User login
The Journal of Clinical Outcomes Management® is an independent, peer-reviewed journal offering evidence-based, practical information for improving the quality, safety, and value of health care.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Doctors’ happiness has not rebounded as pandemic drags on
Physicians reported similar levels of unhappiness in 2022 too.
Fewer than half of physicians said they were currently somewhat or very happy at work, compared with 75% of physicians who said they were somewhat or very happy at work in a previous survey conducted before the pandemic, the new Medscape Physician Lifestyle & Happiness Report 2023 shows.*
“I am not surprised that we’re less happy now,” said Amaryllis Sánchez, MD, a board-certified family medicine physician and a certified physician coach.
“I speak to physicians around the country and I hear that their workplaces are understaffed, they’re overworked and they don’t feel safe. Although we’re in a different phase of the pandemic, doctors feel that the ground beneath them is still shaky,” said Dr. Sánchez, the author of “Recapturing Joy in Medicine.”
Most doctors are seeing more patients than they can handle and are expected to do that consistently. “When you no longer have the capacity to give of yourself, that becomes a nearly impossible task,” said Dr. Sánchez.
Also, physicians in understaffed workplaces often must take on additional work such as administrative or nursing duties, said Katie Cole, DO, a board-certified psychiatrist and a physician coach.
While health systems are aware that physicians need time to rest and recharge, staffing shortages prevent doctors from taking time off because they can’t find coverage, said Dr. Cole.
“While we know that it’s important for physicians to take vacations, more than one-third of doctors still take 2 weeks or less of vacation annually,” said Dr. Cole.
Physicians also tend to have less compassion for themselves and sacrifice self-care compared to other health care workers. “When a patient dies, nurses get together, debrief, and hug each other, whereas doctors have another patient to see. The culture of medicine doesn’t support self-compassion for physicians,” said Dr. Cole.
Physicians also felt less safe at work during the pandemic because of to shortages of personal protective equipment, said Dr. Sánchez. They have also witnessed or experienced an increase in abusive behavior, violence and threats of violence.
Physicians’ personal life suffers
Doctors maintain their mental health primarily by spending time with family members and friends, according to 2022’s Medscape Physician Lifestyle & Happiness Report. Yet half of doctors reported in a survey by the Physicians Foundation that they withdrew from family, friends or coworkers in 2022, said Dr. Sánchez.
“When you exceed your mental, emotional, and physical capacity at work, you have no reserve left for your personal life,” said Dr. Cole.
That may explain why only 58% of doctors reported feeling somewhat or very happy outside of work, compared with 84% who felt that way before the pandemic.
More women doctors said they deal with stronger feelings of conflict in trying to balance parenting responsibilities with a highly demanding job. Nearly one in two women physician-parents reported feeling very conflicted at work, compared with about one in four male physician-parents.
When physicians go home, they may be emotionally drained and tired mentally from making a lot of decisions at work, said Dr. Cole.
“As a woman, if you have children and a husband and you’re responsible for dinner, picking up the kids at daycare or helping them with homework, and making all these decisions when you get home, it’s overwhelming,” said Dr. Cole.
Prioritize your well-being
Doctors need to prioritize their own well-being, said Dr. Sánchez. “That’s not being selfish, that’s doing what’s necessary to stay well and be able to take care of patients. If doctors don’t take care of themselves, no one else will.”
Dr. Sánchez recommended that doctors regularly interact with relatives, friends, trusted colleagues, or clergy to help maintain their well-being, rather than waiting until a crisis to reach out.
A good coach, mentor, or counselor can help physicians gain enough self-awareness to handle their emotions and gain more clarity about what changes need to be made, she said.
Dr. Cole suggested that doctors figure out what makes them happy and fulfilled at work and try to spend more time on that activity. “Knowing what makes you happy and your strengths are foundational for creating a life you love.”
She urged doctors to “start thinking now about what you love about medicine and what is going right at home, and what areas you want to change. Then, start advocating for your needs.”
A version of this article originally appeared on Medscape.com.
Correction, 1/26/23: An earlier version of this article misstated the findings of the survey.
Physicians reported similar levels of unhappiness in 2022 too.
Fewer than half of physicians said they were currently somewhat or very happy at work, compared with 75% of physicians who said they were somewhat or very happy at work in a previous survey conducted before the pandemic, the new Medscape Physician Lifestyle & Happiness Report 2023 shows.*
“I am not surprised that we’re less happy now,” said Amaryllis Sánchez, MD, a board-certified family medicine physician and a certified physician coach.
“I speak to physicians around the country and I hear that their workplaces are understaffed, they’re overworked and they don’t feel safe. Although we’re in a different phase of the pandemic, doctors feel that the ground beneath them is still shaky,” said Dr. Sánchez, the author of “Recapturing Joy in Medicine.”
Most doctors are seeing more patients than they can handle and are expected to do that consistently. “When you no longer have the capacity to give of yourself, that becomes a nearly impossible task,” said Dr. Sánchez.
Also, physicians in understaffed workplaces often must take on additional work such as administrative or nursing duties, said Katie Cole, DO, a board-certified psychiatrist and a physician coach.
While health systems are aware that physicians need time to rest and recharge, staffing shortages prevent doctors from taking time off because they can’t find coverage, said Dr. Cole.
“While we know that it’s important for physicians to take vacations, more than one-third of doctors still take 2 weeks or less of vacation annually,” said Dr. Cole.
Physicians also tend to have less compassion for themselves and sacrifice self-care compared to other health care workers. “When a patient dies, nurses get together, debrief, and hug each other, whereas doctors have another patient to see. The culture of medicine doesn’t support self-compassion for physicians,” said Dr. Cole.
Physicians also felt less safe at work during the pandemic because of to shortages of personal protective equipment, said Dr. Sánchez. They have also witnessed or experienced an increase in abusive behavior, violence and threats of violence.
Physicians’ personal life suffers
Doctors maintain their mental health primarily by spending time with family members and friends, according to 2022’s Medscape Physician Lifestyle & Happiness Report. Yet half of doctors reported in a survey by the Physicians Foundation that they withdrew from family, friends or coworkers in 2022, said Dr. Sánchez.
“When you exceed your mental, emotional, and physical capacity at work, you have no reserve left for your personal life,” said Dr. Cole.
That may explain why only 58% of doctors reported feeling somewhat or very happy outside of work, compared with 84% who felt that way before the pandemic.
More women doctors said they deal with stronger feelings of conflict in trying to balance parenting responsibilities with a highly demanding job. Nearly one in two women physician-parents reported feeling very conflicted at work, compared with about one in four male physician-parents.
When physicians go home, they may be emotionally drained and tired mentally from making a lot of decisions at work, said Dr. Cole.
“As a woman, if you have children and a husband and you’re responsible for dinner, picking up the kids at daycare or helping them with homework, and making all these decisions when you get home, it’s overwhelming,” said Dr. Cole.
Prioritize your well-being
Doctors need to prioritize their own well-being, said Dr. Sánchez. “That’s not being selfish, that’s doing what’s necessary to stay well and be able to take care of patients. If doctors don’t take care of themselves, no one else will.”
Dr. Sánchez recommended that doctors regularly interact with relatives, friends, trusted colleagues, or clergy to help maintain their well-being, rather than waiting until a crisis to reach out.
A good coach, mentor, or counselor can help physicians gain enough self-awareness to handle their emotions and gain more clarity about what changes need to be made, she said.
Dr. Cole suggested that doctors figure out what makes them happy and fulfilled at work and try to spend more time on that activity. “Knowing what makes you happy and your strengths are foundational for creating a life you love.”
She urged doctors to “start thinking now about what you love about medicine and what is going right at home, and what areas you want to change. Then, start advocating for your needs.”
A version of this article originally appeared on Medscape.com.
Correction, 1/26/23: An earlier version of this article misstated the findings of the survey.
Physicians reported similar levels of unhappiness in 2022 too.
Fewer than half of physicians said they were currently somewhat or very happy at work, compared with 75% of physicians who said they were somewhat or very happy at work in a previous survey conducted before the pandemic, the new Medscape Physician Lifestyle & Happiness Report 2023 shows.*
“I am not surprised that we’re less happy now,” said Amaryllis Sánchez, MD, a board-certified family medicine physician and a certified physician coach.
“I speak to physicians around the country and I hear that their workplaces are understaffed, they’re overworked and they don’t feel safe. Although we’re in a different phase of the pandemic, doctors feel that the ground beneath them is still shaky,” said Dr. Sánchez, the author of “Recapturing Joy in Medicine.”
Most doctors are seeing more patients than they can handle and are expected to do that consistently. “When you no longer have the capacity to give of yourself, that becomes a nearly impossible task,” said Dr. Sánchez.
Also, physicians in understaffed workplaces often must take on additional work such as administrative or nursing duties, said Katie Cole, DO, a board-certified psychiatrist and a physician coach.
While health systems are aware that physicians need time to rest and recharge, staffing shortages prevent doctors from taking time off because they can’t find coverage, said Dr. Cole.
“While we know that it’s important for physicians to take vacations, more than one-third of doctors still take 2 weeks or less of vacation annually,” said Dr. Cole.
Physicians also tend to have less compassion for themselves and sacrifice self-care compared to other health care workers. “When a patient dies, nurses get together, debrief, and hug each other, whereas doctors have another patient to see. The culture of medicine doesn’t support self-compassion for physicians,” said Dr. Cole.
Physicians also felt less safe at work during the pandemic because of to shortages of personal protective equipment, said Dr. Sánchez. They have also witnessed or experienced an increase in abusive behavior, violence and threats of violence.
Physicians’ personal life suffers
Doctors maintain their mental health primarily by spending time with family members and friends, according to 2022’s Medscape Physician Lifestyle & Happiness Report. Yet half of doctors reported in a survey by the Physicians Foundation that they withdrew from family, friends or coworkers in 2022, said Dr. Sánchez.
“When you exceed your mental, emotional, and physical capacity at work, you have no reserve left for your personal life,” said Dr. Cole.
That may explain why only 58% of doctors reported feeling somewhat or very happy outside of work, compared with 84% who felt that way before the pandemic.
More women doctors said they deal with stronger feelings of conflict in trying to balance parenting responsibilities with a highly demanding job. Nearly one in two women physician-parents reported feeling very conflicted at work, compared with about one in four male physician-parents.
When physicians go home, they may be emotionally drained and tired mentally from making a lot of decisions at work, said Dr. Cole.
“As a woman, if you have children and a husband and you’re responsible for dinner, picking up the kids at daycare or helping them with homework, and making all these decisions when you get home, it’s overwhelming,” said Dr. Cole.
Prioritize your well-being
Doctors need to prioritize their own well-being, said Dr. Sánchez. “That’s not being selfish, that’s doing what’s necessary to stay well and be able to take care of patients. If doctors don’t take care of themselves, no one else will.”
Dr. Sánchez recommended that doctors regularly interact with relatives, friends, trusted colleagues, or clergy to help maintain their well-being, rather than waiting until a crisis to reach out.
A good coach, mentor, or counselor can help physicians gain enough self-awareness to handle their emotions and gain more clarity about what changes need to be made, she said.
Dr. Cole suggested that doctors figure out what makes them happy and fulfilled at work and try to spend more time on that activity. “Knowing what makes you happy and your strengths are foundational for creating a life you love.”
She urged doctors to “start thinking now about what you love about medicine and what is going right at home, and what areas you want to change. Then, start advocating for your needs.”
A version of this article originally appeared on Medscape.com.
Correction, 1/26/23: An earlier version of this article misstated the findings of the survey.
Diagnostic Errors in Hospitalized Patients
Abstract
Diagnostic errors in hospitalized patients are a leading cause of preventable morbidity and mortality. Significant challenges in defining and measuring diagnostic errors and underlying process failure points have led to considerable variability in reported rates of diagnostic errors and adverse outcomes. In this article, we explore the diagnostic process and its discrete components, emphasizing the centrality of the patient in decision-making as well as the continuous nature of the process. We review the incidence of diagnostic errors in hospitalized patients and different methodological approaches that have been used to arrive at these estimates. We discuss different but interdependent provider- and system-related process-failure points that lead to diagnostic errors. We examine specific challenges related to measurement of diagnostic errors and describe traditional and novel approaches that are being used to obtain the most precise estimates. Finally, we examine various patient-, provider-, and organizational-level interventions that have been proposed to improve diagnostic safety in hospitalized patients.
Keywords: diagnostic error, hospital medicine, patient safety.
Diagnosis is defined as a “pre-existing set of categories agreed upon by the medical profession to designate a specific condition.”1 The diagnostic process involves obtaining a clinical history, performing a physical examination, conducting diagnostic testing, and consulting with other clinical providers to gather data that are relevant to understanding the underlying disease processes. This exercise involves generating hypotheses and updating prior probabilities as more information and evidence become available. Throughout this process of information gathering, integration, and interpretation, there is an ongoing assessment of whether sufficient and necessary knowledge has been obtained to make an accurate diagnosis and provide appropriate treatment.2
Diagnostic error is defined as a missed opportunity to make a timely diagnosis as part of this iterative process, including the failure of communicating the diagnosis to the patient in a timely manner.3 It can be categorized as a missed, delayed, or incorrect diagnosis based on available evidence at the time. Establishing the correct diagnosis has important implications. A timely and precise diagnosis ensures the patient the highest probability of having a positive health outcome that reflects an appropriate understanding of underlying disease processes and is consistent with their overall goals of care.3 When diagnostic errors occur, they can cause patient harm. Adverse events due to medical errors, including diagnostic errors, are estimated to be the third leading cause of death in the United States.4 Most people will experience at least 1 diagnostic error in their lifetime. In the 2015 National Academy of Medicine report Improving Diagnosis in Healthcare, diagnostic errors were identified as a major hazard as well as an opportunity to improve patient outcomes.2
Diagnostic errors during hospitalizations are especially concerning, as they are more likely to be implicated in a wider spectrum of harm, including permanent disability and death. This has become even more relevant for hospital medicine physicians and other clinical providers as they encounter increasing cognitive and administrative workloads, rising dissatisfaction and burnout, and unique obstacles such as night-time scheduling.5
Incidence of Diagnostic Errors in Hospitalized Patients
Several methodological approaches have been used to estimate the incidence of diagnostic errors in hospitalized patients. These include retrospective reviews of a sample of all hospital admissions, evaluations of selected adverse outcomes including autopsy studies, patient and provider surveys, and malpractice claims. Laboratory testing audits and secondary reviews in other diagnostic subspecialities (eg, radiology, pathology, and microbiology) are also essential to improving diagnostic performance in these specialized fields, which in turn affects overall hospital diagnostic error rates.6-8 These diverse approaches provide unique insights regarding our ability to assess the degree to which potential harms, ranging from temporary impairment to permanent disability, to death, are attributable to different failure points in the diagnostic process.
Large retrospective chart reviews of random hospital admissions remain the most accurate way to determine the overall incidence of diagnostic errors in hospitalized patients.9 The Harvard Medical Practice Study, published in 1991, laid the groundwork for measuring the incidence of adverse events in hospitalized patients and assessing their relation to medical error, negligence, and disability. Reviewing 30,121 randomly selected records from 51 randomly selected acute care hospitals in New York State, the study found that adverse events occurred in 3.7% of hospitalizations, diagnostic errors accounted for 13.8% of these events, and these errors were likely attributable to negligence in 74.7% of cases. The study not only outlined individual-level process failures, but also focused attention on some of the systemic causes, setting the agenda for quality improvement research in hospital-based care for years to come.10-12 A recent systematic review and meta-analysis of 22 hospital admission studies found a pooled rate of 0.7% (95% CI, 0.5%-1.1%) for harmful diagnostic errors.9 It found significant variations in the rates of adverse events, diagnostic errors, and range of diagnoses that were missed. This was primarily because of variabilities in pre-test probabilities in detecting diagnostic errors in these specific cohorts, as well as due to heterogeneity in study definitions and methodologies, especially regarding how they defined and measured “diagnostic error.” The analysis, however, did not account for diagnostic errors that were not related to patient harm (missed opportunities); therefore, it likely significantly underestimated the true incidence of diagnostic errors in these study populations. Table 1 summarizes some of key studies that have examined the incidence of harmful diagnostic errors in hospitalized patients.9-21
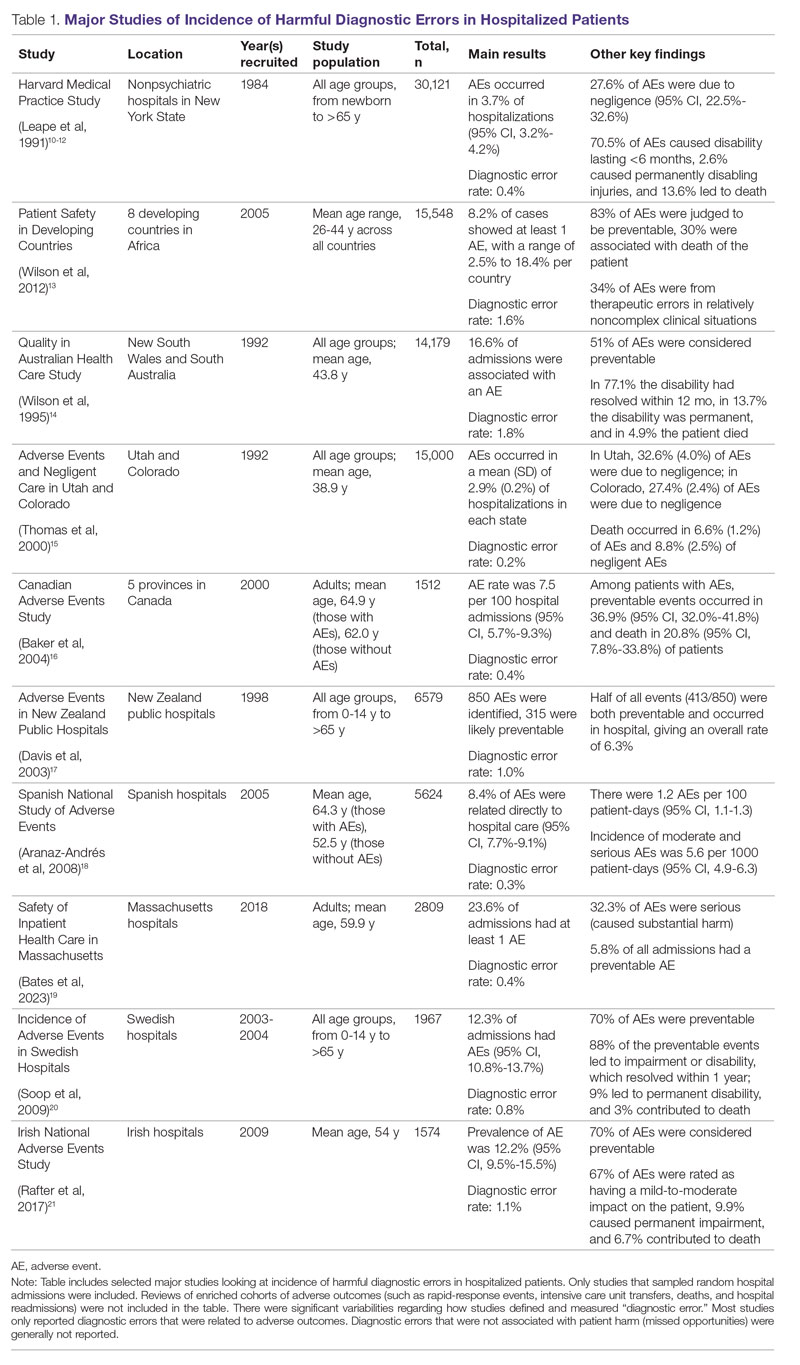
The chief limitation of reviewing random hospital admissions is that, since overall rates of diagnostic errors are still relatively low, a large number of case reviews are required to identify a sufficient sample of adverse outcomes to gain a meaningful understanding of the underlying process failure points and develop tools for remediation. Patient and provider surveys or data from malpractice claims can be high-yield starting points for research on process errors.22,23 Reviews of enriched cohorts of adverse outcomes, such as rapid-response events, intensive care unit (ICU) transfers, deaths, and hospital readmissions, can be an efficient way to identify process failures that lead to greatest harm. Depending on the research approach and the types of underlying patient populations sampled, rates of diagnostic errors in these high-risk groups have been estimated to be approximately 5% to 20%, or even higher.6,24-31 For example, a retrospective study of 391 cases of unplanned 7-day readmissions found that 5.6% of cases contained at least 1 diagnostic error during the index admission.32 In a study conducted at 6 Belgian acute-care hospitals, 56% of patients requiring an unplanned transfer to a higher level of care were determined to have had an adverse event, and of these adverse events, 12.4% of cases were associated with errors in diagnosis.29 A systematic review of 16 hospital-based studies estimated that 3.1% of all inpatient deaths were likely preventable, which corresponded to 22,165 deaths annually in the United States.30 Another such review of 31 autopsy studies reported that 28% of autopsied ICU patients had at least 1 misdiagnosis; of these diagnostic errors, 8% were classified as potentially lethal, and 15% were considered major but not lethal.31 Significant drawbacks of such enriched cohort studies, however, are their poor generalizability and inability to detect failure points that do not lead to patient harm (near-miss events).33
Causes of Diagnostic Errors in Hospitalized Patients
All aspects of the diagnostic process are susceptible to errors. These errors stem from a variety of faulty processes, including failure of the patient to engage with the health care system (eg, due to lack of insurance or transportation, or delay in seeking care); failure in information gathering (eg, missed history or exam findings, ordering wrong tests, laboratory errors); failure in information interpretation (eg, exam finding or test result misinterpretation); inaccurate hypothesis generation (eg, due to suboptimal prioritization or weighing of supporting evidence); and failure in communication (eg, with other team members or with the patient).2,34 Reasons for diagnostic process failures vary widely across different health care settings. While clinician assessment errors (eg, failure to consider or alternatively overweigh competing diagnoses) and errors in testing and the monitoring phase (eg, failure to order or follow up diagnostic tests) can lead to a majority of diagnostic errors in some patient populations, in other settings, social (eg, poor health literacy, punitive cultural practices) and economic factors (eg, lack of access to appropriate diagnostic tests or to specialty expertise) play a more prominent role.34,35
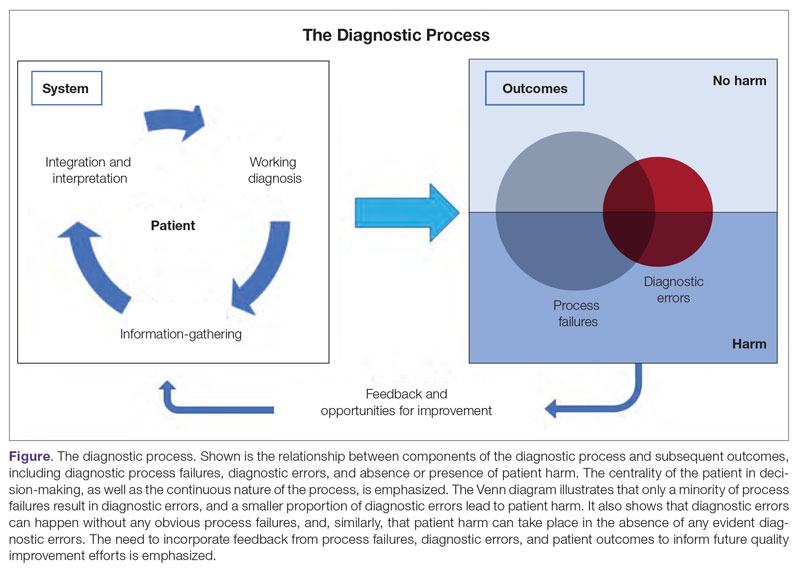
The Figure describes the relationship between components of the diagnostic process and subsequent outcomes, including diagnostic process failures, diagnostic errors, and absence or presence of patient harm.2,36,37 It reemphasizes the centrality of the patient in decision-making and the continuous nature of the process. The Figure also illustrates that only a minority of process failures result in diagnostic errors, and a smaller proportion of diagnostic errors actually lead to patient harm. Conversely, it also shows that diagnostic errors can happen without any obvious process-failure points, and, similarly, patient harm can take place in the absence of any evident diagnostic errors.36-38 Finally, it highlights the need to incorporate feedback from process failures, diagnostic errors, and favorable and unfavorable patient outcomes in order to inform future quality improvement efforts and research.
A significant proportion of diagnostic errors are due to system-related vulnerabilities, such as limitations in availability, adoption or quality of work force training, health informatics resources, and diagnostic capabilities. Lack of institutional culture that promotes safety and transparency also predisposes to diagnostic errors.39,40 The other major domain of process failures is related to cognitive errors in clinician decision-making. Anchoring, confirmation bias, availability bias, and base-rate neglect are some of the common cognitive biases that, along with personality traits (aversion to risk or ambiguity, overconfidence) and affective biases (influence of emotion on decision-making), often determine the degree of utilization of resources and the possibility of suboptimal diagnostic performance.41,42 Further, implicit biases related to age, race, gender, and sexual orientation contribute to disparities in access to health care and outcomes.43 In a large number of cases of preventable adverse outcomes, however, there are multiple interdependent individual and system-related failure points that lead to diagnostic error and patient harm.6,32
Challenges in Defining and Measuring Diagnostic Errors
In order to develop effective, evidence-based interventions to reduce diagnostic errors in hospitalized patients, it is essential to be able to first operationally define, and then accurately measure, diagnostic errors and the process failures that contribute to these errors in a standardized way that is reproducible across different settings.6,44 There are a number of obstacles in this endeavor.
A fundamental problem is that establishing a diagnosis is not a single act but a process. Patterns of symptoms and clinical presentations often differ for the same disease. Information required to make a diagnosis is usually gathered in stages, where the clinician obtains additional data, while considering many possibilities, of which 1 may be ultimately correct. Diagnoses evolve over time and in different care settings. “The most likely diagnosis” is not always the same as “the final correct diagnosis.” Moreover, the diagnostic process is influenced by patients’ individual clinical courses and preferences over time. This makes determination of missed, delayed, or incorrect diagnoses challenging.45,46
For hospitalized patients, generally the goal is to first rule out more serious and acute conditions (eg, pulmonary embolism or stroke), even if their probability is rather low. Conversely, a diagnosis that appears less consequential if delayed (eg, chronic anemia of unclear etiology) might not be pursued on an urgent basis, and is often left to outpatient providers to examine, but still may manifest in downstream harm (eg, delayed diagnosis of gastrointestinal malignancy or recurrent admissions for heart failure due to missed iron-deficiency anemia). Therefore, coming up with disease diagnosis likelihoods in hindsight may turn out to be highly subjective and not always accurate. This can be particularly difficult when clinician and other team deliberations are not recorded in their entirety.47
Another hurdle in the practice of diagnostic medicine is to preserve the balance between underdiagnosing versus pursuing overly aggressive diagnostic approaches. Conducting laboratory, imaging, or other diagnostic studies without a clear shared understanding of how they would affect clinical decision-making (eg, use of prostate-specific antigen to detect prostate cancer) not only leads to increased costs but can also delay appropriate care. Worse, subsequent unnecessary diagnostic tests and treatments can sometimes lead to serious harm.48,49
Finally, retrospective reviews by clinicians are subject to multiple potential limitations that include failure to create well-defined research questions, poorly developed inclusion and exclusion criteria, and issues related to inter- and intra-rater reliability.50 These methodological deficiencies can occur despite following "best practice" guidelines during the study planning, execution, and analysis phases. They further add to the challenge of defining and measuring diagnostic errors.47
Strategies to Improve Measurement of Diagnostic Errors
Development of new methodologies to reliably measure diagnostic errors is an area of active research. The advancement of uniform and universally agreed-upon frameworks to define and identify process failure points and diagnostic errors would help reduce measurement error and support development and testing of interventions that could be generalizable across different health care settings. To more accurately define and measure diagnostic errors, several novel approaches have been proposed (Table 2).
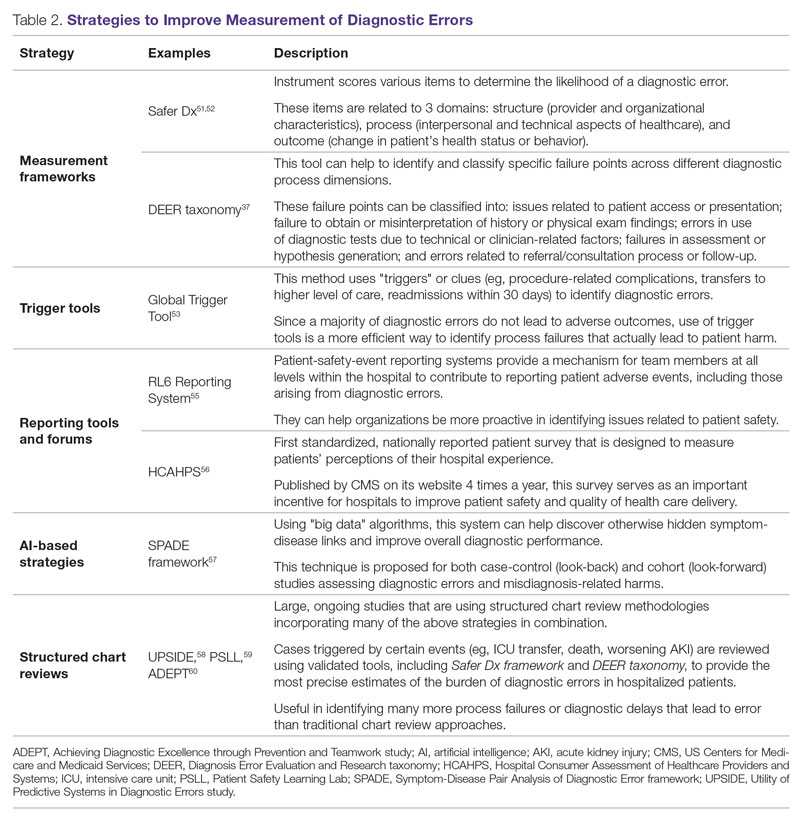
The Safer Dx framework is an all-round tool developed to advance the discipline of measuring diagnostic errors. For an episode of care under review, the instrument scores various items to determine the likelihood of a diagnostic error. These items evaluate multiple dimensions affecting diagnostic performance and measurements across 3 broad domains: structure (provider and organizational characteristics—from everyone involved with patient care, to computing infrastructure, to policies and regulations), process (elements of the patient-provider encounter, diagnostic test performance and follow-up, and subspecialty- and referral-specific factors), and outcome (establishing accurate and timely diagnosis as opposed to missed, delayed, or incorrect diagnosis). This instrument has been revised and can be further modified by a variety of stakeholders, including clinicians, health care organizations, and policymakers, to identify potential diagnostic errors in a standardized way for patient safety and quality improvement research.51,52
Use of standardized tools, such as the Diagnosis Error Evaluation and Research (DEER) taxonomy, can help to identify and classify specific failure points across different diagnostic process dimensions.37 These failure points can be classified into: issues related to patient presentation or access to health care; failure to obtain or misinterpretation of history or physical exam findings; errors in use of diagnostics tests due to technical or clinician-related factors; failures in appropriate weighing of evidence and hypothesis generation; errors associated with referral or consultation process; and failure to monitor the patient or obtain timely follow-up.34 The DEER taxonomy can also be modified based on specific research questions and study populations. Further, it can be recategorized to correspond to Safer Dx framework diagnostic process dimensions to provide insights into reasons for specific process failures and to develop new interventions to mitigate errors and patient harm.6
Since a majority of diagnostic errors do not lead to actual harm, use of “triggers” or clues (eg, procedure-related complications, patient falls, transfers to a higher level of care, readmissions within 30 days) can be a more efficient method to identify diagnostic errors and adverse events that do cause harm. The Global Trigger Tool, developed by the Institute for Healthcare Improvement, uses this strategy. This tool has been shown to identify a significantly higher number of serious adverse events than comparable methods.53 This facilitates selection and development of strategies at the institutional level that are most likely to improve patient outcomes.24
Encouraging and facilitating voluntary or prompted reporting from patients and clinicians can also play an important role in capturing diagnostic errors. Patients and clinicians are not only the key stakeholders but are also uniquely placed within the diagnostic process to detect and report potential errors.25,54 Patient-safety-event reporting systems, such as RL6, play a vital role in reporting near-misses and adverse events. These systems provide a mechanism for team members at all levels within the hospital to contribute toward reporting patient adverse events, including those arising from diagnostic errors.55 The Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey is the first standardized, nationally reported patient survey designed to measure patients’ perceptions of their hospital experience. The US Centers for Medicare and Medicaid Services (CMS) publishes HCAHPS results on its website 4 times a year, which serves as an important incentive for hospitals to improve patient safety and quality of health care delivery.56
Another novel approach links multiple symptoms to a range of target diseases using the Symptom-Disease Pair Analysis of Diagnostic Error (SPADE) framework. Using “big data” technologies, this technique can help discover otherwise hidden symptom-disease links and improve overall diagnostic performance. This approach is proposed for both case-control (look-back) and cohort (look-forward) studies assessing diagnostic errors and misdiagnosis-related harms. For example, starting with a known diagnosis with high potential for harm (eg, stroke), the “look-back” approach can be used to identify high-risk symptoms (eg, dizziness, vertigo). In the “look-forward” approach, a single symptom or exposure risk factor known to be frequently misdiagnosed (eg, dizziness) can be analyzed to identify potential adverse disease outcomes (eg, stroke, migraine).57
Many large ongoing studies looking at diagnostic errors among hospitalized patients, such as Utility of Predictive Systems to identify Inpatient Diagnostic Errors (UPSIDE),58Patient Safety Learning Lab (PSLL),59 and Achieving Diagnostic Excellence through Prevention and Teamwork (ADEPT),60 are using structured chart review methodologies incorporating many of the above strategies in combination. Cases triggered by certain events (eg, ICU transfer, death, rapid response event, new or worsening acute kidney injury) are reviewed using validated tools, including Safer Dx framework and DEER taxonomy, to provide the most precise estimates of the burden of diagnostic errors in hospitalized patients. These estimates may be much higher than previously predicted using traditional chart review approaches.6,24 For example, a recently published study of 2809 random admissions in 11 Massachusetts hospitals identified 978 adverse events but only 10 diagnostic errors (diagnostic error rate, 0.4%).19 This was likely because the trigger method used in the study did not specifically examine the diagnostic process as critically as done by the Safer Dx framework and DEER taxonomy tools, thereby underestimating the total number of diagnostic errors. Further, these ongoing studies (eg, UPSIDE, ADEPT) aim to employ new and upcoming advanced machine-learning methods to create models that can improve overall diagnostic performance. This would pave the way to test and build novel, efficient, and scalable interventions to reduce diagnostic errors and improve patient outcomes.
Strategies to Improve Diagnostic Safety in Hospitalized Patients
Disease-specific biomedical research, as well as advances in laboratory, imaging, and other technologies, play a critical role in improving diagnostic accuracy. However, these technical approaches do not address many of the broader clinician- and system-level failure points and opportunities for improvement. Various patient-, provider-, and organizational-level interventions that could make diagnostic processes more resilient and reduce the risk of error and patient harm have been proposed.61
Among these strategies are approaches to empower patients and their families. Fostering therapeutic relationships between patients and members of the care team is essential to reducing diagnostic errors.62 Facilitating timely access to health records, ensuring transparency in decision making, and tailoring communication strategies to patients’ cultural and educational backgrounds can reduce harm.63 Similarly, at the system level, enhancing communication among different providers by use of tools such as structured handoffs can prevent communication breakdowns and facilitate positive outcomes.64
Interventions targeted at individual health care providers, such as educational programs to improve content-specific knowledge, can enhance diagnostic performance. Regular feedback, strategies to enhance equity, and fostering an environment where all providers are actively encouraged to think critically and participate in the diagnostic process (training programs to use “diagnostic time-outs” and making it a “team sport”) can improve clinical reasoning.65,66 Use of standardized patients can help identify individual-level cognitive failure points and facilitate creation of new interventions to improve clinical decision-making processes.67
Novel health information technologies can further augment these efforts. These include effective documentation by maintaining dynamic and accurate patient histories, problem lists, and medication lists68-70; use of electronic health record–based algorithms to identify potential diagnostic delays for serious conditions71,72; use of telemedicine technologies to improve accessibility and coordination73;application of mobile health and wearable technologies to facilitate data-gathering and care delivery74,75; and use of computerized decision-support tools, including applications to interpret electrocardiograms, imaging studies, and other diagnostic tests.76
Use of precision medicine, powered by new artificial intelligence (AI) tools, is becoming more widespread. Algorithms powered by AI can augment and sometimes even outperform clinician decision-making in areas such as oncology, radiology, and primary care.77 Creation of large biobanks like the All of Us research program can be used to study thousands of environmental and genetic risk factors and health conditions simultaneously, and help identify specific treatments that work best for people of different backgrounds.78 Active research in these areas holds great promise in terms of how and when we diagnose diseases and make appropriate preventative and treatment decisions. Significant scientific, ethical, and regulatory challenges will need to be overcome before these technologies can address some of the most complex problems in health care.79
Finally, diagnostic performance is affected by the external environment, including the functioning of the medical liability system. Diagnostic errors that lead to patient harm are a leading cause of malpractice claims.80 Developing a legal environment, in collaboration with patient advocacy groups and health care organizations, that promotes and facilitates timely disclosure of diagnostic errors could decrease the incentive to hide errors, advance care processes, and improve outcomes.81,82
Conclusion
The burden of diagnostic errors in hospitalized patients is unacceptably high and remains an underemphasized cause of preventable morbidity and mortality. Diagnostic errors often result from a breakdown in multiple interdependent processes that involve patient-, provider-, and system-level factors. Significant challenges remain in defining and identifying diagnostic errors as well as underlying process-failure points. The most effective interventions to reduce diagnostic errors will require greater patient participation in the diagnostic process and a mix of evidence-based interventions that promote individual-provider excellence as well as system-level changes. Further research and collaboration among various stakeholders should help improve diagnostic safety for hospitalized patients.
Corresponding author: Abhishek Goyal, MD, MPH; agoyal4@bwh.harvard.edu
Disclosures: Dr. Dalal disclosed receiving income ≥ $250 from MayaMD.
1. Graber ML, Franklin N, Gordon R. Diagnostic error in internal medicine. Arch Intern Med. 2005;165(13):1493-1499. doi:10.1001/archinte.165.13.1493
2. National Academies of Sciences, Engineering, and Medicine. 2015. Improving Diagnosis in Health Care. The National Academies Press. doi:10.17226/21794
3. Singh H, Graber ML. Improving diagnosis in health care—the next imperative for patient safety. N Engl J Med. 2015;373(26):2493-2495. doi:10.1056/NEJMp1512241
4. Makary MA, Daniel M. Medical error—the third leading cause of death in the US. BMJ. 2016;353:i2139. doi:10.1136/bmj.i2139
5. Flanders SA, Centor B, Weber V, McGinn T, Desalvo K, Auerbach A. Challenges and opportunities in academic hospital medicine: report from the academic hospital medicine summit. J Gen Intern Med. 2009;24(5):636-641. doi:10.1007/s11606-009-0944-6
6. Griffin JA, Carr K, Bersani K, et al. Analyzing diagnostic errors in the acute setting: a process-driven approach. Diagnosis (Berl). 2021;9(1):77-88. doi:10.1515/dx-2021-0033
7. Itri JN, Tappouni RR, McEachern RO, Pesch AJ, Patel SH. Fundamentals of diagnostic error in imaging. RadioGraphics. 2018;38(6):1845-1865. doi:10.1148/rg.2018180021
8. Hammerling JA. A Review of medical errors in laboratory diagnostics and where we are today. Lab Med. 2012;43(2):41-44. doi:10.1309/LM6ER9WJR1IHQAUY
9. Gunderson CG, Bilan VP, Holleck JL, et al. Prevalence of harmful diagnostic errors in hospitalised adults: a systematic review and meta-analysis. BMJ Qual Saf. 2020;29(12):1008-1018. doi:10.1136/bmjqs-2019-010822
10. Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324(6):370-376. doi:10.1056/NEJM199102073240604
11. Leape LL, Brennan TA, Laird N, et al. The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II. N Engl J Med. 1991;324(6):377-384. doi:10.1056/NEJM199102073240605
12. Localio AR, Lawthers AG, Brennan TA, et al. Relation between malpractice claims and adverse events due to negligence. Results of the Harvard Medical Practice Study III. N Engl J Med. 1991;325(4):245-251. doi:10.1056/NEJM199107253250405
13. Wilson RM, Michel P, Olsen S, et al. Patient safety in developing countries: retrospective estimation of scale and nature of harm to patients in hospital. BMJ. 2012;344:e832. doi:10.1136/bmj.e832
14. Wilson RM, Runciman WB, Gibberd RW, Harrison BT, Newby L, Hamilton JD. The Quality in Australian Health Care Study. Med J Aust. 1995;163(9):458-471. doi:10.5694/j.1326-5377.1995.tb124691.x
15. Thomas EJ, Studdert DM, Burstin HR, et al. Incidence and types of adverse events and negligent care in Utah and Colorado. Med Care. 2000;38(3):261-271. doi:10.1097/00005650-200003000-00003
16. Baker GR, Norton PG, Flintoft V, et al. The Canadian Adverse Events Study: the incidence of adverse events among hospital patients in Canada. CMAJ. 2004;170(11):1678-1686. doi:10.1503/cmaj.1040498
17. Davis P, Lay-Yee R, Briant R, Ali W, Scott A, Schug S. Adverse events in New Zealand public hospitals II: preventability and clinical context. N Z Med J. 2003;116(1183):U624.
18. Aranaz-Andrés JM, Aibar-Remón C, Vitaller-Murillo J, et al. Incidence of adverse events related to health care in Spain: results of the Spanish National Study of Adverse Events. J Epidemiol Community Health. 2008;62(12):1022-1029. doi:10.1136/jech.2007.065227
19. Bates DW, Levine DM, Salmasian H, et al. The safety of inpatient health care. N Engl J Med. 2023;388(2):142-153. doi:10.1056/NEJMsa2206117
20. Soop M, Fryksmark U, Köster M, Haglund B. The incidence of adverse events in Swedish hospitals: a retrospective medical record review study. Int J Qual Health Care. 2009;21(4):285-291. doi:10.1093/intqhc/mzp025
21. Rafter N, Hickey A, Conroy RM, et al. The Irish National Adverse Events Study (INAES): the frequency and nature of adverse events in Irish hospitals—a retrospective record review study. BMJ Qual Saf. 2017;26(2):111-119. doi:10.1136/bmjqs-2015-004828
22. Blendon RJ, DesRoches CM, Brodie M, et al. Views of practicing physicians and the public on medical errors. N Engl J Med. 2002;347(24):1933-1940. doi:10.1056/NEJMsa022151
23. Saber Tehrani AS, Lee H, Mathews SC, et al. 25-year summary of US malpractice claims for diagnostic errors 1986-2010: an analysis from the National Practitioner Data Bank. BMJ Qual Saf. 2013;22(8):672-680. doi:10.1136/bmjqs-2012-001550
24. Malik MA, Motta-Calderon D, Piniella N, et al. A structured approach to EHR surveillance of diagnostic error in acute care: an exploratory analysis of two institutionally-defined case cohorts. Diagnosis (Berl). 2022;9(4):446-457. doi:10.1515/dx-2022-0032
25. Graber ML. The incidence of diagnostic error in medicine. BMJ Qual Saf. 2013;22(suppl 2):ii21-ii27. doi:10.1136/bmjqs-2012-001615
26. Bergl PA, Taneja A, El-Kareh R, Singh H, Nanchal RS. Frequency, risk factors, causes, and consequences of diagnostic errors in critically ill medical patients: a retrospective cohort study. Crit Care Med. 2019;47(11):e902-e910. doi:10.1097/CCM.0000000000003976
27. Hogan H, Healey F, Neale G, Thomson R, Vincent C, Black N. Preventable deaths due to problems in care in English acute hospitals: a retrospective case record review study. BMJ Qual Saf. 2012;21(9):737-745. doi:10.1136/bmjqs-2011-001159
28. Bergl PA, Nanchal RS, Singh H. Diagnostic error in the critically ill: defining the problem and exploring next steps to advance intensive care unit safety. Ann Am Thorac Soc. 2018;15(8):903-907. doi:10.1513/AnnalsATS.201801-068PS
29. Marquet K, Claes N, De Troy E, et al. One fourth of unplanned transfers to a higher level of care are associated with a highly preventable adverse event: a patient record review in six Belgian hospitals. Crit Care Med. 2015;43(5):1053-1061. doi:10.1097/CCM.0000000000000932
30. Rodwin BA, Bilan VP, Merchant NB, et al. Rate of preventable mortality in hospitalized patients: a systematic review and meta-analysis. J Gen Intern Med. 2020;35(7):2099-2106. doi:10.1007/s11606-019-05592-5
31. Winters B, Custer J, Galvagno SM, et al. Diagnostic errors in the intensive care unit: a systematic review of autopsy studies. BMJ Qual Saf. 2012;21(11):894-902. doi:10.1136/bmjqs-2012-000803
32. Raffel KE, Kantor MA, Barish P, et al. Prevalence and characterisation of diagnostic error among 7-day all-cause hospital medicine readmissions: a retrospective cohort study. BMJ Qual Saf. 2020;29(12):971-979. doi:10.1136/bmjqs-2020-010896
33. Weingart SN, Pagovich O, Sands DZ, et al. What can hospitalized patients tell us about adverse events? learning from patient-reported incidents. J Gen Intern Med. 2005;20(9):830-836. doi:10.1111/j.1525-1497.2005.0180.x
34. Schiff GD, Hasan O, Kim S, et al. Diagnostic error in medicine: analysis of 583 physician-reported errors. Arch Intern Med. 2009;169(20):1881-1887. doi:10.1001/archinternmed.2009.333
35. Singh H, Schiff GD, Graber ML, Onakpoya I, Thompson MJ. The global burden of diagnostic errors in primary care. BMJ Qual Saf. 2017;26(6):484-494. doi:10.1136/bmjqs-2016-005401
36. Schiff GD, Leape LL. Commentary: how can we make diagnosis safer? Acad Med J Assoc Am Med Coll. 2012;87(2):135-138. doi:10.1097/ACM.0b013e31823f711c
37. Schiff GD, Kim S, Abrams R, et al. Diagnosing diagnosis errors: lessons from a multi-institutional collaborative project. In: Henriksen K, Battles JB, Marks ES, Lewin DI, eds. Advances in Patient Safety: From Research to Implementation. Volume 2: Concepts and Methodology. AHRQ Publication No. 05-0021-2. Agency for Healthcare Research and Quality (US); 2005. Accessed January 16, 2023. http://www.ncbi.nlm.nih.gov/books/NBK20492/
38. Newman-Toker DE. A unified conceptual model for diagnostic errors: underdiagnosis, overdiagnosis, and misdiagnosis. Diagnosis (Berl). 2014;1(1):43-48. doi:10.1515/dx-2013-0027
39. Abimanyi-Ochom J, Bohingamu Mudiyanselage S, Catchpool M, Firipis M, Wanni Arachchige Dona S, Watts JJ. Strategies to reduce diagnostic errors: a systematic review. BMC Med Inform Decis Mak. 2019;19(1):174. doi:10.1186/s12911-019-0901-1
40. Gupta A, Harrod M, Quinn M, et al. Mind the overlap: how system problems contribute to cognitive failure and diagnostic errors. Diagnosis (Berl). 2018;5(3):151-156. doi:10.1515/dx-2018-0014
41. Saposnik G, Redelmeier D, Ruff CC, Tobler PN. Cognitive biases associated with medical decisions: a systematic review. BMC Med Inform Decis Mak. 2016;16:138. doi:10.1186/s12911-016-0377-1
42. Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med. 2003;78(8):775-780. doi: 10.1097/00001888-200308000-00003
43. Chapman EN, Kaatz A, Carnes M. Physicians and implicit bias: how doctors may unwittingly perpetuate health care disparities. J Gen Intern Med. 2013;28(11):1504-1510. doi:10.1007/s11606-013-2441-1
44. Zwaan L, Singh H. The challenges in defining and measuring diagnostic error. Diagnosis (Ber). 2015;2(2):97-103. doi:10.1515/dx-2014-0069
45. Arkes HR, Wortmann RL, Saville PD, Harkness AR. Hindsight bias among physicians weighing the likelihood of diagnoses. J Appl Psychol. 1981;66(2):252-254.
46. Singh H. Editorial: Helping health care organizations to define diagnostic errors as missed opportunities in diagnosis. Jt Comm J Qual Patient Saf. 2014;40(3):99-101. doi:10.1016/s1553-7250(14)40012-6
47. Vassar M, Holzmann M. The retrospective chart review: important methodological considerations. J Educ Eval Health Prof. 2013;10:12. doi:10.3352/jeehp.2013.10.12
48. Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102(9):605-613. doi:10.1093/jnci/djq099
49. Moynihan R, Doust J, Henry D. Preventing overdiagnosis: how to stop harming the healthy. BMJ. 2012;344:e3502. doi:10.1136/bmj.e3502
50. Hayward RA, Hofer TP. Estimating hospital deaths due to medical errors: preventability is in the eye of the reviewer. JAMA. 2001;286(4):415-420. doi:10.1001/jama.286.4.415
51. Singh H, Sittig DF. Advancing the science of measurement of diagnostic errors in healthcare: the Safer Dx framework. BMJ Qual Saf. 2015;24(2):103-110. doi:10.1136/bmjqs-2014-003675
52. Singh H, Khanna A, Spitzmueller C, Meyer AND. Recommendations for using the Revised Safer Dx Instrument to help measure and improve diagnostic safety. Diagnosis (Berl). 2019;6(4):315-323. doi:10.1515/dx-2019-0012
53. Classen DC, Resar R, Griffin F, et al. “Global trigger tool” shows that adverse events in hospitals may be ten times greater than previously measured. Health Aff (Millwood). 2011;30(4):581-589. doi:10.1377/hlthaff.2011.0190
54. Schiff GD. Minimizing diagnostic error: the importance of follow-up and feedback. Am J Med. 2008;121(5 suppl):S38-S42. doi:10.1016/j.amjmed.2008.02.004
55. Mitchell I, Schuster A, Smith K, Pronovost P, Wu A. Patient safety incident reporting: a qualitative study of thoughts and perceptions of experts 15 years after “To Err is Human.” BMJ Qual Saf. 2016;25(2):92-99. doi:10.1136/bmjqs-2015-004405
56. Mazurenko O, Collum T, Ferdinand A, Menachemi N. Predictors of hospital patient satisfaction as measured by HCAHPS: a systematic review. J Healthc Manag. 2017;62(4):272-283. doi:10.1097/JHM-D-15-00050
57. Liberman AL, Newman-Toker DE. Symptom-Disease Pair Analysis of Diagnostic Error (SPADE): a conceptual framework and methodological approach for unearthing misdiagnosis-related harms using big data. BMJ Qual Saf. 2018;27(7):557-566. doi:10.1136/bmjqs-2017-007032
58. Utility of Predictive Systems to Identify Inpatient Diagnostic Errors: the UPSIDE study. NIH RePort/RePORTER. Accessed January 14, 2023. https://reporter.nih.gov/search/rpoHXlEAcEudQV3B9ld8iw/project-details/10020962
59. Overview of Patient Safety Learning Laboratory (PSLL) Projects. Agency for Healthcare Research and Quality. Accessed January 14, 2023. https://www.ahrq.gov/patient-safety/resources/learning-lab/index.html
60. Achieving Diagnostic Excellence through Prevention and Teamwork (ADEPT). NIH RePort/RePORTER. Accessed January 14, 2023. https://reporter.nih.gov/project-details/10642576
61. Zwaan L, Singh H. Diagnostic error in hospitals: finding forests not just the big trees. BMJ Qual Saf. 2020;29(12):961-964. doi:10.1136/bmjqs-2020-011099
62. Longtin Y, Sax H, Leape LL, Sheridan SE, Donaldson L, Pittet D. Patient participation: current knowledge and applicability to patient safety. Mayo Clin Proc. 2010;85(1):53-62. doi:10.4065/mcp.2009.0248
63. Murphy DR, Singh H, Berlin L. Communication breakdowns and diagnostic errors: a radiology perspective. Diagnosis (Berl). 2014;1(4):253-261. doi:10.1515/dx-2014-0035
64. Singh H, Naik AD, Rao R, Petersen LA. Reducing diagnostic errors through effective communication: harnessing the power of information technology. J Gen Intern Med. 2008;23(4):489-494. doi:10.1007/s11606-007-0393-z
65. Singh H, Connor DM, Dhaliwal G. Five strategies for clinicians to advance diagnostic excellence. BMJ. 2022;376:e068044. doi:10.1136/bmj-2021-068044
66. Yale S, Cohen S, Bordini BJ. Diagnostic time-outs to improve diagnosis. Crit Care Clin. 2022;38(2):185-194. doi:10.1016/j.ccc.2021.11.008
67. Schwartz A, Peskin S, Spiro A, Weiner SJ. Impact of unannounced standardized patient audit and feedback on care, documentation, and costs: an experiment and claims analysis. J Gen Intern Med. 2021;36(1):27-34. doi:10.1007/s11606-020-05965-1
68. Carpenter JD, Gorman PN. Using medication list—problem list mismatches as markers of potential error. Proc AMIA Symp. 2002:106-110.
69. Hron JD, Manzi S, Dionne R, et al. Electronic medication reconciliation and medication errors. Int J Qual Health Care. 2015;27(4):314-319. doi:10.1093/intqhc/mzv046
70. Graber ML, Siegal D, Riah H, Johnston D, Kenyon K. Electronic health record–related events in medical malpractice claims. J Patient Saf. 2019;15(2):77-85. doi:10.1097/PTS.0000000000000240
71. Murphy DR, Wu L, Thomas EJ, Forjuoh SN, Meyer AND, Singh H. Electronic trigger-based intervention to reduce delays in diagnostic evaluation for cancer: a cluster randomized controlled trial. J Clin Oncol. 2015;33(31):3560-3567. doi:10.1200/JCO.2015.61.1301
72. Singh H, Giardina TD, Forjuoh SN, et al. Electronic health record-based surveillance of diagnostic errors in primary care. BMJ Qual Saf. 2012;21(2):93-100. doi:10.1136/bmjqs-2011-000304
73. Armaignac DL, Saxena A, Rubens M, et al. Impact of telemedicine on mortality, length of stay, and cost among patients in progressive care units: experience from a large healthcare system. Crit Care Med. 2018;46(5):728-735. doi:10.1097/CCM.0000000000002994
74. MacKinnon GE, Brittain EL. Mobile health technologies in cardiopulmonary disease. Chest. 2020;157(3):654-664. doi:10.1016/j.chest.2019.10.015
75. DeVore AD, Wosik J, Hernandez AF. The future of wearables in heart failure patients. JACC Heart Fail. 2019;7(11):922-932. doi:10.1016/j.jchf.2019.08.008
76. Tsai TL, Fridsma DB, Gatti G. Computer decision support as a source of interpretation error: the case of electrocardiograms. J Am Med Inform Assoc. 2003;10(5):478-483. doi:10.1197/jamia.M1279
77. Lin SY, Mahoney MR, Sinsky CA. Ten ways artificial intelligence will transform primary care. J Gen Intern Med. 2019;34(8):1626-1630. doi:10.1007/s11606-019-05035-1
78. Ramirez AH, Gebo KA, Harris PA. Progress with the All Of Us research program: opening access for researchers. JAMA. 2021;325(24):2441-2442. doi:10.1001/jama.2021.7702
79. Johnson KB, Wei W, Weeraratne D, et al. Precision medicine, AI, and the future of personalized health care. Clin Transl Sci. 2021;14(1):86-93. doi:10.1111/cts.12884
80. Gupta A, Snyder A, Kachalia A, Flanders S, Saint S, Chopra V. Malpractice claims related to diagnostic errors in the hospital. BMJ Qual Saf. 2017;27(1):bmjqs-2017-006774. doi:10.1136/bmjqs-2017-006774
81. Renkema E, Broekhuis M, Ahaus K. Conditions that influence the impact of malpractice litigation risk on physicians’ behavior regarding patient safety. BMC Health Serv Res. 2014;14(1):38. doi:10.1186/1472-6963-14-38
82. Kachalia A, Mello MM, Nallamothu BK, Studdert DM. Legal and policy interventions to improve patient safety. Circulation. 2016;133(7):661-671. doi:10.1161/CIRCULATIONAHA.115.015880
Abstract
Diagnostic errors in hospitalized patients are a leading cause of preventable morbidity and mortality. Significant challenges in defining and measuring diagnostic errors and underlying process failure points have led to considerable variability in reported rates of diagnostic errors and adverse outcomes. In this article, we explore the diagnostic process and its discrete components, emphasizing the centrality of the patient in decision-making as well as the continuous nature of the process. We review the incidence of diagnostic errors in hospitalized patients and different methodological approaches that have been used to arrive at these estimates. We discuss different but interdependent provider- and system-related process-failure points that lead to diagnostic errors. We examine specific challenges related to measurement of diagnostic errors and describe traditional and novel approaches that are being used to obtain the most precise estimates. Finally, we examine various patient-, provider-, and organizational-level interventions that have been proposed to improve diagnostic safety in hospitalized patients.
Keywords: diagnostic error, hospital medicine, patient safety.
Diagnosis is defined as a “pre-existing set of categories agreed upon by the medical profession to designate a specific condition.”1 The diagnostic process involves obtaining a clinical history, performing a physical examination, conducting diagnostic testing, and consulting with other clinical providers to gather data that are relevant to understanding the underlying disease processes. This exercise involves generating hypotheses and updating prior probabilities as more information and evidence become available. Throughout this process of information gathering, integration, and interpretation, there is an ongoing assessment of whether sufficient and necessary knowledge has been obtained to make an accurate diagnosis and provide appropriate treatment.2
Diagnostic error is defined as a missed opportunity to make a timely diagnosis as part of this iterative process, including the failure of communicating the diagnosis to the patient in a timely manner.3 It can be categorized as a missed, delayed, or incorrect diagnosis based on available evidence at the time. Establishing the correct diagnosis has important implications. A timely and precise diagnosis ensures the patient the highest probability of having a positive health outcome that reflects an appropriate understanding of underlying disease processes and is consistent with their overall goals of care.3 When diagnostic errors occur, they can cause patient harm. Adverse events due to medical errors, including diagnostic errors, are estimated to be the third leading cause of death in the United States.4 Most people will experience at least 1 diagnostic error in their lifetime. In the 2015 National Academy of Medicine report Improving Diagnosis in Healthcare, diagnostic errors were identified as a major hazard as well as an opportunity to improve patient outcomes.2
Diagnostic errors during hospitalizations are especially concerning, as they are more likely to be implicated in a wider spectrum of harm, including permanent disability and death. This has become even more relevant for hospital medicine physicians and other clinical providers as they encounter increasing cognitive and administrative workloads, rising dissatisfaction and burnout, and unique obstacles such as night-time scheduling.5
Incidence of Diagnostic Errors in Hospitalized Patients
Several methodological approaches have been used to estimate the incidence of diagnostic errors in hospitalized patients. These include retrospective reviews of a sample of all hospital admissions, evaluations of selected adverse outcomes including autopsy studies, patient and provider surveys, and malpractice claims. Laboratory testing audits and secondary reviews in other diagnostic subspecialities (eg, radiology, pathology, and microbiology) are also essential to improving diagnostic performance in these specialized fields, which in turn affects overall hospital diagnostic error rates.6-8 These diverse approaches provide unique insights regarding our ability to assess the degree to which potential harms, ranging from temporary impairment to permanent disability, to death, are attributable to different failure points in the diagnostic process.
Large retrospective chart reviews of random hospital admissions remain the most accurate way to determine the overall incidence of diagnostic errors in hospitalized patients.9 The Harvard Medical Practice Study, published in 1991, laid the groundwork for measuring the incidence of adverse events in hospitalized patients and assessing their relation to medical error, negligence, and disability. Reviewing 30,121 randomly selected records from 51 randomly selected acute care hospitals in New York State, the study found that adverse events occurred in 3.7% of hospitalizations, diagnostic errors accounted for 13.8% of these events, and these errors were likely attributable to negligence in 74.7% of cases. The study not only outlined individual-level process failures, but also focused attention on some of the systemic causes, setting the agenda for quality improvement research in hospital-based care for years to come.10-12 A recent systematic review and meta-analysis of 22 hospital admission studies found a pooled rate of 0.7% (95% CI, 0.5%-1.1%) for harmful diagnostic errors.9 It found significant variations in the rates of adverse events, diagnostic errors, and range of diagnoses that were missed. This was primarily because of variabilities in pre-test probabilities in detecting diagnostic errors in these specific cohorts, as well as due to heterogeneity in study definitions and methodologies, especially regarding how they defined and measured “diagnostic error.” The analysis, however, did not account for diagnostic errors that were not related to patient harm (missed opportunities); therefore, it likely significantly underestimated the true incidence of diagnostic errors in these study populations. Table 1 summarizes some of key studies that have examined the incidence of harmful diagnostic errors in hospitalized patients.9-21

The chief limitation of reviewing random hospital admissions is that, since overall rates of diagnostic errors are still relatively low, a large number of case reviews are required to identify a sufficient sample of adverse outcomes to gain a meaningful understanding of the underlying process failure points and develop tools for remediation. Patient and provider surveys or data from malpractice claims can be high-yield starting points for research on process errors.22,23 Reviews of enriched cohorts of adverse outcomes, such as rapid-response events, intensive care unit (ICU) transfers, deaths, and hospital readmissions, can be an efficient way to identify process failures that lead to greatest harm. Depending on the research approach and the types of underlying patient populations sampled, rates of diagnostic errors in these high-risk groups have been estimated to be approximately 5% to 20%, or even higher.6,24-31 For example, a retrospective study of 391 cases of unplanned 7-day readmissions found that 5.6% of cases contained at least 1 diagnostic error during the index admission.32 In a study conducted at 6 Belgian acute-care hospitals, 56% of patients requiring an unplanned transfer to a higher level of care were determined to have had an adverse event, and of these adverse events, 12.4% of cases were associated with errors in diagnosis.29 A systematic review of 16 hospital-based studies estimated that 3.1% of all inpatient deaths were likely preventable, which corresponded to 22,165 deaths annually in the United States.30 Another such review of 31 autopsy studies reported that 28% of autopsied ICU patients had at least 1 misdiagnosis; of these diagnostic errors, 8% were classified as potentially lethal, and 15% were considered major but not lethal.31 Significant drawbacks of such enriched cohort studies, however, are their poor generalizability and inability to detect failure points that do not lead to patient harm (near-miss events).33
Causes of Diagnostic Errors in Hospitalized Patients
All aspects of the diagnostic process are susceptible to errors. These errors stem from a variety of faulty processes, including failure of the patient to engage with the health care system (eg, due to lack of insurance or transportation, or delay in seeking care); failure in information gathering (eg, missed history or exam findings, ordering wrong tests, laboratory errors); failure in information interpretation (eg, exam finding or test result misinterpretation); inaccurate hypothesis generation (eg, due to suboptimal prioritization or weighing of supporting evidence); and failure in communication (eg, with other team members or with the patient).2,34 Reasons for diagnostic process failures vary widely across different health care settings. While clinician assessment errors (eg, failure to consider or alternatively overweigh competing diagnoses) and errors in testing and the monitoring phase (eg, failure to order or follow up diagnostic tests) can lead to a majority of diagnostic errors in some patient populations, in other settings, social (eg, poor health literacy, punitive cultural practices) and economic factors (eg, lack of access to appropriate diagnostic tests or to specialty expertise) play a more prominent role.34,35
The Figure describes the relationship between components of the diagnostic process and subsequent outcomes, including diagnostic process failures, diagnostic errors, and absence or presence of patient harm.2,36,37 It reemphasizes the centrality of the patient in decision-making and the continuous nature of the process. The Figure also illustrates that only a minority of process failures result in diagnostic errors, and a smaller proportion of diagnostic errors actually lead to patient harm. Conversely, it also shows that diagnostic errors can happen without any obvious process-failure points, and, similarly, patient harm can take place in the absence of any evident diagnostic errors.36-38 Finally, it highlights the need to incorporate feedback from process failures, diagnostic errors, and favorable and unfavorable patient outcomes in order to inform future quality improvement efforts and research.

A significant proportion of diagnostic errors are due to system-related vulnerabilities, such as limitations in availability, adoption or quality of work force training, health informatics resources, and diagnostic capabilities. Lack of institutional culture that promotes safety and transparency also predisposes to diagnostic errors.39,40 The other major domain of process failures is related to cognitive errors in clinician decision-making. Anchoring, confirmation bias, availability bias, and base-rate neglect are some of the common cognitive biases that, along with personality traits (aversion to risk or ambiguity, overconfidence) and affective biases (influence of emotion on decision-making), often determine the degree of utilization of resources and the possibility of suboptimal diagnostic performance.41,42 Further, implicit biases related to age, race, gender, and sexual orientation contribute to disparities in access to health care and outcomes.43 In a large number of cases of preventable adverse outcomes, however, there are multiple interdependent individual and system-related failure points that lead to diagnostic error and patient harm.6,32
Challenges in Defining and Measuring Diagnostic Errors
In order to develop effective, evidence-based interventions to reduce diagnostic errors in hospitalized patients, it is essential to be able to first operationally define, and then accurately measure, diagnostic errors and the process failures that contribute to these errors in a standardized way that is reproducible across different settings.6,44 There are a number of obstacles in this endeavor.
A fundamental problem is that establishing a diagnosis is not a single act but a process. Patterns of symptoms and clinical presentations often differ for the same disease. Information required to make a diagnosis is usually gathered in stages, where the clinician obtains additional data, while considering many possibilities, of which 1 may be ultimately correct. Diagnoses evolve over time and in different care settings. “The most likely diagnosis” is not always the same as “the final correct diagnosis.” Moreover, the diagnostic process is influenced by patients’ individual clinical courses and preferences over time. This makes determination of missed, delayed, or incorrect diagnoses challenging.45,46
For hospitalized patients, generally the goal is to first rule out more serious and acute conditions (eg, pulmonary embolism or stroke), even if their probability is rather low. Conversely, a diagnosis that appears less consequential if delayed (eg, chronic anemia of unclear etiology) might not be pursued on an urgent basis, and is often left to outpatient providers to examine, but still may manifest in downstream harm (eg, delayed diagnosis of gastrointestinal malignancy or recurrent admissions for heart failure due to missed iron-deficiency anemia). Therefore, coming up with disease diagnosis likelihoods in hindsight may turn out to be highly subjective and not always accurate. This can be particularly difficult when clinician and other team deliberations are not recorded in their entirety.47
Another hurdle in the practice of diagnostic medicine is to preserve the balance between underdiagnosing versus pursuing overly aggressive diagnostic approaches. Conducting laboratory, imaging, or other diagnostic studies without a clear shared understanding of how they would affect clinical decision-making (eg, use of prostate-specific antigen to detect prostate cancer) not only leads to increased costs but can also delay appropriate care. Worse, subsequent unnecessary diagnostic tests and treatments can sometimes lead to serious harm.48,49
Finally, retrospective reviews by clinicians are subject to multiple potential limitations that include failure to create well-defined research questions, poorly developed inclusion and exclusion criteria, and issues related to inter- and intra-rater reliability.50 These methodological deficiencies can occur despite following "best practice" guidelines during the study planning, execution, and analysis phases. They further add to the challenge of defining and measuring diagnostic errors.47
Strategies to Improve Measurement of Diagnostic Errors
Development of new methodologies to reliably measure diagnostic errors is an area of active research. The advancement of uniform and universally agreed-upon frameworks to define and identify process failure points and diagnostic errors would help reduce measurement error and support development and testing of interventions that could be generalizable across different health care settings. To more accurately define and measure diagnostic errors, several novel approaches have been proposed (Table 2).

The Safer Dx framework is an all-round tool developed to advance the discipline of measuring diagnostic errors. For an episode of care under review, the instrument scores various items to determine the likelihood of a diagnostic error. These items evaluate multiple dimensions affecting diagnostic performance and measurements across 3 broad domains: structure (provider and organizational characteristics—from everyone involved with patient care, to computing infrastructure, to policies and regulations), process (elements of the patient-provider encounter, diagnostic test performance and follow-up, and subspecialty- and referral-specific factors), and outcome (establishing accurate and timely diagnosis as opposed to missed, delayed, or incorrect diagnosis). This instrument has been revised and can be further modified by a variety of stakeholders, including clinicians, health care organizations, and policymakers, to identify potential diagnostic errors in a standardized way for patient safety and quality improvement research.51,52
Use of standardized tools, such as the Diagnosis Error Evaluation and Research (DEER) taxonomy, can help to identify and classify specific failure points across different diagnostic process dimensions.37 These failure points can be classified into: issues related to patient presentation or access to health care; failure to obtain or misinterpretation of history or physical exam findings; errors in use of diagnostics tests due to technical or clinician-related factors; failures in appropriate weighing of evidence and hypothesis generation; errors associated with referral or consultation process; and failure to monitor the patient or obtain timely follow-up.34 The DEER taxonomy can also be modified based on specific research questions and study populations. Further, it can be recategorized to correspond to Safer Dx framework diagnostic process dimensions to provide insights into reasons for specific process failures and to develop new interventions to mitigate errors and patient harm.6
Since a majority of diagnostic errors do not lead to actual harm, use of “triggers” or clues (eg, procedure-related complications, patient falls, transfers to a higher level of care, readmissions within 30 days) can be a more efficient method to identify diagnostic errors and adverse events that do cause harm. The Global Trigger Tool, developed by the Institute for Healthcare Improvement, uses this strategy. This tool has been shown to identify a significantly higher number of serious adverse events than comparable methods.53 This facilitates selection and development of strategies at the institutional level that are most likely to improve patient outcomes.24
Encouraging and facilitating voluntary or prompted reporting from patients and clinicians can also play an important role in capturing diagnostic errors. Patients and clinicians are not only the key stakeholders but are also uniquely placed within the diagnostic process to detect and report potential errors.25,54 Patient-safety-event reporting systems, such as RL6, play a vital role in reporting near-misses and adverse events. These systems provide a mechanism for team members at all levels within the hospital to contribute toward reporting patient adverse events, including those arising from diagnostic errors.55 The Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey is the first standardized, nationally reported patient survey designed to measure patients’ perceptions of their hospital experience. The US Centers for Medicare and Medicaid Services (CMS) publishes HCAHPS results on its website 4 times a year, which serves as an important incentive for hospitals to improve patient safety and quality of health care delivery.56
Another novel approach links multiple symptoms to a range of target diseases using the Symptom-Disease Pair Analysis of Diagnostic Error (SPADE) framework. Using “big data” technologies, this technique can help discover otherwise hidden symptom-disease links and improve overall diagnostic performance. This approach is proposed for both case-control (look-back) and cohort (look-forward) studies assessing diagnostic errors and misdiagnosis-related harms. For example, starting with a known diagnosis with high potential for harm (eg, stroke), the “look-back” approach can be used to identify high-risk symptoms (eg, dizziness, vertigo). In the “look-forward” approach, a single symptom or exposure risk factor known to be frequently misdiagnosed (eg, dizziness) can be analyzed to identify potential adverse disease outcomes (eg, stroke, migraine).57
Many large ongoing studies looking at diagnostic errors among hospitalized patients, such as Utility of Predictive Systems to identify Inpatient Diagnostic Errors (UPSIDE),58Patient Safety Learning Lab (PSLL),59 and Achieving Diagnostic Excellence through Prevention and Teamwork (ADEPT),60 are using structured chart review methodologies incorporating many of the above strategies in combination. Cases triggered by certain events (eg, ICU transfer, death, rapid response event, new or worsening acute kidney injury) are reviewed using validated tools, including Safer Dx framework and DEER taxonomy, to provide the most precise estimates of the burden of diagnostic errors in hospitalized patients. These estimates may be much higher than previously predicted using traditional chart review approaches.6,24 For example, a recently published study of 2809 random admissions in 11 Massachusetts hospitals identified 978 adverse events but only 10 diagnostic errors (diagnostic error rate, 0.4%).19 This was likely because the trigger method used in the study did not specifically examine the diagnostic process as critically as done by the Safer Dx framework and DEER taxonomy tools, thereby underestimating the total number of diagnostic errors. Further, these ongoing studies (eg, UPSIDE, ADEPT) aim to employ new and upcoming advanced machine-learning methods to create models that can improve overall diagnostic performance. This would pave the way to test and build novel, efficient, and scalable interventions to reduce diagnostic errors and improve patient outcomes.
Strategies to Improve Diagnostic Safety in Hospitalized Patients
Disease-specific biomedical research, as well as advances in laboratory, imaging, and other technologies, play a critical role in improving diagnostic accuracy. However, these technical approaches do not address many of the broader clinician- and system-level failure points and opportunities for improvement. Various patient-, provider-, and organizational-level interventions that could make diagnostic processes more resilient and reduce the risk of error and patient harm have been proposed.61
Among these strategies are approaches to empower patients and their families. Fostering therapeutic relationships between patients and members of the care team is essential to reducing diagnostic errors.62 Facilitating timely access to health records, ensuring transparency in decision making, and tailoring communication strategies to patients’ cultural and educational backgrounds can reduce harm.63 Similarly, at the system level, enhancing communication among different providers by use of tools such as structured handoffs can prevent communication breakdowns and facilitate positive outcomes.64
Interventions targeted at individual health care providers, such as educational programs to improve content-specific knowledge, can enhance diagnostic performance. Regular feedback, strategies to enhance equity, and fostering an environment where all providers are actively encouraged to think critically and participate in the diagnostic process (training programs to use “diagnostic time-outs” and making it a “team sport”) can improve clinical reasoning.65,66 Use of standardized patients can help identify individual-level cognitive failure points and facilitate creation of new interventions to improve clinical decision-making processes.67
Novel health information technologies can further augment these efforts. These include effective documentation by maintaining dynamic and accurate patient histories, problem lists, and medication lists68-70; use of electronic health record–based algorithms to identify potential diagnostic delays for serious conditions71,72; use of telemedicine technologies to improve accessibility and coordination73;application of mobile health and wearable technologies to facilitate data-gathering and care delivery74,75; and use of computerized decision-support tools, including applications to interpret electrocardiograms, imaging studies, and other diagnostic tests.76
Use of precision medicine, powered by new artificial intelligence (AI) tools, is becoming more widespread. Algorithms powered by AI can augment and sometimes even outperform clinician decision-making in areas such as oncology, radiology, and primary care.77 Creation of large biobanks like the All of Us research program can be used to study thousands of environmental and genetic risk factors and health conditions simultaneously, and help identify specific treatments that work best for people of different backgrounds.78 Active research in these areas holds great promise in terms of how and when we diagnose diseases and make appropriate preventative and treatment decisions. Significant scientific, ethical, and regulatory challenges will need to be overcome before these technologies can address some of the most complex problems in health care.79
Finally, diagnostic performance is affected by the external environment, including the functioning of the medical liability system. Diagnostic errors that lead to patient harm are a leading cause of malpractice claims.80 Developing a legal environment, in collaboration with patient advocacy groups and health care organizations, that promotes and facilitates timely disclosure of diagnostic errors could decrease the incentive to hide errors, advance care processes, and improve outcomes.81,82
Conclusion
The burden of diagnostic errors in hospitalized patients is unacceptably high and remains an underemphasized cause of preventable morbidity and mortality. Diagnostic errors often result from a breakdown in multiple interdependent processes that involve patient-, provider-, and system-level factors. Significant challenges remain in defining and identifying diagnostic errors as well as underlying process-failure points. The most effective interventions to reduce diagnostic errors will require greater patient participation in the diagnostic process and a mix of evidence-based interventions that promote individual-provider excellence as well as system-level changes. Further research and collaboration among various stakeholders should help improve diagnostic safety for hospitalized patients.
Corresponding author: Abhishek Goyal, MD, MPH; agoyal4@bwh.harvard.edu
Disclosures: Dr. Dalal disclosed receiving income ≥ $250 from MayaMD.
Abstract
Diagnostic errors in hospitalized patients are a leading cause of preventable morbidity and mortality. Significant challenges in defining and measuring diagnostic errors and underlying process failure points have led to considerable variability in reported rates of diagnostic errors and adverse outcomes. In this article, we explore the diagnostic process and its discrete components, emphasizing the centrality of the patient in decision-making as well as the continuous nature of the process. We review the incidence of diagnostic errors in hospitalized patients and different methodological approaches that have been used to arrive at these estimates. We discuss different but interdependent provider- and system-related process-failure points that lead to diagnostic errors. We examine specific challenges related to measurement of diagnostic errors and describe traditional and novel approaches that are being used to obtain the most precise estimates. Finally, we examine various patient-, provider-, and organizational-level interventions that have been proposed to improve diagnostic safety in hospitalized patients.
Keywords: diagnostic error, hospital medicine, patient safety.
Diagnosis is defined as a “pre-existing set of categories agreed upon by the medical profession to designate a specific condition.”1 The diagnostic process involves obtaining a clinical history, performing a physical examination, conducting diagnostic testing, and consulting with other clinical providers to gather data that are relevant to understanding the underlying disease processes. This exercise involves generating hypotheses and updating prior probabilities as more information and evidence become available. Throughout this process of information gathering, integration, and interpretation, there is an ongoing assessment of whether sufficient and necessary knowledge has been obtained to make an accurate diagnosis and provide appropriate treatment.2
Diagnostic error is defined as a missed opportunity to make a timely diagnosis as part of this iterative process, including the failure of communicating the diagnosis to the patient in a timely manner.3 It can be categorized as a missed, delayed, or incorrect diagnosis based on available evidence at the time. Establishing the correct diagnosis has important implications. A timely and precise diagnosis ensures the patient the highest probability of having a positive health outcome that reflects an appropriate understanding of underlying disease processes and is consistent with their overall goals of care.3 When diagnostic errors occur, they can cause patient harm. Adverse events due to medical errors, including diagnostic errors, are estimated to be the third leading cause of death in the United States.4 Most people will experience at least 1 diagnostic error in their lifetime. In the 2015 National Academy of Medicine report Improving Diagnosis in Healthcare, diagnostic errors were identified as a major hazard as well as an opportunity to improve patient outcomes.2
Diagnostic errors during hospitalizations are especially concerning, as they are more likely to be implicated in a wider spectrum of harm, including permanent disability and death. This has become even more relevant for hospital medicine physicians and other clinical providers as they encounter increasing cognitive and administrative workloads, rising dissatisfaction and burnout, and unique obstacles such as night-time scheduling.5
Incidence of Diagnostic Errors in Hospitalized Patients
Several methodological approaches have been used to estimate the incidence of diagnostic errors in hospitalized patients. These include retrospective reviews of a sample of all hospital admissions, evaluations of selected adverse outcomes including autopsy studies, patient and provider surveys, and malpractice claims. Laboratory testing audits and secondary reviews in other diagnostic subspecialities (eg, radiology, pathology, and microbiology) are also essential to improving diagnostic performance in these specialized fields, which in turn affects overall hospital diagnostic error rates.6-8 These diverse approaches provide unique insights regarding our ability to assess the degree to which potential harms, ranging from temporary impairment to permanent disability, to death, are attributable to different failure points in the diagnostic process.
Large retrospective chart reviews of random hospital admissions remain the most accurate way to determine the overall incidence of diagnostic errors in hospitalized patients.9 The Harvard Medical Practice Study, published in 1991, laid the groundwork for measuring the incidence of adverse events in hospitalized patients and assessing their relation to medical error, negligence, and disability. Reviewing 30,121 randomly selected records from 51 randomly selected acute care hospitals in New York State, the study found that adverse events occurred in 3.7% of hospitalizations, diagnostic errors accounted for 13.8% of these events, and these errors were likely attributable to negligence in 74.7% of cases. The study not only outlined individual-level process failures, but also focused attention on some of the systemic causes, setting the agenda for quality improvement research in hospital-based care for years to come.10-12 A recent systematic review and meta-analysis of 22 hospital admission studies found a pooled rate of 0.7% (95% CI, 0.5%-1.1%) for harmful diagnostic errors.9 It found significant variations in the rates of adverse events, diagnostic errors, and range of diagnoses that were missed. This was primarily because of variabilities in pre-test probabilities in detecting diagnostic errors in these specific cohorts, as well as due to heterogeneity in study definitions and methodologies, especially regarding how they defined and measured “diagnostic error.” The analysis, however, did not account for diagnostic errors that were not related to patient harm (missed opportunities); therefore, it likely significantly underestimated the true incidence of diagnostic errors in these study populations. Table 1 summarizes some of key studies that have examined the incidence of harmful diagnostic errors in hospitalized patients.9-21

The chief limitation of reviewing random hospital admissions is that, since overall rates of diagnostic errors are still relatively low, a large number of case reviews are required to identify a sufficient sample of adverse outcomes to gain a meaningful understanding of the underlying process failure points and develop tools for remediation. Patient and provider surveys or data from malpractice claims can be high-yield starting points for research on process errors.22,23 Reviews of enriched cohorts of adverse outcomes, such as rapid-response events, intensive care unit (ICU) transfers, deaths, and hospital readmissions, can be an efficient way to identify process failures that lead to greatest harm. Depending on the research approach and the types of underlying patient populations sampled, rates of diagnostic errors in these high-risk groups have been estimated to be approximately 5% to 20%, or even higher.6,24-31 For example, a retrospective study of 391 cases of unplanned 7-day readmissions found that 5.6% of cases contained at least 1 diagnostic error during the index admission.32 In a study conducted at 6 Belgian acute-care hospitals, 56% of patients requiring an unplanned transfer to a higher level of care were determined to have had an adverse event, and of these adverse events, 12.4% of cases were associated with errors in diagnosis.29 A systematic review of 16 hospital-based studies estimated that 3.1% of all inpatient deaths were likely preventable, which corresponded to 22,165 deaths annually in the United States.30 Another such review of 31 autopsy studies reported that 28% of autopsied ICU patients had at least 1 misdiagnosis; of these diagnostic errors, 8% were classified as potentially lethal, and 15% were considered major but not lethal.31 Significant drawbacks of such enriched cohort studies, however, are their poor generalizability and inability to detect failure points that do not lead to patient harm (near-miss events).33
Causes of Diagnostic Errors in Hospitalized Patients
All aspects of the diagnostic process are susceptible to errors. These errors stem from a variety of faulty processes, including failure of the patient to engage with the health care system (eg, due to lack of insurance or transportation, or delay in seeking care); failure in information gathering (eg, missed history or exam findings, ordering wrong tests, laboratory errors); failure in information interpretation (eg, exam finding or test result misinterpretation); inaccurate hypothesis generation (eg, due to suboptimal prioritization or weighing of supporting evidence); and failure in communication (eg, with other team members or with the patient).2,34 Reasons for diagnostic process failures vary widely across different health care settings. While clinician assessment errors (eg, failure to consider or alternatively overweigh competing diagnoses) and errors in testing and the monitoring phase (eg, failure to order or follow up diagnostic tests) can lead to a majority of diagnostic errors in some patient populations, in other settings, social (eg, poor health literacy, punitive cultural practices) and economic factors (eg, lack of access to appropriate diagnostic tests or to specialty expertise) play a more prominent role.34,35
The Figure describes the relationship between components of the diagnostic process and subsequent outcomes, including diagnostic process failures, diagnostic errors, and absence or presence of patient harm.2,36,37 It reemphasizes the centrality of the patient in decision-making and the continuous nature of the process. The Figure also illustrates that only a minority of process failures result in diagnostic errors, and a smaller proportion of diagnostic errors actually lead to patient harm. Conversely, it also shows that diagnostic errors can happen without any obvious process-failure points, and, similarly, patient harm can take place in the absence of any evident diagnostic errors.36-38 Finally, it highlights the need to incorporate feedback from process failures, diagnostic errors, and favorable and unfavorable patient outcomes in order to inform future quality improvement efforts and research.

A significant proportion of diagnostic errors are due to system-related vulnerabilities, such as limitations in availability, adoption or quality of work force training, health informatics resources, and diagnostic capabilities. Lack of institutional culture that promotes safety and transparency also predisposes to diagnostic errors.39,40 The other major domain of process failures is related to cognitive errors in clinician decision-making. Anchoring, confirmation bias, availability bias, and base-rate neglect are some of the common cognitive biases that, along with personality traits (aversion to risk or ambiguity, overconfidence) and affective biases (influence of emotion on decision-making), often determine the degree of utilization of resources and the possibility of suboptimal diagnostic performance.41,42 Further, implicit biases related to age, race, gender, and sexual orientation contribute to disparities in access to health care and outcomes.43 In a large number of cases of preventable adverse outcomes, however, there are multiple interdependent individual and system-related failure points that lead to diagnostic error and patient harm.6,32
Challenges in Defining and Measuring Diagnostic Errors
In order to develop effective, evidence-based interventions to reduce diagnostic errors in hospitalized patients, it is essential to be able to first operationally define, and then accurately measure, diagnostic errors and the process failures that contribute to these errors in a standardized way that is reproducible across different settings.6,44 There are a number of obstacles in this endeavor.
A fundamental problem is that establishing a diagnosis is not a single act but a process. Patterns of symptoms and clinical presentations often differ for the same disease. Information required to make a diagnosis is usually gathered in stages, where the clinician obtains additional data, while considering many possibilities, of which 1 may be ultimately correct. Diagnoses evolve over time and in different care settings. “The most likely diagnosis” is not always the same as “the final correct diagnosis.” Moreover, the diagnostic process is influenced by patients’ individual clinical courses and preferences over time. This makes determination of missed, delayed, or incorrect diagnoses challenging.45,46
For hospitalized patients, generally the goal is to first rule out more serious and acute conditions (eg, pulmonary embolism or stroke), even if their probability is rather low. Conversely, a diagnosis that appears less consequential if delayed (eg, chronic anemia of unclear etiology) might not be pursued on an urgent basis, and is often left to outpatient providers to examine, but still may manifest in downstream harm (eg, delayed diagnosis of gastrointestinal malignancy or recurrent admissions for heart failure due to missed iron-deficiency anemia). Therefore, coming up with disease diagnosis likelihoods in hindsight may turn out to be highly subjective and not always accurate. This can be particularly difficult when clinician and other team deliberations are not recorded in their entirety.47
Another hurdle in the practice of diagnostic medicine is to preserve the balance between underdiagnosing versus pursuing overly aggressive diagnostic approaches. Conducting laboratory, imaging, or other diagnostic studies without a clear shared understanding of how they would affect clinical decision-making (eg, use of prostate-specific antigen to detect prostate cancer) not only leads to increased costs but can also delay appropriate care. Worse, subsequent unnecessary diagnostic tests and treatments can sometimes lead to serious harm.48,49
Finally, retrospective reviews by clinicians are subject to multiple potential limitations that include failure to create well-defined research questions, poorly developed inclusion and exclusion criteria, and issues related to inter- and intra-rater reliability.50 These methodological deficiencies can occur despite following "best practice" guidelines during the study planning, execution, and analysis phases. They further add to the challenge of defining and measuring diagnostic errors.47
Strategies to Improve Measurement of Diagnostic Errors
Development of new methodologies to reliably measure diagnostic errors is an area of active research. The advancement of uniform and universally agreed-upon frameworks to define and identify process failure points and diagnostic errors would help reduce measurement error and support development and testing of interventions that could be generalizable across different health care settings. To more accurately define and measure diagnostic errors, several novel approaches have been proposed (Table 2).

The Safer Dx framework is an all-round tool developed to advance the discipline of measuring diagnostic errors. For an episode of care under review, the instrument scores various items to determine the likelihood of a diagnostic error. These items evaluate multiple dimensions affecting diagnostic performance and measurements across 3 broad domains: structure (provider and organizational characteristics—from everyone involved with patient care, to computing infrastructure, to policies and regulations), process (elements of the patient-provider encounter, diagnostic test performance and follow-up, and subspecialty- and referral-specific factors), and outcome (establishing accurate and timely diagnosis as opposed to missed, delayed, or incorrect diagnosis). This instrument has been revised and can be further modified by a variety of stakeholders, including clinicians, health care organizations, and policymakers, to identify potential diagnostic errors in a standardized way for patient safety and quality improvement research.51,52
Use of standardized tools, such as the Diagnosis Error Evaluation and Research (DEER) taxonomy, can help to identify and classify specific failure points across different diagnostic process dimensions.37 These failure points can be classified into: issues related to patient presentation or access to health care; failure to obtain or misinterpretation of history or physical exam findings; errors in use of diagnostics tests due to technical or clinician-related factors; failures in appropriate weighing of evidence and hypothesis generation; errors associated with referral or consultation process; and failure to monitor the patient or obtain timely follow-up.34 The DEER taxonomy can also be modified based on specific research questions and study populations. Further, it can be recategorized to correspond to Safer Dx framework diagnostic process dimensions to provide insights into reasons for specific process failures and to develop new interventions to mitigate errors and patient harm.6
Since a majority of diagnostic errors do not lead to actual harm, use of “triggers” or clues (eg, procedure-related complications, patient falls, transfers to a higher level of care, readmissions within 30 days) can be a more efficient method to identify diagnostic errors and adverse events that do cause harm. The Global Trigger Tool, developed by the Institute for Healthcare Improvement, uses this strategy. This tool has been shown to identify a significantly higher number of serious adverse events than comparable methods.53 This facilitates selection and development of strategies at the institutional level that are most likely to improve patient outcomes.24
Encouraging and facilitating voluntary or prompted reporting from patients and clinicians can also play an important role in capturing diagnostic errors. Patients and clinicians are not only the key stakeholders but are also uniquely placed within the diagnostic process to detect and report potential errors.25,54 Patient-safety-event reporting systems, such as RL6, play a vital role in reporting near-misses and adverse events. These systems provide a mechanism for team members at all levels within the hospital to contribute toward reporting patient adverse events, including those arising from diagnostic errors.55 The Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey is the first standardized, nationally reported patient survey designed to measure patients’ perceptions of their hospital experience. The US Centers for Medicare and Medicaid Services (CMS) publishes HCAHPS results on its website 4 times a year, which serves as an important incentive for hospitals to improve patient safety and quality of health care delivery.56
Another novel approach links multiple symptoms to a range of target diseases using the Symptom-Disease Pair Analysis of Diagnostic Error (SPADE) framework. Using “big data” technologies, this technique can help discover otherwise hidden symptom-disease links and improve overall diagnostic performance. This approach is proposed for both case-control (look-back) and cohort (look-forward) studies assessing diagnostic errors and misdiagnosis-related harms. For example, starting with a known diagnosis with high potential for harm (eg, stroke), the “look-back” approach can be used to identify high-risk symptoms (eg, dizziness, vertigo). In the “look-forward” approach, a single symptom or exposure risk factor known to be frequently misdiagnosed (eg, dizziness) can be analyzed to identify potential adverse disease outcomes (eg, stroke, migraine).57
Many large ongoing studies looking at diagnostic errors among hospitalized patients, such as Utility of Predictive Systems to identify Inpatient Diagnostic Errors (UPSIDE),58Patient Safety Learning Lab (PSLL),59 and Achieving Diagnostic Excellence through Prevention and Teamwork (ADEPT),60 are using structured chart review methodologies incorporating many of the above strategies in combination. Cases triggered by certain events (eg, ICU transfer, death, rapid response event, new or worsening acute kidney injury) are reviewed using validated tools, including Safer Dx framework and DEER taxonomy, to provide the most precise estimates of the burden of diagnostic errors in hospitalized patients. These estimates may be much higher than previously predicted using traditional chart review approaches.6,24 For example, a recently published study of 2809 random admissions in 11 Massachusetts hospitals identified 978 adverse events but only 10 diagnostic errors (diagnostic error rate, 0.4%).19 This was likely because the trigger method used in the study did not specifically examine the diagnostic process as critically as done by the Safer Dx framework and DEER taxonomy tools, thereby underestimating the total number of diagnostic errors. Further, these ongoing studies (eg, UPSIDE, ADEPT) aim to employ new and upcoming advanced machine-learning methods to create models that can improve overall diagnostic performance. This would pave the way to test and build novel, efficient, and scalable interventions to reduce diagnostic errors and improve patient outcomes.
Strategies to Improve Diagnostic Safety in Hospitalized Patients
Disease-specific biomedical research, as well as advances in laboratory, imaging, and other technologies, play a critical role in improving diagnostic accuracy. However, these technical approaches do not address many of the broader clinician- and system-level failure points and opportunities for improvement. Various patient-, provider-, and organizational-level interventions that could make diagnostic processes more resilient and reduce the risk of error and patient harm have been proposed.61
Among these strategies are approaches to empower patients and their families. Fostering therapeutic relationships between patients and members of the care team is essential to reducing diagnostic errors.62 Facilitating timely access to health records, ensuring transparency in decision making, and tailoring communication strategies to patients’ cultural and educational backgrounds can reduce harm.63 Similarly, at the system level, enhancing communication among different providers by use of tools such as structured handoffs can prevent communication breakdowns and facilitate positive outcomes.64
Interventions targeted at individual health care providers, such as educational programs to improve content-specific knowledge, can enhance diagnostic performance. Regular feedback, strategies to enhance equity, and fostering an environment where all providers are actively encouraged to think critically and participate in the diagnostic process (training programs to use “diagnostic time-outs” and making it a “team sport”) can improve clinical reasoning.65,66 Use of standardized patients can help identify individual-level cognitive failure points and facilitate creation of new interventions to improve clinical decision-making processes.67
Novel health information technologies can further augment these efforts. These include effective documentation by maintaining dynamic and accurate patient histories, problem lists, and medication lists68-70; use of electronic health record–based algorithms to identify potential diagnostic delays for serious conditions71,72; use of telemedicine technologies to improve accessibility and coordination73;application of mobile health and wearable technologies to facilitate data-gathering and care delivery74,75; and use of computerized decision-support tools, including applications to interpret electrocardiograms, imaging studies, and other diagnostic tests.76
Use of precision medicine, powered by new artificial intelligence (AI) tools, is becoming more widespread. Algorithms powered by AI can augment and sometimes even outperform clinician decision-making in areas such as oncology, radiology, and primary care.77 Creation of large biobanks like the All of Us research program can be used to study thousands of environmental and genetic risk factors and health conditions simultaneously, and help identify specific treatments that work best for people of different backgrounds.78 Active research in these areas holds great promise in terms of how and when we diagnose diseases and make appropriate preventative and treatment decisions. Significant scientific, ethical, and regulatory challenges will need to be overcome before these technologies can address some of the most complex problems in health care.79
Finally, diagnostic performance is affected by the external environment, including the functioning of the medical liability system. Diagnostic errors that lead to patient harm are a leading cause of malpractice claims.80 Developing a legal environment, in collaboration with patient advocacy groups and health care organizations, that promotes and facilitates timely disclosure of diagnostic errors could decrease the incentive to hide errors, advance care processes, and improve outcomes.81,82
Conclusion
The burden of diagnostic errors in hospitalized patients is unacceptably high and remains an underemphasized cause of preventable morbidity and mortality. Diagnostic errors often result from a breakdown in multiple interdependent processes that involve patient-, provider-, and system-level factors. Significant challenges remain in defining and identifying diagnostic errors as well as underlying process-failure points. The most effective interventions to reduce diagnostic errors will require greater patient participation in the diagnostic process and a mix of evidence-based interventions that promote individual-provider excellence as well as system-level changes. Further research and collaboration among various stakeholders should help improve diagnostic safety for hospitalized patients.
Corresponding author: Abhishek Goyal, MD, MPH; agoyal4@bwh.harvard.edu
Disclosures: Dr. Dalal disclosed receiving income ≥ $250 from MayaMD.
1. Graber ML, Franklin N, Gordon R. Diagnostic error in internal medicine. Arch Intern Med. 2005;165(13):1493-1499. doi:10.1001/archinte.165.13.1493
2. National Academies of Sciences, Engineering, and Medicine. 2015. Improving Diagnosis in Health Care. The National Academies Press. doi:10.17226/21794
3. Singh H, Graber ML. Improving diagnosis in health care—the next imperative for patient safety. N Engl J Med. 2015;373(26):2493-2495. doi:10.1056/NEJMp1512241
4. Makary MA, Daniel M. Medical error—the third leading cause of death in the US. BMJ. 2016;353:i2139. doi:10.1136/bmj.i2139
5. Flanders SA, Centor B, Weber V, McGinn T, Desalvo K, Auerbach A. Challenges and opportunities in academic hospital medicine: report from the academic hospital medicine summit. J Gen Intern Med. 2009;24(5):636-641. doi:10.1007/s11606-009-0944-6
6. Griffin JA, Carr K, Bersani K, et al. Analyzing diagnostic errors in the acute setting: a process-driven approach. Diagnosis (Berl). 2021;9(1):77-88. doi:10.1515/dx-2021-0033
7. Itri JN, Tappouni RR, McEachern RO, Pesch AJ, Patel SH. Fundamentals of diagnostic error in imaging. RadioGraphics. 2018;38(6):1845-1865. doi:10.1148/rg.2018180021
8. Hammerling JA. A Review of medical errors in laboratory diagnostics and where we are today. Lab Med. 2012;43(2):41-44. doi:10.1309/LM6ER9WJR1IHQAUY
9. Gunderson CG, Bilan VP, Holleck JL, et al. Prevalence of harmful diagnostic errors in hospitalised adults: a systematic review and meta-analysis. BMJ Qual Saf. 2020;29(12):1008-1018. doi:10.1136/bmjqs-2019-010822
10. Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324(6):370-376. doi:10.1056/NEJM199102073240604
11. Leape LL, Brennan TA, Laird N, et al. The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II. N Engl J Med. 1991;324(6):377-384. doi:10.1056/NEJM199102073240605
12. Localio AR, Lawthers AG, Brennan TA, et al. Relation between malpractice claims and adverse events due to negligence. Results of the Harvard Medical Practice Study III. N Engl J Med. 1991;325(4):245-251. doi:10.1056/NEJM199107253250405
13. Wilson RM, Michel P, Olsen S, et al. Patient safety in developing countries: retrospective estimation of scale and nature of harm to patients in hospital. BMJ. 2012;344:e832. doi:10.1136/bmj.e832
14. Wilson RM, Runciman WB, Gibberd RW, Harrison BT, Newby L, Hamilton JD. The Quality in Australian Health Care Study. Med J Aust. 1995;163(9):458-471. doi:10.5694/j.1326-5377.1995.tb124691.x
15. Thomas EJ, Studdert DM, Burstin HR, et al. Incidence and types of adverse events and negligent care in Utah and Colorado. Med Care. 2000;38(3):261-271. doi:10.1097/00005650-200003000-00003
16. Baker GR, Norton PG, Flintoft V, et al. The Canadian Adverse Events Study: the incidence of adverse events among hospital patients in Canada. CMAJ. 2004;170(11):1678-1686. doi:10.1503/cmaj.1040498
17. Davis P, Lay-Yee R, Briant R, Ali W, Scott A, Schug S. Adverse events in New Zealand public hospitals II: preventability and clinical context. N Z Med J. 2003;116(1183):U624.
18. Aranaz-Andrés JM, Aibar-Remón C, Vitaller-Murillo J, et al. Incidence of adverse events related to health care in Spain: results of the Spanish National Study of Adverse Events. J Epidemiol Community Health. 2008;62(12):1022-1029. doi:10.1136/jech.2007.065227
19. Bates DW, Levine DM, Salmasian H, et al. The safety of inpatient health care. N Engl J Med. 2023;388(2):142-153. doi:10.1056/NEJMsa2206117
20. Soop M, Fryksmark U, Köster M, Haglund B. The incidence of adverse events in Swedish hospitals: a retrospective medical record review study. Int J Qual Health Care. 2009;21(4):285-291. doi:10.1093/intqhc/mzp025
21. Rafter N, Hickey A, Conroy RM, et al. The Irish National Adverse Events Study (INAES): the frequency and nature of adverse events in Irish hospitals—a retrospective record review study. BMJ Qual Saf. 2017;26(2):111-119. doi:10.1136/bmjqs-2015-004828
22. Blendon RJ, DesRoches CM, Brodie M, et al. Views of practicing physicians and the public on medical errors. N Engl J Med. 2002;347(24):1933-1940. doi:10.1056/NEJMsa022151
23. Saber Tehrani AS, Lee H, Mathews SC, et al. 25-year summary of US malpractice claims for diagnostic errors 1986-2010: an analysis from the National Practitioner Data Bank. BMJ Qual Saf. 2013;22(8):672-680. doi:10.1136/bmjqs-2012-001550
24. Malik MA, Motta-Calderon D, Piniella N, et al. A structured approach to EHR surveillance of diagnostic error in acute care: an exploratory analysis of two institutionally-defined case cohorts. Diagnosis (Berl). 2022;9(4):446-457. doi:10.1515/dx-2022-0032
25. Graber ML. The incidence of diagnostic error in medicine. BMJ Qual Saf. 2013;22(suppl 2):ii21-ii27. doi:10.1136/bmjqs-2012-001615
26. Bergl PA, Taneja A, El-Kareh R, Singh H, Nanchal RS. Frequency, risk factors, causes, and consequences of diagnostic errors in critically ill medical patients: a retrospective cohort study. Crit Care Med. 2019;47(11):e902-e910. doi:10.1097/CCM.0000000000003976
27. Hogan H, Healey F, Neale G, Thomson R, Vincent C, Black N. Preventable deaths due to problems in care in English acute hospitals: a retrospective case record review study. BMJ Qual Saf. 2012;21(9):737-745. doi:10.1136/bmjqs-2011-001159
28. Bergl PA, Nanchal RS, Singh H. Diagnostic error in the critically ill: defining the problem and exploring next steps to advance intensive care unit safety. Ann Am Thorac Soc. 2018;15(8):903-907. doi:10.1513/AnnalsATS.201801-068PS
29. Marquet K, Claes N, De Troy E, et al. One fourth of unplanned transfers to a higher level of care are associated with a highly preventable adverse event: a patient record review in six Belgian hospitals. Crit Care Med. 2015;43(5):1053-1061. doi:10.1097/CCM.0000000000000932
30. Rodwin BA, Bilan VP, Merchant NB, et al. Rate of preventable mortality in hospitalized patients: a systematic review and meta-analysis. J Gen Intern Med. 2020;35(7):2099-2106. doi:10.1007/s11606-019-05592-5
31. Winters B, Custer J, Galvagno SM, et al. Diagnostic errors in the intensive care unit: a systematic review of autopsy studies. BMJ Qual Saf. 2012;21(11):894-902. doi:10.1136/bmjqs-2012-000803
32. Raffel KE, Kantor MA, Barish P, et al. Prevalence and characterisation of diagnostic error among 7-day all-cause hospital medicine readmissions: a retrospective cohort study. BMJ Qual Saf. 2020;29(12):971-979. doi:10.1136/bmjqs-2020-010896
33. Weingart SN, Pagovich O, Sands DZ, et al. What can hospitalized patients tell us about adverse events? learning from patient-reported incidents. J Gen Intern Med. 2005;20(9):830-836. doi:10.1111/j.1525-1497.2005.0180.x
34. Schiff GD, Hasan O, Kim S, et al. Diagnostic error in medicine: analysis of 583 physician-reported errors. Arch Intern Med. 2009;169(20):1881-1887. doi:10.1001/archinternmed.2009.333
35. Singh H, Schiff GD, Graber ML, Onakpoya I, Thompson MJ. The global burden of diagnostic errors in primary care. BMJ Qual Saf. 2017;26(6):484-494. doi:10.1136/bmjqs-2016-005401
36. Schiff GD, Leape LL. Commentary: how can we make diagnosis safer? Acad Med J Assoc Am Med Coll. 2012;87(2):135-138. doi:10.1097/ACM.0b013e31823f711c
37. Schiff GD, Kim S, Abrams R, et al. Diagnosing diagnosis errors: lessons from a multi-institutional collaborative project. In: Henriksen K, Battles JB, Marks ES, Lewin DI, eds. Advances in Patient Safety: From Research to Implementation. Volume 2: Concepts and Methodology. AHRQ Publication No. 05-0021-2. Agency for Healthcare Research and Quality (US); 2005. Accessed January 16, 2023. http://www.ncbi.nlm.nih.gov/books/NBK20492/
38. Newman-Toker DE. A unified conceptual model for diagnostic errors: underdiagnosis, overdiagnosis, and misdiagnosis. Diagnosis (Berl). 2014;1(1):43-48. doi:10.1515/dx-2013-0027
39. Abimanyi-Ochom J, Bohingamu Mudiyanselage S, Catchpool M, Firipis M, Wanni Arachchige Dona S, Watts JJ. Strategies to reduce diagnostic errors: a systematic review. BMC Med Inform Decis Mak. 2019;19(1):174. doi:10.1186/s12911-019-0901-1
40. Gupta A, Harrod M, Quinn M, et al. Mind the overlap: how system problems contribute to cognitive failure and diagnostic errors. Diagnosis (Berl). 2018;5(3):151-156. doi:10.1515/dx-2018-0014
41. Saposnik G, Redelmeier D, Ruff CC, Tobler PN. Cognitive biases associated with medical decisions: a systematic review. BMC Med Inform Decis Mak. 2016;16:138. doi:10.1186/s12911-016-0377-1
42. Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med. 2003;78(8):775-780. doi: 10.1097/00001888-200308000-00003
43. Chapman EN, Kaatz A, Carnes M. Physicians and implicit bias: how doctors may unwittingly perpetuate health care disparities. J Gen Intern Med. 2013;28(11):1504-1510. doi:10.1007/s11606-013-2441-1
44. Zwaan L, Singh H. The challenges in defining and measuring diagnostic error. Diagnosis (Ber). 2015;2(2):97-103. doi:10.1515/dx-2014-0069
45. Arkes HR, Wortmann RL, Saville PD, Harkness AR. Hindsight bias among physicians weighing the likelihood of diagnoses. J Appl Psychol. 1981;66(2):252-254.
46. Singh H. Editorial: Helping health care organizations to define diagnostic errors as missed opportunities in diagnosis. Jt Comm J Qual Patient Saf. 2014;40(3):99-101. doi:10.1016/s1553-7250(14)40012-6
47. Vassar M, Holzmann M. The retrospective chart review: important methodological considerations. J Educ Eval Health Prof. 2013;10:12. doi:10.3352/jeehp.2013.10.12
48. Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102(9):605-613. doi:10.1093/jnci/djq099
49. Moynihan R, Doust J, Henry D. Preventing overdiagnosis: how to stop harming the healthy. BMJ. 2012;344:e3502. doi:10.1136/bmj.e3502
50. Hayward RA, Hofer TP. Estimating hospital deaths due to medical errors: preventability is in the eye of the reviewer. JAMA. 2001;286(4):415-420. doi:10.1001/jama.286.4.415
51. Singh H, Sittig DF. Advancing the science of measurement of diagnostic errors in healthcare: the Safer Dx framework. BMJ Qual Saf. 2015;24(2):103-110. doi:10.1136/bmjqs-2014-003675
52. Singh H, Khanna A, Spitzmueller C, Meyer AND. Recommendations for using the Revised Safer Dx Instrument to help measure and improve diagnostic safety. Diagnosis (Berl). 2019;6(4):315-323. doi:10.1515/dx-2019-0012
53. Classen DC, Resar R, Griffin F, et al. “Global trigger tool” shows that adverse events in hospitals may be ten times greater than previously measured. Health Aff (Millwood). 2011;30(4):581-589. doi:10.1377/hlthaff.2011.0190
54. Schiff GD. Minimizing diagnostic error: the importance of follow-up and feedback. Am J Med. 2008;121(5 suppl):S38-S42. doi:10.1016/j.amjmed.2008.02.004
55. Mitchell I, Schuster A, Smith K, Pronovost P, Wu A. Patient safety incident reporting: a qualitative study of thoughts and perceptions of experts 15 years after “To Err is Human.” BMJ Qual Saf. 2016;25(2):92-99. doi:10.1136/bmjqs-2015-004405
56. Mazurenko O, Collum T, Ferdinand A, Menachemi N. Predictors of hospital patient satisfaction as measured by HCAHPS: a systematic review. J Healthc Manag. 2017;62(4):272-283. doi:10.1097/JHM-D-15-00050
57. Liberman AL, Newman-Toker DE. Symptom-Disease Pair Analysis of Diagnostic Error (SPADE): a conceptual framework and methodological approach for unearthing misdiagnosis-related harms using big data. BMJ Qual Saf. 2018;27(7):557-566. doi:10.1136/bmjqs-2017-007032
58. Utility of Predictive Systems to Identify Inpatient Diagnostic Errors: the UPSIDE study. NIH RePort/RePORTER. Accessed January 14, 2023. https://reporter.nih.gov/search/rpoHXlEAcEudQV3B9ld8iw/project-details/10020962
59. Overview of Patient Safety Learning Laboratory (PSLL) Projects. Agency for Healthcare Research and Quality. Accessed January 14, 2023. https://www.ahrq.gov/patient-safety/resources/learning-lab/index.html
60. Achieving Diagnostic Excellence through Prevention and Teamwork (ADEPT). NIH RePort/RePORTER. Accessed January 14, 2023. https://reporter.nih.gov/project-details/10642576
61. Zwaan L, Singh H. Diagnostic error in hospitals: finding forests not just the big trees. BMJ Qual Saf. 2020;29(12):961-964. doi:10.1136/bmjqs-2020-011099
62. Longtin Y, Sax H, Leape LL, Sheridan SE, Donaldson L, Pittet D. Patient participation: current knowledge and applicability to patient safety. Mayo Clin Proc. 2010;85(1):53-62. doi:10.4065/mcp.2009.0248
63. Murphy DR, Singh H, Berlin L. Communication breakdowns and diagnostic errors: a radiology perspective. Diagnosis (Berl). 2014;1(4):253-261. doi:10.1515/dx-2014-0035
64. Singh H, Naik AD, Rao R, Petersen LA. Reducing diagnostic errors through effective communication: harnessing the power of information technology. J Gen Intern Med. 2008;23(4):489-494. doi:10.1007/s11606-007-0393-z
65. Singh H, Connor DM, Dhaliwal G. Five strategies for clinicians to advance diagnostic excellence. BMJ. 2022;376:e068044. doi:10.1136/bmj-2021-068044
66. Yale S, Cohen S, Bordini BJ. Diagnostic time-outs to improve diagnosis. Crit Care Clin. 2022;38(2):185-194. doi:10.1016/j.ccc.2021.11.008
67. Schwartz A, Peskin S, Spiro A, Weiner SJ. Impact of unannounced standardized patient audit and feedback on care, documentation, and costs: an experiment and claims analysis. J Gen Intern Med. 2021;36(1):27-34. doi:10.1007/s11606-020-05965-1
68. Carpenter JD, Gorman PN. Using medication list—problem list mismatches as markers of potential error. Proc AMIA Symp. 2002:106-110.
69. Hron JD, Manzi S, Dionne R, et al. Electronic medication reconciliation and medication errors. Int J Qual Health Care. 2015;27(4):314-319. doi:10.1093/intqhc/mzv046
70. Graber ML, Siegal D, Riah H, Johnston D, Kenyon K. Electronic health record–related events in medical malpractice claims. J Patient Saf. 2019;15(2):77-85. doi:10.1097/PTS.0000000000000240
71. Murphy DR, Wu L, Thomas EJ, Forjuoh SN, Meyer AND, Singh H. Electronic trigger-based intervention to reduce delays in diagnostic evaluation for cancer: a cluster randomized controlled trial. J Clin Oncol. 2015;33(31):3560-3567. doi:10.1200/JCO.2015.61.1301
72. Singh H, Giardina TD, Forjuoh SN, et al. Electronic health record-based surveillance of diagnostic errors in primary care. BMJ Qual Saf. 2012;21(2):93-100. doi:10.1136/bmjqs-2011-000304
73. Armaignac DL, Saxena A, Rubens M, et al. Impact of telemedicine on mortality, length of stay, and cost among patients in progressive care units: experience from a large healthcare system. Crit Care Med. 2018;46(5):728-735. doi:10.1097/CCM.0000000000002994
74. MacKinnon GE, Brittain EL. Mobile health technologies in cardiopulmonary disease. Chest. 2020;157(3):654-664. doi:10.1016/j.chest.2019.10.015
75. DeVore AD, Wosik J, Hernandez AF. The future of wearables in heart failure patients. JACC Heart Fail. 2019;7(11):922-932. doi:10.1016/j.jchf.2019.08.008
76. Tsai TL, Fridsma DB, Gatti G. Computer decision support as a source of interpretation error: the case of electrocardiograms. J Am Med Inform Assoc. 2003;10(5):478-483. doi:10.1197/jamia.M1279
77. Lin SY, Mahoney MR, Sinsky CA. Ten ways artificial intelligence will transform primary care. J Gen Intern Med. 2019;34(8):1626-1630. doi:10.1007/s11606-019-05035-1
78. Ramirez AH, Gebo KA, Harris PA. Progress with the All Of Us research program: opening access for researchers. JAMA. 2021;325(24):2441-2442. doi:10.1001/jama.2021.7702
79. Johnson KB, Wei W, Weeraratne D, et al. Precision medicine, AI, and the future of personalized health care. Clin Transl Sci. 2021;14(1):86-93. doi:10.1111/cts.12884
80. Gupta A, Snyder A, Kachalia A, Flanders S, Saint S, Chopra V. Malpractice claims related to diagnostic errors in the hospital. BMJ Qual Saf. 2017;27(1):bmjqs-2017-006774. doi:10.1136/bmjqs-2017-006774
81. Renkema E, Broekhuis M, Ahaus K. Conditions that influence the impact of malpractice litigation risk on physicians’ behavior regarding patient safety. BMC Health Serv Res. 2014;14(1):38. doi:10.1186/1472-6963-14-38
82. Kachalia A, Mello MM, Nallamothu BK, Studdert DM. Legal and policy interventions to improve patient safety. Circulation. 2016;133(7):661-671. doi:10.1161/CIRCULATIONAHA.115.015880
1. Graber ML, Franklin N, Gordon R. Diagnostic error in internal medicine. Arch Intern Med. 2005;165(13):1493-1499. doi:10.1001/archinte.165.13.1493
2. National Academies of Sciences, Engineering, and Medicine. 2015. Improving Diagnosis in Health Care. The National Academies Press. doi:10.17226/21794
3. Singh H, Graber ML. Improving diagnosis in health care—the next imperative for patient safety. N Engl J Med. 2015;373(26):2493-2495. doi:10.1056/NEJMp1512241
4. Makary MA, Daniel M. Medical error—the third leading cause of death in the US. BMJ. 2016;353:i2139. doi:10.1136/bmj.i2139
5. Flanders SA, Centor B, Weber V, McGinn T, Desalvo K, Auerbach A. Challenges and opportunities in academic hospital medicine: report from the academic hospital medicine summit. J Gen Intern Med. 2009;24(5):636-641. doi:10.1007/s11606-009-0944-6
6. Griffin JA, Carr K, Bersani K, et al. Analyzing diagnostic errors in the acute setting: a process-driven approach. Diagnosis (Berl). 2021;9(1):77-88. doi:10.1515/dx-2021-0033
7. Itri JN, Tappouni RR, McEachern RO, Pesch AJ, Patel SH. Fundamentals of diagnostic error in imaging. RadioGraphics. 2018;38(6):1845-1865. doi:10.1148/rg.2018180021
8. Hammerling JA. A Review of medical errors in laboratory diagnostics and where we are today. Lab Med. 2012;43(2):41-44. doi:10.1309/LM6ER9WJR1IHQAUY
9. Gunderson CG, Bilan VP, Holleck JL, et al. Prevalence of harmful diagnostic errors in hospitalised adults: a systematic review and meta-analysis. BMJ Qual Saf. 2020;29(12):1008-1018. doi:10.1136/bmjqs-2019-010822
10. Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324(6):370-376. doi:10.1056/NEJM199102073240604
11. Leape LL, Brennan TA, Laird N, et al. The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II. N Engl J Med. 1991;324(6):377-384. doi:10.1056/NEJM199102073240605
12. Localio AR, Lawthers AG, Brennan TA, et al. Relation between malpractice claims and adverse events due to negligence. Results of the Harvard Medical Practice Study III. N Engl J Med. 1991;325(4):245-251. doi:10.1056/NEJM199107253250405
13. Wilson RM, Michel P, Olsen S, et al. Patient safety in developing countries: retrospective estimation of scale and nature of harm to patients in hospital. BMJ. 2012;344:e832. doi:10.1136/bmj.e832
14. Wilson RM, Runciman WB, Gibberd RW, Harrison BT, Newby L, Hamilton JD. The Quality in Australian Health Care Study. Med J Aust. 1995;163(9):458-471. doi:10.5694/j.1326-5377.1995.tb124691.x
15. Thomas EJ, Studdert DM, Burstin HR, et al. Incidence and types of adverse events and negligent care in Utah and Colorado. Med Care. 2000;38(3):261-271. doi:10.1097/00005650-200003000-00003
16. Baker GR, Norton PG, Flintoft V, et al. The Canadian Adverse Events Study: the incidence of adverse events among hospital patients in Canada. CMAJ. 2004;170(11):1678-1686. doi:10.1503/cmaj.1040498
17. Davis P, Lay-Yee R, Briant R, Ali W, Scott A, Schug S. Adverse events in New Zealand public hospitals II: preventability and clinical context. N Z Med J. 2003;116(1183):U624.
18. Aranaz-Andrés JM, Aibar-Remón C, Vitaller-Murillo J, et al. Incidence of adverse events related to health care in Spain: results of the Spanish National Study of Adverse Events. J Epidemiol Community Health. 2008;62(12):1022-1029. doi:10.1136/jech.2007.065227
19. Bates DW, Levine DM, Salmasian H, et al. The safety of inpatient health care. N Engl J Med. 2023;388(2):142-153. doi:10.1056/NEJMsa2206117
20. Soop M, Fryksmark U, Köster M, Haglund B. The incidence of adverse events in Swedish hospitals: a retrospective medical record review study. Int J Qual Health Care. 2009;21(4):285-291. doi:10.1093/intqhc/mzp025
21. Rafter N, Hickey A, Conroy RM, et al. The Irish National Adverse Events Study (INAES): the frequency and nature of adverse events in Irish hospitals—a retrospective record review study. BMJ Qual Saf. 2017;26(2):111-119. doi:10.1136/bmjqs-2015-004828
22. Blendon RJ, DesRoches CM, Brodie M, et al. Views of practicing physicians and the public on medical errors. N Engl J Med. 2002;347(24):1933-1940. doi:10.1056/NEJMsa022151
23. Saber Tehrani AS, Lee H, Mathews SC, et al. 25-year summary of US malpractice claims for diagnostic errors 1986-2010: an analysis from the National Practitioner Data Bank. BMJ Qual Saf. 2013;22(8):672-680. doi:10.1136/bmjqs-2012-001550
24. Malik MA, Motta-Calderon D, Piniella N, et al. A structured approach to EHR surveillance of diagnostic error in acute care: an exploratory analysis of two institutionally-defined case cohorts. Diagnosis (Berl). 2022;9(4):446-457. doi:10.1515/dx-2022-0032
25. Graber ML. The incidence of diagnostic error in medicine. BMJ Qual Saf. 2013;22(suppl 2):ii21-ii27. doi:10.1136/bmjqs-2012-001615
26. Bergl PA, Taneja A, El-Kareh R, Singh H, Nanchal RS. Frequency, risk factors, causes, and consequences of diagnostic errors in critically ill medical patients: a retrospective cohort study. Crit Care Med. 2019;47(11):e902-e910. doi:10.1097/CCM.0000000000003976
27. Hogan H, Healey F, Neale G, Thomson R, Vincent C, Black N. Preventable deaths due to problems in care in English acute hospitals: a retrospective case record review study. BMJ Qual Saf. 2012;21(9):737-745. doi:10.1136/bmjqs-2011-001159
28. Bergl PA, Nanchal RS, Singh H. Diagnostic error in the critically ill: defining the problem and exploring next steps to advance intensive care unit safety. Ann Am Thorac Soc. 2018;15(8):903-907. doi:10.1513/AnnalsATS.201801-068PS
29. Marquet K, Claes N, De Troy E, et al. One fourth of unplanned transfers to a higher level of care are associated with a highly preventable adverse event: a patient record review in six Belgian hospitals. Crit Care Med. 2015;43(5):1053-1061. doi:10.1097/CCM.0000000000000932
30. Rodwin BA, Bilan VP, Merchant NB, et al. Rate of preventable mortality in hospitalized patients: a systematic review and meta-analysis. J Gen Intern Med. 2020;35(7):2099-2106. doi:10.1007/s11606-019-05592-5
31. Winters B, Custer J, Galvagno SM, et al. Diagnostic errors in the intensive care unit: a systematic review of autopsy studies. BMJ Qual Saf. 2012;21(11):894-902. doi:10.1136/bmjqs-2012-000803
32. Raffel KE, Kantor MA, Barish P, et al. Prevalence and characterisation of diagnostic error among 7-day all-cause hospital medicine readmissions: a retrospective cohort study. BMJ Qual Saf. 2020;29(12):971-979. doi:10.1136/bmjqs-2020-010896
33. Weingart SN, Pagovich O, Sands DZ, et al. What can hospitalized patients tell us about adverse events? learning from patient-reported incidents. J Gen Intern Med. 2005;20(9):830-836. doi:10.1111/j.1525-1497.2005.0180.x
34. Schiff GD, Hasan O, Kim S, et al. Diagnostic error in medicine: analysis of 583 physician-reported errors. Arch Intern Med. 2009;169(20):1881-1887. doi:10.1001/archinternmed.2009.333
35. Singh H, Schiff GD, Graber ML, Onakpoya I, Thompson MJ. The global burden of diagnostic errors in primary care. BMJ Qual Saf. 2017;26(6):484-494. doi:10.1136/bmjqs-2016-005401
36. Schiff GD, Leape LL. Commentary: how can we make diagnosis safer? Acad Med J Assoc Am Med Coll. 2012;87(2):135-138. doi:10.1097/ACM.0b013e31823f711c
37. Schiff GD, Kim S, Abrams R, et al. Diagnosing diagnosis errors: lessons from a multi-institutional collaborative project. In: Henriksen K, Battles JB, Marks ES, Lewin DI, eds. Advances in Patient Safety: From Research to Implementation. Volume 2: Concepts and Methodology. AHRQ Publication No. 05-0021-2. Agency for Healthcare Research and Quality (US); 2005. Accessed January 16, 2023. http://www.ncbi.nlm.nih.gov/books/NBK20492/
38. Newman-Toker DE. A unified conceptual model for diagnostic errors: underdiagnosis, overdiagnosis, and misdiagnosis. Diagnosis (Berl). 2014;1(1):43-48. doi:10.1515/dx-2013-0027
39. Abimanyi-Ochom J, Bohingamu Mudiyanselage S, Catchpool M, Firipis M, Wanni Arachchige Dona S, Watts JJ. Strategies to reduce diagnostic errors: a systematic review. BMC Med Inform Decis Mak. 2019;19(1):174. doi:10.1186/s12911-019-0901-1
40. Gupta A, Harrod M, Quinn M, et al. Mind the overlap: how system problems contribute to cognitive failure and diagnostic errors. Diagnosis (Berl). 2018;5(3):151-156. doi:10.1515/dx-2018-0014
41. Saposnik G, Redelmeier D, Ruff CC, Tobler PN. Cognitive biases associated with medical decisions: a systematic review. BMC Med Inform Decis Mak. 2016;16:138. doi:10.1186/s12911-016-0377-1
42. Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med. 2003;78(8):775-780. doi: 10.1097/00001888-200308000-00003
43. Chapman EN, Kaatz A, Carnes M. Physicians and implicit bias: how doctors may unwittingly perpetuate health care disparities. J Gen Intern Med. 2013;28(11):1504-1510. doi:10.1007/s11606-013-2441-1
44. Zwaan L, Singh H. The challenges in defining and measuring diagnostic error. Diagnosis (Ber). 2015;2(2):97-103. doi:10.1515/dx-2014-0069
45. Arkes HR, Wortmann RL, Saville PD, Harkness AR. Hindsight bias among physicians weighing the likelihood of diagnoses. J Appl Psychol. 1981;66(2):252-254.
46. Singh H. Editorial: Helping health care organizations to define diagnostic errors as missed opportunities in diagnosis. Jt Comm J Qual Patient Saf. 2014;40(3):99-101. doi:10.1016/s1553-7250(14)40012-6
47. Vassar M, Holzmann M. The retrospective chart review: important methodological considerations. J Educ Eval Health Prof. 2013;10:12. doi:10.3352/jeehp.2013.10.12
48. Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102(9):605-613. doi:10.1093/jnci/djq099
49. Moynihan R, Doust J, Henry D. Preventing overdiagnosis: how to stop harming the healthy. BMJ. 2012;344:e3502. doi:10.1136/bmj.e3502
50. Hayward RA, Hofer TP. Estimating hospital deaths due to medical errors: preventability is in the eye of the reviewer. JAMA. 2001;286(4):415-420. doi:10.1001/jama.286.4.415
51. Singh H, Sittig DF. Advancing the science of measurement of diagnostic errors in healthcare: the Safer Dx framework. BMJ Qual Saf. 2015;24(2):103-110. doi:10.1136/bmjqs-2014-003675
52. Singh H, Khanna A, Spitzmueller C, Meyer AND. Recommendations for using the Revised Safer Dx Instrument to help measure and improve diagnostic safety. Diagnosis (Berl). 2019;6(4):315-323. doi:10.1515/dx-2019-0012
53. Classen DC, Resar R, Griffin F, et al. “Global trigger tool” shows that adverse events in hospitals may be ten times greater than previously measured. Health Aff (Millwood). 2011;30(4):581-589. doi:10.1377/hlthaff.2011.0190
54. Schiff GD. Minimizing diagnostic error: the importance of follow-up and feedback. Am J Med. 2008;121(5 suppl):S38-S42. doi:10.1016/j.amjmed.2008.02.004
55. Mitchell I, Schuster A, Smith K, Pronovost P, Wu A. Patient safety incident reporting: a qualitative study of thoughts and perceptions of experts 15 years after “To Err is Human.” BMJ Qual Saf. 2016;25(2):92-99. doi:10.1136/bmjqs-2015-004405
56. Mazurenko O, Collum T, Ferdinand A, Menachemi N. Predictors of hospital patient satisfaction as measured by HCAHPS: a systematic review. J Healthc Manag. 2017;62(4):272-283. doi:10.1097/JHM-D-15-00050
57. Liberman AL, Newman-Toker DE. Symptom-Disease Pair Analysis of Diagnostic Error (SPADE): a conceptual framework and methodological approach for unearthing misdiagnosis-related harms using big data. BMJ Qual Saf. 2018;27(7):557-566. doi:10.1136/bmjqs-2017-007032
58. Utility of Predictive Systems to Identify Inpatient Diagnostic Errors: the UPSIDE study. NIH RePort/RePORTER. Accessed January 14, 2023. https://reporter.nih.gov/search/rpoHXlEAcEudQV3B9ld8iw/project-details/10020962
59. Overview of Patient Safety Learning Laboratory (PSLL) Projects. Agency for Healthcare Research and Quality. Accessed January 14, 2023. https://www.ahrq.gov/patient-safety/resources/learning-lab/index.html
60. Achieving Diagnostic Excellence through Prevention and Teamwork (ADEPT). NIH RePort/RePORTER. Accessed January 14, 2023. https://reporter.nih.gov/project-details/10642576
61. Zwaan L, Singh H. Diagnostic error in hospitals: finding forests not just the big trees. BMJ Qual Saf. 2020;29(12):961-964. doi:10.1136/bmjqs-2020-011099
62. Longtin Y, Sax H, Leape LL, Sheridan SE, Donaldson L, Pittet D. Patient participation: current knowledge and applicability to patient safety. Mayo Clin Proc. 2010;85(1):53-62. doi:10.4065/mcp.2009.0248
63. Murphy DR, Singh H, Berlin L. Communication breakdowns and diagnostic errors: a radiology perspective. Diagnosis (Berl). 2014;1(4):253-261. doi:10.1515/dx-2014-0035
64. Singh H, Naik AD, Rao R, Petersen LA. Reducing diagnostic errors through effective communication: harnessing the power of information technology. J Gen Intern Med. 2008;23(4):489-494. doi:10.1007/s11606-007-0393-z
65. Singh H, Connor DM, Dhaliwal G. Five strategies for clinicians to advance diagnostic excellence. BMJ. 2022;376:e068044. doi:10.1136/bmj-2021-068044
66. Yale S, Cohen S, Bordini BJ. Diagnostic time-outs to improve diagnosis. Crit Care Clin. 2022;38(2):185-194. doi:10.1016/j.ccc.2021.11.008
67. Schwartz A, Peskin S, Spiro A, Weiner SJ. Impact of unannounced standardized patient audit and feedback on care, documentation, and costs: an experiment and claims analysis. J Gen Intern Med. 2021;36(1):27-34. doi:10.1007/s11606-020-05965-1
68. Carpenter JD, Gorman PN. Using medication list—problem list mismatches as markers of potential error. Proc AMIA Symp. 2002:106-110.
69. Hron JD, Manzi S, Dionne R, et al. Electronic medication reconciliation and medication errors. Int J Qual Health Care. 2015;27(4):314-319. doi:10.1093/intqhc/mzv046
70. Graber ML, Siegal D, Riah H, Johnston D, Kenyon K. Electronic health record–related events in medical malpractice claims. J Patient Saf. 2019;15(2):77-85. doi:10.1097/PTS.0000000000000240
71. Murphy DR, Wu L, Thomas EJ, Forjuoh SN, Meyer AND, Singh H. Electronic trigger-based intervention to reduce delays in diagnostic evaluation for cancer: a cluster randomized controlled trial. J Clin Oncol. 2015;33(31):3560-3567. doi:10.1200/JCO.2015.61.1301
72. Singh H, Giardina TD, Forjuoh SN, et al. Electronic health record-based surveillance of diagnostic errors in primary care. BMJ Qual Saf. 2012;21(2):93-100. doi:10.1136/bmjqs-2011-000304
73. Armaignac DL, Saxena A, Rubens M, et al. Impact of telemedicine on mortality, length of stay, and cost among patients in progressive care units: experience from a large healthcare system. Crit Care Med. 2018;46(5):728-735. doi:10.1097/CCM.0000000000002994
74. MacKinnon GE, Brittain EL. Mobile health technologies in cardiopulmonary disease. Chest. 2020;157(3):654-664. doi:10.1016/j.chest.2019.10.015
75. DeVore AD, Wosik J, Hernandez AF. The future of wearables in heart failure patients. JACC Heart Fail. 2019;7(11):922-932. doi:10.1016/j.jchf.2019.08.008
76. Tsai TL, Fridsma DB, Gatti G. Computer decision support as a source of interpretation error: the case of electrocardiograms. J Am Med Inform Assoc. 2003;10(5):478-483. doi:10.1197/jamia.M1279
77. Lin SY, Mahoney MR, Sinsky CA. Ten ways artificial intelligence will transform primary care. J Gen Intern Med. 2019;34(8):1626-1630. doi:10.1007/s11606-019-05035-1
78. Ramirez AH, Gebo KA, Harris PA. Progress with the All Of Us research program: opening access for researchers. JAMA. 2021;325(24):2441-2442. doi:10.1001/jama.2021.7702
79. Johnson KB, Wei W, Weeraratne D, et al. Precision medicine, AI, and the future of personalized health care. Clin Transl Sci. 2021;14(1):86-93. doi:10.1111/cts.12884
80. Gupta A, Snyder A, Kachalia A, Flanders S, Saint S, Chopra V. Malpractice claims related to diagnostic errors in the hospital. BMJ Qual Saf. 2017;27(1):bmjqs-2017-006774. doi:10.1136/bmjqs-2017-006774
81. Renkema E, Broekhuis M, Ahaus K. Conditions that influence the impact of malpractice litigation risk on physicians’ behavior regarding patient safety. BMC Health Serv Res. 2014;14(1):38. doi:10.1186/1472-6963-14-38
82. Kachalia A, Mello MM, Nallamothu BK, Studdert DM. Legal and policy interventions to improve patient safety. Circulation. 2016;133(7):661-671. doi:10.1161/CIRCULATIONAHA.115.015880
Safety in Health Care: An Essential Pillar of Quality
Each year, 40,000 to 98,000 deaths occur due to medical errors.1 The Harvard Medical Practice Study (HMPS), published in 1991, found that 3.7% of hospitalized patients were harmed by adverse events and 1% were harmed by adverse events due to negligence.2 The latest HMPS showed that, despite significant improvements in patient safety over the past 3 decades, patient safety challenges persist. This study found that inpatient care leads to harm in nearly a quarter of patients, and that 1 in 4 of these adverse events are preventable.3
Since the first HMPS study was published, efforts to improve patient safety have focused on identifying causes of medical error and the design and implementation of interventions to mitigate errors. Factors contributing to medical errors have been well documented: the complexity of care delivery from inpatient to outpatient settings, with transitions of care and extensive use of medications; multiple comorbidities; and the fragmentation of care across multiple systems and specialties. Although most errors are related to process or system failure, accountability of each practitioner and clinician is essential to promoting a culture of safety. Many medical errors are preventable through multifaceted approaches employed throughout the phases of the care,4 with medication errors, both prescribing and administration, and diagnostic and treatment errors encompassing most risk prevention areas. Broadly, safety efforts should emphasize building a culture of safety where all safety events are reported, including near-miss events.
Two articles in this issue of JCOM address key elements of patient safety: building a safety culture and diagnostic error. Merchant et al5 report on an initiative designed to promote a safety culture by recognizing and rewarding staff who identify and report near misses. The tiered awards program they designed led to significantly increased staff participation in the safety awards nomination process and was associated with increased reporting of actual and close-call events and greater attendance at monthly safety forums. Goyal et al,6 noting that diagnostic error rates in hospitalized patients remain unacceptably high, provide a concise update on diagnostic error among inpatients, focusing on issues related to defining and measuring diagnostic errors and current strategies to improve diagnostic safety in hospitalized patients. In a third article, Sathi et al report on efforts to teach quality improvement (QI) methods to internal medicine trainees; their project increased residents’ knowledge of their patient panels and comfort with QI approaches and led to improved patient outcomes.
Major progress has been made to improve health care safety since the first HMPS was published. However, the latest HMPS shows that patient safety efforts must continue, given the persistent risk for patient harm in the current health care delivery system. Safety, along with clear accountability for identifying, reporting, and addressing errors, should be a top priority for health care systems throughout the preventive, diagnostic, and therapeutic phases of care.
Corresponding author: Ebrahim Barkoudah, MD, MPH; ebarkoudah@bwh.harvard.edu
1. Clancy C, Munier W, Brady J. National healthcare quality report. Agency for Healthcare Research and Quality; 2013.
2. Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324(6):370-376. doi:10.1056/NEJM199102073240604
3. Bates DW, Levine DM, Salmasian H, et al. The safety of inpatient health care. N Engl J Med. 2023;388(2):142-153. doi:10.1056/NEJMsa2206117
4. Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events: implications for prevention. JAMA. 1995;274(1):29-34.
5. Merchant NB, O’Neal J, Murray JS. Development of a safety awards program at a Veterans Affairs health care system: a quality improvement initiative. J Clin Outcome Manag. 2023;30(1):9-16. doi:10.12788/jcom.0120
6. Goyal A, Martin-Doyle W, Dalal AK. Diagnostic errors in hospitalized patients. J Clin Outcome Manag. 2023;30(1):17-27. doi:10.12788/jcom.0121
7. Sathi K, Huang KTL, Chandler DM, et al. Teaching quality improvement to internal medicine residents to address patient care gaps in ambulatory quality metrics. J Clin Outcome Manag. 2023;30(1):1-6.doi:10.12788/jcom.0119
Each year, 40,000 to 98,000 deaths occur due to medical errors.1 The Harvard Medical Practice Study (HMPS), published in 1991, found that 3.7% of hospitalized patients were harmed by adverse events and 1% were harmed by adverse events due to negligence.2 The latest HMPS showed that, despite significant improvements in patient safety over the past 3 decades, patient safety challenges persist. This study found that inpatient care leads to harm in nearly a quarter of patients, and that 1 in 4 of these adverse events are preventable.3
Since the first HMPS study was published, efforts to improve patient safety have focused on identifying causes of medical error and the design and implementation of interventions to mitigate errors. Factors contributing to medical errors have been well documented: the complexity of care delivery from inpatient to outpatient settings, with transitions of care and extensive use of medications; multiple comorbidities; and the fragmentation of care across multiple systems and specialties. Although most errors are related to process or system failure, accountability of each practitioner and clinician is essential to promoting a culture of safety. Many medical errors are preventable through multifaceted approaches employed throughout the phases of the care,4 with medication errors, both prescribing and administration, and diagnostic and treatment errors encompassing most risk prevention areas. Broadly, safety efforts should emphasize building a culture of safety where all safety events are reported, including near-miss events.
Two articles in this issue of JCOM address key elements of patient safety: building a safety culture and diagnostic error. Merchant et al5 report on an initiative designed to promote a safety culture by recognizing and rewarding staff who identify and report near misses. The tiered awards program they designed led to significantly increased staff participation in the safety awards nomination process and was associated with increased reporting of actual and close-call events and greater attendance at monthly safety forums. Goyal et al,6 noting that diagnostic error rates in hospitalized patients remain unacceptably high, provide a concise update on diagnostic error among inpatients, focusing on issues related to defining and measuring diagnostic errors and current strategies to improve diagnostic safety in hospitalized patients. In a third article, Sathi et al report on efforts to teach quality improvement (QI) methods to internal medicine trainees; their project increased residents’ knowledge of their patient panels and comfort with QI approaches and led to improved patient outcomes.
Major progress has been made to improve health care safety since the first HMPS was published. However, the latest HMPS shows that patient safety efforts must continue, given the persistent risk for patient harm in the current health care delivery system. Safety, along with clear accountability for identifying, reporting, and addressing errors, should be a top priority for health care systems throughout the preventive, diagnostic, and therapeutic phases of care.
Corresponding author: Ebrahim Barkoudah, MD, MPH; ebarkoudah@bwh.harvard.edu
Each year, 40,000 to 98,000 deaths occur due to medical errors.1 The Harvard Medical Practice Study (HMPS), published in 1991, found that 3.7% of hospitalized patients were harmed by adverse events and 1% were harmed by adverse events due to negligence.2 The latest HMPS showed that, despite significant improvements in patient safety over the past 3 decades, patient safety challenges persist. This study found that inpatient care leads to harm in nearly a quarter of patients, and that 1 in 4 of these adverse events are preventable.3
Since the first HMPS study was published, efforts to improve patient safety have focused on identifying causes of medical error and the design and implementation of interventions to mitigate errors. Factors contributing to medical errors have been well documented: the complexity of care delivery from inpatient to outpatient settings, with transitions of care and extensive use of medications; multiple comorbidities; and the fragmentation of care across multiple systems and specialties. Although most errors are related to process or system failure, accountability of each practitioner and clinician is essential to promoting a culture of safety. Many medical errors are preventable through multifaceted approaches employed throughout the phases of the care,4 with medication errors, both prescribing and administration, and diagnostic and treatment errors encompassing most risk prevention areas. Broadly, safety efforts should emphasize building a culture of safety where all safety events are reported, including near-miss events.
Two articles in this issue of JCOM address key elements of patient safety: building a safety culture and diagnostic error. Merchant et al5 report on an initiative designed to promote a safety culture by recognizing and rewarding staff who identify and report near misses. The tiered awards program they designed led to significantly increased staff participation in the safety awards nomination process and was associated with increased reporting of actual and close-call events and greater attendance at monthly safety forums. Goyal et al,6 noting that diagnostic error rates in hospitalized patients remain unacceptably high, provide a concise update on diagnostic error among inpatients, focusing on issues related to defining and measuring diagnostic errors and current strategies to improve diagnostic safety in hospitalized patients. In a third article, Sathi et al report on efforts to teach quality improvement (QI) methods to internal medicine trainees; their project increased residents’ knowledge of their patient panels and comfort with QI approaches and led to improved patient outcomes.
Major progress has been made to improve health care safety since the first HMPS was published. However, the latest HMPS shows that patient safety efforts must continue, given the persistent risk for patient harm in the current health care delivery system. Safety, along with clear accountability for identifying, reporting, and addressing errors, should be a top priority for health care systems throughout the preventive, diagnostic, and therapeutic phases of care.
Corresponding author: Ebrahim Barkoudah, MD, MPH; ebarkoudah@bwh.harvard.edu
1. Clancy C, Munier W, Brady J. National healthcare quality report. Agency for Healthcare Research and Quality; 2013.
2. Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324(6):370-376. doi:10.1056/NEJM199102073240604
3. Bates DW, Levine DM, Salmasian H, et al. The safety of inpatient health care. N Engl J Med. 2023;388(2):142-153. doi:10.1056/NEJMsa2206117
4. Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events: implications for prevention. JAMA. 1995;274(1):29-34.
5. Merchant NB, O’Neal J, Murray JS. Development of a safety awards program at a Veterans Affairs health care system: a quality improvement initiative. J Clin Outcome Manag. 2023;30(1):9-16. doi:10.12788/jcom.0120
6. Goyal A, Martin-Doyle W, Dalal AK. Diagnostic errors in hospitalized patients. J Clin Outcome Manag. 2023;30(1):17-27. doi:10.12788/jcom.0121
7. Sathi K, Huang KTL, Chandler DM, et al. Teaching quality improvement to internal medicine residents to address patient care gaps in ambulatory quality metrics. J Clin Outcome Manag. 2023;30(1):1-6.doi:10.12788/jcom.0119
1. Clancy C, Munier W, Brady J. National healthcare quality report. Agency for Healthcare Research and Quality; 2013.
2. Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324(6):370-376. doi:10.1056/NEJM199102073240604
3. Bates DW, Levine DM, Salmasian H, et al. The safety of inpatient health care. N Engl J Med. 2023;388(2):142-153. doi:10.1056/NEJMsa2206117
4. Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events: implications for prevention. JAMA. 1995;274(1):29-34.
5. Merchant NB, O’Neal J, Murray JS. Development of a safety awards program at a Veterans Affairs health care system: a quality improvement initiative. J Clin Outcome Manag. 2023;30(1):9-16. doi:10.12788/jcom.0120
6. Goyal A, Martin-Doyle W, Dalal AK. Diagnostic errors in hospitalized patients. J Clin Outcome Manag. 2023;30(1):17-27. doi:10.12788/jcom.0121
7. Sathi K, Huang KTL, Chandler DM, et al. Teaching quality improvement to internal medicine residents to address patient care gaps in ambulatory quality metrics. J Clin Outcome Manag. 2023;30(1):1-6.doi:10.12788/jcom.0119
A patient named ‘Settle’ decides to sue instead
On Nov. 1, 2020, Dallas Settle went to Plateau Medical Center, Oak Hill, W.Va., complaining of pain that was later described in court documents as being “in his right mid-abdomen migrating to his right lower abdomen.” Following a CT scan, Mr. Settle was diagnosed with diverticulitis resulting in pneumoperitoneum, which is the presence of air or other gas in the abdominal cavity. The patient, it was decided, required surgery to correct the problem, but Plateau Medical Center didn’t have the staff to perform the procedure.
Mr. Settle was then transferred to another West Virginia hospital, Charleston Area Medical Center (CAMC). Here, he was evaluated by doctors in the facility’s General Division, who initiated treatment with IV fluids and opiate analgesics. He was then placed under the care of a trauma surgeon, who initially decided to treat the patient nonoperatively. If that approach failed, the surgeon believed, Mr. Settle would probably require a laparotomy, bowel resection, and ostomy.
Another surgical team performed an exploratory laparotomy the following day. The team determined that Mr. Settle was suffering from a ruptured appendicitis and allegedly performed an appendectomy. But Mr. Settle’s condition continued to deteriorate the following day.
Another CT scan followed. It revealed various problems – multiple fluid collections, an ileus, distended loops of the patient’s small bowel, a left renal cyst, subcentimeter mesenteric, and retroperitoneal adenopathy. Additional CT scans conducted 4 days later indicated other problems, including fluid collections in the patient’s right- and left-lower quadrants.
Over the next few days, doctors performed further exploratory laparotomies. Finally, on Nov. 22, Mr. Settle was transferred out of the intensive care unit in preparation for his discharge the following day.
His pain continued to worsen, however, and he was readmitted to CAMC a day later. At this point, an examination revealed that his surgical incisions had become infected.
Worse news was on the horizon. On Nov. 28, the trauma surgeon who had first agreed to treat Mr. Settle informed him that, despite claims to the contrary, his appendix hadn’t been removed.
Eventually, Mr. Settle was referred to the Cleveland Clinic, where at press time he was still being treated.
Mr. Settle has hired the firm Calwell Luce diTrapano to sue CAMC, accusing it of medical malpractice, medical negligence, and other lapses in the standard of care. In his complaint, he accused the hospital and its staff of breaching their duty of care “by negligently and improperly treating him” and by failing “to exercise the degree of care, skill, and learning required and expected of reasonable health care providers.”
His suit seeks not only compensatory damages and other relief but also punitive damages.
The content contained in this article is for informational purposes only and does not constitute legal advice. Reliance on any information provided in this article is solely at your own risk.
A version of this article originally appeared on Medscape.com.
On Nov. 1, 2020, Dallas Settle went to Plateau Medical Center, Oak Hill, W.Va., complaining of pain that was later described in court documents as being “in his right mid-abdomen migrating to his right lower abdomen.” Following a CT scan, Mr. Settle was diagnosed with diverticulitis resulting in pneumoperitoneum, which is the presence of air or other gas in the abdominal cavity. The patient, it was decided, required surgery to correct the problem, but Plateau Medical Center didn’t have the staff to perform the procedure.
Mr. Settle was then transferred to another West Virginia hospital, Charleston Area Medical Center (CAMC). Here, he was evaluated by doctors in the facility’s General Division, who initiated treatment with IV fluids and opiate analgesics. He was then placed under the care of a trauma surgeon, who initially decided to treat the patient nonoperatively. If that approach failed, the surgeon believed, Mr. Settle would probably require a laparotomy, bowel resection, and ostomy.
Another surgical team performed an exploratory laparotomy the following day. The team determined that Mr. Settle was suffering from a ruptured appendicitis and allegedly performed an appendectomy. But Mr. Settle’s condition continued to deteriorate the following day.
Another CT scan followed. It revealed various problems – multiple fluid collections, an ileus, distended loops of the patient’s small bowel, a left renal cyst, subcentimeter mesenteric, and retroperitoneal adenopathy. Additional CT scans conducted 4 days later indicated other problems, including fluid collections in the patient’s right- and left-lower quadrants.
Over the next few days, doctors performed further exploratory laparotomies. Finally, on Nov. 22, Mr. Settle was transferred out of the intensive care unit in preparation for his discharge the following day.
His pain continued to worsen, however, and he was readmitted to CAMC a day later. At this point, an examination revealed that his surgical incisions had become infected.
Worse news was on the horizon. On Nov. 28, the trauma surgeon who had first agreed to treat Mr. Settle informed him that, despite claims to the contrary, his appendix hadn’t been removed.
Eventually, Mr. Settle was referred to the Cleveland Clinic, where at press time he was still being treated.
Mr. Settle has hired the firm Calwell Luce diTrapano to sue CAMC, accusing it of medical malpractice, medical negligence, and other lapses in the standard of care. In his complaint, he accused the hospital and its staff of breaching their duty of care “by negligently and improperly treating him” and by failing “to exercise the degree of care, skill, and learning required and expected of reasonable health care providers.”
His suit seeks not only compensatory damages and other relief but also punitive damages.
The content contained in this article is for informational purposes only and does not constitute legal advice. Reliance on any information provided in this article is solely at your own risk.
A version of this article originally appeared on Medscape.com.
On Nov. 1, 2020, Dallas Settle went to Plateau Medical Center, Oak Hill, W.Va., complaining of pain that was later described in court documents as being “in his right mid-abdomen migrating to his right lower abdomen.” Following a CT scan, Mr. Settle was diagnosed with diverticulitis resulting in pneumoperitoneum, which is the presence of air or other gas in the abdominal cavity. The patient, it was decided, required surgery to correct the problem, but Plateau Medical Center didn’t have the staff to perform the procedure.
Mr. Settle was then transferred to another West Virginia hospital, Charleston Area Medical Center (CAMC). Here, he was evaluated by doctors in the facility’s General Division, who initiated treatment with IV fluids and opiate analgesics. He was then placed under the care of a trauma surgeon, who initially decided to treat the patient nonoperatively. If that approach failed, the surgeon believed, Mr. Settle would probably require a laparotomy, bowel resection, and ostomy.
Another surgical team performed an exploratory laparotomy the following day. The team determined that Mr. Settle was suffering from a ruptured appendicitis and allegedly performed an appendectomy. But Mr. Settle’s condition continued to deteriorate the following day.
Another CT scan followed. It revealed various problems – multiple fluid collections, an ileus, distended loops of the patient’s small bowel, a left renal cyst, subcentimeter mesenteric, and retroperitoneal adenopathy. Additional CT scans conducted 4 days later indicated other problems, including fluid collections in the patient’s right- and left-lower quadrants.
Over the next few days, doctors performed further exploratory laparotomies. Finally, on Nov. 22, Mr. Settle was transferred out of the intensive care unit in preparation for his discharge the following day.
His pain continued to worsen, however, and he was readmitted to CAMC a day later. At this point, an examination revealed that his surgical incisions had become infected.
Worse news was on the horizon. On Nov. 28, the trauma surgeon who had first agreed to treat Mr. Settle informed him that, despite claims to the contrary, his appendix hadn’t been removed.
Eventually, Mr. Settle was referred to the Cleveland Clinic, where at press time he was still being treated.
Mr. Settle has hired the firm Calwell Luce diTrapano to sue CAMC, accusing it of medical malpractice, medical negligence, and other lapses in the standard of care. In his complaint, he accused the hospital and its staff of breaching their duty of care “by negligently and improperly treating him” and by failing “to exercise the degree of care, skill, and learning required and expected of reasonable health care providers.”
His suit seeks not only compensatory damages and other relief but also punitive damages.
The content contained in this article is for informational purposes only and does not constitute legal advice. Reliance on any information provided in this article is solely at your own risk.
A version of this article originally appeared on Medscape.com.
Oncologist to insurer: ‘This denial will not stand’
“Is this really the hill you want to die on?” asked Rebecca Shatsky, MD, a medical oncologist at the University of California, San Diego.
It was Nov. 18 and Dr. Shatsky was on the phone with a retired oncologist working for the health insurance company Premera Blue Cross.
Dr. Shatsky was appealing a prior authorization denial for pembrolizumab (Keytruda) to treat her patient with stage IIIc triple-negative breast cancer (TNBC). She hoped the peer-to-peer would reverse the denial. The Food and Drug Administration had approved the immunotherapy for people with high-risk TNBC both in the neoadjuvant setting alongside chemotherapy and, in her patient’s case, as a single-agent adjuvant treatment based on data from the KEYNOTE 522 trial.
In the peer-to-peer, Dr. Shatsky laid out the evidence, but she could tell the physician wasn’t going to budge.
When she pressed him further, asking why he was denying potentially lifesaving care for her patient, he said the data on whether patients really need adjuvant pembrolizumab were not clear yet.
“The man – who was not a breast oncologist – was essentially mansplaining breast oncology to me,” she said in an interview. “I don’t need a nonexpert giving me their misinterpretation of the data.”
Dr. Shatsky informed him that this decision would not stand. She would be escalating the claim.
“I’m not going to let you get in way of my patient’s survival,” Dr. Shatsky told the physician during the peer-to-peer. “We have one shot to cure this, and if we don’t do it now, patients’ average lifespan is 17 months.”
The conversation turned a few heads in her office.
“My whole office stopped and stared. But then they clapped after they realized why I was yelling,” she tweeted later that night.
She continued: “@premera picked the wrong oncologist to mess with today. I will not be letting this go. This denial. Will. Not. Stand. An insurance company should not get to tell me how to practice medicine when Phase III RCT data and @NCCN + @ASCO guideline support my decision!”
A spokesperson for Premera said in a statement that, “while we did see many of the details about the case were posted to Twitter, we cannot comment on the specifics you noted due to privacy policies.”
The spokesperson explained that Premera has “the same goal as our provider partners: ensure our members have access to quality health care,” noting that prior authorization helps health plans evaluate the medical necessity and safety of health care services given that “15%-30% of care is unnecessary.”
“We also understand that providers may not agree with our decisions, which is why we have a robust appeals process,” the spokesperson said, suggesting Dr. Shatsky could have appealed the decision a second time.
And “if the member or provider still disagrees with Premera’s coverage decision after the initial appeal, providers can request review by a medical expert outside Premera who works for an independent review organization,” and the company “will pay for” and “abide by” that decision, the spokesperson added.
The Twitter storm
After Dr. Shatsky tweeted about her experience with Premera, she received a flood of support from the Twitterverse. The thread garnered tens of thousands of likes and hundreds of comments offering support and advice.
Several people suggested asking Merck for help accessing the drug. But Dr. Shatsky said no, “I’m tired of laying down and letting [insurance companies] win. It IS worth fighting for.”
The next morning, Dr. Shatsky got a call. It was the vice president of medical management at Premera.
“We’ve talked again, and we’ll give you the drug,” Dr. Shatsky recalled the Premera vice president saying.
The next day, Monday morning, Dr. Shatsky’s patient received her first infusion of pembrolizumab.
Although relieved, Dr. Shatsky noted that it wasn’t until she posted her experience to Twitter that Premera seemed to take notice.
Plus, “an oncologist without a strong social media following may not have gotten care approved and that’s not how medicine should work,” said Dr. Anderson, assistant professor in the department of clinical pharmacy, University of Colorado at Denver, Aurora.
Tatiana Prowell, MD, expressed similar concerns in a Nov. 20 tweet: “And sadly, the patients with cancer & an even busier, more exhausted doctor who doesn’t have a big [reach] on social media will be denied appropriate care. And that’s bank for insurers.”
But, Dr. Prowell noted sarcastically: “At least a patient with cancer had her care delayed & a dedicated OncTwitter colleague’s Physician Burnout was exacerbated.”
In this case, the prior authorization process took about a week – requiring an initial prior authorization request, an appeal after the request was denied, a peer-to-peer resulting in a second denial, and finally a tweet and a phone call from a top executive at the company.
In fact, these delays have become so common that Dr. Shatsky needs to anticipate and incorporate likely delays into her workflow.
“I learn which drugs will take a long time to get prior authorization for and then plan enough time so that my patient’s care is hopefully not delayed,” Dr. Shatsky said. “It should not be so hard to get appropriate and time-sensitive care for our patients.”
A version of this article first appeared on Medscape.com.
“Is this really the hill you want to die on?” asked Rebecca Shatsky, MD, a medical oncologist at the University of California, San Diego.
It was Nov. 18 and Dr. Shatsky was on the phone with a retired oncologist working for the health insurance company Premera Blue Cross.
Dr. Shatsky was appealing a prior authorization denial for pembrolizumab (Keytruda) to treat her patient with stage IIIc triple-negative breast cancer (TNBC). She hoped the peer-to-peer would reverse the denial. The Food and Drug Administration had approved the immunotherapy for people with high-risk TNBC both in the neoadjuvant setting alongside chemotherapy and, in her patient’s case, as a single-agent adjuvant treatment based on data from the KEYNOTE 522 trial.
In the peer-to-peer, Dr. Shatsky laid out the evidence, but she could tell the physician wasn’t going to budge.
When she pressed him further, asking why he was denying potentially lifesaving care for her patient, he said the data on whether patients really need adjuvant pembrolizumab were not clear yet.
“The man – who was not a breast oncologist – was essentially mansplaining breast oncology to me,” she said in an interview. “I don’t need a nonexpert giving me their misinterpretation of the data.”
Dr. Shatsky informed him that this decision would not stand. She would be escalating the claim.
“I’m not going to let you get in way of my patient’s survival,” Dr. Shatsky told the physician during the peer-to-peer. “We have one shot to cure this, and if we don’t do it now, patients’ average lifespan is 17 months.”
The conversation turned a few heads in her office.
“My whole office stopped and stared. But then they clapped after they realized why I was yelling,” she tweeted later that night.
She continued: “@premera picked the wrong oncologist to mess with today. I will not be letting this go. This denial. Will. Not. Stand. An insurance company should not get to tell me how to practice medicine when Phase III RCT data and @NCCN + @ASCO guideline support my decision!”
A spokesperson for Premera said in a statement that, “while we did see many of the details about the case were posted to Twitter, we cannot comment on the specifics you noted due to privacy policies.”
The spokesperson explained that Premera has “the same goal as our provider partners: ensure our members have access to quality health care,” noting that prior authorization helps health plans evaluate the medical necessity and safety of health care services given that “15%-30% of care is unnecessary.”
“We also understand that providers may not agree with our decisions, which is why we have a robust appeals process,” the spokesperson said, suggesting Dr. Shatsky could have appealed the decision a second time.
And “if the member or provider still disagrees with Premera’s coverage decision after the initial appeal, providers can request review by a medical expert outside Premera who works for an independent review organization,” and the company “will pay for” and “abide by” that decision, the spokesperson added.
The Twitter storm
After Dr. Shatsky tweeted about her experience with Premera, she received a flood of support from the Twitterverse. The thread garnered tens of thousands of likes and hundreds of comments offering support and advice.
Several people suggested asking Merck for help accessing the drug. But Dr. Shatsky said no, “I’m tired of laying down and letting [insurance companies] win. It IS worth fighting for.”
The next morning, Dr. Shatsky got a call. It was the vice president of medical management at Premera.
“We’ve talked again, and we’ll give you the drug,” Dr. Shatsky recalled the Premera vice president saying.
The next day, Monday morning, Dr. Shatsky’s patient received her first infusion of pembrolizumab.
Although relieved, Dr. Shatsky noted that it wasn’t until she posted her experience to Twitter that Premera seemed to take notice.
Plus, “an oncologist without a strong social media following may not have gotten care approved and that’s not how medicine should work,” said Dr. Anderson, assistant professor in the department of clinical pharmacy, University of Colorado at Denver, Aurora.
Tatiana Prowell, MD, expressed similar concerns in a Nov. 20 tweet: “And sadly, the patients with cancer & an even busier, more exhausted doctor who doesn’t have a big [reach] on social media will be denied appropriate care. And that’s bank for insurers.”
But, Dr. Prowell noted sarcastically: “At least a patient with cancer had her care delayed & a dedicated OncTwitter colleague’s Physician Burnout was exacerbated.”
In this case, the prior authorization process took about a week – requiring an initial prior authorization request, an appeal after the request was denied, a peer-to-peer resulting in a second denial, and finally a tweet and a phone call from a top executive at the company.
In fact, these delays have become so common that Dr. Shatsky needs to anticipate and incorporate likely delays into her workflow.
“I learn which drugs will take a long time to get prior authorization for and then plan enough time so that my patient’s care is hopefully not delayed,” Dr. Shatsky said. “It should not be so hard to get appropriate and time-sensitive care for our patients.”
A version of this article first appeared on Medscape.com.
“Is this really the hill you want to die on?” asked Rebecca Shatsky, MD, a medical oncologist at the University of California, San Diego.
It was Nov. 18 and Dr. Shatsky was on the phone with a retired oncologist working for the health insurance company Premera Blue Cross.
Dr. Shatsky was appealing a prior authorization denial for pembrolizumab (Keytruda) to treat her patient with stage IIIc triple-negative breast cancer (TNBC). She hoped the peer-to-peer would reverse the denial. The Food and Drug Administration had approved the immunotherapy for people with high-risk TNBC both in the neoadjuvant setting alongside chemotherapy and, in her patient’s case, as a single-agent adjuvant treatment based on data from the KEYNOTE 522 trial.
In the peer-to-peer, Dr. Shatsky laid out the evidence, but she could tell the physician wasn’t going to budge.
When she pressed him further, asking why he was denying potentially lifesaving care for her patient, he said the data on whether patients really need adjuvant pembrolizumab were not clear yet.
“The man – who was not a breast oncologist – was essentially mansplaining breast oncology to me,” she said in an interview. “I don’t need a nonexpert giving me their misinterpretation of the data.”
Dr. Shatsky informed him that this decision would not stand. She would be escalating the claim.
“I’m not going to let you get in way of my patient’s survival,” Dr. Shatsky told the physician during the peer-to-peer. “We have one shot to cure this, and if we don’t do it now, patients’ average lifespan is 17 months.”
The conversation turned a few heads in her office.
“My whole office stopped and stared. But then they clapped after they realized why I was yelling,” she tweeted later that night.
She continued: “@premera picked the wrong oncologist to mess with today. I will not be letting this go. This denial. Will. Not. Stand. An insurance company should not get to tell me how to practice medicine when Phase III RCT data and @NCCN + @ASCO guideline support my decision!”
A spokesperson for Premera said in a statement that, “while we did see many of the details about the case were posted to Twitter, we cannot comment on the specifics you noted due to privacy policies.”
The spokesperson explained that Premera has “the same goal as our provider partners: ensure our members have access to quality health care,” noting that prior authorization helps health plans evaluate the medical necessity and safety of health care services given that “15%-30% of care is unnecessary.”
“We also understand that providers may not agree with our decisions, which is why we have a robust appeals process,” the spokesperson said, suggesting Dr. Shatsky could have appealed the decision a second time.
And “if the member or provider still disagrees with Premera’s coverage decision after the initial appeal, providers can request review by a medical expert outside Premera who works for an independent review organization,” and the company “will pay for” and “abide by” that decision, the spokesperson added.
The Twitter storm
After Dr. Shatsky tweeted about her experience with Premera, she received a flood of support from the Twitterverse. The thread garnered tens of thousands of likes and hundreds of comments offering support and advice.
Several people suggested asking Merck for help accessing the drug. But Dr. Shatsky said no, “I’m tired of laying down and letting [insurance companies] win. It IS worth fighting for.”
The next morning, Dr. Shatsky got a call. It was the vice president of medical management at Premera.
“We’ve talked again, and we’ll give you the drug,” Dr. Shatsky recalled the Premera vice president saying.
The next day, Monday morning, Dr. Shatsky’s patient received her first infusion of pembrolizumab.
Although relieved, Dr. Shatsky noted that it wasn’t until she posted her experience to Twitter that Premera seemed to take notice.
Plus, “an oncologist without a strong social media following may not have gotten care approved and that’s not how medicine should work,” said Dr. Anderson, assistant professor in the department of clinical pharmacy, University of Colorado at Denver, Aurora.
Tatiana Prowell, MD, expressed similar concerns in a Nov. 20 tweet: “And sadly, the patients with cancer & an even busier, more exhausted doctor who doesn’t have a big [reach] on social media will be denied appropriate care. And that’s bank for insurers.”
But, Dr. Prowell noted sarcastically: “At least a patient with cancer had her care delayed & a dedicated OncTwitter colleague’s Physician Burnout was exacerbated.”
In this case, the prior authorization process took about a week – requiring an initial prior authorization request, an appeal after the request was denied, a peer-to-peer resulting in a second denial, and finally a tweet and a phone call from a top executive at the company.
In fact, these delays have become so common that Dr. Shatsky needs to anticipate and incorporate likely delays into her workflow.
“I learn which drugs will take a long time to get prior authorization for and then plan enough time so that my patient’s care is hopefully not delayed,” Dr. Shatsky said. “It should not be so hard to get appropriate and time-sensitive care for our patients.”
A version of this article first appeared on Medscape.com.
U.S. ketamine poisonings up 81%
Although the overall ketamine exposures were low, researchers say the findings add to a growing body of research that suggests recreational ketamine use may be on the rise.
“Ketamine is by no means the most dangerous drug, but it could be dangerous if combined with drugs such as alcohol or if used in potentially hazardous situations – physically hazardous or socially hazardous,” lead author Joseph Palamar, PhD, associate professor and epidemiologist at New York University Langone Health, New York, told this news organization.
“People who decide to use ketamine recreationally need to be educated about potential risks,” Dr. Palamar said.
The findings were recently published online in the Journal of Psychopharmacology.
More widespread use
Researchers noted that ketamine use has become more widespread in the United States due in part to increasing availability of ketamine in both clinical and nonclinical settings.
Previous work by Dr. Palamar documented an increase in recreational use of ketamine at dance clubs and an increase in ketamine seizures by the Drug Enforcement Administration.
In the current study, investigators analyzed data from the National Poison Control database and included cases reported by 51 of the 55 poison control centers in the United States.
They identified 758 cases involving ketamine exposure between the first quarter of 2019 and the last quarter of 2021 in individuals aged 13 and older, more than half of whom were men.
The number of ketamine exposures increased 81.1% during the study period, rising from 37 to 67 (P = .018).
Nearly 40% of callers reported intentional misuse or abuse of ketamine, while 19.7% involved a suspected suicide or suicide attempt. The ketamine exposure was unintended in 18.9% of cases, and 10.6% of calls involved an adverse drug reaction.
Onep-third of cases involved co-use of other substances, most commonly benzodiazepines, opioids, or alcohol.
The route of administration was ingestion for 44.3%, injection for 18.8%, and inhalation for 17.6%. Another 19.3% involved another route or a combination of routes.
Nearly 20% of cases reported a major adverse effect or death, 42.8% reported a moderate effect, 25.8% a minor effect, and 11.8% no effect. There were seven deaths reported in ketamine-related calls, although Dr. Palamar noted it is unlikely those deaths were due solely to ketamine use.
Researchers didn’t analyze specific harms reported in the calls, but chronic and heavy ketamine use has been previously associated with cognitive impairment, urinary cystitis and other urinary tract issues, and upper gastrointestinal problems.
In addition, using ketamine with gamma-hydroxybutyrate (GHB) or opioids was associated with a significantly higher risk for major adverse effects (P < .001 for both). Injecting ketamine was also linked to a higher prevalence of major adverse effects, although the association did not quite reach significance (P < .05).
Cause for concern
Commenting on the findings, Timothy Wiegand, MD, director of Addiction Toxicology and Toxicology Consult Service and associate professor of emergency medicine at the University of Rochester Medical Center and Strong Memorial Hospital, New York, noted the data on co-use of ketamine with other drugs were cause for concern.
“I think the co-occurring behaviors are critical here with concomitant use of opioids and GHB, intravenous drug use, or that it is used in an attempt to harm one’s self because it allows for identification of these behaviors or use patterns,” said Dr. Wiegand, who was not involved with the research.
He added that it is important for “addiction providers and others in medicine or in the addiction field to be aware of trends” associated with ketamine.
“At the same time, a focus on general prevention, and access to care and treatment, and understanding how to implement harm reduction strategies remain high priorities,” Dr. Wiegand said.
The study was funded by the National Institute on Drug Abuse. Dr. Palamar has reported consulting for Alkermes. Dr. Wiegand has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Although the overall ketamine exposures were low, researchers say the findings add to a growing body of research that suggests recreational ketamine use may be on the rise.
“Ketamine is by no means the most dangerous drug, but it could be dangerous if combined with drugs such as alcohol or if used in potentially hazardous situations – physically hazardous or socially hazardous,” lead author Joseph Palamar, PhD, associate professor and epidemiologist at New York University Langone Health, New York, told this news organization.
“People who decide to use ketamine recreationally need to be educated about potential risks,” Dr. Palamar said.
The findings were recently published online in the Journal of Psychopharmacology.
More widespread use
Researchers noted that ketamine use has become more widespread in the United States due in part to increasing availability of ketamine in both clinical and nonclinical settings.
Previous work by Dr. Palamar documented an increase in recreational use of ketamine at dance clubs and an increase in ketamine seizures by the Drug Enforcement Administration.
In the current study, investigators analyzed data from the National Poison Control database and included cases reported by 51 of the 55 poison control centers in the United States.
They identified 758 cases involving ketamine exposure between the first quarter of 2019 and the last quarter of 2021 in individuals aged 13 and older, more than half of whom were men.
The number of ketamine exposures increased 81.1% during the study period, rising from 37 to 67 (P = .018).
Nearly 40% of callers reported intentional misuse or abuse of ketamine, while 19.7% involved a suspected suicide or suicide attempt. The ketamine exposure was unintended in 18.9% of cases, and 10.6% of calls involved an adverse drug reaction.
Onep-third of cases involved co-use of other substances, most commonly benzodiazepines, opioids, or alcohol.
The route of administration was ingestion for 44.3%, injection for 18.8%, and inhalation for 17.6%. Another 19.3% involved another route or a combination of routes.
Nearly 20% of cases reported a major adverse effect or death, 42.8% reported a moderate effect, 25.8% a minor effect, and 11.8% no effect. There were seven deaths reported in ketamine-related calls, although Dr. Palamar noted it is unlikely those deaths were due solely to ketamine use.
Researchers didn’t analyze specific harms reported in the calls, but chronic and heavy ketamine use has been previously associated with cognitive impairment, urinary cystitis and other urinary tract issues, and upper gastrointestinal problems.
In addition, using ketamine with gamma-hydroxybutyrate (GHB) or opioids was associated with a significantly higher risk for major adverse effects (P < .001 for both). Injecting ketamine was also linked to a higher prevalence of major adverse effects, although the association did not quite reach significance (P < .05).
Cause for concern
Commenting on the findings, Timothy Wiegand, MD, director of Addiction Toxicology and Toxicology Consult Service and associate professor of emergency medicine at the University of Rochester Medical Center and Strong Memorial Hospital, New York, noted the data on co-use of ketamine with other drugs were cause for concern.
“I think the co-occurring behaviors are critical here with concomitant use of opioids and GHB, intravenous drug use, or that it is used in an attempt to harm one’s self because it allows for identification of these behaviors or use patterns,” said Dr. Wiegand, who was not involved with the research.
He added that it is important for “addiction providers and others in medicine or in the addiction field to be aware of trends” associated with ketamine.
“At the same time, a focus on general prevention, and access to care and treatment, and understanding how to implement harm reduction strategies remain high priorities,” Dr. Wiegand said.
The study was funded by the National Institute on Drug Abuse. Dr. Palamar has reported consulting for Alkermes. Dr. Wiegand has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Although the overall ketamine exposures were low, researchers say the findings add to a growing body of research that suggests recreational ketamine use may be on the rise.
“Ketamine is by no means the most dangerous drug, but it could be dangerous if combined with drugs such as alcohol or if used in potentially hazardous situations – physically hazardous or socially hazardous,” lead author Joseph Palamar, PhD, associate professor and epidemiologist at New York University Langone Health, New York, told this news organization.
“People who decide to use ketamine recreationally need to be educated about potential risks,” Dr. Palamar said.
The findings were recently published online in the Journal of Psychopharmacology.
More widespread use
Researchers noted that ketamine use has become more widespread in the United States due in part to increasing availability of ketamine in both clinical and nonclinical settings.
Previous work by Dr. Palamar documented an increase in recreational use of ketamine at dance clubs and an increase in ketamine seizures by the Drug Enforcement Administration.
In the current study, investigators analyzed data from the National Poison Control database and included cases reported by 51 of the 55 poison control centers in the United States.
They identified 758 cases involving ketamine exposure between the first quarter of 2019 and the last quarter of 2021 in individuals aged 13 and older, more than half of whom were men.
The number of ketamine exposures increased 81.1% during the study period, rising from 37 to 67 (P = .018).
Nearly 40% of callers reported intentional misuse or abuse of ketamine, while 19.7% involved a suspected suicide or suicide attempt. The ketamine exposure was unintended in 18.9% of cases, and 10.6% of calls involved an adverse drug reaction.
Onep-third of cases involved co-use of other substances, most commonly benzodiazepines, opioids, or alcohol.
The route of administration was ingestion for 44.3%, injection for 18.8%, and inhalation for 17.6%. Another 19.3% involved another route or a combination of routes.
Nearly 20% of cases reported a major adverse effect or death, 42.8% reported a moderate effect, 25.8% a minor effect, and 11.8% no effect. There were seven deaths reported in ketamine-related calls, although Dr. Palamar noted it is unlikely those deaths were due solely to ketamine use.
Researchers didn’t analyze specific harms reported in the calls, but chronic and heavy ketamine use has been previously associated with cognitive impairment, urinary cystitis and other urinary tract issues, and upper gastrointestinal problems.
In addition, using ketamine with gamma-hydroxybutyrate (GHB) or opioids was associated with a significantly higher risk for major adverse effects (P < .001 for both). Injecting ketamine was also linked to a higher prevalence of major adverse effects, although the association did not quite reach significance (P < .05).
Cause for concern
Commenting on the findings, Timothy Wiegand, MD, director of Addiction Toxicology and Toxicology Consult Service and associate professor of emergency medicine at the University of Rochester Medical Center and Strong Memorial Hospital, New York, noted the data on co-use of ketamine with other drugs were cause for concern.
“I think the co-occurring behaviors are critical here with concomitant use of opioids and GHB, intravenous drug use, or that it is used in an attempt to harm one’s self because it allows for identification of these behaviors or use patterns,” said Dr. Wiegand, who was not involved with the research.
He added that it is important for “addiction providers and others in medicine or in the addiction field to be aware of trends” associated with ketamine.
“At the same time, a focus on general prevention, and access to care and treatment, and understanding how to implement harm reduction strategies remain high priorities,” Dr. Wiegand said.
The study was funded by the National Institute on Drug Abuse. Dr. Palamar has reported consulting for Alkermes. Dr. Wiegand has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF PSYCHOPHARMACOLOGY
COVID dramatically increases death risk during pregnancy: Study
Women infected with COVID-19 during pregnancy are seven times more likely to die during childbirth or during the pregnancy than uninfected pregnant women, a new study shows. The new report also warns of many other severe complications linked with the virus during pregnancy, as well as risks to the baby after birth.
But the researchers said they did not find that COVID-19 infection during pregnancy impacted the risk of stillbirth or a baby’s growth rate during pregnancy.
The study, which was a meta-analysis of previous research, was published Jan. 16 in the journal BMJ Global Health. Data from 12 studies from 12 countries were combined so researchers could analyze outcomes for 13,136 pregnant women.
Babies born to mothers who were infected with COVID during pregnancy had almost double the risk of needing stays in the neonatal intensive care unit and also were more likely to be born preterm, compared with babies who were born to pregnant women who didn’t get COVID.
The researchers also found that pregnant women who got COVID were more likely to be admitted to intensive care units, need a ventilator to help them survive, develop dangerous blood clots, or develop preeclampsia, which is a high blood pressure disorder that can be fatal for the mother or baby.
One of the strengths of the study was that it included women in different trimesters during pregnancy.
“That’s something new here too is that COVID at any time during pregnancy did bring this extra risk onto mom and babies,” said lead author Emily R. Smith, ScD, MPH, assistant professor of global health at the George Washington University, in a video statement.
The report is prompting calls for improved efforts to convince pregnant women to get vaccinated for COVID-19. The rate among them remains low: About 1 in 5 pregnant women had received the most updated COVID-19 booster as of Jan. 7, according to the CDC.
“The implications here are that it’s really important that if you’re pregnant or if you’re thinking about becoming pregnant, to get vaccinated,” Dr. Smith said. “This can really reduce the risk of having some of these bad outcomes for mom or for baby.”
A version of this article first appeared on WebMD.com.
Women infected with COVID-19 during pregnancy are seven times more likely to die during childbirth or during the pregnancy than uninfected pregnant women, a new study shows. The new report also warns of many other severe complications linked with the virus during pregnancy, as well as risks to the baby after birth.
But the researchers said they did not find that COVID-19 infection during pregnancy impacted the risk of stillbirth or a baby’s growth rate during pregnancy.
The study, which was a meta-analysis of previous research, was published Jan. 16 in the journal BMJ Global Health. Data from 12 studies from 12 countries were combined so researchers could analyze outcomes for 13,136 pregnant women.
Babies born to mothers who were infected with COVID during pregnancy had almost double the risk of needing stays in the neonatal intensive care unit and also were more likely to be born preterm, compared with babies who were born to pregnant women who didn’t get COVID.
The researchers also found that pregnant women who got COVID were more likely to be admitted to intensive care units, need a ventilator to help them survive, develop dangerous blood clots, or develop preeclampsia, which is a high blood pressure disorder that can be fatal for the mother or baby.
One of the strengths of the study was that it included women in different trimesters during pregnancy.
“That’s something new here too is that COVID at any time during pregnancy did bring this extra risk onto mom and babies,” said lead author Emily R. Smith, ScD, MPH, assistant professor of global health at the George Washington University, in a video statement.
The report is prompting calls for improved efforts to convince pregnant women to get vaccinated for COVID-19. The rate among them remains low: About 1 in 5 pregnant women had received the most updated COVID-19 booster as of Jan. 7, according to the CDC.
“The implications here are that it’s really important that if you’re pregnant or if you’re thinking about becoming pregnant, to get vaccinated,” Dr. Smith said. “This can really reduce the risk of having some of these bad outcomes for mom or for baby.”
A version of this article first appeared on WebMD.com.
Women infected with COVID-19 during pregnancy are seven times more likely to die during childbirth or during the pregnancy than uninfected pregnant women, a new study shows. The new report also warns of many other severe complications linked with the virus during pregnancy, as well as risks to the baby after birth.
But the researchers said they did not find that COVID-19 infection during pregnancy impacted the risk of stillbirth or a baby’s growth rate during pregnancy.
The study, which was a meta-analysis of previous research, was published Jan. 16 in the journal BMJ Global Health. Data from 12 studies from 12 countries were combined so researchers could analyze outcomes for 13,136 pregnant women.
Babies born to mothers who were infected with COVID during pregnancy had almost double the risk of needing stays in the neonatal intensive care unit and also were more likely to be born preterm, compared with babies who were born to pregnant women who didn’t get COVID.
The researchers also found that pregnant women who got COVID were more likely to be admitted to intensive care units, need a ventilator to help them survive, develop dangerous blood clots, or develop preeclampsia, which is a high blood pressure disorder that can be fatal for the mother or baby.
One of the strengths of the study was that it included women in different trimesters during pregnancy.
“That’s something new here too is that COVID at any time during pregnancy did bring this extra risk onto mom and babies,” said lead author Emily R. Smith, ScD, MPH, assistant professor of global health at the George Washington University, in a video statement.
The report is prompting calls for improved efforts to convince pregnant women to get vaccinated for COVID-19. The rate among them remains low: About 1 in 5 pregnant women had received the most updated COVID-19 booster as of Jan. 7, according to the CDC.
“The implications here are that it’s really important that if you’re pregnant or if you’re thinking about becoming pregnant, to get vaccinated,” Dr. Smith said. “This can really reduce the risk of having some of these bad outcomes for mom or for baby.”
A version of this article first appeared on WebMD.com.
Size of meals, not timing, linked to weight loss
The number of daily meals, but not the timing between first and last daily meals, was significantly associated with weight changes over a 6-year period, in a prospective study of more than 500 adults.
Some studies suggest that timing food intake – through time-restricted eating or intermittent fasting – can promote weight loss, but these strategies have yielded similar weight loss to eating throughout the day in randomized trials, and population-based studies of meal intervals and weight changes are needed, Di Zhao, PhD, of Johns Hopkins University, Baltimore, and colleagues wrote.
“Obesity is an epidemic,” corresponding author Wendy Bennett, MD, also of Johns Hopkins University, said in an interview. “We are interested in identifying ways to prevent weight gain over time and reduce obesity risk, since telling people to ‘just eat less’ doesn’t always work.”
In a study published in the Journal of the American Heart Association, the researchers recruited 1,017 adults who were patients at one of three health systems; of these, complete data were available for 547 individuals.
The participants downloaded an app called Daily24 to record the timing of their meals and sleep for at least 1 day. The researchers used electronic medical records to obtain information on weight and comorbidities of the participants for up to 10 years before study enrollment through 10 months after enrollment.
The mean age of the participants was 51.1 years, 78% were women, and 78% were White; the mean body mass index was 30.8 kg/m2.
The mean interval from first to last meal was 11.5 hours, and this was not associated with change in weight. The mean times from waking up to the first meal and the time from the last meal to sleeping were 1.6 hours and 4.0 hours, respectively, and these were not associated with weight changes over the follow-up period, the researchers wrote. Sleep duration (mean of 7.5 hours) also was not associated with weight change over time.
However, the total daily number of large and medium-sized meals was associated with weight gain over time, while those who reported more smaller meals showed weight loss. A daily increase of one large, medium, or small meal was associated with an average annual weight change of 0.69 kg, 0.97 kg, and –0.30 kg, respectively.
Benefits of time-restricted eating remain unclear
“Animal studies have shown benefits for time restricted feeding, but there are still questions about whether or not it helps prevent weight gain or promotes weight loss in humans,” Dr. Bennett said in an interview.
As for the current study findings, “we were not surprised; humans are more complicated than animals, and we have complicated behaviors, especially with eating,” she said.
“We showed that windows of eating (eating for longer periods of time or less in a day) was not associated with weight change over time among patients from three health systems,” said Dr. Bennett. “The main implication is that restricting your window of eating, such as eating over less time, or having more fasting time, may not reduce weight gain over time, while eating fewer large meals is associated with less weight gain over time.”
The findings were limited by several factors including the exclusion of many younger and less educated individuals, the short follow-up period, and lack of information on weight loss intention at baseline, the researchers noted. Other limitations included the inability to evaluate time-restricted eating or fasting, and the inclusion of individuals currently seeking care, which may limit generalizability.
However, the results were strengthened by the repeated measures of weight, detailed information on obesity risk factors, and real-time assessment of eating behaviors. The results do not support time-restricted eating as a long-term weight-loss strategy, and more studies are needed with a longer follow-up period, the researchers concluded.
However, there may be a role for time restricted eating as a method of total calorie control, Dr. Bennett said.
“Other studies do show that people might be able to use time-restricted eating or intermittent fasting to help them reduce their caloric intake and thus lose weight, so it can still be a helpful weight loss tool for some people who can adhere to it,” she said.
The study was supported by a grant from the American Heart Association to Johns Hopkins University. Dr. Bennett had no financial conflicts to disclose.
The number of daily meals, but not the timing between first and last daily meals, was significantly associated with weight changes over a 6-year period, in a prospective study of more than 500 adults.
Some studies suggest that timing food intake – through time-restricted eating or intermittent fasting – can promote weight loss, but these strategies have yielded similar weight loss to eating throughout the day in randomized trials, and population-based studies of meal intervals and weight changes are needed, Di Zhao, PhD, of Johns Hopkins University, Baltimore, and colleagues wrote.
“Obesity is an epidemic,” corresponding author Wendy Bennett, MD, also of Johns Hopkins University, said in an interview. “We are interested in identifying ways to prevent weight gain over time and reduce obesity risk, since telling people to ‘just eat less’ doesn’t always work.”
In a study published in the Journal of the American Heart Association, the researchers recruited 1,017 adults who were patients at one of three health systems; of these, complete data were available for 547 individuals.
The participants downloaded an app called Daily24 to record the timing of their meals and sleep for at least 1 day. The researchers used electronic medical records to obtain information on weight and comorbidities of the participants for up to 10 years before study enrollment through 10 months after enrollment.
The mean age of the participants was 51.1 years, 78% were women, and 78% were White; the mean body mass index was 30.8 kg/m2.
The mean interval from first to last meal was 11.5 hours, and this was not associated with change in weight. The mean times from waking up to the first meal and the time from the last meal to sleeping were 1.6 hours and 4.0 hours, respectively, and these were not associated with weight changes over the follow-up period, the researchers wrote. Sleep duration (mean of 7.5 hours) also was not associated with weight change over time.
However, the total daily number of large and medium-sized meals was associated with weight gain over time, while those who reported more smaller meals showed weight loss. A daily increase of one large, medium, or small meal was associated with an average annual weight change of 0.69 kg, 0.97 kg, and –0.30 kg, respectively.
Benefits of time-restricted eating remain unclear
“Animal studies have shown benefits for time restricted feeding, but there are still questions about whether or not it helps prevent weight gain or promotes weight loss in humans,” Dr. Bennett said in an interview.
As for the current study findings, “we were not surprised; humans are more complicated than animals, and we have complicated behaviors, especially with eating,” she said.
“We showed that windows of eating (eating for longer periods of time or less in a day) was not associated with weight change over time among patients from three health systems,” said Dr. Bennett. “The main implication is that restricting your window of eating, such as eating over less time, or having more fasting time, may not reduce weight gain over time, while eating fewer large meals is associated with less weight gain over time.”
The findings were limited by several factors including the exclusion of many younger and less educated individuals, the short follow-up period, and lack of information on weight loss intention at baseline, the researchers noted. Other limitations included the inability to evaluate time-restricted eating or fasting, and the inclusion of individuals currently seeking care, which may limit generalizability.
However, the results were strengthened by the repeated measures of weight, detailed information on obesity risk factors, and real-time assessment of eating behaviors. The results do not support time-restricted eating as a long-term weight-loss strategy, and more studies are needed with a longer follow-up period, the researchers concluded.
However, there may be a role for time restricted eating as a method of total calorie control, Dr. Bennett said.
“Other studies do show that people might be able to use time-restricted eating or intermittent fasting to help them reduce their caloric intake and thus lose weight, so it can still be a helpful weight loss tool for some people who can adhere to it,” she said.
The study was supported by a grant from the American Heart Association to Johns Hopkins University. Dr. Bennett had no financial conflicts to disclose.
The number of daily meals, but not the timing between first and last daily meals, was significantly associated with weight changes over a 6-year period, in a prospective study of more than 500 adults.
Some studies suggest that timing food intake – through time-restricted eating or intermittent fasting – can promote weight loss, but these strategies have yielded similar weight loss to eating throughout the day in randomized trials, and population-based studies of meal intervals and weight changes are needed, Di Zhao, PhD, of Johns Hopkins University, Baltimore, and colleagues wrote.
“Obesity is an epidemic,” corresponding author Wendy Bennett, MD, also of Johns Hopkins University, said in an interview. “We are interested in identifying ways to prevent weight gain over time and reduce obesity risk, since telling people to ‘just eat less’ doesn’t always work.”
In a study published in the Journal of the American Heart Association, the researchers recruited 1,017 adults who were patients at one of three health systems; of these, complete data were available for 547 individuals.
The participants downloaded an app called Daily24 to record the timing of their meals and sleep for at least 1 day. The researchers used electronic medical records to obtain information on weight and comorbidities of the participants for up to 10 years before study enrollment through 10 months after enrollment.
The mean age of the participants was 51.1 years, 78% were women, and 78% were White; the mean body mass index was 30.8 kg/m2.
The mean interval from first to last meal was 11.5 hours, and this was not associated with change in weight. The mean times from waking up to the first meal and the time from the last meal to sleeping were 1.6 hours and 4.0 hours, respectively, and these were not associated with weight changes over the follow-up period, the researchers wrote. Sleep duration (mean of 7.5 hours) also was not associated with weight change over time.
However, the total daily number of large and medium-sized meals was associated with weight gain over time, while those who reported more smaller meals showed weight loss. A daily increase of one large, medium, or small meal was associated with an average annual weight change of 0.69 kg, 0.97 kg, and –0.30 kg, respectively.
Benefits of time-restricted eating remain unclear
“Animal studies have shown benefits for time restricted feeding, but there are still questions about whether or not it helps prevent weight gain or promotes weight loss in humans,” Dr. Bennett said in an interview.
As for the current study findings, “we were not surprised; humans are more complicated than animals, and we have complicated behaviors, especially with eating,” she said.
“We showed that windows of eating (eating for longer periods of time or less in a day) was not associated with weight change over time among patients from three health systems,” said Dr. Bennett. “The main implication is that restricting your window of eating, such as eating over less time, or having more fasting time, may not reduce weight gain over time, while eating fewer large meals is associated with less weight gain over time.”
The findings were limited by several factors including the exclusion of many younger and less educated individuals, the short follow-up period, and lack of information on weight loss intention at baseline, the researchers noted. Other limitations included the inability to evaluate time-restricted eating or fasting, and the inclusion of individuals currently seeking care, which may limit generalizability.
However, the results were strengthened by the repeated measures of weight, detailed information on obesity risk factors, and real-time assessment of eating behaviors. The results do not support time-restricted eating as a long-term weight-loss strategy, and more studies are needed with a longer follow-up period, the researchers concluded.
However, there may be a role for time restricted eating as a method of total calorie control, Dr. Bennett said.
“Other studies do show that people might be able to use time-restricted eating or intermittent fasting to help them reduce their caloric intake and thus lose weight, so it can still be a helpful weight loss tool for some people who can adhere to it,” she said.
The study was supported by a grant from the American Heart Association to Johns Hopkins University. Dr. Bennett had no financial conflicts to disclose.
FROM THE JOURNAL OF THE AMERICAN HEART ASSOCIATION
Highly anticipated HIV vaccine fails in large trial
officials announced Wednesday.
The vaccine had been in development since 2019 and was given to 3,900 study participants through October 2022, but data shows it does not protect against HIV compared with a placebo, according to developer Janssen Pharmaceutical.
Experts estimate the failure means there won’t be another potential vaccine on the horizon for 3 to 5 years, the New York Times reported.
“It’s obviously disappointing,” Anthony Fauci, MD, former head of the National Institute of Allergy and Infectious Diseases, told MSNBC, noting that other areas of HIV treatment research are promising. “I don’t think that people should give up on the field of the HIV vaccine.”
No safety issues had been identified with the vaccine during the trial, which studied the experimental treatment in men who have sex with men or with transgender people.
There is no cure for HIV, but disease progression can be managed with existing treatments. HIV attacks the body’s immune system and destroys white blood cells, increasing the risk of other infections. More than 1.5 million people worldwide were infected with HIV in 2021 and 38.4 million people are living with the virus, according to UNAIDS.
A version of this article first appeared on WebMD.com.
officials announced Wednesday.
The vaccine had been in development since 2019 and was given to 3,900 study participants through October 2022, but data shows it does not protect against HIV compared with a placebo, according to developer Janssen Pharmaceutical.
Experts estimate the failure means there won’t be another potential vaccine on the horizon for 3 to 5 years, the New York Times reported.
“It’s obviously disappointing,” Anthony Fauci, MD, former head of the National Institute of Allergy and Infectious Diseases, told MSNBC, noting that other areas of HIV treatment research are promising. “I don’t think that people should give up on the field of the HIV vaccine.”
No safety issues had been identified with the vaccine during the trial, which studied the experimental treatment in men who have sex with men or with transgender people.
There is no cure for HIV, but disease progression can be managed with existing treatments. HIV attacks the body’s immune system and destroys white blood cells, increasing the risk of other infections. More than 1.5 million people worldwide were infected with HIV in 2021 and 38.4 million people are living with the virus, according to UNAIDS.
A version of this article first appeared on WebMD.com.
officials announced Wednesday.
The vaccine had been in development since 2019 and was given to 3,900 study participants through October 2022, but data shows it does not protect against HIV compared with a placebo, according to developer Janssen Pharmaceutical.
Experts estimate the failure means there won’t be another potential vaccine on the horizon for 3 to 5 years, the New York Times reported.
“It’s obviously disappointing,” Anthony Fauci, MD, former head of the National Institute of Allergy and Infectious Diseases, told MSNBC, noting that other areas of HIV treatment research are promising. “I don’t think that people should give up on the field of the HIV vaccine.”
No safety issues had been identified with the vaccine during the trial, which studied the experimental treatment in men who have sex with men or with transgender people.
There is no cure for HIV, but disease progression can be managed with existing treatments. HIV attacks the body’s immune system and destroys white blood cells, increasing the risk of other infections. More than 1.5 million people worldwide were infected with HIV in 2021 and 38.4 million people are living with the virus, according to UNAIDS.
A version of this article first appeared on WebMD.com.
Social isolation hikes dementia risk in older adults
, new research suggests. Results from a longitudinal study that included more than 5,000 United States–based seniors showed that nearly one-quarter were socially isolated.
After adjusting for demographic and health factors, social isolation was found to be associated with a 28% higher risk for developing dementia over a 9-year period, compared with non-isolation. In addition, this finding held true regardless of race or ethnicity.
“Social connections are increasingly understood as a critical factor for the health of individuals as they age,” senior study author Thomas K.M. Cudjoe, MD, Robert and Jane Meyerhoff Endowed Professor and assistant professor of medicine, Division of Geriatric Medicine and Gerontology, Johns Hopkins University School of Medicine, Baltimore, said in a press release. “Our study expands our understanding of the deleterious impact of social isolation on one’s risk for dementia over time,” Dr. Cudjoe added.
The findings were published online in the Journal of the American Geriatric Society.
Upstream resources, downstream outcomes
Social isolation is a “multidimensional construct” characterized by factors such as social connections, social support, resource sharing, and relationship strain. It also affects approximately a quarter of older adults, the investigators noted.
Although prior studies have pointed to an association between socially isolated older adults and increased risk for incident dementia, no study has described this longitudinal association in a nationally representative cohort of U.S. seniors.
Dr. Cudjoe said he was motivated to conduct the current study because he wondered whether or not older adults throughout the United States were similar to some of his patients “who might be at risk for worse cognitive outcomes because they lacked social contact with friends, family, or neighbors.”
The study was also “informed by conceptual foundation that upstream social and personal resources are linked to downstream health outcomes, including cognitive health and function,” the researchers added.
They turned to 2011-2020 data from the National Health and Aging Trends Study, a nationally representative, longitudinal cohort of U.S. Medicare beneficiaries. The sample was drawn from the Medicare enrollment file and incorporated 95 counties and 655 zip codes.
Participants (n = 5,022; mean age, 76.4 years; 57.2% women; 71.7% White, non-Hispanic; 42.4% having more than a college education) were community-dwelling older adults who completed annual 2-hour interviews that included assessment of function, economic health status, and well-being. To be included, they had to attend at least the baseline and first follow-up visits.
NHATS “includes domains that are relevant for the characterization of social isolation,” the investigators wrote. It used a typology of structural social isolation that is informed by the Berkman-Syme Social Network Index.
Included domains were living arrangements, discussion networks, and participation. All are “clinically relevant, practical, and components of a comprehensive social history,” the researchers noted.
They added that individuals classified as “socially isolated” often live alone, have no one or only one person that they can rely upon to discuss important matters, and have limited or no engagement in social or religious groups.
Social isolation in the study was characterized using questions about living with at least one other person, talking to two or more other people about “important matters” in the past year, attending religious services in the past month, and participating in the past month in such things as clubs, meetings, group activities, or volunteer work.
Wake-up call
Study participants received 1 point for each item/domain, with a sum score of 0 or 1 classified as “socially isolated” and 2 or more points considered “not socially isolated.” They were classified as having probable dementia based either on self-report or lower-than-mean performance in 2 or more cognitive domains, or a score indicating probable dementia on the AD8 Dementia Screening Interview.
Covariates included demographic factors, education, and health factors. Mean follow-up was 5.1 years.
Results showed close to one-quarter (23.3%) of the study population was classified as socially isolated, with one-fifth (21.1%) developing dementia by the end of the follow-up period.
Compared with non-isolated older adults, those who were socially isolated were more likely to develop dementia during the follow-up period (19.6% vs. 25.9%, respectively).
After adjusting for demographic factors, social isolation was significantly associated with a higher risk for incident dementia (hazard ratio, 1.33; 95% confidence interval, 1.13-1.56). This association persisted after further adjustment for health factors (HR, 1.27; 95% CI, 1.08-1.49). Race and ethnicity had no bearing on the association.
In addition to the association between social isolation and dementia, the researchers also estimated the cause-specific hazard of death before dementia and found that, overall, 18% of participants died prior to dementia over the follow-up period. In particular, the social isolation–associated cause-specific HR of death before dementia was 1.28 (95% CI, 1.2-1.5).
Dr. Cudjoe noted that the mechanism behind the association between social isolation and dementia in this population needs further study. Still, he hopes that the findings will “serve as a wake-up call for all of us to be more thoughtful of the role of social connections on our cognitive health.”
Clinicians “should be thinking about and assessing the presence or absence of social connections in their patients,” Dr. Cudjoe added.
‘Instrumental role’
Commenting on the study, Nicole Purcell, DO, neurologist and senior director of clinical practice at the Alzheimer’s Association, said the study “contributes to the growing body of evidence that finds social isolation is a serious public health risk for many seniors living in the United States, increasing their risk for dementia and other serious mental conditions.”
Dr. Purcell, who was not involved with the study, added that “health care systems and medical professionals can play an instrumental role in identifying individuals at risk for social isolation.”
She noted that for those experiencing social isolation, “interaction with health care providers may be one of the few opportunities those individuals have for social engagement, [so] using these interactions to identify individuals at risk for social isolation and referring them to local resources and groups that promote engagement, well-being, and access to senior services may help decrease dementia risk for vulnerable seniors.”
Dr. Purcell added that the Alzheimer’s Association offers early-stage programs throughout the country, including support groups, education, art, music, and other socially engaging activities.
The study was funded by the National Institute on Aging, National Institute on Minority Health and Health Disparities, and Secunda Family Foundation. The investigators and Dr. Purcell have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests. Results from a longitudinal study that included more than 5,000 United States–based seniors showed that nearly one-quarter were socially isolated.
After adjusting for demographic and health factors, social isolation was found to be associated with a 28% higher risk for developing dementia over a 9-year period, compared with non-isolation. In addition, this finding held true regardless of race or ethnicity.
“Social connections are increasingly understood as a critical factor for the health of individuals as they age,” senior study author Thomas K.M. Cudjoe, MD, Robert and Jane Meyerhoff Endowed Professor and assistant professor of medicine, Division of Geriatric Medicine and Gerontology, Johns Hopkins University School of Medicine, Baltimore, said in a press release. “Our study expands our understanding of the deleterious impact of social isolation on one’s risk for dementia over time,” Dr. Cudjoe added.
The findings were published online in the Journal of the American Geriatric Society.
Upstream resources, downstream outcomes
Social isolation is a “multidimensional construct” characterized by factors such as social connections, social support, resource sharing, and relationship strain. It also affects approximately a quarter of older adults, the investigators noted.
Although prior studies have pointed to an association between socially isolated older adults and increased risk for incident dementia, no study has described this longitudinal association in a nationally representative cohort of U.S. seniors.
Dr. Cudjoe said he was motivated to conduct the current study because he wondered whether or not older adults throughout the United States were similar to some of his patients “who might be at risk for worse cognitive outcomes because they lacked social contact with friends, family, or neighbors.”
The study was also “informed by conceptual foundation that upstream social and personal resources are linked to downstream health outcomes, including cognitive health and function,” the researchers added.
They turned to 2011-2020 data from the National Health and Aging Trends Study, a nationally representative, longitudinal cohort of U.S. Medicare beneficiaries. The sample was drawn from the Medicare enrollment file and incorporated 95 counties and 655 zip codes.
Participants (n = 5,022; mean age, 76.4 years; 57.2% women; 71.7% White, non-Hispanic; 42.4% having more than a college education) were community-dwelling older adults who completed annual 2-hour interviews that included assessment of function, economic health status, and well-being. To be included, they had to attend at least the baseline and first follow-up visits.
NHATS “includes domains that are relevant for the characterization of social isolation,” the investigators wrote. It used a typology of structural social isolation that is informed by the Berkman-Syme Social Network Index.
Included domains were living arrangements, discussion networks, and participation. All are “clinically relevant, practical, and components of a comprehensive social history,” the researchers noted.
They added that individuals classified as “socially isolated” often live alone, have no one or only one person that they can rely upon to discuss important matters, and have limited or no engagement in social or religious groups.
Social isolation in the study was characterized using questions about living with at least one other person, talking to two or more other people about “important matters” in the past year, attending religious services in the past month, and participating in the past month in such things as clubs, meetings, group activities, or volunteer work.
Wake-up call
Study participants received 1 point for each item/domain, with a sum score of 0 or 1 classified as “socially isolated” and 2 or more points considered “not socially isolated.” They were classified as having probable dementia based either on self-report or lower-than-mean performance in 2 or more cognitive domains, or a score indicating probable dementia on the AD8 Dementia Screening Interview.
Covariates included demographic factors, education, and health factors. Mean follow-up was 5.1 years.
Results showed close to one-quarter (23.3%) of the study population was classified as socially isolated, with one-fifth (21.1%) developing dementia by the end of the follow-up period.
Compared with non-isolated older adults, those who were socially isolated were more likely to develop dementia during the follow-up period (19.6% vs. 25.9%, respectively).
After adjusting for demographic factors, social isolation was significantly associated with a higher risk for incident dementia (hazard ratio, 1.33; 95% confidence interval, 1.13-1.56). This association persisted after further adjustment for health factors (HR, 1.27; 95% CI, 1.08-1.49). Race and ethnicity had no bearing on the association.
In addition to the association between social isolation and dementia, the researchers also estimated the cause-specific hazard of death before dementia and found that, overall, 18% of participants died prior to dementia over the follow-up period. In particular, the social isolation–associated cause-specific HR of death before dementia was 1.28 (95% CI, 1.2-1.5).
Dr. Cudjoe noted that the mechanism behind the association between social isolation and dementia in this population needs further study. Still, he hopes that the findings will “serve as a wake-up call for all of us to be more thoughtful of the role of social connections on our cognitive health.”
Clinicians “should be thinking about and assessing the presence or absence of social connections in their patients,” Dr. Cudjoe added.
‘Instrumental role’
Commenting on the study, Nicole Purcell, DO, neurologist and senior director of clinical practice at the Alzheimer’s Association, said the study “contributes to the growing body of evidence that finds social isolation is a serious public health risk for many seniors living in the United States, increasing their risk for dementia and other serious mental conditions.”
Dr. Purcell, who was not involved with the study, added that “health care systems and medical professionals can play an instrumental role in identifying individuals at risk for social isolation.”
She noted that for those experiencing social isolation, “interaction with health care providers may be one of the few opportunities those individuals have for social engagement, [so] using these interactions to identify individuals at risk for social isolation and referring them to local resources and groups that promote engagement, well-being, and access to senior services may help decrease dementia risk for vulnerable seniors.”
Dr. Purcell added that the Alzheimer’s Association offers early-stage programs throughout the country, including support groups, education, art, music, and other socially engaging activities.
The study was funded by the National Institute on Aging, National Institute on Minority Health and Health Disparities, and Secunda Family Foundation. The investigators and Dr. Purcell have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests. Results from a longitudinal study that included more than 5,000 United States–based seniors showed that nearly one-quarter were socially isolated.
After adjusting for demographic and health factors, social isolation was found to be associated with a 28% higher risk for developing dementia over a 9-year period, compared with non-isolation. In addition, this finding held true regardless of race or ethnicity.
“Social connections are increasingly understood as a critical factor for the health of individuals as they age,” senior study author Thomas K.M. Cudjoe, MD, Robert and Jane Meyerhoff Endowed Professor and assistant professor of medicine, Division of Geriatric Medicine and Gerontology, Johns Hopkins University School of Medicine, Baltimore, said in a press release. “Our study expands our understanding of the deleterious impact of social isolation on one’s risk for dementia over time,” Dr. Cudjoe added.
The findings were published online in the Journal of the American Geriatric Society.
Upstream resources, downstream outcomes
Social isolation is a “multidimensional construct” characterized by factors such as social connections, social support, resource sharing, and relationship strain. It also affects approximately a quarter of older adults, the investigators noted.
Although prior studies have pointed to an association between socially isolated older adults and increased risk for incident dementia, no study has described this longitudinal association in a nationally representative cohort of U.S. seniors.
Dr. Cudjoe said he was motivated to conduct the current study because he wondered whether or not older adults throughout the United States were similar to some of his patients “who might be at risk for worse cognitive outcomes because they lacked social contact with friends, family, or neighbors.”
The study was also “informed by conceptual foundation that upstream social and personal resources are linked to downstream health outcomes, including cognitive health and function,” the researchers added.
They turned to 2011-2020 data from the National Health and Aging Trends Study, a nationally representative, longitudinal cohort of U.S. Medicare beneficiaries. The sample was drawn from the Medicare enrollment file and incorporated 95 counties and 655 zip codes.
Participants (n = 5,022; mean age, 76.4 years; 57.2% women; 71.7% White, non-Hispanic; 42.4% having more than a college education) were community-dwelling older adults who completed annual 2-hour interviews that included assessment of function, economic health status, and well-being. To be included, they had to attend at least the baseline and first follow-up visits.
NHATS “includes domains that are relevant for the characterization of social isolation,” the investigators wrote. It used a typology of structural social isolation that is informed by the Berkman-Syme Social Network Index.
Included domains were living arrangements, discussion networks, and participation. All are “clinically relevant, practical, and components of a comprehensive social history,” the researchers noted.
They added that individuals classified as “socially isolated” often live alone, have no one or only one person that they can rely upon to discuss important matters, and have limited or no engagement in social or religious groups.
Social isolation in the study was characterized using questions about living with at least one other person, talking to two or more other people about “important matters” in the past year, attending religious services in the past month, and participating in the past month in such things as clubs, meetings, group activities, or volunteer work.
Wake-up call
Study participants received 1 point for each item/domain, with a sum score of 0 or 1 classified as “socially isolated” and 2 or more points considered “not socially isolated.” They were classified as having probable dementia based either on self-report or lower-than-mean performance in 2 or more cognitive domains, or a score indicating probable dementia on the AD8 Dementia Screening Interview.
Covariates included demographic factors, education, and health factors. Mean follow-up was 5.1 years.
Results showed close to one-quarter (23.3%) of the study population was classified as socially isolated, with one-fifth (21.1%) developing dementia by the end of the follow-up period.
Compared with non-isolated older adults, those who were socially isolated were more likely to develop dementia during the follow-up period (19.6% vs. 25.9%, respectively).
After adjusting for demographic factors, social isolation was significantly associated with a higher risk for incident dementia (hazard ratio, 1.33; 95% confidence interval, 1.13-1.56). This association persisted after further adjustment for health factors (HR, 1.27; 95% CI, 1.08-1.49). Race and ethnicity had no bearing on the association.
In addition to the association between social isolation and dementia, the researchers also estimated the cause-specific hazard of death before dementia and found that, overall, 18% of participants died prior to dementia over the follow-up period. In particular, the social isolation–associated cause-specific HR of death before dementia was 1.28 (95% CI, 1.2-1.5).
Dr. Cudjoe noted that the mechanism behind the association between social isolation and dementia in this population needs further study. Still, he hopes that the findings will “serve as a wake-up call for all of us to be more thoughtful of the role of social connections on our cognitive health.”
Clinicians “should be thinking about and assessing the presence or absence of social connections in their patients,” Dr. Cudjoe added.
‘Instrumental role’
Commenting on the study, Nicole Purcell, DO, neurologist and senior director of clinical practice at the Alzheimer’s Association, said the study “contributes to the growing body of evidence that finds social isolation is a serious public health risk for many seniors living in the United States, increasing their risk for dementia and other serious mental conditions.”
Dr. Purcell, who was not involved with the study, added that “health care systems and medical professionals can play an instrumental role in identifying individuals at risk for social isolation.”
She noted that for those experiencing social isolation, “interaction with health care providers may be one of the few opportunities those individuals have for social engagement, [so] using these interactions to identify individuals at risk for social isolation and referring them to local resources and groups that promote engagement, well-being, and access to senior services may help decrease dementia risk for vulnerable seniors.”
Dr. Purcell added that the Alzheimer’s Association offers early-stage programs throughout the country, including support groups, education, art, music, and other socially engaging activities.
The study was funded by the National Institute on Aging, National Institute on Minority Health and Health Disparities, and Secunda Family Foundation. The investigators and Dr. Purcell have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.

