User login
MDedge latest news is breaking news from medical conferences, journals, guidelines, the FDA and CDC.
FDA OKs New Drug for Urinary Tract Infections
The Food and Drug Administration (FDA) has approved Orlynvah, a new oral treatment for uncomplicated urinary tract infections (UTIs) in women who have limited options for effective antibiotic therapy.
Uncomplicated UTIs are bladder infections that typically affect women who don’t have other issues like kidney disease or urinary tract abnormalities. These infections are common, affecting around half of all women at least once in their lives.
Treating UTIs can be difficult when standard antibiotics don’t work well, often because of antibiotic resistance or certain health conditions. Orlynvah offers a promising new option by combining two drugs, sulopenem etzadroxil and probenecid, in one oral tablet. This combination helps keep the antibiotic in the body longer, making it work better, especially against bacteria that resist traditional treatments. Orlynvah is specifically approved to target infections from bacteria like Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis, which can be harder to treat.
Marjorie Golden, MD, an infectious disease specialist at Yale New Haven Hospital in Connecticut, described Orlynvah as a much-needed alternative for women struggling with difficult-to-treat UTIs.
“Orlynvah has the potential to be an important treatment option for those who need it,” she said in a news release from Iterum Therapeutics, the drug’s maker.
The FDA approved Orlynvah based on two large clinical trials involving over 3,800 women. In these studies, Orlynvah worked as well as or better than antibiotics like ciprofloxacin and Augmentin. It was generally well-tolerated, though common side effects included diarrhea, nausea, yeast infections, and headaches.
The FDA advises people to discuss their medical history with their doctor before taking Orlynvah, especially if they have conditions like gout, kidney stones, or allergies to other antibiotics.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration (FDA) has approved Orlynvah, a new oral treatment for uncomplicated urinary tract infections (UTIs) in women who have limited options for effective antibiotic therapy.
Uncomplicated UTIs are bladder infections that typically affect women who don’t have other issues like kidney disease or urinary tract abnormalities. These infections are common, affecting around half of all women at least once in their lives.
Treating UTIs can be difficult when standard antibiotics don’t work well, often because of antibiotic resistance or certain health conditions. Orlynvah offers a promising new option by combining two drugs, sulopenem etzadroxil and probenecid, in one oral tablet. This combination helps keep the antibiotic in the body longer, making it work better, especially against bacteria that resist traditional treatments. Orlynvah is specifically approved to target infections from bacteria like Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis, which can be harder to treat.
Marjorie Golden, MD, an infectious disease specialist at Yale New Haven Hospital in Connecticut, described Orlynvah as a much-needed alternative for women struggling with difficult-to-treat UTIs.
“Orlynvah has the potential to be an important treatment option for those who need it,” she said in a news release from Iterum Therapeutics, the drug’s maker.
The FDA approved Orlynvah based on two large clinical trials involving over 3,800 women. In these studies, Orlynvah worked as well as or better than antibiotics like ciprofloxacin and Augmentin. It was generally well-tolerated, though common side effects included diarrhea, nausea, yeast infections, and headaches.
The FDA advises people to discuss their medical history with their doctor before taking Orlynvah, especially if they have conditions like gout, kidney stones, or allergies to other antibiotics.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration (FDA) has approved Orlynvah, a new oral treatment for uncomplicated urinary tract infections (UTIs) in women who have limited options for effective antibiotic therapy.
Uncomplicated UTIs are bladder infections that typically affect women who don’t have other issues like kidney disease or urinary tract abnormalities. These infections are common, affecting around half of all women at least once in their lives.
Treating UTIs can be difficult when standard antibiotics don’t work well, often because of antibiotic resistance or certain health conditions. Orlynvah offers a promising new option by combining two drugs, sulopenem etzadroxil and probenecid, in one oral tablet. This combination helps keep the antibiotic in the body longer, making it work better, especially against bacteria that resist traditional treatments. Orlynvah is specifically approved to target infections from bacteria like Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis, which can be harder to treat.
Marjorie Golden, MD, an infectious disease specialist at Yale New Haven Hospital in Connecticut, described Orlynvah as a much-needed alternative for women struggling with difficult-to-treat UTIs.
“Orlynvah has the potential to be an important treatment option for those who need it,” she said in a news release from Iterum Therapeutics, the drug’s maker.
The FDA approved Orlynvah based on two large clinical trials involving over 3,800 women. In these studies, Orlynvah worked as well as or better than antibiotics like ciprofloxacin and Augmentin. It was generally well-tolerated, though common side effects included diarrhea, nausea, yeast infections, and headaches.
The FDA advises people to discuss their medical history with their doctor before taking Orlynvah, especially if they have conditions like gout, kidney stones, or allergies to other antibiotics.
A version of this article first appeared on Medscape.com.
Treating Digestive Disease Across the Lifespan
Pediatric gastroenterologists are a vital, yet often overlooked segment of the GI workforce and an important part of AGA’s diverse membership. Per the American Board of Pediatrics, 2,232 pediatricians have been board certified in pediatric gastroenterology since formal certification was first offered in 1990, and AGA Institute Council’s Pediatric Gastroenterology and Developmental Biology Section has nearly 1,900 members.
According to a recently published study in the journal Pediatrics, the pediatric GI workforce is expected to double by 2040, growing at a rate faster than that of most other pediatric subspecialties. This is largely due to the increased scope and complexity of the field driven by scientific advances and the increasing prevalence of digestive and liver diseases in children, including inflammatory bowel and other diseases.
In this month’s Member Spotlight, we highlight Dr. Yoyo Zhang, a pediatric gastroenterologist at Stanford Children’s Health specializing in intestinal and liver transplantation. Her passion for her profession and for improving the lives of her patients shines brightly, and her interview provides fascinating insights into the complexities and rewards of the rapidly expanding field of pediatric gastroenterology.
Also in our November issue, we update you on the FDA’s recent approval of the “next-gen” Cologuard test and query a panel of primary care and GI experts on their thoughts regarding the role that newly FDA-approved (but not yet guideline-recommended) Guardant blood-based CRC screening test should play in CRC screening moving forward.
In our Perspectives feature, we offer expert insights on how to appropriately screen patients for certain rare malignancies. Is it worthwhile screening for pancreatic cancer, and if so, how should it be done? Likewise, diagnosing cholangiocarcinoma is challenging; how best should one evaluate for this in higher-risk populations?
We hope you enjoy all the content in our November issue – as always, thanks for reading!
Megan A. Adams, MD, JD, MSc
Editor in Chief
Pediatric gastroenterologists are a vital, yet often overlooked segment of the GI workforce and an important part of AGA’s diverse membership. Per the American Board of Pediatrics, 2,232 pediatricians have been board certified in pediatric gastroenterology since formal certification was first offered in 1990, and AGA Institute Council’s Pediatric Gastroenterology and Developmental Biology Section has nearly 1,900 members.
According to a recently published study in the journal Pediatrics, the pediatric GI workforce is expected to double by 2040, growing at a rate faster than that of most other pediatric subspecialties. This is largely due to the increased scope and complexity of the field driven by scientific advances and the increasing prevalence of digestive and liver diseases in children, including inflammatory bowel and other diseases.
In this month’s Member Spotlight, we highlight Dr. Yoyo Zhang, a pediatric gastroenterologist at Stanford Children’s Health specializing in intestinal and liver transplantation. Her passion for her profession and for improving the lives of her patients shines brightly, and her interview provides fascinating insights into the complexities and rewards of the rapidly expanding field of pediatric gastroenterology.
Also in our November issue, we update you on the FDA’s recent approval of the “next-gen” Cologuard test and query a panel of primary care and GI experts on their thoughts regarding the role that newly FDA-approved (but not yet guideline-recommended) Guardant blood-based CRC screening test should play in CRC screening moving forward.
In our Perspectives feature, we offer expert insights on how to appropriately screen patients for certain rare malignancies. Is it worthwhile screening for pancreatic cancer, and if so, how should it be done? Likewise, diagnosing cholangiocarcinoma is challenging; how best should one evaluate for this in higher-risk populations?
We hope you enjoy all the content in our November issue – as always, thanks for reading!
Megan A. Adams, MD, JD, MSc
Editor in Chief
Pediatric gastroenterologists are a vital, yet often overlooked segment of the GI workforce and an important part of AGA’s diverse membership. Per the American Board of Pediatrics, 2,232 pediatricians have been board certified in pediatric gastroenterology since formal certification was first offered in 1990, and AGA Institute Council’s Pediatric Gastroenterology and Developmental Biology Section has nearly 1,900 members.
According to a recently published study in the journal Pediatrics, the pediatric GI workforce is expected to double by 2040, growing at a rate faster than that of most other pediatric subspecialties. This is largely due to the increased scope and complexity of the field driven by scientific advances and the increasing prevalence of digestive and liver diseases in children, including inflammatory bowel and other diseases.
In this month’s Member Spotlight, we highlight Dr. Yoyo Zhang, a pediatric gastroenterologist at Stanford Children’s Health specializing in intestinal and liver transplantation. Her passion for her profession and for improving the lives of her patients shines brightly, and her interview provides fascinating insights into the complexities and rewards of the rapidly expanding field of pediatric gastroenterology.
Also in our November issue, we update you on the FDA’s recent approval of the “next-gen” Cologuard test and query a panel of primary care and GI experts on their thoughts regarding the role that newly FDA-approved (but not yet guideline-recommended) Guardant blood-based CRC screening test should play in CRC screening moving forward.
In our Perspectives feature, we offer expert insights on how to appropriately screen patients for certain rare malignancies. Is it worthwhile screening for pancreatic cancer, and if so, how should it be done? Likewise, diagnosing cholangiocarcinoma is challenging; how best should one evaluate for this in higher-risk populations?
We hope you enjoy all the content in our November issue – as always, thanks for reading!
Megan A. Adams, MD, JD, MSc
Editor in Chief
Medical, Endoscopic, and Surgical Management of Gastroesophageal Reflux Disease
Introduction
Gastroesophageal reflux disease (GERD) is a frequently encountered condition, and rising annually.1 A recent meta-analysis suggests nearly 14% (1.03 billion) of the population are affected worldwide. Differences may range by region from 12% in Latin America to 20% in North America, and by country from 4% in China to 23% in Turkey.1 In the United States, 21% of the population are afflicted with weekly GERD symptoms.2 Novel medical therapies and endoscopic options provide clinicians with opportunities to help patients with GERD.3
Diagnosis
Definition
GERD was originally defined by the Montreal consensus as a condition that develops when the reflux of stomach contents causes troublesome symptoms and/or complications.4 Heartburn and regurgitation are common symptoms of GERD, with a sensitivity of 30%-76% and specificity of 62%-96% for erosive esophagitis (EE), which occurs when the reflux of stomach content causes esophageal mucosal breaks.5 The presence of characteristic mucosal injury observed during an upper endoscopy or abnormal esophageal acid exposure on ambulatory reflux monitoring are objective evidence of GERD. A trial of a proton pump inhibitor (PPI) may function as a diagnostic test for patients exhibiting the typical symptoms of GERD without any alarm symptoms.3,6
Endoscopic Evaluation and Confirmation
The 2022 American Gastroenterological Association (AGA) clinical practice update recommends diagnostic endoscopy, after PPIs are stopped for 2-4 weeks, in patients whose GERD symptoms do not respond adequately to an empiric trial of a PPI.3 Those with GERD and alarm symptoms such as dysphagia, weight loss, bleeding, and vomiting should undergo endoscopy as soon as possible. Endoscopic findings of EE (Los Angeles Grade B or more severe) and long-segment Barrett’s esophagus (> 3-cm segment with intestinal metaplasia on biopsy) are diagnostic of GERD.3
Reflux Monitoring
With ambulatory reflux monitoring (pH or impedance-pH), esophageal acid exposure (or neutral refluxate in impedance testing) can be measured to confirm GERD diagnosis and to correlate symptoms with reflux episodes. Patients with atypical GERD symptoms or patients with a confirmed diagnosis of GERD whose symptoms have not improved sufficiently with twice-daily PPI therapy should have esophageal impedance-pH monitoring while on PPIs.6,7
Esophageal Manometry
High-resolution esophageal manometry can be used to assess motility abnormalities associated with GERD.
Although no manometric abnormality is unique to GERD, weak lower esophageal sphincter (LES) resting pressure and ineffective esophageal motility frequently coexist with severe GERD.6
Manometry is particularly useful in patients considering surgical or endoscopic anti-reflux procedures to evaluate for achalasia,3 an important contraindication to surgery.
Medical Management
Management of GERD requires a multidisciplinary and personalized approach based on symptom presentation, body mass index, endoscopic findings (e.g., presence of EE, Barrett’s esophagus, hiatal hernia), and physiological abnormalities (e.g., gastroparesis or ineffective motility).3
Lifestyle Modifications
Recommended lifestyle modifications include weight loss for patients with obesity, stress reduction, tobacco and alcohol cessation, elevating the head of the bed, staying upright during and after meals, avoidance of food intake < 3 hours before bedtime, and cessation of foods that potentially aggravate reflux symptoms such as coffee, chocolate, carbonated beverages, spicy foods, acidic foods, and foods with high fat content.6,8
Medications
Pharmacologic therapy for GERD includes medications that primarily aim to neutralize or reduce gastric acid -- we summarize options in Table 1.3,8
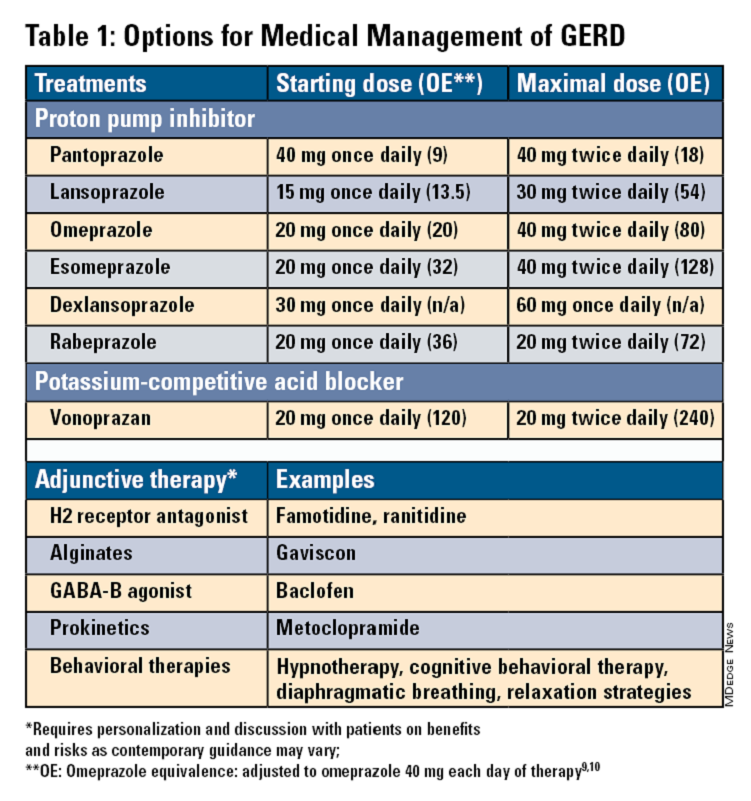
Proton Pump Inhibitors
Most guidelines suggest a trial of 4-8 weeks of once-daily enteric-coated PPI before meals in patients with typical GERD symptoms and no alarm symptoms. Escalation to double-dose PPI may be considered in the case of persistent symptoms. The relative potencies of standard-dose pantoprazole, lansoprazole, esomeprazole, and rabeprazole are presented in Table 1.9 When a PPI switch is needed, rabeprazole may be considered as it is a PPI that does not rely on CYP2C19 for primary metabolism.9
Acid suppression should be weaned down to the lowest effective dose or converted to H2RAs or other antacids once symptoms are sufficiently controlled unless patients have EE, Barrett’s esophagus, or peptic stricture.3 Patients with severe GERD may require long-term PPI therapy or an invasive anti-reflux procedure.
Recent studies have shown that potassium-competitive acid blockers (PCAB) like vonoprazan may offer more effective gastric acid inhibition. While not included in the latest clinical practice update, vonoprazan is thought to be superior to lansoprazole for those with LA Grade C/D esophagitis for both symptom relief and healing at 2 weeks.10
Adjunctive Therapies
Alginates can function as a physical barrier to even neutral reflux and may be helpful for patients with postprandial or nighttime symptoms as well as those with hiatal hernia.3 H2RAs can also help mitigate nighttime symptoms.3 Baclofen is a gamma-aminobutyric acid–B agonist which inhibits transient lower esophageal sphincter relaxation (TLESR) and may be effective for patients with belching.3 Prokinetics may be helpful for GERD with concomitant gastroparesis.3 Sucralfate is a mucosal protective agent, but there is a lack of data supporting its efficacy in GERD treatment. Consider referral to a behavioral therapist for supplemental therapies, hypnotherapy, cognitive-behavior therapy, diaphragmatic breathing, and relaxation strategies for functional heartburn or reflux-associated esophageal hypervigilance or reflux hypersensitivity.3
When to Refer to Higher Level of Care
For patients who do not wish to remain on longer-term pharmacologic therapy or would benefit from anatomic repair, clinicians should have a discussion of risks and benefits prior to consideration of referral for anti-reflux procedures.3,6,8 We advise this conversation should include review of patient health status, postsurgical side effects such as increased flatus, bloating and dysphagia as well as the potential need to still resume PPI post operation.8
Endoscopic Management
Patient Selection And Evaluation
For the groups indicated for a higher level of care, we agree with AGA recommendations, multi-society guidelines, and expert review,3,7,11,12 and highlight potential options in Table 2. Step-up options should be based on patient characteristics and reviewed carefully with patients. Endoscopic therapies are less invasive than surgery and may be considered for those who do not require anatomic repair of hiatal hernia, do not want surgery, or are not suitable for surgery.
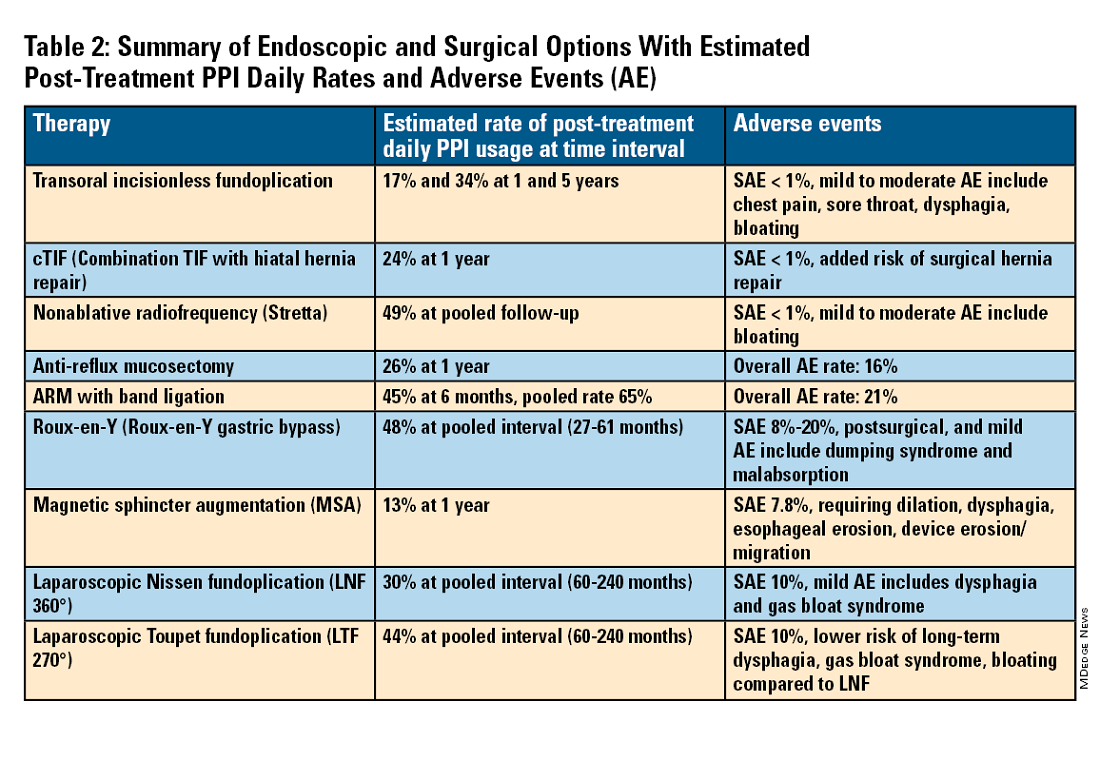
The pathophysiology of GERD is from a loss of the anti-reflux barrier of the esophageal gastric junction (EGJ) at the lower esophageal sphincter (LES) leading to unintended retrograde movement of gastric contents.6 Anatomically, the LES is composed of muscles of the distal esophagus and sling fibers of the proximal stomach, the “external valve” from the diaphragmatic crura, and the “internal valve” from the gastroesophageal flap valve (GEFV). GERD occurs from mechanical failure of the LES. First, there may be disproportional dilation of the diaphragmatic crura as categorized by Hill Grade of the GEFV as seen by a retroflexed view of EGJ after 30-45 seconds of insufflation.13 Second, there may be a migration of the LES away from the diaphragmatic crura as in the case of a hiatal hernia. Provocative maneuvers may reveal a sliding hernia by gentle retraction of the endoscope while under retroflexed view.13 Third, there may be more frequent TLESR associated with GERD.12
The aim of most interventions is to restore competency of the LES by reconstruction of the GEFV via suture or staple-based approximation of tissue.11,12 Intraluminal therapy may only target the GEFV at the internal valve. Therefore, most endoscopic interventions are limited to patients with intact diaphragmatic crura (ie, small to no hiatal hernia and GEFV Hill Grade 1 to 2). Contraindications for endoscopic therapy are moderate to severe reflux (ie, LA Grade C/ D), hiatus hernia 2 cm or larger, strictures, or long-segment Barrett’s esophagus.
Utility, Safety, and Outcomes of TIF
Historically, endoscopic therapy targeting endoscopic fundoplication started with EndoLuminal gastro-gastric fundoplication (ELF, 2005) which was a proof of concept of safe manipulation and suture for gastro-gastric plication to below the Z-line. Transoral incisionless fundoplication (TIF) 1.0 was suggested in 2007 for clinical application by proposing a longitudinal oriented esophago-gastric plication 1 cm above the Z-line.
In 2009, TIF2.0 was proposed as a rotational 270° wrap of the cardia and fundus to a full-thickness esophago-gastric fundoplication around 2-4 cm of the distal esophagus. Like a surgical fundoplication, this reinforces sling fibers, increases the Angle of His and improves the cardiac notch. TIF 2.0 is indicated for those with small (< 2 cm) or no hiatal hernia and a GEFV Hill Grade 1 or 2. The present iteration of TIF2.0 uses EsophyX-Z (EndoGastric Solutions; Redmond, Washington) which features dual fastener deployment and a simplified firing mechanism. Plication is secured via nonresorbable polypropylene T-fasteners with strength equivalence of 3-0 sutures.
Compared with the original, TIF2.0 represents a decrease of severe adverse events from 2%-2.5% to 0.4%-1%.11,14 Based on longitudinal TEMPO data, patient satisfaction ranges between 70% and 90% and rates of patients reverting to daily PPI use are 17% and 34% at 1 and 5 years. A 5% reintervention rate was noted to be comparable with surgical reoperation for fundoplication.15 One retrospective evaluation of patients with failed TIF followed by successful cTIF noted that in all failures there was a documented underestimation of a much larger crura defect at time of index procedure.16 Chest pain is common post procedure and patients and collaborating providers should be counseled on the expected course. In our practice, we admit patients for at least 1 postprocedure day and consider scheduling symptom control medications for those with significant pain.
TIF2.0 for Special Populations
Indications for TIF2.0 continue to evolve. In 2017, concomitant TIF2.0 with hiatal hernia repair (cTIF or HH-TIF) for hernia > 2 cm was accepted for expanded use. In one study, cTIF has been shown to have similar outcomes for postprocedural PPI use, dysphagia, wrap disruption, and hiatal hernia recurrence, compared with hiatal hernia repair paired with laparoscopic Nissen fundoplication with possibly shorter postadmission stay, serious adverse events, and bloating.17 A cTIF may be performed in a single general anesthetic session typically with a surgical hiatal hernia repair followed by TIF2.0.
Other Endoscopic Procedures
Several other endoscopic interventions have been proposed for GERD management. The following procedures are under continuous study and should be considered only by those with expertise.
Stretta
The Stretta device (Restech; Houston, Texas) was approved in 2000 for use of a radiofrequency (RF) generator and catheter applied to the squamocolumnar junction under irrigation. Ideal candidates for this nonablative procedure may include patients with confirmed GERD, low-grade EE, without Barrett’s esophagus, small hiatal hernia, and a competent LES with pressure > 5 mmHg. Meta-analysis has yielded conflicting results in terms of its efficacy, compared with TIF2.0, and recent multi-society guidance suggests fundoplication over Stretta.7
ARM, MASE, and RAP
Anti-reflux mucosectomy (ARM) has been proposed based on the observation that patients undergoing mucosectomy for neoplasms in the cardia had improvement of reflux symptoms.11,12 Systematic review has suggested a clinical response of 80% of either PPI discontinuation or reduction, but 17% of adverse events include development of strictures. Iterations of ARM continue to be studied including ARM with band ligation (L-ARM) and endoscopic submucosal dissection for GERD (ESD-G).12
Experts have proposed incorporating endoscopic suturing of the EGJ to modulate the LES. Mucosal ablation and suturing of the EG junction (MASE) has been proposed by first priming tissue via argon plasma coagulation (APC) prior to endoscopic overstitch of two to three interrupted sutures below the EGJ to narrow and elongate the EGJ. The resection and plication (RAP) procedure performs a mucosal resection prior to full-thickness plication of the LES and cardia.11,12 Expert opinion has suggested that RAP may be used in patients with altered anatomy whereas MASE may be used when resection is not possible (eg, prior scarring, resection or ablation).12
Surgical Management
We agree with a recent multi-society guideline recommending that an interdisciplinary consultation with surgery for indicated patients with refractory GERD and underlying hiatal hernia, or who do not want lifelong medical therapy.
Fundoplication creates a surgical wrap to reinforce the LES and may be performed laparoscopically. Contraindications include body mass index (BMI) >35 kg/m2 and significantly impaired dysmotility. Fundoplication of 180°, 270°, and 360° may achieve comparable outcomes, but a laparoscopic toupet fundoplication (LTF 270°) may have fewer postsurgical issues of dysphagia and bloating. Advantages for both anterior and posterior partial fundoplications have been demonstrated by network meta-analysis. Therefore, a multi-society guideline for GERD suggests partial over complete fundoplication.7 Compared with posterior techniques, anterior fundoplication (Watson fundoplication) led to more recurrent reflux symptoms but less dysphagia and other side effects.19
Magnetic sphincter augmentation (MSA) is a surgical option that strengthens the LES with magnets to improve sphincter competence. In addition to listed contraindications of fundoplication, patients with an allergy to nickel and/or titanium are also contraindicated to receive MSA.7 MSA has been suggested to be equivalent to LNF although there may be less gas bloat and greater ability to belch on follow up.20
Surgical Options for Special Populations
Patients with medically refractory GERD and a BMI ≥ 35 kg/m2 may benefit from either Roux-en-Y gastric bypass (RYGB) or fundoplication, however sleeve gastrectomy is not advised.7 In patients with BMI > 50 kg/m2, RYGB may provide an optimal choice. We agree with consultation with a bariatric surgeon when reviewing these situations.
Conclusion
Patients with GERD are commonly encountered worldwide. Empiric PPI are effective mainstays for medical treatment of GERD. Novel PCABs (e.g., vonoprazan) may present new options for GERD with LA Grade C/D esophagitis EE and merit more study. In refractory cases or for patients who do not want long term medical therapy, step-up therapy may be considered via endoscopic or surgical interventions. Patient anatomy and comorbidities should be considered by the clinician to inform treatment options. Surgery may have the most durable outcomes for those requiring step-up therapy. Improvements in technique, devices and patient selection have allowed TIF2.0 to grow as a viable offering with excellent 5-year outcomes for indicated patients.
Dr. Chang, Dr. Tintara, and Dr. Phan are based in the Division of Gastrointestinal and Liver Disease at the University of Southern California in Los Angeles. They have no conflicts of interest to declare.
References
1. Richter JE andRubenstein JH. Gastroenterology. 2018 Jan. doi: 10.1053/j.gastro.2017.07.045.
2. El-Serag HB et al. Gut. 2014 Jun. doi: 10.1136/gutjnl-2012-304269.
3. Yadlapati R et al. Clin Gastroenterol Hepatol. 2022 May. doi: 10.1016/j.cgh.2022.01.025.
4. Vakil N et al. Am J Gastroenterol. 2006 Aug. doi: 10.1111/j.1572-0241.2006.00630.x.
5. Numans ME et al. Ann Intern Med. 2004 Apr. doi: 10.7326/0003-4819-140-7-200404060-00011.
6. Kahrilas PJ et al. Gastroenterology. 2008 Oct. doi: 10.1053/j.gastro.2008.08.045.
7. Slater BJ et al. Surg Endosc. 2023 Feb. doi: 10.1007/s00464-022-09817-3.
8. Gyawali CP et al. Gut. 2018 Jul. doi:10.1136/gutjnl-2017-314722.
9. Graham DY and Tansel A. Clin Gastroenterol Hepatol. 2018 Jun. doi: 10.1016/j.cgh.2017.09.033.
10. Graham DY and Dore MP. Gastroenterology. 2018 Feb. doi:10.1053/j.gastro.2018.01.018.
11. Haseeb M and Thompson CC. Curr Opin Gastroenterol. 2023 Sep. doi: 10.1097/MOG.0000000000000968.
12. Kolb JM and Chang KJ. Curr Opin Gastroenterol. 2023 Jul. doi:10.1097/MOG.0000000000000944.
13. Nguyen NT et al. Foregut. 2022 Sep. doi: 10.1177/26345161221126961.
14. Mazzoleni G et al. Endosc Int Open. 2021 Feb. doi: 10.1055/a-1322-2209.
15. Trad KS et al. Surg Innov. 2018 Apr. doi: 10.1177/1553350618755214.
16. Kolb JM et al. Gastroenterology. 2021 May. doi: 10.1016/S0016-5085(21)02953-X.
17. Jaruvongvanich VK et al. Endosc Int Open. 2023 Jan. doi: 10.1055/a-1972-9190.
18. Lee Y et al. Surg Endosc. 2023 Jul. doi: 10.1007/s00464-023-10151-5.
19. Andreou A et al. Surg Endosc. 2020 Feb. doi: 10.1007/s00464-019-07208-9.
20. Guidozzi N et al. Dis Esophagus. 2019 Nov. doi: 10.1093/dote/doz031.
Introduction
Gastroesophageal reflux disease (GERD) is a frequently encountered condition, and rising annually.1 A recent meta-analysis suggests nearly 14% (1.03 billion) of the population are affected worldwide. Differences may range by region from 12% in Latin America to 20% in North America, and by country from 4% in China to 23% in Turkey.1 In the United States, 21% of the population are afflicted with weekly GERD symptoms.2 Novel medical therapies and endoscopic options provide clinicians with opportunities to help patients with GERD.3
Diagnosis
Definition
GERD was originally defined by the Montreal consensus as a condition that develops when the reflux of stomach contents causes troublesome symptoms and/or complications.4 Heartburn and regurgitation are common symptoms of GERD, with a sensitivity of 30%-76% and specificity of 62%-96% for erosive esophagitis (EE), which occurs when the reflux of stomach content causes esophageal mucosal breaks.5 The presence of characteristic mucosal injury observed during an upper endoscopy or abnormal esophageal acid exposure on ambulatory reflux monitoring are objective evidence of GERD. A trial of a proton pump inhibitor (PPI) may function as a diagnostic test for patients exhibiting the typical symptoms of GERD without any alarm symptoms.3,6
Endoscopic Evaluation and Confirmation
The 2022 American Gastroenterological Association (AGA) clinical practice update recommends diagnostic endoscopy, after PPIs are stopped for 2-4 weeks, in patients whose GERD symptoms do not respond adequately to an empiric trial of a PPI.3 Those with GERD and alarm symptoms such as dysphagia, weight loss, bleeding, and vomiting should undergo endoscopy as soon as possible. Endoscopic findings of EE (Los Angeles Grade B or more severe) and long-segment Barrett’s esophagus (> 3-cm segment with intestinal metaplasia on biopsy) are diagnostic of GERD.3
Reflux Monitoring
With ambulatory reflux monitoring (pH or impedance-pH), esophageal acid exposure (or neutral refluxate in impedance testing) can be measured to confirm GERD diagnosis and to correlate symptoms with reflux episodes. Patients with atypical GERD symptoms or patients with a confirmed diagnosis of GERD whose symptoms have not improved sufficiently with twice-daily PPI therapy should have esophageal impedance-pH monitoring while on PPIs.6,7
Esophageal Manometry
High-resolution esophageal manometry can be used to assess motility abnormalities associated with GERD.
Although no manometric abnormality is unique to GERD, weak lower esophageal sphincter (LES) resting pressure and ineffective esophageal motility frequently coexist with severe GERD.6
Manometry is particularly useful in patients considering surgical or endoscopic anti-reflux procedures to evaluate for achalasia,3 an important contraindication to surgery.
Medical Management
Management of GERD requires a multidisciplinary and personalized approach based on symptom presentation, body mass index, endoscopic findings (e.g., presence of EE, Barrett’s esophagus, hiatal hernia), and physiological abnormalities (e.g., gastroparesis or ineffective motility).3
Lifestyle Modifications
Recommended lifestyle modifications include weight loss for patients with obesity, stress reduction, tobacco and alcohol cessation, elevating the head of the bed, staying upright during and after meals, avoidance of food intake < 3 hours before bedtime, and cessation of foods that potentially aggravate reflux symptoms such as coffee, chocolate, carbonated beverages, spicy foods, acidic foods, and foods with high fat content.6,8
Medications
Pharmacologic therapy for GERD includes medications that primarily aim to neutralize or reduce gastric acid -- we summarize options in Table 1.3,8

Proton Pump Inhibitors
Most guidelines suggest a trial of 4-8 weeks of once-daily enteric-coated PPI before meals in patients with typical GERD symptoms and no alarm symptoms. Escalation to double-dose PPI may be considered in the case of persistent symptoms. The relative potencies of standard-dose pantoprazole, lansoprazole, esomeprazole, and rabeprazole are presented in Table 1.9 When a PPI switch is needed, rabeprazole may be considered as it is a PPI that does not rely on CYP2C19 for primary metabolism.9
Acid suppression should be weaned down to the lowest effective dose or converted to H2RAs or other antacids once symptoms are sufficiently controlled unless patients have EE, Barrett’s esophagus, or peptic stricture.3 Patients with severe GERD may require long-term PPI therapy or an invasive anti-reflux procedure.
Recent studies have shown that potassium-competitive acid blockers (PCAB) like vonoprazan may offer more effective gastric acid inhibition. While not included in the latest clinical practice update, vonoprazan is thought to be superior to lansoprazole for those with LA Grade C/D esophagitis for both symptom relief and healing at 2 weeks.10
Adjunctive Therapies
Alginates can function as a physical barrier to even neutral reflux and may be helpful for patients with postprandial or nighttime symptoms as well as those with hiatal hernia.3 H2RAs can also help mitigate nighttime symptoms.3 Baclofen is a gamma-aminobutyric acid–B agonist which inhibits transient lower esophageal sphincter relaxation (TLESR) and may be effective for patients with belching.3 Prokinetics may be helpful for GERD with concomitant gastroparesis.3 Sucralfate is a mucosal protective agent, but there is a lack of data supporting its efficacy in GERD treatment. Consider referral to a behavioral therapist for supplemental therapies, hypnotherapy, cognitive-behavior therapy, diaphragmatic breathing, and relaxation strategies for functional heartburn or reflux-associated esophageal hypervigilance or reflux hypersensitivity.3
When to Refer to Higher Level of Care
For patients who do not wish to remain on longer-term pharmacologic therapy or would benefit from anatomic repair, clinicians should have a discussion of risks and benefits prior to consideration of referral for anti-reflux procedures.3,6,8 We advise this conversation should include review of patient health status, postsurgical side effects such as increased flatus, bloating and dysphagia as well as the potential need to still resume PPI post operation.8
Endoscopic Management
Patient Selection And Evaluation
For the groups indicated for a higher level of care, we agree with AGA recommendations, multi-society guidelines, and expert review,3,7,11,12 and highlight potential options in Table 2. Step-up options should be based on patient characteristics and reviewed carefully with patients. Endoscopic therapies are less invasive than surgery and may be considered for those who do not require anatomic repair of hiatal hernia, do not want surgery, or are not suitable for surgery.

The pathophysiology of GERD is from a loss of the anti-reflux barrier of the esophageal gastric junction (EGJ) at the lower esophageal sphincter (LES) leading to unintended retrograde movement of gastric contents.6 Anatomically, the LES is composed of muscles of the distal esophagus and sling fibers of the proximal stomach, the “external valve” from the diaphragmatic crura, and the “internal valve” from the gastroesophageal flap valve (GEFV). GERD occurs from mechanical failure of the LES. First, there may be disproportional dilation of the diaphragmatic crura as categorized by Hill Grade of the GEFV as seen by a retroflexed view of EGJ after 30-45 seconds of insufflation.13 Second, there may be a migration of the LES away from the diaphragmatic crura as in the case of a hiatal hernia. Provocative maneuvers may reveal a sliding hernia by gentle retraction of the endoscope while under retroflexed view.13 Third, there may be more frequent TLESR associated with GERD.12
The aim of most interventions is to restore competency of the LES by reconstruction of the GEFV via suture or staple-based approximation of tissue.11,12 Intraluminal therapy may only target the GEFV at the internal valve. Therefore, most endoscopic interventions are limited to patients with intact diaphragmatic crura (ie, small to no hiatal hernia and GEFV Hill Grade 1 to 2). Contraindications for endoscopic therapy are moderate to severe reflux (ie, LA Grade C/ D), hiatus hernia 2 cm or larger, strictures, or long-segment Barrett’s esophagus.
Utility, Safety, and Outcomes of TIF
Historically, endoscopic therapy targeting endoscopic fundoplication started with EndoLuminal gastro-gastric fundoplication (ELF, 2005) which was a proof of concept of safe manipulation and suture for gastro-gastric plication to below the Z-line. Transoral incisionless fundoplication (TIF) 1.0 was suggested in 2007 for clinical application by proposing a longitudinal oriented esophago-gastric plication 1 cm above the Z-line.
In 2009, TIF2.0 was proposed as a rotational 270° wrap of the cardia and fundus to a full-thickness esophago-gastric fundoplication around 2-4 cm of the distal esophagus. Like a surgical fundoplication, this reinforces sling fibers, increases the Angle of His and improves the cardiac notch. TIF 2.0 is indicated for those with small (< 2 cm) or no hiatal hernia and a GEFV Hill Grade 1 or 2. The present iteration of TIF2.0 uses EsophyX-Z (EndoGastric Solutions; Redmond, Washington) which features dual fastener deployment and a simplified firing mechanism. Plication is secured via nonresorbable polypropylene T-fasteners with strength equivalence of 3-0 sutures.
Compared with the original, TIF2.0 represents a decrease of severe adverse events from 2%-2.5% to 0.4%-1%.11,14 Based on longitudinal TEMPO data, patient satisfaction ranges between 70% and 90% and rates of patients reverting to daily PPI use are 17% and 34% at 1 and 5 years. A 5% reintervention rate was noted to be comparable with surgical reoperation for fundoplication.15 One retrospective evaluation of patients with failed TIF followed by successful cTIF noted that in all failures there was a documented underestimation of a much larger crura defect at time of index procedure.16 Chest pain is common post procedure and patients and collaborating providers should be counseled on the expected course. In our practice, we admit patients for at least 1 postprocedure day and consider scheduling symptom control medications for those with significant pain.
TIF2.0 for Special Populations
Indications for TIF2.0 continue to evolve. In 2017, concomitant TIF2.0 with hiatal hernia repair (cTIF or HH-TIF) for hernia > 2 cm was accepted for expanded use. In one study, cTIF has been shown to have similar outcomes for postprocedural PPI use, dysphagia, wrap disruption, and hiatal hernia recurrence, compared with hiatal hernia repair paired with laparoscopic Nissen fundoplication with possibly shorter postadmission stay, serious adverse events, and bloating.17 A cTIF may be performed in a single general anesthetic session typically with a surgical hiatal hernia repair followed by TIF2.0.
Other Endoscopic Procedures
Several other endoscopic interventions have been proposed for GERD management. The following procedures are under continuous study and should be considered only by those with expertise.
Stretta
The Stretta device (Restech; Houston, Texas) was approved in 2000 for use of a radiofrequency (RF) generator and catheter applied to the squamocolumnar junction under irrigation. Ideal candidates for this nonablative procedure may include patients with confirmed GERD, low-grade EE, without Barrett’s esophagus, small hiatal hernia, and a competent LES with pressure > 5 mmHg. Meta-analysis has yielded conflicting results in terms of its efficacy, compared with TIF2.0, and recent multi-society guidance suggests fundoplication over Stretta.7
ARM, MASE, and RAP
Anti-reflux mucosectomy (ARM) has been proposed based on the observation that patients undergoing mucosectomy for neoplasms in the cardia had improvement of reflux symptoms.11,12 Systematic review has suggested a clinical response of 80% of either PPI discontinuation or reduction, but 17% of adverse events include development of strictures. Iterations of ARM continue to be studied including ARM with band ligation (L-ARM) and endoscopic submucosal dissection for GERD (ESD-G).12
Experts have proposed incorporating endoscopic suturing of the EGJ to modulate the LES. Mucosal ablation and suturing of the EG junction (MASE) has been proposed by first priming tissue via argon plasma coagulation (APC) prior to endoscopic overstitch of two to three interrupted sutures below the EGJ to narrow and elongate the EGJ. The resection and plication (RAP) procedure performs a mucosal resection prior to full-thickness plication of the LES and cardia.11,12 Expert opinion has suggested that RAP may be used in patients with altered anatomy whereas MASE may be used when resection is not possible (eg, prior scarring, resection or ablation).12
Surgical Management
We agree with a recent multi-society guideline recommending that an interdisciplinary consultation with surgery for indicated patients with refractory GERD and underlying hiatal hernia, or who do not want lifelong medical therapy.
Fundoplication creates a surgical wrap to reinforce the LES and may be performed laparoscopically. Contraindications include body mass index (BMI) >35 kg/m2 and significantly impaired dysmotility. Fundoplication of 180°, 270°, and 360° may achieve comparable outcomes, but a laparoscopic toupet fundoplication (LTF 270°) may have fewer postsurgical issues of dysphagia and bloating. Advantages for both anterior and posterior partial fundoplications have been demonstrated by network meta-analysis. Therefore, a multi-society guideline for GERD suggests partial over complete fundoplication.7 Compared with posterior techniques, anterior fundoplication (Watson fundoplication) led to more recurrent reflux symptoms but less dysphagia and other side effects.19
Magnetic sphincter augmentation (MSA) is a surgical option that strengthens the LES with magnets to improve sphincter competence. In addition to listed contraindications of fundoplication, patients with an allergy to nickel and/or titanium are also contraindicated to receive MSA.7 MSA has been suggested to be equivalent to LNF although there may be less gas bloat and greater ability to belch on follow up.20
Surgical Options for Special Populations
Patients with medically refractory GERD and a BMI ≥ 35 kg/m2 may benefit from either Roux-en-Y gastric bypass (RYGB) or fundoplication, however sleeve gastrectomy is not advised.7 In patients with BMI > 50 kg/m2, RYGB may provide an optimal choice. We agree with consultation with a bariatric surgeon when reviewing these situations.
Conclusion
Patients with GERD are commonly encountered worldwide. Empiric PPI are effective mainstays for medical treatment of GERD. Novel PCABs (e.g., vonoprazan) may present new options for GERD with LA Grade C/D esophagitis EE and merit more study. In refractory cases or for patients who do not want long term medical therapy, step-up therapy may be considered via endoscopic or surgical interventions. Patient anatomy and comorbidities should be considered by the clinician to inform treatment options. Surgery may have the most durable outcomes for those requiring step-up therapy. Improvements in technique, devices and patient selection have allowed TIF2.0 to grow as a viable offering with excellent 5-year outcomes for indicated patients.
Dr. Chang, Dr. Tintara, and Dr. Phan are based in the Division of Gastrointestinal and Liver Disease at the University of Southern California in Los Angeles. They have no conflicts of interest to declare.
References
1. Richter JE andRubenstein JH. Gastroenterology. 2018 Jan. doi: 10.1053/j.gastro.2017.07.045.
2. El-Serag HB et al. Gut. 2014 Jun. doi: 10.1136/gutjnl-2012-304269.
3. Yadlapati R et al. Clin Gastroenterol Hepatol. 2022 May. doi: 10.1016/j.cgh.2022.01.025.
4. Vakil N et al. Am J Gastroenterol. 2006 Aug. doi: 10.1111/j.1572-0241.2006.00630.x.
5. Numans ME et al. Ann Intern Med. 2004 Apr. doi: 10.7326/0003-4819-140-7-200404060-00011.
6. Kahrilas PJ et al. Gastroenterology. 2008 Oct. doi: 10.1053/j.gastro.2008.08.045.
7. Slater BJ et al. Surg Endosc. 2023 Feb. doi: 10.1007/s00464-022-09817-3.
8. Gyawali CP et al. Gut. 2018 Jul. doi:10.1136/gutjnl-2017-314722.
9. Graham DY and Tansel A. Clin Gastroenterol Hepatol. 2018 Jun. doi: 10.1016/j.cgh.2017.09.033.
10. Graham DY and Dore MP. Gastroenterology. 2018 Feb. doi:10.1053/j.gastro.2018.01.018.
11. Haseeb M and Thompson CC. Curr Opin Gastroenterol. 2023 Sep. doi: 10.1097/MOG.0000000000000968.
12. Kolb JM and Chang KJ. Curr Opin Gastroenterol. 2023 Jul. doi:10.1097/MOG.0000000000000944.
13. Nguyen NT et al. Foregut. 2022 Sep. doi: 10.1177/26345161221126961.
14. Mazzoleni G et al. Endosc Int Open. 2021 Feb. doi: 10.1055/a-1322-2209.
15. Trad KS et al. Surg Innov. 2018 Apr. doi: 10.1177/1553350618755214.
16. Kolb JM et al. Gastroenterology. 2021 May. doi: 10.1016/S0016-5085(21)02953-X.
17. Jaruvongvanich VK et al. Endosc Int Open. 2023 Jan. doi: 10.1055/a-1972-9190.
18. Lee Y et al. Surg Endosc. 2023 Jul. doi: 10.1007/s00464-023-10151-5.
19. Andreou A et al. Surg Endosc. 2020 Feb. doi: 10.1007/s00464-019-07208-9.
20. Guidozzi N et al. Dis Esophagus. 2019 Nov. doi: 10.1093/dote/doz031.
Introduction
Gastroesophageal reflux disease (GERD) is a frequently encountered condition, and rising annually.1 A recent meta-analysis suggests nearly 14% (1.03 billion) of the population are affected worldwide. Differences may range by region from 12% in Latin America to 20% in North America, and by country from 4% in China to 23% in Turkey.1 In the United States, 21% of the population are afflicted with weekly GERD symptoms.2 Novel medical therapies and endoscopic options provide clinicians with opportunities to help patients with GERD.3
Diagnosis
Definition
GERD was originally defined by the Montreal consensus as a condition that develops when the reflux of stomach contents causes troublesome symptoms and/or complications.4 Heartburn and regurgitation are common symptoms of GERD, with a sensitivity of 30%-76% and specificity of 62%-96% for erosive esophagitis (EE), which occurs when the reflux of stomach content causes esophageal mucosal breaks.5 The presence of characteristic mucosal injury observed during an upper endoscopy or abnormal esophageal acid exposure on ambulatory reflux monitoring are objective evidence of GERD. A trial of a proton pump inhibitor (PPI) may function as a diagnostic test for patients exhibiting the typical symptoms of GERD without any alarm symptoms.3,6
Endoscopic Evaluation and Confirmation
The 2022 American Gastroenterological Association (AGA) clinical practice update recommends diagnostic endoscopy, after PPIs are stopped for 2-4 weeks, in patients whose GERD symptoms do not respond adequately to an empiric trial of a PPI.3 Those with GERD and alarm symptoms such as dysphagia, weight loss, bleeding, and vomiting should undergo endoscopy as soon as possible. Endoscopic findings of EE (Los Angeles Grade B or more severe) and long-segment Barrett’s esophagus (> 3-cm segment with intestinal metaplasia on biopsy) are diagnostic of GERD.3
Reflux Monitoring
With ambulatory reflux monitoring (pH or impedance-pH), esophageal acid exposure (or neutral refluxate in impedance testing) can be measured to confirm GERD diagnosis and to correlate symptoms with reflux episodes. Patients with atypical GERD symptoms or patients with a confirmed diagnosis of GERD whose symptoms have not improved sufficiently with twice-daily PPI therapy should have esophageal impedance-pH monitoring while on PPIs.6,7
Esophageal Manometry
High-resolution esophageal manometry can be used to assess motility abnormalities associated with GERD.
Although no manometric abnormality is unique to GERD, weak lower esophageal sphincter (LES) resting pressure and ineffective esophageal motility frequently coexist with severe GERD.6
Manometry is particularly useful in patients considering surgical or endoscopic anti-reflux procedures to evaluate for achalasia,3 an important contraindication to surgery.
Medical Management
Management of GERD requires a multidisciplinary and personalized approach based on symptom presentation, body mass index, endoscopic findings (e.g., presence of EE, Barrett’s esophagus, hiatal hernia), and physiological abnormalities (e.g., gastroparesis or ineffective motility).3
Lifestyle Modifications
Recommended lifestyle modifications include weight loss for patients with obesity, stress reduction, tobacco and alcohol cessation, elevating the head of the bed, staying upright during and after meals, avoidance of food intake < 3 hours before bedtime, and cessation of foods that potentially aggravate reflux symptoms such as coffee, chocolate, carbonated beverages, spicy foods, acidic foods, and foods with high fat content.6,8
Medications
Pharmacologic therapy for GERD includes medications that primarily aim to neutralize or reduce gastric acid -- we summarize options in Table 1.3,8

Proton Pump Inhibitors
Most guidelines suggest a trial of 4-8 weeks of once-daily enteric-coated PPI before meals in patients with typical GERD symptoms and no alarm symptoms. Escalation to double-dose PPI may be considered in the case of persistent symptoms. The relative potencies of standard-dose pantoprazole, lansoprazole, esomeprazole, and rabeprazole are presented in Table 1.9 When a PPI switch is needed, rabeprazole may be considered as it is a PPI that does not rely on CYP2C19 for primary metabolism.9
Acid suppression should be weaned down to the lowest effective dose or converted to H2RAs or other antacids once symptoms are sufficiently controlled unless patients have EE, Barrett’s esophagus, or peptic stricture.3 Patients with severe GERD may require long-term PPI therapy or an invasive anti-reflux procedure.
Recent studies have shown that potassium-competitive acid blockers (PCAB) like vonoprazan may offer more effective gastric acid inhibition. While not included in the latest clinical practice update, vonoprazan is thought to be superior to lansoprazole for those with LA Grade C/D esophagitis for both symptom relief and healing at 2 weeks.10
Adjunctive Therapies
Alginates can function as a physical barrier to even neutral reflux and may be helpful for patients with postprandial or nighttime symptoms as well as those with hiatal hernia.3 H2RAs can also help mitigate nighttime symptoms.3 Baclofen is a gamma-aminobutyric acid–B agonist which inhibits transient lower esophageal sphincter relaxation (TLESR) and may be effective for patients with belching.3 Prokinetics may be helpful for GERD with concomitant gastroparesis.3 Sucralfate is a mucosal protective agent, but there is a lack of data supporting its efficacy in GERD treatment. Consider referral to a behavioral therapist for supplemental therapies, hypnotherapy, cognitive-behavior therapy, diaphragmatic breathing, and relaxation strategies for functional heartburn or reflux-associated esophageal hypervigilance or reflux hypersensitivity.3
When to Refer to Higher Level of Care
For patients who do not wish to remain on longer-term pharmacologic therapy or would benefit from anatomic repair, clinicians should have a discussion of risks and benefits prior to consideration of referral for anti-reflux procedures.3,6,8 We advise this conversation should include review of patient health status, postsurgical side effects such as increased flatus, bloating and dysphagia as well as the potential need to still resume PPI post operation.8
Endoscopic Management
Patient Selection And Evaluation
For the groups indicated for a higher level of care, we agree with AGA recommendations, multi-society guidelines, and expert review,3,7,11,12 and highlight potential options in Table 2. Step-up options should be based on patient characteristics and reviewed carefully with patients. Endoscopic therapies are less invasive than surgery and may be considered for those who do not require anatomic repair of hiatal hernia, do not want surgery, or are not suitable for surgery.

The pathophysiology of GERD is from a loss of the anti-reflux barrier of the esophageal gastric junction (EGJ) at the lower esophageal sphincter (LES) leading to unintended retrograde movement of gastric contents.6 Anatomically, the LES is composed of muscles of the distal esophagus and sling fibers of the proximal stomach, the “external valve” from the diaphragmatic crura, and the “internal valve” from the gastroesophageal flap valve (GEFV). GERD occurs from mechanical failure of the LES. First, there may be disproportional dilation of the diaphragmatic crura as categorized by Hill Grade of the GEFV as seen by a retroflexed view of EGJ after 30-45 seconds of insufflation.13 Second, there may be a migration of the LES away from the diaphragmatic crura as in the case of a hiatal hernia. Provocative maneuvers may reveal a sliding hernia by gentle retraction of the endoscope while under retroflexed view.13 Third, there may be more frequent TLESR associated with GERD.12
The aim of most interventions is to restore competency of the LES by reconstruction of the GEFV via suture or staple-based approximation of tissue.11,12 Intraluminal therapy may only target the GEFV at the internal valve. Therefore, most endoscopic interventions are limited to patients with intact diaphragmatic crura (ie, small to no hiatal hernia and GEFV Hill Grade 1 to 2). Contraindications for endoscopic therapy are moderate to severe reflux (ie, LA Grade C/ D), hiatus hernia 2 cm or larger, strictures, or long-segment Barrett’s esophagus.
Utility, Safety, and Outcomes of TIF
Historically, endoscopic therapy targeting endoscopic fundoplication started with EndoLuminal gastro-gastric fundoplication (ELF, 2005) which was a proof of concept of safe manipulation and suture for gastro-gastric plication to below the Z-line. Transoral incisionless fundoplication (TIF) 1.0 was suggested in 2007 for clinical application by proposing a longitudinal oriented esophago-gastric plication 1 cm above the Z-line.
In 2009, TIF2.0 was proposed as a rotational 270° wrap of the cardia and fundus to a full-thickness esophago-gastric fundoplication around 2-4 cm of the distal esophagus. Like a surgical fundoplication, this reinforces sling fibers, increases the Angle of His and improves the cardiac notch. TIF 2.0 is indicated for those with small (< 2 cm) or no hiatal hernia and a GEFV Hill Grade 1 or 2. The present iteration of TIF2.0 uses EsophyX-Z (EndoGastric Solutions; Redmond, Washington) which features dual fastener deployment and a simplified firing mechanism. Plication is secured via nonresorbable polypropylene T-fasteners with strength equivalence of 3-0 sutures.
Compared with the original, TIF2.0 represents a decrease of severe adverse events from 2%-2.5% to 0.4%-1%.11,14 Based on longitudinal TEMPO data, patient satisfaction ranges between 70% and 90% and rates of patients reverting to daily PPI use are 17% and 34% at 1 and 5 years. A 5% reintervention rate was noted to be comparable with surgical reoperation for fundoplication.15 One retrospective evaluation of patients with failed TIF followed by successful cTIF noted that in all failures there was a documented underestimation of a much larger crura defect at time of index procedure.16 Chest pain is common post procedure and patients and collaborating providers should be counseled on the expected course. In our practice, we admit patients for at least 1 postprocedure day and consider scheduling symptom control medications for those with significant pain.
TIF2.0 for Special Populations
Indications for TIF2.0 continue to evolve. In 2017, concomitant TIF2.0 with hiatal hernia repair (cTIF or HH-TIF) for hernia > 2 cm was accepted for expanded use. In one study, cTIF has been shown to have similar outcomes for postprocedural PPI use, dysphagia, wrap disruption, and hiatal hernia recurrence, compared with hiatal hernia repair paired with laparoscopic Nissen fundoplication with possibly shorter postadmission stay, serious adverse events, and bloating.17 A cTIF may be performed in a single general anesthetic session typically with a surgical hiatal hernia repair followed by TIF2.0.
Other Endoscopic Procedures
Several other endoscopic interventions have been proposed for GERD management. The following procedures are under continuous study and should be considered only by those with expertise.
Stretta
The Stretta device (Restech; Houston, Texas) was approved in 2000 for use of a radiofrequency (RF) generator and catheter applied to the squamocolumnar junction under irrigation. Ideal candidates for this nonablative procedure may include patients with confirmed GERD, low-grade EE, without Barrett’s esophagus, small hiatal hernia, and a competent LES with pressure > 5 mmHg. Meta-analysis has yielded conflicting results in terms of its efficacy, compared with TIF2.0, and recent multi-society guidance suggests fundoplication over Stretta.7
ARM, MASE, and RAP
Anti-reflux mucosectomy (ARM) has been proposed based on the observation that patients undergoing mucosectomy for neoplasms in the cardia had improvement of reflux symptoms.11,12 Systematic review has suggested a clinical response of 80% of either PPI discontinuation or reduction, but 17% of adverse events include development of strictures. Iterations of ARM continue to be studied including ARM with band ligation (L-ARM) and endoscopic submucosal dissection for GERD (ESD-G).12
Experts have proposed incorporating endoscopic suturing of the EGJ to modulate the LES. Mucosal ablation and suturing of the EG junction (MASE) has been proposed by first priming tissue via argon plasma coagulation (APC) prior to endoscopic overstitch of two to three interrupted sutures below the EGJ to narrow and elongate the EGJ. The resection and plication (RAP) procedure performs a mucosal resection prior to full-thickness plication of the LES and cardia.11,12 Expert opinion has suggested that RAP may be used in patients with altered anatomy whereas MASE may be used when resection is not possible (eg, prior scarring, resection or ablation).12
Surgical Management
We agree with a recent multi-society guideline recommending that an interdisciplinary consultation with surgery for indicated patients with refractory GERD and underlying hiatal hernia, or who do not want lifelong medical therapy.
Fundoplication creates a surgical wrap to reinforce the LES and may be performed laparoscopically. Contraindications include body mass index (BMI) >35 kg/m2 and significantly impaired dysmotility. Fundoplication of 180°, 270°, and 360° may achieve comparable outcomes, but a laparoscopic toupet fundoplication (LTF 270°) may have fewer postsurgical issues of dysphagia and bloating. Advantages for both anterior and posterior partial fundoplications have been demonstrated by network meta-analysis. Therefore, a multi-society guideline for GERD suggests partial over complete fundoplication.7 Compared with posterior techniques, anterior fundoplication (Watson fundoplication) led to more recurrent reflux symptoms but less dysphagia and other side effects.19
Magnetic sphincter augmentation (MSA) is a surgical option that strengthens the LES with magnets to improve sphincter competence. In addition to listed contraindications of fundoplication, patients with an allergy to nickel and/or titanium are also contraindicated to receive MSA.7 MSA has been suggested to be equivalent to LNF although there may be less gas bloat and greater ability to belch on follow up.20
Surgical Options for Special Populations
Patients with medically refractory GERD and a BMI ≥ 35 kg/m2 may benefit from either Roux-en-Y gastric bypass (RYGB) or fundoplication, however sleeve gastrectomy is not advised.7 In patients with BMI > 50 kg/m2, RYGB may provide an optimal choice. We agree with consultation with a bariatric surgeon when reviewing these situations.
Conclusion
Patients with GERD are commonly encountered worldwide. Empiric PPI are effective mainstays for medical treatment of GERD. Novel PCABs (e.g., vonoprazan) may present new options for GERD with LA Grade C/D esophagitis EE and merit more study. In refractory cases or for patients who do not want long term medical therapy, step-up therapy may be considered via endoscopic or surgical interventions. Patient anatomy and comorbidities should be considered by the clinician to inform treatment options. Surgery may have the most durable outcomes for those requiring step-up therapy. Improvements in technique, devices and patient selection have allowed TIF2.0 to grow as a viable offering with excellent 5-year outcomes for indicated patients.
Dr. Chang, Dr. Tintara, and Dr. Phan are based in the Division of Gastrointestinal and Liver Disease at the University of Southern California in Los Angeles. They have no conflicts of interest to declare.
References
1. Richter JE andRubenstein JH. Gastroenterology. 2018 Jan. doi: 10.1053/j.gastro.2017.07.045.
2. El-Serag HB et al. Gut. 2014 Jun. doi: 10.1136/gutjnl-2012-304269.
3. Yadlapati R et al. Clin Gastroenterol Hepatol. 2022 May. doi: 10.1016/j.cgh.2022.01.025.
4. Vakil N et al. Am J Gastroenterol. 2006 Aug. doi: 10.1111/j.1572-0241.2006.00630.x.
5. Numans ME et al. Ann Intern Med. 2004 Apr. doi: 10.7326/0003-4819-140-7-200404060-00011.
6. Kahrilas PJ et al. Gastroenterology. 2008 Oct. doi: 10.1053/j.gastro.2008.08.045.
7. Slater BJ et al. Surg Endosc. 2023 Feb. doi: 10.1007/s00464-022-09817-3.
8. Gyawali CP et al. Gut. 2018 Jul. doi:10.1136/gutjnl-2017-314722.
9. Graham DY and Tansel A. Clin Gastroenterol Hepatol. 2018 Jun. doi: 10.1016/j.cgh.2017.09.033.
10. Graham DY and Dore MP. Gastroenterology. 2018 Feb. doi:10.1053/j.gastro.2018.01.018.
11. Haseeb M and Thompson CC. Curr Opin Gastroenterol. 2023 Sep. doi: 10.1097/MOG.0000000000000968.
12. Kolb JM and Chang KJ. Curr Opin Gastroenterol. 2023 Jul. doi:10.1097/MOG.0000000000000944.
13. Nguyen NT et al. Foregut. 2022 Sep. doi: 10.1177/26345161221126961.
14. Mazzoleni G et al. Endosc Int Open. 2021 Feb. doi: 10.1055/a-1322-2209.
15. Trad KS et al. Surg Innov. 2018 Apr. doi: 10.1177/1553350618755214.
16. Kolb JM et al. Gastroenterology. 2021 May. doi: 10.1016/S0016-5085(21)02953-X.
17. Jaruvongvanich VK et al. Endosc Int Open. 2023 Jan. doi: 10.1055/a-1972-9190.
18. Lee Y et al. Surg Endosc. 2023 Jul. doi: 10.1007/s00464-023-10151-5.
19. Andreou A et al. Surg Endosc. 2020 Feb. doi: 10.1007/s00464-019-07208-9.
20. Guidozzi N et al. Dis Esophagus. 2019 Nov. doi: 10.1093/dote/doz031.
Giving the Smallest GI Transplant Patients a New Lease On Life
The best part about working with kids is that “I get to laugh every day,” said Ke-You (Yoyo) Zhang, MD, clinical assistant professor for pediatrics–gastroenterology and hepatology at Stanford Medicine in California.
Everyday life for them is a challenge.
Dealing with sick children is difficult. “But I think the difference between pediatrics and adults is despite how hard things get, children are the single most resilient people you’re ever going to meet,” she said.
Kids don’t always know they’re sick and they don’t act sick, even when they are. “Every day, I literally get on the floor, I get to play, I get to run around. And truly, I have fun every single day. I get excited to go to work. And I think that’s what makes work not feel like work,” said Dr. Zhang.
In an interview, she discussed the satisfaction of following patients throughout their care continuum and her research to reduce the likelihood of transplant rejection.
She also shared an inspirational story of one young patient who spent his life tied to an IV, and how a transplant exposed him to the normal joys of life, like swimming, going to camp and getting on a plane for the first time.
Q: Why did you choose this subspecialty of pediatric GI?
I think it’s the best subspecialty because I think it combines a lot of the things that I enjoy, which is long-term continuity of care. It’s about growing up with your patients and seeing them through all the various stages of their life, often meeting patients when they’re babies. I get pictures of high school graduations and life milestones and even see some of my patients have families of their own. Becoming a part of their family is very meaningful to me. I also like complexity and acuity, and gastroenterology and hepatology provide those things.
And then lastly, it’s great to be able to exercise procedural skills and constantly learn new procedural skills.
Q: How did you become interested in the field of pediatric intestinal and liver transplantation?
I did all my training here at Stanford. We have one of the largest pediatric transplant centers and we also have a very large intestinal rehabilitation population.
Coming through residency and fellowship, I had a lot of exposure to transplant and intestinal failure, intestinal rehabilitation. I really liked the longitudinal relationship I got to form with my patients. Sometimes they’re in the neonatal ICU, where you’re meeting them in their very first days of life. You follow them through their chronic illness, through transplant and after transplant for many years. You become not just their GI, but the center of their care.
Q: What challenges are unique to this type of transplant work?
Pediatric intestinal failure and intestinal transplant represents an incredibly small subset of children. Oftentimes, they do not get the resources and recognition on a national policy level or even at the hospital level that other gastrointestinal diseases receive. What’s difficult is they are such a small subset but their complexity and their needs are probably in the highest percentile. So that’s a really challenging combination to start with. And there’s only a few centers that specialize in doing intestinal rehabilitation and intestinal transplantation for children in the country.
Developing expertise has been slow. But I think in the last decade or so, our understanding and success with intestinal rehabilitation and intestinal transplantation has really improved, especially at large centers like Stanford. We’ve had a lot of success stories and have not had any graft loss since 2014.
Q: Are these transplants hard to acquire?
Yes, especially when you’re transplanting not just the intestines but the liver as well. You’re waiting for two organs, not just one organ. And on top of that, you’re waiting for an appropriately sized donor; usually a child who’s around the same size or same age who’s passed away. Those organs would have to be a good match. Children can wait multiple years for a transplant.
Q: Is there a success story you’d like to share?
One patient I met in the neonatal ICU had congenital short bowel syndrome. He was born with hardly any intestines. He developed complications of being on long-term intravenous nutrition, which included recurrent central line infections and liver disease. He was never able to eat because he really didn’t have a digestive system that could adequately absorb anything. He had a central line in one of his large veins, so he couldn’t go swimming.
He had to have special adaptive wear to even shower or bathe and couldn’t travel. It’s these types of patients that benefit so much from transplant. Putting any kid through transplant is a massive undertaking and it certainly has risks. But he underwent a successful transplant at the age of 8—not just an intestinal transplant, but a multi-visceral transplant of the liver, intestine, and pancreas. He’s 9 years old now, and no longer needs intravenous nutrition. He ate by mouth for the very first time after transplant. He’s trying all sorts of new foods and he was able to go to a special transplant camp for children. Getting on a plane to Los Angeles, which is where our transplant camp is, was a huge deal.
He was able to swim in the lake. He’s never been able to do that. And he wants to start doing sports this fall. This was really a life-changing story for him.
Q: What advancements lie ahead for this field of work? Have you work on any notable research?
I think our understanding of transplant immunology has really progressed, especially recently. That’s what part of my research is about—using novel therapies to modulate the immune system of pediatric transplant recipients. The No. 1 complication that occurs after intestinal transplant is rejection because obviously you’re implanting somebody else’s organs into a patient.
I am involved in a clinical trial that’s looking at the use of extracellular vesicles that are isolated from hematopoietic stem cells. These vesicles contain various growth factors, anti-inflammatory proteins and tissue repair factors that we are infusing into intestinal transplant patients with the aim to repair the intestinal tissue patients are rejecting.
Q: When you’re not being a GI, how do you spend your free weekend afternoons?
My husband and I have an almost 2-year-old little girl. She keeps us busy and I spend my afternoons chasing after a crazy toddler.
Lightning Round
Texting or talking?
Huge texter
Favorite junk food?
French fries
Cat or dog person?
Dog
Favorite ice cream?
Strawberry
If you weren’t a gastroenterologist, what would you be?Florist
Best place you’ve traveled to?
Thailand
Number of cups of coffee you drink per day?
Too many
Favorite city in the US besides the one you live in?
New York City
Favorite sport?
Tennis
Optimist or pessimist?
Optimist
The best part about working with kids is that “I get to laugh every day,” said Ke-You (Yoyo) Zhang, MD, clinical assistant professor for pediatrics–gastroenterology and hepatology at Stanford Medicine in California.
Everyday life for them is a challenge.
Dealing with sick children is difficult. “But I think the difference between pediatrics and adults is despite how hard things get, children are the single most resilient people you’re ever going to meet,” she said.
Kids don’t always know they’re sick and they don’t act sick, even when they are. “Every day, I literally get on the floor, I get to play, I get to run around. And truly, I have fun every single day. I get excited to go to work. And I think that’s what makes work not feel like work,” said Dr. Zhang.
In an interview, she discussed the satisfaction of following patients throughout their care continuum and her research to reduce the likelihood of transplant rejection.
She also shared an inspirational story of one young patient who spent his life tied to an IV, and how a transplant exposed him to the normal joys of life, like swimming, going to camp and getting on a plane for the first time.
Q: Why did you choose this subspecialty of pediatric GI?
I think it’s the best subspecialty because I think it combines a lot of the things that I enjoy, which is long-term continuity of care. It’s about growing up with your patients and seeing them through all the various stages of their life, often meeting patients when they’re babies. I get pictures of high school graduations and life milestones and even see some of my patients have families of their own. Becoming a part of their family is very meaningful to me. I also like complexity and acuity, and gastroenterology and hepatology provide those things.
And then lastly, it’s great to be able to exercise procedural skills and constantly learn new procedural skills.
Q: How did you become interested in the field of pediatric intestinal and liver transplantation?
I did all my training here at Stanford. We have one of the largest pediatric transplant centers and we also have a very large intestinal rehabilitation population.
Coming through residency and fellowship, I had a lot of exposure to transplant and intestinal failure, intestinal rehabilitation. I really liked the longitudinal relationship I got to form with my patients. Sometimes they’re in the neonatal ICU, where you’re meeting them in their very first days of life. You follow them through their chronic illness, through transplant and after transplant for many years. You become not just their GI, but the center of their care.
Q: What challenges are unique to this type of transplant work?
Pediatric intestinal failure and intestinal transplant represents an incredibly small subset of children. Oftentimes, they do not get the resources and recognition on a national policy level or even at the hospital level that other gastrointestinal diseases receive. What’s difficult is they are such a small subset but their complexity and their needs are probably in the highest percentile. So that’s a really challenging combination to start with. And there’s only a few centers that specialize in doing intestinal rehabilitation and intestinal transplantation for children in the country.
Developing expertise has been slow. But I think in the last decade or so, our understanding and success with intestinal rehabilitation and intestinal transplantation has really improved, especially at large centers like Stanford. We’ve had a lot of success stories and have not had any graft loss since 2014.
Q: Are these transplants hard to acquire?
Yes, especially when you’re transplanting not just the intestines but the liver as well. You’re waiting for two organs, not just one organ. And on top of that, you’re waiting for an appropriately sized donor; usually a child who’s around the same size or same age who’s passed away. Those organs would have to be a good match. Children can wait multiple years for a transplant.
Q: Is there a success story you’d like to share?
One patient I met in the neonatal ICU had congenital short bowel syndrome. He was born with hardly any intestines. He developed complications of being on long-term intravenous nutrition, which included recurrent central line infections and liver disease. He was never able to eat because he really didn’t have a digestive system that could adequately absorb anything. He had a central line in one of his large veins, so he couldn’t go swimming.
He had to have special adaptive wear to even shower or bathe and couldn’t travel. It’s these types of patients that benefit so much from transplant. Putting any kid through transplant is a massive undertaking and it certainly has risks. But he underwent a successful transplant at the age of 8—not just an intestinal transplant, but a multi-visceral transplant of the liver, intestine, and pancreas. He’s 9 years old now, and no longer needs intravenous nutrition. He ate by mouth for the very first time after transplant. He’s trying all sorts of new foods and he was able to go to a special transplant camp for children. Getting on a plane to Los Angeles, which is where our transplant camp is, was a huge deal.
He was able to swim in the lake. He’s never been able to do that. And he wants to start doing sports this fall. This was really a life-changing story for him.
Q: What advancements lie ahead for this field of work? Have you work on any notable research?
I think our understanding of transplant immunology has really progressed, especially recently. That’s what part of my research is about—using novel therapies to modulate the immune system of pediatric transplant recipients. The No. 1 complication that occurs after intestinal transplant is rejection because obviously you’re implanting somebody else’s organs into a patient.
I am involved in a clinical trial that’s looking at the use of extracellular vesicles that are isolated from hematopoietic stem cells. These vesicles contain various growth factors, anti-inflammatory proteins and tissue repair factors that we are infusing into intestinal transplant patients with the aim to repair the intestinal tissue patients are rejecting.
Q: When you’re not being a GI, how do you spend your free weekend afternoons?
My husband and I have an almost 2-year-old little girl. She keeps us busy and I spend my afternoons chasing after a crazy toddler.
Lightning Round
Texting or talking?
Huge texter
Favorite junk food?
French fries
Cat or dog person?
Dog
Favorite ice cream?
Strawberry
If you weren’t a gastroenterologist, what would you be?Florist
Best place you’ve traveled to?
Thailand
Number of cups of coffee you drink per day?
Too many
Favorite city in the US besides the one you live in?
New York City
Favorite sport?
Tennis
Optimist or pessimist?
Optimist
The best part about working with kids is that “I get to laugh every day,” said Ke-You (Yoyo) Zhang, MD, clinical assistant professor for pediatrics–gastroenterology and hepatology at Stanford Medicine in California.
Everyday life for them is a challenge.
Dealing with sick children is difficult. “But I think the difference between pediatrics and adults is despite how hard things get, children are the single most resilient people you’re ever going to meet,” she said.
Kids don’t always know they’re sick and they don’t act sick, even when they are. “Every day, I literally get on the floor, I get to play, I get to run around. And truly, I have fun every single day. I get excited to go to work. And I think that’s what makes work not feel like work,” said Dr. Zhang.
In an interview, she discussed the satisfaction of following patients throughout their care continuum and her research to reduce the likelihood of transplant rejection.
She also shared an inspirational story of one young patient who spent his life tied to an IV, and how a transplant exposed him to the normal joys of life, like swimming, going to camp and getting on a plane for the first time.
Q: Why did you choose this subspecialty of pediatric GI?
I think it’s the best subspecialty because I think it combines a lot of the things that I enjoy, which is long-term continuity of care. It’s about growing up with your patients and seeing them through all the various stages of their life, often meeting patients when they’re babies. I get pictures of high school graduations and life milestones and even see some of my patients have families of their own. Becoming a part of their family is very meaningful to me. I also like complexity and acuity, and gastroenterology and hepatology provide those things.
And then lastly, it’s great to be able to exercise procedural skills and constantly learn new procedural skills.
Q: How did you become interested in the field of pediatric intestinal and liver transplantation?
I did all my training here at Stanford. We have one of the largest pediatric transplant centers and we also have a very large intestinal rehabilitation population.
Coming through residency and fellowship, I had a lot of exposure to transplant and intestinal failure, intestinal rehabilitation. I really liked the longitudinal relationship I got to form with my patients. Sometimes they’re in the neonatal ICU, where you’re meeting them in their very first days of life. You follow them through their chronic illness, through transplant and after transplant for many years. You become not just their GI, but the center of their care.
Q: What challenges are unique to this type of transplant work?
Pediatric intestinal failure and intestinal transplant represents an incredibly small subset of children. Oftentimes, they do not get the resources and recognition on a national policy level or even at the hospital level that other gastrointestinal diseases receive. What’s difficult is they are such a small subset but their complexity and their needs are probably in the highest percentile. So that’s a really challenging combination to start with. And there’s only a few centers that specialize in doing intestinal rehabilitation and intestinal transplantation for children in the country.
Developing expertise has been slow. But I think in the last decade or so, our understanding and success with intestinal rehabilitation and intestinal transplantation has really improved, especially at large centers like Stanford. We’ve had a lot of success stories and have not had any graft loss since 2014.
Q: Are these transplants hard to acquire?
Yes, especially when you’re transplanting not just the intestines but the liver as well. You’re waiting for two organs, not just one organ. And on top of that, you’re waiting for an appropriately sized donor; usually a child who’s around the same size or same age who’s passed away. Those organs would have to be a good match. Children can wait multiple years for a transplant.
Q: Is there a success story you’d like to share?
One patient I met in the neonatal ICU had congenital short bowel syndrome. He was born with hardly any intestines. He developed complications of being on long-term intravenous nutrition, which included recurrent central line infections and liver disease. He was never able to eat because he really didn’t have a digestive system that could adequately absorb anything. He had a central line in one of his large veins, so he couldn’t go swimming.
He had to have special adaptive wear to even shower or bathe and couldn’t travel. It’s these types of patients that benefit so much from transplant. Putting any kid through transplant is a massive undertaking and it certainly has risks. But he underwent a successful transplant at the age of 8—not just an intestinal transplant, but a multi-visceral transplant of the liver, intestine, and pancreas. He’s 9 years old now, and no longer needs intravenous nutrition. He ate by mouth for the very first time after transplant. He’s trying all sorts of new foods and he was able to go to a special transplant camp for children. Getting on a plane to Los Angeles, which is where our transplant camp is, was a huge deal.
He was able to swim in the lake. He’s never been able to do that. And he wants to start doing sports this fall. This was really a life-changing story for him.
Q: What advancements lie ahead for this field of work? Have you work on any notable research?
I think our understanding of transplant immunology has really progressed, especially recently. That’s what part of my research is about—using novel therapies to modulate the immune system of pediatric transplant recipients. The No. 1 complication that occurs after intestinal transplant is rejection because obviously you’re implanting somebody else’s organs into a patient.
I am involved in a clinical trial that’s looking at the use of extracellular vesicles that are isolated from hematopoietic stem cells. These vesicles contain various growth factors, anti-inflammatory proteins and tissue repair factors that we are infusing into intestinal transplant patients with the aim to repair the intestinal tissue patients are rejecting.
Q: When you’re not being a GI, how do you spend your free weekend afternoons?
My husband and I have an almost 2-year-old little girl. She keeps us busy and I spend my afternoons chasing after a crazy toddler.
Lightning Round
Texting or talking?
Huge texter
Favorite junk food?
French fries
Cat or dog person?
Dog
Favorite ice cream?
Strawberry
If you weren’t a gastroenterologist, what would you be?Florist
Best place you’ve traveled to?
Thailand
Number of cups of coffee you drink per day?
Too many
Favorite city in the US besides the one you live in?
New York City
Favorite sport?
Tennis
Optimist or pessimist?
Optimist
Michigan Oncologist Charged in Scheme to Illegally Sell Cancer Drugs
In late October, a federal grand jury charged a Detroit-area medical oncologist Naveed Aslam, MD, in an indictment for his part in a scheme to illegally sell cancer drugs.
According to the indictment, Aslam acquired and sold more than $17 million in cancer drugs and personally netted more than $2.5 million during the scheme.
The charges against Aslam, filed on October 23 in the US District Court for the Eastern District of Michigan, include 10 counts of illegally selling or trading prescription drugs and one count of conspiring to do so.
“Dr. Aslam’s alleged participation in this scheme not only allowed him to profit unlawfully from the sale of cancer drugs but it also posed a serious threat by potentially placing these medications into the wrong hands,” Cheyvoryea Gibson, special agent in charge of the FBI in Michigan, said in a press release announcing the indictment.
The investigation is being conducted jointly by the FBI, the US Food and Drug Administration (FDA), the US Department of Health and Human Services Office of Inspector General, and Homeland Security Investigations.
The indictment alleges that Aslam was recruited by an unnamed operator of a Michigan corporation that engaged in business as a retail pharmacy and in the wholesale distribution of expensive prescription drugs, largely oncology drugs.
According to the indictment, Aslam and the operator came to an agreement where Aslam would purchase these expensive drugs from an authorized distributor under the false pretense that he was going to prescribe them to patients.
Instead, Aslam allegedly “sold and transferred the prescription drugs” to or through the Michigan business, with involvement from the unnamed operator and a second unnamed individual.
The unnamed individuals “identified customers interested in buying prescription cancer drugs” and “communicated with Dr. Aslam about what cancer drugs were requested,” according to the press release. “Dr. Aslam used his access to certain cancer drugs through his medical practice, Somerset Hematology and Oncology, P.C., to order and purchase the cancer drugs from his supplier.”
The indictment lays out that Aslam allegedly profited from this scheme in several ways, which included charging the Michigan business more than he paid the distributor for the drugs, sharing the profits when the business resold the drugs at a markup, and receiving rebates and discounts from the distributor “based on the amount of qualifying drugs he purchased and resold.”
According to the indictment, the scheme ran from early 2019 to mid-2023 and included four antibody drug conjugates — trastuzumab deruxtecan (Enhertu), enfortumab vedotin (Padcev), tisotumab vedotin (Tivdak), and sacituzumab govitecan (Trodelvy) — and the monoclonal antibody mogamulizumab (Poteligeo) for cutaneous T-cell lymphoma.
By working with Aslam, the operatives “obtained prescription drugs from an authorized distributor that they would not otherwise have been permitted to purchase, and which they were able to sell at a profit,” according to the indictment.
Both the prosecuting assistant US attorney, Andrew Lievense, and Aslam’s defense lawyer, Daniel Dena, declined to comment for this news organization.
The prosecutor is seeking to recoup the more than $2.5 million Aslam allegedly pocketed, according to the indictment. The press release also noted that an “indictment is only a charge and is not evidence of guilt.”
A version of this article first appeared on Medscape.com.
In late October, a federal grand jury charged a Detroit-area medical oncologist Naveed Aslam, MD, in an indictment for his part in a scheme to illegally sell cancer drugs.
According to the indictment, Aslam acquired and sold more than $17 million in cancer drugs and personally netted more than $2.5 million during the scheme.
The charges against Aslam, filed on October 23 in the US District Court for the Eastern District of Michigan, include 10 counts of illegally selling or trading prescription drugs and one count of conspiring to do so.
“Dr. Aslam’s alleged participation in this scheme not only allowed him to profit unlawfully from the sale of cancer drugs but it also posed a serious threat by potentially placing these medications into the wrong hands,” Cheyvoryea Gibson, special agent in charge of the FBI in Michigan, said in a press release announcing the indictment.
The investigation is being conducted jointly by the FBI, the US Food and Drug Administration (FDA), the US Department of Health and Human Services Office of Inspector General, and Homeland Security Investigations.
The indictment alleges that Aslam was recruited by an unnamed operator of a Michigan corporation that engaged in business as a retail pharmacy and in the wholesale distribution of expensive prescription drugs, largely oncology drugs.
According to the indictment, Aslam and the operator came to an agreement where Aslam would purchase these expensive drugs from an authorized distributor under the false pretense that he was going to prescribe them to patients.
Instead, Aslam allegedly “sold and transferred the prescription drugs” to or through the Michigan business, with involvement from the unnamed operator and a second unnamed individual.
The unnamed individuals “identified customers interested in buying prescription cancer drugs” and “communicated with Dr. Aslam about what cancer drugs were requested,” according to the press release. “Dr. Aslam used his access to certain cancer drugs through his medical practice, Somerset Hematology and Oncology, P.C., to order and purchase the cancer drugs from his supplier.”
The indictment lays out that Aslam allegedly profited from this scheme in several ways, which included charging the Michigan business more than he paid the distributor for the drugs, sharing the profits when the business resold the drugs at a markup, and receiving rebates and discounts from the distributor “based on the amount of qualifying drugs he purchased and resold.”
According to the indictment, the scheme ran from early 2019 to mid-2023 and included four antibody drug conjugates — trastuzumab deruxtecan (Enhertu), enfortumab vedotin (Padcev), tisotumab vedotin (Tivdak), and sacituzumab govitecan (Trodelvy) — and the monoclonal antibody mogamulizumab (Poteligeo) for cutaneous T-cell lymphoma.
By working with Aslam, the operatives “obtained prescription drugs from an authorized distributor that they would not otherwise have been permitted to purchase, and which they were able to sell at a profit,” according to the indictment.
Both the prosecuting assistant US attorney, Andrew Lievense, and Aslam’s defense lawyer, Daniel Dena, declined to comment for this news organization.
The prosecutor is seeking to recoup the more than $2.5 million Aslam allegedly pocketed, according to the indictment. The press release also noted that an “indictment is only a charge and is not evidence of guilt.”
A version of this article first appeared on Medscape.com.
In late October, a federal grand jury charged a Detroit-area medical oncologist Naveed Aslam, MD, in an indictment for his part in a scheme to illegally sell cancer drugs.
According to the indictment, Aslam acquired and sold more than $17 million in cancer drugs and personally netted more than $2.5 million during the scheme.
The charges against Aslam, filed on October 23 in the US District Court for the Eastern District of Michigan, include 10 counts of illegally selling or trading prescription drugs and one count of conspiring to do so.
“Dr. Aslam’s alleged participation in this scheme not only allowed him to profit unlawfully from the sale of cancer drugs but it also posed a serious threat by potentially placing these medications into the wrong hands,” Cheyvoryea Gibson, special agent in charge of the FBI in Michigan, said in a press release announcing the indictment.
The investigation is being conducted jointly by the FBI, the US Food and Drug Administration (FDA), the US Department of Health and Human Services Office of Inspector General, and Homeland Security Investigations.
The indictment alleges that Aslam was recruited by an unnamed operator of a Michigan corporation that engaged in business as a retail pharmacy and in the wholesale distribution of expensive prescription drugs, largely oncology drugs.
According to the indictment, Aslam and the operator came to an agreement where Aslam would purchase these expensive drugs from an authorized distributor under the false pretense that he was going to prescribe them to patients.
Instead, Aslam allegedly “sold and transferred the prescription drugs” to or through the Michigan business, with involvement from the unnamed operator and a second unnamed individual.
The unnamed individuals “identified customers interested in buying prescription cancer drugs” and “communicated with Dr. Aslam about what cancer drugs were requested,” according to the press release. “Dr. Aslam used his access to certain cancer drugs through his medical practice, Somerset Hematology and Oncology, P.C., to order and purchase the cancer drugs from his supplier.”
The indictment lays out that Aslam allegedly profited from this scheme in several ways, which included charging the Michigan business more than he paid the distributor for the drugs, sharing the profits when the business resold the drugs at a markup, and receiving rebates and discounts from the distributor “based on the amount of qualifying drugs he purchased and resold.”
According to the indictment, the scheme ran from early 2019 to mid-2023 and included four antibody drug conjugates — trastuzumab deruxtecan (Enhertu), enfortumab vedotin (Padcev), tisotumab vedotin (Tivdak), and sacituzumab govitecan (Trodelvy) — and the monoclonal antibody mogamulizumab (Poteligeo) for cutaneous T-cell lymphoma.
By working with Aslam, the operatives “obtained prescription drugs from an authorized distributor that they would not otherwise have been permitted to purchase, and which they were able to sell at a profit,” according to the indictment.
Both the prosecuting assistant US attorney, Andrew Lievense, and Aslam’s defense lawyer, Daniel Dena, declined to comment for this news organization.
The prosecutor is seeking to recoup the more than $2.5 million Aslam allegedly pocketed, according to the indictment. The press release also noted that an “indictment is only a charge and is not evidence of guilt.”
A version of this article first appeared on Medscape.com.
Can Fish Skin Grafts Heal Diabetic Foot Ulcers?
TOPLINE:
METHODOLOGY:
- Standard wound care for diabetic foot ulcers involves vascular assessment, surgical debridement, use of appropriate dressings, infection management, and glycemic control; however, standard care is typically associated with poor outcomes.
- Researchers conducted a multicenter clinical trial in 15 tertiary care centers with diabetic foot units across France, Italy, Germany, and Sweden to evaluate the efficacy and safety of intact fish skin grafts over standard-of-care practices in treating complex diabetic foot ulcers.
- A total of 255 patients aged 18 years or older with diabetes and lower limb wounds penetrating to the tendon, capsule, bone, or joint were randomly assigned to receive either an intact fish skin graft or standard wound care for 14 weeks.
- The primary endpoint was the percentage of wounds achieving complete closure by 16 weeks.
- Wound healing was also assessed at 20 and 24 weeks.
TAKEAWAY:
- The proportion of wounds healed at 16 weeks was higher with intact fish skin grafts than with standard-of-care (44.0% vs 26.4% adjusted odds ratio [aOR], 2.58; 95% CI, 1.48-4.56).
- The fish skin grafts continued to be more effective than standard wound care practices at weeks 20 (aOR, 2.15; 95% CI, 1.27–3.70) and 24 (aOR, 2.19; 95% CI, 1.31–3.70).
- The mean time to healing was 17.31 weeks for the intact fish skin graft group and 19.37 weeks for the standard-of-care group; intact fish skin grafts were also associated with faster healing times than standard wound care (hazard ratio, 1.59; 95% CI, 1.07-2.36).
- Target wound infections were the most common adverse events, occurring in a similar number of patients in both the groups.
IN PRACTICE:
“Our trial demonstrated treatment of complex diabetic foot ulcers with intact fish skin grafts achieved a significantly greater proportion of diabetic foot ulcers healed at 16 weeks than standard of care, and was associated with increased healing at 20 and 24 weeks. That these results were achieved in non-superficial UT [University of Texas diabetic wound classification system] grade 2 and 3 diabetic foot ulcers and included ischemic and/or infected diabetic foot ulcers is of importance,” the authors wrote.
SOURCE:
The study was led by Dured Dardari, MD, PhD, Center Hospitalier Sud Francilien, Corbeil-Essonnes, France, and was published online in NEJM Evidence.
LIMITATIONS:
No limitations were discussed for this study.
DISCLOSURES:
The study was funded by European Commission Fast Track to Innovation Horizon 2020 and Kerecis. Two authors reported being employees with or without stock options at Kerecis, and other authors reported having ties with many sources including Kerecis.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Standard wound care for diabetic foot ulcers involves vascular assessment, surgical debridement, use of appropriate dressings, infection management, and glycemic control; however, standard care is typically associated with poor outcomes.
- Researchers conducted a multicenter clinical trial in 15 tertiary care centers with diabetic foot units across France, Italy, Germany, and Sweden to evaluate the efficacy and safety of intact fish skin grafts over standard-of-care practices in treating complex diabetic foot ulcers.
- A total of 255 patients aged 18 years or older with diabetes and lower limb wounds penetrating to the tendon, capsule, bone, or joint were randomly assigned to receive either an intact fish skin graft or standard wound care for 14 weeks.
- The primary endpoint was the percentage of wounds achieving complete closure by 16 weeks.
- Wound healing was also assessed at 20 and 24 weeks.
TAKEAWAY:
- The proportion of wounds healed at 16 weeks was higher with intact fish skin grafts than with standard-of-care (44.0% vs 26.4% adjusted odds ratio [aOR], 2.58; 95% CI, 1.48-4.56).
- The fish skin grafts continued to be more effective than standard wound care practices at weeks 20 (aOR, 2.15; 95% CI, 1.27–3.70) and 24 (aOR, 2.19; 95% CI, 1.31–3.70).
- The mean time to healing was 17.31 weeks for the intact fish skin graft group and 19.37 weeks for the standard-of-care group; intact fish skin grafts were also associated with faster healing times than standard wound care (hazard ratio, 1.59; 95% CI, 1.07-2.36).
- Target wound infections were the most common adverse events, occurring in a similar number of patients in both the groups.
IN PRACTICE:
“Our trial demonstrated treatment of complex diabetic foot ulcers with intact fish skin grafts achieved a significantly greater proportion of diabetic foot ulcers healed at 16 weeks than standard of care, and was associated with increased healing at 20 and 24 weeks. That these results were achieved in non-superficial UT [University of Texas diabetic wound classification system] grade 2 and 3 diabetic foot ulcers and included ischemic and/or infected diabetic foot ulcers is of importance,” the authors wrote.
SOURCE:
The study was led by Dured Dardari, MD, PhD, Center Hospitalier Sud Francilien, Corbeil-Essonnes, France, and was published online in NEJM Evidence.
LIMITATIONS:
No limitations were discussed for this study.
DISCLOSURES:
The study was funded by European Commission Fast Track to Innovation Horizon 2020 and Kerecis. Two authors reported being employees with or without stock options at Kerecis, and other authors reported having ties with many sources including Kerecis.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Standard wound care for diabetic foot ulcers involves vascular assessment, surgical debridement, use of appropriate dressings, infection management, and glycemic control; however, standard care is typically associated with poor outcomes.
- Researchers conducted a multicenter clinical trial in 15 tertiary care centers with diabetic foot units across France, Italy, Germany, and Sweden to evaluate the efficacy and safety of intact fish skin grafts over standard-of-care practices in treating complex diabetic foot ulcers.
- A total of 255 patients aged 18 years or older with diabetes and lower limb wounds penetrating to the tendon, capsule, bone, or joint were randomly assigned to receive either an intact fish skin graft or standard wound care for 14 weeks.
- The primary endpoint was the percentage of wounds achieving complete closure by 16 weeks.
- Wound healing was also assessed at 20 and 24 weeks.
TAKEAWAY:
- The proportion of wounds healed at 16 weeks was higher with intact fish skin grafts than with standard-of-care (44.0% vs 26.4% adjusted odds ratio [aOR], 2.58; 95% CI, 1.48-4.56).
- The fish skin grafts continued to be more effective than standard wound care practices at weeks 20 (aOR, 2.15; 95% CI, 1.27–3.70) and 24 (aOR, 2.19; 95% CI, 1.31–3.70).
- The mean time to healing was 17.31 weeks for the intact fish skin graft group and 19.37 weeks for the standard-of-care group; intact fish skin grafts were also associated with faster healing times than standard wound care (hazard ratio, 1.59; 95% CI, 1.07-2.36).
- Target wound infections were the most common adverse events, occurring in a similar number of patients in both the groups.
IN PRACTICE:
“Our trial demonstrated treatment of complex diabetic foot ulcers with intact fish skin grafts achieved a significantly greater proportion of diabetic foot ulcers healed at 16 weeks than standard of care, and was associated with increased healing at 20 and 24 weeks. That these results were achieved in non-superficial UT [University of Texas diabetic wound classification system] grade 2 and 3 diabetic foot ulcers and included ischemic and/or infected diabetic foot ulcers is of importance,” the authors wrote.
SOURCE:
The study was led by Dured Dardari, MD, PhD, Center Hospitalier Sud Francilien, Corbeil-Essonnes, France, and was published online in NEJM Evidence.
LIMITATIONS:
No limitations were discussed for this study.
DISCLOSURES:
The study was funded by European Commission Fast Track to Innovation Horizon 2020 and Kerecis. Two authors reported being employees with or without stock options at Kerecis, and other authors reported having ties with many sources including Kerecis.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Patients With Chronic Cough Report Relief With Semen Strychni
If standard therapies don’t give relief to patients with refractory cough associated with interstitial lung disease, maybe a little poison could do the trick.
Among 41 patients with idiopathic interstitial pneumonia with autoimmune features (IPAFs) who had intractable cough, treatment with the traditional Chinese medicine semen strychni was associated with a significant improvement in patient-reported outcomes, reported Mingwan Su, MD, from Guang’anmen Hospital and the China Academy of Chinese Medical Sciences in Beijing, China.
“Semen strychni is associated with reduction in cough and can be an effective drug therapy for refractory cough in association with IPAFs,” she said in an oral abstract session at the American College of Chest Physicians (CHEST) 2024 Annual Meeting.
Semen strychni is derived from the dried seeds of the plant Strychnos nux-vomica L. Its main toxic component is strychnine, the poison said to be favored by legendary mystery writer Agatha Christie.
Semen strychni is a central nervous system agonist that has reported efficacy in the treatment of musculoskeletal and autoimmune conditions, including rheumatoid arthritis, myasthenia gravis, and amyotrophic lateral sclerosis.
The medication also has immunomodulatory properties, Su said, and is thought to have beneficial effects against cough associated with IPAFs by reducing hypersensitivity.
Case-Control Study
To test this, Su and colleagues conducted a single-center retrospective study of the effects of semen strychni on 41 patients with IPAF-associated cough who were treated with low-dose oral semen strychni 300 mg/d for 2 weeks. These patients were paired with 41 control individuals matched for age, sex, and disease course. Control individuals received standard of care therapies.
The investigators found that for the primary endpoint of a change in the visual analog scale (VAS) at 2 weeks, there was a significantly greater reduction from baseline among patients treated with semen strychni compared with control individuals, with a baseline mean VAS score of 4.9 reduced to 2.1 at the end of treatment, vs 4.6 pre- to 3.3 post-treatment for control individuals. This difference translated to an odds ratio (OR) favoring semen strychni of 0.75 (P < .001).
In addition, the toxic compound was also associated with greater patient-reported improvement in the quality of life, as measured using the Leicester Cough Questionnaire, a 19-item scale that measures quality of life for people with chronic cough. Patients in the experimental arm had mean scores of 11.9 before treatment and 19 at the end of therapy compared with 12 and 15.1 points, respectively, among individuals in the control arm. This translated to an OR of 3.8 (P < .001) for patients on semen strychni.
The toxin appeared to be generally safe. There were no reported cases of pain, fainting, or bleeding in either study group, although there was one case of muscle twitching in the semen strychni group, Su reported.
There is evidence to suggest that semen strychni may have a calming effect on cough through action in the STAT3 pathway, considered to be a promising therapeutic target for musculoskeletal conditions, Su noted.
Not Ready for Prime Time
“My feeling is that these kinds of abstracts are welcome, but this is far from reality at this point,” said Vijay Balasubramanian, MD, clinical professor of medicine and director of the Pulmonary Hypertension Program at the University of California San Francisco.
“We need some kind of a regulated way of understanding dose characteristics and pharmacokinetics, and so it should be followed by more systematic studies,” he said in an interview.
Both Balasubramanian and his co-moderator Andrew R. Berman, MD, director of the Division of Pulmonary and Critical Care Medicine and Allergy and Rheumatology at Rutgers Health New Jersey Medical School in Newark, New Jersey, said that they sympathize with clinicians and their patients who seek out unusual therapies such as semen strychni.
“It’s very frustrating to treat chronic cough, especially associated with fibrotic lung disease, and the extent to which researchers will go to find that one product that perhaps can make a difference is understandable,” Berman told this news organization.
Su did not report a study funding source. Su, Balasubramanian, and Berman reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
If standard therapies don’t give relief to patients with refractory cough associated with interstitial lung disease, maybe a little poison could do the trick.
Among 41 patients with idiopathic interstitial pneumonia with autoimmune features (IPAFs) who had intractable cough, treatment with the traditional Chinese medicine semen strychni was associated with a significant improvement in patient-reported outcomes, reported Mingwan Su, MD, from Guang’anmen Hospital and the China Academy of Chinese Medical Sciences in Beijing, China.
“Semen strychni is associated with reduction in cough and can be an effective drug therapy for refractory cough in association with IPAFs,” she said in an oral abstract session at the American College of Chest Physicians (CHEST) 2024 Annual Meeting.
Semen strychni is derived from the dried seeds of the plant Strychnos nux-vomica L. Its main toxic component is strychnine, the poison said to be favored by legendary mystery writer Agatha Christie.
Semen strychni is a central nervous system agonist that has reported efficacy in the treatment of musculoskeletal and autoimmune conditions, including rheumatoid arthritis, myasthenia gravis, and amyotrophic lateral sclerosis.
The medication also has immunomodulatory properties, Su said, and is thought to have beneficial effects against cough associated with IPAFs by reducing hypersensitivity.
Case-Control Study
To test this, Su and colleagues conducted a single-center retrospective study of the effects of semen strychni on 41 patients with IPAF-associated cough who were treated with low-dose oral semen strychni 300 mg/d for 2 weeks. These patients were paired with 41 control individuals matched for age, sex, and disease course. Control individuals received standard of care therapies.
The investigators found that for the primary endpoint of a change in the visual analog scale (VAS) at 2 weeks, there was a significantly greater reduction from baseline among patients treated with semen strychni compared with control individuals, with a baseline mean VAS score of 4.9 reduced to 2.1 at the end of treatment, vs 4.6 pre- to 3.3 post-treatment for control individuals. This difference translated to an odds ratio (OR) favoring semen strychni of 0.75 (P < .001).
In addition, the toxic compound was also associated with greater patient-reported improvement in the quality of life, as measured using the Leicester Cough Questionnaire, a 19-item scale that measures quality of life for people with chronic cough. Patients in the experimental arm had mean scores of 11.9 before treatment and 19 at the end of therapy compared with 12 and 15.1 points, respectively, among individuals in the control arm. This translated to an OR of 3.8 (P < .001) for patients on semen strychni.
The toxin appeared to be generally safe. There were no reported cases of pain, fainting, or bleeding in either study group, although there was one case of muscle twitching in the semen strychni group, Su reported.
There is evidence to suggest that semen strychni may have a calming effect on cough through action in the STAT3 pathway, considered to be a promising therapeutic target for musculoskeletal conditions, Su noted.
Not Ready for Prime Time
“My feeling is that these kinds of abstracts are welcome, but this is far from reality at this point,” said Vijay Balasubramanian, MD, clinical professor of medicine and director of the Pulmonary Hypertension Program at the University of California San Francisco.
“We need some kind of a regulated way of understanding dose characteristics and pharmacokinetics, and so it should be followed by more systematic studies,” he said in an interview.
Both Balasubramanian and his co-moderator Andrew R. Berman, MD, director of the Division of Pulmonary and Critical Care Medicine and Allergy and Rheumatology at Rutgers Health New Jersey Medical School in Newark, New Jersey, said that they sympathize with clinicians and their patients who seek out unusual therapies such as semen strychni.
“It’s very frustrating to treat chronic cough, especially associated with fibrotic lung disease, and the extent to which researchers will go to find that one product that perhaps can make a difference is understandable,” Berman told this news organization.
Su did not report a study funding source. Su, Balasubramanian, and Berman reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
If standard therapies don’t give relief to patients with refractory cough associated with interstitial lung disease, maybe a little poison could do the trick.
Among 41 patients with idiopathic interstitial pneumonia with autoimmune features (IPAFs) who had intractable cough, treatment with the traditional Chinese medicine semen strychni was associated with a significant improvement in patient-reported outcomes, reported Mingwan Su, MD, from Guang’anmen Hospital and the China Academy of Chinese Medical Sciences in Beijing, China.
“Semen strychni is associated with reduction in cough and can be an effective drug therapy for refractory cough in association with IPAFs,” she said in an oral abstract session at the American College of Chest Physicians (CHEST) 2024 Annual Meeting.
Semen strychni is derived from the dried seeds of the plant Strychnos nux-vomica L. Its main toxic component is strychnine, the poison said to be favored by legendary mystery writer Agatha Christie.
Semen strychni is a central nervous system agonist that has reported efficacy in the treatment of musculoskeletal and autoimmune conditions, including rheumatoid arthritis, myasthenia gravis, and amyotrophic lateral sclerosis.
The medication also has immunomodulatory properties, Su said, and is thought to have beneficial effects against cough associated with IPAFs by reducing hypersensitivity.
Case-Control Study
To test this, Su and colleagues conducted a single-center retrospective study of the effects of semen strychni on 41 patients with IPAF-associated cough who were treated with low-dose oral semen strychni 300 mg/d for 2 weeks. These patients were paired with 41 control individuals matched for age, sex, and disease course. Control individuals received standard of care therapies.
The investigators found that for the primary endpoint of a change in the visual analog scale (VAS) at 2 weeks, there was a significantly greater reduction from baseline among patients treated with semen strychni compared with control individuals, with a baseline mean VAS score of 4.9 reduced to 2.1 at the end of treatment, vs 4.6 pre- to 3.3 post-treatment for control individuals. This difference translated to an odds ratio (OR) favoring semen strychni of 0.75 (P < .001).
In addition, the toxic compound was also associated with greater patient-reported improvement in the quality of life, as measured using the Leicester Cough Questionnaire, a 19-item scale that measures quality of life for people with chronic cough. Patients in the experimental arm had mean scores of 11.9 before treatment and 19 at the end of therapy compared with 12 and 15.1 points, respectively, among individuals in the control arm. This translated to an OR of 3.8 (P < .001) for patients on semen strychni.
The toxin appeared to be generally safe. There were no reported cases of pain, fainting, or bleeding in either study group, although there was one case of muscle twitching in the semen strychni group, Su reported.
There is evidence to suggest that semen strychni may have a calming effect on cough through action in the STAT3 pathway, considered to be a promising therapeutic target for musculoskeletal conditions, Su noted.
Not Ready for Prime Time
“My feeling is that these kinds of abstracts are welcome, but this is far from reality at this point,” said Vijay Balasubramanian, MD, clinical professor of medicine and director of the Pulmonary Hypertension Program at the University of California San Francisco.
“We need some kind of a regulated way of understanding dose characteristics and pharmacokinetics, and so it should be followed by more systematic studies,” he said in an interview.
Both Balasubramanian and his co-moderator Andrew R. Berman, MD, director of the Division of Pulmonary and Critical Care Medicine and Allergy and Rheumatology at Rutgers Health New Jersey Medical School in Newark, New Jersey, said that they sympathize with clinicians and their patients who seek out unusual therapies such as semen strychni.
“It’s very frustrating to treat chronic cough, especially associated with fibrotic lung disease, and the extent to which researchers will go to find that one product that perhaps can make a difference is understandable,” Berman told this news organization.
Su did not report a study funding source. Su, Balasubramanian, and Berman reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CHEST 2024
Is This Methadone’s Moment?
Methadone has been shown to be highly effective for opioid use disorder. So why is it still so difficult to prescribe in the United States and is that about to change?
This paper included more than 30,000 patients with opioid use disorder and showed those on methadone were almost 60% significantly less likely to stop treatment at 24 months than their peers assigned to buprenorphine/naloxone (adjusted hazard ratio [aHR], 1.58), with no difference in mortality risk (aHR, 0.57).
“In Canada, unlike the US, methadone and buprenorphine/naloxone are both available in office-based settings. Methadone really outperforms buprenorphine/naloxone in being able to retain people in treatment, which is our main goal and comes with a host of benefits,” Bohdan Nosyk, PhD, with Simon Fraser University in Burnaby, British Columbia, Canada, who worked on the study, said in an interview.
In addition, a recent systematic review and meta-analysis of relevant research involving more than 1 million patients with opioid use disorder also showed better treatment retention with methadone than with buprenorphine.
During the COVID-19 pandemic, relaxed methadone regulations, that included take-home medications, did not lead to an increase in overdoses. Instead, these changes improved treatment retention and patient experiences, highlighting the potential benefits of further deregulation.
‘Atrocious’ Outdated Policies
However, despite methadone’s proven efficacy and safety for opioid use disorder, it remains vastly underutilized because of outdated US policies restricting its use to opioid treatment programs (OTPs).
“It’s absolutely atrocious that methadone policies have not kept up with the evidence. If you look at other countries that have expanded their access to methadone, their overdose rates have fallen dramatically,” said Leslie Suen, MD, with the University of California, San Francisco, and coauthor of a recent JAMA Viewpoint on this topic.
“Methadone is a very good medication that’s been shown over and over to be very effective and safe,” Alan Leshner, PhD, past director of the National Institute on Drug Abuse, said in an interview.
“There is no reason why it couldn’t be administered through pharmacies or through physicians’ offices as long as it’s done in a controlled and careful way,” said Leshner.
Leshner chaired the committee that produced the 2019 report Medications for Opioid Use Disorder Save Lives.
“We learned during COVID that increasing the amount of take-home methadone and increasing access does not lead to an increase in deaths or an increase in overdose, so it’s hard to find a reason not to do it,” he said.
Change Finally on the Horizon?
Several recent and proposed policy changes could revolutionize methadone delivery in the United States.
In March 2022, in response to the pandemic, the Drug Enforcement Administration (DEA) allowed hospitals to dispense up to a 3-day supply of methadone (known as the 72-hour rule) to bridge care transitions without needing OTPs.
In April 2024, the Substance Abuse and Mental Health Services Administration and DEA codified many methadone and buprenorphine delivery flexibilities granted temporarily during the pandemic, including increased use of telehealth assessments and earlier access to take-home methadone doses.
Another contemporary policy change is expansion of the Americans with Disabilities Act mandating that patients taking medications for opioid use disorder, such as methadone, be able to continue treatment when transitioning to settings such as hospitals, jails, and skilled nursing facilities.
At the state level, California Governor Gavin Newsom recently signed a bill, effective immediately, that expands access to methadone treatment in his state.
On the horizon at the federal level is the Modernizing Opioid Treatment Access Act (MOTAA) — the bipartisan and bicameral bill introduced by Sen. Ed Markey (D-MA) and Sen. Rand Paul (R-KY), along with Rep. Donald Norcross (D-NJ) and Rep. Don Bacon, (R-NE) — that would allow methadone to be prescribed by addiction specialists and dispensed in community pharmacies.
An Ethical Imperative
“With only about 2000 OTP clinics clustered in urban areas, less than 25% of people who are diagnosed with opioid use disorder are actually able to access methadone,” Caty Simon, with the National Survivors Union, Greensboro, North Carolina, and coauthor of the JAMA Viewpoint, said in an interview.
While MOTAA represents a major step forward, limiting methadone prescribing to addiction specialists may not fully address the treatment gap, particularly in rural and underserved areas, Simon said.
To optimize methadone’s potential, she’d like to see further expansion of prescribing privileges to general healthcare providers.
“As someone with lived and living experience of opioid use and treatment, and somebody who works nationally and locally in organizations of people impacted by drug use, I know people in my area right now — marginalized people of color — who would have much better chances of survival if they were able to access methadone. If MOTAA passed tomorrow, we could save so many lives. There is an ethical imperative to pass it,” Simon said.
Leshner said he is “always very concerned about access, particularly for underserved populations, poor people, people living in rural areas. If you can access the medications you need, you’re in big trouble.”
Is this methadone’s moment? “I’m a little optimistic, but I haven’t seen the progress I would like to see,” Leshner said.
A version of this article first appeared on Medscape.com.
Methadone has been shown to be highly effective for opioid use disorder. So why is it still so difficult to prescribe in the United States and is that about to change?
This paper included more than 30,000 patients with opioid use disorder and showed those on methadone were almost 60% significantly less likely to stop treatment at 24 months than their peers assigned to buprenorphine/naloxone (adjusted hazard ratio [aHR], 1.58), with no difference in mortality risk (aHR, 0.57).
“In Canada, unlike the US, methadone and buprenorphine/naloxone are both available in office-based settings. Methadone really outperforms buprenorphine/naloxone in being able to retain people in treatment, which is our main goal and comes with a host of benefits,” Bohdan Nosyk, PhD, with Simon Fraser University in Burnaby, British Columbia, Canada, who worked on the study, said in an interview.
In addition, a recent systematic review and meta-analysis of relevant research involving more than 1 million patients with opioid use disorder also showed better treatment retention with methadone than with buprenorphine.
During the COVID-19 pandemic, relaxed methadone regulations, that included take-home medications, did not lead to an increase in overdoses. Instead, these changes improved treatment retention and patient experiences, highlighting the potential benefits of further deregulation.
‘Atrocious’ Outdated Policies
However, despite methadone’s proven efficacy and safety for opioid use disorder, it remains vastly underutilized because of outdated US policies restricting its use to opioid treatment programs (OTPs).
“It’s absolutely atrocious that methadone policies have not kept up with the evidence. If you look at other countries that have expanded their access to methadone, their overdose rates have fallen dramatically,” said Leslie Suen, MD, with the University of California, San Francisco, and coauthor of a recent JAMA Viewpoint on this topic.
“Methadone is a very good medication that’s been shown over and over to be very effective and safe,” Alan Leshner, PhD, past director of the National Institute on Drug Abuse, said in an interview.
“There is no reason why it couldn’t be administered through pharmacies or through physicians’ offices as long as it’s done in a controlled and careful way,” said Leshner.
Leshner chaired the committee that produced the 2019 report Medications for Opioid Use Disorder Save Lives.
“We learned during COVID that increasing the amount of take-home methadone and increasing access does not lead to an increase in deaths or an increase in overdose, so it’s hard to find a reason not to do it,” he said.
Change Finally on the Horizon?
Several recent and proposed policy changes could revolutionize methadone delivery in the United States.
In March 2022, in response to the pandemic, the Drug Enforcement Administration (DEA) allowed hospitals to dispense up to a 3-day supply of methadone (known as the 72-hour rule) to bridge care transitions without needing OTPs.
In April 2024, the Substance Abuse and Mental Health Services Administration and DEA codified many methadone and buprenorphine delivery flexibilities granted temporarily during the pandemic, including increased use of telehealth assessments and earlier access to take-home methadone doses.
Another contemporary policy change is expansion of the Americans with Disabilities Act mandating that patients taking medications for opioid use disorder, such as methadone, be able to continue treatment when transitioning to settings such as hospitals, jails, and skilled nursing facilities.
At the state level, California Governor Gavin Newsom recently signed a bill, effective immediately, that expands access to methadone treatment in his state.
On the horizon at the federal level is the Modernizing Opioid Treatment Access Act (MOTAA) — the bipartisan and bicameral bill introduced by Sen. Ed Markey (D-MA) and Sen. Rand Paul (R-KY), along with Rep. Donald Norcross (D-NJ) and Rep. Don Bacon, (R-NE) — that would allow methadone to be prescribed by addiction specialists and dispensed in community pharmacies.
An Ethical Imperative
“With only about 2000 OTP clinics clustered in urban areas, less than 25% of people who are diagnosed with opioid use disorder are actually able to access methadone,” Caty Simon, with the National Survivors Union, Greensboro, North Carolina, and coauthor of the JAMA Viewpoint, said in an interview.
While MOTAA represents a major step forward, limiting methadone prescribing to addiction specialists may not fully address the treatment gap, particularly in rural and underserved areas, Simon said.
To optimize methadone’s potential, she’d like to see further expansion of prescribing privileges to general healthcare providers.
“As someone with lived and living experience of opioid use and treatment, and somebody who works nationally and locally in organizations of people impacted by drug use, I know people in my area right now — marginalized people of color — who would have much better chances of survival if they were able to access methadone. If MOTAA passed tomorrow, we could save so many lives. There is an ethical imperative to pass it,” Simon said.
Leshner said he is “always very concerned about access, particularly for underserved populations, poor people, people living in rural areas. If you can access the medications you need, you’re in big trouble.”
Is this methadone’s moment? “I’m a little optimistic, but I haven’t seen the progress I would like to see,” Leshner said.
A version of this article first appeared on Medscape.com.
Methadone has been shown to be highly effective for opioid use disorder. So why is it still so difficult to prescribe in the United States and is that about to change?
This paper included more than 30,000 patients with opioid use disorder and showed those on methadone were almost 60% significantly less likely to stop treatment at 24 months than their peers assigned to buprenorphine/naloxone (adjusted hazard ratio [aHR], 1.58), with no difference in mortality risk (aHR, 0.57).
“In Canada, unlike the US, methadone and buprenorphine/naloxone are both available in office-based settings. Methadone really outperforms buprenorphine/naloxone in being able to retain people in treatment, which is our main goal and comes with a host of benefits,” Bohdan Nosyk, PhD, with Simon Fraser University in Burnaby, British Columbia, Canada, who worked on the study, said in an interview.
In addition, a recent systematic review and meta-analysis of relevant research involving more than 1 million patients with opioid use disorder also showed better treatment retention with methadone than with buprenorphine.
During the COVID-19 pandemic, relaxed methadone regulations, that included take-home medications, did not lead to an increase in overdoses. Instead, these changes improved treatment retention and patient experiences, highlighting the potential benefits of further deregulation.
‘Atrocious’ Outdated Policies
However, despite methadone’s proven efficacy and safety for opioid use disorder, it remains vastly underutilized because of outdated US policies restricting its use to opioid treatment programs (OTPs).
“It’s absolutely atrocious that methadone policies have not kept up with the evidence. If you look at other countries that have expanded their access to methadone, their overdose rates have fallen dramatically,” said Leslie Suen, MD, with the University of California, San Francisco, and coauthor of a recent JAMA Viewpoint on this topic.
“Methadone is a very good medication that’s been shown over and over to be very effective and safe,” Alan Leshner, PhD, past director of the National Institute on Drug Abuse, said in an interview.
“There is no reason why it couldn’t be administered through pharmacies or through physicians’ offices as long as it’s done in a controlled and careful way,” said Leshner.
Leshner chaired the committee that produced the 2019 report Medications for Opioid Use Disorder Save Lives.
“We learned during COVID that increasing the amount of take-home methadone and increasing access does not lead to an increase in deaths or an increase in overdose, so it’s hard to find a reason not to do it,” he said.
Change Finally on the Horizon?
Several recent and proposed policy changes could revolutionize methadone delivery in the United States.
In March 2022, in response to the pandemic, the Drug Enforcement Administration (DEA) allowed hospitals to dispense up to a 3-day supply of methadone (known as the 72-hour rule) to bridge care transitions without needing OTPs.
In April 2024, the Substance Abuse and Mental Health Services Administration and DEA codified many methadone and buprenorphine delivery flexibilities granted temporarily during the pandemic, including increased use of telehealth assessments and earlier access to take-home methadone doses.
Another contemporary policy change is expansion of the Americans with Disabilities Act mandating that patients taking medications for opioid use disorder, such as methadone, be able to continue treatment when transitioning to settings such as hospitals, jails, and skilled nursing facilities.
At the state level, California Governor Gavin Newsom recently signed a bill, effective immediately, that expands access to methadone treatment in his state.
On the horizon at the federal level is the Modernizing Opioid Treatment Access Act (MOTAA) — the bipartisan and bicameral bill introduced by Sen. Ed Markey (D-MA) and Sen. Rand Paul (R-KY), along with Rep. Donald Norcross (D-NJ) and Rep. Don Bacon, (R-NE) — that would allow methadone to be prescribed by addiction specialists and dispensed in community pharmacies.
An Ethical Imperative
“With only about 2000 OTP clinics clustered in urban areas, less than 25% of people who are diagnosed with opioid use disorder are actually able to access methadone,” Caty Simon, with the National Survivors Union, Greensboro, North Carolina, and coauthor of the JAMA Viewpoint, said in an interview.
While MOTAA represents a major step forward, limiting methadone prescribing to addiction specialists may not fully address the treatment gap, particularly in rural and underserved areas, Simon said.
To optimize methadone’s potential, she’d like to see further expansion of prescribing privileges to general healthcare providers.
“As someone with lived and living experience of opioid use and treatment, and somebody who works nationally and locally in organizations of people impacted by drug use, I know people in my area right now — marginalized people of color — who would have much better chances of survival if they were able to access methadone. If MOTAA passed tomorrow, we could save so many lives. There is an ethical imperative to pass it,” Simon said.
Leshner said he is “always very concerned about access, particularly for underserved populations, poor people, people living in rural areas. If you can access the medications you need, you’re in big trouble.”
Is this methadone’s moment? “I’m a little optimistic, but I haven’t seen the progress I would like to see,” Leshner said.
A version of this article first appeared on Medscape.com.
The Pediatrician’s Role in Suicide Prevention
When she was 5 years old, Katherine Edson, LCSW, tried to end her life by drowning herself. “I was enduring severe physical and sexual abuse, and it had become unbearable,” she said. “I waded into a lake, knowing there was a point when it would become too deep and I’d go under.”
As she was walking toward the deeper water, it occurred to her that if she died, she wouldn’t be able to eat Rice Krispies again. “I thought, ‘no more Snap, Crackle, and Pop’ — the three little mascots on the cereal box — and I felt sad,” said Edson, a New York–based retired therapist. “But I still kept walking.”
A man on the shore saw her disappear under the water and pulled her out. “I remember vomiting a lot of water and I remember that the man had tattoos, but I don’t remember how I felt to be alive. I was just numb.”
Edson thinks there were clues her pediatrician missed. “We lived in a small Southern town. Everyone knew my parents were alcoholics. I was very dissociated and withdrawn in general and during pediatric visits. My affect broadcasted that something was wrong, but no one asked if I was okay.”
She acknowledged that professionals in those days “weren’t tuned in to mental health issues in kids. At least there’s more awareness today and hopefully more training — especially since it seems like more kids are trying to end their lives today than when I was growing up.”
Alarming Statistics
According to the American Academy of Pediatrics (AAP), suicide is the second leading cause of death for people aged 10-24 years. Data from Children’s Hospital Association’s Pediatric Health Information System revealed that suicide attempts, ideation, and self-injury have become the most common mental health conditions seen in the emergency departments (EDs) of children’s hospitals, with a 166% increase in ED visits for suicide attempts in children aged 5-18 years, between 2016 and 2022.
Psychiatrist Helen Egger, MD, chief medical officer and co-founder of Little Otter, a specialty pediatric and whole family digital mental health company, recently coauthored a report analyzing data on 1434 children who completed a screening session and comprehensive diagnostic assessment at Little Otter from May 2023 to February 2024 (n = 1016 children aged 8-14 years and n = 418 aged 3-7 years).
Almost one fifth of the older children presented with current positive suicide risk (suicidal ideation and/or behavior in the last month), while 6% of the younger age group presented with current suicide risk. The youngest was 5 years old.
Points of Contact
“It’s known that most children who die by suicide had a recent visit with a health professional — a pediatrician or child mental health professional. It’s unlikely that the child was fine and then, a few weeks later, stopped being fine. The likelihood is that the child wasn’t fine during that visit, but the clinician didn’t ask about mental health,” Egger said.
Christine Crawford, MD, MPH, associate medical director of the National Alliance on Mental Illness (NAMI), said that “When you’re working with kids, anything can come up. Be prepared to navigate the conversation. You can never predict who the patient will feel most comfortable disclosing these thoughts to.”
Pediatricians are the physicians most likely to be seen by children, and it’s important for pediatricians to inquire about a child’s mood, especially during child visits, according to Crawford, author of the book You Are Not Alone for Parents and Caregivers: The NAMI Guide to Navigating Your Child’s Mental Health.
Donald E. Greydanus, MD, professor and founding chair, Department of Pediatric and Adolescent Medicine, Homer Stryker MD School of Medicine, Western Michigan University, Kalamazoo, Michigan, said many fellow pediatricians have said the highly compressed exam doesn’t allow enough time to ask questions. “But pediatricians must find a way to make time,” he said. “Asking about depression and potential suicidality is top priority and can help keep your patients alive.”
Some pediatricians have told him, “I’m not prepared to provide counseling.” But “your role isn’t to provide counseling, just to open the conversation, offer hope, and direct the youngster to resources that can help.”
Don’t Be Afraid to Ask
According to the AAP, all children aged 12 years or older should be screened for suicidal risk, and children aged 8-11 years should be screened “when clinically indicated.” AAP also recommends annual screening for depression in children aged 12 years or older. However, Egger thinks that screening for depression should start sooner.
It can be tempting to screen by merely giving a youngster a form to fill out in the waiting room, but Greydanus strongly advises against this approach. “The important thing is having rapport with the child, being in the same room together. You can ask some simple questions. ‘How are you doing? How are things at school? How are things with your family?’”
“When you’re screening for depression and have a kid who’s talking about sadness or low mood for more than 2 weeks and endorsing other symptoms, such as problems with sleep or appetite, difficulty concentrating, anhedonia, losing interest in things they’d usually enjoy, feeling they’re a burden to others, hopelessness about the future, being unable to function the way they used to — that person meets criteria for depression and you should have a high suspicion and concern about potential suicide,” said Crawford, assistant professor of psychiatry, Boston University School of Medicine.
She suggested probing further and being direct. “It sounds like you’ve been having a tough time. You talk about being sad. I wonder if you’re feeling so sad that you might not want to be alive anymore.” Some healthcare providers “tiptoe around when it comes to suicide, but it’s better to be direct and communicate the question in simple, plain language: ‘Have you ever had thoughts about hurting or killing yourself, that life is no longer worth living, or life would be easier for your family if you weren’t alive?’”
It’s a common myth that asking about depression or suicidality will “plant a seed” or “put ideas in people’s heads,” potentially leading to suicidality. “What we know to be true is that asking about suicide doesn’t put lives at risk. In fact, the contrary is true,” according to Crawford. Several studies have refuted this myth.
Two screening tools that might be helpful in ascertaining the presence of depression and suicidality are the PHQ-9 modified for Adolescents and the four-question Ask Suicide-Screening Questions.
Probe for More Details
If a child or adolescent affirms suicidal ideation, it’s important to ask if they have a plan, Crawford advised. “If they say, ‘yes,’ don’t run out of the office or shut down the conversation by picking up the phone and calling the closest child psychiatrist. We want kids to open up as much as possible when they’ve already opened up a little. So continue the conversation.”
If a child has a plan, the risk for following through on that plan is “high,” Crawford emphasized. “You want the maximum amount of information at your fingertips because this will equip you to navigate the next step in getting the child help.”
The suicide plan may not be realistic and, if carried out, might not actually end in death, especially in younger children. “A 6-year-old might say, ‘I’m gonna drink a whole bottle of apple juice and my belly will explode.’ Or ‘I’ll take 10 extra vitamins.’ The objective lethality of the plan doesn’t matter in that moment. What matters is that the child believes it’s going to work, and it provides a window into how depressed that child is.”
Greydanus added that it’s important to understand what might be going on in the child’s life. Could there be abuse in the family? Is the child being bullied? Bullying can take place at school or online, he noted. The overall risk for suicidal thoughts is elevated for youth who are involved in bullying, whether they’re the bully or the one being bullied.
Kirk Smalley, president and co-founder of Stand for the Silent, an organization designed to bring awareness about the devastating effects of bullying, agreed that pediatricians a should ask children if they’re being bullied. “Sometimes, kids will open up to someone who isn’t a parent or a teacher, who might be seen as ‘too close’ to the situation,” Smalley said.
“Let them know you’re a trusted adult they can confide in and you’re willing to help them navigate this — and then follow through,” advised Smalley, whose 11-year-old son died by suicide after being subjected to bullying.
Painting a Complete Picture
Crawford advises clinicians to “look at the whole picture and piece it together.”
For example, “if the child is functioning, going to school, maintaining relationships with other people, and not experiencing symptoms of depression but discloses the desire to kill him/herself, understand the context.” Sometimes, adolescents can be impulsive. Decision-making “can be driven by emotion.” The teen may have experienced emotional distress, such as “conflict with a peer, arguments with a parent, or romantic heartbreak. She might say, ‘I’m going to kill myself if I ever see him holding hands with another girl.’”
In the setting of an acute stressor, such as a breakup, the child might not need a higher level of care such as hospitalization. “But for non-psychiatry providers, it’s unclear if the child might act on it, so it’s important to have the child evaluated; talk to collateral supports, such as parents, teachers, or a therapist if they have one; and see what makes sense for that specific child.”
She also recommended “getting a sense if the kid is future-oriented in thinking. If they’re talking about an upcoming concert this weekend, or wanting to get to basketball practice, that’s reassuring. It suggests the likelihood of following through [on suicide] is low.”
And assess coping strategies. “You can say, ‘I see you’re really going through a lot. I worry that these thoughts will come up in school. What do you think you’d do in the moment if these thoughts come up?’ If there’s a coping strategy — for example, ‘I’d talk to my friend during lunch’ — that’s also reassuring,” Crawford said.
Of course, that doesn’t mean the statement should be ignored or dismissed. Rather, it informs the next preventive steps and how intensive the level of care should be.
Next Steps: Involving the Family, Getting Help
It’s particularly concerning if the child is unable to identify strategies other than suicide, said Crawford. “You can say, ‘I’m concerned because it’s highly likely that you’ll run into this guy and I wouldn’t want you to die. You have so much to live for.’”
Then, you can ask if it’s okay to bring in the parent or caregiver to talk about what the child just revealed. “If the kid says no — especially a teen — you can respond, ‘I hear what you’re saying, but I actually do have to bring your parent in because of your safety and we can discuss together how to keep you safe.’”
In advance, Crawford tells the patient what she plans to share with the parent. “That way, we’re on the same page and the kid has a sense of agency about how the conversation with the parent will go.” If the teen doesn’t want certain information revealed, “you can ask, ‘What would you leave out, and why?’ This lends itself to a helpful conversation about what the child is thinking about.”
Once the provider has received the green light, it’s time to bring the parent into the room. “Especially in the primary care or pediatric setting, the parent is often shocked, worried, and caught off-guard,” Crawford said.
“You can start by thanking the patient for being open and honest. Then you can tell the parent, ‘Your daughter shared she’s been having some difficult emotions and experiences, and she’s thought of ending her life because she doesn’t know how to cope. I wanted to talk to you about this because it’s important to look at resources we can connect her to and effective coping strategies.’”
Further interventions can include referring the patient to a child psychiatrist or therapist, or both. “Have a list of referrals readily available,” Greydanus advised. If you suspect or if the child reveals abuse, you’re a mandated reporter and need to inform Child Protective Services (CPS). “But don’t stop there,” he warned. “Make sure the child is indeed getting help through CPS and appropriate intervention has been taken regarding the abuse and potential suicide attempt.” Or you may send the child to the ED, where ED physicians are “trained in what to do if they suspect abuse. But make sure that when you ‘throw the ball,’ there’s someone who can ‘catch’ it and accept responsibility for the child’s safety.”
Crawford noted that many primary care settings — especially in under-resourced areas — lack child psychiatrists or therapists. “You need to know what’s feasible in the community you’re practicing in,” she advised. “Be aware of the local crisis line — 988 — and mental health resources in the school and community. There are often school psychologists, social workers, or counselors who can become involved.”
Greydanus emphasized that it’s critical to assess for the presence of firearms in the home and address it with the parents. “If a child is sad or angry and gets impulsive, it’s amazingly common for them to get their hands in a firearm and use it.”
As previously reported, pediatricians and other healthcare providers have a valuable role to play in screening parents for firearm ownership and offering counseling on safe storage practices, according to research presented on September 28 at the AAP 2024 National Conference.
Sometimes, Even the Best Efforts Aren’t Successful
“Suicide is complicated, and parents or doctors can take all the ‘right’ steps to get counseling for the child — hospitalization, medication, and support — and children might still take their lives,” said Ronnie Susan Walker, MS, LCPC, founder and executive director of Alliance of Hope for Suicide Loss Survivors. The organization was launched as a “postvention campaign” 7 years ago to provide support to survivors of suicide loss, who are themselves a high-risk population for suicide.
Walker alluded to the concept of a “ suicide trance” — a term coined by Richard Heckler, PhD, in his book Waking Up, Alive. This trance “is a state of mind and body that receives only the kind of input that reinforces the pain and corroborates the person’s conviction that the only way out is through death,” Heckler wrote.
Walker, whose stepson died by suicide, said physicians and other healthcare professionals who have lost a patient to suicide “should focus on postvention — finding support from other professionals and managing their own grief and guilt.”
It’s natural to feel guilt and second-guess yourself, Greydanus said. “You question whether you missed something or could have done more, so acknowledge that even with the best care and intentions, some suicides aren’t preventable,” he said.
Walker recommends reaching out to the family. “When I lost my stepson, his doctor came to the funeral and wrote us a very meaningful note. That meant so much to us.”
Greydanus agreed it’s appropriate for the clinician to offer comfort to the family “if he or she feels it necessary or feels moved to do so.” However, he cautioned, there’s “often a fear of malpractice charges that may interfere in certain cases.”
Egger added that records should always be “very detailed,” with clear documentation of how you interacted with the child and the rationale behind your interventions. “I’m not a legal expert, but I would always err on the side of connecting with family and sharing grief and compassion. My experience with physician-patient relationships is that the more connected, transparent, and empathetic they are, the better the outcome will be for everyone.”
Losing a patient to suicide is traumatic, so give yourself time to grieve, Egger advised. “Unfortunately, this is an experience that almost everyone in the field will likely go through at some point. Reach out for professional counseling or peer support.”
Physicians who have lost a patient to suicide may turn to an online forum, the Coalition of Clinician Survivors, designed to create a safe anonymous space for discussion, education, testimonials, and one-on-one support.
Greydanus emphasized that the most important role in working with suicidal youngsters is to provide hope. “Yes, you can’t help everyone, but you can help most of them. That’s why you’re there.”
Greydanus, Crawford, Egger, Edson, Smalley, and Walker reported no financial conflicts of interest.
A version of this article appeared on Medscape.com.
When she was 5 years old, Katherine Edson, LCSW, tried to end her life by drowning herself. “I was enduring severe physical and sexual abuse, and it had become unbearable,” she said. “I waded into a lake, knowing there was a point when it would become too deep and I’d go under.”
As she was walking toward the deeper water, it occurred to her that if she died, she wouldn’t be able to eat Rice Krispies again. “I thought, ‘no more Snap, Crackle, and Pop’ — the three little mascots on the cereal box — and I felt sad,” said Edson, a New York–based retired therapist. “But I still kept walking.”
A man on the shore saw her disappear under the water and pulled her out. “I remember vomiting a lot of water and I remember that the man had tattoos, but I don’t remember how I felt to be alive. I was just numb.”
Edson thinks there were clues her pediatrician missed. “We lived in a small Southern town. Everyone knew my parents were alcoholics. I was very dissociated and withdrawn in general and during pediatric visits. My affect broadcasted that something was wrong, but no one asked if I was okay.”
She acknowledged that professionals in those days “weren’t tuned in to mental health issues in kids. At least there’s more awareness today and hopefully more training — especially since it seems like more kids are trying to end their lives today than when I was growing up.”
Alarming Statistics
According to the American Academy of Pediatrics (AAP), suicide is the second leading cause of death for people aged 10-24 years. Data from Children’s Hospital Association’s Pediatric Health Information System revealed that suicide attempts, ideation, and self-injury have become the most common mental health conditions seen in the emergency departments (EDs) of children’s hospitals, with a 166% increase in ED visits for suicide attempts in children aged 5-18 years, between 2016 and 2022.
Psychiatrist Helen Egger, MD, chief medical officer and co-founder of Little Otter, a specialty pediatric and whole family digital mental health company, recently coauthored a report analyzing data on 1434 children who completed a screening session and comprehensive diagnostic assessment at Little Otter from May 2023 to February 2024 (n = 1016 children aged 8-14 years and n = 418 aged 3-7 years).
Almost one fifth of the older children presented with current positive suicide risk (suicidal ideation and/or behavior in the last month), while 6% of the younger age group presented with current suicide risk. The youngest was 5 years old.
Points of Contact
“It’s known that most children who die by suicide had a recent visit with a health professional — a pediatrician or child mental health professional. It’s unlikely that the child was fine and then, a few weeks later, stopped being fine. The likelihood is that the child wasn’t fine during that visit, but the clinician didn’t ask about mental health,” Egger said.
Christine Crawford, MD, MPH, associate medical director of the National Alliance on Mental Illness (NAMI), said that “When you’re working with kids, anything can come up. Be prepared to navigate the conversation. You can never predict who the patient will feel most comfortable disclosing these thoughts to.”
Pediatricians are the physicians most likely to be seen by children, and it’s important for pediatricians to inquire about a child’s mood, especially during child visits, according to Crawford, author of the book You Are Not Alone for Parents and Caregivers: The NAMI Guide to Navigating Your Child’s Mental Health.
Donald E. Greydanus, MD, professor and founding chair, Department of Pediatric and Adolescent Medicine, Homer Stryker MD School of Medicine, Western Michigan University, Kalamazoo, Michigan, said many fellow pediatricians have said the highly compressed exam doesn’t allow enough time to ask questions. “But pediatricians must find a way to make time,” he said. “Asking about depression and potential suicidality is top priority and can help keep your patients alive.”
Some pediatricians have told him, “I’m not prepared to provide counseling.” But “your role isn’t to provide counseling, just to open the conversation, offer hope, and direct the youngster to resources that can help.”
Don’t Be Afraid to Ask
According to the AAP, all children aged 12 years or older should be screened for suicidal risk, and children aged 8-11 years should be screened “when clinically indicated.” AAP also recommends annual screening for depression in children aged 12 years or older. However, Egger thinks that screening for depression should start sooner.
It can be tempting to screen by merely giving a youngster a form to fill out in the waiting room, but Greydanus strongly advises against this approach. “The important thing is having rapport with the child, being in the same room together. You can ask some simple questions. ‘How are you doing? How are things at school? How are things with your family?’”
“When you’re screening for depression and have a kid who’s talking about sadness or low mood for more than 2 weeks and endorsing other symptoms, such as problems with sleep or appetite, difficulty concentrating, anhedonia, losing interest in things they’d usually enjoy, feeling they’re a burden to others, hopelessness about the future, being unable to function the way they used to — that person meets criteria for depression and you should have a high suspicion and concern about potential suicide,” said Crawford, assistant professor of psychiatry, Boston University School of Medicine.
She suggested probing further and being direct. “It sounds like you’ve been having a tough time. You talk about being sad. I wonder if you’re feeling so sad that you might not want to be alive anymore.” Some healthcare providers “tiptoe around when it comes to suicide, but it’s better to be direct and communicate the question in simple, plain language: ‘Have you ever had thoughts about hurting or killing yourself, that life is no longer worth living, or life would be easier for your family if you weren’t alive?’”
It’s a common myth that asking about depression or suicidality will “plant a seed” or “put ideas in people’s heads,” potentially leading to suicidality. “What we know to be true is that asking about suicide doesn’t put lives at risk. In fact, the contrary is true,” according to Crawford. Several studies have refuted this myth.
Two screening tools that might be helpful in ascertaining the presence of depression and suicidality are the PHQ-9 modified for Adolescents and the four-question Ask Suicide-Screening Questions.
Probe for More Details
If a child or adolescent affirms suicidal ideation, it’s important to ask if they have a plan, Crawford advised. “If they say, ‘yes,’ don’t run out of the office or shut down the conversation by picking up the phone and calling the closest child psychiatrist. We want kids to open up as much as possible when they’ve already opened up a little. So continue the conversation.”
If a child has a plan, the risk for following through on that plan is “high,” Crawford emphasized. “You want the maximum amount of information at your fingertips because this will equip you to navigate the next step in getting the child help.”
The suicide plan may not be realistic and, if carried out, might not actually end in death, especially in younger children. “A 6-year-old might say, ‘I’m gonna drink a whole bottle of apple juice and my belly will explode.’ Or ‘I’ll take 10 extra vitamins.’ The objective lethality of the plan doesn’t matter in that moment. What matters is that the child believes it’s going to work, and it provides a window into how depressed that child is.”
Greydanus added that it’s important to understand what might be going on in the child’s life. Could there be abuse in the family? Is the child being bullied? Bullying can take place at school or online, he noted. The overall risk for suicidal thoughts is elevated for youth who are involved in bullying, whether they’re the bully or the one being bullied.
Kirk Smalley, president and co-founder of Stand for the Silent, an organization designed to bring awareness about the devastating effects of bullying, agreed that pediatricians a should ask children if they’re being bullied. “Sometimes, kids will open up to someone who isn’t a parent or a teacher, who might be seen as ‘too close’ to the situation,” Smalley said.
“Let them know you’re a trusted adult they can confide in and you’re willing to help them navigate this — and then follow through,” advised Smalley, whose 11-year-old son died by suicide after being subjected to bullying.
Painting a Complete Picture
Crawford advises clinicians to “look at the whole picture and piece it together.”
For example, “if the child is functioning, going to school, maintaining relationships with other people, and not experiencing symptoms of depression but discloses the desire to kill him/herself, understand the context.” Sometimes, adolescents can be impulsive. Decision-making “can be driven by emotion.” The teen may have experienced emotional distress, such as “conflict with a peer, arguments with a parent, or romantic heartbreak. She might say, ‘I’m going to kill myself if I ever see him holding hands with another girl.’”
In the setting of an acute stressor, such as a breakup, the child might not need a higher level of care such as hospitalization. “But for non-psychiatry providers, it’s unclear if the child might act on it, so it’s important to have the child evaluated; talk to collateral supports, such as parents, teachers, or a therapist if they have one; and see what makes sense for that specific child.”
She also recommended “getting a sense if the kid is future-oriented in thinking. If they’re talking about an upcoming concert this weekend, or wanting to get to basketball practice, that’s reassuring. It suggests the likelihood of following through [on suicide] is low.”
And assess coping strategies. “You can say, ‘I see you’re really going through a lot. I worry that these thoughts will come up in school. What do you think you’d do in the moment if these thoughts come up?’ If there’s a coping strategy — for example, ‘I’d talk to my friend during lunch’ — that’s also reassuring,” Crawford said.
Of course, that doesn’t mean the statement should be ignored or dismissed. Rather, it informs the next preventive steps and how intensive the level of care should be.
Next Steps: Involving the Family, Getting Help
It’s particularly concerning if the child is unable to identify strategies other than suicide, said Crawford. “You can say, ‘I’m concerned because it’s highly likely that you’ll run into this guy and I wouldn’t want you to die. You have so much to live for.’”
Then, you can ask if it’s okay to bring in the parent or caregiver to talk about what the child just revealed. “If the kid says no — especially a teen — you can respond, ‘I hear what you’re saying, but I actually do have to bring your parent in because of your safety and we can discuss together how to keep you safe.’”
In advance, Crawford tells the patient what she plans to share with the parent. “That way, we’re on the same page and the kid has a sense of agency about how the conversation with the parent will go.” If the teen doesn’t want certain information revealed, “you can ask, ‘What would you leave out, and why?’ This lends itself to a helpful conversation about what the child is thinking about.”
Once the provider has received the green light, it’s time to bring the parent into the room. “Especially in the primary care or pediatric setting, the parent is often shocked, worried, and caught off-guard,” Crawford said.
“You can start by thanking the patient for being open and honest. Then you can tell the parent, ‘Your daughter shared she’s been having some difficult emotions and experiences, and she’s thought of ending her life because she doesn’t know how to cope. I wanted to talk to you about this because it’s important to look at resources we can connect her to and effective coping strategies.’”
Further interventions can include referring the patient to a child psychiatrist or therapist, or both. “Have a list of referrals readily available,” Greydanus advised. If you suspect or if the child reveals abuse, you’re a mandated reporter and need to inform Child Protective Services (CPS). “But don’t stop there,” he warned. “Make sure the child is indeed getting help through CPS and appropriate intervention has been taken regarding the abuse and potential suicide attempt.” Or you may send the child to the ED, where ED physicians are “trained in what to do if they suspect abuse. But make sure that when you ‘throw the ball,’ there’s someone who can ‘catch’ it and accept responsibility for the child’s safety.”
Crawford noted that many primary care settings — especially in under-resourced areas — lack child psychiatrists or therapists. “You need to know what’s feasible in the community you’re practicing in,” she advised. “Be aware of the local crisis line — 988 — and mental health resources in the school and community. There are often school psychologists, social workers, or counselors who can become involved.”
Greydanus emphasized that it’s critical to assess for the presence of firearms in the home and address it with the parents. “If a child is sad or angry and gets impulsive, it’s amazingly common for them to get their hands in a firearm and use it.”
As previously reported, pediatricians and other healthcare providers have a valuable role to play in screening parents for firearm ownership and offering counseling on safe storage practices, according to research presented on September 28 at the AAP 2024 National Conference.
Sometimes, Even the Best Efforts Aren’t Successful
“Suicide is complicated, and parents or doctors can take all the ‘right’ steps to get counseling for the child — hospitalization, medication, and support — and children might still take their lives,” said Ronnie Susan Walker, MS, LCPC, founder and executive director of Alliance of Hope for Suicide Loss Survivors. The organization was launched as a “postvention campaign” 7 years ago to provide support to survivors of suicide loss, who are themselves a high-risk population for suicide.
Walker alluded to the concept of a “ suicide trance” — a term coined by Richard Heckler, PhD, in his book Waking Up, Alive. This trance “is a state of mind and body that receives only the kind of input that reinforces the pain and corroborates the person’s conviction that the only way out is through death,” Heckler wrote.
Walker, whose stepson died by suicide, said physicians and other healthcare professionals who have lost a patient to suicide “should focus on postvention — finding support from other professionals and managing their own grief and guilt.”
It’s natural to feel guilt and second-guess yourself, Greydanus said. “You question whether you missed something or could have done more, so acknowledge that even with the best care and intentions, some suicides aren’t preventable,” he said.
Walker recommends reaching out to the family. “When I lost my stepson, his doctor came to the funeral and wrote us a very meaningful note. That meant so much to us.”
Greydanus agreed it’s appropriate for the clinician to offer comfort to the family “if he or she feels it necessary or feels moved to do so.” However, he cautioned, there’s “often a fear of malpractice charges that may interfere in certain cases.”
Egger added that records should always be “very detailed,” with clear documentation of how you interacted with the child and the rationale behind your interventions. “I’m not a legal expert, but I would always err on the side of connecting with family and sharing grief and compassion. My experience with physician-patient relationships is that the more connected, transparent, and empathetic they are, the better the outcome will be for everyone.”
Losing a patient to suicide is traumatic, so give yourself time to grieve, Egger advised. “Unfortunately, this is an experience that almost everyone in the field will likely go through at some point. Reach out for professional counseling or peer support.”
Physicians who have lost a patient to suicide may turn to an online forum, the Coalition of Clinician Survivors, designed to create a safe anonymous space for discussion, education, testimonials, and one-on-one support.
Greydanus emphasized that the most important role in working with suicidal youngsters is to provide hope. “Yes, you can’t help everyone, but you can help most of them. That’s why you’re there.”
Greydanus, Crawford, Egger, Edson, Smalley, and Walker reported no financial conflicts of interest.
A version of this article appeared on Medscape.com.
When she was 5 years old, Katherine Edson, LCSW, tried to end her life by drowning herself. “I was enduring severe physical and sexual abuse, and it had become unbearable,” she said. “I waded into a lake, knowing there was a point when it would become too deep and I’d go under.”
As she was walking toward the deeper water, it occurred to her that if she died, she wouldn’t be able to eat Rice Krispies again. “I thought, ‘no more Snap, Crackle, and Pop’ — the three little mascots on the cereal box — and I felt sad,” said Edson, a New York–based retired therapist. “But I still kept walking.”
A man on the shore saw her disappear under the water and pulled her out. “I remember vomiting a lot of water and I remember that the man had tattoos, but I don’t remember how I felt to be alive. I was just numb.”
Edson thinks there were clues her pediatrician missed. “We lived in a small Southern town. Everyone knew my parents were alcoholics. I was very dissociated and withdrawn in general and during pediatric visits. My affect broadcasted that something was wrong, but no one asked if I was okay.”
She acknowledged that professionals in those days “weren’t tuned in to mental health issues in kids. At least there’s more awareness today and hopefully more training — especially since it seems like more kids are trying to end their lives today than when I was growing up.”
Alarming Statistics
According to the American Academy of Pediatrics (AAP), suicide is the second leading cause of death for people aged 10-24 years. Data from Children’s Hospital Association’s Pediatric Health Information System revealed that suicide attempts, ideation, and self-injury have become the most common mental health conditions seen in the emergency departments (EDs) of children’s hospitals, with a 166% increase in ED visits for suicide attempts in children aged 5-18 years, between 2016 and 2022.
Psychiatrist Helen Egger, MD, chief medical officer and co-founder of Little Otter, a specialty pediatric and whole family digital mental health company, recently coauthored a report analyzing data on 1434 children who completed a screening session and comprehensive diagnostic assessment at Little Otter from May 2023 to February 2024 (n = 1016 children aged 8-14 years and n = 418 aged 3-7 years).
Almost one fifth of the older children presented with current positive suicide risk (suicidal ideation and/or behavior in the last month), while 6% of the younger age group presented with current suicide risk. The youngest was 5 years old.
Points of Contact
“It’s known that most children who die by suicide had a recent visit with a health professional — a pediatrician or child mental health professional. It’s unlikely that the child was fine and then, a few weeks later, stopped being fine. The likelihood is that the child wasn’t fine during that visit, but the clinician didn’t ask about mental health,” Egger said.
Christine Crawford, MD, MPH, associate medical director of the National Alliance on Mental Illness (NAMI), said that “When you’re working with kids, anything can come up. Be prepared to navigate the conversation. You can never predict who the patient will feel most comfortable disclosing these thoughts to.”
Pediatricians are the physicians most likely to be seen by children, and it’s important for pediatricians to inquire about a child’s mood, especially during child visits, according to Crawford, author of the book You Are Not Alone for Parents and Caregivers: The NAMI Guide to Navigating Your Child’s Mental Health.
Donald E. Greydanus, MD, professor and founding chair, Department of Pediatric and Adolescent Medicine, Homer Stryker MD School of Medicine, Western Michigan University, Kalamazoo, Michigan, said many fellow pediatricians have said the highly compressed exam doesn’t allow enough time to ask questions. “But pediatricians must find a way to make time,” he said. “Asking about depression and potential suicidality is top priority and can help keep your patients alive.”
Some pediatricians have told him, “I’m not prepared to provide counseling.” But “your role isn’t to provide counseling, just to open the conversation, offer hope, and direct the youngster to resources that can help.”
Don’t Be Afraid to Ask
According to the AAP, all children aged 12 years or older should be screened for suicidal risk, and children aged 8-11 years should be screened “when clinically indicated.” AAP also recommends annual screening for depression in children aged 12 years or older. However, Egger thinks that screening for depression should start sooner.
It can be tempting to screen by merely giving a youngster a form to fill out in the waiting room, but Greydanus strongly advises against this approach. “The important thing is having rapport with the child, being in the same room together. You can ask some simple questions. ‘How are you doing? How are things at school? How are things with your family?’”
“When you’re screening for depression and have a kid who’s talking about sadness or low mood for more than 2 weeks and endorsing other symptoms, such as problems with sleep or appetite, difficulty concentrating, anhedonia, losing interest in things they’d usually enjoy, feeling they’re a burden to others, hopelessness about the future, being unable to function the way they used to — that person meets criteria for depression and you should have a high suspicion and concern about potential suicide,” said Crawford, assistant professor of psychiatry, Boston University School of Medicine.
She suggested probing further and being direct. “It sounds like you’ve been having a tough time. You talk about being sad. I wonder if you’re feeling so sad that you might not want to be alive anymore.” Some healthcare providers “tiptoe around when it comes to suicide, but it’s better to be direct and communicate the question in simple, plain language: ‘Have you ever had thoughts about hurting or killing yourself, that life is no longer worth living, or life would be easier for your family if you weren’t alive?’”
It’s a common myth that asking about depression or suicidality will “plant a seed” or “put ideas in people’s heads,” potentially leading to suicidality. “What we know to be true is that asking about suicide doesn’t put lives at risk. In fact, the contrary is true,” according to Crawford. Several studies have refuted this myth.
Two screening tools that might be helpful in ascertaining the presence of depression and suicidality are the PHQ-9 modified for Adolescents and the four-question Ask Suicide-Screening Questions.
Probe for More Details
If a child or adolescent affirms suicidal ideation, it’s important to ask if they have a plan, Crawford advised. “If they say, ‘yes,’ don’t run out of the office or shut down the conversation by picking up the phone and calling the closest child psychiatrist. We want kids to open up as much as possible when they’ve already opened up a little. So continue the conversation.”
If a child has a plan, the risk for following through on that plan is “high,” Crawford emphasized. “You want the maximum amount of information at your fingertips because this will equip you to navigate the next step in getting the child help.”
The suicide plan may not be realistic and, if carried out, might not actually end in death, especially in younger children. “A 6-year-old might say, ‘I’m gonna drink a whole bottle of apple juice and my belly will explode.’ Or ‘I’ll take 10 extra vitamins.’ The objective lethality of the plan doesn’t matter in that moment. What matters is that the child believes it’s going to work, and it provides a window into how depressed that child is.”
Greydanus added that it’s important to understand what might be going on in the child’s life. Could there be abuse in the family? Is the child being bullied? Bullying can take place at school or online, he noted. The overall risk for suicidal thoughts is elevated for youth who are involved in bullying, whether they’re the bully or the one being bullied.
Kirk Smalley, president and co-founder of Stand for the Silent, an organization designed to bring awareness about the devastating effects of bullying, agreed that pediatricians a should ask children if they’re being bullied. “Sometimes, kids will open up to someone who isn’t a parent or a teacher, who might be seen as ‘too close’ to the situation,” Smalley said.
“Let them know you’re a trusted adult they can confide in and you’re willing to help them navigate this — and then follow through,” advised Smalley, whose 11-year-old son died by suicide after being subjected to bullying.
Painting a Complete Picture
Crawford advises clinicians to “look at the whole picture and piece it together.”
For example, “if the child is functioning, going to school, maintaining relationships with other people, and not experiencing symptoms of depression but discloses the desire to kill him/herself, understand the context.” Sometimes, adolescents can be impulsive. Decision-making “can be driven by emotion.” The teen may have experienced emotional distress, such as “conflict with a peer, arguments with a parent, or romantic heartbreak. She might say, ‘I’m going to kill myself if I ever see him holding hands with another girl.’”
In the setting of an acute stressor, such as a breakup, the child might not need a higher level of care such as hospitalization. “But for non-psychiatry providers, it’s unclear if the child might act on it, so it’s important to have the child evaluated; talk to collateral supports, such as parents, teachers, or a therapist if they have one; and see what makes sense for that specific child.”
She also recommended “getting a sense if the kid is future-oriented in thinking. If they’re talking about an upcoming concert this weekend, or wanting to get to basketball practice, that’s reassuring. It suggests the likelihood of following through [on suicide] is low.”
And assess coping strategies. “You can say, ‘I see you’re really going through a lot. I worry that these thoughts will come up in school. What do you think you’d do in the moment if these thoughts come up?’ If there’s a coping strategy — for example, ‘I’d talk to my friend during lunch’ — that’s also reassuring,” Crawford said.
Of course, that doesn’t mean the statement should be ignored or dismissed. Rather, it informs the next preventive steps and how intensive the level of care should be.
Next Steps: Involving the Family, Getting Help
It’s particularly concerning if the child is unable to identify strategies other than suicide, said Crawford. “You can say, ‘I’m concerned because it’s highly likely that you’ll run into this guy and I wouldn’t want you to die. You have so much to live for.’”
Then, you can ask if it’s okay to bring in the parent or caregiver to talk about what the child just revealed. “If the kid says no — especially a teen — you can respond, ‘I hear what you’re saying, but I actually do have to bring your parent in because of your safety and we can discuss together how to keep you safe.’”
In advance, Crawford tells the patient what she plans to share with the parent. “That way, we’re on the same page and the kid has a sense of agency about how the conversation with the parent will go.” If the teen doesn’t want certain information revealed, “you can ask, ‘What would you leave out, and why?’ This lends itself to a helpful conversation about what the child is thinking about.”
Once the provider has received the green light, it’s time to bring the parent into the room. “Especially in the primary care or pediatric setting, the parent is often shocked, worried, and caught off-guard,” Crawford said.
“You can start by thanking the patient for being open and honest. Then you can tell the parent, ‘Your daughter shared she’s been having some difficult emotions and experiences, and she’s thought of ending her life because she doesn’t know how to cope. I wanted to talk to you about this because it’s important to look at resources we can connect her to and effective coping strategies.’”
Further interventions can include referring the patient to a child psychiatrist or therapist, or both. “Have a list of referrals readily available,” Greydanus advised. If you suspect or if the child reveals abuse, you’re a mandated reporter and need to inform Child Protective Services (CPS). “But don’t stop there,” he warned. “Make sure the child is indeed getting help through CPS and appropriate intervention has been taken regarding the abuse and potential suicide attempt.” Or you may send the child to the ED, where ED physicians are “trained in what to do if they suspect abuse. But make sure that when you ‘throw the ball,’ there’s someone who can ‘catch’ it and accept responsibility for the child’s safety.”
Crawford noted that many primary care settings — especially in under-resourced areas — lack child psychiatrists or therapists. “You need to know what’s feasible in the community you’re practicing in,” she advised. “Be aware of the local crisis line — 988 — and mental health resources in the school and community. There are often school psychologists, social workers, or counselors who can become involved.”
Greydanus emphasized that it’s critical to assess for the presence of firearms in the home and address it with the parents. “If a child is sad or angry and gets impulsive, it’s amazingly common for them to get their hands in a firearm and use it.”
As previously reported, pediatricians and other healthcare providers have a valuable role to play in screening parents for firearm ownership and offering counseling on safe storage practices, according to research presented on September 28 at the AAP 2024 National Conference.
Sometimes, Even the Best Efforts Aren’t Successful
“Suicide is complicated, and parents or doctors can take all the ‘right’ steps to get counseling for the child — hospitalization, medication, and support — and children might still take their lives,” said Ronnie Susan Walker, MS, LCPC, founder and executive director of Alliance of Hope for Suicide Loss Survivors. The organization was launched as a “postvention campaign” 7 years ago to provide support to survivors of suicide loss, who are themselves a high-risk population for suicide.
Walker alluded to the concept of a “ suicide trance” — a term coined by Richard Heckler, PhD, in his book Waking Up, Alive. This trance “is a state of mind and body that receives only the kind of input that reinforces the pain and corroborates the person’s conviction that the only way out is through death,” Heckler wrote.
Walker, whose stepson died by suicide, said physicians and other healthcare professionals who have lost a patient to suicide “should focus on postvention — finding support from other professionals and managing their own grief and guilt.”
It’s natural to feel guilt and second-guess yourself, Greydanus said. “You question whether you missed something or could have done more, so acknowledge that even with the best care and intentions, some suicides aren’t preventable,” he said.
Walker recommends reaching out to the family. “When I lost my stepson, his doctor came to the funeral and wrote us a very meaningful note. That meant so much to us.”
Greydanus agreed it’s appropriate for the clinician to offer comfort to the family “if he or she feels it necessary or feels moved to do so.” However, he cautioned, there’s “often a fear of malpractice charges that may interfere in certain cases.”
Egger added that records should always be “very detailed,” with clear documentation of how you interacted with the child and the rationale behind your interventions. “I’m not a legal expert, but I would always err on the side of connecting with family and sharing grief and compassion. My experience with physician-patient relationships is that the more connected, transparent, and empathetic they are, the better the outcome will be for everyone.”
Losing a patient to suicide is traumatic, so give yourself time to grieve, Egger advised. “Unfortunately, this is an experience that almost everyone in the field will likely go through at some point. Reach out for professional counseling or peer support.”
Physicians who have lost a patient to suicide may turn to an online forum, the Coalition of Clinician Survivors, designed to create a safe anonymous space for discussion, education, testimonials, and one-on-one support.
Greydanus emphasized that the most important role in working with suicidal youngsters is to provide hope. “Yes, you can’t help everyone, but you can help most of them. That’s why you’re there.”
Greydanus, Crawford, Egger, Edson, Smalley, and Walker reported no financial conflicts of interest.
A version of this article appeared on Medscape.com.
FDA Okays Abuse-Deterrent Opioid for Severe Pain
Roxybond, an immediate-release (IR) formulation of oxycodone hydrochloride, is made with Protega’s SentryBond technology, which makes it harder for people to crush, inject, or snort, according to the company.
In a statement from Protega, Paul Howe, the company’s chief commercial officer, said the drug meets an “unmet need for an IR opioid with abuse-deterrent technology that may reduce misuse and abuse while providing pain relief to medically appropriate patients when used as indicated.”
To determine the tablet’s ability to withstand manipulation, more than 2000 in vitro tests were conducted, according to the release. The findings indicate Roxybond reduces — but does not entirely negate — the potential for intranasal and intravenous abuse.
Roxybond was previously approved in 5-, 15-, and 30-mg doses. The 10 mg option provides clinicians with the ability to better modify side effects, manage titration, and provide precision care for patients on opioid therapy, according to Protega.
“For patients, the range of doses can provide better pain control, reduce the risk of side effects, and provide a smoother transition during dosing transitions,” the company stated.
Roxybond is contraindicated in patients with significant respiratory depression, acute or severe bronchial asthma, gastrointestinal obstruction, or hypersensitivity to oxycodone. The drug is not intended for long-term use unless otherwise determined by a clinician. Roxybond also is subject to the FDA’s Risk Evaluation and Mitigation Strategies for opioids.
“The development of Roxybond with SentryBond is a step forward in fighting the national epidemic of prescription opioid overdose,” said Eric Kinzler, PhD, vice president of medical and regulatory affairs for Protega, in a release. “Protega is dedicated to our mission to block the path to abuse and work with healthcare professionals across the continuum of care to reduce misuse and abuse.”
A version of this article first appeared on Medscape.com.
Roxybond, an immediate-release (IR) formulation of oxycodone hydrochloride, is made with Protega’s SentryBond technology, which makes it harder for people to crush, inject, or snort, according to the company.
In a statement from Protega, Paul Howe, the company’s chief commercial officer, said the drug meets an “unmet need for an IR opioid with abuse-deterrent technology that may reduce misuse and abuse while providing pain relief to medically appropriate patients when used as indicated.”
To determine the tablet’s ability to withstand manipulation, more than 2000 in vitro tests were conducted, according to the release. The findings indicate Roxybond reduces — but does not entirely negate — the potential for intranasal and intravenous abuse.
Roxybond was previously approved in 5-, 15-, and 30-mg doses. The 10 mg option provides clinicians with the ability to better modify side effects, manage titration, and provide precision care for patients on opioid therapy, according to Protega.
“For patients, the range of doses can provide better pain control, reduce the risk of side effects, and provide a smoother transition during dosing transitions,” the company stated.
Roxybond is contraindicated in patients with significant respiratory depression, acute or severe bronchial asthma, gastrointestinal obstruction, or hypersensitivity to oxycodone. The drug is not intended for long-term use unless otherwise determined by a clinician. Roxybond also is subject to the FDA’s Risk Evaluation and Mitigation Strategies for opioids.
“The development of Roxybond with SentryBond is a step forward in fighting the national epidemic of prescription opioid overdose,” said Eric Kinzler, PhD, vice president of medical and regulatory affairs for Protega, in a release. “Protega is dedicated to our mission to block the path to abuse and work with healthcare professionals across the continuum of care to reduce misuse and abuse.”
A version of this article first appeared on Medscape.com.
Roxybond, an immediate-release (IR) formulation of oxycodone hydrochloride, is made with Protega’s SentryBond technology, which makes it harder for people to crush, inject, or snort, according to the company.
In a statement from Protega, Paul Howe, the company’s chief commercial officer, said the drug meets an “unmet need for an IR opioid with abuse-deterrent technology that may reduce misuse and abuse while providing pain relief to medically appropriate patients when used as indicated.”
To determine the tablet’s ability to withstand manipulation, more than 2000 in vitro tests were conducted, according to the release. The findings indicate Roxybond reduces — but does not entirely negate — the potential for intranasal and intravenous abuse.
Roxybond was previously approved in 5-, 15-, and 30-mg doses. The 10 mg option provides clinicians with the ability to better modify side effects, manage titration, and provide precision care for patients on opioid therapy, according to Protega.
“For patients, the range of doses can provide better pain control, reduce the risk of side effects, and provide a smoother transition during dosing transitions,” the company stated.
Roxybond is contraindicated in patients with significant respiratory depression, acute or severe bronchial asthma, gastrointestinal obstruction, or hypersensitivity to oxycodone. The drug is not intended for long-term use unless otherwise determined by a clinician. Roxybond also is subject to the FDA’s Risk Evaluation and Mitigation Strategies for opioids.
“The development of Roxybond with SentryBond is a step forward in fighting the national epidemic of prescription opioid overdose,” said Eric Kinzler, PhD, vice president of medical and regulatory affairs for Protega, in a release. “Protega is dedicated to our mission to block the path to abuse and work with healthcare professionals across the continuum of care to reduce misuse and abuse.”
A version of this article first appeared on Medscape.com.











