User login
A Synergistic Antitumor Delivery System
Oncolytic viruses (OVs) are proving to be a promising modality in anticancer therapy. They selectively grow, replicate within, and kill tumor cells while leaving alone untransformed cells. But OVs, such as the respiratory enteric orphan viruses (or reoviruses), have been hampered by neutralizing antireoviral antibodies, which block the viruses from binding to cellular surface receptors, inhibiting viral infection and replication. Researchers from Guizhou Medical University in China and Stanford University in California may have found a solution: a novel strategy using cytokine-induced killer (CIK) cells—which also have antitumor activity—as the delivery system.
Related: Advances in Targeted Therapy for Breast Cancer
Reoviruses have been shown to infect and use peripheral blood mononuclear cells (PBMCs) to reach tumors. Combining cytokine-induced killer cells (developed from PBMCs) with a reovirus could be the way in, the researchers theorized. Their strategy relies on cell carriage—the capacity of some OVs to be “carried” by immune cells to the tumor, where they selectively infect and kill tumor cells.
The researchers found that CIK cells provided cell carriage to the reovirus, which exerted an oncolytic effect on tumor cells but not CIK cells. Moreover, the CIK cells promoted reovirus infection of tumor cells in the presence of neutralizing antibodies. At the same time, the reovirus infection boosted the power of the CIK cells.
Related: Potential New Targeted Treatment for Chondrosarcoma
The researchers’ findings support the idea that reoviruseas and CIK cells are both potent antitumor agents and are “superior as a combination strategy.”
Source:
Zhao X, Ouyang W, Chester C, Long S, Wang N, He Z. PLoS One. 2017;12(9):e0184816.
doi: 10.1371/journal.pone.0184816.
Oncolytic viruses (OVs) are proving to be a promising modality in anticancer therapy. They selectively grow, replicate within, and kill tumor cells while leaving alone untransformed cells. But OVs, such as the respiratory enteric orphan viruses (or reoviruses), have been hampered by neutralizing antireoviral antibodies, which block the viruses from binding to cellular surface receptors, inhibiting viral infection and replication. Researchers from Guizhou Medical University in China and Stanford University in California may have found a solution: a novel strategy using cytokine-induced killer (CIK) cells—which also have antitumor activity—as the delivery system.
Related: Advances in Targeted Therapy for Breast Cancer
Reoviruses have been shown to infect and use peripheral blood mononuclear cells (PBMCs) to reach tumors. Combining cytokine-induced killer cells (developed from PBMCs) with a reovirus could be the way in, the researchers theorized. Their strategy relies on cell carriage—the capacity of some OVs to be “carried” by immune cells to the tumor, where they selectively infect and kill tumor cells.
The researchers found that CIK cells provided cell carriage to the reovirus, which exerted an oncolytic effect on tumor cells but not CIK cells. Moreover, the CIK cells promoted reovirus infection of tumor cells in the presence of neutralizing antibodies. At the same time, the reovirus infection boosted the power of the CIK cells.
Related: Potential New Targeted Treatment for Chondrosarcoma
The researchers’ findings support the idea that reoviruseas and CIK cells are both potent antitumor agents and are “superior as a combination strategy.”
Source:
Zhao X, Ouyang W, Chester C, Long S, Wang N, He Z. PLoS One. 2017;12(9):e0184816.
doi: 10.1371/journal.pone.0184816.
Oncolytic viruses (OVs) are proving to be a promising modality in anticancer therapy. They selectively grow, replicate within, and kill tumor cells while leaving alone untransformed cells. But OVs, such as the respiratory enteric orphan viruses (or reoviruses), have been hampered by neutralizing antireoviral antibodies, which block the viruses from binding to cellular surface receptors, inhibiting viral infection and replication. Researchers from Guizhou Medical University in China and Stanford University in California may have found a solution: a novel strategy using cytokine-induced killer (CIK) cells—which also have antitumor activity—as the delivery system.
Related: Advances in Targeted Therapy for Breast Cancer
Reoviruses have been shown to infect and use peripheral blood mononuclear cells (PBMCs) to reach tumors. Combining cytokine-induced killer cells (developed from PBMCs) with a reovirus could be the way in, the researchers theorized. Their strategy relies on cell carriage—the capacity of some OVs to be “carried” by immune cells to the tumor, where they selectively infect and kill tumor cells.
The researchers found that CIK cells provided cell carriage to the reovirus, which exerted an oncolytic effect on tumor cells but not CIK cells. Moreover, the CIK cells promoted reovirus infection of tumor cells in the presence of neutralizing antibodies. At the same time, the reovirus infection boosted the power of the CIK cells.
Related: Potential New Targeted Treatment for Chondrosarcoma
The researchers’ findings support the idea that reoviruseas and CIK cells are both potent antitumor agents and are “superior as a combination strategy.”
Source:
Zhao X, Ouyang W, Chester C, Long S, Wang N, He Z. PLoS One. 2017;12(9):e0184816.
doi: 10.1371/journal.pone.0184816.
Reminder calls to patients improve fecal test sample response rates
Automated and live phone calls were shown to improve patient return of fecal test samples for both English and Spanish speakers, based on the results of a pilot study.
Colorectal cancer (CRC) is the second deadliest cancer in the United States. In 2017 alone, 50,000 people died from the disease. Screening has been shown to be a very effective tool in decreasing the mortality and incidence of CRC, but screening rates are low with 63% of adults adhering to recommended screening schedules. This problem has been addressed by direct-mail fecal immunochemical testing (FIT) kits with associated reminders, but few studies have evaluated effectiveness of follow-up techniques on FIT return rates until this pilot study.
“While many direct-mail fecal testing programs have delivered patient reminders, ours is the first study to rigorously test the effectiveness of these reminders in a community health center population, and among patients with differing language preferences,” wrote Gloria Coronado, PhD, of the Center for Health Research at Kaiser Permanente and her colleagues. “We observed important variations in return rates based on reminder mode used, and both the overall return rate and reminder mode effectiveness differed according to patients’ language preferences.”
The trial had two groups, one randomized and the other nonrandomized. Nonrandomized patients had active patient portals and received email reminders through the portal. The randomized group was sorted into seven intervention groups: Four of the groups used a unimodal contact method and three groups used a multimodal contact method. The unimodal contact methods were letter reminders, automated call reminders, text reminders, and live call reminders. The multimodal contact methods were a reminder letter plus live call reminders, automated calls plus live call reminders, and text message reminders plus live call reminders. All written materials to contact patients were developed in English and later translated into Spanish and Russian. Phone call scripts were also developed in English and later translated into Spanish but not Russian due to a lack of Russian speaking outreach workers.
After combining early-return FIT samples, those in the nonrandomized patient portal group, and the randomized samples, the overall return rate was 32.7%.
The method of contact for patients strongly influenced return rates for patients. Patients who received live phone calls were 50% more likely to return their FIT kit, compared with those who simply received a reminder email. Both English and Spanish speakers were much more likely to return their FIT kits if they were contacted with live or automated phone calls with odds ratios of 2.17 and 3.45, respectively. All other reminder techniques that did not include a phone call had similar completion rates to that of a reminder letter.
“Automated phone calls and text messages are the least costly options to implement, yet live reminders may allow staff to address or triage other patient health care needs,” they wrote.
Dr. Gloria Coronado was a coinvestigator for a study funded by Epigenomics. All other authors had no conflicts of interest to report.
Automated and live phone calls were shown to improve patient return of fecal test samples for both English and Spanish speakers, based on the results of a pilot study.
Colorectal cancer (CRC) is the second deadliest cancer in the United States. In 2017 alone, 50,000 people died from the disease. Screening has been shown to be a very effective tool in decreasing the mortality and incidence of CRC, but screening rates are low with 63% of adults adhering to recommended screening schedules. This problem has been addressed by direct-mail fecal immunochemical testing (FIT) kits with associated reminders, but few studies have evaluated effectiveness of follow-up techniques on FIT return rates until this pilot study.
“While many direct-mail fecal testing programs have delivered patient reminders, ours is the first study to rigorously test the effectiveness of these reminders in a community health center population, and among patients with differing language preferences,” wrote Gloria Coronado, PhD, of the Center for Health Research at Kaiser Permanente and her colleagues. “We observed important variations in return rates based on reminder mode used, and both the overall return rate and reminder mode effectiveness differed according to patients’ language preferences.”
The trial had two groups, one randomized and the other nonrandomized. Nonrandomized patients had active patient portals and received email reminders through the portal. The randomized group was sorted into seven intervention groups: Four of the groups used a unimodal contact method and three groups used a multimodal contact method. The unimodal contact methods were letter reminders, automated call reminders, text reminders, and live call reminders. The multimodal contact methods were a reminder letter plus live call reminders, automated calls plus live call reminders, and text message reminders plus live call reminders. All written materials to contact patients were developed in English and later translated into Spanish and Russian. Phone call scripts were also developed in English and later translated into Spanish but not Russian due to a lack of Russian speaking outreach workers.
After combining early-return FIT samples, those in the nonrandomized patient portal group, and the randomized samples, the overall return rate was 32.7%.
The method of contact for patients strongly influenced return rates for patients. Patients who received live phone calls were 50% more likely to return their FIT kit, compared with those who simply received a reminder email. Both English and Spanish speakers were much more likely to return their FIT kits if they were contacted with live or automated phone calls with odds ratios of 2.17 and 3.45, respectively. All other reminder techniques that did not include a phone call had similar completion rates to that of a reminder letter.
“Automated phone calls and text messages are the least costly options to implement, yet live reminders may allow staff to address or triage other patient health care needs,” they wrote.
Dr. Gloria Coronado was a coinvestigator for a study funded by Epigenomics. All other authors had no conflicts of interest to report.
Automated and live phone calls were shown to improve patient return of fecal test samples for both English and Spanish speakers, based on the results of a pilot study.
Colorectal cancer (CRC) is the second deadliest cancer in the United States. In 2017 alone, 50,000 people died from the disease. Screening has been shown to be a very effective tool in decreasing the mortality and incidence of CRC, but screening rates are low with 63% of adults adhering to recommended screening schedules. This problem has been addressed by direct-mail fecal immunochemical testing (FIT) kits with associated reminders, but few studies have evaluated effectiveness of follow-up techniques on FIT return rates until this pilot study.
“While many direct-mail fecal testing programs have delivered patient reminders, ours is the first study to rigorously test the effectiveness of these reminders in a community health center population, and among patients with differing language preferences,” wrote Gloria Coronado, PhD, of the Center for Health Research at Kaiser Permanente and her colleagues. “We observed important variations in return rates based on reminder mode used, and both the overall return rate and reminder mode effectiveness differed according to patients’ language preferences.”
The trial had two groups, one randomized and the other nonrandomized. Nonrandomized patients had active patient portals and received email reminders through the portal. The randomized group was sorted into seven intervention groups: Four of the groups used a unimodal contact method and three groups used a multimodal contact method. The unimodal contact methods were letter reminders, automated call reminders, text reminders, and live call reminders. The multimodal contact methods were a reminder letter plus live call reminders, automated calls plus live call reminders, and text message reminders plus live call reminders. All written materials to contact patients were developed in English and later translated into Spanish and Russian. Phone call scripts were also developed in English and later translated into Spanish but not Russian due to a lack of Russian speaking outreach workers.
After combining early-return FIT samples, those in the nonrandomized patient portal group, and the randomized samples, the overall return rate was 32.7%.
The method of contact for patients strongly influenced return rates for patients. Patients who received live phone calls were 50% more likely to return their FIT kit, compared with those who simply received a reminder email. Both English and Spanish speakers were much more likely to return their FIT kits if they were contacted with live or automated phone calls with odds ratios of 2.17 and 3.45, respectively. All other reminder techniques that did not include a phone call had similar completion rates to that of a reminder letter.
“Automated phone calls and text messages are the least costly options to implement, yet live reminders may allow staff to address or triage other patient health care needs,” they wrote.
Dr. Gloria Coronado was a coinvestigator for a study funded by Epigenomics. All other authors had no conflicts of interest to report.
FROM THE JOURNAL OF GENERAL INTERNAL MEDICINE
Key clinical point:
Major finding: 32.7% of patients returned their fecal testing kit after receiving a reminder.
Data source: Patient-randomized control trial of 2,478 adults who were not up to date on their colorectal cancer screening.
Disclosures: Dr. Gloria Coronado was a coinvestigator for a study funded by Epigenomics. All other authors had no conflicts of interest to report.
VA Proposes New Telehealth Rule
Not willing to wait for a pending legislative fix, the VA has proposed a new rule that would allow expanding telehealth offerings across state lines. The Authority of Health Care Providers to Practice Telehealth rule was proposed on October 2, 2017 and comments will remain open through November 1, 2017.
“VA has developed a telehealth program as a modern, beneficiary- and family-centered health care delivery model that leverages information and telecommunication technologies to connect beneficiaries with health care providers, irrespective of the State or location within a State where the health care provider or the beneficiary is physically located at the time the health care is provided,” the rule states. “Telehealth increases the accessibility of VA health care, bringing VA medical services to locations convenient for beneficiaries, including clinics in remote communities and beneficiaries' homes. By providing health care services by telehealth from one State to a beneficiary located in another State or within the same State, whether that beneficiary is located at a VA medical facility or in his or her own home, VA can use its limited health care resources most efficiently.”
The VA has long recognized the importance of telehealth for delivering health care to rural veterans who live far from VAMCs and community-based outpatient clinics. Providing specialty care can be especially challenging, given the dearth of specialists in many rural locations. In addition, many veterans live in a different state than the closest VAMC, creating health care delivery challenges.
The legal standing of telehealth is unclear. Currently, VA requires that health care providers (HCPs) are licensed in the same state as the patient location at time of health care delivery. VA Secretary David J. Shulkin, MD, has called for new legislation clarifying that VA HCPs do have authority to care for patients located in a different state. However, the new rule cites previously granted legislative authority. “In an effort to furnish care to all beneficiaries and use its resources most efficiently,” the rule states, “VA needs to operate its telehealth program with health care providers who will provide services via telehealth to beneficiaries in States in which they are not licensed.”
To date, the rule has received relatively few comments, and none dispute the VA interpretation of its legal authority to change telehealth rules. A few commenters have raised concerns over the quality use of telehealth care, especially for mental health care. Most comments, however, supported the expansion of telehealth to increase access to care for veterans.
House Resolution 2123, the VETS Act of 2017, also contains similar provisions that would streamline interstate telehealth. However, that bill has yet to receive a vote in the House Veterans Affairs committee.
Not willing to wait for a pending legislative fix, the VA has proposed a new rule that would allow expanding telehealth offerings across state lines. The Authority of Health Care Providers to Practice Telehealth rule was proposed on October 2, 2017 and comments will remain open through November 1, 2017.
“VA has developed a telehealth program as a modern, beneficiary- and family-centered health care delivery model that leverages information and telecommunication technologies to connect beneficiaries with health care providers, irrespective of the State or location within a State where the health care provider or the beneficiary is physically located at the time the health care is provided,” the rule states. “Telehealth increases the accessibility of VA health care, bringing VA medical services to locations convenient for beneficiaries, including clinics in remote communities and beneficiaries' homes. By providing health care services by telehealth from one State to a beneficiary located in another State or within the same State, whether that beneficiary is located at a VA medical facility or in his or her own home, VA can use its limited health care resources most efficiently.”
The VA has long recognized the importance of telehealth for delivering health care to rural veterans who live far from VAMCs and community-based outpatient clinics. Providing specialty care can be especially challenging, given the dearth of specialists in many rural locations. In addition, many veterans live in a different state than the closest VAMC, creating health care delivery challenges.
The legal standing of telehealth is unclear. Currently, VA requires that health care providers (HCPs) are licensed in the same state as the patient location at time of health care delivery. VA Secretary David J. Shulkin, MD, has called for new legislation clarifying that VA HCPs do have authority to care for patients located in a different state. However, the new rule cites previously granted legislative authority. “In an effort to furnish care to all beneficiaries and use its resources most efficiently,” the rule states, “VA needs to operate its telehealth program with health care providers who will provide services via telehealth to beneficiaries in States in which they are not licensed.”
To date, the rule has received relatively few comments, and none dispute the VA interpretation of its legal authority to change telehealth rules. A few commenters have raised concerns over the quality use of telehealth care, especially for mental health care. Most comments, however, supported the expansion of telehealth to increase access to care for veterans.
House Resolution 2123, the VETS Act of 2017, also contains similar provisions that would streamline interstate telehealth. However, that bill has yet to receive a vote in the House Veterans Affairs committee.
Not willing to wait for a pending legislative fix, the VA has proposed a new rule that would allow expanding telehealth offerings across state lines. The Authority of Health Care Providers to Practice Telehealth rule was proposed on October 2, 2017 and comments will remain open through November 1, 2017.
“VA has developed a telehealth program as a modern, beneficiary- and family-centered health care delivery model that leverages information and telecommunication technologies to connect beneficiaries with health care providers, irrespective of the State or location within a State where the health care provider or the beneficiary is physically located at the time the health care is provided,” the rule states. “Telehealth increases the accessibility of VA health care, bringing VA medical services to locations convenient for beneficiaries, including clinics in remote communities and beneficiaries' homes. By providing health care services by telehealth from one State to a beneficiary located in another State or within the same State, whether that beneficiary is located at a VA medical facility or in his or her own home, VA can use its limited health care resources most efficiently.”
The VA has long recognized the importance of telehealth for delivering health care to rural veterans who live far from VAMCs and community-based outpatient clinics. Providing specialty care can be especially challenging, given the dearth of specialists in many rural locations. In addition, many veterans live in a different state than the closest VAMC, creating health care delivery challenges.
The legal standing of telehealth is unclear. Currently, VA requires that health care providers (HCPs) are licensed in the same state as the patient location at time of health care delivery. VA Secretary David J. Shulkin, MD, has called for new legislation clarifying that VA HCPs do have authority to care for patients located in a different state. However, the new rule cites previously granted legislative authority. “In an effort to furnish care to all beneficiaries and use its resources most efficiently,” the rule states, “VA needs to operate its telehealth program with health care providers who will provide services via telehealth to beneficiaries in States in which they are not licensed.”
To date, the rule has received relatively few comments, and none dispute the VA interpretation of its legal authority to change telehealth rules. A few commenters have raised concerns over the quality use of telehealth care, especially for mental health care. Most comments, however, supported the expansion of telehealth to increase access to care for veterans.
House Resolution 2123, the VETS Act of 2017, also contains similar provisions that would streamline interstate telehealth. However, that bill has yet to receive a vote in the House Veterans Affairs committee.
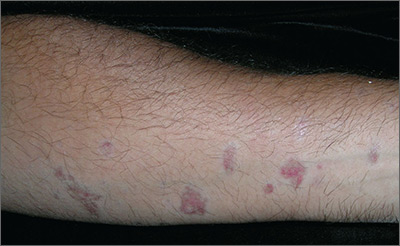
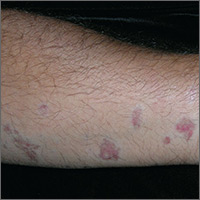
Red and brown lesions
Biopsy results revealed that this was a case of atrophic lichen planus, one of the rarer types of lichen planus that is defined by nonpalpable lesions. The FP examined areas of the patient where lichen planus is commonly found (including the wrist, ankles, and back) and discovered a few similar lesions around the patient’s ankles.
The FP explained the diagnosis to the patient and offered a mid-potency topical corticosteroid—0.1% triamcinolone cream. She advised the patient that if the lesions became more atrophic, they could try to get a topical vitamin D medication approved as an alternative. There is evidence that topical vitamin D may be beneficial, with no risk of skin atrophy. However, these are high-cost medications—despite the fact that some are now generic. A prior authorization for one of these vitamin D medications is more likely to be approved if there has been a failure, or adverse effects, from a first-line topical steroid.
Other treatment options for atrophic lichen planus include oral metronidazole and oral prednisone. The mechanism for the effectiveness of oral metronidazole is unknown, but it is a relatively safe option if topical treatments fail. Oral prednisone taken for 20 to 30 days is more likely to be effective, but is also more likely to cause adverse effects.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Kraft RL, Usatine R. Lichen planus. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 901-909.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
Biopsy results revealed that this was a case of atrophic lichen planus, one of the rarer types of lichen planus that is defined by nonpalpable lesions. The FP examined areas of the patient where lichen planus is commonly found (including the wrist, ankles, and back) and discovered a few similar lesions around the patient’s ankles.
The FP explained the diagnosis to the patient and offered a mid-potency topical corticosteroid—0.1% triamcinolone cream. She advised the patient that if the lesions became more atrophic, they could try to get a topical vitamin D medication approved as an alternative. There is evidence that topical vitamin D may be beneficial, with no risk of skin atrophy. However, these are high-cost medications—despite the fact that some are now generic. A prior authorization for one of these vitamin D medications is more likely to be approved if there has been a failure, or adverse effects, from a first-line topical steroid.
Other treatment options for atrophic lichen planus include oral metronidazole and oral prednisone. The mechanism for the effectiveness of oral metronidazole is unknown, but it is a relatively safe option if topical treatments fail. Oral prednisone taken for 20 to 30 days is more likely to be effective, but is also more likely to cause adverse effects.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Kraft RL, Usatine R. Lichen planus. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 901-909.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
Biopsy results revealed that this was a case of atrophic lichen planus, one of the rarer types of lichen planus that is defined by nonpalpable lesions. The FP examined areas of the patient where lichen planus is commonly found (including the wrist, ankles, and back) and discovered a few similar lesions around the patient’s ankles.
The FP explained the diagnosis to the patient and offered a mid-potency topical corticosteroid—0.1% triamcinolone cream. She advised the patient that if the lesions became more atrophic, they could try to get a topical vitamin D medication approved as an alternative. There is evidence that topical vitamin D may be beneficial, with no risk of skin atrophy. However, these are high-cost medications—despite the fact that some are now generic. A prior authorization for one of these vitamin D medications is more likely to be approved if there has been a failure, or adverse effects, from a first-line topical steroid.
Other treatment options for atrophic lichen planus include oral metronidazole and oral prednisone. The mechanism for the effectiveness of oral metronidazole is unknown, but it is a relatively safe option if topical treatments fail. Oral prednisone taken for 20 to 30 days is more likely to be effective, but is also more likely to cause adverse effects.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Kraft RL, Usatine R. Lichen planus. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 901-909.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
Can’t Quite Put My Finger On It …
ANSWER
The correct answer is perform a shave biopsy (choice “c”). It is a bedrock principle in dermatology that there is no substitute for a correct diagnosis, because correct diagnosis dictates proper treatment. When practical, biopsy is an excellent way to establish the true nature of a lesion and rule out other possibilities; it cuts through all conjecture.
DISCUSSION
The report showed intraepidermal squamous cell carcinoma, also known as Bowen disease. In this case, overexposure to the sun was the probable cause; however, Bowen disease can also develop from non–UV-related triggers, including human papillomavirus, arsenic (usually in contaminated ground water), and radiation treatment.
The differential includes psoriasis (which waxes and wanes), fungal infection (unlikely to last 10 years with so little growth), and superficial basal cell carcinoma.
Treatment success can be achieved by electrodessication and curettage or by the application of 5-fluorouracil or imiquimod cream for a month or two. Rarely, Bowen lesions can become invasive and metastasize if left untreated.
This patient’s prognosis, however, is excellent—at least, as far as this lesion is concerned. His history of sun exposure and numerous skin cancers
ANSWER
The correct answer is perform a shave biopsy (choice “c”). It is a bedrock principle in dermatology that there is no substitute for a correct diagnosis, because correct diagnosis dictates proper treatment. When practical, biopsy is an excellent way to establish the true nature of a lesion and rule out other possibilities; it cuts through all conjecture.
DISCUSSION
The report showed intraepidermal squamous cell carcinoma, also known as Bowen disease. In this case, overexposure to the sun was the probable cause; however, Bowen disease can also develop from non–UV-related triggers, including human papillomavirus, arsenic (usually in contaminated ground water), and radiation treatment.
The differential includes psoriasis (which waxes and wanes), fungal infection (unlikely to last 10 years with so little growth), and superficial basal cell carcinoma.
Treatment success can be achieved by electrodessication and curettage or by the application of 5-fluorouracil or imiquimod cream for a month or two. Rarely, Bowen lesions can become invasive and metastasize if left untreated.
This patient’s prognosis, however, is excellent—at least, as far as this lesion is concerned. His history of sun exposure and numerous skin cancers
ANSWER
The correct answer is perform a shave biopsy (choice “c”). It is a bedrock principle in dermatology that there is no substitute for a correct diagnosis, because correct diagnosis dictates proper treatment. When practical, biopsy is an excellent way to establish the true nature of a lesion and rule out other possibilities; it cuts through all conjecture.
DISCUSSION
The report showed intraepidermal squamous cell carcinoma, also known as Bowen disease. In this case, overexposure to the sun was the probable cause; however, Bowen disease can also develop from non–UV-related triggers, including human papillomavirus, arsenic (usually in contaminated ground water), and radiation treatment.
The differential includes psoriasis (which waxes and wanes), fungal infection (unlikely to last 10 years with so little growth), and superficial basal cell carcinoma.
Treatment success can be achieved by electrodessication and curettage or by the application of 5-fluorouracil or imiquimod cream for a month or two. Rarely, Bowen lesions can become invasive and metastasize if left untreated.
This patient’s prognosis, however, is excellent—at least, as far as this lesion is concerned. His history of sun exposure and numerous skin cancers
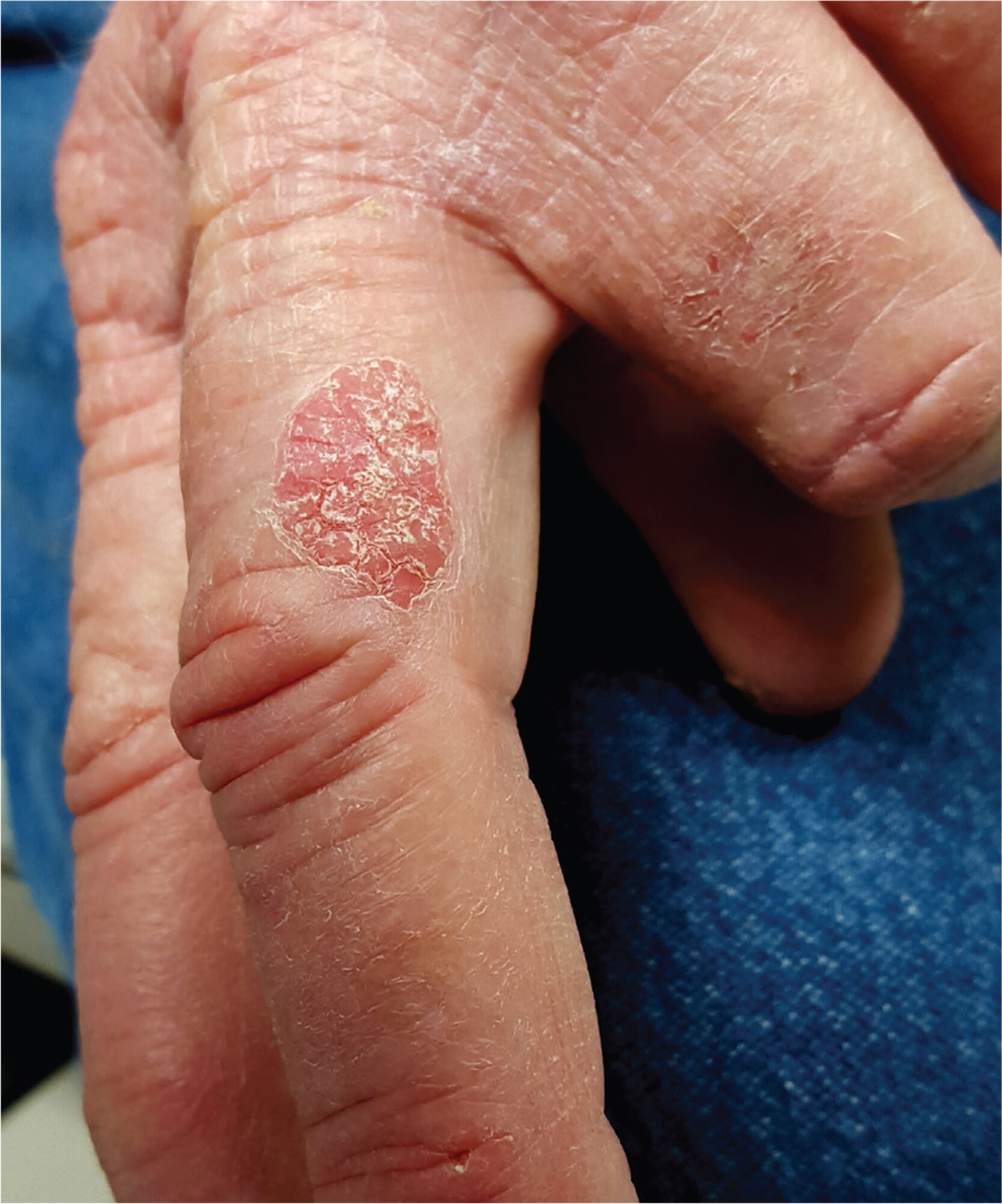
For at least 10 years, a now 70-year-old man has had a lesion on his left third finger. It is asymptomatic but gradually growing larger. Various primary care providers have offered diagnoses, the most recent being “fungal infection,” but treatments including nystatin cream have had no good effect.
The oval, pink, scaly lesion is located on the medial aspect of the proximal phalanx of the patient’s left hand; it measures 2.3 cm with well-defined borders. It is barely palpable and is not at all tender. No nodes can be felt in the arm or axilla.
The patient is otherwise healthy and is not immunosuppressed. His skin elsewhere shows evidence of sun damage—including actinic keratosis, solar lentigines, and telangiectasias—and removal of several basal cell carcinomas from his face and arms. His elbows, knees, scalp, and nails are free of any notable skin changes.
Stroke Deaths: Reversing a Healthy Trend?
After decades of decline, progress has slowed in preventing stroke deaths, according to a CDC Vital Signs report. The report is a “wake-up call,” CDC Director Brenda Fitzgerald says.
About 3 in every 4 states showed stalled rates of decline between 2000 and 2015. In some states, the trend of declining stroke deaths has actually reversed. It is a “disturbing” finding, the researchers say—particularly because 80% of strokes are preventable.
Every 40 seconds, someone in the U.S. has a stroke. Each year, > 140,000 die. Blacks continue to be hardest hit by stroke but stroke deaths are on the rise among Hispanics (by 6% each year between 2013 and 2015) and people living in the South.
Death rates continued to drop steadily between the years 2000 and 2015 among adults aged ≥ 35 years. However, people are dying of stroke at younger ages. Over the past 15 years, stroke hospitalizations have increased among adults aged 18 to 54 years. But the researchers note that risk factors, such as high blood pressure, high cholesterol, obesity, and diabetes are also appearing in younger people. Moreover, those risk factors may not be recognized and treated in middle-aged adults aged 35 to 64 years.
The study categorizes stroke deaths in the U.S. from 2000 to 2015, by age, sex, race/ethnicity, and geographic area. It does not, however, address causes for the slowdown, although it cites other studies that point to obesity and diabetes as contributors. High blood pressure is the single “most important preventable and treatable risk factor for stroke,” the CDC says.
After decades of decline, progress has slowed in preventing stroke deaths, according to a CDC Vital Signs report. The report is a “wake-up call,” CDC Director Brenda Fitzgerald says.
About 3 in every 4 states showed stalled rates of decline between 2000 and 2015. In some states, the trend of declining stroke deaths has actually reversed. It is a “disturbing” finding, the researchers say—particularly because 80% of strokes are preventable.
Every 40 seconds, someone in the U.S. has a stroke. Each year, > 140,000 die. Blacks continue to be hardest hit by stroke but stroke deaths are on the rise among Hispanics (by 6% each year between 2013 and 2015) and people living in the South.
Death rates continued to drop steadily between the years 2000 and 2015 among adults aged ≥ 35 years. However, people are dying of stroke at younger ages. Over the past 15 years, stroke hospitalizations have increased among adults aged 18 to 54 years. But the researchers note that risk factors, such as high blood pressure, high cholesterol, obesity, and diabetes are also appearing in younger people. Moreover, those risk factors may not be recognized and treated in middle-aged adults aged 35 to 64 years.
The study categorizes stroke deaths in the U.S. from 2000 to 2015, by age, sex, race/ethnicity, and geographic area. It does not, however, address causes for the slowdown, although it cites other studies that point to obesity and diabetes as contributors. High blood pressure is the single “most important preventable and treatable risk factor for stroke,” the CDC says.
After decades of decline, progress has slowed in preventing stroke deaths, according to a CDC Vital Signs report. The report is a “wake-up call,” CDC Director Brenda Fitzgerald says.
About 3 in every 4 states showed stalled rates of decline between 2000 and 2015. In some states, the trend of declining stroke deaths has actually reversed. It is a “disturbing” finding, the researchers say—particularly because 80% of strokes are preventable.
Every 40 seconds, someone in the U.S. has a stroke. Each year, > 140,000 die. Blacks continue to be hardest hit by stroke but stroke deaths are on the rise among Hispanics (by 6% each year between 2013 and 2015) and people living in the South.
Death rates continued to drop steadily between the years 2000 and 2015 among adults aged ≥ 35 years. However, people are dying of stroke at younger ages. Over the past 15 years, stroke hospitalizations have increased among adults aged 18 to 54 years. But the researchers note that risk factors, such as high blood pressure, high cholesterol, obesity, and diabetes are also appearing in younger people. Moreover, those risk factors may not be recognized and treated in middle-aged adults aged 35 to 64 years.
The study categorizes stroke deaths in the U.S. from 2000 to 2015, by age, sex, race/ethnicity, and geographic area. It does not, however, address causes for the slowdown, although it cites other studies that point to obesity and diabetes as contributors. High blood pressure is the single “most important preventable and treatable risk factor for stroke,” the CDC says.
Predicting neurotoxicity after CAR T-cell therapy
Researchers say they have identified potential biomarkers that may be used to help identify patients at an increased risk of neurotoxicity after chimeric antigen receptor (CAR) T-cell therapy.
The team also created an algorithm intended to identify patients whose symptoms were most likely to be life-threatening.
The researchers discovered the biomarkers and developed the algorithm based on data from a trial of JCAR014, an anti-CD19 CAR T-cell therapy, in patients with B-cell malignancies.
Cameron J. Turtle, MBBS, PhD, of Fred Hutchinson Cancer Research Center in Seattle, Washington, and his colleagues described this research in Cancer Discovery.
“It’s essential that we understand the potential side effects of CAR T therapies” Dr Turtle said. “While use of these cell therapies is likely to dramatically increase because they’ve been so effective in patients with resistant or refractory B-cell malignancies, there is still much to learn.”
Dr Turtle and his colleagues sought to provide a detailed clinical, radiological, and pathological characterization of neurotoxicity arising from anti-CD19 CAR T-cell therapy.
So the team analyzed data from a phase 1/2 trial of 133 adults with relapsed and/or refractory CD19+ B-cell acute lymphoblastic leukemia, non-Hodgkin lymphoma, or chronic lymphocytic leukemia.
The patients received lymphodepleting chemotherapy followed by an infusion of JCAR014.
Neurotoxicity
Within 28 days of treatment, 53 patients (40%) developed grade 1 or higher neurologic adverse events (AEs), 28 patients (21%) had grade 3 or higher neurotoxicity, and 4 patients (3%) developed fatal neurotoxicity.
Of the 53 patients with any neurologic AE, 48 (91%) also had cytokine release syndrome (CRS). All neurologic AEs in the 5 patients who did not have CRS were mild (grade 1) and transient.
Neurologic AEs included delirium with preserved alertness (66%), headache (55%), language disturbance (34%), decreased level of consciousness (25%), seizures (8%), and macroscopic intracranial hemorrhage (2%).
For most patients, neurotoxicity resolved by day 28 after CAR T-cell infusion. The exceptions were 1 patient in whom a grade 1 neurologic AE resolved 2 months after CAR T-cell infusion and the 4 patients who died of neurotoxicity.
The 4 neurotoxicity-related deaths were due to:
- Acute cerebral edema (n=2)
- Multifocal brainstem hemorrhage and edema associated with disseminated intravascular coagulation (n=1)
- Cortical laminar necrosis with a persistent minimally conscious state until death (n=1).
Potential biomarkers
In a univariate analysis, neurotoxicity was significantly more frequent in patients who:
- Had CRS (P<0.0001)
- Received a high CAR T-cell dose (P<0.0001)
- Had pre-existing neurologic comorbidities at baseline (P=0.0059).
In a multivariable analysis (which did not include CRS as a variable), patients had an increased risk of neurotoxicity if they:
- Had pre-existing neurologic comorbidities (P=0.0023)
- Received cyclophosphamide and fludarabine lymphodepletion (P=0.0259)
- Received a higher CAR T-cell dose (P=0.0009)
- Had a higher burden of malignant CD19+ B cells in the bone marrow (P=0.0165).
The researchers noted that patients who developed grade 3 or higher neurotoxicity had more severe CRS (P<0.0001).
“It appears that cytokine release syndrome is probably necessary for most cases of severe neurotoxicity, but, in terms of what triggers a person with cytokine release syndrome to get neurotoxicity, that’s something we need to investigate further,” said study author Kevin Hay, MD, of Fred Hutchinson Cancer Research Center.
Dr Hay and his colleagues also found that patients with severe neurotoxicity exhibited evidence of endothelial activation, which could contribute to manifestations such as capillary leak, disseminated intravascular coagulation, and disruption of the blood-brain barrier.
Algorithm
The researchers developed a predictive classification tree algorithm to identify patients who have an increased risk of severe neurotoxicity.
The algorithm suggests patients who meet the following criteria in the first 36 hours after CAR T-cell infusion have a high risk of grade 4-5 neurotoxicity:
- Fever of 38.9°C or greater
- Serum levels of IL6 at 16 pg/mL or higher
- Serum levels of MCP1 at 1343.5 pg/mL or higher.
This algorithm predicted severe neurotoxicity with 100% sensitivity and 94% specificity. Eight patients were misclassified, 1 of whom did not subsequently develop grade 2-3 neurotoxicity and/or grade 2 or higher CRS.
Funding
This research was funded by Juno Therapeutics Inc. (the company developing JCAR014), the National Cancer Institute, Life Science Discovery Fund, the Bezos family, the University of British Columbia Clinical Investigator Program, and via institutional funds from Bloodworks Northwest.
Dr Turtle receives research funding from Juno Therapeutics, holds patents licensed by Juno, and has pending patent applications that could be licensed by nonprofit institutions and for-profit companies, including Juno.
The Fred Hutchinson Cancer Research Center has a financial interest in Juno and receives licensing and other payments from the company. ![]()
Researchers say they have identified potential biomarkers that may be used to help identify patients at an increased risk of neurotoxicity after chimeric antigen receptor (CAR) T-cell therapy.
The team also created an algorithm intended to identify patients whose symptoms were most likely to be life-threatening.
The researchers discovered the biomarkers and developed the algorithm based on data from a trial of JCAR014, an anti-CD19 CAR T-cell therapy, in patients with B-cell malignancies.
Cameron J. Turtle, MBBS, PhD, of Fred Hutchinson Cancer Research Center in Seattle, Washington, and his colleagues described this research in Cancer Discovery.
“It’s essential that we understand the potential side effects of CAR T therapies” Dr Turtle said. “While use of these cell therapies is likely to dramatically increase because they’ve been so effective in patients with resistant or refractory B-cell malignancies, there is still much to learn.”
Dr Turtle and his colleagues sought to provide a detailed clinical, radiological, and pathological characterization of neurotoxicity arising from anti-CD19 CAR T-cell therapy.
So the team analyzed data from a phase 1/2 trial of 133 adults with relapsed and/or refractory CD19+ B-cell acute lymphoblastic leukemia, non-Hodgkin lymphoma, or chronic lymphocytic leukemia.
The patients received lymphodepleting chemotherapy followed by an infusion of JCAR014.
Neurotoxicity
Within 28 days of treatment, 53 patients (40%) developed grade 1 or higher neurologic adverse events (AEs), 28 patients (21%) had grade 3 or higher neurotoxicity, and 4 patients (3%) developed fatal neurotoxicity.
Of the 53 patients with any neurologic AE, 48 (91%) also had cytokine release syndrome (CRS). All neurologic AEs in the 5 patients who did not have CRS were mild (grade 1) and transient.
Neurologic AEs included delirium with preserved alertness (66%), headache (55%), language disturbance (34%), decreased level of consciousness (25%), seizures (8%), and macroscopic intracranial hemorrhage (2%).
For most patients, neurotoxicity resolved by day 28 after CAR T-cell infusion. The exceptions were 1 patient in whom a grade 1 neurologic AE resolved 2 months after CAR T-cell infusion and the 4 patients who died of neurotoxicity.
The 4 neurotoxicity-related deaths were due to:
- Acute cerebral edema (n=2)
- Multifocal brainstem hemorrhage and edema associated with disseminated intravascular coagulation (n=1)
- Cortical laminar necrosis with a persistent minimally conscious state until death (n=1).
Potential biomarkers
In a univariate analysis, neurotoxicity was significantly more frequent in patients who:
- Had CRS (P<0.0001)
- Received a high CAR T-cell dose (P<0.0001)
- Had pre-existing neurologic comorbidities at baseline (P=0.0059).
In a multivariable analysis (which did not include CRS as a variable), patients had an increased risk of neurotoxicity if they:
- Had pre-existing neurologic comorbidities (P=0.0023)
- Received cyclophosphamide and fludarabine lymphodepletion (P=0.0259)
- Received a higher CAR T-cell dose (P=0.0009)
- Had a higher burden of malignant CD19+ B cells in the bone marrow (P=0.0165).
The researchers noted that patients who developed grade 3 or higher neurotoxicity had more severe CRS (P<0.0001).
“It appears that cytokine release syndrome is probably necessary for most cases of severe neurotoxicity, but, in terms of what triggers a person with cytokine release syndrome to get neurotoxicity, that’s something we need to investigate further,” said study author Kevin Hay, MD, of Fred Hutchinson Cancer Research Center.
Dr Hay and his colleagues also found that patients with severe neurotoxicity exhibited evidence of endothelial activation, which could contribute to manifestations such as capillary leak, disseminated intravascular coagulation, and disruption of the blood-brain barrier.
Algorithm
The researchers developed a predictive classification tree algorithm to identify patients who have an increased risk of severe neurotoxicity.
The algorithm suggests patients who meet the following criteria in the first 36 hours after CAR T-cell infusion have a high risk of grade 4-5 neurotoxicity:
- Fever of 38.9°C or greater
- Serum levels of IL6 at 16 pg/mL or higher
- Serum levels of MCP1 at 1343.5 pg/mL or higher.
This algorithm predicted severe neurotoxicity with 100% sensitivity and 94% specificity. Eight patients were misclassified, 1 of whom did not subsequently develop grade 2-3 neurotoxicity and/or grade 2 or higher CRS.
Funding
This research was funded by Juno Therapeutics Inc. (the company developing JCAR014), the National Cancer Institute, Life Science Discovery Fund, the Bezos family, the University of British Columbia Clinical Investigator Program, and via institutional funds from Bloodworks Northwest.
Dr Turtle receives research funding from Juno Therapeutics, holds patents licensed by Juno, and has pending patent applications that could be licensed by nonprofit institutions and for-profit companies, including Juno.
The Fred Hutchinson Cancer Research Center has a financial interest in Juno and receives licensing and other payments from the company. ![]()
Researchers say they have identified potential biomarkers that may be used to help identify patients at an increased risk of neurotoxicity after chimeric antigen receptor (CAR) T-cell therapy.
The team also created an algorithm intended to identify patients whose symptoms were most likely to be life-threatening.
The researchers discovered the biomarkers and developed the algorithm based on data from a trial of JCAR014, an anti-CD19 CAR T-cell therapy, in patients with B-cell malignancies.
Cameron J. Turtle, MBBS, PhD, of Fred Hutchinson Cancer Research Center in Seattle, Washington, and his colleagues described this research in Cancer Discovery.
“It’s essential that we understand the potential side effects of CAR T therapies” Dr Turtle said. “While use of these cell therapies is likely to dramatically increase because they’ve been so effective in patients with resistant or refractory B-cell malignancies, there is still much to learn.”
Dr Turtle and his colleagues sought to provide a detailed clinical, radiological, and pathological characterization of neurotoxicity arising from anti-CD19 CAR T-cell therapy.
So the team analyzed data from a phase 1/2 trial of 133 adults with relapsed and/or refractory CD19+ B-cell acute lymphoblastic leukemia, non-Hodgkin lymphoma, or chronic lymphocytic leukemia.
The patients received lymphodepleting chemotherapy followed by an infusion of JCAR014.
Neurotoxicity
Within 28 days of treatment, 53 patients (40%) developed grade 1 or higher neurologic adverse events (AEs), 28 patients (21%) had grade 3 or higher neurotoxicity, and 4 patients (3%) developed fatal neurotoxicity.
Of the 53 patients with any neurologic AE, 48 (91%) also had cytokine release syndrome (CRS). All neurologic AEs in the 5 patients who did not have CRS were mild (grade 1) and transient.
Neurologic AEs included delirium with preserved alertness (66%), headache (55%), language disturbance (34%), decreased level of consciousness (25%), seizures (8%), and macroscopic intracranial hemorrhage (2%).
For most patients, neurotoxicity resolved by day 28 after CAR T-cell infusion. The exceptions were 1 patient in whom a grade 1 neurologic AE resolved 2 months after CAR T-cell infusion and the 4 patients who died of neurotoxicity.
The 4 neurotoxicity-related deaths were due to:
- Acute cerebral edema (n=2)
- Multifocal brainstem hemorrhage and edema associated with disseminated intravascular coagulation (n=1)
- Cortical laminar necrosis with a persistent minimally conscious state until death (n=1).
Potential biomarkers
In a univariate analysis, neurotoxicity was significantly more frequent in patients who:
- Had CRS (P<0.0001)
- Received a high CAR T-cell dose (P<0.0001)
- Had pre-existing neurologic comorbidities at baseline (P=0.0059).
In a multivariable analysis (which did not include CRS as a variable), patients had an increased risk of neurotoxicity if they:
- Had pre-existing neurologic comorbidities (P=0.0023)
- Received cyclophosphamide and fludarabine lymphodepletion (P=0.0259)
- Received a higher CAR T-cell dose (P=0.0009)
- Had a higher burden of malignant CD19+ B cells in the bone marrow (P=0.0165).
The researchers noted that patients who developed grade 3 or higher neurotoxicity had more severe CRS (P<0.0001).
“It appears that cytokine release syndrome is probably necessary for most cases of severe neurotoxicity, but, in terms of what triggers a person with cytokine release syndrome to get neurotoxicity, that’s something we need to investigate further,” said study author Kevin Hay, MD, of Fred Hutchinson Cancer Research Center.
Dr Hay and his colleagues also found that patients with severe neurotoxicity exhibited evidence of endothelial activation, which could contribute to manifestations such as capillary leak, disseminated intravascular coagulation, and disruption of the blood-brain barrier.
Algorithm
The researchers developed a predictive classification tree algorithm to identify patients who have an increased risk of severe neurotoxicity.
The algorithm suggests patients who meet the following criteria in the first 36 hours after CAR T-cell infusion have a high risk of grade 4-5 neurotoxicity:
- Fever of 38.9°C or greater
- Serum levels of IL6 at 16 pg/mL or higher
- Serum levels of MCP1 at 1343.5 pg/mL or higher.
This algorithm predicted severe neurotoxicity with 100% sensitivity and 94% specificity. Eight patients were misclassified, 1 of whom did not subsequently develop grade 2-3 neurotoxicity and/or grade 2 or higher CRS.
Funding
This research was funded by Juno Therapeutics Inc. (the company developing JCAR014), the National Cancer Institute, Life Science Discovery Fund, the Bezos family, the University of British Columbia Clinical Investigator Program, and via institutional funds from Bloodworks Northwest.
Dr Turtle receives research funding from Juno Therapeutics, holds patents licensed by Juno, and has pending patent applications that could be licensed by nonprofit institutions and for-profit companies, including Juno.
The Fred Hutchinson Cancer Research Center has a financial interest in Juno and receives licensing and other payments from the company. ![]()
FDA grants drug fast track designation for rel/ref AML
The US Food and Drug Administration (FDA) has granted fast track designation to gilteritinib for the treatment of adults with FLT3 mutation-positive relapsed or refractory acute myeloid leukemia (AML).
Gilteritinib is a compound that has demonstrated inhibitory activity against FLT3 internal tandem duplication (ITD) and FLT3 tyrosine kinase domain, 2 mutations that are seen in approximately one-third of patients with AML.
Gilteritinib has also demonstrated inhibition of AXL, which is reported to be associated with therapeutic resistance.
Astellas Pharma Inc. is currently investigating gilteritinib in phase 3 trials of AML patients.
Results from a phase 1/2 study of gilteritinib in AML were presented at the 2017 ASCO Annual Meeting.
The goal of the study was to determine the tolerability and antileukemic activity of once-daily gilteritinib in a FLT3-ITD-enriched, relapsed/refractory AML population.
Researchers said the drug exhibited potent FLT3 inhibition at doses greater than 80 mg/day. In patients who received such doses, the greatest overall response rate was 52%, and the longest median overall survival was 31 weeks.
The maximum tolerated dose of gilteritinib was 300 mg/day. Dose-limiting toxicities included diarrhea and liver function abnormalities.
About fast track designation
The FDA’s fast track program is designed to facilitate the development and expedite the review of products intended to treat or prevent serious or life-threatening conditions and address unmet medical need.
Through the fast track program, a product may be eligible for priority review. In addition, the company developing the product may be allowed to submit sections of the new drug application or biologics license application on a rolling basis as data become available.
Fast track designation also provides the company with opportunities for more frequent meetings and written communications with the FDA.
About orphan designation
Gilteritinib also has orphan drug designation for the treatment of AML.
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()
The US Food and Drug Administration (FDA) has granted fast track designation to gilteritinib for the treatment of adults with FLT3 mutation-positive relapsed or refractory acute myeloid leukemia (AML).
Gilteritinib is a compound that has demonstrated inhibitory activity against FLT3 internal tandem duplication (ITD) and FLT3 tyrosine kinase domain, 2 mutations that are seen in approximately one-third of patients with AML.
Gilteritinib has also demonstrated inhibition of AXL, which is reported to be associated with therapeutic resistance.
Astellas Pharma Inc. is currently investigating gilteritinib in phase 3 trials of AML patients.
Results from a phase 1/2 study of gilteritinib in AML were presented at the 2017 ASCO Annual Meeting.
The goal of the study was to determine the tolerability and antileukemic activity of once-daily gilteritinib in a FLT3-ITD-enriched, relapsed/refractory AML population.
Researchers said the drug exhibited potent FLT3 inhibition at doses greater than 80 mg/day. In patients who received such doses, the greatest overall response rate was 52%, and the longest median overall survival was 31 weeks.
The maximum tolerated dose of gilteritinib was 300 mg/day. Dose-limiting toxicities included diarrhea and liver function abnormalities.
About fast track designation
The FDA’s fast track program is designed to facilitate the development and expedite the review of products intended to treat or prevent serious or life-threatening conditions and address unmet medical need.
Through the fast track program, a product may be eligible for priority review. In addition, the company developing the product may be allowed to submit sections of the new drug application or biologics license application on a rolling basis as data become available.
Fast track designation also provides the company with opportunities for more frequent meetings and written communications with the FDA.
About orphan designation
Gilteritinib also has orphan drug designation for the treatment of AML.
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()
The US Food and Drug Administration (FDA) has granted fast track designation to gilteritinib for the treatment of adults with FLT3 mutation-positive relapsed or refractory acute myeloid leukemia (AML).
Gilteritinib is a compound that has demonstrated inhibitory activity against FLT3 internal tandem duplication (ITD) and FLT3 tyrosine kinase domain, 2 mutations that are seen in approximately one-third of patients with AML.
Gilteritinib has also demonstrated inhibition of AXL, which is reported to be associated with therapeutic resistance.
Astellas Pharma Inc. is currently investigating gilteritinib in phase 3 trials of AML patients.
Results from a phase 1/2 study of gilteritinib in AML were presented at the 2017 ASCO Annual Meeting.
The goal of the study was to determine the tolerability and antileukemic activity of once-daily gilteritinib in a FLT3-ITD-enriched, relapsed/refractory AML population.
Researchers said the drug exhibited potent FLT3 inhibition at doses greater than 80 mg/day. In patients who received such doses, the greatest overall response rate was 52%, and the longest median overall survival was 31 weeks.
The maximum tolerated dose of gilteritinib was 300 mg/day. Dose-limiting toxicities included diarrhea and liver function abnormalities.
About fast track designation
The FDA’s fast track program is designed to facilitate the development and expedite the review of products intended to treat or prevent serious or life-threatening conditions and address unmet medical need.
Through the fast track program, a product may be eligible for priority review. In addition, the company developing the product may be allowed to submit sections of the new drug application or biologics license application on a rolling basis as data become available.
Fast track designation also provides the company with opportunities for more frequent meetings and written communications with the FDA.
About orphan designation
Gilteritinib also has orphan drug designation for the treatment of AML.
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()
Antithrombotic agents linked to hematuria-related complications
New research suggests antithrombotic agents increase the risk of hematuria-related complications in older adults.
The study included more than 2.5 million Canadians over the age of 65.
The subjects had an increased risk of emergency department visits, hospitalizations, and urologic procedures to manage visible hematuria if they had received anticoagulants and/or antiplatelet agents.
Robert K. Nam, MD, of Sunnybrook Health Sciences Centre in Toronto, Ontario, Canada, and his colleagues reported these findings in JAMA.
The researchers examined rates of hematuria-related complications in 2,518,064 citizens of Ontario who were 66 and older between 2002 and 2014.
In all, 808,897 patients received at least 1 prescription for an antithrombotic agent over the study period. This included apixaban, dabigatran, rivaroxaban, warfarin, aspirin, and “other” antiplatelet agents.
At a median follow-up of 7.3 years, the incidence density rates (per 1000 person-years) of hematuria-related complications were 123.95 events among patients who were exposed to antithrombotic agents and 80.17 events among patients who were not (difference=43.8; 95% CI, 43.0-44.6; P<0.001; incidence rate ratio [IRR]=1.44; 95% CI, 1.42-1.46).
The incidence density rates of emergency department visits were 7.05 and 2.51, respectively (difference=4.5; 95% CI, 4.3-4.7; P<0.001; IRR=2.80; 95% CI, 2.74-2.86).
The incidence density rates of hospitalizations were 11.12 and 5.42, respectively (difference=5.7; 95% CI, 5.5-5.9; P<0.001; IRR=2.03; 95% CI, 2.00-2.06).
And the incidence density rates of urologic procedures were 105.78 and 72.24, respectively (difference=33.5; 95% CI, 32.8-34.3; P<0.001; IRR=1.37; 95% CI, 1.36-1.39).
The association between antithrombotic agents and hematuria-related complications was present for all the antithrombotic agents examined.
The researchers noted that this study had limitations. In particular, the cohort was restricted to patients age 66 and older because of funding eligibility for prescription medications in Ontario. Given the interaction between age and the association of antithrombotic therapies with hematuria-related complications, these results are not directly applicable to younger patients. ![]()
New research suggests antithrombotic agents increase the risk of hematuria-related complications in older adults.
The study included more than 2.5 million Canadians over the age of 65.
The subjects had an increased risk of emergency department visits, hospitalizations, and urologic procedures to manage visible hematuria if they had received anticoagulants and/or antiplatelet agents.
Robert K. Nam, MD, of Sunnybrook Health Sciences Centre in Toronto, Ontario, Canada, and his colleagues reported these findings in JAMA.
The researchers examined rates of hematuria-related complications in 2,518,064 citizens of Ontario who were 66 and older between 2002 and 2014.
In all, 808,897 patients received at least 1 prescription for an antithrombotic agent over the study period. This included apixaban, dabigatran, rivaroxaban, warfarin, aspirin, and “other” antiplatelet agents.
At a median follow-up of 7.3 years, the incidence density rates (per 1000 person-years) of hematuria-related complications were 123.95 events among patients who were exposed to antithrombotic agents and 80.17 events among patients who were not (difference=43.8; 95% CI, 43.0-44.6; P<0.001; incidence rate ratio [IRR]=1.44; 95% CI, 1.42-1.46).
The incidence density rates of emergency department visits were 7.05 and 2.51, respectively (difference=4.5; 95% CI, 4.3-4.7; P<0.001; IRR=2.80; 95% CI, 2.74-2.86).
The incidence density rates of hospitalizations were 11.12 and 5.42, respectively (difference=5.7; 95% CI, 5.5-5.9; P<0.001; IRR=2.03; 95% CI, 2.00-2.06).
And the incidence density rates of urologic procedures were 105.78 and 72.24, respectively (difference=33.5; 95% CI, 32.8-34.3; P<0.001; IRR=1.37; 95% CI, 1.36-1.39).
The association between antithrombotic agents and hematuria-related complications was present for all the antithrombotic agents examined.
The researchers noted that this study had limitations. In particular, the cohort was restricted to patients age 66 and older because of funding eligibility for prescription medications in Ontario. Given the interaction between age and the association of antithrombotic therapies with hematuria-related complications, these results are not directly applicable to younger patients. ![]()
New research suggests antithrombotic agents increase the risk of hematuria-related complications in older adults.
The study included more than 2.5 million Canadians over the age of 65.
The subjects had an increased risk of emergency department visits, hospitalizations, and urologic procedures to manage visible hematuria if they had received anticoagulants and/or antiplatelet agents.
Robert K. Nam, MD, of Sunnybrook Health Sciences Centre in Toronto, Ontario, Canada, and his colleagues reported these findings in JAMA.
The researchers examined rates of hematuria-related complications in 2,518,064 citizens of Ontario who were 66 and older between 2002 and 2014.
In all, 808,897 patients received at least 1 prescription for an antithrombotic agent over the study period. This included apixaban, dabigatran, rivaroxaban, warfarin, aspirin, and “other” antiplatelet agents.
At a median follow-up of 7.3 years, the incidence density rates (per 1000 person-years) of hematuria-related complications were 123.95 events among patients who were exposed to antithrombotic agents and 80.17 events among patients who were not (difference=43.8; 95% CI, 43.0-44.6; P<0.001; incidence rate ratio [IRR]=1.44; 95% CI, 1.42-1.46).
The incidence density rates of emergency department visits were 7.05 and 2.51, respectively (difference=4.5; 95% CI, 4.3-4.7; P<0.001; IRR=2.80; 95% CI, 2.74-2.86).
The incidence density rates of hospitalizations were 11.12 and 5.42, respectively (difference=5.7; 95% CI, 5.5-5.9; P<0.001; IRR=2.03; 95% CI, 2.00-2.06).
And the incidence density rates of urologic procedures were 105.78 and 72.24, respectively (difference=33.5; 95% CI, 32.8-34.3; P<0.001; IRR=1.37; 95% CI, 1.36-1.39).
The association between antithrombotic agents and hematuria-related complications was present for all the antithrombotic agents examined.
The researchers noted that this study had limitations. In particular, the cohort was restricted to patients age 66 and older because of funding eligibility for prescription medications in Ontario. Given the interaction between age and the association of antithrombotic therapies with hematuria-related complications, these results are not directly applicable to younger patients. ![]()
Clinical trials summary: AGILE
Study of AG-120 (Ivosidenib) vs. Placebo in Combination With Azacitidine in Patients With Previously Untreated Acute Myeloid Leukemia With an IDH1 Mutation (AGILE)
AG120-C-009 (NCT03173248) will evaluate the efficacy and safety of AG-120 (ivosidenib) plus azacitidine vs. placebo plus azacitidine in adult subjects with previously untreated IDH1m AML who are considered appropriate candidates for non-intensive therapy.
The primary endpoint of this global, phase 3, multicenter, double-blind, randomized, placebo-controlled clinical trial is overall survival. Key secondary efficacy endpoints are event-free survival, rate of complete remission, rate of complete remission with partial hematologic recovery, and overall response rate. Subjects will be randomized 1:1 to receive either oral AG-120 or matched placebo, both administered in combination with subcutaneous or intravenous azacitidine. An estimated 392 subjects will participate in the study, which is sponsored by Agios Pharmaceuticals. Estimated completion date is June 2022.
Contact: Medical Affairs Agios Pharmaceuticals, Inc.; (844) 633-2332, e-mail: medinfo@agios.com
Study of AG-120 (Ivosidenib) vs. Placebo in Combination With Azacitidine in Patients With Previously Untreated Acute Myeloid Leukemia With an IDH1 Mutation (AGILE)
AG120-C-009 (NCT03173248) will evaluate the efficacy and safety of AG-120 (ivosidenib) plus azacitidine vs. placebo plus azacitidine in adult subjects with previously untreated IDH1m AML who are considered appropriate candidates for non-intensive therapy.
The primary endpoint of this global, phase 3, multicenter, double-blind, randomized, placebo-controlled clinical trial is overall survival. Key secondary efficacy endpoints are event-free survival, rate of complete remission, rate of complete remission with partial hematologic recovery, and overall response rate. Subjects will be randomized 1:1 to receive either oral AG-120 or matched placebo, both administered in combination with subcutaneous or intravenous azacitidine. An estimated 392 subjects will participate in the study, which is sponsored by Agios Pharmaceuticals. Estimated completion date is June 2022.
Contact: Medical Affairs Agios Pharmaceuticals, Inc.; (844) 633-2332, e-mail: medinfo@agios.com
Study of AG-120 (Ivosidenib) vs. Placebo in Combination With Azacitidine in Patients With Previously Untreated Acute Myeloid Leukemia With an IDH1 Mutation (AGILE)
AG120-C-009 (NCT03173248) will evaluate the efficacy and safety of AG-120 (ivosidenib) plus azacitidine vs. placebo plus azacitidine in adult subjects with previously untreated IDH1m AML who are considered appropriate candidates for non-intensive therapy.
The primary endpoint of this global, phase 3, multicenter, double-blind, randomized, placebo-controlled clinical trial is overall survival. Key secondary efficacy endpoints are event-free survival, rate of complete remission, rate of complete remission with partial hematologic recovery, and overall response rate. Subjects will be randomized 1:1 to receive either oral AG-120 or matched placebo, both administered in combination with subcutaneous or intravenous azacitidine. An estimated 392 subjects will participate in the study, which is sponsored by Agios Pharmaceuticals. Estimated completion date is June 2022.
Contact: Medical Affairs Agios Pharmaceuticals, Inc.; (844) 633-2332, e-mail: medinfo@agios.com