User login
Child of The New Gastroenterologist
Approach to Weight Management in GI Practice
Introduction
The majority of patients in the United States are now overweight or obese, and as gastroenterologists we treat a number of conditions that are caused or worsened by obesity.1 Cirrhosis related to metabolic associated fatty liver disease (MAFLD) is now a leading indication for liver transplantation in the US2 and obesity is a clear risk factor for all major malignancies of the GI tract, including esophageal, gastric cardia, pancreatic, liver, gallbladder, colon, and rectum.3 Obesity is associated with dysbiosis and impacts barrier function: increasing permeability, abnormal gut bacterial translocation, and inflammation.4 It is more common than malnutrition in our patients with inflammatory bowel disease (IBD), where it impacts response to biologic drugs, increases the technical difficulty of surgeries, such as IPAA, and is associated with worse surgical outcomes.5 Furthermore, patients with obesity may be less likely to undergo preventative cancer screenings and are at increased risk related to sedation for endoscopic procedures.6 With over 40% of Americans suffering from obesity, and increasingly effective treatments available,
Understanding the Mechanisms of Obesity
There are complex orexigenic and anorexigenic brain pathways in the hypothalamus which control global energy balance.7 Obesity results when energy intake exceeds energy expenditure. While overeating and a sedentary lifestyle are commonly blamed, there are a number of elements that contribute, including genetics, medical conditions, medications, psychosocial factors, and environmental components. For example, sleep loss contributes to weight gain by several mechanisms including increasing ghrelin and decreasing leptin levels, thereby increasing hunger and appetite, as well as by decreasing insulin sensitivity and increasing cortisol. Subjects exposed to sleep deprivation in research settings take in 550 kcal more the following day.8 Medications used commonly in GI practice including corticosteroids, antihistamines, propranolol, and amitriptyline, are obesogenic9 and cannabis can impact hypothalamic pathways to stimulate hunger.10
When patients diet or exercise to lose weight, as we have traditionally advised, there are strong hormonal changes and metabolic adaptations that occur to preserve the defended fat mass or “set point.” Loss of adipose tissue results in decreased production of leptin, a hormone that stimulates satiety pathways and inhibits orexigenic pathways, greatly increasing hunger and cravings. Increases in ghrelin production by the stomach decreases perceptions of fullness. With weight loss, energy requirements decrease, and muscles become more efficient, meaning fewer kcal are needed to maintain bodily processes.11 Eventually a plateau is reached, while motivation to diet and restraint around food wane, and hedonistic (reward) pathways are activated. These powerful factors result in the regain of lost weight within one year in the majority of patients.
Implementing Weight Management into GI Practice
Given the stigma and bias around obesity, patients often feel shame and vulnerability around the condition. It is important to have empathy in your approach, asking permission to discuss weight and using patient-first language (e.g. “patient with obesity” not “obese patient”). While BMI is predictive of health outcomes, it does not measure body fat percentage and may be misleading, such as in muscular individuals. Other measures of adiposity including waist circumference and body composition testing, such as with DEXA, may provide additional data. A BMI of 30 or above defines obesity, though newer definitions incorporate related symptoms, organ disfunction, and metabolic abnormalities into the term “clinical obesity.”12 Asian patients experience metabolic complications at a lower BMI, and therefore the definition of obese is 27.5kg/m2 in this population.
Begin by taking a weight history. Has this been a lifelong struggle or is there a particular life circumstance, such as working a third shift or recent pregnancy which precipitated weight gain? Patients should be asked about binge eating or eating late into the evening or waking at night to eat, as these disordered eating behaviors are managed with specific medications and behavioral therapies. Inquire about sleep duration and quality and refer for a sleep study if there is suspicion for obstructive sleep apnea. Other weight-related comorbidities including hyperlipidemia, type 2 diabetes mellitus (T2DM), and MAFLD should be considered and merit a more aggressive approach, as does more severe obesity (class III, BMI ≥40). Questions about marijuana and alcohol use as well as review of the medication list for obesogenic medications can provide further insight into modifiable contributing factors.
Pillars of Weight Management
The internet is awash with trendy diet recommendations, and widespread misconceptions about obesity management are even ingrained into how physicians approach the disease. It is critical to remember that this is not a consequence of bad choices or lack of self-control. Exercise alone is insufficient to result in significant weight loss.13 Furthermore, whether it is through low fat, low carb, or intermittent fasting, weight loss will occur with calorie deficit.14 Evidence-based diet and lifestyle recommendations to lay the groundwork for success should be discussed at each visit (see Table 1). The Mediterranean diet is recommended for weight loss as well as for several GI disorders (i.e., MAFLD and IBD) and is the optimal eating strategy for cardiovascular health.15 Patients should be advised to engage in 150 minutes of moderate exercise per week, such as brisk walking, and should incorporate resistance training to build muscle and maintain bone density.
Anti-obesity Medications
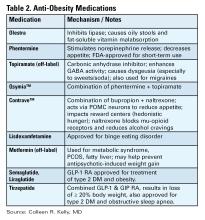
There are a number of medications, either FDA approved or used off label, for treatment of obesity (see Table 2).16 All are indicated for patients with a BMI of ≥ 30 kg/m2 or for those with a BMI between 27-29 kg/m2 with weight-related comorbidities and should be used in combination with diet and lifestyle interventions. None are approved or safe in pregnancy. Mechanisms of action vary by type and include decreased appetite, increased energy expenditure, improved insulin sensitivity, and interfere with absorption.
The newest and most effective anti-obesity medications (AOM), the glucagon-like peptide-1 receptor agonists (GLP-1 RA) are derived from gut hormones secreted in the distal small bowel and colon in response to a meal, which function to delay gastric emptying, increase insulin release from the pancreas, and reduce hepatic gluconeogenesis. Central nervous system effects are not yet entirely understood, but function to decrease appetite and increase satiety. Initially developed for treatment of T2DM, observed weight reduction in patients treated with GLP-1 RA led to clinical trials for treatment of obesity. Semaglutide treatment resulted in weight reduction of 16.9% of total body weight (TBW), and one third of subjects lost ≥ 20% of TBW.17 Tirzepatide combines GLP-1 RA and a gastric inhibitory polypeptide (GIP) receptor agonist, which also has an incretin effect and functions to slow gastric emptying. In the pivotal SURMOUNT trial, approximately 58% of patients achieved ≥20% loss of TBW18 with 15mg weekly dosing of tirzepatide. This class of drugs is a logical choice in patients with T2DM and obesity. Long-term treatment appears necessary, as patients typically regain two-thirds of lost weight within a year after GLP-1 RA are stopped.
Based on tumors observed in rodents, GLP-1 RA are contraindicated in patients with a personal or family history of multiple endocrine neoplasia type 2 (MEN II) or medullary thyroid cancer. These tumors have not been observed in humans treated with GLP-1 RA. They should be used with caution in patients with history of pancreatitis, gastroparesis, or diabetic retinopathy, though a recent systematic review and meta-analysis suggests showed little to no increased risk for biliary events from GLP-1 RA.19 Side effects are most commonly gastrointestinal in nature (nausea, reflux, constipation or diarrhea) and are typically most severe with initiation of the drug and with dose escalation. Side effects can be mitigated by initiating these drugs at lowest doses and gradually titrating up (every four weeks) based on effectiveness and tolerability. Antisecretory, antiemetic, and laxative medications can also be used to help manage GLP-1 RA related side effects.
There is no reason to escalate to highest doses if patients are experiencing weight loss and reduction in food cravings at lower doses. Both semaglutide and tirzepatide are administered subcutaneously every seven days. Once patients have reached goal weight, they can either continue maintenance therapy at that same dose/interval, or if motivated to do so, may gradually reduce the weekly dose in a stepwise approach to determine the minimally effective dose to maintain weight loss. There are not yet published maintenance studies to guide this process. Currently the price of GLP-1 RA and inconsistent insurance coverage make them inaccessible to many patients. The manufacturers of both semaglutide and tirzepatide offer direct to consumer pricing and home delivery.
Bariatric Surgery
In patients with higher BMI (≥35kg/m2) or those with BMI ≥30kg/m2 and obesity-related metabolic disease and the desire to avoid lifelong medications or who fail or are intolerant of AOM, bariatric options should be considered.20 Sleeve gastrectomy has become the most performed surgery for treatment of obesity. It is a restrictive procedure, removing 80% of the stomach, but a drop in circulating levels of ghrelin afterwards also leads to decreased feelings of hunger. It results in weight loss of 25-30% TBW loss. It is not a good choice for patients who suffer from severe GERD, as this typically worsens afterwards; furthermore, de novo Barrett’s has been observed in nearly 6% of patients who undergo sleeve gastrectomy.21
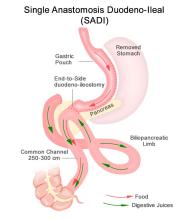
Roux-en-Y gastric bypass is a restrictive and malabsorptive procedure, resulting in 30-35% TBW loss. It has beneficial and immediate metabolic effects, including increased release of endogenous GLP-1, which leads to improvements in weight-related T2DM. The newer single anastomosis duodenal-ileal bypass with sleeve gastrectomy (SADI-S) starts with a sleeve gastrectomy, making a smaller tube-shaped stomach. The duodenum is divided just after the stomach and then a loop of ileum is brought up and connected to the stomach (see Figure 1). This procedure is highly effective, with patients losing 75-95% of excess body weight and is becoming a preferred option for patients with greater BMI (≥50kg/m2). It is also an option for patients who have already had a sleeve gastrectomy and are seeking further weight loss. Because there is only one anastomosis, perioperative complications, such as anastomotic leaks, are reduced. The risk of micronutrient deficiencies is present with all malabsorptive procedures, and these patients must supplement with multivitamins, iron, vitamin D, and calcium.
Endoscopic Therapies
Endoscopic bariatric and metabolic therapies (EBMTs) have been increasingly studied and utilized, and this less invasive option may be more appropriate for or attractive to many patients. Intragastric balloons, which reduce meal volume and delay gastric emptying, can be used short term only (six months) resulting in loss of about 6.9% of total body weight (TBW) greater than lifestyle modification (LM) alone, and may be considered in limited situations, such as need for pre-operative weight loss to reduce risks in very obese individuals.22
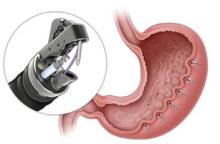
Endoscopic gastric remodeling (EGR), also known as endoscopic sleeve gastrectomy (ESG), is a purely restrictive procedure in which the stomach is cinched to resize and reshape using an endoscopic suturing device (see Figure 2).23 It is an option for patients with class 1 or 2 obesity, with data from a randomized controlled trial in this population demonstrating mean percentage of TBW loss of 13.6% at 52 weeks compared to 0.8% in those treated with LM alone.24 A recent meta-analysis of 21 observational studies, including patients with higher BMIs (32.5 to 49.9 kg/m2) showed pooled average weight loss of 17.3% TBW at 12 months with EGR.22 This procedure has potential advantages of fewer complications, quicker recovery, and much less new-onset GERD compared to laparoscopic sleeve gastrectomy. Furthermore, it may be utilized in combination with AOMs to achieve optimum weight loss and metabolic outcomes.25,26 Potential adverse events include abdominal pain, nausea and vomiting (which may be severe), as well as rare instances of intra/extra luminal bleeding or abdominal abscess requiring drainage.22
Recent joint American/European Gastrointestinal Endoscopy guidelines suggest the use of EBMTs plus lifestyle modification in patients with a BMI of ≥ 30 kg/m2, or with a BMI of 27.0-29.9 kg/m2 with at least 1 obesity-related comorbidity.22 Small bowel interventions including duodenal-jejunal bypass liner and duodenal mucosal resurfacing are being investigated for patients with obesity and type 2 diabetes but not yet commercially available.
Conclusion
Given the overlap of obesity with many GI disorders, it is entirely appropriate for gastroenterologists to consider it worthy of aggressive treatment, particularly in patients with MAFLD and other serious weight related comorbidities. With a compassionate and empathetic approach, and a number of highly effective medical, endoscopic, and surgical therapies now available, weight management has the potential to be extremely rewarding when implemented in GI practice.
Dr. Kelly is based in the Department of Medicine, Division of Gastroenterology, Brigham and Women’s Hospital, and Harvard Medical School, both in Boston, Massachusetts. She serves on the clinical advisory board for OpenBiome (unpaid) and has served on an advisory board for Eli Lilly and Company.
References
1. Hales CM, et al. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017-2018. NCHS Data Brief 2020 Feb:(360):1–8.
2. Pais R, et al. NAFLD and liver transplantation: Current burden and expected challenges. J Hepatol. 2016 Dec. doi: 10.1016/j.jhep.2016.07.033.
3. Lauby-Secretan B, et al. Body Fatness and Cancer--Viewpoint of the IARC Working Group. N Engl J Med. 2016 Aug. doi: 10.1056/NEJMsr1606602.
4. Kim A. Dysbiosis: A Review Highlighting Obesity and Inflammatory Bowel Disease. J Clin Gastroenterol. 2015 Nov-Dec. doi: 10.1097/MCG.0000000000000356.
5. Singh S, et al. Obesity in IBD: epidemiology, pathogenesis, disease course and treatment outcomes. Nat Rev Gastroenterol Hepatol. 2017 Feb. doi: 10.1038/nrgastro.2016.181.
6. Sundararaman L, Goudra B. Sedation for GI Endoscopy in the Morbidly Obese: Challenges and Possible Solutions. J Clin Med. 2024 Aug. doi: 10.3390/jcm13164635.
7. Bombassaro B, et al. The hypothalamus as the central regulator of energy balance and its impact on current and future obesity treatments. Arch Endocrinol Metab. 2024 Nov. doi: 10.20945/2359-4292-2024-0082.
8. Beccuti G, Pannain S. Sleep and obesity. Curr Opin Clin Nutr Metab Care. 2011 Jul. doi: 10.1097/MCO.0b013e3283479109.
9. Desalermos A, et al. Effect of Obesogenic Medications on Weight-Loss Outcomes in a Behavioral Weight-Management Program. Obesity (Silver Spring). 2019 May. doi: 10.1002/oby.22444.
10. Lord MN, Noble EE. Hypothalamic cannabinoid signaling: Consequences for eating behavior. Pharmacol Res Perspect. 2024 Oct. doi: 10.1002/prp2.1251.
11. Farhana A, Rehman A. Metabolic Consequences of Weight Reduction. [Updated 2023 Jul 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK572145/.
12. Rubino F, et al. Definition and diagnostic criteria of clinical obesity. Lancet Diabetes Endocrinol. 2025 Mar. doi: 10.1016/S2213-8587(24)00316-4.
13. Cox CE. Role of Physical Activity for Weight Loss and Weight Maintenance. Diabetes Spectr. 2017 Aug. doi: 10.2337/ds17-0013.
14. Chaput JP, et al. Widespread misconceptions about obesity. Can Fam Physician. 2014 Nov. PMID: 25392431.
15. Muscogiuri G, et al. Mediterranean Diet and Obesity-related Disorders: What is the Evidence? Curr Obes Rep. 2022 Dec. doi: 10.1007/s13679-022-00481-1.
16. Gudzune KA, Kushner RF. Medications for Obesity: A Review. JAMA. 2024 Aug. doi: 10.1001/jama.2024.10816.
17. Wilding JPH, et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N Engl J Med. 2021 Feb. doi: 10.1056/NEJMoa2032183.
18. Jastreboff AM, et al. Tirzepatide Once Weekly for the Treatment of Obesity. N Engl J Med. 2022 Jun. doi: 10.1056/NEJMoa2206038.
19. Chiang CH, et al. Glucagon-Like Peptide-1 Receptor Agonists and Gastrointestinal Adverse Events: A Systematic Review and Meta-Analysis. Gastroenterology. 2025 Nov. doi: 10.1053/j.gastro.2025.06.003.
20. Aderinto N, et al. Recent advances in bariatric surgery: a narrative review of weight loss procedures. Ann Med Surg (Lond). 2023 Nov. doi: 10.1097/MS9.0000000000001472.
21. Chandan S, et al. Risk of De Novo Barrett’s Esophagus Post Sleeve Gastrectomy: A Systematic Review and Meta-Analysis of Studies With Long-Term Follow-Up. Clin Gastroenterol Hepatol. 2025 Jan. doi: 10.1016/j.cgh.2024.06.041.
22. Jirapinyo P, et al. American Society for Gastrointestinal Endoscopy-European Society of Gastrointestinal Endoscopy guideline on primary endoscopic bariatric and metabolic therapies for adults with obesity. Gastrointest Endosc. 2024 Jun. doi: 10.1016/j.gie.2023.12.004.
23. Nduma BN, et al. Endoscopic Gastric Sleeve: A Review of Literature. Cureus. 2023 Mar. doi: 10.7759/cureus.36353.
24. Abu Dayyeh BK, et al. Endoscopic sleeve gastroplasty for treatment of class 1 and 2 obesity (MERIT): a prospective, multicentre, randomised trial. Lancet. 2022 Aug. doi: 10.1016/S0140-6736(22)01280-6.
25. Gala K, et al. Outcomes of concomitant antiobesity medication use with endoscopic sleeve gastroplasty in clinical US settings. Obes Pillars. 2024 May. doi: 10.1016/j.obpill.2024.100112.
26. Chung CS, et al. Endoscopic sleeve gastroplasty combined with anti-obesity medication for better control of weight and diabetes. Clin Endosc. 2025 May. doi: 10.5946/ce.2024.274.
Introduction
The majority of patients in the United States are now overweight or obese, and as gastroenterologists we treat a number of conditions that are caused or worsened by obesity.1 Cirrhosis related to metabolic associated fatty liver disease (MAFLD) is now a leading indication for liver transplantation in the US2 and obesity is a clear risk factor for all major malignancies of the GI tract, including esophageal, gastric cardia, pancreatic, liver, gallbladder, colon, and rectum.3 Obesity is associated with dysbiosis and impacts barrier function: increasing permeability, abnormal gut bacterial translocation, and inflammation.4 It is more common than malnutrition in our patients with inflammatory bowel disease (IBD), where it impacts response to biologic drugs, increases the technical difficulty of surgeries, such as IPAA, and is associated with worse surgical outcomes.5 Furthermore, patients with obesity may be less likely to undergo preventative cancer screenings and are at increased risk related to sedation for endoscopic procedures.6 With over 40% of Americans suffering from obesity, and increasingly effective treatments available,
Understanding the Mechanisms of Obesity
There are complex orexigenic and anorexigenic brain pathways in the hypothalamus which control global energy balance.7 Obesity results when energy intake exceeds energy expenditure. While overeating and a sedentary lifestyle are commonly blamed, there are a number of elements that contribute, including genetics, medical conditions, medications, psychosocial factors, and environmental components. For example, sleep loss contributes to weight gain by several mechanisms including increasing ghrelin and decreasing leptin levels, thereby increasing hunger and appetite, as well as by decreasing insulin sensitivity and increasing cortisol. Subjects exposed to sleep deprivation in research settings take in 550 kcal more the following day.8 Medications used commonly in GI practice including corticosteroids, antihistamines, propranolol, and amitriptyline, are obesogenic9 and cannabis can impact hypothalamic pathways to stimulate hunger.10
When patients diet or exercise to lose weight, as we have traditionally advised, there are strong hormonal changes and metabolic adaptations that occur to preserve the defended fat mass or “set point.” Loss of adipose tissue results in decreased production of leptin, a hormone that stimulates satiety pathways and inhibits orexigenic pathways, greatly increasing hunger and cravings. Increases in ghrelin production by the stomach decreases perceptions of fullness. With weight loss, energy requirements decrease, and muscles become more efficient, meaning fewer kcal are needed to maintain bodily processes.11 Eventually a plateau is reached, while motivation to diet and restraint around food wane, and hedonistic (reward) pathways are activated. These powerful factors result in the regain of lost weight within one year in the majority of patients.
Implementing Weight Management into GI Practice
Given the stigma and bias around obesity, patients often feel shame and vulnerability around the condition. It is important to have empathy in your approach, asking permission to discuss weight and using patient-first language (e.g. “patient with obesity” not “obese patient”). While BMI is predictive of health outcomes, it does not measure body fat percentage and may be misleading, such as in muscular individuals. Other measures of adiposity including waist circumference and body composition testing, such as with DEXA, may provide additional data. A BMI of 30 or above defines obesity, though newer definitions incorporate related symptoms, organ disfunction, and metabolic abnormalities into the term “clinical obesity.”12 Asian patients experience metabolic complications at a lower BMI, and therefore the definition of obese is 27.5kg/m2 in this population.
Begin by taking a weight history. Has this been a lifelong struggle or is there a particular life circumstance, such as working a third shift or recent pregnancy which precipitated weight gain? Patients should be asked about binge eating or eating late into the evening or waking at night to eat, as these disordered eating behaviors are managed with specific medications and behavioral therapies. Inquire about sleep duration and quality and refer for a sleep study if there is suspicion for obstructive sleep apnea. Other weight-related comorbidities including hyperlipidemia, type 2 diabetes mellitus (T2DM), and MAFLD should be considered and merit a more aggressive approach, as does more severe obesity (class III, BMI ≥40). Questions about marijuana and alcohol use as well as review of the medication list for obesogenic medications can provide further insight into modifiable contributing factors.
Pillars of Weight Management
The internet is awash with trendy diet recommendations, and widespread misconceptions about obesity management are even ingrained into how physicians approach the disease. It is critical to remember that this is not a consequence of bad choices or lack of self-control. Exercise alone is insufficient to result in significant weight loss.13 Furthermore, whether it is through low fat, low carb, or intermittent fasting, weight loss will occur with calorie deficit.14 Evidence-based diet and lifestyle recommendations to lay the groundwork for success should be discussed at each visit (see Table 1). The Mediterranean diet is recommended for weight loss as well as for several GI disorders (i.e., MAFLD and IBD) and is the optimal eating strategy for cardiovascular health.15 Patients should be advised to engage in 150 minutes of moderate exercise per week, such as brisk walking, and should incorporate resistance training to build muscle and maintain bone density.
Anti-obesity Medications
There are a number of medications, either FDA approved or used off label, for treatment of obesity (see Table 2).16 All are indicated for patients with a BMI of ≥ 30 kg/m2 or for those with a BMI between 27-29 kg/m2 with weight-related comorbidities and should be used in combination with diet and lifestyle interventions. None are approved or safe in pregnancy. Mechanisms of action vary by type and include decreased appetite, increased energy expenditure, improved insulin sensitivity, and interfere with absorption.
The newest and most effective anti-obesity medications (AOM), the glucagon-like peptide-1 receptor agonists (GLP-1 RA) are derived from gut hormones secreted in the distal small bowel and colon in response to a meal, which function to delay gastric emptying, increase insulin release from the pancreas, and reduce hepatic gluconeogenesis. Central nervous system effects are not yet entirely understood, but function to decrease appetite and increase satiety. Initially developed for treatment of T2DM, observed weight reduction in patients treated with GLP-1 RA led to clinical trials for treatment of obesity. Semaglutide treatment resulted in weight reduction of 16.9% of total body weight (TBW), and one third of subjects lost ≥ 20% of TBW.17 Tirzepatide combines GLP-1 RA and a gastric inhibitory polypeptide (GIP) receptor agonist, which also has an incretin effect and functions to slow gastric emptying. In the pivotal SURMOUNT trial, approximately 58% of patients achieved ≥20% loss of TBW18 with 15mg weekly dosing of tirzepatide. This class of drugs is a logical choice in patients with T2DM and obesity. Long-term treatment appears necessary, as patients typically regain two-thirds of lost weight within a year after GLP-1 RA are stopped.
Based on tumors observed in rodents, GLP-1 RA are contraindicated in patients with a personal or family history of multiple endocrine neoplasia type 2 (MEN II) or medullary thyroid cancer. These tumors have not been observed in humans treated with GLP-1 RA. They should be used with caution in patients with history of pancreatitis, gastroparesis, or diabetic retinopathy, though a recent systematic review and meta-analysis suggests showed little to no increased risk for biliary events from GLP-1 RA.19 Side effects are most commonly gastrointestinal in nature (nausea, reflux, constipation or diarrhea) and are typically most severe with initiation of the drug and with dose escalation. Side effects can be mitigated by initiating these drugs at lowest doses and gradually titrating up (every four weeks) based on effectiveness and tolerability. Antisecretory, antiemetic, and laxative medications can also be used to help manage GLP-1 RA related side effects.
There is no reason to escalate to highest doses if patients are experiencing weight loss and reduction in food cravings at lower doses. Both semaglutide and tirzepatide are administered subcutaneously every seven days. Once patients have reached goal weight, they can either continue maintenance therapy at that same dose/interval, or if motivated to do so, may gradually reduce the weekly dose in a stepwise approach to determine the minimally effective dose to maintain weight loss. There are not yet published maintenance studies to guide this process. Currently the price of GLP-1 RA and inconsistent insurance coverage make them inaccessible to many patients. The manufacturers of both semaglutide and tirzepatide offer direct to consumer pricing and home delivery.
Bariatric Surgery
In patients with higher BMI (≥35kg/m2) or those with BMI ≥30kg/m2 and obesity-related metabolic disease and the desire to avoid lifelong medications or who fail or are intolerant of AOM, bariatric options should be considered.20 Sleeve gastrectomy has become the most performed surgery for treatment of obesity. It is a restrictive procedure, removing 80% of the stomach, but a drop in circulating levels of ghrelin afterwards also leads to decreased feelings of hunger. It results in weight loss of 25-30% TBW loss. It is not a good choice for patients who suffer from severe GERD, as this typically worsens afterwards; furthermore, de novo Barrett’s has been observed in nearly 6% of patients who undergo sleeve gastrectomy.21
Roux-en-Y gastric bypass is a restrictive and malabsorptive procedure, resulting in 30-35% TBW loss. It has beneficial and immediate metabolic effects, including increased release of endogenous GLP-1, which leads to improvements in weight-related T2DM. The newer single anastomosis duodenal-ileal bypass with sleeve gastrectomy (SADI-S) starts with a sleeve gastrectomy, making a smaller tube-shaped stomach. The duodenum is divided just after the stomach and then a loop of ileum is brought up and connected to the stomach (see Figure 1). This procedure is highly effective, with patients losing 75-95% of excess body weight and is becoming a preferred option for patients with greater BMI (≥50kg/m2). It is also an option for patients who have already had a sleeve gastrectomy and are seeking further weight loss. Because there is only one anastomosis, perioperative complications, such as anastomotic leaks, are reduced. The risk of micronutrient deficiencies is present with all malabsorptive procedures, and these patients must supplement with multivitamins, iron, vitamin D, and calcium.
Endoscopic Therapies
Endoscopic bariatric and metabolic therapies (EBMTs) have been increasingly studied and utilized, and this less invasive option may be more appropriate for or attractive to many patients. Intragastric balloons, which reduce meal volume and delay gastric emptying, can be used short term only (six months) resulting in loss of about 6.9% of total body weight (TBW) greater than lifestyle modification (LM) alone, and may be considered in limited situations, such as need for pre-operative weight loss to reduce risks in very obese individuals.22
Endoscopic gastric remodeling (EGR), also known as endoscopic sleeve gastrectomy (ESG), is a purely restrictive procedure in which the stomach is cinched to resize and reshape using an endoscopic suturing device (see Figure 2).23 It is an option for patients with class 1 or 2 obesity, with data from a randomized controlled trial in this population demonstrating mean percentage of TBW loss of 13.6% at 52 weeks compared to 0.8% in those treated with LM alone.24 A recent meta-analysis of 21 observational studies, including patients with higher BMIs (32.5 to 49.9 kg/m2) showed pooled average weight loss of 17.3% TBW at 12 months with EGR.22 This procedure has potential advantages of fewer complications, quicker recovery, and much less new-onset GERD compared to laparoscopic sleeve gastrectomy. Furthermore, it may be utilized in combination with AOMs to achieve optimum weight loss and metabolic outcomes.25,26 Potential adverse events include abdominal pain, nausea and vomiting (which may be severe), as well as rare instances of intra/extra luminal bleeding or abdominal abscess requiring drainage.22
Recent joint American/European Gastrointestinal Endoscopy guidelines suggest the use of EBMTs plus lifestyle modification in patients with a BMI of ≥ 30 kg/m2, or with a BMI of 27.0-29.9 kg/m2 with at least 1 obesity-related comorbidity.22 Small bowel interventions including duodenal-jejunal bypass liner and duodenal mucosal resurfacing are being investigated for patients with obesity and type 2 diabetes but not yet commercially available.
Conclusion
Given the overlap of obesity with many GI disorders, it is entirely appropriate for gastroenterologists to consider it worthy of aggressive treatment, particularly in patients with MAFLD and other serious weight related comorbidities. With a compassionate and empathetic approach, and a number of highly effective medical, endoscopic, and surgical therapies now available, weight management has the potential to be extremely rewarding when implemented in GI practice.
Dr. Kelly is based in the Department of Medicine, Division of Gastroenterology, Brigham and Women’s Hospital, and Harvard Medical School, both in Boston, Massachusetts. She serves on the clinical advisory board for OpenBiome (unpaid) and has served on an advisory board for Eli Lilly and Company.
References
1. Hales CM, et al. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017-2018. NCHS Data Brief 2020 Feb:(360):1–8.
2. Pais R, et al. NAFLD and liver transplantation: Current burden and expected challenges. J Hepatol. 2016 Dec. doi: 10.1016/j.jhep.2016.07.033.
3. Lauby-Secretan B, et al. Body Fatness and Cancer--Viewpoint of the IARC Working Group. N Engl J Med. 2016 Aug. doi: 10.1056/NEJMsr1606602.
4. Kim A. Dysbiosis: A Review Highlighting Obesity and Inflammatory Bowel Disease. J Clin Gastroenterol. 2015 Nov-Dec. doi: 10.1097/MCG.0000000000000356.
5. Singh S, et al. Obesity in IBD: epidemiology, pathogenesis, disease course and treatment outcomes. Nat Rev Gastroenterol Hepatol. 2017 Feb. doi: 10.1038/nrgastro.2016.181.
6. Sundararaman L, Goudra B. Sedation for GI Endoscopy in the Morbidly Obese: Challenges and Possible Solutions. J Clin Med. 2024 Aug. doi: 10.3390/jcm13164635.
7. Bombassaro B, et al. The hypothalamus as the central regulator of energy balance and its impact on current and future obesity treatments. Arch Endocrinol Metab. 2024 Nov. doi: 10.20945/2359-4292-2024-0082.
8. Beccuti G, Pannain S. Sleep and obesity. Curr Opin Clin Nutr Metab Care. 2011 Jul. doi: 10.1097/MCO.0b013e3283479109.
9. Desalermos A, et al. Effect of Obesogenic Medications on Weight-Loss Outcomes in a Behavioral Weight-Management Program. Obesity (Silver Spring). 2019 May. doi: 10.1002/oby.22444.
10. Lord MN, Noble EE. Hypothalamic cannabinoid signaling: Consequences for eating behavior. Pharmacol Res Perspect. 2024 Oct. doi: 10.1002/prp2.1251.
11. Farhana A, Rehman A. Metabolic Consequences of Weight Reduction. [Updated 2023 Jul 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK572145/.
12. Rubino F, et al. Definition and diagnostic criteria of clinical obesity. Lancet Diabetes Endocrinol. 2025 Mar. doi: 10.1016/S2213-8587(24)00316-4.
13. Cox CE. Role of Physical Activity for Weight Loss and Weight Maintenance. Diabetes Spectr. 2017 Aug. doi: 10.2337/ds17-0013.
14. Chaput JP, et al. Widespread misconceptions about obesity. Can Fam Physician. 2014 Nov. PMID: 25392431.
15. Muscogiuri G, et al. Mediterranean Diet and Obesity-related Disorders: What is the Evidence? Curr Obes Rep. 2022 Dec. doi: 10.1007/s13679-022-00481-1.
16. Gudzune KA, Kushner RF. Medications for Obesity: A Review. JAMA. 2024 Aug. doi: 10.1001/jama.2024.10816.
17. Wilding JPH, et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N Engl J Med. 2021 Feb. doi: 10.1056/NEJMoa2032183.
18. Jastreboff AM, et al. Tirzepatide Once Weekly for the Treatment of Obesity. N Engl J Med. 2022 Jun. doi: 10.1056/NEJMoa2206038.
19. Chiang CH, et al. Glucagon-Like Peptide-1 Receptor Agonists and Gastrointestinal Adverse Events: A Systematic Review and Meta-Analysis. Gastroenterology. 2025 Nov. doi: 10.1053/j.gastro.2025.06.003.
20. Aderinto N, et al. Recent advances in bariatric surgery: a narrative review of weight loss procedures. Ann Med Surg (Lond). 2023 Nov. doi: 10.1097/MS9.0000000000001472.
21. Chandan S, et al. Risk of De Novo Barrett’s Esophagus Post Sleeve Gastrectomy: A Systematic Review and Meta-Analysis of Studies With Long-Term Follow-Up. Clin Gastroenterol Hepatol. 2025 Jan. doi: 10.1016/j.cgh.2024.06.041.
22. Jirapinyo P, et al. American Society for Gastrointestinal Endoscopy-European Society of Gastrointestinal Endoscopy guideline on primary endoscopic bariatric and metabolic therapies for adults with obesity. Gastrointest Endosc. 2024 Jun. doi: 10.1016/j.gie.2023.12.004.
23. Nduma BN, et al. Endoscopic Gastric Sleeve: A Review of Literature. Cureus. 2023 Mar. doi: 10.7759/cureus.36353.
24. Abu Dayyeh BK, et al. Endoscopic sleeve gastroplasty for treatment of class 1 and 2 obesity (MERIT): a prospective, multicentre, randomised trial. Lancet. 2022 Aug. doi: 10.1016/S0140-6736(22)01280-6.
25. Gala K, et al. Outcomes of concomitant antiobesity medication use with endoscopic sleeve gastroplasty in clinical US settings. Obes Pillars. 2024 May. doi: 10.1016/j.obpill.2024.100112.
26. Chung CS, et al. Endoscopic sleeve gastroplasty combined with anti-obesity medication for better control of weight and diabetes. Clin Endosc. 2025 May. doi: 10.5946/ce.2024.274.
Introduction
The majority of patients in the United States are now overweight or obese, and as gastroenterologists we treat a number of conditions that are caused or worsened by obesity.1 Cirrhosis related to metabolic associated fatty liver disease (MAFLD) is now a leading indication for liver transplantation in the US2 and obesity is a clear risk factor for all major malignancies of the GI tract, including esophageal, gastric cardia, pancreatic, liver, gallbladder, colon, and rectum.3 Obesity is associated with dysbiosis and impacts barrier function: increasing permeability, abnormal gut bacterial translocation, and inflammation.4 It is more common than malnutrition in our patients with inflammatory bowel disease (IBD), where it impacts response to biologic drugs, increases the technical difficulty of surgeries, such as IPAA, and is associated with worse surgical outcomes.5 Furthermore, patients with obesity may be less likely to undergo preventative cancer screenings and are at increased risk related to sedation for endoscopic procedures.6 With over 40% of Americans suffering from obesity, and increasingly effective treatments available,
Understanding the Mechanisms of Obesity
There are complex orexigenic and anorexigenic brain pathways in the hypothalamus which control global energy balance.7 Obesity results when energy intake exceeds energy expenditure. While overeating and a sedentary lifestyle are commonly blamed, there are a number of elements that contribute, including genetics, medical conditions, medications, psychosocial factors, and environmental components. For example, sleep loss contributes to weight gain by several mechanisms including increasing ghrelin and decreasing leptin levels, thereby increasing hunger and appetite, as well as by decreasing insulin sensitivity and increasing cortisol. Subjects exposed to sleep deprivation in research settings take in 550 kcal more the following day.8 Medications used commonly in GI practice including corticosteroids, antihistamines, propranolol, and amitriptyline, are obesogenic9 and cannabis can impact hypothalamic pathways to stimulate hunger.10
When patients diet or exercise to lose weight, as we have traditionally advised, there are strong hormonal changes and metabolic adaptations that occur to preserve the defended fat mass or “set point.” Loss of adipose tissue results in decreased production of leptin, a hormone that stimulates satiety pathways and inhibits orexigenic pathways, greatly increasing hunger and cravings. Increases in ghrelin production by the stomach decreases perceptions of fullness. With weight loss, energy requirements decrease, and muscles become more efficient, meaning fewer kcal are needed to maintain bodily processes.11 Eventually a plateau is reached, while motivation to diet and restraint around food wane, and hedonistic (reward) pathways are activated. These powerful factors result in the regain of lost weight within one year in the majority of patients.
Implementing Weight Management into GI Practice
Given the stigma and bias around obesity, patients often feel shame and vulnerability around the condition. It is important to have empathy in your approach, asking permission to discuss weight and using patient-first language (e.g. “patient with obesity” not “obese patient”). While BMI is predictive of health outcomes, it does not measure body fat percentage and may be misleading, such as in muscular individuals. Other measures of adiposity including waist circumference and body composition testing, such as with DEXA, may provide additional data. A BMI of 30 or above defines obesity, though newer definitions incorporate related symptoms, organ disfunction, and metabolic abnormalities into the term “clinical obesity.”12 Asian patients experience metabolic complications at a lower BMI, and therefore the definition of obese is 27.5kg/m2 in this population.
Begin by taking a weight history. Has this been a lifelong struggle or is there a particular life circumstance, such as working a third shift or recent pregnancy which precipitated weight gain? Patients should be asked about binge eating or eating late into the evening or waking at night to eat, as these disordered eating behaviors are managed with specific medications and behavioral therapies. Inquire about sleep duration and quality and refer for a sleep study if there is suspicion for obstructive sleep apnea. Other weight-related comorbidities including hyperlipidemia, type 2 diabetes mellitus (T2DM), and MAFLD should be considered and merit a more aggressive approach, as does more severe obesity (class III, BMI ≥40). Questions about marijuana and alcohol use as well as review of the medication list for obesogenic medications can provide further insight into modifiable contributing factors.
Pillars of Weight Management
The internet is awash with trendy diet recommendations, and widespread misconceptions about obesity management are even ingrained into how physicians approach the disease. It is critical to remember that this is not a consequence of bad choices or lack of self-control. Exercise alone is insufficient to result in significant weight loss.13 Furthermore, whether it is through low fat, low carb, or intermittent fasting, weight loss will occur with calorie deficit.14 Evidence-based diet and lifestyle recommendations to lay the groundwork for success should be discussed at each visit (see Table 1). The Mediterranean diet is recommended for weight loss as well as for several GI disorders (i.e., MAFLD and IBD) and is the optimal eating strategy for cardiovascular health.15 Patients should be advised to engage in 150 minutes of moderate exercise per week, such as brisk walking, and should incorporate resistance training to build muscle and maintain bone density.
Anti-obesity Medications
There are a number of medications, either FDA approved or used off label, for treatment of obesity (see Table 2).16 All are indicated for patients with a BMI of ≥ 30 kg/m2 or for those with a BMI between 27-29 kg/m2 with weight-related comorbidities and should be used in combination with diet and lifestyle interventions. None are approved or safe in pregnancy. Mechanisms of action vary by type and include decreased appetite, increased energy expenditure, improved insulin sensitivity, and interfere with absorption.
The newest and most effective anti-obesity medications (AOM), the glucagon-like peptide-1 receptor agonists (GLP-1 RA) are derived from gut hormones secreted in the distal small bowel and colon in response to a meal, which function to delay gastric emptying, increase insulin release from the pancreas, and reduce hepatic gluconeogenesis. Central nervous system effects are not yet entirely understood, but function to decrease appetite and increase satiety. Initially developed for treatment of T2DM, observed weight reduction in patients treated with GLP-1 RA led to clinical trials for treatment of obesity. Semaglutide treatment resulted in weight reduction of 16.9% of total body weight (TBW), and one third of subjects lost ≥ 20% of TBW.17 Tirzepatide combines GLP-1 RA and a gastric inhibitory polypeptide (GIP) receptor agonist, which also has an incretin effect and functions to slow gastric emptying. In the pivotal SURMOUNT trial, approximately 58% of patients achieved ≥20% loss of TBW18 with 15mg weekly dosing of tirzepatide. This class of drugs is a logical choice in patients with T2DM and obesity. Long-term treatment appears necessary, as patients typically regain two-thirds of lost weight within a year after GLP-1 RA are stopped.
Based on tumors observed in rodents, GLP-1 RA are contraindicated in patients with a personal or family history of multiple endocrine neoplasia type 2 (MEN II) or medullary thyroid cancer. These tumors have not been observed in humans treated with GLP-1 RA. They should be used with caution in patients with history of pancreatitis, gastroparesis, or diabetic retinopathy, though a recent systematic review and meta-analysis suggests showed little to no increased risk for biliary events from GLP-1 RA.19 Side effects are most commonly gastrointestinal in nature (nausea, reflux, constipation or diarrhea) and are typically most severe with initiation of the drug and with dose escalation. Side effects can be mitigated by initiating these drugs at lowest doses and gradually titrating up (every four weeks) based on effectiveness and tolerability. Antisecretory, antiemetic, and laxative medications can also be used to help manage GLP-1 RA related side effects.
There is no reason to escalate to highest doses if patients are experiencing weight loss and reduction in food cravings at lower doses. Both semaglutide and tirzepatide are administered subcutaneously every seven days. Once patients have reached goal weight, they can either continue maintenance therapy at that same dose/interval, or if motivated to do so, may gradually reduce the weekly dose in a stepwise approach to determine the minimally effective dose to maintain weight loss. There are not yet published maintenance studies to guide this process. Currently the price of GLP-1 RA and inconsistent insurance coverage make them inaccessible to many patients. The manufacturers of both semaglutide and tirzepatide offer direct to consumer pricing and home delivery.
Bariatric Surgery
In patients with higher BMI (≥35kg/m2) or those with BMI ≥30kg/m2 and obesity-related metabolic disease and the desire to avoid lifelong medications or who fail or are intolerant of AOM, bariatric options should be considered.20 Sleeve gastrectomy has become the most performed surgery for treatment of obesity. It is a restrictive procedure, removing 80% of the stomach, but a drop in circulating levels of ghrelin afterwards also leads to decreased feelings of hunger. It results in weight loss of 25-30% TBW loss. It is not a good choice for patients who suffer from severe GERD, as this typically worsens afterwards; furthermore, de novo Barrett’s has been observed in nearly 6% of patients who undergo sleeve gastrectomy.21
Roux-en-Y gastric bypass is a restrictive and malabsorptive procedure, resulting in 30-35% TBW loss. It has beneficial and immediate metabolic effects, including increased release of endogenous GLP-1, which leads to improvements in weight-related T2DM. The newer single anastomosis duodenal-ileal bypass with sleeve gastrectomy (SADI-S) starts with a sleeve gastrectomy, making a smaller tube-shaped stomach. The duodenum is divided just after the stomach and then a loop of ileum is brought up and connected to the stomach (see Figure 1). This procedure is highly effective, with patients losing 75-95% of excess body weight and is becoming a preferred option for patients with greater BMI (≥50kg/m2). It is also an option for patients who have already had a sleeve gastrectomy and are seeking further weight loss. Because there is only one anastomosis, perioperative complications, such as anastomotic leaks, are reduced. The risk of micronutrient deficiencies is present with all malabsorptive procedures, and these patients must supplement with multivitamins, iron, vitamin D, and calcium.
Endoscopic Therapies
Endoscopic bariatric and metabolic therapies (EBMTs) have been increasingly studied and utilized, and this less invasive option may be more appropriate for or attractive to many patients. Intragastric balloons, which reduce meal volume and delay gastric emptying, can be used short term only (six months) resulting in loss of about 6.9% of total body weight (TBW) greater than lifestyle modification (LM) alone, and may be considered in limited situations, such as need for pre-operative weight loss to reduce risks in very obese individuals.22
Endoscopic gastric remodeling (EGR), also known as endoscopic sleeve gastrectomy (ESG), is a purely restrictive procedure in which the stomach is cinched to resize and reshape using an endoscopic suturing device (see Figure 2).23 It is an option for patients with class 1 or 2 obesity, with data from a randomized controlled trial in this population demonstrating mean percentage of TBW loss of 13.6% at 52 weeks compared to 0.8% in those treated with LM alone.24 A recent meta-analysis of 21 observational studies, including patients with higher BMIs (32.5 to 49.9 kg/m2) showed pooled average weight loss of 17.3% TBW at 12 months with EGR.22 This procedure has potential advantages of fewer complications, quicker recovery, and much less new-onset GERD compared to laparoscopic sleeve gastrectomy. Furthermore, it may be utilized in combination with AOMs to achieve optimum weight loss and metabolic outcomes.25,26 Potential adverse events include abdominal pain, nausea and vomiting (which may be severe), as well as rare instances of intra/extra luminal bleeding or abdominal abscess requiring drainage.22
Recent joint American/European Gastrointestinal Endoscopy guidelines suggest the use of EBMTs plus lifestyle modification in patients with a BMI of ≥ 30 kg/m2, or with a BMI of 27.0-29.9 kg/m2 with at least 1 obesity-related comorbidity.22 Small bowel interventions including duodenal-jejunal bypass liner and duodenal mucosal resurfacing are being investigated for patients with obesity and type 2 diabetes but not yet commercially available.
Conclusion
Given the overlap of obesity with many GI disorders, it is entirely appropriate for gastroenterologists to consider it worthy of aggressive treatment, particularly in patients with MAFLD and other serious weight related comorbidities. With a compassionate and empathetic approach, and a number of highly effective medical, endoscopic, and surgical therapies now available, weight management has the potential to be extremely rewarding when implemented in GI practice.
Dr. Kelly is based in the Department of Medicine, Division of Gastroenterology, Brigham and Women’s Hospital, and Harvard Medical School, both in Boston, Massachusetts. She serves on the clinical advisory board for OpenBiome (unpaid) and has served on an advisory board for Eli Lilly and Company.
References
1. Hales CM, et al. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017-2018. NCHS Data Brief 2020 Feb:(360):1–8.
2. Pais R, et al. NAFLD and liver transplantation: Current burden and expected challenges. J Hepatol. 2016 Dec. doi: 10.1016/j.jhep.2016.07.033.
3. Lauby-Secretan B, et al. Body Fatness and Cancer--Viewpoint of the IARC Working Group. N Engl J Med. 2016 Aug. doi: 10.1056/NEJMsr1606602.
4. Kim A. Dysbiosis: A Review Highlighting Obesity and Inflammatory Bowel Disease. J Clin Gastroenterol. 2015 Nov-Dec. doi: 10.1097/MCG.0000000000000356.
5. Singh S, et al. Obesity in IBD: epidemiology, pathogenesis, disease course and treatment outcomes. Nat Rev Gastroenterol Hepatol. 2017 Feb. doi: 10.1038/nrgastro.2016.181.
6. Sundararaman L, Goudra B. Sedation for GI Endoscopy in the Morbidly Obese: Challenges and Possible Solutions. J Clin Med. 2024 Aug. doi: 10.3390/jcm13164635.
7. Bombassaro B, et al. The hypothalamus as the central regulator of energy balance and its impact on current and future obesity treatments. Arch Endocrinol Metab. 2024 Nov. doi: 10.20945/2359-4292-2024-0082.
8. Beccuti G, Pannain S. Sleep and obesity. Curr Opin Clin Nutr Metab Care. 2011 Jul. doi: 10.1097/MCO.0b013e3283479109.
9. Desalermos A, et al. Effect of Obesogenic Medications on Weight-Loss Outcomes in a Behavioral Weight-Management Program. Obesity (Silver Spring). 2019 May. doi: 10.1002/oby.22444.
10. Lord MN, Noble EE. Hypothalamic cannabinoid signaling: Consequences for eating behavior. Pharmacol Res Perspect. 2024 Oct. doi: 10.1002/prp2.1251.
11. Farhana A, Rehman A. Metabolic Consequences of Weight Reduction. [Updated 2023 Jul 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK572145/.
12. Rubino F, et al. Definition and diagnostic criteria of clinical obesity. Lancet Diabetes Endocrinol. 2025 Mar. doi: 10.1016/S2213-8587(24)00316-4.
13. Cox CE. Role of Physical Activity for Weight Loss and Weight Maintenance. Diabetes Spectr. 2017 Aug. doi: 10.2337/ds17-0013.
14. Chaput JP, et al. Widespread misconceptions about obesity. Can Fam Physician. 2014 Nov. PMID: 25392431.
15. Muscogiuri G, et al. Mediterranean Diet and Obesity-related Disorders: What is the Evidence? Curr Obes Rep. 2022 Dec. doi: 10.1007/s13679-022-00481-1.
16. Gudzune KA, Kushner RF. Medications for Obesity: A Review. JAMA. 2024 Aug. doi: 10.1001/jama.2024.10816.
17. Wilding JPH, et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N Engl J Med. 2021 Feb. doi: 10.1056/NEJMoa2032183.
18. Jastreboff AM, et al. Tirzepatide Once Weekly for the Treatment of Obesity. N Engl J Med. 2022 Jun. doi: 10.1056/NEJMoa2206038.
19. Chiang CH, et al. Glucagon-Like Peptide-1 Receptor Agonists and Gastrointestinal Adverse Events: A Systematic Review and Meta-Analysis. Gastroenterology. 2025 Nov. doi: 10.1053/j.gastro.2025.06.003.
20. Aderinto N, et al. Recent advances in bariatric surgery: a narrative review of weight loss procedures. Ann Med Surg (Lond). 2023 Nov. doi: 10.1097/MS9.0000000000001472.
21. Chandan S, et al. Risk of De Novo Barrett’s Esophagus Post Sleeve Gastrectomy: A Systematic Review and Meta-Analysis of Studies With Long-Term Follow-Up. Clin Gastroenterol Hepatol. 2025 Jan. doi: 10.1016/j.cgh.2024.06.041.
22. Jirapinyo P, et al. American Society for Gastrointestinal Endoscopy-European Society of Gastrointestinal Endoscopy guideline on primary endoscopic bariatric and metabolic therapies for adults with obesity. Gastrointest Endosc. 2024 Jun. doi: 10.1016/j.gie.2023.12.004.
23. Nduma BN, et al. Endoscopic Gastric Sleeve: A Review of Literature. Cureus. 2023 Mar. doi: 10.7759/cureus.36353.
24. Abu Dayyeh BK, et al. Endoscopic sleeve gastroplasty for treatment of class 1 and 2 obesity (MERIT): a prospective, multicentre, randomised trial. Lancet. 2022 Aug. doi: 10.1016/S0140-6736(22)01280-6.
25. Gala K, et al. Outcomes of concomitant antiobesity medication use with endoscopic sleeve gastroplasty in clinical US settings. Obes Pillars. 2024 May. doi: 10.1016/j.obpill.2024.100112.
26. Chung CS, et al. Endoscopic sleeve gastroplasty combined with anti-obesity medication for better control of weight and diabetes. Clin Endosc. 2025 May. doi: 10.5946/ce.2024.274.
An Approach to Exocrine Pancreatic Insufficiency: Considerations in Diagnosis and Treatment
Exocrine pancreatic insufficiency (EPI) is a recognized condition in patients with underlying pancreatic disease. However, it is a disease state that requires a meticulous approach to diagnose, as misdiagnosis can lead to inappropriate testing and unnecessary treatment.
EPI has been defined as “a near total decline in the quantity and/or activity of endogenous pancreatic enzymes to a level that is inadequate to maintain normal digestive capacity leading to steatorrhea.”1 It can lead to complications including malnutrition, micronutrient deficiencies, metabolic bone disease and have significant impact on quality of life. In this article,
EPI Diagnosis
EPI results from ineffective or insufficient pancreatic digestive enzyme secretion. In 2021, a group of experts from the American Gastroenterological Association (AGA) and PancreasFest met and proposed a new mechanistic definition of EPI. This suggests that EPI is the failure of sufficient pancreatic enzymes to effectively reach the intestine in order to allow for optimal digestion of ingested nutrients, leading to downstream macronutrient and micronutrient deficiencies with symptoms of maldigestion including post-prandial abdominal pain, bloating, steatorrhea, loose stools, or weight loss.2
A more pragmatic definition by Khan, et al in 2022 utilized a staging system to distinguish exocrine pancreatic dysfunction (EDP) from EPI. As such EPD occurs when there is a decline in pancreatic function without impaired digestive capacity, while EPI requires digestive capacity impairment leading to objective steatorrhea (coefficient of fat absorption <93 %).3Differential Diagnosis: There are many factors that can impact normal digestion. In approaching EPI, symptoms are often the most common reason to test for disease state in the appropriate clinical context. There can be pancreatic causes of EPI and non-pancreatic (secondary) causes of EPI (see Figure 1), though the latter can be challenging to detect.
The most common parenchymal etiologies for EPI include chronic pancreatitis, recurrent acute pancreatitis, cystic fibrosis, pancreatic cancer or prior pancreatic resections. Non-pancreatic conditions that impact synchronous mixing of endogenous pancreatic enzymes with meals (i.e., Roux-en-Y gastric bypass, short bowel syndrome, delayed gastric emptying), mucosal barriers causing decrease endogenous pancreatic stimulation despite intact parenchyma, such as celiac disease, foregut Crohn’s disease, intraluminal inactivation of pancreatic enzymes (Zollinger-Ellison syndrome), and bile salts de-conjugation with small intestinal bacterial overgrowth (SIBO) can predispose to EPI.4-6 The true prevalence of EPI is difficult to ascertain due to a variety of factors including challenges in diagnosis and misdiagnosis.
Some of the major challenges in the diagnosis and treatment of EPI is that the symptoms of EPI overlap with many other GI conditions including celiac disease, diabetes mellitus, SIBO, irritable bowel syndrome (IBS), bile acid diarrhea, and other functional GI syndromes. These non-pancreatic conditions can also be associated with falsely low FE-1. Hence, ordering FE-1 should be employed with caution when the pretest probability is low. Patients with EPI will generally have a significant response to pancreatic enzyme replacement therapy (PERT) if it is adequately dosed and a lack of response should prompt consideration of an alternative diagnosis. A framework to factors which contribute to EPI is outlined in Figure 2.
Symptoms Screening and Signs: Pancreatic enzymes output estimation is the most reliable indicator for pancreatic digestive capacity. However, EPI diagnosis requires a combination of symptoms screening, stool-based (indirect pancreatic function) testing or direct pancreatic function testing (PFT).
Although symptoms might not correlate with objective disease state, in screening for symptoms of steatorrhea or maldigestion, it is important to ask specific questions regarding bloating, abdominal pain, stool frequency, consistency, and quality. Screening questions should be specific and include question such as, “Is there oil in the toilet bowl or is the stool greasy/shiny?”, “Is the stool sticky and difficult to flush or wipe?”, “Is there malodorous flatus?” If patients screen positive for EPI symptoms and there is a high pre-test probability of EPI such as the presence of severe chronic pancreatitis or significant pancreatic resection (> 90% loss of pancreatic parenchyma), then cautious trial of PERT and assessment for treatment response can be considered without additional stool-based testing. However, this practice end points are unclear and mainly based on subjective response.
Patients with EPI are at increased risk for malnutrition and micronutrient deficiencies. While not required for the diagnosis, low levels of fat-soluble vitamins (vitamin AEDK) or other minerals (zinc, selenium, magnesium, phosphorus) can suggest issues with malabsorption. Once the diagnosis of EPI is made, micronutrient screening should occur annually.
Stool Based Testing: The gold standard clinical test for steatorrhea is measuring coefficient of fat absorption (CFA). With a normal range of 93% fat absorption, the test is performed on a 72-hours fecal fat collection kit. To ensure accurate results, a patient must adhere to a diet with a minimum of 100 grams of fat per day in the three days leading up to the test and during the duration of the test. Patients must also abstain from taking PERT during the duration of the test. This can be incredibly challenging for someone with underlying steatorrhea but can reliably distinguish between EPD and EPI.
A more commonly used stool test is fecal elastase (FE-1). While easier to perform, the test often results in many false positives and false negatives. FE-1 is an ELISA based test, which measures the concentration of the specific isoform CELA3 (chymotrypsin-like elastase family) in the stool sample. The test must be run on a solid stool sample as soft or liquid stool will dilute down elastase concentration falsely. One test advantage is that a patient can continue PERT if needed. FE-1 test measures the concentration of patients’ elastase and PERT is porcine derived. As such, there is no interaction between porcine lipase and human elastase in stool. FE-1 sensitivity and specificity are high for severe disease (<100 mcg/g) if the test is performed properly on patients with a high pretest probability. However, the sensitivity and specificity are poor in mild to moderate pancreatic disease and in the absence of known pancreatic disease.7
Our suggested approach to utilizing FE-1 test is to reserve it for patients with known severe chronic pancreatitis or prior pancreatic surgery in patients with symptoms. In patients without pancreatic disease who are at low risk of EPI, a positive FE-1 can lead to misdiagnosis, further diagnostic testing, and unnecessary treatment. Currently, there is no stool-based test that is accurate, reproducible, and reliable.
Direct Pancreatic Function testing: Secretin stimulated PFT is highly reliable in measuring ductal function with bicarbonate concentration. However, it cannot reliably estimate acinar function as both do not decline at the same rate, unless in severe pancreatic disease. A much more robust test should include cholecystokinin analog to measure pancreatic enzymes concentration. This test involves endoscopy, administration of secretin, and/or a cholecystokinin analog, and subsequent measurement of bicarbonate and digestive enzymes in the pancreatic juice. This test is not routinely offered as it is invasive, cumbersome, and difficult to repeat for reassessment of pancreatic function over time.8
Treatment
The primary goal of treatment is to improve symptoms and nutritional status of the patient. EPI treatment consists of PERT and nutritional counseling. In the United States, there are multiple FDA approved PERT preparations, which include Creon, Zenpep, Pancreaze, Pertzye, Viokase, and Relizorb. While dosing is dependent on lipase concentration, all PERT (aside from Relizorb) preparations have a combination of lipase, proteases, and amylase. All but Viokace and Relizorb are enteric-coated formulations.9
In patients with an inadequate response to enteric coated PERT, non-enteric coated PERT can be added as it may provide a more immediate effect than enteric coated formulations, specially if concern about rapid gut transit with inadequate mixing is raised. If a non-enteric formations is used, acid suppression should be added to prevent inactivation of the PERT. Relizorb is a cartridge system which delivers lipase directly to tube feeds. This cartridge is only utilized in patients receiving enteral nutrition and allows for treatment of EPI even when patients are unable to tolerate oral feeding.
PERT dosing is intended to at least compensate for 10% of the physiologically secreted amount of endogenous lipase after a normal meal (approximately 30,000 IU). Hence, dosing is primarily weight-based. In symptomatic adults, PERT dose of 500-1000 units/kg/meal and half of the amount with snacks is appropriate. Although higher doses of 1500-2000 units/kg/meal may be needed when there is significant steatorrhea, weight loss, or micronutrient deficiencies, PERT doses exceeding 2500 units/kg/meal are not recommended and warrant further investigation.10
Proper counseling is important to ensure compliance with pancreatin preparations. PERT will generally be effective in improving steatorrhea, weight loss, bowel movement frequency, and reversal of nutritional deficiencies, but it does not reliably help symptoms of bloating or abdominal pain. If a patient’s steatorrhea does not respond to PERT, then alternative diagnoses such as SIBO, or diarrhea-predominant IBS should be considered.
PERT must be taken with meals. There are studies that support split dosing as a more effective way of absorbing fat.11 If PERT is ineffective or minimally effective, review of appropriate dosing and timing of PERT to a meal is recommended. Addition of acid suppression may be required to improve treatment efficacy, especially in patients with abnormal intestinal motility or prior pancreatic surgery as PERT is effective at a pH of 4.5. Cost, pill burden, and persistence of certain symptoms may impact adherence to PERT and thus pre-treatment counseling and close follow-up after initiation is important. This aids in assessment of patients’ response to therapy, ensure appropriate PERT administration, and identifying any barriers to therapy adherence.
Nutritional management of EPI consists of an assessment of nutritional status, diet, and lifestyle. An important component of nutritional management is the assessment of micronutrient deficiencies. Patients with a confirmed diagnosis of EPI should be screened for the following micronutrients annually: Vitamins (A, E, D, K, B12), folate, zinc, selenium, magnesium, and iron. Patients with chronic pancreatitis and EPI should also be screened for metabolic bone disease once every two years and for diabetes mellitus annually.4, 12
Conclusion
EPI is a challenging diagnosis as many symptoms overlap with other GI conditions. Pancreas exocrine function is rich with significant reserve to allow for proper digestive capacity, yet EPI occurs when an individual’s pancreatic digestive enzymes are insufficient to meet their nutritional needs. In patients with high likelihood of having EPI, such as those with pre-existing pancreatic disease, diagnosing EPI combines clinical evidence based on subjective symptoms and stool-based testing to support a disease state.
Appropriate dosing and timing of PERT is critical to improve nutritional outcomes and improve certain symptoms of EPI. Failure of PERT requires evaluating for proper dosing/timing, and consideration of additional or alternative diagnosis. EPI morbidity can lead to significant impact on patients’ quality of life, but with counseling, proper PERT use, nutritional consequences can be mediated, and quality of life can improve.
Dr. Hernandez-Barco is based in the Division of Gastroenterology, Massachusetts General Hospital, Boston, Massachusetts. Dr. Ashkar is based in the Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota. Dr. Hernandez-Barco disclosed consulting for AMGEN and served as a scientific advisor for Nestle Health Science. She had project-related funding support or conflicts of interest to disclose. Dr. Ashkar disclosed consulting for AMGEN. He had no project-related funding support or conflicts of interest to disclose.
References
1. Othman MO, et al. Introduction and practical approach to exocrine pancreatic insufficiency for the practicing clinician. Int J Clin Pract. 2018 Feb. doi: 10.1111/ijcp.13066.
2. Whitcomb DC, et al. AGA-PancreasFest Joint Symposium on Exocrine Pancreatitic Insufficiency. Gastro Hep Adv. 2022 Nov. doi: 10.1016/j.gastha.2022.11.008.
3. Khan A, et al. Staging Exocrine Pancreatic Dysfunction. Pancreatology. 2022 Jan. doi: 10.1016/j.pan.2021.11.005.
4. Whitcomb DC, et al. AGA Clinical Practice Update on the Epidemiology, Evaluation, and Management of Exocrine Pancreatitis insufficiency: Expert Review. Gastroenterology. 2023 Nov. doi: 10.1053/j.gastro.2023.07.007.
5. Kunovský L, et al. Causes of Exocrine Pancreatic Insufficiency Other than Chronic Pancreatitis. J Clin Med. 2021 Dec. doi: 10.3390/jcm10245779.
6. Singh VK, et al. Less common etiologies of exocrine pancreatic insufficiency. World J Gastroenterol. 2017 Oct. doi: 10.3748/wjg.v23.i39.7059.
7. Lankisch PG, et al. Faecal elastase 1: not helpful in diagnosing chronic pancreatitis associated with mid to moderate exocrine pancreatic insufficiency. Gut. 1998 Apr. doi: 10.1136/gut.42.4.551.
8. Gardner TB, et al. ACG Clinical Guideline: Chronic Pancreatitis. Am J Gastroenterol. 2020 Mar. doi: 10.14309/ajg.0000000000000535.
9. Lewis DM, et al. Exocrine Pancreatic Insufficiency Dosing Guidelines for Pancreatic Enzyme Replacement Therapy Vary Widely Across Disease Types. Dig Dis Sci. 2024 Feb. doi: 10.1007/s10620-023-08184-w.
10. Borowitz DS, et al. Use of pancreatic enzyme supplements for patients with cystic fibrosis in the context of fibrosing colonopathy. Consensus Committee. J Pediatr. 1995 Nov. doi: 10.1016/s0022-3476(95)70153-2.
11. Domínguez-Muñoz JE, et al. Effect of the Administration Schedule on the therapeutic efficacy of oral pancreatic enzyme supplements in patients with exocrine pancreatic insufficiency: a randomized, three-way crossover study. Aliment Pharmacol Ther. 2005 Apr. doi: 10.1111/j.1365-2036.2005.02390.x.
12. Hart PA and Conwell DL. Chronic Pancreatitis: Managing a Difficult Disease. Am J Gastroenterol. 2020 Jan. doi: 10.14309/ajg.0000000000000421.
Exocrine pancreatic insufficiency (EPI) is a recognized condition in patients with underlying pancreatic disease. However, it is a disease state that requires a meticulous approach to diagnose, as misdiagnosis can lead to inappropriate testing and unnecessary treatment.
EPI has been defined as “a near total decline in the quantity and/or activity of endogenous pancreatic enzymes to a level that is inadequate to maintain normal digestive capacity leading to steatorrhea.”1 It can lead to complications including malnutrition, micronutrient deficiencies, metabolic bone disease and have significant impact on quality of life. In this article,
EPI Diagnosis
EPI results from ineffective or insufficient pancreatic digestive enzyme secretion. In 2021, a group of experts from the American Gastroenterological Association (AGA) and PancreasFest met and proposed a new mechanistic definition of EPI. This suggests that EPI is the failure of sufficient pancreatic enzymes to effectively reach the intestine in order to allow for optimal digestion of ingested nutrients, leading to downstream macronutrient and micronutrient deficiencies with symptoms of maldigestion including post-prandial abdominal pain, bloating, steatorrhea, loose stools, or weight loss.2
A more pragmatic definition by Khan, et al in 2022 utilized a staging system to distinguish exocrine pancreatic dysfunction (EDP) from EPI. As such EPD occurs when there is a decline in pancreatic function without impaired digestive capacity, while EPI requires digestive capacity impairment leading to objective steatorrhea (coefficient of fat absorption <93 %).3Differential Diagnosis: There are many factors that can impact normal digestion. In approaching EPI, symptoms are often the most common reason to test for disease state in the appropriate clinical context. There can be pancreatic causes of EPI and non-pancreatic (secondary) causes of EPI (see Figure 1), though the latter can be challenging to detect.
The most common parenchymal etiologies for EPI include chronic pancreatitis, recurrent acute pancreatitis, cystic fibrosis, pancreatic cancer or prior pancreatic resections. Non-pancreatic conditions that impact synchronous mixing of endogenous pancreatic enzymes with meals (i.e., Roux-en-Y gastric bypass, short bowel syndrome, delayed gastric emptying), mucosal barriers causing decrease endogenous pancreatic stimulation despite intact parenchyma, such as celiac disease, foregut Crohn’s disease, intraluminal inactivation of pancreatic enzymes (Zollinger-Ellison syndrome), and bile salts de-conjugation with small intestinal bacterial overgrowth (SIBO) can predispose to EPI.4-6 The true prevalence of EPI is difficult to ascertain due to a variety of factors including challenges in diagnosis and misdiagnosis.
Some of the major challenges in the diagnosis and treatment of EPI is that the symptoms of EPI overlap with many other GI conditions including celiac disease, diabetes mellitus, SIBO, irritable bowel syndrome (IBS), bile acid diarrhea, and other functional GI syndromes. These non-pancreatic conditions can also be associated with falsely low FE-1. Hence, ordering FE-1 should be employed with caution when the pretest probability is low. Patients with EPI will generally have a significant response to pancreatic enzyme replacement therapy (PERT) if it is adequately dosed and a lack of response should prompt consideration of an alternative diagnosis. A framework to factors which contribute to EPI is outlined in Figure 2.
Symptoms Screening and Signs: Pancreatic enzymes output estimation is the most reliable indicator for pancreatic digestive capacity. However, EPI diagnosis requires a combination of symptoms screening, stool-based (indirect pancreatic function) testing or direct pancreatic function testing (PFT).
Although symptoms might not correlate with objective disease state, in screening for symptoms of steatorrhea or maldigestion, it is important to ask specific questions regarding bloating, abdominal pain, stool frequency, consistency, and quality. Screening questions should be specific and include question such as, “Is there oil in the toilet bowl or is the stool greasy/shiny?”, “Is the stool sticky and difficult to flush or wipe?”, “Is there malodorous flatus?” If patients screen positive for EPI symptoms and there is a high pre-test probability of EPI such as the presence of severe chronic pancreatitis or significant pancreatic resection (> 90% loss of pancreatic parenchyma), then cautious trial of PERT and assessment for treatment response can be considered without additional stool-based testing. However, this practice end points are unclear and mainly based on subjective response.
Patients with EPI are at increased risk for malnutrition and micronutrient deficiencies. While not required for the diagnosis, low levels of fat-soluble vitamins (vitamin AEDK) or other minerals (zinc, selenium, magnesium, phosphorus) can suggest issues with malabsorption. Once the diagnosis of EPI is made, micronutrient screening should occur annually.
Stool Based Testing: The gold standard clinical test for steatorrhea is measuring coefficient of fat absorption (CFA). With a normal range of 93% fat absorption, the test is performed on a 72-hours fecal fat collection kit. To ensure accurate results, a patient must adhere to a diet with a minimum of 100 grams of fat per day in the three days leading up to the test and during the duration of the test. Patients must also abstain from taking PERT during the duration of the test. This can be incredibly challenging for someone with underlying steatorrhea but can reliably distinguish between EPD and EPI.
A more commonly used stool test is fecal elastase (FE-1). While easier to perform, the test often results in many false positives and false negatives. FE-1 is an ELISA based test, which measures the concentration of the specific isoform CELA3 (chymotrypsin-like elastase family) in the stool sample. The test must be run on a solid stool sample as soft or liquid stool will dilute down elastase concentration falsely. One test advantage is that a patient can continue PERT if needed. FE-1 test measures the concentration of patients’ elastase and PERT is porcine derived. As such, there is no interaction between porcine lipase and human elastase in stool. FE-1 sensitivity and specificity are high for severe disease (<100 mcg/g) if the test is performed properly on patients with a high pretest probability. However, the sensitivity and specificity are poor in mild to moderate pancreatic disease and in the absence of known pancreatic disease.7
Our suggested approach to utilizing FE-1 test is to reserve it for patients with known severe chronic pancreatitis or prior pancreatic surgery in patients with symptoms. In patients without pancreatic disease who are at low risk of EPI, a positive FE-1 can lead to misdiagnosis, further diagnostic testing, and unnecessary treatment. Currently, there is no stool-based test that is accurate, reproducible, and reliable.
Direct Pancreatic Function testing: Secretin stimulated PFT is highly reliable in measuring ductal function with bicarbonate concentration. However, it cannot reliably estimate acinar function as both do not decline at the same rate, unless in severe pancreatic disease. A much more robust test should include cholecystokinin analog to measure pancreatic enzymes concentration. This test involves endoscopy, administration of secretin, and/or a cholecystokinin analog, and subsequent measurement of bicarbonate and digestive enzymes in the pancreatic juice. This test is not routinely offered as it is invasive, cumbersome, and difficult to repeat for reassessment of pancreatic function over time.8
Treatment
The primary goal of treatment is to improve symptoms and nutritional status of the patient. EPI treatment consists of PERT and nutritional counseling. In the United States, there are multiple FDA approved PERT preparations, which include Creon, Zenpep, Pancreaze, Pertzye, Viokase, and Relizorb. While dosing is dependent on lipase concentration, all PERT (aside from Relizorb) preparations have a combination of lipase, proteases, and amylase. All but Viokace and Relizorb are enteric-coated formulations.9
In patients with an inadequate response to enteric coated PERT, non-enteric coated PERT can be added as it may provide a more immediate effect than enteric coated formulations, specially if concern about rapid gut transit with inadequate mixing is raised. If a non-enteric formations is used, acid suppression should be added to prevent inactivation of the PERT. Relizorb is a cartridge system which delivers lipase directly to tube feeds. This cartridge is only utilized in patients receiving enteral nutrition and allows for treatment of EPI even when patients are unable to tolerate oral feeding.
PERT dosing is intended to at least compensate for 10% of the physiologically secreted amount of endogenous lipase after a normal meal (approximately 30,000 IU). Hence, dosing is primarily weight-based. In symptomatic adults, PERT dose of 500-1000 units/kg/meal and half of the amount with snacks is appropriate. Although higher doses of 1500-2000 units/kg/meal may be needed when there is significant steatorrhea, weight loss, or micronutrient deficiencies, PERT doses exceeding 2500 units/kg/meal are not recommended and warrant further investigation.10
Proper counseling is important to ensure compliance with pancreatin preparations. PERT will generally be effective in improving steatorrhea, weight loss, bowel movement frequency, and reversal of nutritional deficiencies, but it does not reliably help symptoms of bloating or abdominal pain. If a patient’s steatorrhea does not respond to PERT, then alternative diagnoses such as SIBO, or diarrhea-predominant IBS should be considered.
PERT must be taken with meals. There are studies that support split dosing as a more effective way of absorbing fat.11 If PERT is ineffective or minimally effective, review of appropriate dosing and timing of PERT to a meal is recommended. Addition of acid suppression may be required to improve treatment efficacy, especially in patients with abnormal intestinal motility or prior pancreatic surgery as PERT is effective at a pH of 4.5. Cost, pill burden, and persistence of certain symptoms may impact adherence to PERT and thus pre-treatment counseling and close follow-up after initiation is important. This aids in assessment of patients’ response to therapy, ensure appropriate PERT administration, and identifying any barriers to therapy adherence.
Nutritional management of EPI consists of an assessment of nutritional status, diet, and lifestyle. An important component of nutritional management is the assessment of micronutrient deficiencies. Patients with a confirmed diagnosis of EPI should be screened for the following micronutrients annually: Vitamins (A, E, D, K, B12), folate, zinc, selenium, magnesium, and iron. Patients with chronic pancreatitis and EPI should also be screened for metabolic bone disease once every two years and for diabetes mellitus annually.4, 12
Conclusion
EPI is a challenging diagnosis as many symptoms overlap with other GI conditions. Pancreas exocrine function is rich with significant reserve to allow for proper digestive capacity, yet EPI occurs when an individual’s pancreatic digestive enzymes are insufficient to meet their nutritional needs. In patients with high likelihood of having EPI, such as those with pre-existing pancreatic disease, diagnosing EPI combines clinical evidence based on subjective symptoms and stool-based testing to support a disease state.
Appropriate dosing and timing of PERT is critical to improve nutritional outcomes and improve certain symptoms of EPI. Failure of PERT requires evaluating for proper dosing/timing, and consideration of additional or alternative diagnosis. EPI morbidity can lead to significant impact on patients’ quality of life, but with counseling, proper PERT use, nutritional consequences can be mediated, and quality of life can improve.
Dr. Hernandez-Barco is based in the Division of Gastroenterology, Massachusetts General Hospital, Boston, Massachusetts. Dr. Ashkar is based in the Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota. Dr. Hernandez-Barco disclosed consulting for AMGEN and served as a scientific advisor for Nestle Health Science. She had project-related funding support or conflicts of interest to disclose. Dr. Ashkar disclosed consulting for AMGEN. He had no project-related funding support or conflicts of interest to disclose.
References
1. Othman MO, et al. Introduction and practical approach to exocrine pancreatic insufficiency for the practicing clinician. Int J Clin Pract. 2018 Feb. doi: 10.1111/ijcp.13066.
2. Whitcomb DC, et al. AGA-PancreasFest Joint Symposium on Exocrine Pancreatitic Insufficiency. Gastro Hep Adv. 2022 Nov. doi: 10.1016/j.gastha.2022.11.008.
3. Khan A, et al. Staging Exocrine Pancreatic Dysfunction. Pancreatology. 2022 Jan. doi: 10.1016/j.pan.2021.11.005.
4. Whitcomb DC, et al. AGA Clinical Practice Update on the Epidemiology, Evaluation, and Management of Exocrine Pancreatitis insufficiency: Expert Review. Gastroenterology. 2023 Nov. doi: 10.1053/j.gastro.2023.07.007.
5. Kunovský L, et al. Causes of Exocrine Pancreatic Insufficiency Other than Chronic Pancreatitis. J Clin Med. 2021 Dec. doi: 10.3390/jcm10245779.
6. Singh VK, et al. Less common etiologies of exocrine pancreatic insufficiency. World J Gastroenterol. 2017 Oct. doi: 10.3748/wjg.v23.i39.7059.
7. Lankisch PG, et al. Faecal elastase 1: not helpful in diagnosing chronic pancreatitis associated with mid to moderate exocrine pancreatic insufficiency. Gut. 1998 Apr. doi: 10.1136/gut.42.4.551.
8. Gardner TB, et al. ACG Clinical Guideline: Chronic Pancreatitis. Am J Gastroenterol. 2020 Mar. doi: 10.14309/ajg.0000000000000535.
9. Lewis DM, et al. Exocrine Pancreatic Insufficiency Dosing Guidelines for Pancreatic Enzyme Replacement Therapy Vary Widely Across Disease Types. Dig Dis Sci. 2024 Feb. doi: 10.1007/s10620-023-08184-w.
10. Borowitz DS, et al. Use of pancreatic enzyme supplements for patients with cystic fibrosis in the context of fibrosing colonopathy. Consensus Committee. J Pediatr. 1995 Nov. doi: 10.1016/s0022-3476(95)70153-2.
11. Domínguez-Muñoz JE, et al. Effect of the Administration Schedule on the therapeutic efficacy of oral pancreatic enzyme supplements in patients with exocrine pancreatic insufficiency: a randomized, three-way crossover study. Aliment Pharmacol Ther. 2005 Apr. doi: 10.1111/j.1365-2036.2005.02390.x.
12. Hart PA and Conwell DL. Chronic Pancreatitis: Managing a Difficult Disease. Am J Gastroenterol. 2020 Jan. doi: 10.14309/ajg.0000000000000421.
Exocrine pancreatic insufficiency (EPI) is a recognized condition in patients with underlying pancreatic disease. However, it is a disease state that requires a meticulous approach to diagnose, as misdiagnosis can lead to inappropriate testing and unnecessary treatment.
EPI has been defined as “a near total decline in the quantity and/or activity of endogenous pancreatic enzymes to a level that is inadequate to maintain normal digestive capacity leading to steatorrhea.”1 It can lead to complications including malnutrition, micronutrient deficiencies, metabolic bone disease and have significant impact on quality of life. In this article,
EPI Diagnosis
EPI results from ineffective or insufficient pancreatic digestive enzyme secretion. In 2021, a group of experts from the American Gastroenterological Association (AGA) and PancreasFest met and proposed a new mechanistic definition of EPI. This suggests that EPI is the failure of sufficient pancreatic enzymes to effectively reach the intestine in order to allow for optimal digestion of ingested nutrients, leading to downstream macronutrient and micronutrient deficiencies with symptoms of maldigestion including post-prandial abdominal pain, bloating, steatorrhea, loose stools, or weight loss.2
A more pragmatic definition by Khan, et al in 2022 utilized a staging system to distinguish exocrine pancreatic dysfunction (EDP) from EPI. As such EPD occurs when there is a decline in pancreatic function without impaired digestive capacity, while EPI requires digestive capacity impairment leading to objective steatorrhea (coefficient of fat absorption <93 %).3Differential Diagnosis: There are many factors that can impact normal digestion. In approaching EPI, symptoms are often the most common reason to test for disease state in the appropriate clinical context. There can be pancreatic causes of EPI and non-pancreatic (secondary) causes of EPI (see Figure 1), though the latter can be challenging to detect.
The most common parenchymal etiologies for EPI include chronic pancreatitis, recurrent acute pancreatitis, cystic fibrosis, pancreatic cancer or prior pancreatic resections. Non-pancreatic conditions that impact synchronous mixing of endogenous pancreatic enzymes with meals (i.e., Roux-en-Y gastric bypass, short bowel syndrome, delayed gastric emptying), mucosal barriers causing decrease endogenous pancreatic stimulation despite intact parenchyma, such as celiac disease, foregut Crohn’s disease, intraluminal inactivation of pancreatic enzymes (Zollinger-Ellison syndrome), and bile salts de-conjugation with small intestinal bacterial overgrowth (SIBO) can predispose to EPI.4-6 The true prevalence of EPI is difficult to ascertain due to a variety of factors including challenges in diagnosis and misdiagnosis.
Some of the major challenges in the diagnosis and treatment of EPI is that the symptoms of EPI overlap with many other GI conditions including celiac disease, diabetes mellitus, SIBO, irritable bowel syndrome (IBS), bile acid diarrhea, and other functional GI syndromes. These non-pancreatic conditions can also be associated with falsely low FE-1. Hence, ordering FE-1 should be employed with caution when the pretest probability is low. Patients with EPI will generally have a significant response to pancreatic enzyme replacement therapy (PERT) if it is adequately dosed and a lack of response should prompt consideration of an alternative diagnosis. A framework to factors which contribute to EPI is outlined in Figure 2.
Symptoms Screening and Signs: Pancreatic enzymes output estimation is the most reliable indicator for pancreatic digestive capacity. However, EPI diagnosis requires a combination of symptoms screening, stool-based (indirect pancreatic function) testing or direct pancreatic function testing (PFT).
Although symptoms might not correlate with objective disease state, in screening for symptoms of steatorrhea or maldigestion, it is important to ask specific questions regarding bloating, abdominal pain, stool frequency, consistency, and quality. Screening questions should be specific and include question such as, “Is there oil in the toilet bowl or is the stool greasy/shiny?”, “Is the stool sticky and difficult to flush or wipe?”, “Is there malodorous flatus?” If patients screen positive for EPI symptoms and there is a high pre-test probability of EPI such as the presence of severe chronic pancreatitis or significant pancreatic resection (> 90% loss of pancreatic parenchyma), then cautious trial of PERT and assessment for treatment response can be considered without additional stool-based testing. However, this practice end points are unclear and mainly based on subjective response.
Patients with EPI are at increased risk for malnutrition and micronutrient deficiencies. While not required for the diagnosis, low levels of fat-soluble vitamins (vitamin AEDK) or other minerals (zinc, selenium, magnesium, phosphorus) can suggest issues with malabsorption. Once the diagnosis of EPI is made, micronutrient screening should occur annually.
Stool Based Testing: The gold standard clinical test for steatorrhea is measuring coefficient of fat absorption (CFA). With a normal range of 93% fat absorption, the test is performed on a 72-hours fecal fat collection kit. To ensure accurate results, a patient must adhere to a diet with a minimum of 100 grams of fat per day in the three days leading up to the test and during the duration of the test. Patients must also abstain from taking PERT during the duration of the test. This can be incredibly challenging for someone with underlying steatorrhea but can reliably distinguish between EPD and EPI.
A more commonly used stool test is fecal elastase (FE-1). While easier to perform, the test often results in many false positives and false negatives. FE-1 is an ELISA based test, which measures the concentration of the specific isoform CELA3 (chymotrypsin-like elastase family) in the stool sample. The test must be run on a solid stool sample as soft or liquid stool will dilute down elastase concentration falsely. One test advantage is that a patient can continue PERT if needed. FE-1 test measures the concentration of patients’ elastase and PERT is porcine derived. As such, there is no interaction between porcine lipase and human elastase in stool. FE-1 sensitivity and specificity are high for severe disease (<100 mcg/g) if the test is performed properly on patients with a high pretest probability. However, the sensitivity and specificity are poor in mild to moderate pancreatic disease and in the absence of known pancreatic disease.7
Our suggested approach to utilizing FE-1 test is to reserve it for patients with known severe chronic pancreatitis or prior pancreatic surgery in patients with symptoms. In patients without pancreatic disease who are at low risk of EPI, a positive FE-1 can lead to misdiagnosis, further diagnostic testing, and unnecessary treatment. Currently, there is no stool-based test that is accurate, reproducible, and reliable.
Direct Pancreatic Function testing: Secretin stimulated PFT is highly reliable in measuring ductal function with bicarbonate concentration. However, it cannot reliably estimate acinar function as both do not decline at the same rate, unless in severe pancreatic disease. A much more robust test should include cholecystokinin analog to measure pancreatic enzymes concentration. This test involves endoscopy, administration of secretin, and/or a cholecystokinin analog, and subsequent measurement of bicarbonate and digestive enzymes in the pancreatic juice. This test is not routinely offered as it is invasive, cumbersome, and difficult to repeat for reassessment of pancreatic function over time.8
Treatment
The primary goal of treatment is to improve symptoms and nutritional status of the patient. EPI treatment consists of PERT and nutritional counseling. In the United States, there are multiple FDA approved PERT preparations, which include Creon, Zenpep, Pancreaze, Pertzye, Viokase, and Relizorb. While dosing is dependent on lipase concentration, all PERT (aside from Relizorb) preparations have a combination of lipase, proteases, and amylase. All but Viokace and Relizorb are enteric-coated formulations.9
In patients with an inadequate response to enteric coated PERT, non-enteric coated PERT can be added as it may provide a more immediate effect than enteric coated formulations, specially if concern about rapid gut transit with inadequate mixing is raised. If a non-enteric formations is used, acid suppression should be added to prevent inactivation of the PERT. Relizorb is a cartridge system which delivers lipase directly to tube feeds. This cartridge is only utilized in patients receiving enteral nutrition and allows for treatment of EPI even when patients are unable to tolerate oral feeding.
PERT dosing is intended to at least compensate for 10% of the physiologically secreted amount of endogenous lipase after a normal meal (approximately 30,000 IU). Hence, dosing is primarily weight-based. In symptomatic adults, PERT dose of 500-1000 units/kg/meal and half of the amount with snacks is appropriate. Although higher doses of 1500-2000 units/kg/meal may be needed when there is significant steatorrhea, weight loss, or micronutrient deficiencies, PERT doses exceeding 2500 units/kg/meal are not recommended and warrant further investigation.10
Proper counseling is important to ensure compliance with pancreatin preparations. PERT will generally be effective in improving steatorrhea, weight loss, bowel movement frequency, and reversal of nutritional deficiencies, but it does not reliably help symptoms of bloating or abdominal pain. If a patient’s steatorrhea does not respond to PERT, then alternative diagnoses such as SIBO, or diarrhea-predominant IBS should be considered.
PERT must be taken with meals. There are studies that support split dosing as a more effective way of absorbing fat.11 If PERT is ineffective or minimally effective, review of appropriate dosing and timing of PERT to a meal is recommended. Addition of acid suppression may be required to improve treatment efficacy, especially in patients with abnormal intestinal motility or prior pancreatic surgery as PERT is effective at a pH of 4.5. Cost, pill burden, and persistence of certain symptoms may impact adherence to PERT and thus pre-treatment counseling and close follow-up after initiation is important. This aids in assessment of patients’ response to therapy, ensure appropriate PERT administration, and identifying any barriers to therapy adherence.
Nutritional management of EPI consists of an assessment of nutritional status, diet, and lifestyle. An important component of nutritional management is the assessment of micronutrient deficiencies. Patients with a confirmed diagnosis of EPI should be screened for the following micronutrients annually: Vitamins (A, E, D, K, B12), folate, zinc, selenium, magnesium, and iron. Patients with chronic pancreatitis and EPI should also be screened for metabolic bone disease once every two years and for diabetes mellitus annually.4, 12
Conclusion
EPI is a challenging diagnosis as many symptoms overlap with other GI conditions. Pancreas exocrine function is rich with significant reserve to allow for proper digestive capacity, yet EPI occurs when an individual’s pancreatic digestive enzymes are insufficient to meet their nutritional needs. In patients with high likelihood of having EPI, such as those with pre-existing pancreatic disease, diagnosing EPI combines clinical evidence based on subjective symptoms and stool-based testing to support a disease state.
Appropriate dosing and timing of PERT is critical to improve nutritional outcomes and improve certain symptoms of EPI. Failure of PERT requires evaluating for proper dosing/timing, and consideration of additional or alternative diagnosis. EPI morbidity can lead to significant impact on patients’ quality of life, but with counseling, proper PERT use, nutritional consequences can be mediated, and quality of life can improve.
Dr. Hernandez-Barco is based in the Division of Gastroenterology, Massachusetts General Hospital, Boston, Massachusetts. Dr. Ashkar is based in the Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota. Dr. Hernandez-Barco disclosed consulting for AMGEN and served as a scientific advisor for Nestle Health Science. She had project-related funding support or conflicts of interest to disclose. Dr. Ashkar disclosed consulting for AMGEN. He had no project-related funding support or conflicts of interest to disclose.
References
1. Othman MO, et al. Introduction and practical approach to exocrine pancreatic insufficiency for the practicing clinician. Int J Clin Pract. 2018 Feb. doi: 10.1111/ijcp.13066.
2. Whitcomb DC, et al. AGA-PancreasFest Joint Symposium on Exocrine Pancreatitic Insufficiency. Gastro Hep Adv. 2022 Nov. doi: 10.1016/j.gastha.2022.11.008.
3. Khan A, et al. Staging Exocrine Pancreatic Dysfunction. Pancreatology. 2022 Jan. doi: 10.1016/j.pan.2021.11.005.
4. Whitcomb DC, et al. AGA Clinical Practice Update on the Epidemiology, Evaluation, and Management of Exocrine Pancreatitis insufficiency: Expert Review. Gastroenterology. 2023 Nov. doi: 10.1053/j.gastro.2023.07.007.
5. Kunovský L, et al. Causes of Exocrine Pancreatic Insufficiency Other than Chronic Pancreatitis. J Clin Med. 2021 Dec. doi: 10.3390/jcm10245779.
6. Singh VK, et al. Less common etiologies of exocrine pancreatic insufficiency. World J Gastroenterol. 2017 Oct. doi: 10.3748/wjg.v23.i39.7059.
7. Lankisch PG, et al. Faecal elastase 1: not helpful in diagnosing chronic pancreatitis associated with mid to moderate exocrine pancreatic insufficiency. Gut. 1998 Apr. doi: 10.1136/gut.42.4.551.
8. Gardner TB, et al. ACG Clinical Guideline: Chronic Pancreatitis. Am J Gastroenterol. 2020 Mar. doi: 10.14309/ajg.0000000000000535.
9. Lewis DM, et al. Exocrine Pancreatic Insufficiency Dosing Guidelines for Pancreatic Enzyme Replacement Therapy Vary Widely Across Disease Types. Dig Dis Sci. 2024 Feb. doi: 10.1007/s10620-023-08184-w.
10. Borowitz DS, et al. Use of pancreatic enzyme supplements for patients with cystic fibrosis in the context of fibrosing colonopathy. Consensus Committee. J Pediatr. 1995 Nov. doi: 10.1016/s0022-3476(95)70153-2.
11. Domínguez-Muñoz JE, et al. Effect of the Administration Schedule on the therapeutic efficacy of oral pancreatic enzyme supplements in patients with exocrine pancreatic insufficiency: a randomized, three-way crossover study. Aliment Pharmacol Ther. 2005 Apr. doi: 10.1111/j.1365-2036.2005.02390.x.
12. Hart PA and Conwell DL. Chronic Pancreatitis: Managing a Difficult Disease. Am J Gastroenterol. 2020 Jan. doi: 10.14309/ajg.0000000000000421.
A Practical Approach to Diagnosis and Management of Eosinophilic Esophagitis
Eosinophilic esophagitis (EoE) can be considered a “young” disease, with initial case series reported only about 30 years ago. Since that time, it has become a commonly encountered condition in both emergency and clinic settings. The most recent prevalence study estimates that 1 in 700 people in the U.S. have EoE,1 the volume of EoE-associated ED visits tripped between 2009 and 2019 and is projected to double again by 2030,2 and “new” gastroenterologists undoubtedly have learned about and seen this condition. As a chronic disease, EoE necessitates longitudinal follow-up and optimization of care to prevent complications. With increasing diagnostic delay, EoE progresses in most, but not all, patients from an inflammatory- to fibrostenotic-predominant condition.3
Diagnosis of EoE
The most likely area that you will encounter EoE is during an emergent middle-of-the-night endoscopy for food impaction. If called in for this, EoE will be the cause in more than 50% of patients.4 However, the diagnosis can only be made if esophageal biopsies are obtained at the time of the procedure. This is a critical time to decrease diagnostic delay, as half of patients are lost to follow-up after a food impaction.5 Unfortunately, although taking biopsies during index food impaction is guideline-recommended, a quality metric, and safe to obtain after the food bolus is cleared, this is infrequently done in practice.6, 7
The next most likely area for EoE detection is in the endoscopy suite where 15-23% of patients with dysphagia and 5-7% of patients undergoing upper endoscopy for any indication will have EoE.4 Sometimes EoE will be detected “incidentally” during an open-access case (for example, in a patient with diarrhea undergoing evaluation for celiac). In these cases, it is important to perform a careful history (as noted below) as subtle EoE symptoms can frequently be identified. Finally, when patients are seen in clinic for solid food dysphagia, EoE is clearly on the differential. A few percent of patients with refractory heartburn or chest pain will have EoE causing the symptoms rather than reflux,4 and all patients under consideration for antireflux surgery should have an endoscopy to assess for EoE.
When talking to patients with known or suspected EoE, the history must go beyond general questions about dysphagia or trouble swallowing. Many patients with EoE have overtly or subconsciously modified their eating behaviors over many years to minimize symptoms, may have adapted to chronic dysphagia, and will answer “no” when asked if they have trouble swallowing. Instead, use the acronym “IMPACT” to delve deeper into possible symptoms.8 Do they “Imbibe” fluids or liquids between each bite to help get food down? Do they “Modify” the way they eat (cut food into small bites; puree foods)? Do they “Prolong” mealtimes? Do they “Avoid” certain foods that stick? Do they “Chew’ until their food is a mush to get it down? And do they “Turn away” tablets or pills? Pill dysphagia is often a subtle symptom, and sometimes the only symptom elicited.
Additionally, it may be important to ask a partner or family member (if present) about their observations. They may provide insight (e.g. “yes – he chokes with every bite but never says it bothers him”) that the patient might not otherwise provide. The suspicion for EoE should also be increased in patients with concomitant atopic diseases and in those with a family history of dysphagia or who have family members needing esophageal dilation. It is important to remember that EoE can be seen across all ages, sexes, and races/ethnicities.
Diagnosis of EoE is based on the AGREE consensus,9 which is also echoed in the recently updated American College of Gastroenterology (ACG) guidelines.10 Diagnosis requires three steps. First, symptoms of esophageal dysfunction must be present. This will most typically be dysphagia in adolescents and adults, but symptoms are non-specific in children (e.g. poor growth and feeding, abdominal pain, vomiting, regurgitation, heartburn).
Second, at least 15 eosinophils per high-power field (eos/hpf) are required on esophageal biopsy, which implies that an endoscopy be performed. A high-quality endoscopic exam in EoE is of the utmost importance. The approach has been described elsewhere,11 but enough time on insertion should be taken to fully insufflate and examine the esophagus, including the areas of the gastroesophageal junction and upper esophageal sphincter where strictures can be missed, to gently wash debris, and to assess the endoscopic findings of EoE. Endoscopic findings should be reported using the validated EoE Endoscopy Reference Score (EREFS),12 which grades five key features. EREFS is reproducible, is responsive to treatment, and is guideline-recommended (see Figure 1).6, 10 The features are edema (present=1), rings (mild=1; moderate=2; severe=3), exudates (mild=1; severe=2), furrows (mild=1; severe=2), and stricture (present=1; also estimate diameter in mm) and are incorporated into many endoscopic reporting programs. Additionally, diffuse luminal narrowing and mucosal fragility (“crepe-paper” mucosa) should be assessed.
After this, biopsies should be obtained with at least 6 biopsy fragments from different locations in the esophagus. Any visible endoscopic abnormalities should be targeted (the highest yield is in exudates and furrows). The rationale is that EoE is patchy and at least 6 biopsies will maximize diagnostic yield.10 Ideally the initial endoscopy for EoE should be done off of treatments (like PPI or diet restriction) as these could mask the diagnosis. If a patient with suspected EoE has an endoscopy while on PPI, and the endoscopy is normal, a diagnosis of EoE cannot be made. In this case, consideration should be given as to stopping the PPI, allowing a wash out period (at least 1-2 months), and then repeating the endoscopy to confirm the diagnosis. This is important as EoE is a chronic condition necessitating life-long treatment and monitoring, so a definitive diagnosis is critical.
The third and final step in diagnosis is assessing for other conditions that could cause esophageal eosinophilia.9 The most common differential diagnosis is gastroesophageal reflux disease (GERD). In some cases, EoE and GERD overlap or can have a complex relationship.13 Unfortunately the location of the eosinophilia (i.e. distal only) and the level of the eosinophil counts are not useful in making this distinction, so all clinical features (symptoms, presence of erosive esophagitis, or a hiatal hernia endoscopically), and ancillary reflex testing when indicated may be required prior to a formal EoE diagnosis. After the diagnosis is established, there should be direct communication with the patient to review the diagnosis and select treatments. While it is possible to convey results electronically in a messaging portal or with a letter, a more formal interaction, such as a clinic visit, is recommended because this is a new diagnosis of a chronic condition. Similarly, a new diagnosis of inflammatory bowel disease would never be made in a pathology follow-up letter alone.
Treatment of EoE
When it comes to treatment, the new guidelines emphasize several points.10 First, there is the concept that anti-inflammatory treatment should be paired with assessment of fibrostenosis and esophageal dilation; to do either in isolation is incomplete treatment. It is safe to perform dilation both prior to anti-inflammatory treatment (for example, with a critical stricture in a patient with dysphagia) and after anti-inflammatory treatment has been prescribed (for example, during an endoscopy to assess treatment response).
Second, proton pump inhibitors (PPIs), swallowed topical corticosteroids (tCS), or dietary elimination are all acceptable first-line treatment options for EoE. A shared decision-making framework should be used for this discussion. If dietary elimination is selected,14 based on new clinical trial data, guidelines recommend using empiric elimination and starting with a less restrictive diet (either a one-food elimination diet with dairy alone or a two-food elimination with dairy and wheat elimination). If PPIs are selected, the dose should be double the standard reflux dose. Data are mixed as to whether to use twice daily dosing (i.e., omeprazole 20 mg twice daily) or once a day dosing (i.e., omeprazole 40 mg daily), but total dose and adherence may be more important than frequency.10
For tCS use, either budesonide or fluticasone can be selected, but budesonide oral suspension is the only FDA-approved tCS for EoE.15 Initial treatment length is usually 6-8 weeks for diet elimination and, 12 weeks for PPI and tCS. In general, it is best to pick a single treatment to start, and reserve combining therapies for patients who do not have a complete response to a single modality as there are few data to support combination therapy.
After initial treatment, it is critical to assess for treatment response.16 Goals of EoE treatment include improvement in symptoms, but also improvement in endoscopic and histologic features to prevent complications. Symptoms in EoE do not always correlate with underlying biologic disease activity: patients can minimize symptoms with careful eating; they may perceive no difference in symptoms despite histologic improvement if a stricture persists; and they may have minimal symptoms after esophageal dilation despite ongoing inflammation. Because of this, performing a follow-up endoscopy after initial treatment is guideline-recommended.10, 17 This allows assessing for endoscopic improvement, re-assessing for fibrostenosis and performing dilation if indicated, and obtaining esophageal biopsies. If there is non-response, options include switching between other first line treatments or considering “stepping-up” to dupilumab which is also an FDA-approved option for EoE that is recommended in the guidelines.10, 18 In some cases where patients have multiple severe atopic conditions such as asthma or eczema that would warrant dupilumab use, or if patients are intolerant to PPIs or tCS, dupilumab could be considered as an earlier treatment for EoE.
Long-Term Maintenance
If a patient has a good response (for example, improved symptoms, improved endoscopic features, and <15 eos/hpf on biopsy), treatment can be maintained long-term. In almost all cases, if treatment is stopped, EoE disease activity recurs.19 Patients could be seen back in clinic in 6-12 months, and then a discussion can be conducted about a follow-up endoscopy, with timing to be determined based on their individual disease features and severity.17
Patients with more severe strictures, however, may have to be seen in endoscopy for serial dilations. Continued follow-up is essential for optimal care. Just as patients can progress in their disease course with diagnostic delay, there are data that show they can also progress after diagnosis when there are gaps in care without regular follow-up.20 Unlike other chronic esophageal disorders such as GERD and Barrett’s esophagus and other chronic GI inflammatory conditions like inflammatory bowel disease, however, EoE is not associated with an increased risk of esophageal cancer.21, 22
Given its increasing frequency, EoE will be commonly encountered by gastroenterologists both new and established. Having a systematic approach for diagnosis, understanding how to elicit subtle symptoms, implementing a shared decision-making framework for treatment with a structured algorithm for assessing response, performing follow-up, maintaining treatment, and monitoring patients long-term will allow the large majority of EoE patients to be successfully managed.
Dr. Dellon is based at the Center for Esophageal Diseases and Swallowing, Center for Gastrointestinal Biology and Disease, Division of Gastroenterology and Hepatology, University of North Carolina School of Medicine, Chapel Hill. He disclosed research funding, consultant fees, and educational grants from multiple companies.
References
1. Thel HL, et al. Prevalence and Costs of Eosinophilic Esophagitis in the United States. Clin Gastroenterol Hepatol. 2025 Feb. doi: 10.1016/j.cgh.2024.09.031.
2. Lam AY, et al. Epidemiologic Burden and Projections for Eosinophilic Esophagitis-Associated Emergency Department Visits in the United States: 2009-2030. Clin Gastroenterol Hepatol. 2023 Nov. doi: 10.1016/j.cgh.2023.04.028.
3. Schoepfer AM, et al. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology. 2013 Dec. doi: 10.1053/j.gastro.2013.08.015.
4. Dellon ES, Hirano I. Epidemiology and Natural History of Eosinophilic Esophagitis. Gastroenterology. 2018 Jan. doi: 10.1053/j.gastro.2017.06.067.
5. Chang JW, et al. Loss to follow-up after food impaction among patients with and without eosinophilic esophagitis. Dis Esophagus. 2019 Dec. doi: 10.1093/dote/doz056.
6. Aceves SS, et al. Endoscopic approach to eosinophilic esophagitis: American Society for Gastrointestinal Endoscopy Consensus Conference. Gastrointest Endosc. 2022 Aug. doi: 10.1016/j.gie.2022.05.013.
7. Leiman DA, et al. Quality Indicators for the Diagnosis and Management of Eosinophilic Esophagitis. Am J Gastroenterol. 2023 Jun. doi: 10.14309/ajg.0000000000002138.
8. Hirano I, Furuta GT. Approaches and Challenges to Management of Pediatric and Adult Patients With Eosinophilic Esophagitis. Gastroenterology. 2020 Mar. doi: 10.1053/j.gastro.2019.09.052.
9. Dellon ES, et al. Updated international consensus diagnostic criteria for eosinophilic esophagitis: Proceedings of the AGREE conference. Gastroenterology. 2018 Oct. doi: 10.1053/j.gastro.2018.07.009.
10. Dellon ES, et al. ACG Clinical Guideline: Diagnosis and Management of Eosinophilic Esophagitis. Am J Gastroenterol. 2025 Jan. doi: 10.14309/ajg.0000000000003194.
11. Dellon ES. Optimizing the Endoscopic Examination in Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2021 Dec. doi: 10.1016/j.cgh.2021.07.011.
12. Hirano I, et al. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut. 2012 May. doi: 10.1136/gutjnl-2011-301817.
13. Spechler SJ, et al. Thoughts on the complex relationship between gastroesophageal reflux disease and eosinophilic esophagitis. Am J Gastroenterol. 2007 Jun. doi: 10.1111/j.1572-0241.2007.01179.x.
14. Chang JW, et al. Development of a Practical Guide to Implement and Monitor Diet Therapy for Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2023 Jul. doi: 10.1016/j.cgh.2023.03.006.
15. Hirano I, et al. Budesonide Oral Suspension Improves Outcomes in Patients With Eosinophilic Esophagitis: Results from a Phase 3 Trial. Clin Gastroenterol Hepatol. 2022 Mar. doi: 10.1016/j.cgh.2021.04.022.
16. Dellon ES, Gupta SK. A conceptual approach to understanding treatment response in eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2019 Oct. doi: 10.1016/j.cgh.2019.01.030.
17. von Arnim U, et al. Monitoring Patients With Eosinophilic Esophagitis in Routine Clinical Practice - International Expert Recommendations. Clin Gastroenterol Hepatol. 2023 Sep. doi: 10.1016/j.cgh.2022.12.018.
18. Dellon ES, et al. Dupilumab in Adults and Adolescents with Eosinophilic Esophagitis. N Engl J Med. 2022 Dec. doi: 10.1056/NEJMoa220598.
19. Dellon ES, et al. Rapid Recurrence of Eosinophilic Esophagitis Activity After Successful Treatment in the Observation Phase of a Randomized, Double-Blind, Double-Dummy Trial. Clin Gastroenterol Hepatol. 2020 Jun. doi: 10.1016/j.cgh.2019.08.050.
20. Chang NC, et al. A Gap in Care Leads to Progression of Fibrosis in Eosinophilic Esophagitis Patients. Clin Gastroenterol Hepatol. 2022 Aug. doi: 10.1016/j.cgh.2021.10.028.
21. Syed A, et al. The relationship between eosinophilic esophagitis and esophageal cancer. Dis Esophagus. 2017 Jul. doi: 10.1093/dote/dox050.
22. Albaneze N, et al. No Association Between Eosinophilic Oesophagitis and Oesophageal Cancer in US Adults: A Case-Control Study. Aliment Pharmacol Ther. 2025 Jan. doi: 10.1111/apt.18431.
Eosinophilic esophagitis (EoE) can be considered a “young” disease, with initial case series reported only about 30 years ago. Since that time, it has become a commonly encountered condition in both emergency and clinic settings. The most recent prevalence study estimates that 1 in 700 people in the U.S. have EoE,1 the volume of EoE-associated ED visits tripped between 2009 and 2019 and is projected to double again by 2030,2 and “new” gastroenterologists undoubtedly have learned about and seen this condition. As a chronic disease, EoE necessitates longitudinal follow-up and optimization of care to prevent complications. With increasing diagnostic delay, EoE progresses in most, but not all, patients from an inflammatory- to fibrostenotic-predominant condition.3
Diagnosis of EoE
The most likely area that you will encounter EoE is during an emergent middle-of-the-night endoscopy for food impaction. If called in for this, EoE will be the cause in more than 50% of patients.4 However, the diagnosis can only be made if esophageal biopsies are obtained at the time of the procedure. This is a critical time to decrease diagnostic delay, as half of patients are lost to follow-up after a food impaction.5 Unfortunately, although taking biopsies during index food impaction is guideline-recommended, a quality metric, and safe to obtain after the food bolus is cleared, this is infrequently done in practice.6, 7
The next most likely area for EoE detection is in the endoscopy suite where 15-23% of patients with dysphagia and 5-7% of patients undergoing upper endoscopy for any indication will have EoE.4 Sometimes EoE will be detected “incidentally” during an open-access case (for example, in a patient with diarrhea undergoing evaluation for celiac). In these cases, it is important to perform a careful history (as noted below) as subtle EoE symptoms can frequently be identified. Finally, when patients are seen in clinic for solid food dysphagia, EoE is clearly on the differential. A few percent of patients with refractory heartburn or chest pain will have EoE causing the symptoms rather than reflux,4 and all patients under consideration for antireflux surgery should have an endoscopy to assess for EoE.
When talking to patients with known or suspected EoE, the history must go beyond general questions about dysphagia or trouble swallowing. Many patients with EoE have overtly or subconsciously modified their eating behaviors over many years to minimize symptoms, may have adapted to chronic dysphagia, and will answer “no” when asked if they have trouble swallowing. Instead, use the acronym “IMPACT” to delve deeper into possible symptoms.8 Do they “Imbibe” fluids or liquids between each bite to help get food down? Do they “Modify” the way they eat (cut food into small bites; puree foods)? Do they “Prolong” mealtimes? Do they “Avoid” certain foods that stick? Do they “Chew’ until their food is a mush to get it down? And do they “Turn away” tablets or pills? Pill dysphagia is often a subtle symptom, and sometimes the only symptom elicited.
Additionally, it may be important to ask a partner or family member (if present) about their observations. They may provide insight (e.g. “yes – he chokes with every bite but never says it bothers him”) that the patient might not otherwise provide. The suspicion for EoE should also be increased in patients with concomitant atopic diseases and in those with a family history of dysphagia or who have family members needing esophageal dilation. It is important to remember that EoE can be seen across all ages, sexes, and races/ethnicities.
Diagnosis of EoE is based on the AGREE consensus,9 which is also echoed in the recently updated American College of Gastroenterology (ACG) guidelines.10 Diagnosis requires three steps. First, symptoms of esophageal dysfunction must be present. This will most typically be dysphagia in adolescents and adults, but symptoms are non-specific in children (e.g. poor growth and feeding, abdominal pain, vomiting, regurgitation, heartburn).
Second, at least 15 eosinophils per high-power field (eos/hpf) are required on esophageal biopsy, which implies that an endoscopy be performed. A high-quality endoscopic exam in EoE is of the utmost importance. The approach has been described elsewhere,11 but enough time on insertion should be taken to fully insufflate and examine the esophagus, including the areas of the gastroesophageal junction and upper esophageal sphincter where strictures can be missed, to gently wash debris, and to assess the endoscopic findings of EoE. Endoscopic findings should be reported using the validated EoE Endoscopy Reference Score (EREFS),12 which grades five key features. EREFS is reproducible, is responsive to treatment, and is guideline-recommended (see Figure 1).6, 10 The features are edema (present=1), rings (mild=1; moderate=2; severe=3), exudates (mild=1; severe=2), furrows (mild=1; severe=2), and stricture (present=1; also estimate diameter in mm) and are incorporated into many endoscopic reporting programs. Additionally, diffuse luminal narrowing and mucosal fragility (“crepe-paper” mucosa) should be assessed.
After this, biopsies should be obtained with at least 6 biopsy fragments from different locations in the esophagus. Any visible endoscopic abnormalities should be targeted (the highest yield is in exudates and furrows). The rationale is that EoE is patchy and at least 6 biopsies will maximize diagnostic yield.10 Ideally the initial endoscopy for EoE should be done off of treatments (like PPI or diet restriction) as these could mask the diagnosis. If a patient with suspected EoE has an endoscopy while on PPI, and the endoscopy is normal, a diagnosis of EoE cannot be made. In this case, consideration should be given as to stopping the PPI, allowing a wash out period (at least 1-2 months), and then repeating the endoscopy to confirm the diagnosis. This is important as EoE is a chronic condition necessitating life-long treatment and monitoring, so a definitive diagnosis is critical.
The third and final step in diagnosis is assessing for other conditions that could cause esophageal eosinophilia.9 The most common differential diagnosis is gastroesophageal reflux disease (GERD). In some cases, EoE and GERD overlap or can have a complex relationship.13 Unfortunately the location of the eosinophilia (i.e. distal only) and the level of the eosinophil counts are not useful in making this distinction, so all clinical features (symptoms, presence of erosive esophagitis, or a hiatal hernia endoscopically), and ancillary reflex testing when indicated may be required prior to a formal EoE diagnosis. After the diagnosis is established, there should be direct communication with the patient to review the diagnosis and select treatments. While it is possible to convey results electronically in a messaging portal or with a letter, a more formal interaction, such as a clinic visit, is recommended because this is a new diagnosis of a chronic condition. Similarly, a new diagnosis of inflammatory bowel disease would never be made in a pathology follow-up letter alone.
Treatment of EoE
When it comes to treatment, the new guidelines emphasize several points.10 First, there is the concept that anti-inflammatory treatment should be paired with assessment of fibrostenosis and esophageal dilation; to do either in isolation is incomplete treatment. It is safe to perform dilation both prior to anti-inflammatory treatment (for example, with a critical stricture in a patient with dysphagia) and after anti-inflammatory treatment has been prescribed (for example, during an endoscopy to assess treatment response).
Second, proton pump inhibitors (PPIs), swallowed topical corticosteroids (tCS), or dietary elimination are all acceptable first-line treatment options for EoE. A shared decision-making framework should be used for this discussion. If dietary elimination is selected,14 based on new clinical trial data, guidelines recommend using empiric elimination and starting with a less restrictive diet (either a one-food elimination diet with dairy alone or a two-food elimination with dairy and wheat elimination). If PPIs are selected, the dose should be double the standard reflux dose. Data are mixed as to whether to use twice daily dosing (i.e., omeprazole 20 mg twice daily) or once a day dosing (i.e., omeprazole 40 mg daily), but total dose and adherence may be more important than frequency.10
For tCS use, either budesonide or fluticasone can be selected, but budesonide oral suspension is the only FDA-approved tCS for EoE.15 Initial treatment length is usually 6-8 weeks for diet elimination and, 12 weeks for PPI and tCS. In general, it is best to pick a single treatment to start, and reserve combining therapies for patients who do not have a complete response to a single modality as there are few data to support combination therapy.
After initial treatment, it is critical to assess for treatment response.16 Goals of EoE treatment include improvement in symptoms, but also improvement in endoscopic and histologic features to prevent complications. Symptoms in EoE do not always correlate with underlying biologic disease activity: patients can minimize symptoms with careful eating; they may perceive no difference in symptoms despite histologic improvement if a stricture persists; and they may have minimal symptoms after esophageal dilation despite ongoing inflammation. Because of this, performing a follow-up endoscopy after initial treatment is guideline-recommended.10, 17 This allows assessing for endoscopic improvement, re-assessing for fibrostenosis and performing dilation if indicated, and obtaining esophageal biopsies. If there is non-response, options include switching between other first line treatments or considering “stepping-up” to dupilumab which is also an FDA-approved option for EoE that is recommended in the guidelines.10, 18 In some cases where patients have multiple severe atopic conditions such as asthma or eczema that would warrant dupilumab use, or if patients are intolerant to PPIs or tCS, dupilumab could be considered as an earlier treatment for EoE.
Long-Term Maintenance
If a patient has a good response (for example, improved symptoms, improved endoscopic features, and <15 eos/hpf on biopsy), treatment can be maintained long-term. In almost all cases, if treatment is stopped, EoE disease activity recurs.19 Patients could be seen back in clinic in 6-12 months, and then a discussion can be conducted about a follow-up endoscopy, with timing to be determined based on their individual disease features and severity.17
Patients with more severe strictures, however, may have to be seen in endoscopy for serial dilations. Continued follow-up is essential for optimal care. Just as patients can progress in their disease course with diagnostic delay, there are data that show they can also progress after diagnosis when there are gaps in care without regular follow-up.20 Unlike other chronic esophageal disorders such as GERD and Barrett’s esophagus and other chronic GI inflammatory conditions like inflammatory bowel disease, however, EoE is not associated with an increased risk of esophageal cancer.21, 22
Given its increasing frequency, EoE will be commonly encountered by gastroenterologists both new and established. Having a systematic approach for diagnosis, understanding how to elicit subtle symptoms, implementing a shared decision-making framework for treatment with a structured algorithm for assessing response, performing follow-up, maintaining treatment, and monitoring patients long-term will allow the large majority of EoE patients to be successfully managed.
Dr. Dellon is based at the Center for Esophageal Diseases and Swallowing, Center for Gastrointestinal Biology and Disease, Division of Gastroenterology and Hepatology, University of North Carolina School of Medicine, Chapel Hill. He disclosed research funding, consultant fees, and educational grants from multiple companies.
References
1. Thel HL, et al. Prevalence and Costs of Eosinophilic Esophagitis in the United States. Clin Gastroenterol Hepatol. 2025 Feb. doi: 10.1016/j.cgh.2024.09.031.
2. Lam AY, et al. Epidemiologic Burden and Projections for Eosinophilic Esophagitis-Associated Emergency Department Visits in the United States: 2009-2030. Clin Gastroenterol Hepatol. 2023 Nov. doi: 10.1016/j.cgh.2023.04.028.
3. Schoepfer AM, et al. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology. 2013 Dec. doi: 10.1053/j.gastro.2013.08.015.
4. Dellon ES, Hirano I. Epidemiology and Natural History of Eosinophilic Esophagitis. Gastroenterology. 2018 Jan. doi: 10.1053/j.gastro.2017.06.067.
5. Chang JW, et al. Loss to follow-up after food impaction among patients with and without eosinophilic esophagitis. Dis Esophagus. 2019 Dec. doi: 10.1093/dote/doz056.
6. Aceves SS, et al. Endoscopic approach to eosinophilic esophagitis: American Society for Gastrointestinal Endoscopy Consensus Conference. Gastrointest Endosc. 2022 Aug. doi: 10.1016/j.gie.2022.05.013.
7. Leiman DA, et al. Quality Indicators for the Diagnosis and Management of Eosinophilic Esophagitis. Am J Gastroenterol. 2023 Jun. doi: 10.14309/ajg.0000000000002138.
8. Hirano I, Furuta GT. Approaches and Challenges to Management of Pediatric and Adult Patients With Eosinophilic Esophagitis. Gastroenterology. 2020 Mar. doi: 10.1053/j.gastro.2019.09.052.
9. Dellon ES, et al. Updated international consensus diagnostic criteria for eosinophilic esophagitis: Proceedings of the AGREE conference. Gastroenterology. 2018 Oct. doi: 10.1053/j.gastro.2018.07.009.
10. Dellon ES, et al. ACG Clinical Guideline: Diagnosis and Management of Eosinophilic Esophagitis. Am J Gastroenterol. 2025 Jan. doi: 10.14309/ajg.0000000000003194.
11. Dellon ES. Optimizing the Endoscopic Examination in Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2021 Dec. doi: 10.1016/j.cgh.2021.07.011.
12. Hirano I, et al. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut. 2012 May. doi: 10.1136/gutjnl-2011-301817.
13. Spechler SJ, et al. Thoughts on the complex relationship between gastroesophageal reflux disease and eosinophilic esophagitis. Am J Gastroenterol. 2007 Jun. doi: 10.1111/j.1572-0241.2007.01179.x.
14. Chang JW, et al. Development of a Practical Guide to Implement and Monitor Diet Therapy for Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2023 Jul. doi: 10.1016/j.cgh.2023.03.006.
15. Hirano I, et al. Budesonide Oral Suspension Improves Outcomes in Patients With Eosinophilic Esophagitis: Results from a Phase 3 Trial. Clin Gastroenterol Hepatol. 2022 Mar. doi: 10.1016/j.cgh.2021.04.022.
16. Dellon ES, Gupta SK. A conceptual approach to understanding treatment response in eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2019 Oct. doi: 10.1016/j.cgh.2019.01.030.
17. von Arnim U, et al. Monitoring Patients With Eosinophilic Esophagitis in Routine Clinical Practice - International Expert Recommendations. Clin Gastroenterol Hepatol. 2023 Sep. doi: 10.1016/j.cgh.2022.12.018.
18. Dellon ES, et al. Dupilumab in Adults and Adolescents with Eosinophilic Esophagitis. N Engl J Med. 2022 Dec. doi: 10.1056/NEJMoa220598.
19. Dellon ES, et al. Rapid Recurrence of Eosinophilic Esophagitis Activity After Successful Treatment in the Observation Phase of a Randomized, Double-Blind, Double-Dummy Trial. Clin Gastroenterol Hepatol. 2020 Jun. doi: 10.1016/j.cgh.2019.08.050.
20. Chang NC, et al. A Gap in Care Leads to Progression of Fibrosis in Eosinophilic Esophagitis Patients. Clin Gastroenterol Hepatol. 2022 Aug. doi: 10.1016/j.cgh.2021.10.028.
21. Syed A, et al. The relationship between eosinophilic esophagitis and esophageal cancer. Dis Esophagus. 2017 Jul. doi: 10.1093/dote/dox050.
22. Albaneze N, et al. No Association Between Eosinophilic Oesophagitis and Oesophageal Cancer in US Adults: A Case-Control Study. Aliment Pharmacol Ther. 2025 Jan. doi: 10.1111/apt.18431.
Eosinophilic esophagitis (EoE) can be considered a “young” disease, with initial case series reported only about 30 years ago. Since that time, it has become a commonly encountered condition in both emergency and clinic settings. The most recent prevalence study estimates that 1 in 700 people in the U.S. have EoE,1 the volume of EoE-associated ED visits tripped between 2009 and 2019 and is projected to double again by 2030,2 and “new” gastroenterologists undoubtedly have learned about and seen this condition. As a chronic disease, EoE necessitates longitudinal follow-up and optimization of care to prevent complications. With increasing diagnostic delay, EoE progresses in most, but not all, patients from an inflammatory- to fibrostenotic-predominant condition.3
Diagnosis of EoE
The most likely area that you will encounter EoE is during an emergent middle-of-the-night endoscopy for food impaction. If called in for this, EoE will be the cause in more than 50% of patients.4 However, the diagnosis can only be made if esophageal biopsies are obtained at the time of the procedure. This is a critical time to decrease diagnostic delay, as half of patients are lost to follow-up after a food impaction.5 Unfortunately, although taking biopsies during index food impaction is guideline-recommended, a quality metric, and safe to obtain after the food bolus is cleared, this is infrequently done in practice.6, 7
The next most likely area for EoE detection is in the endoscopy suite where 15-23% of patients with dysphagia and 5-7% of patients undergoing upper endoscopy for any indication will have EoE.4 Sometimes EoE will be detected “incidentally” during an open-access case (for example, in a patient with diarrhea undergoing evaluation for celiac). In these cases, it is important to perform a careful history (as noted below) as subtle EoE symptoms can frequently be identified. Finally, when patients are seen in clinic for solid food dysphagia, EoE is clearly on the differential. A few percent of patients with refractory heartburn or chest pain will have EoE causing the symptoms rather than reflux,4 and all patients under consideration for antireflux surgery should have an endoscopy to assess for EoE.
When talking to patients with known or suspected EoE, the history must go beyond general questions about dysphagia or trouble swallowing. Many patients with EoE have overtly or subconsciously modified their eating behaviors over many years to minimize symptoms, may have adapted to chronic dysphagia, and will answer “no” when asked if they have trouble swallowing. Instead, use the acronym “IMPACT” to delve deeper into possible symptoms.8 Do they “Imbibe” fluids or liquids between each bite to help get food down? Do they “Modify” the way they eat (cut food into small bites; puree foods)? Do they “Prolong” mealtimes? Do they “Avoid” certain foods that stick? Do they “Chew’ until their food is a mush to get it down? And do they “Turn away” tablets or pills? Pill dysphagia is often a subtle symptom, and sometimes the only symptom elicited.
Additionally, it may be important to ask a partner or family member (if present) about their observations. They may provide insight (e.g. “yes – he chokes with every bite but never says it bothers him”) that the patient might not otherwise provide. The suspicion for EoE should also be increased in patients with concomitant atopic diseases and in those with a family history of dysphagia or who have family members needing esophageal dilation. It is important to remember that EoE can be seen across all ages, sexes, and races/ethnicities.
Diagnosis of EoE is based on the AGREE consensus,9 which is also echoed in the recently updated American College of Gastroenterology (ACG) guidelines.10 Diagnosis requires three steps. First, symptoms of esophageal dysfunction must be present. This will most typically be dysphagia in adolescents and adults, but symptoms are non-specific in children (e.g. poor growth and feeding, abdominal pain, vomiting, regurgitation, heartburn).
Second, at least 15 eosinophils per high-power field (eos/hpf) are required on esophageal biopsy, which implies that an endoscopy be performed. A high-quality endoscopic exam in EoE is of the utmost importance. The approach has been described elsewhere,11 but enough time on insertion should be taken to fully insufflate and examine the esophagus, including the areas of the gastroesophageal junction and upper esophageal sphincter where strictures can be missed, to gently wash debris, and to assess the endoscopic findings of EoE. Endoscopic findings should be reported using the validated EoE Endoscopy Reference Score (EREFS),12 which grades five key features. EREFS is reproducible, is responsive to treatment, and is guideline-recommended (see Figure 1).6, 10 The features are edema (present=1), rings (mild=1; moderate=2; severe=3), exudates (mild=1; severe=2), furrows (mild=1; severe=2), and stricture (present=1; also estimate diameter in mm) and are incorporated into many endoscopic reporting programs. Additionally, diffuse luminal narrowing and mucosal fragility (“crepe-paper” mucosa) should be assessed.
After this, biopsies should be obtained with at least 6 biopsy fragments from different locations in the esophagus. Any visible endoscopic abnormalities should be targeted (the highest yield is in exudates and furrows). The rationale is that EoE is patchy and at least 6 biopsies will maximize diagnostic yield.10 Ideally the initial endoscopy for EoE should be done off of treatments (like PPI or diet restriction) as these could mask the diagnosis. If a patient with suspected EoE has an endoscopy while on PPI, and the endoscopy is normal, a diagnosis of EoE cannot be made. In this case, consideration should be given as to stopping the PPI, allowing a wash out period (at least 1-2 months), and then repeating the endoscopy to confirm the diagnosis. This is important as EoE is a chronic condition necessitating life-long treatment and monitoring, so a definitive diagnosis is critical.
The third and final step in diagnosis is assessing for other conditions that could cause esophageal eosinophilia.9 The most common differential diagnosis is gastroesophageal reflux disease (GERD). In some cases, EoE and GERD overlap or can have a complex relationship.13 Unfortunately the location of the eosinophilia (i.e. distal only) and the level of the eosinophil counts are not useful in making this distinction, so all clinical features (symptoms, presence of erosive esophagitis, or a hiatal hernia endoscopically), and ancillary reflex testing when indicated may be required prior to a formal EoE diagnosis. After the diagnosis is established, there should be direct communication with the patient to review the diagnosis and select treatments. While it is possible to convey results electronically in a messaging portal or with a letter, a more formal interaction, such as a clinic visit, is recommended because this is a new diagnosis of a chronic condition. Similarly, a new diagnosis of inflammatory bowel disease would never be made in a pathology follow-up letter alone.
Treatment of EoE
When it comes to treatment, the new guidelines emphasize several points.10 First, there is the concept that anti-inflammatory treatment should be paired with assessment of fibrostenosis and esophageal dilation; to do either in isolation is incomplete treatment. It is safe to perform dilation both prior to anti-inflammatory treatment (for example, with a critical stricture in a patient with dysphagia) and after anti-inflammatory treatment has been prescribed (for example, during an endoscopy to assess treatment response).
Second, proton pump inhibitors (PPIs), swallowed topical corticosteroids (tCS), or dietary elimination are all acceptable first-line treatment options for EoE. A shared decision-making framework should be used for this discussion. If dietary elimination is selected,14 based on new clinical trial data, guidelines recommend using empiric elimination and starting with a less restrictive diet (either a one-food elimination diet with dairy alone or a two-food elimination with dairy and wheat elimination). If PPIs are selected, the dose should be double the standard reflux dose. Data are mixed as to whether to use twice daily dosing (i.e., omeprazole 20 mg twice daily) or once a day dosing (i.e., omeprazole 40 mg daily), but total dose and adherence may be more important than frequency.10
For tCS use, either budesonide or fluticasone can be selected, but budesonide oral suspension is the only FDA-approved tCS for EoE.15 Initial treatment length is usually 6-8 weeks for diet elimination and, 12 weeks for PPI and tCS. In general, it is best to pick a single treatment to start, and reserve combining therapies for patients who do not have a complete response to a single modality as there are few data to support combination therapy.
After initial treatment, it is critical to assess for treatment response.16 Goals of EoE treatment include improvement in symptoms, but also improvement in endoscopic and histologic features to prevent complications. Symptoms in EoE do not always correlate with underlying biologic disease activity: patients can minimize symptoms with careful eating; they may perceive no difference in symptoms despite histologic improvement if a stricture persists; and they may have minimal symptoms after esophageal dilation despite ongoing inflammation. Because of this, performing a follow-up endoscopy after initial treatment is guideline-recommended.10, 17 This allows assessing for endoscopic improvement, re-assessing for fibrostenosis and performing dilation if indicated, and obtaining esophageal biopsies. If there is non-response, options include switching between other first line treatments or considering “stepping-up” to dupilumab which is also an FDA-approved option for EoE that is recommended in the guidelines.10, 18 In some cases where patients have multiple severe atopic conditions such as asthma or eczema that would warrant dupilumab use, or if patients are intolerant to PPIs or tCS, dupilumab could be considered as an earlier treatment for EoE.
Long-Term Maintenance
If a patient has a good response (for example, improved symptoms, improved endoscopic features, and <15 eos/hpf on biopsy), treatment can be maintained long-term. In almost all cases, if treatment is stopped, EoE disease activity recurs.19 Patients could be seen back in clinic in 6-12 months, and then a discussion can be conducted about a follow-up endoscopy, with timing to be determined based on their individual disease features and severity.17
Patients with more severe strictures, however, may have to be seen in endoscopy for serial dilations. Continued follow-up is essential for optimal care. Just as patients can progress in their disease course with diagnostic delay, there are data that show they can also progress after diagnosis when there are gaps in care without regular follow-up.20 Unlike other chronic esophageal disorders such as GERD and Barrett’s esophagus and other chronic GI inflammatory conditions like inflammatory bowel disease, however, EoE is not associated with an increased risk of esophageal cancer.21, 22
Given its increasing frequency, EoE will be commonly encountered by gastroenterologists both new and established. Having a systematic approach for diagnosis, understanding how to elicit subtle symptoms, implementing a shared decision-making framework for treatment with a structured algorithm for assessing response, performing follow-up, maintaining treatment, and monitoring patients long-term will allow the large majority of EoE patients to be successfully managed.
Dr. Dellon is based at the Center for Esophageal Diseases and Swallowing, Center for Gastrointestinal Biology and Disease, Division of Gastroenterology and Hepatology, University of North Carolina School of Medicine, Chapel Hill. He disclosed research funding, consultant fees, and educational grants from multiple companies.
References
1. Thel HL, et al. Prevalence and Costs of Eosinophilic Esophagitis in the United States. Clin Gastroenterol Hepatol. 2025 Feb. doi: 10.1016/j.cgh.2024.09.031.
2. Lam AY, et al. Epidemiologic Burden and Projections for Eosinophilic Esophagitis-Associated Emergency Department Visits in the United States: 2009-2030. Clin Gastroenterol Hepatol. 2023 Nov. doi: 10.1016/j.cgh.2023.04.028.
3. Schoepfer AM, et al. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology. 2013 Dec. doi: 10.1053/j.gastro.2013.08.015.
4. Dellon ES, Hirano I. Epidemiology and Natural History of Eosinophilic Esophagitis. Gastroenterology. 2018 Jan. doi: 10.1053/j.gastro.2017.06.067.
5. Chang JW, et al. Loss to follow-up after food impaction among patients with and without eosinophilic esophagitis. Dis Esophagus. 2019 Dec. doi: 10.1093/dote/doz056.
6. Aceves SS, et al. Endoscopic approach to eosinophilic esophagitis: American Society for Gastrointestinal Endoscopy Consensus Conference. Gastrointest Endosc. 2022 Aug. doi: 10.1016/j.gie.2022.05.013.
7. Leiman DA, et al. Quality Indicators for the Diagnosis and Management of Eosinophilic Esophagitis. Am J Gastroenterol. 2023 Jun. doi: 10.14309/ajg.0000000000002138.
8. Hirano I, Furuta GT. Approaches and Challenges to Management of Pediatric and Adult Patients With Eosinophilic Esophagitis. Gastroenterology. 2020 Mar. doi: 10.1053/j.gastro.2019.09.052.
9. Dellon ES, et al. Updated international consensus diagnostic criteria for eosinophilic esophagitis: Proceedings of the AGREE conference. Gastroenterology. 2018 Oct. doi: 10.1053/j.gastro.2018.07.009.
10. Dellon ES, et al. ACG Clinical Guideline: Diagnosis and Management of Eosinophilic Esophagitis. Am J Gastroenterol. 2025 Jan. doi: 10.14309/ajg.0000000000003194.
11. Dellon ES. Optimizing the Endoscopic Examination in Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2021 Dec. doi: 10.1016/j.cgh.2021.07.011.
12. Hirano I, et al. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut. 2012 May. doi: 10.1136/gutjnl-2011-301817.
13. Spechler SJ, et al. Thoughts on the complex relationship between gastroesophageal reflux disease and eosinophilic esophagitis. Am J Gastroenterol. 2007 Jun. doi: 10.1111/j.1572-0241.2007.01179.x.
14. Chang JW, et al. Development of a Practical Guide to Implement and Monitor Diet Therapy for Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2023 Jul. doi: 10.1016/j.cgh.2023.03.006.
15. Hirano I, et al. Budesonide Oral Suspension Improves Outcomes in Patients With Eosinophilic Esophagitis: Results from a Phase 3 Trial. Clin Gastroenterol Hepatol. 2022 Mar. doi: 10.1016/j.cgh.2021.04.022.
16. Dellon ES, Gupta SK. A conceptual approach to understanding treatment response in eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2019 Oct. doi: 10.1016/j.cgh.2019.01.030.
17. von Arnim U, et al. Monitoring Patients With Eosinophilic Esophagitis in Routine Clinical Practice - International Expert Recommendations. Clin Gastroenterol Hepatol. 2023 Sep. doi: 10.1016/j.cgh.2022.12.018.
18. Dellon ES, et al. Dupilumab in Adults and Adolescents with Eosinophilic Esophagitis. N Engl J Med. 2022 Dec. doi: 10.1056/NEJMoa220598.
19. Dellon ES, et al. Rapid Recurrence of Eosinophilic Esophagitis Activity After Successful Treatment in the Observation Phase of a Randomized, Double-Blind, Double-Dummy Trial. Clin Gastroenterol Hepatol. 2020 Jun. doi: 10.1016/j.cgh.2019.08.050.
20. Chang NC, et al. A Gap in Care Leads to Progression of Fibrosis in Eosinophilic Esophagitis Patients. Clin Gastroenterol Hepatol. 2022 Aug. doi: 10.1016/j.cgh.2021.10.028.
21. Syed A, et al. The relationship between eosinophilic esophagitis and esophageal cancer. Dis Esophagus. 2017 Jul. doi: 10.1093/dote/dox050.
22. Albaneze N, et al. No Association Between Eosinophilic Oesophagitis and Oesophageal Cancer in US Adults: A Case-Control Study. Aliment Pharmacol Ther. 2025 Jan. doi: 10.1111/apt.18431.
Avoid Getting Stuck: A Practical Guide to Managing Chronic Constipation
Introduction
Constipation affects one in six people worldwide and accounts for one third of outpatient visits.1 Chronic constipation is defined by difficult, infrequent, and/or incomplete defecation, quantified by less than three spontaneous bowel movements per week, persisting for at least 3 months. Patients may complain of straining during defecation, incomplete evacuation, hard stools (Bristol stool scale [BSS] type 1-2), and fullness or bloating. Chronic constipation can be subclassified as either a primary or secondary disorder.1,2
Primary Constipation Disorders
Primary constipation includes disorders of the colon or anorectum. This includes irritable bowel syndrome with constipation (IBS-C), chronic idiopathic constipation (CIC), slow transit constipation (STC), dyssynergic defecation, and pelvic floor disorders (see Figure 1).
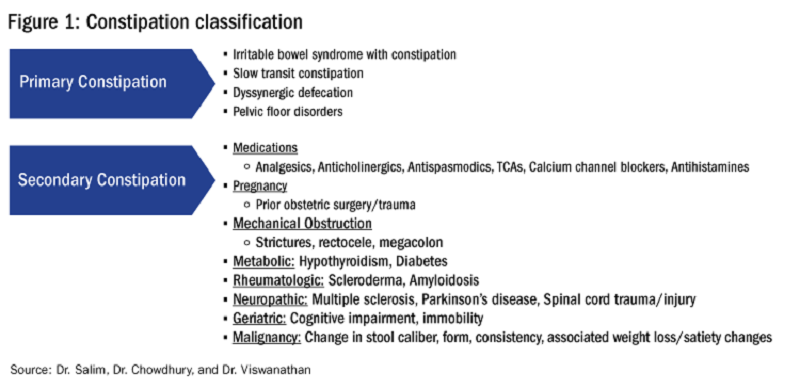
IBS-C
IBS-C is a chronic disorder of the gut-brain axis with a worldwide prevalence of 1.3% and a prevalence of 6%-16% in the United States, United Kingdom, and Canada, with females more likely to seek care than males.2 The economic impact of IBS-C is estimated to be $1.5 billion–$10 billion per year in the United States alone.3 The distinguishing characteristic is abdominal pain, however IBS-C can present with a constellation of symptoms. The diagnostic paradigm has shifted from IBS being a diagnosis of exclusion to now using a positive diagnostic strategy.2 Using this Rome IV criteria, one can make the diagnosis with > 95% accuracy.2,4
CIC
CIC, previously defined as functional constipation, is a disorder defined by incomplete defecation and difficult or infrequent stool. CIC is diagnosed in patients without an underlying anatomic or structural abnormality. Rome IV Criteria helps further classify the defining characteristics of chronic idiopathic constipation.2
Slow Transit Constipation
STC is characterized by impaired colonic transit time in the absence of pelvic floor dysfunction. It presents with infrequent bowel movements, diminished urgency, and/or straining with defecation.
Defecatory Disorders: Dyssynergic Defecation and Pelvic Floor Dysfunction
Defecatory disorders (DDs) result from alterations in the colonic-neural pathway with an unclear pathogenesis. A firm understanding of colonic physiology is necessary to identify DDs. The right colon helps to store and mix stool contents, the left colon helps add water to the stool, and the anal canal and rectum enable defecation and maintain continence. Any alteration along this physiologic pathway results in DDs.5
DDs primarily develop via maladaptive pelvic floor contraction during defecation or from muscle or nerve injury and include functional outlet obstruction, anorectal dyssynergia, and pelvic floor dysfunction. Increased resistance to defecation results from anismus, paradoxical anal sphincter contraction, or incomplete relaxation of the pelvic floor and external anal sphincter. This muscle incoordination is described as dyssynergia. DDs can involve either muscle or nerve dysfunction or a combination of the two. Reduced rectal sensation caused by reduced sensory triggers can cause stasis of stool, thus propagating the cycle of constipation. Over time, excessive straining can weaken the pelvic floor, increasing the risk of excessive perineal descent, rectal intussusception, solitary rectal ulcer syndrome, and pudendal neuropathy.5 Thus, identification of DDs is crucial in patients with chronic constipation.
Secondary Constipation Disorders
Secondary constipation disorders are a result of an alternate process and warrant a thorough review of outpatient medications and past medical history. Figure 1 outlines the most common causes of secondary constipation, which span a wide differential.
Clinical Evaluation
The evaluation of constipation begins with a thorough history. Description of bowel habits should include frequency, duration, straining, stool consistency using a Bristol stool chart, complete vs incomplete evacuation, pain, bloating, and use of digital maneuvers (vaginal splinting or digital stool removal). One should inquire about back trauma/surgeries and obstetric history to include vaginal forceps injury or episiotomy.
With increased smartphone use, toilet time on average has increased and can contribute to maladaptive bowel habits.6 Patients may not realize they are constipated, so patient education is critical. A patient with daily bowel movements ranging between BSS type 1-6 with incomplete evacuation might complain of diarrhea but may in fact have constipation with overflow diarrhea, for example. Past medical history is also clinically relevant, as systemic conditions can cause secondary constipation. A constipated patient should also be asked what therapies he/she has tried prior to gastroenterology referral as primary care referrals for constipation account for 8 million visits to gastroenterology per year.7
While a sensitive topic, inquire about abuse history, especially in those with childhood constipation symptoms. There is a positive correlation between childhood constipation and physical, emotional, and sexual abuse and, for any number of reasons, your patient may be reluctant to share this or undergo a digital rectal exam (DRE).8 In such cases, be sensitive in asking for this history in private rather than with other family members around and always perform this exam with a chaperone present.
A detailed physical exam is an indispensable tool all gastroenterologists must master when evaluating a constipated patient. Some key exam findings include abdominal distention, high-pitched bowel sounds, and presence of a succussion splash indicating obstructive pathology. Dry skin and brittle hair indicate hypothyroidism while hypermobile joints and skin laxity suggest connective tissue disease. Finally, a physical examination is incomplete without a DRE.
DRE
DRE is an often-overlooked physical exam component which provides helpful insight that can guide management. An informed DRE can help identify structural disorders such as fissure, hemorrhoids, anorectal mass, fecal impaction, rectal prolapse, and excessive perineal descent syndrome.9 Unless contraindicated, DRE should be a standard part of the workup of a patient with chronic constipation.
Workup
Colonoscopy
The role of colonoscopy in chronic constipation is low yield and only indicated if alarm signs are present.2 When no organic causes can be identified, the patient is deemed to have a functional bowel or motility disorder leading to constipation.
Colonic Transit Time
Colonic transit time (CTT) can be evaluated by assessing the presence of radio-opaque sitz markers in the colon with an abdominal x-ray 5 days after ingestion. The presence of five or more sitz markers may indicate STC. However, this can also signal an obstructive defecatory disorder. Colon scintigraphy can determine whether there is diffuse colonic dysmotility or dysfunction in a specific segment of the colon.10
Anorectal Function Testing (AFT)
AFT can evaluate DDs, such as fecal incontinence, dyssynergic defecation, rectal sensory disorders, anorectal pain, and rectal prolapse. AFT comprises three tests: anorectal manometry (ARM), balloon expulsion test (BET), and rectal sensory testing. These assess the defecation, continence, and sensory mechanisms of the rectum, respectively.
ARM testing employs a thin, flexible probe with an attached sensor that is inserted into the rectum to measure internal and external sphincter pressures while at rest, squeezing, and bearing down to give a functional assessment of sphincter tone.11 Cough or party balloon test assesses continence and sphincter strength. Rectal sensation is assessed by inflating a balloon incrementally and asking the patient to indicate first sensation, urgency to defecate, and discomfort. If both ARM and BET are abnormal, the patient meets diagnostic criteria for dyssynergic defecation.12
Pelvic floor disorders can be further assessed by MR defecography or barium defecography. Barium defecography is the more widely available of the two. MR defecography is a dynamic study that directly assesses pelvic floor muscles and endopelvic fascia during various stages of defecation and considered superior. This testing modality can distinguish between functional causes such as dyssynergia or pelvic floor dysfunction and structural causes of obstruction such as rectocele, rectal prolapse, or rectal intussusception. MR defecography is helpful when dyssynergia is suggested by ARM with a normal BET or if there is an absent recto-anal inhibitory reflex on ARM, which may suggest rectal intussusception.
Management
CIC
Incorporating 20-30 g of total soluble fiber, such as psyllium in individuals with low dietary fiber intake is the first-line recommendation for CIC.13 If response to a trial of fiber supplementation is inadequate, over-the-counter (OTC) osmotic laxatives such as polyethylene glycol and magnesium oxide can be incorporated. In the event of failure of OTC osmotic laxatives, lactulose can be considered. Stimulant laxatives such as senna, bisacodyl, or sodium picosulfate can be added as an adjunctive measure for short periods of time, defined as daily for 4 weeks or less.
If these measures are inadequate, pharmacological therapy with secretagogues and 5HT agonists can be considered. Prucalopride, a selective agonist of serotonin 5-HT4 receptors, is approved for CIC, prescribed 2 mg daily.14 It can also be used in patients with global motility delays, such as gastroparetics with constipation. The mechanism of action of secretagogues and specific dosing of these medications are discussed in Figure 2.15 Vibrant is a non-pharmacologic, orally ingested, vibrating, and programmable capsule device that has recently received Food and Drug Administration approval for treatment of chronic constipation by stimulating the intestinal wall, thereby promoting colonic contractile activity to achieve more spontaneous bowel movements. Further studies are required to assess its efficacy.16 Additionally, if there is inadequate response to all the above, it would be prudent to evaluate for the presence of pelvic floor dysfunction as well.
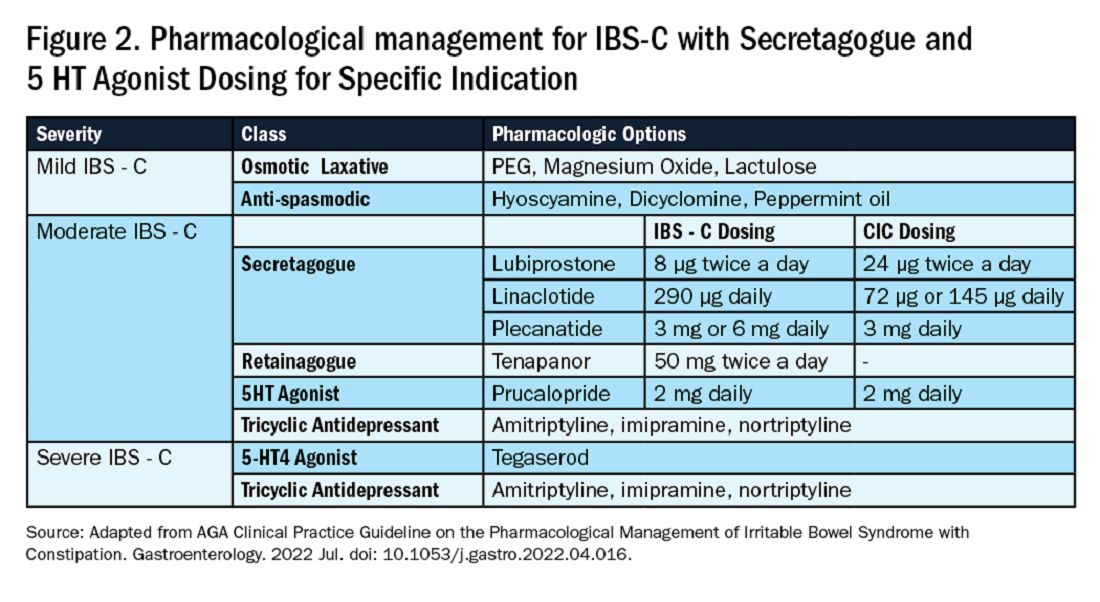
IBS-C
Similar to CIC, treatment for mild IBS-C starts with osmotic laxatives with the additional component of pain control. Antispasmodics can be used to manage the abdominal pain, cramping, and spasms associated with IBS-C. Antispasmodics available in the United States include anticholinergic agents that cause smooth muscle relaxation, such as dicyclomine or hyoscyamine or direct smooth muscle relaxants such as peppermint oil.17 IBS-C patients with moderate symptoms may need escalation of therapy to secretagogues or 5HT agonists (see Figure 2). Secretagogues increase fluid retention in the colonic lumen to promote bowel movements and improve visceral hypersensitivity. Lubiprostone is an intestinal chloride channel activator, indicated only for adult women with IBS-C. Linaclotide and plecanatide are guanylate cyclase-C activators which increase intestinal chloride and bicarbonate secretion, and both are indicated in IBS-C and CIC. Tenapanor inhibits the sodium/hydrogen exchanger in the GI tract, leading to increased water secretion, and is recommended for IBS-C in adults who have failed secretagogues.
All four of these drugs can be considered for moderate to severe IBS-C symptoms. In the case of severe IBS-C symptoms, Tegaserod, a 5-HT4 receptor partial agonist has been approved in women under 65 without significant cardiovascular or cerebrovascular disease.18 Regardless of IBS-C symptom severity, persistent visceral hypersensitivity can be treated with low-dose neuromodulators.19 Figure 2 provides treatment recommendations for IBS-C based on symptom severity.
Opioid-Induced Constipation (OIC)
In patients with OIC, peripherally acting mu-opioid receptor antagonists such as methylnaltrexone and naloxegol can be beneficial where stimulant laxatives are insufficient. Additionally, lubiprostone is indicated in OIC in non-cancer patients. At present, there are no head-to-head trials comparing efficacy of these medications.
Defecatory Disorders
Biofeedback therapy is the cornerstone of treatment for dyssynergic defecation, focusing on neuromuscular training to restore a normal pattern of defecation by teaching patients to tense the abdomen and relax the pelvic floor muscles and anal sphincter. It retrains the body to coordinate abdominal, rectal, and anal muscles to achieve synchronous contraction to achieve complete evacuation. It also increases awareness and response to rectal fullness or the need to defecate.
Biofeedback makes patients aware of counterproductive subconscious actions such as contracting of their anal sphincter during defecation followed by simulated defecation training with focus on how to tighten abdominal muscles and relax pelvic floor muscles to initiate and complete defecation.20 This is performed in the office with a physiotherapist or trained nurse for at least six sessions or at home where patients are encouraged to perform the exercises for 20 minutes, twice a day. These sessions utilize tools such as manometry probes, electromyography probes, simulated balloon, or home biofeedback training devices to provide visual feedback while practicing abdominophrenic breathing. Biofeedback is particularly helpful in patients suffering from constipation. Patients with defecatory disorders can also benefit from pelvic floor physical therapy which focuses on strengthening the pelvic and puborectal muscles, external anal sphincter, and pelvic muscles. This is more useful in patients with fecal incontinence. Despite all these treatments, a subset of patients may still not respond and may qualify for surgical evaluation.
Conclusion
While constipation is seldom life-threatening, it has a negative impact on patient quality of life and poses a significant financial burden on our overall healthcare system. The complexity of this condition should be appreciated and understood in order for a complete and thorough evaluation. We trust that our practical guide should serve as a useful tool in the evaluation of a chronically constipated patient.
Dr. Salim (@hamsalim07 on X) is based in the Department of Internal Medicine, University of Texas Medical Branch, Galveston. Dr. Chowdhury (annicho.med on Instagram) is a fellow in the Department of Gastroenterology, Hepatology, and Nutrition, University of Texas MD Anderson Cancer Center, Houston. Dr. Viswanathan (@LavanyaMD on X) is Associate Professor, University of Texas MD Anderson Cancer Center. The authors declare no conflict of interest.
References
1. Mugie S et al. Best Pract Res Clin Gastroenterol. 2011 Feb. doi: 10.1016/j.bpg.2010.12.010.
2. Almario CV et al. Gastroenterology. 2023 Dec. doi: 10.1053/j.gastro.2023.08.010.
3. Canavan C et al. Clin Epidemiol. 2014 Feb. doi: 10.2147/CLEP.S40245.
4. Rao SSC. Gastroenterol Clin North Am. 2007 Sep. doi: 10.1016/j.gtc.2007.07.013.
5. Bharucha AE et al. Gastroenterology. 2020 Apr. doi: 10.1053/j.gastro.2019.12.034.
6. Cinquetti M et al. Clin Exp Pediatr. 2021 Sep. doi: 10.3345/cep.2020.01326.
7. Shah ND et al. Am J Gastroenterol. 2008 Jul. doi: 10.1111/j.1572-0241.2008.01910.x.
8. Rajindrajith S et al. J Pediatr Gastroenterol Nutr. 2014 Apr. doi: 10.1097/MPG.0000000000000249.
9. Talley NJ. Am J Gastroenterol. 2008 Apr. doi: 10.1111/j.1572-0241.2008.01832.x.
10. Maurer AH. J Nucl Med. 2015 Sep. doi: 10.2967/jnumed.113.134551.
11. Frye J et al. Am J Gastroenterol. 2024 Aug. doi: 10.14309/ajg.0000000000002670.
12. Rao SSC et al. J Neurogastroenterol Motil. 2016 Jun. doi: 10.5056/jnm16060.
13. Chang L et al. Gastroenterology. 2023 Jun. doi: 10.1053/j.gastro.2023.03.214.
14. Brenner DM et al. Am J Gastroenterol. 2021 Aug. doi: 10.14309/ajg.0000000000001266.
15. Chang L et al. Gastroenterology. 2022 Jul. doi: 10.1053/j.gastro.2022.04.016.
16. Rao SSC et al. Gastroenterology. 2023 Jun. doi: 10.1053/j.gastro.2023.02.013.
17. Lacy BE et al. Am J Gastroenterol. 2021 Jan. doi: 10.14309/ajg.0000000000001036.
18. Anderson JL et al. J Cardiovasc Pharmacol Ther. 2009 Sep. doi: 10.1177/1074248409340158.
19. Rahimi R et al. World J Gastroenterol. 2009 Apr. doi: 10.3748/wjg.15.1548.
20. Rao SSC. Best Pract Res Clin Gastroenterol. 2011 Feb. doi: 10.1016/j.bpg.2011.01.004.
Introduction
Constipation affects one in six people worldwide and accounts for one third of outpatient visits.1 Chronic constipation is defined by difficult, infrequent, and/or incomplete defecation, quantified by less than three spontaneous bowel movements per week, persisting for at least 3 months. Patients may complain of straining during defecation, incomplete evacuation, hard stools (Bristol stool scale [BSS] type 1-2), and fullness or bloating. Chronic constipation can be subclassified as either a primary or secondary disorder.1,2
Primary Constipation Disorders
Primary constipation includes disorders of the colon or anorectum. This includes irritable bowel syndrome with constipation (IBS-C), chronic idiopathic constipation (CIC), slow transit constipation (STC), dyssynergic defecation, and pelvic floor disorders (see Figure 1).

IBS-C
IBS-C is a chronic disorder of the gut-brain axis with a worldwide prevalence of 1.3% and a prevalence of 6%-16% in the United States, United Kingdom, and Canada, with females more likely to seek care than males.2 The economic impact of IBS-C is estimated to be $1.5 billion–$10 billion per year in the United States alone.3 The distinguishing characteristic is abdominal pain, however IBS-C can present with a constellation of symptoms. The diagnostic paradigm has shifted from IBS being a diagnosis of exclusion to now using a positive diagnostic strategy.2 Using this Rome IV criteria, one can make the diagnosis with > 95% accuracy.2,4
CIC
CIC, previously defined as functional constipation, is a disorder defined by incomplete defecation and difficult or infrequent stool. CIC is diagnosed in patients without an underlying anatomic or structural abnormality. Rome IV Criteria helps further classify the defining characteristics of chronic idiopathic constipation.2
Slow Transit Constipation
STC is characterized by impaired colonic transit time in the absence of pelvic floor dysfunction. It presents with infrequent bowel movements, diminished urgency, and/or straining with defecation.
Defecatory Disorders: Dyssynergic Defecation and Pelvic Floor Dysfunction
Defecatory disorders (DDs) result from alterations in the colonic-neural pathway with an unclear pathogenesis. A firm understanding of colonic physiology is necessary to identify DDs. The right colon helps to store and mix stool contents, the left colon helps add water to the stool, and the anal canal and rectum enable defecation and maintain continence. Any alteration along this physiologic pathway results in DDs.5
DDs primarily develop via maladaptive pelvic floor contraction during defecation or from muscle or nerve injury and include functional outlet obstruction, anorectal dyssynergia, and pelvic floor dysfunction. Increased resistance to defecation results from anismus, paradoxical anal sphincter contraction, or incomplete relaxation of the pelvic floor and external anal sphincter. This muscle incoordination is described as dyssynergia. DDs can involve either muscle or nerve dysfunction or a combination of the two. Reduced rectal sensation caused by reduced sensory triggers can cause stasis of stool, thus propagating the cycle of constipation. Over time, excessive straining can weaken the pelvic floor, increasing the risk of excessive perineal descent, rectal intussusception, solitary rectal ulcer syndrome, and pudendal neuropathy.5 Thus, identification of DDs is crucial in patients with chronic constipation.
Secondary Constipation Disorders
Secondary constipation disorders are a result of an alternate process and warrant a thorough review of outpatient medications and past medical history. Figure 1 outlines the most common causes of secondary constipation, which span a wide differential.
Clinical Evaluation
The evaluation of constipation begins with a thorough history. Description of bowel habits should include frequency, duration, straining, stool consistency using a Bristol stool chart, complete vs incomplete evacuation, pain, bloating, and use of digital maneuvers (vaginal splinting or digital stool removal). One should inquire about back trauma/surgeries and obstetric history to include vaginal forceps injury or episiotomy.
With increased smartphone use, toilet time on average has increased and can contribute to maladaptive bowel habits.6 Patients may not realize they are constipated, so patient education is critical. A patient with daily bowel movements ranging between BSS type 1-6 with incomplete evacuation might complain of diarrhea but may in fact have constipation with overflow diarrhea, for example. Past medical history is also clinically relevant, as systemic conditions can cause secondary constipation. A constipated patient should also be asked what therapies he/she has tried prior to gastroenterology referral as primary care referrals for constipation account for 8 million visits to gastroenterology per year.7
While a sensitive topic, inquire about abuse history, especially in those with childhood constipation symptoms. There is a positive correlation between childhood constipation and physical, emotional, and sexual abuse and, for any number of reasons, your patient may be reluctant to share this or undergo a digital rectal exam (DRE).8 In such cases, be sensitive in asking for this history in private rather than with other family members around and always perform this exam with a chaperone present.
A detailed physical exam is an indispensable tool all gastroenterologists must master when evaluating a constipated patient. Some key exam findings include abdominal distention, high-pitched bowel sounds, and presence of a succussion splash indicating obstructive pathology. Dry skin and brittle hair indicate hypothyroidism while hypermobile joints and skin laxity suggest connective tissue disease. Finally, a physical examination is incomplete without a DRE.
DRE
DRE is an often-overlooked physical exam component which provides helpful insight that can guide management. An informed DRE can help identify structural disorders such as fissure, hemorrhoids, anorectal mass, fecal impaction, rectal prolapse, and excessive perineal descent syndrome.9 Unless contraindicated, DRE should be a standard part of the workup of a patient with chronic constipation.
Workup
Colonoscopy
The role of colonoscopy in chronic constipation is low yield and only indicated if alarm signs are present.2 When no organic causes can be identified, the patient is deemed to have a functional bowel or motility disorder leading to constipation.
Colonic Transit Time
Colonic transit time (CTT) can be evaluated by assessing the presence of radio-opaque sitz markers in the colon with an abdominal x-ray 5 days after ingestion. The presence of five or more sitz markers may indicate STC. However, this can also signal an obstructive defecatory disorder. Colon scintigraphy can determine whether there is diffuse colonic dysmotility or dysfunction in a specific segment of the colon.10
Anorectal Function Testing (AFT)
AFT can evaluate DDs, such as fecal incontinence, dyssynergic defecation, rectal sensory disorders, anorectal pain, and rectal prolapse. AFT comprises three tests: anorectal manometry (ARM), balloon expulsion test (BET), and rectal sensory testing. These assess the defecation, continence, and sensory mechanisms of the rectum, respectively.
ARM testing employs a thin, flexible probe with an attached sensor that is inserted into the rectum to measure internal and external sphincter pressures while at rest, squeezing, and bearing down to give a functional assessment of sphincter tone.11 Cough or party balloon test assesses continence and sphincter strength. Rectal sensation is assessed by inflating a balloon incrementally and asking the patient to indicate first sensation, urgency to defecate, and discomfort. If both ARM and BET are abnormal, the patient meets diagnostic criteria for dyssynergic defecation.12
Pelvic floor disorders can be further assessed by MR defecography or barium defecography. Barium defecography is the more widely available of the two. MR defecography is a dynamic study that directly assesses pelvic floor muscles and endopelvic fascia during various stages of defecation and considered superior. This testing modality can distinguish between functional causes such as dyssynergia or pelvic floor dysfunction and structural causes of obstruction such as rectocele, rectal prolapse, or rectal intussusception. MR defecography is helpful when dyssynergia is suggested by ARM with a normal BET or if there is an absent recto-anal inhibitory reflex on ARM, which may suggest rectal intussusception.
Management
CIC
Incorporating 20-30 g of total soluble fiber, such as psyllium in individuals with low dietary fiber intake is the first-line recommendation for CIC.13 If response to a trial of fiber supplementation is inadequate, over-the-counter (OTC) osmotic laxatives such as polyethylene glycol and magnesium oxide can be incorporated. In the event of failure of OTC osmotic laxatives, lactulose can be considered. Stimulant laxatives such as senna, bisacodyl, or sodium picosulfate can be added as an adjunctive measure for short periods of time, defined as daily for 4 weeks or less.
If these measures are inadequate, pharmacological therapy with secretagogues and 5HT agonists can be considered. Prucalopride, a selective agonist of serotonin 5-HT4 receptors, is approved for CIC, prescribed 2 mg daily.14 It can also be used in patients with global motility delays, such as gastroparetics with constipation. The mechanism of action of secretagogues and specific dosing of these medications are discussed in Figure 2.15 Vibrant is a non-pharmacologic, orally ingested, vibrating, and programmable capsule device that has recently received Food and Drug Administration approval for treatment of chronic constipation by stimulating the intestinal wall, thereby promoting colonic contractile activity to achieve more spontaneous bowel movements. Further studies are required to assess its efficacy.16 Additionally, if there is inadequate response to all the above, it would be prudent to evaluate for the presence of pelvic floor dysfunction as well.

IBS-C
Similar to CIC, treatment for mild IBS-C starts with osmotic laxatives with the additional component of pain control. Antispasmodics can be used to manage the abdominal pain, cramping, and spasms associated with IBS-C. Antispasmodics available in the United States include anticholinergic agents that cause smooth muscle relaxation, such as dicyclomine or hyoscyamine or direct smooth muscle relaxants such as peppermint oil.17 IBS-C patients with moderate symptoms may need escalation of therapy to secretagogues or 5HT agonists (see Figure 2). Secretagogues increase fluid retention in the colonic lumen to promote bowel movements and improve visceral hypersensitivity. Lubiprostone is an intestinal chloride channel activator, indicated only for adult women with IBS-C. Linaclotide and plecanatide are guanylate cyclase-C activators which increase intestinal chloride and bicarbonate secretion, and both are indicated in IBS-C and CIC. Tenapanor inhibits the sodium/hydrogen exchanger in the GI tract, leading to increased water secretion, and is recommended for IBS-C in adults who have failed secretagogues.
All four of these drugs can be considered for moderate to severe IBS-C symptoms. In the case of severe IBS-C symptoms, Tegaserod, a 5-HT4 receptor partial agonist has been approved in women under 65 without significant cardiovascular or cerebrovascular disease.18 Regardless of IBS-C symptom severity, persistent visceral hypersensitivity can be treated with low-dose neuromodulators.19 Figure 2 provides treatment recommendations for IBS-C based on symptom severity.
Opioid-Induced Constipation (OIC)
In patients with OIC, peripherally acting mu-opioid receptor antagonists such as methylnaltrexone and naloxegol can be beneficial where stimulant laxatives are insufficient. Additionally, lubiprostone is indicated in OIC in non-cancer patients. At present, there are no head-to-head trials comparing efficacy of these medications.
Defecatory Disorders
Biofeedback therapy is the cornerstone of treatment for dyssynergic defecation, focusing on neuromuscular training to restore a normal pattern of defecation by teaching patients to tense the abdomen and relax the pelvic floor muscles and anal sphincter. It retrains the body to coordinate abdominal, rectal, and anal muscles to achieve synchronous contraction to achieve complete evacuation. It also increases awareness and response to rectal fullness or the need to defecate.
Biofeedback makes patients aware of counterproductive subconscious actions such as contracting of their anal sphincter during defecation followed by simulated defecation training with focus on how to tighten abdominal muscles and relax pelvic floor muscles to initiate and complete defecation.20 This is performed in the office with a physiotherapist or trained nurse for at least six sessions or at home where patients are encouraged to perform the exercises for 20 minutes, twice a day. These sessions utilize tools such as manometry probes, electromyography probes, simulated balloon, or home biofeedback training devices to provide visual feedback while practicing abdominophrenic breathing. Biofeedback is particularly helpful in patients suffering from constipation. Patients with defecatory disorders can also benefit from pelvic floor physical therapy which focuses on strengthening the pelvic and puborectal muscles, external anal sphincter, and pelvic muscles. This is more useful in patients with fecal incontinence. Despite all these treatments, a subset of patients may still not respond and may qualify for surgical evaluation.
Conclusion
While constipation is seldom life-threatening, it has a negative impact on patient quality of life and poses a significant financial burden on our overall healthcare system. The complexity of this condition should be appreciated and understood in order for a complete and thorough evaluation. We trust that our practical guide should serve as a useful tool in the evaluation of a chronically constipated patient.
Dr. Salim (@hamsalim07 on X) is based in the Department of Internal Medicine, University of Texas Medical Branch, Galveston. Dr. Chowdhury (annicho.med on Instagram) is a fellow in the Department of Gastroenterology, Hepatology, and Nutrition, University of Texas MD Anderson Cancer Center, Houston. Dr. Viswanathan (@LavanyaMD on X) is Associate Professor, University of Texas MD Anderson Cancer Center. The authors declare no conflict of interest.
References
1. Mugie S et al. Best Pract Res Clin Gastroenterol. 2011 Feb. doi: 10.1016/j.bpg.2010.12.010.
2. Almario CV et al. Gastroenterology. 2023 Dec. doi: 10.1053/j.gastro.2023.08.010.
3. Canavan C et al. Clin Epidemiol. 2014 Feb. doi: 10.2147/CLEP.S40245.
4. Rao SSC. Gastroenterol Clin North Am. 2007 Sep. doi: 10.1016/j.gtc.2007.07.013.
5. Bharucha AE et al. Gastroenterology. 2020 Apr. doi: 10.1053/j.gastro.2019.12.034.
6. Cinquetti M et al. Clin Exp Pediatr. 2021 Sep. doi: 10.3345/cep.2020.01326.
7. Shah ND et al. Am J Gastroenterol. 2008 Jul. doi: 10.1111/j.1572-0241.2008.01910.x.
8. Rajindrajith S et al. J Pediatr Gastroenterol Nutr. 2014 Apr. doi: 10.1097/MPG.0000000000000249.
9. Talley NJ. Am J Gastroenterol. 2008 Apr. doi: 10.1111/j.1572-0241.2008.01832.x.
10. Maurer AH. J Nucl Med. 2015 Sep. doi: 10.2967/jnumed.113.134551.
11. Frye J et al. Am J Gastroenterol. 2024 Aug. doi: 10.14309/ajg.0000000000002670.
12. Rao SSC et al. J Neurogastroenterol Motil. 2016 Jun. doi: 10.5056/jnm16060.
13. Chang L et al. Gastroenterology. 2023 Jun. doi: 10.1053/j.gastro.2023.03.214.
14. Brenner DM et al. Am J Gastroenterol. 2021 Aug. doi: 10.14309/ajg.0000000000001266.
15. Chang L et al. Gastroenterology. 2022 Jul. doi: 10.1053/j.gastro.2022.04.016.
16. Rao SSC et al. Gastroenterology. 2023 Jun. doi: 10.1053/j.gastro.2023.02.013.
17. Lacy BE et al. Am J Gastroenterol. 2021 Jan. doi: 10.14309/ajg.0000000000001036.
18. Anderson JL et al. J Cardiovasc Pharmacol Ther. 2009 Sep. doi: 10.1177/1074248409340158.
19. Rahimi R et al. World J Gastroenterol. 2009 Apr. doi: 10.3748/wjg.15.1548.
20. Rao SSC. Best Pract Res Clin Gastroenterol. 2011 Feb. doi: 10.1016/j.bpg.2011.01.004.
Introduction
Constipation affects one in six people worldwide and accounts for one third of outpatient visits.1 Chronic constipation is defined by difficult, infrequent, and/or incomplete defecation, quantified by less than three spontaneous bowel movements per week, persisting for at least 3 months. Patients may complain of straining during defecation, incomplete evacuation, hard stools (Bristol stool scale [BSS] type 1-2), and fullness or bloating. Chronic constipation can be subclassified as either a primary or secondary disorder.1,2
Primary Constipation Disorders
Primary constipation includes disorders of the colon or anorectum. This includes irritable bowel syndrome with constipation (IBS-C), chronic idiopathic constipation (CIC), slow transit constipation (STC), dyssynergic defecation, and pelvic floor disorders (see Figure 1).

IBS-C
IBS-C is a chronic disorder of the gut-brain axis with a worldwide prevalence of 1.3% and a prevalence of 6%-16% in the United States, United Kingdom, and Canada, with females more likely to seek care than males.2 The economic impact of IBS-C is estimated to be $1.5 billion–$10 billion per year in the United States alone.3 The distinguishing characteristic is abdominal pain, however IBS-C can present with a constellation of symptoms. The diagnostic paradigm has shifted from IBS being a diagnosis of exclusion to now using a positive diagnostic strategy.2 Using this Rome IV criteria, one can make the diagnosis with > 95% accuracy.2,4
CIC
CIC, previously defined as functional constipation, is a disorder defined by incomplete defecation and difficult or infrequent stool. CIC is diagnosed in patients without an underlying anatomic or structural abnormality. Rome IV Criteria helps further classify the defining characteristics of chronic idiopathic constipation.2
Slow Transit Constipation
STC is characterized by impaired colonic transit time in the absence of pelvic floor dysfunction. It presents with infrequent bowel movements, diminished urgency, and/or straining with defecation.
Defecatory Disorders: Dyssynergic Defecation and Pelvic Floor Dysfunction
Defecatory disorders (DDs) result from alterations in the colonic-neural pathway with an unclear pathogenesis. A firm understanding of colonic physiology is necessary to identify DDs. The right colon helps to store and mix stool contents, the left colon helps add water to the stool, and the anal canal and rectum enable defecation and maintain continence. Any alteration along this physiologic pathway results in DDs.5
DDs primarily develop via maladaptive pelvic floor contraction during defecation or from muscle or nerve injury and include functional outlet obstruction, anorectal dyssynergia, and pelvic floor dysfunction. Increased resistance to defecation results from anismus, paradoxical anal sphincter contraction, or incomplete relaxation of the pelvic floor and external anal sphincter. This muscle incoordination is described as dyssynergia. DDs can involve either muscle or nerve dysfunction or a combination of the two. Reduced rectal sensation caused by reduced sensory triggers can cause stasis of stool, thus propagating the cycle of constipation. Over time, excessive straining can weaken the pelvic floor, increasing the risk of excessive perineal descent, rectal intussusception, solitary rectal ulcer syndrome, and pudendal neuropathy.5 Thus, identification of DDs is crucial in patients with chronic constipation.
Secondary Constipation Disorders
Secondary constipation disorders are a result of an alternate process and warrant a thorough review of outpatient medications and past medical history. Figure 1 outlines the most common causes of secondary constipation, which span a wide differential.
Clinical Evaluation
The evaluation of constipation begins with a thorough history. Description of bowel habits should include frequency, duration, straining, stool consistency using a Bristol stool chart, complete vs incomplete evacuation, pain, bloating, and use of digital maneuvers (vaginal splinting or digital stool removal). One should inquire about back trauma/surgeries and obstetric history to include vaginal forceps injury or episiotomy.
With increased smartphone use, toilet time on average has increased and can contribute to maladaptive bowel habits.6 Patients may not realize they are constipated, so patient education is critical. A patient with daily bowel movements ranging between BSS type 1-6 with incomplete evacuation might complain of diarrhea but may in fact have constipation with overflow diarrhea, for example. Past medical history is also clinically relevant, as systemic conditions can cause secondary constipation. A constipated patient should also be asked what therapies he/she has tried prior to gastroenterology referral as primary care referrals for constipation account for 8 million visits to gastroenterology per year.7
While a sensitive topic, inquire about abuse history, especially in those with childhood constipation symptoms. There is a positive correlation between childhood constipation and physical, emotional, and sexual abuse and, for any number of reasons, your patient may be reluctant to share this or undergo a digital rectal exam (DRE).8 In such cases, be sensitive in asking for this history in private rather than with other family members around and always perform this exam with a chaperone present.
A detailed physical exam is an indispensable tool all gastroenterologists must master when evaluating a constipated patient. Some key exam findings include abdominal distention, high-pitched bowel sounds, and presence of a succussion splash indicating obstructive pathology. Dry skin and brittle hair indicate hypothyroidism while hypermobile joints and skin laxity suggest connective tissue disease. Finally, a physical examination is incomplete without a DRE.
DRE
DRE is an often-overlooked physical exam component which provides helpful insight that can guide management. An informed DRE can help identify structural disorders such as fissure, hemorrhoids, anorectal mass, fecal impaction, rectal prolapse, and excessive perineal descent syndrome.9 Unless contraindicated, DRE should be a standard part of the workup of a patient with chronic constipation.
Workup
Colonoscopy
The role of colonoscopy in chronic constipation is low yield and only indicated if alarm signs are present.2 When no organic causes can be identified, the patient is deemed to have a functional bowel or motility disorder leading to constipation.
Colonic Transit Time
Colonic transit time (CTT) can be evaluated by assessing the presence of radio-opaque sitz markers in the colon with an abdominal x-ray 5 days after ingestion. The presence of five or more sitz markers may indicate STC. However, this can also signal an obstructive defecatory disorder. Colon scintigraphy can determine whether there is diffuse colonic dysmotility or dysfunction in a specific segment of the colon.10
Anorectal Function Testing (AFT)
AFT can evaluate DDs, such as fecal incontinence, dyssynergic defecation, rectal sensory disorders, anorectal pain, and rectal prolapse. AFT comprises three tests: anorectal manometry (ARM), balloon expulsion test (BET), and rectal sensory testing. These assess the defecation, continence, and sensory mechanisms of the rectum, respectively.
ARM testing employs a thin, flexible probe with an attached sensor that is inserted into the rectum to measure internal and external sphincter pressures while at rest, squeezing, and bearing down to give a functional assessment of sphincter tone.11 Cough or party balloon test assesses continence and sphincter strength. Rectal sensation is assessed by inflating a balloon incrementally and asking the patient to indicate first sensation, urgency to defecate, and discomfort. If both ARM and BET are abnormal, the patient meets diagnostic criteria for dyssynergic defecation.12
Pelvic floor disorders can be further assessed by MR defecography or barium defecography. Barium defecography is the more widely available of the two. MR defecography is a dynamic study that directly assesses pelvic floor muscles and endopelvic fascia during various stages of defecation and considered superior. This testing modality can distinguish between functional causes such as dyssynergia or pelvic floor dysfunction and structural causes of obstruction such as rectocele, rectal prolapse, or rectal intussusception. MR defecography is helpful when dyssynergia is suggested by ARM with a normal BET or if there is an absent recto-anal inhibitory reflex on ARM, which may suggest rectal intussusception.
Management
CIC
Incorporating 20-30 g of total soluble fiber, such as psyllium in individuals with low dietary fiber intake is the first-line recommendation for CIC.13 If response to a trial of fiber supplementation is inadequate, over-the-counter (OTC) osmotic laxatives such as polyethylene glycol and magnesium oxide can be incorporated. In the event of failure of OTC osmotic laxatives, lactulose can be considered. Stimulant laxatives such as senna, bisacodyl, or sodium picosulfate can be added as an adjunctive measure for short periods of time, defined as daily for 4 weeks or less.
If these measures are inadequate, pharmacological therapy with secretagogues and 5HT agonists can be considered. Prucalopride, a selective agonist of serotonin 5-HT4 receptors, is approved for CIC, prescribed 2 mg daily.14 It can also be used in patients with global motility delays, such as gastroparetics with constipation. The mechanism of action of secretagogues and specific dosing of these medications are discussed in Figure 2.15 Vibrant is a non-pharmacologic, orally ingested, vibrating, and programmable capsule device that has recently received Food and Drug Administration approval for treatment of chronic constipation by stimulating the intestinal wall, thereby promoting colonic contractile activity to achieve more spontaneous bowel movements. Further studies are required to assess its efficacy.16 Additionally, if there is inadequate response to all the above, it would be prudent to evaluate for the presence of pelvic floor dysfunction as well.

IBS-C
Similar to CIC, treatment for mild IBS-C starts with osmotic laxatives with the additional component of pain control. Antispasmodics can be used to manage the abdominal pain, cramping, and spasms associated with IBS-C. Antispasmodics available in the United States include anticholinergic agents that cause smooth muscle relaxation, such as dicyclomine or hyoscyamine or direct smooth muscle relaxants such as peppermint oil.17 IBS-C patients with moderate symptoms may need escalation of therapy to secretagogues or 5HT agonists (see Figure 2). Secretagogues increase fluid retention in the colonic lumen to promote bowel movements and improve visceral hypersensitivity. Lubiprostone is an intestinal chloride channel activator, indicated only for adult women with IBS-C. Linaclotide and plecanatide are guanylate cyclase-C activators which increase intestinal chloride and bicarbonate secretion, and both are indicated in IBS-C and CIC. Tenapanor inhibits the sodium/hydrogen exchanger in the GI tract, leading to increased water secretion, and is recommended for IBS-C in adults who have failed secretagogues.
All four of these drugs can be considered for moderate to severe IBS-C symptoms. In the case of severe IBS-C symptoms, Tegaserod, a 5-HT4 receptor partial agonist has been approved in women under 65 without significant cardiovascular or cerebrovascular disease.18 Regardless of IBS-C symptom severity, persistent visceral hypersensitivity can be treated with low-dose neuromodulators.19 Figure 2 provides treatment recommendations for IBS-C based on symptom severity.
Opioid-Induced Constipation (OIC)
In patients with OIC, peripherally acting mu-opioid receptor antagonists such as methylnaltrexone and naloxegol can be beneficial where stimulant laxatives are insufficient. Additionally, lubiprostone is indicated in OIC in non-cancer patients. At present, there are no head-to-head trials comparing efficacy of these medications.
Defecatory Disorders
Biofeedback therapy is the cornerstone of treatment for dyssynergic defecation, focusing on neuromuscular training to restore a normal pattern of defecation by teaching patients to tense the abdomen and relax the pelvic floor muscles and anal sphincter. It retrains the body to coordinate abdominal, rectal, and anal muscles to achieve synchronous contraction to achieve complete evacuation. It also increases awareness and response to rectal fullness or the need to defecate.
Biofeedback makes patients aware of counterproductive subconscious actions such as contracting of their anal sphincter during defecation followed by simulated defecation training with focus on how to tighten abdominal muscles and relax pelvic floor muscles to initiate and complete defecation.20 This is performed in the office with a physiotherapist or trained nurse for at least six sessions or at home where patients are encouraged to perform the exercises for 20 minutes, twice a day. These sessions utilize tools such as manometry probes, electromyography probes, simulated balloon, or home biofeedback training devices to provide visual feedback while practicing abdominophrenic breathing. Biofeedback is particularly helpful in patients suffering from constipation. Patients with defecatory disorders can also benefit from pelvic floor physical therapy which focuses on strengthening the pelvic and puborectal muscles, external anal sphincter, and pelvic muscles. This is more useful in patients with fecal incontinence. Despite all these treatments, a subset of patients may still not respond and may qualify for surgical evaluation.
Conclusion
While constipation is seldom life-threatening, it has a negative impact on patient quality of life and poses a significant financial burden on our overall healthcare system. The complexity of this condition should be appreciated and understood in order for a complete and thorough evaluation. We trust that our practical guide should serve as a useful tool in the evaluation of a chronically constipated patient.
Dr. Salim (@hamsalim07 on X) is based in the Department of Internal Medicine, University of Texas Medical Branch, Galveston. Dr. Chowdhury (annicho.med on Instagram) is a fellow in the Department of Gastroenterology, Hepatology, and Nutrition, University of Texas MD Anderson Cancer Center, Houston. Dr. Viswanathan (@LavanyaMD on X) is Associate Professor, University of Texas MD Anderson Cancer Center. The authors declare no conflict of interest.
References
1. Mugie S et al. Best Pract Res Clin Gastroenterol. 2011 Feb. doi: 10.1016/j.bpg.2010.12.010.
2. Almario CV et al. Gastroenterology. 2023 Dec. doi: 10.1053/j.gastro.2023.08.010.
3. Canavan C et al. Clin Epidemiol. 2014 Feb. doi: 10.2147/CLEP.S40245.
4. Rao SSC. Gastroenterol Clin North Am. 2007 Sep. doi: 10.1016/j.gtc.2007.07.013.
5. Bharucha AE et al. Gastroenterology. 2020 Apr. doi: 10.1053/j.gastro.2019.12.034.
6. Cinquetti M et al. Clin Exp Pediatr. 2021 Sep. doi: 10.3345/cep.2020.01326.
7. Shah ND et al. Am J Gastroenterol. 2008 Jul. doi: 10.1111/j.1572-0241.2008.01910.x.
8. Rajindrajith S et al. J Pediatr Gastroenterol Nutr. 2014 Apr. doi: 10.1097/MPG.0000000000000249.
9. Talley NJ. Am J Gastroenterol. 2008 Apr. doi: 10.1111/j.1572-0241.2008.01832.x.
10. Maurer AH. J Nucl Med. 2015 Sep. doi: 10.2967/jnumed.113.134551.
11. Frye J et al. Am J Gastroenterol. 2024 Aug. doi: 10.14309/ajg.0000000000002670.
12. Rao SSC et al. J Neurogastroenterol Motil. 2016 Jun. doi: 10.5056/jnm16060.
13. Chang L et al. Gastroenterology. 2023 Jun. doi: 10.1053/j.gastro.2023.03.214.
14. Brenner DM et al. Am J Gastroenterol. 2021 Aug. doi: 10.14309/ajg.0000000000001266.
15. Chang L et al. Gastroenterology. 2022 Jul. doi: 10.1053/j.gastro.2022.04.016.
16. Rao SSC et al. Gastroenterology. 2023 Jun. doi: 10.1053/j.gastro.2023.02.013.
17. Lacy BE et al. Am J Gastroenterol. 2021 Jan. doi: 10.14309/ajg.0000000000001036.
18. Anderson JL et al. J Cardiovasc Pharmacol Ther. 2009 Sep. doi: 10.1177/1074248409340158.
19. Rahimi R et al. World J Gastroenterol. 2009 Apr. doi: 10.3748/wjg.15.1548.
20. Rao SSC. Best Pract Res Clin Gastroenterol. 2011 Feb. doi: 10.1016/j.bpg.2011.01.004.
Medical, Endoscopic, and Surgical Management of Gastroesophageal Reflux Disease
Introduction
Gastroesophageal reflux disease (GERD) is a frequently encountered condition, and rising annually.1 A recent meta-analysis suggests nearly 14% (1.03 billion) of the population are affected worldwide. Differences may range by region from 12% in Latin America to 20% in North America, and by country from 4% in China to 23% in Turkey.1 In the United States, 21% of the population are afflicted with weekly GERD symptoms.2 Novel medical therapies and endoscopic options provide clinicians with opportunities to help patients with GERD.3
Diagnosis
Definition
GERD was originally defined by the Montreal consensus as a condition that develops when the reflux of stomach contents causes troublesome symptoms and/or complications.4 Heartburn and regurgitation are common symptoms of GERD, with a sensitivity of 30%-76% and specificity of 62%-96% for erosive esophagitis (EE), which occurs when the reflux of stomach content causes esophageal mucosal breaks.5 The presence of characteristic mucosal injury observed during an upper endoscopy or abnormal esophageal acid exposure on ambulatory reflux monitoring are objective evidence of GERD. A trial of a proton pump inhibitor (PPI) may function as a diagnostic test for patients exhibiting the typical symptoms of GERD without any alarm symptoms.3,6
Endoscopic Evaluation and Confirmation
The 2022 American Gastroenterological Association (AGA) clinical practice update recommends diagnostic endoscopy, after PPIs are stopped for 2-4 weeks, in patients whose GERD symptoms do not respond adequately to an empiric trial of a PPI.3 Those with GERD and alarm symptoms such as dysphagia, weight loss, bleeding, and vomiting should undergo endoscopy as soon as possible. Endoscopic findings of EE (Los Angeles Grade B or more severe) and long-segment Barrett’s esophagus (> 3-cm segment with intestinal metaplasia on biopsy) are diagnostic of GERD.3
Reflux Monitoring
With ambulatory reflux monitoring (pH or impedance-pH), esophageal acid exposure (or neutral refluxate in impedance testing) can be measured to confirm GERD diagnosis and to correlate symptoms with reflux episodes. Patients with atypical GERD symptoms or patients with a confirmed diagnosis of GERD whose symptoms have not improved sufficiently with twice-daily PPI therapy should have esophageal impedance-pH monitoring while on PPIs.6,7
Esophageal Manometry
High-resolution esophageal manometry can be used to assess motility abnormalities associated with GERD.
Although no manometric abnormality is unique to GERD, weak lower esophageal sphincter (LES) resting pressure and ineffective esophageal motility frequently coexist with severe GERD.6
Manometry is particularly useful in patients considering surgical or endoscopic anti-reflux procedures to evaluate for achalasia,3 an important contraindication to surgery.
Medical Management
Management of GERD requires a multidisciplinary and personalized approach based on symptom presentation, body mass index, endoscopic findings (e.g., presence of EE, Barrett’s esophagus, hiatal hernia), and physiological abnormalities (e.g., gastroparesis or ineffective motility).3
Lifestyle Modifications
Recommended lifestyle modifications include weight loss for patients with obesity, stress reduction, tobacco and alcohol cessation, elevating the head of the bed, staying upright during and after meals, avoidance of food intake < 3 hours before bedtime, and cessation of foods that potentially aggravate reflux symptoms such as coffee, chocolate, carbonated beverages, spicy foods, acidic foods, and foods with high fat content.6,8
Medications
Pharmacologic therapy for GERD includes medications that primarily aim to neutralize or reduce gastric acid -- we summarize options in Table 1.3,8
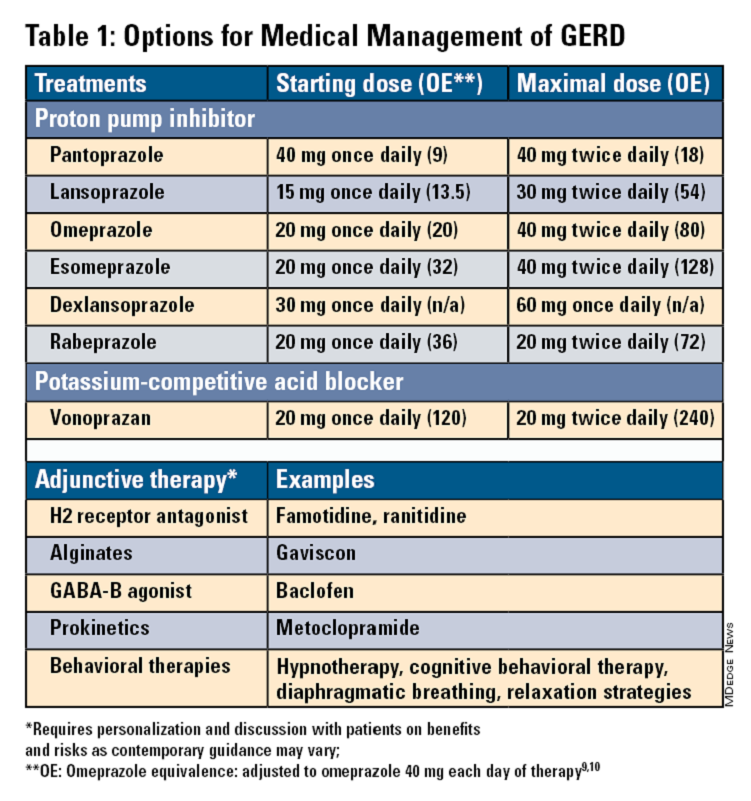
Proton Pump Inhibitors
Most guidelines suggest a trial of 4-8 weeks of once-daily enteric-coated PPI before meals in patients with typical GERD symptoms and no alarm symptoms. Escalation to double-dose PPI may be considered in the case of persistent symptoms. The relative potencies of standard-dose pantoprazole, lansoprazole, esomeprazole, and rabeprazole are presented in Table 1.9 When a PPI switch is needed, rabeprazole may be considered as it is a PPI that does not rely on CYP2C19 for primary metabolism.9
Acid suppression should be weaned down to the lowest effective dose or converted to H2RAs or other antacids once symptoms are sufficiently controlled unless patients have EE, Barrett’s esophagus, or peptic stricture.3 Patients with severe GERD may require long-term PPI therapy or an invasive anti-reflux procedure.
Recent studies have shown that potassium-competitive acid blockers (PCAB) like vonoprazan may offer more effective gastric acid inhibition. While not included in the latest clinical practice update, vonoprazan is thought to be superior to lansoprazole for those with LA Grade C/D esophagitis for both symptom relief and healing at 2 weeks.10
Adjunctive Therapies
Alginates can function as a physical barrier to even neutral reflux and may be helpful for patients with postprandial or nighttime symptoms as well as those with hiatal hernia.3 H2RAs can also help mitigate nighttime symptoms.3 Baclofen is a gamma-aminobutyric acid–B agonist which inhibits transient lower esophageal sphincter relaxation (TLESR) and may be effective for patients with belching.3 Prokinetics may be helpful for GERD with concomitant gastroparesis.3 Sucralfate is a mucosal protective agent, but there is a lack of data supporting its efficacy in GERD treatment. Consider referral to a behavioral therapist for supplemental therapies, hypnotherapy, cognitive-behavior therapy, diaphragmatic breathing, and relaxation strategies for functional heartburn or reflux-associated esophageal hypervigilance or reflux hypersensitivity.3
When to Refer to Higher Level of Care
For patients who do not wish to remain on longer-term pharmacologic therapy or would benefit from anatomic repair, clinicians should have a discussion of risks and benefits prior to consideration of referral for anti-reflux procedures.3,6,8 We advise this conversation should include review of patient health status, postsurgical side effects such as increased flatus, bloating and dysphagia as well as the potential need to still resume PPI post operation.8
Endoscopic Management
Patient Selection And Evaluation
For the groups indicated for a higher level of care, we agree with AGA recommendations, multi-society guidelines, and expert review,3,7,11,12 and highlight potential options in Table 2. Step-up options should be based on patient characteristics and reviewed carefully with patients. Endoscopic therapies are less invasive than surgery and may be considered for those who do not require anatomic repair of hiatal hernia, do not want surgery, or are not suitable for surgery.
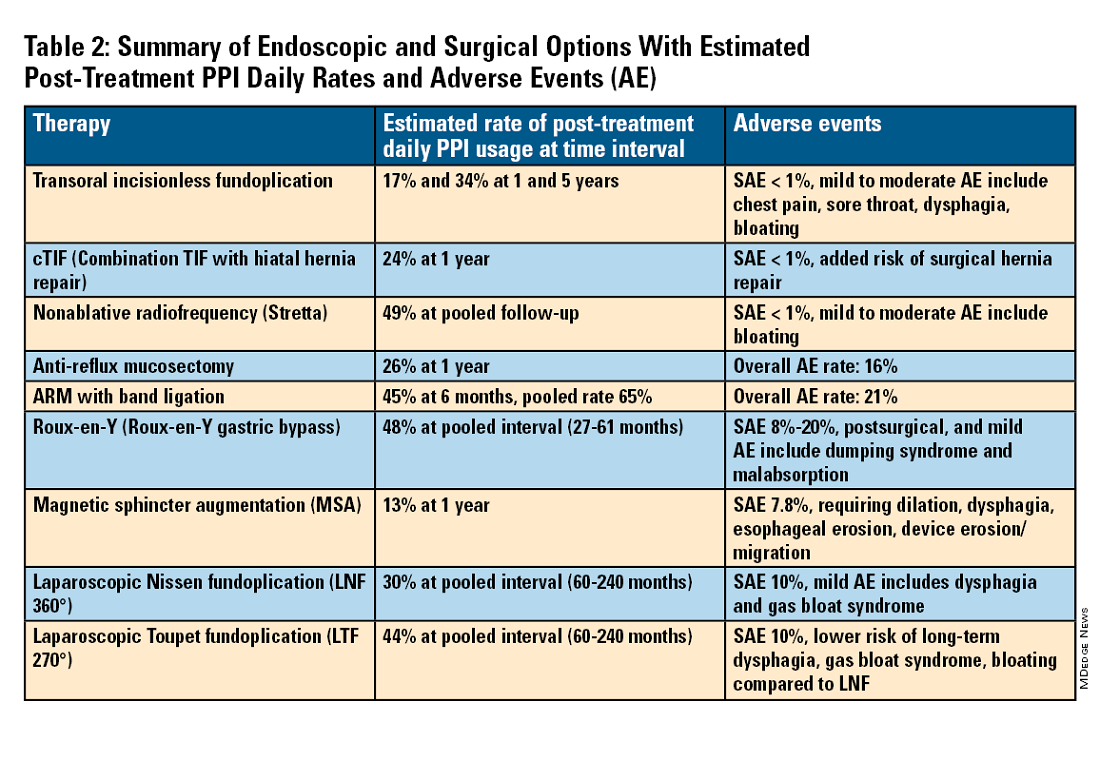
The pathophysiology of GERD is from a loss of the anti-reflux barrier of the esophageal gastric junction (EGJ) at the lower esophageal sphincter (LES) leading to unintended retrograde movement of gastric contents.6 Anatomically, the LES is composed of muscles of the distal esophagus and sling fibers of the proximal stomach, the “external valve” from the diaphragmatic crura, and the “internal valve” from the gastroesophageal flap valve (GEFV). GERD occurs from mechanical failure of the LES. First, there may be disproportional dilation of the diaphragmatic crura as categorized by Hill Grade of the GEFV as seen by a retroflexed view of EGJ after 30-45 seconds of insufflation.13 Second, there may be a migration of the LES away from the diaphragmatic crura as in the case of a hiatal hernia. Provocative maneuvers may reveal a sliding hernia by gentle retraction of the endoscope while under retroflexed view.13 Third, there may be more frequent TLESR associated with GERD.12
The aim of most interventions is to restore competency of the LES by reconstruction of the GEFV via suture or staple-based approximation of tissue.11,12 Intraluminal therapy may only target the GEFV at the internal valve. Therefore, most endoscopic interventions are limited to patients with intact diaphragmatic crura (ie, small to no hiatal hernia and GEFV Hill Grade 1 to 2). Contraindications for endoscopic therapy are moderate to severe reflux (ie, LA Grade C/ D), hiatus hernia 2 cm or larger, strictures, or long-segment Barrett’s esophagus.
Utility, Safety, and Outcomes of TIF
Historically, endoscopic therapy targeting endoscopic fundoplication started with EndoLuminal gastro-gastric fundoplication (ELF, 2005) which was a proof of concept of safe manipulation and suture for gastro-gastric plication to below the Z-line. Transoral incisionless fundoplication (TIF) 1.0 was suggested in 2007 for clinical application by proposing a longitudinal oriented esophago-gastric plication 1 cm above the Z-line.
In 2009, TIF2.0 was proposed as a rotational 270° wrap of the cardia and fundus to a full-thickness esophago-gastric fundoplication around 2-4 cm of the distal esophagus. Like a surgical fundoplication, this reinforces sling fibers, increases the Angle of His and improves the cardiac notch. TIF 2.0 is indicated for those with small (< 2 cm) or no hiatal hernia and a GEFV Hill Grade 1 or 2. The present iteration of TIF2.0 uses EsophyX-Z (EndoGastric Solutions; Redmond, Washington) which features dual fastener deployment and a simplified firing mechanism. Plication is secured via nonresorbable polypropylene T-fasteners with strength equivalence of 3-0 sutures.
Compared with the original, TIF2.0 represents a decrease of severe adverse events from 2%-2.5% to 0.4%-1%.11,14 Based on longitudinal TEMPO data, patient satisfaction ranges between 70% and 90% and rates of patients reverting to daily PPI use are 17% and 34% at 1 and 5 years. A 5% reintervention rate was noted to be comparable with surgical reoperation for fundoplication.15 One retrospective evaluation of patients with failed TIF followed by successful cTIF noted that in all failures there was a documented underestimation of a much larger crura defect at time of index procedure.16 Chest pain is common post procedure and patients and collaborating providers should be counseled on the expected course. In our practice, we admit patients for at least 1 postprocedure day and consider scheduling symptom control medications for those with significant pain.
TIF2.0 for Special Populations
Indications for TIF2.0 continue to evolve. In 2017, concomitant TIF2.0 with hiatal hernia repair (cTIF or HH-TIF) for hernia > 2 cm was accepted for expanded use. In one study, cTIF has been shown to have similar outcomes for postprocedural PPI use, dysphagia, wrap disruption, and hiatal hernia recurrence, compared with hiatal hernia repair paired with laparoscopic Nissen fundoplication with possibly shorter postadmission stay, serious adverse events, and bloating.17 A cTIF may be performed in a single general anesthetic session typically with a surgical hiatal hernia repair followed by TIF2.0.
Other Endoscopic Procedures
Several other endoscopic interventions have been proposed for GERD management. The following procedures are under continuous study and should be considered only by those with expertise.
Stretta
The Stretta device (Restech; Houston, Texas) was approved in 2000 for use of a radiofrequency (RF) generator and catheter applied to the squamocolumnar junction under irrigation. Ideal candidates for this nonablative procedure may include patients with confirmed GERD, low-grade EE, without Barrett’s esophagus, small hiatal hernia, and a competent LES with pressure > 5 mmHg. Meta-analysis has yielded conflicting results in terms of its efficacy, compared with TIF2.0, and recent multi-society guidance suggests fundoplication over Stretta.7
ARM, MASE, and RAP
Anti-reflux mucosectomy (ARM) has been proposed based on the observation that patients undergoing mucosectomy for neoplasms in the cardia had improvement of reflux symptoms.11,12 Systematic review has suggested a clinical response of 80% of either PPI discontinuation or reduction, but 17% of adverse events include development of strictures. Iterations of ARM continue to be studied including ARM with band ligation (L-ARM) and endoscopic submucosal dissection for GERD (ESD-G).12
Experts have proposed incorporating endoscopic suturing of the EGJ to modulate the LES. Mucosal ablation and suturing of the EG junction (MASE) has been proposed by first priming tissue via argon plasma coagulation (APC) prior to endoscopic overstitch of two to three interrupted sutures below the EGJ to narrow and elongate the EGJ. The resection and plication (RAP) procedure performs a mucosal resection prior to full-thickness plication of the LES and cardia.11,12 Expert opinion has suggested that RAP may be used in patients with altered anatomy whereas MASE may be used when resection is not possible (eg, prior scarring, resection or ablation).12
Surgical Management
We agree with a recent multi-society guideline recommending that an interdisciplinary consultation with surgery for indicated patients with refractory GERD and underlying hiatal hernia, or who do not want lifelong medical therapy.
Fundoplication creates a surgical wrap to reinforce the LES and may be performed laparoscopically. Contraindications include body mass index (BMI) >35 kg/m2 and significantly impaired dysmotility. Fundoplication of 180°, 270°, and 360° may achieve comparable outcomes, but a laparoscopic toupet fundoplication (LTF 270°) may have fewer postsurgical issues of dysphagia and bloating. Advantages for both anterior and posterior partial fundoplications have been demonstrated by network meta-analysis. Therefore, a multi-society guideline for GERD suggests partial over complete fundoplication.7 Compared with posterior techniques, anterior fundoplication (Watson fundoplication) led to more recurrent reflux symptoms but less dysphagia and other side effects.19
Magnetic sphincter augmentation (MSA) is a surgical option that strengthens the LES with magnets to improve sphincter competence. In addition to listed contraindications of fundoplication, patients with an allergy to nickel and/or titanium are also contraindicated to receive MSA.7 MSA has been suggested to be equivalent to LNF although there may be less gas bloat and greater ability to belch on follow up.20
Surgical Options for Special Populations
Patients with medically refractory GERD and a BMI ≥ 35 kg/m2 may benefit from either Roux-en-Y gastric bypass (RYGB) or fundoplication, however sleeve gastrectomy is not advised.7 In patients with BMI > 50 kg/m2, RYGB may provide an optimal choice. We agree with consultation with a bariatric surgeon when reviewing these situations.
Conclusion
Patients with GERD are commonly encountered worldwide. Empiric PPI are effective mainstays for medical treatment of GERD. Novel PCABs (e.g., vonoprazan) may present new options for GERD with LA Grade C/D esophagitis EE and merit more study. In refractory cases or for patients who do not want long term medical therapy, step-up therapy may be considered via endoscopic or surgical interventions. Patient anatomy and comorbidities should be considered by the clinician to inform treatment options. Surgery may have the most durable outcomes for those requiring step-up therapy. Improvements in technique, devices and patient selection have allowed TIF2.0 to grow as a viable offering with excellent 5-year outcomes for indicated patients.
Dr. Chang, Dr. Tintara, and Dr. Phan are based in the Division of Gastrointestinal and Liver Disease at the University of Southern California in Los Angeles. They have no conflicts of interest to declare.
References
1. Richter JE andRubenstein JH. Gastroenterology. 2018 Jan. doi: 10.1053/j.gastro.2017.07.045.
2. El-Serag HB et al. Gut. 2014 Jun. doi: 10.1136/gutjnl-2012-304269.
3. Yadlapati R et al. Clin Gastroenterol Hepatol. 2022 May. doi: 10.1016/j.cgh.2022.01.025.
4. Vakil N et al. Am J Gastroenterol. 2006 Aug. doi: 10.1111/j.1572-0241.2006.00630.x.
5. Numans ME et al. Ann Intern Med. 2004 Apr. doi: 10.7326/0003-4819-140-7-200404060-00011.
6. Kahrilas PJ et al. Gastroenterology. 2008 Oct. doi: 10.1053/j.gastro.2008.08.045.
7. Slater BJ et al. Surg Endosc. 2023 Feb. doi: 10.1007/s00464-022-09817-3.
8. Gyawali CP et al. Gut. 2018 Jul. doi:10.1136/gutjnl-2017-314722.
9. Graham DY and Tansel A. Clin Gastroenterol Hepatol. 2018 Jun. doi: 10.1016/j.cgh.2017.09.033.
10. Graham DY and Dore MP. Gastroenterology. 2018 Feb. doi:10.1053/j.gastro.2018.01.018.
11. Haseeb M and Thompson CC. Curr Opin Gastroenterol. 2023 Sep. doi: 10.1097/MOG.0000000000000968.
12. Kolb JM and Chang KJ. Curr Opin Gastroenterol. 2023 Jul. doi:10.1097/MOG.0000000000000944.
13. Nguyen NT et al. Foregut. 2022 Sep. doi: 10.1177/26345161221126961.
14. Mazzoleni G et al. Endosc Int Open. 2021 Feb. doi: 10.1055/a-1322-2209.
15. Trad KS et al. Surg Innov. 2018 Apr. doi: 10.1177/1553350618755214.
16. Kolb JM et al. Gastroenterology. 2021 May. doi: 10.1016/S0016-5085(21)02953-X.
17. Jaruvongvanich VK et al. Endosc Int Open. 2023 Jan. doi: 10.1055/a-1972-9190.
18. Lee Y et al. Surg Endosc. 2023 Jul. doi: 10.1007/s00464-023-10151-5.
19. Andreou A et al. Surg Endosc. 2020 Feb. doi: 10.1007/s00464-019-07208-9.
20. Guidozzi N et al. Dis Esophagus. 2019 Nov. doi: 10.1093/dote/doz031.
Introduction
Gastroesophageal reflux disease (GERD) is a frequently encountered condition, and rising annually.1 A recent meta-analysis suggests nearly 14% (1.03 billion) of the population are affected worldwide. Differences may range by region from 12% in Latin America to 20% in North America, and by country from 4% in China to 23% in Turkey.1 In the United States, 21% of the population are afflicted with weekly GERD symptoms.2 Novel medical therapies and endoscopic options provide clinicians with opportunities to help patients with GERD.3
Diagnosis
Definition
GERD was originally defined by the Montreal consensus as a condition that develops when the reflux of stomach contents causes troublesome symptoms and/or complications.4 Heartburn and regurgitation are common symptoms of GERD, with a sensitivity of 30%-76% and specificity of 62%-96% for erosive esophagitis (EE), which occurs when the reflux of stomach content causes esophageal mucosal breaks.5 The presence of characteristic mucosal injury observed during an upper endoscopy or abnormal esophageal acid exposure on ambulatory reflux monitoring are objective evidence of GERD. A trial of a proton pump inhibitor (PPI) may function as a diagnostic test for patients exhibiting the typical symptoms of GERD without any alarm symptoms.3,6
Endoscopic Evaluation and Confirmation
The 2022 American Gastroenterological Association (AGA) clinical practice update recommends diagnostic endoscopy, after PPIs are stopped for 2-4 weeks, in patients whose GERD symptoms do not respond adequately to an empiric trial of a PPI.3 Those with GERD and alarm symptoms such as dysphagia, weight loss, bleeding, and vomiting should undergo endoscopy as soon as possible. Endoscopic findings of EE (Los Angeles Grade B or more severe) and long-segment Barrett’s esophagus (> 3-cm segment with intestinal metaplasia on biopsy) are diagnostic of GERD.3
Reflux Monitoring
With ambulatory reflux monitoring (pH or impedance-pH), esophageal acid exposure (or neutral refluxate in impedance testing) can be measured to confirm GERD diagnosis and to correlate symptoms with reflux episodes. Patients with atypical GERD symptoms or patients with a confirmed diagnosis of GERD whose symptoms have not improved sufficiently with twice-daily PPI therapy should have esophageal impedance-pH monitoring while on PPIs.6,7
Esophageal Manometry
High-resolution esophageal manometry can be used to assess motility abnormalities associated with GERD.
Although no manometric abnormality is unique to GERD, weak lower esophageal sphincter (LES) resting pressure and ineffective esophageal motility frequently coexist with severe GERD.6
Manometry is particularly useful in patients considering surgical or endoscopic anti-reflux procedures to evaluate for achalasia,3 an important contraindication to surgery.
Medical Management
Management of GERD requires a multidisciplinary and personalized approach based on symptom presentation, body mass index, endoscopic findings (e.g., presence of EE, Barrett’s esophagus, hiatal hernia), and physiological abnormalities (e.g., gastroparesis or ineffective motility).3
Lifestyle Modifications
Recommended lifestyle modifications include weight loss for patients with obesity, stress reduction, tobacco and alcohol cessation, elevating the head of the bed, staying upright during and after meals, avoidance of food intake < 3 hours before bedtime, and cessation of foods that potentially aggravate reflux symptoms such as coffee, chocolate, carbonated beverages, spicy foods, acidic foods, and foods with high fat content.6,8
Medications
Pharmacologic therapy for GERD includes medications that primarily aim to neutralize or reduce gastric acid -- we summarize options in Table 1.3,8

Proton Pump Inhibitors
Most guidelines suggest a trial of 4-8 weeks of once-daily enteric-coated PPI before meals in patients with typical GERD symptoms and no alarm symptoms. Escalation to double-dose PPI may be considered in the case of persistent symptoms. The relative potencies of standard-dose pantoprazole, lansoprazole, esomeprazole, and rabeprazole are presented in Table 1.9 When a PPI switch is needed, rabeprazole may be considered as it is a PPI that does not rely on CYP2C19 for primary metabolism.9
Acid suppression should be weaned down to the lowest effective dose or converted to H2RAs or other antacids once symptoms are sufficiently controlled unless patients have EE, Barrett’s esophagus, or peptic stricture.3 Patients with severe GERD may require long-term PPI therapy or an invasive anti-reflux procedure.
Recent studies have shown that potassium-competitive acid blockers (PCAB) like vonoprazan may offer more effective gastric acid inhibition. While not included in the latest clinical practice update, vonoprazan is thought to be superior to lansoprazole for those with LA Grade C/D esophagitis for both symptom relief and healing at 2 weeks.10
Adjunctive Therapies
Alginates can function as a physical barrier to even neutral reflux and may be helpful for patients with postprandial or nighttime symptoms as well as those with hiatal hernia.3 H2RAs can also help mitigate nighttime symptoms.3 Baclofen is a gamma-aminobutyric acid–B agonist which inhibits transient lower esophageal sphincter relaxation (TLESR) and may be effective for patients with belching.3 Prokinetics may be helpful for GERD with concomitant gastroparesis.3 Sucralfate is a mucosal protective agent, but there is a lack of data supporting its efficacy in GERD treatment. Consider referral to a behavioral therapist for supplemental therapies, hypnotherapy, cognitive-behavior therapy, diaphragmatic breathing, and relaxation strategies for functional heartburn or reflux-associated esophageal hypervigilance or reflux hypersensitivity.3
When to Refer to Higher Level of Care
For patients who do not wish to remain on longer-term pharmacologic therapy or would benefit from anatomic repair, clinicians should have a discussion of risks and benefits prior to consideration of referral for anti-reflux procedures.3,6,8 We advise this conversation should include review of patient health status, postsurgical side effects such as increased flatus, bloating and dysphagia as well as the potential need to still resume PPI post operation.8
Endoscopic Management
Patient Selection And Evaluation
For the groups indicated for a higher level of care, we agree with AGA recommendations, multi-society guidelines, and expert review,3,7,11,12 and highlight potential options in Table 2. Step-up options should be based on patient characteristics and reviewed carefully with patients. Endoscopic therapies are less invasive than surgery and may be considered for those who do not require anatomic repair of hiatal hernia, do not want surgery, or are not suitable for surgery.

The pathophysiology of GERD is from a loss of the anti-reflux barrier of the esophageal gastric junction (EGJ) at the lower esophageal sphincter (LES) leading to unintended retrograde movement of gastric contents.6 Anatomically, the LES is composed of muscles of the distal esophagus and sling fibers of the proximal stomach, the “external valve” from the diaphragmatic crura, and the “internal valve” from the gastroesophageal flap valve (GEFV). GERD occurs from mechanical failure of the LES. First, there may be disproportional dilation of the diaphragmatic crura as categorized by Hill Grade of the GEFV as seen by a retroflexed view of EGJ after 30-45 seconds of insufflation.13 Second, there may be a migration of the LES away from the diaphragmatic crura as in the case of a hiatal hernia. Provocative maneuvers may reveal a sliding hernia by gentle retraction of the endoscope while under retroflexed view.13 Third, there may be more frequent TLESR associated with GERD.12
The aim of most interventions is to restore competency of the LES by reconstruction of the GEFV via suture or staple-based approximation of tissue.11,12 Intraluminal therapy may only target the GEFV at the internal valve. Therefore, most endoscopic interventions are limited to patients with intact diaphragmatic crura (ie, small to no hiatal hernia and GEFV Hill Grade 1 to 2). Contraindications for endoscopic therapy are moderate to severe reflux (ie, LA Grade C/ D), hiatus hernia 2 cm or larger, strictures, or long-segment Barrett’s esophagus.
Utility, Safety, and Outcomes of TIF
Historically, endoscopic therapy targeting endoscopic fundoplication started with EndoLuminal gastro-gastric fundoplication (ELF, 2005) which was a proof of concept of safe manipulation and suture for gastro-gastric plication to below the Z-line. Transoral incisionless fundoplication (TIF) 1.0 was suggested in 2007 for clinical application by proposing a longitudinal oriented esophago-gastric plication 1 cm above the Z-line.
In 2009, TIF2.0 was proposed as a rotational 270° wrap of the cardia and fundus to a full-thickness esophago-gastric fundoplication around 2-4 cm of the distal esophagus. Like a surgical fundoplication, this reinforces sling fibers, increases the Angle of His and improves the cardiac notch. TIF 2.0 is indicated for those with small (< 2 cm) or no hiatal hernia and a GEFV Hill Grade 1 or 2. The present iteration of TIF2.0 uses EsophyX-Z (EndoGastric Solutions; Redmond, Washington) which features dual fastener deployment and a simplified firing mechanism. Plication is secured via nonresorbable polypropylene T-fasteners with strength equivalence of 3-0 sutures.
Compared with the original, TIF2.0 represents a decrease of severe adverse events from 2%-2.5% to 0.4%-1%.11,14 Based on longitudinal TEMPO data, patient satisfaction ranges between 70% and 90% and rates of patients reverting to daily PPI use are 17% and 34% at 1 and 5 years. A 5% reintervention rate was noted to be comparable with surgical reoperation for fundoplication.15 One retrospective evaluation of patients with failed TIF followed by successful cTIF noted that in all failures there was a documented underestimation of a much larger crura defect at time of index procedure.16 Chest pain is common post procedure and patients and collaborating providers should be counseled on the expected course. In our practice, we admit patients for at least 1 postprocedure day and consider scheduling symptom control medications for those with significant pain.
TIF2.0 for Special Populations
Indications for TIF2.0 continue to evolve. In 2017, concomitant TIF2.0 with hiatal hernia repair (cTIF or HH-TIF) for hernia > 2 cm was accepted for expanded use. In one study, cTIF has been shown to have similar outcomes for postprocedural PPI use, dysphagia, wrap disruption, and hiatal hernia recurrence, compared with hiatal hernia repair paired with laparoscopic Nissen fundoplication with possibly shorter postadmission stay, serious adverse events, and bloating.17 A cTIF may be performed in a single general anesthetic session typically with a surgical hiatal hernia repair followed by TIF2.0.
Other Endoscopic Procedures
Several other endoscopic interventions have been proposed for GERD management. The following procedures are under continuous study and should be considered only by those with expertise.
Stretta
The Stretta device (Restech; Houston, Texas) was approved in 2000 for use of a radiofrequency (RF) generator and catheter applied to the squamocolumnar junction under irrigation. Ideal candidates for this nonablative procedure may include patients with confirmed GERD, low-grade EE, without Barrett’s esophagus, small hiatal hernia, and a competent LES with pressure > 5 mmHg. Meta-analysis has yielded conflicting results in terms of its efficacy, compared with TIF2.0, and recent multi-society guidance suggests fundoplication over Stretta.7
ARM, MASE, and RAP
Anti-reflux mucosectomy (ARM) has been proposed based on the observation that patients undergoing mucosectomy for neoplasms in the cardia had improvement of reflux symptoms.11,12 Systematic review has suggested a clinical response of 80% of either PPI discontinuation or reduction, but 17% of adverse events include development of strictures. Iterations of ARM continue to be studied including ARM with band ligation (L-ARM) and endoscopic submucosal dissection for GERD (ESD-G).12
Experts have proposed incorporating endoscopic suturing of the EGJ to modulate the LES. Mucosal ablation and suturing of the EG junction (MASE) has been proposed by first priming tissue via argon plasma coagulation (APC) prior to endoscopic overstitch of two to three interrupted sutures below the EGJ to narrow and elongate the EGJ. The resection and plication (RAP) procedure performs a mucosal resection prior to full-thickness plication of the LES and cardia.11,12 Expert opinion has suggested that RAP may be used in patients with altered anatomy whereas MASE may be used when resection is not possible (eg, prior scarring, resection or ablation).12
Surgical Management
We agree with a recent multi-society guideline recommending that an interdisciplinary consultation with surgery for indicated patients with refractory GERD and underlying hiatal hernia, or who do not want lifelong medical therapy.
Fundoplication creates a surgical wrap to reinforce the LES and may be performed laparoscopically. Contraindications include body mass index (BMI) >35 kg/m2 and significantly impaired dysmotility. Fundoplication of 180°, 270°, and 360° may achieve comparable outcomes, but a laparoscopic toupet fundoplication (LTF 270°) may have fewer postsurgical issues of dysphagia and bloating. Advantages for both anterior and posterior partial fundoplications have been demonstrated by network meta-analysis. Therefore, a multi-society guideline for GERD suggests partial over complete fundoplication.7 Compared with posterior techniques, anterior fundoplication (Watson fundoplication) led to more recurrent reflux symptoms but less dysphagia and other side effects.19
Magnetic sphincter augmentation (MSA) is a surgical option that strengthens the LES with magnets to improve sphincter competence. In addition to listed contraindications of fundoplication, patients with an allergy to nickel and/or titanium are also contraindicated to receive MSA.7 MSA has been suggested to be equivalent to LNF although there may be less gas bloat and greater ability to belch on follow up.20
Surgical Options for Special Populations
Patients with medically refractory GERD and a BMI ≥ 35 kg/m2 may benefit from either Roux-en-Y gastric bypass (RYGB) or fundoplication, however sleeve gastrectomy is not advised.7 In patients with BMI > 50 kg/m2, RYGB may provide an optimal choice. We agree with consultation with a bariatric surgeon when reviewing these situations.
Conclusion
Patients with GERD are commonly encountered worldwide. Empiric PPI are effective mainstays for medical treatment of GERD. Novel PCABs (e.g., vonoprazan) may present new options for GERD with LA Grade C/D esophagitis EE and merit more study. In refractory cases or for patients who do not want long term medical therapy, step-up therapy may be considered via endoscopic or surgical interventions. Patient anatomy and comorbidities should be considered by the clinician to inform treatment options. Surgery may have the most durable outcomes for those requiring step-up therapy. Improvements in technique, devices and patient selection have allowed TIF2.0 to grow as a viable offering with excellent 5-year outcomes for indicated patients.
Dr. Chang, Dr. Tintara, and Dr. Phan are based in the Division of Gastrointestinal and Liver Disease at the University of Southern California in Los Angeles. They have no conflicts of interest to declare.
References
1. Richter JE andRubenstein JH. Gastroenterology. 2018 Jan. doi: 10.1053/j.gastro.2017.07.045.
2. El-Serag HB et al. Gut. 2014 Jun. doi: 10.1136/gutjnl-2012-304269.
3. Yadlapati R et al. Clin Gastroenterol Hepatol. 2022 May. doi: 10.1016/j.cgh.2022.01.025.
4. Vakil N et al. Am J Gastroenterol. 2006 Aug. doi: 10.1111/j.1572-0241.2006.00630.x.
5. Numans ME et al. Ann Intern Med. 2004 Apr. doi: 10.7326/0003-4819-140-7-200404060-00011.
6. Kahrilas PJ et al. Gastroenterology. 2008 Oct. doi: 10.1053/j.gastro.2008.08.045.
7. Slater BJ et al. Surg Endosc. 2023 Feb. doi: 10.1007/s00464-022-09817-3.
8. Gyawali CP et al. Gut. 2018 Jul. doi:10.1136/gutjnl-2017-314722.
9. Graham DY and Tansel A. Clin Gastroenterol Hepatol. 2018 Jun. doi: 10.1016/j.cgh.2017.09.033.
10. Graham DY and Dore MP. Gastroenterology. 2018 Feb. doi:10.1053/j.gastro.2018.01.018.
11. Haseeb M and Thompson CC. Curr Opin Gastroenterol. 2023 Sep. doi: 10.1097/MOG.0000000000000968.
12. Kolb JM and Chang KJ. Curr Opin Gastroenterol. 2023 Jul. doi:10.1097/MOG.0000000000000944.
13. Nguyen NT et al. Foregut. 2022 Sep. doi: 10.1177/26345161221126961.
14. Mazzoleni G et al. Endosc Int Open. 2021 Feb. doi: 10.1055/a-1322-2209.
15. Trad KS et al. Surg Innov. 2018 Apr. doi: 10.1177/1553350618755214.
16. Kolb JM et al. Gastroenterology. 2021 May. doi: 10.1016/S0016-5085(21)02953-X.
17. Jaruvongvanich VK et al. Endosc Int Open. 2023 Jan. doi: 10.1055/a-1972-9190.
18. Lee Y et al. Surg Endosc. 2023 Jul. doi: 10.1007/s00464-023-10151-5.
19. Andreou A et al. Surg Endosc. 2020 Feb. doi: 10.1007/s00464-019-07208-9.
20. Guidozzi N et al. Dis Esophagus. 2019 Nov. doi: 10.1093/dote/doz031.
Introduction
Gastroesophageal reflux disease (GERD) is a frequently encountered condition, and rising annually.1 A recent meta-analysis suggests nearly 14% (1.03 billion) of the population are affected worldwide. Differences may range by region from 12% in Latin America to 20% in North America, and by country from 4% in China to 23% in Turkey.1 In the United States, 21% of the population are afflicted with weekly GERD symptoms.2 Novel medical therapies and endoscopic options provide clinicians with opportunities to help patients with GERD.3
Diagnosis
Definition
GERD was originally defined by the Montreal consensus as a condition that develops when the reflux of stomach contents causes troublesome symptoms and/or complications.4 Heartburn and regurgitation are common symptoms of GERD, with a sensitivity of 30%-76% and specificity of 62%-96% for erosive esophagitis (EE), which occurs when the reflux of stomach content causes esophageal mucosal breaks.5 The presence of characteristic mucosal injury observed during an upper endoscopy or abnormal esophageal acid exposure on ambulatory reflux monitoring are objective evidence of GERD. A trial of a proton pump inhibitor (PPI) may function as a diagnostic test for patients exhibiting the typical symptoms of GERD without any alarm symptoms.3,6
Endoscopic Evaluation and Confirmation
The 2022 American Gastroenterological Association (AGA) clinical practice update recommends diagnostic endoscopy, after PPIs are stopped for 2-4 weeks, in patients whose GERD symptoms do not respond adequately to an empiric trial of a PPI.3 Those with GERD and alarm symptoms such as dysphagia, weight loss, bleeding, and vomiting should undergo endoscopy as soon as possible. Endoscopic findings of EE (Los Angeles Grade B or more severe) and long-segment Barrett’s esophagus (> 3-cm segment with intestinal metaplasia on biopsy) are diagnostic of GERD.3
Reflux Monitoring
With ambulatory reflux monitoring (pH or impedance-pH), esophageal acid exposure (or neutral refluxate in impedance testing) can be measured to confirm GERD diagnosis and to correlate symptoms with reflux episodes. Patients with atypical GERD symptoms or patients with a confirmed diagnosis of GERD whose symptoms have not improved sufficiently with twice-daily PPI therapy should have esophageal impedance-pH monitoring while on PPIs.6,7
Esophageal Manometry
High-resolution esophageal manometry can be used to assess motility abnormalities associated with GERD.
Although no manometric abnormality is unique to GERD, weak lower esophageal sphincter (LES) resting pressure and ineffective esophageal motility frequently coexist with severe GERD.6
Manometry is particularly useful in patients considering surgical or endoscopic anti-reflux procedures to evaluate for achalasia,3 an important contraindication to surgery.
Medical Management
Management of GERD requires a multidisciplinary and personalized approach based on symptom presentation, body mass index, endoscopic findings (e.g., presence of EE, Barrett’s esophagus, hiatal hernia), and physiological abnormalities (e.g., gastroparesis or ineffective motility).3
Lifestyle Modifications
Recommended lifestyle modifications include weight loss for patients with obesity, stress reduction, tobacco and alcohol cessation, elevating the head of the bed, staying upright during and after meals, avoidance of food intake < 3 hours before bedtime, and cessation of foods that potentially aggravate reflux symptoms such as coffee, chocolate, carbonated beverages, spicy foods, acidic foods, and foods with high fat content.6,8
Medications
Pharmacologic therapy for GERD includes medications that primarily aim to neutralize or reduce gastric acid -- we summarize options in Table 1.3,8

Proton Pump Inhibitors
Most guidelines suggest a trial of 4-8 weeks of once-daily enteric-coated PPI before meals in patients with typical GERD symptoms and no alarm symptoms. Escalation to double-dose PPI may be considered in the case of persistent symptoms. The relative potencies of standard-dose pantoprazole, lansoprazole, esomeprazole, and rabeprazole are presented in Table 1.9 When a PPI switch is needed, rabeprazole may be considered as it is a PPI that does not rely on CYP2C19 for primary metabolism.9
Acid suppression should be weaned down to the lowest effective dose or converted to H2RAs or other antacids once symptoms are sufficiently controlled unless patients have EE, Barrett’s esophagus, or peptic stricture.3 Patients with severe GERD may require long-term PPI therapy or an invasive anti-reflux procedure.
Recent studies have shown that potassium-competitive acid blockers (PCAB) like vonoprazan may offer more effective gastric acid inhibition. While not included in the latest clinical practice update, vonoprazan is thought to be superior to lansoprazole for those with LA Grade C/D esophagitis for both symptom relief and healing at 2 weeks.10
Adjunctive Therapies
Alginates can function as a physical barrier to even neutral reflux and may be helpful for patients with postprandial or nighttime symptoms as well as those with hiatal hernia.3 H2RAs can also help mitigate nighttime symptoms.3 Baclofen is a gamma-aminobutyric acid–B agonist which inhibits transient lower esophageal sphincter relaxation (TLESR) and may be effective for patients with belching.3 Prokinetics may be helpful for GERD with concomitant gastroparesis.3 Sucralfate is a mucosal protective agent, but there is a lack of data supporting its efficacy in GERD treatment. Consider referral to a behavioral therapist for supplemental therapies, hypnotherapy, cognitive-behavior therapy, diaphragmatic breathing, and relaxation strategies for functional heartburn or reflux-associated esophageal hypervigilance or reflux hypersensitivity.3
When to Refer to Higher Level of Care
For patients who do not wish to remain on longer-term pharmacologic therapy or would benefit from anatomic repair, clinicians should have a discussion of risks and benefits prior to consideration of referral for anti-reflux procedures.3,6,8 We advise this conversation should include review of patient health status, postsurgical side effects such as increased flatus, bloating and dysphagia as well as the potential need to still resume PPI post operation.8
Endoscopic Management
Patient Selection And Evaluation
For the groups indicated for a higher level of care, we agree with AGA recommendations, multi-society guidelines, and expert review,3,7,11,12 and highlight potential options in Table 2. Step-up options should be based on patient characteristics and reviewed carefully with patients. Endoscopic therapies are less invasive than surgery and may be considered for those who do not require anatomic repair of hiatal hernia, do not want surgery, or are not suitable for surgery.

The pathophysiology of GERD is from a loss of the anti-reflux barrier of the esophageal gastric junction (EGJ) at the lower esophageal sphincter (LES) leading to unintended retrograde movement of gastric contents.6 Anatomically, the LES is composed of muscles of the distal esophagus and sling fibers of the proximal stomach, the “external valve” from the diaphragmatic crura, and the “internal valve” from the gastroesophageal flap valve (GEFV). GERD occurs from mechanical failure of the LES. First, there may be disproportional dilation of the diaphragmatic crura as categorized by Hill Grade of the GEFV as seen by a retroflexed view of EGJ after 30-45 seconds of insufflation.13 Second, there may be a migration of the LES away from the diaphragmatic crura as in the case of a hiatal hernia. Provocative maneuvers may reveal a sliding hernia by gentle retraction of the endoscope while under retroflexed view.13 Third, there may be more frequent TLESR associated with GERD.12
The aim of most interventions is to restore competency of the LES by reconstruction of the GEFV via suture or staple-based approximation of tissue.11,12 Intraluminal therapy may only target the GEFV at the internal valve. Therefore, most endoscopic interventions are limited to patients with intact diaphragmatic crura (ie, small to no hiatal hernia and GEFV Hill Grade 1 to 2). Contraindications for endoscopic therapy are moderate to severe reflux (ie, LA Grade C/ D), hiatus hernia 2 cm or larger, strictures, or long-segment Barrett’s esophagus.
Utility, Safety, and Outcomes of TIF
Historically, endoscopic therapy targeting endoscopic fundoplication started with EndoLuminal gastro-gastric fundoplication (ELF, 2005) which was a proof of concept of safe manipulation and suture for gastro-gastric plication to below the Z-line. Transoral incisionless fundoplication (TIF) 1.0 was suggested in 2007 for clinical application by proposing a longitudinal oriented esophago-gastric plication 1 cm above the Z-line.
In 2009, TIF2.0 was proposed as a rotational 270° wrap of the cardia and fundus to a full-thickness esophago-gastric fundoplication around 2-4 cm of the distal esophagus. Like a surgical fundoplication, this reinforces sling fibers, increases the Angle of His and improves the cardiac notch. TIF 2.0 is indicated for those with small (< 2 cm) or no hiatal hernia and a GEFV Hill Grade 1 or 2. The present iteration of TIF2.0 uses EsophyX-Z (EndoGastric Solutions; Redmond, Washington) which features dual fastener deployment and a simplified firing mechanism. Plication is secured via nonresorbable polypropylene T-fasteners with strength equivalence of 3-0 sutures.
Compared with the original, TIF2.0 represents a decrease of severe adverse events from 2%-2.5% to 0.4%-1%.11,14 Based on longitudinal TEMPO data, patient satisfaction ranges between 70% and 90% and rates of patients reverting to daily PPI use are 17% and 34% at 1 and 5 years. A 5% reintervention rate was noted to be comparable with surgical reoperation for fundoplication.15 One retrospective evaluation of patients with failed TIF followed by successful cTIF noted that in all failures there was a documented underestimation of a much larger crura defect at time of index procedure.16 Chest pain is common post procedure and patients and collaborating providers should be counseled on the expected course. In our practice, we admit patients for at least 1 postprocedure day and consider scheduling symptom control medications for those with significant pain.
TIF2.0 for Special Populations
Indications for TIF2.0 continue to evolve. In 2017, concomitant TIF2.0 with hiatal hernia repair (cTIF or HH-TIF) for hernia > 2 cm was accepted for expanded use. In one study, cTIF has been shown to have similar outcomes for postprocedural PPI use, dysphagia, wrap disruption, and hiatal hernia recurrence, compared with hiatal hernia repair paired with laparoscopic Nissen fundoplication with possibly shorter postadmission stay, serious adverse events, and bloating.17 A cTIF may be performed in a single general anesthetic session typically with a surgical hiatal hernia repair followed by TIF2.0.
Other Endoscopic Procedures
Several other endoscopic interventions have been proposed for GERD management. The following procedures are under continuous study and should be considered only by those with expertise.
Stretta
The Stretta device (Restech; Houston, Texas) was approved in 2000 for use of a radiofrequency (RF) generator and catheter applied to the squamocolumnar junction under irrigation. Ideal candidates for this nonablative procedure may include patients with confirmed GERD, low-grade EE, without Barrett’s esophagus, small hiatal hernia, and a competent LES with pressure > 5 mmHg. Meta-analysis has yielded conflicting results in terms of its efficacy, compared with TIF2.0, and recent multi-society guidance suggests fundoplication over Stretta.7
ARM, MASE, and RAP
Anti-reflux mucosectomy (ARM) has been proposed based on the observation that patients undergoing mucosectomy for neoplasms in the cardia had improvement of reflux symptoms.11,12 Systematic review has suggested a clinical response of 80% of either PPI discontinuation or reduction, but 17% of adverse events include development of strictures. Iterations of ARM continue to be studied including ARM with band ligation (L-ARM) and endoscopic submucosal dissection for GERD (ESD-G).12
Experts have proposed incorporating endoscopic suturing of the EGJ to modulate the LES. Mucosal ablation and suturing of the EG junction (MASE) has been proposed by first priming tissue via argon plasma coagulation (APC) prior to endoscopic overstitch of two to three interrupted sutures below the EGJ to narrow and elongate the EGJ. The resection and plication (RAP) procedure performs a mucosal resection prior to full-thickness plication of the LES and cardia.11,12 Expert opinion has suggested that RAP may be used in patients with altered anatomy whereas MASE may be used when resection is not possible (eg, prior scarring, resection or ablation).12
Surgical Management
We agree with a recent multi-society guideline recommending that an interdisciplinary consultation with surgery for indicated patients with refractory GERD and underlying hiatal hernia, or who do not want lifelong medical therapy.
Fundoplication creates a surgical wrap to reinforce the LES and may be performed laparoscopically. Contraindications include body mass index (BMI) >35 kg/m2 and significantly impaired dysmotility. Fundoplication of 180°, 270°, and 360° may achieve comparable outcomes, but a laparoscopic toupet fundoplication (LTF 270°) may have fewer postsurgical issues of dysphagia and bloating. Advantages for both anterior and posterior partial fundoplications have been demonstrated by network meta-analysis. Therefore, a multi-society guideline for GERD suggests partial over complete fundoplication.7 Compared with posterior techniques, anterior fundoplication (Watson fundoplication) led to more recurrent reflux symptoms but less dysphagia and other side effects.19
Magnetic sphincter augmentation (MSA) is a surgical option that strengthens the LES with magnets to improve sphincter competence. In addition to listed contraindications of fundoplication, patients with an allergy to nickel and/or titanium are also contraindicated to receive MSA.7 MSA has been suggested to be equivalent to LNF although there may be less gas bloat and greater ability to belch on follow up.20
Surgical Options for Special Populations
Patients with medically refractory GERD and a BMI ≥ 35 kg/m2 may benefit from either Roux-en-Y gastric bypass (RYGB) or fundoplication, however sleeve gastrectomy is not advised.7 In patients with BMI > 50 kg/m2, RYGB may provide an optimal choice. We agree with consultation with a bariatric surgeon when reviewing these situations.
Conclusion
Patients with GERD are commonly encountered worldwide. Empiric PPI are effective mainstays for medical treatment of GERD. Novel PCABs (e.g., vonoprazan) may present new options for GERD with LA Grade C/D esophagitis EE and merit more study. In refractory cases or for patients who do not want long term medical therapy, step-up therapy may be considered via endoscopic or surgical interventions. Patient anatomy and comorbidities should be considered by the clinician to inform treatment options. Surgery may have the most durable outcomes for those requiring step-up therapy. Improvements in technique, devices and patient selection have allowed TIF2.0 to grow as a viable offering with excellent 5-year outcomes for indicated patients.
Dr. Chang, Dr. Tintara, and Dr. Phan are based in the Division of Gastrointestinal and Liver Disease at the University of Southern California in Los Angeles. They have no conflicts of interest to declare.
References
1. Richter JE andRubenstein JH. Gastroenterology. 2018 Jan. doi: 10.1053/j.gastro.2017.07.045.
2. El-Serag HB et al. Gut. 2014 Jun. doi: 10.1136/gutjnl-2012-304269.
3. Yadlapati R et al. Clin Gastroenterol Hepatol. 2022 May. doi: 10.1016/j.cgh.2022.01.025.
4. Vakil N et al. Am J Gastroenterol. 2006 Aug. doi: 10.1111/j.1572-0241.2006.00630.x.
5. Numans ME et al. Ann Intern Med. 2004 Apr. doi: 10.7326/0003-4819-140-7-200404060-00011.
6. Kahrilas PJ et al. Gastroenterology. 2008 Oct. doi: 10.1053/j.gastro.2008.08.045.
7. Slater BJ et al. Surg Endosc. 2023 Feb. doi: 10.1007/s00464-022-09817-3.
8. Gyawali CP et al. Gut. 2018 Jul. doi:10.1136/gutjnl-2017-314722.
9. Graham DY and Tansel A. Clin Gastroenterol Hepatol. 2018 Jun. doi: 10.1016/j.cgh.2017.09.033.
10. Graham DY and Dore MP. Gastroenterology. 2018 Feb. doi:10.1053/j.gastro.2018.01.018.
11. Haseeb M and Thompson CC. Curr Opin Gastroenterol. 2023 Sep. doi: 10.1097/MOG.0000000000000968.
12. Kolb JM and Chang KJ. Curr Opin Gastroenterol. 2023 Jul. doi:10.1097/MOG.0000000000000944.
13. Nguyen NT et al. Foregut. 2022 Sep. doi: 10.1177/26345161221126961.
14. Mazzoleni G et al. Endosc Int Open. 2021 Feb. doi: 10.1055/a-1322-2209.
15. Trad KS et al. Surg Innov. 2018 Apr. doi: 10.1177/1553350618755214.
16. Kolb JM et al. Gastroenterology. 2021 May. doi: 10.1016/S0016-5085(21)02953-X.
17. Jaruvongvanich VK et al. Endosc Int Open. 2023 Jan. doi: 10.1055/a-1972-9190.
18. Lee Y et al. Surg Endosc. 2023 Jul. doi: 10.1007/s00464-023-10151-5.
19. Andreou A et al. Surg Endosc. 2020 Feb. doi: 10.1007/s00464-019-07208-9.
20. Guidozzi N et al. Dis Esophagus. 2019 Nov. doi: 10.1093/dote/doz031.
A Paradigm Shift in Evaluating and Investigating the Etiology of Bloating
Introduction
Abdominal bloating is a common condition affecting up to 3.5% of people globally (4.6% in women and 2.4% in men),1 with 13.9% of the US population reporting bloating in the past 7 days.2 The prevalence of bloating and distention exceeds 50% when linked to disorders of gut-brain interaction (DGBIs) such as irritable bowel syndrome (IBS), constipation, gastroparesis, and functional dyspepsia (FD).3,4 According to the Rome IV criteria, functional bloating and distention (FABD) patients are characterized by recurrent symptoms of abdominal fullness or pressure (bloating), or a visible increase in abdominal girth (distention) occurring at least 1 day per week for 3 consecutive months with an onset of 6 months and without predominant pain or altered bowel habits.5
Prolonged abdominal bloating and distention (ABD) can significantly impact quality of life and work productivity and can lead to increased medical consultations.2 Multiple pathophysiological mechanisms are involved in ABD that complicate the clinical management.4 There is an unmet need to understand the underlying mechanisms that lead to the development of ABD such as, food intolerance, abnormal viscerosomatic reflex, visceral hypersensitivity, and gut microbial dysbiosis. Recent advancements and acceptance of a multidisciplinary management of ABD have shifted the paradigm from merely treating symptoms to subtyping the condition and identifying overlaps with other DGBIs in order to individualize treatment that addresses the underlying pathophysiological mechanism. The recent American Gastroenterological Association (AGA) clinical update provided insights into the best practice advice for evaluating and managing ABD based on a review of current literature and on expert opinion of coauthors.6 This article aims to deliberate a practical approach to diagnostic strategies and treatment options based on etiology to refine clinical care of patients with ABD.
Pathophysiological Mechanisms
ABD can result from various pathophysiological mechanisms. This section highlights the major causes (illustrated in Figure 1).
Food intolerances
Understanding food intolerances is crucial for diagnosing and managing patients with ABD. Disaccharidase deficiency is common (e.g., lactase deficiency is found in 35%-40% of adults).7 It can be undiagnosed in patients presenting with IBS symptoms, given the overlap in presentation with a prevalence of 9% of pan-disaccharidase deficiency. Sucrase-deficient patients must often adjust sugar and carbohydrate/starch intake to relieve symptoms.7 Deficiencies in lactase and sucrase activity, along with the consumption of some artificial sweeteners (e.g., sugar alcohols and sorbitol) and fructans can lead to bloating and distention. These substances increase osmotic load, fluid retention, microbial fermentation, and visceral hypersensitivity, leading to gas production and abdominal distention. One prospective study of symptomatic patients with various DGBIs (n = 1372) reported a prevalence of lactose intolerance and malabsorption at 51% and 32%, respectively.8 Furthermore, fructose intolerance and malabsorption prevalence were 60% and 45%, respectively.8 Notably, lactase deficiency does not always cause ABD, as not all individuals with lactase deficiency experience these symptoms after consuming lactose. Patients with celiac disease (CD), non-celiac gluten sensitivity (NCGS), and gluten intolerance can also experience bloating and distention, with or without changes in bowel habits.9 In some patients with self-reported NCGS, symptoms may be due to fructans in gluten-rich foods rather than gluten itself, thus recommending the elimination of fructans may help improve symptoms.9
Visceral hypersensitivity
Visceral hypersensitivity is explained by an increased perception of gut mechano-chemical stimulation, which typically manifests in an aggravated feeling of pain, nausea, distension, and ABD.10 In the gut, food particles and gut bacteria and their derived molecules interact with neuroimmune and enteroendocrine cells causing visceral sensitivity by the proximity of gut’s neurons to immune cells activated by them and leading to inflammatory reactions (Figure 1).
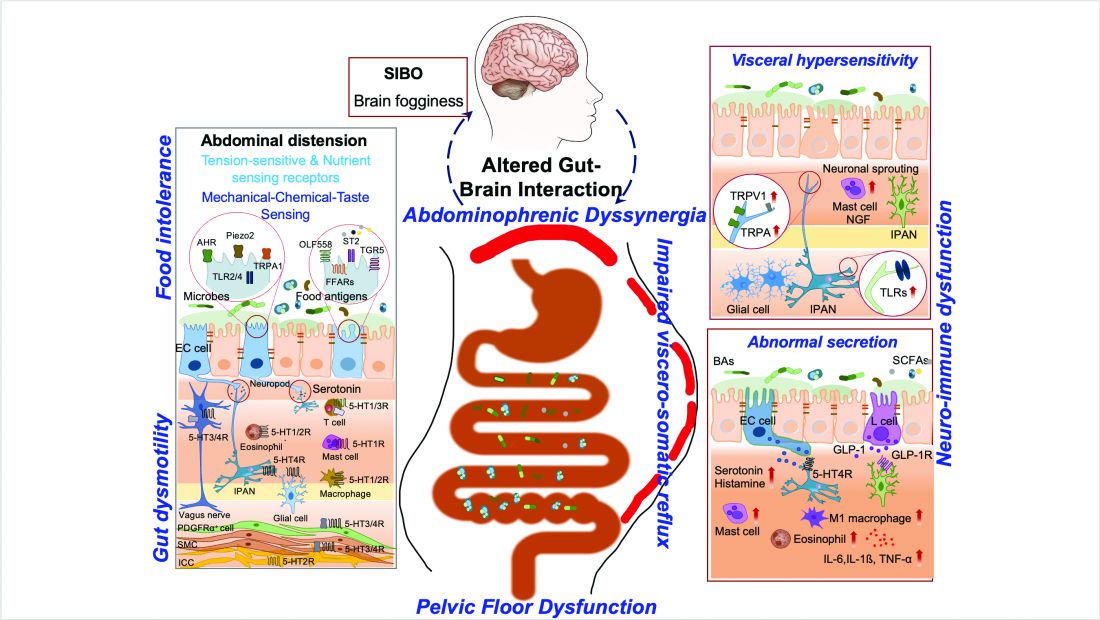
Pelvic floor dysfunction
Patients with anorectal motor dysfunction often experience difficulty in effectively evacuating both gas and stool, leading to ABD.12 Impaired ability to expel gas and stool results in prolonged balloon expulsion times, which correlates with symptoms of distention in patients with constipation.
Abdominophrenic dyssynergia
Abdominophrenic dyssynergia is characterized as a paradoxical viscerosomatic reflex response to minimal gaseous distention in individuals with FABD.13 In this condition, the diaphragm contracts (descends), and the anterior abdominal wall muscles relax in response to the presence of gas. This response is opposite to the normal physiological response to increased intraluminal gas, where the diaphragm relaxes and the anterior abdominal muscles contract to increase the craniocaudal capacity of the abdominal cavity without causing abdominal protrusion.13 Patients with FABD exhibit significant abdominal wall protrusion and diaphragmatic descent even with relatively small increases in intraluminal gas.11 Understanding the role of abdominophrenic dyssynergia in abdominal bloating and distention is essential for effective diagnosis and management of the patients.
Gut dysmotility
Gut dysmotility is a crucial factor that can contribute to FABD. Gut dysmotility affects the movement of contents through the GI tract, accumulating gas and stool, directly contributing to bloating and distention. A prospective study involving over 2000 patients with functional constipation and constipation predominant-IBS (IBS-C) found that more than 90% of these patients reported symptoms of bloating.14 Furthermore, in IBS-C patients, those with prolonged colonic transit exhibited greater abdominal distention compared to those with normal gut transit times. In patients with gastroparesis, delayed gastric emptying resulting in prolonged retention of stomach contents is the main factor in the generation of bloating symptoms.4
Small intestinal bacterial overgrowth (SIBO)
SIBO is overrepresented in various conditions, including IBS, FD, diabetes, gastrointestinal (GI) surgery patients and obesity, and can play an important role in generating ABD. Excess bacteria in the small intestine ferment carbohydrates, producing gas that stretches and distends the small intestine, leading to these symptoms. Additionally, altered sensation and abnormal viscerosomatic reflexes may contribute to SIBO-related bloating.4 One recent study noted decreased duodenal phylogenetic diversity in individuals who developed postprandial bloating.15 Increased methane levels caused by intestinal methanogen overgrowth, primarily the archaea Methanobrevibacter smithii, is possibly responsible for ABD in patients with IBS-C.16 Testing for SIBO in patients with ABD is generally only recommended if there are clear risk factors or severe symptoms warranting a test-and-treat approach.
Practical Diagnosis
Diagnosing ABD typically does not require extensive laboratory testing, imaging, or endoscopy unless there are alarm features or significant changes in symptoms. Here is the AGA clinical update on best practice advice6 for when to conduct further testing:
Diagnostic tests should be considered if patients exhibit:
- Recent onset or worsening of dyspepsia or abdominal pain
- Vomiting
- GI bleeding
- Unintentional weight loss exceeding 10% of body weight
- Chronic diarrhea
- Family history of GI malignancy, celiac disease, or inflammatory bowel disease
Physical examination
If visible abdominal distention is present, a thorough abdominal examination can help identify potential issues:
- Tympany to percussion suggests bowel dilation.
- Abnormal bowel sounds may indicate obstruction or ileus.
- A succussion splash could indicate the presence of ascites and obstruction.
- Any abnormalities discovered during the physical exam should prompt further investigation with imaging, such as a computed tomography (CT) scan or ultrasound, to evaluate for ascites, masses, or increased bowel gas due to ileus, obstruction, or pseudo-obstruction.
Radiologic imaging, laboratory testing and endoscopy
- An abdominal x-ray may reveal an increased stool burden, suggesting the need for further evaluation of slow transit constipation or a pelvic floor disorder, particularly in patients with functional constipation, IBS-mixed, or IBS-C.
- Hyperglycemia, weight gain, and bloating can be a presenting sign of ovarian cancer therefore all women should continue pelvic exams as dictated by the gynecologic societies. The need for an annual pelvic exam should be discussed with health care professionals especially in those with family history of ovarian cancer.
- An upper endoscopy may be warranted for patients over 40 years old with dyspeptic symptoms and abdominal bloating or distention, especially in regions with a high prevalence of Helicobacter pylori.
- Chronic pancreatitis, indicated by bloating and pain, may necessitate fecal elastase testing to assess pancreatic function.
The expert review in the AGA clinical update provides step-by-step advice regarding the best practices6 for diagnosis and identifying who to test for ABD.
Treatment Options
The following sections highlight recent best practice advice on therapeutic approaches for treating ABD.
Dietary interventions
Specific foods may trigger bloating and abdominal distention, especially in patients with overlapping DGBIs. However, only a few studies have evaluated dietary restriction specifically for patients with primary ABD. Restricting non-absorbable sugars led to symptomatic improvement in 81% of patients with FABD who had documented sugar malabsorption.17 Two studies have shown that IBS patients treated with a low-fermentable, oligo-, di-, and monosaccharides (FODMAP) diet noted improvement in ABD and that restricting fructans initially may be the most optimal.18 A recent study showed that the Mediterranean diet improved IBS symptoms, including abdominal pain and bloating.19 It should be noted restrictive diets are efficacious but come with short- and long-term challenges. If empiric treatment and/or therapeutic testing do not resolve symptoms, a referral to a dietitian can be useful. Dietitians can provide tailored dietary advice, ensuring patients avoid trigger foods while maintaining a balanced and nutritious diet.
Prokinetics and laxatives
Prokinetic agents are used to treat symptoms of FD, gastroparesis, chronic idiopathic constipation (CIC), and IBS. A meta-analysis of 13 trials found all constipation medications superior to placebo for treating abdominal bloating in patients with IBS-C.20
Probiotics
Treatment with probiotics is recommended for bloating or distention. One double-blind placebo-controlled trial with two separate probiotics, Bifidobacterium lactis and Lactobacillus acidophilus, showed improvements in global GI symptoms of patients with DGBI at 8 weeks versus placebo, with improvements in bloating symptoms.21
Antibiotics
The most commonly studied antibiotic for treating bloating is rifaximin.22 Global symptomatic improvement in IBS patients treated with antibiotics has correlated with the normalization of hydrogen levels in lactulose hydrogen breath tests.22 Patients with non-constipation IBS randomized to rifaximin 550 mg three times daily for 14 days had a greater proportion of relief of IBS-related bloating compared to placebo for at least 2 of the first 4 weeks after treatment.22 Future research warrants use of narrow-spectrum antibiotics study for FABD as the use of broad-spectrum antibiotics may deplete commensals forever, resulting in metabolic disorders.
Biofeedback therapy
Anorectal biofeedback therapy may help with ABD, particularly in patients with IBS-C and chronic constipation. One study noted that post-biofeedback therapy, myoelectric activity of the intercostals and diaphragm decreased, and internal oblique myoelectric activity increased.23 This study also showed ascent of the diaphragm and decreased girth, improving distention.
Central neuromodulators
As bloating results from multiple disturbed mechanisms, including altered gut-brain interaction, these symptoms can be amplified by psychological states such as anxiety, depression, or somatization. Central neuromodulators reduce the perception of visceral signals, re-regulate brain-gut control mechanisms, and improve psychological comorbidities.6 A large study of FD patients demonstrated that both amitriptyline (50 mg daily) and escitalopram (10 mg daily) significantly improved postprandial bloating compared to placebo.24 Antidepressants that activate noradrenergic and serotonergic pathways, including tricyclic antidepressants (e.g., amitriptyline) and serotonin-norepinephrine reuptake inhibitors (e.g., duloxetine and venlafaxine), show the greatest benefit in reducing visceral sensations.6
Brain-gut behavioral therapies
A recent multidisciplinary consensus report supports a myriad of potential brain-gut behavioral therapies (BGBTs) for treating DGBI.25 These therapies, including hypnotherapy, cognitive behavioral therapy (CBT), and other modalities, may be combined with central neuromodulators and other GI treatments in a safe, noninvasive, and complementary fashion. BGBTs do not need to be symptom-specific, as they improve overall quality of life, anxiety, stress, and the burden associated with DGBIs. To date, none of the BGBTs have focused exclusively on FABD; however, prescription-based psychological therapies are now FDA-approved for use on smart apps, improving global symptoms that include bloating in IBS and FD.
Recent AGA clinical update best practices should be considered for the clinical care of patients with ABD.6
Conclusion and Future Perspectives
ABD are highly prevalent and significantly impact patients with various GI and metabolic disorders. Although our understanding of these symptoms is still evolving, evidence increasingly points to the dysregulation of the gut-brain axis and supports the application of the biopsychosocial model in treatment. This model addresses diet, motility, visceral sensitivity, pelvic floor disorders and psychosocial factors, providing a comprehensive approach to patient care.
Physician-scientists around the globe face numerous challenges when evaluating patients with these symptoms. However, the recent AGA clinical update on the best practice guidelines offers step-by-step diagnostic tests and treatment options to assist physicians in making informed decisions. . More comprehensive, large-scale, and longitudinal studies using metabolomics, capsule technologies for discovery of dysbiosis, mass spectrometry, and imaging data are needed to identify the exact contributors to disease pathogenesis, particularly those that can be targeted with pharmacologic agents. Collaborative work between gastroenterologists, dietitians, gut-brain behavioral therapists, endocrinologists, is crucial for clinical care of patients with ABD.
Careful attention to the patient’s primary symptoms and physical examination, combined with advancements in targeted diagnostics like the analysis of microbial markers, metabolites, and molecular signals, can significantly enhance patient clinical outcomes. Additionally, education and effective communication using a patient-centered care model are essential for guiding practical evaluation and individualized treatment.
Dr. Singh is assistant professor (research) at the University of Nevada, Reno, School of Medicine. Dr. Moshiree is director of motility at Atrium Health, and clinical professor of medicine, Wake Forest Medical University, Charlotte, North Carolina.
References
1. Ballou S et al. Prevalence and associated factors of bloating: Results from the Rome Foundation Global Epidemiology Study. Gastroenterology. 2023 June. doi: 10.1053/j.gastro.2023.05.049.
2. Oh JE et al. Abdominal bloating in the United States: Results of a survey of 88,795 Americans examining prevalence and healthcare seeking. Clin Gastroenterol Hepatol. 2023 Aug. doi: 10.1016/j.cgh.2022.10.031.
3. Drossman DA et al. Neuromodulators for functional gastrointestinal disorders (disorders of gut-brain interaction): A Rome Foundation Working Team Report. Gastroenterology. 2018 Mar. doi: 10.1053/j.gastro.2017.11.279.
4. Lacy BE et al. Management of chronic abdominal distension and bloating. Clin Gastroenterol Hepatol. 2021 Feb. doi: 10.1016/j.cgh.2020.03.056.
5. Mearin F et al. Bowel disorders. Gastroenterology. 2016 Feb. doi: 10.1053/j.gastro.2016.02.031.
6. Moshiree B et al. AGA Clinical Practice Update on evaluation and management of belching, abdominal bloating, and distention: expert review. Gastroenterology. 2023 Sep. doi: 10.1053/j.gastro.2023.04.039.
7. Viswanathan L and Rao SS. Intestinal disaccharidase deficiency in adults: evaluation and treatment. Curr Gastroenterol Rep 2023 May. doi: 10.1007/s11894-023-00870-z.
8. Wilder-Smith CH et al. Fructose and lactose intolerance and malabsorption testing: the relationship with symptoms in functional gastrointestinal disorders. Aliment Pharmacol Ther. 2013 Jun. doi: 10.1111/apt.12306.
9. Skodje GI et al. Fructan, rather than gluten, induces symptoms in patients with self-reported non-celiac gluten sensitivity. Gastroenterology. 2018 Feb. doi: 10.1053/j.gastro.2017.10.040.
10. Singh R et al. Current treatment options and therapeutic insights for gastrointestinal dysmotility and functional gastrointestinal disorders. Front Pharmacol. 2022 Jan. doi: 10.3389/fphar.2022.808195.
11. Accarino A et al. Abdominal distention results from caudo-ventral redistribution of contents. Gastroenterology 2009 May. doi: 10.1053/j.gastro.2009.01.067.
12. Shim L et al. Prolonged balloon expulsion is predictive of abdominal distension in bloating. Am J Gastroenterol. 2010 Apr. doi: 10.1038/ajg.2010.54.
13. Villoria A et al. Abdomino-phrenic dyssynergia in patients with abdominal bloating and distension. Am J Gastroenterol. 2011 May. doi: 10.1038/ajg.2010.408.
14. Neri L and Iovino P. Laxative Inadequate Relief Survey Group. Bloating is associated with worse quality of life, treatment satisfaction, and treatment responsiveness among patients with constipation-predominant irritable bowel syndrome and functional constipation. Neurogastroenterol Motil. 2016 Apr. doi: 10.1111/nmo.12758.
15. Saffouri GB et al. Small intestinal microbial dysbiosis underlies symptoms associated with functional gastrointestinal disorders. Nat Commun. 2019 May. doi: 10.1038/s41467-019-09964-7.
16. Villanueva-Millan MJ et al. Methanogens and hydrogen sulfide producing bacteria guide distinct gut microbe profiles and irritable bowel syndrome subtypes. Am J Gastroenterol. 2022 Dec. doi: 10.14309/ajg.0000000000001997.
17. Fernández-Bañares F et al. Sugar malabsorption in functional abdominal bloating: a pilot study on the long-term effect of dietary treatment. Clin Nutr. 2006 Oct. doi: 10.1016/j.clnu.2005.11.010.
18. Böhn L et al. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: a randomized controlled trial. Gastroenterology. 2015 Nov. doi: 10.1053/j.gastro.2015.07.054.
19. Staudacher HM et al. Clinical trial: A Mediterranean diet is feasible and improves gastrointestinal and psychological symptoms in irritable bowel syndrome. Aliment Pharmacol Ther. 2024 Feb. doi: 10.1111/apt.17791.
20. Nelson AD et al. Systematic review and network meta-analysis: efficacy of licensed drugs for abdominal bloating in irritable bowel syndrome with constipation. Aliment Pharmacol Ther. 2021 Jul. doi: 10.1111/apt.16437.
21. Ringel-Kulka T et al. Probiotic bacteria Lactobacillus acidophilus NCFM and Bifidobacterium lactis Bi-07 versus placebo for the symptoms of bloating in patients with functional bowel disorders: a double-blind study. J Clin Gastroenterol. 2011 Jul. doi: 10.1097/MCG.0b013e31820ca4d6.
22. Pimentel M et al. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011 Jan. doi: 10.1056/NEJMoa1004409.
23. Iovino P et al. Pelvic floor biofeedback is an effective treatment for severe bloating in disorders of gut-brain interaction with outlet dysfunction. Neurogastroenterol Motil 2022 May. doi: 10.1111/nmo.14264.
24. Talley NJ et al. Effect of amitriptyline and escitalopram on functional dyspepsia: A multicenter, randomized controlled study. Gastroenterology. 2015 Aug. doi: 10.1053/j.gastro.2015.04.020.
25. Keefer L et al. A Rome Working Team Report on brain-gut behavior therapies for disorders of gut-brain interaction. Gastroenterology. 2022 Jan. doi: 10.1053/j.gastro.2021.09.015.
Introduction
Abdominal bloating is a common condition affecting up to 3.5% of people globally (4.6% in women and 2.4% in men),1 with 13.9% of the US population reporting bloating in the past 7 days.2 The prevalence of bloating and distention exceeds 50% when linked to disorders of gut-brain interaction (DGBIs) such as irritable bowel syndrome (IBS), constipation, gastroparesis, and functional dyspepsia (FD).3,4 According to the Rome IV criteria, functional bloating and distention (FABD) patients are characterized by recurrent symptoms of abdominal fullness or pressure (bloating), or a visible increase in abdominal girth (distention) occurring at least 1 day per week for 3 consecutive months with an onset of 6 months and without predominant pain or altered bowel habits.5
Prolonged abdominal bloating and distention (ABD) can significantly impact quality of life and work productivity and can lead to increased medical consultations.2 Multiple pathophysiological mechanisms are involved in ABD that complicate the clinical management.4 There is an unmet need to understand the underlying mechanisms that lead to the development of ABD such as, food intolerance, abnormal viscerosomatic reflex, visceral hypersensitivity, and gut microbial dysbiosis. Recent advancements and acceptance of a multidisciplinary management of ABD have shifted the paradigm from merely treating symptoms to subtyping the condition and identifying overlaps with other DGBIs in order to individualize treatment that addresses the underlying pathophysiological mechanism. The recent American Gastroenterological Association (AGA) clinical update provided insights into the best practice advice for evaluating and managing ABD based on a review of current literature and on expert opinion of coauthors.6 This article aims to deliberate a practical approach to diagnostic strategies and treatment options based on etiology to refine clinical care of patients with ABD.
Pathophysiological Mechanisms
ABD can result from various pathophysiological mechanisms. This section highlights the major causes (illustrated in Figure 1).
Food intolerances
Understanding food intolerances is crucial for diagnosing and managing patients with ABD. Disaccharidase deficiency is common (e.g., lactase deficiency is found in 35%-40% of adults).7 It can be undiagnosed in patients presenting with IBS symptoms, given the overlap in presentation with a prevalence of 9% of pan-disaccharidase deficiency. Sucrase-deficient patients must often adjust sugar and carbohydrate/starch intake to relieve symptoms.7 Deficiencies in lactase and sucrase activity, along with the consumption of some artificial sweeteners (e.g., sugar alcohols and sorbitol) and fructans can lead to bloating and distention. These substances increase osmotic load, fluid retention, microbial fermentation, and visceral hypersensitivity, leading to gas production and abdominal distention. One prospective study of symptomatic patients with various DGBIs (n = 1372) reported a prevalence of lactose intolerance and malabsorption at 51% and 32%, respectively.8 Furthermore, fructose intolerance and malabsorption prevalence were 60% and 45%, respectively.8 Notably, lactase deficiency does not always cause ABD, as not all individuals with lactase deficiency experience these symptoms after consuming lactose. Patients with celiac disease (CD), non-celiac gluten sensitivity (NCGS), and gluten intolerance can also experience bloating and distention, with or without changes in bowel habits.9 In some patients with self-reported NCGS, symptoms may be due to fructans in gluten-rich foods rather than gluten itself, thus recommending the elimination of fructans may help improve symptoms.9
Visceral hypersensitivity
Visceral hypersensitivity is explained by an increased perception of gut mechano-chemical stimulation, which typically manifests in an aggravated feeling of pain, nausea, distension, and ABD.10 In the gut, food particles and gut bacteria and their derived molecules interact with neuroimmune and enteroendocrine cells causing visceral sensitivity by the proximity of gut’s neurons to immune cells activated by them and leading to inflammatory reactions (Figure 1).

Pelvic floor dysfunction
Patients with anorectal motor dysfunction often experience difficulty in effectively evacuating both gas and stool, leading to ABD.12 Impaired ability to expel gas and stool results in prolonged balloon expulsion times, which correlates with symptoms of distention in patients with constipation.
Abdominophrenic dyssynergia
Abdominophrenic dyssynergia is characterized as a paradoxical viscerosomatic reflex response to minimal gaseous distention in individuals with FABD.13 In this condition, the diaphragm contracts (descends), and the anterior abdominal wall muscles relax in response to the presence of gas. This response is opposite to the normal physiological response to increased intraluminal gas, where the diaphragm relaxes and the anterior abdominal muscles contract to increase the craniocaudal capacity of the abdominal cavity without causing abdominal protrusion.13 Patients with FABD exhibit significant abdominal wall protrusion and diaphragmatic descent even with relatively small increases in intraluminal gas.11 Understanding the role of abdominophrenic dyssynergia in abdominal bloating and distention is essential for effective diagnosis and management of the patients.
Gut dysmotility
Gut dysmotility is a crucial factor that can contribute to FABD. Gut dysmotility affects the movement of contents through the GI tract, accumulating gas and stool, directly contributing to bloating and distention. A prospective study involving over 2000 patients with functional constipation and constipation predominant-IBS (IBS-C) found that more than 90% of these patients reported symptoms of bloating.14 Furthermore, in IBS-C patients, those with prolonged colonic transit exhibited greater abdominal distention compared to those with normal gut transit times. In patients with gastroparesis, delayed gastric emptying resulting in prolonged retention of stomach contents is the main factor in the generation of bloating symptoms.4
Small intestinal bacterial overgrowth (SIBO)
SIBO is overrepresented in various conditions, including IBS, FD, diabetes, gastrointestinal (GI) surgery patients and obesity, and can play an important role in generating ABD. Excess bacteria in the small intestine ferment carbohydrates, producing gas that stretches and distends the small intestine, leading to these symptoms. Additionally, altered sensation and abnormal viscerosomatic reflexes may contribute to SIBO-related bloating.4 One recent study noted decreased duodenal phylogenetic diversity in individuals who developed postprandial bloating.15 Increased methane levels caused by intestinal methanogen overgrowth, primarily the archaea Methanobrevibacter smithii, is possibly responsible for ABD in patients with IBS-C.16 Testing for SIBO in patients with ABD is generally only recommended if there are clear risk factors or severe symptoms warranting a test-and-treat approach.
Practical Diagnosis
Diagnosing ABD typically does not require extensive laboratory testing, imaging, or endoscopy unless there are alarm features or significant changes in symptoms. Here is the AGA clinical update on best practice advice6 for when to conduct further testing:
Diagnostic tests should be considered if patients exhibit:
- Recent onset or worsening of dyspepsia or abdominal pain
- Vomiting
- GI bleeding
- Unintentional weight loss exceeding 10% of body weight
- Chronic diarrhea
- Family history of GI malignancy, celiac disease, or inflammatory bowel disease
Physical examination
If visible abdominal distention is present, a thorough abdominal examination can help identify potential issues:
- Tympany to percussion suggests bowel dilation.
- Abnormal bowel sounds may indicate obstruction or ileus.
- A succussion splash could indicate the presence of ascites and obstruction.
- Any abnormalities discovered during the physical exam should prompt further investigation with imaging, such as a computed tomography (CT) scan or ultrasound, to evaluate for ascites, masses, or increased bowel gas due to ileus, obstruction, or pseudo-obstruction.
Radiologic imaging, laboratory testing and endoscopy
- An abdominal x-ray may reveal an increased stool burden, suggesting the need for further evaluation of slow transit constipation or a pelvic floor disorder, particularly in patients with functional constipation, IBS-mixed, or IBS-C.
- Hyperglycemia, weight gain, and bloating can be a presenting sign of ovarian cancer therefore all women should continue pelvic exams as dictated by the gynecologic societies. The need for an annual pelvic exam should be discussed with health care professionals especially in those with family history of ovarian cancer.
- An upper endoscopy may be warranted for patients over 40 years old with dyspeptic symptoms and abdominal bloating or distention, especially in regions with a high prevalence of Helicobacter pylori.
- Chronic pancreatitis, indicated by bloating and pain, may necessitate fecal elastase testing to assess pancreatic function.
The expert review in the AGA clinical update provides step-by-step advice regarding the best practices6 for diagnosis and identifying who to test for ABD.
Treatment Options
The following sections highlight recent best practice advice on therapeutic approaches for treating ABD.
Dietary interventions
Specific foods may trigger bloating and abdominal distention, especially in patients with overlapping DGBIs. However, only a few studies have evaluated dietary restriction specifically for patients with primary ABD. Restricting non-absorbable sugars led to symptomatic improvement in 81% of patients with FABD who had documented sugar malabsorption.17 Two studies have shown that IBS patients treated with a low-fermentable, oligo-, di-, and monosaccharides (FODMAP) diet noted improvement in ABD and that restricting fructans initially may be the most optimal.18 A recent study showed that the Mediterranean diet improved IBS symptoms, including abdominal pain and bloating.19 It should be noted restrictive diets are efficacious but come with short- and long-term challenges. If empiric treatment and/or therapeutic testing do not resolve symptoms, a referral to a dietitian can be useful. Dietitians can provide tailored dietary advice, ensuring patients avoid trigger foods while maintaining a balanced and nutritious diet.
Prokinetics and laxatives
Prokinetic agents are used to treat symptoms of FD, gastroparesis, chronic idiopathic constipation (CIC), and IBS. A meta-analysis of 13 trials found all constipation medications superior to placebo for treating abdominal bloating in patients with IBS-C.20
Probiotics
Treatment with probiotics is recommended for bloating or distention. One double-blind placebo-controlled trial with two separate probiotics, Bifidobacterium lactis and Lactobacillus acidophilus, showed improvements in global GI symptoms of patients with DGBI at 8 weeks versus placebo, with improvements in bloating symptoms.21
Antibiotics
The most commonly studied antibiotic for treating bloating is rifaximin.22 Global symptomatic improvement in IBS patients treated with antibiotics has correlated with the normalization of hydrogen levels in lactulose hydrogen breath tests.22 Patients with non-constipation IBS randomized to rifaximin 550 mg three times daily for 14 days had a greater proportion of relief of IBS-related bloating compared to placebo for at least 2 of the first 4 weeks after treatment.22 Future research warrants use of narrow-spectrum antibiotics study for FABD as the use of broad-spectrum antibiotics may deplete commensals forever, resulting in metabolic disorders.
Biofeedback therapy
Anorectal biofeedback therapy may help with ABD, particularly in patients with IBS-C and chronic constipation. One study noted that post-biofeedback therapy, myoelectric activity of the intercostals and diaphragm decreased, and internal oblique myoelectric activity increased.23 This study also showed ascent of the diaphragm and decreased girth, improving distention.
Central neuromodulators
As bloating results from multiple disturbed mechanisms, including altered gut-brain interaction, these symptoms can be amplified by psychological states such as anxiety, depression, or somatization. Central neuromodulators reduce the perception of visceral signals, re-regulate brain-gut control mechanisms, and improve psychological comorbidities.6 A large study of FD patients demonstrated that both amitriptyline (50 mg daily) and escitalopram (10 mg daily) significantly improved postprandial bloating compared to placebo.24 Antidepressants that activate noradrenergic and serotonergic pathways, including tricyclic antidepressants (e.g., amitriptyline) and serotonin-norepinephrine reuptake inhibitors (e.g., duloxetine and venlafaxine), show the greatest benefit in reducing visceral sensations.6
Brain-gut behavioral therapies
A recent multidisciplinary consensus report supports a myriad of potential brain-gut behavioral therapies (BGBTs) for treating DGBI.25 These therapies, including hypnotherapy, cognitive behavioral therapy (CBT), and other modalities, may be combined with central neuromodulators and other GI treatments in a safe, noninvasive, and complementary fashion. BGBTs do not need to be symptom-specific, as they improve overall quality of life, anxiety, stress, and the burden associated with DGBIs. To date, none of the BGBTs have focused exclusively on FABD; however, prescription-based psychological therapies are now FDA-approved for use on smart apps, improving global symptoms that include bloating in IBS and FD.
Recent AGA clinical update best practices should be considered for the clinical care of patients with ABD.6
Conclusion and Future Perspectives
ABD are highly prevalent and significantly impact patients with various GI and metabolic disorders. Although our understanding of these symptoms is still evolving, evidence increasingly points to the dysregulation of the gut-brain axis and supports the application of the biopsychosocial model in treatment. This model addresses diet, motility, visceral sensitivity, pelvic floor disorders and psychosocial factors, providing a comprehensive approach to patient care.
Physician-scientists around the globe face numerous challenges when evaluating patients with these symptoms. However, the recent AGA clinical update on the best practice guidelines offers step-by-step diagnostic tests and treatment options to assist physicians in making informed decisions. . More comprehensive, large-scale, and longitudinal studies using metabolomics, capsule technologies for discovery of dysbiosis, mass spectrometry, and imaging data are needed to identify the exact contributors to disease pathogenesis, particularly those that can be targeted with pharmacologic agents. Collaborative work between gastroenterologists, dietitians, gut-brain behavioral therapists, endocrinologists, is crucial for clinical care of patients with ABD.
Careful attention to the patient’s primary symptoms and physical examination, combined with advancements in targeted diagnostics like the analysis of microbial markers, metabolites, and molecular signals, can significantly enhance patient clinical outcomes. Additionally, education and effective communication using a patient-centered care model are essential for guiding practical evaluation and individualized treatment.
Dr. Singh is assistant professor (research) at the University of Nevada, Reno, School of Medicine. Dr. Moshiree is director of motility at Atrium Health, and clinical professor of medicine, Wake Forest Medical University, Charlotte, North Carolina.
References
1. Ballou S et al. Prevalence and associated factors of bloating: Results from the Rome Foundation Global Epidemiology Study. Gastroenterology. 2023 June. doi: 10.1053/j.gastro.2023.05.049.
2. Oh JE et al. Abdominal bloating in the United States: Results of a survey of 88,795 Americans examining prevalence and healthcare seeking. Clin Gastroenterol Hepatol. 2023 Aug. doi: 10.1016/j.cgh.2022.10.031.
3. Drossman DA et al. Neuromodulators for functional gastrointestinal disorders (disorders of gut-brain interaction): A Rome Foundation Working Team Report. Gastroenterology. 2018 Mar. doi: 10.1053/j.gastro.2017.11.279.
4. Lacy BE et al. Management of chronic abdominal distension and bloating. Clin Gastroenterol Hepatol. 2021 Feb. doi: 10.1016/j.cgh.2020.03.056.
5. Mearin F et al. Bowel disorders. Gastroenterology. 2016 Feb. doi: 10.1053/j.gastro.2016.02.031.
6. Moshiree B et al. AGA Clinical Practice Update on evaluation and management of belching, abdominal bloating, and distention: expert review. Gastroenterology. 2023 Sep. doi: 10.1053/j.gastro.2023.04.039.
7. Viswanathan L and Rao SS. Intestinal disaccharidase deficiency in adults: evaluation and treatment. Curr Gastroenterol Rep 2023 May. doi: 10.1007/s11894-023-00870-z.
8. Wilder-Smith CH et al. Fructose and lactose intolerance and malabsorption testing: the relationship with symptoms in functional gastrointestinal disorders. Aliment Pharmacol Ther. 2013 Jun. doi: 10.1111/apt.12306.
9. Skodje GI et al. Fructan, rather than gluten, induces symptoms in patients with self-reported non-celiac gluten sensitivity. Gastroenterology. 2018 Feb. doi: 10.1053/j.gastro.2017.10.040.
10. Singh R et al. Current treatment options and therapeutic insights for gastrointestinal dysmotility and functional gastrointestinal disorders. Front Pharmacol. 2022 Jan. doi: 10.3389/fphar.2022.808195.
11. Accarino A et al. Abdominal distention results from caudo-ventral redistribution of contents. Gastroenterology 2009 May. doi: 10.1053/j.gastro.2009.01.067.
12. Shim L et al. Prolonged balloon expulsion is predictive of abdominal distension in bloating. Am J Gastroenterol. 2010 Apr. doi: 10.1038/ajg.2010.54.
13. Villoria A et al. Abdomino-phrenic dyssynergia in patients with abdominal bloating and distension. Am J Gastroenterol. 2011 May. doi: 10.1038/ajg.2010.408.
14. Neri L and Iovino P. Laxative Inadequate Relief Survey Group. Bloating is associated with worse quality of life, treatment satisfaction, and treatment responsiveness among patients with constipation-predominant irritable bowel syndrome and functional constipation. Neurogastroenterol Motil. 2016 Apr. doi: 10.1111/nmo.12758.
15. Saffouri GB et al. Small intestinal microbial dysbiosis underlies symptoms associated with functional gastrointestinal disorders. Nat Commun. 2019 May. doi: 10.1038/s41467-019-09964-7.
16. Villanueva-Millan MJ et al. Methanogens and hydrogen sulfide producing bacteria guide distinct gut microbe profiles and irritable bowel syndrome subtypes. Am J Gastroenterol. 2022 Dec. doi: 10.14309/ajg.0000000000001997.
17. Fernández-Bañares F et al. Sugar malabsorption in functional abdominal bloating: a pilot study on the long-term effect of dietary treatment. Clin Nutr. 2006 Oct. doi: 10.1016/j.clnu.2005.11.010.
18. Böhn L et al. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: a randomized controlled trial. Gastroenterology. 2015 Nov. doi: 10.1053/j.gastro.2015.07.054.
19. Staudacher HM et al. Clinical trial: A Mediterranean diet is feasible and improves gastrointestinal and psychological symptoms in irritable bowel syndrome. Aliment Pharmacol Ther. 2024 Feb. doi: 10.1111/apt.17791.
20. Nelson AD et al. Systematic review and network meta-analysis: efficacy of licensed drugs for abdominal bloating in irritable bowel syndrome with constipation. Aliment Pharmacol Ther. 2021 Jul. doi: 10.1111/apt.16437.
21. Ringel-Kulka T et al. Probiotic bacteria Lactobacillus acidophilus NCFM and Bifidobacterium lactis Bi-07 versus placebo for the symptoms of bloating in patients with functional bowel disorders: a double-blind study. J Clin Gastroenterol. 2011 Jul. doi: 10.1097/MCG.0b013e31820ca4d6.
22. Pimentel M et al. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011 Jan. doi: 10.1056/NEJMoa1004409.
23. Iovino P et al. Pelvic floor biofeedback is an effective treatment for severe bloating in disorders of gut-brain interaction with outlet dysfunction. Neurogastroenterol Motil 2022 May. doi: 10.1111/nmo.14264.
24. Talley NJ et al. Effect of amitriptyline and escitalopram on functional dyspepsia: A multicenter, randomized controlled study. Gastroenterology. 2015 Aug. doi: 10.1053/j.gastro.2015.04.020.
25. Keefer L et al. A Rome Working Team Report on brain-gut behavior therapies for disorders of gut-brain interaction. Gastroenterology. 2022 Jan. doi: 10.1053/j.gastro.2021.09.015.
Introduction
Abdominal bloating is a common condition affecting up to 3.5% of people globally (4.6% in women and 2.4% in men),1 with 13.9% of the US population reporting bloating in the past 7 days.2 The prevalence of bloating and distention exceeds 50% when linked to disorders of gut-brain interaction (DGBIs) such as irritable bowel syndrome (IBS), constipation, gastroparesis, and functional dyspepsia (FD).3,4 According to the Rome IV criteria, functional bloating and distention (FABD) patients are characterized by recurrent symptoms of abdominal fullness or pressure (bloating), or a visible increase in abdominal girth (distention) occurring at least 1 day per week for 3 consecutive months with an onset of 6 months and without predominant pain or altered bowel habits.5
Prolonged abdominal bloating and distention (ABD) can significantly impact quality of life and work productivity and can lead to increased medical consultations.2 Multiple pathophysiological mechanisms are involved in ABD that complicate the clinical management.4 There is an unmet need to understand the underlying mechanisms that lead to the development of ABD such as, food intolerance, abnormal viscerosomatic reflex, visceral hypersensitivity, and gut microbial dysbiosis. Recent advancements and acceptance of a multidisciplinary management of ABD have shifted the paradigm from merely treating symptoms to subtyping the condition and identifying overlaps with other DGBIs in order to individualize treatment that addresses the underlying pathophysiological mechanism. The recent American Gastroenterological Association (AGA) clinical update provided insights into the best practice advice for evaluating and managing ABD based on a review of current literature and on expert opinion of coauthors.6 This article aims to deliberate a practical approach to diagnostic strategies and treatment options based on etiology to refine clinical care of patients with ABD.
Pathophysiological Mechanisms
ABD can result from various pathophysiological mechanisms. This section highlights the major causes (illustrated in Figure 1).
Food intolerances
Understanding food intolerances is crucial for diagnosing and managing patients with ABD. Disaccharidase deficiency is common (e.g., lactase deficiency is found in 35%-40% of adults).7 It can be undiagnosed in patients presenting with IBS symptoms, given the overlap in presentation with a prevalence of 9% of pan-disaccharidase deficiency. Sucrase-deficient patients must often adjust sugar and carbohydrate/starch intake to relieve symptoms.7 Deficiencies in lactase and sucrase activity, along with the consumption of some artificial sweeteners (e.g., sugar alcohols and sorbitol) and fructans can lead to bloating and distention. These substances increase osmotic load, fluid retention, microbial fermentation, and visceral hypersensitivity, leading to gas production and abdominal distention. One prospective study of symptomatic patients with various DGBIs (n = 1372) reported a prevalence of lactose intolerance and malabsorption at 51% and 32%, respectively.8 Furthermore, fructose intolerance and malabsorption prevalence were 60% and 45%, respectively.8 Notably, lactase deficiency does not always cause ABD, as not all individuals with lactase deficiency experience these symptoms after consuming lactose. Patients with celiac disease (CD), non-celiac gluten sensitivity (NCGS), and gluten intolerance can also experience bloating and distention, with or without changes in bowel habits.9 In some patients with self-reported NCGS, symptoms may be due to fructans in gluten-rich foods rather than gluten itself, thus recommending the elimination of fructans may help improve symptoms.9
Visceral hypersensitivity
Visceral hypersensitivity is explained by an increased perception of gut mechano-chemical stimulation, which typically manifests in an aggravated feeling of pain, nausea, distension, and ABD.10 In the gut, food particles and gut bacteria and their derived molecules interact with neuroimmune and enteroendocrine cells causing visceral sensitivity by the proximity of gut’s neurons to immune cells activated by them and leading to inflammatory reactions (Figure 1).

Pelvic floor dysfunction
Patients with anorectal motor dysfunction often experience difficulty in effectively evacuating both gas and stool, leading to ABD.12 Impaired ability to expel gas and stool results in prolonged balloon expulsion times, which correlates with symptoms of distention in patients with constipation.
Abdominophrenic dyssynergia
Abdominophrenic dyssynergia is characterized as a paradoxical viscerosomatic reflex response to minimal gaseous distention in individuals with FABD.13 In this condition, the diaphragm contracts (descends), and the anterior abdominal wall muscles relax in response to the presence of gas. This response is opposite to the normal physiological response to increased intraluminal gas, where the diaphragm relaxes and the anterior abdominal muscles contract to increase the craniocaudal capacity of the abdominal cavity without causing abdominal protrusion.13 Patients with FABD exhibit significant abdominal wall protrusion and diaphragmatic descent even with relatively small increases in intraluminal gas.11 Understanding the role of abdominophrenic dyssynergia in abdominal bloating and distention is essential for effective diagnosis and management of the patients.
Gut dysmotility
Gut dysmotility is a crucial factor that can contribute to FABD. Gut dysmotility affects the movement of contents through the GI tract, accumulating gas and stool, directly contributing to bloating and distention. A prospective study involving over 2000 patients with functional constipation and constipation predominant-IBS (IBS-C) found that more than 90% of these patients reported symptoms of bloating.14 Furthermore, in IBS-C patients, those with prolonged colonic transit exhibited greater abdominal distention compared to those with normal gut transit times. In patients with gastroparesis, delayed gastric emptying resulting in prolonged retention of stomach contents is the main factor in the generation of bloating symptoms.4
Small intestinal bacterial overgrowth (SIBO)
SIBO is overrepresented in various conditions, including IBS, FD, diabetes, gastrointestinal (GI) surgery patients and obesity, and can play an important role in generating ABD. Excess bacteria in the small intestine ferment carbohydrates, producing gas that stretches and distends the small intestine, leading to these symptoms. Additionally, altered sensation and abnormal viscerosomatic reflexes may contribute to SIBO-related bloating.4 One recent study noted decreased duodenal phylogenetic diversity in individuals who developed postprandial bloating.15 Increased methane levels caused by intestinal methanogen overgrowth, primarily the archaea Methanobrevibacter smithii, is possibly responsible for ABD in patients with IBS-C.16 Testing for SIBO in patients with ABD is generally only recommended if there are clear risk factors or severe symptoms warranting a test-and-treat approach.
Practical Diagnosis
Diagnosing ABD typically does not require extensive laboratory testing, imaging, or endoscopy unless there are alarm features or significant changes in symptoms. Here is the AGA clinical update on best practice advice6 for when to conduct further testing:
Diagnostic tests should be considered if patients exhibit:
- Recent onset or worsening of dyspepsia or abdominal pain
- Vomiting
- GI bleeding
- Unintentional weight loss exceeding 10% of body weight
- Chronic diarrhea
- Family history of GI malignancy, celiac disease, or inflammatory bowel disease
Physical examination
If visible abdominal distention is present, a thorough abdominal examination can help identify potential issues:
- Tympany to percussion suggests bowel dilation.
- Abnormal bowel sounds may indicate obstruction or ileus.
- A succussion splash could indicate the presence of ascites and obstruction.
- Any abnormalities discovered during the physical exam should prompt further investigation with imaging, such as a computed tomography (CT) scan or ultrasound, to evaluate for ascites, masses, or increased bowel gas due to ileus, obstruction, or pseudo-obstruction.
Radiologic imaging, laboratory testing and endoscopy
- An abdominal x-ray may reveal an increased stool burden, suggesting the need for further evaluation of slow transit constipation or a pelvic floor disorder, particularly in patients with functional constipation, IBS-mixed, or IBS-C.
- Hyperglycemia, weight gain, and bloating can be a presenting sign of ovarian cancer therefore all women should continue pelvic exams as dictated by the gynecologic societies. The need for an annual pelvic exam should be discussed with health care professionals especially in those with family history of ovarian cancer.
- An upper endoscopy may be warranted for patients over 40 years old with dyspeptic symptoms and abdominal bloating or distention, especially in regions with a high prevalence of Helicobacter pylori.
- Chronic pancreatitis, indicated by bloating and pain, may necessitate fecal elastase testing to assess pancreatic function.
The expert review in the AGA clinical update provides step-by-step advice regarding the best practices6 for diagnosis and identifying who to test for ABD.
Treatment Options
The following sections highlight recent best practice advice on therapeutic approaches for treating ABD.
Dietary interventions
Specific foods may trigger bloating and abdominal distention, especially in patients with overlapping DGBIs. However, only a few studies have evaluated dietary restriction specifically for patients with primary ABD. Restricting non-absorbable sugars led to symptomatic improvement in 81% of patients with FABD who had documented sugar malabsorption.17 Two studies have shown that IBS patients treated with a low-fermentable, oligo-, di-, and monosaccharides (FODMAP) diet noted improvement in ABD and that restricting fructans initially may be the most optimal.18 A recent study showed that the Mediterranean diet improved IBS symptoms, including abdominal pain and bloating.19 It should be noted restrictive diets are efficacious but come with short- and long-term challenges. If empiric treatment and/or therapeutic testing do not resolve symptoms, a referral to a dietitian can be useful. Dietitians can provide tailored dietary advice, ensuring patients avoid trigger foods while maintaining a balanced and nutritious diet.
Prokinetics and laxatives
Prokinetic agents are used to treat symptoms of FD, gastroparesis, chronic idiopathic constipation (CIC), and IBS. A meta-analysis of 13 trials found all constipation medications superior to placebo for treating abdominal bloating in patients with IBS-C.20
Probiotics
Treatment with probiotics is recommended for bloating or distention. One double-blind placebo-controlled trial with two separate probiotics, Bifidobacterium lactis and Lactobacillus acidophilus, showed improvements in global GI symptoms of patients with DGBI at 8 weeks versus placebo, with improvements in bloating symptoms.21
Antibiotics
The most commonly studied antibiotic for treating bloating is rifaximin.22 Global symptomatic improvement in IBS patients treated with antibiotics has correlated with the normalization of hydrogen levels in lactulose hydrogen breath tests.22 Patients with non-constipation IBS randomized to rifaximin 550 mg three times daily for 14 days had a greater proportion of relief of IBS-related bloating compared to placebo for at least 2 of the first 4 weeks after treatment.22 Future research warrants use of narrow-spectrum antibiotics study for FABD as the use of broad-spectrum antibiotics may deplete commensals forever, resulting in metabolic disorders.
Biofeedback therapy
Anorectal biofeedback therapy may help with ABD, particularly in patients with IBS-C and chronic constipation. One study noted that post-biofeedback therapy, myoelectric activity of the intercostals and diaphragm decreased, and internal oblique myoelectric activity increased.23 This study also showed ascent of the diaphragm and decreased girth, improving distention.
Central neuromodulators
As bloating results from multiple disturbed mechanisms, including altered gut-brain interaction, these symptoms can be amplified by psychological states such as anxiety, depression, or somatization. Central neuromodulators reduce the perception of visceral signals, re-regulate brain-gut control mechanisms, and improve psychological comorbidities.6 A large study of FD patients demonstrated that both amitriptyline (50 mg daily) and escitalopram (10 mg daily) significantly improved postprandial bloating compared to placebo.24 Antidepressants that activate noradrenergic and serotonergic pathways, including tricyclic antidepressants (e.g., amitriptyline) and serotonin-norepinephrine reuptake inhibitors (e.g., duloxetine and venlafaxine), show the greatest benefit in reducing visceral sensations.6
Brain-gut behavioral therapies
A recent multidisciplinary consensus report supports a myriad of potential brain-gut behavioral therapies (BGBTs) for treating DGBI.25 These therapies, including hypnotherapy, cognitive behavioral therapy (CBT), and other modalities, may be combined with central neuromodulators and other GI treatments in a safe, noninvasive, and complementary fashion. BGBTs do not need to be symptom-specific, as they improve overall quality of life, anxiety, stress, and the burden associated with DGBIs. To date, none of the BGBTs have focused exclusively on FABD; however, prescription-based psychological therapies are now FDA-approved for use on smart apps, improving global symptoms that include bloating in IBS and FD.
Recent AGA clinical update best practices should be considered for the clinical care of patients with ABD.6
Conclusion and Future Perspectives
ABD are highly prevalent and significantly impact patients with various GI and metabolic disorders. Although our understanding of these symptoms is still evolving, evidence increasingly points to the dysregulation of the gut-brain axis and supports the application of the biopsychosocial model in treatment. This model addresses diet, motility, visceral sensitivity, pelvic floor disorders and psychosocial factors, providing a comprehensive approach to patient care.
Physician-scientists around the globe face numerous challenges when evaluating patients with these symptoms. However, the recent AGA clinical update on the best practice guidelines offers step-by-step diagnostic tests and treatment options to assist physicians in making informed decisions. . More comprehensive, large-scale, and longitudinal studies using metabolomics, capsule technologies for discovery of dysbiosis, mass spectrometry, and imaging data are needed to identify the exact contributors to disease pathogenesis, particularly those that can be targeted with pharmacologic agents. Collaborative work between gastroenterologists, dietitians, gut-brain behavioral therapists, endocrinologists, is crucial for clinical care of patients with ABD.
Careful attention to the patient’s primary symptoms and physical examination, combined with advancements in targeted diagnostics like the analysis of microbial markers, metabolites, and molecular signals, can significantly enhance patient clinical outcomes. Additionally, education and effective communication using a patient-centered care model are essential for guiding practical evaluation and individualized treatment.
Dr. Singh is assistant professor (research) at the University of Nevada, Reno, School of Medicine. Dr. Moshiree is director of motility at Atrium Health, and clinical professor of medicine, Wake Forest Medical University, Charlotte, North Carolina.
References
1. Ballou S et al. Prevalence and associated factors of bloating: Results from the Rome Foundation Global Epidemiology Study. Gastroenterology. 2023 June. doi: 10.1053/j.gastro.2023.05.049.
2. Oh JE et al. Abdominal bloating in the United States: Results of a survey of 88,795 Americans examining prevalence and healthcare seeking. Clin Gastroenterol Hepatol. 2023 Aug. doi: 10.1016/j.cgh.2022.10.031.
3. Drossman DA et al. Neuromodulators for functional gastrointestinal disorders (disorders of gut-brain interaction): A Rome Foundation Working Team Report. Gastroenterology. 2018 Mar. doi: 10.1053/j.gastro.2017.11.279.
4. Lacy BE et al. Management of chronic abdominal distension and bloating. Clin Gastroenterol Hepatol. 2021 Feb. doi: 10.1016/j.cgh.2020.03.056.
5. Mearin F et al. Bowel disorders. Gastroenterology. 2016 Feb. doi: 10.1053/j.gastro.2016.02.031.
6. Moshiree B et al. AGA Clinical Practice Update on evaluation and management of belching, abdominal bloating, and distention: expert review. Gastroenterology. 2023 Sep. doi: 10.1053/j.gastro.2023.04.039.
7. Viswanathan L and Rao SS. Intestinal disaccharidase deficiency in adults: evaluation and treatment. Curr Gastroenterol Rep 2023 May. doi: 10.1007/s11894-023-00870-z.
8. Wilder-Smith CH et al. Fructose and lactose intolerance and malabsorption testing: the relationship with symptoms in functional gastrointestinal disorders. Aliment Pharmacol Ther. 2013 Jun. doi: 10.1111/apt.12306.
9. Skodje GI et al. Fructan, rather than gluten, induces symptoms in patients with self-reported non-celiac gluten sensitivity. Gastroenterology. 2018 Feb. doi: 10.1053/j.gastro.2017.10.040.
10. Singh R et al. Current treatment options and therapeutic insights for gastrointestinal dysmotility and functional gastrointestinal disorders. Front Pharmacol. 2022 Jan. doi: 10.3389/fphar.2022.808195.
11. Accarino A et al. Abdominal distention results from caudo-ventral redistribution of contents. Gastroenterology 2009 May. doi: 10.1053/j.gastro.2009.01.067.
12. Shim L et al. Prolonged balloon expulsion is predictive of abdominal distension in bloating. Am J Gastroenterol. 2010 Apr. doi: 10.1038/ajg.2010.54.
13. Villoria A et al. Abdomino-phrenic dyssynergia in patients with abdominal bloating and distension. Am J Gastroenterol. 2011 May. doi: 10.1038/ajg.2010.408.
14. Neri L and Iovino P. Laxative Inadequate Relief Survey Group. Bloating is associated with worse quality of life, treatment satisfaction, and treatment responsiveness among patients with constipation-predominant irritable bowel syndrome and functional constipation. Neurogastroenterol Motil. 2016 Apr. doi: 10.1111/nmo.12758.
15. Saffouri GB et al. Small intestinal microbial dysbiosis underlies symptoms associated with functional gastrointestinal disorders. Nat Commun. 2019 May. doi: 10.1038/s41467-019-09964-7.
16. Villanueva-Millan MJ et al. Methanogens and hydrogen sulfide producing bacteria guide distinct gut microbe profiles and irritable bowel syndrome subtypes. Am J Gastroenterol. 2022 Dec. doi: 10.14309/ajg.0000000000001997.
17. Fernández-Bañares F et al. Sugar malabsorption in functional abdominal bloating: a pilot study on the long-term effect of dietary treatment. Clin Nutr. 2006 Oct. doi: 10.1016/j.clnu.2005.11.010.
18. Böhn L et al. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: a randomized controlled trial. Gastroenterology. 2015 Nov. doi: 10.1053/j.gastro.2015.07.054.
19. Staudacher HM et al. Clinical trial: A Mediterranean diet is feasible and improves gastrointestinal and psychological symptoms in irritable bowel syndrome. Aliment Pharmacol Ther. 2024 Feb. doi: 10.1111/apt.17791.
20. Nelson AD et al. Systematic review and network meta-analysis: efficacy of licensed drugs for abdominal bloating in irritable bowel syndrome with constipation. Aliment Pharmacol Ther. 2021 Jul. doi: 10.1111/apt.16437.
21. Ringel-Kulka T et al. Probiotic bacteria Lactobacillus acidophilus NCFM and Bifidobacterium lactis Bi-07 versus placebo for the symptoms of bloating in patients with functional bowel disorders: a double-blind study. J Clin Gastroenterol. 2011 Jul. doi: 10.1097/MCG.0b013e31820ca4d6.
22. Pimentel M et al. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011 Jan. doi: 10.1056/NEJMoa1004409.
23. Iovino P et al. Pelvic floor biofeedback is an effective treatment for severe bloating in disorders of gut-brain interaction with outlet dysfunction. Neurogastroenterol Motil 2022 May. doi: 10.1111/nmo.14264.
24. Talley NJ et al. Effect of amitriptyline and escitalopram on functional dyspepsia: A multicenter, randomized controlled study. Gastroenterology. 2015 Aug. doi: 10.1053/j.gastro.2015.04.020.
25. Keefer L et al. A Rome Working Team Report on brain-gut behavior therapies for disorders of gut-brain interaction. Gastroenterology. 2022 Jan. doi: 10.1053/j.gastro.2021.09.015.
Endoscopic Management of Barrett’s Esophagus
Introduction
Barrett’s esophagus (BE) is characterized by the replacement of squamous epithelium by columnar metaplasia of the distal esophagus (>1 cm length). It is a precancerous condition, with 3%-5% of patients with BE developing esophageal adenocarcinoma (EAC) in their lifetime. EAC is one of the cancers with high morbidity and mortality (5-year survival < 20%), and its incidence has been on the rise. Studies examining the natural history of BE have demonstrated that the progression happens through a metaplasia-dysplasia-neoplasia sequence. Therefore, early detection of BE and timely management to prevent progression to EAC is crucial.
Grades of Dysplasia
The current gold standard for the diagnosis of BE neoplasia includes a high-quality endoscopic evaluation and biopsies. Biopsies should be obtained from any visible lesions (nodules, ulcers) followed by a random 4-quadrant fashion (Seattle protocol) interval of the entire length of the BE segment. It is essential to pay attention to the results of the biopsy that have been obtained since it will not only determine the surveillance interval but is crucial in planning any necessary endoscopic therapy. The possible results of the biopsy and its implications are:
- No intestinal metaplasia (IM): This would rule out Barrett’s esophagus and no further surveillance would be necessary. A recent population-based study of over 1 million patients showed a 55% and 61% reduced risk of upper gastrointestinal (UGI) cancer and deaths respectively after a negative endoscopy.1
- Intestinal metaplasia with no dysplasia (non-dysplastic BE): Biopsies confirm presence of intestinal metaplasia in the biopsies without any evidence of dysplasia. While the rate of progression to EAC is low (0.07%-0.25%), it is not absent and thus surveillance would be indicated. Current guidelines suggest repeating an endoscopy with biopsy in 5 years if the length of BE is < 3 cm or 3 years if length of BE ≥ 3 cm.2
- Indeterminate for dysplasia (BE-IND): Biopsies confirm IM but are not able to definitively rule out dysplasia. This can be seen in about 4%-8% of the biopsies obtained. The progression rates to EAC are reported to be comparable or lower to low-grade dysplasia (LGD), so the current recommendation is to intensify acid reduction therapy and repeat endoscopy in 6 months. If repeat endoscopy downgrades to non-dysplastic, then can follow surveillance according to NDBE protocol; otherwise recommend continuing surveillance every 12 months.
- Low-grade dysplasia (BE-LGD): Biopsies confirm IM but also show tightly packed overlapping basal nuclei with hyperchromasia and irregular contours, basal stratification of nuclei, and diminished goblet and columnar cell mucus. There is significant inter-observer variability reported,3 and thus the slides must be reviewed by a second pathologist with experience in BE to confirm the findings. Once confirmed, based on risk factors such as presence of multifocal LGD, persistence of LGD, presence of visible lesions, etc., the patient can be offered Barrett’s endoscopic therapy (BET) or undergo continued surveillance. The decision of pursuing one or the other would be dependent on patient preference and shared decision-making between the patient and the provider.
- High-grade dysplasia (BE-HGD): Biopsies confirm IM with cells showing greater degree of cytologic and architectural alterations of dysplasia than LGD but without overt neoplastic features. Over 40% of the patients would progress to EAC and thus the current recommendations would be to recommend BET in these patients.4
- Esophageal adenocarcinoma (EAC): Biopsies demonstrate neoplasia. If the neoplastic changes are limited to the mucosa (T1a) on endoscopic ultrasound or cross-sectional imaging, then BET is suggested. If there is involvement of submucosa, then depending on the depth of invasion, absence of high-risk features (poor differentiation, lymphovascular invasion), BET can be considered as an alternative to esophagectomy.
Lesion Detection on Endoscopy
Data from large population-based studies with at least 3 years of follow-up reported that 58%-66% of EAC detected during endoscopy were diagnosed within 1 year of an index Barrett’s esophagus screening endoscopy, or post-endoscopy Barrett’s neoplasia, and were considered likely to have been missed during index endoscopy.5 This underscores the importance of careful and systematic endoscopic examination during an upper endoscopy.
Studies have also demonstrated that longer examination time was associated with significantly higher detection of HGD/EAC.6,7 Careful examination of the tubular esophagus and gastroesophageal junction (GEJ) should be performed in forward and retroflexed views looking for any subtle areas of nodularity, loop distortion, variability in vascular patterns, mucosal changes concerning for dysplasia or neoplasia. Use of high-definition white light endoscopy (HD-WLE) and virtual chromoendoscopy techniques such as narrow banding imaging (NBI) or blue laser imaging (BLI) are currently recommended in the guidelines.2 Spray chromoendoscopy using acetic acid can also be utilized. Another exciting development is the use of artificial intelligence (AI) in detecting and diagnosing BE associated lesions and neoplasia.
Barrett’s Endoscopic Therapy (BET)
Patients with visible lesions, dysplasia, or early EAC are candidates for BET (Table 1). 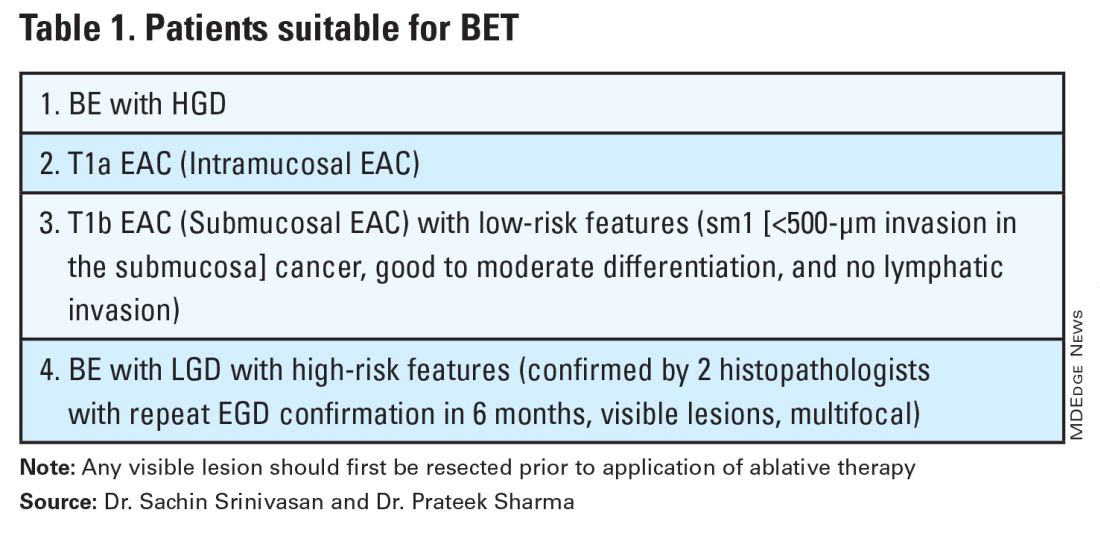
BET involves resective and ablative modalities. The resective modalities include endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) and are the modalities of choice for nodular or raised lesions.
EMR involves endoscopic resection of abnormal mucosa using either lift-assisted technique or multi-band ligation (Figure 1). 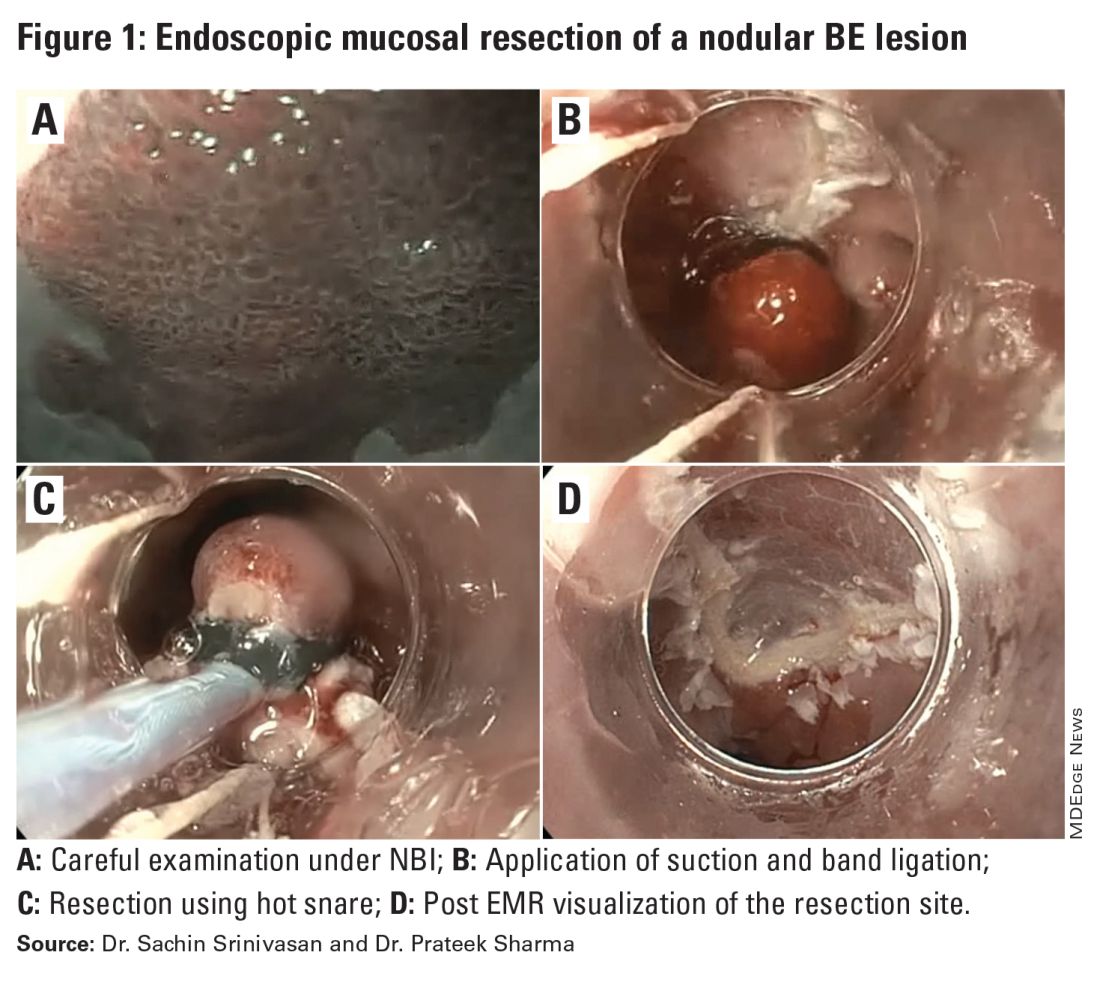
ESD, on the other hand, involves submucosal dissection and perimeter resection of the lesion, thus providing the advantage of an en-bloc resection. In a recent randomized controlled trial (RCT) of 40 patients undergoing ESD vs EMR for HGD/EAC, ESD was better for curative resection (R0) (58%) compared with EMR (12%); however, the remission rates at 3 months were comparable with two perforations reported in the ESD group while there were no complications in the EMR group.8
There is an apparent learning curve when it comes to these advanced techniques, and with more experience, we are seeing comparable results for both these modalities. However, given the complexity and time required for the procedure, current practices typically involve preserving ESD for lesions > 2 cm, those having a likelihood of cancer in the superficial submucosa, or those that EMR cannot remove due to underlying fibrosis or post-EMR recurrence.
The ablative modalities include radiofrequency ablation (RFA), cryotherapy, and hybrid argon plasma coagulation (hybrid APC). These modalities are used for flat lesions, and as therapy following endoscopic resection of nodular lesions to treat residual flat segment of BE. RFA, one of the earliest introduced endoscopic modalities, involves applying directed and controlled heat energy to ablate lesions. Current devices allow circumferential or focal application of RFA. It is a safe and effective modality with good complete eradication of IM (CE-IM) (71%-93%) and complete eradication of dysplasia (CE-D) (91%-100%) rates. These results have been sustained even at 2 years, with the most recent long-term data from a registry study showing a relapse rate of 6% for dysplasia and 19% for IM after 8 years, suggesting durability of this treatment.9
Cryotherapy involves the application of liquid nitrogen or rapidly expanding CO2 to the abnormal mucosa, leading to the rapid freezing and thawing that leads to the death of the cells. Cryogen can be applied as a spray or using a balloon with the spray nozzle in the center. This modality can be used to treat focal lesions and/or larger segments. While it has not been systematically compared with RFA, rates of CE-IM up to 81% and CE-D up to 97% are reported. Hybrid APC involves the use of submucosal saline injection to provide a protective cushion before APC is applied. It has CE-IM rate of 69% and CE-D rate of 67%-86%.10 In a recent RCT of 101 patients randomized to RFA or hybrid APC, CE-IM rates were similar (RFA:74.2% vs hAPC: 82.9%).11
Recently, another technique called radiofrequency vapor ablation (RFVA) is being evaluated, which involves ablating BE segment using vapor at 100° C generated with an RF electrode. A proof-of-concept study of 15 patients showed median squamous conversion of 55% (IQR 33-74) and 98% (IQR 56-99) for 1- and 3-second applications, respectively, with no reported adverse events.12
Barrett’s Refractory to Endoscopic Therapy
Failure of BET is defined as persistent columnar lined epithelium (intestinal metaplasia) with inadequate response, after adequate attempts at endoscopic ablation therapy (after resection) with at least four ablation sessions.13 If encountered, special attention must be given to check compliance with proton pump inhibitors (PPIs), previous incomplete resection, and presence of large hiatal hernia. If CE-IM is not achieved after multiple sessions, change of ablative modality is typically considered. In addition, careful examination for visible lesions should be performed and even if a small one is noted, this should be first resected prior to application of any ablative therapy.
Currently there are no guideline recommendations regarding the preference of one endoscopic modality over another or consideration of potential endoscopic or surgical fundoplication. These modalities primarily rely on technologies available at an institution and the preference of a provider based on their training and experience. Most studies indicate 1-3 sessions (~ 3 months apart) of ablative treatment before achieving CE-IM.
Success and Adverse Events of BET
In a recent real-world study of over 27,000 patients with dysplastic BE, 5295 underwent BET. Analysis showed that patients with HGD/EAC who had BET had a significantly lower 3-year mortality (HGD: RR, 0.59; 95%CI, 0.49-0.71; EAC: RR, 0.53; 95% CI, 0.44-0.65) compared with those who did not undergo BET. Esophageal strictures were the most common adverse event and were noted in 6.5%, followed by chest pain (1.8%), upper GI bleeding (0.47%), and esophageal perforation (0.2%).14
In general, adverse events can be divided into immediate and delayed adverse events. Immediate adverse events typically involve bleeding and perforation that can occur during or shortly after the procedure. These are reported at higher rates with resective modalities compared with ablative therapies. Standard endoscopic techniques involving coagulation grasper or clips can be used to achieve hemostasis. Endoscopic suturing devices offer the ability to contain any perforation. The need for surgical intervention is small and limited to adverse events not detected during the procedure.
Delayed adverse events such as stricture and stenosis are higher for resective modalities (up to 30%), especially when involving more significant than 75% of the esophageal circumference. Post-procedural pain/dysphagia is most common after ablative therapies. Dysphagia reported after any endoscopic therapy should be promptly evaluated, and sequential dilation until the goal esophageal lumen is achieved should be performed every 2-4 weeks.
Recurrences and Surveillance After BET
What is established is that recurrences can occur and may be subtle, therefore detailed endoscopic surveillance is required. In a prospective study, recurrence rates of 15%-16% for IM and 3%-5% for any dysplasia were reported, with the majority being in the first 2 years after achieving CE-IM.15 A systematic review of 21 studies looking at the location of recurrences suggested that the majority (56%) occur in the distal esophagus. Of those that occur in the esophagus, about 80% of them were in the distal 2 cm of the esophagus and only 50% of the recurrences were visible recurrences, thus reiterating the importance of meticulous examination and systematic biopsies.16
On the contrary, a recent single-center study of 217 patients who had achieved CE-IM with 5.5 years of follow-up demonstrated a 26% and 8% recurrence of IM and dysplasia, respectively. One hundred percent of the recurrence in the esophagus was reported as visible.17 Therefore, follow-up endoscopy surveillance protocol after CE-IM should still involve meticulous examination, biopsy of visible lesions, and systematic biopsies for non-visible lesions from the original BE segment, similar to those patients who have not needed BET.
Current guidelines based on expert consensus and evidence recommend surveillance after CE-IM based on original most advanced histology:2
1. LGD: 1 year, 3 years, and every 2 years after that.
2. HGD/EAC: 3 months, 6 months, 12 months, and annually after that.
There is no clear guideline on when to stop surveillance since the longest available follow-up is around 10 years, and recurrences are still detected. A potential surveillance endpoint may be based on age and comorbidities, especially those that would preclude a patient from being a candidate for BET.
When Should a Patient Be Referred?
BE patients with visible lesions and/or dysplastic changes in the biopsy who would require BET should be considered for referral to high-volume centers. Studies have shown higher success for CE-IM and lower rates of adverse events and recurrences in these patients managed at expert centers. The presence of a multidisciplinary team involving pathologists, surgeons, and oncologists is critical and offers a timely opportunity in case of need for a high-risk patient.
Conclusion
BE is a precursor to EAC, with rising incidence and poor 5-year survival. Endoscopic diagnosis is the gold standard and requires a high-quality examination and biopsies. Based on histopathology, a systematic surveillance and BET plan should be performed to achieve CE-IM in patients with dysplasia. Once CE-IM is achieved, regular surveillance should be performed with careful attention to recurrences and complications from the BET modalities.
Dr. Srinivasan and Dr. Sharma are based at the University of Kansas Medical Center, Kansas City, Kansas, and the Kansas City Veterans Affairs Medical Center, Kansas City, Missouri. Dr. Srinivasan has no relevant disclosures. Dr. Sharma disclosed research grants from ERBE, Ironwood Pharmaceuticals, Olympus, and Medtronic. He has served as a consultant for Takeda, Samsung Bioepis, Olympus, and Lumendi, and reports other funding from Medtronic, Fujifilm Medical Systems USA, and Salix.
References
1. Holmberg D, et al. Incidence and mortality in upper gastrointestinal cancer after negative endoscopy for gastroesophageal reflux disease. Gastroenterology. 2022;162(2):431-438.e4.
2. Shaheen NJ, et al. Diagnosis and management of Barrett’s esophagus: An updated ACG guideline. Am J Gastroenterol. 2022 Apr;117(4):559-587.
3. Pech O, et al. Inter-observer variability in the diagnosis of low-grade dysplasia in pathologists: A comparison between experienced and inexperienced pathologists. Gastrointest Endosc. 2006 Apr;63(5):AB130.
4. Krishnamoorthi R, et al. Factors associated with progression of Barrett’s esophagus: A systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2018 Jul;16(7):1046-1055.e8.
5. Visrodia K, et al. Magnitude of missed esophageal adenocarcinoma after Barrett’s esophagus diagnosis: A systematic review and meta-analysis. Gastroenterology. 2016 Mar;150(3):599-607.e7; quiz e14-5.
6. Perisetti A, Sharma P. Tips for improving the identification of neoplastic visible lesions in Barrett’s esophagus. Gastrointest Endosc. 2023 Feb;97(2):248-250.
7. Gupta N, et al. Longer inspection time is associated with increased detection of high-grade dysplasia and esophageal adenocarcinoma in Barrett’s esophagus. Gastrointest Endosc. 2012 Sep;76(3):531-538.
8. Terheggen G, et al. A randomised trial of endoscopic submucosal dissection versus endoscopic mucosal resection for early Barrett’s neoplasia. Gut. 2017 May;66(5):783-793.
9. Wolfson P, et al. Endoscopic eradication therapy for Barrett’s esophagus-related neoplasia: A final 10-year report from the UK National HALO Radiofrequency Ablation Registry. Gastrointest Endosc. 2022 Aug;96(2):223-233.
10. Rösch T, et al. 1151 Multicenter feasibility study of combined injection and argon plasma coagulation (hybrid-APC) in the ablation therapy of neoplastic Barrett esophagus. Gastrointest Endosc. 2017;85(5):AB154.
11. Knabe M, et al. Radiofrequency ablation versus hybrid argon plasma coagulation in Barrett’s esophagus: A prospective randomised trial. Surg Endosc. 2023;37(10):7803-7811.
12. Van Munster SN, et al. Radiofrequency vapor ablation for Barrett’s esophagus: Feasibility, safety, and proof of concept in a stepwise study with in vitro, animal, and the first in-human application. Endoscopy. 2021 Nov;53(11):1162-1168.
13. Emura F, et al. Rio de Janeiro global consensus on landmarks, definitions, and classifications in Barrett’s esophagus: World Endoscopy Organization Delphi study. Gastroenterology. 2022 Jul;163(1):84-96.e2.
14. Singh RR, et al. Real-world evidence of safety and effectiveness of Barrett’s endoscopic therapy. Gastrointest Endosc. 2023 Aug;98(2):155-161.e1.
15. Wani S, et al. Recurrence Is rare following complete eradication of intestinal metaplasia in patients with Barrett’s esophagus and peaks at 18 months. Clin Gastroenterol Hepatol. 2020 Oct;18(11):2609-2617.e2.
16. Duvvuri A, et al. Mo1273 Location and pattern of recurrences in patients with Barrett’s esophagus after endoscopic therapy: A systematic review and critical analysis of the published literature. Gastrointest Endosc. 2020;91(6):AB410-1.
17. He T, et al. Location and appearance of dysplastic Barrett’s esophagus recurrence after endoscopic eradication therapy: No additional yield from random biopsy sampling neosquamous mucosa. Gastrointest Endosc. 2023 Nov;98(5):722-732.
Introduction
Barrett’s esophagus (BE) is characterized by the replacement of squamous epithelium by columnar metaplasia of the distal esophagus (>1 cm length). It is a precancerous condition, with 3%-5% of patients with BE developing esophageal adenocarcinoma (EAC) in their lifetime. EAC is one of the cancers with high morbidity and mortality (5-year survival < 20%), and its incidence has been on the rise. Studies examining the natural history of BE have demonstrated that the progression happens through a metaplasia-dysplasia-neoplasia sequence. Therefore, early detection of BE and timely management to prevent progression to EAC is crucial.
Grades of Dysplasia
The current gold standard for the diagnosis of BE neoplasia includes a high-quality endoscopic evaluation and biopsies. Biopsies should be obtained from any visible lesions (nodules, ulcers) followed by a random 4-quadrant fashion (Seattle protocol) interval of the entire length of the BE segment. It is essential to pay attention to the results of the biopsy that have been obtained since it will not only determine the surveillance interval but is crucial in planning any necessary endoscopic therapy. The possible results of the biopsy and its implications are:
- No intestinal metaplasia (IM): This would rule out Barrett’s esophagus and no further surveillance would be necessary. A recent population-based study of over 1 million patients showed a 55% and 61% reduced risk of upper gastrointestinal (UGI) cancer and deaths respectively after a negative endoscopy.1
- Intestinal metaplasia with no dysplasia (non-dysplastic BE): Biopsies confirm presence of intestinal metaplasia in the biopsies without any evidence of dysplasia. While the rate of progression to EAC is low (0.07%-0.25%), it is not absent and thus surveillance would be indicated. Current guidelines suggest repeating an endoscopy with biopsy in 5 years if the length of BE is < 3 cm or 3 years if length of BE ≥ 3 cm.2
- Indeterminate for dysplasia (BE-IND): Biopsies confirm IM but are not able to definitively rule out dysplasia. This can be seen in about 4%-8% of the biopsies obtained. The progression rates to EAC are reported to be comparable or lower to low-grade dysplasia (LGD), so the current recommendation is to intensify acid reduction therapy and repeat endoscopy in 6 months. If repeat endoscopy downgrades to non-dysplastic, then can follow surveillance according to NDBE protocol; otherwise recommend continuing surveillance every 12 months.
- Low-grade dysplasia (BE-LGD): Biopsies confirm IM but also show tightly packed overlapping basal nuclei with hyperchromasia and irregular contours, basal stratification of nuclei, and diminished goblet and columnar cell mucus. There is significant inter-observer variability reported,3 and thus the slides must be reviewed by a second pathologist with experience in BE to confirm the findings. Once confirmed, based on risk factors such as presence of multifocal LGD, persistence of LGD, presence of visible lesions, etc., the patient can be offered Barrett’s endoscopic therapy (BET) or undergo continued surveillance. The decision of pursuing one or the other would be dependent on patient preference and shared decision-making between the patient and the provider.
- High-grade dysplasia (BE-HGD): Biopsies confirm IM with cells showing greater degree of cytologic and architectural alterations of dysplasia than LGD but without overt neoplastic features. Over 40% of the patients would progress to EAC and thus the current recommendations would be to recommend BET in these patients.4
- Esophageal adenocarcinoma (EAC): Biopsies demonstrate neoplasia. If the neoplastic changes are limited to the mucosa (T1a) on endoscopic ultrasound or cross-sectional imaging, then BET is suggested. If there is involvement of submucosa, then depending on the depth of invasion, absence of high-risk features (poor differentiation, lymphovascular invasion), BET can be considered as an alternative to esophagectomy.
Lesion Detection on Endoscopy
Data from large population-based studies with at least 3 years of follow-up reported that 58%-66% of EAC detected during endoscopy were diagnosed within 1 year of an index Barrett’s esophagus screening endoscopy, or post-endoscopy Barrett’s neoplasia, and were considered likely to have been missed during index endoscopy.5 This underscores the importance of careful and systematic endoscopic examination during an upper endoscopy.
Studies have also demonstrated that longer examination time was associated with significantly higher detection of HGD/EAC.6,7 Careful examination of the tubular esophagus and gastroesophageal junction (GEJ) should be performed in forward and retroflexed views looking for any subtle areas of nodularity, loop distortion, variability in vascular patterns, mucosal changes concerning for dysplasia or neoplasia. Use of high-definition white light endoscopy (HD-WLE) and virtual chromoendoscopy techniques such as narrow banding imaging (NBI) or blue laser imaging (BLI) are currently recommended in the guidelines.2 Spray chromoendoscopy using acetic acid can also be utilized. Another exciting development is the use of artificial intelligence (AI) in detecting and diagnosing BE associated lesions and neoplasia.
Barrett’s Endoscopic Therapy (BET)
Patients with visible lesions, dysplasia, or early EAC are candidates for BET (Table 1). 
BET involves resective and ablative modalities. The resective modalities include endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) and are the modalities of choice for nodular or raised lesions.
EMR involves endoscopic resection of abnormal mucosa using either lift-assisted technique or multi-band ligation (Figure 1). 
ESD, on the other hand, involves submucosal dissection and perimeter resection of the lesion, thus providing the advantage of an en-bloc resection. In a recent randomized controlled trial (RCT) of 40 patients undergoing ESD vs EMR for HGD/EAC, ESD was better for curative resection (R0) (58%) compared with EMR (12%); however, the remission rates at 3 months were comparable with two perforations reported in the ESD group while there were no complications in the EMR group.8
There is an apparent learning curve when it comes to these advanced techniques, and with more experience, we are seeing comparable results for both these modalities. However, given the complexity and time required for the procedure, current practices typically involve preserving ESD for lesions > 2 cm, those having a likelihood of cancer in the superficial submucosa, or those that EMR cannot remove due to underlying fibrosis or post-EMR recurrence.
The ablative modalities include radiofrequency ablation (RFA), cryotherapy, and hybrid argon plasma coagulation (hybrid APC). These modalities are used for flat lesions, and as therapy following endoscopic resection of nodular lesions to treat residual flat segment of BE. RFA, one of the earliest introduced endoscopic modalities, involves applying directed and controlled heat energy to ablate lesions. Current devices allow circumferential or focal application of RFA. It is a safe and effective modality with good complete eradication of IM (CE-IM) (71%-93%) and complete eradication of dysplasia (CE-D) (91%-100%) rates. These results have been sustained even at 2 years, with the most recent long-term data from a registry study showing a relapse rate of 6% for dysplasia and 19% for IM after 8 years, suggesting durability of this treatment.9
Cryotherapy involves the application of liquid nitrogen or rapidly expanding CO2 to the abnormal mucosa, leading to the rapid freezing and thawing that leads to the death of the cells. Cryogen can be applied as a spray or using a balloon with the spray nozzle in the center. This modality can be used to treat focal lesions and/or larger segments. While it has not been systematically compared with RFA, rates of CE-IM up to 81% and CE-D up to 97% are reported. Hybrid APC involves the use of submucosal saline injection to provide a protective cushion before APC is applied. It has CE-IM rate of 69% and CE-D rate of 67%-86%.10 In a recent RCT of 101 patients randomized to RFA or hybrid APC, CE-IM rates were similar (RFA:74.2% vs hAPC: 82.9%).11
Recently, another technique called radiofrequency vapor ablation (RFVA) is being evaluated, which involves ablating BE segment using vapor at 100° C generated with an RF electrode. A proof-of-concept study of 15 patients showed median squamous conversion of 55% (IQR 33-74) and 98% (IQR 56-99) for 1- and 3-second applications, respectively, with no reported adverse events.12
Barrett’s Refractory to Endoscopic Therapy
Failure of BET is defined as persistent columnar lined epithelium (intestinal metaplasia) with inadequate response, after adequate attempts at endoscopic ablation therapy (after resection) with at least four ablation sessions.13 If encountered, special attention must be given to check compliance with proton pump inhibitors (PPIs), previous incomplete resection, and presence of large hiatal hernia. If CE-IM is not achieved after multiple sessions, change of ablative modality is typically considered. In addition, careful examination for visible lesions should be performed and even if a small one is noted, this should be first resected prior to application of any ablative therapy.
Currently there are no guideline recommendations regarding the preference of one endoscopic modality over another or consideration of potential endoscopic or surgical fundoplication. These modalities primarily rely on technologies available at an institution and the preference of a provider based on their training and experience. Most studies indicate 1-3 sessions (~ 3 months apart) of ablative treatment before achieving CE-IM.
Success and Adverse Events of BET
In a recent real-world study of over 27,000 patients with dysplastic BE, 5295 underwent BET. Analysis showed that patients with HGD/EAC who had BET had a significantly lower 3-year mortality (HGD: RR, 0.59; 95%CI, 0.49-0.71; EAC: RR, 0.53; 95% CI, 0.44-0.65) compared with those who did not undergo BET. Esophageal strictures were the most common adverse event and were noted in 6.5%, followed by chest pain (1.8%), upper GI bleeding (0.47%), and esophageal perforation (0.2%).14
In general, adverse events can be divided into immediate and delayed adverse events. Immediate adverse events typically involve bleeding and perforation that can occur during or shortly after the procedure. These are reported at higher rates with resective modalities compared with ablative therapies. Standard endoscopic techniques involving coagulation grasper or clips can be used to achieve hemostasis. Endoscopic suturing devices offer the ability to contain any perforation. The need for surgical intervention is small and limited to adverse events not detected during the procedure.
Delayed adverse events such as stricture and stenosis are higher for resective modalities (up to 30%), especially when involving more significant than 75% of the esophageal circumference. Post-procedural pain/dysphagia is most common after ablative therapies. Dysphagia reported after any endoscopic therapy should be promptly evaluated, and sequential dilation until the goal esophageal lumen is achieved should be performed every 2-4 weeks.
Recurrences and Surveillance After BET
What is established is that recurrences can occur and may be subtle, therefore detailed endoscopic surveillance is required. In a prospective study, recurrence rates of 15%-16% for IM and 3%-5% for any dysplasia were reported, with the majority being in the first 2 years after achieving CE-IM.15 A systematic review of 21 studies looking at the location of recurrences suggested that the majority (56%) occur in the distal esophagus. Of those that occur in the esophagus, about 80% of them were in the distal 2 cm of the esophagus and only 50% of the recurrences were visible recurrences, thus reiterating the importance of meticulous examination and systematic biopsies.16
On the contrary, a recent single-center study of 217 patients who had achieved CE-IM with 5.5 years of follow-up demonstrated a 26% and 8% recurrence of IM and dysplasia, respectively. One hundred percent of the recurrence in the esophagus was reported as visible.17 Therefore, follow-up endoscopy surveillance protocol after CE-IM should still involve meticulous examination, biopsy of visible lesions, and systematic biopsies for non-visible lesions from the original BE segment, similar to those patients who have not needed BET.
Current guidelines based on expert consensus and evidence recommend surveillance after CE-IM based on original most advanced histology:2
1. LGD: 1 year, 3 years, and every 2 years after that.
2. HGD/EAC: 3 months, 6 months, 12 months, and annually after that.
There is no clear guideline on when to stop surveillance since the longest available follow-up is around 10 years, and recurrences are still detected. A potential surveillance endpoint may be based on age and comorbidities, especially those that would preclude a patient from being a candidate for BET.
When Should a Patient Be Referred?
BE patients with visible lesions and/or dysplastic changes in the biopsy who would require BET should be considered for referral to high-volume centers. Studies have shown higher success for CE-IM and lower rates of adverse events and recurrences in these patients managed at expert centers. The presence of a multidisciplinary team involving pathologists, surgeons, and oncologists is critical and offers a timely opportunity in case of need for a high-risk patient.
Conclusion
BE is a precursor to EAC, with rising incidence and poor 5-year survival. Endoscopic diagnosis is the gold standard and requires a high-quality examination and biopsies. Based on histopathology, a systematic surveillance and BET plan should be performed to achieve CE-IM in patients with dysplasia. Once CE-IM is achieved, regular surveillance should be performed with careful attention to recurrences and complications from the BET modalities.
Dr. Srinivasan and Dr. Sharma are based at the University of Kansas Medical Center, Kansas City, Kansas, and the Kansas City Veterans Affairs Medical Center, Kansas City, Missouri. Dr. Srinivasan has no relevant disclosures. Dr. Sharma disclosed research grants from ERBE, Ironwood Pharmaceuticals, Olympus, and Medtronic. He has served as a consultant for Takeda, Samsung Bioepis, Olympus, and Lumendi, and reports other funding from Medtronic, Fujifilm Medical Systems USA, and Salix.
References
1. Holmberg D, et al. Incidence and mortality in upper gastrointestinal cancer after negative endoscopy for gastroesophageal reflux disease. Gastroenterology. 2022;162(2):431-438.e4.
2. Shaheen NJ, et al. Diagnosis and management of Barrett’s esophagus: An updated ACG guideline. Am J Gastroenterol. 2022 Apr;117(4):559-587.
3. Pech O, et al. Inter-observer variability in the diagnosis of low-grade dysplasia in pathologists: A comparison between experienced and inexperienced pathologists. Gastrointest Endosc. 2006 Apr;63(5):AB130.
4. Krishnamoorthi R, et al. Factors associated with progression of Barrett’s esophagus: A systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2018 Jul;16(7):1046-1055.e8.
5. Visrodia K, et al. Magnitude of missed esophageal adenocarcinoma after Barrett’s esophagus diagnosis: A systematic review and meta-analysis. Gastroenterology. 2016 Mar;150(3):599-607.e7; quiz e14-5.
6. Perisetti A, Sharma P. Tips for improving the identification of neoplastic visible lesions in Barrett’s esophagus. Gastrointest Endosc. 2023 Feb;97(2):248-250.
7. Gupta N, et al. Longer inspection time is associated with increased detection of high-grade dysplasia and esophageal adenocarcinoma in Barrett’s esophagus. Gastrointest Endosc. 2012 Sep;76(3):531-538.
8. Terheggen G, et al. A randomised trial of endoscopic submucosal dissection versus endoscopic mucosal resection for early Barrett’s neoplasia. Gut. 2017 May;66(5):783-793.
9. Wolfson P, et al. Endoscopic eradication therapy for Barrett’s esophagus-related neoplasia: A final 10-year report from the UK National HALO Radiofrequency Ablation Registry. Gastrointest Endosc. 2022 Aug;96(2):223-233.
10. Rösch T, et al. 1151 Multicenter feasibility study of combined injection and argon plasma coagulation (hybrid-APC) in the ablation therapy of neoplastic Barrett esophagus. Gastrointest Endosc. 2017;85(5):AB154.
11. Knabe M, et al. Radiofrequency ablation versus hybrid argon plasma coagulation in Barrett’s esophagus: A prospective randomised trial. Surg Endosc. 2023;37(10):7803-7811.
12. Van Munster SN, et al. Radiofrequency vapor ablation for Barrett’s esophagus: Feasibility, safety, and proof of concept in a stepwise study with in vitro, animal, and the first in-human application. Endoscopy. 2021 Nov;53(11):1162-1168.
13. Emura F, et al. Rio de Janeiro global consensus on landmarks, definitions, and classifications in Barrett’s esophagus: World Endoscopy Organization Delphi study. Gastroenterology. 2022 Jul;163(1):84-96.e2.
14. Singh RR, et al. Real-world evidence of safety and effectiveness of Barrett’s endoscopic therapy. Gastrointest Endosc. 2023 Aug;98(2):155-161.e1.
15. Wani S, et al. Recurrence Is rare following complete eradication of intestinal metaplasia in patients with Barrett’s esophagus and peaks at 18 months. Clin Gastroenterol Hepatol. 2020 Oct;18(11):2609-2617.e2.
16. Duvvuri A, et al. Mo1273 Location and pattern of recurrences in patients with Barrett’s esophagus after endoscopic therapy: A systematic review and critical analysis of the published literature. Gastrointest Endosc. 2020;91(6):AB410-1.
17. He T, et al. Location and appearance of dysplastic Barrett’s esophagus recurrence after endoscopic eradication therapy: No additional yield from random biopsy sampling neosquamous mucosa. Gastrointest Endosc. 2023 Nov;98(5):722-732.
Introduction
Barrett’s esophagus (BE) is characterized by the replacement of squamous epithelium by columnar metaplasia of the distal esophagus (>1 cm length). It is a precancerous condition, with 3%-5% of patients with BE developing esophageal adenocarcinoma (EAC) in their lifetime. EAC is one of the cancers with high morbidity and mortality (5-year survival < 20%), and its incidence has been on the rise. Studies examining the natural history of BE have demonstrated that the progression happens through a metaplasia-dysplasia-neoplasia sequence. Therefore, early detection of BE and timely management to prevent progression to EAC is crucial.
Grades of Dysplasia
The current gold standard for the diagnosis of BE neoplasia includes a high-quality endoscopic evaluation and biopsies. Biopsies should be obtained from any visible lesions (nodules, ulcers) followed by a random 4-quadrant fashion (Seattle protocol) interval of the entire length of the BE segment. It is essential to pay attention to the results of the biopsy that have been obtained since it will not only determine the surveillance interval but is crucial in planning any necessary endoscopic therapy. The possible results of the biopsy and its implications are:
- No intestinal metaplasia (IM): This would rule out Barrett’s esophagus and no further surveillance would be necessary. A recent population-based study of over 1 million patients showed a 55% and 61% reduced risk of upper gastrointestinal (UGI) cancer and deaths respectively after a negative endoscopy.1
- Intestinal metaplasia with no dysplasia (non-dysplastic BE): Biopsies confirm presence of intestinal metaplasia in the biopsies without any evidence of dysplasia. While the rate of progression to EAC is low (0.07%-0.25%), it is not absent and thus surveillance would be indicated. Current guidelines suggest repeating an endoscopy with biopsy in 5 years if the length of BE is < 3 cm or 3 years if length of BE ≥ 3 cm.2
- Indeterminate for dysplasia (BE-IND): Biopsies confirm IM but are not able to definitively rule out dysplasia. This can be seen in about 4%-8% of the biopsies obtained. The progression rates to EAC are reported to be comparable or lower to low-grade dysplasia (LGD), so the current recommendation is to intensify acid reduction therapy and repeat endoscopy in 6 months. If repeat endoscopy downgrades to non-dysplastic, then can follow surveillance according to NDBE protocol; otherwise recommend continuing surveillance every 12 months.
- Low-grade dysplasia (BE-LGD): Biopsies confirm IM but also show tightly packed overlapping basal nuclei with hyperchromasia and irregular contours, basal stratification of nuclei, and diminished goblet and columnar cell mucus. There is significant inter-observer variability reported,3 and thus the slides must be reviewed by a second pathologist with experience in BE to confirm the findings. Once confirmed, based on risk factors such as presence of multifocal LGD, persistence of LGD, presence of visible lesions, etc., the patient can be offered Barrett’s endoscopic therapy (BET) or undergo continued surveillance. The decision of pursuing one or the other would be dependent on patient preference and shared decision-making between the patient and the provider.
- High-grade dysplasia (BE-HGD): Biopsies confirm IM with cells showing greater degree of cytologic and architectural alterations of dysplasia than LGD but without overt neoplastic features. Over 40% of the patients would progress to EAC and thus the current recommendations would be to recommend BET in these patients.4
- Esophageal adenocarcinoma (EAC): Biopsies demonstrate neoplasia. If the neoplastic changes are limited to the mucosa (T1a) on endoscopic ultrasound or cross-sectional imaging, then BET is suggested. If there is involvement of submucosa, then depending on the depth of invasion, absence of high-risk features (poor differentiation, lymphovascular invasion), BET can be considered as an alternative to esophagectomy.
Lesion Detection on Endoscopy
Data from large population-based studies with at least 3 years of follow-up reported that 58%-66% of EAC detected during endoscopy were diagnosed within 1 year of an index Barrett’s esophagus screening endoscopy, or post-endoscopy Barrett’s neoplasia, and were considered likely to have been missed during index endoscopy.5 This underscores the importance of careful and systematic endoscopic examination during an upper endoscopy.
Studies have also demonstrated that longer examination time was associated with significantly higher detection of HGD/EAC.6,7 Careful examination of the tubular esophagus and gastroesophageal junction (GEJ) should be performed in forward and retroflexed views looking for any subtle areas of nodularity, loop distortion, variability in vascular patterns, mucosal changes concerning for dysplasia or neoplasia. Use of high-definition white light endoscopy (HD-WLE) and virtual chromoendoscopy techniques such as narrow banding imaging (NBI) or blue laser imaging (BLI) are currently recommended in the guidelines.2 Spray chromoendoscopy using acetic acid can also be utilized. Another exciting development is the use of artificial intelligence (AI) in detecting and diagnosing BE associated lesions and neoplasia.
Barrett’s Endoscopic Therapy (BET)
Patients with visible lesions, dysplasia, or early EAC are candidates for BET (Table 1). 
BET involves resective and ablative modalities. The resective modalities include endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) and are the modalities of choice for nodular or raised lesions.
EMR involves endoscopic resection of abnormal mucosa using either lift-assisted technique or multi-band ligation (Figure 1). 
ESD, on the other hand, involves submucosal dissection and perimeter resection of the lesion, thus providing the advantage of an en-bloc resection. In a recent randomized controlled trial (RCT) of 40 patients undergoing ESD vs EMR for HGD/EAC, ESD was better for curative resection (R0) (58%) compared with EMR (12%); however, the remission rates at 3 months were comparable with two perforations reported in the ESD group while there were no complications in the EMR group.8
There is an apparent learning curve when it comes to these advanced techniques, and with more experience, we are seeing comparable results for both these modalities. However, given the complexity and time required for the procedure, current practices typically involve preserving ESD for lesions > 2 cm, those having a likelihood of cancer in the superficial submucosa, or those that EMR cannot remove due to underlying fibrosis or post-EMR recurrence.
The ablative modalities include radiofrequency ablation (RFA), cryotherapy, and hybrid argon plasma coagulation (hybrid APC). These modalities are used for flat lesions, and as therapy following endoscopic resection of nodular lesions to treat residual flat segment of BE. RFA, one of the earliest introduced endoscopic modalities, involves applying directed and controlled heat energy to ablate lesions. Current devices allow circumferential or focal application of RFA. It is a safe and effective modality with good complete eradication of IM (CE-IM) (71%-93%) and complete eradication of dysplasia (CE-D) (91%-100%) rates. These results have been sustained even at 2 years, with the most recent long-term data from a registry study showing a relapse rate of 6% for dysplasia and 19% for IM after 8 years, suggesting durability of this treatment.9
Cryotherapy involves the application of liquid nitrogen or rapidly expanding CO2 to the abnormal mucosa, leading to the rapid freezing and thawing that leads to the death of the cells. Cryogen can be applied as a spray or using a balloon with the spray nozzle in the center. This modality can be used to treat focal lesions and/or larger segments. While it has not been systematically compared with RFA, rates of CE-IM up to 81% and CE-D up to 97% are reported. Hybrid APC involves the use of submucosal saline injection to provide a protective cushion before APC is applied. It has CE-IM rate of 69% and CE-D rate of 67%-86%.10 In a recent RCT of 101 patients randomized to RFA or hybrid APC, CE-IM rates were similar (RFA:74.2% vs hAPC: 82.9%).11
Recently, another technique called radiofrequency vapor ablation (RFVA) is being evaluated, which involves ablating BE segment using vapor at 100° C generated with an RF electrode. A proof-of-concept study of 15 patients showed median squamous conversion of 55% (IQR 33-74) and 98% (IQR 56-99) for 1- and 3-second applications, respectively, with no reported adverse events.12
Barrett’s Refractory to Endoscopic Therapy
Failure of BET is defined as persistent columnar lined epithelium (intestinal metaplasia) with inadequate response, after adequate attempts at endoscopic ablation therapy (after resection) with at least four ablation sessions.13 If encountered, special attention must be given to check compliance with proton pump inhibitors (PPIs), previous incomplete resection, and presence of large hiatal hernia. If CE-IM is not achieved after multiple sessions, change of ablative modality is typically considered. In addition, careful examination for visible lesions should be performed and even if a small one is noted, this should be first resected prior to application of any ablative therapy.
Currently there are no guideline recommendations regarding the preference of one endoscopic modality over another or consideration of potential endoscopic or surgical fundoplication. These modalities primarily rely on technologies available at an institution and the preference of a provider based on their training and experience. Most studies indicate 1-3 sessions (~ 3 months apart) of ablative treatment before achieving CE-IM.
Success and Adverse Events of BET
In a recent real-world study of over 27,000 patients with dysplastic BE, 5295 underwent BET. Analysis showed that patients with HGD/EAC who had BET had a significantly lower 3-year mortality (HGD: RR, 0.59; 95%CI, 0.49-0.71; EAC: RR, 0.53; 95% CI, 0.44-0.65) compared with those who did not undergo BET. Esophageal strictures were the most common adverse event and were noted in 6.5%, followed by chest pain (1.8%), upper GI bleeding (0.47%), and esophageal perforation (0.2%).14
In general, adverse events can be divided into immediate and delayed adverse events. Immediate adverse events typically involve bleeding and perforation that can occur during or shortly after the procedure. These are reported at higher rates with resective modalities compared with ablative therapies. Standard endoscopic techniques involving coagulation grasper or clips can be used to achieve hemostasis. Endoscopic suturing devices offer the ability to contain any perforation. The need for surgical intervention is small and limited to adverse events not detected during the procedure.
Delayed adverse events such as stricture and stenosis are higher for resective modalities (up to 30%), especially when involving more significant than 75% of the esophageal circumference. Post-procedural pain/dysphagia is most common after ablative therapies. Dysphagia reported after any endoscopic therapy should be promptly evaluated, and sequential dilation until the goal esophageal lumen is achieved should be performed every 2-4 weeks.
Recurrences and Surveillance After BET
What is established is that recurrences can occur and may be subtle, therefore detailed endoscopic surveillance is required. In a prospective study, recurrence rates of 15%-16% for IM and 3%-5% for any dysplasia were reported, with the majority being in the first 2 years after achieving CE-IM.15 A systematic review of 21 studies looking at the location of recurrences suggested that the majority (56%) occur in the distal esophagus. Of those that occur in the esophagus, about 80% of them were in the distal 2 cm of the esophagus and only 50% of the recurrences were visible recurrences, thus reiterating the importance of meticulous examination and systematic biopsies.16
On the contrary, a recent single-center study of 217 patients who had achieved CE-IM with 5.5 years of follow-up demonstrated a 26% and 8% recurrence of IM and dysplasia, respectively. One hundred percent of the recurrence in the esophagus was reported as visible.17 Therefore, follow-up endoscopy surveillance protocol after CE-IM should still involve meticulous examination, biopsy of visible lesions, and systematic biopsies for non-visible lesions from the original BE segment, similar to those patients who have not needed BET.
Current guidelines based on expert consensus and evidence recommend surveillance after CE-IM based on original most advanced histology:2
1. LGD: 1 year, 3 years, and every 2 years after that.
2. HGD/EAC: 3 months, 6 months, 12 months, and annually after that.
There is no clear guideline on when to stop surveillance since the longest available follow-up is around 10 years, and recurrences are still detected. A potential surveillance endpoint may be based on age and comorbidities, especially those that would preclude a patient from being a candidate for BET.
When Should a Patient Be Referred?
BE patients with visible lesions and/or dysplastic changes in the biopsy who would require BET should be considered for referral to high-volume centers. Studies have shown higher success for CE-IM and lower rates of adverse events and recurrences in these patients managed at expert centers. The presence of a multidisciplinary team involving pathologists, surgeons, and oncologists is critical and offers a timely opportunity in case of need for a high-risk patient.
Conclusion
BE is a precursor to EAC, with rising incidence and poor 5-year survival. Endoscopic diagnosis is the gold standard and requires a high-quality examination and biopsies. Based on histopathology, a systematic surveillance and BET plan should be performed to achieve CE-IM in patients with dysplasia. Once CE-IM is achieved, regular surveillance should be performed with careful attention to recurrences and complications from the BET modalities.
Dr. Srinivasan and Dr. Sharma are based at the University of Kansas Medical Center, Kansas City, Kansas, and the Kansas City Veterans Affairs Medical Center, Kansas City, Missouri. Dr. Srinivasan has no relevant disclosures. Dr. Sharma disclosed research grants from ERBE, Ironwood Pharmaceuticals, Olympus, and Medtronic. He has served as a consultant for Takeda, Samsung Bioepis, Olympus, and Lumendi, and reports other funding from Medtronic, Fujifilm Medical Systems USA, and Salix.
References
1. Holmberg D, et al. Incidence and mortality in upper gastrointestinal cancer after negative endoscopy for gastroesophageal reflux disease. Gastroenterology. 2022;162(2):431-438.e4.
2. Shaheen NJ, et al. Diagnosis and management of Barrett’s esophagus: An updated ACG guideline. Am J Gastroenterol. 2022 Apr;117(4):559-587.
3. Pech O, et al. Inter-observer variability in the diagnosis of low-grade dysplasia in pathologists: A comparison between experienced and inexperienced pathologists. Gastrointest Endosc. 2006 Apr;63(5):AB130.
4. Krishnamoorthi R, et al. Factors associated with progression of Barrett’s esophagus: A systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2018 Jul;16(7):1046-1055.e8.
5. Visrodia K, et al. Magnitude of missed esophageal adenocarcinoma after Barrett’s esophagus diagnosis: A systematic review and meta-analysis. Gastroenterology. 2016 Mar;150(3):599-607.e7; quiz e14-5.
6. Perisetti A, Sharma P. Tips for improving the identification of neoplastic visible lesions in Barrett’s esophagus. Gastrointest Endosc. 2023 Feb;97(2):248-250.
7. Gupta N, et al. Longer inspection time is associated with increased detection of high-grade dysplasia and esophageal adenocarcinoma in Barrett’s esophagus. Gastrointest Endosc. 2012 Sep;76(3):531-538.
8. Terheggen G, et al. A randomised trial of endoscopic submucosal dissection versus endoscopic mucosal resection for early Barrett’s neoplasia. Gut. 2017 May;66(5):783-793.
9. Wolfson P, et al. Endoscopic eradication therapy for Barrett’s esophagus-related neoplasia: A final 10-year report from the UK National HALO Radiofrequency Ablation Registry. Gastrointest Endosc. 2022 Aug;96(2):223-233.
10. Rösch T, et al. 1151 Multicenter feasibility study of combined injection and argon plasma coagulation (hybrid-APC) in the ablation therapy of neoplastic Barrett esophagus. Gastrointest Endosc. 2017;85(5):AB154.
11. Knabe M, et al. Radiofrequency ablation versus hybrid argon plasma coagulation in Barrett’s esophagus: A prospective randomised trial. Surg Endosc. 2023;37(10):7803-7811.
12. Van Munster SN, et al. Radiofrequency vapor ablation for Barrett’s esophagus: Feasibility, safety, and proof of concept in a stepwise study with in vitro, animal, and the first in-human application. Endoscopy. 2021 Nov;53(11):1162-1168.
13. Emura F, et al. Rio de Janeiro global consensus on landmarks, definitions, and classifications in Barrett’s esophagus: World Endoscopy Organization Delphi study. Gastroenterology. 2022 Jul;163(1):84-96.e2.
14. Singh RR, et al. Real-world evidence of safety and effectiveness of Barrett’s endoscopic therapy. Gastrointest Endosc. 2023 Aug;98(2):155-161.e1.
15. Wani S, et al. Recurrence Is rare following complete eradication of intestinal metaplasia in patients with Barrett’s esophagus and peaks at 18 months. Clin Gastroenterol Hepatol. 2020 Oct;18(11):2609-2617.e2.
16. Duvvuri A, et al. Mo1273 Location and pattern of recurrences in patients with Barrett’s esophagus after endoscopic therapy: A systematic review and critical analysis of the published literature. Gastrointest Endosc. 2020;91(6):AB410-1.
17. He T, et al. Location and appearance of dysplastic Barrett’s esophagus recurrence after endoscopic eradication therapy: No additional yield from random biopsy sampling neosquamous mucosa. Gastrointest Endosc. 2023 Nov;98(5):722-732.
Endoscopic Management of Benign Gallbladder Disease
Introduction
The treatment of benign gallbladder disease has changed substantially in the past decade, but this represents only a snapshot in the evolutionary history of the management of this organ. What began as a problem managed exclusively by open cholecystectomy (CCY) transitioned into a race toward minimally invasive approaches in the 1980s, with advances from gastroenterology, surgery, and radiology.
The opening strides were made in 1980 with the first description of percutaneous cholecystostomy (PC) by Dr. R.W. Radder.1 Shortly thereafter, in 1984, Dr. Richard Kozarek first reported the feasibility of selective cystic duct cannulation during endoscopic retrograde cholangiopancreatography (ERCP).2 Subsequent stenting for the treatment of acute cholecystitis (endoscopic transpapillary gallbladder drainage, ET-GBD) was then reported by Tamada et. al. in 1991.3 Not to be outdone, the first laparoscopic cholecystectomy (LC) was completed by Dr. Med Erich Mühe of Germany in 1985.4 More recently, with the expansion of interventional endoscopic ultrasound (EUS), the first transmural EUS-guided gallbladder drainage (EUS-GBD) was described by Dr. Baron and Dr. Topazian in 2007.5
The subsequent advent of lumen apposing metal stents (LAMS) has cemented EUS-GBD in the toolbox of treatment for benign gallbladder disease. Results of a recent prospective multicenter trial, with a Food and Drug Administration–approved protocol and investigational device exemption, have been published, opening the door for the expansion of FDA approved indications for this device.6
Benign gallbladder disease encompasses both polyps (benign and premalignant) and cholecystitis (acute/chronic, calculous/acalculous), in addition to others. The four management techniques (LC, PC, ET-GBD, and EUS-GBD) have filled integral niches in the management of these patients. Even gallbladder polyps have not been able to escape the reach of endoscopic approaches with the recent description of LAMS-assisted polypectomy as part of a gallbladder preserving strategy.7,8 While EUS-GBD also has been used for biliary decompression in the presence of a patent cystic duct and absence of cholecystitis, .9 Both of these techniques have gained wide recognition and/or guideline support for their use from the American Society for Gastrointestinal Endoscopy (ASGE) and the European Society of Gastrointestinal Endoscopy (ESGE).10,11 In addition, there is now one FDA-approved stent device for treatment of acute cholecystitis in patients unfit for surgery.
Techniques & Tips
ET-GBD
- During ERCP, after successful cannulation of the bile duct, attempted wire cannulation of the cystic duct is performed.
A cholangiogram, which clearly delineates the insertion of the cystic duct into the main bile duct, can enhance cannulation success. Rotatable fluoroscopy can facilitate identification.
- After anatomy is clear, wire access is often best achieved using a sphincterotome or stone retrieval (occlusion) balloon.
The balloon, once inflated, can be pulled downward to establish traction on the main bile duct, which can straighten the approach.
- After superficial wire engagement into the cystic duct, the accessory used can be slowly advanced into the cystic duct to stabilize the catheter and then navigate the valves of Heister to reach the gallbladder lumen.
Use of a sphincterotome, which directs toward the patient’s right (most often direction of cystic duct takeoff), is helpful. Angled guidewires are preferable. We often use a 0.035-inch, 260-cm angled hydrophilic wire (GLIDEWIRE; Terumo, Somerset, NJ) to overcome this challenging portion of ET-GBD.
If despite the above maneuvers the guidewire has failed to enter the cystic duct, cholangioscopy can be used to identify the orifice and/or stabilize deep wire cannulation. This is often cumbersome, time consuming, does not always produce success, and requires additional expertise.
- If a stone is encountered that cannot be extracted or traversed by a guidewire, cholangioscopy with electrohydraulic lithotripsy can be pursued.
- After the guidewire has entered the gallbladder, a 5 French or 7 French plastic double pigtail stent is placed. Typical lengths are 9-15 cm.
Some authors prefer to use two side-by-side plastic stents.12 This has been shown retrospectively to enhance the long term clinical success of ET-GBD but with additional technical difficulty.
- This stent can remain in place indefinitely and need not be exchanged, though it should be removed just prior to CCY if pursued. Alternatively, the surgeon can be alerted to its presence and, if comfortable, it can be removed intraoperatively.
EUS-GBD
- Use of fluoroscopy is optional but can enhance technical success in selected situations.
- Conversion, or internalization, of PC is reasonable and can enhance patient quality of life.13
- If the gallbladder wall is not in close apposition to the duodenal (or gastric) wall, consider measuring the distance.
We preferentially use 10-mm diameter by 10-mm saddle length LAMS for EUS-GBD, unless the above distance warrants use of a 15-mm by 15-mm LAMS (AXIOS, Boston Scientific, Marlborough, MA). If the distance is greater than 15 mm, consider searching for an alternative site, using a traditional biliary fully covered self-expandable metal stent (FCSEMS) for longer length, or converting to ET-GBD. Smaller diameter (8 mm) with an 8-mm saddle length can be used as well. The optimal diameter is unknown and also dependent on whether transluminal endoscopic diagnosis or therapy is a consideration.
- If there is difficulty locating the gallbladder, it may be decompressed or small (particularly if PC or a partial CCY has already been performed).
If a cholecystostomy tube is in place, instillation of sterile water via the tube can sometimes improve the target for LAMS placement, though caution should be made to not over-distend the gallbladder. ERCP with placement of a nasobiliary tube into the gallbladder can also serve this purpose and has been previously described.14
The gallbladder can be punctured with a 19-gauge FNA needle to instill sterile water and distend the gallbladder with the added benefit of being able to pass a guidewire, which may enhance procedural safety in difficult cases. However, success of this technique is contingent on fluid remaining within the gallbladder and not transiting out via the cystic duct. Expedient exchange of the FNA needle for the LAMS device may be necessary.
- Attempt to confirm location within the duodenum prior to puncture, as gastric origins can pose unique ramifications (i.e. potential for partial gastric outlet obstruction, obstruction of LAMS with food debris, etc.).
It can be easy to mistake an unintentional pre-pyloric position for a position within the duodenum since the working channel is behind (proximal to) the echoprobe.
- Turning off Doppler flow prior to advancement of the cautery enhanced LAMS can reduce obscurement of views on entry into the gallbladder. Lack of certainty about entry or misdeployment after presumed entry herald the most challenging aspect of EUS-GBD.
Utilization of a previously placed guidewire or advancement of one preloaded into the LAMS can aid in both enhancing confidence in location and assist with salvage maneuvers, if needed.
- After successful deployment of the LAMS we routinely place a double pigtail plastic stent through it (typically 7 French by 4 cm) to maintain patency. This may also prevent bleeding from the LAMS flange abrading the wall of either lumen.
- We routinely exchange the LAMS for two double pigtail plastic stents (typically 7 French by 4 cm) 4 weeks after initial placement especially when there is a more than modest residual stone burden (data in press). These plastic stents can remain in place indefinitely.
This exchange can be deferred if the patient is not expected to survive until the one-year anniversary of LAMS deployment. After one year the LAMS plastic covering may degrade and pose additional problems.15
LAMS Misdeployment Salvage Tips
- Salvage techniques can vary from simple to complex.
- If a wire is in place, it can be used to balloon or catheter dilate the tract and place a FCSEMS traversing the gallbladder and duodenal/gastric lumens. A similar approach can be used if the LAMS deployed on only one side (gallbladder or duodenum/stomach) and the other flange is within the peritoneum.
- The most challenging scenario to salvage is if the LAMS is misdeployed or becomes dislodged and no wire is present. This is why the use of a guidewire, even if preloaded into the LAMS and placement is freehand, is essential for EUS-GBD. A potential technique is to balloon dilate the duodenal/gastric defect and drive the endoscope into the peritoneum to reconnect that lumen to the gallbladder defect or LAMS, depending on the site of misdeployment. Doing so requires a high degree of commitment and skill and should not be done casually.
- If uncertainty remains or if misdeployment has occurred and salvage attempts have failed, consider closure of the duodenal/gastric defect and conversion to ET-GBD.
This may both treat the initial procedural indication and assist with what is essentially a large bile leak, which might also require percutaneous therapy for non-surgical management.
- For endoscopists with limited experience at salvage techniques, it is reasonable for the threshold for conversion to be low, assuming experience with and confidence in ET-GBD is high.
- If salvage is successful but ambiguity remains, consider obtaining a cholangiogram via the LAMS to confirm positioning and absence of leak.
Adverse Events
Both ET-GBD and EUS-GBD should be performed by an endoscopist comfortable with their techniques and the management of their adverse events (AEs). Rates for EUS-GBD AEs in patients at high risk for LC were reported in one international multicenter registry to be 15.3% with a 30-day mortality of 9.2%, with a significant predictor of AE being endoscopist experience less than 25 procedures.16 A meta-analysis also found an overall AE rate of 18.31%, with rates for perforation and stent related AEs (i.e. migration, occlusion, pneumoperitoneum) being 6.71% and 8.16%, respectively.17 For this reason, we recommend that patients with cholecystitis who are deemed to be poor surgical candidates be transferred to a tertiary referral center with expertise in these approaches. Rates of AEs for ET-GBD are similar to that for standard ERCP, with reported ranges of 5%-10.3%.10
Comparisons Between Techniques
The decision on which technique to utilize for endoscopic management of cholecystitis or symptomatic cholelithiasis depends first and foremost on the expertise and comfort level of the endoscopist. Given the additional training that an advanced endoscopist needs to perform EUS-GBD, combined with the perhaps slightly higher AE rate and permanency of endoscopic cholecystostomy, it is reasonable to proceed with a trial of ET-GBD if confidence is insufficient. However, ET-GBD can certainly be more technically challenging and less effective than EUS-GBD, with lower reported technical and clinical success rates (technical 85.3% vs 93.0%, clinical 95.2% vs 97.3%).18 Despite this, the rate of recurrence of cholecystitis is similar between ET-GBD and EUS-GBD (4.6% vs 4.2%).19 As stated above in the Techniques & Tips section, some authors utilize two plastic stents for ET-GBD for this purpose, though with increased technical difficulty. It is important to remember that these numbers, when paired with AE rates, represent the achievements of expert endoscopists.
Discussion with your surgery team is important when deciding modality. If the patient is felt to be a potential candidate for CCY, and EUS-GBD is not being used as a destination therapy, the surgeon may prefer ET-GBD. EUS-GBD may enhance the difficulty of CCY, though at least one study demonstrated that this was no different than PC with similar rates of conversion from LC to open CCY.20 This conversation is most critical for patients who are potential liver transplant candidates. For patients where this is not a consideration there is some evidence to suggest equivalency between LC and EUS-GBD, though certainly EUS-GBD has not yet supplanted LC as the treatment of choice.21
While there may eventually be a shift towards EUS-GBD instead of LC in certain patient groups, what is clearer are the advantages of EUS-GBD over PC. One recent meta-analysis revealed that EUS-GBD has significantly favorable odds of overall adverse events (OR 0.43, 95% CI 0.18-1.00), shorter hospital stay (2.76 less days, 95% CI 0.31-5.20 less days), reinterventions (OR 0.15, 95% CI 0.02-0.98), and unplanned readmissions (OR 0.14, 95% CI 0.03-0.70) compared to PC.22 Beyond the data, though, are the emotional and psychological impacts an external drain can have on a patient.
Conclusion
When expertise is available, endoscopic treatment of benign gallbladder disease has a definite role but should be undertaken only by those with the experience and skill to safely do so. Decision to proceed, especially with EUS-GBD, should be accompanied by conversation and collaboration with surgical teams. If a patient is under consideration for PC instead of LC, it may be worthwhile to seek consultation with a local center with expertise in EUS-GBD or ET-GBD. The adoption of these techniques is part of the paradigm shift, seen broadly throughout medicine, towards minimally invasive interventions, particularly in advanced endoscopy.
Dr. Gilman (X @a_gilman) and Dr. Baron (X @EndoTx) are with the University of North Carolina, Chapel Hill, Division of Gastroenterology & Hepatology. Dr. Gilman has no relevant financial disclosures. Dr. Baron is a consultant and speaker for Ambu, Boston Scientific, Cook Endoscopy, Medtronic, Olympus America, and W.L. Gore.
References
1. Radder RW. Ultrasonically guided percutaneous catheter drainage for gallbladder empyema. Diagn Imaging. 1980;49:330-333.
2. Kozarek RA. Selective cannulation of the cystic duct at time of ERCP. J Clin Gastroenterol. 1984;6:37-40.
3. Tamada K et al. Efficacy of endoscopic retrograde cholecystoendoprosthesis (ERCCE) for cholecystitis. Endoscopy. 1991;23:2-3.
4. Reynolds W. The first laparoscopic cholecystectomy. JSLS. 2001;5:89-94.
5. Baron TH, Topazian MD. Endoscopic transduodenal drainage of the gallbladder: Implications for endoluminal treatment of gallbladder disease. Gastrointest Endosc. 2007 Apr;65(4):735-7. doi: 10.1016/j.gie.2006.07.041.
6. Irani SS et al. Endoscopic ultrasound-guided transluminal gallbladder drainage in patients with acute cholecystitis: A prospective multicenter trial. Ann Surg. 2023 Sep 1;278(3):e556-e562. doi: 10.1097/SLA.0000000000005784.
7. Shen Y et al. Endoscopic ultrasound-guided cholecystostomy for resection of gallbladder polyps with lumen-apposing metal stent. Medicine (Baltimore). 2020 Oct 23;99(43):e22903. doi: 10.1097/MD.0000000000022903.
8. Pang H et al. Endoscopic ultrasound-guided gallbladder endoscopic mucosal resection: A pilot porcine study. Minim Invasive Ther Allied Technol. 2023 Feb;32(1):24-32. doi: 10.1080/13645706.2022.2153228.
9. Imai H et al. EUS-guided gallbladder drainage for rescue treatment of malignant distal biliary obstruction after unsuccessful ERCP. Gastrointest Endosc. 2016 Jul;84(1):147-51. doi: 10.1016/j.gie.2015.12.024.
10. Saumoy M et al. Endoscopic therapies for gallbladder drainage. Gastrointest Endosc. 2021 Oct;94(4):671-84. doi: 10.1016/j.gie.2021.05.031.
11. Van der Merwe SW et al. Therapeutic endoscopic ultrasound: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2022 Feb;54(2):185-205. doi: 10.1055/a-1717-1391.
12. Storm AC et al. Transpapillary gallbladder stent placement for long-term therapy of acute cholecystitis. Gastrointest Endosc. 2021 Oct;94(4):742-8 e1. doi: 10.1016/j.gie.2021.03.025.
13. James TW, Baron TH. Converting percutaneous gallbladder drainage to internal drainage using EUS-guided therapy: A review of current practices and procedures. Endosc Ultrasound. 2018 Mar-Apr;7(2):93-6. doi: 10.4103/eus.eus_110_17.
14. James TW, Baron TH. Transpapillary nasocystic tube placement to allow gallbladder distention for EUS-guided cholecystoduodenostomy. VideoGIE. 2019 Dec;4(12):561-2. doi: 10.1016/j.vgie.2019.08.009.
15. Gilman AJ, Baron TH. Delamination of a lumen-apposing metal stent with tissue ingrowth and stent-in-stent removal. Gastrointest Endosc. 2023 Sep;98(3):451-3. doi: 10.1016/j.gie.2023.04.2087.
16. Teoh AY et al. Outcomes of an international multicenter registry on EUS-guided gallbladder drainage in patients at high risk for cholecystectomy. Endosc Int Open. 2019 Aug;7(8):E964-E973. doi: 10.1055/a-0915-2098.
17. Kalva NR et al. Efficacy and safety of lumen apposing self-expandable metal stents for EUS guided cholecystostomy: A meta-analysis and systematic review. Can J Gastroenterol Hepatol. 2018;2018:7070961. doi: 10.1155/2018/7070961.
18. Khan MA et al. Efficacy and safety of endoscopic gallbladder drainage in acute cholecystitis: Is it better than percutaneous gallbladder drainage? Gastrointest Endosc. 2017 Jan;85(1):76-87 e3. doi: 10.1016/j.gie.2016.06.032.
19. Mohan BP et al. Endoscopic ultrasound-guided gallbladder drainage, transpapillary drainage, or percutaneous drainage in high risk acute cholecystitis patients: a systematic review and comparative meta-analysis. Endoscopy. 2020 Feb;52(2):96-106. doi: 10.1055/a-1020-3932.
20. Jang JW et al. Endoscopic ultrasound-guided transmural and percutaneous transhepatic gallbladder drainage are comparable for acute cholecystitis. Gastroenterology. 2012 Apr;142(4):805-11. doi: 10.1053/j.gastro.2011.12.051.
21. Teoh AYB et al. EUS-guided gallbladder drainage versus laparoscopic cholecystectomy for acute cholecystitis: a propensity score analysis with 1-year follow-up data. Gastrointest Endosc. 2021 Mar;93(3):577-83. doi: 10.1016/j.gie.2020.06.066.
22. Luk SW et al. Endoscopic ultrasound-guided gallbladder drainage versus percutaneous cholecystostomy for high risk surgical patients with acute cholecystitis: a systematic review and meta-analysis. Endoscopy. 2019 Aug;51(8):722-32. doi: 10.1055/a-0929-6603.
Introduction
The treatment of benign gallbladder disease has changed substantially in the past decade, but this represents only a snapshot in the evolutionary history of the management of this organ. What began as a problem managed exclusively by open cholecystectomy (CCY) transitioned into a race toward minimally invasive approaches in the 1980s, with advances from gastroenterology, surgery, and radiology.
The opening strides were made in 1980 with the first description of percutaneous cholecystostomy (PC) by Dr. R.W. Radder.1 Shortly thereafter, in 1984, Dr. Richard Kozarek first reported the feasibility of selective cystic duct cannulation during endoscopic retrograde cholangiopancreatography (ERCP).2 Subsequent stenting for the treatment of acute cholecystitis (endoscopic transpapillary gallbladder drainage, ET-GBD) was then reported by Tamada et. al. in 1991.3 Not to be outdone, the first laparoscopic cholecystectomy (LC) was completed by Dr. Med Erich Mühe of Germany in 1985.4 More recently, with the expansion of interventional endoscopic ultrasound (EUS), the first transmural EUS-guided gallbladder drainage (EUS-GBD) was described by Dr. Baron and Dr. Topazian in 2007.5
The subsequent advent of lumen apposing metal stents (LAMS) has cemented EUS-GBD in the toolbox of treatment for benign gallbladder disease. Results of a recent prospective multicenter trial, with a Food and Drug Administration–approved protocol and investigational device exemption, have been published, opening the door for the expansion of FDA approved indications for this device.6
Benign gallbladder disease encompasses both polyps (benign and premalignant) and cholecystitis (acute/chronic, calculous/acalculous), in addition to others. The four management techniques (LC, PC, ET-GBD, and EUS-GBD) have filled integral niches in the management of these patients. Even gallbladder polyps have not been able to escape the reach of endoscopic approaches with the recent description of LAMS-assisted polypectomy as part of a gallbladder preserving strategy.7,8 While EUS-GBD also has been used for biliary decompression in the presence of a patent cystic duct and absence of cholecystitis, .9 Both of these techniques have gained wide recognition and/or guideline support for their use from the American Society for Gastrointestinal Endoscopy (ASGE) and the European Society of Gastrointestinal Endoscopy (ESGE).10,11 In addition, there is now one FDA-approved stent device for treatment of acute cholecystitis in patients unfit for surgery.
Techniques & Tips
ET-GBD
- During ERCP, after successful cannulation of the bile duct, attempted wire cannulation of the cystic duct is performed.
A cholangiogram, which clearly delineates the insertion of the cystic duct into the main bile duct, can enhance cannulation success. Rotatable fluoroscopy can facilitate identification.
- After anatomy is clear, wire access is often best achieved using a sphincterotome or stone retrieval (occlusion) balloon.
The balloon, once inflated, can be pulled downward to establish traction on the main bile duct, which can straighten the approach.
- After superficial wire engagement into the cystic duct, the accessory used can be slowly advanced into the cystic duct to stabilize the catheter and then navigate the valves of Heister to reach the gallbladder lumen.
Use of a sphincterotome, which directs toward the patient’s right (most often direction of cystic duct takeoff), is helpful. Angled guidewires are preferable. We often use a 0.035-inch, 260-cm angled hydrophilic wire (GLIDEWIRE; Terumo, Somerset, NJ) to overcome this challenging portion of ET-GBD.
If despite the above maneuvers the guidewire has failed to enter the cystic duct, cholangioscopy can be used to identify the orifice and/or stabilize deep wire cannulation. This is often cumbersome, time consuming, does not always produce success, and requires additional expertise.
- If a stone is encountered that cannot be extracted or traversed by a guidewire, cholangioscopy with electrohydraulic lithotripsy can be pursued.
- After the guidewire has entered the gallbladder, a 5 French or 7 French plastic double pigtail stent is placed. Typical lengths are 9-15 cm.
Some authors prefer to use two side-by-side plastic stents.12 This has been shown retrospectively to enhance the long term clinical success of ET-GBD but with additional technical difficulty.
- This stent can remain in place indefinitely and need not be exchanged, though it should be removed just prior to CCY if pursued. Alternatively, the surgeon can be alerted to its presence and, if comfortable, it can be removed intraoperatively.
EUS-GBD
- Use of fluoroscopy is optional but can enhance technical success in selected situations.
- Conversion, or internalization, of PC is reasonable and can enhance patient quality of life.13
- If the gallbladder wall is not in close apposition to the duodenal (or gastric) wall, consider measuring the distance.
We preferentially use 10-mm diameter by 10-mm saddle length LAMS for EUS-GBD, unless the above distance warrants use of a 15-mm by 15-mm LAMS (AXIOS, Boston Scientific, Marlborough, MA). If the distance is greater than 15 mm, consider searching for an alternative site, using a traditional biliary fully covered self-expandable metal stent (FCSEMS) for longer length, or converting to ET-GBD. Smaller diameter (8 mm) with an 8-mm saddle length can be used as well. The optimal diameter is unknown and also dependent on whether transluminal endoscopic diagnosis or therapy is a consideration.
- If there is difficulty locating the gallbladder, it may be decompressed or small (particularly if PC or a partial CCY has already been performed).
If a cholecystostomy tube is in place, instillation of sterile water via the tube can sometimes improve the target for LAMS placement, though caution should be made to not over-distend the gallbladder. ERCP with placement of a nasobiliary tube into the gallbladder can also serve this purpose and has been previously described.14
The gallbladder can be punctured with a 19-gauge FNA needle to instill sterile water and distend the gallbladder with the added benefit of being able to pass a guidewire, which may enhance procedural safety in difficult cases. However, success of this technique is contingent on fluid remaining within the gallbladder and not transiting out via the cystic duct. Expedient exchange of the FNA needle for the LAMS device may be necessary.
- Attempt to confirm location within the duodenum prior to puncture, as gastric origins can pose unique ramifications (i.e. potential for partial gastric outlet obstruction, obstruction of LAMS with food debris, etc.).
It can be easy to mistake an unintentional pre-pyloric position for a position within the duodenum since the working channel is behind (proximal to) the echoprobe.
- Turning off Doppler flow prior to advancement of the cautery enhanced LAMS can reduce obscurement of views on entry into the gallbladder. Lack of certainty about entry or misdeployment after presumed entry herald the most challenging aspect of EUS-GBD.
Utilization of a previously placed guidewire or advancement of one preloaded into the LAMS can aid in both enhancing confidence in location and assist with salvage maneuvers, if needed.
- After successful deployment of the LAMS we routinely place a double pigtail plastic stent through it (typically 7 French by 4 cm) to maintain patency. This may also prevent bleeding from the LAMS flange abrading the wall of either lumen.
- We routinely exchange the LAMS for two double pigtail plastic stents (typically 7 French by 4 cm) 4 weeks after initial placement especially when there is a more than modest residual stone burden (data in press). These plastic stents can remain in place indefinitely.
This exchange can be deferred if the patient is not expected to survive until the one-year anniversary of LAMS deployment. After one year the LAMS plastic covering may degrade and pose additional problems.15
LAMS Misdeployment Salvage Tips
- Salvage techniques can vary from simple to complex.
- If a wire is in place, it can be used to balloon or catheter dilate the tract and place a FCSEMS traversing the gallbladder and duodenal/gastric lumens. A similar approach can be used if the LAMS deployed on only one side (gallbladder or duodenum/stomach) and the other flange is within the peritoneum.
- The most challenging scenario to salvage is if the LAMS is misdeployed or becomes dislodged and no wire is present. This is why the use of a guidewire, even if preloaded into the LAMS and placement is freehand, is essential for EUS-GBD. A potential technique is to balloon dilate the duodenal/gastric defect and drive the endoscope into the peritoneum to reconnect that lumen to the gallbladder defect or LAMS, depending on the site of misdeployment. Doing so requires a high degree of commitment and skill and should not be done casually.
- If uncertainty remains or if misdeployment has occurred and salvage attempts have failed, consider closure of the duodenal/gastric defect and conversion to ET-GBD.
This may both treat the initial procedural indication and assist with what is essentially a large bile leak, which might also require percutaneous therapy for non-surgical management.
- For endoscopists with limited experience at salvage techniques, it is reasonable for the threshold for conversion to be low, assuming experience with and confidence in ET-GBD is high.
- If salvage is successful but ambiguity remains, consider obtaining a cholangiogram via the LAMS to confirm positioning and absence of leak.
Adverse Events
Both ET-GBD and EUS-GBD should be performed by an endoscopist comfortable with their techniques and the management of their adverse events (AEs). Rates for EUS-GBD AEs in patients at high risk for LC were reported in one international multicenter registry to be 15.3% with a 30-day mortality of 9.2%, with a significant predictor of AE being endoscopist experience less than 25 procedures.16 A meta-analysis also found an overall AE rate of 18.31%, with rates for perforation and stent related AEs (i.e. migration, occlusion, pneumoperitoneum) being 6.71% and 8.16%, respectively.17 For this reason, we recommend that patients with cholecystitis who are deemed to be poor surgical candidates be transferred to a tertiary referral center with expertise in these approaches. Rates of AEs for ET-GBD are similar to that for standard ERCP, with reported ranges of 5%-10.3%.10
Comparisons Between Techniques
The decision on which technique to utilize for endoscopic management of cholecystitis or symptomatic cholelithiasis depends first and foremost on the expertise and comfort level of the endoscopist. Given the additional training that an advanced endoscopist needs to perform EUS-GBD, combined with the perhaps slightly higher AE rate and permanency of endoscopic cholecystostomy, it is reasonable to proceed with a trial of ET-GBD if confidence is insufficient. However, ET-GBD can certainly be more technically challenging and less effective than EUS-GBD, with lower reported technical and clinical success rates (technical 85.3% vs 93.0%, clinical 95.2% vs 97.3%).18 Despite this, the rate of recurrence of cholecystitis is similar between ET-GBD and EUS-GBD (4.6% vs 4.2%).19 As stated above in the Techniques & Tips section, some authors utilize two plastic stents for ET-GBD for this purpose, though with increased technical difficulty. It is important to remember that these numbers, when paired with AE rates, represent the achievements of expert endoscopists.
Discussion with your surgery team is important when deciding modality. If the patient is felt to be a potential candidate for CCY, and EUS-GBD is not being used as a destination therapy, the surgeon may prefer ET-GBD. EUS-GBD may enhance the difficulty of CCY, though at least one study demonstrated that this was no different than PC with similar rates of conversion from LC to open CCY.20 This conversation is most critical for patients who are potential liver transplant candidates. For patients where this is not a consideration there is some evidence to suggest equivalency between LC and EUS-GBD, though certainly EUS-GBD has not yet supplanted LC as the treatment of choice.21
While there may eventually be a shift towards EUS-GBD instead of LC in certain patient groups, what is clearer are the advantages of EUS-GBD over PC. One recent meta-analysis revealed that EUS-GBD has significantly favorable odds of overall adverse events (OR 0.43, 95% CI 0.18-1.00), shorter hospital stay (2.76 less days, 95% CI 0.31-5.20 less days), reinterventions (OR 0.15, 95% CI 0.02-0.98), and unplanned readmissions (OR 0.14, 95% CI 0.03-0.70) compared to PC.22 Beyond the data, though, are the emotional and psychological impacts an external drain can have on a patient.
Conclusion
When expertise is available, endoscopic treatment of benign gallbladder disease has a definite role but should be undertaken only by those with the experience and skill to safely do so. Decision to proceed, especially with EUS-GBD, should be accompanied by conversation and collaboration with surgical teams. If a patient is under consideration for PC instead of LC, it may be worthwhile to seek consultation with a local center with expertise in EUS-GBD or ET-GBD. The adoption of these techniques is part of the paradigm shift, seen broadly throughout medicine, towards minimally invasive interventions, particularly in advanced endoscopy.
Dr. Gilman (X @a_gilman) and Dr. Baron (X @EndoTx) are with the University of North Carolina, Chapel Hill, Division of Gastroenterology & Hepatology. Dr. Gilman has no relevant financial disclosures. Dr. Baron is a consultant and speaker for Ambu, Boston Scientific, Cook Endoscopy, Medtronic, Olympus America, and W.L. Gore.
References
1. Radder RW. Ultrasonically guided percutaneous catheter drainage for gallbladder empyema. Diagn Imaging. 1980;49:330-333.
2. Kozarek RA. Selective cannulation of the cystic duct at time of ERCP. J Clin Gastroenterol. 1984;6:37-40.
3. Tamada K et al. Efficacy of endoscopic retrograde cholecystoendoprosthesis (ERCCE) for cholecystitis. Endoscopy. 1991;23:2-3.
4. Reynolds W. The first laparoscopic cholecystectomy. JSLS. 2001;5:89-94.
5. Baron TH, Topazian MD. Endoscopic transduodenal drainage of the gallbladder: Implications for endoluminal treatment of gallbladder disease. Gastrointest Endosc. 2007 Apr;65(4):735-7. doi: 10.1016/j.gie.2006.07.041.
6. Irani SS et al. Endoscopic ultrasound-guided transluminal gallbladder drainage in patients with acute cholecystitis: A prospective multicenter trial. Ann Surg. 2023 Sep 1;278(3):e556-e562. doi: 10.1097/SLA.0000000000005784.
7. Shen Y et al. Endoscopic ultrasound-guided cholecystostomy for resection of gallbladder polyps with lumen-apposing metal stent. Medicine (Baltimore). 2020 Oct 23;99(43):e22903. doi: 10.1097/MD.0000000000022903.
8. Pang H et al. Endoscopic ultrasound-guided gallbladder endoscopic mucosal resection: A pilot porcine study. Minim Invasive Ther Allied Technol. 2023 Feb;32(1):24-32. doi: 10.1080/13645706.2022.2153228.
9. Imai H et al. EUS-guided gallbladder drainage for rescue treatment of malignant distal biliary obstruction after unsuccessful ERCP. Gastrointest Endosc. 2016 Jul;84(1):147-51. doi: 10.1016/j.gie.2015.12.024.
10. Saumoy M et al. Endoscopic therapies for gallbladder drainage. Gastrointest Endosc. 2021 Oct;94(4):671-84. doi: 10.1016/j.gie.2021.05.031.
11. Van der Merwe SW et al. Therapeutic endoscopic ultrasound: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2022 Feb;54(2):185-205. doi: 10.1055/a-1717-1391.
12. Storm AC et al. Transpapillary gallbladder stent placement for long-term therapy of acute cholecystitis. Gastrointest Endosc. 2021 Oct;94(4):742-8 e1. doi: 10.1016/j.gie.2021.03.025.
13. James TW, Baron TH. Converting percutaneous gallbladder drainage to internal drainage using EUS-guided therapy: A review of current practices and procedures. Endosc Ultrasound. 2018 Mar-Apr;7(2):93-6. doi: 10.4103/eus.eus_110_17.
14. James TW, Baron TH. Transpapillary nasocystic tube placement to allow gallbladder distention for EUS-guided cholecystoduodenostomy. VideoGIE. 2019 Dec;4(12):561-2. doi: 10.1016/j.vgie.2019.08.009.
15. Gilman AJ, Baron TH. Delamination of a lumen-apposing metal stent with tissue ingrowth and stent-in-stent removal. Gastrointest Endosc. 2023 Sep;98(3):451-3. doi: 10.1016/j.gie.2023.04.2087.
16. Teoh AY et al. Outcomes of an international multicenter registry on EUS-guided gallbladder drainage in patients at high risk for cholecystectomy. Endosc Int Open. 2019 Aug;7(8):E964-E973. doi: 10.1055/a-0915-2098.
17. Kalva NR et al. Efficacy and safety of lumen apposing self-expandable metal stents for EUS guided cholecystostomy: A meta-analysis and systematic review. Can J Gastroenterol Hepatol. 2018;2018:7070961. doi: 10.1155/2018/7070961.
18. Khan MA et al. Efficacy and safety of endoscopic gallbladder drainage in acute cholecystitis: Is it better than percutaneous gallbladder drainage? Gastrointest Endosc. 2017 Jan;85(1):76-87 e3. doi: 10.1016/j.gie.2016.06.032.
19. Mohan BP et al. Endoscopic ultrasound-guided gallbladder drainage, transpapillary drainage, or percutaneous drainage in high risk acute cholecystitis patients: a systematic review and comparative meta-analysis. Endoscopy. 2020 Feb;52(2):96-106. doi: 10.1055/a-1020-3932.
20. Jang JW et al. Endoscopic ultrasound-guided transmural and percutaneous transhepatic gallbladder drainage are comparable for acute cholecystitis. Gastroenterology. 2012 Apr;142(4):805-11. doi: 10.1053/j.gastro.2011.12.051.
21. Teoh AYB et al. EUS-guided gallbladder drainage versus laparoscopic cholecystectomy for acute cholecystitis: a propensity score analysis with 1-year follow-up data. Gastrointest Endosc. 2021 Mar;93(3):577-83. doi: 10.1016/j.gie.2020.06.066.
22. Luk SW et al. Endoscopic ultrasound-guided gallbladder drainage versus percutaneous cholecystostomy for high risk surgical patients with acute cholecystitis: a systematic review and meta-analysis. Endoscopy. 2019 Aug;51(8):722-32. doi: 10.1055/a-0929-6603.
Introduction
The treatment of benign gallbladder disease has changed substantially in the past decade, but this represents only a snapshot in the evolutionary history of the management of this organ. What began as a problem managed exclusively by open cholecystectomy (CCY) transitioned into a race toward minimally invasive approaches in the 1980s, with advances from gastroenterology, surgery, and radiology.
The opening strides were made in 1980 with the first description of percutaneous cholecystostomy (PC) by Dr. R.W. Radder.1 Shortly thereafter, in 1984, Dr. Richard Kozarek first reported the feasibility of selective cystic duct cannulation during endoscopic retrograde cholangiopancreatography (ERCP).2 Subsequent stenting for the treatment of acute cholecystitis (endoscopic transpapillary gallbladder drainage, ET-GBD) was then reported by Tamada et. al. in 1991.3 Not to be outdone, the first laparoscopic cholecystectomy (LC) was completed by Dr. Med Erich Mühe of Germany in 1985.4 More recently, with the expansion of interventional endoscopic ultrasound (EUS), the first transmural EUS-guided gallbladder drainage (EUS-GBD) was described by Dr. Baron and Dr. Topazian in 2007.5
The subsequent advent of lumen apposing metal stents (LAMS) has cemented EUS-GBD in the toolbox of treatment for benign gallbladder disease. Results of a recent prospective multicenter trial, with a Food and Drug Administration–approved protocol and investigational device exemption, have been published, opening the door for the expansion of FDA approved indications for this device.6
Benign gallbladder disease encompasses both polyps (benign and premalignant) and cholecystitis (acute/chronic, calculous/acalculous), in addition to others. The four management techniques (LC, PC, ET-GBD, and EUS-GBD) have filled integral niches in the management of these patients. Even gallbladder polyps have not been able to escape the reach of endoscopic approaches with the recent description of LAMS-assisted polypectomy as part of a gallbladder preserving strategy.7,8 While EUS-GBD also has been used for biliary decompression in the presence of a patent cystic duct and absence of cholecystitis, .9 Both of these techniques have gained wide recognition and/or guideline support for their use from the American Society for Gastrointestinal Endoscopy (ASGE) and the European Society of Gastrointestinal Endoscopy (ESGE).10,11 In addition, there is now one FDA-approved stent device for treatment of acute cholecystitis in patients unfit for surgery.
Techniques & Tips
ET-GBD
- During ERCP, after successful cannulation of the bile duct, attempted wire cannulation of the cystic duct is performed.
A cholangiogram, which clearly delineates the insertion of the cystic duct into the main bile duct, can enhance cannulation success. Rotatable fluoroscopy can facilitate identification.
- After anatomy is clear, wire access is often best achieved using a sphincterotome or stone retrieval (occlusion) balloon.
The balloon, once inflated, can be pulled downward to establish traction on the main bile duct, which can straighten the approach.
- After superficial wire engagement into the cystic duct, the accessory used can be slowly advanced into the cystic duct to stabilize the catheter and then navigate the valves of Heister to reach the gallbladder lumen.
Use of a sphincterotome, which directs toward the patient’s right (most often direction of cystic duct takeoff), is helpful. Angled guidewires are preferable. We often use a 0.035-inch, 260-cm angled hydrophilic wire (GLIDEWIRE; Terumo, Somerset, NJ) to overcome this challenging portion of ET-GBD.
If despite the above maneuvers the guidewire has failed to enter the cystic duct, cholangioscopy can be used to identify the orifice and/or stabilize deep wire cannulation. This is often cumbersome, time consuming, does not always produce success, and requires additional expertise.
- If a stone is encountered that cannot be extracted or traversed by a guidewire, cholangioscopy with electrohydraulic lithotripsy can be pursued.
- After the guidewire has entered the gallbladder, a 5 French or 7 French plastic double pigtail stent is placed. Typical lengths are 9-15 cm.
Some authors prefer to use two side-by-side plastic stents.12 This has been shown retrospectively to enhance the long term clinical success of ET-GBD but with additional technical difficulty.
- This stent can remain in place indefinitely and need not be exchanged, though it should be removed just prior to CCY if pursued. Alternatively, the surgeon can be alerted to its presence and, if comfortable, it can be removed intraoperatively.
EUS-GBD
- Use of fluoroscopy is optional but can enhance technical success in selected situations.
- Conversion, or internalization, of PC is reasonable and can enhance patient quality of life.13
- If the gallbladder wall is not in close apposition to the duodenal (or gastric) wall, consider measuring the distance.
We preferentially use 10-mm diameter by 10-mm saddle length LAMS for EUS-GBD, unless the above distance warrants use of a 15-mm by 15-mm LAMS (AXIOS, Boston Scientific, Marlborough, MA). If the distance is greater than 15 mm, consider searching for an alternative site, using a traditional biliary fully covered self-expandable metal stent (FCSEMS) for longer length, or converting to ET-GBD. Smaller diameter (8 mm) with an 8-mm saddle length can be used as well. The optimal diameter is unknown and also dependent on whether transluminal endoscopic diagnosis or therapy is a consideration.
- If there is difficulty locating the gallbladder, it may be decompressed or small (particularly if PC or a partial CCY has already been performed).
If a cholecystostomy tube is in place, instillation of sterile water via the tube can sometimes improve the target for LAMS placement, though caution should be made to not over-distend the gallbladder. ERCP with placement of a nasobiliary tube into the gallbladder can also serve this purpose and has been previously described.14
The gallbladder can be punctured with a 19-gauge FNA needle to instill sterile water and distend the gallbladder with the added benefit of being able to pass a guidewire, which may enhance procedural safety in difficult cases. However, success of this technique is contingent on fluid remaining within the gallbladder and not transiting out via the cystic duct. Expedient exchange of the FNA needle for the LAMS device may be necessary.
- Attempt to confirm location within the duodenum prior to puncture, as gastric origins can pose unique ramifications (i.e. potential for partial gastric outlet obstruction, obstruction of LAMS with food debris, etc.).
It can be easy to mistake an unintentional pre-pyloric position for a position within the duodenum since the working channel is behind (proximal to) the echoprobe.
- Turning off Doppler flow prior to advancement of the cautery enhanced LAMS can reduce obscurement of views on entry into the gallbladder. Lack of certainty about entry or misdeployment after presumed entry herald the most challenging aspect of EUS-GBD.
Utilization of a previously placed guidewire or advancement of one preloaded into the LAMS can aid in both enhancing confidence in location and assist with salvage maneuvers, if needed.
- After successful deployment of the LAMS we routinely place a double pigtail plastic stent through it (typically 7 French by 4 cm) to maintain patency. This may also prevent bleeding from the LAMS flange abrading the wall of either lumen.
- We routinely exchange the LAMS for two double pigtail plastic stents (typically 7 French by 4 cm) 4 weeks after initial placement especially when there is a more than modest residual stone burden (data in press). These plastic stents can remain in place indefinitely.
This exchange can be deferred if the patient is not expected to survive until the one-year anniversary of LAMS deployment. After one year the LAMS plastic covering may degrade and pose additional problems.15
LAMS Misdeployment Salvage Tips
- Salvage techniques can vary from simple to complex.
- If a wire is in place, it can be used to balloon or catheter dilate the tract and place a FCSEMS traversing the gallbladder and duodenal/gastric lumens. A similar approach can be used if the LAMS deployed on only one side (gallbladder or duodenum/stomach) and the other flange is within the peritoneum.
- The most challenging scenario to salvage is if the LAMS is misdeployed or becomes dislodged and no wire is present. This is why the use of a guidewire, even if preloaded into the LAMS and placement is freehand, is essential for EUS-GBD. A potential technique is to balloon dilate the duodenal/gastric defect and drive the endoscope into the peritoneum to reconnect that lumen to the gallbladder defect or LAMS, depending on the site of misdeployment. Doing so requires a high degree of commitment and skill and should not be done casually.
- If uncertainty remains or if misdeployment has occurred and salvage attempts have failed, consider closure of the duodenal/gastric defect and conversion to ET-GBD.
This may both treat the initial procedural indication and assist with what is essentially a large bile leak, which might also require percutaneous therapy for non-surgical management.
- For endoscopists with limited experience at salvage techniques, it is reasonable for the threshold for conversion to be low, assuming experience with and confidence in ET-GBD is high.
- If salvage is successful but ambiguity remains, consider obtaining a cholangiogram via the LAMS to confirm positioning and absence of leak.
Adverse Events
Both ET-GBD and EUS-GBD should be performed by an endoscopist comfortable with their techniques and the management of their adverse events (AEs). Rates for EUS-GBD AEs in patients at high risk for LC were reported in one international multicenter registry to be 15.3% with a 30-day mortality of 9.2%, with a significant predictor of AE being endoscopist experience less than 25 procedures.16 A meta-analysis also found an overall AE rate of 18.31%, with rates for perforation and stent related AEs (i.e. migration, occlusion, pneumoperitoneum) being 6.71% and 8.16%, respectively.17 For this reason, we recommend that patients with cholecystitis who are deemed to be poor surgical candidates be transferred to a tertiary referral center with expertise in these approaches. Rates of AEs for ET-GBD are similar to that for standard ERCP, with reported ranges of 5%-10.3%.10
Comparisons Between Techniques
The decision on which technique to utilize for endoscopic management of cholecystitis or symptomatic cholelithiasis depends first and foremost on the expertise and comfort level of the endoscopist. Given the additional training that an advanced endoscopist needs to perform EUS-GBD, combined with the perhaps slightly higher AE rate and permanency of endoscopic cholecystostomy, it is reasonable to proceed with a trial of ET-GBD if confidence is insufficient. However, ET-GBD can certainly be more technically challenging and less effective than EUS-GBD, with lower reported technical and clinical success rates (technical 85.3% vs 93.0%, clinical 95.2% vs 97.3%).18 Despite this, the rate of recurrence of cholecystitis is similar between ET-GBD and EUS-GBD (4.6% vs 4.2%).19 As stated above in the Techniques & Tips section, some authors utilize two plastic stents for ET-GBD for this purpose, though with increased technical difficulty. It is important to remember that these numbers, when paired with AE rates, represent the achievements of expert endoscopists.
Discussion with your surgery team is important when deciding modality. If the patient is felt to be a potential candidate for CCY, and EUS-GBD is not being used as a destination therapy, the surgeon may prefer ET-GBD. EUS-GBD may enhance the difficulty of CCY, though at least one study demonstrated that this was no different than PC with similar rates of conversion from LC to open CCY.20 This conversation is most critical for patients who are potential liver transplant candidates. For patients where this is not a consideration there is some evidence to suggest equivalency between LC and EUS-GBD, though certainly EUS-GBD has not yet supplanted LC as the treatment of choice.21
While there may eventually be a shift towards EUS-GBD instead of LC in certain patient groups, what is clearer are the advantages of EUS-GBD over PC. One recent meta-analysis revealed that EUS-GBD has significantly favorable odds of overall adverse events (OR 0.43, 95% CI 0.18-1.00), shorter hospital stay (2.76 less days, 95% CI 0.31-5.20 less days), reinterventions (OR 0.15, 95% CI 0.02-0.98), and unplanned readmissions (OR 0.14, 95% CI 0.03-0.70) compared to PC.22 Beyond the data, though, are the emotional and psychological impacts an external drain can have on a patient.
Conclusion
When expertise is available, endoscopic treatment of benign gallbladder disease has a definite role but should be undertaken only by those with the experience and skill to safely do so. Decision to proceed, especially with EUS-GBD, should be accompanied by conversation and collaboration with surgical teams. If a patient is under consideration for PC instead of LC, it may be worthwhile to seek consultation with a local center with expertise in EUS-GBD or ET-GBD. The adoption of these techniques is part of the paradigm shift, seen broadly throughout medicine, towards minimally invasive interventions, particularly in advanced endoscopy.
Dr. Gilman (X @a_gilman) and Dr. Baron (X @EndoTx) are with the University of North Carolina, Chapel Hill, Division of Gastroenterology & Hepatology. Dr. Gilman has no relevant financial disclosures. Dr. Baron is a consultant and speaker for Ambu, Boston Scientific, Cook Endoscopy, Medtronic, Olympus America, and W.L. Gore.
References
1. Radder RW. Ultrasonically guided percutaneous catheter drainage for gallbladder empyema. Diagn Imaging. 1980;49:330-333.
2. Kozarek RA. Selective cannulation of the cystic duct at time of ERCP. J Clin Gastroenterol. 1984;6:37-40.
3. Tamada K et al. Efficacy of endoscopic retrograde cholecystoendoprosthesis (ERCCE) for cholecystitis. Endoscopy. 1991;23:2-3.
4. Reynolds W. The first laparoscopic cholecystectomy. JSLS. 2001;5:89-94.
5. Baron TH, Topazian MD. Endoscopic transduodenal drainage of the gallbladder: Implications for endoluminal treatment of gallbladder disease. Gastrointest Endosc. 2007 Apr;65(4):735-7. doi: 10.1016/j.gie.2006.07.041.
6. Irani SS et al. Endoscopic ultrasound-guided transluminal gallbladder drainage in patients with acute cholecystitis: A prospective multicenter trial. Ann Surg. 2023 Sep 1;278(3):e556-e562. doi: 10.1097/SLA.0000000000005784.
7. Shen Y et al. Endoscopic ultrasound-guided cholecystostomy for resection of gallbladder polyps with lumen-apposing metal stent. Medicine (Baltimore). 2020 Oct 23;99(43):e22903. doi: 10.1097/MD.0000000000022903.
8. Pang H et al. Endoscopic ultrasound-guided gallbladder endoscopic mucosal resection: A pilot porcine study. Minim Invasive Ther Allied Technol. 2023 Feb;32(1):24-32. doi: 10.1080/13645706.2022.2153228.
9. Imai H et al. EUS-guided gallbladder drainage for rescue treatment of malignant distal biliary obstruction after unsuccessful ERCP. Gastrointest Endosc. 2016 Jul;84(1):147-51. doi: 10.1016/j.gie.2015.12.024.
10. Saumoy M et al. Endoscopic therapies for gallbladder drainage. Gastrointest Endosc. 2021 Oct;94(4):671-84. doi: 10.1016/j.gie.2021.05.031.
11. Van der Merwe SW et al. Therapeutic endoscopic ultrasound: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2022 Feb;54(2):185-205. doi: 10.1055/a-1717-1391.
12. Storm AC et al. Transpapillary gallbladder stent placement for long-term therapy of acute cholecystitis. Gastrointest Endosc. 2021 Oct;94(4):742-8 e1. doi: 10.1016/j.gie.2021.03.025.
13. James TW, Baron TH. Converting percutaneous gallbladder drainage to internal drainage using EUS-guided therapy: A review of current practices and procedures. Endosc Ultrasound. 2018 Mar-Apr;7(2):93-6. doi: 10.4103/eus.eus_110_17.
14. James TW, Baron TH. Transpapillary nasocystic tube placement to allow gallbladder distention for EUS-guided cholecystoduodenostomy. VideoGIE. 2019 Dec;4(12):561-2. doi: 10.1016/j.vgie.2019.08.009.
15. Gilman AJ, Baron TH. Delamination of a lumen-apposing metal stent with tissue ingrowth and stent-in-stent removal. Gastrointest Endosc. 2023 Sep;98(3):451-3. doi: 10.1016/j.gie.2023.04.2087.
16. Teoh AY et al. Outcomes of an international multicenter registry on EUS-guided gallbladder drainage in patients at high risk for cholecystectomy. Endosc Int Open. 2019 Aug;7(8):E964-E973. doi: 10.1055/a-0915-2098.
17. Kalva NR et al. Efficacy and safety of lumen apposing self-expandable metal stents for EUS guided cholecystostomy: A meta-analysis and systematic review. Can J Gastroenterol Hepatol. 2018;2018:7070961. doi: 10.1155/2018/7070961.
18. Khan MA et al. Efficacy and safety of endoscopic gallbladder drainage in acute cholecystitis: Is it better than percutaneous gallbladder drainage? Gastrointest Endosc. 2017 Jan;85(1):76-87 e3. doi: 10.1016/j.gie.2016.06.032.
19. Mohan BP et al. Endoscopic ultrasound-guided gallbladder drainage, transpapillary drainage, or percutaneous drainage in high risk acute cholecystitis patients: a systematic review and comparative meta-analysis. Endoscopy. 2020 Feb;52(2):96-106. doi: 10.1055/a-1020-3932.
20. Jang JW et al. Endoscopic ultrasound-guided transmural and percutaneous transhepatic gallbladder drainage are comparable for acute cholecystitis. Gastroenterology. 2012 Apr;142(4):805-11. doi: 10.1053/j.gastro.2011.12.051.
21. Teoh AYB et al. EUS-guided gallbladder drainage versus laparoscopic cholecystectomy for acute cholecystitis: a propensity score analysis with 1-year follow-up data. Gastrointest Endosc. 2021 Mar;93(3):577-83. doi: 10.1016/j.gie.2020.06.066.
22. Luk SW et al. Endoscopic ultrasound-guided gallbladder drainage versus percutaneous cholecystostomy for high risk surgical patients with acute cholecystitis: a systematic review and meta-analysis. Endoscopy. 2019 Aug;51(8):722-32. doi: 10.1055/a-0929-6603.
Selecting therapies in moderate to severe inflammatory bowel disease: Key factors in decision making
Despite new advances in treatment, head to head clinical trials, which are considered the gold standard when comparing therapies, remain limited. Other comparative effectiveness studies and network meta-analyses are the currently available substitutes to guide decision making.1
While efficacy is often considered first when choosing a drug, other critical factors play a role in tailoring a treatment plan. This article focuses on key considerations to help guide clinical decision making when treating patients with moderate to severe IBD (Figure 1).

Disease activity versus severity
Both disease activity and disease severity should be considered when evaluating a patient for treatment. Disease activity is a cross-sectional view of one’s signs and symptoms which can vary visit to visit. Standardized indices measure disease activity in both Crohn’s disease (CD) and ulcerative colitis (UC).2,3 Disease severity encompasses the overall prognosis of disease over time and includes factors such as the presence or absence of high risk features, prior medication exposure, history of surgery, hospitalizations and the impact on quality of life.4
To prevent disease complications, the goals of treatment should be aimed at both reducing active symptoms (disease activity) but also healing mucosal inflammation, preventing disease progression (disease severity) and downstream sequelae including cancer, hospitalization or surgery.5 Determining the best treatment option takes disease activity and severity into account, in addition to the other key factors listed below (Figure 2).
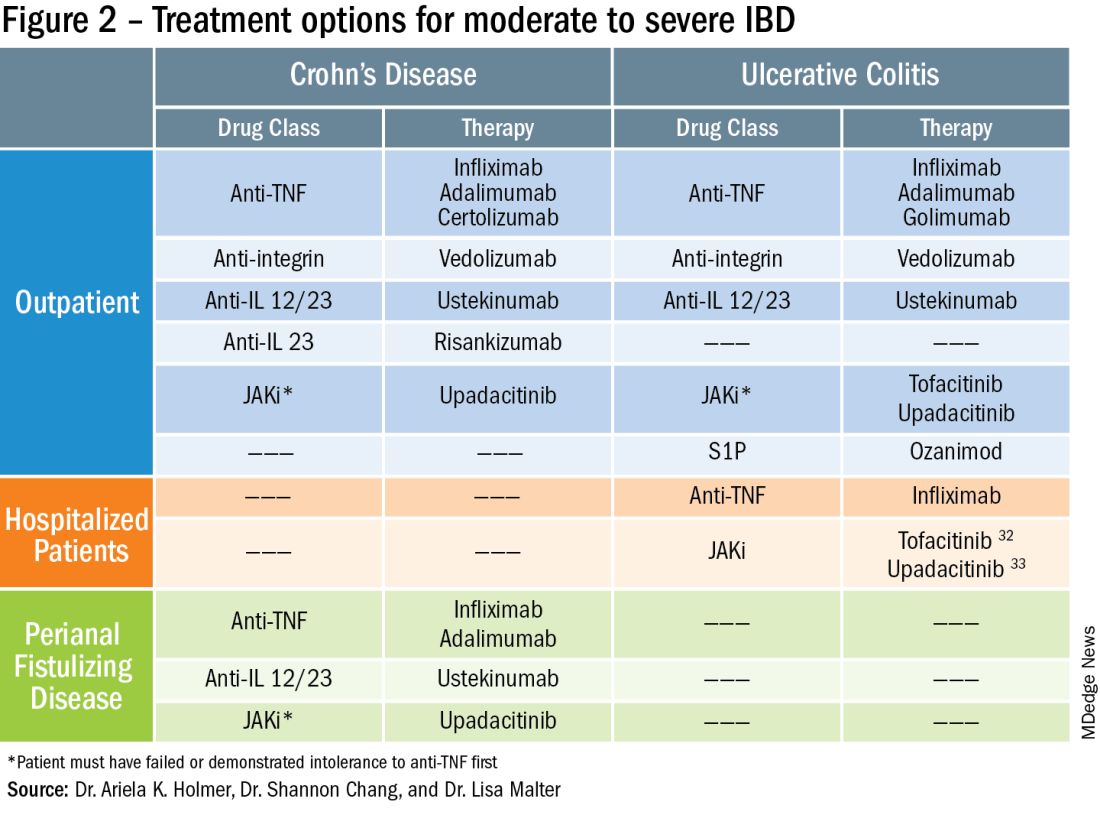
Extraintestinal manifestations
Inflammation of organs outside of the gastrointestinal tract is common and can occur in up to 50% of patients with IBD.6 The most prevalent extraintestinal manifestations (EIMs) involve the skin and joints, which will be the primary focus in this article. We will also focus on treatment options with the most evidence supporting their use. Peripheral arthritis is often associated with intestinal inflammation, and treatment of underlying IBD can simultaneously improve joint symptoms. Conversely, axial spondyloarthritis does not commonly parallel intestinal inflammation. Anti–tumor necrosis factor (TNF) agents including infliximab and adalimumab are effective for the treatment of both peripheral and axial disease.6
Ustekinumab, an interleukin (IL)-12/23 inhibitor, may be effective for peripheral arthritis, however is ineffective for the treatment of axial spondyloarthritis.6 Janus kinase (JAK) inhibitors which include tofacitinib and upadacitinib are oral small molecules used to treat peripheral and axial spondyloarthritis and have more recently been approved for moderate to severe IBD.6,7
Erythema nodosum (EN) and pyoderma gangrenosum (PG) are skin manifestations seen in patients with IBD. EN appears as subcutaneous nodules and parallels intestinal inflammation, while PG consists of violaceous, ulcerated plaques, and presents with more significant pain. Anti-TNFs are effective for both EN and PG, with infliximab being the only biologic studied in a randomized control trial of patients with PG.8 In addition, small case reports have described some benefit from ustekinumab and upadacitinib in the treatment of PG.9,10
Safety
The safety of IBD therapies is a key consideration and often the most important factor to patients when choosing a treatment option. It is important to note that untreated disease is associated with significant morbidity, and should be weighed when discussing risks of medications with patients. In general, anti-TNFs and JAK inhibitors may be associated with an increased risk of infection and malignancy, while ustekinumab, vedolizumab, risankizumab and ozanimod offer a more favorable safety profile.11 In large registries and observational studies, infliximab was associated with up to a two times greater risk of serious infection as compared to nonbiologic medications, with the most common infections being pneumonia, sepsis and herpes zoster.12 JAK inhibitors are associated with an increased risk of herpes zoster infection, with a dose dependent effect seen in the maintenance clinical trials with tofacitinib.7
Ozanimod may be associated with atrioventricular conduction delays and bradycardia, however long-term safety data has reported a low incidence of serious cardiac related adverse events.13 Overall, though risks of infection may vary with different therapies, other consistent risk factors associated with greater rates of serious infection include prolonged corticosteroid use, combination therapy with thiopurines, and disease severity. Anti-TNFs have also been associated with a somewhat increased risk of lymphoma, increased when used in combination with thiopurines. Reassuringly, however, in patients with a prior history of cancer, anti-TNFs and non-TNF biologics have not been found to increase the risk of new or recurrent cancer.14
Ultimately, in patients with a prior history of cancer, the choice of biologic or small molecule should be made in collaboration with a patient’s oncologist.
Anti-TNF exposure
Anti-TNFs were the first available biologics for the treatment of IBD. After the approval of vedolizumab in 2014, the first non-TNF biologic, many patients enrolled in clinical trials thereafter had already tried and failed anti-TNFs. In general, exposure to anti-TNFs may reduce the efficacy of a future biologic. In patients treated with vedolizumab, endoscopic and clinical outcomes were negatively impacted by prior anti-TNF exposure.15 However, in VARSITY, a head-to-head clinical trial where 20% of patients with UC were previously exposed to anti-TNFs other than adalimumab, vedolizumab had significantly higher rates of clinical remission and endoscopic improvement compared to adalimumab.16 Clinical remission rates with tofacitinib were not impacted by exposure to anti-TNF treatment, and similar findings were observed with ustekinumab.7,17 Risankizumab, a newly approved selective anti-IL23, also does not appear to be impacted by prior anti-TNF exposure by demonstrating similar rates of clinical remission regardless of biologic exposure status.18 Therefore, in patients with prior history of anti-TNF use, consideration of ustekinumab, risankizumab or JAK inhibitors as second line agents may be more favorable as compared to vedolizumab.
Perianal fistulizing disease
Perianal fistulizing disease can affect up to one-third of patients with CD and significantly impact a patient’s quality of life.19 The most robust data for the treatment of perianal fistulizing disease includes the use of infliximab with up to one-third of patients on maintenance therapy achieving complete resolution of fistula drainage. While no head-to-head trials compare combination therapy with infliximab plus immunomodulators versus infliximab alone for this indication specifically, one observational study demonstrated higher rates of fistula closure with combination therapy as compared to infliximab mono-therapy.19 In a post hoc analysis, higher infliximab concentrations at week 14 were associated with greater fistula response and remission rates.20 In patients with perianal disease, ustekinumab and vedolizumab may also be an effective treatment option by promoting resolution of fistula drainage.21
More recently, emerging data demonstrate that upadacitinib may be an excellent option as a second-line treatment for perianal disease in patients who have failed anti-TNF therapy. Use of upadacitinib was associated with greater rates of complete resolution of fistula drainage and higher rates of external fistula closure (Figure 2).22 Lastly, as an alternative to medical therapy, mesenchymal stem cell therapy has also shown to improve fistula drainage and improve external fistula openings in patients with CD.23 Stem cell therapy is only available through clinical trials at this time.
Patient preferences
Overall, data are lacking for evaluating patient preferences in treatment options for IBD especially with the recent increase in therapeutic options. One survey demonstrated that patient preferences were most impacted by the possibility of improving abdominal pain, with patients accepting additional risk of treatment side effects in order to reduce their abdominal pain.24 An oral route of administration and improving fatigue and bowel urgency were similarly important to patients. Patient preferences can also be highly variable with some valuing avoidance of corticosteroid use while others valuing avoidance of symptoms or risks of medication side effects and surgery. It is important to tailor the discussion on treatment strategies to each individual patient and inquire about the patient’s lifestyle, medical history, and value system, which may impact their treatment preferences utilizing shared decision making.
Access to treatment including the role of social determinants of health
The expanded therapeutic armamentarium has the potential to help patients achieve the current goals of care in IBD. However, these medications are not available to all patients due to numerous barriers including step therapy payer policies, prohibitive costs, insurance prior authorizations, and the role of social determinants of health and proximity to IBD expertise.25 While clinicians work with patients to determine the best treatment option, more often than not, the decision lies with the insurance payer. Step therapy is the protocol used by insurance companies that requires patients to try a lower-cost medication and fail to respond before they approve the originally requested treatment. This can lead to treatment delays, progression of disease, and disease complications. The option to incorporate the use of biosimilars, currently available for anti-TNFs, and other biologics in the near future, will reduce cost and potentially increase access.26 Additionally, working with a clinical pharmacist to navigate access and utilize patient assistance programs may help overcome cost related barriers to treatment and prevent delays in care.
Socioeconomic status has been shown to impact IBD disease outcomes, and compliance rates in treatment vary depending on race and ethnicity.27 Certain racial and ethnic groups remain vulnerable and may require additional support to achieve treatment goals. For example, disparities in health literacy in patients with IBD have been demonstrated with older black men at risk.28 Additionally, the patient’s proximity to their health care facility may impact treatment options. Most IBD centers are located in metropolitan areas and numerous “IBD deserts” exist, potentially limiting therapies for patients from more remote/rural settings.29 Access to treatment and the interplay of social determinants of health can have a large role in therapy selection.
Special considerations: Pregnancy and older adults
Certain patient populations warrant special consideration when approaching treatment strategies. Pregnancy in IBD will not be addressed in full depth in this article, however a key takeaway is that planning is critical and providers should emphasize the importance of steroid-free clinical remission for at least 3 months before conception.30 Additionally, biologic use during pregnancy has not been shown to increase adverse fetal outcomes, thus should be continued to minimize disease flare. Newer novel small molecules are generally avoided during pregnancy due to limited available safety data.
Older adults are the largest growing patient population with IBD. Frailty, or a state of decreased reserve, is more commonly observed in older patients and has been shown to increase adverse events including hospitalization and mortaility.31 Ultimately reducing polypharmacy, ensuring adequate nutrition, minimizing corticosteroid exposure and avoiding undertreatment of active IBD are all key in optimizing outcomes in an older patient with IBD.
Conclusion
When discussing treatment options with patients with IBD, it is important to individualize care and share the decision-making process with patients. Goals include improving symptoms and quality of life while working to achieve the goal of healing intestinal inflammation. In summary, this article can serve as a guide to clinicians for key factors in decision making when selecting therapies in moderate to severe IBD.
Dr. Holmer is a gastroenterologist with NYU Langone Health specializing in inflammatory bowel disease. Dr. Chang is director of clinical operations for the NYU Langone Health Inflammatory Bowel Disease Center. Dr. Malter is director of education for the Inflammatory Bowel Disease Center at NYU Langone Health and director of the inflammatory bowel disease program at Bellevue Hospital Center. Follow Dr. Holmer on X (formerly Twitter) at @HolmerMd and Dr. Chang @shannonchangmd. Dr. Holmer disclosed affiliations with Pfizer, Bristol Myers Squibb, and AvevoRx. Dr. Chang disclosed affiliations with Pfizer and Bristol Myers Squibb. Dr. Malter disclosed receiving educational grants form Abbvie, Janssen, Pfizer and Takeda, and serving on the advisory boards of AbbVie, Bristol Myers Squibb, Celltrion, Janssen, Merck, and Takeda.
References
1. Chang S et al. Am J Gastroenterol. 2023 Aug 24. doi: 10.14309/ajg.0000000000002485.
2. Harvey RF et al. The Lancet. 1980;1:514.
3. Lewis JD et al. Inflammatory Bowel Diseases. 2008;14:1660-1666.
4. Siegel CA et al. Gut. 2018;67(2):244-54.
5. Peyrin-Biroulet L et al. Am J Gastroenterol. 2015;110:1324-38
6. Rogler G et al. Gastroenterology. 2021;161:1118-32.
7. Sandborn WJ et al. N Engl J Med. 2017;376:1723-36.
8. Brooklyn TN et al. Gut. 2006;55:505-9.
9. Fahmy M et al. Am J Gastroenterol. 2012;107:794-5.
10. Van Eycken L et al. JAAD Case Rep. 2023;37:89-91.
11. Lasa JS et al. Lancet Gastroenterol Hepatol. 2022;7:161-70.
12. Lichtenstein GR et al. Inflamm Bowel Dis. 2018;24:490-501.
13. Long MD et al. Gastroenterology. 2022;162:S-5-S-6.
14. Holmer AK et al. Clin Gastroenterol Hepatol.2023;21:1598-1606.e5.
15. Sands BE et al. Gastroenterology. 2014;147:618-27.e3.
16. Sands BE et al. N Engl J Med. 2019;381:1215-26.
17. Sands BE et al. N Engl J Med. 2019;381:1201-14.
18. D’Haens G et al. Lancet. 2022;399:2015-30.
19. Bouguen G et al. Clin Gastroenterol Hepatol. 2013;11:975-81.e1-4.
20. Papamichael K et al. Am J Gastroenterol. 2021;116:1007-14.
21. Shehab M et al. Inflamm Bowel Dis. 2023;29:367-75.
22. Colombel JF et al. J Crohns Colitis. 2023;17:i620-i623.
23. Garcia-Olmo D et al. Dis Colon Rectum. 2022;65:713-20.
24. Louis E et al. J Crohns Colitis. 2023;17:231-9.
25. Rubin DT et al. Inflamm Bowel Dis. 2017;23:224-32.
26. Gulacsi L et al. Curr Med Chem. 2019;26:259-69.
27. Cai Q et al. BMC Gastroenterol. 2022;22:545.
28. Dos Santos Marques IC et al. Crohns Colitis 360. 2020 Oct;2(4):otaa076.
29. Deepak P et al. Gastroenterology. 2023;165:11-15.
30. Mahadevan U et al. Gastroenterology. 2019;156:1508-24.
31. Faye AS et al. Inflamm Bowel Dis. 2022;28:126-32.
32. Berinstein JA et al. Clin Gastroenterol Hepatol. 2021;19:2112-20.e1.
33. Levine J et al. Gastroenterology. 2023;164:S103-S104.
Despite new advances in treatment, head to head clinical trials, which are considered the gold standard when comparing therapies, remain limited. Other comparative effectiveness studies and network meta-analyses are the currently available substitutes to guide decision making.1
While efficacy is often considered first when choosing a drug, other critical factors play a role in tailoring a treatment plan. This article focuses on key considerations to help guide clinical decision making when treating patients with moderate to severe IBD (Figure 1).

Disease activity versus severity
Both disease activity and disease severity should be considered when evaluating a patient for treatment. Disease activity is a cross-sectional view of one’s signs and symptoms which can vary visit to visit. Standardized indices measure disease activity in both Crohn’s disease (CD) and ulcerative colitis (UC).2,3 Disease severity encompasses the overall prognosis of disease over time and includes factors such as the presence or absence of high risk features, prior medication exposure, history of surgery, hospitalizations and the impact on quality of life.4
To prevent disease complications, the goals of treatment should be aimed at both reducing active symptoms (disease activity) but also healing mucosal inflammation, preventing disease progression (disease severity) and downstream sequelae including cancer, hospitalization or surgery.5 Determining the best treatment option takes disease activity and severity into account, in addition to the other key factors listed below (Figure 2).

Extraintestinal manifestations
Inflammation of organs outside of the gastrointestinal tract is common and can occur in up to 50% of patients with IBD.6 The most prevalent extraintestinal manifestations (EIMs) involve the skin and joints, which will be the primary focus in this article. We will also focus on treatment options with the most evidence supporting their use. Peripheral arthritis is often associated with intestinal inflammation, and treatment of underlying IBD can simultaneously improve joint symptoms. Conversely, axial spondyloarthritis does not commonly parallel intestinal inflammation. Anti–tumor necrosis factor (TNF) agents including infliximab and adalimumab are effective for the treatment of both peripheral and axial disease.6
Ustekinumab, an interleukin (IL)-12/23 inhibitor, may be effective for peripheral arthritis, however is ineffective for the treatment of axial spondyloarthritis.6 Janus kinase (JAK) inhibitors which include tofacitinib and upadacitinib are oral small molecules used to treat peripheral and axial spondyloarthritis and have more recently been approved for moderate to severe IBD.6,7
Erythema nodosum (EN) and pyoderma gangrenosum (PG) are skin manifestations seen in patients with IBD. EN appears as subcutaneous nodules and parallels intestinal inflammation, while PG consists of violaceous, ulcerated plaques, and presents with more significant pain. Anti-TNFs are effective for both EN and PG, with infliximab being the only biologic studied in a randomized control trial of patients with PG.8 In addition, small case reports have described some benefit from ustekinumab and upadacitinib in the treatment of PG.9,10
Safety
The safety of IBD therapies is a key consideration and often the most important factor to patients when choosing a treatment option. It is important to note that untreated disease is associated with significant morbidity, and should be weighed when discussing risks of medications with patients. In general, anti-TNFs and JAK inhibitors may be associated with an increased risk of infection and malignancy, while ustekinumab, vedolizumab, risankizumab and ozanimod offer a more favorable safety profile.11 In large registries and observational studies, infliximab was associated with up to a two times greater risk of serious infection as compared to nonbiologic medications, with the most common infections being pneumonia, sepsis and herpes zoster.12 JAK inhibitors are associated with an increased risk of herpes zoster infection, with a dose dependent effect seen in the maintenance clinical trials with tofacitinib.7
Ozanimod may be associated with atrioventricular conduction delays and bradycardia, however long-term safety data has reported a low incidence of serious cardiac related adverse events.13 Overall, though risks of infection may vary with different therapies, other consistent risk factors associated with greater rates of serious infection include prolonged corticosteroid use, combination therapy with thiopurines, and disease severity. Anti-TNFs have also been associated with a somewhat increased risk of lymphoma, increased when used in combination with thiopurines. Reassuringly, however, in patients with a prior history of cancer, anti-TNFs and non-TNF biologics have not been found to increase the risk of new or recurrent cancer.14
Ultimately, in patients with a prior history of cancer, the choice of biologic or small molecule should be made in collaboration with a patient’s oncologist.
Anti-TNF exposure
Anti-TNFs were the first available biologics for the treatment of IBD. After the approval of vedolizumab in 2014, the first non-TNF biologic, many patients enrolled in clinical trials thereafter had already tried and failed anti-TNFs. In general, exposure to anti-TNFs may reduce the efficacy of a future biologic. In patients treated with vedolizumab, endoscopic and clinical outcomes were negatively impacted by prior anti-TNF exposure.15 However, in VARSITY, a head-to-head clinical trial where 20% of patients with UC were previously exposed to anti-TNFs other than adalimumab, vedolizumab had significantly higher rates of clinical remission and endoscopic improvement compared to adalimumab.16 Clinical remission rates with tofacitinib were not impacted by exposure to anti-TNF treatment, and similar findings were observed with ustekinumab.7,17 Risankizumab, a newly approved selective anti-IL23, also does not appear to be impacted by prior anti-TNF exposure by demonstrating similar rates of clinical remission regardless of biologic exposure status.18 Therefore, in patients with prior history of anti-TNF use, consideration of ustekinumab, risankizumab or JAK inhibitors as second line agents may be more favorable as compared to vedolizumab.
Perianal fistulizing disease
Perianal fistulizing disease can affect up to one-third of patients with CD and significantly impact a patient’s quality of life.19 The most robust data for the treatment of perianal fistulizing disease includes the use of infliximab with up to one-third of patients on maintenance therapy achieving complete resolution of fistula drainage. While no head-to-head trials compare combination therapy with infliximab plus immunomodulators versus infliximab alone for this indication specifically, one observational study demonstrated higher rates of fistula closure with combination therapy as compared to infliximab mono-therapy.19 In a post hoc analysis, higher infliximab concentrations at week 14 were associated with greater fistula response and remission rates.20 In patients with perianal disease, ustekinumab and vedolizumab may also be an effective treatment option by promoting resolution of fistula drainage.21
More recently, emerging data demonstrate that upadacitinib may be an excellent option as a second-line treatment for perianal disease in patients who have failed anti-TNF therapy. Use of upadacitinib was associated with greater rates of complete resolution of fistula drainage and higher rates of external fistula closure (Figure 2).22 Lastly, as an alternative to medical therapy, mesenchymal stem cell therapy has also shown to improve fistula drainage and improve external fistula openings in patients with CD.23 Stem cell therapy is only available through clinical trials at this time.
Patient preferences
Overall, data are lacking for evaluating patient preferences in treatment options for IBD especially with the recent increase in therapeutic options. One survey demonstrated that patient preferences were most impacted by the possibility of improving abdominal pain, with patients accepting additional risk of treatment side effects in order to reduce their abdominal pain.24 An oral route of administration and improving fatigue and bowel urgency were similarly important to patients. Patient preferences can also be highly variable with some valuing avoidance of corticosteroid use while others valuing avoidance of symptoms or risks of medication side effects and surgery. It is important to tailor the discussion on treatment strategies to each individual patient and inquire about the patient’s lifestyle, medical history, and value system, which may impact their treatment preferences utilizing shared decision making.
Access to treatment including the role of social determinants of health
The expanded therapeutic armamentarium has the potential to help patients achieve the current goals of care in IBD. However, these medications are not available to all patients due to numerous barriers including step therapy payer policies, prohibitive costs, insurance prior authorizations, and the role of social determinants of health and proximity to IBD expertise.25 While clinicians work with patients to determine the best treatment option, more often than not, the decision lies with the insurance payer. Step therapy is the protocol used by insurance companies that requires patients to try a lower-cost medication and fail to respond before they approve the originally requested treatment. This can lead to treatment delays, progression of disease, and disease complications. The option to incorporate the use of biosimilars, currently available for anti-TNFs, and other biologics in the near future, will reduce cost and potentially increase access.26 Additionally, working with a clinical pharmacist to navigate access and utilize patient assistance programs may help overcome cost related barriers to treatment and prevent delays in care.
Socioeconomic status has been shown to impact IBD disease outcomes, and compliance rates in treatment vary depending on race and ethnicity.27 Certain racial and ethnic groups remain vulnerable and may require additional support to achieve treatment goals. For example, disparities in health literacy in patients with IBD have been demonstrated with older black men at risk.28 Additionally, the patient’s proximity to their health care facility may impact treatment options. Most IBD centers are located in metropolitan areas and numerous “IBD deserts” exist, potentially limiting therapies for patients from more remote/rural settings.29 Access to treatment and the interplay of social determinants of health can have a large role in therapy selection.
Special considerations: Pregnancy and older adults
Certain patient populations warrant special consideration when approaching treatment strategies. Pregnancy in IBD will not be addressed in full depth in this article, however a key takeaway is that planning is critical and providers should emphasize the importance of steroid-free clinical remission for at least 3 months before conception.30 Additionally, biologic use during pregnancy has not been shown to increase adverse fetal outcomes, thus should be continued to minimize disease flare. Newer novel small molecules are generally avoided during pregnancy due to limited available safety data.
Older adults are the largest growing patient population with IBD. Frailty, or a state of decreased reserve, is more commonly observed in older patients and has been shown to increase adverse events including hospitalization and mortaility.31 Ultimately reducing polypharmacy, ensuring adequate nutrition, minimizing corticosteroid exposure and avoiding undertreatment of active IBD are all key in optimizing outcomes in an older patient with IBD.
Conclusion
When discussing treatment options with patients with IBD, it is important to individualize care and share the decision-making process with patients. Goals include improving symptoms and quality of life while working to achieve the goal of healing intestinal inflammation. In summary, this article can serve as a guide to clinicians for key factors in decision making when selecting therapies in moderate to severe IBD.
Dr. Holmer is a gastroenterologist with NYU Langone Health specializing in inflammatory bowel disease. Dr. Chang is director of clinical operations for the NYU Langone Health Inflammatory Bowel Disease Center. Dr. Malter is director of education for the Inflammatory Bowel Disease Center at NYU Langone Health and director of the inflammatory bowel disease program at Bellevue Hospital Center. Follow Dr. Holmer on X (formerly Twitter) at @HolmerMd and Dr. Chang @shannonchangmd. Dr. Holmer disclosed affiliations with Pfizer, Bristol Myers Squibb, and AvevoRx. Dr. Chang disclosed affiliations with Pfizer and Bristol Myers Squibb. Dr. Malter disclosed receiving educational grants form Abbvie, Janssen, Pfizer and Takeda, and serving on the advisory boards of AbbVie, Bristol Myers Squibb, Celltrion, Janssen, Merck, and Takeda.
References
1. Chang S et al. Am J Gastroenterol. 2023 Aug 24. doi: 10.14309/ajg.0000000000002485.
2. Harvey RF et al. The Lancet. 1980;1:514.
3. Lewis JD et al. Inflammatory Bowel Diseases. 2008;14:1660-1666.
4. Siegel CA et al. Gut. 2018;67(2):244-54.
5. Peyrin-Biroulet L et al. Am J Gastroenterol. 2015;110:1324-38
6. Rogler G et al. Gastroenterology. 2021;161:1118-32.
7. Sandborn WJ et al. N Engl J Med. 2017;376:1723-36.
8. Brooklyn TN et al. Gut. 2006;55:505-9.
9. Fahmy M et al. Am J Gastroenterol. 2012;107:794-5.
10. Van Eycken L et al. JAAD Case Rep. 2023;37:89-91.
11. Lasa JS et al. Lancet Gastroenterol Hepatol. 2022;7:161-70.
12. Lichtenstein GR et al. Inflamm Bowel Dis. 2018;24:490-501.
13. Long MD et al. Gastroenterology. 2022;162:S-5-S-6.
14. Holmer AK et al. Clin Gastroenterol Hepatol.2023;21:1598-1606.e5.
15. Sands BE et al. Gastroenterology. 2014;147:618-27.e3.
16. Sands BE et al. N Engl J Med. 2019;381:1215-26.
17. Sands BE et al. N Engl J Med. 2019;381:1201-14.
18. D’Haens G et al. Lancet. 2022;399:2015-30.
19. Bouguen G et al. Clin Gastroenterol Hepatol. 2013;11:975-81.e1-4.
20. Papamichael K et al. Am J Gastroenterol. 2021;116:1007-14.
21. Shehab M et al. Inflamm Bowel Dis. 2023;29:367-75.
22. Colombel JF et al. J Crohns Colitis. 2023;17:i620-i623.
23. Garcia-Olmo D et al. Dis Colon Rectum. 2022;65:713-20.
24. Louis E et al. J Crohns Colitis. 2023;17:231-9.
25. Rubin DT et al. Inflamm Bowel Dis. 2017;23:224-32.
26. Gulacsi L et al. Curr Med Chem. 2019;26:259-69.
27. Cai Q et al. BMC Gastroenterol. 2022;22:545.
28. Dos Santos Marques IC et al. Crohns Colitis 360. 2020 Oct;2(4):otaa076.
29. Deepak P et al. Gastroenterology. 2023;165:11-15.
30. Mahadevan U et al. Gastroenterology. 2019;156:1508-24.
31. Faye AS et al. Inflamm Bowel Dis. 2022;28:126-32.
32. Berinstein JA et al. Clin Gastroenterol Hepatol. 2021;19:2112-20.e1.
33. Levine J et al. Gastroenterology. 2023;164:S103-S104.
Despite new advances in treatment, head to head clinical trials, which are considered the gold standard when comparing therapies, remain limited. Other comparative effectiveness studies and network meta-analyses are the currently available substitutes to guide decision making.1
While efficacy is often considered first when choosing a drug, other critical factors play a role in tailoring a treatment plan. This article focuses on key considerations to help guide clinical decision making when treating patients with moderate to severe IBD (Figure 1).

Disease activity versus severity
Both disease activity and disease severity should be considered when evaluating a patient for treatment. Disease activity is a cross-sectional view of one’s signs and symptoms which can vary visit to visit. Standardized indices measure disease activity in both Crohn’s disease (CD) and ulcerative colitis (UC).2,3 Disease severity encompasses the overall prognosis of disease over time and includes factors such as the presence or absence of high risk features, prior medication exposure, history of surgery, hospitalizations and the impact on quality of life.4
To prevent disease complications, the goals of treatment should be aimed at both reducing active symptoms (disease activity) but also healing mucosal inflammation, preventing disease progression (disease severity) and downstream sequelae including cancer, hospitalization or surgery.5 Determining the best treatment option takes disease activity and severity into account, in addition to the other key factors listed below (Figure 2).

Extraintestinal manifestations
Inflammation of organs outside of the gastrointestinal tract is common and can occur in up to 50% of patients with IBD.6 The most prevalent extraintestinal manifestations (EIMs) involve the skin and joints, which will be the primary focus in this article. We will also focus on treatment options with the most evidence supporting their use. Peripheral arthritis is often associated with intestinal inflammation, and treatment of underlying IBD can simultaneously improve joint symptoms. Conversely, axial spondyloarthritis does not commonly parallel intestinal inflammation. Anti–tumor necrosis factor (TNF) agents including infliximab and adalimumab are effective for the treatment of both peripheral and axial disease.6
Ustekinumab, an interleukin (IL)-12/23 inhibitor, may be effective for peripheral arthritis, however is ineffective for the treatment of axial spondyloarthritis.6 Janus kinase (JAK) inhibitors which include tofacitinib and upadacitinib are oral small molecules used to treat peripheral and axial spondyloarthritis and have more recently been approved for moderate to severe IBD.6,7
Erythema nodosum (EN) and pyoderma gangrenosum (PG) are skin manifestations seen in patients with IBD. EN appears as subcutaneous nodules and parallels intestinal inflammation, while PG consists of violaceous, ulcerated plaques, and presents with more significant pain. Anti-TNFs are effective for both EN and PG, with infliximab being the only biologic studied in a randomized control trial of patients with PG.8 In addition, small case reports have described some benefit from ustekinumab and upadacitinib in the treatment of PG.9,10
Safety
The safety of IBD therapies is a key consideration and often the most important factor to patients when choosing a treatment option. It is important to note that untreated disease is associated with significant morbidity, and should be weighed when discussing risks of medications with patients. In general, anti-TNFs and JAK inhibitors may be associated with an increased risk of infection and malignancy, while ustekinumab, vedolizumab, risankizumab and ozanimod offer a more favorable safety profile.11 In large registries and observational studies, infliximab was associated with up to a two times greater risk of serious infection as compared to nonbiologic medications, with the most common infections being pneumonia, sepsis and herpes zoster.12 JAK inhibitors are associated with an increased risk of herpes zoster infection, with a dose dependent effect seen in the maintenance clinical trials with tofacitinib.7
Ozanimod may be associated with atrioventricular conduction delays and bradycardia, however long-term safety data has reported a low incidence of serious cardiac related adverse events.13 Overall, though risks of infection may vary with different therapies, other consistent risk factors associated with greater rates of serious infection include prolonged corticosteroid use, combination therapy with thiopurines, and disease severity. Anti-TNFs have also been associated with a somewhat increased risk of lymphoma, increased when used in combination with thiopurines. Reassuringly, however, in patients with a prior history of cancer, anti-TNFs and non-TNF biologics have not been found to increase the risk of new or recurrent cancer.14
Ultimately, in patients with a prior history of cancer, the choice of biologic or small molecule should be made in collaboration with a patient’s oncologist.
Anti-TNF exposure
Anti-TNFs were the first available biologics for the treatment of IBD. After the approval of vedolizumab in 2014, the first non-TNF biologic, many patients enrolled in clinical trials thereafter had already tried and failed anti-TNFs. In general, exposure to anti-TNFs may reduce the efficacy of a future biologic. In patients treated with vedolizumab, endoscopic and clinical outcomes were negatively impacted by prior anti-TNF exposure.15 However, in VARSITY, a head-to-head clinical trial where 20% of patients with UC were previously exposed to anti-TNFs other than adalimumab, vedolizumab had significantly higher rates of clinical remission and endoscopic improvement compared to adalimumab.16 Clinical remission rates with tofacitinib were not impacted by exposure to anti-TNF treatment, and similar findings were observed with ustekinumab.7,17 Risankizumab, a newly approved selective anti-IL23, also does not appear to be impacted by prior anti-TNF exposure by demonstrating similar rates of clinical remission regardless of biologic exposure status.18 Therefore, in patients with prior history of anti-TNF use, consideration of ustekinumab, risankizumab or JAK inhibitors as second line agents may be more favorable as compared to vedolizumab.
Perianal fistulizing disease
Perianal fistulizing disease can affect up to one-third of patients with CD and significantly impact a patient’s quality of life.19 The most robust data for the treatment of perianal fistulizing disease includes the use of infliximab with up to one-third of patients on maintenance therapy achieving complete resolution of fistula drainage. While no head-to-head trials compare combination therapy with infliximab plus immunomodulators versus infliximab alone for this indication specifically, one observational study demonstrated higher rates of fistula closure with combination therapy as compared to infliximab mono-therapy.19 In a post hoc analysis, higher infliximab concentrations at week 14 were associated with greater fistula response and remission rates.20 In patients with perianal disease, ustekinumab and vedolizumab may also be an effective treatment option by promoting resolution of fistula drainage.21
More recently, emerging data demonstrate that upadacitinib may be an excellent option as a second-line treatment for perianal disease in patients who have failed anti-TNF therapy. Use of upadacitinib was associated with greater rates of complete resolution of fistula drainage and higher rates of external fistula closure (Figure 2).22 Lastly, as an alternative to medical therapy, mesenchymal stem cell therapy has also shown to improve fistula drainage and improve external fistula openings in patients with CD.23 Stem cell therapy is only available through clinical trials at this time.
Patient preferences
Overall, data are lacking for evaluating patient preferences in treatment options for IBD especially with the recent increase in therapeutic options. One survey demonstrated that patient preferences were most impacted by the possibility of improving abdominal pain, with patients accepting additional risk of treatment side effects in order to reduce their abdominal pain.24 An oral route of administration and improving fatigue and bowel urgency were similarly important to patients. Patient preferences can also be highly variable with some valuing avoidance of corticosteroid use while others valuing avoidance of symptoms or risks of medication side effects and surgery. It is important to tailor the discussion on treatment strategies to each individual patient and inquire about the patient’s lifestyle, medical history, and value system, which may impact their treatment preferences utilizing shared decision making.
Access to treatment including the role of social determinants of health
The expanded therapeutic armamentarium has the potential to help patients achieve the current goals of care in IBD. However, these medications are not available to all patients due to numerous barriers including step therapy payer policies, prohibitive costs, insurance prior authorizations, and the role of social determinants of health and proximity to IBD expertise.25 While clinicians work with patients to determine the best treatment option, more often than not, the decision lies with the insurance payer. Step therapy is the protocol used by insurance companies that requires patients to try a lower-cost medication and fail to respond before they approve the originally requested treatment. This can lead to treatment delays, progression of disease, and disease complications. The option to incorporate the use of biosimilars, currently available for anti-TNFs, and other biologics in the near future, will reduce cost and potentially increase access.26 Additionally, working with a clinical pharmacist to navigate access and utilize patient assistance programs may help overcome cost related barriers to treatment and prevent delays in care.
Socioeconomic status has been shown to impact IBD disease outcomes, and compliance rates in treatment vary depending on race and ethnicity.27 Certain racial and ethnic groups remain vulnerable and may require additional support to achieve treatment goals. For example, disparities in health literacy in patients with IBD have been demonstrated with older black men at risk.28 Additionally, the patient’s proximity to their health care facility may impact treatment options. Most IBD centers are located in metropolitan areas and numerous “IBD deserts” exist, potentially limiting therapies for patients from more remote/rural settings.29 Access to treatment and the interplay of social determinants of health can have a large role in therapy selection.
Special considerations: Pregnancy and older adults
Certain patient populations warrant special consideration when approaching treatment strategies. Pregnancy in IBD will not be addressed in full depth in this article, however a key takeaway is that planning is critical and providers should emphasize the importance of steroid-free clinical remission for at least 3 months before conception.30 Additionally, biologic use during pregnancy has not been shown to increase adverse fetal outcomes, thus should be continued to minimize disease flare. Newer novel small molecules are generally avoided during pregnancy due to limited available safety data.
Older adults are the largest growing patient population with IBD. Frailty, or a state of decreased reserve, is more commonly observed in older patients and has been shown to increase adverse events including hospitalization and mortaility.31 Ultimately reducing polypharmacy, ensuring adequate nutrition, minimizing corticosteroid exposure and avoiding undertreatment of active IBD are all key in optimizing outcomes in an older patient with IBD.
Conclusion
When discussing treatment options with patients with IBD, it is important to individualize care and share the decision-making process with patients. Goals include improving symptoms and quality of life while working to achieve the goal of healing intestinal inflammation. In summary, this article can serve as a guide to clinicians for key factors in decision making when selecting therapies in moderate to severe IBD.
Dr. Holmer is a gastroenterologist with NYU Langone Health specializing in inflammatory bowel disease. Dr. Chang is director of clinical operations for the NYU Langone Health Inflammatory Bowel Disease Center. Dr. Malter is director of education for the Inflammatory Bowel Disease Center at NYU Langone Health and director of the inflammatory bowel disease program at Bellevue Hospital Center. Follow Dr. Holmer on X (formerly Twitter) at @HolmerMd and Dr. Chang @shannonchangmd. Dr. Holmer disclosed affiliations with Pfizer, Bristol Myers Squibb, and AvevoRx. Dr. Chang disclosed affiliations with Pfizer and Bristol Myers Squibb. Dr. Malter disclosed receiving educational grants form Abbvie, Janssen, Pfizer and Takeda, and serving on the advisory boards of AbbVie, Bristol Myers Squibb, Celltrion, Janssen, Merck, and Takeda.
References
1. Chang S et al. Am J Gastroenterol. 2023 Aug 24. doi: 10.14309/ajg.0000000000002485.
2. Harvey RF et al. The Lancet. 1980;1:514.
3. Lewis JD et al. Inflammatory Bowel Diseases. 2008;14:1660-1666.
4. Siegel CA et al. Gut. 2018;67(2):244-54.
5. Peyrin-Biroulet L et al. Am J Gastroenterol. 2015;110:1324-38
6. Rogler G et al. Gastroenterology. 2021;161:1118-32.
7. Sandborn WJ et al. N Engl J Med. 2017;376:1723-36.
8. Brooklyn TN et al. Gut. 2006;55:505-9.
9. Fahmy M et al. Am J Gastroenterol. 2012;107:794-5.
10. Van Eycken L et al. JAAD Case Rep. 2023;37:89-91.
11. Lasa JS et al. Lancet Gastroenterol Hepatol. 2022;7:161-70.
12. Lichtenstein GR et al. Inflamm Bowel Dis. 2018;24:490-501.
13. Long MD et al. Gastroenterology. 2022;162:S-5-S-6.
14. Holmer AK et al. Clin Gastroenterol Hepatol.2023;21:1598-1606.e5.
15. Sands BE et al. Gastroenterology. 2014;147:618-27.e3.
16. Sands BE et al. N Engl J Med. 2019;381:1215-26.
17. Sands BE et al. N Engl J Med. 2019;381:1201-14.
18. D’Haens G et al. Lancet. 2022;399:2015-30.
19. Bouguen G et al. Clin Gastroenterol Hepatol. 2013;11:975-81.e1-4.
20. Papamichael K et al. Am J Gastroenterol. 2021;116:1007-14.
21. Shehab M et al. Inflamm Bowel Dis. 2023;29:367-75.
22. Colombel JF et al. J Crohns Colitis. 2023;17:i620-i623.
23. Garcia-Olmo D et al. Dis Colon Rectum. 2022;65:713-20.
24. Louis E et al. J Crohns Colitis. 2023;17:231-9.
25. Rubin DT et al. Inflamm Bowel Dis. 2017;23:224-32.
26. Gulacsi L et al. Curr Med Chem. 2019;26:259-69.
27. Cai Q et al. BMC Gastroenterol. 2022;22:545.
28. Dos Santos Marques IC et al. Crohns Colitis 360. 2020 Oct;2(4):otaa076.
29. Deepak P et al. Gastroenterology. 2023;165:11-15.
30. Mahadevan U et al. Gastroenterology. 2019;156:1508-24.
31. Faye AS et al. Inflamm Bowel Dis. 2022;28:126-32.
32. Berinstein JA et al. Clin Gastroenterol Hepatol. 2021;19:2112-20.e1.
33. Levine J et al. Gastroenterology. 2023;164:S103-S104.
Navigating NAFLD: Unveiling the approach to mitigate the impact of NAFLD
Burden of NAFLD in the U.S.
NAFLD is a manifestation of systemic metabolic abnormalities, including insulin resistance, dyslipidemia, central obesity, and hypertension. In this short review, we summarize data on the burden of NAFLD in the U.S. and its prognostic determinants and review what clinical and public health approaches may be needed to mitigating its impact.
Epidemiology of NAFLD
Worldwide, the prevalence of NAFLD is estimated at 6% to 35%, with biopsy-based studies reporting NASH in 3% to 5%.1 U.S. estimates for the prevalence of NAFLD range from 10% to 46%.2 In our own analysis of the National Health and Nutrition Examination Survey (NHANES) data, transient elastography-detected steatosis was found in 36%, which projected to a minimum of 73 million American adults.3
NAFLD represents a spectrum of disorders ranging from simple steatosis to nonalcoholic steatohepatitis (NASH), the latter leading, in some cases, to progressive hepatic fibrosis and cirrhosis.4 Out of a large number of subjects with NAFLD, the proportions of NASH patients that develop severe liver problems such as end-stage liver disease (ESLD) or hepatocellular carcinoma (HCC) are progressively smaller. For example, we recently reported that less than 2,000 liver-related deaths are attributable to NAFLD in the U.S. per annum, which corresponds to a crude case fatality rate of < 0.005% per year.5
According to the Centers for Disease Control and Prevention (CDC), there have been substantial increases in liver-related deaths over the last 2 decades. Mortality from liver disease including hepatobiliary cancers more than doubled from 41,966 deaths (including 15,321 women and 26,645 men) in 2000 to 85,884 deaths (33,000 women and 52,884 men) in 2020. The proportion of deaths specifically attributed to NAFLD among liver-related deaths was miniscule in 2000, accounting for 1.1% in women and 0.7% in men. By 2020, the proportions increased several folds in both sexes (7.4% in women and 2.7% in men).6 Moreover, it is likely that a substantial portion of deaths from chronic liver disease from unknown causes (“cryptogenic”) are likely end-stage NAFLD, making these figures underestimates of the true impact of NAFLD in the U.S.
From a comparative epidemiologic perspective, there are significant racial and ethnic and socioeconomic disparities in NAFLD prevalence, wherein Hispanic persons and individuals experiencing food insecurity – independent of poverty status, education level, race and ethnicity – are disproportionately more affected by NAFLD.7,8 Furthermore, these disparities persist when examining long-term complications of NAFLD, such as developing HCC.
Prognosis in NAFLD: NASH versus fibrosis
Given the enormous prevalence and increasing public health burden of NAFLD, systematic interventions to mitigate its impact are urgently needed. Clearly, patients who already have developed advanced liver disease need to be directed to specialty care so the disease progression may be halted and complications of ESLD may be prevented or managed. On the other hand, in order to mitigate the future impact of ESLD, prompt identification of at-risk patients and proactive interventions to improve liver health are needed.
In the assessment of disease progression, prior data have shown that the presence of NASH and increasing stages of liver fibrosis are important predictors of disease progression. Fibrosis is a component of NASH, while NASH is thought to be a prerequisite for fibrosis. In a prospective, multicenter follow-up study of NAFLD evaluated by liver biopsies (n = 1,773), over a median follow-up of 4 years, 37 (2%) developed hepatic decompensation, while 47 (3%) died from any cause, which included ESLD (n = 12), cardiovascular complications (n = 4), and malignancies (n = 12), including HCC (n = 9).9 It is not entirely surprising that advanced fibrosis and cirrhosis was highly associated with the development of hepatic decompensation. In their multivariable analysis, patients with F3-4 had a 13.8-fold (95% confidence interval [CI]: 4.6, 41.0) increase in the hazard of reaching a MELD score of 15 compared to those with F0-2. In addition, all-cause mortality was 17.2-fold (95% CI: 5.2, 56.6) higher with F3-4 compared to F0-2.
These data have been borne out by a larger body of literature on the topic. In a recent meta-analysis assessing the relation between liver fibrosis and future mortality, which included 17,301 subjects with NAFLD, patients with at least stage 2 fibrosis experience a significantly increased risk of liver-related and overall mortality, a trend that accelerates at higher fibrosis stages.10 These point to liver fibrosis as the singular determinant of long-term prognosis, in comparison, for example, with the diagnosis of NASH. Hagström conducted a retrospective cohort study of patients with biopsy-proven NAFLD in Sweden. When fibrosis stage and histological diagnosis of NASH were considered together, NASH did not have an impact on overall mortality (hazard ratio [HR] = 0.83, P = .29) or liver morbidity (HR = 0.62, P = .25).11
On an individual level, factors that affect fibrosis progression are not as well studied. It is commonly believed that demographic factors (e.g., age, sex and race), genetic polymorphisms (e.g., PNPLA3, TM6SF2), clinical comorbidities (e.g., obesity, DM, and sleep apnea), and environmental factors (e.g., smoking) may accelerate fibrosis and disease outcomes, although prospective data are sparse to estimate the extent these individual variables affect progression.12 Recent guidelines remain silent about whether and how these data may be incorporated in screening for NAFLD in the population.
Assessment of liver fibrosis
The traditional means to detect liver fibrosis is liver histology, which also assesses steatosis, individual components of NASH and, often importantly, other concomitant liver pathology. In reality, however, liver biopsies have several limitations including the risk of complications, patient discomfort, economic costs, and sampling variability. Increasingly, “noninvasive” methods have been used to estimate liver fibrosis in patients with NAFLD. Liver elastography estimates the physical stiffness of the organ, which may be measured by MRI or ultrasound. Among ultrasound-based technologies, vibration-controlled transient elastography (VCTE) is more widely accepted and affordable although it may not be as accurate as MR elastography.13
In general, these elastographic tests are not readily accessible to most physicians outside hepatology specialty practices. Instead, blood test-based markers have been developed and widely recommended as the initial modality to assess liver fibrosis. Figure 1 represents a partial list of blood test-based markers. Traditionally, FIB-4 and NFS have been considered the most widely recommended by society guidelines. The AGA Pathway for evaluation of patients with NAFLD recommends first to apply the FIB-4 score and, in patients considered to be at intermediate risk of fibrosis for advanced fibrosis (stage 3 or 4, FIB-4 = 1.3-2.67), to assess liver stiffness by VCTE.14
More recently, the accumulating natural history data have highlighted the inflection in the risk of future outcomes coinciding with F2 and therapeutic trials that target patients with “at risk NASH,” thus more attention has been paid to the identification of patients with stage 2 (or higher). The steatosis-associated fibrosis estimator (SAFE) was developed for this specific purpose. The score has been validated in multiple data sets, in all of which SAFE outperformed FIB-4 and NFS (Figure 1). When the score was applied to assess overall survival in participants of the NHANES, patients with NAFLD deemed to be high risk (SAFE > 100) had significantly lower survival (37% Kaplan-Meier survival at 20 years), compared to those with intermediate (SAFE 0-100, 61% survival) and low (SAFE < 0, 86% survival). In comparison, the 20-year survival of subjects without NAFLD survival was 79%.15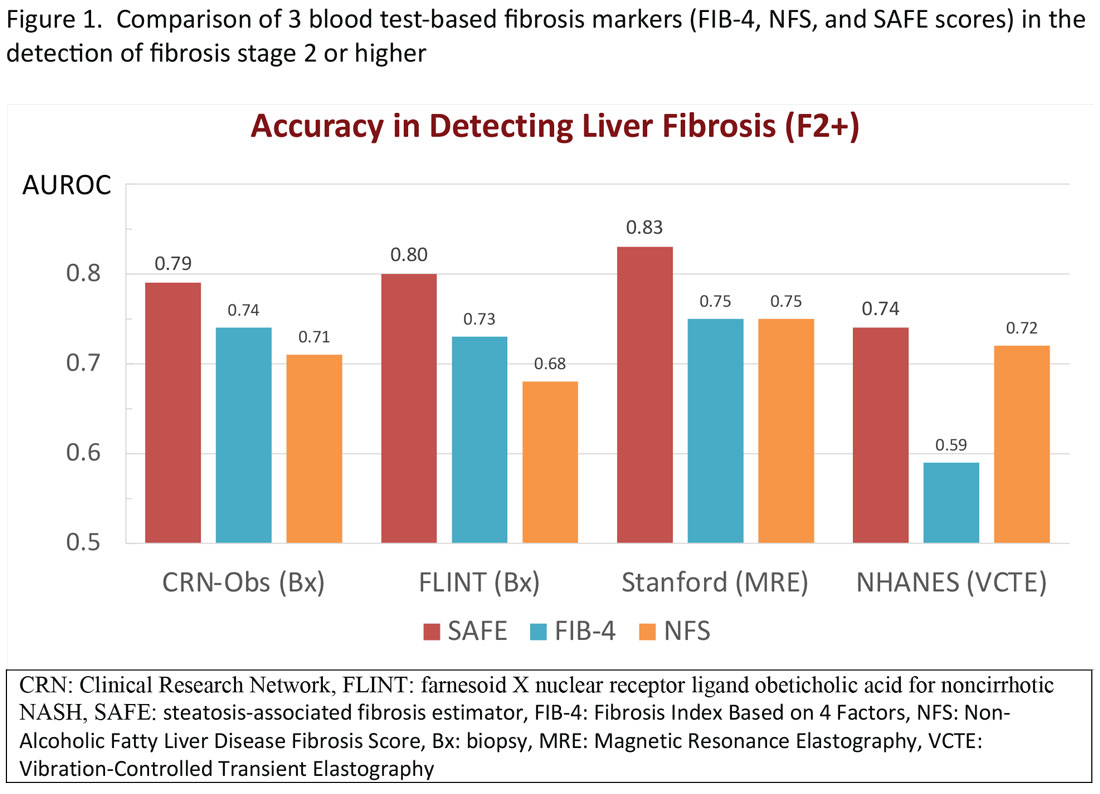
Regardless of the modality for initial stratification, it is widely accepted that mechanical elastography constitutes the next step in prognosticating the patient. In the AGA Pathway, liver stiffness of < 8 kPa is considered low risk, which corresponds in most analysis with lack of stage 2 fibrosis, whereas stiffness of > 12 kPa may be indicative of stage 3 or 4. These recommendations are consistent with those from the latest Baveno Consensus Conference (“Baveno 7”). Figure 2 expands on the so-called “rule of 5” from the consensus document and correlates liver stiffness (by VCTE) with progression of liver fibrosis as well as clinical presentation. For example, liver stiffness < 15 kPa is associated with a low risk of clinically significant portal hypertension (CSPH). Similarly, in patients with a normal platelet count (>150,000/mm3) and liver stiffness < 20 kPa, the probability of gastroesophageal varices is sufficiently low that a screening endoscopy may be avoided. On the other hand, liver stiffness > 25 kPa is associated with increasing risk of decompensated cirrhosis and portal hypertension.16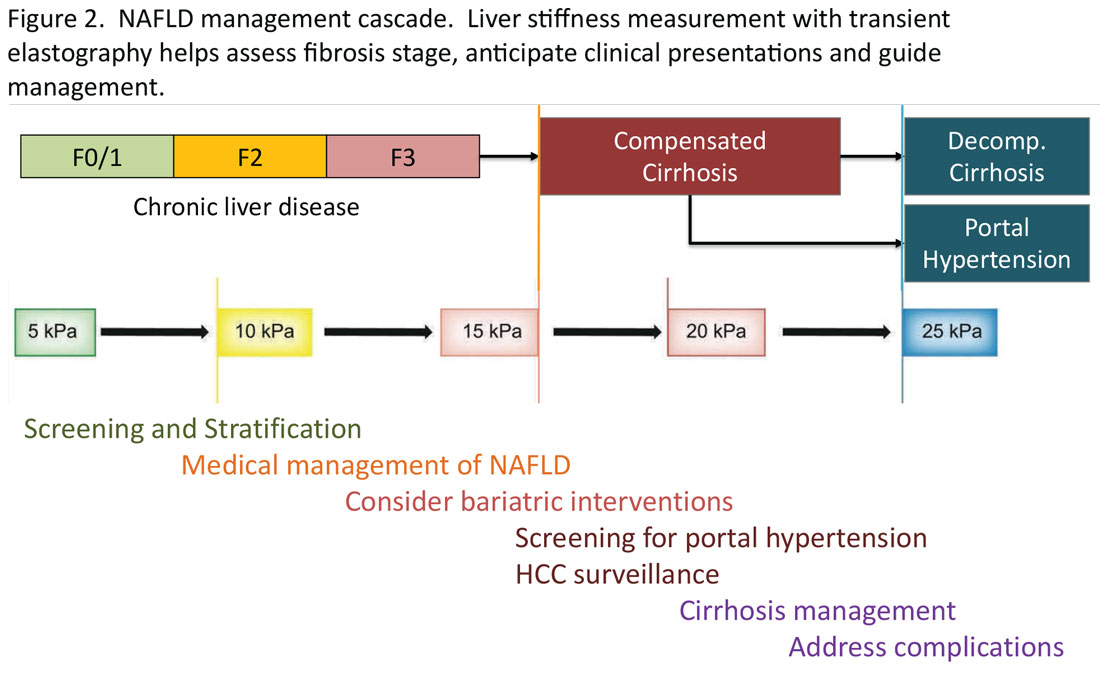
Partnership between primary care and specialty
The insights expressed in Figure 2 can be utilized to guide management decisions. In patients without evidence of liver fibrosis, emphasis may primarily be on screening, stratification and management of metabolic syndrome. For patients with evidence of incipient liver fibrosis, medical management of NAFLD needs to be implemented including lifestyle changes and pharmacological interventions as appropriate. For patients unresponsive to medical therapy, an endoscopic or surgical bariatric procedure should be considered. Management of patients with evidence of cirrhosis includes screening for portal hypertension, surveillance for HCC, medical management of cirrhosis, and finally, in suitable cases, referral for liver transplant evaluation. The reader is referred to the latest treatment guidelines for detailed discussion of these individual management modalities [ref, AGA and AASLD guidelines].14,17
Given the spectrum of management modalities needed to successfully manage patients with NAFLD, it is unrealistic to expect that hepatologists and gastroenterologists are able to manage the large number of patients with NAFLD. In general, clinical activities on the left side of the figure are in the domain of primary care providers, whereas management of patients with progressive liver fibrosis is conducted by the specialist. An important aspect of the overall management of these patients is risk management in terms of the metabolic syndrome, including cardiovascular risk reduction and diabetes management, as appropriate. Many patients with NAFLD are burdened with several comorbidities and likely to benefit from a multidisciplinary team consisting of primary care, endocrinology, preventive cardiology, pharmacy, nutrition/dietetics, social services, and addiction specialists, as well as hepatology and gastroenterology. Prospective, high-quality data to define these teams and their function are yet to be generated.
Conclusion
NAFLD is an important and increasing public health concern in the U.S. Once diagnosed, assessing liver fibrosis and evaluating the presence of the components of metabolic syndrome in these patients, constitute the key components in the care in terms of risk stratification, medical management, and referral decisions. Noninvasive tests have been increasingly utilized including liver stiffness measurements and various blood test-based indicators. For patients in specialty GI/hepatology care, transient elastography is a widely accepted tool, with which standardized recommendations may be made for screening, stratification, and medical and surgical interventions in patients with NAFLD.
Mai Sedki, MD, MPH, is a doctoral candidate at the University of California, San Francisco. W. Ray Kim, MD, is professor of medicine (gastroenterology and hepatology) at Stanford (Calif.) University. Address correspondence to: wrkim@stanford.edu. The authors disclosed no conflicts of interest. Twitter: @SedkiMD and @WRayKimMD.
References
1. Younossi ZM et al. Epidemiology of chronic liver diseases in the USA in the past three decades. Gut. 2020 Mar;69(3):564-8.
2. Lazo M et al. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988-1994. Am J Epidemiol. 2013 Jul 1;178(1):38-45.
3. Kim D et al. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology. 2013 Apr;57:1357-65.
4. Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002 Apr 18;346:1221-31.
5. Kim D et al. Changing trends in etiology-based annual mortality from chronic liver disease, from 2007 through 2016. Gastroenterology. 2018;155(4):1154-63.e3.
6. FastStats. Chronic Liver Disease and Cirrhosis. Centers for Disease Control and Prevention.
7. Rich NE et al. Racial and ethnic disparities in nonalcoholic fatty liver disease prevalence, severity, and outcomes in the United States: A systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2018;16(2):198-210. e2.
8. Coleman-Jensen A et al. Household food security in the United States in 2020 (ERR-298). Washington, DC: U.S. Department of Agriculture; Sep 2021.
9. Sanyal AJ et al. Prospective study of outcomes in adults with nonalcoholic fatty liver disease. N Engl J Med. 2021 Oct 21;385(17):1559-69.
10. Ng CH et al. Mortality outcomes by fibrosis stage in nonalcoholic fatty liver disease: A systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2023 Apr;21(4):931-9.e5.
11. Hagström H et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol. 2017;67(6):1265-73.
12. Rinella ME et al. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023 May 1;77(5):1797-835.
13. Singh S et al. Diagnostic performance of magnetic resonance elastography in staging liver fibrosis: A systematic review and meta-analysis of individual participant data. Clin Gastroenterol Hepatol. 2015 Mar;13(3):440-51.e6.
14. Kanwal F et al. Clinical Care Pathway for the risk stratification and management of patients with nonalcoholic fatty liver disease. Gastroenterology. 2021 Nov;161(5):1657-69.
15. Sripongpun P et al. The steatosis-associated fibrosis estimator (SAFE) score: A tool to detect low-risk NAFLD in primary care. .
16. de Franchis R et al. Baveno VII: Renewing consensus in portal hypertension. J Hepatol. 2022 Apr;76(4):959-74.
17. Rinella ME et al. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023 May 1;77(5):1797-835.
Burden of NAFLD in the U.S.
NAFLD is a manifestation of systemic metabolic abnormalities, including insulin resistance, dyslipidemia, central obesity, and hypertension. In this short review, we summarize data on the burden of NAFLD in the U.S. and its prognostic determinants and review what clinical and public health approaches may be needed to mitigating its impact.
Epidemiology of NAFLD
Worldwide, the prevalence of NAFLD is estimated at 6% to 35%, with biopsy-based studies reporting NASH in 3% to 5%.1 U.S. estimates for the prevalence of NAFLD range from 10% to 46%.2 In our own analysis of the National Health and Nutrition Examination Survey (NHANES) data, transient elastography-detected steatosis was found in 36%, which projected to a minimum of 73 million American adults.3
NAFLD represents a spectrum of disorders ranging from simple steatosis to nonalcoholic steatohepatitis (NASH), the latter leading, in some cases, to progressive hepatic fibrosis and cirrhosis.4 Out of a large number of subjects with NAFLD, the proportions of NASH patients that develop severe liver problems such as end-stage liver disease (ESLD) or hepatocellular carcinoma (HCC) are progressively smaller. For example, we recently reported that less than 2,000 liver-related deaths are attributable to NAFLD in the U.S. per annum, which corresponds to a crude case fatality rate of < 0.005% per year.5
According to the Centers for Disease Control and Prevention (CDC), there have been substantial increases in liver-related deaths over the last 2 decades. Mortality from liver disease including hepatobiliary cancers more than doubled from 41,966 deaths (including 15,321 women and 26,645 men) in 2000 to 85,884 deaths (33,000 women and 52,884 men) in 2020. The proportion of deaths specifically attributed to NAFLD among liver-related deaths was miniscule in 2000, accounting for 1.1% in women and 0.7% in men. By 2020, the proportions increased several folds in both sexes (7.4% in women and 2.7% in men).6 Moreover, it is likely that a substantial portion of deaths from chronic liver disease from unknown causes (“cryptogenic”) are likely end-stage NAFLD, making these figures underestimates of the true impact of NAFLD in the U.S.
From a comparative epidemiologic perspective, there are significant racial and ethnic and socioeconomic disparities in NAFLD prevalence, wherein Hispanic persons and individuals experiencing food insecurity – independent of poverty status, education level, race and ethnicity – are disproportionately more affected by NAFLD.7,8 Furthermore, these disparities persist when examining long-term complications of NAFLD, such as developing HCC.
Prognosis in NAFLD: NASH versus fibrosis
Given the enormous prevalence and increasing public health burden of NAFLD, systematic interventions to mitigate its impact are urgently needed. Clearly, patients who already have developed advanced liver disease need to be directed to specialty care so the disease progression may be halted and complications of ESLD may be prevented or managed. On the other hand, in order to mitigate the future impact of ESLD, prompt identification of at-risk patients and proactive interventions to improve liver health are needed.
In the assessment of disease progression, prior data have shown that the presence of NASH and increasing stages of liver fibrosis are important predictors of disease progression. Fibrosis is a component of NASH, while NASH is thought to be a prerequisite for fibrosis. In a prospective, multicenter follow-up study of NAFLD evaluated by liver biopsies (n = 1,773), over a median follow-up of 4 years, 37 (2%) developed hepatic decompensation, while 47 (3%) died from any cause, which included ESLD (n = 12), cardiovascular complications (n = 4), and malignancies (n = 12), including HCC (n = 9).9 It is not entirely surprising that advanced fibrosis and cirrhosis was highly associated with the development of hepatic decompensation. In their multivariable analysis, patients with F3-4 had a 13.8-fold (95% confidence interval [CI]: 4.6, 41.0) increase in the hazard of reaching a MELD score of 15 compared to those with F0-2. In addition, all-cause mortality was 17.2-fold (95% CI: 5.2, 56.6) higher with F3-4 compared to F0-2.
These data have been borne out by a larger body of literature on the topic. In a recent meta-analysis assessing the relation between liver fibrosis and future mortality, which included 17,301 subjects with NAFLD, patients with at least stage 2 fibrosis experience a significantly increased risk of liver-related and overall mortality, a trend that accelerates at higher fibrosis stages.10 These point to liver fibrosis as the singular determinant of long-term prognosis, in comparison, for example, with the diagnosis of NASH. Hagström conducted a retrospective cohort study of patients with biopsy-proven NAFLD in Sweden. When fibrosis stage and histological diagnosis of NASH were considered together, NASH did not have an impact on overall mortality (hazard ratio [HR] = 0.83, P = .29) or liver morbidity (HR = 0.62, P = .25).11
On an individual level, factors that affect fibrosis progression are not as well studied. It is commonly believed that demographic factors (e.g., age, sex and race), genetic polymorphisms (e.g., PNPLA3, TM6SF2), clinical comorbidities (e.g., obesity, DM, and sleep apnea), and environmental factors (e.g., smoking) may accelerate fibrosis and disease outcomes, although prospective data are sparse to estimate the extent these individual variables affect progression.12 Recent guidelines remain silent about whether and how these data may be incorporated in screening for NAFLD in the population.
Assessment of liver fibrosis
The traditional means to detect liver fibrosis is liver histology, which also assesses steatosis, individual components of NASH and, often importantly, other concomitant liver pathology. In reality, however, liver biopsies have several limitations including the risk of complications, patient discomfort, economic costs, and sampling variability. Increasingly, “noninvasive” methods have been used to estimate liver fibrosis in patients with NAFLD. Liver elastography estimates the physical stiffness of the organ, which may be measured by MRI or ultrasound. Among ultrasound-based technologies, vibration-controlled transient elastography (VCTE) is more widely accepted and affordable although it may not be as accurate as MR elastography.13
In general, these elastographic tests are not readily accessible to most physicians outside hepatology specialty practices. Instead, blood test-based markers have been developed and widely recommended as the initial modality to assess liver fibrosis. Figure 1 represents a partial list of blood test-based markers. Traditionally, FIB-4 and NFS have been considered the most widely recommended by society guidelines. The AGA Pathway for evaluation of patients with NAFLD recommends first to apply the FIB-4 score and, in patients considered to be at intermediate risk of fibrosis for advanced fibrosis (stage 3 or 4, FIB-4 = 1.3-2.67), to assess liver stiffness by VCTE.14
More recently, the accumulating natural history data have highlighted the inflection in the risk of future outcomes coinciding with F2 and therapeutic trials that target patients with “at risk NASH,” thus more attention has been paid to the identification of patients with stage 2 (or higher). The steatosis-associated fibrosis estimator (SAFE) was developed for this specific purpose. The score has been validated in multiple data sets, in all of which SAFE outperformed FIB-4 and NFS (Figure 1). When the score was applied to assess overall survival in participants of the NHANES, patients with NAFLD deemed to be high risk (SAFE > 100) had significantly lower survival (37% Kaplan-Meier survival at 20 years), compared to those with intermediate (SAFE 0-100, 61% survival) and low (SAFE < 0, 86% survival). In comparison, the 20-year survival of subjects without NAFLD survival was 79%.15
Regardless of the modality for initial stratification, it is widely accepted that mechanical elastography constitutes the next step in prognosticating the patient. In the AGA Pathway, liver stiffness of < 8 kPa is considered low risk, which corresponds in most analysis with lack of stage 2 fibrosis, whereas stiffness of > 12 kPa may be indicative of stage 3 or 4. These recommendations are consistent with those from the latest Baveno Consensus Conference (“Baveno 7”). Figure 2 expands on the so-called “rule of 5” from the consensus document and correlates liver stiffness (by VCTE) with progression of liver fibrosis as well as clinical presentation. For example, liver stiffness < 15 kPa is associated with a low risk of clinically significant portal hypertension (CSPH). Similarly, in patients with a normal platelet count (>150,000/mm3) and liver stiffness < 20 kPa, the probability of gastroesophageal varices is sufficiently low that a screening endoscopy may be avoided. On the other hand, liver stiffness > 25 kPa is associated with increasing risk of decompensated cirrhosis and portal hypertension.16
Partnership between primary care and specialty
The insights expressed in Figure 2 can be utilized to guide management decisions. In patients without evidence of liver fibrosis, emphasis may primarily be on screening, stratification and management of metabolic syndrome. For patients with evidence of incipient liver fibrosis, medical management of NAFLD needs to be implemented including lifestyle changes and pharmacological interventions as appropriate. For patients unresponsive to medical therapy, an endoscopic or surgical bariatric procedure should be considered. Management of patients with evidence of cirrhosis includes screening for portal hypertension, surveillance for HCC, medical management of cirrhosis, and finally, in suitable cases, referral for liver transplant evaluation. The reader is referred to the latest treatment guidelines for detailed discussion of these individual management modalities [ref, AGA and AASLD guidelines].14,17
Given the spectrum of management modalities needed to successfully manage patients with NAFLD, it is unrealistic to expect that hepatologists and gastroenterologists are able to manage the large number of patients with NAFLD. In general, clinical activities on the left side of the figure are in the domain of primary care providers, whereas management of patients with progressive liver fibrosis is conducted by the specialist. An important aspect of the overall management of these patients is risk management in terms of the metabolic syndrome, including cardiovascular risk reduction and diabetes management, as appropriate. Many patients with NAFLD are burdened with several comorbidities and likely to benefit from a multidisciplinary team consisting of primary care, endocrinology, preventive cardiology, pharmacy, nutrition/dietetics, social services, and addiction specialists, as well as hepatology and gastroenterology. Prospective, high-quality data to define these teams and their function are yet to be generated.
Conclusion
NAFLD is an important and increasing public health concern in the U.S. Once diagnosed, assessing liver fibrosis and evaluating the presence of the components of metabolic syndrome in these patients, constitute the key components in the care in terms of risk stratification, medical management, and referral decisions. Noninvasive tests have been increasingly utilized including liver stiffness measurements and various blood test-based indicators. For patients in specialty GI/hepatology care, transient elastography is a widely accepted tool, with which standardized recommendations may be made for screening, stratification, and medical and surgical interventions in patients with NAFLD.
Mai Sedki, MD, MPH, is a doctoral candidate at the University of California, San Francisco. W. Ray Kim, MD, is professor of medicine (gastroenterology and hepatology) at Stanford (Calif.) University. Address correspondence to: wrkim@stanford.edu. The authors disclosed no conflicts of interest. Twitter: @SedkiMD and @WRayKimMD.
References
1. Younossi ZM et al. Epidemiology of chronic liver diseases in the USA in the past three decades. Gut. 2020 Mar;69(3):564-8.
2. Lazo M et al. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988-1994. Am J Epidemiol. 2013 Jul 1;178(1):38-45.
3. Kim D et al. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology. 2013 Apr;57:1357-65.
4. Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002 Apr 18;346:1221-31.
5. Kim D et al. Changing trends in etiology-based annual mortality from chronic liver disease, from 2007 through 2016. Gastroenterology. 2018;155(4):1154-63.e3.
6. FastStats. Chronic Liver Disease and Cirrhosis. Centers for Disease Control and Prevention.
7. Rich NE et al. Racial and ethnic disparities in nonalcoholic fatty liver disease prevalence, severity, and outcomes in the United States: A systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2018;16(2):198-210. e2.
8. Coleman-Jensen A et al. Household food security in the United States in 2020 (ERR-298). Washington, DC: U.S. Department of Agriculture; Sep 2021.
9. Sanyal AJ et al. Prospective study of outcomes in adults with nonalcoholic fatty liver disease. N Engl J Med. 2021 Oct 21;385(17):1559-69.
10. Ng CH et al. Mortality outcomes by fibrosis stage in nonalcoholic fatty liver disease: A systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2023 Apr;21(4):931-9.e5.
11. Hagström H et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol. 2017;67(6):1265-73.
12. Rinella ME et al. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023 May 1;77(5):1797-835.
13. Singh S et al. Diagnostic performance of magnetic resonance elastography in staging liver fibrosis: A systematic review and meta-analysis of individual participant data. Clin Gastroenterol Hepatol. 2015 Mar;13(3):440-51.e6.
14. Kanwal F et al. Clinical Care Pathway for the risk stratification and management of patients with nonalcoholic fatty liver disease. Gastroenterology. 2021 Nov;161(5):1657-69.
15. Sripongpun P et al. The steatosis-associated fibrosis estimator (SAFE) score: A tool to detect low-risk NAFLD in primary care. .
16. de Franchis R et al. Baveno VII: Renewing consensus in portal hypertension. J Hepatol. 2022 Apr;76(4):959-74.
17. Rinella ME et al. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023 May 1;77(5):1797-835.
Burden of NAFLD in the U.S.
NAFLD is a manifestation of systemic metabolic abnormalities, including insulin resistance, dyslipidemia, central obesity, and hypertension. In this short review, we summarize data on the burden of NAFLD in the U.S. and its prognostic determinants and review what clinical and public health approaches may be needed to mitigating its impact.
Epidemiology of NAFLD
Worldwide, the prevalence of NAFLD is estimated at 6% to 35%, with biopsy-based studies reporting NASH in 3% to 5%.1 U.S. estimates for the prevalence of NAFLD range from 10% to 46%.2 In our own analysis of the National Health and Nutrition Examination Survey (NHANES) data, transient elastography-detected steatosis was found in 36%, which projected to a minimum of 73 million American adults.3
NAFLD represents a spectrum of disorders ranging from simple steatosis to nonalcoholic steatohepatitis (NASH), the latter leading, in some cases, to progressive hepatic fibrosis and cirrhosis.4 Out of a large number of subjects with NAFLD, the proportions of NASH patients that develop severe liver problems such as end-stage liver disease (ESLD) or hepatocellular carcinoma (HCC) are progressively smaller. For example, we recently reported that less than 2,000 liver-related deaths are attributable to NAFLD in the U.S. per annum, which corresponds to a crude case fatality rate of < 0.005% per year.5
According to the Centers for Disease Control and Prevention (CDC), there have been substantial increases in liver-related deaths over the last 2 decades. Mortality from liver disease including hepatobiliary cancers more than doubled from 41,966 deaths (including 15,321 women and 26,645 men) in 2000 to 85,884 deaths (33,000 women and 52,884 men) in 2020. The proportion of deaths specifically attributed to NAFLD among liver-related deaths was miniscule in 2000, accounting for 1.1% in women and 0.7% in men. By 2020, the proportions increased several folds in both sexes (7.4% in women and 2.7% in men).6 Moreover, it is likely that a substantial portion of deaths from chronic liver disease from unknown causes (“cryptogenic”) are likely end-stage NAFLD, making these figures underestimates of the true impact of NAFLD in the U.S.
From a comparative epidemiologic perspective, there are significant racial and ethnic and socioeconomic disparities in NAFLD prevalence, wherein Hispanic persons and individuals experiencing food insecurity – independent of poverty status, education level, race and ethnicity – are disproportionately more affected by NAFLD.7,8 Furthermore, these disparities persist when examining long-term complications of NAFLD, such as developing HCC.
Prognosis in NAFLD: NASH versus fibrosis
Given the enormous prevalence and increasing public health burden of NAFLD, systematic interventions to mitigate its impact are urgently needed. Clearly, patients who already have developed advanced liver disease need to be directed to specialty care so the disease progression may be halted and complications of ESLD may be prevented or managed. On the other hand, in order to mitigate the future impact of ESLD, prompt identification of at-risk patients and proactive interventions to improve liver health are needed.
In the assessment of disease progression, prior data have shown that the presence of NASH and increasing stages of liver fibrosis are important predictors of disease progression. Fibrosis is a component of NASH, while NASH is thought to be a prerequisite for fibrosis. In a prospective, multicenter follow-up study of NAFLD evaluated by liver biopsies (n = 1,773), over a median follow-up of 4 years, 37 (2%) developed hepatic decompensation, while 47 (3%) died from any cause, which included ESLD (n = 12), cardiovascular complications (n = 4), and malignancies (n = 12), including HCC (n = 9).9 It is not entirely surprising that advanced fibrosis and cirrhosis was highly associated with the development of hepatic decompensation. In their multivariable analysis, patients with F3-4 had a 13.8-fold (95% confidence interval [CI]: 4.6, 41.0) increase in the hazard of reaching a MELD score of 15 compared to those with F0-2. In addition, all-cause mortality was 17.2-fold (95% CI: 5.2, 56.6) higher with F3-4 compared to F0-2.
These data have been borne out by a larger body of literature on the topic. In a recent meta-analysis assessing the relation between liver fibrosis and future mortality, which included 17,301 subjects with NAFLD, patients with at least stage 2 fibrosis experience a significantly increased risk of liver-related and overall mortality, a trend that accelerates at higher fibrosis stages.10 These point to liver fibrosis as the singular determinant of long-term prognosis, in comparison, for example, with the diagnosis of NASH. Hagström conducted a retrospective cohort study of patients with biopsy-proven NAFLD in Sweden. When fibrosis stage and histological diagnosis of NASH were considered together, NASH did not have an impact on overall mortality (hazard ratio [HR] = 0.83, P = .29) or liver morbidity (HR = 0.62, P = .25).11
On an individual level, factors that affect fibrosis progression are not as well studied. It is commonly believed that demographic factors (e.g., age, sex and race), genetic polymorphisms (e.g., PNPLA3, TM6SF2), clinical comorbidities (e.g., obesity, DM, and sleep apnea), and environmental factors (e.g., smoking) may accelerate fibrosis and disease outcomes, although prospective data are sparse to estimate the extent these individual variables affect progression.12 Recent guidelines remain silent about whether and how these data may be incorporated in screening for NAFLD in the population.
Assessment of liver fibrosis
The traditional means to detect liver fibrosis is liver histology, which also assesses steatosis, individual components of NASH and, often importantly, other concomitant liver pathology. In reality, however, liver biopsies have several limitations including the risk of complications, patient discomfort, economic costs, and sampling variability. Increasingly, “noninvasive” methods have been used to estimate liver fibrosis in patients with NAFLD. Liver elastography estimates the physical stiffness of the organ, which may be measured by MRI or ultrasound. Among ultrasound-based technologies, vibration-controlled transient elastography (VCTE) is more widely accepted and affordable although it may not be as accurate as MR elastography.13
In general, these elastographic tests are not readily accessible to most physicians outside hepatology specialty practices. Instead, blood test-based markers have been developed and widely recommended as the initial modality to assess liver fibrosis. Figure 1 represents a partial list of blood test-based markers. Traditionally, FIB-4 and NFS have been considered the most widely recommended by society guidelines. The AGA Pathway for evaluation of patients with NAFLD recommends first to apply the FIB-4 score and, in patients considered to be at intermediate risk of fibrosis for advanced fibrosis (stage 3 or 4, FIB-4 = 1.3-2.67), to assess liver stiffness by VCTE.14
More recently, the accumulating natural history data have highlighted the inflection in the risk of future outcomes coinciding with F2 and therapeutic trials that target patients with “at risk NASH,” thus more attention has been paid to the identification of patients with stage 2 (or higher). The steatosis-associated fibrosis estimator (SAFE) was developed for this specific purpose. The score has been validated in multiple data sets, in all of which SAFE outperformed FIB-4 and NFS (Figure 1). When the score was applied to assess overall survival in participants of the NHANES, patients with NAFLD deemed to be high risk (SAFE > 100) had significantly lower survival (37% Kaplan-Meier survival at 20 years), compared to those with intermediate (SAFE 0-100, 61% survival) and low (SAFE < 0, 86% survival). In comparison, the 20-year survival of subjects without NAFLD survival was 79%.15
Regardless of the modality for initial stratification, it is widely accepted that mechanical elastography constitutes the next step in prognosticating the patient. In the AGA Pathway, liver stiffness of < 8 kPa is considered low risk, which corresponds in most analysis with lack of stage 2 fibrosis, whereas stiffness of > 12 kPa may be indicative of stage 3 or 4. These recommendations are consistent with those from the latest Baveno Consensus Conference (“Baveno 7”). Figure 2 expands on the so-called “rule of 5” from the consensus document and correlates liver stiffness (by VCTE) with progression of liver fibrosis as well as clinical presentation. For example, liver stiffness < 15 kPa is associated with a low risk of clinically significant portal hypertension (CSPH). Similarly, in patients with a normal platelet count (>150,000/mm3) and liver stiffness < 20 kPa, the probability of gastroesophageal varices is sufficiently low that a screening endoscopy may be avoided. On the other hand, liver stiffness > 25 kPa is associated with increasing risk of decompensated cirrhosis and portal hypertension.16
Partnership between primary care and specialty
The insights expressed in Figure 2 can be utilized to guide management decisions. In patients without evidence of liver fibrosis, emphasis may primarily be on screening, stratification and management of metabolic syndrome. For patients with evidence of incipient liver fibrosis, medical management of NAFLD needs to be implemented including lifestyle changes and pharmacological interventions as appropriate. For patients unresponsive to medical therapy, an endoscopic or surgical bariatric procedure should be considered. Management of patients with evidence of cirrhosis includes screening for portal hypertension, surveillance for HCC, medical management of cirrhosis, and finally, in suitable cases, referral for liver transplant evaluation. The reader is referred to the latest treatment guidelines for detailed discussion of these individual management modalities [ref, AGA and AASLD guidelines].14,17
Given the spectrum of management modalities needed to successfully manage patients with NAFLD, it is unrealistic to expect that hepatologists and gastroenterologists are able to manage the large number of patients with NAFLD. In general, clinical activities on the left side of the figure are in the domain of primary care providers, whereas management of patients with progressive liver fibrosis is conducted by the specialist. An important aspect of the overall management of these patients is risk management in terms of the metabolic syndrome, including cardiovascular risk reduction and diabetes management, as appropriate. Many patients with NAFLD are burdened with several comorbidities and likely to benefit from a multidisciplinary team consisting of primary care, endocrinology, preventive cardiology, pharmacy, nutrition/dietetics, social services, and addiction specialists, as well as hepatology and gastroenterology. Prospective, high-quality data to define these teams and their function are yet to be generated.
Conclusion
NAFLD is an important and increasing public health concern in the U.S. Once diagnosed, assessing liver fibrosis and evaluating the presence of the components of metabolic syndrome in these patients, constitute the key components in the care in terms of risk stratification, medical management, and referral decisions. Noninvasive tests have been increasingly utilized including liver stiffness measurements and various blood test-based indicators. For patients in specialty GI/hepatology care, transient elastography is a widely accepted tool, with which standardized recommendations may be made for screening, stratification, and medical and surgical interventions in patients with NAFLD.
Mai Sedki, MD, MPH, is a doctoral candidate at the University of California, San Francisco. W. Ray Kim, MD, is professor of medicine (gastroenterology and hepatology) at Stanford (Calif.) University. Address correspondence to: wrkim@stanford.edu. The authors disclosed no conflicts of interest. Twitter: @SedkiMD and @WRayKimMD.
References
1. Younossi ZM et al. Epidemiology of chronic liver diseases in the USA in the past three decades. Gut. 2020 Mar;69(3):564-8.
2. Lazo M et al. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988-1994. Am J Epidemiol. 2013 Jul 1;178(1):38-45.
3. Kim D et al. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology. 2013 Apr;57:1357-65.
4. Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002 Apr 18;346:1221-31.
5. Kim D et al. Changing trends in etiology-based annual mortality from chronic liver disease, from 2007 through 2016. Gastroenterology. 2018;155(4):1154-63.e3.
6. FastStats. Chronic Liver Disease and Cirrhosis. Centers for Disease Control and Prevention.
7. Rich NE et al. Racial and ethnic disparities in nonalcoholic fatty liver disease prevalence, severity, and outcomes in the United States: A systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2018;16(2):198-210. e2.
8. Coleman-Jensen A et al. Household food security in the United States in 2020 (ERR-298). Washington, DC: U.S. Department of Agriculture; Sep 2021.
9. Sanyal AJ et al. Prospective study of outcomes in adults with nonalcoholic fatty liver disease. N Engl J Med. 2021 Oct 21;385(17):1559-69.
10. Ng CH et al. Mortality outcomes by fibrosis stage in nonalcoholic fatty liver disease: A systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2023 Apr;21(4):931-9.e5.
11. Hagström H et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol. 2017;67(6):1265-73.
12. Rinella ME et al. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023 May 1;77(5):1797-835.
13. Singh S et al. Diagnostic performance of magnetic resonance elastography in staging liver fibrosis: A systematic review and meta-analysis of individual participant data. Clin Gastroenterol Hepatol. 2015 Mar;13(3):440-51.e6.
14. Kanwal F et al. Clinical Care Pathway for the risk stratification and management of patients with nonalcoholic fatty liver disease. Gastroenterology. 2021 Nov;161(5):1657-69.
15. Sripongpun P et al. The steatosis-associated fibrosis estimator (SAFE) score: A tool to detect low-risk NAFLD in primary care. .
16. de Franchis R et al. Baveno VII: Renewing consensus in portal hypertension. J Hepatol. 2022 Apr;76(4):959-74.
17. Rinella ME et al. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023 May 1;77(5):1797-835.