User login
Introduction
Barrett’s esophagus (BE) is characterized by the replacement of squamous epithelium by columnar metaplasia of the distal esophagus (>1 cm length). It is a precancerous condition, with 3%-5% of patients with BE developing esophageal adenocarcinoma (EAC) in their lifetime. EAC is one of the cancers with high morbidity and mortality (5-year survival < 20%), and its incidence has been on the rise. Studies examining the natural history of BE have demonstrated that the progression happens through a metaplasia-dysplasia-neoplasia sequence. Therefore, early detection of BE and timely management to prevent progression to EAC is crucial.
Grades of Dysplasia
The current gold standard for the diagnosis of BE neoplasia includes a high-quality endoscopic evaluation and biopsies. Biopsies should be obtained from any visible lesions (nodules, ulcers) followed by a random 4-quadrant fashion (Seattle protocol) interval of the entire length of the BE segment. It is essential to pay attention to the results of the biopsy that have been obtained since it will not only determine the surveillance interval but is crucial in planning any necessary endoscopic therapy. The possible results of the biopsy and its implications are:
- No intestinal metaplasia (IM): This would rule out Barrett’s esophagus and no further surveillance would be necessary. A recent population-based study of over 1 million patients showed a 55% and 61% reduced risk of upper gastrointestinal (UGI) cancer and deaths respectively after a negative endoscopy.1
- Intestinal metaplasia with no dysplasia (non-dysplastic BE): Biopsies confirm presence of intestinal metaplasia in the biopsies without any evidence of dysplasia. While the rate of progression to EAC is low (0.07%-0.25%), it is not absent and thus surveillance would be indicated. Current guidelines suggest repeating an endoscopy with biopsy in 5 years if the length of BE is < 3 cm or 3 years if length of BE ≥ 3 cm.2
- Indeterminate for dysplasia (BE-IND): Biopsies confirm IM but are not able to definitively rule out dysplasia. This can be seen in about 4%-8% of the biopsies obtained. The progression rates to EAC are reported to be comparable or lower to low-grade dysplasia (LGD), so the current recommendation is to intensify acid reduction therapy and repeat endoscopy in 6 months. If repeat endoscopy downgrades to non-dysplastic, then can follow surveillance according to NDBE protocol; otherwise recommend continuing surveillance every 12 months.
- Low-grade dysplasia (BE-LGD): Biopsies confirm IM but also show tightly packed overlapping basal nuclei with hyperchromasia and irregular contours, basal stratification of nuclei, and diminished goblet and columnar cell mucus. There is significant inter-observer variability reported,3 and thus the slides must be reviewed by a second pathologist with experience in BE to confirm the findings. Once confirmed, based on risk factors such as presence of multifocal LGD, persistence of LGD, presence of visible lesions, etc., the patient can be offered Barrett’s endoscopic therapy (BET) or undergo continued surveillance. The decision of pursuing one or the other would be dependent on patient preference and shared decision-making between the patient and the provider.
- High-grade dysplasia (BE-HGD): Biopsies confirm IM with cells showing greater degree of cytologic and architectural alterations of dysplasia than LGD but without overt neoplastic features. Over 40% of the patients would progress to EAC and thus the current recommendations would be to recommend BET in these patients.4
- Esophageal adenocarcinoma (EAC): Biopsies demonstrate neoplasia. If the neoplastic changes are limited to the mucosa (T1a) on endoscopic ultrasound or cross-sectional imaging, then BET is suggested. If there is involvement of submucosa, then depending on the depth of invasion, absence of high-risk features (poor differentiation, lymphovascular invasion), BET can be considered as an alternative to esophagectomy.
Lesion Detection on Endoscopy
Data from large population-based studies with at least 3 years of follow-up reported that 58%-66% of EAC detected during endoscopy were diagnosed within 1 year of an index Barrett’s esophagus screening endoscopy, or post-endoscopy Barrett’s neoplasia, and were considered likely to have been missed during index endoscopy.5 This underscores the importance of careful and systematic endoscopic examination during an upper endoscopy.
Studies have also demonstrated that longer examination time was associated with significantly higher detection of HGD/EAC.6,7 Careful examination of the tubular esophagus and gastroesophageal junction (GEJ) should be performed in forward and retroflexed views looking for any subtle areas of nodularity, loop distortion, variability in vascular patterns, mucosal changes concerning for dysplasia or neoplasia. Use of high-definition white light endoscopy (HD-WLE) and virtual chromoendoscopy techniques such as narrow banding imaging (NBI) or blue laser imaging (BLI) are currently recommended in the guidelines.2 Spray chromoendoscopy using acetic acid can also be utilized. Another exciting development is the use of artificial intelligence (AI) in detecting and diagnosing BE associated lesions and neoplasia.
Barrett’s Endoscopic Therapy (BET)
Patients with visible lesions, dysplasia, or early EAC are candidates for BET (Table 1). 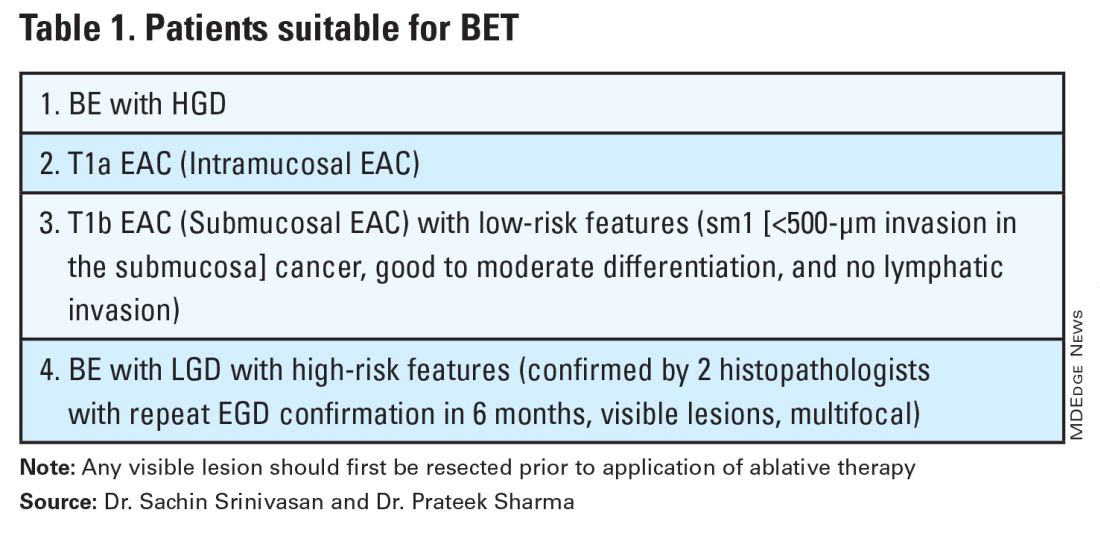
BET involves resective and ablative modalities. The resective modalities include endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) and are the modalities of choice for nodular or raised lesions.
EMR involves endoscopic resection of abnormal mucosa using either lift-assisted technique or multi-band ligation (Figure 1). 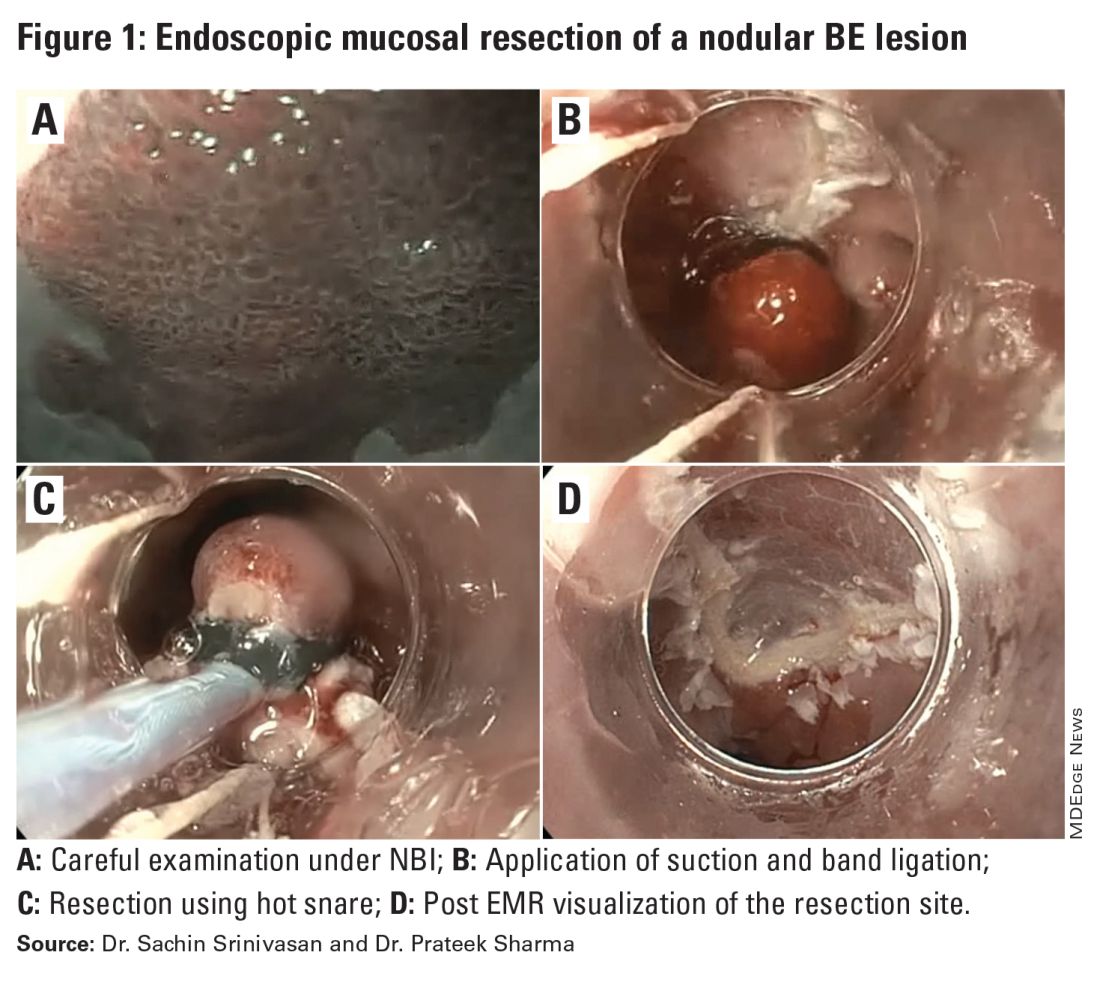
ESD, on the other hand, involves submucosal dissection and perimeter resection of the lesion, thus providing the advantage of an en-bloc resection. In a recent randomized controlled trial (RCT) of 40 patients undergoing ESD vs EMR for HGD/EAC, ESD was better for curative resection (R0) (58%) compared with EMR (12%); however, the remission rates at 3 months were comparable with two perforations reported in the ESD group while there were no complications in the EMR group.8
There is an apparent learning curve when it comes to these advanced techniques, and with more experience, we are seeing comparable results for both these modalities. However, given the complexity and time required for the procedure, current practices typically involve preserving ESD for lesions > 2 cm, those having a likelihood of cancer in the superficial submucosa, or those that EMR cannot remove due to underlying fibrosis or post-EMR recurrence.
The ablative modalities include radiofrequency ablation (RFA), cryotherapy, and hybrid argon plasma coagulation (hybrid APC). These modalities are used for flat lesions, and as therapy following endoscopic resection of nodular lesions to treat residual flat segment of BE. RFA, one of the earliest introduced endoscopic modalities, involves applying directed and controlled heat energy to ablate lesions. Current devices allow circumferential or focal application of RFA. It is a safe and effective modality with good complete eradication of IM (CE-IM) (71%-93%) and complete eradication of dysplasia (CE-D) (91%-100%) rates. These results have been sustained even at 2 years, with the most recent long-term data from a registry study showing a relapse rate of 6% for dysplasia and 19% for IM after 8 years, suggesting durability of this treatment.9
Cryotherapy involves the application of liquid nitrogen or rapidly expanding CO2 to the abnormal mucosa, leading to the rapid freezing and thawing that leads to the death of the cells. Cryogen can be applied as a spray or using a balloon with the spray nozzle in the center. This modality can be used to treat focal lesions and/or larger segments. While it has not been systematically compared with RFA, rates of CE-IM up to 81% and CE-D up to 97% are reported. Hybrid APC involves the use of submucosal saline injection to provide a protective cushion before APC is applied. It has CE-IM rate of 69% and CE-D rate of 67%-86%.10 In a recent RCT of 101 patients randomized to RFA or hybrid APC, CE-IM rates were similar (RFA:74.2% vs hAPC: 82.9%).11
Recently, another technique called radiofrequency vapor ablation (RFVA) is being evaluated, which involves ablating BE segment using vapor at 100° C generated with an RF electrode. A proof-of-concept study of 15 patients showed median squamous conversion of 55% (IQR 33-74) and 98% (IQR 56-99) for 1- and 3-second applications, respectively, with no reported adverse events.12
Barrett’s Refractory to Endoscopic Therapy
Failure of BET is defined as persistent columnar lined epithelium (intestinal metaplasia) with inadequate response, after adequate attempts at endoscopic ablation therapy (after resection) with at least four ablation sessions.13 If encountered, special attention must be given to check compliance with proton pump inhibitors (PPIs), previous incomplete resection, and presence of large hiatal hernia. If CE-IM is not achieved after multiple sessions, change of ablative modality is typically considered. In addition, careful examination for visible lesions should be performed and even if a small one is noted, this should be first resected prior to application of any ablative therapy.
Currently there are no guideline recommendations regarding the preference of one endoscopic modality over another or consideration of potential endoscopic or surgical fundoplication. These modalities primarily rely on technologies available at an institution and the preference of a provider based on their training and experience. Most studies indicate 1-3 sessions (~ 3 months apart) of ablative treatment before achieving CE-IM.
Success and Adverse Events of BET
In a recent real-world study of over 27,000 patients with dysplastic BE, 5295 underwent BET. Analysis showed that patients with HGD/EAC who had BET had a significantly lower 3-year mortality (HGD: RR, 0.59; 95%CI, 0.49-0.71; EAC: RR, 0.53; 95% CI, 0.44-0.65) compared with those who did not undergo BET. Esophageal strictures were the most common adverse event and were noted in 6.5%, followed by chest pain (1.8%), upper GI bleeding (0.47%), and esophageal perforation (0.2%).14
In general, adverse events can be divided into immediate and delayed adverse events. Immediate adverse events typically involve bleeding and perforation that can occur during or shortly after the procedure. These are reported at higher rates with resective modalities compared with ablative therapies. Standard endoscopic techniques involving coagulation grasper or clips can be used to achieve hemostasis. Endoscopic suturing devices offer the ability to contain any perforation. The need for surgical intervention is small and limited to adverse events not detected during the procedure.
Delayed adverse events such as stricture and stenosis are higher for resective modalities (up to 30%), especially when involving more significant than 75% of the esophageal circumference. Post-procedural pain/dysphagia is most common after ablative therapies. Dysphagia reported after any endoscopic therapy should be promptly evaluated, and sequential dilation until the goal esophageal lumen is achieved should be performed every 2-4 weeks.
Recurrences and Surveillance After BET
What is established is that recurrences can occur and may be subtle, therefore detailed endoscopic surveillance is required. In a prospective study, recurrence rates of 15%-16% for IM and 3%-5% for any dysplasia were reported, with the majority being in the first 2 years after achieving CE-IM.15 A systematic review of 21 studies looking at the location of recurrences suggested that the majority (56%) occur in the distal esophagus. Of those that occur in the esophagus, about 80% of them were in the distal 2 cm of the esophagus and only 50% of the recurrences were visible recurrences, thus reiterating the importance of meticulous examination and systematic biopsies.16
On the contrary, a recent single-center study of 217 patients who had achieved CE-IM with 5.5 years of follow-up demonstrated a 26% and 8% recurrence of IM and dysplasia, respectively. One hundred percent of the recurrence in the esophagus was reported as visible.17 Therefore, follow-up endoscopy surveillance protocol after CE-IM should still involve meticulous examination, biopsy of visible lesions, and systematic biopsies for non-visible lesions from the original BE segment, similar to those patients who have not needed BET.
Current guidelines based on expert consensus and evidence recommend surveillance after CE-IM based on original most advanced histology:2
1. LGD: 1 year, 3 years, and every 2 years after that.
2. HGD/EAC: 3 months, 6 months, 12 months, and annually after that.
There is no clear guideline on when to stop surveillance since the longest available follow-up is around 10 years, and recurrences are still detected. A potential surveillance endpoint may be based on age and comorbidities, especially those that would preclude a patient from being a candidate for BET.
When Should a Patient Be Referred?
BE patients with visible lesions and/or dysplastic changes in the biopsy who would require BET should be considered for referral to high-volume centers. Studies have shown higher success for CE-IM and lower rates of adverse events and recurrences in these patients managed at expert centers. The presence of a multidisciplinary team involving pathologists, surgeons, and oncologists is critical and offers a timely opportunity in case of need for a high-risk patient.
Conclusion
BE is a precursor to EAC, with rising incidence and poor 5-year survival. Endoscopic diagnosis is the gold standard and requires a high-quality examination and biopsies. Based on histopathology, a systematic surveillance and BET plan should be performed to achieve CE-IM in patients with dysplasia. Once CE-IM is achieved, regular surveillance should be performed with careful attention to recurrences and complications from the BET modalities.
Dr. Srinivasan and Dr. Sharma are based at the University of Kansas Medical Center, Kansas City, Kansas, and the Kansas City Veterans Affairs Medical Center, Kansas City, Missouri. Dr. Srinivasan has no relevant disclosures. Dr. Sharma disclosed research grants from ERBE, Ironwood Pharmaceuticals, Olympus, and Medtronic. He has served as a consultant for Takeda, Samsung Bioepis, Olympus, and Lumendi, and reports other funding from Medtronic, Fujifilm Medical Systems USA, and Salix.
References
1. Holmberg D, et al. Incidence and mortality in upper gastrointestinal cancer after negative endoscopy for gastroesophageal reflux disease. Gastroenterology. 2022;162(2):431-438.e4.
2. Shaheen NJ, et al. Diagnosis and management of Barrett’s esophagus: An updated ACG guideline. Am J Gastroenterol. 2022 Apr;117(4):559-587.
3. Pech O, et al. Inter-observer variability in the diagnosis of low-grade dysplasia in pathologists: A comparison between experienced and inexperienced pathologists. Gastrointest Endosc. 2006 Apr;63(5):AB130.
4. Krishnamoorthi R, et al. Factors associated with progression of Barrett’s esophagus: A systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2018 Jul;16(7):1046-1055.e8.
5. Visrodia K, et al. Magnitude of missed esophageal adenocarcinoma after Barrett’s esophagus diagnosis: A systematic review and meta-analysis. Gastroenterology. 2016 Mar;150(3):599-607.e7; quiz e14-5.
6. Perisetti A, Sharma P. Tips for improving the identification of neoplastic visible lesions in Barrett’s esophagus. Gastrointest Endosc. 2023 Feb;97(2):248-250.
7. Gupta N, et al. Longer inspection time is associated with increased detection of high-grade dysplasia and esophageal adenocarcinoma in Barrett’s esophagus. Gastrointest Endosc. 2012 Sep;76(3):531-538.
8. Terheggen G, et al. A randomised trial of endoscopic submucosal dissection versus endoscopic mucosal resection for early Barrett’s neoplasia. Gut. 2017 May;66(5):783-793.
9. Wolfson P, et al. Endoscopic eradication therapy for Barrett’s esophagus-related neoplasia: A final 10-year report from the UK National HALO Radiofrequency Ablation Registry. Gastrointest Endosc. 2022 Aug;96(2):223-233.
10. Rösch T, et al. 1151 Multicenter feasibility study of combined injection and argon plasma coagulation (hybrid-APC) in the ablation therapy of neoplastic Barrett esophagus. Gastrointest Endosc. 2017;85(5):AB154.
11. Knabe M, et al. Radiofrequency ablation versus hybrid argon plasma coagulation in Barrett’s esophagus: A prospective randomised trial. Surg Endosc. 2023;37(10):7803-7811.
12. Van Munster SN, et al. Radiofrequency vapor ablation for Barrett’s esophagus: Feasibility, safety, and proof of concept in a stepwise study with in vitro, animal, and the first in-human application. Endoscopy. 2021 Nov;53(11):1162-1168.
13. Emura F, et al. Rio de Janeiro global consensus on landmarks, definitions, and classifications in Barrett’s esophagus: World Endoscopy Organization Delphi study. Gastroenterology. 2022 Jul;163(1):84-96.e2.
14. Singh RR, et al. Real-world evidence of safety and effectiveness of Barrett’s endoscopic therapy. Gastrointest Endosc. 2023 Aug;98(2):155-161.e1.
15. Wani S, et al. Recurrence Is rare following complete eradication of intestinal metaplasia in patients with Barrett’s esophagus and peaks at 18 months. Clin Gastroenterol Hepatol. 2020 Oct;18(11):2609-2617.e2.
16. Duvvuri A, et al. Mo1273 Location and pattern of recurrences in patients with Barrett’s esophagus after endoscopic therapy: A systematic review and critical analysis of the published literature. Gastrointest Endosc. 2020;91(6):AB410-1.
17. He T, et al. Location and appearance of dysplastic Barrett’s esophagus recurrence after endoscopic eradication therapy: No additional yield from random biopsy sampling neosquamous mucosa. Gastrointest Endosc. 2023 Nov;98(5):722-732.
Introduction
Barrett’s esophagus (BE) is characterized by the replacement of squamous epithelium by columnar metaplasia of the distal esophagus (>1 cm length). It is a precancerous condition, with 3%-5% of patients with BE developing esophageal adenocarcinoma (EAC) in their lifetime. EAC is one of the cancers with high morbidity and mortality (5-year survival < 20%), and its incidence has been on the rise. Studies examining the natural history of BE have demonstrated that the progression happens through a metaplasia-dysplasia-neoplasia sequence. Therefore, early detection of BE and timely management to prevent progression to EAC is crucial.
Grades of Dysplasia
The current gold standard for the diagnosis of BE neoplasia includes a high-quality endoscopic evaluation and biopsies. Biopsies should be obtained from any visible lesions (nodules, ulcers) followed by a random 4-quadrant fashion (Seattle protocol) interval of the entire length of the BE segment. It is essential to pay attention to the results of the biopsy that have been obtained since it will not only determine the surveillance interval but is crucial in planning any necessary endoscopic therapy. The possible results of the biopsy and its implications are:
- No intestinal metaplasia (IM): This would rule out Barrett’s esophagus and no further surveillance would be necessary. A recent population-based study of over 1 million patients showed a 55% and 61% reduced risk of upper gastrointestinal (UGI) cancer and deaths respectively after a negative endoscopy.1
- Intestinal metaplasia with no dysplasia (non-dysplastic BE): Biopsies confirm presence of intestinal metaplasia in the biopsies without any evidence of dysplasia. While the rate of progression to EAC is low (0.07%-0.25%), it is not absent and thus surveillance would be indicated. Current guidelines suggest repeating an endoscopy with biopsy in 5 years if the length of BE is < 3 cm or 3 years if length of BE ≥ 3 cm.2
- Indeterminate for dysplasia (BE-IND): Biopsies confirm IM but are not able to definitively rule out dysplasia. This can be seen in about 4%-8% of the biopsies obtained. The progression rates to EAC are reported to be comparable or lower to low-grade dysplasia (LGD), so the current recommendation is to intensify acid reduction therapy and repeat endoscopy in 6 months. If repeat endoscopy downgrades to non-dysplastic, then can follow surveillance according to NDBE protocol; otherwise recommend continuing surveillance every 12 months.
- Low-grade dysplasia (BE-LGD): Biopsies confirm IM but also show tightly packed overlapping basal nuclei with hyperchromasia and irregular contours, basal stratification of nuclei, and diminished goblet and columnar cell mucus. There is significant inter-observer variability reported,3 and thus the slides must be reviewed by a second pathologist with experience in BE to confirm the findings. Once confirmed, based on risk factors such as presence of multifocal LGD, persistence of LGD, presence of visible lesions, etc., the patient can be offered Barrett’s endoscopic therapy (BET) or undergo continued surveillance. The decision of pursuing one or the other would be dependent on patient preference and shared decision-making between the patient and the provider.
- High-grade dysplasia (BE-HGD): Biopsies confirm IM with cells showing greater degree of cytologic and architectural alterations of dysplasia than LGD but without overt neoplastic features. Over 40% of the patients would progress to EAC and thus the current recommendations would be to recommend BET in these patients.4
- Esophageal adenocarcinoma (EAC): Biopsies demonstrate neoplasia. If the neoplastic changes are limited to the mucosa (T1a) on endoscopic ultrasound or cross-sectional imaging, then BET is suggested. If there is involvement of submucosa, then depending on the depth of invasion, absence of high-risk features (poor differentiation, lymphovascular invasion), BET can be considered as an alternative to esophagectomy.
Lesion Detection on Endoscopy
Data from large population-based studies with at least 3 years of follow-up reported that 58%-66% of EAC detected during endoscopy were diagnosed within 1 year of an index Barrett’s esophagus screening endoscopy, or post-endoscopy Barrett’s neoplasia, and were considered likely to have been missed during index endoscopy.5 This underscores the importance of careful and systematic endoscopic examination during an upper endoscopy.
Studies have also demonstrated that longer examination time was associated with significantly higher detection of HGD/EAC.6,7 Careful examination of the tubular esophagus and gastroesophageal junction (GEJ) should be performed in forward and retroflexed views looking for any subtle areas of nodularity, loop distortion, variability in vascular patterns, mucosal changes concerning for dysplasia or neoplasia. Use of high-definition white light endoscopy (HD-WLE) and virtual chromoendoscopy techniques such as narrow banding imaging (NBI) or blue laser imaging (BLI) are currently recommended in the guidelines.2 Spray chromoendoscopy using acetic acid can also be utilized. Another exciting development is the use of artificial intelligence (AI) in detecting and diagnosing BE associated lesions and neoplasia.
Barrett’s Endoscopic Therapy (BET)
Patients with visible lesions, dysplasia, or early EAC are candidates for BET (Table 1). 
BET involves resective and ablative modalities. The resective modalities include endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) and are the modalities of choice for nodular or raised lesions.
EMR involves endoscopic resection of abnormal mucosa using either lift-assisted technique or multi-band ligation (Figure 1). 
ESD, on the other hand, involves submucosal dissection and perimeter resection of the lesion, thus providing the advantage of an en-bloc resection. In a recent randomized controlled trial (RCT) of 40 patients undergoing ESD vs EMR for HGD/EAC, ESD was better for curative resection (R0) (58%) compared with EMR (12%); however, the remission rates at 3 months were comparable with two perforations reported in the ESD group while there were no complications in the EMR group.8
There is an apparent learning curve when it comes to these advanced techniques, and with more experience, we are seeing comparable results for both these modalities. However, given the complexity and time required for the procedure, current practices typically involve preserving ESD for lesions > 2 cm, those having a likelihood of cancer in the superficial submucosa, or those that EMR cannot remove due to underlying fibrosis or post-EMR recurrence.
The ablative modalities include radiofrequency ablation (RFA), cryotherapy, and hybrid argon plasma coagulation (hybrid APC). These modalities are used for flat lesions, and as therapy following endoscopic resection of nodular lesions to treat residual flat segment of BE. RFA, one of the earliest introduced endoscopic modalities, involves applying directed and controlled heat energy to ablate lesions. Current devices allow circumferential or focal application of RFA. It is a safe and effective modality with good complete eradication of IM (CE-IM) (71%-93%) and complete eradication of dysplasia (CE-D) (91%-100%) rates. These results have been sustained even at 2 years, with the most recent long-term data from a registry study showing a relapse rate of 6% for dysplasia and 19% for IM after 8 years, suggesting durability of this treatment.9
Cryotherapy involves the application of liquid nitrogen or rapidly expanding CO2 to the abnormal mucosa, leading to the rapid freezing and thawing that leads to the death of the cells. Cryogen can be applied as a spray or using a balloon with the spray nozzle in the center. This modality can be used to treat focal lesions and/or larger segments. While it has not been systematically compared with RFA, rates of CE-IM up to 81% and CE-D up to 97% are reported. Hybrid APC involves the use of submucosal saline injection to provide a protective cushion before APC is applied. It has CE-IM rate of 69% and CE-D rate of 67%-86%.10 In a recent RCT of 101 patients randomized to RFA or hybrid APC, CE-IM rates were similar (RFA:74.2% vs hAPC: 82.9%).11
Recently, another technique called radiofrequency vapor ablation (RFVA) is being evaluated, which involves ablating BE segment using vapor at 100° C generated with an RF electrode. A proof-of-concept study of 15 patients showed median squamous conversion of 55% (IQR 33-74) and 98% (IQR 56-99) for 1- and 3-second applications, respectively, with no reported adverse events.12
Barrett’s Refractory to Endoscopic Therapy
Failure of BET is defined as persistent columnar lined epithelium (intestinal metaplasia) with inadequate response, after adequate attempts at endoscopic ablation therapy (after resection) with at least four ablation sessions.13 If encountered, special attention must be given to check compliance with proton pump inhibitors (PPIs), previous incomplete resection, and presence of large hiatal hernia. If CE-IM is not achieved after multiple sessions, change of ablative modality is typically considered. In addition, careful examination for visible lesions should be performed and even if a small one is noted, this should be first resected prior to application of any ablative therapy.
Currently there are no guideline recommendations regarding the preference of one endoscopic modality over another or consideration of potential endoscopic or surgical fundoplication. These modalities primarily rely on technologies available at an institution and the preference of a provider based on their training and experience. Most studies indicate 1-3 sessions (~ 3 months apart) of ablative treatment before achieving CE-IM.
Success and Adverse Events of BET
In a recent real-world study of over 27,000 patients with dysplastic BE, 5295 underwent BET. Analysis showed that patients with HGD/EAC who had BET had a significantly lower 3-year mortality (HGD: RR, 0.59; 95%CI, 0.49-0.71; EAC: RR, 0.53; 95% CI, 0.44-0.65) compared with those who did not undergo BET. Esophageal strictures were the most common adverse event and were noted in 6.5%, followed by chest pain (1.8%), upper GI bleeding (0.47%), and esophageal perforation (0.2%).14
In general, adverse events can be divided into immediate and delayed adverse events. Immediate adverse events typically involve bleeding and perforation that can occur during or shortly after the procedure. These are reported at higher rates with resective modalities compared with ablative therapies. Standard endoscopic techniques involving coagulation grasper or clips can be used to achieve hemostasis. Endoscopic suturing devices offer the ability to contain any perforation. The need for surgical intervention is small and limited to adverse events not detected during the procedure.
Delayed adverse events such as stricture and stenosis are higher for resective modalities (up to 30%), especially when involving more significant than 75% of the esophageal circumference. Post-procedural pain/dysphagia is most common after ablative therapies. Dysphagia reported after any endoscopic therapy should be promptly evaluated, and sequential dilation until the goal esophageal lumen is achieved should be performed every 2-4 weeks.
Recurrences and Surveillance After BET
What is established is that recurrences can occur and may be subtle, therefore detailed endoscopic surveillance is required. In a prospective study, recurrence rates of 15%-16% for IM and 3%-5% for any dysplasia were reported, with the majority being in the first 2 years after achieving CE-IM.15 A systematic review of 21 studies looking at the location of recurrences suggested that the majority (56%) occur in the distal esophagus. Of those that occur in the esophagus, about 80% of them were in the distal 2 cm of the esophagus and only 50% of the recurrences were visible recurrences, thus reiterating the importance of meticulous examination and systematic biopsies.16
On the contrary, a recent single-center study of 217 patients who had achieved CE-IM with 5.5 years of follow-up demonstrated a 26% and 8% recurrence of IM and dysplasia, respectively. One hundred percent of the recurrence in the esophagus was reported as visible.17 Therefore, follow-up endoscopy surveillance protocol after CE-IM should still involve meticulous examination, biopsy of visible lesions, and systematic biopsies for non-visible lesions from the original BE segment, similar to those patients who have not needed BET.
Current guidelines based on expert consensus and evidence recommend surveillance after CE-IM based on original most advanced histology:2
1. LGD: 1 year, 3 years, and every 2 years after that.
2. HGD/EAC: 3 months, 6 months, 12 months, and annually after that.
There is no clear guideline on when to stop surveillance since the longest available follow-up is around 10 years, and recurrences are still detected. A potential surveillance endpoint may be based on age and comorbidities, especially those that would preclude a patient from being a candidate for BET.
When Should a Patient Be Referred?
BE patients with visible lesions and/or dysplastic changes in the biopsy who would require BET should be considered for referral to high-volume centers. Studies have shown higher success for CE-IM and lower rates of adverse events and recurrences in these patients managed at expert centers. The presence of a multidisciplinary team involving pathologists, surgeons, and oncologists is critical and offers a timely opportunity in case of need for a high-risk patient.
Conclusion
BE is a precursor to EAC, with rising incidence and poor 5-year survival. Endoscopic diagnosis is the gold standard and requires a high-quality examination and biopsies. Based on histopathology, a systematic surveillance and BET plan should be performed to achieve CE-IM in patients with dysplasia. Once CE-IM is achieved, regular surveillance should be performed with careful attention to recurrences and complications from the BET modalities.
Dr. Srinivasan and Dr. Sharma are based at the University of Kansas Medical Center, Kansas City, Kansas, and the Kansas City Veterans Affairs Medical Center, Kansas City, Missouri. Dr. Srinivasan has no relevant disclosures. Dr. Sharma disclosed research grants from ERBE, Ironwood Pharmaceuticals, Olympus, and Medtronic. He has served as a consultant for Takeda, Samsung Bioepis, Olympus, and Lumendi, and reports other funding from Medtronic, Fujifilm Medical Systems USA, and Salix.
References
1. Holmberg D, et al. Incidence and mortality in upper gastrointestinal cancer after negative endoscopy for gastroesophageal reflux disease. Gastroenterology. 2022;162(2):431-438.e4.
2. Shaheen NJ, et al. Diagnosis and management of Barrett’s esophagus: An updated ACG guideline. Am J Gastroenterol. 2022 Apr;117(4):559-587.
3. Pech O, et al. Inter-observer variability in the diagnosis of low-grade dysplasia in pathologists: A comparison between experienced and inexperienced pathologists. Gastrointest Endosc. 2006 Apr;63(5):AB130.
4. Krishnamoorthi R, et al. Factors associated with progression of Barrett’s esophagus: A systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2018 Jul;16(7):1046-1055.e8.
5. Visrodia K, et al. Magnitude of missed esophageal adenocarcinoma after Barrett’s esophagus diagnosis: A systematic review and meta-analysis. Gastroenterology. 2016 Mar;150(3):599-607.e7; quiz e14-5.
6. Perisetti A, Sharma P. Tips for improving the identification of neoplastic visible lesions in Barrett’s esophagus. Gastrointest Endosc. 2023 Feb;97(2):248-250.
7. Gupta N, et al. Longer inspection time is associated with increased detection of high-grade dysplasia and esophageal adenocarcinoma in Barrett’s esophagus. Gastrointest Endosc. 2012 Sep;76(3):531-538.
8. Terheggen G, et al. A randomised trial of endoscopic submucosal dissection versus endoscopic mucosal resection for early Barrett’s neoplasia. Gut. 2017 May;66(5):783-793.
9. Wolfson P, et al. Endoscopic eradication therapy for Barrett’s esophagus-related neoplasia: A final 10-year report from the UK National HALO Radiofrequency Ablation Registry. Gastrointest Endosc. 2022 Aug;96(2):223-233.
10. Rösch T, et al. 1151 Multicenter feasibility study of combined injection and argon plasma coagulation (hybrid-APC) in the ablation therapy of neoplastic Barrett esophagus. Gastrointest Endosc. 2017;85(5):AB154.
11. Knabe M, et al. Radiofrequency ablation versus hybrid argon plasma coagulation in Barrett’s esophagus: A prospective randomised trial. Surg Endosc. 2023;37(10):7803-7811.
12. Van Munster SN, et al. Radiofrequency vapor ablation for Barrett’s esophagus: Feasibility, safety, and proof of concept in a stepwise study with in vitro, animal, and the first in-human application. Endoscopy. 2021 Nov;53(11):1162-1168.
13. Emura F, et al. Rio de Janeiro global consensus on landmarks, definitions, and classifications in Barrett’s esophagus: World Endoscopy Organization Delphi study. Gastroenterology. 2022 Jul;163(1):84-96.e2.
14. Singh RR, et al. Real-world evidence of safety and effectiveness of Barrett’s endoscopic therapy. Gastrointest Endosc. 2023 Aug;98(2):155-161.e1.
15. Wani S, et al. Recurrence Is rare following complete eradication of intestinal metaplasia in patients with Barrett’s esophagus and peaks at 18 months. Clin Gastroenterol Hepatol. 2020 Oct;18(11):2609-2617.e2.
16. Duvvuri A, et al. Mo1273 Location and pattern of recurrences in patients with Barrett’s esophagus after endoscopic therapy: A systematic review and critical analysis of the published literature. Gastrointest Endosc. 2020;91(6):AB410-1.
17. He T, et al. Location and appearance of dysplastic Barrett’s esophagus recurrence after endoscopic eradication therapy: No additional yield from random biopsy sampling neosquamous mucosa. Gastrointest Endosc. 2023 Nov;98(5):722-732.
Introduction
Barrett’s esophagus (BE) is characterized by the replacement of squamous epithelium by columnar metaplasia of the distal esophagus (>1 cm length). It is a precancerous condition, with 3%-5% of patients with BE developing esophageal adenocarcinoma (EAC) in their lifetime. EAC is one of the cancers with high morbidity and mortality (5-year survival < 20%), and its incidence has been on the rise. Studies examining the natural history of BE have demonstrated that the progression happens through a metaplasia-dysplasia-neoplasia sequence. Therefore, early detection of BE and timely management to prevent progression to EAC is crucial.
Grades of Dysplasia
The current gold standard for the diagnosis of BE neoplasia includes a high-quality endoscopic evaluation and biopsies. Biopsies should be obtained from any visible lesions (nodules, ulcers) followed by a random 4-quadrant fashion (Seattle protocol) interval of the entire length of the BE segment. It is essential to pay attention to the results of the biopsy that have been obtained since it will not only determine the surveillance interval but is crucial in planning any necessary endoscopic therapy. The possible results of the biopsy and its implications are:
- No intestinal metaplasia (IM): This would rule out Barrett’s esophagus and no further surveillance would be necessary. A recent population-based study of over 1 million patients showed a 55% and 61% reduced risk of upper gastrointestinal (UGI) cancer and deaths respectively after a negative endoscopy.1
- Intestinal metaplasia with no dysplasia (non-dysplastic BE): Biopsies confirm presence of intestinal metaplasia in the biopsies without any evidence of dysplasia. While the rate of progression to EAC is low (0.07%-0.25%), it is not absent and thus surveillance would be indicated. Current guidelines suggest repeating an endoscopy with biopsy in 5 years if the length of BE is < 3 cm or 3 years if length of BE ≥ 3 cm.2
- Indeterminate for dysplasia (BE-IND): Biopsies confirm IM but are not able to definitively rule out dysplasia. This can be seen in about 4%-8% of the biopsies obtained. The progression rates to EAC are reported to be comparable or lower to low-grade dysplasia (LGD), so the current recommendation is to intensify acid reduction therapy and repeat endoscopy in 6 months. If repeat endoscopy downgrades to non-dysplastic, then can follow surveillance according to NDBE protocol; otherwise recommend continuing surveillance every 12 months.
- Low-grade dysplasia (BE-LGD): Biopsies confirm IM but also show tightly packed overlapping basal nuclei with hyperchromasia and irregular contours, basal stratification of nuclei, and diminished goblet and columnar cell mucus. There is significant inter-observer variability reported,3 and thus the slides must be reviewed by a second pathologist with experience in BE to confirm the findings. Once confirmed, based on risk factors such as presence of multifocal LGD, persistence of LGD, presence of visible lesions, etc., the patient can be offered Barrett’s endoscopic therapy (BET) or undergo continued surveillance. The decision of pursuing one or the other would be dependent on patient preference and shared decision-making between the patient and the provider.
- High-grade dysplasia (BE-HGD): Biopsies confirm IM with cells showing greater degree of cytologic and architectural alterations of dysplasia than LGD but without overt neoplastic features. Over 40% of the patients would progress to EAC and thus the current recommendations would be to recommend BET in these patients.4
- Esophageal adenocarcinoma (EAC): Biopsies demonstrate neoplasia. If the neoplastic changes are limited to the mucosa (T1a) on endoscopic ultrasound or cross-sectional imaging, then BET is suggested. If there is involvement of submucosa, then depending on the depth of invasion, absence of high-risk features (poor differentiation, lymphovascular invasion), BET can be considered as an alternative to esophagectomy.
Lesion Detection on Endoscopy
Data from large population-based studies with at least 3 years of follow-up reported that 58%-66% of EAC detected during endoscopy were diagnosed within 1 year of an index Barrett’s esophagus screening endoscopy, or post-endoscopy Barrett’s neoplasia, and were considered likely to have been missed during index endoscopy.5 This underscores the importance of careful and systematic endoscopic examination during an upper endoscopy.
Studies have also demonstrated that longer examination time was associated with significantly higher detection of HGD/EAC.6,7 Careful examination of the tubular esophagus and gastroesophageal junction (GEJ) should be performed in forward and retroflexed views looking for any subtle areas of nodularity, loop distortion, variability in vascular patterns, mucosal changes concerning for dysplasia or neoplasia. Use of high-definition white light endoscopy (HD-WLE) and virtual chromoendoscopy techniques such as narrow banding imaging (NBI) or blue laser imaging (BLI) are currently recommended in the guidelines.2 Spray chromoendoscopy using acetic acid can also be utilized. Another exciting development is the use of artificial intelligence (AI) in detecting and diagnosing BE associated lesions and neoplasia.
Barrett’s Endoscopic Therapy (BET)
Patients with visible lesions, dysplasia, or early EAC are candidates for BET (Table 1). 
BET involves resective and ablative modalities. The resective modalities include endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) and are the modalities of choice for nodular or raised lesions.
EMR involves endoscopic resection of abnormal mucosa using either lift-assisted technique or multi-band ligation (Figure 1). 
ESD, on the other hand, involves submucosal dissection and perimeter resection of the lesion, thus providing the advantage of an en-bloc resection. In a recent randomized controlled trial (RCT) of 40 patients undergoing ESD vs EMR for HGD/EAC, ESD was better for curative resection (R0) (58%) compared with EMR (12%); however, the remission rates at 3 months were comparable with two perforations reported in the ESD group while there were no complications in the EMR group.8
There is an apparent learning curve when it comes to these advanced techniques, and with more experience, we are seeing comparable results for both these modalities. However, given the complexity and time required for the procedure, current practices typically involve preserving ESD for lesions > 2 cm, those having a likelihood of cancer in the superficial submucosa, or those that EMR cannot remove due to underlying fibrosis or post-EMR recurrence.
The ablative modalities include radiofrequency ablation (RFA), cryotherapy, and hybrid argon plasma coagulation (hybrid APC). These modalities are used for flat lesions, and as therapy following endoscopic resection of nodular lesions to treat residual flat segment of BE. RFA, one of the earliest introduced endoscopic modalities, involves applying directed and controlled heat energy to ablate lesions. Current devices allow circumferential or focal application of RFA. It is a safe and effective modality with good complete eradication of IM (CE-IM) (71%-93%) and complete eradication of dysplasia (CE-D) (91%-100%) rates. These results have been sustained even at 2 years, with the most recent long-term data from a registry study showing a relapse rate of 6% for dysplasia and 19% for IM after 8 years, suggesting durability of this treatment.9
Cryotherapy involves the application of liquid nitrogen or rapidly expanding CO2 to the abnormal mucosa, leading to the rapid freezing and thawing that leads to the death of the cells. Cryogen can be applied as a spray or using a balloon with the spray nozzle in the center. This modality can be used to treat focal lesions and/or larger segments. While it has not been systematically compared with RFA, rates of CE-IM up to 81% and CE-D up to 97% are reported. Hybrid APC involves the use of submucosal saline injection to provide a protective cushion before APC is applied. It has CE-IM rate of 69% and CE-D rate of 67%-86%.10 In a recent RCT of 101 patients randomized to RFA or hybrid APC, CE-IM rates were similar (RFA:74.2% vs hAPC: 82.9%).11
Recently, another technique called radiofrequency vapor ablation (RFVA) is being evaluated, which involves ablating BE segment using vapor at 100° C generated with an RF electrode. A proof-of-concept study of 15 patients showed median squamous conversion of 55% (IQR 33-74) and 98% (IQR 56-99) for 1- and 3-second applications, respectively, with no reported adverse events.12
Barrett’s Refractory to Endoscopic Therapy
Failure of BET is defined as persistent columnar lined epithelium (intestinal metaplasia) with inadequate response, after adequate attempts at endoscopic ablation therapy (after resection) with at least four ablation sessions.13 If encountered, special attention must be given to check compliance with proton pump inhibitors (PPIs), previous incomplete resection, and presence of large hiatal hernia. If CE-IM is not achieved after multiple sessions, change of ablative modality is typically considered. In addition, careful examination for visible lesions should be performed and even if a small one is noted, this should be first resected prior to application of any ablative therapy.
Currently there are no guideline recommendations regarding the preference of one endoscopic modality over another or consideration of potential endoscopic or surgical fundoplication. These modalities primarily rely on technologies available at an institution and the preference of a provider based on their training and experience. Most studies indicate 1-3 sessions (~ 3 months apart) of ablative treatment before achieving CE-IM.
Success and Adverse Events of BET
In a recent real-world study of over 27,000 patients with dysplastic BE, 5295 underwent BET. Analysis showed that patients with HGD/EAC who had BET had a significantly lower 3-year mortality (HGD: RR, 0.59; 95%CI, 0.49-0.71; EAC: RR, 0.53; 95% CI, 0.44-0.65) compared with those who did not undergo BET. Esophageal strictures were the most common adverse event and were noted in 6.5%, followed by chest pain (1.8%), upper GI bleeding (0.47%), and esophageal perforation (0.2%).14
In general, adverse events can be divided into immediate and delayed adverse events. Immediate adverse events typically involve bleeding and perforation that can occur during or shortly after the procedure. These are reported at higher rates with resective modalities compared with ablative therapies. Standard endoscopic techniques involving coagulation grasper or clips can be used to achieve hemostasis. Endoscopic suturing devices offer the ability to contain any perforation. The need for surgical intervention is small and limited to adverse events not detected during the procedure.
Delayed adverse events such as stricture and stenosis are higher for resective modalities (up to 30%), especially when involving more significant than 75% of the esophageal circumference. Post-procedural pain/dysphagia is most common after ablative therapies. Dysphagia reported after any endoscopic therapy should be promptly evaluated, and sequential dilation until the goal esophageal lumen is achieved should be performed every 2-4 weeks.
Recurrences and Surveillance After BET
What is established is that recurrences can occur and may be subtle, therefore detailed endoscopic surveillance is required. In a prospective study, recurrence rates of 15%-16% for IM and 3%-5% for any dysplasia were reported, with the majority being in the first 2 years after achieving CE-IM.15 A systematic review of 21 studies looking at the location of recurrences suggested that the majority (56%) occur in the distal esophagus. Of those that occur in the esophagus, about 80% of them were in the distal 2 cm of the esophagus and only 50% of the recurrences were visible recurrences, thus reiterating the importance of meticulous examination and systematic biopsies.16
On the contrary, a recent single-center study of 217 patients who had achieved CE-IM with 5.5 years of follow-up demonstrated a 26% and 8% recurrence of IM and dysplasia, respectively. One hundred percent of the recurrence in the esophagus was reported as visible.17 Therefore, follow-up endoscopy surveillance protocol after CE-IM should still involve meticulous examination, biopsy of visible lesions, and systematic biopsies for non-visible lesions from the original BE segment, similar to those patients who have not needed BET.
Current guidelines based on expert consensus and evidence recommend surveillance after CE-IM based on original most advanced histology:2
1. LGD: 1 year, 3 years, and every 2 years after that.
2. HGD/EAC: 3 months, 6 months, 12 months, and annually after that.
There is no clear guideline on when to stop surveillance since the longest available follow-up is around 10 years, and recurrences are still detected. A potential surveillance endpoint may be based on age and comorbidities, especially those that would preclude a patient from being a candidate for BET.
When Should a Patient Be Referred?
BE patients with visible lesions and/or dysplastic changes in the biopsy who would require BET should be considered for referral to high-volume centers. Studies have shown higher success for CE-IM and lower rates of adverse events and recurrences in these patients managed at expert centers. The presence of a multidisciplinary team involving pathologists, surgeons, and oncologists is critical and offers a timely opportunity in case of need for a high-risk patient.
Conclusion
BE is a precursor to EAC, with rising incidence and poor 5-year survival. Endoscopic diagnosis is the gold standard and requires a high-quality examination and biopsies. Based on histopathology, a systematic surveillance and BET plan should be performed to achieve CE-IM in patients with dysplasia. Once CE-IM is achieved, regular surveillance should be performed with careful attention to recurrences and complications from the BET modalities.
Dr. Srinivasan and Dr. Sharma are based at the University of Kansas Medical Center, Kansas City, Kansas, and the Kansas City Veterans Affairs Medical Center, Kansas City, Missouri. Dr. Srinivasan has no relevant disclosures. Dr. Sharma disclosed research grants from ERBE, Ironwood Pharmaceuticals, Olympus, and Medtronic. He has served as a consultant for Takeda, Samsung Bioepis, Olympus, and Lumendi, and reports other funding from Medtronic, Fujifilm Medical Systems USA, and Salix.
References
1. Holmberg D, et al. Incidence and mortality in upper gastrointestinal cancer after negative endoscopy for gastroesophageal reflux disease. Gastroenterology. 2022;162(2):431-438.e4.
2. Shaheen NJ, et al. Diagnosis and management of Barrett’s esophagus: An updated ACG guideline. Am J Gastroenterol. 2022 Apr;117(4):559-587.
3. Pech O, et al. Inter-observer variability in the diagnosis of low-grade dysplasia in pathologists: A comparison between experienced and inexperienced pathologists. Gastrointest Endosc. 2006 Apr;63(5):AB130.
4. Krishnamoorthi R, et al. Factors associated with progression of Barrett’s esophagus: A systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2018 Jul;16(7):1046-1055.e8.
5. Visrodia K, et al. Magnitude of missed esophageal adenocarcinoma after Barrett’s esophagus diagnosis: A systematic review and meta-analysis. Gastroenterology. 2016 Mar;150(3):599-607.e7; quiz e14-5.
6. Perisetti A, Sharma P. Tips for improving the identification of neoplastic visible lesions in Barrett’s esophagus. Gastrointest Endosc. 2023 Feb;97(2):248-250.
7. Gupta N, et al. Longer inspection time is associated with increased detection of high-grade dysplasia and esophageal adenocarcinoma in Barrett’s esophagus. Gastrointest Endosc. 2012 Sep;76(3):531-538.
8. Terheggen G, et al. A randomised trial of endoscopic submucosal dissection versus endoscopic mucosal resection for early Barrett’s neoplasia. Gut. 2017 May;66(5):783-793.
9. Wolfson P, et al. Endoscopic eradication therapy for Barrett’s esophagus-related neoplasia: A final 10-year report from the UK National HALO Radiofrequency Ablation Registry. Gastrointest Endosc. 2022 Aug;96(2):223-233.
10. Rösch T, et al. 1151 Multicenter feasibility study of combined injection and argon plasma coagulation (hybrid-APC) in the ablation therapy of neoplastic Barrett esophagus. Gastrointest Endosc. 2017;85(5):AB154.
11. Knabe M, et al. Radiofrequency ablation versus hybrid argon plasma coagulation in Barrett’s esophagus: A prospective randomised trial. Surg Endosc. 2023;37(10):7803-7811.
12. Van Munster SN, et al. Radiofrequency vapor ablation for Barrett’s esophagus: Feasibility, safety, and proof of concept in a stepwise study with in vitro, animal, and the first in-human application. Endoscopy. 2021 Nov;53(11):1162-1168.
13. Emura F, et al. Rio de Janeiro global consensus on landmarks, definitions, and classifications in Barrett’s esophagus: World Endoscopy Organization Delphi study. Gastroenterology. 2022 Jul;163(1):84-96.e2.
14. Singh RR, et al. Real-world evidence of safety and effectiveness of Barrett’s endoscopic therapy. Gastrointest Endosc. 2023 Aug;98(2):155-161.e1.
15. Wani S, et al. Recurrence Is rare following complete eradication of intestinal metaplasia in patients with Barrett’s esophagus and peaks at 18 months. Clin Gastroenterol Hepatol. 2020 Oct;18(11):2609-2617.e2.
16. Duvvuri A, et al. Mo1273 Location and pattern of recurrences in patients with Barrett’s esophagus after endoscopic therapy: A systematic review and critical analysis of the published literature. Gastrointest Endosc. 2020;91(6):AB410-1.
17. He T, et al. Location and appearance of dysplastic Barrett’s esophagus recurrence after endoscopic eradication therapy: No additional yield from random biopsy sampling neosquamous mucosa. Gastrointest Endosc. 2023 Nov;98(5):722-732.

