User login
Nevus Lipomatosis Deemed Suspicious by Airport Security
To the Editor:
A 47-year-old man presented at the dermatology clinic with a growing lesion on the left medial thigh.
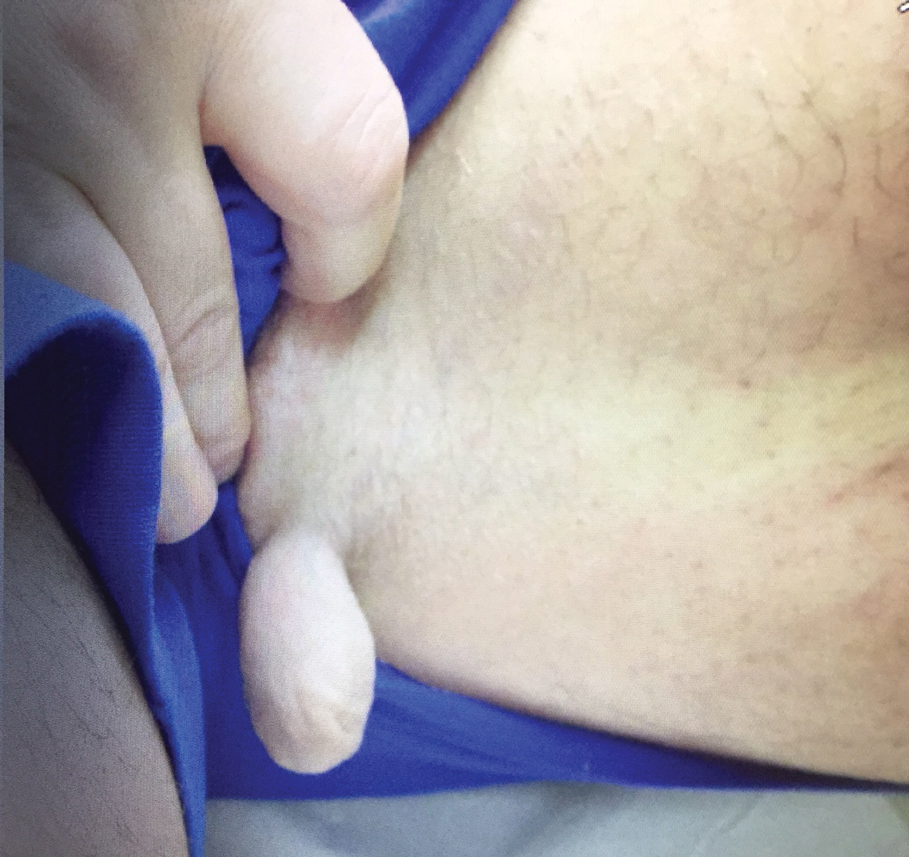
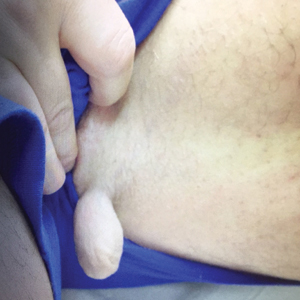
Physical examination revealed a 5-cm, pedunculated, fatty nodule on the left medial thigh that was clinically consistent with nevus lipomatosis (NL)(Figure). Although benign, trouble traveling through airport security prompted the patient to request shave removal, which subsequently was performed. Histology showed a large pedunculated nodule with prominent adipose tissue, consistent with NL. At 3-month follow-up, the patient reported getting through airport security multiple times without incident.
Nevus lipomatosis is a benign fatty lesion most commonly found on the medial thighs or trunk of adults. The lesion usually is asymptomatic but can become irritated by rubbing or catching on clothing. Our patient had symptomatic NL that caused delays getting through airport security; he experienced full resolution after simple shave removal. In rare instances, both benign and malignant skin conditions have been seen on airport scanning devices since the introduction of increased security measures following September 11, 2001. In 2016, Heymann1 reported a man with a 1.5-cm epidermal inclusion cyst detected by airport security scanners, prompting the traveler to request and carry a medically explanatory letter used to get through security. In 2015 Mayer and Adams2 described a case of nodular melanoma that was detected 20 times over a period of 2 months by airport scanners, and in 2016, Caine et al3 reported a case of desmoplastic melanoma that was detected by airport security, but after its removal was not identified by security for the next 40 flights. Noncutaneous pathology also can be detected by airport scanners. In 2013, Naraynsingh et al4 reported a man with a large left reducible inguinal hernia who was stopped by airport security and subjected to an invasive physical examination of the area. These instances demonstrate the breadth of conditions that can be cumbersome when individuals are traveling by airplane in our current security climate.
Our patient had to go through the trouble of having the benign NL lesion removed to avoid the hassle of repeatedly being stopped by airport security. The patient had the lesion removed and is doing well, but the procedure could have been avoided if systems existed to help patients with dermatologic and medical conditions at airport security. Our patient likely will never be stopped again for the suspicious lump on the left inner thigh, but many others will be stopped for similar reasons.
- Heymann WR. A cyst misinterpreted on airport scan as security threat. JAMA Dermatol. 2016;152:1388. doi:10.1001/jamadermatol.2016.3329
- Mayer JE, Adams BB. Nodular melanoma serendipitously detected by airport full body scanners. Dermatology. 2015;230:16-17. doi:10.1159/000368045
- Caine P, Javed MU, Karoo ROS. A desmoplastic melanoma detected by an airport security scanner. J Plast Reconstr Aesthet Surg. 2016;69:874-876. doi:10.1016/j.bjps.2016.02.022
- Naraynsingh V, Cawich SO, Maharaj R, et al. Inguinal hernia and airport scanners: an emerging indication for repair? 2013;2013:952835. Case Rep Med. doi:10.1155/2013/952835
To the Editor:
A 47-year-old man presented at the dermatology clinic with a growing lesion on the left medial thigh.
Physical examination revealed a 5-cm, pedunculated, fatty nodule on the left medial thigh that was clinically consistent with nevus lipomatosis (NL)(Figure). Although benign, trouble traveling through airport security prompted the patient to request shave removal, which subsequently was performed. Histology showed a large pedunculated nodule with prominent adipose tissue, consistent with NL. At 3-month follow-up, the patient reported getting through airport security multiple times without incident.
Nevus lipomatosis is a benign fatty lesion most commonly found on the medial thighs or trunk of adults. The lesion usually is asymptomatic but can become irritated by rubbing or catching on clothing. Our patient had symptomatic NL that caused delays getting through airport security; he experienced full resolution after simple shave removal. In rare instances, both benign and malignant skin conditions have been seen on airport scanning devices since the introduction of increased security measures following September 11, 2001. In 2016, Heymann1 reported a man with a 1.5-cm epidermal inclusion cyst detected by airport security scanners, prompting the traveler to request and carry a medically explanatory letter used to get through security. In 2015 Mayer and Adams2 described a case of nodular melanoma that was detected 20 times over a period of 2 months by airport scanners, and in 2016, Caine et al3 reported a case of desmoplastic melanoma that was detected by airport security, but after its removal was not identified by security for the next 40 flights. Noncutaneous pathology also can be detected by airport scanners. In 2013, Naraynsingh et al4 reported a man with a large left reducible inguinal hernia who was stopped by airport security and subjected to an invasive physical examination of the area. These instances demonstrate the breadth of conditions that can be cumbersome when individuals are traveling by airplane in our current security climate.
Our patient had to go through the trouble of having the benign NL lesion removed to avoid the hassle of repeatedly being stopped by airport security. The patient had the lesion removed and is doing well, but the procedure could have been avoided if systems existed to help patients with dermatologic and medical conditions at airport security. Our patient likely will never be stopped again for the suspicious lump on the left inner thigh, but many others will be stopped for similar reasons.
To the Editor:
A 47-year-old man presented at the dermatology clinic with a growing lesion on the left medial thigh.
Physical examination revealed a 5-cm, pedunculated, fatty nodule on the left medial thigh that was clinically consistent with nevus lipomatosis (NL)(Figure). Although benign, trouble traveling through airport security prompted the patient to request shave removal, which subsequently was performed. Histology showed a large pedunculated nodule with prominent adipose tissue, consistent with NL. At 3-month follow-up, the patient reported getting through airport security multiple times without incident.
Nevus lipomatosis is a benign fatty lesion most commonly found on the medial thighs or trunk of adults. The lesion usually is asymptomatic but can become irritated by rubbing or catching on clothing. Our patient had symptomatic NL that caused delays getting through airport security; he experienced full resolution after simple shave removal. In rare instances, both benign and malignant skin conditions have been seen on airport scanning devices since the introduction of increased security measures following September 11, 2001. In 2016, Heymann1 reported a man with a 1.5-cm epidermal inclusion cyst detected by airport security scanners, prompting the traveler to request and carry a medically explanatory letter used to get through security. In 2015 Mayer and Adams2 described a case of nodular melanoma that was detected 20 times over a period of 2 months by airport scanners, and in 2016, Caine et al3 reported a case of desmoplastic melanoma that was detected by airport security, but after its removal was not identified by security for the next 40 flights. Noncutaneous pathology also can be detected by airport scanners. In 2013, Naraynsingh et al4 reported a man with a large left reducible inguinal hernia who was stopped by airport security and subjected to an invasive physical examination of the area. These instances demonstrate the breadth of conditions that can be cumbersome when individuals are traveling by airplane in our current security climate.
Our patient had to go through the trouble of having the benign NL lesion removed to avoid the hassle of repeatedly being stopped by airport security. The patient had the lesion removed and is doing well, but the procedure could have been avoided if systems existed to help patients with dermatologic and medical conditions at airport security. Our patient likely will never be stopped again for the suspicious lump on the left inner thigh, but many others will be stopped for similar reasons.
- Heymann WR. A cyst misinterpreted on airport scan as security threat. JAMA Dermatol. 2016;152:1388. doi:10.1001/jamadermatol.2016.3329
- Mayer JE, Adams BB. Nodular melanoma serendipitously detected by airport full body scanners. Dermatology. 2015;230:16-17. doi:10.1159/000368045
- Caine P, Javed MU, Karoo ROS. A desmoplastic melanoma detected by an airport security scanner. J Plast Reconstr Aesthet Surg. 2016;69:874-876. doi:10.1016/j.bjps.2016.02.022
- Naraynsingh V, Cawich SO, Maharaj R, et al. Inguinal hernia and airport scanners: an emerging indication for repair? 2013;2013:952835. Case Rep Med. doi:10.1155/2013/952835
- Heymann WR. A cyst misinterpreted on airport scan as security threat. JAMA Dermatol. 2016;152:1388. doi:10.1001/jamadermatol.2016.3329
- Mayer JE, Adams BB. Nodular melanoma serendipitously detected by airport full body scanners. Dermatology. 2015;230:16-17. doi:10.1159/000368045
- Caine P, Javed MU, Karoo ROS. A desmoplastic melanoma detected by an airport security scanner. J Plast Reconstr Aesthet Surg. 2016;69:874-876. doi:10.1016/j.bjps.2016.02.022
- Naraynsingh V, Cawich SO, Maharaj R, et al. Inguinal hernia and airport scanners: an emerging indication for repair? 2013;2013:952835. Case Rep Med. doi:10.1155/2013/952835
Practice Points
- Nevus lipomatosis is a benign fatty lesion that most commonly is found on the medial thighs or trunk of adults.
- Both benign and malignant skin conditions have been detected on airport scanning devices.
- At times, patients must go through the hassle of having the benign lesions removed to avoid repeated problems at airport security.
Lawmakers argue for changes in prior authorization processes
Republican and Democratic members of the House called for changes in how insurer-run Medicare plans manage the prior authorization process, following testimony from a federal watchdog organization about improper denials of payment for care.
About 18% of payment denials in a sample examined by the Office of Inspector General (OIG) of the Department of Health and Human Services (HHS) either met Medicare coverage rules or the rules of the insurance plan.
As such, they should not have been denied, according to the OIG. That was the finding of an April OIG report, based on a sample of 2019 denials from large insurer-run Medicare plans.
Erin Bliss, an assistant inspector general with the OIG, appeared as a witness at a June 28 Energy and Commerce Subcommittee on Oversight and Investigations hearing to discuss this investigation and other issues with prior authorization and insurer-run Medicare, also known as the Advantage plans.
Most of these payment denials of appropriate services were due to human error during manual claims-processing reviews, Ms. Bliss told the subcommittee, such as overlooking a document, and to system processing errors, such as a Medicare insurance plan failing to program or update a system correctly.
In many cases, these denials were reversed, but patient care was still disrupted and clinicians lost time chasing clearances for services that plans already had covered, Ms. Bliss said in her testimony.
The April report was not the OIG’s first look into concerns about insurer-run plans inappropriately denying care through prior authorizations. The OIG in 2018 reported that insurer-run Medicare plans overturned 75% of their own denials during 2014-2016 when patients and clinicians appealed these decisions, overturning approximately 216,000 denials each year.
‘Numerous hoops’ unnecessary for doctors, patients
Lawmakers at the hearing supported the idea of the need for prior authorization as a screening tool to prevent unneeded care.
But they chided insurance companies for their execution of this process, with clinicians and patients often frustrated by complex steps needed. Medicare Advantage plans sometimes require prior authorization for “relatively standard medical services,” said Subcommittee on Oversight and Investigations Chair Diana DeGette (D-Colo.).
“Our seniors and their doctors should not be required to jump through numerous hoops to ensure coverage for straightforward and medically necessary procedures,” Rep. DeGette said.
Several lawmakers spoke at the hearing about the need for changes to prior authorization, including calling for action on a pending bill intended to compel insurers to streamline the review process. The Improving Seniors’ Timely Access to Care Act of 2021 already has attracted more than 300 bipartisan sponsors. A companion Senate bill has more than 30 sponsors.
The bill’s aim is to shift this process away from faxes and phone calls while also encouraging plans to adhere to evidence-based medical guidelines in consultation with physicians. The bill calls for the establishment of an electronic prior authorization program that could issue real-time decisions.
“The result will be less administrative burden for providers and more information in the hands of patients. It will allow more patients to receive care when they need it, reducing the likelihood of additional, often more severe complications,” said Rep. Larry Bucshon, MD, (R-Ind.) who is among the active sponsors of the bill.
“In the long term, I believe it would also result in cost savings for the health care system at large by identifying problems earlier and getting them treated before their patients have more complications,” Rep. Bucshon added.
Finding ‘room for improvement’ for prior authorizations
There’s strong bipartisan support in Congress for insurer-run Medicare, which has grown by 10% per year over the last several years and has doubled since 2010, according to the Medicare Payment Advisory Commission (MedPAC). About 27 million people are now enrolled in these plans.
But for that reason, insurer-run Medicare may also need more careful watching, lawmakers made clear at the hearing.
“We’ve heard quite a bit of evidence today that there is room for improvement,” said Rep. Bucshon, a strong supporter of insurer-run Medicare, which can offer patients added benefits such as dental coverage.
Rep. Ann Kuster (D-N.H.) said simplifying prior authorization would reduce stress on clinicians already dealing with burnout.
“They’re just so tired of all this paperwork and red tape,” Rep. Kuster said. “In 2022 can’t we at least consider electronic prior authorization?”
At the hearing, Rep. Michael C. Burgess, MD, (R-Tex.) noted that his home state already has taken a step toward reducing the burden of prior authorization with its “gold card” program.
In 2021, a new Texas law called on the state department of insurance to develop rules to require health plans to provide an exemption from preauthorization requirements for a particular health care service if the issuer has approved, or would have approved, at least 90% of the preauthorization requests submitted by the physician or provider for that service. The law also mandates that a physician participating in a peer-to-peer review on behalf of a health benefit plan issuer must be a Texas-licensed physician who has the same or similar specialty as the physician or clinician requesting the service, according to the state insurance department.
Separately, Rep. Suzan DelBene (D-Wash.), the sponsor of the Improving Seniors’ Timely Access to Care Act, told the American Medical Association in a recent interview that she expects the House Ways and Means Committee, on which she serves, to mark up her bill in July. (A mark-up is the process by which a House or Senate committee considers and often amends a bill and then sends it to the chamber’s leadership for a floor vote.)
In a statement issued about the hearing, America’s Health Insurance Plans (AHIP) noted that there has been work in recent years toward streamlining prior authorization. AHIP said it launched the Fast Prior Authorization Technology Highway (Fast PATH) initiative in 2020 to study electronic procedures for handling these reviews.
“The findings of this study showed that ePA delivered improvements with a strong majority of experienced providers reporting faster time to patient care, fewer phone calls and faxes, better understanding of [prior authorization] requirements, and faster time to decisions,” AHIP said.
A version of this article first appeared on Medscape.com.
Republican and Democratic members of the House called for changes in how insurer-run Medicare plans manage the prior authorization process, following testimony from a federal watchdog organization about improper denials of payment for care.
About 18% of payment denials in a sample examined by the Office of Inspector General (OIG) of the Department of Health and Human Services (HHS) either met Medicare coverage rules or the rules of the insurance plan.
As such, they should not have been denied, according to the OIG. That was the finding of an April OIG report, based on a sample of 2019 denials from large insurer-run Medicare plans.
Erin Bliss, an assistant inspector general with the OIG, appeared as a witness at a June 28 Energy and Commerce Subcommittee on Oversight and Investigations hearing to discuss this investigation and other issues with prior authorization and insurer-run Medicare, also known as the Advantage plans.
Most of these payment denials of appropriate services were due to human error during manual claims-processing reviews, Ms. Bliss told the subcommittee, such as overlooking a document, and to system processing errors, such as a Medicare insurance plan failing to program or update a system correctly.
In many cases, these denials were reversed, but patient care was still disrupted and clinicians lost time chasing clearances for services that plans already had covered, Ms. Bliss said in her testimony.
The April report was not the OIG’s first look into concerns about insurer-run plans inappropriately denying care through prior authorizations. The OIG in 2018 reported that insurer-run Medicare plans overturned 75% of their own denials during 2014-2016 when patients and clinicians appealed these decisions, overturning approximately 216,000 denials each year.
‘Numerous hoops’ unnecessary for doctors, patients
Lawmakers at the hearing supported the idea of the need for prior authorization as a screening tool to prevent unneeded care.
But they chided insurance companies for their execution of this process, with clinicians and patients often frustrated by complex steps needed. Medicare Advantage plans sometimes require prior authorization for “relatively standard medical services,” said Subcommittee on Oversight and Investigations Chair Diana DeGette (D-Colo.).
“Our seniors and their doctors should not be required to jump through numerous hoops to ensure coverage for straightforward and medically necessary procedures,” Rep. DeGette said.
Several lawmakers spoke at the hearing about the need for changes to prior authorization, including calling for action on a pending bill intended to compel insurers to streamline the review process. The Improving Seniors’ Timely Access to Care Act of 2021 already has attracted more than 300 bipartisan sponsors. A companion Senate bill has more than 30 sponsors.
The bill’s aim is to shift this process away from faxes and phone calls while also encouraging plans to adhere to evidence-based medical guidelines in consultation with physicians. The bill calls for the establishment of an electronic prior authorization program that could issue real-time decisions.
“The result will be less administrative burden for providers and more information in the hands of patients. It will allow more patients to receive care when they need it, reducing the likelihood of additional, often more severe complications,” said Rep. Larry Bucshon, MD, (R-Ind.) who is among the active sponsors of the bill.
“In the long term, I believe it would also result in cost savings for the health care system at large by identifying problems earlier and getting them treated before their patients have more complications,” Rep. Bucshon added.
Finding ‘room for improvement’ for prior authorizations
There’s strong bipartisan support in Congress for insurer-run Medicare, which has grown by 10% per year over the last several years and has doubled since 2010, according to the Medicare Payment Advisory Commission (MedPAC). About 27 million people are now enrolled in these plans.
But for that reason, insurer-run Medicare may also need more careful watching, lawmakers made clear at the hearing.
“We’ve heard quite a bit of evidence today that there is room for improvement,” said Rep. Bucshon, a strong supporter of insurer-run Medicare, which can offer patients added benefits such as dental coverage.
Rep. Ann Kuster (D-N.H.) said simplifying prior authorization would reduce stress on clinicians already dealing with burnout.
“They’re just so tired of all this paperwork and red tape,” Rep. Kuster said. “In 2022 can’t we at least consider electronic prior authorization?”
At the hearing, Rep. Michael C. Burgess, MD, (R-Tex.) noted that his home state already has taken a step toward reducing the burden of prior authorization with its “gold card” program.
In 2021, a new Texas law called on the state department of insurance to develop rules to require health plans to provide an exemption from preauthorization requirements for a particular health care service if the issuer has approved, or would have approved, at least 90% of the preauthorization requests submitted by the physician or provider for that service. The law also mandates that a physician participating in a peer-to-peer review on behalf of a health benefit plan issuer must be a Texas-licensed physician who has the same or similar specialty as the physician or clinician requesting the service, according to the state insurance department.
Separately, Rep. Suzan DelBene (D-Wash.), the sponsor of the Improving Seniors’ Timely Access to Care Act, told the American Medical Association in a recent interview that she expects the House Ways and Means Committee, on which she serves, to mark up her bill in July. (A mark-up is the process by which a House or Senate committee considers and often amends a bill and then sends it to the chamber’s leadership for a floor vote.)
In a statement issued about the hearing, America’s Health Insurance Plans (AHIP) noted that there has been work in recent years toward streamlining prior authorization. AHIP said it launched the Fast Prior Authorization Technology Highway (Fast PATH) initiative in 2020 to study electronic procedures for handling these reviews.
“The findings of this study showed that ePA delivered improvements with a strong majority of experienced providers reporting faster time to patient care, fewer phone calls and faxes, better understanding of [prior authorization] requirements, and faster time to decisions,” AHIP said.
A version of this article first appeared on Medscape.com.
Republican and Democratic members of the House called for changes in how insurer-run Medicare plans manage the prior authorization process, following testimony from a federal watchdog organization about improper denials of payment for care.
About 18% of payment denials in a sample examined by the Office of Inspector General (OIG) of the Department of Health and Human Services (HHS) either met Medicare coverage rules or the rules of the insurance plan.
As such, they should not have been denied, according to the OIG. That was the finding of an April OIG report, based on a sample of 2019 denials from large insurer-run Medicare plans.
Erin Bliss, an assistant inspector general with the OIG, appeared as a witness at a June 28 Energy and Commerce Subcommittee on Oversight and Investigations hearing to discuss this investigation and other issues with prior authorization and insurer-run Medicare, also known as the Advantage plans.
Most of these payment denials of appropriate services were due to human error during manual claims-processing reviews, Ms. Bliss told the subcommittee, such as overlooking a document, and to system processing errors, such as a Medicare insurance plan failing to program or update a system correctly.
In many cases, these denials were reversed, but patient care was still disrupted and clinicians lost time chasing clearances for services that plans already had covered, Ms. Bliss said in her testimony.
The April report was not the OIG’s first look into concerns about insurer-run plans inappropriately denying care through prior authorizations. The OIG in 2018 reported that insurer-run Medicare plans overturned 75% of their own denials during 2014-2016 when patients and clinicians appealed these decisions, overturning approximately 216,000 denials each year.
‘Numerous hoops’ unnecessary for doctors, patients
Lawmakers at the hearing supported the idea of the need for prior authorization as a screening tool to prevent unneeded care.
But they chided insurance companies for their execution of this process, with clinicians and patients often frustrated by complex steps needed. Medicare Advantage plans sometimes require prior authorization for “relatively standard medical services,” said Subcommittee on Oversight and Investigations Chair Diana DeGette (D-Colo.).
“Our seniors and their doctors should not be required to jump through numerous hoops to ensure coverage for straightforward and medically necessary procedures,” Rep. DeGette said.
Several lawmakers spoke at the hearing about the need for changes to prior authorization, including calling for action on a pending bill intended to compel insurers to streamline the review process. The Improving Seniors’ Timely Access to Care Act of 2021 already has attracted more than 300 bipartisan sponsors. A companion Senate bill has more than 30 sponsors.
The bill’s aim is to shift this process away from faxes and phone calls while also encouraging plans to adhere to evidence-based medical guidelines in consultation with physicians. The bill calls for the establishment of an electronic prior authorization program that could issue real-time decisions.
“The result will be less administrative burden for providers and more information in the hands of patients. It will allow more patients to receive care when they need it, reducing the likelihood of additional, often more severe complications,” said Rep. Larry Bucshon, MD, (R-Ind.) who is among the active sponsors of the bill.
“In the long term, I believe it would also result in cost savings for the health care system at large by identifying problems earlier and getting them treated before their patients have more complications,” Rep. Bucshon added.
Finding ‘room for improvement’ for prior authorizations
There’s strong bipartisan support in Congress for insurer-run Medicare, which has grown by 10% per year over the last several years and has doubled since 2010, according to the Medicare Payment Advisory Commission (MedPAC). About 27 million people are now enrolled in these plans.
But for that reason, insurer-run Medicare may also need more careful watching, lawmakers made clear at the hearing.
“We’ve heard quite a bit of evidence today that there is room for improvement,” said Rep. Bucshon, a strong supporter of insurer-run Medicare, which can offer patients added benefits such as dental coverage.
Rep. Ann Kuster (D-N.H.) said simplifying prior authorization would reduce stress on clinicians already dealing with burnout.
“They’re just so tired of all this paperwork and red tape,” Rep. Kuster said. “In 2022 can’t we at least consider electronic prior authorization?”
At the hearing, Rep. Michael C. Burgess, MD, (R-Tex.) noted that his home state already has taken a step toward reducing the burden of prior authorization with its “gold card” program.
In 2021, a new Texas law called on the state department of insurance to develop rules to require health plans to provide an exemption from preauthorization requirements for a particular health care service if the issuer has approved, or would have approved, at least 90% of the preauthorization requests submitted by the physician or provider for that service. The law also mandates that a physician participating in a peer-to-peer review on behalf of a health benefit plan issuer must be a Texas-licensed physician who has the same or similar specialty as the physician or clinician requesting the service, according to the state insurance department.
Separately, Rep. Suzan DelBene (D-Wash.), the sponsor of the Improving Seniors’ Timely Access to Care Act, told the American Medical Association in a recent interview that she expects the House Ways and Means Committee, on which she serves, to mark up her bill in July. (A mark-up is the process by which a House or Senate committee considers and often amends a bill and then sends it to the chamber’s leadership for a floor vote.)
In a statement issued about the hearing, America’s Health Insurance Plans (AHIP) noted that there has been work in recent years toward streamlining prior authorization. AHIP said it launched the Fast Prior Authorization Technology Highway (Fast PATH) initiative in 2020 to study electronic procedures for handling these reviews.
“The findings of this study showed that ePA delivered improvements with a strong majority of experienced providers reporting faster time to patient care, fewer phone calls and faxes, better understanding of [prior authorization] requirements, and faster time to decisions,” AHIP said.
A version of this article first appeared on Medscape.com.
FDA unveils 5-year plan for ALS and other neurodegenerative diseases
The agency’s Action Plan for Rare Neurodegenerative Diseases including Amyotrophic Lateral Sclerosis (ALS) aims to advance the development of safe and effective medical products and facilitate patient access to novel treatments.
“The effects of rare neurodegenerative diseases are devastating, with very few effective therapeutic options available to patients. We recognize the urgent need for new treatments that can both improve and extend the lives of people diagnosed with these diseases,” FDA Commissioner Robert M. Califf, MD, said in a news release.
“To face that challenge and to accelerate drug development, we need innovative approaches to better understand these diseases while also building on current scientific and research capabilities,” Dr. Califf acknowledged.
“This action plan, especially including the use of public-private partnerships and direct involvement of patients, will ensure the FDA is working toward meeting the task set forth by Congress to enhance the quality of life for those suffering by facilitating access to new therapies,” Dr. Califf added.
Blueprint to ‘aggressively’ move forward
The action plan represents a “blueprint” for how the agency will “aggressively” move forward to address challenges in drug development for rare neurodegenerative diseases to improve patient health, the FDA said.
The plan was created in accordance with provisions in the Accelerating Access to Critical Therapies for ALS Act (ACT for ALS) that President Biden signed into law in late 2021.
Targeted activities include establishing the FDA Rare Neurodegenerative Diseases Task Force and the public-private partnership for rare neurodegenerative diseases, developing disease-specific science strategies over the next 5 years, and leveraging ongoing FDA regulatory science efforts.
The ALS Science Strategy is part of the plan focused specifically on ALS. It provides a “forward-leaning” framework for FDA activities, which include efforts to improve characterization of disease pathogenesis and natural history, boost clinical trial infrastructure and agility to enable early selection of promising therapeutic candidates for further development, optimize clinical trial design, improve access to the trials, streamline clinical trial operations, and reduce the time and cost of drug development.
The FDA says patient engagement, public workshops, research projects, coordination across FDA centers and offices, and collaboration with the National Institutes of Health will be key to the success of implementation of the ALS Science Strategy.
A version of this article first appeared on Medscape.com.
The agency’s Action Plan for Rare Neurodegenerative Diseases including Amyotrophic Lateral Sclerosis (ALS) aims to advance the development of safe and effective medical products and facilitate patient access to novel treatments.
“The effects of rare neurodegenerative diseases are devastating, with very few effective therapeutic options available to patients. We recognize the urgent need for new treatments that can both improve and extend the lives of people diagnosed with these diseases,” FDA Commissioner Robert M. Califf, MD, said in a news release.
“To face that challenge and to accelerate drug development, we need innovative approaches to better understand these diseases while also building on current scientific and research capabilities,” Dr. Califf acknowledged.
“This action plan, especially including the use of public-private partnerships and direct involvement of patients, will ensure the FDA is working toward meeting the task set forth by Congress to enhance the quality of life for those suffering by facilitating access to new therapies,” Dr. Califf added.
Blueprint to ‘aggressively’ move forward
The action plan represents a “blueprint” for how the agency will “aggressively” move forward to address challenges in drug development for rare neurodegenerative diseases to improve patient health, the FDA said.
The plan was created in accordance with provisions in the Accelerating Access to Critical Therapies for ALS Act (ACT for ALS) that President Biden signed into law in late 2021.
Targeted activities include establishing the FDA Rare Neurodegenerative Diseases Task Force and the public-private partnership for rare neurodegenerative diseases, developing disease-specific science strategies over the next 5 years, and leveraging ongoing FDA regulatory science efforts.
The ALS Science Strategy is part of the plan focused specifically on ALS. It provides a “forward-leaning” framework for FDA activities, which include efforts to improve characterization of disease pathogenesis and natural history, boost clinical trial infrastructure and agility to enable early selection of promising therapeutic candidates for further development, optimize clinical trial design, improve access to the trials, streamline clinical trial operations, and reduce the time and cost of drug development.
The FDA says patient engagement, public workshops, research projects, coordination across FDA centers and offices, and collaboration with the National Institutes of Health will be key to the success of implementation of the ALS Science Strategy.
A version of this article first appeared on Medscape.com.
The agency’s Action Plan for Rare Neurodegenerative Diseases including Amyotrophic Lateral Sclerosis (ALS) aims to advance the development of safe and effective medical products and facilitate patient access to novel treatments.
“The effects of rare neurodegenerative diseases are devastating, with very few effective therapeutic options available to patients. We recognize the urgent need for new treatments that can both improve and extend the lives of people diagnosed with these diseases,” FDA Commissioner Robert M. Califf, MD, said in a news release.
“To face that challenge and to accelerate drug development, we need innovative approaches to better understand these diseases while also building on current scientific and research capabilities,” Dr. Califf acknowledged.
“This action plan, especially including the use of public-private partnerships and direct involvement of patients, will ensure the FDA is working toward meeting the task set forth by Congress to enhance the quality of life for those suffering by facilitating access to new therapies,” Dr. Califf added.
Blueprint to ‘aggressively’ move forward
The action plan represents a “blueprint” for how the agency will “aggressively” move forward to address challenges in drug development for rare neurodegenerative diseases to improve patient health, the FDA said.
The plan was created in accordance with provisions in the Accelerating Access to Critical Therapies for ALS Act (ACT for ALS) that President Biden signed into law in late 2021.
Targeted activities include establishing the FDA Rare Neurodegenerative Diseases Task Force and the public-private partnership for rare neurodegenerative diseases, developing disease-specific science strategies over the next 5 years, and leveraging ongoing FDA regulatory science efforts.
The ALS Science Strategy is part of the plan focused specifically on ALS. It provides a “forward-leaning” framework for FDA activities, which include efforts to improve characterization of disease pathogenesis and natural history, boost clinical trial infrastructure and agility to enable early selection of promising therapeutic candidates for further development, optimize clinical trial design, improve access to the trials, streamline clinical trial operations, and reduce the time and cost of drug development.
The FDA says patient engagement, public workshops, research projects, coordination across FDA centers and offices, and collaboration with the National Institutes of Health will be key to the success of implementation of the ALS Science Strategy.
A version of this article first appeared on Medscape.com.
LGBTQ students would get new protections under Biden plan
On the 50th anniversary of Title IX’s inception, the Biden administration has proposed changes to the law that would protect transgender students and assault survivors on college and university campuses.
With these changes, the protections provided by Title IX – a civil rights law that prohibits sex-based discrimination in schools that receive federal funding – would now be extended to students who identify as trans. The update would ensure that government-funded schools make proper accommodations for a trans student population, such as allowing students to use bathrooms and other facilities that align with their gender identity, and enforcing the use of students’ correct pronouns.
The revisions also seek to undo amendments made to the law by Betsy DeVos, who was secretary of education during the Trump presidency, which strengthened due process protections for students accused of sexual assault and narrowed the definition of sexual harassment. These rules “weakened protections for survivors of sexual assault and diminished the promise of an education free from discrimination,” the Biden administration said.
“Our proposed changes will allow us to continue that progress and ensure all our nation’s students – no matter where they live, who they are, or whom they love – can learn, grow, and thrive in school,” Education Secretary Miguel Cardona, PhD, said in a news release. “We welcome public comment on these critical regulations so we can further the Biden-Harris Administration’s mission of creating educational environments free from sex discrimination and sexual violence.”
The revisions will go through a long period of public comment before they are set into law. Still, the proposed changes mark a way forward for trans students who are not explicitly protected under Title IX, and they offer solace to assault survivors who may have felt discouraged to come forward and report under Ms. DeVos’s rules.
“The proposed regulations reflect the [Education] Department’s commitment to give full effect to Title IX, ensuring that no person experiences sex discrimination in education, and that school procedures for addressing complaints of sex discrimination, including sexual violence and other forms of sex-based harassment, are clear, effective, and fair to all involved,” said Catherine Lhamon, JD, assistant secretary for the Education Department’s Office Of Civil Rights.
More specific rules about transgender students’ participation in school sports are still to come.
A version of this article first appeared on WebMD.com.
On the 50th anniversary of Title IX’s inception, the Biden administration has proposed changes to the law that would protect transgender students and assault survivors on college and university campuses.
With these changes, the protections provided by Title IX – a civil rights law that prohibits sex-based discrimination in schools that receive federal funding – would now be extended to students who identify as trans. The update would ensure that government-funded schools make proper accommodations for a trans student population, such as allowing students to use bathrooms and other facilities that align with their gender identity, and enforcing the use of students’ correct pronouns.
The revisions also seek to undo amendments made to the law by Betsy DeVos, who was secretary of education during the Trump presidency, which strengthened due process protections for students accused of sexual assault and narrowed the definition of sexual harassment. These rules “weakened protections for survivors of sexual assault and diminished the promise of an education free from discrimination,” the Biden administration said.
“Our proposed changes will allow us to continue that progress and ensure all our nation’s students – no matter where they live, who they are, or whom they love – can learn, grow, and thrive in school,” Education Secretary Miguel Cardona, PhD, said in a news release. “We welcome public comment on these critical regulations so we can further the Biden-Harris Administration’s mission of creating educational environments free from sex discrimination and sexual violence.”
The revisions will go through a long period of public comment before they are set into law. Still, the proposed changes mark a way forward for trans students who are not explicitly protected under Title IX, and they offer solace to assault survivors who may have felt discouraged to come forward and report under Ms. DeVos’s rules.
“The proposed regulations reflect the [Education] Department’s commitment to give full effect to Title IX, ensuring that no person experiences sex discrimination in education, and that school procedures for addressing complaints of sex discrimination, including sexual violence and other forms of sex-based harassment, are clear, effective, and fair to all involved,” said Catherine Lhamon, JD, assistant secretary for the Education Department’s Office Of Civil Rights.
More specific rules about transgender students’ participation in school sports are still to come.
A version of this article first appeared on WebMD.com.
On the 50th anniversary of Title IX’s inception, the Biden administration has proposed changes to the law that would protect transgender students and assault survivors on college and university campuses.
With these changes, the protections provided by Title IX – a civil rights law that prohibits sex-based discrimination in schools that receive federal funding – would now be extended to students who identify as trans. The update would ensure that government-funded schools make proper accommodations for a trans student population, such as allowing students to use bathrooms and other facilities that align with their gender identity, and enforcing the use of students’ correct pronouns.
The revisions also seek to undo amendments made to the law by Betsy DeVos, who was secretary of education during the Trump presidency, which strengthened due process protections for students accused of sexual assault and narrowed the definition of sexual harassment. These rules “weakened protections for survivors of sexual assault and diminished the promise of an education free from discrimination,” the Biden administration said.
“Our proposed changes will allow us to continue that progress and ensure all our nation’s students – no matter where they live, who they are, or whom they love – can learn, grow, and thrive in school,” Education Secretary Miguel Cardona, PhD, said in a news release. “We welcome public comment on these critical regulations so we can further the Biden-Harris Administration’s mission of creating educational environments free from sex discrimination and sexual violence.”
The revisions will go through a long period of public comment before they are set into law. Still, the proposed changes mark a way forward for trans students who are not explicitly protected under Title IX, and they offer solace to assault survivors who may have felt discouraged to come forward and report under Ms. DeVos’s rules.
“The proposed regulations reflect the [Education] Department’s commitment to give full effect to Title IX, ensuring that no person experiences sex discrimination in education, and that school procedures for addressing complaints of sex discrimination, including sexual violence and other forms of sex-based harassment, are clear, effective, and fair to all involved,” said Catherine Lhamon, JD, assistant secretary for the Education Department’s Office Of Civil Rights.
More specific rules about transgender students’ participation in school sports are still to come.
A version of this article first appeared on WebMD.com.
$3 billion in cancer drug waste: Can it be salvaged?
Three billion dollars: It’s enough to finance the annual out-of-pocket costs for 1 in 7 patients with cancer. It would cover almost half of the National Cancer Institute’s annual budget. And it could fund President Biden’s entire Cancer Moonshot program, with more than a billion to spare.
It’s also how much the United States spends on unused cancer drugs each year, some experts estimate.
Drug companies typically sell infused drugs in one or two single-dose vial sizes, but patients don’t come in such neat packages. A patient may need 300 mg of a drug that is only sold as 200 mg vials, which means half of a vial will go to waste.
Although most oncology drugs don’t incur substantial waste, even small volumes can translate to millions of dollars a year.
But can this money be saved or reallocated, if only we delivered drugs more efficiently?
Some experts don’t believe that’s possible.
“Attempts to recoup money for discarded drugs wouldn’t happen in a vacuum,” said Robin Yabroff, PhD, MBA, an epidemiologist and scientific vice president of Health Services Research at the American Cancer Society, who was part of a committee commissioned to evaluate the costs associated with discarded drugs.
The potential catch of any widespread effort to seek repayment or reduce the amount of discarded drugs, Dr. Yabroff and colleagues note, is that manufacturers would “simply increase the price of the vial.”
In other words, attempting to fix one problem may lead to another — essentially a whack-a-mole of cancer costs, which are projected to balloon to $246 billion by 2030.
What this means is without sweeping policies to rein in cancer care costs, oncologists can only do so much. And every little bit counts.
“We are left chipping away at this monster of cancer care costs,” said Adam Binder, MD, a medical oncologist at the Sidney Kimmel Cancer Center at Jefferson Health in Philadelphia.
Millions spent on “reasonable amount” of waste
Michal Sarfaty, MD, was excited when enfortumab vedotin came on the market to treat advanced urothelial cancer in late 2019.
The cost of the drug, however, tempered her enthusiasm.
Enfortumab vedotin is a “great drug,” said Dr. Sarfaty, an oncologist at the Sheba Medical Center, Ramat Gan, Israel. But it can cost upwards of $500,000 a year for an average-weight man.
Given the expense, Dr. Sarfaty wanted to understand how much of the drug gets thrown away. During a fellowship at Memorial Sloan Kettering (MSK) Cancer Center in New York, Dr. Sarfaty explored the amount of unused enfortumab vedotin among the 64 patients who received the drug in 2020. She, along with a team at MSK, calculated the price tag of that waste and extrapolated those estimates for patients across the country.
Although waste occurred in almost half of administered doses (367 of 793), only a small volume got discarded — 2.9% per dose, on average.
Multiplying unused milligrams by the cost per milligram, Dr. Sarfaty and colleagues estimated that, for each patient, $3,127 of the drug got discarded. When calculated over the year, the cost came to just over $200,000 at MSK, and nearly $15 million when projected across the approved patient population in the United States.
“Ultimately, we did not see a lot of waste with this specific drug,” Dr. Sarfaty said. “Under 2.9% is considered a reasonable amount, below the 3% threshold Peter Bach, MD, and colleagues recommend. But even with this small amount of waste, the cost per patient and to the system remains notable.”
The problem with recouping drug waste
Estimates from the Centers for Medicare & Medicaid Services (CMS), which tracks costs associated with discarded weight-based drugs covered under Medicare Part B, support the notion that small quantities of discarded drugs can still translate to big bucks.
Since 2017, CMS has required healthcare providers to report the volume of drugs discarded from a single-dose vial using a code, known as the JW modifier. The JW modifier means that providers can be reimbursed for the entire vial amount, not just the quantity the patient used.
In 2019, claims data from Medicare Part B showed that 1.85% of discarded rituximab came to $33.3 million. For infliximab, the 1.55% of discarded liquid translated to $15 million, and just 0.36% of discarded pembrolizumab reached $10 million.
However, experts question whether the JW modifier accurately reflects the quantity of drugs discarded.
According to the 2021 report from the National Academies of Sciences, Engineering, and Medicine (NASEM), most physicians don’t use the JW modifier. Among Medicare claims, 16.2% included the JW modifier in 2017 and 16.9% did in 2018.
The rate was significantly lower for private insurance. Of more than 4 million private insurance claims on 77 drugs made in 2017 and 2018, only 3.6% included the JW modifier; 15 of these drugs had no JW claims.
“Although we found that most physicians don’t use the JW modifier, even those who do, don’t use it consistently, even for the same patient,” said Dr. Yabroff, a co-author on the report.
Going a step further, Dr. Yabroff and colleagues argue that even if everyone used the JW modifier as intended, manufacturers would probably increase the price of drugs to compensate for any loss, potentially eliminating savings for payers.
That’s because, in the United States, manufacturers typically base drug prices on a patient and payers’ “willingness to pay for better health,” not on the volume of liquid used. Take a patient who pays $2,000 to receive the dose they need. If that dose is 600 mg but requires using two vials of 400 mg, then “to the patient, the 600-mg dose is worth $2,000, and the remainder has no value whatsoever,” the NASEM authors argue.
The authors parallel this scenario to purchasing a designer coat or dress. If that item requires alterations that remove a section of material, “the customer does not typically get a rebate because all the fabric was not needed,” the NASEM team writes.
But there’s a flaw in this rationale, argues Daniel Goldstein, MD, a medical oncologist at the Rabin Medical Center, Petah Tikva, Israel. A person’s willingness to pay for better health assumes that the price of a drug is based on proper market forces, where a drug’s cost and its effectiveness are in harmony.
“The problem is we’re operating in a broken market where the prices of oncology drugs have no real bearing on their efficacy,” said Dr. Goldstein.
And, as Dr. Bach noted in a 2021 Health Affairs piece, willingness to pay also requires that consumers know what they’re paying and allows them to walk away from an excessively high price.
But neither is a reality.
For one, Dr. Bach explains, companies may lowball the monthly price of a drug. In 2020, GlaxoSmithKline (GSK) announced that its new drug Blenrep would carry a list price of $8,277 per vial, or about $23,900 per month for an average 79 kg (175 lb) patient. That price accounts for two vials of the drug. But, according to Dr. Bach, “what GSK left out is that 44% of U.S. adults weigh more than 80 kg, and above that weight, three vials are needed per dose.” That would raise the average monthly cost to $30,479.
Perhaps more importantly, consumers can’t easily walk away.
“Medicare can’t negotiate prices and is forced to pay what a drug company says,” Dr. Goldstein said. “This is very different to when I buy a coat. If the price is too high, I can walk away.”
Fixed dosing: A solution or a new problem?
Efforts to reduce the financial impact of discarded cancer drugs can blow back on physicians, patients, and payers in other unanticipated ways. Take fixed dosing. Although chemotherapy dosing remains weight-based, many targeted therapies — such as nivolumab and pembrolizumab — recently transitioned to a fixed dosing regimen.
Administering a fixed, instead of weight-based, dose eliminates waste but can create new problems.
“Patients with cancer not only tend to get too high a dose of the drug, but costs go up significantly,” said Dr. Goldstein. In a 2017 analysis, Dr. Goldstein and colleagues compared dosing strategies in patients with metastatic non–small cell lung cancer who received pembrolizumab. The team found that the total annual cost of weight-based dosing was $2.6 billion, whereas the cost of the fixed dosing strategy was $3.44 billion — 24% more. In other words, personalized weight-based dosing would save more than $825 million dollars in the United States each year.
A 2020 analysis based in France found a similar cost increase of 26% for fixed dosing of pembrolizumab as well as nivolumab.
“I’ve argued we should go back to weight-based dosing,” Dr. Goldstein said. “Why should we give a higher dose with the same efficacy when that dose will cost significantly more and has the potential to increase adverse events?”
Does dose rounding work?
Rose DiMarco, PharmD, BCPS, BCOP, keeps a tight watch on patients being treated at the Sidney Kimmel Cancer Center at Jefferson Health in Philadelphia.
Dr. DiMarco educates patients about their treatment plan, reviews their lab results, and monitors them for side effects and drug interactions.
She also thinks a lot about costs.
“We spend about $100,000 a day on oncology drugs, and we want to make sure we’re not being wasteful,” Dr. DiMarco said in an interview.
One major initiative to curb waste and reduce costs at Jefferson has centered on dose rounding, which calculates whether a specific dose can be altered slightly to conserve vials and prevent waste. According to the Hematology/Oncology Pharmacy Association, a patient can receive up to 10% more or less of a weight-based dose without impacting treatment efficacy.
If, for instance, a patient requires 380 mg, but two vials come to 400 mg, rounding up that dose by approximately 5% means eliminating 20 mg that would go unused. But if that patient requires 420 mg, rounding down about 5% means substantial savings from not opening a new vial.
At Jefferson, Dr. DiMarco and her pharmacy colleagues map out dose ranges for all patients. Anyone who falls inside the 10% may be eligible for dose rounding. Anyone who doesn’t will receive the usual dose.
Although it is a challenge to implement, dose rounding has become standard of care at many cancer centers across the United States and is linked to substantial savings.
A 2018 analysis projected annual savings of $865,000 associated with rounding down eight monoclonal antibodies for patients with metastatic disease at a community cancer center. A more recent analysis from the Mayo Clinic found that dose rounding saved a total of 9,814 drug vials — 4485 of which were cancer drugs and 5329 of which were biologics — and resulted in $7.3 million in savings over 6 months in 2019 — $1.56 million from oncology agents and $5.7 from biologics.
And in a small 2019 analysis, researchers at Jefferson showed dose rounding of one monoclonal antibody saved approximately $30,000 in just 3 months, Dr. DiMarco noted.
“Not only does this process reduce costs and waste, but it also standardizes the preparation of hazardous medications, which can help prevent medication errors,” Dr. DiMarco said.
Nibbling around the edges
Despite estimates that scale into the billions of dollars, “drug wastage is just a small part of overall cancer costs,” Dr. Sarfaty said.
Fumiko Chino, MD, a radiation oncologist at MSK, agrees. “When we talk about affordability and cost, we can nibble around the edges of what’s really important,” Dr. Chino said. “Discarded drugs may cost a lot when you consider them in aggregate, but they are not as important as negotiated drug prices, which could substantially reduce overall costs.”
And until drug prices are addressed on a broader policy level, the cost of cancer care likely won’t improve in a meaningful way.
“But for the patient sitting in front of me, my focus will always be to provide the best care possible,” Dr. Binder said.
A version of this article first appeared on Medscape.com.
Three billion dollars: It’s enough to finance the annual out-of-pocket costs for 1 in 7 patients with cancer. It would cover almost half of the National Cancer Institute’s annual budget. And it could fund President Biden’s entire Cancer Moonshot program, with more than a billion to spare.
It’s also how much the United States spends on unused cancer drugs each year, some experts estimate.
Drug companies typically sell infused drugs in one or two single-dose vial sizes, but patients don’t come in such neat packages. A patient may need 300 mg of a drug that is only sold as 200 mg vials, which means half of a vial will go to waste.
Although most oncology drugs don’t incur substantial waste, even small volumes can translate to millions of dollars a year.
But can this money be saved or reallocated, if only we delivered drugs more efficiently?
Some experts don’t believe that’s possible.
“Attempts to recoup money for discarded drugs wouldn’t happen in a vacuum,” said Robin Yabroff, PhD, MBA, an epidemiologist and scientific vice president of Health Services Research at the American Cancer Society, who was part of a committee commissioned to evaluate the costs associated with discarded drugs.
The potential catch of any widespread effort to seek repayment or reduce the amount of discarded drugs, Dr. Yabroff and colleagues note, is that manufacturers would “simply increase the price of the vial.”
In other words, attempting to fix one problem may lead to another — essentially a whack-a-mole of cancer costs, which are projected to balloon to $246 billion by 2030.
What this means is without sweeping policies to rein in cancer care costs, oncologists can only do so much. And every little bit counts.
“We are left chipping away at this monster of cancer care costs,” said Adam Binder, MD, a medical oncologist at the Sidney Kimmel Cancer Center at Jefferson Health in Philadelphia.
Millions spent on “reasonable amount” of waste
Michal Sarfaty, MD, was excited when enfortumab vedotin came on the market to treat advanced urothelial cancer in late 2019.
The cost of the drug, however, tempered her enthusiasm.
Enfortumab vedotin is a “great drug,” said Dr. Sarfaty, an oncologist at the Sheba Medical Center, Ramat Gan, Israel. But it can cost upwards of $500,000 a year for an average-weight man.
Given the expense, Dr. Sarfaty wanted to understand how much of the drug gets thrown away. During a fellowship at Memorial Sloan Kettering (MSK) Cancer Center in New York, Dr. Sarfaty explored the amount of unused enfortumab vedotin among the 64 patients who received the drug in 2020. She, along with a team at MSK, calculated the price tag of that waste and extrapolated those estimates for patients across the country.
Although waste occurred in almost half of administered doses (367 of 793), only a small volume got discarded — 2.9% per dose, on average.
Multiplying unused milligrams by the cost per milligram, Dr. Sarfaty and colleagues estimated that, for each patient, $3,127 of the drug got discarded. When calculated over the year, the cost came to just over $200,000 at MSK, and nearly $15 million when projected across the approved patient population in the United States.
“Ultimately, we did not see a lot of waste with this specific drug,” Dr. Sarfaty said. “Under 2.9% is considered a reasonable amount, below the 3% threshold Peter Bach, MD, and colleagues recommend. But even with this small amount of waste, the cost per patient and to the system remains notable.”
The problem with recouping drug waste
Estimates from the Centers for Medicare & Medicaid Services (CMS), which tracks costs associated with discarded weight-based drugs covered under Medicare Part B, support the notion that small quantities of discarded drugs can still translate to big bucks.
Since 2017, CMS has required healthcare providers to report the volume of drugs discarded from a single-dose vial using a code, known as the JW modifier. The JW modifier means that providers can be reimbursed for the entire vial amount, not just the quantity the patient used.
In 2019, claims data from Medicare Part B showed that 1.85% of discarded rituximab came to $33.3 million. For infliximab, the 1.55% of discarded liquid translated to $15 million, and just 0.36% of discarded pembrolizumab reached $10 million.
However, experts question whether the JW modifier accurately reflects the quantity of drugs discarded.
According to the 2021 report from the National Academies of Sciences, Engineering, and Medicine (NASEM), most physicians don’t use the JW modifier. Among Medicare claims, 16.2% included the JW modifier in 2017 and 16.9% did in 2018.
The rate was significantly lower for private insurance. Of more than 4 million private insurance claims on 77 drugs made in 2017 and 2018, only 3.6% included the JW modifier; 15 of these drugs had no JW claims.
“Although we found that most physicians don’t use the JW modifier, even those who do, don’t use it consistently, even for the same patient,” said Dr. Yabroff, a co-author on the report.
Going a step further, Dr. Yabroff and colleagues argue that even if everyone used the JW modifier as intended, manufacturers would probably increase the price of drugs to compensate for any loss, potentially eliminating savings for payers.
That’s because, in the United States, manufacturers typically base drug prices on a patient and payers’ “willingness to pay for better health,” not on the volume of liquid used. Take a patient who pays $2,000 to receive the dose they need. If that dose is 600 mg but requires using two vials of 400 mg, then “to the patient, the 600-mg dose is worth $2,000, and the remainder has no value whatsoever,” the NASEM authors argue.
The authors parallel this scenario to purchasing a designer coat or dress. If that item requires alterations that remove a section of material, “the customer does not typically get a rebate because all the fabric was not needed,” the NASEM team writes.
But there’s a flaw in this rationale, argues Daniel Goldstein, MD, a medical oncologist at the Rabin Medical Center, Petah Tikva, Israel. A person’s willingness to pay for better health assumes that the price of a drug is based on proper market forces, where a drug’s cost and its effectiveness are in harmony.
“The problem is we’re operating in a broken market where the prices of oncology drugs have no real bearing on their efficacy,” said Dr. Goldstein.
And, as Dr. Bach noted in a 2021 Health Affairs piece, willingness to pay also requires that consumers know what they’re paying and allows them to walk away from an excessively high price.
But neither is a reality.
For one, Dr. Bach explains, companies may lowball the monthly price of a drug. In 2020, GlaxoSmithKline (GSK) announced that its new drug Blenrep would carry a list price of $8,277 per vial, or about $23,900 per month for an average 79 kg (175 lb) patient. That price accounts for two vials of the drug. But, according to Dr. Bach, “what GSK left out is that 44% of U.S. adults weigh more than 80 kg, and above that weight, three vials are needed per dose.” That would raise the average monthly cost to $30,479.
Perhaps more importantly, consumers can’t easily walk away.
“Medicare can’t negotiate prices and is forced to pay what a drug company says,” Dr. Goldstein said. “This is very different to when I buy a coat. If the price is too high, I can walk away.”
Fixed dosing: A solution or a new problem?
Efforts to reduce the financial impact of discarded cancer drugs can blow back on physicians, patients, and payers in other unanticipated ways. Take fixed dosing. Although chemotherapy dosing remains weight-based, many targeted therapies — such as nivolumab and pembrolizumab — recently transitioned to a fixed dosing regimen.
Administering a fixed, instead of weight-based, dose eliminates waste but can create new problems.
“Patients with cancer not only tend to get too high a dose of the drug, but costs go up significantly,” said Dr. Goldstein. In a 2017 analysis, Dr. Goldstein and colleagues compared dosing strategies in patients with metastatic non–small cell lung cancer who received pembrolizumab. The team found that the total annual cost of weight-based dosing was $2.6 billion, whereas the cost of the fixed dosing strategy was $3.44 billion — 24% more. In other words, personalized weight-based dosing would save more than $825 million dollars in the United States each year.
A 2020 analysis based in France found a similar cost increase of 26% for fixed dosing of pembrolizumab as well as nivolumab.
“I’ve argued we should go back to weight-based dosing,” Dr. Goldstein said. “Why should we give a higher dose with the same efficacy when that dose will cost significantly more and has the potential to increase adverse events?”
Does dose rounding work?
Rose DiMarco, PharmD, BCPS, BCOP, keeps a tight watch on patients being treated at the Sidney Kimmel Cancer Center at Jefferson Health in Philadelphia.
Dr. DiMarco educates patients about their treatment plan, reviews their lab results, and monitors them for side effects and drug interactions.
She also thinks a lot about costs.
“We spend about $100,000 a day on oncology drugs, and we want to make sure we’re not being wasteful,” Dr. DiMarco said in an interview.
One major initiative to curb waste and reduce costs at Jefferson has centered on dose rounding, which calculates whether a specific dose can be altered slightly to conserve vials and prevent waste. According to the Hematology/Oncology Pharmacy Association, a patient can receive up to 10% more or less of a weight-based dose without impacting treatment efficacy.
If, for instance, a patient requires 380 mg, but two vials come to 400 mg, rounding up that dose by approximately 5% means eliminating 20 mg that would go unused. But if that patient requires 420 mg, rounding down about 5% means substantial savings from not opening a new vial.
At Jefferson, Dr. DiMarco and her pharmacy colleagues map out dose ranges for all patients. Anyone who falls inside the 10% may be eligible for dose rounding. Anyone who doesn’t will receive the usual dose.
Although it is a challenge to implement, dose rounding has become standard of care at many cancer centers across the United States and is linked to substantial savings.
A 2018 analysis projected annual savings of $865,000 associated with rounding down eight monoclonal antibodies for patients with metastatic disease at a community cancer center. A more recent analysis from the Mayo Clinic found that dose rounding saved a total of 9,814 drug vials — 4485 of which were cancer drugs and 5329 of which were biologics — and resulted in $7.3 million in savings over 6 months in 2019 — $1.56 million from oncology agents and $5.7 from biologics.
And in a small 2019 analysis, researchers at Jefferson showed dose rounding of one monoclonal antibody saved approximately $30,000 in just 3 months, Dr. DiMarco noted.
“Not only does this process reduce costs and waste, but it also standardizes the preparation of hazardous medications, which can help prevent medication errors,” Dr. DiMarco said.
Nibbling around the edges
Despite estimates that scale into the billions of dollars, “drug wastage is just a small part of overall cancer costs,” Dr. Sarfaty said.
Fumiko Chino, MD, a radiation oncologist at MSK, agrees. “When we talk about affordability and cost, we can nibble around the edges of what’s really important,” Dr. Chino said. “Discarded drugs may cost a lot when you consider them in aggregate, but they are not as important as negotiated drug prices, which could substantially reduce overall costs.”
And until drug prices are addressed on a broader policy level, the cost of cancer care likely won’t improve in a meaningful way.
“But for the patient sitting in front of me, my focus will always be to provide the best care possible,” Dr. Binder said.
A version of this article first appeared on Medscape.com.
Three billion dollars: It’s enough to finance the annual out-of-pocket costs for 1 in 7 patients with cancer. It would cover almost half of the National Cancer Institute’s annual budget. And it could fund President Biden’s entire Cancer Moonshot program, with more than a billion to spare.
It’s also how much the United States spends on unused cancer drugs each year, some experts estimate.
Drug companies typically sell infused drugs in one or two single-dose vial sizes, but patients don’t come in such neat packages. A patient may need 300 mg of a drug that is only sold as 200 mg vials, which means half of a vial will go to waste.
Although most oncology drugs don’t incur substantial waste, even small volumes can translate to millions of dollars a year.
But can this money be saved or reallocated, if only we delivered drugs more efficiently?
Some experts don’t believe that’s possible.
“Attempts to recoup money for discarded drugs wouldn’t happen in a vacuum,” said Robin Yabroff, PhD, MBA, an epidemiologist and scientific vice president of Health Services Research at the American Cancer Society, who was part of a committee commissioned to evaluate the costs associated with discarded drugs.
The potential catch of any widespread effort to seek repayment or reduce the amount of discarded drugs, Dr. Yabroff and colleagues note, is that manufacturers would “simply increase the price of the vial.”
In other words, attempting to fix one problem may lead to another — essentially a whack-a-mole of cancer costs, which are projected to balloon to $246 billion by 2030.
What this means is without sweeping policies to rein in cancer care costs, oncologists can only do so much. And every little bit counts.
“We are left chipping away at this monster of cancer care costs,” said Adam Binder, MD, a medical oncologist at the Sidney Kimmel Cancer Center at Jefferson Health in Philadelphia.
Millions spent on “reasonable amount” of waste
Michal Sarfaty, MD, was excited when enfortumab vedotin came on the market to treat advanced urothelial cancer in late 2019.
The cost of the drug, however, tempered her enthusiasm.
Enfortumab vedotin is a “great drug,” said Dr. Sarfaty, an oncologist at the Sheba Medical Center, Ramat Gan, Israel. But it can cost upwards of $500,000 a year for an average-weight man.
Given the expense, Dr. Sarfaty wanted to understand how much of the drug gets thrown away. During a fellowship at Memorial Sloan Kettering (MSK) Cancer Center in New York, Dr. Sarfaty explored the amount of unused enfortumab vedotin among the 64 patients who received the drug in 2020. She, along with a team at MSK, calculated the price tag of that waste and extrapolated those estimates for patients across the country.
Although waste occurred in almost half of administered doses (367 of 793), only a small volume got discarded — 2.9% per dose, on average.
Multiplying unused milligrams by the cost per milligram, Dr. Sarfaty and colleagues estimated that, for each patient, $3,127 of the drug got discarded. When calculated over the year, the cost came to just over $200,000 at MSK, and nearly $15 million when projected across the approved patient population in the United States.
“Ultimately, we did not see a lot of waste with this specific drug,” Dr. Sarfaty said. “Under 2.9% is considered a reasonable amount, below the 3% threshold Peter Bach, MD, and colleagues recommend. But even with this small amount of waste, the cost per patient and to the system remains notable.”
The problem with recouping drug waste
Estimates from the Centers for Medicare & Medicaid Services (CMS), which tracks costs associated with discarded weight-based drugs covered under Medicare Part B, support the notion that small quantities of discarded drugs can still translate to big bucks.
Since 2017, CMS has required healthcare providers to report the volume of drugs discarded from a single-dose vial using a code, known as the JW modifier. The JW modifier means that providers can be reimbursed for the entire vial amount, not just the quantity the patient used.
In 2019, claims data from Medicare Part B showed that 1.85% of discarded rituximab came to $33.3 million. For infliximab, the 1.55% of discarded liquid translated to $15 million, and just 0.36% of discarded pembrolizumab reached $10 million.
However, experts question whether the JW modifier accurately reflects the quantity of drugs discarded.
According to the 2021 report from the National Academies of Sciences, Engineering, and Medicine (NASEM), most physicians don’t use the JW modifier. Among Medicare claims, 16.2% included the JW modifier in 2017 and 16.9% did in 2018.
The rate was significantly lower for private insurance. Of more than 4 million private insurance claims on 77 drugs made in 2017 and 2018, only 3.6% included the JW modifier; 15 of these drugs had no JW claims.
“Although we found that most physicians don’t use the JW modifier, even those who do, don’t use it consistently, even for the same patient,” said Dr. Yabroff, a co-author on the report.
Going a step further, Dr. Yabroff and colleagues argue that even if everyone used the JW modifier as intended, manufacturers would probably increase the price of drugs to compensate for any loss, potentially eliminating savings for payers.
That’s because, in the United States, manufacturers typically base drug prices on a patient and payers’ “willingness to pay for better health,” not on the volume of liquid used. Take a patient who pays $2,000 to receive the dose they need. If that dose is 600 mg but requires using two vials of 400 mg, then “to the patient, the 600-mg dose is worth $2,000, and the remainder has no value whatsoever,” the NASEM authors argue.
The authors parallel this scenario to purchasing a designer coat or dress. If that item requires alterations that remove a section of material, “the customer does not typically get a rebate because all the fabric was not needed,” the NASEM team writes.
But there’s a flaw in this rationale, argues Daniel Goldstein, MD, a medical oncologist at the Rabin Medical Center, Petah Tikva, Israel. A person’s willingness to pay for better health assumes that the price of a drug is based on proper market forces, where a drug’s cost and its effectiveness are in harmony.
“The problem is we’re operating in a broken market where the prices of oncology drugs have no real bearing on their efficacy,” said Dr. Goldstein.
And, as Dr. Bach noted in a 2021 Health Affairs piece, willingness to pay also requires that consumers know what they’re paying and allows them to walk away from an excessively high price.
But neither is a reality.
For one, Dr. Bach explains, companies may lowball the monthly price of a drug. In 2020, GlaxoSmithKline (GSK) announced that its new drug Blenrep would carry a list price of $8,277 per vial, or about $23,900 per month for an average 79 kg (175 lb) patient. That price accounts for two vials of the drug. But, according to Dr. Bach, “what GSK left out is that 44% of U.S. adults weigh more than 80 kg, and above that weight, three vials are needed per dose.” That would raise the average monthly cost to $30,479.
Perhaps more importantly, consumers can’t easily walk away.
“Medicare can’t negotiate prices and is forced to pay what a drug company says,” Dr. Goldstein said. “This is very different to when I buy a coat. If the price is too high, I can walk away.”
Fixed dosing: A solution or a new problem?
Efforts to reduce the financial impact of discarded cancer drugs can blow back on physicians, patients, and payers in other unanticipated ways. Take fixed dosing. Although chemotherapy dosing remains weight-based, many targeted therapies — such as nivolumab and pembrolizumab — recently transitioned to a fixed dosing regimen.
Administering a fixed, instead of weight-based, dose eliminates waste but can create new problems.
“Patients with cancer not only tend to get too high a dose of the drug, but costs go up significantly,” said Dr. Goldstein. In a 2017 analysis, Dr. Goldstein and colleagues compared dosing strategies in patients with metastatic non–small cell lung cancer who received pembrolizumab. The team found that the total annual cost of weight-based dosing was $2.6 billion, whereas the cost of the fixed dosing strategy was $3.44 billion — 24% more. In other words, personalized weight-based dosing would save more than $825 million dollars in the United States each year.
A 2020 analysis based in France found a similar cost increase of 26% for fixed dosing of pembrolizumab as well as nivolumab.
“I’ve argued we should go back to weight-based dosing,” Dr. Goldstein said. “Why should we give a higher dose with the same efficacy when that dose will cost significantly more and has the potential to increase adverse events?”
Does dose rounding work?
Rose DiMarco, PharmD, BCPS, BCOP, keeps a tight watch on patients being treated at the Sidney Kimmel Cancer Center at Jefferson Health in Philadelphia.
Dr. DiMarco educates patients about their treatment plan, reviews their lab results, and monitors them for side effects and drug interactions.
She also thinks a lot about costs.
“We spend about $100,000 a day on oncology drugs, and we want to make sure we’re not being wasteful,” Dr. DiMarco said in an interview.
One major initiative to curb waste and reduce costs at Jefferson has centered on dose rounding, which calculates whether a specific dose can be altered slightly to conserve vials and prevent waste. According to the Hematology/Oncology Pharmacy Association, a patient can receive up to 10% more or less of a weight-based dose without impacting treatment efficacy.
If, for instance, a patient requires 380 mg, but two vials come to 400 mg, rounding up that dose by approximately 5% means eliminating 20 mg that would go unused. But if that patient requires 420 mg, rounding down about 5% means substantial savings from not opening a new vial.
At Jefferson, Dr. DiMarco and her pharmacy colleagues map out dose ranges for all patients. Anyone who falls inside the 10% may be eligible for dose rounding. Anyone who doesn’t will receive the usual dose.
Although it is a challenge to implement, dose rounding has become standard of care at many cancer centers across the United States and is linked to substantial savings.
A 2018 analysis projected annual savings of $865,000 associated with rounding down eight monoclonal antibodies for patients with metastatic disease at a community cancer center. A more recent analysis from the Mayo Clinic found that dose rounding saved a total of 9,814 drug vials — 4485 of which were cancer drugs and 5329 of which were biologics — and resulted in $7.3 million in savings over 6 months in 2019 — $1.56 million from oncology agents and $5.7 from biologics.
And in a small 2019 analysis, researchers at Jefferson showed dose rounding of one monoclonal antibody saved approximately $30,000 in just 3 months, Dr. DiMarco noted.
“Not only does this process reduce costs and waste, but it also standardizes the preparation of hazardous medications, which can help prevent medication errors,” Dr. DiMarco said.
Nibbling around the edges
Despite estimates that scale into the billions of dollars, “drug wastage is just a small part of overall cancer costs,” Dr. Sarfaty said.
Fumiko Chino, MD, a radiation oncologist at MSK, agrees. “When we talk about affordability and cost, we can nibble around the edges of what’s really important,” Dr. Chino said. “Discarded drugs may cost a lot when you consider them in aggregate, but they are not as important as negotiated drug prices, which could substantially reduce overall costs.”
And until drug prices are addressed on a broader policy level, the cost of cancer care likely won’t improve in a meaningful way.
“But for the patient sitting in front of me, my focus will always be to provide the best care possible,” Dr. Binder said.
A version of this article first appeared on Medscape.com.
FDA orders Juul to stop selling E-cigarettes
The marketing denial order covers all the company’s products in the United States, which means Juul must stop distributing the products and remove everything on the market. That includes the Juul device and flavor replacement pods in the tobacco and menthol flavors.
“Today’s action is further progress on the FDA’s commitment to ensuring that all e-cigarette and electronic nicotine delivery system products currently being marketed to consumers meet our public health standards,” Robert Califf, MD, the FDA commissioner, said in the announcement.
“The agency has dedicated significant resources to review products from the companies that account for most of the U.S. market,” he said. “We recognize these make up a significant part of the available products and many have played a disproportionate role in the rise in youth vaping.”
The marketing denial order covers only the commercial distribution and retail sale of Juul’s products and doesn’t restrict consumer possession or use. The FDA “cannot and will not” enforce actions against consumers, the agency said.
The order comes after a 2-year review of the company’s application seeking authorization to continue selling non–fruit-flavored products, such as menthol and tobacco. The FDA determined the application “lacked sufficient evidence regarding the toxicological profile of the products to demonstrate that marketing of the products would be appropriate for the protection of the public health.”
Some of Juul’s study findings raised concerns because of “insufficient and conflicting data,” the FDA said, including potentially harmful chemicals leaching from the Juul liquid replacement pods.
“To date, the FDA has not received clinical information to suggest an immediate hazard associated with the use of the JUUL device or JUUL pods,” the agency said. “However, the [orders] issued today reflect FDA’s determination that there is insufficient evidence to assess the potential toxicological risks of using the JUUL products.”
Juul is expected to appeal the FDA’s decision, according to The New York Times.
In recent years, the FDA has reviewed marketing applications from Juul and other e-cigarette companies as anti-tobacco groups have called for new rules to limit products that led to a surge in youth vaping during the past decade. At the same time, advocates of e-cigarettes and nicotine-delivery devices have said the products help adult smokers to quit cigarettes and other tobacco products.
Juul, in particular, has been blamed for fueling the surge in underage vaping due to fruity flavors and hip marketing, according to The Wall Street Journal. The company removed sweet and fruity flavors from shelves in 2019 and has been trying to repair its reputation by limiting its marketing and focusing on adult cigarette smokers.
In 2020, all e-cigarette manufacturers in the United States were required to submit their products for FDA review to stay on the market, the newspaper reported. The agency has been weighing the potential benefits for adult cigarette smokers against the harms for young people.
The FDA banned the sale of fruit- and mint-flavored cartridges and juice pods in 2020, but menthol and tobacco-flavored products were left on the market, according to USA Today. In September 2021, the agency also banned the sale of hundreds of thousands of vaping and e-cigarette products but didn’t rule on Juul.
Meanwhile, the FDA has cleared Reynolds American and NJOY Holdings – two of Juul’s biggest rivals – to keep tobacco-flavored products on the market. Industry experts expected Juul to receive similar clearance, the Journal reported.
Juul, which was at the top of the U.S. e-cigarette market in 2018, has moved to second place behind Reynolds’s Vuse brand, the newspaper reported. The United States represents most of the company’s revenue, though its products are also available in Canada, the United Kingdom, France, Italy, and the Philippines.
Underage vaping has fallen in the United States since federal restrictions raised the legal purchase age for tobacco products to 21 and banned the sale of sweet and fruity cartridges, according to the Journal. Juul’s popularity has also dropped among youth, with other products such as Puff Bar, Vuse, and Smok becoming more popular among e-cigarette users in high school.
In a separate decision announced this week, the FDA is also moving forward with a plan to reduce the amount of nicotine in cigarettes. The decision, which has been years in the making, is aimed at prompting millions of cigarette users to quit smoking or switch to alternatives such as e-cigarettes, as well as limit the number of users who pick up smoking at an early age.
A version of this article first appeared on WebMD.com .
The marketing denial order covers all the company’s products in the United States, which means Juul must stop distributing the products and remove everything on the market. That includes the Juul device and flavor replacement pods in the tobacco and menthol flavors.
“Today’s action is further progress on the FDA’s commitment to ensuring that all e-cigarette and electronic nicotine delivery system products currently being marketed to consumers meet our public health standards,” Robert Califf, MD, the FDA commissioner, said in the announcement.
“The agency has dedicated significant resources to review products from the companies that account for most of the U.S. market,” he said. “We recognize these make up a significant part of the available products and many have played a disproportionate role in the rise in youth vaping.”
The marketing denial order covers only the commercial distribution and retail sale of Juul’s products and doesn’t restrict consumer possession or use. The FDA “cannot and will not” enforce actions against consumers, the agency said.
The order comes after a 2-year review of the company’s application seeking authorization to continue selling non–fruit-flavored products, such as menthol and tobacco. The FDA determined the application “lacked sufficient evidence regarding the toxicological profile of the products to demonstrate that marketing of the products would be appropriate for the protection of the public health.”
Some of Juul’s study findings raised concerns because of “insufficient and conflicting data,” the FDA said, including potentially harmful chemicals leaching from the Juul liquid replacement pods.
“To date, the FDA has not received clinical information to suggest an immediate hazard associated with the use of the JUUL device or JUUL pods,” the agency said. “However, the [orders] issued today reflect FDA’s determination that there is insufficient evidence to assess the potential toxicological risks of using the JUUL products.”
Juul is expected to appeal the FDA’s decision, according to The New York Times.
In recent years, the FDA has reviewed marketing applications from Juul and other e-cigarette companies as anti-tobacco groups have called for new rules to limit products that led to a surge in youth vaping during the past decade. At the same time, advocates of e-cigarettes and nicotine-delivery devices have said the products help adult smokers to quit cigarettes and other tobacco products.
Juul, in particular, has been blamed for fueling the surge in underage vaping due to fruity flavors and hip marketing, according to The Wall Street Journal. The company removed sweet and fruity flavors from shelves in 2019 and has been trying to repair its reputation by limiting its marketing and focusing on adult cigarette smokers.
In 2020, all e-cigarette manufacturers in the United States were required to submit their products for FDA review to stay on the market, the newspaper reported. The agency has been weighing the potential benefits for adult cigarette smokers against the harms for young people.
The FDA banned the sale of fruit- and mint-flavored cartridges and juice pods in 2020, but menthol and tobacco-flavored products were left on the market, according to USA Today. In September 2021, the agency also banned the sale of hundreds of thousands of vaping and e-cigarette products but didn’t rule on Juul.
Meanwhile, the FDA has cleared Reynolds American and NJOY Holdings – two of Juul’s biggest rivals – to keep tobacco-flavored products on the market. Industry experts expected Juul to receive similar clearance, the Journal reported.
Juul, which was at the top of the U.S. e-cigarette market in 2018, has moved to second place behind Reynolds’s Vuse brand, the newspaper reported. The United States represents most of the company’s revenue, though its products are also available in Canada, the United Kingdom, France, Italy, and the Philippines.
Underage vaping has fallen in the United States since federal restrictions raised the legal purchase age for tobacco products to 21 and banned the sale of sweet and fruity cartridges, according to the Journal. Juul’s popularity has also dropped among youth, with other products such as Puff Bar, Vuse, and Smok becoming more popular among e-cigarette users in high school.
In a separate decision announced this week, the FDA is also moving forward with a plan to reduce the amount of nicotine in cigarettes. The decision, which has been years in the making, is aimed at prompting millions of cigarette users to quit smoking or switch to alternatives such as e-cigarettes, as well as limit the number of users who pick up smoking at an early age.
A version of this article first appeared on WebMD.com .
The marketing denial order covers all the company’s products in the United States, which means Juul must stop distributing the products and remove everything on the market. That includes the Juul device and flavor replacement pods in the tobacco and menthol flavors.
“Today’s action is further progress on the FDA’s commitment to ensuring that all e-cigarette and electronic nicotine delivery system products currently being marketed to consumers meet our public health standards,” Robert Califf, MD, the FDA commissioner, said in the announcement.
“The agency has dedicated significant resources to review products from the companies that account for most of the U.S. market,” he said. “We recognize these make up a significant part of the available products and many have played a disproportionate role in the rise in youth vaping.”
The marketing denial order covers only the commercial distribution and retail sale of Juul’s products and doesn’t restrict consumer possession or use. The FDA “cannot and will not” enforce actions against consumers, the agency said.
The order comes after a 2-year review of the company’s application seeking authorization to continue selling non–fruit-flavored products, such as menthol and tobacco. The FDA determined the application “lacked sufficient evidence regarding the toxicological profile of the products to demonstrate that marketing of the products would be appropriate for the protection of the public health.”
Some of Juul’s study findings raised concerns because of “insufficient and conflicting data,” the FDA said, including potentially harmful chemicals leaching from the Juul liquid replacement pods.
“To date, the FDA has not received clinical information to suggest an immediate hazard associated with the use of the JUUL device or JUUL pods,” the agency said. “However, the [orders] issued today reflect FDA’s determination that there is insufficient evidence to assess the potential toxicological risks of using the JUUL products.”
Juul is expected to appeal the FDA’s decision, according to The New York Times.
In recent years, the FDA has reviewed marketing applications from Juul and other e-cigarette companies as anti-tobacco groups have called for new rules to limit products that led to a surge in youth vaping during the past decade. At the same time, advocates of e-cigarettes and nicotine-delivery devices have said the products help adult smokers to quit cigarettes and other tobacco products.
Juul, in particular, has been blamed for fueling the surge in underage vaping due to fruity flavors and hip marketing, according to The Wall Street Journal. The company removed sweet and fruity flavors from shelves in 2019 and has been trying to repair its reputation by limiting its marketing and focusing on adult cigarette smokers.
In 2020, all e-cigarette manufacturers in the United States were required to submit their products for FDA review to stay on the market, the newspaper reported. The agency has been weighing the potential benefits for adult cigarette smokers against the harms for young people.
The FDA banned the sale of fruit- and mint-flavored cartridges and juice pods in 2020, but menthol and tobacco-flavored products were left on the market, according to USA Today. In September 2021, the agency also banned the sale of hundreds of thousands of vaping and e-cigarette products but didn’t rule on Juul.
Meanwhile, the FDA has cleared Reynolds American and NJOY Holdings – two of Juul’s biggest rivals – to keep tobacco-flavored products on the market. Industry experts expected Juul to receive similar clearance, the Journal reported.
Juul, which was at the top of the U.S. e-cigarette market in 2018, has moved to second place behind Reynolds’s Vuse brand, the newspaper reported. The United States represents most of the company’s revenue, though its products are also available in Canada, the United Kingdom, France, Italy, and the Philippines.
Underage vaping has fallen in the United States since federal restrictions raised the legal purchase age for tobacco products to 21 and banned the sale of sweet and fruity cartridges, according to the Journal. Juul’s popularity has also dropped among youth, with other products such as Puff Bar, Vuse, and Smok becoming more popular among e-cigarette users in high school.
In a separate decision announced this week, the FDA is also moving forward with a plan to reduce the amount of nicotine in cigarettes. The decision, which has been years in the making, is aimed at prompting millions of cigarette users to quit smoking or switch to alternatives such as e-cigarettes, as well as limit the number of users who pick up smoking at an early age.
A version of this article first appeared on WebMD.com .
Biden boosts LGBTQIA+ protections, bans conversion therapy
President Joe Biden issued an executive order on June 15 banning conversion therapy and offering other LBGTQIA+ protections as part of White House efforts to advance equality during Pride Month.
“My order will use the full force of the federal government to end inhumane practices of conversion therapy,” President Biden said in a speech before signing the order. “This is the first time the federal government is making a coordinated effort against this dangerous and discredited practice.”
Conversion therapy is any emotional or physical therapy used to “cure” or “repair” a person’s attraction to the same sex, or their gender identity and expression. Providers claim these therapies can make someone heterosexual or “straight.” But there’s no evidence to support this.
Medical and mental health experts have rejected conversion therapy practices as dangerous and discriminatory for decades.
The executive order also addresses:
- The LGBTQIA+ youth mental health crisis, in part by expanding suicide prevention resources for that at-risk population.
- Discrimination within the foster care system against LGBTQIA+ children and parents.
- Discrimination, poverty and isolation challenges faced by LGBTQIA+ seniors.
- Efforts to strengthen federal data collection in this population to counter homelessness, housing insecurity and barriers to health care access.
Enforcement of executive order will rely on legal experts, including the Justice Department.
President Biden’s order comes at a time when multiple states are promoting or passing anti-LGBTQIA+ laws.
“I don’t have to tell you about the ultra-MAGA agenda attacking our freedoms. There are more than 300 discriminatory bills introduced in states across this country,” President Biden said. “In Texas, they are knocking on front doors to investigate parents who are raising transgender children, and in Florida they are going after Mickey Mouse for God’s sake.”
First Lady Jill Biden, PhD, said the order will not solve all problems. “Prejudice and discrimination still lurk. We will not let the progress we fought for slip away. Pride is a celebration of the courage it takes to stand up for what’s right.”
The American Psychiatric Association applauded President Biden’s action. This executive order will “protect the mental health of LGBTQ+ people, particularly children. APA has long condemned the practice of so-called ‘conversion therapy’ and we welcome the federal government’s efforts to raise public awareness about its harms, alongside other practices that will help to end it.”
The goal of the order is to “improve the health, wellbeing, and safety of countless families across the country,” senior White House administration officials said in a June 15 media call. “And they will send a powerful signal from the president of the United States to LGBTQIA+ kids across the country – who may be feeling scared and hopeless – that their president has their back.”
Biden also called on Congress to pass the Equality Act “to enshrine the long overdue civil rights to protect all Americans.”
The event was held in the East Room of the White House at a Pride event attended by Vice President Kamala Harris and her husband, the first lady, Transportation Secretary Pete Buttigieg, and hundreds of LGBTQIA+ leaders.
Guidance on starting transgender treatment
In other LGBTQIA+-related news, an international group focusing on transgender health lowered the minimum ages they recommend for starting hormone therapy or surgery for transgender youth.
The World Professional Association for Transgender Health said that hormones could be started at 14, 2 years earlier than the group’s previous advice. The association also said some surgeries can be performed at age 15 or 17, a year or so earlier than their previous recommendations.
The group acknowledged potential risks but said it is unethical and harmful to withhold early treatment, according to a report from The Associated Press.
Transgender treatment for teens has been a controversial issue, with experts disagreeing about whether teenagers can fully understand the ramifications of such life-altering decisions.
During the White House background media call, senior administration officials pointed to existing policy regarding transgender care. “We’ve already put out guidance through HHS about civil rights protections and making clear that the denial of medical care based on someone’s gender identity is discriminatory and have invited the members of the public to file complaints with the Office of Civil Rights.”
A version of this article first appeared on WebMD.com.
President Joe Biden issued an executive order on June 15 banning conversion therapy and offering other LBGTQIA+ protections as part of White House efforts to advance equality during Pride Month.
“My order will use the full force of the federal government to end inhumane practices of conversion therapy,” President Biden said in a speech before signing the order. “This is the first time the federal government is making a coordinated effort against this dangerous and discredited practice.”
Conversion therapy is any emotional or physical therapy used to “cure” or “repair” a person’s attraction to the same sex, or their gender identity and expression. Providers claim these therapies can make someone heterosexual or “straight.” But there’s no evidence to support this.
Medical and mental health experts have rejected conversion therapy practices as dangerous and discriminatory for decades.
The executive order also addresses:
- The LGBTQIA+ youth mental health crisis, in part by expanding suicide prevention resources for that at-risk population.
- Discrimination within the foster care system against LGBTQIA+ children and parents.
- Discrimination, poverty and isolation challenges faced by LGBTQIA+ seniors.
- Efforts to strengthen federal data collection in this population to counter homelessness, housing insecurity and barriers to health care access.
Enforcement of executive order will rely on legal experts, including the Justice Department.
President Biden’s order comes at a time when multiple states are promoting or passing anti-LGBTQIA+ laws.
“I don’t have to tell you about the ultra-MAGA agenda attacking our freedoms. There are more than 300 discriminatory bills introduced in states across this country,” President Biden said. “In Texas, they are knocking on front doors to investigate parents who are raising transgender children, and in Florida they are going after Mickey Mouse for God’s sake.”
First Lady Jill Biden, PhD, said the order will not solve all problems. “Prejudice and discrimination still lurk. We will not let the progress we fought for slip away. Pride is a celebration of the courage it takes to stand up for what’s right.”
The American Psychiatric Association applauded President Biden’s action. This executive order will “protect the mental health of LGBTQ+ people, particularly children. APA has long condemned the practice of so-called ‘conversion therapy’ and we welcome the federal government’s efforts to raise public awareness about its harms, alongside other practices that will help to end it.”
The goal of the order is to “improve the health, wellbeing, and safety of countless families across the country,” senior White House administration officials said in a June 15 media call. “And they will send a powerful signal from the president of the United States to LGBTQIA+ kids across the country – who may be feeling scared and hopeless – that their president has their back.”
Biden also called on Congress to pass the Equality Act “to enshrine the long overdue civil rights to protect all Americans.”
The event was held in the East Room of the White House at a Pride event attended by Vice President Kamala Harris and her husband, the first lady, Transportation Secretary Pete Buttigieg, and hundreds of LGBTQIA+ leaders.
Guidance on starting transgender treatment
In other LGBTQIA+-related news, an international group focusing on transgender health lowered the minimum ages they recommend for starting hormone therapy or surgery for transgender youth.
The World Professional Association for Transgender Health said that hormones could be started at 14, 2 years earlier than the group’s previous advice. The association also said some surgeries can be performed at age 15 or 17, a year or so earlier than their previous recommendations.
The group acknowledged potential risks but said it is unethical and harmful to withhold early treatment, according to a report from The Associated Press.
Transgender treatment for teens has been a controversial issue, with experts disagreeing about whether teenagers can fully understand the ramifications of such life-altering decisions.
During the White House background media call, senior administration officials pointed to existing policy regarding transgender care. “We’ve already put out guidance through HHS about civil rights protections and making clear that the denial of medical care based on someone’s gender identity is discriminatory and have invited the members of the public to file complaints with the Office of Civil Rights.”
A version of this article first appeared on WebMD.com.
President Joe Biden issued an executive order on June 15 banning conversion therapy and offering other LBGTQIA+ protections as part of White House efforts to advance equality during Pride Month.
“My order will use the full force of the federal government to end inhumane practices of conversion therapy,” President Biden said in a speech before signing the order. “This is the first time the federal government is making a coordinated effort against this dangerous and discredited practice.”
Conversion therapy is any emotional or physical therapy used to “cure” or “repair” a person’s attraction to the same sex, or their gender identity and expression. Providers claim these therapies can make someone heterosexual or “straight.” But there’s no evidence to support this.
Medical and mental health experts have rejected conversion therapy practices as dangerous and discriminatory for decades.
The executive order also addresses:
- The LGBTQIA+ youth mental health crisis, in part by expanding suicide prevention resources for that at-risk population.
- Discrimination within the foster care system against LGBTQIA+ children and parents.
- Discrimination, poverty and isolation challenges faced by LGBTQIA+ seniors.
- Efforts to strengthen federal data collection in this population to counter homelessness, housing insecurity and barriers to health care access.
Enforcement of executive order will rely on legal experts, including the Justice Department.
President Biden’s order comes at a time when multiple states are promoting or passing anti-LGBTQIA+ laws.
“I don’t have to tell you about the ultra-MAGA agenda attacking our freedoms. There are more than 300 discriminatory bills introduced in states across this country,” President Biden said. “In Texas, they are knocking on front doors to investigate parents who are raising transgender children, and in Florida they are going after Mickey Mouse for God’s sake.”
First Lady Jill Biden, PhD, said the order will not solve all problems. “Prejudice and discrimination still lurk. We will not let the progress we fought for slip away. Pride is a celebration of the courage it takes to stand up for what’s right.”
The American Psychiatric Association applauded President Biden’s action. This executive order will “protect the mental health of LGBTQ+ people, particularly children. APA has long condemned the practice of so-called ‘conversion therapy’ and we welcome the federal government’s efforts to raise public awareness about its harms, alongside other practices that will help to end it.”
The goal of the order is to “improve the health, wellbeing, and safety of countless families across the country,” senior White House administration officials said in a June 15 media call. “And they will send a powerful signal from the president of the United States to LGBTQIA+ kids across the country – who may be feeling scared and hopeless – that their president has their back.”
Biden also called on Congress to pass the Equality Act “to enshrine the long overdue civil rights to protect all Americans.”
The event was held in the East Room of the White House at a Pride event attended by Vice President Kamala Harris and her husband, the first lady, Transportation Secretary Pete Buttigieg, and hundreds of LGBTQIA+ leaders.
Guidance on starting transgender treatment
In other LGBTQIA+-related news, an international group focusing on transgender health lowered the minimum ages they recommend for starting hormone therapy or surgery for transgender youth.
The World Professional Association for Transgender Health said that hormones could be started at 14, 2 years earlier than the group’s previous advice. The association also said some surgeries can be performed at age 15 or 17, a year or so earlier than their previous recommendations.
The group acknowledged potential risks but said it is unethical and harmful to withhold early treatment, according to a report from The Associated Press.
Transgender treatment for teens has been a controversial issue, with experts disagreeing about whether teenagers can fully understand the ramifications of such life-altering decisions.
During the White House background media call, senior administration officials pointed to existing policy regarding transgender care. “We’ve already put out guidance through HHS about civil rights protections and making clear that the denial of medical care based on someone’s gender identity is discriminatory and have invited the members of the public to file complaints with the Office of Civil Rights.”
A version of this article first appeared on WebMD.com.
New law aims to meet crushing need for mental health care professionals
, say state leaders.
Governor J.B. Pritzker (D) signed the legislation, which took effect on June 10.
The law seeks to attract psychologists, social workers, and counselors who have left the workforce within the past 5 years by temporarily ending relicensing requirements, including the need for continuing education credit completion, passing new exams, and fee payments. It also eases the process for those practicing in other states to become licensed in Illinois.
State legislators said there is currently a crushing need for mental health providers, estimating that there are only 14 behavioral health care professionals for every 10,000 Illinois residents. The preamble to the law noted that there will be 8,353 unfilled mental health care jobs in Illinois by 2026.
“We need a mental health care workforce that is robust enough to get people help when they need it – not after months on a waiting list,” Governor Pritzker said in news release. “This legislation invests in mental health infrastructure – and that infrastructure is people,” he added.
Grant pathway
“Being told you have to wait weeks – or months – for care is extremely discouraging,” State Senator Laura Fine (D), a lead sponsor of the legislation, noted in the release.
“We need to support people struggling with mental and behavioral health issues, as well as address difficulties our mental health providers are facing trying to see as many patients as possible,” said Senator Fine.
Marvin Lindsey, CEO of the Community Behavioral Healthcare Association, added that the law would “accelerate the process for out-of-state professionals to obtain their Illinois licensure and [increase] the pipeline and diversity of the behavioral health workforce by implementing a funding mechanism that supports new or existing licensure training of interns.”
The law sets up a grant pathway for community mental health centers, which often serve as training sites. The grants would provide funds to establish or enhance training and supervision of interns and behavioral health providers-in-training seeking to become licensed clinical social workers, licensed clinical professional counselors, or licensed marriage and family therapists.
The money for those grants still has to be appropriated.
The law will also allow patient visits at Specialized Mental Health Rehabilitation Facilities conducted by either a psychiatrist or an advanced practice registered mental health or psychiatric nurse.
Finally, it would establish tax credits for employers who hire individuals in recovery from a substance use disorder or a behavioral disorder. Beginning in January 2023, employers will be eligible for up to $2,000 in credits per employee hired.
A version of this article first appeared on Medscape.com.
, say state leaders.
Governor J.B. Pritzker (D) signed the legislation, which took effect on June 10.
The law seeks to attract psychologists, social workers, and counselors who have left the workforce within the past 5 years by temporarily ending relicensing requirements, including the need for continuing education credit completion, passing new exams, and fee payments. It also eases the process for those practicing in other states to become licensed in Illinois.
State legislators said there is currently a crushing need for mental health providers, estimating that there are only 14 behavioral health care professionals for every 10,000 Illinois residents. The preamble to the law noted that there will be 8,353 unfilled mental health care jobs in Illinois by 2026.
“We need a mental health care workforce that is robust enough to get people help when they need it – not after months on a waiting list,” Governor Pritzker said in news release. “This legislation invests in mental health infrastructure – and that infrastructure is people,” he added.
Grant pathway
“Being told you have to wait weeks – or months – for care is extremely discouraging,” State Senator Laura Fine (D), a lead sponsor of the legislation, noted in the release.
“We need to support people struggling with mental and behavioral health issues, as well as address difficulties our mental health providers are facing trying to see as many patients as possible,” said Senator Fine.
Marvin Lindsey, CEO of the Community Behavioral Healthcare Association, added that the law would “accelerate the process for out-of-state professionals to obtain their Illinois licensure and [increase] the pipeline and diversity of the behavioral health workforce by implementing a funding mechanism that supports new or existing licensure training of interns.”
The law sets up a grant pathway for community mental health centers, which often serve as training sites. The grants would provide funds to establish or enhance training and supervision of interns and behavioral health providers-in-training seeking to become licensed clinical social workers, licensed clinical professional counselors, or licensed marriage and family therapists.
The money for those grants still has to be appropriated.
The law will also allow patient visits at Specialized Mental Health Rehabilitation Facilities conducted by either a psychiatrist or an advanced practice registered mental health or psychiatric nurse.
Finally, it would establish tax credits for employers who hire individuals in recovery from a substance use disorder or a behavioral disorder. Beginning in January 2023, employers will be eligible for up to $2,000 in credits per employee hired.
A version of this article first appeared on Medscape.com.
, say state leaders.
Governor J.B. Pritzker (D) signed the legislation, which took effect on June 10.
The law seeks to attract psychologists, social workers, and counselors who have left the workforce within the past 5 years by temporarily ending relicensing requirements, including the need for continuing education credit completion, passing new exams, and fee payments. It also eases the process for those practicing in other states to become licensed in Illinois.
State legislators said there is currently a crushing need for mental health providers, estimating that there are only 14 behavioral health care professionals for every 10,000 Illinois residents. The preamble to the law noted that there will be 8,353 unfilled mental health care jobs in Illinois by 2026.
“We need a mental health care workforce that is robust enough to get people help when they need it – not after months on a waiting list,” Governor Pritzker said in news release. “This legislation invests in mental health infrastructure – and that infrastructure is people,” he added.
Grant pathway
“Being told you have to wait weeks – or months – for care is extremely discouraging,” State Senator Laura Fine (D), a lead sponsor of the legislation, noted in the release.
“We need to support people struggling with mental and behavioral health issues, as well as address difficulties our mental health providers are facing trying to see as many patients as possible,” said Senator Fine.
Marvin Lindsey, CEO of the Community Behavioral Healthcare Association, added that the law would “accelerate the process for out-of-state professionals to obtain their Illinois licensure and [increase] the pipeline and diversity of the behavioral health workforce by implementing a funding mechanism that supports new or existing licensure training of interns.”
The law sets up a grant pathway for community mental health centers, which often serve as training sites. The grants would provide funds to establish or enhance training and supervision of interns and behavioral health providers-in-training seeking to become licensed clinical social workers, licensed clinical professional counselors, or licensed marriage and family therapists.
The money for those grants still has to be appropriated.
The law will also allow patient visits at Specialized Mental Health Rehabilitation Facilities conducted by either a psychiatrist or an advanced practice registered mental health or psychiatric nurse.
Finally, it would establish tax credits for employers who hire individuals in recovery from a substance use disorder or a behavioral disorder. Beginning in January 2023, employers will be eligible for up to $2,000 in credits per employee hired.
A version of this article first appeared on Medscape.com.
WHO to rename monkeypox because of stigma concerns
The virus has infected more than 1,600 people in 39 countries so far this year, the WHO said, including 32 countries where the virus isn’t typically detected.
“WHO is working with partners and experts from around the world on changing the name of monkeypox virus, its clades, and the disease it causes,” Tedros Adhanom Ghebreyesus, PhD, the WHO’s director-general, said during a press briefing.
“We will make announcements about the new names as soon as possible,” he said.
Last week, more than 30 international scientists urged the public health community to change the name of the virus. The scientists posted a letter on June 10, which included support from the Africa Centres for Disease Control and Prevention, noting that the name should change with the ongoing transmission among humans this year.
“The prevailing perception in the international media and scientific literature is that MPXV is endemic in people in some African countries. However, it is well established that nearly all MPXV outbreaks in Africa prior to the 2022 outbreak have been the result of spillover from animals and humans and only rarely have there been reports of sustained human-to-human transmissions,” they wrote.
“In the context of the current global outbreak, continued reference to, and nomenclature of this virus being African is not only inaccurate but is also discriminatory and stigmatizing,” they added.
As one example, they noted, news outlets have used images of African patients to depict the pox lesions, although most stories about the current outbreak have focused on the global north. The Foreign Press Association of Africa has urged the global media to stop using images of Black people to highlight the outbreak in Europe.
“Although the origin of the new global MPXV outbreak is still unknown, there is growing evidence that the most likely scenario is that cross-continent, cryptic human transmission has been ongoing for longer than previously thought,” they wrote.
The WHO has listed two known clades of the monkeypox virus in recent updates – “one identified in West Africa and one in the Congo Basin region.” The group of scientists wrote that this approach is “counter to the best practice of avoiding geographic locations in the nomenclature of diseases and disease groups.”
The scientists proposed a new classification that would name three clades in order of detection – 1, 2, and 3 – for the viral genomes detected in Central Africa, Western Africa, and the localized spillover events detected this year in global north countries. More genome sequencing could uncover additional clades, they noted.
Even within the most recent clade, there is already notable diversity among the genomes, the scientists said. Like the new naming convention adopted for the coronavirus pandemic, the nomenclature for human monkeypox could be donated as “A.1, A.2, A.1.1,” they wrote.
The largest current outbreak is in the United Kingdom, where health officials have detected 524 cases, according to the latest update from the U.K. Health Security Agency.
As of June 15, 72 cases have been reported in the United States, including 15 in California and 15 in New York, according to the latest Centers for Disease Control and Prevention data.
Also on June 15, the WHO published interim guidance on the use of smallpox vaccines for monkeypox. The WHO doesn’t recommend mass vaccination against monkeypox and said vaccines should be used on a case-by-case basis.
The WHO will convene an emergency meeting next week to determine whether the spread of the virus should be considered a global public health emergency.
“The global outbreak of monkeypox is clearly unusual and concerning,” Dr. Tedros said June 15. “It’s for that reason that I have decided to convene the emergency committee under the International Health Regulations next week to assess whether this outbreak represents a public health emergency of international concern.”
A version of this article first appeared on WebMD.com.
The virus has infected more than 1,600 people in 39 countries so far this year, the WHO said, including 32 countries where the virus isn’t typically detected.
“WHO is working with partners and experts from around the world on changing the name of monkeypox virus, its clades, and the disease it causes,” Tedros Adhanom Ghebreyesus, PhD, the WHO’s director-general, said during a press briefing.
“We will make announcements about the new names as soon as possible,” he said.
Last week, more than 30 international scientists urged the public health community to change the name of the virus. The scientists posted a letter on June 10, which included support from the Africa Centres for Disease Control and Prevention, noting that the name should change with the ongoing transmission among humans this year.
“The prevailing perception in the international media and scientific literature is that MPXV is endemic in people in some African countries. However, it is well established that nearly all MPXV outbreaks in Africa prior to the 2022 outbreak have been the result of spillover from animals and humans and only rarely have there been reports of sustained human-to-human transmissions,” they wrote.
“In the context of the current global outbreak, continued reference to, and nomenclature of this virus being African is not only inaccurate but is also discriminatory and stigmatizing,” they added.
As one example, they noted, news outlets have used images of African patients to depict the pox lesions, although most stories about the current outbreak have focused on the global north. The Foreign Press Association of Africa has urged the global media to stop using images of Black people to highlight the outbreak in Europe.
“Although the origin of the new global MPXV outbreak is still unknown, there is growing evidence that the most likely scenario is that cross-continent, cryptic human transmission has been ongoing for longer than previously thought,” they wrote.
The WHO has listed two known clades of the monkeypox virus in recent updates – “one identified in West Africa and one in the Congo Basin region.” The group of scientists wrote that this approach is “counter to the best practice of avoiding geographic locations in the nomenclature of diseases and disease groups.”
The scientists proposed a new classification that would name three clades in order of detection – 1, 2, and 3 – for the viral genomes detected in Central Africa, Western Africa, and the localized spillover events detected this year in global north countries. More genome sequencing could uncover additional clades, they noted.
Even within the most recent clade, there is already notable diversity among the genomes, the scientists said. Like the new naming convention adopted for the coronavirus pandemic, the nomenclature for human monkeypox could be donated as “A.1, A.2, A.1.1,” they wrote.
The largest current outbreak is in the United Kingdom, where health officials have detected 524 cases, according to the latest update from the U.K. Health Security Agency.
As of June 15, 72 cases have been reported in the United States, including 15 in California and 15 in New York, according to the latest Centers for Disease Control and Prevention data.
Also on June 15, the WHO published interim guidance on the use of smallpox vaccines for monkeypox. The WHO doesn’t recommend mass vaccination against monkeypox and said vaccines should be used on a case-by-case basis.
The WHO will convene an emergency meeting next week to determine whether the spread of the virus should be considered a global public health emergency.
“The global outbreak of monkeypox is clearly unusual and concerning,” Dr. Tedros said June 15. “It’s for that reason that I have decided to convene the emergency committee under the International Health Regulations next week to assess whether this outbreak represents a public health emergency of international concern.”
A version of this article first appeared on WebMD.com.
The virus has infected more than 1,600 people in 39 countries so far this year, the WHO said, including 32 countries where the virus isn’t typically detected.
“WHO is working with partners and experts from around the world on changing the name of monkeypox virus, its clades, and the disease it causes,” Tedros Adhanom Ghebreyesus, PhD, the WHO’s director-general, said during a press briefing.
“We will make announcements about the new names as soon as possible,” he said.
Last week, more than 30 international scientists urged the public health community to change the name of the virus. The scientists posted a letter on June 10, which included support from the Africa Centres for Disease Control and Prevention, noting that the name should change with the ongoing transmission among humans this year.
“The prevailing perception in the international media and scientific literature is that MPXV is endemic in people in some African countries. However, it is well established that nearly all MPXV outbreaks in Africa prior to the 2022 outbreak have been the result of spillover from animals and humans and only rarely have there been reports of sustained human-to-human transmissions,” they wrote.
“In the context of the current global outbreak, continued reference to, and nomenclature of this virus being African is not only inaccurate but is also discriminatory and stigmatizing,” they added.
As one example, they noted, news outlets have used images of African patients to depict the pox lesions, although most stories about the current outbreak have focused on the global north. The Foreign Press Association of Africa has urged the global media to stop using images of Black people to highlight the outbreak in Europe.
“Although the origin of the new global MPXV outbreak is still unknown, there is growing evidence that the most likely scenario is that cross-continent, cryptic human transmission has been ongoing for longer than previously thought,” they wrote.
The WHO has listed two known clades of the monkeypox virus in recent updates – “one identified in West Africa and one in the Congo Basin region.” The group of scientists wrote that this approach is “counter to the best practice of avoiding geographic locations in the nomenclature of diseases and disease groups.”
The scientists proposed a new classification that would name three clades in order of detection – 1, 2, and 3 – for the viral genomes detected in Central Africa, Western Africa, and the localized spillover events detected this year in global north countries. More genome sequencing could uncover additional clades, they noted.
Even within the most recent clade, there is already notable diversity among the genomes, the scientists said. Like the new naming convention adopted for the coronavirus pandemic, the nomenclature for human monkeypox could be donated as “A.1, A.2, A.1.1,” they wrote.
The largest current outbreak is in the United Kingdom, where health officials have detected 524 cases, according to the latest update from the U.K. Health Security Agency.
As of June 15, 72 cases have been reported in the United States, including 15 in California and 15 in New York, according to the latest Centers for Disease Control and Prevention data.
Also on June 15, the WHO published interim guidance on the use of smallpox vaccines for monkeypox. The WHO doesn’t recommend mass vaccination against monkeypox and said vaccines should be used on a case-by-case basis.
The WHO will convene an emergency meeting next week to determine whether the spread of the virus should be considered a global public health emergency.
“The global outbreak of monkeypox is clearly unusual and concerning,” Dr. Tedros said June 15. “It’s for that reason that I have decided to convene the emergency committee under the International Health Regulations next week to assess whether this outbreak represents a public health emergency of international concern.”
A version of this article first appeared on WebMD.com.
FDA panel votes unanimously for COVID shots for youngest kids
Federal advisers to the U.S. Food and Drug Administration voted unanimously June 15 to recommend the use of the Moderna and Pfizer-BioNTech COVID-19 vaccines in infants and young children.
The Vaccines and Related Biological Products Advisory Committee (VRBPAC) of the FDA voted 21-0 to say that benefits of a two-dose series of Moderna’s mRNA vaccine outweigh risk for use in infants and children 6 months through 5 years of age.
The panel then voted 21-0 to say that benefits of a three-dose series of the Pfizer-BioNTech mRNA vaccine outweigh risk for use in infants and children 6 months through 4 years of age.
The FDA is not bound to follow the suggestions of its advisory committees, but it often does. Moderna and Pfizer are seeking to expand emergency use authorization (EUA) for their vaccines. EUAs are special clearances used to allow use of products in connection with public health crises such as the pandemic.
The Pfizer vaccine has standard, nonemergency FDA approval for use in people 16 years of age and older. The FDA also has granted EUA clearance for use of the shot in people ages 5 to 15.
The VRBPAC on June 15 recommended granting EUA clearance for Moderna’s COVID-19 vaccine for people ages 6 to 17. The Moderna vaccine already has full approval for use in people 18 years of age and older.
Many parents have been waiting for a clearance of COVID vaccines for their infants and young children, seeking protection for them at a time of continued spread of the virus.
The White House on June 9 outlined plans for making 10 million doses of COVID vaccines available for children under the age of 5 in the coming weeks.
The Centers for Disease Control and Prevention (CDC) has scheduled a June 18 meeting of its Advisory Committee on Immunization Practices, where members of that panel will vote on recommendations about use of the Moderna and Pfizer-BioNTech vaccines in infants and young children. The last step in the approval process to get shots into arms will be endorsement by the CDC director if the committee votes in favor of the vaccines.
For and against
During the public session during the June 15 FDA meeting, speakers offered varied opinions.
Some urged the panel to vote against the EUA expansion, citing concerns about risks of COVID vaccines in general.
But at the close of the meeting, top FDA vaccine official Peter Marks, MD, PhD, urged the public to be cautious about drawing conclusions from reading incident reports of side effects.
He said he has seen a “Twitter storm” during the day about claims of side effects. but stressed that the FDA has reported to the public on the rare side effects linked to the COVID vaccines, such as myocarditis, with advisories based on a review of reports of side effects. But many of these reports, gathered from the Vaccine Adverse Event Reporting System (VAERS) system, will turn out on further inspection not to be related to vaccination.
Many other speakers urged members of the panel to support expanded use of the vaccines for infants and young children. These speakers emphasized how lack of a vaccine to date has isolated young children who remain unprotected, even with about 83% of those age 5 and older in the United States having received at least one COVID shot.
Dr. Marks noted that there have been 442 deaths from COVID among children under 4 years of age during the pandemic, a number that he compared with the 78 deaths reported in the H1N1 flu. He urged the panel “to be careful that we don’t become numb to the number of pediatric deaths because of the overwhelming number of older deaths here.”
Panelist H. Cody Meissner, MD, a pediatric infectious disease specialist from Tufts University, said the vaccine should be made available -- particularly for children considered to be at high risk for complications from COVID --but health officials need to present a clear picture of the relatively low risks to children of harm from the vaccines-- and from COVID.
“That has to be communicated clearly to parents so that they can participate in the decision about vaccinating a child in this age group,” Dr. Meissner said.
The results presented June 15 from studies of the shots in younger children were less impressive than those from the initial COVID vaccine trials done in adults. This was not a surprise to panelists given the rise of the omicron variant and the evolution of the pandemic, but it still led to comments about the need for further continued study of the vaccines in young children even if they are authorized.
Consider that in 2020, Pfizer won the first EUA for a COVID vaccine of any kind with data that pegged the shot’s efficacy rate at 95%. Statisticians estimated a likely possible range, or 95% confidence interval, for the vaccine efficacy rate at 90.3% to 97.6%.
Those estimates were based on finding eight cases of COVID reported among 18,198 study participants who got the Pfizer-BioNTech shot, compared with 162 cases among the 18,325 people in the placebo group, according to the FDA review of Pifzer’s initial application.
Study data
But on June 15, FDA advisers had to consider an EUA application for which the data did not make as strong a case for the vaccine’s benefit among younger patients.
Pfizer presented what the FDA called a “preliminary descriptive analysis” of vaccine efficacy among participants in Study C4591007 who received three study vaccinations, following accrual of 10 total confirmed COVID-19 cases occurring at least 7 days after the third dose.
Looking at results for study participants ages 6 to 23 months of age, there was one case in the group that got the Pfizer-BioNTech shot and two in the placebo group, pegged as a 75.6% vaccine efficacy rate -- but one with caveats to the small numbers of cases. The 95% confidence interval for this vaccine efficacy rate was reported as-369.1% to 99.6% according to the FDA staff review.
For participants 2-4 years of age with and without evidence of prior SARS-CoV-
2 infection, there were two cases in the group that got the shot and five in the placebo group showing a vaccine efficacy rate of 82.4%, with a 95% confidence interval estimated ranging between -7.6% and 98.3%. For the combined analysis of both age groups, the efficacy rate was estimated at 80.4%, with a 95% confidence interval of 14.1% and 96.7%.
Doran Fink, MD, PhD, a top official in the FDA’s vaccines division, noted that the current EUA application for expanded pediatric use involved “some very preliminary” results that involved “a small number of cases and limited follow up time.”
But he stressed that the evidence gathered to date for the Pifzer application for use of its COVID shot in infants and young children met the threshold for conditional clearance during a crisis.
“We do feel very confident that the evidentiary standard for benefit for an EUA has been met here,” but added that more data would be needed to address questions about the efficacy of the vaccine beyond a third dose and whether an additional dose may be needed.
Pfizer also used a comparison known as “immunobridging” in support of the application. This looked at SARS- CoV-2 50% neutralizing antibody titers for the children in the age group covered by the EUA application and compared them to a randomly selected subset of 16-25-year-old participants in another study,
Key data for the pending Moderna EUA for use of its shot in infants and young children came from study P204. In it, Moderna found 51 cases of COVID among 1,511 children ages 6 months to 23 months who got the vaccines, versus 34 cases among 513 children who received a placebo, according to an FDA staff review.
That resulted in a vaccine efficacy rate pegged at 50.6%, with a 95% confidence interval of 21.4% to 68.6%.
Looking at the children ages 2 to 5 years in the P204 study, there were 119 cases out of 2,594 participants who got the shot, versus 61 cases of 858 in the placebo arm, or 7.1%. That translated to a 36.8% vaccine efficacy rate, with a confidence interval 12.5% to 54.0%.
Panelist Jay Portnoy, MD, of Children’s Mercy Hospital in Kansas City said all of the pediatricians he knows are waiting for the FDA to authorize the new uses of these vaccines in infants and young children.
“The death rate from COVID in young children may not be extremely high, but it’s absolutely terrifying to parents to have their child be sick, have to go to the hospital or even go to the emergency room or their primary care doctor because they’re sick and having trouble breathing,” said Dr. Portnoy, who served as the panel’s consumer representative.
A version of this article first appeared on WebMD.com.
This article was updated on 6/16/22.
Federal advisers to the U.S. Food and Drug Administration voted unanimously June 15 to recommend the use of the Moderna and Pfizer-BioNTech COVID-19 vaccines in infants and young children.
The Vaccines and Related Biological Products Advisory Committee (VRBPAC) of the FDA voted 21-0 to say that benefits of a two-dose series of Moderna’s mRNA vaccine outweigh risk for use in infants and children 6 months through 5 years of age.
The panel then voted 21-0 to say that benefits of a three-dose series of the Pfizer-BioNTech mRNA vaccine outweigh risk for use in infants and children 6 months through 4 years of age.
The FDA is not bound to follow the suggestions of its advisory committees, but it often does. Moderna and Pfizer are seeking to expand emergency use authorization (EUA) for their vaccines. EUAs are special clearances used to allow use of products in connection with public health crises such as the pandemic.
The Pfizer vaccine has standard, nonemergency FDA approval for use in people 16 years of age and older. The FDA also has granted EUA clearance for use of the shot in people ages 5 to 15.
The VRBPAC on June 15 recommended granting EUA clearance for Moderna’s COVID-19 vaccine for people ages 6 to 17. The Moderna vaccine already has full approval for use in people 18 years of age and older.
Many parents have been waiting for a clearance of COVID vaccines for their infants and young children, seeking protection for them at a time of continued spread of the virus.
The White House on June 9 outlined plans for making 10 million doses of COVID vaccines available for children under the age of 5 in the coming weeks.
The Centers for Disease Control and Prevention (CDC) has scheduled a June 18 meeting of its Advisory Committee on Immunization Practices, where members of that panel will vote on recommendations about use of the Moderna and Pfizer-BioNTech vaccines in infants and young children. The last step in the approval process to get shots into arms will be endorsement by the CDC director if the committee votes in favor of the vaccines.
For and against
During the public session during the June 15 FDA meeting, speakers offered varied opinions.
Some urged the panel to vote against the EUA expansion, citing concerns about risks of COVID vaccines in general.
But at the close of the meeting, top FDA vaccine official Peter Marks, MD, PhD, urged the public to be cautious about drawing conclusions from reading incident reports of side effects.
He said he has seen a “Twitter storm” during the day about claims of side effects. but stressed that the FDA has reported to the public on the rare side effects linked to the COVID vaccines, such as myocarditis, with advisories based on a review of reports of side effects. But many of these reports, gathered from the Vaccine Adverse Event Reporting System (VAERS) system, will turn out on further inspection not to be related to vaccination.
Many other speakers urged members of the panel to support expanded use of the vaccines for infants and young children. These speakers emphasized how lack of a vaccine to date has isolated young children who remain unprotected, even with about 83% of those age 5 and older in the United States having received at least one COVID shot.
Dr. Marks noted that there have been 442 deaths from COVID among children under 4 years of age during the pandemic, a number that he compared with the 78 deaths reported in the H1N1 flu. He urged the panel “to be careful that we don’t become numb to the number of pediatric deaths because of the overwhelming number of older deaths here.”
Panelist H. Cody Meissner, MD, a pediatric infectious disease specialist from Tufts University, said the vaccine should be made available -- particularly for children considered to be at high risk for complications from COVID --but health officials need to present a clear picture of the relatively low risks to children of harm from the vaccines-- and from COVID.
“That has to be communicated clearly to parents so that they can participate in the decision about vaccinating a child in this age group,” Dr. Meissner said.
The results presented June 15 from studies of the shots in younger children were less impressive than those from the initial COVID vaccine trials done in adults. This was not a surprise to panelists given the rise of the omicron variant and the evolution of the pandemic, but it still led to comments about the need for further continued study of the vaccines in young children even if they are authorized.
Consider that in 2020, Pfizer won the first EUA for a COVID vaccine of any kind with data that pegged the shot’s efficacy rate at 95%. Statisticians estimated a likely possible range, or 95% confidence interval, for the vaccine efficacy rate at 90.3% to 97.6%.
Those estimates were based on finding eight cases of COVID reported among 18,198 study participants who got the Pfizer-BioNTech shot, compared with 162 cases among the 18,325 people in the placebo group, according to the FDA review of Pifzer’s initial application.
Study data
But on June 15, FDA advisers had to consider an EUA application for which the data did not make as strong a case for the vaccine’s benefit among younger patients.
Pfizer presented what the FDA called a “preliminary descriptive analysis” of vaccine efficacy among participants in Study C4591007 who received three study vaccinations, following accrual of 10 total confirmed COVID-19 cases occurring at least 7 days after the third dose.
Looking at results for study participants ages 6 to 23 months of age, there was one case in the group that got the Pfizer-BioNTech shot and two in the placebo group, pegged as a 75.6% vaccine efficacy rate -- but one with caveats to the small numbers of cases. The 95% confidence interval for this vaccine efficacy rate was reported as-369.1% to 99.6% according to the FDA staff review.
For participants 2-4 years of age with and without evidence of prior SARS-CoV-
2 infection, there were two cases in the group that got the shot and five in the placebo group showing a vaccine efficacy rate of 82.4%, with a 95% confidence interval estimated ranging between -7.6% and 98.3%. For the combined analysis of both age groups, the efficacy rate was estimated at 80.4%, with a 95% confidence interval of 14.1% and 96.7%.
Doran Fink, MD, PhD, a top official in the FDA’s vaccines division, noted that the current EUA application for expanded pediatric use involved “some very preliminary” results that involved “a small number of cases and limited follow up time.”
But he stressed that the evidence gathered to date for the Pifzer application for use of its COVID shot in infants and young children met the threshold for conditional clearance during a crisis.
“We do feel very confident that the evidentiary standard for benefit for an EUA has been met here,” but added that more data would be needed to address questions about the efficacy of the vaccine beyond a third dose and whether an additional dose may be needed.
Pfizer also used a comparison known as “immunobridging” in support of the application. This looked at SARS- CoV-2 50% neutralizing antibody titers for the children in the age group covered by the EUA application and compared them to a randomly selected subset of 16-25-year-old participants in another study,
Key data for the pending Moderna EUA for use of its shot in infants and young children came from study P204. In it, Moderna found 51 cases of COVID among 1,511 children ages 6 months to 23 months who got the vaccines, versus 34 cases among 513 children who received a placebo, according to an FDA staff review.
That resulted in a vaccine efficacy rate pegged at 50.6%, with a 95% confidence interval of 21.4% to 68.6%.
Looking at the children ages 2 to 5 years in the P204 study, there were 119 cases out of 2,594 participants who got the shot, versus 61 cases of 858 in the placebo arm, or 7.1%. That translated to a 36.8% vaccine efficacy rate, with a confidence interval 12.5% to 54.0%.
Panelist Jay Portnoy, MD, of Children’s Mercy Hospital in Kansas City said all of the pediatricians he knows are waiting for the FDA to authorize the new uses of these vaccines in infants and young children.
“The death rate from COVID in young children may not be extremely high, but it’s absolutely terrifying to parents to have their child be sick, have to go to the hospital or even go to the emergency room or their primary care doctor because they’re sick and having trouble breathing,” said Dr. Portnoy, who served as the panel’s consumer representative.
A version of this article first appeared on WebMD.com.
This article was updated on 6/16/22.
Federal advisers to the U.S. Food and Drug Administration voted unanimously June 15 to recommend the use of the Moderna and Pfizer-BioNTech COVID-19 vaccines in infants and young children.
The Vaccines and Related Biological Products Advisory Committee (VRBPAC) of the FDA voted 21-0 to say that benefits of a two-dose series of Moderna’s mRNA vaccine outweigh risk for use in infants and children 6 months through 5 years of age.
The panel then voted 21-0 to say that benefits of a three-dose series of the Pfizer-BioNTech mRNA vaccine outweigh risk for use in infants and children 6 months through 4 years of age.
The FDA is not bound to follow the suggestions of its advisory committees, but it often does. Moderna and Pfizer are seeking to expand emergency use authorization (EUA) for their vaccines. EUAs are special clearances used to allow use of products in connection with public health crises such as the pandemic.
The Pfizer vaccine has standard, nonemergency FDA approval for use in people 16 years of age and older. The FDA also has granted EUA clearance for use of the shot in people ages 5 to 15.
The VRBPAC on June 15 recommended granting EUA clearance for Moderna’s COVID-19 vaccine for people ages 6 to 17. The Moderna vaccine already has full approval for use in people 18 years of age and older.
Many parents have been waiting for a clearance of COVID vaccines for their infants and young children, seeking protection for them at a time of continued spread of the virus.
The White House on June 9 outlined plans for making 10 million doses of COVID vaccines available for children under the age of 5 in the coming weeks.
The Centers for Disease Control and Prevention (CDC) has scheduled a June 18 meeting of its Advisory Committee on Immunization Practices, where members of that panel will vote on recommendations about use of the Moderna and Pfizer-BioNTech vaccines in infants and young children. The last step in the approval process to get shots into arms will be endorsement by the CDC director if the committee votes in favor of the vaccines.
For and against
During the public session during the June 15 FDA meeting, speakers offered varied opinions.
Some urged the panel to vote against the EUA expansion, citing concerns about risks of COVID vaccines in general.
But at the close of the meeting, top FDA vaccine official Peter Marks, MD, PhD, urged the public to be cautious about drawing conclusions from reading incident reports of side effects.
He said he has seen a “Twitter storm” during the day about claims of side effects. but stressed that the FDA has reported to the public on the rare side effects linked to the COVID vaccines, such as myocarditis, with advisories based on a review of reports of side effects. But many of these reports, gathered from the Vaccine Adverse Event Reporting System (VAERS) system, will turn out on further inspection not to be related to vaccination.
Many other speakers urged members of the panel to support expanded use of the vaccines for infants and young children. These speakers emphasized how lack of a vaccine to date has isolated young children who remain unprotected, even with about 83% of those age 5 and older in the United States having received at least one COVID shot.
Dr. Marks noted that there have been 442 deaths from COVID among children under 4 years of age during the pandemic, a number that he compared with the 78 deaths reported in the H1N1 flu. He urged the panel “to be careful that we don’t become numb to the number of pediatric deaths because of the overwhelming number of older deaths here.”
Panelist H. Cody Meissner, MD, a pediatric infectious disease specialist from Tufts University, said the vaccine should be made available -- particularly for children considered to be at high risk for complications from COVID --but health officials need to present a clear picture of the relatively low risks to children of harm from the vaccines-- and from COVID.
“That has to be communicated clearly to parents so that they can participate in the decision about vaccinating a child in this age group,” Dr. Meissner said.
The results presented June 15 from studies of the shots in younger children were less impressive than those from the initial COVID vaccine trials done in adults. This was not a surprise to panelists given the rise of the omicron variant and the evolution of the pandemic, but it still led to comments about the need for further continued study of the vaccines in young children even if they are authorized.
Consider that in 2020, Pfizer won the first EUA for a COVID vaccine of any kind with data that pegged the shot’s efficacy rate at 95%. Statisticians estimated a likely possible range, or 95% confidence interval, for the vaccine efficacy rate at 90.3% to 97.6%.
Those estimates were based on finding eight cases of COVID reported among 18,198 study participants who got the Pfizer-BioNTech shot, compared with 162 cases among the 18,325 people in the placebo group, according to the FDA review of Pifzer’s initial application.
Study data
But on June 15, FDA advisers had to consider an EUA application for which the data did not make as strong a case for the vaccine’s benefit among younger patients.
Pfizer presented what the FDA called a “preliminary descriptive analysis” of vaccine efficacy among participants in Study C4591007 who received three study vaccinations, following accrual of 10 total confirmed COVID-19 cases occurring at least 7 days after the third dose.
Looking at results for study participants ages 6 to 23 months of age, there was one case in the group that got the Pfizer-BioNTech shot and two in the placebo group, pegged as a 75.6% vaccine efficacy rate -- but one with caveats to the small numbers of cases. The 95% confidence interval for this vaccine efficacy rate was reported as-369.1% to 99.6% according to the FDA staff review.
For participants 2-4 years of age with and without evidence of prior SARS-CoV-
2 infection, there were two cases in the group that got the shot and five in the placebo group showing a vaccine efficacy rate of 82.4%, with a 95% confidence interval estimated ranging between -7.6% and 98.3%. For the combined analysis of both age groups, the efficacy rate was estimated at 80.4%, with a 95% confidence interval of 14.1% and 96.7%.
Doran Fink, MD, PhD, a top official in the FDA’s vaccines division, noted that the current EUA application for expanded pediatric use involved “some very preliminary” results that involved “a small number of cases and limited follow up time.”
But he stressed that the evidence gathered to date for the Pifzer application for use of its COVID shot in infants and young children met the threshold for conditional clearance during a crisis.
“We do feel very confident that the evidentiary standard for benefit for an EUA has been met here,” but added that more data would be needed to address questions about the efficacy of the vaccine beyond a third dose and whether an additional dose may be needed.
Pfizer also used a comparison known as “immunobridging” in support of the application. This looked at SARS- CoV-2 50% neutralizing antibody titers for the children in the age group covered by the EUA application and compared them to a randomly selected subset of 16-25-year-old participants in another study,
Key data for the pending Moderna EUA for use of its shot in infants and young children came from study P204. In it, Moderna found 51 cases of COVID among 1,511 children ages 6 months to 23 months who got the vaccines, versus 34 cases among 513 children who received a placebo, according to an FDA staff review.
That resulted in a vaccine efficacy rate pegged at 50.6%, with a 95% confidence interval of 21.4% to 68.6%.
Looking at the children ages 2 to 5 years in the P204 study, there were 119 cases out of 2,594 participants who got the shot, versus 61 cases of 858 in the placebo arm, or 7.1%. That translated to a 36.8% vaccine efficacy rate, with a confidence interval 12.5% to 54.0%.
Panelist Jay Portnoy, MD, of Children’s Mercy Hospital in Kansas City said all of the pediatricians he knows are waiting for the FDA to authorize the new uses of these vaccines in infants and young children.
“The death rate from COVID in young children may not be extremely high, but it’s absolutely terrifying to parents to have their child be sick, have to go to the hospital or even go to the emergency room or their primary care doctor because they’re sick and having trouble breathing,” said Dr. Portnoy, who served as the panel’s consumer representative.
A version of this article first appeared on WebMD.com.
This article was updated on 6/16/22.