User login
Therapeutic management of NAFLD
Nonalcoholic fatty liver disease (NAFLD) is defined by the presence of hepatic steatosis detected on either imaging or histology in the absence of secondary causes of fatty liver (e.g., excessive alcohol consumption) or other chronic liver diseases.1 For practical NAFLD diagnosis purposes, excessive alcohol intake can be defined as an active or recent history of more than 21 standard drinks per week in men and more than14 standard drinks per week in women. For the sake of terminology, NAFLD is characterized by fatty liver infiltration, affecting at least 5% of hepatocytes, with no evidence of hepatocyte injury, whereas nonalcoholic steatohepatitis (NASH) is defined as the presence of necroinflammation with or without fibrosis in a background of fatty liver.1
Natural history
NASH and the degree of fibrosis are the two most important determinants of the natural history of NAFLD. NASH can evolve into fibrosis and cirrhosis, whereas advanced fibrosis and cirrhosis (stages 3 or 4 of fibrosis) significantly increase the risk of liver-related decompensation and mortality. NAFLD, per se, has been associated with an increased risk of overall mortality, compared with that of the general population.2 The three most common causes of mortality for patients with NAFLD are cardiovascular diseases (CVD), extrahepatic malignancies, and liver-related deaths. Mortality and liver-related events, including hepatic decompensation and hepatocellular carcinoma (HCC), may significantly increase in a dose-dependent manner with increasing fibrosis stages, and stages 3 or 4 of fibrosis may display the highest rates of all-cause mortality and liver-related events.3,4 It is important to note, however, that almost 15% of HCCs occur in patients with NAFLD who do not have cirrhosis.5 The presence of commonly associated comorbidities such as obesity, insulin resistance or diabetes, dyslipidemia, hypothyroidism, polycystic ovary syndrome, and sleep apnea may contribute to an increased risk of NASH and advanced fibrosis and, therefore, an accelerated clinical course of NAFLD.
Nonpharmacological interventions
Lifestyle modification
Lifestyle modification to achieve weight loss remains a first-line intervention in patients with NAFLD. Weight loss achieved either by hypocaloric diet alone or in conjunction with increased physical activity can be beneficial for all patients with NAFLD. The benefits extend not only to those who are overweight and obese but also to those within normal body weight (lean NAFLD).1,6,7 Weight loss of approximately 3%-5% is necessary to improve hepatic steatosis, but a greater weight loss (7%-10%) is required to improve other histopathological features like necroinflammatory lesions and fibrosis.8-10 Individuals with higher BMI and/or type 2 diabetes (T2D) will require a larger weight reduction to achieve a similar benefit on NAFLD-related features.7,8 Weight loss via lifestyle changes can also decrease hepatic venous pressure gradient (HVPG), with greater declines reported among those with more than 10% weight loss.11
Weight loss can be achieved through a variety of modalities, but long-term maintenance of lost weight is much more challenging. A combination of a hypocaloric diet with a caloric deficit of 500-1,000 kcal/d, alongside moderate-intensity exercise and intensive on-site behavioral treatment, will likely increase the possibility of a sustained weight loss over time.1,12 A growing body of scientific evidence indicates that a healthy diet that includes a reduction of high-glycemic-index foods and refined carbohydrates; increased consumption of monounsaturated fatty acids, omega-3 fatty acids, and fibers; and high intakes of olive oil, nuts, vegetables, fruits, legumes, whole grains, and fish can have beneficial effects on NAFLD and its severity.13-16 Adherence to these healthy dietary patterns has been associated with a marked reduction in CVD morbidity and mortality and is, thus, a strategic lifestyle recommendation for patients with NAFLD in whom the leading cause of morbidity and death is CVD.1,3
Exercise alone in adults with NAFLD may reduce hepatic steatosis, but its ability to improve inflammation and fibrosis has not been proven in well-designed RCTs.17,18 Physical activity and exercise have been shown to curb both the development and the progression of NAFLD, and beneficial effects could be achieved independent of weight loss.17,19,20 Most importantly, moderate-to-vigorous physical activity is likely associated with lower all-cause and cardiovascular mortality in patients with NAFLD.21
Heavy alcohol intake should be avoided by patients with NAFLD or NASH, and those with cirrhotic NASH should avoid any alcohol consumption given the risk of HCC and hepatic decompensation.1,4,22 Limiting light-to-moderate alcohol intake among patients without cirrhosis is still under debate.1 People with NAFLD may be advised to drink an equivalent of two to three 8-oz cups of regular brewed coffee daily as it has shown certain antifibrotic effects in NAFLD patients.23
Bariatric surgery
Bariatric surgery is an attractive therapeutic option for eligible obese patients with NAFLD. Bariatric surgery has the potential for inducing great weight loss and, therefore, reverses not only the steatosis, inflammation, and fibrosis among NAFLD individuals but also important comorbid conditions like T2D. A recent systematic review and meta-analysis examining data on the effects of bariatric surgery on histologic features of NAFLD from 32 cohort studies (no RCTs included) showed that bariatric surgery was associated with significant improvements in steatosis (66%), lobular inflammation (50%), ballooning degeneration (76%), and fibrosis (40%), and the benefits were significantly higher in those who underwent Roux-en-Y gastric bypass (RYGB). Of note, worsening of liver histology, including fibrosis, could be seen in up to 12% of patients who underwent bariatric surgery.24 The postsurgical weight regained after RYGB could explain partly the lack of fibrosis improvement or even worsening of fibrosis, although further research is needed to clarify these controversial findings.
RYGB and sleeve gastrectomy (SG) are the most commonly performed bariatric surgeries worldwide. Patients who undergo RYGB achieve higher weight loss when compared with those treated with SG.25 Among all bariatric procedures, RYGB could result in a higher proportion of complete resolution of NAFLD than SG, although evidence is inconclusive on fibrosis improvement rates.24,26 Most recently, a single-center RCT has compared the effects of RYGB vs. SG on liver fat content and fibrosis in patients with severe obesity and T2D.27 Data showed that both surgical procedures were highly and equally effective in reducing fatty liver content (quantified by magnetic resonance imaging), with an almost complete resolution of the fatty liver at 1 year of both surgical interventions. The beneficial effects of both GB and SG on fibrosis (assessed by enhanced liver test [ELF]) were less evident with no substantial difference between the two groups. Importantly, 69% of participants had an increase in their ELF scores during the study, despite the majority of participants achieving significant reductions in their body weights and better glycemic control at the end of the study. These findings might be considered with caution as several factors, such as the duration of the study (only 1 year) and lack of a liver biopsy to confirm fibrosis changes over time, could be influencing the study results.
Among all NAFLD phenotypes, those with cirrhosis and, most importantly, hepatic decompensation appear to be at increased risk of perioperative mortality and inpatient hospital stays than those without cirrhosis.28-29 Bariatric surgery is an absolute contraindication in patients with decompensated cirrhosis (Child B and Child C). Among compensated -Child A- cirrhotics, those with portal hypertension are at increased risk of morbidity and perioperative mortality.30 A recent analysis of National Inpatient Sample data suggested that the rates of complications in those with cirrhosis have decreased with time, which could be due to a better selection process and the use of more restrictive bariatric surgery in those with cirrhosis. Low volume centers (defined as less than 50 procedures per year) and nonrestrictive bariatric surgery were associated with a higher mortality rate. These data may suggest that patients with cirrhosis should undergo bariatric surgery only in high-volume centers after a multidisciplinary evaluation.31 Bariatric endoscopy is emerging as a new treatment for obesity, but the long-term durability of its effects remains to be determined.
A recent retrospective cohort study, including 1,158 adult patients with biopsy-proven NASH, has investigated the benefits of bariatric surgery on the occurrence of major adverse liver and cardiovascular outcomes in 650 patients who underwent bariatric surgery, compared with 508 patients who received nonsurgical usual care. This study showed that bariatric surgery was associated with 88% lower risk of progression of fatty liver to cirrhosis, liver cancer, or liver-related death, and 70% lower risk of serious CVD events during a follow-up period of 10 years.32 Within 1 year after surgery, 0.6% of patients died from surgical complications. The potential benefits of bariatric surgery in patients with NAFLD must be balanced against surgical risk, especially in eligible obese individuals with established cirrhosis. Data from a retrospective cohort study have shown that bariatric surgery in obese cirrhotic patients does not seem to associate with excessive mortality, compared with noncirrhotic obese patients.33 More data on immediate complication rates and long-term outcomes in patients with NAFLD by type of bariatric surgery is also required.
NAFLD as a standalone is not an indication for bariatric surgery. However, it could be considered in NAFLD patients who have a BMI of 40 kg/m2 or more without coexisting comorbidities or with a BMI of 35 kg/m2 or more and one or more severe obesity-related comorbidities, including T2D, hypertension, hyperlipidemia, or obstructive sleep apnea. Bariatric surgery must always be offered in centers with an experienced bariatric surgery program.1
Management of comorbidities
Given the multiple comorbidities associated with NAFLD and the potential to influence its severity, a comprehensive and multidisciplinary approach is needed to ameliorate not only the progression of liver disease but also those complications related to metabolic syndrome, hyperlipidemia, hypertension, diabetes, and other related conditions. Of note, all patients with NAFLD should receive aggressive management of comorbidities regardless of the severity of NAFLD. Ideally, a multidisciplinary team – including a primary care provider, an endocrinologist for patients with T2D, and a gastroenterologist/hepatologist – is needed to successfully manage patients with NAFLD.
It is well recognized that individuals with biopsy-proven NAFLD are at a higher risk of coronary heart disease, stroke, congestive heart failure, and death resulting from CVD when compared with the non-NAFLD population, and excess in CVD morbidity and mortality is evident across all stages of NAFLD and increases with worsening disease severity.34 The strong association between CVD and NAFLD has important clinical implications that may influence the decision to initiate treatment for primary prevention, including lipid-lowering, antihypertensive, or antiplatelet therapies.35 Statins are widely used to reduce LDL cholesterol and have been proven to be safe in NAFLD, including for those with elevated liver enzymes and even in compensated cirrhosis, in several studies conducted during the last 15 years.36 Statins are characterized by anti-inflammatory, anti-oxidative, antifibrotic, and plaque-stabilizing effects, whereby they may improve vascular and hepatic function among patients with NAFLD and reduce cardiovascular risk.37 Statin use for the treatment of NAFLD is still controversial and off-label and is not specifically recommended to treat NASH, but positive results have been shown for reductions in liver enzymes.1 A recent meta-analysis of 13 studies showed that continued use of statin in cirrhosis was associated with a 46% and 44% risk reduction in hepatic decompensation and mortality, respectively.38
The Food and Drug Administration has approved omega-3 (n-3) fatty acid agents and fibrates for the treatment of very high triglycerides (500 mg/dL or higher); however, no specific indications exist to treat NAFLD.1 Fenofibrate is related to mild aminotransferase elevations and, in some cases, severe liver injury, so caution must be paid, especially within 2 days of taking the drug.39-40
NAFLD phenotypes that need liver pharmacotherapy
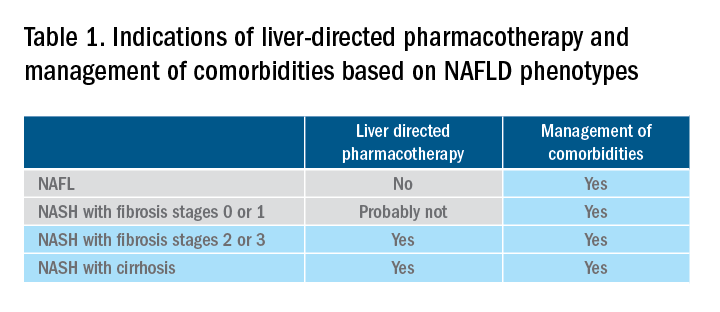
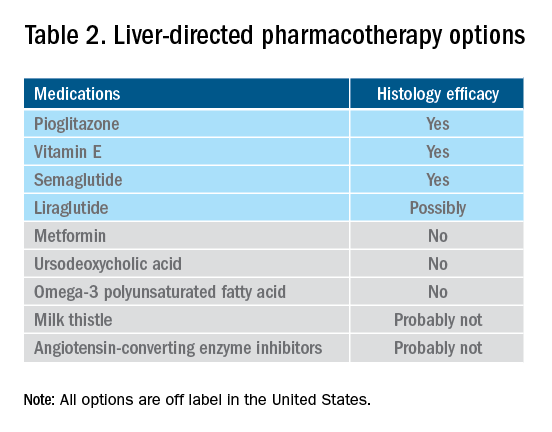
There are still no FDA-approved drugs or biological treatments for NASH. Pharmacological interventions aiming primarily at improving liver disease should generally be limited to those with biopsy-proven NASH and clinically significant fibrosis (fibrosis stages of 2 or greater).1,4 For FDA approval, medications used for treating NAFLD with fibrosis need to meet one of the following endpoint criteria: resolution of NASH without worsening of fibrosis, improvement in fibrosis without worsening of NASH, or both. In addition to those criteria, a new medication might improve the metabolic profile and have a tolerable safety profile. Table 1 displays those NAFLD phenotypes that will likely benefit from liver-directed therapy.
Obeticholic acid as an experimental therapy for NASH
A planned month-18 interim analysis of a multicentre, phase III RCT examined the efficacy and safety of obeticholic acid (OCA), a farnesoid X receptor agonist, in patients with NASH and stages 1-3 of fibrosis. The primary endpoint (fibrosis reduction 1 stage or more with no worsening of NASH) was met by 12% of patients in the placebo group, 18% of patients receiving OCA 10 mg (P = .045), and 23% of those receiving OCA 25 mg (P = .0002). An alternative primary endpoint of NASH resolution with no worsening of fibrosis was not met. OCA 25 mg led to the highest rates of pruritus and hyperlipidemia, compared with OCA 10 mg.42 These side effects seem to be related to the activation of the farnesoid X receptor.43
Currently available but off label medications
Vitamin E, an antioxidant, administered at a daily dose of 800 IU/day improves steatosis, inflammation, and ballooning, but not fibrosis in nondiabetic adults with biopsy-proven NASH.44 Vitamin E for 96 weeks was associated with a significantly higher rate of improvement in NASH (43% vs. 19%, P less than .01), compared with placebo.44 In the Treatment of Nonalcoholic Fatty Liver Disease in Children trial (TONIC), which examined vitamin E (800 IU/day) or metformin (500 mg twice daily) against placebo in children with biopsy-proven NAFLD, resolution of NASH was significantly greater in children treated with vitamin E than in children treated with placebo (58% vs. 28%, P less than .01). Metformin did not significantly improve the NASH resolution rates, compared with placebo (41% vs. 28%, P = .23). Vitamin E could be recommended for nondiabetic adults or children if lifestyle modifications do not produce the expected results as a result of noncompliance or ineffectiveness. Since continued use of vitamin E has been suggested to be associated with a very small increase in the risk for prostate cancer (an absolute increase of 1.6 per 1,000 person-years of vitamin E use) in men, risks and benefits should be discussed with each patient before starting therapy. A meta-analysis of nine placebo-controlled trials including roughly 119,000 patients reported that vitamin E supplementation increases the risk of hemorrhagic stroke by 20% while reducing ischemic stroke by 10%. It was estimated that vitamin E supplementation would prevent one ischemic stroke per 476 treated patients while inducing one hemorrhagic stroke for every 1,250 patients. It is noteworthy that the combination of vitamin E with anticoagulant and/or antiplatelet therapy was not examined in this trial, so we could not determine how combination therapy might affect the risk of ischemic or hemorrhagic stroke.45
Thiazolidinediones drugs have been reported to be effective in improving NAFLD in many human studies. Evidence from RCTs suggests that pioglitazone could significantly improve glucose metabolism, alanine aminotransferase, and liver histology – such as hepatic steatosis, lobular inflammation, and ballooning degeneration – among patients with or without T2D. However, the beneficial effects on improving fibrosis remain to be verified.1,46 Because of safety concerns, the risk/benefit balance of using pioglitazone to treat NASH should be discussed with each patient.47-48 Pioglitazone has been associated with long-term risk of bladder cancer,49 congestive heart failure,50 and bone fractures.51 Data from the Pioglitazone, Vitamin E, or Placebo for Nonalcoholic Steatohepatitis (PIVENS) trial showed that pioglitazone was significantly associated with weight gain but with no other serious adverse events. However, this study was not powered to test any safety-related hypotheses.44
Glucagon-like peptide 1 analogs have been reported to induce weight loss and reduce insulin resistance, which may lead to improvements in NAFLD. Phase II RCTs of glucagon-like peptide 1 receptor agonists (liraglutide and semaglutide) for the treatment of biopsy-proven NASH showed significant improvements in serum liver enzymes, steatosis, and inflammation, as well as NASH resolution without worsening liver fibrosis, although no direct benefit was observed in reversing fibrosis.52-53 One of these studies explores the efficacy and safety of different doses of daily subcutaneous semaglutide vs. placebo on the rates of resolution of NASH with no worsening of fibrosis. The highest dose (0.4 mg) showed the greatest difference (59% vs. 17%, P less than .01), compared with the placebo arm. However, there was no difference in improvement in fibrosis stage between the two groups (43% in the 0.4-mg group vs. 33% in the placebo group, P = .48).53 Gastrointestinal adverse events were common in the semaglutide arm.
“Spontaneous” NASH resolution and fibrosis improvement are commonly seen in participants assigned to placebo arms in clinical trials. A recent meta-analysis of 43 RCTs including 2649 placebo-treated patients showed a pooled estimate of NASH resolution without worsening of fibrosis and 1 stage reduction or more in fibrosis of 12% and 19%, respectively. Relevant factors involved in “spontaneous” NASH improvement are unknown but could be related to changes in BMI resulting from lifestyle changes, race and ethnicity, age, and, likely, NAFLD-related genetic variations, although more data is needed to better understand the histologic response in placebo-treated patients.54
Semaglutide injections (2.4 mg once weekly) or (2.0 mg once weekly) have been recently approved by the FDA for chronic weight management in adults with obesity or overweight with at least one weight-related condition or glucose control of T2D, respectively. Of note, the semaglutide dose used in the NASH trial is not currently available for the treatment of patients who are overweight/obese or have T2D, but the beneficial effects on body weight reductions and glucose control are similar overall to the effects seen with currently available doses for management of obesity or diabetes. One may consider using semaglutide in patients who are overweight/obese or have T2D with NASH, but in the senior author’s experience, it has been quite challenging to receive the payer’s approval, as its use is not specifically approved to treat liver disease.1
How to follow patients with NAFLD in the clinic
Once a diagnosis of NAFLD is made, the use of noninvasive testing may aid to identify which patients are at high risk of fibrosis. Easy to use clinical tools, such as the NAFLD Fibrosis Score and the Fib-4 index, and liver stiffness measurements using vibration-controlled transient elastography (FibroScan) or magnetic resonance elastography (MRE) are clinically useful noninvasive tools for identifying patients with NAFLD who have a higher likelihood of progressing to advanced fibrosis.1,55 The use of either NAFLD Fibrosis Score (less than -1.455) or Fib-4 index (less than 1.30) low cutoffs may be particularly useful to rule out advanced fibrosis. People with a NAFLD Fibrosis Score (greater than –1.455) or Fib-4 index (greater than 1.30) should undergo liver stiffness measurement (LSM) via FibroScan. Those with an LSM of 8 kPa or higher should be referred to specialized care, where a decision to perform a liver biopsy and initiate monitoring and therapy will be taken. MRE is the most accurate noninvasive method for the estimation of liver fibrosis. When MRE is available, it can be a diagnostic alternative to accurately rule in and rule out patients with advanced fibrosis. This technique can be preferred in clinical trials, but it is rarely used in clinical practice because it is expensive and not easily available. Reassessment by noninvasive scores at 1-3 years’ follow-up will be considered for those with an LSM less than 8 kPa. Patients with NASH cirrhosis should be screened for both gastroesophageal varix and HCC according to the American Association for the Study of Liver Diseases guidelines.56-57
Dr. Vilar-Gomez is assistant professor in the division of gastroenterology and hepatology at Indiana University, Indianapolis. Dr. Chalasani is vice president for academic affairs at Indiana University Health, Indianapolis, and the David W. Crabb Professor of Gastroenterology and Hepatology and an adjunct professor of anatomy, cell biology, and physiology in the division of gastroenterology and hepatology at Indiana University. Dr. Vilar-Gomez reports no financial conflicts of interest. Dr. Chalasani serves as a paid consultant to AbbVie, Boehringer-Ingelheim, Altimmune, Madrigal, Lilly, Zydus, and Galectin. He receives research support from Galectin and DSM.
References
1. Chalasani N et al. Hepatology 2018;67:328-57.
2. Söderberg C et al. Hepatology 2010;51:595-602.
3. Sanyal AJ et al. N Engl J Med 2021;385:1559-69.
4. Vilar-Gomez E et al. Gastroenterology 2018;155:443-57.e17.
5. Younossi ZM et al. Hepatology 2016;64:73-84.
6. EASL-EASD-EASO. J Hepatol 2016;64:1388-402.
7. Wong VW et al. J Hepatol 2018; 69:1349-56.
8. Vilar-Gomez E et al. Gastroenterology 2015;149:367-78.e5; quiz e14-5.
9. Promrat K et al. Hepatology 2010;51:121-9.
10. Wong VW et al. J Hepatol 2013;59:536-42.
11. Berzigotti A et al. Hepatology 2017;65:1293-1305.
12. Sacks FM et al. N Engl J Med 2009;360:859-73.
13. Vilar-Gomez E et al. Hepatology 2022 Jun;75(6):1491-1506.
14. Zelber-Sagi S et al. Liver Int 2017;37:936-49.
15. Hassani Zadeh S et al. J Gastroenterol Hepatol 2021;36:1470-8.
16. Yaskolka Meir A et al. Gut 2021;70:2085-95.
17. Sung KC et al. J Hepatol 2016;65:791-7.
18. Orci LA et al. Clin Gastroenterol Hepatol 2016;14:1398-411.
19. Ryu S et al. J Hepatol 2015;63:1229-37.
20. Kim D et al. Hepatology 2020;72:1556-68.
21. Kim D et al. Clin Gastroenterol Hepatol 2021;19:1240-7.e5.
22. Ascha MS et al. Hepatology 2010;51:1972-8.
23. Bambha K et al. Liver Int 2014;34:1250-8.
24. Lee Y et al. Clin Gastroenterol Hepatol 2019;17:1040-60.e11.
25. Grönroos S et al. JAMA Surg 2021;156:137-46.
26. Fakhry TK et al. Surg Obes Relat Dis 2019;15:502-11.
27. Seeberg KA et al. Ann Intern Med 2022;175:74-83.
28. Bower G et al. Obes Surg 2015;25:2280-9.
29. Jan A et al. Obes Surg 2015;25:1518-26.
30. Hanipah ZN et al. Obes Surg 2018;28:3431-8.
31. Are VS et al. Am J Gastroenterol 2020;115:1849-56.
32. Aminian A et al. JAMA 2021;326:2031-42.
33. Vuppalanchi R et al. Ann Surg 2022;275:e174-80.
34. Simon TG et al. Gut 2021. doi: 10.1136/gutjnl-2021-325724.
35. Lonardo A et al. J Hepatol 2018;68:335-52.
36. Chalasani N et al. Gastroenterology 2004;126:1287-92.
37. Pastori D et al. Dig Liver Dis 2015;47:4-11.
38. Kim RG et al. Clin Gastroenterol Hepatol 2017;15:1521-30.e8.
39. Ahmad J et al. Dig Dis Sci 2017;62:3596-604.
40. Chalasani NP et al. Am J Gastroenterol 2021;116(5):878-98.
41. Rinella ME et al. Hepatology 2019;70:1424-36.
42. Younossi ZM et al. Lancet 2019;394:2184-96.
43. Ratziu V. Clin Liver Dis (Hoboken) 2021;17:398-400.
44. Sanyal AJ et al. N Engl J Med 2010;341:1675-85.
45. Schürks M et al. BMJ 2010;341:c5702.
46. Cusi K et al. Ann Intern Med 2016;165:305-15.
47. Lewis JD et al. JAMA 2015;314:265-77.
48. Billington EO et al. Diabetologia 2015;58:2238-46.
49. Lewis JD et al. Diabetes Care 2011;34:916-22.
50. Erdmann E et al. Diabetes Care 2007;30:2773-8.
51. Viscoli CM et al. J Clin Endocrinol Metab 2017;102:914-22.
52. Armstong MJ et al. Lancet 2016;387:679-90.
53. Newsome PN et al. N Engl J Med 2021;384:1113-24.
54. Ng CH et al. Hepatology 2022;75:1647-61.
55. Kanwal F et al. Gastroenterology 2021;161:1030-1042.e8.
56. Garcia-Tsao G et al. Hepatology 2017;65:310-35.
57. Heimbach JK et al. Hepatology 2018;67:358-80.
Nonalcoholic fatty liver disease (NAFLD) is defined by the presence of hepatic steatosis detected on either imaging or histology in the absence of secondary causes of fatty liver (e.g., excessive alcohol consumption) or other chronic liver diseases.1 For practical NAFLD diagnosis purposes, excessive alcohol intake can be defined as an active or recent history of more than 21 standard drinks per week in men and more than14 standard drinks per week in women. For the sake of terminology, NAFLD is characterized by fatty liver infiltration, affecting at least 5% of hepatocytes, with no evidence of hepatocyte injury, whereas nonalcoholic steatohepatitis (NASH) is defined as the presence of necroinflammation with or without fibrosis in a background of fatty liver.1
Natural history
NASH and the degree of fibrosis are the two most important determinants of the natural history of NAFLD. NASH can evolve into fibrosis and cirrhosis, whereas advanced fibrosis and cirrhosis (stages 3 or 4 of fibrosis) significantly increase the risk of liver-related decompensation and mortality. NAFLD, per se, has been associated with an increased risk of overall mortality, compared with that of the general population.2 The three most common causes of mortality for patients with NAFLD are cardiovascular diseases (CVD), extrahepatic malignancies, and liver-related deaths. Mortality and liver-related events, including hepatic decompensation and hepatocellular carcinoma (HCC), may significantly increase in a dose-dependent manner with increasing fibrosis stages, and stages 3 or 4 of fibrosis may display the highest rates of all-cause mortality and liver-related events.3,4 It is important to note, however, that almost 15% of HCCs occur in patients with NAFLD who do not have cirrhosis.5 The presence of commonly associated comorbidities such as obesity, insulin resistance or diabetes, dyslipidemia, hypothyroidism, polycystic ovary syndrome, and sleep apnea may contribute to an increased risk of NASH and advanced fibrosis and, therefore, an accelerated clinical course of NAFLD.
Nonpharmacological interventions
Lifestyle modification
Lifestyle modification to achieve weight loss remains a first-line intervention in patients with NAFLD. Weight loss achieved either by hypocaloric diet alone or in conjunction with increased physical activity can be beneficial for all patients with NAFLD. The benefits extend not only to those who are overweight and obese but also to those within normal body weight (lean NAFLD).1,6,7 Weight loss of approximately 3%-5% is necessary to improve hepatic steatosis, but a greater weight loss (7%-10%) is required to improve other histopathological features like necroinflammatory lesions and fibrosis.8-10 Individuals with higher BMI and/or type 2 diabetes (T2D) will require a larger weight reduction to achieve a similar benefit on NAFLD-related features.7,8 Weight loss via lifestyle changes can also decrease hepatic venous pressure gradient (HVPG), with greater declines reported among those with more than 10% weight loss.11
Weight loss can be achieved through a variety of modalities, but long-term maintenance of lost weight is much more challenging. A combination of a hypocaloric diet with a caloric deficit of 500-1,000 kcal/d, alongside moderate-intensity exercise and intensive on-site behavioral treatment, will likely increase the possibility of a sustained weight loss over time.1,12 A growing body of scientific evidence indicates that a healthy diet that includes a reduction of high-glycemic-index foods and refined carbohydrates; increased consumption of monounsaturated fatty acids, omega-3 fatty acids, and fibers; and high intakes of olive oil, nuts, vegetables, fruits, legumes, whole grains, and fish can have beneficial effects on NAFLD and its severity.13-16 Adherence to these healthy dietary patterns has been associated with a marked reduction in CVD morbidity and mortality and is, thus, a strategic lifestyle recommendation for patients with NAFLD in whom the leading cause of morbidity and death is CVD.1,3
Exercise alone in adults with NAFLD may reduce hepatic steatosis, but its ability to improve inflammation and fibrosis has not been proven in well-designed RCTs.17,18 Physical activity and exercise have been shown to curb both the development and the progression of NAFLD, and beneficial effects could be achieved independent of weight loss.17,19,20 Most importantly, moderate-to-vigorous physical activity is likely associated with lower all-cause and cardiovascular mortality in patients with NAFLD.21
Heavy alcohol intake should be avoided by patients with NAFLD or NASH, and those with cirrhotic NASH should avoid any alcohol consumption given the risk of HCC and hepatic decompensation.1,4,22 Limiting light-to-moderate alcohol intake among patients without cirrhosis is still under debate.1 People with NAFLD may be advised to drink an equivalent of two to three 8-oz cups of regular brewed coffee daily as it has shown certain antifibrotic effects in NAFLD patients.23
Bariatric surgery
Bariatric surgery is an attractive therapeutic option for eligible obese patients with NAFLD. Bariatric surgery has the potential for inducing great weight loss and, therefore, reverses not only the steatosis, inflammation, and fibrosis among NAFLD individuals but also important comorbid conditions like T2D. A recent systematic review and meta-analysis examining data on the effects of bariatric surgery on histologic features of NAFLD from 32 cohort studies (no RCTs included) showed that bariatric surgery was associated with significant improvements in steatosis (66%), lobular inflammation (50%), ballooning degeneration (76%), and fibrosis (40%), and the benefits were significantly higher in those who underwent Roux-en-Y gastric bypass (RYGB). Of note, worsening of liver histology, including fibrosis, could be seen in up to 12% of patients who underwent bariatric surgery.24 The postsurgical weight regained after RYGB could explain partly the lack of fibrosis improvement or even worsening of fibrosis, although further research is needed to clarify these controversial findings.
RYGB and sleeve gastrectomy (SG) are the most commonly performed bariatric surgeries worldwide. Patients who undergo RYGB achieve higher weight loss when compared with those treated with SG.25 Among all bariatric procedures, RYGB could result in a higher proportion of complete resolution of NAFLD than SG, although evidence is inconclusive on fibrosis improvement rates.24,26 Most recently, a single-center RCT has compared the effects of RYGB vs. SG on liver fat content and fibrosis in patients with severe obesity and T2D.27 Data showed that both surgical procedures were highly and equally effective in reducing fatty liver content (quantified by magnetic resonance imaging), with an almost complete resolution of the fatty liver at 1 year of both surgical interventions. The beneficial effects of both GB and SG on fibrosis (assessed by enhanced liver test [ELF]) were less evident with no substantial difference between the two groups. Importantly, 69% of participants had an increase in their ELF scores during the study, despite the majority of participants achieving significant reductions in their body weights and better glycemic control at the end of the study. These findings might be considered with caution as several factors, such as the duration of the study (only 1 year) and lack of a liver biopsy to confirm fibrosis changes over time, could be influencing the study results.
Among all NAFLD phenotypes, those with cirrhosis and, most importantly, hepatic decompensation appear to be at increased risk of perioperative mortality and inpatient hospital stays than those without cirrhosis.28-29 Bariatric surgery is an absolute contraindication in patients with decompensated cirrhosis (Child B and Child C). Among compensated -Child A- cirrhotics, those with portal hypertension are at increased risk of morbidity and perioperative mortality.30 A recent analysis of National Inpatient Sample data suggested that the rates of complications in those with cirrhosis have decreased with time, which could be due to a better selection process and the use of more restrictive bariatric surgery in those with cirrhosis. Low volume centers (defined as less than 50 procedures per year) and nonrestrictive bariatric surgery were associated with a higher mortality rate. These data may suggest that patients with cirrhosis should undergo bariatric surgery only in high-volume centers after a multidisciplinary evaluation.31 Bariatric endoscopy is emerging as a new treatment for obesity, but the long-term durability of its effects remains to be determined.
A recent retrospective cohort study, including 1,158 adult patients with biopsy-proven NASH, has investigated the benefits of bariatric surgery on the occurrence of major adverse liver and cardiovascular outcomes in 650 patients who underwent bariatric surgery, compared with 508 patients who received nonsurgical usual care. This study showed that bariatric surgery was associated with 88% lower risk of progression of fatty liver to cirrhosis, liver cancer, or liver-related death, and 70% lower risk of serious CVD events during a follow-up period of 10 years.32 Within 1 year after surgery, 0.6% of patients died from surgical complications. The potential benefits of bariatric surgery in patients with NAFLD must be balanced against surgical risk, especially in eligible obese individuals with established cirrhosis. Data from a retrospective cohort study have shown that bariatric surgery in obese cirrhotic patients does not seem to associate with excessive mortality, compared with noncirrhotic obese patients.33 More data on immediate complication rates and long-term outcomes in patients with NAFLD by type of bariatric surgery is also required.
NAFLD as a standalone is not an indication for bariatric surgery. However, it could be considered in NAFLD patients who have a BMI of 40 kg/m2 or more without coexisting comorbidities or with a BMI of 35 kg/m2 or more and one or more severe obesity-related comorbidities, including T2D, hypertension, hyperlipidemia, or obstructive sleep apnea. Bariatric surgery must always be offered in centers with an experienced bariatric surgery program.1
Management of comorbidities
Given the multiple comorbidities associated with NAFLD and the potential to influence its severity, a comprehensive and multidisciplinary approach is needed to ameliorate not only the progression of liver disease but also those complications related to metabolic syndrome, hyperlipidemia, hypertension, diabetes, and other related conditions. Of note, all patients with NAFLD should receive aggressive management of comorbidities regardless of the severity of NAFLD. Ideally, a multidisciplinary team – including a primary care provider, an endocrinologist for patients with T2D, and a gastroenterologist/hepatologist – is needed to successfully manage patients with NAFLD.
It is well recognized that individuals with biopsy-proven NAFLD are at a higher risk of coronary heart disease, stroke, congestive heart failure, and death resulting from CVD when compared with the non-NAFLD population, and excess in CVD morbidity and mortality is evident across all stages of NAFLD and increases with worsening disease severity.34 The strong association between CVD and NAFLD has important clinical implications that may influence the decision to initiate treatment for primary prevention, including lipid-lowering, antihypertensive, or antiplatelet therapies.35 Statins are widely used to reduce LDL cholesterol and have been proven to be safe in NAFLD, including for those with elevated liver enzymes and even in compensated cirrhosis, in several studies conducted during the last 15 years.36 Statins are characterized by anti-inflammatory, anti-oxidative, antifibrotic, and plaque-stabilizing effects, whereby they may improve vascular and hepatic function among patients with NAFLD and reduce cardiovascular risk.37 Statin use for the treatment of NAFLD is still controversial and off-label and is not specifically recommended to treat NASH, but positive results have been shown for reductions in liver enzymes.1 A recent meta-analysis of 13 studies showed that continued use of statin in cirrhosis was associated with a 46% and 44% risk reduction in hepatic decompensation and mortality, respectively.38
The Food and Drug Administration has approved omega-3 (n-3) fatty acid agents and fibrates for the treatment of very high triglycerides (500 mg/dL or higher); however, no specific indications exist to treat NAFLD.1 Fenofibrate is related to mild aminotransferase elevations and, in some cases, severe liver injury, so caution must be paid, especially within 2 days of taking the drug.39-40
NAFLD phenotypes that need liver pharmacotherapy
There are still no FDA-approved drugs or biological treatments for NASH. Pharmacological interventions aiming primarily at improving liver disease should generally be limited to those with biopsy-proven NASH and clinically significant fibrosis (fibrosis stages of 2 or greater).1,4 For FDA approval, medications used for treating NAFLD with fibrosis need to meet one of the following endpoint criteria: resolution of NASH without worsening of fibrosis, improvement in fibrosis without worsening of NASH, or both. In addition to those criteria, a new medication might improve the metabolic profile and have a tolerable safety profile. Table 1 displays those NAFLD phenotypes that will likely benefit from liver-directed therapy.

Obeticholic acid as an experimental therapy for NASH
A planned month-18 interim analysis of a multicentre, phase III RCT examined the efficacy and safety of obeticholic acid (OCA), a farnesoid X receptor agonist, in patients with NASH and stages 1-3 of fibrosis. The primary endpoint (fibrosis reduction 1 stage or more with no worsening of NASH) was met by 12% of patients in the placebo group, 18% of patients receiving OCA 10 mg (P = .045), and 23% of those receiving OCA 25 mg (P = .0002). An alternative primary endpoint of NASH resolution with no worsening of fibrosis was not met. OCA 25 mg led to the highest rates of pruritus and hyperlipidemia, compared with OCA 10 mg.42 These side effects seem to be related to the activation of the farnesoid X receptor.43
Currently available but off label medications
Vitamin E, an antioxidant, administered at a daily dose of 800 IU/day improves steatosis, inflammation, and ballooning, but not fibrosis in nondiabetic adults with biopsy-proven NASH.44 Vitamin E for 96 weeks was associated with a significantly higher rate of improvement in NASH (43% vs. 19%, P less than .01), compared with placebo.44 In the Treatment of Nonalcoholic Fatty Liver Disease in Children trial (TONIC), which examined vitamin E (800 IU/day) or metformin (500 mg twice daily) against placebo in children with biopsy-proven NAFLD, resolution of NASH was significantly greater in children treated with vitamin E than in children treated with placebo (58% vs. 28%, P less than .01). Metformin did not significantly improve the NASH resolution rates, compared with placebo (41% vs. 28%, P = .23). Vitamin E could be recommended for nondiabetic adults or children if lifestyle modifications do not produce the expected results as a result of noncompliance or ineffectiveness. Since continued use of vitamin E has been suggested to be associated with a very small increase in the risk for prostate cancer (an absolute increase of 1.6 per 1,000 person-years of vitamin E use) in men, risks and benefits should be discussed with each patient before starting therapy. A meta-analysis of nine placebo-controlled trials including roughly 119,000 patients reported that vitamin E supplementation increases the risk of hemorrhagic stroke by 20% while reducing ischemic stroke by 10%. It was estimated that vitamin E supplementation would prevent one ischemic stroke per 476 treated patients while inducing one hemorrhagic stroke for every 1,250 patients. It is noteworthy that the combination of vitamin E with anticoagulant and/or antiplatelet therapy was not examined in this trial, so we could not determine how combination therapy might affect the risk of ischemic or hemorrhagic stroke.45

Thiazolidinediones drugs have been reported to be effective in improving NAFLD in many human studies. Evidence from RCTs suggests that pioglitazone could significantly improve glucose metabolism, alanine aminotransferase, and liver histology – such as hepatic steatosis, lobular inflammation, and ballooning degeneration – among patients with or without T2D. However, the beneficial effects on improving fibrosis remain to be verified.1,46 Because of safety concerns, the risk/benefit balance of using pioglitazone to treat NASH should be discussed with each patient.47-48 Pioglitazone has been associated with long-term risk of bladder cancer,49 congestive heart failure,50 and bone fractures.51 Data from the Pioglitazone, Vitamin E, or Placebo for Nonalcoholic Steatohepatitis (PIVENS) trial showed that pioglitazone was significantly associated with weight gain but with no other serious adverse events. However, this study was not powered to test any safety-related hypotheses.44
Glucagon-like peptide 1 analogs have been reported to induce weight loss and reduce insulin resistance, which may lead to improvements in NAFLD. Phase II RCTs of glucagon-like peptide 1 receptor agonists (liraglutide and semaglutide) for the treatment of biopsy-proven NASH showed significant improvements in serum liver enzymes, steatosis, and inflammation, as well as NASH resolution without worsening liver fibrosis, although no direct benefit was observed in reversing fibrosis.52-53 One of these studies explores the efficacy and safety of different doses of daily subcutaneous semaglutide vs. placebo on the rates of resolution of NASH with no worsening of fibrosis. The highest dose (0.4 mg) showed the greatest difference (59% vs. 17%, P less than .01), compared with the placebo arm. However, there was no difference in improvement in fibrosis stage between the two groups (43% in the 0.4-mg group vs. 33% in the placebo group, P = .48).53 Gastrointestinal adverse events were common in the semaglutide arm.
“Spontaneous” NASH resolution and fibrosis improvement are commonly seen in participants assigned to placebo arms in clinical trials. A recent meta-analysis of 43 RCTs including 2649 placebo-treated patients showed a pooled estimate of NASH resolution without worsening of fibrosis and 1 stage reduction or more in fibrosis of 12% and 19%, respectively. Relevant factors involved in “spontaneous” NASH improvement are unknown but could be related to changes in BMI resulting from lifestyle changes, race and ethnicity, age, and, likely, NAFLD-related genetic variations, although more data is needed to better understand the histologic response in placebo-treated patients.54
Semaglutide injections (2.4 mg once weekly) or (2.0 mg once weekly) have been recently approved by the FDA for chronic weight management in adults with obesity or overweight with at least one weight-related condition or glucose control of T2D, respectively. Of note, the semaglutide dose used in the NASH trial is not currently available for the treatment of patients who are overweight/obese or have T2D, but the beneficial effects on body weight reductions and glucose control are similar overall to the effects seen with currently available doses for management of obesity or diabetes. One may consider using semaglutide in patients who are overweight/obese or have T2D with NASH, but in the senior author’s experience, it has been quite challenging to receive the payer’s approval, as its use is not specifically approved to treat liver disease.1
How to follow patients with NAFLD in the clinic
Once a diagnosis of NAFLD is made, the use of noninvasive testing may aid to identify which patients are at high risk of fibrosis. Easy to use clinical tools, such as the NAFLD Fibrosis Score and the Fib-4 index, and liver stiffness measurements using vibration-controlled transient elastography (FibroScan) or magnetic resonance elastography (MRE) are clinically useful noninvasive tools for identifying patients with NAFLD who have a higher likelihood of progressing to advanced fibrosis.1,55 The use of either NAFLD Fibrosis Score (less than -1.455) or Fib-4 index (less than 1.30) low cutoffs may be particularly useful to rule out advanced fibrosis. People with a NAFLD Fibrosis Score (greater than –1.455) or Fib-4 index (greater than 1.30) should undergo liver stiffness measurement (LSM) via FibroScan. Those with an LSM of 8 kPa or higher should be referred to specialized care, where a decision to perform a liver biopsy and initiate monitoring and therapy will be taken. MRE is the most accurate noninvasive method for the estimation of liver fibrosis. When MRE is available, it can be a diagnostic alternative to accurately rule in and rule out patients with advanced fibrosis. This technique can be preferred in clinical trials, but it is rarely used in clinical practice because it is expensive and not easily available. Reassessment by noninvasive scores at 1-3 years’ follow-up will be considered for those with an LSM less than 8 kPa. Patients with NASH cirrhosis should be screened for both gastroesophageal varix and HCC according to the American Association for the Study of Liver Diseases guidelines.56-57
Dr. Vilar-Gomez is assistant professor in the division of gastroenterology and hepatology at Indiana University, Indianapolis. Dr. Chalasani is vice president for academic affairs at Indiana University Health, Indianapolis, and the David W. Crabb Professor of Gastroenterology and Hepatology and an adjunct professor of anatomy, cell biology, and physiology in the division of gastroenterology and hepatology at Indiana University. Dr. Vilar-Gomez reports no financial conflicts of interest. Dr. Chalasani serves as a paid consultant to AbbVie, Boehringer-Ingelheim, Altimmune, Madrigal, Lilly, Zydus, and Galectin. He receives research support from Galectin and DSM.
References
1. Chalasani N et al. Hepatology 2018;67:328-57.
2. Söderberg C et al. Hepatology 2010;51:595-602.
3. Sanyal AJ et al. N Engl J Med 2021;385:1559-69.
4. Vilar-Gomez E et al. Gastroenterology 2018;155:443-57.e17.
5. Younossi ZM et al. Hepatology 2016;64:73-84.
6. EASL-EASD-EASO. J Hepatol 2016;64:1388-402.
7. Wong VW et al. J Hepatol 2018; 69:1349-56.
8. Vilar-Gomez E et al. Gastroenterology 2015;149:367-78.e5; quiz e14-5.
9. Promrat K et al. Hepatology 2010;51:121-9.
10. Wong VW et al. J Hepatol 2013;59:536-42.
11. Berzigotti A et al. Hepatology 2017;65:1293-1305.
12. Sacks FM et al. N Engl J Med 2009;360:859-73.
13. Vilar-Gomez E et al. Hepatology 2022 Jun;75(6):1491-1506.
14. Zelber-Sagi S et al. Liver Int 2017;37:936-49.
15. Hassani Zadeh S et al. J Gastroenterol Hepatol 2021;36:1470-8.
16. Yaskolka Meir A et al. Gut 2021;70:2085-95.
17. Sung KC et al. J Hepatol 2016;65:791-7.
18. Orci LA et al. Clin Gastroenterol Hepatol 2016;14:1398-411.
19. Ryu S et al. J Hepatol 2015;63:1229-37.
20. Kim D et al. Hepatology 2020;72:1556-68.
21. Kim D et al. Clin Gastroenterol Hepatol 2021;19:1240-7.e5.
22. Ascha MS et al. Hepatology 2010;51:1972-8.
23. Bambha K et al. Liver Int 2014;34:1250-8.
24. Lee Y et al. Clin Gastroenterol Hepatol 2019;17:1040-60.e11.
25. Grönroos S et al. JAMA Surg 2021;156:137-46.
26. Fakhry TK et al. Surg Obes Relat Dis 2019;15:502-11.
27. Seeberg KA et al. Ann Intern Med 2022;175:74-83.
28. Bower G et al. Obes Surg 2015;25:2280-9.
29. Jan A et al. Obes Surg 2015;25:1518-26.
30. Hanipah ZN et al. Obes Surg 2018;28:3431-8.
31. Are VS et al. Am J Gastroenterol 2020;115:1849-56.
32. Aminian A et al. JAMA 2021;326:2031-42.
33. Vuppalanchi R et al. Ann Surg 2022;275:e174-80.
34. Simon TG et al. Gut 2021. doi: 10.1136/gutjnl-2021-325724.
35. Lonardo A et al. J Hepatol 2018;68:335-52.
36. Chalasani N et al. Gastroenterology 2004;126:1287-92.
37. Pastori D et al. Dig Liver Dis 2015;47:4-11.
38. Kim RG et al. Clin Gastroenterol Hepatol 2017;15:1521-30.e8.
39. Ahmad J et al. Dig Dis Sci 2017;62:3596-604.
40. Chalasani NP et al. Am J Gastroenterol 2021;116(5):878-98.
41. Rinella ME et al. Hepatology 2019;70:1424-36.
42. Younossi ZM et al. Lancet 2019;394:2184-96.
43. Ratziu V. Clin Liver Dis (Hoboken) 2021;17:398-400.
44. Sanyal AJ et al. N Engl J Med 2010;341:1675-85.
45. Schürks M et al. BMJ 2010;341:c5702.
46. Cusi K et al. Ann Intern Med 2016;165:305-15.
47. Lewis JD et al. JAMA 2015;314:265-77.
48. Billington EO et al. Diabetologia 2015;58:2238-46.
49. Lewis JD et al. Diabetes Care 2011;34:916-22.
50. Erdmann E et al. Diabetes Care 2007;30:2773-8.
51. Viscoli CM et al. J Clin Endocrinol Metab 2017;102:914-22.
52. Armstong MJ et al. Lancet 2016;387:679-90.
53. Newsome PN et al. N Engl J Med 2021;384:1113-24.
54. Ng CH et al. Hepatology 2022;75:1647-61.
55. Kanwal F et al. Gastroenterology 2021;161:1030-1042.e8.
56. Garcia-Tsao G et al. Hepatology 2017;65:310-35.
57. Heimbach JK et al. Hepatology 2018;67:358-80.
Nonalcoholic fatty liver disease (NAFLD) is defined by the presence of hepatic steatosis detected on either imaging or histology in the absence of secondary causes of fatty liver (e.g., excessive alcohol consumption) or other chronic liver diseases.1 For practical NAFLD diagnosis purposes, excessive alcohol intake can be defined as an active or recent history of more than 21 standard drinks per week in men and more than14 standard drinks per week in women. For the sake of terminology, NAFLD is characterized by fatty liver infiltration, affecting at least 5% of hepatocytes, with no evidence of hepatocyte injury, whereas nonalcoholic steatohepatitis (NASH) is defined as the presence of necroinflammation with or without fibrosis in a background of fatty liver.1
Natural history
NASH and the degree of fibrosis are the two most important determinants of the natural history of NAFLD. NASH can evolve into fibrosis and cirrhosis, whereas advanced fibrosis and cirrhosis (stages 3 or 4 of fibrosis) significantly increase the risk of liver-related decompensation and mortality. NAFLD, per se, has been associated with an increased risk of overall mortality, compared with that of the general population.2 The three most common causes of mortality for patients with NAFLD are cardiovascular diseases (CVD), extrahepatic malignancies, and liver-related deaths. Mortality and liver-related events, including hepatic decompensation and hepatocellular carcinoma (HCC), may significantly increase in a dose-dependent manner with increasing fibrosis stages, and stages 3 or 4 of fibrosis may display the highest rates of all-cause mortality and liver-related events.3,4 It is important to note, however, that almost 15% of HCCs occur in patients with NAFLD who do not have cirrhosis.5 The presence of commonly associated comorbidities such as obesity, insulin resistance or diabetes, dyslipidemia, hypothyroidism, polycystic ovary syndrome, and sleep apnea may contribute to an increased risk of NASH and advanced fibrosis and, therefore, an accelerated clinical course of NAFLD.
Nonpharmacological interventions
Lifestyle modification
Lifestyle modification to achieve weight loss remains a first-line intervention in patients with NAFLD. Weight loss achieved either by hypocaloric diet alone or in conjunction with increased physical activity can be beneficial for all patients with NAFLD. The benefits extend not only to those who are overweight and obese but also to those within normal body weight (lean NAFLD).1,6,7 Weight loss of approximately 3%-5% is necessary to improve hepatic steatosis, but a greater weight loss (7%-10%) is required to improve other histopathological features like necroinflammatory lesions and fibrosis.8-10 Individuals with higher BMI and/or type 2 diabetes (T2D) will require a larger weight reduction to achieve a similar benefit on NAFLD-related features.7,8 Weight loss via lifestyle changes can also decrease hepatic venous pressure gradient (HVPG), with greater declines reported among those with more than 10% weight loss.11
Weight loss can be achieved through a variety of modalities, but long-term maintenance of lost weight is much more challenging. A combination of a hypocaloric diet with a caloric deficit of 500-1,000 kcal/d, alongside moderate-intensity exercise and intensive on-site behavioral treatment, will likely increase the possibility of a sustained weight loss over time.1,12 A growing body of scientific evidence indicates that a healthy diet that includes a reduction of high-glycemic-index foods and refined carbohydrates; increased consumption of monounsaturated fatty acids, omega-3 fatty acids, and fibers; and high intakes of olive oil, nuts, vegetables, fruits, legumes, whole grains, and fish can have beneficial effects on NAFLD and its severity.13-16 Adherence to these healthy dietary patterns has been associated with a marked reduction in CVD morbidity and mortality and is, thus, a strategic lifestyle recommendation for patients with NAFLD in whom the leading cause of morbidity and death is CVD.1,3
Exercise alone in adults with NAFLD may reduce hepatic steatosis, but its ability to improve inflammation and fibrosis has not been proven in well-designed RCTs.17,18 Physical activity and exercise have been shown to curb both the development and the progression of NAFLD, and beneficial effects could be achieved independent of weight loss.17,19,20 Most importantly, moderate-to-vigorous physical activity is likely associated with lower all-cause and cardiovascular mortality in patients with NAFLD.21
Heavy alcohol intake should be avoided by patients with NAFLD or NASH, and those with cirrhotic NASH should avoid any alcohol consumption given the risk of HCC and hepatic decompensation.1,4,22 Limiting light-to-moderate alcohol intake among patients without cirrhosis is still under debate.1 People with NAFLD may be advised to drink an equivalent of two to three 8-oz cups of regular brewed coffee daily as it has shown certain antifibrotic effects in NAFLD patients.23
Bariatric surgery
Bariatric surgery is an attractive therapeutic option for eligible obese patients with NAFLD. Bariatric surgery has the potential for inducing great weight loss and, therefore, reverses not only the steatosis, inflammation, and fibrosis among NAFLD individuals but also important comorbid conditions like T2D. A recent systematic review and meta-analysis examining data on the effects of bariatric surgery on histologic features of NAFLD from 32 cohort studies (no RCTs included) showed that bariatric surgery was associated with significant improvements in steatosis (66%), lobular inflammation (50%), ballooning degeneration (76%), and fibrosis (40%), and the benefits were significantly higher in those who underwent Roux-en-Y gastric bypass (RYGB). Of note, worsening of liver histology, including fibrosis, could be seen in up to 12% of patients who underwent bariatric surgery.24 The postsurgical weight regained after RYGB could explain partly the lack of fibrosis improvement or even worsening of fibrosis, although further research is needed to clarify these controversial findings.
RYGB and sleeve gastrectomy (SG) are the most commonly performed bariatric surgeries worldwide. Patients who undergo RYGB achieve higher weight loss when compared with those treated with SG.25 Among all bariatric procedures, RYGB could result in a higher proportion of complete resolution of NAFLD than SG, although evidence is inconclusive on fibrosis improvement rates.24,26 Most recently, a single-center RCT has compared the effects of RYGB vs. SG on liver fat content and fibrosis in patients with severe obesity and T2D.27 Data showed that both surgical procedures were highly and equally effective in reducing fatty liver content (quantified by magnetic resonance imaging), with an almost complete resolution of the fatty liver at 1 year of both surgical interventions. The beneficial effects of both GB and SG on fibrosis (assessed by enhanced liver test [ELF]) were less evident with no substantial difference between the two groups. Importantly, 69% of participants had an increase in their ELF scores during the study, despite the majority of participants achieving significant reductions in their body weights and better glycemic control at the end of the study. These findings might be considered with caution as several factors, such as the duration of the study (only 1 year) and lack of a liver biopsy to confirm fibrosis changes over time, could be influencing the study results.
Among all NAFLD phenotypes, those with cirrhosis and, most importantly, hepatic decompensation appear to be at increased risk of perioperative mortality and inpatient hospital stays than those without cirrhosis.28-29 Bariatric surgery is an absolute contraindication in patients with decompensated cirrhosis (Child B and Child C). Among compensated -Child A- cirrhotics, those with portal hypertension are at increased risk of morbidity and perioperative mortality.30 A recent analysis of National Inpatient Sample data suggested that the rates of complications in those with cirrhosis have decreased with time, which could be due to a better selection process and the use of more restrictive bariatric surgery in those with cirrhosis. Low volume centers (defined as less than 50 procedures per year) and nonrestrictive bariatric surgery were associated with a higher mortality rate. These data may suggest that patients with cirrhosis should undergo bariatric surgery only in high-volume centers after a multidisciplinary evaluation.31 Bariatric endoscopy is emerging as a new treatment for obesity, but the long-term durability of its effects remains to be determined.
A recent retrospective cohort study, including 1,158 adult patients with biopsy-proven NASH, has investigated the benefits of bariatric surgery on the occurrence of major adverse liver and cardiovascular outcomes in 650 patients who underwent bariatric surgery, compared with 508 patients who received nonsurgical usual care. This study showed that bariatric surgery was associated with 88% lower risk of progression of fatty liver to cirrhosis, liver cancer, or liver-related death, and 70% lower risk of serious CVD events during a follow-up period of 10 years.32 Within 1 year after surgery, 0.6% of patients died from surgical complications. The potential benefits of bariatric surgery in patients with NAFLD must be balanced against surgical risk, especially in eligible obese individuals with established cirrhosis. Data from a retrospective cohort study have shown that bariatric surgery in obese cirrhotic patients does not seem to associate with excessive mortality, compared with noncirrhotic obese patients.33 More data on immediate complication rates and long-term outcomes in patients with NAFLD by type of bariatric surgery is also required.
NAFLD as a standalone is not an indication for bariatric surgery. However, it could be considered in NAFLD patients who have a BMI of 40 kg/m2 or more without coexisting comorbidities or with a BMI of 35 kg/m2 or more and one or more severe obesity-related comorbidities, including T2D, hypertension, hyperlipidemia, or obstructive sleep apnea. Bariatric surgery must always be offered in centers with an experienced bariatric surgery program.1
Management of comorbidities
Given the multiple comorbidities associated with NAFLD and the potential to influence its severity, a comprehensive and multidisciplinary approach is needed to ameliorate not only the progression of liver disease but also those complications related to metabolic syndrome, hyperlipidemia, hypertension, diabetes, and other related conditions. Of note, all patients with NAFLD should receive aggressive management of comorbidities regardless of the severity of NAFLD. Ideally, a multidisciplinary team – including a primary care provider, an endocrinologist for patients with T2D, and a gastroenterologist/hepatologist – is needed to successfully manage patients with NAFLD.
It is well recognized that individuals with biopsy-proven NAFLD are at a higher risk of coronary heart disease, stroke, congestive heart failure, and death resulting from CVD when compared with the non-NAFLD population, and excess in CVD morbidity and mortality is evident across all stages of NAFLD and increases with worsening disease severity.34 The strong association between CVD and NAFLD has important clinical implications that may influence the decision to initiate treatment for primary prevention, including lipid-lowering, antihypertensive, or antiplatelet therapies.35 Statins are widely used to reduce LDL cholesterol and have been proven to be safe in NAFLD, including for those with elevated liver enzymes and even in compensated cirrhosis, in several studies conducted during the last 15 years.36 Statins are characterized by anti-inflammatory, anti-oxidative, antifibrotic, and plaque-stabilizing effects, whereby they may improve vascular and hepatic function among patients with NAFLD and reduce cardiovascular risk.37 Statin use for the treatment of NAFLD is still controversial and off-label and is not specifically recommended to treat NASH, but positive results have been shown for reductions in liver enzymes.1 A recent meta-analysis of 13 studies showed that continued use of statin in cirrhosis was associated with a 46% and 44% risk reduction in hepatic decompensation and mortality, respectively.38
The Food and Drug Administration has approved omega-3 (n-3) fatty acid agents and fibrates for the treatment of very high triglycerides (500 mg/dL or higher); however, no specific indications exist to treat NAFLD.1 Fenofibrate is related to mild aminotransferase elevations and, in some cases, severe liver injury, so caution must be paid, especially within 2 days of taking the drug.39-40
NAFLD phenotypes that need liver pharmacotherapy
There are still no FDA-approved drugs or biological treatments for NASH. Pharmacological interventions aiming primarily at improving liver disease should generally be limited to those with biopsy-proven NASH and clinically significant fibrosis (fibrosis stages of 2 or greater).1,4 For FDA approval, medications used for treating NAFLD with fibrosis need to meet one of the following endpoint criteria: resolution of NASH without worsening of fibrosis, improvement in fibrosis without worsening of NASH, or both. In addition to those criteria, a new medication might improve the metabolic profile and have a tolerable safety profile. Table 1 displays those NAFLD phenotypes that will likely benefit from liver-directed therapy.

Obeticholic acid as an experimental therapy for NASH
A planned month-18 interim analysis of a multicentre, phase III RCT examined the efficacy and safety of obeticholic acid (OCA), a farnesoid X receptor agonist, in patients with NASH and stages 1-3 of fibrosis. The primary endpoint (fibrosis reduction 1 stage or more with no worsening of NASH) was met by 12% of patients in the placebo group, 18% of patients receiving OCA 10 mg (P = .045), and 23% of those receiving OCA 25 mg (P = .0002). An alternative primary endpoint of NASH resolution with no worsening of fibrosis was not met. OCA 25 mg led to the highest rates of pruritus and hyperlipidemia, compared with OCA 10 mg.42 These side effects seem to be related to the activation of the farnesoid X receptor.43
Currently available but off label medications
Vitamin E, an antioxidant, administered at a daily dose of 800 IU/day improves steatosis, inflammation, and ballooning, but not fibrosis in nondiabetic adults with biopsy-proven NASH.44 Vitamin E for 96 weeks was associated with a significantly higher rate of improvement in NASH (43% vs. 19%, P less than .01), compared with placebo.44 In the Treatment of Nonalcoholic Fatty Liver Disease in Children trial (TONIC), which examined vitamin E (800 IU/day) or metformin (500 mg twice daily) against placebo in children with biopsy-proven NAFLD, resolution of NASH was significantly greater in children treated with vitamin E than in children treated with placebo (58% vs. 28%, P less than .01). Metformin did not significantly improve the NASH resolution rates, compared with placebo (41% vs. 28%, P = .23). Vitamin E could be recommended for nondiabetic adults or children if lifestyle modifications do not produce the expected results as a result of noncompliance or ineffectiveness. Since continued use of vitamin E has been suggested to be associated with a very small increase in the risk for prostate cancer (an absolute increase of 1.6 per 1,000 person-years of vitamin E use) in men, risks and benefits should be discussed with each patient before starting therapy. A meta-analysis of nine placebo-controlled trials including roughly 119,000 patients reported that vitamin E supplementation increases the risk of hemorrhagic stroke by 20% while reducing ischemic stroke by 10%. It was estimated that vitamin E supplementation would prevent one ischemic stroke per 476 treated patients while inducing one hemorrhagic stroke for every 1,250 patients. It is noteworthy that the combination of vitamin E with anticoagulant and/or antiplatelet therapy was not examined in this trial, so we could not determine how combination therapy might affect the risk of ischemic or hemorrhagic stroke.45

Thiazolidinediones drugs have been reported to be effective in improving NAFLD in many human studies. Evidence from RCTs suggests that pioglitazone could significantly improve glucose metabolism, alanine aminotransferase, and liver histology – such as hepatic steatosis, lobular inflammation, and ballooning degeneration – among patients with or without T2D. However, the beneficial effects on improving fibrosis remain to be verified.1,46 Because of safety concerns, the risk/benefit balance of using pioglitazone to treat NASH should be discussed with each patient.47-48 Pioglitazone has been associated with long-term risk of bladder cancer,49 congestive heart failure,50 and bone fractures.51 Data from the Pioglitazone, Vitamin E, or Placebo for Nonalcoholic Steatohepatitis (PIVENS) trial showed that pioglitazone was significantly associated with weight gain but with no other serious adverse events. However, this study was not powered to test any safety-related hypotheses.44
Glucagon-like peptide 1 analogs have been reported to induce weight loss and reduce insulin resistance, which may lead to improvements in NAFLD. Phase II RCTs of glucagon-like peptide 1 receptor agonists (liraglutide and semaglutide) for the treatment of biopsy-proven NASH showed significant improvements in serum liver enzymes, steatosis, and inflammation, as well as NASH resolution without worsening liver fibrosis, although no direct benefit was observed in reversing fibrosis.52-53 One of these studies explores the efficacy and safety of different doses of daily subcutaneous semaglutide vs. placebo on the rates of resolution of NASH with no worsening of fibrosis. The highest dose (0.4 mg) showed the greatest difference (59% vs. 17%, P less than .01), compared with the placebo arm. However, there was no difference in improvement in fibrosis stage between the two groups (43% in the 0.4-mg group vs. 33% in the placebo group, P = .48).53 Gastrointestinal adverse events were common in the semaglutide arm.
“Spontaneous” NASH resolution and fibrosis improvement are commonly seen in participants assigned to placebo arms in clinical trials. A recent meta-analysis of 43 RCTs including 2649 placebo-treated patients showed a pooled estimate of NASH resolution without worsening of fibrosis and 1 stage reduction or more in fibrosis of 12% and 19%, respectively. Relevant factors involved in “spontaneous” NASH improvement are unknown but could be related to changes in BMI resulting from lifestyle changes, race and ethnicity, age, and, likely, NAFLD-related genetic variations, although more data is needed to better understand the histologic response in placebo-treated patients.54
Semaglutide injections (2.4 mg once weekly) or (2.0 mg once weekly) have been recently approved by the FDA for chronic weight management in adults with obesity or overweight with at least one weight-related condition or glucose control of T2D, respectively. Of note, the semaglutide dose used in the NASH trial is not currently available for the treatment of patients who are overweight/obese or have T2D, but the beneficial effects on body weight reductions and glucose control are similar overall to the effects seen with currently available doses for management of obesity or diabetes. One may consider using semaglutide in patients who are overweight/obese or have T2D with NASH, but in the senior author’s experience, it has been quite challenging to receive the payer’s approval, as its use is not specifically approved to treat liver disease.1
How to follow patients with NAFLD in the clinic
Once a diagnosis of NAFLD is made, the use of noninvasive testing may aid to identify which patients are at high risk of fibrosis. Easy to use clinical tools, such as the NAFLD Fibrosis Score and the Fib-4 index, and liver stiffness measurements using vibration-controlled transient elastography (FibroScan) or magnetic resonance elastography (MRE) are clinically useful noninvasive tools for identifying patients with NAFLD who have a higher likelihood of progressing to advanced fibrosis.1,55 The use of either NAFLD Fibrosis Score (less than -1.455) or Fib-4 index (less than 1.30) low cutoffs may be particularly useful to rule out advanced fibrosis. People with a NAFLD Fibrosis Score (greater than –1.455) or Fib-4 index (greater than 1.30) should undergo liver stiffness measurement (LSM) via FibroScan. Those with an LSM of 8 kPa or higher should be referred to specialized care, where a decision to perform a liver biopsy and initiate monitoring and therapy will be taken. MRE is the most accurate noninvasive method for the estimation of liver fibrosis. When MRE is available, it can be a diagnostic alternative to accurately rule in and rule out patients with advanced fibrosis. This technique can be preferred in clinical trials, but it is rarely used in clinical practice because it is expensive and not easily available. Reassessment by noninvasive scores at 1-3 years’ follow-up will be considered for those with an LSM less than 8 kPa. Patients with NASH cirrhosis should be screened for both gastroesophageal varix and HCC according to the American Association for the Study of Liver Diseases guidelines.56-57
Dr. Vilar-Gomez is assistant professor in the division of gastroenterology and hepatology at Indiana University, Indianapolis. Dr. Chalasani is vice president for academic affairs at Indiana University Health, Indianapolis, and the David W. Crabb Professor of Gastroenterology and Hepatology and an adjunct professor of anatomy, cell biology, and physiology in the division of gastroenterology and hepatology at Indiana University. Dr. Vilar-Gomez reports no financial conflicts of interest. Dr. Chalasani serves as a paid consultant to AbbVie, Boehringer-Ingelheim, Altimmune, Madrigal, Lilly, Zydus, and Galectin. He receives research support from Galectin and DSM.
References
1. Chalasani N et al. Hepatology 2018;67:328-57.
2. Söderberg C et al. Hepatology 2010;51:595-602.
3. Sanyal AJ et al. N Engl J Med 2021;385:1559-69.
4. Vilar-Gomez E et al. Gastroenterology 2018;155:443-57.e17.
5. Younossi ZM et al. Hepatology 2016;64:73-84.
6. EASL-EASD-EASO. J Hepatol 2016;64:1388-402.
7. Wong VW et al. J Hepatol 2018; 69:1349-56.
8. Vilar-Gomez E et al. Gastroenterology 2015;149:367-78.e5; quiz e14-5.
9. Promrat K et al. Hepatology 2010;51:121-9.
10. Wong VW et al. J Hepatol 2013;59:536-42.
11. Berzigotti A et al. Hepatology 2017;65:1293-1305.
12. Sacks FM et al. N Engl J Med 2009;360:859-73.
13. Vilar-Gomez E et al. Hepatology 2022 Jun;75(6):1491-1506.
14. Zelber-Sagi S et al. Liver Int 2017;37:936-49.
15. Hassani Zadeh S et al. J Gastroenterol Hepatol 2021;36:1470-8.
16. Yaskolka Meir A et al. Gut 2021;70:2085-95.
17. Sung KC et al. J Hepatol 2016;65:791-7.
18. Orci LA et al. Clin Gastroenterol Hepatol 2016;14:1398-411.
19. Ryu S et al. J Hepatol 2015;63:1229-37.
20. Kim D et al. Hepatology 2020;72:1556-68.
21. Kim D et al. Clin Gastroenterol Hepatol 2021;19:1240-7.e5.
22. Ascha MS et al. Hepatology 2010;51:1972-8.
23. Bambha K et al. Liver Int 2014;34:1250-8.
24. Lee Y et al. Clin Gastroenterol Hepatol 2019;17:1040-60.e11.
25. Grönroos S et al. JAMA Surg 2021;156:137-46.
26. Fakhry TK et al. Surg Obes Relat Dis 2019;15:502-11.
27. Seeberg KA et al. Ann Intern Med 2022;175:74-83.
28. Bower G et al. Obes Surg 2015;25:2280-9.
29. Jan A et al. Obes Surg 2015;25:1518-26.
30. Hanipah ZN et al. Obes Surg 2018;28:3431-8.
31. Are VS et al. Am J Gastroenterol 2020;115:1849-56.
32. Aminian A et al. JAMA 2021;326:2031-42.
33. Vuppalanchi R et al. Ann Surg 2022;275:e174-80.
34. Simon TG et al. Gut 2021. doi: 10.1136/gutjnl-2021-325724.
35. Lonardo A et al. J Hepatol 2018;68:335-52.
36. Chalasani N et al. Gastroenterology 2004;126:1287-92.
37. Pastori D et al. Dig Liver Dis 2015;47:4-11.
38. Kim RG et al. Clin Gastroenterol Hepatol 2017;15:1521-30.e8.
39. Ahmad J et al. Dig Dis Sci 2017;62:3596-604.
40. Chalasani NP et al. Am J Gastroenterol 2021;116(5):878-98.
41. Rinella ME et al. Hepatology 2019;70:1424-36.
42. Younossi ZM et al. Lancet 2019;394:2184-96.
43. Ratziu V. Clin Liver Dis (Hoboken) 2021;17:398-400.
44. Sanyal AJ et al. N Engl J Med 2010;341:1675-85.
45. Schürks M et al. BMJ 2010;341:c5702.
46. Cusi K et al. Ann Intern Med 2016;165:305-15.
47. Lewis JD et al. JAMA 2015;314:265-77.
48. Billington EO et al. Diabetologia 2015;58:2238-46.
49. Lewis JD et al. Diabetes Care 2011;34:916-22.
50. Erdmann E et al. Diabetes Care 2007;30:2773-8.
51. Viscoli CM et al. J Clin Endocrinol Metab 2017;102:914-22.
52. Armstong MJ et al. Lancet 2016;387:679-90.
53. Newsome PN et al. N Engl J Med 2021;384:1113-24.
54. Ng CH et al. Hepatology 2022;75:1647-61.
55. Kanwal F et al. Gastroenterology 2021;161:1030-1042.e8.
56. Garcia-Tsao G et al. Hepatology 2017;65:310-35.
57. Heimbach JK et al. Hepatology 2018;67:358-80.
Hospital Readmissions in Patients with Cirrhosis: A Systematic Review
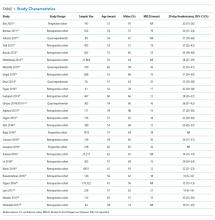
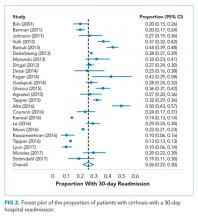
Cirrhosis is a morbid condition characterized by complications such as ascites, gastrointestinal bleeding, and hepatic encephalopathy. These complications frequently require hospitalization, which is a substantial burden to the healthcare system. In 2012, liver disease was responsible for nearly 250,000 admissions across the United States, costing $3 billion.1 Despite this substantial resource utilization, outcomes remain poor, with an inpatient mortality of 6%. For those that survive, many experience hospital readmission.
More generally, early readmission reflects poor quality of care in the US. In 2004, 30-day readmissions occurred in nearly 20% of Medicare beneficiaries and costed over $17 billion.2 In response to this problem, the Affordable Care Act established the Hospital Readmissions Reduction Program (HRRP), which reduces Centers for Medicare & Medicaid Services (CMS) payments to hospitals with excess 30-day readmissions for high-risk conditions, including pneumonia and heart failure.3 Heart failure, in particular, has been the subject of numerous studies detailing risk factors and interventions to predict and prevent readmission.4-6 Based on this extensive evidence, guidelines recommend disease management programs to reduce readmissions in this population.7 In contrast, readmission in the cirrhosis population has received limited attention.
We therefore conducted a systematic review aiming to examine the range of readmission risk noted in the literature, with a focus on the model for end-stage liver disease (MELD) score as a risk factor for readmission.
METHODS
Search Strategy
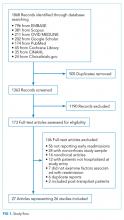
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines for conducting and reporting systematic reviews.8 A literature search was performed by a medical librarian using the following databases: Ovid MEDLINE, PubMed, EMBASE, CINAHL, the full Cochrane Library, Scopus, Google Scholar, and ClinicalTrials.gov. All the databases were searched from 2000 to May 2017. We did not include older reports because the review focused on contemporary care; earlier studies may not reflect current cirrhosis management. To ensure literature saturation, included articles’ reference lists were reviewed.
Search strategies were developed by combining database-specific subject headings and keywords for readmissions with those for cirrhosis or its complications (Supplementary Material). Google Scholar and ClinicalTrials.gov were searched using keywords only. All results were limited to the English language and those published in 2000 or later, but no other limits were applied.
Identified records were reviewed based on strict criteria. We excluded case reports, case series, reviews, editorials, letters, and meeting abstracts without final peer-reviewed publication. We also excluded studies of pediatric populations (age < 18 years), patients without cirrhosis, and patients with liver transplants. We excluded studies in which patients were not hospitalized at study onset and those where the index admission was for an elective procedure. Because our interest was to identify factors associated with early readmission, we excluded studies that did not report readmissions within 90 days or those with a mean or median follow-up of less than 30 days. We also excluded studies that did not examine the association between readmission and at least 1 independent variable or intervention. Duplicate reports of a common sample were excluded unless the duplicate provided additional information, and such reports were examined together in our synthesis.
Two authors identified potentially eligible records by independently screening titles and abstracts. At this stage, records that did not meet the eligibility criteria were excluded, and the reasons for exclusion were not recorded. Records with disagreement were retained for full-text review. After this initial exclusion of records, the remaining full-text records were reviewed independently. For this full-text review, we recorded exclusion reasons and disagreements were resolved through discussion.
Data Collection
Data were abstracted from each study by 2 authors independently and recorded in a REDCap database.9 Discrepancies were resolved through discussion. We recorded study characteristics, including study design, setting, population (including the inclusion/exclusion criteria, sample size, and patient and hospitalization characteristics), interventions, and comparisons. To facilitate comparisons across studies, we employed validated methods to approximate means and standard deviations (SD).10 We recorded detailed information on outcomes including readmissions, preventability, independent variables, and mortality. Studies that focused on a single independent factor or intervention were classified as “focused,” while those that examined multiple factors were classified as “broad.” We used the Newcastle–Ottawa Scale to assess the risk of bias in each study.11 This instrument uses a 9-point scale to gauge methodological quality based on selection, group comparability, and exposure/outcome assessment.
Statistical Analysis
Analyses were performed using Stata 13.1 (StataCorp LP, College Station, Texas). We determined the pooled proportion of patients with 30-day readmission using a random-effects model, with the Freeman–Tukey double-arcsine transformation for meta-analysis of proportions.12 We investigated the heterogeneity by stratifying analyses according to prespecified study characteristics, including “broad” versus “focused.” However, the readmission risk was not different in the stratified analysis; therefore, we chose to pool the findings. For point estimates, 95% confidence intervals (CIs) were calculated, and a P-value < .05 was considered statistically significant.
RESULTS
Search Results
Study Characteristics
Two studies were performed in Australia, 4 in Europe, and the remainder in North America. Twenty one of the 26 studies were retrospective cohort studies (Table 1). Twenty studies were single-center studies (of which half were performed at transplant centers), and 4 of the 6 multicenter studies were based on administrative data with large samples (173,254 patients). The inclusion/exclusion criteria varied widely (Supplementary Material). Some studies only included patients admitted for specific cirrhosis complications, while others included those admitted for any reason. Two studies excluded patients admitted in the prior 30 days, and 6 excluded patients discharged to hospice. The mean risk of bias score was 7.5 (SD 1.3) out of a possible 9 points, with most lacking an adequate description of follow-up and several lacking adjustment for confounders.
Outcomes
Readmission and MELD
DISCUSSION
Hospital readmission is a costly and common problem in the US.2 In addition to the negative impact that readmissions have on patients’ lives,40 readmissions are increasingly being used to measure quality. Unplanned 30-day readmissions are posted publicly, and excess readmissions for high-risk conditions are penalized through HRRP.3 Although HRRP does not currently include cirrhosis, the program has expanded to include several conditions that were not included in the initial iteration. Whether cirrhosis will be included in future iterations remains to be seen; however, increasing scrutiny is likely to continue. Of specific populations at risk, patients with cirrhosis are particularly vulnerable due to several features. Ascites management often requires hospitalization due to diuretic titration and poor access to paracentesis, and hepatic encephalopathy treatment requires complex lactulose titration.16 Other features of cirrhosis, such as gastrointestinal bleeding, infections, and renal failure, also place patients at risk of poor outcomes. The resulting readmission burden is high, with a pooled 30-day readmission rate of 26%. Other associated outcomes are also poor, with a consistent relationship between readmission and subsequent mortality.
We found striking heterogeneity in various aspects. First, the inclusion/exclusion criteria varied widely, both cirrhosis-specific (eg, spontaneous bacterial peritonitis) and more general (patients admitted within the prior 30 days). Some of these criteria may bias readmission estimates; the risk of readmission may be reduced in those on hospice, as patients forgo curative therapy. Additionally, an established risk factor for readmission is prior hospitalization41; excluding patients with prior admissions prohibits analysis of this variable. Another aspect is the capture of readmissions: readmissions outside of the index hospital were not included in most studies. In those that did include outside readmissions, the burden was sizeable: 17% in 1 single-center study and 23% in a multistate administrative database.16,36 These outside readmissions must be included in future studies; they are as important as same-center readmissions both to patients and CMS.3 Despite this heterogeneity, the studies scored relatively high on the Newcastle–Ottawa risk of bias scale, with the only common deficiency being an inadequate description of follow-up.
Building on the findings of this review, an important step will be the design of interventions to reduce readmissions. Such interventions require a full understanding of this population’s characteristics and needs. Critically, we found a lack of data on social determinants of health. Impairments in these factors are well-established contributors to readmission risk in other populations,4,40 and are highly prevalent in cirrhosis.42 Indeed, CMS has focused resources toward social determinants of health in the effort to reduce utilization and improve outcomes. This lack of data on social determinants of health, as well as other understudied factors, represents an important opportunity for future research efforts to better define the modifiable features that could be targeted in the future to prevent readmissions. Such research is urgently needed and will likely require prospective studies to gather these important factors. Notably, most studies in this systematic review were retrospective and therefore unable to examine many of these understudied factors. Another important aspect that has received little attention is readmission preventability: only 2 studies assessed preventability, both through unstructured chart review. Preventability assessments in noncirrhotic populations have used wide-ranging methodologies, yielding inconsistent results.43 This variability prompted recommendations that preventability should be assessed by multiple reviewers guided by explicit parameters.43 Such detailed attention to preventability is urgently needed to better inform interventions.
In contrast to the lack of data on social factors, we found that the MELD score was examined in most studies and was frequently associated with readmission. Despite this consistent association, differences in the MELD scores between studies limit inferences into specific cutoff values that could identify the highest risk patients. Because of its existing widespread clinical use, the MELD score may prove to be important in readmission risk stratification. Efforts to develop a useful model including the MELD score are needed to target interventions to the highest risk patients.
This review has several limitations. Although we used a broad search strategy to capture studies, some may not have been included due to our selection criteria. For instance, 1 retrospective paper described factors associated with high admission density during 1 year but did not specifically report the frequency of early readmissions.44 Similarly, a randomized trial of a disease management program did not specifically examine early readmissions.45 Another quasi-experimental study of a quality improvement initiative was not included because a large proportion of their subjects was post liver transplant.46 However, the inclusion of these papers is unlikely to change our conclusions; the retrospective study identified factors similar to those in the included studies, and the quasi-experimental study overlapped with the included study that assessed frailty.27 Another potential limitation is the exclusion of studies published in abstract form only. Such studies may be important, as the field of cirrhosis readmissions is relatively young. However, including only full-paper publications ensures the inclusion of only higher quality studies scrutinized during the peer-review process. Similarly, newer published studies may have been missed due to the abundant interest in this topic and ongoing research. Lastly, the significant heterogeneity of the studies limits conclusions that can be made regarding the pooled readmission rates.
In summary, we found that patients with cirrhosis experience a high incidence of hospital readmissions. Several processes of care may be associated with readmissions, suggesting room for improvement in caring for this population and reducing readmissions. However, we identified several gaps in the literature, which does not adequately describe social factors and is lacking details on readmission preventability assessment. Future studies should attempt to address these issues so that interventions can be targeted to the highest risk patients and designed to best meet the needs of patients with cirrhosis.
Disclosures
Dr. Orman, Dr. Ghabril, and Dr. Emmett report no potential conflicts of interest. Dr. Chalasani reports personal fees from Lilly, personal fees from Abbvie, personal fees from Tobira/Allergan, personal fees from Ardelyx, personal fees from Amarin, personal fees from Shire, personal fees from Madrigal, personal fees from DS Biopharma (Afimmune), personal fees from Cempra, personal fees from NuSirt, grants from Galectin, grants from Gilead, grants from Intercept, grants from Cumberland, grants from Conatus, personal fees from Immuron, and personal fees from Axovant, outside the submitted work.
Funding Information
This work was supported, in part, by the National Institutes of Health, KL2 TR001106 and K23 DK109202
1. Peery AF, Crockett SD, Barritt AS, et al. Burden of gastrointestinal, liver, and pancreatic diseases in the United States. Gastroenterology. 2015;149(7):1731-1741.e3. DOI: 10.1053/j.gastro.2015.08.045. PubMed
13. Bini EJ, Weinshel EH, Generoso R, et al. Impact of gastroenterology consultation on the outcomes of patients admitted to the hospital with decompensated cirrhosis. Hepatology. 2001;34(6):1089-1095. DOI: 10.1053/jhep.2001.29204. PubMed
14. Berman K, Tandra S, Forssell K, et al. Incidence and predictors of 30-day readmission among patients hospitalized for advanced liver disease. Clin Gastroenterol Hepatol. 2011;9(3):254-259. DOI: 10.1016/j.cgh.2010.10.035. PubMed
15. Johnson EA, Spier BJ, Leff JA, Lucey MR, Said A. Optimising the care of patients with cirrhosis and gastrointestinal haemorrhage: a quality improvement study. Aliment Pharmacol Ther. 2011;34(1):76-82. DOI: 10.1111/j.1365-2036.2011.04692.x. PubMed
16. Volk ML, Tocco RS, Bazick J, Rakoski MO, Lok AS. Hospital readmissions among patients with decompensated cirrhosis. Am J Gastroenterol. 2012;107(2):247-252. DOI: 10.1038/ajg.2011.314. PubMed
17. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Clinical outcomes after bedside and interventional radiology paracentesis procedures. Am J Med. 2013;126(4):349-356. DOI: 10.1016/j.amjmed.2012.09.016. PubMed
18. Deitelzweig S, Amin A, Christian R, Friend K, Lin J, Lowe TJ. Hyponatremia-associated healthcare burden among US patients hospitalized for cirrhosis. Adv Ther. 2013;30(1):71-80. DOI: 10.1007/s12325-012-0073-1. PubMed
19. Morando F, Maresio G, Piano S, et al. How to improve care in outpatients with cirrhosis and ascites: a new model of care coordination by consultant hepatologists. J Hepatol. 2013;59(2):257-264. DOI: 10.1016/j.jhep.2013.03.010. PubMed
20. Singal AG, Rahimi RS, Clark C, et al. An automated model using electronic medical record data identifies patients with cirrhosis at high risk for readmission. Clin Gastroenterol Hepatol. 2013;11(10):1335-1341.e1. DOI: 10.1016/j.cgh.2013.03.022. PubMed
21. Desai AP, Satoskar R, Appannagari A, et al. Co-management between hospitalist and hepatologist improves the quality of care of inpatients with chronic liver disease. J Clin Gastroenterol. 2014;48(4):e30-e36. DOI: 10.1097/MCG.0b013e3182a87f70. PubMed
22. Fagan KJ, Zhao EY, Horsfall LU, et al. Burden of decompensated cirrhosis and ascites on hospital services in a tertiary care facility: time for change? Intern Med J. 2014;44(9):865-872. DOI: 10.1111/imj.12491. PubMed
23. Gaduputi V, Chandrala C, Abbas N, Tariq H, Chilimuri S, Balar B. Prognostic significance of hypokalemia in hepatic encephalopathy. Hepatogastroenterology. 2014;61(133):1170-1174. PubMed
26. Agrawal K, Kumar P, Markert R, Agrawal S. Risk factors for 30-day readmissions of individuals with decompensated cirrhosis. South Med J. 2015;108(11):682-687. DOI: 10.14423/SMJ.0000000000000371. PubMed
27. Tapper EB, Finkelstein D, Mittleman MA, Piatkowski G, Lai M. Standard assessments of frailty are validated predictors of mortality in hospitalized patients with cirrhosis. Hepatology. 2015;62(2):584-590. DOI: 10.1002/hep.27830. PubMed
28. Atla PR, Sheikh MY, Gill F, Kundu R, Choudhury J. Predictors of hospital re-admissions among Hispanics with hepatitis C-related cirrhosis. Ann Gastroenterol. 2016;29(4):515-520. DOI: 10.20524/aog.2016.0072. PubMed
29. Bajaj JS, Reddy KR, Tandon P, et al. The 3-month readmission rate remains unacceptably high in a large North American cohort of patients with cirrhosis. Hepatology. 2016;64(1):200-208. DOI: 10.1002/hep.28414. PubMed
30. Courson A, Jones GM, Twilla JD. Treatment of acute hepatic encephalopathy: comparing the effects of adding rifaximin to lactulose on patient outcomes. J Pharm Pract. 2016;29(3):212-217. DOI: 10.1177/0897190014566312. PubMed
31. Graupera I, Solà E, Fabrellas N, et al. Urine monocyte chemoattractant protein-1 is an independent predictive factor of hospital readmission and survival in cirrhosis. PLOS ONE. 2016;11(6):e0157371. DOI: 10.1371/journal.pone.0157371. PubMed
32. Kanwal F, Asch SM, Kramer JR, Cao Y, Asrani S, El-Serag HB. Early outpatient follow-up and 30-day outcomes in patients hospitalized with cirrhosis. Hepatology. 2016;64(2):569-581. DOI: 10.1002/hep.28558. PubMed
46. Tapper EB, Finkelstein D, Mittleman MA, Piatkowski G, Chang M, Lai M. A quality improvement initiative reduces 30-day rate of readmission for patients with cirrhosis. Clin Gastroenterol Hepatol. 2016;14(5):753-759. DOI: 10.1016/j.cgh.2015.08.041. PubMed
45. Wigg AJ, McCormick R, Wundke R, Woodman RJ. Efficacy of a chronic disease management model for patients with chronic liver failure. Clin Gastroenterol Hepatol. 2013;11(7):850-8.e1. DOI: 10.1016/j.cgh.2013.01.014. PubMed
44. Ganesh S, Rogal SS, Yadav D, Humar A, Behari J. Risk factors for frequent readmissions and barriers to transplantation in patients with cirrhosis. PLOS ONE. 2013;8(1):e55140. DOI: 10.1371/journal.pone.0055140. PubMed
43. van Walraven C, Bennett C, Jennings A, Austin PC, Forster AJ. Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ. 2011;183(7):E391-E402. DOI: 10.1503/cmaj.101860. PubMed
42. Bajaj JS, Wade JB, Gibson DP, et al. The multi-dimensional burden of cirrhosis and hepatic encephalopathy on patients and caregivers. Am J Gastroenterol. 2011;106(9):1646-1653. DOI: 10.1038/ajg.2011.157. PubMed
41. van Walraven C, Dhalla IA, Bell C, et al. Derivation and validation of an index to predict early death or unplanned readmission after discharge from hospital to the community. CMAJ. 2010;182(6):551-557. DOI: 10.1503/cmaj.091117. PubMed
40. Rodríguez-Artalejo F, Guallar-Castillón P, Pascual CR, et al. Health-related quality of life as a predictor of hospital readmission and death among patients with heart failure. Arch Intern Med. 2005;165(11):1274-1279. DOI: 10.1001/archinte.165.11.1274. PubMed
39. Strömdahl M, Helgeson J, Kalaitzakis E. Emergency readmission following acute upper gastrointestinal bleeding. Eur J Gastroenterol Hepatol. 2017;29(1):73-77. DOI: 10.1097/MEG.0000000000000746. PubMed
38. Morales BP, Planas R, Bartoli R, et al. Early hospital readmission in decompensated cirrhosis: incidence, impact on mortality, and predictive factors. Dig Liver Dis. 2017;49(8):903-909. DOI: 10.1016/j.dld.2017.03.005. PubMed
37. Lyon KC, Likar E, Martello JL, Regier M. Retrospective cross-sectional pilot study of rifaximin dosing for the prevention of recurrent hepatic encephalopathy. J Gastroenterol Hepatol. 2017;32(9):1548-1552. DOI: 10.1111/jgh.13759. PubMed
36. Tapper EB, Halbert B, Mellinger J. Rates of and reasons for hospital readmissions in patients with cirrhosis: a multistate population-based cohort study. Clin Gastroenterol Hepatol. 2016;14(8):1181-1188.e2. DOI: 10.1016/j.cgh.2016.04.009. PubMed
35. Rassameehiran S, Mankongpaisarnrung C, Sutamtewagul G, Klomjit S, Rakvit A. Predictor of 90-day readmission rate for hepatic encephalopathy. South Med J. 2016;109(6):365-369. DOI: 10.14423/SMJ.0000000000000475. PubMed
34. Moon AM, Dominitz JA, Ioannou GN, Lowy E, Beste LA. Use of antibiotics among patients with cirrhosis and upper gastrointestinal bleeding is associated with reduced mortality. Clin Gastroenterol Hepatol. 2016;14(11):1629-1637.e1. DOI: 10.1016/j.cgh.2016.05.040. PubMed
33. Le S, Spelman T, Chong CP, et al. Could adherence to quality of care indicators for hospitalized patients with cirrhosis-related ascites improve clinical outcomes? Am J Gastroenterol. 2016;111(1):87-92. DOI: .10.1038/ajg.2015.402. PubMed
Cirrhosis is a morbid condition characterized by complications such as ascites, gastrointestinal bleeding, and hepatic encephalopathy. These complications frequently require hospitalization, which is a substantial burden to the healthcare system. In 2012, liver disease was responsible for nearly 250,000 admissions across the United States, costing $3 billion.1 Despite this substantial resource utilization, outcomes remain poor, with an inpatient mortality of 6%. For those that survive, many experience hospital readmission.
More generally, early readmission reflects poor quality of care in the US. In 2004, 30-day readmissions occurred in nearly 20% of Medicare beneficiaries and costed over $17 billion.2 In response to this problem, the Affordable Care Act established the Hospital Readmissions Reduction Program (HRRP), which reduces Centers for Medicare & Medicaid Services (CMS) payments to hospitals with excess 30-day readmissions for high-risk conditions, including pneumonia and heart failure.3 Heart failure, in particular, has been the subject of numerous studies detailing risk factors and interventions to predict and prevent readmission.4-6 Based on this extensive evidence, guidelines recommend disease management programs to reduce readmissions in this population.7 In contrast, readmission in the cirrhosis population has received limited attention.
We therefore conducted a systematic review aiming to examine the range of readmission risk noted in the literature, with a focus on the model for end-stage liver disease (MELD) score as a risk factor for readmission.
METHODS
Search Strategy
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines for conducting and reporting systematic reviews.8 A literature search was performed by a medical librarian using the following databases: Ovid MEDLINE, PubMed, EMBASE, CINAHL, the full Cochrane Library, Scopus, Google Scholar, and ClinicalTrials.gov. All the databases were searched from 2000 to May 2017. We did not include older reports because the review focused on contemporary care; earlier studies may not reflect current cirrhosis management. To ensure literature saturation, included articles’ reference lists were reviewed.
Search strategies were developed by combining database-specific subject headings and keywords for readmissions with those for cirrhosis or its complications (Supplementary Material). Google Scholar and ClinicalTrials.gov were searched using keywords only. All results were limited to the English language and those published in 2000 or later, but no other limits were applied.
Identified records were reviewed based on strict criteria. We excluded case reports, case series, reviews, editorials, letters, and meeting abstracts without final peer-reviewed publication. We also excluded studies of pediatric populations (age < 18 years), patients without cirrhosis, and patients with liver transplants. We excluded studies in which patients were not hospitalized at study onset and those where the index admission was for an elective procedure. Because our interest was to identify factors associated with early readmission, we excluded studies that did not report readmissions within 90 days or those with a mean or median follow-up of less than 30 days. We also excluded studies that did not examine the association between readmission and at least 1 independent variable or intervention. Duplicate reports of a common sample were excluded unless the duplicate provided additional information, and such reports were examined together in our synthesis.
Two authors identified potentially eligible records by independently screening titles and abstracts. At this stage, records that did not meet the eligibility criteria were excluded, and the reasons for exclusion were not recorded. Records with disagreement were retained for full-text review. After this initial exclusion of records, the remaining full-text records were reviewed independently. For this full-text review, we recorded exclusion reasons and disagreements were resolved through discussion.
Data Collection
Data were abstracted from each study by 2 authors independently and recorded in a REDCap database.9 Discrepancies were resolved through discussion. We recorded study characteristics, including study design, setting, population (including the inclusion/exclusion criteria, sample size, and patient and hospitalization characteristics), interventions, and comparisons. To facilitate comparisons across studies, we employed validated methods to approximate means and standard deviations (SD).10 We recorded detailed information on outcomes including readmissions, preventability, independent variables, and mortality. Studies that focused on a single independent factor or intervention were classified as “focused,” while those that examined multiple factors were classified as “broad.” We used the Newcastle–Ottawa Scale to assess the risk of bias in each study.11 This instrument uses a 9-point scale to gauge methodological quality based on selection, group comparability, and exposure/outcome assessment.
Statistical Analysis
Analyses were performed using Stata 13.1 (StataCorp LP, College Station, Texas). We determined the pooled proportion of patients with 30-day readmission using a random-effects model, with the Freeman–Tukey double-arcsine transformation for meta-analysis of proportions.12 We investigated the heterogeneity by stratifying analyses according to prespecified study characteristics, including “broad” versus “focused.” However, the readmission risk was not different in the stratified analysis; therefore, we chose to pool the findings. For point estimates, 95% confidence intervals (CIs) were calculated, and a P-value < .05 was considered statistically significant.
RESULTS
Search Results
Study Characteristics
Two studies were performed in Australia, 4 in Europe, and the remainder in North America. Twenty one of the 26 studies were retrospective cohort studies (Table 1). Twenty studies were single-center studies (of which half were performed at transplant centers), and 4 of the 6 multicenter studies were based on administrative data with large samples (173,254 patients). The inclusion/exclusion criteria varied widely (Supplementary Material). Some studies only included patients admitted for specific cirrhosis complications, while others included those admitted for any reason. Two studies excluded patients admitted in the prior 30 days, and 6 excluded patients discharged to hospice. The mean risk of bias score was 7.5 (SD 1.3) out of a possible 9 points, with most lacking an adequate description of follow-up and several lacking adjustment for confounders.
Outcomes
Readmission and MELD
DISCUSSION
Hospital readmission is a costly and common problem in the US.2 In addition to the negative impact that readmissions have on patients’ lives,40 readmissions are increasingly being used to measure quality. Unplanned 30-day readmissions are posted publicly, and excess readmissions for high-risk conditions are penalized through HRRP.3 Although HRRP does not currently include cirrhosis, the program has expanded to include several conditions that were not included in the initial iteration. Whether cirrhosis will be included in future iterations remains to be seen; however, increasing scrutiny is likely to continue. Of specific populations at risk, patients with cirrhosis are particularly vulnerable due to several features. Ascites management often requires hospitalization due to diuretic titration and poor access to paracentesis, and hepatic encephalopathy treatment requires complex lactulose titration.16 Other features of cirrhosis, such as gastrointestinal bleeding, infections, and renal failure, also place patients at risk of poor outcomes. The resulting readmission burden is high, with a pooled 30-day readmission rate of 26%. Other associated outcomes are also poor, with a consistent relationship between readmission and subsequent mortality.
We found striking heterogeneity in various aspects. First, the inclusion/exclusion criteria varied widely, both cirrhosis-specific (eg, spontaneous bacterial peritonitis) and more general (patients admitted within the prior 30 days). Some of these criteria may bias readmission estimates; the risk of readmission may be reduced in those on hospice, as patients forgo curative therapy. Additionally, an established risk factor for readmission is prior hospitalization41; excluding patients with prior admissions prohibits analysis of this variable. Another aspect is the capture of readmissions: readmissions outside of the index hospital were not included in most studies. In those that did include outside readmissions, the burden was sizeable: 17% in 1 single-center study and 23% in a multistate administrative database.16,36 These outside readmissions must be included in future studies; they are as important as same-center readmissions both to patients and CMS.3 Despite this heterogeneity, the studies scored relatively high on the Newcastle–Ottawa risk of bias scale, with the only common deficiency being an inadequate description of follow-up.
Building on the findings of this review, an important step will be the design of interventions to reduce readmissions. Such interventions require a full understanding of this population’s characteristics and needs. Critically, we found a lack of data on social determinants of health. Impairments in these factors are well-established contributors to readmission risk in other populations,4,40 and are highly prevalent in cirrhosis.42 Indeed, CMS has focused resources toward social determinants of health in the effort to reduce utilization and improve outcomes. This lack of data on social determinants of health, as well as other understudied factors, represents an important opportunity for future research efforts to better define the modifiable features that could be targeted in the future to prevent readmissions. Such research is urgently needed and will likely require prospective studies to gather these important factors. Notably, most studies in this systematic review were retrospective and therefore unable to examine many of these understudied factors. Another important aspect that has received little attention is readmission preventability: only 2 studies assessed preventability, both through unstructured chart review. Preventability assessments in noncirrhotic populations have used wide-ranging methodologies, yielding inconsistent results.43 This variability prompted recommendations that preventability should be assessed by multiple reviewers guided by explicit parameters.43 Such detailed attention to preventability is urgently needed to better inform interventions.
In contrast to the lack of data on social factors, we found that the MELD score was examined in most studies and was frequently associated with readmission. Despite this consistent association, differences in the MELD scores between studies limit inferences into specific cutoff values that could identify the highest risk patients. Because of its existing widespread clinical use, the MELD score may prove to be important in readmission risk stratification. Efforts to develop a useful model including the MELD score are needed to target interventions to the highest risk patients.
This review has several limitations. Although we used a broad search strategy to capture studies, some may not have been included due to our selection criteria. For instance, 1 retrospective paper described factors associated with high admission density during 1 year but did not specifically report the frequency of early readmissions.44 Similarly, a randomized trial of a disease management program did not specifically examine early readmissions.45 Another quasi-experimental study of a quality improvement initiative was not included because a large proportion of their subjects was post liver transplant.46 However, the inclusion of these papers is unlikely to change our conclusions; the retrospective study identified factors similar to those in the included studies, and the quasi-experimental study overlapped with the included study that assessed frailty.27 Another potential limitation is the exclusion of studies published in abstract form only. Such studies may be important, as the field of cirrhosis readmissions is relatively young. However, including only full-paper publications ensures the inclusion of only higher quality studies scrutinized during the peer-review process. Similarly, newer published studies may have been missed due to the abundant interest in this topic and ongoing research. Lastly, the significant heterogeneity of the studies limits conclusions that can be made regarding the pooled readmission rates.
In summary, we found that patients with cirrhosis experience a high incidence of hospital readmissions. Several processes of care may be associated with readmissions, suggesting room for improvement in caring for this population and reducing readmissions. However, we identified several gaps in the literature, which does not adequately describe social factors and is lacking details on readmission preventability assessment. Future studies should attempt to address these issues so that interventions can be targeted to the highest risk patients and designed to best meet the needs of patients with cirrhosis.
Disclosures
Dr. Orman, Dr. Ghabril, and Dr. Emmett report no potential conflicts of interest. Dr. Chalasani reports personal fees from Lilly, personal fees from Abbvie, personal fees from Tobira/Allergan, personal fees from Ardelyx, personal fees from Amarin, personal fees from Shire, personal fees from Madrigal, personal fees from DS Biopharma (Afimmune), personal fees from Cempra, personal fees from NuSirt, grants from Galectin, grants from Gilead, grants from Intercept, grants from Cumberland, grants from Conatus, personal fees from Immuron, and personal fees from Axovant, outside the submitted work.
Funding Information
This work was supported, in part, by the National Institutes of Health, KL2 TR001106 and K23 DK109202
Cirrhosis is a morbid condition characterized by complications such as ascites, gastrointestinal bleeding, and hepatic encephalopathy. These complications frequently require hospitalization, which is a substantial burden to the healthcare system. In 2012, liver disease was responsible for nearly 250,000 admissions across the United States, costing $3 billion.1 Despite this substantial resource utilization, outcomes remain poor, with an inpatient mortality of 6%. For those that survive, many experience hospital readmission.
More generally, early readmission reflects poor quality of care in the US. In 2004, 30-day readmissions occurred in nearly 20% of Medicare beneficiaries and costed over $17 billion.2 In response to this problem, the Affordable Care Act established the Hospital Readmissions Reduction Program (HRRP), which reduces Centers for Medicare & Medicaid Services (CMS) payments to hospitals with excess 30-day readmissions for high-risk conditions, including pneumonia and heart failure.3 Heart failure, in particular, has been the subject of numerous studies detailing risk factors and interventions to predict and prevent readmission.4-6 Based on this extensive evidence, guidelines recommend disease management programs to reduce readmissions in this population.7 In contrast, readmission in the cirrhosis population has received limited attention.
We therefore conducted a systematic review aiming to examine the range of readmission risk noted in the literature, with a focus on the model for end-stage liver disease (MELD) score as a risk factor for readmission.
METHODS
Search Strategy
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines for conducting and reporting systematic reviews.8 A literature search was performed by a medical librarian using the following databases: Ovid MEDLINE, PubMed, EMBASE, CINAHL, the full Cochrane Library, Scopus, Google Scholar, and ClinicalTrials.gov. All the databases were searched from 2000 to May 2017. We did not include older reports because the review focused on contemporary care; earlier studies may not reflect current cirrhosis management. To ensure literature saturation, included articles’ reference lists were reviewed.
Search strategies were developed by combining database-specific subject headings and keywords for readmissions with those for cirrhosis or its complications (Supplementary Material). Google Scholar and ClinicalTrials.gov were searched using keywords only. All results were limited to the English language and those published in 2000 or later, but no other limits were applied.
Identified records were reviewed based on strict criteria. We excluded case reports, case series, reviews, editorials, letters, and meeting abstracts without final peer-reviewed publication. We also excluded studies of pediatric populations (age < 18 years), patients without cirrhosis, and patients with liver transplants. We excluded studies in which patients were not hospitalized at study onset and those where the index admission was for an elective procedure. Because our interest was to identify factors associated with early readmission, we excluded studies that did not report readmissions within 90 days or those with a mean or median follow-up of less than 30 days. We also excluded studies that did not examine the association between readmission and at least 1 independent variable or intervention. Duplicate reports of a common sample were excluded unless the duplicate provided additional information, and such reports were examined together in our synthesis.
Two authors identified potentially eligible records by independently screening titles and abstracts. At this stage, records that did not meet the eligibility criteria were excluded, and the reasons for exclusion were not recorded. Records with disagreement were retained for full-text review. After this initial exclusion of records, the remaining full-text records were reviewed independently. For this full-text review, we recorded exclusion reasons and disagreements were resolved through discussion.
Data Collection
Data were abstracted from each study by 2 authors independently and recorded in a REDCap database.9 Discrepancies were resolved through discussion. We recorded study characteristics, including study design, setting, population (including the inclusion/exclusion criteria, sample size, and patient and hospitalization characteristics), interventions, and comparisons. To facilitate comparisons across studies, we employed validated methods to approximate means and standard deviations (SD).10 We recorded detailed information on outcomes including readmissions, preventability, independent variables, and mortality. Studies that focused on a single independent factor or intervention were classified as “focused,” while those that examined multiple factors were classified as “broad.” We used the Newcastle–Ottawa Scale to assess the risk of bias in each study.11 This instrument uses a 9-point scale to gauge methodological quality based on selection, group comparability, and exposure/outcome assessment.
Statistical Analysis
Analyses were performed using Stata 13.1 (StataCorp LP, College Station, Texas). We determined the pooled proportion of patients with 30-day readmission using a random-effects model, with the Freeman–Tukey double-arcsine transformation for meta-analysis of proportions.12 We investigated the heterogeneity by stratifying analyses according to prespecified study characteristics, including “broad” versus “focused.” However, the readmission risk was not different in the stratified analysis; therefore, we chose to pool the findings. For point estimates, 95% confidence intervals (CIs) were calculated, and a P-value < .05 was considered statistically significant.
RESULTS
Search Results
Study Characteristics
Two studies were performed in Australia, 4 in Europe, and the remainder in North America. Twenty one of the 26 studies were retrospective cohort studies (Table 1). Twenty studies were single-center studies (of which half were performed at transplant centers), and 4 of the 6 multicenter studies were based on administrative data with large samples (173,254 patients). The inclusion/exclusion criteria varied widely (Supplementary Material). Some studies only included patients admitted for specific cirrhosis complications, while others included those admitted for any reason. Two studies excluded patients admitted in the prior 30 days, and 6 excluded patients discharged to hospice. The mean risk of bias score was 7.5 (SD 1.3) out of a possible 9 points, with most lacking an adequate description of follow-up and several lacking adjustment for confounders.
Outcomes
Readmission and MELD
DISCUSSION
Hospital readmission is a costly and common problem in the US.2 In addition to the negative impact that readmissions have on patients’ lives,40 readmissions are increasingly being used to measure quality. Unplanned 30-day readmissions are posted publicly, and excess readmissions for high-risk conditions are penalized through HRRP.3 Although HRRP does not currently include cirrhosis, the program has expanded to include several conditions that were not included in the initial iteration. Whether cirrhosis will be included in future iterations remains to be seen; however, increasing scrutiny is likely to continue. Of specific populations at risk, patients with cirrhosis are particularly vulnerable due to several features. Ascites management often requires hospitalization due to diuretic titration and poor access to paracentesis, and hepatic encephalopathy treatment requires complex lactulose titration.16 Other features of cirrhosis, such as gastrointestinal bleeding, infections, and renal failure, also place patients at risk of poor outcomes. The resulting readmission burden is high, with a pooled 30-day readmission rate of 26%. Other associated outcomes are also poor, with a consistent relationship between readmission and subsequent mortality.
We found striking heterogeneity in various aspects. First, the inclusion/exclusion criteria varied widely, both cirrhosis-specific (eg, spontaneous bacterial peritonitis) and more general (patients admitted within the prior 30 days). Some of these criteria may bias readmission estimates; the risk of readmission may be reduced in those on hospice, as patients forgo curative therapy. Additionally, an established risk factor for readmission is prior hospitalization41; excluding patients with prior admissions prohibits analysis of this variable. Another aspect is the capture of readmissions: readmissions outside of the index hospital were not included in most studies. In those that did include outside readmissions, the burden was sizeable: 17% in 1 single-center study and 23% in a multistate administrative database.16,36 These outside readmissions must be included in future studies; they are as important as same-center readmissions both to patients and CMS.3 Despite this heterogeneity, the studies scored relatively high on the Newcastle–Ottawa risk of bias scale, with the only common deficiency being an inadequate description of follow-up.
Building on the findings of this review, an important step will be the design of interventions to reduce readmissions. Such interventions require a full understanding of this population’s characteristics and needs. Critically, we found a lack of data on social determinants of health. Impairments in these factors are well-established contributors to readmission risk in other populations,4,40 and are highly prevalent in cirrhosis.42 Indeed, CMS has focused resources toward social determinants of health in the effort to reduce utilization and improve outcomes. This lack of data on social determinants of health, as well as other understudied factors, represents an important opportunity for future research efforts to better define the modifiable features that could be targeted in the future to prevent readmissions. Such research is urgently needed and will likely require prospective studies to gather these important factors. Notably, most studies in this systematic review were retrospective and therefore unable to examine many of these understudied factors. Another important aspect that has received little attention is readmission preventability: only 2 studies assessed preventability, both through unstructured chart review. Preventability assessments in noncirrhotic populations have used wide-ranging methodologies, yielding inconsistent results.43 This variability prompted recommendations that preventability should be assessed by multiple reviewers guided by explicit parameters.43 Such detailed attention to preventability is urgently needed to better inform interventions.
In contrast to the lack of data on social factors, we found that the MELD score was examined in most studies and was frequently associated with readmission. Despite this consistent association, differences in the MELD scores between studies limit inferences into specific cutoff values that could identify the highest risk patients. Because of its existing widespread clinical use, the MELD score may prove to be important in readmission risk stratification. Efforts to develop a useful model including the MELD score are needed to target interventions to the highest risk patients.
This review has several limitations. Although we used a broad search strategy to capture studies, some may not have been included due to our selection criteria. For instance, 1 retrospective paper described factors associated with high admission density during 1 year but did not specifically report the frequency of early readmissions.44 Similarly, a randomized trial of a disease management program did not specifically examine early readmissions.45 Another quasi-experimental study of a quality improvement initiative was not included because a large proportion of their subjects was post liver transplant.46 However, the inclusion of these papers is unlikely to change our conclusions; the retrospective study identified factors similar to those in the included studies, and the quasi-experimental study overlapped with the included study that assessed frailty.27 Another potential limitation is the exclusion of studies published in abstract form only. Such studies may be important, as the field of cirrhosis readmissions is relatively young. However, including only full-paper publications ensures the inclusion of only higher quality studies scrutinized during the peer-review process. Similarly, newer published studies may have been missed due to the abundant interest in this topic and ongoing research. Lastly, the significant heterogeneity of the studies limits conclusions that can be made regarding the pooled readmission rates.
In summary, we found that patients with cirrhosis experience a high incidence of hospital readmissions. Several processes of care may be associated with readmissions, suggesting room for improvement in caring for this population and reducing readmissions. However, we identified several gaps in the literature, which does not adequately describe social factors and is lacking details on readmission preventability assessment. Future studies should attempt to address these issues so that interventions can be targeted to the highest risk patients and designed to best meet the needs of patients with cirrhosis.
Disclosures
Dr. Orman, Dr. Ghabril, and Dr. Emmett report no potential conflicts of interest. Dr. Chalasani reports personal fees from Lilly, personal fees from Abbvie, personal fees from Tobira/Allergan, personal fees from Ardelyx, personal fees from Amarin, personal fees from Shire, personal fees from Madrigal, personal fees from DS Biopharma (Afimmune), personal fees from Cempra, personal fees from NuSirt, grants from Galectin, grants from Gilead, grants from Intercept, grants from Cumberland, grants from Conatus, personal fees from Immuron, and personal fees from Axovant, outside the submitted work.
Funding Information
This work was supported, in part, by the National Institutes of Health, KL2 TR001106 and K23 DK109202
1. Peery AF, Crockett SD, Barritt AS, et al. Burden of gastrointestinal, liver, and pancreatic diseases in the United States. Gastroenterology. 2015;149(7):1731-1741.e3. DOI: 10.1053/j.gastro.2015.08.045. PubMed
13. Bini EJ, Weinshel EH, Generoso R, et al. Impact of gastroenterology consultation on the outcomes of patients admitted to the hospital with decompensated cirrhosis. Hepatology. 2001;34(6):1089-1095. DOI: 10.1053/jhep.2001.29204. PubMed
14. Berman K, Tandra S, Forssell K, et al. Incidence and predictors of 30-day readmission among patients hospitalized for advanced liver disease. Clin Gastroenterol Hepatol. 2011;9(3):254-259. DOI: 10.1016/j.cgh.2010.10.035. PubMed
15. Johnson EA, Spier BJ, Leff JA, Lucey MR, Said A. Optimising the care of patients with cirrhosis and gastrointestinal haemorrhage: a quality improvement study. Aliment Pharmacol Ther. 2011;34(1):76-82. DOI: 10.1111/j.1365-2036.2011.04692.x. PubMed
16. Volk ML, Tocco RS, Bazick J, Rakoski MO, Lok AS. Hospital readmissions among patients with decompensated cirrhosis. Am J Gastroenterol. 2012;107(2):247-252. DOI: 10.1038/ajg.2011.314. PubMed
17. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Clinical outcomes after bedside and interventional radiology paracentesis procedures. Am J Med. 2013;126(4):349-356. DOI: 10.1016/j.amjmed.2012.09.016. PubMed
18. Deitelzweig S, Amin A, Christian R, Friend K, Lin J, Lowe TJ. Hyponatremia-associated healthcare burden among US patients hospitalized for cirrhosis. Adv Ther. 2013;30(1):71-80. DOI: 10.1007/s12325-012-0073-1. PubMed
19. Morando F, Maresio G, Piano S, et al. How to improve care in outpatients with cirrhosis and ascites: a new model of care coordination by consultant hepatologists. J Hepatol. 2013;59(2):257-264. DOI: 10.1016/j.jhep.2013.03.010. PubMed
20. Singal AG, Rahimi RS, Clark C, et al. An automated model using electronic medical record data identifies patients with cirrhosis at high risk for readmission. Clin Gastroenterol Hepatol. 2013;11(10):1335-1341.e1. DOI: 10.1016/j.cgh.2013.03.022. PubMed
21. Desai AP, Satoskar R, Appannagari A, et al. Co-management between hospitalist and hepatologist improves the quality of care of inpatients with chronic liver disease. J Clin Gastroenterol. 2014;48(4):e30-e36. DOI: 10.1097/MCG.0b013e3182a87f70. PubMed
22. Fagan KJ, Zhao EY, Horsfall LU, et al. Burden of decompensated cirrhosis and ascites on hospital services in a tertiary care facility: time for change? Intern Med J. 2014;44(9):865-872. DOI: 10.1111/imj.12491. PubMed
23. Gaduputi V, Chandrala C, Abbas N, Tariq H, Chilimuri S, Balar B. Prognostic significance of hypokalemia in hepatic encephalopathy. Hepatogastroenterology. 2014;61(133):1170-1174. PubMed
26. Agrawal K, Kumar P, Markert R, Agrawal S. Risk factors for 30-day readmissions of individuals with decompensated cirrhosis. South Med J. 2015;108(11):682-687. DOI: 10.14423/SMJ.0000000000000371. PubMed
27. Tapper EB, Finkelstein D, Mittleman MA, Piatkowski G, Lai M. Standard assessments of frailty are validated predictors of mortality in hospitalized patients with cirrhosis. Hepatology. 2015;62(2):584-590. DOI: 10.1002/hep.27830. PubMed
28. Atla PR, Sheikh MY, Gill F, Kundu R, Choudhury J. Predictors of hospital re-admissions among Hispanics with hepatitis C-related cirrhosis. Ann Gastroenterol. 2016;29(4):515-520. DOI: 10.20524/aog.2016.0072. PubMed
29. Bajaj JS, Reddy KR, Tandon P, et al. The 3-month readmission rate remains unacceptably high in a large North American cohort of patients with cirrhosis. Hepatology. 2016;64(1):200-208. DOI: 10.1002/hep.28414. PubMed
30. Courson A, Jones GM, Twilla JD. Treatment of acute hepatic encephalopathy: comparing the effects of adding rifaximin to lactulose on patient outcomes. J Pharm Pract. 2016;29(3):212-217. DOI: 10.1177/0897190014566312. PubMed
31. Graupera I, Solà E, Fabrellas N, et al. Urine monocyte chemoattractant protein-1 is an independent predictive factor of hospital readmission and survival in cirrhosis. PLOS ONE. 2016;11(6):e0157371. DOI: 10.1371/journal.pone.0157371. PubMed
32. Kanwal F, Asch SM, Kramer JR, Cao Y, Asrani S, El-Serag HB. Early outpatient follow-up and 30-day outcomes in patients hospitalized with cirrhosis. Hepatology. 2016;64(2):569-581. DOI: 10.1002/hep.28558. PubMed
46. Tapper EB, Finkelstein D, Mittleman MA, Piatkowski G, Chang M, Lai M. A quality improvement initiative reduces 30-day rate of readmission for patients with cirrhosis. Clin Gastroenterol Hepatol. 2016;14(5):753-759. DOI: 10.1016/j.cgh.2015.08.041. PubMed
45. Wigg AJ, McCormick R, Wundke R, Woodman RJ. Efficacy of a chronic disease management model for patients with chronic liver failure. Clin Gastroenterol Hepatol. 2013;11(7):850-8.e1. DOI: 10.1016/j.cgh.2013.01.014. PubMed
44. Ganesh S, Rogal SS, Yadav D, Humar A, Behari J. Risk factors for frequent readmissions and barriers to transplantation in patients with cirrhosis. PLOS ONE. 2013;8(1):e55140. DOI: 10.1371/journal.pone.0055140. PubMed
43. van Walraven C, Bennett C, Jennings A, Austin PC, Forster AJ. Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ. 2011;183(7):E391-E402. DOI: 10.1503/cmaj.101860. PubMed
42. Bajaj JS, Wade JB, Gibson DP, et al. The multi-dimensional burden of cirrhosis and hepatic encephalopathy on patients and caregivers. Am J Gastroenterol. 2011;106(9):1646-1653. DOI: 10.1038/ajg.2011.157. PubMed
41. van Walraven C, Dhalla IA, Bell C, et al. Derivation and validation of an index to predict early death or unplanned readmission after discharge from hospital to the community. CMAJ. 2010;182(6):551-557. DOI: 10.1503/cmaj.091117. PubMed
40. Rodríguez-Artalejo F, Guallar-Castillón P, Pascual CR, et al. Health-related quality of life as a predictor of hospital readmission and death among patients with heart failure. Arch Intern Med. 2005;165(11):1274-1279. DOI: 10.1001/archinte.165.11.1274. PubMed
39. Strömdahl M, Helgeson J, Kalaitzakis E. Emergency readmission following acute upper gastrointestinal bleeding. Eur J Gastroenterol Hepatol. 2017;29(1):73-77. DOI: 10.1097/MEG.0000000000000746. PubMed
38. Morales BP, Planas R, Bartoli R, et al. Early hospital readmission in decompensated cirrhosis: incidence, impact on mortality, and predictive factors. Dig Liver Dis. 2017;49(8):903-909. DOI: 10.1016/j.dld.2017.03.005. PubMed
37. Lyon KC, Likar E, Martello JL, Regier M. Retrospective cross-sectional pilot study of rifaximin dosing for the prevention of recurrent hepatic encephalopathy. J Gastroenterol Hepatol. 2017;32(9):1548-1552. DOI: 10.1111/jgh.13759. PubMed
36. Tapper EB, Halbert B, Mellinger J. Rates of and reasons for hospital readmissions in patients with cirrhosis: a multistate population-based cohort study. Clin Gastroenterol Hepatol. 2016;14(8):1181-1188.e2. DOI: 10.1016/j.cgh.2016.04.009. PubMed
35. Rassameehiran S, Mankongpaisarnrung C, Sutamtewagul G, Klomjit S, Rakvit A. Predictor of 90-day readmission rate for hepatic encephalopathy. South Med J. 2016;109(6):365-369. DOI: 10.14423/SMJ.0000000000000475. PubMed
34. Moon AM, Dominitz JA, Ioannou GN, Lowy E, Beste LA. Use of antibiotics among patients with cirrhosis and upper gastrointestinal bleeding is associated with reduced mortality. Clin Gastroenterol Hepatol. 2016;14(11):1629-1637.e1. DOI: 10.1016/j.cgh.2016.05.040. PubMed
33. Le S, Spelman T, Chong CP, et al. Could adherence to quality of care indicators for hospitalized patients with cirrhosis-related ascites improve clinical outcomes? Am J Gastroenterol. 2016;111(1):87-92. DOI: .10.1038/ajg.2015.402. PubMed
1. Peery AF, Crockett SD, Barritt AS, et al. Burden of gastrointestinal, liver, and pancreatic diseases in the United States. Gastroenterology. 2015;149(7):1731-1741.e3. DOI: 10.1053/j.gastro.2015.08.045. PubMed
13. Bini EJ, Weinshel EH, Generoso R, et al. Impact of gastroenterology consultation on the outcomes of patients admitted to the hospital with decompensated cirrhosis. Hepatology. 2001;34(6):1089-1095. DOI: 10.1053/jhep.2001.29204. PubMed
14. Berman K, Tandra S, Forssell K, et al. Incidence and predictors of 30-day readmission among patients hospitalized for advanced liver disease. Clin Gastroenterol Hepatol. 2011;9(3):254-259. DOI: 10.1016/j.cgh.2010.10.035. PubMed
15. Johnson EA, Spier BJ, Leff JA, Lucey MR, Said A. Optimising the care of patients with cirrhosis and gastrointestinal haemorrhage: a quality improvement study. Aliment Pharmacol Ther. 2011;34(1):76-82. DOI: 10.1111/j.1365-2036.2011.04692.x. PubMed
16. Volk ML, Tocco RS, Bazick J, Rakoski MO, Lok AS. Hospital readmissions among patients with decompensated cirrhosis. Am J Gastroenterol. 2012;107(2):247-252. DOI: 10.1038/ajg.2011.314. PubMed
17. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Clinical outcomes after bedside and interventional radiology paracentesis procedures. Am J Med. 2013;126(4):349-356. DOI: 10.1016/j.amjmed.2012.09.016. PubMed
18. Deitelzweig S, Amin A, Christian R, Friend K, Lin J, Lowe TJ. Hyponatremia-associated healthcare burden among US patients hospitalized for cirrhosis. Adv Ther. 2013;30(1):71-80. DOI: 10.1007/s12325-012-0073-1. PubMed
19. Morando F, Maresio G, Piano S, et al. How to improve care in outpatients with cirrhosis and ascites: a new model of care coordination by consultant hepatologists. J Hepatol. 2013;59(2):257-264. DOI: 10.1016/j.jhep.2013.03.010. PubMed
20. Singal AG, Rahimi RS, Clark C, et al. An automated model using electronic medical record data identifies patients with cirrhosis at high risk for readmission. Clin Gastroenterol Hepatol. 2013;11(10):1335-1341.e1. DOI: 10.1016/j.cgh.2013.03.022. PubMed
21. Desai AP, Satoskar R, Appannagari A, et al. Co-management between hospitalist and hepatologist improves the quality of care of inpatients with chronic liver disease. J Clin Gastroenterol. 2014;48(4):e30-e36. DOI: 10.1097/MCG.0b013e3182a87f70. PubMed
22. Fagan KJ, Zhao EY, Horsfall LU, et al. Burden of decompensated cirrhosis and ascites on hospital services in a tertiary care facility: time for change? Intern Med J. 2014;44(9):865-872. DOI: 10.1111/imj.12491. PubMed
23. Gaduputi V, Chandrala C, Abbas N, Tariq H, Chilimuri S, Balar B. Prognostic significance of hypokalemia in hepatic encephalopathy. Hepatogastroenterology. 2014;61(133):1170-1174. PubMed
26. Agrawal K, Kumar P, Markert R, Agrawal S. Risk factors for 30-day readmissions of individuals with decompensated cirrhosis. South Med J. 2015;108(11):682-687. DOI: 10.14423/SMJ.0000000000000371. PubMed
27. Tapper EB, Finkelstein D, Mittleman MA, Piatkowski G, Lai M. Standard assessments of frailty are validated predictors of mortality in hospitalized patients with cirrhosis. Hepatology. 2015;62(2):584-590. DOI: 10.1002/hep.27830. PubMed
28. Atla PR, Sheikh MY, Gill F, Kundu R, Choudhury J. Predictors of hospital re-admissions among Hispanics with hepatitis C-related cirrhosis. Ann Gastroenterol. 2016;29(4):515-520. DOI: 10.20524/aog.2016.0072. PubMed
29. Bajaj JS, Reddy KR, Tandon P, et al. The 3-month readmission rate remains unacceptably high in a large North American cohort of patients with cirrhosis. Hepatology. 2016;64(1):200-208. DOI: 10.1002/hep.28414. PubMed
30. Courson A, Jones GM, Twilla JD. Treatment of acute hepatic encephalopathy: comparing the effects of adding rifaximin to lactulose on patient outcomes. J Pharm Pract. 2016;29(3):212-217. DOI: 10.1177/0897190014566312. PubMed
31. Graupera I, Solà E, Fabrellas N, et al. Urine monocyte chemoattractant protein-1 is an independent predictive factor of hospital readmission and survival in cirrhosis. PLOS ONE. 2016;11(6):e0157371. DOI: 10.1371/journal.pone.0157371. PubMed
32. Kanwal F, Asch SM, Kramer JR, Cao Y, Asrani S, El-Serag HB. Early outpatient follow-up and 30-day outcomes in patients hospitalized with cirrhosis. Hepatology. 2016;64(2):569-581. DOI: 10.1002/hep.28558. PubMed
46. Tapper EB, Finkelstein D, Mittleman MA, Piatkowski G, Chang M, Lai M. A quality improvement initiative reduces 30-day rate of readmission for patients with cirrhosis. Clin Gastroenterol Hepatol. 2016;14(5):753-759. DOI: 10.1016/j.cgh.2015.08.041. PubMed
45. Wigg AJ, McCormick R, Wundke R, Woodman RJ. Efficacy of a chronic disease management model for patients with chronic liver failure. Clin Gastroenterol Hepatol. 2013;11(7):850-8.e1. DOI: 10.1016/j.cgh.2013.01.014. PubMed
44. Ganesh S, Rogal SS, Yadav D, Humar A, Behari J. Risk factors for frequent readmissions and barriers to transplantation in patients with cirrhosis. PLOS ONE. 2013;8(1):e55140. DOI: 10.1371/journal.pone.0055140. PubMed
43. van Walraven C, Bennett C, Jennings A, Austin PC, Forster AJ. Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ. 2011;183(7):E391-E402. DOI: 10.1503/cmaj.101860. PubMed
42. Bajaj JS, Wade JB, Gibson DP, et al. The multi-dimensional burden of cirrhosis and hepatic encephalopathy on patients and caregivers. Am J Gastroenterol. 2011;106(9):1646-1653. DOI: 10.1038/ajg.2011.157. PubMed
41. van Walraven C, Dhalla IA, Bell C, et al. Derivation and validation of an index to predict early death or unplanned readmission after discharge from hospital to the community. CMAJ. 2010;182(6):551-557. DOI: 10.1503/cmaj.091117. PubMed
40. Rodríguez-Artalejo F, Guallar-Castillón P, Pascual CR, et al. Health-related quality of life as a predictor of hospital readmission and death among patients with heart failure. Arch Intern Med. 2005;165(11):1274-1279. DOI: 10.1001/archinte.165.11.1274. PubMed
39. Strömdahl M, Helgeson J, Kalaitzakis E. Emergency readmission following acute upper gastrointestinal bleeding. Eur J Gastroenterol Hepatol. 2017;29(1):73-77. DOI: 10.1097/MEG.0000000000000746. PubMed
38. Morales BP, Planas R, Bartoli R, et al. Early hospital readmission in decompensated cirrhosis: incidence, impact on mortality, and predictive factors. Dig Liver Dis. 2017;49(8):903-909. DOI: 10.1016/j.dld.2017.03.005. PubMed
37. Lyon KC, Likar E, Martello JL, Regier M. Retrospective cross-sectional pilot study of rifaximin dosing for the prevention of recurrent hepatic encephalopathy. J Gastroenterol Hepatol. 2017;32(9):1548-1552. DOI: 10.1111/jgh.13759. PubMed
36. Tapper EB, Halbert B, Mellinger J. Rates of and reasons for hospital readmissions in patients with cirrhosis: a multistate population-based cohort study. Clin Gastroenterol Hepatol. 2016;14(8):1181-1188.e2. DOI: 10.1016/j.cgh.2016.04.009. PubMed
35. Rassameehiran S, Mankongpaisarnrung C, Sutamtewagul G, Klomjit S, Rakvit A. Predictor of 90-day readmission rate for hepatic encephalopathy. South Med J. 2016;109(6):365-369. DOI: 10.14423/SMJ.0000000000000475. PubMed
34. Moon AM, Dominitz JA, Ioannou GN, Lowy E, Beste LA. Use of antibiotics among patients with cirrhosis and upper gastrointestinal bleeding is associated with reduced mortality. Clin Gastroenterol Hepatol. 2016;14(11):1629-1637.e1. DOI: 10.1016/j.cgh.2016.05.040. PubMed
33. Le S, Spelman T, Chong CP, et al. Could adherence to quality of care indicators for hospitalized patients with cirrhosis-related ascites improve clinical outcomes? Am J Gastroenterol. 2016;111(1):87-92. DOI: .10.1038/ajg.2015.402. PubMed
© 2018 Society of Hospital Medicine