User login
ORBITA: PCI no better than meds for stable angina
DENVER – The first-ever blinded, sham-controlled randomized trial of percutaneous coronary intervention for stable angina failed to show a significant improvement in exercise time for PCI, compared with placebo PCI, Rasha Al-Lamee, MD, reported at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
The blockbuster results of the ORBITA trial, published online in the Lancet simultaneously with Dr. Al-Lamee’s presentation in Denver, quickly went viral, with a story splashed across the front page of the New York Times under the headline “‘Unbelievable’: Heart Stents Fail to Ease Chest Pain.” Interventional cardiology thought leaders at TCT said the newspaper piece, and a Lancet editorial commentary entitled “Last nail in the coffin for PCI in stable angina?” that accompanied publication of ORBITA, failed to convey the study’s major limitations, drawbacks that Dr. Al-Lamee readily acknowledged.
What ORBITA did
ORBITA (Objective Randomized Blinded Investigation with Optimal Medical Therapy of Angioplasty in Stable Angina) included 200 patients referred to five U.K. cardiac catheterization labs for diagnostic angiography. Participants had to have stable angina, single-vessel disease, and at least one 70% or greater stenosis; in fact, their stenotic severity averaged 84.4% by quantitative coronary angiography.
The patients received 6 weeks of intensive medical therapy during which they were uptitrated to an average of three antianginal medications. They then underwent either real or sham PCI followed by 6 weeks of recovery, during which both the patients and care team remained blinded. Then the same assessments done before randomization were repeated, including exercise treadmill testing, the Seattle Angina Questionnaire, and dobutamine stress echocardiography, explained Dr. Al-Lamee of Imperial College London.
The primary outcome was achievement of at least a 30-second greater improvement in total exercise time following PCI, compared with sham PCI, an effect size chosen based on placebo-controlled studies of antianginal drugs. The PCI group improved by a mean of 28.4 seconds, the controls by 11.8 seconds, and the resultant 16.6-second difference made for a negative result (Lancet. 2017 Nov 2;doi: 10.1016/S0140-6736[17]32714-9).
PCI did, however, result in significant improvement in the secondary endpoint of ischemia reduction as assessed by blinded evaluation of dobutamine stress echocardiography results. The PCI group’s mean peak stress wall motion index score improved from 1.11 prerandomization to 1.03 – that is, normal – at follow-up 6 weeks post procedure while remaining unchanged in the sham PCI group, Dr. Al-Lamee noted at the meeting, sponsored by the Cardiovascular Research Foundation.
What the results mean
Dr. Al-Lamee said the ORBITA results should enable cardiologists to sit with patients similar to those in the trial and have a more informed, patient-centered discussion in which intensive medical management can be offered as an initial first-line option with an understanding that it will likely improve their symptoms to the same degree as angioplasty.
“There will be those patients who would rather avoid having to take high doses of antianginal medications with the side effects they involve, who may well prefer to have an upfront procedure with a small risk in order to reduce their pill count, and who also would rather have improved blood flow to the heart, which may have prognostic implications,” Dr. Al-Lamee said.
Carl L. Tommaso, MD, part of the panel of discussants at the late-breaking clinical trials session in which Dr. Al-Lamee presented the ORBITA findings, applauded the investigators for their ingenious study design, which included elaborate blinding techniques involving music played through headphones throughout the procedure, heavy sedation, separate angioplasty and clinical care teams, the same postprocedural instructions and discharge letter, and dual-antiplatelet therapy in both study arms.
“This is a great study. I don’t think any of us could get this study past an institutional review board in the United States,” commented Dr. Tommaso, director of the cardiac catheterization laboratory at Skokie (Ill.) Hospital.
He added, however, that he wouldn’t have performed PCI on the basis of angiographic findings alone in stable angina patients with a 9-minute treadmill exercise time.
Where OPTIMA fell short
Angiography vs. functional testing
“Twenty-nine percent of patients, we’d all agree, should not have had angioplasty because they had no ischemia,” said Dr. Stone, professor of medicine at Columbia University, New York, and director of the TCT conference.
All subjects in ORBITA did indeed undergo measurement of both FFR and instant Wave-Free Ratio (iFR) while on the table immediately before and after their real or sham PCI. The mean stenosis severity was 0.69 by FFR and 0.76 by iFR, readings indicative of significantly impaired flow. However, the operators were blinded as to those results. The rationale for withholding that information was that, even though it has been shown to be clinically useful, studies show that 80% of angioplasties are done based upon angiography alone, and the ORBITA investigators wanted the study to reflect routine clinical practice, Dr. Al-Lamee explained.
“I think one of the many lessons coming out of this trial is to see the discrepancy between the angiogram and functional testing. We cannot guide our therapy solely by the angiogram. We have to get physiologic data and consider that together with symptoms in the patient’s clinical context,” said panelist Allen Jeremias, MD, director of interventional cardiology research at St. Francis Hospital in Rosyln, N.Y.
Commentary goes too far
The “last-nail-in-the-coffin” Lancet commentary (2017 Nov 2. doi: 10.1016/S0140-6736[17]32757-5) penned by David L. Brown, MD, of Washington University in St. Louis and Rita F. Redberg, MD, of the University of California, San Francisco, emphatically declared that the ORBITA results mean all cardiology guidelines should be revised to downgrade the recommendation for PCI in patients with angina despite medical therapy. Dr. Al-Lamee was one of many at TCT 2017 who took strong exception to that.
“This is the first trial of its kind. I think it would be very easy to take the results of this trial and overextrapolate. To downgrade the guideline recommendations based on this study would be an incredibly large overreach,” she said.
Ajay J. Kirtane, MD, who chaired a press conference in which Dr. Al-Lamee presented the ORBITA results, had a further criticism of the editorial.
“Some of the risks of PCI as described in the editorial are just factually inaccurate. An MI rate of 15%, an acute kidney injury rate of 13% – those are simply factually incorrect,” said Dr. Kirtane, director of the cardiac catheterization laboratories at New York-Presbyterian/Columbia University Medical Center.
The ORBITA trial was sponsored by Imperial College London and funded by grants from the National Institute of Health Research Imperial Biomedical Research Center and charity organizations. Dr. Al-Lamee reported serving as a paid consultant to Philips Volcano, which supplied the coronary pressure wires for physiologic testing.
DENVER – The first-ever blinded, sham-controlled randomized trial of percutaneous coronary intervention for stable angina failed to show a significant improvement in exercise time for PCI, compared with placebo PCI, Rasha Al-Lamee, MD, reported at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
The blockbuster results of the ORBITA trial, published online in the Lancet simultaneously with Dr. Al-Lamee’s presentation in Denver, quickly went viral, with a story splashed across the front page of the New York Times under the headline “‘Unbelievable’: Heart Stents Fail to Ease Chest Pain.” Interventional cardiology thought leaders at TCT said the newspaper piece, and a Lancet editorial commentary entitled “Last nail in the coffin for PCI in stable angina?” that accompanied publication of ORBITA, failed to convey the study’s major limitations, drawbacks that Dr. Al-Lamee readily acknowledged.
What ORBITA did
ORBITA (Objective Randomized Blinded Investigation with Optimal Medical Therapy of Angioplasty in Stable Angina) included 200 patients referred to five U.K. cardiac catheterization labs for diagnostic angiography. Participants had to have stable angina, single-vessel disease, and at least one 70% or greater stenosis; in fact, their stenotic severity averaged 84.4% by quantitative coronary angiography.
The patients received 6 weeks of intensive medical therapy during which they were uptitrated to an average of three antianginal medications. They then underwent either real or sham PCI followed by 6 weeks of recovery, during which both the patients and care team remained blinded. Then the same assessments done before randomization were repeated, including exercise treadmill testing, the Seattle Angina Questionnaire, and dobutamine stress echocardiography, explained Dr. Al-Lamee of Imperial College London.
The primary outcome was achievement of at least a 30-second greater improvement in total exercise time following PCI, compared with sham PCI, an effect size chosen based on placebo-controlled studies of antianginal drugs. The PCI group improved by a mean of 28.4 seconds, the controls by 11.8 seconds, and the resultant 16.6-second difference made for a negative result (Lancet. 2017 Nov 2;doi: 10.1016/S0140-6736[17]32714-9).
PCI did, however, result in significant improvement in the secondary endpoint of ischemia reduction as assessed by blinded evaluation of dobutamine stress echocardiography results. The PCI group’s mean peak stress wall motion index score improved from 1.11 prerandomization to 1.03 – that is, normal – at follow-up 6 weeks post procedure while remaining unchanged in the sham PCI group, Dr. Al-Lamee noted at the meeting, sponsored by the Cardiovascular Research Foundation.
What the results mean
Dr. Al-Lamee said the ORBITA results should enable cardiologists to sit with patients similar to those in the trial and have a more informed, patient-centered discussion in which intensive medical management can be offered as an initial first-line option with an understanding that it will likely improve their symptoms to the same degree as angioplasty.
“There will be those patients who would rather avoid having to take high doses of antianginal medications with the side effects they involve, who may well prefer to have an upfront procedure with a small risk in order to reduce their pill count, and who also would rather have improved blood flow to the heart, which may have prognostic implications,” Dr. Al-Lamee said.
Carl L. Tommaso, MD, part of the panel of discussants at the late-breaking clinical trials session in which Dr. Al-Lamee presented the ORBITA findings, applauded the investigators for their ingenious study design, which included elaborate blinding techniques involving music played through headphones throughout the procedure, heavy sedation, separate angioplasty and clinical care teams, the same postprocedural instructions and discharge letter, and dual-antiplatelet therapy in both study arms.
“This is a great study. I don’t think any of us could get this study past an institutional review board in the United States,” commented Dr. Tommaso, director of the cardiac catheterization laboratory at Skokie (Ill.) Hospital.
He added, however, that he wouldn’t have performed PCI on the basis of angiographic findings alone in stable angina patients with a 9-minute treadmill exercise time.
Where OPTIMA fell short
Angiography vs. functional testing
“Twenty-nine percent of patients, we’d all agree, should not have had angioplasty because they had no ischemia,” said Dr. Stone, professor of medicine at Columbia University, New York, and director of the TCT conference.
All subjects in ORBITA did indeed undergo measurement of both FFR and instant Wave-Free Ratio (iFR) while on the table immediately before and after their real or sham PCI. The mean stenosis severity was 0.69 by FFR and 0.76 by iFR, readings indicative of significantly impaired flow. However, the operators were blinded as to those results. The rationale for withholding that information was that, even though it has been shown to be clinically useful, studies show that 80% of angioplasties are done based upon angiography alone, and the ORBITA investigators wanted the study to reflect routine clinical practice, Dr. Al-Lamee explained.
“I think one of the many lessons coming out of this trial is to see the discrepancy between the angiogram and functional testing. We cannot guide our therapy solely by the angiogram. We have to get physiologic data and consider that together with symptoms in the patient’s clinical context,” said panelist Allen Jeremias, MD, director of interventional cardiology research at St. Francis Hospital in Rosyln, N.Y.
Commentary goes too far
The “last-nail-in-the-coffin” Lancet commentary (2017 Nov 2. doi: 10.1016/S0140-6736[17]32757-5) penned by David L. Brown, MD, of Washington University in St. Louis and Rita F. Redberg, MD, of the University of California, San Francisco, emphatically declared that the ORBITA results mean all cardiology guidelines should be revised to downgrade the recommendation for PCI in patients with angina despite medical therapy. Dr. Al-Lamee was one of many at TCT 2017 who took strong exception to that.
“This is the first trial of its kind. I think it would be very easy to take the results of this trial and overextrapolate. To downgrade the guideline recommendations based on this study would be an incredibly large overreach,” she said.
Ajay J. Kirtane, MD, who chaired a press conference in which Dr. Al-Lamee presented the ORBITA results, had a further criticism of the editorial.
“Some of the risks of PCI as described in the editorial are just factually inaccurate. An MI rate of 15%, an acute kidney injury rate of 13% – those are simply factually incorrect,” said Dr. Kirtane, director of the cardiac catheterization laboratories at New York-Presbyterian/Columbia University Medical Center.
The ORBITA trial was sponsored by Imperial College London and funded by grants from the National Institute of Health Research Imperial Biomedical Research Center and charity organizations. Dr. Al-Lamee reported serving as a paid consultant to Philips Volcano, which supplied the coronary pressure wires for physiologic testing.
DENVER – The first-ever blinded, sham-controlled randomized trial of percutaneous coronary intervention for stable angina failed to show a significant improvement in exercise time for PCI, compared with placebo PCI, Rasha Al-Lamee, MD, reported at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
The blockbuster results of the ORBITA trial, published online in the Lancet simultaneously with Dr. Al-Lamee’s presentation in Denver, quickly went viral, with a story splashed across the front page of the New York Times under the headline “‘Unbelievable’: Heart Stents Fail to Ease Chest Pain.” Interventional cardiology thought leaders at TCT said the newspaper piece, and a Lancet editorial commentary entitled “Last nail in the coffin for PCI in stable angina?” that accompanied publication of ORBITA, failed to convey the study’s major limitations, drawbacks that Dr. Al-Lamee readily acknowledged.
What ORBITA did
ORBITA (Objective Randomized Blinded Investigation with Optimal Medical Therapy of Angioplasty in Stable Angina) included 200 patients referred to five U.K. cardiac catheterization labs for diagnostic angiography. Participants had to have stable angina, single-vessel disease, and at least one 70% or greater stenosis; in fact, their stenotic severity averaged 84.4% by quantitative coronary angiography.
The patients received 6 weeks of intensive medical therapy during which they were uptitrated to an average of three antianginal medications. They then underwent either real or sham PCI followed by 6 weeks of recovery, during which both the patients and care team remained blinded. Then the same assessments done before randomization were repeated, including exercise treadmill testing, the Seattle Angina Questionnaire, and dobutamine stress echocardiography, explained Dr. Al-Lamee of Imperial College London.
The primary outcome was achievement of at least a 30-second greater improvement in total exercise time following PCI, compared with sham PCI, an effect size chosen based on placebo-controlled studies of antianginal drugs. The PCI group improved by a mean of 28.4 seconds, the controls by 11.8 seconds, and the resultant 16.6-second difference made for a negative result (Lancet. 2017 Nov 2;doi: 10.1016/S0140-6736[17]32714-9).
PCI did, however, result in significant improvement in the secondary endpoint of ischemia reduction as assessed by blinded evaluation of dobutamine stress echocardiography results. The PCI group’s mean peak stress wall motion index score improved from 1.11 prerandomization to 1.03 – that is, normal – at follow-up 6 weeks post procedure while remaining unchanged in the sham PCI group, Dr. Al-Lamee noted at the meeting, sponsored by the Cardiovascular Research Foundation.
What the results mean
Dr. Al-Lamee said the ORBITA results should enable cardiologists to sit with patients similar to those in the trial and have a more informed, patient-centered discussion in which intensive medical management can be offered as an initial first-line option with an understanding that it will likely improve their symptoms to the same degree as angioplasty.
“There will be those patients who would rather avoid having to take high doses of antianginal medications with the side effects they involve, who may well prefer to have an upfront procedure with a small risk in order to reduce their pill count, and who also would rather have improved blood flow to the heart, which may have prognostic implications,” Dr. Al-Lamee said.
Carl L. Tommaso, MD, part of the panel of discussants at the late-breaking clinical trials session in which Dr. Al-Lamee presented the ORBITA findings, applauded the investigators for their ingenious study design, which included elaborate blinding techniques involving music played through headphones throughout the procedure, heavy sedation, separate angioplasty and clinical care teams, the same postprocedural instructions and discharge letter, and dual-antiplatelet therapy in both study arms.
“This is a great study. I don’t think any of us could get this study past an institutional review board in the United States,” commented Dr. Tommaso, director of the cardiac catheterization laboratory at Skokie (Ill.) Hospital.
He added, however, that he wouldn’t have performed PCI on the basis of angiographic findings alone in stable angina patients with a 9-minute treadmill exercise time.
Where OPTIMA fell short
Angiography vs. functional testing
“Twenty-nine percent of patients, we’d all agree, should not have had angioplasty because they had no ischemia,” said Dr. Stone, professor of medicine at Columbia University, New York, and director of the TCT conference.
All subjects in ORBITA did indeed undergo measurement of both FFR and instant Wave-Free Ratio (iFR) while on the table immediately before and after their real or sham PCI. The mean stenosis severity was 0.69 by FFR and 0.76 by iFR, readings indicative of significantly impaired flow. However, the operators were blinded as to those results. The rationale for withholding that information was that, even though it has been shown to be clinically useful, studies show that 80% of angioplasties are done based upon angiography alone, and the ORBITA investigators wanted the study to reflect routine clinical practice, Dr. Al-Lamee explained.
“I think one of the many lessons coming out of this trial is to see the discrepancy between the angiogram and functional testing. We cannot guide our therapy solely by the angiogram. We have to get physiologic data and consider that together with symptoms in the patient’s clinical context,” said panelist Allen Jeremias, MD, director of interventional cardiology research at St. Francis Hospital in Rosyln, N.Y.
Commentary goes too far
The “last-nail-in-the-coffin” Lancet commentary (2017 Nov 2. doi: 10.1016/S0140-6736[17]32757-5) penned by David L. Brown, MD, of Washington University in St. Louis and Rita F. Redberg, MD, of the University of California, San Francisco, emphatically declared that the ORBITA results mean all cardiology guidelines should be revised to downgrade the recommendation for PCI in patients with angina despite medical therapy. Dr. Al-Lamee was one of many at TCT 2017 who took strong exception to that.
“This is the first trial of its kind. I think it would be very easy to take the results of this trial and overextrapolate. To downgrade the guideline recommendations based on this study would be an incredibly large overreach,” she said.
Ajay J. Kirtane, MD, who chaired a press conference in which Dr. Al-Lamee presented the ORBITA results, had a further criticism of the editorial.
“Some of the risks of PCI as described in the editorial are just factually inaccurate. An MI rate of 15%, an acute kidney injury rate of 13% – those are simply factually incorrect,” said Dr. Kirtane, director of the cardiac catheterization laboratories at New York-Presbyterian/Columbia University Medical Center.
The ORBITA trial was sponsored by Imperial College London and funded by grants from the National Institute of Health Research Imperial Biomedical Research Center and charity organizations. Dr. Al-Lamee reported serving as a paid consultant to Philips Volcano, which supplied the coronary pressure wires for physiologic testing.
AT TCT 2017
Key clinical point:
Major finding: PCI on top of intensive antianginal medications was not significantly more effective at improving exercise tolerance than sham PCI.
Data source: ORBITA, a randomized, multicenter, blinded, sham-controlled study of 200 patients with mild angina and single-vessel CAD.
Disclosures: ORBITA was sponsored by Imperial College London and funded by grants from the National Institute of Health Research Imperial Biomedical Research Center and charity organizations. The presenter reported serving as a paid consultant to Philips Volcano, which supplied the coronary pressure wires for physiologic testing.
CV outcomes better with SGLT2 inhibitor than DPP4 inhibitor in T2DM study
LISBON – The sodium-glucose co-transporter 2 (SGLT-2) inhibitor dapagliflozin (Farxiga) posed a lower risk of cardiovascular events and all-cause mortality in patients with type 2 diabetes mellitus (T2DM) compared with dipeptidyl peptidase-4 inhibitors in a large real-world, observational study.
In CVD-REAL NORDIC, treatment with the SGLT-2 inhibitor reduced the incidence of major cardiovascular adverse events by 21% as compared to DDP-4 inhibitors (hazard ratio, 0.79; 95% confidence interval, 0.67-0.94; P = .006).
Anna Norhammar, MD, PhD, of the Institute of Medicine, Cardiology Unit, at Karolinska Institutet in Stockholm, presented the findings at the annual meeting of the European Association for the Study of Diabetes.
She said: “These results are in line with previous clinical trials and meta-analyses, but extend the results to a broader CV risk population and with a commonly used comparator.”
Indeed, the findings build on those from the widely reported EMPA-REG Outcome (New Engl J Med. 2015;373;2117-8) and CANVAS (New Engl J Med. 2017;377:644-7) randomized controlled trials. These trials respectively showed empagliflozin and canagliflozin significantly reduced the risk for MACE and heart failure in patients with T2DM versus placebo. As the majority of patients in these trials had established CV disease, this suggested a class effect for SGLT2 inhibitors, Dr. Norhammar explained.
CVD-REAL NORDIC is part of a larger, multinational, observational, comparative effectiveness study looking at the real-world effect of SGLT2 inhibitors versus other glucose-lowering drugs on CV outcomes in patients with T2DM. Altogether around 90,000 patients have been recruited in Sweden, Norway, and Denmark.
Previously, Dr. Norhammar and co-investigators have reported that SGLT2 inhibitors lowered MACE (HR, 0.78; 95% CI 0.69–0.87, P less than .0001) and heart failure hospitalization (HR, 0.70; 95% CI 0.61–0.81, P less than .0001) relative to all glucose-lowering drugs (Lancet Diabetes Endocrinol. 2017;709–17).
“However, the comparator group used in that study, other [glucose-lowering drugs], consisted of almost 50% patients with T2DM treated with insulin or sul[f]onylureas,” they wrote in Diabetes, Obesity and Metabolism (8 Sep., doi: 10.1111/dom.13077). Insulin or sulfonylureas, they add, “have been shown to have increased associated CV risks” compared with DPP4 inhibitors and it is “not fully clear to what extent this could have influenced risk estimates.”
Furthermore, the CVD-NORDIC investigators note that the comparator group reflected real-world practice and it is important to look at treatment strategies more specifically, hence why they decided to do an analysis comparing SGLT2 and DPP4 inhibitors.
The study population for the current analysis consisted of 40,909 patients with T2DM who were newly initiated on either dapagliflozin (n=10,227) or a DPP4 inhibitor (n=30,682) between 2012 and 2015. The mean age was 61 years, and around 23% had prior CV disease and 5% had previous heart failure.
After a mean follow-up of 11.3 months, 177 MACE events had occurred among the 10,227 dapagliflozin-treated patients and 695 among the 30,681 DPP4 inhibitor-treated patients, giving respective event rates of 1.86 and 2.34 per 100 patient years. MACE was defined as a composite of nonfatal myocardial infarction, nonfatal stroke, and CV mortality.
“Dapagliflozin is the most commonly used SGLT2 inhibitor in the Nordic countries,” Dr. Norhammar said, explaining why this particular SGLT2 inhibitor was used in the analysis.
While there was a clear benefit of using dapagliflozin over DPP4 inhibitors in terms of MACE, heart failure hospitalization, and all-cause mortality, there was a “neutral” effect on atrial fibrillation and severe hypoglycaemia, with respective HRs of 0.92 (95% CI 0.76–1.12; P = .41) and 0.94 (95% CI 0.74–1.19; P = .62).
Dr. Norhammar said these “neutral results for atrial fibrillation and severe hypoglycaemia,” were “in line with expectations, and suggest a low likelihood of confounding.”
As this was an observational study, one of the limitations is that could be confounding factors that could not be adequately matched in the analysis. The events were not adjudicated and the study didn’t look at safety.
Dr. Norhammar noted that the results of the DECLARE-TIMI 58 trials were now needed to see if the potential CV benefits of using an SGLT2 inhibitor over other gluc0se-lowering medications might extend into patients at lower CV risk.
AstraZeneca supported the study.
Dr. Norhammar disclosed acting as a consultant to and receiving honoraria from AstraZeneca, Boehringer-Ingelheim, Eli Lilly, Novo Nordisk, and MSD Sweden.
LISBON – The sodium-glucose co-transporter 2 (SGLT-2) inhibitor dapagliflozin (Farxiga) posed a lower risk of cardiovascular events and all-cause mortality in patients with type 2 diabetes mellitus (T2DM) compared with dipeptidyl peptidase-4 inhibitors in a large real-world, observational study.
In CVD-REAL NORDIC, treatment with the SGLT-2 inhibitor reduced the incidence of major cardiovascular adverse events by 21% as compared to DDP-4 inhibitors (hazard ratio, 0.79; 95% confidence interval, 0.67-0.94; P = .006).
Anna Norhammar, MD, PhD, of the Institute of Medicine, Cardiology Unit, at Karolinska Institutet in Stockholm, presented the findings at the annual meeting of the European Association for the Study of Diabetes.
She said: “These results are in line with previous clinical trials and meta-analyses, but extend the results to a broader CV risk population and with a commonly used comparator.”
Indeed, the findings build on those from the widely reported EMPA-REG Outcome (New Engl J Med. 2015;373;2117-8) and CANVAS (New Engl J Med. 2017;377:644-7) randomized controlled trials. These trials respectively showed empagliflozin and canagliflozin significantly reduced the risk for MACE and heart failure in patients with T2DM versus placebo. As the majority of patients in these trials had established CV disease, this suggested a class effect for SGLT2 inhibitors, Dr. Norhammar explained.
CVD-REAL NORDIC is part of a larger, multinational, observational, comparative effectiveness study looking at the real-world effect of SGLT2 inhibitors versus other glucose-lowering drugs on CV outcomes in patients with T2DM. Altogether around 90,000 patients have been recruited in Sweden, Norway, and Denmark.
Previously, Dr. Norhammar and co-investigators have reported that SGLT2 inhibitors lowered MACE (HR, 0.78; 95% CI 0.69–0.87, P less than .0001) and heart failure hospitalization (HR, 0.70; 95% CI 0.61–0.81, P less than .0001) relative to all glucose-lowering drugs (Lancet Diabetes Endocrinol. 2017;709–17).
“However, the comparator group used in that study, other [glucose-lowering drugs], consisted of almost 50% patients with T2DM treated with insulin or sul[f]onylureas,” they wrote in Diabetes, Obesity and Metabolism (8 Sep., doi: 10.1111/dom.13077). Insulin or sulfonylureas, they add, “have been shown to have increased associated CV risks” compared with DPP4 inhibitors and it is “not fully clear to what extent this could have influenced risk estimates.”
Furthermore, the CVD-NORDIC investigators note that the comparator group reflected real-world practice and it is important to look at treatment strategies more specifically, hence why they decided to do an analysis comparing SGLT2 and DPP4 inhibitors.
The study population for the current analysis consisted of 40,909 patients with T2DM who were newly initiated on either dapagliflozin (n=10,227) or a DPP4 inhibitor (n=30,682) between 2012 and 2015. The mean age was 61 years, and around 23% had prior CV disease and 5% had previous heart failure.
After a mean follow-up of 11.3 months, 177 MACE events had occurred among the 10,227 dapagliflozin-treated patients and 695 among the 30,681 DPP4 inhibitor-treated patients, giving respective event rates of 1.86 and 2.34 per 100 patient years. MACE was defined as a composite of nonfatal myocardial infarction, nonfatal stroke, and CV mortality.
“Dapagliflozin is the most commonly used SGLT2 inhibitor in the Nordic countries,” Dr. Norhammar said, explaining why this particular SGLT2 inhibitor was used in the analysis.
While there was a clear benefit of using dapagliflozin over DPP4 inhibitors in terms of MACE, heart failure hospitalization, and all-cause mortality, there was a “neutral” effect on atrial fibrillation and severe hypoglycaemia, with respective HRs of 0.92 (95% CI 0.76–1.12; P = .41) and 0.94 (95% CI 0.74–1.19; P = .62).
Dr. Norhammar said these “neutral results for atrial fibrillation and severe hypoglycaemia,” were “in line with expectations, and suggest a low likelihood of confounding.”
As this was an observational study, one of the limitations is that could be confounding factors that could not be adequately matched in the analysis. The events were not adjudicated and the study didn’t look at safety.
Dr. Norhammar noted that the results of the DECLARE-TIMI 58 trials were now needed to see if the potential CV benefits of using an SGLT2 inhibitor over other gluc0se-lowering medications might extend into patients at lower CV risk.
AstraZeneca supported the study.
Dr. Norhammar disclosed acting as a consultant to and receiving honoraria from AstraZeneca, Boehringer-Ingelheim, Eli Lilly, Novo Nordisk, and MSD Sweden.
LISBON – The sodium-glucose co-transporter 2 (SGLT-2) inhibitor dapagliflozin (Farxiga) posed a lower risk of cardiovascular events and all-cause mortality in patients with type 2 diabetes mellitus (T2DM) compared with dipeptidyl peptidase-4 inhibitors in a large real-world, observational study.
In CVD-REAL NORDIC, treatment with the SGLT-2 inhibitor reduced the incidence of major cardiovascular adverse events by 21% as compared to DDP-4 inhibitors (hazard ratio, 0.79; 95% confidence interval, 0.67-0.94; P = .006).
Anna Norhammar, MD, PhD, of the Institute of Medicine, Cardiology Unit, at Karolinska Institutet in Stockholm, presented the findings at the annual meeting of the European Association for the Study of Diabetes.
She said: “These results are in line with previous clinical trials and meta-analyses, but extend the results to a broader CV risk population and with a commonly used comparator.”
Indeed, the findings build on those from the widely reported EMPA-REG Outcome (New Engl J Med. 2015;373;2117-8) and CANVAS (New Engl J Med. 2017;377:644-7) randomized controlled trials. These trials respectively showed empagliflozin and canagliflozin significantly reduced the risk for MACE and heart failure in patients with T2DM versus placebo. As the majority of patients in these trials had established CV disease, this suggested a class effect for SGLT2 inhibitors, Dr. Norhammar explained.
CVD-REAL NORDIC is part of a larger, multinational, observational, comparative effectiveness study looking at the real-world effect of SGLT2 inhibitors versus other glucose-lowering drugs on CV outcomes in patients with T2DM. Altogether around 90,000 patients have been recruited in Sweden, Norway, and Denmark.
Previously, Dr. Norhammar and co-investigators have reported that SGLT2 inhibitors lowered MACE (HR, 0.78; 95% CI 0.69–0.87, P less than .0001) and heart failure hospitalization (HR, 0.70; 95% CI 0.61–0.81, P less than .0001) relative to all glucose-lowering drugs (Lancet Diabetes Endocrinol. 2017;709–17).
“However, the comparator group used in that study, other [glucose-lowering drugs], consisted of almost 50% patients with T2DM treated with insulin or sul[f]onylureas,” they wrote in Diabetes, Obesity and Metabolism (8 Sep., doi: 10.1111/dom.13077). Insulin or sulfonylureas, they add, “have been shown to have increased associated CV risks” compared with DPP4 inhibitors and it is “not fully clear to what extent this could have influenced risk estimates.”
Furthermore, the CVD-NORDIC investigators note that the comparator group reflected real-world practice and it is important to look at treatment strategies more specifically, hence why they decided to do an analysis comparing SGLT2 and DPP4 inhibitors.
The study population for the current analysis consisted of 40,909 patients with T2DM who were newly initiated on either dapagliflozin (n=10,227) or a DPP4 inhibitor (n=30,682) between 2012 and 2015. The mean age was 61 years, and around 23% had prior CV disease and 5% had previous heart failure.
After a mean follow-up of 11.3 months, 177 MACE events had occurred among the 10,227 dapagliflozin-treated patients and 695 among the 30,681 DPP4 inhibitor-treated patients, giving respective event rates of 1.86 and 2.34 per 100 patient years. MACE was defined as a composite of nonfatal myocardial infarction, nonfatal stroke, and CV mortality.
“Dapagliflozin is the most commonly used SGLT2 inhibitor in the Nordic countries,” Dr. Norhammar said, explaining why this particular SGLT2 inhibitor was used in the analysis.
While there was a clear benefit of using dapagliflozin over DPP4 inhibitors in terms of MACE, heart failure hospitalization, and all-cause mortality, there was a “neutral” effect on atrial fibrillation and severe hypoglycaemia, with respective HRs of 0.92 (95% CI 0.76–1.12; P = .41) and 0.94 (95% CI 0.74–1.19; P = .62).
Dr. Norhammar said these “neutral results for atrial fibrillation and severe hypoglycaemia,” were “in line with expectations, and suggest a low likelihood of confounding.”
As this was an observational study, one of the limitations is that could be confounding factors that could not be adequately matched in the analysis. The events were not adjudicated and the study didn’t look at safety.
Dr. Norhammar noted that the results of the DECLARE-TIMI 58 trials were now needed to see if the potential CV benefits of using an SGLT2 inhibitor over other gluc0se-lowering medications might extend into patients at lower CV risk.
AstraZeneca supported the study.
Dr. Norhammar disclosed acting as a consultant to and receiving honoraria from AstraZeneca, Boehringer-Ingelheim, Eli Lilly, Novo Nordisk, and MSD Sweden.
AT EASD 2017
Key clinical point:
Major finding: Hazard ratios for MACE, heart failure hospitalization, and all-cause mortality were a respective 0.79, 0.62, and 0.59, comparing SGLT2 with DPP4 inhibitors.
Data source: CVD-NORDIC, part of a multinational, observational study comparing the real-world effect of SGLT2 inhibitors versus other glucose-lowering drugs on CV outcomes in patients with T2DM.
Disclosures: AstraZeneca supported the study. The presenting author disclosed acting as a consultant to and receiving honoraria from AstraZeneca, Boehringer-Ingelheim, Eli Lilly, Novo Nordisk, and MSD Sweden.
Product can improve joint health in hemophilia A
New research suggests prophylaxis with a recombinant factor VIII Fc fusion protein (rFVIIIFc) can improve joint health over time in patients with hemophilia A.
Patients saw continuous improvement in joint health over a nearly 3-year period, regardless of prior treatment regimen, severity of joint damage, or target joints.
“Gradual joint destruction, which is the leading cause of morbidity for people with hemophilia, remains a significant challenge in the treatment of hemophilia A,” said Johannes Oldenburg, MD, of University Clinic Bonn in Germany.
“This is the first study to show that functional joint health can continue to improve using prophylactic treatment with an extended half-life factor therapy, even for those who have severe joint disease at the start of treatment.”
Dr Oldenburg and his colleagues reported these findings in Haemophilia. The research was sponsored by Biogen/Bioverativ and Sobi, the companies marketing rFVIIIFc (or efmoroctocog alfa) as Eloctate or Elocta.
This interim post hoc analysis was an evaluation of joint health in adults and adolescents who received rFVIIIFc prophylaxis in the A-LONG and ASPIRE studies.
In A-LONG, patients age 12 and older who had severe hemophilia A received rFVIIIFc at 25-65 IU/kg every 3 to 5 days (arm 1), at 65 IU/kg weekly (arm 2), or as episodic treatment (arm 3). Patients who completed A-LONG could then enroll in the ASPIRE extension study.
For the current analysis, Dr Oldenburg and his colleagues assessed joint health in ASPIRE enrollees using a modified version of the Hemophilia Joint Health Score (mHJHS). This tool grades joints by specific domains, including swelling, muscle atrophy, alignment, range of motion, joint pain, strength, and global gait.
The researchers examined mHJHS measurements (a decrease in score reflecting improvement) taken at A-LONG baseline, ASPIRE baseline, and annually thereafter for roughly 2.8 years of treatment.
There were 47 patients who had mHJHS data at both study baselines, ASPIRE year 1, and ASPIRE year 2.
These patients had a mean improvement in joint health score of -4.1 at ASPIRE year 2, compared with A-LONG baseline (P=0.001).
The mean improvement was -2.4 (P=0.09) for patients who received pre-study prophylaxis and -7.2 (P=0.003) for those who received pre-study episodic treatment.
The mean improvement was -5.6 (P=0.005) in patients with target joints and -8.8 (P=0.02) in those with severe joint destruction.
The mHJHS components with the greatest improvement at ASPIRE year 2 were swelling (-1.4, P=0.008), range of motion (-1.1, P=0.03), and strength (-0.8, P=0.04). ![]()
New research suggests prophylaxis with a recombinant factor VIII Fc fusion protein (rFVIIIFc) can improve joint health over time in patients with hemophilia A.
Patients saw continuous improvement in joint health over a nearly 3-year period, regardless of prior treatment regimen, severity of joint damage, or target joints.
“Gradual joint destruction, which is the leading cause of morbidity for people with hemophilia, remains a significant challenge in the treatment of hemophilia A,” said Johannes Oldenburg, MD, of University Clinic Bonn in Germany.
“This is the first study to show that functional joint health can continue to improve using prophylactic treatment with an extended half-life factor therapy, even for those who have severe joint disease at the start of treatment.”
Dr Oldenburg and his colleagues reported these findings in Haemophilia. The research was sponsored by Biogen/Bioverativ and Sobi, the companies marketing rFVIIIFc (or efmoroctocog alfa) as Eloctate or Elocta.
This interim post hoc analysis was an evaluation of joint health in adults and adolescents who received rFVIIIFc prophylaxis in the A-LONG and ASPIRE studies.
In A-LONG, patients age 12 and older who had severe hemophilia A received rFVIIIFc at 25-65 IU/kg every 3 to 5 days (arm 1), at 65 IU/kg weekly (arm 2), or as episodic treatment (arm 3). Patients who completed A-LONG could then enroll in the ASPIRE extension study.
For the current analysis, Dr Oldenburg and his colleagues assessed joint health in ASPIRE enrollees using a modified version of the Hemophilia Joint Health Score (mHJHS). This tool grades joints by specific domains, including swelling, muscle atrophy, alignment, range of motion, joint pain, strength, and global gait.
The researchers examined mHJHS measurements (a decrease in score reflecting improvement) taken at A-LONG baseline, ASPIRE baseline, and annually thereafter for roughly 2.8 years of treatment.
There were 47 patients who had mHJHS data at both study baselines, ASPIRE year 1, and ASPIRE year 2.
These patients had a mean improvement in joint health score of -4.1 at ASPIRE year 2, compared with A-LONG baseline (P=0.001).
The mean improvement was -2.4 (P=0.09) for patients who received pre-study prophylaxis and -7.2 (P=0.003) for those who received pre-study episodic treatment.
The mean improvement was -5.6 (P=0.005) in patients with target joints and -8.8 (P=0.02) in those with severe joint destruction.
The mHJHS components with the greatest improvement at ASPIRE year 2 were swelling (-1.4, P=0.008), range of motion (-1.1, P=0.03), and strength (-0.8, P=0.04). ![]()
New research suggests prophylaxis with a recombinant factor VIII Fc fusion protein (rFVIIIFc) can improve joint health over time in patients with hemophilia A.
Patients saw continuous improvement in joint health over a nearly 3-year period, regardless of prior treatment regimen, severity of joint damage, or target joints.
“Gradual joint destruction, which is the leading cause of morbidity for people with hemophilia, remains a significant challenge in the treatment of hemophilia A,” said Johannes Oldenburg, MD, of University Clinic Bonn in Germany.
“This is the first study to show that functional joint health can continue to improve using prophylactic treatment with an extended half-life factor therapy, even for those who have severe joint disease at the start of treatment.”
Dr Oldenburg and his colleagues reported these findings in Haemophilia. The research was sponsored by Biogen/Bioverativ and Sobi, the companies marketing rFVIIIFc (or efmoroctocog alfa) as Eloctate or Elocta.
This interim post hoc analysis was an evaluation of joint health in adults and adolescents who received rFVIIIFc prophylaxis in the A-LONG and ASPIRE studies.
In A-LONG, patients age 12 and older who had severe hemophilia A received rFVIIIFc at 25-65 IU/kg every 3 to 5 days (arm 1), at 65 IU/kg weekly (arm 2), or as episodic treatment (arm 3). Patients who completed A-LONG could then enroll in the ASPIRE extension study.
For the current analysis, Dr Oldenburg and his colleagues assessed joint health in ASPIRE enrollees using a modified version of the Hemophilia Joint Health Score (mHJHS). This tool grades joints by specific domains, including swelling, muscle atrophy, alignment, range of motion, joint pain, strength, and global gait.
The researchers examined mHJHS measurements (a decrease in score reflecting improvement) taken at A-LONG baseline, ASPIRE baseline, and annually thereafter for roughly 2.8 years of treatment.
There were 47 patients who had mHJHS data at both study baselines, ASPIRE year 1, and ASPIRE year 2.
These patients had a mean improvement in joint health score of -4.1 at ASPIRE year 2, compared with A-LONG baseline (P=0.001).
The mean improvement was -2.4 (P=0.09) for patients who received pre-study prophylaxis and -7.2 (P=0.003) for those who received pre-study episodic treatment.
The mean improvement was -5.6 (P=0.005) in patients with target joints and -8.8 (P=0.02) in those with severe joint destruction.
The mHJHS components with the greatest improvement at ASPIRE year 2 were swelling (-1.4, P=0.008), range of motion (-1.1, P=0.03), and strength (-0.8, P=0.04). ![]()
Birthdays, Booze, and … Broken Bones?
ANSWER
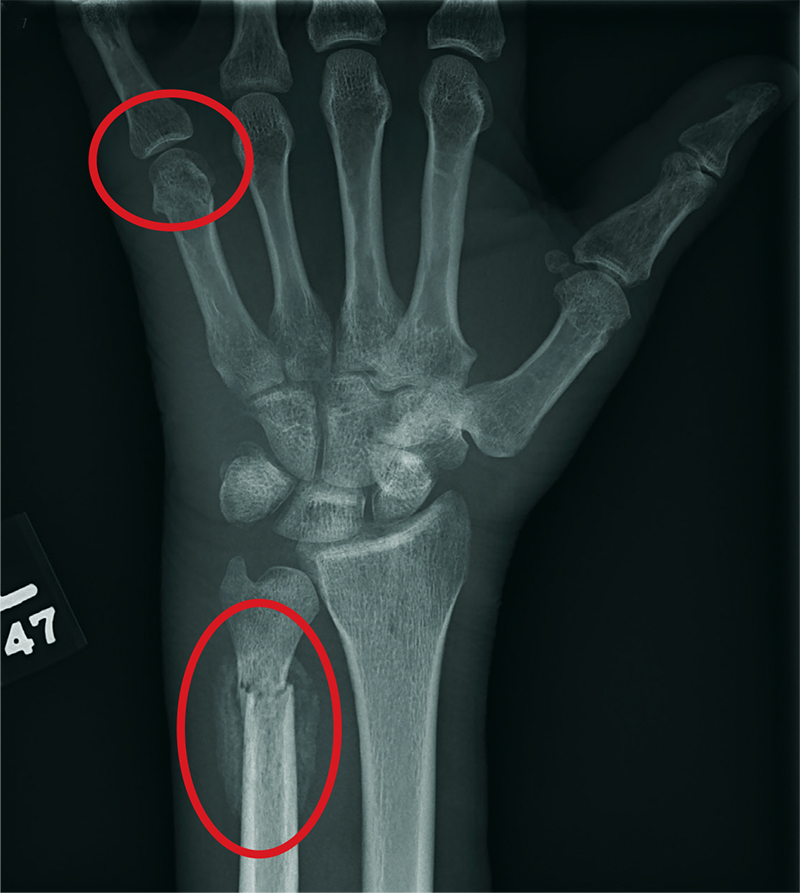
The radiograph shows two fractures: one within the distal ulna and one within the fifth metacarpal. On closer examination, you can see that a bony callous surrounds each of the fracture lines, making these injuries more likely to be subacute or remote than acute.
Review of the patient's electronic health record showed he had presented three months earlier for a left hand and wrist injury, at which time an acute fracture was diagnosed. Nonetheless, he was placed in a splint and referred to orthopedics for outpatient follow-up.

ANSWER
The radiograph shows two fractures: one within the distal ulna and one within the fifth metacarpal. On closer examination, you can see that a bony callous surrounds each of the fracture lines, making these injuries more likely to be subacute or remote than acute.
Review of the patient's electronic health record showed he had presented three months earlier for a left hand and wrist injury, at which time an acute fracture was diagnosed. Nonetheless, he was placed in a splint and referred to orthopedics for outpatient follow-up.

ANSWER
The radiograph shows two fractures: one within the distal ulna and one within the fifth metacarpal. On closer examination, you can see that a bony callous surrounds each of the fracture lines, making these injuries more likely to be subacute or remote than acute.
Review of the patient's electronic health record showed he had presented three months earlier for a left hand and wrist injury, at which time an acute fracture was diagnosed. Nonetheless, he was placed in a splint and referred to orthopedics for outpatient follow-up.
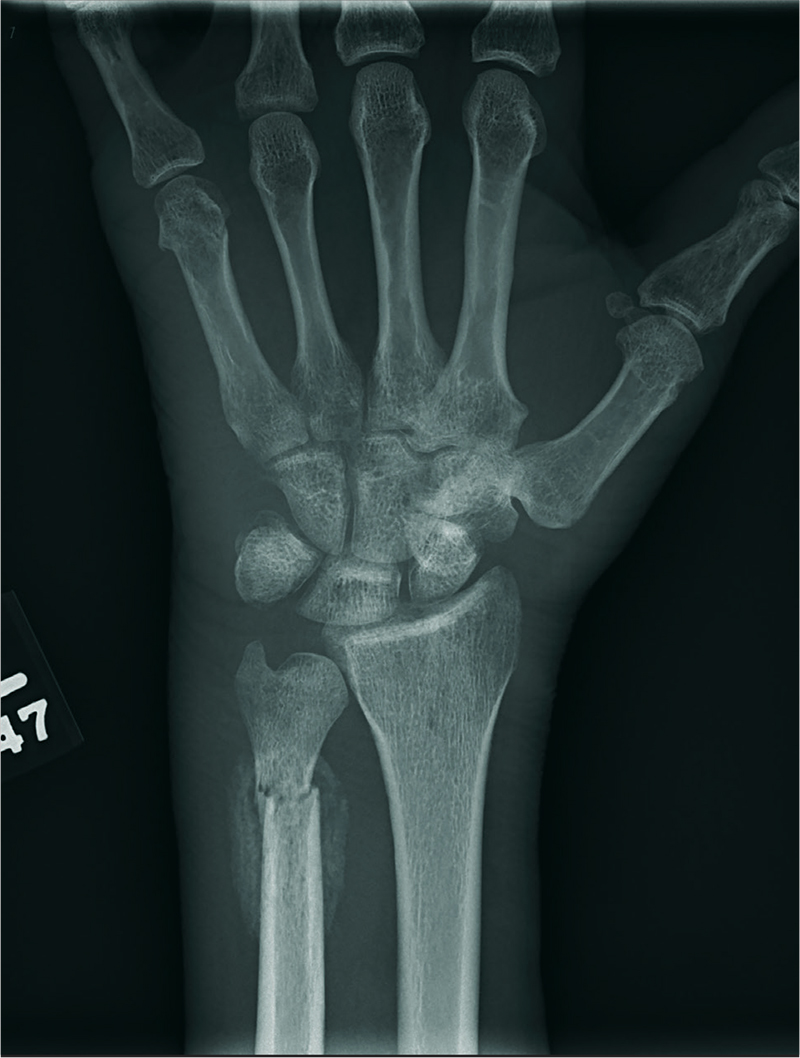
A 60-year-old man is brought to your facility emergently for decreased consciousness secondary to alcohol intoxication. He is somewhat incoherent, but from what you gather, he was attending a birthday celebration. He does not know how much he drank.
The patient complains of a headache and pain in his left wrist. You ask if he fell or was assaulted, but he does not respond. His medical history is otherwise unknown.
His initial vital signs are stable, and primary survey does not show any major injuries. He appears to spontaneously move all four extremities.
Closer examination of his left wrist shows no deformity or swelling, but the dorsolateral aspect of his hand is tender. Radiograph of his left wrist is obtained (shown). What is your impression?
VIDEO: Treating vascular lesions in children
LAS VEGAS – Clinicians should not shy away from light-based treatment of vascular lesions in children, for reasons that include achieving better results when treated early, according to Kristen M. Kelly, MD.
Special considerations include addressing children’s fears. “One of the strategies we use is we have child life specialists who help us” create a friendly and welcoming environment, Dr. Kelly said in a video interview at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar. Consequently, “many of our children actually come in, they’re excited about their visit ... and are looking forward to seeing us at the next visit,” she noted.
Which type of anesthesia to use is another important consideration when treating children, said Dr. Kelly of the University of California, Irvine, in Orange. “For a larger procedure ... one definitely could consider general anesthesia,” but there are risks and benefits to general anesthesia in very young children, and options should be discussed with patients and their families, she said.
Dr. Kelly disclosed relationships with multiple companies including Allergan, MundiPharma, Syneron-Candela, Light Sciences Oncology, Novartis, Sciton, and ThermiRF.
SDEF and this news organization are owned by the same parent company.
LAS VEGAS – Clinicians should not shy away from light-based treatment of vascular lesions in children, for reasons that include achieving better results when treated early, according to Kristen M. Kelly, MD.
Special considerations include addressing children’s fears. “One of the strategies we use is we have child life specialists who help us” create a friendly and welcoming environment, Dr. Kelly said in a video interview at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar. Consequently, “many of our children actually come in, they’re excited about their visit ... and are looking forward to seeing us at the next visit,” she noted.
Which type of anesthesia to use is another important consideration when treating children, said Dr. Kelly of the University of California, Irvine, in Orange. “For a larger procedure ... one definitely could consider general anesthesia,” but there are risks and benefits to general anesthesia in very young children, and options should be discussed with patients and their families, she said.
Dr. Kelly disclosed relationships with multiple companies including Allergan, MundiPharma, Syneron-Candela, Light Sciences Oncology, Novartis, Sciton, and ThermiRF.
SDEF and this news organization are owned by the same parent company.
LAS VEGAS – Clinicians should not shy away from light-based treatment of vascular lesions in children, for reasons that include achieving better results when treated early, according to Kristen M. Kelly, MD.
Special considerations include addressing children’s fears. “One of the strategies we use is we have child life specialists who help us” create a friendly and welcoming environment, Dr. Kelly said in a video interview at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar. Consequently, “many of our children actually come in, they’re excited about their visit ... and are looking forward to seeing us at the next visit,” she noted.
Which type of anesthesia to use is another important consideration when treating children, said Dr. Kelly of the University of California, Irvine, in Orange. “For a larger procedure ... one definitely could consider general anesthesia,” but there are risks and benefits to general anesthesia in very young children, and options should be discussed with patients and their families, she said.
Dr. Kelly disclosed relationships with multiple companies including Allergan, MundiPharma, Syneron-Candela, Light Sciences Oncology, Novartis, Sciton, and ThermiRF.
SDEF and this news organization are owned by the same parent company.
AT SDEF LAS VEGAS DERMATOLOGY SEMINAR
Your patient has a large symptomatic fibroid: Tools for decision making
VIDEO: A challenging case of lichen planus
LAS VEGAS – For challenging cases of oral or cutaneous lichen planus, bullous pemphigoid, or lupus, Miriam S. Bettencourt, MD, recommends thinking outside the box and considering off-label treatments.
At the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar, Dr. Bettencourt discussed such cases, including a series of patients with oral lichen planus who improved with apremilast, an oral phosphodiesterase 4 inhibitor approved for psoriasis.
In a video interview at the meeting, she described one of those patients, a 73-year-old woman with mouth ulcers who was diagnosed with oral lichen planus. Multiple topical and oral therapies proved unsuccessful, and her condition was eventually controlled with apremilast, and the patient is doing well, “with occasional flares,” said Dr. Bettencourt, of the University of Nevada, Las Vegas.
She described this case in her annual presentation at the meeting, titled “Great Cases From the Las Vegas Dermatology Society.”
Dr. Bettencourt disclosed relationships with multiple companies including AbbVie, Aclaris, Celgene, IntraDerm, Pfizer, Promium, Sun Pharma, and Valeant.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS – For challenging cases of oral or cutaneous lichen planus, bullous pemphigoid, or lupus, Miriam S. Bettencourt, MD, recommends thinking outside the box and considering off-label treatments.
At the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar, Dr. Bettencourt discussed such cases, including a series of patients with oral lichen planus who improved with apremilast, an oral phosphodiesterase 4 inhibitor approved for psoriasis.
In a video interview at the meeting, she described one of those patients, a 73-year-old woman with mouth ulcers who was diagnosed with oral lichen planus. Multiple topical and oral therapies proved unsuccessful, and her condition was eventually controlled with apremilast, and the patient is doing well, “with occasional flares,” said Dr. Bettencourt, of the University of Nevada, Las Vegas.
She described this case in her annual presentation at the meeting, titled “Great Cases From the Las Vegas Dermatology Society.”
Dr. Bettencourt disclosed relationships with multiple companies including AbbVie, Aclaris, Celgene, IntraDerm, Pfizer, Promium, Sun Pharma, and Valeant.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS – For challenging cases of oral or cutaneous lichen planus, bullous pemphigoid, or lupus, Miriam S. Bettencourt, MD, recommends thinking outside the box and considering off-label treatments.
At the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar, Dr. Bettencourt discussed such cases, including a series of patients with oral lichen planus who improved with apremilast, an oral phosphodiesterase 4 inhibitor approved for psoriasis.
In a video interview at the meeting, she described one of those patients, a 73-year-old woman with mouth ulcers who was diagnosed with oral lichen planus. Multiple topical and oral therapies proved unsuccessful, and her condition was eventually controlled with apremilast, and the patient is doing well, “with occasional flares,” said Dr. Bettencourt, of the University of Nevada, Las Vegas.
She described this case in her annual presentation at the meeting, titled “Great Cases From the Las Vegas Dermatology Society.”
Dr. Bettencourt disclosed relationships with multiple companies including AbbVie, Aclaris, Celgene, IntraDerm, Pfizer, Promium, Sun Pharma, and Valeant.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SDEF LAS VEGAS DERMATOLOGY SEMINAR
Aducanumab continues to rack up positive numbers in phase 1b open-label extension
BOSTON – The antiamyloid antibody aducanumab continued its slide around the bases this week, revealing more positive imaging and cognitive data at 36 months into the phase 1b PRIME trial.
Patients who have been taking the highest dose of aducanumab, 10 mg/kg, for the duration of the study improved the most on two measures of cognition, the Mini Mental State Exam (MMSE) and the Clinical Dementia Rating Scale–sum of boxes (CDR-sb). On the pathology side, at least some of the 10-mg/kg patients dropped below the threshold of PET amyloid positivity by 24 months and stayed at that low level up to 36 months, Samantha Budd Haeberlein, PhD, said at the Clinical Trials on Alzheimer’s Disease conference.
The 36-month data support the continued development of aducanumab, she said. The antibody is now being tested in two phase 3 studies, ENGAGE and EMERGE.
“The aducanumab data reported at CTAD is good news for safety and good news for the signals we need to see in the phase 3 trials,” Maria Carillo, PhD, chief science officer of the Alzheimer’s Association said when asked to comment on the latest data. “These are hopeful signs, but – based on what we’ve learned from past Alzheimer’s studies – we need to wait for the phase 3 trial results.”
Aducanumab is a monoclonal human antibody derived from B cells collected from a cohort of cognitively normal elderly subjects and cognitively impaired elderly subjects who exhibited unusually slow decline, according to Biogen. It binds to fibrillar and oligomeric amyloid aggregates, thus directly reducing amyloid plaque in the brain.
PRIME enrolled 165 patients with prodromal or mild Alzheimer’s disease. Importantly, all of the subjects had brain amyloid proven by PET imaging. PRIME is the first randomized trial of an antiamyloid compound to enroll a purely PET-proven amyloid-positive cohort. These subjects were randomized to placebo or aducanumab at 1, 3, 6, or 10 mg/kg for 1 year. This was followed by a 2-year open-label extension period. Patients who were randomized to placebo or 1 mg/kg were switched to aducanumab 3 mg/kg or to a 3- to 6-mg/kg titration regimen in the long-term extension. Patients randomized to aducanumab at 3, 6 or 10 mg/kg or titration in the placebo-controlled period continued in the same dose group.
Dr. Haeberlein presented only the fixed-dose data; the titration group data will be presented later in the conference.
PRIME’s primary outcomes are safety and tolerability. The cognitive and functional outcomes, not usually assessed in a phase 1b study, are exploratory. This is important to remember, Dr. Haeberlein said. She also stressed that the numbers in each dosing group are quite small. Of the original cohort, 117 entered the extension study and just 50 made it to 166 weeks, at which time 10-16 patients were in each of the dosage cohorts.
At 36 months, the mean change in amyloid plaque level was greatest for the 10-mg/kg group, which, on average, fell below the threshold of amyloid positivity on florbetapir PET scan. The 6-mg/kg group approached the threshold, but did not fall below it. The 1- and 3-mg/kg groups declined similarly to each other, although not as dramatically as the higher-dose group.
Everyone in the trial declined on both cognitive measures, the MMSE and CDR-sb. However, the decline was clearly attenuated in some of the active groups, where the best results were seen in the 10 patients who took 10 mg/kg. The average decline from baseline on the CDR-sb was 2.84 points among those patients. In the other groups, declines from baseline on the CDR-sb were:
- 5.28 points in those who switched from placebo to 3 mg/kg.
- 6.11 points in those who switched from 1 mg/kg to 3 mg/kg.
- 3.86 points in the 3-mg/kg treatment group.
- 4.49 points in the 6-mg/kg treatment group.
Patients taking 10 mg/kg also fared best on the MMSE, declining 4.10 points on average. Declines in the other groups were:
- 7.98 points in those who switched from placebo to 3 mg/kg.
- 6.35 points in those who switched from 1 mg/kg to 3 mg/kg.
- 4.83 points in the 3-mg/kg treatment group.
- 8.97 points in the 6 mg/kg treatment group.
During the presentation, Dr. Haeberlein said these differences were not statistically significant. In an interview, she said, “In this extension trial, we aren’t talking about statistical significance. We are beyond that.”
The incidence of ARIA (Amyloid-Related Imaging Abnormalities), however, did not follow this dose-dependent pattern. All eight cases of ARIA-E (the edematous form) in the long-term extension phase occurred in the placebo group that switched to 1 mg/kg or in the 1-mg/kg group that titrated up to 3 mg/kg. All cases occurred early in the extension phase, with no new cases during the last year, and all but one occurred in APOE4 allele carriers.
Hemorrhagic ARIA was more sporadic, occurring in two placebo switchers, five taking 3 mg/kg, two taking 6 mg/kg, and one patient taking the highest 10 mg/kg dose. Again, these cases occurred early in the trial. All of the ARIA cases, regardless of etiology, were considered mild and resolved spontaneously.
In all of PRIME, 46 patients have experienced ARIA, with 6 experiencing more than one episode.
The most common adverse events in the long-term extension phase were falls, headache, and ARIA. Two patients in the extension phase died – one taking 6 mg/kg and one taking 10 mg/kg. Neither death was related to the study medication.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
BOSTON – The antiamyloid antibody aducanumab continued its slide around the bases this week, revealing more positive imaging and cognitive data at 36 months into the phase 1b PRIME trial.
Patients who have been taking the highest dose of aducanumab, 10 mg/kg, for the duration of the study improved the most on two measures of cognition, the Mini Mental State Exam (MMSE) and the Clinical Dementia Rating Scale–sum of boxes (CDR-sb). On the pathology side, at least some of the 10-mg/kg patients dropped below the threshold of PET amyloid positivity by 24 months and stayed at that low level up to 36 months, Samantha Budd Haeberlein, PhD, said at the Clinical Trials on Alzheimer’s Disease conference.
The 36-month data support the continued development of aducanumab, she said. The antibody is now being tested in two phase 3 studies, ENGAGE and EMERGE.
“The aducanumab data reported at CTAD is good news for safety and good news for the signals we need to see in the phase 3 trials,” Maria Carillo, PhD, chief science officer of the Alzheimer’s Association said when asked to comment on the latest data. “These are hopeful signs, but – based on what we’ve learned from past Alzheimer’s studies – we need to wait for the phase 3 trial results.”
Aducanumab is a monoclonal human antibody derived from B cells collected from a cohort of cognitively normal elderly subjects and cognitively impaired elderly subjects who exhibited unusually slow decline, according to Biogen. It binds to fibrillar and oligomeric amyloid aggregates, thus directly reducing amyloid plaque in the brain.
PRIME enrolled 165 patients with prodromal or mild Alzheimer’s disease. Importantly, all of the subjects had brain amyloid proven by PET imaging. PRIME is the first randomized trial of an antiamyloid compound to enroll a purely PET-proven amyloid-positive cohort. These subjects were randomized to placebo or aducanumab at 1, 3, 6, or 10 mg/kg for 1 year. This was followed by a 2-year open-label extension period. Patients who were randomized to placebo or 1 mg/kg were switched to aducanumab 3 mg/kg or to a 3- to 6-mg/kg titration regimen in the long-term extension. Patients randomized to aducanumab at 3, 6 or 10 mg/kg or titration in the placebo-controlled period continued in the same dose group.
Dr. Haeberlein presented only the fixed-dose data; the titration group data will be presented later in the conference.
PRIME’s primary outcomes are safety and tolerability. The cognitive and functional outcomes, not usually assessed in a phase 1b study, are exploratory. This is important to remember, Dr. Haeberlein said. She also stressed that the numbers in each dosing group are quite small. Of the original cohort, 117 entered the extension study and just 50 made it to 166 weeks, at which time 10-16 patients were in each of the dosage cohorts.
At 36 months, the mean change in amyloid plaque level was greatest for the 10-mg/kg group, which, on average, fell below the threshold of amyloid positivity on florbetapir PET scan. The 6-mg/kg group approached the threshold, but did not fall below it. The 1- and 3-mg/kg groups declined similarly to each other, although not as dramatically as the higher-dose group.
Everyone in the trial declined on both cognitive measures, the MMSE and CDR-sb. However, the decline was clearly attenuated in some of the active groups, where the best results were seen in the 10 patients who took 10 mg/kg. The average decline from baseline on the CDR-sb was 2.84 points among those patients. In the other groups, declines from baseline on the CDR-sb were:
- 5.28 points in those who switched from placebo to 3 mg/kg.
- 6.11 points in those who switched from 1 mg/kg to 3 mg/kg.
- 3.86 points in the 3-mg/kg treatment group.
- 4.49 points in the 6-mg/kg treatment group.
Patients taking 10 mg/kg also fared best on the MMSE, declining 4.10 points on average. Declines in the other groups were:
- 7.98 points in those who switched from placebo to 3 mg/kg.
- 6.35 points in those who switched from 1 mg/kg to 3 mg/kg.
- 4.83 points in the 3-mg/kg treatment group.
- 8.97 points in the 6 mg/kg treatment group.
During the presentation, Dr. Haeberlein said these differences were not statistically significant. In an interview, she said, “In this extension trial, we aren’t talking about statistical significance. We are beyond that.”
The incidence of ARIA (Amyloid-Related Imaging Abnormalities), however, did not follow this dose-dependent pattern. All eight cases of ARIA-E (the edematous form) in the long-term extension phase occurred in the placebo group that switched to 1 mg/kg or in the 1-mg/kg group that titrated up to 3 mg/kg. All cases occurred early in the extension phase, with no new cases during the last year, and all but one occurred in APOE4 allele carriers.
Hemorrhagic ARIA was more sporadic, occurring in two placebo switchers, five taking 3 mg/kg, two taking 6 mg/kg, and one patient taking the highest 10 mg/kg dose. Again, these cases occurred early in the trial. All of the ARIA cases, regardless of etiology, were considered mild and resolved spontaneously.
In all of PRIME, 46 patients have experienced ARIA, with 6 experiencing more than one episode.
The most common adverse events in the long-term extension phase were falls, headache, and ARIA. Two patients in the extension phase died – one taking 6 mg/kg and one taking 10 mg/kg. Neither death was related to the study medication.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
BOSTON – The antiamyloid antibody aducanumab continued its slide around the bases this week, revealing more positive imaging and cognitive data at 36 months into the phase 1b PRIME trial.
Patients who have been taking the highest dose of aducanumab, 10 mg/kg, for the duration of the study improved the most on two measures of cognition, the Mini Mental State Exam (MMSE) and the Clinical Dementia Rating Scale–sum of boxes (CDR-sb). On the pathology side, at least some of the 10-mg/kg patients dropped below the threshold of PET amyloid positivity by 24 months and stayed at that low level up to 36 months, Samantha Budd Haeberlein, PhD, said at the Clinical Trials on Alzheimer’s Disease conference.
The 36-month data support the continued development of aducanumab, she said. The antibody is now being tested in two phase 3 studies, ENGAGE and EMERGE.
“The aducanumab data reported at CTAD is good news for safety and good news for the signals we need to see in the phase 3 trials,” Maria Carillo, PhD, chief science officer of the Alzheimer’s Association said when asked to comment on the latest data. “These are hopeful signs, but – based on what we’ve learned from past Alzheimer’s studies – we need to wait for the phase 3 trial results.”
Aducanumab is a monoclonal human antibody derived from B cells collected from a cohort of cognitively normal elderly subjects and cognitively impaired elderly subjects who exhibited unusually slow decline, according to Biogen. It binds to fibrillar and oligomeric amyloid aggregates, thus directly reducing amyloid plaque in the brain.
PRIME enrolled 165 patients with prodromal or mild Alzheimer’s disease. Importantly, all of the subjects had brain amyloid proven by PET imaging. PRIME is the first randomized trial of an antiamyloid compound to enroll a purely PET-proven amyloid-positive cohort. These subjects were randomized to placebo or aducanumab at 1, 3, 6, or 10 mg/kg for 1 year. This was followed by a 2-year open-label extension period. Patients who were randomized to placebo or 1 mg/kg were switched to aducanumab 3 mg/kg or to a 3- to 6-mg/kg titration regimen in the long-term extension. Patients randomized to aducanumab at 3, 6 or 10 mg/kg or titration in the placebo-controlled period continued in the same dose group.
Dr. Haeberlein presented only the fixed-dose data; the titration group data will be presented later in the conference.
PRIME’s primary outcomes are safety and tolerability. The cognitive and functional outcomes, not usually assessed in a phase 1b study, are exploratory. This is important to remember, Dr. Haeberlein said. She also stressed that the numbers in each dosing group are quite small. Of the original cohort, 117 entered the extension study and just 50 made it to 166 weeks, at which time 10-16 patients were in each of the dosage cohorts.
At 36 months, the mean change in amyloid plaque level was greatest for the 10-mg/kg group, which, on average, fell below the threshold of amyloid positivity on florbetapir PET scan. The 6-mg/kg group approached the threshold, but did not fall below it. The 1- and 3-mg/kg groups declined similarly to each other, although not as dramatically as the higher-dose group.
Everyone in the trial declined on both cognitive measures, the MMSE and CDR-sb. However, the decline was clearly attenuated in some of the active groups, where the best results were seen in the 10 patients who took 10 mg/kg. The average decline from baseline on the CDR-sb was 2.84 points among those patients. In the other groups, declines from baseline on the CDR-sb were:
- 5.28 points in those who switched from placebo to 3 mg/kg.
- 6.11 points in those who switched from 1 mg/kg to 3 mg/kg.
- 3.86 points in the 3-mg/kg treatment group.
- 4.49 points in the 6-mg/kg treatment group.
Patients taking 10 mg/kg also fared best on the MMSE, declining 4.10 points on average. Declines in the other groups were:
- 7.98 points in those who switched from placebo to 3 mg/kg.
- 6.35 points in those who switched from 1 mg/kg to 3 mg/kg.
- 4.83 points in the 3-mg/kg treatment group.
- 8.97 points in the 6 mg/kg treatment group.
During the presentation, Dr. Haeberlein said these differences were not statistically significant. In an interview, she said, “In this extension trial, we aren’t talking about statistical significance. We are beyond that.”
The incidence of ARIA (Amyloid-Related Imaging Abnormalities), however, did not follow this dose-dependent pattern. All eight cases of ARIA-E (the edematous form) in the long-term extension phase occurred in the placebo group that switched to 1 mg/kg or in the 1-mg/kg group that titrated up to 3 mg/kg. All cases occurred early in the extension phase, with no new cases during the last year, and all but one occurred in APOE4 allele carriers.
Hemorrhagic ARIA was more sporadic, occurring in two placebo switchers, five taking 3 mg/kg, two taking 6 mg/kg, and one patient taking the highest 10 mg/kg dose. Again, these cases occurred early in the trial. All of the ARIA cases, regardless of etiology, were considered mild and resolved spontaneously.
In all of PRIME, 46 patients have experienced ARIA, with 6 experiencing more than one episode.
The most common adverse events in the long-term extension phase were falls, headache, and ARIA. Two patients in the extension phase died – one taking 6 mg/kg and one taking 10 mg/kg. Neither death was related to the study medication.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
EXPERT ANALYSIS FROM CTAD
Key clinical point:
Major finding: At 36 months, the mean change in amyloid plaque level was greatest for the 10-mg/kg group, which, on average, fell below the threshold of amyloid positivity on florbetapir PET scan.
Data source: 3-year extension phase data from the phase 1B PRIME trial.
Disclosures: The PRIME trial is sponsored by Biogen. The presenter is an employee of the company.
VIDEO: Metabolic regulator FGF21 improves fibrosis in NASH patients
WASHINGTON – Fibroblast growth factor 21 (FGF21), a nonmitogenic hormone, improved fibrosis, liver injury, and steatosis in patients with nonalcoholic steatohepatitis (NASH), according to a study presented at the American Association for the Study of Liver Disease’s annual meeting.
There is no drug therapy currently available for NASH, the most advanced form of nonalcoholic fatty liver disease (NAFLD), creating a strong need for effective treatments, according to Arun Sanyal, MD, of the Virginia Commonwealth University, Richmond, said in a video interview.
This treatment “relative to placebo was associated with improvements in biomarkers of fibrosis, metabolic parameters, and markers of hepatic injury,” said Dr. Sanyal. “These results suggest BMS-986036 [FGF21] has beneficial effects on steatosis, liver injury, and fibrosis in NASH.”
Investigators conducted a phase 2 multicenter, double-blind, placebo-controlled study of 74 NASH patients to test BMS-986036, a pegylated version of FGF21.
Patients were an average of 51 years old, most were women (64%), who were predominantly white (96%), with a mean hepatic fat fraction of 19%.
Patients received either a 10-mg treatment daily, a 20-mg treatment weekly, or placebo, over the course of 16 weeks, with patients distributed equally among the three arms.
Overall hepatic fat fraction among the daily and weekly treatment groups reduced by 6.8% and 5.2%, respectively, compared with the placebo group, which reduced by 1.3% (P less than .001).
Patients in the treatment arms also saw improvement in average adiponectin levels, growing 15.3% in the daily arm and 15.7% in the weekly arm. Meanwhile, adiponectin levels dropped by an average of 3.5% in the placebo group.
In investigating serum Pro-C3 levels, which are associated with fibrosis, patients in the daily and weekly treatment group saw an average drop of 29% and 19%, respectively, as opposed to an increase of 2% in the placebo group (P less than .0001).
Patients in the treatment groups saw no serious adverse effects, and no patients died during the study.
Dr. Sanyal received funding for this study from Bristol-Myers Squibb and reported receiving financial compensation from Pfizer, Nimbus, Novartis, AstraZeneca, and other similar companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ezimmerman@frontlinemedcom.com
On Twitter @eaztweets
WASHINGTON – Fibroblast growth factor 21 (FGF21), a nonmitogenic hormone, improved fibrosis, liver injury, and steatosis in patients with nonalcoholic steatohepatitis (NASH), according to a study presented at the American Association for the Study of Liver Disease’s annual meeting.
There is no drug therapy currently available for NASH, the most advanced form of nonalcoholic fatty liver disease (NAFLD), creating a strong need for effective treatments, according to Arun Sanyal, MD, of the Virginia Commonwealth University, Richmond, said in a video interview.
This treatment “relative to placebo was associated with improvements in biomarkers of fibrosis, metabolic parameters, and markers of hepatic injury,” said Dr. Sanyal. “These results suggest BMS-986036 [FGF21] has beneficial effects on steatosis, liver injury, and fibrosis in NASH.”
Investigators conducted a phase 2 multicenter, double-blind, placebo-controlled study of 74 NASH patients to test BMS-986036, a pegylated version of FGF21.
Patients were an average of 51 years old, most were women (64%), who were predominantly white (96%), with a mean hepatic fat fraction of 19%.
Patients received either a 10-mg treatment daily, a 20-mg treatment weekly, or placebo, over the course of 16 weeks, with patients distributed equally among the three arms.
Overall hepatic fat fraction among the daily and weekly treatment groups reduced by 6.8% and 5.2%, respectively, compared with the placebo group, which reduced by 1.3% (P less than .001).
Patients in the treatment arms also saw improvement in average adiponectin levels, growing 15.3% in the daily arm and 15.7% in the weekly arm. Meanwhile, adiponectin levels dropped by an average of 3.5% in the placebo group.
In investigating serum Pro-C3 levels, which are associated with fibrosis, patients in the daily and weekly treatment group saw an average drop of 29% and 19%, respectively, as opposed to an increase of 2% in the placebo group (P less than .0001).
Patients in the treatment groups saw no serious adverse effects, and no patients died during the study.
Dr. Sanyal received funding for this study from Bristol-Myers Squibb and reported receiving financial compensation from Pfizer, Nimbus, Novartis, AstraZeneca, and other similar companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ezimmerman@frontlinemedcom.com
On Twitter @eaztweets
WASHINGTON – Fibroblast growth factor 21 (FGF21), a nonmitogenic hormone, improved fibrosis, liver injury, and steatosis in patients with nonalcoholic steatohepatitis (NASH), according to a study presented at the American Association for the Study of Liver Disease’s annual meeting.
There is no drug therapy currently available for NASH, the most advanced form of nonalcoholic fatty liver disease (NAFLD), creating a strong need for effective treatments, according to Arun Sanyal, MD, of the Virginia Commonwealth University, Richmond, said in a video interview.
This treatment “relative to placebo was associated with improvements in biomarkers of fibrosis, metabolic parameters, and markers of hepatic injury,” said Dr. Sanyal. “These results suggest BMS-986036 [FGF21] has beneficial effects on steatosis, liver injury, and fibrosis in NASH.”
Investigators conducted a phase 2 multicenter, double-blind, placebo-controlled study of 74 NASH patients to test BMS-986036, a pegylated version of FGF21.
Patients were an average of 51 years old, most were women (64%), who were predominantly white (96%), with a mean hepatic fat fraction of 19%.
Patients received either a 10-mg treatment daily, a 20-mg treatment weekly, or placebo, over the course of 16 weeks, with patients distributed equally among the three arms.
Overall hepatic fat fraction among the daily and weekly treatment groups reduced by 6.8% and 5.2%, respectively, compared with the placebo group, which reduced by 1.3% (P less than .001).
Patients in the treatment arms also saw improvement in average adiponectin levels, growing 15.3% in the daily arm and 15.7% in the weekly arm. Meanwhile, adiponectin levels dropped by an average of 3.5% in the placebo group.
In investigating serum Pro-C3 levels, which are associated with fibrosis, patients in the daily and weekly treatment group saw an average drop of 29% and 19%, respectively, as opposed to an increase of 2% in the placebo group (P less than .0001).
Patients in the treatment groups saw no serious adverse effects, and no patients died during the study.
Dr. Sanyal received funding for this study from Bristol-Myers Squibb and reported receiving financial compensation from Pfizer, Nimbus, Novartis, AstraZeneca, and other similar companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ezimmerman@frontlinemedcom.com
On Twitter @eaztweets
AT THE LIVER MEETING 2017
More physicians excluded from MIPS under final CMS rule
More doctors will be exempt from participation in the Merit-Based Incentive Payment System in 2018, under a final rule issued by the Health & Human Service department.
The final rule excludes from MIPS participation any health care providers who are part of an advanced alternative payment model (APM), those who have $90,000 or less in Medicare Part B billings or who see 200 or fewer Medicare patients. For the 2017 reporting year, those levels were $30,000 and 100 patients.
In comments when the rule was a draft, many organizations suggested that CMS allow clinicians who are ready to participate in MIPS to opt in even if they fall into the MIPS low-volume threshold category. While the agency did not codify this suggestion, officials noted that they intend “revisit this policy in future rule making and are seeking comment on methods to implement this policy in a low-burden manner.”
Medical societies were generally in favor of the new higher threshold, but it was met with resistance from associations representing group practices.
“The transition to value is challenging and CMS understandably want to ease providers into value,” Jerry Penso, MD, president and CEO of the American Medical Group Association said in a statement. “But excluding providers isn’t the same as learning how to deliver care in a value-based world. Taking accountability for the quality and cost of care requires years of experience. Despite CMS’ intentions to ensure a smooth transition, AMGA is concerned that this rule actually hinders the prospects for value-based care.”
CMS is providing a number of enhancements for small practices participating in MIPS.
Small practices (15 or fewer providers) will get five bonus points under MIPS and will continue to earn points for partial data reporting of quality measures. They also will be able to join virtual groups to help aggregate their reporting and improve abilities to access payment bonuses. (Find a link to download a virtual group toolkit on page 4 of the CMS fact sheet for the final rule.)
CMS also is slowly phasing into the cost performance category, which will account for 10% of a MIPS score and will include Medicare spending per beneficiary and total per capita cost measures. These measures are carried over from the Value Modifier program and will require no action from providers to calculate. CMS will measure the performance in this category.
Finally, the agency included a hardship exemption for those affected by major hurricanes in the Gulf Coast and Puerto Rico in 2017. Currently, those who lost access to their EHRs because of the hurricanes, other natural disasters, or public health emergencies, they can file a hardship exemption to have their Advancing Care Information (formerly the meaningful use program) score reweighted to reflect the issues. Applications must be filed by Dec. 31, 2017. The final rule extends the reweighting policy to the other three categories (quality, cost, and improvement activities) through the 2018 performance year, with a deadline of Dec. 31, 2018, to file for a hardship exemption.
“Because our policies relating to reweighting the quality, cost, and improvement activities performance categories are not effective until next year, we are issuing an interim final rule for automatic extreme and uncontrollable circumstances where clinicians can be exempt form these categories in the transition year without submitting a hardship exception application,” CMS noted in the fact sheet. For 2017, that means clinicians in areas affected by the hurricanes who do not submit data will not receive any negative adjustment, Clinicians who do submit data will be scored as usual.
On the advanced APM track, under which physicians take on more risk in exchange for a potential for greater bonus payments, CMS said it is making it easier for clinicians to participate, including extending certain revenue and expenditure provisions for an additional 2 years that are used to determine nominal risk, changing the medical home models to slower the increase of the minimal amount of financial risk taken on, and making it easier for clinicians to earn bonus payments for APMs that begin or end mid-year.
The final rule is scheduled for publication in the Federal Register on Nov. 16.
More doctors will be exempt from participation in the Merit-Based Incentive Payment System in 2018, under a final rule issued by the Health & Human Service department.
The final rule excludes from MIPS participation any health care providers who are part of an advanced alternative payment model (APM), those who have $90,000 or less in Medicare Part B billings or who see 200 or fewer Medicare patients. For the 2017 reporting year, those levels were $30,000 and 100 patients.
In comments when the rule was a draft, many organizations suggested that CMS allow clinicians who are ready to participate in MIPS to opt in even if they fall into the MIPS low-volume threshold category. While the agency did not codify this suggestion, officials noted that they intend “revisit this policy in future rule making and are seeking comment on methods to implement this policy in a low-burden manner.”
Medical societies were generally in favor of the new higher threshold, but it was met with resistance from associations representing group practices.
“The transition to value is challenging and CMS understandably want to ease providers into value,” Jerry Penso, MD, president and CEO of the American Medical Group Association said in a statement. “But excluding providers isn’t the same as learning how to deliver care in a value-based world. Taking accountability for the quality and cost of care requires years of experience. Despite CMS’ intentions to ensure a smooth transition, AMGA is concerned that this rule actually hinders the prospects for value-based care.”
CMS is providing a number of enhancements for small practices participating in MIPS.
Small practices (15 or fewer providers) will get five bonus points under MIPS and will continue to earn points for partial data reporting of quality measures. They also will be able to join virtual groups to help aggregate their reporting and improve abilities to access payment bonuses. (Find a link to download a virtual group toolkit on page 4 of the CMS fact sheet for the final rule.)
CMS also is slowly phasing into the cost performance category, which will account for 10% of a MIPS score and will include Medicare spending per beneficiary and total per capita cost measures. These measures are carried over from the Value Modifier program and will require no action from providers to calculate. CMS will measure the performance in this category.
Finally, the agency included a hardship exemption for those affected by major hurricanes in the Gulf Coast and Puerto Rico in 2017. Currently, those who lost access to their EHRs because of the hurricanes, other natural disasters, or public health emergencies, they can file a hardship exemption to have their Advancing Care Information (formerly the meaningful use program) score reweighted to reflect the issues. Applications must be filed by Dec. 31, 2017. The final rule extends the reweighting policy to the other three categories (quality, cost, and improvement activities) through the 2018 performance year, with a deadline of Dec. 31, 2018, to file for a hardship exemption.
“Because our policies relating to reweighting the quality, cost, and improvement activities performance categories are not effective until next year, we are issuing an interim final rule for automatic extreme and uncontrollable circumstances where clinicians can be exempt form these categories in the transition year without submitting a hardship exception application,” CMS noted in the fact sheet. For 2017, that means clinicians in areas affected by the hurricanes who do not submit data will not receive any negative adjustment, Clinicians who do submit data will be scored as usual.
On the advanced APM track, under which physicians take on more risk in exchange for a potential for greater bonus payments, CMS said it is making it easier for clinicians to participate, including extending certain revenue and expenditure provisions for an additional 2 years that are used to determine nominal risk, changing the medical home models to slower the increase of the minimal amount of financial risk taken on, and making it easier for clinicians to earn bonus payments for APMs that begin or end mid-year.
The final rule is scheduled for publication in the Federal Register on Nov. 16.
More doctors will be exempt from participation in the Merit-Based Incentive Payment System in 2018, under a final rule issued by the Health & Human Service department.
The final rule excludes from MIPS participation any health care providers who are part of an advanced alternative payment model (APM), those who have $90,000 or less in Medicare Part B billings or who see 200 or fewer Medicare patients. For the 2017 reporting year, those levels were $30,000 and 100 patients.
In comments when the rule was a draft, many organizations suggested that CMS allow clinicians who are ready to participate in MIPS to opt in even if they fall into the MIPS low-volume threshold category. While the agency did not codify this suggestion, officials noted that they intend “revisit this policy in future rule making and are seeking comment on methods to implement this policy in a low-burden manner.”
Medical societies were generally in favor of the new higher threshold, but it was met with resistance from associations representing group practices.
“The transition to value is challenging and CMS understandably want to ease providers into value,” Jerry Penso, MD, president and CEO of the American Medical Group Association said in a statement. “But excluding providers isn’t the same as learning how to deliver care in a value-based world. Taking accountability for the quality and cost of care requires years of experience. Despite CMS’ intentions to ensure a smooth transition, AMGA is concerned that this rule actually hinders the prospects for value-based care.”
CMS is providing a number of enhancements for small practices participating in MIPS.
Small practices (15 or fewer providers) will get five bonus points under MIPS and will continue to earn points for partial data reporting of quality measures. They also will be able to join virtual groups to help aggregate their reporting and improve abilities to access payment bonuses. (Find a link to download a virtual group toolkit on page 4 of the CMS fact sheet for the final rule.)
CMS also is slowly phasing into the cost performance category, which will account for 10% of a MIPS score and will include Medicare spending per beneficiary and total per capita cost measures. These measures are carried over from the Value Modifier program and will require no action from providers to calculate. CMS will measure the performance in this category.
Finally, the agency included a hardship exemption for those affected by major hurricanes in the Gulf Coast and Puerto Rico in 2017. Currently, those who lost access to their EHRs because of the hurricanes, other natural disasters, or public health emergencies, they can file a hardship exemption to have their Advancing Care Information (formerly the meaningful use program) score reweighted to reflect the issues. Applications must be filed by Dec. 31, 2017. The final rule extends the reweighting policy to the other three categories (quality, cost, and improvement activities) through the 2018 performance year, with a deadline of Dec. 31, 2018, to file for a hardship exemption.
“Because our policies relating to reweighting the quality, cost, and improvement activities performance categories are not effective until next year, we are issuing an interim final rule for automatic extreme and uncontrollable circumstances where clinicians can be exempt form these categories in the transition year without submitting a hardship exception application,” CMS noted in the fact sheet. For 2017, that means clinicians in areas affected by the hurricanes who do not submit data will not receive any negative adjustment, Clinicians who do submit data will be scored as usual.
On the advanced APM track, under which physicians take on more risk in exchange for a potential for greater bonus payments, CMS said it is making it easier for clinicians to participate, including extending certain revenue and expenditure provisions for an additional 2 years that are used to determine nominal risk, changing the medical home models to slower the increase of the minimal amount of financial risk taken on, and making it easier for clinicians to earn bonus payments for APMs that begin or end mid-year.
The final rule is scheduled for publication in the Federal Register on Nov. 16.