User login
Non-Invasive Blood and Stool CRC Screening Tests: Available Modalities and Their Clinical Application
Introduction
Colorectal cancer (CRC) screening significantly reduces CRC incidence and mortality, but only 65% of eligible individuals report being up-to-date with screening.1 Colonoscopy is the most widely used opportunistic screening method in the United States and is associated with many barriers to uptake. Providing patients a choice of colonoscopy and/or stool-based tests, improves screening adherence in randomized controlled trials.2,3 Non-invasive screening options have expanded from stool occult blood and multi-target DNA tests, to multi-target stool RNA tests, and novel blood-based tests, the latter only U.S. Food and Drug Administration (FDA) approved for patients who refuse colonoscopy and stool-based tests.
Stool Occult Blood Tests
Guaiac-based fecal occult blood testing (gFOBT) significantly reduces CRC mortality by 33%-35% when implemented on an annual or biennial basis.4,5 Fecal immunochemical testing (FIT) has supplanted gFOBT with advantages including independence from dietary restriction and medication-related interference, use of antibodies specific to human globin, and the need for only a single stool sample.
The most common threshold for a positive FIT in the U.S. is ≥ 20 micrograms (μg) of hemoglobin per gram (g) of stool. FIT is approved by the FDA as a qualitative positive or negative result based on a threshold value.6 A meta-analysis summarized test characteristics of commercially available FITs at various detection thresholds.7 The CRC sensitivity and specificity was 75% and 95% for ≥ 20 ug hemoglobin/g stool, and 91% and 90% for 10 ug hemoglobin/g stool, respectively. The sensitivity for advanced adenomas ranged from 25% at 20 μg/g to 40% at a 10 μg/g. Programmatic use of FIT in adults ages ≥ 50 years at 20 ug/g of stool, in cohort and case control studies, has been shown to significantly reduce CRC mortality by 33%-40% and advanced stage CRC by 34%.8,9
Over 57,000 average-risk individuals ages 50–69 years were randomized to biennial FIT or one-time colonoscopy and followed for 10 years.10 CRC mortality and incidence was similar between the groups: 0.22% with FIT vs. 0.24% with colonoscopy and 1.13% with FIT vs. 1.22% with colonoscopy, respectively. Thus, confirming biennial FIT screening is non-inferior to one-time colonoscopy in important CRC-related outcomes.
Multi-Target Stool Tests
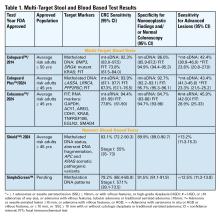
Two multitarget stool DNA tests (mt-sDNA) known as Cologuard™ and Cologuard Plus™ have been approved by the FDA. Both tests include a FIT (with a positivity threshold of 20 μg hemoglobin per gram of stool) combined with DNA methylation markers. The test result is qualitative, reported as a positive or negative. Cologuard™ markers include methylated BMP3, NDRG4, and mutant KRAS while Cologuard Plus™ assesses methylated LASS4, LRRC4, and PPP2R5C. The respective mt-sDNA tests were studied in 9989 of 12,776 and 20,176 of 26,758 average-risk individuals undergoing colonoscopy and the results were compared to a commercially available FIT (with a positivity threshold of 20 μg hemoglobin/gram of stool).11,12 In both trials, the sensitivity for CRC and advanced precancerous lesions was higher with the mt-sDNA tests compared to FIT but had a significantly lower specificity for advanced precancerous lesions versus FIT (see Table 1). An age-related decline in specificity was noted in both trials with mt-sDNA, a trend not observed with FIT. This reduction may be attributed to age-related DNA methylation.
Multi-Target Stool RNA Test
A multi-target stool RNA test (mt-sRNA) commercially available as ColoSense™ is FDA-approved. It combines FIT (at a positivity threshold of 20 μg hemoglobin/gram of stool) with RNA-based stool markers. The combined results of the RNA markers, FIT, and smoking status provide a qualitative single test result. In the trial, 8,920 adults aged ≥45 underwent the mt-sRNA test and FIT followed by colonoscopy (13). The mt-sRNA showed higher sensitivity for CRC than FIT (94.4% versus 77.8%) and advanced adenomas (45.9% versus 28.9%) but lower CRC specificity (84.7% vs 94.7%) (Table 1). Unlike mt-sDNA-based tests, mt-sRNA showed consistent performance across age groups, addressing concerns about age-related declines in specificity attributed to DNA methylation.
Blood-Based Tests
In 2014, the first blood-based (BBT) CRC screening test known as Epi proColon™ was FDA but not Centers for Medicare & Medicaid Services (CMS) approved for average-risk adults ≥50 years of age who are offered and refused other U.S Preventive Services Task Force (USPSTF) endorsed CRC screening tests. It is a qualitative test for detection of circulating methylated Septin 9 (mSeptin9). The accuracy of mSeptin9 to detect CRC was assessed in a subset of 7941 asymptomatic average risk adults undergoing screening colonoscopy.14 The sensitivity and specificity for CRC were 48% and 91.5%, respectively. The sensitivity for advanced adenomas was 11.2%. An increase in sensitivity to 63.9% and reduction in specificity to 88.4% for CRC was demonstrated in a sub-analysis of available samples where an additional (third) polymerase chain replicate was performed. Epi proColon™ is not currently reimbursed by Medicare and not endorsed in the latest USPSTF guidelines.
Technologic advancements have improved the detection of circulating tumor markers in the blood. The Shield™ BBT approved by the FDA in 2024 for average risk adults ≥ 45 years integrates three types of cfDNA data (epigenetic changes resulting in the aberrant methylation or fragmentation patterns, and genomic changes resulting in somatic mutations) into a positive or negative test result. In the trial, 22,877 average-risk, asymptomatic individuals ages 45–84 were enrolled and clinical validation was performed in 7,861 of the participants.15 The sensitivity for CRC was 83.1% which decreased to 55% for stage I tumors (see Table 1). CRC specificity was 89.6% and the sensitivity for advanced adenomas and large sessile serrated lesions was 13.2%.
Another BBT SimpleScreen™, which is not yet FDA-approved, analyzed circulating, cell-free DNA methylation patterns in 27,010 evaluable average-risk, asymptomatic adults ages 45–85 years undergoing screening colonoscopy.16 The sensitivity and specificity for CRC was 79.2% and 91.5%, respectively. Similar to Shield, the sensitivity for stage I CRC was low at 57.1%. The sensitivity for advanced precancerous lesions, a secondary endpoint, was 12.5% which did not meet the prespecified study criteria.
Effectiveness and Cost Effectiveness
Modeling studies have evaluated novel noninvasive CRC screening tests compared to FIT and colonoscopy.17-20 One compared a hypothetical BBT performed every 3 years that meets the minimum CMS threshold CRC sensitivity and specificity of 74% and 90%, respectively, to other established CRC screening tests beginning at age 45.17 Every 3-year BBT reduced CRC incidence and mortality by 40% and 52%, respectively compared to no screening. However, the reductions were much lower than yearly FIT (72% and 76%, respectively), every 10 year colonoscopy (79% and 81%, respectively), and triennial mt-sDNA (68% and 73%, respectively). The BBT resulted in fewer quality-adjusted life-years per person compared to the alternatives.
Additionally, FIT, colonoscopy, and mt-sDNA were less costly and more effective. Advanced precancerous lesion detection was a key measure for a test’s effectiveness. BBT characteristics would require a CRC sensitivity and specificity of >90% and 90%, respectively, and 80% sensitivity for advanced precancerous lesions at a cost of ≤$120–$140 to be cost-effective compared to FIT at comparable participation rates.
Another analysis simulated colorectal neoplasia progression and compared clinical effectiveness and cost between annual FIT, every 3 year stool mt-sRNA, every 3 year stool mt-sDNA tests, every 3 year stool Shield™; these outcomes were compared to colonoscopy every 10 years and no screening in adults ≥ age 45 over different adherence rates.19 At real-world adherence rates of 60%, colonoscopy prevented most CRC cases and associated deaths. FIT was the most cost-effective strategy at all adherence levels. Between the multi-target stool tests and Shield™, mt-sRNA was the most cost-effective. Compared to FIT, mt-sRNA reduced CRC cases and deaths by 1% and 14%.
The third study evaluated CRC incidence and mortality, quality-adjusted life-years and costs with annual FIT, colonoscopy every 10 years, mt- sDNA tests, mt-sRNA test, and BBTs.20 The latest mt-sDNA (Colguard plus™) and mt-sRNA achieved benefits approaching FIT but the Shield™ test was substantially less effective. The authors hypothesized that if 15% of the population substituted Shield™ for current effective CRC screening strategies, an increase in CRC deaths would occur and require 9-10% of the unscreened population to uptake screening with Shield to avert the increases in CRC deaths due to the substitution effect.
Clinical Implications
The effectiveness of non-invasive screening strategies depends on their diagnostic performance, adherence, and ensuring a timely colonoscopy after a positive test. Two claims-based studies found 47.9% and 49% of patients underwent follow-up colonoscopy within 6 months of an abnormal stool or BBT CRC screening test, respectively.21-22
Conclusions
Non-invasive stool mt-sDNA and mt-sRNA have higher effectiveness than the new BBTs. BBTs can lead to increased CRC mortality if substituted for the FDA and CMS-approved, USPSTF-endorsed, CRC screening modalities. If future BBTs increase their sensitivity for CRC (including early-stage CRC) and advanced precancerous lesions and decrease their cost, they may prove to have similar cost-effectiveness to stool-based tests. Currently, BBTs are not a substitute for colonoscopy or other stool tests and should be offered to patients who refuse other CRC screening modalities. A personalized, risk-adapted approach, paired with improved adherence and follow-up are essential to optimize the population-level impact of CRC screening and ensure equitable, effective cancer prevention.
Dr. Gupta is based at the Division of Gastroenterology and Hepatology, Department of Medicine, University of Maryland School of Medicine, Baltimore. Dr. Burke and Dr. Macaron are based at the Department of Gastroenterology, Hepatology, and Nutrition, Cleveland Clinic, Cleveland, Ohio. Dr. Gupta and Dr. Macaron declared no conflicts of interest in regard to this article. Dr. Burke declared research support from Emtora Biosciences. She is a current consultant for Lumabridge, and has been a consultant for Sebela and Almirall. She also disclosed support from Myriad, Genzyme, Ferring, Merck, Sharp and Dohme, Abbvie, Salix, and Natera.
References
1. Benavidez GA, Sedani AE, Felder TM, Asare M, Rogers CR. Rural-urban disparities and trends in cancer screening: an analysis of Behavioral Risk Factor Surveillance System data (2018-2022). JNCI Cancer Spectr. 2024 Nov 1;8(6):pkae113
2. Galoosian A, Dai H, Croymans D, et al. Population Health Colorectal Cancer Screening Strategies in Adults Aged 45 to 49 Years: A Randomized Clinical Trial. JAMA. 2025 Aug 4:e2512049. doi: 10.1001/jama.2025.12049. Epub ahead of print.
3. Pilonis ND, Bugajski M, Wieszczy P, et al. Participation in Competing Strategies for Colorectal Cancer Screening: A Randomized Health Services Study (PICCOLINO Study). Gastroenterology. 2021 Mar;160(4):1097-1105.
4. Shaukat A, Mongin SJ, Geisser MS, et al. Long-term mortality after screening for colorectal cancer. N Engl J Med. 2013;369(12):1106–1114.
5. Kronborg O, Fenger C, Olsen J, Jørgensen OD, Søndergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996 Nov 30;348(9040):1467-71. doi: 10.1016/S0140-6736(96)03430-7. PMID: 8942774.
6. Burke CA, Lieberman D, Feuerstein JD. AGA Clinical Practice Update on Approach to the Use of Noninvasive Colorectal Cancer Screening Options: Commentary. Gastroenterology. 2022 Mar;162(3):952-956. doi: 10.1053/j.gastro.2021.09.075. Epub 2022 Jan 28. PMID: 35094786.
7. Imperiale TF, Gruber RN Stump TE, et al. Performance characteristics of fecal immunochemical tests for colorectal cancer and advanced adenomatous polyps: a systematic review and meta-analysis. Ann Intern Med 2019; 170(5):319-329
8. Doubeni CA, Corley DA, Jensen CD, et al. Fecal Immunochemical Test Screening and Risk of Colorectal Cancer Death. JAMA Netw Open. 2024 Jul 1;7(7):e2423671. doi: 10.1001/jamanetworkopen.2024.23671.
9. Chiu HM, Jen GH, Wang YW, et al. Long-term effectiveness of faecal immunochemical test screening for proximal and distal colorectal cancers. Gut. 2021 Dec;70(12):2321-2329. doi: 10.1136/gutjnl-2020-322545. Epub 2021 Jan 25.
10. Castells A, Quintero E, Bujanda L, et al; COLONPREV study investigators. Effect of invitation to colonoscopy versus fecal immunochemical test screening on colorectal cancer mortality (COLONPREV): a pragmatic, randomised, controlled, non-inferiority trial. Lancet. 2025;405(10486):1231–1239
11. Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014;370(14):1287-1297
12. Imperiale TF, Porter K, Zella J, et al. Next-Generation Multitarget Stool DNA Test for Colorectal Cancer Screening. N Engl J Med. 2024 Mar 14;390(11):984-993
13. Barnell EK, Wurtzler EM, La Rocca J, et al. Multitarget Stool RNA Test for Colorectal Cancer Screening. JAMA. 2023 Nov 14;330(18):1760-1768.
14. Church TR, Wandell M, Lofton-Day C, et al. Prospective evaluation of methylated SEPT9 in plasma for detection of asymptomatic colorectal cancer. Gut 2014; 63:317–325.
15. Chung DC, Gray DM 2nd, Singh H, et al. A Cell-free DNA Blood-Based Test for Colorectal Cancer Screening. N Engl J Med. 2024 Mar 14;390(11):973-983.
16. Shaukat A, Burke CA, Chan AT, et al. Clinical Validation of a Circulating Tumor DNA-Based Blood Test to Screen for Colorectal Cancer. JAMA. 2025 Jul 1;334(1):56-63.
17. Ladabaum U, Mannalithara A, Weng Y, et al. Comparative Effectiveness and Cost-Effectiveness of Colorectal Cancer Screening with Blood-Based Biomarkers (Liquid Biopsy) vs Fecal Tests or Colonoscopy. Gastroenterology. 2024 Jul;167(2):378-391.
18. van den Puttelaar R, Nascimento de Lima P, Knudsen AB, et al. Effectiveness and cost-effectiveness of colorectal cancer screening with a blood test that meets the Centers for Medicare & Medicaid Services coverage decision. Gastroenterology 2024;167:368–377.
19. Shaukat A, Levin TR, Liang PS. Cost-effectiveness of Novel Noninvasive Screening Tests for Colorectal Neoplasia. Clin Gastroenterol Hepatol. 2025 Jun 23:S1542-3565(25)00525-7. doi: 10.1016/j.cgh.2025.06.006. Epub ahead of print. PMID: 40562290.
20. Ladabaum U, Mannalithara A, Schoen RE, Dominitz JA, Lieberman D. Projected Impact and Cost-Effectiveness of Novel Molecular Blood-Based or Stool-Based Screening Tests for Colorectal Cancer. Ann Intern Med. 2024 Dec;177(12):1610-1620.
20. Ciemins EL, Mohl JT, Moreno CA, Colangelo F, Smith RA, Barton M. Development of a Follow-Up Measure to Ensure Complete Screening for Colorectal Cancer. JAMA Netw Open. 2024 Mar 4;7(3):e242693. doi: 10.1001/jamanetworkopen.2024.2693.
21. Zaki TA, Zhang NJ, Forbes SP, Raymond VM, Das AK, May FP. Colonoscopic Follow-up After Abnormal Blood-Based Colorectal Cancer Screening Results. Gastroenterology. 2025 Jul 21:S0016-5085(25)05775-0. doi: 10.1053/j.gastro.2025.07.019. Epub ahead of print. PMID: 40744392.
Introduction
Colorectal cancer (CRC) screening significantly reduces CRC incidence and mortality, but only 65% of eligible individuals report being up-to-date with screening.1 Colonoscopy is the most widely used opportunistic screening method in the United States and is associated with many barriers to uptake. Providing patients a choice of colonoscopy and/or stool-based tests, improves screening adherence in randomized controlled trials.2,3 Non-invasive screening options have expanded from stool occult blood and multi-target DNA tests, to multi-target stool RNA tests, and novel blood-based tests, the latter only U.S. Food and Drug Administration (FDA) approved for patients who refuse colonoscopy and stool-based tests.
Stool Occult Blood Tests
Guaiac-based fecal occult blood testing (gFOBT) significantly reduces CRC mortality by 33%-35% when implemented on an annual or biennial basis.4,5 Fecal immunochemical testing (FIT) has supplanted gFOBT with advantages including independence from dietary restriction and medication-related interference, use of antibodies specific to human globin, and the need for only a single stool sample.
The most common threshold for a positive FIT in the U.S. is ≥ 20 micrograms (μg) of hemoglobin per gram (g) of stool. FIT is approved by the FDA as a qualitative positive or negative result based on a threshold value.6 A meta-analysis summarized test characteristics of commercially available FITs at various detection thresholds.7 The CRC sensitivity and specificity was 75% and 95% for ≥ 20 ug hemoglobin/g stool, and 91% and 90% for 10 ug hemoglobin/g stool, respectively. The sensitivity for advanced adenomas ranged from 25% at 20 μg/g to 40% at a 10 μg/g. Programmatic use of FIT in adults ages ≥ 50 years at 20 ug/g of stool, in cohort and case control studies, has been shown to significantly reduce CRC mortality by 33%-40% and advanced stage CRC by 34%.8,9
Over 57,000 average-risk individuals ages 50–69 years were randomized to biennial FIT or one-time colonoscopy and followed for 10 years.10 CRC mortality and incidence was similar between the groups: 0.22% with FIT vs. 0.24% with colonoscopy and 1.13% with FIT vs. 1.22% with colonoscopy, respectively. Thus, confirming biennial FIT screening is non-inferior to one-time colonoscopy in important CRC-related outcomes.
Multi-Target Stool Tests
Two multitarget stool DNA tests (mt-sDNA) known as Cologuard™ and Cologuard Plus™ have been approved by the FDA. Both tests include a FIT (with a positivity threshold of 20 μg hemoglobin per gram of stool) combined with DNA methylation markers. The test result is qualitative, reported as a positive or negative. Cologuard™ markers include methylated BMP3, NDRG4, and mutant KRAS while Cologuard Plus™ assesses methylated LASS4, LRRC4, and PPP2R5C. The respective mt-sDNA tests were studied in 9989 of 12,776 and 20,176 of 26,758 average-risk individuals undergoing colonoscopy and the results were compared to a commercially available FIT (with a positivity threshold of 20 μg hemoglobin/gram of stool).11,12 In both trials, the sensitivity for CRC and advanced precancerous lesions was higher with the mt-sDNA tests compared to FIT but had a significantly lower specificity for advanced precancerous lesions versus FIT (see Table 1). An age-related decline in specificity was noted in both trials with mt-sDNA, a trend not observed with FIT. This reduction may be attributed to age-related DNA methylation.
Multi-Target Stool RNA Test
A multi-target stool RNA test (mt-sRNA) commercially available as ColoSense™ is FDA-approved. It combines FIT (at a positivity threshold of 20 μg hemoglobin/gram of stool) with RNA-based stool markers. The combined results of the RNA markers, FIT, and smoking status provide a qualitative single test result. In the trial, 8,920 adults aged ≥45 underwent the mt-sRNA test and FIT followed by colonoscopy (13). The mt-sRNA showed higher sensitivity for CRC than FIT (94.4% versus 77.8%) and advanced adenomas (45.9% versus 28.9%) but lower CRC specificity (84.7% vs 94.7%) (Table 1). Unlike mt-sDNA-based tests, mt-sRNA showed consistent performance across age groups, addressing concerns about age-related declines in specificity attributed to DNA methylation.
Blood-Based Tests
In 2014, the first blood-based (BBT) CRC screening test known as Epi proColon™ was FDA but not Centers for Medicare & Medicaid Services (CMS) approved for average-risk adults ≥50 years of age who are offered and refused other U.S Preventive Services Task Force (USPSTF) endorsed CRC screening tests. It is a qualitative test for detection of circulating methylated Septin 9 (mSeptin9). The accuracy of mSeptin9 to detect CRC was assessed in a subset of 7941 asymptomatic average risk adults undergoing screening colonoscopy.14 The sensitivity and specificity for CRC were 48% and 91.5%, respectively. The sensitivity for advanced adenomas was 11.2%. An increase in sensitivity to 63.9% and reduction in specificity to 88.4% for CRC was demonstrated in a sub-analysis of available samples where an additional (third) polymerase chain replicate was performed. Epi proColon™ is not currently reimbursed by Medicare and not endorsed in the latest USPSTF guidelines.
Technologic advancements have improved the detection of circulating tumor markers in the blood. The Shield™ BBT approved by the FDA in 2024 for average risk adults ≥ 45 years integrates three types of cfDNA data (epigenetic changes resulting in the aberrant methylation or fragmentation patterns, and genomic changes resulting in somatic mutations) into a positive or negative test result. In the trial, 22,877 average-risk, asymptomatic individuals ages 45–84 were enrolled and clinical validation was performed in 7,861 of the participants.15 The sensitivity for CRC was 83.1% which decreased to 55% for stage I tumors (see Table 1). CRC specificity was 89.6% and the sensitivity for advanced adenomas and large sessile serrated lesions was 13.2%.
Another BBT SimpleScreen™, which is not yet FDA-approved, analyzed circulating, cell-free DNA methylation patterns in 27,010 evaluable average-risk, asymptomatic adults ages 45–85 years undergoing screening colonoscopy.16 The sensitivity and specificity for CRC was 79.2% and 91.5%, respectively. Similar to Shield, the sensitivity for stage I CRC was low at 57.1%. The sensitivity for advanced precancerous lesions, a secondary endpoint, was 12.5% which did not meet the prespecified study criteria.
Effectiveness and Cost Effectiveness
Modeling studies have evaluated novel noninvasive CRC screening tests compared to FIT and colonoscopy.17-20 One compared a hypothetical BBT performed every 3 years that meets the minimum CMS threshold CRC sensitivity and specificity of 74% and 90%, respectively, to other established CRC screening tests beginning at age 45.17 Every 3-year BBT reduced CRC incidence and mortality by 40% and 52%, respectively compared to no screening. However, the reductions were much lower than yearly FIT (72% and 76%, respectively), every 10 year colonoscopy (79% and 81%, respectively), and triennial mt-sDNA (68% and 73%, respectively). The BBT resulted in fewer quality-adjusted life-years per person compared to the alternatives.
Additionally, FIT, colonoscopy, and mt-sDNA were less costly and more effective. Advanced precancerous lesion detection was a key measure for a test’s effectiveness. BBT characteristics would require a CRC sensitivity and specificity of >90% and 90%, respectively, and 80% sensitivity for advanced precancerous lesions at a cost of ≤$120–$140 to be cost-effective compared to FIT at comparable participation rates.
Another analysis simulated colorectal neoplasia progression and compared clinical effectiveness and cost between annual FIT, every 3 year stool mt-sRNA, every 3 year stool mt-sDNA tests, every 3 year stool Shield™; these outcomes were compared to colonoscopy every 10 years and no screening in adults ≥ age 45 over different adherence rates.19 At real-world adherence rates of 60%, colonoscopy prevented most CRC cases and associated deaths. FIT was the most cost-effective strategy at all adherence levels. Between the multi-target stool tests and Shield™, mt-sRNA was the most cost-effective. Compared to FIT, mt-sRNA reduced CRC cases and deaths by 1% and 14%.
The third study evaluated CRC incidence and mortality, quality-adjusted life-years and costs with annual FIT, colonoscopy every 10 years, mt- sDNA tests, mt-sRNA test, and BBTs.20 The latest mt-sDNA (Colguard plus™) and mt-sRNA achieved benefits approaching FIT but the Shield™ test was substantially less effective. The authors hypothesized that if 15% of the population substituted Shield™ for current effective CRC screening strategies, an increase in CRC deaths would occur and require 9-10% of the unscreened population to uptake screening with Shield to avert the increases in CRC deaths due to the substitution effect.
Clinical Implications
The effectiveness of non-invasive screening strategies depends on their diagnostic performance, adherence, and ensuring a timely colonoscopy after a positive test. Two claims-based studies found 47.9% and 49% of patients underwent follow-up colonoscopy within 6 months of an abnormal stool or BBT CRC screening test, respectively.21-22
Conclusions
Non-invasive stool mt-sDNA and mt-sRNA have higher effectiveness than the new BBTs. BBTs can lead to increased CRC mortality if substituted for the FDA and CMS-approved, USPSTF-endorsed, CRC screening modalities. If future BBTs increase their sensitivity for CRC (including early-stage CRC) and advanced precancerous lesions and decrease their cost, they may prove to have similar cost-effectiveness to stool-based tests. Currently, BBTs are not a substitute for colonoscopy or other stool tests and should be offered to patients who refuse other CRC screening modalities. A personalized, risk-adapted approach, paired with improved adherence and follow-up are essential to optimize the population-level impact of CRC screening and ensure equitable, effective cancer prevention.
Dr. Gupta is based at the Division of Gastroenterology and Hepatology, Department of Medicine, University of Maryland School of Medicine, Baltimore. Dr. Burke and Dr. Macaron are based at the Department of Gastroenterology, Hepatology, and Nutrition, Cleveland Clinic, Cleveland, Ohio. Dr. Gupta and Dr. Macaron declared no conflicts of interest in regard to this article. Dr. Burke declared research support from Emtora Biosciences. She is a current consultant for Lumabridge, and has been a consultant for Sebela and Almirall. She also disclosed support from Myriad, Genzyme, Ferring, Merck, Sharp and Dohme, Abbvie, Salix, and Natera.
References
1. Benavidez GA, Sedani AE, Felder TM, Asare M, Rogers CR. Rural-urban disparities and trends in cancer screening: an analysis of Behavioral Risk Factor Surveillance System data (2018-2022). JNCI Cancer Spectr. 2024 Nov 1;8(6):pkae113
2. Galoosian A, Dai H, Croymans D, et al. Population Health Colorectal Cancer Screening Strategies in Adults Aged 45 to 49 Years: A Randomized Clinical Trial. JAMA. 2025 Aug 4:e2512049. doi: 10.1001/jama.2025.12049. Epub ahead of print.
3. Pilonis ND, Bugajski M, Wieszczy P, et al. Participation in Competing Strategies for Colorectal Cancer Screening: A Randomized Health Services Study (PICCOLINO Study). Gastroenterology. 2021 Mar;160(4):1097-1105.
4. Shaukat A, Mongin SJ, Geisser MS, et al. Long-term mortality after screening for colorectal cancer. N Engl J Med. 2013;369(12):1106–1114.
5. Kronborg O, Fenger C, Olsen J, Jørgensen OD, Søndergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996 Nov 30;348(9040):1467-71. doi: 10.1016/S0140-6736(96)03430-7. PMID: 8942774.
6. Burke CA, Lieberman D, Feuerstein JD. AGA Clinical Practice Update on Approach to the Use of Noninvasive Colorectal Cancer Screening Options: Commentary. Gastroenterology. 2022 Mar;162(3):952-956. doi: 10.1053/j.gastro.2021.09.075. Epub 2022 Jan 28. PMID: 35094786.
7. Imperiale TF, Gruber RN Stump TE, et al. Performance characteristics of fecal immunochemical tests for colorectal cancer and advanced adenomatous polyps: a systematic review and meta-analysis. Ann Intern Med 2019; 170(5):319-329
8. Doubeni CA, Corley DA, Jensen CD, et al. Fecal Immunochemical Test Screening and Risk of Colorectal Cancer Death. JAMA Netw Open. 2024 Jul 1;7(7):e2423671. doi: 10.1001/jamanetworkopen.2024.23671.
9. Chiu HM, Jen GH, Wang YW, et al. Long-term effectiveness of faecal immunochemical test screening for proximal and distal colorectal cancers. Gut. 2021 Dec;70(12):2321-2329. doi: 10.1136/gutjnl-2020-322545. Epub 2021 Jan 25.
10. Castells A, Quintero E, Bujanda L, et al; COLONPREV study investigators. Effect of invitation to colonoscopy versus fecal immunochemical test screening on colorectal cancer mortality (COLONPREV): a pragmatic, randomised, controlled, non-inferiority trial. Lancet. 2025;405(10486):1231–1239
11. Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014;370(14):1287-1297
12. Imperiale TF, Porter K, Zella J, et al. Next-Generation Multitarget Stool DNA Test for Colorectal Cancer Screening. N Engl J Med. 2024 Mar 14;390(11):984-993
13. Barnell EK, Wurtzler EM, La Rocca J, et al. Multitarget Stool RNA Test for Colorectal Cancer Screening. JAMA. 2023 Nov 14;330(18):1760-1768.
14. Church TR, Wandell M, Lofton-Day C, et al. Prospective evaluation of methylated SEPT9 in plasma for detection of asymptomatic colorectal cancer. Gut 2014; 63:317–325.
15. Chung DC, Gray DM 2nd, Singh H, et al. A Cell-free DNA Blood-Based Test for Colorectal Cancer Screening. N Engl J Med. 2024 Mar 14;390(11):973-983.
16. Shaukat A, Burke CA, Chan AT, et al. Clinical Validation of a Circulating Tumor DNA-Based Blood Test to Screen for Colorectal Cancer. JAMA. 2025 Jul 1;334(1):56-63.
17. Ladabaum U, Mannalithara A, Weng Y, et al. Comparative Effectiveness and Cost-Effectiveness of Colorectal Cancer Screening with Blood-Based Biomarkers (Liquid Biopsy) vs Fecal Tests or Colonoscopy. Gastroenterology. 2024 Jul;167(2):378-391.
18. van den Puttelaar R, Nascimento de Lima P, Knudsen AB, et al. Effectiveness and cost-effectiveness of colorectal cancer screening with a blood test that meets the Centers for Medicare & Medicaid Services coverage decision. Gastroenterology 2024;167:368–377.
19. Shaukat A, Levin TR, Liang PS. Cost-effectiveness of Novel Noninvasive Screening Tests for Colorectal Neoplasia. Clin Gastroenterol Hepatol. 2025 Jun 23:S1542-3565(25)00525-7. doi: 10.1016/j.cgh.2025.06.006. Epub ahead of print. PMID: 40562290.
20. Ladabaum U, Mannalithara A, Schoen RE, Dominitz JA, Lieberman D. Projected Impact and Cost-Effectiveness of Novel Molecular Blood-Based or Stool-Based Screening Tests for Colorectal Cancer. Ann Intern Med. 2024 Dec;177(12):1610-1620.
20. Ciemins EL, Mohl JT, Moreno CA, Colangelo F, Smith RA, Barton M. Development of a Follow-Up Measure to Ensure Complete Screening for Colorectal Cancer. JAMA Netw Open. 2024 Mar 4;7(3):e242693. doi: 10.1001/jamanetworkopen.2024.2693.
21. Zaki TA, Zhang NJ, Forbes SP, Raymond VM, Das AK, May FP. Colonoscopic Follow-up After Abnormal Blood-Based Colorectal Cancer Screening Results. Gastroenterology. 2025 Jul 21:S0016-5085(25)05775-0. doi: 10.1053/j.gastro.2025.07.019. Epub ahead of print. PMID: 40744392.
Introduction
Colorectal cancer (CRC) screening significantly reduces CRC incidence and mortality, but only 65% of eligible individuals report being up-to-date with screening.1 Colonoscopy is the most widely used opportunistic screening method in the United States and is associated with many barriers to uptake. Providing patients a choice of colonoscopy and/or stool-based tests, improves screening adherence in randomized controlled trials.2,3 Non-invasive screening options have expanded from stool occult blood and multi-target DNA tests, to multi-target stool RNA tests, and novel blood-based tests, the latter only U.S. Food and Drug Administration (FDA) approved for patients who refuse colonoscopy and stool-based tests.
Stool Occult Blood Tests
Guaiac-based fecal occult blood testing (gFOBT) significantly reduces CRC mortality by 33%-35% when implemented on an annual or biennial basis.4,5 Fecal immunochemical testing (FIT) has supplanted gFOBT with advantages including independence from dietary restriction and medication-related interference, use of antibodies specific to human globin, and the need for only a single stool sample.
The most common threshold for a positive FIT in the U.S. is ≥ 20 micrograms (μg) of hemoglobin per gram (g) of stool. FIT is approved by the FDA as a qualitative positive or negative result based on a threshold value.6 A meta-analysis summarized test characteristics of commercially available FITs at various detection thresholds.7 The CRC sensitivity and specificity was 75% and 95% for ≥ 20 ug hemoglobin/g stool, and 91% and 90% for 10 ug hemoglobin/g stool, respectively. The sensitivity for advanced adenomas ranged from 25% at 20 μg/g to 40% at a 10 μg/g. Programmatic use of FIT in adults ages ≥ 50 years at 20 ug/g of stool, in cohort and case control studies, has been shown to significantly reduce CRC mortality by 33%-40% and advanced stage CRC by 34%.8,9
Over 57,000 average-risk individuals ages 50–69 years were randomized to biennial FIT or one-time colonoscopy and followed for 10 years.10 CRC mortality and incidence was similar between the groups: 0.22% with FIT vs. 0.24% with colonoscopy and 1.13% with FIT vs. 1.22% with colonoscopy, respectively. Thus, confirming biennial FIT screening is non-inferior to one-time colonoscopy in important CRC-related outcomes.
Multi-Target Stool Tests
Two multitarget stool DNA tests (mt-sDNA) known as Cologuard™ and Cologuard Plus™ have been approved by the FDA. Both tests include a FIT (with a positivity threshold of 20 μg hemoglobin per gram of stool) combined with DNA methylation markers. The test result is qualitative, reported as a positive or negative. Cologuard™ markers include methylated BMP3, NDRG4, and mutant KRAS while Cologuard Plus™ assesses methylated LASS4, LRRC4, and PPP2R5C. The respective mt-sDNA tests were studied in 9989 of 12,776 and 20,176 of 26,758 average-risk individuals undergoing colonoscopy and the results were compared to a commercially available FIT (with a positivity threshold of 20 μg hemoglobin/gram of stool).11,12 In both trials, the sensitivity for CRC and advanced precancerous lesions was higher with the mt-sDNA tests compared to FIT but had a significantly lower specificity for advanced precancerous lesions versus FIT (see Table 1). An age-related decline in specificity was noted in both trials with mt-sDNA, a trend not observed with FIT. This reduction may be attributed to age-related DNA methylation.
Multi-Target Stool RNA Test
A multi-target stool RNA test (mt-sRNA) commercially available as ColoSense™ is FDA-approved. It combines FIT (at a positivity threshold of 20 μg hemoglobin/gram of stool) with RNA-based stool markers. The combined results of the RNA markers, FIT, and smoking status provide a qualitative single test result. In the trial, 8,920 adults aged ≥45 underwent the mt-sRNA test and FIT followed by colonoscopy (13). The mt-sRNA showed higher sensitivity for CRC than FIT (94.4% versus 77.8%) and advanced adenomas (45.9% versus 28.9%) but lower CRC specificity (84.7% vs 94.7%) (Table 1). Unlike mt-sDNA-based tests, mt-sRNA showed consistent performance across age groups, addressing concerns about age-related declines in specificity attributed to DNA methylation.
Blood-Based Tests
In 2014, the first blood-based (BBT) CRC screening test known as Epi proColon™ was FDA but not Centers for Medicare & Medicaid Services (CMS) approved for average-risk adults ≥50 years of age who are offered and refused other U.S Preventive Services Task Force (USPSTF) endorsed CRC screening tests. It is a qualitative test for detection of circulating methylated Septin 9 (mSeptin9). The accuracy of mSeptin9 to detect CRC was assessed in a subset of 7941 asymptomatic average risk adults undergoing screening colonoscopy.14 The sensitivity and specificity for CRC were 48% and 91.5%, respectively. The sensitivity for advanced adenomas was 11.2%. An increase in sensitivity to 63.9% and reduction in specificity to 88.4% for CRC was demonstrated in a sub-analysis of available samples where an additional (third) polymerase chain replicate was performed. Epi proColon™ is not currently reimbursed by Medicare and not endorsed in the latest USPSTF guidelines.
Technologic advancements have improved the detection of circulating tumor markers in the blood. The Shield™ BBT approved by the FDA in 2024 for average risk adults ≥ 45 years integrates three types of cfDNA data (epigenetic changes resulting in the aberrant methylation or fragmentation patterns, and genomic changes resulting in somatic mutations) into a positive or negative test result. In the trial, 22,877 average-risk, asymptomatic individuals ages 45–84 were enrolled and clinical validation was performed in 7,861 of the participants.15 The sensitivity for CRC was 83.1% which decreased to 55% for stage I tumors (see Table 1). CRC specificity was 89.6% and the sensitivity for advanced adenomas and large sessile serrated lesions was 13.2%.
Another BBT SimpleScreen™, which is not yet FDA-approved, analyzed circulating, cell-free DNA methylation patterns in 27,010 evaluable average-risk, asymptomatic adults ages 45–85 years undergoing screening colonoscopy.16 The sensitivity and specificity for CRC was 79.2% and 91.5%, respectively. Similar to Shield, the sensitivity for stage I CRC was low at 57.1%. The sensitivity for advanced precancerous lesions, a secondary endpoint, was 12.5% which did not meet the prespecified study criteria.
Effectiveness and Cost Effectiveness
Modeling studies have evaluated novel noninvasive CRC screening tests compared to FIT and colonoscopy.17-20 One compared a hypothetical BBT performed every 3 years that meets the minimum CMS threshold CRC sensitivity and specificity of 74% and 90%, respectively, to other established CRC screening tests beginning at age 45.17 Every 3-year BBT reduced CRC incidence and mortality by 40% and 52%, respectively compared to no screening. However, the reductions were much lower than yearly FIT (72% and 76%, respectively), every 10 year colonoscopy (79% and 81%, respectively), and triennial mt-sDNA (68% and 73%, respectively). The BBT resulted in fewer quality-adjusted life-years per person compared to the alternatives.
Additionally, FIT, colonoscopy, and mt-sDNA were less costly and more effective. Advanced precancerous lesion detection was a key measure for a test’s effectiveness. BBT characteristics would require a CRC sensitivity and specificity of >90% and 90%, respectively, and 80% sensitivity for advanced precancerous lesions at a cost of ≤$120–$140 to be cost-effective compared to FIT at comparable participation rates.
Another analysis simulated colorectal neoplasia progression and compared clinical effectiveness and cost between annual FIT, every 3 year stool mt-sRNA, every 3 year stool mt-sDNA tests, every 3 year stool Shield™; these outcomes were compared to colonoscopy every 10 years and no screening in adults ≥ age 45 over different adherence rates.19 At real-world adherence rates of 60%, colonoscopy prevented most CRC cases and associated deaths. FIT was the most cost-effective strategy at all adherence levels. Between the multi-target stool tests and Shield™, mt-sRNA was the most cost-effective. Compared to FIT, mt-sRNA reduced CRC cases and deaths by 1% and 14%.
The third study evaluated CRC incidence and mortality, quality-adjusted life-years and costs with annual FIT, colonoscopy every 10 years, mt- sDNA tests, mt-sRNA test, and BBTs.20 The latest mt-sDNA (Colguard plus™) and mt-sRNA achieved benefits approaching FIT but the Shield™ test was substantially less effective. The authors hypothesized that if 15% of the population substituted Shield™ for current effective CRC screening strategies, an increase in CRC deaths would occur and require 9-10% of the unscreened population to uptake screening with Shield to avert the increases in CRC deaths due to the substitution effect.
Clinical Implications
The effectiveness of non-invasive screening strategies depends on their diagnostic performance, adherence, and ensuring a timely colonoscopy after a positive test. Two claims-based studies found 47.9% and 49% of patients underwent follow-up colonoscopy within 6 months of an abnormal stool or BBT CRC screening test, respectively.21-22
Conclusions
Non-invasive stool mt-sDNA and mt-sRNA have higher effectiveness than the new BBTs. BBTs can lead to increased CRC mortality if substituted for the FDA and CMS-approved, USPSTF-endorsed, CRC screening modalities. If future BBTs increase their sensitivity for CRC (including early-stage CRC) and advanced precancerous lesions and decrease their cost, they may prove to have similar cost-effectiveness to stool-based tests. Currently, BBTs are not a substitute for colonoscopy or other stool tests and should be offered to patients who refuse other CRC screening modalities. A personalized, risk-adapted approach, paired with improved adherence and follow-up are essential to optimize the population-level impact of CRC screening and ensure equitable, effective cancer prevention.
Dr. Gupta is based at the Division of Gastroenterology and Hepatology, Department of Medicine, University of Maryland School of Medicine, Baltimore. Dr. Burke and Dr. Macaron are based at the Department of Gastroenterology, Hepatology, and Nutrition, Cleveland Clinic, Cleveland, Ohio. Dr. Gupta and Dr. Macaron declared no conflicts of interest in regard to this article. Dr. Burke declared research support from Emtora Biosciences. She is a current consultant for Lumabridge, and has been a consultant for Sebela and Almirall. She also disclosed support from Myriad, Genzyme, Ferring, Merck, Sharp and Dohme, Abbvie, Salix, and Natera.
References
1. Benavidez GA, Sedani AE, Felder TM, Asare M, Rogers CR. Rural-urban disparities and trends in cancer screening: an analysis of Behavioral Risk Factor Surveillance System data (2018-2022). JNCI Cancer Spectr. 2024 Nov 1;8(6):pkae113
2. Galoosian A, Dai H, Croymans D, et al. Population Health Colorectal Cancer Screening Strategies in Adults Aged 45 to 49 Years: A Randomized Clinical Trial. JAMA. 2025 Aug 4:e2512049. doi: 10.1001/jama.2025.12049. Epub ahead of print.
3. Pilonis ND, Bugajski M, Wieszczy P, et al. Participation in Competing Strategies for Colorectal Cancer Screening: A Randomized Health Services Study (PICCOLINO Study). Gastroenterology. 2021 Mar;160(4):1097-1105.
4. Shaukat A, Mongin SJ, Geisser MS, et al. Long-term mortality after screening for colorectal cancer. N Engl J Med. 2013;369(12):1106–1114.
5. Kronborg O, Fenger C, Olsen J, Jørgensen OD, Søndergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996 Nov 30;348(9040):1467-71. doi: 10.1016/S0140-6736(96)03430-7. PMID: 8942774.
6. Burke CA, Lieberman D, Feuerstein JD. AGA Clinical Practice Update on Approach to the Use of Noninvasive Colorectal Cancer Screening Options: Commentary. Gastroenterology. 2022 Mar;162(3):952-956. doi: 10.1053/j.gastro.2021.09.075. Epub 2022 Jan 28. PMID: 35094786.
7. Imperiale TF, Gruber RN Stump TE, et al. Performance characteristics of fecal immunochemical tests for colorectal cancer and advanced adenomatous polyps: a systematic review and meta-analysis. Ann Intern Med 2019; 170(5):319-329
8. Doubeni CA, Corley DA, Jensen CD, et al. Fecal Immunochemical Test Screening and Risk of Colorectal Cancer Death. JAMA Netw Open. 2024 Jul 1;7(7):e2423671. doi: 10.1001/jamanetworkopen.2024.23671.
9. Chiu HM, Jen GH, Wang YW, et al. Long-term effectiveness of faecal immunochemical test screening for proximal and distal colorectal cancers. Gut. 2021 Dec;70(12):2321-2329. doi: 10.1136/gutjnl-2020-322545. Epub 2021 Jan 25.
10. Castells A, Quintero E, Bujanda L, et al; COLONPREV study investigators. Effect of invitation to colonoscopy versus fecal immunochemical test screening on colorectal cancer mortality (COLONPREV): a pragmatic, randomised, controlled, non-inferiority trial. Lancet. 2025;405(10486):1231–1239
11. Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014;370(14):1287-1297
12. Imperiale TF, Porter K, Zella J, et al. Next-Generation Multitarget Stool DNA Test for Colorectal Cancer Screening. N Engl J Med. 2024 Mar 14;390(11):984-993
13. Barnell EK, Wurtzler EM, La Rocca J, et al. Multitarget Stool RNA Test for Colorectal Cancer Screening. JAMA. 2023 Nov 14;330(18):1760-1768.
14. Church TR, Wandell M, Lofton-Day C, et al. Prospective evaluation of methylated SEPT9 in plasma for detection of asymptomatic colorectal cancer. Gut 2014; 63:317–325.
15. Chung DC, Gray DM 2nd, Singh H, et al. A Cell-free DNA Blood-Based Test for Colorectal Cancer Screening. N Engl J Med. 2024 Mar 14;390(11):973-983.
16. Shaukat A, Burke CA, Chan AT, et al. Clinical Validation of a Circulating Tumor DNA-Based Blood Test to Screen for Colorectal Cancer. JAMA. 2025 Jul 1;334(1):56-63.
17. Ladabaum U, Mannalithara A, Weng Y, et al. Comparative Effectiveness and Cost-Effectiveness of Colorectal Cancer Screening with Blood-Based Biomarkers (Liquid Biopsy) vs Fecal Tests or Colonoscopy. Gastroenterology. 2024 Jul;167(2):378-391.
18. van den Puttelaar R, Nascimento de Lima P, Knudsen AB, et al. Effectiveness and cost-effectiveness of colorectal cancer screening with a blood test that meets the Centers for Medicare & Medicaid Services coverage decision. Gastroenterology 2024;167:368–377.
19. Shaukat A, Levin TR, Liang PS. Cost-effectiveness of Novel Noninvasive Screening Tests for Colorectal Neoplasia. Clin Gastroenterol Hepatol. 2025 Jun 23:S1542-3565(25)00525-7. doi: 10.1016/j.cgh.2025.06.006. Epub ahead of print. PMID: 40562290.
20. Ladabaum U, Mannalithara A, Schoen RE, Dominitz JA, Lieberman D. Projected Impact and Cost-Effectiveness of Novel Molecular Blood-Based or Stool-Based Screening Tests for Colorectal Cancer. Ann Intern Med. 2024 Dec;177(12):1610-1620.
20. Ciemins EL, Mohl JT, Moreno CA, Colangelo F, Smith RA, Barton M. Development of a Follow-Up Measure to Ensure Complete Screening for Colorectal Cancer. JAMA Netw Open. 2024 Mar 4;7(3):e242693. doi: 10.1001/jamanetworkopen.2024.2693.
21. Zaki TA, Zhang NJ, Forbes SP, Raymond VM, Das AK, May FP. Colonoscopic Follow-up After Abnormal Blood-Based Colorectal Cancer Screening Results. Gastroenterology. 2025 Jul 21:S0016-5085(25)05775-0. doi: 10.1053/j.gastro.2025.07.019. Epub ahead of print. PMID: 40744392.
GLP-1 Receptor Agonist Use in Gastrointestinal Endoscopy: A Review of Current Evidence and Guidelines
The use of glucagon-like peptide-1 receptor agonists (GLP-1 RAs) has increased over the past several years and has become a cornerstone in both diabetes and weight loss management, particularly because of its unique combination of glucose control, weight reduction potential, and cardiac and metabolic benefits. However, increased use of these agents presents a dilemma in gastrointestinal endoscopy as it pertains to their safety and management during the periprocedural period.
highlighting gaps and future directions.
Pharmacology and Mechanisms of Action
GLP-1 RAs have several mechanisms of action that make them relevant in gastrointestinal endoscopy. These medications modulate glucose control via enhancement of glucose-dependent insulin secretion and reduction of postprandial glucagon, which promotes satiety and delays gastric emptying. This delay in gastric emptying mediated by vagal pathways has been postulated to increase gastric residuals, posing a risk for aspiration during anesthesia.1
It is important to also consider the pharmacokinetics of GLP-1 RAs, as some have shorter half-lives on the order of several hours, like exenatide, while others, like semaglutide, are dosed weekly. Additionally, common side effects of GLP-1 RAs include nausea, vomiting, bloating, and early satiety, which pose challenges for patients undergoing endoscopic procedures.
Current Guidelines
Various societies have published guidelines on the periprocedural use of GLP-1 RAs. The American Society of Anesthesiologist (ASA) in 2023 presented early recommendations to hold GLP-1 RAs either day of procedure or week prior depending on pharmacokinetics, because of the risk of delayed gastric emptying and increased potential for aspiration.2 Soon thereafter, a multi-gastroenterology society guideline was released stating more data is needed to decide if GLP-1 RAs need to be held prior to endoscopic procedures.3
In early 2024, the American Gastroenterological Association (AGA) published a rapid clinical update that advocated for a more individualized approach, particularly in light of limited overall data for GLP-1 RAs and endoscopic procedures.4 In asymptomatic patients who follow typical fasting protocols for procedures, it is generally safe to proceed with endoscopy without holding GLP-1 RAs. In symptomatic patients (nausea, abdominal distension, etc), the AGA advises additional precautions, including performing transabdominal ultrasound if feasible to assess retained gastric contents. The AGA also suggests placing a patient on a clear liquid diet the day prior to the procedure — rather than holding GLP-1 RAs — as another reasonable strategy.
The guidelines continue to evolve with newer multi-society guidelines establishing best practices. While initially in 2023 the ASA did recommend holding these medications prior to endoscopy, the initial guidance was based on expert opinion with limited evidence. Newer multi-society guidance published jointly by the ASA along with various gastroenterology societies, including the AGA in December 2024, takes a more nuanced approach.5
The newer guidelines include two main recommendations:
1. Periprocedural management of GLP-1 RAs should be a joint decision among the procedural, anesthesia, and prescribing team balancing metabolic needs vs patient risks.
- In a low-risk patient, one that is asymptomatic and on standard dosing, among other factors, the guidance states that GLP-1 RAs can be continued.
- In higher-risk patients, the original guidance of holding a day or a week prior to endoscopic procedures should be followed.
2. Periprocedural management of GLP-1 RAs should attempt to minimize the aspiration risks loosely associated with delayed gastric emptying.
- Consider a 24-hour clear liquid diet a day prior to the procedure and transabdominal ultrasound to check gastric contents.
- It is acknowledged that this guidance is based on limited evidence and will be evolving as new medications and data are released.
Recent Clinical Studies
Although there is very little data to guide clinicians, several recent studies have been published that can direct clinical decision-making as guidelines continue to be refined and updated.
A multicenter trial of approximately 800 patients undergoing upper endoscopy found a significant difference in rates of retained gastric contents between those that underwent endoscopy who did and did not follow the ASA guidance on periprocedural management of GLP-1 RAs (12.7% vs 4.4%; P < .0001). However, there were no significant differences in rates of aborted procedures or unplanned intubations.
Furthermore, a multivariable analysis was performed controlling for GLP-1 RA type and other factors, which found the likelihood of gastric retention increased by 36% for every 1% increase in hemoglobin A1c. This study suggests that a more individualized approach to holding GLP-1 RA would be applicable rather than a universal periprocedural hold.6
More recently, a single-center study of nearly 600 patients undergoing upper endoscopy showed that while there were slightly increased rates of retained gastric contents (OR 3.80; P = .003) and aborted procedures (1.3% vs 0%; P = .02), the rates of adverse anesthesia events (hypoxia, etc) were similar between the groups and no cases of pulmonary aspiration were noted.7
One single-center study of 57 patients evaluated the safety of GLP-1 RAs in those undergoing endoscopic sleeve gastrectomy. GLP-1 RAs were continued on all patients, but all adhered to a liquid only diet for at least 24 hours prior to the procedure. There were no instances of retained gastric solids, aspiration, or hypoxia. This study suggests that with a 24-hour clear liquid diet and routine NPO recommendations prior to endoscopy, it would be safe to continue GLP-1 RAs. This study provides rationale for the AGA recommendation for a clear liquid diet 24 hours prior to endoscopic procedures for those on GLP-1 RAs.8
A study looking at those who underwent emergency surgery and endoscopy with claims data of use of GLP-1 RAs found an overall incidence of postoperative respiratory complications of 3.5% for those with GLP-1 RAs fill history vs 4.0% for those without (P = .12). Approximately 800 of the 24,000 patients identified had undergone endoscopic procedures for GI bleeding or food impaction. The study overall showed that preoperative use of GLP-1 RAs in patients undergoing surgery or endoscopy, evaluated as a combined group, was not associated with an increased risk of pulmonary complications.9
Lastly, a systematic review and meta-analysis that included 15 studies that quantified gastric emptying using various methods, including gastric emptying scintigraphy and acetaminophen absorption test, found that there was a quantifiable delay in gastric emptying of about 36 minutes, compared to placebo (P < .01), in patients using GLP-1 RAs. However, compared to standard periprocedural fasting, this delay is clinically insignificant and standard fasting protocols would still be appropriate for patients on GLP-1 RAs.10
These studies taken together suggest that while GLP-1 RAs can mildly increase the likelihood of retained gastric contents, there is no statistically significant increase in the risk of aspiration or other anesthesia complications. Furthermore, while decreased gastric emptying is a known effect of GLP-1 RAs, this effect may not be clinically significant in the context of standard periprocedural fasting protocols particularly when combined with a 24-hour clear liquid diet. These findings support at a minimum a more patient-specific strategy for periprocedural management of GLP-1 RAs.
Clinical Implications
These most recent studies, as well as prior studies and guidelines by various societies lead to a dilemma among endoscopists on proper patient counseling on GLP-1 RAs use before endoscopic procedures. Clinicians must balance the metabolic benefits of GLP-1 RAs with potential endoscopic complications and risks.
Holding therapy theoretically decreases aspiration risk and pulmonary complications, though evidence remains low to support this. Holding medication, however, affects glycemic control leading to potential rebound hyperglycemia which may impact and delay plans for endoscopy. With growing indications for the use of GLP-1 RAs, a more tailored patient-centered treatment plan may be required, especially with consideration of procedure indication and comorbidities.
Currently, practice patterns at different institutions vary widely, making standardization much more difficult. Some centers have opted to follow ASA guidelines of holding these medications up to 1 week prior to procedures, while others have continued therapy with no pre-procedural adjustments. This leaves endoscopists to deal with the downstream effects of inconvenience to patients, care delays, and financial considerations if procedures are postponed related to GLP-1 RAs use.
Future Directions
Future studies are needed to make further evidence-based recommendations. Studies should focus on stratifying risks and recommendations based on procedure type (EGD, colonoscopy, etc). More widespread implementation of gastric ultrasound can assist in real-time decision-making, albeit this would require expertise and dedicated time within the pre-procedural workflow. Randomized controlled trials comparing outcomes of patients who continue GLP-1 RAs vs those who discontinue stratified by baseline risk will be instrumental for making concrete guidelines that provide clarity on periprocedural management of GLP-1 RAs.
Conclusion
The periprocedural management of GLP-1 RAs remains a controversial topic that presents unique challenges in endoscopy. Several guidelines have been released by various stakeholders including anesthesiologists, gastroenterologists, and other prescribing providers. Clinical data remains limited with no robust evidence available to suggest that gastric emptying delays caused by GLP-1 RAs prior to endoscopic procedures significantly increases risk of aspiration, pulmonary complications, or other comorbidities. Evolving multi-society guidelines will be important to establish more consistent practices with reassessment of the data as new studies emerge. A multidisciplinary, individualized patient approach may be the best strategy for managing GLP-1 RAs for patients undergoing endoscopic procedures.
Dr. Sekar and Dr. Asamoah are based in the department of gastroenterology at MedStar Georgetown University Hospital, Washington, D.C. Dr. Sekar reports no conflicts of interest in regard to this article. Dr. Asamoah serves on the Johnson & Johnson advisory board for inflammatory bowel disease–related therapies.
References
1. Halim MA et al. Glucagon-Like Peptide-1 Inhibits Prandial Gastrointestinal Motility Through Myenteric Neuronal Mechanisms in Humans. J Clin Endocrinol Metab. 2018 Feb. doi: 10.1210/jc.2017-02006.
2. American Society of Anesthesiologists. American Society of Anesthesiologists releases consensus-based guidance on preoperative use of GLP-1 receptor agonists. 2023 Jun 20. www.asahq.org/about-asa/newsroom/news-releases/2023/06/american-society-of-anesthesiologists-consensus-based-guidance-on-preoperative
3. American Gastroenterological Association. GI multi-society statement regarding GLP-1 agonists and endoscopy. 2023 Jul 25. gastro.org/news/gi-multi-society-statement-regarding-glp-1-agonists-and-endoscopy/.
4. Hashash JG et al. AGA Rapid Clinical Practice Update on the Management of Patients Taking GLP-1 Receptor Agonists Prior to Endoscopy: Communication. Clin Gastroenterol Hepatol. 2024 Apr. doi: 10.1016/j.cgh.2023.11.002.
5. Kindel TL et al; American Gastroenterological Association; American Society for Metabolic and Bariatric Surgery; American Society of Anesthesiologists; International Society of Perioperative Care of Patients with Obesity; Society of American Gastrointestinal and Endoscopic Surgeons. Multi-society Clinical Practice Guidance for the Safe Use of Glucagon-like Peptide-1 Receptor Agonists in the Perioperative Period. Clin Gastroenterol Hepatol. 2024 Oct. doi: 10.1016/j.cgh.2024.10.003.
6. Phan J et al. Glucagon-Like Peptide Receptor Agonists Use Before Endoscopy Is Associated With Low Retained Gastric Contents: A Multicenter Cross-Sectional Analysis. Am J Gastroenterol. 2025 Mar. doi: 10.14309/ajg.0000000000002969.
7. Panchal S et al. Endoscopy and Anesthesia Outcomes Associated With Glucagon-like Peptide-1 Receptor Agonist use in Patients Undergoing Outpatient Upper Endoscopy. Gastrointest Endosc. 2025 Aug. doi:10.1016/j.gie.2025.01.004.
8. Maselli DB et al. Safe Continuation of glucagon-like Peptide 1 Receptor Agonists at Endoscopy: A Case Series of 57 Adults Undergoing Endoscopic Sleeve Gastroplasty. Obes Surg. 2024 Jul. doi: 10.1007/s11695-024-07278-2.
9. Dixit AA et al. Preoperative GLP-1 Receptor Agonist Use and Risk of Postoperative Respiratory Complications. JAMA. 2024 Apr. doi: 10.1001/jama.2024.5003.
10. Hiramoto B et al. Quantified Metrics of Gastric Emptying Delay by Glucagon-Like Peptide-1 Agonists: A systematic review and meta-analysis with insights for periprocedural management. Am J Gastroenterol. 2024 Jun. doi: 10.14309/ajg.0000000000002820.
The use of glucagon-like peptide-1 receptor agonists (GLP-1 RAs) has increased over the past several years and has become a cornerstone in both diabetes and weight loss management, particularly because of its unique combination of glucose control, weight reduction potential, and cardiac and metabolic benefits. However, increased use of these agents presents a dilemma in gastrointestinal endoscopy as it pertains to their safety and management during the periprocedural period.
highlighting gaps and future directions.
Pharmacology and Mechanisms of Action
GLP-1 RAs have several mechanisms of action that make them relevant in gastrointestinal endoscopy. These medications modulate glucose control via enhancement of glucose-dependent insulin secretion and reduction of postprandial glucagon, which promotes satiety and delays gastric emptying. This delay in gastric emptying mediated by vagal pathways has been postulated to increase gastric residuals, posing a risk for aspiration during anesthesia.1
It is important to also consider the pharmacokinetics of GLP-1 RAs, as some have shorter half-lives on the order of several hours, like exenatide, while others, like semaglutide, are dosed weekly. Additionally, common side effects of GLP-1 RAs include nausea, vomiting, bloating, and early satiety, which pose challenges for patients undergoing endoscopic procedures.
Current Guidelines
Various societies have published guidelines on the periprocedural use of GLP-1 RAs. The American Society of Anesthesiologist (ASA) in 2023 presented early recommendations to hold GLP-1 RAs either day of procedure or week prior depending on pharmacokinetics, because of the risk of delayed gastric emptying and increased potential for aspiration.2 Soon thereafter, a multi-gastroenterology society guideline was released stating more data is needed to decide if GLP-1 RAs need to be held prior to endoscopic procedures.3
In early 2024, the American Gastroenterological Association (AGA) published a rapid clinical update that advocated for a more individualized approach, particularly in light of limited overall data for GLP-1 RAs and endoscopic procedures.4 In asymptomatic patients who follow typical fasting protocols for procedures, it is generally safe to proceed with endoscopy without holding GLP-1 RAs. In symptomatic patients (nausea, abdominal distension, etc), the AGA advises additional precautions, including performing transabdominal ultrasound if feasible to assess retained gastric contents. The AGA also suggests placing a patient on a clear liquid diet the day prior to the procedure — rather than holding GLP-1 RAs — as another reasonable strategy.
The guidelines continue to evolve with newer multi-society guidelines establishing best practices. While initially in 2023 the ASA did recommend holding these medications prior to endoscopy, the initial guidance was based on expert opinion with limited evidence. Newer multi-society guidance published jointly by the ASA along with various gastroenterology societies, including the AGA in December 2024, takes a more nuanced approach.5
The newer guidelines include two main recommendations:
1. Periprocedural management of GLP-1 RAs should be a joint decision among the procedural, anesthesia, and prescribing team balancing metabolic needs vs patient risks.
- In a low-risk patient, one that is asymptomatic and on standard dosing, among other factors, the guidance states that GLP-1 RAs can be continued.
- In higher-risk patients, the original guidance of holding a day or a week prior to endoscopic procedures should be followed.
2. Periprocedural management of GLP-1 RAs should attempt to minimize the aspiration risks loosely associated with delayed gastric emptying.
- Consider a 24-hour clear liquid diet a day prior to the procedure and transabdominal ultrasound to check gastric contents.
- It is acknowledged that this guidance is based on limited evidence and will be evolving as new medications and data are released.
Recent Clinical Studies
Although there is very little data to guide clinicians, several recent studies have been published that can direct clinical decision-making as guidelines continue to be refined and updated.
A multicenter trial of approximately 800 patients undergoing upper endoscopy found a significant difference in rates of retained gastric contents between those that underwent endoscopy who did and did not follow the ASA guidance on periprocedural management of GLP-1 RAs (12.7% vs 4.4%; P < .0001). However, there were no significant differences in rates of aborted procedures or unplanned intubations.
Furthermore, a multivariable analysis was performed controlling for GLP-1 RA type and other factors, which found the likelihood of gastric retention increased by 36% for every 1% increase in hemoglobin A1c. This study suggests that a more individualized approach to holding GLP-1 RA would be applicable rather than a universal periprocedural hold.6
More recently, a single-center study of nearly 600 patients undergoing upper endoscopy showed that while there were slightly increased rates of retained gastric contents (OR 3.80; P = .003) and aborted procedures (1.3% vs 0%; P = .02), the rates of adverse anesthesia events (hypoxia, etc) were similar between the groups and no cases of pulmonary aspiration were noted.7
One single-center study of 57 patients evaluated the safety of GLP-1 RAs in those undergoing endoscopic sleeve gastrectomy. GLP-1 RAs were continued on all patients, but all adhered to a liquid only diet for at least 24 hours prior to the procedure. There were no instances of retained gastric solids, aspiration, or hypoxia. This study suggests that with a 24-hour clear liquid diet and routine NPO recommendations prior to endoscopy, it would be safe to continue GLP-1 RAs. This study provides rationale for the AGA recommendation for a clear liquid diet 24 hours prior to endoscopic procedures for those on GLP-1 RAs.8
A study looking at those who underwent emergency surgery and endoscopy with claims data of use of GLP-1 RAs found an overall incidence of postoperative respiratory complications of 3.5% for those with GLP-1 RAs fill history vs 4.0% for those without (P = .12). Approximately 800 of the 24,000 patients identified had undergone endoscopic procedures for GI bleeding or food impaction. The study overall showed that preoperative use of GLP-1 RAs in patients undergoing surgery or endoscopy, evaluated as a combined group, was not associated with an increased risk of pulmonary complications.9
Lastly, a systematic review and meta-analysis that included 15 studies that quantified gastric emptying using various methods, including gastric emptying scintigraphy and acetaminophen absorption test, found that there was a quantifiable delay in gastric emptying of about 36 minutes, compared to placebo (P < .01), in patients using GLP-1 RAs. However, compared to standard periprocedural fasting, this delay is clinically insignificant and standard fasting protocols would still be appropriate for patients on GLP-1 RAs.10
These studies taken together suggest that while GLP-1 RAs can mildly increase the likelihood of retained gastric contents, there is no statistically significant increase in the risk of aspiration or other anesthesia complications. Furthermore, while decreased gastric emptying is a known effect of GLP-1 RAs, this effect may not be clinically significant in the context of standard periprocedural fasting protocols particularly when combined with a 24-hour clear liquid diet. These findings support at a minimum a more patient-specific strategy for periprocedural management of GLP-1 RAs.
Clinical Implications
These most recent studies, as well as prior studies and guidelines by various societies lead to a dilemma among endoscopists on proper patient counseling on GLP-1 RAs use before endoscopic procedures. Clinicians must balance the metabolic benefits of GLP-1 RAs with potential endoscopic complications and risks.
Holding therapy theoretically decreases aspiration risk and pulmonary complications, though evidence remains low to support this. Holding medication, however, affects glycemic control leading to potential rebound hyperglycemia which may impact and delay plans for endoscopy. With growing indications for the use of GLP-1 RAs, a more tailored patient-centered treatment plan may be required, especially with consideration of procedure indication and comorbidities.
Currently, practice patterns at different institutions vary widely, making standardization much more difficult. Some centers have opted to follow ASA guidelines of holding these medications up to 1 week prior to procedures, while others have continued therapy with no pre-procedural adjustments. This leaves endoscopists to deal with the downstream effects of inconvenience to patients, care delays, and financial considerations if procedures are postponed related to GLP-1 RAs use.
Future Directions
Future studies are needed to make further evidence-based recommendations. Studies should focus on stratifying risks and recommendations based on procedure type (EGD, colonoscopy, etc). More widespread implementation of gastric ultrasound can assist in real-time decision-making, albeit this would require expertise and dedicated time within the pre-procedural workflow. Randomized controlled trials comparing outcomes of patients who continue GLP-1 RAs vs those who discontinue stratified by baseline risk will be instrumental for making concrete guidelines that provide clarity on periprocedural management of GLP-1 RAs.
Conclusion
The periprocedural management of GLP-1 RAs remains a controversial topic that presents unique challenges in endoscopy. Several guidelines have been released by various stakeholders including anesthesiologists, gastroenterologists, and other prescribing providers. Clinical data remains limited with no robust evidence available to suggest that gastric emptying delays caused by GLP-1 RAs prior to endoscopic procedures significantly increases risk of aspiration, pulmonary complications, or other comorbidities. Evolving multi-society guidelines will be important to establish more consistent practices with reassessment of the data as new studies emerge. A multidisciplinary, individualized patient approach may be the best strategy for managing GLP-1 RAs for patients undergoing endoscopic procedures.
Dr. Sekar and Dr. Asamoah are based in the department of gastroenterology at MedStar Georgetown University Hospital, Washington, D.C. Dr. Sekar reports no conflicts of interest in regard to this article. Dr. Asamoah serves on the Johnson & Johnson advisory board for inflammatory bowel disease–related therapies.
References
1. Halim MA et al. Glucagon-Like Peptide-1 Inhibits Prandial Gastrointestinal Motility Through Myenteric Neuronal Mechanisms in Humans. J Clin Endocrinol Metab. 2018 Feb. doi: 10.1210/jc.2017-02006.
2. American Society of Anesthesiologists. American Society of Anesthesiologists releases consensus-based guidance on preoperative use of GLP-1 receptor agonists. 2023 Jun 20. www.asahq.org/about-asa/newsroom/news-releases/2023/06/american-society-of-anesthesiologists-consensus-based-guidance-on-preoperative
3. American Gastroenterological Association. GI multi-society statement regarding GLP-1 agonists and endoscopy. 2023 Jul 25. gastro.org/news/gi-multi-society-statement-regarding-glp-1-agonists-and-endoscopy/.
4. Hashash JG et al. AGA Rapid Clinical Practice Update on the Management of Patients Taking GLP-1 Receptor Agonists Prior to Endoscopy: Communication. Clin Gastroenterol Hepatol. 2024 Apr. doi: 10.1016/j.cgh.2023.11.002.
5. Kindel TL et al; American Gastroenterological Association; American Society for Metabolic and Bariatric Surgery; American Society of Anesthesiologists; International Society of Perioperative Care of Patients with Obesity; Society of American Gastrointestinal and Endoscopic Surgeons. Multi-society Clinical Practice Guidance for the Safe Use of Glucagon-like Peptide-1 Receptor Agonists in the Perioperative Period. Clin Gastroenterol Hepatol. 2024 Oct. doi: 10.1016/j.cgh.2024.10.003.
6. Phan J et al. Glucagon-Like Peptide Receptor Agonists Use Before Endoscopy Is Associated With Low Retained Gastric Contents: A Multicenter Cross-Sectional Analysis. Am J Gastroenterol. 2025 Mar. doi: 10.14309/ajg.0000000000002969.
7. Panchal S et al. Endoscopy and Anesthesia Outcomes Associated With Glucagon-like Peptide-1 Receptor Agonist use in Patients Undergoing Outpatient Upper Endoscopy. Gastrointest Endosc. 2025 Aug. doi:10.1016/j.gie.2025.01.004.
8. Maselli DB et al. Safe Continuation of glucagon-like Peptide 1 Receptor Agonists at Endoscopy: A Case Series of 57 Adults Undergoing Endoscopic Sleeve Gastroplasty. Obes Surg. 2024 Jul. doi: 10.1007/s11695-024-07278-2.
9. Dixit AA et al. Preoperative GLP-1 Receptor Agonist Use and Risk of Postoperative Respiratory Complications. JAMA. 2024 Apr. doi: 10.1001/jama.2024.5003.
10. Hiramoto B et al. Quantified Metrics of Gastric Emptying Delay by Glucagon-Like Peptide-1 Agonists: A systematic review and meta-analysis with insights for periprocedural management. Am J Gastroenterol. 2024 Jun. doi: 10.14309/ajg.0000000000002820.
The use of glucagon-like peptide-1 receptor agonists (GLP-1 RAs) has increased over the past several years and has become a cornerstone in both diabetes and weight loss management, particularly because of its unique combination of glucose control, weight reduction potential, and cardiac and metabolic benefits. However, increased use of these agents presents a dilemma in gastrointestinal endoscopy as it pertains to their safety and management during the periprocedural period.
highlighting gaps and future directions.
Pharmacology and Mechanisms of Action
GLP-1 RAs have several mechanisms of action that make them relevant in gastrointestinal endoscopy. These medications modulate glucose control via enhancement of glucose-dependent insulin secretion and reduction of postprandial glucagon, which promotes satiety and delays gastric emptying. This delay in gastric emptying mediated by vagal pathways has been postulated to increase gastric residuals, posing a risk for aspiration during anesthesia.1
It is important to also consider the pharmacokinetics of GLP-1 RAs, as some have shorter half-lives on the order of several hours, like exenatide, while others, like semaglutide, are dosed weekly. Additionally, common side effects of GLP-1 RAs include nausea, vomiting, bloating, and early satiety, which pose challenges for patients undergoing endoscopic procedures.
Current Guidelines
Various societies have published guidelines on the periprocedural use of GLP-1 RAs. The American Society of Anesthesiologist (ASA) in 2023 presented early recommendations to hold GLP-1 RAs either day of procedure or week prior depending on pharmacokinetics, because of the risk of delayed gastric emptying and increased potential for aspiration.2 Soon thereafter, a multi-gastroenterology society guideline was released stating more data is needed to decide if GLP-1 RAs need to be held prior to endoscopic procedures.3
In early 2024, the American Gastroenterological Association (AGA) published a rapid clinical update that advocated for a more individualized approach, particularly in light of limited overall data for GLP-1 RAs and endoscopic procedures.4 In asymptomatic patients who follow typical fasting protocols for procedures, it is generally safe to proceed with endoscopy without holding GLP-1 RAs. In symptomatic patients (nausea, abdominal distension, etc), the AGA advises additional precautions, including performing transabdominal ultrasound if feasible to assess retained gastric contents. The AGA also suggests placing a patient on a clear liquid diet the day prior to the procedure — rather than holding GLP-1 RAs — as another reasonable strategy.
The guidelines continue to evolve with newer multi-society guidelines establishing best practices. While initially in 2023 the ASA did recommend holding these medications prior to endoscopy, the initial guidance was based on expert opinion with limited evidence. Newer multi-society guidance published jointly by the ASA along with various gastroenterology societies, including the AGA in December 2024, takes a more nuanced approach.5
The newer guidelines include two main recommendations:
1. Periprocedural management of GLP-1 RAs should be a joint decision among the procedural, anesthesia, and prescribing team balancing metabolic needs vs patient risks.
- In a low-risk patient, one that is asymptomatic and on standard dosing, among other factors, the guidance states that GLP-1 RAs can be continued.
- In higher-risk patients, the original guidance of holding a day or a week prior to endoscopic procedures should be followed.
2. Periprocedural management of GLP-1 RAs should attempt to minimize the aspiration risks loosely associated with delayed gastric emptying.
- Consider a 24-hour clear liquid diet a day prior to the procedure and transabdominal ultrasound to check gastric contents.
- It is acknowledged that this guidance is based on limited evidence and will be evolving as new medications and data are released.
Recent Clinical Studies
Although there is very little data to guide clinicians, several recent studies have been published that can direct clinical decision-making as guidelines continue to be refined and updated.
A multicenter trial of approximately 800 patients undergoing upper endoscopy found a significant difference in rates of retained gastric contents between those that underwent endoscopy who did and did not follow the ASA guidance on periprocedural management of GLP-1 RAs (12.7% vs 4.4%; P < .0001). However, there were no significant differences in rates of aborted procedures or unplanned intubations.
Furthermore, a multivariable analysis was performed controlling for GLP-1 RA type and other factors, which found the likelihood of gastric retention increased by 36% for every 1% increase in hemoglobin A1c. This study suggests that a more individualized approach to holding GLP-1 RA would be applicable rather than a universal periprocedural hold.6
More recently, a single-center study of nearly 600 patients undergoing upper endoscopy showed that while there were slightly increased rates of retained gastric contents (OR 3.80; P = .003) and aborted procedures (1.3% vs 0%; P = .02), the rates of adverse anesthesia events (hypoxia, etc) were similar between the groups and no cases of pulmonary aspiration were noted.7
One single-center study of 57 patients evaluated the safety of GLP-1 RAs in those undergoing endoscopic sleeve gastrectomy. GLP-1 RAs were continued on all patients, but all adhered to a liquid only diet for at least 24 hours prior to the procedure. There were no instances of retained gastric solids, aspiration, or hypoxia. This study suggests that with a 24-hour clear liquid diet and routine NPO recommendations prior to endoscopy, it would be safe to continue GLP-1 RAs. This study provides rationale for the AGA recommendation for a clear liquid diet 24 hours prior to endoscopic procedures for those on GLP-1 RAs.8
A study looking at those who underwent emergency surgery and endoscopy with claims data of use of GLP-1 RAs found an overall incidence of postoperative respiratory complications of 3.5% for those with GLP-1 RAs fill history vs 4.0% for those without (P = .12). Approximately 800 of the 24,000 patients identified had undergone endoscopic procedures for GI bleeding or food impaction. The study overall showed that preoperative use of GLP-1 RAs in patients undergoing surgery or endoscopy, evaluated as a combined group, was not associated with an increased risk of pulmonary complications.9
Lastly, a systematic review and meta-analysis that included 15 studies that quantified gastric emptying using various methods, including gastric emptying scintigraphy and acetaminophen absorption test, found that there was a quantifiable delay in gastric emptying of about 36 minutes, compared to placebo (P < .01), in patients using GLP-1 RAs. However, compared to standard periprocedural fasting, this delay is clinically insignificant and standard fasting protocols would still be appropriate for patients on GLP-1 RAs.10
These studies taken together suggest that while GLP-1 RAs can mildly increase the likelihood of retained gastric contents, there is no statistically significant increase in the risk of aspiration or other anesthesia complications. Furthermore, while decreased gastric emptying is a known effect of GLP-1 RAs, this effect may not be clinically significant in the context of standard periprocedural fasting protocols particularly when combined with a 24-hour clear liquid diet. These findings support at a minimum a more patient-specific strategy for periprocedural management of GLP-1 RAs.
Clinical Implications
These most recent studies, as well as prior studies and guidelines by various societies lead to a dilemma among endoscopists on proper patient counseling on GLP-1 RAs use before endoscopic procedures. Clinicians must balance the metabolic benefits of GLP-1 RAs with potential endoscopic complications and risks.
Holding therapy theoretically decreases aspiration risk and pulmonary complications, though evidence remains low to support this. Holding medication, however, affects glycemic control leading to potential rebound hyperglycemia which may impact and delay plans for endoscopy. With growing indications for the use of GLP-1 RAs, a more tailored patient-centered treatment plan may be required, especially with consideration of procedure indication and comorbidities.
Currently, practice patterns at different institutions vary widely, making standardization much more difficult. Some centers have opted to follow ASA guidelines of holding these medications up to 1 week prior to procedures, while others have continued therapy with no pre-procedural adjustments. This leaves endoscopists to deal with the downstream effects of inconvenience to patients, care delays, and financial considerations if procedures are postponed related to GLP-1 RAs use.
Future Directions
Future studies are needed to make further evidence-based recommendations. Studies should focus on stratifying risks and recommendations based on procedure type (EGD, colonoscopy, etc). More widespread implementation of gastric ultrasound can assist in real-time decision-making, albeit this would require expertise and dedicated time within the pre-procedural workflow. Randomized controlled trials comparing outcomes of patients who continue GLP-1 RAs vs those who discontinue stratified by baseline risk will be instrumental for making concrete guidelines that provide clarity on periprocedural management of GLP-1 RAs.
Conclusion
The periprocedural management of GLP-1 RAs remains a controversial topic that presents unique challenges in endoscopy. Several guidelines have been released by various stakeholders including anesthesiologists, gastroenterologists, and other prescribing providers. Clinical data remains limited with no robust evidence available to suggest that gastric emptying delays caused by GLP-1 RAs prior to endoscopic procedures significantly increases risk of aspiration, pulmonary complications, or other comorbidities. Evolving multi-society guidelines will be important to establish more consistent practices with reassessment of the data as new studies emerge. A multidisciplinary, individualized patient approach may be the best strategy for managing GLP-1 RAs for patients undergoing endoscopic procedures.
Dr. Sekar and Dr. Asamoah are based in the department of gastroenterology at MedStar Georgetown University Hospital, Washington, D.C. Dr. Sekar reports no conflicts of interest in regard to this article. Dr. Asamoah serves on the Johnson & Johnson advisory board for inflammatory bowel disease–related therapies.
References
1. Halim MA et al. Glucagon-Like Peptide-1 Inhibits Prandial Gastrointestinal Motility Through Myenteric Neuronal Mechanisms in Humans. J Clin Endocrinol Metab. 2018 Feb. doi: 10.1210/jc.2017-02006.
2. American Society of Anesthesiologists. American Society of Anesthesiologists releases consensus-based guidance on preoperative use of GLP-1 receptor agonists. 2023 Jun 20. www.asahq.org/about-asa/newsroom/news-releases/2023/06/american-society-of-anesthesiologists-consensus-based-guidance-on-preoperative
3. American Gastroenterological Association. GI multi-society statement regarding GLP-1 agonists and endoscopy. 2023 Jul 25. gastro.org/news/gi-multi-society-statement-regarding-glp-1-agonists-and-endoscopy/.
4. Hashash JG et al. AGA Rapid Clinical Practice Update on the Management of Patients Taking GLP-1 Receptor Agonists Prior to Endoscopy: Communication. Clin Gastroenterol Hepatol. 2024 Apr. doi: 10.1016/j.cgh.2023.11.002.
5. Kindel TL et al; American Gastroenterological Association; American Society for Metabolic and Bariatric Surgery; American Society of Anesthesiologists; International Society of Perioperative Care of Patients with Obesity; Society of American Gastrointestinal and Endoscopic Surgeons. Multi-society Clinical Practice Guidance for the Safe Use of Glucagon-like Peptide-1 Receptor Agonists in the Perioperative Period. Clin Gastroenterol Hepatol. 2024 Oct. doi: 10.1016/j.cgh.2024.10.003.
6. Phan J et al. Glucagon-Like Peptide Receptor Agonists Use Before Endoscopy Is Associated With Low Retained Gastric Contents: A Multicenter Cross-Sectional Analysis. Am J Gastroenterol. 2025 Mar. doi: 10.14309/ajg.0000000000002969.
7. Panchal S et al. Endoscopy and Anesthesia Outcomes Associated With Glucagon-like Peptide-1 Receptor Agonist use in Patients Undergoing Outpatient Upper Endoscopy. Gastrointest Endosc. 2025 Aug. doi:10.1016/j.gie.2025.01.004.
8. Maselli DB et al. Safe Continuation of glucagon-like Peptide 1 Receptor Agonists at Endoscopy: A Case Series of 57 Adults Undergoing Endoscopic Sleeve Gastroplasty. Obes Surg. 2024 Jul. doi: 10.1007/s11695-024-07278-2.
9. Dixit AA et al. Preoperative GLP-1 Receptor Agonist Use and Risk of Postoperative Respiratory Complications. JAMA. 2024 Apr. doi: 10.1001/jama.2024.5003.
10. Hiramoto B et al. Quantified Metrics of Gastric Emptying Delay by Glucagon-Like Peptide-1 Agonists: A systematic review and meta-analysis with insights for periprocedural management. Am J Gastroenterol. 2024 Jun. doi: 10.14309/ajg.0000000000002820.
Improving Care for Patients from Historically Minoritized and Marginalized Communities with Disorders of Gut-Brain Interaction
Introduction: Cases
Patient 1: A 57-year-old man with post-prandial distress variant functional dyspepsia (FD) was recommended to start nortriptyline. He previously established primary care with a physician he met at a barbershop health fair in Harlem, who referred him for specialty evaluation. Today, he presents for follow-up and reports he did not take this medication because he heard it is an antidepressant. How would you counsel him?
Patient 2: A 61-year-old woman was previously diagnosed with mixed variant irritable bowel syndrome (IBS-M). Her symptoms have not significantly changed. Her prior workup has been reassuring and consistent with IBS-M. Despite this, the patient pushes to repeat a colonoscopy, fearful that something is being missed or that she is not being offered care because of her undocumented status. How do you respond?
Patient 3: A 36-year-old man is followed for the management of generalized anxiety disorder and functional heartburn. He was started on low-dose amitriptyline with some benefit, but follow-up has been sporadic. On further discussion, he reports financial stressors, time barriers, and difficulty scheduling a meeting with his union representative for work accommodations as he lives in a more rural community. How do you reply?
Patient 4: A 74-year-old man with Parkinson’s disease who uses a wheelchair has functional constipation that is well controlled on his current regimen. He has never undergone colon cancer screening. He occasionally notices blood in his stool, so a colonoscopy was recommended to confirm that his hematochezia reflects functional constipation complicated by hemorrhoids. He is concerned about the bowel preparation required for a colonoscopy given his limited mobility, as his insurance does not cover assistance at home. He does not have family members to help him. How can you assist him?
Social determinants of health, health disparities, and DGBIs
Social determinants of health affect all aspects of patient care, with an increasing body of published work looking at potential disparities in organ-based and structural diseases.1,2,3,4 However, little has been done to explore their influence on disorders of gut-brain interaction or DGBIs.
. As DGBIs cannot be diagnosed with a single laboratory or endoscopic test, the patient history is of the utmost importance and physician-patient rapport is paramount in their treatment. Such rapport may be more difficult to establish in patients coming from historically marginalized and minoritized communities who may be distrustful of healthcare as an institution of (discriminatory) power.
Potential DGBI management pitfalls in historically marginalized or minoritized communities
For racial and ethnic minorities in the United States, disparities in healthcare take on many forms. People from racial and ethnic minority communities are less likely to receive a gastroenterology consultation and those with IBS are more likely to undergo procedures as compared to White patients with IBS.6 Implicit bias may lead to fewer specialist referrals, and specialty care may be limited or unavailable in some areas. Patients may prefer seeing providers in their own community, with whom they share racial or ethnic identities, which could lead to fewer referrals to specialists outside of the community.
Historical discrimination contributes to a lack of trust in healthcare professionals, which may lead patients to favor more objective diagnostics such as endoscopy or view being counseled against invasive procedures as having necessary care denied. Due to a broader cultural stigma surrounding mental illness, patients may be more hesitant to utilize neuromodulators, which have historically been used for psychiatric diagnoses, as it may lead them to conflate their GI illness with mental illness.7,8
Since DGBIs cannot be diagnosed with a single test or managed with a single treatment modality, providing excellent care for patients with DGBIs requires clear communication. For patients with limited English proficiency (LEP), access to high-quality language assistance is the foundation of comprehensive care. Interpreter use (or lack thereof) may limit the ability to obtain a complete and accurate clinical history, which can lead to fewer referrals to specialists and increased reliance on endoscopic evaluations that may not be clinically indicated.
These language barriers affect patients on many levels – in their ability to understand instructions for medication administration, preparation for procedures, and return precautions – which may ultimately lead to poorer responses to therapy or delays in care. LEP alone is broadly associated with fewer referrals for outpatient follow-up, adverse health outcomes and complications, and longer hospital stays.9 These disparities can be mitigated by investing in high-quality interpreter services, providing instructions and forms in multiple languages, and engaging the patient’s family and social supports according to their preferences.
People experiencing poverty (urban and rural) face challenges across multiple domains including access to healthcare, health insurance, stable housing and employment, and more. Many patients seek care at federally qualified health centers, which may face greater difficulties coordinating care with external gastroenterologists.10
Insurance barriers limit access to essential medications, tests, and procedures, and create delays in establishing care with specialists. Significant psychological stress and higher rates of comorbid anxiety and depression contribute to increased IBS severity.11 Financial limitations may limit dietary choices, which can further exacerbate DGBI symptoms. Long work hours with limited flexibility may prohibit them from presenting for regular follow-ups and establishing advanced DGBI care such as with a dietitian or psychologist.
Patients with disabilities face many of the health inequities previously discussed, as well as additional challenges with physical accessibility, transportation, exclusion from education and employment, discrimination, and stigma. Higher prevalence of comorbid mental illness and higher rates of intimate partner violence and interpersonal violence all contribute to DGBI severity and challenges with access to care.12,13 Patients with disabilities may struggle to arrive at appointments, maneuver through the building or exam room, and ultimately follow recommended care plans.
How to approach DGBIs in historically marginalized and minoritized communities
Returning to the patients from the introduction, how would you counsel each of them?
Patient 1: We can discuss with the patient how nortriptyline and other typical antidepressants can and often are used for indications other than depression. These medications modify centrally-mediated pain signaling and many patients with functional dyspepsia experience a significant benefit. It is critical to build on the rapport that was established at the community health outreach event and to explore the patient’s concerns thoroughly.
Patient 2: We would begin by inquiring about her underlying fears associated with her symptoms and seek to understand her goals for repeat intervention. We can review the risks of endoscopy and shift the focus to improving her symptoms. If we can improve her bowel habits or her pain, her desire for further interventions may lessen.
Patient 3: It will be important to work within the realistic time and monetary constraints in this patient’s life. We can validate him and the challenges he is facing, provide positive reinforcement for the progress he has made so far, and avoid disparaging him for the aspects of the treatment plan he has been unable to follow through with. As he reported a benefit from amitriptyline, we can consider increasing his dose as a feasible next step.
Patient 4: We can encourage the patient to discuss with his primary care physician how they may be able to coordinate an inpatient admission for colonoscopy preparation. Given his co-morbidities, this avenue will provide him dedicated support to help him adequately prep to ensure a higher quality examination and limit the need for repeat procedures.
DGBI care in historically marginalized and minoritized communities: A call to action
Understanding cultural differences and existing disparities in care is essential to improving care for patients from historically minoritized communities with DGBIs. Motivational interviewing and shared decision-making, with acknowledgment of social and cultural differences, allow us to work together with patients and their support systems to set and achieve feasible goals.14
To address known health disparities, offices can take steps to ensure the accessibility of language, forms, physical space, providers, and care teams. Providing culturally sensitive care and lowering barriers to care are the first steps to effecting meaningful change for patients with DGBIs from historically minoritized communities.
Dr. Yu is based at Division of Gastroenterology and Hepatology, Boston Medical Center and Boston University, both in Boston, Massachusetts. Dr. Dimino and Dr. Vélez are based at the Division of Gastroenterology, Massachusetts General Hospital and Harvard Medical School, both in Boston, Massachusetts. Dr. Yu, Dr. Dimino, and Dr. Vélez do not have any conflicts of interest for this article.
Additional Online Resources
Form Accessibility
- Intake Form Guidance for Providers
- Making Your Clinic Welcoming to LGBTQ Patients
- Transgender data collection in the electronic health record: Current concepts and issues
Language Accessibility
Physical Accessibility
- Access to Medical Care for Individuals with Mobility Disabilities
- Making your medical office accessible
References
1. Zavala VA, et al. Cancer health disparities in racial/ethnic minorities in the United States. Br J Cancer. 2021 Jan. doi: 10.1038/s41416-020-01038-6.
2. Kardashian A, et al. Health disparities in chronic liver disease. Hepatology. 2023 Apr. doi: 10.1002/hep.32743.
3. Nephew LD, Serper M. Racial, Gender, and Socioeconomic Disparities in Liver Transplantation. Liver Transpl. 2021 Jun. doi: 10.1002/lt.25996.
4. Anyane-Yeboa A, et al. The Impact of the Social Determinants of Health on Disparities in Inflammatory Bowel Disease. Clin Gastroenterol Hepatol. 2022 Nov. doi: 10.1016/j.cgh.2022.03.011.
5. Drossman DA. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features and Rome IV. Gastroenterology. 2016 Feb. doi: 10.1053/j.gastro.2016.02.032.
6. Silvernale C, et al. Racial disparity in healthcare utilization among patients with Irritable Bowel Syndrome: results from a multicenter cohort. Neurogastroenterol Motil. 2021 May. doi: 10.1111/nmo.14039.
7. Hearn M, et al. Stigma and irritable bowel syndrome: a taboo subject? Lancet Gastroenterol Hepatol. 2020 Jun. doi: 10.1016/S2468-1253(19)30348-6.
8. Yan XJ, et al. The impact of stigma on medication adherence in patients with functional dyspepsia. Neurogastroenterol Motil. 2021 Feb. doi: 10.1111/nmo.13956.
9. Twersky SE, et al. The Impact of Limited English Proficiency on Healthcare Access and Outcomes in the U.S.: A Scoping Review. Healthcare (Basel). 2024 Jan. doi: 10.3390/healthcare12030364.
10. Bayly JE, et al. Limited English proficiency and reported receipt of colorectal cancer screening among adults 45-75 in 2019 and 2021. Prev Med Rep. 2024 Feb. doi: 10.1016/j.pmedr.2024.102638.
11. Cheng K, et al. Epidemiology of Irritable Bowel Syndrome in a Large Academic Safety-Net Hospital. J Clin Med. 2024 Feb. doi: 10.3390/jcm13051314.
12. Breiding MJ, Armour BS. The association between disability and intimate partner violence in the United States. Ann Epidemiol. 2015 Jun. doi: 10.1016/j.annepidem.2015.03.017.
13. Mitra M, et al. Prevalence and characteristics of sexual violence against men with disabilities. Am J Prev Med. 2016 Mar. doi: 10.1016/j.amepre.2015.07.030.
14. Bahafzallah L, et al. Motivational Interviewing in Ethnic Populations. J Immigr Minor Health. 2020 Aug. doi: 10.1007/s10903-019-00940-3.
Introduction: Cases
Patient 1: A 57-year-old man with post-prandial distress variant functional dyspepsia (FD) was recommended to start nortriptyline. He previously established primary care with a physician he met at a barbershop health fair in Harlem, who referred him for specialty evaluation. Today, he presents for follow-up and reports he did not take this medication because he heard it is an antidepressant. How would you counsel him?
Patient 2: A 61-year-old woman was previously diagnosed with mixed variant irritable bowel syndrome (IBS-M). Her symptoms have not significantly changed. Her prior workup has been reassuring and consistent with IBS-M. Despite this, the patient pushes to repeat a colonoscopy, fearful that something is being missed or that she is not being offered care because of her undocumented status. How do you respond?
Patient 3: A 36-year-old man is followed for the management of generalized anxiety disorder and functional heartburn. He was started on low-dose amitriptyline with some benefit, but follow-up has been sporadic. On further discussion, he reports financial stressors, time barriers, and difficulty scheduling a meeting with his union representative for work accommodations as he lives in a more rural community. How do you reply?
Patient 4: A 74-year-old man with Parkinson’s disease who uses a wheelchair has functional constipation that is well controlled on his current regimen. He has never undergone colon cancer screening. He occasionally notices blood in his stool, so a colonoscopy was recommended to confirm that his hematochezia reflects functional constipation complicated by hemorrhoids. He is concerned about the bowel preparation required for a colonoscopy given his limited mobility, as his insurance does not cover assistance at home. He does not have family members to help him. How can you assist him?
Social determinants of health, health disparities, and DGBIs
Social determinants of health affect all aspects of patient care, with an increasing body of published work looking at potential disparities in organ-based and structural diseases.1,2,3,4 However, little has been done to explore their influence on disorders of gut-brain interaction or DGBIs.
. As DGBIs cannot be diagnosed with a single laboratory or endoscopic test, the patient history is of the utmost importance and physician-patient rapport is paramount in their treatment. Such rapport may be more difficult to establish in patients coming from historically marginalized and minoritized communities who may be distrustful of healthcare as an institution of (discriminatory) power.
Potential DGBI management pitfalls in historically marginalized or minoritized communities
For racial and ethnic minorities in the United States, disparities in healthcare take on many forms. People from racial and ethnic minority communities are less likely to receive a gastroenterology consultation and those with IBS are more likely to undergo procedures as compared to White patients with IBS.6 Implicit bias may lead to fewer specialist referrals, and specialty care may be limited or unavailable in some areas. Patients may prefer seeing providers in their own community, with whom they share racial or ethnic identities, which could lead to fewer referrals to specialists outside of the community.
Historical discrimination contributes to a lack of trust in healthcare professionals, which may lead patients to favor more objective diagnostics such as endoscopy or view being counseled against invasive procedures as having necessary care denied. Due to a broader cultural stigma surrounding mental illness, patients may be more hesitant to utilize neuromodulators, which have historically been used for psychiatric diagnoses, as it may lead them to conflate their GI illness with mental illness.7,8
Since DGBIs cannot be diagnosed with a single test or managed with a single treatment modality, providing excellent care for patients with DGBIs requires clear communication. For patients with limited English proficiency (LEP), access to high-quality language assistance is the foundation of comprehensive care. Interpreter use (or lack thereof) may limit the ability to obtain a complete and accurate clinical history, which can lead to fewer referrals to specialists and increased reliance on endoscopic evaluations that may not be clinically indicated.
These language barriers affect patients on many levels – in their ability to understand instructions for medication administration, preparation for procedures, and return precautions – which may ultimately lead to poorer responses to therapy or delays in care. LEP alone is broadly associated with fewer referrals for outpatient follow-up, adverse health outcomes and complications, and longer hospital stays.9 These disparities can be mitigated by investing in high-quality interpreter services, providing instructions and forms in multiple languages, and engaging the patient’s family and social supports according to their preferences.
People experiencing poverty (urban and rural) face challenges across multiple domains including access to healthcare, health insurance, stable housing and employment, and more. Many patients seek care at federally qualified health centers, which may face greater difficulties coordinating care with external gastroenterologists.10
Insurance barriers limit access to essential medications, tests, and procedures, and create delays in establishing care with specialists. Significant psychological stress and higher rates of comorbid anxiety and depression contribute to increased IBS severity.11 Financial limitations may limit dietary choices, which can further exacerbate DGBI symptoms. Long work hours with limited flexibility may prohibit them from presenting for regular follow-ups and establishing advanced DGBI care such as with a dietitian or psychologist.
Patients with disabilities face many of the health inequities previously discussed, as well as additional challenges with physical accessibility, transportation, exclusion from education and employment, discrimination, and stigma. Higher prevalence of comorbid mental illness and higher rates of intimate partner violence and interpersonal violence all contribute to DGBI severity and challenges with access to care.12,13 Patients with disabilities may struggle to arrive at appointments, maneuver through the building or exam room, and ultimately follow recommended care plans.
How to approach DGBIs in historically marginalized and minoritized communities
Returning to the patients from the introduction, how would you counsel each of them?
Patient 1: We can discuss with the patient how nortriptyline and other typical antidepressants can and often are used for indications other than depression. These medications modify centrally-mediated pain signaling and many patients with functional dyspepsia experience a significant benefit. It is critical to build on the rapport that was established at the community health outreach event and to explore the patient’s concerns thoroughly.
Patient 2: We would begin by inquiring about her underlying fears associated with her symptoms and seek to understand her goals for repeat intervention. We can review the risks of endoscopy and shift the focus to improving her symptoms. If we can improve her bowel habits or her pain, her desire for further interventions may lessen.
Patient 3: It will be important to work within the realistic time and monetary constraints in this patient’s life. We can validate him and the challenges he is facing, provide positive reinforcement for the progress he has made so far, and avoid disparaging him for the aspects of the treatment plan he has been unable to follow through with. As he reported a benefit from amitriptyline, we can consider increasing his dose as a feasible next step.
Patient 4: We can encourage the patient to discuss with his primary care physician how they may be able to coordinate an inpatient admission for colonoscopy preparation. Given his co-morbidities, this avenue will provide him dedicated support to help him adequately prep to ensure a higher quality examination and limit the need for repeat procedures.
DGBI care in historically marginalized and minoritized communities: A call to action
Understanding cultural differences and existing disparities in care is essential to improving care for patients from historically minoritized communities with DGBIs. Motivational interviewing and shared decision-making, with acknowledgment of social and cultural differences, allow us to work together with patients and their support systems to set and achieve feasible goals.14
To address known health disparities, offices can take steps to ensure the accessibility of language, forms, physical space, providers, and care teams. Providing culturally sensitive care and lowering barriers to care are the first steps to effecting meaningful change for patients with DGBIs from historically minoritized communities.
Dr. Yu is based at Division of Gastroenterology and Hepatology, Boston Medical Center and Boston University, both in Boston, Massachusetts. Dr. Dimino and Dr. Vélez are based at the Division of Gastroenterology, Massachusetts General Hospital and Harvard Medical School, both in Boston, Massachusetts. Dr. Yu, Dr. Dimino, and Dr. Vélez do not have any conflicts of interest for this article.
Additional Online Resources
Form Accessibility
- Intake Form Guidance for Providers
- Making Your Clinic Welcoming to LGBTQ Patients
- Transgender data collection in the electronic health record: Current concepts and issues
Language Accessibility
Physical Accessibility
- Access to Medical Care for Individuals with Mobility Disabilities
- Making your medical office accessible
References
1. Zavala VA, et al. Cancer health disparities in racial/ethnic minorities in the United States. Br J Cancer. 2021 Jan. doi: 10.1038/s41416-020-01038-6.
2. Kardashian A, et al. Health disparities in chronic liver disease. Hepatology. 2023 Apr. doi: 10.1002/hep.32743.
3. Nephew LD, Serper M. Racial, Gender, and Socioeconomic Disparities in Liver Transplantation. Liver Transpl. 2021 Jun. doi: 10.1002/lt.25996.
4. Anyane-Yeboa A, et al. The Impact of the Social Determinants of Health on Disparities in Inflammatory Bowel Disease. Clin Gastroenterol Hepatol. 2022 Nov. doi: 10.1016/j.cgh.2022.03.011.
5. Drossman DA. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features and Rome IV. Gastroenterology. 2016 Feb. doi: 10.1053/j.gastro.2016.02.032.
6. Silvernale C, et al. Racial disparity in healthcare utilization among patients with Irritable Bowel Syndrome: results from a multicenter cohort. Neurogastroenterol Motil. 2021 May. doi: 10.1111/nmo.14039.
7. Hearn M, et al. Stigma and irritable bowel syndrome: a taboo subject? Lancet Gastroenterol Hepatol. 2020 Jun. doi: 10.1016/S2468-1253(19)30348-6.
8. Yan XJ, et al. The impact of stigma on medication adherence in patients with functional dyspepsia. Neurogastroenterol Motil. 2021 Feb. doi: 10.1111/nmo.13956.
9. Twersky SE, et al. The Impact of Limited English Proficiency on Healthcare Access and Outcomes in the U.S.: A Scoping Review. Healthcare (Basel). 2024 Jan. doi: 10.3390/healthcare12030364.
10. Bayly JE, et al. Limited English proficiency and reported receipt of colorectal cancer screening among adults 45-75 in 2019 and 2021. Prev Med Rep. 2024 Feb. doi: 10.1016/j.pmedr.2024.102638.
11. Cheng K, et al. Epidemiology of Irritable Bowel Syndrome in a Large Academic Safety-Net Hospital. J Clin Med. 2024 Feb. doi: 10.3390/jcm13051314.
12. Breiding MJ, Armour BS. The association between disability and intimate partner violence in the United States. Ann Epidemiol. 2015 Jun. doi: 10.1016/j.annepidem.2015.03.017.
13. Mitra M, et al. Prevalence and characteristics of sexual violence against men with disabilities. Am J Prev Med. 2016 Mar. doi: 10.1016/j.amepre.2015.07.030.
14. Bahafzallah L, et al. Motivational Interviewing in Ethnic Populations. J Immigr Minor Health. 2020 Aug. doi: 10.1007/s10903-019-00940-3.
Introduction: Cases
Patient 1: A 57-year-old man with post-prandial distress variant functional dyspepsia (FD) was recommended to start nortriptyline. He previously established primary care with a physician he met at a barbershop health fair in Harlem, who referred him for specialty evaluation. Today, he presents for follow-up and reports he did not take this medication because he heard it is an antidepressant. How would you counsel him?
Patient 2: A 61-year-old woman was previously diagnosed with mixed variant irritable bowel syndrome (IBS-M). Her symptoms have not significantly changed. Her prior workup has been reassuring and consistent with IBS-M. Despite this, the patient pushes to repeat a colonoscopy, fearful that something is being missed or that she is not being offered care because of her undocumented status. How do you respond?
Patient 3: A 36-year-old man is followed for the management of generalized anxiety disorder and functional heartburn. He was started on low-dose amitriptyline with some benefit, but follow-up has been sporadic. On further discussion, he reports financial stressors, time barriers, and difficulty scheduling a meeting with his union representative for work accommodations as he lives in a more rural community. How do you reply?
Patient 4: A 74-year-old man with Parkinson’s disease who uses a wheelchair has functional constipation that is well controlled on his current regimen. He has never undergone colon cancer screening. He occasionally notices blood in his stool, so a colonoscopy was recommended to confirm that his hematochezia reflects functional constipation complicated by hemorrhoids. He is concerned about the bowel preparation required for a colonoscopy given his limited mobility, as his insurance does not cover assistance at home. He does not have family members to help him. How can you assist him?
Social determinants of health, health disparities, and DGBIs
Social determinants of health affect all aspects of patient care, with an increasing body of published work looking at potential disparities in organ-based and structural diseases.1,2,3,4 However, little has been done to explore their influence on disorders of gut-brain interaction or DGBIs.
. As DGBIs cannot be diagnosed with a single laboratory or endoscopic test, the patient history is of the utmost importance and physician-patient rapport is paramount in their treatment. Such rapport may be more difficult to establish in patients coming from historically marginalized and minoritized communities who may be distrustful of healthcare as an institution of (discriminatory) power.
Potential DGBI management pitfalls in historically marginalized or minoritized communities
For racial and ethnic minorities in the United States, disparities in healthcare take on many forms. People from racial and ethnic minority communities are less likely to receive a gastroenterology consultation and those with IBS are more likely to undergo procedures as compared to White patients with IBS.6 Implicit bias may lead to fewer specialist referrals, and specialty care may be limited or unavailable in some areas. Patients may prefer seeing providers in their own community, with whom they share racial or ethnic identities, which could lead to fewer referrals to specialists outside of the community.
Historical discrimination contributes to a lack of trust in healthcare professionals, which may lead patients to favor more objective diagnostics such as endoscopy or view being counseled against invasive procedures as having necessary care denied. Due to a broader cultural stigma surrounding mental illness, patients may be more hesitant to utilize neuromodulators, which have historically been used for psychiatric diagnoses, as it may lead them to conflate their GI illness with mental illness.7,8
Since DGBIs cannot be diagnosed with a single test or managed with a single treatment modality, providing excellent care for patients with DGBIs requires clear communication. For patients with limited English proficiency (LEP), access to high-quality language assistance is the foundation of comprehensive care. Interpreter use (or lack thereof) may limit the ability to obtain a complete and accurate clinical history, which can lead to fewer referrals to specialists and increased reliance on endoscopic evaluations that may not be clinically indicated.
These language barriers affect patients on many levels – in their ability to understand instructions for medication administration, preparation for procedures, and return precautions – which may ultimately lead to poorer responses to therapy or delays in care. LEP alone is broadly associated with fewer referrals for outpatient follow-up, adverse health outcomes and complications, and longer hospital stays.9 These disparities can be mitigated by investing in high-quality interpreter services, providing instructions and forms in multiple languages, and engaging the patient’s family and social supports according to their preferences.
People experiencing poverty (urban and rural) face challenges across multiple domains including access to healthcare, health insurance, stable housing and employment, and more. Many patients seek care at federally qualified health centers, which may face greater difficulties coordinating care with external gastroenterologists.10
Insurance barriers limit access to essential medications, tests, and procedures, and create delays in establishing care with specialists. Significant psychological stress and higher rates of comorbid anxiety and depression contribute to increased IBS severity.11 Financial limitations may limit dietary choices, which can further exacerbate DGBI symptoms. Long work hours with limited flexibility may prohibit them from presenting for regular follow-ups and establishing advanced DGBI care such as with a dietitian or psychologist.
Patients with disabilities face many of the health inequities previously discussed, as well as additional challenges with physical accessibility, transportation, exclusion from education and employment, discrimination, and stigma. Higher prevalence of comorbid mental illness and higher rates of intimate partner violence and interpersonal violence all contribute to DGBI severity and challenges with access to care.12,13 Patients with disabilities may struggle to arrive at appointments, maneuver through the building or exam room, and ultimately follow recommended care plans.
How to approach DGBIs in historically marginalized and minoritized communities
Returning to the patients from the introduction, how would you counsel each of them?
Patient 1: We can discuss with the patient how nortriptyline and other typical antidepressants can and often are used for indications other than depression. These medications modify centrally-mediated pain signaling and many patients with functional dyspepsia experience a significant benefit. It is critical to build on the rapport that was established at the community health outreach event and to explore the patient’s concerns thoroughly.
Patient 2: We would begin by inquiring about her underlying fears associated with her symptoms and seek to understand her goals for repeat intervention. We can review the risks of endoscopy and shift the focus to improving her symptoms. If we can improve her bowel habits or her pain, her desire for further interventions may lessen.
Patient 3: It will be important to work within the realistic time and monetary constraints in this patient’s life. We can validate him and the challenges he is facing, provide positive reinforcement for the progress he has made so far, and avoid disparaging him for the aspects of the treatment plan he has been unable to follow through with. As he reported a benefit from amitriptyline, we can consider increasing his dose as a feasible next step.
Patient 4: We can encourage the patient to discuss with his primary care physician how they may be able to coordinate an inpatient admission for colonoscopy preparation. Given his co-morbidities, this avenue will provide him dedicated support to help him adequately prep to ensure a higher quality examination and limit the need for repeat procedures.
DGBI care in historically marginalized and minoritized communities: A call to action
Understanding cultural differences and existing disparities in care is essential to improving care for patients from historically minoritized communities with DGBIs. Motivational interviewing and shared decision-making, with acknowledgment of social and cultural differences, allow us to work together with patients and their support systems to set and achieve feasible goals.14
To address known health disparities, offices can take steps to ensure the accessibility of language, forms, physical space, providers, and care teams. Providing culturally sensitive care and lowering barriers to care are the first steps to effecting meaningful change for patients with DGBIs from historically minoritized communities.
Dr. Yu is based at Division of Gastroenterology and Hepatology, Boston Medical Center and Boston University, both in Boston, Massachusetts. Dr. Dimino and Dr. Vélez are based at the Division of Gastroenterology, Massachusetts General Hospital and Harvard Medical School, both in Boston, Massachusetts. Dr. Yu, Dr. Dimino, and Dr. Vélez do not have any conflicts of interest for this article.
Additional Online Resources
Form Accessibility
- Intake Form Guidance for Providers
- Making Your Clinic Welcoming to LGBTQ Patients
- Transgender data collection in the electronic health record: Current concepts and issues
Language Accessibility
Physical Accessibility
- Access to Medical Care for Individuals with Mobility Disabilities
- Making your medical office accessible
References
1. Zavala VA, et al. Cancer health disparities in racial/ethnic minorities in the United States. Br J Cancer. 2021 Jan. doi: 10.1038/s41416-020-01038-6.
2. Kardashian A, et al. Health disparities in chronic liver disease. Hepatology. 2023 Apr. doi: 10.1002/hep.32743.
3. Nephew LD, Serper M. Racial, Gender, and Socioeconomic Disparities in Liver Transplantation. Liver Transpl. 2021 Jun. doi: 10.1002/lt.25996.
4. Anyane-Yeboa A, et al. The Impact of the Social Determinants of Health on Disparities in Inflammatory Bowel Disease. Clin Gastroenterol Hepatol. 2022 Nov. doi: 10.1016/j.cgh.2022.03.011.
5. Drossman DA. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features and Rome IV. Gastroenterology. 2016 Feb. doi: 10.1053/j.gastro.2016.02.032.
6. Silvernale C, et al. Racial disparity in healthcare utilization among patients with Irritable Bowel Syndrome: results from a multicenter cohort. Neurogastroenterol Motil. 2021 May. doi: 10.1111/nmo.14039.
7. Hearn M, et al. Stigma and irritable bowel syndrome: a taboo subject? Lancet Gastroenterol Hepatol. 2020 Jun. doi: 10.1016/S2468-1253(19)30348-6.
8. Yan XJ, et al. The impact of stigma on medication adherence in patients with functional dyspepsia. Neurogastroenterol Motil. 2021 Feb. doi: 10.1111/nmo.13956.
9. Twersky SE, et al. The Impact of Limited English Proficiency on Healthcare Access and Outcomes in the U.S.: A Scoping Review. Healthcare (Basel). 2024 Jan. doi: 10.3390/healthcare12030364.
10. Bayly JE, et al. Limited English proficiency and reported receipt of colorectal cancer screening among adults 45-75 in 2019 and 2021. Prev Med Rep. 2024 Feb. doi: 10.1016/j.pmedr.2024.102638.
11. Cheng K, et al. Epidemiology of Irritable Bowel Syndrome in a Large Academic Safety-Net Hospital. J Clin Med. 2024 Feb. doi: 10.3390/jcm13051314.
12. Breiding MJ, Armour BS. The association between disability and intimate partner violence in the United States. Ann Epidemiol. 2015 Jun. doi: 10.1016/j.annepidem.2015.03.017.
13. Mitra M, et al. Prevalence and characteristics of sexual violence against men with disabilities. Am J Prev Med. 2016 Mar. doi: 10.1016/j.amepre.2015.07.030.
14. Bahafzallah L, et al. Motivational Interviewing in Ethnic Populations. J Immigr Minor Health. 2020 Aug. doi: 10.1007/s10903-019-00940-3.
Improving Care for Sexual and Gender Minority Patients with Disorders of Gut-Brain Interaction
Brief Introduction to the SGM Communities
The sexual and gender minority (SGM) communities (see Table 1), also termed “LGBTQIA+ community” (lesbian, gay, bisexual, transgender, queer, intersex, asexual, plus — including two spirit) are historically minoritized with unique risks for inequities in gastrointestinal health outcomes.1 These potential disparities remain largely uninvestigated because of continued systemic discrimination and inadequate collection of sexual orientation and gender identity (SOGI) data,2 with the National Institutes of Health Sexual & Gender Minority Research Office (SGMRO) having been instructed to address these failures. There is increased SGM self-identification (7.1% of all people in the United States and 20.8% of generation Z).3 Given the high worldwide prevalence of disorders of gut-brain interaction (DGBIs)and the influence of biopsychosocial determinants of health in DGBI incidence,4 it becomes increasingly likely that research in DGBI-related factors in SGM people will be fruitful.
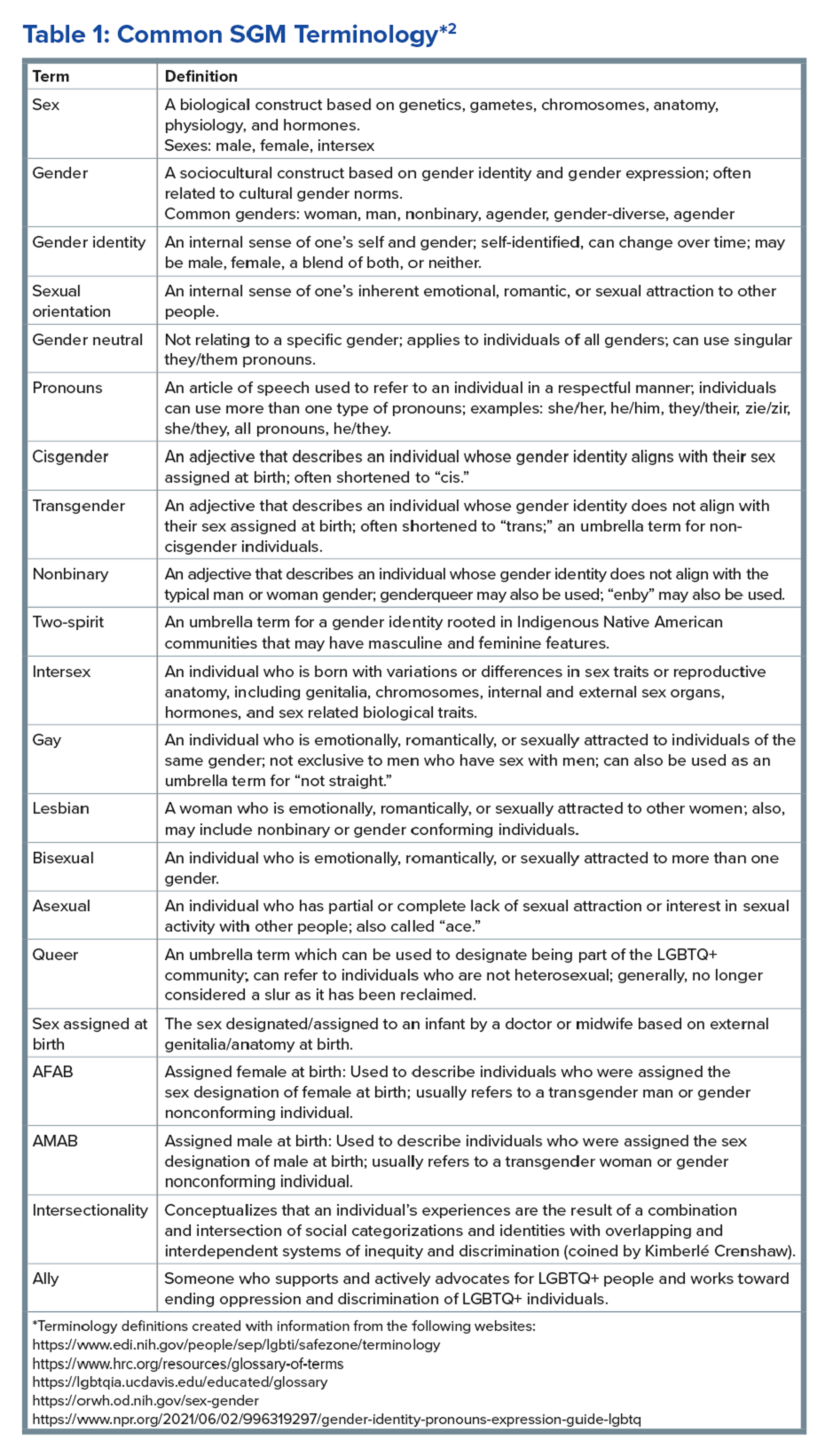
Disorders of Gut-Brain Interaction and the Potential Minority Stress Link in SGM People
DGBIs are gastrointestinal conditions that occur because of brain-gut axis dysregulation. There is evidence that chronic stress and trauma negatively influence brain-gut interaction, which likely results in minority communities who face increased levels of trauma, stress, discrimination, and social injustice being at higher risk of DGBI development.5-7 Given increased rates of trauma in the SGM community, practicing trauma-informed care is essential to increase patient comfort and decrease the chance of retraumatization in medical settings.8 Trauma-informed care focuses on how trauma influences a patient’s life and response to medical care. To practice trauma-informed care, screening for trauma when appropriate, actively creating a supportive environment with active listening and communication, with informing the patient of planned actions prior to doing them, like physical exams, is key.
Trauma-Informed Care: Examples of Verbiage
Asking about Identity
- Begin by introducing yourself with your pronouns to create a safe environment for patient disclosure. Example: “Hello, I am Dr. Kara Jencks, and my pronouns are she/her. I am one of the gastroenterologists here at XYZ Clinic. How would you prefer to be addressed?”
- You can also wear a pronoun lapel pin or a pronoun button on your ID badge to indicate you are someone who your patient can be themselves around.
- The easiest way to obtain sexual orientation and gender identity is through intake forms. Below are examples of how to ask these questions on intake forms. It is important to offer the option to select more than one option when applicable and to opt out of answering if the patient is not comfortable answering these questions.
Sample Questions for Intake Forms
1. What is your sex assigned at birth? (Select one)
- Female
- Male
- Intersex
- Do not know
- Prefer not to disclose
2. What is your gender identity? (Select all that apply)
- Nonbinary
- Gender queer
- Woman
- Man
- Transwoman
- Transman
- Gender fluid
- Two-spirit
- Agender
- Intersex
- Other: type in response
- Prefer not to disclose
3. What are your pronouns? (Select all that apply)
- They/them/theirs
- She/her/hers
- He/him/his
- Zie/zir/zirs
- Other: type in response
- Prefer not to disclose
4. What is your sexual orientation? (Select all that apply)
- Bisexual
- Pansexual
- Queer
- Lesbian
- Gay
- Asexual
- Demisexual
- Heterosexual or straight
- Other: type in response
- Prefer not to disclose
Screening for Trauma
While there are questionnaires that exist to ask about trauma history, if time allows, it can be helpful to screen verbally with the patient. See reference number 8, for additional prompts and actions to practice trauma-informed care.
- Example: “Many patients with gastrointestinal symptoms and disorders have experienced trauma in the past. We do our best to ensure we are keeping you as comfortable as possible while caring for you. Are you comfortable sharing this information? [if yes->] Do you have a history of trauma, including physical, emotional, or sexual abuse? ... Have these experiences impacted the way in which you navigate your healthcare? ... Is there anything we can do to make you more comfortable today?”
General Physical Examination
Provide details for what you are going to do before you do it. Ask for permission for the examination. Here are two examples:
- “I would like to perform a physical exam to help better understand your symptoms. Is that okay with you?”
- “I would like to examine your abdomen with my stethoscope and my hands. Here is a sheet that we can use to help with your privacy. Please let me know if and when you feel any tenderness or pain.”
Rectal Physical Examination
Let the patient know why it would be helpful to perform a rectal exam, what the rectal exam will entail, and the benefits and risks to doing a rectal exam. An example follows:
- “Based on the symptoms you are describing, I think it would be helpful to perform a rectal exam to make sure you don’t have any fissures or hemorrhoids on the outside around the anus, any blockages or major issues inside the rectum, and to assess the strength and ability of your nerves and muscles or the pelvic floor to coordinate bowel movements. There are no risks aside from discomfort. If it is painful, and you would like me to stop, you tell me to stop, and I will stop right away. What questions do you have? Are we okay to proceed with the rectal exam?”
- “Please pull down your undergarments and your pants to either midthigh, your ankles, or all the way off, whatever your preference is, lie down on the left side on the exam table, and cover yourself with this sheet. In the meantime, I will be getting a chaperone to keep us safe and serve as a patient advocate during the procedure.”
- Upon returning to the exam room: “Here is Sara, who will be chaperoning today. Let myself or Sara know if you are uncomfortable or having pain during this exam. I will be lifting up the sheet to get a good look around the anus. [lifts up sheet] You will feel my hand helping to spread apart the buttocks. I am looking around the anus, and I do not see any fissures, hemorrhoids, or anything else concerning. Please squeeze in like you are trying to hold in gas. Please bear down like you are trying to have a bowel movement or let out gas. Okay, now you may feel some cold gel around the anus, and you will feel my finger go inside. Take a deep breath in. Do you feel any pain as I palpate? Please squeeze in like you are trying to hold in gas. Please bear down like you are trying to have a bowel movement or let out gas. I will be stopping the exam now.”
- You would then wash your hands and allow the patient to get dressed, and then disclose the exam findings and the rest of your visit.
Ilan H. Meyer coined the minority stress model when discussing mental health disorders in SGM patients in the early 2000s.9 With it being well known that DGBIs can overlap with (but are not necessarily caused by) mental health disorders, this model can easily apply to unify multiple individual and societal factors that can combine to result in disorders of brain-gut interaction (see Figure 1) in SGM communities. Let us keep this framework in mind when evaluating the following cases.
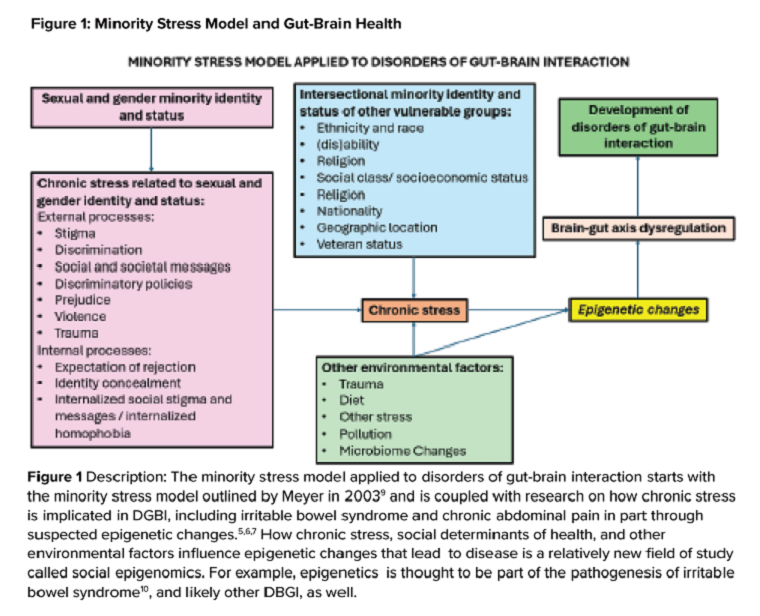
Case Presentations
Case 1
A 56-year-old man (pronouns: he/him) assigned male sex at birth, who identifies as gay, presents to your gastroenterology clinic for treatment-refractory constipation-predominant irritable bowel syndrome. It has impacted his sexual function. Outside hospital records report a normal colonoscopy 1 year ago and an unremarkable abdominal computerized tomography 4 months ago, aside from increased stool burden in the entire colon. He has tried to use enemas prior to sex, though these do not always help. Fiber-rich diet and fermentable food avoidance has not been successful. He is currently taking two capfuls of polyethylene glycol 3350 twice per day, as well as senna at night and continues to have a bowel movement every 2-3 days that is Bristol stool form scale type 1-2 unless he uses enemas. How do you counsel this patient about his IBS-C and rectal discomfort?
After assessing for sexual violence or other potential trauma-related factors, your digital rectal examination suggests that an anorectal defecatory disorder is less likely with normal relaxation and perineal movement. You recommend linaclotide. He notices improvement within 1 week, with improved comfort during anoreceptive sex.
Case 2
A 30-year-old woman (pronouns: she/her) assigned male sex at birth who has sex with men underwent vaginoplasty 2 years ago and is referred to the gastroenterology clinic for fecal incontinence and diarrhea. On review of her anatomic inventory, her vaginoplasty was a penile inversion vaginoplasty (no intestinal tissue was used for creation), and her prostate was left intact. The vaginal vault was created in between the urethra and rectum, similar to the pelvic floor anatomy of a woman assigned female sex at birth. Blood, imaging, and endoscopic workup has been negative. She is also not taking any medications associated with diarrhea, only taking estrogen and spironolactone. The diarrhea is not daily, but when present, about once per week, can be up to 10 episodes per day, and she has a sense of incomplete evacuation regularly. She notes having a rectal exam in the past but is not sure if her pelvic floor muscles have ever been assessed. How do you manage this patient?
To complete her evaluation in the office, you perform a trauma-informed rectal exam which reveals a decreased resting anal sphincter tone and paradoxical defecatory maneuvers without tenderness to the puborectalis muscle. Augmentation of the squeeze is also weak. Given her pelvic floor related surgical history, her symptoms, and her rectal exam, you recommend anorectal manometry which is abnormal and send her for anorectal biofeedback pelvic floor physical therapy, which improves her symptoms significantly.
Case 3
A 36-year-old woman (pronouns: she/her) assigned female sex at birth, who identifies as a lesbian, has a history of posttraumatic stress disorder and chronic nausea and vomiting that has begun to affect her quality of life. She notes the nausea and vomiting used to be managed well with evening cannabis gummies, though in the past 3 months, the nausea and vomiting has worsened, and she has lost 20 pounds as a result. As symptom predated cannabis usage, cannabis hyperemesis syndrome (CHS) was less likely (an important point as she has been stigmatized during prior encounters for her cannabis usage). Her primary care physician recommended a gastroscopy which was normal, aside from some residual solid food material in the stomach. Her bowel movements are normal, and she doesn’t have other gastrointestinal symptoms. She and her wife are considering having a third child, so she is worried about medications that may affect pregnancy or breast-feeding. How do you manage her nausea and vomiting?
After validating her concerns and performing a trauma-informed physical exam and encounter, you recommend a 4-hour gastric emptying test with a standard radiolabeled egg meal. Her gastric emptying does reveal significantly delayed gastric emptying at 2 and 4 hours. You discuss the risks and benefits of lifestyle modification (smaller frequent meals), initiating medications (erythromycin and metoclopramide) or cessation of cannabis (despite low likelihood of CHS). Desiring to avoid starting medications around initiation of pregnancy, she opts for the dietary approach and cessation of cannabis. You see her at a follow-up visit in 6 months, and her nausea is now only once a month, and she is excited to begin planning for a pregnancy using assisted reproductive technology.
Case 4
A 20-year-old nonbinary intersex individual (pronouns: he/they) (incorrectly assigned female at birth — is intersex with congenital adrenal hyperplasia) presents to the gastroenterology clinic with 8 years of heartburn, acid reflux, postprandial bloating, alternating diarrhea and constipation, nausea, and vomiting, complicated by avoidant restrictive food intake disorder. They have a history of bipolar II disorder with prior suicidal ideation. He has not yet had diagnostic workup as he previously had a bad encounter with a gastroenterologist where the gastroenterologist blamed his symptoms on his gender-affirming therapy, misgendered the patient, and told the patient their symptoms were “all in her [sic] head.”
You recognize that affirming their gender and using proper pronouns is the best first way to start rapport and help break the cycle of medicalized trauma. You then recommend a holistic work up with interdisciplinary management because of the complexity of his symptoms. For testing, you recommend a colonoscopy, upper endoscopy, a gastric emptying test with a 48-hour transit scintigraphy test, anorectal manometry, a dietitian referral, and a gastrointestinal psychology referral. Their anorectal manometry is consistent with an evacuation disorder. The rest of the work up is unremarkable. You diagnose them with anorectal pelvic floor dysfunction and functional dyspepsia, recommending biofeedback pelvic floor physical therapy, a proton-pump inhibitor, and neuromodulation in coordination with psychiatry and psychology to start with a plan for follow-up. The patient appreciates you for helping them and listening to their symptoms.
Discussion
When approaching DGBIs in the SGM community, it is vital to validate their concerns and be inclusive with diagnostic and treatment modalities. The diagnostic tools and treatments for DGBI are not different for patients in the SGM community. Like with other patients, trauma-informed care should be utilized, particularly given higher rates of trauma and discrimination in this community. Importantly, their DGBI is not a result of their sexual orientation or gender identity, and hormone therapy is not the cause of their DGBI. Recommending cessation of gender-affirming care or recommending lifestyle measures against their identity is generally not appropriate or necessary. among members of the SGM communities.
Dr. Jencks (@karajencks) is based in the division of gastroenterology and hepatology, Mayo Clinic, Rochester, Minnesota. Dr. Vélez (@Chris_Velez_MD) is based in the division of gastroenterology, Massachusetts General Hospital and Harvard Medical School, both in Boston. Both authors do not have any conflicts of interest for this article.
References
1. Duong N et al. 2023 Apr. doi: 10.1016/S2468-1253(23)00005-5.
2. Vélez C et al. Am J Gastroenterol. 2022 Jun. doi: 10.14309/ajg.0000000000001804.
3. Jones JM. Gallup. LGBTQ+ identification in U.S. now at 7.6%. 2024 Mar 13. https://news.gallup.com/poll/611864/lgbtq-identification.aspx
4. Sperber AD et al. Gastroenterology. 2021 Jan. doi: 10.1053/j.gastro.2020.04.014.
5. Wiley JW et al. Neurogastroenterol Motil. 2016 Jan. doi: 10.1111/nmo.12706.
6. Labanski A et al. Psychoneuroendocrinology. 2020 Jan. doi: 10.1016/j.psyneuen.2019.104501.
7. Khlevner J et al. Gastroenterol Clin North Am. 2018 Dec. doi: 10.1016/j.gtc.2018.07.002.
8. Jagielski CH and Harer KN. Gastroenterol Clin North Am. 2022 Dec. doi: 10.1016/j.gtc.2022.07.012.
9. Meyer IH. Psychol Bull. 2003 Sep. doi: 10.1037/0033-2909.129.5.674.
10. Mahurkar-Joshi S and Chang L. Front Psychiatry. 2020 Aug. doi: 10.3389/fpsyt.2020.00805.
Brief Introduction to the SGM Communities
The sexual and gender minority (SGM) communities (see Table 1), also termed “LGBTQIA+ community” (lesbian, gay, bisexual, transgender, queer, intersex, asexual, plus — including two spirit) are historically minoritized with unique risks for inequities in gastrointestinal health outcomes.1 These potential disparities remain largely uninvestigated because of continued systemic discrimination and inadequate collection of sexual orientation and gender identity (SOGI) data,2 with the National Institutes of Health Sexual & Gender Minority Research Office (SGMRO) having been instructed to address these failures. There is increased SGM self-identification (7.1% of all people in the United States and 20.8% of generation Z).3 Given the high worldwide prevalence of disorders of gut-brain interaction (DGBIs)and the influence of biopsychosocial determinants of health in DGBI incidence,4 it becomes increasingly likely that research in DGBI-related factors in SGM people will be fruitful.

Disorders of Gut-Brain Interaction and the Potential Minority Stress Link in SGM People
DGBIs are gastrointestinal conditions that occur because of brain-gut axis dysregulation. There is evidence that chronic stress and trauma negatively influence brain-gut interaction, which likely results in minority communities who face increased levels of trauma, stress, discrimination, and social injustice being at higher risk of DGBI development.5-7 Given increased rates of trauma in the SGM community, practicing trauma-informed care is essential to increase patient comfort and decrease the chance of retraumatization in medical settings.8 Trauma-informed care focuses on how trauma influences a patient’s life and response to medical care. To practice trauma-informed care, screening for trauma when appropriate, actively creating a supportive environment with active listening and communication, with informing the patient of planned actions prior to doing them, like physical exams, is key.
Trauma-Informed Care: Examples of Verbiage
Asking about Identity
- Begin by introducing yourself with your pronouns to create a safe environment for patient disclosure. Example: “Hello, I am Dr. Kara Jencks, and my pronouns are she/her. I am one of the gastroenterologists here at XYZ Clinic. How would you prefer to be addressed?”
- You can also wear a pronoun lapel pin or a pronoun button on your ID badge to indicate you are someone who your patient can be themselves around.
- The easiest way to obtain sexual orientation and gender identity is through intake forms. Below are examples of how to ask these questions on intake forms. It is important to offer the option to select more than one option when applicable and to opt out of answering if the patient is not comfortable answering these questions.
Sample Questions for Intake Forms
1. What is your sex assigned at birth? (Select one)
- Female
- Male
- Intersex
- Do not know
- Prefer not to disclose
2. What is your gender identity? (Select all that apply)
- Nonbinary
- Gender queer
- Woman
- Man
- Transwoman
- Transman
- Gender fluid
- Two-spirit
- Agender
- Intersex
- Other: type in response
- Prefer not to disclose
3. What are your pronouns? (Select all that apply)
- They/them/theirs
- She/her/hers
- He/him/his
- Zie/zir/zirs
- Other: type in response
- Prefer not to disclose
4. What is your sexual orientation? (Select all that apply)
- Bisexual
- Pansexual
- Queer
- Lesbian
- Gay
- Asexual
- Demisexual
- Heterosexual or straight
- Other: type in response
- Prefer not to disclose
Screening for Trauma
While there are questionnaires that exist to ask about trauma history, if time allows, it can be helpful to screen verbally with the patient. See reference number 8, for additional prompts and actions to practice trauma-informed care.
- Example: “Many patients with gastrointestinal symptoms and disorders have experienced trauma in the past. We do our best to ensure we are keeping you as comfortable as possible while caring for you. Are you comfortable sharing this information? [if yes->] Do you have a history of trauma, including physical, emotional, or sexual abuse? ... Have these experiences impacted the way in which you navigate your healthcare? ... Is there anything we can do to make you more comfortable today?”
General Physical Examination
Provide details for what you are going to do before you do it. Ask for permission for the examination. Here are two examples:
- “I would like to perform a physical exam to help better understand your symptoms. Is that okay with you?”
- “I would like to examine your abdomen with my stethoscope and my hands. Here is a sheet that we can use to help with your privacy. Please let me know if and when you feel any tenderness or pain.”
Rectal Physical Examination
Let the patient know why it would be helpful to perform a rectal exam, what the rectal exam will entail, and the benefits and risks to doing a rectal exam. An example follows:
- “Based on the symptoms you are describing, I think it would be helpful to perform a rectal exam to make sure you don’t have any fissures or hemorrhoids on the outside around the anus, any blockages or major issues inside the rectum, and to assess the strength and ability of your nerves and muscles or the pelvic floor to coordinate bowel movements. There are no risks aside from discomfort. If it is painful, and you would like me to stop, you tell me to stop, and I will stop right away. What questions do you have? Are we okay to proceed with the rectal exam?”
- “Please pull down your undergarments and your pants to either midthigh, your ankles, or all the way off, whatever your preference is, lie down on the left side on the exam table, and cover yourself with this sheet. In the meantime, I will be getting a chaperone to keep us safe and serve as a patient advocate during the procedure.”
- Upon returning to the exam room: “Here is Sara, who will be chaperoning today. Let myself or Sara know if you are uncomfortable or having pain during this exam. I will be lifting up the sheet to get a good look around the anus. [lifts up sheet] You will feel my hand helping to spread apart the buttocks. I am looking around the anus, and I do not see any fissures, hemorrhoids, or anything else concerning. Please squeeze in like you are trying to hold in gas. Please bear down like you are trying to have a bowel movement or let out gas. Okay, now you may feel some cold gel around the anus, and you will feel my finger go inside. Take a deep breath in. Do you feel any pain as I palpate? Please squeeze in like you are trying to hold in gas. Please bear down like you are trying to have a bowel movement or let out gas. I will be stopping the exam now.”
- You would then wash your hands and allow the patient to get dressed, and then disclose the exam findings and the rest of your visit.
Ilan H. Meyer coined the minority stress model when discussing mental health disorders in SGM patients in the early 2000s.9 With it being well known that DGBIs can overlap with (but are not necessarily caused by) mental health disorders, this model can easily apply to unify multiple individual and societal factors that can combine to result in disorders of brain-gut interaction (see Figure 1) in SGM communities. Let us keep this framework in mind when evaluating the following cases.

Case Presentations
Case 1
A 56-year-old man (pronouns: he/him) assigned male sex at birth, who identifies as gay, presents to your gastroenterology clinic for treatment-refractory constipation-predominant irritable bowel syndrome. It has impacted his sexual function. Outside hospital records report a normal colonoscopy 1 year ago and an unremarkable abdominal computerized tomography 4 months ago, aside from increased stool burden in the entire colon. He has tried to use enemas prior to sex, though these do not always help. Fiber-rich diet and fermentable food avoidance has not been successful. He is currently taking two capfuls of polyethylene glycol 3350 twice per day, as well as senna at night and continues to have a bowel movement every 2-3 days that is Bristol stool form scale type 1-2 unless he uses enemas. How do you counsel this patient about his IBS-C and rectal discomfort?
After assessing for sexual violence or other potential trauma-related factors, your digital rectal examination suggests that an anorectal defecatory disorder is less likely with normal relaxation and perineal movement. You recommend linaclotide. He notices improvement within 1 week, with improved comfort during anoreceptive sex.
Case 2
A 30-year-old woman (pronouns: she/her) assigned male sex at birth who has sex with men underwent vaginoplasty 2 years ago and is referred to the gastroenterology clinic for fecal incontinence and diarrhea. On review of her anatomic inventory, her vaginoplasty was a penile inversion vaginoplasty (no intestinal tissue was used for creation), and her prostate was left intact. The vaginal vault was created in between the urethra and rectum, similar to the pelvic floor anatomy of a woman assigned female sex at birth. Blood, imaging, and endoscopic workup has been negative. She is also not taking any medications associated with diarrhea, only taking estrogen and spironolactone. The diarrhea is not daily, but when present, about once per week, can be up to 10 episodes per day, and she has a sense of incomplete evacuation regularly. She notes having a rectal exam in the past but is not sure if her pelvic floor muscles have ever been assessed. How do you manage this patient?
To complete her evaluation in the office, you perform a trauma-informed rectal exam which reveals a decreased resting anal sphincter tone and paradoxical defecatory maneuvers without tenderness to the puborectalis muscle. Augmentation of the squeeze is also weak. Given her pelvic floor related surgical history, her symptoms, and her rectal exam, you recommend anorectal manometry which is abnormal and send her for anorectal biofeedback pelvic floor physical therapy, which improves her symptoms significantly.
Case 3
A 36-year-old woman (pronouns: she/her) assigned female sex at birth, who identifies as a lesbian, has a history of posttraumatic stress disorder and chronic nausea and vomiting that has begun to affect her quality of life. She notes the nausea and vomiting used to be managed well with evening cannabis gummies, though in the past 3 months, the nausea and vomiting has worsened, and she has lost 20 pounds as a result. As symptom predated cannabis usage, cannabis hyperemesis syndrome (CHS) was less likely (an important point as she has been stigmatized during prior encounters for her cannabis usage). Her primary care physician recommended a gastroscopy which was normal, aside from some residual solid food material in the stomach. Her bowel movements are normal, and she doesn’t have other gastrointestinal symptoms. She and her wife are considering having a third child, so she is worried about medications that may affect pregnancy or breast-feeding. How do you manage her nausea and vomiting?
After validating her concerns and performing a trauma-informed physical exam and encounter, you recommend a 4-hour gastric emptying test with a standard radiolabeled egg meal. Her gastric emptying does reveal significantly delayed gastric emptying at 2 and 4 hours. You discuss the risks and benefits of lifestyle modification (smaller frequent meals), initiating medications (erythromycin and metoclopramide) or cessation of cannabis (despite low likelihood of CHS). Desiring to avoid starting medications around initiation of pregnancy, she opts for the dietary approach and cessation of cannabis. You see her at a follow-up visit in 6 months, and her nausea is now only once a month, and she is excited to begin planning for a pregnancy using assisted reproductive technology.
Case 4
A 20-year-old nonbinary intersex individual (pronouns: he/they) (incorrectly assigned female at birth — is intersex with congenital adrenal hyperplasia) presents to the gastroenterology clinic with 8 years of heartburn, acid reflux, postprandial bloating, alternating diarrhea and constipation, nausea, and vomiting, complicated by avoidant restrictive food intake disorder. They have a history of bipolar II disorder with prior suicidal ideation. He has not yet had diagnostic workup as he previously had a bad encounter with a gastroenterologist where the gastroenterologist blamed his symptoms on his gender-affirming therapy, misgendered the patient, and told the patient their symptoms were “all in her [sic] head.”
You recognize that affirming their gender and using proper pronouns is the best first way to start rapport and help break the cycle of medicalized trauma. You then recommend a holistic work up with interdisciplinary management because of the complexity of his symptoms. For testing, you recommend a colonoscopy, upper endoscopy, a gastric emptying test with a 48-hour transit scintigraphy test, anorectal manometry, a dietitian referral, and a gastrointestinal psychology referral. Their anorectal manometry is consistent with an evacuation disorder. The rest of the work up is unremarkable. You diagnose them with anorectal pelvic floor dysfunction and functional dyspepsia, recommending biofeedback pelvic floor physical therapy, a proton-pump inhibitor, and neuromodulation in coordination with psychiatry and psychology to start with a plan for follow-up. The patient appreciates you for helping them and listening to their symptoms.
Discussion
When approaching DGBIs in the SGM community, it is vital to validate their concerns and be inclusive with diagnostic and treatment modalities. The diagnostic tools and treatments for DGBI are not different for patients in the SGM community. Like with other patients, trauma-informed care should be utilized, particularly given higher rates of trauma and discrimination in this community. Importantly, their DGBI is not a result of their sexual orientation or gender identity, and hormone therapy is not the cause of their DGBI. Recommending cessation of gender-affirming care or recommending lifestyle measures against their identity is generally not appropriate or necessary. among members of the SGM communities.
Dr. Jencks (@karajencks) is based in the division of gastroenterology and hepatology, Mayo Clinic, Rochester, Minnesota. Dr. Vélez (@Chris_Velez_MD) is based in the division of gastroenterology, Massachusetts General Hospital and Harvard Medical School, both in Boston. Both authors do not have any conflicts of interest for this article.
References
1. Duong N et al. 2023 Apr. doi: 10.1016/S2468-1253(23)00005-5.
2. Vélez C et al. Am J Gastroenterol. 2022 Jun. doi: 10.14309/ajg.0000000000001804.
3. Jones JM. Gallup. LGBTQ+ identification in U.S. now at 7.6%. 2024 Mar 13. https://news.gallup.com/poll/611864/lgbtq-identification.aspx
4. Sperber AD et al. Gastroenterology. 2021 Jan. doi: 10.1053/j.gastro.2020.04.014.
5. Wiley JW et al. Neurogastroenterol Motil. 2016 Jan. doi: 10.1111/nmo.12706.
6. Labanski A et al. Psychoneuroendocrinology. 2020 Jan. doi: 10.1016/j.psyneuen.2019.104501.
7. Khlevner J et al. Gastroenterol Clin North Am. 2018 Dec. doi: 10.1016/j.gtc.2018.07.002.
8. Jagielski CH and Harer KN. Gastroenterol Clin North Am. 2022 Dec. doi: 10.1016/j.gtc.2022.07.012.
9. Meyer IH. Psychol Bull. 2003 Sep. doi: 10.1037/0033-2909.129.5.674.
10. Mahurkar-Joshi S and Chang L. Front Psychiatry. 2020 Aug. doi: 10.3389/fpsyt.2020.00805.
Brief Introduction to the SGM Communities
The sexual and gender minority (SGM) communities (see Table 1), also termed “LGBTQIA+ community” (lesbian, gay, bisexual, transgender, queer, intersex, asexual, plus — including two spirit) are historically minoritized with unique risks for inequities in gastrointestinal health outcomes.1 These potential disparities remain largely uninvestigated because of continued systemic discrimination and inadequate collection of sexual orientation and gender identity (SOGI) data,2 with the National Institutes of Health Sexual & Gender Minority Research Office (SGMRO) having been instructed to address these failures. There is increased SGM self-identification (7.1% of all people in the United States and 20.8% of generation Z).3 Given the high worldwide prevalence of disorders of gut-brain interaction (DGBIs)and the influence of biopsychosocial determinants of health in DGBI incidence,4 it becomes increasingly likely that research in DGBI-related factors in SGM people will be fruitful.

Disorders of Gut-Brain Interaction and the Potential Minority Stress Link in SGM People
DGBIs are gastrointestinal conditions that occur because of brain-gut axis dysregulation. There is evidence that chronic stress and trauma negatively influence brain-gut interaction, which likely results in minority communities who face increased levels of trauma, stress, discrimination, and social injustice being at higher risk of DGBI development.5-7 Given increased rates of trauma in the SGM community, practicing trauma-informed care is essential to increase patient comfort and decrease the chance of retraumatization in medical settings.8 Trauma-informed care focuses on how trauma influences a patient’s life and response to medical care. To practice trauma-informed care, screening for trauma when appropriate, actively creating a supportive environment with active listening and communication, with informing the patient of planned actions prior to doing them, like physical exams, is key.
Trauma-Informed Care: Examples of Verbiage
Asking about Identity
- Begin by introducing yourself with your pronouns to create a safe environment for patient disclosure. Example: “Hello, I am Dr. Kara Jencks, and my pronouns are she/her. I am one of the gastroenterologists here at XYZ Clinic. How would you prefer to be addressed?”
- You can also wear a pronoun lapel pin or a pronoun button on your ID badge to indicate you are someone who your patient can be themselves around.
- The easiest way to obtain sexual orientation and gender identity is through intake forms. Below are examples of how to ask these questions on intake forms. It is important to offer the option to select more than one option when applicable and to opt out of answering if the patient is not comfortable answering these questions.
Sample Questions for Intake Forms
1. What is your sex assigned at birth? (Select one)
- Female
- Male
- Intersex
- Do not know
- Prefer not to disclose
2. What is your gender identity? (Select all that apply)
- Nonbinary
- Gender queer
- Woman
- Man
- Transwoman
- Transman
- Gender fluid
- Two-spirit
- Agender
- Intersex
- Other: type in response
- Prefer not to disclose
3. What are your pronouns? (Select all that apply)
- They/them/theirs
- She/her/hers
- He/him/his
- Zie/zir/zirs
- Other: type in response
- Prefer not to disclose
4. What is your sexual orientation? (Select all that apply)
- Bisexual
- Pansexual
- Queer
- Lesbian
- Gay
- Asexual
- Demisexual
- Heterosexual or straight
- Other: type in response
- Prefer not to disclose
Screening for Trauma
While there are questionnaires that exist to ask about trauma history, if time allows, it can be helpful to screen verbally with the patient. See reference number 8, for additional prompts and actions to practice trauma-informed care.
- Example: “Many patients with gastrointestinal symptoms and disorders have experienced trauma in the past. We do our best to ensure we are keeping you as comfortable as possible while caring for you. Are you comfortable sharing this information? [if yes->] Do you have a history of trauma, including physical, emotional, or sexual abuse? ... Have these experiences impacted the way in which you navigate your healthcare? ... Is there anything we can do to make you more comfortable today?”
General Physical Examination
Provide details for what you are going to do before you do it. Ask for permission for the examination. Here are two examples:
- “I would like to perform a physical exam to help better understand your symptoms. Is that okay with you?”
- “I would like to examine your abdomen with my stethoscope and my hands. Here is a sheet that we can use to help with your privacy. Please let me know if and when you feel any tenderness or pain.”
Rectal Physical Examination
Let the patient know why it would be helpful to perform a rectal exam, what the rectal exam will entail, and the benefits and risks to doing a rectal exam. An example follows:
- “Based on the symptoms you are describing, I think it would be helpful to perform a rectal exam to make sure you don’t have any fissures or hemorrhoids on the outside around the anus, any blockages or major issues inside the rectum, and to assess the strength and ability of your nerves and muscles or the pelvic floor to coordinate bowel movements. There are no risks aside from discomfort. If it is painful, and you would like me to stop, you tell me to stop, and I will stop right away. What questions do you have? Are we okay to proceed with the rectal exam?”
- “Please pull down your undergarments and your pants to either midthigh, your ankles, or all the way off, whatever your preference is, lie down on the left side on the exam table, and cover yourself with this sheet. In the meantime, I will be getting a chaperone to keep us safe and serve as a patient advocate during the procedure.”
- Upon returning to the exam room: “Here is Sara, who will be chaperoning today. Let myself or Sara know if you are uncomfortable or having pain during this exam. I will be lifting up the sheet to get a good look around the anus. [lifts up sheet] You will feel my hand helping to spread apart the buttocks. I am looking around the anus, and I do not see any fissures, hemorrhoids, or anything else concerning. Please squeeze in like you are trying to hold in gas. Please bear down like you are trying to have a bowel movement or let out gas. Okay, now you may feel some cold gel around the anus, and you will feel my finger go inside. Take a deep breath in. Do you feel any pain as I palpate? Please squeeze in like you are trying to hold in gas. Please bear down like you are trying to have a bowel movement or let out gas. I will be stopping the exam now.”
- You would then wash your hands and allow the patient to get dressed, and then disclose the exam findings and the rest of your visit.
Ilan H. Meyer coined the minority stress model when discussing mental health disorders in SGM patients in the early 2000s.9 With it being well known that DGBIs can overlap with (but are not necessarily caused by) mental health disorders, this model can easily apply to unify multiple individual and societal factors that can combine to result in disorders of brain-gut interaction (see Figure 1) in SGM communities. Let us keep this framework in mind when evaluating the following cases.

Case Presentations
Case 1
A 56-year-old man (pronouns: he/him) assigned male sex at birth, who identifies as gay, presents to your gastroenterology clinic for treatment-refractory constipation-predominant irritable bowel syndrome. It has impacted his sexual function. Outside hospital records report a normal colonoscopy 1 year ago and an unremarkable abdominal computerized tomography 4 months ago, aside from increased stool burden in the entire colon. He has tried to use enemas prior to sex, though these do not always help. Fiber-rich diet and fermentable food avoidance has not been successful. He is currently taking two capfuls of polyethylene glycol 3350 twice per day, as well as senna at night and continues to have a bowel movement every 2-3 days that is Bristol stool form scale type 1-2 unless he uses enemas. How do you counsel this patient about his IBS-C and rectal discomfort?
After assessing for sexual violence or other potential trauma-related factors, your digital rectal examination suggests that an anorectal defecatory disorder is less likely with normal relaxation and perineal movement. You recommend linaclotide. He notices improvement within 1 week, with improved comfort during anoreceptive sex.
Case 2
A 30-year-old woman (pronouns: she/her) assigned male sex at birth who has sex with men underwent vaginoplasty 2 years ago and is referred to the gastroenterology clinic for fecal incontinence and diarrhea. On review of her anatomic inventory, her vaginoplasty was a penile inversion vaginoplasty (no intestinal tissue was used for creation), and her prostate was left intact. The vaginal vault was created in between the urethra and rectum, similar to the pelvic floor anatomy of a woman assigned female sex at birth. Blood, imaging, and endoscopic workup has been negative. She is also not taking any medications associated with diarrhea, only taking estrogen and spironolactone. The diarrhea is not daily, but when present, about once per week, can be up to 10 episodes per day, and she has a sense of incomplete evacuation regularly. She notes having a rectal exam in the past but is not sure if her pelvic floor muscles have ever been assessed. How do you manage this patient?
To complete her evaluation in the office, you perform a trauma-informed rectal exam which reveals a decreased resting anal sphincter tone and paradoxical defecatory maneuvers without tenderness to the puborectalis muscle. Augmentation of the squeeze is also weak. Given her pelvic floor related surgical history, her symptoms, and her rectal exam, you recommend anorectal manometry which is abnormal and send her for anorectal biofeedback pelvic floor physical therapy, which improves her symptoms significantly.
Case 3
A 36-year-old woman (pronouns: she/her) assigned female sex at birth, who identifies as a lesbian, has a history of posttraumatic stress disorder and chronic nausea and vomiting that has begun to affect her quality of life. She notes the nausea and vomiting used to be managed well with evening cannabis gummies, though in the past 3 months, the nausea and vomiting has worsened, and she has lost 20 pounds as a result. As symptom predated cannabis usage, cannabis hyperemesis syndrome (CHS) was less likely (an important point as she has been stigmatized during prior encounters for her cannabis usage). Her primary care physician recommended a gastroscopy which was normal, aside from some residual solid food material in the stomach. Her bowel movements are normal, and she doesn’t have other gastrointestinal symptoms. She and her wife are considering having a third child, so she is worried about medications that may affect pregnancy or breast-feeding. How do you manage her nausea and vomiting?
After validating her concerns and performing a trauma-informed physical exam and encounter, you recommend a 4-hour gastric emptying test with a standard radiolabeled egg meal. Her gastric emptying does reveal significantly delayed gastric emptying at 2 and 4 hours. You discuss the risks and benefits of lifestyle modification (smaller frequent meals), initiating medications (erythromycin and metoclopramide) or cessation of cannabis (despite low likelihood of CHS). Desiring to avoid starting medications around initiation of pregnancy, she opts for the dietary approach and cessation of cannabis. You see her at a follow-up visit in 6 months, and her nausea is now only once a month, and she is excited to begin planning for a pregnancy using assisted reproductive technology.
Case 4
A 20-year-old nonbinary intersex individual (pronouns: he/they) (incorrectly assigned female at birth — is intersex with congenital adrenal hyperplasia) presents to the gastroenterology clinic with 8 years of heartburn, acid reflux, postprandial bloating, alternating diarrhea and constipation, nausea, and vomiting, complicated by avoidant restrictive food intake disorder. They have a history of bipolar II disorder with prior suicidal ideation. He has not yet had diagnostic workup as he previously had a bad encounter with a gastroenterologist where the gastroenterologist blamed his symptoms on his gender-affirming therapy, misgendered the patient, and told the patient their symptoms were “all in her [sic] head.”
You recognize that affirming their gender and using proper pronouns is the best first way to start rapport and help break the cycle of medicalized trauma. You then recommend a holistic work up with interdisciplinary management because of the complexity of his symptoms. For testing, you recommend a colonoscopy, upper endoscopy, a gastric emptying test with a 48-hour transit scintigraphy test, anorectal manometry, a dietitian referral, and a gastrointestinal psychology referral. Their anorectal manometry is consistent with an evacuation disorder. The rest of the work up is unremarkable. You diagnose them with anorectal pelvic floor dysfunction and functional dyspepsia, recommending biofeedback pelvic floor physical therapy, a proton-pump inhibitor, and neuromodulation in coordination with psychiatry and psychology to start with a plan for follow-up. The patient appreciates you for helping them and listening to their symptoms.
Discussion
When approaching DGBIs in the SGM community, it is vital to validate their concerns and be inclusive with diagnostic and treatment modalities. The diagnostic tools and treatments for DGBI are not different for patients in the SGM community. Like with other patients, trauma-informed care should be utilized, particularly given higher rates of trauma and discrimination in this community. Importantly, their DGBI is not a result of their sexual orientation or gender identity, and hormone therapy is not the cause of their DGBI. Recommending cessation of gender-affirming care or recommending lifestyle measures against their identity is generally not appropriate or necessary. among members of the SGM communities.
Dr. Jencks (@karajencks) is based in the division of gastroenterology and hepatology, Mayo Clinic, Rochester, Minnesota. Dr. Vélez (@Chris_Velez_MD) is based in the division of gastroenterology, Massachusetts General Hospital and Harvard Medical School, both in Boston. Both authors do not have any conflicts of interest for this article.
References
1. Duong N et al. 2023 Apr. doi: 10.1016/S2468-1253(23)00005-5.
2. Vélez C et al. Am J Gastroenterol. 2022 Jun. doi: 10.14309/ajg.0000000000001804.
3. Jones JM. Gallup. LGBTQ+ identification in U.S. now at 7.6%. 2024 Mar 13. https://news.gallup.com/poll/611864/lgbtq-identification.aspx
4. Sperber AD et al. Gastroenterology. 2021 Jan. doi: 10.1053/j.gastro.2020.04.014.
5. Wiley JW et al. Neurogastroenterol Motil. 2016 Jan. doi: 10.1111/nmo.12706.
6. Labanski A et al. Psychoneuroendocrinology. 2020 Jan. doi: 10.1016/j.psyneuen.2019.104501.
7. Khlevner J et al. Gastroenterol Clin North Am. 2018 Dec. doi: 10.1016/j.gtc.2018.07.002.
8. Jagielski CH and Harer KN. Gastroenterol Clin North Am. 2022 Dec. doi: 10.1016/j.gtc.2022.07.012.
9. Meyer IH. Psychol Bull. 2003 Sep. doi: 10.1037/0033-2909.129.5.674.
10. Mahurkar-Joshi S and Chang L. Front Psychiatry. 2020 Aug. doi: 10.3389/fpsyt.2020.00805.
How to Discuss Lifestyle Modifications in MASLD
Metabolic dysfunction–associated steatotic liver disease (MASLD) is a spectrum of hepatic disorders closely linked to insulin resistance, dyslipidemia, hypertension, and obesity.1 An increasingly prevalent cause of liver disease and liver-related deaths worldwide, MASLD affects at least 38% of the global population.2 The immense burden of MASLD and its complications demands attention and action from the medical community.
Lifestyle modifications involving weight management and dietary composition adjustments are the foundation of addressing MASLD, with a critical emphasis on early intervention.3 Healthy dietary indices and weight loss can lower enzyme levels, reduce hepatic fat content, improve insulin resistance, and overall, reduce the risk of MASLD.3 Given the abundance of literature that exists on the benefits of lifestyle modifications on liver and general health outcomes, clinicians should be prepared to have informed, individualized, and culturally concordant conversations with their patients about these modifications. This Short Clinical Review aims to
Initiate the Conversation
Conversations about lifestyle modifications can be challenging and complex. If patients themselves are not initiating conversations about dietary composition and physical activity, then it is important for clinicians to start a productive discussion.
The use of non-stigmatizing, open-ended questions can begin this process. For example, clinicians can consider asking patients: “How would you describe your lifestyle habits, such as foods you usually eat and your physical activity levels? What do you usually look for when you are grocery shopping or thinking of a meal to cook? Are there ways in which you stay physically active throughout the day or week?”4 (see Table 1).
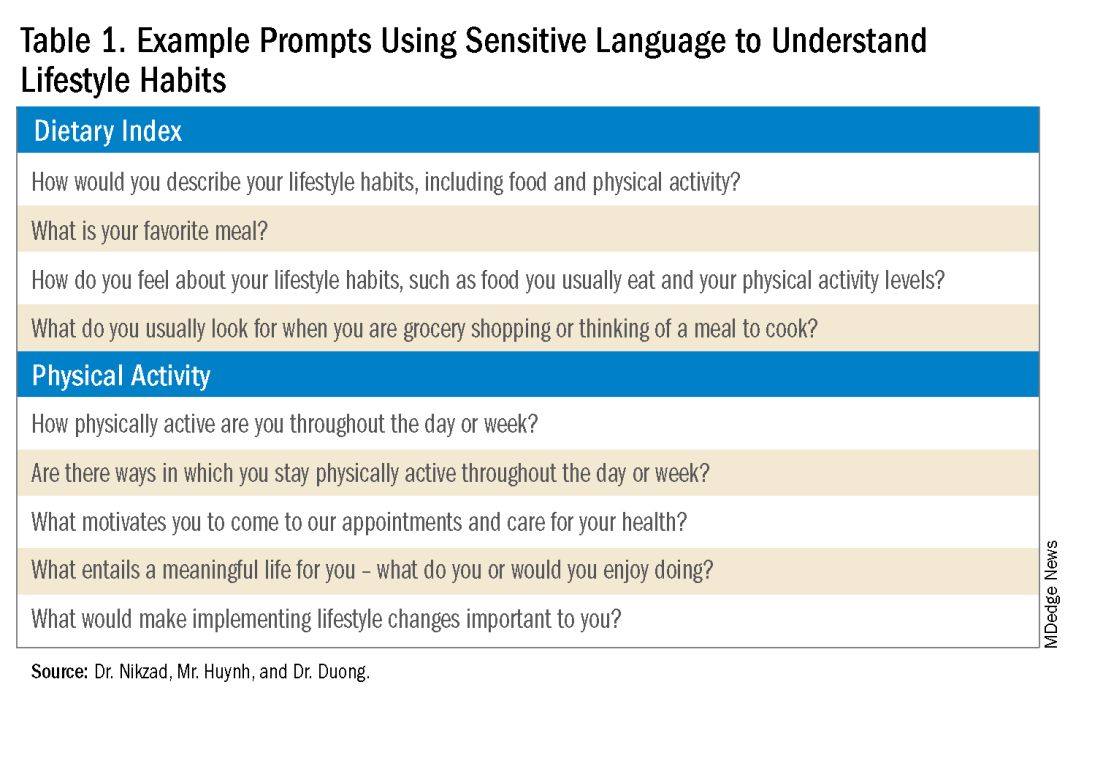
Such questions can provide significant insight into patients’ activity and eating patterns. They also eliminate the utilization of words such as “diet” or “exercise” that may have associated stigma, pressure, or negative connotations.4
Regardless, some patients may not feel prepared or willing to discuss lifestyle modifications during a visit, especially if it is the first clinical encounter when rapport has yet to even be established.4 Lifestyle modifications are implemented at various paces, and patients have their individual timelines for achieving these adjustments. Building rapport with patients and creating spaces in which they feel safe discussing and incorporating changes to various components of their lives can take time. Patients want to trust their providers while being vulnerable. They want to trust that their providers will guide them in what can sometimes be a life altering journey. It is important for clinicians to acknowledge and respect this reality when caring for patients with MASLD. Dr. Duong often utilizes this phrase, “It may seem like you are about to walk through fire, but we are here to walk with you. Remember, what doesn’t challenge you, doesn’t change you.”
Identify Motivators of Engagement
Identifying patients’ motivators of engagement will allow clinicians to guide patients through not only the introduction, but also the maintenance of such changes. Improvements in dietary composition and physical activity are often recommended by clinicians who are inevitably and understandably concerned about the consequences of MASLD. Liver diseases, specifically cirrhosis and hepatocellular carcinoma, as well as associated metabolic disorders, are consequences that could result from poorly controlled MASLD. Though these consequences should be conveyed to patients, this tactic may not always serve as an impetus for patients to engage in behavioral changes.5
Clinicians can shed light on motivators by utilizing these suggested prompts: “What motivates you to come to our appointments and care for your health? What entails a meaningful life for you — what do or would you enjoy doing? What would make implementing lifestyle changes important to you?” Patient goals may include “being able to keep up with their grandchildren,” “becoming a runner,” or “providing healthy meals for their families.”5,6 Engagement is more likely to be feasible and sustainable when lifestyle modifications are tied to goals that are personally meaningful and relevant to patients.
Within the realm of physical activity specifically, exercise can be individualized to optimize motivation as well. Both aerobic exercise and resistance training are associated independently with benefits such as weight loss and decreased hepatic adipose content.3 Currently, there is no consensus regarding the optimal type of physical activity for patients with MASLD; therefore, clinicians should encourage patients to personalize physical activity.3 While some patients may prefer aerobic activities such as running and swimming, others may find more fulfillment in weightlifting or high intensity interval training. Furthermore, patients with cardiopulmonary or musculoskeletal health contraindications may be limited to specific types of exercise. It is appropriate and helpful for clinicians to ask patients, “What types of physical activity feel achievable and realistic for you at this time?” If physicians can guide patients with MASLD in identifying types of exercise that are safe and enjoyable, their patients may be more motivated to implement such lifestyle changes.
It is also crucial to recognize that lifestyle changes demand active effort from patients. While sustained improvements in body weight and dietary composition are the foundation of MASLD management, they can initially feel cumbersome and abstract to patients. Physicians can help their patients remain motivated by developing small, tangible goals such as “reducing daily caloric intake by 500 kcal” or “participating in three 30-minute fitness classes per week.” These goals should be developed jointly with patients, primarily to ensure that they are tangible, feasible, and productive.
A Culturally Safe Approach
Additionally, acknowledging a patient’s cultural background can be conducive to incorporating patient-specific care into MASLD management. For example, qualitative studies have shown that people from Mexican heritage traditionally complement dinners with soft drinks. While meal portion sizes vary amongst households, families of Mexican origin believe larger portion sizes may be perceived as healthier than Western diets since their cuisine incorporates more vegetables into each dish.7
Eating rituals should also be considered since some families expect the absence of leftovers on the plate.7 Therefore, it is appropriate to consider questions such as, “What are common ingredients in your culture? What are some of your family traditions when it comes to meals?” By integrating cultural considerations, clinicians can adopt a culturally safe approach, empowering patients to make lifestyle modifications tailored toward their unique social identities. Clinicians should avoid generalizations or stereotypes about cultural values regarding lifestyle practices, as these can vary among individuals.
Identify Barriers to Lifestyle Changes and Social Determinants of Health
Even with delicate language from providers and immense motivation from patients, barriers to lifestyle changes persist. Studies have shown that patients with MASLD perceive a lack of self-efficacy and knowledge as major barriers to adopting lifestyle modifications.8,9 Patients have reported challenges in interpreting nutritional data, identifying caloric intake and portion sizes. Physicians can effectively guide patients through lifestyle changes by identifying each patient’s unique knowledge gap and determining the most effective, accessible form of education. For example, some patients may benefit from jointly interpreting a nutritional label with their healthcare providers, while others may require educational materials and interventions provided by a registered dietitian.
Understanding patients’ professional or other commitments can help physicians further individualize recommendations. Questions such as, “Do you have work or other responsibilities that take up some of your time during the day?” minimize presumptive language about employment status. It can reveal whether patients have schedules that make certain lifestyle changes more challenging than others. For example, a patient who is an overnight delivery associate at a warehouse may have a different routine from another patient who is a family member’s caretaker. This framework allows physicians to build rapport with their patients and ultimately, make lifestyle recommendations that are more accessible.
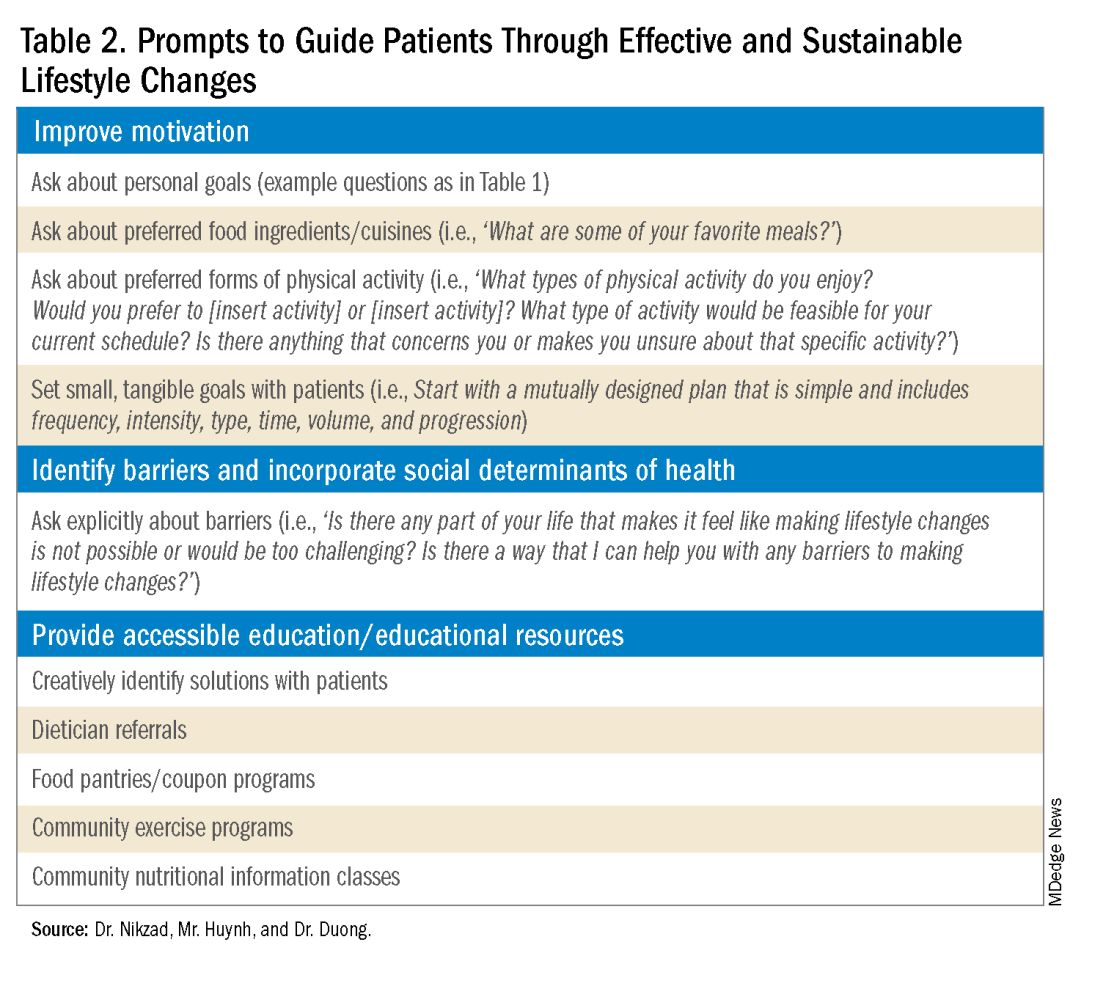
Though MASLD is driven by inflammation and metabolic dysregulation, social determinants of health play an equally important role in disease development and progression.10 As previously discussed, health literacy can deeply influence patients’ abilities to implement lifestyle changes. Furthermore, economic stability, neighborhood and built environment (i.e., access to fresh produce and sidewalks), community, and social support also impact lifestyle modifications. It is paramount to understand the tangible social factors in which patients live. Such factors can be ascertained by beginning the dialogue with “Which grocery stores do you find most convenient? How do you travel to obtain food/attend community exercise programs?” These questions may offer insight into physical barriers to lifestyle changes. Physicians must utilize an intersectional lens that incorporates patients’ unique circumstances of existence into their individualized health care plans to address MASLD.
Summary
- Communication preferences, cultural backgrounds, and sociocultural contexts of patient existence must be considered when treating a patient with MASLD.
- The utilization of an intersectional and culturally safe approach to communication with patients can lead to more sustainable lifestyle changes and improved health outcomes.
- Equipping and empowering physicians to have meaningful discussions about MASLD is crucial to combating a spectrum of diseases that is rapidly affecting a substantial proportion of patients worldwide.
Dr. Nikzad is based in the Department of Internal Medicine at University of Chicago Medicine (@NewshaN27). Mr. Huynh is a medical student at Stony Brook University Renaissance School of Medicine, Stony Brook, N.Y. (@danielhuynhhh). Dr. Duong is an assistant professor of medicine and transplant hepatologist at Stanford University, Palo Alto, Calif. (@doctornikkid). They have no conflicts of interest to declare.
References
1. Mohanty A. MASLD/MASH and Weight Loss. GI & Hepatology News. 2023 Oct. Data Trends 2023:9-13.
2. Wong VW, et al. Changing epidemiology, global trends and implications for outcomes of NAFLD. J Hepatol. 2023 Sep. doi: 10.1016/j.jhep.2023.04.036.
3. Zeng J, et al. Therapeutic management of metabolic dysfunction associated steatotic liver disease. United European Gastroenterol J. 2024 Mar. doi: 10.1002/ueg2.12525.
4. Berg S. How patients can start—and stick with—key lifestyle changes. AMA Public Health. 2020 Jan.
5. Berg S. 3 ways to get patients engaged in lasting lifestyle change. AMA Diabetes. 2019 Jan.
6. Teixeira PJ, et al. Motivation, self-determination, and long-term weight control. Int J Behav Nutr Phys Act. 2012 Mar. doi: 10.1186/1479-5868-9-22.
7. Aceves-Martins M, et al. Cultural factors related to childhood and adolescent obesity in Mexico: A systematic review of qualitative studies. Obes Rev. 2022 Sep. doi: 10.1111/obr.13461.
8. Figueroa G, et al. Low health literacy, lack of knowledge, and self-control hinder healthy lifestyles in diverse patients with steatotic liver disease. Dig Dis Sci. 2024 Feb. doi: 10.1007/s10620-023-08212-9.
9. Wang L, et al. Factors influencing adherence to lifestyle prescriptions among patients with nonalcoholic fatty liver disease: A qualitative study using the health action process approach framework. Front Public Health. 2023 Mar. doi: 10.3389/fpubh.2023.1131827.
10. Andermann A, CLEAR Collaboration. Taking action on the social determinants of health in clinical practice: a framework for health professionals. CMAJ. 2016 Dec. doi: 10.1503/cmaj.160177.
Metabolic dysfunction–associated steatotic liver disease (MASLD) is a spectrum of hepatic disorders closely linked to insulin resistance, dyslipidemia, hypertension, and obesity.1 An increasingly prevalent cause of liver disease and liver-related deaths worldwide, MASLD affects at least 38% of the global population.2 The immense burden of MASLD and its complications demands attention and action from the medical community.
Lifestyle modifications involving weight management and dietary composition adjustments are the foundation of addressing MASLD, with a critical emphasis on early intervention.3 Healthy dietary indices and weight loss can lower enzyme levels, reduce hepatic fat content, improve insulin resistance, and overall, reduce the risk of MASLD.3 Given the abundance of literature that exists on the benefits of lifestyle modifications on liver and general health outcomes, clinicians should be prepared to have informed, individualized, and culturally concordant conversations with their patients about these modifications. This Short Clinical Review aims to
Initiate the Conversation
Conversations about lifestyle modifications can be challenging and complex. If patients themselves are not initiating conversations about dietary composition and physical activity, then it is important for clinicians to start a productive discussion.
The use of non-stigmatizing, open-ended questions can begin this process. For example, clinicians can consider asking patients: “How would you describe your lifestyle habits, such as foods you usually eat and your physical activity levels? What do you usually look for when you are grocery shopping or thinking of a meal to cook? Are there ways in which you stay physically active throughout the day or week?”4 (see Table 1).

Such questions can provide significant insight into patients’ activity and eating patterns. They also eliminate the utilization of words such as “diet” or “exercise” that may have associated stigma, pressure, or negative connotations.4
Regardless, some patients may not feel prepared or willing to discuss lifestyle modifications during a visit, especially if it is the first clinical encounter when rapport has yet to even be established.4 Lifestyle modifications are implemented at various paces, and patients have their individual timelines for achieving these adjustments. Building rapport with patients and creating spaces in which they feel safe discussing and incorporating changes to various components of their lives can take time. Patients want to trust their providers while being vulnerable. They want to trust that their providers will guide them in what can sometimes be a life altering journey. It is important for clinicians to acknowledge and respect this reality when caring for patients with MASLD. Dr. Duong often utilizes this phrase, “It may seem like you are about to walk through fire, but we are here to walk with you. Remember, what doesn’t challenge you, doesn’t change you.”
Identify Motivators of Engagement
Identifying patients’ motivators of engagement will allow clinicians to guide patients through not only the introduction, but also the maintenance of such changes. Improvements in dietary composition and physical activity are often recommended by clinicians who are inevitably and understandably concerned about the consequences of MASLD. Liver diseases, specifically cirrhosis and hepatocellular carcinoma, as well as associated metabolic disorders, are consequences that could result from poorly controlled MASLD. Though these consequences should be conveyed to patients, this tactic may not always serve as an impetus for patients to engage in behavioral changes.5
Clinicians can shed light on motivators by utilizing these suggested prompts: “What motivates you to come to our appointments and care for your health? What entails a meaningful life for you — what do or would you enjoy doing? What would make implementing lifestyle changes important to you?” Patient goals may include “being able to keep up with their grandchildren,” “becoming a runner,” or “providing healthy meals for their families.”5,6 Engagement is more likely to be feasible and sustainable when lifestyle modifications are tied to goals that are personally meaningful and relevant to patients.
Within the realm of physical activity specifically, exercise can be individualized to optimize motivation as well. Both aerobic exercise and resistance training are associated independently with benefits such as weight loss and decreased hepatic adipose content.3 Currently, there is no consensus regarding the optimal type of physical activity for patients with MASLD; therefore, clinicians should encourage patients to personalize physical activity.3 While some patients may prefer aerobic activities such as running and swimming, others may find more fulfillment in weightlifting or high intensity interval training. Furthermore, patients with cardiopulmonary or musculoskeletal health contraindications may be limited to specific types of exercise. It is appropriate and helpful for clinicians to ask patients, “What types of physical activity feel achievable and realistic for you at this time?” If physicians can guide patients with MASLD in identifying types of exercise that are safe and enjoyable, their patients may be more motivated to implement such lifestyle changes.
It is also crucial to recognize that lifestyle changes demand active effort from patients. While sustained improvements in body weight and dietary composition are the foundation of MASLD management, they can initially feel cumbersome and abstract to patients. Physicians can help their patients remain motivated by developing small, tangible goals such as “reducing daily caloric intake by 500 kcal” or “participating in three 30-minute fitness classes per week.” These goals should be developed jointly with patients, primarily to ensure that they are tangible, feasible, and productive.
A Culturally Safe Approach
Additionally, acknowledging a patient’s cultural background can be conducive to incorporating patient-specific care into MASLD management. For example, qualitative studies have shown that people from Mexican heritage traditionally complement dinners with soft drinks. While meal portion sizes vary amongst households, families of Mexican origin believe larger portion sizes may be perceived as healthier than Western diets since their cuisine incorporates more vegetables into each dish.7
Eating rituals should also be considered since some families expect the absence of leftovers on the plate.7 Therefore, it is appropriate to consider questions such as, “What are common ingredients in your culture? What are some of your family traditions when it comes to meals?” By integrating cultural considerations, clinicians can adopt a culturally safe approach, empowering patients to make lifestyle modifications tailored toward their unique social identities. Clinicians should avoid generalizations or stereotypes about cultural values regarding lifestyle practices, as these can vary among individuals.
Identify Barriers to Lifestyle Changes and Social Determinants of Health
Even with delicate language from providers and immense motivation from patients, barriers to lifestyle changes persist. Studies have shown that patients with MASLD perceive a lack of self-efficacy and knowledge as major barriers to adopting lifestyle modifications.8,9 Patients have reported challenges in interpreting nutritional data, identifying caloric intake and portion sizes. Physicians can effectively guide patients through lifestyle changes by identifying each patient’s unique knowledge gap and determining the most effective, accessible form of education. For example, some patients may benefit from jointly interpreting a nutritional label with their healthcare providers, while others may require educational materials and interventions provided by a registered dietitian.
Understanding patients’ professional or other commitments can help physicians further individualize recommendations. Questions such as, “Do you have work or other responsibilities that take up some of your time during the day?” minimize presumptive language about employment status. It can reveal whether patients have schedules that make certain lifestyle changes more challenging than others. For example, a patient who is an overnight delivery associate at a warehouse may have a different routine from another patient who is a family member’s caretaker. This framework allows physicians to build rapport with their patients and ultimately, make lifestyle recommendations that are more accessible.

Though MASLD is driven by inflammation and metabolic dysregulation, social determinants of health play an equally important role in disease development and progression.10 As previously discussed, health literacy can deeply influence patients’ abilities to implement lifestyle changes. Furthermore, economic stability, neighborhood and built environment (i.e., access to fresh produce and sidewalks), community, and social support also impact lifestyle modifications. It is paramount to understand the tangible social factors in which patients live. Such factors can be ascertained by beginning the dialogue with “Which grocery stores do you find most convenient? How do you travel to obtain food/attend community exercise programs?” These questions may offer insight into physical barriers to lifestyle changes. Physicians must utilize an intersectional lens that incorporates patients’ unique circumstances of existence into their individualized health care plans to address MASLD.
Summary
- Communication preferences, cultural backgrounds, and sociocultural contexts of patient existence must be considered when treating a patient with MASLD.
- The utilization of an intersectional and culturally safe approach to communication with patients can lead to more sustainable lifestyle changes and improved health outcomes.
- Equipping and empowering physicians to have meaningful discussions about MASLD is crucial to combating a spectrum of diseases that is rapidly affecting a substantial proportion of patients worldwide.
Dr. Nikzad is based in the Department of Internal Medicine at University of Chicago Medicine (@NewshaN27). Mr. Huynh is a medical student at Stony Brook University Renaissance School of Medicine, Stony Brook, N.Y. (@danielhuynhhh). Dr. Duong is an assistant professor of medicine and transplant hepatologist at Stanford University, Palo Alto, Calif. (@doctornikkid). They have no conflicts of interest to declare.
References
1. Mohanty A. MASLD/MASH and Weight Loss. GI & Hepatology News. 2023 Oct. Data Trends 2023:9-13.
2. Wong VW, et al. Changing epidemiology, global trends and implications for outcomes of NAFLD. J Hepatol. 2023 Sep. doi: 10.1016/j.jhep.2023.04.036.
3. Zeng J, et al. Therapeutic management of metabolic dysfunction associated steatotic liver disease. United European Gastroenterol J. 2024 Mar. doi: 10.1002/ueg2.12525.
4. Berg S. How patients can start—and stick with—key lifestyle changes. AMA Public Health. 2020 Jan.
5. Berg S. 3 ways to get patients engaged in lasting lifestyle change. AMA Diabetes. 2019 Jan.
6. Teixeira PJ, et al. Motivation, self-determination, and long-term weight control. Int J Behav Nutr Phys Act. 2012 Mar. doi: 10.1186/1479-5868-9-22.
7. Aceves-Martins M, et al. Cultural factors related to childhood and adolescent obesity in Mexico: A systematic review of qualitative studies. Obes Rev. 2022 Sep. doi: 10.1111/obr.13461.
8. Figueroa G, et al. Low health literacy, lack of knowledge, and self-control hinder healthy lifestyles in diverse patients with steatotic liver disease. Dig Dis Sci. 2024 Feb. doi: 10.1007/s10620-023-08212-9.
9. Wang L, et al. Factors influencing adherence to lifestyle prescriptions among patients with nonalcoholic fatty liver disease: A qualitative study using the health action process approach framework. Front Public Health. 2023 Mar. doi: 10.3389/fpubh.2023.1131827.
10. Andermann A, CLEAR Collaboration. Taking action on the social determinants of health in clinical practice: a framework for health professionals. CMAJ. 2016 Dec. doi: 10.1503/cmaj.160177.
Metabolic dysfunction–associated steatotic liver disease (MASLD) is a spectrum of hepatic disorders closely linked to insulin resistance, dyslipidemia, hypertension, and obesity.1 An increasingly prevalent cause of liver disease and liver-related deaths worldwide, MASLD affects at least 38% of the global population.2 The immense burden of MASLD and its complications demands attention and action from the medical community.
Lifestyle modifications involving weight management and dietary composition adjustments are the foundation of addressing MASLD, with a critical emphasis on early intervention.3 Healthy dietary indices and weight loss can lower enzyme levels, reduce hepatic fat content, improve insulin resistance, and overall, reduce the risk of MASLD.3 Given the abundance of literature that exists on the benefits of lifestyle modifications on liver and general health outcomes, clinicians should be prepared to have informed, individualized, and culturally concordant conversations with their patients about these modifications. This Short Clinical Review aims to
Initiate the Conversation
Conversations about lifestyle modifications can be challenging and complex. If patients themselves are not initiating conversations about dietary composition and physical activity, then it is important for clinicians to start a productive discussion.
The use of non-stigmatizing, open-ended questions can begin this process. For example, clinicians can consider asking patients: “How would you describe your lifestyle habits, such as foods you usually eat and your physical activity levels? What do you usually look for when you are grocery shopping or thinking of a meal to cook? Are there ways in which you stay physically active throughout the day or week?”4 (see Table 1).

Such questions can provide significant insight into patients’ activity and eating patterns. They also eliminate the utilization of words such as “diet” or “exercise” that may have associated stigma, pressure, or negative connotations.4
Regardless, some patients may not feel prepared or willing to discuss lifestyle modifications during a visit, especially if it is the first clinical encounter when rapport has yet to even be established.4 Lifestyle modifications are implemented at various paces, and patients have their individual timelines for achieving these adjustments. Building rapport with patients and creating spaces in which they feel safe discussing and incorporating changes to various components of their lives can take time. Patients want to trust their providers while being vulnerable. They want to trust that their providers will guide them in what can sometimes be a life altering journey. It is important for clinicians to acknowledge and respect this reality when caring for patients with MASLD. Dr. Duong often utilizes this phrase, “It may seem like you are about to walk through fire, but we are here to walk with you. Remember, what doesn’t challenge you, doesn’t change you.”
Identify Motivators of Engagement
Identifying patients’ motivators of engagement will allow clinicians to guide patients through not only the introduction, but also the maintenance of such changes. Improvements in dietary composition and physical activity are often recommended by clinicians who are inevitably and understandably concerned about the consequences of MASLD. Liver diseases, specifically cirrhosis and hepatocellular carcinoma, as well as associated metabolic disorders, are consequences that could result from poorly controlled MASLD. Though these consequences should be conveyed to patients, this tactic may not always serve as an impetus for patients to engage in behavioral changes.5
Clinicians can shed light on motivators by utilizing these suggested prompts: “What motivates you to come to our appointments and care for your health? What entails a meaningful life for you — what do or would you enjoy doing? What would make implementing lifestyle changes important to you?” Patient goals may include “being able to keep up with their grandchildren,” “becoming a runner,” or “providing healthy meals for their families.”5,6 Engagement is more likely to be feasible and sustainable when lifestyle modifications are tied to goals that are personally meaningful and relevant to patients.
Within the realm of physical activity specifically, exercise can be individualized to optimize motivation as well. Both aerobic exercise and resistance training are associated independently with benefits such as weight loss and decreased hepatic adipose content.3 Currently, there is no consensus regarding the optimal type of physical activity for patients with MASLD; therefore, clinicians should encourage patients to personalize physical activity.3 While some patients may prefer aerobic activities such as running and swimming, others may find more fulfillment in weightlifting or high intensity interval training. Furthermore, patients with cardiopulmonary or musculoskeletal health contraindications may be limited to specific types of exercise. It is appropriate and helpful for clinicians to ask patients, “What types of physical activity feel achievable and realistic for you at this time?” If physicians can guide patients with MASLD in identifying types of exercise that are safe and enjoyable, their patients may be more motivated to implement such lifestyle changes.
It is also crucial to recognize that lifestyle changes demand active effort from patients. While sustained improvements in body weight and dietary composition are the foundation of MASLD management, they can initially feel cumbersome and abstract to patients. Physicians can help their patients remain motivated by developing small, tangible goals such as “reducing daily caloric intake by 500 kcal” or “participating in three 30-minute fitness classes per week.” These goals should be developed jointly with patients, primarily to ensure that they are tangible, feasible, and productive.
A Culturally Safe Approach
Additionally, acknowledging a patient’s cultural background can be conducive to incorporating patient-specific care into MASLD management. For example, qualitative studies have shown that people from Mexican heritage traditionally complement dinners with soft drinks. While meal portion sizes vary amongst households, families of Mexican origin believe larger portion sizes may be perceived as healthier than Western diets since their cuisine incorporates more vegetables into each dish.7
Eating rituals should also be considered since some families expect the absence of leftovers on the plate.7 Therefore, it is appropriate to consider questions such as, “What are common ingredients in your culture? What are some of your family traditions when it comes to meals?” By integrating cultural considerations, clinicians can adopt a culturally safe approach, empowering patients to make lifestyle modifications tailored toward their unique social identities. Clinicians should avoid generalizations or stereotypes about cultural values regarding lifestyle practices, as these can vary among individuals.
Identify Barriers to Lifestyle Changes and Social Determinants of Health
Even with delicate language from providers and immense motivation from patients, barriers to lifestyle changes persist. Studies have shown that patients with MASLD perceive a lack of self-efficacy and knowledge as major barriers to adopting lifestyle modifications.8,9 Patients have reported challenges in interpreting nutritional data, identifying caloric intake and portion sizes. Physicians can effectively guide patients through lifestyle changes by identifying each patient’s unique knowledge gap and determining the most effective, accessible form of education. For example, some patients may benefit from jointly interpreting a nutritional label with their healthcare providers, while others may require educational materials and interventions provided by a registered dietitian.
Understanding patients’ professional or other commitments can help physicians further individualize recommendations. Questions such as, “Do you have work or other responsibilities that take up some of your time during the day?” minimize presumptive language about employment status. It can reveal whether patients have schedules that make certain lifestyle changes more challenging than others. For example, a patient who is an overnight delivery associate at a warehouse may have a different routine from another patient who is a family member’s caretaker. This framework allows physicians to build rapport with their patients and ultimately, make lifestyle recommendations that are more accessible.

Though MASLD is driven by inflammation and metabolic dysregulation, social determinants of health play an equally important role in disease development and progression.10 As previously discussed, health literacy can deeply influence patients’ abilities to implement lifestyle changes. Furthermore, economic stability, neighborhood and built environment (i.e., access to fresh produce and sidewalks), community, and social support also impact lifestyle modifications. It is paramount to understand the tangible social factors in which patients live. Such factors can be ascertained by beginning the dialogue with “Which grocery stores do you find most convenient? How do you travel to obtain food/attend community exercise programs?” These questions may offer insight into physical barriers to lifestyle changes. Physicians must utilize an intersectional lens that incorporates patients’ unique circumstances of existence into their individualized health care plans to address MASLD.
Summary
- Communication preferences, cultural backgrounds, and sociocultural contexts of patient existence must be considered when treating a patient with MASLD.
- The utilization of an intersectional and culturally safe approach to communication with patients can lead to more sustainable lifestyle changes and improved health outcomes.
- Equipping and empowering physicians to have meaningful discussions about MASLD is crucial to combating a spectrum of diseases that is rapidly affecting a substantial proportion of patients worldwide.
Dr. Nikzad is based in the Department of Internal Medicine at University of Chicago Medicine (@NewshaN27). Mr. Huynh is a medical student at Stony Brook University Renaissance School of Medicine, Stony Brook, N.Y. (@danielhuynhhh). Dr. Duong is an assistant professor of medicine and transplant hepatologist at Stanford University, Palo Alto, Calif. (@doctornikkid). They have no conflicts of interest to declare.
References
1. Mohanty A. MASLD/MASH and Weight Loss. GI & Hepatology News. 2023 Oct. Data Trends 2023:9-13.
2. Wong VW, et al. Changing epidemiology, global trends and implications for outcomes of NAFLD. J Hepatol. 2023 Sep. doi: 10.1016/j.jhep.2023.04.036.
3. Zeng J, et al. Therapeutic management of metabolic dysfunction associated steatotic liver disease. United European Gastroenterol J. 2024 Mar. doi: 10.1002/ueg2.12525.
4. Berg S. How patients can start—and stick with—key lifestyle changes. AMA Public Health. 2020 Jan.
5. Berg S. 3 ways to get patients engaged in lasting lifestyle change. AMA Diabetes. 2019 Jan.
6. Teixeira PJ, et al. Motivation, self-determination, and long-term weight control. Int J Behav Nutr Phys Act. 2012 Mar. doi: 10.1186/1479-5868-9-22.
7. Aceves-Martins M, et al. Cultural factors related to childhood and adolescent obesity in Mexico: A systematic review of qualitative studies. Obes Rev. 2022 Sep. doi: 10.1111/obr.13461.
8. Figueroa G, et al. Low health literacy, lack of knowledge, and self-control hinder healthy lifestyles in diverse patients with steatotic liver disease. Dig Dis Sci. 2024 Feb. doi: 10.1007/s10620-023-08212-9.
9. Wang L, et al. Factors influencing adherence to lifestyle prescriptions among patients with nonalcoholic fatty liver disease: A qualitative study using the health action process approach framework. Front Public Health. 2023 Mar. doi: 10.3389/fpubh.2023.1131827.
10. Andermann A, CLEAR Collaboration. Taking action on the social determinants of health in clinical practice: a framework for health professionals. CMAJ. 2016 Dec. doi: 10.1503/cmaj.160177.
Advanced Tissue Resection in Gastroenterology: Indications, Role, and Outcomes
Endoscopists are often faced with unique challenges in the management and resection of various gastrointestinal tract lesions. These challenges could be lesion-related, endoscopist-related, or practice-related (see Table 1). (ATR). Not only does this organ-sparing approach offer a less invasive alternative to surgery, but it has also proved to have outcomes comparable to those of surgical standard of practice in specific scenarios. 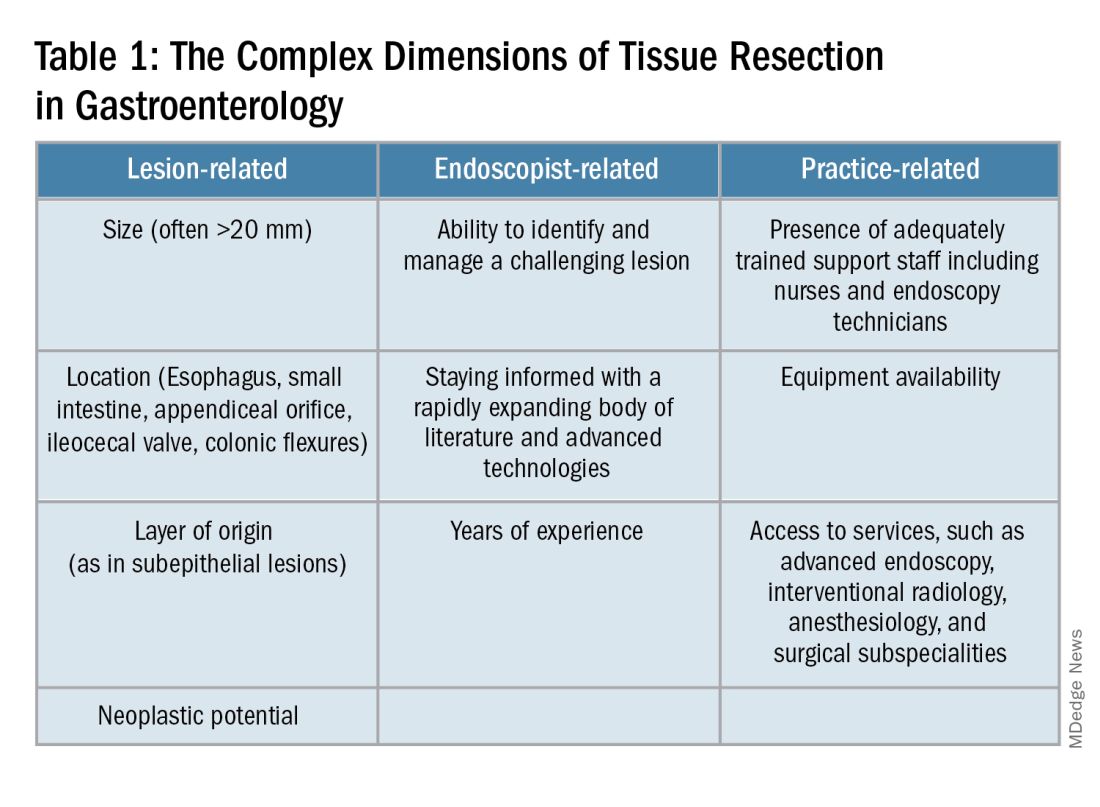
When Do You Refer to an Advanced Endoscopist?
One of the most critical steps in caring for patients with complex lesions is the ability to accurately determine whether a referral to an advanced endoscopist is warranted. The initial assessment of a lesion should always involve a careful assessment that risk stratifies the lesion depending on the location, size, neoplastic potential, and the feasibility of standard endoscopic resection compared to the need for surgical input.
A practical example in the case of colonic polyps is highlighted by the American Gastroenterology Association (AGA) guidelines recommending the referral of patients with polyps’ size ≥ 20 mm, challenging polypectomy location, or recurrent polyp at a prior polypectomy site to an endoscopic referral center.1 In the case of subepithelial lesions without endoscopic characteristics of benign etiology (i.e., lipomas, pancreatic rests, etc.), the threshold for referral to advanced endoscopists for further diagnostic testing by means of endoscopic ultrasonography or for therapeutic ATR should be lower.
Endoscopic tissue resection follows a spectrum, which often involves deeper layers of the gastrointestinal tract (GIT) as we progress along this spectrum (see Figure 1).
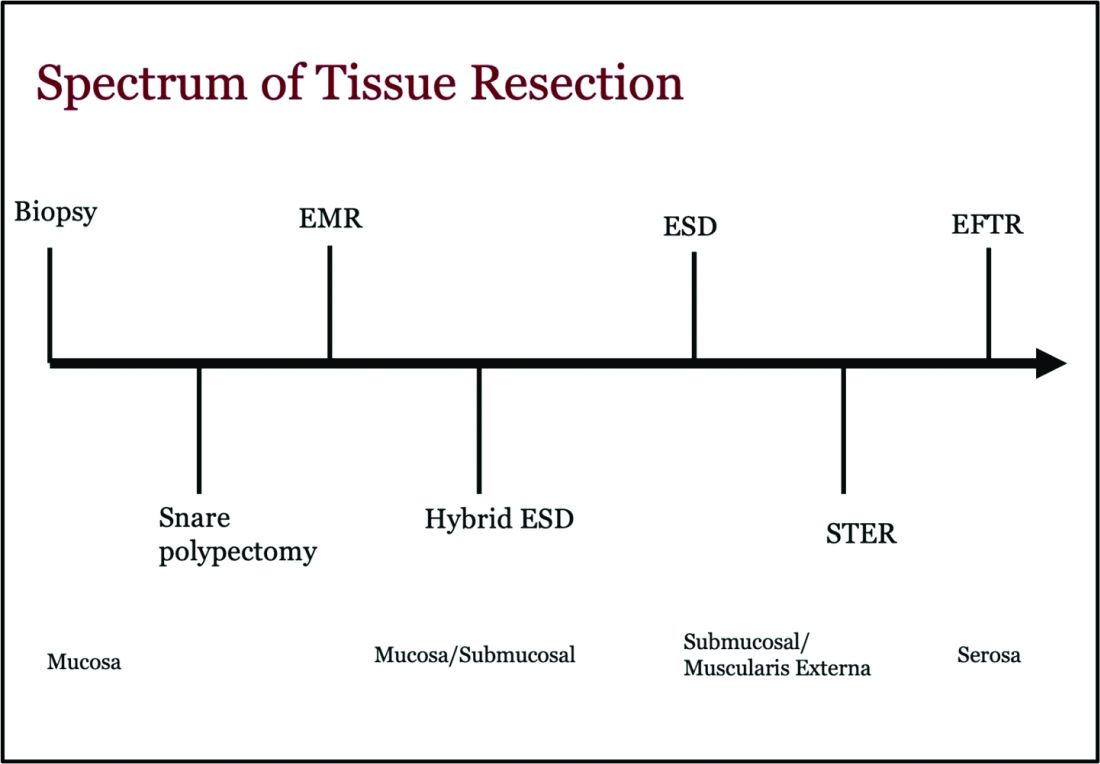
ATR, a term encompassing a variety of endoscopic techniques ranging from endoscopic mucosal resection to full thickness resection, has gained traction over the last years given the ability to effectively remove various lesions in a precise time and cost-effective manner while maintaining the integrity of the GIT and avoiding major surgery. The indications for ATR vary depending on the technique, but generally include the presence of large or poorly positioned lesions, particularly in high-risk areas of the GIT such as the esophagus and small intestine, lesions extending beyond the mucosal layer or originating from deeper layers, and when en bloc resection of select lesions is necessary.
For providers referring patients for ATR, we recommend a few important endoscopic pearls when caring for these patients.
1) Biopsy the lesion if there is concern for malignancy — While some studies have noted increased fibrosis during endoscopic submucosal dissection (ESD) and some guidelines recommend against biopsies pre ESD, we believe that when there is high pretest probability for malignancy, a biopsy should be obtained. This should involve the area that is most concerning for malignancy (at the margin or center).2
2) While marking a lesion with tattoo is helpful for surgical planning and for lesions difficult to locate endoscopically, we stress the importance of placing tattoos 3 to 5 centimeters distal to the lesion and avoiding tattooing the lesion itself, which has been shown to induce fibrosis and can make resection challenging. Based on an international Delphi consensus, expert recommendations on when and how to endoscopically tattoo a lesion can be instrumental in adequately localizing the lesion, allowing for endoscopic resection, and preventing unnecessary surgeries.3
3) If you encounter a lesion that you are not sure can be resected safely and efficaciously, we recommend against attempting resection that may result in partial resection. This can also induce fibrosis and scarring and limit future attempts at resection.
Endoscopic Mucosal Resection (EMR)
EMR is currently utilized for curative treatment of a wide array of GIT lesions limited to the mucosal layer, whether metaplastic, dysplastic, or even in cases with early mucosal cancer, where the risk of submucosal and lymphatic invasion is minimal.4 This makes EMR a versatile and proven therapy, often serving as the first-line treatment for many GIT lesions.
EMR has various techniques that could be categorized into suction or non-suction (lift and cut) techniques. In the suction technique, devices like multiband mucosectomy (MBM) are commonly used, especially in nodular Barrett’s dysplasia, forming a pseudopolyp for subsequent resection. The procedure is characterized by its safety, efficacy, and cost-effectiveness, contributing to its widespread adoption in clinical practice. In the lift and cut approach, a submucosal injection is utilized to separate the muscularis propria from the lesion, thereby reducing the risk of perforation. Different solutions, such as normal saline, hypertonic saline, 50% dextrose, or proprietary submucosal injection solutions, are employed for submucosal injection.5
The non-suction technique using a snare to resect polyps after injection is more often used in colonic and small intestinal EMR. Resection can be done via thermal energy in the form of cut or coagulation; however, there is rising data on the use of piecemeal cold snare resection for select flat polyps of the colon.6 There is also promising data on the role of underwater EMR, a common technique employed for colonic lesions, particularly if the lesion does not lift well with submucosal injection.7
Adverse events associated with EMR include bleeding (7%-8%) and perforation (0.9%-2%).8-9 Adequate submucosal fluid injection is crucial to prevent perforations. However, the main limitation of EMR is the piecemeal nature of resections for lesions larger than 20 mm, leading to compromised histopathologic evaluation for complete excision, especially in cases with superficial submucosal invasion (SMI). This can result in residual or recurrent tissue, reportedly 8% to 20%.10 Despite this limitation, EMR remains a reliable strategy, and recurrent lesions are generally manageable through repeat sessions. The importance of EMR as a therapeutic modality lies in its role in addressing lesions with favorable characteristics, where the risk of SMI is low.
Endoscopic Submucosal Dissection (ESD)
ESD is an evolving technique that can be utilized for submucosal lesions of the GIT, lesions not amenable to EMR due to submucosal fibrosis, when en bloc removal of a lesion is needed for accurate histopathological diagnosis, and when other techniques fail.11-12
ESD was only recently adopted in the United States, requires specialized training, and usually is a lengthier procedure than EMR.13 Compared to EMR, it has higher en bloc resection rates and lower recurrence rates, making it curative for lesions with superficial SMI and favorable histologic features.4,14 The safety profile of ESD appears favorable, with most of the adverse events managed successfully by endoscopic methods. Major complications include intraoperative and delayed perforation, intraoperative and delayed bleeding, aspiration pneumonia, thromboembolism, and stricture formation in the case of circumferential lesions.15
Despite being technically challenging, ESD may provide a cost-effective long-term solution by avoiding surgery, reducing the need for additional interventions by minimizing recurrence rates. Given the technical complexity of ESD, particularly the submucosal dissection portion, techniques such as hybrid ESD developed. Hybrid ESD combines snaring with circumferential mucosal incision and partial submucosal dissection. Although it promises shorter procedure times, reduced complication rates like perforation, and similar recurrence rates compared to traditional ESD, studies have shown lower success rates in en bloc resection.16-17
Both EMR and ESD are considered complementary strategies, and the choice between them should be dictated by lesion characteristics, patient preferences, and local expertise.
Submucosal Tunneling Endoscopic Resection (STER)
STER has emerged as a well-established technique for the endoscopic resection of GI subepithelial tumors (SETs) originating from the muscularis propria layer. The standard STER procedure involves a series of steps including submucosal elevation proximal to the SET, mucosotomy, creation of a submucosal tunnel, dissection of the SET within the tunnel, enucleation from the deep muscle layer, and subsequent specimen retrieval followed by mucosal closure.
This technique is typically recommended for SETs smaller than 3.5 cm, particularly those located in the mid or distal esophagus, cardia, or along the greater curvature of the gastric body.18 However, STER may pose technical challenges for larger SETs or lesions in anatomically difficult locations, where surgical resection is recommended instead.19 Notably, recent large-scale meta-analyses have showcased the favorable complete resection and en bloc resection rates of STER in treating GI SETs.20
Endoscopic Full Thickness Resection (EFTR)
EFTR has emerged as a valuable technique in the endoscopic management of gastrointestinal lesions, particularly SETs and lesions not amenable to EMR or ESD due to fibrosis. EFTR involves the resection of all layers of the GIT from mucosa to serosa, and therefore is well-suited for SETs arising from the muscularis propria (MP).20
EFTR entails two main concepts: tissue resection and complete defect closure. Conventional EFTR consists of several steps, which include mucosal and submucosal pre-cutting, circumferential incision, and dissection through the MP or serosa. This results in a full thickness defect, for which closure of the wall defect is achieved using standard endoscopic clips or a combination of clips and endoloops or endoscopic suturing.21 For lesions less than 2 cm, EFTR can be performed in a single step using a cap-mounted full thickness resection device (FTRD). This results in deployment of over-the-scope clip over the target lesion followed by snaring the lesions above the clip.21
Location of the SET generally dictates the specific modality of ATR. For example, esophageal SETs may be more amenable to STER given that the lesion typically runs parallel with the lumen of the tubular esophagus, which allows for easier dissection without the need of full or partial retroflexion. While gastric SETs can be resected with STER, it may be challenging and more effectively addressed with EFTR, particularly when the entire lesion can be grasped into the full-thickness resection device.22 Limited data exists for duodenal EFTR, and colorectal SETs closure is particularly challenging.
Conclusion
It is key to emphasize that ATR cannot be safely established in practice without the incorporation of a multidisciplinary team (surgeons, radiologists, etc.), specialized tools, and trained personnel. This requires dedicated endoscopic rooms, careful patient selection, and a comprehensive approach to patient care before, during, and after these procedures.
Moreover, it is important to note that some patients may require post-procedure hospitalization for observation to ensure no early complications are encountered. Optimal surveillance strategies after ATR rely heavily on the potential for residual or recurrent disease, underlying pathology, and the expertise of the advanced endoscopist. As the field continues to evolve, ongoing research and technological advances of devices will further enhance the efficacy and safety of ATR in gastroenterology.
Dr. Madi (@MahMadi90) is based in the Division of Gastroenterology and Hepatology, Saint Louis University School of Medicine, Saint Louis, Missouri. Dr. Rengarajan (@ArvindRenga) and Dr. Bazarbashi (@AhmadBazarbashi) are based in the Division of Gastroenterology, Washington University in St. Louis. The authors have no conflicts of interest to disclose, and no funding was required for this project.
References
1. Copland AP, et al. AGA Clinical Practice Update on appropriate and tailored polypectomy: Expert review. Clin Gastroenterol Hepatol. 2024 Mar. doi: 10.1016/j.cgh.2023.10.012.
2. Lee SP, et al. Effect of preceding biopsy on the results of endoscopic submucosal dissection for colorectal laterally spreading tumor. Dig Dis Sci. 2019 Oct. doi: 10.1007/s10620-019-05625-3.
3. Medina-Prado L, et al. When and how to use endoscopic tattooing in the colon: An international Delphi agreement. Clin Gastroenterol Hepatol. 2021 May. doi: 10.1016/j.cgh.2021.01.024.
4. Rashid MU, et al. EMR and ESD: Indications, techniques and results. Surg Oncol. 2022 Aug. doi: 10.1016/j.suronc.2022.101742.
5. Castro R, et al. Solutions for submucosal injection: What to choose and how to do it. World J Gastroenterol. 2019 Feb. doi: 10.3748/wjg.v25.i7.777.
6. Rex DK. Best practices for resection of diminutive and small polyps in the colorectum. Gastrointest Endosc Clin N Am. 2019 Oct. doi: 10.1016/j.giec.2019.06.004.
7. Lv XH, et al. Underwater EMR for nonpedunculated colorectal lesions. Gastrointest Endosc. 2023 Apr. doi: 10.1016/j.gie.2022.10.044.
8. Fujiya M, et al. Efficacy and adverse events of EMR and endoscopic submucosal dissection for the treatment of colon neoplasms: a meta-analysis of studies comparing EMR and endoscopic submucosal dissection. Gastrointest Endosc. 2015 Mar. doi: 10.1016/j.gie.2014.07.034.
9. Kandel P, Wallace MB. Colorectal endoscopic mucosal resection (EMR). Best Pract Res Clin Gastroenterol. 2017 Aug. doi: 10.1016/j.bpg.2017.05.006.
10. Kemper G, et al; ENDOCARE Study Group. Endoscopic techniques to reduce recurrence rates after colorectal EMR: systematic review and meta-analysis. Surg Endosc. 2021 Oct. doi: 10.1007/s00464-021-08574-z.
11. Goto O, et al. Expanding indications for ESD: submucosal disease (SMT/carcinoid tumors). Gastrointest Endosc Clin N Am. 2014 Apr. doi: 10.1016/j.giec.2013.11.006.
12. Wang K, et al. Endoscopic full-thickness resection, indication, methods and perspectives. Dig Endosc. 2023 Jan. doi: 10.1111/den.14474.
13. Herreros de Tejada A. ESD training: A challenging path to excellence. World J Gastrointest Endosc. 2014 Apr 16. doi: 10.4253/wjge.v6.i4.112.
14. Chiba H, et al. Safety and efficacy of simultaneous colorectal ESD for large synchronous colorectal lesions. Endosc Int Open. 2017 Jul. doi: 10.1055/s-0043-110567.
15. Mannath J, Ragunath K. Endoscopic mucosal resection: who and how? Therap Adv Gastroenterol. 2011 Sep. doi: 10.1177/1756283X10388683.
16. Wang XY, et al. Hybrid endoscopic submucosal dissection: An alternative resection modality for large laterally spreading tumors in the cecum? BMC Gastroenterol. 2021 May. doi: 10.1186/s12876-021-01766-w.
17. McCarty TR, et al. Hybrid endoscopic submucosal dissection (ESD) compared with conventional ESD for colorectal lesions: a systematic review and meta-analysis. Endoscopy. 2021 Oct. doi: 10.1055/a-1266-1855.
18. Jain D, et al. Submucosal tunneling endoscopic resection of upper gastrointestinal tract tumors arising from muscularis propria. Ann Gastroenterol. 2017 Feb. doi: 10.20524/aog.2017.0128.
19. Lv XH, et al. Efficacy and safety of submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors: a systematic review and meta-analysis. Surg Endosc. 2017 Jan. doi: 10.1007/s00464-016-4978-7.
20. Cao B, et al. Efficacy and safety of submucosal tunneling endoscopic resection for gastric submucosal tumors: a systematic review and meta-analysis. Rev Esp Enferm Dig. 2021 Jan. doi: 10.17235/reed.2020.6989/2020.
21. Cai M, et al. Endoscopic full-thickness resection (EFTR) for gastrointestinal subepithelial tumors. Gastrointest Endosc Clin N Am. 2016 Apr. doi: 10.1016/j.giec.2015.12.013.
22. Brigic A, et al. A systematic review regarding the feasibility and safety of endoscopic full thickness resection (EFTR) for colonic lesions. Surg Endosc. 2013 Oct. doi: 10.1007/s00464-013-2946-z.
Endoscopists are often faced with unique challenges in the management and resection of various gastrointestinal tract lesions. These challenges could be lesion-related, endoscopist-related, or practice-related (see Table 1). (ATR). Not only does this organ-sparing approach offer a less invasive alternative to surgery, but it has also proved to have outcomes comparable to those of surgical standard of practice in specific scenarios. 
When Do You Refer to an Advanced Endoscopist?
One of the most critical steps in caring for patients with complex lesions is the ability to accurately determine whether a referral to an advanced endoscopist is warranted. The initial assessment of a lesion should always involve a careful assessment that risk stratifies the lesion depending on the location, size, neoplastic potential, and the feasibility of standard endoscopic resection compared to the need for surgical input.
A practical example in the case of colonic polyps is highlighted by the American Gastroenterology Association (AGA) guidelines recommending the referral of patients with polyps’ size ≥ 20 mm, challenging polypectomy location, or recurrent polyp at a prior polypectomy site to an endoscopic referral center.1 In the case of subepithelial lesions without endoscopic characteristics of benign etiology (i.e., lipomas, pancreatic rests, etc.), the threshold for referral to advanced endoscopists for further diagnostic testing by means of endoscopic ultrasonography or for therapeutic ATR should be lower.
Endoscopic tissue resection follows a spectrum, which often involves deeper layers of the gastrointestinal tract (GIT) as we progress along this spectrum (see Figure 1).

ATR, a term encompassing a variety of endoscopic techniques ranging from endoscopic mucosal resection to full thickness resection, has gained traction over the last years given the ability to effectively remove various lesions in a precise time and cost-effective manner while maintaining the integrity of the GIT and avoiding major surgery. The indications for ATR vary depending on the technique, but generally include the presence of large or poorly positioned lesions, particularly in high-risk areas of the GIT such as the esophagus and small intestine, lesions extending beyond the mucosal layer or originating from deeper layers, and when en bloc resection of select lesions is necessary.
For providers referring patients for ATR, we recommend a few important endoscopic pearls when caring for these patients.
1) Biopsy the lesion if there is concern for malignancy — While some studies have noted increased fibrosis during endoscopic submucosal dissection (ESD) and some guidelines recommend against biopsies pre ESD, we believe that when there is high pretest probability for malignancy, a biopsy should be obtained. This should involve the area that is most concerning for malignancy (at the margin or center).2
2) While marking a lesion with tattoo is helpful for surgical planning and for lesions difficult to locate endoscopically, we stress the importance of placing tattoos 3 to 5 centimeters distal to the lesion and avoiding tattooing the lesion itself, which has been shown to induce fibrosis and can make resection challenging. Based on an international Delphi consensus, expert recommendations on when and how to endoscopically tattoo a lesion can be instrumental in adequately localizing the lesion, allowing for endoscopic resection, and preventing unnecessary surgeries.3
3) If you encounter a lesion that you are not sure can be resected safely and efficaciously, we recommend against attempting resection that may result in partial resection. This can also induce fibrosis and scarring and limit future attempts at resection.
Endoscopic Mucosal Resection (EMR)
EMR is currently utilized for curative treatment of a wide array of GIT lesions limited to the mucosal layer, whether metaplastic, dysplastic, or even in cases with early mucosal cancer, where the risk of submucosal and lymphatic invasion is minimal.4 This makes EMR a versatile and proven therapy, often serving as the first-line treatment for many GIT lesions.
EMR has various techniques that could be categorized into suction or non-suction (lift and cut) techniques. In the suction technique, devices like multiband mucosectomy (MBM) are commonly used, especially in nodular Barrett’s dysplasia, forming a pseudopolyp for subsequent resection. The procedure is characterized by its safety, efficacy, and cost-effectiveness, contributing to its widespread adoption in clinical practice. In the lift and cut approach, a submucosal injection is utilized to separate the muscularis propria from the lesion, thereby reducing the risk of perforation. Different solutions, such as normal saline, hypertonic saline, 50% dextrose, or proprietary submucosal injection solutions, are employed for submucosal injection.5
The non-suction technique using a snare to resect polyps after injection is more often used in colonic and small intestinal EMR. Resection can be done via thermal energy in the form of cut or coagulation; however, there is rising data on the use of piecemeal cold snare resection for select flat polyps of the colon.6 There is also promising data on the role of underwater EMR, a common technique employed for colonic lesions, particularly if the lesion does not lift well with submucosal injection.7
Adverse events associated with EMR include bleeding (7%-8%) and perforation (0.9%-2%).8-9 Adequate submucosal fluid injection is crucial to prevent perforations. However, the main limitation of EMR is the piecemeal nature of resections for lesions larger than 20 mm, leading to compromised histopathologic evaluation for complete excision, especially in cases with superficial submucosal invasion (SMI). This can result in residual or recurrent tissue, reportedly 8% to 20%.10 Despite this limitation, EMR remains a reliable strategy, and recurrent lesions are generally manageable through repeat sessions. The importance of EMR as a therapeutic modality lies in its role in addressing lesions with favorable characteristics, where the risk of SMI is low.
Endoscopic Submucosal Dissection (ESD)
ESD is an evolving technique that can be utilized for submucosal lesions of the GIT, lesions not amenable to EMR due to submucosal fibrosis, when en bloc removal of a lesion is needed for accurate histopathological diagnosis, and when other techniques fail.11-12
ESD was only recently adopted in the United States, requires specialized training, and usually is a lengthier procedure than EMR.13 Compared to EMR, it has higher en bloc resection rates and lower recurrence rates, making it curative for lesions with superficial SMI and favorable histologic features.4,14 The safety profile of ESD appears favorable, with most of the adverse events managed successfully by endoscopic methods. Major complications include intraoperative and delayed perforation, intraoperative and delayed bleeding, aspiration pneumonia, thromboembolism, and stricture formation in the case of circumferential lesions.15
Despite being technically challenging, ESD may provide a cost-effective long-term solution by avoiding surgery, reducing the need for additional interventions by minimizing recurrence rates. Given the technical complexity of ESD, particularly the submucosal dissection portion, techniques such as hybrid ESD developed. Hybrid ESD combines snaring with circumferential mucosal incision and partial submucosal dissection. Although it promises shorter procedure times, reduced complication rates like perforation, and similar recurrence rates compared to traditional ESD, studies have shown lower success rates in en bloc resection.16-17
Both EMR and ESD are considered complementary strategies, and the choice between them should be dictated by lesion characteristics, patient preferences, and local expertise.
Submucosal Tunneling Endoscopic Resection (STER)
STER has emerged as a well-established technique for the endoscopic resection of GI subepithelial tumors (SETs) originating from the muscularis propria layer. The standard STER procedure involves a series of steps including submucosal elevation proximal to the SET, mucosotomy, creation of a submucosal tunnel, dissection of the SET within the tunnel, enucleation from the deep muscle layer, and subsequent specimen retrieval followed by mucosal closure.
This technique is typically recommended for SETs smaller than 3.5 cm, particularly those located in the mid or distal esophagus, cardia, or along the greater curvature of the gastric body.18 However, STER may pose technical challenges for larger SETs or lesions in anatomically difficult locations, where surgical resection is recommended instead.19 Notably, recent large-scale meta-analyses have showcased the favorable complete resection and en bloc resection rates of STER in treating GI SETs.20
Endoscopic Full Thickness Resection (EFTR)
EFTR has emerged as a valuable technique in the endoscopic management of gastrointestinal lesions, particularly SETs and lesions not amenable to EMR or ESD due to fibrosis. EFTR involves the resection of all layers of the GIT from mucosa to serosa, and therefore is well-suited for SETs arising from the muscularis propria (MP).20
EFTR entails two main concepts: tissue resection and complete defect closure. Conventional EFTR consists of several steps, which include mucosal and submucosal pre-cutting, circumferential incision, and dissection through the MP or serosa. This results in a full thickness defect, for which closure of the wall defect is achieved using standard endoscopic clips or a combination of clips and endoloops or endoscopic suturing.21 For lesions less than 2 cm, EFTR can be performed in a single step using a cap-mounted full thickness resection device (FTRD). This results in deployment of over-the-scope clip over the target lesion followed by snaring the lesions above the clip.21
Location of the SET generally dictates the specific modality of ATR. For example, esophageal SETs may be more amenable to STER given that the lesion typically runs parallel with the lumen of the tubular esophagus, which allows for easier dissection without the need of full or partial retroflexion. While gastric SETs can be resected with STER, it may be challenging and more effectively addressed with EFTR, particularly when the entire lesion can be grasped into the full-thickness resection device.22 Limited data exists for duodenal EFTR, and colorectal SETs closure is particularly challenging.
Conclusion
It is key to emphasize that ATR cannot be safely established in practice without the incorporation of a multidisciplinary team (surgeons, radiologists, etc.), specialized tools, and trained personnel. This requires dedicated endoscopic rooms, careful patient selection, and a comprehensive approach to patient care before, during, and after these procedures.
Moreover, it is important to note that some patients may require post-procedure hospitalization for observation to ensure no early complications are encountered. Optimal surveillance strategies after ATR rely heavily on the potential for residual or recurrent disease, underlying pathology, and the expertise of the advanced endoscopist. As the field continues to evolve, ongoing research and technological advances of devices will further enhance the efficacy and safety of ATR in gastroenterology.
Dr. Madi (@MahMadi90) is based in the Division of Gastroenterology and Hepatology, Saint Louis University School of Medicine, Saint Louis, Missouri. Dr. Rengarajan (@ArvindRenga) and Dr. Bazarbashi (@AhmadBazarbashi) are based in the Division of Gastroenterology, Washington University in St. Louis. The authors have no conflicts of interest to disclose, and no funding was required for this project.
References
1. Copland AP, et al. AGA Clinical Practice Update on appropriate and tailored polypectomy: Expert review. Clin Gastroenterol Hepatol. 2024 Mar. doi: 10.1016/j.cgh.2023.10.012.
2. Lee SP, et al. Effect of preceding biopsy on the results of endoscopic submucosal dissection for colorectal laterally spreading tumor. Dig Dis Sci. 2019 Oct. doi: 10.1007/s10620-019-05625-3.
3. Medina-Prado L, et al. When and how to use endoscopic tattooing in the colon: An international Delphi agreement. Clin Gastroenterol Hepatol. 2021 May. doi: 10.1016/j.cgh.2021.01.024.
4. Rashid MU, et al. EMR and ESD: Indications, techniques and results. Surg Oncol. 2022 Aug. doi: 10.1016/j.suronc.2022.101742.
5. Castro R, et al. Solutions for submucosal injection: What to choose and how to do it. World J Gastroenterol. 2019 Feb. doi: 10.3748/wjg.v25.i7.777.
6. Rex DK. Best practices for resection of diminutive and small polyps in the colorectum. Gastrointest Endosc Clin N Am. 2019 Oct. doi: 10.1016/j.giec.2019.06.004.
7. Lv XH, et al. Underwater EMR for nonpedunculated colorectal lesions. Gastrointest Endosc. 2023 Apr. doi: 10.1016/j.gie.2022.10.044.
8. Fujiya M, et al. Efficacy and adverse events of EMR and endoscopic submucosal dissection for the treatment of colon neoplasms: a meta-analysis of studies comparing EMR and endoscopic submucosal dissection. Gastrointest Endosc. 2015 Mar. doi: 10.1016/j.gie.2014.07.034.
9. Kandel P, Wallace MB. Colorectal endoscopic mucosal resection (EMR). Best Pract Res Clin Gastroenterol. 2017 Aug. doi: 10.1016/j.bpg.2017.05.006.
10. Kemper G, et al; ENDOCARE Study Group. Endoscopic techniques to reduce recurrence rates after colorectal EMR: systematic review and meta-analysis. Surg Endosc. 2021 Oct. doi: 10.1007/s00464-021-08574-z.
11. Goto O, et al. Expanding indications for ESD: submucosal disease (SMT/carcinoid tumors). Gastrointest Endosc Clin N Am. 2014 Apr. doi: 10.1016/j.giec.2013.11.006.
12. Wang K, et al. Endoscopic full-thickness resection, indication, methods and perspectives. Dig Endosc. 2023 Jan. doi: 10.1111/den.14474.
13. Herreros de Tejada A. ESD training: A challenging path to excellence. World J Gastrointest Endosc. 2014 Apr 16. doi: 10.4253/wjge.v6.i4.112.
14. Chiba H, et al. Safety and efficacy of simultaneous colorectal ESD for large synchronous colorectal lesions. Endosc Int Open. 2017 Jul. doi: 10.1055/s-0043-110567.
15. Mannath J, Ragunath K. Endoscopic mucosal resection: who and how? Therap Adv Gastroenterol. 2011 Sep. doi: 10.1177/1756283X10388683.
16. Wang XY, et al. Hybrid endoscopic submucosal dissection: An alternative resection modality for large laterally spreading tumors in the cecum? BMC Gastroenterol. 2021 May. doi: 10.1186/s12876-021-01766-w.
17. McCarty TR, et al. Hybrid endoscopic submucosal dissection (ESD) compared with conventional ESD for colorectal lesions: a systematic review and meta-analysis. Endoscopy. 2021 Oct. doi: 10.1055/a-1266-1855.
18. Jain D, et al. Submucosal tunneling endoscopic resection of upper gastrointestinal tract tumors arising from muscularis propria. Ann Gastroenterol. 2017 Feb. doi: 10.20524/aog.2017.0128.
19. Lv XH, et al. Efficacy and safety of submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors: a systematic review and meta-analysis. Surg Endosc. 2017 Jan. doi: 10.1007/s00464-016-4978-7.
20. Cao B, et al. Efficacy and safety of submucosal tunneling endoscopic resection for gastric submucosal tumors: a systematic review and meta-analysis. Rev Esp Enferm Dig. 2021 Jan. doi: 10.17235/reed.2020.6989/2020.
21. Cai M, et al. Endoscopic full-thickness resection (EFTR) for gastrointestinal subepithelial tumors. Gastrointest Endosc Clin N Am. 2016 Apr. doi: 10.1016/j.giec.2015.12.013.
22. Brigic A, et al. A systematic review regarding the feasibility and safety of endoscopic full thickness resection (EFTR) for colonic lesions. Surg Endosc. 2013 Oct. doi: 10.1007/s00464-013-2946-z.
Endoscopists are often faced with unique challenges in the management and resection of various gastrointestinal tract lesions. These challenges could be lesion-related, endoscopist-related, or practice-related (see Table 1). (ATR). Not only does this organ-sparing approach offer a less invasive alternative to surgery, but it has also proved to have outcomes comparable to those of surgical standard of practice in specific scenarios. 
When Do You Refer to an Advanced Endoscopist?
One of the most critical steps in caring for patients with complex lesions is the ability to accurately determine whether a referral to an advanced endoscopist is warranted. The initial assessment of a lesion should always involve a careful assessment that risk stratifies the lesion depending on the location, size, neoplastic potential, and the feasibility of standard endoscopic resection compared to the need for surgical input.
A practical example in the case of colonic polyps is highlighted by the American Gastroenterology Association (AGA) guidelines recommending the referral of patients with polyps’ size ≥ 20 mm, challenging polypectomy location, or recurrent polyp at a prior polypectomy site to an endoscopic referral center.1 In the case of subepithelial lesions without endoscopic characteristics of benign etiology (i.e., lipomas, pancreatic rests, etc.), the threshold for referral to advanced endoscopists for further diagnostic testing by means of endoscopic ultrasonography or for therapeutic ATR should be lower.
Endoscopic tissue resection follows a spectrum, which often involves deeper layers of the gastrointestinal tract (GIT) as we progress along this spectrum (see Figure 1).

ATR, a term encompassing a variety of endoscopic techniques ranging from endoscopic mucosal resection to full thickness resection, has gained traction over the last years given the ability to effectively remove various lesions in a precise time and cost-effective manner while maintaining the integrity of the GIT and avoiding major surgery. The indications for ATR vary depending on the technique, but generally include the presence of large or poorly positioned lesions, particularly in high-risk areas of the GIT such as the esophagus and small intestine, lesions extending beyond the mucosal layer or originating from deeper layers, and when en bloc resection of select lesions is necessary.
For providers referring patients for ATR, we recommend a few important endoscopic pearls when caring for these patients.
1) Biopsy the lesion if there is concern for malignancy — While some studies have noted increased fibrosis during endoscopic submucosal dissection (ESD) and some guidelines recommend against biopsies pre ESD, we believe that when there is high pretest probability for malignancy, a biopsy should be obtained. This should involve the area that is most concerning for malignancy (at the margin or center).2
2) While marking a lesion with tattoo is helpful for surgical planning and for lesions difficult to locate endoscopically, we stress the importance of placing tattoos 3 to 5 centimeters distal to the lesion and avoiding tattooing the lesion itself, which has been shown to induce fibrosis and can make resection challenging. Based on an international Delphi consensus, expert recommendations on when and how to endoscopically tattoo a lesion can be instrumental in adequately localizing the lesion, allowing for endoscopic resection, and preventing unnecessary surgeries.3
3) If you encounter a lesion that you are not sure can be resected safely and efficaciously, we recommend against attempting resection that may result in partial resection. This can also induce fibrosis and scarring and limit future attempts at resection.
Endoscopic Mucosal Resection (EMR)
EMR is currently utilized for curative treatment of a wide array of GIT lesions limited to the mucosal layer, whether metaplastic, dysplastic, or even in cases with early mucosal cancer, where the risk of submucosal and lymphatic invasion is minimal.4 This makes EMR a versatile and proven therapy, often serving as the first-line treatment for many GIT lesions.
EMR has various techniques that could be categorized into suction or non-suction (lift and cut) techniques. In the suction technique, devices like multiband mucosectomy (MBM) are commonly used, especially in nodular Barrett’s dysplasia, forming a pseudopolyp for subsequent resection. The procedure is characterized by its safety, efficacy, and cost-effectiveness, contributing to its widespread adoption in clinical practice. In the lift and cut approach, a submucosal injection is utilized to separate the muscularis propria from the lesion, thereby reducing the risk of perforation. Different solutions, such as normal saline, hypertonic saline, 50% dextrose, or proprietary submucosal injection solutions, are employed for submucosal injection.5
The non-suction technique using a snare to resect polyps after injection is more often used in colonic and small intestinal EMR. Resection can be done via thermal energy in the form of cut or coagulation; however, there is rising data on the use of piecemeal cold snare resection for select flat polyps of the colon.6 There is also promising data on the role of underwater EMR, a common technique employed for colonic lesions, particularly if the lesion does not lift well with submucosal injection.7
Adverse events associated with EMR include bleeding (7%-8%) and perforation (0.9%-2%).8-9 Adequate submucosal fluid injection is crucial to prevent perforations. However, the main limitation of EMR is the piecemeal nature of resections for lesions larger than 20 mm, leading to compromised histopathologic evaluation for complete excision, especially in cases with superficial submucosal invasion (SMI). This can result in residual or recurrent tissue, reportedly 8% to 20%.10 Despite this limitation, EMR remains a reliable strategy, and recurrent lesions are generally manageable through repeat sessions. The importance of EMR as a therapeutic modality lies in its role in addressing lesions with favorable characteristics, where the risk of SMI is low.
Endoscopic Submucosal Dissection (ESD)
ESD is an evolving technique that can be utilized for submucosal lesions of the GIT, lesions not amenable to EMR due to submucosal fibrosis, when en bloc removal of a lesion is needed for accurate histopathological diagnosis, and when other techniques fail.11-12
ESD was only recently adopted in the United States, requires specialized training, and usually is a lengthier procedure than EMR.13 Compared to EMR, it has higher en bloc resection rates and lower recurrence rates, making it curative for lesions with superficial SMI and favorable histologic features.4,14 The safety profile of ESD appears favorable, with most of the adverse events managed successfully by endoscopic methods. Major complications include intraoperative and delayed perforation, intraoperative and delayed bleeding, aspiration pneumonia, thromboembolism, and stricture formation in the case of circumferential lesions.15
Despite being technically challenging, ESD may provide a cost-effective long-term solution by avoiding surgery, reducing the need for additional interventions by minimizing recurrence rates. Given the technical complexity of ESD, particularly the submucosal dissection portion, techniques such as hybrid ESD developed. Hybrid ESD combines snaring with circumferential mucosal incision and partial submucosal dissection. Although it promises shorter procedure times, reduced complication rates like perforation, and similar recurrence rates compared to traditional ESD, studies have shown lower success rates in en bloc resection.16-17
Both EMR and ESD are considered complementary strategies, and the choice between them should be dictated by lesion characteristics, patient preferences, and local expertise.
Submucosal Tunneling Endoscopic Resection (STER)
STER has emerged as a well-established technique for the endoscopic resection of GI subepithelial tumors (SETs) originating from the muscularis propria layer. The standard STER procedure involves a series of steps including submucosal elevation proximal to the SET, mucosotomy, creation of a submucosal tunnel, dissection of the SET within the tunnel, enucleation from the deep muscle layer, and subsequent specimen retrieval followed by mucosal closure.
This technique is typically recommended for SETs smaller than 3.5 cm, particularly those located in the mid or distal esophagus, cardia, or along the greater curvature of the gastric body.18 However, STER may pose technical challenges for larger SETs or lesions in anatomically difficult locations, where surgical resection is recommended instead.19 Notably, recent large-scale meta-analyses have showcased the favorable complete resection and en bloc resection rates of STER in treating GI SETs.20
Endoscopic Full Thickness Resection (EFTR)
EFTR has emerged as a valuable technique in the endoscopic management of gastrointestinal lesions, particularly SETs and lesions not amenable to EMR or ESD due to fibrosis. EFTR involves the resection of all layers of the GIT from mucosa to serosa, and therefore is well-suited for SETs arising from the muscularis propria (MP).20
EFTR entails two main concepts: tissue resection and complete defect closure. Conventional EFTR consists of several steps, which include mucosal and submucosal pre-cutting, circumferential incision, and dissection through the MP or serosa. This results in a full thickness defect, for which closure of the wall defect is achieved using standard endoscopic clips or a combination of clips and endoloops or endoscopic suturing.21 For lesions less than 2 cm, EFTR can be performed in a single step using a cap-mounted full thickness resection device (FTRD). This results in deployment of over-the-scope clip over the target lesion followed by snaring the lesions above the clip.21
Location of the SET generally dictates the specific modality of ATR. For example, esophageal SETs may be more amenable to STER given that the lesion typically runs parallel with the lumen of the tubular esophagus, which allows for easier dissection without the need of full or partial retroflexion. While gastric SETs can be resected with STER, it may be challenging and more effectively addressed with EFTR, particularly when the entire lesion can be grasped into the full-thickness resection device.22 Limited data exists for duodenal EFTR, and colorectal SETs closure is particularly challenging.
Conclusion
It is key to emphasize that ATR cannot be safely established in practice without the incorporation of a multidisciplinary team (surgeons, radiologists, etc.), specialized tools, and trained personnel. This requires dedicated endoscopic rooms, careful patient selection, and a comprehensive approach to patient care before, during, and after these procedures.
Moreover, it is important to note that some patients may require post-procedure hospitalization for observation to ensure no early complications are encountered. Optimal surveillance strategies after ATR rely heavily on the potential for residual or recurrent disease, underlying pathology, and the expertise of the advanced endoscopist. As the field continues to evolve, ongoing research and technological advances of devices will further enhance the efficacy and safety of ATR in gastroenterology.
Dr. Madi (@MahMadi90) is based in the Division of Gastroenterology and Hepatology, Saint Louis University School of Medicine, Saint Louis, Missouri. Dr. Rengarajan (@ArvindRenga) and Dr. Bazarbashi (@AhmadBazarbashi) are based in the Division of Gastroenterology, Washington University in St. Louis. The authors have no conflicts of interest to disclose, and no funding was required for this project.
References
1. Copland AP, et al. AGA Clinical Practice Update on appropriate and tailored polypectomy: Expert review. Clin Gastroenterol Hepatol. 2024 Mar. doi: 10.1016/j.cgh.2023.10.012.
2. Lee SP, et al. Effect of preceding biopsy on the results of endoscopic submucosal dissection for colorectal laterally spreading tumor. Dig Dis Sci. 2019 Oct. doi: 10.1007/s10620-019-05625-3.
3. Medina-Prado L, et al. When and how to use endoscopic tattooing in the colon: An international Delphi agreement. Clin Gastroenterol Hepatol. 2021 May. doi: 10.1016/j.cgh.2021.01.024.
4. Rashid MU, et al. EMR and ESD: Indications, techniques and results. Surg Oncol. 2022 Aug. doi: 10.1016/j.suronc.2022.101742.
5. Castro R, et al. Solutions for submucosal injection: What to choose and how to do it. World J Gastroenterol. 2019 Feb. doi: 10.3748/wjg.v25.i7.777.
6. Rex DK. Best practices for resection of diminutive and small polyps in the colorectum. Gastrointest Endosc Clin N Am. 2019 Oct. doi: 10.1016/j.giec.2019.06.004.
7. Lv XH, et al. Underwater EMR for nonpedunculated colorectal lesions. Gastrointest Endosc. 2023 Apr. doi: 10.1016/j.gie.2022.10.044.
8. Fujiya M, et al. Efficacy and adverse events of EMR and endoscopic submucosal dissection for the treatment of colon neoplasms: a meta-analysis of studies comparing EMR and endoscopic submucosal dissection. Gastrointest Endosc. 2015 Mar. doi: 10.1016/j.gie.2014.07.034.
9. Kandel P, Wallace MB. Colorectal endoscopic mucosal resection (EMR). Best Pract Res Clin Gastroenterol. 2017 Aug. doi: 10.1016/j.bpg.2017.05.006.
10. Kemper G, et al; ENDOCARE Study Group. Endoscopic techniques to reduce recurrence rates after colorectal EMR: systematic review and meta-analysis. Surg Endosc. 2021 Oct. doi: 10.1007/s00464-021-08574-z.
11. Goto O, et al. Expanding indications for ESD: submucosal disease (SMT/carcinoid tumors). Gastrointest Endosc Clin N Am. 2014 Apr. doi: 10.1016/j.giec.2013.11.006.
12. Wang K, et al. Endoscopic full-thickness resection, indication, methods and perspectives. Dig Endosc. 2023 Jan. doi: 10.1111/den.14474.
13. Herreros de Tejada A. ESD training: A challenging path to excellence. World J Gastrointest Endosc. 2014 Apr 16. doi: 10.4253/wjge.v6.i4.112.
14. Chiba H, et al. Safety and efficacy of simultaneous colorectal ESD for large synchronous colorectal lesions. Endosc Int Open. 2017 Jul. doi: 10.1055/s-0043-110567.
15. Mannath J, Ragunath K. Endoscopic mucosal resection: who and how? Therap Adv Gastroenterol. 2011 Sep. doi: 10.1177/1756283X10388683.
16. Wang XY, et al. Hybrid endoscopic submucosal dissection: An alternative resection modality for large laterally spreading tumors in the cecum? BMC Gastroenterol. 2021 May. doi: 10.1186/s12876-021-01766-w.
17. McCarty TR, et al. Hybrid endoscopic submucosal dissection (ESD) compared with conventional ESD for colorectal lesions: a systematic review and meta-analysis. Endoscopy. 2021 Oct. doi: 10.1055/a-1266-1855.
18. Jain D, et al. Submucosal tunneling endoscopic resection of upper gastrointestinal tract tumors arising from muscularis propria. Ann Gastroenterol. 2017 Feb. doi: 10.20524/aog.2017.0128.
19. Lv XH, et al. Efficacy and safety of submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors: a systematic review and meta-analysis. Surg Endosc. 2017 Jan. doi: 10.1007/s00464-016-4978-7.
20. Cao B, et al. Efficacy and safety of submucosal tunneling endoscopic resection for gastric submucosal tumors: a systematic review and meta-analysis. Rev Esp Enferm Dig. 2021 Jan. doi: 10.17235/reed.2020.6989/2020.
21. Cai M, et al. Endoscopic full-thickness resection (EFTR) for gastrointestinal subepithelial tumors. Gastrointest Endosc Clin N Am. 2016 Apr. doi: 10.1016/j.giec.2015.12.013.
22. Brigic A, et al. A systematic review regarding the feasibility and safety of endoscopic full thickness resection (EFTR) for colonic lesions. Surg Endosc. 2013 Oct. doi: 10.1007/s00464-013-2946-z.
A Simplified Approach to Pelvic Floor Dysfunction
Pelvic floor dysfunction (PFD) represents a spectrum of symptoms involving sensory and emptying abnormalities of the bowel and bladder and pelvic organ prolapse. The pelvic floor refers to a group of muscles that spans the pelvic outlet, providing support to the pelvic organs and coordinating constrictor mechanisms to control urination and defecation. Symptoms reported by patients experiencing PFD include involuntary loss of stool or urine, incomplete emptying of the bowel and bladder, a sensation of fullness, bulging in the vagina, and sexual dysfunction.1
As such, symptoms related to PFD are very common concerns raised by patients to their gastroenterologists. Data from the National Health and Nutrition Examination Survey show that 23.7% of women over the age of 20 had at least one symptom of PFD.2 Unfortunately, patients experiencing pelvic floor dysfunction often are hesitant to seek care because of embarrassment or perception that limited treatment options exist for their symptoms.
Pelvic Floor Anatomy
Regions of the pelvis are often referred to by anatomic compartment: anterior (bladder and urethra), middle (vagina and uterus or prostate), and posterior (colon, rectum, and anal canal). Supporting these compartments is the levator ani, a muscle group that is used synonymously with the term “pelvic diaphragm.”
Continence of stool is provided by the anal sphincter muscles and the puborectalis muscle, which wraps around the posterior aspect of the anorectal canal. Damage to the musculature or sensory perception to this area may result in fecal incontinence. Defecation is a coordinated process during which the abdominal and rectal muscles contract, while the anal sphincter muscles and puborectalis simultaneously relax. A disturbance in neuromuscular coordination (dyssynergic defecation) or structural pathology such as pelvic organ prolapse may lead to obstructed defecation.
PFD is thought to be a result of one or more insults to the pelvic floor such as chronic straining, childbirth, iatrogenic injury, or systemic disease such as diabetes.3
Evaluation of PFD Symptoms
Patients presenting with suspected PFD necessitate a comprehensive interdisciplinary assessment. In addition to obtaining a medical, surgical, and obstetric history, details about symptoms and lifestyle should include toileting habits, diet, and physical activity. The Pelvic Floor Distress Inventory (PFDI-20) is a commonly used tool that can be employed in the clinical setting.4
A pelvic exam can reveal pelvic organ prolapse and other mucosal pathology. The Pelvic Organ Prolapse Quantification System (POP-Q) is a widely used classification system for describing pelvic organ prolapse.5 Protrusion of the rectal wall into the vagina is referred to as a rectocele, while prolapse of small bowel into the upper posterior wall of the vagina is called an enterocele. While the finding of a rectocele on exam is common in parous women and may not cause any symptoms, a larger rectocele may cause a sensation of incomplete evacuation of stool.
A digital rectal exam (DRE) should be performed to assess pelvic floor function and help identify structural abnormalities.
Initial Management
A stepwise approach to the management of PFD can allow many patients to be effectively treated without the need for surgical intervention. For patients reporting liquid stool consistency, the evaluation should pivot toward the workup and management of diarrhea, which can easily overwhelm continence mechanisms and cause fecal incontinence. Fiber supplementation to normalize stool consistency is considered first-line therapy for patients presenting with both fecal incontinence and obstructed defecation. Other tools for fecal incontinence include avoiding foods that trigger diarrhea and use of loperamide.6 For patients with obstructed defecation, a trial of laxatives can be followed by a prescription agent if needed, such as a secretagogue or prokinetic.7
Vaginal splinting is a technique that can be used in patients with rectocele, whereby a finger is inserted into the vagina and pressure is applied on the posterior vaginal wall toward the rectum. Reducing the rectocele can facilitate emptying stool from the rectum and prevent leakage of retained stool.8 Similarly, use of rectal irrigation enemas can also help clear retained stool.
Pelvic floor physical therapists examine the strength, coordination, and tone of the pelvic floor muscles. When hypertonic musculature is present, manual interventions may be performed including trigger point release, myofascial release, and dry needling.9 When hypotonic musculature or dyssynergia is present, strengthening and neuromuscular re-education are recommended. Biofeedback can be administered via surface electromyography and/or balloon training to improve rectal sensitivity. Proper defecation techniques, including positioning, breathing, and behavioral modifications, improve clinical outcomes.
Diagnostic Testing
For patients who do not improve with conservative management, further testing is recommended to characterize the underlying pathology. Typically, anorectal manometry (ARM) is performed in conjunction with the balloon expulsion test and imaging. Each modality has its strengths and limitations (see Table 1).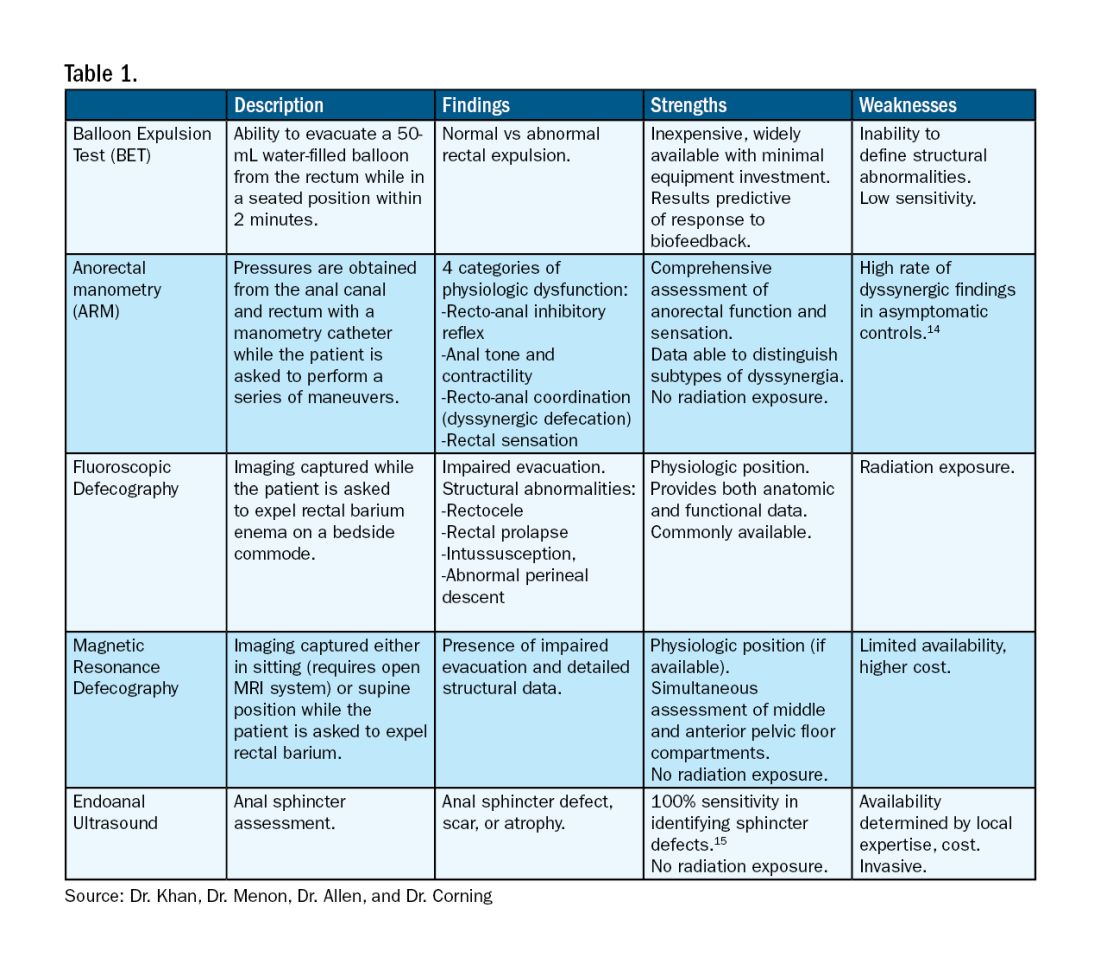
ARM allows for the assessment of rectal sensation and recto-anal pressures and coordination.10
Dynamic imaging, by barium defecography under fluoroscopy or MRI, captures anatomy at rest and with simulated defecation to identify pelvic organ prolapse, compartmental defects, and organ mobility.11 Endoanal ultrasonography is considered in patients experiencing fecal incontinence to evaluate the integrity of the anal sphincter muscles.
Minimally Invasive Procedures and Surgical Options for PFD
Functional abnormalities such as dyssynergia often coexist with structural abnormalities. Because structural abnormalities are commonly found in asymptomatic patients, noninvasive functional therapy, such as pelvic floor physical therapy and anorectal biofeedback, are preferred prior to surgical repair of a structural finding. For patients with fecal incontinence, sacral nerve stimulation (SNS) has emerged as a preferred therapy due to demonstrated efficacy in symptom improvement.12 Sphincteroplasty is reserved for those with acute sphincter injury or failure of SNS.
In patients with findings of intussusception, prolapse, or rectocele that have not responded to conservative therapy, referral for surgical repair may be considered. While the specific surgical approach will depend on many factors, the goal is typically excision and/or suspension of rectal tissue and reinforcement of the rectovaginal septum.
It is critical that we are equipped with the available knowledge and tools to provide these patients with optimal care.
Dr. Khan, Dr. Menon, Dr. Allen, and Dr. Corning are based at the University of Texas Medical Branch in Galveston, Texas. They report no conflicts of interest.
References
1. Grimes WR and Stratton M. Pelvic floor dysfunction. 2023 Jun 26. In: StatPearls [Internet]. Treasure Island (Fla.): StatPearls Publishing; 2024 Jan. PMID: 32644672.
2. Nygaard I et al. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008 Sep 17. doi: 10.1001/jama.300.11.1311.
3. Lawrence JM et al. Pelvic floor disorders, diabetes, and obesity in women: Findings from the Kaiser Permanente Continence Associated Risk Epidemiology Study. Diabetes Care. 2007 Oct. doi: 10.2337/dc07-0262.
4. Barber MD et al. Short forms of two condition-specific quality-of-life questionnaires for women with pelvic floor disorders (PFDI-20 and PFIQ-7). Am J Obstet Gynecol. 2005 Jul. doi: 10.1016/j.ajog.2004.12.025.
5. Persu C et al. Pelvic Organ Prolapse Quantification System (POP-Q) — A new era in pelvic prolapse staging. J Med Life. 2011 Jan-Mar. PMID: 21505577.
6. Wald A et al. ACG Clinical Guidelines: Management of benign anorectal disorders. Am J Gastroenterol. 2021 Oct 1. doi: 10.14309/ajg.0000000000001507.
7. Bharucha AE and Lacy BE. Mechanisms, evaluation, and management of chronic constipation. Gastroenterology. 2020 Apr. doi: 10.1053/j.gastro.2019.12.034.
8. Menees S and Chey WD. Fecal incontinence: Pathogenesis, diagnosis, and updated treatment strategies. Gastroenterol Clin North Am. 2022 Mar. doi: 10.1016/j.gtc.2021.10.005.
9. Wallace SL et al. Pelvic floor physical therapy in the treatment of pelvic floor dysfunction in women. Curr Opin Obstet Gynecol. 2019 Dec. doi: 10.1097/GCO.0000000000000584.
10. Carrington EV et al. The international anorectal physiology working group (IAPWG) recommendations: Standardized testing protocol and the London classification for disorders of anorectal function. Neurogastroenterol Motil. 2020 Jan. doi: 10.1111/nmo.13679.
11. El Sayed RF et al. Magnetic resonance imaging of pelvic floor dysfunction — Joint recommendations of the ESUR and ESGAR Pelvic Floor Working Group. Eur Radiol. 2017 May. doi: 10.1007/s00330-016-4471-7.
12. Thaha MA et al. Sacral nerve stimulation for faecal incontinence and constipation in adults. Cochrane Database Syst Rev. 2015 Aug 24. doi: 10.1002/14651858.CD004464.pub3.
13. Chiarioni G et al. Biofeedback benefits only patients with outlet dysfunction, not patients with isolated slow transit constipation. Gastroenterology. 2005 Jul. doi: 10.1053/j.gastro.2005.05.015.
14. Grossi U et al. Diagnostic accuracy study of anorectal manometry for diagnosis of dyssynergic defecation. Gut. 2016 Mar. doi: 10.1136/gutjnl-2014-308835.
15. Albuquerque A. Endoanal ultrasonography in fecal incontinence: Current and future perspectives. World J Gastrointest Endosc. 2015 Jun 10. doi: 10.4253/wjge.v7.i6.575.
Pelvic floor dysfunction (PFD) represents a spectrum of symptoms involving sensory and emptying abnormalities of the bowel and bladder and pelvic organ prolapse. The pelvic floor refers to a group of muscles that spans the pelvic outlet, providing support to the pelvic organs and coordinating constrictor mechanisms to control urination and defecation. Symptoms reported by patients experiencing PFD include involuntary loss of stool or urine, incomplete emptying of the bowel and bladder, a sensation of fullness, bulging in the vagina, and sexual dysfunction.1
As such, symptoms related to PFD are very common concerns raised by patients to their gastroenterologists. Data from the National Health and Nutrition Examination Survey show that 23.7% of women over the age of 20 had at least one symptom of PFD.2 Unfortunately, patients experiencing pelvic floor dysfunction often are hesitant to seek care because of embarrassment or perception that limited treatment options exist for their symptoms.
Pelvic Floor Anatomy
Regions of the pelvis are often referred to by anatomic compartment: anterior (bladder and urethra), middle (vagina and uterus or prostate), and posterior (colon, rectum, and anal canal). Supporting these compartments is the levator ani, a muscle group that is used synonymously with the term “pelvic diaphragm.”
Continence of stool is provided by the anal sphincter muscles and the puborectalis muscle, which wraps around the posterior aspect of the anorectal canal. Damage to the musculature or sensory perception to this area may result in fecal incontinence. Defecation is a coordinated process during which the abdominal and rectal muscles contract, while the anal sphincter muscles and puborectalis simultaneously relax. A disturbance in neuromuscular coordination (dyssynergic defecation) or structural pathology such as pelvic organ prolapse may lead to obstructed defecation.
PFD is thought to be a result of one or more insults to the pelvic floor such as chronic straining, childbirth, iatrogenic injury, or systemic disease such as diabetes.3
Evaluation of PFD Symptoms
Patients presenting with suspected PFD necessitate a comprehensive interdisciplinary assessment. In addition to obtaining a medical, surgical, and obstetric history, details about symptoms and lifestyle should include toileting habits, diet, and physical activity. The Pelvic Floor Distress Inventory (PFDI-20) is a commonly used tool that can be employed in the clinical setting.4
A pelvic exam can reveal pelvic organ prolapse and other mucosal pathology. The Pelvic Organ Prolapse Quantification System (POP-Q) is a widely used classification system for describing pelvic organ prolapse.5 Protrusion of the rectal wall into the vagina is referred to as a rectocele, while prolapse of small bowel into the upper posterior wall of the vagina is called an enterocele. While the finding of a rectocele on exam is common in parous women and may not cause any symptoms, a larger rectocele may cause a sensation of incomplete evacuation of stool.
A digital rectal exam (DRE) should be performed to assess pelvic floor function and help identify structural abnormalities.
Initial Management
A stepwise approach to the management of PFD can allow many patients to be effectively treated without the need for surgical intervention. For patients reporting liquid stool consistency, the evaluation should pivot toward the workup and management of diarrhea, which can easily overwhelm continence mechanisms and cause fecal incontinence. Fiber supplementation to normalize stool consistency is considered first-line therapy for patients presenting with both fecal incontinence and obstructed defecation. Other tools for fecal incontinence include avoiding foods that trigger diarrhea and use of loperamide.6 For patients with obstructed defecation, a trial of laxatives can be followed by a prescription agent if needed, such as a secretagogue or prokinetic.7
Vaginal splinting is a technique that can be used in patients with rectocele, whereby a finger is inserted into the vagina and pressure is applied on the posterior vaginal wall toward the rectum. Reducing the rectocele can facilitate emptying stool from the rectum and prevent leakage of retained stool.8 Similarly, use of rectal irrigation enemas can also help clear retained stool.
Pelvic floor physical therapists examine the strength, coordination, and tone of the pelvic floor muscles. When hypertonic musculature is present, manual interventions may be performed including trigger point release, myofascial release, and dry needling.9 When hypotonic musculature or dyssynergia is present, strengthening and neuromuscular re-education are recommended. Biofeedback can be administered via surface electromyography and/or balloon training to improve rectal sensitivity. Proper defecation techniques, including positioning, breathing, and behavioral modifications, improve clinical outcomes.
Diagnostic Testing
For patients who do not improve with conservative management, further testing is recommended to characterize the underlying pathology. Typically, anorectal manometry (ARM) is performed in conjunction with the balloon expulsion test and imaging. Each modality has its strengths and limitations (see Table 1).
ARM allows for the assessment of rectal sensation and recto-anal pressures and coordination.10
Dynamic imaging, by barium defecography under fluoroscopy or MRI, captures anatomy at rest and with simulated defecation to identify pelvic organ prolapse, compartmental defects, and organ mobility.11 Endoanal ultrasonography is considered in patients experiencing fecal incontinence to evaluate the integrity of the anal sphincter muscles.
Minimally Invasive Procedures and Surgical Options for PFD
Functional abnormalities such as dyssynergia often coexist with structural abnormalities. Because structural abnormalities are commonly found in asymptomatic patients, noninvasive functional therapy, such as pelvic floor physical therapy and anorectal biofeedback, are preferred prior to surgical repair of a structural finding. For patients with fecal incontinence, sacral nerve stimulation (SNS) has emerged as a preferred therapy due to demonstrated efficacy in symptom improvement.12 Sphincteroplasty is reserved for those with acute sphincter injury or failure of SNS.
In patients with findings of intussusception, prolapse, or rectocele that have not responded to conservative therapy, referral for surgical repair may be considered. While the specific surgical approach will depend on many factors, the goal is typically excision and/or suspension of rectal tissue and reinforcement of the rectovaginal septum.
It is critical that we are equipped with the available knowledge and tools to provide these patients with optimal care.
Dr. Khan, Dr. Menon, Dr. Allen, and Dr. Corning are based at the University of Texas Medical Branch in Galveston, Texas. They report no conflicts of interest.
References
1. Grimes WR and Stratton M. Pelvic floor dysfunction. 2023 Jun 26. In: StatPearls [Internet]. Treasure Island (Fla.): StatPearls Publishing; 2024 Jan. PMID: 32644672.
2. Nygaard I et al. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008 Sep 17. doi: 10.1001/jama.300.11.1311.
3. Lawrence JM et al. Pelvic floor disorders, diabetes, and obesity in women: Findings from the Kaiser Permanente Continence Associated Risk Epidemiology Study. Diabetes Care. 2007 Oct. doi: 10.2337/dc07-0262.
4. Barber MD et al. Short forms of two condition-specific quality-of-life questionnaires for women with pelvic floor disorders (PFDI-20 and PFIQ-7). Am J Obstet Gynecol. 2005 Jul. doi: 10.1016/j.ajog.2004.12.025.
5. Persu C et al. Pelvic Organ Prolapse Quantification System (POP-Q) — A new era in pelvic prolapse staging. J Med Life. 2011 Jan-Mar. PMID: 21505577.
6. Wald A et al. ACG Clinical Guidelines: Management of benign anorectal disorders. Am J Gastroenterol. 2021 Oct 1. doi: 10.14309/ajg.0000000000001507.
7. Bharucha AE and Lacy BE. Mechanisms, evaluation, and management of chronic constipation. Gastroenterology. 2020 Apr. doi: 10.1053/j.gastro.2019.12.034.
8. Menees S and Chey WD. Fecal incontinence: Pathogenesis, diagnosis, and updated treatment strategies. Gastroenterol Clin North Am. 2022 Mar. doi: 10.1016/j.gtc.2021.10.005.
9. Wallace SL et al. Pelvic floor physical therapy in the treatment of pelvic floor dysfunction in women. Curr Opin Obstet Gynecol. 2019 Dec. doi: 10.1097/GCO.0000000000000584.
10. Carrington EV et al. The international anorectal physiology working group (IAPWG) recommendations: Standardized testing protocol and the London classification for disorders of anorectal function. Neurogastroenterol Motil. 2020 Jan. doi: 10.1111/nmo.13679.
11. El Sayed RF et al. Magnetic resonance imaging of pelvic floor dysfunction — Joint recommendations of the ESUR and ESGAR Pelvic Floor Working Group. Eur Radiol. 2017 May. doi: 10.1007/s00330-016-4471-7.
12. Thaha MA et al. Sacral nerve stimulation for faecal incontinence and constipation in adults. Cochrane Database Syst Rev. 2015 Aug 24. doi: 10.1002/14651858.CD004464.pub3.
13. Chiarioni G et al. Biofeedback benefits only patients with outlet dysfunction, not patients with isolated slow transit constipation. Gastroenterology. 2005 Jul. doi: 10.1053/j.gastro.2005.05.015.
14. Grossi U et al. Diagnostic accuracy study of anorectal manometry for diagnosis of dyssynergic defecation. Gut. 2016 Mar. doi: 10.1136/gutjnl-2014-308835.
15. Albuquerque A. Endoanal ultrasonography in fecal incontinence: Current and future perspectives. World J Gastrointest Endosc. 2015 Jun 10. doi: 10.4253/wjge.v7.i6.575.
Pelvic floor dysfunction (PFD) represents a spectrum of symptoms involving sensory and emptying abnormalities of the bowel and bladder and pelvic organ prolapse. The pelvic floor refers to a group of muscles that spans the pelvic outlet, providing support to the pelvic organs and coordinating constrictor mechanisms to control urination and defecation. Symptoms reported by patients experiencing PFD include involuntary loss of stool or urine, incomplete emptying of the bowel and bladder, a sensation of fullness, bulging in the vagina, and sexual dysfunction.1
As such, symptoms related to PFD are very common concerns raised by patients to their gastroenterologists. Data from the National Health and Nutrition Examination Survey show that 23.7% of women over the age of 20 had at least one symptom of PFD.2 Unfortunately, patients experiencing pelvic floor dysfunction often are hesitant to seek care because of embarrassment or perception that limited treatment options exist for their symptoms.
Pelvic Floor Anatomy
Regions of the pelvis are often referred to by anatomic compartment: anterior (bladder and urethra), middle (vagina and uterus or prostate), and posterior (colon, rectum, and anal canal). Supporting these compartments is the levator ani, a muscle group that is used synonymously with the term “pelvic diaphragm.”
Continence of stool is provided by the anal sphincter muscles and the puborectalis muscle, which wraps around the posterior aspect of the anorectal canal. Damage to the musculature or sensory perception to this area may result in fecal incontinence. Defecation is a coordinated process during which the abdominal and rectal muscles contract, while the anal sphincter muscles and puborectalis simultaneously relax. A disturbance in neuromuscular coordination (dyssynergic defecation) or structural pathology such as pelvic organ prolapse may lead to obstructed defecation.
PFD is thought to be a result of one or more insults to the pelvic floor such as chronic straining, childbirth, iatrogenic injury, or systemic disease such as diabetes.3
Evaluation of PFD Symptoms
Patients presenting with suspected PFD necessitate a comprehensive interdisciplinary assessment. In addition to obtaining a medical, surgical, and obstetric history, details about symptoms and lifestyle should include toileting habits, diet, and physical activity. The Pelvic Floor Distress Inventory (PFDI-20) is a commonly used tool that can be employed in the clinical setting.4
A pelvic exam can reveal pelvic organ prolapse and other mucosal pathology. The Pelvic Organ Prolapse Quantification System (POP-Q) is a widely used classification system for describing pelvic organ prolapse.5 Protrusion of the rectal wall into the vagina is referred to as a rectocele, while prolapse of small bowel into the upper posterior wall of the vagina is called an enterocele. While the finding of a rectocele on exam is common in parous women and may not cause any symptoms, a larger rectocele may cause a sensation of incomplete evacuation of stool.
A digital rectal exam (DRE) should be performed to assess pelvic floor function and help identify structural abnormalities.
Initial Management
A stepwise approach to the management of PFD can allow many patients to be effectively treated without the need for surgical intervention. For patients reporting liquid stool consistency, the evaluation should pivot toward the workup and management of diarrhea, which can easily overwhelm continence mechanisms and cause fecal incontinence. Fiber supplementation to normalize stool consistency is considered first-line therapy for patients presenting with both fecal incontinence and obstructed defecation. Other tools for fecal incontinence include avoiding foods that trigger diarrhea and use of loperamide.6 For patients with obstructed defecation, a trial of laxatives can be followed by a prescription agent if needed, such as a secretagogue or prokinetic.7
Vaginal splinting is a technique that can be used in patients with rectocele, whereby a finger is inserted into the vagina and pressure is applied on the posterior vaginal wall toward the rectum. Reducing the rectocele can facilitate emptying stool from the rectum and prevent leakage of retained stool.8 Similarly, use of rectal irrigation enemas can also help clear retained stool.
Pelvic floor physical therapists examine the strength, coordination, and tone of the pelvic floor muscles. When hypertonic musculature is present, manual interventions may be performed including trigger point release, myofascial release, and dry needling.9 When hypotonic musculature or dyssynergia is present, strengthening and neuromuscular re-education are recommended. Biofeedback can be administered via surface electromyography and/or balloon training to improve rectal sensitivity. Proper defecation techniques, including positioning, breathing, and behavioral modifications, improve clinical outcomes.
Diagnostic Testing
For patients who do not improve with conservative management, further testing is recommended to characterize the underlying pathology. Typically, anorectal manometry (ARM) is performed in conjunction with the balloon expulsion test and imaging. Each modality has its strengths and limitations (see Table 1).
ARM allows for the assessment of rectal sensation and recto-anal pressures and coordination.10
Dynamic imaging, by barium defecography under fluoroscopy or MRI, captures anatomy at rest and with simulated defecation to identify pelvic organ prolapse, compartmental defects, and organ mobility.11 Endoanal ultrasonography is considered in patients experiencing fecal incontinence to evaluate the integrity of the anal sphincter muscles.
Minimally Invasive Procedures and Surgical Options for PFD
Functional abnormalities such as dyssynergia often coexist with structural abnormalities. Because structural abnormalities are commonly found in asymptomatic patients, noninvasive functional therapy, such as pelvic floor physical therapy and anorectal biofeedback, are preferred prior to surgical repair of a structural finding. For patients with fecal incontinence, sacral nerve stimulation (SNS) has emerged as a preferred therapy due to demonstrated efficacy in symptom improvement.12 Sphincteroplasty is reserved for those with acute sphincter injury or failure of SNS.
In patients with findings of intussusception, prolapse, or rectocele that have not responded to conservative therapy, referral for surgical repair may be considered. While the specific surgical approach will depend on many factors, the goal is typically excision and/or suspension of rectal tissue and reinforcement of the rectovaginal septum.
It is critical that we are equipped with the available knowledge and tools to provide these patients with optimal care.
Dr. Khan, Dr. Menon, Dr. Allen, and Dr. Corning are based at the University of Texas Medical Branch in Galveston, Texas. They report no conflicts of interest.
References
1. Grimes WR and Stratton M. Pelvic floor dysfunction. 2023 Jun 26. In: StatPearls [Internet]. Treasure Island (Fla.): StatPearls Publishing; 2024 Jan. PMID: 32644672.
2. Nygaard I et al. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008 Sep 17. doi: 10.1001/jama.300.11.1311.
3. Lawrence JM et al. Pelvic floor disorders, diabetes, and obesity in women: Findings from the Kaiser Permanente Continence Associated Risk Epidemiology Study. Diabetes Care. 2007 Oct. doi: 10.2337/dc07-0262.
4. Barber MD et al. Short forms of two condition-specific quality-of-life questionnaires for women with pelvic floor disorders (PFDI-20 and PFIQ-7). Am J Obstet Gynecol. 2005 Jul. doi: 10.1016/j.ajog.2004.12.025.
5. Persu C et al. Pelvic Organ Prolapse Quantification System (POP-Q) — A new era in pelvic prolapse staging. J Med Life. 2011 Jan-Mar. PMID: 21505577.
6. Wald A et al. ACG Clinical Guidelines: Management of benign anorectal disorders. Am J Gastroenterol. 2021 Oct 1. doi: 10.14309/ajg.0000000000001507.
7. Bharucha AE and Lacy BE. Mechanisms, evaluation, and management of chronic constipation. Gastroenterology. 2020 Apr. doi: 10.1053/j.gastro.2019.12.034.
8. Menees S and Chey WD. Fecal incontinence: Pathogenesis, diagnosis, and updated treatment strategies. Gastroenterol Clin North Am. 2022 Mar. doi: 10.1016/j.gtc.2021.10.005.
9. Wallace SL et al. Pelvic floor physical therapy in the treatment of pelvic floor dysfunction in women. Curr Opin Obstet Gynecol. 2019 Dec. doi: 10.1097/GCO.0000000000000584.
10. Carrington EV et al. The international anorectal physiology working group (IAPWG) recommendations: Standardized testing protocol and the London classification for disorders of anorectal function. Neurogastroenterol Motil. 2020 Jan. doi: 10.1111/nmo.13679.
11. El Sayed RF et al. Magnetic resonance imaging of pelvic floor dysfunction — Joint recommendations of the ESUR and ESGAR Pelvic Floor Working Group. Eur Radiol. 2017 May. doi: 10.1007/s00330-016-4471-7.
12. Thaha MA et al. Sacral nerve stimulation for faecal incontinence and constipation in adults. Cochrane Database Syst Rev. 2015 Aug 24. doi: 10.1002/14651858.CD004464.pub3.
13. Chiarioni G et al. Biofeedback benefits only patients with outlet dysfunction, not patients with isolated slow transit constipation. Gastroenterology. 2005 Jul. doi: 10.1053/j.gastro.2005.05.015.
14. Grossi U et al. Diagnostic accuracy study of anorectal manometry for diagnosis of dyssynergic defecation. Gut. 2016 Mar. doi: 10.1136/gutjnl-2014-308835.
15. Albuquerque A. Endoanal ultrasonography in fecal incontinence: Current and future perspectives. World J Gastrointest Endosc. 2015 Jun 10. doi: 10.4253/wjge.v7.i6.575.
Caring for LGBTQ+ Patients with IBD
Cases
Patient 1: 55-year-old cis-male, who identifies as gay, has ulcerative colitis that has been refractory to multiple biologic therapies. His provider recommends a total proctocolectomy with ileal pouch anal anastomosis (TPC with IPAA), but the patient has questions regarding sexual function following surgery. Specifically, he is wondering when, or if, he can resume receptive anal intercourse. How would you counsel him?
Patient 2: 25-year-old, trans-female, status-post vaginoplasty with use of sigmoid colon and with well-controlled ulcerative colitis, presents with vaginal discharge, weight loss, and rectal bleeding. How do you explain what has happened to her? During your discussion, she also asks you why her chart continues to use her “dead name.” How do you respond?
Patient 3: 32-year-old, cis-female, G2P2, who identifies as a lesbian, has active ulcerative colitis. She wants to discuss medical or surgical therapy and future pregnancies. How would you counsel her?
Many gastroenterologists would likely know how to address patient 3’s concerns, but the concerns of patients 1 and 2 often go unaddressed or dismissed. Numerous studies and surveys have been conducted on patients with inflammatory bowel disease (IBD), but the focus of these studies has always been through a heteronormative cisgender lens. The focus of many studies is on fertility or sexual health and function in cisgender, heteronormative individuals.1-3 In the last few years, however, there has been increasing awareness of the health disparities, stigma, and discrimination that sexual and gender minorities (SGM) experience.4-6 For the purposes of this discussion, individuals within the lesbian, gay, bisexual, transgender, queer/questioning, intersex, and asexual (LGBTQIA+) community will be referred to as SGM. We recognize that even this exhaustive listing above does not acknowledge the full spectrum of diversity within the SGM community.
Clinical Care/Competency for SGM with IBD is Lacking
Almost 10% of the US population identifies as some form of SGM, and that number can be higher within the younger generations.4 SGM patients tend to delay or avoid seeking health care due to concern for provider mistreatment or lack of regard for their individual concerns. Additionally, there are several gaps in clinical knowledge about caring for SGM individuals. Little is known regarding the incidence or prevalence of IBD in SGM populations, but it is perceived to be similar to cisgender heterosexual individuals. Furthermore, as Newman et al. highlighted in their systematic review published in May 2023, there is a lack of guidance regarding sexual activity in the setting of IBD in SGM individuals.5 There is also a significant lack of knowledge on the impact of gender-affirming care on the natural history and treatments of IBD in transgender and gender non-conforming (TGNC) individuals. This can impact providers’ comfort and competence in caring for TGNC individuals.
Another important point to make is that the SGM community still faces discrimination due to sexual orientation or gender identity to this day, which impacts the quality and delivery of their care.7 Culturally-competent care should include care that is free from stigma, implicit and explicit biases, and discrimination. In 2011, an Institute of Medicine report documented, among other issues, provider discomfort in delivering care to SGM patients.8 While SGM individuals prefer a provider who acknowledges their sexual orientation and gender identity and treats them with the dignity and respect they deserve, many SGM individuals share valid concerns regarding their safety, which impact their desire to disclose their identity to health care providers.9 This certainly can have an impact on the quality of care they receive, including important health maintenance milestones and cancer screenings.10
An internal survey at our institution of providers (nurses, physician assistants, surgeons, and physicians) found that among 85 responders, 70% have cared for SGM who have undergone TPC with ileal pouch anal anastomosis (IPAA). Of these, 75% did not ask about sexual orientation or practices before pouch formation (though almost all of them agreed it would be important to ask). A total of 55% were comfortable in discussing SGM-related concerns; 53% did not feel comfortable discussing sexual orientation or practices; and in particular when it came to anoreceptive intercourse (ARI), 73% did not feel confident discussing recommendations.11
All of these issues highlight the importance of developing curricula that focus on reducing implicit and explicit biases towards SGM individuals and increasing the competence of providers to take care of SGM individuals in a safe space.
Additionally, it further justifies the need for ethical research that focuses on the needs of SGM individuals to guide evidence-based approaches to care. Given the implicit and explicit heterosexism and transphobia in society and many health care systems, Rainbows in Gastro was formed as an advocacy group for SGM patients, trainees, and staff in gastroenterology and hepatology.4
Research in SGM and IBD is lacking
There are additional needs for research in IBD and how it pertains to the needs of SGM individuals. Figure 1 highlights the lack of PubMed results for the search terms “IBD + LGBT,” “IBD + LGBTQ,” or “IBD + queer.” In contrast, the search terms “IBD + fertility” and “IBD + sexual dysfunction” generate many results. Even a systemic review conducted by Newman et al. of multiple databases in 2022 found only seven articles that demonstrated appropriately performed studies on SGM patients with IBD.5 This highlights the significant dearth of research in the realm of SGM health in IBD.
Newman and colleagues have recently published research considerations for SGM individuals. They highlighted the need to include understanding the “unique combination of psychosocial, biomedical, and legal experiences” that results in different needs and outcomes. There were several areas identified, including minority stress, which comes from existence of being SGM, especially as transgender individuals face increasing legal challenges in a variety of settings, not just healthcare.6 In a retrospective chart review investigating social determinants of health in SGM-IBD populations,12 36% of patients reported some level of social isolation, and almost 50% reported some level of stress. A total of 40% of them self-reported some perceived level of risk with respect to employment, and 17% reported depression. Given that this was a chart review and not a strict questionnaire, this study was certainly limited, and we would hypothesize that these numbers are therefore underestimating the true proportion of SGM-IBD patients who deal with employment concerns, social isolation, or psychological distress.
What Next? Back to the Patients
Circling back to our patients from the introduction, how would you counsel each of them? In patient 1’s case, we would inform him that pelvic surgery can increase the risk for sexual dysfunction, such as erectile dysfunction. He additionally would be advised during a staged TPC with IPAA, he may experience issues with body image. However, should he desire to participate in receptive anal intercourse after completion of his surgeries, the general recommendation would be to wait at least 6 months and with proven remission. It should further be noted that these are not formalized recommendations, only highlighting the need for more research and consensus on standards of care for SGM patients. He should finally be told that because he has ulcerative colitis, removal of the colon does not remove the risk for future intestinal involvement such as possible pouchitis.
In patient 2’s case, she is likely experiencing diversion vaginitis related to use of her colon for her neo-vagina. She should undergo colonoscopy and vaginoscopy in addition to standard work-up for her known ulcerative colitis.13 Management should be done in a multidisciplinary approach between the IBD provider, gynecologist, and gender-affirming provider. The electronic medical record should be updated to reflect the patient’s preferred name, pronouns, and gender identity, and her medical records, including automated clinical reports, should be updated accordingly.
As for patient 3, she would be counseled according to well-documented guidelines on pregnancy and IBD, including risks of medications (such as Jak inhibitors or methotrexate) versus the risk of uncontrolled IBD during pregnancy.1
Regardless of a patient’s gender identity or sexual orientation, patient-centered, culturally competent, and sensitive care should be provided. At Mayo Clinic in Rochester, we started one of the first Pride in IBD Clinics, which focuses on the care of SGM individuals with IBD. Our focus is to address the needs of patients who belong to the SGM community in a wholistic approach within a safe space (https://www.youtube.com/watch?v=pYa_zYaCA6M; https://www.mayoclinic.org/departments-centers/inflammatory-bowel-disease-clinic/overview/ovc-20357763). Our process of developing the clinic included training all staff on proper communication and cultural sensitivity for the SGM community.

Furthermore, providing welcoming and affirming signs of inclusivity for SGM individuals at the provider’s office — including but not limited to rainbow progressive flags, gender-neutral bathroom signs, or pronoun pins on provider identification badges (see Figure 2) — are usually appreciated by patients. Ensuring that patient education materials do not assume gender (for example, using the term “parents” rather than “mother and father”) and using gender neutral terms on intake forms is very important. Inclusive communication includes providers introducing themselves by preferred name and pronouns, asking the patients to introduce themselves, and welcoming them to share their pronouns. These simple actions can provide an atmosphere of safety for SGM patients, which would serve to enhance the quality of care we can provide for them.
For Resources and Further Reading: CDC,14 the Fenway Institute’s National LGBTQIA+ Health Education Center,15 and US Department of Health and Human Services.16
Dr. Chiang and Dr. Chedid are both in the Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota. Dr. Chedid is also with the Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery, Mayo Clinic. Neither of the authors have any relevant conflicts of interest. They are on X, formerly Twitter: @dr_davidchiang , @VictorChedidMD .
CITATIONS
1. Mahadevan U et al. Inflammatory bowel disease in pregnancy clinical care pathway: A report from the American Gastroenterological Association IBD Parenthood Project Working Group. Gastroenterology. 2019;156:1508-24.
2. Pires F et al. A survey on the impact of IBD in sexual health: Into intimacy. Medicine (Baltimore). 2022;101:e32279.
3. Mules TC et al. The impact of disease activity on sexual and erectile dysfunction in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2023;29:1244-54.
4. Duong N et al. Overcoming disparities for sexual and gender minority patients and providers in gastroenterology and hepatology: Introduction to Rainbows in Gastro. Lancet Gastroenterol Hepatol. 2023;8:299-301.
5. Newman KL et al. A systematic review of inflammatory bowel disease epidemiology and health outcomes in sexual and gender minority individuals. Gastroenterology. 2023;164:866-71.
6. Newman KL et al. Research considerations in Digestive and liver disease in transgender and gender-diverse populations. Gastroenterology. 2023;165:523-28 e1.
7. Velez C et al. Digestive health in sexual and gender minority populations. Am J Gastroenterol. 2022;117:865-75.
8. Medicine Io. Washington (DC): The National Academies Press, 2011.
9. Austin EL. Sexual orientation disclosure to health care providers among urban and non-urban southern lesbians. Women Health. 2013;53:41-55.
10. Oladeru OT et al. Breast and cervical cancer screening disparities in transgender people. Am J Clin Oncol. 2022;45:116-21.
11. Vinsard DG et al. Healthcare providers’ perspectives on anoreceptive intercourse in sexual and gender minorities with ileal pouch anal anastomosis. Digestive Disease Week (DDW). Chicago, IL, 2023.
12. Ghusn W et al. Social determinants of health in LGBTQIA+ patients with inflammatory bowel disease. American College of Gastroenterology (ACG). Charlotte, NC, 2022.
13. Grasman ME et al. Neovaginal sparing in a transgender woman with ulcerative colitis. Clin Gastroenterol Hepatol. 2016;14:e73-4.
14. Prevention CfDCa. Lesbian, Gay, Bisexual, and Transgender Health — https://www.cdc.gov/lgbthealth/index.htm.
15. Institute TF. National LGBTQIA+ Health Education Center — https://www.lgbtqiahealtheducation.org/.
16. Services UDoHaH. LGBTQI+ Resources — https://www.hhs.gov/programs/topic-sites/lgbtqi/resources/index.html.
Cases
Patient 1: 55-year-old cis-male, who identifies as gay, has ulcerative colitis that has been refractory to multiple biologic therapies. His provider recommends a total proctocolectomy with ileal pouch anal anastomosis (TPC with IPAA), but the patient has questions regarding sexual function following surgery. Specifically, he is wondering when, or if, he can resume receptive anal intercourse. How would you counsel him?
Patient 2: 25-year-old, trans-female, status-post vaginoplasty with use of sigmoid colon and with well-controlled ulcerative colitis, presents with vaginal discharge, weight loss, and rectal bleeding. How do you explain what has happened to her? During your discussion, she also asks you why her chart continues to use her “dead name.” How do you respond?
Patient 3: 32-year-old, cis-female, G2P2, who identifies as a lesbian, has active ulcerative colitis. She wants to discuss medical or surgical therapy and future pregnancies. How would you counsel her?
Many gastroenterologists would likely know how to address patient 3’s concerns, but the concerns of patients 1 and 2 often go unaddressed or dismissed. Numerous studies and surveys have been conducted on patients with inflammatory bowel disease (IBD), but the focus of these studies has always been through a heteronormative cisgender lens. The focus of many studies is on fertility or sexual health and function in cisgender, heteronormative individuals.1-3 In the last few years, however, there has been increasing awareness of the health disparities, stigma, and discrimination that sexual and gender minorities (SGM) experience.4-6 For the purposes of this discussion, individuals within the lesbian, gay, bisexual, transgender, queer/questioning, intersex, and asexual (LGBTQIA+) community will be referred to as SGM. We recognize that even this exhaustive listing above does not acknowledge the full spectrum of diversity within the SGM community.
Clinical Care/Competency for SGM with IBD is Lacking
Almost 10% of the US population identifies as some form of SGM, and that number can be higher within the younger generations.4 SGM patients tend to delay or avoid seeking health care due to concern for provider mistreatment or lack of regard for their individual concerns. Additionally, there are several gaps in clinical knowledge about caring for SGM individuals. Little is known regarding the incidence or prevalence of IBD in SGM populations, but it is perceived to be similar to cisgender heterosexual individuals. Furthermore, as Newman et al. highlighted in their systematic review published in May 2023, there is a lack of guidance regarding sexual activity in the setting of IBD in SGM individuals.5 There is also a significant lack of knowledge on the impact of gender-affirming care on the natural history and treatments of IBD in transgender and gender non-conforming (TGNC) individuals. This can impact providers’ comfort and competence in caring for TGNC individuals.
Another important point to make is that the SGM community still faces discrimination due to sexual orientation or gender identity to this day, which impacts the quality and delivery of their care.7 Culturally-competent care should include care that is free from stigma, implicit and explicit biases, and discrimination. In 2011, an Institute of Medicine report documented, among other issues, provider discomfort in delivering care to SGM patients.8 While SGM individuals prefer a provider who acknowledges their sexual orientation and gender identity and treats them with the dignity and respect they deserve, many SGM individuals share valid concerns regarding their safety, which impact their desire to disclose their identity to health care providers.9 This certainly can have an impact on the quality of care they receive, including important health maintenance milestones and cancer screenings.10
An internal survey at our institution of providers (nurses, physician assistants, surgeons, and physicians) found that among 85 responders, 70% have cared for SGM who have undergone TPC with ileal pouch anal anastomosis (IPAA). Of these, 75% did not ask about sexual orientation or practices before pouch formation (though almost all of them agreed it would be important to ask). A total of 55% were comfortable in discussing SGM-related concerns; 53% did not feel comfortable discussing sexual orientation or practices; and in particular when it came to anoreceptive intercourse (ARI), 73% did not feel confident discussing recommendations.11
All of these issues highlight the importance of developing curricula that focus on reducing implicit and explicit biases towards SGM individuals and increasing the competence of providers to take care of SGM individuals in a safe space.
Additionally, it further justifies the need for ethical research that focuses on the needs of SGM individuals to guide evidence-based approaches to care. Given the implicit and explicit heterosexism and transphobia in society and many health care systems, Rainbows in Gastro was formed as an advocacy group for SGM patients, trainees, and staff in gastroenterology and hepatology.4
Research in SGM and IBD is lacking
There are additional needs for research in IBD and how it pertains to the needs of SGM individuals. Figure 1 highlights the lack of PubMed results for the search terms “IBD + LGBT,” “IBD + LGBTQ,” or “IBD + queer.” In contrast, the search terms “IBD + fertility” and “IBD + sexual dysfunction” generate many results. Even a systemic review conducted by Newman et al. of multiple databases in 2022 found only seven articles that demonstrated appropriately performed studies on SGM patients with IBD.5 This highlights the significant dearth of research in the realm of SGM health in IBD.

Newman and colleagues have recently published research considerations for SGM individuals. They highlighted the need to include understanding the “unique combination of psychosocial, biomedical, and legal experiences” that results in different needs and outcomes. There were several areas identified, including minority stress, which comes from existence of being SGM, especially as transgender individuals face increasing legal challenges in a variety of settings, not just healthcare.6 In a retrospective chart review investigating social determinants of health in SGM-IBD populations,12 36% of patients reported some level of social isolation, and almost 50% reported some level of stress. A total of 40% of them self-reported some perceived level of risk with respect to employment, and 17% reported depression. Given that this was a chart review and not a strict questionnaire, this study was certainly limited, and we would hypothesize that these numbers are therefore underestimating the true proportion of SGM-IBD patients who deal with employment concerns, social isolation, or psychological distress.
What Next? Back to the Patients
Circling back to our patients from the introduction, how would you counsel each of them? In patient 1’s case, we would inform him that pelvic surgery can increase the risk for sexual dysfunction, such as erectile dysfunction. He additionally would be advised during a staged TPC with IPAA, he may experience issues with body image. However, should he desire to participate in receptive anal intercourse after completion of his surgeries, the general recommendation would be to wait at least 6 months and with proven remission. It should further be noted that these are not formalized recommendations, only highlighting the need for more research and consensus on standards of care for SGM patients. He should finally be told that because he has ulcerative colitis, removal of the colon does not remove the risk for future intestinal involvement such as possible pouchitis.
In patient 2’s case, she is likely experiencing diversion vaginitis related to use of her colon for her neo-vagina. She should undergo colonoscopy and vaginoscopy in addition to standard work-up for her known ulcerative colitis.13 Management should be done in a multidisciplinary approach between the IBD provider, gynecologist, and gender-affirming provider. The electronic medical record should be updated to reflect the patient’s preferred name, pronouns, and gender identity, and her medical records, including automated clinical reports, should be updated accordingly.
As for patient 3, she would be counseled according to well-documented guidelines on pregnancy and IBD, including risks of medications (such as Jak inhibitors or methotrexate) versus the risk of uncontrolled IBD during pregnancy.1
Regardless of a patient’s gender identity or sexual orientation, patient-centered, culturally competent, and sensitive care should be provided. At Mayo Clinic in Rochester, we started one of the first Pride in IBD Clinics, which focuses on the care of SGM individuals with IBD. Our focus is to address the needs of patients who belong to the SGM community in a wholistic approach within a safe space (https://www.youtube.com/watch?v=pYa_zYaCA6M; https://www.mayoclinic.org/departments-centers/inflammatory-bowel-disease-clinic/overview/ovc-20357763). Our process of developing the clinic included training all staff on proper communication and cultural sensitivity for the SGM community.

Furthermore, providing welcoming and affirming signs of inclusivity for SGM individuals at the provider’s office — including but not limited to rainbow progressive flags, gender-neutral bathroom signs, or pronoun pins on provider identification badges (see Figure 2) — are usually appreciated by patients. Ensuring that patient education materials do not assume gender (for example, using the term “parents” rather than “mother and father”) and using gender neutral terms on intake forms is very important. Inclusive communication includes providers introducing themselves by preferred name and pronouns, asking the patients to introduce themselves, and welcoming them to share their pronouns. These simple actions can provide an atmosphere of safety for SGM patients, which would serve to enhance the quality of care we can provide for them.
For Resources and Further Reading: CDC,14 the Fenway Institute’s National LGBTQIA+ Health Education Center,15 and US Department of Health and Human Services.16
Dr. Chiang and Dr. Chedid are both in the Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota. Dr. Chedid is also with the Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery, Mayo Clinic. Neither of the authors have any relevant conflicts of interest. They are on X, formerly Twitter: @dr_davidchiang , @VictorChedidMD .
CITATIONS
1. Mahadevan U et al. Inflammatory bowel disease in pregnancy clinical care pathway: A report from the American Gastroenterological Association IBD Parenthood Project Working Group. Gastroenterology. 2019;156:1508-24.
2. Pires F et al. A survey on the impact of IBD in sexual health: Into intimacy. Medicine (Baltimore). 2022;101:e32279.
3. Mules TC et al. The impact of disease activity on sexual and erectile dysfunction in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2023;29:1244-54.
4. Duong N et al. Overcoming disparities for sexual and gender minority patients and providers in gastroenterology and hepatology: Introduction to Rainbows in Gastro. Lancet Gastroenterol Hepatol. 2023;8:299-301.
5. Newman KL et al. A systematic review of inflammatory bowel disease epidemiology and health outcomes in sexual and gender minority individuals. Gastroenterology. 2023;164:866-71.
6. Newman KL et al. Research considerations in Digestive and liver disease in transgender and gender-diverse populations. Gastroenterology. 2023;165:523-28 e1.
7. Velez C et al. Digestive health in sexual and gender minority populations. Am J Gastroenterol. 2022;117:865-75.
8. Medicine Io. Washington (DC): The National Academies Press, 2011.
9. Austin EL. Sexual orientation disclosure to health care providers among urban and non-urban southern lesbians. Women Health. 2013;53:41-55.
10. Oladeru OT et al. Breast and cervical cancer screening disparities in transgender people. Am J Clin Oncol. 2022;45:116-21.
11. Vinsard DG et al. Healthcare providers’ perspectives on anoreceptive intercourse in sexual and gender minorities with ileal pouch anal anastomosis. Digestive Disease Week (DDW). Chicago, IL, 2023.
12. Ghusn W et al. Social determinants of health in LGBTQIA+ patients with inflammatory bowel disease. American College of Gastroenterology (ACG). Charlotte, NC, 2022.
13. Grasman ME et al. Neovaginal sparing in a transgender woman with ulcerative colitis. Clin Gastroenterol Hepatol. 2016;14:e73-4.
14. Prevention CfDCa. Lesbian, Gay, Bisexual, and Transgender Health — https://www.cdc.gov/lgbthealth/index.htm.
15. Institute TF. National LGBTQIA+ Health Education Center — https://www.lgbtqiahealtheducation.org/.
16. Services UDoHaH. LGBTQI+ Resources — https://www.hhs.gov/programs/topic-sites/lgbtqi/resources/index.html.
Cases
Patient 1: 55-year-old cis-male, who identifies as gay, has ulcerative colitis that has been refractory to multiple biologic therapies. His provider recommends a total proctocolectomy with ileal pouch anal anastomosis (TPC with IPAA), but the patient has questions regarding sexual function following surgery. Specifically, he is wondering when, or if, he can resume receptive anal intercourse. How would you counsel him?
Patient 2: 25-year-old, trans-female, status-post vaginoplasty with use of sigmoid colon and with well-controlled ulcerative colitis, presents with vaginal discharge, weight loss, and rectal bleeding. How do you explain what has happened to her? During your discussion, she also asks you why her chart continues to use her “dead name.” How do you respond?
Patient 3: 32-year-old, cis-female, G2P2, who identifies as a lesbian, has active ulcerative colitis. She wants to discuss medical or surgical therapy and future pregnancies. How would you counsel her?
Many gastroenterologists would likely know how to address patient 3’s concerns, but the concerns of patients 1 and 2 often go unaddressed or dismissed. Numerous studies and surveys have been conducted on patients with inflammatory bowel disease (IBD), but the focus of these studies has always been through a heteronormative cisgender lens. The focus of many studies is on fertility or sexual health and function in cisgender, heteronormative individuals.1-3 In the last few years, however, there has been increasing awareness of the health disparities, stigma, and discrimination that sexual and gender minorities (SGM) experience.4-6 For the purposes of this discussion, individuals within the lesbian, gay, bisexual, transgender, queer/questioning, intersex, and asexual (LGBTQIA+) community will be referred to as SGM. We recognize that even this exhaustive listing above does not acknowledge the full spectrum of diversity within the SGM community.
Clinical Care/Competency for SGM with IBD is Lacking
Almost 10% of the US population identifies as some form of SGM, and that number can be higher within the younger generations.4 SGM patients tend to delay or avoid seeking health care due to concern for provider mistreatment or lack of regard for their individual concerns. Additionally, there are several gaps in clinical knowledge about caring for SGM individuals. Little is known regarding the incidence or prevalence of IBD in SGM populations, but it is perceived to be similar to cisgender heterosexual individuals. Furthermore, as Newman et al. highlighted in their systematic review published in May 2023, there is a lack of guidance regarding sexual activity in the setting of IBD in SGM individuals.5 There is also a significant lack of knowledge on the impact of gender-affirming care on the natural history and treatments of IBD in transgender and gender non-conforming (TGNC) individuals. This can impact providers’ comfort and competence in caring for TGNC individuals.
Another important point to make is that the SGM community still faces discrimination due to sexual orientation or gender identity to this day, which impacts the quality and delivery of their care.7 Culturally-competent care should include care that is free from stigma, implicit and explicit biases, and discrimination. In 2011, an Institute of Medicine report documented, among other issues, provider discomfort in delivering care to SGM patients.8 While SGM individuals prefer a provider who acknowledges their sexual orientation and gender identity and treats them with the dignity and respect they deserve, many SGM individuals share valid concerns regarding their safety, which impact their desire to disclose their identity to health care providers.9 This certainly can have an impact on the quality of care they receive, including important health maintenance milestones and cancer screenings.10
An internal survey at our institution of providers (nurses, physician assistants, surgeons, and physicians) found that among 85 responders, 70% have cared for SGM who have undergone TPC with ileal pouch anal anastomosis (IPAA). Of these, 75% did not ask about sexual orientation or practices before pouch formation (though almost all of them agreed it would be important to ask). A total of 55% were comfortable in discussing SGM-related concerns; 53% did not feel comfortable discussing sexual orientation or practices; and in particular when it came to anoreceptive intercourse (ARI), 73% did not feel confident discussing recommendations.11
All of these issues highlight the importance of developing curricula that focus on reducing implicit and explicit biases towards SGM individuals and increasing the competence of providers to take care of SGM individuals in a safe space.
Additionally, it further justifies the need for ethical research that focuses on the needs of SGM individuals to guide evidence-based approaches to care. Given the implicit and explicit heterosexism and transphobia in society and many health care systems, Rainbows in Gastro was formed as an advocacy group for SGM patients, trainees, and staff in gastroenterology and hepatology.4
Research in SGM and IBD is lacking
There are additional needs for research in IBD and how it pertains to the needs of SGM individuals. Figure 1 highlights the lack of PubMed results for the search terms “IBD + LGBT,” “IBD + LGBTQ,” or “IBD + queer.” In contrast, the search terms “IBD + fertility” and “IBD + sexual dysfunction” generate many results. Even a systemic review conducted by Newman et al. of multiple databases in 2022 found only seven articles that demonstrated appropriately performed studies on SGM patients with IBD.5 This highlights the significant dearth of research in the realm of SGM health in IBD.

Newman and colleagues have recently published research considerations for SGM individuals. They highlighted the need to include understanding the “unique combination of psychosocial, biomedical, and legal experiences” that results in different needs and outcomes. There were several areas identified, including minority stress, which comes from existence of being SGM, especially as transgender individuals face increasing legal challenges in a variety of settings, not just healthcare.6 In a retrospective chart review investigating social determinants of health in SGM-IBD populations,12 36% of patients reported some level of social isolation, and almost 50% reported some level of stress. A total of 40% of them self-reported some perceived level of risk with respect to employment, and 17% reported depression. Given that this was a chart review and not a strict questionnaire, this study was certainly limited, and we would hypothesize that these numbers are therefore underestimating the true proportion of SGM-IBD patients who deal with employment concerns, social isolation, or psychological distress.
What Next? Back to the Patients
Circling back to our patients from the introduction, how would you counsel each of them? In patient 1’s case, we would inform him that pelvic surgery can increase the risk for sexual dysfunction, such as erectile dysfunction. He additionally would be advised during a staged TPC with IPAA, he may experience issues with body image. However, should he desire to participate in receptive anal intercourse after completion of his surgeries, the general recommendation would be to wait at least 6 months and with proven remission. It should further be noted that these are not formalized recommendations, only highlighting the need for more research and consensus on standards of care for SGM patients. He should finally be told that because he has ulcerative colitis, removal of the colon does not remove the risk for future intestinal involvement such as possible pouchitis.
In patient 2’s case, she is likely experiencing diversion vaginitis related to use of her colon for her neo-vagina. She should undergo colonoscopy and vaginoscopy in addition to standard work-up for her known ulcerative colitis.13 Management should be done in a multidisciplinary approach between the IBD provider, gynecologist, and gender-affirming provider. The electronic medical record should be updated to reflect the patient’s preferred name, pronouns, and gender identity, and her medical records, including automated clinical reports, should be updated accordingly.
As for patient 3, she would be counseled according to well-documented guidelines on pregnancy and IBD, including risks of medications (such as Jak inhibitors or methotrexate) versus the risk of uncontrolled IBD during pregnancy.1
Regardless of a patient’s gender identity or sexual orientation, patient-centered, culturally competent, and sensitive care should be provided. At Mayo Clinic in Rochester, we started one of the first Pride in IBD Clinics, which focuses on the care of SGM individuals with IBD. Our focus is to address the needs of patients who belong to the SGM community in a wholistic approach within a safe space (https://www.youtube.com/watch?v=pYa_zYaCA6M; https://www.mayoclinic.org/departments-centers/inflammatory-bowel-disease-clinic/overview/ovc-20357763). Our process of developing the clinic included training all staff on proper communication and cultural sensitivity for the SGM community.

Furthermore, providing welcoming and affirming signs of inclusivity for SGM individuals at the provider’s office — including but not limited to rainbow progressive flags, gender-neutral bathroom signs, or pronoun pins on provider identification badges (see Figure 2) — are usually appreciated by patients. Ensuring that patient education materials do not assume gender (for example, using the term “parents” rather than “mother and father”) and using gender neutral terms on intake forms is very important. Inclusive communication includes providers introducing themselves by preferred name and pronouns, asking the patients to introduce themselves, and welcoming them to share their pronouns. These simple actions can provide an atmosphere of safety for SGM patients, which would serve to enhance the quality of care we can provide for them.
For Resources and Further Reading: CDC,14 the Fenway Institute’s National LGBTQIA+ Health Education Center,15 and US Department of Health and Human Services.16
Dr. Chiang and Dr. Chedid are both in the Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota. Dr. Chedid is also with the Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery, Mayo Clinic. Neither of the authors have any relevant conflicts of interest. They are on X, formerly Twitter: @dr_davidchiang , @VictorChedidMD .
CITATIONS
1. Mahadevan U et al. Inflammatory bowel disease in pregnancy clinical care pathway: A report from the American Gastroenterological Association IBD Parenthood Project Working Group. Gastroenterology. 2019;156:1508-24.
2. Pires F et al. A survey on the impact of IBD in sexual health: Into intimacy. Medicine (Baltimore). 2022;101:e32279.
3. Mules TC et al. The impact of disease activity on sexual and erectile dysfunction in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2023;29:1244-54.
4. Duong N et al. Overcoming disparities for sexual and gender minority patients and providers in gastroenterology and hepatology: Introduction to Rainbows in Gastro. Lancet Gastroenterol Hepatol. 2023;8:299-301.
5. Newman KL et al. A systematic review of inflammatory bowel disease epidemiology and health outcomes in sexual and gender minority individuals. Gastroenterology. 2023;164:866-71.
6. Newman KL et al. Research considerations in Digestive and liver disease in transgender and gender-diverse populations. Gastroenterology. 2023;165:523-28 e1.
7. Velez C et al. Digestive health in sexual and gender minority populations. Am J Gastroenterol. 2022;117:865-75.
8. Medicine Io. Washington (DC): The National Academies Press, 2011.
9. Austin EL. Sexual orientation disclosure to health care providers among urban and non-urban southern lesbians. Women Health. 2013;53:41-55.
10. Oladeru OT et al. Breast and cervical cancer screening disparities in transgender people. Am J Clin Oncol. 2022;45:116-21.
11. Vinsard DG et al. Healthcare providers’ perspectives on anoreceptive intercourse in sexual and gender minorities with ileal pouch anal anastomosis. Digestive Disease Week (DDW). Chicago, IL, 2023.
12. Ghusn W et al. Social determinants of health in LGBTQIA+ patients with inflammatory bowel disease. American College of Gastroenterology (ACG). Charlotte, NC, 2022.
13. Grasman ME et al. Neovaginal sparing in a transgender woman with ulcerative colitis. Clin Gastroenterol Hepatol. 2016;14:e73-4.
14. Prevention CfDCa. Lesbian, Gay, Bisexual, and Transgender Health — https://www.cdc.gov/lgbthealth/index.htm.
15. Institute TF. National LGBTQIA+ Health Education Center — https://www.lgbtqiahealtheducation.org/.
16. Services UDoHaH. LGBTQI+ Resources — https://www.hhs.gov/programs/topic-sites/lgbtqi/resources/index.html.
Advances in endoscopic therapies in inflammatory bowel disease
Introduction
Inflammatory bowel disease (IBD) is a chronic, relapsing and remitting disorder that is becoming increasingly prevalent worldwide.1 Despite major advances in this area, many patients with moderate to severe IBD do not achieve disease remission with immunosuppressive therapy.2 Dysplasia and fibrostenosis are two common consequences of uncontrolled chronic inflammation and these structural complications are often the primary reasons for surgical interventions.3 While there is certainly a time and a place for surgery in IBD, this approach is invasive and postoperative recrudescence of disease is common.4 Moreover patients with complex surgical or medical histories may not make optimal surgical candidates.
Thanks to advancements in a variety of endoscopic technologies, Over the last several years, applications of endoscopic therapies in IBD have been gaining traction and the need for these therapies is expected to continue to rise over time. As such, understanding the domains of available endoscopic options in IBD is important for the modern-day gastroenterologist. In this article, we will discuss some of the recent advancements in endoscopic therapies for IBD and how we may position these in clinical practice.
Protecting against colitis dysplasia and colon cancer
IBD is a risk factor for colorectal cancer because of the dysplasia-carcinoma sequence arising from chronic colitis. Endoscopic resection is the first-line treatment for conventional colitis-associated dysplasia (CAD).5,6 However, larger or complex lesions may not have been previously amenable to this organ-preserving approach. The application of newer techniques has extended the indication for endoscopic resection to include most CAD lesions, as an alternative to proctocolectomy. Endoscopic mucosal resection (EMR) is the most commonly used technique and its outcomes for CAD greater than 2 cm have been excellent (Figure 1).7 However, employing EMR for lesions greater than 2 cm in size may require piecemeal resection and this has been associated with a small risk of local recurrence.8 Endoscopic submucosal dissection (ESD) is an alternate method of endoscopic tissue resection that can reliably achieve en bloc (single specimen) resections even in larger lesions.9
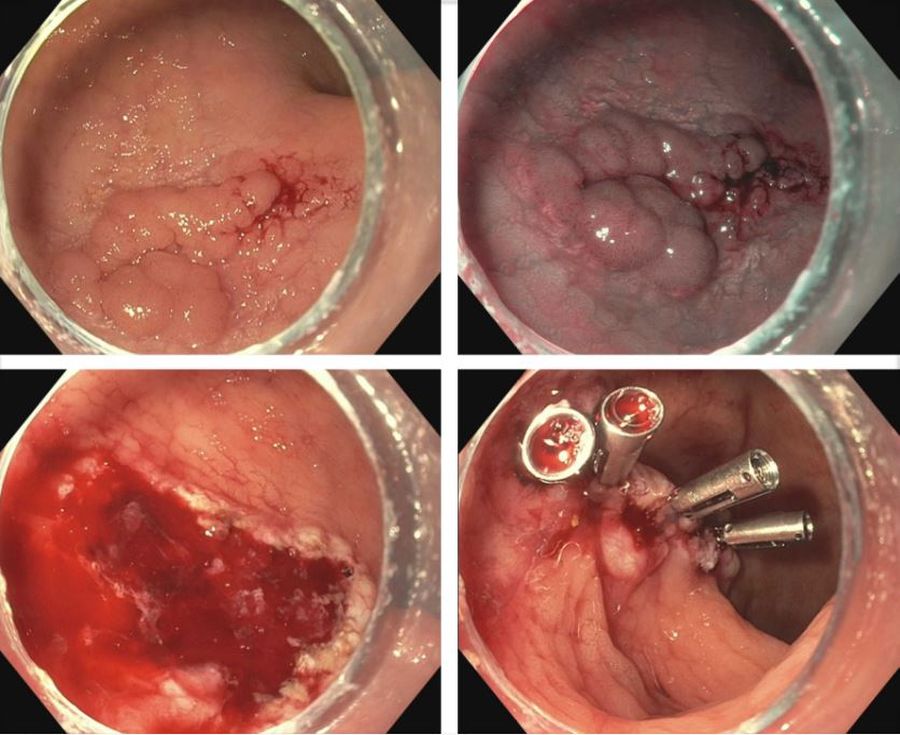
These technical advantages, however, have not been proven to result in broad clinical superiority of ESD over EMR for advanced lesions.10 The other consideration is that ESD is associated with greater risk of perforation and is more technically complex to perform.10 Yet, recent data supporting ESD in larger lesions is amounting and it may be more suitable for situations where conventional techniques fall short.11 To that end, dense submucosal fibrosis is a common characteristic of CAD and may prohibit successful EMR or ESD as a single modality. Different therapeutic methods can be incorporated in these circumstances, including combined ESD and EMR technique, tissue thermal ablation, or even full-thickness resection has been described.11-13
Taken together, we have many effective options for how we can effectively deal with CAD endoscopically and maintain our patients free of colorectal cancer. The method in which this is done may not matter as much at this juncture and may be more dependent on available local clinical expertise. Moreover, we can’t forget that metachronous lesions and neoplastic recurrence after endoscopic resection are not uncommon and a structured, vigilant endoscopic surveillance program for all patients undergoing endoscopic management of CAD is mandated.7,10
Restoring gastrointestinal tract transit
Crohn’s strictures may lead to acute intestinal obstructions or facilitate the onset of penetrating disease, such as fistula formation or abscess. These strictures are often characterized by a combination of inflammation and layered fibrosis, which requires the application of medical therapies alongside structural remodeling to successfully manage. Not all strictures may be clinically overt due to variances in visceral sensitivity, yet experts believe that treatment of all strictures should be considered to avoid occurrence of delayed complications.14 Endoscopic balloon dilation (EBD) is a well-established treatment for Crohn’s strictures up to 4-5 cm in length (Figure 2). This treatment involves inflating a balloon within the narrowed section of intestine, thereby stretching and disrupting the layered fibrotic bands to widen the stricture. EBD improves symptoms 70% of the time and successfully avoids the medium-term need for surgery in most, although it often requires repeat endoscopic procedures.15 In fact, up to 74% of patients will require repeat dilation over 2 years and 43% will require salvage surgery after EBD.16
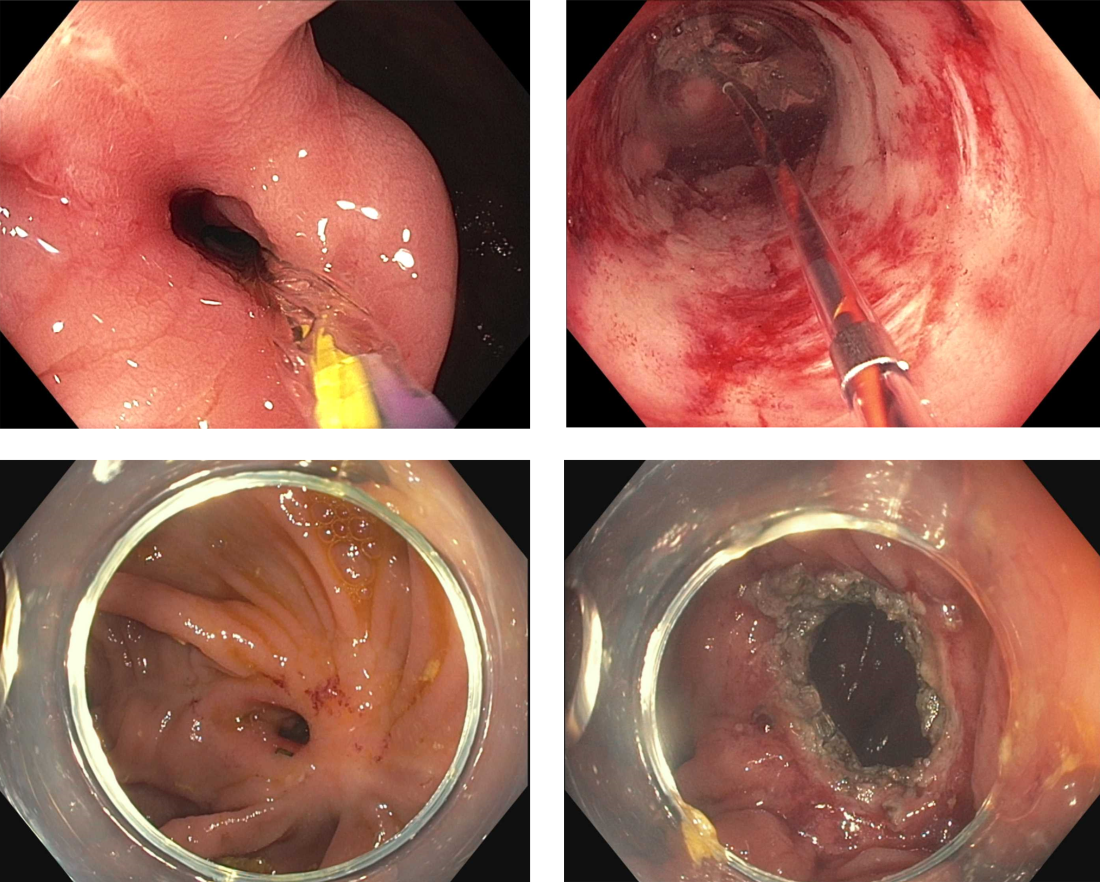
Endoscopic stricturotomy (Est) is a newer technique that involves making radial and longitudinal incisions within the stricture using an endoscopic knife (Figure 2). The ability to excise fibrotic bands allows for more advanced remodeling and thus a lower need for reintervention or surgery (9%-22.5%) in comparison with EBD, while maintaining similar technical and clinical success rates.17 Est also carries a lower risk of perforation, but a higher risk of delayed bleeding.17 Refinements in Est are ongoing as the technique continues to develop, including the application of prophylactic clips after Est or use of other hemostatic agents such as gels or powders to minimizing bleeding risk. Despite this, Est has clear benefit in durability for treating strictures especially anastomotic subtype or those refractory to balloon dilation.
Stenting is a third option for treating strictures in Crohn’s disease that is reserved for specific situations. This approach involves endoscopic implantation of a covered metallic stent within the stricture in order to promote remodeling throughout a selected dwell time (generally 2-4 weeks). Stents may be considered in nonoperative candidates with strictures longer than 5 cm, which are generally too long for EBD or Est, or in EBD-refractory strictures in which there is no clear plane for Est excision. However, given the risk of migration, stents are currently not considered a first-line treatment of IBD-related strictures.18 Perhaps with further modifications in design and availability of stent-fixation methods, their use may become more practical in the future.19
The future for endoscopic therapy is bright
Structural complications of IBD are common and can pose a significant detriment to quality of life and general well-being for patients. From mucosal resection of CAD to surgery-sparing therapies for intestinal strictures, endoscopic therapies are valuable and effective options for managing disease-related sequelae within the scope of interventional IBD practice. We can expect the availability of these options to grow as the scope of endoscopy training incorporates principles of interventional IBD, along with the concurrent development of additional therapeutic applications beyond the categories discussed here (including perianal disease, fistulas, and abscess formation). It is noteworthy to mention that while endoscopic therapies are separate treatment modalities, should not be considered mutually exclusive; endotherapies are best viewed as a complement to existing medical and surgical approaches. Thus, Interventional IBD endoscopy can serve as an integral part of the multidisciplinary IBD framework to provide comprehensive care for our patients with IBD.
Juan Reyes Genere, MD, is an assistant professor of medicine in gastroenterology at Washington University in St. Louis. He served as the corresponding author of this article. Michael Rubeiz, MD, is a physician in the internal medicine residency program at Washington University in St. Louis. Kemmian Johnson, MD, MPH, is a gastroenterologist at Washington University in St. Louis specializing in inflammatory bowel disease. Dr. Genere is a consultant for Edulis Therapeutics. Dr. Rubeiz and Dr. Johnson had no personal or financial conflicts of interest. Dr. Johnson can be reached on Instagram @KJ.1906; Dr. Rubeiz is on X @MichaelRubeiz1 and Dr. Genere can be reached via X @JPGenereMD.
References
1. Ng SC et al. Lancet. 2017;390(10114):2769-78.
2. Gordon JP et al. Eur J Gastroenterol Hepatol. 2015;27(7):804-12.
3. Sica GS and Biancone L. World J Gastroenterol. 2013;19(16):2445-8.
4. Iborra M et al. Gastroenterol Rep (Oxf). 2019;7(6):411-8.
5. Annese V et al. J Crohns Colitis. 2013;7(12):982-1018.
6. Laine L et al. Gastrointest Endosc. 2015;81(3):489-501.e426.
7. Mohan BP et al. Gastrointest Endosc. 2021;93(1):59-67.e10.
8. Briedigkeit A et al. World J Gastrointest Endosc. 2016;8(5):276-81.
9. Manta R et al. J Crohns Colitis. 2021;15(1):165-8.
10. Mohapatra S et al. Endosc Int Open. 2022;10(5):E593-601.
11. Ngamruengphong S et al. Endosc Int Open. 2022;10(4):E354-60.
12. Baker G et al. Cureus. 2022 May 3;14(5):e24688.
13. Yadav S et al. Endosc Int Open. 2019;7(8):E994-1001.
14. Schwartz DA. Gastrointestinal Endoscopy. 2023;97(5):974-6.
15. Morar PS et al. Aliment Pharmacol Ther. 2015;42(10):1137-48.
16. Bettenworth D et al. Inflamm Bowel Dis. 2017;23(1):133-42.
17. Lan N and Shen B. Inflamm Bowel Dis. 2018;24(4):897-907.
18. Loras C et al. Lancet Gastroenterol Hepatol. 2022;7(4):332-41.
19. Genere JR et al. Lancet Gastroenterol Hepatol. 2022;7(6):503-4.
Introduction
Inflammatory bowel disease (IBD) is a chronic, relapsing and remitting disorder that is becoming increasingly prevalent worldwide.1 Despite major advances in this area, many patients with moderate to severe IBD do not achieve disease remission with immunosuppressive therapy.2 Dysplasia and fibrostenosis are two common consequences of uncontrolled chronic inflammation and these structural complications are often the primary reasons for surgical interventions.3 While there is certainly a time and a place for surgery in IBD, this approach is invasive and postoperative recrudescence of disease is common.4 Moreover patients with complex surgical or medical histories may not make optimal surgical candidates.
Thanks to advancements in a variety of endoscopic technologies, Over the last several years, applications of endoscopic therapies in IBD have been gaining traction and the need for these therapies is expected to continue to rise over time. As such, understanding the domains of available endoscopic options in IBD is important for the modern-day gastroenterologist. In this article, we will discuss some of the recent advancements in endoscopic therapies for IBD and how we may position these in clinical practice.
Protecting against colitis dysplasia and colon cancer
IBD is a risk factor for colorectal cancer because of the dysplasia-carcinoma sequence arising from chronic colitis. Endoscopic resection is the first-line treatment for conventional colitis-associated dysplasia (CAD).5,6 However, larger or complex lesions may not have been previously amenable to this organ-preserving approach. The application of newer techniques has extended the indication for endoscopic resection to include most CAD lesions, as an alternative to proctocolectomy. Endoscopic mucosal resection (EMR) is the most commonly used technique and its outcomes for CAD greater than 2 cm have been excellent (Figure 1).7 However, employing EMR for lesions greater than 2 cm in size may require piecemeal resection and this has been associated with a small risk of local recurrence.8 Endoscopic submucosal dissection (ESD) is an alternate method of endoscopic tissue resection that can reliably achieve en bloc (single specimen) resections even in larger lesions.9

These technical advantages, however, have not been proven to result in broad clinical superiority of ESD over EMR for advanced lesions.10 The other consideration is that ESD is associated with greater risk of perforation and is more technically complex to perform.10 Yet, recent data supporting ESD in larger lesions is amounting and it may be more suitable for situations where conventional techniques fall short.11 To that end, dense submucosal fibrosis is a common characteristic of CAD and may prohibit successful EMR or ESD as a single modality. Different therapeutic methods can be incorporated in these circumstances, including combined ESD and EMR technique, tissue thermal ablation, or even full-thickness resection has been described.11-13
Taken together, we have many effective options for how we can effectively deal with CAD endoscopically and maintain our patients free of colorectal cancer. The method in which this is done may not matter as much at this juncture and may be more dependent on available local clinical expertise. Moreover, we can’t forget that metachronous lesions and neoplastic recurrence after endoscopic resection are not uncommon and a structured, vigilant endoscopic surveillance program for all patients undergoing endoscopic management of CAD is mandated.7,10
Restoring gastrointestinal tract transit
Crohn’s strictures may lead to acute intestinal obstructions or facilitate the onset of penetrating disease, such as fistula formation or abscess. These strictures are often characterized by a combination of inflammation and layered fibrosis, which requires the application of medical therapies alongside structural remodeling to successfully manage. Not all strictures may be clinically overt due to variances in visceral sensitivity, yet experts believe that treatment of all strictures should be considered to avoid occurrence of delayed complications.14 Endoscopic balloon dilation (EBD) is a well-established treatment for Crohn’s strictures up to 4-5 cm in length (Figure 2). This treatment involves inflating a balloon within the narrowed section of intestine, thereby stretching and disrupting the layered fibrotic bands to widen the stricture. EBD improves symptoms 70% of the time and successfully avoids the medium-term need for surgery in most, although it often requires repeat endoscopic procedures.15 In fact, up to 74% of patients will require repeat dilation over 2 years and 43% will require salvage surgery after EBD.16

Endoscopic stricturotomy (Est) is a newer technique that involves making radial and longitudinal incisions within the stricture using an endoscopic knife (Figure 2). The ability to excise fibrotic bands allows for more advanced remodeling and thus a lower need for reintervention or surgery (9%-22.5%) in comparison with EBD, while maintaining similar technical and clinical success rates.17 Est also carries a lower risk of perforation, but a higher risk of delayed bleeding.17 Refinements in Est are ongoing as the technique continues to develop, including the application of prophylactic clips after Est or use of other hemostatic agents such as gels or powders to minimizing bleeding risk. Despite this, Est has clear benefit in durability for treating strictures especially anastomotic subtype or those refractory to balloon dilation.
Stenting is a third option for treating strictures in Crohn’s disease that is reserved for specific situations. This approach involves endoscopic implantation of a covered metallic stent within the stricture in order to promote remodeling throughout a selected dwell time (generally 2-4 weeks). Stents may be considered in nonoperative candidates with strictures longer than 5 cm, which are generally too long for EBD or Est, or in EBD-refractory strictures in which there is no clear plane for Est excision. However, given the risk of migration, stents are currently not considered a first-line treatment of IBD-related strictures.18 Perhaps with further modifications in design and availability of stent-fixation methods, their use may become more practical in the future.19
The future for endoscopic therapy is bright
Structural complications of IBD are common and can pose a significant detriment to quality of life and general well-being for patients. From mucosal resection of CAD to surgery-sparing therapies for intestinal strictures, endoscopic therapies are valuable and effective options for managing disease-related sequelae within the scope of interventional IBD practice. We can expect the availability of these options to grow as the scope of endoscopy training incorporates principles of interventional IBD, along with the concurrent development of additional therapeutic applications beyond the categories discussed here (including perianal disease, fistulas, and abscess formation). It is noteworthy to mention that while endoscopic therapies are separate treatment modalities, should not be considered mutually exclusive; endotherapies are best viewed as a complement to existing medical and surgical approaches. Thus, Interventional IBD endoscopy can serve as an integral part of the multidisciplinary IBD framework to provide comprehensive care for our patients with IBD.
Juan Reyes Genere, MD, is an assistant professor of medicine in gastroenterology at Washington University in St. Louis. He served as the corresponding author of this article. Michael Rubeiz, MD, is a physician in the internal medicine residency program at Washington University in St. Louis. Kemmian Johnson, MD, MPH, is a gastroenterologist at Washington University in St. Louis specializing in inflammatory bowel disease. Dr. Genere is a consultant for Edulis Therapeutics. Dr. Rubeiz and Dr. Johnson had no personal or financial conflicts of interest. Dr. Johnson can be reached on Instagram @KJ.1906; Dr. Rubeiz is on X @MichaelRubeiz1 and Dr. Genere can be reached via X @JPGenereMD.
References
1. Ng SC et al. Lancet. 2017;390(10114):2769-78.
2. Gordon JP et al. Eur J Gastroenterol Hepatol. 2015;27(7):804-12.
3. Sica GS and Biancone L. World J Gastroenterol. 2013;19(16):2445-8.
4. Iborra M et al. Gastroenterol Rep (Oxf). 2019;7(6):411-8.
5. Annese V et al. J Crohns Colitis. 2013;7(12):982-1018.
6. Laine L et al. Gastrointest Endosc. 2015;81(3):489-501.e426.
7. Mohan BP et al. Gastrointest Endosc. 2021;93(1):59-67.e10.
8. Briedigkeit A et al. World J Gastrointest Endosc. 2016;8(5):276-81.
9. Manta R et al. J Crohns Colitis. 2021;15(1):165-8.
10. Mohapatra S et al. Endosc Int Open. 2022;10(5):E593-601.
11. Ngamruengphong S et al. Endosc Int Open. 2022;10(4):E354-60.
12. Baker G et al. Cureus. 2022 May 3;14(5):e24688.
13. Yadav S et al. Endosc Int Open. 2019;7(8):E994-1001.
14. Schwartz DA. Gastrointestinal Endoscopy. 2023;97(5):974-6.
15. Morar PS et al. Aliment Pharmacol Ther. 2015;42(10):1137-48.
16. Bettenworth D et al. Inflamm Bowel Dis. 2017;23(1):133-42.
17. Lan N and Shen B. Inflamm Bowel Dis. 2018;24(4):897-907.
18. Loras C et al. Lancet Gastroenterol Hepatol. 2022;7(4):332-41.
19. Genere JR et al. Lancet Gastroenterol Hepatol. 2022;7(6):503-4.
Introduction
Inflammatory bowel disease (IBD) is a chronic, relapsing and remitting disorder that is becoming increasingly prevalent worldwide.1 Despite major advances in this area, many patients with moderate to severe IBD do not achieve disease remission with immunosuppressive therapy.2 Dysplasia and fibrostenosis are two common consequences of uncontrolled chronic inflammation and these structural complications are often the primary reasons for surgical interventions.3 While there is certainly a time and a place for surgery in IBD, this approach is invasive and postoperative recrudescence of disease is common.4 Moreover patients with complex surgical or medical histories may not make optimal surgical candidates.
Thanks to advancements in a variety of endoscopic technologies, Over the last several years, applications of endoscopic therapies in IBD have been gaining traction and the need for these therapies is expected to continue to rise over time. As such, understanding the domains of available endoscopic options in IBD is important for the modern-day gastroenterologist. In this article, we will discuss some of the recent advancements in endoscopic therapies for IBD and how we may position these in clinical practice.
Protecting against colitis dysplasia and colon cancer
IBD is a risk factor for colorectal cancer because of the dysplasia-carcinoma sequence arising from chronic colitis. Endoscopic resection is the first-line treatment for conventional colitis-associated dysplasia (CAD).5,6 However, larger or complex lesions may not have been previously amenable to this organ-preserving approach. The application of newer techniques has extended the indication for endoscopic resection to include most CAD lesions, as an alternative to proctocolectomy. Endoscopic mucosal resection (EMR) is the most commonly used technique and its outcomes for CAD greater than 2 cm have been excellent (Figure 1).7 However, employing EMR for lesions greater than 2 cm in size may require piecemeal resection and this has been associated with a small risk of local recurrence.8 Endoscopic submucosal dissection (ESD) is an alternate method of endoscopic tissue resection that can reliably achieve en bloc (single specimen) resections even in larger lesions.9

These technical advantages, however, have not been proven to result in broad clinical superiority of ESD over EMR for advanced lesions.10 The other consideration is that ESD is associated with greater risk of perforation and is more technically complex to perform.10 Yet, recent data supporting ESD in larger lesions is amounting and it may be more suitable for situations where conventional techniques fall short.11 To that end, dense submucosal fibrosis is a common characteristic of CAD and may prohibit successful EMR or ESD as a single modality. Different therapeutic methods can be incorporated in these circumstances, including combined ESD and EMR technique, tissue thermal ablation, or even full-thickness resection has been described.11-13
Taken together, we have many effective options for how we can effectively deal with CAD endoscopically and maintain our patients free of colorectal cancer. The method in which this is done may not matter as much at this juncture and may be more dependent on available local clinical expertise. Moreover, we can’t forget that metachronous lesions and neoplastic recurrence after endoscopic resection are not uncommon and a structured, vigilant endoscopic surveillance program for all patients undergoing endoscopic management of CAD is mandated.7,10
Restoring gastrointestinal tract transit
Crohn’s strictures may lead to acute intestinal obstructions or facilitate the onset of penetrating disease, such as fistula formation or abscess. These strictures are often characterized by a combination of inflammation and layered fibrosis, which requires the application of medical therapies alongside structural remodeling to successfully manage. Not all strictures may be clinically overt due to variances in visceral sensitivity, yet experts believe that treatment of all strictures should be considered to avoid occurrence of delayed complications.14 Endoscopic balloon dilation (EBD) is a well-established treatment for Crohn’s strictures up to 4-5 cm in length (Figure 2). This treatment involves inflating a balloon within the narrowed section of intestine, thereby stretching and disrupting the layered fibrotic bands to widen the stricture. EBD improves symptoms 70% of the time and successfully avoids the medium-term need for surgery in most, although it often requires repeat endoscopic procedures.15 In fact, up to 74% of patients will require repeat dilation over 2 years and 43% will require salvage surgery after EBD.16

Endoscopic stricturotomy (Est) is a newer technique that involves making radial and longitudinal incisions within the stricture using an endoscopic knife (Figure 2). The ability to excise fibrotic bands allows for more advanced remodeling and thus a lower need for reintervention or surgery (9%-22.5%) in comparison with EBD, while maintaining similar technical and clinical success rates.17 Est also carries a lower risk of perforation, but a higher risk of delayed bleeding.17 Refinements in Est are ongoing as the technique continues to develop, including the application of prophylactic clips after Est or use of other hemostatic agents such as gels or powders to minimizing bleeding risk. Despite this, Est has clear benefit in durability for treating strictures especially anastomotic subtype or those refractory to balloon dilation.
Stenting is a third option for treating strictures in Crohn’s disease that is reserved for specific situations. This approach involves endoscopic implantation of a covered metallic stent within the stricture in order to promote remodeling throughout a selected dwell time (generally 2-4 weeks). Stents may be considered in nonoperative candidates with strictures longer than 5 cm, which are generally too long for EBD or Est, or in EBD-refractory strictures in which there is no clear plane for Est excision. However, given the risk of migration, stents are currently not considered a first-line treatment of IBD-related strictures.18 Perhaps with further modifications in design and availability of stent-fixation methods, their use may become more practical in the future.19
The future for endoscopic therapy is bright
Structural complications of IBD are common and can pose a significant detriment to quality of life and general well-being for patients. From mucosal resection of CAD to surgery-sparing therapies for intestinal strictures, endoscopic therapies are valuable and effective options for managing disease-related sequelae within the scope of interventional IBD practice. We can expect the availability of these options to grow as the scope of endoscopy training incorporates principles of interventional IBD, along with the concurrent development of additional therapeutic applications beyond the categories discussed here (including perianal disease, fistulas, and abscess formation). It is noteworthy to mention that while endoscopic therapies are separate treatment modalities, should not be considered mutually exclusive; endotherapies are best viewed as a complement to existing medical and surgical approaches. Thus, Interventional IBD endoscopy can serve as an integral part of the multidisciplinary IBD framework to provide comprehensive care for our patients with IBD.
Juan Reyes Genere, MD, is an assistant professor of medicine in gastroenterology at Washington University in St. Louis. He served as the corresponding author of this article. Michael Rubeiz, MD, is a physician in the internal medicine residency program at Washington University in St. Louis. Kemmian Johnson, MD, MPH, is a gastroenterologist at Washington University in St. Louis specializing in inflammatory bowel disease. Dr. Genere is a consultant for Edulis Therapeutics. Dr. Rubeiz and Dr. Johnson had no personal or financial conflicts of interest. Dr. Johnson can be reached on Instagram @KJ.1906; Dr. Rubeiz is on X @MichaelRubeiz1 and Dr. Genere can be reached via X @JPGenereMD.
References
1. Ng SC et al. Lancet. 2017;390(10114):2769-78.
2. Gordon JP et al. Eur J Gastroenterol Hepatol. 2015;27(7):804-12.
3. Sica GS and Biancone L. World J Gastroenterol. 2013;19(16):2445-8.
4. Iborra M et al. Gastroenterol Rep (Oxf). 2019;7(6):411-8.
5. Annese V et al. J Crohns Colitis. 2013;7(12):982-1018.
6. Laine L et al. Gastrointest Endosc. 2015;81(3):489-501.e426.
7. Mohan BP et al. Gastrointest Endosc. 2021;93(1):59-67.e10.
8. Briedigkeit A et al. World J Gastrointest Endosc. 2016;8(5):276-81.
9. Manta R et al. J Crohns Colitis. 2021;15(1):165-8.
10. Mohapatra S et al. Endosc Int Open. 2022;10(5):E593-601.
11. Ngamruengphong S et al. Endosc Int Open. 2022;10(4):E354-60.
12. Baker G et al. Cureus. 2022 May 3;14(5):e24688.
13. Yadav S et al. Endosc Int Open. 2019;7(8):E994-1001.
14. Schwartz DA. Gastrointestinal Endoscopy. 2023;97(5):974-6.
15. Morar PS et al. Aliment Pharmacol Ther. 2015;42(10):1137-48.
16. Bettenworth D et al. Inflamm Bowel Dis. 2017;23(1):133-42.
17. Lan N and Shen B. Inflamm Bowel Dis. 2018;24(4):897-907.
18. Loras C et al. Lancet Gastroenterol Hepatol. 2022;7(4):332-41.
19. Genere JR et al. Lancet Gastroenterol Hepatol. 2022;7(6):503-4.
Advances in endohepatology
Introduction
Historically, the role of endoscopy in hepatology has been limited to intraluminal and bile duct interventions, primarily for the management of varices and biliary strictures. Recently, endoscopic ultrasound (EUS) has broadened the range of endoscopic treatment by enabling transluminal access to the liver parenchyma and associated vasculature. In this review, we will address recent advances in the expanding field of endohepatology.
Endoscopic-ultrasound guided liver biopsy
Liver biopsies are a critical tool in the diagnostic evaluation and management of patients with liver disease. Conventional approaches for obtaining liver tissue have been most commonly through the percutaneous or vascular approaches. In 2007, the first EUS-guided liver biopsy (EUS-LB) was described.1 EUS-LB is performed by advancing a line-array echoendoscope to the duodenal bulb to access the right lobe of the liver or proximal stomach to sample the left lobe. Doppler is first used to identify a pathway with few intervening vessels. Then a 19G or 20G needle is passed and slowly withdrawn to capture tissue (Figure 1). Careful evaluation with Doppler ultrasound to evaluate for bleeding is recommended after EUS-LB and if persistent, a small amount of clot may be reinjected as a blood or “Chang” patch akin to technique to control oozing postlumbar puncture.2
While large prospective studies are needed to compare the methods, it appears that specimen adequacy acquired via EUS-LB are comparable to percutaneous and transjugular approaches.3-5 Utilization of specific needle types and suction may optimize samples. Namely, 19G needles may provide better samples than smaller sizes and contemporary fine-needle biopsy needles with Franseen tips are superior to conventional spring-loaded cutting needles and fork tip needles.6-8 The use of dry suction has been shown to increase the yield of tissue, but at the expense of increased bloodiness. Wet suction, which involves the presence of fluid, rather than air, in the needle lumen to lubricate and improve transmission of negative pressure to the needle tip, is the preferred technique for EUS-LB given improvement in the likelihood of intact liver biopsy cores and increased specimen adequacy.9
There are several advantages to EUS-LB (Table 1). When compared with percutaneous liver biopsy (PC-LB) and transjugular liver biopsy (TJ-LB), EUS-LB is uniquely able to access both liver lobes in a single setting, which minimizes sampling error.3 EUS-LB may also have an advantage in sampling focal liver lesions given the close proximity of the transducer to the liver.10 Another advantage over PC-LB is that EUS-LB can be performed in patients with a large body habitus. Additionally, EUS-LB is better tolerated than PC-LB, with less postprocedure pain and shorter postprocedure monitoring time.4,5
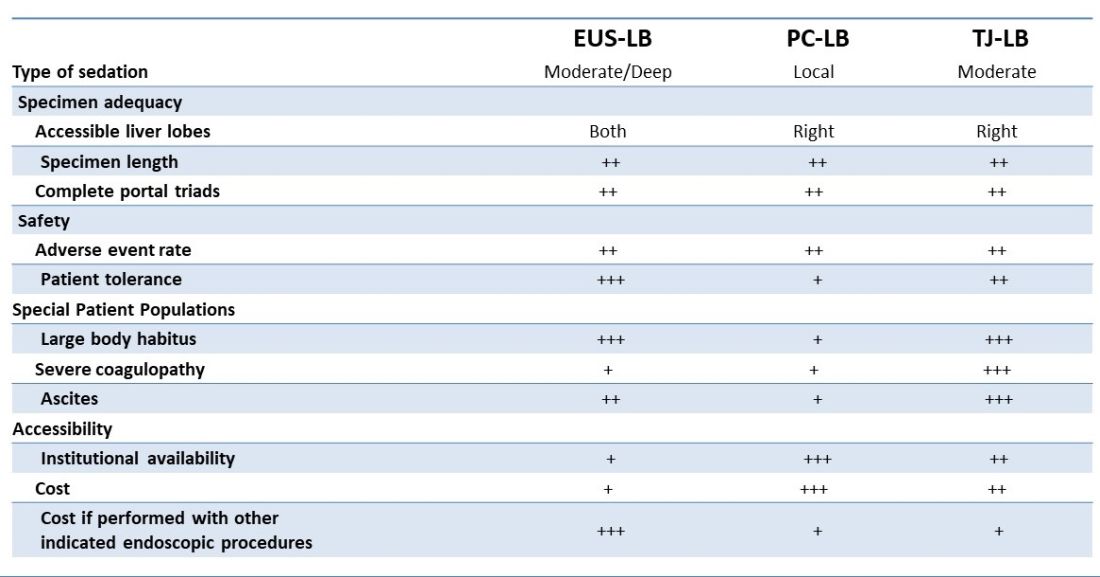
Rates of adverse events appear to be similar between the three methods. Similar to PC-LB, EUS-LB requires capsular puncture, which can lead to intraperitoneal hemorrhage. Therefore, TJ-LB is preferred in patients with significant coagulopathy. While small ascites is not an absolute contraindication for EUS-LB, large ascites can obscure a safe window from the proximal stomach or duodenum to the liver, and thus TJLB is also preferred in these patients.11 Given its relative novelty and logistic challenges, other disadvantages of EUS-LB include limited provider availability and increased cost, especially compared with PC-LB. The most significant limitation is that it requires moderate or deep sedation, as opposed to local anesthetics. However, if there is another indication for endoscopy (that is, variceal screening), then “one-stop shop” procedures including EUS-LB may be more convenient and cost-effective than traditional methods. Nevertheless, rigorous comparative studies are needed.
EUS-guided portal pressure gradient measurement
The presence of clinically significant portal hypertension (CSPH), defined as hepatic venous pressure gradient (HVPG) greater than or equal to 10 is a potent predictor of decompensation. There is growing evidence to support the use of beta-blockers to mitigate this risk.12 Therefore, early identification of patients with CSPH has important diagnostic and therapeutic implications. The current gold standard for diagnosing CSPH is with wedged HVPG measurements performed by interventional radiology.
Since its introduction in 2016, EUS-guided portal pressure gradient measurement (EUS-PPG) has emerged as an alternative to wedged HVPG.13,14 Using a linear echoendoscope, the portal vein is directly accessed with a 25G fine-needle aspiration needle, and three direct measurements are taken using a compact manometer to determine the mean pressure. The hepatic vein, or less commonly the inferior vena cava, pressure is also measured. The direct measurement of portal pressure provides a significant advantage of EUS-PPG over HVPG in patients with presinusoidal and prehepatic portal hypertension. Wedged HVPG, which utilizes the difference between the wedged and free hepatic venous pressure to indirectly estimate the portal venous pressure gradient, yields erroneously low gradients in patients with noncirrhotic portal hypertension.15 An additional advantage of EUS-PPG is that it obviates the need for a central venous line placement, which is associated with thrombosis and, in rare cases, air embolus.16
Observational studies indicate that EUS-PPG has a high degree of consistency with HVPG measurements and a strong correlation between other clinical findings of portal hyper-tension including esophageal varices and thrombocytopenia.13,14 Nevertheless, EUS-PPG is performed under moderate or deep sedation which may impact HVPG measurements.17 In addition, the real-world application of EUS-PPG measurement on clinical care is undefined, but it is the topic of an ongoing clinical trial (ClinicalTrials.gov – NCT05357599).
EUS-guided interventions of gastric varices
Compared with esophageal varices, current approaches to the treatment and prophylaxis of gastric varices are more controversial.18 The most common approach to bleeding gastric varices in the United States is the placement of a transjugular intrahepatic portosystemic shunt (TIPS). Nevertheless, in addition to risks associated with central venous line placement, 5%-35% of individuals develop hepatic encephalopathy after TIPS and ischemic acute liver failure can occur in rare situations.19 Cyanoacrylate (CYA) glue injection is the recommended first-line endoscopic therapy for the treatment of bleeding gastric varices, but use has not been widely adopted in the United States because of a lack of an approved Food and Drug Administration CYA formulation, limited expertise, and risk of serious complications. In particular systemic embolization may result in pulmonary or cerebral infarct.12,18 EUS-guided interventions have been developed to mitigate these safety concerns. EUS-guided coil embolization can be performed, either alone or in combination with CYA injection.20 In the latter approach it acts as a scaffold to prevent migration of the glue bolus. Doppler assessment enables direct visualization of the gastric varix for identification of feeder vessels, more controlled deployment of hemostatic agents, and real-time confirmation of varix obliteration. Fluoroscopy can be used as an adjunct.
EUS-guided interventions in the management of gastric varices appear to be effective and superior to CYA injection under direct endoscopic visualization with improved likelihood of obliteration and lower rebleeding rates, without increase in adverse events.21 Additionally, EUS-guided combination therapy improves technical outcomes and reduces adverse events relative to EUS-guided coil or EUS-guided glue injection therapy alone.21-23 Nevertheless, large-scale prospective trials are needed to determine whether EUS-guided interventions should be considered over TIPS. The role of EUS-guided interventions as primary prophylaxis to prevent bleeding from large gastric varices also requires additional study.24
Future directions
with the goal of optimizing care and increasing efficiency. In addition to new endoscopic procedures to optimize liver biopsy, portal pressure measurement, and gastric variceal treatment, there are a number of emerging technologies including EUS-guided liver elastography, portal venous sampling, liver tumor chemoembolization, and intrahepatic portosystemic shunts.25 However, the practice of endohepatology faces a number of challenges before widespread adoption, including limited provider expertise and institutional availability. Additionally, more robust, multicenter outcomes and cost-effective analyses comparing these novel procedures with traditional approaches are needed to define their clinical impact.
Dr. Bui is a fellow in gastroenterology in the division of gastroenterology and hepatology, University of Southern California, Los Angeles. Dr. Buxbaum is associate professor of medicine (clinical scholar) in the division of gastroenterology and hepatology, University of Southern California. Dr. Buxbaum is a consultant for Cook Medical, Boston Scientific, and Olympus. Dr. Bui has no disclosures.
References
1. Mathew A. Am J Gastroenterol. 2007;102(10):2354-5.
2. Sowa P et al. VideoGIE. 2021;6(11):487-8.
3. Pineda JJ et al. Gastrointest Endosc. 2016;83(2):360-5.
4. Ali AH et al. J Ultrasound. 2020;23(2):157-67.
5. Shuja A et al. Dig Liver Dis. 2019;51(6):826-30.
6. Schulman AR et al. Gastrointest Endosc. 2017;85(2):419-26.
7. DeWitt J et al. Endosc Int Open. 2015;3(5):E471-8.
8. Aggarwal SN et al. Gastrointest Endosc. 2021;93(5):1133-8.
9. Mok SRS et al. Gastrointest Endosc. 2018;88(6):919-25.
10. Lee YN et al. J Gastroenterol Hepatol. 2015;30(7):1161-6.
11. Kalambokis G et al. J Hepatol. 2007;47(2):284-94.
12. de Franchis R et al. J Hepatol. 2022;76(4):959-74.
13. Choi AY et al. J Gastroenterol Hepatol. 2022;37(7):1373-9.
14. Zhang W et al. Gastrointest Endosc. 2021;93(3):565-72.
15. Seijo S et al. Dig Liver Dis. 2012;44(10):855-60.
16. Vesely TM. J Vasc Interv Radiol. 2001;12(11):1291-5.
17. Reverter E et al. Liver Int. 2014;34(1):16-25.
18. Henry Z et al. Clin Gastroenterol Hepatol. 2021;19(6):1098-107.e1091.
19. Ripamonti R et al. Semin Intervent Radiol. 2006;23(2):165-76.
20. Rengstorff DS and Binmoeller KF. Gastrointest Endosc. 2004;59(4):553-8.
21. Mohan BP et al. Endoscopy. 2020;52(4):259-67.
22. Robles-Medranda C et al. Endoscopy. 2020;52(4):268-75.
23. McCarty TR et al. Endosc Ultrasound. 2020;9(1):6-15.
24. Kouanda A et al. Gastrointest Endosc. 2021;94(2):291-6.
25. Bazarbashi AN et al. 2022;24(1):98-107.
Introduction
Historically, the role of endoscopy in hepatology has been limited to intraluminal and bile duct interventions, primarily for the management of varices and biliary strictures. Recently, endoscopic ultrasound (EUS) has broadened the range of endoscopic treatment by enabling transluminal access to the liver parenchyma and associated vasculature. In this review, we will address recent advances in the expanding field of endohepatology.
Endoscopic-ultrasound guided liver biopsy
Liver biopsies are a critical tool in the diagnostic evaluation and management of patients with liver disease. Conventional approaches for obtaining liver tissue have been most commonly through the percutaneous or vascular approaches. In 2007, the first EUS-guided liver biopsy (EUS-LB) was described.1 EUS-LB is performed by advancing a line-array echoendoscope to the duodenal bulb to access the right lobe of the liver or proximal stomach to sample the left lobe. Doppler is first used to identify a pathway with few intervening vessels. Then a 19G or 20G needle is passed and slowly withdrawn to capture tissue (Figure 1). Careful evaluation with Doppler ultrasound to evaluate for bleeding is recommended after EUS-LB and if persistent, a small amount of clot may be reinjected as a blood or “Chang” patch akin to technique to control oozing postlumbar puncture.2

While large prospective studies are needed to compare the methods, it appears that specimen adequacy acquired via EUS-LB are comparable to percutaneous and transjugular approaches.3-5 Utilization of specific needle types and suction may optimize samples. Namely, 19G needles may provide better samples than smaller sizes and contemporary fine-needle biopsy needles with Franseen tips are superior to conventional spring-loaded cutting needles and fork tip needles.6-8 The use of dry suction has been shown to increase the yield of tissue, but at the expense of increased bloodiness. Wet suction, which involves the presence of fluid, rather than air, in the needle lumen to lubricate and improve transmission of negative pressure to the needle tip, is the preferred technique for EUS-LB given improvement in the likelihood of intact liver biopsy cores and increased specimen adequacy.9
There are several advantages to EUS-LB (Table 1). When compared with percutaneous liver biopsy (PC-LB) and transjugular liver biopsy (TJ-LB), EUS-LB is uniquely able to access both liver lobes in a single setting, which minimizes sampling error.3 EUS-LB may also have an advantage in sampling focal liver lesions given the close proximity of the transducer to the liver.10 Another advantage over PC-LB is that EUS-LB can be performed in patients with a large body habitus. Additionally, EUS-LB is better tolerated than PC-LB, with less postprocedure pain and shorter postprocedure monitoring time.4,5

Rates of adverse events appear to be similar between the three methods. Similar to PC-LB, EUS-LB requires capsular puncture, which can lead to intraperitoneal hemorrhage. Therefore, TJ-LB is preferred in patients with significant coagulopathy. While small ascites is not an absolute contraindication for EUS-LB, large ascites can obscure a safe window from the proximal stomach or duodenum to the liver, and thus TJLB is also preferred in these patients.11 Given its relative novelty and logistic challenges, other disadvantages of EUS-LB include limited provider availability and increased cost, especially compared with PC-LB. The most significant limitation is that it requires moderate or deep sedation, as opposed to local anesthetics. However, if there is another indication for endoscopy (that is, variceal screening), then “one-stop shop” procedures including EUS-LB may be more convenient and cost-effective than traditional methods. Nevertheless, rigorous comparative studies are needed.
EUS-guided portal pressure gradient measurement
The presence of clinically significant portal hypertension (CSPH), defined as hepatic venous pressure gradient (HVPG) greater than or equal to 10 is a potent predictor of decompensation. There is growing evidence to support the use of beta-blockers to mitigate this risk.12 Therefore, early identification of patients with CSPH has important diagnostic and therapeutic implications. The current gold standard for diagnosing CSPH is with wedged HVPG measurements performed by interventional radiology.
Since its introduction in 2016, EUS-guided portal pressure gradient measurement (EUS-PPG) has emerged as an alternative to wedged HVPG.13,14 Using a linear echoendoscope, the portal vein is directly accessed with a 25G fine-needle aspiration needle, and three direct measurements are taken using a compact manometer to determine the mean pressure. The hepatic vein, or less commonly the inferior vena cava, pressure is also measured. The direct measurement of portal pressure provides a significant advantage of EUS-PPG over HVPG in patients with presinusoidal and prehepatic portal hypertension. Wedged HVPG, which utilizes the difference between the wedged and free hepatic venous pressure to indirectly estimate the portal venous pressure gradient, yields erroneously low gradients in patients with noncirrhotic portal hypertension.15 An additional advantage of EUS-PPG is that it obviates the need for a central venous line placement, which is associated with thrombosis and, in rare cases, air embolus.16
Observational studies indicate that EUS-PPG has a high degree of consistency with HVPG measurements and a strong correlation between other clinical findings of portal hyper-tension including esophageal varices and thrombocytopenia.13,14 Nevertheless, EUS-PPG is performed under moderate or deep sedation which may impact HVPG measurements.17 In addition, the real-world application of EUS-PPG measurement on clinical care is undefined, but it is the topic of an ongoing clinical trial (ClinicalTrials.gov – NCT05357599).
EUS-guided interventions of gastric varices
Compared with esophageal varices, current approaches to the treatment and prophylaxis of gastric varices are more controversial.18 The most common approach to bleeding gastric varices in the United States is the placement of a transjugular intrahepatic portosystemic shunt (TIPS). Nevertheless, in addition to risks associated with central venous line placement, 5%-35% of individuals develop hepatic encephalopathy after TIPS and ischemic acute liver failure can occur in rare situations.19 Cyanoacrylate (CYA) glue injection is the recommended first-line endoscopic therapy for the treatment of bleeding gastric varices, but use has not been widely adopted in the United States because of a lack of an approved Food and Drug Administration CYA formulation, limited expertise, and risk of serious complications. In particular systemic embolization may result in pulmonary or cerebral infarct.12,18 EUS-guided interventions have been developed to mitigate these safety concerns. EUS-guided coil embolization can be performed, either alone or in combination with CYA injection.20 In the latter approach it acts as a scaffold to prevent migration of the glue bolus. Doppler assessment enables direct visualization of the gastric varix for identification of feeder vessels, more controlled deployment of hemostatic agents, and real-time confirmation of varix obliteration. Fluoroscopy can be used as an adjunct.
EUS-guided interventions in the management of gastric varices appear to be effective and superior to CYA injection under direct endoscopic visualization with improved likelihood of obliteration and lower rebleeding rates, without increase in adverse events.21 Additionally, EUS-guided combination therapy improves technical outcomes and reduces adverse events relative to EUS-guided coil or EUS-guided glue injection therapy alone.21-23 Nevertheless, large-scale prospective trials are needed to determine whether EUS-guided interventions should be considered over TIPS. The role of EUS-guided interventions as primary prophylaxis to prevent bleeding from large gastric varices also requires additional study.24
Future directions
with the goal of optimizing care and increasing efficiency. In addition to new endoscopic procedures to optimize liver biopsy, portal pressure measurement, and gastric variceal treatment, there are a number of emerging technologies including EUS-guided liver elastography, portal venous sampling, liver tumor chemoembolization, and intrahepatic portosystemic shunts.25 However, the practice of endohepatology faces a number of challenges before widespread adoption, including limited provider expertise and institutional availability. Additionally, more robust, multicenter outcomes and cost-effective analyses comparing these novel procedures with traditional approaches are needed to define their clinical impact.
Dr. Bui is a fellow in gastroenterology in the division of gastroenterology and hepatology, University of Southern California, Los Angeles. Dr. Buxbaum is associate professor of medicine (clinical scholar) in the division of gastroenterology and hepatology, University of Southern California. Dr. Buxbaum is a consultant for Cook Medical, Boston Scientific, and Olympus. Dr. Bui has no disclosures.
References
1. Mathew A. Am J Gastroenterol. 2007;102(10):2354-5.
2. Sowa P et al. VideoGIE. 2021;6(11):487-8.
3. Pineda JJ et al. Gastrointest Endosc. 2016;83(2):360-5.
4. Ali AH et al. J Ultrasound. 2020;23(2):157-67.
5. Shuja A et al. Dig Liver Dis. 2019;51(6):826-30.
6. Schulman AR et al. Gastrointest Endosc. 2017;85(2):419-26.
7. DeWitt J et al. Endosc Int Open. 2015;3(5):E471-8.
8. Aggarwal SN et al. Gastrointest Endosc. 2021;93(5):1133-8.
9. Mok SRS et al. Gastrointest Endosc. 2018;88(6):919-25.
10. Lee YN et al. J Gastroenterol Hepatol. 2015;30(7):1161-6.
11. Kalambokis G et al. J Hepatol. 2007;47(2):284-94.
12. de Franchis R et al. J Hepatol. 2022;76(4):959-74.
13. Choi AY et al. J Gastroenterol Hepatol. 2022;37(7):1373-9.
14. Zhang W et al. Gastrointest Endosc. 2021;93(3):565-72.
15. Seijo S et al. Dig Liver Dis. 2012;44(10):855-60.
16. Vesely TM. J Vasc Interv Radiol. 2001;12(11):1291-5.
17. Reverter E et al. Liver Int. 2014;34(1):16-25.
18. Henry Z et al. Clin Gastroenterol Hepatol. 2021;19(6):1098-107.e1091.
19. Ripamonti R et al. Semin Intervent Radiol. 2006;23(2):165-76.
20. Rengstorff DS and Binmoeller KF. Gastrointest Endosc. 2004;59(4):553-8.
21. Mohan BP et al. Endoscopy. 2020;52(4):259-67.
22. Robles-Medranda C et al. Endoscopy. 2020;52(4):268-75.
23. McCarty TR et al. Endosc Ultrasound. 2020;9(1):6-15.
24. Kouanda A et al. Gastrointest Endosc. 2021;94(2):291-6.
25. Bazarbashi AN et al. 2022;24(1):98-107.
Introduction
Historically, the role of endoscopy in hepatology has been limited to intraluminal and bile duct interventions, primarily for the management of varices and biliary strictures. Recently, endoscopic ultrasound (EUS) has broadened the range of endoscopic treatment by enabling transluminal access to the liver parenchyma and associated vasculature. In this review, we will address recent advances in the expanding field of endohepatology.
Endoscopic-ultrasound guided liver biopsy
Liver biopsies are a critical tool in the diagnostic evaluation and management of patients with liver disease. Conventional approaches for obtaining liver tissue have been most commonly through the percutaneous or vascular approaches. In 2007, the first EUS-guided liver biopsy (EUS-LB) was described.1 EUS-LB is performed by advancing a line-array echoendoscope to the duodenal bulb to access the right lobe of the liver or proximal stomach to sample the left lobe. Doppler is first used to identify a pathway with few intervening vessels. Then a 19G or 20G needle is passed and slowly withdrawn to capture tissue (Figure 1). Careful evaluation with Doppler ultrasound to evaluate for bleeding is recommended after EUS-LB and if persistent, a small amount of clot may be reinjected as a blood or “Chang” patch akin to technique to control oozing postlumbar puncture.2

While large prospective studies are needed to compare the methods, it appears that specimen adequacy acquired via EUS-LB are comparable to percutaneous and transjugular approaches.3-5 Utilization of specific needle types and suction may optimize samples. Namely, 19G needles may provide better samples than smaller sizes and contemporary fine-needle biopsy needles with Franseen tips are superior to conventional spring-loaded cutting needles and fork tip needles.6-8 The use of dry suction has been shown to increase the yield of tissue, but at the expense of increased bloodiness. Wet suction, which involves the presence of fluid, rather than air, in the needle lumen to lubricate and improve transmission of negative pressure to the needle tip, is the preferred technique for EUS-LB given improvement in the likelihood of intact liver biopsy cores and increased specimen adequacy.9
There are several advantages to EUS-LB (Table 1). When compared with percutaneous liver biopsy (PC-LB) and transjugular liver biopsy (TJ-LB), EUS-LB is uniquely able to access both liver lobes in a single setting, which minimizes sampling error.3 EUS-LB may also have an advantage in sampling focal liver lesions given the close proximity of the transducer to the liver.10 Another advantage over PC-LB is that EUS-LB can be performed in patients with a large body habitus. Additionally, EUS-LB is better tolerated than PC-LB, with less postprocedure pain and shorter postprocedure monitoring time.4,5

Rates of adverse events appear to be similar between the three methods. Similar to PC-LB, EUS-LB requires capsular puncture, which can lead to intraperitoneal hemorrhage. Therefore, TJ-LB is preferred in patients with significant coagulopathy. While small ascites is not an absolute contraindication for EUS-LB, large ascites can obscure a safe window from the proximal stomach or duodenum to the liver, and thus TJLB is also preferred in these patients.11 Given its relative novelty and logistic challenges, other disadvantages of EUS-LB include limited provider availability and increased cost, especially compared with PC-LB. The most significant limitation is that it requires moderate or deep sedation, as opposed to local anesthetics. However, if there is another indication for endoscopy (that is, variceal screening), then “one-stop shop” procedures including EUS-LB may be more convenient and cost-effective than traditional methods. Nevertheless, rigorous comparative studies are needed.
EUS-guided portal pressure gradient measurement
The presence of clinically significant portal hypertension (CSPH), defined as hepatic venous pressure gradient (HVPG) greater than or equal to 10 is a potent predictor of decompensation. There is growing evidence to support the use of beta-blockers to mitigate this risk.12 Therefore, early identification of patients with CSPH has important diagnostic and therapeutic implications. The current gold standard for diagnosing CSPH is with wedged HVPG measurements performed by interventional radiology.
Since its introduction in 2016, EUS-guided portal pressure gradient measurement (EUS-PPG) has emerged as an alternative to wedged HVPG.13,14 Using a linear echoendoscope, the portal vein is directly accessed with a 25G fine-needle aspiration needle, and three direct measurements are taken using a compact manometer to determine the mean pressure. The hepatic vein, or less commonly the inferior vena cava, pressure is also measured. The direct measurement of portal pressure provides a significant advantage of EUS-PPG over HVPG in patients with presinusoidal and prehepatic portal hypertension. Wedged HVPG, which utilizes the difference between the wedged and free hepatic venous pressure to indirectly estimate the portal venous pressure gradient, yields erroneously low gradients in patients with noncirrhotic portal hypertension.15 An additional advantage of EUS-PPG is that it obviates the need for a central venous line placement, which is associated with thrombosis and, in rare cases, air embolus.16
Observational studies indicate that EUS-PPG has a high degree of consistency with HVPG measurements and a strong correlation between other clinical findings of portal hyper-tension including esophageal varices and thrombocytopenia.13,14 Nevertheless, EUS-PPG is performed under moderate or deep sedation which may impact HVPG measurements.17 In addition, the real-world application of EUS-PPG measurement on clinical care is undefined, but it is the topic of an ongoing clinical trial (ClinicalTrials.gov – NCT05357599).
EUS-guided interventions of gastric varices
Compared with esophageal varices, current approaches to the treatment and prophylaxis of gastric varices are more controversial.18 The most common approach to bleeding gastric varices in the United States is the placement of a transjugular intrahepatic portosystemic shunt (TIPS). Nevertheless, in addition to risks associated with central venous line placement, 5%-35% of individuals develop hepatic encephalopathy after TIPS and ischemic acute liver failure can occur in rare situations.19 Cyanoacrylate (CYA) glue injection is the recommended first-line endoscopic therapy for the treatment of bleeding gastric varices, but use has not been widely adopted in the United States because of a lack of an approved Food and Drug Administration CYA formulation, limited expertise, and risk of serious complications. In particular systemic embolization may result in pulmonary or cerebral infarct.12,18 EUS-guided interventions have been developed to mitigate these safety concerns. EUS-guided coil embolization can be performed, either alone or in combination with CYA injection.20 In the latter approach it acts as a scaffold to prevent migration of the glue bolus. Doppler assessment enables direct visualization of the gastric varix for identification of feeder vessels, more controlled deployment of hemostatic agents, and real-time confirmation of varix obliteration. Fluoroscopy can be used as an adjunct.
EUS-guided interventions in the management of gastric varices appear to be effective and superior to CYA injection under direct endoscopic visualization with improved likelihood of obliteration and lower rebleeding rates, without increase in adverse events.21 Additionally, EUS-guided combination therapy improves technical outcomes and reduces adverse events relative to EUS-guided coil or EUS-guided glue injection therapy alone.21-23 Nevertheless, large-scale prospective trials are needed to determine whether EUS-guided interventions should be considered over TIPS. The role of EUS-guided interventions as primary prophylaxis to prevent bleeding from large gastric varices also requires additional study.24
Future directions
with the goal of optimizing care and increasing efficiency. In addition to new endoscopic procedures to optimize liver biopsy, portal pressure measurement, and gastric variceal treatment, there are a number of emerging technologies including EUS-guided liver elastography, portal venous sampling, liver tumor chemoembolization, and intrahepatic portosystemic shunts.25 However, the practice of endohepatology faces a number of challenges before widespread adoption, including limited provider expertise and institutional availability. Additionally, more robust, multicenter outcomes and cost-effective analyses comparing these novel procedures with traditional approaches are needed to define their clinical impact.
Dr. Bui is a fellow in gastroenterology in the division of gastroenterology and hepatology, University of Southern California, Los Angeles. Dr. Buxbaum is associate professor of medicine (clinical scholar) in the division of gastroenterology and hepatology, University of Southern California. Dr. Buxbaum is a consultant for Cook Medical, Boston Scientific, and Olympus. Dr. Bui has no disclosures.
References
1. Mathew A. Am J Gastroenterol. 2007;102(10):2354-5.
2. Sowa P et al. VideoGIE. 2021;6(11):487-8.
3. Pineda JJ et al. Gastrointest Endosc. 2016;83(2):360-5.
4. Ali AH et al. J Ultrasound. 2020;23(2):157-67.
5. Shuja A et al. Dig Liver Dis. 2019;51(6):826-30.
6. Schulman AR et al. Gastrointest Endosc. 2017;85(2):419-26.
7. DeWitt J et al. Endosc Int Open. 2015;3(5):E471-8.
8. Aggarwal SN et al. Gastrointest Endosc. 2021;93(5):1133-8.
9. Mok SRS et al. Gastrointest Endosc. 2018;88(6):919-25.
10. Lee YN et al. J Gastroenterol Hepatol. 2015;30(7):1161-6.
11. Kalambokis G et al. J Hepatol. 2007;47(2):284-94.
12. de Franchis R et al. J Hepatol. 2022;76(4):959-74.
13. Choi AY et al. J Gastroenterol Hepatol. 2022;37(7):1373-9.
14. Zhang W et al. Gastrointest Endosc. 2021;93(3):565-72.
15. Seijo S et al. Dig Liver Dis. 2012;44(10):855-60.
16. Vesely TM. J Vasc Interv Radiol. 2001;12(11):1291-5.
17. Reverter E et al. Liver Int. 2014;34(1):16-25.
18. Henry Z et al. Clin Gastroenterol Hepatol. 2021;19(6):1098-107.e1091.
19. Ripamonti R et al. Semin Intervent Radiol. 2006;23(2):165-76.
20. Rengstorff DS and Binmoeller KF. Gastrointest Endosc. 2004;59(4):553-8.
21. Mohan BP et al. Endoscopy. 2020;52(4):259-67.
22. Robles-Medranda C et al. Endoscopy. 2020;52(4):268-75.
23. McCarty TR et al. Endosc Ultrasound. 2020;9(1):6-15.
24. Kouanda A et al. Gastrointest Endosc. 2021;94(2):291-6.
25. Bazarbashi AN et al. 2022;24(1):98-107.