User login
How Common Is Pediatric Emergency Mistriage?
, according to a multicenter retrospective study published in JAMA Pediatrics. Researchers also identified gender, age, race, ethnicity, and comorbidity disparities in those who were undertriaged.
The researchers found that only 34.1% of visits were correctly triaged while 58.5% were overtriaged and 7.4% were undertriaged. The findings were based on analysis of more than 1 million pediatric emergency visits over a 5-year period that used the Emergency Severity Index (ESI) version 4 for triage.
“The ESI had poor sensitivity in identifying a critically ill pediatric patient, and undertriage occurred in 1 in 14 children,” wrote Dana R. Sax, MD, a senior emergency physician at The Permanente Medical Group in northern California, and her colleagues.

“More than 90% of pediatric visits were assigned a mid to low triage acuity category, and actual resource use and care intensity frequently did not align with ESI predictions,” the authors wrote. “Our findings highlight an opportunity to improve triage for pediatric patients to mitigate critical undertriage, optimize resource decisions, standardize processes across time and setting, and promote more equitable care.”
The authors added that the study findings are currently being used by the Permanente system “to develop standardized triage education across centers to improve early identification of high-risk patients.”
Disparities in Emergency Care
The results underscore the need for more work to address disparities in emergency care, wrote Warren D. Frankenberger, PhD, RN, a nurse scientist at Children’s Hospital of Philadelphia, and two colleagues in an accompanying editorial.
“Decisions in triage can have significant downstream effects on subsequent care during the ED visit,” they wrote in their editorial. “Given that the triage process in most instances is fully executed by nurses, nurse researchers are in a key position to evaluate these and other covariates to influence further improvements in triage.” They suggested that use of clinical decision support tools and artificial intelligence (AI) may improve the triage process, albeit with the caveat that AI often relies on models with pre-existing historical bias that may perpetuate structural inequalities.
Study Methodology
The researchers analyzed 1,016,816 pediatric visits at 21 emergency departments in Kaiser Permanente Northern California between January 2016 and December 2020. The patients were an average 7 years old, and 47% were female. The researchers excluded visits that lacked ESI data or had incomplete ED time variables as well as those with patients who left against medical advice, were not seen, or were transferred from another ED.
The study relied on novel definitions of ESI undertriage and overtriage developed through a modified Delphi process by a team of four emergency physicians, one pediatric emergency physician, two emergency nurses, and one pediatric ICU physician. The definition involved comparing ESI levels to the clinical outcomes and resource use.
Resources included laboratory analysis, electrocardiography, radiography, CT, MRI, diagnostic ultrasonography (not point of care), angiography, IV fluids, and IV, intramuscular, or nebulized medications. Resources did not include “oral medications, tetanus immunizations, point-of-care testing, history and physical examination, saline or heparin lock, prescription refills, simple wound care, crutches, splints, and slings.”
Level 1 events were those requiring time-sensitive, critical intervention, including high-risk sepsis. Level 2 events included most level 1 events that occurred after the first hour (except operating room admission or hospital transfer) as well as respiratory therapy, toxicology consult, lumbar puncture, suicidality as chief concern, at least 2 doses of albuterol or continuous albuterol nebulization, a skeletal survey x-ray order, and medical social work consult with an ED length of stay of at least 2 hours. Level 3 events included IV mediation order, any CT order, OR admission or hospital transfer after one hour, or any pediatric hospitalist consult.
Analyzing the ED Visits
Overtriaged cases were ESI level 1 or 2 cases in which fewer than 2 resources were used; level 3 cases where fewer than 2 resources were used and no level 1 or 2 events occurred; and level 4 cases where no resources were used.
Undertriaged cases were defined as the following:
- ESI level 5 cases where any resource was used and any level 1, 2, or 3 events occurred.
- Level 4 cases where more than 1 resource was used and any level 1, 2, or 3 events occurred.
- Level 3 cases where any level 1 event occurred, more than one level 2 event occurred, or any level 2 event occurred and more than one additional ED resource type was used.
- Level 2 cases where any level 1 event occurred.
About half the visits (51%) were assigned ESI 3, which was the category with the highest proportion of mistriage. After adjusting for study facility and triage vital signs, the researchers found that children age 6 and older were more likely to be undertriaged than those younger than 6, particularly those age 15 and older (relative risk [RR], 1.36).
Undertriage was also modestly more likely with male patients (female patients’ RR, 0.93), patients with comorbidities (RR, 1.11-1.2), patients who arrived by ambulance (RR, 1.04), and patients who were Asian (RR, 1.10), Black (RR, 1.05), or Hispanic (RR, 1.04). Undertriage became gradually less likely with each additional year in the study period, with an RR of 0.89 in 2019 and 2020.
Among the study’s limitations were use of ESI version 4, instead of the currently used 5, and the omission of common procedures from the outcome definition that “may systematically bias the analysis toward overtriage,” the editorial noted. The authors also did not include pain as a variable in the analysis, which can often indicate patient acuity.
Further, this study was unable to include covariates identified in other research that may influence clinical decision-making, such as “the presenting illness or injury, children with complex medical needs, and language proficiency,” Dr. Frankenberger and colleagues wrote. “Furthermore, environmental stressors, such as ED volume and crowding, can influence how a nurse prioritizes care and may increase bias in decision-making and/or increase practice variability.”
The study was funded by the Kaiser Permanente Northern California (KPNC) Community Health program. One author had consulting payments from CSL Behring and Abbott Point-of-Care, and six of the authors have received grant funding from the KPNC Community Health program. The editorial authors reported no conflicts of interest.
, according to a multicenter retrospective study published in JAMA Pediatrics. Researchers also identified gender, age, race, ethnicity, and comorbidity disparities in those who were undertriaged.
The researchers found that only 34.1% of visits were correctly triaged while 58.5% were overtriaged and 7.4% were undertriaged. The findings were based on analysis of more than 1 million pediatric emergency visits over a 5-year period that used the Emergency Severity Index (ESI) version 4 for triage.
“The ESI had poor sensitivity in identifying a critically ill pediatric patient, and undertriage occurred in 1 in 14 children,” wrote Dana R. Sax, MD, a senior emergency physician at The Permanente Medical Group in northern California, and her colleagues.

“More than 90% of pediatric visits were assigned a mid to low triage acuity category, and actual resource use and care intensity frequently did not align with ESI predictions,” the authors wrote. “Our findings highlight an opportunity to improve triage for pediatric patients to mitigate critical undertriage, optimize resource decisions, standardize processes across time and setting, and promote more equitable care.”
The authors added that the study findings are currently being used by the Permanente system “to develop standardized triage education across centers to improve early identification of high-risk patients.”
Disparities in Emergency Care
The results underscore the need for more work to address disparities in emergency care, wrote Warren D. Frankenberger, PhD, RN, a nurse scientist at Children’s Hospital of Philadelphia, and two colleagues in an accompanying editorial.
“Decisions in triage can have significant downstream effects on subsequent care during the ED visit,” they wrote in their editorial. “Given that the triage process in most instances is fully executed by nurses, nurse researchers are in a key position to evaluate these and other covariates to influence further improvements in triage.” They suggested that use of clinical decision support tools and artificial intelligence (AI) may improve the triage process, albeit with the caveat that AI often relies on models with pre-existing historical bias that may perpetuate structural inequalities.
Study Methodology
The researchers analyzed 1,016,816 pediatric visits at 21 emergency departments in Kaiser Permanente Northern California between January 2016 and December 2020. The patients were an average 7 years old, and 47% were female. The researchers excluded visits that lacked ESI data or had incomplete ED time variables as well as those with patients who left against medical advice, were not seen, or were transferred from another ED.
The study relied on novel definitions of ESI undertriage and overtriage developed through a modified Delphi process by a team of four emergency physicians, one pediatric emergency physician, two emergency nurses, and one pediatric ICU physician. The definition involved comparing ESI levels to the clinical outcomes and resource use.
Resources included laboratory analysis, electrocardiography, radiography, CT, MRI, diagnostic ultrasonography (not point of care), angiography, IV fluids, and IV, intramuscular, or nebulized medications. Resources did not include “oral medications, tetanus immunizations, point-of-care testing, history and physical examination, saline or heparin lock, prescription refills, simple wound care, crutches, splints, and slings.”
Level 1 events were those requiring time-sensitive, critical intervention, including high-risk sepsis. Level 2 events included most level 1 events that occurred after the first hour (except operating room admission or hospital transfer) as well as respiratory therapy, toxicology consult, lumbar puncture, suicidality as chief concern, at least 2 doses of albuterol or continuous albuterol nebulization, a skeletal survey x-ray order, and medical social work consult with an ED length of stay of at least 2 hours. Level 3 events included IV mediation order, any CT order, OR admission or hospital transfer after one hour, or any pediatric hospitalist consult.
Analyzing the ED Visits
Overtriaged cases were ESI level 1 or 2 cases in which fewer than 2 resources were used; level 3 cases where fewer than 2 resources were used and no level 1 or 2 events occurred; and level 4 cases where no resources were used.
Undertriaged cases were defined as the following:
- ESI level 5 cases where any resource was used and any level 1, 2, or 3 events occurred.
- Level 4 cases where more than 1 resource was used and any level 1, 2, or 3 events occurred.
- Level 3 cases where any level 1 event occurred, more than one level 2 event occurred, or any level 2 event occurred and more than one additional ED resource type was used.
- Level 2 cases where any level 1 event occurred.
About half the visits (51%) were assigned ESI 3, which was the category with the highest proportion of mistriage. After adjusting for study facility and triage vital signs, the researchers found that children age 6 and older were more likely to be undertriaged than those younger than 6, particularly those age 15 and older (relative risk [RR], 1.36).
Undertriage was also modestly more likely with male patients (female patients’ RR, 0.93), patients with comorbidities (RR, 1.11-1.2), patients who arrived by ambulance (RR, 1.04), and patients who were Asian (RR, 1.10), Black (RR, 1.05), or Hispanic (RR, 1.04). Undertriage became gradually less likely with each additional year in the study period, with an RR of 0.89 in 2019 and 2020.
Among the study’s limitations were use of ESI version 4, instead of the currently used 5, and the omission of common procedures from the outcome definition that “may systematically bias the analysis toward overtriage,” the editorial noted. The authors also did not include pain as a variable in the analysis, which can often indicate patient acuity.
Further, this study was unable to include covariates identified in other research that may influence clinical decision-making, such as “the presenting illness or injury, children with complex medical needs, and language proficiency,” Dr. Frankenberger and colleagues wrote. “Furthermore, environmental stressors, such as ED volume and crowding, can influence how a nurse prioritizes care and may increase bias in decision-making and/or increase practice variability.”
The study was funded by the Kaiser Permanente Northern California (KPNC) Community Health program. One author had consulting payments from CSL Behring and Abbott Point-of-Care, and six of the authors have received grant funding from the KPNC Community Health program. The editorial authors reported no conflicts of interest.
, according to a multicenter retrospective study published in JAMA Pediatrics. Researchers also identified gender, age, race, ethnicity, and comorbidity disparities in those who were undertriaged.
The researchers found that only 34.1% of visits were correctly triaged while 58.5% were overtriaged and 7.4% were undertriaged. The findings were based on analysis of more than 1 million pediatric emergency visits over a 5-year period that used the Emergency Severity Index (ESI) version 4 for triage.
“The ESI had poor sensitivity in identifying a critically ill pediatric patient, and undertriage occurred in 1 in 14 children,” wrote Dana R. Sax, MD, a senior emergency physician at The Permanente Medical Group in northern California, and her colleagues.

“More than 90% of pediatric visits were assigned a mid to low triage acuity category, and actual resource use and care intensity frequently did not align with ESI predictions,” the authors wrote. “Our findings highlight an opportunity to improve triage for pediatric patients to mitigate critical undertriage, optimize resource decisions, standardize processes across time and setting, and promote more equitable care.”
The authors added that the study findings are currently being used by the Permanente system “to develop standardized triage education across centers to improve early identification of high-risk patients.”
Disparities in Emergency Care
The results underscore the need for more work to address disparities in emergency care, wrote Warren D. Frankenberger, PhD, RN, a nurse scientist at Children’s Hospital of Philadelphia, and two colleagues in an accompanying editorial.
“Decisions in triage can have significant downstream effects on subsequent care during the ED visit,” they wrote in their editorial. “Given that the triage process in most instances is fully executed by nurses, nurse researchers are in a key position to evaluate these and other covariates to influence further improvements in triage.” They suggested that use of clinical decision support tools and artificial intelligence (AI) may improve the triage process, albeit with the caveat that AI often relies on models with pre-existing historical bias that may perpetuate structural inequalities.
Study Methodology
The researchers analyzed 1,016,816 pediatric visits at 21 emergency departments in Kaiser Permanente Northern California between January 2016 and December 2020. The patients were an average 7 years old, and 47% were female. The researchers excluded visits that lacked ESI data or had incomplete ED time variables as well as those with patients who left against medical advice, were not seen, or were transferred from another ED.
The study relied on novel definitions of ESI undertriage and overtriage developed through a modified Delphi process by a team of four emergency physicians, one pediatric emergency physician, two emergency nurses, and one pediatric ICU physician. The definition involved comparing ESI levels to the clinical outcomes and resource use.
Resources included laboratory analysis, electrocardiography, radiography, CT, MRI, diagnostic ultrasonography (not point of care), angiography, IV fluids, and IV, intramuscular, or nebulized medications. Resources did not include “oral medications, tetanus immunizations, point-of-care testing, history and physical examination, saline or heparin lock, prescription refills, simple wound care, crutches, splints, and slings.”
Level 1 events were those requiring time-sensitive, critical intervention, including high-risk sepsis. Level 2 events included most level 1 events that occurred after the first hour (except operating room admission or hospital transfer) as well as respiratory therapy, toxicology consult, lumbar puncture, suicidality as chief concern, at least 2 doses of albuterol or continuous albuterol nebulization, a skeletal survey x-ray order, and medical social work consult with an ED length of stay of at least 2 hours. Level 3 events included IV mediation order, any CT order, OR admission or hospital transfer after one hour, or any pediatric hospitalist consult.
Analyzing the ED Visits
Overtriaged cases were ESI level 1 or 2 cases in which fewer than 2 resources were used; level 3 cases where fewer than 2 resources were used and no level 1 or 2 events occurred; and level 4 cases where no resources were used.
Undertriaged cases were defined as the following:
- ESI level 5 cases where any resource was used and any level 1, 2, or 3 events occurred.
- Level 4 cases where more than 1 resource was used and any level 1, 2, or 3 events occurred.
- Level 3 cases where any level 1 event occurred, more than one level 2 event occurred, or any level 2 event occurred and more than one additional ED resource type was used.
- Level 2 cases where any level 1 event occurred.
About half the visits (51%) were assigned ESI 3, which was the category with the highest proportion of mistriage. After adjusting for study facility and triage vital signs, the researchers found that children age 6 and older were more likely to be undertriaged than those younger than 6, particularly those age 15 and older (relative risk [RR], 1.36).
Undertriage was also modestly more likely with male patients (female patients’ RR, 0.93), patients with comorbidities (RR, 1.11-1.2), patients who arrived by ambulance (RR, 1.04), and patients who were Asian (RR, 1.10), Black (RR, 1.05), or Hispanic (RR, 1.04). Undertriage became gradually less likely with each additional year in the study period, with an RR of 0.89 in 2019 and 2020.
Among the study’s limitations were use of ESI version 4, instead of the currently used 5, and the omission of common procedures from the outcome definition that “may systematically bias the analysis toward overtriage,” the editorial noted. The authors also did not include pain as a variable in the analysis, which can often indicate patient acuity.
Further, this study was unable to include covariates identified in other research that may influence clinical decision-making, such as “the presenting illness or injury, children with complex medical needs, and language proficiency,” Dr. Frankenberger and colleagues wrote. “Furthermore, environmental stressors, such as ED volume and crowding, can influence how a nurse prioritizes care and may increase bias in decision-making and/or increase practice variability.”
The study was funded by the Kaiser Permanente Northern California (KPNC) Community Health program. One author had consulting payments from CSL Behring and Abbott Point-of-Care, and six of the authors have received grant funding from the KPNC Community Health program. The editorial authors reported no conflicts of interest.
FROM JAMA PEDIATRICS
I*DEA in the VA: Optimizing the Physician Workforce to Enhance Quality of Care
Enhancing the quality of care for the evolving American veteran population is critical: many are vulnerable as a result of unique psychological and physical exposures, and many are increasingly coming from populations the federal government considers “potentially vulnerable.”1 To ensure that the needs of veterans enrolled in the Veterans Health Administration (VHA) are met, the US Department of Veterans Affairs (VA) workforce must be aware of shifts in the demographics of those who served.
The I*DEA (inclusion, diversity, equity, and access) Council is a new VHA equity team that aims to eliminate gaps in health care and benefits to ensure that historically underserved veteran communities receive the treatment they need. The Council is the oversight body for veteran and employee-facing I*DEA programs, policies, and initiatives.2 One strategy to achieve better health outcomes for enrolled veterans is to prioritize the VA health care workforce. In this capacity, the I*DEA Council examines obstacles to hiring, promoting, and retaining employees from underserved communities.
This article discusses how diversity encompasses more than gender and ethnicity and proposes applying the following I*DEA strategies to leadership positions within the VA health care workforce: inclusion of diverse perspectives and ideas, equity of opportunities, and accessibility to leadership roles within VHA facilities. Implementing these actions may help attract and retain qualified clinicians as health care leaders and enable the VHA to better serve the diverse veteran population.
Veteran Demographics
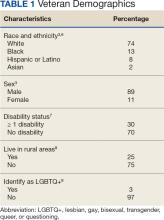
Characteristics of the current population of veterans differ significantly from those of individuals who served in previous eras. Since 2016, Gulf War era veterans have comprised the largest share of the veteran population, even larger than the share of Vietnam War era veterans.3 Among Gulf War veterans, 47% of women and 39% of men are aged < 35 years.4 Another notable change is the increase in the number of female veterans. In 1992, only 4% of veterans were female.5 Now, about 11% of veterans are female, a number projected to grow to 18% by 2046 (Table 1).3
With respect to race and ethnicity, about 74% of the current veteran population identifies as White, 13% as Black, 8% as Hispanic or Latino, and 2% as Asian.3,6 In addition, about 30% of veterans have ≥ 1 disability.7 About 1 million current veterans (3%) identify as lesbian, gay, bisexual, transgender, queer, and/or questioning (LGBTQ+).8 Almost 1 in 4 veterans—about 4.4 million—reside in rural communities, and 55% of these rural veterans are aged > 65 years.9 Of the 4.4 million veterans who live in rural areas, 61% are enrolled in VA health care, and among those individuals 8% are women and 10% are minorities.9
Studies have found that age, sex, race and ethnicity, disability status, and LGBTQ+ identification all significantly affect health care access and outcomes in the general population.10-16 Female patients are more likely to have their symptoms downplayed or dismissed, and are often less likely to receive aggressive treatments when compared with male patients. They are also frequently underrepresented or even excluded from clinical trials.11 Female veterans have unique health care needs and report preferences for being treated by female clinicians.17,18
Higher rates of chronic health conditions and reduced access to mental health services are found among Black Americans compared to White Americans.13 Black veterans are also denied VHA benefits more often than White veterans.19 Patients with disabilities have barriers to accessing care, including difficulty with transportation and a lack of knowledge among clinicians regarding the best course of care.14 Additionally, veterans who identify as LGBTQ+ are less likely than veterans who are cisgender and heterosexual to access Veterans Health Administration (VHA) care.20 Veterans in rural communities experience more challenges to accessing health care; up to one-third of veterans in this population are unable to access the internet at home.9
To optimize care for the evolving veteran population, VHA clinicians and leaders need to be aware of the changing demographic characteristics and unique health care needs of the veteran population. Increased inclusion, diversity, and equity within the health care workforce is associated with improved quality of care, improved clinical outcomes, and have had positive financial effects on health care institutions.21-25
VA Workforce Demographics
According to the VA Office of Resolution Management, Diversity, and Inclusion, at the end of fiscal year 2020 57% of VA employees identified as White, 25% as Black, 8% as Asian, 7% as Hispanic or Latino, 2% as American Indian or Alaskan Native, and 1% belonged to ≥ 2 races.26 Women comprise about 60% of the permanent VA workforce.27 About 12% of VA employees report having a disability, which is similar to the rate of disability among noninstitutionalized civilians in the US (12.7%).28 Five percent of VA employees identified as LGBTQ+.29
Although the general workforce is relatively diverse, there is not as much diversity within VA leadership, and little data exist about the demographic characteristics of VHA physicians. As of September 2020, there were 494 senior executive service and Title 38 (health care workers) senior executive service equivalent leaders in the VHA.26 Almost 78% of these leadership positions belonged to white men and women: about 50% to white men and 28% to white women. In contrast, 8% of these positions were occupied by Black men, 7% by Black women, 3% by Asian men, 2% by Asian women, and 2% by Hispanic or Latino men.26
I*DEA in the VA
The I*DEA Council seeks to eliminate gaps in VHA care and benefits to ensure that historically underserved veteran communities receive fair treatment.30 In addition to continued attention to racial disparities, the new initiative will also examine challenges experienced by other groups, including women, individuals who identify as LGBTQ+, tribal communities, and veterans who live in rural areas, aiming to eliminate disparities that exist within the VHA.
Published in 2021, the I*DEA Action Plan discusses recommendations to enhance inclusion, diversity, equity, and accessibility within the VHA. Its mission statement states that the Council aims to “advance an inclusive environment that values and supports the diverse communities we serve” and “cultivates equitable access to care, benefits and services for all” from 2021 to 2025.31 To achieve better health outcomes for veterans, the I*DEA Council plans to focus on the VHA workforce and examine and address obstacles to hiring, promoting, and retaining employees.31
There are several potential benefits of increased I*DEA integration into the health care workforce.21-25 The inclusion of ideas and perspectives from diverse backgrounds, establishing equity of opportunities for all who are appropriately qualified, and accessibility to leadership roles that enable decision making by fostering culture change are direct components of I*DEA that may be beneficial. Diversity encompasses more than race, ethnicity, and gender, and creating a more diverse workforce involves recruiting qualified clinicians with diverse backgrounds and perspectives. Doing so would better reflect the diversity of veteran patients and could enhance the ability of clinicians to learn from each other and be inclusive, while understanding veterans’ unique barriers to accessing health care.
I*DEA integration may reduce the incidence of microaggressions and help transform workplace culture.32 This would be particularly beneficial for patients, as microaggressions can decrease patient satisfaction and may potentially negatively affect health outcomes.33,34 In addition, health care professionals (HCPs) would benefit from fewer microaggressions in the workplace and this would foster a more positive, supportive work environment and improve morale.
Current VHA workforce data reflect changes in the veteran population. The workforce is relatively diverse regarding race and ethnicity, gender, disability, and LGBTQ+ status. However, room for improvement remains with respect to greater inclusion, diversity of perspectives, equity, and accessibility to leadership positions and decision making roles. This would ultimately benefit and improve care for veterans. Prioritizing this within the VHA, as reflected in one of the I*DEA Task Force recommendations, is of great significance.31
It can be difficult to accurately assess the progress made in implementing I*DEA strategies at individual institutions within the VHA. While demographic diversity can be gauged using employee statistics, assessing perceptions of inclusion, incorporation of diverse perspectives, equity, and accessibility is more challenging. We recommend continuing to administer questions focusing specifically on these perceptions to current HCPs via the VHA annual All Employee Survey.35
Implementation
The VA has begun initiating I*DEA concepts in its workforce, starting with the establishment and usage of Special Emphasis Programs.36 The goal of these programs is to increase the employment of historically marginalized groups, including women, people belonging to racial and ethnic minorities, people with disabilities, and individuals identifying as LGBTQ+.28,37-42 For example, each federal agency has a designated Federal Women’s Program whose responsibilities include helping with the recruitment and advancement of female employees.37
The VHA also has an affirmative action plan with goals for recruiting and retaining individuals with disabilities.28 To strengthen equity and inclusion, the VHA offers multiple educational courses (some mandatory), both virtual and in-person, on topics such as understanding microaggressions, managing implicit bias, and understanding the importance of gender and generational diversity.43 Creating awareness and addressing misconceptions about veteran demographics at VA medical centers is important, as is enhancing awareness among the physician workforce about VA strategies and action plans to increase I*DEA. The VHA has hired officers specifically tasked with focusing on these initiatives.
Workforce Strategies
It is important to recognize overlaps between organizational ethics, quality improvement, and I*DEA initiatives. Establishing an I*DEA Council to ensure the delivery of quality care to veterans is commendable. At the facility level, individual I*DEA officers can make observations and recommendations but are not empowered to effect change. Without participation and buy-in from individuals in leadership positions, the efficacy of I*DEA initiatives is limited.
We propose implementing simple strategies to enhance the inclusion of diverse ideas and perspectives, equity of opportunities, and accessibility to clinical leadership roles within the VHA (Table 2). A competitive selection process with specific, objective criteria to enable the selection of qualified clinical leaders is vital. Specific achievements in or contributions to quality improvement, education, research, professional publications, or diversity enhancing efforts should be required qualifications for clinical leadership roles.44
Establishing term limits for clinical leadership positions—something already being implemented at the National Institutes of Health—would be of tremendous value in the VHA.45-47 Term limits would facilitate I*DEA initiatives and accessibility of leadership roles to qualified clinicians fromvarious demographics. Improving diversity of thought among clinical leaders is especially important, given how buy-in from leadership is critical in transforming the culture of an organization. Term limits would enable access to leadership roles for forward thinking, qualified clinical leaders who could institute and support changes that would promote continuous process improvement initiatives. Leaders could have the option to reapply following the completion of a term, with the ability to demonstrate specific achievements.
Another strategy for increasing equity is to ensure transparency of committee structures, with the rotation of committee members and term limits set for committee chairs whenever possible. This provides access to leadership roles, which enables participation in decision making processes. Residents and fellows who work and train at VA hospitals should have awareness of the facility’s organizational structure and the ability to participate in certain committees. The VHA workforce should be regularly informed about educational opportunities, leadership openings, and I*DEA initiatives to increase their access and use.
Exit interviews for clinicians leaving the VA would enable feedback, provide focused reviews of any problematic issues that need to be addressed, and serve as assessments of organizational ethics.48 Transparency and truth telling could be encouraged by having these exit interviews conducted by staff in the human resources department or others outside the home department of the departing clinician.
Mentorship has played a significant role in exposing individuals from historically underrepresented groups to careers in health care, while also advancing and enhancing their careers after they become health care professionals.49-51 Implementing and publicizing VA and veteran health care-focused mentorship and volunteer programs targeted at local communities, rural areas, schools, undergraduate programs, and medical students could increase the likelihood that students and trainees from these groups are exposed to the VHA which may lead them to join the workforce.
Conclusions
Veterans receiving care from the VHA are becoming increasingly diverse. I*DEA strategies could optimize the VHA workforce and enhance the provision of quality care for veterans. The inclusion of diverse perspectives and backgrounds, equity of opportunities, and accessibility to leadership positions is important. Careful selection of qualified clinical leaders within the VHA—with established term limits for leadership positions, rotation of committee chairs and members, and exit interviews to obtain insights from clinicians who leave the VHA—all align with these strategies. This will foster energy and culture change, create an environment conducive to collaboration, learning, and professional growth and will enable continuous process improvement within individual VA medical centers.
1. US Department of Veterans Affairs, Office of Research & Development. Health equity. Accessed July 1, 2024. https://www.research.va.gov/topics/health_equity.cfm
2. US Department of Veterans Affairs. Equity action plan. Accessed July 1, 2024. https://department.va.gov/wp-content/uploads/2024/02/Department-of-Veterans-Affairs-Equity-Action-Plan.pdf
3. Schaeffer K. The changing face of America’s veteran population. Pew Research Center. March 2021. Updated November 8, 2023. Accessed May 23, 2024. https://www.pewresearch.org/short-reads/2021/04/05/the-changing-face-of-americas-veteran-population/
4. US Department of Labor, Veterans’ Employment and Training Service. 2021 employment situation of women veterans. Accessed May 23, 2024. http://www.dol.gov/agencies/vets/womenveterans/womenveterans-employment
5. US Department of Veterans Affairs, National Center for Veteran Analysis and Statistics. National survey of veterans (NSV9503). Accessed June 20, 2024. https://www.va.gov/vetdata/docs/surveysandstudies/vetpop.pdf
6. US Census Bureau. Veterans Day 2022: November 11. News release. October 26, 2022. Updated April 4, 2024. Accessed May 23, 2024. https://www.census.gov/newsroom/facts-for-features/2022/veterans-day.html
7. ADA National Network. Employment data for veterans with disabilities. 2017. Accessed June 23, 2024. https://adata.org/factsheet/employment-data-veterans-disabilities
8. LGBTQ+ Veterans. DAV. Accessed July 26, 2024. https://www.dav.org/get-help-now/veteran-topics-resources/lgbtq-veterans/
9. US Department of Veterans Affairs, Office of Rural Health. Rural Veterans. Updated May 14, 2024. Accessed June 20, 2024. https://www.ruralhealth.va.gov/aboutus/ruralvets.asp
10. Mikton C, de la Fuente-Núñez V, Officer A, Krug E. Ageism: a social determinant of health that has come of age. Lancet. 2021;397(10282):1333-1334.
doi:10.1016/S0140-6736(21)00524-9
11. Heise L, Greene ME, Opper N, et al. Gender inequality and restrictive gender norms: framing the challenges to health. Lancet. 2019;393(10189):2440-2454.
doi:10.1016/S0140-6736(19)30652-X
12. Egede LE. Race, ethnicity, culture, and disparities in health care. J Gen Intern Med. 2006;21(6):667-669. doi:10.1111/j.1525-1497.2006.0512.x
13. Carratala S, Maxwell C. Health disparities by race and ethnicity. Center for American Progress. Updated May 11, 2020. Accessed June 23, 2024. https://www.americanprogress.org/article/health-disparities-race-ethnicity/
14. Clemente KAP, Silva SVD, Vieira GI, et al. Barriers to the access of people with disabilities to health services: a scoping review. Rev Saude Publica. 2022;56:64.
doi:10.11606/s1518-8787.2022056003893
15. Krehely J. How to close the LGBT health disparities gap. Center for American Progress. December 21, 2009. Accessed May 23, 2024. https://www.americanprogress.org/article/how-to-close-the-lgbt-health-disparities-gap/
16. Dawson L, Frederiksen B, Long M, Ranji U, Kates J. LGBT+ people’s health and experiences accessing care. KFF. July 22, 2021. Accessed May 23, 2024. https://www.kff.org/womens-health-policy/report/lgbt-peoples-health-and-experiences-accessing-care
17. Disabled American Veterans. DAV report spotlights issues facing women veterans. September 12, 2018. Accessed June 23, 2024. https://www.dav.org/learn-more/news/2018/new-report-spotlights-continuing-challenges-facing-women-veterans/
18. Sheahan KL, Goldstein KM, Than CT, et al. Women veterans’ healthcare needs, utilization, and preferences in veterans affairs primary care settings. J Gen Intern Med. 2022;37(Suppl 3):791-798.
doi:10.1007/s11606-022-07585-3
19. Habeshian S. VA denied Black veterans health benefits more often than White vets, data shows. Axios. June 23, 2023. Accessed June 20, 2024. https://www.axios.com/2023/06/23/veterans-benefits-black-white-rate-disproportionate
20. Shipherd JC, Darling JE, Klap RS, Rose D, Yano EM. Experiences in the Veterans Health Administration and impact on healthcare utilization: comparisons between LGBT and non‐LGBT women veterans. LGBT Health. 2018;5(5):303‐311. doi:10.1089/lgbt.2017.0179
21. Gomez LE, Bernet P. Diversity improves performance and outcomes. J Natl Med Assoc. 2019;111(4):383-392. doi:10.1016/j.jnma.2019.01.006
22. Gill GK, McNally MJ, Berman V. Effective diversity, equity, and inclusion practices. Healthc Manage Forum. 2018;31(5):196-199. doi:10.1177/0840470418773785
23. Balinda IG, Reza N. Diversity, equity, inclusion, and belonging in cardiovascular disease fellowship training. Methodist DeBakey Cardiovasc J. 2022;18(3):67-77. doi:10.14797/mdcvj.1080
24. Parsons SK, Fineberg IC, Lin M, Singer M, Tang M, Erban JK. Promoting high-quality cancer care and equity through disciplinary diversity in team composition. J Oncol Pract. 2016;12(11):1141-1147. doi:10.1200/JOP.2016.013920
25. Stanford FC. The importance of diversity and inclusion in the healthcare workforce. J Natl Med Assoc. 2020;112(3):247-249. doi:10.1016/j.jnma.2020.03.014
26. US Department of Veterans Affairs. Diversity and inclusion strategic plan, fiscal years 2021-2022. Accessed May 23, 2024. https://www.va.gov/ORMDI/docs/StrategicPlan.pdf
27. US Department of Veterans Affairs (VA). US EEOC. Accessed July 1, 2024. https://www.eeoc.gov/federal-sector/department-veterans-affairs-va-0
28. US Department of Veterans Affairs, Office of Resolution Management, Diversity & Inclusion (ORMDI). Individuals with disabilities employment program. Updated August 15, 2022. Accessed June 20, 2024. https://www.va.gov/ORMDI/DiversityInclusion/IWD.asp
29. US Department of Veterans Affairs, Office of Resolution Management, Diversity & Inclusion (ORMDI). VA workforce diversity: FY 2022. Accessed July 1, 2024. https://www.va.gov/ORMDI/Diversity_Inclusion.asp
30. US Department of Veterans Affairs. Same mission, new I-DEA: VA supports inclusion, diversity, equity and access. News release. April 28, 2023. Accessed June 20, 2024. https://news.va.gov/118609/same-mission-va-supports-inclusion-diversity/
31. US Department of Veterans Affairs, Office of Resolution Management, Diversity & Inclusion. Inclusion, diversity, equity, & access (I-DEA) action plan. September 2021. Accessed June 20, 2024. https://www.va.gov/ORMDI/docs/VA_I-DEA_Action_Plan-SIGNED.pdf
32. Sue DW, Alsaidi S, Awad MN, Glaeser E, Calle CZ. Disarming racial microaggressions: microintervention strategies for targets, White allies, and bystanders. Am Psychol. 2019;74(1):128-142. doi:10.1037/amp0000296
33. Cruz D, Rodriguez Y, Mastropaolo C. Perceived microaggressions in health care: a measurement study. PLoS One. 2019;14(2):e0211620. doi:10.1371/journal.pone.0211620
 34. Ehie O, Muse I, Hill L, Bastien A. Professionalism: microaggression in the healthcare setting. Curr Opin Anaesthesiol. 2021;34(2):131-136. doi:10.1097/ACO.0000000000000966
34. Ehie O, Muse I, Hill L, Bastien A. Professionalism: microaggression in the healthcare setting. Curr Opin Anaesthesiol. 2021;34(2):131-136. doi:10.1097/ACO.0000000000000966
35. US Department of Veterans Affairs. VA all employee survey. Accessed May 23, 2024. https://www.data.va.gov/stories/s/VA-All-Employee-Survey-AES-/r32e-j4vj/
36. US Department of Veterans Affairs, Office of Resolution Management, Diversity & Inclusion. Special emphasis programs (ORMDI). Updated May 3, 2023. Accessed June 20, 2024. https://www.va.gov/ORMDI/DiversityInclusion/Special_Emphasis_Programs.asp
37. US Department of Veterans Affairs, Office of Resolution Management, Diversity & Inclusion (ORMDI). Federal women’s program. Updated August 9, 2022. Accessed June 20, 2024. https://www.va.gov/ORMDI/DiversityInclusion/FWP.asp
38. US Department of Veterans Affairs, Office of Resolution Management, Diversity & Inclusion (ORMDI). Hispanic Employment program. Updated May 16, 2024. Accessed June 20, 2024. https://www.va.gov/ORMDI/DiversityInclusion/HEP.asp
39. US Department of Veterans Affairs, Office of Resolution Management, Diversity & Inclusion (ORMDI). American Indian & Alaska Native Program. Updated September 27, 2023. Accessed June 20, 2024. https://www.va.gov/ORMDI/DiversityInclusion/AIAN.asp
40. US Department of Veterans Affairs, Office of Resolution Management, Diversity & Inclusion (ORMDI). Asian American, Native Hawaiian and Pacific Islander program. Updated September 27, 2023. Accessed June 20, 2024. https://www.va.gov/ORMDI/DiversityInclusion/AAPI.asp
41. US Department of Veterans Affairs, Office of Resolution Management, Diversity & Inclusion (ORMDI). Black/African American program. Updated May 3, 2023. Accessed June 20, 2024. https://www.va.gov/ORMDI/DiversityInclusion/Black_African_American.asp
42. US Department of Veterans Affairs, Office of Resolution Management, Diversity & Inclusion (ORMDI). LGBTQ+ program. Updated May 21, 2024. Accessed June 20, 2024. https://www.va.gov/ORMDI/DiversityInclusion/LGBT.asp
43. US Department of Veterans Affairs, Office of Resolution Management, Diversity & Inclusion (ORMDI). Diversity, equity and inclusion training. Updated March 18, 2024. Accessed June 20, 2024. https://www.va.gov/ORMDI/DiversityInclusion/Diversity_Inclusion_Training.asp
44. Rotenstein LS, Reede JY, Jena AB. Addressing workforce diversity - a quality-improvement framework. N Engl J Med. 2021;384(12):1083-1086. doi:10.1056/NEJMp2032224
45. Beeler WH, Mangurian C, Jagsi R. Unplugging the pipeline - a call for term limits in academic medicine. N Engl J Med. 2019;381(16):1508-1511. doi:10.1056/NEJMp1906832
46. Smith DG. Term limits in academic public health administration. Public Health Rep. 2020;135(6):859-863. doi:10.1177/0033354920954495
47. Kaiser J. Shake-up at NIH: Term limits for important positions would open new opportunities for women, minorities. science.org. May 2, 2019. Accessed May 23, 2024. https://www.science.org/content/article/shakeup-nih-term-limits-important-positions-would-open-new-opportunities-women
48. Giacalone RA, Jurkiewicz CL, Knouse SB. Exit surveys as assessments of organizational ethicality. Public Pers Manage. 2003;32(3):397-410. doi:10.1177/009102600303200306
49. Bonifacino E, Ufomata EO, Farkas AH, Turner R, Corbelli JA. Mentorship of underrepresented physicians and trainees in academic medicine: a systematic review. J Gen Intern Med. 2021;36(4):1023-1034. doi:10.1007/s11606-020-06478-7
50. Brown IM. Diversity matters: mentorship is the missing ingredient in DEI. Emergency Medicine News. 2021;43(8):28. doi:10.1097/01.EEM.0000771148.76632.35
51. Sinha A, Kuy S. The future of surgery - increasing diversity, equity, and inclusion through early mentorship. Am J Surg. 2023;225(4):800-802. doi:10.1016/j.amjsurg.2022.12.011
Enhancing the quality of care for the evolving American veteran population is critical: many are vulnerable as a result of unique psychological and physical exposures, and many are increasingly coming from populations the federal government considers “potentially vulnerable.”1 To ensure that the needs of veterans enrolled in the Veterans Health Administration (VHA) are met, the US Department of Veterans Affairs (VA) workforce must be aware of shifts in the demographics of those who served.
The I*DEA (inclusion, diversity, equity, and access) Council is a new VHA equity team that aims to eliminate gaps in health care and benefits to ensure that historically underserved veteran communities receive the treatment they need. The Council is the oversight body for veteran and employee-facing I*DEA programs, policies, and initiatives.2 One strategy to achieve better health outcomes for enrolled veterans is to prioritize the VA health care workforce. In this capacity, the I*DEA Council examines obstacles to hiring, promoting, and retaining employees from underserved communities.
This article discusses how diversity encompasses more than gender and ethnicity and proposes applying the following I*DEA strategies to leadership positions within the VA health care workforce: inclusion of diverse perspectives and ideas, equity of opportunities, and accessibility to leadership roles within VHA facilities. Implementing these actions may help attract and retain qualified clinicians as health care leaders and enable the VHA to better serve the diverse veteran population.
Veteran Demographics
Characteristics of the current population of veterans differ significantly from those of individuals who served in previous eras. Since 2016, Gulf War era veterans have comprised the largest share of the veteran population, even larger than the share of Vietnam War era veterans.3 Among Gulf War veterans, 47% of women and 39% of men are aged < 35 years.4 Another notable change is the increase in the number of female veterans. In 1992, only 4% of veterans were female.5 Now, about 11% of veterans are female, a number projected to grow to 18% by 2046 (Table 1).3
With respect to race and ethnicity, about 74% of the current veteran population identifies as White, 13% as Black, 8% as Hispanic or Latino, and 2% as Asian.3,6 In addition, about 30% of veterans have ≥ 1 disability.7 About 1 million current veterans (3%) identify as lesbian, gay, bisexual, transgender, queer, and/or questioning (LGBTQ+).8 Almost 1 in 4 veterans—about 4.4 million—reside in rural communities, and 55% of these rural veterans are aged > 65 years.9 Of the 4.4 million veterans who live in rural areas, 61% are enrolled in VA health care, and among those individuals 8% are women and 10% are minorities.9
Studies have found that age, sex, race and ethnicity, disability status, and LGBTQ+ identification all significantly affect health care access and outcomes in the general population.10-16 Female patients are more likely to have their symptoms downplayed or dismissed, and are often less likely to receive aggressive treatments when compared with male patients. They are also frequently underrepresented or even excluded from clinical trials.11 Female veterans have unique health care needs and report preferences for being treated by female clinicians.17,18
Higher rates of chronic health conditions and reduced access to mental health services are found among Black Americans compared to White Americans.13 Black veterans are also denied VHA benefits more often than White veterans.19 Patients with disabilities have barriers to accessing care, including difficulty with transportation and a lack of knowledge among clinicians regarding the best course of care.14 Additionally, veterans who identify as LGBTQ+ are less likely than veterans who are cisgender and heterosexual to access Veterans Health Administration (VHA) care.20 Veterans in rural communities experience more challenges to accessing health care; up to one-third of veterans in this population are unable to access the internet at home.9
To optimize care for the evolving veteran population, VHA clinicians and leaders need to be aware of the changing demographic characteristics and unique health care needs of the veteran population. Increased inclusion, diversity, and equity within the health care workforce is associated with improved quality of care, improved clinical outcomes, and have had positive financial effects on health care institutions.21-25
VA Workforce Demographics
According to the VA Office of Resolution Management, Diversity, and Inclusion, at the end of fiscal year 2020 57% of VA employees identified as White, 25% as Black, 8% as Asian, 7% as Hispanic or Latino, 2% as American Indian or Alaskan Native, and 1% belonged to ≥ 2 races.26 Women comprise about 60% of the permanent VA workforce.27 About 12% of VA employees report having a disability, which is similar to the rate of disability among noninstitutionalized civilians in the US (12.7%).28 Five percent of VA employees identified as LGBTQ+.29
Although the general workforce is relatively diverse, there is not as much diversity within VA leadership, and little data exist about the demographic characteristics of VHA physicians. As of September 2020, there were 494 senior executive service and Title 38 (health care workers) senior executive service equivalent leaders in the VHA.26 Almost 78% of these leadership positions belonged to white men and women: about 50% to white men and 28% to white women. In contrast, 8% of these positions were occupied by Black men, 7% by Black women, 3% by Asian men, 2% by Asian women, and 2% by Hispanic or Latino men.26
I*DEA in the VA
The I*DEA Council seeks to eliminate gaps in VHA care and benefits to ensure that historically underserved veteran communities receive fair treatment.30 In addition to continued attention to racial disparities, the new initiative will also examine challenges experienced by other groups, including women, individuals who identify as LGBTQ+, tribal communities, and veterans who live in rural areas, aiming to eliminate disparities that exist within the VHA.
Published in 2021, the I*DEA Action Plan discusses recommendations to enhance inclusion, diversity, equity, and accessibility within the VHA. Its mission statement states that the Council aims to “advance an inclusive environment that values and supports the diverse communities we serve” and “cultivates equitable access to care, benefits and services for all” from 2021 to 2025.31 To achieve better health outcomes for veterans, the I*DEA Council plans to focus on the VHA workforce and examine and address obstacles to hiring, promoting, and retaining employees.31
There are several potential benefits of increased I*DEA integration into the health care workforce.21-25 The inclusion of ideas and perspectives from diverse backgrounds, establishing equity of opportunities for all who are appropriately qualified, and accessibility to leadership roles that enable decision making by fostering culture change are direct components of I*DEA that may be beneficial. Diversity encompasses more than race, ethnicity, and gender, and creating a more diverse workforce involves recruiting qualified clinicians with diverse backgrounds and perspectives. Doing so would better reflect the diversity of veteran patients and could enhance the ability of clinicians to learn from each other and be inclusive, while understanding veterans’ unique barriers to accessing health care.
I*DEA integration may reduce the incidence of microaggressions and help transform workplace culture.32 This would be particularly beneficial for patients, as microaggressions can decrease patient satisfaction and may potentially negatively affect health outcomes.33,34 In addition, health care professionals (HCPs) would benefit from fewer microaggressions in the workplace and this would foster a more positive, supportive work environment and improve morale.
Current VHA workforce data reflect changes in the veteran population. The workforce is relatively diverse regarding race and ethnicity, gender, disability, and LGBTQ+ status. However, room for improvement remains with respect to greater inclusion, diversity of perspectives, equity, and accessibility to leadership positions and decision making roles. This would ultimately benefit and improve care for veterans. Prioritizing this within the VHA, as reflected in one of the I*DEA Task Force recommendations, is of great significance.31
It can be difficult to accurately assess the progress made in implementing I*DEA strategies at individual institutions within the VHA. While demographic diversity can be gauged using employee statistics, assessing perceptions of inclusion, incorporation of diverse perspectives, equity, and accessibility is more challenging. We recommend continuing to administer questions focusing specifically on these perceptions to current HCPs via the VHA annual All Employee Survey.35
Implementation
The VA has begun initiating I*DEA concepts in its workforce, starting with the establishment and usage of Special Emphasis Programs.36 The goal of these programs is to increase the employment of historically marginalized groups, including women, people belonging to racial and ethnic minorities, people with disabilities, and individuals identifying as LGBTQ+.28,37-42 For example, each federal agency has a designated Federal Women’s Program whose responsibilities include helping with the recruitment and advancement of female employees.37
The VHA also has an affirmative action plan with goals for recruiting and retaining individuals with disabilities.28 To strengthen equity and inclusion, the VHA offers multiple educational courses (some mandatory), both virtual and in-person, on topics such as understanding microaggressions, managing implicit bias, and understanding the importance of gender and generational diversity.43 Creating awareness and addressing misconceptions about veteran demographics at VA medical centers is important, as is enhancing awareness among the physician workforce about VA strategies and action plans to increase I*DEA. The VHA has hired officers specifically tasked with focusing on these initiatives.
Workforce Strategies
It is important to recognize overlaps between organizational ethics, quality improvement, and I*DEA initiatives. Establishing an I*DEA Council to ensure the delivery of quality care to veterans is commendable. At the facility level, individual I*DEA officers can make observations and recommendations but are not empowered to effect change. Without participation and buy-in from individuals in leadership positions, the efficacy of I*DEA initiatives is limited.
We propose implementing simple strategies to enhance the inclusion of diverse ideas and perspectives, equity of opportunities, and accessibility to clinical leadership roles within the VHA (Table 2). A competitive selection process with specific, objective criteria to enable the selection of qualified clinical leaders is vital. Specific achievements in or contributions to quality improvement, education, research, professional publications, or diversity enhancing efforts should be required qualifications for clinical leadership roles.44
Establishing term limits for clinical leadership positions—something already being implemented at the National Institutes of Health—would be of tremendous value in the VHA.45-47 Term limits would facilitate I*DEA initiatives and accessibility of leadership roles to qualified clinicians fromvarious demographics. Improving diversity of thought among clinical leaders is especially important, given how buy-in from leadership is critical in transforming the culture of an organization. Term limits would enable access to leadership roles for forward thinking, qualified clinical leaders who could institute and support changes that would promote continuous process improvement initiatives. Leaders could have the option to reapply following the completion of a term, with the ability to demonstrate specific achievements.
Another strategy for increasing equity is to ensure transparency of committee structures, with the rotation of committee members and term limits set for committee chairs whenever possible. This provides access to leadership roles, which enables participation in decision making processes. Residents and fellows who work and train at VA hospitals should have awareness of the facility’s organizational structure and the ability to participate in certain committees. The VHA workforce should be regularly informed about educational opportunities, leadership openings, and I*DEA initiatives to increase their access and use.
Exit interviews for clinicians leaving the VA would enable feedback, provide focused reviews of any problematic issues that need to be addressed, and serve as assessments of organizational ethics.48 Transparency and truth telling could be encouraged by having these exit interviews conducted by staff in the human resources department or others outside the home department of the departing clinician.
Mentorship has played a significant role in exposing individuals from historically underrepresented groups to careers in health care, while also advancing and enhancing their careers after they become health care professionals.49-51 Implementing and publicizing VA and veteran health care-focused mentorship and volunteer programs targeted at local communities, rural areas, schools, undergraduate programs, and medical students could increase the likelihood that students and trainees from these groups are exposed to the VHA which may lead them to join the workforce.
Conclusions
Veterans receiving care from the VHA are becoming increasingly diverse. I*DEA strategies could optimize the VHA workforce and enhance the provision of quality care for veterans. The inclusion of diverse perspectives and backgrounds, equity of opportunities, and accessibility to leadership positions is important. Careful selection of qualified clinical leaders within the VHA—with established term limits for leadership positions, rotation of committee chairs and members, and exit interviews to obtain insights from clinicians who leave the VHA—all align with these strategies. This will foster energy and culture change, create an environment conducive to collaboration, learning, and professional growth and will enable continuous process improvement within individual VA medical centers.
Enhancing the quality of care for the evolving American veteran population is critical: many are vulnerable as a result of unique psychological and physical exposures, and many are increasingly coming from populations the federal government considers “potentially vulnerable.”1 To ensure that the needs of veterans enrolled in the Veterans Health Administration (VHA) are met, the US Department of Veterans Affairs (VA) workforce must be aware of shifts in the demographics of those who served.
The I*DEA (inclusion, diversity, equity, and access) Council is a new VHA equity team that aims to eliminate gaps in health care and benefits to ensure that historically underserved veteran communities receive the treatment they need. The Council is the oversight body for veteran and employee-facing I*DEA programs, policies, and initiatives.2 One strategy to achieve better health outcomes for enrolled veterans is to prioritize the VA health care workforce. In this capacity, the I*DEA Council examines obstacles to hiring, promoting, and retaining employees from underserved communities.
This article discusses how diversity encompasses more than gender and ethnicity and proposes applying the following I*DEA strategies to leadership positions within the VA health care workforce: inclusion of diverse perspectives and ideas, equity of opportunities, and accessibility to leadership roles within VHA facilities. Implementing these actions may help attract and retain qualified clinicians as health care leaders and enable the VHA to better serve the diverse veteran population.
Veteran Demographics
Characteristics of the current population of veterans differ significantly from those of individuals who served in previous eras. Since 2016, Gulf War era veterans have comprised the largest share of the veteran population, even larger than the share of Vietnam War era veterans.3 Among Gulf War veterans, 47% of women and 39% of men are aged < 35 years.4 Another notable change is the increase in the number of female veterans. In 1992, only 4% of veterans were female.5 Now, about 11% of veterans are female, a number projected to grow to 18% by 2046 (Table 1).3
With respect to race and ethnicity, about 74% of the current veteran population identifies as White, 13% as Black, 8% as Hispanic or Latino, and 2% as Asian.3,6 In addition, about 30% of veterans have ≥ 1 disability.7 About 1 million current veterans (3%) identify as lesbian, gay, bisexual, transgender, queer, and/or questioning (LGBTQ+).8 Almost 1 in 4 veterans—about 4.4 million—reside in rural communities, and 55% of these rural veterans are aged > 65 years.9 Of the 4.4 million veterans who live in rural areas, 61% are enrolled in VA health care, and among those individuals 8% are women and 10% are minorities.9
Studies have found that age, sex, race and ethnicity, disability status, and LGBTQ+ identification all significantly affect health care access and outcomes in the general population.10-16 Female patients are more likely to have their symptoms downplayed or dismissed, and are often less likely to receive aggressive treatments when compared with male patients. They are also frequently underrepresented or even excluded from clinical trials.11 Female veterans have unique health care needs and report preferences for being treated by female clinicians.17,18
Higher rates of chronic health conditions and reduced access to mental health services are found among Black Americans compared to White Americans.13 Black veterans are also denied VHA benefits more often than White veterans.19 Patients with disabilities have barriers to accessing care, including difficulty with transportation and a lack of knowledge among clinicians regarding the best course of care.14 Additionally, veterans who identify as LGBTQ+ are less likely than veterans who are cisgender and heterosexual to access Veterans Health Administration (VHA) care.20 Veterans in rural communities experience more challenges to accessing health care; up to one-third of veterans in this population are unable to access the internet at home.9
To optimize care for the evolving veteran population, VHA clinicians and leaders need to be aware of the changing demographic characteristics and unique health care needs of the veteran population. Increased inclusion, diversity, and equity within the health care workforce is associated with improved quality of care, improved clinical outcomes, and have had positive financial effects on health care institutions.21-25
VA Workforce Demographics
According to the VA Office of Resolution Management, Diversity, and Inclusion, at the end of fiscal year 2020 57% of VA employees identified as White, 25% as Black, 8% as Asian, 7% as Hispanic or Latino, 2% as American Indian or Alaskan Native, and 1% belonged to ≥ 2 races.26 Women comprise about 60% of the permanent VA workforce.27 About 12% of VA employees report having a disability, which is similar to the rate of disability among noninstitutionalized civilians in the US (12.7%).28 Five percent of VA employees identified as LGBTQ+.29
Although the general workforce is relatively diverse, there is not as much diversity within VA leadership, and little data exist about the demographic characteristics of VHA physicians. As of September 2020, there were 494 senior executive service and Title 38 (health care workers) senior executive service equivalent leaders in the VHA.26 Almost 78% of these leadership positions belonged to white men and women: about 50% to white men and 28% to white women. In contrast, 8% of these positions were occupied by Black men, 7% by Black women, 3% by Asian men, 2% by Asian women, and 2% by Hispanic or Latino men.26
I*DEA in the VA
The I*DEA Council seeks to eliminate gaps in VHA care and benefits to ensure that historically underserved veteran communities receive fair treatment.30 In addition to continued attention to racial disparities, the new initiative will also examine challenges experienced by other groups, including women, individuals who identify as LGBTQ+, tribal communities, and veterans who live in rural areas, aiming to eliminate disparities that exist within the VHA.
Published in 2021, the I*DEA Action Plan discusses recommendations to enhance inclusion, diversity, equity, and accessibility within the VHA. Its mission statement states that the Council aims to “advance an inclusive environment that values and supports the diverse communities we serve” and “cultivates equitable access to care, benefits and services for all” from 2021 to 2025.31 To achieve better health outcomes for veterans, the I*DEA Council plans to focus on the VHA workforce and examine and address obstacles to hiring, promoting, and retaining employees.31
There are several potential benefits of increased I*DEA integration into the health care workforce.21-25 The inclusion of ideas and perspectives from diverse backgrounds, establishing equity of opportunities for all who are appropriately qualified, and accessibility to leadership roles that enable decision making by fostering culture change are direct components of I*DEA that may be beneficial. Diversity encompasses more than race, ethnicity, and gender, and creating a more diverse workforce involves recruiting qualified clinicians with diverse backgrounds and perspectives. Doing so would better reflect the diversity of veteran patients and could enhance the ability of clinicians to learn from each other and be inclusive, while understanding veterans’ unique barriers to accessing health care.
I*DEA integration may reduce the incidence of microaggressions and help transform workplace culture.32 This would be particularly beneficial for patients, as microaggressions can decrease patient satisfaction and may potentially negatively affect health outcomes.33,34 In addition, health care professionals (HCPs) would benefit from fewer microaggressions in the workplace and this would foster a more positive, supportive work environment and improve morale.
Current VHA workforce data reflect changes in the veteran population. The workforce is relatively diverse regarding race and ethnicity, gender, disability, and LGBTQ+ status. However, room for improvement remains with respect to greater inclusion, diversity of perspectives, equity, and accessibility to leadership positions and decision making roles. This would ultimately benefit and improve care for veterans. Prioritizing this within the VHA, as reflected in one of the I*DEA Task Force recommendations, is of great significance.31
It can be difficult to accurately assess the progress made in implementing I*DEA strategies at individual institutions within the VHA. While demographic diversity can be gauged using employee statistics, assessing perceptions of inclusion, incorporation of diverse perspectives, equity, and accessibility is more challenging. We recommend continuing to administer questions focusing specifically on these perceptions to current HCPs via the VHA annual All Employee Survey.35
Implementation
The VA has begun initiating I*DEA concepts in its workforce, starting with the establishment and usage of Special Emphasis Programs.36 The goal of these programs is to increase the employment of historically marginalized groups, including women, people belonging to racial and ethnic minorities, people with disabilities, and individuals identifying as LGBTQ+.28,37-42 For example, each federal agency has a designated Federal Women’s Program whose responsibilities include helping with the recruitment and advancement of female employees.37
The VHA also has an affirmative action plan with goals for recruiting and retaining individuals with disabilities.28 To strengthen equity and inclusion, the VHA offers multiple educational courses (some mandatory), both virtual and in-person, on topics such as understanding microaggressions, managing implicit bias, and understanding the importance of gender and generational diversity.43 Creating awareness and addressing misconceptions about veteran demographics at VA medical centers is important, as is enhancing awareness among the physician workforce about VA strategies and action plans to increase I*DEA. The VHA has hired officers specifically tasked with focusing on these initiatives.
Workforce Strategies
It is important to recognize overlaps between organizational ethics, quality improvement, and I*DEA initiatives. Establishing an I*DEA Council to ensure the delivery of quality care to veterans is commendable. At the facility level, individual I*DEA officers can make observations and recommendations but are not empowered to effect change. Without participation and buy-in from individuals in leadership positions, the efficacy of I*DEA initiatives is limited.
We propose implementing simple strategies to enhance the inclusion of diverse ideas and perspectives, equity of opportunities, and accessibility to clinical leadership roles within the VHA (Table 2). A competitive selection process with specific, objective criteria to enable the selection of qualified clinical leaders is vital. Specific achievements in or contributions to quality improvement, education, research, professional publications, or diversity enhancing efforts should be required qualifications for clinical leadership roles.44
Establishing term limits for clinical leadership positions—something already being implemented at the National Institutes of Health—would be of tremendous value in the VHA.45-47 Term limits would facilitate I*DEA initiatives and accessibility of leadership roles to qualified clinicians fromvarious demographics. Improving diversity of thought among clinical leaders is especially important, given how buy-in from leadership is critical in transforming the culture of an organization. Term limits would enable access to leadership roles for forward thinking, qualified clinical leaders who could institute and support changes that would promote continuous process improvement initiatives. Leaders could have the option to reapply following the completion of a term, with the ability to demonstrate specific achievements.
Another strategy for increasing equity is to ensure transparency of committee structures, with the rotation of committee members and term limits set for committee chairs whenever possible. This provides access to leadership roles, which enables participation in decision making processes. Residents and fellows who work and train at VA hospitals should have awareness of the facility’s organizational structure and the ability to participate in certain committees. The VHA workforce should be regularly informed about educational opportunities, leadership openings, and I*DEA initiatives to increase their access and use.
Exit interviews for clinicians leaving the VA would enable feedback, provide focused reviews of any problematic issues that need to be addressed, and serve as assessments of organizational ethics.48 Transparency and truth telling could be encouraged by having these exit interviews conducted by staff in the human resources department or others outside the home department of the departing clinician.
Mentorship has played a significant role in exposing individuals from historically underrepresented groups to careers in health care, while also advancing and enhancing their careers after they become health care professionals.49-51 Implementing and publicizing VA and veteran health care-focused mentorship and volunteer programs targeted at local communities, rural areas, schools, undergraduate programs, and medical students could increase the likelihood that students and trainees from these groups are exposed to the VHA which may lead them to join the workforce.
Conclusions
Veterans receiving care from the VHA are becoming increasingly diverse. I*DEA strategies could optimize the VHA workforce and enhance the provision of quality care for veterans. The inclusion of diverse perspectives and backgrounds, equity of opportunities, and accessibility to leadership positions is important. Careful selection of qualified clinical leaders within the VHA—with established term limits for leadership positions, rotation of committee chairs and members, and exit interviews to obtain insights from clinicians who leave the VHA—all align with these strategies. This will foster energy and culture change, create an environment conducive to collaboration, learning, and professional growth and will enable continuous process improvement within individual VA medical centers.
1. US Department of Veterans Affairs, Office of Research & Development. Health equity. Accessed July 1, 2024. https://www.research.va.gov/topics/health_equity.cfm
2. US Department of Veterans Affairs. Equity action plan. Accessed July 1, 2024. https://department.va.gov/wp-content/uploads/2024/02/Department-of-Veterans-Affairs-Equity-Action-Plan.pdf
3. Schaeffer K. The changing face of America’s veteran population. Pew Research Center. March 2021. Updated November 8, 2023. Accessed May 23, 2024. https://www.pewresearch.org/short-reads/2021/04/05/the-changing-face-of-americas-veteran-population/
4. US Department of Labor, Veterans’ Employment and Training Service. 2021 employment situation of women veterans. Accessed May 23, 2024. http://www.dol.gov/agencies/vets/womenveterans/womenveterans-employment
5. US Department of Veterans Affairs, National Center for Veteran Analysis and Statistics. National survey of veterans (NSV9503). Accessed June 20, 2024. https://www.va.gov/vetdata/docs/surveysandstudies/vetpop.pdf
6. US Census Bureau. Veterans Day 2022: November 11. News release. October 26, 2022. Updated April 4, 2024. Accessed May 23, 2024. https://www.census.gov/newsroom/facts-for-features/2022/veterans-day.html
7. ADA National Network. Employment data for veterans with disabilities. 2017. Accessed June 23, 2024. https://adata.org/factsheet/employment-data-veterans-disabilities
8. LGBTQ+ Veterans. DAV. Accessed July 26, 2024. https://www.dav.org/get-help-now/veteran-topics-resources/lgbtq-veterans/
9. US Department of Veterans Affairs, Office of Rural Health. Rural Veterans. Updated May 14, 2024. Accessed June 20, 2024. https://www.ruralhealth.va.gov/aboutus/ruralvets.asp
10. Mikton C, de la Fuente-Núñez V, Officer A, Krug E. Ageism: a social determinant of health that has come of age. Lancet. 2021;397(10282):1333-1334.
doi:10.1016/S0140-6736(21)00524-9
11. Heise L, Greene ME, Opper N, et al. Gender inequality and restrictive gender norms: framing the challenges to health. Lancet. 2019;393(10189):2440-2454.
doi:10.1016/S0140-6736(19)30652-X
12. Egede LE. Race, ethnicity, culture, and disparities in health care. J Gen Intern Med. 2006;21(6):667-669. doi:10.1111/j.1525-1497.2006.0512.x
13. Carratala S, Maxwell C. Health disparities by race and ethnicity. Center for American Progress. Updated May 11, 2020. Accessed June 23, 2024. https://www.americanprogress.org/article/health-disparities-race-ethnicity/
14. Clemente KAP, Silva SVD, Vieira GI, et al. Barriers to the access of people with disabilities to health services: a scoping review. Rev Saude Publica. 2022;56:64.
doi:10.11606/s1518-8787.2022056003893
15. Krehely J. How to close the LGBT health disparities gap. Center for American Progress. December 21, 2009. Accessed May 23, 2024. https://www.americanprogress.org/article/how-to-close-the-lgbt-health-disparities-gap/
16. Dawson L, Frederiksen B, Long M, Ranji U, Kates J. LGBT+ people’s health and experiences accessing care. KFF. July 22, 2021. Accessed May 23, 2024. https://www.kff.org/womens-health-policy/report/lgbt-peoples-health-and-experiences-accessing-care
17. Disabled American Veterans. DAV report spotlights issues facing women veterans. September 12, 2018. Accessed June 23, 2024. https://www.dav.org/learn-more/news/2018/new-report-spotlights-continuing-challenges-facing-women-veterans/
18. Sheahan KL, Goldstein KM, Than CT, et al. Women veterans’ healthcare needs, utilization, and preferences in veterans affairs primary care settings. J Gen Intern Med. 2022;37(Suppl 3):791-798.
doi:10.1007/s11606-022-07585-3
19. Habeshian S. VA denied Black veterans health benefits more often than White vets, data shows. Axios. June 23, 2023. Accessed June 20, 2024. https://www.axios.com/2023/06/23/veterans-benefits-black-white-rate-disproportionate
20. Shipherd JC, Darling JE, Klap RS, Rose D, Yano EM. Experiences in the Veterans Health Administration and impact on healthcare utilization: comparisons between LGBT and non‐LGBT women veterans. LGBT Health. 2018;5(5):303‐311. doi:10.1089/lgbt.2017.0179
21. Gomez LE, Bernet P. Diversity improves performance and outcomes. J Natl Med Assoc. 2019;111(4):383-392. doi:10.1016/j.jnma.2019.01.006
22. Gill GK, McNally MJ, Berman V. Effective diversity, equity, and inclusion practices. Healthc Manage Forum. 2018;31(5):196-199. doi:10.1177/0840470418773785
23. Balinda IG, Reza N. Diversity, equity, inclusion, and belonging in cardiovascular disease fellowship training. Methodist DeBakey Cardiovasc J. 2022;18(3):67-77. doi:10.14797/mdcvj.1080
24. Parsons SK, Fineberg IC, Lin M, Singer M, Tang M, Erban JK. Promoting high-quality cancer care and equity through disciplinary diversity in team composition. J Oncol Pract. 2016;12(11):1141-1147. doi:10.1200/JOP.2016.013920
25. Stanford FC. The importance of diversity and inclusion in the healthcare workforce. J Natl Med Assoc. 2020;112(3):247-249. doi:10.1016/j.jnma.2020.03.014
26. US Department of Veterans Affairs. Diversity and inclusion strategic plan, fiscal years 2021-2022. Accessed May 23, 2024. https://www.va.gov/ORMDI/docs/StrategicPlan.pdf
27. US Department of Veterans Affairs (VA). US EEOC. Accessed July 1, 2024. https://www.eeoc.gov/federal-sector/department-veterans-affairs-va-0
28. US Department of Veterans Affairs, Office of Resolution Management, Diversity & Inclusion (ORMDI). Individuals with disabilities employment program. Updated August 15, 2022. Accessed June 20, 2024. https://www.va.gov/ORMDI/DiversityInclusion/IWD.asp
29. US Department of Veterans Affairs, Office of Resolution Management, Diversity & Inclusion (ORMDI). VA workforce diversity: FY 2022. Accessed July 1, 2024. https://www.va.gov/ORMDI/Diversity_Inclusion.asp
30. US Department of Veterans Affairs. Same mission, new I-DEA: VA supports inclusion, diversity, equity and access. News release. April 28, 2023. Accessed June 20, 2024. https://news.va.gov/118609/same-mission-va-supports-inclusion-diversity/
31. US Department of Veterans Affairs, Office of Resolution Management, Diversity & Inclusion. Inclusion, diversity, equity, & access (I-DEA) action plan. September 2021. Accessed June 20, 2024. https://www.va.gov/ORMDI/docs/VA_I-DEA_Action_Plan-SIGNED.pdf
32. Sue DW, Alsaidi S, Awad MN, Glaeser E, Calle CZ. Disarming racial microaggressions: microintervention strategies for targets, White allies, and bystanders. Am Psychol. 2019;74(1):128-142. doi:10.1037/amp0000296
33. Cruz D, Rodriguez Y, Mastropaolo C. Perceived microaggressions in health care: a measurement study. PLoS One. 2019;14(2):e0211620. doi:10.1371/journal.pone.0211620
 34. Ehie O, Muse I, Hill L, Bastien A. Professionalism: microaggression in the healthcare setting. Curr Opin Anaesthesiol. 2021;34(2):131-136. doi:10.1097/ACO.0000000000000966
34. Ehie O, Muse I, Hill L, Bastien A. Professionalism: microaggression in the healthcare setting. Curr Opin Anaesthesiol. 2021;34(2):131-136. doi:10.1097/ACO.0000000000000966
35. US Department of Veterans Affairs. VA all employee survey. Accessed May 23, 2024. https://www.data.va.gov/stories/s/VA-All-Employee-Survey-AES-/r32e-j4vj/
36. US Department of Veterans Affairs, Office of Resolution Management, Diversity & Inclusion. Special emphasis programs (ORMDI). Updated May 3, 2023. Accessed June 20, 2024. https://www.va.gov/ORMDI/DiversityInclusion/Special_Emphasis_Programs.asp
37. US Department of Veterans Affairs, Office of Resolution Management, Diversity & Inclusion (ORMDI). Federal women’s program. Updated August 9, 2022. Accessed June 20, 2024. https://www.va.gov/ORMDI/DiversityInclusion/FWP.asp
38. US Department of Veterans Affairs, Office of Resolution Management, Diversity & Inclusion (ORMDI). Hispanic Employment program. Updated May 16, 2024. Accessed June 20, 2024. https://www.va.gov/ORMDI/DiversityInclusion/HEP.asp
39. US Department of Veterans Affairs, Office of Resolution Management, Diversity & Inclusion (ORMDI). American Indian & Alaska Native Program. Updated September 27, 2023. Accessed June 20, 2024. https://www.va.gov/ORMDI/DiversityInclusion/AIAN.asp
40. US Department of Veterans Affairs, Office of Resolution Management, Diversity & Inclusion (ORMDI). Asian American, Native Hawaiian and Pacific Islander program. Updated September 27, 2023. Accessed June 20, 2024. https://www.va.gov/ORMDI/DiversityInclusion/AAPI.asp
41. US Department of Veterans Affairs, Office of Resolution Management, Diversity & Inclusion (ORMDI). Black/African American program. Updated May 3, 2023. Accessed June 20, 2024. https://www.va.gov/ORMDI/DiversityInclusion/Black_African_American.asp
42. US Department of Veterans Affairs, Office of Resolution Management, Diversity & Inclusion (ORMDI). LGBTQ+ program. Updated May 21, 2024. Accessed June 20, 2024. https://www.va.gov/ORMDI/DiversityInclusion/LGBT.asp
43. US Department of Veterans Affairs, Office of Resolution Management, Diversity & Inclusion (ORMDI). Diversity, equity and inclusion training. Updated March 18, 2024. Accessed June 20, 2024. https://www.va.gov/ORMDI/DiversityInclusion/Diversity_Inclusion_Training.asp
44. Rotenstein LS, Reede JY, Jena AB. Addressing workforce diversity - a quality-improvement framework. N Engl J Med. 2021;384(12):1083-1086. doi:10.1056/NEJMp2032224
45. Beeler WH, Mangurian C, Jagsi R. Unplugging the pipeline - a call for term limits in academic medicine. N Engl J Med. 2019;381(16):1508-1511. doi:10.1056/NEJMp1906832
46. Smith DG. Term limits in academic public health administration. Public Health Rep. 2020;135(6):859-863. doi:10.1177/0033354920954495
47. Kaiser J. Shake-up at NIH: Term limits for important positions would open new opportunities for women, minorities. science.org. May 2, 2019. Accessed May 23, 2024. https://www.science.org/content/article/shakeup-nih-term-limits-important-positions-would-open-new-opportunities-women
48. Giacalone RA, Jurkiewicz CL, Knouse SB. Exit surveys as assessments of organizational ethicality. Public Pers Manage. 2003;32(3):397-410. doi:10.1177/009102600303200306
49. Bonifacino E, Ufomata EO, Farkas AH, Turner R, Corbelli JA. Mentorship of underrepresented physicians and trainees in academic medicine: a systematic review. J Gen Intern Med. 2021;36(4):1023-1034. doi:10.1007/s11606-020-06478-7
50. Brown IM. Diversity matters: mentorship is the missing ingredient in DEI. Emergency Medicine News. 2021;43(8):28. doi:10.1097/01.EEM.0000771148.76632.35
51. Sinha A, Kuy S. The future of surgery - increasing diversity, equity, and inclusion through early mentorship. Am J Surg. 2023;225(4):800-802. doi:10.1016/j.amjsurg.2022.12.011
1. US Department of Veterans Affairs, Office of Research & Development. Health equity. Accessed July 1, 2024. https://www.research.va.gov/topics/health_equity.cfm
2. US Department of Veterans Affairs. Equity action plan. Accessed July 1, 2024. https://department.va.gov/wp-content/uploads/2024/02/Department-of-Veterans-Affairs-Equity-Action-Plan.pdf
3. Schaeffer K. The changing face of America’s veteran population. Pew Research Center. March 2021. Updated November 8, 2023. Accessed May 23, 2024. https://www.pewresearch.org/short-reads/2021/04/05/the-changing-face-of-americas-veteran-population/
4. US Department of Labor, Veterans’ Employment and Training Service. 2021 employment situation of women veterans. Accessed May 23, 2024. http://www.dol.gov/agencies/vets/womenveterans/womenveterans-employment
5. US Department of Veterans Affairs, National Center for Veteran Analysis and Statistics. National survey of veterans (NSV9503). Accessed June 20, 2024. https://www.va.gov/vetdata/docs/surveysandstudies/vetpop.pdf
6. US Census Bureau. Veterans Day 2022: November 11. News release. October 26, 2022. Updated April 4, 2024. Accessed May 23, 2024. https://www.census.gov/newsroom/facts-for-features/2022/veterans-day.html
7. ADA National Network. Employment data for veterans with disabilities. 2017. Accessed June 23, 2024. https://adata.org/factsheet/employment-data-veterans-disabilities
8. LGBTQ+ Veterans. DAV. Accessed July 26, 2024. https://www.dav.org/get-help-now/veteran-topics-resources/lgbtq-veterans/
9. US Department of Veterans Affairs, Office of Rural Health. Rural Veterans. Updated May 14, 2024. Accessed June 20, 2024. https://www.ruralhealth.va.gov/aboutus/ruralvets.asp
10. Mikton C, de la Fuente-Núñez V, Officer A, Krug E. Ageism: a social determinant of health that has come of age. Lancet. 2021;397(10282):1333-1334.
doi:10.1016/S0140-6736(21)00524-9
11. Heise L, Greene ME, Opper N, et al. Gender inequality and restrictive gender norms: framing the challenges to health. Lancet. 2019;393(10189):2440-2454.
doi:10.1016/S0140-6736(19)30652-X
12. Egede LE. Race, ethnicity, culture, and disparities in health care. J Gen Intern Med. 2006;21(6):667-669. doi:10.1111/j.1525-1497.2006.0512.x
13. Carratala S, Maxwell C. Health disparities by race and ethnicity. Center for American Progress. Updated May 11, 2020. Accessed June 23, 2024. https://www.americanprogress.org/article/health-disparities-race-ethnicity/
14. Clemente KAP, Silva SVD, Vieira GI, et al. Barriers to the access of people with disabilities to health services: a scoping review. Rev Saude Publica. 2022;56:64.
doi:10.11606/s1518-8787.2022056003893
15. Krehely J. How to close the LGBT health disparities gap. Center for American Progress. December 21, 2009. Accessed May 23, 2024. https://www.americanprogress.org/article/how-to-close-the-lgbt-health-disparities-gap/
16. Dawson L, Frederiksen B, Long M, Ranji U, Kates J. LGBT+ people’s health and experiences accessing care. KFF. July 22, 2021. Accessed May 23, 2024. https://www.kff.org/womens-health-policy/report/lgbt-peoples-health-and-experiences-accessing-care
17. Disabled American Veterans. DAV report spotlights issues facing women veterans. September 12, 2018. Accessed June 23, 2024. https://www.dav.org/learn-more/news/2018/new-report-spotlights-continuing-challenges-facing-women-veterans/
18. Sheahan KL, Goldstein KM, Than CT, et al. Women veterans’ healthcare needs, utilization, and preferences in veterans affairs primary care settings. J Gen Intern Med. 2022;37(Suppl 3):791-798.
doi:10.1007/s11606-022-07585-3
19. Habeshian S. VA denied Black veterans health benefits more often than White vets, data shows. Axios. June 23, 2023. Accessed June 20, 2024. https://www.axios.com/2023/06/23/veterans-benefits-black-white-rate-disproportionate
20. Shipherd JC, Darling JE, Klap RS, Rose D, Yano EM. Experiences in the Veterans Health Administration and impact on healthcare utilization: comparisons between LGBT and non‐LGBT women veterans. LGBT Health. 2018;5(5):303‐311. doi:10.1089/lgbt.2017.0179
21. Gomez LE, Bernet P. Diversity improves performance and outcomes. J Natl Med Assoc. 2019;111(4):383-392. doi:10.1016/j.jnma.2019.01.006
22. Gill GK, McNally MJ, Berman V. Effective diversity, equity, and inclusion practices. Healthc Manage Forum. 2018;31(5):196-199. doi:10.1177/0840470418773785
23. Balinda IG, Reza N. Diversity, equity, inclusion, and belonging in cardiovascular disease fellowship training. Methodist DeBakey Cardiovasc J. 2022;18(3):67-77. doi:10.14797/mdcvj.1080
24. Parsons SK, Fineberg IC, Lin M, Singer M, Tang M, Erban JK. Promoting high-quality cancer care and equity through disciplinary diversity in team composition. J Oncol Pract. 2016;12(11):1141-1147. doi:10.1200/JOP.2016.013920
25. Stanford FC. The importance of diversity and inclusion in the healthcare workforce. J Natl Med Assoc. 2020;112(3):247-249. doi:10.1016/j.jnma.2020.03.014
26. US Department of Veterans Affairs. Diversity and inclusion strategic plan, fiscal years 2021-2022. Accessed May 23, 2024. https://www.va.gov/ORMDI/docs/StrategicPlan.pdf
27. US Department of Veterans Affairs (VA). US EEOC. Accessed July 1, 2024. https://www.eeoc.gov/federal-sector/department-veterans-affairs-va-0
28. US Department of Veterans Affairs, Office of Resolution Management, Diversity & Inclusion (ORMDI). Individuals with disabilities employment program. Updated August 15, 2022. Accessed June 20, 2024. https://www.va.gov/ORMDI/DiversityInclusion/IWD.asp
29. US Department of Veterans Affairs, Office of Resolution Management, Diversity & Inclusion (ORMDI). VA workforce diversity: FY 2022. Accessed July 1, 2024. https://www.va.gov/ORMDI/Diversity_Inclusion.asp
30. US Department of Veterans Affairs. Same mission, new I-DEA: VA supports inclusion, diversity, equity and access. News release. April 28, 2023. Accessed June 20, 2024. https://news.va.gov/118609/same-mission-va-supports-inclusion-diversity/
31. US Department of Veterans Affairs, Office of Resolution Management, Diversity & Inclusion. Inclusion, diversity, equity, & access (I-DEA) action plan. September 2021. Accessed June 20, 2024. https://www.va.gov/ORMDI/docs/VA_I-DEA_Action_Plan-SIGNED.pdf
32. Sue DW, Alsaidi S, Awad MN, Glaeser E, Calle CZ. Disarming racial microaggressions: microintervention strategies for targets, White allies, and bystanders. Am Psychol. 2019;74(1):128-142. doi:10.1037/amp0000296
33. Cruz D, Rodriguez Y, Mastropaolo C. Perceived microaggressions in health care: a measurement study. PLoS One. 2019;14(2):e0211620. doi:10.1371/journal.pone.0211620
 34. Ehie O, Muse I, Hill L, Bastien A. Professionalism: microaggression in the healthcare setting. Curr Opin Anaesthesiol. 2021;34(2):131-136. doi:10.1097/ACO.0000000000000966
34. Ehie O, Muse I, Hill L, Bastien A. Professionalism: microaggression in the healthcare setting. Curr Opin Anaesthesiol. 2021;34(2):131-136. doi:10.1097/ACO.0000000000000966
35. US Department of Veterans Affairs. VA all employee survey. Accessed May 23, 2024. https://www.data.va.gov/stories/s/VA-All-Employee-Survey-AES-/r32e-j4vj/
36. US Department of Veterans Affairs, Office of Resolution Management, Diversity & Inclusion. Special emphasis programs (ORMDI). Updated May 3, 2023. Accessed June 20, 2024. https://www.va.gov/ORMDI/DiversityInclusion/Special_Emphasis_Programs.asp
37. US Department of Veterans Affairs, Office of Resolution Management, Diversity & Inclusion (ORMDI). Federal women’s program. Updated August 9, 2022. Accessed June 20, 2024. https://www.va.gov/ORMDI/DiversityInclusion/FWP.asp
38. US Department of Veterans Affairs, Office of Resolution Management, Diversity & Inclusion (ORMDI). Hispanic Employment program. Updated May 16, 2024. Accessed June 20, 2024. https://www.va.gov/ORMDI/DiversityInclusion/HEP.asp
39. US Department of Veterans Affairs, Office of Resolution Management, Diversity & Inclusion (ORMDI). American Indian & Alaska Native Program. Updated September 27, 2023. Accessed June 20, 2024. https://www.va.gov/ORMDI/DiversityInclusion/AIAN.asp
40. US Department of Veterans Affairs, Office of Resolution Management, Diversity & Inclusion (ORMDI). Asian American, Native Hawaiian and Pacific Islander program. Updated September 27, 2023. Accessed June 20, 2024. https://www.va.gov/ORMDI/DiversityInclusion/AAPI.asp
41. US Department of Veterans Affairs, Office of Resolution Management, Diversity & Inclusion (ORMDI). Black/African American program. Updated May 3, 2023. Accessed June 20, 2024. https://www.va.gov/ORMDI/DiversityInclusion/Black_African_American.asp
42. US Department of Veterans Affairs, Office of Resolution Management, Diversity & Inclusion (ORMDI). LGBTQ+ program. Updated May 21, 2024. Accessed June 20, 2024. https://www.va.gov/ORMDI/DiversityInclusion/LGBT.asp
43. US Department of Veterans Affairs, Office of Resolution Management, Diversity & Inclusion (ORMDI). Diversity, equity and inclusion training. Updated March 18, 2024. Accessed June 20, 2024. https://www.va.gov/ORMDI/DiversityInclusion/Diversity_Inclusion_Training.asp
44. Rotenstein LS, Reede JY, Jena AB. Addressing workforce diversity - a quality-improvement framework. N Engl J Med. 2021;384(12):1083-1086. doi:10.1056/NEJMp2032224
45. Beeler WH, Mangurian C, Jagsi R. Unplugging the pipeline - a call for term limits in academic medicine. N Engl J Med. 2019;381(16):1508-1511. doi:10.1056/NEJMp1906832
46. Smith DG. Term limits in academic public health administration. Public Health Rep. 2020;135(6):859-863. doi:10.1177/0033354920954495
47. Kaiser J. Shake-up at NIH: Term limits for important positions would open new opportunities for women, minorities. science.org. May 2, 2019. Accessed May 23, 2024. https://www.science.org/content/article/shakeup-nih-term-limits-important-positions-would-open-new-opportunities-women
48. Giacalone RA, Jurkiewicz CL, Knouse SB. Exit surveys as assessments of organizational ethicality. Public Pers Manage. 2003;32(3):397-410. doi:10.1177/009102600303200306
49. Bonifacino E, Ufomata EO, Farkas AH, Turner R, Corbelli JA. Mentorship of underrepresented physicians and trainees in academic medicine: a systematic review. J Gen Intern Med. 2021;36(4):1023-1034. doi:10.1007/s11606-020-06478-7
50. Brown IM. Diversity matters: mentorship is the missing ingredient in DEI. Emergency Medicine News. 2021;43(8):28. doi:10.1097/01.EEM.0000771148.76632.35
51. Sinha A, Kuy S. The future of surgery - increasing diversity, equity, and inclusion through early mentorship. Am J Surg. 2023;225(4):800-802. doi:10.1016/j.amjsurg.2022.12.011
Black Women With Breast Cancer Face Clinical Inequities
Black metastatic breast cancer patients with PIK3CA mutations were less likely to receive targeted therapy and less likely to be enrolled in clinical trials than White patients and had shorter overall survival, according to a retrospective cohort study. Black and White patients were equally likely to receive other drugs that did not require genomic testing.
“These clinical inequities in the use of targeted therapies and clinical trials ... must be a focus going forward,” said lead investigator Emily Podany, MD, a clinical fellow in hematology-oncology at Washington University in St. Louis, Missouri. “Our consortium is looking for paths forward in order to try and decrease these striking inequities. And it’s a focus of future research for us and future implementation [of] science interventions, hopefully, across the country.”
The study results were presented at the annual meeting of the American Society of Clinical Oncology.
Black Women Underrepresented
Black women are generally underrepresented in clinical trials, noted Dr. Podany. “They make up about 2%-5% of the patients in breast cancer clinical trials, and there are documented inequities in treatment and in outcomes for Black patients with metastatic breast cancer. This includes longer treatment delays, it includes fewer sentinel lymph node biopsies, and unfortunately, they’re more likely to discontinue treatment early.”
In terms of PI3K inhibition, PIK3CA mutations are found in about 40% of patients with HR-positive HER2-negative metastatic breast cancer. Alpelisib is FDA-approved as a targeted therapy for these patients, she said.
The study evaluated records of 1327 patients with metastatic breast cancer who also had circulating tumor DNA (ctDNA) results and were treated at Washington University, Massachusetts General Hospital in Boston, and Northwestern University in Chicago. Of these, 795 had an ER-positive, HER2-negative subtype and were included in the analysis. Most (89%) of the patients were White (n = 708), while 11% (n = 87) were Black, and the only baseline difference between patients was that Black patients had significantly more de novo metastatic breast cancer (31% versus 22%).
Use of PI3K, CDK4/6, or mTOR inhibitors was evaluated using manual electronic medical review, and genomic differences were evaluated using logistic regression.
The analysis showed inequities in both treatment and clinical trial enrollment. There were no differences between groups in the use of CDK4/6 or mTOR inhibitors, which do not require a genomic profile, the researchers noted, but Black patients with PIK3CA single nucleotide variants (SNV) were significantly less likely than White patients to use PI3K inhibitors (5.9% versus 28.8%; P = .045), despite no difference in PIK3CA mutations between groups (36% and 34% respectively). Similarly, 11% of White patients with PIK3CA mutations were enrolled in clinical trials, but none of the Black patients was.
Genomic differences were also found, Dr. Podany reported. Black patients with estrogen/progesterone receptor (ER/PR) positive, HER2-negative disease were more likely to have a CCND1 copy number variant. And for ER-positive PR-negative HER2-negative patients, Black patients were more likely to have a GATA3 SNV, while White patients were more likely to have a KRAS copy number variant.
Black Survival Less Than Half
The analysis also found significant differences in overall survival from the time of the first liquid biopsy, with White ER-positive, PR-negative, HER2-negative patients living a median of 21 months, versus 9.1 months for Black patients.
There were several limitations to the study beyond its retrospective nature, “so, we may be underestimating the true inequity,” noted Dr. Podany. “These are large urban academic centers, so our patients have access to these treatments. They have access to care. They have access to ctDNA liquid biopsy testing. And the timing of ctDNA, especially the first ctDNA test, is variable and provider-dependant. We were also unable to assess receipt of PI3 kinase inhibitors at future time points after the end of this cohort study.”
Asked for comment, Giuseppe Del Priore, MD, MPH, from Morehouse School of Medicine in Atlanta, Georgia, approved of the study design “with subjects limited to three distinctive institutions. That parameter alone can control for several unknown variables among the studied comparison groups, ie, Black women versus others.”
However, Dr. Del Priore, who is adjunct professor of obstetrics and gynecology, with a specialty in oncology, added, “retrospective studies are not reliable except for generating hypotheses. Therefore, I would like to see a rapid implementation of an intervention trial at these same institutions to ensure equal consideration of, and access to, targeted therapies. Too often a retrospective correlation is reported, but the solution is elusive due to unknown factors. In this case, knowing there is a mutation is far from alleviating the disproportionate burden of disease that many communities face.”
Dr. Podany had no relevant disclosures. Dr. Del Priore reported no conflicts of interest and disclosed that he is chief medical officer at BriaCell.
Black metastatic breast cancer patients with PIK3CA mutations were less likely to receive targeted therapy and less likely to be enrolled in clinical trials than White patients and had shorter overall survival, according to a retrospective cohort study. Black and White patients were equally likely to receive other drugs that did not require genomic testing.
“These clinical inequities in the use of targeted therapies and clinical trials ... must be a focus going forward,” said lead investigator Emily Podany, MD, a clinical fellow in hematology-oncology at Washington University in St. Louis, Missouri. “Our consortium is looking for paths forward in order to try and decrease these striking inequities. And it’s a focus of future research for us and future implementation [of] science interventions, hopefully, across the country.”
The study results were presented at the annual meeting of the American Society of Clinical Oncology.
Black Women Underrepresented
Black women are generally underrepresented in clinical trials, noted Dr. Podany. “They make up about 2%-5% of the patients in breast cancer clinical trials, and there are documented inequities in treatment and in outcomes for Black patients with metastatic breast cancer. This includes longer treatment delays, it includes fewer sentinel lymph node biopsies, and unfortunately, they’re more likely to discontinue treatment early.”
In terms of PI3K inhibition, PIK3CA mutations are found in about 40% of patients with HR-positive HER2-negative metastatic breast cancer. Alpelisib is FDA-approved as a targeted therapy for these patients, she said.
The study evaluated records of 1327 patients with metastatic breast cancer who also had circulating tumor DNA (ctDNA) results and were treated at Washington University, Massachusetts General Hospital in Boston, and Northwestern University in Chicago. Of these, 795 had an ER-positive, HER2-negative subtype and were included in the analysis. Most (89%) of the patients were White (n = 708), while 11% (n = 87) were Black, and the only baseline difference between patients was that Black patients had significantly more de novo metastatic breast cancer (31% versus 22%).
Use of PI3K, CDK4/6, or mTOR inhibitors was evaluated using manual electronic medical review, and genomic differences were evaluated using logistic regression.
The analysis showed inequities in both treatment and clinical trial enrollment. There were no differences between groups in the use of CDK4/6 or mTOR inhibitors, which do not require a genomic profile, the researchers noted, but Black patients with PIK3CA single nucleotide variants (SNV) were significantly less likely than White patients to use PI3K inhibitors (5.9% versus 28.8%; P = .045), despite no difference in PIK3CA mutations between groups (36% and 34% respectively). Similarly, 11% of White patients with PIK3CA mutations were enrolled in clinical trials, but none of the Black patients was.
Genomic differences were also found, Dr. Podany reported. Black patients with estrogen/progesterone receptor (ER/PR) positive, HER2-negative disease were more likely to have a CCND1 copy number variant. And for ER-positive PR-negative HER2-negative patients, Black patients were more likely to have a GATA3 SNV, while White patients were more likely to have a KRAS copy number variant.
Black Survival Less Than Half
The analysis also found significant differences in overall survival from the time of the first liquid biopsy, with White ER-positive, PR-negative, HER2-negative patients living a median of 21 months, versus 9.1 months for Black patients.
There were several limitations to the study beyond its retrospective nature, “so, we may be underestimating the true inequity,” noted Dr. Podany. “These are large urban academic centers, so our patients have access to these treatments. They have access to care. They have access to ctDNA liquid biopsy testing. And the timing of ctDNA, especially the first ctDNA test, is variable and provider-dependant. We were also unable to assess receipt of PI3 kinase inhibitors at future time points after the end of this cohort study.”
Asked for comment, Giuseppe Del Priore, MD, MPH, from Morehouse School of Medicine in Atlanta, Georgia, approved of the study design “with subjects limited to three distinctive institutions. That parameter alone can control for several unknown variables among the studied comparison groups, ie, Black women versus others.”
However, Dr. Del Priore, who is adjunct professor of obstetrics and gynecology, with a specialty in oncology, added, “retrospective studies are not reliable except for generating hypotheses. Therefore, I would like to see a rapid implementation of an intervention trial at these same institutions to ensure equal consideration of, and access to, targeted therapies. Too often a retrospective correlation is reported, but the solution is elusive due to unknown factors. In this case, knowing there is a mutation is far from alleviating the disproportionate burden of disease that many communities face.”
Dr. Podany had no relevant disclosures. Dr. Del Priore reported no conflicts of interest and disclosed that he is chief medical officer at BriaCell.
Black metastatic breast cancer patients with PIK3CA mutations were less likely to receive targeted therapy and less likely to be enrolled in clinical trials than White patients and had shorter overall survival, according to a retrospective cohort study. Black and White patients were equally likely to receive other drugs that did not require genomic testing.
“These clinical inequities in the use of targeted therapies and clinical trials ... must be a focus going forward,” said lead investigator Emily Podany, MD, a clinical fellow in hematology-oncology at Washington University in St. Louis, Missouri. “Our consortium is looking for paths forward in order to try and decrease these striking inequities. And it’s a focus of future research for us and future implementation [of] science interventions, hopefully, across the country.”
The study results were presented at the annual meeting of the American Society of Clinical Oncology.
Black Women Underrepresented
Black women are generally underrepresented in clinical trials, noted Dr. Podany. “They make up about 2%-5% of the patients in breast cancer clinical trials, and there are documented inequities in treatment and in outcomes for Black patients with metastatic breast cancer. This includes longer treatment delays, it includes fewer sentinel lymph node biopsies, and unfortunately, they’re more likely to discontinue treatment early.”
In terms of PI3K inhibition, PIK3CA mutations are found in about 40% of patients with HR-positive HER2-negative metastatic breast cancer. Alpelisib is FDA-approved as a targeted therapy for these patients, she said.
The study evaluated records of 1327 patients with metastatic breast cancer who also had circulating tumor DNA (ctDNA) results and were treated at Washington University, Massachusetts General Hospital in Boston, and Northwestern University in Chicago. Of these, 795 had an ER-positive, HER2-negative subtype and were included in the analysis. Most (89%) of the patients were White (n = 708), while 11% (n = 87) were Black, and the only baseline difference between patients was that Black patients had significantly more de novo metastatic breast cancer (31% versus 22%).
Use of PI3K, CDK4/6, or mTOR inhibitors was evaluated using manual electronic medical review, and genomic differences were evaluated using logistic regression.
The analysis showed inequities in both treatment and clinical trial enrollment. There were no differences between groups in the use of CDK4/6 or mTOR inhibitors, which do not require a genomic profile, the researchers noted, but Black patients with PIK3CA single nucleotide variants (SNV) were significantly less likely than White patients to use PI3K inhibitors (5.9% versus 28.8%; P = .045), despite no difference in PIK3CA mutations between groups (36% and 34% respectively). Similarly, 11% of White patients with PIK3CA mutations were enrolled in clinical trials, but none of the Black patients was.
Genomic differences were also found, Dr. Podany reported. Black patients with estrogen/progesterone receptor (ER/PR) positive, HER2-negative disease were more likely to have a CCND1 copy number variant. And for ER-positive PR-negative HER2-negative patients, Black patients were more likely to have a GATA3 SNV, while White patients were more likely to have a KRAS copy number variant.
Black Survival Less Than Half
The analysis also found significant differences in overall survival from the time of the first liquid biopsy, with White ER-positive, PR-negative, HER2-negative patients living a median of 21 months, versus 9.1 months for Black patients.
There were several limitations to the study beyond its retrospective nature, “so, we may be underestimating the true inequity,” noted Dr. Podany. “These are large urban academic centers, so our patients have access to these treatments. They have access to care. They have access to ctDNA liquid biopsy testing. And the timing of ctDNA, especially the first ctDNA test, is variable and provider-dependant. We were also unable to assess receipt of PI3 kinase inhibitors at future time points after the end of this cohort study.”
Asked for comment, Giuseppe Del Priore, MD, MPH, from Morehouse School of Medicine in Atlanta, Georgia, approved of the study design “with subjects limited to three distinctive institutions. That parameter alone can control for several unknown variables among the studied comparison groups, ie, Black women versus others.”
However, Dr. Del Priore, who is adjunct professor of obstetrics and gynecology, with a specialty in oncology, added, “retrospective studies are not reliable except for generating hypotheses. Therefore, I would like to see a rapid implementation of an intervention trial at these same institutions to ensure equal consideration of, and access to, targeted therapies. Too often a retrospective correlation is reported, but the solution is elusive due to unknown factors. In this case, knowing there is a mutation is far from alleviating the disproportionate burden of disease that many communities face.”
Dr. Podany had no relevant disclosures. Dr. Del Priore reported no conflicts of interest and disclosed that he is chief medical officer at BriaCell.
FROM ASCO 2024
Study Finds Varying Skin Cancer Rates Based on Sexual Orientation
Addressing dynamics of each SM subgroup will require increasingly tailored prevention, screening, and research efforts, the study authors said.
“We identified specific subgroups within the sexual minority community who are at higher risk for skin cancer, specifically White gay males and Hispanic and non-Hispanic Black SM men and women — particularly individuals who identify as bisexual,” senior author Matthew Mansh, MD, said in an interview. He is an assistant professor of dermatology at the University of California, San Francisco. The study was published online in JAMA Dermatology.
Using data of adults in the US general population from the Behavioral Risk Factor Surveillance System from January 2014 to December 2021, investigators included more than 1.5 million respondents. The proportions of SM women and men (who self-identified as bisexual, lesbian, gay, “something else,” or other) were 2.6% and 2.0%, respectively.
Lifetime skin cancer prevalence was higher among SM men than among heterosexual men (7.4% vs 6.8%; adjusted odds ratio [aOR], 1.16). In analyses stratified by racial and ethnic group, AORs for non-Hispanic Black and Hispanic SM men vs their heterosexual counterparts were 2.18 and 3.81, respectively. The corresponding figures for non-Hispanic Black and Hispanic SM women were 2.33 and 2.46, respectively.
When investigators combined all minority respondents along gender lines, lifetime skin cancer prevalence was higher in bisexual men (aOR, 3.94), bisexual women (aOR, 1.51), and women identifying as something else or other (aOR, 2.70) than in their heterosexual peers.
“I wasn’t expecting that Hispanic or non-Hispanic Black SMs would be at higher risk for skin cancer,” Dr. Mansh said. Even if these groups have more behavioral risk factors for UV radiation (UVR) exposure, he explained, UVR exposure is less strongly linked with skin cancer in darker skin than in lighter skin. Reasons for the counterintuitive finding could include different screening habits among SM people of different racial and ethnic groups, he said, and analyzing such factors will require further research.
Although some effect sizes were modest, the authors wrote, their findings may have important implications for population-based research and public health efforts aimed at early skin cancer detection and prevention. Presently, the United States lacks established guidelines for skin cancer screening. In a 2023 statement published in JAMA, the US Preventive Services Task Force said that there is insufficient evidence to determine the benefit-harm balance of skin cancer screening in asymptomatic people.
“So there has been a lot of recent talk and a need to identify which subset groups of patients might be higher risk for skin cancer and might benefit from more screening,” Dr. Mansh said in an interview. “Understanding more about the high-risk demographic and clinical features that predispose someone to skin cancer helps identify these high-risk populations that could be used to develop better screening guidelines.”
Identifying groups at a higher risk for skin cancer also allows experts to design more targeted counseling or public health interventions focused on these groups, Dr. Mansh added. Absent screening guidelines, experts emphasize changing modifiable risk factors such as UVR exposure, smoking, and alcohol use. “And we know that the message that might change behaviors in a cisgender heterosexual man might be different than in a gay White male or a Hispanic bisexual male.”
A 2017 review showed that interventions to reduce behaviors involving UVR exposure, such as indoor tanning, among young cisgender women focused largely on aging and appearance-based concerns. A 2019 study showed that messages focused on avoiding skin cancer may help motivate SM men to reduce tanning behaviors.
Furthermore, said Dr. Mansh, all electronic health record products available in the United States must provide data fields for sexual orientation. “I don’t believe many dermatologists, depending on the setting, collect that information routinely. Integrating sexual orientation and/or gender identity data into patient intake forms so that it can be integrated into the electronic health record is probably very helpful, not only for your clinical practice but also for future research studies.”
Asked to comment on the results, Rebecca I. Hartman, MD, MPH, who was not involved with the study, said that its impact on clinical practice will be challenging to ascertain. She is chief of dermatology with the VA Boston Healthcare System, assistant professor of dermatology at Harvard Medical School, and director of melanoma epidemiology at Brigham and Women’s Hospital, all in Boston, Massachusetts.
“The study found significant adjusted odds ratios,” Dr. Hartman explained, “but for some of the different populations, the overall lifetime rate of skin cancer is still quite low.” For example, 1.0% for SM non-Hispanic Black men or a difference of 2.1% vs 1.8% in SM Hispanic women. “Thus, I am not sure specific screening recommendations are warranted, although some populations, such as Hispanic sexual minority males, seemed to have a much higher risk (3.8-fold on adjusted analysis) that warrants further investigation.”
For now, she advised assessing patients’ risks for skin cancer based on well-established risk factors such as sun exposure/indoor tanning, skin phototype, immunosuppression, and age.
Dr. Mansh reported no relevant conflicts or funding sources for the study. Dr. Hartman reported no relevant conflicts.
A version of this article appeared on Medscape.com.
Addressing dynamics of each SM subgroup will require increasingly tailored prevention, screening, and research efforts, the study authors said.
“We identified specific subgroups within the sexual minority community who are at higher risk for skin cancer, specifically White gay males and Hispanic and non-Hispanic Black SM men and women — particularly individuals who identify as bisexual,” senior author Matthew Mansh, MD, said in an interview. He is an assistant professor of dermatology at the University of California, San Francisco. The study was published online in JAMA Dermatology.
Using data of adults in the US general population from the Behavioral Risk Factor Surveillance System from January 2014 to December 2021, investigators included more than 1.5 million respondents. The proportions of SM women and men (who self-identified as bisexual, lesbian, gay, “something else,” or other) were 2.6% and 2.0%, respectively.
Lifetime skin cancer prevalence was higher among SM men than among heterosexual men (7.4% vs 6.8%; adjusted odds ratio [aOR], 1.16). In analyses stratified by racial and ethnic group, AORs for non-Hispanic Black and Hispanic SM men vs their heterosexual counterparts were 2.18 and 3.81, respectively. The corresponding figures for non-Hispanic Black and Hispanic SM women were 2.33 and 2.46, respectively.
When investigators combined all minority respondents along gender lines, lifetime skin cancer prevalence was higher in bisexual men (aOR, 3.94), bisexual women (aOR, 1.51), and women identifying as something else or other (aOR, 2.70) than in their heterosexual peers.
“I wasn’t expecting that Hispanic or non-Hispanic Black SMs would be at higher risk for skin cancer,” Dr. Mansh said. Even if these groups have more behavioral risk factors for UV radiation (UVR) exposure, he explained, UVR exposure is less strongly linked with skin cancer in darker skin than in lighter skin. Reasons for the counterintuitive finding could include different screening habits among SM people of different racial and ethnic groups, he said, and analyzing such factors will require further research.
Although some effect sizes were modest, the authors wrote, their findings may have important implications for population-based research and public health efforts aimed at early skin cancer detection and prevention. Presently, the United States lacks established guidelines for skin cancer screening. In a 2023 statement published in JAMA, the US Preventive Services Task Force said that there is insufficient evidence to determine the benefit-harm balance of skin cancer screening in asymptomatic people.
“So there has been a lot of recent talk and a need to identify which subset groups of patients might be higher risk for skin cancer and might benefit from more screening,” Dr. Mansh said in an interview. “Understanding more about the high-risk demographic and clinical features that predispose someone to skin cancer helps identify these high-risk populations that could be used to develop better screening guidelines.”
Identifying groups at a higher risk for skin cancer also allows experts to design more targeted counseling or public health interventions focused on these groups, Dr. Mansh added. Absent screening guidelines, experts emphasize changing modifiable risk factors such as UVR exposure, smoking, and alcohol use. “And we know that the message that might change behaviors in a cisgender heterosexual man might be different than in a gay White male or a Hispanic bisexual male.”
A 2017 review showed that interventions to reduce behaviors involving UVR exposure, such as indoor tanning, among young cisgender women focused largely on aging and appearance-based concerns. A 2019 study showed that messages focused on avoiding skin cancer may help motivate SM men to reduce tanning behaviors.
Furthermore, said Dr. Mansh, all electronic health record products available in the United States must provide data fields for sexual orientation. “I don’t believe many dermatologists, depending on the setting, collect that information routinely. Integrating sexual orientation and/or gender identity data into patient intake forms so that it can be integrated into the electronic health record is probably very helpful, not only for your clinical practice but also for future research studies.”
Asked to comment on the results, Rebecca I. Hartman, MD, MPH, who was not involved with the study, said that its impact on clinical practice will be challenging to ascertain. She is chief of dermatology with the VA Boston Healthcare System, assistant professor of dermatology at Harvard Medical School, and director of melanoma epidemiology at Brigham and Women’s Hospital, all in Boston, Massachusetts.
“The study found significant adjusted odds ratios,” Dr. Hartman explained, “but for some of the different populations, the overall lifetime rate of skin cancer is still quite low.” For example, 1.0% for SM non-Hispanic Black men or a difference of 2.1% vs 1.8% in SM Hispanic women. “Thus, I am not sure specific screening recommendations are warranted, although some populations, such as Hispanic sexual minority males, seemed to have a much higher risk (3.8-fold on adjusted analysis) that warrants further investigation.”
For now, she advised assessing patients’ risks for skin cancer based on well-established risk factors such as sun exposure/indoor tanning, skin phototype, immunosuppression, and age.
Dr. Mansh reported no relevant conflicts or funding sources for the study. Dr. Hartman reported no relevant conflicts.
A version of this article appeared on Medscape.com.
Addressing dynamics of each SM subgroup will require increasingly tailored prevention, screening, and research efforts, the study authors said.
“We identified specific subgroups within the sexual minority community who are at higher risk for skin cancer, specifically White gay males and Hispanic and non-Hispanic Black SM men and women — particularly individuals who identify as bisexual,” senior author Matthew Mansh, MD, said in an interview. He is an assistant professor of dermatology at the University of California, San Francisco. The study was published online in JAMA Dermatology.
Using data of adults in the US general population from the Behavioral Risk Factor Surveillance System from January 2014 to December 2021, investigators included more than 1.5 million respondents. The proportions of SM women and men (who self-identified as bisexual, lesbian, gay, “something else,” or other) were 2.6% and 2.0%, respectively.
Lifetime skin cancer prevalence was higher among SM men than among heterosexual men (7.4% vs 6.8%; adjusted odds ratio [aOR], 1.16). In analyses stratified by racial and ethnic group, AORs for non-Hispanic Black and Hispanic SM men vs their heterosexual counterparts were 2.18 and 3.81, respectively. The corresponding figures for non-Hispanic Black and Hispanic SM women were 2.33 and 2.46, respectively.
When investigators combined all minority respondents along gender lines, lifetime skin cancer prevalence was higher in bisexual men (aOR, 3.94), bisexual women (aOR, 1.51), and women identifying as something else or other (aOR, 2.70) than in their heterosexual peers.
“I wasn’t expecting that Hispanic or non-Hispanic Black SMs would be at higher risk for skin cancer,” Dr. Mansh said. Even if these groups have more behavioral risk factors for UV radiation (UVR) exposure, he explained, UVR exposure is less strongly linked with skin cancer in darker skin than in lighter skin. Reasons for the counterintuitive finding could include different screening habits among SM people of different racial and ethnic groups, he said, and analyzing such factors will require further research.
Although some effect sizes were modest, the authors wrote, their findings may have important implications for population-based research and public health efforts aimed at early skin cancer detection and prevention. Presently, the United States lacks established guidelines for skin cancer screening. In a 2023 statement published in JAMA, the US Preventive Services Task Force said that there is insufficient evidence to determine the benefit-harm balance of skin cancer screening in asymptomatic people.
“So there has been a lot of recent talk and a need to identify which subset groups of patients might be higher risk for skin cancer and might benefit from more screening,” Dr. Mansh said in an interview. “Understanding more about the high-risk demographic and clinical features that predispose someone to skin cancer helps identify these high-risk populations that could be used to develop better screening guidelines.”
Identifying groups at a higher risk for skin cancer also allows experts to design more targeted counseling or public health interventions focused on these groups, Dr. Mansh added. Absent screening guidelines, experts emphasize changing modifiable risk factors such as UVR exposure, smoking, and alcohol use. “And we know that the message that might change behaviors in a cisgender heterosexual man might be different than in a gay White male or a Hispanic bisexual male.”
A 2017 review showed that interventions to reduce behaviors involving UVR exposure, such as indoor tanning, among young cisgender women focused largely on aging and appearance-based concerns. A 2019 study showed that messages focused on avoiding skin cancer may help motivate SM men to reduce tanning behaviors.
Furthermore, said Dr. Mansh, all electronic health record products available in the United States must provide data fields for sexual orientation. “I don’t believe many dermatologists, depending on the setting, collect that information routinely. Integrating sexual orientation and/or gender identity data into patient intake forms so that it can be integrated into the electronic health record is probably very helpful, not only for your clinical practice but also for future research studies.”
Asked to comment on the results, Rebecca I. Hartman, MD, MPH, who was not involved with the study, said that its impact on clinical practice will be challenging to ascertain. She is chief of dermatology with the VA Boston Healthcare System, assistant professor of dermatology at Harvard Medical School, and director of melanoma epidemiology at Brigham and Women’s Hospital, all in Boston, Massachusetts.
“The study found significant adjusted odds ratios,” Dr. Hartman explained, “but for some of the different populations, the overall lifetime rate of skin cancer is still quite low.” For example, 1.0% for SM non-Hispanic Black men or a difference of 2.1% vs 1.8% in SM Hispanic women. “Thus, I am not sure specific screening recommendations are warranted, although some populations, such as Hispanic sexual minority males, seemed to have a much higher risk (3.8-fold on adjusted analysis) that warrants further investigation.”
For now, she advised assessing patients’ risks for skin cancer based on well-established risk factors such as sun exposure/indoor tanning, skin phototype, immunosuppression, and age.
Dr. Mansh reported no relevant conflicts or funding sources for the study. Dr. Hartman reported no relevant conflicts.
A version of this article appeared on Medscape.com.
FROM JAMA DERMATOLOGY
Greater Transparency of Oncologists’ Pharma Relationships Needed
The findings reflect limited awareness in low-income countries about what scenarios constitute a conflict of interest, first author, Khalid El Bairi, MD, said during an interview. “There is a lack of training in ethics and integrity in medical schools [in countries in Africa], so people are not informed about conflicts of interest,” continued Dr. El Bairi, who presented the new research at the annual meeting of the American Society of Clinical Oncology. “There is also a lack of policies in universities and hospitals to guide clinicians about conflict of interest reporting.”
Overall, 58.5% of survey participants categorized honoraria as a conflict of interest that required disclosure, while 50% said the same of gifts from pharmaceutical representatives, and 44.5% identified travel grants for attending conferences as conflicts of interests. The report was published in JCO Global Oncology. Less often considered conflicts of interest were personal and institutional research funding, trips to conferences, consulting or advisory roles, food and beverages, expert testimony, and sample drugs provided by the pharmaceutical industry.
Just 24% of participants indicated that all of the listed items were deemed conflicts of interest. The survey — called Oncology Transparency Under Scrutiny and Tracking, or ONCOTRUST-1 — considered the perceptions of 200 oncologists, about 70% of whom practice in low- and middle-income countries.
What’s more, 37.5% of respondents identified fear of losing financial support as a reason not to report a conflict of interest. Still, 75% indicated that industry-sponsored speaking does not affect treatment decisions, and 60% said conflicts of interest do not impair objective appraisal of clinical trials.
Dr. El Bairi, a research associate in the department of medical oncology at Mohammed VI University Hospital, Oujda, Morocco, and his colleagues undertook the study in part because of an editorial published in The Lancet Oncology last year. First author Fidel Rubagumya, MD, a consultant oncologist and director of research at Rwanda Military Hospital, Kigali, and colleagues called for more research on the ties between oncologists and industry in Africa. The ONCOTRUST-1 findings set the stage for a planned follow-up study, which aims to compare views surrounding conflicts of interests between oncologists in different economic settings.
Open Payments Houses US Physicians’ Conflicts of Interest
To be sure, many authors of research published in major US journals are based outside of the United States. According to JAMA Network Open, 69% of submissions to the journal are from international authors. However, Dr. El Bairi also raised other potential signs of industry influence that he said need global discussion, such as the role of pharmaceutical companies in presentations of clinical trial findings at large cancer societies’ conferences, a shift toward progression-free survival as the endpoint in clinical cancer trials, and the rise of third-party writing assistance.
“There are two sides of the story,” Dr. El Bairi said. “The good side is that unfortunately, sometimes [industry money is] the only way for African oncologists to go abroad for training, to conferences for their continuous medical education. The bad is now we may harm patients, we might harm science by having conflicts of interest not reported.”
Unlike other countries, the United States has plentiful data on the scale of physicians’ financial conflicts of interest in the form of the Open Payments platform. Championed by Sen. Chuck Grassley (R-Iowa), the federal repository of payments to doctors and teaching hospitals by drug and medical device companies was established as part of the Affordable Care Act (ACA).
The health care reform law, which passed in 2010, requires pharmaceutical companies and medical device makers to report this information.
From 2013 to 2021, the pharmaceutical and medical device industry paid physicians $12.1 billion, according to a research letter published in JAMA in March of 2024 that reviewed Open Payments data.
Ranked by specialty, hematologists and oncologists received the fourth-largest amount of money in aggregate, the study shows. Their total of $825.8 million trailed only physicians in orthopedics ($1.36 billion), neurology and psychiatry ($1.32 billion) and cardiology ($1.29 billion). What’s more, this specialty had the biggest share of physicians taking industry money, with 74.2% of hematologists and oncologists receiving payments.
The payments from industry include fees for consulting services and speaking, as well as food and beverages, travel and lodging, education, gifts, grants, and honoraria.
Joseph S. Ross, MD, MHS, one of the JAMA study’s coauthors, said in an interview that the continued prevalence of such funding runs counter to the expectation behind the measure, which was that transparency would lead to physicians’ becoming less likely to accept a payment.
“We as a profession need to take a cold hard look in the mirror,” he said, referring to physicians in general.
Dr. Ross, professor of medicine at Yale University School of Medicine, New Haven, Connecticut, said he hopes that the profession will self-police, and that patients will make a bigger deal of the issue. Still, he acknowledged that “the vast majority” of patient advocacy groups, too, are funded by the pharmaceutical industry.
Exposing Industry Payments May Have Perverse Effect
A growing body of research explores the effect that physicians’ financial relationships with pharmaceutical companies can have on their prescribing practices. Indeed, oncologists taking industry payments seem to be more likely to prescribe nonrecommended and low-value drugs in some clinical settings, according to a study published in The BMJ last year.
That study’s first author, Aaron P. Mitchell, MD, a medical oncologist and assistant attending physician at Memorial Sloan Kettering Cancer Center, New York City, suggested in an interview that exposing industry payments to the sunlight may have had a perverse effect on physicians.
“There’s this idea of having license to do something,” Dr. Mitchell said, speaking broadly about human psychology rather than drawing on empirical data. “You might feel a little less bad about then prescribing more of that company’s drug, because the disclosure has already been done.”
The influence of pharmaceutical industry money on oncologists goes beyond what’s prescribed to which treatments get studied, approved, and recommended by guidelines, Dr. Mitchell said. He was also first author of a 2016 paper published in JAMA Oncology that found 86% of authors of the National Comprehensive Cancer Network guidelines had at least one conflict of interest reported on Open Systems in 2014.
Meanwhile, the fact that physicians’ payments from industry are a matter of public record on Open Systems has not guaranteed that doctors will disclose their conflicts of interest in other forums. A study published in JAMA earlier this year, for which Dr. Mitchell served as first author, found that almost one in three physicians endorsing drugs and devices on the social media platform X failed to disclose that the manufacturer paid them.
The lack of disclosure seems to extend beyond social media. A 2018 study published in JAMA Oncology found that 32% of oncologist authors of clinical drug trials for drugs approved over a 20-month period from 2016 to 2017 did not fully disclose payments from the trial sponsor when checked against the Open Payments database.
A lion’s share of industry payments within oncology appears to be going to a small group of high-profile physicians, suggested a 2022 study published in JCO Oncology Practice. It found that just 1% of all US oncologists accounted for 37% of industry payments, with each receiving more than $100,000 a year.
Experts: Professional Societies Should Further Limit Industry Payments
While partnerships between drug companies and physicians are necessary and have often been positive, more than disclosure is needed to minimize the risk of patient harm, according to an editorial published in March in JCO Oncology Practice. In it, Nina Niu Sanford, MD, a radiation oncologist UT Southwestern Medical Center, Dallas, and Bishal Gyawali, MD, PhD, a medical oncologist at Queen’s University, Kingston, Ontario, Canada, argue that following a specific blueprint could help mitigate financial conflicts of interest.
For starters, Dr. Sanford and Dr. Gyawali contend in the editorial that the maximum general payment NCCN members are allowed to receive from industry should be $0, compared with a current bar of $20,000 from a single entity or $50,000 from all external entities combined. They also urge professional societies to follow the current policy of the American Society of Clinical Oncology and ban members serving in their leadership from receiving any general payments from the industry.
The authors further suggest that investigators of clinical trials should be barred from holding stock for the drug or product while it is under study and that editorialists should not have conflicts of interest with the company whose drug or product they are discussing.
Pharmaceutical money can harm patients in ways that are not always obvious, Dr. Gyawali said in an interview.
“It can dominate the conversation by removing critical viewpoints from these top people about certain drugs,” he said. “It’s not always about saying good things about the drug.”
For instance, he suggested, a doctor receiving payments from Pfizer might openly criticize perceived flaws in drugs from other companies but refrain from weighing in negatively on a Pfizer drug.
From 2016 to 2018, industry made general payments to more than 52,000 physicians for 137 unique cancer drugs, according to a separate 2021 study published in the Journal of Cancer Policy, for which Dr. Gyawali served as one of the coauthors.
The results suggest that pharmaceutical money affects the entire cancer system, not relatively few oncology leaders. The amounts and dollar values grew each year covered by the study, to nearly 466,000 payments totaling $98.5 million in 2018.
Adriane Fugh-Berman, MD, professor of pharmacology and physiology at Georgetown University, Washington, DC, and director of PharmedOut, a Georgetown-based project that advances evidence-based prescribing and educates healthcare professionals about pharmaceutical marketing practices, has called for a ban on industry gifts to physicians.
When a publication asks physicians to disclose relevant conflicts of interest, physicians may choose not to disclose, because they don’t feel that their conflicts are relevant, Dr. Fugh-Berman said. Drug and device makers have also grown sophisticated about how they work with physicians, she suggested. “It’s illegal to market a drug before it comes on the market, but it’s not illegal to market the disease,” said Dr. Fugh-Berman, noting that drugmakers often work on long timelines.
“The doctor is going around saying we don’t have good therapies. They’re not pushing a drug. And so they feel totally fine about it.”
Anecdotally, Dr. Fugh-Berman noted that, if anything, speaking fees and similar payments only improve doctors’ reputations. She said that’s especially true if the physicians are paid by multiple companies, on the supposed theory that their conflicts of interest cancel each other out.
“I’m not defending this,” added Dr. Fugh-Berman, observing that, at the end of the day, such conflicts may go against the interests of patients.
“Sometimes the best drugs are older, generic, cheap drugs, and if oncologists or other specialists are only choosing among the most promoted drugs, they’re not necessarily choosing the best drugs.”
Beyond any prestige, doctors have other possible nonfinancial incentives for receiving industry payments. “It’s the relationships,” Dr. Fugh-Berman said. “Companies are very good at offering friendship.”
Dr. El Bairi reported NCODA leadership and honoraria along with expert testimony through techspert.io. Dr. Ross reported that he is a deputy editor of JAMA but was not involved in decisions regarding acceptance of or the review of the manuscript he authored and discussed in this article. Dr. Ross also reported receiving grants from the Food and Drug Administration, Johnson & Johnson, the Medical Device Innovation Consortium, the Agency for Healthcare Research and Quality, and the National Heart, Lung, and Blood Institute. He was an expert witness in a qui tam suit alleging violations of the False Claims Act and Anti-Kickback Statute against Biogen that was settled in 2022. Dr. Mitchell reported no relevant financial relationships. Dr. Gyawali reported a consulting or advisory role with Vivio Health. Dr. Fugh-Berman reported being an expert witness for plaintiffs in complaints about drug and device marketing practices.
The findings reflect limited awareness in low-income countries about what scenarios constitute a conflict of interest, first author, Khalid El Bairi, MD, said during an interview. “There is a lack of training in ethics and integrity in medical schools [in countries in Africa], so people are not informed about conflicts of interest,” continued Dr. El Bairi, who presented the new research at the annual meeting of the American Society of Clinical Oncology. “There is also a lack of policies in universities and hospitals to guide clinicians about conflict of interest reporting.”
Overall, 58.5% of survey participants categorized honoraria as a conflict of interest that required disclosure, while 50% said the same of gifts from pharmaceutical representatives, and 44.5% identified travel grants for attending conferences as conflicts of interests. The report was published in JCO Global Oncology. Less often considered conflicts of interest were personal and institutional research funding, trips to conferences, consulting or advisory roles, food and beverages, expert testimony, and sample drugs provided by the pharmaceutical industry.
Just 24% of participants indicated that all of the listed items were deemed conflicts of interest. The survey — called Oncology Transparency Under Scrutiny and Tracking, or ONCOTRUST-1 — considered the perceptions of 200 oncologists, about 70% of whom practice in low- and middle-income countries.
What’s more, 37.5% of respondents identified fear of losing financial support as a reason not to report a conflict of interest. Still, 75% indicated that industry-sponsored speaking does not affect treatment decisions, and 60% said conflicts of interest do not impair objective appraisal of clinical trials.
Dr. El Bairi, a research associate in the department of medical oncology at Mohammed VI University Hospital, Oujda, Morocco, and his colleagues undertook the study in part because of an editorial published in The Lancet Oncology last year. First author Fidel Rubagumya, MD, a consultant oncologist and director of research at Rwanda Military Hospital, Kigali, and colleagues called for more research on the ties between oncologists and industry in Africa. The ONCOTRUST-1 findings set the stage for a planned follow-up study, which aims to compare views surrounding conflicts of interests between oncologists in different economic settings.
Open Payments Houses US Physicians’ Conflicts of Interest
To be sure, many authors of research published in major US journals are based outside of the United States. According to JAMA Network Open, 69% of submissions to the journal are from international authors. However, Dr. El Bairi also raised other potential signs of industry influence that he said need global discussion, such as the role of pharmaceutical companies in presentations of clinical trial findings at large cancer societies’ conferences, a shift toward progression-free survival as the endpoint in clinical cancer trials, and the rise of third-party writing assistance.
“There are two sides of the story,” Dr. El Bairi said. “The good side is that unfortunately, sometimes [industry money is] the only way for African oncologists to go abroad for training, to conferences for their continuous medical education. The bad is now we may harm patients, we might harm science by having conflicts of interest not reported.”
Unlike other countries, the United States has plentiful data on the scale of physicians’ financial conflicts of interest in the form of the Open Payments platform. Championed by Sen. Chuck Grassley (R-Iowa), the federal repository of payments to doctors and teaching hospitals by drug and medical device companies was established as part of the Affordable Care Act (ACA).
The health care reform law, which passed in 2010, requires pharmaceutical companies and medical device makers to report this information.
From 2013 to 2021, the pharmaceutical and medical device industry paid physicians $12.1 billion, according to a research letter published in JAMA in March of 2024 that reviewed Open Payments data.
Ranked by specialty, hematologists and oncologists received the fourth-largest amount of money in aggregate, the study shows. Their total of $825.8 million trailed only physicians in orthopedics ($1.36 billion), neurology and psychiatry ($1.32 billion) and cardiology ($1.29 billion). What’s more, this specialty had the biggest share of physicians taking industry money, with 74.2% of hematologists and oncologists receiving payments.
The payments from industry include fees for consulting services and speaking, as well as food and beverages, travel and lodging, education, gifts, grants, and honoraria.
Joseph S. Ross, MD, MHS, one of the JAMA study’s coauthors, said in an interview that the continued prevalence of such funding runs counter to the expectation behind the measure, which was that transparency would lead to physicians’ becoming less likely to accept a payment.
“We as a profession need to take a cold hard look in the mirror,” he said, referring to physicians in general.
Dr. Ross, professor of medicine at Yale University School of Medicine, New Haven, Connecticut, said he hopes that the profession will self-police, and that patients will make a bigger deal of the issue. Still, he acknowledged that “the vast majority” of patient advocacy groups, too, are funded by the pharmaceutical industry.
Exposing Industry Payments May Have Perverse Effect
A growing body of research explores the effect that physicians’ financial relationships with pharmaceutical companies can have on their prescribing practices. Indeed, oncologists taking industry payments seem to be more likely to prescribe nonrecommended and low-value drugs in some clinical settings, according to a study published in The BMJ last year.
That study’s first author, Aaron P. Mitchell, MD, a medical oncologist and assistant attending physician at Memorial Sloan Kettering Cancer Center, New York City, suggested in an interview that exposing industry payments to the sunlight may have had a perverse effect on physicians.
“There’s this idea of having license to do something,” Dr. Mitchell said, speaking broadly about human psychology rather than drawing on empirical data. “You might feel a little less bad about then prescribing more of that company’s drug, because the disclosure has already been done.”
The influence of pharmaceutical industry money on oncologists goes beyond what’s prescribed to which treatments get studied, approved, and recommended by guidelines, Dr. Mitchell said. He was also first author of a 2016 paper published in JAMA Oncology that found 86% of authors of the National Comprehensive Cancer Network guidelines had at least one conflict of interest reported on Open Systems in 2014.
Meanwhile, the fact that physicians’ payments from industry are a matter of public record on Open Systems has not guaranteed that doctors will disclose their conflicts of interest in other forums. A study published in JAMA earlier this year, for which Dr. Mitchell served as first author, found that almost one in three physicians endorsing drugs and devices on the social media platform X failed to disclose that the manufacturer paid them.
The lack of disclosure seems to extend beyond social media. A 2018 study published in JAMA Oncology found that 32% of oncologist authors of clinical drug trials for drugs approved over a 20-month period from 2016 to 2017 did not fully disclose payments from the trial sponsor when checked against the Open Payments database.
A lion’s share of industry payments within oncology appears to be going to a small group of high-profile physicians, suggested a 2022 study published in JCO Oncology Practice. It found that just 1% of all US oncologists accounted for 37% of industry payments, with each receiving more than $100,000 a year.
Experts: Professional Societies Should Further Limit Industry Payments
While partnerships between drug companies and physicians are necessary and have often been positive, more than disclosure is needed to minimize the risk of patient harm, according to an editorial published in March in JCO Oncology Practice. In it, Nina Niu Sanford, MD, a radiation oncologist UT Southwestern Medical Center, Dallas, and Bishal Gyawali, MD, PhD, a medical oncologist at Queen’s University, Kingston, Ontario, Canada, argue that following a specific blueprint could help mitigate financial conflicts of interest.
For starters, Dr. Sanford and Dr. Gyawali contend in the editorial that the maximum general payment NCCN members are allowed to receive from industry should be $0, compared with a current bar of $20,000 from a single entity or $50,000 from all external entities combined. They also urge professional societies to follow the current policy of the American Society of Clinical Oncology and ban members serving in their leadership from receiving any general payments from the industry.
The authors further suggest that investigators of clinical trials should be barred from holding stock for the drug or product while it is under study and that editorialists should not have conflicts of interest with the company whose drug or product they are discussing.
Pharmaceutical money can harm patients in ways that are not always obvious, Dr. Gyawali said in an interview.
“It can dominate the conversation by removing critical viewpoints from these top people about certain drugs,” he said. “It’s not always about saying good things about the drug.”
For instance, he suggested, a doctor receiving payments from Pfizer might openly criticize perceived flaws in drugs from other companies but refrain from weighing in negatively on a Pfizer drug.
From 2016 to 2018, industry made general payments to more than 52,000 physicians for 137 unique cancer drugs, according to a separate 2021 study published in the Journal of Cancer Policy, for which Dr. Gyawali served as one of the coauthors.
The results suggest that pharmaceutical money affects the entire cancer system, not relatively few oncology leaders. The amounts and dollar values grew each year covered by the study, to nearly 466,000 payments totaling $98.5 million in 2018.
Adriane Fugh-Berman, MD, professor of pharmacology and physiology at Georgetown University, Washington, DC, and director of PharmedOut, a Georgetown-based project that advances evidence-based prescribing and educates healthcare professionals about pharmaceutical marketing practices, has called for a ban on industry gifts to physicians.
When a publication asks physicians to disclose relevant conflicts of interest, physicians may choose not to disclose, because they don’t feel that their conflicts are relevant, Dr. Fugh-Berman said. Drug and device makers have also grown sophisticated about how they work with physicians, she suggested. “It’s illegal to market a drug before it comes on the market, but it’s not illegal to market the disease,” said Dr. Fugh-Berman, noting that drugmakers often work on long timelines.
“The doctor is going around saying we don’t have good therapies. They’re not pushing a drug. And so they feel totally fine about it.”
Anecdotally, Dr. Fugh-Berman noted that, if anything, speaking fees and similar payments only improve doctors’ reputations. She said that’s especially true if the physicians are paid by multiple companies, on the supposed theory that their conflicts of interest cancel each other out.
“I’m not defending this,” added Dr. Fugh-Berman, observing that, at the end of the day, such conflicts may go against the interests of patients.
“Sometimes the best drugs are older, generic, cheap drugs, and if oncologists or other specialists are only choosing among the most promoted drugs, they’re not necessarily choosing the best drugs.”
Beyond any prestige, doctors have other possible nonfinancial incentives for receiving industry payments. “It’s the relationships,” Dr. Fugh-Berman said. “Companies are very good at offering friendship.”
Dr. El Bairi reported NCODA leadership and honoraria along with expert testimony through techspert.io. Dr. Ross reported that he is a deputy editor of JAMA but was not involved in decisions regarding acceptance of or the review of the manuscript he authored and discussed in this article. Dr. Ross also reported receiving grants from the Food and Drug Administration, Johnson & Johnson, the Medical Device Innovation Consortium, the Agency for Healthcare Research and Quality, and the National Heart, Lung, and Blood Institute. He was an expert witness in a qui tam suit alleging violations of the False Claims Act and Anti-Kickback Statute against Biogen that was settled in 2022. Dr. Mitchell reported no relevant financial relationships. Dr. Gyawali reported a consulting or advisory role with Vivio Health. Dr. Fugh-Berman reported being an expert witness for plaintiffs in complaints about drug and device marketing practices.
The findings reflect limited awareness in low-income countries about what scenarios constitute a conflict of interest, first author, Khalid El Bairi, MD, said during an interview. “There is a lack of training in ethics and integrity in medical schools [in countries in Africa], so people are not informed about conflicts of interest,” continued Dr. El Bairi, who presented the new research at the annual meeting of the American Society of Clinical Oncology. “There is also a lack of policies in universities and hospitals to guide clinicians about conflict of interest reporting.”
Overall, 58.5% of survey participants categorized honoraria as a conflict of interest that required disclosure, while 50% said the same of gifts from pharmaceutical representatives, and 44.5% identified travel grants for attending conferences as conflicts of interests. The report was published in JCO Global Oncology. Less often considered conflicts of interest were personal and institutional research funding, trips to conferences, consulting or advisory roles, food and beverages, expert testimony, and sample drugs provided by the pharmaceutical industry.
Just 24% of participants indicated that all of the listed items were deemed conflicts of interest. The survey — called Oncology Transparency Under Scrutiny and Tracking, or ONCOTRUST-1 — considered the perceptions of 200 oncologists, about 70% of whom practice in low- and middle-income countries.
What’s more, 37.5% of respondents identified fear of losing financial support as a reason not to report a conflict of interest. Still, 75% indicated that industry-sponsored speaking does not affect treatment decisions, and 60% said conflicts of interest do not impair objective appraisal of clinical trials.
Dr. El Bairi, a research associate in the department of medical oncology at Mohammed VI University Hospital, Oujda, Morocco, and his colleagues undertook the study in part because of an editorial published in The Lancet Oncology last year. First author Fidel Rubagumya, MD, a consultant oncologist and director of research at Rwanda Military Hospital, Kigali, and colleagues called for more research on the ties between oncologists and industry in Africa. The ONCOTRUST-1 findings set the stage for a planned follow-up study, which aims to compare views surrounding conflicts of interests between oncologists in different economic settings.
Open Payments Houses US Physicians’ Conflicts of Interest
To be sure, many authors of research published in major US journals are based outside of the United States. According to JAMA Network Open, 69% of submissions to the journal are from international authors. However, Dr. El Bairi also raised other potential signs of industry influence that he said need global discussion, such as the role of pharmaceutical companies in presentations of clinical trial findings at large cancer societies’ conferences, a shift toward progression-free survival as the endpoint in clinical cancer trials, and the rise of third-party writing assistance.
“There are two sides of the story,” Dr. El Bairi said. “The good side is that unfortunately, sometimes [industry money is] the only way for African oncologists to go abroad for training, to conferences for their continuous medical education. The bad is now we may harm patients, we might harm science by having conflicts of interest not reported.”
Unlike other countries, the United States has plentiful data on the scale of physicians’ financial conflicts of interest in the form of the Open Payments platform. Championed by Sen. Chuck Grassley (R-Iowa), the federal repository of payments to doctors and teaching hospitals by drug and medical device companies was established as part of the Affordable Care Act (ACA).
The health care reform law, which passed in 2010, requires pharmaceutical companies and medical device makers to report this information.
From 2013 to 2021, the pharmaceutical and medical device industry paid physicians $12.1 billion, according to a research letter published in JAMA in March of 2024 that reviewed Open Payments data.
Ranked by specialty, hematologists and oncologists received the fourth-largest amount of money in aggregate, the study shows. Their total of $825.8 million trailed only physicians in orthopedics ($1.36 billion), neurology and psychiatry ($1.32 billion) and cardiology ($1.29 billion). What’s more, this specialty had the biggest share of physicians taking industry money, with 74.2% of hematologists and oncologists receiving payments.
The payments from industry include fees for consulting services and speaking, as well as food and beverages, travel and lodging, education, gifts, grants, and honoraria.
Joseph S. Ross, MD, MHS, one of the JAMA study’s coauthors, said in an interview that the continued prevalence of such funding runs counter to the expectation behind the measure, which was that transparency would lead to physicians’ becoming less likely to accept a payment.
“We as a profession need to take a cold hard look in the mirror,” he said, referring to physicians in general.
Dr. Ross, professor of medicine at Yale University School of Medicine, New Haven, Connecticut, said he hopes that the profession will self-police, and that patients will make a bigger deal of the issue. Still, he acknowledged that “the vast majority” of patient advocacy groups, too, are funded by the pharmaceutical industry.
Exposing Industry Payments May Have Perverse Effect
A growing body of research explores the effect that physicians’ financial relationships with pharmaceutical companies can have on their prescribing practices. Indeed, oncologists taking industry payments seem to be more likely to prescribe nonrecommended and low-value drugs in some clinical settings, according to a study published in The BMJ last year.
That study’s first author, Aaron P. Mitchell, MD, a medical oncologist and assistant attending physician at Memorial Sloan Kettering Cancer Center, New York City, suggested in an interview that exposing industry payments to the sunlight may have had a perverse effect on physicians.
“There’s this idea of having license to do something,” Dr. Mitchell said, speaking broadly about human psychology rather than drawing on empirical data. “You might feel a little less bad about then prescribing more of that company’s drug, because the disclosure has already been done.”
The influence of pharmaceutical industry money on oncologists goes beyond what’s prescribed to which treatments get studied, approved, and recommended by guidelines, Dr. Mitchell said. He was also first author of a 2016 paper published in JAMA Oncology that found 86% of authors of the National Comprehensive Cancer Network guidelines had at least one conflict of interest reported on Open Systems in 2014.
Meanwhile, the fact that physicians’ payments from industry are a matter of public record on Open Systems has not guaranteed that doctors will disclose their conflicts of interest in other forums. A study published in JAMA earlier this year, for which Dr. Mitchell served as first author, found that almost one in three physicians endorsing drugs and devices on the social media platform X failed to disclose that the manufacturer paid them.
The lack of disclosure seems to extend beyond social media. A 2018 study published in JAMA Oncology found that 32% of oncologist authors of clinical drug trials for drugs approved over a 20-month period from 2016 to 2017 did not fully disclose payments from the trial sponsor when checked against the Open Payments database.
A lion’s share of industry payments within oncology appears to be going to a small group of high-profile physicians, suggested a 2022 study published in JCO Oncology Practice. It found that just 1% of all US oncologists accounted for 37% of industry payments, with each receiving more than $100,000 a year.
Experts: Professional Societies Should Further Limit Industry Payments
While partnerships between drug companies and physicians are necessary and have often been positive, more than disclosure is needed to minimize the risk of patient harm, according to an editorial published in March in JCO Oncology Practice. In it, Nina Niu Sanford, MD, a radiation oncologist UT Southwestern Medical Center, Dallas, and Bishal Gyawali, MD, PhD, a medical oncologist at Queen’s University, Kingston, Ontario, Canada, argue that following a specific blueprint could help mitigate financial conflicts of interest.
For starters, Dr. Sanford and Dr. Gyawali contend in the editorial that the maximum general payment NCCN members are allowed to receive from industry should be $0, compared with a current bar of $20,000 from a single entity or $50,000 from all external entities combined. They also urge professional societies to follow the current policy of the American Society of Clinical Oncology and ban members serving in their leadership from receiving any general payments from the industry.
The authors further suggest that investigators of clinical trials should be barred from holding stock for the drug or product while it is under study and that editorialists should not have conflicts of interest with the company whose drug or product they are discussing.
Pharmaceutical money can harm patients in ways that are not always obvious, Dr. Gyawali said in an interview.
“It can dominate the conversation by removing critical viewpoints from these top people about certain drugs,” he said. “It’s not always about saying good things about the drug.”
For instance, he suggested, a doctor receiving payments from Pfizer might openly criticize perceived flaws in drugs from other companies but refrain from weighing in negatively on a Pfizer drug.
From 2016 to 2018, industry made general payments to more than 52,000 physicians for 137 unique cancer drugs, according to a separate 2021 study published in the Journal of Cancer Policy, for which Dr. Gyawali served as one of the coauthors.
The results suggest that pharmaceutical money affects the entire cancer system, not relatively few oncology leaders. The amounts and dollar values grew each year covered by the study, to nearly 466,000 payments totaling $98.5 million in 2018.
Adriane Fugh-Berman, MD, professor of pharmacology and physiology at Georgetown University, Washington, DC, and director of PharmedOut, a Georgetown-based project that advances evidence-based prescribing and educates healthcare professionals about pharmaceutical marketing practices, has called for a ban on industry gifts to physicians.
When a publication asks physicians to disclose relevant conflicts of interest, physicians may choose not to disclose, because they don’t feel that their conflicts are relevant, Dr. Fugh-Berman said. Drug and device makers have also grown sophisticated about how they work with physicians, she suggested. “It’s illegal to market a drug before it comes on the market, but it’s not illegal to market the disease,” said Dr. Fugh-Berman, noting that drugmakers often work on long timelines.
“The doctor is going around saying we don’t have good therapies. They’re not pushing a drug. And so they feel totally fine about it.”
Anecdotally, Dr. Fugh-Berman noted that, if anything, speaking fees and similar payments only improve doctors’ reputations. She said that’s especially true if the physicians are paid by multiple companies, on the supposed theory that their conflicts of interest cancel each other out.
“I’m not defending this,” added Dr. Fugh-Berman, observing that, at the end of the day, such conflicts may go against the interests of patients.
“Sometimes the best drugs are older, generic, cheap drugs, and if oncologists or other specialists are only choosing among the most promoted drugs, they’re not necessarily choosing the best drugs.”
Beyond any prestige, doctors have other possible nonfinancial incentives for receiving industry payments. “It’s the relationships,” Dr. Fugh-Berman said. “Companies are very good at offering friendship.”
Dr. El Bairi reported NCODA leadership and honoraria along with expert testimony through techspert.io. Dr. Ross reported that he is a deputy editor of JAMA but was not involved in decisions regarding acceptance of or the review of the manuscript he authored and discussed in this article. Dr. Ross also reported receiving grants from the Food and Drug Administration, Johnson & Johnson, the Medical Device Innovation Consortium, the Agency for Healthcare Research and Quality, and the National Heart, Lung, and Blood Institute. He was an expert witness in a qui tam suit alleging violations of the False Claims Act and Anti-Kickback Statute against Biogen that was settled in 2022. Dr. Mitchell reported no relevant financial relationships. Dr. Gyawali reported a consulting or advisory role with Vivio Health. Dr. Fugh-Berman reported being an expert witness for plaintiffs in complaints about drug and device marketing practices.
FROM ASCO 2024
Barriers to Mohs Micrographic Surgery in Japanese Patients With Basal Cell Carcinoma
Margin-controlled surgery for squamous cell carcinoma (SCC) on the lower lip was first performed by Dr. Frederic Mohs on June 30, 1936. Since then, thousands of skin cancer surgeons have refined and adopted the technique. Due to the high cure rate and sparing of normal tissue, Mohs micrographic surgery (MMS) has become the gold standard treatment for facial and special-site nonmelanoma skin cancer worldwide. Mohs micrographic surgery is performed on more than 876,000 tumors annually in the United States.1 Among 3.5 million Americans diagnosed with nonmelanoma skin cancer in 2006, one-quarter were treated with MMS.2 In Japan, basal cell carcinoma (BCC) is the most common skin malignancy, with an incidence of 3.34 cases per 100,000 individuals; SCC is the second most common, with an incidence of 2.5 cases per 100,000 individuals.3
The essential element that makes MMS unique is the careful microscopic examination of the entire margin of the removed specimen. Tissue processing is done with careful en face orientation to ensure that circumferential and deep margins are entirely visible. The surgeon interprets the slides and proceeds to remove the additional tumor as necessary. Because the same physician performs both the surgery and the pathologic assessment throughout the procedure, a precise correlation between the microscopic and surgical findings can be made. The surgeon can begin with smaller margins, removing minimal healthy tissue while removing all the cancer cells, which results in the smallest-possible skin defect and the best prognosis for the malignancy (Figure 1).
At the only facility in Japan offering MMS, the lead author (S.S.) has treated 52 lesions with MMS in 46 patients (2020-2022). Of these patients, 40 were White, 5 were Japanese, and 1 was of African descent. In this case series, we present 5 Japanese patients who had BCC treated with MMS.
Case Series
Patient 1—A 50-year-old Japanese woman presented to dermatology with a brown papule on the nasal tip of 1.25 year’s duration (Figure 2). A biopsy revealed infiltrative BCC (Figure 3), and the patient was referred to the dermatology department at a nearby university hospital. Because the BCC was an aggressive variant, wide local excision (WLE) with subsequent flap reconstruction was recommended as well as radiation therapy. The patient learned about MMS through an internet search and refused both options, seeking MMS treatment at our clinic. Although Japanese health insurance does not cover MMS, the patient had supplemental private insurance that did cover the cost. She provided consent to undergo the procedure. Physical examination revealed a 7.5×6-mm, brown-red macule with ill-defined borders on the tip of the nose. We used a 1.5-mm margin for the first stage of MMS (Figure 4A). The frozen section revealed that the tumor had been entirely excised in the first stage, leaving only a 10.5×9-mm skin defect that was reconstructed with a Dufourmentel flap (Figure 4B). No signs of recurrence were noted at 3.5-year follow-up, and the cosmetic outcome was favorable (Figure 4C). National Comprehensive Cancer Network guidelines recommend a margin greater than 4 mm for infiltrative BCCs4; therefore, our technique reduced the total defect by at least 4 mm in a cosmetically sensitive area. The patient also did not need radiation therapy, which reduced morbidity. She continues to be recurrence free at 3.5-year follow-up.
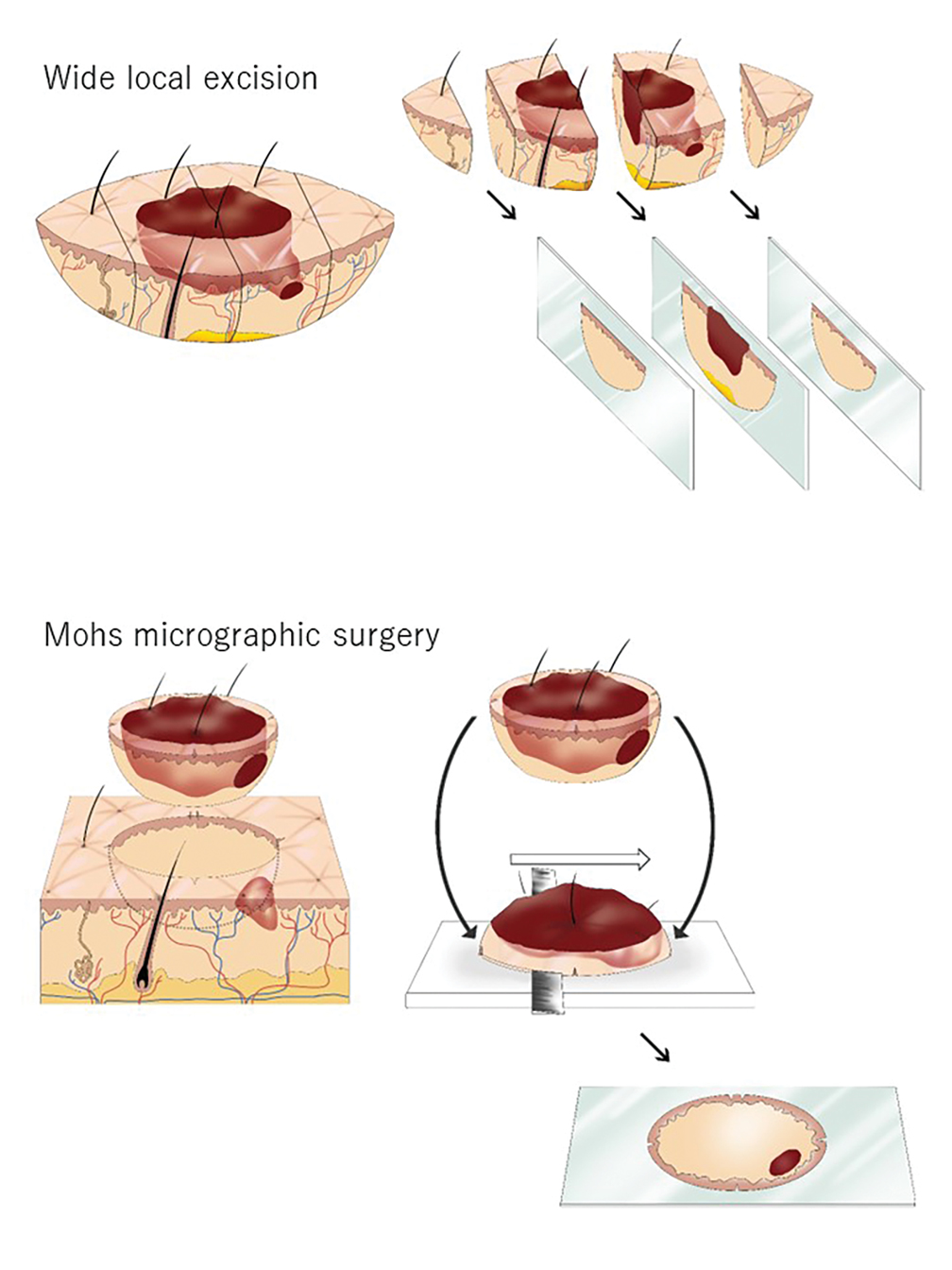
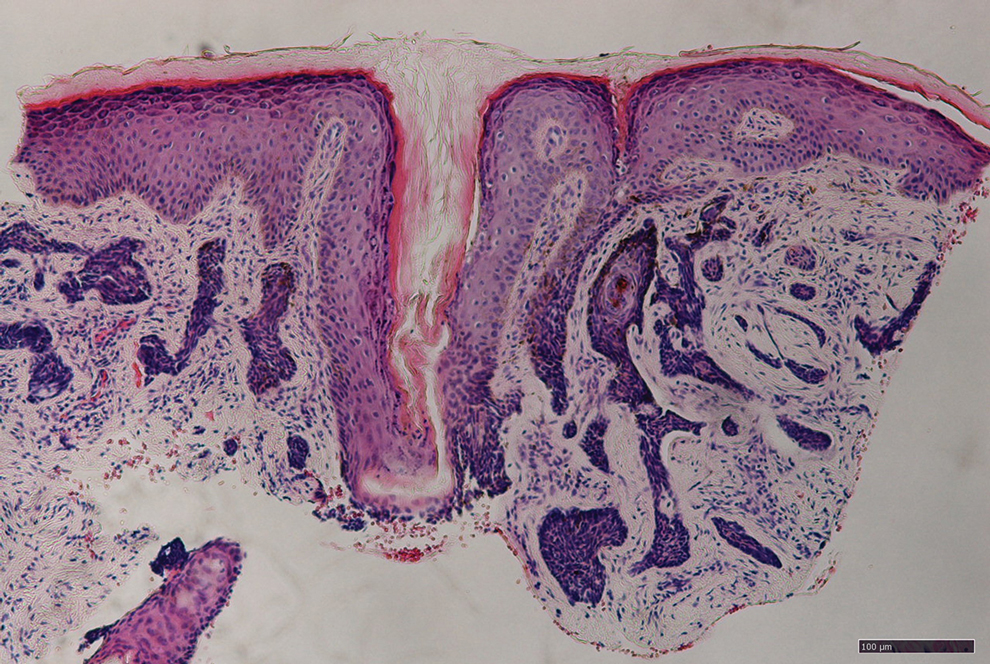
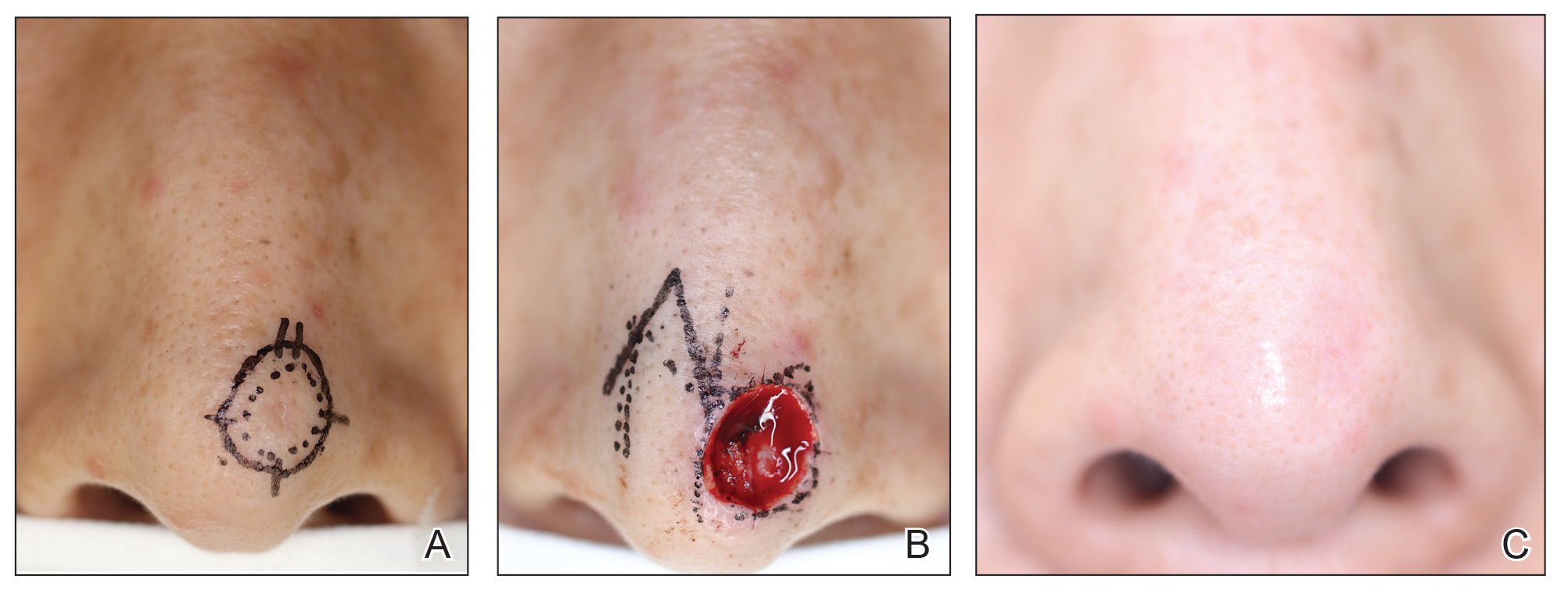
Patient 2—A 63-year-old Japanese man presented to dermatology with a brown macule on the right lower eyelid of 2 years’ duration. A biopsy of the lesion was positive for nodular BCC. After being advised to undergo WLE and extensive reconstruction with plastic surgery, the patient learned of MMS through an internet search and found our clinic. Physical examination revealed a 7×5-mm brown macule on the right lower eyelid. The patient had supplemental private insurance that covered the cost of MMS, and he provided consent for the procedure. A 1.5-mm margin was taken for the first stage, resulting in a 10×8-mm defect superficial to the orbicularis oculi muscle. The frozen section revealed residual tumor exposure in the dermis at the 9- to 10-o’clock position. A second-stage excision was performed to remove an additional 1.5 mm of skin at the 9- to 12-o’clock position with a thin layer of the orbicularis oculi muscle. The subsequent histologic examination revealed no residual BCC, and the final 13×9-mm skin defect was reconstructed with a rotation flap. There were no signs of recurrence at 2.5-year follow-up with an excellent cosmetic outcome.
Patient 3—A 73-year-old Japanese man presented to a local university dermatology clinic with a new papule on the nose. The dermatologist suggested WLE with 4-mm margins and reconstruction of the skin defect 2 weeks later by a plastic surgeon. The patient was not satisfied with the proposed surgical plan, which led him to learn about MMS on the internet; he subsequently found our clinic. Physical examination revealed a 4×3.5-mm brown papule on the tip of the nose. He understood the nature of MMS and chose to pay out-of-pocket because Japanese health insurance did not cover the procedure. We used a 2-mm margin for the first stage, which created a 7.5×7-mm skin defect. The frozen section pathology revealed no residual BCC at the cut surface. The skin defect was reconstructed with a Limberg rhombic flap. There were no signs of recurrence at 1.5-year follow-up with a favorable cosmetic outcome.
Patient 4—A 45-year-old man presented to a dermatology clinic with a papule on the right side of the nose of 1 year’s duration. A biopsy revealed the lesion was a nodular BCC. The dermatologist recommended WLE at a general hospital, but the patient refused after learning about MMS. He subsequently made an appointment with our clinic. Physical examination revealed a 7×4-mm white papule on the right side of the nose. The patient had private insurance that covered the cost of MMS. The first stage was performed with 1.5-mm margins and was clear of residual tumor. A Limberg rhombic flap from the adjacent cheek was used to repair the final 10×7-mm skin defect. There were no signs of recurrence at 1 year and 9 months’ follow-up with a favorable cosmetic outcome.
Patient 5—A 76-year-old Japanese woman presented to a university hospital near Tokyo with a black papule on the left cutaneous lip of 5 years’ duration. A biopsy revealed nodular BCC, and WLE with flap reconstruction was recommended. The patient’s son learned about MMS through internet research and referred her to our clinic. Physical examination revealed a 7×5-mm black papule on the left upper lip. The patient’s private insurance covered the cost of MMS, and she consented to the procedure. We used a 2-mm initial margin, and the immediate frozen section revealed no signs of BCC at the cut surface. The 11×9-mm skin defect was reconstructed with a Limberg rhombic flap. There were no signs of recurrence at 1.5-year follow-up with a favorable cosmetic outcome.
Comment
We presented 5 cases of MMS in Japanese patients with BCC. More than 7000 new cases of nonmelanoma skin cancer occur every year in Japan.3 Only 0.04% of these cases—the 5 cases presented here—were treated with MMS in Japan in 2020 and 2021, in contrast to 25% in the United States in 2006.2
MMS vs Other BCC Treatments—Mohs micrographic surgery offers 2 distinct advantages over conventional excision: an improved cure rate while achieving a smaller final defect size, generally leading to better cosmetic outcomes. Overall 5-year recurrence rates of BCC are 10% for conventional surgical excision vs 1% for MMS, while the recurrence rates for SCC are 8% and 3%, respectively.5 A study of well-demarcated BCCs smaller than 2 cm that were treated with MMS with 2-mm increments revealed that 95% of the cases were free of malignancy within a 4-mm margin of the normal-appearing skin surrounding the tumor.6 Several articles have reported a 95% cure rate or higher with conventional excision of localized BCC,7 but 4- to 5-mm excision margins are required, resulting in a greater skin defect and a lower cure rate compared to MMS.
Aggressive subtypes of BCC have a higher recurrence rate. Rowe et al8 reported the following 5-year recurrence rates: 5.6% for MMS, 17.4% for conventional surgical excision, 40.0% for curettage and electrodesiccation, and 9.8% for radiation therapy. Primary BCCs with high-risk histologic subtypes has a 10-year recurrence rate of 4.4% with MMS vs 12.2% with conventional excision.9 These findings reveal that MMS yields a better prognosis compared to traditional treatment methods for recurrent BCCs and BCCs of high-risk histologic subtypes.
The primary reason for the excellent cure rate seen in MMS is the ability to perform complete margin assessment. Peripheral and deep en face margin assessment (PDEMA) is crucial in achieving high cure rates with narrow margins. In WLE (Figure 1), vertical sectioning (also known as bread-loafing) does not achieve direct visualization of the entire surgical margin, as this technique only evaluates random sections and does not achieve PDEMA.10 The bread-loafing method is used almost exclusively in Japan and visualizes only 0.1% of the entire margin compared to 100% with MMS.11 Beyond the superior cure rate, the MMS technique often yields smaller final defects compared to WLE. All 5 of our patients achieved complete tumor removal while sparing more normal tissue compared to conventional WLE, which takes at least a 4-mm margin in all directions.
Barriers to Adopting MMS in Japan—There are many barriers to the broader adoption of MMS in Japan. A guideline of the Japanese Dermatological Association says, MMS “is complicated, requires special training for acquisition, and requires time and labor for implementation of a series of processes, and it has not gained wide acceptance in Japan because of these disadvantages.”3 There currently are no MMS training programs in Japan. We refute this statement from the Japanese Dermatological Association because, in our experience, only 1 surgeon plus a single histotechnician familiar with MMS is sufficient for a facility to offer the procedure (the lead author of this study [S.S.] acts as both the surgeon and the histotechnician). Another misconception among some physicians in Japan is that cancer on ethnically Japanese skin is uniquely suited to excision without microscopic verification of tumor clearance because the borders of the tumors are easily identified, which was based on good cure rates for the excision of well-demarcated pigmented BCCs in a Japanese cohort. This study of a Japanese cohort investigated the specimens with the conventional bread-loafing technique but not with the PDEMA.12
Eighty percent (4/5) of our patients presented with nodular BCC, and only 1 required a second stage. In comparison, we also treated 16 White patients with nodular BCC with MMS during the same period, and 31% (5/16) required more than 1 stage, with 1 patient requiring 3 stages. This cohort, however, is too small to demonstrate a statistically significant difference (S.S., unpublished data, 2020-2022).
A study in Singapore reported the postsurgical complication rate and 5-year recurrence rate for 481 tumors (92% BCC and 7.5% SCC). The median follow-up duration after MMS was 36 months, and the recurrence rate was 0.6%. The postsurgical complications included 11 (2.3%) cases with superficial tip necrosis of surgical flaps/grafts, 2 (0.4%) with mild wound dehiscence, 1 (0.2%) with minor surgical site bleeding, and 1 (0.2%) with minor wound infection.13 This study supports the notion that MMS is equally effective for Asian patients.
Awareness of MMS in Japan is lacking, and most Japanese dermatologists do not know about the technique. All 5 patients in our case series asked their dermatologists about alternative treatment options and were not offered MMS. In each case, the patients learned of the technique through internet research.
The lack of insurance reimbursement for MMS in Japan is another barrier. Because the national health insurance does not reimburse for MMS, the procedure is relatively unavailable to most Japanese citizens who cannot pay out-of-pocket for the treatment and do not have supplemental insurance. Mohs micrographic surgery may seem expensive compared to WLE followed by repair; however, in the authors’ experience, in Japan, excision without MMS may require general sedation and multiple surgeries to reconstruct larger skin defects, leading to greater morbidity and risk for the patient.
Conclusion
Mohs micrographic surgery in Japan is in its infancy, and further studies showing recurrence rates and long-term prognosis are needed. Such data should help increase awareness of MMS among Japanese physicians as an excellent treatment option for their patients. Furthermore, as Japan becomes more heterogenous as a society and the US Military increases its presence in the region, the need for MMS is likely to increase.
Acknowledgments—We appreciate the proofreading support by Mark Bivens, MBA, MSc (Tokyo, Japan), as well as the technical support from Ben Tallon, MBChB, and Robyn Mason (both in Tauranga, New Zealand) to start MMS at our clinic.
- Asgari MM, Olson J, Alam M. Needs assessment for Mohs micrographic surgery. Dermatol Clin. 2012;30:167-175. doi:10.1016/j.det.2011.08.010
- Connolly SM, Baker DR, Baker DR, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Ansai SI, Umebayashi Y, Katsumata N, et al. Japanese Dermatological Association Guidelines: outlines of guidelines for cutaneous squamous cell carcinoma 2020. J Dermatol. 2021;48:E288-E311.
- Schmults CD, Blitzblau R, Aasi SZ, et at. Basal cell skin cancer, version 2.2024, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2023;21:1181-1203. doi:10.6004/jncn.2023.0056
- Snow SN, Gunkel J. Mohs surgery. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017:2445-2455. doi:10.1016/b978-0-070-94171-3.00041-7
- Wolf DJ, Zitelli JA. Surgical margins for basal cell carcinoma. Arch Dermatol. 1987;123:340-344.
- Quazi SJ, Aslam N, Saleem H, et al. Surgical margin of excision in basal cell carcinoma: a systematic review of literature. Cureus. 2020;12:E9211.
- Rowe DE, Carroll RJ, Day Jus CL. Mohs surgery is the treatment of choice for recurrent (previously treated) basal cell carcinoma. J Dermatol Surg Oncol. 1989;15:424-431.
- Van Loo, Mosterd K, Krekels GA. Surgical excision versus Mohs’ micrographic surgery for basal cell carcinoma of the face. Eur J Cancer. 2014;50:3011-3020.
- Schmults CD, Blitzblau R, Aasi SZ, et al. NCCN Guidelines Insights: Squamous Cell Skin Cancer, Version 1.2022. J Natl Compr Canc Netw. 2021;19:1382-1394.
- Hui AM, Jacobson M, Markowitz O, et al. Mohs micrographic surgery for the treatment of melanoma. Dermatol Clin. 2012;30:503-515.
- Ito T, Inatomi Y, Nagae K, et al. Narrow-margin excision is a safe, reliable treatment for well-defined, primary pigmented basal cell carcinoma: an analysis of 288 lesions in Japan. J Eur Acad Dermatol Venereol. 2015;29:1828-1831.
- Ho WYB, Zhao X, Tan WPM. Mohs micrographic surgery in Singapore: a long-term follow-up review. Ann Acad Med Singap. 2021;50:922-923.
Margin-controlled surgery for squamous cell carcinoma (SCC) on the lower lip was first performed by Dr. Frederic Mohs on June 30, 1936. Since then, thousands of skin cancer surgeons have refined and adopted the technique. Due to the high cure rate and sparing of normal tissue, Mohs micrographic surgery (MMS) has become the gold standard treatment for facial and special-site nonmelanoma skin cancer worldwide. Mohs micrographic surgery is performed on more than 876,000 tumors annually in the United States.1 Among 3.5 million Americans diagnosed with nonmelanoma skin cancer in 2006, one-quarter were treated with MMS.2 In Japan, basal cell carcinoma (BCC) is the most common skin malignancy, with an incidence of 3.34 cases per 100,000 individuals; SCC is the second most common, with an incidence of 2.5 cases per 100,000 individuals.3
The essential element that makes MMS unique is the careful microscopic examination of the entire margin of the removed specimen. Tissue processing is done with careful en face orientation to ensure that circumferential and deep margins are entirely visible. The surgeon interprets the slides and proceeds to remove the additional tumor as necessary. Because the same physician performs both the surgery and the pathologic assessment throughout the procedure, a precise correlation between the microscopic and surgical findings can be made. The surgeon can begin with smaller margins, removing minimal healthy tissue while removing all the cancer cells, which results in the smallest-possible skin defect and the best prognosis for the malignancy (Figure 1).
At the only facility in Japan offering MMS, the lead author (S.S.) has treated 52 lesions with MMS in 46 patients (2020-2022). Of these patients, 40 were White, 5 were Japanese, and 1 was of African descent. In this case series, we present 5 Japanese patients who had BCC treated with MMS.
Case Series
Patient 1—A 50-year-old Japanese woman presented to dermatology with a brown papule on the nasal tip of 1.25 year’s duration (Figure 2). A biopsy revealed infiltrative BCC (Figure 3), and the patient was referred to the dermatology department at a nearby university hospital. Because the BCC was an aggressive variant, wide local excision (WLE) with subsequent flap reconstruction was recommended as well as radiation therapy. The patient learned about MMS through an internet search and refused both options, seeking MMS treatment at our clinic. Although Japanese health insurance does not cover MMS, the patient had supplemental private insurance that did cover the cost. She provided consent to undergo the procedure. Physical examination revealed a 7.5×6-mm, brown-red macule with ill-defined borders on the tip of the nose. We used a 1.5-mm margin for the first stage of MMS (Figure 4A). The frozen section revealed that the tumor had been entirely excised in the first stage, leaving only a 10.5×9-mm skin defect that was reconstructed with a Dufourmentel flap (Figure 4B). No signs of recurrence were noted at 3.5-year follow-up, and the cosmetic outcome was favorable (Figure 4C). National Comprehensive Cancer Network guidelines recommend a margin greater than 4 mm for infiltrative BCCs4; therefore, our technique reduced the total defect by at least 4 mm in a cosmetically sensitive area. The patient also did not need radiation therapy, which reduced morbidity. She continues to be recurrence free at 3.5-year follow-up.




Patient 2—A 63-year-old Japanese man presented to dermatology with a brown macule on the right lower eyelid of 2 years’ duration. A biopsy of the lesion was positive for nodular BCC. After being advised to undergo WLE and extensive reconstruction with plastic surgery, the patient learned of MMS through an internet search and found our clinic. Physical examination revealed a 7×5-mm brown macule on the right lower eyelid. The patient had supplemental private insurance that covered the cost of MMS, and he provided consent for the procedure. A 1.5-mm margin was taken for the first stage, resulting in a 10×8-mm defect superficial to the orbicularis oculi muscle. The frozen section revealed residual tumor exposure in the dermis at the 9- to 10-o’clock position. A second-stage excision was performed to remove an additional 1.5 mm of skin at the 9- to 12-o’clock position with a thin layer of the orbicularis oculi muscle. The subsequent histologic examination revealed no residual BCC, and the final 13×9-mm skin defect was reconstructed with a rotation flap. There were no signs of recurrence at 2.5-year follow-up with an excellent cosmetic outcome.
Patient 3—A 73-year-old Japanese man presented to a local university dermatology clinic with a new papule on the nose. The dermatologist suggested WLE with 4-mm margins and reconstruction of the skin defect 2 weeks later by a plastic surgeon. The patient was not satisfied with the proposed surgical plan, which led him to learn about MMS on the internet; he subsequently found our clinic. Physical examination revealed a 4×3.5-mm brown papule on the tip of the nose. He understood the nature of MMS and chose to pay out-of-pocket because Japanese health insurance did not cover the procedure. We used a 2-mm margin for the first stage, which created a 7.5×7-mm skin defect. The frozen section pathology revealed no residual BCC at the cut surface. The skin defect was reconstructed with a Limberg rhombic flap. There were no signs of recurrence at 1.5-year follow-up with a favorable cosmetic outcome.
Patient 4—A 45-year-old man presented to a dermatology clinic with a papule on the right side of the nose of 1 year’s duration. A biopsy revealed the lesion was a nodular BCC. The dermatologist recommended WLE at a general hospital, but the patient refused after learning about MMS. He subsequently made an appointment with our clinic. Physical examination revealed a 7×4-mm white papule on the right side of the nose. The patient had private insurance that covered the cost of MMS. The first stage was performed with 1.5-mm margins and was clear of residual tumor. A Limberg rhombic flap from the adjacent cheek was used to repair the final 10×7-mm skin defect. There were no signs of recurrence at 1 year and 9 months’ follow-up with a favorable cosmetic outcome.
Patient 5—A 76-year-old Japanese woman presented to a university hospital near Tokyo with a black papule on the left cutaneous lip of 5 years’ duration. A biopsy revealed nodular BCC, and WLE with flap reconstruction was recommended. The patient’s son learned about MMS through internet research and referred her to our clinic. Physical examination revealed a 7×5-mm black papule on the left upper lip. The patient’s private insurance covered the cost of MMS, and she consented to the procedure. We used a 2-mm initial margin, and the immediate frozen section revealed no signs of BCC at the cut surface. The 11×9-mm skin defect was reconstructed with a Limberg rhombic flap. There were no signs of recurrence at 1.5-year follow-up with a favorable cosmetic outcome.
Comment
We presented 5 cases of MMS in Japanese patients with BCC. More than 7000 new cases of nonmelanoma skin cancer occur every year in Japan.3 Only 0.04% of these cases—the 5 cases presented here—were treated with MMS in Japan in 2020 and 2021, in contrast to 25% in the United States in 2006.2
MMS vs Other BCC Treatments—Mohs micrographic surgery offers 2 distinct advantages over conventional excision: an improved cure rate while achieving a smaller final defect size, generally leading to better cosmetic outcomes. Overall 5-year recurrence rates of BCC are 10% for conventional surgical excision vs 1% for MMS, while the recurrence rates for SCC are 8% and 3%, respectively.5 A study of well-demarcated BCCs smaller than 2 cm that were treated with MMS with 2-mm increments revealed that 95% of the cases were free of malignancy within a 4-mm margin of the normal-appearing skin surrounding the tumor.6 Several articles have reported a 95% cure rate or higher with conventional excision of localized BCC,7 but 4- to 5-mm excision margins are required, resulting in a greater skin defect and a lower cure rate compared to MMS.
Aggressive subtypes of BCC have a higher recurrence rate. Rowe et al8 reported the following 5-year recurrence rates: 5.6% for MMS, 17.4% for conventional surgical excision, 40.0% for curettage and electrodesiccation, and 9.8% for radiation therapy. Primary BCCs with high-risk histologic subtypes has a 10-year recurrence rate of 4.4% with MMS vs 12.2% with conventional excision.9 These findings reveal that MMS yields a better prognosis compared to traditional treatment methods for recurrent BCCs and BCCs of high-risk histologic subtypes.
The primary reason for the excellent cure rate seen in MMS is the ability to perform complete margin assessment. Peripheral and deep en face margin assessment (PDEMA) is crucial in achieving high cure rates with narrow margins. In WLE (Figure 1), vertical sectioning (also known as bread-loafing) does not achieve direct visualization of the entire surgical margin, as this technique only evaluates random sections and does not achieve PDEMA.10 The bread-loafing method is used almost exclusively in Japan and visualizes only 0.1% of the entire margin compared to 100% with MMS.11 Beyond the superior cure rate, the MMS technique often yields smaller final defects compared to WLE. All 5 of our patients achieved complete tumor removal while sparing more normal tissue compared to conventional WLE, which takes at least a 4-mm margin in all directions.
Barriers to Adopting MMS in Japan—There are many barriers to the broader adoption of MMS in Japan. A guideline of the Japanese Dermatological Association says, MMS “is complicated, requires special training for acquisition, and requires time and labor for implementation of a series of processes, and it has not gained wide acceptance in Japan because of these disadvantages.”3 There currently are no MMS training programs in Japan. We refute this statement from the Japanese Dermatological Association because, in our experience, only 1 surgeon plus a single histotechnician familiar with MMS is sufficient for a facility to offer the procedure (the lead author of this study [S.S.] acts as both the surgeon and the histotechnician). Another misconception among some physicians in Japan is that cancer on ethnically Japanese skin is uniquely suited to excision without microscopic verification of tumor clearance because the borders of the tumors are easily identified, which was based on good cure rates for the excision of well-demarcated pigmented BCCs in a Japanese cohort. This study of a Japanese cohort investigated the specimens with the conventional bread-loafing technique but not with the PDEMA.12
Eighty percent (4/5) of our patients presented with nodular BCC, and only 1 required a second stage. In comparison, we also treated 16 White patients with nodular BCC with MMS during the same period, and 31% (5/16) required more than 1 stage, with 1 patient requiring 3 stages. This cohort, however, is too small to demonstrate a statistically significant difference (S.S., unpublished data, 2020-2022).
A study in Singapore reported the postsurgical complication rate and 5-year recurrence rate for 481 tumors (92% BCC and 7.5% SCC). The median follow-up duration after MMS was 36 months, and the recurrence rate was 0.6%. The postsurgical complications included 11 (2.3%) cases with superficial tip necrosis of surgical flaps/grafts, 2 (0.4%) with mild wound dehiscence, 1 (0.2%) with minor surgical site bleeding, and 1 (0.2%) with minor wound infection.13 This study supports the notion that MMS is equally effective for Asian patients.
Awareness of MMS in Japan is lacking, and most Japanese dermatologists do not know about the technique. All 5 patients in our case series asked their dermatologists about alternative treatment options and were not offered MMS. In each case, the patients learned of the technique through internet research.
The lack of insurance reimbursement for MMS in Japan is another barrier. Because the national health insurance does not reimburse for MMS, the procedure is relatively unavailable to most Japanese citizens who cannot pay out-of-pocket for the treatment and do not have supplemental insurance. Mohs micrographic surgery may seem expensive compared to WLE followed by repair; however, in the authors’ experience, in Japan, excision without MMS may require general sedation and multiple surgeries to reconstruct larger skin defects, leading to greater morbidity and risk for the patient.
Conclusion
Mohs micrographic surgery in Japan is in its infancy, and further studies showing recurrence rates and long-term prognosis are needed. Such data should help increase awareness of MMS among Japanese physicians as an excellent treatment option for their patients. Furthermore, as Japan becomes more heterogenous as a society and the US Military increases its presence in the region, the need for MMS is likely to increase.
Acknowledgments—We appreciate the proofreading support by Mark Bivens, MBA, MSc (Tokyo, Japan), as well as the technical support from Ben Tallon, MBChB, and Robyn Mason (both in Tauranga, New Zealand) to start MMS at our clinic.
Margin-controlled surgery for squamous cell carcinoma (SCC) on the lower lip was first performed by Dr. Frederic Mohs on June 30, 1936. Since then, thousands of skin cancer surgeons have refined and adopted the technique. Due to the high cure rate and sparing of normal tissue, Mohs micrographic surgery (MMS) has become the gold standard treatment for facial and special-site nonmelanoma skin cancer worldwide. Mohs micrographic surgery is performed on more than 876,000 tumors annually in the United States.1 Among 3.5 million Americans diagnosed with nonmelanoma skin cancer in 2006, one-quarter were treated with MMS.2 In Japan, basal cell carcinoma (BCC) is the most common skin malignancy, with an incidence of 3.34 cases per 100,000 individuals; SCC is the second most common, with an incidence of 2.5 cases per 100,000 individuals.3
The essential element that makes MMS unique is the careful microscopic examination of the entire margin of the removed specimen. Tissue processing is done with careful en face orientation to ensure that circumferential and deep margins are entirely visible. The surgeon interprets the slides and proceeds to remove the additional tumor as necessary. Because the same physician performs both the surgery and the pathologic assessment throughout the procedure, a precise correlation between the microscopic and surgical findings can be made. The surgeon can begin with smaller margins, removing minimal healthy tissue while removing all the cancer cells, which results in the smallest-possible skin defect and the best prognosis for the malignancy (Figure 1).
At the only facility in Japan offering MMS, the lead author (S.S.) has treated 52 lesions with MMS in 46 patients (2020-2022). Of these patients, 40 were White, 5 were Japanese, and 1 was of African descent. In this case series, we present 5 Japanese patients who had BCC treated with MMS.
Case Series
Patient 1—A 50-year-old Japanese woman presented to dermatology with a brown papule on the nasal tip of 1.25 year’s duration (Figure 2). A biopsy revealed infiltrative BCC (Figure 3), and the patient was referred to the dermatology department at a nearby university hospital. Because the BCC was an aggressive variant, wide local excision (WLE) with subsequent flap reconstruction was recommended as well as radiation therapy. The patient learned about MMS through an internet search and refused both options, seeking MMS treatment at our clinic. Although Japanese health insurance does not cover MMS, the patient had supplemental private insurance that did cover the cost. She provided consent to undergo the procedure. Physical examination revealed a 7.5×6-mm, brown-red macule with ill-defined borders on the tip of the nose. We used a 1.5-mm margin for the first stage of MMS (Figure 4A). The frozen section revealed that the tumor had been entirely excised in the first stage, leaving only a 10.5×9-mm skin defect that was reconstructed with a Dufourmentel flap (Figure 4B). No signs of recurrence were noted at 3.5-year follow-up, and the cosmetic outcome was favorable (Figure 4C). National Comprehensive Cancer Network guidelines recommend a margin greater than 4 mm for infiltrative BCCs4; therefore, our technique reduced the total defect by at least 4 mm in a cosmetically sensitive area. The patient also did not need radiation therapy, which reduced morbidity. She continues to be recurrence free at 3.5-year follow-up.




Patient 2—A 63-year-old Japanese man presented to dermatology with a brown macule on the right lower eyelid of 2 years’ duration. A biopsy of the lesion was positive for nodular BCC. After being advised to undergo WLE and extensive reconstruction with plastic surgery, the patient learned of MMS through an internet search and found our clinic. Physical examination revealed a 7×5-mm brown macule on the right lower eyelid. The patient had supplemental private insurance that covered the cost of MMS, and he provided consent for the procedure. A 1.5-mm margin was taken for the first stage, resulting in a 10×8-mm defect superficial to the orbicularis oculi muscle. The frozen section revealed residual tumor exposure in the dermis at the 9- to 10-o’clock position. A second-stage excision was performed to remove an additional 1.5 mm of skin at the 9- to 12-o’clock position with a thin layer of the orbicularis oculi muscle. The subsequent histologic examination revealed no residual BCC, and the final 13×9-mm skin defect was reconstructed with a rotation flap. There were no signs of recurrence at 2.5-year follow-up with an excellent cosmetic outcome.
Patient 3—A 73-year-old Japanese man presented to a local university dermatology clinic with a new papule on the nose. The dermatologist suggested WLE with 4-mm margins and reconstruction of the skin defect 2 weeks later by a plastic surgeon. The patient was not satisfied with the proposed surgical plan, which led him to learn about MMS on the internet; he subsequently found our clinic. Physical examination revealed a 4×3.5-mm brown papule on the tip of the nose. He understood the nature of MMS and chose to pay out-of-pocket because Japanese health insurance did not cover the procedure. We used a 2-mm margin for the first stage, which created a 7.5×7-mm skin defect. The frozen section pathology revealed no residual BCC at the cut surface. The skin defect was reconstructed with a Limberg rhombic flap. There were no signs of recurrence at 1.5-year follow-up with a favorable cosmetic outcome.
Patient 4—A 45-year-old man presented to a dermatology clinic with a papule on the right side of the nose of 1 year’s duration. A biopsy revealed the lesion was a nodular BCC. The dermatologist recommended WLE at a general hospital, but the patient refused after learning about MMS. He subsequently made an appointment with our clinic. Physical examination revealed a 7×4-mm white papule on the right side of the nose. The patient had private insurance that covered the cost of MMS. The first stage was performed with 1.5-mm margins and was clear of residual tumor. A Limberg rhombic flap from the adjacent cheek was used to repair the final 10×7-mm skin defect. There were no signs of recurrence at 1 year and 9 months’ follow-up with a favorable cosmetic outcome.
Patient 5—A 76-year-old Japanese woman presented to a university hospital near Tokyo with a black papule on the left cutaneous lip of 5 years’ duration. A biopsy revealed nodular BCC, and WLE with flap reconstruction was recommended. The patient’s son learned about MMS through internet research and referred her to our clinic. Physical examination revealed a 7×5-mm black papule on the left upper lip. The patient’s private insurance covered the cost of MMS, and she consented to the procedure. We used a 2-mm initial margin, and the immediate frozen section revealed no signs of BCC at the cut surface. The 11×9-mm skin defect was reconstructed with a Limberg rhombic flap. There were no signs of recurrence at 1.5-year follow-up with a favorable cosmetic outcome.
Comment
We presented 5 cases of MMS in Japanese patients with BCC. More than 7000 new cases of nonmelanoma skin cancer occur every year in Japan.3 Only 0.04% of these cases—the 5 cases presented here—were treated with MMS in Japan in 2020 and 2021, in contrast to 25% in the United States in 2006.2
MMS vs Other BCC Treatments—Mohs micrographic surgery offers 2 distinct advantages over conventional excision: an improved cure rate while achieving a smaller final defect size, generally leading to better cosmetic outcomes. Overall 5-year recurrence rates of BCC are 10% for conventional surgical excision vs 1% for MMS, while the recurrence rates for SCC are 8% and 3%, respectively.5 A study of well-demarcated BCCs smaller than 2 cm that were treated with MMS with 2-mm increments revealed that 95% of the cases were free of malignancy within a 4-mm margin of the normal-appearing skin surrounding the tumor.6 Several articles have reported a 95% cure rate or higher with conventional excision of localized BCC,7 but 4- to 5-mm excision margins are required, resulting in a greater skin defect and a lower cure rate compared to MMS.
Aggressive subtypes of BCC have a higher recurrence rate. Rowe et al8 reported the following 5-year recurrence rates: 5.6% for MMS, 17.4% for conventional surgical excision, 40.0% for curettage and electrodesiccation, and 9.8% for radiation therapy. Primary BCCs with high-risk histologic subtypes has a 10-year recurrence rate of 4.4% with MMS vs 12.2% with conventional excision.9 These findings reveal that MMS yields a better prognosis compared to traditional treatment methods for recurrent BCCs and BCCs of high-risk histologic subtypes.
The primary reason for the excellent cure rate seen in MMS is the ability to perform complete margin assessment. Peripheral and deep en face margin assessment (PDEMA) is crucial in achieving high cure rates with narrow margins. In WLE (Figure 1), vertical sectioning (also known as bread-loafing) does not achieve direct visualization of the entire surgical margin, as this technique only evaluates random sections and does not achieve PDEMA.10 The bread-loafing method is used almost exclusively in Japan and visualizes only 0.1% of the entire margin compared to 100% with MMS.11 Beyond the superior cure rate, the MMS technique often yields smaller final defects compared to WLE. All 5 of our patients achieved complete tumor removal while sparing more normal tissue compared to conventional WLE, which takes at least a 4-mm margin in all directions.
Barriers to Adopting MMS in Japan—There are many barriers to the broader adoption of MMS in Japan. A guideline of the Japanese Dermatological Association says, MMS “is complicated, requires special training for acquisition, and requires time and labor for implementation of a series of processes, and it has not gained wide acceptance in Japan because of these disadvantages.”3 There currently are no MMS training programs in Japan. We refute this statement from the Japanese Dermatological Association because, in our experience, only 1 surgeon plus a single histotechnician familiar with MMS is sufficient for a facility to offer the procedure (the lead author of this study [S.S.] acts as both the surgeon and the histotechnician). Another misconception among some physicians in Japan is that cancer on ethnically Japanese skin is uniquely suited to excision without microscopic verification of tumor clearance because the borders of the tumors are easily identified, which was based on good cure rates for the excision of well-demarcated pigmented BCCs in a Japanese cohort. This study of a Japanese cohort investigated the specimens with the conventional bread-loafing technique but not with the PDEMA.12
Eighty percent (4/5) of our patients presented with nodular BCC, and only 1 required a second stage. In comparison, we also treated 16 White patients with nodular BCC with MMS during the same period, and 31% (5/16) required more than 1 stage, with 1 patient requiring 3 stages. This cohort, however, is too small to demonstrate a statistically significant difference (S.S., unpublished data, 2020-2022).
A study in Singapore reported the postsurgical complication rate and 5-year recurrence rate for 481 tumors (92% BCC and 7.5% SCC). The median follow-up duration after MMS was 36 months, and the recurrence rate was 0.6%. The postsurgical complications included 11 (2.3%) cases with superficial tip necrosis of surgical flaps/grafts, 2 (0.4%) with mild wound dehiscence, 1 (0.2%) with minor surgical site bleeding, and 1 (0.2%) with minor wound infection.13 This study supports the notion that MMS is equally effective for Asian patients.
Awareness of MMS in Japan is lacking, and most Japanese dermatologists do not know about the technique. All 5 patients in our case series asked their dermatologists about alternative treatment options and were not offered MMS. In each case, the patients learned of the technique through internet research.
The lack of insurance reimbursement for MMS in Japan is another barrier. Because the national health insurance does not reimburse for MMS, the procedure is relatively unavailable to most Japanese citizens who cannot pay out-of-pocket for the treatment and do not have supplemental insurance. Mohs micrographic surgery may seem expensive compared to WLE followed by repair; however, in the authors’ experience, in Japan, excision without MMS may require general sedation and multiple surgeries to reconstruct larger skin defects, leading to greater morbidity and risk for the patient.
Conclusion
Mohs micrographic surgery in Japan is in its infancy, and further studies showing recurrence rates and long-term prognosis are needed. Such data should help increase awareness of MMS among Japanese physicians as an excellent treatment option for their patients. Furthermore, as Japan becomes more heterogenous as a society and the US Military increases its presence in the region, the need for MMS is likely to increase.
Acknowledgments—We appreciate the proofreading support by Mark Bivens, MBA, MSc (Tokyo, Japan), as well as the technical support from Ben Tallon, MBChB, and Robyn Mason (both in Tauranga, New Zealand) to start MMS at our clinic.
- Asgari MM, Olson J, Alam M. Needs assessment for Mohs micrographic surgery. Dermatol Clin. 2012;30:167-175. doi:10.1016/j.det.2011.08.010
- Connolly SM, Baker DR, Baker DR, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Ansai SI, Umebayashi Y, Katsumata N, et al. Japanese Dermatological Association Guidelines: outlines of guidelines for cutaneous squamous cell carcinoma 2020. J Dermatol. 2021;48:E288-E311.
- Schmults CD, Blitzblau R, Aasi SZ, et at. Basal cell skin cancer, version 2.2024, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2023;21:1181-1203. doi:10.6004/jncn.2023.0056
- Snow SN, Gunkel J. Mohs surgery. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017:2445-2455. doi:10.1016/b978-0-070-94171-3.00041-7
- Wolf DJ, Zitelli JA. Surgical margins for basal cell carcinoma. Arch Dermatol. 1987;123:340-344.
- Quazi SJ, Aslam N, Saleem H, et al. Surgical margin of excision in basal cell carcinoma: a systematic review of literature. Cureus. 2020;12:E9211.
- Rowe DE, Carroll RJ, Day Jus CL. Mohs surgery is the treatment of choice for recurrent (previously treated) basal cell carcinoma. J Dermatol Surg Oncol. 1989;15:424-431.
- Van Loo, Mosterd K, Krekels GA. Surgical excision versus Mohs’ micrographic surgery for basal cell carcinoma of the face. Eur J Cancer. 2014;50:3011-3020.
- Schmults CD, Blitzblau R, Aasi SZ, et al. NCCN Guidelines Insights: Squamous Cell Skin Cancer, Version 1.2022. J Natl Compr Canc Netw. 2021;19:1382-1394.
- Hui AM, Jacobson M, Markowitz O, et al. Mohs micrographic surgery for the treatment of melanoma. Dermatol Clin. 2012;30:503-515.
- Ito T, Inatomi Y, Nagae K, et al. Narrow-margin excision is a safe, reliable treatment for well-defined, primary pigmented basal cell carcinoma: an analysis of 288 lesions in Japan. J Eur Acad Dermatol Venereol. 2015;29:1828-1831.
- Ho WYB, Zhao X, Tan WPM. Mohs micrographic surgery in Singapore: a long-term follow-up review. Ann Acad Med Singap. 2021;50:922-923.
- Asgari MM, Olson J, Alam M. Needs assessment for Mohs micrographic surgery. Dermatol Clin. 2012;30:167-175. doi:10.1016/j.det.2011.08.010
- Connolly SM, Baker DR, Baker DR, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Ansai SI, Umebayashi Y, Katsumata N, et al. Japanese Dermatological Association Guidelines: outlines of guidelines for cutaneous squamous cell carcinoma 2020. J Dermatol. 2021;48:E288-E311.
- Schmults CD, Blitzblau R, Aasi SZ, et at. Basal cell skin cancer, version 2.2024, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2023;21:1181-1203. doi:10.6004/jncn.2023.0056
- Snow SN, Gunkel J. Mohs surgery. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017:2445-2455. doi:10.1016/b978-0-070-94171-3.00041-7
- Wolf DJ, Zitelli JA. Surgical margins for basal cell carcinoma. Arch Dermatol. 1987;123:340-344.
- Quazi SJ, Aslam N, Saleem H, et al. Surgical margin of excision in basal cell carcinoma: a systematic review of literature. Cureus. 2020;12:E9211.
- Rowe DE, Carroll RJ, Day Jus CL. Mohs surgery is the treatment of choice for recurrent (previously treated) basal cell carcinoma. J Dermatol Surg Oncol. 1989;15:424-431.
- Van Loo, Mosterd K, Krekels GA. Surgical excision versus Mohs’ micrographic surgery for basal cell carcinoma of the face. Eur J Cancer. 2014;50:3011-3020.
- Schmults CD, Blitzblau R, Aasi SZ, et al. NCCN Guidelines Insights: Squamous Cell Skin Cancer, Version 1.2022. J Natl Compr Canc Netw. 2021;19:1382-1394.
- Hui AM, Jacobson M, Markowitz O, et al. Mohs micrographic surgery for the treatment of melanoma. Dermatol Clin. 2012;30:503-515.
- Ito T, Inatomi Y, Nagae K, et al. Narrow-margin excision is a safe, reliable treatment for well-defined, primary pigmented basal cell carcinoma: an analysis of 288 lesions in Japan. J Eur Acad Dermatol Venereol. 2015;29:1828-1831.
- Ho WYB, Zhao X, Tan WPM. Mohs micrographic surgery in Singapore: a long-term follow-up review. Ann Acad Med Singap. 2021;50:922-923.
Practice Points
- Mohs micrographic surgery (MMS) is a safe and effective treatment method for nonmelanoma skin cancer. In some cases, this procedure is superior to standard wide local excision and repair.
- For the broader adaptation of this vital technique in Japan—where MMS is not well established—increased awareness of treatment outcomes among Japanese physicians is needed.
Act Fast With Traction Alopecia to Avoid Permanent Hair Loss
Traction alopecia (TA) is a common type of alopecia that ultimately can result in permanent hair loss. It often is caused or worsened by repetitive and prolonged hairstyling practices such as tight ponytails, braids, or locs, or use of wigs or weaves.1 Use of headwear, as in certain religious or ethnic groups, also can be contributory.2 Individuals participating in or training for occupations involving military service or ballet are at risk for TA due to hairstyling-specific policies. Early stages of TA are reversible with proper treatment and avoidance of exacerbating factors, emphasizing the importance of prompt recognition.3
Epidemiology
Data on the true prevalence of TA are lacking. It can occur in individuals of any race or any hair type. However, it is most common in women of African descent, affecting approximately one-third of this population.4 Other commonly affected groups include ballerinas and active-duty service members due to tight ponytails and buns, as well as the Sikh population due to the use of turbans as a part of their religious practice.2,5,6
Traction alopecia also impacts children, particularly those of African descent. A 2007 study of schoolchildren in South Africa determined that more than 17% of young African girls had evidence of TA—even some as young as 6 years of age.7
Traction alopecia can be caused or exacerbated by the use of hair clips and bobby pins that aid holding styles in place.8 Hair shaft morphology may contribute to the risk for TA, with more tightly coiled hair types being more susceptible.8 Variables such as use of chemical relaxers also increase the risk for disease, especially when combined with high-tension styling methods such as braids.9
Key clinical features
Patients with TA clinically present with hair loss and breakage in areas with tension, most commonly the marginal areas of the scalp as well as the frontal hairline and temporal scalp. Hair loss can result in a “fringe sign,” in which a patient may have preservation of a thin line of hairs at the frontal aspect of the hairline with a band of hair loss behind.10 This presentation may be used to differentiate TA from other forms of alopecia, including frontal fibrosing alopecia and female pattern hair loss. When the hair loss is not marginal, it may mimic other forms of patchy hair loss including alopecia areata and trichotillomania. Other clinical findings in TA may include broken hairs, pustules, and follicular papules.10 Patients also may describe symptoms such as scalp tenderness with specific hairstyles or headaches,11 or they may be completely asymptomatic.
Trichoscopy can be helpful in guiding diagnosis and treatment. Patients with TA often have perifollicular erythema and hair casts (cylindrical structures that encircle the proximal hair shafts) in the earlier stages of the disease, with eventual loss of follicular ostia in the later stages.10,12 Hair casts also may indicate ongoing traction.12 The flambeau sign—white tracks seen on trichoscopy in the direction the hair is pulled—resembles a lit torch.13
Worth noting
Early-stage TA can be reversed by avoiding hair tension. However, patients may not be amenable to this due to personal hairstyling preferences, job duties, or religious practices. Treatment with topical or intralesional steroids or even oral antibiotics such as doxycycline for its anti-inflammatory ability may result in regrowth of lost hair if the follicles are not permanently lost and exacerbating factors are avoided.3,14 Both topical and oral minoxidil have been used with success, with minoxidil thought to increase hair density by extending the anagen (growth) phase of hair follicles.3,15 Culturally sensitive patient counseling on the condition and potential exacerbating factors is critical.16
At later stages of the disease—after loss of follicular ostia has occurred—surgical interventions should be considered,17 such as hair transplantation, which can be successful but remains a technical challenge due to variability in hair shaft curvature.18 Additionally, the cost of the procedure can limit use, and some patients may not be optimal candidates due to the extent of their hair loss. Traction alopecia may not be the only hair loss condition present. Examining the scalp is important even if the chief area of concern is the marginal scalp.
Health disparity highlight
Prevention, early identification, and treatment initiated in a timely fashion are crucial to prevent permanent hair loss. There are added societal and cultural pressures that impact hairstyle and hair care practices, especially for those with tightly coiled hair.19 Historically, tightly coiled hair has been unfairly viewed as “unprofessional,” “unkempt,” and a challenge to “manage” by some. Thus, heat, chemical relaxers, and tight hairstyles holding hair in one position have been used to straighten the hair permanently or temporarily or to keep it maintained in a style that did not necessitate excessive manipulation—often contributing to further tension on the hair.
Military service branches have evaluated and changed some hair-related policies to reflect the diverse hair types of military personnel.20 The CROWN Act (www.thecrownact.com/about)—“Creating a Respectful and Open World for Natural Hair”—is a model law passed by 26 states that prohibits race-based hair discrimination, which is the denial of employment and educational opportunities because of hair texture. Although the law has not been passed in every state, it may help individuals with tightly coiled hair to embrace natural hairstyles. However, even hairstyles with one’s own natural curl pattern can contribute to tension and thus potential development of TA.
1. Larrondo J, McMichael AJ. Traction alopecia. JAMA Dermatol. 2023;159:676. doi:10.1001/jamadermatol.2022.6298
2. James J, Saladi RN, Fox JL. Traction alopecia in Sikh male patients. J Am Board Fam Med. 2007;20:497-498. doi:10.3122/jabfm.2007.05.070076
3. Callender VD, McMichael AJ, Cohen GF. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17:164-176.
4. Loussouarn G, El Rawadi C, Genain G. Diversity of hair growth profiles. Int J Dermatol. 2005;44(suppl 1):6-9.
5. Samrao AChen CZedek Det al. Traction alopecia in a ballerina: clinicopathologic features. Arch Dermatol. 2010;146:918-935. doi:10.1001/archdermatol.2010.183
6. Korona-Bailey J, Banaag A, Nguyen DR, et al. Free the bun: prevalence of alopecia among active duty service women, fiscal years 2010-2019. Mil Med. 2023;188:e492-e496. doi:10.1093/milmed/usab274
7. Khumalo NP, Jessop S, Gumedze F, et al. Hairdressing is associated with scalp disease in African schoolchildren. Br J Dermatol. 2007;157:106-110. doi:10.1111/j.1365-2133.2007.07987.x
8. Billero V, Miteva M. Traction alopecia: the root of the problem. Clin Cosmet Investig Dermatol. 2018;11:149-159. doi:10.2147/CCID.S137296
9. Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia (TA). J Am Acad Dermatol. 2016;75:606-611. doi:10.1016/j.jaad.2016.02.1162
10. Samrao A, Price VH, Zedek D, et al. The “fringe sign”—a useful clinical finding in traction alopecia of the marginal hair line. Dermatol Online J. 2011;17:1.
11. Kararizou E, Bougea AM, Giotopoulou D, et al. An update on the less-known group of other primary headaches—a review. Eur Neurol Rev. 2014;9:71-77. doi:10.17925/ENR.2014.09.01.71
12. Tosti A, Miteva M, Torres F, et al. Hair casts are a dermoscopic clue for the diagnosis of traction alopecia. Br J Dermatol. 2010;163:1353-1355.
13. Agrawal S, Daruwalla SB, Dhurat RS. The flambeau sign—a new dermoscopy finding in a case of marginal traction alopecia. Australas J Dermatol. 2020;61:49-50. doi:10.1111/ajd.13187
14. Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3:S21-S37.
15. Awad A, Chim I, Sharma P, et al. Low-dose oral minoxidil improves hair density in traction alopecia. J Am Acad Dermatol. 2023;89:157-159. doi:10.1016/j.jaad.2023.02.024
16. Grayson C, Heath CR. Counseling about traction alopecia: a “compliment, discuss, and suggest” method. Cutis. 2021;108:20-22.
17. Ozçelik D. Extensive traction alopecia attributable to ponytail hairstyle and its treatment with hair transplantation. Aesthetic Plast Surg. 2005;29:325-327. doi:10.1007/s00266-005-0004-5
18. Singh MK, Avram MR. Technical considerations for follicular unit extraction in African-American hair. Dermatol Surg. 2013;39:1282-1284. doi:10.1111/dsu.12229
19. Jones NL, Heath CR. Hair at the intersection of dermatology and anthropology: a conversation on race and relationships. Pediatr Dermatol. 2021;38(suppl 2):158-160.
20. Franklin JMM, Wohltmann WE, Wong EB. From buns to braids and ponytails: entering a new era of female military hair-grooming standards. Cutis. 2021;108:31-35. doi:10.12788/cutis.0296
Traction alopecia (TA) is a common type of alopecia that ultimately can result in permanent hair loss. It often is caused or worsened by repetitive and prolonged hairstyling practices such as tight ponytails, braids, or locs, or use of wigs or weaves.1 Use of headwear, as in certain religious or ethnic groups, also can be contributory.2 Individuals participating in or training for occupations involving military service or ballet are at risk for TA due to hairstyling-specific policies. Early stages of TA are reversible with proper treatment and avoidance of exacerbating factors, emphasizing the importance of prompt recognition.3
Epidemiology
Data on the true prevalence of TA are lacking. It can occur in individuals of any race or any hair type. However, it is most common in women of African descent, affecting approximately one-third of this population.4 Other commonly affected groups include ballerinas and active-duty service members due to tight ponytails and buns, as well as the Sikh population due to the use of turbans as a part of their religious practice.2,5,6
Traction alopecia also impacts children, particularly those of African descent. A 2007 study of schoolchildren in South Africa determined that more than 17% of young African girls had evidence of TA—even some as young as 6 years of age.7
Traction alopecia can be caused or exacerbated by the use of hair clips and bobby pins that aid holding styles in place.8 Hair shaft morphology may contribute to the risk for TA, with more tightly coiled hair types being more susceptible.8 Variables such as use of chemical relaxers also increase the risk for disease, especially when combined with high-tension styling methods such as braids.9
Key clinical features
Patients with TA clinically present with hair loss and breakage in areas with tension, most commonly the marginal areas of the scalp as well as the frontal hairline and temporal scalp. Hair loss can result in a “fringe sign,” in which a patient may have preservation of a thin line of hairs at the frontal aspect of the hairline with a band of hair loss behind.10 This presentation may be used to differentiate TA from other forms of alopecia, including frontal fibrosing alopecia and female pattern hair loss. When the hair loss is not marginal, it may mimic other forms of patchy hair loss including alopecia areata and trichotillomania. Other clinical findings in TA may include broken hairs, pustules, and follicular papules.10 Patients also may describe symptoms such as scalp tenderness with specific hairstyles or headaches,11 or they may be completely asymptomatic.
Trichoscopy can be helpful in guiding diagnosis and treatment. Patients with TA often have perifollicular erythema and hair casts (cylindrical structures that encircle the proximal hair shafts) in the earlier stages of the disease, with eventual loss of follicular ostia in the later stages.10,12 Hair casts also may indicate ongoing traction.12 The flambeau sign—white tracks seen on trichoscopy in the direction the hair is pulled—resembles a lit torch.13
Worth noting
Early-stage TA can be reversed by avoiding hair tension. However, patients may not be amenable to this due to personal hairstyling preferences, job duties, or religious practices. Treatment with topical or intralesional steroids or even oral antibiotics such as doxycycline for its anti-inflammatory ability may result in regrowth of lost hair if the follicles are not permanently lost and exacerbating factors are avoided.3,14 Both topical and oral minoxidil have been used with success, with minoxidil thought to increase hair density by extending the anagen (growth) phase of hair follicles.3,15 Culturally sensitive patient counseling on the condition and potential exacerbating factors is critical.16
At later stages of the disease—after loss of follicular ostia has occurred—surgical interventions should be considered,17 such as hair transplantation, which can be successful but remains a technical challenge due to variability in hair shaft curvature.18 Additionally, the cost of the procedure can limit use, and some patients may not be optimal candidates due to the extent of their hair loss. Traction alopecia may not be the only hair loss condition present. Examining the scalp is important even if the chief area of concern is the marginal scalp.
Health disparity highlight
Prevention, early identification, and treatment initiated in a timely fashion are crucial to prevent permanent hair loss. There are added societal and cultural pressures that impact hairstyle and hair care practices, especially for those with tightly coiled hair.19 Historically, tightly coiled hair has been unfairly viewed as “unprofessional,” “unkempt,” and a challenge to “manage” by some. Thus, heat, chemical relaxers, and tight hairstyles holding hair in one position have been used to straighten the hair permanently or temporarily or to keep it maintained in a style that did not necessitate excessive manipulation—often contributing to further tension on the hair.
Military service branches have evaluated and changed some hair-related policies to reflect the diverse hair types of military personnel.20 The CROWN Act (www.thecrownact.com/about)—“Creating a Respectful and Open World for Natural Hair”—is a model law passed by 26 states that prohibits race-based hair discrimination, which is the denial of employment and educational opportunities because of hair texture. Although the law has not been passed in every state, it may help individuals with tightly coiled hair to embrace natural hairstyles. However, even hairstyles with one’s own natural curl pattern can contribute to tension and thus potential development of TA.
Traction alopecia (TA) is a common type of alopecia that ultimately can result in permanent hair loss. It often is caused or worsened by repetitive and prolonged hairstyling practices such as tight ponytails, braids, or locs, or use of wigs or weaves.1 Use of headwear, as in certain religious or ethnic groups, also can be contributory.2 Individuals participating in or training for occupations involving military service or ballet are at risk for TA due to hairstyling-specific policies. Early stages of TA are reversible with proper treatment and avoidance of exacerbating factors, emphasizing the importance of prompt recognition.3
Epidemiology
Data on the true prevalence of TA are lacking. It can occur in individuals of any race or any hair type. However, it is most common in women of African descent, affecting approximately one-third of this population.4 Other commonly affected groups include ballerinas and active-duty service members due to tight ponytails and buns, as well as the Sikh population due to the use of turbans as a part of their religious practice.2,5,6
Traction alopecia also impacts children, particularly those of African descent. A 2007 study of schoolchildren in South Africa determined that more than 17% of young African girls had evidence of TA—even some as young as 6 years of age.7
Traction alopecia can be caused or exacerbated by the use of hair clips and bobby pins that aid holding styles in place.8 Hair shaft morphology may contribute to the risk for TA, with more tightly coiled hair types being more susceptible.8 Variables such as use of chemical relaxers also increase the risk for disease, especially when combined with high-tension styling methods such as braids.9
Key clinical features
Patients with TA clinically present with hair loss and breakage in areas with tension, most commonly the marginal areas of the scalp as well as the frontal hairline and temporal scalp. Hair loss can result in a “fringe sign,” in which a patient may have preservation of a thin line of hairs at the frontal aspect of the hairline with a band of hair loss behind.10 This presentation may be used to differentiate TA from other forms of alopecia, including frontal fibrosing alopecia and female pattern hair loss. When the hair loss is not marginal, it may mimic other forms of patchy hair loss including alopecia areata and trichotillomania. Other clinical findings in TA may include broken hairs, pustules, and follicular papules.10 Patients also may describe symptoms such as scalp tenderness with specific hairstyles or headaches,11 or they may be completely asymptomatic.
Trichoscopy can be helpful in guiding diagnosis and treatment. Patients with TA often have perifollicular erythema and hair casts (cylindrical structures that encircle the proximal hair shafts) in the earlier stages of the disease, with eventual loss of follicular ostia in the later stages.10,12 Hair casts also may indicate ongoing traction.12 The flambeau sign—white tracks seen on trichoscopy in the direction the hair is pulled—resembles a lit torch.13
Worth noting
Early-stage TA can be reversed by avoiding hair tension. However, patients may not be amenable to this due to personal hairstyling preferences, job duties, or religious practices. Treatment with topical or intralesional steroids or even oral antibiotics such as doxycycline for its anti-inflammatory ability may result in regrowth of lost hair if the follicles are not permanently lost and exacerbating factors are avoided.3,14 Both topical and oral minoxidil have been used with success, with minoxidil thought to increase hair density by extending the anagen (growth) phase of hair follicles.3,15 Culturally sensitive patient counseling on the condition and potential exacerbating factors is critical.16
At later stages of the disease—after loss of follicular ostia has occurred—surgical interventions should be considered,17 such as hair transplantation, which can be successful but remains a technical challenge due to variability in hair shaft curvature.18 Additionally, the cost of the procedure can limit use, and some patients may not be optimal candidates due to the extent of their hair loss. Traction alopecia may not be the only hair loss condition present. Examining the scalp is important even if the chief area of concern is the marginal scalp.
Health disparity highlight
Prevention, early identification, and treatment initiated in a timely fashion are crucial to prevent permanent hair loss. There are added societal and cultural pressures that impact hairstyle and hair care practices, especially for those with tightly coiled hair.19 Historically, tightly coiled hair has been unfairly viewed as “unprofessional,” “unkempt,” and a challenge to “manage” by some. Thus, heat, chemical relaxers, and tight hairstyles holding hair in one position have been used to straighten the hair permanently or temporarily or to keep it maintained in a style that did not necessitate excessive manipulation—often contributing to further tension on the hair.
Military service branches have evaluated and changed some hair-related policies to reflect the diverse hair types of military personnel.20 The CROWN Act (www.thecrownact.com/about)—“Creating a Respectful and Open World for Natural Hair”—is a model law passed by 26 states that prohibits race-based hair discrimination, which is the denial of employment and educational opportunities because of hair texture. Although the law has not been passed in every state, it may help individuals with tightly coiled hair to embrace natural hairstyles. However, even hairstyles with one’s own natural curl pattern can contribute to tension and thus potential development of TA.
1. Larrondo J, McMichael AJ. Traction alopecia. JAMA Dermatol. 2023;159:676. doi:10.1001/jamadermatol.2022.6298
2. James J, Saladi RN, Fox JL. Traction alopecia in Sikh male patients. J Am Board Fam Med. 2007;20:497-498. doi:10.3122/jabfm.2007.05.070076
3. Callender VD, McMichael AJ, Cohen GF. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17:164-176.
4. Loussouarn G, El Rawadi C, Genain G. Diversity of hair growth profiles. Int J Dermatol. 2005;44(suppl 1):6-9.
5. Samrao AChen CZedek Det al. Traction alopecia in a ballerina: clinicopathologic features. Arch Dermatol. 2010;146:918-935. doi:10.1001/archdermatol.2010.183
6. Korona-Bailey J, Banaag A, Nguyen DR, et al. Free the bun: prevalence of alopecia among active duty service women, fiscal years 2010-2019. Mil Med. 2023;188:e492-e496. doi:10.1093/milmed/usab274
7. Khumalo NP, Jessop S, Gumedze F, et al. Hairdressing is associated with scalp disease in African schoolchildren. Br J Dermatol. 2007;157:106-110. doi:10.1111/j.1365-2133.2007.07987.x
8. Billero V, Miteva M. Traction alopecia: the root of the problem. Clin Cosmet Investig Dermatol. 2018;11:149-159. doi:10.2147/CCID.S137296
9. Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia (TA). J Am Acad Dermatol. 2016;75:606-611. doi:10.1016/j.jaad.2016.02.1162
10. Samrao A, Price VH, Zedek D, et al. The “fringe sign”—a useful clinical finding in traction alopecia of the marginal hair line. Dermatol Online J. 2011;17:1.
11. Kararizou E, Bougea AM, Giotopoulou D, et al. An update on the less-known group of other primary headaches—a review. Eur Neurol Rev. 2014;9:71-77. doi:10.17925/ENR.2014.09.01.71
12. Tosti A, Miteva M, Torres F, et al. Hair casts are a dermoscopic clue for the diagnosis of traction alopecia. Br J Dermatol. 2010;163:1353-1355.
13. Agrawal S, Daruwalla SB, Dhurat RS. The flambeau sign—a new dermoscopy finding in a case of marginal traction alopecia. Australas J Dermatol. 2020;61:49-50. doi:10.1111/ajd.13187
14. Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3:S21-S37.
15. Awad A, Chim I, Sharma P, et al. Low-dose oral minoxidil improves hair density in traction alopecia. J Am Acad Dermatol. 2023;89:157-159. doi:10.1016/j.jaad.2023.02.024
16. Grayson C, Heath CR. Counseling about traction alopecia: a “compliment, discuss, and suggest” method. Cutis. 2021;108:20-22.
17. Ozçelik D. Extensive traction alopecia attributable to ponytail hairstyle and its treatment with hair transplantation. Aesthetic Plast Surg. 2005;29:325-327. doi:10.1007/s00266-005-0004-5
18. Singh MK, Avram MR. Technical considerations for follicular unit extraction in African-American hair. Dermatol Surg. 2013;39:1282-1284. doi:10.1111/dsu.12229
19. Jones NL, Heath CR. Hair at the intersection of dermatology and anthropology: a conversation on race and relationships. Pediatr Dermatol. 2021;38(suppl 2):158-160.
20. Franklin JMM, Wohltmann WE, Wong EB. From buns to braids and ponytails: entering a new era of female military hair-grooming standards. Cutis. 2021;108:31-35. doi:10.12788/cutis.0296
1. Larrondo J, McMichael AJ. Traction alopecia. JAMA Dermatol. 2023;159:676. doi:10.1001/jamadermatol.2022.6298
2. James J, Saladi RN, Fox JL. Traction alopecia in Sikh male patients. J Am Board Fam Med. 2007;20:497-498. doi:10.3122/jabfm.2007.05.070076
3. Callender VD, McMichael AJ, Cohen GF. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17:164-176.
4. Loussouarn G, El Rawadi C, Genain G. Diversity of hair growth profiles. Int J Dermatol. 2005;44(suppl 1):6-9.
5. Samrao AChen CZedek Det al. Traction alopecia in a ballerina: clinicopathologic features. Arch Dermatol. 2010;146:918-935. doi:10.1001/archdermatol.2010.183
6. Korona-Bailey J, Banaag A, Nguyen DR, et al. Free the bun: prevalence of alopecia among active duty service women, fiscal years 2010-2019. Mil Med. 2023;188:e492-e496. doi:10.1093/milmed/usab274
7. Khumalo NP, Jessop S, Gumedze F, et al. Hairdressing is associated with scalp disease in African schoolchildren. Br J Dermatol. 2007;157:106-110. doi:10.1111/j.1365-2133.2007.07987.x
8. Billero V, Miteva M. Traction alopecia: the root of the problem. Clin Cosmet Investig Dermatol. 2018;11:149-159. doi:10.2147/CCID.S137296
9. Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia (TA). J Am Acad Dermatol. 2016;75:606-611. doi:10.1016/j.jaad.2016.02.1162
10. Samrao A, Price VH, Zedek D, et al. The “fringe sign”—a useful clinical finding in traction alopecia of the marginal hair line. Dermatol Online J. 2011;17:1.
11. Kararizou E, Bougea AM, Giotopoulou D, et al. An update on the less-known group of other primary headaches—a review. Eur Neurol Rev. 2014;9:71-77. doi:10.17925/ENR.2014.09.01.71
12. Tosti A, Miteva M, Torres F, et al. Hair casts are a dermoscopic clue for the diagnosis of traction alopecia. Br J Dermatol. 2010;163:1353-1355.
13. Agrawal S, Daruwalla SB, Dhurat RS. The flambeau sign—a new dermoscopy finding in a case of marginal traction alopecia. Australas J Dermatol. 2020;61:49-50. doi:10.1111/ajd.13187
14. Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3:S21-S37.
15. Awad A, Chim I, Sharma P, et al. Low-dose oral minoxidil improves hair density in traction alopecia. J Am Acad Dermatol. 2023;89:157-159. doi:10.1016/j.jaad.2023.02.024
16. Grayson C, Heath CR. Counseling about traction alopecia: a “compliment, discuss, and suggest” method. Cutis. 2021;108:20-22.
17. Ozçelik D. Extensive traction alopecia attributable to ponytail hairstyle and its treatment with hair transplantation. Aesthetic Plast Surg. 2005;29:325-327. doi:10.1007/s00266-005-0004-5
18. Singh MK, Avram MR. Technical considerations for follicular unit extraction in African-American hair. Dermatol Surg. 2013;39:1282-1284. doi:10.1111/dsu.12229
19. Jones NL, Heath CR. Hair at the intersection of dermatology and anthropology: a conversation on race and relationships. Pediatr Dermatol. 2021;38(suppl 2):158-160.
20. Franklin JMM, Wohltmann WE, Wong EB. From buns to braids and ponytails: entering a new era of female military hair-grooming standards. Cutis. 2021;108:31-35. doi:10.12788/cutis.0296
The State of Skin of Color Centers in the United States: A Cross-Sectional Survey Study
Although individuals with skin of color (SoC) are expected to become at least half of the US population by the year 2044, there remains a paucity of education and exposure to treatment of patients with SoC at many dermatology residency programs across the country.1 One way to improve SoC education has been the formation of specialized clinics, centers, and programs. The first SoC center (SoCC) was established in 1999 at Mount Sinai–St. Luke’s Roosevelt in New York, New York2; since then, at least 13 additional formal SoCCs or SoC specialty clinics (SoCSCs) at US academic dermatology programs have been established.
Skin of color centers serve several important purposes: they improve dermatologic care in patients with SoC, increase research efforts focused on SoC dermatologic conditions, and educate dermatology resident and fellow trainees about SoC. Improving dermatologic care of patients with SoC in the United States is important in providing equitable health care and improving health disparities. Studies have shown that patient-physician racial and cultural concordance can positively impact patient care, increase patient trust and rapport, and improve patient-physician communication, and it can even influence patient decision-making to seek care.3,4 Unfortunately, even though the US population continues to diversify, the racial/ethnic backgrounds of dermatologists do not parallel this trend; Hispanic and Black physicians comprise 18.9% and 13.6% of the general population, respectively, but represent only 4.2% and 3.0% of dermatologists, respectively.5-7 This deficit is mirrored by resident and faculty representation, with Black and Latino representation ranging from 3% to 7%.8-10
Many SoCC’s engage in research focused on dermatologic conditions affecting patients with SoC, which is vital to improving the dermatologic care in this underserved population. Despite increasing recognition of the importance of SoC research, there remains a paucity of clinical trials and research specifically focused on or demonstrating equitable representation of SoC.11,12
The education and training of future dermatologists is another important area that can be improved by SoCCs. A 2008 study involving 63 chief residents showed that approximately half (52.4% [33/63]) of graduating dermatology residents reported receiving SoC-specific didactics, sessions, or lectures, and 30.2% (19/63) reported having a dedicated rotation where they gained specific experience treating patients with SoC.13 A later study in 2022 (N=125) found that 63.2% of graduating dermatology residents reported receiving SoC-specific didactics, sessions, or lectures, and only 11.2% reported having a dedicated rotation where they gained experience treating patients with SoC.14 These findings suggest that in the last 14 years, formal SoC education—specifically SoC clinical training—has not increased sufficiently.
We conducted a cross-sectional survey study to provide an in-depth analysis of SoCCs and SoCSCs in the United States, including their patient care focus, research, and program diversity.
Methods
We conducted an investigator-initiated, multicenter, cross-sectional survey study of all SoCCs in the United States and their respective academic residency programs. Fifteen formal SoCCs and/or SoCSCs were identified by dermatology program websites and an article by Tull et al2 on the state of ethnic skin centers. All programs and centers identified were associated with a dermatology residency program accredited by the Accreditation Council for Graduate Medical Education.
A 42-item questionnaire was sent via email to the directors of these centers and clinics with the intent to collect descriptive information about each of the SoCCs, the diversity of the faculty and residents of the associated dermatology department, current research and funding, diversity and inclusion initiatives, and trainee education from March through April 2020. Data were analyzed using Excel and SPSS statistical software to obtain descriptive statistics including the mean value numeric trends across programs.
This study underwent expedited review and was approved by the University of Southern California (Los Angeles, California) institutional review board (IRB #HS-20-00113). Patient consent was not applicable, as no information was collected about patients.
Results
Fourteen directors from SoCCs/SoCSCs completed the questionnaire (93.3% response rate). Most centers were located in urban areas (12/14 [85.71%]), except for 2 in rural or suburban settings (Table). Most of the SoCCs/SoCSCs were located in the South (5/14 [35.71%]), followed by the Northeast (4/14 [28.57%]), West (3/14 [21.43%]), and Midwest (2/14 [14.29%])(Table). Six (42.86%) of the programs had a SoCSC, 3 (21.43%) had a formal SoCC, and 5 (35.71%) had both. Across all centers, the most common population seen and treated was Black/African American followed by Hispanic/Latino and Asian, respectively. The most commonly seen dermatologic conditions were acne, pigmentary disorders, alopecia, and atopic dermatitis (Figure). The most common cosmetic practice performed for patients with SoC was dermatosis papulosa nigra/seborrheic keratosis removal, followed by laser treatments, skin tag removal, chemical peels, and neuromodulator injections, respectively.
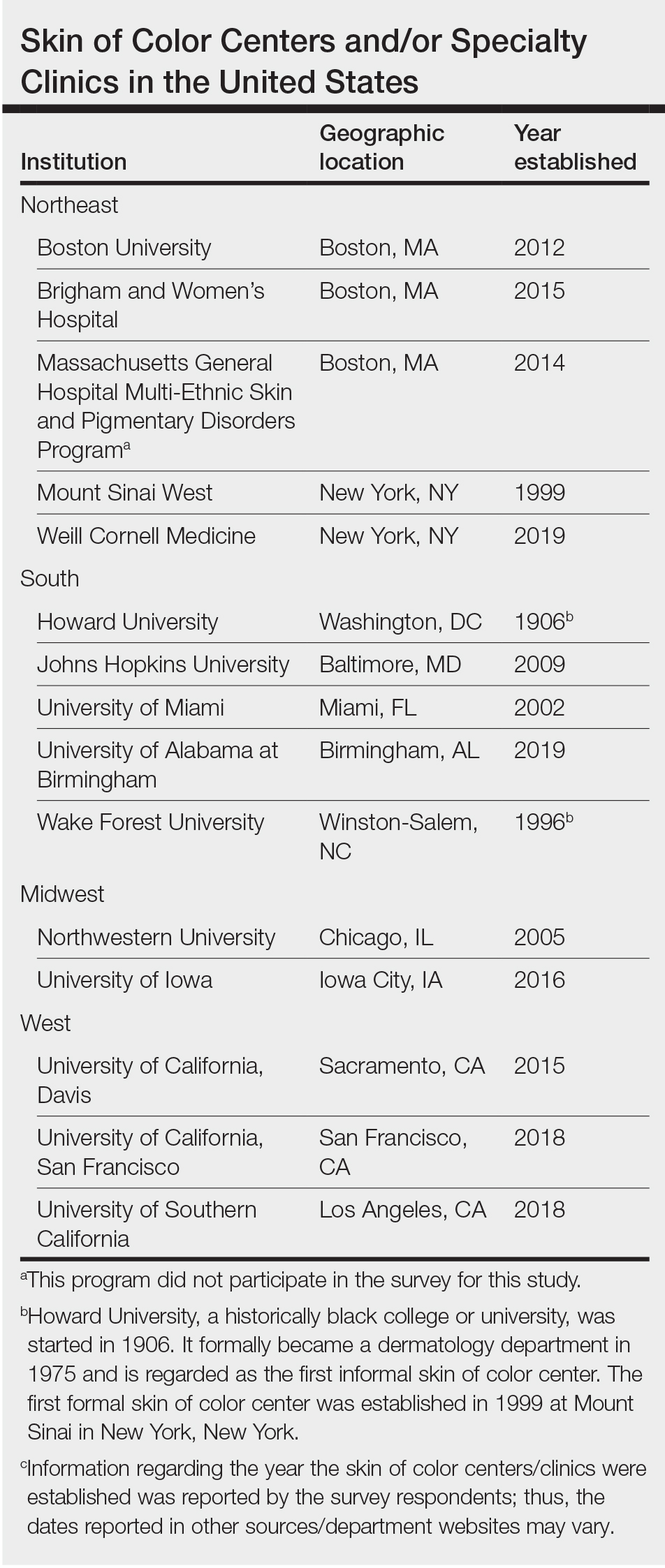
Faculty and Resident Demographics and Areas of Focus—The demographics and diversity of the dermatology faculty and residents at each individual institution also were assessed. The average number of full-time faculty at each institution was 19.4 (range, 2–48), while the average number of full-time faculty who identified as underrepresented in medicine (URiM) was 2.1 (range, 0–5). The average number of residents at each institution was 17.1 (range, 10–31), while the average number of URiM residents was 1.7 (range, 1–3).
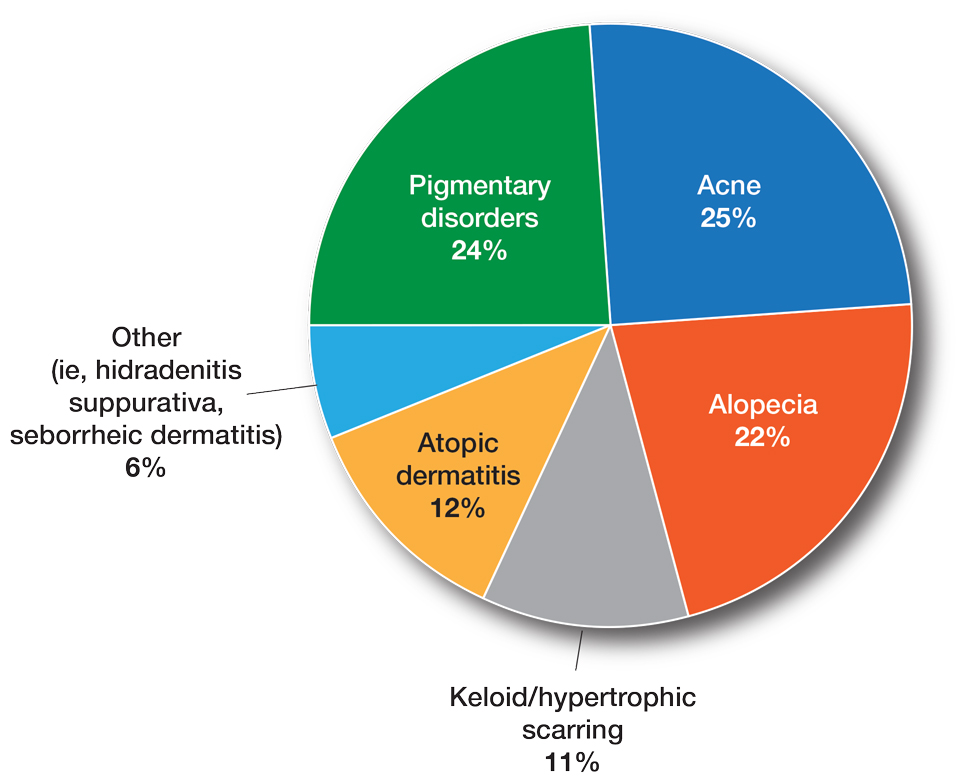
The average number of full-time faculty members at each SoCC was 1.6 (range, 1–4). The majority of program directors reported having other specialists in their department that also treated dermatologic conditions predominantly affecting patients with SoC (10/14 [71.43%]). The 3 most common areas of expertise were alopecia, including central centrifugal cicatricial alopecia (CCCA); cutaneous lupus; and traction alopecia (eTable 1).
Faculty SoC Research—Only a minority of programs had active clinical trials related to SoC (5/14 [35.71%]). Clinical research was the most common type of research being conducted (11/14 [78.57%]), followed by basic science/translational (4/14 [28.57%]) and epidemiologic research (2/14 [14.29%]). The most commonly investigated conditions for observational studies included CCCA, keloids/hypertrophic scarring, and atopic dermatitis (eTable 2). Only 8 of 14 programs had formal SoC research opportunities for residents (57.14%), while 9 had opportunities for medical students (64.29%).
Few institutions had internal funding (3/14 [21.43%]) or external funding (4/14 [28.57%]) for SoC research. Extramural fun ding sources included the Skin of Color Society, the Dermatology Foundation, and the Radiation Oncology Institute, as well as industry funding. No federal funding was received by any of the sites.
Skin of Color Education and Diversity Initiatives—All 14 programs had residents rotating through their SoCC and/or SoCSCs. The vast majority (12/14 [85.71%]) indicated resident exposure to clinical training at the SoCC and/or SoCSC during all 3 years of training. Residents at most of the programs spent 1 to 3 months rotating at the SoCC/SoCSC (6/14 [42.86%]). The other programs indicated residents spent 3 to 6 months (3/14 [21.43%]) or longer than 6 months (3/14 [21.4%]), and only 2 programs (14.29%) indicated that residents spent less than 1 month in the SoCC/SoCSC.
The majority of programs offered a SoC didactic curriculum for residents (10/14 [71.43%]), with an average of 3.3 SoC-related lectures per year (range, 0–5). Almost all programs (13/14 [92.86%]) invited SoC specialists from outside institutions as guest lecturers. Half of the programs (7/14 [50.0%]) used a SoC textbook for resident education. Only 3 programs (21.43%) offered at least 1 introductory SoC dermatology lecture as part of the preclinical medical student dermatology curriculum.
Home institution medical students were able to rotate at their respective SoCC/SoCSC at 11 of 14 institutions (78.57%), while visiting students were able to rotate at half of the programs (7/14 [50.0%]). At some programs, rotating at the SoCC/SoCSC was optional and was not formally integrated into the medical student rotation schedule for both home and visiting students (1/14 [7.14%] and 4/14 [28.57%], respectively). A majority of the programs (8/14 [57.14%]) offered scholarships and/or grants for home and/or visiting URiM students to help fund away rotations.
Despite their SoC focus, only half of the programs with SoCCs/SoCSCs had a formal committee focused on diversity and inclusion (7/14 [50.0%]) Additionally, only 5 of 14 (35.71%) programs had any URiM outreach programs with the medical school and/or the local community.
Comment
As the number of SoCCs/SoCSCs in the United States continues to grow, it is important to highlight their programmatic, research, and educational accomplishments to show the benefits of such programs, including their ability to increase access to culturally competent and inclusive care for diverse patient populations. One study found that nearly 92% of patients in the United States seen by dermatologists are White.15 Although studies have shown that Hispanic/Latino and Black patients are less likely to seek care from a dermatologist,16,17 there is no indication that these patients have a lesser need for such specialty care. Additionally, outcomes of common dermatologic conditions often are poorer in SoC populations.15 The dermatologists leading SoCCs/SoCSCs are actively working to reverse these trends, with Black and Hispanic/Latino patients representing the majority of their patients.
Faculty and Resident Demographics and Areas of Focus—Although there are increased diversity efforts in dermatology and the medical profession more broadly, there still is much work to be done. While individuals with SoC now comprise more than 35% of the US population, only 12% of dermatology residents and 6% of academic dermatology faculty identify as either Black or Hispanic/Latino.5,8,10 These numbers are even more discouraging when considering other URiM racial groups such as Pacific Islander/Native Hawaiians or Native American/American Indians who represent 0% and 0.1% of dermatology faculty, respectively.8,10 Academic programs with SoCCs/SoCSCs are working to create a space in which these discrepancies in representation can begin to be addressed. Compared to the national 6.8% rate of URiM faculty at academic institutions, those with SoCCs/SoCSCs report closer to 10% of faculty identifying as URiM.18 Moreover, almost all programs had faculty specialized in at least 1 condition that predominantly affects patients with SoC. This is of critical importance, as the conditions that most commonly affect SoC populations—such as CCCA, hidradenitis suppurativa, and cutaneous lupus—often are understudied, underfunded, underdiagnosed, and undertreated.19-22
Faculty SoC Research—An important step in narrowing the knowledge gap and improving health care disparities in patients with SoC is to increase SoC research and/or to increase the representation of patients with SoC in research studies. In a 2021 study, a PubMed search of articles indexed for MEDLINE using the terms race/ethnicity, dyschromia, atopic dermatitis, and acne was conducted to investigate publications pertaining to the top 3 most common chief concerns in patients with SoC. Only 1.6% of studies analyzed (N=74,941) had a specific focus on SoC.12 A similar study found that among the top 5 dermatology-focused research journals, only 3.4% of all research (N=11,003) on the top 3 most common chief concerns in patients with SOC was conducted in patients with SoC.23 Research efforts focused on dermatologic issues that affect patients with SoC are a priority at SoCCs/SoCSCs. In our study, all respondents indicated that they had at least 1 ongoing observational study; the most commonly studied conditions were CCCA, keloids/hypertrophic scarring, and atopic dermatitis, all of which are conditions that either occur in high frequency or primarily occur in SoC. Only 35.71% (5/14) of respondents had active clinical trials related to SoC, and only 21.43% (3/14) and 28.57% (4/14) had internal and external funding, respectively. Although research efforts are a priority at SoCCs/SoCSCs, our survey study highlights the continued paucity of formal clinical trials as well as funding for SoC-focused research. Improved research efforts for SoC must address these deficits in funding, academic support, and other resources.
It also is of great importance for institutions to provide support for trainees wanting to pursue SoC research. Encouragingly, more than half (57.14%) of SoCCs/SoCSCs have developed formal research opportunities for residents, and nearly 64.29% have formal opportunities for medical students. These efforts to provide early experiences in SoC research are especially impactful by cultivating interest in working with populations with SoC and hopefully inspiring future dermatologists to engage in further SoC research.
SoC Education and Diversity Initiatives—Although it is important to increase representation of URiM physicians in dermatology and to train more SoC specialists, it is imperative that all dermatologists feel comfortable recognizing and treating dermatologic conditions in patients of all skin tones and all racial/ethnic backgrounds; however, many studies suggest that residents not only lack formal didactics and education in SoC, but even more unsettling, they also lack confidence in treating SoC.13,24 However, one study showed that this can be changed; Mhlaba et al25 assessed a SoC curriculum for dermatology residents, and indeed all of the residents indicated that the curriculum improved their ability to treat SoC patients. This deficit in dermatology residency training is specifically addressed by SoCCs/SoCSCs. In our study, all respondents indicated that residents rotate through their centers. Moreover, our study found that most of the academic institutions with SoCCs/SoCSCs provide a SoC didactic curriculum for residents, and almost all of the programs invited SoC specialists to give guest lectures. This is in contrast to a 2022 study showing that 63.2% (N=125) of graduating dermatology residents reported receiving SoC-specific didactics, sessions, or lectures.14 These findings highlight the critical role that SoCCs/SoCSCs can provide in dermatology residency training.
Although SoCCs/SoCSCs have made considerable progress, there is still much room for improvement. Namely, only half of the respondents in our study indicated that their program has formally incorporated a SoC textbook into resident education (eTable 3). Representation of SoC in the textbooks that dermatology residents use is critically important because these images form the foundation of the morphologic aids of diagnosis. Numerous studies have analyzed popular dermatologic textbooks used by residency programs nationwide, finding the number of SoC images across dermatology textbooks ranging from 4% to 18%.26,27 The use of standard dermatology textbooks is not enough to train residents to be competent in diagnosing and treating patients with SoC. There should be a concerted effort across the field of dermatology to encourage the development of a SoC educational curriculum at every academic dermatology program, including SoC textbooks, Kodachromes, and online/electronic resources.
Efforts to increase diversity in dermatology and dermatologic training should start in medical school preclinical curriculums and medical student rotations. Although our survey did not assess current medical student curricula, the benefits of academic institutions with SoCCs/SoCSCs are highlighted by the ability for both home and visiting medical students to rotate through the centers and gain early exposure to SoC dermatology. Most of the programs even provide scholarships and/or grants for URiM students to help fund their rotations, which is of critical importance considering the mounting data that the financial burden of visiting rotations disproportionately affects URiM students.28
Study Limitations—Although we did an extensive search and believe to have correctly identified all 15 formal SoCCs/SoCSCs with a high response rate (93.3%), there are institutions that do not have formalized SoCCs/SoCSCs but are known to serve SoC populations. Likewise, there are private dermatology practices not associated with academic centers that have SoC specialists and positively contribute to SoC patient care, research, and education that were not included in this study. Additionally, the data for this study were collected in 2020 and analyzed in 2021, so it is possible that not all SoCCs, divisions, or clinics were included in this study, particularly if established after 2021.
Conclusion
As the United States continues to diversify, the proportion of patients with SoC will continue to grow, and it is imperative that this racial, ethnic, and cultural diversity is reflected in the dermatology workforce as well as research and training. The current deficits in medical training related to SoC populations and the importance for patients with SoC to find dermatologists who can appropriately treat them is well known.29 Skin of color centers/SoCSCs strive to increase access to care for patients with SoC, improve cultural competency, promote diversity among faculty and trainees, and encourage SoC research and education at all levels. We urge academic dermatology training programs to make SoC education, research, and patient care a departmental priority. Important first steps include departmental diversification at all levels, incorporating SoC into curricula for residents, providing and securing funding for SoC research, and supporting the establishment of more formal SoCCs and/or SoCSCs to help reduce dermatologic health care disparities among patients with SoC and improve health equity.
Appendix
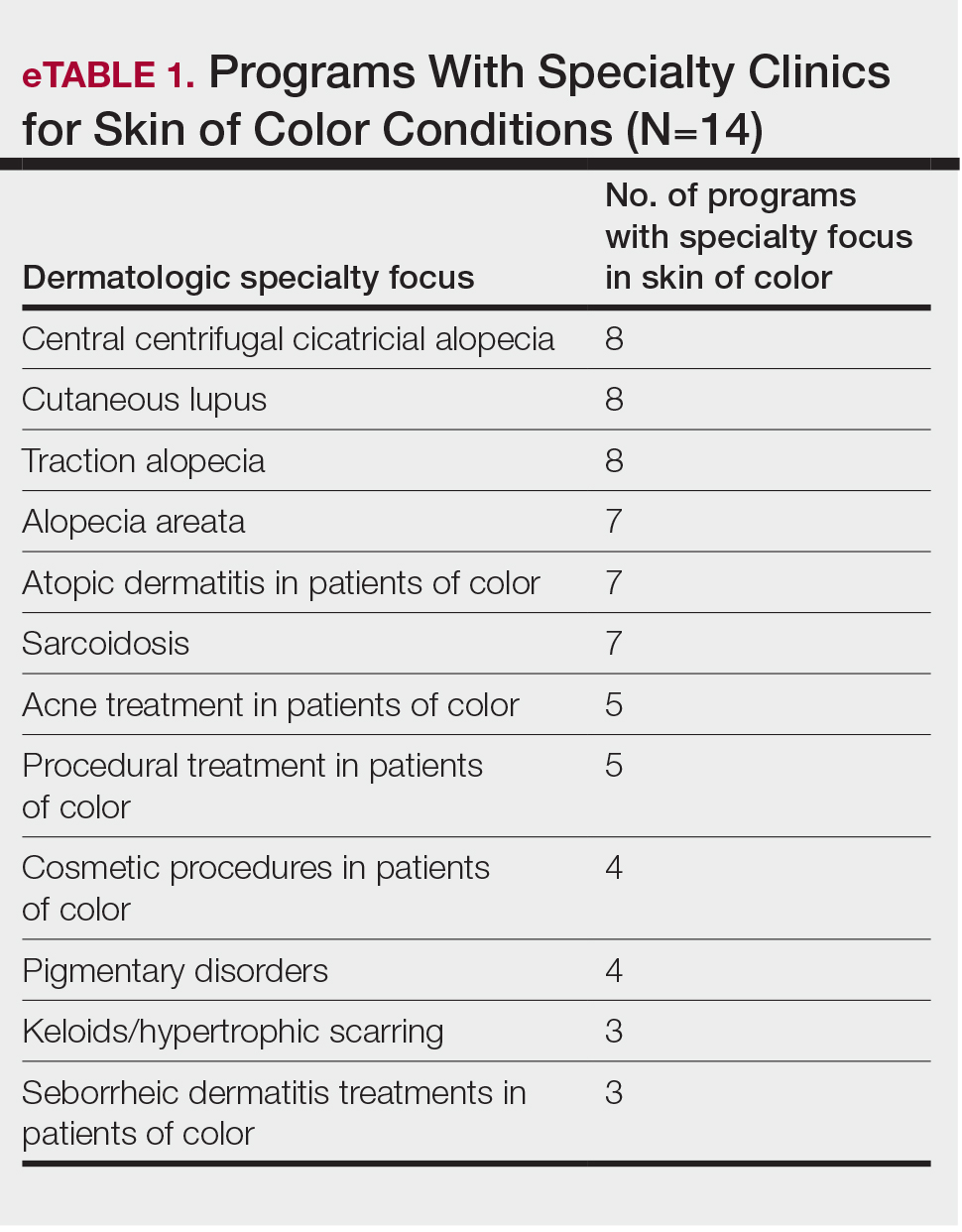
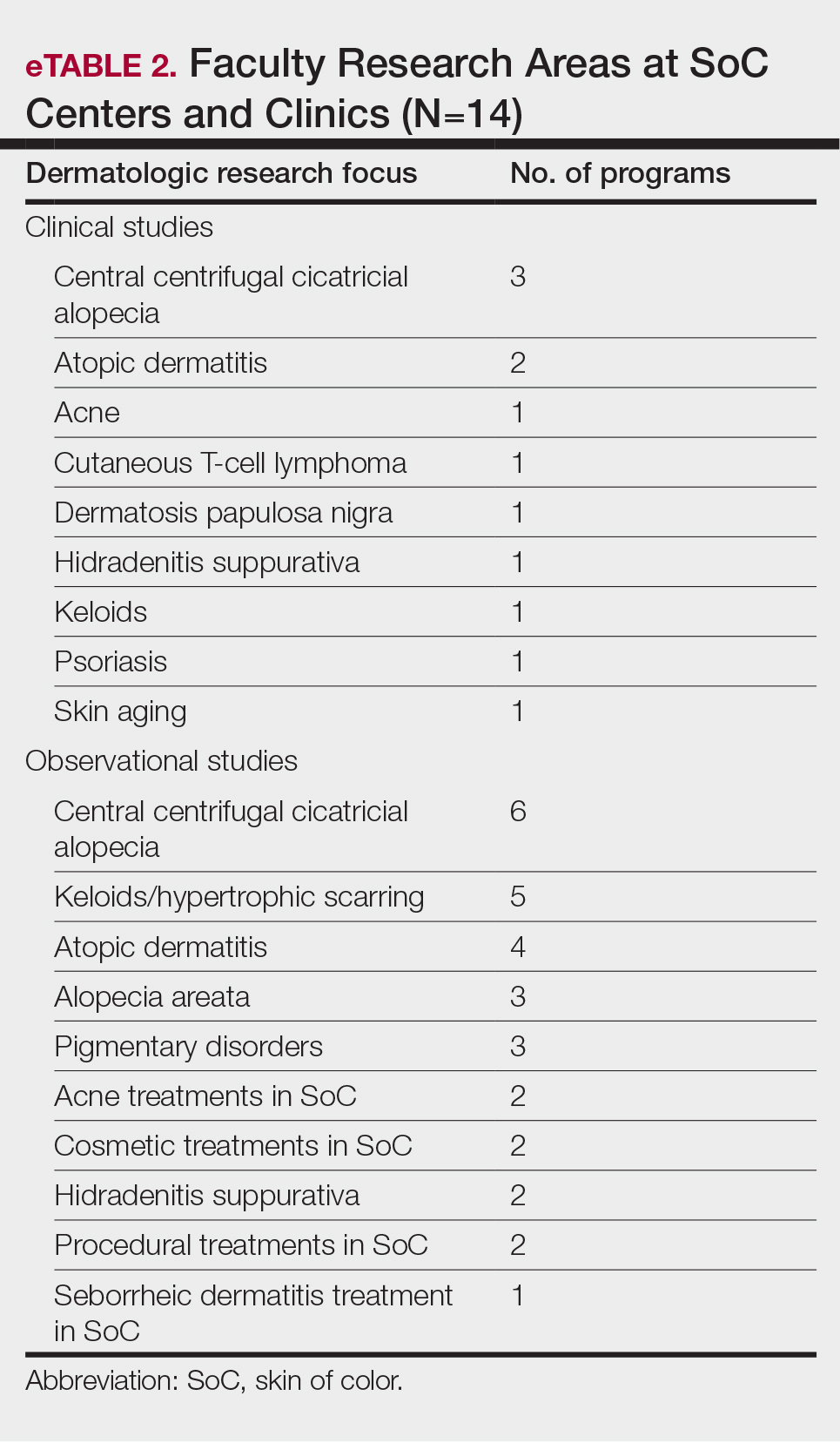

- Colby SL, Jennifer JM. Projections of the size and composition of the U.S. population: 2014 to 2060. United States Census Bureau website. March 3, 2015. Accessed June 18, 2024. https://www.census.gov/library/publications/2015/demo/p25-1143.html
- Tull RZ, Kerby E, Subash JJ, et al. Ethnic skin centers in the United States: where are we in 2020? J Am Acad Dermatol. 2020;83:1757-1759. doi:10.1016/j.jaad.2020.03.054
- Shen MJ, Peterson EB, Costas-Muñiz R, et al. The effects of race and racial concordance on patient-physician communication: a systematic review of the literature. J Racial Ethn Health Disparities. 2018;5:117-140. doi:10.1007/s40615-017-0350-4
- Saha S, Beach MC. Impact of physician race on patient decision-making and ratings of physicians: a randomized experiment using video vignettes. J Gen Intern Med. 2020;35:1084-1091. doi:10.1007/s11606-020-05646-z
- Quick Facts: United States. US Census Bureau website. Accessed June 18, 2024. https://www.census.gov/quickfacts/fact/table/US/PST045221
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587. doi:10.1016/j.jaad.2015.10.044
- Van Voorhees AS, Enos CW. Diversity in dermatology residency programs. J Investig Dermatol Symp Proc. 2017;18:S46-S49. doi:10.1016/j.jisp.2017.07.001
- Association of American Medical Colleges. Table B5. number of active MD residents, by race/ethnicity (alone or in combination) and GME specialty. Accessed June 18, 2024. https://www.aamc.org/data-reports/students-residents/interactive-data/report-residents/2022/table-b5-md-residents-race-ethnicity-and-specialty
- Association of American Medical Colleges. Table B6. number of active DO residents, by race/ethnicity (alone or in combination) and GME specialty. Accessed June 18, 2024. https://www.aamc.org/data-reports/students-residents/interactive-data/report-residents/2022/table-b6-do-residents-race-ethnicity-and-specialty
- Association of American Medical Colleges. Table 16. U.S. medical school faculty by gender, race/ethnicity, and department, 2022. Accessed June 24, 2024. https://www.aamc.org/media/8456/download
- Chen V, Akhtar S, Zheng C, et al. Assessment of changes in diversity in dermatology clinical trials between 2010-2015 and 2015-2020: a systematic review. JAMA Dermatol. 2022;158:288-292. doi:10.1001/jamadermatol.2021.5596
- Montgomery SNB, Elbuluk N. A quantitative analysis of research publications focused on the top chief complaints in patients withskinof color. J Am Acad Dermatol. 2021;85:241-242. doi:10.1016/j.jaad.2020.08.031
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618. doi:10.1016/j.jaad.2008.06.024
- Ibraheim MK, Gupta R, Dao H, et al. Evaluating skin of color education in dermatology residency programs: data from a national survey. Clin Dermatol. 2022;40:228-233. doi:10.1016/j.clindermatol.2021.11.015
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59, viii. doi:10.1016/j.det.2011.08.002
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291. doi:10.1001/jamadermatol.2018.3114
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 202;156:312-319. doi:10.1001/jamadermatol.2019.4818
- Dlova NC, Salkey KS, Callender VD, et al. Central centrifugal cicatricial alopecia: new insights and a call for action. J Investig Dermatol Symp Proc. 2017;18:S54-S56. doi:10.1016/j.jisp.2017.01.004
- Okeke CAV, Perry JD, Simmonds FC, et al. Clinical trials and skin of color: the example of hidradenitis suppurativa. dermatology. 2022;238:180-184. doi:10.1159/000516467
- Robles J, Anim T, Wusu MH, et al. An Approach to Faculty Development for Underrepresented Minorities in Medicine. South Med J. 2021;114(9):579-582. doi:10.14423/SMJ.0000000000001290
- Serrano L, Ulschmid C, Szabo A, et al. Racial disparities of delay in diagnosis and dermatologic care for hidradenitis suppurativa. J Natl Med Assoc. 2022;114:613-616. doi:10.1016/j.jnma.2022.08.002
- Drenkard C, Lim SS. Update on lupus epidemiology: advancinghealth disparities research through the study of minority populations. Curr Opin Rheumatol. 2019;31:689-696. doi:10.1097/BOR.0000000000000646
- Militello M, Szeto MD, Presley CL, et al. A quantitative analysis of research publications focused on skin of color: representation in academic dermatology journals. J Am Acad Dermatol. 2021;85:E189-E192. doi:10.1016/j.jaad.2021.04.053
- Cline A, Winter RP, Kourosh S, et al. Multiethnic training in residency: a survey of dermatology residents. Cutis. 2020;105:310-313.
- Mhlaba JM, Pontes DS, Patterson SS, et al. Evaluation of a skin of color curriculum for dermatology residents. J Drugs Dermatol. 2021;20:786-789. doi:10.36849/JDD.6193
- Adelekun A, Onyekaba G, Lipoff JB. Skin color in dermatology textbooks: an updated evaluation and analysis. J Am Acad Dermatol. 2021;84:194-196. doi:10.1016/j.jaad.2020.04.084
- Harp T, Militello M, McCarver V, et al. Further analysis of skin of color representation in dermatology textbooks used by residents. J Am Acad Dermatol. 2022;87:E39-E41. doi:10.1016/j.jaad.2022.02.069
- Muzumdar S, Grant-Kels JM, Feng H. Strategies to improve medical student visiting rotations. Clin Dermatol. 2021;39:727-728. doi:10.1016/j.clindermatol.2020.11.001
- Gorbatenko-Roth K, Prose N, Kundu RV, et al. Assessment of Black patients’ perception of their dermatology care. JAMA Dermatol. 2019;155:1129-1134. doi:10.1001/jamadermatol.2019.2063
Although individuals with skin of color (SoC) are expected to become at least half of the US population by the year 2044, there remains a paucity of education and exposure to treatment of patients with SoC at many dermatology residency programs across the country.1 One way to improve SoC education has been the formation of specialized clinics, centers, and programs. The first SoC center (SoCC) was established in 1999 at Mount Sinai–St. Luke’s Roosevelt in New York, New York2; since then, at least 13 additional formal SoCCs or SoC specialty clinics (SoCSCs) at US academic dermatology programs have been established.
Skin of color centers serve several important purposes: they improve dermatologic care in patients with SoC, increase research efforts focused on SoC dermatologic conditions, and educate dermatology resident and fellow trainees about SoC. Improving dermatologic care of patients with SoC in the United States is important in providing equitable health care and improving health disparities. Studies have shown that patient-physician racial and cultural concordance can positively impact patient care, increase patient trust and rapport, and improve patient-physician communication, and it can even influence patient decision-making to seek care.3,4 Unfortunately, even though the US population continues to diversify, the racial/ethnic backgrounds of dermatologists do not parallel this trend; Hispanic and Black physicians comprise 18.9% and 13.6% of the general population, respectively, but represent only 4.2% and 3.0% of dermatologists, respectively.5-7 This deficit is mirrored by resident and faculty representation, with Black and Latino representation ranging from 3% to 7%.8-10
Many SoCC’s engage in research focused on dermatologic conditions affecting patients with SoC, which is vital to improving the dermatologic care in this underserved population. Despite increasing recognition of the importance of SoC research, there remains a paucity of clinical trials and research specifically focused on or demonstrating equitable representation of SoC.11,12
The education and training of future dermatologists is another important area that can be improved by SoCCs. A 2008 study involving 63 chief residents showed that approximately half (52.4% [33/63]) of graduating dermatology residents reported receiving SoC-specific didactics, sessions, or lectures, and 30.2% (19/63) reported having a dedicated rotation where they gained specific experience treating patients with SoC.13 A later study in 2022 (N=125) found that 63.2% of graduating dermatology residents reported receiving SoC-specific didactics, sessions, or lectures, and only 11.2% reported having a dedicated rotation where they gained experience treating patients with SoC.14 These findings suggest that in the last 14 years, formal SoC education—specifically SoC clinical training—has not increased sufficiently.
We conducted a cross-sectional survey study to provide an in-depth analysis of SoCCs and SoCSCs in the United States, including their patient care focus, research, and program diversity.
Methods
We conducted an investigator-initiated, multicenter, cross-sectional survey study of all SoCCs in the United States and their respective academic residency programs. Fifteen formal SoCCs and/or SoCSCs were identified by dermatology program websites and an article by Tull et al2 on the state of ethnic skin centers. All programs and centers identified were associated with a dermatology residency program accredited by the Accreditation Council for Graduate Medical Education.
A 42-item questionnaire was sent via email to the directors of these centers and clinics with the intent to collect descriptive information about each of the SoCCs, the diversity of the faculty and residents of the associated dermatology department, current research and funding, diversity and inclusion initiatives, and trainee education from March through April 2020. Data were analyzed using Excel and SPSS statistical software to obtain descriptive statistics including the mean value numeric trends across programs.
This study underwent expedited review and was approved by the University of Southern California (Los Angeles, California) institutional review board (IRB #HS-20-00113). Patient consent was not applicable, as no information was collected about patients.
Results
Fourteen directors from SoCCs/SoCSCs completed the questionnaire (93.3% response rate). Most centers were located in urban areas (12/14 [85.71%]), except for 2 in rural or suburban settings (Table). Most of the SoCCs/SoCSCs were located in the South (5/14 [35.71%]), followed by the Northeast (4/14 [28.57%]), West (3/14 [21.43%]), and Midwest (2/14 [14.29%])(Table). Six (42.86%) of the programs had a SoCSC, 3 (21.43%) had a formal SoCC, and 5 (35.71%) had both. Across all centers, the most common population seen and treated was Black/African American followed by Hispanic/Latino and Asian, respectively. The most commonly seen dermatologic conditions were acne, pigmentary disorders, alopecia, and atopic dermatitis (Figure). The most common cosmetic practice performed for patients with SoC was dermatosis papulosa nigra/seborrheic keratosis removal, followed by laser treatments, skin tag removal, chemical peels, and neuromodulator injections, respectively.

Faculty and Resident Demographics and Areas of Focus—The demographics and diversity of the dermatology faculty and residents at each individual institution also were assessed. The average number of full-time faculty at each institution was 19.4 (range, 2–48), while the average number of full-time faculty who identified as underrepresented in medicine (URiM) was 2.1 (range, 0–5). The average number of residents at each institution was 17.1 (range, 10–31), while the average number of URiM residents was 1.7 (range, 1–3).

The average number of full-time faculty members at each SoCC was 1.6 (range, 1–4). The majority of program directors reported having other specialists in their department that also treated dermatologic conditions predominantly affecting patients with SoC (10/14 [71.43%]). The 3 most common areas of expertise were alopecia, including central centrifugal cicatricial alopecia (CCCA); cutaneous lupus; and traction alopecia (eTable 1).
Faculty SoC Research—Only a minority of programs had active clinical trials related to SoC (5/14 [35.71%]). Clinical research was the most common type of research being conducted (11/14 [78.57%]), followed by basic science/translational (4/14 [28.57%]) and epidemiologic research (2/14 [14.29%]). The most commonly investigated conditions for observational studies included CCCA, keloids/hypertrophic scarring, and atopic dermatitis (eTable 2). Only 8 of 14 programs had formal SoC research opportunities for residents (57.14%), while 9 had opportunities for medical students (64.29%).
Few institutions had internal funding (3/14 [21.43%]) or external funding (4/14 [28.57%]) for SoC research. Extramural fun ding sources included the Skin of Color Society, the Dermatology Foundation, and the Radiation Oncology Institute, as well as industry funding. No federal funding was received by any of the sites.
Skin of Color Education and Diversity Initiatives—All 14 programs had residents rotating through their SoCC and/or SoCSCs. The vast majority (12/14 [85.71%]) indicated resident exposure to clinical training at the SoCC and/or SoCSC during all 3 years of training. Residents at most of the programs spent 1 to 3 months rotating at the SoCC/SoCSC (6/14 [42.86%]). The other programs indicated residents spent 3 to 6 months (3/14 [21.43%]) or longer than 6 months (3/14 [21.4%]), and only 2 programs (14.29%) indicated that residents spent less than 1 month in the SoCC/SoCSC.
The majority of programs offered a SoC didactic curriculum for residents (10/14 [71.43%]), with an average of 3.3 SoC-related lectures per year (range, 0–5). Almost all programs (13/14 [92.86%]) invited SoC specialists from outside institutions as guest lecturers. Half of the programs (7/14 [50.0%]) used a SoC textbook for resident education. Only 3 programs (21.43%) offered at least 1 introductory SoC dermatology lecture as part of the preclinical medical student dermatology curriculum.
Home institution medical students were able to rotate at their respective SoCC/SoCSC at 11 of 14 institutions (78.57%), while visiting students were able to rotate at half of the programs (7/14 [50.0%]). At some programs, rotating at the SoCC/SoCSC was optional and was not formally integrated into the medical student rotation schedule for both home and visiting students (1/14 [7.14%] and 4/14 [28.57%], respectively). A majority of the programs (8/14 [57.14%]) offered scholarships and/or grants for home and/or visiting URiM students to help fund away rotations.
Despite their SoC focus, only half of the programs with SoCCs/SoCSCs had a formal committee focused on diversity and inclusion (7/14 [50.0%]) Additionally, only 5 of 14 (35.71%) programs had any URiM outreach programs with the medical school and/or the local community.
Comment
As the number of SoCCs/SoCSCs in the United States continues to grow, it is important to highlight their programmatic, research, and educational accomplishments to show the benefits of such programs, including their ability to increase access to culturally competent and inclusive care for diverse patient populations. One study found that nearly 92% of patients in the United States seen by dermatologists are White.15 Although studies have shown that Hispanic/Latino and Black patients are less likely to seek care from a dermatologist,16,17 there is no indication that these patients have a lesser need for such specialty care. Additionally, outcomes of common dermatologic conditions often are poorer in SoC populations.15 The dermatologists leading SoCCs/SoCSCs are actively working to reverse these trends, with Black and Hispanic/Latino patients representing the majority of their patients.
Faculty and Resident Demographics and Areas of Focus—Although there are increased diversity efforts in dermatology and the medical profession more broadly, there still is much work to be done. While individuals with SoC now comprise more than 35% of the US population, only 12% of dermatology residents and 6% of academic dermatology faculty identify as either Black or Hispanic/Latino.5,8,10 These numbers are even more discouraging when considering other URiM racial groups such as Pacific Islander/Native Hawaiians or Native American/American Indians who represent 0% and 0.1% of dermatology faculty, respectively.8,10 Academic programs with SoCCs/SoCSCs are working to create a space in which these discrepancies in representation can begin to be addressed. Compared to the national 6.8% rate of URiM faculty at academic institutions, those with SoCCs/SoCSCs report closer to 10% of faculty identifying as URiM.18 Moreover, almost all programs had faculty specialized in at least 1 condition that predominantly affects patients with SoC. This is of critical importance, as the conditions that most commonly affect SoC populations—such as CCCA, hidradenitis suppurativa, and cutaneous lupus—often are understudied, underfunded, underdiagnosed, and undertreated.19-22
Faculty SoC Research—An important step in narrowing the knowledge gap and improving health care disparities in patients with SoC is to increase SoC research and/or to increase the representation of patients with SoC in research studies. In a 2021 study, a PubMed search of articles indexed for MEDLINE using the terms race/ethnicity, dyschromia, atopic dermatitis, and acne was conducted to investigate publications pertaining to the top 3 most common chief concerns in patients with SoC. Only 1.6% of studies analyzed (N=74,941) had a specific focus on SoC.12 A similar study found that among the top 5 dermatology-focused research journals, only 3.4% of all research (N=11,003) on the top 3 most common chief concerns in patients with SOC was conducted in patients with SoC.23 Research efforts focused on dermatologic issues that affect patients with SoC are a priority at SoCCs/SoCSCs. In our study, all respondents indicated that they had at least 1 ongoing observational study; the most commonly studied conditions were CCCA, keloids/hypertrophic scarring, and atopic dermatitis, all of which are conditions that either occur in high frequency or primarily occur in SoC. Only 35.71% (5/14) of respondents had active clinical trials related to SoC, and only 21.43% (3/14) and 28.57% (4/14) had internal and external funding, respectively. Although research efforts are a priority at SoCCs/SoCSCs, our survey study highlights the continued paucity of formal clinical trials as well as funding for SoC-focused research. Improved research efforts for SoC must address these deficits in funding, academic support, and other resources.
It also is of great importance for institutions to provide support for trainees wanting to pursue SoC research. Encouragingly, more than half (57.14%) of SoCCs/SoCSCs have developed formal research opportunities for residents, and nearly 64.29% have formal opportunities for medical students. These efforts to provide early experiences in SoC research are especially impactful by cultivating interest in working with populations with SoC and hopefully inspiring future dermatologists to engage in further SoC research.
SoC Education and Diversity Initiatives—Although it is important to increase representation of URiM physicians in dermatology and to train more SoC specialists, it is imperative that all dermatologists feel comfortable recognizing and treating dermatologic conditions in patients of all skin tones and all racial/ethnic backgrounds; however, many studies suggest that residents not only lack formal didactics and education in SoC, but even more unsettling, they also lack confidence in treating SoC.13,24 However, one study showed that this can be changed; Mhlaba et al25 assessed a SoC curriculum for dermatology residents, and indeed all of the residents indicated that the curriculum improved their ability to treat SoC patients. This deficit in dermatology residency training is specifically addressed by SoCCs/SoCSCs. In our study, all respondents indicated that residents rotate through their centers. Moreover, our study found that most of the academic institutions with SoCCs/SoCSCs provide a SoC didactic curriculum for residents, and almost all of the programs invited SoC specialists to give guest lectures. This is in contrast to a 2022 study showing that 63.2% (N=125) of graduating dermatology residents reported receiving SoC-specific didactics, sessions, or lectures.14 These findings highlight the critical role that SoCCs/SoCSCs can provide in dermatology residency training.
Although SoCCs/SoCSCs have made considerable progress, there is still much room for improvement. Namely, only half of the respondents in our study indicated that their program has formally incorporated a SoC textbook into resident education (eTable 3). Representation of SoC in the textbooks that dermatology residents use is critically important because these images form the foundation of the morphologic aids of diagnosis. Numerous studies have analyzed popular dermatologic textbooks used by residency programs nationwide, finding the number of SoC images across dermatology textbooks ranging from 4% to 18%.26,27 The use of standard dermatology textbooks is not enough to train residents to be competent in diagnosing and treating patients with SoC. There should be a concerted effort across the field of dermatology to encourage the development of a SoC educational curriculum at every academic dermatology program, including SoC textbooks, Kodachromes, and online/electronic resources.
Efforts to increase diversity in dermatology and dermatologic training should start in medical school preclinical curriculums and medical student rotations. Although our survey did not assess current medical student curricula, the benefits of academic institutions with SoCCs/SoCSCs are highlighted by the ability for both home and visiting medical students to rotate through the centers and gain early exposure to SoC dermatology. Most of the programs even provide scholarships and/or grants for URiM students to help fund their rotations, which is of critical importance considering the mounting data that the financial burden of visiting rotations disproportionately affects URiM students.28
Study Limitations—Although we did an extensive search and believe to have correctly identified all 15 formal SoCCs/SoCSCs with a high response rate (93.3%), there are institutions that do not have formalized SoCCs/SoCSCs but are known to serve SoC populations. Likewise, there are private dermatology practices not associated with academic centers that have SoC specialists and positively contribute to SoC patient care, research, and education that were not included in this study. Additionally, the data for this study were collected in 2020 and analyzed in 2021, so it is possible that not all SoCCs, divisions, or clinics were included in this study, particularly if established after 2021.
Conclusion
As the United States continues to diversify, the proportion of patients with SoC will continue to grow, and it is imperative that this racial, ethnic, and cultural diversity is reflected in the dermatology workforce as well as research and training. The current deficits in medical training related to SoC populations and the importance for patients with SoC to find dermatologists who can appropriately treat them is well known.29 Skin of color centers/SoCSCs strive to increase access to care for patients with SoC, improve cultural competency, promote diversity among faculty and trainees, and encourage SoC research and education at all levels. We urge academic dermatology training programs to make SoC education, research, and patient care a departmental priority. Important first steps include departmental diversification at all levels, incorporating SoC into curricula for residents, providing and securing funding for SoC research, and supporting the establishment of more formal SoCCs and/or SoCSCs to help reduce dermatologic health care disparities among patients with SoC and improve health equity.
Appendix



Although individuals with skin of color (SoC) are expected to become at least half of the US population by the year 2044, there remains a paucity of education and exposure to treatment of patients with SoC at many dermatology residency programs across the country.1 One way to improve SoC education has been the formation of specialized clinics, centers, and programs. The first SoC center (SoCC) was established in 1999 at Mount Sinai–St. Luke’s Roosevelt in New York, New York2; since then, at least 13 additional formal SoCCs or SoC specialty clinics (SoCSCs) at US academic dermatology programs have been established.
Skin of color centers serve several important purposes: they improve dermatologic care in patients with SoC, increase research efforts focused on SoC dermatologic conditions, and educate dermatology resident and fellow trainees about SoC. Improving dermatologic care of patients with SoC in the United States is important in providing equitable health care and improving health disparities. Studies have shown that patient-physician racial and cultural concordance can positively impact patient care, increase patient trust and rapport, and improve patient-physician communication, and it can even influence patient decision-making to seek care.3,4 Unfortunately, even though the US population continues to diversify, the racial/ethnic backgrounds of dermatologists do not parallel this trend; Hispanic and Black physicians comprise 18.9% and 13.6% of the general population, respectively, but represent only 4.2% and 3.0% of dermatologists, respectively.5-7 This deficit is mirrored by resident and faculty representation, with Black and Latino representation ranging from 3% to 7%.8-10
Many SoCC’s engage in research focused on dermatologic conditions affecting patients with SoC, which is vital to improving the dermatologic care in this underserved population. Despite increasing recognition of the importance of SoC research, there remains a paucity of clinical trials and research specifically focused on or demonstrating equitable representation of SoC.11,12
The education and training of future dermatologists is another important area that can be improved by SoCCs. A 2008 study involving 63 chief residents showed that approximately half (52.4% [33/63]) of graduating dermatology residents reported receiving SoC-specific didactics, sessions, or lectures, and 30.2% (19/63) reported having a dedicated rotation where they gained specific experience treating patients with SoC.13 A later study in 2022 (N=125) found that 63.2% of graduating dermatology residents reported receiving SoC-specific didactics, sessions, or lectures, and only 11.2% reported having a dedicated rotation where they gained experience treating patients with SoC.14 These findings suggest that in the last 14 years, formal SoC education—specifically SoC clinical training—has not increased sufficiently.
We conducted a cross-sectional survey study to provide an in-depth analysis of SoCCs and SoCSCs in the United States, including their patient care focus, research, and program diversity.
Methods
We conducted an investigator-initiated, multicenter, cross-sectional survey study of all SoCCs in the United States and their respective academic residency programs. Fifteen formal SoCCs and/or SoCSCs were identified by dermatology program websites and an article by Tull et al2 on the state of ethnic skin centers. All programs and centers identified were associated with a dermatology residency program accredited by the Accreditation Council for Graduate Medical Education.
A 42-item questionnaire was sent via email to the directors of these centers and clinics with the intent to collect descriptive information about each of the SoCCs, the diversity of the faculty and residents of the associated dermatology department, current research and funding, diversity and inclusion initiatives, and trainee education from March through April 2020. Data were analyzed using Excel and SPSS statistical software to obtain descriptive statistics including the mean value numeric trends across programs.
This study underwent expedited review and was approved by the University of Southern California (Los Angeles, California) institutional review board (IRB #HS-20-00113). Patient consent was not applicable, as no information was collected about patients.
Results
Fourteen directors from SoCCs/SoCSCs completed the questionnaire (93.3% response rate). Most centers were located in urban areas (12/14 [85.71%]), except for 2 in rural or suburban settings (Table). Most of the SoCCs/SoCSCs were located in the South (5/14 [35.71%]), followed by the Northeast (4/14 [28.57%]), West (3/14 [21.43%]), and Midwest (2/14 [14.29%])(Table). Six (42.86%) of the programs had a SoCSC, 3 (21.43%) had a formal SoCC, and 5 (35.71%) had both. Across all centers, the most common population seen and treated was Black/African American followed by Hispanic/Latino and Asian, respectively. The most commonly seen dermatologic conditions were acne, pigmentary disorders, alopecia, and atopic dermatitis (Figure). The most common cosmetic practice performed for patients with SoC was dermatosis papulosa nigra/seborrheic keratosis removal, followed by laser treatments, skin tag removal, chemical peels, and neuromodulator injections, respectively.

Faculty and Resident Demographics and Areas of Focus—The demographics and diversity of the dermatology faculty and residents at each individual institution also were assessed. The average number of full-time faculty at each institution was 19.4 (range, 2–48), while the average number of full-time faculty who identified as underrepresented in medicine (URiM) was 2.1 (range, 0–5). The average number of residents at each institution was 17.1 (range, 10–31), while the average number of URiM residents was 1.7 (range, 1–3).

The average number of full-time faculty members at each SoCC was 1.6 (range, 1–4). The majority of program directors reported having other specialists in their department that also treated dermatologic conditions predominantly affecting patients with SoC (10/14 [71.43%]). The 3 most common areas of expertise were alopecia, including central centrifugal cicatricial alopecia (CCCA); cutaneous lupus; and traction alopecia (eTable 1).
Faculty SoC Research—Only a minority of programs had active clinical trials related to SoC (5/14 [35.71%]). Clinical research was the most common type of research being conducted (11/14 [78.57%]), followed by basic science/translational (4/14 [28.57%]) and epidemiologic research (2/14 [14.29%]). The most commonly investigated conditions for observational studies included CCCA, keloids/hypertrophic scarring, and atopic dermatitis (eTable 2). Only 8 of 14 programs had formal SoC research opportunities for residents (57.14%), while 9 had opportunities for medical students (64.29%).
Few institutions had internal funding (3/14 [21.43%]) or external funding (4/14 [28.57%]) for SoC research. Extramural fun ding sources included the Skin of Color Society, the Dermatology Foundation, and the Radiation Oncology Institute, as well as industry funding. No federal funding was received by any of the sites.
Skin of Color Education and Diversity Initiatives—All 14 programs had residents rotating through their SoCC and/or SoCSCs. The vast majority (12/14 [85.71%]) indicated resident exposure to clinical training at the SoCC and/or SoCSC during all 3 years of training. Residents at most of the programs spent 1 to 3 months rotating at the SoCC/SoCSC (6/14 [42.86%]). The other programs indicated residents spent 3 to 6 months (3/14 [21.43%]) or longer than 6 months (3/14 [21.4%]), and only 2 programs (14.29%) indicated that residents spent less than 1 month in the SoCC/SoCSC.
The majority of programs offered a SoC didactic curriculum for residents (10/14 [71.43%]), with an average of 3.3 SoC-related lectures per year (range, 0–5). Almost all programs (13/14 [92.86%]) invited SoC specialists from outside institutions as guest lecturers. Half of the programs (7/14 [50.0%]) used a SoC textbook for resident education. Only 3 programs (21.43%) offered at least 1 introductory SoC dermatology lecture as part of the preclinical medical student dermatology curriculum.
Home institution medical students were able to rotate at their respective SoCC/SoCSC at 11 of 14 institutions (78.57%), while visiting students were able to rotate at half of the programs (7/14 [50.0%]). At some programs, rotating at the SoCC/SoCSC was optional and was not formally integrated into the medical student rotation schedule for both home and visiting students (1/14 [7.14%] and 4/14 [28.57%], respectively). A majority of the programs (8/14 [57.14%]) offered scholarships and/or grants for home and/or visiting URiM students to help fund away rotations.
Despite their SoC focus, only half of the programs with SoCCs/SoCSCs had a formal committee focused on diversity and inclusion (7/14 [50.0%]) Additionally, only 5 of 14 (35.71%) programs had any URiM outreach programs with the medical school and/or the local community.
Comment
As the number of SoCCs/SoCSCs in the United States continues to grow, it is important to highlight their programmatic, research, and educational accomplishments to show the benefits of such programs, including their ability to increase access to culturally competent and inclusive care for diverse patient populations. One study found that nearly 92% of patients in the United States seen by dermatologists are White.15 Although studies have shown that Hispanic/Latino and Black patients are less likely to seek care from a dermatologist,16,17 there is no indication that these patients have a lesser need for such specialty care. Additionally, outcomes of common dermatologic conditions often are poorer in SoC populations.15 The dermatologists leading SoCCs/SoCSCs are actively working to reverse these trends, with Black and Hispanic/Latino patients representing the majority of their patients.
Faculty and Resident Demographics and Areas of Focus—Although there are increased diversity efforts in dermatology and the medical profession more broadly, there still is much work to be done. While individuals with SoC now comprise more than 35% of the US population, only 12% of dermatology residents and 6% of academic dermatology faculty identify as either Black or Hispanic/Latino.5,8,10 These numbers are even more discouraging when considering other URiM racial groups such as Pacific Islander/Native Hawaiians or Native American/American Indians who represent 0% and 0.1% of dermatology faculty, respectively.8,10 Academic programs with SoCCs/SoCSCs are working to create a space in which these discrepancies in representation can begin to be addressed. Compared to the national 6.8% rate of URiM faculty at academic institutions, those with SoCCs/SoCSCs report closer to 10% of faculty identifying as URiM.18 Moreover, almost all programs had faculty specialized in at least 1 condition that predominantly affects patients with SoC. This is of critical importance, as the conditions that most commonly affect SoC populations—such as CCCA, hidradenitis suppurativa, and cutaneous lupus—often are understudied, underfunded, underdiagnosed, and undertreated.19-22
Faculty SoC Research—An important step in narrowing the knowledge gap and improving health care disparities in patients with SoC is to increase SoC research and/or to increase the representation of patients with SoC in research studies. In a 2021 study, a PubMed search of articles indexed for MEDLINE using the terms race/ethnicity, dyschromia, atopic dermatitis, and acne was conducted to investigate publications pertaining to the top 3 most common chief concerns in patients with SoC. Only 1.6% of studies analyzed (N=74,941) had a specific focus on SoC.12 A similar study found that among the top 5 dermatology-focused research journals, only 3.4% of all research (N=11,003) on the top 3 most common chief concerns in patients with SOC was conducted in patients with SoC.23 Research efforts focused on dermatologic issues that affect patients with SoC are a priority at SoCCs/SoCSCs. In our study, all respondents indicated that they had at least 1 ongoing observational study; the most commonly studied conditions were CCCA, keloids/hypertrophic scarring, and atopic dermatitis, all of which are conditions that either occur in high frequency or primarily occur in SoC. Only 35.71% (5/14) of respondents had active clinical trials related to SoC, and only 21.43% (3/14) and 28.57% (4/14) had internal and external funding, respectively. Although research efforts are a priority at SoCCs/SoCSCs, our survey study highlights the continued paucity of formal clinical trials as well as funding for SoC-focused research. Improved research efforts for SoC must address these deficits in funding, academic support, and other resources.
It also is of great importance for institutions to provide support for trainees wanting to pursue SoC research. Encouragingly, more than half (57.14%) of SoCCs/SoCSCs have developed formal research opportunities for residents, and nearly 64.29% have formal opportunities for medical students. These efforts to provide early experiences in SoC research are especially impactful by cultivating interest in working with populations with SoC and hopefully inspiring future dermatologists to engage in further SoC research.
SoC Education and Diversity Initiatives—Although it is important to increase representation of URiM physicians in dermatology and to train more SoC specialists, it is imperative that all dermatologists feel comfortable recognizing and treating dermatologic conditions in patients of all skin tones and all racial/ethnic backgrounds; however, many studies suggest that residents not only lack formal didactics and education in SoC, but even more unsettling, they also lack confidence in treating SoC.13,24 However, one study showed that this can be changed; Mhlaba et al25 assessed a SoC curriculum for dermatology residents, and indeed all of the residents indicated that the curriculum improved their ability to treat SoC patients. This deficit in dermatology residency training is specifically addressed by SoCCs/SoCSCs. In our study, all respondents indicated that residents rotate through their centers. Moreover, our study found that most of the academic institutions with SoCCs/SoCSCs provide a SoC didactic curriculum for residents, and almost all of the programs invited SoC specialists to give guest lectures. This is in contrast to a 2022 study showing that 63.2% (N=125) of graduating dermatology residents reported receiving SoC-specific didactics, sessions, or lectures.14 These findings highlight the critical role that SoCCs/SoCSCs can provide in dermatology residency training.
Although SoCCs/SoCSCs have made considerable progress, there is still much room for improvement. Namely, only half of the respondents in our study indicated that their program has formally incorporated a SoC textbook into resident education (eTable 3). Representation of SoC in the textbooks that dermatology residents use is critically important because these images form the foundation of the morphologic aids of diagnosis. Numerous studies have analyzed popular dermatologic textbooks used by residency programs nationwide, finding the number of SoC images across dermatology textbooks ranging from 4% to 18%.26,27 The use of standard dermatology textbooks is not enough to train residents to be competent in diagnosing and treating patients with SoC. There should be a concerted effort across the field of dermatology to encourage the development of a SoC educational curriculum at every academic dermatology program, including SoC textbooks, Kodachromes, and online/electronic resources.
Efforts to increase diversity in dermatology and dermatologic training should start in medical school preclinical curriculums and medical student rotations. Although our survey did not assess current medical student curricula, the benefits of academic institutions with SoCCs/SoCSCs are highlighted by the ability for both home and visiting medical students to rotate through the centers and gain early exposure to SoC dermatology. Most of the programs even provide scholarships and/or grants for URiM students to help fund their rotations, which is of critical importance considering the mounting data that the financial burden of visiting rotations disproportionately affects URiM students.28
Study Limitations—Although we did an extensive search and believe to have correctly identified all 15 formal SoCCs/SoCSCs with a high response rate (93.3%), there are institutions that do not have formalized SoCCs/SoCSCs but are known to serve SoC populations. Likewise, there are private dermatology practices not associated with academic centers that have SoC specialists and positively contribute to SoC patient care, research, and education that were not included in this study. Additionally, the data for this study were collected in 2020 and analyzed in 2021, so it is possible that not all SoCCs, divisions, or clinics were included in this study, particularly if established after 2021.
Conclusion
As the United States continues to diversify, the proportion of patients with SoC will continue to grow, and it is imperative that this racial, ethnic, and cultural diversity is reflected in the dermatology workforce as well as research and training. The current deficits in medical training related to SoC populations and the importance for patients with SoC to find dermatologists who can appropriately treat them is well known.29 Skin of color centers/SoCSCs strive to increase access to care for patients with SoC, improve cultural competency, promote diversity among faculty and trainees, and encourage SoC research and education at all levels. We urge academic dermatology training programs to make SoC education, research, and patient care a departmental priority. Important first steps include departmental diversification at all levels, incorporating SoC into curricula for residents, providing and securing funding for SoC research, and supporting the establishment of more formal SoCCs and/or SoCSCs to help reduce dermatologic health care disparities among patients with SoC and improve health equity.
Appendix



- Colby SL, Jennifer JM. Projections of the size and composition of the U.S. population: 2014 to 2060. United States Census Bureau website. March 3, 2015. Accessed June 18, 2024. https://www.census.gov/library/publications/2015/demo/p25-1143.html
- Tull RZ, Kerby E, Subash JJ, et al. Ethnic skin centers in the United States: where are we in 2020? J Am Acad Dermatol. 2020;83:1757-1759. doi:10.1016/j.jaad.2020.03.054
- Shen MJ, Peterson EB, Costas-Muñiz R, et al. The effects of race and racial concordance on patient-physician communication: a systematic review of the literature. J Racial Ethn Health Disparities. 2018;5:117-140. doi:10.1007/s40615-017-0350-4
- Saha S, Beach MC. Impact of physician race on patient decision-making and ratings of physicians: a randomized experiment using video vignettes. J Gen Intern Med. 2020;35:1084-1091. doi:10.1007/s11606-020-05646-z
- Quick Facts: United States. US Census Bureau website. Accessed June 18, 2024. https://www.census.gov/quickfacts/fact/table/US/PST045221
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587. doi:10.1016/j.jaad.2015.10.044
- Van Voorhees AS, Enos CW. Diversity in dermatology residency programs. J Investig Dermatol Symp Proc. 2017;18:S46-S49. doi:10.1016/j.jisp.2017.07.001
- Association of American Medical Colleges. Table B5. number of active MD residents, by race/ethnicity (alone or in combination) and GME specialty. Accessed June 18, 2024. https://www.aamc.org/data-reports/students-residents/interactive-data/report-residents/2022/table-b5-md-residents-race-ethnicity-and-specialty
- Association of American Medical Colleges. Table B6. number of active DO residents, by race/ethnicity (alone or in combination) and GME specialty. Accessed June 18, 2024. https://www.aamc.org/data-reports/students-residents/interactive-data/report-residents/2022/table-b6-do-residents-race-ethnicity-and-specialty
- Association of American Medical Colleges. Table 16. U.S. medical school faculty by gender, race/ethnicity, and department, 2022. Accessed June 24, 2024. https://www.aamc.org/media/8456/download
- Chen V, Akhtar S, Zheng C, et al. Assessment of changes in diversity in dermatology clinical trials between 2010-2015 and 2015-2020: a systematic review. JAMA Dermatol. 2022;158:288-292. doi:10.1001/jamadermatol.2021.5596
- Montgomery SNB, Elbuluk N. A quantitative analysis of research publications focused on the top chief complaints in patients withskinof color. J Am Acad Dermatol. 2021;85:241-242. doi:10.1016/j.jaad.2020.08.031
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618. doi:10.1016/j.jaad.2008.06.024
- Ibraheim MK, Gupta R, Dao H, et al. Evaluating skin of color education in dermatology residency programs: data from a national survey. Clin Dermatol. 2022;40:228-233. doi:10.1016/j.clindermatol.2021.11.015
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59, viii. doi:10.1016/j.det.2011.08.002
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291. doi:10.1001/jamadermatol.2018.3114
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 202;156:312-319. doi:10.1001/jamadermatol.2019.4818
- Dlova NC, Salkey KS, Callender VD, et al. Central centrifugal cicatricial alopecia: new insights and a call for action. J Investig Dermatol Symp Proc. 2017;18:S54-S56. doi:10.1016/j.jisp.2017.01.004
- Okeke CAV, Perry JD, Simmonds FC, et al. Clinical trials and skin of color: the example of hidradenitis suppurativa. dermatology. 2022;238:180-184. doi:10.1159/000516467
- Robles J, Anim T, Wusu MH, et al. An Approach to Faculty Development for Underrepresented Minorities in Medicine. South Med J. 2021;114(9):579-582. doi:10.14423/SMJ.0000000000001290
- Serrano L, Ulschmid C, Szabo A, et al. Racial disparities of delay in diagnosis and dermatologic care for hidradenitis suppurativa. J Natl Med Assoc. 2022;114:613-616. doi:10.1016/j.jnma.2022.08.002
- Drenkard C, Lim SS. Update on lupus epidemiology: advancinghealth disparities research through the study of minority populations. Curr Opin Rheumatol. 2019;31:689-696. doi:10.1097/BOR.0000000000000646
- Militello M, Szeto MD, Presley CL, et al. A quantitative analysis of research publications focused on skin of color: representation in academic dermatology journals. J Am Acad Dermatol. 2021;85:E189-E192. doi:10.1016/j.jaad.2021.04.053
- Cline A, Winter RP, Kourosh S, et al. Multiethnic training in residency: a survey of dermatology residents. Cutis. 2020;105:310-313.
- Mhlaba JM, Pontes DS, Patterson SS, et al. Evaluation of a skin of color curriculum for dermatology residents. J Drugs Dermatol. 2021;20:786-789. doi:10.36849/JDD.6193
- Adelekun A, Onyekaba G, Lipoff JB. Skin color in dermatology textbooks: an updated evaluation and analysis. J Am Acad Dermatol. 2021;84:194-196. doi:10.1016/j.jaad.2020.04.084
- Harp T, Militello M, McCarver V, et al. Further analysis of skin of color representation in dermatology textbooks used by residents. J Am Acad Dermatol. 2022;87:E39-E41. doi:10.1016/j.jaad.2022.02.069
- Muzumdar S, Grant-Kels JM, Feng H. Strategies to improve medical student visiting rotations. Clin Dermatol. 2021;39:727-728. doi:10.1016/j.clindermatol.2020.11.001
- Gorbatenko-Roth K, Prose N, Kundu RV, et al. Assessment of Black patients’ perception of their dermatology care. JAMA Dermatol. 2019;155:1129-1134. doi:10.1001/jamadermatol.2019.2063
- Colby SL, Jennifer JM. Projections of the size and composition of the U.S. population: 2014 to 2060. United States Census Bureau website. March 3, 2015. Accessed June 18, 2024. https://www.census.gov/library/publications/2015/demo/p25-1143.html
- Tull RZ, Kerby E, Subash JJ, et al. Ethnic skin centers in the United States: where are we in 2020? J Am Acad Dermatol. 2020;83:1757-1759. doi:10.1016/j.jaad.2020.03.054
- Shen MJ, Peterson EB, Costas-Muñiz R, et al. The effects of race and racial concordance on patient-physician communication: a systematic review of the literature. J Racial Ethn Health Disparities. 2018;5:117-140. doi:10.1007/s40615-017-0350-4
- Saha S, Beach MC. Impact of physician race on patient decision-making and ratings of physicians: a randomized experiment using video vignettes. J Gen Intern Med. 2020;35:1084-1091. doi:10.1007/s11606-020-05646-z
- Quick Facts: United States. US Census Bureau website. Accessed June 18, 2024. https://www.census.gov/quickfacts/fact/table/US/PST045221
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587. doi:10.1016/j.jaad.2015.10.044
- Van Voorhees AS, Enos CW. Diversity in dermatology residency programs. J Investig Dermatol Symp Proc. 2017;18:S46-S49. doi:10.1016/j.jisp.2017.07.001
- Association of American Medical Colleges. Table B5. number of active MD residents, by race/ethnicity (alone or in combination) and GME specialty. Accessed June 18, 2024. https://www.aamc.org/data-reports/students-residents/interactive-data/report-residents/2022/table-b5-md-residents-race-ethnicity-and-specialty
- Association of American Medical Colleges. Table B6. number of active DO residents, by race/ethnicity (alone or in combination) and GME specialty. Accessed June 18, 2024. https://www.aamc.org/data-reports/students-residents/interactive-data/report-residents/2022/table-b6-do-residents-race-ethnicity-and-specialty
- Association of American Medical Colleges. Table 16. U.S. medical school faculty by gender, race/ethnicity, and department, 2022. Accessed June 24, 2024. https://www.aamc.org/media/8456/download
- Chen V, Akhtar S, Zheng C, et al. Assessment of changes in diversity in dermatology clinical trials between 2010-2015 and 2015-2020: a systematic review. JAMA Dermatol. 2022;158:288-292. doi:10.1001/jamadermatol.2021.5596
- Montgomery SNB, Elbuluk N. A quantitative analysis of research publications focused on the top chief complaints in patients withskinof color. J Am Acad Dermatol. 2021;85:241-242. doi:10.1016/j.jaad.2020.08.031
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618. doi:10.1016/j.jaad.2008.06.024
- Ibraheim MK, Gupta R, Dao H, et al. Evaluating skin of color education in dermatology residency programs: data from a national survey. Clin Dermatol. 2022;40:228-233. doi:10.1016/j.clindermatol.2021.11.015
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59, viii. doi:10.1016/j.det.2011.08.002
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291. doi:10.1001/jamadermatol.2018.3114
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 202;156:312-319. doi:10.1001/jamadermatol.2019.4818
- Dlova NC, Salkey KS, Callender VD, et al. Central centrifugal cicatricial alopecia: new insights and a call for action. J Investig Dermatol Symp Proc. 2017;18:S54-S56. doi:10.1016/j.jisp.2017.01.004
- Okeke CAV, Perry JD, Simmonds FC, et al. Clinical trials and skin of color: the example of hidradenitis suppurativa. dermatology. 2022;238:180-184. doi:10.1159/000516467
- Robles J, Anim T, Wusu MH, et al. An Approach to Faculty Development for Underrepresented Minorities in Medicine. South Med J. 2021;114(9):579-582. doi:10.14423/SMJ.0000000000001290
- Serrano L, Ulschmid C, Szabo A, et al. Racial disparities of delay in diagnosis and dermatologic care for hidradenitis suppurativa. J Natl Med Assoc. 2022;114:613-616. doi:10.1016/j.jnma.2022.08.002
- Drenkard C, Lim SS. Update on lupus epidemiology: advancinghealth disparities research through the study of minority populations. Curr Opin Rheumatol. 2019;31:689-696. doi:10.1097/BOR.0000000000000646
- Militello M, Szeto MD, Presley CL, et al. A quantitative analysis of research publications focused on skin of color: representation in academic dermatology journals. J Am Acad Dermatol. 2021;85:E189-E192. doi:10.1016/j.jaad.2021.04.053
- Cline A, Winter RP, Kourosh S, et al. Multiethnic training in residency: a survey of dermatology residents. Cutis. 2020;105:310-313.
- Mhlaba JM, Pontes DS, Patterson SS, et al. Evaluation of a skin of color curriculum for dermatology residents. J Drugs Dermatol. 2021;20:786-789. doi:10.36849/JDD.6193
- Adelekun A, Onyekaba G, Lipoff JB. Skin color in dermatology textbooks: an updated evaluation and analysis. J Am Acad Dermatol. 2021;84:194-196. doi:10.1016/j.jaad.2020.04.084
- Harp T, Militello M, McCarver V, et al. Further analysis of skin of color representation in dermatology textbooks used by residents. J Am Acad Dermatol. 2022;87:E39-E41. doi:10.1016/j.jaad.2022.02.069
- Muzumdar S, Grant-Kels JM, Feng H. Strategies to improve medical student visiting rotations. Clin Dermatol. 2021;39:727-728. doi:10.1016/j.clindermatol.2020.11.001
- Gorbatenko-Roth K, Prose N, Kundu RV, et al. Assessment of Black patients’ perception of their dermatology care. JAMA Dermatol. 2019;155:1129-1134. doi:10.1001/jamadermatol.2019.2063
Practice Points
- Skin of color centers in the United States work to reverse the paucity of research, education, and training in skin of color dermatology and promote the diversification of residents and faculty.
- Skin of color centers expand access to culturally competent and inclusive care for diverse patient populations.
Generational Differences in Isotretinoin Prescribing Habits: A Cross-Sectional Analysis
To the Editor:
Prescriptions for isotretinoin may be influenced by patient demographics, medical comorbidities, and drug safety programs.1,2 In 1982, isotretinoin was approved by the US Food and Drug Administration for treatment of severe recalcitrant nodulocystic acne that is nonresponsive to conventional therapies such as antibiotics; however, prescriber beliefs regarding the necessity of oral antibiotic failure before isotretinoin is prescribed may be influenced by the provider’s generational age.3 Currently, there is a knowledge gap regarding the impact of provider characteristics, including the year providers completed training, on isotretinoin utilization. The aim of our cross-sectional study was to characterize generational isotretinoin prescribing habits in a large-scale midwestern private practice dermatology group.
Modernizing Medicine (https://www.modmed.com), an electronic medical record software, was queried for all encounters that included both an International Classification of Diseases, Tenth Revision, Clinical Modification diagnosis code L70.0 (acne vulgaris) and a medication prescription from May 2021 to May 2022. Data were collected from a large private practice group with locations across the state of Ohio. Exclusion criteria included provider-patient prescription pairs that included non–acne medication prescriptions, patients seen by multiple providers, and providers who treated fewer than 5 patients with acne during the study period. A mixed-effect multiple logistic regression was performed to analyze whether a patient was ever prescribed isotretinoin, adjusting for individual prescriber, prescriber generation (millennial [1981–1996], Generation X [1965–1980], and baby boomer [1946–1964]),4 and patient sex; spironolactone and oral antibiotic prescriptions during the study period were included as additional covariates in a subsequent post hoc analysis. This study utilized data that was fully deidentified in accordance with the US Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule. Approval from an institutional review board was not required.
A total of 18,089 provider-patient prescription pairs were included in our analysis (Table). In our most robust model, female patients were significantly less likely to receive isotretinoin compared with male patients (adjusted OR [aOR], 0.394; P<.01). Millennial providers were significantly more likely to utilize isotretinoin in patients who did not receive antibiotics compared with patients who did receive antibiotics (aOR, 1.693; P<.01). When compared with both Generation X and baby boomers, millennial providers were more likely to prescribe isotretinoin in patients who received antibiotics (aOR, 2.227 [P=.02] and 3.638 [P<.01], respectively).
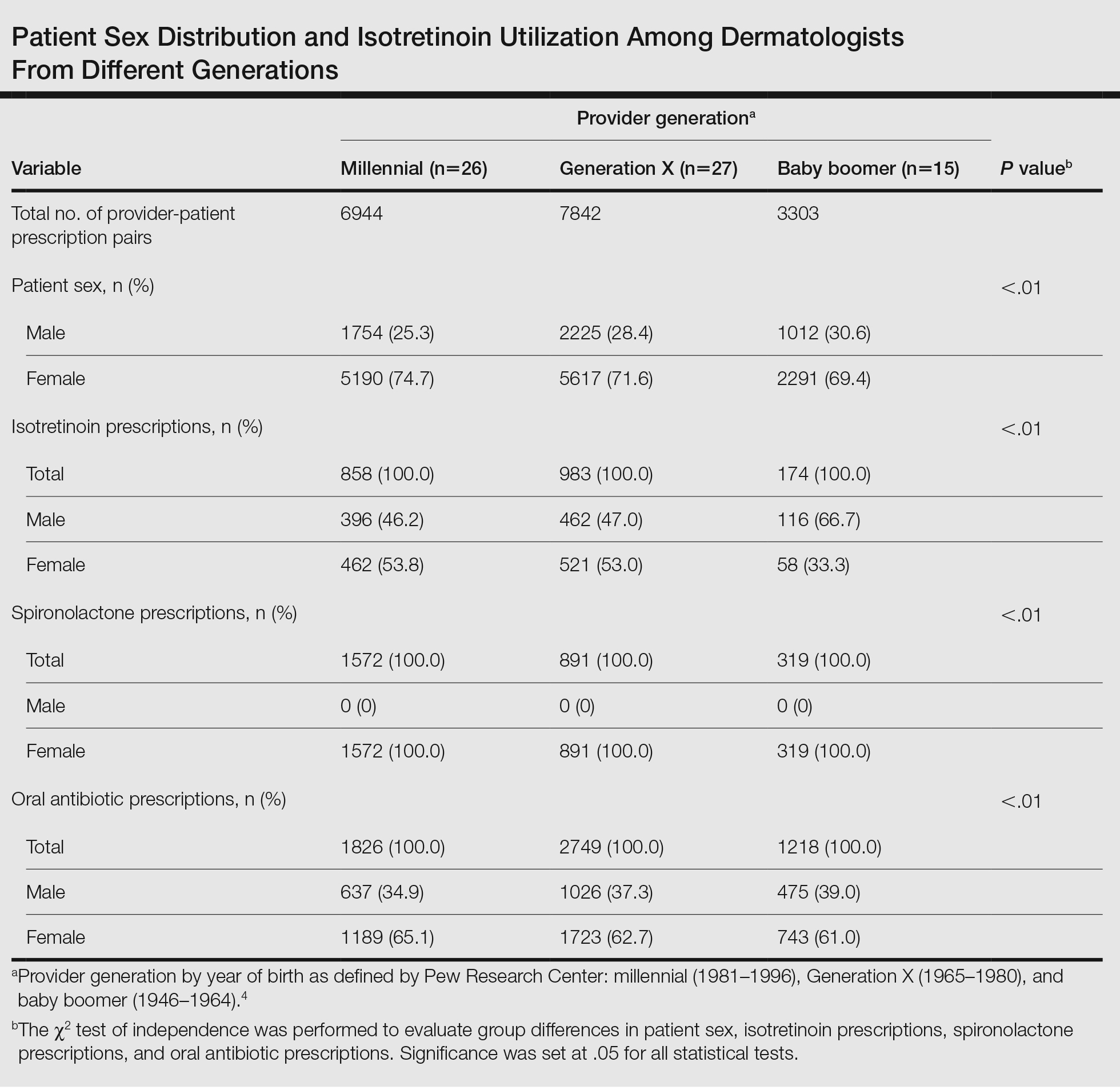
In 2018, the American Academy of Dermatology and the Global Alliance to Improve Outcomes in Acne updated thir guidelines to recommend isotretinoin as a first-line therapy for severe nodular acne, treatment-resistant moderate acne, or acne that produces scarring or psychosocial distress.5 Our study results suggest that millennial providers are adhering to these guidelines and readily prescribing isotretinoin in patients who did not receive antibiotics, which corroborates survey findings by Nagler and Orlow.3 Our results also revealed that prescriber generation may influence isotretinoin usage, with millennials utilizing isotretinoin more in patients who received oral antibiotic therapy than their older counterparts. In part, this may be due to beliefs among older generations that failure of oral antibiotics is necessary before pursuing isotretinoin.3 Additionally, this finding suggests that millennials, if utilizing antibiotics for acne, may have a lower threshold for starting isotretinoin in patients who received oral antibiotic therapy.
Generational prescribing variation appears not to be unique to isotretinoin and also may be present in the use of spironolactone. Over the past decade, utilization of spironolactone for acne treatment has increased, likely in response to new data demonstrating that routine use is safe and effective.6 Several large cohort and retrospective studies have debunked the historical concerns for tumorigenicity in those with breast cancer history as well as the need for routine laboratory monitoring for hyperkalemia.7,8 Although spironolactone use for the treatment of acne has increased, it still remains relatively underutilized,6 suggesting there may be a knowledge gap similar to that of isotretinoin, with younger generations utilizing spironolactone more readily than older generations.
Our study analyzed generational differences in isotretinoin utilization for acne over 1 calendar year. Limitations include sampling from a midwestern patient cohort and private practice–based providers. Due to limitations of our data set, we were unable to capture acne medication usage prior to May 2021, temporal sequencing of acne medication usage, and stratification of patients by acne severity. Furthermore, we were unable to capture female patients who were pregnant or planning pregnancy at the time of their encounter, which would exclude isotretinoin usage.
Overall, millennial providers may be utilizing isotretinoin more in line with the updated acne guidelines5 compared with providers from older generations. Further research is necessary to elucidate how these prescribing habits may change based on acne severity.
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319. doi:10.1001/jamadermatol.2019.4818
- Barbieri JS, Frieden IJ, Nagler AR. Isotretinoin, patient safety, and patient-centered care-time to reform iPLEDGE. JAMA Dermatol. 2020;156:21-22. doi:10.1001/jamadermatol.2019.3270
- Nagler AR, Orlow SJ. Dermatologists’ attitudes, prescription, and counseling patterns for isotretinoin: a questionnaire-based study. J Drugs Dermatol. 2015;14:184-189.
- Dimock M. Where Millennials end and Generation Z begins. Pew Research Center website. January 17, 2019. Accessed June 17, 2024. https://www.pewresearch.org/fact-tank/2019/01/17/where-millennials-end-and-generation-z-begins/
- Thiboutot DM, Dréno B, Abanmi A, et al. Practical management of acne for clinicians: an international consensus from the Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol. 2018;78(2 suppl 1):S1-S23.e1. doi:10.1016/j.jaad.2017.09.078
- Guzman AK, Barbieri JS. Comparative analysis of prescribing patterns of tetracycline class antibiotics and spironolactone between advanced practice providers and physicians in the treatment of acne vulgaris. J Am Acad Dermatol. 2021;84:1119-1121. doi:10.1016/j.jaad.2020.06.044
- Wei C, Bovonratwet P, Gu A, et al. Spironolactone use does not increase the risk of female breast cancer recurrence: a retrospective analysis. J Am Acad Dermatol. 2020;83:1021-1027. doi:10.1016/j.jaad.2020.05.081
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944. doi:10.1001/jamadermatol.2015.34
To the Editor:
Prescriptions for isotretinoin may be influenced by patient demographics, medical comorbidities, and drug safety programs.1,2 In 1982, isotretinoin was approved by the US Food and Drug Administration for treatment of severe recalcitrant nodulocystic acne that is nonresponsive to conventional therapies such as antibiotics; however, prescriber beliefs regarding the necessity of oral antibiotic failure before isotretinoin is prescribed may be influenced by the provider’s generational age.3 Currently, there is a knowledge gap regarding the impact of provider characteristics, including the year providers completed training, on isotretinoin utilization. The aim of our cross-sectional study was to characterize generational isotretinoin prescribing habits in a large-scale midwestern private practice dermatology group.
Modernizing Medicine (https://www.modmed.com), an electronic medical record software, was queried for all encounters that included both an International Classification of Diseases, Tenth Revision, Clinical Modification diagnosis code L70.0 (acne vulgaris) and a medication prescription from May 2021 to May 2022. Data were collected from a large private practice group with locations across the state of Ohio. Exclusion criteria included provider-patient prescription pairs that included non–acne medication prescriptions, patients seen by multiple providers, and providers who treated fewer than 5 patients with acne during the study period. A mixed-effect multiple logistic regression was performed to analyze whether a patient was ever prescribed isotretinoin, adjusting for individual prescriber, prescriber generation (millennial [1981–1996], Generation X [1965–1980], and baby boomer [1946–1964]),4 and patient sex; spironolactone and oral antibiotic prescriptions during the study period were included as additional covariates in a subsequent post hoc analysis. This study utilized data that was fully deidentified in accordance with the US Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule. Approval from an institutional review board was not required.
A total of 18,089 provider-patient prescription pairs were included in our analysis (Table). In our most robust model, female patients were significantly less likely to receive isotretinoin compared with male patients (adjusted OR [aOR], 0.394; P<.01). Millennial providers were significantly more likely to utilize isotretinoin in patients who did not receive antibiotics compared with patients who did receive antibiotics (aOR, 1.693; P<.01). When compared with both Generation X and baby boomers, millennial providers were more likely to prescribe isotretinoin in patients who received antibiotics (aOR, 2.227 [P=.02] and 3.638 [P<.01], respectively).

In 2018, the American Academy of Dermatology and the Global Alliance to Improve Outcomes in Acne updated thir guidelines to recommend isotretinoin as a first-line therapy for severe nodular acne, treatment-resistant moderate acne, or acne that produces scarring or psychosocial distress.5 Our study results suggest that millennial providers are adhering to these guidelines and readily prescribing isotretinoin in patients who did not receive antibiotics, which corroborates survey findings by Nagler and Orlow.3 Our results also revealed that prescriber generation may influence isotretinoin usage, with millennials utilizing isotretinoin more in patients who received oral antibiotic therapy than their older counterparts. In part, this may be due to beliefs among older generations that failure of oral antibiotics is necessary before pursuing isotretinoin.3 Additionally, this finding suggests that millennials, if utilizing antibiotics for acne, may have a lower threshold for starting isotretinoin in patients who received oral antibiotic therapy.
Generational prescribing variation appears not to be unique to isotretinoin and also may be present in the use of spironolactone. Over the past decade, utilization of spironolactone for acne treatment has increased, likely in response to new data demonstrating that routine use is safe and effective.6 Several large cohort and retrospective studies have debunked the historical concerns for tumorigenicity in those with breast cancer history as well as the need for routine laboratory monitoring for hyperkalemia.7,8 Although spironolactone use for the treatment of acne has increased, it still remains relatively underutilized,6 suggesting there may be a knowledge gap similar to that of isotretinoin, with younger generations utilizing spironolactone more readily than older generations.
Our study analyzed generational differences in isotretinoin utilization for acne over 1 calendar year. Limitations include sampling from a midwestern patient cohort and private practice–based providers. Due to limitations of our data set, we were unable to capture acne medication usage prior to May 2021, temporal sequencing of acne medication usage, and stratification of patients by acne severity. Furthermore, we were unable to capture female patients who were pregnant or planning pregnancy at the time of their encounter, which would exclude isotretinoin usage.
Overall, millennial providers may be utilizing isotretinoin more in line with the updated acne guidelines5 compared with providers from older generations. Further research is necessary to elucidate how these prescribing habits may change based on acne severity.
To the Editor:
Prescriptions for isotretinoin may be influenced by patient demographics, medical comorbidities, and drug safety programs.1,2 In 1982, isotretinoin was approved by the US Food and Drug Administration for treatment of severe recalcitrant nodulocystic acne that is nonresponsive to conventional therapies such as antibiotics; however, prescriber beliefs regarding the necessity of oral antibiotic failure before isotretinoin is prescribed may be influenced by the provider’s generational age.3 Currently, there is a knowledge gap regarding the impact of provider characteristics, including the year providers completed training, on isotretinoin utilization. The aim of our cross-sectional study was to characterize generational isotretinoin prescribing habits in a large-scale midwestern private practice dermatology group.
Modernizing Medicine (https://www.modmed.com), an electronic medical record software, was queried for all encounters that included both an International Classification of Diseases, Tenth Revision, Clinical Modification diagnosis code L70.0 (acne vulgaris) and a medication prescription from May 2021 to May 2022. Data were collected from a large private practice group with locations across the state of Ohio. Exclusion criteria included provider-patient prescription pairs that included non–acne medication prescriptions, patients seen by multiple providers, and providers who treated fewer than 5 patients with acne during the study period. A mixed-effect multiple logistic regression was performed to analyze whether a patient was ever prescribed isotretinoin, adjusting for individual prescriber, prescriber generation (millennial [1981–1996], Generation X [1965–1980], and baby boomer [1946–1964]),4 and patient sex; spironolactone and oral antibiotic prescriptions during the study period were included as additional covariates in a subsequent post hoc analysis. This study utilized data that was fully deidentified in accordance with the US Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule. Approval from an institutional review board was not required.
A total of 18,089 provider-patient prescription pairs were included in our analysis (Table). In our most robust model, female patients were significantly less likely to receive isotretinoin compared with male patients (adjusted OR [aOR], 0.394; P<.01). Millennial providers were significantly more likely to utilize isotretinoin in patients who did not receive antibiotics compared with patients who did receive antibiotics (aOR, 1.693; P<.01). When compared with both Generation X and baby boomers, millennial providers were more likely to prescribe isotretinoin in patients who received antibiotics (aOR, 2.227 [P=.02] and 3.638 [P<.01], respectively).

In 2018, the American Academy of Dermatology and the Global Alliance to Improve Outcomes in Acne updated thir guidelines to recommend isotretinoin as a first-line therapy for severe nodular acne, treatment-resistant moderate acne, or acne that produces scarring or psychosocial distress.5 Our study results suggest that millennial providers are adhering to these guidelines and readily prescribing isotretinoin in patients who did not receive antibiotics, which corroborates survey findings by Nagler and Orlow.3 Our results also revealed that prescriber generation may influence isotretinoin usage, with millennials utilizing isotretinoin more in patients who received oral antibiotic therapy than their older counterparts. In part, this may be due to beliefs among older generations that failure of oral antibiotics is necessary before pursuing isotretinoin.3 Additionally, this finding suggests that millennials, if utilizing antibiotics for acne, may have a lower threshold for starting isotretinoin in patients who received oral antibiotic therapy.
Generational prescribing variation appears not to be unique to isotretinoin and also may be present in the use of spironolactone. Over the past decade, utilization of spironolactone for acne treatment has increased, likely in response to new data demonstrating that routine use is safe and effective.6 Several large cohort and retrospective studies have debunked the historical concerns for tumorigenicity in those with breast cancer history as well as the need for routine laboratory monitoring for hyperkalemia.7,8 Although spironolactone use for the treatment of acne has increased, it still remains relatively underutilized,6 suggesting there may be a knowledge gap similar to that of isotretinoin, with younger generations utilizing spironolactone more readily than older generations.
Our study analyzed generational differences in isotretinoin utilization for acne over 1 calendar year. Limitations include sampling from a midwestern patient cohort and private practice–based providers. Due to limitations of our data set, we were unable to capture acne medication usage prior to May 2021, temporal sequencing of acne medication usage, and stratification of patients by acne severity. Furthermore, we were unable to capture female patients who were pregnant or planning pregnancy at the time of their encounter, which would exclude isotretinoin usage.
Overall, millennial providers may be utilizing isotretinoin more in line with the updated acne guidelines5 compared with providers from older generations. Further research is necessary to elucidate how these prescribing habits may change based on acne severity.
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319. doi:10.1001/jamadermatol.2019.4818
- Barbieri JS, Frieden IJ, Nagler AR. Isotretinoin, patient safety, and patient-centered care-time to reform iPLEDGE. JAMA Dermatol. 2020;156:21-22. doi:10.1001/jamadermatol.2019.3270
- Nagler AR, Orlow SJ. Dermatologists’ attitudes, prescription, and counseling patterns for isotretinoin: a questionnaire-based study. J Drugs Dermatol. 2015;14:184-189.
- Dimock M. Where Millennials end and Generation Z begins. Pew Research Center website. January 17, 2019. Accessed June 17, 2024. https://www.pewresearch.org/fact-tank/2019/01/17/where-millennials-end-and-generation-z-begins/
- Thiboutot DM, Dréno B, Abanmi A, et al. Practical management of acne for clinicians: an international consensus from the Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol. 2018;78(2 suppl 1):S1-S23.e1. doi:10.1016/j.jaad.2017.09.078
- Guzman AK, Barbieri JS. Comparative analysis of prescribing patterns of tetracycline class antibiotics and spironolactone between advanced practice providers and physicians in the treatment of acne vulgaris. J Am Acad Dermatol. 2021;84:1119-1121. doi:10.1016/j.jaad.2020.06.044
- Wei C, Bovonratwet P, Gu A, et al. Spironolactone use does not increase the risk of female breast cancer recurrence: a retrospective analysis. J Am Acad Dermatol. 2020;83:1021-1027. doi:10.1016/j.jaad.2020.05.081
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944. doi:10.1001/jamadermatol.2015.34
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319. doi:10.1001/jamadermatol.2019.4818
- Barbieri JS, Frieden IJ, Nagler AR. Isotretinoin, patient safety, and patient-centered care-time to reform iPLEDGE. JAMA Dermatol. 2020;156:21-22. doi:10.1001/jamadermatol.2019.3270
- Nagler AR, Orlow SJ. Dermatologists’ attitudes, prescription, and counseling patterns for isotretinoin: a questionnaire-based study. J Drugs Dermatol. 2015;14:184-189.
- Dimock M. Where Millennials end and Generation Z begins. Pew Research Center website. January 17, 2019. Accessed June 17, 2024. https://www.pewresearch.org/fact-tank/2019/01/17/where-millennials-end-and-generation-z-begins/
- Thiboutot DM, Dréno B, Abanmi A, et al. Practical management of acne for clinicians: an international consensus from the Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol. 2018;78(2 suppl 1):S1-S23.e1. doi:10.1016/j.jaad.2017.09.078
- Guzman AK, Barbieri JS. Comparative analysis of prescribing patterns of tetracycline class antibiotics and spironolactone between advanced practice providers and physicians in the treatment of acne vulgaris. J Am Acad Dermatol. 2021;84:1119-1121. doi:10.1016/j.jaad.2020.06.044
- Wei C, Bovonratwet P, Gu A, et al. Spironolactone use does not increase the risk of female breast cancer recurrence: a retrospective analysis. J Am Acad Dermatol. 2020;83:1021-1027. doi:10.1016/j.jaad.2020.05.081
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944. doi:10.1001/jamadermatol.2015.34
Practice Points
- Provider generational age appears to impact utilization of isotretinoin for the treatment of acne.
- Millennial providers seem to adhere more readily to guidelines for precribing isotretinoin vs older generations and also may have a lower threshold for starting isotretinoin in patients who received oral antibiotic therapy for acne treatment.
Dermatofibrosarcoma Protuberans More Common In Black Patients, Analysis Finds
TOPLINE:
that also found that larger tumor size and older age were associated with survival outcomes.
METHODOLOGY:
- Researchers used the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) registry from 2000 through 2018 to provide a comprehensive report on the incidence of DFSP, a rare, low-grade cutaneous soft tissue sarcoma, and factors associated with metastatic progression, overall survival (OS), and cancer-specific survival.
- A total of 7748 patients (mean age, 43.5 years; 53.3% women; 52% non-Hispanic White) were diagnosed with histologically confirmed DFSP of the skin and connective tissue and were included in the study.
- DFSP incidence was reported as cases per million person-years and age-adjusted to the 2000 US Standard Population, and factors influencing metastasis were assessed.
TAKEAWAY:
- The overall DFSP incidence rate was 6.25 cases per million person-years, with a higher incidence in Black individuals than in White individuals (8.74 vs 4.53).
- The 5-year OS rate was 95.8%. Older age (≥ 60 years; hazard ratio [HR], 6.66), male gender assigned at birth (HR, 1.79), and larger tumor size (≥ 3 cm; HR, 2.02) were associated with poorer OS (P < .001 for all).
- The 1-year and 5-year DFSP-specific survival rates were 99.9% and 99.2%, respectively. Older age (HR, 3.47; P < .001) and larger tumor size (≥ 3 cm; HR, 5.34; P = .002) were associated with significantly worse cancer-specific survival.
- Large tumor size (odds ratio [OR], 2.24) and DFSP located on the head and neck (OR, 4.88), or genitalia (OR, 3.16) were significantly associated with increased metastasis risk. Higher socioeconomic status was linked to a lower risk for metastasis.
IN PRACTICE:
“Our findings highlight the increased incidence rates of DFSP among Black patients. We demonstrate the interplay between patient demographics and clinical factors in influencing DFSP metastasis, OS, and cancer-specific survival,” the authors wrote. The results, they added, “may be useful for further evaluation of proposed causes, which will ultimately lead to further understanding and prevention of this disease.”
SOURCE:
The study was led by Jalal Maghfour, MD, Department of Dermatology, Henry Ford Health, Detroit, and was published online on June 20 in the Journal of the American Academy of Dermatology.
LIMITATIONS:
Details on specific cases in the SEER registry are limited. For 1752 patients, tumor size was not included, increasing the risk for misclassification bias. Because specific pathology reports were not available, the analysis did not address histologic grade.
DISCLOSURES:
The study did not receive any funding support. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
that also found that larger tumor size and older age were associated with survival outcomes.
METHODOLOGY:
- Researchers used the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) registry from 2000 through 2018 to provide a comprehensive report on the incidence of DFSP, a rare, low-grade cutaneous soft tissue sarcoma, and factors associated with metastatic progression, overall survival (OS), and cancer-specific survival.
- A total of 7748 patients (mean age, 43.5 years; 53.3% women; 52% non-Hispanic White) were diagnosed with histologically confirmed DFSP of the skin and connective tissue and were included in the study.
- DFSP incidence was reported as cases per million person-years and age-adjusted to the 2000 US Standard Population, and factors influencing metastasis were assessed.
TAKEAWAY:
- The overall DFSP incidence rate was 6.25 cases per million person-years, with a higher incidence in Black individuals than in White individuals (8.74 vs 4.53).
- The 5-year OS rate was 95.8%. Older age (≥ 60 years; hazard ratio [HR], 6.66), male gender assigned at birth (HR, 1.79), and larger tumor size (≥ 3 cm; HR, 2.02) were associated with poorer OS (P < .001 for all).
- The 1-year and 5-year DFSP-specific survival rates were 99.9% and 99.2%, respectively. Older age (HR, 3.47; P < .001) and larger tumor size (≥ 3 cm; HR, 5.34; P = .002) were associated with significantly worse cancer-specific survival.
- Large tumor size (odds ratio [OR], 2.24) and DFSP located on the head and neck (OR, 4.88), or genitalia (OR, 3.16) were significantly associated with increased metastasis risk. Higher socioeconomic status was linked to a lower risk for metastasis.
IN PRACTICE:
“Our findings highlight the increased incidence rates of DFSP among Black patients. We demonstrate the interplay between patient demographics and clinical factors in influencing DFSP metastasis, OS, and cancer-specific survival,” the authors wrote. The results, they added, “may be useful for further evaluation of proposed causes, which will ultimately lead to further understanding and prevention of this disease.”
SOURCE:
The study was led by Jalal Maghfour, MD, Department of Dermatology, Henry Ford Health, Detroit, and was published online on June 20 in the Journal of the American Academy of Dermatology.
LIMITATIONS:
Details on specific cases in the SEER registry are limited. For 1752 patients, tumor size was not included, increasing the risk for misclassification bias. Because specific pathology reports were not available, the analysis did not address histologic grade.
DISCLOSURES:
The study did not receive any funding support. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
that also found that larger tumor size and older age were associated with survival outcomes.
METHODOLOGY:
- Researchers used the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) registry from 2000 through 2018 to provide a comprehensive report on the incidence of DFSP, a rare, low-grade cutaneous soft tissue sarcoma, and factors associated with metastatic progression, overall survival (OS), and cancer-specific survival.
- A total of 7748 patients (mean age, 43.5 years; 53.3% women; 52% non-Hispanic White) were diagnosed with histologically confirmed DFSP of the skin and connective tissue and were included in the study.
- DFSP incidence was reported as cases per million person-years and age-adjusted to the 2000 US Standard Population, and factors influencing metastasis were assessed.
TAKEAWAY:
- The overall DFSP incidence rate was 6.25 cases per million person-years, with a higher incidence in Black individuals than in White individuals (8.74 vs 4.53).
- The 5-year OS rate was 95.8%. Older age (≥ 60 years; hazard ratio [HR], 6.66), male gender assigned at birth (HR, 1.79), and larger tumor size (≥ 3 cm; HR, 2.02) were associated with poorer OS (P < .001 for all).
- The 1-year and 5-year DFSP-specific survival rates were 99.9% and 99.2%, respectively. Older age (HR, 3.47; P < .001) and larger tumor size (≥ 3 cm; HR, 5.34; P = .002) were associated with significantly worse cancer-specific survival.
- Large tumor size (odds ratio [OR], 2.24) and DFSP located on the head and neck (OR, 4.88), or genitalia (OR, 3.16) were significantly associated with increased metastasis risk. Higher socioeconomic status was linked to a lower risk for metastasis.
IN PRACTICE:
“Our findings highlight the increased incidence rates of DFSP among Black patients. We demonstrate the interplay between patient demographics and clinical factors in influencing DFSP metastasis, OS, and cancer-specific survival,” the authors wrote. The results, they added, “may be useful for further evaluation of proposed causes, which will ultimately lead to further understanding and prevention of this disease.”
SOURCE:
The study was led by Jalal Maghfour, MD, Department of Dermatology, Henry Ford Health, Detroit, and was published online on June 20 in the Journal of the American Academy of Dermatology.
LIMITATIONS:
Details on specific cases in the SEER registry are limited. For 1752 patients, tumor size was not included, increasing the risk for misclassification bias. Because specific pathology reports were not available, the analysis did not address histologic grade.
DISCLOSURES:
The study did not receive any funding support. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.