User login
Journal Highlights: July-November 2025
Endoscopy
Barkun AN, et al. Canadian Association of Gastroenterology Clinical Practice Guideline for the Endoscopic Management of Nonvariceal Nonpeptic Ulcer Upper Gastrointestinal Bleeding. Gastroenterology. 2025 Oct. doi: 10.1053/j.gastro.2025.04.041.
Kindel TL, et al. Multisociety Clinical Practice Guidance for the Safe Use of Glucagon-like Peptide-1 Receptor Agonists in the Perioperative Period. Clin Gastroenterol Hepatol. 2025 Nov. doi: 10.1016/j.cgh.2024.10.003.
Roy A, et al. Endohepatology: Evolving Indications, Challenges, Unmet Needs and Opportunities. Gastro Hep Advances. 2025 Oct. doi: 10.1016/j.gastha.2025.100838.
Esophagus
Wani S, et al. AGA Clinical Practice Guideline on Surveillance of Barrett’s Esophagus. Gastroenterology. 2025 Nov. doi: 10.1053/j.gastro.2025.09.012.
Reed CC, et al. Worsening Disease Severity as Measured by I-SEE Associates With Decreased Treatment Response to Topical Steroids in Eosinophilic Esophagitis Patients. Clin Gastroenterol Hepatol. 2025 Sep. doi: 10.1016/j.cgh.2025.01.015.
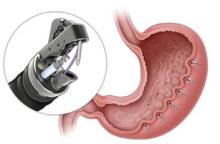
Kagzi Y, et al. Safety and Efficacy of Transoral Incisionless Fundoplication for Post–Esophageal Peroral Endoscopic Myotomy Gastroesophageal Reflux Disease With Esophagitis: A Meta-Analysis. Tech Innov Gastrointest Endosc. 2025 Oct. doi:10.1016/j.tige.2025.250953.
Stomach
Staller K, et al. AGA Clinical Practice Guideline on Management of Gastroparesis. Gastroenterology. 2025 Oct. doi: 10.1053/j.gastro.2025.08.004.
Colon
Bergman D, et al. Cholecystectomy Is a Risk Factor for Microscopic Colitis: A Nationwide Population-based Matched Case Control Study. Clin Gastroenterol Hepatol. 2025 Nov. doi: 10.1016/j.cgh.2024.12.032.
Liver
Younossi ZM, et al. Global Consensus Recommendations for Metabolic Dysfunction-Associated Steatotic Liver Disease and Steatohepatitis. Gastroenterology. 2025 Oct. doi: 10.1053/j.gastro.2025.02.044.
Kabelitz MA, et al. Early Occurrence of Hepatic Encephalopathy Following Transjugular Intrahepatic Portosystemic Shunt Insertion is Linked to Impaired Survival: A Multicenter Cohort Study. Clin Gastroenterol Hepatol. 2025 Nov. doi: 10.1016/j.cgh.2025.01.024.
Brar G, et al. Association of Cirrhosis Etiology with Outcomes After TIPS: A National Cohort Study. Gastro Hep Advances. 2025 Nov. doi: 10.1016/j.gastha.2025.100850.
IBD
Kucharzik T, et al. Role of Noninvasive Imaging in the Diagnosis and Management of Patients With Suspected and Established Inflammatory Bowel Disease. Gastroenterology. 2025 Nov. doi: 10.1053/j.gastro.2025.06.002.
Griffiths BJ, et al. Hypercoagulation After Hospital Discharge in Acute Severe Ulcerative Colitis: A Prospective Study. Clin Gastroenterol Hepatol. 2025 Sep. doi: 10.1016/j.cgh.2024.10.031.
Disorders of Gut-Brain Interaction
Trindade IA, et al. Implications of Shame for Patient-Reported Outcomes in Bowel Disorders of Gut-Brain Interaction. Gastroenterology. 2025 Aug. doi: 10.1053/j.gastro.2025.06.030.
Salwen-Deremer JK, et al. A Practical Guide to Incorporating a Psychologist Into a Gastroenterology Practice. Gastroenterology. 2025 Nov. doi: 10.1053/j.gastro.2025.05.014.
Misc
Monahan K, et al. In Our Scope of Practice: Genetic Risk Assessment and Testing for Gastrointestinal Cancers and Polyposis in Gastroenterology. Gastroenterology. 2025 Nov. doi: 10.1053/j.gastro.2025.06.001.
Dr. Trieu is assistant professor of medicine, interventional endoscopy, in the Division of Gastroenterology at Washington University in St. Louis School of Medicine, Missouri.
Endoscopy
Barkun AN, et al. Canadian Association of Gastroenterology Clinical Practice Guideline for the Endoscopic Management of Nonvariceal Nonpeptic Ulcer Upper Gastrointestinal Bleeding. Gastroenterology. 2025 Oct. doi: 10.1053/j.gastro.2025.04.041.
Kindel TL, et al. Multisociety Clinical Practice Guidance for the Safe Use of Glucagon-like Peptide-1 Receptor Agonists in the Perioperative Period. Clin Gastroenterol Hepatol. 2025 Nov. doi: 10.1016/j.cgh.2024.10.003.
Roy A, et al. Endohepatology: Evolving Indications, Challenges, Unmet Needs and Opportunities. Gastro Hep Advances. 2025 Oct. doi: 10.1016/j.gastha.2025.100838.
Esophagus
Wani S, et al. AGA Clinical Practice Guideline on Surveillance of Barrett’s Esophagus. Gastroenterology. 2025 Nov. doi: 10.1053/j.gastro.2025.09.012.
Reed CC, et al. Worsening Disease Severity as Measured by I-SEE Associates With Decreased Treatment Response to Topical Steroids in Eosinophilic Esophagitis Patients. Clin Gastroenterol Hepatol. 2025 Sep. doi: 10.1016/j.cgh.2025.01.015.
Kagzi Y, et al. Safety and Efficacy of Transoral Incisionless Fundoplication for Post–Esophageal Peroral Endoscopic Myotomy Gastroesophageal Reflux Disease With Esophagitis: A Meta-Analysis. Tech Innov Gastrointest Endosc. 2025 Oct. doi:10.1016/j.tige.2025.250953.
Stomach
Staller K, et al. AGA Clinical Practice Guideline on Management of Gastroparesis. Gastroenterology. 2025 Oct. doi: 10.1053/j.gastro.2025.08.004.
Colon
Bergman D, et al. Cholecystectomy Is a Risk Factor for Microscopic Colitis: A Nationwide Population-based Matched Case Control Study. Clin Gastroenterol Hepatol. 2025 Nov. doi: 10.1016/j.cgh.2024.12.032.
Liver
Younossi ZM, et al. Global Consensus Recommendations for Metabolic Dysfunction-Associated Steatotic Liver Disease and Steatohepatitis. Gastroenterology. 2025 Oct. doi: 10.1053/j.gastro.2025.02.044.
Kabelitz MA, et al. Early Occurrence of Hepatic Encephalopathy Following Transjugular Intrahepatic Portosystemic Shunt Insertion is Linked to Impaired Survival: A Multicenter Cohort Study. Clin Gastroenterol Hepatol. 2025 Nov. doi: 10.1016/j.cgh.2025.01.024.
Brar G, et al. Association of Cirrhosis Etiology with Outcomes After TIPS: A National Cohort Study. Gastro Hep Advances. 2025 Nov. doi: 10.1016/j.gastha.2025.100850.
IBD
Kucharzik T, et al. Role of Noninvasive Imaging in the Diagnosis and Management of Patients With Suspected and Established Inflammatory Bowel Disease. Gastroenterology. 2025 Nov. doi: 10.1053/j.gastro.2025.06.002.
Griffiths BJ, et al. Hypercoagulation After Hospital Discharge in Acute Severe Ulcerative Colitis: A Prospective Study. Clin Gastroenterol Hepatol. 2025 Sep. doi: 10.1016/j.cgh.2024.10.031.
Disorders of Gut-Brain Interaction
Trindade IA, et al. Implications of Shame for Patient-Reported Outcomes in Bowel Disorders of Gut-Brain Interaction. Gastroenterology. 2025 Aug. doi: 10.1053/j.gastro.2025.06.030.
Salwen-Deremer JK, et al. A Practical Guide to Incorporating a Psychologist Into a Gastroenterology Practice. Gastroenterology. 2025 Nov. doi: 10.1053/j.gastro.2025.05.014.
Misc
Monahan K, et al. In Our Scope of Practice: Genetic Risk Assessment and Testing for Gastrointestinal Cancers and Polyposis in Gastroenterology. Gastroenterology. 2025 Nov. doi: 10.1053/j.gastro.2025.06.001.
Dr. Trieu is assistant professor of medicine, interventional endoscopy, in the Division of Gastroenterology at Washington University in St. Louis School of Medicine, Missouri.
Endoscopy
Barkun AN, et al. Canadian Association of Gastroenterology Clinical Practice Guideline for the Endoscopic Management of Nonvariceal Nonpeptic Ulcer Upper Gastrointestinal Bleeding. Gastroenterology. 2025 Oct. doi: 10.1053/j.gastro.2025.04.041.
Kindel TL, et al. Multisociety Clinical Practice Guidance for the Safe Use of Glucagon-like Peptide-1 Receptor Agonists in the Perioperative Period. Clin Gastroenterol Hepatol. 2025 Nov. doi: 10.1016/j.cgh.2024.10.003.
Roy A, et al. Endohepatology: Evolving Indications, Challenges, Unmet Needs and Opportunities. Gastro Hep Advances. 2025 Oct. doi: 10.1016/j.gastha.2025.100838.
Esophagus
Wani S, et al. AGA Clinical Practice Guideline on Surveillance of Barrett’s Esophagus. Gastroenterology. 2025 Nov. doi: 10.1053/j.gastro.2025.09.012.
Reed CC, et al. Worsening Disease Severity as Measured by I-SEE Associates With Decreased Treatment Response to Topical Steroids in Eosinophilic Esophagitis Patients. Clin Gastroenterol Hepatol. 2025 Sep. doi: 10.1016/j.cgh.2025.01.015.
Kagzi Y, et al. Safety and Efficacy of Transoral Incisionless Fundoplication for Post–Esophageal Peroral Endoscopic Myotomy Gastroesophageal Reflux Disease With Esophagitis: A Meta-Analysis. Tech Innov Gastrointest Endosc. 2025 Oct. doi:10.1016/j.tige.2025.250953.
Stomach
Staller K, et al. AGA Clinical Practice Guideline on Management of Gastroparesis. Gastroenterology. 2025 Oct. doi: 10.1053/j.gastro.2025.08.004.
Colon
Bergman D, et al. Cholecystectomy Is a Risk Factor for Microscopic Colitis: A Nationwide Population-based Matched Case Control Study. Clin Gastroenterol Hepatol. 2025 Nov. doi: 10.1016/j.cgh.2024.12.032.
Liver
Younossi ZM, et al. Global Consensus Recommendations for Metabolic Dysfunction-Associated Steatotic Liver Disease and Steatohepatitis. Gastroenterology. 2025 Oct. doi: 10.1053/j.gastro.2025.02.044.
Kabelitz MA, et al. Early Occurrence of Hepatic Encephalopathy Following Transjugular Intrahepatic Portosystemic Shunt Insertion is Linked to Impaired Survival: A Multicenter Cohort Study. Clin Gastroenterol Hepatol. 2025 Nov. doi: 10.1016/j.cgh.2025.01.024.
Brar G, et al. Association of Cirrhosis Etiology with Outcomes After TIPS: A National Cohort Study. Gastro Hep Advances. 2025 Nov. doi: 10.1016/j.gastha.2025.100850.
IBD
Kucharzik T, et al. Role of Noninvasive Imaging in the Diagnosis and Management of Patients With Suspected and Established Inflammatory Bowel Disease. Gastroenterology. 2025 Nov. doi: 10.1053/j.gastro.2025.06.002.
Griffiths BJ, et al. Hypercoagulation After Hospital Discharge in Acute Severe Ulcerative Colitis: A Prospective Study. Clin Gastroenterol Hepatol. 2025 Sep. doi: 10.1016/j.cgh.2024.10.031.
Disorders of Gut-Brain Interaction
Trindade IA, et al. Implications of Shame for Patient-Reported Outcomes in Bowel Disorders of Gut-Brain Interaction. Gastroenterology. 2025 Aug. doi: 10.1053/j.gastro.2025.06.030.
Salwen-Deremer JK, et al. A Practical Guide to Incorporating a Psychologist Into a Gastroenterology Practice. Gastroenterology. 2025 Nov. doi: 10.1053/j.gastro.2025.05.014.
Misc
Monahan K, et al. In Our Scope of Practice: Genetic Risk Assessment and Testing for Gastrointestinal Cancers and Polyposis in Gastroenterology. Gastroenterology. 2025 Nov. doi: 10.1053/j.gastro.2025.06.001.
Dr. Trieu is assistant professor of medicine, interventional endoscopy, in the Division of Gastroenterology at Washington University in St. Louis School of Medicine, Missouri.
Finding Your Voice in Advocacy
Dear Friends,
Since moving to Missouri a little over 2 years ago, I got involved with the Missouri GI Society. They held their inaugural in-person meeting in September, and it was exciting to see and meet gastroenterologists and associates from all over the state. The meeting sparked conversations about challenges in practices and ways to improve patient care. It was incredibly inspiring to see the beginnings and bright future of a society motivated to mobilize change in the community. On a national scale, AGA Advocacy Day 2025 this fall was another example of how to make an impact for the field. I am grateful that local and national GI communities can be a platform for our voices.
In this issue’s “In Focus,” Dr. Colleen R. Kelly discusses the approach for weight management for the gastroenterologist, including how to discuss lifestyle modifications, anti-obesity medications, endoscopic therapies, and bariatric surgeries. In the “Short Clinical Review,” Dr. Ekta Gupta, Dr. Carol Burke, and Dr. Carole Macaron review available non-invasive blood and stool tests for colorectal cancer screening, including guidelines recommendations and evidence supporting each modality.
In the “Early Career” section, Dr. Mayada Ismail shares her personal journey in making the difficult decision of leaving her first job as an early career gastroenterologist, outlining the challenges and lessons learned along the way.
Dr. Alicia Muratore, Dr. Emily V. Wechsler, and Dr. Eric D. Shah provide a practical guide to tech and device development in the “Finance/Legal” section of this issue, outlining everything from intellectual property ownership to building the right team, and selecting the right incubator.
If you are interested in contributing or have ideas for future TNG topics, please contact me (tjudy@wustl.edu) or Danielle Kiefer (dkiefer@gastro.org), Communications/Managing Editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: screening colonoscopy for colorectal cancer was only first introduced in the mid-1990s with Medicare coverage for high-risk individuals starting in 1998, followed by coverage for average-risk patients in 2001.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Assistant Professor of Medicine
Interventional Endoscopy, Division of Gastroenterology
Washington University School of Medicine in St. Louis
Dear Friends,
Since moving to Missouri a little over 2 years ago, I got involved with the Missouri GI Society. They held their inaugural in-person meeting in September, and it was exciting to see and meet gastroenterologists and associates from all over the state. The meeting sparked conversations about challenges in practices and ways to improve patient care. It was incredibly inspiring to see the beginnings and bright future of a society motivated to mobilize change in the community. On a national scale, AGA Advocacy Day 2025 this fall was another example of how to make an impact for the field. I am grateful that local and national GI communities can be a platform for our voices.
In this issue’s “In Focus,” Dr. Colleen R. Kelly discusses the approach for weight management for the gastroenterologist, including how to discuss lifestyle modifications, anti-obesity medications, endoscopic therapies, and bariatric surgeries. In the “Short Clinical Review,” Dr. Ekta Gupta, Dr. Carol Burke, and Dr. Carole Macaron review available non-invasive blood and stool tests for colorectal cancer screening, including guidelines recommendations and evidence supporting each modality.
In the “Early Career” section, Dr. Mayada Ismail shares her personal journey in making the difficult decision of leaving her first job as an early career gastroenterologist, outlining the challenges and lessons learned along the way.
Dr. Alicia Muratore, Dr. Emily V. Wechsler, and Dr. Eric D. Shah provide a practical guide to tech and device development in the “Finance/Legal” section of this issue, outlining everything from intellectual property ownership to building the right team, and selecting the right incubator.
If you are interested in contributing or have ideas for future TNG topics, please contact me (tjudy@wustl.edu) or Danielle Kiefer (dkiefer@gastro.org), Communications/Managing Editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: screening colonoscopy for colorectal cancer was only first introduced in the mid-1990s with Medicare coverage for high-risk individuals starting in 1998, followed by coverage for average-risk patients in 2001.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Assistant Professor of Medicine
Interventional Endoscopy, Division of Gastroenterology
Washington University School of Medicine in St. Louis
Dear Friends,
Since moving to Missouri a little over 2 years ago, I got involved with the Missouri GI Society. They held their inaugural in-person meeting in September, and it was exciting to see and meet gastroenterologists and associates from all over the state. The meeting sparked conversations about challenges in practices and ways to improve patient care. It was incredibly inspiring to see the beginnings and bright future of a society motivated to mobilize change in the community. On a national scale, AGA Advocacy Day 2025 this fall was another example of how to make an impact for the field. I am grateful that local and national GI communities can be a platform for our voices.
In this issue’s “In Focus,” Dr. Colleen R. Kelly discusses the approach for weight management for the gastroenterologist, including how to discuss lifestyle modifications, anti-obesity medications, endoscopic therapies, and bariatric surgeries. In the “Short Clinical Review,” Dr. Ekta Gupta, Dr. Carol Burke, and Dr. Carole Macaron review available non-invasive blood and stool tests for colorectal cancer screening, including guidelines recommendations and evidence supporting each modality.
In the “Early Career” section, Dr. Mayada Ismail shares her personal journey in making the difficult decision of leaving her first job as an early career gastroenterologist, outlining the challenges and lessons learned along the way.
Dr. Alicia Muratore, Dr. Emily V. Wechsler, and Dr. Eric D. Shah provide a practical guide to tech and device development in the “Finance/Legal” section of this issue, outlining everything from intellectual property ownership to building the right team, and selecting the right incubator.
If you are interested in contributing or have ideas for future TNG topics, please contact me (tjudy@wustl.edu) or Danielle Kiefer (dkiefer@gastro.org), Communications/Managing Editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: screening colonoscopy for colorectal cancer was only first introduced in the mid-1990s with Medicare coverage for high-risk individuals starting in 1998, followed by coverage for average-risk patients in 2001.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Assistant Professor of Medicine
Interventional Endoscopy, Division of Gastroenterology
Washington University School of Medicine in St. Louis
Non-Invasive Blood and Stool CRC Screening Tests: Available Modalities and Their Clinical Application
Introduction
Colorectal cancer (CRC) screening significantly reduces CRC incidence and mortality, but only 65% of eligible individuals report being up-to-date with screening.1 Colonoscopy is the most widely used opportunistic screening method in the United States and is associated with many barriers to uptake. Providing patients a choice of colonoscopy and/or stool-based tests, improves screening adherence in randomized controlled trials.2,3 Non-invasive screening options have expanded from stool occult blood and multi-target DNA tests, to multi-target stool RNA tests, and novel blood-based tests, the latter only U.S. Food and Drug Administration (FDA) approved for patients who refuse colonoscopy and stool-based tests.
Stool Occult Blood Tests
Guaiac-based fecal occult blood testing (gFOBT) significantly reduces CRC mortality by 33%-35% when implemented on an annual or biennial basis.4,5 Fecal immunochemical testing (FIT) has supplanted gFOBT with advantages including independence from dietary restriction and medication-related interference, use of antibodies specific to human globin, and the need for only a single stool sample.
The most common threshold for a positive FIT in the U.S. is ≥ 20 micrograms (μg) of hemoglobin per gram (g) of stool. FIT is approved by the FDA as a qualitative positive or negative result based on a threshold value.6 A meta-analysis summarized test characteristics of commercially available FITs at various detection thresholds.7 The CRC sensitivity and specificity was 75% and 95% for ≥ 20 ug hemoglobin/g stool, and 91% and 90% for 10 ug hemoglobin/g stool, respectively. The sensitivity for advanced adenomas ranged from 25% at 20 μg/g to 40% at a 10 μg/g. Programmatic use of FIT in adults ages ≥ 50 years at 20 ug/g of stool, in cohort and case control studies, has been shown to significantly reduce CRC mortality by 33%-40% and advanced stage CRC by 34%.8,9
Over 57,000 average-risk individuals ages 50–69 years were randomized to biennial FIT or one-time colonoscopy and followed for 10 years.10 CRC mortality and incidence was similar between the groups: 0.22% with FIT vs. 0.24% with colonoscopy and 1.13% with FIT vs. 1.22% with colonoscopy, respectively. Thus, confirming biennial FIT screening is non-inferior to one-time colonoscopy in important CRC-related outcomes.
Multi-Target Stool Tests
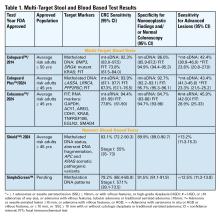
Two multitarget stool DNA tests (mt-sDNA) known as Cologuard™ and Cologuard Plus™ have been approved by the FDA. Both tests include a FIT (with a positivity threshold of 20 μg hemoglobin per gram of stool) combined with DNA methylation markers. The test result is qualitative, reported as a positive or negative. Cologuard™ markers include methylated BMP3, NDRG4, and mutant KRAS while Cologuard Plus™ assesses methylated LASS4, LRRC4, and PPP2R5C. The respective mt-sDNA tests were studied in 9989 of 12,776 and 20,176 of 26,758 average-risk individuals undergoing colonoscopy and the results were compared to a commercially available FIT (with a positivity threshold of 20 μg hemoglobin/gram of stool).11,12 In both trials, the sensitivity for CRC and advanced precancerous lesions was higher with the mt-sDNA tests compared to FIT but had a significantly lower specificity for advanced precancerous lesions versus FIT (see Table 1). An age-related decline in specificity was noted in both trials with mt-sDNA, a trend not observed with FIT. This reduction may be attributed to age-related DNA methylation.
Multi-Target Stool RNA Test
A multi-target stool RNA test (mt-sRNA) commercially available as ColoSense™ is FDA-approved. It combines FIT (at a positivity threshold of 20 μg hemoglobin/gram of stool) with RNA-based stool markers. The combined results of the RNA markers, FIT, and smoking status provide a qualitative single test result. In the trial, 8,920 adults aged ≥45 underwent the mt-sRNA test and FIT followed by colonoscopy (13). The mt-sRNA showed higher sensitivity for CRC than FIT (94.4% versus 77.8%) and advanced adenomas (45.9% versus 28.9%) but lower CRC specificity (84.7% vs 94.7%) (Table 1). Unlike mt-sDNA-based tests, mt-sRNA showed consistent performance across age groups, addressing concerns about age-related declines in specificity attributed to DNA methylation.
Blood-Based Tests
In 2014, the first blood-based (BBT) CRC screening test known as Epi proColon™ was FDA but not Centers for Medicare & Medicaid Services (CMS) approved for average-risk adults ≥50 years of age who are offered and refused other U.S Preventive Services Task Force (USPSTF) endorsed CRC screening tests. It is a qualitative test for detection of circulating methylated Septin 9 (mSeptin9). The accuracy of mSeptin9 to detect CRC was assessed in a subset of 7941 asymptomatic average risk adults undergoing screening colonoscopy.14 The sensitivity and specificity for CRC were 48% and 91.5%, respectively. The sensitivity for advanced adenomas was 11.2%. An increase in sensitivity to 63.9% and reduction in specificity to 88.4% for CRC was demonstrated in a sub-analysis of available samples where an additional (third) polymerase chain replicate was performed. Epi proColon™ is not currently reimbursed by Medicare and not endorsed in the latest USPSTF guidelines.
Technologic advancements have improved the detection of circulating tumor markers in the blood. The Shield™ BBT approved by the FDA in 2024 for average risk adults ≥ 45 years integrates three types of cfDNA data (epigenetic changes resulting in the aberrant methylation or fragmentation patterns, and genomic changes resulting in somatic mutations) into a positive or negative test result. In the trial, 22,877 average-risk, asymptomatic individuals ages 45–84 were enrolled and clinical validation was performed in 7,861 of the participants.15 The sensitivity for CRC was 83.1% which decreased to 55% for stage I tumors (see Table 1). CRC specificity was 89.6% and the sensitivity for advanced adenomas and large sessile serrated lesions was 13.2%.
Another BBT SimpleScreen™, which is not yet FDA-approved, analyzed circulating, cell-free DNA methylation patterns in 27,010 evaluable average-risk, asymptomatic adults ages 45–85 years undergoing screening colonoscopy.16 The sensitivity and specificity for CRC was 79.2% and 91.5%, respectively. Similar to Shield, the sensitivity for stage I CRC was low at 57.1%. The sensitivity for advanced precancerous lesions, a secondary endpoint, was 12.5% which did not meet the prespecified study criteria.
Effectiveness and Cost Effectiveness
Modeling studies have evaluated novel noninvasive CRC screening tests compared to FIT and colonoscopy.17-20 One compared a hypothetical BBT performed every 3 years that meets the minimum CMS threshold CRC sensitivity and specificity of 74% and 90%, respectively, to other established CRC screening tests beginning at age 45.17 Every 3-year BBT reduced CRC incidence and mortality by 40% and 52%, respectively compared to no screening. However, the reductions were much lower than yearly FIT (72% and 76%, respectively), every 10 year colonoscopy (79% and 81%, respectively), and triennial mt-sDNA (68% and 73%, respectively). The BBT resulted in fewer quality-adjusted life-years per person compared to the alternatives.
Additionally, FIT, colonoscopy, and mt-sDNA were less costly and more effective. Advanced precancerous lesion detection was a key measure for a test’s effectiveness. BBT characteristics would require a CRC sensitivity and specificity of >90% and 90%, respectively, and 80% sensitivity for advanced precancerous lesions at a cost of ≤$120–$140 to be cost-effective compared to FIT at comparable participation rates.
Another analysis simulated colorectal neoplasia progression and compared clinical effectiveness and cost between annual FIT, every 3 year stool mt-sRNA, every 3 year stool mt-sDNA tests, every 3 year stool Shield™; these outcomes were compared to colonoscopy every 10 years and no screening in adults ≥ age 45 over different adherence rates.19 At real-world adherence rates of 60%, colonoscopy prevented most CRC cases and associated deaths. FIT was the most cost-effective strategy at all adherence levels. Between the multi-target stool tests and Shield™, mt-sRNA was the most cost-effective. Compared to FIT, mt-sRNA reduced CRC cases and deaths by 1% and 14%.
The third study evaluated CRC incidence and mortality, quality-adjusted life-years and costs with annual FIT, colonoscopy every 10 years, mt- sDNA tests, mt-sRNA test, and BBTs.20 The latest mt-sDNA (Colguard plus™) and mt-sRNA achieved benefits approaching FIT but the Shield™ test was substantially less effective. The authors hypothesized that if 15% of the population substituted Shield™ for current effective CRC screening strategies, an increase in CRC deaths would occur and require 9-10% of the unscreened population to uptake screening with Shield to avert the increases in CRC deaths due to the substitution effect.
Clinical Implications
The effectiveness of non-invasive screening strategies depends on their diagnostic performance, adherence, and ensuring a timely colonoscopy after a positive test. Two claims-based studies found 47.9% and 49% of patients underwent follow-up colonoscopy within 6 months of an abnormal stool or BBT CRC screening test, respectively.21-22
Conclusions
Non-invasive stool mt-sDNA and mt-sRNA have higher effectiveness than the new BBTs. BBTs can lead to increased CRC mortality if substituted for the FDA and CMS-approved, USPSTF-endorsed, CRC screening modalities. If future BBTs increase their sensitivity for CRC (including early-stage CRC) and advanced precancerous lesions and decrease their cost, they may prove to have similar cost-effectiveness to stool-based tests. Currently, BBTs are not a substitute for colonoscopy or other stool tests and should be offered to patients who refuse other CRC screening modalities. A personalized, risk-adapted approach, paired with improved adherence and follow-up are essential to optimize the population-level impact of CRC screening and ensure equitable, effective cancer prevention.
Dr. Gupta is based at the Division of Gastroenterology and Hepatology, Department of Medicine, University of Maryland School of Medicine, Baltimore. Dr. Burke and Dr. Macaron are based at the Department of Gastroenterology, Hepatology, and Nutrition, Cleveland Clinic, Cleveland, Ohio. Dr. Gupta and Dr. Macaron declared no conflicts of interest in regard to this article. Dr. Burke declared research support from Emtora Biosciences. She is a current consultant for Lumabridge, and has been a consultant for Sebela and Almirall. She also disclosed support from Myriad, Genzyme, Ferring, Merck, Sharp and Dohme, Abbvie, Salix, and Natera.
References
1. Benavidez GA, Sedani AE, Felder TM, Asare M, Rogers CR. Rural-urban disparities and trends in cancer screening: an analysis of Behavioral Risk Factor Surveillance System data (2018-2022). JNCI Cancer Spectr. 2024 Nov 1;8(6):pkae113
2. Galoosian A, Dai H, Croymans D, et al. Population Health Colorectal Cancer Screening Strategies in Adults Aged 45 to 49 Years: A Randomized Clinical Trial. JAMA. 2025 Aug 4:e2512049. doi: 10.1001/jama.2025.12049. Epub ahead of print.
3. Pilonis ND, Bugajski M, Wieszczy P, et al. Participation in Competing Strategies for Colorectal Cancer Screening: A Randomized Health Services Study (PICCOLINO Study). Gastroenterology. 2021 Mar;160(4):1097-1105.
4. Shaukat A, Mongin SJ, Geisser MS, et al. Long-term mortality after screening for colorectal cancer. N Engl J Med. 2013;369(12):1106–1114.
5. Kronborg O, Fenger C, Olsen J, Jørgensen OD, Søndergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996 Nov 30;348(9040):1467-71. doi: 10.1016/S0140-6736(96)03430-7. PMID: 8942774.
6. Burke CA, Lieberman D, Feuerstein JD. AGA Clinical Practice Update on Approach to the Use of Noninvasive Colorectal Cancer Screening Options: Commentary. Gastroenterology. 2022 Mar;162(3):952-956. doi: 10.1053/j.gastro.2021.09.075. Epub 2022 Jan 28. PMID: 35094786.
7. Imperiale TF, Gruber RN Stump TE, et al. Performance characteristics of fecal immunochemical tests for colorectal cancer and advanced adenomatous polyps: a systematic review and meta-analysis. Ann Intern Med 2019; 170(5):319-329
8. Doubeni CA, Corley DA, Jensen CD, et al. Fecal Immunochemical Test Screening and Risk of Colorectal Cancer Death. JAMA Netw Open. 2024 Jul 1;7(7):e2423671. doi: 10.1001/jamanetworkopen.2024.23671.
9. Chiu HM, Jen GH, Wang YW, et al. Long-term effectiveness of faecal immunochemical test screening for proximal and distal colorectal cancers. Gut. 2021 Dec;70(12):2321-2329. doi: 10.1136/gutjnl-2020-322545. Epub 2021 Jan 25.
10. Castells A, Quintero E, Bujanda L, et al; COLONPREV study investigators. Effect of invitation to colonoscopy versus fecal immunochemical test screening on colorectal cancer mortality (COLONPREV): a pragmatic, randomised, controlled, non-inferiority trial. Lancet. 2025;405(10486):1231–1239
11. Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014;370(14):1287-1297
12. Imperiale TF, Porter K, Zella J, et al. Next-Generation Multitarget Stool DNA Test for Colorectal Cancer Screening. N Engl J Med. 2024 Mar 14;390(11):984-993
13. Barnell EK, Wurtzler EM, La Rocca J, et al. Multitarget Stool RNA Test for Colorectal Cancer Screening. JAMA. 2023 Nov 14;330(18):1760-1768.
14. Church TR, Wandell M, Lofton-Day C, et al. Prospective evaluation of methylated SEPT9 in plasma for detection of asymptomatic colorectal cancer. Gut 2014; 63:317–325.
15. Chung DC, Gray DM 2nd, Singh H, et al. A Cell-free DNA Blood-Based Test for Colorectal Cancer Screening. N Engl J Med. 2024 Mar 14;390(11):973-983.
16. Shaukat A, Burke CA, Chan AT, et al. Clinical Validation of a Circulating Tumor DNA-Based Blood Test to Screen for Colorectal Cancer. JAMA. 2025 Jul 1;334(1):56-63.
17. Ladabaum U, Mannalithara A, Weng Y, et al. Comparative Effectiveness and Cost-Effectiveness of Colorectal Cancer Screening with Blood-Based Biomarkers (Liquid Biopsy) vs Fecal Tests or Colonoscopy. Gastroenterology. 2024 Jul;167(2):378-391.
18. van den Puttelaar R, Nascimento de Lima P, Knudsen AB, et al. Effectiveness and cost-effectiveness of colorectal cancer screening with a blood test that meets the Centers for Medicare & Medicaid Services coverage decision. Gastroenterology 2024;167:368–377.
19. Shaukat A, Levin TR, Liang PS. Cost-effectiveness of Novel Noninvasive Screening Tests for Colorectal Neoplasia. Clin Gastroenterol Hepatol. 2025 Jun 23:S1542-3565(25)00525-7. doi: 10.1016/j.cgh.2025.06.006. Epub ahead of print. PMID: 40562290.
20. Ladabaum U, Mannalithara A, Schoen RE, Dominitz JA, Lieberman D. Projected Impact and Cost-Effectiveness of Novel Molecular Blood-Based or Stool-Based Screening Tests for Colorectal Cancer. Ann Intern Med. 2024 Dec;177(12):1610-1620.
20. Ciemins EL, Mohl JT, Moreno CA, Colangelo F, Smith RA, Barton M. Development of a Follow-Up Measure to Ensure Complete Screening for Colorectal Cancer. JAMA Netw Open. 2024 Mar 4;7(3):e242693. doi: 10.1001/jamanetworkopen.2024.2693.
21. Zaki TA, Zhang NJ, Forbes SP, Raymond VM, Das AK, May FP. Colonoscopic Follow-up After Abnormal Blood-Based Colorectal Cancer Screening Results. Gastroenterology. 2025 Jul 21:S0016-5085(25)05775-0. doi: 10.1053/j.gastro.2025.07.019. Epub ahead of print. PMID: 40744392.
Introduction
Colorectal cancer (CRC) screening significantly reduces CRC incidence and mortality, but only 65% of eligible individuals report being up-to-date with screening.1 Colonoscopy is the most widely used opportunistic screening method in the United States and is associated with many barriers to uptake. Providing patients a choice of colonoscopy and/or stool-based tests, improves screening adherence in randomized controlled trials.2,3 Non-invasive screening options have expanded from stool occult blood and multi-target DNA tests, to multi-target stool RNA tests, and novel blood-based tests, the latter only U.S. Food and Drug Administration (FDA) approved for patients who refuse colonoscopy and stool-based tests.
Stool Occult Blood Tests
Guaiac-based fecal occult blood testing (gFOBT) significantly reduces CRC mortality by 33%-35% when implemented on an annual or biennial basis.4,5 Fecal immunochemical testing (FIT) has supplanted gFOBT with advantages including independence from dietary restriction and medication-related interference, use of antibodies specific to human globin, and the need for only a single stool sample.
The most common threshold for a positive FIT in the U.S. is ≥ 20 micrograms (μg) of hemoglobin per gram (g) of stool. FIT is approved by the FDA as a qualitative positive or negative result based on a threshold value.6 A meta-analysis summarized test characteristics of commercially available FITs at various detection thresholds.7 The CRC sensitivity and specificity was 75% and 95% for ≥ 20 ug hemoglobin/g stool, and 91% and 90% for 10 ug hemoglobin/g stool, respectively. The sensitivity for advanced adenomas ranged from 25% at 20 μg/g to 40% at a 10 μg/g. Programmatic use of FIT in adults ages ≥ 50 years at 20 ug/g of stool, in cohort and case control studies, has been shown to significantly reduce CRC mortality by 33%-40% and advanced stage CRC by 34%.8,9
Over 57,000 average-risk individuals ages 50–69 years were randomized to biennial FIT or one-time colonoscopy and followed for 10 years.10 CRC mortality and incidence was similar between the groups: 0.22% with FIT vs. 0.24% with colonoscopy and 1.13% with FIT vs. 1.22% with colonoscopy, respectively. Thus, confirming biennial FIT screening is non-inferior to one-time colonoscopy in important CRC-related outcomes.
Multi-Target Stool Tests
Two multitarget stool DNA tests (mt-sDNA) known as Cologuard™ and Cologuard Plus™ have been approved by the FDA. Both tests include a FIT (with a positivity threshold of 20 μg hemoglobin per gram of stool) combined with DNA methylation markers. The test result is qualitative, reported as a positive or negative. Cologuard™ markers include methylated BMP3, NDRG4, and mutant KRAS while Cologuard Plus™ assesses methylated LASS4, LRRC4, and PPP2R5C. The respective mt-sDNA tests were studied in 9989 of 12,776 and 20,176 of 26,758 average-risk individuals undergoing colonoscopy and the results were compared to a commercially available FIT (with a positivity threshold of 20 μg hemoglobin/gram of stool).11,12 In both trials, the sensitivity for CRC and advanced precancerous lesions was higher with the mt-sDNA tests compared to FIT but had a significantly lower specificity for advanced precancerous lesions versus FIT (see Table 1). An age-related decline in specificity was noted in both trials with mt-sDNA, a trend not observed with FIT. This reduction may be attributed to age-related DNA methylation.
Multi-Target Stool RNA Test
A multi-target stool RNA test (mt-sRNA) commercially available as ColoSense™ is FDA-approved. It combines FIT (at a positivity threshold of 20 μg hemoglobin/gram of stool) with RNA-based stool markers. The combined results of the RNA markers, FIT, and smoking status provide a qualitative single test result. In the trial, 8,920 adults aged ≥45 underwent the mt-sRNA test and FIT followed by colonoscopy (13). The mt-sRNA showed higher sensitivity for CRC than FIT (94.4% versus 77.8%) and advanced adenomas (45.9% versus 28.9%) but lower CRC specificity (84.7% vs 94.7%) (Table 1). Unlike mt-sDNA-based tests, mt-sRNA showed consistent performance across age groups, addressing concerns about age-related declines in specificity attributed to DNA methylation.
Blood-Based Tests
In 2014, the first blood-based (BBT) CRC screening test known as Epi proColon™ was FDA but not Centers for Medicare & Medicaid Services (CMS) approved for average-risk adults ≥50 years of age who are offered and refused other U.S Preventive Services Task Force (USPSTF) endorsed CRC screening tests. It is a qualitative test for detection of circulating methylated Septin 9 (mSeptin9). The accuracy of mSeptin9 to detect CRC was assessed in a subset of 7941 asymptomatic average risk adults undergoing screening colonoscopy.14 The sensitivity and specificity for CRC were 48% and 91.5%, respectively. The sensitivity for advanced adenomas was 11.2%. An increase in sensitivity to 63.9% and reduction in specificity to 88.4% for CRC was demonstrated in a sub-analysis of available samples where an additional (third) polymerase chain replicate was performed. Epi proColon™ is not currently reimbursed by Medicare and not endorsed in the latest USPSTF guidelines.
Technologic advancements have improved the detection of circulating tumor markers in the blood. The Shield™ BBT approved by the FDA in 2024 for average risk adults ≥ 45 years integrates three types of cfDNA data (epigenetic changes resulting in the aberrant methylation or fragmentation patterns, and genomic changes resulting in somatic mutations) into a positive or negative test result. In the trial, 22,877 average-risk, asymptomatic individuals ages 45–84 were enrolled and clinical validation was performed in 7,861 of the participants.15 The sensitivity for CRC was 83.1% which decreased to 55% for stage I tumors (see Table 1). CRC specificity was 89.6% and the sensitivity for advanced adenomas and large sessile serrated lesions was 13.2%.
Another BBT SimpleScreen™, which is not yet FDA-approved, analyzed circulating, cell-free DNA methylation patterns in 27,010 evaluable average-risk, asymptomatic adults ages 45–85 years undergoing screening colonoscopy.16 The sensitivity and specificity for CRC was 79.2% and 91.5%, respectively. Similar to Shield, the sensitivity for stage I CRC was low at 57.1%. The sensitivity for advanced precancerous lesions, a secondary endpoint, was 12.5% which did not meet the prespecified study criteria.
Effectiveness and Cost Effectiveness
Modeling studies have evaluated novel noninvasive CRC screening tests compared to FIT and colonoscopy.17-20 One compared a hypothetical BBT performed every 3 years that meets the minimum CMS threshold CRC sensitivity and specificity of 74% and 90%, respectively, to other established CRC screening tests beginning at age 45.17 Every 3-year BBT reduced CRC incidence and mortality by 40% and 52%, respectively compared to no screening. However, the reductions were much lower than yearly FIT (72% and 76%, respectively), every 10 year colonoscopy (79% and 81%, respectively), and triennial mt-sDNA (68% and 73%, respectively). The BBT resulted in fewer quality-adjusted life-years per person compared to the alternatives.
Additionally, FIT, colonoscopy, and mt-sDNA were less costly and more effective. Advanced precancerous lesion detection was a key measure for a test’s effectiveness. BBT characteristics would require a CRC sensitivity and specificity of >90% and 90%, respectively, and 80% sensitivity for advanced precancerous lesions at a cost of ≤$120–$140 to be cost-effective compared to FIT at comparable participation rates.
Another analysis simulated colorectal neoplasia progression and compared clinical effectiveness and cost between annual FIT, every 3 year stool mt-sRNA, every 3 year stool mt-sDNA tests, every 3 year stool Shield™; these outcomes were compared to colonoscopy every 10 years and no screening in adults ≥ age 45 over different adherence rates.19 At real-world adherence rates of 60%, colonoscopy prevented most CRC cases and associated deaths. FIT was the most cost-effective strategy at all adherence levels. Between the multi-target stool tests and Shield™, mt-sRNA was the most cost-effective. Compared to FIT, mt-sRNA reduced CRC cases and deaths by 1% and 14%.
The third study evaluated CRC incidence and mortality, quality-adjusted life-years and costs with annual FIT, colonoscopy every 10 years, mt- sDNA tests, mt-sRNA test, and BBTs.20 The latest mt-sDNA (Colguard plus™) and mt-sRNA achieved benefits approaching FIT but the Shield™ test was substantially less effective. The authors hypothesized that if 15% of the population substituted Shield™ for current effective CRC screening strategies, an increase in CRC deaths would occur and require 9-10% of the unscreened population to uptake screening with Shield to avert the increases in CRC deaths due to the substitution effect.
Clinical Implications
The effectiveness of non-invasive screening strategies depends on their diagnostic performance, adherence, and ensuring a timely colonoscopy after a positive test. Two claims-based studies found 47.9% and 49% of patients underwent follow-up colonoscopy within 6 months of an abnormal stool or BBT CRC screening test, respectively.21-22
Conclusions
Non-invasive stool mt-sDNA and mt-sRNA have higher effectiveness than the new BBTs. BBTs can lead to increased CRC mortality if substituted for the FDA and CMS-approved, USPSTF-endorsed, CRC screening modalities. If future BBTs increase their sensitivity for CRC (including early-stage CRC) and advanced precancerous lesions and decrease their cost, they may prove to have similar cost-effectiveness to stool-based tests. Currently, BBTs are not a substitute for colonoscopy or other stool tests and should be offered to patients who refuse other CRC screening modalities. A personalized, risk-adapted approach, paired with improved adherence and follow-up are essential to optimize the population-level impact of CRC screening and ensure equitable, effective cancer prevention.
Dr. Gupta is based at the Division of Gastroenterology and Hepatology, Department of Medicine, University of Maryland School of Medicine, Baltimore. Dr. Burke and Dr. Macaron are based at the Department of Gastroenterology, Hepatology, and Nutrition, Cleveland Clinic, Cleveland, Ohio. Dr. Gupta and Dr. Macaron declared no conflicts of interest in regard to this article. Dr. Burke declared research support from Emtora Biosciences. She is a current consultant for Lumabridge, and has been a consultant for Sebela and Almirall. She also disclosed support from Myriad, Genzyme, Ferring, Merck, Sharp and Dohme, Abbvie, Salix, and Natera.
References
1. Benavidez GA, Sedani AE, Felder TM, Asare M, Rogers CR. Rural-urban disparities and trends in cancer screening: an analysis of Behavioral Risk Factor Surveillance System data (2018-2022). JNCI Cancer Spectr. 2024 Nov 1;8(6):pkae113
2. Galoosian A, Dai H, Croymans D, et al. Population Health Colorectal Cancer Screening Strategies in Adults Aged 45 to 49 Years: A Randomized Clinical Trial. JAMA. 2025 Aug 4:e2512049. doi: 10.1001/jama.2025.12049. Epub ahead of print.
3. Pilonis ND, Bugajski M, Wieszczy P, et al. Participation in Competing Strategies for Colorectal Cancer Screening: A Randomized Health Services Study (PICCOLINO Study). Gastroenterology. 2021 Mar;160(4):1097-1105.
4. Shaukat A, Mongin SJ, Geisser MS, et al. Long-term mortality after screening for colorectal cancer. N Engl J Med. 2013;369(12):1106–1114.
5. Kronborg O, Fenger C, Olsen J, Jørgensen OD, Søndergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996 Nov 30;348(9040):1467-71. doi: 10.1016/S0140-6736(96)03430-7. PMID: 8942774.
6. Burke CA, Lieberman D, Feuerstein JD. AGA Clinical Practice Update on Approach to the Use of Noninvasive Colorectal Cancer Screening Options: Commentary. Gastroenterology. 2022 Mar;162(3):952-956. doi: 10.1053/j.gastro.2021.09.075. Epub 2022 Jan 28. PMID: 35094786.
7. Imperiale TF, Gruber RN Stump TE, et al. Performance characteristics of fecal immunochemical tests for colorectal cancer and advanced adenomatous polyps: a systematic review and meta-analysis. Ann Intern Med 2019; 170(5):319-329
8. Doubeni CA, Corley DA, Jensen CD, et al. Fecal Immunochemical Test Screening and Risk of Colorectal Cancer Death. JAMA Netw Open. 2024 Jul 1;7(7):e2423671. doi: 10.1001/jamanetworkopen.2024.23671.
9. Chiu HM, Jen GH, Wang YW, et al. Long-term effectiveness of faecal immunochemical test screening for proximal and distal colorectal cancers. Gut. 2021 Dec;70(12):2321-2329. doi: 10.1136/gutjnl-2020-322545. Epub 2021 Jan 25.
10. Castells A, Quintero E, Bujanda L, et al; COLONPREV study investigators. Effect of invitation to colonoscopy versus fecal immunochemical test screening on colorectal cancer mortality (COLONPREV): a pragmatic, randomised, controlled, non-inferiority trial. Lancet. 2025;405(10486):1231–1239
11. Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014;370(14):1287-1297
12. Imperiale TF, Porter K, Zella J, et al. Next-Generation Multitarget Stool DNA Test for Colorectal Cancer Screening. N Engl J Med. 2024 Mar 14;390(11):984-993
13. Barnell EK, Wurtzler EM, La Rocca J, et al. Multitarget Stool RNA Test for Colorectal Cancer Screening. JAMA. 2023 Nov 14;330(18):1760-1768.
14. Church TR, Wandell M, Lofton-Day C, et al. Prospective evaluation of methylated SEPT9 in plasma for detection of asymptomatic colorectal cancer. Gut 2014; 63:317–325.
15. Chung DC, Gray DM 2nd, Singh H, et al. A Cell-free DNA Blood-Based Test for Colorectal Cancer Screening. N Engl J Med. 2024 Mar 14;390(11):973-983.
16. Shaukat A, Burke CA, Chan AT, et al. Clinical Validation of a Circulating Tumor DNA-Based Blood Test to Screen for Colorectal Cancer. JAMA. 2025 Jul 1;334(1):56-63.
17. Ladabaum U, Mannalithara A, Weng Y, et al. Comparative Effectiveness and Cost-Effectiveness of Colorectal Cancer Screening with Blood-Based Biomarkers (Liquid Biopsy) vs Fecal Tests or Colonoscopy. Gastroenterology. 2024 Jul;167(2):378-391.
18. van den Puttelaar R, Nascimento de Lima P, Knudsen AB, et al. Effectiveness and cost-effectiveness of colorectal cancer screening with a blood test that meets the Centers for Medicare & Medicaid Services coverage decision. Gastroenterology 2024;167:368–377.
19. Shaukat A, Levin TR, Liang PS. Cost-effectiveness of Novel Noninvasive Screening Tests for Colorectal Neoplasia. Clin Gastroenterol Hepatol. 2025 Jun 23:S1542-3565(25)00525-7. doi: 10.1016/j.cgh.2025.06.006. Epub ahead of print. PMID: 40562290.
20. Ladabaum U, Mannalithara A, Schoen RE, Dominitz JA, Lieberman D. Projected Impact and Cost-Effectiveness of Novel Molecular Blood-Based or Stool-Based Screening Tests for Colorectal Cancer. Ann Intern Med. 2024 Dec;177(12):1610-1620.
20. Ciemins EL, Mohl JT, Moreno CA, Colangelo F, Smith RA, Barton M. Development of a Follow-Up Measure to Ensure Complete Screening for Colorectal Cancer. JAMA Netw Open. 2024 Mar 4;7(3):e242693. doi: 10.1001/jamanetworkopen.2024.2693.
21. Zaki TA, Zhang NJ, Forbes SP, Raymond VM, Das AK, May FP. Colonoscopic Follow-up After Abnormal Blood-Based Colorectal Cancer Screening Results. Gastroenterology. 2025 Jul 21:S0016-5085(25)05775-0. doi: 10.1053/j.gastro.2025.07.019. Epub ahead of print. PMID: 40744392.
Introduction
Colorectal cancer (CRC) screening significantly reduces CRC incidence and mortality, but only 65% of eligible individuals report being up-to-date with screening.1 Colonoscopy is the most widely used opportunistic screening method in the United States and is associated with many barriers to uptake. Providing patients a choice of colonoscopy and/or stool-based tests, improves screening adherence in randomized controlled trials.2,3 Non-invasive screening options have expanded from stool occult blood and multi-target DNA tests, to multi-target stool RNA tests, and novel blood-based tests, the latter only U.S. Food and Drug Administration (FDA) approved for patients who refuse colonoscopy and stool-based tests.
Stool Occult Blood Tests
Guaiac-based fecal occult blood testing (gFOBT) significantly reduces CRC mortality by 33%-35% when implemented on an annual or biennial basis.4,5 Fecal immunochemical testing (FIT) has supplanted gFOBT with advantages including independence from dietary restriction and medication-related interference, use of antibodies specific to human globin, and the need for only a single stool sample.
The most common threshold for a positive FIT in the U.S. is ≥ 20 micrograms (μg) of hemoglobin per gram (g) of stool. FIT is approved by the FDA as a qualitative positive or negative result based on a threshold value.6 A meta-analysis summarized test characteristics of commercially available FITs at various detection thresholds.7 The CRC sensitivity and specificity was 75% and 95% for ≥ 20 ug hemoglobin/g stool, and 91% and 90% for 10 ug hemoglobin/g stool, respectively. The sensitivity for advanced adenomas ranged from 25% at 20 μg/g to 40% at a 10 μg/g. Programmatic use of FIT in adults ages ≥ 50 years at 20 ug/g of stool, in cohort and case control studies, has been shown to significantly reduce CRC mortality by 33%-40% and advanced stage CRC by 34%.8,9
Over 57,000 average-risk individuals ages 50–69 years were randomized to biennial FIT or one-time colonoscopy and followed for 10 years.10 CRC mortality and incidence was similar between the groups: 0.22% with FIT vs. 0.24% with colonoscopy and 1.13% with FIT vs. 1.22% with colonoscopy, respectively. Thus, confirming biennial FIT screening is non-inferior to one-time colonoscopy in important CRC-related outcomes.
Multi-Target Stool Tests
Two multitarget stool DNA tests (mt-sDNA) known as Cologuard™ and Cologuard Plus™ have been approved by the FDA. Both tests include a FIT (with a positivity threshold of 20 μg hemoglobin per gram of stool) combined with DNA methylation markers. The test result is qualitative, reported as a positive or negative. Cologuard™ markers include methylated BMP3, NDRG4, and mutant KRAS while Cologuard Plus™ assesses methylated LASS4, LRRC4, and PPP2R5C. The respective mt-sDNA tests were studied in 9989 of 12,776 and 20,176 of 26,758 average-risk individuals undergoing colonoscopy and the results were compared to a commercially available FIT (with a positivity threshold of 20 μg hemoglobin/gram of stool).11,12 In both trials, the sensitivity for CRC and advanced precancerous lesions was higher with the mt-sDNA tests compared to FIT but had a significantly lower specificity for advanced precancerous lesions versus FIT (see Table 1). An age-related decline in specificity was noted in both trials with mt-sDNA, a trend not observed with FIT. This reduction may be attributed to age-related DNA methylation.
Multi-Target Stool RNA Test
A multi-target stool RNA test (mt-sRNA) commercially available as ColoSense™ is FDA-approved. It combines FIT (at a positivity threshold of 20 μg hemoglobin/gram of stool) with RNA-based stool markers. The combined results of the RNA markers, FIT, and smoking status provide a qualitative single test result. In the trial, 8,920 adults aged ≥45 underwent the mt-sRNA test and FIT followed by colonoscopy (13). The mt-sRNA showed higher sensitivity for CRC than FIT (94.4% versus 77.8%) and advanced adenomas (45.9% versus 28.9%) but lower CRC specificity (84.7% vs 94.7%) (Table 1). Unlike mt-sDNA-based tests, mt-sRNA showed consistent performance across age groups, addressing concerns about age-related declines in specificity attributed to DNA methylation.
Blood-Based Tests
In 2014, the first blood-based (BBT) CRC screening test known as Epi proColon™ was FDA but not Centers for Medicare & Medicaid Services (CMS) approved for average-risk adults ≥50 years of age who are offered and refused other U.S Preventive Services Task Force (USPSTF) endorsed CRC screening tests. It is a qualitative test for detection of circulating methylated Septin 9 (mSeptin9). The accuracy of mSeptin9 to detect CRC was assessed in a subset of 7941 asymptomatic average risk adults undergoing screening colonoscopy.14 The sensitivity and specificity for CRC were 48% and 91.5%, respectively. The sensitivity for advanced adenomas was 11.2%. An increase in sensitivity to 63.9% and reduction in specificity to 88.4% for CRC was demonstrated in a sub-analysis of available samples where an additional (third) polymerase chain replicate was performed. Epi proColon™ is not currently reimbursed by Medicare and not endorsed in the latest USPSTF guidelines.
Technologic advancements have improved the detection of circulating tumor markers in the blood. The Shield™ BBT approved by the FDA in 2024 for average risk adults ≥ 45 years integrates three types of cfDNA data (epigenetic changes resulting in the aberrant methylation or fragmentation patterns, and genomic changes resulting in somatic mutations) into a positive or negative test result. In the trial, 22,877 average-risk, asymptomatic individuals ages 45–84 were enrolled and clinical validation was performed in 7,861 of the participants.15 The sensitivity for CRC was 83.1% which decreased to 55% for stage I tumors (see Table 1). CRC specificity was 89.6% and the sensitivity for advanced adenomas and large sessile serrated lesions was 13.2%.
Another BBT SimpleScreen™, which is not yet FDA-approved, analyzed circulating, cell-free DNA methylation patterns in 27,010 evaluable average-risk, asymptomatic adults ages 45–85 years undergoing screening colonoscopy.16 The sensitivity and specificity for CRC was 79.2% and 91.5%, respectively. Similar to Shield, the sensitivity for stage I CRC was low at 57.1%. The sensitivity for advanced precancerous lesions, a secondary endpoint, was 12.5% which did not meet the prespecified study criteria.
Effectiveness and Cost Effectiveness
Modeling studies have evaluated novel noninvasive CRC screening tests compared to FIT and colonoscopy.17-20 One compared a hypothetical BBT performed every 3 years that meets the minimum CMS threshold CRC sensitivity and specificity of 74% and 90%, respectively, to other established CRC screening tests beginning at age 45.17 Every 3-year BBT reduced CRC incidence and mortality by 40% and 52%, respectively compared to no screening. However, the reductions were much lower than yearly FIT (72% and 76%, respectively), every 10 year colonoscopy (79% and 81%, respectively), and triennial mt-sDNA (68% and 73%, respectively). The BBT resulted in fewer quality-adjusted life-years per person compared to the alternatives.
Additionally, FIT, colonoscopy, and mt-sDNA were less costly and more effective. Advanced precancerous lesion detection was a key measure for a test’s effectiveness. BBT characteristics would require a CRC sensitivity and specificity of >90% and 90%, respectively, and 80% sensitivity for advanced precancerous lesions at a cost of ≤$120–$140 to be cost-effective compared to FIT at comparable participation rates.
Another analysis simulated colorectal neoplasia progression and compared clinical effectiveness and cost between annual FIT, every 3 year stool mt-sRNA, every 3 year stool mt-sDNA tests, every 3 year stool Shield™; these outcomes were compared to colonoscopy every 10 years and no screening in adults ≥ age 45 over different adherence rates.19 At real-world adherence rates of 60%, colonoscopy prevented most CRC cases and associated deaths. FIT was the most cost-effective strategy at all adherence levels. Between the multi-target stool tests and Shield™, mt-sRNA was the most cost-effective. Compared to FIT, mt-sRNA reduced CRC cases and deaths by 1% and 14%.
The third study evaluated CRC incidence and mortality, quality-adjusted life-years and costs with annual FIT, colonoscopy every 10 years, mt- sDNA tests, mt-sRNA test, and BBTs.20 The latest mt-sDNA (Colguard plus™) and mt-sRNA achieved benefits approaching FIT but the Shield™ test was substantially less effective. The authors hypothesized that if 15% of the population substituted Shield™ for current effective CRC screening strategies, an increase in CRC deaths would occur and require 9-10% of the unscreened population to uptake screening with Shield to avert the increases in CRC deaths due to the substitution effect.
Clinical Implications
The effectiveness of non-invasive screening strategies depends on their diagnostic performance, adherence, and ensuring a timely colonoscopy after a positive test. Two claims-based studies found 47.9% and 49% of patients underwent follow-up colonoscopy within 6 months of an abnormal stool or BBT CRC screening test, respectively.21-22
Conclusions
Non-invasive stool mt-sDNA and mt-sRNA have higher effectiveness than the new BBTs. BBTs can lead to increased CRC mortality if substituted for the FDA and CMS-approved, USPSTF-endorsed, CRC screening modalities. If future BBTs increase their sensitivity for CRC (including early-stage CRC) and advanced precancerous lesions and decrease their cost, they may prove to have similar cost-effectiveness to stool-based tests. Currently, BBTs are not a substitute for colonoscopy or other stool tests and should be offered to patients who refuse other CRC screening modalities. A personalized, risk-adapted approach, paired with improved adherence and follow-up are essential to optimize the population-level impact of CRC screening and ensure equitable, effective cancer prevention.
Dr. Gupta is based at the Division of Gastroenterology and Hepatology, Department of Medicine, University of Maryland School of Medicine, Baltimore. Dr. Burke and Dr. Macaron are based at the Department of Gastroenterology, Hepatology, and Nutrition, Cleveland Clinic, Cleveland, Ohio. Dr. Gupta and Dr. Macaron declared no conflicts of interest in regard to this article. Dr. Burke declared research support from Emtora Biosciences. She is a current consultant for Lumabridge, and has been a consultant for Sebela and Almirall. She also disclosed support from Myriad, Genzyme, Ferring, Merck, Sharp and Dohme, Abbvie, Salix, and Natera.
References
1. Benavidez GA, Sedani AE, Felder TM, Asare M, Rogers CR. Rural-urban disparities and trends in cancer screening: an analysis of Behavioral Risk Factor Surveillance System data (2018-2022). JNCI Cancer Spectr. 2024 Nov 1;8(6):pkae113
2. Galoosian A, Dai H, Croymans D, et al. Population Health Colorectal Cancer Screening Strategies in Adults Aged 45 to 49 Years: A Randomized Clinical Trial. JAMA. 2025 Aug 4:e2512049. doi: 10.1001/jama.2025.12049. Epub ahead of print.
3. Pilonis ND, Bugajski M, Wieszczy P, et al. Participation in Competing Strategies for Colorectal Cancer Screening: A Randomized Health Services Study (PICCOLINO Study). Gastroenterology. 2021 Mar;160(4):1097-1105.
4. Shaukat A, Mongin SJ, Geisser MS, et al. Long-term mortality after screening for colorectal cancer. N Engl J Med. 2013;369(12):1106–1114.
5. Kronborg O, Fenger C, Olsen J, Jørgensen OD, Søndergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996 Nov 30;348(9040):1467-71. doi: 10.1016/S0140-6736(96)03430-7. PMID: 8942774.
6. Burke CA, Lieberman D, Feuerstein JD. AGA Clinical Practice Update on Approach to the Use of Noninvasive Colorectal Cancer Screening Options: Commentary. Gastroenterology. 2022 Mar;162(3):952-956. doi: 10.1053/j.gastro.2021.09.075. Epub 2022 Jan 28. PMID: 35094786.
7. Imperiale TF, Gruber RN Stump TE, et al. Performance characteristics of fecal immunochemical tests for colorectal cancer and advanced adenomatous polyps: a systematic review and meta-analysis. Ann Intern Med 2019; 170(5):319-329
8. Doubeni CA, Corley DA, Jensen CD, et al. Fecal Immunochemical Test Screening and Risk of Colorectal Cancer Death. JAMA Netw Open. 2024 Jul 1;7(7):e2423671. doi: 10.1001/jamanetworkopen.2024.23671.
9. Chiu HM, Jen GH, Wang YW, et al. Long-term effectiveness of faecal immunochemical test screening for proximal and distal colorectal cancers. Gut. 2021 Dec;70(12):2321-2329. doi: 10.1136/gutjnl-2020-322545. Epub 2021 Jan 25.
10. Castells A, Quintero E, Bujanda L, et al; COLONPREV study investigators. Effect of invitation to colonoscopy versus fecal immunochemical test screening on colorectal cancer mortality (COLONPREV): a pragmatic, randomised, controlled, non-inferiority trial. Lancet. 2025;405(10486):1231–1239
11. Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014;370(14):1287-1297
12. Imperiale TF, Porter K, Zella J, et al. Next-Generation Multitarget Stool DNA Test for Colorectal Cancer Screening. N Engl J Med. 2024 Mar 14;390(11):984-993
13. Barnell EK, Wurtzler EM, La Rocca J, et al. Multitarget Stool RNA Test for Colorectal Cancer Screening. JAMA. 2023 Nov 14;330(18):1760-1768.
14. Church TR, Wandell M, Lofton-Day C, et al. Prospective evaluation of methylated SEPT9 in plasma for detection of asymptomatic colorectal cancer. Gut 2014; 63:317–325.
15. Chung DC, Gray DM 2nd, Singh H, et al. A Cell-free DNA Blood-Based Test for Colorectal Cancer Screening. N Engl J Med. 2024 Mar 14;390(11):973-983.
16. Shaukat A, Burke CA, Chan AT, et al. Clinical Validation of a Circulating Tumor DNA-Based Blood Test to Screen for Colorectal Cancer. JAMA. 2025 Jul 1;334(1):56-63.
17. Ladabaum U, Mannalithara A, Weng Y, et al. Comparative Effectiveness and Cost-Effectiveness of Colorectal Cancer Screening with Blood-Based Biomarkers (Liquid Biopsy) vs Fecal Tests or Colonoscopy. Gastroenterology. 2024 Jul;167(2):378-391.
18. van den Puttelaar R, Nascimento de Lima P, Knudsen AB, et al. Effectiveness and cost-effectiveness of colorectal cancer screening with a blood test that meets the Centers for Medicare & Medicaid Services coverage decision. Gastroenterology 2024;167:368–377.
19. Shaukat A, Levin TR, Liang PS. Cost-effectiveness of Novel Noninvasive Screening Tests for Colorectal Neoplasia. Clin Gastroenterol Hepatol. 2025 Jun 23:S1542-3565(25)00525-7. doi: 10.1016/j.cgh.2025.06.006. Epub ahead of print. PMID: 40562290.
20. Ladabaum U, Mannalithara A, Schoen RE, Dominitz JA, Lieberman D. Projected Impact and Cost-Effectiveness of Novel Molecular Blood-Based or Stool-Based Screening Tests for Colorectal Cancer. Ann Intern Med. 2024 Dec;177(12):1610-1620.
20. Ciemins EL, Mohl JT, Moreno CA, Colangelo F, Smith RA, Barton M. Development of a Follow-Up Measure to Ensure Complete Screening for Colorectal Cancer. JAMA Netw Open. 2024 Mar 4;7(3):e242693. doi: 10.1001/jamanetworkopen.2024.2693.
21. Zaki TA, Zhang NJ, Forbes SP, Raymond VM, Das AK, May FP. Colonoscopic Follow-up After Abnormal Blood-Based Colorectal Cancer Screening Results. Gastroenterology. 2025 Jul 21:S0016-5085(25)05775-0. doi: 10.1053/j.gastro.2025.07.019. Epub ahead of print. PMID: 40744392.
‘So You Have an Idea…’: A Practical Guide to Tech and Device Development for the Early Career GI
You are in the middle of a busy clinic day and think, “there has to be a better way to do this.” Suddenly, a better way to do something becomes obvious. Maybe it’s a tool that simplifies documentation, a device that improves patient comfort, or an app that bridges a clinical gap. Many physicians, especially early career gastroenterologists, have ideas like this, but few know what to do next.
This article is for the curious innovator at the beginning of their clinical career. It offers practical, real-world guidance on developing a clinical product: whether that be hardware, software, or a hybrid. It outlines what questions to ask, who to consult, and how to protect your work, using personal insights and business principles learned through lived experience.
1. Understand Intellectual Property (IP): Know Its Value and Ownership
What is IP?
Intellectual property refers to your original creations: inventions, designs, software, and more. This is what you want to protect legally through patents, trademarks, or copyrights.
Who owns your idea?
This is the first and most important question to ask. If you are employed (especially by a hospital or academic center), your contract may already give your employer rights to any inventions you create, even those developed in your personal time.
What to ask:
- Does my employment contract include an “assignment of inventions” clause?
- Does the institution claim rights to anything developed with institutional resources?
- Are there moonlighting or external activity policies that affect this?
If you are developing an idea on your personal time, with your own resources, and outside your scope of clinical duties, it might still be considered “theirs” under some contracts. Early legal consultation is critical. A specialized IP attorney can help you understand what you own and how to protect it. This should be done early, ideally before you start building anything.
2. Lawyers Aren’t Optional: They’re Essential Early Partners
You do not need a full legal team, but you do need a lawyer early. An early consultation with an IP attorney can clarify your rights, guide your filing process (e.g. provisional patents), and help you avoid costly missteps.
Do this before sharing your idea publicly, including in academic presentations, pitch competitions, or even on social media. Public disclosure can start a clock ticking for application to protect your IP.
3. Build a Founding Team with Intent
Think of your startup team like a long-term relationship: you’re committing to build something together through uncertainty, tension, and change.
Strong early-stage teams often include:
- The Visionary – understands the clinical need and vision
- The Builder – engineer, developer, or designer
- The Doer – project manager or operations lead
Before forming a company, clearly define:
- Ownership (equity percentages)
- Roles and responsibilities
- Time commitments
- What happens if someone exits
Have these discussions early and document your agreements. Avoid informal “handshake” deals that can lead to serious disputes later.
4. You Don’t Need to Know Everything on Day One
You do not need to know how to write code, build a prototype, or get FDA clearance on day one. Successful innovators are humble learners. Use a Minimum Viable Product (MVP), a simple, functional version of your idea, to test assumptions and gather feedback. Iterate based on what you learn. Do not chase perfection; pursue progress. Consider using online accelerators like Y Combinator’s startup school or AGA’s Center for GI Innovation and Technology.
5. Incubators: Use them Strategically
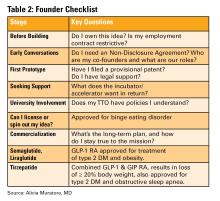
Incubators can offer mentorship, seed funding, legal support, and technical resources, but they vary widely in value (see Table 1). Many may want equity, and not all offer when you truly need.
Ask Yourself:
- Do I need technical help, business mentorship, or just accountability?
- What does this incubator offer in terms of IP protection, exposure, and connections?
- Do I understand the equity trade-off?
- What services and funding do they provide?
- Do they take equity? How much and when?
- What’s their track record with similar ventures?
- Are their incentives aligned with your vision?
6. Academic Institutions: Partners or Pitfalls?
Universities can provide credibility, resources, and early funding through their tech transfer office (TTO).
Key Questions to Ask:
- Will my IP be managed by the TTO?
- How much say do I have in licensing decisions?
- Are there royalty-sharing agreements in place?
- Can I form a startup while employed here?
You may need to negotiate if you want to commercialize your idea independently.
7. Do it for Purpose, Not Payday
Most founders end up owning only a small percentage of their company by the time a product reaches the market. Do not expect to get rich. Do it because it solves a problem you care about. If it happens to come with a nice paycheck, then that is an added bonus.
Your clinical training and insight give you a unique edge. You already know what’s broken. Use that as your compass.
Conclusion
Innovation isn’t about brilliance, it’s about curiosity, structure, and tenacity (see Table 2). Start small. Protect your work. Choose the right partners. Most importantly, stay anchored in your mission to make GI care better.
Dr. Muratore is based at UNC Rex Digestive Health, Raleigh, North Carolina. She has no conflicts related to this article. Dr. Wechsler is based at the University of North Carolina at Chapel Hill, Chapel Hill, North Carolina. She holds a patent assigned to Trustees of Dartmouth College. Dr. Shah is based at the University of Michigan, Ann Arbor, Michigan. He consults for Ardelyx, Laborie, Neuraxis, Salix, Sanofi, and Takeda and holds a patent with the Regents of the University of Michigan.
You are in the middle of a busy clinic day and think, “there has to be a better way to do this.” Suddenly, a better way to do something becomes obvious. Maybe it’s a tool that simplifies documentation, a device that improves patient comfort, or an app that bridges a clinical gap. Many physicians, especially early career gastroenterologists, have ideas like this, but few know what to do next.
This article is for the curious innovator at the beginning of their clinical career. It offers practical, real-world guidance on developing a clinical product: whether that be hardware, software, or a hybrid. It outlines what questions to ask, who to consult, and how to protect your work, using personal insights and business principles learned through lived experience.
1. Understand Intellectual Property (IP): Know Its Value and Ownership
What is IP?
Intellectual property refers to your original creations: inventions, designs, software, and more. This is what you want to protect legally through patents, trademarks, or copyrights.
Who owns your idea?
This is the first and most important question to ask. If you are employed (especially by a hospital or academic center), your contract may already give your employer rights to any inventions you create, even those developed in your personal time.
What to ask:
- Does my employment contract include an “assignment of inventions” clause?
- Does the institution claim rights to anything developed with institutional resources?
- Are there moonlighting or external activity policies that affect this?
If you are developing an idea on your personal time, with your own resources, and outside your scope of clinical duties, it might still be considered “theirs” under some contracts. Early legal consultation is critical. A specialized IP attorney can help you understand what you own and how to protect it. This should be done early, ideally before you start building anything.
2. Lawyers Aren’t Optional: They’re Essential Early Partners
You do not need a full legal team, but you do need a lawyer early. An early consultation with an IP attorney can clarify your rights, guide your filing process (e.g. provisional patents), and help you avoid costly missteps.
Do this before sharing your idea publicly, including in academic presentations, pitch competitions, or even on social media. Public disclosure can start a clock ticking for application to protect your IP.
3. Build a Founding Team with Intent
Think of your startup team like a long-term relationship: you’re committing to build something together through uncertainty, tension, and change.
Strong early-stage teams often include:
- The Visionary – understands the clinical need and vision
- The Builder – engineer, developer, or designer
- The Doer – project manager or operations lead
Before forming a company, clearly define:
- Ownership (equity percentages)
- Roles and responsibilities
- Time commitments
- What happens if someone exits
Have these discussions early and document your agreements. Avoid informal “handshake” deals that can lead to serious disputes later.
4. You Don’t Need to Know Everything on Day One
You do not need to know how to write code, build a prototype, or get FDA clearance on day one. Successful innovators are humble learners. Use a Minimum Viable Product (MVP), a simple, functional version of your idea, to test assumptions and gather feedback. Iterate based on what you learn. Do not chase perfection; pursue progress. Consider using online accelerators like Y Combinator’s startup school or AGA’s Center for GI Innovation and Technology.
5. Incubators: Use them Strategically
Incubators can offer mentorship, seed funding, legal support, and technical resources, but they vary widely in value (see Table 1). Many may want equity, and not all offer when you truly need.
Ask Yourself:
- Do I need technical help, business mentorship, or just accountability?
- What does this incubator offer in terms of IP protection, exposure, and connections?
- Do I understand the equity trade-off?
- What services and funding do they provide?
- Do they take equity? How much and when?
- What’s their track record with similar ventures?
- Are their incentives aligned with your vision?
6. Academic Institutions: Partners or Pitfalls?
Universities can provide credibility, resources, and early funding through their tech transfer office (TTO).
Key Questions to Ask:
- Will my IP be managed by the TTO?
- How much say do I have in licensing decisions?
- Are there royalty-sharing agreements in place?
- Can I form a startup while employed here?
You may need to negotiate if you want to commercialize your idea independently.
7. Do it for Purpose, Not Payday
Most founders end up owning only a small percentage of their company by the time a product reaches the market. Do not expect to get rich. Do it because it solves a problem you care about. If it happens to come with a nice paycheck, then that is an added bonus.
Your clinical training and insight give you a unique edge. You already know what’s broken. Use that as your compass.
Conclusion
Innovation isn’t about brilliance, it’s about curiosity, structure, and tenacity (see Table 2). Start small. Protect your work. Choose the right partners. Most importantly, stay anchored in your mission to make GI care better.
Dr. Muratore is based at UNC Rex Digestive Health, Raleigh, North Carolina. She has no conflicts related to this article. Dr. Wechsler is based at the University of North Carolina at Chapel Hill, Chapel Hill, North Carolina. She holds a patent assigned to Trustees of Dartmouth College. Dr. Shah is based at the University of Michigan, Ann Arbor, Michigan. He consults for Ardelyx, Laborie, Neuraxis, Salix, Sanofi, and Takeda and holds a patent with the Regents of the University of Michigan.
You are in the middle of a busy clinic day and think, “there has to be a better way to do this.” Suddenly, a better way to do something becomes obvious. Maybe it’s a tool that simplifies documentation, a device that improves patient comfort, or an app that bridges a clinical gap. Many physicians, especially early career gastroenterologists, have ideas like this, but few know what to do next.
This article is for the curious innovator at the beginning of their clinical career. It offers practical, real-world guidance on developing a clinical product: whether that be hardware, software, or a hybrid. It outlines what questions to ask, who to consult, and how to protect your work, using personal insights and business principles learned through lived experience.
1. Understand Intellectual Property (IP): Know Its Value and Ownership
What is IP?
Intellectual property refers to your original creations: inventions, designs, software, and more. This is what you want to protect legally through patents, trademarks, or copyrights.
Who owns your idea?
This is the first and most important question to ask. If you are employed (especially by a hospital or academic center), your contract may already give your employer rights to any inventions you create, even those developed in your personal time.
What to ask:
- Does my employment contract include an “assignment of inventions” clause?
- Does the institution claim rights to anything developed with institutional resources?
- Are there moonlighting or external activity policies that affect this?
If you are developing an idea on your personal time, with your own resources, and outside your scope of clinical duties, it might still be considered “theirs” under some contracts. Early legal consultation is critical. A specialized IP attorney can help you understand what you own and how to protect it. This should be done early, ideally before you start building anything.
2. Lawyers Aren’t Optional: They’re Essential Early Partners
You do not need a full legal team, but you do need a lawyer early. An early consultation with an IP attorney can clarify your rights, guide your filing process (e.g. provisional patents), and help you avoid costly missteps.
Do this before sharing your idea publicly, including in academic presentations, pitch competitions, or even on social media. Public disclosure can start a clock ticking for application to protect your IP.
3. Build a Founding Team with Intent
Think of your startup team like a long-term relationship: you’re committing to build something together through uncertainty, tension, and change.
Strong early-stage teams often include:
- The Visionary – understands the clinical need and vision
- The Builder – engineer, developer, or designer
- The Doer – project manager or operations lead
Before forming a company, clearly define:
- Ownership (equity percentages)
- Roles and responsibilities
- Time commitments
- What happens if someone exits
Have these discussions early and document your agreements. Avoid informal “handshake” deals that can lead to serious disputes later.
4. You Don’t Need to Know Everything on Day One
You do not need to know how to write code, build a prototype, or get FDA clearance on day one. Successful innovators are humble learners. Use a Minimum Viable Product (MVP), a simple, functional version of your idea, to test assumptions and gather feedback. Iterate based on what you learn. Do not chase perfection; pursue progress. Consider using online accelerators like Y Combinator’s startup school or AGA’s Center for GI Innovation and Technology.
5. Incubators: Use them Strategically
Incubators can offer mentorship, seed funding, legal support, and technical resources, but they vary widely in value (see Table 1). Many may want equity, and not all offer when you truly need.
Ask Yourself:
- Do I need technical help, business mentorship, or just accountability?
- What does this incubator offer in terms of IP protection, exposure, and connections?
- Do I understand the equity trade-off?
- What services and funding do they provide?
- Do they take equity? How much and when?
- What’s their track record with similar ventures?
- Are their incentives aligned with your vision?
6. Academic Institutions: Partners or Pitfalls?
Universities can provide credibility, resources, and early funding through their tech transfer office (TTO).
Key Questions to Ask:
- Will my IP be managed by the TTO?
- How much say do I have in licensing decisions?
- Are there royalty-sharing agreements in place?
- Can I form a startup while employed here?
You may need to negotiate if you want to commercialize your idea independently.
7. Do it for Purpose, Not Payday
Most founders end up owning only a small percentage of their company by the time a product reaches the market. Do not expect to get rich. Do it because it solves a problem you care about. If it happens to come with a nice paycheck, then that is an added bonus.
Your clinical training and insight give you a unique edge. You already know what’s broken. Use that as your compass.
Conclusion
Innovation isn’t about brilliance, it’s about curiosity, structure, and tenacity (see Table 2). Start small. Protect your work. Choose the right partners. Most importantly, stay anchored in your mission to make GI care better.
Dr. Muratore is based at UNC Rex Digestive Health, Raleigh, North Carolina. She has no conflicts related to this article. Dr. Wechsler is based at the University of North Carolina at Chapel Hill, Chapel Hill, North Carolina. She holds a patent assigned to Trustees of Dartmouth College. Dr. Shah is based at the University of Michigan, Ann Arbor, Michigan. He consults for Ardelyx, Laborie, Neuraxis, Salix, Sanofi, and Takeda and holds a patent with the Regents of the University of Michigan.
When Your First Job Isn’t Forever: Lessons from My Journey and What Early-Career GIs Need to Know
Introduction
For many of us in gastroenterology, landing that first attending job feels like the ultimate victory lap — the reward for all those years of training. We sign the contract, relocate, and imagine this will be our “forever job.” Reality often plays out differently.
In fact, 43% of physicians change jobs within five years, while 83% changed employers at least once in their careers.1 Even within our field — which is always in demand — turnover is high; 1 in 3 gastroenterologists are planning to leave their current role within two years.2 Why does this happen? More importantly, how do we navigate this transition with clarity and confidence as an early-career GI?
My Story: When I Dared to Change My “Forever Job”
When I signed my first attending contract, I didn’t negotiate a single thing. My priorities were simple: family in Toronto and visa requirements. After a decade of medical school, residency, and fellowship, everything else felt secondary. I was happy to be back home.
The job itself was good — reasonable hours, flexible colleagues, and ample opportunity to enhance my procedural skills. As I started carving out my niche in endobariatrics, the support I needed to grow further was not there. I kept telling myself that this job fulfilled my values and I needed to be patient: “this is my forever job. I am close to my family and that’s what matters.”
Then, during a suturing course at the American Society of Gastrointestinal Endoscopy, I had a casual chat with the course director (now my boss). It took me by surprise, but as the conversation continued, he offered me a job. It was tempting: the chance to build my own endobariatrics program with real institutional backing. The catch? It was in a city I had never been to, with no family or friends around. I politely said “no, thank you, I can’t.” He smiled, gave me his number, and said, “think about it.”
For the first time, I allowed myself to ask, “could I really leave my forever job?”
The Power of a Circle and a Spreadsheet
I leaned on my circle — a close group of fellowship friends who each took a turn being someone’s lifeline. We have monthly Zoom calls to talk about jobs, family, and career aspirations. When I shared my dilemma, I realized I wasn’t alone; one friend was also unhappy with her first job. Suddenly, we were asking one another, “can we really leave?”
I hired a career consultant familiar with physician visa issues — hands down, the best money I ever invested. The job search felt like dating: each interview was a first date; some needed a second or third date before I knew if it could be a match.
After every interview, I’d jump on Zoom with my circle. We’d screen-share my giant Excel spreadsheet — our decision matrix — with columns for everything I cared about:
- Institute
- Administrative Time
- Endobariatric support
- Director Title
- Salary
- On-call
- Vacation
- Proximity to airport
- Cost of living
- RVU percentage
- Endoscopy center buy-in
- Contract duration
- Support staff
- CME
We scored each job, line by line, and not a single job checked all the boxes. As I sat there in a state of decision paralysis, it became clear that this was not a simple decision.
The GI Community: A Small, Supportive World
The GI community is incredibly close-knit and kind-hearted. At every conference, I made a point to chat with as many colleagues as I could, to hear their perspectives on jobs and how they made tough career moves. Those conversations were real — no Google search or Excel sheet could offer the perspective and insight I gained by simply asking and leaning on the GI community.
Meanwhile, the person who had first offered me that job kept checking in, catching up at conferences, and bonding over our love for food and baking. With him, I never felt like I was being ‘interviewed’ — I felt valued. It did not feel like he was trying to fill a position with just anyone to improve the call pool. He genuinely wanted to understand what my goals were and how I envisioned my future. Through those conversations, he reminded me of my original passions, which were sidelined when so immersed in the daily routine.
I’ve learned that feeling valued doesn’t come from grand gestures in recruitment. It’s in the quiet signs of respect, trust, and being seen. He wasn’t looking for just anyone; he was looking for someone whose goals aligned with his group’s and someone in whom he wanted to invest. While others might chase the highest salary, the most flexible schedule, or the strongest ancillary support, I realized I valued something I did not realize that I was lacking until then: mentorship.
What I Learned: There is No Such Thing As “The Perfect Job”
After a full year of spreadsheets, Zoom calls, conference chats, and overthinking, I came to a big realization: there’s no perfect job — there’s no such thing as an ideal “forever job.” The only constant for humans is change. Our circumstances change, our priorities shift, our interests shuffle, and our finances evolve. The best job is simply the one that fits the stage of life you’re in at that given moment. For me, mentorship and growth became my top priorities, even if it meant moving away from family.
What Physicians Value Most in a Second Job
After their first job, early-career gastroenterologists often reevaluate what really matters. Recent surveys highlight four key priorities:
- Work-life balance:
In a 2022 CompHealth Group healthcare survey, 85% of physicians ranked work-life balance as their top job priority.3
- Mentorship and growth:
Nearly 1 in 3 physicians cited lack of mentorship or career advancement as their reason for leaving a first job, per the 2023 MGMA/Jackson Physician Search report.4
- Compensation:
While not always the main reason for leaving, 77% of physicians now list compensation as a top priority — a big jump from prior years.3
- Practice support:
Poor infrastructure, administrative overload, or understaffed teams are common dealbreakers. In the second job, physicians look for well-run practices with solid support staff and reduced burnout risk.5
Conclusion
Welcome the uncertainty, talk to your circle, lean on your community, and use a spreadsheet if you need to — but don’t forget to trust your gut. There’s no forever job or the perfect path, only the next move that feels most true to who you are in that moment.
Dr. Ismail (@mayyismail) is Assistant Professor of Clinical Medicine (Gastroenterology) at Temple University in Philadelphia, Pennsylvania. She declares no conflicts of interest.
References
1. CHG Healthcare. Survey: 62% of physicians made a career change in the last two years. CHG Healthcare blog. June 10, 2024. Accessed August 5, 2025.
2. Berg S. Physicians in these 10 specialties are less likely to quit. AMA News. Published June 24, 2025. Accessed July 2025.
3. Saley C. Survey: Work/life balance is #1 priority in physicians’ job search. CHG Healthcare Insights. March 10, 2022. Accessed August 2025.
4. Medical Group Management Association; Jackson Physician Search. Early‑Career Physician Recruiting & Retention Playbook. October 23, 2023. Accessed August 2025.
5. Von Rosenvinge EC, et al. A crisis in scope: Recruitment and retention challenges reported by VA gastroenterology section chiefs. Fed Pract. 2024 Aug. doi:10.12788/fp.0504.
Introduction
For many of us in gastroenterology, landing that first attending job feels like the ultimate victory lap — the reward for all those years of training. We sign the contract, relocate, and imagine this will be our “forever job.” Reality often plays out differently.
In fact, 43% of physicians change jobs within five years, while 83% changed employers at least once in their careers.1 Even within our field — which is always in demand — turnover is high; 1 in 3 gastroenterologists are planning to leave their current role within two years.2 Why does this happen? More importantly, how do we navigate this transition with clarity and confidence as an early-career GI?
My Story: When I Dared to Change My “Forever Job”
When I signed my first attending contract, I didn’t negotiate a single thing. My priorities were simple: family in Toronto and visa requirements. After a decade of medical school, residency, and fellowship, everything else felt secondary. I was happy to be back home.
The job itself was good — reasonable hours, flexible colleagues, and ample opportunity to enhance my procedural skills. As I started carving out my niche in endobariatrics, the support I needed to grow further was not there. I kept telling myself that this job fulfilled my values and I needed to be patient: “this is my forever job. I am close to my family and that’s what matters.”
Then, during a suturing course at the American Society of Gastrointestinal Endoscopy, I had a casual chat with the course director (now my boss). It took me by surprise, but as the conversation continued, he offered me a job. It was tempting: the chance to build my own endobariatrics program with real institutional backing. The catch? It was in a city I had never been to, with no family or friends around. I politely said “no, thank you, I can’t.” He smiled, gave me his number, and said, “think about it.”
For the first time, I allowed myself to ask, “could I really leave my forever job?”
The Power of a Circle and a Spreadsheet
I leaned on my circle — a close group of fellowship friends who each took a turn being someone’s lifeline. We have monthly Zoom calls to talk about jobs, family, and career aspirations. When I shared my dilemma, I realized I wasn’t alone; one friend was also unhappy with her first job. Suddenly, we were asking one another, “can we really leave?”
I hired a career consultant familiar with physician visa issues — hands down, the best money I ever invested. The job search felt like dating: each interview was a first date; some needed a second or third date before I knew if it could be a match.
After every interview, I’d jump on Zoom with my circle. We’d screen-share my giant Excel spreadsheet — our decision matrix — with columns for everything I cared about:
- Institute
- Administrative Time
- Endobariatric support
- Director Title
- Salary
- On-call
- Vacation
- Proximity to airport
- Cost of living
- RVU percentage
- Endoscopy center buy-in
- Contract duration
- Support staff
- CME
We scored each job, line by line, and not a single job checked all the boxes. As I sat there in a state of decision paralysis, it became clear that this was not a simple decision.
The GI Community: A Small, Supportive World
The GI community is incredibly close-knit and kind-hearted. At every conference, I made a point to chat with as many colleagues as I could, to hear their perspectives on jobs and how they made tough career moves. Those conversations were real — no Google search or Excel sheet could offer the perspective and insight I gained by simply asking and leaning on the GI community.
Meanwhile, the person who had first offered me that job kept checking in, catching up at conferences, and bonding over our love for food and baking. With him, I never felt like I was being ‘interviewed’ — I felt valued. It did not feel like he was trying to fill a position with just anyone to improve the call pool. He genuinely wanted to understand what my goals were and how I envisioned my future. Through those conversations, he reminded me of my original passions, which were sidelined when so immersed in the daily routine.
I’ve learned that feeling valued doesn’t come from grand gestures in recruitment. It’s in the quiet signs of respect, trust, and being seen. He wasn’t looking for just anyone; he was looking for someone whose goals aligned with his group’s and someone in whom he wanted to invest. While others might chase the highest salary, the most flexible schedule, or the strongest ancillary support, I realized I valued something I did not realize that I was lacking until then: mentorship.
What I Learned: There is No Such Thing As “The Perfect Job”
After a full year of spreadsheets, Zoom calls, conference chats, and overthinking, I came to a big realization: there’s no perfect job — there’s no such thing as an ideal “forever job.” The only constant for humans is change. Our circumstances change, our priorities shift, our interests shuffle, and our finances evolve. The best job is simply the one that fits the stage of life you’re in at that given moment. For me, mentorship and growth became my top priorities, even if it meant moving away from family.
What Physicians Value Most in a Second Job
After their first job, early-career gastroenterologists often reevaluate what really matters. Recent surveys highlight four key priorities:
- Work-life balance:
In a 2022 CompHealth Group healthcare survey, 85% of physicians ranked work-life balance as their top job priority.3
- Mentorship and growth:
Nearly 1 in 3 physicians cited lack of mentorship or career advancement as their reason for leaving a first job, per the 2023 MGMA/Jackson Physician Search report.4
- Compensation:
While not always the main reason for leaving, 77% of physicians now list compensation as a top priority — a big jump from prior years.3
- Practice support:
Poor infrastructure, administrative overload, or understaffed teams are common dealbreakers. In the second job, physicians look for well-run practices with solid support staff and reduced burnout risk.5
Conclusion
Welcome the uncertainty, talk to your circle, lean on your community, and use a spreadsheet if you need to — but don’t forget to trust your gut. There’s no forever job or the perfect path, only the next move that feels most true to who you are in that moment.
Dr. Ismail (@mayyismail) is Assistant Professor of Clinical Medicine (Gastroenterology) at Temple University in Philadelphia, Pennsylvania. She declares no conflicts of interest.
References
1. CHG Healthcare. Survey: 62% of physicians made a career change in the last two years. CHG Healthcare blog. June 10, 2024. Accessed August 5, 2025.
2. Berg S. Physicians in these 10 specialties are less likely to quit. AMA News. Published June 24, 2025. Accessed July 2025.
3. Saley C. Survey: Work/life balance is #1 priority in physicians’ job search. CHG Healthcare Insights. March 10, 2022. Accessed August 2025.
4. Medical Group Management Association; Jackson Physician Search. Early‑Career Physician Recruiting & Retention Playbook. October 23, 2023. Accessed August 2025.
5. Von Rosenvinge EC, et al. A crisis in scope: Recruitment and retention challenges reported by VA gastroenterology section chiefs. Fed Pract. 2024 Aug. doi:10.12788/fp.0504.
Introduction
For many of us in gastroenterology, landing that first attending job feels like the ultimate victory lap — the reward for all those years of training. We sign the contract, relocate, and imagine this will be our “forever job.” Reality often plays out differently.
In fact, 43% of physicians change jobs within five years, while 83% changed employers at least once in their careers.1 Even within our field — which is always in demand — turnover is high; 1 in 3 gastroenterologists are planning to leave their current role within two years.2 Why does this happen? More importantly, how do we navigate this transition with clarity and confidence as an early-career GI?
My Story: When I Dared to Change My “Forever Job”
When I signed my first attending contract, I didn’t negotiate a single thing. My priorities were simple: family in Toronto and visa requirements. After a decade of medical school, residency, and fellowship, everything else felt secondary. I was happy to be back home.
The job itself was good — reasonable hours, flexible colleagues, and ample opportunity to enhance my procedural skills. As I started carving out my niche in endobariatrics, the support I needed to grow further was not there. I kept telling myself that this job fulfilled my values and I needed to be patient: “this is my forever job. I am close to my family and that’s what matters.”
Then, during a suturing course at the American Society of Gastrointestinal Endoscopy, I had a casual chat with the course director (now my boss). It took me by surprise, but as the conversation continued, he offered me a job. It was tempting: the chance to build my own endobariatrics program with real institutional backing. The catch? It was in a city I had never been to, with no family or friends around. I politely said “no, thank you, I can’t.” He smiled, gave me his number, and said, “think about it.”
For the first time, I allowed myself to ask, “could I really leave my forever job?”
The Power of a Circle and a Spreadsheet
I leaned on my circle — a close group of fellowship friends who each took a turn being someone’s lifeline. We have monthly Zoom calls to talk about jobs, family, and career aspirations. When I shared my dilemma, I realized I wasn’t alone; one friend was also unhappy with her first job. Suddenly, we were asking one another, “can we really leave?”
I hired a career consultant familiar with physician visa issues — hands down, the best money I ever invested. The job search felt like dating: each interview was a first date; some needed a second or third date before I knew if it could be a match.
After every interview, I’d jump on Zoom with my circle. We’d screen-share my giant Excel spreadsheet — our decision matrix — with columns for everything I cared about:
- Institute
- Administrative Time
- Endobariatric support
- Director Title
- Salary
- On-call
- Vacation
- Proximity to airport
- Cost of living
- RVU percentage
- Endoscopy center buy-in
- Contract duration
- Support staff
- CME
We scored each job, line by line, and not a single job checked all the boxes. As I sat there in a state of decision paralysis, it became clear that this was not a simple decision.
The GI Community: A Small, Supportive World
The GI community is incredibly close-knit and kind-hearted. At every conference, I made a point to chat with as many colleagues as I could, to hear their perspectives on jobs and how they made tough career moves. Those conversations were real — no Google search or Excel sheet could offer the perspective and insight I gained by simply asking and leaning on the GI community.
Meanwhile, the person who had first offered me that job kept checking in, catching up at conferences, and bonding over our love for food and baking. With him, I never felt like I was being ‘interviewed’ — I felt valued. It did not feel like he was trying to fill a position with just anyone to improve the call pool. He genuinely wanted to understand what my goals were and how I envisioned my future. Through those conversations, he reminded me of my original passions, which were sidelined when so immersed in the daily routine.
I’ve learned that feeling valued doesn’t come from grand gestures in recruitment. It’s in the quiet signs of respect, trust, and being seen. He wasn’t looking for just anyone; he was looking for someone whose goals aligned with his group’s and someone in whom he wanted to invest. While others might chase the highest salary, the most flexible schedule, or the strongest ancillary support, I realized I valued something I did not realize that I was lacking until then: mentorship.
What I Learned: There is No Such Thing As “The Perfect Job”
After a full year of spreadsheets, Zoom calls, conference chats, and overthinking, I came to a big realization: there’s no perfect job — there’s no such thing as an ideal “forever job.” The only constant for humans is change. Our circumstances change, our priorities shift, our interests shuffle, and our finances evolve. The best job is simply the one that fits the stage of life you’re in at that given moment. For me, mentorship and growth became my top priorities, even if it meant moving away from family.
What Physicians Value Most in a Second Job
After their first job, early-career gastroenterologists often reevaluate what really matters. Recent surveys highlight four key priorities:
- Work-life balance:
In a 2022 CompHealth Group healthcare survey, 85% of physicians ranked work-life balance as their top job priority.3
- Mentorship and growth:
Nearly 1 in 3 physicians cited lack of mentorship or career advancement as their reason for leaving a first job, per the 2023 MGMA/Jackson Physician Search report.4
- Compensation:
While not always the main reason for leaving, 77% of physicians now list compensation as a top priority — a big jump from prior years.3
- Practice support:
Poor infrastructure, administrative overload, or understaffed teams are common dealbreakers. In the second job, physicians look for well-run practices with solid support staff and reduced burnout risk.5
Conclusion
Welcome the uncertainty, talk to your circle, lean on your community, and use a spreadsheet if you need to — but don’t forget to trust your gut. There’s no forever job or the perfect path, only the next move that feels most true to who you are in that moment.
Dr. Ismail (@mayyismail) is Assistant Professor of Clinical Medicine (Gastroenterology) at Temple University in Philadelphia, Pennsylvania. She declares no conflicts of interest.
References
1. CHG Healthcare. Survey: 62% of physicians made a career change in the last two years. CHG Healthcare blog. June 10, 2024. Accessed August 5, 2025.
2. Berg S. Physicians in these 10 specialties are less likely to quit. AMA News. Published June 24, 2025. Accessed July 2025.
3. Saley C. Survey: Work/life balance is #1 priority in physicians’ job search. CHG Healthcare Insights. March 10, 2022. Accessed August 2025.
4. Medical Group Management Association; Jackson Physician Search. Early‑Career Physician Recruiting & Retention Playbook. October 23, 2023. Accessed August 2025.
5. Von Rosenvinge EC, et al. A crisis in scope: Recruitment and retention challenges reported by VA gastroenterology section chiefs. Fed Pract. 2024 Aug. doi:10.12788/fp.0504.
Developing the Next Generation of GI Leaders

In this episode of Private Practice Perspectives, Dr. Naresh Gunaratnam, current president and board chair of Digestive Health Physician Association, speaks with Dr. Larry Kim, current president of AGA, about .

In this episode of Private Practice Perspectives, Dr. Naresh Gunaratnam, current president and board chair of Digestive Health Physician Association, speaks with Dr. Larry Kim, current president of AGA, about .

In this episode of Private Practice Perspectives, Dr. Naresh Gunaratnam, current president and board chair of Digestive Health Physician Association, speaks with Dr. Larry Kim, current president of AGA, about .
Approach to Weight Management in GI Practice
Introduction
The majority of patients in the United States are now overweight or obese, and as gastroenterologists we treat a number of conditions that are caused or worsened by obesity.1 Cirrhosis related to metabolic associated fatty liver disease (MAFLD) is now a leading indication for liver transplantation in the US2 and obesity is a clear risk factor for all major malignancies of the GI tract, including esophageal, gastric cardia, pancreatic, liver, gallbladder, colon, and rectum.3 Obesity is associated with dysbiosis and impacts barrier function: increasing permeability, abnormal gut bacterial translocation, and inflammation.4 It is more common than malnutrition in our patients with inflammatory bowel disease (IBD), where it impacts response to biologic drugs, increases the technical difficulty of surgeries, such as IPAA, and is associated with worse surgical outcomes.5 Furthermore, patients with obesity may be less likely to undergo preventative cancer screenings and are at increased risk related to sedation for endoscopic procedures.6 With over 40% of Americans suffering from obesity, and increasingly effective treatments available,
Understanding the Mechanisms of Obesity
There are complex orexigenic and anorexigenic brain pathways in the hypothalamus which control global energy balance.7 Obesity results when energy intake exceeds energy expenditure. While overeating and a sedentary lifestyle are commonly blamed, there are a number of elements that contribute, including genetics, medical conditions, medications, psychosocial factors, and environmental components. For example, sleep loss contributes to weight gain by several mechanisms including increasing ghrelin and decreasing leptin levels, thereby increasing hunger and appetite, as well as by decreasing insulin sensitivity and increasing cortisol. Subjects exposed to sleep deprivation in research settings take in 550 kcal more the following day.8 Medications used commonly in GI practice including corticosteroids, antihistamines, propranolol, and amitriptyline, are obesogenic9 and cannabis can impact hypothalamic pathways to stimulate hunger.10
When patients diet or exercise to lose weight, as we have traditionally advised, there are strong hormonal changes and metabolic adaptations that occur to preserve the defended fat mass or “set point.” Loss of adipose tissue results in decreased production of leptin, a hormone that stimulates satiety pathways and inhibits orexigenic pathways, greatly increasing hunger and cravings. Increases in ghrelin production by the stomach decreases perceptions of fullness. With weight loss, energy requirements decrease, and muscles become more efficient, meaning fewer kcal are needed to maintain bodily processes.11 Eventually a plateau is reached, while motivation to diet and restraint around food wane, and hedonistic (reward) pathways are activated. These powerful factors result in the regain of lost weight within one year in the majority of patients.
Implementing Weight Management into GI Practice
Given the stigma and bias around obesity, patients often feel shame and vulnerability around the condition. It is important to have empathy in your approach, asking permission to discuss weight and using patient-first language (e.g. “patient with obesity” not “obese patient”). While BMI is predictive of health outcomes, it does not measure body fat percentage and may be misleading, such as in muscular individuals. Other measures of adiposity including waist circumference and body composition testing, such as with DEXA, may provide additional data. A BMI of 30 or above defines obesity, though newer definitions incorporate related symptoms, organ disfunction, and metabolic abnormalities into the term “clinical obesity.”12 Asian patients experience metabolic complications at a lower BMI, and therefore the definition of obese is 27.5kg/m2 in this population.
Begin by taking a weight history. Has this been a lifelong struggle or is there a particular life circumstance, such as working a third shift or recent pregnancy which precipitated weight gain? Patients should be asked about binge eating or eating late into the evening or waking at night to eat, as these disordered eating behaviors are managed with specific medications and behavioral therapies. Inquire about sleep duration and quality and refer for a sleep study if there is suspicion for obstructive sleep apnea. Other weight-related comorbidities including hyperlipidemia, type 2 diabetes mellitus (T2DM), and MAFLD should be considered and merit a more aggressive approach, as does more severe obesity (class III, BMI ≥40). Questions about marijuana and alcohol use as well as review of the medication list for obesogenic medications can provide further insight into modifiable contributing factors.
Pillars of Weight Management
The internet is awash with trendy diet recommendations, and widespread misconceptions about obesity management are even ingrained into how physicians approach the disease. It is critical to remember that this is not a consequence of bad choices or lack of self-control. Exercise alone is insufficient to result in significant weight loss.13 Furthermore, whether it is through low fat, low carb, or intermittent fasting, weight loss will occur with calorie deficit.14 Evidence-based diet and lifestyle recommendations to lay the groundwork for success should be discussed at each visit (see Table 1). The Mediterranean diet is recommended for weight loss as well as for several GI disorders (i.e., MAFLD and IBD) and is the optimal eating strategy for cardiovascular health.15 Patients should be advised to engage in 150 minutes of moderate exercise per week, such as brisk walking, and should incorporate resistance training to build muscle and maintain bone density.
Anti-obesity Medications
There are a number of medications, either FDA approved or used off label, for treatment of obesity (see Table 2).16 All are indicated for patients with a BMI of ≥ 30 kg/m2 or for those with a BMI between 27-29 kg/m2 with weight-related comorbidities and should be used in combination with diet and lifestyle interventions. None are approved or safe in pregnancy. Mechanisms of action vary by type and include decreased appetite, increased energy expenditure, improved insulin sensitivity, and interfere with absorption.
The newest and most effective anti-obesity medications (AOM), the glucagon-like peptide-1 receptor agonists (GLP-1 RA) are derived from gut hormones secreted in the distal small bowel and colon in response to a meal, which function to delay gastric emptying, increase insulin release from the pancreas, and reduce hepatic gluconeogenesis. Central nervous system effects are not yet entirely understood, but function to decrease appetite and increase satiety. Initially developed for treatment of T2DM, observed weight reduction in patients treated with GLP-1 RA led to clinical trials for treatment of obesity. Semaglutide treatment resulted in weight reduction of 16.9% of total body weight (TBW), and one third of subjects lost ≥ 20% of TBW.17 Tirzepatide combines GLP-1 RA and a gastric inhibitory polypeptide (GIP) receptor agonist, which also has an incretin effect and functions to slow gastric emptying. In the pivotal SURMOUNT trial, approximately 58% of patients achieved ≥20% loss of TBW18 with 15mg weekly dosing of tirzepatide. This class of drugs is a logical choice in patients with T2DM and obesity. Long-term treatment appears necessary, as patients typically regain two-thirds of lost weight within a year after GLP-1 RA are stopped.
Based on tumors observed in rodents, GLP-1 RA are contraindicated in patients with a personal or family history of multiple endocrine neoplasia type 2 (MEN II) or medullary thyroid cancer. These tumors have not been observed in humans treated with GLP-1 RA. They should be used with caution in patients with history of pancreatitis, gastroparesis, or diabetic retinopathy, though a recent systematic review and meta-analysis suggests showed little to no increased risk for biliary events from GLP-1 RA.19 Side effects are most commonly gastrointestinal in nature (nausea, reflux, constipation or diarrhea) and are typically most severe with initiation of the drug and with dose escalation. Side effects can be mitigated by initiating these drugs at lowest doses and gradually titrating up (every four weeks) based on effectiveness and tolerability. Antisecretory, antiemetic, and laxative medications can also be used to help manage GLP-1 RA related side effects.
There is no reason to escalate to highest doses if patients are experiencing weight loss and reduction in food cravings at lower doses. Both semaglutide and tirzepatide are administered subcutaneously every seven days. Once patients have reached goal weight, they can either continue maintenance therapy at that same dose/interval, or if motivated to do so, may gradually reduce the weekly dose in a stepwise approach to determine the minimally effective dose to maintain weight loss. There are not yet published maintenance studies to guide this process. Currently the price of GLP-1 RA and inconsistent insurance coverage make them inaccessible to many patients. The manufacturers of both semaglutide and tirzepatide offer direct to consumer pricing and home delivery.
Bariatric Surgery
In patients with higher BMI (≥35kg/m2) or those with BMI ≥30kg/m2 and obesity-related metabolic disease and the desire to avoid lifelong medications or who fail or are intolerant of AOM, bariatric options should be considered.20 Sleeve gastrectomy has become the most performed surgery for treatment of obesity. It is a restrictive procedure, removing 80% of the stomach, but a drop in circulating levels of ghrelin afterwards also leads to decreased feelings of hunger. It results in weight loss of 25-30% TBW loss. It is not a good choice for patients who suffer from severe GERD, as this typically worsens afterwards; furthermore, de novo Barrett’s has been observed in nearly 6% of patients who undergo sleeve gastrectomy.21
Roux-en-Y gastric bypass is a restrictive and malabsorptive procedure, resulting in 30-35% TBW loss. It has beneficial and immediate metabolic effects, including increased release of endogenous GLP-1, which leads to improvements in weight-related T2DM. The newer single anastomosis duodenal-ileal bypass with sleeve gastrectomy (SADI-S) starts with a sleeve gastrectomy, making a smaller tube-shaped stomach. The duodenum is divided just after the stomach and then a loop of ileum is brought up and connected to the stomach (see Figure 1). This procedure is highly effective, with patients losing 75-95% of excess body weight and is becoming a preferred option for patients with greater BMI (≥50kg/m2). It is also an option for patients who have already had a sleeve gastrectomy and are seeking further weight loss. Because there is only one anastomosis, perioperative complications, such as anastomotic leaks, are reduced. The risk of micronutrient deficiencies is present with all malabsorptive procedures, and these patients must supplement with multivitamins, iron, vitamin D, and calcium.
Endoscopic Therapies
Endoscopic bariatric and metabolic therapies (EBMTs) have been increasingly studied and utilized, and this less invasive option may be more appropriate for or attractive to many patients. Intragastric balloons, which reduce meal volume and delay gastric emptying, can be used short term only (six months) resulting in loss of about 6.9% of total body weight (TBW) greater than lifestyle modification (LM) alone, and may be considered in limited situations, such as need for pre-operative weight loss to reduce risks in very obese individuals.22
Endoscopic gastric remodeling (EGR), also known as endoscopic sleeve gastrectomy (ESG), is a purely restrictive procedure in which the stomach is cinched to resize and reshape using an endoscopic suturing device (see Figure 2).23 It is an option for patients with class 1 or 2 obesity, with data from a randomized controlled trial in this population demonstrating mean percentage of TBW loss of 13.6% at 52 weeks compared to 0.8% in those treated with LM alone.24 A recent meta-analysis of 21 observational studies, including patients with higher BMIs (32.5 to 49.9 kg/m2) showed pooled average weight loss of 17.3% TBW at 12 months with EGR.22 This procedure has potential advantages of fewer complications, quicker recovery, and much less new-onset GERD compared to laparoscopic sleeve gastrectomy. Furthermore, it may be utilized in combination with AOMs to achieve optimum weight loss and metabolic outcomes.25,26 Potential adverse events include abdominal pain, nausea and vomiting (which may be severe), as well as rare instances of intra/extra luminal bleeding or abdominal abscess requiring drainage.22
Recent joint American/European Gastrointestinal Endoscopy guidelines suggest the use of EBMTs plus lifestyle modification in patients with a BMI of ≥ 30 kg/m2, or with a BMI of 27.0-29.9 kg/m2 with at least 1 obesity-related comorbidity.22 Small bowel interventions including duodenal-jejunal bypass liner and duodenal mucosal resurfacing are being investigated for patients with obesity and type 2 diabetes but not yet commercially available.
Conclusion
Given the overlap of obesity with many GI disorders, it is entirely appropriate for gastroenterologists to consider it worthy of aggressive treatment, particularly in patients with MAFLD and other serious weight related comorbidities. With a compassionate and empathetic approach, and a number of highly effective medical, endoscopic, and surgical therapies now available, weight management has the potential to be extremely rewarding when implemented in GI practice.
Dr. Kelly is based in the Department of Medicine, Division of Gastroenterology, Brigham and Women’s Hospital, and Harvard Medical School, both in Boston, Massachusetts. She serves on the clinical advisory board for OpenBiome (unpaid) and has served on an advisory board for Eli Lilly and Company.
References
1. Hales CM, et al. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017-2018. NCHS Data Brief 2020 Feb:(360):1–8.
2. Pais R, et al. NAFLD and liver transplantation: Current burden and expected challenges. J Hepatol. 2016 Dec. doi: 10.1016/j.jhep.2016.07.033.
3. Lauby-Secretan B, et al. Body Fatness and Cancer--Viewpoint of the IARC Working Group. N Engl J Med. 2016 Aug. doi: 10.1056/NEJMsr1606602.
4. Kim A. Dysbiosis: A Review Highlighting Obesity and Inflammatory Bowel Disease. J Clin Gastroenterol. 2015 Nov-Dec. doi: 10.1097/MCG.0000000000000356.
5. Singh S, et al. Obesity in IBD: epidemiology, pathogenesis, disease course and treatment outcomes. Nat Rev Gastroenterol Hepatol. 2017 Feb. doi: 10.1038/nrgastro.2016.181.
6. Sundararaman L, Goudra B. Sedation for GI Endoscopy in the Morbidly Obese: Challenges and Possible Solutions. J Clin Med. 2024 Aug. doi: 10.3390/jcm13164635.
7. Bombassaro B, et al. The hypothalamus as the central regulator of energy balance and its impact on current and future obesity treatments. Arch Endocrinol Metab. 2024 Nov. doi: 10.20945/2359-4292-2024-0082.
8. Beccuti G, Pannain S. Sleep and obesity. Curr Opin Clin Nutr Metab Care. 2011 Jul. doi: 10.1097/MCO.0b013e3283479109.
9. Desalermos A, et al. Effect of Obesogenic Medications on Weight-Loss Outcomes in a Behavioral Weight-Management Program. Obesity (Silver Spring). 2019 May. doi: 10.1002/oby.22444.
10. Lord MN, Noble EE. Hypothalamic cannabinoid signaling: Consequences for eating behavior. Pharmacol Res Perspect. 2024 Oct. doi: 10.1002/prp2.1251.
11. Farhana A, Rehman A. Metabolic Consequences of Weight Reduction. [Updated 2023 Jul 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK572145/.
12. Rubino F, et al. Definition and diagnostic criteria of clinical obesity. Lancet Diabetes Endocrinol. 2025 Mar. doi: 10.1016/S2213-8587(24)00316-4.
13. Cox CE. Role of Physical Activity for Weight Loss and Weight Maintenance. Diabetes Spectr. 2017 Aug. doi: 10.2337/ds17-0013.
14. Chaput JP, et al. Widespread misconceptions about obesity. Can Fam Physician. 2014 Nov. PMID: 25392431.
15. Muscogiuri G, et al. Mediterranean Diet and Obesity-related Disorders: What is the Evidence? Curr Obes Rep. 2022 Dec. doi: 10.1007/s13679-022-00481-1.
16. Gudzune KA, Kushner RF. Medications for Obesity: A Review. JAMA. 2024 Aug. doi: 10.1001/jama.2024.10816.
17. Wilding JPH, et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N Engl J Med. 2021 Feb. doi: 10.1056/NEJMoa2032183.
18. Jastreboff AM, et al. Tirzepatide Once Weekly for the Treatment of Obesity. N Engl J Med. 2022 Jun. doi: 10.1056/NEJMoa2206038.
19. Chiang CH, et al. Glucagon-Like Peptide-1 Receptor Agonists and Gastrointestinal Adverse Events: A Systematic Review and Meta-Analysis. Gastroenterology. 2025 Nov. doi: 10.1053/j.gastro.2025.06.003.
20. Aderinto N, et al. Recent advances in bariatric surgery: a narrative review of weight loss procedures. Ann Med Surg (Lond). 2023 Nov. doi: 10.1097/MS9.0000000000001472.
21. Chandan S, et al. Risk of De Novo Barrett’s Esophagus Post Sleeve Gastrectomy: A Systematic Review and Meta-Analysis of Studies With Long-Term Follow-Up. Clin Gastroenterol Hepatol. 2025 Jan. doi: 10.1016/j.cgh.2024.06.041.
22. Jirapinyo P, et al. American Society for Gastrointestinal Endoscopy-European Society of Gastrointestinal Endoscopy guideline on primary endoscopic bariatric and metabolic therapies for adults with obesity. Gastrointest Endosc. 2024 Jun. doi: 10.1016/j.gie.2023.12.004.
23. Nduma BN, et al. Endoscopic Gastric Sleeve: A Review of Literature. Cureus. 2023 Mar. doi: 10.7759/cureus.36353.
24. Abu Dayyeh BK, et al. Endoscopic sleeve gastroplasty for treatment of class 1 and 2 obesity (MERIT): a prospective, multicentre, randomised trial. Lancet. 2022 Aug. doi: 10.1016/S0140-6736(22)01280-6.
25. Gala K, et al. Outcomes of concomitant antiobesity medication use with endoscopic sleeve gastroplasty in clinical US settings. Obes Pillars. 2024 May. doi: 10.1016/j.obpill.2024.100112.
26. Chung CS, et al. Endoscopic sleeve gastroplasty combined with anti-obesity medication for better control of weight and diabetes. Clin Endosc. 2025 May. doi: 10.5946/ce.2024.274.
Introduction
The majority of patients in the United States are now overweight or obese, and as gastroenterologists we treat a number of conditions that are caused or worsened by obesity.1 Cirrhosis related to metabolic associated fatty liver disease (MAFLD) is now a leading indication for liver transplantation in the US2 and obesity is a clear risk factor for all major malignancies of the GI tract, including esophageal, gastric cardia, pancreatic, liver, gallbladder, colon, and rectum.3 Obesity is associated with dysbiosis and impacts barrier function: increasing permeability, abnormal gut bacterial translocation, and inflammation.4 It is more common than malnutrition in our patients with inflammatory bowel disease (IBD), where it impacts response to biologic drugs, increases the technical difficulty of surgeries, such as IPAA, and is associated with worse surgical outcomes.5 Furthermore, patients with obesity may be less likely to undergo preventative cancer screenings and are at increased risk related to sedation for endoscopic procedures.6 With over 40% of Americans suffering from obesity, and increasingly effective treatments available,
Understanding the Mechanisms of Obesity
There are complex orexigenic and anorexigenic brain pathways in the hypothalamus which control global energy balance.7 Obesity results when energy intake exceeds energy expenditure. While overeating and a sedentary lifestyle are commonly blamed, there are a number of elements that contribute, including genetics, medical conditions, medications, psychosocial factors, and environmental components. For example, sleep loss contributes to weight gain by several mechanisms including increasing ghrelin and decreasing leptin levels, thereby increasing hunger and appetite, as well as by decreasing insulin sensitivity and increasing cortisol. Subjects exposed to sleep deprivation in research settings take in 550 kcal more the following day.8 Medications used commonly in GI practice including corticosteroids, antihistamines, propranolol, and amitriptyline, are obesogenic9 and cannabis can impact hypothalamic pathways to stimulate hunger.10
When patients diet or exercise to lose weight, as we have traditionally advised, there are strong hormonal changes and metabolic adaptations that occur to preserve the defended fat mass or “set point.” Loss of adipose tissue results in decreased production of leptin, a hormone that stimulates satiety pathways and inhibits orexigenic pathways, greatly increasing hunger and cravings. Increases in ghrelin production by the stomach decreases perceptions of fullness. With weight loss, energy requirements decrease, and muscles become more efficient, meaning fewer kcal are needed to maintain bodily processes.11 Eventually a plateau is reached, while motivation to diet and restraint around food wane, and hedonistic (reward) pathways are activated. These powerful factors result in the regain of lost weight within one year in the majority of patients.
Implementing Weight Management into GI Practice
Given the stigma and bias around obesity, patients often feel shame and vulnerability around the condition. It is important to have empathy in your approach, asking permission to discuss weight and using patient-first language (e.g. “patient with obesity” not “obese patient”). While BMI is predictive of health outcomes, it does not measure body fat percentage and may be misleading, such as in muscular individuals. Other measures of adiposity including waist circumference and body composition testing, such as with DEXA, may provide additional data. A BMI of 30 or above defines obesity, though newer definitions incorporate related symptoms, organ disfunction, and metabolic abnormalities into the term “clinical obesity.”12 Asian patients experience metabolic complications at a lower BMI, and therefore the definition of obese is 27.5kg/m2 in this population.
Begin by taking a weight history. Has this been a lifelong struggle or is there a particular life circumstance, such as working a third shift or recent pregnancy which precipitated weight gain? Patients should be asked about binge eating or eating late into the evening or waking at night to eat, as these disordered eating behaviors are managed with specific medications and behavioral therapies. Inquire about sleep duration and quality and refer for a sleep study if there is suspicion for obstructive sleep apnea. Other weight-related comorbidities including hyperlipidemia, type 2 diabetes mellitus (T2DM), and MAFLD should be considered and merit a more aggressive approach, as does more severe obesity (class III, BMI ≥40). Questions about marijuana and alcohol use as well as review of the medication list for obesogenic medications can provide further insight into modifiable contributing factors.
Pillars of Weight Management
The internet is awash with trendy diet recommendations, and widespread misconceptions about obesity management are even ingrained into how physicians approach the disease. It is critical to remember that this is not a consequence of bad choices or lack of self-control. Exercise alone is insufficient to result in significant weight loss.13 Furthermore, whether it is through low fat, low carb, or intermittent fasting, weight loss will occur with calorie deficit.14 Evidence-based diet and lifestyle recommendations to lay the groundwork for success should be discussed at each visit (see Table 1). The Mediterranean diet is recommended for weight loss as well as for several GI disorders (i.e., MAFLD and IBD) and is the optimal eating strategy for cardiovascular health.15 Patients should be advised to engage in 150 minutes of moderate exercise per week, such as brisk walking, and should incorporate resistance training to build muscle and maintain bone density.
Anti-obesity Medications
There are a number of medications, either FDA approved or used off label, for treatment of obesity (see Table 2).16 All are indicated for patients with a BMI of ≥ 30 kg/m2 or for those with a BMI between 27-29 kg/m2 with weight-related comorbidities and should be used in combination with diet and lifestyle interventions. None are approved or safe in pregnancy. Mechanisms of action vary by type and include decreased appetite, increased energy expenditure, improved insulin sensitivity, and interfere with absorption.
The newest and most effective anti-obesity medications (AOM), the glucagon-like peptide-1 receptor agonists (GLP-1 RA) are derived from gut hormones secreted in the distal small bowel and colon in response to a meal, which function to delay gastric emptying, increase insulin release from the pancreas, and reduce hepatic gluconeogenesis. Central nervous system effects are not yet entirely understood, but function to decrease appetite and increase satiety. Initially developed for treatment of T2DM, observed weight reduction in patients treated with GLP-1 RA led to clinical trials for treatment of obesity. Semaglutide treatment resulted in weight reduction of 16.9% of total body weight (TBW), and one third of subjects lost ≥ 20% of TBW.17 Tirzepatide combines GLP-1 RA and a gastric inhibitory polypeptide (GIP) receptor agonist, which also has an incretin effect and functions to slow gastric emptying. In the pivotal SURMOUNT trial, approximately 58% of patients achieved ≥20% loss of TBW18 with 15mg weekly dosing of tirzepatide. This class of drugs is a logical choice in patients with T2DM and obesity. Long-term treatment appears necessary, as patients typically regain two-thirds of lost weight within a year after GLP-1 RA are stopped.
Based on tumors observed in rodents, GLP-1 RA are contraindicated in patients with a personal or family history of multiple endocrine neoplasia type 2 (MEN II) or medullary thyroid cancer. These tumors have not been observed in humans treated with GLP-1 RA. They should be used with caution in patients with history of pancreatitis, gastroparesis, or diabetic retinopathy, though a recent systematic review and meta-analysis suggests showed little to no increased risk for biliary events from GLP-1 RA.19 Side effects are most commonly gastrointestinal in nature (nausea, reflux, constipation or diarrhea) and are typically most severe with initiation of the drug and with dose escalation. Side effects can be mitigated by initiating these drugs at lowest doses and gradually titrating up (every four weeks) based on effectiveness and tolerability. Antisecretory, antiemetic, and laxative medications can also be used to help manage GLP-1 RA related side effects.
There is no reason to escalate to highest doses if patients are experiencing weight loss and reduction in food cravings at lower doses. Both semaglutide and tirzepatide are administered subcutaneously every seven days. Once patients have reached goal weight, they can either continue maintenance therapy at that same dose/interval, or if motivated to do so, may gradually reduce the weekly dose in a stepwise approach to determine the minimally effective dose to maintain weight loss. There are not yet published maintenance studies to guide this process. Currently the price of GLP-1 RA and inconsistent insurance coverage make them inaccessible to many patients. The manufacturers of both semaglutide and tirzepatide offer direct to consumer pricing and home delivery.
Bariatric Surgery
In patients with higher BMI (≥35kg/m2) or those with BMI ≥30kg/m2 and obesity-related metabolic disease and the desire to avoid lifelong medications or who fail or are intolerant of AOM, bariatric options should be considered.20 Sleeve gastrectomy has become the most performed surgery for treatment of obesity. It is a restrictive procedure, removing 80% of the stomach, but a drop in circulating levels of ghrelin afterwards also leads to decreased feelings of hunger. It results in weight loss of 25-30% TBW loss. It is not a good choice for patients who suffer from severe GERD, as this typically worsens afterwards; furthermore, de novo Barrett’s has been observed in nearly 6% of patients who undergo sleeve gastrectomy.21
Roux-en-Y gastric bypass is a restrictive and malabsorptive procedure, resulting in 30-35% TBW loss. It has beneficial and immediate metabolic effects, including increased release of endogenous GLP-1, which leads to improvements in weight-related T2DM. The newer single anastomosis duodenal-ileal bypass with sleeve gastrectomy (SADI-S) starts with a sleeve gastrectomy, making a smaller tube-shaped stomach. The duodenum is divided just after the stomach and then a loop of ileum is brought up and connected to the stomach (see Figure 1). This procedure is highly effective, with patients losing 75-95% of excess body weight and is becoming a preferred option for patients with greater BMI (≥50kg/m2). It is also an option for patients who have already had a sleeve gastrectomy and are seeking further weight loss. Because there is only one anastomosis, perioperative complications, such as anastomotic leaks, are reduced. The risk of micronutrient deficiencies is present with all malabsorptive procedures, and these patients must supplement with multivitamins, iron, vitamin D, and calcium.
Endoscopic Therapies
Endoscopic bariatric and metabolic therapies (EBMTs) have been increasingly studied and utilized, and this less invasive option may be more appropriate for or attractive to many patients. Intragastric balloons, which reduce meal volume and delay gastric emptying, can be used short term only (six months) resulting in loss of about 6.9% of total body weight (TBW) greater than lifestyle modification (LM) alone, and may be considered in limited situations, such as need for pre-operative weight loss to reduce risks in very obese individuals.22
Endoscopic gastric remodeling (EGR), also known as endoscopic sleeve gastrectomy (ESG), is a purely restrictive procedure in which the stomach is cinched to resize and reshape using an endoscopic suturing device (see Figure 2).23 It is an option for patients with class 1 or 2 obesity, with data from a randomized controlled trial in this population demonstrating mean percentage of TBW loss of 13.6% at 52 weeks compared to 0.8% in those treated with LM alone.24 A recent meta-analysis of 21 observational studies, including patients with higher BMIs (32.5 to 49.9 kg/m2) showed pooled average weight loss of 17.3% TBW at 12 months with EGR.22 This procedure has potential advantages of fewer complications, quicker recovery, and much less new-onset GERD compared to laparoscopic sleeve gastrectomy. Furthermore, it may be utilized in combination with AOMs to achieve optimum weight loss and metabolic outcomes.25,26 Potential adverse events include abdominal pain, nausea and vomiting (which may be severe), as well as rare instances of intra/extra luminal bleeding or abdominal abscess requiring drainage.22
Recent joint American/European Gastrointestinal Endoscopy guidelines suggest the use of EBMTs plus lifestyle modification in patients with a BMI of ≥ 30 kg/m2, or with a BMI of 27.0-29.9 kg/m2 with at least 1 obesity-related comorbidity.22 Small bowel interventions including duodenal-jejunal bypass liner and duodenal mucosal resurfacing are being investigated for patients with obesity and type 2 diabetes but not yet commercially available.
Conclusion
Given the overlap of obesity with many GI disorders, it is entirely appropriate for gastroenterologists to consider it worthy of aggressive treatment, particularly in patients with MAFLD and other serious weight related comorbidities. With a compassionate and empathetic approach, and a number of highly effective medical, endoscopic, and surgical therapies now available, weight management has the potential to be extremely rewarding when implemented in GI practice.
Dr. Kelly is based in the Department of Medicine, Division of Gastroenterology, Brigham and Women’s Hospital, and Harvard Medical School, both in Boston, Massachusetts. She serves on the clinical advisory board for OpenBiome (unpaid) and has served on an advisory board for Eli Lilly and Company.
References
1. Hales CM, et al. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017-2018. NCHS Data Brief 2020 Feb:(360):1–8.
2. Pais R, et al. NAFLD and liver transplantation: Current burden and expected challenges. J Hepatol. 2016 Dec. doi: 10.1016/j.jhep.2016.07.033.
3. Lauby-Secretan B, et al. Body Fatness and Cancer--Viewpoint of the IARC Working Group. N Engl J Med. 2016 Aug. doi: 10.1056/NEJMsr1606602.
4. Kim A. Dysbiosis: A Review Highlighting Obesity and Inflammatory Bowel Disease. J Clin Gastroenterol. 2015 Nov-Dec. doi: 10.1097/MCG.0000000000000356.
5. Singh S, et al. Obesity in IBD: epidemiology, pathogenesis, disease course and treatment outcomes. Nat Rev Gastroenterol Hepatol. 2017 Feb. doi: 10.1038/nrgastro.2016.181.
6. Sundararaman L, Goudra B. Sedation for GI Endoscopy in the Morbidly Obese: Challenges and Possible Solutions. J Clin Med. 2024 Aug. doi: 10.3390/jcm13164635.
7. Bombassaro B, et al. The hypothalamus as the central regulator of energy balance and its impact on current and future obesity treatments. Arch Endocrinol Metab. 2024 Nov. doi: 10.20945/2359-4292-2024-0082.
8. Beccuti G, Pannain S. Sleep and obesity. Curr Opin Clin Nutr Metab Care. 2011 Jul. doi: 10.1097/MCO.0b013e3283479109.
9. Desalermos A, et al. Effect of Obesogenic Medications on Weight-Loss Outcomes in a Behavioral Weight-Management Program. Obesity (Silver Spring). 2019 May. doi: 10.1002/oby.22444.
10. Lord MN, Noble EE. Hypothalamic cannabinoid signaling: Consequences for eating behavior. Pharmacol Res Perspect. 2024 Oct. doi: 10.1002/prp2.1251.
11. Farhana A, Rehman A. Metabolic Consequences of Weight Reduction. [Updated 2023 Jul 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK572145/.
12. Rubino F, et al. Definition and diagnostic criteria of clinical obesity. Lancet Diabetes Endocrinol. 2025 Mar. doi: 10.1016/S2213-8587(24)00316-4.
13. Cox CE. Role of Physical Activity for Weight Loss and Weight Maintenance. Diabetes Spectr. 2017 Aug. doi: 10.2337/ds17-0013.
14. Chaput JP, et al. Widespread misconceptions about obesity. Can Fam Physician. 2014 Nov. PMID: 25392431.
15. Muscogiuri G, et al. Mediterranean Diet and Obesity-related Disorders: What is the Evidence? Curr Obes Rep. 2022 Dec. doi: 10.1007/s13679-022-00481-1.
16. Gudzune KA, Kushner RF. Medications for Obesity: A Review. JAMA. 2024 Aug. doi: 10.1001/jama.2024.10816.
17. Wilding JPH, et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N Engl J Med. 2021 Feb. doi: 10.1056/NEJMoa2032183.
18. Jastreboff AM, et al. Tirzepatide Once Weekly for the Treatment of Obesity. N Engl J Med. 2022 Jun. doi: 10.1056/NEJMoa2206038.
19. Chiang CH, et al. Glucagon-Like Peptide-1 Receptor Agonists and Gastrointestinal Adverse Events: A Systematic Review and Meta-Analysis. Gastroenterology. 2025 Nov. doi: 10.1053/j.gastro.2025.06.003.
20. Aderinto N, et al. Recent advances in bariatric surgery: a narrative review of weight loss procedures. Ann Med Surg (Lond). 2023 Nov. doi: 10.1097/MS9.0000000000001472.
21. Chandan S, et al. Risk of De Novo Barrett’s Esophagus Post Sleeve Gastrectomy: A Systematic Review and Meta-Analysis of Studies With Long-Term Follow-Up. Clin Gastroenterol Hepatol. 2025 Jan. doi: 10.1016/j.cgh.2024.06.041.
22. Jirapinyo P, et al. American Society for Gastrointestinal Endoscopy-European Society of Gastrointestinal Endoscopy guideline on primary endoscopic bariatric and metabolic therapies for adults with obesity. Gastrointest Endosc. 2024 Jun. doi: 10.1016/j.gie.2023.12.004.
23. Nduma BN, et al. Endoscopic Gastric Sleeve: A Review of Literature. Cureus. 2023 Mar. doi: 10.7759/cureus.36353.
24. Abu Dayyeh BK, et al. Endoscopic sleeve gastroplasty for treatment of class 1 and 2 obesity (MERIT): a prospective, multicentre, randomised trial. Lancet. 2022 Aug. doi: 10.1016/S0140-6736(22)01280-6.
25. Gala K, et al. Outcomes of concomitant antiobesity medication use with endoscopic sleeve gastroplasty in clinical US settings. Obes Pillars. 2024 May. doi: 10.1016/j.obpill.2024.100112.
26. Chung CS, et al. Endoscopic sleeve gastroplasty combined with anti-obesity medication for better control of weight and diabetes. Clin Endosc. 2025 May. doi: 10.5946/ce.2024.274.
Introduction
The majority of patients in the United States are now overweight or obese, and as gastroenterologists we treat a number of conditions that are caused or worsened by obesity.1 Cirrhosis related to metabolic associated fatty liver disease (MAFLD) is now a leading indication for liver transplantation in the US2 and obesity is a clear risk factor for all major malignancies of the GI tract, including esophageal, gastric cardia, pancreatic, liver, gallbladder, colon, and rectum.3 Obesity is associated with dysbiosis and impacts barrier function: increasing permeability, abnormal gut bacterial translocation, and inflammation.4 It is more common than malnutrition in our patients with inflammatory bowel disease (IBD), where it impacts response to biologic drugs, increases the technical difficulty of surgeries, such as IPAA, and is associated with worse surgical outcomes.5 Furthermore, patients with obesity may be less likely to undergo preventative cancer screenings and are at increased risk related to sedation for endoscopic procedures.6 With over 40% of Americans suffering from obesity, and increasingly effective treatments available,
Understanding the Mechanisms of Obesity
There are complex orexigenic and anorexigenic brain pathways in the hypothalamus which control global energy balance.7 Obesity results when energy intake exceeds energy expenditure. While overeating and a sedentary lifestyle are commonly blamed, there are a number of elements that contribute, including genetics, medical conditions, medications, psychosocial factors, and environmental components. For example, sleep loss contributes to weight gain by several mechanisms including increasing ghrelin and decreasing leptin levels, thereby increasing hunger and appetite, as well as by decreasing insulin sensitivity and increasing cortisol. Subjects exposed to sleep deprivation in research settings take in 550 kcal more the following day.8 Medications used commonly in GI practice including corticosteroids, antihistamines, propranolol, and amitriptyline, are obesogenic9 and cannabis can impact hypothalamic pathways to stimulate hunger.10
When patients diet or exercise to lose weight, as we have traditionally advised, there are strong hormonal changes and metabolic adaptations that occur to preserve the defended fat mass or “set point.” Loss of adipose tissue results in decreased production of leptin, a hormone that stimulates satiety pathways and inhibits orexigenic pathways, greatly increasing hunger and cravings. Increases in ghrelin production by the stomach decreases perceptions of fullness. With weight loss, energy requirements decrease, and muscles become more efficient, meaning fewer kcal are needed to maintain bodily processes.11 Eventually a plateau is reached, while motivation to diet and restraint around food wane, and hedonistic (reward) pathways are activated. These powerful factors result in the regain of lost weight within one year in the majority of patients.
Implementing Weight Management into GI Practice
Given the stigma and bias around obesity, patients often feel shame and vulnerability around the condition. It is important to have empathy in your approach, asking permission to discuss weight and using patient-first language (e.g. “patient with obesity” not “obese patient”). While BMI is predictive of health outcomes, it does not measure body fat percentage and may be misleading, such as in muscular individuals. Other measures of adiposity including waist circumference and body composition testing, such as with DEXA, may provide additional data. A BMI of 30 or above defines obesity, though newer definitions incorporate related symptoms, organ disfunction, and metabolic abnormalities into the term “clinical obesity.”12 Asian patients experience metabolic complications at a lower BMI, and therefore the definition of obese is 27.5kg/m2 in this population.
Begin by taking a weight history. Has this been a lifelong struggle or is there a particular life circumstance, such as working a third shift or recent pregnancy which precipitated weight gain? Patients should be asked about binge eating or eating late into the evening or waking at night to eat, as these disordered eating behaviors are managed with specific medications and behavioral therapies. Inquire about sleep duration and quality and refer for a sleep study if there is suspicion for obstructive sleep apnea. Other weight-related comorbidities including hyperlipidemia, type 2 diabetes mellitus (T2DM), and MAFLD should be considered and merit a more aggressive approach, as does more severe obesity (class III, BMI ≥40). Questions about marijuana and alcohol use as well as review of the medication list for obesogenic medications can provide further insight into modifiable contributing factors.
Pillars of Weight Management
The internet is awash with trendy diet recommendations, and widespread misconceptions about obesity management are even ingrained into how physicians approach the disease. It is critical to remember that this is not a consequence of bad choices or lack of self-control. Exercise alone is insufficient to result in significant weight loss.13 Furthermore, whether it is through low fat, low carb, or intermittent fasting, weight loss will occur with calorie deficit.14 Evidence-based diet and lifestyle recommendations to lay the groundwork for success should be discussed at each visit (see Table 1). The Mediterranean diet is recommended for weight loss as well as for several GI disorders (i.e., MAFLD and IBD) and is the optimal eating strategy for cardiovascular health.15 Patients should be advised to engage in 150 minutes of moderate exercise per week, such as brisk walking, and should incorporate resistance training to build muscle and maintain bone density.
Anti-obesity Medications
There are a number of medications, either FDA approved or used off label, for treatment of obesity (see Table 2).16 All are indicated for patients with a BMI of ≥ 30 kg/m2 or for those with a BMI between 27-29 kg/m2 with weight-related comorbidities and should be used in combination with diet and lifestyle interventions. None are approved or safe in pregnancy. Mechanisms of action vary by type and include decreased appetite, increased energy expenditure, improved insulin sensitivity, and interfere with absorption.
The newest and most effective anti-obesity medications (AOM), the glucagon-like peptide-1 receptor agonists (GLP-1 RA) are derived from gut hormones secreted in the distal small bowel and colon in response to a meal, which function to delay gastric emptying, increase insulin release from the pancreas, and reduce hepatic gluconeogenesis. Central nervous system effects are not yet entirely understood, but function to decrease appetite and increase satiety. Initially developed for treatment of T2DM, observed weight reduction in patients treated with GLP-1 RA led to clinical trials for treatment of obesity. Semaglutide treatment resulted in weight reduction of 16.9% of total body weight (TBW), and one third of subjects lost ≥ 20% of TBW.17 Tirzepatide combines GLP-1 RA and a gastric inhibitory polypeptide (GIP) receptor agonist, which also has an incretin effect and functions to slow gastric emptying. In the pivotal SURMOUNT trial, approximately 58% of patients achieved ≥20% loss of TBW18 with 15mg weekly dosing of tirzepatide. This class of drugs is a logical choice in patients with T2DM and obesity. Long-term treatment appears necessary, as patients typically regain two-thirds of lost weight within a year after GLP-1 RA are stopped.
Based on tumors observed in rodents, GLP-1 RA are contraindicated in patients with a personal or family history of multiple endocrine neoplasia type 2 (MEN II) or medullary thyroid cancer. These tumors have not been observed in humans treated with GLP-1 RA. They should be used with caution in patients with history of pancreatitis, gastroparesis, or diabetic retinopathy, though a recent systematic review and meta-analysis suggests showed little to no increased risk for biliary events from GLP-1 RA.19 Side effects are most commonly gastrointestinal in nature (nausea, reflux, constipation or diarrhea) and are typically most severe with initiation of the drug and with dose escalation. Side effects can be mitigated by initiating these drugs at lowest doses and gradually titrating up (every four weeks) based on effectiveness and tolerability. Antisecretory, antiemetic, and laxative medications can also be used to help manage GLP-1 RA related side effects.
There is no reason to escalate to highest doses if patients are experiencing weight loss and reduction in food cravings at lower doses. Both semaglutide and tirzepatide are administered subcutaneously every seven days. Once patients have reached goal weight, they can either continue maintenance therapy at that same dose/interval, or if motivated to do so, may gradually reduce the weekly dose in a stepwise approach to determine the minimally effective dose to maintain weight loss. There are not yet published maintenance studies to guide this process. Currently the price of GLP-1 RA and inconsistent insurance coverage make them inaccessible to many patients. The manufacturers of both semaglutide and tirzepatide offer direct to consumer pricing and home delivery.
Bariatric Surgery
In patients with higher BMI (≥35kg/m2) or those with BMI ≥30kg/m2 and obesity-related metabolic disease and the desire to avoid lifelong medications or who fail or are intolerant of AOM, bariatric options should be considered.20 Sleeve gastrectomy has become the most performed surgery for treatment of obesity. It is a restrictive procedure, removing 80% of the stomach, but a drop in circulating levels of ghrelin afterwards also leads to decreased feelings of hunger. It results in weight loss of 25-30% TBW loss. It is not a good choice for patients who suffer from severe GERD, as this typically worsens afterwards; furthermore, de novo Barrett’s has been observed in nearly 6% of patients who undergo sleeve gastrectomy.21
Roux-en-Y gastric bypass is a restrictive and malabsorptive procedure, resulting in 30-35% TBW loss. It has beneficial and immediate metabolic effects, including increased release of endogenous GLP-1, which leads to improvements in weight-related T2DM. The newer single anastomosis duodenal-ileal bypass with sleeve gastrectomy (SADI-S) starts with a sleeve gastrectomy, making a smaller tube-shaped stomach. The duodenum is divided just after the stomach and then a loop of ileum is brought up and connected to the stomach (see Figure 1). This procedure is highly effective, with patients losing 75-95% of excess body weight and is becoming a preferred option for patients with greater BMI (≥50kg/m2). It is also an option for patients who have already had a sleeve gastrectomy and are seeking further weight loss. Because there is only one anastomosis, perioperative complications, such as anastomotic leaks, are reduced. The risk of micronutrient deficiencies is present with all malabsorptive procedures, and these patients must supplement with multivitamins, iron, vitamin D, and calcium.
Endoscopic Therapies
Endoscopic bariatric and metabolic therapies (EBMTs) have been increasingly studied and utilized, and this less invasive option may be more appropriate for or attractive to many patients. Intragastric balloons, which reduce meal volume and delay gastric emptying, can be used short term only (six months) resulting in loss of about 6.9% of total body weight (TBW) greater than lifestyle modification (LM) alone, and may be considered in limited situations, such as need for pre-operative weight loss to reduce risks in very obese individuals.22
Endoscopic gastric remodeling (EGR), also known as endoscopic sleeve gastrectomy (ESG), is a purely restrictive procedure in which the stomach is cinched to resize and reshape using an endoscopic suturing device (see Figure 2).23 It is an option for patients with class 1 or 2 obesity, with data from a randomized controlled trial in this population demonstrating mean percentage of TBW loss of 13.6% at 52 weeks compared to 0.8% in those treated with LM alone.24 A recent meta-analysis of 21 observational studies, including patients with higher BMIs (32.5 to 49.9 kg/m2) showed pooled average weight loss of 17.3% TBW at 12 months with EGR.22 This procedure has potential advantages of fewer complications, quicker recovery, and much less new-onset GERD compared to laparoscopic sleeve gastrectomy. Furthermore, it may be utilized in combination with AOMs to achieve optimum weight loss and metabolic outcomes.25,26 Potential adverse events include abdominal pain, nausea and vomiting (which may be severe), as well as rare instances of intra/extra luminal bleeding or abdominal abscess requiring drainage.22
Recent joint American/European Gastrointestinal Endoscopy guidelines suggest the use of EBMTs plus lifestyle modification in patients with a BMI of ≥ 30 kg/m2, or with a BMI of 27.0-29.9 kg/m2 with at least 1 obesity-related comorbidity.22 Small bowel interventions including duodenal-jejunal bypass liner and duodenal mucosal resurfacing are being investigated for patients with obesity and type 2 diabetes but not yet commercially available.
Conclusion
Given the overlap of obesity with many GI disorders, it is entirely appropriate for gastroenterologists to consider it worthy of aggressive treatment, particularly in patients with MAFLD and other serious weight related comorbidities. With a compassionate and empathetic approach, and a number of highly effective medical, endoscopic, and surgical therapies now available, weight management has the potential to be extremely rewarding when implemented in GI practice.
Dr. Kelly is based in the Department of Medicine, Division of Gastroenterology, Brigham and Women’s Hospital, and Harvard Medical School, both in Boston, Massachusetts. She serves on the clinical advisory board for OpenBiome (unpaid) and has served on an advisory board for Eli Lilly and Company.
References
1. Hales CM, et al. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017-2018. NCHS Data Brief 2020 Feb:(360):1–8.
2. Pais R, et al. NAFLD and liver transplantation: Current burden and expected challenges. J Hepatol. 2016 Dec. doi: 10.1016/j.jhep.2016.07.033.
3. Lauby-Secretan B, et al. Body Fatness and Cancer--Viewpoint of the IARC Working Group. N Engl J Med. 2016 Aug. doi: 10.1056/NEJMsr1606602.
4. Kim A. Dysbiosis: A Review Highlighting Obesity and Inflammatory Bowel Disease. J Clin Gastroenterol. 2015 Nov-Dec. doi: 10.1097/MCG.0000000000000356.
5. Singh S, et al. Obesity in IBD: epidemiology, pathogenesis, disease course and treatment outcomes. Nat Rev Gastroenterol Hepatol. 2017 Feb. doi: 10.1038/nrgastro.2016.181.
6. Sundararaman L, Goudra B. Sedation for GI Endoscopy in the Morbidly Obese: Challenges and Possible Solutions. J Clin Med. 2024 Aug. doi: 10.3390/jcm13164635.
7. Bombassaro B, et al. The hypothalamus as the central regulator of energy balance and its impact on current and future obesity treatments. Arch Endocrinol Metab. 2024 Nov. doi: 10.20945/2359-4292-2024-0082.
8. Beccuti G, Pannain S. Sleep and obesity. Curr Opin Clin Nutr Metab Care. 2011 Jul. doi: 10.1097/MCO.0b013e3283479109.
9. Desalermos A, et al. Effect of Obesogenic Medications on Weight-Loss Outcomes in a Behavioral Weight-Management Program. Obesity (Silver Spring). 2019 May. doi: 10.1002/oby.22444.
10. Lord MN, Noble EE. Hypothalamic cannabinoid signaling: Consequences for eating behavior. Pharmacol Res Perspect. 2024 Oct. doi: 10.1002/prp2.1251.
11. Farhana A, Rehman A. Metabolic Consequences of Weight Reduction. [Updated 2023 Jul 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK572145/.
12. Rubino F, et al. Definition and diagnostic criteria of clinical obesity. Lancet Diabetes Endocrinol. 2025 Mar. doi: 10.1016/S2213-8587(24)00316-4.
13. Cox CE. Role of Physical Activity for Weight Loss and Weight Maintenance. Diabetes Spectr. 2017 Aug. doi: 10.2337/ds17-0013.
14. Chaput JP, et al. Widespread misconceptions about obesity. Can Fam Physician. 2014 Nov. PMID: 25392431.
15. Muscogiuri G, et al. Mediterranean Diet and Obesity-related Disorders: What is the Evidence? Curr Obes Rep. 2022 Dec. doi: 10.1007/s13679-022-00481-1.
16. Gudzune KA, Kushner RF. Medications for Obesity: A Review. JAMA. 2024 Aug. doi: 10.1001/jama.2024.10816.
17. Wilding JPH, et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N Engl J Med. 2021 Feb. doi: 10.1056/NEJMoa2032183.
18. Jastreboff AM, et al. Tirzepatide Once Weekly for the Treatment of Obesity. N Engl J Med. 2022 Jun. doi: 10.1056/NEJMoa2206038.
19. Chiang CH, et al. Glucagon-Like Peptide-1 Receptor Agonists and Gastrointestinal Adverse Events: A Systematic Review and Meta-Analysis. Gastroenterology. 2025 Nov. doi: 10.1053/j.gastro.2025.06.003.
20. Aderinto N, et al. Recent advances in bariatric surgery: a narrative review of weight loss procedures. Ann Med Surg (Lond). 2023 Nov. doi: 10.1097/MS9.0000000000001472.
21. Chandan S, et al. Risk of De Novo Barrett’s Esophagus Post Sleeve Gastrectomy: A Systematic Review and Meta-Analysis of Studies With Long-Term Follow-Up. Clin Gastroenterol Hepatol. 2025 Jan. doi: 10.1016/j.cgh.2024.06.041.
22. Jirapinyo P, et al. American Society for Gastrointestinal Endoscopy-European Society of Gastrointestinal Endoscopy guideline on primary endoscopic bariatric and metabolic therapies for adults with obesity. Gastrointest Endosc. 2024 Jun. doi: 10.1016/j.gie.2023.12.004.
23. Nduma BN, et al. Endoscopic Gastric Sleeve: A Review of Literature. Cureus. 2023 Mar. doi: 10.7759/cureus.36353.
24. Abu Dayyeh BK, et al. Endoscopic sleeve gastroplasty for treatment of class 1 and 2 obesity (MERIT): a prospective, multicentre, randomised trial. Lancet. 2022 Aug. doi: 10.1016/S0140-6736(22)01280-6.
25. Gala K, et al. Outcomes of concomitant antiobesity medication use with endoscopic sleeve gastroplasty in clinical US settings. Obes Pillars. 2024 May. doi: 10.1016/j.obpill.2024.100112.
26. Chung CS, et al. Endoscopic sleeve gastroplasty combined with anti-obesity medication for better control of weight and diabetes. Clin Endosc. 2025 May. doi: 10.5946/ce.2024.274.
Medical Liability for the Gastroenterologist
While nearly 75% of physicians in low-risk specialties and 99% of physicians in high-risk specialties may face a malpractice claim in their careers,1 malpractice is rarely discussed openly in medical school, residency, fellowship, or even amongst colleagues. Indeed, one study suggested that more than 10% of practicing gastroenterologists may face a malpractice claim,2 with gastroenterologists expected to spend 10-15% of their careers with an outstanding malpractice claim3 as cases may take 27-29 months to resolve on average.4
Believing that if a physician is sued, one must have done something “wrong” or that speaking about one’s experience may implicate a colleague, creates an intense stigma and isolation that only serves to aggravate the “second victim syndrome” (SVS) that is well documented in the surgical literature.2 Herein,
What is Malpractice? Why Do Physicians Get Sued?
Malpractice is defined as negligence on the part of a physician which causes physical or emotional damage to the patient. This requires a variety of legal issues to be evaluated (e.g. breach of duty between the physicians and patient, breach of standard of care), that often center around the question: would a “reasonable, careful, and prudent” doctor behave in the same manner in the same circumstance?
While some fields of medicine lend themselves better to algorithmic applications of highly evidence-based guidelines, many aspects of GI care and endoscopic practice are highly physician/patient-specific, dependent on local expertise, and based on low-quality evidence. As a result, an assessment of negligence may be quite subjective, depending on the expert retained by a plaintiff. Conflicting expert testimony on what professional custom is and whether practice deviates may hinge on technical details that may or may not be appreciated by a lay jury.
Plaintiffs must prove both that they have sustained an injury and that the injury (emotional or physical) was due to the physician’s negligence. While this may be straightforward in a “slip-and-fall” tort claim, medical malpractice claims usually involve sick patients with multiple comorbidities, where assigning causality to a single intervention/misinterpretation/missed opportunity is difficult to weigh against competing causes of adverse outcomes. Assessing a specific liability requires that the plaintiff prove this to a “more likely than not” standard which may be part of the reason why only 30% of cases are closed with indemnity payments, a figure that has not changed significantly in the past decade.4
While the perception amongst physicians is that tort legislation is ever increasing, data from the National Practitioner Data Bank (NPDB) demonstrates that the number of paid claims against physicians has decreased by 75% in the last 20 years.5 This may reflect a progressive improvement in the quality of care delivered or success of “tort reform” on the state level to limit damages and “nuisance” lawsuits. However, another more problematic possibility is that with the corporatization of medicine, an untold number of physicians may be removed from litigation as a named party, with their institution shielding them from reporting. While the number of cases may or may not be declining, the average indemnity payment appears to be rising to $330,000 on average,4 with one study suggesting a significant growth in paid claims in gastroenterology.6
Historically, studies of closed malpractice claims have demonstrated that 59% involved diagnostic errors involving a cancer diagnosis,7 though why this actually happens may be for a wide variety of reasons including errors in the development of a differential diagnosis, ordering of an appropriate diagnostic test, interpretation of the diagnostic test, or follow-up of an abnormality identified.
What are the Intended/Unintended Consequences of Litigation?
The objective of our tort system is to compensate patients for economic damages (medical costs and lost wages) and non-economic damages (pain and suffering), and to ideally deter negligent behavior of providers. Interestingly, data from the NPDB have suggested that approximately 1% of all physicians account for 32% of all paid claims, with the same study showing that among physicians with paid claims, 4% had at least 3.8
While certain fields are obviously more prone to litigation, the risk of additional claims on a physician with 3 prior claims was more than 3 times that of physicians with 1 lifetime claim. One would assume that the system was built to drive out a small proportion of “bad actors.” Indeed, similar data from the NPDB has demonstrated that the number of claims against physicians was associated both with their leaving the practice of medicine and relocating to smaller practice settings.9
Another frequent question is whether the threat of litigation drives “defensive medicine” (i.e. medical care that is not beneficial) or avoidance medicine (i.e. excluding high risk patients and procedures from ones’ practice). These behaviors have been well documented in physicians around the world,10 as well as several surveys of gastroenterologists specifically suggesting regular ordering of unnecessary imaging/endoscopy and referrals of patients to specialists that may not be necessary.11,12
However, does defensive medicine work: does spending more prevent you from being the target of a lawsuit? In an observational study in Florida from 2000-2009, researchers demonstrated that across specialties, greater average spending by physicians was associated with a reduced risk of incurring a malpractice claim. Indeed, the likelihood of a top quintile spending internist having a malpractice incident vs a bottom quintile spending internist was 0.3% vs 1.5%.13
Approximately 10.4-43.3% of physicians may experience SVS, experiencing trauma after an adverse patient event/medical error, manifesting with psychological trauma (shame, guilt, anxiety) and cognitive limitations (burnout, stress).2 Significant emotional consequences are common on the part of the physician and have well-documented stages to recovery,14 which if ignored may lead to long-term detrimental mental/emotional health of the physician and their future patients.
Specifically, in one study, 80.8% of physicians who had a closed malpractice claim reported significant emotional distress (regardless of the legal outcome), with frequent reports of mood symptoms that affected professional conduct.15 Recognizing these effects and implementing peer counseling and institutional support may help to expedite recovery and mitigate future adverse career outcomes.14
Anatomy/Timeline of a Liability Lawsuit
Medical malpractice cases are heard in state courts, in the jurisdictions where the care was provided. From the time an event occurs to when a jury verdict may be rendered may take 4-5 years or more depending on the local statute of limitations, discovery process, backlog of the local case docket, and specific circumstances of the case. The length of time is important to consider given the likelihood that a physician may advance in training or move practice locations during the course of litigation. Several common myths surrounding this process are summarized in the accompanying box, titled “Myths Surrounding Medical Liability Litigation.”
The plaintiff faces a statute of limitations to file a lawsuit that may range from 1-6 years depending on the state. The first indication that legal action may be pending will generally be a plaintiff’s formal request for medical records. After these records are reviewed, the plaintiff’s attorney will consult one or more experts (often credentialed in the same specialty) to assess if the case is viable and to ultimately form the basis of an affidavit of merit from a plaintiff expert.
Once the lawsuit is filed, the physician(s) named will be assigned an attorney by their employer/insurance company. A state medical board malpractice questionnaire will generally follow that will seek to independently evaluate the alleged malpractice with interrogatives to determine if censure is warranted. There is a formal response to the plaintiff’s petition by the defense and then the discovery phase begins where both sides depose the defendants/plaintiffs and retain medical experts that are favorable to their arguments.
In choosing potential “experts,” physicians must ensure that they are willing/able to be present for a potential trial, do not have any personal/professional/academic conflicts with the defendants, and are willing to provide compelling testimony to a jury. A pre-trial conference and trial date is set which may be >12 months away depending on the local docket. While the amount of time a trial may take is variable, it may be up to 5-7 days that the defendants are expected to be in court in addition to days where depositions are being taken.
During the discovery process, dismissal of the physician from the lawsuit is pursued. In addition, settlement negotiations generally proceed in parallel with discovery process and may result in a pre-trial/pre-verdict settlement. Once a verdict is reached, whether for the plaintiff or the defendant, the case may be appealed, and the trial preparation process may be repeated.
Conclusions
Awareness of the medical liability process is critical for trainees and attendings alike, given the high likelihood of litigation in a gastroenterologist’s career. Specific considerations like local tort law and malpractice coverage are important to be familiar. Ongoing health services research help to shape our understanding on the intended and unintended consequences of litigation on medicine, though detailed data on outcomes/settlements are limited by confidentiality agreements, which may hamper efforts to improve patient safety.
Dr. Das is associate professor of medicine in the Division of Gastroenterology at Washington University School of Medicine, St. Louis, Missouri. He has served as a consultant for Olympus, but has no other relevant conflicts.
References
1. Jena AB, et al. Malpractice Risk According to Physician Specialty. N Engl J Med. 2011 Aug. doi: 10.1056/NEJMsa1012370.
2. Chong RIH, et al. Scoping review of the second victim syndrome among surgeons: Understanding the impact, responses, and support systems. Am J Surg 2024 Mar. doi: 10.1016/j.amjsurg.2023.09.045.
3. Seabury S, et al. On Average, Physicians Spend Nearly 11 Percent Of Their 40-Year Careers With An Open, Unresolved Malpractice Claim. Health Aff Proj Hope. 2013 Jan. doi: 10.1377/hlthaff.2012.0967.
4. CRICO Strategies. Medical Malpractice in America: A 10-Year Asessment with Insights. 2018. Accessed Apr 28, 2025.
5. Studdert DM, Hall MA. Medical Malpractice Law — Doctrine and Dynamics. N Engl J Med 2022 Oct. doi: 10.1056/NEJMp2201675.
6. Schaffer AC, et al. Rates and Characteristics of Paid Malpractice Claims Among US Physicians by Specialty, 1992-2014. JAMA Intern Med. 2017 May. doi: 10.1001/jamainternmed.2017.0311.
7. Gandhi TK, et al. Missed and Delayed Diagnoses in the Ambulatory Setting: A Study of Closed Malpractice Claims. Ann Intern Med. 2006 Oct. doi: 10.7326/0003-4819-145-7-200610030-00006.
8. Studdert DM, et al. Prevalence and Characteristics of Physicians Prone to Malpractice Claims. N Engl J Med. 2016 Jan. doi: 10.1056/NEJMsa1506137.
9. Studdert DM, et al. Changes in Practice among Physicians with Malpractice Claims. N Engl J Med. 2019 Mar. doi: 10.1056/NEJMsa1809981.
10. Ries NM, Jansen J. Physicians’ views and experiences of defensive medicine: An international review of empirical research. Health Policy. 2021 May. doi: 10.1016/j.healthpol.2021.02.005.
11. Hiyama T, et al. Defensive medicine practices among gastroenterologists in Japan. World J Gastroenterol. 2006 Dec. doi: 10.3748/wjg.v12.i47.7671.
12. Elli L, et al. Defensive medicine practices among gastroenterologists in Lombardy: Between lawsuits and the economic crisis. Dig Liver Dis. 2013 Jun. doi: 10.1016/j.dld.2013.01.004.
13. Jena AB, et al. Physician spending and subsequent risk of malpractice claims: observational study. BMJ. 2015 Nov. doi: 10.1136/bmj.h5516.
14. Scott SD, et al. The natural history of recovery for the healthcare provider “second victim” after adverse patient events. BMJ Qual Saf. 2009 Oct. doi: 10.1136/qshc.2009.032870.
15. Gómez-Durán EL, et al. Physicians as second victims after a malpractice claim: An important issue in need of attention. J Healthc Qual Res. 2018 Oct. doi: 10.1016/j.jhqr.2018.06.002.
While nearly 75% of physicians in low-risk specialties and 99% of physicians in high-risk specialties may face a malpractice claim in their careers,1 malpractice is rarely discussed openly in medical school, residency, fellowship, or even amongst colleagues. Indeed, one study suggested that more than 10% of practicing gastroenterologists may face a malpractice claim,2 with gastroenterologists expected to spend 10-15% of their careers with an outstanding malpractice claim3 as cases may take 27-29 months to resolve on average.4
Believing that if a physician is sued, one must have done something “wrong” or that speaking about one’s experience may implicate a colleague, creates an intense stigma and isolation that only serves to aggravate the “second victim syndrome” (SVS) that is well documented in the surgical literature.2 Herein,
What is Malpractice? Why Do Physicians Get Sued?
Malpractice is defined as negligence on the part of a physician which causes physical or emotional damage to the patient. This requires a variety of legal issues to be evaluated (e.g. breach of duty between the physicians and patient, breach of standard of care), that often center around the question: would a “reasonable, careful, and prudent” doctor behave in the same manner in the same circumstance?
While some fields of medicine lend themselves better to algorithmic applications of highly evidence-based guidelines, many aspects of GI care and endoscopic practice are highly physician/patient-specific, dependent on local expertise, and based on low-quality evidence. As a result, an assessment of negligence may be quite subjective, depending on the expert retained by a plaintiff. Conflicting expert testimony on what professional custom is and whether practice deviates may hinge on technical details that may or may not be appreciated by a lay jury.
Plaintiffs must prove both that they have sustained an injury and that the injury (emotional or physical) was due to the physician’s negligence. While this may be straightforward in a “slip-and-fall” tort claim, medical malpractice claims usually involve sick patients with multiple comorbidities, where assigning causality to a single intervention/misinterpretation/missed opportunity is difficult to weigh against competing causes of adverse outcomes. Assessing a specific liability requires that the plaintiff prove this to a “more likely than not” standard which may be part of the reason why only 30% of cases are closed with indemnity payments, a figure that has not changed significantly in the past decade.4
While the perception amongst physicians is that tort legislation is ever increasing, data from the National Practitioner Data Bank (NPDB) demonstrates that the number of paid claims against physicians has decreased by 75% in the last 20 years.5 This may reflect a progressive improvement in the quality of care delivered or success of “tort reform” on the state level to limit damages and “nuisance” lawsuits. However, another more problematic possibility is that with the corporatization of medicine, an untold number of physicians may be removed from litigation as a named party, with their institution shielding them from reporting. While the number of cases may or may not be declining, the average indemnity payment appears to be rising to $330,000 on average,4 with one study suggesting a significant growth in paid claims in gastroenterology.6
Historically, studies of closed malpractice claims have demonstrated that 59% involved diagnostic errors involving a cancer diagnosis,7 though why this actually happens may be for a wide variety of reasons including errors in the development of a differential diagnosis, ordering of an appropriate diagnostic test, interpretation of the diagnostic test, or follow-up of an abnormality identified.
What are the Intended/Unintended Consequences of Litigation?
The objective of our tort system is to compensate patients for economic damages (medical costs and lost wages) and non-economic damages (pain and suffering), and to ideally deter negligent behavior of providers. Interestingly, data from the NPDB have suggested that approximately 1% of all physicians account for 32% of all paid claims, with the same study showing that among physicians with paid claims, 4% had at least 3.8
While certain fields are obviously more prone to litigation, the risk of additional claims on a physician with 3 prior claims was more than 3 times that of physicians with 1 lifetime claim. One would assume that the system was built to drive out a small proportion of “bad actors.” Indeed, similar data from the NPDB has demonstrated that the number of claims against physicians was associated both with their leaving the practice of medicine and relocating to smaller practice settings.9
Another frequent question is whether the threat of litigation drives “defensive medicine” (i.e. medical care that is not beneficial) or avoidance medicine (i.e. excluding high risk patients and procedures from ones’ practice). These behaviors have been well documented in physicians around the world,10 as well as several surveys of gastroenterologists specifically suggesting regular ordering of unnecessary imaging/endoscopy and referrals of patients to specialists that may not be necessary.11,12
However, does defensive medicine work: does spending more prevent you from being the target of a lawsuit? In an observational study in Florida from 2000-2009, researchers demonstrated that across specialties, greater average spending by physicians was associated with a reduced risk of incurring a malpractice claim. Indeed, the likelihood of a top quintile spending internist having a malpractice incident vs a bottom quintile spending internist was 0.3% vs 1.5%.13
Approximately 10.4-43.3% of physicians may experience SVS, experiencing trauma after an adverse patient event/medical error, manifesting with psychological trauma (shame, guilt, anxiety) and cognitive limitations (burnout, stress).2 Significant emotional consequences are common on the part of the physician and have well-documented stages to recovery,14 which if ignored may lead to long-term detrimental mental/emotional health of the physician and their future patients.
Specifically, in one study, 80.8% of physicians who had a closed malpractice claim reported significant emotional distress (regardless of the legal outcome), with frequent reports of mood symptoms that affected professional conduct.15 Recognizing these effects and implementing peer counseling and institutional support may help to expedite recovery and mitigate future adverse career outcomes.14
Anatomy/Timeline of a Liability Lawsuit
Medical malpractice cases are heard in state courts, in the jurisdictions where the care was provided. From the time an event occurs to when a jury verdict may be rendered may take 4-5 years or more depending on the local statute of limitations, discovery process, backlog of the local case docket, and specific circumstances of the case. The length of time is important to consider given the likelihood that a physician may advance in training or move practice locations during the course of litigation. Several common myths surrounding this process are summarized in the accompanying box, titled “Myths Surrounding Medical Liability Litigation.”
The plaintiff faces a statute of limitations to file a lawsuit that may range from 1-6 years depending on the state. The first indication that legal action may be pending will generally be a plaintiff’s formal request for medical records. After these records are reviewed, the plaintiff’s attorney will consult one or more experts (often credentialed in the same specialty) to assess if the case is viable and to ultimately form the basis of an affidavit of merit from a plaintiff expert.
Once the lawsuit is filed, the physician(s) named will be assigned an attorney by their employer/insurance company. A state medical board malpractice questionnaire will generally follow that will seek to independently evaluate the alleged malpractice with interrogatives to determine if censure is warranted. There is a formal response to the plaintiff’s petition by the defense and then the discovery phase begins where both sides depose the defendants/plaintiffs and retain medical experts that are favorable to their arguments.
In choosing potential “experts,” physicians must ensure that they are willing/able to be present for a potential trial, do not have any personal/professional/academic conflicts with the defendants, and are willing to provide compelling testimony to a jury. A pre-trial conference and trial date is set which may be >12 months away depending on the local docket. While the amount of time a trial may take is variable, it may be up to 5-7 days that the defendants are expected to be in court in addition to days where depositions are being taken.
During the discovery process, dismissal of the physician from the lawsuit is pursued. In addition, settlement negotiations generally proceed in parallel with discovery process and may result in a pre-trial/pre-verdict settlement. Once a verdict is reached, whether for the plaintiff or the defendant, the case may be appealed, and the trial preparation process may be repeated.
Conclusions
Awareness of the medical liability process is critical for trainees and attendings alike, given the high likelihood of litigation in a gastroenterologist’s career. Specific considerations like local tort law and malpractice coverage are important to be familiar. Ongoing health services research help to shape our understanding on the intended and unintended consequences of litigation on medicine, though detailed data on outcomes/settlements are limited by confidentiality agreements, which may hamper efforts to improve patient safety.
Dr. Das is associate professor of medicine in the Division of Gastroenterology at Washington University School of Medicine, St. Louis, Missouri. He has served as a consultant for Olympus, but has no other relevant conflicts.
References
1. Jena AB, et al. Malpractice Risk According to Physician Specialty. N Engl J Med. 2011 Aug. doi: 10.1056/NEJMsa1012370.
2. Chong RIH, et al. Scoping review of the second victim syndrome among surgeons: Understanding the impact, responses, and support systems. Am J Surg 2024 Mar. doi: 10.1016/j.amjsurg.2023.09.045.
3. Seabury S, et al. On Average, Physicians Spend Nearly 11 Percent Of Their 40-Year Careers With An Open, Unresolved Malpractice Claim. Health Aff Proj Hope. 2013 Jan. doi: 10.1377/hlthaff.2012.0967.
4. CRICO Strategies. Medical Malpractice in America: A 10-Year Asessment with Insights. 2018. Accessed Apr 28, 2025.
5. Studdert DM, Hall MA. Medical Malpractice Law — Doctrine and Dynamics. N Engl J Med 2022 Oct. doi: 10.1056/NEJMp2201675.
6. Schaffer AC, et al. Rates and Characteristics of Paid Malpractice Claims Among US Physicians by Specialty, 1992-2014. JAMA Intern Med. 2017 May. doi: 10.1001/jamainternmed.2017.0311.
7. Gandhi TK, et al. Missed and Delayed Diagnoses in the Ambulatory Setting: A Study of Closed Malpractice Claims. Ann Intern Med. 2006 Oct. doi: 10.7326/0003-4819-145-7-200610030-00006.
8. Studdert DM, et al. Prevalence and Characteristics of Physicians Prone to Malpractice Claims. N Engl J Med. 2016 Jan. doi: 10.1056/NEJMsa1506137.
9. Studdert DM, et al. Changes in Practice among Physicians with Malpractice Claims. N Engl J Med. 2019 Mar. doi: 10.1056/NEJMsa1809981.
10. Ries NM, Jansen J. Physicians’ views and experiences of defensive medicine: An international review of empirical research. Health Policy. 2021 May. doi: 10.1016/j.healthpol.2021.02.005.
11. Hiyama T, et al. Defensive medicine practices among gastroenterologists in Japan. World J Gastroenterol. 2006 Dec. doi: 10.3748/wjg.v12.i47.7671.
12. Elli L, et al. Defensive medicine practices among gastroenterologists in Lombardy: Between lawsuits and the economic crisis. Dig Liver Dis. 2013 Jun. doi: 10.1016/j.dld.2013.01.004.
13. Jena AB, et al. Physician spending and subsequent risk of malpractice claims: observational study. BMJ. 2015 Nov. doi: 10.1136/bmj.h5516.
14. Scott SD, et al. The natural history of recovery for the healthcare provider “second victim” after adverse patient events. BMJ Qual Saf. 2009 Oct. doi: 10.1136/qshc.2009.032870.
15. Gómez-Durán EL, et al. Physicians as second victims after a malpractice claim: An important issue in need of attention. J Healthc Qual Res. 2018 Oct. doi: 10.1016/j.jhqr.2018.06.002.
While nearly 75% of physicians in low-risk specialties and 99% of physicians in high-risk specialties may face a malpractice claim in their careers,1 malpractice is rarely discussed openly in medical school, residency, fellowship, or even amongst colleagues. Indeed, one study suggested that more than 10% of practicing gastroenterologists may face a malpractice claim,2 with gastroenterologists expected to spend 10-15% of their careers with an outstanding malpractice claim3 as cases may take 27-29 months to resolve on average.4
Believing that if a physician is sued, one must have done something “wrong” or that speaking about one’s experience may implicate a colleague, creates an intense stigma and isolation that only serves to aggravate the “second victim syndrome” (SVS) that is well documented in the surgical literature.2 Herein,
What is Malpractice? Why Do Physicians Get Sued?
Malpractice is defined as negligence on the part of a physician which causes physical or emotional damage to the patient. This requires a variety of legal issues to be evaluated (e.g. breach of duty between the physicians and patient, breach of standard of care), that often center around the question: would a “reasonable, careful, and prudent” doctor behave in the same manner in the same circumstance?
While some fields of medicine lend themselves better to algorithmic applications of highly evidence-based guidelines, many aspects of GI care and endoscopic practice are highly physician/patient-specific, dependent on local expertise, and based on low-quality evidence. As a result, an assessment of negligence may be quite subjective, depending on the expert retained by a plaintiff. Conflicting expert testimony on what professional custom is and whether practice deviates may hinge on technical details that may or may not be appreciated by a lay jury.
Plaintiffs must prove both that they have sustained an injury and that the injury (emotional or physical) was due to the physician’s negligence. While this may be straightforward in a “slip-and-fall” tort claim, medical malpractice claims usually involve sick patients with multiple comorbidities, where assigning causality to a single intervention/misinterpretation/missed opportunity is difficult to weigh against competing causes of adverse outcomes. Assessing a specific liability requires that the plaintiff prove this to a “more likely than not” standard which may be part of the reason why only 30% of cases are closed with indemnity payments, a figure that has not changed significantly in the past decade.4
While the perception amongst physicians is that tort legislation is ever increasing, data from the National Practitioner Data Bank (NPDB) demonstrates that the number of paid claims against physicians has decreased by 75% in the last 20 years.5 This may reflect a progressive improvement in the quality of care delivered or success of “tort reform” on the state level to limit damages and “nuisance” lawsuits. However, another more problematic possibility is that with the corporatization of medicine, an untold number of physicians may be removed from litigation as a named party, with their institution shielding them from reporting. While the number of cases may or may not be declining, the average indemnity payment appears to be rising to $330,000 on average,4 with one study suggesting a significant growth in paid claims in gastroenterology.6
Historically, studies of closed malpractice claims have demonstrated that 59% involved diagnostic errors involving a cancer diagnosis,7 though why this actually happens may be for a wide variety of reasons including errors in the development of a differential diagnosis, ordering of an appropriate diagnostic test, interpretation of the diagnostic test, or follow-up of an abnormality identified.
What are the Intended/Unintended Consequences of Litigation?
The objective of our tort system is to compensate patients for economic damages (medical costs and lost wages) and non-economic damages (pain and suffering), and to ideally deter negligent behavior of providers. Interestingly, data from the NPDB have suggested that approximately 1% of all physicians account for 32% of all paid claims, with the same study showing that among physicians with paid claims, 4% had at least 3.8
While certain fields are obviously more prone to litigation, the risk of additional claims on a physician with 3 prior claims was more than 3 times that of physicians with 1 lifetime claim. One would assume that the system was built to drive out a small proportion of “bad actors.” Indeed, similar data from the NPDB has demonstrated that the number of claims against physicians was associated both with their leaving the practice of medicine and relocating to smaller practice settings.9
Another frequent question is whether the threat of litigation drives “defensive medicine” (i.e. medical care that is not beneficial) or avoidance medicine (i.e. excluding high risk patients and procedures from ones’ practice). These behaviors have been well documented in physicians around the world,10 as well as several surveys of gastroenterologists specifically suggesting regular ordering of unnecessary imaging/endoscopy and referrals of patients to specialists that may not be necessary.11,12
However, does defensive medicine work: does spending more prevent you from being the target of a lawsuit? In an observational study in Florida from 2000-2009, researchers demonstrated that across specialties, greater average spending by physicians was associated with a reduced risk of incurring a malpractice claim. Indeed, the likelihood of a top quintile spending internist having a malpractice incident vs a bottom quintile spending internist was 0.3% vs 1.5%.13
Approximately 10.4-43.3% of physicians may experience SVS, experiencing trauma after an adverse patient event/medical error, manifesting with psychological trauma (shame, guilt, anxiety) and cognitive limitations (burnout, stress).2 Significant emotional consequences are common on the part of the physician and have well-documented stages to recovery,14 which if ignored may lead to long-term detrimental mental/emotional health of the physician and their future patients.
Specifically, in one study, 80.8% of physicians who had a closed malpractice claim reported significant emotional distress (regardless of the legal outcome), with frequent reports of mood symptoms that affected professional conduct.15 Recognizing these effects and implementing peer counseling and institutional support may help to expedite recovery and mitigate future adverse career outcomes.14
Anatomy/Timeline of a Liability Lawsuit
Medical malpractice cases are heard in state courts, in the jurisdictions where the care was provided. From the time an event occurs to when a jury verdict may be rendered may take 4-5 years or more depending on the local statute of limitations, discovery process, backlog of the local case docket, and specific circumstances of the case. The length of time is important to consider given the likelihood that a physician may advance in training or move practice locations during the course of litigation. Several common myths surrounding this process are summarized in the accompanying box, titled “Myths Surrounding Medical Liability Litigation.”
The plaintiff faces a statute of limitations to file a lawsuit that may range from 1-6 years depending on the state. The first indication that legal action may be pending will generally be a plaintiff’s formal request for medical records. After these records are reviewed, the plaintiff’s attorney will consult one or more experts (often credentialed in the same specialty) to assess if the case is viable and to ultimately form the basis of an affidavit of merit from a plaintiff expert.
Once the lawsuit is filed, the physician(s) named will be assigned an attorney by their employer/insurance company. A state medical board malpractice questionnaire will generally follow that will seek to independently evaluate the alleged malpractice with interrogatives to determine if censure is warranted. There is a formal response to the plaintiff’s petition by the defense and then the discovery phase begins where both sides depose the defendants/plaintiffs and retain medical experts that are favorable to their arguments.
In choosing potential “experts,” physicians must ensure that they are willing/able to be present for a potential trial, do not have any personal/professional/academic conflicts with the defendants, and are willing to provide compelling testimony to a jury. A pre-trial conference and trial date is set which may be >12 months away depending on the local docket. While the amount of time a trial may take is variable, it may be up to 5-7 days that the defendants are expected to be in court in addition to days where depositions are being taken.
During the discovery process, dismissal of the physician from the lawsuit is pursued. In addition, settlement negotiations generally proceed in parallel with discovery process and may result in a pre-trial/pre-verdict settlement. Once a verdict is reached, whether for the plaintiff or the defendant, the case may be appealed, and the trial preparation process may be repeated.
Conclusions
Awareness of the medical liability process is critical for trainees and attendings alike, given the high likelihood of litigation in a gastroenterologist’s career. Specific considerations like local tort law and malpractice coverage are important to be familiar. Ongoing health services research help to shape our understanding on the intended and unintended consequences of litigation on medicine, though detailed data on outcomes/settlements are limited by confidentiality agreements, which may hamper efforts to improve patient safety.
Dr. Das is associate professor of medicine in the Division of Gastroenterology at Washington University School of Medicine, St. Louis, Missouri. He has served as a consultant for Olympus, but has no other relevant conflicts.
References
1. Jena AB, et al. Malpractice Risk According to Physician Specialty. N Engl J Med. 2011 Aug. doi: 10.1056/NEJMsa1012370.
2. Chong RIH, et al. Scoping review of the second victim syndrome among surgeons: Understanding the impact, responses, and support systems. Am J Surg 2024 Mar. doi: 10.1016/j.amjsurg.2023.09.045.
3. Seabury S, et al. On Average, Physicians Spend Nearly 11 Percent Of Their 40-Year Careers With An Open, Unresolved Malpractice Claim. Health Aff Proj Hope. 2013 Jan. doi: 10.1377/hlthaff.2012.0967.
4. CRICO Strategies. Medical Malpractice in America: A 10-Year Asessment with Insights. 2018. Accessed Apr 28, 2025.
5. Studdert DM, Hall MA. Medical Malpractice Law — Doctrine and Dynamics. N Engl J Med 2022 Oct. doi: 10.1056/NEJMp2201675.
6. Schaffer AC, et al. Rates and Characteristics of Paid Malpractice Claims Among US Physicians by Specialty, 1992-2014. JAMA Intern Med. 2017 May. doi: 10.1001/jamainternmed.2017.0311.
7. Gandhi TK, et al. Missed and Delayed Diagnoses in the Ambulatory Setting: A Study of Closed Malpractice Claims. Ann Intern Med. 2006 Oct. doi: 10.7326/0003-4819-145-7-200610030-00006.
8. Studdert DM, et al. Prevalence and Characteristics of Physicians Prone to Malpractice Claims. N Engl J Med. 2016 Jan. doi: 10.1056/NEJMsa1506137.
9. Studdert DM, et al. Changes in Practice among Physicians with Malpractice Claims. N Engl J Med. 2019 Mar. doi: 10.1056/NEJMsa1809981.
10. Ries NM, Jansen J. Physicians’ views and experiences of defensive medicine: An international review of empirical research. Health Policy. 2021 May. doi: 10.1016/j.healthpol.2021.02.005.
11. Hiyama T, et al. Defensive medicine practices among gastroenterologists in Japan. World J Gastroenterol. 2006 Dec. doi: 10.3748/wjg.v12.i47.7671.
12. Elli L, et al. Defensive medicine practices among gastroenterologists in Lombardy: Between lawsuits and the economic crisis. Dig Liver Dis. 2013 Jun. doi: 10.1016/j.dld.2013.01.004.
13. Jena AB, et al. Physician spending and subsequent risk of malpractice claims: observational study. BMJ. 2015 Nov. doi: 10.1136/bmj.h5516.
14. Scott SD, et al. The natural history of recovery for the healthcare provider “second victim” after adverse patient events. BMJ Qual Saf. 2009 Oct. doi: 10.1136/qshc.2009.032870.
15. Gómez-Durán EL, et al. Physicians as second victims after a malpractice claim: An important issue in need of attention. J Healthc Qual Res. 2018 Oct. doi: 10.1016/j.jhqr.2018.06.002.
Remembering Why We Are In Medicine
Dear Friends,
There have been recent policy changes that may be affecting trainees and practicing physicians, whether directly impacting our current practices or influencing the decisions that shape our careers. During these challenging times, I am trying to remind myself more often of why I am in medicine – my patients. I will continue to advocate for my patients on Hill Days to affect change in policy. I will continue to provide the best care I can and fight for resources to do so. I will continue to adapt to the changing climate and do what is best for my practice so that I can deliver the care I think my patients need. By remembering why I am in medicine, I can fight for a future of medicine and science that is still bright.
In this issue’s “In Focus” article, Dr. Yasmin G. Hernandez-Barco and Dr. Motaz Ashkar review the diagnostic and treatment approaches to exocrine pancreatic insufficiency, including common symptoms, differential diagnoses, and the different pancreatic enzyme replacement therapies.
Medications for weight loss are becoming more widely available; however, the literature on what to do with these medications in gastrointestinal endoscopy is still lacking. Dr. Sitharthan Sekar and Dr. Nikiya Asamoah summarize the current data and available guidelines in our “Short Clinical Review.”
With another new academic year upon us, this issue’s “Early Career” section features Dr. Allon Kahn’s top tips for becoming an effective gastroenterology consultant. He describes the 5 principles that would improve patient care and relationships with referring providers.
In the “Finance/Legal” section, Dr. Koushik Das dissects what happens when a physician gets sued, including the basis of malpractice suits, consequences, and anticipated timeline.
If you are interested in contributing or have ideas for future TNG topics, please contact me (tjudy@wustl.edu) or Danielle Kiefer (dkiefer@gastro.org), Communications/Managing Editor of TNG.
Until next time, I leave you with a historical fun fact, because we would not be where we are now without appreciating where we were: the pancreas was first discovered by a Greek surgeon, Herophilus, in 336 BC, but its exocrine and endocrine functions were not described until the 1850s-1860s by D. Moyse in Paris and Paul Langerhans in Berlin, respectively.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Assistant Professor of Medicine
Interventional Endoscopy, Division of Gastroenterology
Washington University School of Medicine in St. Louis
Dear Friends,
There have been recent policy changes that may be affecting trainees and practicing physicians, whether directly impacting our current practices or influencing the decisions that shape our careers. During these challenging times, I am trying to remind myself more often of why I am in medicine – my patients. I will continue to advocate for my patients on Hill Days to affect change in policy. I will continue to provide the best care I can and fight for resources to do so. I will continue to adapt to the changing climate and do what is best for my practice so that I can deliver the care I think my patients need. By remembering why I am in medicine, I can fight for a future of medicine and science that is still bright.
In this issue’s “In Focus” article, Dr. Yasmin G. Hernandez-Barco and Dr. Motaz Ashkar review the diagnostic and treatment approaches to exocrine pancreatic insufficiency, including common symptoms, differential diagnoses, and the different pancreatic enzyme replacement therapies.
Medications for weight loss are becoming more widely available; however, the literature on what to do with these medications in gastrointestinal endoscopy is still lacking. Dr. Sitharthan Sekar and Dr. Nikiya Asamoah summarize the current data and available guidelines in our “Short Clinical Review.”
With another new academic year upon us, this issue’s “Early Career” section features Dr. Allon Kahn’s top tips for becoming an effective gastroenterology consultant. He describes the 5 principles that would improve patient care and relationships with referring providers.
In the “Finance/Legal” section, Dr. Koushik Das dissects what happens when a physician gets sued, including the basis of malpractice suits, consequences, and anticipated timeline.
If you are interested in contributing or have ideas for future TNG topics, please contact me (tjudy@wustl.edu) or Danielle Kiefer (dkiefer@gastro.org), Communications/Managing Editor of TNG.
Until next time, I leave you with a historical fun fact, because we would not be where we are now without appreciating where we were: the pancreas was first discovered by a Greek surgeon, Herophilus, in 336 BC, but its exocrine and endocrine functions were not described until the 1850s-1860s by D. Moyse in Paris and Paul Langerhans in Berlin, respectively.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Assistant Professor of Medicine
Interventional Endoscopy, Division of Gastroenterology
Washington University School of Medicine in St. Louis
Dear Friends,
There have been recent policy changes that may be affecting trainees and practicing physicians, whether directly impacting our current practices or influencing the decisions that shape our careers. During these challenging times, I am trying to remind myself more often of why I am in medicine – my patients. I will continue to advocate for my patients on Hill Days to affect change in policy. I will continue to provide the best care I can and fight for resources to do so. I will continue to adapt to the changing climate and do what is best for my practice so that I can deliver the care I think my patients need. By remembering why I am in medicine, I can fight for a future of medicine and science that is still bright.
In this issue’s “In Focus” article, Dr. Yasmin G. Hernandez-Barco and Dr. Motaz Ashkar review the diagnostic and treatment approaches to exocrine pancreatic insufficiency, including common symptoms, differential diagnoses, and the different pancreatic enzyme replacement therapies.
Medications for weight loss are becoming more widely available; however, the literature on what to do with these medications in gastrointestinal endoscopy is still lacking. Dr. Sitharthan Sekar and Dr. Nikiya Asamoah summarize the current data and available guidelines in our “Short Clinical Review.”
With another new academic year upon us, this issue’s “Early Career” section features Dr. Allon Kahn’s top tips for becoming an effective gastroenterology consultant. He describes the 5 principles that would improve patient care and relationships with referring providers.
In the “Finance/Legal” section, Dr. Koushik Das dissects what happens when a physician gets sued, including the basis of malpractice suits, consequences, and anticipated timeline.
If you are interested in contributing or have ideas for future TNG topics, please contact me (tjudy@wustl.edu) or Danielle Kiefer (dkiefer@gastro.org), Communications/Managing Editor of TNG.
Until next time, I leave you with a historical fun fact, because we would not be where we are now without appreciating where we were: the pancreas was first discovered by a Greek surgeon, Herophilus, in 336 BC, but its exocrine and endocrine functions were not described until the 1850s-1860s by D. Moyse in Paris and Paul Langerhans in Berlin, respectively.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Assistant Professor of Medicine
Interventional Endoscopy, Division of Gastroenterology
Washington University School of Medicine in St. Louis
Journal Highlights: May-July 2025
Esophagus/Motility
Nguyen AD, et al. AGA Clinical Practice Update on Incorporating Functional Lumen Imaging Probe Into Esophageal Clinical Practice: Expert Review. Gastroenterology. 2025 Jul. doi: 10.1053/j.gastro.2025.05.011.
Hartnett DA, et al. Distribution of Esophageal Eosinophilia as a Predictor of Proton Pump Inhibitor Response in Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2025 Jul. doi: 10.1016/j.cgh.2025.06.032.
Gyawali CP, et al. pH Impedance Monitoring on Proton Pump Inhibitor Therapy Impacts Management Decisions in Proven GERD but not in Unproven GERD. Clin Gastroenterol Hepatol. 2025 May. doi: 10.1016/j.cgh.2025.02.032.
Stomach
Wiklund AK, et al. Risk of Gastric Adenocarcinoma After Eradication of Helicobacter pylori. Gastroenterology. 2025 Feb. doi: 10.1053/j.gastro.2025.01.239.
Sonaiya S, et al. Over-the-Scope Clip versus Standard Endoscopic Therapy as First-Line Intervention for Nonvariceal Upper Gastrointestinal Bleeding: A Cost-Effectiveness Analysis. Tech Innov Gastrointest. 2025 Jun. doi: 10.1016/j.tige.2025.250935.
Colon
Hassan C, et al. Colon Cancer Screening, Surveillance, and Treatment: Novel Artificial Intelligence Driving Strategies in the Management of Colon Lesions. Gastroenterology. 2025 Mar. doi: 10.1053/j.gastro.2025.02.021.
Pancreas
Wilcox CM, et al; US Pancreatic Disease Study Group. Management of the Disconnected Pancreatic Duct in Pancreatic Necrosis. Clin Gastroenterol Hepatol. 2025 Jul. doi: 10.1016/j.cgh.2025.05.024.
Ghimire C, et al. The effect of advances in pancreatic cancer treatment in population mortality: A SEER-based study. Gastro Hep Adv. 2025 Jul. doi: 10.1016/j.gastha.2025.100739.
Hepatology
Canivet CM, et al. Validation of the AASLD/EASL Multi-Step Screening Strategies for MASLD. Gastro Hep Adv. 2025 Jul. doi: 10.1016/j.gastha.2025.100747.
Miscellaneous
Chang L, et al. Gut Feelings: The Critical Role of Interoception in Obesity and Disorders of Gut-Brain Interaction. Gastroenterology. 2025 Aug. doi: 10.1053/j.gastro.2025.04.002.
Bashiri K, et al. Advancing Hemostatic Powder Technologies for Management of Gastrointestinal Bleeding: Challenges and Solutions. Tech Innov Gastrointest. 2025 Jul. doi: 10.1016/j.tige.2025.250940.
Dr. Trieu is assistant professor of medicine, interventional endoscopy, in the Division of Gastroenterology at Washington University in St. Louis School of Medicine, Missouri.
Esophagus/Motility
Nguyen AD, et al. AGA Clinical Practice Update on Incorporating Functional Lumen Imaging Probe Into Esophageal Clinical Practice: Expert Review. Gastroenterology. 2025 Jul. doi: 10.1053/j.gastro.2025.05.011.
Hartnett DA, et al. Distribution of Esophageal Eosinophilia as a Predictor of Proton Pump Inhibitor Response in Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2025 Jul. doi: 10.1016/j.cgh.2025.06.032.
Gyawali CP, et al. pH Impedance Monitoring on Proton Pump Inhibitor Therapy Impacts Management Decisions in Proven GERD but not in Unproven GERD. Clin Gastroenterol Hepatol. 2025 May. doi: 10.1016/j.cgh.2025.02.032.
Stomach
Wiklund AK, et al. Risk of Gastric Adenocarcinoma After Eradication of Helicobacter pylori. Gastroenterology. 2025 Feb. doi: 10.1053/j.gastro.2025.01.239.
Sonaiya S, et al. Over-the-Scope Clip versus Standard Endoscopic Therapy as First-Line Intervention for Nonvariceal Upper Gastrointestinal Bleeding: A Cost-Effectiveness Analysis. Tech Innov Gastrointest. 2025 Jun. doi: 10.1016/j.tige.2025.250935.
Colon
Hassan C, et al. Colon Cancer Screening, Surveillance, and Treatment: Novel Artificial Intelligence Driving Strategies in the Management of Colon Lesions. Gastroenterology. 2025 Mar. doi: 10.1053/j.gastro.2025.02.021.
Pancreas
Wilcox CM, et al; US Pancreatic Disease Study Group. Management of the Disconnected Pancreatic Duct in Pancreatic Necrosis. Clin Gastroenterol Hepatol. 2025 Jul. doi: 10.1016/j.cgh.2025.05.024.
Ghimire C, et al. The effect of advances in pancreatic cancer treatment in population mortality: A SEER-based study. Gastro Hep Adv. 2025 Jul. doi: 10.1016/j.gastha.2025.100739.
Hepatology
Canivet CM, et al. Validation of the AASLD/EASL Multi-Step Screening Strategies for MASLD. Gastro Hep Adv. 2025 Jul. doi: 10.1016/j.gastha.2025.100747.
Miscellaneous
Chang L, et al. Gut Feelings: The Critical Role of Interoception in Obesity and Disorders of Gut-Brain Interaction. Gastroenterology. 2025 Aug. doi: 10.1053/j.gastro.2025.04.002.
Bashiri K, et al. Advancing Hemostatic Powder Technologies for Management of Gastrointestinal Bleeding: Challenges and Solutions. Tech Innov Gastrointest. 2025 Jul. doi: 10.1016/j.tige.2025.250940.
Dr. Trieu is assistant professor of medicine, interventional endoscopy, in the Division of Gastroenterology at Washington University in St. Louis School of Medicine, Missouri.
Esophagus/Motility
Nguyen AD, et al. AGA Clinical Practice Update on Incorporating Functional Lumen Imaging Probe Into Esophageal Clinical Practice: Expert Review. Gastroenterology. 2025 Jul. doi: 10.1053/j.gastro.2025.05.011.
Hartnett DA, et al. Distribution of Esophageal Eosinophilia as a Predictor of Proton Pump Inhibitor Response in Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2025 Jul. doi: 10.1016/j.cgh.2025.06.032.
Gyawali CP, et al. pH Impedance Monitoring on Proton Pump Inhibitor Therapy Impacts Management Decisions in Proven GERD but not in Unproven GERD. Clin Gastroenterol Hepatol. 2025 May. doi: 10.1016/j.cgh.2025.02.032.
Stomach
Wiklund AK, et al. Risk of Gastric Adenocarcinoma After Eradication of Helicobacter pylori. Gastroenterology. 2025 Feb. doi: 10.1053/j.gastro.2025.01.239.
Sonaiya S, et al. Over-the-Scope Clip versus Standard Endoscopic Therapy as First-Line Intervention for Nonvariceal Upper Gastrointestinal Bleeding: A Cost-Effectiveness Analysis. Tech Innov Gastrointest. 2025 Jun. doi: 10.1016/j.tige.2025.250935.
Colon
Hassan C, et al. Colon Cancer Screening, Surveillance, and Treatment: Novel Artificial Intelligence Driving Strategies in the Management of Colon Lesions. Gastroenterology. 2025 Mar. doi: 10.1053/j.gastro.2025.02.021.
Pancreas
Wilcox CM, et al; US Pancreatic Disease Study Group. Management of the Disconnected Pancreatic Duct in Pancreatic Necrosis. Clin Gastroenterol Hepatol. 2025 Jul. doi: 10.1016/j.cgh.2025.05.024.
Ghimire C, et al. The effect of advances in pancreatic cancer treatment in population mortality: A SEER-based study. Gastro Hep Adv. 2025 Jul. doi: 10.1016/j.gastha.2025.100739.
Hepatology
Canivet CM, et al. Validation of the AASLD/EASL Multi-Step Screening Strategies for MASLD. Gastro Hep Adv. 2025 Jul. doi: 10.1016/j.gastha.2025.100747.
Miscellaneous
Chang L, et al. Gut Feelings: The Critical Role of Interoception in Obesity and Disorders of Gut-Brain Interaction. Gastroenterology. 2025 Aug. doi: 10.1053/j.gastro.2025.04.002.
Bashiri K, et al. Advancing Hemostatic Powder Technologies for Management of Gastrointestinal Bleeding: Challenges and Solutions. Tech Innov Gastrointest. 2025 Jul. doi: 10.1016/j.tige.2025.250940.
Dr. Trieu is assistant professor of medicine, interventional endoscopy, in the Division of Gastroenterology at Washington University in St. Louis School of Medicine, Missouri.