qual Rev Bull. 1990;16(7):246-251. doi:10.1016/s0097-5990(16)30377-3
11. Volkar JK, Phrampus P, English D, et al. Institution of just culture physician peer review in an academic medical center. J Patient Saf. 2021;17(7):e689-e693. doi:10.1097/PTS.0000000000000449
12. Burns J, Miller T, Weiss JM, Erdfarb A, Silber D, Goldberg-Stein S. Just culture: practical implementation for radiologist peer review. J Am Coll Radiol. 2019;16(3):384-388. doi:10.1016/j.jacr.2018.10.021
13. Deyo-Svendsen ME, Phillips MR, Albright JK, et al. A systematic approach to clinical peer review in a critical access hospital. Qual Manag Health Care. 2016;25(4):213-218. doi:10.1097/QMH.0000000000000113
Hospital leaders report the most common aim of peer review (PR) is to improve quality and patient safety, thus it is a potentially powerful quality improvement (QI) driver.1 “When conducted systematically and credibly, peer review for quality management can result in both short-term and long-term improvements in patient care by revealing areas for improvement in the provision of care,” Veterans Health Administration (VHA) Directive 1190 states. “This ultimately contributes to organizational improvements.” At the same time, there are anecdotal concerns that PR may be used punitively and driven by case outcomes rather than by accepted best practices supporting QI.
Studies of the PR process suggest these concerns are valid. A key tenet of QI is standardization. PR is problematic in that regard; studies show poor interrater reliability for judgments on care, as well as hindsight bias—the fact that raters are strongly influenced by the outcome of care, not the process of care.2-5 There are concerns that case selection or review process when not standardized may be wielded as punitive too.6 In this study, we sought to identify the relationship between PR findings and subsequent institution quality metrics. If PR does lead to an improvement in quality, or if quality concerns are managed within the PR committee, it should be possible to identify a measurable relationship between the PR process and a facility’s subsequent quality measures.
A handful of studies describe the association between PR and quality of care. Itri and colleagues noted that random, not standardized PR in radiology does not achieve reductions in diagnostic error rate.7 However, adoption of just culture principles in PR resulted in a significant improvement in facility leaders’ self-reports of quality measures at surveyed institutions.8 The same author reported that increases in PR standardization and integration with performance improvement activities could explain up to 18% of objective quality measure variation.9
We sought to determine whether a specific aspect of the PR process, the PR committee judgment of quality of care by clinicians, was related to medical center quality in a cross-sectional study of 136 Veterans Health Administration (VHA) medical centers. The VHA is a good source of study because there are standardized PR processes and training for committee members and reviewers. Our hypothesis was that medical centers with a higher number of Level 2 (“most experienced and competent clinicians might have managed the case differently”) and Level 3 (“most experienced and competent providers would have managed the case differently”) PR findings would also have lower quality metric scores for processes and outcomes of care.
Methods
We used PR data from fiscal year 2018 and 2019. VHA PR data are available quarterly and are self-reported by each facility to the VHA Office of Clinical Risk Management. These data are broken down by facility. The following data, when available in both fiscal years 2018 and 2019, were used for this analysis: percent and number of PR that are ranked as level 1, 2, or 3; medical center group (MCG) acuity measure assigned by the VHA (1 is highest, 3 is lowest); and number of PR per 100,000 unique veteran encounters in 2019. Measures of facility quality are drawn from Strategic Analytics for Improvement and Learning (SAIL) data from 2019, which are available quarterly by facility and are rolling for 12 months. SAIL measures processes and outcomes of care. Table 1 indicates which measures are focused on outcomes vs quality processes.
SAS Version 9.2 was used to perform statistical analyses. We used Spearman correlation to estimate the PR and quality relationship.
Results
There were 136 facilities with 2 years of PR data available. The majority of these facilities (89) were highest complexity MCG 1 facilities; 19 were MCG 2, and 28 were MCG 3. Of 13,515 PRs, most of the 9555 PR findings were level 1 (70.7%). The between-facility range of level 2 and 3 findings was large, varying from 3.5% to nearly 70% in 2019 (Table 2). Findings were similar in 2018; facilities level 2 and 3 ratings ranged from 3.6% to 73.5% of all PR findings.
There was no correlation between most quality measures and facility PR findings (Table 3). The only exception was for Global Measures (GM90), an inpatient process of care measure. Unexpectedly, the correlation was positive—facilities with a higher percentage of level 2 and 3 PR findings had better inpatient processes of care SAIL score. The strongest correlation was between 2018 and 2019 PR findings.
Discussion
We hypothesized that a high percentage of level 2 and 3 PR findings would be negatively associated with objective facility measures of care processes in SAIL but we did not see this association. The only quality measure associated with PR findings was GM90, a score of inpatient care processes. However, the association was positive, with better performance associated with more level 2 and 3 PR findings.
The best predictor of the proportion of a facility’s PR findings is the previous year’s PR findings. With an R = 0.59, the previous year findings explain about 35% of the variability in level assignment. Our analysis may describe a new bias in PR, in which committees consistently assign either low or high proportions of level 2 and 3 findings. This correlation could be due to individual PR committee culture or composition, but it does not relate to objective quality measures.
Strengths
For this study we use objective measures of PR processes, the assignment of levels of care.
Limitations
Facilities self-report PR outcomes, so there could be errors in reporting. In addition, this study was cross sectional and not longitudinal and it is possible that change in quality measures over time are correlated with PR findings. Future studies using the VHA PR and SAIL data could evaluate whether changes over time, and perhaps in response to level 2 and 3 findings, would be a more sensitive indicator of the impact of the PR process on quality metrics. Future studies could incorporate the relationship between findings from the All Employee Survey, which is conducted annually, such as psychologic safety, as well as the distance the facility has gone on the high reliability organization journey, with PR findings and SAIL metrics. Finally, PR is focused on the practice of an individual clinician, while SAIL quality metrics reflect facility performance. Interventions possibly stay at the clinician level and do not drive subsequent QI processes.
What does this mean for PR? Since the early 1990s, there have been exhortations from experts to improve PR, by adopting a QI model, or for a deeper integration of PR and QI.1,2,10 Just culture tools, which include QI, are promoted as a means to improve PR.8,11,12 Other studies show PR remains problematic in terms of standardization, incorporation of best practices, redesigning systems of care, or demonstrable improvements to facility safety and care quality.1,4,6,8 Several publications have described interventions to improve PR. Deyo-Svedson discussed a program with standardized training and triggers, much like VHA.13 Itri and colleagues standardized PR in radiology to target areas of known diagnostic error, as well as use the issues assessed in PR to perform QI and education. One example of a successful QI effort involved changing the radiology reporting template to make sure areas that are prone to diagnostic error are addressed.7
Conclusions
Since 35% of PR level variance is correlated with prior year’s results, PR committees should look at increased standardization in reviews and findings. We endorse a strong focus on standardization, application of just culture tools to case reviews, and tighter linkage between process and outcome metrics measured by SAIL and PR case finding. Studies should be performed to pilot interventions to improve the linkage between PR and quality, so that greater and faster gains can be made in quality processes and, leading from this, outcomes. Additionally, future research should investigate why some facilities consistently choose higher or lower PR ratings.
Acknowledgments
We acknowledge Dr. George “Web” Ross for his helpful edits.
Hospital leaders report the most common aim of peer review (PR) is to improve quality and patient safety, thus it is a potentially powerful quality improvement (QI) driver.1 “When conducted systematically and credibly, peer review for quality management can result in both short-term and long-term improvements in patient care by revealing areas for improvement in the provision of care,” Veterans Health Administration (VHA) Directive 1190 states. “This ultimately contributes to organizational improvements.” At the same time, there are anecdotal concerns that PR may be used punitively and driven by case outcomes rather than by accepted best practices supporting QI.
Studies of the PR process suggest these concerns are valid. A key tenet of QI is standardization. PR is problematic in that regard; studies show poor interrater reliability for judgments on care, as well as hindsight bias—the fact that raters are strongly influenced by the outcome of care, not the process of care.2-5 There are concerns that case selection or review process when not standardized may be wielded as punitive too.6 In this study, we sought to identify the relationship between PR findings and subsequent institution quality metrics. If PR does lead to an improvement in quality, or if quality concerns are managed within the PR committee, it should be possible to identify a measurable relationship between the PR process and a facility’s subsequent quality measures.
A handful of studies describe the association between PR and quality of care. Itri and colleagues noted that random, not standardized PR in radiology does not achieve reductions in diagnostic error rate.7 However, adoption of just culture principles in PR resulted in a significant improvement in facility leaders’ self-reports of quality measures at surveyed institutions.8 The same author reported that increases in PR standardization and integration with performance improvement activities could explain up to 18% of objective quality measure variation.9
We sought to determine whether a specific aspect of the PR process, the PR committee judgment of quality of care by clinicians, was related to medical center quality in a cross-sectional study of 136 Veterans Health Administration (VHA) medical centers. The VHA is a good source of study because there are standardized PR processes and training for committee members and reviewers. Our hypothesis was that medical centers with a higher number of Level 2 (“most experienced and competent clinicians might have managed the case differently”) and Level 3 (“most experienced and competent providers would have managed the case differently”) PR findings would also have lower quality metric scores for processes and outcomes of care.
Methods
We used PR data from fiscal year 2018 and 2019. VHA PR data are available quarterly and are self-reported by each facility to the VHA Office of Clinical Risk Management. These data are broken down by facility. The following data, when available in both fiscal years 2018 and 2019, were used for this analysis: percent and number of PR that are ranked as level 1, 2, or 3; medical center group (MCG) acuity measure assigned by the VHA (1 is highest, 3 is lowest); and number of PR per 100,000 unique veteran encounters in 2019. Measures of facility quality are drawn from Strategic Analytics for Improvement and Learning (SAIL) data from 2019, which are available quarterly by facility and are rolling for 12 months. SAIL measures processes and outcomes of care. Table 1 indicates which measures are focused on outcomes vs quality processes.
SAS Version 9.2 was used to perform statistical analyses. We used Spearman correlation to estimate the PR and quality relationship.
Results
There were 136 facilities with 2 years of PR data available. The majority of these facilities (89) were highest complexity MCG 1 facilities; 19 were MCG 2, and 28 were MCG 3. Of 13,515 PRs, most of the 9555 PR findings were level 1 (70.7%). The between-facility range of level 2 and 3 findings was large, varying from 3.5% to nearly 70% in 2019 (Table 2). Findings were similar in 2018; facilities level 2 and 3 ratings ranged from 3.6% to 73.5% of all PR findings.
There was no correlation between most quality measures and facility PR findings (Table 3). The only exception was for Global Measures (GM90), an inpatient process of care measure. Unexpectedly, the correlation was positive—facilities with a higher percentage of level 2 and 3 PR findings had better inpatient processes of care SAIL score. The strongest correlation was between 2018 and 2019 PR findings.
Discussion
We hypothesized that a high percentage of level 2 and 3 PR findings would be negatively associated with objective facility measures of care processes in SAIL but we did not see this association. The only quality measure associated with PR findings was GM90, a score of inpatient care processes. However, the association was positive, with better performance associated with more level 2 and 3 PR findings.
The best predictor of the proportion of a facility’s PR findings is the previous year’s PR findings. With an R = 0.59, the previous year findings explain about 35% of the variability in level assignment. Our analysis may describe a new bias in PR, in which committees consistently assign either low or high proportions of level 2 and 3 findings. This correlation could be due to individual PR committee culture or composition, but it does not relate to objective quality measures.
Strengths
For this study we use objective measures of PR processes, the assignment of levels of care.
Limitations
Facilities self-report PR outcomes, so there could be errors in reporting. In addition, this study was cross sectional and not longitudinal and it is possible that change in quality measures over time are correlated with PR findings. Future studies using the VHA PR and SAIL data could evaluate whether changes over time, and perhaps in response to level 2 and 3 findings, would be a more sensitive indicator of the impact of the PR process on quality metrics. Future studies could incorporate the relationship between findings from the All Employee Survey, which is conducted annually, such as psychologic safety, as well as the distance the facility has gone on the high reliability organization journey, with PR findings and SAIL metrics. Finally, PR is focused on the practice of an individual clinician, while SAIL quality metrics reflect facility performance. Interventions possibly stay at the clinician level and do not drive subsequent QI processes.
What does this mean for PR? Since the early 1990s, there have been exhortations from experts to improve PR, by adopting a QI model, or for a deeper integration of PR and QI.1,2,10 Just culture tools, which include QI, are promoted as a means to improve PR.8,11,12 Other studies show PR remains problematic in terms of standardization, incorporation of best practices, redesigning systems of care, or demonstrable improvements to facility safety and care quality.1,4,6,8 Several publications have described interventions to improve PR. Deyo-Svedson discussed a program with standardized training and triggers, much like VHA.13 Itri and colleagues standardized PR in radiology to target areas of known diagnostic error, as well as use the issues assessed in PR to perform QI and education. One example of a successful QI effort involved changing the radiology reporting template to make sure areas that are prone to diagnostic error are addressed.7
Conclusions
Since 35% of PR level variance is correlated with prior year’s results, PR committees should look at increased standardization in reviews and findings. We endorse a strong focus on standardization, application of just culture tools to case reviews, and tighter linkage between process and outcome metrics measured by SAIL and PR case finding. Studies should be performed to pilot interventions to improve the linkage between PR and quality, so that greater and faster gains can be made in quality processes and, leading from this, outcomes. Additionally, future research should investigate why some facilities consistently choose higher or lower PR ratings.
Acknowledgments
We acknowledge Dr. George “Web” Ross for his helpful edits.
1. Edwards MT. In pursuit of quality and safety: an 8-year study of clinical peer review best practices in US hospitals. Int J Qual Health Care. 2018;30(8):602-607. doi:10.1093/intqhc/mzy069
2. Dans PE. Clinical peer Review: burnishing a tarnished icon. Ann Intern Med. 1993;118(7):566-568. doi:10.7326/0003-4819-118-7-199304010-00014
3. Goldman RL. The reliability of peer assessments of quality of care. JAMA. 1992;267(7):958-960. doi:10.1001/jama.1992.03480070074034
4. Swaroop R. Disrupting physician clinical practice peer review. Perm J. 2019;23:18-207. doi:10.7812/TPP/18-207
5. Caplan RA, Posner KL, Cheney FW. Effect of outcome on physician judgments of appropriateness of care. JAMA. 1991;265(15):1957–1960. doi:10.1001/jama.1991.03460150061024
6. Vyas D, Hozain AE. Clinical peer review in the United States: history, legal development and subsequent abuse. World J Gastroenterol. 2014;20(21):6357-6363. doi:10.3748/wjg.v20.i21.6357
7. Itri JN, Donithan A, Patel SH. Random versus nonrandom peer review: a case for more meaningful peer review. J Am Coll Radiol. 2018;15(7):1045-1052. doi:10.1016/j.jacr.2018.03.054
8. Edwards MT. An assessment of the impact of just culture on quality and safety in US hospitals. Am J Med Qual. 2018; 33(5):502-508. doi:10.1177/1062860618768057
9. Edwards MT. The objective impact of clinical peer review on hospital quality and safety. Am J Med Qual. 2011;26(2);110-119. doi:10.1177/1062860610380732
10. Berwick DM. Peer review and quality management: are they compatible?. QRB Qual Rev Bull. 1990;16(7):246-251. doi:10.1016/s0097-5990(16)30377-3
11. Volkar JK, Phrampus P, English D, et al. Institution of just culture physician peer review in an academic medical center. J Patient Saf. 2021;17(7):e689-e693. doi:10.1097/PTS.0000000000000449
12. Burns J, Miller T, Weiss JM, Erdfarb A, Silber D, Goldberg-Stein S. Just culture: practical implementation for radiologist peer review. J Am Coll Radiol. 2019;16(3):384-388. doi:10.1016/j.jacr.2018.10.021
13. Deyo-Svendsen ME, Phillips MR, Albright JK, et al. A systematic approach to clinical peer review in a critical access hospital. Qual Manag Health Care. 2016;25(4):213-218. doi:10.1097/QMH.0000000000000113
1. Edwards MT. In pursuit of quality and safety: an 8-year study of clinical peer review best practices in US hospitals. Int J Qual Health Care. 2018;30(8):602-607. doi:10.1093/intqhc/mzy069
2. Dans PE. Clinical peer Review: burnishing a tarnished icon. Ann Intern Med. 1993;118(7):566-568. doi:10.7326/0003-4819-118-7-199304010-00014
3. Goldman RL. The reliability of peer assessments of quality of care. JAMA. 1992;267(7):958-960. doi:10.1001/jama.1992.03480070074034
4. Swaroop R. Disrupting physician clinical practice peer review. Perm J. 2019;23:18-207. doi:10.7812/TPP/18-207
5. Caplan RA, Posner KL, Cheney FW. Effect of outcome on physician judgments of appropriateness of care. JAMA. 1991;265(15):1957–1960. doi:10.1001/jama.1991.03460150061024
6. Vyas D, Hozain AE. Clinical peer review in the United States: history, legal development and subsequent abuse. World J Gastroenterol. 2014;20(21):6357-6363. doi:10.3748/wjg.v20.i21.6357
7. Itri JN, Donithan A, Patel SH. Random versus nonrandom peer review: a case for more meaningful peer review. J Am Coll Radiol. 2018;15(7):1045-1052. doi:10.1016/j.jacr.2018.03.054
8. Edwards MT. An assessment of the impact of just culture on quality and safety in US hospitals. Am J Med Qual. 2018; 33(5):502-508. doi:10.1177/1062860618768057
9. Edwards MT. The objective impact of clinical peer review on hospital quality and safety. Am J Med Qual. 2011;26(2);110-119. doi:10.1177/1062860610380732
10. Berwick DM. Peer review and quality management: are they compatible?. QRB Qual Rev Bull. 1990;16(7):246-251. doi:10.1016/s0097-5990(16)30377-3
11. Volkar JK, Phrampus P, English D, et al. Institution of just culture physician peer review in an academic medical center. J Patient Saf. 2021;17(7):e689-e693. doi:10.1097/PTS.0000000000000449
12. Burns J, Miller T, Weiss JM, Erdfarb A, Silber D, Goldberg-Stein S. Just culture: practical implementation for radiologist peer review. J Am Coll Radiol. 2019;16(3):384-388. doi:10.1016/j.jacr.2018.10.021
13. Deyo-Svendsen ME, Phillips MR, Albright JK, et al. A systematic approach to clinical peer review in a critical access hospital. Qual Manag Health Care. 2016;25(4):213-218. doi:10.1097/QMH.0000000000000113
My choice? Unvaccinated pose outsize risk to vaccinated
according to a mathematical modeling study.
The study, which simulated patterns of infection among vaccinated and unvaccinated populations, showed that, as the populations mixed less, attack rates decreased among vaccinated people (from 15% to 10%) and increased among unvaccinated people (from 62% to 79%). The unvaccinated increasingly became the source of infection, however.
“When the vaccinated and unvaccinated mix, indirect protection is conferred upon the unvaccinated by the buffering effect of vaccinated individuals, and by contrast, risk in the vaccinated goes up,” lead author David Fisman, MD, professor of epidemiology at the University of Toronto, told this news organization.
As the groups mix less and less, the size of the epidemic increases among the unvaccinated and decreases among the vaccinated. “But the impact of the unvaccinated on risk in the vaccinated is disproportionate to the numbers of contacts between the two groups,” said Dr. Fisman.
The study was published online in the Canadian Medical Association Journal.
Relative contributions to risk
The researchers used a model of a respiratory viral disease “similar to SARS-CoV-2 infection with Delta variant.” They included reproduction values to capture the dynamics of the Omicron variant, which was emerging at the time. In the study, vaccines ranged in effectiveness from 40% to 80%. The study incorporated various levels of mixing between a partially vaccinated and an unvaccinated population. The mixing ranged from random mixing to like-with-like mixing (“assortativity”). There were three possible “compartments” of people in the model: those considered susceptible to infection, those considered infected and infectious, and those considered immune because of recovery.
The model showed that, as mixing between the vaccinated and the unvaccinated populations increased, case numbers rose, “with cases in the unvaccinated subpopulation accounting for a substantial proportion of infections.” However, as mixing between the populations decreased, the final attack rate decreased among vaccinated people, but the relative “contribution of risk to vaccinated people caused by infection acquired from contact with unvaccinated people ... increased.”
When the vaccination rate was increased in the model, case numbers among the vaccinated declined “as expected, owing to indirect protective effects,” the researchers noted. But this also “further increased the relative contribution to risk in vaccinated people by those who were unvaccinated.”
Self-regarding risk?
The findings show that “choices made by people who forgo vaccination contribute disproportionately to risk among those who do get vaccinated,” the researchers wrote. “Although risk associated with avoiding vaccination during a virulent pandemic accrues chiefly to those who are unvaccinated, the choice of some individuals to refuse vaccination is likely to affect the health and safety of vaccinated people in a manner disproportionate to the fraction of unvaccinated people in the population.”
The fact that like-with-like mixing cannot mitigate the risk to vaccinated people “undermines the assertion that vaccine choice is best left to the individual and supports strong public actions aimed at enhancing vaccine uptake and limiting access to public spaces for unvaccinated people,” they wrote.
Mandates and passports
“Our model provides support for vaccine mandates and passports during epidemics, such that vaccination is required for people to take part in nonessential activities,” said Dr. Fisman. The choice to not be vaccinated against COVID-19 should not be considered “self-regarding,” he added. “Risk is self-regarding when it only impacts the person engaging in the activity. Something like smoking cigarettes (alone, without others around) creates a lot of risk over time, but if nobody is breathing your secondhand smoke, you’re only creating risk for yourself. By contrast, we regulate, in Ontario, your right to smoke in public indoor spaces such as restaurants, because once other people are around, the risk isn’t self-regarding anymore. You’re creating risk for others.”
The authors also noted that the risks created by the unvaccinated extend beyond those of infection by “creating a risk that those around them may not be able to obtain the care they need.” They recommended that considerations of equity and justice for people who do choose to be vaccinated, as well as those who choose not to be, need to be included in formulating vaccination policy.
Illuminating the discussion
Asked to comment on the study, Matthew Oughton, MD, assistant professor of medicine at McGill University, Montreal, said: “It is easy to dismiss a mathematical model as a series of assumptions that leads to an implausible conclusion. ... However, they can serve to illustrate and, to an extent, quantify the results of complex interactions, and this study does just that.” Dr. Oughton was not involved in the research.
During the past 2 years, the scientific press and the general press have often discussed the individual and collective effects of disease-prevention methods, including nonpharmaceutical interventions. “Models like this can help illuminate those discussions by highlighting important consequences of preventive measures,” said Dr. Oughton, who also works in the division of infectious diseases at the Jewish General Hospital, Montreal.
It’s worth noting that the authors modeled vaccine effectiveness against all infection, “rather than the generally greater and more durable effects we have seen for vaccines in prevention of severe infection,” said Dr. Oughton. He added that the authors did not include the effect of vaccination in reducing forward transmission. “Inclusion of this effect would presumably have reduced overall infectious burden in mixed populations and increased the difference between groups at lower levels of mixing between populations.”
The research was supported by a grant from the Canadian Institutes of Health Research. Dr. Fisman has served on advisory boards related to influenza and SARS-CoV-2 vaccines for Seqirus, Pfizer, AstraZeneca, and Sanofi-Pasteur Vaccines and has served as a legal expert on issues related to COVID-19 epidemiology for the Elementary Teachers Federation of Ontario and the Registered Nurses Association of Ontario. Dr. Oughton disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a mathematical modeling study.
The study, which simulated patterns of infection among vaccinated and unvaccinated populations, showed that, as the populations mixed less, attack rates decreased among vaccinated people (from 15% to 10%) and increased among unvaccinated people (from 62% to 79%). The unvaccinated increasingly became the source of infection, however.
“When the vaccinated and unvaccinated mix, indirect protection is conferred upon the unvaccinated by the buffering effect of vaccinated individuals, and by contrast, risk in the vaccinated goes up,” lead author David Fisman, MD, professor of epidemiology at the University of Toronto, told this news organization.
As the groups mix less and less, the size of the epidemic increases among the unvaccinated and decreases among the vaccinated. “But the impact of the unvaccinated on risk in the vaccinated is disproportionate to the numbers of contacts between the two groups,” said Dr. Fisman.
The study was published online in the Canadian Medical Association Journal.
Relative contributions to risk
The researchers used a model of a respiratory viral disease “similar to SARS-CoV-2 infection with Delta variant.” They included reproduction values to capture the dynamics of the Omicron variant, which was emerging at the time. In the study, vaccines ranged in effectiveness from 40% to 80%. The study incorporated various levels of mixing between a partially vaccinated and an unvaccinated population. The mixing ranged from random mixing to like-with-like mixing (“assortativity”). There were three possible “compartments” of people in the model: those considered susceptible to infection, those considered infected and infectious, and those considered immune because of recovery.
The model showed that, as mixing between the vaccinated and the unvaccinated populations increased, case numbers rose, “with cases in the unvaccinated subpopulation accounting for a substantial proportion of infections.” However, as mixing between the populations decreased, the final attack rate decreased among vaccinated people, but the relative “contribution of risk to vaccinated people caused by infection acquired from contact with unvaccinated people ... increased.”
When the vaccination rate was increased in the model, case numbers among the vaccinated declined “as expected, owing to indirect protective effects,” the researchers noted. But this also “further increased the relative contribution to risk in vaccinated people by those who were unvaccinated.”
Self-regarding risk?
The findings show that “choices made by people who forgo vaccination contribute disproportionately to risk among those who do get vaccinated,” the researchers wrote. “Although risk associated with avoiding vaccination during a virulent pandemic accrues chiefly to those who are unvaccinated, the choice of some individuals to refuse vaccination is likely to affect the health and safety of vaccinated people in a manner disproportionate to the fraction of unvaccinated people in the population.”
The fact that like-with-like mixing cannot mitigate the risk to vaccinated people “undermines the assertion that vaccine choice is best left to the individual and supports strong public actions aimed at enhancing vaccine uptake and limiting access to public spaces for unvaccinated people,” they wrote.
Mandates and passports
“Our model provides support for vaccine mandates and passports during epidemics, such that vaccination is required for people to take part in nonessential activities,” said Dr. Fisman. The choice to not be vaccinated against COVID-19 should not be considered “self-regarding,” he added. “Risk is self-regarding when it only impacts the person engaging in the activity. Something like smoking cigarettes (alone, without others around) creates a lot of risk over time, but if nobody is breathing your secondhand smoke, you’re only creating risk for yourself. By contrast, we regulate, in Ontario, your right to smoke in public indoor spaces such as restaurants, because once other people are around, the risk isn’t self-regarding anymore. You’re creating risk for others.”
The authors also noted that the risks created by the unvaccinated extend beyond those of infection by “creating a risk that those around them may not be able to obtain the care they need.” They recommended that considerations of equity and justice for people who do choose to be vaccinated, as well as those who choose not to be, need to be included in formulating vaccination policy.
Illuminating the discussion
Asked to comment on the study, Matthew Oughton, MD, assistant professor of medicine at McGill University, Montreal, said: “It is easy to dismiss a mathematical model as a series of assumptions that leads to an implausible conclusion. ... However, they can serve to illustrate and, to an extent, quantify the results of complex interactions, and this study does just that.” Dr. Oughton was not involved in the research.
During the past 2 years, the scientific press and the general press have often discussed the individual and collective effects of disease-prevention methods, including nonpharmaceutical interventions. “Models like this can help illuminate those discussions by highlighting important consequences of preventive measures,” said Dr. Oughton, who also works in the division of infectious diseases at the Jewish General Hospital, Montreal.
It’s worth noting that the authors modeled vaccine effectiveness against all infection, “rather than the generally greater and more durable effects we have seen for vaccines in prevention of severe infection,” said Dr. Oughton. He added that the authors did not include the effect of vaccination in reducing forward transmission. “Inclusion of this effect would presumably have reduced overall infectious burden in mixed populations and increased the difference between groups at lower levels of mixing between populations.”
The research was supported by a grant from the Canadian Institutes of Health Research. Dr. Fisman has served on advisory boards related to influenza and SARS-CoV-2 vaccines for Seqirus, Pfizer, AstraZeneca, and Sanofi-Pasteur Vaccines and has served as a legal expert on issues related to COVID-19 epidemiology for the Elementary Teachers Federation of Ontario and the Registered Nurses Association of Ontario. Dr. Oughton disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a mathematical modeling study.
The study, which simulated patterns of infection among vaccinated and unvaccinated populations, showed that, as the populations mixed less, attack rates decreased among vaccinated people (from 15% to 10%) and increased among unvaccinated people (from 62% to 79%). The unvaccinated increasingly became the source of infection, however.
“When the vaccinated and unvaccinated mix, indirect protection is conferred upon the unvaccinated by the buffering effect of vaccinated individuals, and by contrast, risk in the vaccinated goes up,” lead author David Fisman, MD, professor of epidemiology at the University of Toronto, told this news organization.
As the groups mix less and less, the size of the epidemic increases among the unvaccinated and decreases among the vaccinated. “But the impact of the unvaccinated on risk in the vaccinated is disproportionate to the numbers of contacts between the two groups,” said Dr. Fisman.
The study was published online in the Canadian Medical Association Journal.
Relative contributions to risk
The researchers used a model of a respiratory viral disease “similar to SARS-CoV-2 infection with Delta variant.” They included reproduction values to capture the dynamics of the Omicron variant, which was emerging at the time. In the study, vaccines ranged in effectiveness from 40% to 80%. The study incorporated various levels of mixing between a partially vaccinated and an unvaccinated population. The mixing ranged from random mixing to like-with-like mixing (“assortativity”). There were three possible “compartments” of people in the model: those considered susceptible to infection, those considered infected and infectious, and those considered immune because of recovery.
The model showed that, as mixing between the vaccinated and the unvaccinated populations increased, case numbers rose, “with cases in the unvaccinated subpopulation accounting for a substantial proportion of infections.” However, as mixing between the populations decreased, the final attack rate decreased among vaccinated people, but the relative “contribution of risk to vaccinated people caused by infection acquired from contact with unvaccinated people ... increased.”
When the vaccination rate was increased in the model, case numbers among the vaccinated declined “as expected, owing to indirect protective effects,” the researchers noted. But this also “further increased the relative contribution to risk in vaccinated people by those who were unvaccinated.”
Self-regarding risk?
The findings show that “choices made by people who forgo vaccination contribute disproportionately to risk among those who do get vaccinated,” the researchers wrote. “Although risk associated with avoiding vaccination during a virulent pandemic accrues chiefly to those who are unvaccinated, the choice of some individuals to refuse vaccination is likely to affect the health and safety of vaccinated people in a manner disproportionate to the fraction of unvaccinated people in the population.”
The fact that like-with-like mixing cannot mitigate the risk to vaccinated people “undermines the assertion that vaccine choice is best left to the individual and supports strong public actions aimed at enhancing vaccine uptake and limiting access to public spaces for unvaccinated people,” they wrote.
Mandates and passports
“Our model provides support for vaccine mandates and passports during epidemics, such that vaccination is required for people to take part in nonessential activities,” said Dr. Fisman. The choice to not be vaccinated against COVID-19 should not be considered “self-regarding,” he added. “Risk is self-regarding when it only impacts the person engaging in the activity. Something like smoking cigarettes (alone, without others around) creates a lot of risk over time, but if nobody is breathing your secondhand smoke, you’re only creating risk for yourself. By contrast, we regulate, in Ontario, your right to smoke in public indoor spaces such as restaurants, because once other people are around, the risk isn’t self-regarding anymore. You’re creating risk for others.”
The authors also noted that the risks created by the unvaccinated extend beyond those of infection by “creating a risk that those around them may not be able to obtain the care they need.” They recommended that considerations of equity and justice for people who do choose to be vaccinated, as well as those who choose not to be, need to be included in formulating vaccination policy.
Illuminating the discussion
Asked to comment on the study, Matthew Oughton, MD, assistant professor of medicine at McGill University, Montreal, said: “It is easy to dismiss a mathematical model as a series of assumptions that leads to an implausible conclusion. ... However, they can serve to illustrate and, to an extent, quantify the results of complex interactions, and this study does just that.” Dr. Oughton was not involved in the research.
During the past 2 years, the scientific press and the general press have often discussed the individual and collective effects of disease-prevention methods, including nonpharmaceutical interventions. “Models like this can help illuminate those discussions by highlighting important consequences of preventive measures,” said Dr. Oughton, who also works in the division of infectious diseases at the Jewish General Hospital, Montreal.
It’s worth noting that the authors modeled vaccine effectiveness against all infection, “rather than the generally greater and more durable effects we have seen for vaccines in prevention of severe infection,” said Dr. Oughton. He added that the authors did not include the effect of vaccination in reducing forward transmission. “Inclusion of this effect would presumably have reduced overall infectious burden in mixed populations and increased the difference between groups at lower levels of mixing between populations.”
The research was supported by a grant from the Canadian Institutes of Health Research. Dr. Fisman has served on advisory boards related to influenza and SARS-CoV-2 vaccines for Seqirus, Pfizer, AstraZeneca, and Sanofi-Pasteur Vaccines and has served as a legal expert on issues related to COVID-19 epidemiology for the Elementary Teachers Federation of Ontario and the Registered Nurses Association of Ontario. Dr. Oughton disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE CANADIAN MEDICAL ASSOCIATION JOURNAL
Hospital factors tied to lower maternal morbidity
A new study of hospitals in New York City suggests ways to reduce severe maternal morbidity (SMM). The researchers interviewed health care professionals in four institutions with low performance and four with high performance, and identified various themes associated with good performance.
“Our results raise the hypothesis that hospital learning collaboratives focused on optimizing organizational practices and policies, increasing clinician and staff awareness and education on maternal health disparities, and addressing structural racism may be important tools for improving equity in maternal outcomes,” the authors wrote in the study, published in Obstetrics & Gynecology.
The researchers conducted 50 semistructured interviews with health care professionals at lower-performing and higher-performing New York City hospitals, which were selected based on risk-adjusted morbidity metrics. The interviews explored various topics, including structural characteristics like staffing, organizational characteristics like culture and communication, labor and delivery practices such as teamwork and use of evidence-based practices, and racial and ethnic disparities.
The analysis revealed six broad areas that were stronger in high-performing hospitals: day-to-day involvement of leadership in quality activities, an emphasis on standards and standardized care, good communication and teamwork between nurses and physicians, good staffing and supervision among physicians and nurses, sharing of performance data with health care workers, and acknowledgment of the existence of racial and ethnic disparities and that bias can cause treatment differences.
“I think this qualitative approach is an important lens to pair with the quantitative approach. With such variability in severe maternal morbidity between hospitals in New York, it is not enough to just look at the quantitative data. To understand how to improve you must examine structures and processes. The structures, which are the physical and organizational characteristics in health care, and the process, which is how health care is delivered,” Veronica Gillispie-Bell, MD, wrote in a comment. Dr. Gillispie-Bell is medical director at Louisiana Perinatal Quality Collaborative and the Pregnancy-Associated Mortality Review for the Louisiana Department of Health.
“We know that high reliability organizations are those who are preoccupied with quality and safety. That means accountability from leadership (structure) and stability in standardization of care (processes). However, none of this matters if you do not have a culture that promotes safety. Based on the key findings of the high-performing hospitals, there was a culture that promoted safety and quality evidenced in the nurse-physician communication and the transparency around data through a lens of equity,” wrote Dr. Gillispie-Bell.
She noted that the study should encourage low-performing hospitals, since it illustrates avenues for improvement. Her personal experience reflects that, though she said that hospitals need help. The Louisiana Perinatal Quality Collaborative addressed severe maternal morbidity at birthing centers by implementing evidence-based best practices for management of hypertension and hemorrhage along with health equity measures. The team conducted coaching calls, in-person learning sessions, and in-person visits through a “Listening Tour.”
The result was a 35% reduction in hemorrhage overall and a reduction of 49% in hemorrhage in Black women, as well as hypertension by 12% overall between August 2018 and May 2020. Not all the news was good, as Black women still had an increase in severe maternal morbidity, possibly because of the COVID epidemic, since it is a risk factor for hypertension during pregnancy and infection rates are higher among Black individuals. “We need support for state based perinatal quality collaboratives to do this work and we need accountability as we are now seeing from metrics being implemented by [the Centers for Medicare & Medicaid Services]. Hospitals need to stratify their data by race and ethnicity to see where there are disparities in their outcomes,” said Dr. Gillispie-Bell.
The improvements are needed, given that the United States has the highest rates of maternal mortality and morbidity among developed countries, “most of which is preventable, and we have significant inequities by race and ethnicity,” said Laurie Zephyrin, MD, vice president for advancing health equity at the Commonwealth Fund. The question becomes how to effect change, and “there’s a lot happening in the policy space. Some of this policy change is directed at expanding insurance coverage, including more opportunities, including funding for community health workers and doulas, and thinking about how to incorporate midwives. There’s also work around how do we actually improve the care delivered by our health system.” Dr. Zephyrin added that the Department of Health & Human Services has contracted with the health improvement company Premier to use data and best-practices to improve maternal health.
The new work has the potential to be complementary to such approaches. “It provides some structure around how to approach some of the solutions, none of which I think is rocket science. It’s just something that needs to be focused on more intentionally,” said Dr. Zephyrin.
For example, the report found that high-performing hospitals had leaders who collaborated with frontline clinicians to share performance data, and this occurred in person, at departmental quality meetings, and during grand rounds. In contrast, staff in low-performing hospitals did not mention data feedback and some said that their institution made little effort to communicate performance metrics to frontline staff.
“One of the key lessons from the pandemic is that we need to have better data, and we need to have data around race and ethnicity to be able to understand the impact on marginalized communities. This study highlights that there’s more to be done around data to ensure that we can truly move the needle on advancing health equity,” said Dr. Zephyrin.
The researchers also found that clinicians in low-performing institutions did not acknowledge the presence of structural racism or differences in care associated with race or ethnicity. When they acknowledge differences in care, they attributed them to factors outside of the hospital’s control, such as patients not seeking out health care or not maintaining a healthy weight. Clinicians at high-performing hospitals were more likely to explicitly mention racism and bias and acknowledged that these factors could contribute to differences in care.
Dr. Gillispie-Bell and Dr. Zephyrin have no relevant financial disclosures.
A new study of hospitals in New York City suggests ways to reduce severe maternal morbidity (SMM). The researchers interviewed health care professionals in four institutions with low performance and four with high performance, and identified various themes associated with good performance.
“Our results raise the hypothesis that hospital learning collaboratives focused on optimizing organizational practices and policies, increasing clinician and staff awareness and education on maternal health disparities, and addressing structural racism may be important tools for improving equity in maternal outcomes,” the authors wrote in the study, published in Obstetrics & Gynecology.
The researchers conducted 50 semistructured interviews with health care professionals at lower-performing and higher-performing New York City hospitals, which were selected based on risk-adjusted morbidity metrics. The interviews explored various topics, including structural characteristics like staffing, organizational characteristics like culture and communication, labor and delivery practices such as teamwork and use of evidence-based practices, and racial and ethnic disparities.
The analysis revealed six broad areas that were stronger in high-performing hospitals: day-to-day involvement of leadership in quality activities, an emphasis on standards and standardized care, good communication and teamwork between nurses and physicians, good staffing and supervision among physicians and nurses, sharing of performance data with health care workers, and acknowledgment of the existence of racial and ethnic disparities and that bias can cause treatment differences.
“I think this qualitative approach is an important lens to pair with the quantitative approach. With such variability in severe maternal morbidity between hospitals in New York, it is not enough to just look at the quantitative data. To understand how to improve you must examine structures and processes. The structures, which are the physical and organizational characteristics in health care, and the process, which is how health care is delivered,” Veronica Gillispie-Bell, MD, wrote in a comment. Dr. Gillispie-Bell is medical director at Louisiana Perinatal Quality Collaborative and the Pregnancy-Associated Mortality Review for the Louisiana Department of Health.
“We know that high reliability organizations are those who are preoccupied with quality and safety. That means accountability from leadership (structure) and stability in standardization of care (processes). However, none of this matters if you do not have a culture that promotes safety. Based on the key findings of the high-performing hospitals, there was a culture that promoted safety and quality evidenced in the nurse-physician communication and the transparency around data through a lens of equity,” wrote Dr. Gillispie-Bell.
She noted that the study should encourage low-performing hospitals, since it illustrates avenues for improvement. Her personal experience reflects that, though she said that hospitals need help. The Louisiana Perinatal Quality Collaborative addressed severe maternal morbidity at birthing centers by implementing evidence-based best practices for management of hypertension and hemorrhage along with health equity measures. The team conducted coaching calls, in-person learning sessions, and in-person visits through a “Listening Tour.”
The result was a 35% reduction in hemorrhage overall and a reduction of 49% in hemorrhage in Black women, as well as hypertension by 12% overall between August 2018 and May 2020. Not all the news was good, as Black women still had an increase in severe maternal morbidity, possibly because of the COVID epidemic, since it is a risk factor for hypertension during pregnancy and infection rates are higher among Black individuals. “We need support for state based perinatal quality collaboratives to do this work and we need accountability as we are now seeing from metrics being implemented by [the Centers for Medicare & Medicaid Services]. Hospitals need to stratify their data by race and ethnicity to see where there are disparities in their outcomes,” said Dr. Gillispie-Bell.
The improvements are needed, given that the United States has the highest rates of maternal mortality and morbidity among developed countries, “most of which is preventable, and we have significant inequities by race and ethnicity,” said Laurie Zephyrin, MD, vice president for advancing health equity at the Commonwealth Fund. The question becomes how to effect change, and “there’s a lot happening in the policy space. Some of this policy change is directed at expanding insurance coverage, including more opportunities, including funding for community health workers and doulas, and thinking about how to incorporate midwives. There’s also work around how do we actually improve the care delivered by our health system.” Dr. Zephyrin added that the Department of Health & Human Services has contracted with the health improvement company Premier to use data and best-practices to improve maternal health.
The new work has the potential to be complementary to such approaches. “It provides some structure around how to approach some of the solutions, none of which I think is rocket science. It’s just something that needs to be focused on more intentionally,” said Dr. Zephyrin.
For example, the report found that high-performing hospitals had leaders who collaborated with frontline clinicians to share performance data, and this occurred in person, at departmental quality meetings, and during grand rounds. In contrast, staff in low-performing hospitals did not mention data feedback and some said that their institution made little effort to communicate performance metrics to frontline staff.
“One of the key lessons from the pandemic is that we need to have better data, and we need to have data around race and ethnicity to be able to understand the impact on marginalized communities. This study highlights that there’s more to be done around data to ensure that we can truly move the needle on advancing health equity,” said Dr. Zephyrin.
The researchers also found that clinicians in low-performing institutions did not acknowledge the presence of structural racism or differences in care associated with race or ethnicity. When they acknowledge differences in care, they attributed them to factors outside of the hospital’s control, such as patients not seeking out health care or not maintaining a healthy weight. Clinicians at high-performing hospitals were more likely to explicitly mention racism and bias and acknowledged that these factors could contribute to differences in care.
Dr. Gillispie-Bell and Dr. Zephyrin have no relevant financial disclosures.
A new study of hospitals in New York City suggests ways to reduce severe maternal morbidity (SMM). The researchers interviewed health care professionals in four institutions with low performance and four with high performance, and identified various themes associated with good performance.
“Our results raise the hypothesis that hospital learning collaboratives focused on optimizing organizational practices and policies, increasing clinician and staff awareness and education on maternal health disparities, and addressing structural racism may be important tools for improving equity in maternal outcomes,” the authors wrote in the study, published in Obstetrics & Gynecology.
The researchers conducted 50 semistructured interviews with health care professionals at lower-performing and higher-performing New York City hospitals, which were selected based on risk-adjusted morbidity metrics. The interviews explored various topics, including structural characteristics like staffing, organizational characteristics like culture and communication, labor and delivery practices such as teamwork and use of evidence-based practices, and racial and ethnic disparities.
The analysis revealed six broad areas that were stronger in high-performing hospitals: day-to-day involvement of leadership in quality activities, an emphasis on standards and standardized care, good communication and teamwork between nurses and physicians, good staffing and supervision among physicians and nurses, sharing of performance data with health care workers, and acknowledgment of the existence of racial and ethnic disparities and that bias can cause treatment differences.
“I think this qualitative approach is an important lens to pair with the quantitative approach. With such variability in severe maternal morbidity between hospitals in New York, it is not enough to just look at the quantitative data. To understand how to improve you must examine structures and processes. The structures, which are the physical and organizational characteristics in health care, and the process, which is how health care is delivered,” Veronica Gillispie-Bell, MD, wrote in a comment. Dr. Gillispie-Bell is medical director at Louisiana Perinatal Quality Collaborative and the Pregnancy-Associated Mortality Review for the Louisiana Department of Health.
“We know that high reliability organizations are those who are preoccupied with quality and safety. That means accountability from leadership (structure) and stability in standardization of care (processes). However, none of this matters if you do not have a culture that promotes safety. Based on the key findings of the high-performing hospitals, there was a culture that promoted safety and quality evidenced in the nurse-physician communication and the transparency around data through a lens of equity,” wrote Dr. Gillispie-Bell.
She noted that the study should encourage low-performing hospitals, since it illustrates avenues for improvement. Her personal experience reflects that, though she said that hospitals need help. The Louisiana Perinatal Quality Collaborative addressed severe maternal morbidity at birthing centers by implementing evidence-based best practices for management of hypertension and hemorrhage along with health equity measures. The team conducted coaching calls, in-person learning sessions, and in-person visits through a “Listening Tour.”
The result was a 35% reduction in hemorrhage overall and a reduction of 49% in hemorrhage in Black women, as well as hypertension by 12% overall between August 2018 and May 2020. Not all the news was good, as Black women still had an increase in severe maternal morbidity, possibly because of the COVID epidemic, since it is a risk factor for hypertension during pregnancy and infection rates are higher among Black individuals. “We need support for state based perinatal quality collaboratives to do this work and we need accountability as we are now seeing from metrics being implemented by [the Centers for Medicare & Medicaid Services]. Hospitals need to stratify their data by race and ethnicity to see where there are disparities in their outcomes,” said Dr. Gillispie-Bell.
The improvements are needed, given that the United States has the highest rates of maternal mortality and morbidity among developed countries, “most of which is preventable, and we have significant inequities by race and ethnicity,” said Laurie Zephyrin, MD, vice president for advancing health equity at the Commonwealth Fund. The question becomes how to effect change, and “there’s a lot happening in the policy space. Some of this policy change is directed at expanding insurance coverage, including more opportunities, including funding for community health workers and doulas, and thinking about how to incorporate midwives. There’s also work around how do we actually improve the care delivered by our health system.” Dr. Zephyrin added that the Department of Health & Human Services has contracted with the health improvement company Premier to use data and best-practices to improve maternal health.
The new work has the potential to be complementary to such approaches. “It provides some structure around how to approach some of the solutions, none of which I think is rocket science. It’s just something that needs to be focused on more intentionally,” said Dr. Zephyrin.
For example, the report found that high-performing hospitals had leaders who collaborated with frontline clinicians to share performance data, and this occurred in person, at departmental quality meetings, and during grand rounds. In contrast, staff in low-performing hospitals did not mention data feedback and some said that their institution made little effort to communicate performance metrics to frontline staff.
“One of the key lessons from the pandemic is that we need to have better data, and we need to have data around race and ethnicity to be able to understand the impact on marginalized communities. This study highlights that there’s more to be done around data to ensure that we can truly move the needle on advancing health equity,” said Dr. Zephyrin.
The researchers also found that clinicians in low-performing institutions did not acknowledge the presence of structural racism or differences in care associated with race or ethnicity. When they acknowledge differences in care, they attributed them to factors outside of the hospital’s control, such as patients not seeking out health care or not maintaining a healthy weight. Clinicians at high-performing hospitals were more likely to explicitly mention racism and bias and acknowledged that these factors could contribute to differences in care.
Dr. Gillispie-Bell and Dr. Zephyrin have no relevant financial disclosures.
FROM OBSTETRICS & GYNECOLOGY
Experts decry CDC’s long pause on neglected tropical disease testing
The Centers for Disease Control and Prevention has long been the premier reference lab for the United States and, for some diseases, internationally.
In September 2021, the CDC stated on its website that it would stop testing for parasites, herpesvirus encephalitis, human herpesvirus 6 and 7, Epstein-Barr virus, and other viruses, saying, “We are working diligently to implement laboratory system improvements.”
At the time, the CDC said testing would be halted only for a few months.
In response to a query from this news organization, a CDC spokesperson replied, “While at present we are unable to share a detailed timeline, our highest priority is to resume high-quality testing operations in a phased, prioritized approach as soon as possible and to offer the same tests that were available before the pause.”
Several global health clinicians told this news organization that they were not aware of the halt and that they are now uncertain about the specific diagnosis and best treatment for some patients. Other patients have been lost to follow-up.
In response, a group of tropical disease specialists who focus on neglected tropical diseases (NTDs) wrote an editorial, “Neglected Testing for Neglected Tropical Diseases at the CDC,” which recently appeared in the American Journal of Tropical Medicine and Hygiene (AJTMH).
NTDs are caused by viruses, bacteria, and parasites. They include leprosy and worms; many such diseases are disfiguring, such as filariasis (which causes the hugely swollen extremities of elephantiasis) and onchocerciasis (river blindness). They also include some viral and bacterial diseases. Their common denominator is that they are diseases of poverty, primarily in Africa, Asia, and Latin America, so they garner little attention from “first world” countries.
The loss of testing for two devastating parasites – Chagas and Leishmania – was particularly significant. Few other labs in the United States test for these, and the tests can be expensive and of variable quality, experts said.
Norman Beatty, MD, a global health physician at the University of Florida, told this news organization, “Chagas confirmatory testing is only available at the CDC and is the most reliable testing we have access to in the United States. Leishmania species identification is also only available at the CDC and is important in determining which antiparasitic medications we will use.”
Chagas disease is caused by the parasite Trypanosoma cruzi and is transmitted by triatomine bugs, also known as kissing bugs. Chagas is a major cause of an enlarged heart and congestive heart failure, as well as a dramatically enlarged esophagus or colon.
Prior to the cuts and before COVID-19, the CDC reported that they ran 10,000 to 15,000 tests for parasitic diseases annually. Testing requests declined during COVID. In 2021, they ran 1,003 tests for Chagas.
Dr. Beatty said that he first became aware of the CDC’s testing cuts last fall when he sought care for a patient. He was first told the delay would be 2-3 weeks, then another 2-3 weeks. It’s now been 7 months, and only three tests have been resumed.
Dr. Beatty added that for Chagas disease in particular, there is urgency in testing because cardiac complications can be life-threatening. He said that “a lot of these diseases can be considered rare, but they also have a tremendous ability to cause morbidity and mortality.”
Leishmania infections are also serious. Following the bite of an infected sandfly, they can cause disfiguring skin infections, but, more importantly, they can affect the liver, spleen, and bone marrow. Dr. Beatty said that since testing was dropped at the CDC, some colleagues had to send specimens outside of the country.
Dr. Beatty emphasized that the cuts in testing at the CDC highlight disparities in our society. “There are other commercial reference laboratories who may have some of these tests available, but the vast majority of people who suffer from diseases are underserved and vulnerable. [My patients] most definitely will not have access to advanced testing commercial laboratories,” Dr. Beatty said. Those laboratories include Associated Regional University Pathologists laboratories, Quest Diagnostics, and LabCorp Diagnostics. But for some parasitic infections, there will simply be no testing, and patients will not receive appropriate therapy.
The CDC’s website says, “USAID and CDC work together on a shared agenda to advance global progress towards the control and elimination of NTDs that can be addressed with preventive chemotherapy. ... CDC has strong working relationships with WHO, regional reference laboratories/bodies, [and] national NTD programs ... working with these partners through the provision of unique laboratory, diagnostic, and epidemiological technical assistance.”
The WHO Roadmap for 2030 aims to prevent and control many NTDs, in part by “providing new interventions and effective, standardized, and affordable diagnostics.” Last year, the CDC said that they “will continue working with WHO and other global partners to meet the established goals.”
But testing for a number of NTDs is not currently available at the CDC. In response to questions from this news organization, a CDC spokesperson said the agency “supports the development of country capacity for NTD testing required ... but does not perform testing related to the WHO Roadmap.”
A group of CDC officials wrote an editorial response that was published in AJTMH, saying the agency has “three main priorities: reducing parasitic disease-related death, illness, and disability in the United States; reducing the global burden of malaria; and eliminating targeted neglected tropical diseases.”
In response to this news organization’s interview request, a CDC spokesperson wrote, “CDC is unwavering in our commitment to provide the highest quality laboratory diagnostic services for parasitic diseases. We understand the concerns expressed in the editorial and the challenges the pause in testing for parasitic diseases presents for health care providers, particularly those treating people at elevated risk for parasitic diseases.”
Michael Reich, PhD, Dr. Beatty’s co-author, is an international health policy expert at Harvard. He and the physicians had approached CDC about the elimination of services. He said in an interview, “We’re still unable to get clear responses except for something along the lines of, ‘We are working on it. It is complicated. It takes time. We’re doing our best.’”
Dr. Reich added, “For me, this raises troubling issues both of transparency and accountability – transparency about what is going on and what the problems are, and accountability in terms of who’s being held responsible for the closures and the impacts on both public health and patient treatment.”
Dr. Beatty concluded, “I think the goal of our group was to bring more awareness to the importance of having a national laboratory that can service all people, even the most underserved and vulnerable populations.” He added, “Chagas disease is a disease of inequity in Latin Americans. Without having access to an appropriate laboratory such as the CDC, we would be taking a backwards approach to tackle neglected tropical diseases in our country and worldwide.”
Dr. Beatty and Dr. Reich report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention has long been the premier reference lab for the United States and, for some diseases, internationally.
In September 2021, the CDC stated on its website that it would stop testing for parasites, herpesvirus encephalitis, human herpesvirus 6 and 7, Epstein-Barr virus, and other viruses, saying, “We are working diligently to implement laboratory system improvements.”
At the time, the CDC said testing would be halted only for a few months.
In response to a query from this news organization, a CDC spokesperson replied, “While at present we are unable to share a detailed timeline, our highest priority is to resume high-quality testing operations in a phased, prioritized approach as soon as possible and to offer the same tests that were available before the pause.”
Several global health clinicians told this news organization that they were not aware of the halt and that they are now uncertain about the specific diagnosis and best treatment for some patients. Other patients have been lost to follow-up.
In response, a group of tropical disease specialists who focus on neglected tropical diseases (NTDs) wrote an editorial, “Neglected Testing for Neglected Tropical Diseases at the CDC,” which recently appeared in the American Journal of Tropical Medicine and Hygiene (AJTMH).
NTDs are caused by viruses, bacteria, and parasites. They include leprosy and worms; many such diseases are disfiguring, such as filariasis (which causes the hugely swollen extremities of elephantiasis) and onchocerciasis (river blindness). They also include some viral and bacterial diseases. Their common denominator is that they are diseases of poverty, primarily in Africa, Asia, and Latin America, so they garner little attention from “first world” countries.
The loss of testing for two devastating parasites – Chagas and Leishmania – was particularly significant. Few other labs in the United States test for these, and the tests can be expensive and of variable quality, experts said.
Norman Beatty, MD, a global health physician at the University of Florida, told this news organization, “Chagas confirmatory testing is only available at the CDC and is the most reliable testing we have access to in the United States. Leishmania species identification is also only available at the CDC and is important in determining which antiparasitic medications we will use.”
Chagas disease is caused by the parasite Trypanosoma cruzi and is transmitted by triatomine bugs, also known as kissing bugs. Chagas is a major cause of an enlarged heart and congestive heart failure, as well as a dramatically enlarged esophagus or colon.
Prior to the cuts and before COVID-19, the CDC reported that they ran 10,000 to 15,000 tests for parasitic diseases annually. Testing requests declined during COVID. In 2021, they ran 1,003 tests for Chagas.
Dr. Beatty said that he first became aware of the CDC’s testing cuts last fall when he sought care for a patient. He was first told the delay would be 2-3 weeks, then another 2-3 weeks. It’s now been 7 months, and only three tests have been resumed.
Dr. Beatty added that for Chagas disease in particular, there is urgency in testing because cardiac complications can be life-threatening. He said that “a lot of these diseases can be considered rare, but they also have a tremendous ability to cause morbidity and mortality.”
Leishmania infections are also serious. Following the bite of an infected sandfly, they can cause disfiguring skin infections, but, more importantly, they can affect the liver, spleen, and bone marrow. Dr. Beatty said that since testing was dropped at the CDC, some colleagues had to send specimens outside of the country.
Dr. Beatty emphasized that the cuts in testing at the CDC highlight disparities in our society. “There are other commercial reference laboratories who may have some of these tests available, but the vast majority of people who suffer from diseases are underserved and vulnerable. [My patients] most definitely will not have access to advanced testing commercial laboratories,” Dr. Beatty said. Those laboratories include Associated Regional University Pathologists laboratories, Quest Diagnostics, and LabCorp Diagnostics. But for some parasitic infections, there will simply be no testing, and patients will not receive appropriate therapy.
The CDC’s website says, “USAID and CDC work together on a shared agenda to advance global progress towards the control and elimination of NTDs that can be addressed with preventive chemotherapy. ... CDC has strong working relationships with WHO, regional reference laboratories/bodies, [and] national NTD programs ... working with these partners through the provision of unique laboratory, diagnostic, and epidemiological technical assistance.”
The WHO Roadmap for 2030 aims to prevent and control many NTDs, in part by “providing new interventions and effective, standardized, and affordable diagnostics.” Last year, the CDC said that they “will continue working with WHO and other global partners to meet the established goals.”
But testing for a number of NTDs is not currently available at the CDC. In response to questions from this news organization, a CDC spokesperson said the agency “supports the development of country capacity for NTD testing required ... but does not perform testing related to the WHO Roadmap.”
A group of CDC officials wrote an editorial response that was published in AJTMH, saying the agency has “three main priorities: reducing parasitic disease-related death, illness, and disability in the United States; reducing the global burden of malaria; and eliminating targeted neglected tropical diseases.”
In response to this news organization’s interview request, a CDC spokesperson wrote, “CDC is unwavering in our commitment to provide the highest quality laboratory diagnostic services for parasitic diseases. We understand the concerns expressed in the editorial and the challenges the pause in testing for parasitic diseases presents for health care providers, particularly those treating people at elevated risk for parasitic diseases.”
Michael Reich, PhD, Dr. Beatty’s co-author, is an international health policy expert at Harvard. He and the physicians had approached CDC about the elimination of services. He said in an interview, “We’re still unable to get clear responses except for something along the lines of, ‘We are working on it. It is complicated. It takes time. We’re doing our best.’”
Dr. Reich added, “For me, this raises troubling issues both of transparency and accountability – transparency about what is going on and what the problems are, and accountability in terms of who’s being held responsible for the closures and the impacts on both public health and patient treatment.”
Dr. Beatty concluded, “I think the goal of our group was to bring more awareness to the importance of having a national laboratory that can service all people, even the most underserved and vulnerable populations.” He added, “Chagas disease is a disease of inequity in Latin Americans. Without having access to an appropriate laboratory such as the CDC, we would be taking a backwards approach to tackle neglected tropical diseases in our country and worldwide.”
Dr. Beatty and Dr. Reich report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention has long been the premier reference lab for the United States and, for some diseases, internationally.
In September 2021, the CDC stated on its website that it would stop testing for parasites, herpesvirus encephalitis, human herpesvirus 6 and 7, Epstein-Barr virus, and other viruses, saying, “We are working diligently to implement laboratory system improvements.”
At the time, the CDC said testing would be halted only for a few months.
In response to a query from this news organization, a CDC spokesperson replied, “While at present we are unable to share a detailed timeline, our highest priority is to resume high-quality testing operations in a phased, prioritized approach as soon as possible and to offer the same tests that were available before the pause.”
Several global health clinicians told this news organization that they were not aware of the halt and that they are now uncertain about the specific diagnosis and best treatment for some patients. Other patients have been lost to follow-up.
In response, a group of tropical disease specialists who focus on neglected tropical diseases (NTDs) wrote an editorial, “Neglected Testing for Neglected Tropical Diseases at the CDC,” which recently appeared in the American Journal of Tropical Medicine and Hygiene (AJTMH).
NTDs are caused by viruses, bacteria, and parasites. They include leprosy and worms; many such diseases are disfiguring, such as filariasis (which causes the hugely swollen extremities of elephantiasis) and onchocerciasis (river blindness). They also include some viral and bacterial diseases. Their common denominator is that they are diseases of poverty, primarily in Africa, Asia, and Latin America, so they garner little attention from “first world” countries.
The loss of testing for two devastating parasites – Chagas and Leishmania – was particularly significant. Few other labs in the United States test for these, and the tests can be expensive and of variable quality, experts said.
Norman Beatty, MD, a global health physician at the University of Florida, told this news organization, “Chagas confirmatory testing is only available at the CDC and is the most reliable testing we have access to in the United States. Leishmania species identification is also only available at the CDC and is important in determining which antiparasitic medications we will use.”
Chagas disease is caused by the parasite Trypanosoma cruzi and is transmitted by triatomine bugs, also known as kissing bugs. Chagas is a major cause of an enlarged heart and congestive heart failure, as well as a dramatically enlarged esophagus or colon.
Prior to the cuts and before COVID-19, the CDC reported that they ran 10,000 to 15,000 tests for parasitic diseases annually. Testing requests declined during COVID. In 2021, they ran 1,003 tests for Chagas.
Dr. Beatty said that he first became aware of the CDC’s testing cuts last fall when he sought care for a patient. He was first told the delay would be 2-3 weeks, then another 2-3 weeks. It’s now been 7 months, and only three tests have been resumed.
Dr. Beatty added that for Chagas disease in particular, there is urgency in testing because cardiac complications can be life-threatening. He said that “a lot of these diseases can be considered rare, but they also have a tremendous ability to cause morbidity and mortality.”
Leishmania infections are also serious. Following the bite of an infected sandfly, they can cause disfiguring skin infections, but, more importantly, they can affect the liver, spleen, and bone marrow. Dr. Beatty said that since testing was dropped at the CDC, some colleagues had to send specimens outside of the country.
Dr. Beatty emphasized that the cuts in testing at the CDC highlight disparities in our society. “There are other commercial reference laboratories who may have some of these tests available, but the vast majority of people who suffer from diseases are underserved and vulnerable. [My patients] most definitely will not have access to advanced testing commercial laboratories,” Dr. Beatty said. Those laboratories include Associated Regional University Pathologists laboratories, Quest Diagnostics, and LabCorp Diagnostics. But for some parasitic infections, there will simply be no testing, and patients will not receive appropriate therapy.
The CDC’s website says, “USAID and CDC work together on a shared agenda to advance global progress towards the control and elimination of NTDs that can be addressed with preventive chemotherapy. ... CDC has strong working relationships with WHO, regional reference laboratories/bodies, [and] national NTD programs ... working with these partners through the provision of unique laboratory, diagnostic, and epidemiological technical assistance.”
The WHO Roadmap for 2030 aims to prevent and control many NTDs, in part by “providing new interventions and effective, standardized, and affordable diagnostics.” Last year, the CDC said that they “will continue working with WHO and other global partners to meet the established goals.”
But testing for a number of NTDs is not currently available at the CDC. In response to questions from this news organization, a CDC spokesperson said the agency “supports the development of country capacity for NTD testing required ... but does not perform testing related to the WHO Roadmap.”
A group of CDC officials wrote an editorial response that was published in AJTMH, saying the agency has “three main priorities: reducing parasitic disease-related death, illness, and disability in the United States; reducing the global burden of malaria; and eliminating targeted neglected tropical diseases.”
In response to this news organization’s interview request, a CDC spokesperson wrote, “CDC is unwavering in our commitment to provide the highest quality laboratory diagnostic services for parasitic diseases. We understand the concerns expressed in the editorial and the challenges the pause in testing for parasitic diseases presents for health care providers, particularly those treating people at elevated risk for parasitic diseases.”
Michael Reich, PhD, Dr. Beatty’s co-author, is an international health policy expert at Harvard. He and the physicians had approached CDC about the elimination of services. He said in an interview, “We’re still unable to get clear responses except for something along the lines of, ‘We are working on it. It is complicated. It takes time. We’re doing our best.’”
Dr. Reich added, “For me, this raises troubling issues both of transparency and accountability – transparency about what is going on and what the problems are, and accountability in terms of who’s being held responsible for the closures and the impacts on both public health and patient treatment.”
Dr. Beatty concluded, “I think the goal of our group was to bring more awareness to the importance of having a national laboratory that can service all people, even the most underserved and vulnerable populations.” He added, “Chagas disease is a disease of inequity in Latin Americans. Without having access to an appropriate laboratory such as the CDC, we would be taking a backwards approach to tackle neglected tropical diseases in our country and worldwide.”
Dr. Beatty and Dr. Reich report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CDC panel lists reasons to get second COVID booster
The Centers for Disease Control and Prevention is considering what to tell the public about second booster shots with mRNA vaccinations for COVID-19.
The U.S. Food and Drug Administration in March authorized a second booster dose of either the Pfizer-BioNTech or the Moderna COVID-19 vaccines for people aged 50 and older and certain immunocompromised adults, even though many top infectious disease experts questioned the need before the agency’s decision.
In a meeting April 20, the CDC asked its Advisory Committee on Immunization Practices to discuss second booster shots, but did not ask the group of experts to vote on formal recommendations.
Instead,
ACIP member Beth Bell, MD, MPH, of the University of Washington, Seattle, said she’s concerned about the potential for “booster fatigue.”
“A vaccination program that’s going to require boosting large proportions of the population every 4-6 months is really not sustainable and probably not something that most people want to participate in,” she said.
The benefit of additional COVID-19 shots for now appears to be smaller than what people get from the initial doses, Dr. Bell said.
Earlier in the meeting, CDC staff presented estimates about how well the COVID-19 vaccines work to prevent one case of hospitalization from the disease over 4 months among people aged 50 and older.
The major gain in preventing hospitalizations occurs with the first vaccination series and then wanes, the CDC said.
It appears that one hospitalization is prevented for every 135 people who get the first round of COVID-19 vaccinations. But it takes 674 people getting a first booster dose to prevent one hospitalization. A second booster prevents one hospitalization for every 1,205 people vaccinated.
Dr. Bell said she’s concerned about considering additional doses for “smaller and smaller return and creating an impression that we don’t have a very effective vaccination program,” even though the CDC’s data show a clear benefit.
Reasons to get a second booster
Elisha Hall, PhD, RD, of the CDC presented slides with some factors to help determine the urgency for a person to get a second booster:
- Having certain underlying medical conditions that increase the risk of severe COVID-19 illness.
- Being moderately or severely immunocompromised.
- Living with someone who is immunocompromised, at increased risk for severe disease, or who cannot be vaccinated because of age or contraindication.
- Being at increased risk of exposure to SARS-CoV-2, the virus that causes COVID-19, such as through occupational, institutional, or other activities (e.g., travel or large gatherings).
- Living or working in an area where there is a medium or high level of COVID-19 in the community.
In contrast, people might want to wait if they had been infected with SARS-CoV-2 within the past 3 months, Dr. Hall said in her presentation. Another reason for delay might be a concern that a booster dose may be more important later in the year.
The experts also addressed public confusion over boosters. For the Pfizer and Moderna mRNA vaccines, a second booster is a fourth dose, but for those who received the one-shot J&J vaccine, the second booster is a third dose.
Going forward, it may be easier to refer to subsequent doses as “annual boosters,” the CDC’s Sara Oliver, MD, MSPH, told the panel. It will be important to keep language about subsequent vaccinations clear and easy for the public to follow, she said.
Dr. Oliver also said there’s already been a drop-off in the acceptance of second rounds of COVID-19 vaccinations. CDC data show that 77% of people in the United States have had at least one dose of a COVID-19 vaccine, but only 66% of the population is fully vaccinated, and only 45% have had a first booster dose.
In her presentation, Dr. Oliver said the top priority in COVID-19 vaccination efforts remains initial vaccinations for people who haven’t gotten them.
Kids younger than 5
During the public comment session of the CDC meeting, several people called on the FDA to move quickly to expand authorization of COVID-19 vaccines to children aged 5 years and younger.
“We know that many parents and caregivers and health care providers are anxious to have COVID vaccines available” for young children, said Doran Fink, MD, PhD, a deputy director of the FDA’s vaccines division.
He said the agency is working to be ready to authorize the shots for young children while it awaits research results from the manufacturers.
A version of this article first appeared on WebMD.com.
The Centers for Disease Control and Prevention is considering what to tell the public about second booster shots with mRNA vaccinations for COVID-19.
The U.S. Food and Drug Administration in March authorized a second booster dose of either the Pfizer-BioNTech or the Moderna COVID-19 vaccines for people aged 50 and older and certain immunocompromised adults, even though many top infectious disease experts questioned the need before the agency’s decision.
In a meeting April 20, the CDC asked its Advisory Committee on Immunization Practices to discuss second booster shots, but did not ask the group of experts to vote on formal recommendations.
Instead,
ACIP member Beth Bell, MD, MPH, of the University of Washington, Seattle, said she’s concerned about the potential for “booster fatigue.”
“A vaccination program that’s going to require boosting large proportions of the population every 4-6 months is really not sustainable and probably not something that most people want to participate in,” she said.
The benefit of additional COVID-19 shots for now appears to be smaller than what people get from the initial doses, Dr. Bell said.
Earlier in the meeting, CDC staff presented estimates about how well the COVID-19 vaccines work to prevent one case of hospitalization from the disease over 4 months among people aged 50 and older.
The major gain in preventing hospitalizations occurs with the first vaccination series and then wanes, the CDC said.
It appears that one hospitalization is prevented for every 135 people who get the first round of COVID-19 vaccinations. But it takes 674 people getting a first booster dose to prevent one hospitalization. A second booster prevents one hospitalization for every 1,205 people vaccinated.
Dr. Bell said she’s concerned about considering additional doses for “smaller and smaller return and creating an impression that we don’t have a very effective vaccination program,” even though the CDC’s data show a clear benefit.
Reasons to get a second booster
Elisha Hall, PhD, RD, of the CDC presented slides with some factors to help determine the urgency for a person to get a second booster:
- Having certain underlying medical conditions that increase the risk of severe COVID-19 illness.
- Being moderately or severely immunocompromised.
- Living with someone who is immunocompromised, at increased risk for severe disease, or who cannot be vaccinated because of age or contraindication.
- Being at increased risk of exposure to SARS-CoV-2, the virus that causes COVID-19, such as through occupational, institutional, or other activities (e.g., travel or large gatherings).
- Living or working in an area where there is a medium or high level of COVID-19 in the community.
In contrast, people might want to wait if they had been infected with SARS-CoV-2 within the past 3 months, Dr. Hall said in her presentation. Another reason for delay might be a concern that a booster dose may be more important later in the year.
The experts also addressed public confusion over boosters. For the Pfizer and Moderna mRNA vaccines, a second booster is a fourth dose, but for those who received the one-shot J&J vaccine, the second booster is a third dose.
Going forward, it may be easier to refer to subsequent doses as “annual boosters,” the CDC’s Sara Oliver, MD, MSPH, told the panel. It will be important to keep language about subsequent vaccinations clear and easy for the public to follow, she said.
Dr. Oliver also said there’s already been a drop-off in the acceptance of second rounds of COVID-19 vaccinations. CDC data show that 77% of people in the United States have had at least one dose of a COVID-19 vaccine, but only 66% of the population is fully vaccinated, and only 45% have had a first booster dose.
In her presentation, Dr. Oliver said the top priority in COVID-19 vaccination efforts remains initial vaccinations for people who haven’t gotten them.
Kids younger than 5
During the public comment session of the CDC meeting, several people called on the FDA to move quickly to expand authorization of COVID-19 vaccines to children aged 5 years and younger.
“We know that many parents and caregivers and health care providers are anxious to have COVID vaccines available” for young children, said Doran Fink, MD, PhD, a deputy director of the FDA’s vaccines division.
He said the agency is working to be ready to authorize the shots for young children while it awaits research results from the manufacturers.
A version of this article first appeared on WebMD.com.
The Centers for Disease Control and Prevention is considering what to tell the public about second booster shots with mRNA vaccinations for COVID-19.
The U.S. Food and Drug Administration in March authorized a second booster dose of either the Pfizer-BioNTech or the Moderna COVID-19 vaccines for people aged 50 and older and certain immunocompromised adults, even though many top infectious disease experts questioned the need before the agency’s decision.
In a meeting April 20, the CDC asked its Advisory Committee on Immunization Practices to discuss second booster shots, but did not ask the group of experts to vote on formal recommendations.
Instead,
ACIP member Beth Bell, MD, MPH, of the University of Washington, Seattle, said she’s concerned about the potential for “booster fatigue.”
“A vaccination program that’s going to require boosting large proportions of the population every 4-6 months is really not sustainable and probably not something that most people want to participate in,” she said.
The benefit of additional COVID-19 shots for now appears to be smaller than what people get from the initial doses, Dr. Bell said.
Earlier in the meeting, CDC staff presented estimates about how well the COVID-19 vaccines work to prevent one case of hospitalization from the disease over 4 months among people aged 50 and older.
The major gain in preventing hospitalizations occurs with the first vaccination series and then wanes, the CDC said.
It appears that one hospitalization is prevented for every 135 people who get the first round of COVID-19 vaccinations. But it takes 674 people getting a first booster dose to prevent one hospitalization. A second booster prevents one hospitalization for every 1,205 people vaccinated.
Dr. Bell said she’s concerned about considering additional doses for “smaller and smaller return and creating an impression that we don’t have a very effective vaccination program,” even though the CDC’s data show a clear benefit.
Reasons to get a second booster
Elisha Hall, PhD, RD, of the CDC presented slides with some factors to help determine the urgency for a person to get a second booster:
- Having certain underlying medical conditions that increase the risk of severe COVID-19 illness.
- Being moderately or severely immunocompromised.
- Living with someone who is immunocompromised, at increased risk for severe disease, or who cannot be vaccinated because of age or contraindication.
- Being at increased risk of exposure to SARS-CoV-2, the virus that causes COVID-19, such as through occupational, institutional, or other activities (e.g., travel or large gatherings).
- Living or working in an area where there is a medium or high level of COVID-19 in the community.
In contrast, people might want to wait if they had been infected with SARS-CoV-2 within the past 3 months, Dr. Hall said in her presentation. Another reason for delay might be a concern that a booster dose may be more important later in the year.
The experts also addressed public confusion over boosters. For the Pfizer and Moderna mRNA vaccines, a second booster is a fourth dose, but for those who received the one-shot J&J vaccine, the second booster is a third dose.
Going forward, it may be easier to refer to subsequent doses as “annual boosters,” the CDC’s Sara Oliver, MD, MSPH, told the panel. It will be important to keep language about subsequent vaccinations clear and easy for the public to follow, she said.
Dr. Oliver also said there’s already been a drop-off in the acceptance of second rounds of COVID-19 vaccinations. CDC data show that 77% of people in the United States have had at least one dose of a COVID-19 vaccine, but only 66% of the population is fully vaccinated, and only 45% have had a first booster dose.
In her presentation, Dr. Oliver said the top priority in COVID-19 vaccination efforts remains initial vaccinations for people who haven’t gotten them.
Kids younger than 5
During the public comment session of the CDC meeting, several people called on the FDA to move quickly to expand authorization of COVID-19 vaccines to children aged 5 years and younger.
“We know that many parents and caregivers and health care providers are anxious to have COVID vaccines available” for young children, said Doran Fink, MD, PhD, a deputy director of the FDA’s vaccines division.
He said the agency is working to be ready to authorize the shots for young children while it awaits research results from the manufacturers.
A version of this article first appeared on WebMD.com.
FDA warns companies selling OTC skin lighteners
The as the active ingredient, and don’t meet the requirements to be sold legally over the counter. The letters were dated April 13.
The 12 products with hydroquinone are “unapproved drugs and are not generally recognized as safe and effective” (abbreviated as GRASE), the FDA said.
Among the side effects associated with hydroquinone products reported to the FDA are skin rashes, facial swelling, and skin discoloration or ochronosis. The discoloration can be permanent, the FDA said. The lighteners are marketed for use on age or dark spots on the skin associated with melasma.
Tri-Luma, a prescription product for the treatment of moderate to severe melasma of the face, is the only FDA-approved drug containing hydroquinone, according to the FDA. It contains 4% hydroquinone and two other ingredients. It is meant to be used under the supervision of a health care professional. Tri-Luma is indicated for up to 8 weeks of treatment for moderate to severe melasma of the face. The OTC products contain up to 2%. (Generic versions of 4% hydroquinone are available by prescription, dermatologists said.)
“Hydroquinone is a very effective medication, and that’s exactly what it is, a medication,” said Lily Talakoub, MD, a dermatologist in McLean, Va., who supports the FDA action. “It’s very effective and very safe to use in the right hands, but when it is overused or used in the wrong situation, it can cause problems.” Those problems often occur, she said, when there is no health care professional overseeing the use of the OTC products, and when people use them over the long term.
The FDA action to ban the OTC products is “very appropriate,” said dermatologist Pooja Sodha, MD, assistant professor and director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington. “We know patients pick this up [an OTC product] and use it without physician oversight.” When patients use the products longer than is appropriate, which is also common, it can worsen the initial skin issue, she said.
The action follows reforms finalized under the CARES Act (Coronavirus Aid, Relief and Economic Security Act), which included not only COVID-19 response efforts but also updated the method in which certain OTC drugs are regulated. Manufacturers of the skin lightening products that don’t have FDA approval had been told to remove the products from the market by September 2020.
The recent letters were sent to a dozen companies still marketing their products without an FDA new drug approval. The agency asked the companies to take prompt action and respond with 15 days, stating what they have done to correct the violations.
The 12 companies are AMBI Enterprises, Clinical Formula, Elements Brands Inc., Genomma Lab USA, Intilight/Dr Thomas Balshi, M&M Beauty and Wellness, Neoteric Cosmetics/Scott’s Liquid Gold, Skin Authority, Skin Pro, Skin PS Brands, True Earth Health Products, and Ultimark Products.
Health care professionals and consumers can report adverse reactions associated with these products to the FDA’s MedWatch Adverse Event Reporting program.
A version of this article first appeared on Medscape.com.
The as the active ingredient, and don’t meet the requirements to be sold legally over the counter. The letters were dated April 13.
The 12 products with hydroquinone are “unapproved drugs and are not generally recognized as safe and effective” (abbreviated as GRASE), the FDA said.
Among the side effects associated with hydroquinone products reported to the FDA are skin rashes, facial swelling, and skin discoloration or ochronosis. The discoloration can be permanent, the FDA said. The lighteners are marketed for use on age or dark spots on the skin associated with melasma.
Tri-Luma, a prescription product for the treatment of moderate to severe melasma of the face, is the only FDA-approved drug containing hydroquinone, according to the FDA. It contains 4% hydroquinone and two other ingredients. It is meant to be used under the supervision of a health care professional. Tri-Luma is indicated for up to 8 weeks of treatment for moderate to severe melasma of the face. The OTC products contain up to 2%. (Generic versions of 4% hydroquinone are available by prescription, dermatologists said.)
“Hydroquinone is a very effective medication, and that’s exactly what it is, a medication,” said Lily Talakoub, MD, a dermatologist in McLean, Va., who supports the FDA action. “It’s very effective and very safe to use in the right hands, but when it is overused or used in the wrong situation, it can cause problems.” Those problems often occur, she said, when there is no health care professional overseeing the use of the OTC products, and when people use them over the long term.
The FDA action to ban the OTC products is “very appropriate,” said dermatologist Pooja Sodha, MD, assistant professor and director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington. “We know patients pick this up [an OTC product] and use it without physician oversight.” When patients use the products longer than is appropriate, which is also common, it can worsen the initial skin issue, she said.
The action follows reforms finalized under the CARES Act (Coronavirus Aid, Relief and Economic Security Act), which included not only COVID-19 response efforts but also updated the method in which certain OTC drugs are regulated. Manufacturers of the skin lightening products that don’t have FDA approval had been told to remove the products from the market by September 2020.
The recent letters were sent to a dozen companies still marketing their products without an FDA new drug approval. The agency asked the companies to take prompt action and respond with 15 days, stating what they have done to correct the violations.
The 12 companies are AMBI Enterprises, Clinical Formula, Elements Brands Inc., Genomma Lab USA, Intilight/Dr Thomas Balshi, M&M Beauty and Wellness, Neoteric Cosmetics/Scott’s Liquid Gold, Skin Authority, Skin Pro, Skin PS Brands, True Earth Health Products, and Ultimark Products.
Health care professionals and consumers can report adverse reactions associated with these products to the FDA’s MedWatch Adverse Event Reporting program.
A version of this article first appeared on Medscape.com.
The as the active ingredient, and don’t meet the requirements to be sold legally over the counter. The letters were dated April 13.
The 12 products with hydroquinone are “unapproved drugs and are not generally recognized as safe and effective” (abbreviated as GRASE), the FDA said.
Among the side effects associated with hydroquinone products reported to the FDA are skin rashes, facial swelling, and skin discoloration or ochronosis. The discoloration can be permanent, the FDA said. The lighteners are marketed for use on age or dark spots on the skin associated with melasma.
Tri-Luma, a prescription product for the treatment of moderate to severe melasma of the face, is the only FDA-approved drug containing hydroquinone, according to the FDA. It contains 4% hydroquinone and two other ingredients. It is meant to be used under the supervision of a health care professional. Tri-Luma is indicated for up to 8 weeks of treatment for moderate to severe melasma of the face. The OTC products contain up to 2%. (Generic versions of 4% hydroquinone are available by prescription, dermatologists said.)
“Hydroquinone is a very effective medication, and that’s exactly what it is, a medication,” said Lily Talakoub, MD, a dermatologist in McLean, Va., who supports the FDA action. “It’s very effective and very safe to use in the right hands, but when it is overused or used in the wrong situation, it can cause problems.” Those problems often occur, she said, when there is no health care professional overseeing the use of the OTC products, and when people use them over the long term.
The FDA action to ban the OTC products is “very appropriate,” said dermatologist Pooja Sodha, MD, assistant professor and director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington. “We know patients pick this up [an OTC product] and use it without physician oversight.” When patients use the products longer than is appropriate, which is also common, it can worsen the initial skin issue, she said.
The action follows reforms finalized under the CARES Act (Coronavirus Aid, Relief and Economic Security Act), which included not only COVID-19 response efforts but also updated the method in which certain OTC drugs are regulated. Manufacturers of the skin lightening products that don’t have FDA approval had been told to remove the products from the market by September 2020.
The recent letters were sent to a dozen companies still marketing their products without an FDA new drug approval. The agency asked the companies to take prompt action and respond with 15 days, stating what they have done to correct the violations.
The 12 companies are AMBI Enterprises, Clinical Formula, Elements Brands Inc., Genomma Lab USA, Intilight/Dr Thomas Balshi, M&M Beauty and Wellness, Neoteric Cosmetics/Scott’s Liquid Gold, Skin Authority, Skin Pro, Skin PS Brands, True Earth Health Products, and Ultimark Products.
Health care professionals and consumers can report adverse reactions associated with these products to the FDA’s MedWatch Adverse Event Reporting program.
A version of this article first appeared on Medscape.com.
FDA to decide by June on future of COVID vaccines
April 6.
But members of the panel also acknowledged that it will be an uphill battle to reach that goal, especially given how quickly the virus continues to change.
The members of the Vaccines and Related Biological Products Advisory Committee said they want to find the balance that makes sure Americans are protected against severe illness and death but doesn’t wear them out with constant recommendations for boosters.
“We don’t feel comfortable with multiple boosters every 8 weeks,” said committee chairman Arnold Monto, MD, professor emeritus of public health at the University of Michigan, Ann Arbor. “We’d love to see an annual vaccination similar to influenza but realize that the evolution of the virus will dictate how we respond in terms of additional vaccine doses.”
The virus itself will dictate vaccination plans, he said.
The government must also keep its focus on convincing Americans who haven’t been vaccinated to join the club, said committee member Henry H. Bernstein, DO, given that “it seems quite obvious that those who are vaccinated do better than those who aren’t vaccinated.”
The government should clearly communicate to the public the goals of vaccination, he said.
“I would suggest that our overall aim is to prevent severe disease, hospitalization, and death more than just infection prevention,” said Dr. Bernstein, professor of pediatrics at Hofstra University, Hempstead, N.Y.
The FDA called the meeting of its advisers to discuss overall booster and vaccine strategy, even though it already authorized a fourth dose of the Pfizer and Moderna vaccines for certain immune compromised adults and for everyone over age 50.
Early in the all-day meeting, temporary committee member James Hildreth, MD, the president of Meharry Medical College, Nashville, Tenn., asked why that authorization was given without the panel’s input. Peter Marks, MD, the director of FDA’s Center for Biologics Evaluation and Research, said the decision was based on data from the United Kingdom and Israel that suggested immunity from a third shot was already waning.
Dr. Marks later said the fourth dose was “authorized as a stopgap measure until we could get something else in place,” because the aim was to protect older Americans who had died at a higher rate than younger individuals.
“I think we’re very much on board that we simply can’t be boosting people as frequently as we are,” said Dr. Marks.
Not enough information to make broader plan
The meeting was meant to be a larger conversation about how to keep pace with the evolving virus and to set up a vaccine selection and development process to better and more quickly respond to changes, such as new variants.
But committee members said they felt stymied by a lack of information. They wanted more data from vaccine manufacturers’ clinical trials. And they noted that so far, there’s no objective, reliable lab-based measurement of COVID-19 vaccine effectiveness – known as a correlate of immunity. Instead, public health officials have looked at rates of hospitalizations and deaths to measure whether the vaccine is still offering protection.
“The question is, what is insufficient protection?” asked H. Cody Meissner, MD, director of pediatric infectious disease at Tufts Medical Center in Boston. “At what point will we say the vaccine isn’t working well enough?”
Centers for Disease Control and Prevention officials presented data showing that a third shot has been more effective than a two-shot regimen in preventing serious disease and death, and that the three shots were significantly more protective than being unvaccinated.
In February, as the Omicron variant continued to rage, unvaccinated Americans aged 5 years and older had an almost three times higher risk of testing positive, and nine times higher risk of dying, compared with those who were considered fully vaccinated, said Heather Scobie, PhD, MPH, a member of the CDC’s COVID-19 Emergency Response team.
But only 98 million Americans – about half of those aged 12 years or older – have received a third dose, Dr. Scobie said.
It’s also still not clear how much more protection a fourth shot adds, or how long it will last. The committee heard data on a just-published study of a fourth dose of the Pfizer vaccine given to some 600,000 Israelis during the Omicron wave from January to March. The rate of severe COVID-19 was 3.5 times lower in the group that received a fourth dose, compared with those who had gotten only three shots, and protection lasted for at least 12 weeks.
Still, study authors said, any protection against infection itself was “short lived.”
More like flu vaccine?
The advisers discussed the possibility of making COVID-19 vaccine development similar to the process for the flu vaccine but acknowledged many difficulties.
The flu predictably hits during the winter in each hemisphere and a global surveillance network helps the World Health Organization decide on the vaccine strains each year. Then each nation’s regulatory and public health officials choose the strains for their shot and vaccine makers begin what is typically a 6-month-long manufacturing process.
COVID outbreaks have happened during all seasons and new variants haven’t always hit every country in a similar fashion. The COVID virus has mutated at five times the speed of the flu virus – producing a new dominant strain in a year, compared with the 3-5 years it takes for the flu virus to do so, said Trevor Bedford, PhD, a professor in the vaccine and infectious disease division at the Fred Hutchinson Cancer Research Center in Seattle.
Global COVID surveillance is patchy and the WHO has not yet created a program to help select strains for a COVID-19 vaccine but is working on a process. Currently, vaccine makers seem to be driving vaccine strain selection, said panelist Paul Offit, MD, professor of paediatrics at Children’s Hospital of Philadelphia. “I feel like to some extent the companies dictate the conversation. It shouldn’t come from them. It should come from us.”
“The important thing is that the public understands how complex this is,” said temporary committee member Oveta A. Fuller, PhD, associate professor of microbiology and immunology at the University of Michigan. “We didn’t get to understand influenza in 2 years. It’s taken years to get an imperfect but useful process to deal with flu.”
A version of this article first appeared on WebMD.com.
April 6.
But members of the panel also acknowledged that it will be an uphill battle to reach that goal, especially given how quickly the virus continues to change.
The members of the Vaccines and Related Biological Products Advisory Committee said they want to find the balance that makes sure Americans are protected against severe illness and death but doesn’t wear them out with constant recommendations for boosters.
“We don’t feel comfortable with multiple boosters every 8 weeks,” said committee chairman Arnold Monto, MD, professor emeritus of public health at the University of Michigan, Ann Arbor. “We’d love to see an annual vaccination similar to influenza but realize that the evolution of the virus will dictate how we respond in terms of additional vaccine doses.”
The virus itself will dictate vaccination plans, he said.
The government must also keep its focus on convincing Americans who haven’t been vaccinated to join the club, said committee member Henry H. Bernstein, DO, given that “it seems quite obvious that those who are vaccinated do better than those who aren’t vaccinated.”
The government should clearly communicate to the public the goals of vaccination, he said.
“I would suggest that our overall aim is to prevent severe disease, hospitalization, and death more than just infection prevention,” said Dr. Bernstein, professor of pediatrics at Hofstra University, Hempstead, N.Y.
The FDA called the meeting of its advisers to discuss overall booster and vaccine strategy, even though it already authorized a fourth dose of the Pfizer and Moderna vaccines for certain immune compromised adults and for everyone over age 50.
Early in the all-day meeting, temporary committee member James Hildreth, MD, the president of Meharry Medical College, Nashville, Tenn., asked why that authorization was given without the panel’s input. Peter Marks, MD, the director of FDA’s Center for Biologics Evaluation and Research, said the decision was based on data from the United Kingdom and Israel that suggested immunity from a third shot was already waning.
Dr. Marks later said the fourth dose was “authorized as a stopgap measure until we could get something else in place,” because the aim was to protect older Americans who had died at a higher rate than younger individuals.
“I think we’re very much on board that we simply can’t be boosting people as frequently as we are,” said Dr. Marks.
Not enough information to make broader plan
The meeting was meant to be a larger conversation about how to keep pace with the evolving virus and to set up a vaccine selection and development process to better and more quickly respond to changes, such as new variants.
But committee members said they felt stymied by a lack of information. They wanted more data from vaccine manufacturers’ clinical trials. And they noted that so far, there’s no objective, reliable lab-based measurement of COVID-19 vaccine effectiveness – known as a correlate of immunity. Instead, public health officials have looked at rates of hospitalizations and deaths to measure whether the vaccine is still offering protection.
“The question is, what is insufficient protection?” asked H. Cody Meissner, MD, director of pediatric infectious disease at Tufts Medical Center in Boston. “At what point will we say the vaccine isn’t working well enough?”
Centers for Disease Control and Prevention officials presented data showing that a third shot has been more effective than a two-shot regimen in preventing serious disease and death, and that the three shots were significantly more protective than being unvaccinated.
In February, as the Omicron variant continued to rage, unvaccinated Americans aged 5 years and older had an almost three times higher risk of testing positive, and nine times higher risk of dying, compared with those who were considered fully vaccinated, said Heather Scobie, PhD, MPH, a member of the CDC’s COVID-19 Emergency Response team.
But only 98 million Americans – about half of those aged 12 years or older – have received a third dose, Dr. Scobie said.
It’s also still not clear how much more protection a fourth shot adds, or how long it will last. The committee heard data on a just-published study of a fourth dose of the Pfizer vaccine given to some 600,000 Israelis during the Omicron wave from January to March. The rate of severe COVID-19 was 3.5 times lower in the group that received a fourth dose, compared with those who had gotten only three shots, and protection lasted for at least 12 weeks.
Still, study authors said, any protection against infection itself was “short lived.”
More like flu vaccine?
The advisers discussed the possibility of making COVID-19 vaccine development similar to the process for the flu vaccine but acknowledged many difficulties.
The flu predictably hits during the winter in each hemisphere and a global surveillance network helps the World Health Organization decide on the vaccine strains each year. Then each nation’s regulatory and public health officials choose the strains for their shot and vaccine makers begin what is typically a 6-month-long manufacturing process.
COVID outbreaks have happened during all seasons and new variants haven’t always hit every country in a similar fashion. The COVID virus has mutated at five times the speed of the flu virus – producing a new dominant strain in a year, compared with the 3-5 years it takes for the flu virus to do so, said Trevor Bedford, PhD, a professor in the vaccine and infectious disease division at the Fred Hutchinson Cancer Research Center in Seattle.
Global COVID surveillance is patchy and the WHO has not yet created a program to help select strains for a COVID-19 vaccine but is working on a process. Currently, vaccine makers seem to be driving vaccine strain selection, said panelist Paul Offit, MD, professor of paediatrics at Children’s Hospital of Philadelphia. “I feel like to some extent the companies dictate the conversation. It shouldn’t come from them. It should come from us.”
“The important thing is that the public understands how complex this is,” said temporary committee member Oveta A. Fuller, PhD, associate professor of microbiology and immunology at the University of Michigan. “We didn’t get to understand influenza in 2 years. It’s taken years to get an imperfect but useful process to deal with flu.”
A version of this article first appeared on WebMD.com.
April 6.
But members of the panel also acknowledged that it will be an uphill battle to reach that goal, especially given how quickly the virus continues to change.
The members of the Vaccines and Related Biological Products Advisory Committee said they want to find the balance that makes sure Americans are protected against severe illness and death but doesn’t wear them out with constant recommendations for boosters.
“We don’t feel comfortable with multiple boosters every 8 weeks,” said committee chairman Arnold Monto, MD, professor emeritus of public health at the University of Michigan, Ann Arbor. “We’d love to see an annual vaccination similar to influenza but realize that the evolution of the virus will dictate how we respond in terms of additional vaccine doses.”
The virus itself will dictate vaccination plans, he said.
The government must also keep its focus on convincing Americans who haven’t been vaccinated to join the club, said committee member Henry H. Bernstein, DO, given that “it seems quite obvious that those who are vaccinated do better than those who aren’t vaccinated.”
The government should clearly communicate to the public the goals of vaccination, he said.
“I would suggest that our overall aim is to prevent severe disease, hospitalization, and death more than just infection prevention,” said Dr. Bernstein, professor of pediatrics at Hofstra University, Hempstead, N.Y.
The FDA called the meeting of its advisers to discuss overall booster and vaccine strategy, even though it already authorized a fourth dose of the Pfizer and Moderna vaccines for certain immune compromised adults and for everyone over age 50.
Early in the all-day meeting, temporary committee member James Hildreth, MD, the president of Meharry Medical College, Nashville, Tenn., asked why that authorization was given without the panel’s input. Peter Marks, MD, the director of FDA’s Center for Biologics Evaluation and Research, said the decision was based on data from the United Kingdom and Israel that suggested immunity from a third shot was already waning.
Dr. Marks later said the fourth dose was “authorized as a stopgap measure until we could get something else in place,” because the aim was to protect older Americans who had died at a higher rate than younger individuals.
“I think we’re very much on board that we simply can’t be boosting people as frequently as we are,” said Dr. Marks.
Not enough information to make broader plan
The meeting was meant to be a larger conversation about how to keep pace with the evolving virus and to set up a vaccine selection and development process to better and more quickly respond to changes, such as new variants.
But committee members said they felt stymied by a lack of information. They wanted more data from vaccine manufacturers’ clinical trials. And they noted that so far, there’s no objective, reliable lab-based measurement of COVID-19 vaccine effectiveness – known as a correlate of immunity. Instead, public health officials have looked at rates of hospitalizations and deaths to measure whether the vaccine is still offering protection.
“The question is, what is insufficient protection?” asked H. Cody Meissner, MD, director of pediatric infectious disease at Tufts Medical Center in Boston. “At what point will we say the vaccine isn’t working well enough?”
Centers for Disease Control and Prevention officials presented data showing that a third shot has been more effective than a two-shot regimen in preventing serious disease and death, and that the three shots were significantly more protective than being unvaccinated.
In February, as the Omicron variant continued to rage, unvaccinated Americans aged 5 years and older had an almost three times higher risk of testing positive, and nine times higher risk of dying, compared with those who were considered fully vaccinated, said Heather Scobie, PhD, MPH, a member of the CDC’s COVID-19 Emergency Response team.
But only 98 million Americans – about half of those aged 12 years or older – have received a third dose, Dr. Scobie said.
It’s also still not clear how much more protection a fourth shot adds, or how long it will last. The committee heard data on a just-published study of a fourth dose of the Pfizer vaccine given to some 600,000 Israelis during the Omicron wave from January to March. The rate of severe COVID-19 was 3.5 times lower in the group that received a fourth dose, compared with those who had gotten only three shots, and protection lasted for at least 12 weeks.
Still, study authors said, any protection against infection itself was “short lived.”
More like flu vaccine?
The advisers discussed the possibility of making COVID-19 vaccine development similar to the process for the flu vaccine but acknowledged many difficulties.
The flu predictably hits during the winter in each hemisphere and a global surveillance network helps the World Health Organization decide on the vaccine strains each year. Then each nation’s regulatory and public health officials choose the strains for their shot and vaccine makers begin what is typically a 6-month-long manufacturing process.
COVID outbreaks have happened during all seasons and new variants haven’t always hit every country in a similar fashion. The COVID virus has mutated at five times the speed of the flu virus – producing a new dominant strain in a year, compared with the 3-5 years it takes for the flu virus to do so, said Trevor Bedford, PhD, a professor in the vaccine and infectious disease division at the Fred Hutchinson Cancer Research Center in Seattle.
Global COVID surveillance is patchy and the WHO has not yet created a program to help select strains for a COVID-19 vaccine but is working on a process. Currently, vaccine makers seem to be driving vaccine strain selection, said panelist Paul Offit, MD, professor of paediatrics at Children’s Hospital of Philadelphia. “I feel like to some extent the companies dictate the conversation. It shouldn’t come from them. It should come from us.”
“The important thing is that the public understands how complex this is,” said temporary committee member Oveta A. Fuller, PhD, associate professor of microbiology and immunology at the University of Michigan. “We didn’t get to understand influenza in 2 years. It’s taken years to get an imperfect but useful process to deal with flu.”
A version of this article first appeared on WebMD.com.
White House announces long-COVID action plan
The National Research Action Plan on Long COVID will gather experts from various agencies, including the Department of Defense and the Department of Veterans Affairs, to expand existing long-COVID clinics and broaden research on symptoms of the virus that persist long after infection.
“We’ll collaborate with academic, industry, state and local partners to better understand long COVID,” Health and Human Services Secretary Xavier Becerra said at a White House briefing April 5. “We need to work as aggressively as we can to make sure no American is left behind.”
The plan will build on the RECOVER Initiative, a $1.15 billion effort announced last year that will study long COVID.
The COVID-19 Response Team also announced that the United States will donate tens of millions of pediatric coronavirus vaccines to other countries. More than 20 countries have asked for the donations, the team said.
The United States has delivered more than 500 million vaccine doses to 114 countries.
Meanwhile, national COVID-19 numbers continue to fall. CDC Director Rochelle Walensky, MD, reported that average daily cases are down 4% this week to 25,000; hospitalizations have dropped 17% to 1,400 per day; and daily deaths are down to 570 a day, which is a decrease of about 17%.
New national estimates show that Omicron’s subvariant BA.2 now accounts for 72% of circulating variants nationally, she said.
Top infectious disease expert Anthony Fauci, MD, reported that recent data supports the need for a second booster among certain people 50 and older – a move authorized by the Food and Drug Administration and Centers for Disease Control and Prevention last week.
“The effectiveness of the first booster dose we know wanes over time, and growing evidence shows a second dose can restore vaccine effectiveness for certain populations,” he said.
Dr. Fauci reported findings from an Israeli study of more than 1 million people 60 and older, which showed that an additional booster dose after 4 months lowered the rate of infection by two times and lowered the rate of severe infection by more than four times.
Another study from Israeli scientists showed that out of half a million people 60 and older, a second booster after 4 months brought a 78% reduction in death, compared to those who received only the first boost.
A version of this article first appeared on WebMD.com.
The National Research Action Plan on Long COVID will gather experts from various agencies, including the Department of Defense and the Department of Veterans Affairs, to expand existing long-COVID clinics and broaden research on symptoms of the virus that persist long after infection.
“We’ll collaborate with academic, industry, state and local partners to better understand long COVID,” Health and Human Services Secretary Xavier Becerra said at a White House briefing April 5. “We need to work as aggressively as we can to make sure no American is left behind.”
The plan will build on the RECOVER Initiative, a $1.15 billion effort announced last year that will study long COVID.
The COVID-19 Response Team also announced that the United States will donate tens of millions of pediatric coronavirus vaccines to other countries. More than 20 countries have asked for the donations, the team said.
The United States has delivered more than 500 million vaccine doses to 114 countries.
Meanwhile, national COVID-19 numbers continue to fall. CDC Director Rochelle Walensky, MD, reported that average daily cases are down 4% this week to 25,000; hospitalizations have dropped 17% to 1,400 per day; and daily deaths are down to 570 a day, which is a decrease of about 17%.
New national estimates show that Omicron’s subvariant BA.2 now accounts for 72% of circulating variants nationally, she said.
Top infectious disease expert Anthony Fauci, MD, reported that recent data supports the need for a second booster among certain people 50 and older – a move authorized by the Food and Drug Administration and Centers for Disease Control and Prevention last week.
“The effectiveness of the first booster dose we know wanes over time, and growing evidence shows a second dose can restore vaccine effectiveness for certain populations,” he said.
Dr. Fauci reported findings from an Israeli study of more than 1 million people 60 and older, which showed that an additional booster dose after 4 months lowered the rate of infection by two times and lowered the rate of severe infection by more than four times.
Another study from Israeli scientists showed that out of half a million people 60 and older, a second booster after 4 months brought a 78% reduction in death, compared to those who received only the first boost.
A version of this article first appeared on WebMD.com.
The National Research Action Plan on Long COVID will gather experts from various agencies, including the Department of Defense and the Department of Veterans Affairs, to expand existing long-COVID clinics and broaden research on symptoms of the virus that persist long after infection.
“We’ll collaborate with academic, industry, state and local partners to better understand long COVID,” Health and Human Services Secretary Xavier Becerra said at a White House briefing April 5. “We need to work as aggressively as we can to make sure no American is left behind.”
The plan will build on the RECOVER Initiative, a $1.15 billion effort announced last year that will study long COVID.
The COVID-19 Response Team also announced that the United States will donate tens of millions of pediatric coronavirus vaccines to other countries. More than 20 countries have asked for the donations, the team said.
The United States has delivered more than 500 million vaccine doses to 114 countries.
Meanwhile, national COVID-19 numbers continue to fall. CDC Director Rochelle Walensky, MD, reported that average daily cases are down 4% this week to 25,000; hospitalizations have dropped 17% to 1,400 per day; and daily deaths are down to 570 a day, which is a decrease of about 17%.
New national estimates show that Omicron’s subvariant BA.2 now accounts for 72% of circulating variants nationally, she said.
Top infectious disease expert Anthony Fauci, MD, reported that recent data supports the need for a second booster among certain people 50 and older – a move authorized by the Food and Drug Administration and Centers for Disease Control and Prevention last week.
“The effectiveness of the first booster dose we know wanes over time, and growing evidence shows a second dose can restore vaccine effectiveness for certain populations,” he said.
Dr. Fauci reported findings from an Israeli study of more than 1 million people 60 and older, which showed that an additional booster dose after 4 months lowered the rate of infection by two times and lowered the rate of severe infection by more than four times.
Another study from Israeli scientists showed that out of half a million people 60 and older, a second booster after 4 months brought a 78% reduction in death, compared to those who received only the first boost.
A version of this article first appeared on WebMD.com.
CDC recommends hep B vaccination for most adults
It also added that adults aged 60 years or older without known risk factors for hepatitis B may get vaccinated.
The agency earlier recommended the vaccination for all infants and children under the age of 19 years and for adults aged 60 years or older with known risk factors.
The CDC said it wants to expand vaccinations because, after decades of progress, the number of new hepatitis B infections is increasing among adults. Acute hepatitis B infections among adults lead to chronic hepatitis B disease in an estimated 2%-6% of cases, and can result in cirrhosis, liver cancer, and death.
Among adults aged 40-49 years, the rate of cases increased from 1.9 per 100,000 people in 2011 to 2.7 per 100,000 in 2019. Among adults aged 50-59 years, the rate increased during this period from 1.1 to 1.6 per 100,000.
Most adults aren’t vaccinated. Among adults aged 19 years or older, only 30.0% reported that they’d received at least the three recommended doses of the vaccine. The rate was 40.3% for adults aged 19-49 years, and 19.1% for adults aged 50 years or older.
Hepatitis B infection rates are particularly elevated among African Americans.
Even among adults with chronic liver disease, the vaccination rate is only 33.0%. And, among travelers to countries where the virus has been endemic since 1995, only 38.9% were vaccinated.
In a 2018 survey of internal medicine and family physicians, 68% said their patients had not told them about risk factors, making it difficult to assess whether the patients needed the vaccine according to the recommendations at the time. These risk factors include injection drug use, incarceration, and multiple sex partners, experiences the patients may not have been willing to discuss.
CDC researchers calculated that universal adult hepatitis B vaccination would cost $153,000 for every quality-adjusted life-year (QALY) gained. For adults aged 19-59 years, a QALY would cost $117,000 because infections are more prevalent in that age group.
The CDC specified that it intends its new guidelines to prompt physicians to offer the vaccine to adults aged 60 years or older rather than wait for them to request it.
The Food and Drug Administration has approved both three-dose and two-dose hepatitis B vaccines, with evidence showing similar seroprotection and adverse events.
People who have already completed their vaccination or have a history of hepatitis B infection should only receive additional vaccinations in specific cases, as detailed in the CDC’s 2018 recommendations.
A version of this article first appeared on Medscape.com.
It also added that adults aged 60 years or older without known risk factors for hepatitis B may get vaccinated.
The agency earlier recommended the vaccination for all infants and children under the age of 19 years and for adults aged 60 years or older with known risk factors.
The CDC said it wants to expand vaccinations because, after decades of progress, the number of new hepatitis B infections is increasing among adults. Acute hepatitis B infections among adults lead to chronic hepatitis B disease in an estimated 2%-6% of cases, and can result in cirrhosis, liver cancer, and death.
Among adults aged 40-49 years, the rate of cases increased from 1.9 per 100,000 people in 2011 to 2.7 per 100,000 in 2019. Among adults aged 50-59 years, the rate increased during this period from 1.1 to 1.6 per 100,000.
Most adults aren’t vaccinated. Among adults aged 19 years or older, only 30.0% reported that they’d received at least the three recommended doses of the vaccine. The rate was 40.3% for adults aged 19-49 years, and 19.1% for adults aged 50 years or older.
Hepatitis B infection rates are particularly elevated among African Americans.
Even among adults with chronic liver disease, the vaccination rate is only 33.0%. And, among travelers to countries where the virus has been endemic since 1995, only 38.9% were vaccinated.
In a 2018 survey of internal medicine and family physicians, 68% said their patients had not told them about risk factors, making it difficult to assess whether the patients needed the vaccine according to the recommendations at the time. These risk factors include injection drug use, incarceration, and multiple sex partners, experiences the patients may not have been willing to discuss.
CDC researchers calculated that universal adult hepatitis B vaccination would cost $153,000 for every quality-adjusted life-year (QALY) gained. For adults aged 19-59 years, a QALY would cost $117,000 because infections are more prevalent in that age group.
The CDC specified that it intends its new guidelines to prompt physicians to offer the vaccine to adults aged 60 years or older rather than wait for them to request it.
The Food and Drug Administration has approved both three-dose and two-dose hepatitis B vaccines, with evidence showing similar seroprotection and adverse events.
People who have already completed their vaccination or have a history of hepatitis B infection should only receive additional vaccinations in specific cases, as detailed in the CDC’s 2018 recommendations.
A version of this article first appeared on Medscape.com.
It also added that adults aged 60 years or older without known risk factors for hepatitis B may get vaccinated.
The agency earlier recommended the vaccination for all infants and children under the age of 19 years and for adults aged 60 years or older with known risk factors.
The CDC said it wants to expand vaccinations because, after decades of progress, the number of new hepatitis B infections is increasing among adults. Acute hepatitis B infections among adults lead to chronic hepatitis B disease in an estimated 2%-6% of cases, and can result in cirrhosis, liver cancer, and death.
Among adults aged 40-49 years, the rate of cases increased from 1.9 per 100,000 people in 2011 to 2.7 per 100,000 in 2019. Among adults aged 50-59 years, the rate increased during this period from 1.1 to 1.6 per 100,000.
Most adults aren’t vaccinated. Among adults aged 19 years or older, only 30.0% reported that they’d received at least the three recommended doses of the vaccine. The rate was 40.3% for adults aged 19-49 years, and 19.1% for adults aged 50 years or older.
Hepatitis B infection rates are particularly elevated among African Americans.
Even among adults with chronic liver disease, the vaccination rate is only 33.0%. And, among travelers to countries where the virus has been endemic since 1995, only 38.9% were vaccinated.
In a 2018 survey of internal medicine and family physicians, 68% said their patients had not told them about risk factors, making it difficult to assess whether the patients needed the vaccine according to the recommendations at the time. These risk factors include injection drug use, incarceration, and multiple sex partners, experiences the patients may not have been willing to discuss.
CDC researchers calculated that universal adult hepatitis B vaccination would cost $153,000 for every quality-adjusted life-year (QALY) gained. For adults aged 19-59 years, a QALY would cost $117,000 because infections are more prevalent in that age group.
The CDC specified that it intends its new guidelines to prompt physicians to offer the vaccine to adults aged 60 years or older rather than wait for them to request it.
The Food and Drug Administration has approved both three-dose and two-dose hepatitis B vaccines, with evidence showing similar seroprotection and adverse events.
People who have already completed their vaccination or have a history of hepatitis B infection should only receive additional vaccinations in specific cases, as detailed in the CDC’s 2018 recommendations.
A version of this article first appeared on Medscape.com.
FROM THE MMWR
As FDA OKs another COVID booster, some experts question need
, even though many top infectious disease experts questioned the need before the agency’s decision.
The FDA granted emergency use authorization for both Pfizer and Moderna to offer the second booster – and fourth shot overall – for adults over 50 as well as those over 18 with compromised immune systems.
The Centers for Control and Prevention must still sign off before those doses start reaching American arms. That approval could come at any time.
“The general consensus, certainly the CDC’s consensus, is that the current vaccines are still really quite effective against Omicron and this new BA.2 variant in keeping people out of the hospital, and preventing the development of severe disease,” William Schaffner, MD, an infectious disease specialist at Vanderbilt University in Nashville said prior to the FDA’s announcement March 29.
Of the 217.4 million Americans who are “fully vaccinated,” i.e., received two doses of either Pfizer or Moderna’s vaccines or one dose of the Johnson & Johnson vaccine, only 45% have also received a booster shot, according to the CDC.
“Given that, there’s no need at the moment for the general population to get a fourth inoculation,” Dr. Schaffner says. “Our current focus ought to be on making sure that as many people as possible get that [first] booster who are eligible.”
Monica Gandhi, MD, an infectious disease specialist at the University of California, San Francisco, agreed that another booster for everyone was unnecessary. The only people who would need a fourth shot (or third, if they had the Johnson & Johnson vaccine initially) are those over age 65 or 70 years, Dr. Gandhi says.
“Older people need those antibodies up high because they’re more susceptible to severe breakthroughs,” she said, also before the latest development.
To boost or not to boost
Daniel Kuritzkes, MD, chief of infectious diseases at Brigham & Women’s Hospital in Boston, said the timing of a booster and who should be eligible depends on what the nation is trying to achieve with its vaccination strategy.
“Is the goal to prevent any symptomatic infection with COVID-19, is the goal to prevent the spread of COVID-19, or is the goal to prevent severe disease that requires hospitalization?” asked Dr. Kuritzkes.
The current vaccine — with a booster — has prevented severe disease, he said.
An Israeli study showed, for instance, that a third Pfizer dose was 93% effective against hospitalization, 92% effective against severe illness, and 81% effective against death.
A just-published study in the New England Journal of Medicine found that a booster of the Pfizer vaccine was 95% effective against COVID-19 infection and that it did not raise any new safety issues.
A small Israeli study, also published in NEJM, of a fourth Pfizer dose given to health care workers found that it prevented symptomatic infection and illness, but that it was much less effective than previous doses — maybe 65% effective against symptomatic illness, the authors write.
Giving Americans another booster now — which has been shown to lose some effectiveness after about 4 months — means it might not offer protection this fall and winter, when there could be a seasonal surge of the virus, Dr. Kuritzkes says.
And, even if people receive boosters every few months, they are still likely to get a mild respiratory virus infection, he said.
“I’m pretty convinced that we cannot boost ourselves out of this pandemic,” said Dr. Kuritzkes. “We need to first of all ensure there’s global immunization so that all the people who have not been vaccinated at all get vaccinated. That’s far more important than boosting people a fourth time.”
Booster confusion
The April 6 FDA meeting of the agency’s Vaccines and Related Biological Products Advisory Committee comes as the two major COVID vaccine makers — Pfizer and Moderna — have applied for emergency use authorization for an additional booster.
Pfizer had asked for authorization for a fourth shot in patients over age 65 years, while Moderna wanted a booster to be available to all Americans over 18. The FDA instead granted authorization to both companies for those over 50 and anyone 18 or older who is immunocompromised.
What this means for the committee’s April 6 meeting is not clear. The original agenda says the committee will consider the evidence on safety and effectiveness of the additional vaccine doses and discuss how to set up a process — similar to that used for the influenza vaccine — to be able to determine the makeup of COVID vaccines as new variants emerge. That could lay the groundwork for an annual COVID shot, if needed.
The FDA advisers will not make recommendations nor vote on whether — and which — Americans should get a COVID booster. That is the job of the CDC’s Advisory Committee on Immunization Practices (ACIP).
The last time a booster was considered, CDC Director Rochelle Walensky, MD, overrode the committee and recommended that all Americans — not just older individuals — get an additional COVID shot, which became the first booster.
That past action worries Dr. Gandhi, who calls it confusing, and says it may have contributed to the fact that less than half of Americans have since chosen to get a booster.
Dr. Schaffner says he expects the FDA to authorize emergency use for fourth doses of the Pfizer and Moderna vaccines, but he doesn’t think the CDC committee will recommend routine use. As was seen before, however, the CDC director does not have to follow the committee’s advice.
The members of ACIP “might be more conservative or narrower in scope in terms of recommending who needs to be boosted and when boosting is appropriate,” Dr. Kuritzkes says.
Dr. Gandhi says she’s concerned the FDA’s deliberations could be swayed by Moderna and Pfizer’s influence and that “pharmaceutical companies are going to have more of a say than they should in the scientific process.”
There are similar worries for Dr. Schaffner. He says he’s “a bit grumpy” that the vaccine makers have been using press releases to argue for boosters.
“Press releases are no way to make vaccine recommendations,” Dr. Schaffner said, adding that he “would advise [vaccine makers] to sit down and be quiet and let the FDA and CDC advisory committee do their thing.”
Moderna Chief Medical Officer Paul Burton, MD, however, told WebMD last week that the signs point to why a fourth shot may be needed.
“We see waning of effectiveness, antibody levels come down, and certainly effectiveness against Omicron comes down in 3 to 6 months,” Burton said. “The natural history, from what we’re seeing around the world, is that BA.2 is definitely here, it’s highly transmissible, and I think we are going to get an additional wave of BA.2 here in the United States.”
Another wave is coming, he said, and “I think there will be waning of effectiveness. We need to be prepared for that, so that’s why we need the fourth dose.”
Supply issues?
Meanwhile, the United Kingdom has begun offering boosters to anyone over 75, and Sweden’s health authority has recommended a fourth shot to people over age 80.
That puts pressure on the United States — at least on its politicians and policymakers — to, in a sense, keep up, said the infectious disease specialists.
Indeed, the White House has been keeping fourth shots in the news, warning that it is running out of money to ensure that all Americans would have access to one, if recommended.
On March 23, outgoing White House COVID-19 Response Coordinator Jeff Zients said the federal government had enough vaccine for the immunocompromised to get a fourth dose “and, if authorized in the coming weeks, enough supply for fourth doses for our most vulnerable, including seniors.”
But he warned that without congressional approval of a COVID-19 funding package, “We can’t procure the necessary vaccine supply to support fourth shots for all Americans.”
Mr. Zients also noted that other countries, including Japan, Vietnam, and the Philippines had already secured future booster doses and added, “We should be securing additional supply right now.”
Dr. Schaffner says that while it would be nice to “have a booster on the shelf,” the United States needs to put more effort into creating a globally-coordinated process for ensuring that vaccines match circulating strains and that they are manufactured on a timely basis.
He says he and others “have been reminding the public that the COVID pandemic may indeed be diminishing and moving into the endemic, but that doesn’t mean COVID is over or finished or disappeared.”
Dr. Schaffner says that it may be that “perhaps we’d need a periodic reminder to our immune system to remain protected. In other words, we might have to get boosted perhaps annually like we do with influenza.”
A version of this article first appeared on WebMD.com.
, even though many top infectious disease experts questioned the need before the agency’s decision.
The FDA granted emergency use authorization for both Pfizer and Moderna to offer the second booster – and fourth shot overall – for adults over 50 as well as those over 18 with compromised immune systems.
The Centers for Control and Prevention must still sign off before those doses start reaching American arms. That approval could come at any time.
“The general consensus, certainly the CDC’s consensus, is that the current vaccines are still really quite effective against Omicron and this new BA.2 variant in keeping people out of the hospital, and preventing the development of severe disease,” William Schaffner, MD, an infectious disease specialist at Vanderbilt University in Nashville said prior to the FDA’s announcement March 29.
Of the 217.4 million Americans who are “fully vaccinated,” i.e., received two doses of either Pfizer or Moderna’s vaccines or one dose of the Johnson & Johnson vaccine, only 45% have also received a booster shot, according to the CDC.
“Given that, there’s no need at the moment for the general population to get a fourth inoculation,” Dr. Schaffner says. “Our current focus ought to be on making sure that as many people as possible get that [first] booster who are eligible.”
Monica Gandhi, MD, an infectious disease specialist at the University of California, San Francisco, agreed that another booster for everyone was unnecessary. The only people who would need a fourth shot (or third, if they had the Johnson & Johnson vaccine initially) are those over age 65 or 70 years, Dr. Gandhi says.
“Older people need those antibodies up high because they’re more susceptible to severe breakthroughs,” she said, also before the latest development.
To boost or not to boost
Daniel Kuritzkes, MD, chief of infectious diseases at Brigham & Women’s Hospital in Boston, said the timing of a booster and who should be eligible depends on what the nation is trying to achieve with its vaccination strategy.
“Is the goal to prevent any symptomatic infection with COVID-19, is the goal to prevent the spread of COVID-19, or is the goal to prevent severe disease that requires hospitalization?” asked Dr. Kuritzkes.
The current vaccine — with a booster — has prevented severe disease, he said.
An Israeli study showed, for instance, that a third Pfizer dose was 93% effective against hospitalization, 92% effective against severe illness, and 81% effective against death.
A just-published study in the New England Journal of Medicine found that a booster of the Pfizer vaccine was 95% effective against COVID-19 infection and that it did not raise any new safety issues.
A small Israeli study, also published in NEJM, of a fourth Pfizer dose given to health care workers found that it prevented symptomatic infection and illness, but that it was much less effective than previous doses — maybe 65% effective against symptomatic illness, the authors write.
Giving Americans another booster now — which has been shown to lose some effectiveness after about 4 months — means it might not offer protection this fall and winter, when there could be a seasonal surge of the virus, Dr. Kuritzkes says.
And, even if people receive boosters every few months, they are still likely to get a mild respiratory virus infection, he said.
“I’m pretty convinced that we cannot boost ourselves out of this pandemic,” said Dr. Kuritzkes. “We need to first of all ensure there’s global immunization so that all the people who have not been vaccinated at all get vaccinated. That’s far more important than boosting people a fourth time.”
Booster confusion
The April 6 FDA meeting of the agency’s Vaccines and Related Biological Products Advisory Committee comes as the two major COVID vaccine makers — Pfizer and Moderna — have applied for emergency use authorization for an additional booster.
Pfizer had asked for authorization for a fourth shot in patients over age 65 years, while Moderna wanted a booster to be available to all Americans over 18. The FDA instead granted authorization to both companies for those over 50 and anyone 18 or older who is immunocompromised.
What this means for the committee’s April 6 meeting is not clear. The original agenda says the committee will consider the evidence on safety and effectiveness of the additional vaccine doses and discuss how to set up a process — similar to that used for the influenza vaccine — to be able to determine the makeup of COVID vaccines as new variants emerge. That could lay the groundwork for an annual COVID shot, if needed.
The FDA advisers will not make recommendations nor vote on whether — and which — Americans should get a COVID booster. That is the job of the CDC’s Advisory Committee on Immunization Practices (ACIP).
The last time a booster was considered, CDC Director Rochelle Walensky, MD, overrode the committee and recommended that all Americans — not just older individuals — get an additional COVID shot, which became the first booster.
That past action worries Dr. Gandhi, who calls it confusing, and says it may have contributed to the fact that less than half of Americans have since chosen to get a booster.
Dr. Schaffner says he expects the FDA to authorize emergency use for fourth doses of the Pfizer and Moderna vaccines, but he doesn’t think the CDC committee will recommend routine use. As was seen before, however, the CDC director does not have to follow the committee’s advice.
The members of ACIP “might be more conservative or narrower in scope in terms of recommending who needs to be boosted and when boosting is appropriate,” Dr. Kuritzkes says.
Dr. Gandhi says she’s concerned the FDA’s deliberations could be swayed by Moderna and Pfizer’s influence and that “pharmaceutical companies are going to have more of a say than they should in the scientific process.”
There are similar worries for Dr. Schaffner. He says he’s “a bit grumpy” that the vaccine makers have been using press releases to argue for boosters.
“Press releases are no way to make vaccine recommendations,” Dr. Schaffner said, adding that he “would advise [vaccine makers] to sit down and be quiet and let the FDA and CDC advisory committee do their thing.”
Moderna Chief Medical Officer Paul Burton, MD, however, told WebMD last week that the signs point to why a fourth shot may be needed.
“We see waning of effectiveness, antibody levels come down, and certainly effectiveness against Omicron comes down in 3 to 6 months,” Burton said. “The natural history, from what we’re seeing around the world, is that BA.2 is definitely here, it’s highly transmissible, and I think we are going to get an additional wave of BA.2 here in the United States.”
Another wave is coming, he said, and “I think there will be waning of effectiveness. We need to be prepared for that, so that’s why we need the fourth dose.”
Supply issues?
Meanwhile, the United Kingdom has begun offering boosters to anyone over 75, and Sweden’s health authority has recommended a fourth shot to people over age 80.
That puts pressure on the United States — at least on its politicians and policymakers — to, in a sense, keep up, said the infectious disease specialists.
Indeed, the White House has been keeping fourth shots in the news, warning that it is running out of money to ensure that all Americans would have access to one, if recommended.
On March 23, outgoing White House COVID-19 Response Coordinator Jeff Zients said the federal government had enough vaccine for the immunocompromised to get a fourth dose “and, if authorized in the coming weeks, enough supply for fourth doses for our most vulnerable, including seniors.”
But he warned that without congressional approval of a COVID-19 funding package, “We can’t procure the necessary vaccine supply to support fourth shots for all Americans.”
Mr. Zients also noted that other countries, including Japan, Vietnam, and the Philippines had already secured future booster doses and added, “We should be securing additional supply right now.”
Dr. Schaffner says that while it would be nice to “have a booster on the shelf,” the United States needs to put more effort into creating a globally-coordinated process for ensuring that vaccines match circulating strains and that they are manufactured on a timely basis.
He says he and others “have been reminding the public that the COVID pandemic may indeed be diminishing and moving into the endemic, but that doesn’t mean COVID is over or finished or disappeared.”
Dr. Schaffner says that it may be that “perhaps we’d need a periodic reminder to our immune system to remain protected. In other words, we might have to get boosted perhaps annually like we do with influenza.”
A version of this article first appeared on WebMD.com.
, even though many top infectious disease experts questioned the need before the agency’s decision.
The FDA granted emergency use authorization for both Pfizer and Moderna to offer the second booster – and fourth shot overall – for adults over 50 as well as those over 18 with compromised immune systems.
The Centers for Control and Prevention must still sign off before those doses start reaching American arms. That approval could come at any time.
“The general consensus, certainly the CDC’s consensus, is that the current vaccines are still really quite effective against Omicron and this new BA.2 variant in keeping people out of the hospital, and preventing the development of severe disease,” William Schaffner, MD, an infectious disease specialist at Vanderbilt University in Nashville said prior to the FDA’s announcement March 29.
Of the 217.4 million Americans who are “fully vaccinated,” i.e., received two doses of either Pfizer or Moderna’s vaccines or one dose of the Johnson & Johnson vaccine, only 45% have also received a booster shot, according to the CDC.
“Given that, there’s no need at the moment for the general population to get a fourth inoculation,” Dr. Schaffner says. “Our current focus ought to be on making sure that as many people as possible get that [first] booster who are eligible.”
Monica Gandhi, MD, an infectious disease specialist at the University of California, San Francisco, agreed that another booster for everyone was unnecessary. The only people who would need a fourth shot (or third, if they had the Johnson & Johnson vaccine initially) are those over age 65 or 70 years, Dr. Gandhi says.
“Older people need those antibodies up high because they’re more susceptible to severe breakthroughs,” she said, also before the latest development.
To boost or not to boost
Daniel Kuritzkes, MD, chief of infectious diseases at Brigham & Women’s Hospital in Boston, said the timing of a booster and who should be eligible depends on what the nation is trying to achieve with its vaccination strategy.
“Is the goal to prevent any symptomatic infection with COVID-19, is the goal to prevent the spread of COVID-19, or is the goal to prevent severe disease that requires hospitalization?” asked Dr. Kuritzkes.
The current vaccine — with a booster — has prevented severe disease, he said.
An Israeli study showed, for instance, that a third Pfizer dose was 93% effective against hospitalization, 92% effective against severe illness, and 81% effective against death.
A just-published study in the New England Journal of Medicine found that a booster of the Pfizer vaccine was 95% effective against COVID-19 infection and that it did not raise any new safety issues.
A small Israeli study, also published in NEJM, of a fourth Pfizer dose given to health care workers found that it prevented symptomatic infection and illness, but that it was much less effective than previous doses — maybe 65% effective against symptomatic illness, the authors write.
Giving Americans another booster now — which has been shown to lose some effectiveness after about 4 months — means it might not offer protection this fall and winter, when there could be a seasonal surge of the virus, Dr. Kuritzkes says.
And, even if people receive boosters every few months, they are still likely to get a mild respiratory virus infection, he said.
“I’m pretty convinced that we cannot boost ourselves out of this pandemic,” said Dr. Kuritzkes. “We need to first of all ensure there’s global immunization so that all the people who have not been vaccinated at all get vaccinated. That’s far more important than boosting people a fourth time.”
Booster confusion
The April 6 FDA meeting of the agency’s Vaccines and Related Biological Products Advisory Committee comes as the two major COVID vaccine makers — Pfizer and Moderna — have applied for emergency use authorization for an additional booster.
Pfizer had asked for authorization for a fourth shot in patients over age 65 years, while Moderna wanted a booster to be available to all Americans over 18. The FDA instead granted authorization to both companies for those over 50 and anyone 18 or older who is immunocompromised.
What this means for the committee’s April 6 meeting is not clear. The original agenda says the committee will consider the evidence on safety and effectiveness of the additional vaccine doses and discuss how to set up a process — similar to that used for the influenza vaccine — to be able to determine the makeup of COVID vaccines as new variants emerge. That could lay the groundwork for an annual COVID shot, if needed.
The FDA advisers will not make recommendations nor vote on whether — and which — Americans should get a COVID booster. That is the job of the CDC’s Advisory Committee on Immunization Practices (ACIP).
The last time a booster was considered, CDC Director Rochelle Walensky, MD, overrode the committee and recommended that all Americans — not just older individuals — get an additional COVID shot, which became the first booster.
That past action worries Dr. Gandhi, who calls it confusing, and says it may have contributed to the fact that less than half of Americans have since chosen to get a booster.
Dr. Schaffner says he expects the FDA to authorize emergency use for fourth doses of the Pfizer and Moderna vaccines, but he doesn’t think the CDC committee will recommend routine use. As was seen before, however, the CDC director does not have to follow the committee’s advice.
The members of ACIP “might be more conservative or narrower in scope in terms of recommending who needs to be boosted and when boosting is appropriate,” Dr. Kuritzkes says.
Dr. Gandhi says she’s concerned the FDA’s deliberations could be swayed by Moderna and Pfizer’s influence and that “pharmaceutical companies are going to have more of a say than they should in the scientific process.”
There are similar worries for Dr. Schaffner. He says he’s “a bit grumpy” that the vaccine makers have been using press releases to argue for boosters.
“Press releases are no way to make vaccine recommendations,” Dr. Schaffner said, adding that he “would advise [vaccine makers] to sit down and be quiet and let the FDA and CDC advisory committee do their thing.”
Moderna Chief Medical Officer Paul Burton, MD, however, told WebMD last week that the signs point to why a fourth shot may be needed.
“We see waning of effectiveness, antibody levels come down, and certainly effectiveness against Omicron comes down in 3 to 6 months,” Burton said. “The natural history, from what we’re seeing around the world, is that BA.2 is definitely here, it’s highly transmissible, and I think we are going to get an additional wave of BA.2 here in the United States.”
Another wave is coming, he said, and “I think there will be waning of effectiveness. We need to be prepared for that, so that’s why we need the fourth dose.”
Supply issues?
Meanwhile, the United Kingdom has begun offering boosters to anyone over 75, and Sweden’s health authority has recommended a fourth shot to people over age 80.
That puts pressure on the United States — at least on its politicians and policymakers — to, in a sense, keep up, said the infectious disease specialists.
Indeed, the White House has been keeping fourth shots in the news, warning that it is running out of money to ensure that all Americans would have access to one, if recommended.
On March 23, outgoing White House COVID-19 Response Coordinator Jeff Zients said the federal government had enough vaccine for the immunocompromised to get a fourth dose “and, if authorized in the coming weeks, enough supply for fourth doses for our most vulnerable, including seniors.”
But he warned that without congressional approval of a COVID-19 funding package, “We can’t procure the necessary vaccine supply to support fourth shots for all Americans.”
Mr. Zients also noted that other countries, including Japan, Vietnam, and the Philippines had already secured future booster doses and added, “We should be securing additional supply right now.”
Dr. Schaffner says that while it would be nice to “have a booster on the shelf,” the United States needs to put more effort into creating a globally-coordinated process for ensuring that vaccines match circulating strains and that they are manufactured on a timely basis.
He says he and others “have been reminding the public that the COVID pandemic may indeed be diminishing and moving into the endemic, but that doesn’t mean COVID is over or finished or disappeared.”
Dr. Schaffner says that it may be that “perhaps we’d need a periodic reminder to our immune system to remain protected. In other words, we might have to get boosted perhaps annually like we do with influenza.”
A version of this article first appeared on WebMD.com.