User login
Payment gaps seen for child visits
, according to the Agency for Healthcare Research and Quality.
Child visits covered by private insurance brought in a mean $214 for office-based physicians of all specialties in 2015, which was $88 more than the $126 they received for Medicaid-covered visits, the AHRQ said.
That gap has been consistent since 2010, even as payments rose from 2010 to 2013 and fell in 2014 and 2015. The payment gap was even larger in 2015 when looking at the most expensive 10% of child visits: $406 for those that were privately insured and $215 for those covered by Medicaid, the AHRQ reported in a recent Statistical Brief.
Variations in payments also were seen between geographic regions and among the different physician specialties. Mean payments to physicians in the Midwest were highest for both private insurance ($249) and Medicaid ($152), while the lowest payments – $192 from private insurance and $120 from Medicaid – went to physicians in the South. The South also had the smallest gap between private and Medicaid coverage at $72, and the West had the largest gap at an even $100, but none of the variation across regions was significant, the AHRQ said.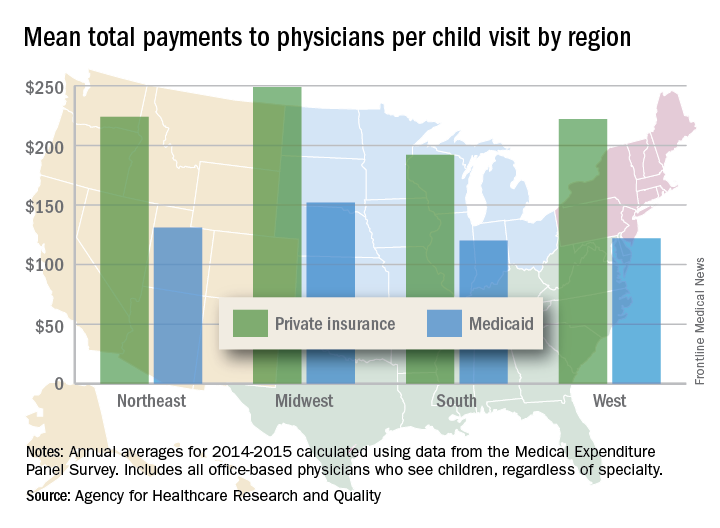
There were statistically significant gaps between private and Medicaid payments by specialty, although only for visits to orthopedists ($423 private – $162 Medicaid = $261) and primary care physicians such as family physicians and internists ($174 private – $122 Medicaid = $52). Payments to pediatricians were $190 private/$115 Medicaid, and the psychiatrists’ $111 mean payment from Medicaid represented the lowest for any specialty, data from Medical Expenditure Panel Survey’s household component show.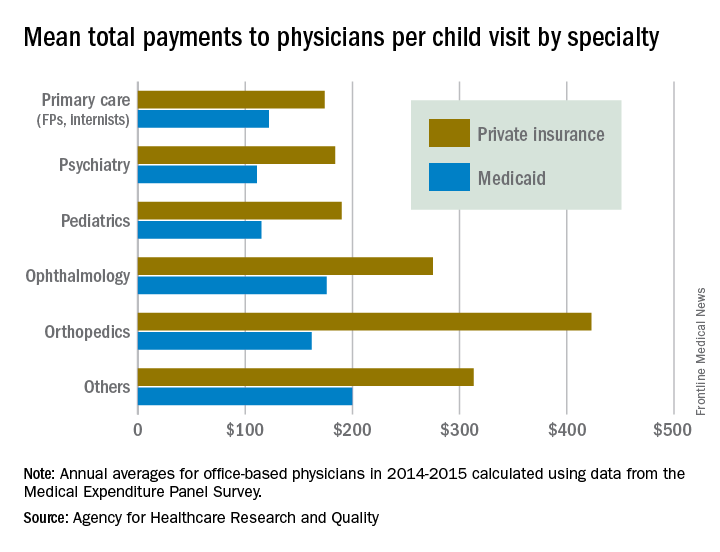
, according to the Agency for Healthcare Research and Quality.
Child visits covered by private insurance brought in a mean $214 for office-based physicians of all specialties in 2015, which was $88 more than the $126 they received for Medicaid-covered visits, the AHRQ said.
That gap has been consistent since 2010, even as payments rose from 2010 to 2013 and fell in 2014 and 2015. The payment gap was even larger in 2015 when looking at the most expensive 10% of child visits: $406 for those that were privately insured and $215 for those covered by Medicaid, the AHRQ reported in a recent Statistical Brief.
Variations in payments also were seen between geographic regions and among the different physician specialties. Mean payments to physicians in the Midwest were highest for both private insurance ($249) and Medicaid ($152), while the lowest payments – $192 from private insurance and $120 from Medicaid – went to physicians in the South. The South also had the smallest gap between private and Medicaid coverage at $72, and the West had the largest gap at an even $100, but none of the variation across regions was significant, the AHRQ said.
There were statistically significant gaps between private and Medicaid payments by specialty, although only for visits to orthopedists ($423 private – $162 Medicaid = $261) and primary care physicians such as family physicians and internists ($174 private – $122 Medicaid = $52). Payments to pediatricians were $190 private/$115 Medicaid, and the psychiatrists’ $111 mean payment from Medicaid represented the lowest for any specialty, data from Medical Expenditure Panel Survey’s household component show.
, according to the Agency for Healthcare Research and Quality.
Child visits covered by private insurance brought in a mean $214 for office-based physicians of all specialties in 2015, which was $88 more than the $126 they received for Medicaid-covered visits, the AHRQ said.
That gap has been consistent since 2010, even as payments rose from 2010 to 2013 and fell in 2014 and 2015. The payment gap was even larger in 2015 when looking at the most expensive 10% of child visits: $406 for those that were privately insured and $215 for those covered by Medicaid, the AHRQ reported in a recent Statistical Brief.
Variations in payments also were seen between geographic regions and among the different physician specialties. Mean payments to physicians in the Midwest were highest for both private insurance ($249) and Medicaid ($152), while the lowest payments – $192 from private insurance and $120 from Medicaid – went to physicians in the South. The South also had the smallest gap between private and Medicaid coverage at $72, and the West had the largest gap at an even $100, but none of the variation across regions was significant, the AHRQ said.
There were statistically significant gaps between private and Medicaid payments by specialty, although only for visits to orthopedists ($423 private – $162 Medicaid = $261) and primary care physicians such as family physicians and internists ($174 private – $122 Medicaid = $52). Payments to pediatricians were $190 private/$115 Medicaid, and the psychiatrists’ $111 mean payment from Medicaid represented the lowest for any specialty, data from Medical Expenditure Panel Survey’s household component show.
Tdap during pregnancy, or before, offers infants pertussis protection
during the early months of life, according to Tami H. Skoff of the Centers for Disease Control and Prevention, Atlanta, and her associates.
In an analysis of 240 infants younger than 2 months with pertussis cough onset between 2011 and 2015 and 535 control infants, 57% of case mothers and 67% of control mothers had at least one valid Tdap dose; 13% of vaccinated case mothers and 14% of vaccinated control mothers had more than one valid dose of Tdap reported.
Of Tdap doses received during pregnancy in 22 cases and 117 controls, 77% were received during the third trimester, most during the Advisory Committee on Immunization Practices’ recommended 27-36 weeks of gestation. Of the Tdap doses received before pregnancy in mothers of 24 cases and 67 controls, 25% of the case mothers and 67% of the control mothers received Tdap 2 or fewer years before pregnancy.
The effectiveness of Tdap vaccination during the third trimester of pregnancy was 78%, and effectiveness during the first or second trimester was 64%. Effectiveness of Tdap given 2 or fewer years before pregnancy was 83%. This study was not powered to determine a difference if the vaccine was administered in the ACIP-recommended time period during the third trimester.
A reported 49% of U.S. pregnant women received Tdap during the 2015-2016 flu season, an increase of 22% from the 2013-2014 season, according to a CDC Internet panel survey.
“While maternal immunization during pregnancy will help bridge the gap until next-generation pertussis vaccines are licensed and available for use, this highly effective strategy will likely remain an integral component of pertussis prevention and control, even in the setting of new vaccines,” the investigators said.
Read more in Clinical Infectious Diseases (2017 Sep 28. doi: 10.1093/cid/cix724).
during the early months of life, according to Tami H. Skoff of the Centers for Disease Control and Prevention, Atlanta, and her associates.
In an analysis of 240 infants younger than 2 months with pertussis cough onset between 2011 and 2015 and 535 control infants, 57% of case mothers and 67% of control mothers had at least one valid Tdap dose; 13% of vaccinated case mothers and 14% of vaccinated control mothers had more than one valid dose of Tdap reported.
Of Tdap doses received during pregnancy in 22 cases and 117 controls, 77% were received during the third trimester, most during the Advisory Committee on Immunization Practices’ recommended 27-36 weeks of gestation. Of the Tdap doses received before pregnancy in mothers of 24 cases and 67 controls, 25% of the case mothers and 67% of the control mothers received Tdap 2 or fewer years before pregnancy.
The effectiveness of Tdap vaccination during the third trimester of pregnancy was 78%, and effectiveness during the first or second trimester was 64%. Effectiveness of Tdap given 2 or fewer years before pregnancy was 83%. This study was not powered to determine a difference if the vaccine was administered in the ACIP-recommended time period during the third trimester.
A reported 49% of U.S. pregnant women received Tdap during the 2015-2016 flu season, an increase of 22% from the 2013-2014 season, according to a CDC Internet panel survey.
“While maternal immunization during pregnancy will help bridge the gap until next-generation pertussis vaccines are licensed and available for use, this highly effective strategy will likely remain an integral component of pertussis prevention and control, even in the setting of new vaccines,” the investigators said.
Read more in Clinical Infectious Diseases (2017 Sep 28. doi: 10.1093/cid/cix724).
during the early months of life, according to Tami H. Skoff of the Centers for Disease Control and Prevention, Atlanta, and her associates.
In an analysis of 240 infants younger than 2 months with pertussis cough onset between 2011 and 2015 and 535 control infants, 57% of case mothers and 67% of control mothers had at least one valid Tdap dose; 13% of vaccinated case mothers and 14% of vaccinated control mothers had more than one valid dose of Tdap reported.
Of Tdap doses received during pregnancy in 22 cases and 117 controls, 77% were received during the third trimester, most during the Advisory Committee on Immunization Practices’ recommended 27-36 weeks of gestation. Of the Tdap doses received before pregnancy in mothers of 24 cases and 67 controls, 25% of the case mothers and 67% of the control mothers received Tdap 2 or fewer years before pregnancy.
The effectiveness of Tdap vaccination during the third trimester of pregnancy was 78%, and effectiveness during the first or second trimester was 64%. Effectiveness of Tdap given 2 or fewer years before pregnancy was 83%. This study was not powered to determine a difference if the vaccine was administered in the ACIP-recommended time period during the third trimester.
A reported 49% of U.S. pregnant women received Tdap during the 2015-2016 flu season, an increase of 22% from the 2013-2014 season, according to a CDC Internet panel survey.
“While maternal immunization during pregnancy will help bridge the gap until next-generation pertussis vaccines are licensed and available for use, this highly effective strategy will likely remain an integral component of pertussis prevention and control, even in the setting of new vaccines,” the investigators said.
Read more in Clinical Infectious Diseases (2017 Sep 28. doi: 10.1093/cid/cix724).
FROM CLINICAL INFECTIOUS DISEASES
Avoid sildenafil for pulmonary hypertension after corrected valvular disease
BARCELONA – Off-label use of the phosphodiesterase-5 inhibitor sildenafil to treat residual pulmonary hypertension after successful correction of valvular heart disease is not merely ineffective, it’s counterproductive, according to the results of the randomized, placebo-controlled SIOVAC study.
“We believe based upon our results that off-label use of sildenafil in patients with left heart disease-pulmonary hypertension due to valvular disease should be discouraged,” Javier Bermejo, MD, declared at the annual congress of the European Society of Cardiology. 
Sildenafil is approved with a solid, evidence-based indication for treating some other types of pulmonary hypertension. Many cardiologists also prescribe the drug off label for residual pulmonary hypertension in patients with corrected valve disease, hoping that it will be of benefit, since there is currently no approved treatment for this common and serious condition associated with increased mortality. But because the anecdotal literature on sildenafil for this specific type of pulmonary hypertension is mixed, Dr. Bermejo and his coinvestigators in the Spanish Network Center for Cardiovascular Research decided to conduct a multicenter randomized trial.
SIOVAC (Sildenafil for Improving Clinical Outcomes After Valvular Correction) comprised 200 patients with residual pulmonary hypertension after corrected valvular heart disease at 17 Spanish general hospitals. The patients were randomized to receive sildenafil at 40 mg t.i.d. or placebo for 6 months in this double-blind trial.
The primary endpoint was a standardized composite clinical score widely used in heart failure trials. It consists of all-cause mortality, hospital admission for heart failure, worsening exercise tolerance, and deterioration in a global self-assessment rating.
The shocker for the investigators – who had expected a positive study – was that 33% of patients in the sildenafil group worsened significantly on the composite clinical score at 6 months, compared with 14% of placebo-treated controls, said Dr. Bermejo, a cardiologist at Gregorio Marañón University Hospital in Madrid.
Moreover, only 27% of the sildenafil group improved, compared with 44% of controls. About one-third of patients in both groups remained unchanged over the course of the 6-month trial.
Dr. Bermejo noted that valvular disease is considered the next cardiac epidemic because of its strong association with advancing age and the rapid aging of the population worldwide. Pulmonary hypertension occurs is virtually all patients with severe mitral disease and in up to two-thirds of those with asymptomatic aortic stenosis. Regression of the pulmonary hypertension is often incomplete after successful surgical or transcatheter correction of the valvular lesion.
Discussant Irene M. Lang, MD, called SIOVAC “a very clear study.” It convincingly establishes that sildenafil – a vasodilator – is ineffective for the treatment of what the current ESC/European Respiratory Society guidelines on pulmonary hypertension call isolated post-capillary pulmonary hypertension, a condition defined hemodynamically by a diastolic pulmonary vascular pressure gradient of less than 7 mm Hg and/or a pulmonary vascular resistance below 3 Wood units (Eur Heart J. 2016 Jan 1;37[1]:67-119.)
The SIOVAC findings underscore the strong IIIC recommendation in the European guidelines that the use of approved therapies for pulmonary arterial hypertension is not recommended in patients with left heart disease-pulmonary hypertension, added Dr. Lang, a coauthor of the guidelines and professor of vascular biology at the Medical University of Vienna.
The Spanish government funded SIOVAC. Dr. Bermejo reported having no financial conflicts of interest.
BARCELONA – Off-label use of the phosphodiesterase-5 inhibitor sildenafil to treat residual pulmonary hypertension after successful correction of valvular heart disease is not merely ineffective, it’s counterproductive, according to the results of the randomized, placebo-controlled SIOVAC study.
“We believe based upon our results that off-label use of sildenafil in patients with left heart disease-pulmonary hypertension due to valvular disease should be discouraged,” Javier Bermejo, MD, declared at the annual congress of the European Society of Cardiology. 
Sildenafil is approved with a solid, evidence-based indication for treating some other types of pulmonary hypertension. Many cardiologists also prescribe the drug off label for residual pulmonary hypertension in patients with corrected valve disease, hoping that it will be of benefit, since there is currently no approved treatment for this common and serious condition associated with increased mortality. But because the anecdotal literature on sildenafil for this specific type of pulmonary hypertension is mixed, Dr. Bermejo and his coinvestigators in the Spanish Network Center for Cardiovascular Research decided to conduct a multicenter randomized trial.
SIOVAC (Sildenafil for Improving Clinical Outcomes After Valvular Correction) comprised 200 patients with residual pulmonary hypertension after corrected valvular heart disease at 17 Spanish general hospitals. The patients were randomized to receive sildenafil at 40 mg t.i.d. or placebo for 6 months in this double-blind trial.
The primary endpoint was a standardized composite clinical score widely used in heart failure trials. It consists of all-cause mortality, hospital admission for heart failure, worsening exercise tolerance, and deterioration in a global self-assessment rating.
The shocker for the investigators – who had expected a positive study – was that 33% of patients in the sildenafil group worsened significantly on the composite clinical score at 6 months, compared with 14% of placebo-treated controls, said Dr. Bermejo, a cardiologist at Gregorio Marañón University Hospital in Madrid.
Moreover, only 27% of the sildenafil group improved, compared with 44% of controls. About one-third of patients in both groups remained unchanged over the course of the 6-month trial.
Dr. Bermejo noted that valvular disease is considered the next cardiac epidemic because of its strong association with advancing age and the rapid aging of the population worldwide. Pulmonary hypertension occurs is virtually all patients with severe mitral disease and in up to two-thirds of those with asymptomatic aortic stenosis. Regression of the pulmonary hypertension is often incomplete after successful surgical or transcatheter correction of the valvular lesion.
Discussant Irene M. Lang, MD, called SIOVAC “a very clear study.” It convincingly establishes that sildenafil – a vasodilator – is ineffective for the treatment of what the current ESC/European Respiratory Society guidelines on pulmonary hypertension call isolated post-capillary pulmonary hypertension, a condition defined hemodynamically by a diastolic pulmonary vascular pressure gradient of less than 7 mm Hg and/or a pulmonary vascular resistance below 3 Wood units (Eur Heart J. 2016 Jan 1;37[1]:67-119.)
The SIOVAC findings underscore the strong IIIC recommendation in the European guidelines that the use of approved therapies for pulmonary arterial hypertension is not recommended in patients with left heart disease-pulmonary hypertension, added Dr. Lang, a coauthor of the guidelines and professor of vascular biology at the Medical University of Vienna.
The Spanish government funded SIOVAC. Dr. Bermejo reported having no financial conflicts of interest.
BARCELONA – Off-label use of the phosphodiesterase-5 inhibitor sildenafil to treat residual pulmonary hypertension after successful correction of valvular heart disease is not merely ineffective, it’s counterproductive, according to the results of the randomized, placebo-controlled SIOVAC study.
“We believe based upon our results that off-label use of sildenafil in patients with left heart disease-pulmonary hypertension due to valvular disease should be discouraged,” Javier Bermejo, MD, declared at the annual congress of the European Society of Cardiology. 
Sildenafil is approved with a solid, evidence-based indication for treating some other types of pulmonary hypertension. Many cardiologists also prescribe the drug off label for residual pulmonary hypertension in patients with corrected valve disease, hoping that it will be of benefit, since there is currently no approved treatment for this common and serious condition associated with increased mortality. But because the anecdotal literature on sildenafil for this specific type of pulmonary hypertension is mixed, Dr. Bermejo and his coinvestigators in the Spanish Network Center for Cardiovascular Research decided to conduct a multicenter randomized trial.
SIOVAC (Sildenafil for Improving Clinical Outcomes After Valvular Correction) comprised 200 patients with residual pulmonary hypertension after corrected valvular heart disease at 17 Spanish general hospitals. The patients were randomized to receive sildenafil at 40 mg t.i.d. or placebo for 6 months in this double-blind trial.
The primary endpoint was a standardized composite clinical score widely used in heart failure trials. It consists of all-cause mortality, hospital admission for heart failure, worsening exercise tolerance, and deterioration in a global self-assessment rating.
The shocker for the investigators – who had expected a positive study – was that 33% of patients in the sildenafil group worsened significantly on the composite clinical score at 6 months, compared with 14% of placebo-treated controls, said Dr. Bermejo, a cardiologist at Gregorio Marañón University Hospital in Madrid.
Moreover, only 27% of the sildenafil group improved, compared with 44% of controls. About one-third of patients in both groups remained unchanged over the course of the 6-month trial.
Dr. Bermejo noted that valvular disease is considered the next cardiac epidemic because of its strong association with advancing age and the rapid aging of the population worldwide. Pulmonary hypertension occurs is virtually all patients with severe mitral disease and in up to two-thirds of those with asymptomatic aortic stenosis. Regression of the pulmonary hypertension is often incomplete after successful surgical or transcatheter correction of the valvular lesion.
Discussant Irene M. Lang, MD, called SIOVAC “a very clear study.” It convincingly establishes that sildenafil – a vasodilator – is ineffective for the treatment of what the current ESC/European Respiratory Society guidelines on pulmonary hypertension call isolated post-capillary pulmonary hypertension, a condition defined hemodynamically by a diastolic pulmonary vascular pressure gradient of less than 7 mm Hg and/or a pulmonary vascular resistance below 3 Wood units (Eur Heart J. 2016 Jan 1;37[1]:67-119.)
The SIOVAC findings underscore the strong IIIC recommendation in the European guidelines that the use of approved therapies for pulmonary arterial hypertension is not recommended in patients with left heart disease-pulmonary hypertension, added Dr. Lang, a coauthor of the guidelines and professor of vascular biology at the Medical University of Vienna.
The Spanish government funded SIOVAC. Dr. Bermejo reported having no financial conflicts of interest.
AT THE ESC CONGRESS 2017
Key clinical point:
Major finding: One-third of patients with residual pulmonary hypertension after successful correction of valvular heart disease experienced significant clinical worsening during 6 months of sildenafil therapy, compared with 14% on placebo.
Data source: SIOVAC, a 6-month, double-blind, placebo-controlled study of 200 patients with residual pulmonary hypertension after correction for valvular heart disease.
Disclosures: The Spanish government funded SIOVAC. The presenter reported having no financial conflicts of interest.
Walking the halls of power
Hospital medicine may be a young specialty, but it is already playing a significant role in both front-line patient care and, increasingly, in shaping public policy. Case in point: Two hospitalists serving currently in key roles in the federal government, and two former top civil servants, each of whom are examples of the growing influence of the hospitalist perspective.
“The hospitalist viewpoint of the health care system is a unique one, and it lends itself very well to the challenges of our current delivery system reform. We’re reforming the health care system to deliver care more cost effectively,” said Ron Greeno, MD, FCCP, MHM, SHM president and chair of the SHM Public Policy committee. “Hospitalists are trained to do that – they go to work every day to do that.”
Leading the FDA
“He’s the perfect person for that job and is looking to shake things up,” Dr. Greeno said. “There are a lot of things that can improve in terms of how drugs get to market, including lower cost generic drugs.” That’s an issue Dr. Gottlieb has been championing for years, and his understanding of the issue also makes him well prepared to take this position now, Dr. Greeno said.
“Dr. Gottlieb’s nomination comes at a momentous time for the agency, which Mr. Trump has promised to significantly remake,” the New York Times wrote on March 29, prior to his confirmation. “The next commissioner will be charged with putting into practice a far-reaching law, passed in December, aimed at bringing drugs to market more quickly.”
In addition to his work at the AEI, Dr. Gottlieb served on SHM’s Public Policy committee. He was a clinical assistant professor at New York University School of Medicine and advised the U.S. Department of Health and Human Services as a member of the Federal Health IT Policy committee.
Steering national quality programs
Kate Goodrich’s preparation for her government role included experience with several sides of the health care system: Dr. Goodrich, MD, MHS, was the director of the Division of Hospital Medicine at George Washington University Hospital, one of the first hospitalist programs in the Washington area. She worked at an inpatient rehab facility and has practiced in ambulatory care.
Now, as chief medical officer of CMS and director of the Center for Clinical Standards and Quality (CCSQ), she’s helping drive those policy decisions, overseeing multiple quality measurement and value-based purchasing programs and health and safety standards for hospitals.
Dr. Goodrich still makes rounds at George Washington Hospital on weekends. “It allows me to have a sort of in-your-bones understanding of the challenges of frontline providers,” she said. “I’m able to understand the clinician point of view in our policy decisions.” She’s also able to see first-hand the effects of those policy decisions on clinicians, patients, and health care systems.
As physician leaders within their organizations, hospitalists fit naturally into other leadership positions, she said. “Hospitalists often take leadership roles around quality of care and efficiency and flow and those sorts of thing,” Dr. Goodrich said. “I think it is a very natural progression for hospitalists to get interested in health care and medicine from that viewpoint, which then might allow them to make a leap into another type of field.”
An innovator at CMS
Until very recently, pediatric hospitalist Patrick Conway, MD, FAAP, MHM, served as deputy administrator for Innovation and Quality at the Centers for Medicare & Medicaid Services and director of the Center for Medicare and Medicaid Innovation. On Oct. 1, he took on a new challenge, becoming president and CEO of Blue Cross and Blue Shield of North Carolina (Blue Cross NC).
Dr. Conway was selected as a Master of Hospital Medicine by SHM, and received the HHS Secretary’s Award for Distinguished Service, the Secretary’s highest distinction for excellence. The Patient Safety Movement Foundation gave him their Humanitarian Award, and in February 2017, he received the AMA’s Dr. Nathan Davis Award for Outstanding Government Service. He also was elected to the Health and Medicine Division of the National Academies of Sciences, Engineering, and Medicine in 2014.
Prior to joining CMS, Dr. Conway oversaw clinical operations and research at Cincinnati Children’s Hospital Medical Center as director of hospital medicine, with a focus on improving patient outcomes across the health system.
Improving the country’s health
Obesity, tobacco-related disease, mental illness, and addiction are some of the issues Vivek H. Murthy, MD, MBA, targeted while serving as the 19th U.S. Surgeon General. He was appointed to the position by President Obama in 2014, and was relieved of his duties by President Trump in April 2017.
Dr. Murthy has said that addiction should be seen as a chronic illness, not a character flaw, and last year sent a letter to 2.3 million health care providers nationwide, encouraging them to join a national effort to reform prescribing practices.
According to Dr. Greeno, each of these hospitalists illuminates new paths for others in the field. “I think for young people who are trying to identify what career path they want to pursue, this is something that can’t be anything but good for our specialty – and good for the health system,” he said. “Hospitalists have the perfect clinical background and mindset to help our health care system get to where it needs to go. It’s a huge challenge. It’s going to be a ton of work, and the stakes are very, very high.”
Reference
1. Thomas K. F.D.A. Nominee, Paid Millions by Industry, Says He’ll Recuse Himself if Needed. New York Times. March 29, 2017. https://www.nytimes.com/2017/03/29/health/fda-nominee-scott-gottlieb-recuse-conflicts.html?_r=0. Accessed March 31, 2017.
Hospital medicine may be a young specialty, but it is already playing a significant role in both front-line patient care and, increasingly, in shaping public policy. Case in point: Two hospitalists serving currently in key roles in the federal government, and two former top civil servants, each of whom are examples of the growing influence of the hospitalist perspective.
“The hospitalist viewpoint of the health care system is a unique one, and it lends itself very well to the challenges of our current delivery system reform. We’re reforming the health care system to deliver care more cost effectively,” said Ron Greeno, MD, FCCP, MHM, SHM president and chair of the SHM Public Policy committee. “Hospitalists are trained to do that – they go to work every day to do that.”
Leading the FDA
“He’s the perfect person for that job and is looking to shake things up,” Dr. Greeno said. “There are a lot of things that can improve in terms of how drugs get to market, including lower cost generic drugs.” That’s an issue Dr. Gottlieb has been championing for years, and his understanding of the issue also makes him well prepared to take this position now, Dr. Greeno said.
“Dr. Gottlieb’s nomination comes at a momentous time for the agency, which Mr. Trump has promised to significantly remake,” the New York Times wrote on March 29, prior to his confirmation. “The next commissioner will be charged with putting into practice a far-reaching law, passed in December, aimed at bringing drugs to market more quickly.”
In addition to his work at the AEI, Dr. Gottlieb served on SHM’s Public Policy committee. He was a clinical assistant professor at New York University School of Medicine and advised the U.S. Department of Health and Human Services as a member of the Federal Health IT Policy committee.
Steering national quality programs
Kate Goodrich’s preparation for her government role included experience with several sides of the health care system: Dr. Goodrich, MD, MHS, was the director of the Division of Hospital Medicine at George Washington University Hospital, one of the first hospitalist programs in the Washington area. She worked at an inpatient rehab facility and has practiced in ambulatory care.
Now, as chief medical officer of CMS and director of the Center for Clinical Standards and Quality (CCSQ), she’s helping drive those policy decisions, overseeing multiple quality measurement and value-based purchasing programs and health and safety standards for hospitals.
Dr. Goodrich still makes rounds at George Washington Hospital on weekends. “It allows me to have a sort of in-your-bones understanding of the challenges of frontline providers,” she said. “I’m able to understand the clinician point of view in our policy decisions.” She’s also able to see first-hand the effects of those policy decisions on clinicians, patients, and health care systems.
As physician leaders within their organizations, hospitalists fit naturally into other leadership positions, she said. “Hospitalists often take leadership roles around quality of care and efficiency and flow and those sorts of thing,” Dr. Goodrich said. “I think it is a very natural progression for hospitalists to get interested in health care and medicine from that viewpoint, which then might allow them to make a leap into another type of field.”
An innovator at CMS
Until very recently, pediatric hospitalist Patrick Conway, MD, FAAP, MHM, served as deputy administrator for Innovation and Quality at the Centers for Medicare & Medicaid Services and director of the Center for Medicare and Medicaid Innovation. On Oct. 1, he took on a new challenge, becoming president and CEO of Blue Cross and Blue Shield of North Carolina (Blue Cross NC).
Dr. Conway was selected as a Master of Hospital Medicine by SHM, and received the HHS Secretary’s Award for Distinguished Service, the Secretary’s highest distinction for excellence. The Patient Safety Movement Foundation gave him their Humanitarian Award, and in February 2017, he received the AMA’s Dr. Nathan Davis Award for Outstanding Government Service. He also was elected to the Health and Medicine Division of the National Academies of Sciences, Engineering, and Medicine in 2014.
Prior to joining CMS, Dr. Conway oversaw clinical operations and research at Cincinnati Children’s Hospital Medical Center as director of hospital medicine, with a focus on improving patient outcomes across the health system.
Improving the country’s health
Obesity, tobacco-related disease, mental illness, and addiction are some of the issues Vivek H. Murthy, MD, MBA, targeted while serving as the 19th U.S. Surgeon General. He was appointed to the position by President Obama in 2014, and was relieved of his duties by President Trump in April 2017.
Dr. Murthy has said that addiction should be seen as a chronic illness, not a character flaw, and last year sent a letter to 2.3 million health care providers nationwide, encouraging them to join a national effort to reform prescribing practices.
According to Dr. Greeno, each of these hospitalists illuminates new paths for others in the field. “I think for young people who are trying to identify what career path they want to pursue, this is something that can’t be anything but good for our specialty – and good for the health system,” he said. “Hospitalists have the perfect clinical background and mindset to help our health care system get to where it needs to go. It’s a huge challenge. It’s going to be a ton of work, and the stakes are very, very high.”
Reference
1. Thomas K. F.D.A. Nominee, Paid Millions by Industry, Says He’ll Recuse Himself if Needed. New York Times. March 29, 2017. https://www.nytimes.com/2017/03/29/health/fda-nominee-scott-gottlieb-recuse-conflicts.html?_r=0. Accessed March 31, 2017.
Hospital medicine may be a young specialty, but it is already playing a significant role in both front-line patient care and, increasingly, in shaping public policy. Case in point: Two hospitalists serving currently in key roles in the federal government, and two former top civil servants, each of whom are examples of the growing influence of the hospitalist perspective.
“The hospitalist viewpoint of the health care system is a unique one, and it lends itself very well to the challenges of our current delivery system reform. We’re reforming the health care system to deliver care more cost effectively,” said Ron Greeno, MD, FCCP, MHM, SHM president and chair of the SHM Public Policy committee. “Hospitalists are trained to do that – they go to work every day to do that.”
Leading the FDA
“He’s the perfect person for that job and is looking to shake things up,” Dr. Greeno said. “There are a lot of things that can improve in terms of how drugs get to market, including lower cost generic drugs.” That’s an issue Dr. Gottlieb has been championing for years, and his understanding of the issue also makes him well prepared to take this position now, Dr. Greeno said.
“Dr. Gottlieb’s nomination comes at a momentous time for the agency, which Mr. Trump has promised to significantly remake,” the New York Times wrote on March 29, prior to his confirmation. “The next commissioner will be charged with putting into practice a far-reaching law, passed in December, aimed at bringing drugs to market more quickly.”
In addition to his work at the AEI, Dr. Gottlieb served on SHM’s Public Policy committee. He was a clinical assistant professor at New York University School of Medicine and advised the U.S. Department of Health and Human Services as a member of the Federal Health IT Policy committee.
Steering national quality programs
Kate Goodrich’s preparation for her government role included experience with several sides of the health care system: Dr. Goodrich, MD, MHS, was the director of the Division of Hospital Medicine at George Washington University Hospital, one of the first hospitalist programs in the Washington area. She worked at an inpatient rehab facility and has practiced in ambulatory care.
Now, as chief medical officer of CMS and director of the Center for Clinical Standards and Quality (CCSQ), she’s helping drive those policy decisions, overseeing multiple quality measurement and value-based purchasing programs and health and safety standards for hospitals.
Dr. Goodrich still makes rounds at George Washington Hospital on weekends. “It allows me to have a sort of in-your-bones understanding of the challenges of frontline providers,” she said. “I’m able to understand the clinician point of view in our policy decisions.” She’s also able to see first-hand the effects of those policy decisions on clinicians, patients, and health care systems.
As physician leaders within their organizations, hospitalists fit naturally into other leadership positions, she said. “Hospitalists often take leadership roles around quality of care and efficiency and flow and those sorts of thing,” Dr. Goodrich said. “I think it is a very natural progression for hospitalists to get interested in health care and medicine from that viewpoint, which then might allow them to make a leap into another type of field.”
An innovator at CMS
Until very recently, pediatric hospitalist Patrick Conway, MD, FAAP, MHM, served as deputy administrator for Innovation and Quality at the Centers for Medicare & Medicaid Services and director of the Center for Medicare and Medicaid Innovation. On Oct. 1, he took on a new challenge, becoming president and CEO of Blue Cross and Blue Shield of North Carolina (Blue Cross NC).
Dr. Conway was selected as a Master of Hospital Medicine by SHM, and received the HHS Secretary’s Award for Distinguished Service, the Secretary’s highest distinction for excellence. The Patient Safety Movement Foundation gave him their Humanitarian Award, and in February 2017, he received the AMA’s Dr. Nathan Davis Award for Outstanding Government Service. He also was elected to the Health and Medicine Division of the National Academies of Sciences, Engineering, and Medicine in 2014.
Prior to joining CMS, Dr. Conway oversaw clinical operations and research at Cincinnati Children’s Hospital Medical Center as director of hospital medicine, with a focus on improving patient outcomes across the health system.
Improving the country’s health
Obesity, tobacco-related disease, mental illness, and addiction are some of the issues Vivek H. Murthy, MD, MBA, targeted while serving as the 19th U.S. Surgeon General. He was appointed to the position by President Obama in 2014, and was relieved of his duties by President Trump in April 2017.
Dr. Murthy has said that addiction should be seen as a chronic illness, not a character flaw, and last year sent a letter to 2.3 million health care providers nationwide, encouraging them to join a national effort to reform prescribing practices.
According to Dr. Greeno, each of these hospitalists illuminates new paths for others in the field. “I think for young people who are trying to identify what career path they want to pursue, this is something that can’t be anything but good for our specialty – and good for the health system,” he said. “Hospitalists have the perfect clinical background and mindset to help our health care system get to where it needs to go. It’s a huge challenge. It’s going to be a ton of work, and the stakes are very, very high.”
Reference
1. Thomas K. F.D.A. Nominee, Paid Millions by Industry, Says He’ll Recuse Himself if Needed. New York Times. March 29, 2017. https://www.nytimes.com/2017/03/29/health/fda-nominee-scott-gottlieb-recuse-conflicts.html?_r=0. Accessed March 31, 2017.
Febuxostat prevents early gout flares
The urate-lowering therapy febuxostat reduced the number of disease flares that people with early gout experienced relative to placebo in a double-blind, placebo-controlled study.
Febuxostat (Uloric) produced a 12.1% reduction in the overall percentage of patients who experienced at least one gout flare in comparison to placebo (29.3% vs. 41.4%; P less than .05).
Treatment with febuxostat also significantly improved control of serum uric acid, compared with the placebo-treated patients (64.3% vs. 5.7%; P less than .001), as well as synovitis as measured via the rheumatoid arthritis magnetic resonance imaging score (RAMRIS) score (–0.43 vs. –0.07; P less than .001).
“This study indicates that even for people who have only had one or two prior gout flares, urate-lowering therapy to reduce serum urate below 6 mg/dL may have benefit in reduction future flares,” said Dr. Dalbeth of the University of Auckland (New Zealand).
Febuxostat is a xanthine oxidase inhibitor indicated for the chronic management of excess uric acid in the blood in people diagnosed with gout. It is not indicated for use in people who have high serum uric acid levels without a history of gout.
“Although acute self-limiting flares are the most common clinical presentation of gout, joint damage is a frequent complication of the disease,” Dr. Dalbeth and her associates wrote.
“Joint damage is typically a late feature of long-standing gout,” they added, but “longitudinal observational data have indicated that joint damage can occur in some patients with early disease.” Increased urate crystal deposition could be associated with bone erosions, they explained, and the primary aim of the study was to look the rate of bone erosions over the course of 2 years’ treatment.
However, despite this reasoning and some prior clinical evidence, the researchers found no noticeable differences between the study groups in terms of radiologic joint erosion. This was assessed over 2 years in a single-affected joint. The mean change from baseline to month 24 in modified Sharp-van der Heijde erosion scores was 0.01 for both febuxostat and placebo (P = .47).
This was a phase 2 study conducted in 56 centers in the United States that screened 798 subjects and enrolled 314 adults with early gout. For inclusion, patients had to have hyperuricemia, defined as a serum uric acid level of 7.0 mg/dL or higher, and they had to have experienced no more than two gout flares in the past year.
Patients were randomized to once-daily treatment with febuxostat or placebo, with those randomized to the active treatment started at a dose of 40 mg, which could be increased to 80 mg after 2 weeks if serum uric acid levels remained high, at 6.0 mg/dL or above.
“To our knowledge, this is the first randomized, controlled trial examining the effects of [urate-lowering therapy] in patients with early gout,” the authors observed.
Although the primary endpoint was not met, the study “provides important new information about the natural history of joint damage in subjects with gout,” they suggested. In contrast to other inflammatory joint diseases, such as rheumatoid arthritis, joint erosions on plain radiographs were “infrequently observed at baseline” and over the 2-year study period. The fact the there was a change in MRI synovitis suggests that perhaps there may be some underlying joint damage occurring, although the clinical significance of this is uncertain.
Takeda, which makes febuxostat, funded the study. Dr. Dalbeth has received consulting fees, speaker fees, or research grants from Takeda, CymaBay, Ardea Biosciences, AstraZeneca, and Horizon. A couple of her coauthors disclosed acting as consultants to or receiving research funding from Takeda, and three were current or past employees of the company.
The urate-lowering therapy febuxostat reduced the number of disease flares that people with early gout experienced relative to placebo in a double-blind, placebo-controlled study.
Febuxostat (Uloric) produced a 12.1% reduction in the overall percentage of patients who experienced at least one gout flare in comparison to placebo (29.3% vs. 41.4%; P less than .05).
Treatment with febuxostat also significantly improved control of serum uric acid, compared with the placebo-treated patients (64.3% vs. 5.7%; P less than .001), as well as synovitis as measured via the rheumatoid arthritis magnetic resonance imaging score (RAMRIS) score (–0.43 vs. –0.07; P less than .001).
“This study indicates that even for people who have only had one or two prior gout flares, urate-lowering therapy to reduce serum urate below 6 mg/dL may have benefit in reduction future flares,” said Dr. Dalbeth of the University of Auckland (New Zealand).
Febuxostat is a xanthine oxidase inhibitor indicated for the chronic management of excess uric acid in the blood in people diagnosed with gout. It is not indicated for use in people who have high serum uric acid levels without a history of gout.
“Although acute self-limiting flares are the most common clinical presentation of gout, joint damage is a frequent complication of the disease,” Dr. Dalbeth and her associates wrote.
“Joint damage is typically a late feature of long-standing gout,” they added, but “longitudinal observational data have indicated that joint damage can occur in some patients with early disease.” Increased urate crystal deposition could be associated with bone erosions, they explained, and the primary aim of the study was to look the rate of bone erosions over the course of 2 years’ treatment.
However, despite this reasoning and some prior clinical evidence, the researchers found no noticeable differences between the study groups in terms of radiologic joint erosion. This was assessed over 2 years in a single-affected joint. The mean change from baseline to month 24 in modified Sharp-van der Heijde erosion scores was 0.01 for both febuxostat and placebo (P = .47).
This was a phase 2 study conducted in 56 centers in the United States that screened 798 subjects and enrolled 314 adults with early gout. For inclusion, patients had to have hyperuricemia, defined as a serum uric acid level of 7.0 mg/dL or higher, and they had to have experienced no more than two gout flares in the past year.
Patients were randomized to once-daily treatment with febuxostat or placebo, with those randomized to the active treatment started at a dose of 40 mg, which could be increased to 80 mg after 2 weeks if serum uric acid levels remained high, at 6.0 mg/dL or above.
“To our knowledge, this is the first randomized, controlled trial examining the effects of [urate-lowering therapy] in patients with early gout,” the authors observed.
Although the primary endpoint was not met, the study “provides important new information about the natural history of joint damage in subjects with gout,” they suggested. In contrast to other inflammatory joint diseases, such as rheumatoid arthritis, joint erosions on plain radiographs were “infrequently observed at baseline” and over the 2-year study period. The fact the there was a change in MRI synovitis suggests that perhaps there may be some underlying joint damage occurring, although the clinical significance of this is uncertain.
Takeda, which makes febuxostat, funded the study. Dr. Dalbeth has received consulting fees, speaker fees, or research grants from Takeda, CymaBay, Ardea Biosciences, AstraZeneca, and Horizon. A couple of her coauthors disclosed acting as consultants to or receiving research funding from Takeda, and three were current or past employees of the company.
The urate-lowering therapy febuxostat reduced the number of disease flares that people with early gout experienced relative to placebo in a double-blind, placebo-controlled study.
Febuxostat (Uloric) produced a 12.1% reduction in the overall percentage of patients who experienced at least one gout flare in comparison to placebo (29.3% vs. 41.4%; P less than .05).
Treatment with febuxostat also significantly improved control of serum uric acid, compared with the placebo-treated patients (64.3% vs. 5.7%; P less than .001), as well as synovitis as measured via the rheumatoid arthritis magnetic resonance imaging score (RAMRIS) score (–0.43 vs. –0.07; P less than .001).
“This study indicates that even for people who have only had one or two prior gout flares, urate-lowering therapy to reduce serum urate below 6 mg/dL may have benefit in reduction future flares,” said Dr. Dalbeth of the University of Auckland (New Zealand).
Febuxostat is a xanthine oxidase inhibitor indicated for the chronic management of excess uric acid in the blood in people diagnosed with gout. It is not indicated for use in people who have high serum uric acid levels without a history of gout.
“Although acute self-limiting flares are the most common clinical presentation of gout, joint damage is a frequent complication of the disease,” Dr. Dalbeth and her associates wrote.
“Joint damage is typically a late feature of long-standing gout,” they added, but “longitudinal observational data have indicated that joint damage can occur in some patients with early disease.” Increased urate crystal deposition could be associated with bone erosions, they explained, and the primary aim of the study was to look the rate of bone erosions over the course of 2 years’ treatment.
However, despite this reasoning and some prior clinical evidence, the researchers found no noticeable differences between the study groups in terms of radiologic joint erosion. This was assessed over 2 years in a single-affected joint. The mean change from baseline to month 24 in modified Sharp-van der Heijde erosion scores was 0.01 for both febuxostat and placebo (P = .47).
This was a phase 2 study conducted in 56 centers in the United States that screened 798 subjects and enrolled 314 adults with early gout. For inclusion, patients had to have hyperuricemia, defined as a serum uric acid level of 7.0 mg/dL or higher, and they had to have experienced no more than two gout flares in the past year.
Patients were randomized to once-daily treatment with febuxostat or placebo, with those randomized to the active treatment started at a dose of 40 mg, which could be increased to 80 mg after 2 weeks if serum uric acid levels remained high, at 6.0 mg/dL or above.
“To our knowledge, this is the first randomized, controlled trial examining the effects of [urate-lowering therapy] in patients with early gout,” the authors observed.
Although the primary endpoint was not met, the study “provides important new information about the natural history of joint damage in subjects with gout,” they suggested. In contrast to other inflammatory joint diseases, such as rheumatoid arthritis, joint erosions on plain radiographs were “infrequently observed at baseline” and over the 2-year study period. The fact the there was a change in MRI synovitis suggests that perhaps there may be some underlying joint damage occurring, although the clinical significance of this is uncertain.
Takeda, which makes febuxostat, funded the study. Dr. Dalbeth has received consulting fees, speaker fees, or research grants from Takeda, CymaBay, Ardea Biosciences, AstraZeneca, and Horizon. A couple of her coauthors disclosed acting as consultants to or receiving research funding from Takeda, and three were current or past employees of the company.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point:
Major finding: Gout flares over 2 years occurred in 29.3% of subjects treated with febuxostat versus 41.4% of those given placebo (P less than .05)
Data source Randomized, double-blind, placebo-controlled study assessing febuxostat versus placebo effects on joint damage in 314 hyperuricemic subjects with early gout.
Disclosures: Takeda, which makes febuxostat, funded the study. Dr. Dalbeth has received consulting fees, speaker fees, or research grants from Takeda, CymaBay, Ardea Biosciences, AstraZeneca, and Horizon. A couple of her coauthors disclosed acting as consultants to or receiving research funding from Takeda, and three were current or past employees of the company.
Innovations in Dermatology: Brodalumab for Plaque Psoriasis
Study supports routine rapid HCV testing for at-risk youth
Routine finger-stick testing for hepatitis C virus infection is the best screening strategy for 15- to 30-year-olds, provided that at least 6 in every 1,000 have injected drugs, according to the results of a modeling study.
“Currently, nearly all hepatitis C virus (HCV) transmission in the United States occurs among young persons who inject drugs,” wrote Sabrina A. Assoumou, MD, of Boston Medical Center and Boston University and her associates. “We show that routine testing provides the most clinical benefit and best value for money in an urban community health setting where HCV prevalence is high.”
Rapid routine testing consistently yielded more quality-adjusted life years (QALYs) at a lower cost than did the current practice of reflexive, risk-based venipuncture testing, the researchers said. They recommended that urban health centers either replace venipuncture diagnostics with routine finger-stick testing or that they ensure follow-up RNA testing when needed so they can link HCV-positive patients to treatment (Clin Infect Dis. 2017 Sep 9. doi: 10.1093/cid/cix798).
The Centers for Disease Control and Prevention has recommended risk-based HCV testing, but studies indicate that primary care providers often miss the chance to test and have trouble identifying high-risk patients, Dr. Assoumou and her associates said. The standard HCV test is a blood draw for antibody testing followed by confirmatory RNA testing, but a two-step process complicates follow-up.
To compare one-time HCV screening strategies in high-risk settings, the researchers created a decision analytic model using TreeAge Pro 2014 software and input data on prevalence, mortality, treatment costs, and efficacy from an extensive literature review.
Compared with targeted risk-based HCV testing, routine rapid testing performed by dedicated counselors yielded an incremental cost-effectiveness ratio of less than $100,000 per quality-adjusted life year unless the prevalence of injection drug use was less than 0.59%, the prevalence of HCV infection among injection drug users was less than 16%, the reinfection rate exceeded 26 cases per 100 person-years, or all venipuncture antibody tests were followed by confirmatory testing. Routine rapid testing identified 20% of HCV infections in the model, which is four times the rate under current practice. Rates of sustained virologic response were 18% with routine rapid testing and 2% with standard practice.
Routine rapid testing did not dramatically boost QALYs at a population level, the researchers acknowledged, but diagnosing and treating an injection drug user increased life span by an average of 2 years and saved $214,000 per patient in additional costs.
“Rapid testing always provided greater life expectancy than venipuncture testing at either a lower lifetime medical cost or a lower cost/QALY gained,” the investigators concluded. “Future studies are needed to define the programmatic effectiveness of HCV treatment among youth, and testing and treatment acceptability in this population.”
The National Institute on Drug Abuse and the National Institute of Allergy and Infectious Diseases provided funding. The researchers reported having no conflicts of interest.
Routine finger-stick testing for hepatitis C virus infection is the best screening strategy for 15- to 30-year-olds, provided that at least 6 in every 1,000 have injected drugs, according to the results of a modeling study.
“Currently, nearly all hepatitis C virus (HCV) transmission in the United States occurs among young persons who inject drugs,” wrote Sabrina A. Assoumou, MD, of Boston Medical Center and Boston University and her associates. “We show that routine testing provides the most clinical benefit and best value for money in an urban community health setting where HCV prevalence is high.”
Rapid routine testing consistently yielded more quality-adjusted life years (QALYs) at a lower cost than did the current practice of reflexive, risk-based venipuncture testing, the researchers said. They recommended that urban health centers either replace venipuncture diagnostics with routine finger-stick testing or that they ensure follow-up RNA testing when needed so they can link HCV-positive patients to treatment (Clin Infect Dis. 2017 Sep 9. doi: 10.1093/cid/cix798).
The Centers for Disease Control and Prevention has recommended risk-based HCV testing, but studies indicate that primary care providers often miss the chance to test and have trouble identifying high-risk patients, Dr. Assoumou and her associates said. The standard HCV test is a blood draw for antibody testing followed by confirmatory RNA testing, but a two-step process complicates follow-up.
To compare one-time HCV screening strategies in high-risk settings, the researchers created a decision analytic model using TreeAge Pro 2014 software and input data on prevalence, mortality, treatment costs, and efficacy from an extensive literature review.
Compared with targeted risk-based HCV testing, routine rapid testing performed by dedicated counselors yielded an incremental cost-effectiveness ratio of less than $100,000 per quality-adjusted life year unless the prevalence of injection drug use was less than 0.59%, the prevalence of HCV infection among injection drug users was less than 16%, the reinfection rate exceeded 26 cases per 100 person-years, or all venipuncture antibody tests were followed by confirmatory testing. Routine rapid testing identified 20% of HCV infections in the model, which is four times the rate under current practice. Rates of sustained virologic response were 18% with routine rapid testing and 2% with standard practice.
Routine rapid testing did not dramatically boost QALYs at a population level, the researchers acknowledged, but diagnosing and treating an injection drug user increased life span by an average of 2 years and saved $214,000 per patient in additional costs.
“Rapid testing always provided greater life expectancy than venipuncture testing at either a lower lifetime medical cost or a lower cost/QALY gained,” the investigators concluded. “Future studies are needed to define the programmatic effectiveness of HCV treatment among youth, and testing and treatment acceptability in this population.”
The National Institute on Drug Abuse and the National Institute of Allergy and Infectious Diseases provided funding. The researchers reported having no conflicts of interest.
Routine finger-stick testing for hepatitis C virus infection is the best screening strategy for 15- to 30-year-olds, provided that at least 6 in every 1,000 have injected drugs, according to the results of a modeling study.
“Currently, nearly all hepatitis C virus (HCV) transmission in the United States occurs among young persons who inject drugs,” wrote Sabrina A. Assoumou, MD, of Boston Medical Center and Boston University and her associates. “We show that routine testing provides the most clinical benefit and best value for money in an urban community health setting where HCV prevalence is high.”
Rapid routine testing consistently yielded more quality-adjusted life years (QALYs) at a lower cost than did the current practice of reflexive, risk-based venipuncture testing, the researchers said. They recommended that urban health centers either replace venipuncture diagnostics with routine finger-stick testing or that they ensure follow-up RNA testing when needed so they can link HCV-positive patients to treatment (Clin Infect Dis. 2017 Sep 9. doi: 10.1093/cid/cix798).
The Centers for Disease Control and Prevention has recommended risk-based HCV testing, but studies indicate that primary care providers often miss the chance to test and have trouble identifying high-risk patients, Dr. Assoumou and her associates said. The standard HCV test is a blood draw for antibody testing followed by confirmatory RNA testing, but a two-step process complicates follow-up.
To compare one-time HCV screening strategies in high-risk settings, the researchers created a decision analytic model using TreeAge Pro 2014 software and input data on prevalence, mortality, treatment costs, and efficacy from an extensive literature review.
Compared with targeted risk-based HCV testing, routine rapid testing performed by dedicated counselors yielded an incremental cost-effectiveness ratio of less than $100,000 per quality-adjusted life year unless the prevalence of injection drug use was less than 0.59%, the prevalence of HCV infection among injection drug users was less than 16%, the reinfection rate exceeded 26 cases per 100 person-years, or all venipuncture antibody tests were followed by confirmatory testing. Routine rapid testing identified 20% of HCV infections in the model, which is four times the rate under current practice. Rates of sustained virologic response were 18% with routine rapid testing and 2% with standard practice.
Routine rapid testing did not dramatically boost QALYs at a population level, the researchers acknowledged, but diagnosing and treating an injection drug user increased life span by an average of 2 years and saved $214,000 per patient in additional costs.
“Rapid testing always provided greater life expectancy than venipuncture testing at either a lower lifetime medical cost or a lower cost/QALY gained,” the investigators concluded. “Future studies are needed to define the programmatic effectiveness of HCV treatment among youth, and testing and treatment acceptability in this population.”
The National Institute on Drug Abuse and the National Institute of Allergy and Infectious Diseases provided funding. The researchers reported having no conflicts of interest.
FROM CLINICAL INFECTIOUS DISEASES
Key clinical point: Routine finger-stick testing was the most cost-effective way to screen urban adolescents and young adults for hepatitis C virus infection.
Major finding: The incremental cost-effectiveness ratio was less than $100,000 per quality-adjusted life year unless prevalence of injection drug use was less than 0.59%, less than 16% of injection drug users had HCV infection, the reinfection rate exceeded 26 cases per 100 person-years, or all venipuncture antibody tests were followed by confirmatory testing.
Data source: A decision analytic model created with TreeAge Pro 2014 and data from an extensive literature review.
Disclosures: The National Institute on Drug Abuse and the National Institute of Allergy and Infectious Diseases provided funding. The researchers reported having no conflicts of interest.
FDA approves implantable device for central sleep apnea
The U.S. Food and Drug Administration on Oct. 6 approved an implantable device for the treatment of moderate to severe central sleep apnea.
The remedē System consists of a battery pack and small, thin wires placed under the skin in the upper chest area. The wires are inserted into the blood vessels in the chest to stimulate the phrenic nerve. The system monitors respiratory signals and, when it stimulates the nerve, the diaphragm moves to restore normal breathing.
The agency’s approval comes on the basis of study results showing that the system reduced the apnea–hypopnea index scores by 50% or more in 51% of patients studied. Control patients in the study saw an 11% reduction in their score.
Adverse events reported in the study included concomitant device interaction, implant site infection, and swelling and local tissue damage or pocket erosion. The remedē System is contraindicated for patients with active infection or who are known to require an MRI.
The U.S. Food and Drug Administration on Oct. 6 approved an implantable device for the treatment of moderate to severe central sleep apnea.
The remedē System consists of a battery pack and small, thin wires placed under the skin in the upper chest area. The wires are inserted into the blood vessels in the chest to stimulate the phrenic nerve. The system monitors respiratory signals and, when it stimulates the nerve, the diaphragm moves to restore normal breathing.
The agency’s approval comes on the basis of study results showing that the system reduced the apnea–hypopnea index scores by 50% or more in 51% of patients studied. Control patients in the study saw an 11% reduction in their score.
Adverse events reported in the study included concomitant device interaction, implant site infection, and swelling and local tissue damage or pocket erosion. The remedē System is contraindicated for patients with active infection or who are known to require an MRI.
The U.S. Food and Drug Administration on Oct. 6 approved an implantable device for the treatment of moderate to severe central sleep apnea.
The remedē System consists of a battery pack and small, thin wires placed under the skin in the upper chest area. The wires are inserted into the blood vessels in the chest to stimulate the phrenic nerve. The system monitors respiratory signals and, when it stimulates the nerve, the diaphragm moves to restore normal breathing.
The agency’s approval comes on the basis of study results showing that the system reduced the apnea–hypopnea index scores by 50% or more in 51% of patients studied. Control patients in the study saw an 11% reduction in their score.
Adverse events reported in the study included concomitant device interaction, implant site infection, and swelling and local tissue damage or pocket erosion. The remedē System is contraindicated for patients with active infection or who are known to require an MRI.
Spotting Sepsis Sooner
More than 1.5 million Americans develop sepsis each year, and at least 250,000 die of it. “Detecting sepsis early and starting immediate treatment is often the difference between life and death. It starts with preventing the infections that lead to sepsis,” said CDC Director Brenda Fitzgerald, MD, introducing the CDC’s Get Ahead of Sepsis campaign, which launched in August. “We created Get Ahead of Sepsis to give people the resources they need to help stop this medical emergency in its tracks.”
The campaign is an educational initiative for both the public and health care professionals in hospitals, home care, long-term care, and urgent care. For many patients, the CDC says, sepsis develops from an infection that begins outside the hospital. Health care professionals are not only in prime positions to monitor for signs and symptoms of sepsis in the health care setting—they can also help educate patients about things they can do to prevent sepsis. For instance, people with chronic conditions can take good care to avoid infections that could lead to sepsis.
The campaign website, www.cdc.gov/sepsis, provides fact sheets, infographics, brochures, and other materials to help spread the word.
More than 1.5 million Americans develop sepsis each year, and at least 250,000 die of it. “Detecting sepsis early and starting immediate treatment is often the difference between life and death. It starts with preventing the infections that lead to sepsis,” said CDC Director Brenda Fitzgerald, MD, introducing the CDC’s Get Ahead of Sepsis campaign, which launched in August. “We created Get Ahead of Sepsis to give people the resources they need to help stop this medical emergency in its tracks.”
The campaign is an educational initiative for both the public and health care professionals in hospitals, home care, long-term care, and urgent care. For many patients, the CDC says, sepsis develops from an infection that begins outside the hospital. Health care professionals are not only in prime positions to monitor for signs and symptoms of sepsis in the health care setting—they can also help educate patients about things they can do to prevent sepsis. For instance, people with chronic conditions can take good care to avoid infections that could lead to sepsis.
The campaign website, www.cdc.gov/sepsis, provides fact sheets, infographics, brochures, and other materials to help spread the word.
More than 1.5 million Americans develop sepsis each year, and at least 250,000 die of it. “Detecting sepsis early and starting immediate treatment is often the difference between life and death. It starts with preventing the infections that lead to sepsis,” said CDC Director Brenda Fitzgerald, MD, introducing the CDC’s Get Ahead of Sepsis campaign, which launched in August. “We created Get Ahead of Sepsis to give people the resources they need to help stop this medical emergency in its tracks.”
The campaign is an educational initiative for both the public and health care professionals in hospitals, home care, long-term care, and urgent care. For many patients, the CDC says, sepsis develops from an infection that begins outside the hospital. Health care professionals are not only in prime positions to monitor for signs and symptoms of sepsis in the health care setting—they can also help educate patients about things they can do to prevent sepsis. For instance, people with chronic conditions can take good care to avoid infections that could lead to sepsis.
The campaign website, www.cdc.gov/sepsis, provides fact sheets, infographics, brochures, and other materials to help spread the word.
Nutrition Index Helps Identify High-Risk Elderly Heart Patients
A “wealth of evidence” suggests that nutrition and immunologic status on admission is closely associated with the outcome of patients with cardiovascular disease—especially high-risk elderly patients, say researchers from Chinese People’s Liberation Army General Hospital, Beijing. The researchers note that malnutrition is an independent factor influencing post myocardial infarction complications and mortality in geriatric patients with coronary artery disease (CAD). According to their study of 336 hospitalized patients with hypertension, the Controlling Nutritional Status (CONUT) score can help predict who is at highest risk.
Nutrition indexes are widely used. CONUT scores, which are calculated based on serum albumin concentration, total peripheral lymphocyte count, and total cholesterol concentration, have been found useful in a variety of areas, including cancer. The Geriatric Nutritional Risk Index (GNRI), although a relatively new index for nutrition assessment in the elderly, is the most-used tool to evaluate patients with chronic kidney disease, the researchers say. Both indexes are “widely applied” in evaluation of patients with tumors who are also undergoing dialysis. Some studies also have reported on GNRI as a prognostic factor in cardiovascular diseases.
The researchers conducted their study to assess the effect of nutrition status on survival in patients aged ≥ 80 years, with hypertension, measuring outcomes at 90 days postadmission. All patients had a history of CAD, 167 had type 2 diabetes, and 124 had anemia. Of the enrolled patients, 192 were admitted for respiratory tract infection, with a significantly high proportion of poor nutrition status. Five patients scored > 9 on the CONUT scale. A score of ≥ 5 indicated moderate to severe malnutrition.
During the 90-day follow-up, 27 patients died. No differences in systolic blood pressure were found. The surviving patients, however, showed increased body mass index, hemoglobin, and albumin levels, as well as lower diastolic blood pressure and fasting blood glucose. Surviving patients had improved GRNI scores and reduced CONUT scores, both of which indicated improved nutrition status. Respiratory tract infection, CONUT, and albumin were independent predictors of all-cause mortality.
However, only CONUT accurately predicted all-cause mortality among patients with hypertension during the 90-day follow-up. A CONUT score above 3.0 at admission predicted all-cause mortality with a sensitivity of 77.8% and specificity of 64.7%.
Source:
Sun X, Luo L, Zhao X, Ye P. BMJ Open. 2017;7(9):e015649.
doi: 10.1136/bmjopen-2016-015649.
A “wealth of evidence” suggests that nutrition and immunologic status on admission is closely associated with the outcome of patients with cardiovascular disease—especially high-risk elderly patients, say researchers from Chinese People’s Liberation Army General Hospital, Beijing. The researchers note that malnutrition is an independent factor influencing post myocardial infarction complications and mortality in geriatric patients with coronary artery disease (CAD). According to their study of 336 hospitalized patients with hypertension, the Controlling Nutritional Status (CONUT) score can help predict who is at highest risk.
Nutrition indexes are widely used. CONUT scores, which are calculated based on serum albumin concentration, total peripheral lymphocyte count, and total cholesterol concentration, have been found useful in a variety of areas, including cancer. The Geriatric Nutritional Risk Index (GNRI), although a relatively new index for nutrition assessment in the elderly, is the most-used tool to evaluate patients with chronic kidney disease, the researchers say. Both indexes are “widely applied” in evaluation of patients with tumors who are also undergoing dialysis. Some studies also have reported on GNRI as a prognostic factor in cardiovascular diseases.
The researchers conducted their study to assess the effect of nutrition status on survival in patients aged ≥ 80 years, with hypertension, measuring outcomes at 90 days postadmission. All patients had a history of CAD, 167 had type 2 diabetes, and 124 had anemia. Of the enrolled patients, 192 were admitted for respiratory tract infection, with a significantly high proportion of poor nutrition status. Five patients scored > 9 on the CONUT scale. A score of ≥ 5 indicated moderate to severe malnutrition.
During the 90-day follow-up, 27 patients died. No differences in systolic blood pressure were found. The surviving patients, however, showed increased body mass index, hemoglobin, and albumin levels, as well as lower diastolic blood pressure and fasting blood glucose. Surviving patients had improved GRNI scores and reduced CONUT scores, both of which indicated improved nutrition status. Respiratory tract infection, CONUT, and albumin were independent predictors of all-cause mortality.
However, only CONUT accurately predicted all-cause mortality among patients with hypertension during the 90-day follow-up. A CONUT score above 3.0 at admission predicted all-cause mortality with a sensitivity of 77.8% and specificity of 64.7%.
Source:
Sun X, Luo L, Zhao X, Ye P. BMJ Open. 2017;7(9):e015649.
doi: 10.1136/bmjopen-2016-015649.
A “wealth of evidence” suggests that nutrition and immunologic status on admission is closely associated with the outcome of patients with cardiovascular disease—especially high-risk elderly patients, say researchers from Chinese People’s Liberation Army General Hospital, Beijing. The researchers note that malnutrition is an independent factor influencing post myocardial infarction complications and mortality in geriatric patients with coronary artery disease (CAD). According to their study of 336 hospitalized patients with hypertension, the Controlling Nutritional Status (CONUT) score can help predict who is at highest risk.
Nutrition indexes are widely used. CONUT scores, which are calculated based on serum albumin concentration, total peripheral lymphocyte count, and total cholesterol concentration, have been found useful in a variety of areas, including cancer. The Geriatric Nutritional Risk Index (GNRI), although a relatively new index for nutrition assessment in the elderly, is the most-used tool to evaluate patients with chronic kidney disease, the researchers say. Both indexes are “widely applied” in evaluation of patients with tumors who are also undergoing dialysis. Some studies also have reported on GNRI as a prognostic factor in cardiovascular diseases.
The researchers conducted their study to assess the effect of nutrition status on survival in patients aged ≥ 80 years, with hypertension, measuring outcomes at 90 days postadmission. All patients had a history of CAD, 167 had type 2 diabetes, and 124 had anemia. Of the enrolled patients, 192 were admitted for respiratory tract infection, with a significantly high proportion of poor nutrition status. Five patients scored > 9 on the CONUT scale. A score of ≥ 5 indicated moderate to severe malnutrition.
During the 90-day follow-up, 27 patients died. No differences in systolic blood pressure were found. The surviving patients, however, showed increased body mass index, hemoglobin, and albumin levels, as well as lower diastolic blood pressure and fasting blood glucose. Surviving patients had improved GRNI scores and reduced CONUT scores, both of which indicated improved nutrition status. Respiratory tract infection, CONUT, and albumin were independent predictors of all-cause mortality.
However, only CONUT accurately predicted all-cause mortality among patients with hypertension during the 90-day follow-up. A CONUT score above 3.0 at admission predicted all-cause mortality with a sensitivity of 77.8% and specificity of 64.7%.
Source:
Sun X, Luo L, Zhao X, Ye P. BMJ Open. 2017;7(9):e015649.
doi: 10.1136/bmjopen-2016-015649.