User login
Evaluation of the Prostate Cancer Molecular Testing Pathway (PCMTP) Within the Veterans Health Administration (VHA)
Purpose
The PCMTP was developed to provide standardized decision support for molecular testing for veterans with prostate cancer.
Background
Prior to the precision medicine era, molecular tumor testing in prostate cancer was not standard of care. Field practitioners were unfamiliar with the role of molecular testing in clinical care. The PCMTP provides direction for germline and tumor testing in appropriate patients with prostate cancer. The expectation is that at least 80% of veterans will be pathway adherent. The PCMTP is an Oncology Clinical Pathway (OCP) that supports evidence-based practice providing highquality, safe, and cost-effective care for veterans reducing variability of care in the VHA.
Methods
The National Oncology Program Office assembled a Prostate Cancer Team (PCT) to develop OCPs. The pathways were incorporated into note templates that record clinical decisions using text and metadata (Health Factors [HF]), and record pathway adherence for the 4 key nodes of the PCMTP. The templates were pilot-tested and improved using an iterative process over a 3-month period. Further evaluation was conducted by the Office of Human Factors Engineering and the National Clinical Template Workgroup, utilizing a heuristic evaluation to ensure standardization, interoperability, and reduce duplication. HF data were retrieved from the Corporate Data Warehouse using a custom-built dashboard. Descriptive statistics of PCMTP use are presented.
Results
Between 4/1/2021 and 6/22/2022, 6276 health factors were generated from 1707 unique veterans in whom this clinical pathway was accessed. 328 distinct providers participated at 61 sites. Average veteran age was 73 years. (range 45-100) including 42% Black and 56% White. Of 1243 veterans considered for germline testing, 96.6% had germline testing ordered and for 1102 veterans considered for tumor testing, 93.3% had tumor testing ordered.
Conclusions
Pathway adherence exceeded the 80% benchmark. Race representation was diverse and reflective of the VA prostate cancer population. About 46% of VA oncology practices have used the PCMTP for ~11% of the estimated 15,000 veterans with metastatic prostate cancer in VHA. Increased use of this pathway is expected to improve outcomes for veterans with prostate cancer
Purpose
The PCMTP was developed to provide standardized decision support for molecular testing for veterans with prostate cancer.
Background
Prior to the precision medicine era, molecular tumor testing in prostate cancer was not standard of care. Field practitioners were unfamiliar with the role of molecular testing in clinical care. The PCMTP provides direction for germline and tumor testing in appropriate patients with prostate cancer. The expectation is that at least 80% of veterans will be pathway adherent. The PCMTP is an Oncology Clinical Pathway (OCP) that supports evidence-based practice providing highquality, safe, and cost-effective care for veterans reducing variability of care in the VHA.
Methods
The National Oncology Program Office assembled a Prostate Cancer Team (PCT) to develop OCPs. The pathways were incorporated into note templates that record clinical decisions using text and metadata (Health Factors [HF]), and record pathway adherence for the 4 key nodes of the PCMTP. The templates were pilot-tested and improved using an iterative process over a 3-month period. Further evaluation was conducted by the Office of Human Factors Engineering and the National Clinical Template Workgroup, utilizing a heuristic evaluation to ensure standardization, interoperability, and reduce duplication. HF data were retrieved from the Corporate Data Warehouse using a custom-built dashboard. Descriptive statistics of PCMTP use are presented.
Results
Between 4/1/2021 and 6/22/2022, 6276 health factors were generated from 1707 unique veterans in whom this clinical pathway was accessed. 328 distinct providers participated at 61 sites. Average veteran age was 73 years. (range 45-100) including 42% Black and 56% White. Of 1243 veterans considered for germline testing, 96.6% had germline testing ordered and for 1102 veterans considered for tumor testing, 93.3% had tumor testing ordered.
Conclusions
Pathway adherence exceeded the 80% benchmark. Race representation was diverse and reflective of the VA prostate cancer population. About 46% of VA oncology practices have used the PCMTP for ~11% of the estimated 15,000 veterans with metastatic prostate cancer in VHA. Increased use of this pathway is expected to improve outcomes for veterans with prostate cancer
Purpose
The PCMTP was developed to provide standardized decision support for molecular testing for veterans with prostate cancer.
Background
Prior to the precision medicine era, molecular tumor testing in prostate cancer was not standard of care. Field practitioners were unfamiliar with the role of molecular testing in clinical care. The PCMTP provides direction for germline and tumor testing in appropriate patients with prostate cancer. The expectation is that at least 80% of veterans will be pathway adherent. The PCMTP is an Oncology Clinical Pathway (OCP) that supports evidence-based practice providing highquality, safe, and cost-effective care for veterans reducing variability of care in the VHA.
Methods
The National Oncology Program Office assembled a Prostate Cancer Team (PCT) to develop OCPs. The pathways were incorporated into note templates that record clinical decisions using text and metadata (Health Factors [HF]), and record pathway adherence for the 4 key nodes of the PCMTP. The templates were pilot-tested and improved using an iterative process over a 3-month period. Further evaluation was conducted by the Office of Human Factors Engineering and the National Clinical Template Workgroup, utilizing a heuristic evaluation to ensure standardization, interoperability, and reduce duplication. HF data were retrieved from the Corporate Data Warehouse using a custom-built dashboard. Descriptive statistics of PCMTP use are presented.
Results
Between 4/1/2021 and 6/22/2022, 6276 health factors were generated from 1707 unique veterans in whom this clinical pathway was accessed. 328 distinct providers participated at 61 sites. Average veteran age was 73 years. (range 45-100) including 42% Black and 56% White. Of 1243 veterans considered for germline testing, 96.6% had germline testing ordered and for 1102 veterans considered for tumor testing, 93.3% had tumor testing ordered.
Conclusions
Pathway adherence exceeded the 80% benchmark. Race representation was diverse and reflective of the VA prostate cancer population. About 46% of VA oncology practices have used the PCMTP for ~11% of the estimated 15,000 veterans with metastatic prostate cancer in VHA. Increased use of this pathway is expected to improve outcomes for veterans with prostate cancer
New Delivery Models Improve Access to Germline Testing for Patients With Advanced Prostate Cancer
Objectives
The VA Oncology Clinical Pathway for Prostate Cancer is the first to include both tumor and germline testing to inform treatment and clinical trial eligibility for advanced disease. Anticipating increased germline testing demand, new germline testing delivery models were created to augment the existing traditional model of referring patients to genetics providers (VA or non-VA) for germline testing. The new models include: a non-traditional model where oncology clinicians perform all pre- and post-test activities and consult genetics when needed, and a hybrid model where oncology clinicians obtain informed consent and place e-consults for germline test ordering, results disclosure, and genetics follow-up, as needed. We sought to assess germline testing by delivery model.
Methods
Data sources included the National Precision Oncology Program (NPOP) dashboard and NPOP-contracted germline testing laboratories. Patient inclusion criteria: living as of 5/2/2021 with VA oncology or urology visits after 5/2/2021. We used multivariate regression to assess associations between patient characteristics and germline testing between 5/3/2021 (pathway launch) and 5/2/2022, accounting for clustering of patients within ordering clinicians.
Results
We identified 16,041 patients from 129 VA facilities with average age 75 years (SD, 8.2; range, 36- 102), 28.7% Black and 60.0% White. Only 5.6% had germline testing ordered by 60 clinicians at 67 facilities with 52.2% of orders by the hybrid model, 32.1% the non-traditional model, and 15.4% the traditional model. Patient characteristics positively associated with germline testing included care at hybrid model (OR, 6.03; 95% CI, 4.62-7.88) or non-traditional model facilities (OR, 5.66; 95% CI, 4.24-7.56) compared to the traditional model, completing tumor molecular testing (OR, 5.80; 95%CI, 4.98-6.75), and Black compared with White race (OR, 1.24; 95%CI, 1.06-1.45). Compared to patients aged < 66 years, patients aged 66-75 years and 76-85 years were less likely to have germline testing (OR, 0.74; 95%CI, 0.60-0.90; and OR, 0.67; 95%CI, 0.53-0.84, respectively).
Conclusions/Implications
Though only a small percentage of patients with advanced prostate cancer had NPOP-supported germline testing since the pathway launch, the new delivery models were instrumental to improving access to germline testing. Ongoing evaluation will help to understand observed demographic differences in germline testing. Implementation and evaluation of strategies that promote adoption of the new germline testing delivery models is needed. 0922FED AVAHO_Abstracts.indd 15 8
Objectives
The VA Oncology Clinical Pathway for Prostate Cancer is the first to include both tumor and germline testing to inform treatment and clinical trial eligibility for advanced disease. Anticipating increased germline testing demand, new germline testing delivery models were created to augment the existing traditional model of referring patients to genetics providers (VA or non-VA) for germline testing. The new models include: a non-traditional model where oncology clinicians perform all pre- and post-test activities and consult genetics when needed, and a hybrid model where oncology clinicians obtain informed consent and place e-consults for germline test ordering, results disclosure, and genetics follow-up, as needed. We sought to assess germline testing by delivery model.
Methods
Data sources included the National Precision Oncology Program (NPOP) dashboard and NPOP-contracted germline testing laboratories. Patient inclusion criteria: living as of 5/2/2021 with VA oncology or urology visits after 5/2/2021. We used multivariate regression to assess associations between patient characteristics and germline testing between 5/3/2021 (pathway launch) and 5/2/2022, accounting for clustering of patients within ordering clinicians.
Results
We identified 16,041 patients from 129 VA facilities with average age 75 years (SD, 8.2; range, 36- 102), 28.7% Black and 60.0% White. Only 5.6% had germline testing ordered by 60 clinicians at 67 facilities with 52.2% of orders by the hybrid model, 32.1% the non-traditional model, and 15.4% the traditional model. Patient characteristics positively associated with germline testing included care at hybrid model (OR, 6.03; 95% CI, 4.62-7.88) or non-traditional model facilities (OR, 5.66; 95% CI, 4.24-7.56) compared to the traditional model, completing tumor molecular testing (OR, 5.80; 95%CI, 4.98-6.75), and Black compared with White race (OR, 1.24; 95%CI, 1.06-1.45). Compared to patients aged < 66 years, patients aged 66-75 years and 76-85 years were less likely to have germline testing (OR, 0.74; 95%CI, 0.60-0.90; and OR, 0.67; 95%CI, 0.53-0.84, respectively).
Conclusions/Implications
Though only a small percentage of patients with advanced prostate cancer had NPOP-supported germline testing since the pathway launch, the new delivery models were instrumental to improving access to germline testing. Ongoing evaluation will help to understand observed demographic differences in germline testing. Implementation and evaluation of strategies that promote adoption of the new germline testing delivery models is needed. 0922FED AVAHO_Abstracts.indd 15 8
Objectives
The VA Oncology Clinical Pathway for Prostate Cancer is the first to include both tumor and germline testing to inform treatment and clinical trial eligibility for advanced disease. Anticipating increased germline testing demand, new germline testing delivery models were created to augment the existing traditional model of referring patients to genetics providers (VA or non-VA) for germline testing. The new models include: a non-traditional model where oncology clinicians perform all pre- and post-test activities and consult genetics when needed, and a hybrid model where oncology clinicians obtain informed consent and place e-consults for germline test ordering, results disclosure, and genetics follow-up, as needed. We sought to assess germline testing by delivery model.
Methods
Data sources included the National Precision Oncology Program (NPOP) dashboard and NPOP-contracted germline testing laboratories. Patient inclusion criteria: living as of 5/2/2021 with VA oncology or urology visits after 5/2/2021. We used multivariate regression to assess associations between patient characteristics and germline testing between 5/3/2021 (pathway launch) and 5/2/2022, accounting for clustering of patients within ordering clinicians.
Results
We identified 16,041 patients from 129 VA facilities with average age 75 years (SD, 8.2; range, 36- 102), 28.7% Black and 60.0% White. Only 5.6% had germline testing ordered by 60 clinicians at 67 facilities with 52.2% of orders by the hybrid model, 32.1% the non-traditional model, and 15.4% the traditional model. Patient characteristics positively associated with germline testing included care at hybrid model (OR, 6.03; 95% CI, 4.62-7.88) or non-traditional model facilities (OR, 5.66; 95% CI, 4.24-7.56) compared to the traditional model, completing tumor molecular testing (OR, 5.80; 95%CI, 4.98-6.75), and Black compared with White race (OR, 1.24; 95%CI, 1.06-1.45). Compared to patients aged < 66 years, patients aged 66-75 years and 76-85 years were less likely to have germline testing (OR, 0.74; 95%CI, 0.60-0.90; and OR, 0.67; 95%CI, 0.53-0.84, respectively).
Conclusions/Implications
Though only a small percentage of patients with advanced prostate cancer had NPOP-supported germline testing since the pathway launch, the new delivery models were instrumental to improving access to germline testing. Ongoing evaluation will help to understand observed demographic differences in germline testing. Implementation and evaluation of strategies that promote adoption of the new germline testing delivery models is needed. 0922FED AVAHO_Abstracts.indd 15 8
Utilization of Next Generation Sequencing in Metastatic Colorectal Cancer
Introduction
Metastatic colorectal cancer (mCRC) is one of the most common and lethal cancers. Nextgeneration sequencing (NGS) has been recommended as a tool to help guide treatment by identifying actionable genetic mutations. However, data regarding realworld usage of NGS in a Veterans Affairs (VA) health care system is lacking. We conducted a retrospective observational study of the patterns of NGS usage in patients with mCRC at the South Texas Veterans Affairs Healthcare System (STVAHCS).
Methods
We identified patients with a diagnosis of mCRC evaluated and treated at STVAHCS between January 1, 2018 and June 1, 2022. We assessed the prevalence of utilizing NGS on solid tumor samples performed by Foundation One and identified the presence of different molecular aberrations detected by NGS.
Results
65 patients were identified. Median age was 68 years. 63 (96.9%) were males and 2 (3.1%) were females. 29 (44.6%) were Hispanic, 25 (38.5%) were White, 10 (15.4%) were African American and 1 (1.5%) was Pacific Islander. NGS was performed in 34 (52.3%) patients. The most common reasons for not performing NGS were unknown/not documented (54.8%), early mortality (29%), lack of adequate tissue (12.9%) and patient refusal of treatment (3.2%). The most common molecular aberrations identified in patients who had NGS were TP53 (73.5%), APC (64.7%), KRAS (47.1%), ATM (20.6%), SMAD4 (14.7%) and BRAF (14.7%). All patients who had NGS were found to have at least one identifiable mutation.
Conclusions
Approximately 50% of patients with mCRC did not have NGS performed on their tissue sample. This rate is similar to other real-world studies in non-VA settings. Documented reasons for lack of NGS testing included inadequate tissue and early patient mortality. Other potential reasons could be lack of efficient VA clinical testing protocols, use of simple molecular testing rather than comprehensive NGS testing and limited knowledge of availability of NGS among providers. Measures that can be taken to increase utilization of NGS include incorporating NGS testing early in the disease course, incorporating testing into VA clinical pathways, improving physician education, increasing the size of solid tissue samples and ordering liquid biopsies where solid tissue is deficient.
Introduction
Metastatic colorectal cancer (mCRC) is one of the most common and lethal cancers. Nextgeneration sequencing (NGS) has been recommended as a tool to help guide treatment by identifying actionable genetic mutations. However, data regarding realworld usage of NGS in a Veterans Affairs (VA) health care system is lacking. We conducted a retrospective observational study of the patterns of NGS usage in patients with mCRC at the South Texas Veterans Affairs Healthcare System (STVAHCS).
Methods
We identified patients with a diagnosis of mCRC evaluated and treated at STVAHCS between January 1, 2018 and June 1, 2022. We assessed the prevalence of utilizing NGS on solid tumor samples performed by Foundation One and identified the presence of different molecular aberrations detected by NGS.
Results
65 patients were identified. Median age was 68 years. 63 (96.9%) were males and 2 (3.1%) were females. 29 (44.6%) were Hispanic, 25 (38.5%) were White, 10 (15.4%) were African American and 1 (1.5%) was Pacific Islander. NGS was performed in 34 (52.3%) patients. The most common reasons for not performing NGS were unknown/not documented (54.8%), early mortality (29%), lack of adequate tissue (12.9%) and patient refusal of treatment (3.2%). The most common molecular aberrations identified in patients who had NGS were TP53 (73.5%), APC (64.7%), KRAS (47.1%), ATM (20.6%), SMAD4 (14.7%) and BRAF (14.7%). All patients who had NGS were found to have at least one identifiable mutation.
Conclusions
Approximately 50% of patients with mCRC did not have NGS performed on their tissue sample. This rate is similar to other real-world studies in non-VA settings. Documented reasons for lack of NGS testing included inadequate tissue and early patient mortality. Other potential reasons could be lack of efficient VA clinical testing protocols, use of simple molecular testing rather than comprehensive NGS testing and limited knowledge of availability of NGS among providers. Measures that can be taken to increase utilization of NGS include incorporating NGS testing early in the disease course, incorporating testing into VA clinical pathways, improving physician education, increasing the size of solid tissue samples and ordering liquid biopsies where solid tissue is deficient.
Introduction
Metastatic colorectal cancer (mCRC) is one of the most common and lethal cancers. Nextgeneration sequencing (NGS) has been recommended as a tool to help guide treatment by identifying actionable genetic mutations. However, data regarding realworld usage of NGS in a Veterans Affairs (VA) health care system is lacking. We conducted a retrospective observational study of the patterns of NGS usage in patients with mCRC at the South Texas Veterans Affairs Healthcare System (STVAHCS).
Methods
We identified patients with a diagnosis of mCRC evaluated and treated at STVAHCS between January 1, 2018 and June 1, 2022. We assessed the prevalence of utilizing NGS on solid tumor samples performed by Foundation One and identified the presence of different molecular aberrations detected by NGS.
Results
65 patients were identified. Median age was 68 years. 63 (96.9%) were males and 2 (3.1%) were females. 29 (44.6%) were Hispanic, 25 (38.5%) were White, 10 (15.4%) were African American and 1 (1.5%) was Pacific Islander. NGS was performed in 34 (52.3%) patients. The most common reasons for not performing NGS were unknown/not documented (54.8%), early mortality (29%), lack of adequate tissue (12.9%) and patient refusal of treatment (3.2%). The most common molecular aberrations identified in patients who had NGS were TP53 (73.5%), APC (64.7%), KRAS (47.1%), ATM (20.6%), SMAD4 (14.7%) and BRAF (14.7%). All patients who had NGS were found to have at least one identifiable mutation.
Conclusions
Approximately 50% of patients with mCRC did not have NGS performed on their tissue sample. This rate is similar to other real-world studies in non-VA settings. Documented reasons for lack of NGS testing included inadequate tissue and early patient mortality. Other potential reasons could be lack of efficient VA clinical testing protocols, use of simple molecular testing rather than comprehensive NGS testing and limited knowledge of availability of NGS among providers. Measures that can be taken to increase utilization of NGS include incorporating NGS testing early in the disease course, incorporating testing into VA clinical pathways, improving physician education, increasing the size of solid tissue samples and ordering liquid biopsies where solid tissue is deficient.
Impact of Race on Outcomes of High-Risk Patients With Prostate Cancer Treated With Moderately Hypofractionated Radiotherapy in an Equal Access Setting
Although moderately hypofractionated radiotherapy (MHRT) is an accepted treatment for localized prostate cancer, its adaptation remains limited in the United States.1,2 MHRT theoretically exploits α/β ratio differences between the prostate (1.5 Gy), bladder (5-10 Gy), and rectum (3 Gy), thereby reducing late treatment-related adverse effects compared with those of conventional fractionation at biologically equivalent doses.3-8 Multiple randomized noninferiority trials have demonstrated equivalent outcomes between MHRT and conventional fraction with no appreciable increase in patient-reported toxicity.9-14 Although these studies have led to the acceptance of MHRT as a standard treatment, the majority of these trials involve individuals with low- and intermediate-risk disease.
There are less phase 3 data addressing MHRT for high-risk prostate cancer (HRPC).10,12,14-17 Only 2 studies examined predominately high-risk populations, accounting for 83 and 292 patients, respectively.15,16 Additional phase 3 trials with small proportions of high-risk patients (n = 126, 12%; n = 53, 35%) offer limited additional information regarding clinical outcomes and toxicity rates specific to high-risk disease.10-12 Numerous phase 1 and 2 studies report various field designs and fractionation plans for MHRT in the context of high-risk disease, although the applicability of these data to off-trial populations remains limited.18-20
Furthermore, African American individuals are underrepresented in the trials establishing the role of MHRT despite higher rates of prostate cancer incidence, more advanced disease stage at diagnosis, and higher rates of prostate cancer–specific survival (PCSS) when compared with White patients.21 Racial disparities across patients with prostate cancer and their management are multifactorial across health care literacy, education level, access to care (including transportation issues), and issues of adherence and distrust.22-25 Correlation of patient race to prostate cancer outcomes varies greatly across health care systems, with the US Department of Veterans Affairs (VA) equal access system providing robust mental health services and transportation services for some patients, while demonstrating similar rates of stage-adjusted PCSS between African American and White patients across a broad range of treatment modalities.26-28 Given the paucity of data exploring outcomes following MHRT for African American patients with HRPC, the present analysis provides long-term clinical outcomes and toxicity profiles for an off-trial majority African American population with HRPC treated with MHRT within the VA.
Methods
Records were retrospectively reviewed under an institutional review board–approved protocol for all patients with HRPC treated with definitive MHRT at the Durham Veterans Affairs Healthcare System in North Carolina between November 2008 and August 2018. Exclusion criteria included < 12 months of follow-up or elective nodal irradiation. Demographic variables obtained included age at diagnosis, race, clinical T stage, pre-MHRT prostate-specific antigen (PSA), Gleason grade group at diagnosis, favorable vs unfavorable high-risk disease, pre-MHRT international prostate symptom score (IPSS), and pre-MHRT urinary medication usage (yes/no).29
Concurrent androgen deprivation therapy (ADT) was initiated 6 to 8 weeks before MHRT unless medically contraindicated per the discretion of the treating radiation oncologist. Patients generally received 18 to 24 months of ADT, with those with favorable HRPC (ie, T1c disease with either Gleason 4+4 and PSA < 10 mg/mL or Gleason 3+3 and PSA > 20 ng/mL) receiving 6 months after 2015.29 Patients were simulated supine in either standard or custom immobilization with a full bladder and empty rectum. MHRT fractionation plans included 70 Gy at 2.5 Gy per fraction and 60 Gy at 3 Gy per fraction. Radiotherapy targets included the prostate and seminal vesicles without elective nodal coverage per institutional practice. Treatments were delivered following image guidance, either prostate matching with cone beam computed tomography or fiducial matching with kilo voltage imaging. All patients received intensity-modulated radiotherapy. For plans delivering 70 Gy at 2.5 Gy per fraction, constraints included bladder V (volume receiving) 70 < 10 cc, V65 ≤ 15%, V40 ≤ 35%, rectum V70 < 10 cc, V65 ≤ 10%, V40 ≤ 35%, femoral heads maximum point dose ≤ 40 Gy, penile bulb mean dose ≤ 50 Gy, and small bowel V40 ≤ 1%. For plans delivering 60 Gy at 3 Gy per fraction, constraints included rectum V57 ≤ 15%, V46 ≤ 30%, V37 ≤ 50%, bladder V60 ≤ 5%, V46 ≤ 30%, V37 ≤ 50%, and femoral heads V43 ≤ 5%.
Gastrointestinal (GI) and genitourinary (GU) toxicities were graded using Common Terminology Criteria for Adverse Events (CTCAE), version 5.0, with acute toxicity defined as on-treatment < 3 months following completion of MHRT. Late toxicity was defined as ≥ 3 months following completion of MHRT. Individuals were seen in follow-up at 6 weeks and 3 months with PSA and testosterone after MHRT completion, then every 6 to 12 months for 5 years and annually thereafter. Each follow-up visit included history, physical examination, IPSS, and CTCAE grading for GI and GU toxicity.
The Wilcoxon rank sum test and χ2 test were used to compare differences in demographic data, dosimetric parameters, and frequency of toxicity events with respect to patient race. Clinical endpoints including biochemical recurrence-free survival (BRFS; defined by Phoenix criteria as 2.0 above PSA nadir), distant metastases-free survival (DMFS), PCSS, and overall survival (OS) were estimated from time of radiotherapy completion by the Kaplan-Meier method and compared between African American and White race by log-rank testing.30 Late GI and GU toxicity-free survival were estimated by Kaplan-Meier plots and compared between African American and White patients by the log-rank test. Statistical analysis was performed using SAS 9.4.
Results
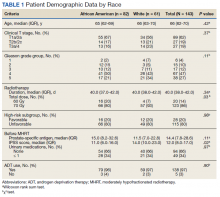
We identified 143 patients with HRPC treated with definitive MHRT between November 2008 and August 2018 (Table 1). Mean age was 65 years (range, 36-80 years); 57% were African American men. Eighty percent of individuals had unfavorable high-risk disease. Median (IQR) PSA was 14.4 (7.8-28.6). Twenty-six percent had grade group 1-3 disease, 47% had grade group 4 disease, and 27% had grade group 5 disease. African American patients had significantly lower pre-MHRT IPSS scores than White patients (mean IPSS, 11 vs 14, respectively; P = .02) despite similar rates of preradiotherapy urinary medication usage (66% and 66%, respectively).
Eighty-six percent received 70 Gy over 28 fractions, with institutional protocol shifting to 60 Gy over 20 fractions (14%) in June 2017. The median (IQR) duration of radiotherapy was 39 (38-42) days, with 97% of individuals undergoing ADT for a median (IQR) duration of 24 (24-36) months. The median follow-up time was 38 months, with 57 (40%) patients followed for at least 60 months.
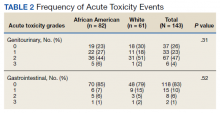
Grade 3 GI and GU acute toxicity events were observed in 1% and 4% of all individuals, respectively (Table 2). No acute GI or GU grade 4+ events were observed. No significant differences in acute GU or GI toxicity were observed between African American and White patients.
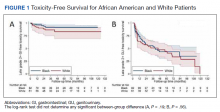
No significant differences between African American and White patients were observed for late grade 2+ GI (P = .19) or GU (P = .55) toxicity. Late grade 2+ GI toxicity was observed in 17 (12%) patients overall (Figure 1A). One grade 3 and 1 grade 4 late GI event were observed following MHRT completion: The latter involved hospitalization for bleeding secondary to radiation proctitis in the context of cirrhosis predating MHRT. Late grade 2+ GU toxicity was observed in 80 (56%) patients, with late grade 2 events steadily increasing over time (Figure 1B). Nine late grade 3 GU toxicity events were observed at a median of 13 months following completion of MHRT, 2 of which occurred more than 24 months after MHRT completion. No late grade 4 or 5 GU events were observed. IPSS values both before MHRT and at time of last follow-up were available for 65 (40%) patients, with a median (IQR) IPSS of 10 (6-16) before MHRT and 12 (8-16) at last follow-up at a median (IQR) interval of 36 months (26-76) from radiation completion.
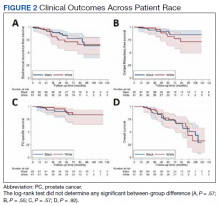
No significant differences were observed between African American and White patients with respect to BRFS, DMFS, PCSS, or OS (Figure 2). Overall, 21 of 143 (15%) patients experienced biochemical recurrence: 5-year BRFS was 77% (95% CI, 67%-85%) for all patients, 83% (95% CI, 70%-91%) for African American patients, and 71% (95% CI, 53%-82%) for White patients. Five-year DMFS was 87% (95% CI, 77%-92%) for all individuals, 91% (95% CI, 80%-96%) for African American patients, and 81% (95% CI, 62%-91%) for White patients. Five-year PCSS was 89% (95% CI, 80%-94%) for all patients, with 5-year PCSS rates of 90% (95% CI, 79%-95%) for African American patients and 87% (95% CI, 70%-95%) for White patients. Five-year OS was 75% overall (95% CI, 64%-82%), with 5-year OS rates of 73% (95% CI, 58%-83%) for African American patients and 77% (95% CI, 60%-87%) for White patients.
Discussion
In this study, we reported acute and late GI and GU toxicity rates as well as clinical outcomes for a majority African American population with predominately unfavorable HRPC treated with MHRT in an equal access health care environment. We found that MHRT was well tolerated with high rates of biochemical control, PCSS, and OS. Additionally, outcomes were not significantly different across patient race. To our knowledge, this is the first report of MHRT for HRPC in a majority African American population.
We found that MHRT was an effective treatment for patients with HRPC, in particular those with unfavorable high-risk disease. While prior prospective and randomized studies have investigated the use of MHRT, our series was larger than most and had a predominately unfavorable high-risk population.12,15-17 Our biochemical and PCSS rates compare favorably with those of HRPC trial populations, particularly given the high proportion of unfavorable high-risk disease.12,15,16 Despite similar rates of biochemical control, OS was lower in the present cohort than in HRPC trial populations, even with a younger median age at diagnosis. The similarly high rates of non–HRPC-related death across race may reflect differences in baseline comorbidities compared with trial populations as well as reported differences between individuals in the VA and the private sector.31 This suggests that MHRT can be an effective treatment for patients with unfavorable HRPC.
We did not find any differences in outcomes between African American and White individuals with HRPC treated with MHRT. Furthermore, our study demonstrates long-term rates of BRFS and PCSS in a majority African American population with predominately unfavorable HRPC that are comparable with those of prior randomized MHRT studies in high-risk, predominately White populations.12,15,16 Prior reports have found that African American men with HRPC may be at increased risk for inferior clinical outcomes due to a number of socioeconomic, biologic, and cultural mediators.26,27,32 Such individuals may disproportionally benefit from shorter treatment courses that improve access to radiotherapy, a well-documented disparity for African American men with localized prostate cancer.33-36 The VA is an ideal system for studying racial disparities within prostate cancer, as accessibility of mental health and transportation services, income, and insurance status are not barriers to preventative or acute care.37 Our results are concordant with those previously seen for African American patients with prostate cancer seen in the VA, which similarly demonstrate equal outcomes with those of other races.28,36 Incorporation of the earlier mentioned VA services into oncologic care across other health care systems could better characterize determinants of racial disparities in prostate cancer, including the prognostic significance of shortening treatment duration and number of patient visits via MHRT.
Despite widespread acceptance in prostate cancer radiotherapy guidelines, routine use of MHRT seems limited across all stages of localized prostate cancer.1,2 Late toxicity is a frequently noted concern regarding MHRT use. Higher rates of late grade 2+ GI toxicity were observed in the hypofractionation arm of the HYPRO trial.17 While RTOG 0415 did not include patients with HRPC, significantly higher rates of physician-reported (but not patient-reported) late grade 2+ GI and GU toxicity were observed using the same MHRT fractionation regimen used for the majority of individuals in our cohort.9 In our study, the steady increase in late grade 2 GU toxicity is consistent with what is seen following conventionally fractionated radiotherapy and is likely multifactorial.38 The mean IPSS difference of 2/35 from pre-MHRT baseline to the time of last follow-up suggests minimal quality of life decline. The relatively stable IPSSs over time alongside the > 50% prevalence of late grade 2 GU toxicity per CTCAE grading seems consistent with the discrepancy noted in RTOG 0415 between increased physician-reported late toxicity and favorable patient-reported quality of life scores.9 Moreover, significant variance exists in toxicity grading across scoring systems, revised editions of CTCAE, and physician-specific toxicity classification, particularly with regard to the use of adrenergic receptor blocker medications. In light of these factors, the high rate of late grade 2 GU toxicity in our study should be interpreted in the context of largely stable post-MHRT IPSSs and favorable rates of late GI grade 2+ and late GU grade 3+ toxicity.
Limitations
This study has several inherent limitations. While the size of the current HRPC cohort is notably larger than similar populations within the majority of phase 3 MHRT trials, these data derive from a single VA hospital. It is unclear whether these outcomes would be representative in a similar high-risk population receiving care outside of the VA equal access system. Follow-up data beyond 5 years was available for less than half of patients, partially due to nonprostate cancer–related mortality at a higher rate than observed in HRPC trial populations.12,15,16 Furthermore, all GI toxicity events were exclusively physician reported, and GU toxicity reporting was limited in the off-trial setting with not all patients routinely completing IPSS questionnaires following MHRT completion. However, all patients were treated similarly, and radiation quality was verified over the treatment period with mandated accreditation, frequent standardized output checks, and systematic treatment review.39
Conclusions
Patients with HRPC treated with MHRT in an equal access, off-trial setting demonstrated favorable rates of biochemical control with acceptable rates of acute and late GI and GU toxicities. Clinical outcomes, including biochemical control, were not significantly different between African American and White patients, which may reflect equal access to care within the VA irrespective of income and insurance status. Incorporating VA services, such as access to primary care, mental health services, and transportation across other health care systems may aid in characterizing and mitigating racial and gender disparities in oncologic care.
Acknowledgments
Portions of this work were presented at the November 2020 ASTRO conference. 40
1. Stokes WA, Kavanagh BD, Raben D, Pugh TJ. Implementation of hypofractionated prostate radiation therapy in the United States: a National Cancer Database analysis. Pract Radiat Oncol. 2017;7:270-278. doi:10.1016/j.prro.2017.03.011
2. Jaworski L, Dominello MM, Heimburger DK, et al. Contemporary practice patterns for intact and post-operative prostate cancer: results from a statewide collaborative. Int J Radiat Oncol Biol Phys. 2019;105(1):E282. doi:10.1016/j.ijrobp.2019.06.1915
3. Miralbell R, Roberts SA, Zubizarreta E, Hendry JH. Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5,969 patients in seven international institutional datasets: α/β = 1.4 (0.9-2.2) Gy. Int J Radiat Oncol Biol Phys. 2012;82(1):e17-e24. doi:10.1016/j.ijrobp.2010.10.075
4. Tree AC, Khoo VS, van As NJ, Partridge M. Is biochemical relapse-free survival after profoundly hypofractionated radiotherapy consistent with current radiobiological models? Clin Oncol (R Coll Radiol). 2014;26(4):216-229. doi:10.1016/j.clon.2014.01.008
5. Brenner DJ. Fractionation and late rectal toxicity. Int J Radiat Oncol Biol Phys. 2004;60(4):1013-1015. doi:10.1016/j.ijrobp.2004.04.014
6. Tucker SL, Thames HD, Michalski JM, et al. Estimation of α/β for late rectal toxicity based on RTOG 94-06. Int J Radiat Oncol Biol Phys. 2011;81(2):600-605. doi:10.1016/j.ijrobp.2010.11.080
7. Dasu A, Toma-Dasu I. Prostate alpha/beta revisited—an analysis of clinical results from 14 168 patients. Acta Oncol. 2012;51(8):963-974. doi:10.3109/0284186X.2012.719635 start
8. Proust-Lima C, Taylor JMG, Sécher S, et al. Confirmation of a Low α/β ratio for prostate cancer treated by external beam radiation therapy alone using a post-treatment repeated-measures model for PSA dynamics. Int J Radiat Oncol Biol Phys. 2011;79(1):195-201. doi:10.1016/j.ijrobp.2009.10.008
9. Lee WR, Dignam JJ, Amin MB, et al. Randomized phase III noninferiority study comparing two radiotherapy fractionation schedules in patients with low-risk prostate cancer. J Clin Oncol. 2016;34(20): 2325-2332. doi:10.1200/JCO.2016.67.0448
10. Dearnaley D, Syndikus I, Mossop H, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17(8):1047-1060. doi:10.1016/S1470-2045(16)30102-4
11. Catton CN, Lukka H, Gu C-S, et al. Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. J Clin Oncol. 2017;35(17):1884-1890. doi:10.1200/JCO.2016.71.7397
12. Pollack A, Walker G, Horwitz EM, et al. Randomized trial of hypofractionated external-beam radiotherapy for prostate cancer. J Clin Oncol. 2013;31(31):3860-3868. doi:10.1200/JCO.2013.51.1972
13. Hoffman KE, Voong KR, Levy LB, et al. Randomized trial of hypofractionated, dose-escalated, intensity-modulated radiation therapy (IMRT) versus conventionally fractionated IMRT for localized prostate cancer. J Clin Oncol. 2018;36(29):2943-2949. doi:10.1200/JCO.2018.77.9868
14. Wilkins A, Mossop H, Syndikus I, et al. Hypofractionated radiotherapy versus conventionally fractionated radiotherapy for patients with intermediate-risk localised prostate cancer: 2-year patient-reported outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2015;16(16):1605-1616. doi:10.1016/S1470-2045(15)00280-6
15. Incrocci L, Wortel RC, Alemayehu WG, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with localised prostate cancer (HYPRO): final efficacy results from a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2016;17(8):1061-1069. doi.10.1016/S1470-2045(16)30070-5
16. Arcangeli G, Saracino B, Arcangeli S, et al. Moderate hypofractionation in high-risk, organ-confined prostate cancer: final results of a phase III randomized trial. J Clin Oncol. 2017;35(17):1891-1897. doi:10.1200/JCO.2016.70.4189
17. Aluwini S, Pos F, Schimmel E, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): late toxicity results from a randomised, non-inferiority, phase 3 trial. Lancet Oncol. 2016;17(4):464-474. doi:10.1016/S1470-2045(15)00567-7
18. Pervez N, Small C, MacKenzie M, et al. Acute toxicity in high-risk prostate cancer patients treated with androgen suppression and hypofractionated intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;76(1):57-64. doi:10.1016/j.ijrobp.2009.01.048
19. Magli A, Moretti E, Tullio A, Giannarini G. Hypofractionated simultaneous integrated boost (IMRT- cancer: results of a prospective phase II trial SIB) with pelvic nodal irradiation and concurrent androgen deprivation therapy for high-risk prostate cancer: results of a prospective phase II trial. Prostate Cancer Prostatic Dis. 2018;21(2):269-276. doi:10.1038/s41391-018-0034-0
20. Di Muzio NG, Fodor A, Noris Chiorda B, et al. Moderate hypofractionation with simultaneous integrated boost in prostate cancer: long-term results of a phase I–II study. Clin Oncol (R Coll Radiol). 2016;28(8):490-500. doi:10.1016/j.clon.2016.02.005
21. DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA Cancer J Clin. 2019;69(3):21-233. doi:10.3322/caac.21555
22. Wolf MS, Knight SJ, Lyons EA, et al. Literacy, race, and PSA level among low-income men newly diagnosed with prostate cancer. Urology. 2006(1);68:89-93. doi:10.1016/j.urology.2006.01.064
23. Rebbeck TR. Prostate cancer disparities by race and ethnicity: from nucleotide to neighborhood. Cold Spring Harb Perspect Med. 2018;8(9):a030387. doi:10.1101/cshperspect.a030387
24. Guidry JJ, Aday LA, Zhang D, Winn RJ. Transportation as a barrier to cancer treatment. Cancer Pract. 1997;5(6):361-366.
25. Friedman DB, Corwin SJ, Dominick GM, Rose ID. African American men’s understanding and perceptions about prostate cancer: why multiple dimensions of health literacy are important in cancer communication. J Community Health. 2009;34(5):449-460. doi:10.1007/s10900-009-9167-3
26. Connell PP, Ignacio L, Haraf D, et al. Equivalent racial outcome after conformal radiotherapy for prostate cancer: a single departmental experience. J Clin Oncol. 2001;19(1):54-61. doi:10.1200/JCO.2001.19.1.54
27. Dess RT, Hartman HE, Mahal BA, et al. Association of black race with prostate cancer-specific and other-cause mortality. JAMA Oncol. 2019;5(1):975-983. doi:10.1200/JCO.2001.19.1.54
28. McKay RR, Sarkar RR, Kumar A, et al. Outcomes of Black men with prostate cancer treated with radiation therapy in the Veterans Health Administration. Cancer. 2021;127(3):403-411. doi:10.1002/cncr.33224

29. Muralidhar V, Chen M-H, Reznor G, et al. Definition and validation of “favorable high-risk prostate cancer”: implications for personalizing treatment of radiation-managed patients. Int J Radiat Oncol Biol Phys. 2015;93(4):828-835. doi:10.1016/j.ijrobp.2015.07.2281
30. Roach M 3rd, Hanks G, Thames H Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65(4):965-974. doi:10.1016/j.ijrobp.2006.04.029
31. Freeman VL, Durazo-Arvizu R, Arozullah AM, Keys LC. Determinants of mortality following a diagnosis of prostate cancer in Veterans Affairs and private sector health care systems. Am J Public Health. 2003;93(100):1706-1712. doi:10.2105/ajph.93.10.1706
32. Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54(2):78-93. doi:10.3322/canjclin.54.2.78
33. Zemplenyi AT, Kaló Z, Kovacs G, et al. Cost-effectiveness analysis of intensity-modulated radiation therapy with normal and hypofractionated schemes for the treatment of localised prostate cancer. Eur J Cancer Care. 2018;27(1):e12430. doi:10.1111/ecc.12430
34. Klabunde CN, Potosky AL, Harlan LC, Kramer BS. Trends and black/white differences in treatment for nonmetastatic prostate cancer. Med Care. 1998;36(9):1337-1348. doi:10.1097/00005650-199809000-00006
35. Harlan L, Brawley O, Pommerenke F, Wali P, Kramer B. Geographic, age, and racial variation in the treatment of local/regional carcinoma of the prostate. J Clin Oncol. 1995;13(1):93-100. doi:10.1200/JCO.1995.13.1.93
36. Riviere P, Luterstein E, Kumar A, et al. Racial equity among African-American and non-Hispanic white men diagnosed with prostate cancer in the veterans affairs healthcare system. Int J Radiat Oncol Biol Phys. 2019;105:E305.
37. Peterson K, Anderson J, Boundy E, Ferguson L, McCleery E, Waldrip K. Mortality disparities in racial/ethnic minority groups in the Veterans Health Administration: an evidence review and map. Am J Public Health. 2018;108(3):e1-e11. doi:10.2105/AJPH.2017.304246
38. Zietman AL, DeSilvio ML, Slater JD, et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA. 2005;294(10):1233-1239. doi:10.1001/jama.294.10.1233
39. Hagan M, Kapoor R, Michalski J, et al. VA-Radiation Oncology Quality Surveillance program. Int J Radiat Oncol Biol Phys. 2020;106(3):639-647. doi.10.1016/j.ijrobp.2019.08.064
40. Carpenter DJ, Natesan D, Floyd W, et al. Long-term experience in an equal access health care system using moderately hypofractionated radiotherapy for high risk prostate cancer in a predominately African American population with unfavorable disease. Int J Radiat Oncol Biol Phys. 2020;108(3):E417. https://www.redjournal.org/article/S0360-3016(20)33923-7/fulltext
Although moderately hypofractionated radiotherapy (MHRT) is an accepted treatment for localized prostate cancer, its adaptation remains limited in the United States.1,2 MHRT theoretically exploits α/β ratio differences between the prostate (1.5 Gy), bladder (5-10 Gy), and rectum (3 Gy), thereby reducing late treatment-related adverse effects compared with those of conventional fractionation at biologically equivalent doses.3-8 Multiple randomized noninferiority trials have demonstrated equivalent outcomes between MHRT and conventional fraction with no appreciable increase in patient-reported toxicity.9-14 Although these studies have led to the acceptance of MHRT as a standard treatment, the majority of these trials involve individuals with low- and intermediate-risk disease.
There are less phase 3 data addressing MHRT for high-risk prostate cancer (HRPC).10,12,14-17 Only 2 studies examined predominately high-risk populations, accounting for 83 and 292 patients, respectively.15,16 Additional phase 3 trials with small proportions of high-risk patients (n = 126, 12%; n = 53, 35%) offer limited additional information regarding clinical outcomes and toxicity rates specific to high-risk disease.10-12 Numerous phase 1 and 2 studies report various field designs and fractionation plans for MHRT in the context of high-risk disease, although the applicability of these data to off-trial populations remains limited.18-20
Furthermore, African American individuals are underrepresented in the trials establishing the role of MHRT despite higher rates of prostate cancer incidence, more advanced disease stage at diagnosis, and higher rates of prostate cancer–specific survival (PCSS) when compared with White patients.21 Racial disparities across patients with prostate cancer and their management are multifactorial across health care literacy, education level, access to care (including transportation issues), and issues of adherence and distrust.22-25 Correlation of patient race to prostate cancer outcomes varies greatly across health care systems, with the US Department of Veterans Affairs (VA) equal access system providing robust mental health services and transportation services for some patients, while demonstrating similar rates of stage-adjusted PCSS between African American and White patients across a broad range of treatment modalities.26-28 Given the paucity of data exploring outcomes following MHRT for African American patients with HRPC, the present analysis provides long-term clinical outcomes and toxicity profiles for an off-trial majority African American population with HRPC treated with MHRT within the VA.
Methods
Records were retrospectively reviewed under an institutional review board–approved protocol for all patients with HRPC treated with definitive MHRT at the Durham Veterans Affairs Healthcare System in North Carolina between November 2008 and August 2018. Exclusion criteria included < 12 months of follow-up or elective nodal irradiation. Demographic variables obtained included age at diagnosis, race, clinical T stage, pre-MHRT prostate-specific antigen (PSA), Gleason grade group at diagnosis, favorable vs unfavorable high-risk disease, pre-MHRT international prostate symptom score (IPSS), and pre-MHRT urinary medication usage (yes/no).29
Concurrent androgen deprivation therapy (ADT) was initiated 6 to 8 weeks before MHRT unless medically contraindicated per the discretion of the treating radiation oncologist. Patients generally received 18 to 24 months of ADT, with those with favorable HRPC (ie, T1c disease with either Gleason 4+4 and PSA < 10 mg/mL or Gleason 3+3 and PSA > 20 ng/mL) receiving 6 months after 2015.29 Patients were simulated supine in either standard or custom immobilization with a full bladder and empty rectum. MHRT fractionation plans included 70 Gy at 2.5 Gy per fraction and 60 Gy at 3 Gy per fraction. Radiotherapy targets included the prostate and seminal vesicles without elective nodal coverage per institutional practice. Treatments were delivered following image guidance, either prostate matching with cone beam computed tomography or fiducial matching with kilo voltage imaging. All patients received intensity-modulated radiotherapy. For plans delivering 70 Gy at 2.5 Gy per fraction, constraints included bladder V (volume receiving) 70 < 10 cc, V65 ≤ 15%, V40 ≤ 35%, rectum V70 < 10 cc, V65 ≤ 10%, V40 ≤ 35%, femoral heads maximum point dose ≤ 40 Gy, penile bulb mean dose ≤ 50 Gy, and small bowel V40 ≤ 1%. For plans delivering 60 Gy at 3 Gy per fraction, constraints included rectum V57 ≤ 15%, V46 ≤ 30%, V37 ≤ 50%, bladder V60 ≤ 5%, V46 ≤ 30%, V37 ≤ 50%, and femoral heads V43 ≤ 5%.
Gastrointestinal (GI) and genitourinary (GU) toxicities were graded using Common Terminology Criteria for Adverse Events (CTCAE), version 5.0, with acute toxicity defined as on-treatment < 3 months following completion of MHRT. Late toxicity was defined as ≥ 3 months following completion of MHRT. Individuals were seen in follow-up at 6 weeks and 3 months with PSA and testosterone after MHRT completion, then every 6 to 12 months for 5 years and annually thereafter. Each follow-up visit included history, physical examination, IPSS, and CTCAE grading for GI and GU toxicity.
The Wilcoxon rank sum test and χ2 test were used to compare differences in demographic data, dosimetric parameters, and frequency of toxicity events with respect to patient race. Clinical endpoints including biochemical recurrence-free survival (BRFS; defined by Phoenix criteria as 2.0 above PSA nadir), distant metastases-free survival (DMFS), PCSS, and overall survival (OS) were estimated from time of radiotherapy completion by the Kaplan-Meier method and compared between African American and White race by log-rank testing.30 Late GI and GU toxicity-free survival were estimated by Kaplan-Meier plots and compared between African American and White patients by the log-rank test. Statistical analysis was performed using SAS 9.4.
Results
We identified 143 patients with HRPC treated with definitive MHRT between November 2008 and August 2018 (Table 1). Mean age was 65 years (range, 36-80 years); 57% were African American men. Eighty percent of individuals had unfavorable high-risk disease. Median (IQR) PSA was 14.4 (7.8-28.6). Twenty-six percent had grade group 1-3 disease, 47% had grade group 4 disease, and 27% had grade group 5 disease. African American patients had significantly lower pre-MHRT IPSS scores than White patients (mean IPSS, 11 vs 14, respectively; P = .02) despite similar rates of preradiotherapy urinary medication usage (66% and 66%, respectively).
Eighty-six percent received 70 Gy over 28 fractions, with institutional protocol shifting to 60 Gy over 20 fractions (14%) in June 2017. The median (IQR) duration of radiotherapy was 39 (38-42) days, with 97% of individuals undergoing ADT for a median (IQR) duration of 24 (24-36) months. The median follow-up time was 38 months, with 57 (40%) patients followed for at least 60 months.
Grade 3 GI and GU acute toxicity events were observed in 1% and 4% of all individuals, respectively (Table 2). No acute GI or GU grade 4+ events were observed. No significant differences in acute GU or GI toxicity were observed between African American and White patients.
No significant differences between African American and White patients were observed for late grade 2+ GI (P = .19) or GU (P = .55) toxicity. Late grade 2+ GI toxicity was observed in 17 (12%) patients overall (Figure 1A). One grade 3 and 1 grade 4 late GI event were observed following MHRT completion: The latter involved hospitalization for bleeding secondary to radiation proctitis in the context of cirrhosis predating MHRT. Late grade 2+ GU toxicity was observed in 80 (56%) patients, with late grade 2 events steadily increasing over time (Figure 1B). Nine late grade 3 GU toxicity events were observed at a median of 13 months following completion of MHRT, 2 of which occurred more than 24 months after MHRT completion. No late grade 4 or 5 GU events were observed. IPSS values both before MHRT and at time of last follow-up were available for 65 (40%) patients, with a median (IQR) IPSS of 10 (6-16) before MHRT and 12 (8-16) at last follow-up at a median (IQR) interval of 36 months (26-76) from radiation completion.
No significant differences were observed between African American and White patients with respect to BRFS, DMFS, PCSS, or OS (Figure 2). Overall, 21 of 143 (15%) patients experienced biochemical recurrence: 5-year BRFS was 77% (95% CI, 67%-85%) for all patients, 83% (95% CI, 70%-91%) for African American patients, and 71% (95% CI, 53%-82%) for White patients. Five-year DMFS was 87% (95% CI, 77%-92%) for all individuals, 91% (95% CI, 80%-96%) for African American patients, and 81% (95% CI, 62%-91%) for White patients. Five-year PCSS was 89% (95% CI, 80%-94%) for all patients, with 5-year PCSS rates of 90% (95% CI, 79%-95%) for African American patients and 87% (95% CI, 70%-95%) for White patients. Five-year OS was 75% overall (95% CI, 64%-82%), with 5-year OS rates of 73% (95% CI, 58%-83%) for African American patients and 77% (95% CI, 60%-87%) for White patients.
Discussion
In this study, we reported acute and late GI and GU toxicity rates as well as clinical outcomes for a majority African American population with predominately unfavorable HRPC treated with MHRT in an equal access health care environment. We found that MHRT was well tolerated with high rates of biochemical control, PCSS, and OS. Additionally, outcomes were not significantly different across patient race. To our knowledge, this is the first report of MHRT for HRPC in a majority African American population.
We found that MHRT was an effective treatment for patients with HRPC, in particular those with unfavorable high-risk disease. While prior prospective and randomized studies have investigated the use of MHRT, our series was larger than most and had a predominately unfavorable high-risk population.12,15-17 Our biochemical and PCSS rates compare favorably with those of HRPC trial populations, particularly given the high proportion of unfavorable high-risk disease.12,15,16 Despite similar rates of biochemical control, OS was lower in the present cohort than in HRPC trial populations, even with a younger median age at diagnosis. The similarly high rates of non–HRPC-related death across race may reflect differences in baseline comorbidities compared with trial populations as well as reported differences between individuals in the VA and the private sector.31 This suggests that MHRT can be an effective treatment for patients with unfavorable HRPC.
We did not find any differences in outcomes between African American and White individuals with HRPC treated with MHRT. Furthermore, our study demonstrates long-term rates of BRFS and PCSS in a majority African American population with predominately unfavorable HRPC that are comparable with those of prior randomized MHRT studies in high-risk, predominately White populations.12,15,16 Prior reports have found that African American men with HRPC may be at increased risk for inferior clinical outcomes due to a number of socioeconomic, biologic, and cultural mediators.26,27,32 Such individuals may disproportionally benefit from shorter treatment courses that improve access to radiotherapy, a well-documented disparity for African American men with localized prostate cancer.33-36 The VA is an ideal system for studying racial disparities within prostate cancer, as accessibility of mental health and transportation services, income, and insurance status are not barriers to preventative or acute care.37 Our results are concordant with those previously seen for African American patients with prostate cancer seen in the VA, which similarly demonstrate equal outcomes with those of other races.28,36 Incorporation of the earlier mentioned VA services into oncologic care across other health care systems could better characterize determinants of racial disparities in prostate cancer, including the prognostic significance of shortening treatment duration and number of patient visits via MHRT.
Despite widespread acceptance in prostate cancer radiotherapy guidelines, routine use of MHRT seems limited across all stages of localized prostate cancer.1,2 Late toxicity is a frequently noted concern regarding MHRT use. Higher rates of late grade 2+ GI toxicity were observed in the hypofractionation arm of the HYPRO trial.17 While RTOG 0415 did not include patients with HRPC, significantly higher rates of physician-reported (but not patient-reported) late grade 2+ GI and GU toxicity were observed using the same MHRT fractionation regimen used for the majority of individuals in our cohort.9 In our study, the steady increase in late grade 2 GU toxicity is consistent with what is seen following conventionally fractionated radiotherapy and is likely multifactorial.38 The mean IPSS difference of 2/35 from pre-MHRT baseline to the time of last follow-up suggests minimal quality of life decline. The relatively stable IPSSs over time alongside the > 50% prevalence of late grade 2 GU toxicity per CTCAE grading seems consistent with the discrepancy noted in RTOG 0415 between increased physician-reported late toxicity and favorable patient-reported quality of life scores.9 Moreover, significant variance exists in toxicity grading across scoring systems, revised editions of CTCAE, and physician-specific toxicity classification, particularly with regard to the use of adrenergic receptor blocker medications. In light of these factors, the high rate of late grade 2 GU toxicity in our study should be interpreted in the context of largely stable post-MHRT IPSSs and favorable rates of late GI grade 2+ and late GU grade 3+ toxicity.
Limitations
This study has several inherent limitations. While the size of the current HRPC cohort is notably larger than similar populations within the majority of phase 3 MHRT trials, these data derive from a single VA hospital. It is unclear whether these outcomes would be representative in a similar high-risk population receiving care outside of the VA equal access system. Follow-up data beyond 5 years was available for less than half of patients, partially due to nonprostate cancer–related mortality at a higher rate than observed in HRPC trial populations.12,15,16 Furthermore, all GI toxicity events were exclusively physician reported, and GU toxicity reporting was limited in the off-trial setting with not all patients routinely completing IPSS questionnaires following MHRT completion. However, all patients were treated similarly, and radiation quality was verified over the treatment period with mandated accreditation, frequent standardized output checks, and systematic treatment review.39
Conclusions
Patients with HRPC treated with MHRT in an equal access, off-trial setting demonstrated favorable rates of biochemical control with acceptable rates of acute and late GI and GU toxicities. Clinical outcomes, including biochemical control, were not significantly different between African American and White patients, which may reflect equal access to care within the VA irrespective of income and insurance status. Incorporating VA services, such as access to primary care, mental health services, and transportation across other health care systems may aid in characterizing and mitigating racial and gender disparities in oncologic care.
Acknowledgments
Portions of this work were presented at the November 2020 ASTRO conference. 40
Although moderately hypofractionated radiotherapy (MHRT) is an accepted treatment for localized prostate cancer, its adaptation remains limited in the United States.1,2 MHRT theoretically exploits α/β ratio differences between the prostate (1.5 Gy), bladder (5-10 Gy), and rectum (3 Gy), thereby reducing late treatment-related adverse effects compared with those of conventional fractionation at biologically equivalent doses.3-8 Multiple randomized noninferiority trials have demonstrated equivalent outcomes between MHRT and conventional fraction with no appreciable increase in patient-reported toxicity.9-14 Although these studies have led to the acceptance of MHRT as a standard treatment, the majority of these trials involve individuals with low- and intermediate-risk disease.
There are less phase 3 data addressing MHRT for high-risk prostate cancer (HRPC).10,12,14-17 Only 2 studies examined predominately high-risk populations, accounting for 83 and 292 patients, respectively.15,16 Additional phase 3 trials with small proportions of high-risk patients (n = 126, 12%; n = 53, 35%) offer limited additional information regarding clinical outcomes and toxicity rates specific to high-risk disease.10-12 Numerous phase 1 and 2 studies report various field designs and fractionation plans for MHRT in the context of high-risk disease, although the applicability of these data to off-trial populations remains limited.18-20
Furthermore, African American individuals are underrepresented in the trials establishing the role of MHRT despite higher rates of prostate cancer incidence, more advanced disease stage at diagnosis, and higher rates of prostate cancer–specific survival (PCSS) when compared with White patients.21 Racial disparities across patients with prostate cancer and their management are multifactorial across health care literacy, education level, access to care (including transportation issues), and issues of adherence and distrust.22-25 Correlation of patient race to prostate cancer outcomes varies greatly across health care systems, with the US Department of Veterans Affairs (VA) equal access system providing robust mental health services and transportation services for some patients, while demonstrating similar rates of stage-adjusted PCSS between African American and White patients across a broad range of treatment modalities.26-28 Given the paucity of data exploring outcomes following MHRT for African American patients with HRPC, the present analysis provides long-term clinical outcomes and toxicity profiles for an off-trial majority African American population with HRPC treated with MHRT within the VA.
Methods
Records were retrospectively reviewed under an institutional review board–approved protocol for all patients with HRPC treated with definitive MHRT at the Durham Veterans Affairs Healthcare System in North Carolina between November 2008 and August 2018. Exclusion criteria included < 12 months of follow-up or elective nodal irradiation. Demographic variables obtained included age at diagnosis, race, clinical T stage, pre-MHRT prostate-specific antigen (PSA), Gleason grade group at diagnosis, favorable vs unfavorable high-risk disease, pre-MHRT international prostate symptom score (IPSS), and pre-MHRT urinary medication usage (yes/no).29
Concurrent androgen deprivation therapy (ADT) was initiated 6 to 8 weeks before MHRT unless medically contraindicated per the discretion of the treating radiation oncologist. Patients generally received 18 to 24 months of ADT, with those with favorable HRPC (ie, T1c disease with either Gleason 4+4 and PSA < 10 mg/mL or Gleason 3+3 and PSA > 20 ng/mL) receiving 6 months after 2015.29 Patients were simulated supine in either standard or custom immobilization with a full bladder and empty rectum. MHRT fractionation plans included 70 Gy at 2.5 Gy per fraction and 60 Gy at 3 Gy per fraction. Radiotherapy targets included the prostate and seminal vesicles without elective nodal coverage per institutional practice. Treatments were delivered following image guidance, either prostate matching with cone beam computed tomography or fiducial matching with kilo voltage imaging. All patients received intensity-modulated radiotherapy. For plans delivering 70 Gy at 2.5 Gy per fraction, constraints included bladder V (volume receiving) 70 < 10 cc, V65 ≤ 15%, V40 ≤ 35%, rectum V70 < 10 cc, V65 ≤ 10%, V40 ≤ 35%, femoral heads maximum point dose ≤ 40 Gy, penile bulb mean dose ≤ 50 Gy, and small bowel V40 ≤ 1%. For plans delivering 60 Gy at 3 Gy per fraction, constraints included rectum V57 ≤ 15%, V46 ≤ 30%, V37 ≤ 50%, bladder V60 ≤ 5%, V46 ≤ 30%, V37 ≤ 50%, and femoral heads V43 ≤ 5%.
Gastrointestinal (GI) and genitourinary (GU) toxicities were graded using Common Terminology Criteria for Adverse Events (CTCAE), version 5.0, with acute toxicity defined as on-treatment < 3 months following completion of MHRT. Late toxicity was defined as ≥ 3 months following completion of MHRT. Individuals were seen in follow-up at 6 weeks and 3 months with PSA and testosterone after MHRT completion, then every 6 to 12 months for 5 years and annually thereafter. Each follow-up visit included history, physical examination, IPSS, and CTCAE grading for GI and GU toxicity.
The Wilcoxon rank sum test and χ2 test were used to compare differences in demographic data, dosimetric parameters, and frequency of toxicity events with respect to patient race. Clinical endpoints including biochemical recurrence-free survival (BRFS; defined by Phoenix criteria as 2.0 above PSA nadir), distant metastases-free survival (DMFS), PCSS, and overall survival (OS) were estimated from time of radiotherapy completion by the Kaplan-Meier method and compared between African American and White race by log-rank testing.30 Late GI and GU toxicity-free survival were estimated by Kaplan-Meier plots and compared between African American and White patients by the log-rank test. Statistical analysis was performed using SAS 9.4.
Results
We identified 143 patients with HRPC treated with definitive MHRT between November 2008 and August 2018 (Table 1). Mean age was 65 years (range, 36-80 years); 57% were African American men. Eighty percent of individuals had unfavorable high-risk disease. Median (IQR) PSA was 14.4 (7.8-28.6). Twenty-six percent had grade group 1-3 disease, 47% had grade group 4 disease, and 27% had grade group 5 disease. African American patients had significantly lower pre-MHRT IPSS scores than White patients (mean IPSS, 11 vs 14, respectively; P = .02) despite similar rates of preradiotherapy urinary medication usage (66% and 66%, respectively).
Eighty-six percent received 70 Gy over 28 fractions, with institutional protocol shifting to 60 Gy over 20 fractions (14%) in June 2017. The median (IQR) duration of radiotherapy was 39 (38-42) days, with 97% of individuals undergoing ADT for a median (IQR) duration of 24 (24-36) months. The median follow-up time was 38 months, with 57 (40%) patients followed for at least 60 months.
Grade 3 GI and GU acute toxicity events were observed in 1% and 4% of all individuals, respectively (Table 2). No acute GI or GU grade 4+ events were observed. No significant differences in acute GU or GI toxicity were observed between African American and White patients.
No significant differences between African American and White patients were observed for late grade 2+ GI (P = .19) or GU (P = .55) toxicity. Late grade 2+ GI toxicity was observed in 17 (12%) patients overall (Figure 1A). One grade 3 and 1 grade 4 late GI event were observed following MHRT completion: The latter involved hospitalization for bleeding secondary to radiation proctitis in the context of cirrhosis predating MHRT. Late grade 2+ GU toxicity was observed in 80 (56%) patients, with late grade 2 events steadily increasing over time (Figure 1B). Nine late grade 3 GU toxicity events were observed at a median of 13 months following completion of MHRT, 2 of which occurred more than 24 months after MHRT completion. No late grade 4 or 5 GU events were observed. IPSS values both before MHRT and at time of last follow-up were available for 65 (40%) patients, with a median (IQR) IPSS of 10 (6-16) before MHRT and 12 (8-16) at last follow-up at a median (IQR) interval of 36 months (26-76) from radiation completion.
No significant differences were observed between African American and White patients with respect to BRFS, DMFS, PCSS, or OS (Figure 2). Overall, 21 of 143 (15%) patients experienced biochemical recurrence: 5-year BRFS was 77% (95% CI, 67%-85%) for all patients, 83% (95% CI, 70%-91%) for African American patients, and 71% (95% CI, 53%-82%) for White patients. Five-year DMFS was 87% (95% CI, 77%-92%) for all individuals, 91% (95% CI, 80%-96%) for African American patients, and 81% (95% CI, 62%-91%) for White patients. Five-year PCSS was 89% (95% CI, 80%-94%) for all patients, with 5-year PCSS rates of 90% (95% CI, 79%-95%) for African American patients and 87% (95% CI, 70%-95%) for White patients. Five-year OS was 75% overall (95% CI, 64%-82%), with 5-year OS rates of 73% (95% CI, 58%-83%) for African American patients and 77% (95% CI, 60%-87%) for White patients.
Discussion
In this study, we reported acute and late GI and GU toxicity rates as well as clinical outcomes for a majority African American population with predominately unfavorable HRPC treated with MHRT in an equal access health care environment. We found that MHRT was well tolerated with high rates of biochemical control, PCSS, and OS. Additionally, outcomes were not significantly different across patient race. To our knowledge, this is the first report of MHRT for HRPC in a majority African American population.
We found that MHRT was an effective treatment for patients with HRPC, in particular those with unfavorable high-risk disease. While prior prospective and randomized studies have investigated the use of MHRT, our series was larger than most and had a predominately unfavorable high-risk population.12,15-17 Our biochemical and PCSS rates compare favorably with those of HRPC trial populations, particularly given the high proportion of unfavorable high-risk disease.12,15,16 Despite similar rates of biochemical control, OS was lower in the present cohort than in HRPC trial populations, even with a younger median age at diagnosis. The similarly high rates of non–HRPC-related death across race may reflect differences in baseline comorbidities compared with trial populations as well as reported differences between individuals in the VA and the private sector.31 This suggests that MHRT can be an effective treatment for patients with unfavorable HRPC.
We did not find any differences in outcomes between African American and White individuals with HRPC treated with MHRT. Furthermore, our study demonstrates long-term rates of BRFS and PCSS in a majority African American population with predominately unfavorable HRPC that are comparable with those of prior randomized MHRT studies in high-risk, predominately White populations.12,15,16 Prior reports have found that African American men with HRPC may be at increased risk for inferior clinical outcomes due to a number of socioeconomic, biologic, and cultural mediators.26,27,32 Such individuals may disproportionally benefit from shorter treatment courses that improve access to radiotherapy, a well-documented disparity for African American men with localized prostate cancer.33-36 The VA is an ideal system for studying racial disparities within prostate cancer, as accessibility of mental health and transportation services, income, and insurance status are not barriers to preventative or acute care.37 Our results are concordant with those previously seen for African American patients with prostate cancer seen in the VA, which similarly demonstrate equal outcomes with those of other races.28,36 Incorporation of the earlier mentioned VA services into oncologic care across other health care systems could better characterize determinants of racial disparities in prostate cancer, including the prognostic significance of shortening treatment duration and number of patient visits via MHRT.
Despite widespread acceptance in prostate cancer radiotherapy guidelines, routine use of MHRT seems limited across all stages of localized prostate cancer.1,2 Late toxicity is a frequently noted concern regarding MHRT use. Higher rates of late grade 2+ GI toxicity were observed in the hypofractionation arm of the HYPRO trial.17 While RTOG 0415 did not include patients with HRPC, significantly higher rates of physician-reported (but not patient-reported) late grade 2+ GI and GU toxicity were observed using the same MHRT fractionation regimen used for the majority of individuals in our cohort.9 In our study, the steady increase in late grade 2 GU toxicity is consistent with what is seen following conventionally fractionated radiotherapy and is likely multifactorial.38 The mean IPSS difference of 2/35 from pre-MHRT baseline to the time of last follow-up suggests minimal quality of life decline. The relatively stable IPSSs over time alongside the > 50% prevalence of late grade 2 GU toxicity per CTCAE grading seems consistent with the discrepancy noted in RTOG 0415 between increased physician-reported late toxicity and favorable patient-reported quality of life scores.9 Moreover, significant variance exists in toxicity grading across scoring systems, revised editions of CTCAE, and physician-specific toxicity classification, particularly with regard to the use of adrenergic receptor blocker medications. In light of these factors, the high rate of late grade 2 GU toxicity in our study should be interpreted in the context of largely stable post-MHRT IPSSs and favorable rates of late GI grade 2+ and late GU grade 3+ toxicity.
Limitations
This study has several inherent limitations. While the size of the current HRPC cohort is notably larger than similar populations within the majority of phase 3 MHRT trials, these data derive from a single VA hospital. It is unclear whether these outcomes would be representative in a similar high-risk population receiving care outside of the VA equal access system. Follow-up data beyond 5 years was available for less than half of patients, partially due to nonprostate cancer–related mortality at a higher rate than observed in HRPC trial populations.12,15,16 Furthermore, all GI toxicity events were exclusively physician reported, and GU toxicity reporting was limited in the off-trial setting with not all patients routinely completing IPSS questionnaires following MHRT completion. However, all patients were treated similarly, and radiation quality was verified over the treatment period with mandated accreditation, frequent standardized output checks, and systematic treatment review.39
Conclusions
Patients with HRPC treated with MHRT in an equal access, off-trial setting demonstrated favorable rates of biochemical control with acceptable rates of acute and late GI and GU toxicities. Clinical outcomes, including biochemical control, were not significantly different between African American and White patients, which may reflect equal access to care within the VA irrespective of income and insurance status. Incorporating VA services, such as access to primary care, mental health services, and transportation across other health care systems may aid in characterizing and mitigating racial and gender disparities in oncologic care.
Acknowledgments
Portions of this work were presented at the November 2020 ASTRO conference. 40
1. Stokes WA, Kavanagh BD, Raben D, Pugh TJ. Implementation of hypofractionated prostate radiation therapy in the United States: a National Cancer Database analysis. Pract Radiat Oncol. 2017;7:270-278. doi:10.1016/j.prro.2017.03.011
2. Jaworski L, Dominello MM, Heimburger DK, et al. Contemporary practice patterns for intact and post-operative prostate cancer: results from a statewide collaborative. Int J Radiat Oncol Biol Phys. 2019;105(1):E282. doi:10.1016/j.ijrobp.2019.06.1915
3. Miralbell R, Roberts SA, Zubizarreta E, Hendry JH. Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5,969 patients in seven international institutional datasets: α/β = 1.4 (0.9-2.2) Gy. Int J Radiat Oncol Biol Phys. 2012;82(1):e17-e24. doi:10.1016/j.ijrobp.2010.10.075
4. Tree AC, Khoo VS, van As NJ, Partridge M. Is biochemical relapse-free survival after profoundly hypofractionated radiotherapy consistent with current radiobiological models? Clin Oncol (R Coll Radiol). 2014;26(4):216-229. doi:10.1016/j.clon.2014.01.008
5. Brenner DJ. Fractionation and late rectal toxicity. Int J Radiat Oncol Biol Phys. 2004;60(4):1013-1015. doi:10.1016/j.ijrobp.2004.04.014
6. Tucker SL, Thames HD, Michalski JM, et al. Estimation of α/β for late rectal toxicity based on RTOG 94-06. Int J Radiat Oncol Biol Phys. 2011;81(2):600-605. doi:10.1016/j.ijrobp.2010.11.080
7. Dasu A, Toma-Dasu I. Prostate alpha/beta revisited—an analysis of clinical results from 14 168 patients. Acta Oncol. 2012;51(8):963-974. doi:10.3109/0284186X.2012.719635 start
8. Proust-Lima C, Taylor JMG, Sécher S, et al. Confirmation of a Low α/β ratio for prostate cancer treated by external beam radiation therapy alone using a post-treatment repeated-measures model for PSA dynamics. Int J Radiat Oncol Biol Phys. 2011;79(1):195-201. doi:10.1016/j.ijrobp.2009.10.008
9. Lee WR, Dignam JJ, Amin MB, et al. Randomized phase III noninferiority study comparing two radiotherapy fractionation schedules in patients with low-risk prostate cancer. J Clin Oncol. 2016;34(20): 2325-2332. doi:10.1200/JCO.2016.67.0448
10. Dearnaley D, Syndikus I, Mossop H, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17(8):1047-1060. doi:10.1016/S1470-2045(16)30102-4
11. Catton CN, Lukka H, Gu C-S, et al. Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. J Clin Oncol. 2017;35(17):1884-1890. doi:10.1200/JCO.2016.71.7397
12. Pollack A, Walker G, Horwitz EM, et al. Randomized trial of hypofractionated external-beam radiotherapy for prostate cancer. J Clin Oncol. 2013;31(31):3860-3868. doi:10.1200/JCO.2013.51.1972
13. Hoffman KE, Voong KR, Levy LB, et al. Randomized trial of hypofractionated, dose-escalated, intensity-modulated radiation therapy (IMRT) versus conventionally fractionated IMRT for localized prostate cancer. J Clin Oncol. 2018;36(29):2943-2949. doi:10.1200/JCO.2018.77.9868
14. Wilkins A, Mossop H, Syndikus I, et al. Hypofractionated radiotherapy versus conventionally fractionated radiotherapy for patients with intermediate-risk localised prostate cancer: 2-year patient-reported outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2015;16(16):1605-1616. doi:10.1016/S1470-2045(15)00280-6
15. Incrocci L, Wortel RC, Alemayehu WG, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with localised prostate cancer (HYPRO): final efficacy results from a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2016;17(8):1061-1069. doi.10.1016/S1470-2045(16)30070-5
16. Arcangeli G, Saracino B, Arcangeli S, et al. Moderate hypofractionation in high-risk, organ-confined prostate cancer: final results of a phase III randomized trial. J Clin Oncol. 2017;35(17):1891-1897. doi:10.1200/JCO.2016.70.4189
17. Aluwini S, Pos F, Schimmel E, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): late toxicity results from a randomised, non-inferiority, phase 3 trial. Lancet Oncol. 2016;17(4):464-474. doi:10.1016/S1470-2045(15)00567-7
18. Pervez N, Small C, MacKenzie M, et al. Acute toxicity in high-risk prostate cancer patients treated with androgen suppression and hypofractionated intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;76(1):57-64. doi:10.1016/j.ijrobp.2009.01.048
19. Magli A, Moretti E, Tullio A, Giannarini G. Hypofractionated simultaneous integrated boost (IMRT- cancer: results of a prospective phase II trial SIB) with pelvic nodal irradiation and concurrent androgen deprivation therapy for high-risk prostate cancer: results of a prospective phase II trial. Prostate Cancer Prostatic Dis. 2018;21(2):269-276. doi:10.1038/s41391-018-0034-0
20. Di Muzio NG, Fodor A, Noris Chiorda B, et al. Moderate hypofractionation with simultaneous integrated boost in prostate cancer: long-term results of a phase I–II study. Clin Oncol (R Coll Radiol). 2016;28(8):490-500. doi:10.1016/j.clon.2016.02.005
21. DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA Cancer J Clin. 2019;69(3):21-233. doi:10.3322/caac.21555
22. Wolf MS, Knight SJ, Lyons EA, et al. Literacy, race, and PSA level among low-income men newly diagnosed with prostate cancer. Urology. 2006(1);68:89-93. doi:10.1016/j.urology.2006.01.064
23. Rebbeck TR. Prostate cancer disparities by race and ethnicity: from nucleotide to neighborhood. Cold Spring Harb Perspect Med. 2018;8(9):a030387. doi:10.1101/cshperspect.a030387
24. Guidry JJ, Aday LA, Zhang D, Winn RJ. Transportation as a barrier to cancer treatment. Cancer Pract. 1997;5(6):361-366.
25. Friedman DB, Corwin SJ, Dominick GM, Rose ID. African American men’s understanding and perceptions about prostate cancer: why multiple dimensions of health literacy are important in cancer communication. J Community Health. 2009;34(5):449-460. doi:10.1007/s10900-009-9167-3
26. Connell PP, Ignacio L, Haraf D, et al. Equivalent racial outcome after conformal radiotherapy for prostate cancer: a single departmental experience. J Clin Oncol. 2001;19(1):54-61. doi:10.1200/JCO.2001.19.1.54
27. Dess RT, Hartman HE, Mahal BA, et al. Association of black race with prostate cancer-specific and other-cause mortality. JAMA Oncol. 2019;5(1):975-983. doi:10.1200/JCO.2001.19.1.54
28. McKay RR, Sarkar RR, Kumar A, et al. Outcomes of Black men with prostate cancer treated with radiation therapy in the Veterans Health Administration. Cancer. 2021;127(3):403-411. doi:10.1002/cncr.33224

29. Muralidhar V, Chen M-H, Reznor G, et al. Definition and validation of “favorable high-risk prostate cancer”: implications for personalizing treatment of radiation-managed patients. Int J Radiat Oncol Biol Phys. 2015;93(4):828-835. doi:10.1016/j.ijrobp.2015.07.2281
30. Roach M 3rd, Hanks G, Thames H Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65(4):965-974. doi:10.1016/j.ijrobp.2006.04.029
31. Freeman VL, Durazo-Arvizu R, Arozullah AM, Keys LC. Determinants of mortality following a diagnosis of prostate cancer in Veterans Affairs and private sector health care systems. Am J Public Health. 2003;93(100):1706-1712. doi:10.2105/ajph.93.10.1706
32. Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54(2):78-93. doi:10.3322/canjclin.54.2.78
33. Zemplenyi AT, Kaló Z, Kovacs G, et al. Cost-effectiveness analysis of intensity-modulated radiation therapy with normal and hypofractionated schemes for the treatment of localised prostate cancer. Eur J Cancer Care. 2018;27(1):e12430. doi:10.1111/ecc.12430
34. Klabunde CN, Potosky AL, Harlan LC, Kramer BS. Trends and black/white differences in treatment for nonmetastatic prostate cancer. Med Care. 1998;36(9):1337-1348. doi:10.1097/00005650-199809000-00006
35. Harlan L, Brawley O, Pommerenke F, Wali P, Kramer B. Geographic, age, and racial variation in the treatment of local/regional carcinoma of the prostate. J Clin Oncol. 1995;13(1):93-100. doi:10.1200/JCO.1995.13.1.93
36. Riviere P, Luterstein E, Kumar A, et al. Racial equity among African-American and non-Hispanic white men diagnosed with prostate cancer in the veterans affairs healthcare system. Int J Radiat Oncol Biol Phys. 2019;105:E305.
37. Peterson K, Anderson J, Boundy E, Ferguson L, McCleery E, Waldrip K. Mortality disparities in racial/ethnic minority groups in the Veterans Health Administration: an evidence review and map. Am J Public Health. 2018;108(3):e1-e11. doi:10.2105/AJPH.2017.304246
38. Zietman AL, DeSilvio ML, Slater JD, et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA. 2005;294(10):1233-1239. doi:10.1001/jama.294.10.1233
39. Hagan M, Kapoor R, Michalski J, et al. VA-Radiation Oncology Quality Surveillance program. Int J Radiat Oncol Biol Phys. 2020;106(3):639-647. doi.10.1016/j.ijrobp.2019.08.064
40. Carpenter DJ, Natesan D, Floyd W, et al. Long-term experience in an equal access health care system using moderately hypofractionated radiotherapy for high risk prostate cancer in a predominately African American population with unfavorable disease. Int J Radiat Oncol Biol Phys. 2020;108(3):E417. https://www.redjournal.org/article/S0360-3016(20)33923-7/fulltext
1. Stokes WA, Kavanagh BD, Raben D, Pugh TJ. Implementation of hypofractionated prostate radiation therapy in the United States: a National Cancer Database analysis. Pract Radiat Oncol. 2017;7:270-278. doi:10.1016/j.prro.2017.03.011
2. Jaworski L, Dominello MM, Heimburger DK, et al. Contemporary practice patterns for intact and post-operative prostate cancer: results from a statewide collaborative. Int J Radiat Oncol Biol Phys. 2019;105(1):E282. doi:10.1016/j.ijrobp.2019.06.1915
3. Miralbell R, Roberts SA, Zubizarreta E, Hendry JH. Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5,969 patients in seven international institutional datasets: α/β = 1.4 (0.9-2.2) Gy. Int J Radiat Oncol Biol Phys. 2012;82(1):e17-e24. doi:10.1016/j.ijrobp.2010.10.075
4. Tree AC, Khoo VS, van As NJ, Partridge M. Is biochemical relapse-free survival after profoundly hypofractionated radiotherapy consistent with current radiobiological models? Clin Oncol (R Coll Radiol). 2014;26(4):216-229. doi:10.1016/j.clon.2014.01.008
5. Brenner DJ. Fractionation and late rectal toxicity. Int J Radiat Oncol Biol Phys. 2004;60(4):1013-1015. doi:10.1016/j.ijrobp.2004.04.014
6. Tucker SL, Thames HD, Michalski JM, et al. Estimation of α/β for late rectal toxicity based on RTOG 94-06. Int J Radiat Oncol Biol Phys. 2011;81(2):600-605. doi:10.1016/j.ijrobp.2010.11.080
7. Dasu A, Toma-Dasu I. Prostate alpha/beta revisited—an analysis of clinical results from 14 168 patients. Acta Oncol. 2012;51(8):963-974. doi:10.3109/0284186X.2012.719635 start
8. Proust-Lima C, Taylor JMG, Sécher S, et al. Confirmation of a Low α/β ratio for prostate cancer treated by external beam radiation therapy alone using a post-treatment repeated-measures model for PSA dynamics. Int J Radiat Oncol Biol Phys. 2011;79(1):195-201. doi:10.1016/j.ijrobp.2009.10.008
9. Lee WR, Dignam JJ, Amin MB, et al. Randomized phase III noninferiority study comparing two radiotherapy fractionation schedules in patients with low-risk prostate cancer. J Clin Oncol. 2016;34(20): 2325-2332. doi:10.1200/JCO.2016.67.0448
10. Dearnaley D, Syndikus I, Mossop H, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17(8):1047-1060. doi:10.1016/S1470-2045(16)30102-4
11. Catton CN, Lukka H, Gu C-S, et al. Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. J Clin Oncol. 2017;35(17):1884-1890. doi:10.1200/JCO.2016.71.7397
12. Pollack A, Walker G, Horwitz EM, et al. Randomized trial of hypofractionated external-beam radiotherapy for prostate cancer. J Clin Oncol. 2013;31(31):3860-3868. doi:10.1200/JCO.2013.51.1972
13. Hoffman KE, Voong KR, Levy LB, et al. Randomized trial of hypofractionated, dose-escalated, intensity-modulated radiation therapy (IMRT) versus conventionally fractionated IMRT for localized prostate cancer. J Clin Oncol. 2018;36(29):2943-2949. doi:10.1200/JCO.2018.77.9868
14. Wilkins A, Mossop H, Syndikus I, et al. Hypofractionated radiotherapy versus conventionally fractionated radiotherapy for patients with intermediate-risk localised prostate cancer: 2-year patient-reported outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2015;16(16):1605-1616. doi:10.1016/S1470-2045(15)00280-6
15. Incrocci L, Wortel RC, Alemayehu WG, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with localised prostate cancer (HYPRO): final efficacy results from a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2016;17(8):1061-1069. doi.10.1016/S1470-2045(16)30070-5
16. Arcangeli G, Saracino B, Arcangeli S, et al. Moderate hypofractionation in high-risk, organ-confined prostate cancer: final results of a phase III randomized trial. J Clin Oncol. 2017;35(17):1891-1897. doi:10.1200/JCO.2016.70.4189
17. Aluwini S, Pos F, Schimmel E, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): late toxicity results from a randomised, non-inferiority, phase 3 trial. Lancet Oncol. 2016;17(4):464-474. doi:10.1016/S1470-2045(15)00567-7
18. Pervez N, Small C, MacKenzie M, et al. Acute toxicity in high-risk prostate cancer patients treated with androgen suppression and hypofractionated intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;76(1):57-64. doi:10.1016/j.ijrobp.2009.01.048
19. Magli A, Moretti E, Tullio A, Giannarini G. Hypofractionated simultaneous integrated boost (IMRT- cancer: results of a prospective phase II trial SIB) with pelvic nodal irradiation and concurrent androgen deprivation therapy for high-risk prostate cancer: results of a prospective phase II trial. Prostate Cancer Prostatic Dis. 2018;21(2):269-276. doi:10.1038/s41391-018-0034-0
20. Di Muzio NG, Fodor A, Noris Chiorda B, et al. Moderate hypofractionation with simultaneous integrated boost in prostate cancer: long-term results of a phase I–II study. Clin Oncol (R Coll Radiol). 2016;28(8):490-500. doi:10.1016/j.clon.2016.02.005
21. DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA Cancer J Clin. 2019;69(3):21-233. doi:10.3322/caac.21555
22. Wolf MS, Knight SJ, Lyons EA, et al. Literacy, race, and PSA level among low-income men newly diagnosed with prostate cancer. Urology. 2006(1);68:89-93. doi:10.1016/j.urology.2006.01.064
23. Rebbeck TR. Prostate cancer disparities by race and ethnicity: from nucleotide to neighborhood. Cold Spring Harb Perspect Med. 2018;8(9):a030387. doi:10.1101/cshperspect.a030387
24. Guidry JJ, Aday LA, Zhang D, Winn RJ. Transportation as a barrier to cancer treatment. Cancer Pract. 1997;5(6):361-366.
25. Friedman DB, Corwin SJ, Dominick GM, Rose ID. African American men’s understanding and perceptions about prostate cancer: why multiple dimensions of health literacy are important in cancer communication. J Community Health. 2009;34(5):449-460. doi:10.1007/s10900-009-9167-3
26. Connell PP, Ignacio L, Haraf D, et al. Equivalent racial outcome after conformal radiotherapy for prostate cancer: a single departmental experience. J Clin Oncol. 2001;19(1):54-61. doi:10.1200/JCO.2001.19.1.54
27. Dess RT, Hartman HE, Mahal BA, et al. Association of black race with prostate cancer-specific and other-cause mortality. JAMA Oncol. 2019;5(1):975-983. doi:10.1200/JCO.2001.19.1.54
28. McKay RR, Sarkar RR, Kumar A, et al. Outcomes of Black men with prostate cancer treated with radiation therapy in the Veterans Health Administration. Cancer. 2021;127(3):403-411. doi:10.1002/cncr.33224

29. Muralidhar V, Chen M-H, Reznor G, et al. Definition and validation of “favorable high-risk prostate cancer”: implications for personalizing treatment of radiation-managed patients. Int J Radiat Oncol Biol Phys. 2015;93(4):828-835. doi:10.1016/j.ijrobp.2015.07.2281
30. Roach M 3rd, Hanks G, Thames H Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65(4):965-974. doi:10.1016/j.ijrobp.2006.04.029
31. Freeman VL, Durazo-Arvizu R, Arozullah AM, Keys LC. Determinants of mortality following a diagnosis of prostate cancer in Veterans Affairs and private sector health care systems. Am J Public Health. 2003;93(100):1706-1712. doi:10.2105/ajph.93.10.1706
32. Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54(2):78-93. doi:10.3322/canjclin.54.2.78
33. Zemplenyi AT, Kaló Z, Kovacs G, et al. Cost-effectiveness analysis of intensity-modulated radiation therapy with normal and hypofractionated schemes for the treatment of localised prostate cancer. Eur J Cancer Care. 2018;27(1):e12430. doi:10.1111/ecc.12430
34. Klabunde CN, Potosky AL, Harlan LC, Kramer BS. Trends and black/white differences in treatment for nonmetastatic prostate cancer. Med Care. 1998;36(9):1337-1348. doi:10.1097/00005650-199809000-00006
35. Harlan L, Brawley O, Pommerenke F, Wali P, Kramer B. Geographic, age, and racial variation in the treatment of local/regional carcinoma of the prostate. J Clin Oncol. 1995;13(1):93-100. doi:10.1200/JCO.1995.13.1.93
36. Riviere P, Luterstein E, Kumar A, et al. Racial equity among African-American and non-Hispanic white men diagnosed with prostate cancer in the veterans affairs healthcare system. Int J Radiat Oncol Biol Phys. 2019;105:E305.
37. Peterson K, Anderson J, Boundy E, Ferguson L, McCleery E, Waldrip K. Mortality disparities in racial/ethnic minority groups in the Veterans Health Administration: an evidence review and map. Am J Public Health. 2018;108(3):e1-e11. doi:10.2105/AJPH.2017.304246
38. Zietman AL, DeSilvio ML, Slater JD, et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA. 2005;294(10):1233-1239. doi:10.1001/jama.294.10.1233
39. Hagan M, Kapoor R, Michalski J, et al. VA-Radiation Oncology Quality Surveillance program. Int J Radiat Oncol Biol Phys. 2020;106(3):639-647. doi.10.1016/j.ijrobp.2019.08.064
40. Carpenter DJ, Natesan D, Floyd W, et al. Long-term experience in an equal access health care system using moderately hypofractionated radiotherapy for high risk prostate cancer in a predominately African American population with unfavorable disease. Int J Radiat Oncol Biol Phys. 2020;108(3):E417. https://www.redjournal.org/article/S0360-3016(20)33923-7/fulltext
Colonic Crohn’s: Segmental vs. total colectomy
Segmental rather than total colectomy may be a safe and effective choice for some patients with colonic Crohn’s disease (cCD), showing significantly lower rates of repeat surgery and reduced need for stoma, according to long-term data.
Gianluca Pellino, MD, with the department of advanced medical and surgical sciences, Università degli Studi della Campania “Luigi Vanvitelli” in Naples, Italy, led the study, which was published in the Journal of Crohn’s and Colitis.
CD of the colon has gotten less attention than the more prevalent small bowel disease, according to the authors, but it can be debilitating and permanently reduce quality of life. Isolated cCD incidence ranges between 14% and 32% of all CD cases from the start of disease. Historically, extensive resection has been linked with longer disease-free intervals, and reduced repeat surgeries compared with segmental resections. However, most of the data have included low-quality evidence and reports typically have not adequately considered the role of biologics or advances in perioperative management of patients with cCD, the authors wrote.
The Segmental Colectomy for Crohn’s disease (SCOTCH) international study included a retrospective analysis of data from six European Inflammatory Bowel Disease referral centers on patients operated on between 2000 and 2019 who had either segmental or total colectomy for cCD.
Among 687 patients (301 male; 386 female), segmental colectomy was performed in 285 (41.5%) of cases and total colectomy in 402 (58.5%). The 15-year surgical recurrence rate was 44% among patients who had TC and 27% for patients with segmental colectomy (P = .006).
The SCOTCH study found that segmental colectomy may be performed safely and effectively and reduce the need for stoma in cCD patients without increasing risk of repeat surgeries compared with total colectomy, which was the primary measure investigators studied.
The findings of this study also suggest that biologics, when used early and correctly, may allow more conservative options for cCD, with a fivefold reduction in surgical recurrence risk in patients who have one to three large bowel locations.
Morbidity and mortality were similar in the SC and TC groups.
Among the limitations of the study are that the total colectomy patients in the study had indications for total colectomy that were also higher risk factors for recurrence – for instance, perianal disease.
The authors wrote, “The differences between patients who underwent SC vs TC might have accounted for the choice of one treatment over the other. It is however difficult to obtain a homogenous population of cCD patients.” They also cite the difficulties in gathering enough patients for randomized trials.
“These findings need to be discussed with the patients, and the choice of operation should be individualised,” they concluded. “Multidisciplinary management of patients with cCD is of critical importance to achieve optimal long-term results of bowel-sparing approaches.”
Miguel Regueiro, MD, chair of the department of gastroenterology, hepatology, and nutrition at the Cleveland Clinic, who was not part of the study, told this publication the findings should be considered confirmatory rather than suggestive of practice change.
“If a patient has a limited segment of Crohn’s, for example ileocecal Crohn’s – a common phenotype – then the standard of care is a segmental resection and primary anastomosis,” he said. “If the patient has more extensive CD – perianal fistula, colonic-only CD – they’re more likely to undergo a total colectomy. This study confirms that.“
The authors and Dr. Regueiro declared no relevant financial relationships.
Segmental rather than total colectomy may be a safe and effective choice for some patients with colonic Crohn’s disease (cCD), showing significantly lower rates of repeat surgery and reduced need for stoma, according to long-term data.
Gianluca Pellino, MD, with the department of advanced medical and surgical sciences, Università degli Studi della Campania “Luigi Vanvitelli” in Naples, Italy, led the study, which was published in the Journal of Crohn’s and Colitis.
CD of the colon has gotten less attention than the more prevalent small bowel disease, according to the authors, but it can be debilitating and permanently reduce quality of life. Isolated cCD incidence ranges between 14% and 32% of all CD cases from the start of disease. Historically, extensive resection has been linked with longer disease-free intervals, and reduced repeat surgeries compared with segmental resections. However, most of the data have included low-quality evidence and reports typically have not adequately considered the role of biologics or advances in perioperative management of patients with cCD, the authors wrote.
The Segmental Colectomy for Crohn’s disease (SCOTCH) international study included a retrospective analysis of data from six European Inflammatory Bowel Disease referral centers on patients operated on between 2000 and 2019 who had either segmental or total colectomy for cCD.
Among 687 patients (301 male; 386 female), segmental colectomy was performed in 285 (41.5%) of cases and total colectomy in 402 (58.5%). The 15-year surgical recurrence rate was 44% among patients who had TC and 27% for patients with segmental colectomy (P = .006).
The SCOTCH study found that segmental colectomy may be performed safely and effectively and reduce the need for stoma in cCD patients without increasing risk of repeat surgeries compared with total colectomy, which was the primary measure investigators studied.
The findings of this study also suggest that biologics, when used early and correctly, may allow more conservative options for cCD, with a fivefold reduction in surgical recurrence risk in patients who have one to three large bowel locations.
Morbidity and mortality were similar in the SC and TC groups.
Among the limitations of the study are that the total colectomy patients in the study had indications for total colectomy that were also higher risk factors for recurrence – for instance, perianal disease.
The authors wrote, “The differences between patients who underwent SC vs TC might have accounted for the choice of one treatment over the other. It is however difficult to obtain a homogenous population of cCD patients.” They also cite the difficulties in gathering enough patients for randomized trials.
“These findings need to be discussed with the patients, and the choice of operation should be individualised,” they concluded. “Multidisciplinary management of patients with cCD is of critical importance to achieve optimal long-term results of bowel-sparing approaches.”
Miguel Regueiro, MD, chair of the department of gastroenterology, hepatology, and nutrition at the Cleveland Clinic, who was not part of the study, told this publication the findings should be considered confirmatory rather than suggestive of practice change.
“If a patient has a limited segment of Crohn’s, for example ileocecal Crohn’s – a common phenotype – then the standard of care is a segmental resection and primary anastomosis,” he said. “If the patient has more extensive CD – perianal fistula, colonic-only CD – they’re more likely to undergo a total colectomy. This study confirms that.“
The authors and Dr. Regueiro declared no relevant financial relationships.
Segmental rather than total colectomy may be a safe and effective choice for some patients with colonic Crohn’s disease (cCD), showing significantly lower rates of repeat surgery and reduced need for stoma, according to long-term data.
Gianluca Pellino, MD, with the department of advanced medical and surgical sciences, Università degli Studi della Campania “Luigi Vanvitelli” in Naples, Italy, led the study, which was published in the Journal of Crohn’s and Colitis.
CD of the colon has gotten less attention than the more prevalent small bowel disease, according to the authors, but it can be debilitating and permanently reduce quality of life. Isolated cCD incidence ranges between 14% and 32% of all CD cases from the start of disease. Historically, extensive resection has been linked with longer disease-free intervals, and reduced repeat surgeries compared with segmental resections. However, most of the data have included low-quality evidence and reports typically have not adequately considered the role of biologics or advances in perioperative management of patients with cCD, the authors wrote.
The Segmental Colectomy for Crohn’s disease (SCOTCH) international study included a retrospective analysis of data from six European Inflammatory Bowel Disease referral centers on patients operated on between 2000 and 2019 who had either segmental or total colectomy for cCD.
Among 687 patients (301 male; 386 female), segmental colectomy was performed in 285 (41.5%) of cases and total colectomy in 402 (58.5%). The 15-year surgical recurrence rate was 44% among patients who had TC and 27% for patients with segmental colectomy (P = .006).
The SCOTCH study found that segmental colectomy may be performed safely and effectively and reduce the need for stoma in cCD patients without increasing risk of repeat surgeries compared with total colectomy, which was the primary measure investigators studied.
The findings of this study also suggest that biologics, when used early and correctly, may allow more conservative options for cCD, with a fivefold reduction in surgical recurrence risk in patients who have one to three large bowel locations.
Morbidity and mortality were similar in the SC and TC groups.
Among the limitations of the study are that the total colectomy patients in the study had indications for total colectomy that were also higher risk factors for recurrence – for instance, perianal disease.
The authors wrote, “The differences between patients who underwent SC vs TC might have accounted for the choice of one treatment over the other. It is however difficult to obtain a homogenous population of cCD patients.” They also cite the difficulties in gathering enough patients for randomized trials.
“These findings need to be discussed with the patients, and the choice of operation should be individualised,” they concluded. “Multidisciplinary management of patients with cCD is of critical importance to achieve optimal long-term results of bowel-sparing approaches.”
Miguel Regueiro, MD, chair of the department of gastroenterology, hepatology, and nutrition at the Cleveland Clinic, who was not part of the study, told this publication the findings should be considered confirmatory rather than suggestive of practice change.
“If a patient has a limited segment of Crohn’s, for example ileocecal Crohn’s – a common phenotype – then the standard of care is a segmental resection and primary anastomosis,” he said. “If the patient has more extensive CD – perianal fistula, colonic-only CD – they’re more likely to undergo a total colectomy. This study confirms that.“
The authors and Dr. Regueiro declared no relevant financial relationships.
FROM JOURNAL OF CROHN’S AND COLITIS
Marital status plays modest role in gastric cancer overall survival
, according to research published in the Journal of Investigative Medicine.
Tumor size remained the largest contributor to overall survival, but marital status was among several other significant factors, such as age, race, gender, treatment style, and pathologic stage, that can provide insight into a patient’s likelihood of overall survival, as it does with several other cancers.
“Married patients had the best prognosis, followed by single patients, and the prognosis of separated patients was the worst,” write Lixiang Zhang and colleagues at the First Affiliated Hospital of Anhui Medical University, Hefei, China. “We speculate that this might be due to the fact that married patients had better financial conditions and emotional encouragement, while separated patients might be more likely to experience financial difficulties [and] emotional loss.”
The results were not necessarily surprising to Richard M. Peek, Jr., MD, director of the division of gastroenterology and a professor of medicine at Vanderbilt University Medical Center, who was not involved in the research.
“Marital status is a reflection of support systems, and a strong support system is a prognosticator for increased compliance with medical appointments and medical therapies,” Dr. Peek told this news organization. “It is something to consider when somebody is being treated for gastric cancer, because if they don’t have a strong support system – and marital status can be a proxy for that – then they may need more intensive follow-up and surveillance, for example, than somebody who does not have that support system.”
Exploring the marital status–cancer survival connection
Gastric cancer is the third leading cause of cancer deaths across the world, causing 780,000 deaths in 2018, the authors note. Yet it’s difficult to accurately predict the prognosis in patients who undergo treatment for early stage gastric cancer. Previous research has found marital status to be associated with survival in prostate, cervical, and rectal cancers.
Mark A. Lewis, MD, director of gastrointestinal oncology at Intermountain Healthcare Cancer Center, Utah, told this news organization that the connection between marital status and cancer outcomes has been described previously, including in an even larger analysis using the U.S. Surveillance, Epidemiology, and End Results (SEER) database from 2013. That study found that “unmarried patients are at significantly higher risk of presentation with metastatic cancer, undertreatment, and death resulting from their cancer.”
In this study, the researchers compared marital status and survival rates among 3,647 patients with early-stage gastric cancer, using data from the SEER database. The study only included patients with tumors in the lamina propria, mucosa, and submucosa and excluded those with distant metastasis or distant lymph node metastases, a second cancer, no data on chemotherapy received, or unknown survival time.
Because they were using a nomogram and building a new predictive nomogram that would include marital status, the researchers divided the patient population into a training set of 2,719 patients and a testing set of 928 patients. Using overall survival as the primary endpoint, the analysis included the variables of “age at diagnosis, race, gender, tumor location, histology, grade, stage_T and stage_N, surgery in the primary site, lymph node dissection, chemotherapy, radiation, tumor size, insurance, and marital status,” the authors report.
Among the study population, 53.7% were married, 17.3% were widowed, 14% were single and never married, 7.5% were divorced, 1.1% were separated, and the status of 6.4% was unknown. Age at diagnosis, race, gender, histology, tumor grade, stage T, stage N, surgery type, tumor size, and insurance status were all significantly different between the marital status subgroups.
Married patients had the best prognosis, with an average overall survival of 72 months, compared with an average 60 months in widowed persons, the group with the poorest overall survival. Overall survival was higher in married women (76 months) than in married men (69 months). The same pattern held for women (62 months) and men (52 months) who had been widowed.
“It is worthy to note that survival was significantly better in divorced female patients than in divorced male patients,” the authors report. “Survival was better in female patients than in male patients” across all marital groups.
What long-term relationships reveal
These findings do not mean that simply getting married changes one’s likelihood of survival, however. Rather, a long-term relationship is revealing about other aspects in a person’s life.
“I think it represents more stability in the supportive relationship that you need to really deal with a serious disease like cancer,” Dr. Peek said.
If a patient does not have a long-term partner, their care team can ask other questions to get a sense of what their support network is like, Dr. Peek added. “We want to know, does anybody else live in the house with them? Do they have adequate transportation? Can they make medical appointments? Do they have somebody who can help with the medical issues that are going to come up? Do they have family in the area?”
Cancer treatment requires a multidisciplinary approach, and having someone other than just the patient around to help bring together the different aspects of care from different care teams can make a difference in how the patient fares, Dr. Peek explained. Patients without a strong support system may need closer follow-up and other accommodations, he said.
Providers “may schedule their clinical appointments closer together if they don’t have a support system, or they may be able to reach out and offer transportation assistance and those kinds of things that somebody living alone may need,” Dr. Peek said. Outside resources may be a higher priority for those who lack a support system at home, he added.
Dr. Peek also noted other factors that may play a role in a patient’s survival that these researchers did not have the data to address, such as socioeconomic status, employment, alcohol use, smoking, and infection with Helicobacter pylori, the strongest known risk factor for gastric cancer.
A potentially relevant limitation of the study is that it probably has some selection bias, because the patients who were included probably had the means to have received an earlier diagnosis, said Dr. Lewis, who was not involved in the research.
“Furthermore, just in terms of the group sizes, the baseline characteristics section makes it clear that the preponderance of patients were married, lending that group more statistical weight,” Dr. Lewis said.
“Of the seven attributes in the nomogram, the impact of the marital status seems comparatively meager relative to conventional clinicopathology risk factors like T stage,” he added.
“All in all, I think this study reinforces our awareness that socioeconomic status and social determinants of health play a huge role in cancer outcomes, but it’s not entirely clear that’s modifiable just by getting married,” Dr. Lewis said. “There is a saying in oncology that ‘expensive liquor causes less cancer than cheap liquor,’ which is not negating the carcinogenicity of alcohol but rather identifying different outcomes by socioeconomic status.”
The research was funded by the Natural Science Foundation of Anhui Province. The authors report no relevant financial relationships. Dr. Peek reports no relevant financial relationships. Dr. Lewis reports receiving speaking fees for AstraZeneca/Daiichi Sankyo and having done educational videos for Astellas.
A version of this article first appeared on Medscape.com.
, according to research published in the Journal of Investigative Medicine.
Tumor size remained the largest contributor to overall survival, but marital status was among several other significant factors, such as age, race, gender, treatment style, and pathologic stage, that can provide insight into a patient’s likelihood of overall survival, as it does with several other cancers.
“Married patients had the best prognosis, followed by single patients, and the prognosis of separated patients was the worst,” write Lixiang Zhang and colleagues at the First Affiliated Hospital of Anhui Medical University, Hefei, China. “We speculate that this might be due to the fact that married patients had better financial conditions and emotional encouragement, while separated patients might be more likely to experience financial difficulties [and] emotional loss.”
The results were not necessarily surprising to Richard M. Peek, Jr., MD, director of the division of gastroenterology and a professor of medicine at Vanderbilt University Medical Center, who was not involved in the research.
“Marital status is a reflection of support systems, and a strong support system is a prognosticator for increased compliance with medical appointments and medical therapies,” Dr. Peek told this news organization. “It is something to consider when somebody is being treated for gastric cancer, because if they don’t have a strong support system – and marital status can be a proxy for that – then they may need more intensive follow-up and surveillance, for example, than somebody who does not have that support system.”
Exploring the marital status–cancer survival connection
Gastric cancer is the third leading cause of cancer deaths across the world, causing 780,000 deaths in 2018, the authors note. Yet it’s difficult to accurately predict the prognosis in patients who undergo treatment for early stage gastric cancer. Previous research has found marital status to be associated with survival in prostate, cervical, and rectal cancers.
Mark A. Lewis, MD, director of gastrointestinal oncology at Intermountain Healthcare Cancer Center, Utah, told this news organization that the connection between marital status and cancer outcomes has been described previously, including in an even larger analysis using the U.S. Surveillance, Epidemiology, and End Results (SEER) database from 2013. That study found that “unmarried patients are at significantly higher risk of presentation with metastatic cancer, undertreatment, and death resulting from their cancer.”
In this study, the researchers compared marital status and survival rates among 3,647 patients with early-stage gastric cancer, using data from the SEER database. The study only included patients with tumors in the lamina propria, mucosa, and submucosa and excluded those with distant metastasis or distant lymph node metastases, a second cancer, no data on chemotherapy received, or unknown survival time.
Because they were using a nomogram and building a new predictive nomogram that would include marital status, the researchers divided the patient population into a training set of 2,719 patients and a testing set of 928 patients. Using overall survival as the primary endpoint, the analysis included the variables of “age at diagnosis, race, gender, tumor location, histology, grade, stage_T and stage_N, surgery in the primary site, lymph node dissection, chemotherapy, radiation, tumor size, insurance, and marital status,” the authors report.
Among the study population, 53.7% were married, 17.3% were widowed, 14% were single and never married, 7.5% were divorced, 1.1% were separated, and the status of 6.4% was unknown. Age at diagnosis, race, gender, histology, tumor grade, stage T, stage N, surgery type, tumor size, and insurance status were all significantly different between the marital status subgroups.
Married patients had the best prognosis, with an average overall survival of 72 months, compared with an average 60 months in widowed persons, the group with the poorest overall survival. Overall survival was higher in married women (76 months) than in married men (69 months). The same pattern held for women (62 months) and men (52 months) who had been widowed.
“It is worthy to note that survival was significantly better in divorced female patients than in divorced male patients,” the authors report. “Survival was better in female patients than in male patients” across all marital groups.
What long-term relationships reveal
These findings do not mean that simply getting married changes one’s likelihood of survival, however. Rather, a long-term relationship is revealing about other aspects in a person’s life.
“I think it represents more stability in the supportive relationship that you need to really deal with a serious disease like cancer,” Dr. Peek said.
If a patient does not have a long-term partner, their care team can ask other questions to get a sense of what their support network is like, Dr. Peek added. “We want to know, does anybody else live in the house with them? Do they have adequate transportation? Can they make medical appointments? Do they have somebody who can help with the medical issues that are going to come up? Do they have family in the area?”
Cancer treatment requires a multidisciplinary approach, and having someone other than just the patient around to help bring together the different aspects of care from different care teams can make a difference in how the patient fares, Dr. Peek explained. Patients without a strong support system may need closer follow-up and other accommodations, he said.
Providers “may schedule their clinical appointments closer together if they don’t have a support system, or they may be able to reach out and offer transportation assistance and those kinds of things that somebody living alone may need,” Dr. Peek said. Outside resources may be a higher priority for those who lack a support system at home, he added.
Dr. Peek also noted other factors that may play a role in a patient’s survival that these researchers did not have the data to address, such as socioeconomic status, employment, alcohol use, smoking, and infection with Helicobacter pylori, the strongest known risk factor for gastric cancer.
A potentially relevant limitation of the study is that it probably has some selection bias, because the patients who were included probably had the means to have received an earlier diagnosis, said Dr. Lewis, who was not involved in the research.
“Furthermore, just in terms of the group sizes, the baseline characteristics section makes it clear that the preponderance of patients were married, lending that group more statistical weight,” Dr. Lewis said.
“Of the seven attributes in the nomogram, the impact of the marital status seems comparatively meager relative to conventional clinicopathology risk factors like T stage,” he added.
“All in all, I think this study reinforces our awareness that socioeconomic status and social determinants of health play a huge role in cancer outcomes, but it’s not entirely clear that’s modifiable just by getting married,” Dr. Lewis said. “There is a saying in oncology that ‘expensive liquor causes less cancer than cheap liquor,’ which is not negating the carcinogenicity of alcohol but rather identifying different outcomes by socioeconomic status.”
The research was funded by the Natural Science Foundation of Anhui Province. The authors report no relevant financial relationships. Dr. Peek reports no relevant financial relationships. Dr. Lewis reports receiving speaking fees for AstraZeneca/Daiichi Sankyo and having done educational videos for Astellas.
A version of this article first appeared on Medscape.com.
, according to research published in the Journal of Investigative Medicine.
Tumor size remained the largest contributor to overall survival, but marital status was among several other significant factors, such as age, race, gender, treatment style, and pathologic stage, that can provide insight into a patient’s likelihood of overall survival, as it does with several other cancers.
“Married patients had the best prognosis, followed by single patients, and the prognosis of separated patients was the worst,” write Lixiang Zhang and colleagues at the First Affiliated Hospital of Anhui Medical University, Hefei, China. “We speculate that this might be due to the fact that married patients had better financial conditions and emotional encouragement, while separated patients might be more likely to experience financial difficulties [and] emotional loss.”
The results were not necessarily surprising to Richard M. Peek, Jr., MD, director of the division of gastroenterology and a professor of medicine at Vanderbilt University Medical Center, who was not involved in the research.
“Marital status is a reflection of support systems, and a strong support system is a prognosticator for increased compliance with medical appointments and medical therapies,” Dr. Peek told this news organization. “It is something to consider when somebody is being treated for gastric cancer, because if they don’t have a strong support system – and marital status can be a proxy for that – then they may need more intensive follow-up and surveillance, for example, than somebody who does not have that support system.”
Exploring the marital status–cancer survival connection
Gastric cancer is the third leading cause of cancer deaths across the world, causing 780,000 deaths in 2018, the authors note. Yet it’s difficult to accurately predict the prognosis in patients who undergo treatment for early stage gastric cancer. Previous research has found marital status to be associated with survival in prostate, cervical, and rectal cancers.
Mark A. Lewis, MD, director of gastrointestinal oncology at Intermountain Healthcare Cancer Center, Utah, told this news organization that the connection between marital status and cancer outcomes has been described previously, including in an even larger analysis using the U.S. Surveillance, Epidemiology, and End Results (SEER) database from 2013. That study found that “unmarried patients are at significantly higher risk of presentation with metastatic cancer, undertreatment, and death resulting from their cancer.”
In this study, the researchers compared marital status and survival rates among 3,647 patients with early-stage gastric cancer, using data from the SEER database. The study only included patients with tumors in the lamina propria, mucosa, and submucosa and excluded those with distant metastasis or distant lymph node metastases, a second cancer, no data on chemotherapy received, or unknown survival time.
Because they were using a nomogram and building a new predictive nomogram that would include marital status, the researchers divided the patient population into a training set of 2,719 patients and a testing set of 928 patients. Using overall survival as the primary endpoint, the analysis included the variables of “age at diagnosis, race, gender, tumor location, histology, grade, stage_T and stage_N, surgery in the primary site, lymph node dissection, chemotherapy, radiation, tumor size, insurance, and marital status,” the authors report.
Among the study population, 53.7% were married, 17.3% were widowed, 14% were single and never married, 7.5% were divorced, 1.1% were separated, and the status of 6.4% was unknown. Age at diagnosis, race, gender, histology, tumor grade, stage T, stage N, surgery type, tumor size, and insurance status were all significantly different between the marital status subgroups.
Married patients had the best prognosis, with an average overall survival of 72 months, compared with an average 60 months in widowed persons, the group with the poorest overall survival. Overall survival was higher in married women (76 months) than in married men (69 months). The same pattern held for women (62 months) and men (52 months) who had been widowed.
“It is worthy to note that survival was significantly better in divorced female patients than in divorced male patients,” the authors report. “Survival was better in female patients than in male patients” across all marital groups.
What long-term relationships reveal
These findings do not mean that simply getting married changes one’s likelihood of survival, however. Rather, a long-term relationship is revealing about other aspects in a person’s life.
“I think it represents more stability in the supportive relationship that you need to really deal with a serious disease like cancer,” Dr. Peek said.
If a patient does not have a long-term partner, their care team can ask other questions to get a sense of what their support network is like, Dr. Peek added. “We want to know, does anybody else live in the house with them? Do they have adequate transportation? Can they make medical appointments? Do they have somebody who can help with the medical issues that are going to come up? Do they have family in the area?”
Cancer treatment requires a multidisciplinary approach, and having someone other than just the patient around to help bring together the different aspects of care from different care teams can make a difference in how the patient fares, Dr. Peek explained. Patients without a strong support system may need closer follow-up and other accommodations, he said.
Providers “may schedule their clinical appointments closer together if they don’t have a support system, or they may be able to reach out and offer transportation assistance and those kinds of things that somebody living alone may need,” Dr. Peek said. Outside resources may be a higher priority for those who lack a support system at home, he added.
Dr. Peek also noted other factors that may play a role in a patient’s survival that these researchers did not have the data to address, such as socioeconomic status, employment, alcohol use, smoking, and infection with Helicobacter pylori, the strongest known risk factor for gastric cancer.
A potentially relevant limitation of the study is that it probably has some selection bias, because the patients who were included probably had the means to have received an earlier diagnosis, said Dr. Lewis, who was not involved in the research.
“Furthermore, just in terms of the group sizes, the baseline characteristics section makes it clear that the preponderance of patients were married, lending that group more statistical weight,” Dr. Lewis said.
“Of the seven attributes in the nomogram, the impact of the marital status seems comparatively meager relative to conventional clinicopathology risk factors like T stage,” he added.
“All in all, I think this study reinforces our awareness that socioeconomic status and social determinants of health play a huge role in cancer outcomes, but it’s not entirely clear that’s modifiable just by getting married,” Dr. Lewis said. “There is a saying in oncology that ‘expensive liquor causes less cancer than cheap liquor,’ which is not negating the carcinogenicity of alcohol but rather identifying different outcomes by socioeconomic status.”
The research was funded by the Natural Science Foundation of Anhui Province. The authors report no relevant financial relationships. Dr. Peek reports no relevant financial relationships. Dr. Lewis reports receiving speaking fees for AstraZeneca/Daiichi Sankyo and having done educational videos for Astellas.
A version of this article first appeared on Medscape.com.
FROM JOURNAL OF INVESTIGATIVE MEDICINE
CRC screening disparities greatest among those under 55
Adults younger than 55 years were least likely to get screened for colorectal cancer over the past 2 decades, particularly if they were Hispanic or Asian or had a low income, lower education level, or no health insurance, according to a new study published online in Cancer Epidemiology, Biomarkers & Prevention.
The findings have raised concerns that disparities in screening rates will be even greater in adults aged 45-49 years, prompting the need for increased awareness and outreach to ensure that underserved groups have access to screenings.
“Differences in prevalence of screening by race and ethnicity, educational attainment, household income, and health insurance were most pronounced for those ages 50-54 years, whereas older adults experienced larger increases in prevalence across these groups,” wrote Po-Hong Liu, MD, MPH, a clinical investigator at Harvard University, Boston, and his colleagues. “The persistent and worsening disparities we observed in adults 50-54 years may extend to those ages 45-49 as they become eligible for screening.”
The U.S. Preventive Services Task Force shifted their recommendation for colorectal cancer screening in May 2021 to 5 years earlier, advising people to start screenings at 45 instead of 50, which aligns with the recommendations the American Cancer Society made 3 years earlier.
Both organizations made the change because of increasing rates of colorectal cancer in adults under age 50 and research indicating that beginning screenings at age 45 results in fewer cases, fewer deaths, and more life years gained.
“Across all age groups, colorectal cancer screening participation remains below national goals, and the benefits of screening are not equally realized across populations,” senior author Caitlin Murphy, PhD, MPH, associate professor, UTHealth School of Public Health, Houston, said in a prepared statement. “Extra care must be taken to ensure that expanding screening to younger ages does not negatively impact efforts to eliminate disparities in colorectal screening and outcomes nor jeopardize efforts to increase screening initiation among older adults who remain unscreened.”
Data analyzed from 8 years over 2 decades
The researchers analyzed data from the CDC’s cross-sectional National Health Interview Survey during 8 years over the past 2 decades: 2000, 2003, 2005, 2008, 2010, 2013, 2015, and 2018.
The number of participants each year ranged from a low of 21,781 in 2008 to a high of 34,557 in 2013. After excluding participants with a history of colorectal cancer or missing information on screenings, the total population sample included 80,220 participants 50-75 years old.
The researchers considered a person as having been screened if they received at least one recommended screening test within the year covered by the survey, regardless of why they underwent the test.
Recommended tests included sigmoidoscopy, colonoscopy, and stool-based tests for all survey years. In addition, the surveys for 2010, 2015, and 2018 included CT colonography, and the 2018 survey included FIT-DNA.
Screening across population groups
Colorectal cancer screening rates have doubled in the past 2 decades, from 36.7% in 2000 to 66.1% in 2018.
Rates are considerably lower, however, for several key groups, including the youngest group. Less than half (47.6%) of those aged 50-54 years received screenings in 2018, though this was still a nearly 20-point improvement over the 28.2% in this age group who were screened in 2000.
Separate from age, several other groups continue to have low screening rates in general, including Hispanics (56.5%, up from 25.9% in 2000), Asians (57.1%, up from 22.6% in 2000), those who have not received a high school degree (53.6%, up from 26.8% in 2000), and those from low income families (56.6%, up from 30.2% in 2000).
The group with the greatest need for more outreach and screenings are people without insurance, only 39.7% of whom were screened in 2018, a modest increase from 30.2% in 2000.
The biggest increase in screenings over time occurred in those aged 70-75 years, from 46.4% in 2000 to 78% in 2018 overall.
Racial/ethnic, economic, education, and insurance-based disparities were particularly evident the younger people were, including in terms of progress made over time.
For example, screenings of non-Hispanic White people aged 50-54 years improved 21 points (30.3% to 51%) between 2000 and 2018, compared with 19 points in Hispanics (16.7% to 35.5%) and 15 points in Asians (17.3% to 32.3%). Fortunately, Black Americans made even greater strides than White Americans with a 27-point increase during that time (23.4% to 50%).
Similarly, income correlated with expansion in screening rates for 50- to 54-year-olds: Those earning at least 400% over the federal poverty line improved 20 points (from 33.5% to 53.8%), compared with a 16-point improvement in those earning less than 200% above the poverty line (from 19.3% to 35%).
Those with private insurance likewise improved 21 points (from 30.7% to 51.7%), while those in this age group without insurance declined, with just 21.2% getting screened in 2018, compared with 28.2% in 2000. Those on public insurance saw a 15-point improvement, from 27.8% in 2000 to 43.1% in 2018.
“The individual and societal burden of colorectal cancer is especially great among younger adults,” the authors wrote.
The reasons for the much lower prevalence of screening in those under 55, the authors suggested, is likely due to less concern about colorectal cancer, less access to medical care (including being underinsured or uninsured), and the barriers created by competing priorities, such as work schedules, family responsibilities, and caregiving. The latter may be particularly true in underserved populations, the authors noted.
“Screening programs must consider the barriers unique to younger adults, ensuring the benefits of screening are equally realized by all population groups,” the authors concluded.
The research was funded by the National Institutes of Health and the Cancer Prevention and Research Institute of Texas. One author reported grants from Epigenomics and Freenome and personal fees from Guardant Health. Another author reported personal fees from Freenome, and a third author reported personal fees from Exact Sciences. No other authors had industry disclosures.
A version of this article first appeared on Medscape.com.
Adults younger than 55 years were least likely to get screened for colorectal cancer over the past 2 decades, particularly if they were Hispanic or Asian or had a low income, lower education level, or no health insurance, according to a new study published online in Cancer Epidemiology, Biomarkers & Prevention.
The findings have raised concerns that disparities in screening rates will be even greater in adults aged 45-49 years, prompting the need for increased awareness and outreach to ensure that underserved groups have access to screenings.
“Differences in prevalence of screening by race and ethnicity, educational attainment, household income, and health insurance were most pronounced for those ages 50-54 years, whereas older adults experienced larger increases in prevalence across these groups,” wrote Po-Hong Liu, MD, MPH, a clinical investigator at Harvard University, Boston, and his colleagues. “The persistent and worsening disparities we observed in adults 50-54 years may extend to those ages 45-49 as they become eligible for screening.”
The U.S. Preventive Services Task Force shifted their recommendation for colorectal cancer screening in May 2021 to 5 years earlier, advising people to start screenings at 45 instead of 50, which aligns with the recommendations the American Cancer Society made 3 years earlier.
Both organizations made the change because of increasing rates of colorectal cancer in adults under age 50 and research indicating that beginning screenings at age 45 results in fewer cases, fewer deaths, and more life years gained.
“Across all age groups, colorectal cancer screening participation remains below national goals, and the benefits of screening are not equally realized across populations,” senior author Caitlin Murphy, PhD, MPH, associate professor, UTHealth School of Public Health, Houston, said in a prepared statement. “Extra care must be taken to ensure that expanding screening to younger ages does not negatively impact efforts to eliminate disparities in colorectal screening and outcomes nor jeopardize efforts to increase screening initiation among older adults who remain unscreened.”
Data analyzed from 8 years over 2 decades
The researchers analyzed data from the CDC’s cross-sectional National Health Interview Survey during 8 years over the past 2 decades: 2000, 2003, 2005, 2008, 2010, 2013, 2015, and 2018.
The number of participants each year ranged from a low of 21,781 in 2008 to a high of 34,557 in 2013. After excluding participants with a history of colorectal cancer or missing information on screenings, the total population sample included 80,220 participants 50-75 years old.
The researchers considered a person as having been screened if they received at least one recommended screening test within the year covered by the survey, regardless of why they underwent the test.
Recommended tests included sigmoidoscopy, colonoscopy, and stool-based tests for all survey years. In addition, the surveys for 2010, 2015, and 2018 included CT colonography, and the 2018 survey included FIT-DNA.
Screening across population groups
Colorectal cancer screening rates have doubled in the past 2 decades, from 36.7% in 2000 to 66.1% in 2018.
Rates are considerably lower, however, for several key groups, including the youngest group. Less than half (47.6%) of those aged 50-54 years received screenings in 2018, though this was still a nearly 20-point improvement over the 28.2% in this age group who were screened in 2000.
Separate from age, several other groups continue to have low screening rates in general, including Hispanics (56.5%, up from 25.9% in 2000), Asians (57.1%, up from 22.6% in 2000), those who have not received a high school degree (53.6%, up from 26.8% in 2000), and those from low income families (56.6%, up from 30.2% in 2000).
The group with the greatest need for more outreach and screenings are people without insurance, only 39.7% of whom were screened in 2018, a modest increase from 30.2% in 2000.
The biggest increase in screenings over time occurred in those aged 70-75 years, from 46.4% in 2000 to 78% in 2018 overall.
Racial/ethnic, economic, education, and insurance-based disparities were particularly evident the younger people were, including in terms of progress made over time.
For example, screenings of non-Hispanic White people aged 50-54 years improved 21 points (30.3% to 51%) between 2000 and 2018, compared with 19 points in Hispanics (16.7% to 35.5%) and 15 points in Asians (17.3% to 32.3%). Fortunately, Black Americans made even greater strides than White Americans with a 27-point increase during that time (23.4% to 50%).
Similarly, income correlated with expansion in screening rates for 50- to 54-year-olds: Those earning at least 400% over the federal poverty line improved 20 points (from 33.5% to 53.8%), compared with a 16-point improvement in those earning less than 200% above the poverty line (from 19.3% to 35%).
Those with private insurance likewise improved 21 points (from 30.7% to 51.7%), while those in this age group without insurance declined, with just 21.2% getting screened in 2018, compared with 28.2% in 2000. Those on public insurance saw a 15-point improvement, from 27.8% in 2000 to 43.1% in 2018.
“The individual and societal burden of colorectal cancer is especially great among younger adults,” the authors wrote.
The reasons for the much lower prevalence of screening in those under 55, the authors suggested, is likely due to less concern about colorectal cancer, less access to medical care (including being underinsured or uninsured), and the barriers created by competing priorities, such as work schedules, family responsibilities, and caregiving. The latter may be particularly true in underserved populations, the authors noted.
“Screening programs must consider the barriers unique to younger adults, ensuring the benefits of screening are equally realized by all population groups,” the authors concluded.
The research was funded by the National Institutes of Health and the Cancer Prevention and Research Institute of Texas. One author reported grants from Epigenomics and Freenome and personal fees from Guardant Health. Another author reported personal fees from Freenome, and a third author reported personal fees from Exact Sciences. No other authors had industry disclosures.
A version of this article first appeared on Medscape.com.
Adults younger than 55 years were least likely to get screened for colorectal cancer over the past 2 decades, particularly if they were Hispanic or Asian or had a low income, lower education level, or no health insurance, according to a new study published online in Cancer Epidemiology, Biomarkers & Prevention.
The findings have raised concerns that disparities in screening rates will be even greater in adults aged 45-49 years, prompting the need for increased awareness and outreach to ensure that underserved groups have access to screenings.
“Differences in prevalence of screening by race and ethnicity, educational attainment, household income, and health insurance were most pronounced for those ages 50-54 years, whereas older adults experienced larger increases in prevalence across these groups,” wrote Po-Hong Liu, MD, MPH, a clinical investigator at Harvard University, Boston, and his colleagues. “The persistent and worsening disparities we observed in adults 50-54 years may extend to those ages 45-49 as they become eligible for screening.”
The U.S. Preventive Services Task Force shifted their recommendation for colorectal cancer screening in May 2021 to 5 years earlier, advising people to start screenings at 45 instead of 50, which aligns with the recommendations the American Cancer Society made 3 years earlier.
Both organizations made the change because of increasing rates of colorectal cancer in adults under age 50 and research indicating that beginning screenings at age 45 results in fewer cases, fewer deaths, and more life years gained.
“Across all age groups, colorectal cancer screening participation remains below national goals, and the benefits of screening are not equally realized across populations,” senior author Caitlin Murphy, PhD, MPH, associate professor, UTHealth School of Public Health, Houston, said in a prepared statement. “Extra care must be taken to ensure that expanding screening to younger ages does not negatively impact efforts to eliminate disparities in colorectal screening and outcomes nor jeopardize efforts to increase screening initiation among older adults who remain unscreened.”
Data analyzed from 8 years over 2 decades
The researchers analyzed data from the CDC’s cross-sectional National Health Interview Survey during 8 years over the past 2 decades: 2000, 2003, 2005, 2008, 2010, 2013, 2015, and 2018.
The number of participants each year ranged from a low of 21,781 in 2008 to a high of 34,557 in 2013. After excluding participants with a history of colorectal cancer or missing information on screenings, the total population sample included 80,220 participants 50-75 years old.
The researchers considered a person as having been screened if they received at least one recommended screening test within the year covered by the survey, regardless of why they underwent the test.
Recommended tests included sigmoidoscopy, colonoscopy, and stool-based tests for all survey years. In addition, the surveys for 2010, 2015, and 2018 included CT colonography, and the 2018 survey included FIT-DNA.
Screening across population groups
Colorectal cancer screening rates have doubled in the past 2 decades, from 36.7% in 2000 to 66.1% in 2018.
Rates are considerably lower, however, for several key groups, including the youngest group. Less than half (47.6%) of those aged 50-54 years received screenings in 2018, though this was still a nearly 20-point improvement over the 28.2% in this age group who were screened in 2000.
Separate from age, several other groups continue to have low screening rates in general, including Hispanics (56.5%, up from 25.9% in 2000), Asians (57.1%, up from 22.6% in 2000), those who have not received a high school degree (53.6%, up from 26.8% in 2000), and those from low income families (56.6%, up from 30.2% in 2000).
The group with the greatest need for more outreach and screenings are people without insurance, only 39.7% of whom were screened in 2018, a modest increase from 30.2% in 2000.
The biggest increase in screenings over time occurred in those aged 70-75 years, from 46.4% in 2000 to 78% in 2018 overall.
Racial/ethnic, economic, education, and insurance-based disparities were particularly evident the younger people were, including in terms of progress made over time.
For example, screenings of non-Hispanic White people aged 50-54 years improved 21 points (30.3% to 51%) between 2000 and 2018, compared with 19 points in Hispanics (16.7% to 35.5%) and 15 points in Asians (17.3% to 32.3%). Fortunately, Black Americans made even greater strides than White Americans with a 27-point increase during that time (23.4% to 50%).
Similarly, income correlated with expansion in screening rates for 50- to 54-year-olds: Those earning at least 400% over the federal poverty line improved 20 points (from 33.5% to 53.8%), compared with a 16-point improvement in those earning less than 200% above the poverty line (from 19.3% to 35%).
Those with private insurance likewise improved 21 points (from 30.7% to 51.7%), while those in this age group without insurance declined, with just 21.2% getting screened in 2018, compared with 28.2% in 2000. Those on public insurance saw a 15-point improvement, from 27.8% in 2000 to 43.1% in 2018.
“The individual and societal burden of colorectal cancer is especially great among younger adults,” the authors wrote.
The reasons for the much lower prevalence of screening in those under 55, the authors suggested, is likely due to less concern about colorectal cancer, less access to medical care (including being underinsured or uninsured), and the barriers created by competing priorities, such as work schedules, family responsibilities, and caregiving. The latter may be particularly true in underserved populations, the authors noted.
“Screening programs must consider the barriers unique to younger adults, ensuring the benefits of screening are equally realized by all population groups,” the authors concluded.
The research was funded by the National Institutes of Health and the Cancer Prevention and Research Institute of Texas. One author reported grants from Epigenomics and Freenome and personal fees from Guardant Health. Another author reported personal fees from Freenome, and a third author reported personal fees from Exact Sciences. No other authors had industry disclosures.
A version of this article first appeared on Medscape.com.
FROM CANCER EPIDEMIOLOGY, BIOMARKERS AN PREVENTION
Evidence still lacking that vitamins prevent CVD, cancer: USPSTF
There is not enough evidence to recommend for or against taking most vitamin and mineral supplements to prevent heart disease, stroke, and cancer, a new report by the U.S. Preventive Services Task Force concludes.
However, there are two vitamins – vitamin E and beta-carotene – that the task force recommends against for the prevention of heart disease, stroke, and cancer. Evidence shows that there is no benefit to taking vitamin E and that beta-carotene can increase the risk for lung cancer in people already at risk, such as smokers and those with occupational exposure to asbestos.
These are the main findings of the USPSTF’s final recommendation statement on vitamin, mineral, and multivitamin supplementation to prevent cardiovascular disease and cancer. The statement was published in JAMA.
“This is essentially the same recommendation that the task force made in 2014,” USPSTF member John Wong, MD, professor of medicine at Tufts University, Boston, said in an interview.
“We recognize that over half of people in the U.S. take a vitamin supplement of some sort every day and 30% take a vitamin/mineral combination. We wanted to review the evidence again to see if there was any benefit in terms of reducing the risk of cardiovascular disease or cancer or increasing the chances of living longer,” Dr. Wong explained.
“We looked hard for evidence, reviewing 84 studies in total. But we did not find sufficient evidence in favor of taking or not taking vitamins, with the two exceptions of beta-carotene and vitamin E, which we recommend against taking,” he noted.
Although there is evidence of some harm with beta-carotene, the main reason behind the recommendation against taking vitamin E is the consistent evidence of no benefit, Dr. Wong explained.
“While the evidence for some other vitamins is conflicting, there is more consistent evidence of no benefit for vitamin E,” he said.
The bulk of new evidence since the last review in 2014 was predominately for vitamin D supplementation, but despite the inclusion of 32 new randomized, controlled trials and two cohort studies, pooled estimates for all-cause mortality were similar to those in the previous review, with confidence intervals only slightly crossing 1, and point estimates that suggest at most a very small benefit, the task force noted.
“Apart from beta-carotene and vitamin E, after reviewing 84 studies – including 78 randomized controlled trials – in over a million patients, we can find no clear demonstration of benefit or harm of taking vitamins in terms of developing cardiovascular disease or cancer or the effect on all-cause mortality. So, we don’t know whether people should take vitamins or not, and we need more research,” Dr. Wong added.
On the use of a multivitamin supplement, Dr. Wong noted that the complete body of evidence did not find any benefit of taking a multivitamin on cardiovascular or cancer mortality. But there was a small reduction in cancer incidence.
However, he pointed out that the three studies that suggested a reduction in cancer incidence all had issues regarding generalizability.
“The recently published COSMOS trial had an average follow-up of only 3.6 years, which isn’t really long enough when thinking about the prevention of cancer, one of the other studies only used antioxidants, and the third study was conducted only in U.S. male physicians. So those limitations regarding generalizability limited our confidence in making recommendations about multivitamins,” Dr. Wong explained.
But he noted that the task force did not find any significant harms from taking multivitamins.
“There are possible harms from taking high doses of vitamin A and vitamin D, but generally the doses contained in a multivitamin tablet are lower than these. But if the goal for taking a multivitamin is to lower your risk of cancer or cardiovascular disease, we didn’t find sufficient evidence to be able to make a recommendation,” he said.
Asked what he would say to all the people currently taking multivitamins, Dr. Wong responded that he would advise them to have a conversation with a trusted health care professional about their particular circumstances.
“Our statement has quite a narrow focus. It is directed toward community-dwelling, nonpregnant adults. This recommendation does not apply to children, persons who are pregnant or may become pregnant, or persons who are chronically ill, are hospitalized, or have a known nutritional deficiency,” he commented.
‘Any benefit likely to be small’
In an editorial accompanying the publication of the USPSTF statement, Jenny Jia, MD; Natalie Cameron, MD; and Jeffrey Linder, MD – all from Northwestern University, Chicago – noted that the current evidence base includes 52 additional studies not available when the last USPSTF recommendation on this topic was published in 2014.
The editorialists pointed out that for multivitamins, proving the absence of a benefit is challenging, but at best, current evidence suggests that any potential benefits of a multivitamin to reduce mortality are likely to be small.
They gave an example of a healthy 65-year-old woman with a 9-year estimated mortality risk of about 8%, and note that taking a multivitamin for 5-10 years might reduce her estimated mortality risk to 7.5% (based on an odds ratio of 0.94).
“In addition to showing small potential benefit, this estimate is based on imperfect evidence, is imprecise, and is highly sensitive to how the data are interpreted and analyzed,” they said.
The editorialists recommended that lifestyle counseling to prevent chronic diseases should continue to focus on evidence-based approaches, including balanced diets that are high in fruits and vegetables and physical activity.
However, they added that healthy eating can be a challenge when the American industrialized food system does not prioritize health, and healthy foods tend to be more expensive, leading to access problems and food insecurity.
The editorialists suggested that, rather than focusing money, time, and attention on supplements, it would be better to emphasize lower-risk, higher-benefit activities, such as getting exercise, maintaining a healthy weight, and avoiding smoking, in addition to following a healthful diet.
Possible benefit for older adults?
Commenting on the USPSTF statement, JoAnn Manson, MD, chief, division of preventive medicine, Brigham and Women’s Hospital, Boston, who led the recent COSMOS study, said that vitamin and mineral supplements should not be perceived as a substitute for a healthful diet.
“The emphasis needs to be on getting nutritional needs from a healthy diet that is high in plant-based and whole foods that don’t strip the vitamins and minerals through excessive processing,” she said. “Although it’s easier to pop a pill each day than to focus on healthful dietary patterns, the mixture of phytochemicals, fiber, and all the other nutrients in actual foods just can’t be packaged into a pill. Also, vitamins and minerals tend to be better absorbed from food than from supplements and healthy foods can replace calories from less healthy foods, such as red meat and processed foods.”
However, Dr. Manson noted that the evidence is mounting that taking a tablet containing moderate doses of a wide range of vitamins and minerals is safe and may actually have benefits for some people.
She pointed out that the COSMOS and COSMOS-Mind studies showed benefits of multivitamins in slowing cognitive decline in older adults, but the findings need to be replicated.
“The USPSTF did see a statistically significant 7% reduction in cancer with multivitamins in their meta-analysis of four randomized trials and a borderline 6% reduction in all-cause mortality,” she noted. “Plus, multivitamins have been shown to be quite safe in several large and long-term randomized trials. I agree the evidence is not sufficient to make a blanket recommendation for everyone to take multivitamins, but the evidence is mounting that this would be a prudent approach for many older adults,” Dr. Manson said.
“Many people view multivitamins as a form of insurance, as a way to hedge their bets,” she added. “Although this is a rational approach, especially for those who have concerns about the adequacy of their diet, it’s important that this mindset not lead to complacency about following healthy lifestyle practices, including healthy eating, regular physical activity, not smoking, making sure that blood pressure and cholesterol levels are well controlled, and many other practices that critically important for health but are more challenging than simply popping a pill each day.”
A version of this article first appeared on Medscape.com.
There is not enough evidence to recommend for or against taking most vitamin and mineral supplements to prevent heart disease, stroke, and cancer, a new report by the U.S. Preventive Services Task Force concludes.
However, there are two vitamins – vitamin E and beta-carotene – that the task force recommends against for the prevention of heart disease, stroke, and cancer. Evidence shows that there is no benefit to taking vitamin E and that beta-carotene can increase the risk for lung cancer in people already at risk, such as smokers and those with occupational exposure to asbestos.
These are the main findings of the USPSTF’s final recommendation statement on vitamin, mineral, and multivitamin supplementation to prevent cardiovascular disease and cancer. The statement was published in JAMA.
“This is essentially the same recommendation that the task force made in 2014,” USPSTF member John Wong, MD, professor of medicine at Tufts University, Boston, said in an interview.
“We recognize that over half of people in the U.S. take a vitamin supplement of some sort every day and 30% take a vitamin/mineral combination. We wanted to review the evidence again to see if there was any benefit in terms of reducing the risk of cardiovascular disease or cancer or increasing the chances of living longer,” Dr. Wong explained.
“We looked hard for evidence, reviewing 84 studies in total. But we did not find sufficient evidence in favor of taking or not taking vitamins, with the two exceptions of beta-carotene and vitamin E, which we recommend against taking,” he noted.
Although there is evidence of some harm with beta-carotene, the main reason behind the recommendation against taking vitamin E is the consistent evidence of no benefit, Dr. Wong explained.
“While the evidence for some other vitamins is conflicting, there is more consistent evidence of no benefit for vitamin E,” he said.
The bulk of new evidence since the last review in 2014 was predominately for vitamin D supplementation, but despite the inclusion of 32 new randomized, controlled trials and two cohort studies, pooled estimates for all-cause mortality were similar to those in the previous review, with confidence intervals only slightly crossing 1, and point estimates that suggest at most a very small benefit, the task force noted.
“Apart from beta-carotene and vitamin E, after reviewing 84 studies – including 78 randomized controlled trials – in over a million patients, we can find no clear demonstration of benefit or harm of taking vitamins in terms of developing cardiovascular disease or cancer or the effect on all-cause mortality. So, we don’t know whether people should take vitamins or not, and we need more research,” Dr. Wong added.
On the use of a multivitamin supplement, Dr. Wong noted that the complete body of evidence did not find any benefit of taking a multivitamin on cardiovascular or cancer mortality. But there was a small reduction in cancer incidence.
However, he pointed out that the three studies that suggested a reduction in cancer incidence all had issues regarding generalizability.
“The recently published COSMOS trial had an average follow-up of only 3.6 years, which isn’t really long enough when thinking about the prevention of cancer, one of the other studies only used antioxidants, and the third study was conducted only in U.S. male physicians. So those limitations regarding generalizability limited our confidence in making recommendations about multivitamins,” Dr. Wong explained.
But he noted that the task force did not find any significant harms from taking multivitamins.
“There are possible harms from taking high doses of vitamin A and vitamin D, but generally the doses contained in a multivitamin tablet are lower than these. But if the goal for taking a multivitamin is to lower your risk of cancer or cardiovascular disease, we didn’t find sufficient evidence to be able to make a recommendation,” he said.
Asked what he would say to all the people currently taking multivitamins, Dr. Wong responded that he would advise them to have a conversation with a trusted health care professional about their particular circumstances.
“Our statement has quite a narrow focus. It is directed toward community-dwelling, nonpregnant adults. This recommendation does not apply to children, persons who are pregnant or may become pregnant, or persons who are chronically ill, are hospitalized, or have a known nutritional deficiency,” he commented.
‘Any benefit likely to be small’
In an editorial accompanying the publication of the USPSTF statement, Jenny Jia, MD; Natalie Cameron, MD; and Jeffrey Linder, MD – all from Northwestern University, Chicago – noted that the current evidence base includes 52 additional studies not available when the last USPSTF recommendation on this topic was published in 2014.
The editorialists pointed out that for multivitamins, proving the absence of a benefit is challenging, but at best, current evidence suggests that any potential benefits of a multivitamin to reduce mortality are likely to be small.
They gave an example of a healthy 65-year-old woman with a 9-year estimated mortality risk of about 8%, and note that taking a multivitamin for 5-10 years might reduce her estimated mortality risk to 7.5% (based on an odds ratio of 0.94).
“In addition to showing small potential benefit, this estimate is based on imperfect evidence, is imprecise, and is highly sensitive to how the data are interpreted and analyzed,” they said.
The editorialists recommended that lifestyle counseling to prevent chronic diseases should continue to focus on evidence-based approaches, including balanced diets that are high in fruits and vegetables and physical activity.
However, they added that healthy eating can be a challenge when the American industrialized food system does not prioritize health, and healthy foods tend to be more expensive, leading to access problems and food insecurity.
The editorialists suggested that, rather than focusing money, time, and attention on supplements, it would be better to emphasize lower-risk, higher-benefit activities, such as getting exercise, maintaining a healthy weight, and avoiding smoking, in addition to following a healthful diet.
Possible benefit for older adults?
Commenting on the USPSTF statement, JoAnn Manson, MD, chief, division of preventive medicine, Brigham and Women’s Hospital, Boston, who led the recent COSMOS study, said that vitamin and mineral supplements should not be perceived as a substitute for a healthful diet.
“The emphasis needs to be on getting nutritional needs from a healthy diet that is high in plant-based and whole foods that don’t strip the vitamins and minerals through excessive processing,” she said. “Although it’s easier to pop a pill each day than to focus on healthful dietary patterns, the mixture of phytochemicals, fiber, and all the other nutrients in actual foods just can’t be packaged into a pill. Also, vitamins and minerals tend to be better absorbed from food than from supplements and healthy foods can replace calories from less healthy foods, such as red meat and processed foods.”
However, Dr. Manson noted that the evidence is mounting that taking a tablet containing moderate doses of a wide range of vitamins and minerals is safe and may actually have benefits for some people.
She pointed out that the COSMOS and COSMOS-Mind studies showed benefits of multivitamins in slowing cognitive decline in older adults, but the findings need to be replicated.
“The USPSTF did see a statistically significant 7% reduction in cancer with multivitamins in their meta-analysis of four randomized trials and a borderline 6% reduction in all-cause mortality,” she noted. “Plus, multivitamins have been shown to be quite safe in several large and long-term randomized trials. I agree the evidence is not sufficient to make a blanket recommendation for everyone to take multivitamins, but the evidence is mounting that this would be a prudent approach for many older adults,” Dr. Manson said.
“Many people view multivitamins as a form of insurance, as a way to hedge their bets,” she added. “Although this is a rational approach, especially for those who have concerns about the adequacy of their diet, it’s important that this mindset not lead to complacency about following healthy lifestyle practices, including healthy eating, regular physical activity, not smoking, making sure that blood pressure and cholesterol levels are well controlled, and many other practices that critically important for health but are more challenging than simply popping a pill each day.”
A version of this article first appeared on Medscape.com.
There is not enough evidence to recommend for or against taking most vitamin and mineral supplements to prevent heart disease, stroke, and cancer, a new report by the U.S. Preventive Services Task Force concludes.
However, there are two vitamins – vitamin E and beta-carotene – that the task force recommends against for the prevention of heart disease, stroke, and cancer. Evidence shows that there is no benefit to taking vitamin E and that beta-carotene can increase the risk for lung cancer in people already at risk, such as smokers and those with occupational exposure to asbestos.
These are the main findings of the USPSTF’s final recommendation statement on vitamin, mineral, and multivitamin supplementation to prevent cardiovascular disease and cancer. The statement was published in JAMA.
“This is essentially the same recommendation that the task force made in 2014,” USPSTF member John Wong, MD, professor of medicine at Tufts University, Boston, said in an interview.
“We recognize that over half of people in the U.S. take a vitamin supplement of some sort every day and 30% take a vitamin/mineral combination. We wanted to review the evidence again to see if there was any benefit in terms of reducing the risk of cardiovascular disease or cancer or increasing the chances of living longer,” Dr. Wong explained.
“We looked hard for evidence, reviewing 84 studies in total. But we did not find sufficient evidence in favor of taking or not taking vitamins, with the two exceptions of beta-carotene and vitamin E, which we recommend against taking,” he noted.
Although there is evidence of some harm with beta-carotene, the main reason behind the recommendation against taking vitamin E is the consistent evidence of no benefit, Dr. Wong explained.
“While the evidence for some other vitamins is conflicting, there is more consistent evidence of no benefit for vitamin E,” he said.
The bulk of new evidence since the last review in 2014 was predominately for vitamin D supplementation, but despite the inclusion of 32 new randomized, controlled trials and two cohort studies, pooled estimates for all-cause mortality were similar to those in the previous review, with confidence intervals only slightly crossing 1, and point estimates that suggest at most a very small benefit, the task force noted.
“Apart from beta-carotene and vitamin E, after reviewing 84 studies – including 78 randomized controlled trials – in over a million patients, we can find no clear demonstration of benefit or harm of taking vitamins in terms of developing cardiovascular disease or cancer or the effect on all-cause mortality. So, we don’t know whether people should take vitamins or not, and we need more research,” Dr. Wong added.
On the use of a multivitamin supplement, Dr. Wong noted that the complete body of evidence did not find any benefit of taking a multivitamin on cardiovascular or cancer mortality. But there was a small reduction in cancer incidence.
However, he pointed out that the three studies that suggested a reduction in cancer incidence all had issues regarding generalizability.
“The recently published COSMOS trial had an average follow-up of only 3.6 years, which isn’t really long enough when thinking about the prevention of cancer, one of the other studies only used antioxidants, and the third study was conducted only in U.S. male physicians. So those limitations regarding generalizability limited our confidence in making recommendations about multivitamins,” Dr. Wong explained.
But he noted that the task force did not find any significant harms from taking multivitamins.
“There are possible harms from taking high doses of vitamin A and vitamin D, but generally the doses contained in a multivitamin tablet are lower than these. But if the goal for taking a multivitamin is to lower your risk of cancer or cardiovascular disease, we didn’t find sufficient evidence to be able to make a recommendation,” he said.
Asked what he would say to all the people currently taking multivitamins, Dr. Wong responded that he would advise them to have a conversation with a trusted health care professional about their particular circumstances.
“Our statement has quite a narrow focus. It is directed toward community-dwelling, nonpregnant adults. This recommendation does not apply to children, persons who are pregnant or may become pregnant, or persons who are chronically ill, are hospitalized, or have a known nutritional deficiency,” he commented.
‘Any benefit likely to be small’
In an editorial accompanying the publication of the USPSTF statement, Jenny Jia, MD; Natalie Cameron, MD; and Jeffrey Linder, MD – all from Northwestern University, Chicago – noted that the current evidence base includes 52 additional studies not available when the last USPSTF recommendation on this topic was published in 2014.
The editorialists pointed out that for multivitamins, proving the absence of a benefit is challenging, but at best, current evidence suggests that any potential benefits of a multivitamin to reduce mortality are likely to be small.
They gave an example of a healthy 65-year-old woman with a 9-year estimated mortality risk of about 8%, and note that taking a multivitamin for 5-10 years might reduce her estimated mortality risk to 7.5% (based on an odds ratio of 0.94).
“In addition to showing small potential benefit, this estimate is based on imperfect evidence, is imprecise, and is highly sensitive to how the data are interpreted and analyzed,” they said.
The editorialists recommended that lifestyle counseling to prevent chronic diseases should continue to focus on evidence-based approaches, including balanced diets that are high in fruits and vegetables and physical activity.
However, they added that healthy eating can be a challenge when the American industrialized food system does not prioritize health, and healthy foods tend to be more expensive, leading to access problems and food insecurity.
The editorialists suggested that, rather than focusing money, time, and attention on supplements, it would be better to emphasize lower-risk, higher-benefit activities, such as getting exercise, maintaining a healthy weight, and avoiding smoking, in addition to following a healthful diet.
Possible benefit for older adults?
Commenting on the USPSTF statement, JoAnn Manson, MD, chief, division of preventive medicine, Brigham and Women’s Hospital, Boston, who led the recent COSMOS study, said that vitamin and mineral supplements should not be perceived as a substitute for a healthful diet.
“The emphasis needs to be on getting nutritional needs from a healthy diet that is high in plant-based and whole foods that don’t strip the vitamins and minerals through excessive processing,” she said. “Although it’s easier to pop a pill each day than to focus on healthful dietary patterns, the mixture of phytochemicals, fiber, and all the other nutrients in actual foods just can’t be packaged into a pill. Also, vitamins and minerals tend to be better absorbed from food than from supplements and healthy foods can replace calories from less healthy foods, such as red meat and processed foods.”
However, Dr. Manson noted that the evidence is mounting that taking a tablet containing moderate doses of a wide range of vitamins and minerals is safe and may actually have benefits for some people.
She pointed out that the COSMOS and COSMOS-Mind studies showed benefits of multivitamins in slowing cognitive decline in older adults, but the findings need to be replicated.
“The USPSTF did see a statistically significant 7% reduction in cancer with multivitamins in their meta-analysis of four randomized trials and a borderline 6% reduction in all-cause mortality,” she noted. “Plus, multivitamins have been shown to be quite safe in several large and long-term randomized trials. I agree the evidence is not sufficient to make a blanket recommendation for everyone to take multivitamins, but the evidence is mounting that this would be a prudent approach for many older adults,” Dr. Manson said.
“Many people view multivitamins as a form of insurance, as a way to hedge their bets,” she added. “Although this is a rational approach, especially for those who have concerns about the adequacy of their diet, it’s important that this mindset not lead to complacency about following healthy lifestyle practices, including healthy eating, regular physical activity, not smoking, making sure that blood pressure and cholesterol levels are well controlled, and many other practices that critically important for health but are more challenging than simply popping a pill each day.”
A version of this article first appeared on Medscape.com.
FROM JAMA
Add AFib to noncardiac surgery risk evaluation: New support
Practice has gone back and forth on whether atrial fibrillation (AFib) should be considered in the preoperative cardiovascular risk (CV) evaluation of patients slated for noncardiac surgery, and the Revised Cardiac Risk Index (RCRI), currently widely used as an assessment tool, doesn’t include the arrhythmia.
But consideration of preexisting AFib along with the RCRI predicted 30-day mortality more sharply than the RCRI alone in an analysis of data covering several million patients slated for such procedures.
Indeed, AFib emerged as a significant, independent risk factor for a number of bad postoperative outcomes. Mortality within a month of the procedure climbed about 30% for patients with AFib before the noncardiac surgery. Their 30-day risks for stroke and for heart failure hospitalization went up similarly.
The addition of AFib to the RCRI significantly improved its ability to discriminate 30-day postoperative risk levels regardless of age, sex, and type of noncardiac surgery, Amgad Mentias, MD, Cleveland Clinic, told this news organization. And “it was able to correctly up-classify patients to high risk, if AFib was there, and it was able to down-classify some patients to lower risk if it wasn’t there.”
“I think [the findings] are convincing evidence that atrial fib should at least be part of the thought process for the surgical team and the medical team taking care of the patient,” said Dr. Mentias, who is senior author on the study published in the Journal of the American College of Cardiology, with lead author Sameer Prasada, MD, also of the Cleveland Clinic.
The results “call for incorporating AFib as a risk factor in perioperative risk scores for cardiovascular morbidity and mortality,” the published report states.
Supraventricular arrhythmias had been part of the Goldman Risk Index once widely used preoperatively to assess cardiac risk before practice adopted the RCRI in the past decade, observe Anne B. Curtis, MD, and Sai Krishna C. Korada, MD, University at Buffalo, New York, in an accompanying editorial.
The current findings “demonstrate improved prediction of adverse postsurgical outcomes” from supplementing the RCRI with AFib, they write. Given associations between preexisting AFib and serious cardiac events, “it is time to ‘re-revise’ the RCRI and acknowledge the importance of AFib in predicting adverse outcomes” after noncardiac surgery.
The new findings, however, aren’t all straightforward. In one result that remains a bit of a head-scratcher, postoperative risk of myocardial infarction (MI) in patients with preexisting AFib went in the opposite direction of risk for death and other CV outcomes, falling by almost 20%.
That is “hard to explain with the available data,” the report states, but “the use of anticoagulation, whether oral or parenteral (as a bridge therapy in the perioperative period), is a plausible explanation” given the frequent role of thrombosis in triggering MIs.
Consistent with such a mechanism, the group argues, the MI risk reduction was seen primarily among patients with AFib and a CHA2DS2-VASc score of 2 or higher – that is, those at highest risk for stroke and therefore most likely to be on oral anticoagulation. The MI risk reduction wasn’t seen in such patients with a CHA2DS2-VASc score of 0 or 1.
“I think that’s part of the explanation, that anticoagulation can reduce risk of MI. But it’s not the whole explanation,” Dr. Mentias said in an interview. If it were the sole mechanism, he said, then the same oral anticoagulation that protected against MI should have also cut the postoperative stroke risk. Yet that risk climbed 40% among patients with preexisting AFib.
The analysis started with 8.6 million Medicare patients with planned noncardiac surgery, seen from 2015 to 2019, of whom 16.4% had preexisting AFib. Propensity matching for demographics, urgency and type of surgery, CHA2DS2-VASc score, and RCRI index created two cohorts for comparison: 1.13 million patients with and 1.92 million without preexisting AFib.
Preexisting AFib was associated with a higher 30-day risk for death from any cause, the primary endpoint being 8.3% versus 5.8% for those without such AFib (P < .001), for an odds ratio of 1.31 (95% confidence interval, 1.30-1.32).
Corresponding 30-day ORs for other events, all significant at P < .001, were:
- 1.31 (95% CI, 1.30-1.33) for heart failure
- 1.40 (95% CI, 1.37-1.43) for stroke
- 1.59 (95% CI, 1.43-1.75) for systemic embolism
- 1.14 (95% CI, 1.13-1.16) for major bleeding
- 0.81 (95% CI, 0.79-0.82) for MI
Those with preexisting AFib also had longer hospitalizations at a median 5 days, compared with 4 days for those without such AFib (P < .001).
The study has the limitations of most any retrospective cohort analysis. Other limitations, the report notes, include lack of information on any antiarrhythmic meds given during hospitalization or type of AFib.
For example, AFib that is permanent – compared with paroxysmal or persistent – may be associated with more atrial fibrosis, greater atrial dilatation, “and probably higher pressures inside the heart,” Dr. Mentias observed.
“That’s not always the case, but that’s the notion. So presumably people with persistent or permanent atrial fib would have more advanced heart disease, and that could imply more risk. But we did not have that kind of data.”
Dr. Mentias and Dr. Prasada report no relevant financial relationships; disclosures for the other authors are in the report. Dr. Curtis discloses serving on advisory boards for Abbott, Janssen Pharmaceuticals, Sanofi, and Milestone Pharmaceuticals; receiving honoraria for speaking from Medtronic and Zoll; and serving on a data-monitoring board for Medtronic. Dr. Korada reports he has no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Practice has gone back and forth on whether atrial fibrillation (AFib) should be considered in the preoperative cardiovascular risk (CV) evaluation of patients slated for noncardiac surgery, and the Revised Cardiac Risk Index (RCRI), currently widely used as an assessment tool, doesn’t include the arrhythmia.
But consideration of preexisting AFib along with the RCRI predicted 30-day mortality more sharply than the RCRI alone in an analysis of data covering several million patients slated for such procedures.
Indeed, AFib emerged as a significant, independent risk factor for a number of bad postoperative outcomes. Mortality within a month of the procedure climbed about 30% for patients with AFib before the noncardiac surgery. Their 30-day risks for stroke and for heart failure hospitalization went up similarly.
The addition of AFib to the RCRI significantly improved its ability to discriminate 30-day postoperative risk levels regardless of age, sex, and type of noncardiac surgery, Amgad Mentias, MD, Cleveland Clinic, told this news organization. And “it was able to correctly up-classify patients to high risk, if AFib was there, and it was able to down-classify some patients to lower risk if it wasn’t there.”
“I think [the findings] are convincing evidence that atrial fib should at least be part of the thought process for the surgical team and the medical team taking care of the patient,” said Dr. Mentias, who is senior author on the study published in the Journal of the American College of Cardiology, with lead author Sameer Prasada, MD, also of the Cleveland Clinic.
The results “call for incorporating AFib as a risk factor in perioperative risk scores for cardiovascular morbidity and mortality,” the published report states.
Supraventricular arrhythmias had been part of the Goldman Risk Index once widely used preoperatively to assess cardiac risk before practice adopted the RCRI in the past decade, observe Anne B. Curtis, MD, and Sai Krishna C. Korada, MD, University at Buffalo, New York, in an accompanying editorial.
The current findings “demonstrate improved prediction of adverse postsurgical outcomes” from supplementing the RCRI with AFib, they write. Given associations between preexisting AFib and serious cardiac events, “it is time to ‘re-revise’ the RCRI and acknowledge the importance of AFib in predicting adverse outcomes” after noncardiac surgery.
The new findings, however, aren’t all straightforward. In one result that remains a bit of a head-scratcher, postoperative risk of myocardial infarction (MI) in patients with preexisting AFib went in the opposite direction of risk for death and other CV outcomes, falling by almost 20%.
That is “hard to explain with the available data,” the report states, but “the use of anticoagulation, whether oral or parenteral (as a bridge therapy in the perioperative period), is a plausible explanation” given the frequent role of thrombosis in triggering MIs.
Consistent with such a mechanism, the group argues, the MI risk reduction was seen primarily among patients with AFib and a CHA2DS2-VASc score of 2 or higher – that is, those at highest risk for stroke and therefore most likely to be on oral anticoagulation. The MI risk reduction wasn’t seen in such patients with a CHA2DS2-VASc score of 0 or 1.
“I think that’s part of the explanation, that anticoagulation can reduce risk of MI. But it’s not the whole explanation,” Dr. Mentias said in an interview. If it were the sole mechanism, he said, then the same oral anticoagulation that protected against MI should have also cut the postoperative stroke risk. Yet that risk climbed 40% among patients with preexisting AFib.
The analysis started with 8.6 million Medicare patients with planned noncardiac surgery, seen from 2015 to 2019, of whom 16.4% had preexisting AFib. Propensity matching for demographics, urgency and type of surgery, CHA2DS2-VASc score, and RCRI index created two cohorts for comparison: 1.13 million patients with and 1.92 million without preexisting AFib.
Preexisting AFib was associated with a higher 30-day risk for death from any cause, the primary endpoint being 8.3% versus 5.8% for those without such AFib (P < .001), for an odds ratio of 1.31 (95% confidence interval, 1.30-1.32).
Corresponding 30-day ORs for other events, all significant at P < .001, were:
- 1.31 (95% CI, 1.30-1.33) for heart failure
- 1.40 (95% CI, 1.37-1.43) for stroke
- 1.59 (95% CI, 1.43-1.75) for systemic embolism
- 1.14 (95% CI, 1.13-1.16) for major bleeding
- 0.81 (95% CI, 0.79-0.82) for MI
Those with preexisting AFib also had longer hospitalizations at a median 5 days, compared with 4 days for those without such AFib (P < .001).
The study has the limitations of most any retrospective cohort analysis. Other limitations, the report notes, include lack of information on any antiarrhythmic meds given during hospitalization or type of AFib.
For example, AFib that is permanent – compared with paroxysmal or persistent – may be associated with more atrial fibrosis, greater atrial dilatation, “and probably higher pressures inside the heart,” Dr. Mentias observed.
“That’s not always the case, but that’s the notion. So presumably people with persistent or permanent atrial fib would have more advanced heart disease, and that could imply more risk. But we did not have that kind of data.”
Dr. Mentias and Dr. Prasada report no relevant financial relationships; disclosures for the other authors are in the report. Dr. Curtis discloses serving on advisory boards for Abbott, Janssen Pharmaceuticals, Sanofi, and Milestone Pharmaceuticals; receiving honoraria for speaking from Medtronic and Zoll; and serving on a data-monitoring board for Medtronic. Dr. Korada reports he has no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Practice has gone back and forth on whether atrial fibrillation (AFib) should be considered in the preoperative cardiovascular risk (CV) evaluation of patients slated for noncardiac surgery, and the Revised Cardiac Risk Index (RCRI), currently widely used as an assessment tool, doesn’t include the arrhythmia.
But consideration of preexisting AFib along with the RCRI predicted 30-day mortality more sharply than the RCRI alone in an analysis of data covering several million patients slated for such procedures.
Indeed, AFib emerged as a significant, independent risk factor for a number of bad postoperative outcomes. Mortality within a month of the procedure climbed about 30% for patients with AFib before the noncardiac surgery. Their 30-day risks for stroke and for heart failure hospitalization went up similarly.
The addition of AFib to the RCRI significantly improved its ability to discriminate 30-day postoperative risk levels regardless of age, sex, and type of noncardiac surgery, Amgad Mentias, MD, Cleveland Clinic, told this news organization. And “it was able to correctly up-classify patients to high risk, if AFib was there, and it was able to down-classify some patients to lower risk if it wasn’t there.”
“I think [the findings] are convincing evidence that atrial fib should at least be part of the thought process for the surgical team and the medical team taking care of the patient,” said Dr. Mentias, who is senior author on the study published in the Journal of the American College of Cardiology, with lead author Sameer Prasada, MD, also of the Cleveland Clinic.
The results “call for incorporating AFib as a risk factor in perioperative risk scores for cardiovascular morbidity and mortality,” the published report states.
Supraventricular arrhythmias had been part of the Goldman Risk Index once widely used preoperatively to assess cardiac risk before practice adopted the RCRI in the past decade, observe Anne B. Curtis, MD, and Sai Krishna C. Korada, MD, University at Buffalo, New York, in an accompanying editorial.
The current findings “demonstrate improved prediction of adverse postsurgical outcomes” from supplementing the RCRI with AFib, they write. Given associations between preexisting AFib and serious cardiac events, “it is time to ‘re-revise’ the RCRI and acknowledge the importance of AFib in predicting adverse outcomes” after noncardiac surgery.
The new findings, however, aren’t all straightforward. In one result that remains a bit of a head-scratcher, postoperative risk of myocardial infarction (MI) in patients with preexisting AFib went in the opposite direction of risk for death and other CV outcomes, falling by almost 20%.
That is “hard to explain with the available data,” the report states, but “the use of anticoagulation, whether oral or parenteral (as a bridge therapy in the perioperative period), is a plausible explanation” given the frequent role of thrombosis in triggering MIs.
Consistent with such a mechanism, the group argues, the MI risk reduction was seen primarily among patients with AFib and a CHA2DS2-VASc score of 2 or higher – that is, those at highest risk for stroke and therefore most likely to be on oral anticoagulation. The MI risk reduction wasn’t seen in such patients with a CHA2DS2-VASc score of 0 or 1.
“I think that’s part of the explanation, that anticoagulation can reduce risk of MI. But it’s not the whole explanation,” Dr. Mentias said in an interview. If it were the sole mechanism, he said, then the same oral anticoagulation that protected against MI should have also cut the postoperative stroke risk. Yet that risk climbed 40% among patients with preexisting AFib.
The analysis started with 8.6 million Medicare patients with planned noncardiac surgery, seen from 2015 to 2019, of whom 16.4% had preexisting AFib. Propensity matching for demographics, urgency and type of surgery, CHA2DS2-VASc score, and RCRI index created two cohorts for comparison: 1.13 million patients with and 1.92 million without preexisting AFib.
Preexisting AFib was associated with a higher 30-day risk for death from any cause, the primary endpoint being 8.3% versus 5.8% for those without such AFib (P < .001), for an odds ratio of 1.31 (95% confidence interval, 1.30-1.32).
Corresponding 30-day ORs for other events, all significant at P < .001, were:
- 1.31 (95% CI, 1.30-1.33) for heart failure
- 1.40 (95% CI, 1.37-1.43) for stroke
- 1.59 (95% CI, 1.43-1.75) for systemic embolism
- 1.14 (95% CI, 1.13-1.16) for major bleeding
- 0.81 (95% CI, 0.79-0.82) for MI
Those with preexisting AFib also had longer hospitalizations at a median 5 days, compared with 4 days for those without such AFib (P < .001).
The study has the limitations of most any retrospective cohort analysis. Other limitations, the report notes, include lack of information on any antiarrhythmic meds given during hospitalization or type of AFib.
For example, AFib that is permanent – compared with paroxysmal or persistent – may be associated with more atrial fibrosis, greater atrial dilatation, “and probably higher pressures inside the heart,” Dr. Mentias observed.
“That’s not always the case, but that’s the notion. So presumably people with persistent or permanent atrial fib would have more advanced heart disease, and that could imply more risk. But we did not have that kind of data.”
Dr. Mentias and Dr. Prasada report no relevant financial relationships; disclosures for the other authors are in the report. Dr. Curtis discloses serving on advisory boards for Abbott, Janssen Pharmaceuticals, Sanofi, and Milestone Pharmaceuticals; receiving honoraria for speaking from Medtronic and Zoll; and serving on a data-monitoring board for Medtronic. Dr. Korada reports he has no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Care gaps common after anal sphincter injuries from childbirth
Postpartum complications may go unrecognized in women who incur anal sphincter injuries during childbirth, a review of electronic medical records at one academic health system suggests.
In the first 3 months after delivery, few patients with an obstetric anal sphincter injury (OASI) had documented pelvic floor problems, compared with higher rates documented in medical literature, the researchers found.
“Lack of identified pelvic floor dysfunction in this population differs from the incidence in previously published data and may reflect lack of identification by obstetric providers,” the researchers reported. The findings “highlight a gap in health care that, when addressed, could significantly improve postpartum quality of life.”
The findings are scheduled to be presented at the annual scientific meeting of the American Urogynecologic Society and International Urogynecological Association.
Anal sphincter injuries occur in about 4.4% of vaginal deliveries and are the most common cause of anal incontinence in women of reproductive age.
For the new study, researchers reviewed records of 287 women who underwent a vaginal birth that resulted in an anal sphincter injury at five Ohio hospitals affiliated with Cleveland Clinic from 2013 to 2015.
Of those who met eligibility criteria, 209 (72.8%) were White, 262 (91.3%) were non-Hispanic, and 249 (86.8%) were aged 20-34 years. Most had an epidural (92%), did not require a blood transfusion (97.9%), did not develop a vaginal hematoma (98.9%), and did not have their injury repaired in an operating room (97.2%), the researchers reported.
Among pelvic floor disorders, urinary incontinence was not reported in 96% of patients, fecal incontinence was not reported in 97.1%, and pelvic organ prolapse was not reported in 99.3%. Most had no recorded complications from their lacerations (87.8%) or postpartum depression (92%), the researchers found.
However, a 2015 study found that, 12 weeks after delivery, women with OASIs commonly reported symptoms of incontinence, with 26% reporting urinary stress incontinence, 21.4% urinary urgency incontinence, 59% anal incontinence, and 15% fecal incontinence.
Depression was also seldom identified despite higher risk of mood disorders among women with OASI, the researchers found.
The team also examined interpregnancy intervals, defined as the time between a woman’s first vaginal delivery and conception of a subsequent pregnancy. Of 178 women for whom data were available, the median interval was 26.4 months (95% confidence interval, 23.7-29.9), similar to the median for births nationally.
Lead researcher Alexandra Nutaitis, DO, a resident in obstetrics and gynecology at Cleveland Clinic Akron General, said in an interview that it’s unclear whether physicians did not inquire about symptoms or didn’t record them. She noted that anal sphincter injuries are a “stigmatized topic.”
Not asked, not told
Carolyn Swenson, MD, an associate professor in urogynecology at the University of Utah, Salt Lake City, said physicians in the study may have relied on patients to bring up their symptoms rather than using questionnaires to screen for problems.
“What we know is that if you don’t ask women about pelvic floor disorders, they often don’t tell you that they are experiencing symptoms,” said Dr. Swenson, who was not involved in the new research.
Dr. Swenson called for validated questionnaires to assess pelvic floor symptoms in postpartum patients.
Regarding interpregnancy intervals, Dr. Nutaitis said she would be surprised if women who experienced an OASI didn’t delay having another child longer than women who did not undergo that physical and psychological trauma – but other factors such as societal pressures may override any reluctance to proceed with another pregnancy.
Dr. Swenson said it’s possible that a subgroup of women who have severe complications, such as those with a fourth-degree tear, might put off having another child. However, more research is needed to find out, she said.
Dr. Nutaitis and Dr. Swenson disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Postpartum complications may go unrecognized in women who incur anal sphincter injuries during childbirth, a review of electronic medical records at one academic health system suggests.
In the first 3 months after delivery, few patients with an obstetric anal sphincter injury (OASI) had documented pelvic floor problems, compared with higher rates documented in medical literature, the researchers found.
“Lack of identified pelvic floor dysfunction in this population differs from the incidence in previously published data and may reflect lack of identification by obstetric providers,” the researchers reported. The findings “highlight a gap in health care that, when addressed, could significantly improve postpartum quality of life.”
The findings are scheduled to be presented at the annual scientific meeting of the American Urogynecologic Society and International Urogynecological Association.
Anal sphincter injuries occur in about 4.4% of vaginal deliveries and are the most common cause of anal incontinence in women of reproductive age.
For the new study, researchers reviewed records of 287 women who underwent a vaginal birth that resulted in an anal sphincter injury at five Ohio hospitals affiliated with Cleveland Clinic from 2013 to 2015.
Of those who met eligibility criteria, 209 (72.8%) were White, 262 (91.3%) were non-Hispanic, and 249 (86.8%) were aged 20-34 years. Most had an epidural (92%), did not require a blood transfusion (97.9%), did not develop a vaginal hematoma (98.9%), and did not have their injury repaired in an operating room (97.2%), the researchers reported.
Among pelvic floor disorders, urinary incontinence was not reported in 96% of patients, fecal incontinence was not reported in 97.1%, and pelvic organ prolapse was not reported in 99.3%. Most had no recorded complications from their lacerations (87.8%) or postpartum depression (92%), the researchers found.
However, a 2015 study found that, 12 weeks after delivery, women with OASIs commonly reported symptoms of incontinence, with 26% reporting urinary stress incontinence, 21.4% urinary urgency incontinence, 59% anal incontinence, and 15% fecal incontinence.
Depression was also seldom identified despite higher risk of mood disorders among women with OASI, the researchers found.
The team also examined interpregnancy intervals, defined as the time between a woman’s first vaginal delivery and conception of a subsequent pregnancy. Of 178 women for whom data were available, the median interval was 26.4 months (95% confidence interval, 23.7-29.9), similar to the median for births nationally.
Lead researcher Alexandra Nutaitis, DO, a resident in obstetrics and gynecology at Cleveland Clinic Akron General, said in an interview that it’s unclear whether physicians did not inquire about symptoms or didn’t record them. She noted that anal sphincter injuries are a “stigmatized topic.”
Not asked, not told
Carolyn Swenson, MD, an associate professor in urogynecology at the University of Utah, Salt Lake City, said physicians in the study may have relied on patients to bring up their symptoms rather than using questionnaires to screen for problems.
“What we know is that if you don’t ask women about pelvic floor disorders, they often don’t tell you that they are experiencing symptoms,” said Dr. Swenson, who was not involved in the new research.
Dr. Swenson called for validated questionnaires to assess pelvic floor symptoms in postpartum patients.
Regarding interpregnancy intervals, Dr. Nutaitis said she would be surprised if women who experienced an OASI didn’t delay having another child longer than women who did not undergo that physical and psychological trauma – but other factors such as societal pressures may override any reluctance to proceed with another pregnancy.
Dr. Swenson said it’s possible that a subgroup of women who have severe complications, such as those with a fourth-degree tear, might put off having another child. However, more research is needed to find out, she said.
Dr. Nutaitis and Dr. Swenson disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Postpartum complications may go unrecognized in women who incur anal sphincter injuries during childbirth, a review of electronic medical records at one academic health system suggests.
In the first 3 months after delivery, few patients with an obstetric anal sphincter injury (OASI) had documented pelvic floor problems, compared with higher rates documented in medical literature, the researchers found.
“Lack of identified pelvic floor dysfunction in this population differs from the incidence in previously published data and may reflect lack of identification by obstetric providers,” the researchers reported. The findings “highlight a gap in health care that, when addressed, could significantly improve postpartum quality of life.”
The findings are scheduled to be presented at the annual scientific meeting of the American Urogynecologic Society and International Urogynecological Association.
Anal sphincter injuries occur in about 4.4% of vaginal deliveries and are the most common cause of anal incontinence in women of reproductive age.
For the new study, researchers reviewed records of 287 women who underwent a vaginal birth that resulted in an anal sphincter injury at five Ohio hospitals affiliated with Cleveland Clinic from 2013 to 2015.
Of those who met eligibility criteria, 209 (72.8%) were White, 262 (91.3%) were non-Hispanic, and 249 (86.8%) were aged 20-34 years. Most had an epidural (92%), did not require a blood transfusion (97.9%), did not develop a vaginal hematoma (98.9%), and did not have their injury repaired in an operating room (97.2%), the researchers reported.
Among pelvic floor disorders, urinary incontinence was not reported in 96% of patients, fecal incontinence was not reported in 97.1%, and pelvic organ prolapse was not reported in 99.3%. Most had no recorded complications from their lacerations (87.8%) or postpartum depression (92%), the researchers found.
However, a 2015 study found that, 12 weeks after delivery, women with OASIs commonly reported symptoms of incontinence, with 26% reporting urinary stress incontinence, 21.4% urinary urgency incontinence, 59% anal incontinence, and 15% fecal incontinence.
Depression was also seldom identified despite higher risk of mood disorders among women with OASI, the researchers found.
The team also examined interpregnancy intervals, defined as the time between a woman’s first vaginal delivery and conception of a subsequent pregnancy. Of 178 women for whom data were available, the median interval was 26.4 months (95% confidence interval, 23.7-29.9), similar to the median for births nationally.
Lead researcher Alexandra Nutaitis, DO, a resident in obstetrics and gynecology at Cleveland Clinic Akron General, said in an interview that it’s unclear whether physicians did not inquire about symptoms or didn’t record them. She noted that anal sphincter injuries are a “stigmatized topic.”
Not asked, not told
Carolyn Swenson, MD, an associate professor in urogynecology at the University of Utah, Salt Lake City, said physicians in the study may have relied on patients to bring up their symptoms rather than using questionnaires to screen for problems.
“What we know is that if you don’t ask women about pelvic floor disorders, they often don’t tell you that they are experiencing symptoms,” said Dr. Swenson, who was not involved in the new research.
Dr. Swenson called for validated questionnaires to assess pelvic floor symptoms in postpartum patients.
Regarding interpregnancy intervals, Dr. Nutaitis said she would be surprised if women who experienced an OASI didn’t delay having another child longer than women who did not undergo that physical and psychological trauma – but other factors such as societal pressures may override any reluctance to proceed with another pregnancy.
Dr. Swenson said it’s possible that a subgroup of women who have severe complications, such as those with a fourth-degree tear, might put off having another child. However, more research is needed to find out, she said.
Dr. Nutaitis and Dr. Swenson disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AUGS 2022