User login
Interval FITs could cut colonoscopies in those at above-average risk
In a new retrospective analysis of patients with above-average risk of colorectal cancer, multiple negative fecal immunohistochemical tests (FITs) were associated with a lower risk of advanced neoplasia. The findings suggest that multiple negative FITs could potentially identify individuals in high-risk surveillance who aren’t truly high risk, which could in turn ease the logjam of colonoscopies and free resources for truly high-risk individuals.
The study, conducted in Australia, was published online in Clinical Gastroenterology and Hepatology. It included patients who completed at least two FIT exams between surveillance colonoscopies and had no neoplasia or nonadvanced adenoma at prior colonoscopy. Above-average risk was defined as a family history or by findings at surveillance colonoscopy.
The study has some limitations. It is a retrospective analysis between the years 2008 and 2019, and colonoscopy guidelines in the United States have since changed, with a recommendation of surveillance colonoscopy at 7-10 years following 1-2 adenomas discovered at surveillance colonoscopy, and the current study includes follow-up colonoscopy at 5 years. “These data are informative for patients up to 5 years, but they’re not really informative afterwards. They just don’t have those data yet,” said Reed Ness, MD, who was asked to comment on the study.
The authors also don’t describe what they mean by a family history of colorectal cancer risk. “My take was that it’s an interesting result which would seem to support the possibility of returning some patients with a family history or adenoma history to a noncolonoscopy screening regimen after a negative surveillance colonoscopy. We’ll need to see where the data lead us in the future,” said Dr. Ness, who is an associate professor of medicine at Vanderbilt University Medical Center, Nashville, Tenn.
“We’re letting people go 10 years now, and some people are uncomfortable with allowing patients to go 10 years. So you could think of a scenario where you use FIT to try to find people that might have higher-risk lesions that need to come back for colonoscopy within that 10 years,” said Dr. Ness. That issue is particularly relevant given the wide range of adenoma detection rates among gastroenterologists, because FIT could detect a polyp that was missed during a colonoscopy.
The study included two groups with increased risk – those with a family history of colon cancer, and those with previously detected adenomas. The family history cohort may be useful for clinical practice, according to Priyanka Kanth, MD, who was also asked to comment on the study. “Some people may not need [a colonoscopy] at 5 years if they have no polyps found and negative FIT,” said Dr. Kanth, who is an associate professor of gastroenterology at Georgetown University, Washington.
She feels less certain about the group with previously detected adenomas, given the change in U.S. guidelines. “We have already changed that, so I don’t think we need to really do FIT intervals for that cohort,” said Dr. Kanth. “I think this is a good study that has a lot of information and also reassures us that we don’t need such frequent colonoscopy surveillance,” she added.
Steve Serrao, MD, PhD, who was also asked for comment, emphasized the importance of high-quality colonoscopies that reach the cecum 95% of the time, and achieving high adenoma-detection rates. The system can get overwhelmed conducting colonoscopies on patients with good insurance coverage who have already undergone high-quality colonoscopies. “That pushes out patients that haven’t necessarily had a colonoscopy or a FIT. People who don’t have access are kind of crowded out by these false-positive tests. The best modality is actually to do a high-quality colonoscopy and then to have a really well-directed strategy following that colonoscopy,” said Dr. Serrao, who is division chief of gastroenterology and hepatology at Riverside University Health System, Moreno Valley, Calif.
The researchers analyzed data from 4,021 surveillance intervals and 3,369 participants. A total of 1,436 had no neoplasia at the prior colonoscopy, 1,704 had nonadvanced adenoma, and 880 had advanced adenoma. Participants completed no or one to four FIT tests between colonoscopies, with the final colonoscopy performed within 2 years of FIT tests. The median age was 63.9 years; 53.6% were female; 71.1% had a prior adenoma; and 28.9% had a family history of colorectal cancer. A total of 29.4% of participants had one negative FIT; 6.9% had four negative FITs during the interval period; and 31.0% did not complete any FIT tests.
Of follow-up colonoscopies, 9.9% revealed advanced adenomas. Among the patients with no prior neoplasia, those with one negative FIT had a cumulative index function for advanced neoplasia at 5 years of 8.5% (95% confidence interval, 4.9%-13.3%). This was higher than for those with three negative FITs (4.5%; 95% CI, 2.0%-8.6%) or four negative FITs (1.9%; 95% CI, 0.5%-5.0%). The association held for individuals with prior nonadvanced adenoma but not those with advanced adenoma.
Over the 5-year interval, three or more negative FIT tests were associated with a 50%-70% reduction in advanced neoplasia risk at follow-up colonoscopy (P < .001). There was no significant association over a 3-year interval. Dr. Kanth, Dr. Serrao, and Dr. Ness have no relevant financial disclosures.
In a new retrospective analysis of patients with above-average risk of colorectal cancer, multiple negative fecal immunohistochemical tests (FITs) were associated with a lower risk of advanced neoplasia. The findings suggest that multiple negative FITs could potentially identify individuals in high-risk surveillance who aren’t truly high risk, which could in turn ease the logjam of colonoscopies and free resources for truly high-risk individuals.
The study, conducted in Australia, was published online in Clinical Gastroenterology and Hepatology. It included patients who completed at least two FIT exams between surveillance colonoscopies and had no neoplasia or nonadvanced adenoma at prior colonoscopy. Above-average risk was defined as a family history or by findings at surveillance colonoscopy.
The study has some limitations. It is a retrospective analysis between the years 2008 and 2019, and colonoscopy guidelines in the United States have since changed, with a recommendation of surveillance colonoscopy at 7-10 years following 1-2 adenomas discovered at surveillance colonoscopy, and the current study includes follow-up colonoscopy at 5 years. “These data are informative for patients up to 5 years, but they’re not really informative afterwards. They just don’t have those data yet,” said Reed Ness, MD, who was asked to comment on the study.
The authors also don’t describe what they mean by a family history of colorectal cancer risk. “My take was that it’s an interesting result which would seem to support the possibility of returning some patients with a family history or adenoma history to a noncolonoscopy screening regimen after a negative surveillance colonoscopy. We’ll need to see where the data lead us in the future,” said Dr. Ness, who is an associate professor of medicine at Vanderbilt University Medical Center, Nashville, Tenn.
“We’re letting people go 10 years now, and some people are uncomfortable with allowing patients to go 10 years. So you could think of a scenario where you use FIT to try to find people that might have higher-risk lesions that need to come back for colonoscopy within that 10 years,” said Dr. Ness. That issue is particularly relevant given the wide range of adenoma detection rates among gastroenterologists, because FIT could detect a polyp that was missed during a colonoscopy.
The study included two groups with increased risk – those with a family history of colon cancer, and those with previously detected adenomas. The family history cohort may be useful for clinical practice, according to Priyanka Kanth, MD, who was also asked to comment on the study. “Some people may not need [a colonoscopy] at 5 years if they have no polyps found and negative FIT,” said Dr. Kanth, who is an associate professor of gastroenterology at Georgetown University, Washington.
She feels less certain about the group with previously detected adenomas, given the change in U.S. guidelines. “We have already changed that, so I don’t think we need to really do FIT intervals for that cohort,” said Dr. Kanth. “I think this is a good study that has a lot of information and also reassures us that we don’t need such frequent colonoscopy surveillance,” she added.
Steve Serrao, MD, PhD, who was also asked for comment, emphasized the importance of high-quality colonoscopies that reach the cecum 95% of the time, and achieving high adenoma-detection rates. The system can get overwhelmed conducting colonoscopies on patients with good insurance coverage who have already undergone high-quality colonoscopies. “That pushes out patients that haven’t necessarily had a colonoscopy or a FIT. People who don’t have access are kind of crowded out by these false-positive tests. The best modality is actually to do a high-quality colonoscopy and then to have a really well-directed strategy following that colonoscopy,” said Dr. Serrao, who is division chief of gastroenterology and hepatology at Riverside University Health System, Moreno Valley, Calif.
The researchers analyzed data from 4,021 surveillance intervals and 3,369 participants. A total of 1,436 had no neoplasia at the prior colonoscopy, 1,704 had nonadvanced adenoma, and 880 had advanced adenoma. Participants completed no or one to four FIT tests between colonoscopies, with the final colonoscopy performed within 2 years of FIT tests. The median age was 63.9 years; 53.6% were female; 71.1% had a prior adenoma; and 28.9% had a family history of colorectal cancer. A total of 29.4% of participants had one negative FIT; 6.9% had four negative FITs during the interval period; and 31.0% did not complete any FIT tests.
Of follow-up colonoscopies, 9.9% revealed advanced adenomas. Among the patients with no prior neoplasia, those with one negative FIT had a cumulative index function for advanced neoplasia at 5 years of 8.5% (95% confidence interval, 4.9%-13.3%). This was higher than for those with three negative FITs (4.5%; 95% CI, 2.0%-8.6%) or four negative FITs (1.9%; 95% CI, 0.5%-5.0%). The association held for individuals with prior nonadvanced adenoma but not those with advanced adenoma.
Over the 5-year interval, three or more negative FIT tests were associated with a 50%-70% reduction in advanced neoplasia risk at follow-up colonoscopy (P < .001). There was no significant association over a 3-year interval. Dr. Kanth, Dr. Serrao, and Dr. Ness have no relevant financial disclosures.
In a new retrospective analysis of patients with above-average risk of colorectal cancer, multiple negative fecal immunohistochemical tests (FITs) were associated with a lower risk of advanced neoplasia. The findings suggest that multiple negative FITs could potentially identify individuals in high-risk surveillance who aren’t truly high risk, which could in turn ease the logjam of colonoscopies and free resources for truly high-risk individuals.
The study, conducted in Australia, was published online in Clinical Gastroenterology and Hepatology. It included patients who completed at least two FIT exams between surveillance colonoscopies and had no neoplasia or nonadvanced adenoma at prior colonoscopy. Above-average risk was defined as a family history or by findings at surveillance colonoscopy.
The study has some limitations. It is a retrospective analysis between the years 2008 and 2019, and colonoscopy guidelines in the United States have since changed, with a recommendation of surveillance colonoscopy at 7-10 years following 1-2 adenomas discovered at surveillance colonoscopy, and the current study includes follow-up colonoscopy at 5 years. “These data are informative for patients up to 5 years, but they’re not really informative afterwards. They just don’t have those data yet,” said Reed Ness, MD, who was asked to comment on the study.
The authors also don’t describe what they mean by a family history of colorectal cancer risk. “My take was that it’s an interesting result which would seem to support the possibility of returning some patients with a family history or adenoma history to a noncolonoscopy screening regimen after a negative surveillance colonoscopy. We’ll need to see where the data lead us in the future,” said Dr. Ness, who is an associate professor of medicine at Vanderbilt University Medical Center, Nashville, Tenn.
“We’re letting people go 10 years now, and some people are uncomfortable with allowing patients to go 10 years. So you could think of a scenario where you use FIT to try to find people that might have higher-risk lesions that need to come back for colonoscopy within that 10 years,” said Dr. Ness. That issue is particularly relevant given the wide range of adenoma detection rates among gastroenterologists, because FIT could detect a polyp that was missed during a colonoscopy.
The study included two groups with increased risk – those with a family history of colon cancer, and those with previously detected adenomas. The family history cohort may be useful for clinical practice, according to Priyanka Kanth, MD, who was also asked to comment on the study. “Some people may not need [a colonoscopy] at 5 years if they have no polyps found and negative FIT,” said Dr. Kanth, who is an associate professor of gastroenterology at Georgetown University, Washington.
She feels less certain about the group with previously detected adenomas, given the change in U.S. guidelines. “We have already changed that, so I don’t think we need to really do FIT intervals for that cohort,” said Dr. Kanth. “I think this is a good study that has a lot of information and also reassures us that we don’t need such frequent colonoscopy surveillance,” she added.
Steve Serrao, MD, PhD, who was also asked for comment, emphasized the importance of high-quality colonoscopies that reach the cecum 95% of the time, and achieving high adenoma-detection rates. The system can get overwhelmed conducting colonoscopies on patients with good insurance coverage who have already undergone high-quality colonoscopies. “That pushes out patients that haven’t necessarily had a colonoscopy or a FIT. People who don’t have access are kind of crowded out by these false-positive tests. The best modality is actually to do a high-quality colonoscopy and then to have a really well-directed strategy following that colonoscopy,” said Dr. Serrao, who is division chief of gastroenterology and hepatology at Riverside University Health System, Moreno Valley, Calif.
The researchers analyzed data from 4,021 surveillance intervals and 3,369 participants. A total of 1,436 had no neoplasia at the prior colonoscopy, 1,704 had nonadvanced adenoma, and 880 had advanced adenoma. Participants completed no or one to four FIT tests between colonoscopies, with the final colonoscopy performed within 2 years of FIT tests. The median age was 63.9 years; 53.6% were female; 71.1% had a prior adenoma; and 28.9% had a family history of colorectal cancer. A total of 29.4% of participants had one negative FIT; 6.9% had four negative FITs during the interval period; and 31.0% did not complete any FIT tests.
Of follow-up colonoscopies, 9.9% revealed advanced adenomas. Among the patients with no prior neoplasia, those with one negative FIT had a cumulative index function for advanced neoplasia at 5 years of 8.5% (95% confidence interval, 4.9%-13.3%). This was higher than for those with three negative FITs (4.5%; 95% CI, 2.0%-8.6%) or four negative FITs (1.9%; 95% CI, 0.5%-5.0%). The association held for individuals with prior nonadvanced adenoma but not those with advanced adenoma.
Over the 5-year interval, three or more negative FIT tests were associated with a 50%-70% reduction in advanced neoplasia risk at follow-up colonoscopy (P < .001). There was no significant association over a 3-year interval. Dr. Kanth, Dr. Serrao, and Dr. Ness have no relevant financial disclosures.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
A Novel Text Message Protocol to Improve Bowel Preparation for Outpatient Colonoscopies in Veterans
Colorectal cancer is the third leading cause of cancer-related death in both men and women.1 Colonoscopy is the current gold standard for screening due to the ability to remove precancerous lesions but remains highly dependent on the quality of bowel preparation.2 Poor bowel preparation has been associated with impaired adenoma detection as well as increased health care utilization due to the need for a repeat colonoscopy.3
Multiple patient factors are associated with increased risk of poor bowel preparation, including age > 60 years, male sex, diabetes mellitus, and presence of a mental health diagnosis, factors that are prevalent among the veteran population.3-5 Text messages have been shown to improve the quality of bowel preparation by increasing patients' understanding and adherence with the preparation process. Improved adherence with bowel preparation directions is associated with a cleaner colon prior to colonoscopy, leading to a thorough examination. Studies using text messaging instructions prior to colonoscopies have also shown measurable improvement in adenoma detection rate, patient preparation-associated discomfort, and completion of colonoscopy.6-10
In 2016, the Veterans Health Administration (VHA) introduced Annie, one of the first automated text messaging services, named after Army Lieutenant Annie Fox, the first woman to receive the Purple Heart for combat. The Annie platform allows for notifications, instructions, and simple data collection. The development of this platform allows VHA practitioners to engage and educate veterans in a similar way to other health care systems using text messaging protocols. Annie text messages have been piloted for the use of hepatitis C treatment, demonstrating promise of improved medication adherence and patient satisfaction.11 We aimed to develop and pilot the Annie bowel preparation protocol to improve the quality of colonoscopy bowel preparation for outpatients at the Minneapolis Veterans Affairs Medical Center (MVAMC) in Minnesota. A secondary goal included measuring patient satisfaction with the text messaging instructions for outpatient colonoscopy preparation.
Methods
We conducted a single center, prospective, endoscopist-blinded, study with two 3-month long Plan-Do-Study-Act (PDSA) cycles to improve the text messaging bowel preparation protocol at MVAMC between January 2019 and April 2020. The MVAMC Institutional Review Board determined the quality improvement project was exempt. Veterans who had outpatient colonoscopies scheduled were included. Veterans undergoing inpatient colonoscopies or outpatients who could not be reached to obtain informed consent, lacked text message capability, declined participation, or required extended colonoscopy preparation were excluded. Per MVAMC procedures, extended colonoscopy preparation was provided to patients receiving general or monitored anesthesia care, with a history of poor bowel preparation, or with risk factors for poor preparation as determined by the ordering health care professional (HCP). Standard bowel preparation involves ingestion of 4 L of polyethylene glycol 3350 with electrolytes; extended bowel preparation requires ingestion of an additional 2 L to total 6 L and uses a different set of instructions. Additionally, the patient population requiring extended bowel preparation also includes patients with spinal cord injuries, who often are admitted for assistance with extended preparation. Patients who consented to receiving text messages were placed in the Annie intervention group, and all others were placed in the control group.
The control group received standardized patient education, including a mailed copy of bowel preparation instructions and a phone call from a gastroenterology service nurse about 1 to 2 weeks before the procedure. Current MVAMC standard of care involves a phone call from a nurse to confirm that patients have received the polyethylene glycol preparation solution, the mailed instructions, have an escort and transportation, and to answer any questions. Both the usual care and intervention group received the phone call. During this call, the Annie text messaging bowel preparation protocol was introduced; if the veteran chose to participate, consent and enrollment were completed.
On the day of the colonoscopy, veterans in the intervention group were surveyed in the waiting room about their experience receiving the text messages and soliciting feedback for improvement or surveyed via telephone call within 3 days of their procedure. Patient satisfaction was quantified with a scale from 1 (low) to 10 (high), including questions about how helpful the texts were in relation to total number, timing, and content of messages as well as whether veterans would like to receive the text messages again for future procedures.
We reviewed individual charts and collected Boston Bowel Preparation Scale (BBPS) scores to determine adequate preparation. BBPS assigns a score of 0 to 3 for the right, transverse, and left colon applied upon withdrawal after flushing and suctioning have been completed.12 Adequate preparation is considered a total score of ≥ 6 with no segment scoring < 2. This method of preparation assessment is preferred due to its ability to account for difference in preparation quality among colonic segments, well-defined scoring characteristics, and several studies validating its use showing inter- and intraobserver reliability.12 Follow-up studies have shown validity of the BBPS when compared with relevant outcomes such as polyp detection rate and recommended timing for repeat procedure.13 Variables associated with poor bowel preparation (ie, gender, prior abdominal surgery, impaired mobility, high body mass index, diabetes mellitus, stroke, dementia, any neurologic diagnosis, cirrhosis, smoking, polypharmacy [> 8 active medications], and narcotic or tricyclic antidepressant medication use) were also collected through chart review.3-5 We note that immobility was defined by International Classification of Diseases (ICD)-9 and ICD-10 codes and prescriptions for assistive devices (ie, canes, wheelchairs, 4-wheeled walkers).
Veterans assent to be enrolled in Annie. After enrollment, veterans must text back a specific word response to an initial text message to receive the protocolized messages from the Annie program. A contact phone number to the gastrointestinal nurse line was provided for questions during business hours. The start date for the text message protocol is 6 days prior to the procedure date. If a patient rescheduled their colonoscopy, the Annie database was updated manually.
Statistical Analysis
We used both Pearson χ2 test and 2-sample t test analyses to compare demographic information and patient satisfaction scores between the control and intervention groups. We compared continuous BBPS scores between Annie intervention vs control group using parametric and nonparametric independent t tests using the Mann-Whitney U test. We repeated this analysis controlling for both mental health diagnoses and age using linear regression. We were unable to survey 61 of the 187 veterans who received Annie text messages.
RESULTS
During PDSA cycles 1 and 2, 640 veterans were scheduled for outpatient colonoscopy: 453 veterans were in the control group; 187 veterans were in the intervention group, of which 126 were surveyed. A significant percentage of veterans declined participation because they felt like they did not need reinforced education; others were not eligible for Annie due to requirement for extended bowel preparation, cancelled colonoscopy, inability to physically read text messages, or lack of cell phone.
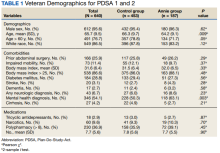
The mean (SD) age was 65 (8) years; 184 (28.8%) had a diabetes mellitus diagnosis, and the mean (SD) body mass index was 31.6 (6.4). The Annie group was slightly more likely to have mental health diagnoses and lower age compared with the control group (Table 1).
Patient Feedback
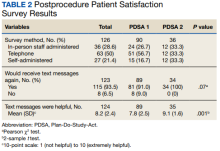
We collected feedback from veterans after each PDSA cycle to identify areas for improvement by both in-person and telephone surveys. Based on feedback from PDSA cycle 1, we decreased the total number of text messages to create a more succinct set of instructions. The most frequently requested change involved timing the text messages to align with the exact morning a specific instruction should take place.
Patient satisfaction with the Annie text messaging service was high.
DISCUSSION
To our knowledge, this is the first report of using Annie at a VAMC for colonoscopy bowel preparation improvement. We found a statistically significant improvement in the average BBPS in those receiving Annie text messages compared with the routine care control group. We also found high levels of patient satisfaction with most patients requesting to receive them again for future procedures.
The clinical significance of a BBPS of 7.8 vs 8.2 is unclear, although any score > 6 is considered to be adequate. However, subjectively speaking, the higher the BBPS the cleaner the colon, and theoretically the easier it is to see small or flat polyps. Future steps could include calculating adenoma detection rates for those enrolled in the Annie program vs the control group.
We have received inquiries regarding potential program implementation at other facilities. Success and sustainability of the program will require long-term commitment and ideally protected time for staff. It is helpful to remember that for each person who chooses to enroll in the intervention, the program currently requires that a brief consent note is placed in the patient’s chart. Thus, depending on the facilities’ resources, it is ideal for one staff member to be the designated lead to help oversee, troubleshoot, and train additional personnel. Surveys can be intermittently used to obtain feedback for improvement but are not required for sustainability. Automated text messaging is a promising addition to medicine for clinical education and communication. Future studies should examine the clinical significance (ie, adenoma detection rates) of text messaging bowel preparation protocols.
Limitations
Our study has several limitations. First, this was a single center study, thus generalizability is limited. MVAMC represents a predominantly White, male, and rural population. Second, data are likely an underestimation of the true impact of intervention, because results do not account for patients who were turned away on day of procedure (typically still reporting brown stools at time of check-in for procedure) due to poor preparation or aborted procedures secondary to poor preparation. Only about one-third of the 640 veterans opted to receive Annie text messages.
Studies have shown veterans are willing to use technology for health care; however, access to technology and lack of training remain barriers to use.14 This has been most robustly studied at the VA in veterans experiencing mental illness and homelessness. Targeted strategies to improve veteran adoption of technology within their health care include supplying veterans with cell phones and paid data plans and providing training on specific technology-based resources.15-17 Future improvement for the Annie platform should include improved integration with CPRS. Integration will facilitate automatic import of key information such as mobile phone number or colonoscopy procedure date. Unfortunately, this is not currently an automated process, and the manual workload of staff limits sustainability. Since our study ended, the Annie database now allows an “event date” to be programmed in to center the text message series around. This will be entered at the time of Annie enrollment and eliminate manual activation of the protocol. The issue of updating information for rescheduled procedures remains.
Conclusions
There is increasing evidence that automated text messaging is a promising addition to medicine for clinical education and communication. It continues to gain traction as a readily available and acceptable option, and many patients are willing to incorporate the technology platform into their care plan. We found high patient satisfaction with our protocol, and Annie patients had cleaner bowel preparations compared with control patients. Our study supports the use of text message reminders as an effective intervention for improving patient adherence with bowel preparation instructions. We suspect that creation of a text messaging protocol designed for patients requiring outpatient extended bowel preparation will yield great benefit. As technology continues to improve, future implementation of Annie text messaging will become increasingly seamless within the field of gastroenterology and beyond.
1. Centers for Disease Control and Prevention. Colorectal cancer statistics. Updated June 6, 2022. Accessed September 8, 2022. https://www.cdc.gov/cancer/colorectal/statistics
2. Lieberman D, Ladabaum U, Cruz-Correa M, et al. Screening for colorectal cancer and evolving issues for physicians and patients: a review. JAMA. 2016;316(20):2135-2145. doi:10.1001/jama.2016.17418
3. Nguyen DL, Wieland M. Risk factors predictive of poor quality preparation during average risk colonoscopy screening: the importance of health literacy. J Gastrointestin Liver Dis. 2010;19(4):369-372.
4. Mahmood S, Farooqui SM, Madhoun MF. Predictors of inadequate bowel preparation for colonoscopy: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2018;30(8):819-826. doi:10.1097/MEG.0000000000001175
5. Harrington KM, Nguyen XT, Song RJ, et al. Gender differences in demographic and health characteristics of the Million Veteran Program cohort. Womens Health Issues. 2019;29(suppl 1):S56-S66. doi:10.1016/j.whi.2019.04.012
6. Zhang QX, Li J, Zhang Q, et al. Effect of education by messaging software on the quality of bowel preparation for colonoscopy. Chin Med J (Engl). 2018;131(14):1750-1752. doi:10.4103/0366-6999.235881
7. Walter B, Klare P, Strehle K, et al. Improving the quality and acceptance of colonoscopy preparation by reinforced patient education with short message service: results from a randomized, multicenter study (PERICLES-II). Gastrointest Endosc. 2019;89(3):506-513.e4. doi:10.1016/j.gie.2018.08.014
8. Nadim MM, Doshi S, Coniglio M, et al. Automated text message navigation to improve preparation quality and show rate for colonoscopy. Am J Gastroenterol. 2018;113:S64-S66.
9. Walter B, Frank R, Ludwig L, et al. Smartphone application to reinforce education increases high-quality preparation for colorectal cancer screening colonoscopies in a randomized trial. Clin Gastroenterol Hepatol. 2021;19(2):331-338.e5. doi:10.1016/j.cgh.2020.03.051
10. Guo B, Zuo X, Li Z, et al. Improving the quality of bowel preparation through an app for inpatients undergoing colonoscopy: a randomized controlled trial. J Adv Nurs. 2020;76(4):1037-1045. doi:10.1111/jan.14295
11. Yakovchenko V, Hogan TP, Houston TK, et al. Automated text messaging with patients in department of veterans affairs specialty clinics: cluster randomized trial. J Med Internet Res. 2019;21(8):e14750. doi:10.2196/14750
12. Lai EJ, Calderwood AH, Doros G, Fix OK, Jacobson BC. The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc. 2009;69(3 Pt 2):620-625. doi:10.1016/j.gie.2008.05.057
13. Calderwood AH, Jacobson BC. Comprehensive validation of the Boston Bowel Preparation Scale. Gastrointest Endosc. 2010;72(4):686-692. doi:10.1016/j.gie.2010.06.068
14. Duan-Porter W, Van Houtven CH, Mahanna EP, et al. Internet use and technology-related attitudes of veterans and informal caregivers of veterans. Telemed J E Health. 2018;24(7):471-480. doi:10.1089/tmj.2017.0015
15. Boston University School of Public Health. how mobile technology can increase veteran healthcare and wellbeing. November 10, 2021. Accessed November 1, 2022. https://www.ideahub.org/research-data/how-mobile-technology-increases-veteran-healthcare-and-wellbeing/
16. Klee A, Stacy M, Rosenheck R, Harkness L, Tsai J. Interest in technology-based therapies hampered by access: A survey of veterans with serious mental illnesses. Psychiatr Rehabil J. 2016;39(2):173-179. doi:10.1037/prj0000180
17. Berrouiguet S, Baca-García E, Brandt S, Walter M, Courtet P. Fundamentals for future mobile-health (mHealth): a systematic review of mobile phone and web-based text messaging in mental health. J Med Internet Res. 2016;18(6):e135. Published 2016 Jun 10. doi:10.2196/jmir.5066
Colorectal cancer is the third leading cause of cancer-related death in both men and women.1 Colonoscopy is the current gold standard for screening due to the ability to remove precancerous lesions but remains highly dependent on the quality of bowel preparation.2 Poor bowel preparation has been associated with impaired adenoma detection as well as increased health care utilization due to the need for a repeat colonoscopy.3
Multiple patient factors are associated with increased risk of poor bowel preparation, including age > 60 years, male sex, diabetes mellitus, and presence of a mental health diagnosis, factors that are prevalent among the veteran population.3-5 Text messages have been shown to improve the quality of bowel preparation by increasing patients' understanding and adherence with the preparation process. Improved adherence with bowel preparation directions is associated with a cleaner colon prior to colonoscopy, leading to a thorough examination. Studies using text messaging instructions prior to colonoscopies have also shown measurable improvement in adenoma detection rate, patient preparation-associated discomfort, and completion of colonoscopy.6-10
In 2016, the Veterans Health Administration (VHA) introduced Annie, one of the first automated text messaging services, named after Army Lieutenant Annie Fox, the first woman to receive the Purple Heart for combat. The Annie platform allows for notifications, instructions, and simple data collection. The development of this platform allows VHA practitioners to engage and educate veterans in a similar way to other health care systems using text messaging protocols. Annie text messages have been piloted for the use of hepatitis C treatment, demonstrating promise of improved medication adherence and patient satisfaction.11 We aimed to develop and pilot the Annie bowel preparation protocol to improve the quality of colonoscopy bowel preparation for outpatients at the Minneapolis Veterans Affairs Medical Center (MVAMC) in Minnesota. A secondary goal included measuring patient satisfaction with the text messaging instructions for outpatient colonoscopy preparation.
Methods
We conducted a single center, prospective, endoscopist-blinded, study with two 3-month long Plan-Do-Study-Act (PDSA) cycles to improve the text messaging bowel preparation protocol at MVAMC between January 2019 and April 2020. The MVAMC Institutional Review Board determined the quality improvement project was exempt. Veterans who had outpatient colonoscopies scheduled were included. Veterans undergoing inpatient colonoscopies or outpatients who could not be reached to obtain informed consent, lacked text message capability, declined participation, or required extended colonoscopy preparation were excluded. Per MVAMC procedures, extended colonoscopy preparation was provided to patients receiving general or monitored anesthesia care, with a history of poor bowel preparation, or with risk factors for poor preparation as determined by the ordering health care professional (HCP). Standard bowel preparation involves ingestion of 4 L of polyethylene glycol 3350 with electrolytes; extended bowel preparation requires ingestion of an additional 2 L to total 6 L and uses a different set of instructions. Additionally, the patient population requiring extended bowel preparation also includes patients with spinal cord injuries, who often are admitted for assistance with extended preparation. Patients who consented to receiving text messages were placed in the Annie intervention group, and all others were placed in the control group.
The control group received standardized patient education, including a mailed copy of bowel preparation instructions and a phone call from a gastroenterology service nurse about 1 to 2 weeks before the procedure. Current MVAMC standard of care involves a phone call from a nurse to confirm that patients have received the polyethylene glycol preparation solution, the mailed instructions, have an escort and transportation, and to answer any questions. Both the usual care and intervention group received the phone call. During this call, the Annie text messaging bowel preparation protocol was introduced; if the veteran chose to participate, consent and enrollment were completed.
On the day of the colonoscopy, veterans in the intervention group were surveyed in the waiting room about their experience receiving the text messages and soliciting feedback for improvement or surveyed via telephone call within 3 days of their procedure. Patient satisfaction was quantified with a scale from 1 (low) to 10 (high), including questions about how helpful the texts were in relation to total number, timing, and content of messages as well as whether veterans would like to receive the text messages again for future procedures.
We reviewed individual charts and collected Boston Bowel Preparation Scale (BBPS) scores to determine adequate preparation. BBPS assigns a score of 0 to 3 for the right, transverse, and left colon applied upon withdrawal after flushing and suctioning have been completed.12 Adequate preparation is considered a total score of ≥ 6 with no segment scoring < 2. This method of preparation assessment is preferred due to its ability to account for difference in preparation quality among colonic segments, well-defined scoring characteristics, and several studies validating its use showing inter- and intraobserver reliability.12 Follow-up studies have shown validity of the BBPS when compared with relevant outcomes such as polyp detection rate and recommended timing for repeat procedure.13 Variables associated with poor bowel preparation (ie, gender, prior abdominal surgery, impaired mobility, high body mass index, diabetes mellitus, stroke, dementia, any neurologic diagnosis, cirrhosis, smoking, polypharmacy [> 8 active medications], and narcotic or tricyclic antidepressant medication use) were also collected through chart review.3-5 We note that immobility was defined by International Classification of Diseases (ICD)-9 and ICD-10 codes and prescriptions for assistive devices (ie, canes, wheelchairs, 4-wheeled walkers).
Veterans assent to be enrolled in Annie. After enrollment, veterans must text back a specific word response to an initial text message to receive the protocolized messages from the Annie program. A contact phone number to the gastrointestinal nurse line was provided for questions during business hours. The start date for the text message protocol is 6 days prior to the procedure date. If a patient rescheduled their colonoscopy, the Annie database was updated manually.
Statistical Analysis
We used both Pearson χ2 test and 2-sample t test analyses to compare demographic information and patient satisfaction scores between the control and intervention groups. We compared continuous BBPS scores between Annie intervention vs control group using parametric and nonparametric independent t tests using the Mann-Whitney U test. We repeated this analysis controlling for both mental health diagnoses and age using linear regression. We were unable to survey 61 of the 187 veterans who received Annie text messages.
RESULTS
During PDSA cycles 1 and 2, 640 veterans were scheduled for outpatient colonoscopy: 453 veterans were in the control group; 187 veterans were in the intervention group, of which 126 were surveyed. A significant percentage of veterans declined participation because they felt like they did not need reinforced education; others were not eligible for Annie due to requirement for extended bowel preparation, cancelled colonoscopy, inability to physically read text messages, or lack of cell phone.
The mean (SD) age was 65 (8) years; 184 (28.8%) had a diabetes mellitus diagnosis, and the mean (SD) body mass index was 31.6 (6.4). The Annie group was slightly more likely to have mental health diagnoses and lower age compared with the control group (Table 1).
Patient Feedback
We collected feedback from veterans after each PDSA cycle to identify areas for improvement by both in-person and telephone surveys. Based on feedback from PDSA cycle 1, we decreased the total number of text messages to create a more succinct set of instructions. The most frequently requested change involved timing the text messages to align with the exact morning a specific instruction should take place.
Patient satisfaction with the Annie text messaging service was high.
DISCUSSION
To our knowledge, this is the first report of using Annie at a VAMC for colonoscopy bowel preparation improvement. We found a statistically significant improvement in the average BBPS in those receiving Annie text messages compared with the routine care control group. We also found high levels of patient satisfaction with most patients requesting to receive them again for future procedures.
The clinical significance of a BBPS of 7.8 vs 8.2 is unclear, although any score > 6 is considered to be adequate. However, subjectively speaking, the higher the BBPS the cleaner the colon, and theoretically the easier it is to see small or flat polyps. Future steps could include calculating adenoma detection rates for those enrolled in the Annie program vs the control group.
We have received inquiries regarding potential program implementation at other facilities. Success and sustainability of the program will require long-term commitment and ideally protected time for staff. It is helpful to remember that for each person who chooses to enroll in the intervention, the program currently requires that a brief consent note is placed in the patient’s chart. Thus, depending on the facilities’ resources, it is ideal for one staff member to be the designated lead to help oversee, troubleshoot, and train additional personnel. Surveys can be intermittently used to obtain feedback for improvement but are not required for sustainability. Automated text messaging is a promising addition to medicine for clinical education and communication. Future studies should examine the clinical significance (ie, adenoma detection rates) of text messaging bowel preparation protocols.
Limitations
Our study has several limitations. First, this was a single center study, thus generalizability is limited. MVAMC represents a predominantly White, male, and rural population. Second, data are likely an underestimation of the true impact of intervention, because results do not account for patients who were turned away on day of procedure (typically still reporting brown stools at time of check-in for procedure) due to poor preparation or aborted procedures secondary to poor preparation. Only about one-third of the 640 veterans opted to receive Annie text messages.
Studies have shown veterans are willing to use technology for health care; however, access to technology and lack of training remain barriers to use.14 This has been most robustly studied at the VA in veterans experiencing mental illness and homelessness. Targeted strategies to improve veteran adoption of technology within their health care include supplying veterans with cell phones and paid data plans and providing training on specific technology-based resources.15-17 Future improvement for the Annie platform should include improved integration with CPRS. Integration will facilitate automatic import of key information such as mobile phone number or colonoscopy procedure date. Unfortunately, this is not currently an automated process, and the manual workload of staff limits sustainability. Since our study ended, the Annie database now allows an “event date” to be programmed in to center the text message series around. This will be entered at the time of Annie enrollment and eliminate manual activation of the protocol. The issue of updating information for rescheduled procedures remains.
Conclusions
There is increasing evidence that automated text messaging is a promising addition to medicine for clinical education and communication. It continues to gain traction as a readily available and acceptable option, and many patients are willing to incorporate the technology platform into their care plan. We found high patient satisfaction with our protocol, and Annie patients had cleaner bowel preparations compared with control patients. Our study supports the use of text message reminders as an effective intervention for improving patient adherence with bowel preparation instructions. We suspect that creation of a text messaging protocol designed for patients requiring outpatient extended bowel preparation will yield great benefit. As technology continues to improve, future implementation of Annie text messaging will become increasingly seamless within the field of gastroenterology and beyond.
Colorectal cancer is the third leading cause of cancer-related death in both men and women.1 Colonoscopy is the current gold standard for screening due to the ability to remove precancerous lesions but remains highly dependent on the quality of bowel preparation.2 Poor bowel preparation has been associated with impaired adenoma detection as well as increased health care utilization due to the need for a repeat colonoscopy.3
Multiple patient factors are associated with increased risk of poor bowel preparation, including age > 60 years, male sex, diabetes mellitus, and presence of a mental health diagnosis, factors that are prevalent among the veteran population.3-5 Text messages have been shown to improve the quality of bowel preparation by increasing patients' understanding and adherence with the preparation process. Improved adherence with bowel preparation directions is associated with a cleaner colon prior to colonoscopy, leading to a thorough examination. Studies using text messaging instructions prior to colonoscopies have also shown measurable improvement in adenoma detection rate, patient preparation-associated discomfort, and completion of colonoscopy.6-10
In 2016, the Veterans Health Administration (VHA) introduced Annie, one of the first automated text messaging services, named after Army Lieutenant Annie Fox, the first woman to receive the Purple Heart for combat. The Annie platform allows for notifications, instructions, and simple data collection. The development of this platform allows VHA practitioners to engage and educate veterans in a similar way to other health care systems using text messaging protocols. Annie text messages have been piloted for the use of hepatitis C treatment, demonstrating promise of improved medication adherence and patient satisfaction.11 We aimed to develop and pilot the Annie bowel preparation protocol to improve the quality of colonoscopy bowel preparation for outpatients at the Minneapolis Veterans Affairs Medical Center (MVAMC) in Minnesota. A secondary goal included measuring patient satisfaction with the text messaging instructions for outpatient colonoscopy preparation.
Methods
We conducted a single center, prospective, endoscopist-blinded, study with two 3-month long Plan-Do-Study-Act (PDSA) cycles to improve the text messaging bowel preparation protocol at MVAMC between January 2019 and April 2020. The MVAMC Institutional Review Board determined the quality improvement project was exempt. Veterans who had outpatient colonoscopies scheduled were included. Veterans undergoing inpatient colonoscopies or outpatients who could not be reached to obtain informed consent, lacked text message capability, declined participation, or required extended colonoscopy preparation were excluded. Per MVAMC procedures, extended colonoscopy preparation was provided to patients receiving general or monitored anesthesia care, with a history of poor bowel preparation, or with risk factors for poor preparation as determined by the ordering health care professional (HCP). Standard bowel preparation involves ingestion of 4 L of polyethylene glycol 3350 with electrolytes; extended bowel preparation requires ingestion of an additional 2 L to total 6 L and uses a different set of instructions. Additionally, the patient population requiring extended bowel preparation also includes patients with spinal cord injuries, who often are admitted for assistance with extended preparation. Patients who consented to receiving text messages were placed in the Annie intervention group, and all others were placed in the control group.
The control group received standardized patient education, including a mailed copy of bowel preparation instructions and a phone call from a gastroenterology service nurse about 1 to 2 weeks before the procedure. Current MVAMC standard of care involves a phone call from a nurse to confirm that patients have received the polyethylene glycol preparation solution, the mailed instructions, have an escort and transportation, and to answer any questions. Both the usual care and intervention group received the phone call. During this call, the Annie text messaging bowel preparation protocol was introduced; if the veteran chose to participate, consent and enrollment were completed.
On the day of the colonoscopy, veterans in the intervention group were surveyed in the waiting room about their experience receiving the text messages and soliciting feedback for improvement or surveyed via telephone call within 3 days of their procedure. Patient satisfaction was quantified with a scale from 1 (low) to 10 (high), including questions about how helpful the texts were in relation to total number, timing, and content of messages as well as whether veterans would like to receive the text messages again for future procedures.
We reviewed individual charts and collected Boston Bowel Preparation Scale (BBPS) scores to determine adequate preparation. BBPS assigns a score of 0 to 3 for the right, transverse, and left colon applied upon withdrawal after flushing and suctioning have been completed.12 Adequate preparation is considered a total score of ≥ 6 with no segment scoring < 2. This method of preparation assessment is preferred due to its ability to account for difference in preparation quality among colonic segments, well-defined scoring characteristics, and several studies validating its use showing inter- and intraobserver reliability.12 Follow-up studies have shown validity of the BBPS when compared with relevant outcomes such as polyp detection rate and recommended timing for repeat procedure.13 Variables associated with poor bowel preparation (ie, gender, prior abdominal surgery, impaired mobility, high body mass index, diabetes mellitus, stroke, dementia, any neurologic diagnosis, cirrhosis, smoking, polypharmacy [> 8 active medications], and narcotic or tricyclic antidepressant medication use) were also collected through chart review.3-5 We note that immobility was defined by International Classification of Diseases (ICD)-9 and ICD-10 codes and prescriptions for assistive devices (ie, canes, wheelchairs, 4-wheeled walkers).
Veterans assent to be enrolled in Annie. After enrollment, veterans must text back a specific word response to an initial text message to receive the protocolized messages from the Annie program. A contact phone number to the gastrointestinal nurse line was provided for questions during business hours. The start date for the text message protocol is 6 days prior to the procedure date. If a patient rescheduled their colonoscopy, the Annie database was updated manually.
Statistical Analysis
We used both Pearson χ2 test and 2-sample t test analyses to compare demographic information and patient satisfaction scores between the control and intervention groups. We compared continuous BBPS scores between Annie intervention vs control group using parametric and nonparametric independent t tests using the Mann-Whitney U test. We repeated this analysis controlling for both mental health diagnoses and age using linear regression. We were unable to survey 61 of the 187 veterans who received Annie text messages.
RESULTS
During PDSA cycles 1 and 2, 640 veterans were scheduled for outpatient colonoscopy: 453 veterans were in the control group; 187 veterans were in the intervention group, of which 126 were surveyed. A significant percentage of veterans declined participation because they felt like they did not need reinforced education; others were not eligible for Annie due to requirement for extended bowel preparation, cancelled colonoscopy, inability to physically read text messages, or lack of cell phone.
The mean (SD) age was 65 (8) years; 184 (28.8%) had a diabetes mellitus diagnosis, and the mean (SD) body mass index was 31.6 (6.4). The Annie group was slightly more likely to have mental health diagnoses and lower age compared with the control group (Table 1).
Patient Feedback
We collected feedback from veterans after each PDSA cycle to identify areas for improvement by both in-person and telephone surveys. Based on feedback from PDSA cycle 1, we decreased the total number of text messages to create a more succinct set of instructions. The most frequently requested change involved timing the text messages to align with the exact morning a specific instruction should take place.
Patient satisfaction with the Annie text messaging service was high.
DISCUSSION
To our knowledge, this is the first report of using Annie at a VAMC for colonoscopy bowel preparation improvement. We found a statistically significant improvement in the average BBPS in those receiving Annie text messages compared with the routine care control group. We also found high levels of patient satisfaction with most patients requesting to receive them again for future procedures.
The clinical significance of a BBPS of 7.8 vs 8.2 is unclear, although any score > 6 is considered to be adequate. However, subjectively speaking, the higher the BBPS the cleaner the colon, and theoretically the easier it is to see small or flat polyps. Future steps could include calculating adenoma detection rates for those enrolled in the Annie program vs the control group.
We have received inquiries regarding potential program implementation at other facilities. Success and sustainability of the program will require long-term commitment and ideally protected time for staff. It is helpful to remember that for each person who chooses to enroll in the intervention, the program currently requires that a brief consent note is placed in the patient’s chart. Thus, depending on the facilities’ resources, it is ideal for one staff member to be the designated lead to help oversee, troubleshoot, and train additional personnel. Surveys can be intermittently used to obtain feedback for improvement but are not required for sustainability. Automated text messaging is a promising addition to medicine for clinical education and communication. Future studies should examine the clinical significance (ie, adenoma detection rates) of text messaging bowel preparation protocols.
Limitations
Our study has several limitations. First, this was a single center study, thus generalizability is limited. MVAMC represents a predominantly White, male, and rural population. Second, data are likely an underestimation of the true impact of intervention, because results do not account for patients who were turned away on day of procedure (typically still reporting brown stools at time of check-in for procedure) due to poor preparation or aborted procedures secondary to poor preparation. Only about one-third of the 640 veterans opted to receive Annie text messages.
Studies have shown veterans are willing to use technology for health care; however, access to technology and lack of training remain barriers to use.14 This has been most robustly studied at the VA in veterans experiencing mental illness and homelessness. Targeted strategies to improve veteran adoption of technology within their health care include supplying veterans with cell phones and paid data plans and providing training on specific technology-based resources.15-17 Future improvement for the Annie platform should include improved integration with CPRS. Integration will facilitate automatic import of key information such as mobile phone number or colonoscopy procedure date. Unfortunately, this is not currently an automated process, and the manual workload of staff limits sustainability. Since our study ended, the Annie database now allows an “event date” to be programmed in to center the text message series around. This will be entered at the time of Annie enrollment and eliminate manual activation of the protocol. The issue of updating information for rescheduled procedures remains.
Conclusions
There is increasing evidence that automated text messaging is a promising addition to medicine for clinical education and communication. It continues to gain traction as a readily available and acceptable option, and many patients are willing to incorporate the technology platform into their care plan. We found high patient satisfaction with our protocol, and Annie patients had cleaner bowel preparations compared with control patients. Our study supports the use of text message reminders as an effective intervention for improving patient adherence with bowel preparation instructions. We suspect that creation of a text messaging protocol designed for patients requiring outpatient extended bowel preparation will yield great benefit. As technology continues to improve, future implementation of Annie text messaging will become increasingly seamless within the field of gastroenterology and beyond.
1. Centers for Disease Control and Prevention. Colorectal cancer statistics. Updated June 6, 2022. Accessed September 8, 2022. https://www.cdc.gov/cancer/colorectal/statistics
2. Lieberman D, Ladabaum U, Cruz-Correa M, et al. Screening for colorectal cancer and evolving issues for physicians and patients: a review. JAMA. 2016;316(20):2135-2145. doi:10.1001/jama.2016.17418
3. Nguyen DL, Wieland M. Risk factors predictive of poor quality preparation during average risk colonoscopy screening: the importance of health literacy. J Gastrointestin Liver Dis. 2010;19(4):369-372.
4. Mahmood S, Farooqui SM, Madhoun MF. Predictors of inadequate bowel preparation for colonoscopy: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2018;30(8):819-826. doi:10.1097/MEG.0000000000001175
5. Harrington KM, Nguyen XT, Song RJ, et al. Gender differences in demographic and health characteristics of the Million Veteran Program cohort. Womens Health Issues. 2019;29(suppl 1):S56-S66. doi:10.1016/j.whi.2019.04.012
6. Zhang QX, Li J, Zhang Q, et al. Effect of education by messaging software on the quality of bowel preparation for colonoscopy. Chin Med J (Engl). 2018;131(14):1750-1752. doi:10.4103/0366-6999.235881
7. Walter B, Klare P, Strehle K, et al. Improving the quality and acceptance of colonoscopy preparation by reinforced patient education with short message service: results from a randomized, multicenter study (PERICLES-II). Gastrointest Endosc. 2019;89(3):506-513.e4. doi:10.1016/j.gie.2018.08.014
8. Nadim MM, Doshi S, Coniglio M, et al. Automated text message navigation to improve preparation quality and show rate for colonoscopy. Am J Gastroenterol. 2018;113:S64-S66.
9. Walter B, Frank R, Ludwig L, et al. Smartphone application to reinforce education increases high-quality preparation for colorectal cancer screening colonoscopies in a randomized trial. Clin Gastroenterol Hepatol. 2021;19(2):331-338.e5. doi:10.1016/j.cgh.2020.03.051
10. Guo B, Zuo X, Li Z, et al. Improving the quality of bowel preparation through an app for inpatients undergoing colonoscopy: a randomized controlled trial. J Adv Nurs. 2020;76(4):1037-1045. doi:10.1111/jan.14295
11. Yakovchenko V, Hogan TP, Houston TK, et al. Automated text messaging with patients in department of veterans affairs specialty clinics: cluster randomized trial. J Med Internet Res. 2019;21(8):e14750. doi:10.2196/14750
12. Lai EJ, Calderwood AH, Doros G, Fix OK, Jacobson BC. The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc. 2009;69(3 Pt 2):620-625. doi:10.1016/j.gie.2008.05.057
13. Calderwood AH, Jacobson BC. Comprehensive validation of the Boston Bowel Preparation Scale. Gastrointest Endosc. 2010;72(4):686-692. doi:10.1016/j.gie.2010.06.068
14. Duan-Porter W, Van Houtven CH, Mahanna EP, et al. Internet use and technology-related attitudes of veterans and informal caregivers of veterans. Telemed J E Health. 2018;24(7):471-480. doi:10.1089/tmj.2017.0015
15. Boston University School of Public Health. how mobile technology can increase veteran healthcare and wellbeing. November 10, 2021. Accessed November 1, 2022. https://www.ideahub.org/research-data/how-mobile-technology-increases-veteran-healthcare-and-wellbeing/
16. Klee A, Stacy M, Rosenheck R, Harkness L, Tsai J. Interest in technology-based therapies hampered by access: A survey of veterans with serious mental illnesses. Psychiatr Rehabil J. 2016;39(2):173-179. doi:10.1037/prj0000180
17. Berrouiguet S, Baca-García E, Brandt S, Walter M, Courtet P. Fundamentals for future mobile-health (mHealth): a systematic review of mobile phone and web-based text messaging in mental health. J Med Internet Res. 2016;18(6):e135. Published 2016 Jun 10. doi:10.2196/jmir.5066
1. Centers for Disease Control and Prevention. Colorectal cancer statistics. Updated June 6, 2022. Accessed September 8, 2022. https://www.cdc.gov/cancer/colorectal/statistics
2. Lieberman D, Ladabaum U, Cruz-Correa M, et al. Screening for colorectal cancer and evolving issues for physicians and patients: a review. JAMA. 2016;316(20):2135-2145. doi:10.1001/jama.2016.17418
3. Nguyen DL, Wieland M. Risk factors predictive of poor quality preparation during average risk colonoscopy screening: the importance of health literacy. J Gastrointestin Liver Dis. 2010;19(4):369-372.
4. Mahmood S, Farooqui SM, Madhoun MF. Predictors of inadequate bowel preparation for colonoscopy: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2018;30(8):819-826. doi:10.1097/MEG.0000000000001175
5. Harrington KM, Nguyen XT, Song RJ, et al. Gender differences in demographic and health characteristics of the Million Veteran Program cohort. Womens Health Issues. 2019;29(suppl 1):S56-S66. doi:10.1016/j.whi.2019.04.012
6. Zhang QX, Li J, Zhang Q, et al. Effect of education by messaging software on the quality of bowel preparation for colonoscopy. Chin Med J (Engl). 2018;131(14):1750-1752. doi:10.4103/0366-6999.235881
7. Walter B, Klare P, Strehle K, et al. Improving the quality and acceptance of colonoscopy preparation by reinforced patient education with short message service: results from a randomized, multicenter study (PERICLES-II). Gastrointest Endosc. 2019;89(3):506-513.e4. doi:10.1016/j.gie.2018.08.014
8. Nadim MM, Doshi S, Coniglio M, et al. Automated text message navigation to improve preparation quality and show rate for colonoscopy. Am J Gastroenterol. 2018;113:S64-S66.
9. Walter B, Frank R, Ludwig L, et al. Smartphone application to reinforce education increases high-quality preparation for colorectal cancer screening colonoscopies in a randomized trial. Clin Gastroenterol Hepatol. 2021;19(2):331-338.e5. doi:10.1016/j.cgh.2020.03.051
10. Guo B, Zuo X, Li Z, et al. Improving the quality of bowel preparation through an app for inpatients undergoing colonoscopy: a randomized controlled trial. J Adv Nurs. 2020;76(4):1037-1045. doi:10.1111/jan.14295
11. Yakovchenko V, Hogan TP, Houston TK, et al. Automated text messaging with patients in department of veterans affairs specialty clinics: cluster randomized trial. J Med Internet Res. 2019;21(8):e14750. doi:10.2196/14750
12. Lai EJ, Calderwood AH, Doros G, Fix OK, Jacobson BC. The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc. 2009;69(3 Pt 2):620-625. doi:10.1016/j.gie.2008.05.057
13. Calderwood AH, Jacobson BC. Comprehensive validation of the Boston Bowel Preparation Scale. Gastrointest Endosc. 2010;72(4):686-692. doi:10.1016/j.gie.2010.06.068
14. Duan-Porter W, Van Houtven CH, Mahanna EP, et al. Internet use and technology-related attitudes of veterans and informal caregivers of veterans. Telemed J E Health. 2018;24(7):471-480. doi:10.1089/tmj.2017.0015
15. Boston University School of Public Health. how mobile technology can increase veteran healthcare and wellbeing. November 10, 2021. Accessed November 1, 2022. https://www.ideahub.org/research-data/how-mobile-technology-increases-veteran-healthcare-and-wellbeing/
16. Klee A, Stacy M, Rosenheck R, Harkness L, Tsai J. Interest in technology-based therapies hampered by access: A survey of veterans with serious mental illnesses. Psychiatr Rehabil J. 2016;39(2):173-179. doi:10.1037/prj0000180
17. Berrouiguet S, Baca-García E, Brandt S, Walter M, Courtet P. Fundamentals for future mobile-health (mHealth): a systematic review of mobile phone and web-based text messaging in mental health. J Med Internet Res. 2016;18(6):e135. Published 2016 Jun 10. doi:10.2196/jmir.5066
Randomized, Double-Blind Placebo-Controlled Trial to Assess the Effect of Probiotics on Irritable Bowel Syndrome in Veterans With Gulf War Illness
About 700,000 US military personnel were deployed in Operation Desert Storm (August 1990 to March 1991).1 Almost 30 years since the war, a large number of these veterans continue to experience a complex of symptoms of unknown etiology called Gulf War illness (GWI), which significantly affects health and quality of life (QOL). The lack of clear etiology of the illness has impaired research to find specific treatments and has further exacerbated the stress among veterans. GWI typically includes a mixture of chronic headache, cognitive difficulties, widespread pain, unexplained fatigue, memory and concentration problems, as well as chronic respiratory and gastrointestinal (GI) symptoms.2 Abdominal pain and alteration of bowel habits are also symptoms typical of irritable bowel syndrome (IBS). It has been estimated that IBS occurs in up to 30% of Gulf War veterans.3
The etiology of IBS is unknown. Possible mechanisms include visceral hypersensitivity, altered gut motor function, aberrant brain-gut interaction, and psychological factors, perhaps with a genetic predisposition.4 Gastroenteritis has been reported as a triggering mechanism in up to one-third of patients with IBS.5 Gastroenteritis can alter the gut microbiota and has been reported to be a significant risk factor for the development of IBS.6 In one study of Operation Desert Shield soldiers, > 50% of military personnel developed acute gastroenteritis while on duty.7 A high prevalence of extra-intestinal symptoms also has been reported, including fatigue, headache, joint pains, and anxiety, in Gulf War veterans with IBS. These extra-intestinal symptoms of IBS are consistent with the reported GWI symptoms. Change in gut microbiota also has been associated with many of the extra-intestinal symptoms of IBS, especially fatigue.8,9 Gut microbiota are known to change with travel, stress, and a change in diet, all potential factors that are relevant to Gulf War veterans. This would suggest that an imbalance in the gut microbiota, ie, dysbiosis, may play a role in the pathogenesis of both IBS and GWI. Dysbiosis could be a risk factor for or alternatively a consequence of GWI.
A systematic review highlighted the heterogeneity of the gut microbiota in patients with IBS.10 Overall, Enterobacteriaceae, Lactobacillaceae, and Bacteroides were increased, whereas Clostridiales, Faecalibacterium, and Bifidobacterium were decreased in patients with IBS compared with controls. Gut microbiota also has been associated with cognitive changes, anxiety, and depression—symptoms associated with IBS and are part of the GWI.
If altered gut microbiota contributes to the etiopathogenesis of IBS, its restoration of with probiotics should help. Probiotics are live organisms that when ingested may improve health by promoting the growth of naturally occurring flora and establishing a healthy gut flora. Probiotics have several mechanisms of actions. Probiotics work in the lumen of the gut by producing antibacterial molecules and enhancing the mucosal barrier.11 Probiotics also may produce metabolic compounds that alter the intestinal microbiota and improve intestinal barrier function.12 Probiotics also have been shown to activate receptors in the enteric nervous system with the potential to promote pain relief in the setting of visceral hyperalgesia.13,14 The anti-inflammatory properties of probiotics potentially could modulate the basic pathophysiology of IBS and improve motility, visceral hypersensitivity, and brain-gut interaction.15 Furthermore, significant gut dysbiosis has been shown with GWI; suggesting that probiotics may have a role in its management.16,17
Probiotics have not been studied in Gulf War veterans with IBS. We performed a prospective, double-blind placebo-controlled study to determine the efficacy of a commercially available probiotic containing 8 strains of bacteria (De Simone Formulation; formally known as VSL#3 and Visbiome) on symptoms of IBS and GWI. This probiotic was selected as the overall literature suggested benefit of combination probiotics in IBS, and VSL#3 has been shown to be efficacious in ulcerative colitis and microscopic colitis.18-20
Methods
Veterans who served in Operation Desert Storm (August 1990 to March 1991) and enrolled at the George E. Wahlen Veterans Affairs (VA) Medical Center (GEWVAMC), Salt Lake City, Utah, were eligible for the study. The inclusion criteria were: veterans aged ≥ 35 years; ≥ 2 nonintestinal GWI symptoms (eg, fatigue, joint pains, insomnia, general stiffness, and headache); IBS diagnosis based on the Rome III criteria; IBS symptoms > 6 months; normal gross appearance of the colonic mucosa; negative markers for celiac disease and inflammatory bowel disease (IBD); normal thyroid function; and serum calcium levels.21 Those who had a clinically significant cardiac, pulmonary, hepatic or renal dysfunction; history of/or presence of systemic malignancy; current evidence of celiac disease or IBD; unstable/significant psychiatric disease; recent change in GI medications; current pregnancy; or use of antibiotics or probiotics within the past 1 month were excluded. Subjects were enrolled from a list of veterans with GWI from the GEWVAMC Gulf War registry; referrals to gastroenterology clinics for IBS from internal medicine clinics; and posted advertisements.
Protocol
After written informed consent was obtained, each veteran was verified to have IBS and ≥ 2 GWI symptoms. All veterans had the following tests and panels: complete blood count, erythrocyte sedimentation rate, serum comprehensive metabolic panel, thyroid-stimulating hormone, tissue transglutaminase, stool test for ova and parasite, giardia antigen, and clostridia toxins to exclude organic cause of GI symptoms. Colonoscopy was performed in all veterans to exclude IBD, and to rule out microscopic or lymphocytic colitis.
Randomization was computer generated and maintained by the study pharmacist so that study personnel and patients were blinded to the trial groups. All investigators were blinded and allocation was concealed. The medication was supplied in a numbered container by the pharmacist after patient enrollment. After a 2-week run-in period, veterans were randomized (1:1) to receive either 1 sachet of probiotic (De Simone Formulation; formally known as VSL#3 and Visbiome) or placebo once daily for 8 weeks.
Each probiotic packet contains 900 billion probiotic bacteria per sachet.11 This formulation contained 8 viable strains of bacteria: 4 strains of Lactobacillus (L acidophilus, L plantarum, L paracasei, L delbrueckii subsp. bulgaricus); 3 strains of Bifidobacteria (Bifidobacterium breve, B lactis, B infantis); and 1 strain of Streptococcus thermophilus. This formulation had been commercialized and studied as VSL#3 and is currently available in the United States under the Visbiome trade name. While branding changed during the study, the formulation did not. The investigational medicine (VSL#3, Visbiome, and placebo) were shipped from the manufacturer Dupont/Danisco in Madison, Wisconsin. The subjects received placebo or probiotic (VSL#3/Visbiome) and both were identical in appearance. The medication was supplied in a numbered container by the pharmacist after patient enrollment.
Measures
Veterans completed the bowel disease questionnaire to record baseline bowel habits.22 All veterans recorded daily bowel symptoms to confirm the presence of IBS during the 2-week pretreatment period, at baseline, and at the end of the 8-week treatment. The symptoms assessed included severity of abdominal pain (0, none to 100, severe); severity of bloating (0, none to 100, severe); stool frequency; Bristol stool scale (1, very hard to 7, watery); severity of diarrhea (0, none to 100, severe); severity of constipation (0, none to 100, severe); satisfaction with bowel habits (0, none to 100, severe); and IBS affecting or interfering with life (0, none to 100, severe). The bowel symptom score is the sum of the 5 symptom scores.23,24
IBS-specific QOL (IBS-QOL) was recorded at baseline and at the end of treatment.25 The IBS-QOL consists of a 34-item validated disease-specific questionnaire that measures 8 domains relevant to subjects with IBS: dysphoria, interference with activity, body image, health worry, food avoidance, social reaction, sexual life, and relationships. We used the Somatic Symptom Checklist to detect the following extra-intestinal symptoms that are common among veterans with GWI: headache, backache, wheeziness, insomnia, bad breath, fatigue, general stiffness, dizziness, weakness, sensitivity to hot and cold, palpitation, and tightness in chest. Subjects rated symptoms on a scale of 1 to 5: how often (1, none; 2, monthly; 3, once weekly; 4, several times weekly; 5, daily), and how bothersome (1, not at all to 5, extremely).26
Subjects completed the Posttraumatic Stress Disorder (PTSD) Checklist–Military, which is specific to military experience with 17 items on a 1 to 5 scale (1, not at all to 5, extremely). Scores were summed to produce a total symptom severity score (range, 17-85).27 Subjects also completed the Brief Symptom Inventory 18 (BSI-18) during the baseline evaluation.28 BSI-18 measures subjects’ reported overall psychological distress. It assesses 3 symptoms dimensions (somatization, depression, and anxiety) and a global severity index. The raw scores were transferred to normative T scores based on samples of nonpatient normal men and women.
Symptom data were compared after 8 weeks of treatment. The primary study endpoint was change in bowel symptom score. The secondary endpoints were mean change in symptoms, QOL, extra-intestinal symptoms, and PTSD score. The study was approved by the Salt Lake City Veterans Affairs Medical Center and the University of Utah Institutional Review Board and registered in ClinicalTrials.gov (NCT03078530).
Statistical Methods
Comparisons of the probiotic vs placebo groups for demographic variable were analyzed using a 2-sample t test for continuous variables, and with a χ2 test or Fisher exact test for categorical variables. The primary and secondary outcome variables were recorded daily for 2 weeks as pretreatment baseline and for 2 weeks at the end of treatment. These symptoms were recorded as ordered categorical variables, which were then averaged across the week to produce a continuous measurement for statistical analysis. For the primary outcome of GI symptoms, posttreatment comparisons were made between the study groups using a 2-sample t test of the baseline vs posttreatment values. All P values were calculated for 2-sided comparisons. The planned sample size in our study protocol was to recruit 40 individuals per group in order to achieve 80% power to detect a 30% improvement between baseline and end of treatment in the primary bowel symptom score. This study recruited 53 subjects. With this sample size, the study had 80% power to detect a 0.8 SD in any of the outcomes.
Results
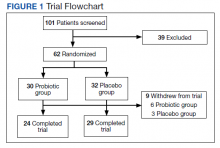
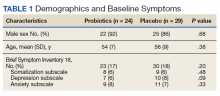
We screened 101 veterans with IBS and GWI; 39 veterans did not fulfill the inclusion/exclusion criteria, 22 declined to participate or did not complete the screening questionnaires and tests, and 9 were lost to follow-up. Sixty-two participants were randomized in a double-blind placebo-controlled study design; 9 dropped out before the end of the study. Data were analyzed from 53 veterans who completed the study, 29 in the placebo group and 24 in the probiotic group (Figure 1). The cohort was primarily male with a mean (SD) age of 55 (8) years (range, 42-73) (Table 1).
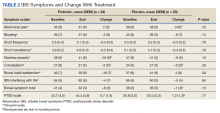
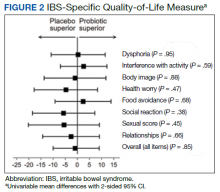
Overall, the treatment was well tolerated. All subjects were contacted every 2 weeks during the study to check for adverse effects, but no serious events were reported. There were no differences at baseline in any of the BSI-18 subscale scores in veterans between the groups. There was a greater mean (SEM) improvement of diarrhea severity in the probiotic group compared with the placebo group: 18 (6), a 31% improvement, vs 6 (5), a 13% improvement, respectively; however, the difference was not statistically significance (P = .13) (Table 2). There also was a greater mean (SEM) improvement in satisfaction of bowel habits in the probiotic group compared with the placebo group: 16 (7), a 35% improvement vs 4 (9), an 8% worsening; this also was not statistically significant (P = .09). There was no difference in the change of IBS-QOL before and after treatment in either group (Figure 2). There was no improvement in any of the symptoms of GWI (all P ≥ .06) (Appendix).
Discussion
GWI is a complex multisystem illness of unknown etiology. There was high prevalence of diarrhea during deployment, and veterans were exposed to several physical, environmental, and mental stresses of the war.3 A change in gut microbiota can occur during deployment due to diet changes, environmental and physical stress, and GI infections.29 These changes would suggest that manipulation of gut microbiota might offer a new modality of treatment of IBS and GWI. We evaluated the effect of a high-potency multistrain probiotic in veterans with IBS and GWI. We did not detect any statistically significant differences between the probiotic and placebo groups on bowel symptom score and individual symptoms of IBS and on QOL. Also, there was no improvement for the other symptoms of GWI. To our knowledge, this is the first study evaluating the effect of probiotics in veterans with IBS and GWI. Our results are consistent with the literature on probiotics and IBS.
The probiotic formulation used in our study has been evaluated in patients with IBS previously. Kim and colleagues found that after 8 weeks of treatment of patients with diarrhea-predominant IBS with VSL#3, there was improvement in bloating, but no effect was found on abdominal pain, gas, or urgency.30 A subsequent study by the same investigators on patients with all types of IBS found that VSL#3 showed no effect on abdominal pain, stool frequency and consistency, or on bloating, but there was improvement in flatulence.31 Another study that evaluated the effect of VSL#3 on symptoms of diarrhea-predominant IBS and QOL found improvement in IBS symptoms from baseline in both the probiotic and the placebo groups, but the difference between the 2 groups was not statistically significant.32 Similarly, Wong and colleagues performed a double-blind, placebo-controlled mechanistic study to evaluate the effect of VSL#3. They found improvement in bowel symptom score, abdominal pain intensity, and satisfaction with bowel habits with both the VSL#3 and placebo group but similar to our study, the differences were not statistically significant.
Several reviews have evaluated the efficacy of probiotics for IBS. A 2010 review found evidence that probiotics trended toward improved IBS symptoms compared with placebo.33 The 2014 follow-up by the same authors demonstrated that overall, probiotics improved global symptoms of IBS and multistrain probiotics were more effective.20 A third meta-analysis from the same group found evidence that multistrain probiotics seemed to have a beneficial effect but could not definitively conclude that probiotics are efficacious in improving IBS symptoms.34 Other authors also have seen inconsistent effects of probiotics compared with placebo on global symptoms, abdominal pain, and bloating after performing systematic reviews of the literature.35-38 Although several reviews support that multistrain probiotics are more effective, they fail to conclude which combinations are more efficacious.
The effect of probiotics on QOL has not been investigated by many studies.37 In our study, we did not find significant improvement in QOL in the probiotic group, which is in line with 2 previous studies that showed no effect on IBS QOL of VSL#3 vs placebo.32,39 Most of the research reports that multistrain probiotics are more effective than using a single strain.34,35,40Bifidobacterium and Lactobacillus are the most commonly used bacteria in the multistrain probiotics that have shown their positive effect on IBS.35,41 The probiotic used in our study contained other species along with these 2 microorganisms.
The dose and duration of treatment of probiotics also has been debated. In one meta-analysis, the investigators found that studies of ≥ 8 weeks were more likely to show a positive effect; 4 of the 7 studies with statistically significant improvement in IBS symptoms were longer than 8 weeks.35 However, another meta-analysis based on 35 randomized controlled trials found that there was not a statistically significant difference between groups treated for > 4 weeks vs < 4 weeks.42 In addition, another meta-analysis of VSL#3 on IBS in children and adults also found no difference in results based on the duration of treatment of probiotics.43 Similar to our study, 3 other studies of VSL#3 treated patients for 8 weeks and found no statistically significant effect.30-32 In the past, VSL#3 has been used at dosages of 450 or 900 billion bacteria per day.
An individual’s response to probiotics may depend on the subtype of IBS. However, most of the studies, like ours, included groups of all subtypes. It may be that probiotics are more effective in patients with moderate-to-severe symptoms. Most of our patients had milder symptoms, and we cannot discount how subjects with more severe disease may have responded to the drug. Interestingly, one study demonstrated that Lactobacillus was more effective in patients with moderately severe abdominal pain compared with mild symptoms.44
In our study, the probiotic did not improve PTSD symptoms or other extra-intestinal symptoms common in IBS and GWI. Similar to our study, Wong and colleagues did not find significant improvement of psychological and sleep scores after treatment with VSL#3.6 Similarly, there is evidence that alteration in gut microbiota is associated with health and diseases, but what specific alterations occur and whether they can be improved with probiotics remains unknown.45
Limitations
The inconsistent response to probiotics in various studies may be due to IBS heterogeneity. Furthermore, there are demographic differences between Gulf War veterans and patients enrolled in other studies: Gulf War veterans are predominantly male, many were deployed abroad and had a history of gastroenteritis during deployment, and were exposed to stressful situations.46 These factors may be involved in triggering or maintaining IBS in Gulf War veterans. A further limitation of our randomized trial is the relatively small sample size.
Conclusions
This study did not demonstrate statistically significant improvement in symptoms of IBS or improvement in QOL after treatment with a multistrain probiotic. We also did not find any improvement in symptoms of GWI or PTSD. There was no difference in psychological scores between the placebo and treatment groups, and it is unlikely that psychological factors confounded the response to treatment in this study.
The effectiveness of a probiotic may depend on the baseline gut microbiome of the individual and depend on the strain, amount, and frequency of bacteria used. A lack of response of the probiotics does not exclude gut viruses and fungi having a role in exacerbating GWI symptoms. It is also possible that the bacteria present or the dose of the probiotic used was not sufficient to improve symptoms. So far, the definitive benefit of probiotics has been demonstrated for only a few preparations, and none are approved by the US Food and Drug Administration for any disease. More research is needed to determine whether probiotics have any role in the treatment of IBS and GWI.
Acknowledgments
AKT received grant support from the US Department of Veterans Affairs and the US Department of Defense (W81XWH-10-1-0593, W81XWH-15-1-0636). We thank Keith G. Tolman, MD, for assistance in editing the initial proposal and for periodic consultation. We thank the manufacturer of the probiotic for supplying the active drug and the placebo. The manufacture of the probiotic had no role in the design and conduct of the study, analysis and interpretation of the data, and in the preparation of the manuscript.
1. O’Shea EF, Cotter PD, Stanton C, Ross RP, Hill C. Production of bioactive substances by intestinal bacteria as a basis for explaining probiotic mechanisms: bacteriocins and conjugated linoleic acid. Int J Food Microbiol. 2012;152(3):189-205. doi:10.1016/j.ijfoodmicro.2011.05.025.
2. Kamiya T, Wang L, Forsythe P, et al. Inhibitory effects of Lactobacillus reuteri on visceral pain induced by colorectal distension in Sprague-Dawley rats. Gut. 2006;55(2):191-196. doi:10.1136/gut.2005.070987.
3. Verdu EF, Bercik P, Verma-Gandhu M, et al. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut. 2006;55(2):182-190. doi:10.1136/gut.2005.066100
4. Ford AC, Harris LA, Lacy BE, Quigley EMM, Moayyedi P. Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharmacol Ther. 2018;48(10):1044-1060. doi:10.1111/apt.15001.
5. Niu HL, Xiao JY. The efficacy and safety of probiotics in patients with irritable bowel syndrome: Evidence based on 35 randomized controlled trials. Int J Surg. 2020;75:116-127. doi:10.1016/j.ijsu.2020.01.142.
6. Wong RK, Yang C, Song GH, Wong J, Ho KY. Melatonin regulation as a possible mechanism for probiotic (VSL#3) in irritable bowel syndrome: a randomized double-blinded placebo study. Dig Dis Sci. 2015;60(1):186-194. doi:10.1007/s10620-014-3299-8.
7. Hyams KC, Bourgeois AL, Merrell BR, et al. Diarrheal disease during Operation Desert Shield. N Engl J Med. 1991;325(20):1423-1428. doi:10.1056/NEJM199111143252006 8. Clancy RL, Gleeson M, Cox A, et al. Reversal in fatigued athletes of a defect in interferon gamma secretion after administration of Lactobacillus acidophilus. Br J Sports Med. 2006;40(4):351-354. doi:10.1136/bjsm.2005.024364
9. Sullivan A, Nord CE, Evengard B. Effect of supplement with lactic-acid producing bacteria on fatigue and physical activity in patients with chronic fatigue syndrome. Nutr J. 2009;8:4. doi:10.1186/1475-2891-8-4
10. Pittayanon R, Lau JT, Yuan Y, et al. Gut microbiota in patients with irritable bowel syndrome—a systematic review. Gastroenterology. 2019;157(1):97-108. doi:10.1053/j.gastro.2019.03.049
11. Rao RK, Samak G. Protection and restitution of gut barrier by probiotics: nutritional and clinical implications. Curr Nutr Food Sci. 2013;9(2):99-107. doi:10.2174/1573401311309020004
12. O´Shea EF, Cotter PD, Stanton C, Ross RP, Hill C. Production of bioactive substances by intestinal bacteria as a basis for explaining probiotic mechanisms: bacteriocins and conjugated linoleic acid. Int J Food Microbiol. 2012;152(3):189-205. doi:10.1016/j.ijfoodmicro.2011.05.025
13. Kamiya T, Wang L, Forsythe P, et al. Inhibitory effects of Lactobacillus reuteri on visceral pain induced by colorectal distension in Sprague-Dawley rats. Gut. 2006;55(2):191-196. doi:10.1136/gut.2005.070987
14. Verdu EF, Bercik P, Verma-Gandhu M, et al. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut. 2006;55(2):182-190. doi:10.1136/gut.2005.06610015. O´Mahony L, McCarthy J, Kelly P, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128(3):541-551. doi:10.1053/j.gastro.2004.11.050
16. Alhasson F, Das S, Seth R, et al. Altered gut microbiome in a mouse model of Gulf War Illness causes neuroinflammation and intestinal injury via leaky gut and TLR4 activation. PLoS One. 2017;12(3):e0172914. doi:10.1371/journal.pone.0172914.17. Janulewicz PA, Seth RK, Carlson JM, et al. The gut-microbiome in Gulf War veterans: a preliminary report. Int J Environ Res Public Health. 2019;16(19). doi:10.3390/ijerph16193751
18. Dang X, Xu M, Liu D, Zhou D, Yang W. Assessing the efficacy and safety of fecal microbiota transplantation and probiotic VSL#3 for active ulcerative colitis: a systematic review and meta-analysis. PLoS One. 2020;15(3):e0228846. doi:10.1371/journal.pone.0228846
19. Ford AC, Quigley EM, Lacy BE, et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol. 2014;109(10):1547-1561; quiz 1546, 1562. doi:10.1038/ajg.2014.202
20. Rohatgi S, Ahuja V, Makharia GK, et al. VSL#3 induces and maintains short-term clinical response in patients with active microscopic colitis: a two-phase randomised clinical trial. BMJ Open Gastroenterol. 2015;2(1):e000018. doi:10.1136/bmjgast-2014-000018
21. Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130(5):1480-1491. doi:10.1053/j.gastro.2005.11.061
22. Talley NJ, Phillips SF, Melton J, 3rd, Wiltgen C, Zinsmeister AR. A patient questionnaire to identify bowel disease. Ann Intern Med. 1989;111(8):671-674. doi:10.7326/0003-4819-111-8-671
23. Bensoussan A, Talley NJ, Hing M, Menzies R, Guo A, Ngu M. Treatment of irritable bowel syndrome with Chinese herbal medicine: a randomized controlled trial. JAMA. 1998;280(18):1585-1589. doi:10.1001/jama.280.18.1585
24. Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11(2):395-402. doi:10.1046/j.1365-2036.1997.142318000.x
25. Patrick DL, Drossman DA, Frederick IO, DiCesare J, Puder KL. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Dig Dis Sci. 1998;43(2):400-411. doi:10.1023/a:1018831127942
26. Attanasio V, Andrasik F, Blanchard EB, Arena JG. Psychometric properties of the SUNYA revision of the Psychosomatic Symptom Checklist. J Behav Med. 1984;7(2):247-257. doi:10.1007/BF00845390
27. Weathers F, Litz B, Herman D, Huska J, Keane T. The PTSD Checklist (PCL): reliability, validity, and diagnostic utility. Accessed August 25, 2022. https://www.researchgate.net/publication/291448760_The_PTSD_Checklist_PCL_Reliability_validity_and_diagnostic_utility
28. Derogatis L. Brief Symptom Inventory-18 (BSI-18): Administration, Scoring, and Procedure Manual. Ed 3 ed. National Computer Systems; 2000.
29. Stamps BW, Lyon WJ, Irvin AP, Kelley-Loughnane N, Goodson MS. A pilot study of the effect of deployment on the gut microbiome and traveler´s diarrhea susceptibility. Front Cell Infect Microbiol. 2020;10:589297. doi:10.3389/fcimb.2020.589297
30. Kim HJ, Camilleri M, McKinzie S, et al. A randomized controlled trial of a probiotic, VSL#3, on gut transit and symptoms in diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2003;17(7):895-904. doi:10.1046/j.1365-2036.2003.01543.x
31. Kim HJ, Vazquez Roque MI, Camilleri M, et al. A randomized controlled trial of a probiotic combination VSL# 3 and placebo in irritable bowel syndrome with bloating. Neurogastroenterol Motil. 2005;17(5):687-696. doi:10.1111/j.1365-2982.2005.00695.x32. Michail S, Kenche H. Gut microbiota is not modified by randomized, double-blind, placebo-controlled trial of vsl#3 in diarrhea-predominant irritable bowel syndrome. Probiotics Antimicrob Proteins. 2011;3(1):1-7. doi:10.1007/s12602-010-9059-y
33. Moayyedi P, Ford AC, Talley NJ, et al. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010;59(3):325-332. doi:10.1136/gut.2008.167270

34. Ford AC, Harris LA, Lacy BE, Quigley EMM, Moayyedi P. Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharmacol Ther. 2018;48(10):1044-1060. doi:10.1111/apt.15001
35. Dale HF, Rasmussen SH, Asiller OO, Lied GA. Probiotics in irritable bowel syndrome: an up-to-date systematic review. Nutrients. 2019;11(9). doi:10.3390/nu11092048
36. Didari T, Mozaffari S, Nikfar S, Abdollahi M. Effectiveness of probiotics in irritable bowel syndrome: Updated systematic review with meta-analysis. World J Gastroenterol. 2015;21(10):3072-84. doi:10.3748/wjg.v21.i10.3072
37. Hungin APS, Mitchell CR, Whorwell P, et al. Systematic review: probiotics in the management of lower gastrointestinal symptoms—an updated evidence-based international consensus. Aliment Pharmacol Ther. 2018;47(8):1054-1070. doi:10.1111/apt.14539
38. Niu HL, Xiao JY. The efficacy and safety of probiotics in patients with irritable bowel syndrome: evidence based on 35 randomized controlled trials. Int J Surg. 2020;75:116-127. doi:10.1016/j.ijsu.2020.01.142
39. Wong RK, Yang C, Song GH, Wong J, Ho KY. Melatonin regulation as a possible mechanism for probiotic (VSL#3) in irritable bowel syndrome: a randomized double-blinded placebo study. Dig Dis Sci. 2015;60(1):186-194. doi:10.1007/s10620-014-3299-8
40. Ford AC, Moayyedi P, Lacy BE, et al. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol. 2014;109(suppl 1):S2-26; quiz S27. doi: 10.1038/ajg.2014.187
41. Simren M, Barbara G, Flint HJ, et al. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut. 2013;62(1):159-76. doi:10.1136/gutjnl-2012-302167
42. Ki Cha B, Mun Jung S, Hwan Choi C, et al. The effect of a multispecies probiotic mixture on the symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Clin Gastroenterol. 2012;46(3):220-7. doi:10.1097/MCG.0b013e31823712b1
43. Connell M, Shin A, James-Stevenson T, Xu H, Imperiale TF, Herron J. Systematic review and meta-analysis: Efficacy of patented probiotic, VSL#3, in irritable bowel syndrome. Neurogastroenterol Motil. 2018;30(12):e13427. doi:10.1111/nmo.13427
44. Lyra A, Hillila M, Huttunen T, et al. Irritable bowel syndrome symptom severity improves equally with probiotic and placebo. World J Gastroenterol. 2016;22(48):10631-10642. doi:10.3748/wjg.v22.i48.10631
45. Sanders ME, Guarner F, Guerrant R, et al. An update on the use and investigation of probiotics in health and disease. Gut. 2013;62(5):787-796. doi:10.1136/gutjnl-2012-302504
46. Tuteja AK. Deployment-associated functional gastrointestinal disorders: do we know the etiology? Dig Dis Sci. 2011;56(11):3109-3111. doi:10.1007/s10620-011-1856-y
About 700,000 US military personnel were deployed in Operation Desert Storm (August 1990 to March 1991).1 Almost 30 years since the war, a large number of these veterans continue to experience a complex of symptoms of unknown etiology called Gulf War illness (GWI), which significantly affects health and quality of life (QOL). The lack of clear etiology of the illness has impaired research to find specific treatments and has further exacerbated the stress among veterans. GWI typically includes a mixture of chronic headache, cognitive difficulties, widespread pain, unexplained fatigue, memory and concentration problems, as well as chronic respiratory and gastrointestinal (GI) symptoms.2 Abdominal pain and alteration of bowel habits are also symptoms typical of irritable bowel syndrome (IBS). It has been estimated that IBS occurs in up to 30% of Gulf War veterans.3
The etiology of IBS is unknown. Possible mechanisms include visceral hypersensitivity, altered gut motor function, aberrant brain-gut interaction, and psychological factors, perhaps with a genetic predisposition.4 Gastroenteritis has been reported as a triggering mechanism in up to one-third of patients with IBS.5 Gastroenteritis can alter the gut microbiota and has been reported to be a significant risk factor for the development of IBS.6 In one study of Operation Desert Shield soldiers, > 50% of military personnel developed acute gastroenteritis while on duty.7 A high prevalence of extra-intestinal symptoms also has been reported, including fatigue, headache, joint pains, and anxiety, in Gulf War veterans with IBS. These extra-intestinal symptoms of IBS are consistent with the reported GWI symptoms. Change in gut microbiota also has been associated with many of the extra-intestinal symptoms of IBS, especially fatigue.8,9 Gut microbiota are known to change with travel, stress, and a change in diet, all potential factors that are relevant to Gulf War veterans. This would suggest that an imbalance in the gut microbiota, ie, dysbiosis, may play a role in the pathogenesis of both IBS and GWI. Dysbiosis could be a risk factor for or alternatively a consequence of GWI.
A systematic review highlighted the heterogeneity of the gut microbiota in patients with IBS.10 Overall, Enterobacteriaceae, Lactobacillaceae, and Bacteroides were increased, whereas Clostridiales, Faecalibacterium, and Bifidobacterium were decreased in patients with IBS compared with controls. Gut microbiota also has been associated with cognitive changes, anxiety, and depression—symptoms associated with IBS and are part of the GWI.
If altered gut microbiota contributes to the etiopathogenesis of IBS, its restoration of with probiotics should help. Probiotics are live organisms that when ingested may improve health by promoting the growth of naturally occurring flora and establishing a healthy gut flora. Probiotics have several mechanisms of actions. Probiotics work in the lumen of the gut by producing antibacterial molecules and enhancing the mucosal barrier.11 Probiotics also may produce metabolic compounds that alter the intestinal microbiota and improve intestinal barrier function.12 Probiotics also have been shown to activate receptors in the enteric nervous system with the potential to promote pain relief in the setting of visceral hyperalgesia.13,14 The anti-inflammatory properties of probiotics potentially could modulate the basic pathophysiology of IBS and improve motility, visceral hypersensitivity, and brain-gut interaction.15 Furthermore, significant gut dysbiosis has been shown with GWI; suggesting that probiotics may have a role in its management.16,17
Probiotics have not been studied in Gulf War veterans with IBS. We performed a prospective, double-blind placebo-controlled study to determine the efficacy of a commercially available probiotic containing 8 strains of bacteria (De Simone Formulation; formally known as VSL#3 and Visbiome) on symptoms of IBS and GWI. This probiotic was selected as the overall literature suggested benefit of combination probiotics in IBS, and VSL#3 has been shown to be efficacious in ulcerative colitis and microscopic colitis.18-20
Methods
Veterans who served in Operation Desert Storm (August 1990 to March 1991) and enrolled at the George E. Wahlen Veterans Affairs (VA) Medical Center (GEWVAMC), Salt Lake City, Utah, were eligible for the study. The inclusion criteria were: veterans aged ≥ 35 years; ≥ 2 nonintestinal GWI symptoms (eg, fatigue, joint pains, insomnia, general stiffness, and headache); IBS diagnosis based on the Rome III criteria; IBS symptoms > 6 months; normal gross appearance of the colonic mucosa; negative markers for celiac disease and inflammatory bowel disease (IBD); normal thyroid function; and serum calcium levels.21 Those who had a clinically significant cardiac, pulmonary, hepatic or renal dysfunction; history of/or presence of systemic malignancy; current evidence of celiac disease or IBD; unstable/significant psychiatric disease; recent change in GI medications; current pregnancy; or use of antibiotics or probiotics within the past 1 month were excluded. Subjects were enrolled from a list of veterans with GWI from the GEWVAMC Gulf War registry; referrals to gastroenterology clinics for IBS from internal medicine clinics; and posted advertisements.
Protocol
After written informed consent was obtained, each veteran was verified to have IBS and ≥ 2 GWI symptoms. All veterans had the following tests and panels: complete blood count, erythrocyte sedimentation rate, serum comprehensive metabolic panel, thyroid-stimulating hormone, tissue transglutaminase, stool test for ova and parasite, giardia antigen, and clostridia toxins to exclude organic cause of GI symptoms. Colonoscopy was performed in all veterans to exclude IBD, and to rule out microscopic or lymphocytic colitis.
Randomization was computer generated and maintained by the study pharmacist so that study personnel and patients were blinded to the trial groups. All investigators were blinded and allocation was concealed. The medication was supplied in a numbered container by the pharmacist after patient enrollment. After a 2-week run-in period, veterans were randomized (1:1) to receive either 1 sachet of probiotic (De Simone Formulation; formally known as VSL#3 and Visbiome) or placebo once daily for 8 weeks.
Each probiotic packet contains 900 billion probiotic bacteria per sachet.11 This formulation contained 8 viable strains of bacteria: 4 strains of Lactobacillus (L acidophilus, L plantarum, L paracasei, L delbrueckii subsp. bulgaricus); 3 strains of Bifidobacteria (Bifidobacterium breve, B lactis, B infantis); and 1 strain of Streptococcus thermophilus. This formulation had been commercialized and studied as VSL#3 and is currently available in the United States under the Visbiome trade name. While branding changed during the study, the formulation did not. The investigational medicine (VSL#3, Visbiome, and placebo) were shipped from the manufacturer Dupont/Danisco in Madison, Wisconsin. The subjects received placebo or probiotic (VSL#3/Visbiome) and both were identical in appearance. The medication was supplied in a numbered container by the pharmacist after patient enrollment.
Measures
Veterans completed the bowel disease questionnaire to record baseline bowel habits.22 All veterans recorded daily bowel symptoms to confirm the presence of IBS during the 2-week pretreatment period, at baseline, and at the end of the 8-week treatment. The symptoms assessed included severity of abdominal pain (0, none to 100, severe); severity of bloating (0, none to 100, severe); stool frequency; Bristol stool scale (1, very hard to 7, watery); severity of diarrhea (0, none to 100, severe); severity of constipation (0, none to 100, severe); satisfaction with bowel habits (0, none to 100, severe); and IBS affecting or interfering with life (0, none to 100, severe). The bowel symptom score is the sum of the 5 symptom scores.23,24
IBS-specific QOL (IBS-QOL) was recorded at baseline and at the end of treatment.25 The IBS-QOL consists of a 34-item validated disease-specific questionnaire that measures 8 domains relevant to subjects with IBS: dysphoria, interference with activity, body image, health worry, food avoidance, social reaction, sexual life, and relationships. We used the Somatic Symptom Checklist to detect the following extra-intestinal symptoms that are common among veterans with GWI: headache, backache, wheeziness, insomnia, bad breath, fatigue, general stiffness, dizziness, weakness, sensitivity to hot and cold, palpitation, and tightness in chest. Subjects rated symptoms on a scale of 1 to 5: how often (1, none; 2, monthly; 3, once weekly; 4, several times weekly; 5, daily), and how bothersome (1, not at all to 5, extremely).26
Subjects completed the Posttraumatic Stress Disorder (PTSD) Checklist–Military, which is specific to military experience with 17 items on a 1 to 5 scale (1, not at all to 5, extremely). Scores were summed to produce a total symptom severity score (range, 17-85).27 Subjects also completed the Brief Symptom Inventory 18 (BSI-18) during the baseline evaluation.28 BSI-18 measures subjects’ reported overall psychological distress. It assesses 3 symptoms dimensions (somatization, depression, and anxiety) and a global severity index. The raw scores were transferred to normative T scores based on samples of nonpatient normal men and women.
Symptom data were compared after 8 weeks of treatment. The primary study endpoint was change in bowel symptom score. The secondary endpoints were mean change in symptoms, QOL, extra-intestinal symptoms, and PTSD score. The study was approved by the Salt Lake City Veterans Affairs Medical Center and the University of Utah Institutional Review Board and registered in ClinicalTrials.gov (NCT03078530).
Statistical Methods
Comparisons of the probiotic vs placebo groups for demographic variable were analyzed using a 2-sample t test for continuous variables, and with a χ2 test or Fisher exact test for categorical variables. The primary and secondary outcome variables were recorded daily for 2 weeks as pretreatment baseline and for 2 weeks at the end of treatment. These symptoms were recorded as ordered categorical variables, which were then averaged across the week to produce a continuous measurement for statistical analysis. For the primary outcome of GI symptoms, posttreatment comparisons were made between the study groups using a 2-sample t test of the baseline vs posttreatment values. All P values were calculated for 2-sided comparisons. The planned sample size in our study protocol was to recruit 40 individuals per group in order to achieve 80% power to detect a 30% improvement between baseline and end of treatment in the primary bowel symptom score. This study recruited 53 subjects. With this sample size, the study had 80% power to detect a 0.8 SD in any of the outcomes.
Results
We screened 101 veterans with IBS and GWI; 39 veterans did not fulfill the inclusion/exclusion criteria, 22 declined to participate or did not complete the screening questionnaires and tests, and 9 were lost to follow-up. Sixty-two participants were randomized in a double-blind placebo-controlled study design; 9 dropped out before the end of the study. Data were analyzed from 53 veterans who completed the study, 29 in the placebo group and 24 in the probiotic group (Figure 1). The cohort was primarily male with a mean (SD) age of 55 (8) years (range, 42-73) (Table 1).
Overall, the treatment was well tolerated. All subjects were contacted every 2 weeks during the study to check for adverse effects, but no serious events were reported. There were no differences at baseline in any of the BSI-18 subscale scores in veterans between the groups. There was a greater mean (SEM) improvement of diarrhea severity in the probiotic group compared with the placebo group: 18 (6), a 31% improvement, vs 6 (5), a 13% improvement, respectively; however, the difference was not statistically significance (P = .13) (Table 2). There also was a greater mean (SEM) improvement in satisfaction of bowel habits in the probiotic group compared with the placebo group: 16 (7), a 35% improvement vs 4 (9), an 8% worsening; this also was not statistically significant (P = .09). There was no difference in the change of IBS-QOL before and after treatment in either group (Figure 2). There was no improvement in any of the symptoms of GWI (all P ≥ .06) (Appendix).
Discussion
GWI is a complex multisystem illness of unknown etiology. There was high prevalence of diarrhea during deployment, and veterans were exposed to several physical, environmental, and mental stresses of the war.3 A change in gut microbiota can occur during deployment due to diet changes, environmental and physical stress, and GI infections.29 These changes would suggest that manipulation of gut microbiota might offer a new modality of treatment of IBS and GWI. We evaluated the effect of a high-potency multistrain probiotic in veterans with IBS and GWI. We did not detect any statistically significant differences between the probiotic and placebo groups on bowel symptom score and individual symptoms of IBS and on QOL. Also, there was no improvement for the other symptoms of GWI. To our knowledge, this is the first study evaluating the effect of probiotics in veterans with IBS and GWI. Our results are consistent with the literature on probiotics and IBS.
The probiotic formulation used in our study has been evaluated in patients with IBS previously. Kim and colleagues found that after 8 weeks of treatment of patients with diarrhea-predominant IBS with VSL#3, there was improvement in bloating, but no effect was found on abdominal pain, gas, or urgency.30 A subsequent study by the same investigators on patients with all types of IBS found that VSL#3 showed no effect on abdominal pain, stool frequency and consistency, or on bloating, but there was improvement in flatulence.31 Another study that evaluated the effect of VSL#3 on symptoms of diarrhea-predominant IBS and QOL found improvement in IBS symptoms from baseline in both the probiotic and the placebo groups, but the difference between the 2 groups was not statistically significant.32 Similarly, Wong and colleagues performed a double-blind, placebo-controlled mechanistic study to evaluate the effect of VSL#3. They found improvement in bowel symptom score, abdominal pain intensity, and satisfaction with bowel habits with both the VSL#3 and placebo group but similar to our study, the differences were not statistically significant.
Several reviews have evaluated the efficacy of probiotics for IBS. A 2010 review found evidence that probiotics trended toward improved IBS symptoms compared with placebo.33 The 2014 follow-up by the same authors demonstrated that overall, probiotics improved global symptoms of IBS and multistrain probiotics were more effective.20 A third meta-analysis from the same group found evidence that multistrain probiotics seemed to have a beneficial effect but could not definitively conclude that probiotics are efficacious in improving IBS symptoms.34 Other authors also have seen inconsistent effects of probiotics compared with placebo on global symptoms, abdominal pain, and bloating after performing systematic reviews of the literature.35-38 Although several reviews support that multistrain probiotics are more effective, they fail to conclude which combinations are more efficacious.
The effect of probiotics on QOL has not been investigated by many studies.37 In our study, we did not find significant improvement in QOL in the probiotic group, which is in line with 2 previous studies that showed no effect on IBS QOL of VSL#3 vs placebo.32,39 Most of the research reports that multistrain probiotics are more effective than using a single strain.34,35,40Bifidobacterium and Lactobacillus are the most commonly used bacteria in the multistrain probiotics that have shown their positive effect on IBS.35,41 The probiotic used in our study contained other species along with these 2 microorganisms.
The dose and duration of treatment of probiotics also has been debated. In one meta-analysis, the investigators found that studies of ≥ 8 weeks were more likely to show a positive effect; 4 of the 7 studies with statistically significant improvement in IBS symptoms were longer than 8 weeks.35 However, another meta-analysis based on 35 randomized controlled trials found that there was not a statistically significant difference between groups treated for > 4 weeks vs < 4 weeks.42 In addition, another meta-analysis of VSL#3 on IBS in children and adults also found no difference in results based on the duration of treatment of probiotics.43 Similar to our study, 3 other studies of VSL#3 treated patients for 8 weeks and found no statistically significant effect.30-32 In the past, VSL#3 has been used at dosages of 450 or 900 billion bacteria per day.
An individual’s response to probiotics may depend on the subtype of IBS. However, most of the studies, like ours, included groups of all subtypes. It may be that probiotics are more effective in patients with moderate-to-severe symptoms. Most of our patients had milder symptoms, and we cannot discount how subjects with more severe disease may have responded to the drug. Interestingly, one study demonstrated that Lactobacillus was more effective in patients with moderately severe abdominal pain compared with mild symptoms.44
In our study, the probiotic did not improve PTSD symptoms or other extra-intestinal symptoms common in IBS and GWI. Similar to our study, Wong and colleagues did not find significant improvement of psychological and sleep scores after treatment with VSL#3.6 Similarly, there is evidence that alteration in gut microbiota is associated with health and diseases, but what specific alterations occur and whether they can be improved with probiotics remains unknown.45
Limitations
The inconsistent response to probiotics in various studies may be due to IBS heterogeneity. Furthermore, there are demographic differences between Gulf War veterans and patients enrolled in other studies: Gulf War veterans are predominantly male, many were deployed abroad and had a history of gastroenteritis during deployment, and were exposed to stressful situations.46 These factors may be involved in triggering or maintaining IBS in Gulf War veterans. A further limitation of our randomized trial is the relatively small sample size.
Conclusions
This study did not demonstrate statistically significant improvement in symptoms of IBS or improvement in QOL after treatment with a multistrain probiotic. We also did not find any improvement in symptoms of GWI or PTSD. There was no difference in psychological scores between the placebo and treatment groups, and it is unlikely that psychological factors confounded the response to treatment in this study.
The effectiveness of a probiotic may depend on the baseline gut microbiome of the individual and depend on the strain, amount, and frequency of bacteria used. A lack of response of the probiotics does not exclude gut viruses and fungi having a role in exacerbating GWI symptoms. It is also possible that the bacteria present or the dose of the probiotic used was not sufficient to improve symptoms. So far, the definitive benefit of probiotics has been demonstrated for only a few preparations, and none are approved by the US Food and Drug Administration for any disease. More research is needed to determine whether probiotics have any role in the treatment of IBS and GWI.
Acknowledgments
AKT received grant support from the US Department of Veterans Affairs and the US Department of Defense (W81XWH-10-1-0593, W81XWH-15-1-0636). We thank Keith G. Tolman, MD, for assistance in editing the initial proposal and for periodic consultation. We thank the manufacturer of the probiotic for supplying the active drug and the placebo. The manufacture of the probiotic had no role in the design and conduct of the study, analysis and interpretation of the data, and in the preparation of the manuscript.
About 700,000 US military personnel were deployed in Operation Desert Storm (August 1990 to March 1991).1 Almost 30 years since the war, a large number of these veterans continue to experience a complex of symptoms of unknown etiology called Gulf War illness (GWI), which significantly affects health and quality of life (QOL). The lack of clear etiology of the illness has impaired research to find specific treatments and has further exacerbated the stress among veterans. GWI typically includes a mixture of chronic headache, cognitive difficulties, widespread pain, unexplained fatigue, memory and concentration problems, as well as chronic respiratory and gastrointestinal (GI) symptoms.2 Abdominal pain and alteration of bowel habits are also symptoms typical of irritable bowel syndrome (IBS). It has been estimated that IBS occurs in up to 30% of Gulf War veterans.3
The etiology of IBS is unknown. Possible mechanisms include visceral hypersensitivity, altered gut motor function, aberrant brain-gut interaction, and psychological factors, perhaps with a genetic predisposition.4 Gastroenteritis has been reported as a triggering mechanism in up to one-third of patients with IBS.5 Gastroenteritis can alter the gut microbiota and has been reported to be a significant risk factor for the development of IBS.6 In one study of Operation Desert Shield soldiers, > 50% of military personnel developed acute gastroenteritis while on duty.7 A high prevalence of extra-intestinal symptoms also has been reported, including fatigue, headache, joint pains, and anxiety, in Gulf War veterans with IBS. These extra-intestinal symptoms of IBS are consistent with the reported GWI symptoms. Change in gut microbiota also has been associated with many of the extra-intestinal symptoms of IBS, especially fatigue.8,9 Gut microbiota are known to change with travel, stress, and a change in diet, all potential factors that are relevant to Gulf War veterans. This would suggest that an imbalance in the gut microbiota, ie, dysbiosis, may play a role in the pathogenesis of both IBS and GWI. Dysbiosis could be a risk factor for or alternatively a consequence of GWI.
A systematic review highlighted the heterogeneity of the gut microbiota in patients with IBS.10 Overall, Enterobacteriaceae, Lactobacillaceae, and Bacteroides were increased, whereas Clostridiales, Faecalibacterium, and Bifidobacterium were decreased in patients with IBS compared with controls. Gut microbiota also has been associated with cognitive changes, anxiety, and depression—symptoms associated with IBS and are part of the GWI.
If altered gut microbiota contributes to the etiopathogenesis of IBS, its restoration of with probiotics should help. Probiotics are live organisms that when ingested may improve health by promoting the growth of naturally occurring flora and establishing a healthy gut flora. Probiotics have several mechanisms of actions. Probiotics work in the lumen of the gut by producing antibacterial molecules and enhancing the mucosal barrier.11 Probiotics also may produce metabolic compounds that alter the intestinal microbiota and improve intestinal barrier function.12 Probiotics also have been shown to activate receptors in the enteric nervous system with the potential to promote pain relief in the setting of visceral hyperalgesia.13,14 The anti-inflammatory properties of probiotics potentially could modulate the basic pathophysiology of IBS and improve motility, visceral hypersensitivity, and brain-gut interaction.15 Furthermore, significant gut dysbiosis has been shown with GWI; suggesting that probiotics may have a role in its management.16,17
Probiotics have not been studied in Gulf War veterans with IBS. We performed a prospective, double-blind placebo-controlled study to determine the efficacy of a commercially available probiotic containing 8 strains of bacteria (De Simone Formulation; formally known as VSL#3 and Visbiome) on symptoms of IBS and GWI. This probiotic was selected as the overall literature suggested benefit of combination probiotics in IBS, and VSL#3 has been shown to be efficacious in ulcerative colitis and microscopic colitis.18-20
Methods
Veterans who served in Operation Desert Storm (August 1990 to March 1991) and enrolled at the George E. Wahlen Veterans Affairs (VA) Medical Center (GEWVAMC), Salt Lake City, Utah, were eligible for the study. The inclusion criteria were: veterans aged ≥ 35 years; ≥ 2 nonintestinal GWI symptoms (eg, fatigue, joint pains, insomnia, general stiffness, and headache); IBS diagnosis based on the Rome III criteria; IBS symptoms > 6 months; normal gross appearance of the colonic mucosa; negative markers for celiac disease and inflammatory bowel disease (IBD); normal thyroid function; and serum calcium levels.21 Those who had a clinically significant cardiac, pulmonary, hepatic or renal dysfunction; history of/or presence of systemic malignancy; current evidence of celiac disease or IBD; unstable/significant psychiatric disease; recent change in GI medications; current pregnancy; or use of antibiotics or probiotics within the past 1 month were excluded. Subjects were enrolled from a list of veterans with GWI from the GEWVAMC Gulf War registry; referrals to gastroenterology clinics for IBS from internal medicine clinics; and posted advertisements.
Protocol
After written informed consent was obtained, each veteran was verified to have IBS and ≥ 2 GWI symptoms. All veterans had the following tests and panels: complete blood count, erythrocyte sedimentation rate, serum comprehensive metabolic panel, thyroid-stimulating hormone, tissue transglutaminase, stool test for ova and parasite, giardia antigen, and clostridia toxins to exclude organic cause of GI symptoms. Colonoscopy was performed in all veterans to exclude IBD, and to rule out microscopic or lymphocytic colitis.
Randomization was computer generated and maintained by the study pharmacist so that study personnel and patients were blinded to the trial groups. All investigators were blinded and allocation was concealed. The medication was supplied in a numbered container by the pharmacist after patient enrollment. After a 2-week run-in period, veterans were randomized (1:1) to receive either 1 sachet of probiotic (De Simone Formulation; formally known as VSL#3 and Visbiome) or placebo once daily for 8 weeks.
Each probiotic packet contains 900 billion probiotic bacteria per sachet.11 This formulation contained 8 viable strains of bacteria: 4 strains of Lactobacillus (L acidophilus, L plantarum, L paracasei, L delbrueckii subsp. bulgaricus); 3 strains of Bifidobacteria (Bifidobacterium breve, B lactis, B infantis); and 1 strain of Streptococcus thermophilus. This formulation had been commercialized and studied as VSL#3 and is currently available in the United States under the Visbiome trade name. While branding changed during the study, the formulation did not. The investigational medicine (VSL#3, Visbiome, and placebo) were shipped from the manufacturer Dupont/Danisco in Madison, Wisconsin. The subjects received placebo or probiotic (VSL#3/Visbiome) and both were identical in appearance. The medication was supplied in a numbered container by the pharmacist after patient enrollment.
Measures
Veterans completed the bowel disease questionnaire to record baseline bowel habits.22 All veterans recorded daily bowel symptoms to confirm the presence of IBS during the 2-week pretreatment period, at baseline, and at the end of the 8-week treatment. The symptoms assessed included severity of abdominal pain (0, none to 100, severe); severity of bloating (0, none to 100, severe); stool frequency; Bristol stool scale (1, very hard to 7, watery); severity of diarrhea (0, none to 100, severe); severity of constipation (0, none to 100, severe); satisfaction with bowel habits (0, none to 100, severe); and IBS affecting or interfering with life (0, none to 100, severe). The bowel symptom score is the sum of the 5 symptom scores.23,24
IBS-specific QOL (IBS-QOL) was recorded at baseline and at the end of treatment.25 The IBS-QOL consists of a 34-item validated disease-specific questionnaire that measures 8 domains relevant to subjects with IBS: dysphoria, interference with activity, body image, health worry, food avoidance, social reaction, sexual life, and relationships. We used the Somatic Symptom Checklist to detect the following extra-intestinal symptoms that are common among veterans with GWI: headache, backache, wheeziness, insomnia, bad breath, fatigue, general stiffness, dizziness, weakness, sensitivity to hot and cold, palpitation, and tightness in chest. Subjects rated symptoms on a scale of 1 to 5: how often (1, none; 2, monthly; 3, once weekly; 4, several times weekly; 5, daily), and how bothersome (1, not at all to 5, extremely).26
Subjects completed the Posttraumatic Stress Disorder (PTSD) Checklist–Military, which is specific to military experience with 17 items on a 1 to 5 scale (1, not at all to 5, extremely). Scores were summed to produce a total symptom severity score (range, 17-85).27 Subjects also completed the Brief Symptom Inventory 18 (BSI-18) during the baseline evaluation.28 BSI-18 measures subjects’ reported overall psychological distress. It assesses 3 symptoms dimensions (somatization, depression, and anxiety) and a global severity index. The raw scores were transferred to normative T scores based on samples of nonpatient normal men and women.
Symptom data were compared after 8 weeks of treatment. The primary study endpoint was change in bowel symptom score. The secondary endpoints were mean change in symptoms, QOL, extra-intestinal symptoms, and PTSD score. The study was approved by the Salt Lake City Veterans Affairs Medical Center and the University of Utah Institutional Review Board and registered in ClinicalTrials.gov (NCT03078530).
Statistical Methods
Comparisons of the probiotic vs placebo groups for demographic variable were analyzed using a 2-sample t test for continuous variables, and with a χ2 test or Fisher exact test for categorical variables. The primary and secondary outcome variables were recorded daily for 2 weeks as pretreatment baseline and for 2 weeks at the end of treatment. These symptoms were recorded as ordered categorical variables, which were then averaged across the week to produce a continuous measurement for statistical analysis. For the primary outcome of GI symptoms, posttreatment comparisons were made between the study groups using a 2-sample t test of the baseline vs posttreatment values. All P values were calculated for 2-sided comparisons. The planned sample size in our study protocol was to recruit 40 individuals per group in order to achieve 80% power to detect a 30% improvement between baseline and end of treatment in the primary bowel symptom score. This study recruited 53 subjects. With this sample size, the study had 80% power to detect a 0.8 SD in any of the outcomes.
Results
We screened 101 veterans with IBS and GWI; 39 veterans did not fulfill the inclusion/exclusion criteria, 22 declined to participate or did not complete the screening questionnaires and tests, and 9 were lost to follow-up. Sixty-two participants were randomized in a double-blind placebo-controlled study design; 9 dropped out before the end of the study. Data were analyzed from 53 veterans who completed the study, 29 in the placebo group and 24 in the probiotic group (Figure 1). The cohort was primarily male with a mean (SD) age of 55 (8) years (range, 42-73) (Table 1).
Overall, the treatment was well tolerated. All subjects were contacted every 2 weeks during the study to check for adverse effects, but no serious events were reported. There were no differences at baseline in any of the BSI-18 subscale scores in veterans between the groups. There was a greater mean (SEM) improvement of diarrhea severity in the probiotic group compared with the placebo group: 18 (6), a 31% improvement, vs 6 (5), a 13% improvement, respectively; however, the difference was not statistically significance (P = .13) (Table 2). There also was a greater mean (SEM) improvement in satisfaction of bowel habits in the probiotic group compared with the placebo group: 16 (7), a 35% improvement vs 4 (9), an 8% worsening; this also was not statistically significant (P = .09). There was no difference in the change of IBS-QOL before and after treatment in either group (Figure 2). There was no improvement in any of the symptoms of GWI (all P ≥ .06) (Appendix).
Discussion
GWI is a complex multisystem illness of unknown etiology. There was high prevalence of diarrhea during deployment, and veterans were exposed to several physical, environmental, and mental stresses of the war.3 A change in gut microbiota can occur during deployment due to diet changes, environmental and physical stress, and GI infections.29 These changes would suggest that manipulation of gut microbiota might offer a new modality of treatment of IBS and GWI. We evaluated the effect of a high-potency multistrain probiotic in veterans with IBS and GWI. We did not detect any statistically significant differences between the probiotic and placebo groups on bowel symptom score and individual symptoms of IBS and on QOL. Also, there was no improvement for the other symptoms of GWI. To our knowledge, this is the first study evaluating the effect of probiotics in veterans with IBS and GWI. Our results are consistent with the literature on probiotics and IBS.
The probiotic formulation used in our study has been evaluated in patients with IBS previously. Kim and colleagues found that after 8 weeks of treatment of patients with diarrhea-predominant IBS with VSL#3, there was improvement in bloating, but no effect was found on abdominal pain, gas, or urgency.30 A subsequent study by the same investigators on patients with all types of IBS found that VSL#3 showed no effect on abdominal pain, stool frequency and consistency, or on bloating, but there was improvement in flatulence.31 Another study that evaluated the effect of VSL#3 on symptoms of diarrhea-predominant IBS and QOL found improvement in IBS symptoms from baseline in both the probiotic and the placebo groups, but the difference between the 2 groups was not statistically significant.32 Similarly, Wong and colleagues performed a double-blind, placebo-controlled mechanistic study to evaluate the effect of VSL#3. They found improvement in bowel symptom score, abdominal pain intensity, and satisfaction with bowel habits with both the VSL#3 and placebo group but similar to our study, the differences were not statistically significant.
Several reviews have evaluated the efficacy of probiotics for IBS. A 2010 review found evidence that probiotics trended toward improved IBS symptoms compared with placebo.33 The 2014 follow-up by the same authors demonstrated that overall, probiotics improved global symptoms of IBS and multistrain probiotics were more effective.20 A third meta-analysis from the same group found evidence that multistrain probiotics seemed to have a beneficial effect but could not definitively conclude that probiotics are efficacious in improving IBS symptoms.34 Other authors also have seen inconsistent effects of probiotics compared with placebo on global symptoms, abdominal pain, and bloating after performing systematic reviews of the literature.35-38 Although several reviews support that multistrain probiotics are more effective, they fail to conclude which combinations are more efficacious.
The effect of probiotics on QOL has not been investigated by many studies.37 In our study, we did not find significant improvement in QOL in the probiotic group, which is in line with 2 previous studies that showed no effect on IBS QOL of VSL#3 vs placebo.32,39 Most of the research reports that multistrain probiotics are more effective than using a single strain.34,35,40Bifidobacterium and Lactobacillus are the most commonly used bacteria in the multistrain probiotics that have shown their positive effect on IBS.35,41 The probiotic used in our study contained other species along with these 2 microorganisms.
The dose and duration of treatment of probiotics also has been debated. In one meta-analysis, the investigators found that studies of ≥ 8 weeks were more likely to show a positive effect; 4 of the 7 studies with statistically significant improvement in IBS symptoms were longer than 8 weeks.35 However, another meta-analysis based on 35 randomized controlled trials found that there was not a statistically significant difference between groups treated for > 4 weeks vs < 4 weeks.42 In addition, another meta-analysis of VSL#3 on IBS in children and adults also found no difference in results based on the duration of treatment of probiotics.43 Similar to our study, 3 other studies of VSL#3 treated patients for 8 weeks and found no statistically significant effect.30-32 In the past, VSL#3 has been used at dosages of 450 or 900 billion bacteria per day.
An individual’s response to probiotics may depend on the subtype of IBS. However, most of the studies, like ours, included groups of all subtypes. It may be that probiotics are more effective in patients with moderate-to-severe symptoms. Most of our patients had milder symptoms, and we cannot discount how subjects with more severe disease may have responded to the drug. Interestingly, one study demonstrated that Lactobacillus was more effective in patients with moderately severe abdominal pain compared with mild symptoms.44
In our study, the probiotic did not improve PTSD symptoms or other extra-intestinal symptoms common in IBS and GWI. Similar to our study, Wong and colleagues did not find significant improvement of psychological and sleep scores after treatment with VSL#3.6 Similarly, there is evidence that alteration in gut microbiota is associated with health and diseases, but what specific alterations occur and whether they can be improved with probiotics remains unknown.45
Limitations
The inconsistent response to probiotics in various studies may be due to IBS heterogeneity. Furthermore, there are demographic differences between Gulf War veterans and patients enrolled in other studies: Gulf War veterans are predominantly male, many were deployed abroad and had a history of gastroenteritis during deployment, and were exposed to stressful situations.46 These factors may be involved in triggering or maintaining IBS in Gulf War veterans. A further limitation of our randomized trial is the relatively small sample size.
Conclusions
This study did not demonstrate statistically significant improvement in symptoms of IBS or improvement in QOL after treatment with a multistrain probiotic. We also did not find any improvement in symptoms of GWI or PTSD. There was no difference in psychological scores between the placebo and treatment groups, and it is unlikely that psychological factors confounded the response to treatment in this study.
The effectiveness of a probiotic may depend on the baseline gut microbiome of the individual and depend on the strain, amount, and frequency of bacteria used. A lack of response of the probiotics does not exclude gut viruses and fungi having a role in exacerbating GWI symptoms. It is also possible that the bacteria present or the dose of the probiotic used was not sufficient to improve symptoms. So far, the definitive benefit of probiotics has been demonstrated for only a few preparations, and none are approved by the US Food and Drug Administration for any disease. More research is needed to determine whether probiotics have any role in the treatment of IBS and GWI.
Acknowledgments
AKT received grant support from the US Department of Veterans Affairs and the US Department of Defense (W81XWH-10-1-0593, W81XWH-15-1-0636). We thank Keith G. Tolman, MD, for assistance in editing the initial proposal and for periodic consultation. We thank the manufacturer of the probiotic for supplying the active drug and the placebo. The manufacture of the probiotic had no role in the design and conduct of the study, analysis and interpretation of the data, and in the preparation of the manuscript.
1. O’Shea EF, Cotter PD, Stanton C, Ross RP, Hill C. Production of bioactive substances by intestinal bacteria as a basis for explaining probiotic mechanisms: bacteriocins and conjugated linoleic acid. Int J Food Microbiol. 2012;152(3):189-205. doi:10.1016/j.ijfoodmicro.2011.05.025.
2. Kamiya T, Wang L, Forsythe P, et al. Inhibitory effects of Lactobacillus reuteri on visceral pain induced by colorectal distension in Sprague-Dawley rats. Gut. 2006;55(2):191-196. doi:10.1136/gut.2005.070987.
3. Verdu EF, Bercik P, Verma-Gandhu M, et al. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut. 2006;55(2):182-190. doi:10.1136/gut.2005.066100
4. Ford AC, Harris LA, Lacy BE, Quigley EMM, Moayyedi P. Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharmacol Ther. 2018;48(10):1044-1060. doi:10.1111/apt.15001.
5. Niu HL, Xiao JY. The efficacy and safety of probiotics in patients with irritable bowel syndrome: Evidence based on 35 randomized controlled trials. Int J Surg. 2020;75:116-127. doi:10.1016/j.ijsu.2020.01.142.
6. Wong RK, Yang C, Song GH, Wong J, Ho KY. Melatonin regulation as a possible mechanism for probiotic (VSL#3) in irritable bowel syndrome: a randomized double-blinded placebo study. Dig Dis Sci. 2015;60(1):186-194. doi:10.1007/s10620-014-3299-8.
7. Hyams KC, Bourgeois AL, Merrell BR, et al. Diarrheal disease during Operation Desert Shield. N Engl J Med. 1991;325(20):1423-1428. doi:10.1056/NEJM199111143252006 8. Clancy RL, Gleeson M, Cox A, et al. Reversal in fatigued athletes of a defect in interferon gamma secretion after administration of Lactobacillus acidophilus. Br J Sports Med. 2006;40(4):351-354. doi:10.1136/bjsm.2005.024364
9. Sullivan A, Nord CE, Evengard B. Effect of supplement with lactic-acid producing bacteria on fatigue and physical activity in patients with chronic fatigue syndrome. Nutr J. 2009;8:4. doi:10.1186/1475-2891-8-4
10. Pittayanon R, Lau JT, Yuan Y, et al. Gut microbiota in patients with irritable bowel syndrome—a systematic review. Gastroenterology. 2019;157(1):97-108. doi:10.1053/j.gastro.2019.03.049
11. Rao RK, Samak G. Protection and restitution of gut barrier by probiotics: nutritional and clinical implications. Curr Nutr Food Sci. 2013;9(2):99-107. doi:10.2174/1573401311309020004
12. O´Shea EF, Cotter PD, Stanton C, Ross RP, Hill C. Production of bioactive substances by intestinal bacteria as a basis for explaining probiotic mechanisms: bacteriocins and conjugated linoleic acid. Int J Food Microbiol. 2012;152(3):189-205. doi:10.1016/j.ijfoodmicro.2011.05.025
13. Kamiya T, Wang L, Forsythe P, et al. Inhibitory effects of Lactobacillus reuteri on visceral pain induced by colorectal distension in Sprague-Dawley rats. Gut. 2006;55(2):191-196. doi:10.1136/gut.2005.070987
14. Verdu EF, Bercik P, Verma-Gandhu M, et al. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut. 2006;55(2):182-190. doi:10.1136/gut.2005.06610015. O´Mahony L, McCarthy J, Kelly P, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128(3):541-551. doi:10.1053/j.gastro.2004.11.050
16. Alhasson F, Das S, Seth R, et al. Altered gut microbiome in a mouse model of Gulf War Illness causes neuroinflammation and intestinal injury via leaky gut and TLR4 activation. PLoS One. 2017;12(3):e0172914. doi:10.1371/journal.pone.0172914.17. Janulewicz PA, Seth RK, Carlson JM, et al. The gut-microbiome in Gulf War veterans: a preliminary report. Int J Environ Res Public Health. 2019;16(19). doi:10.3390/ijerph16193751
18. Dang X, Xu M, Liu D, Zhou D, Yang W. Assessing the efficacy and safety of fecal microbiota transplantation and probiotic VSL#3 for active ulcerative colitis: a systematic review and meta-analysis. PLoS One. 2020;15(3):e0228846. doi:10.1371/journal.pone.0228846
19. Ford AC, Quigley EM, Lacy BE, et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol. 2014;109(10):1547-1561; quiz 1546, 1562. doi:10.1038/ajg.2014.202
20. Rohatgi S, Ahuja V, Makharia GK, et al. VSL#3 induces and maintains short-term clinical response in patients with active microscopic colitis: a two-phase randomised clinical trial. BMJ Open Gastroenterol. 2015;2(1):e000018. doi:10.1136/bmjgast-2014-000018
21. Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130(5):1480-1491. doi:10.1053/j.gastro.2005.11.061
22. Talley NJ, Phillips SF, Melton J, 3rd, Wiltgen C, Zinsmeister AR. A patient questionnaire to identify bowel disease. Ann Intern Med. 1989;111(8):671-674. doi:10.7326/0003-4819-111-8-671
23. Bensoussan A, Talley NJ, Hing M, Menzies R, Guo A, Ngu M. Treatment of irritable bowel syndrome with Chinese herbal medicine: a randomized controlled trial. JAMA. 1998;280(18):1585-1589. doi:10.1001/jama.280.18.1585
24. Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11(2):395-402. doi:10.1046/j.1365-2036.1997.142318000.x
25. Patrick DL, Drossman DA, Frederick IO, DiCesare J, Puder KL. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Dig Dis Sci. 1998;43(2):400-411. doi:10.1023/a:1018831127942
26. Attanasio V, Andrasik F, Blanchard EB, Arena JG. Psychometric properties of the SUNYA revision of the Psychosomatic Symptom Checklist. J Behav Med. 1984;7(2):247-257. doi:10.1007/BF00845390
27. Weathers F, Litz B, Herman D, Huska J, Keane T. The PTSD Checklist (PCL): reliability, validity, and diagnostic utility. Accessed August 25, 2022. https://www.researchgate.net/publication/291448760_The_PTSD_Checklist_PCL_Reliability_validity_and_diagnostic_utility
28. Derogatis L. Brief Symptom Inventory-18 (BSI-18): Administration, Scoring, and Procedure Manual. Ed 3 ed. National Computer Systems; 2000.
29. Stamps BW, Lyon WJ, Irvin AP, Kelley-Loughnane N, Goodson MS. A pilot study of the effect of deployment on the gut microbiome and traveler´s diarrhea susceptibility. Front Cell Infect Microbiol. 2020;10:589297. doi:10.3389/fcimb.2020.589297
30. Kim HJ, Camilleri M, McKinzie S, et al. A randomized controlled trial of a probiotic, VSL#3, on gut transit and symptoms in diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2003;17(7):895-904. doi:10.1046/j.1365-2036.2003.01543.x
31. Kim HJ, Vazquez Roque MI, Camilleri M, et al. A randomized controlled trial of a probiotic combination VSL# 3 and placebo in irritable bowel syndrome with bloating. Neurogastroenterol Motil. 2005;17(5):687-696. doi:10.1111/j.1365-2982.2005.00695.x32. Michail S, Kenche H. Gut microbiota is not modified by randomized, double-blind, placebo-controlled trial of vsl#3 in diarrhea-predominant irritable bowel syndrome. Probiotics Antimicrob Proteins. 2011;3(1):1-7. doi:10.1007/s12602-010-9059-y
33. Moayyedi P, Ford AC, Talley NJ, et al. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010;59(3):325-332. doi:10.1136/gut.2008.167270

34. Ford AC, Harris LA, Lacy BE, Quigley EMM, Moayyedi P. Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharmacol Ther. 2018;48(10):1044-1060. doi:10.1111/apt.15001
35. Dale HF, Rasmussen SH, Asiller OO, Lied GA. Probiotics in irritable bowel syndrome: an up-to-date systematic review. Nutrients. 2019;11(9). doi:10.3390/nu11092048
36. Didari T, Mozaffari S, Nikfar S, Abdollahi M. Effectiveness of probiotics in irritable bowel syndrome: Updated systematic review with meta-analysis. World J Gastroenterol. 2015;21(10):3072-84. doi:10.3748/wjg.v21.i10.3072
37. Hungin APS, Mitchell CR, Whorwell P, et al. Systematic review: probiotics in the management of lower gastrointestinal symptoms—an updated evidence-based international consensus. Aliment Pharmacol Ther. 2018;47(8):1054-1070. doi:10.1111/apt.14539
38. Niu HL, Xiao JY. The efficacy and safety of probiotics in patients with irritable bowel syndrome: evidence based on 35 randomized controlled trials. Int J Surg. 2020;75:116-127. doi:10.1016/j.ijsu.2020.01.142
39. Wong RK, Yang C, Song GH, Wong J, Ho KY. Melatonin regulation as a possible mechanism for probiotic (VSL#3) in irritable bowel syndrome: a randomized double-blinded placebo study. Dig Dis Sci. 2015;60(1):186-194. doi:10.1007/s10620-014-3299-8
40. Ford AC, Moayyedi P, Lacy BE, et al. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol. 2014;109(suppl 1):S2-26; quiz S27. doi: 10.1038/ajg.2014.187
41. Simren M, Barbara G, Flint HJ, et al. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut. 2013;62(1):159-76. doi:10.1136/gutjnl-2012-302167
42. Ki Cha B, Mun Jung S, Hwan Choi C, et al. The effect of a multispecies probiotic mixture on the symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Clin Gastroenterol. 2012;46(3):220-7. doi:10.1097/MCG.0b013e31823712b1
43. Connell M, Shin A, James-Stevenson T, Xu H, Imperiale TF, Herron J. Systematic review and meta-analysis: Efficacy of patented probiotic, VSL#3, in irritable bowel syndrome. Neurogastroenterol Motil. 2018;30(12):e13427. doi:10.1111/nmo.13427
44. Lyra A, Hillila M, Huttunen T, et al. Irritable bowel syndrome symptom severity improves equally with probiotic and placebo. World J Gastroenterol. 2016;22(48):10631-10642. doi:10.3748/wjg.v22.i48.10631
45. Sanders ME, Guarner F, Guerrant R, et al. An update on the use and investigation of probiotics in health and disease. Gut. 2013;62(5):787-796. doi:10.1136/gutjnl-2012-302504
46. Tuteja AK. Deployment-associated functional gastrointestinal disorders: do we know the etiology? Dig Dis Sci. 2011;56(11):3109-3111. doi:10.1007/s10620-011-1856-y
1. O’Shea EF, Cotter PD, Stanton C, Ross RP, Hill C. Production of bioactive substances by intestinal bacteria as a basis for explaining probiotic mechanisms: bacteriocins and conjugated linoleic acid. Int J Food Microbiol. 2012;152(3):189-205. doi:10.1016/j.ijfoodmicro.2011.05.025.
2. Kamiya T, Wang L, Forsythe P, et al. Inhibitory effects of Lactobacillus reuteri on visceral pain induced by colorectal distension in Sprague-Dawley rats. Gut. 2006;55(2):191-196. doi:10.1136/gut.2005.070987.
3. Verdu EF, Bercik P, Verma-Gandhu M, et al. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut. 2006;55(2):182-190. doi:10.1136/gut.2005.066100
4. Ford AC, Harris LA, Lacy BE, Quigley EMM, Moayyedi P. Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharmacol Ther. 2018;48(10):1044-1060. doi:10.1111/apt.15001.
5. Niu HL, Xiao JY. The efficacy and safety of probiotics in patients with irritable bowel syndrome: Evidence based on 35 randomized controlled trials. Int J Surg. 2020;75:116-127. doi:10.1016/j.ijsu.2020.01.142.
6. Wong RK, Yang C, Song GH, Wong J, Ho KY. Melatonin regulation as a possible mechanism for probiotic (VSL#3) in irritable bowel syndrome: a randomized double-blinded placebo study. Dig Dis Sci. 2015;60(1):186-194. doi:10.1007/s10620-014-3299-8.
7. Hyams KC, Bourgeois AL, Merrell BR, et al. Diarrheal disease during Operation Desert Shield. N Engl J Med. 1991;325(20):1423-1428. doi:10.1056/NEJM199111143252006 8. Clancy RL, Gleeson M, Cox A, et al. Reversal in fatigued athletes of a defect in interferon gamma secretion after administration of Lactobacillus acidophilus. Br J Sports Med. 2006;40(4):351-354. doi:10.1136/bjsm.2005.024364
9. Sullivan A, Nord CE, Evengard B. Effect of supplement with lactic-acid producing bacteria on fatigue and physical activity in patients with chronic fatigue syndrome. Nutr J. 2009;8:4. doi:10.1186/1475-2891-8-4
10. Pittayanon R, Lau JT, Yuan Y, et al. Gut microbiota in patients with irritable bowel syndrome—a systematic review. Gastroenterology. 2019;157(1):97-108. doi:10.1053/j.gastro.2019.03.049
11. Rao RK, Samak G. Protection and restitution of gut barrier by probiotics: nutritional and clinical implications. Curr Nutr Food Sci. 2013;9(2):99-107. doi:10.2174/1573401311309020004
12. O´Shea EF, Cotter PD, Stanton C, Ross RP, Hill C. Production of bioactive substances by intestinal bacteria as a basis for explaining probiotic mechanisms: bacteriocins and conjugated linoleic acid. Int J Food Microbiol. 2012;152(3):189-205. doi:10.1016/j.ijfoodmicro.2011.05.025
13. Kamiya T, Wang L, Forsythe P, et al. Inhibitory effects of Lactobacillus reuteri on visceral pain induced by colorectal distension in Sprague-Dawley rats. Gut. 2006;55(2):191-196. doi:10.1136/gut.2005.070987
14. Verdu EF, Bercik P, Verma-Gandhu M, et al. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut. 2006;55(2):182-190. doi:10.1136/gut.2005.06610015. O´Mahony L, McCarthy J, Kelly P, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128(3):541-551. doi:10.1053/j.gastro.2004.11.050
16. Alhasson F, Das S, Seth R, et al. Altered gut microbiome in a mouse model of Gulf War Illness causes neuroinflammation and intestinal injury via leaky gut and TLR4 activation. PLoS One. 2017;12(3):e0172914. doi:10.1371/journal.pone.0172914.17. Janulewicz PA, Seth RK, Carlson JM, et al. The gut-microbiome in Gulf War veterans: a preliminary report. Int J Environ Res Public Health. 2019;16(19). doi:10.3390/ijerph16193751
18. Dang X, Xu M, Liu D, Zhou D, Yang W. Assessing the efficacy and safety of fecal microbiota transplantation and probiotic VSL#3 for active ulcerative colitis: a systematic review and meta-analysis. PLoS One. 2020;15(3):e0228846. doi:10.1371/journal.pone.0228846
19. Ford AC, Quigley EM, Lacy BE, et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol. 2014;109(10):1547-1561; quiz 1546, 1562. doi:10.1038/ajg.2014.202
20. Rohatgi S, Ahuja V, Makharia GK, et al. VSL#3 induces and maintains short-term clinical response in patients with active microscopic colitis: a two-phase randomised clinical trial. BMJ Open Gastroenterol. 2015;2(1):e000018. doi:10.1136/bmjgast-2014-000018
21. Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130(5):1480-1491. doi:10.1053/j.gastro.2005.11.061
22. Talley NJ, Phillips SF, Melton J, 3rd, Wiltgen C, Zinsmeister AR. A patient questionnaire to identify bowel disease. Ann Intern Med. 1989;111(8):671-674. doi:10.7326/0003-4819-111-8-671
23. Bensoussan A, Talley NJ, Hing M, Menzies R, Guo A, Ngu M. Treatment of irritable bowel syndrome with Chinese herbal medicine: a randomized controlled trial. JAMA. 1998;280(18):1585-1589. doi:10.1001/jama.280.18.1585
24. Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11(2):395-402. doi:10.1046/j.1365-2036.1997.142318000.x
25. Patrick DL, Drossman DA, Frederick IO, DiCesare J, Puder KL. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Dig Dis Sci. 1998;43(2):400-411. doi:10.1023/a:1018831127942
26. Attanasio V, Andrasik F, Blanchard EB, Arena JG. Psychometric properties of the SUNYA revision of the Psychosomatic Symptom Checklist. J Behav Med. 1984;7(2):247-257. doi:10.1007/BF00845390
27. Weathers F, Litz B, Herman D, Huska J, Keane T. The PTSD Checklist (PCL): reliability, validity, and diagnostic utility. Accessed August 25, 2022. https://www.researchgate.net/publication/291448760_The_PTSD_Checklist_PCL_Reliability_validity_and_diagnostic_utility
28. Derogatis L. Brief Symptom Inventory-18 (BSI-18): Administration, Scoring, and Procedure Manual. Ed 3 ed. National Computer Systems; 2000.
29. Stamps BW, Lyon WJ, Irvin AP, Kelley-Loughnane N, Goodson MS. A pilot study of the effect of deployment on the gut microbiome and traveler´s diarrhea susceptibility. Front Cell Infect Microbiol. 2020;10:589297. doi:10.3389/fcimb.2020.589297
30. Kim HJ, Camilleri M, McKinzie S, et al. A randomized controlled trial of a probiotic, VSL#3, on gut transit and symptoms in diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2003;17(7):895-904. doi:10.1046/j.1365-2036.2003.01543.x
31. Kim HJ, Vazquez Roque MI, Camilleri M, et al. A randomized controlled trial of a probiotic combination VSL# 3 and placebo in irritable bowel syndrome with bloating. Neurogastroenterol Motil. 2005;17(5):687-696. doi:10.1111/j.1365-2982.2005.00695.x32. Michail S, Kenche H. Gut microbiota is not modified by randomized, double-blind, placebo-controlled trial of vsl#3 in diarrhea-predominant irritable bowel syndrome. Probiotics Antimicrob Proteins. 2011;3(1):1-7. doi:10.1007/s12602-010-9059-y
33. Moayyedi P, Ford AC, Talley NJ, et al. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010;59(3):325-332. doi:10.1136/gut.2008.167270

34. Ford AC, Harris LA, Lacy BE, Quigley EMM, Moayyedi P. Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharmacol Ther. 2018;48(10):1044-1060. doi:10.1111/apt.15001
35. Dale HF, Rasmussen SH, Asiller OO, Lied GA. Probiotics in irritable bowel syndrome: an up-to-date systematic review. Nutrients. 2019;11(9). doi:10.3390/nu11092048
36. Didari T, Mozaffari S, Nikfar S, Abdollahi M. Effectiveness of probiotics in irritable bowel syndrome: Updated systematic review with meta-analysis. World J Gastroenterol. 2015;21(10):3072-84. doi:10.3748/wjg.v21.i10.3072
37. Hungin APS, Mitchell CR, Whorwell P, et al. Systematic review: probiotics in the management of lower gastrointestinal symptoms—an updated evidence-based international consensus. Aliment Pharmacol Ther. 2018;47(8):1054-1070. doi:10.1111/apt.14539
38. Niu HL, Xiao JY. The efficacy and safety of probiotics in patients with irritable bowel syndrome: evidence based on 35 randomized controlled trials. Int J Surg. 2020;75:116-127. doi:10.1016/j.ijsu.2020.01.142
39. Wong RK, Yang C, Song GH, Wong J, Ho KY. Melatonin regulation as a possible mechanism for probiotic (VSL#3) in irritable bowel syndrome: a randomized double-blinded placebo study. Dig Dis Sci. 2015;60(1):186-194. doi:10.1007/s10620-014-3299-8
40. Ford AC, Moayyedi P, Lacy BE, et al. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol. 2014;109(suppl 1):S2-26; quiz S27. doi: 10.1038/ajg.2014.187
41. Simren M, Barbara G, Flint HJ, et al. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut. 2013;62(1):159-76. doi:10.1136/gutjnl-2012-302167
42. Ki Cha B, Mun Jung S, Hwan Choi C, et al. The effect of a multispecies probiotic mixture on the symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Clin Gastroenterol. 2012;46(3):220-7. doi:10.1097/MCG.0b013e31823712b1
43. Connell M, Shin A, James-Stevenson T, Xu H, Imperiale TF, Herron J. Systematic review and meta-analysis: Efficacy of patented probiotic, VSL#3, in irritable bowel syndrome. Neurogastroenterol Motil. 2018;30(12):e13427. doi:10.1111/nmo.13427
44. Lyra A, Hillila M, Huttunen T, et al. Irritable bowel syndrome symptom severity improves equally with probiotic and placebo. World J Gastroenterol. 2016;22(48):10631-10642. doi:10.3748/wjg.v22.i48.10631
45. Sanders ME, Guarner F, Guerrant R, et al. An update on the use and investigation of probiotics in health and disease. Gut. 2013;62(5):787-796. doi:10.1136/gutjnl-2012-302504
46. Tuteja AK. Deployment-associated functional gastrointestinal disorders: do we know the etiology? Dig Dis Sci. 2011;56(11):3109-3111. doi:10.1007/s10620-011-1856-y
Despite benefits, extended-interval pembro uptake remains low
In April 2020, the Food and Drug Administration approved extended dosing for standalone pembrolizumab – 400 mg every 6 weeks instead of the standard dosing of 200 mg every 3 weeks. The shift came, in part, to reduce patient health care encounters during the early days of the COVID-19 pandemic, but also because fewer infusions save patients time and out-of-pocket costs and reduce the burden on the health care system.
The FDA deemed this move safe after pharmacologic studies and a small melanoma study found that responses and adverse events were equivalent in comparison with standard dosing.
Given the benefits, one would expect “brisk adoption” of extended-interval dosing, Garth Strohbehn, MD, an oncologist at the VA Medical Center in Ann Arbor, Mich., and colleagues wrote in a recent report in JAMA Oncology.
However, when the team reviewed data on 835 veterans from the Veterans Health Administration who began taking single-agent pembrolizumab between April 1, 2020, and July 1, 2021, only about one-third received extended-interval dosing.
Between April and January 2021, use of extended-interval dosing rose steadily to about 35% of patients but then hovered in that range through August 2021.
Among the patients, age, sex, Charlson comorbidity index, and pembrolizumab indications were well balanced between the standard-dosing and the extended-interval dosing groups.
Notably, Dr. Strohbehn and colleagues also found no difference in time-to-treatment discontinuation between patients receiving extended dosing in comparison with patients receiving standard dosing, which is “a real-world measure of clinical effectiveness,” the team said.
And there was no difference in immune-related side effects between the two regimens, as assessed by incident levothyroxine and prednisone prescriptions.
The real-world near equivalence of extended and standard dosing intervals that was demonstrated in the study is “reassuring” and helps make the case for considering it “as a best practice” for single-agent pembrolizumab, the investigators wrote.
Dr. Strohbehn remained somewhat puzzled by the low uptake of the extended-dosing option.
“I was frankly surprised by the small number of patients who received the extended-interval regimen,” Dr. Strohbehn said in an interview.
“Admittedly, there are patients who would prefer to receive standard-interval therapy, and that preference should of course be accommodated whenever possible, but in my experience, those numbers are small,” at least in the VA system, he noted.
In addition, the authors noted, there is no direct financial incentive for more frequent dosing in the VA system.
It’s possible that low uptake could stem from clinicians’ doubts about switching to an extended-interval dose, given that the FDA’s approval was based largely on a study of 44 patients with melanoma in a single-arm trial.
If that is indeed the case, the new findings – which represent the first health system–level, real-world comparative effectiveness data for standard vs. extended-interval pembrolizumab – should help address these concerns, the team said.
“This observational dataset lends further credence to [the dosing] regimens being clinically equivalent,” said Zachery Reichert, MD, PhD, a urologic oncologist at the University of Michigan, Ann Arbor, who was not involved in the study.
To address the issue, Dr. Strohbehn and his team suggested “clinical guideline promotion to overcome some of the barriers to the adoption of extended-interval pembrolizumab.”
Dr. Riechert suggested further validation of equivalent outcomes for the two regimens, more advocacy to encourage patients to ask about the 6-week option, as well as incentives from insurers to adopt it.
Dr. Strohbehn added that the situation highlights a broader issue in oncology, namely that many drugs “end up on the market with dosing regimens that haven’t necessarily been optimized.”
Across the world, investigators are conducting clinical trials “to identify the minimum dosages, frequencies, and durations patients need in order to achieve their best outcome,” Dr. Strohbehn said. In oncology, much of this effort is being led by Project Optimus, from the FDA’s Oncology Center of Excellence, he said.
The study was funded by the VA National Oncology Program. Dr. Reichert and Dr. Strohbehn have disclosed no relevant financial relationships. One investigator has received grants from Novartis, Bristol-Myers Squibb, Regeneron, and Genentech.
A version of this article first appeared on Medscape.com.
In April 2020, the Food and Drug Administration approved extended dosing for standalone pembrolizumab – 400 mg every 6 weeks instead of the standard dosing of 200 mg every 3 weeks. The shift came, in part, to reduce patient health care encounters during the early days of the COVID-19 pandemic, but also because fewer infusions save patients time and out-of-pocket costs and reduce the burden on the health care system.
The FDA deemed this move safe after pharmacologic studies and a small melanoma study found that responses and adverse events were equivalent in comparison with standard dosing.
Given the benefits, one would expect “brisk adoption” of extended-interval dosing, Garth Strohbehn, MD, an oncologist at the VA Medical Center in Ann Arbor, Mich., and colleagues wrote in a recent report in JAMA Oncology.
However, when the team reviewed data on 835 veterans from the Veterans Health Administration who began taking single-agent pembrolizumab between April 1, 2020, and July 1, 2021, only about one-third received extended-interval dosing.
Between April and January 2021, use of extended-interval dosing rose steadily to about 35% of patients but then hovered in that range through August 2021.
Among the patients, age, sex, Charlson comorbidity index, and pembrolizumab indications were well balanced between the standard-dosing and the extended-interval dosing groups.
Notably, Dr. Strohbehn and colleagues also found no difference in time-to-treatment discontinuation between patients receiving extended dosing in comparison with patients receiving standard dosing, which is “a real-world measure of clinical effectiveness,” the team said.
And there was no difference in immune-related side effects between the two regimens, as assessed by incident levothyroxine and prednisone prescriptions.
The real-world near equivalence of extended and standard dosing intervals that was demonstrated in the study is “reassuring” and helps make the case for considering it “as a best practice” for single-agent pembrolizumab, the investigators wrote.
Dr. Strohbehn remained somewhat puzzled by the low uptake of the extended-dosing option.
“I was frankly surprised by the small number of patients who received the extended-interval regimen,” Dr. Strohbehn said in an interview.
“Admittedly, there are patients who would prefer to receive standard-interval therapy, and that preference should of course be accommodated whenever possible, but in my experience, those numbers are small,” at least in the VA system, he noted.
In addition, the authors noted, there is no direct financial incentive for more frequent dosing in the VA system.
It’s possible that low uptake could stem from clinicians’ doubts about switching to an extended-interval dose, given that the FDA’s approval was based largely on a study of 44 patients with melanoma in a single-arm trial.
If that is indeed the case, the new findings – which represent the first health system–level, real-world comparative effectiveness data for standard vs. extended-interval pembrolizumab – should help address these concerns, the team said.
“This observational dataset lends further credence to [the dosing] regimens being clinically equivalent,” said Zachery Reichert, MD, PhD, a urologic oncologist at the University of Michigan, Ann Arbor, who was not involved in the study.
To address the issue, Dr. Strohbehn and his team suggested “clinical guideline promotion to overcome some of the barriers to the adoption of extended-interval pembrolizumab.”
Dr. Riechert suggested further validation of equivalent outcomes for the two regimens, more advocacy to encourage patients to ask about the 6-week option, as well as incentives from insurers to adopt it.
Dr. Strohbehn added that the situation highlights a broader issue in oncology, namely that many drugs “end up on the market with dosing regimens that haven’t necessarily been optimized.”
Across the world, investigators are conducting clinical trials “to identify the minimum dosages, frequencies, and durations patients need in order to achieve their best outcome,” Dr. Strohbehn said. In oncology, much of this effort is being led by Project Optimus, from the FDA’s Oncology Center of Excellence, he said.
The study was funded by the VA National Oncology Program. Dr. Reichert and Dr. Strohbehn have disclosed no relevant financial relationships. One investigator has received grants from Novartis, Bristol-Myers Squibb, Regeneron, and Genentech.
A version of this article first appeared on Medscape.com.
In April 2020, the Food and Drug Administration approved extended dosing for standalone pembrolizumab – 400 mg every 6 weeks instead of the standard dosing of 200 mg every 3 weeks. The shift came, in part, to reduce patient health care encounters during the early days of the COVID-19 pandemic, but also because fewer infusions save patients time and out-of-pocket costs and reduce the burden on the health care system.
The FDA deemed this move safe after pharmacologic studies and a small melanoma study found that responses and adverse events were equivalent in comparison with standard dosing.
Given the benefits, one would expect “brisk adoption” of extended-interval dosing, Garth Strohbehn, MD, an oncologist at the VA Medical Center in Ann Arbor, Mich., and colleagues wrote in a recent report in JAMA Oncology.
However, when the team reviewed data on 835 veterans from the Veterans Health Administration who began taking single-agent pembrolizumab between April 1, 2020, and July 1, 2021, only about one-third received extended-interval dosing.
Between April and January 2021, use of extended-interval dosing rose steadily to about 35% of patients but then hovered in that range through August 2021.
Among the patients, age, sex, Charlson comorbidity index, and pembrolizumab indications were well balanced between the standard-dosing and the extended-interval dosing groups.
Notably, Dr. Strohbehn and colleagues also found no difference in time-to-treatment discontinuation between patients receiving extended dosing in comparison with patients receiving standard dosing, which is “a real-world measure of clinical effectiveness,” the team said.
And there was no difference in immune-related side effects between the two regimens, as assessed by incident levothyroxine and prednisone prescriptions.
The real-world near equivalence of extended and standard dosing intervals that was demonstrated in the study is “reassuring” and helps make the case for considering it “as a best practice” for single-agent pembrolizumab, the investigators wrote.
Dr. Strohbehn remained somewhat puzzled by the low uptake of the extended-dosing option.
“I was frankly surprised by the small number of patients who received the extended-interval regimen,” Dr. Strohbehn said in an interview.
“Admittedly, there are patients who would prefer to receive standard-interval therapy, and that preference should of course be accommodated whenever possible, but in my experience, those numbers are small,” at least in the VA system, he noted.
In addition, the authors noted, there is no direct financial incentive for more frequent dosing in the VA system.
It’s possible that low uptake could stem from clinicians’ doubts about switching to an extended-interval dose, given that the FDA’s approval was based largely on a study of 44 patients with melanoma in a single-arm trial.
If that is indeed the case, the new findings – which represent the first health system–level, real-world comparative effectiveness data for standard vs. extended-interval pembrolizumab – should help address these concerns, the team said.
“This observational dataset lends further credence to [the dosing] regimens being clinically equivalent,” said Zachery Reichert, MD, PhD, a urologic oncologist at the University of Michigan, Ann Arbor, who was not involved in the study.
To address the issue, Dr. Strohbehn and his team suggested “clinical guideline promotion to overcome some of the barriers to the adoption of extended-interval pembrolizumab.”
Dr. Riechert suggested further validation of equivalent outcomes for the two regimens, more advocacy to encourage patients to ask about the 6-week option, as well as incentives from insurers to adopt it.
Dr. Strohbehn added that the situation highlights a broader issue in oncology, namely that many drugs “end up on the market with dosing regimens that haven’t necessarily been optimized.”
Across the world, investigators are conducting clinical trials “to identify the minimum dosages, frequencies, and durations patients need in order to achieve their best outcome,” Dr. Strohbehn said. In oncology, much of this effort is being led by Project Optimus, from the FDA’s Oncology Center of Excellence, he said.
The study was funded by the VA National Oncology Program. Dr. Reichert and Dr. Strohbehn have disclosed no relevant financial relationships. One investigator has received grants from Novartis, Bristol-Myers Squibb, Regeneron, and Genentech.
A version of this article first appeared on Medscape.com.
FROM JAMA ONCOLOGY
New ESC guidelines for cutting CV risk in noncardiac surgery
The European Society of Cardiology guidelines on cardiovascular assessment and management of patients undergoing noncardiac surgery have seen extensive revision since the 2014 version.
They still have the same aim – to prevent surgery-related bleeding complications, perioperative myocardial infarction/injury (PMI), stent thrombosis, acute heart failure, arrhythmias, pulmonary embolism, ischemic stroke, and cardiovascular (CV) death.
Cochairpersons Sigrun Halvorsen, MD, PhD, and Julinda Mehilli, MD, presented highlights from the guidelines at the annual congress of the European Society of Cardiology and the document was simultaneously published online in the European Heart Journal.
The document classifies noncardiac surgery into three levels of 30-day risk of CV death, MI, or stroke. Low (< 1%) risk includes eye or thyroid surgery; intermediate (1%-5%) risk includes knee or hip replacement or renal transplant; and high (> 5%) risk includes aortic aneurysm, lung transplant, or pancreatic or bladder cancer surgery (see more examples below).
It classifies patients as low risk if they are younger than 65 without CV disease or CV risk factors (smoking, hypertension, diabetes, dyslipidemia, family history); intermediate risk if they are 65 or older or have CV risk factors; and high risk if they have CVD.
In an interview, Dr. Halvorsen, professor in cardiology, University of Oslo, zeroed in on three important revisions:
First, recommendations for preoperative ECG and biomarkers are more specific, he noted.
The guidelines advise that before intermediate- or high-risk noncardiac surgery, in patients who have known CVD, CV risk factors (including age 65 or older), or symptoms suggestive of CVD:
- It is recommended to obtain a preoperative 12-lead ECG (class I).
- It is recommended to measure high-sensitivity cardiac troponin T (hs-cTn T) or high-sensitivity cardiac troponin I (hs-cTn I). It is also recommended to measure these biomarkers at 24 hours and 48 hours post surgery (class I).
- It should be considered to measure B-type natriuretic peptide or N-terminal of the prohormone BNP (NT-proBNP).
However, for low-risk patients undergoing low- and intermediate-risk noncardiac surgery, it is not recommended to routinely obtain preoperative ECG, hs-cTn T/I, or BNP/NT-proBNP concentrations (class III).
Troponins have a stronger class I recommendation, compared with the IIA recommendation for BNP, because they are useful for preoperative risk stratification and for diagnosis of PMI, Dr. Halvorsen explained. “Patients receive painkillers after surgery and may have no pain,” she noted, but they may have PMI, which has a bad prognosis.
Second, the guidelines recommend that “all patients should stop smoking 4 weeks before noncardiac surgery [class I],” she noted. Clinicians should also “measure hemoglobin, and if the patient is anemic, treat the anemia.”
Third, the sections on antithrombotic treatment have been significantly revised. “Bridging – stopping an oral antithrombotic drug and switching to a subcutaneous or IV drug – has been common,” Dr. Halvorsen said, “but recently we have new evidence that in most cases that increases the risk of bleeding.”
“We are [now] much more restrictive with respect to bridging” with unfractionated heparin or low-molecular-weight heparin, she said. “We recommend against bridging in patients with low to moderate thrombotic risk,” and bridging should only be considered in patients with mechanical prosthetic heart valves or with very high thrombotic risk.
More preoperative recommendations
In the guideline overview session at the congress, Dr. Halverson highlighted some of the new recommendations for preoperative risk assessment.
If time allows, it is recommended to optimize guideline-recommended treatment of CVD and control of CV risk factors including blood pressure, dyslipidemia, and diabetes, before noncardiac surgery (class I).
Patients commonly have “murmurs, chest pain, dyspnea, and edema that may suggest severe CVD, but may also be caused by noncardiac disease,” she noted. The guidelines state that “for patients with a newly detected murmur and symptoms or signs of CVD, transthoracic echocardiography is recommended before noncardiac surgery (class I).
“Many studies have been performed to try to find out if initiation of specific drugs before surgery could reduce the risk of complications,” Dr. Halvorsen noted. However, few have shown any benefit and “the question of presurgery initiation of beta-blockers has been greatly debated,” she said. “We have again reviewed the literature and concluded ‘Routine initiation of beta-blockers perioperatively is not recommended (class IIIA).’ “
“We adhere to the guidelines on acute and chronic coronary syndrome recommending 6-12 months of dual antiplatelet treatment as a standard before elective surgery,” she said. “However, in case of time-sensitive surgery, the duration of that treatment can be shortened down to a minimum of 1 month after elective PCI and a minimum of 3 months after PCI and ACS.”
Patients with specific types of CVD
Dr. Mehilli, a professor at Landshut-Achdorf (Germany) Hospital, highlighted some new guideline recommendations for patients who have specific types of cardiovascular disease.
Coronary artery disease (CAD). “For chronic coronary syndrome, a cardiac workup is recommended only for patients undergoing intermediate risk or high-risk noncardiac surgery.”
“Stress imaging should be considered before any high risk, noncardiac surgery in asymptomatic patients with poor functional capacity and prior PCI or coronary artery bypass graft (new recommendation, class IIa).”
Mitral valve regurgitation. For patients undergoing scheduled noncardiac surgery, who remain symptomatic despite guideline-directed medical treatment for mitral valve regurgitation (including resynchronization and myocardial revascularization), consider a valve intervention – either transcatheter or surgical – before noncardiac surgery in eligible patients with acceptable procedural risk (new recommendation).
Cardiac implantable electronic devices (CIED). For high-risk patients with CIEDs undergoing noncardiac surgery with high probability of electromagnetic interference, a CIED checkup and necessary reprogramming immediately before the procedure should be considered (new recommendation).
Arrhythmias. “I want only to stress,” Dr. Mehilli said, “in patients with atrial fibrillation with acute or worsening hemodynamic instability undergoing noncardiac surgery, an emergency electrical cardioversion is recommended (class I).”
Peripheral artery disease (PAD) and abdominal aortic aneurysm. For these patients “we do not recommend a routine referral for a cardiac workup. But we recommend it for patients with poor functional capacity or with significant risk factors or symptoms (new recommendations).”
Chronic arterial hypertension. “We have modified the recommendation, recommending avoidance of large perioperative fluctuations in blood pressure, and we do not recommend deferring noncardiac surgery in patients with stage 1 or 2 hypertension,” she said.
Postoperative cardiovascular complications
The most frequent postoperative cardiovascular complication is PMI, Dr. Mehilli noted.
“In the BASEL-PMI registry, the incidence of this complication around intermediate or high-risk noncardiac surgery was up to 15% among patients older than 65 years or with a history of CAD or PAD, which makes this kind of complication really important to prevent, to assess, and to know how to treat.”
“It is recommended to have a high awareness for perioperative cardiovascular complications, combined with surveillance for PMI in patients undergoing intermediate- or high-risk noncardiac surgery” based on serial measurements of high-sensitivity cardiac troponin.
The guidelines define PMI as “an increase in the delta of high-sensitivity troponin more than the upper level of normal,” Dr. Mehilli said. “It’s different from the one used in a rule-in algorithm for non-STEMI acute coronary syndrome.”
Postoperative atrial fibrillation (AFib) is observed in 2%-30% of noncardiac surgery patients in different registries, particularly in patients undergoing intermediate or high-risk noncardiac surgery, she noted.
“We propose an algorithm on how to prevent and treat this complication. I want to highlight that in patients with hemodynamic unstable postoperative AF[ib], an emergency cardioversion is indicated. For the others, a rate control with the target heart rate of less than 110 beats per minute is indicated.”
In patients with postoperative AFib, long-term oral anticoagulation therapy should be considered in all patients at risk for stroke, considering the anticipated net clinical benefit of oral anticoagulation therapy as well as informed patient preference (new recommendations).
Routine use of beta-blockers to prevent postoperative AFib in patients undergoing noncardiac surgery is not recommended.
The document also covers the management of patients with kidney disease, diabetes, cancer, obesity, and COVID-19. In general, elective noncardiac surgery should be postponed after a patient has COVID-19, until he or she recovers completely, and coexisting conditions are optimized.
The guidelines are available from the ESC website in several formats: pocket guidelines, pocket guidelines smartphone app, guidelines slide set, essential messages, and the European Heart Journal article.
Noncardiac surgery risk categories
The guideline includes a table that classifies noncardiac surgeries into three groups, based on the associated 30-day risk of death, MI, or stroke:
- Low (< 1%): breast, dental, eye, thyroid, and minor gynecologic, orthopedic, and urologic surgery.
- Intermediate (1%-5%): carotid surgery, endovascular aortic aneurysm repair, gallbladder surgery, head or neck surgery, hernia repair, peripheral arterial angioplasty, renal transplant, major gynecologic, orthopedic, or neurologic (hip or spine) surgery, or urologic surgery
- High (> 5%): aortic and major vascular surgery (including aortic aneurysm), bladder removal (usually as a result of cancer), limb amputation, lung or liver transplant, pancreatic surgery, or perforated bowel repair.
The guidelines were endorsed by the European Society of Anaesthesiology and Intensive Care. The guideline authors reported numerous disclosures.
A version of this article first appeared on Medscape.com.
The European Society of Cardiology guidelines on cardiovascular assessment and management of patients undergoing noncardiac surgery have seen extensive revision since the 2014 version.
They still have the same aim – to prevent surgery-related bleeding complications, perioperative myocardial infarction/injury (PMI), stent thrombosis, acute heart failure, arrhythmias, pulmonary embolism, ischemic stroke, and cardiovascular (CV) death.
Cochairpersons Sigrun Halvorsen, MD, PhD, and Julinda Mehilli, MD, presented highlights from the guidelines at the annual congress of the European Society of Cardiology and the document was simultaneously published online in the European Heart Journal.
The document classifies noncardiac surgery into three levels of 30-day risk of CV death, MI, or stroke. Low (< 1%) risk includes eye or thyroid surgery; intermediate (1%-5%) risk includes knee or hip replacement or renal transplant; and high (> 5%) risk includes aortic aneurysm, lung transplant, or pancreatic or bladder cancer surgery (see more examples below).
It classifies patients as low risk if they are younger than 65 without CV disease or CV risk factors (smoking, hypertension, diabetes, dyslipidemia, family history); intermediate risk if they are 65 or older or have CV risk factors; and high risk if they have CVD.
In an interview, Dr. Halvorsen, professor in cardiology, University of Oslo, zeroed in on three important revisions:
First, recommendations for preoperative ECG and biomarkers are more specific, he noted.
The guidelines advise that before intermediate- or high-risk noncardiac surgery, in patients who have known CVD, CV risk factors (including age 65 or older), or symptoms suggestive of CVD:
- It is recommended to obtain a preoperative 12-lead ECG (class I).
- It is recommended to measure high-sensitivity cardiac troponin T (hs-cTn T) or high-sensitivity cardiac troponin I (hs-cTn I). It is also recommended to measure these biomarkers at 24 hours and 48 hours post surgery (class I).
- It should be considered to measure B-type natriuretic peptide or N-terminal of the prohormone BNP (NT-proBNP).
However, for low-risk patients undergoing low- and intermediate-risk noncardiac surgery, it is not recommended to routinely obtain preoperative ECG, hs-cTn T/I, or BNP/NT-proBNP concentrations (class III).
Troponins have a stronger class I recommendation, compared with the IIA recommendation for BNP, because they are useful for preoperative risk stratification and for diagnosis of PMI, Dr. Halvorsen explained. “Patients receive painkillers after surgery and may have no pain,” she noted, but they may have PMI, which has a bad prognosis.
Second, the guidelines recommend that “all patients should stop smoking 4 weeks before noncardiac surgery [class I],” she noted. Clinicians should also “measure hemoglobin, and if the patient is anemic, treat the anemia.”
Third, the sections on antithrombotic treatment have been significantly revised. “Bridging – stopping an oral antithrombotic drug and switching to a subcutaneous or IV drug – has been common,” Dr. Halvorsen said, “but recently we have new evidence that in most cases that increases the risk of bleeding.”
“We are [now] much more restrictive with respect to bridging” with unfractionated heparin or low-molecular-weight heparin, she said. “We recommend against bridging in patients with low to moderate thrombotic risk,” and bridging should only be considered in patients with mechanical prosthetic heart valves or with very high thrombotic risk.
More preoperative recommendations
In the guideline overview session at the congress, Dr. Halverson highlighted some of the new recommendations for preoperative risk assessment.
If time allows, it is recommended to optimize guideline-recommended treatment of CVD and control of CV risk factors including blood pressure, dyslipidemia, and diabetes, before noncardiac surgery (class I).
Patients commonly have “murmurs, chest pain, dyspnea, and edema that may suggest severe CVD, but may also be caused by noncardiac disease,” she noted. The guidelines state that “for patients with a newly detected murmur and symptoms or signs of CVD, transthoracic echocardiography is recommended before noncardiac surgery (class I).
“Many studies have been performed to try to find out if initiation of specific drugs before surgery could reduce the risk of complications,” Dr. Halvorsen noted. However, few have shown any benefit and “the question of presurgery initiation of beta-blockers has been greatly debated,” she said. “We have again reviewed the literature and concluded ‘Routine initiation of beta-blockers perioperatively is not recommended (class IIIA).’ “
“We adhere to the guidelines on acute and chronic coronary syndrome recommending 6-12 months of dual antiplatelet treatment as a standard before elective surgery,” she said. “However, in case of time-sensitive surgery, the duration of that treatment can be shortened down to a minimum of 1 month after elective PCI and a minimum of 3 months after PCI and ACS.”
Patients with specific types of CVD
Dr. Mehilli, a professor at Landshut-Achdorf (Germany) Hospital, highlighted some new guideline recommendations for patients who have specific types of cardiovascular disease.
Coronary artery disease (CAD). “For chronic coronary syndrome, a cardiac workup is recommended only for patients undergoing intermediate risk or high-risk noncardiac surgery.”
“Stress imaging should be considered before any high risk, noncardiac surgery in asymptomatic patients with poor functional capacity and prior PCI or coronary artery bypass graft (new recommendation, class IIa).”
Mitral valve regurgitation. For patients undergoing scheduled noncardiac surgery, who remain symptomatic despite guideline-directed medical treatment for mitral valve regurgitation (including resynchronization and myocardial revascularization), consider a valve intervention – either transcatheter or surgical – before noncardiac surgery in eligible patients with acceptable procedural risk (new recommendation).
Cardiac implantable electronic devices (CIED). For high-risk patients with CIEDs undergoing noncardiac surgery with high probability of electromagnetic interference, a CIED checkup and necessary reprogramming immediately before the procedure should be considered (new recommendation).
Arrhythmias. “I want only to stress,” Dr. Mehilli said, “in patients with atrial fibrillation with acute or worsening hemodynamic instability undergoing noncardiac surgery, an emergency electrical cardioversion is recommended (class I).”
Peripheral artery disease (PAD) and abdominal aortic aneurysm. For these patients “we do not recommend a routine referral for a cardiac workup. But we recommend it for patients with poor functional capacity or with significant risk factors or symptoms (new recommendations).”
Chronic arterial hypertension. “We have modified the recommendation, recommending avoidance of large perioperative fluctuations in blood pressure, and we do not recommend deferring noncardiac surgery in patients with stage 1 or 2 hypertension,” she said.
Postoperative cardiovascular complications
The most frequent postoperative cardiovascular complication is PMI, Dr. Mehilli noted.
“In the BASEL-PMI registry, the incidence of this complication around intermediate or high-risk noncardiac surgery was up to 15% among patients older than 65 years or with a history of CAD or PAD, which makes this kind of complication really important to prevent, to assess, and to know how to treat.”
“It is recommended to have a high awareness for perioperative cardiovascular complications, combined with surveillance for PMI in patients undergoing intermediate- or high-risk noncardiac surgery” based on serial measurements of high-sensitivity cardiac troponin.
The guidelines define PMI as “an increase in the delta of high-sensitivity troponin more than the upper level of normal,” Dr. Mehilli said. “It’s different from the one used in a rule-in algorithm for non-STEMI acute coronary syndrome.”
Postoperative atrial fibrillation (AFib) is observed in 2%-30% of noncardiac surgery patients in different registries, particularly in patients undergoing intermediate or high-risk noncardiac surgery, she noted.
“We propose an algorithm on how to prevent and treat this complication. I want to highlight that in patients with hemodynamic unstable postoperative AF[ib], an emergency cardioversion is indicated. For the others, a rate control with the target heart rate of less than 110 beats per minute is indicated.”
In patients with postoperative AFib, long-term oral anticoagulation therapy should be considered in all patients at risk for stroke, considering the anticipated net clinical benefit of oral anticoagulation therapy as well as informed patient preference (new recommendations).
Routine use of beta-blockers to prevent postoperative AFib in patients undergoing noncardiac surgery is not recommended.
The document also covers the management of patients with kidney disease, diabetes, cancer, obesity, and COVID-19. In general, elective noncardiac surgery should be postponed after a patient has COVID-19, until he or she recovers completely, and coexisting conditions are optimized.
The guidelines are available from the ESC website in several formats: pocket guidelines, pocket guidelines smartphone app, guidelines slide set, essential messages, and the European Heart Journal article.
Noncardiac surgery risk categories
The guideline includes a table that classifies noncardiac surgeries into three groups, based on the associated 30-day risk of death, MI, or stroke:
- Low (< 1%): breast, dental, eye, thyroid, and minor gynecologic, orthopedic, and urologic surgery.
- Intermediate (1%-5%): carotid surgery, endovascular aortic aneurysm repair, gallbladder surgery, head or neck surgery, hernia repair, peripheral arterial angioplasty, renal transplant, major gynecologic, orthopedic, or neurologic (hip or spine) surgery, or urologic surgery
- High (> 5%): aortic and major vascular surgery (including aortic aneurysm), bladder removal (usually as a result of cancer), limb amputation, lung or liver transplant, pancreatic surgery, or perforated bowel repair.
The guidelines were endorsed by the European Society of Anaesthesiology and Intensive Care. The guideline authors reported numerous disclosures.
A version of this article first appeared on Medscape.com.
The European Society of Cardiology guidelines on cardiovascular assessment and management of patients undergoing noncardiac surgery have seen extensive revision since the 2014 version.
They still have the same aim – to prevent surgery-related bleeding complications, perioperative myocardial infarction/injury (PMI), stent thrombosis, acute heart failure, arrhythmias, pulmonary embolism, ischemic stroke, and cardiovascular (CV) death.
Cochairpersons Sigrun Halvorsen, MD, PhD, and Julinda Mehilli, MD, presented highlights from the guidelines at the annual congress of the European Society of Cardiology and the document was simultaneously published online in the European Heart Journal.
The document classifies noncardiac surgery into three levels of 30-day risk of CV death, MI, or stroke. Low (< 1%) risk includes eye or thyroid surgery; intermediate (1%-5%) risk includes knee or hip replacement or renal transplant; and high (> 5%) risk includes aortic aneurysm, lung transplant, or pancreatic or bladder cancer surgery (see more examples below).
It classifies patients as low risk if they are younger than 65 without CV disease or CV risk factors (smoking, hypertension, diabetes, dyslipidemia, family history); intermediate risk if they are 65 or older or have CV risk factors; and high risk if they have CVD.
In an interview, Dr. Halvorsen, professor in cardiology, University of Oslo, zeroed in on three important revisions:
First, recommendations for preoperative ECG and biomarkers are more specific, he noted.
The guidelines advise that before intermediate- or high-risk noncardiac surgery, in patients who have known CVD, CV risk factors (including age 65 or older), or symptoms suggestive of CVD:
- It is recommended to obtain a preoperative 12-lead ECG (class I).
- It is recommended to measure high-sensitivity cardiac troponin T (hs-cTn T) or high-sensitivity cardiac troponin I (hs-cTn I). It is also recommended to measure these biomarkers at 24 hours and 48 hours post surgery (class I).
- It should be considered to measure B-type natriuretic peptide or N-terminal of the prohormone BNP (NT-proBNP).
However, for low-risk patients undergoing low- and intermediate-risk noncardiac surgery, it is not recommended to routinely obtain preoperative ECG, hs-cTn T/I, or BNP/NT-proBNP concentrations (class III).
Troponins have a stronger class I recommendation, compared with the IIA recommendation for BNP, because they are useful for preoperative risk stratification and for diagnosis of PMI, Dr. Halvorsen explained. “Patients receive painkillers after surgery and may have no pain,” she noted, but they may have PMI, which has a bad prognosis.
Second, the guidelines recommend that “all patients should stop smoking 4 weeks before noncardiac surgery [class I],” she noted. Clinicians should also “measure hemoglobin, and if the patient is anemic, treat the anemia.”
Third, the sections on antithrombotic treatment have been significantly revised. “Bridging – stopping an oral antithrombotic drug and switching to a subcutaneous or IV drug – has been common,” Dr. Halvorsen said, “but recently we have new evidence that in most cases that increases the risk of bleeding.”
“We are [now] much more restrictive with respect to bridging” with unfractionated heparin or low-molecular-weight heparin, she said. “We recommend against bridging in patients with low to moderate thrombotic risk,” and bridging should only be considered in patients with mechanical prosthetic heart valves or with very high thrombotic risk.
More preoperative recommendations
In the guideline overview session at the congress, Dr. Halverson highlighted some of the new recommendations for preoperative risk assessment.
If time allows, it is recommended to optimize guideline-recommended treatment of CVD and control of CV risk factors including blood pressure, dyslipidemia, and diabetes, before noncardiac surgery (class I).
Patients commonly have “murmurs, chest pain, dyspnea, and edema that may suggest severe CVD, but may also be caused by noncardiac disease,” she noted. The guidelines state that “for patients with a newly detected murmur and symptoms or signs of CVD, transthoracic echocardiography is recommended before noncardiac surgery (class I).
“Many studies have been performed to try to find out if initiation of specific drugs before surgery could reduce the risk of complications,” Dr. Halvorsen noted. However, few have shown any benefit and “the question of presurgery initiation of beta-blockers has been greatly debated,” she said. “We have again reviewed the literature and concluded ‘Routine initiation of beta-blockers perioperatively is not recommended (class IIIA).’ “
“We adhere to the guidelines on acute and chronic coronary syndrome recommending 6-12 months of dual antiplatelet treatment as a standard before elective surgery,” she said. “However, in case of time-sensitive surgery, the duration of that treatment can be shortened down to a minimum of 1 month after elective PCI and a minimum of 3 months after PCI and ACS.”
Patients with specific types of CVD
Dr. Mehilli, a professor at Landshut-Achdorf (Germany) Hospital, highlighted some new guideline recommendations for patients who have specific types of cardiovascular disease.
Coronary artery disease (CAD). “For chronic coronary syndrome, a cardiac workup is recommended only for patients undergoing intermediate risk or high-risk noncardiac surgery.”
“Stress imaging should be considered before any high risk, noncardiac surgery in asymptomatic patients with poor functional capacity and prior PCI or coronary artery bypass graft (new recommendation, class IIa).”
Mitral valve regurgitation. For patients undergoing scheduled noncardiac surgery, who remain symptomatic despite guideline-directed medical treatment for mitral valve regurgitation (including resynchronization and myocardial revascularization), consider a valve intervention – either transcatheter or surgical – before noncardiac surgery in eligible patients with acceptable procedural risk (new recommendation).
Cardiac implantable electronic devices (CIED). For high-risk patients with CIEDs undergoing noncardiac surgery with high probability of electromagnetic interference, a CIED checkup and necessary reprogramming immediately before the procedure should be considered (new recommendation).
Arrhythmias. “I want only to stress,” Dr. Mehilli said, “in patients with atrial fibrillation with acute or worsening hemodynamic instability undergoing noncardiac surgery, an emergency electrical cardioversion is recommended (class I).”
Peripheral artery disease (PAD) and abdominal aortic aneurysm. For these patients “we do not recommend a routine referral for a cardiac workup. But we recommend it for patients with poor functional capacity or with significant risk factors or symptoms (new recommendations).”
Chronic arterial hypertension. “We have modified the recommendation, recommending avoidance of large perioperative fluctuations in blood pressure, and we do not recommend deferring noncardiac surgery in patients with stage 1 or 2 hypertension,” she said.
Postoperative cardiovascular complications
The most frequent postoperative cardiovascular complication is PMI, Dr. Mehilli noted.
“In the BASEL-PMI registry, the incidence of this complication around intermediate or high-risk noncardiac surgery was up to 15% among patients older than 65 years or with a history of CAD or PAD, which makes this kind of complication really important to prevent, to assess, and to know how to treat.”
“It is recommended to have a high awareness for perioperative cardiovascular complications, combined with surveillance for PMI in patients undergoing intermediate- or high-risk noncardiac surgery” based on serial measurements of high-sensitivity cardiac troponin.
The guidelines define PMI as “an increase in the delta of high-sensitivity troponin more than the upper level of normal,” Dr. Mehilli said. “It’s different from the one used in a rule-in algorithm for non-STEMI acute coronary syndrome.”
Postoperative atrial fibrillation (AFib) is observed in 2%-30% of noncardiac surgery patients in different registries, particularly in patients undergoing intermediate or high-risk noncardiac surgery, she noted.
“We propose an algorithm on how to prevent and treat this complication. I want to highlight that in patients with hemodynamic unstable postoperative AF[ib], an emergency cardioversion is indicated. For the others, a rate control with the target heart rate of less than 110 beats per minute is indicated.”
In patients with postoperative AFib, long-term oral anticoagulation therapy should be considered in all patients at risk for stroke, considering the anticipated net clinical benefit of oral anticoagulation therapy as well as informed patient preference (new recommendations).
Routine use of beta-blockers to prevent postoperative AFib in patients undergoing noncardiac surgery is not recommended.
The document also covers the management of patients with kidney disease, diabetes, cancer, obesity, and COVID-19. In general, elective noncardiac surgery should be postponed after a patient has COVID-19, until he or she recovers completely, and coexisting conditions are optimized.
The guidelines are available from the ESC website in several formats: pocket guidelines, pocket guidelines smartphone app, guidelines slide set, essential messages, and the European Heart Journal article.
Noncardiac surgery risk categories
The guideline includes a table that classifies noncardiac surgeries into three groups, based on the associated 30-day risk of death, MI, or stroke:
- Low (< 1%): breast, dental, eye, thyroid, and minor gynecologic, orthopedic, and urologic surgery.
- Intermediate (1%-5%): carotid surgery, endovascular aortic aneurysm repair, gallbladder surgery, head or neck surgery, hernia repair, peripheral arterial angioplasty, renal transplant, major gynecologic, orthopedic, or neurologic (hip or spine) surgery, or urologic surgery
- High (> 5%): aortic and major vascular surgery (including aortic aneurysm), bladder removal (usually as a result of cancer), limb amputation, lung or liver transplant, pancreatic surgery, or perforated bowel repair.
The guidelines were endorsed by the European Society of Anaesthesiology and Intensive Care. The guideline authors reported numerous disclosures.
A version of this article first appeared on Medscape.com.
FROM ESC CONGRESS 2022
Post Pandemic Return to Colorectal Cancer Screening
Purpose/Background
Colorectal cancer (CRC) screening was significantly curtailed due to the COVID-19 pandemic and Hines VA Medical Center in Illinois performed 50% fewer screening colonoscopies in 2020 compared to 2019 (pre-pandemic). This quality study aimed to increase use of fecal immunochemical tests (FIT) as an alternative screening method while in-person screening was limited. The primary goal was to return to pre-pandemic rates of screening (colonoscopy + FIT) and the secondary goal was to increase monthly screenings by 10% to address the backlog of patients not screened early in the pandemic.
Methods/Data Analysis
Using Plan-Do-StudyAct (PDSA) quality improvement methodology, a multidisciplinary team led by Primary Care, Gastroenterology and Laboratory/Pathology services, standardized processes for dissemination and processing of FIT tests. The first PDSA cycle implemented utilization of Colorectal Cancer Screening & Surveillance Clinical Reports (CRCS/S) to identify average-risk patients due or overdue for screening, devised plain language patient instructions for FIT-based testing, and formalized a mechanism for tracking FIT test kits.
Results
Baseline number of CRC screenings in 2019 was 2,808 (750 colonoscopy + 2,058 FIT). After the first PDSA cycle, CRC screenings were recorded during the 12-month period from April 2021 to March 2022. Colonoscopy + FIT increased to 3,558, largely due to an increase in completed FIT tests (362 colonoscopy + 3,196 FIT tests). While the number of screening colonoscopies was 52% lower compared to 2019, the number of patients screened with FIT increased by 55% after the intervention. Colonoscopy + FIT in the 12 month period starting in April of 2021 exceeded that of 2019, supporting the fact that stoolbased FIT testing was a feasible approach to screening average risk patients while in-person screening activities were restricted.
Conclusions
This quality improvement study met the primary goal of returning to pre-pandemic rates of colonoscopy + FIT and the secondary goal of increasing average number of monthly screenings by 10% to address the backlog of patients not screened early in the pandemic. Interventions directed at optimizing the FIT test process were associated with an increase in completed FIT tests. Planned PDSA cycle two will implement a mailed FIT Outreach pilot to reach additional patients for CRC screening.
Purpose/Background
Colorectal cancer (CRC) screening was significantly curtailed due to the COVID-19 pandemic and Hines VA Medical Center in Illinois performed 50% fewer screening colonoscopies in 2020 compared to 2019 (pre-pandemic). This quality study aimed to increase use of fecal immunochemical tests (FIT) as an alternative screening method while in-person screening was limited. The primary goal was to return to pre-pandemic rates of screening (colonoscopy + FIT) and the secondary goal was to increase monthly screenings by 10% to address the backlog of patients not screened early in the pandemic.
Methods/Data Analysis
Using Plan-Do-StudyAct (PDSA) quality improvement methodology, a multidisciplinary team led by Primary Care, Gastroenterology and Laboratory/Pathology services, standardized processes for dissemination and processing of FIT tests. The first PDSA cycle implemented utilization of Colorectal Cancer Screening & Surveillance Clinical Reports (CRCS/S) to identify average-risk patients due or overdue for screening, devised plain language patient instructions for FIT-based testing, and formalized a mechanism for tracking FIT test kits.
Results
Baseline number of CRC screenings in 2019 was 2,808 (750 colonoscopy + 2,058 FIT). After the first PDSA cycle, CRC screenings were recorded during the 12-month period from April 2021 to March 2022. Colonoscopy + FIT increased to 3,558, largely due to an increase in completed FIT tests (362 colonoscopy + 3,196 FIT tests). While the number of screening colonoscopies was 52% lower compared to 2019, the number of patients screened with FIT increased by 55% after the intervention. Colonoscopy + FIT in the 12 month period starting in April of 2021 exceeded that of 2019, supporting the fact that stoolbased FIT testing was a feasible approach to screening average risk patients while in-person screening activities were restricted.
Conclusions
This quality improvement study met the primary goal of returning to pre-pandemic rates of colonoscopy + FIT and the secondary goal of increasing average number of monthly screenings by 10% to address the backlog of patients not screened early in the pandemic. Interventions directed at optimizing the FIT test process were associated with an increase in completed FIT tests. Planned PDSA cycle two will implement a mailed FIT Outreach pilot to reach additional patients for CRC screening.
Purpose/Background
Colorectal cancer (CRC) screening was significantly curtailed due to the COVID-19 pandemic and Hines VA Medical Center in Illinois performed 50% fewer screening colonoscopies in 2020 compared to 2019 (pre-pandemic). This quality study aimed to increase use of fecal immunochemical tests (FIT) as an alternative screening method while in-person screening was limited. The primary goal was to return to pre-pandemic rates of screening (colonoscopy + FIT) and the secondary goal was to increase monthly screenings by 10% to address the backlog of patients not screened early in the pandemic.
Methods/Data Analysis
Using Plan-Do-StudyAct (PDSA) quality improvement methodology, a multidisciplinary team led by Primary Care, Gastroenterology and Laboratory/Pathology services, standardized processes for dissemination and processing of FIT tests. The first PDSA cycle implemented utilization of Colorectal Cancer Screening & Surveillance Clinical Reports (CRCS/S) to identify average-risk patients due or overdue for screening, devised plain language patient instructions for FIT-based testing, and formalized a mechanism for tracking FIT test kits.
Results
Baseline number of CRC screenings in 2019 was 2,808 (750 colonoscopy + 2,058 FIT). After the first PDSA cycle, CRC screenings were recorded during the 12-month period from April 2021 to March 2022. Colonoscopy + FIT increased to 3,558, largely due to an increase in completed FIT tests (362 colonoscopy + 3,196 FIT tests). While the number of screening colonoscopies was 52% lower compared to 2019, the number of patients screened with FIT increased by 55% after the intervention. Colonoscopy + FIT in the 12 month period starting in April of 2021 exceeded that of 2019, supporting the fact that stoolbased FIT testing was a feasible approach to screening average risk patients while in-person screening activities were restricted.
Conclusions
This quality improvement study met the primary goal of returning to pre-pandemic rates of colonoscopy + FIT and the secondary goal of increasing average number of monthly screenings by 10% to address the backlog of patients not screened early in the pandemic. Interventions directed at optimizing the FIT test process were associated with an increase in completed FIT tests. Planned PDSA cycle two will implement a mailed FIT Outreach pilot to reach additional patients for CRC screening.
Utilization and Clinical Benefit of Immune Checkpoint Inhibitor in Veterans With Microsatellite Instability-High Prostate Cancer
Background
The utilization of immune checkpoint inhibitors (ICI) in prostate cancer (PC) can be very effective for patients with mismatch repair-deficiency (as identified by MSI-H by PCR/NGS or dMMR IHC). The use of ICI in this patient population has been associated with high rates of durable response. There is limited published data on factors that may influence patient response and outcomes. The aim of this study is to describe the utilization of and tumor response to ICI in this patient population.
Methods
This is a retrospective study of men with MSI-H PC reported by somatic genomic testing from April 1, 2015 to March 31, 2022 through the VA National Precision Oncology Program (NPOP), who received at least one dose of ICI. The primary objectives are to describe the incidence of MSI-H PC and the utilization of ICI. Descriptive statistics and Kaplan- Meier estimator were used for secondary objectives to determine the prostate-specific antigen decline of at least 50% (PSA50), clinical progression free survival (cPFS), time on ICI as a function of number of prior therapies, the extent of metastasis prior to initiation of ICI, and the correlation of MMR genetic alterations with treatment response.
Results
66 patients with MSI-H PC were identified (1.5% of a total of 4267 patients with PC tested through NPOP). 23 patients (35%) received at least one dose of ICI. 12 of 23 patients (52%) had PSA response. PSA50 responses occurred in 6 patients (50%) and 5 continued to have durable PSA50 at six months. Median cPFS was 280 days (95% CI: 105 days-not reached) and the estimated PFS at six months was 72.2% (95% CI: 35.7%-90.2%). 8 of 12 (67%) responders have received multiple lines of therapy for M1 PC. 8 of 12 patients (67%) had high-volume disease at ICI initiation. Of those patients with a MMR genetic alteration, patients with MLH1 (3/3) and MSH2 (6/8) alterations responded more frequently than those with MSH6 alterations (1/4).
Conclusions
MSI-H PC is rare but response rates to ICI are high and durable. Patients with MLH1 and MSH2 alterations appeared to respond more frequently than those with MSH6. Additional follow-up is ongoing.
Background
The utilization of immune checkpoint inhibitors (ICI) in prostate cancer (PC) can be very effective for patients with mismatch repair-deficiency (as identified by MSI-H by PCR/NGS or dMMR IHC). The use of ICI in this patient population has been associated with high rates of durable response. There is limited published data on factors that may influence patient response and outcomes. The aim of this study is to describe the utilization of and tumor response to ICI in this patient population.
Methods
This is a retrospective study of men with MSI-H PC reported by somatic genomic testing from April 1, 2015 to March 31, 2022 through the VA National Precision Oncology Program (NPOP), who received at least one dose of ICI. The primary objectives are to describe the incidence of MSI-H PC and the utilization of ICI. Descriptive statistics and Kaplan- Meier estimator were used for secondary objectives to determine the prostate-specific antigen decline of at least 50% (PSA50), clinical progression free survival (cPFS), time on ICI as a function of number of prior therapies, the extent of metastasis prior to initiation of ICI, and the correlation of MMR genetic alterations with treatment response.
Results
66 patients with MSI-H PC were identified (1.5% of a total of 4267 patients with PC tested through NPOP). 23 patients (35%) received at least one dose of ICI. 12 of 23 patients (52%) had PSA response. PSA50 responses occurred in 6 patients (50%) and 5 continued to have durable PSA50 at six months. Median cPFS was 280 days (95% CI: 105 days-not reached) and the estimated PFS at six months was 72.2% (95% CI: 35.7%-90.2%). 8 of 12 (67%) responders have received multiple lines of therapy for M1 PC. 8 of 12 patients (67%) had high-volume disease at ICI initiation. Of those patients with a MMR genetic alteration, patients with MLH1 (3/3) and MSH2 (6/8) alterations responded more frequently than those with MSH6 alterations (1/4).
Conclusions
MSI-H PC is rare but response rates to ICI are high and durable. Patients with MLH1 and MSH2 alterations appeared to respond more frequently than those with MSH6. Additional follow-up is ongoing.
Background
The utilization of immune checkpoint inhibitors (ICI) in prostate cancer (PC) can be very effective for patients with mismatch repair-deficiency (as identified by MSI-H by PCR/NGS or dMMR IHC). The use of ICI in this patient population has been associated with high rates of durable response. There is limited published data on factors that may influence patient response and outcomes. The aim of this study is to describe the utilization of and tumor response to ICI in this patient population.
Methods
This is a retrospective study of men with MSI-H PC reported by somatic genomic testing from April 1, 2015 to March 31, 2022 through the VA National Precision Oncology Program (NPOP), who received at least one dose of ICI. The primary objectives are to describe the incidence of MSI-H PC and the utilization of ICI. Descriptive statistics and Kaplan- Meier estimator were used for secondary objectives to determine the prostate-specific antigen decline of at least 50% (PSA50), clinical progression free survival (cPFS), time on ICI as a function of number of prior therapies, the extent of metastasis prior to initiation of ICI, and the correlation of MMR genetic alterations with treatment response.
Results
66 patients with MSI-H PC were identified (1.5% of a total of 4267 patients with PC tested through NPOP). 23 patients (35%) received at least one dose of ICI. 12 of 23 patients (52%) had PSA response. PSA50 responses occurred in 6 patients (50%) and 5 continued to have durable PSA50 at six months. Median cPFS was 280 days (95% CI: 105 days-not reached) and the estimated PFS at six months was 72.2% (95% CI: 35.7%-90.2%). 8 of 12 (67%) responders have received multiple lines of therapy for M1 PC. 8 of 12 patients (67%) had high-volume disease at ICI initiation. Of those patients with a MMR genetic alteration, patients with MLH1 (3/3) and MSH2 (6/8) alterations responded more frequently than those with MSH6 alterations (1/4).
Conclusions
MSI-H PC is rare but response rates to ICI are high and durable. Patients with MLH1 and MSH2 alterations appeared to respond more frequently than those with MSH6. Additional follow-up is ongoing.
Financial Toxicity in Colorectal Cancer Patient Who Received Localized Treatment in the Veterans Affairs Health System
Purpose
To describe patient-reported financial toxicity for patients who received localized colorectal cancer (CRC) treatment in the Veterans Health Administration (VHA).
Background
CRC is the 2nd leading cause of cancer-related death. In the private sector, many patients suffer economic hardship from CRC and its treatment. This leads to financial toxicity, or the negative impact of medical expenses, which is a strong independent predictor of quality of life. In the VHA patients access cancer care based on a sliding fee scale; however, there is a knowledge gap regarding financial toxicity for CRC patients in the VHA whose out of pocket costs have largely been subsidized.
Methods
We performed a descriptive, retrospective analysis of a survey administered at a VHA facility to patients with colorectal cancer who received localized treatment (ie, surgery or chemoradiotherapy). The survey consisted of 49 items assessing several clinical and psychosocial domains including subjective financial burden and use of financial coping strategies. Additionally, we used the validated Confusion, Hubbub and Order Scale (CHAOS) measure, which was designed to assess the level of confusion and disorganization in homes.
Results
Between November 2015 and September 2016, we mailed surveys to 265 patients diagnosed with CRC, 133 responded, for a response rate of 50%. For financial strain, 24% (n=32) of participants reported reduced spending on basics like food or clothing to pay for their cancer treatment, 17% (n=23) reported using all or a portion of their savings to pay for their cancer care,14% (n=18) noted borrowing money or using a credit card to pay for care, and 9% (n=12) of participants noted they did not fill a prescription because it was too expensive.
Conclusions/Implications
Despite policies to reduce out-of-pocket costs for VHA patients with CRC, patients reported significant financial toxicity. In the continued movement for value-based care centered on whole person care delivery, identifying persistent financial toxicity for vulnerable cancer patients is important data as we try and improve the infrastructure to impact quality of life and healthcare delivery for this population.
Purpose
To describe patient-reported financial toxicity for patients who received localized colorectal cancer (CRC) treatment in the Veterans Health Administration (VHA).
Background
CRC is the 2nd leading cause of cancer-related death. In the private sector, many patients suffer economic hardship from CRC and its treatment. This leads to financial toxicity, or the negative impact of medical expenses, which is a strong independent predictor of quality of life. In the VHA patients access cancer care based on a sliding fee scale; however, there is a knowledge gap regarding financial toxicity for CRC patients in the VHA whose out of pocket costs have largely been subsidized.
Methods
We performed a descriptive, retrospective analysis of a survey administered at a VHA facility to patients with colorectal cancer who received localized treatment (ie, surgery or chemoradiotherapy). The survey consisted of 49 items assessing several clinical and psychosocial domains including subjective financial burden and use of financial coping strategies. Additionally, we used the validated Confusion, Hubbub and Order Scale (CHAOS) measure, which was designed to assess the level of confusion and disorganization in homes.
Results
Between November 2015 and September 2016, we mailed surveys to 265 patients diagnosed with CRC, 133 responded, for a response rate of 50%. For financial strain, 24% (n=32) of participants reported reduced spending on basics like food or clothing to pay for their cancer treatment, 17% (n=23) reported using all or a portion of their savings to pay for their cancer care,14% (n=18) noted borrowing money or using a credit card to pay for care, and 9% (n=12) of participants noted they did not fill a prescription because it was too expensive.
Conclusions/Implications
Despite policies to reduce out-of-pocket costs for VHA patients with CRC, patients reported significant financial toxicity. In the continued movement for value-based care centered on whole person care delivery, identifying persistent financial toxicity for vulnerable cancer patients is important data as we try and improve the infrastructure to impact quality of life and healthcare delivery for this population.
Purpose
To describe patient-reported financial toxicity for patients who received localized colorectal cancer (CRC) treatment in the Veterans Health Administration (VHA).
Background
CRC is the 2nd leading cause of cancer-related death. In the private sector, many patients suffer economic hardship from CRC and its treatment. This leads to financial toxicity, or the negative impact of medical expenses, which is a strong independent predictor of quality of life. In the VHA patients access cancer care based on a sliding fee scale; however, there is a knowledge gap regarding financial toxicity for CRC patients in the VHA whose out of pocket costs have largely been subsidized.
Methods
We performed a descriptive, retrospective analysis of a survey administered at a VHA facility to patients with colorectal cancer who received localized treatment (ie, surgery or chemoradiotherapy). The survey consisted of 49 items assessing several clinical and psychosocial domains including subjective financial burden and use of financial coping strategies. Additionally, we used the validated Confusion, Hubbub and Order Scale (CHAOS) measure, which was designed to assess the level of confusion and disorganization in homes.
Results
Between November 2015 and September 2016, we mailed surveys to 265 patients diagnosed with CRC, 133 responded, for a response rate of 50%. For financial strain, 24% (n=32) of participants reported reduced spending on basics like food or clothing to pay for their cancer treatment, 17% (n=23) reported using all or a portion of their savings to pay for their cancer care,14% (n=18) noted borrowing money or using a credit card to pay for care, and 9% (n=12) of participants noted they did not fill a prescription because it was too expensive.
Conclusions/Implications
Despite policies to reduce out-of-pocket costs for VHA patients with CRC, patients reported significant financial toxicity. In the continued movement for value-based care centered on whole person care delivery, identifying persistent financial toxicity for vulnerable cancer patients is important data as we try and improve the infrastructure to impact quality of life and healthcare delivery for this population.
Castration-Resistant Prostate Cancer—Not Only Challenging to Treat, but Difficult to Define
Purpose
Examine the impact of different definitions of castration resistance used to identify patients with castration-resistant prostate cancer (CRPC) using electronic health records (EHR).
Background
CRPC is a form of prostate cancer that is resistant to treatment with androgen deprivation therapy (ADT) and is associated with higher morbidity and mortality. Widely used guidelines like the Prostate Cancer Working Group 3 (PCWG 3), the American Urological Association (AUA), and many others differ in their definitions of castration-resistance. Until now, the feasibility of identifying CRPC using different definitions from EHR data has not been studied.
Methods/Data Analyisis
EHR data from the Veterans Health Administration (01/2006-12/2020) were used to identify veterans with CRPC according to the following criteria: 1) PCWG 3—a PSA increase ?25% from the nadir with a minimum rise of 2 ng/mL, while castrate (testosterone < 50 ng/mL); 2) AUA—2 consecutive PSA rises of ?0.2 ng/mL; 3) CRPC screening—a PSA rise of > 0.0 ng/mL within a window of 7–90 days.
Results
36,101 unique patients were identified using 1 of (or a combination of) the 3 CRPC criteria. Approximately 12,775 (35%) patients met all 3 criteria, while 8,589 (24%) were identified by AUA, 4,785 (13%) by CRPC screening, and 145 (0.4%) by PCWG3. A total of 8,377 (23%) patients met both the AUA and CRPC screening criteria, 1,219 (3%) patients met the AUA and PCWG3 criteria, and 211 (1%) met the PCWG3 and CRPC screening criteria.
Conculsions/Implications
Although several definitions can be used to identify CRPC patients, a combination of these definitions results in the greatest yield of CRPC patients identified using EHR data. Even though the PCWG3 criterion is frequently used in both clinical trials research and retrospective observational research, PCWG3 may miss many patients meeting other criteria and should not be used by itself when studying patients with CRPC identified from EHR data.
Purpose
Examine the impact of different definitions of castration resistance used to identify patients with castration-resistant prostate cancer (CRPC) using electronic health records (EHR).
Background
CRPC is a form of prostate cancer that is resistant to treatment with androgen deprivation therapy (ADT) and is associated with higher morbidity and mortality. Widely used guidelines like the Prostate Cancer Working Group 3 (PCWG 3), the American Urological Association (AUA), and many others differ in their definitions of castration-resistance. Until now, the feasibility of identifying CRPC using different definitions from EHR data has not been studied.
Methods/Data Analyisis
EHR data from the Veterans Health Administration (01/2006-12/2020) were used to identify veterans with CRPC according to the following criteria: 1) PCWG 3—a PSA increase ?25% from the nadir with a minimum rise of 2 ng/mL, while castrate (testosterone < 50 ng/mL); 2) AUA—2 consecutive PSA rises of ?0.2 ng/mL; 3) CRPC screening—a PSA rise of > 0.0 ng/mL within a window of 7–90 days.
Results
36,101 unique patients were identified using 1 of (or a combination of) the 3 CRPC criteria. Approximately 12,775 (35%) patients met all 3 criteria, while 8,589 (24%) were identified by AUA, 4,785 (13%) by CRPC screening, and 145 (0.4%) by PCWG3. A total of 8,377 (23%) patients met both the AUA and CRPC screening criteria, 1,219 (3%) patients met the AUA and PCWG3 criteria, and 211 (1%) met the PCWG3 and CRPC screening criteria.
Conculsions/Implications
Although several definitions can be used to identify CRPC patients, a combination of these definitions results in the greatest yield of CRPC patients identified using EHR data. Even though the PCWG3 criterion is frequently used in both clinical trials research and retrospective observational research, PCWG3 may miss many patients meeting other criteria and should not be used by itself when studying patients with CRPC identified from EHR data.
Purpose
Examine the impact of different definitions of castration resistance used to identify patients with castration-resistant prostate cancer (CRPC) using electronic health records (EHR).
Background
CRPC is a form of prostate cancer that is resistant to treatment with androgen deprivation therapy (ADT) and is associated with higher morbidity and mortality. Widely used guidelines like the Prostate Cancer Working Group 3 (PCWG 3), the American Urological Association (AUA), and many others differ in their definitions of castration-resistance. Until now, the feasibility of identifying CRPC using different definitions from EHR data has not been studied.
Methods/Data Analyisis
EHR data from the Veterans Health Administration (01/2006-12/2020) were used to identify veterans with CRPC according to the following criteria: 1) PCWG 3—a PSA increase ?25% from the nadir with a minimum rise of 2 ng/mL, while castrate (testosterone < 50 ng/mL); 2) AUA—2 consecutive PSA rises of ?0.2 ng/mL; 3) CRPC screening—a PSA rise of > 0.0 ng/mL within a window of 7–90 days.
Results
36,101 unique patients were identified using 1 of (or a combination of) the 3 CRPC criteria. Approximately 12,775 (35%) patients met all 3 criteria, while 8,589 (24%) were identified by AUA, 4,785 (13%) by CRPC screening, and 145 (0.4%) by PCWG3. A total of 8,377 (23%) patients met both the AUA and CRPC screening criteria, 1,219 (3%) patients met the AUA and PCWG3 criteria, and 211 (1%) met the PCWG3 and CRPC screening criteria.
Conculsions/Implications
Although several definitions can be used to identify CRPC patients, a combination of these definitions results in the greatest yield of CRPC patients identified using EHR data. Even though the PCWG3 criterion is frequently used in both clinical trials research and retrospective observational research, PCWG3 may miss many patients meeting other criteria and should not be used by itself when studying patients with CRPC identified from EHR data.
Identification of Clinically Actionable Genomic Alterations in Colorectal Cancer Patients From the VA National Precision Oncology Program (NPOP)
Purpose
Colorectal cancer (CRC) is the fourth most common cancer at VA and the third leading cause of cancer-related death in the USA. The VA National Precision Oncology Program (NPOP) was established in 2016 with the goal of implementing standardized, streamlined methods for molecular testing of veterans with cancer and has enabled comprehensive genomic profiling (CGP) and precision medicine as part of routine cancer care. Obtaining CGP of predictive biomarkers in cancer tissue, including mutations in genes (e.g., KRAS, NRAS and BRAF), tumor mutation burden (TMB) and microsatellite instability status (MSI) can be used to support treatment decisions with targeted and immunotherapies.
Methods
In this study we describe the frequencies of these clinical biomarkers in colon adenocarcinoma (COAD), rectal adenocarcinoma (READ), and other colon or rectum histologies (CROT); and compare these frequencies to a published cohort of metastatic CRC using Chi-square test (Yaeger et al., 2018).
Results
A total of 1802 patients with CRC were included in this study. COAD was the most frequent disease site (76.9%) followed by READ (19.1%). Approximately 52.9% of COAD patients harbored at least one highly actionable biomarker (defined as having an FDA-approved indication) including NRAS/ KRAS/BRAF wildtype (38.0%), TMB-H (12.9%), BRAF V600E (9.7%), MSI-H (8.9%), and NTRK fusion or rearrangement (0.3%). About 52.0% of patients with READ had these biomarkers, while this rate was (16.4%) in CROT. Among patients with COAD and READ, those with BRAF V600E mutations were more likely to be older, White, not Hispanic or Latino, and lived in urban areas compared to those without BRAF V600E. Relative to those with NRAS/KRAS/BRAF mutations, patients with NRAS/KRAS/BRAF wildtype were frequently younger. Relative to the frequency of biomarkers from a cBioPortal cohort of metastatic CRC, the frequency of NRAS wildtype was significantly lower in patients with COAD and READ tested through NPOP.
Consulsions
In this cohort, ~53 % of patients with COAD and 52% of patients with READ have highly actionable biomarkers and are potentially eligible for FDAapproved targeted therapies. Future studies examining cancer outcomes with regard to the use of targeted therapies in the setting of actionable gene alterations, TMB, and MSI are warranted.
Purpose
Colorectal cancer (CRC) is the fourth most common cancer at VA and the third leading cause of cancer-related death in the USA. The VA National Precision Oncology Program (NPOP) was established in 2016 with the goal of implementing standardized, streamlined methods for molecular testing of veterans with cancer and has enabled comprehensive genomic profiling (CGP) and precision medicine as part of routine cancer care. Obtaining CGP of predictive biomarkers in cancer tissue, including mutations in genes (e.g., KRAS, NRAS and BRAF), tumor mutation burden (TMB) and microsatellite instability status (MSI) can be used to support treatment decisions with targeted and immunotherapies.
Methods
In this study we describe the frequencies of these clinical biomarkers in colon adenocarcinoma (COAD), rectal adenocarcinoma (READ), and other colon or rectum histologies (CROT); and compare these frequencies to a published cohort of metastatic CRC using Chi-square test (Yaeger et al., 2018).
Results
A total of 1802 patients with CRC were included in this study. COAD was the most frequent disease site (76.9%) followed by READ (19.1%). Approximately 52.9% of COAD patients harbored at least one highly actionable biomarker (defined as having an FDA-approved indication) including NRAS/ KRAS/BRAF wildtype (38.0%), TMB-H (12.9%), BRAF V600E (9.7%), MSI-H (8.9%), and NTRK fusion or rearrangement (0.3%). About 52.0% of patients with READ had these biomarkers, while this rate was (16.4%) in CROT. Among patients with COAD and READ, those with BRAF V600E mutations were more likely to be older, White, not Hispanic or Latino, and lived in urban areas compared to those without BRAF V600E. Relative to those with NRAS/KRAS/BRAF mutations, patients with NRAS/KRAS/BRAF wildtype were frequently younger. Relative to the frequency of biomarkers from a cBioPortal cohort of metastatic CRC, the frequency of NRAS wildtype was significantly lower in patients with COAD and READ tested through NPOP.
Consulsions
In this cohort, ~53 % of patients with COAD and 52% of patients with READ have highly actionable biomarkers and are potentially eligible for FDAapproved targeted therapies. Future studies examining cancer outcomes with regard to the use of targeted therapies in the setting of actionable gene alterations, TMB, and MSI are warranted.
Purpose
Colorectal cancer (CRC) is the fourth most common cancer at VA and the third leading cause of cancer-related death in the USA. The VA National Precision Oncology Program (NPOP) was established in 2016 with the goal of implementing standardized, streamlined methods for molecular testing of veterans with cancer and has enabled comprehensive genomic profiling (CGP) and precision medicine as part of routine cancer care. Obtaining CGP of predictive biomarkers in cancer tissue, including mutations in genes (e.g., KRAS, NRAS and BRAF), tumor mutation burden (TMB) and microsatellite instability status (MSI) can be used to support treatment decisions with targeted and immunotherapies.
Methods
In this study we describe the frequencies of these clinical biomarkers in colon adenocarcinoma (COAD), rectal adenocarcinoma (READ), and other colon or rectum histologies (CROT); and compare these frequencies to a published cohort of metastatic CRC using Chi-square test (Yaeger et al., 2018).
Results
A total of 1802 patients with CRC were included in this study. COAD was the most frequent disease site (76.9%) followed by READ (19.1%). Approximately 52.9% of COAD patients harbored at least one highly actionable biomarker (defined as having an FDA-approved indication) including NRAS/ KRAS/BRAF wildtype (38.0%), TMB-H (12.9%), BRAF V600E (9.7%), MSI-H (8.9%), and NTRK fusion or rearrangement (0.3%). About 52.0% of patients with READ had these biomarkers, while this rate was (16.4%) in CROT. Among patients with COAD and READ, those with BRAF V600E mutations were more likely to be older, White, not Hispanic or Latino, and lived in urban areas compared to those without BRAF V600E. Relative to those with NRAS/KRAS/BRAF mutations, patients with NRAS/KRAS/BRAF wildtype were frequently younger. Relative to the frequency of biomarkers from a cBioPortal cohort of metastatic CRC, the frequency of NRAS wildtype was significantly lower in patients with COAD and READ tested through NPOP.
Consulsions
In this cohort, ~53 % of patients with COAD and 52% of patients with READ have highly actionable biomarkers and are potentially eligible for FDAapproved targeted therapies. Future studies examining cancer outcomes with regard to the use of targeted therapies in the setting of actionable gene alterations, TMB, and MSI are warranted.