User login
ASCO 2024: Treating Myeloma Just Got More Complicated
For brevity’s sake, I’ll focus on trials about newly diagnosed MM and myeloma at first relapse. Here’s my take on how to interpret those studies in light of broader evidence, what I view as their key limitations, and how what came out of ASCO 2024 changes my approach.
The Return of Belantamab
Belantamab, a BCMA targeting antibody-drug conjugate, previously had shown a response rate of 34% in a single-arm, heavily pretreated population, albeit with modest progression free survival (PFS), only to fail its confirmatory randomized study against pomalidomide/dexamethasone. Given the ocular toxicity associated with belantamab, many — including myself — had written off this drug (save in exceptional/unique circumstances), especially with the rise of novel immunotherapies targeting BCMA, such as chimeric antigen receptor (CAR T-cell) therapy and bispecific antibodies.
However, this year at ASCO, two key randomized trials were presented with concurrent publications, a trial of belantamab/bortezomib/dexamethasone versus daratumumab/bortezomib/dexamethasone (DVd) (DREAMM-7), and a trial of belantamab/pomalidomide/dexamethasone versus bortezomib/pomalidomide/dexamethasone (DREAMM-8). Both trials evaluated patients with myeloma who had relapsed disease and had received at least one prior line of therapy.
In both trials, the belantamab triplet beat the other triplets for the endpoint of PFS (median PFS 36.6 vs 13 months for DREAMM-7, and 12 months PFS 71% vs 51% for DREAMM-8). We must commend the bold three-versus-three design and a convincing result.
What are the caveats? Some censoring of information happened in DREAMM-7, which helped make the intervention arm look better than reality and the control arm look even worse than reality. To illustrate this point: the control arm of DVd (PFS 13 months) underperformed, compared to the CASTOR trial, where DVd led to a PFS of 16.7 months. The drug remains toxic, with high rates of keratopathy and vision problems in its current dosing schema. (Perhaps the future lies in less frequent dosing.) This toxicity is almost always reversible, but it is a huge problem to deal with, and our current quality-of-life instruments fail miserably at capturing this.
Furthermore, DVd is now emerging as perhaps the weakest daratumumab triplet that exists. Almost all patients in this trial had disease sensitivity to lenalidomide, and daratumumab/lenalidomide/dexamethasone (PFS of 45 months in the POLLUX trial) is unequivocally easier to use and handle (in my opinion) than this belantamab triplet--which is quite literally “an eyesore.” Would belantamab-based triplets beat dara/len/dex for patients with lenalidomide sensitive disease? Or, for that matter, would belantamab combos beat anti-CD38+carfilzomib+dex combinations, or cilta-cel (which is also now approved for first relapse)?
How do I foresee the future of belantamab? Despite these unequivocally positive results, I am not enthused about using it for most patients at first relapse. When trials for bispecifics at first relapse read out, my enthusiasm will likely wane even more. Still, it is useful to have belantamab in the armamentarium. For some patients perceived to be at very high risk of infection, belantamab-based triplets may indeed prove to be a better option than bispecifics. However, I suspect that with better dosing strategies for bispecifics, perhaps even that trend may be mitigated. Since we do not yet have bispecifics available in this line, my suggested algorithm for first relapse is as follows: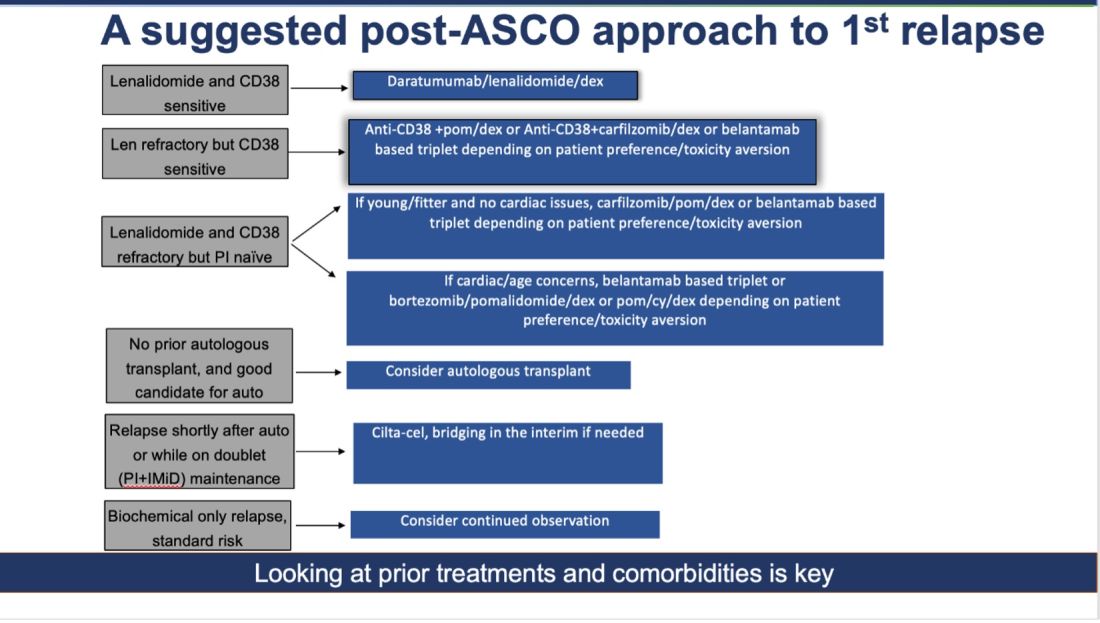
Newly Diagnosed MM: The Era of Quads Solidifies
At ASCO 2024, two key trials with concurrent publications assessed the role of quadruplets (without the use of transplant): the IMROZ trial of a quadruplet of isatuximab/bortezomib/lenalidomide/dexamethasone versus bortezomib/lenalidomide/dexamethasone (VRd), and the BENEFIT trial (isatuximab/lenalidomide/bortezomib/dexamethasone versus isatuximab/lenalidomide/dexamethasone).
The IMROZ trial tested the addition of an anti-CD38 antibody to a triplet backbone, and the results are compelling. The PFS was not reached for the quad vs 54 months for VRd. Unlike in the belantamab trial (where the control arm underperformed), here the control arm really overperformed. In this case, we have never seen such a compelling PFS of 54 months for VRd before. (Based on other trials, VRd PFS has been more in the ballpark of 35-43 months.) This speaks to the fitness and biology of the patients enrolled in this trial, and perhaps to how we will not see such stellar results with this quad recreated in real life.
The addition of isatuximab did not seem to impair quality of life, and although there were more treatment-related deaths with isatuximab, those higher numbers seem to have been driven by longer treatment durations. For this study, the upper age limit was 80 years, and most patients enrolled had an excellent functional status--making it clear that frail patients were greatly underrepresented.
What can we conclude from this study? For fit, older patients (who would have been transplant-eligible in the United States), this study provides excellent proof of concept that very good outcomes can be obtained without the use of transplantation. In treating frail patients, we do not know if quads are safe (or even necessary, compared to gentler sequencing), so these data are not applicable.
High-risk cytogenetics were underrepresented, and although the subgroup analysis for such patients did not show a benefit, it is hard to draw conclusions either way. For me, this trial is further evidence that for many older patients with MM, even if you “can” do a transplant, you probably “shouldn’t, they will experience increasingly better outcomes.
The standard for newly diagnosed MM in older patients for whom transplant is not intended is currently dara/len/dex. Is isa/bort/len/dex better? I do not know. It may give a better PFS, but the addition of bortezomib will lead to more neuropathy: 60% of patients developed neuropathy here, with 7% developing Grade III/IV peripheral neuropathy.
To resolve this issue, highly individualized discussions with patients will be needed. The BENEFIT trial evaluated this question more directly, with a randomized comparison of Isa-VRd versus Isa-Rd (the role of bortezomib being the main variable assessed here) with a primary endpoint of MRD negativity at 10-5 at 18 months. Although MRD negativity allows for a quick read-out, having MRD as an endpoint is a foregone conclusion. Adding another drug will almost certainly lead to deeper responses. But is it worth it?
In the BENEFIT trial, the MRD negativity at 10-5 was 26% versus 53% with the quad. However, peripheral neuropathy rates were much higher with the quad (28% vs 52%). Without longer-term data such as PFS and OS, I do not know whether it is worth the extra risks of neuropathy for older patients. Their priority may not be eradication of cancer cells at all costs. Instead, it may be better quality of life and functioning while preserving survival.
To sum up: Post-ASCO 2024, the approach to newly diagnosed MM just got a lot more complicated. For fit, older patients willing to endure extra toxicities of neuropathy (and acknowledging that we do not know whether survival will be any better with this approach), a quad is a very reasonable option to offer while forgoing transplant, in resource-rich areas of the world, such as the United States. Omitting a transplant now seems very reasonable for most older adults. However, a nuanced and individualized approach remains paramount. And given the speed of new developments, even this suggested approach will be outdated soon!
Dr. Mohyuddin is assistant professor in the multiple myeloma program at the Huntsman Cancer Institute at the University of Utah in Salt Lake City.
For brevity’s sake, I’ll focus on trials about newly diagnosed MM and myeloma at first relapse. Here’s my take on how to interpret those studies in light of broader evidence, what I view as their key limitations, and how what came out of ASCO 2024 changes my approach.
The Return of Belantamab
Belantamab, a BCMA targeting antibody-drug conjugate, previously had shown a response rate of 34% in a single-arm, heavily pretreated population, albeit with modest progression free survival (PFS), only to fail its confirmatory randomized study against pomalidomide/dexamethasone. Given the ocular toxicity associated with belantamab, many — including myself — had written off this drug (save in exceptional/unique circumstances), especially with the rise of novel immunotherapies targeting BCMA, such as chimeric antigen receptor (CAR T-cell) therapy and bispecific antibodies.
However, this year at ASCO, two key randomized trials were presented with concurrent publications, a trial of belantamab/bortezomib/dexamethasone versus daratumumab/bortezomib/dexamethasone (DVd) (DREAMM-7), and a trial of belantamab/pomalidomide/dexamethasone versus bortezomib/pomalidomide/dexamethasone (DREAMM-8). Both trials evaluated patients with myeloma who had relapsed disease and had received at least one prior line of therapy.
In both trials, the belantamab triplet beat the other triplets for the endpoint of PFS (median PFS 36.6 vs 13 months for DREAMM-7, and 12 months PFS 71% vs 51% for DREAMM-8). We must commend the bold three-versus-three design and a convincing result.
What are the caveats? Some censoring of information happened in DREAMM-7, which helped make the intervention arm look better than reality and the control arm look even worse than reality. To illustrate this point: the control arm of DVd (PFS 13 months) underperformed, compared to the CASTOR trial, where DVd led to a PFS of 16.7 months. The drug remains toxic, with high rates of keratopathy and vision problems in its current dosing schema. (Perhaps the future lies in less frequent dosing.) This toxicity is almost always reversible, but it is a huge problem to deal with, and our current quality-of-life instruments fail miserably at capturing this.
Furthermore, DVd is now emerging as perhaps the weakest daratumumab triplet that exists. Almost all patients in this trial had disease sensitivity to lenalidomide, and daratumumab/lenalidomide/dexamethasone (PFS of 45 months in the POLLUX trial) is unequivocally easier to use and handle (in my opinion) than this belantamab triplet--which is quite literally “an eyesore.” Would belantamab-based triplets beat dara/len/dex for patients with lenalidomide sensitive disease? Or, for that matter, would belantamab combos beat anti-CD38+carfilzomib+dex combinations, or cilta-cel (which is also now approved for first relapse)?
How do I foresee the future of belantamab? Despite these unequivocally positive results, I am not enthused about using it for most patients at first relapse. When trials for bispecifics at first relapse read out, my enthusiasm will likely wane even more. Still, it is useful to have belantamab in the armamentarium. For some patients perceived to be at very high risk of infection, belantamab-based triplets may indeed prove to be a better option than bispecifics. However, I suspect that with better dosing strategies for bispecifics, perhaps even that trend may be mitigated. Since we do not yet have bispecifics available in this line, my suggested algorithm for first relapse is as follows:
Newly Diagnosed MM: The Era of Quads Solidifies
At ASCO 2024, two key trials with concurrent publications assessed the role of quadruplets (without the use of transplant): the IMROZ trial of a quadruplet of isatuximab/bortezomib/lenalidomide/dexamethasone versus bortezomib/lenalidomide/dexamethasone (VRd), and the BENEFIT trial (isatuximab/lenalidomide/bortezomib/dexamethasone versus isatuximab/lenalidomide/dexamethasone).
The IMROZ trial tested the addition of an anti-CD38 antibody to a triplet backbone, and the results are compelling. The PFS was not reached for the quad vs 54 months for VRd. Unlike in the belantamab trial (where the control arm underperformed), here the control arm really overperformed. In this case, we have never seen such a compelling PFS of 54 months for VRd before. (Based on other trials, VRd PFS has been more in the ballpark of 35-43 months.) This speaks to the fitness and biology of the patients enrolled in this trial, and perhaps to how we will not see such stellar results with this quad recreated in real life.
The addition of isatuximab did not seem to impair quality of life, and although there were more treatment-related deaths with isatuximab, those higher numbers seem to have been driven by longer treatment durations. For this study, the upper age limit was 80 years, and most patients enrolled had an excellent functional status--making it clear that frail patients were greatly underrepresented.
What can we conclude from this study? For fit, older patients (who would have been transplant-eligible in the United States), this study provides excellent proof of concept that very good outcomes can be obtained without the use of transplantation. In treating frail patients, we do not know if quads are safe (or even necessary, compared to gentler sequencing), so these data are not applicable.
High-risk cytogenetics were underrepresented, and although the subgroup analysis for such patients did not show a benefit, it is hard to draw conclusions either way. For me, this trial is further evidence that for many older patients with MM, even if you “can” do a transplant, you probably “shouldn’t, they will experience increasingly better outcomes.
The standard for newly diagnosed MM in older patients for whom transplant is not intended is currently dara/len/dex. Is isa/bort/len/dex better? I do not know. It may give a better PFS, but the addition of bortezomib will lead to more neuropathy: 60% of patients developed neuropathy here, with 7% developing Grade III/IV peripheral neuropathy.
To resolve this issue, highly individualized discussions with patients will be needed. The BENEFIT trial evaluated this question more directly, with a randomized comparison of Isa-VRd versus Isa-Rd (the role of bortezomib being the main variable assessed here) with a primary endpoint of MRD negativity at 10-5 at 18 months. Although MRD negativity allows for a quick read-out, having MRD as an endpoint is a foregone conclusion. Adding another drug will almost certainly lead to deeper responses. But is it worth it?
In the BENEFIT trial, the MRD negativity at 10-5 was 26% versus 53% with the quad. However, peripheral neuropathy rates were much higher with the quad (28% vs 52%). Without longer-term data such as PFS and OS, I do not know whether it is worth the extra risks of neuropathy for older patients. Their priority may not be eradication of cancer cells at all costs. Instead, it may be better quality of life and functioning while preserving survival.
To sum up: Post-ASCO 2024, the approach to newly diagnosed MM just got a lot more complicated. For fit, older patients willing to endure extra toxicities of neuropathy (and acknowledging that we do not know whether survival will be any better with this approach), a quad is a very reasonable option to offer while forgoing transplant, in resource-rich areas of the world, such as the United States. Omitting a transplant now seems very reasonable for most older adults. However, a nuanced and individualized approach remains paramount. And given the speed of new developments, even this suggested approach will be outdated soon!
Dr. Mohyuddin is assistant professor in the multiple myeloma program at the Huntsman Cancer Institute at the University of Utah in Salt Lake City.
For brevity’s sake, I’ll focus on trials about newly diagnosed MM and myeloma at first relapse. Here’s my take on how to interpret those studies in light of broader evidence, what I view as their key limitations, and how what came out of ASCO 2024 changes my approach.
The Return of Belantamab
Belantamab, a BCMA targeting antibody-drug conjugate, previously had shown a response rate of 34% in a single-arm, heavily pretreated population, albeit with modest progression free survival (PFS), only to fail its confirmatory randomized study against pomalidomide/dexamethasone. Given the ocular toxicity associated with belantamab, many — including myself — had written off this drug (save in exceptional/unique circumstances), especially with the rise of novel immunotherapies targeting BCMA, such as chimeric antigen receptor (CAR T-cell) therapy and bispecific antibodies.
However, this year at ASCO, two key randomized trials were presented with concurrent publications, a trial of belantamab/bortezomib/dexamethasone versus daratumumab/bortezomib/dexamethasone (DVd) (DREAMM-7), and a trial of belantamab/pomalidomide/dexamethasone versus bortezomib/pomalidomide/dexamethasone (DREAMM-8). Both trials evaluated patients with myeloma who had relapsed disease and had received at least one prior line of therapy.
In both trials, the belantamab triplet beat the other triplets for the endpoint of PFS (median PFS 36.6 vs 13 months for DREAMM-7, and 12 months PFS 71% vs 51% for DREAMM-8). We must commend the bold three-versus-three design and a convincing result.
What are the caveats? Some censoring of information happened in DREAMM-7, which helped make the intervention arm look better than reality and the control arm look even worse than reality. To illustrate this point: the control arm of DVd (PFS 13 months) underperformed, compared to the CASTOR trial, where DVd led to a PFS of 16.7 months. The drug remains toxic, with high rates of keratopathy and vision problems in its current dosing schema. (Perhaps the future lies in less frequent dosing.) This toxicity is almost always reversible, but it is a huge problem to deal with, and our current quality-of-life instruments fail miserably at capturing this.
Furthermore, DVd is now emerging as perhaps the weakest daratumumab triplet that exists. Almost all patients in this trial had disease sensitivity to lenalidomide, and daratumumab/lenalidomide/dexamethasone (PFS of 45 months in the POLLUX trial) is unequivocally easier to use and handle (in my opinion) than this belantamab triplet--which is quite literally “an eyesore.” Would belantamab-based triplets beat dara/len/dex for patients with lenalidomide sensitive disease? Or, for that matter, would belantamab combos beat anti-CD38+carfilzomib+dex combinations, or cilta-cel (which is also now approved for first relapse)?
How do I foresee the future of belantamab? Despite these unequivocally positive results, I am not enthused about using it for most patients at first relapse. When trials for bispecifics at first relapse read out, my enthusiasm will likely wane even more. Still, it is useful to have belantamab in the armamentarium. For some patients perceived to be at very high risk of infection, belantamab-based triplets may indeed prove to be a better option than bispecifics. However, I suspect that with better dosing strategies for bispecifics, perhaps even that trend may be mitigated. Since we do not yet have bispecifics available in this line, my suggested algorithm for first relapse is as follows:
Newly Diagnosed MM: The Era of Quads Solidifies
At ASCO 2024, two key trials with concurrent publications assessed the role of quadruplets (without the use of transplant): the IMROZ trial of a quadruplet of isatuximab/bortezomib/lenalidomide/dexamethasone versus bortezomib/lenalidomide/dexamethasone (VRd), and the BENEFIT trial (isatuximab/lenalidomide/bortezomib/dexamethasone versus isatuximab/lenalidomide/dexamethasone).
The IMROZ trial tested the addition of an anti-CD38 antibody to a triplet backbone, and the results are compelling. The PFS was not reached for the quad vs 54 months for VRd. Unlike in the belantamab trial (where the control arm underperformed), here the control arm really overperformed. In this case, we have never seen such a compelling PFS of 54 months for VRd before. (Based on other trials, VRd PFS has been more in the ballpark of 35-43 months.) This speaks to the fitness and biology of the patients enrolled in this trial, and perhaps to how we will not see such stellar results with this quad recreated in real life.
The addition of isatuximab did not seem to impair quality of life, and although there were more treatment-related deaths with isatuximab, those higher numbers seem to have been driven by longer treatment durations. For this study, the upper age limit was 80 years, and most patients enrolled had an excellent functional status--making it clear that frail patients were greatly underrepresented.
What can we conclude from this study? For fit, older patients (who would have been transplant-eligible in the United States), this study provides excellent proof of concept that very good outcomes can be obtained without the use of transplantation. In treating frail patients, we do not know if quads are safe (or even necessary, compared to gentler sequencing), so these data are not applicable.
High-risk cytogenetics were underrepresented, and although the subgroup analysis for such patients did not show a benefit, it is hard to draw conclusions either way. For me, this trial is further evidence that for many older patients with MM, even if you “can” do a transplant, you probably “shouldn’t, they will experience increasingly better outcomes.
The standard for newly diagnosed MM in older patients for whom transplant is not intended is currently dara/len/dex. Is isa/bort/len/dex better? I do not know. It may give a better PFS, but the addition of bortezomib will lead to more neuropathy: 60% of patients developed neuropathy here, with 7% developing Grade III/IV peripheral neuropathy.
To resolve this issue, highly individualized discussions with patients will be needed. The BENEFIT trial evaluated this question more directly, with a randomized comparison of Isa-VRd versus Isa-Rd (the role of bortezomib being the main variable assessed here) with a primary endpoint of MRD negativity at 10-5 at 18 months. Although MRD negativity allows for a quick read-out, having MRD as an endpoint is a foregone conclusion. Adding another drug will almost certainly lead to deeper responses. But is it worth it?
In the BENEFIT trial, the MRD negativity at 10-5 was 26% versus 53% with the quad. However, peripheral neuropathy rates were much higher with the quad (28% vs 52%). Without longer-term data such as PFS and OS, I do not know whether it is worth the extra risks of neuropathy for older patients. Their priority may not be eradication of cancer cells at all costs. Instead, it may be better quality of life and functioning while preserving survival.
To sum up: Post-ASCO 2024, the approach to newly diagnosed MM just got a lot more complicated. For fit, older patients willing to endure extra toxicities of neuropathy (and acknowledging that we do not know whether survival will be any better with this approach), a quad is a very reasonable option to offer while forgoing transplant, in resource-rich areas of the world, such as the United States. Omitting a transplant now seems very reasonable for most older adults. However, a nuanced and individualized approach remains paramount. And given the speed of new developments, even this suggested approach will be outdated soon!
Dr. Mohyuddin is assistant professor in the multiple myeloma program at the Huntsman Cancer Institute at the University of Utah in Salt Lake City.
Cardiovascular Health Becoming a Major Risk Factor for Dementia
That’s according to researchers from University College London (UCL) in the United Kingdom who analyzed 27 papers about dementia that had data collected over more than 70 years. They calculated what share of dementia cases were due to different risk factors. Their findings were recently published in the Lancet Public Health.
Top risk factors for dementia over the years have been hypertension, obesity, diabetes, education, and smoking, according to a news release on the findings. But the prevalence of risk factors has changed over the decades.
Researchers said smoking and education have become less important risk factors because of “population-level interventions,” such as stop-smoking campaigns and compulsory public education. On the other hand, obesity and diabetes rates have increased and become bigger risk factors.
Hypertension remains the greatest risk factor, even though doctors and public health groups are putting more emphasis on managing the condition, the study said.
“Cardiovascular risk factors may have contributed more to dementia risk over time, so these deserve more targeted action for future dementia prevention efforts,” said Naaheed Mukadam, PhD, an associate professor at UCL and the lead author of the study.
Eliminating modifiable risk factors could theoretically prevent 40% of dementia cases, the release said.
The CDC says that an estimated 5.8 million people in the United States have Alzheimer’s disease and related dementias, including 5.6 million people ages 65 and older and about 200,000 under age 65. The UCL release said an estimated 944,000 in the U.K. have dementia.
A version of this article first appeared on WebMD.com.
That’s according to researchers from University College London (UCL) in the United Kingdom who analyzed 27 papers about dementia that had data collected over more than 70 years. They calculated what share of dementia cases were due to different risk factors. Their findings were recently published in the Lancet Public Health.
Top risk factors for dementia over the years have been hypertension, obesity, diabetes, education, and smoking, according to a news release on the findings. But the prevalence of risk factors has changed over the decades.
Researchers said smoking and education have become less important risk factors because of “population-level interventions,” such as stop-smoking campaigns and compulsory public education. On the other hand, obesity and diabetes rates have increased and become bigger risk factors.
Hypertension remains the greatest risk factor, even though doctors and public health groups are putting more emphasis on managing the condition, the study said.
“Cardiovascular risk factors may have contributed more to dementia risk over time, so these deserve more targeted action for future dementia prevention efforts,” said Naaheed Mukadam, PhD, an associate professor at UCL and the lead author of the study.
Eliminating modifiable risk factors could theoretically prevent 40% of dementia cases, the release said.
The CDC says that an estimated 5.8 million people in the United States have Alzheimer’s disease and related dementias, including 5.6 million people ages 65 and older and about 200,000 under age 65. The UCL release said an estimated 944,000 in the U.K. have dementia.
A version of this article first appeared on WebMD.com.
That’s according to researchers from University College London (UCL) in the United Kingdom who analyzed 27 papers about dementia that had data collected over more than 70 years. They calculated what share of dementia cases were due to different risk factors. Their findings were recently published in the Lancet Public Health.
Top risk factors for dementia over the years have been hypertension, obesity, diabetes, education, and smoking, according to a news release on the findings. But the prevalence of risk factors has changed over the decades.
Researchers said smoking and education have become less important risk factors because of “population-level interventions,” such as stop-smoking campaigns and compulsory public education. On the other hand, obesity and diabetes rates have increased and become bigger risk factors.
Hypertension remains the greatest risk factor, even though doctors and public health groups are putting more emphasis on managing the condition, the study said.
“Cardiovascular risk factors may have contributed more to dementia risk over time, so these deserve more targeted action for future dementia prevention efforts,” said Naaheed Mukadam, PhD, an associate professor at UCL and the lead author of the study.
Eliminating modifiable risk factors could theoretically prevent 40% of dementia cases, the release said.
The CDC says that an estimated 5.8 million people in the United States have Alzheimer’s disease and related dementias, including 5.6 million people ages 65 and older and about 200,000 under age 65. The UCL release said an estimated 944,000 in the U.K. have dementia.
A version of this article first appeared on WebMD.com.
FROM THE LANCET PUBLIC HEALTH
Revised Criteria for Alzheimer’s Diagnosis, Staging Released
, including a new biomarker classification system that incorporates fluid and imaging biomarkers as well as an updated disease staging system.
“Plasma markers are here now, and it’s very important to incorporate them into the criteria for diagnosis,” said senior author Maria C. Carrillo, PhD, Alzheimer’s Association chief science officer and medical affairs lead.
The revised criteria are the first updates since 2018.
“Defining diseases biologically, rather than based on syndromic presentation, has long been standard in many areas of medicine — including cancer, heart disease, and diabetes — and is becoming a unifying concept common to all neurodegenerative diseases,” lead author Clifford Jack Jr, MD, with Mayo Clinic, Rochester, Minnesota, said in a news release from the Alzheimer’s Association.
“These updates to the diagnostic criteria are needed now because we know more about the underlying biology of Alzheimer’s and we are able to measure those changes,” Dr. Jack added.
The 2024 revised criteria for diagnosis and staging of Alzheimer’s disease were published online in Alzheimer’s & Dementia.
Core Biomarkers Defined
The revised criteria define Alzheimer’s disease as a biologic process that begins with the appearance of Alzheimer’s disease neuropathologic change (ADNPC) in the absence of symptoms. Progression of the neuropathologic burden leads to the later appearance and progression of clinical symptoms.
The work group organized Alzheimer’s disease biomarkers into three broad categories: (1) core biomarkers of ADNPC, (2) nonspecific biomarkers that are important in Alzheimer’s disease but are also involved in other brain diseases, and (3) biomarkers of diseases or conditions that commonly coexist with Alzheimer’s disease.
Core Alzheimer’s biomarkers are subdivided into Core 1 and Core 2.
Core 1 biomarkers become abnormal early in the disease course and directly measure either amyloid plaques or phosphorylated tau (p-tau). They include amyloid PET; cerebrospinal fluid (CSF) amyloid beta 42/40 ratio, CSF p-tau181/amyloid beta 42 ratio, and CSF total (t)-tau/amyloid beta 42 ratio; and “accurate” plasma biomarkers, such as p-tau217.
“An abnormal Core 1 biomarker result is sufficient to establish a diagnosis of Alzheimer’s disease and to inform clinical decision making [sic] throughout the disease continuum,” the work group wrote.
Core 2 biomarkers become abnormal later in the disease process and are more closely linked with the onset of symptoms. Core 2 biomarkers include tau PET and certain soluble tau fragments associated with tau proteinopathy (eg, MTBR-tau243) but also pT205 and nonphosphorylated mid-region tau fragments.
Core 2 biomarkers, when combined with Core 1, may be used to stage biologic disease severity; abnormal Core 2 biomarkers “increase confidence that Alzheimer’s disease is contributing to symptoms,” the work group noted.
The revised criteria give clinicians “the flexibility to use plasma or PET scans or CSF,” Dr. Carrillo said. “They will have several tools that they can choose from and offer this variety of tools to their patients. We need different tools for different individuals. There will be differences in coverage and access to these diagnostics.”
The revised criteria also include an integrated biologic and clinical staging scheme that acknowledges the fact that common co-pathologies, cognitive reserve, and resistance may modify relationships between clinical and biologic Alzheimer’s disease stages.
Formal Guidelines to Come
The work group noted that currently, the clinical use of Alzheimer’s disease biomarkers is intended for the evaluation of symptomatic patients, not cognitively unimpaired individuals.
Disease-targeted therapies have not yet been approved for cognitively unimpaired individuals. For this reason, the work group currently recommends against diagnostic testing in cognitively unimpaired individuals outside the context of observational or therapeutic research studies.
This recommendation would change in the future if disease-targeted therapies that are currently being evaluated in trials demonstrate a benefit in preventing cognitive decline and are approved for use in preclinical Alzheimer’s disease, they wrote.
They emphasize that the revised criteria are not intended to provide step-by-step clinical practice guidelines for clinicians. Rather, they provide general principles to inform diagnosis and staging of Alzheimer’s disease that reflect current science.
“This is just the beginning,” said Dr. Carrillo. “This is a gathering of the evidence to date and putting it in one place so we can have a consensus and actually a way to test it and make it better as we add new science.”
This also serves as a “springboard” for the Alzheimer’s Association to create formal clinical guidelines. “That will come, hopefully, over the next 12 months. We’ll be working on it, and we hope to have that in 2025,” Dr. Carrillo said.
The revised criteria also emphasize the role of the clinician.
“The biologically based diagnosis of Alzheimer’s disease is meant to assist, rather than supplant, the clinical evaluation of individuals with cognitive impairment,” the work group wrote in a related commentary published online in Nature Medicine.
Recent diagnostics and therapeutic developments “herald a virtuous cycle in which improvements in diagnostic methods enable more sophisticated treatment approaches, which in turn steer advances in diagnostic methods,” they continued. “An unchanging principle, however, is that effective treatment will always rely on the ability to diagnose and stage the biology driving the disease process.”
Funding for this research was provided by the National Institutes of Health, Alexander family professorship, GHR Foundation, Alzheimer’s Association, Veterans Administration, Life Molecular Imaging, Michael J. Fox Foundation for Parkinson’s Research, Avid Radiopharmaceuticals, Eli Lilly, Gates Foundation, Biogen, C2N Diagnostics, Eisai, Fujirebio, GE Healthcare, Roche, National Institute on Aging, Roche/Genentech, BrightFocus Foundation, Hoffmann-La Roche, Novo Nordisk, Toyama, National MS Society, Alzheimer Drug Discovery Foundation, and others. A complete list of donors and disclosures is included in the original article.
A version of this article appeared on Medscape.com.
, including a new biomarker classification system that incorporates fluid and imaging biomarkers as well as an updated disease staging system.
“Plasma markers are here now, and it’s very important to incorporate them into the criteria for diagnosis,” said senior author Maria C. Carrillo, PhD, Alzheimer’s Association chief science officer and medical affairs lead.
The revised criteria are the first updates since 2018.
“Defining diseases biologically, rather than based on syndromic presentation, has long been standard in many areas of medicine — including cancer, heart disease, and diabetes — and is becoming a unifying concept common to all neurodegenerative diseases,” lead author Clifford Jack Jr, MD, with Mayo Clinic, Rochester, Minnesota, said in a news release from the Alzheimer’s Association.
“These updates to the diagnostic criteria are needed now because we know more about the underlying biology of Alzheimer’s and we are able to measure those changes,” Dr. Jack added.
The 2024 revised criteria for diagnosis and staging of Alzheimer’s disease were published online in Alzheimer’s & Dementia.
Core Biomarkers Defined
The revised criteria define Alzheimer’s disease as a biologic process that begins with the appearance of Alzheimer’s disease neuropathologic change (ADNPC) in the absence of symptoms. Progression of the neuropathologic burden leads to the later appearance and progression of clinical symptoms.
The work group organized Alzheimer’s disease biomarkers into three broad categories: (1) core biomarkers of ADNPC, (2) nonspecific biomarkers that are important in Alzheimer’s disease but are also involved in other brain diseases, and (3) biomarkers of diseases or conditions that commonly coexist with Alzheimer’s disease.
Core Alzheimer’s biomarkers are subdivided into Core 1 and Core 2.
Core 1 biomarkers become abnormal early in the disease course and directly measure either amyloid plaques or phosphorylated tau (p-tau). They include amyloid PET; cerebrospinal fluid (CSF) amyloid beta 42/40 ratio, CSF p-tau181/amyloid beta 42 ratio, and CSF total (t)-tau/amyloid beta 42 ratio; and “accurate” plasma biomarkers, such as p-tau217.
“An abnormal Core 1 biomarker result is sufficient to establish a diagnosis of Alzheimer’s disease and to inform clinical decision making [sic] throughout the disease continuum,” the work group wrote.
Core 2 biomarkers become abnormal later in the disease process and are more closely linked with the onset of symptoms. Core 2 biomarkers include tau PET and certain soluble tau fragments associated with tau proteinopathy (eg, MTBR-tau243) but also pT205 and nonphosphorylated mid-region tau fragments.
Core 2 biomarkers, when combined with Core 1, may be used to stage biologic disease severity; abnormal Core 2 biomarkers “increase confidence that Alzheimer’s disease is contributing to symptoms,” the work group noted.
The revised criteria give clinicians “the flexibility to use plasma or PET scans or CSF,” Dr. Carrillo said. “They will have several tools that they can choose from and offer this variety of tools to their patients. We need different tools for different individuals. There will be differences in coverage and access to these diagnostics.”
The revised criteria also include an integrated biologic and clinical staging scheme that acknowledges the fact that common co-pathologies, cognitive reserve, and resistance may modify relationships between clinical and biologic Alzheimer’s disease stages.
Formal Guidelines to Come
The work group noted that currently, the clinical use of Alzheimer’s disease biomarkers is intended for the evaluation of symptomatic patients, not cognitively unimpaired individuals.
Disease-targeted therapies have not yet been approved for cognitively unimpaired individuals. For this reason, the work group currently recommends against diagnostic testing in cognitively unimpaired individuals outside the context of observational or therapeutic research studies.
This recommendation would change in the future if disease-targeted therapies that are currently being evaluated in trials demonstrate a benefit in preventing cognitive decline and are approved for use in preclinical Alzheimer’s disease, they wrote.
They emphasize that the revised criteria are not intended to provide step-by-step clinical practice guidelines for clinicians. Rather, they provide general principles to inform diagnosis and staging of Alzheimer’s disease that reflect current science.
“This is just the beginning,” said Dr. Carrillo. “This is a gathering of the evidence to date and putting it in one place so we can have a consensus and actually a way to test it and make it better as we add new science.”
This also serves as a “springboard” for the Alzheimer’s Association to create formal clinical guidelines. “That will come, hopefully, over the next 12 months. We’ll be working on it, and we hope to have that in 2025,” Dr. Carrillo said.
The revised criteria also emphasize the role of the clinician.
“The biologically based diagnosis of Alzheimer’s disease is meant to assist, rather than supplant, the clinical evaluation of individuals with cognitive impairment,” the work group wrote in a related commentary published online in Nature Medicine.
Recent diagnostics and therapeutic developments “herald a virtuous cycle in which improvements in diagnostic methods enable more sophisticated treatment approaches, which in turn steer advances in diagnostic methods,” they continued. “An unchanging principle, however, is that effective treatment will always rely on the ability to diagnose and stage the biology driving the disease process.”
Funding for this research was provided by the National Institutes of Health, Alexander family professorship, GHR Foundation, Alzheimer’s Association, Veterans Administration, Life Molecular Imaging, Michael J. Fox Foundation for Parkinson’s Research, Avid Radiopharmaceuticals, Eli Lilly, Gates Foundation, Biogen, C2N Diagnostics, Eisai, Fujirebio, GE Healthcare, Roche, National Institute on Aging, Roche/Genentech, BrightFocus Foundation, Hoffmann-La Roche, Novo Nordisk, Toyama, National MS Society, Alzheimer Drug Discovery Foundation, and others. A complete list of donors and disclosures is included in the original article.
A version of this article appeared on Medscape.com.
, including a new biomarker classification system that incorporates fluid and imaging biomarkers as well as an updated disease staging system.
“Plasma markers are here now, and it’s very important to incorporate them into the criteria for diagnosis,” said senior author Maria C. Carrillo, PhD, Alzheimer’s Association chief science officer and medical affairs lead.
The revised criteria are the first updates since 2018.
“Defining diseases biologically, rather than based on syndromic presentation, has long been standard in many areas of medicine — including cancer, heart disease, and diabetes — and is becoming a unifying concept common to all neurodegenerative diseases,” lead author Clifford Jack Jr, MD, with Mayo Clinic, Rochester, Minnesota, said in a news release from the Alzheimer’s Association.
“These updates to the diagnostic criteria are needed now because we know more about the underlying biology of Alzheimer’s and we are able to measure those changes,” Dr. Jack added.
The 2024 revised criteria for diagnosis and staging of Alzheimer’s disease were published online in Alzheimer’s & Dementia.
Core Biomarkers Defined
The revised criteria define Alzheimer’s disease as a biologic process that begins with the appearance of Alzheimer’s disease neuropathologic change (ADNPC) in the absence of symptoms. Progression of the neuropathologic burden leads to the later appearance and progression of clinical symptoms.
The work group organized Alzheimer’s disease biomarkers into three broad categories: (1) core biomarkers of ADNPC, (2) nonspecific biomarkers that are important in Alzheimer’s disease but are also involved in other brain diseases, and (3) biomarkers of diseases or conditions that commonly coexist with Alzheimer’s disease.
Core Alzheimer’s biomarkers are subdivided into Core 1 and Core 2.
Core 1 biomarkers become abnormal early in the disease course and directly measure either amyloid plaques or phosphorylated tau (p-tau). They include amyloid PET; cerebrospinal fluid (CSF) amyloid beta 42/40 ratio, CSF p-tau181/amyloid beta 42 ratio, and CSF total (t)-tau/amyloid beta 42 ratio; and “accurate” plasma biomarkers, such as p-tau217.
“An abnormal Core 1 biomarker result is sufficient to establish a diagnosis of Alzheimer’s disease and to inform clinical decision making [sic] throughout the disease continuum,” the work group wrote.
Core 2 biomarkers become abnormal later in the disease process and are more closely linked with the onset of symptoms. Core 2 biomarkers include tau PET and certain soluble tau fragments associated with tau proteinopathy (eg, MTBR-tau243) but also pT205 and nonphosphorylated mid-region tau fragments.
Core 2 biomarkers, when combined with Core 1, may be used to stage biologic disease severity; abnormal Core 2 biomarkers “increase confidence that Alzheimer’s disease is contributing to symptoms,” the work group noted.
The revised criteria give clinicians “the flexibility to use plasma or PET scans or CSF,” Dr. Carrillo said. “They will have several tools that they can choose from and offer this variety of tools to their patients. We need different tools for different individuals. There will be differences in coverage and access to these diagnostics.”
The revised criteria also include an integrated biologic and clinical staging scheme that acknowledges the fact that common co-pathologies, cognitive reserve, and resistance may modify relationships between clinical and biologic Alzheimer’s disease stages.
Formal Guidelines to Come
The work group noted that currently, the clinical use of Alzheimer’s disease biomarkers is intended for the evaluation of symptomatic patients, not cognitively unimpaired individuals.
Disease-targeted therapies have not yet been approved for cognitively unimpaired individuals. For this reason, the work group currently recommends against diagnostic testing in cognitively unimpaired individuals outside the context of observational or therapeutic research studies.
This recommendation would change in the future if disease-targeted therapies that are currently being evaluated in trials demonstrate a benefit in preventing cognitive decline and are approved for use in preclinical Alzheimer’s disease, they wrote.
They emphasize that the revised criteria are not intended to provide step-by-step clinical practice guidelines for clinicians. Rather, they provide general principles to inform diagnosis and staging of Alzheimer’s disease that reflect current science.
“This is just the beginning,” said Dr. Carrillo. “This is a gathering of the evidence to date and putting it in one place so we can have a consensus and actually a way to test it and make it better as we add new science.”
This also serves as a “springboard” for the Alzheimer’s Association to create formal clinical guidelines. “That will come, hopefully, over the next 12 months. We’ll be working on it, and we hope to have that in 2025,” Dr. Carrillo said.
The revised criteria also emphasize the role of the clinician.
“The biologically based diagnosis of Alzheimer’s disease is meant to assist, rather than supplant, the clinical evaluation of individuals with cognitive impairment,” the work group wrote in a related commentary published online in Nature Medicine.
Recent diagnostics and therapeutic developments “herald a virtuous cycle in which improvements in diagnostic methods enable more sophisticated treatment approaches, which in turn steer advances in diagnostic methods,” they continued. “An unchanging principle, however, is that effective treatment will always rely on the ability to diagnose and stage the biology driving the disease process.”
Funding for this research was provided by the National Institutes of Health, Alexander family professorship, GHR Foundation, Alzheimer’s Association, Veterans Administration, Life Molecular Imaging, Michael J. Fox Foundation for Parkinson’s Research, Avid Radiopharmaceuticals, Eli Lilly, Gates Foundation, Biogen, C2N Diagnostics, Eisai, Fujirebio, GE Healthcare, Roche, National Institute on Aging, Roche/Genentech, BrightFocus Foundation, Hoffmann-La Roche, Novo Nordisk, Toyama, National MS Society, Alzheimer Drug Discovery Foundation, and others. A complete list of donors and disclosures is included in the original article.
A version of this article appeared on Medscape.com.
FROM ALZHEIMER’S & DEMENTIA
Common Cognitive Test Falls Short for Concussion Diagnosis
, a new study showed.
Investigators found that almost half of athletes diagnosed with a concussion tested normally on the Sports Concussion Assessment Tool 5 (SCAT5), the recommended tool for measuring cognitive skills in concussion evaluations. The most accurate measure of concussion was symptoms reported by the athletes.
“If you don’t do well on the cognitive exam, it suggests you have a concussion. But many people who are concussed do fine on the exam,” lead author Kimberly Harmon, MD, professor of family medicine and section head of sports medicine at the University of Washington School of Medicine, Seattle, said in a news release.
The study was published online in JAMA Network Open.
Introduced in 2004, the SCAT was created to standardize the collection of information clinicians use to diagnose concussion, including evaluation of symptoms, orientation, and balance. It also uses a 10-word list to assess immediate memory and delayed recall.
Dr. Harmon’s own experiences as a team physician led her to wonder about the accuracy of the cognitive screening portion of the SCAT. She saw that “some people were concussed, and they did well on the recall test. Some people weren’t concussed, and they didn’t do well. So I thought we should study it,” she said.
Investigators compared 92 National Collegiate Athletic Association (NCAA) Division 1 athletes who had sustained a concussion between 2020 and 2022 and had a concussion evaluation within 48 hours to 92 matched nonconcussed teammates (overall cohort, 52% men). Most concussions occurred in those who played football, followed by volleyball.
All athletes had previously completed NCAA-required baseline concussion screenings. Participants completed the SCAT5 screening test within 2 weeks of the incident concussion.
No significant differences were found between the baseline scores of athletes with and without concussion. Moreover, responses on the word recall section of the SCAT5 held little predictive value for concussion.
Nearly half (45%) of athletes with concussion performed at or even above their baseline cognitive report, which the authors said highlights the limitations of the cognitive components of SCAT5.
The most accurate predictor of concussion was participants’ responses to questions about their symptoms.
“If you get hit in the head and go to the sideline and say, ‘I have a headache, I’m dizzy, I don’t feel right,’ I can say with pretty good assurance that you have a concussion,” Dr. Harmon continued. “I don’t need to do any testing.”
Unfortunately, the problem is “that some athletes don’t want to come out. They don’t report their symptoms or may not recognize their symptoms. So then you need an objective, accurate test to tell you whether you can safely put the athlete back on the field. We don’t have that right now.”
The study did not control for concussion history, and the all–Division 1 cohort means the findings may not be generalizable to other athletes.
Nevertheless, investigators said the study “affirms that reported symptoms are the most sensitive indicator of concussion, and there are limitations to the objective cognitive testing included in the SCAT.” They concluded that concussion “remains a clinical diagnosis that should be based on a thorough review of signs, symptoms, and clinical findings.”
This study was funded in part by donations from University of Washington alumni Jack and Luellen Cherneski and the Chisholm Foundation. Dr. Harmon reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a new study showed.
Investigators found that almost half of athletes diagnosed with a concussion tested normally on the Sports Concussion Assessment Tool 5 (SCAT5), the recommended tool for measuring cognitive skills in concussion evaluations. The most accurate measure of concussion was symptoms reported by the athletes.
“If you don’t do well on the cognitive exam, it suggests you have a concussion. But many people who are concussed do fine on the exam,” lead author Kimberly Harmon, MD, professor of family medicine and section head of sports medicine at the University of Washington School of Medicine, Seattle, said in a news release.
The study was published online in JAMA Network Open.
Introduced in 2004, the SCAT was created to standardize the collection of information clinicians use to diagnose concussion, including evaluation of symptoms, orientation, and balance. It also uses a 10-word list to assess immediate memory and delayed recall.
Dr. Harmon’s own experiences as a team physician led her to wonder about the accuracy of the cognitive screening portion of the SCAT. She saw that “some people were concussed, and they did well on the recall test. Some people weren’t concussed, and they didn’t do well. So I thought we should study it,” she said.
Investigators compared 92 National Collegiate Athletic Association (NCAA) Division 1 athletes who had sustained a concussion between 2020 and 2022 and had a concussion evaluation within 48 hours to 92 matched nonconcussed teammates (overall cohort, 52% men). Most concussions occurred in those who played football, followed by volleyball.
All athletes had previously completed NCAA-required baseline concussion screenings. Participants completed the SCAT5 screening test within 2 weeks of the incident concussion.
No significant differences were found between the baseline scores of athletes with and without concussion. Moreover, responses on the word recall section of the SCAT5 held little predictive value for concussion.
Nearly half (45%) of athletes with concussion performed at or even above their baseline cognitive report, which the authors said highlights the limitations of the cognitive components of SCAT5.
The most accurate predictor of concussion was participants’ responses to questions about their symptoms.
“If you get hit in the head and go to the sideline and say, ‘I have a headache, I’m dizzy, I don’t feel right,’ I can say with pretty good assurance that you have a concussion,” Dr. Harmon continued. “I don’t need to do any testing.”
Unfortunately, the problem is “that some athletes don’t want to come out. They don’t report their symptoms or may not recognize their symptoms. So then you need an objective, accurate test to tell you whether you can safely put the athlete back on the field. We don’t have that right now.”
The study did not control for concussion history, and the all–Division 1 cohort means the findings may not be generalizable to other athletes.
Nevertheless, investigators said the study “affirms that reported symptoms are the most sensitive indicator of concussion, and there are limitations to the objective cognitive testing included in the SCAT.” They concluded that concussion “remains a clinical diagnosis that should be based on a thorough review of signs, symptoms, and clinical findings.”
This study was funded in part by donations from University of Washington alumni Jack and Luellen Cherneski and the Chisholm Foundation. Dr. Harmon reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a new study showed.
Investigators found that almost half of athletes diagnosed with a concussion tested normally on the Sports Concussion Assessment Tool 5 (SCAT5), the recommended tool for measuring cognitive skills in concussion evaluations. The most accurate measure of concussion was symptoms reported by the athletes.
“If you don’t do well on the cognitive exam, it suggests you have a concussion. But many people who are concussed do fine on the exam,” lead author Kimberly Harmon, MD, professor of family medicine and section head of sports medicine at the University of Washington School of Medicine, Seattle, said in a news release.
The study was published online in JAMA Network Open.
Introduced in 2004, the SCAT was created to standardize the collection of information clinicians use to diagnose concussion, including evaluation of symptoms, orientation, and balance. It also uses a 10-word list to assess immediate memory and delayed recall.
Dr. Harmon’s own experiences as a team physician led her to wonder about the accuracy of the cognitive screening portion of the SCAT. She saw that “some people were concussed, and they did well on the recall test. Some people weren’t concussed, and they didn’t do well. So I thought we should study it,” she said.
Investigators compared 92 National Collegiate Athletic Association (NCAA) Division 1 athletes who had sustained a concussion between 2020 and 2022 and had a concussion evaluation within 48 hours to 92 matched nonconcussed teammates (overall cohort, 52% men). Most concussions occurred in those who played football, followed by volleyball.
All athletes had previously completed NCAA-required baseline concussion screenings. Participants completed the SCAT5 screening test within 2 weeks of the incident concussion.
No significant differences were found between the baseline scores of athletes with and without concussion. Moreover, responses on the word recall section of the SCAT5 held little predictive value for concussion.
Nearly half (45%) of athletes with concussion performed at or even above their baseline cognitive report, which the authors said highlights the limitations of the cognitive components of SCAT5.
The most accurate predictor of concussion was participants’ responses to questions about their symptoms.
“If you get hit in the head and go to the sideline and say, ‘I have a headache, I’m dizzy, I don’t feel right,’ I can say with pretty good assurance that you have a concussion,” Dr. Harmon continued. “I don’t need to do any testing.”
Unfortunately, the problem is “that some athletes don’t want to come out. They don’t report their symptoms or may not recognize their symptoms. So then you need an objective, accurate test to tell you whether you can safely put the athlete back on the field. We don’t have that right now.”
The study did not control for concussion history, and the all–Division 1 cohort means the findings may not be generalizable to other athletes.
Nevertheless, investigators said the study “affirms that reported symptoms are the most sensitive indicator of concussion, and there are limitations to the objective cognitive testing included in the SCAT.” They concluded that concussion “remains a clinical diagnosis that should be based on a thorough review of signs, symptoms, and clinical findings.”
This study was funded in part by donations from University of Washington alumni Jack and Luellen Cherneski and the Chisholm Foundation. Dr. Harmon reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Form of B12 Deficiency Affecting the Central Nervous System May Be New Autoimmune Disease
Discovered while studying a puzzling case of one patient with inexplicable neurological systems, the same autoantibody was detected in a small percentage of healthy individuals and was nearly four times as prevalent in patients with neuropsychiatric systemic lupus erythematosus (SLE).
“I didn’t think this single investigation was going to yield a broader phenomenon with other patients,” lead author John V. Pluvinage, MD, PhD, a neurology resident at the University of California San Francisco, said in an interview. “It started as an N-of-one study just based on scientific curiosity.”
“It’s a beautifully done study,” added Betty Diamond, MD, director of the Institute of Molecular Medicine at the Feinstein Institutes for Medical Research in Manhasset, New York, commenting on the research. It uncovers “yet another example of a disease where antibodies getting into the brain are the problem.”
The research was published in Science Translational Medicine.
The Patient
The investigation began in 2014 with a 67-year-old woman presenting with difficulty speaking, ataxia, and tremor. Her blood tests showed no signs of B12 deficiency, and testing for known autoantibodies came back negative.
Solving this mystery required a more exhaustive approach. The patient enrolled in a research study focused on identifying novel autoantibodies in suspected neuroinflammatory disease, using a screening technology called phage immunoprecipitation sequencing.
“We adapted this technology to screen for autoantibodies in an unbiased manner by displaying every peptide across the human proteome and then mixing those peptides with patient antibodies in order to figure out what the antibodies are binding to,” explained Dr. Pluvinage.
Using this method, he and colleagues discovered that this woman had autoantibodies that target CD320 — a receptor important in the cellular uptake of B12. While her blood tests were normal, B12 in the patient’s cerebral spinal fluid (CSF) was “nearly undetectable,” Dr. Pluvinage said. Using an in vitro model of the blood-brain barrier (BBB), the researchers determined that anti-CD320 impaired the transport of B12 across the BBB by targeting receptors on the cell surface.
Treating the patient with a combination of immunosuppressant medication and high-dose B12 supplementation increased B12 levels in the patient’s CSF and improved clinical symptoms.
Identifying More Cases
Dr. Pluvinage and colleagues tested the 254 other individuals enrolled in the neuroinflammatory disease study and identified seven participants with CSF anti-CD320 autoantibodies — four of whom had low B12 in the CSF.
In a group of healthy controls, anti-CD320 seropositivity was 6%, similar to the positivity rate in 132 paired serum and CSF samples from a cohort of patients with multiple sclerosis (5.7%). In this group of patients with multiple sclerosis, anti-CD320 presence in the blood was highly predictive of high levels of CSF methylmalonic acid, a metabolic marker of B12 deficiency.
Researchers also screened for anti-CD320 seropositivity in 408 patients with non-neurologic SLE and 28 patients with neuropsychiatric SLE and found that the autoantibody was nearly four times as prevalent in patients with neurologic symptoms (21.4%) compared with in those with non-neurologic SLE (5.6%).
“The clinical relevance of anti-CD320 in healthy controls remains uncertain,” the authors wrote. However, it is not uncommon to have healthy patients with known autoantibodies.
“There are always people who have autoantibodies who don’t get disease, and why that is we don’t know,” said Dr. Diamond. Some individuals may develop clinical symptoms later, or there may be other reasons why they are protected against disease.
Pluvinage is eager to follow some seropositive healthy individuals to track their neurologic health overtime, to see if the presence of anti-CD320 “alters their neurologic trajectories.”
Alternative Pathways
Lastly, Dr. Pluvinage and colleagues set out to explain why patients with anti-CD320 in their blood did not show any signs of B12 deficiency. They hypothesized that another receptor may be compensating and still allowing blood cells to take up B12. Using CRISPR screening, the team identified the low-density lipoprotein receptor as an alternative pathway to B12 uptake.
“These findings suggest a model in which anti-CD320 impairs transport of B12 across the BBB, leading to autoimmune B12 central deficiency (ABCD) with varied neurologic manifestations but sparing peripheral manifestations of B12 deficiency,” the authors wrote.
The work was supported by the National Institute of Mental Health, National Center for Chronic Disease Prevention and Health Promotion, Department of Defense, UCSF Helen Diller Family Comprehensive Cancer Center Laboratory for Cell Analysis Shared Resource Facility, National Multiple Sclerosis Society, Valhalla Foundation, and the Westridge Foundation. Dr. Pluvinage is a co-inventor on a patent application related to this work. Dr. Diamond had no relevant disclosures.
A version of this article first appeared on Medscape.com.
Discovered while studying a puzzling case of one patient with inexplicable neurological systems, the same autoantibody was detected in a small percentage of healthy individuals and was nearly four times as prevalent in patients with neuropsychiatric systemic lupus erythematosus (SLE).
“I didn’t think this single investigation was going to yield a broader phenomenon with other patients,” lead author John V. Pluvinage, MD, PhD, a neurology resident at the University of California San Francisco, said in an interview. “It started as an N-of-one study just based on scientific curiosity.”
“It’s a beautifully done study,” added Betty Diamond, MD, director of the Institute of Molecular Medicine at the Feinstein Institutes for Medical Research in Manhasset, New York, commenting on the research. It uncovers “yet another example of a disease where antibodies getting into the brain are the problem.”
The research was published in Science Translational Medicine.
The Patient
The investigation began in 2014 with a 67-year-old woman presenting with difficulty speaking, ataxia, and tremor. Her blood tests showed no signs of B12 deficiency, and testing for known autoantibodies came back negative.
Solving this mystery required a more exhaustive approach. The patient enrolled in a research study focused on identifying novel autoantibodies in suspected neuroinflammatory disease, using a screening technology called phage immunoprecipitation sequencing.
“We adapted this technology to screen for autoantibodies in an unbiased manner by displaying every peptide across the human proteome and then mixing those peptides with patient antibodies in order to figure out what the antibodies are binding to,” explained Dr. Pluvinage.
Using this method, he and colleagues discovered that this woman had autoantibodies that target CD320 — a receptor important in the cellular uptake of B12. While her blood tests were normal, B12 in the patient’s cerebral spinal fluid (CSF) was “nearly undetectable,” Dr. Pluvinage said. Using an in vitro model of the blood-brain barrier (BBB), the researchers determined that anti-CD320 impaired the transport of B12 across the BBB by targeting receptors on the cell surface.
Treating the patient with a combination of immunosuppressant medication and high-dose B12 supplementation increased B12 levels in the patient’s CSF and improved clinical symptoms.
Identifying More Cases
Dr. Pluvinage and colleagues tested the 254 other individuals enrolled in the neuroinflammatory disease study and identified seven participants with CSF anti-CD320 autoantibodies — four of whom had low B12 in the CSF.
In a group of healthy controls, anti-CD320 seropositivity was 6%, similar to the positivity rate in 132 paired serum and CSF samples from a cohort of patients with multiple sclerosis (5.7%). In this group of patients with multiple sclerosis, anti-CD320 presence in the blood was highly predictive of high levels of CSF methylmalonic acid, a metabolic marker of B12 deficiency.
Researchers also screened for anti-CD320 seropositivity in 408 patients with non-neurologic SLE and 28 patients with neuropsychiatric SLE and found that the autoantibody was nearly four times as prevalent in patients with neurologic symptoms (21.4%) compared with in those with non-neurologic SLE (5.6%).
“The clinical relevance of anti-CD320 in healthy controls remains uncertain,” the authors wrote. However, it is not uncommon to have healthy patients with known autoantibodies.
“There are always people who have autoantibodies who don’t get disease, and why that is we don’t know,” said Dr. Diamond. Some individuals may develop clinical symptoms later, or there may be other reasons why they are protected against disease.
Pluvinage is eager to follow some seropositive healthy individuals to track their neurologic health overtime, to see if the presence of anti-CD320 “alters their neurologic trajectories.”
Alternative Pathways
Lastly, Dr. Pluvinage and colleagues set out to explain why patients with anti-CD320 in their blood did not show any signs of B12 deficiency. They hypothesized that another receptor may be compensating and still allowing blood cells to take up B12. Using CRISPR screening, the team identified the low-density lipoprotein receptor as an alternative pathway to B12 uptake.
“These findings suggest a model in which anti-CD320 impairs transport of B12 across the BBB, leading to autoimmune B12 central deficiency (ABCD) with varied neurologic manifestations but sparing peripheral manifestations of B12 deficiency,” the authors wrote.
The work was supported by the National Institute of Mental Health, National Center for Chronic Disease Prevention and Health Promotion, Department of Defense, UCSF Helen Diller Family Comprehensive Cancer Center Laboratory for Cell Analysis Shared Resource Facility, National Multiple Sclerosis Society, Valhalla Foundation, and the Westridge Foundation. Dr. Pluvinage is a co-inventor on a patent application related to this work. Dr. Diamond had no relevant disclosures.
A version of this article first appeared on Medscape.com.
Discovered while studying a puzzling case of one patient with inexplicable neurological systems, the same autoantibody was detected in a small percentage of healthy individuals and was nearly four times as prevalent in patients with neuropsychiatric systemic lupus erythematosus (SLE).
“I didn’t think this single investigation was going to yield a broader phenomenon with other patients,” lead author John V. Pluvinage, MD, PhD, a neurology resident at the University of California San Francisco, said in an interview. “It started as an N-of-one study just based on scientific curiosity.”
“It’s a beautifully done study,” added Betty Diamond, MD, director of the Institute of Molecular Medicine at the Feinstein Institutes for Medical Research in Manhasset, New York, commenting on the research. It uncovers “yet another example of a disease where antibodies getting into the brain are the problem.”
The research was published in Science Translational Medicine.
The Patient
The investigation began in 2014 with a 67-year-old woman presenting with difficulty speaking, ataxia, and tremor. Her blood tests showed no signs of B12 deficiency, and testing for known autoantibodies came back negative.
Solving this mystery required a more exhaustive approach. The patient enrolled in a research study focused on identifying novel autoantibodies in suspected neuroinflammatory disease, using a screening technology called phage immunoprecipitation sequencing.
“We adapted this technology to screen for autoantibodies in an unbiased manner by displaying every peptide across the human proteome and then mixing those peptides with patient antibodies in order to figure out what the antibodies are binding to,” explained Dr. Pluvinage.
Using this method, he and colleagues discovered that this woman had autoantibodies that target CD320 — a receptor important in the cellular uptake of B12. While her blood tests were normal, B12 in the patient’s cerebral spinal fluid (CSF) was “nearly undetectable,” Dr. Pluvinage said. Using an in vitro model of the blood-brain barrier (BBB), the researchers determined that anti-CD320 impaired the transport of B12 across the BBB by targeting receptors on the cell surface.
Treating the patient with a combination of immunosuppressant medication and high-dose B12 supplementation increased B12 levels in the patient’s CSF and improved clinical symptoms.
Identifying More Cases
Dr. Pluvinage and colleagues tested the 254 other individuals enrolled in the neuroinflammatory disease study and identified seven participants with CSF anti-CD320 autoantibodies — four of whom had low B12 in the CSF.
In a group of healthy controls, anti-CD320 seropositivity was 6%, similar to the positivity rate in 132 paired serum and CSF samples from a cohort of patients with multiple sclerosis (5.7%). In this group of patients with multiple sclerosis, anti-CD320 presence in the blood was highly predictive of high levels of CSF methylmalonic acid, a metabolic marker of B12 deficiency.
Researchers also screened for anti-CD320 seropositivity in 408 patients with non-neurologic SLE and 28 patients with neuropsychiatric SLE and found that the autoantibody was nearly four times as prevalent in patients with neurologic symptoms (21.4%) compared with in those with non-neurologic SLE (5.6%).
“The clinical relevance of anti-CD320 in healthy controls remains uncertain,” the authors wrote. However, it is not uncommon to have healthy patients with known autoantibodies.
“There are always people who have autoantibodies who don’t get disease, and why that is we don’t know,” said Dr. Diamond. Some individuals may develop clinical symptoms later, or there may be other reasons why they are protected against disease.
Pluvinage is eager to follow some seropositive healthy individuals to track their neurologic health overtime, to see if the presence of anti-CD320 “alters their neurologic trajectories.”
Alternative Pathways
Lastly, Dr. Pluvinage and colleagues set out to explain why patients with anti-CD320 in their blood did not show any signs of B12 deficiency. They hypothesized that another receptor may be compensating and still allowing blood cells to take up B12. Using CRISPR screening, the team identified the low-density lipoprotein receptor as an alternative pathway to B12 uptake.
“These findings suggest a model in which anti-CD320 impairs transport of B12 across the BBB, leading to autoimmune B12 central deficiency (ABCD) with varied neurologic manifestations but sparing peripheral manifestations of B12 deficiency,” the authors wrote.
The work was supported by the National Institute of Mental Health, National Center for Chronic Disease Prevention and Health Promotion, Department of Defense, UCSF Helen Diller Family Comprehensive Cancer Center Laboratory for Cell Analysis Shared Resource Facility, National Multiple Sclerosis Society, Valhalla Foundation, and the Westridge Foundation. Dr. Pluvinage is a co-inventor on a patent application related to this work. Dr. Diamond had no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM SCIENCE TRANSLATIONAL MEDICINE
Is Screen Time to Blame for Rising Rates of Myopia in Children?
TOPLINE:
More time spent exposed to screens is associated with a higher risk for myopia in children and adolescents; the use of computers and televisions appears to have the most significant effects on eye health.
METHODOLOGY:
- Researchers conducted a meta-analysis of 19 studies involving 102,360 children and adolescents to assess the association between screen time and myopia.
- Data were collected from studies published before June 1, 2023, in three databases: PubMed, Embase, and Web of Science.
- Screen time was categorized by device type, including computers, televisions, and smartphones, and analyzed using random or fixed-effect models.
- The analysis included both cohort and cross-sectional studies.
TAKEAWAY:
- High exposure to screen time was significantly associated with myopia in both cross-sectional (odds ratio [OR], 2.24; 95% confidence interval (CI), 1.47-3.42) and cohort studies (OR, 2.39; 95% CI, 2.07-2.76).
- In cohort studies, each extra hour per day spent using screens increased the risk for myopia by 7% (95% CI, 1.01-1.13).
- Subgroup analyses revealed significant associations between myopia and screen time on computers (OR, 8.19; 95% CI, 4.78-14.04) and televisions (OR, 1.46; 95% CI, 1.02-2.10), whereas time spent using smartphones was not significantly associated with myopia.
IN PRACTICE:
“With the development of technology and GDP [gross domestic product], educational pressure may lead students to use screen devices such as smartphones and computers for long periods of time to learn online courses, receive additional tutoring or practice, and increase the incidence of myopia,” wrote the authors.
SOURCE:
The study was led by Zhiqiang Zong of Anhui Medical University in Hefei, China. It was published online in BMC Public Health.
LIMITATIONS:
The majority of the studies included were cross-sectional, which cannot establish causality. High heterogeneity was found among the included studies, possibly due to differences in research design, population characteristics, and exposure levels. Some studies did not adjust for important confounding factors such as outdoor activities.
DISCLOSURES:
The study was supported by grants from the Educational Commission of Anhui Province of China, Research Fund of Anhui Institute of Translational Medicine, and National Natural Science Foundation of China. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
More time spent exposed to screens is associated with a higher risk for myopia in children and adolescents; the use of computers and televisions appears to have the most significant effects on eye health.
METHODOLOGY:
- Researchers conducted a meta-analysis of 19 studies involving 102,360 children and adolescents to assess the association between screen time and myopia.
- Data were collected from studies published before June 1, 2023, in three databases: PubMed, Embase, and Web of Science.
- Screen time was categorized by device type, including computers, televisions, and smartphones, and analyzed using random or fixed-effect models.
- The analysis included both cohort and cross-sectional studies.
TAKEAWAY:
- High exposure to screen time was significantly associated with myopia in both cross-sectional (odds ratio [OR], 2.24; 95% confidence interval (CI), 1.47-3.42) and cohort studies (OR, 2.39; 95% CI, 2.07-2.76).
- In cohort studies, each extra hour per day spent using screens increased the risk for myopia by 7% (95% CI, 1.01-1.13).
- Subgroup analyses revealed significant associations between myopia and screen time on computers (OR, 8.19; 95% CI, 4.78-14.04) and televisions (OR, 1.46; 95% CI, 1.02-2.10), whereas time spent using smartphones was not significantly associated with myopia.
IN PRACTICE:
“With the development of technology and GDP [gross domestic product], educational pressure may lead students to use screen devices such as smartphones and computers for long periods of time to learn online courses, receive additional tutoring or practice, and increase the incidence of myopia,” wrote the authors.
SOURCE:
The study was led by Zhiqiang Zong of Anhui Medical University in Hefei, China. It was published online in BMC Public Health.
LIMITATIONS:
The majority of the studies included were cross-sectional, which cannot establish causality. High heterogeneity was found among the included studies, possibly due to differences in research design, population characteristics, and exposure levels. Some studies did not adjust for important confounding factors such as outdoor activities.
DISCLOSURES:
The study was supported by grants from the Educational Commission of Anhui Province of China, Research Fund of Anhui Institute of Translational Medicine, and National Natural Science Foundation of China. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
More time spent exposed to screens is associated with a higher risk for myopia in children and adolescents; the use of computers and televisions appears to have the most significant effects on eye health.
METHODOLOGY:
- Researchers conducted a meta-analysis of 19 studies involving 102,360 children and adolescents to assess the association between screen time and myopia.
- Data were collected from studies published before June 1, 2023, in three databases: PubMed, Embase, and Web of Science.
- Screen time was categorized by device type, including computers, televisions, and smartphones, and analyzed using random or fixed-effect models.
- The analysis included both cohort and cross-sectional studies.
TAKEAWAY:
- High exposure to screen time was significantly associated with myopia in both cross-sectional (odds ratio [OR], 2.24; 95% confidence interval (CI), 1.47-3.42) and cohort studies (OR, 2.39; 95% CI, 2.07-2.76).
- In cohort studies, each extra hour per day spent using screens increased the risk for myopia by 7% (95% CI, 1.01-1.13).
- Subgroup analyses revealed significant associations between myopia and screen time on computers (OR, 8.19; 95% CI, 4.78-14.04) and televisions (OR, 1.46; 95% CI, 1.02-2.10), whereas time spent using smartphones was not significantly associated with myopia.
IN PRACTICE:
“With the development of technology and GDP [gross domestic product], educational pressure may lead students to use screen devices such as smartphones and computers for long periods of time to learn online courses, receive additional tutoring or practice, and increase the incidence of myopia,” wrote the authors.
SOURCE:
The study was led by Zhiqiang Zong of Anhui Medical University in Hefei, China. It was published online in BMC Public Health.
LIMITATIONS:
The majority of the studies included were cross-sectional, which cannot establish causality. High heterogeneity was found among the included studies, possibly due to differences in research design, population characteristics, and exposure levels. Some studies did not adjust for important confounding factors such as outdoor activities.
DISCLOSURES:
The study was supported by grants from the Educational Commission of Anhui Province of China, Research Fund of Anhui Institute of Translational Medicine, and National Natural Science Foundation of China. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
AML: Shorter Venetoclax Course Shows Promise for Some
However, the azacitidine plus venetoclax therapy — the “7+7” regimen — was associated with lower platelet transfusion requirements and lower 8-week mortality, suggesting the regimen might be preferable in certain patient populations, Alexandre Bazinet, MD, of the University of Texas MD Anderson Cancer Center, Houston, reported at the American Society of Clinical Oncology (ASCO) annual meeting.
The composite complete remission (CRc) rate, including complete remission with or without complete count recovery, was identical at 72% among 82 patients treated with the 7+7 regimen and 166 treated with standard therapy, and the complete remission (CR) rate was 57% and 55%, respectively, Dr. Bazinet said.
The median number of cycles to first response was one in both groups, but 42% of responders in the 7+7 group required more than one cycle to achieve their first response, compared with just 1% of those in the standard therapy group, he noted, adding that the median number of cycles to achieve best response was two in the 7+7 group and one in the standard therapy group.
The mortality rate at 4 weeks was similar in the groups (2% vs 5% for 7+7 vs standard therapy), but at 8 weeks, the mortality rate was significantly higher in the standard therapy group (6% vs 16%, respectively). Median overall survival (OS) was 11.2 months versus 10.3 months, and median 2-year survival was 27.7% versus 33.6% in the groups, respectively.
Event-free survival was 6.5 versus 7.4 months, and 2-year event-free survival was 24.5% versus 27.0%, respectively.
Of note, fewer patients in the 7+7 group required platelet transfusions during cycle 1 (62% vs 77%) and the cycle 1 rates of neutropenic fever and red cell transfusion requirements were similar in the two treatment groups, Dr. Bazinet said.
Study participants were 82 adults from seven centers in France who received the 7+7 regimen, and 166 adults from MD Anderson who received standard therapy with a hypomethylating agent plus venetoclax doublets given for 21-28 days during induction. Preliminary data on the 7+7 regimen in patients from the French centers were reported previously and “suggested preserved efficacy with potentially less toxicity,” he noted.
“A hypomethylating agent plus venetoclax doublets are standard-of-care in patients with AML who are older or ineligible for chemotherapy due to comorbidities,” Dr. Bazinet explained, adding that although the venetoclax label calls for 28 days of drug per cycle, shorter courses of 14 to 21 days are commonly used.
These findings are limited by the retrospective study design and by small patient numbers in many subgroups, he said.
“In addition, the cohorts were heterogeneous, consisting of patients treated with a variety of different regimens and across multiple centers and countries. The distribution of FLT3-ITD and NRAS/KRAS mutations differed significantly between cohorts,” he explained, also noting that prophylactic azole use differed across the cohort. “Furthermore, analysis of the toxicity results was also limited by likely differing transfusion polices in different centers.”
Overall, however, the findings suggest that reducing the duration of venetoclax is safe and results in similar CRc rates, although responses may be faster with standard dosing, he said, adding that “7+7 is potentially less toxic and is attractive in patients who are more frail or at risk for complications.”
“Our data support further study of shorter venetoclax duration, within emerging triplet regimens in patients with intermediate or low predictive benefit to mitigate toxicity,” he concluded.
Dr. Bazinet reported having no disclosures.
However, the azacitidine plus venetoclax therapy — the “7+7” regimen — was associated with lower platelet transfusion requirements and lower 8-week mortality, suggesting the regimen might be preferable in certain patient populations, Alexandre Bazinet, MD, of the University of Texas MD Anderson Cancer Center, Houston, reported at the American Society of Clinical Oncology (ASCO) annual meeting.
The composite complete remission (CRc) rate, including complete remission with or without complete count recovery, was identical at 72% among 82 patients treated with the 7+7 regimen and 166 treated with standard therapy, and the complete remission (CR) rate was 57% and 55%, respectively, Dr. Bazinet said.
The median number of cycles to first response was one in both groups, but 42% of responders in the 7+7 group required more than one cycle to achieve their first response, compared with just 1% of those in the standard therapy group, he noted, adding that the median number of cycles to achieve best response was two in the 7+7 group and one in the standard therapy group.
The mortality rate at 4 weeks was similar in the groups (2% vs 5% for 7+7 vs standard therapy), but at 8 weeks, the mortality rate was significantly higher in the standard therapy group (6% vs 16%, respectively). Median overall survival (OS) was 11.2 months versus 10.3 months, and median 2-year survival was 27.7% versus 33.6% in the groups, respectively.
Event-free survival was 6.5 versus 7.4 months, and 2-year event-free survival was 24.5% versus 27.0%, respectively.
Of note, fewer patients in the 7+7 group required platelet transfusions during cycle 1 (62% vs 77%) and the cycle 1 rates of neutropenic fever and red cell transfusion requirements were similar in the two treatment groups, Dr. Bazinet said.
Study participants were 82 adults from seven centers in France who received the 7+7 regimen, and 166 adults from MD Anderson who received standard therapy with a hypomethylating agent plus venetoclax doublets given for 21-28 days during induction. Preliminary data on the 7+7 regimen in patients from the French centers were reported previously and “suggested preserved efficacy with potentially less toxicity,” he noted.
“A hypomethylating agent plus venetoclax doublets are standard-of-care in patients with AML who are older or ineligible for chemotherapy due to comorbidities,” Dr. Bazinet explained, adding that although the venetoclax label calls for 28 days of drug per cycle, shorter courses of 14 to 21 days are commonly used.
These findings are limited by the retrospective study design and by small patient numbers in many subgroups, he said.
“In addition, the cohorts were heterogeneous, consisting of patients treated with a variety of different regimens and across multiple centers and countries. The distribution of FLT3-ITD and NRAS/KRAS mutations differed significantly between cohorts,” he explained, also noting that prophylactic azole use differed across the cohort. “Furthermore, analysis of the toxicity results was also limited by likely differing transfusion polices in different centers.”
Overall, however, the findings suggest that reducing the duration of venetoclax is safe and results in similar CRc rates, although responses may be faster with standard dosing, he said, adding that “7+7 is potentially less toxic and is attractive in patients who are more frail or at risk for complications.”
“Our data support further study of shorter venetoclax duration, within emerging triplet regimens in patients with intermediate or low predictive benefit to mitigate toxicity,” he concluded.
Dr. Bazinet reported having no disclosures.
However, the azacitidine plus venetoclax therapy — the “7+7” regimen — was associated with lower platelet transfusion requirements and lower 8-week mortality, suggesting the regimen might be preferable in certain patient populations, Alexandre Bazinet, MD, of the University of Texas MD Anderson Cancer Center, Houston, reported at the American Society of Clinical Oncology (ASCO) annual meeting.
The composite complete remission (CRc) rate, including complete remission with or without complete count recovery, was identical at 72% among 82 patients treated with the 7+7 regimen and 166 treated with standard therapy, and the complete remission (CR) rate was 57% and 55%, respectively, Dr. Bazinet said.
The median number of cycles to first response was one in both groups, but 42% of responders in the 7+7 group required more than one cycle to achieve their first response, compared with just 1% of those in the standard therapy group, he noted, adding that the median number of cycles to achieve best response was two in the 7+7 group and one in the standard therapy group.
The mortality rate at 4 weeks was similar in the groups (2% vs 5% for 7+7 vs standard therapy), but at 8 weeks, the mortality rate was significantly higher in the standard therapy group (6% vs 16%, respectively). Median overall survival (OS) was 11.2 months versus 10.3 months, and median 2-year survival was 27.7% versus 33.6% in the groups, respectively.
Event-free survival was 6.5 versus 7.4 months, and 2-year event-free survival was 24.5% versus 27.0%, respectively.
Of note, fewer patients in the 7+7 group required platelet transfusions during cycle 1 (62% vs 77%) and the cycle 1 rates of neutropenic fever and red cell transfusion requirements were similar in the two treatment groups, Dr. Bazinet said.
Study participants were 82 adults from seven centers in France who received the 7+7 regimen, and 166 adults from MD Anderson who received standard therapy with a hypomethylating agent plus venetoclax doublets given for 21-28 days during induction. Preliminary data on the 7+7 regimen in patients from the French centers were reported previously and “suggested preserved efficacy with potentially less toxicity,” he noted.
“A hypomethylating agent plus venetoclax doublets are standard-of-care in patients with AML who are older or ineligible for chemotherapy due to comorbidities,” Dr. Bazinet explained, adding that although the venetoclax label calls for 28 days of drug per cycle, shorter courses of 14 to 21 days are commonly used.
These findings are limited by the retrospective study design and by small patient numbers in many subgroups, he said.
“In addition, the cohorts were heterogeneous, consisting of patients treated with a variety of different regimens and across multiple centers and countries. The distribution of FLT3-ITD and NRAS/KRAS mutations differed significantly between cohorts,” he explained, also noting that prophylactic azole use differed across the cohort. “Furthermore, analysis of the toxicity results was also limited by likely differing transfusion polices in different centers.”
Overall, however, the findings suggest that reducing the duration of venetoclax is safe and results in similar CRc rates, although responses may be faster with standard dosing, he said, adding that “7+7 is potentially less toxic and is attractive in patients who are more frail or at risk for complications.”
“Our data support further study of shorter venetoclax duration, within emerging triplet regimens in patients with intermediate or low predictive benefit to mitigate toxicity,” he concluded.
Dr. Bazinet reported having no disclosures.
FROM ASCO 2024
Climate Change, Climate Anxiety, Climate Hope
Clinical Case: Sol is a 10 year-old cisgender White girl who appears sad at her annual well visit. On further inquiry she describes that her father is angry that there is no snow, her mother keeps talking about the forests disappearing, and local flooding closed down her favorite family restaurant for good. She is worried “the planet is in trouble and there’s nothing we can do” so much that she gets stomachaches when she thinks about it.
Climate Anxiety
Climate change is a complex phenomenon that has been subject to decades of political disagreement. Lobbying by groups like the fossil fuel industry, state legislation to implement recycling, oil spills and pollution disasters, and outspoken icons like former US Vice President Al Gore and Swedish activist Greta Thunberg have kept the climate crisis a hot topic. What was once a slow burn has begun to boil as climate-related disasters occur — wildfires, droughts, floods, and increasingly powerful and frequent severe weather events — alongside increasing temperatures globally. With heroic efforts, the UN-convened Paris Agreement was adopted by 196 nations in 2015 with ambitious goals to reduce global greenhouse emissions and limit Earth’s rising temperature.1 Yet doomsday headlines on this topic remain a regular occurrence.
Between sensationalized news coverage, political controversy, and international disasters, it is no wonder some youth are overwhelmed. .2 Direct effects could include a family losing their home to flooding or wildfires, resulting in post-traumatic stress symptoms or an anxiety disorder. Indirect effects might include a drought that results in loss of agricultural income leading to a forced migration, family stress and/or separation, and disordered substance use.
Add to these direct and indirect effects the cultural and media pressures, such as frequent debate about the consequences of failure to reduce greenhouse gas emissions by 2030,3 and youth can encounter a sense of existential dread that intersects squarely with their developmental trajectory. “Climate anxiety,” also called eco-anxiety or solastalgia, refers to “distress about climate change and its impacts on the landscape and human existence.”4 Eco-anxiety is not a formal psychiatric diagnosis and is not found in the DSM-5-TR.
In practice, existential climate-centered fears range from worrying about what to do to help with the climate crisis all the way to being overwhelmed about humanity’s future to the point of dysfunction. Some argue that this is not pathological, but rather a practical response to real-world phenomena.5 An international survey of youth found 59% were “very or extremely” worried about climate change with a mix of associated emotions, and almost half described eco-anxiety as something that affects their daily functioning.6 The climate crisis often amplifies the inequities already experienced by youth from historically marginalized groups.
Managing Climate Anxiety
Climate anxiety presents with many of the typical features of other anxieties. These include worries that cycle repetitively and intrusively through the mind, somatic distress such as headaches or stomachaches, and avoidance of things that remind one of the uncertainty and distress associated with climate change. Because the climate crisis is so global and complex, hopelessness and fatigue are not uncommon.
However, climate anxiety can often be ameliorated with the typical approaches to treating anxiety. Borrowing from cognitive-behavioral and mindfulness-based interventions, many recommendations have been offered to help with eco-anxiety. External validation of youth’s concerns and fears is a starting point that might build a teen’s capacity to tolerate distressing emotions about global warming.
Once reactions to climate change are acknowledged and accepted, space is created for reflection. This might include a balance of hope and pragmatic action. For example, renewable energy sources have made up an increasing share of the market over time with the world adding 50% more renewable capacity in 2023.7 Seventy-two percent of Americans acknowledge global warming, 75% feel schools should teach about consequences and solutions for global warming, and 79% support investment in renewable energy.8
Climate activism itself has been shown to buffer climate anxiety, particularly when implemented collectively rather than individually.4 Nature connectedness, or cognitive and emotional connections with nature, not only has many direct mental health benefits, but is also associated with climate activism.9 Many other integrative interventions can improve well-being while reducing ecological harm. Nutrition, physical activity, mindfulness, and sleep are youth mental health interventions with a strong evidence base that also reduce the carbon footprint and pollution attributable to psychiatric pharmaceuticals. Moreover, these climate-friendly interventions can improve family-connectedness, thus boosting resilience.
Without needing to become eco-warriors, healthcare providers can model sustainable practices while caring for patients. This might include having more plants in the office, recycling and composting at work, adding solar panels to the rooftop, or joining local parks prescription programs (see mygreendoctor.org, a nonprofit owned by the Florida Medical Association).
Next Steps
Sol is relieved to hear that many kids her age share her family’s concerns. A conversation about how to manage distressing emotions and physical feelings leads to a referral for brief cognitive behavioral interventions. Her parents join your visit to hear her concerns. They want to begin a family plan for climate action. You recommend the books How to Change Everything: The Young Human’s Guide to Protecting the Planet and Each Other by Naomi Klein and The Parents’ Guide to Climate Revolution: 100 Ways to Build a Fossil-Free Future, Raise Empowered Kids, and Still Get a Good Night’s Sleep by Mary DeMocker.
Dr. Rosenfeld is associate professor of psychiatry and pediatrics at the University of Vermont, Burlington.
References
1. Maizland L. Global Climate Agreements: Successes and Failures. Council on Foreign Relations. https://www.cfr.org/backgrounder/paris-global-climate-change-agreements.
2. van Nieuwenhuizen A et al. The effects of climate change on child and adolescent mental health: Clinical considerations. Curr Psychiatry Rep. 2021 Dec 7;23(12):88. doi: 10.1007/s11920-021-01296-y.
3. Window to Reach Climate Goals ‘Rapidly Closing’, UN Report Warns. United Nations. https://news.un.org/en/story/2023/09/1140527.
4. Schwartz SEO et al. Climate change anxiety and mental health: Environmental activism as buffer. Curr Psychol. 2022 Feb 28:1-14. doi: 10.1007/s12144-022-02735-6.
5. Pihkala P. Anxiety and the ecological crisis: an analysis of eco-anxiety and climate anxiety. Sustainability. 2020;12:7836. doi: 10.3390/su12197836.
6. Hickman C et al. Climate Anxiety in Children and Young People and Their Beliefs About Government Responses to Climate Change: A Global Survey. Lancet Planet Health. 2021 Dec;5(12):e863-e873. doi: 10.1016/S2542-5196(21)00278-3.
7. IEA (2021), Global Energy Review 2021, IEA, Paris. https://www.iea.org/reports/global-energy-review-2021/renewables.
8. Marlon J et al. Yale Climate Opinion Maps 2023. https://climatecommunication.yale.edu/visualizations-data/ycom-us/.
9. Thomson EE, Roach SP. The Relationships Among Nature Connectedness, Climate Anxiety, Climate Action, Climate Knowledge, and Mental Health. Front Psychol. 2023 Nov 15:14:1241400. doi: 10.3389/fpsyg.2023.1241400.
Clinical Case: Sol is a 10 year-old cisgender White girl who appears sad at her annual well visit. On further inquiry she describes that her father is angry that there is no snow, her mother keeps talking about the forests disappearing, and local flooding closed down her favorite family restaurant for good. She is worried “the planet is in trouble and there’s nothing we can do” so much that she gets stomachaches when she thinks about it.
Climate Anxiety
Climate change is a complex phenomenon that has been subject to decades of political disagreement. Lobbying by groups like the fossil fuel industry, state legislation to implement recycling, oil spills and pollution disasters, and outspoken icons like former US Vice President Al Gore and Swedish activist Greta Thunberg have kept the climate crisis a hot topic. What was once a slow burn has begun to boil as climate-related disasters occur — wildfires, droughts, floods, and increasingly powerful and frequent severe weather events — alongside increasing temperatures globally. With heroic efforts, the UN-convened Paris Agreement was adopted by 196 nations in 2015 with ambitious goals to reduce global greenhouse emissions and limit Earth’s rising temperature.1 Yet doomsday headlines on this topic remain a regular occurrence.
Between sensationalized news coverage, political controversy, and international disasters, it is no wonder some youth are overwhelmed. .2 Direct effects could include a family losing their home to flooding or wildfires, resulting in post-traumatic stress symptoms or an anxiety disorder. Indirect effects might include a drought that results in loss of agricultural income leading to a forced migration, family stress and/or separation, and disordered substance use.
Add to these direct and indirect effects the cultural and media pressures, such as frequent debate about the consequences of failure to reduce greenhouse gas emissions by 2030,3 and youth can encounter a sense of existential dread that intersects squarely with their developmental trajectory. “Climate anxiety,” also called eco-anxiety or solastalgia, refers to “distress about climate change and its impacts on the landscape and human existence.”4 Eco-anxiety is not a formal psychiatric diagnosis and is not found in the DSM-5-TR.
In practice, existential climate-centered fears range from worrying about what to do to help with the climate crisis all the way to being overwhelmed about humanity’s future to the point of dysfunction. Some argue that this is not pathological, but rather a practical response to real-world phenomena.5 An international survey of youth found 59% were “very or extremely” worried about climate change with a mix of associated emotions, and almost half described eco-anxiety as something that affects their daily functioning.6 The climate crisis often amplifies the inequities already experienced by youth from historically marginalized groups.
Managing Climate Anxiety
Climate anxiety presents with many of the typical features of other anxieties. These include worries that cycle repetitively and intrusively through the mind, somatic distress such as headaches or stomachaches, and avoidance of things that remind one of the uncertainty and distress associated with climate change. Because the climate crisis is so global and complex, hopelessness and fatigue are not uncommon.
However, climate anxiety can often be ameliorated with the typical approaches to treating anxiety. Borrowing from cognitive-behavioral and mindfulness-based interventions, many recommendations have been offered to help with eco-anxiety. External validation of youth’s concerns and fears is a starting point that might build a teen’s capacity to tolerate distressing emotions about global warming.
Once reactions to climate change are acknowledged and accepted, space is created for reflection. This might include a balance of hope and pragmatic action. For example, renewable energy sources have made up an increasing share of the market over time with the world adding 50% more renewable capacity in 2023.7 Seventy-two percent of Americans acknowledge global warming, 75% feel schools should teach about consequences and solutions for global warming, and 79% support investment in renewable energy.8
Climate activism itself has been shown to buffer climate anxiety, particularly when implemented collectively rather than individually.4 Nature connectedness, or cognitive and emotional connections with nature, not only has many direct mental health benefits, but is also associated with climate activism.9 Many other integrative interventions can improve well-being while reducing ecological harm. Nutrition, physical activity, mindfulness, and sleep are youth mental health interventions with a strong evidence base that also reduce the carbon footprint and pollution attributable to psychiatric pharmaceuticals. Moreover, these climate-friendly interventions can improve family-connectedness, thus boosting resilience.
Without needing to become eco-warriors, healthcare providers can model sustainable practices while caring for patients. This might include having more plants in the office, recycling and composting at work, adding solar panels to the rooftop, or joining local parks prescription programs (see mygreendoctor.org, a nonprofit owned by the Florida Medical Association).
Next Steps
Sol is relieved to hear that many kids her age share her family’s concerns. A conversation about how to manage distressing emotions and physical feelings leads to a referral for brief cognitive behavioral interventions. Her parents join your visit to hear her concerns. They want to begin a family plan for climate action. You recommend the books How to Change Everything: The Young Human’s Guide to Protecting the Planet and Each Other by Naomi Klein and The Parents’ Guide to Climate Revolution: 100 Ways to Build a Fossil-Free Future, Raise Empowered Kids, and Still Get a Good Night’s Sleep by Mary DeMocker.
Dr. Rosenfeld is associate professor of psychiatry and pediatrics at the University of Vermont, Burlington.
References
1. Maizland L. Global Climate Agreements: Successes and Failures. Council on Foreign Relations. https://www.cfr.org/backgrounder/paris-global-climate-change-agreements.
2. van Nieuwenhuizen A et al. The effects of climate change on child and adolescent mental health: Clinical considerations. Curr Psychiatry Rep. 2021 Dec 7;23(12):88. doi: 10.1007/s11920-021-01296-y.
3. Window to Reach Climate Goals ‘Rapidly Closing’, UN Report Warns. United Nations. https://news.un.org/en/story/2023/09/1140527.
4. Schwartz SEO et al. Climate change anxiety and mental health: Environmental activism as buffer. Curr Psychol. 2022 Feb 28:1-14. doi: 10.1007/s12144-022-02735-6.
5. Pihkala P. Anxiety and the ecological crisis: an analysis of eco-anxiety and climate anxiety. Sustainability. 2020;12:7836. doi: 10.3390/su12197836.
6. Hickman C et al. Climate Anxiety in Children and Young People and Their Beliefs About Government Responses to Climate Change: A Global Survey. Lancet Planet Health. 2021 Dec;5(12):e863-e873. doi: 10.1016/S2542-5196(21)00278-3.
7. IEA (2021), Global Energy Review 2021, IEA, Paris. https://www.iea.org/reports/global-energy-review-2021/renewables.
8. Marlon J et al. Yale Climate Opinion Maps 2023. https://climatecommunication.yale.edu/visualizations-data/ycom-us/.
9. Thomson EE, Roach SP. The Relationships Among Nature Connectedness, Climate Anxiety, Climate Action, Climate Knowledge, and Mental Health. Front Psychol. 2023 Nov 15:14:1241400. doi: 10.3389/fpsyg.2023.1241400.
Clinical Case: Sol is a 10 year-old cisgender White girl who appears sad at her annual well visit. On further inquiry she describes that her father is angry that there is no snow, her mother keeps talking about the forests disappearing, and local flooding closed down her favorite family restaurant for good. She is worried “the planet is in trouble and there’s nothing we can do” so much that she gets stomachaches when she thinks about it.
Climate Anxiety
Climate change is a complex phenomenon that has been subject to decades of political disagreement. Lobbying by groups like the fossil fuel industry, state legislation to implement recycling, oil spills and pollution disasters, and outspoken icons like former US Vice President Al Gore and Swedish activist Greta Thunberg have kept the climate crisis a hot topic. What was once a slow burn has begun to boil as climate-related disasters occur — wildfires, droughts, floods, and increasingly powerful and frequent severe weather events — alongside increasing temperatures globally. With heroic efforts, the UN-convened Paris Agreement was adopted by 196 nations in 2015 with ambitious goals to reduce global greenhouse emissions and limit Earth’s rising temperature.1 Yet doomsday headlines on this topic remain a regular occurrence.
Between sensationalized news coverage, political controversy, and international disasters, it is no wonder some youth are overwhelmed. .2 Direct effects could include a family losing their home to flooding or wildfires, resulting in post-traumatic stress symptoms or an anxiety disorder. Indirect effects might include a drought that results in loss of agricultural income leading to a forced migration, family stress and/or separation, and disordered substance use.
Add to these direct and indirect effects the cultural and media pressures, such as frequent debate about the consequences of failure to reduce greenhouse gas emissions by 2030,3 and youth can encounter a sense of existential dread that intersects squarely with their developmental trajectory. “Climate anxiety,” also called eco-anxiety or solastalgia, refers to “distress about climate change and its impacts on the landscape and human existence.”4 Eco-anxiety is not a formal psychiatric diagnosis and is not found in the DSM-5-TR.
In practice, existential climate-centered fears range from worrying about what to do to help with the climate crisis all the way to being overwhelmed about humanity’s future to the point of dysfunction. Some argue that this is not pathological, but rather a practical response to real-world phenomena.5 An international survey of youth found 59% were “very or extremely” worried about climate change with a mix of associated emotions, and almost half described eco-anxiety as something that affects their daily functioning.6 The climate crisis often amplifies the inequities already experienced by youth from historically marginalized groups.
Managing Climate Anxiety
Climate anxiety presents with many of the typical features of other anxieties. These include worries that cycle repetitively and intrusively through the mind, somatic distress such as headaches or stomachaches, and avoidance of things that remind one of the uncertainty and distress associated with climate change. Because the climate crisis is so global and complex, hopelessness and fatigue are not uncommon.
However, climate anxiety can often be ameliorated with the typical approaches to treating anxiety. Borrowing from cognitive-behavioral and mindfulness-based interventions, many recommendations have been offered to help with eco-anxiety. External validation of youth’s concerns and fears is a starting point that might build a teen’s capacity to tolerate distressing emotions about global warming.
Once reactions to climate change are acknowledged and accepted, space is created for reflection. This might include a balance of hope and pragmatic action. For example, renewable energy sources have made up an increasing share of the market over time with the world adding 50% more renewable capacity in 2023.7 Seventy-two percent of Americans acknowledge global warming, 75% feel schools should teach about consequences and solutions for global warming, and 79% support investment in renewable energy.8
Climate activism itself has been shown to buffer climate anxiety, particularly when implemented collectively rather than individually.4 Nature connectedness, or cognitive and emotional connections with nature, not only has many direct mental health benefits, but is also associated with climate activism.9 Many other integrative interventions can improve well-being while reducing ecological harm. Nutrition, physical activity, mindfulness, and sleep are youth mental health interventions with a strong evidence base that also reduce the carbon footprint and pollution attributable to psychiatric pharmaceuticals. Moreover, these climate-friendly interventions can improve family-connectedness, thus boosting resilience.
Without needing to become eco-warriors, healthcare providers can model sustainable practices while caring for patients. This might include having more plants in the office, recycling and composting at work, adding solar panels to the rooftop, or joining local parks prescription programs (see mygreendoctor.org, a nonprofit owned by the Florida Medical Association).
Next Steps
Sol is relieved to hear that many kids her age share her family’s concerns. A conversation about how to manage distressing emotions and physical feelings leads to a referral for brief cognitive behavioral interventions. Her parents join your visit to hear her concerns. They want to begin a family plan for climate action. You recommend the books How to Change Everything: The Young Human’s Guide to Protecting the Planet and Each Other by Naomi Klein and The Parents’ Guide to Climate Revolution: 100 Ways to Build a Fossil-Free Future, Raise Empowered Kids, and Still Get a Good Night’s Sleep by Mary DeMocker.
Dr. Rosenfeld is associate professor of psychiatry and pediatrics at the University of Vermont, Burlington.
References
1. Maizland L. Global Climate Agreements: Successes and Failures. Council on Foreign Relations. https://www.cfr.org/backgrounder/paris-global-climate-change-agreements.
2. van Nieuwenhuizen A et al. The effects of climate change on child and adolescent mental health: Clinical considerations. Curr Psychiatry Rep. 2021 Dec 7;23(12):88. doi: 10.1007/s11920-021-01296-y.
3. Window to Reach Climate Goals ‘Rapidly Closing’, UN Report Warns. United Nations. https://news.un.org/en/story/2023/09/1140527.
4. Schwartz SEO et al. Climate change anxiety and mental health: Environmental activism as buffer. Curr Psychol. 2022 Feb 28:1-14. doi: 10.1007/s12144-022-02735-6.
5. Pihkala P. Anxiety and the ecological crisis: an analysis of eco-anxiety and climate anxiety. Sustainability. 2020;12:7836. doi: 10.3390/su12197836.
6. Hickman C et al. Climate Anxiety in Children and Young People and Their Beliefs About Government Responses to Climate Change: A Global Survey. Lancet Planet Health. 2021 Dec;5(12):e863-e873. doi: 10.1016/S2542-5196(21)00278-3.
7. IEA (2021), Global Energy Review 2021, IEA, Paris. https://www.iea.org/reports/global-energy-review-2021/renewables.
8. Marlon J et al. Yale Climate Opinion Maps 2023. https://climatecommunication.yale.edu/visualizations-data/ycom-us/.
9. Thomson EE, Roach SP. The Relationships Among Nature Connectedness, Climate Anxiety, Climate Action, Climate Knowledge, and Mental Health. Front Psychol. 2023 Nov 15:14:1241400. doi: 10.3389/fpsyg.2023.1241400.
Study Links Suicide to Missed Early Care After Discharge
TOPLINE:
A study found that patients who die by suicide within a year after discharge from inpatient mental health care are less likely to have primary care consultation in the first 2 weeks, highlighting a gap during the high-risk transition period.
METHODOLOGY:
- Researchers used a nested case-control study design, analyzing the records of 613 people who died by suicide within a year of being discharged from an inpatient psychiatric facility in England between 2001 and 2019.
- Of these, 93 (15.4%) died within 2 weeks of discharge.
- Each patient was matched with up to 20 control individuals who were discharged at a similar time but were living.
- Researchers evaluated primary care consultations after discharge.
TAKEAWAY:
- People who died by suicide within a year were less likely to have had a primary care consultation within 2 weeks of discharge (adjusted odds ratio [aOR], 0.61; P = .01).
- Those who died by suicide had higher odds for a consultation in the week preceding their death (aOR, 1.71; P < .001) and the prescription of three or more psychotropic medications (aOR, 1.73; P < .001).
- Evidence of discharge communication between the facility and primary care clinician was infrequent, highlighting a gap in continuity of care.
- Approximately 40% of people who died within 2 weeks of discharge had a documented visit with a primary care clinician during that period.
IN PRACTICE:
“Primary care clinicians have opportunities to intervene and should prioritize patients experiencing transition from inpatient care,” the authors wrote.
SOURCE:
The study was led by Rebecca Musgrove, PhD, of the Centre for Mental Health and Safety at The University of Manchester in England, and published online on June 12 in BJGP Open.
LIMITATIONS:
The study’s reliance on individuals registered with the Clinical Practice Research Datalink may have caused some suicide cases to be excluded, limiting generalizability. Lack of linked up-to-date mental health records may have led to the omission of significant post-discharge care data. Incomplete discharge documentation may undercount informational continuity, affecting multivariable analysis.
DISCLOSURES:
The study was supported by the National Institute of Health and Care Research. Some authors declared serving as members of advisory groups and receiving grants and personal fees from various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
A study found that patients who die by suicide within a year after discharge from inpatient mental health care are less likely to have primary care consultation in the first 2 weeks, highlighting a gap during the high-risk transition period.
METHODOLOGY:
- Researchers used a nested case-control study design, analyzing the records of 613 people who died by suicide within a year of being discharged from an inpatient psychiatric facility in England between 2001 and 2019.
- Of these, 93 (15.4%) died within 2 weeks of discharge.
- Each patient was matched with up to 20 control individuals who were discharged at a similar time but were living.
- Researchers evaluated primary care consultations after discharge.
TAKEAWAY:
- People who died by suicide within a year were less likely to have had a primary care consultation within 2 weeks of discharge (adjusted odds ratio [aOR], 0.61; P = .01).
- Those who died by suicide had higher odds for a consultation in the week preceding their death (aOR, 1.71; P < .001) and the prescription of three or more psychotropic medications (aOR, 1.73; P < .001).
- Evidence of discharge communication between the facility and primary care clinician was infrequent, highlighting a gap in continuity of care.
- Approximately 40% of people who died within 2 weeks of discharge had a documented visit with a primary care clinician during that period.
IN PRACTICE:
“Primary care clinicians have opportunities to intervene and should prioritize patients experiencing transition from inpatient care,” the authors wrote.
SOURCE:
The study was led by Rebecca Musgrove, PhD, of the Centre for Mental Health and Safety at The University of Manchester in England, and published online on June 12 in BJGP Open.
LIMITATIONS:
The study’s reliance on individuals registered with the Clinical Practice Research Datalink may have caused some suicide cases to be excluded, limiting generalizability. Lack of linked up-to-date mental health records may have led to the omission of significant post-discharge care data. Incomplete discharge documentation may undercount informational continuity, affecting multivariable analysis.
DISCLOSURES:
The study was supported by the National Institute of Health and Care Research. Some authors declared serving as members of advisory groups and receiving grants and personal fees from various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
A study found that patients who die by suicide within a year after discharge from inpatient mental health care are less likely to have primary care consultation in the first 2 weeks, highlighting a gap during the high-risk transition period.
METHODOLOGY:
- Researchers used a nested case-control study design, analyzing the records of 613 people who died by suicide within a year of being discharged from an inpatient psychiatric facility in England between 2001 and 2019.
- Of these, 93 (15.4%) died within 2 weeks of discharge.
- Each patient was matched with up to 20 control individuals who were discharged at a similar time but were living.
- Researchers evaluated primary care consultations after discharge.
TAKEAWAY:
- People who died by suicide within a year were less likely to have had a primary care consultation within 2 weeks of discharge (adjusted odds ratio [aOR], 0.61; P = .01).
- Those who died by suicide had higher odds for a consultation in the week preceding their death (aOR, 1.71; P < .001) and the prescription of three or more psychotropic medications (aOR, 1.73; P < .001).
- Evidence of discharge communication between the facility and primary care clinician was infrequent, highlighting a gap in continuity of care.
- Approximately 40% of people who died within 2 weeks of discharge had a documented visit with a primary care clinician during that period.
IN PRACTICE:
“Primary care clinicians have opportunities to intervene and should prioritize patients experiencing transition from inpatient care,” the authors wrote.
SOURCE:
The study was led by Rebecca Musgrove, PhD, of the Centre for Mental Health and Safety at The University of Manchester in England, and published online on June 12 in BJGP Open.
LIMITATIONS:
The study’s reliance on individuals registered with the Clinical Practice Research Datalink may have caused some suicide cases to be excluded, limiting generalizability. Lack of linked up-to-date mental health records may have led to the omission of significant post-discharge care data. Incomplete discharge documentation may undercount informational continuity, affecting multivariable analysis.
DISCLOSURES:
The study was supported by the National Institute of Health and Care Research. Some authors declared serving as members of advisory groups and receiving grants and personal fees from various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Thanks, But No Thanks
She was young, neatly dressed, professional. I don’t remember her name, though she handed me a business card as soon as I stepped up to the front window.
I thought she was a new drug rep to my territory, and I usually try to say “hi” when they first come in. They’re just doing their job, and I don’t mind chatting for a few minutes.
But she, as it turned out, was here for a whole new thing. Taking out a glossy brochure, she dived into a spiel about my offering a medical credit card through my office. I would get paid quickly, I might even get some extra money from patient interest payments, it is convenient for patients, win-win situation all around, yadda yadda yadda.
I smiled, thanked her for coming in, but told her this wasn’t a good fit for my practice.
I’m well aware that keeping a small practice afloat ain’t easy. Medicine is one of the few fields (unless you’re strictly doing cash pay) where we can’t raise prices to keep up with inflation. Well, we can, but what we get paid won’t change. That’s the nature of dealing with Medicare and insurance. What you charge and what you’ll get (and have to accept) are generally not the same.
But even so, I try to stick with what I know — being a neurologist. I’m not here to offer a range of financial services. I have neither the time, nor interest, to run a patient’s copay while trying to sell them on a medical credit card.
For that matter I’m not going to set up shop selling vitamin supplements, hangover-curing infusions, endorsing products on X, or any of the other dubious things touted as “thinking outside the box” ways to increase revenue.
I suppose some will say I’m old-fashioned, or this is why my practice operates on a thin margin, or that I’m focusing more on patients than business. I don’t mind. Caring for patients is why I’m here.
I also hear the argument that if I don’t market a medical credit card (or whatever), someone else will. That’s fine. Let them. I wish them good luck. It’s just not for me.
Like I’ve said in the past, I’m an old dog, but a happy one. I’ll leave the new tricks to someone else.
Dr. Block has a solo neurology practice in Scottsdale, Arizona.
She was young, neatly dressed, professional. I don’t remember her name, though she handed me a business card as soon as I stepped up to the front window.
I thought she was a new drug rep to my territory, and I usually try to say “hi” when they first come in. They’re just doing their job, and I don’t mind chatting for a few minutes.
But she, as it turned out, was here for a whole new thing. Taking out a glossy brochure, she dived into a spiel about my offering a medical credit card through my office. I would get paid quickly, I might even get some extra money from patient interest payments, it is convenient for patients, win-win situation all around, yadda yadda yadda.
I smiled, thanked her for coming in, but told her this wasn’t a good fit for my practice.
I’m well aware that keeping a small practice afloat ain’t easy. Medicine is one of the few fields (unless you’re strictly doing cash pay) where we can’t raise prices to keep up with inflation. Well, we can, but what we get paid won’t change. That’s the nature of dealing with Medicare and insurance. What you charge and what you’ll get (and have to accept) are generally not the same.
But even so, I try to stick with what I know — being a neurologist. I’m not here to offer a range of financial services. I have neither the time, nor interest, to run a patient’s copay while trying to sell them on a medical credit card.
For that matter I’m not going to set up shop selling vitamin supplements, hangover-curing infusions, endorsing products on X, or any of the other dubious things touted as “thinking outside the box” ways to increase revenue.
I suppose some will say I’m old-fashioned, or this is why my practice operates on a thin margin, or that I’m focusing more on patients than business. I don’t mind. Caring for patients is why I’m here.
I also hear the argument that if I don’t market a medical credit card (or whatever), someone else will. That’s fine. Let them. I wish them good luck. It’s just not for me.
Like I’ve said in the past, I’m an old dog, but a happy one. I’ll leave the new tricks to someone else.
Dr. Block has a solo neurology practice in Scottsdale, Arizona.
She was young, neatly dressed, professional. I don’t remember her name, though she handed me a business card as soon as I stepped up to the front window.
I thought she was a new drug rep to my territory, and I usually try to say “hi” when they first come in. They’re just doing their job, and I don’t mind chatting for a few minutes.
But she, as it turned out, was here for a whole new thing. Taking out a glossy brochure, she dived into a spiel about my offering a medical credit card through my office. I would get paid quickly, I might even get some extra money from patient interest payments, it is convenient for patients, win-win situation all around, yadda yadda yadda.
I smiled, thanked her for coming in, but told her this wasn’t a good fit for my practice.
I’m well aware that keeping a small practice afloat ain’t easy. Medicine is one of the few fields (unless you’re strictly doing cash pay) where we can’t raise prices to keep up with inflation. Well, we can, but what we get paid won’t change. That’s the nature of dealing with Medicare and insurance. What you charge and what you’ll get (and have to accept) are generally not the same.
But even so, I try to stick with what I know — being a neurologist. I’m not here to offer a range of financial services. I have neither the time, nor interest, to run a patient’s copay while trying to sell them on a medical credit card.
For that matter I’m not going to set up shop selling vitamin supplements, hangover-curing infusions, endorsing products on X, or any of the other dubious things touted as “thinking outside the box” ways to increase revenue.
I suppose some will say I’m old-fashioned, or this is why my practice operates on a thin margin, or that I’m focusing more on patients than business. I don’t mind. Caring for patients is why I’m here.
I also hear the argument that if I don’t market a medical credit card (or whatever), someone else will. That’s fine. Let them. I wish them good luck. It’s just not for me.
Like I’ve said in the past, I’m an old dog, but a happy one. I’ll leave the new tricks to someone else.
Dr. Block has a solo neurology practice in Scottsdale, Arizona.





