User login
The Journal of Clinical Outcomes Management® is an independent, peer-reviewed journal offering evidence-based, practical information for improving the quality, safety, and value of health care.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Is it time for yet another COVID booster? It’s complicated
For some people who have received a two-dose primary series and all the recommended boosters, that could mean a sixth shot since COVID-19 vaccines became available. But is even that enough (or too much)?
At this point, no one knows for sure, but new guidance may be on the docket.
On Jan. 26, the FDA’s Vaccines and Related Biological Products Advisory Committee is meeting. On the agenda is discussion about plans for future vaccinations for COVID-19.The committee, made up of external advisers, evaluates data on vaccines and other products for the agency.
According to the FDA announcement, after the meeting, “the FDA will consider whether to recommend adjustments to the current authorizations and approvals, and the FDA will consider the most efficient and transparent process to use for selection of strains for inclusion in the primary and booster vaccines.”
From there, the CDC will take up the issue and decide on recommendations.
The issue is important, as more than 550 Americans a day are still dying from COVID-19, as of the week ending Jan. 13, the CDC reported. That’s up from 346 a day for the week ending Dec. 28.
Yet, uptake of the newest vaccine, the bivalent booster, has been slow. As of Jan. 11, just 15.9% of the population 5 years and up has gotten it; for those most vulnerable to COVID19 – those 65 and up – the number is just 39%.
COVID vaccines, 2023 and beyond
Meanwhile, infectious disease experts have widely differing views on what the vaccination landscape of 2023 and beyond should look like. Among the areas of disagreement are how effective the bivalent vaccine is, which people most need another shot, and what type of vaccine is best.
“I think we probably will need another booster,” says Peter Hotez, MD, PhD, dean of the National School of Tropical Medicine at Baylor College of Medicine, and codirector of the Center for Vaccine Development at Texas Children’s Hospital in Houston. “The question is, what is it going to be? Is it going to be the same bivalent that we just got, or will it be a new bivalent or even a trivalent?”
The trivalent booster, he suggested, might include something more protective against XBB.1.5.
The bivalent booster gives “broadened immunity” that is improved from the original booster shots, says Eric Topol, MD, founder and director of the Scripps Research Translational Institute in La Jolla, Calif., and editor-in-chief of Medscape, WebMD’s sister site for health professionals.
In his publication Ground Truths, Dr. Topol on Jan. 11 explained how new data caused him to reverse his previously skeptical view of how the FDA authorized the bivalent vaccine in September without data on how it affected humans at the time.
Paul Offit, MD, director of the Vaccine Education Center and a professor of pediatrics at the Children’s Hospital of Philadelphia, is a member of the FDA advisory committee for vaccines. He still takes a dimmer view of more bivalent booster vaccines, at least as a blanket recommendation.
While he acknowledges that boosters can help some groups – such as older adults, people with multiple health conditions, and those with compromised immune systems – he opposes a recommendation that’s population-wide.
“People who fall into those three groups do benefit,” he says, “but the recommendation is everyone over 6 months get the bivalent, and what I’m asking is, ‘Where is the data that a healthy 12-year-old boy needs a booster to stay out of the hospital?’ ”
Evolving research
“We are trying to understand how to stay one step ahead rather than several steps behind [the virus],“ says Michael Osterholm, PhD, director of the Center for Infectious Disease Research and Policy at the University of Minnesota.
Among the key questions: How well can a vaccine work against a single subvariant, when no one can say for sure what the next predominant subvariant will be?
Much more research has become available recently about the bivalent vaccine and its effectiveness, Dr. Osterholm says. “The bivalent vaccine is working as well as we could have expected,” he says, especially in high-risk people and in those over age 65. “The challenge we have is, what does that mean going forward?”
In his review, Dr. Topol concludes: “There is now more than ample, highly consistent evidence via lab studies and clinical outcomes to support the bivalent’s benefit over the original booster.”
Among other evidence, he looked at eight studies, including four that used a live virus as part of the research. Six of the eight studies showed the bivalent booster is more effective against the BA.5 variant, compared with the original booster shots. Two others showed no real difference.
“The four live virus studies offer consistent evidence of broadened immunity for the BA.5 vaccine that is improved over the original booster shots,” Dr. Topol wrote. The evidence also found the bivalent antibody response superior against XBB, he wrote.
Dr. Topol also cited CDC data that supports the benefits of the bivalent shot on hospitalization in older adults. During November, hospitalization of adults 65 and above was 2.5 times higher for those vaccinated who did not get the booster, compared to those who got the updated bivalent booster.
Boosters do matter, Dr. Offit says. “But not for all.” In a perspective published Jan. 11 in the New England Journal of Medicine – the same issue that published the two studies finding few differences between the original and bivalent – Dr. Offit wrote that boosting is best reserved for vulnerable groups.
Chasing the variants with a bivalent vaccine, he says, “has not panned out. There remains no evidence that a bivalent vaccine is any better than what we had. Please, show me the data that one is better than the other.”
Dr. Offit believes the goal should not be to prevent all symptomatic infections in healthy, young people by boosting them “with vaccines containing mRNA from strains that might disappear a few months later.”
The CDC needs to parse the data by subgroups, Dr. Offit says. “The critical question is, ‘Who gets hospitalized and who is dying? Who are they?’ ”
That data should take into account age, ethnicity, vaccine history, and other factors, Dr. Offit says, because right now, there is no great data to say, “OK, everyone gets a boost.”
Future vaccine costs
Another debate – for not only current boosters but future ones, too – centers on cost. Without congressional action to fund more vaccines, vaccine makers have suggested their prices may reach $130 a dose, compared with the average $20-per-dose cost the federal government pays now, according to a Kaiser Family Foundation report.
The government has spent more than $30 billion on COVID-19 vaccines, including the bivalent, to provide them free of charge.
The suggested price increase infuriated many. On Jan. 10, Sen. Bernie Sanders (I-Vt.), incoming chair of the Senate Committee on Health, Education, Labor and Pensions, sent a letter to Moderna CEO Stéphane Bancel, urging him to reconsider and refrain from any price increase.
“The huge increase in price that you have proposed will have a significantly negative impact on the budgets of Medicaid, Medicare and other government programs that will continue covering the vaccine without cost-sharing for patients.”
He pointed out, too, the $19 billion in profits Moderna has made over the past 2 years.
While most people with health insurance would likely still get the vaccines and booster for free, according to the Kaiser analysis, will a higher price discourage people from keeping up with recommended vaccinations, including a possible new booster?
“I think so, yes,” Dr. Hotez says, noting that vaccine reluctance is high as it is, even with free vaccinations and easy access.
“The government is balking at paying for the boosters,” he says. “I think it’s very tone deaf from the pharmaceutical companies [to increase the price]. Given all the help they’ve gotten from the American people, I think they should not be gouging at this point.”
He noted that the federal government provided not just money to the companies for the vaccines, but a “glide path” through the FDA for the vaccine approvals.
Are new, variant-specific boosters coming?
Are Moderna, Pfizer-BioNTech, and others developing more variant-specific vaccines, boosters, or other advances?
Novavax, approved in July 2022 as a primary series and in some cases as a booster, is “also developing an Omicron-containing bivalent vaccine at the direction of public health agencies,” says spokesperson Alison Chartan.
Pfizer responded: “When and if we have something to share we will let you know.”
Moderna did not respond.
A version of this article first appeared on WebMD.com.
For some people who have received a two-dose primary series and all the recommended boosters, that could mean a sixth shot since COVID-19 vaccines became available. But is even that enough (or too much)?
At this point, no one knows for sure, but new guidance may be on the docket.
On Jan. 26, the FDA’s Vaccines and Related Biological Products Advisory Committee is meeting. On the agenda is discussion about plans for future vaccinations for COVID-19.The committee, made up of external advisers, evaluates data on vaccines and other products for the agency.
According to the FDA announcement, after the meeting, “the FDA will consider whether to recommend adjustments to the current authorizations and approvals, and the FDA will consider the most efficient and transparent process to use for selection of strains for inclusion in the primary and booster vaccines.”
From there, the CDC will take up the issue and decide on recommendations.
The issue is important, as more than 550 Americans a day are still dying from COVID-19, as of the week ending Jan. 13, the CDC reported. That’s up from 346 a day for the week ending Dec. 28.
Yet, uptake of the newest vaccine, the bivalent booster, has been slow. As of Jan. 11, just 15.9% of the population 5 years and up has gotten it; for those most vulnerable to COVID19 – those 65 and up – the number is just 39%.
COVID vaccines, 2023 and beyond
Meanwhile, infectious disease experts have widely differing views on what the vaccination landscape of 2023 and beyond should look like. Among the areas of disagreement are how effective the bivalent vaccine is, which people most need another shot, and what type of vaccine is best.
“I think we probably will need another booster,” says Peter Hotez, MD, PhD, dean of the National School of Tropical Medicine at Baylor College of Medicine, and codirector of the Center for Vaccine Development at Texas Children’s Hospital in Houston. “The question is, what is it going to be? Is it going to be the same bivalent that we just got, or will it be a new bivalent or even a trivalent?”
The trivalent booster, he suggested, might include something more protective against XBB.1.5.
The bivalent booster gives “broadened immunity” that is improved from the original booster shots, says Eric Topol, MD, founder and director of the Scripps Research Translational Institute in La Jolla, Calif., and editor-in-chief of Medscape, WebMD’s sister site for health professionals.
In his publication Ground Truths, Dr. Topol on Jan. 11 explained how new data caused him to reverse his previously skeptical view of how the FDA authorized the bivalent vaccine in September without data on how it affected humans at the time.
Paul Offit, MD, director of the Vaccine Education Center and a professor of pediatrics at the Children’s Hospital of Philadelphia, is a member of the FDA advisory committee for vaccines. He still takes a dimmer view of more bivalent booster vaccines, at least as a blanket recommendation.
While he acknowledges that boosters can help some groups – such as older adults, people with multiple health conditions, and those with compromised immune systems – he opposes a recommendation that’s population-wide.
“People who fall into those three groups do benefit,” he says, “but the recommendation is everyone over 6 months get the bivalent, and what I’m asking is, ‘Where is the data that a healthy 12-year-old boy needs a booster to stay out of the hospital?’ ”
Evolving research
“We are trying to understand how to stay one step ahead rather than several steps behind [the virus],“ says Michael Osterholm, PhD, director of the Center for Infectious Disease Research and Policy at the University of Minnesota.
Among the key questions: How well can a vaccine work against a single subvariant, when no one can say for sure what the next predominant subvariant will be?
Much more research has become available recently about the bivalent vaccine and its effectiveness, Dr. Osterholm says. “The bivalent vaccine is working as well as we could have expected,” he says, especially in high-risk people and in those over age 65. “The challenge we have is, what does that mean going forward?”
In his review, Dr. Topol concludes: “There is now more than ample, highly consistent evidence via lab studies and clinical outcomes to support the bivalent’s benefit over the original booster.”
Among other evidence, he looked at eight studies, including four that used a live virus as part of the research. Six of the eight studies showed the bivalent booster is more effective against the BA.5 variant, compared with the original booster shots. Two others showed no real difference.
“The four live virus studies offer consistent evidence of broadened immunity for the BA.5 vaccine that is improved over the original booster shots,” Dr. Topol wrote. The evidence also found the bivalent antibody response superior against XBB, he wrote.
Dr. Topol also cited CDC data that supports the benefits of the bivalent shot on hospitalization in older adults. During November, hospitalization of adults 65 and above was 2.5 times higher for those vaccinated who did not get the booster, compared to those who got the updated bivalent booster.
Boosters do matter, Dr. Offit says. “But not for all.” In a perspective published Jan. 11 in the New England Journal of Medicine – the same issue that published the two studies finding few differences between the original and bivalent – Dr. Offit wrote that boosting is best reserved for vulnerable groups.
Chasing the variants with a bivalent vaccine, he says, “has not panned out. There remains no evidence that a bivalent vaccine is any better than what we had. Please, show me the data that one is better than the other.”
Dr. Offit believes the goal should not be to prevent all symptomatic infections in healthy, young people by boosting them “with vaccines containing mRNA from strains that might disappear a few months later.”
The CDC needs to parse the data by subgroups, Dr. Offit says. “The critical question is, ‘Who gets hospitalized and who is dying? Who are they?’ ”
That data should take into account age, ethnicity, vaccine history, and other factors, Dr. Offit says, because right now, there is no great data to say, “OK, everyone gets a boost.”
Future vaccine costs
Another debate – for not only current boosters but future ones, too – centers on cost. Without congressional action to fund more vaccines, vaccine makers have suggested their prices may reach $130 a dose, compared with the average $20-per-dose cost the federal government pays now, according to a Kaiser Family Foundation report.
The government has spent more than $30 billion on COVID-19 vaccines, including the bivalent, to provide them free of charge.
The suggested price increase infuriated many. On Jan. 10, Sen. Bernie Sanders (I-Vt.), incoming chair of the Senate Committee on Health, Education, Labor and Pensions, sent a letter to Moderna CEO Stéphane Bancel, urging him to reconsider and refrain from any price increase.
“The huge increase in price that you have proposed will have a significantly negative impact on the budgets of Medicaid, Medicare and other government programs that will continue covering the vaccine without cost-sharing for patients.”
He pointed out, too, the $19 billion in profits Moderna has made over the past 2 years.
While most people with health insurance would likely still get the vaccines and booster for free, according to the Kaiser analysis, will a higher price discourage people from keeping up with recommended vaccinations, including a possible new booster?
“I think so, yes,” Dr. Hotez says, noting that vaccine reluctance is high as it is, even with free vaccinations and easy access.
“The government is balking at paying for the boosters,” he says. “I think it’s very tone deaf from the pharmaceutical companies [to increase the price]. Given all the help they’ve gotten from the American people, I think they should not be gouging at this point.”
He noted that the federal government provided not just money to the companies for the vaccines, but a “glide path” through the FDA for the vaccine approvals.
Are new, variant-specific boosters coming?
Are Moderna, Pfizer-BioNTech, and others developing more variant-specific vaccines, boosters, or other advances?
Novavax, approved in July 2022 as a primary series and in some cases as a booster, is “also developing an Omicron-containing bivalent vaccine at the direction of public health agencies,” says spokesperson Alison Chartan.
Pfizer responded: “When and if we have something to share we will let you know.”
Moderna did not respond.
A version of this article first appeared on WebMD.com.
For some people who have received a two-dose primary series and all the recommended boosters, that could mean a sixth shot since COVID-19 vaccines became available. But is even that enough (or too much)?
At this point, no one knows for sure, but new guidance may be on the docket.
On Jan. 26, the FDA’s Vaccines and Related Biological Products Advisory Committee is meeting. On the agenda is discussion about plans for future vaccinations for COVID-19.The committee, made up of external advisers, evaluates data on vaccines and other products for the agency.
According to the FDA announcement, after the meeting, “the FDA will consider whether to recommend adjustments to the current authorizations and approvals, and the FDA will consider the most efficient and transparent process to use for selection of strains for inclusion in the primary and booster vaccines.”
From there, the CDC will take up the issue and decide on recommendations.
The issue is important, as more than 550 Americans a day are still dying from COVID-19, as of the week ending Jan. 13, the CDC reported. That’s up from 346 a day for the week ending Dec. 28.
Yet, uptake of the newest vaccine, the bivalent booster, has been slow. As of Jan. 11, just 15.9% of the population 5 years and up has gotten it; for those most vulnerable to COVID19 – those 65 and up – the number is just 39%.
COVID vaccines, 2023 and beyond
Meanwhile, infectious disease experts have widely differing views on what the vaccination landscape of 2023 and beyond should look like. Among the areas of disagreement are how effective the bivalent vaccine is, which people most need another shot, and what type of vaccine is best.
“I think we probably will need another booster,” says Peter Hotez, MD, PhD, dean of the National School of Tropical Medicine at Baylor College of Medicine, and codirector of the Center for Vaccine Development at Texas Children’s Hospital in Houston. “The question is, what is it going to be? Is it going to be the same bivalent that we just got, or will it be a new bivalent or even a trivalent?”
The trivalent booster, he suggested, might include something more protective against XBB.1.5.
The bivalent booster gives “broadened immunity” that is improved from the original booster shots, says Eric Topol, MD, founder and director of the Scripps Research Translational Institute in La Jolla, Calif., and editor-in-chief of Medscape, WebMD’s sister site for health professionals.
In his publication Ground Truths, Dr. Topol on Jan. 11 explained how new data caused him to reverse his previously skeptical view of how the FDA authorized the bivalent vaccine in September without data on how it affected humans at the time.
Paul Offit, MD, director of the Vaccine Education Center and a professor of pediatrics at the Children’s Hospital of Philadelphia, is a member of the FDA advisory committee for vaccines. He still takes a dimmer view of more bivalent booster vaccines, at least as a blanket recommendation.
While he acknowledges that boosters can help some groups – such as older adults, people with multiple health conditions, and those with compromised immune systems – he opposes a recommendation that’s population-wide.
“People who fall into those three groups do benefit,” he says, “but the recommendation is everyone over 6 months get the bivalent, and what I’m asking is, ‘Where is the data that a healthy 12-year-old boy needs a booster to stay out of the hospital?’ ”
Evolving research
“We are trying to understand how to stay one step ahead rather than several steps behind [the virus],“ says Michael Osterholm, PhD, director of the Center for Infectious Disease Research and Policy at the University of Minnesota.
Among the key questions: How well can a vaccine work against a single subvariant, when no one can say for sure what the next predominant subvariant will be?
Much more research has become available recently about the bivalent vaccine and its effectiveness, Dr. Osterholm says. “The bivalent vaccine is working as well as we could have expected,” he says, especially in high-risk people and in those over age 65. “The challenge we have is, what does that mean going forward?”
In his review, Dr. Topol concludes: “There is now more than ample, highly consistent evidence via lab studies and clinical outcomes to support the bivalent’s benefit over the original booster.”
Among other evidence, he looked at eight studies, including four that used a live virus as part of the research. Six of the eight studies showed the bivalent booster is more effective against the BA.5 variant, compared with the original booster shots. Two others showed no real difference.
“The four live virus studies offer consistent evidence of broadened immunity for the BA.5 vaccine that is improved over the original booster shots,” Dr. Topol wrote. The evidence also found the bivalent antibody response superior against XBB, he wrote.
Dr. Topol also cited CDC data that supports the benefits of the bivalent shot on hospitalization in older adults. During November, hospitalization of adults 65 and above was 2.5 times higher for those vaccinated who did not get the booster, compared to those who got the updated bivalent booster.
Boosters do matter, Dr. Offit says. “But not for all.” In a perspective published Jan. 11 in the New England Journal of Medicine – the same issue that published the two studies finding few differences between the original and bivalent – Dr. Offit wrote that boosting is best reserved for vulnerable groups.
Chasing the variants with a bivalent vaccine, he says, “has not panned out. There remains no evidence that a bivalent vaccine is any better than what we had. Please, show me the data that one is better than the other.”
Dr. Offit believes the goal should not be to prevent all symptomatic infections in healthy, young people by boosting them “with vaccines containing mRNA from strains that might disappear a few months later.”
The CDC needs to parse the data by subgroups, Dr. Offit says. “The critical question is, ‘Who gets hospitalized and who is dying? Who are they?’ ”
That data should take into account age, ethnicity, vaccine history, and other factors, Dr. Offit says, because right now, there is no great data to say, “OK, everyone gets a boost.”
Future vaccine costs
Another debate – for not only current boosters but future ones, too – centers on cost. Without congressional action to fund more vaccines, vaccine makers have suggested their prices may reach $130 a dose, compared with the average $20-per-dose cost the federal government pays now, according to a Kaiser Family Foundation report.
The government has spent more than $30 billion on COVID-19 vaccines, including the bivalent, to provide them free of charge.
The suggested price increase infuriated many. On Jan. 10, Sen. Bernie Sanders (I-Vt.), incoming chair of the Senate Committee on Health, Education, Labor and Pensions, sent a letter to Moderna CEO Stéphane Bancel, urging him to reconsider and refrain from any price increase.
“The huge increase in price that you have proposed will have a significantly negative impact on the budgets of Medicaid, Medicare and other government programs that will continue covering the vaccine without cost-sharing for patients.”
He pointed out, too, the $19 billion in profits Moderna has made over the past 2 years.
While most people with health insurance would likely still get the vaccines and booster for free, according to the Kaiser analysis, will a higher price discourage people from keeping up with recommended vaccinations, including a possible new booster?
“I think so, yes,” Dr. Hotez says, noting that vaccine reluctance is high as it is, even with free vaccinations and easy access.
“The government is balking at paying for the boosters,” he says. “I think it’s very tone deaf from the pharmaceutical companies [to increase the price]. Given all the help they’ve gotten from the American people, I think they should not be gouging at this point.”
He noted that the federal government provided not just money to the companies for the vaccines, but a “glide path” through the FDA for the vaccine approvals.
Are new, variant-specific boosters coming?
Are Moderna, Pfizer-BioNTech, and others developing more variant-specific vaccines, boosters, or other advances?
Novavax, approved in July 2022 as a primary series and in some cases as a booster, is “also developing an Omicron-containing bivalent vaccine at the direction of public health agencies,” says spokesperson Alison Chartan.
Pfizer responded: “When and if we have something to share we will let you know.”
Moderna did not respond.
A version of this article first appeared on WebMD.com.
FAST score appears accurate for diagnosis of fibrotic NASH
The FAST score had an overall sensitivity of 89% and an overall specificity of 89% with a defined rule-out cutoff of .35 or lower and rule-in cutoff of .67 or higher, respectively, Federico Ravaioli, MD, PhD, a gastroenterologist at the University of Modena & Reggio Emilia in Italy, and colleagues, wrote in Gut.
“These results could be used in clinical screening studies to efficiently identify patients at risk of progressive NASH, who should be referred for a conclusive liver biopsy, and who might benefit from treatment with emerging pharmacotherapies,” the authors wrote.
The research team analyzed 12 observational studies with 5,835 participants with biopsy-confirmed nonalcoholic fatty liver disease (NAFLD) between February 2020 and April 2022. They included articles that reported data for the calculation of sensitivity and specificity of the FAST score for identifying adult patients with fibrotic NASH based on a defined rule-out cutoff of .35 or lower and rule-in cutoff of .67 or higher. Fibrotic NASH was defined as patients with NASH plus a NAFLD activity score of 4 or greater and fibrosis stage 2 or higher.
The pooled prevalence of fibrotic NASH was 28%. The mean age of participants ranged from 40 to 60, and the proportion of men ranged from 23% to 91%. The mean body mass index ranged from 23 kg/m2 to 41 kg/m2, with a prevalence of obesity ranging from 23% to 100% and preexisting type 2 diabetes ranging from 18% to 60%. Nine studies included patients with biopsy-proven NAFLD from tertiary care liver centers, and three studies included patients from bariatric clinics or bariatric surgery centers with available liver biopsy data.
Fibrotic NASH was ruled out in 2,723 patients (45.5%) by a FAST score of .35 or lower and ruled in 1,287 patients (21.5%) by a FAST score of .67 or higher. In addition, 1,979 patients (33%) had a FAST score in the so-called “grey” intermediate zone.
Overall, the FAST score pooled sensitivity was 89%, and the pooled specificity was 89%. By the rule-out cutoff of .35, the sensitivity was 89% and the specificity was 56%. By the rule-in cutoff of .67, the sensitivity was 46% and the specificity was 89%.
At an expected prevalence of fibrotic NASH of 30%, the negative predictive value of the .35 cutoff was 92%, and the positive predictive value of the .67 cutoff was 65%. Across the included studies, the negative predictive value ranged from 77% to 98%, and the positive predictive value ranged from 32% to 87%.
For the rule-in cutoff of .67, at a pretest probability of 10%, 20%, 26.3%, and 30%, there was an increasing likelihood of detecting fibrotic NASH by FAST score at 32%, 52%, 60%, and 65%, respectively. For the rule-out cutoff of .35, at the same pretest probability levels, the likelihood of someone not having fibrotic NASH and not being detected by FAST score was 2%, 5%, 7%, and 8%, respectively.
In subgroup analyses, the sensitivity of the rule-out cutoff was significantly affected by the study design. In addition, age and BMI above the median both affected pooled sensitivity but not pooled specificity. On the other hand, the rule-in cutoff was significantly affected by study design, BMI above the median, and presence of preexisting type 2 diabetes above the median.
“Today, we stand on the cusp of a revolutionary time to treat NASH. This is due in part to the fact that many exciting, novel precision metabolic treatments are in the pipeline to combat this disease,” said Brian DeBosch, MD, PhD, associate professor of cell biology and physiology at the Washington University in St. Louis, who was not involved with this study.
“A major barrier in clinical NASH management is a rapid, noninvasive, and precise means by which to clinically stage such patients,” Dr. DeBosch said. “We now approach as closely as ever the sensitivity and specificity required to stratify the highest-risk patients, identify candidates for advanced therapy, and meaningfully reduce biopsies through using noninvasive testing.”
Dr. DeBosch noted the importance of pretest probability and specific subpopulations when deciding whether to use the FAST score. For instance, he said, a tertiary academic liver transplant center will see a different patient population than in a primary care setting. Also, in this study, the presence or absence of diabetes and a BMI above 30 significantly altered sensitivity and specificity.
“One important remaining question stemming from these data is whether FAST can also be used as a surrogate measure to follow disease regression over time following intervention,” Dr. DeBosch said. “Even if FAST is not useful in that way, defining individuals who most need to undergo biopsy and/or those who need to undergo treatment remain important uses for this test.”
The study authors did not declare a specific funding source or report any competing interests. DeBosch reported no relevant disclosures.
The FAST score had an overall sensitivity of 89% and an overall specificity of 89% with a defined rule-out cutoff of .35 or lower and rule-in cutoff of .67 or higher, respectively, Federico Ravaioli, MD, PhD, a gastroenterologist at the University of Modena & Reggio Emilia in Italy, and colleagues, wrote in Gut.
“These results could be used in clinical screening studies to efficiently identify patients at risk of progressive NASH, who should be referred for a conclusive liver biopsy, and who might benefit from treatment with emerging pharmacotherapies,” the authors wrote.
The research team analyzed 12 observational studies with 5,835 participants with biopsy-confirmed nonalcoholic fatty liver disease (NAFLD) between February 2020 and April 2022. They included articles that reported data for the calculation of sensitivity and specificity of the FAST score for identifying adult patients with fibrotic NASH based on a defined rule-out cutoff of .35 or lower and rule-in cutoff of .67 or higher. Fibrotic NASH was defined as patients with NASH plus a NAFLD activity score of 4 or greater and fibrosis stage 2 or higher.
The pooled prevalence of fibrotic NASH was 28%. The mean age of participants ranged from 40 to 60, and the proportion of men ranged from 23% to 91%. The mean body mass index ranged from 23 kg/m2 to 41 kg/m2, with a prevalence of obesity ranging from 23% to 100% and preexisting type 2 diabetes ranging from 18% to 60%. Nine studies included patients with biopsy-proven NAFLD from tertiary care liver centers, and three studies included patients from bariatric clinics or bariatric surgery centers with available liver biopsy data.
Fibrotic NASH was ruled out in 2,723 patients (45.5%) by a FAST score of .35 or lower and ruled in 1,287 patients (21.5%) by a FAST score of .67 or higher. In addition, 1,979 patients (33%) had a FAST score in the so-called “grey” intermediate zone.
Overall, the FAST score pooled sensitivity was 89%, and the pooled specificity was 89%. By the rule-out cutoff of .35, the sensitivity was 89% and the specificity was 56%. By the rule-in cutoff of .67, the sensitivity was 46% and the specificity was 89%.
At an expected prevalence of fibrotic NASH of 30%, the negative predictive value of the .35 cutoff was 92%, and the positive predictive value of the .67 cutoff was 65%. Across the included studies, the negative predictive value ranged from 77% to 98%, and the positive predictive value ranged from 32% to 87%.
For the rule-in cutoff of .67, at a pretest probability of 10%, 20%, 26.3%, and 30%, there was an increasing likelihood of detecting fibrotic NASH by FAST score at 32%, 52%, 60%, and 65%, respectively. For the rule-out cutoff of .35, at the same pretest probability levels, the likelihood of someone not having fibrotic NASH and not being detected by FAST score was 2%, 5%, 7%, and 8%, respectively.
In subgroup analyses, the sensitivity of the rule-out cutoff was significantly affected by the study design. In addition, age and BMI above the median both affected pooled sensitivity but not pooled specificity. On the other hand, the rule-in cutoff was significantly affected by study design, BMI above the median, and presence of preexisting type 2 diabetes above the median.
“Today, we stand on the cusp of a revolutionary time to treat NASH. This is due in part to the fact that many exciting, novel precision metabolic treatments are in the pipeline to combat this disease,” said Brian DeBosch, MD, PhD, associate professor of cell biology and physiology at the Washington University in St. Louis, who was not involved with this study.
“A major barrier in clinical NASH management is a rapid, noninvasive, and precise means by which to clinically stage such patients,” Dr. DeBosch said. “We now approach as closely as ever the sensitivity and specificity required to stratify the highest-risk patients, identify candidates for advanced therapy, and meaningfully reduce biopsies through using noninvasive testing.”
Dr. DeBosch noted the importance of pretest probability and specific subpopulations when deciding whether to use the FAST score. For instance, he said, a tertiary academic liver transplant center will see a different patient population than in a primary care setting. Also, in this study, the presence or absence of diabetes and a BMI above 30 significantly altered sensitivity and specificity.
“One important remaining question stemming from these data is whether FAST can also be used as a surrogate measure to follow disease regression over time following intervention,” Dr. DeBosch said. “Even if FAST is not useful in that way, defining individuals who most need to undergo biopsy and/or those who need to undergo treatment remain important uses for this test.”
The study authors did not declare a specific funding source or report any competing interests. DeBosch reported no relevant disclosures.
The FAST score had an overall sensitivity of 89% and an overall specificity of 89% with a defined rule-out cutoff of .35 or lower and rule-in cutoff of .67 or higher, respectively, Federico Ravaioli, MD, PhD, a gastroenterologist at the University of Modena & Reggio Emilia in Italy, and colleagues, wrote in Gut.
“These results could be used in clinical screening studies to efficiently identify patients at risk of progressive NASH, who should be referred for a conclusive liver biopsy, and who might benefit from treatment with emerging pharmacotherapies,” the authors wrote.
The research team analyzed 12 observational studies with 5,835 participants with biopsy-confirmed nonalcoholic fatty liver disease (NAFLD) between February 2020 and April 2022. They included articles that reported data for the calculation of sensitivity and specificity of the FAST score for identifying adult patients with fibrotic NASH based on a defined rule-out cutoff of .35 or lower and rule-in cutoff of .67 or higher. Fibrotic NASH was defined as patients with NASH plus a NAFLD activity score of 4 or greater and fibrosis stage 2 or higher.
The pooled prevalence of fibrotic NASH was 28%. The mean age of participants ranged from 40 to 60, and the proportion of men ranged from 23% to 91%. The mean body mass index ranged from 23 kg/m2 to 41 kg/m2, with a prevalence of obesity ranging from 23% to 100% and preexisting type 2 diabetes ranging from 18% to 60%. Nine studies included patients with biopsy-proven NAFLD from tertiary care liver centers, and three studies included patients from bariatric clinics or bariatric surgery centers with available liver biopsy data.
Fibrotic NASH was ruled out in 2,723 patients (45.5%) by a FAST score of .35 or lower and ruled in 1,287 patients (21.5%) by a FAST score of .67 or higher. In addition, 1,979 patients (33%) had a FAST score in the so-called “grey” intermediate zone.
Overall, the FAST score pooled sensitivity was 89%, and the pooled specificity was 89%. By the rule-out cutoff of .35, the sensitivity was 89% and the specificity was 56%. By the rule-in cutoff of .67, the sensitivity was 46% and the specificity was 89%.
At an expected prevalence of fibrotic NASH of 30%, the negative predictive value of the .35 cutoff was 92%, and the positive predictive value of the .67 cutoff was 65%. Across the included studies, the negative predictive value ranged from 77% to 98%, and the positive predictive value ranged from 32% to 87%.
For the rule-in cutoff of .67, at a pretest probability of 10%, 20%, 26.3%, and 30%, there was an increasing likelihood of detecting fibrotic NASH by FAST score at 32%, 52%, 60%, and 65%, respectively. For the rule-out cutoff of .35, at the same pretest probability levels, the likelihood of someone not having fibrotic NASH and not being detected by FAST score was 2%, 5%, 7%, and 8%, respectively.
In subgroup analyses, the sensitivity of the rule-out cutoff was significantly affected by the study design. In addition, age and BMI above the median both affected pooled sensitivity but not pooled specificity. On the other hand, the rule-in cutoff was significantly affected by study design, BMI above the median, and presence of preexisting type 2 diabetes above the median.
“Today, we stand on the cusp of a revolutionary time to treat NASH. This is due in part to the fact that many exciting, novel precision metabolic treatments are in the pipeline to combat this disease,” said Brian DeBosch, MD, PhD, associate professor of cell biology and physiology at the Washington University in St. Louis, who was not involved with this study.
“A major barrier in clinical NASH management is a rapid, noninvasive, and precise means by which to clinically stage such patients,” Dr. DeBosch said. “We now approach as closely as ever the sensitivity and specificity required to stratify the highest-risk patients, identify candidates for advanced therapy, and meaningfully reduce biopsies through using noninvasive testing.”
Dr. DeBosch noted the importance of pretest probability and specific subpopulations when deciding whether to use the FAST score. For instance, he said, a tertiary academic liver transplant center will see a different patient population than in a primary care setting. Also, in this study, the presence or absence of diabetes and a BMI above 30 significantly altered sensitivity and specificity.
“One important remaining question stemming from these data is whether FAST can also be used as a surrogate measure to follow disease regression over time following intervention,” Dr. DeBosch said. “Even if FAST is not useful in that way, defining individuals who most need to undergo biopsy and/or those who need to undergo treatment remain important uses for this test.”
The study authors did not declare a specific funding source or report any competing interests. DeBosch reported no relevant disclosures.
FROM GUT
Post-birth hospitalizations dropped with Medicaid expansion
Women living in states that expanded Medicaid over the past decade were nearly 20% less likely to be hospitalized within 2 months of giving birth, according to a first-of-its-kind study published in Health Affairs.
Researchers analyzed patient records from eight states – four that expanded Medicaid insurance to include a broader swath of residents following the implementation of the Affordable Care Act, and four states that did not.
Hospitalizations in the 60 days after a woman gave birth fell by 17% in states that expanded Medicaid. The analysis also revealed an 8% drop in hospitalizations between 61 days and 6 months post partum.
“This is a very meaningful decline in hospitalization rates,” said Laura Wherry, PhD, a professor of economics and public service at New York University and a co-author of the study.
Women in states that chose not to expand Medicaid experienced a 7% increase in postpartum hospitalizations during that same time frame, the researchers report.
Many states raised income eligibility thresholds to 138% of the federal poverty level in 2014 with the implementation of the Affordable Care Act, which resulted in more coverage for low-income expectant mothers. To date, a dozen states have not implemented Medicaid expansion.
Dr. Wherry and her colleague wanted to take a closer look at outcomes for pregnant women during the postpartum period, both before and after states chose to expand Medicaid.
“A lot of prior work looking at the Medicaid program examined huge expansions to cover pregnant women during pregnancy, but often other periods of a woman’s life have been overlooked,” Dr. Wherry said. “What we were interested in is how that changed with the Affordable Care Act. You no longer needed to be pregnant to qualify.”
The researchers analyzed hospital discharge data between 2010 and 2017 before and after expansion in Iowa, Maryland, New Mexico, and Washington, which expanded coverage under Medicaid, and Florida, Georgia, Mississippi, and Utah, which did not do so.
Prior to 2014, fewer than 2% of births resulted in a postpartum hospitalization during the 60-day period in Medicaid expansion states. But in states that expanded Medicaid, hospitalizations decreased by 0.289 percentage points (P = .052), or 17% during the 60-day post-birth period.
Approximately 75% of the decline was attributed to diagnoses related to complications in pregnancy, childbirth, and the postpartum period.
Dr. Wherry said a variety of factors possibly drove down hospitalizations for new mothers who were able to obtain Medicaid coverage, including access to robust prenatal care, preconception counseling, and improved management of postpartum conditions outside the hospital.
The study provides a strategy for tackling the rising rate of maternal mortality in the United States, an increase largely attributed to postpartum deaths, said Lindsay Admon, MD, an ob.gyn. at the University of Michigan Medical School in Ann Arbor.
“This is one of the first studies showing or suggesting that Medicaid expansion not only led to improvements in Medicaid insurance but health outcomes as well,” said Dr. Admon, who is also researching maternal health and expanded Medicaid coverage.
Federal law has long required states to provide coverage for pregnant women up to 60 days post partum.
The 2021 American Rescue Act allowed states to extend coverage for pregnant women beyond the federal requirement to a year. More than half of states have chosen to do so. Since the study indicates that Medicaid expansion improves outcomes for these enrollees, Dr. Wherry and Dr. Admon said they hope state officials will consider the new findings during discussions to utilize the Rescue Act Coverage for pregnant women.
Dr. Wherry received support for the study from the Robert Wood Johnson Foundation Policies for Action Program and grant funding from the National Institute on Aging and the National Institute of Child Health and Human Development. Another author received grants from the Agency for Healthcare Research and Quality and the National Institute of Child Health and Human Development.
A version of this article first appeared on Medscape.com.
Women living in states that expanded Medicaid over the past decade were nearly 20% less likely to be hospitalized within 2 months of giving birth, according to a first-of-its-kind study published in Health Affairs.
Researchers analyzed patient records from eight states – four that expanded Medicaid insurance to include a broader swath of residents following the implementation of the Affordable Care Act, and four states that did not.
Hospitalizations in the 60 days after a woman gave birth fell by 17% in states that expanded Medicaid. The analysis also revealed an 8% drop in hospitalizations between 61 days and 6 months post partum.
“This is a very meaningful decline in hospitalization rates,” said Laura Wherry, PhD, a professor of economics and public service at New York University and a co-author of the study.
Women in states that chose not to expand Medicaid experienced a 7% increase in postpartum hospitalizations during that same time frame, the researchers report.
Many states raised income eligibility thresholds to 138% of the federal poverty level in 2014 with the implementation of the Affordable Care Act, which resulted in more coverage for low-income expectant mothers. To date, a dozen states have not implemented Medicaid expansion.
Dr. Wherry and her colleague wanted to take a closer look at outcomes for pregnant women during the postpartum period, both before and after states chose to expand Medicaid.
“A lot of prior work looking at the Medicaid program examined huge expansions to cover pregnant women during pregnancy, but often other periods of a woman’s life have been overlooked,” Dr. Wherry said. “What we were interested in is how that changed with the Affordable Care Act. You no longer needed to be pregnant to qualify.”
The researchers analyzed hospital discharge data between 2010 and 2017 before and after expansion in Iowa, Maryland, New Mexico, and Washington, which expanded coverage under Medicaid, and Florida, Georgia, Mississippi, and Utah, which did not do so.
Prior to 2014, fewer than 2% of births resulted in a postpartum hospitalization during the 60-day period in Medicaid expansion states. But in states that expanded Medicaid, hospitalizations decreased by 0.289 percentage points (P = .052), or 17% during the 60-day post-birth period.
Approximately 75% of the decline was attributed to diagnoses related to complications in pregnancy, childbirth, and the postpartum period.
Dr. Wherry said a variety of factors possibly drove down hospitalizations for new mothers who were able to obtain Medicaid coverage, including access to robust prenatal care, preconception counseling, and improved management of postpartum conditions outside the hospital.
The study provides a strategy for tackling the rising rate of maternal mortality in the United States, an increase largely attributed to postpartum deaths, said Lindsay Admon, MD, an ob.gyn. at the University of Michigan Medical School in Ann Arbor.
“This is one of the first studies showing or suggesting that Medicaid expansion not only led to improvements in Medicaid insurance but health outcomes as well,” said Dr. Admon, who is also researching maternal health and expanded Medicaid coverage.
Federal law has long required states to provide coverage for pregnant women up to 60 days post partum.
The 2021 American Rescue Act allowed states to extend coverage for pregnant women beyond the federal requirement to a year. More than half of states have chosen to do so. Since the study indicates that Medicaid expansion improves outcomes for these enrollees, Dr. Wherry and Dr. Admon said they hope state officials will consider the new findings during discussions to utilize the Rescue Act Coverage for pregnant women.
Dr. Wherry received support for the study from the Robert Wood Johnson Foundation Policies for Action Program and grant funding from the National Institute on Aging and the National Institute of Child Health and Human Development. Another author received grants from the Agency for Healthcare Research and Quality and the National Institute of Child Health and Human Development.
A version of this article first appeared on Medscape.com.
Women living in states that expanded Medicaid over the past decade were nearly 20% less likely to be hospitalized within 2 months of giving birth, according to a first-of-its-kind study published in Health Affairs.
Researchers analyzed patient records from eight states – four that expanded Medicaid insurance to include a broader swath of residents following the implementation of the Affordable Care Act, and four states that did not.
Hospitalizations in the 60 days after a woman gave birth fell by 17% in states that expanded Medicaid. The analysis also revealed an 8% drop in hospitalizations between 61 days and 6 months post partum.
“This is a very meaningful decline in hospitalization rates,” said Laura Wherry, PhD, a professor of economics and public service at New York University and a co-author of the study.
Women in states that chose not to expand Medicaid experienced a 7% increase in postpartum hospitalizations during that same time frame, the researchers report.
Many states raised income eligibility thresholds to 138% of the federal poverty level in 2014 with the implementation of the Affordable Care Act, which resulted in more coverage for low-income expectant mothers. To date, a dozen states have not implemented Medicaid expansion.
Dr. Wherry and her colleague wanted to take a closer look at outcomes for pregnant women during the postpartum period, both before and after states chose to expand Medicaid.
“A lot of prior work looking at the Medicaid program examined huge expansions to cover pregnant women during pregnancy, but often other periods of a woman’s life have been overlooked,” Dr. Wherry said. “What we were interested in is how that changed with the Affordable Care Act. You no longer needed to be pregnant to qualify.”
The researchers analyzed hospital discharge data between 2010 and 2017 before and after expansion in Iowa, Maryland, New Mexico, and Washington, which expanded coverage under Medicaid, and Florida, Georgia, Mississippi, and Utah, which did not do so.
Prior to 2014, fewer than 2% of births resulted in a postpartum hospitalization during the 60-day period in Medicaid expansion states. But in states that expanded Medicaid, hospitalizations decreased by 0.289 percentage points (P = .052), or 17% during the 60-day post-birth period.
Approximately 75% of the decline was attributed to diagnoses related to complications in pregnancy, childbirth, and the postpartum period.
Dr. Wherry said a variety of factors possibly drove down hospitalizations for new mothers who were able to obtain Medicaid coverage, including access to robust prenatal care, preconception counseling, and improved management of postpartum conditions outside the hospital.
The study provides a strategy for tackling the rising rate of maternal mortality in the United States, an increase largely attributed to postpartum deaths, said Lindsay Admon, MD, an ob.gyn. at the University of Michigan Medical School in Ann Arbor.
“This is one of the first studies showing or suggesting that Medicaid expansion not only led to improvements in Medicaid insurance but health outcomes as well,” said Dr. Admon, who is also researching maternal health and expanded Medicaid coverage.
Federal law has long required states to provide coverage for pregnant women up to 60 days post partum.
The 2021 American Rescue Act allowed states to extend coverage for pregnant women beyond the federal requirement to a year. More than half of states have chosen to do so. Since the study indicates that Medicaid expansion improves outcomes for these enrollees, Dr. Wherry and Dr. Admon said they hope state officials will consider the new findings during discussions to utilize the Rescue Act Coverage for pregnant women.
Dr. Wherry received support for the study from the Robert Wood Johnson Foundation Policies for Action Program and grant funding from the National Institute on Aging and the National Institute of Child Health and Human Development. Another author received grants from the Agency for Healthcare Research and Quality and the National Institute of Child Health and Human Development.
A version of this article first appeared on Medscape.com.
Adverse events reported in one-quarter of inpatient admissions
as indicated from data from 2,809 admissions at 11 hospitals.
The 1991 Harvard Medical Practice Study, which focused on medical injury and litigation, documented an adverse event rate of 3.7 events per 100 admissions; 28% of those events were attributed to negligence, write David W. Bates, MD, of Brigham and Women’s Hospital, Boston, and colleagues.
Although patient safety has changed significantly since 1991, documenting improvements has been challenging, the researchers say. Several reports have shown a decrease in health care–associated infections. However, other aspects of safety – notably, adverse drug events, defined as injuries resulting from drugs taken – are not easily measured and tracked, the researchers say.
“We have not had good estimates of how much harm is being caused by care in hospitals in an ongoing way that looked across all types of adverse events,” and the current review is therefore important, Dr. Bates said in an interview.
In a study recently published in the New England Journal of Medicine, the researchers analyzed a random sample of 2,809 hospital admissions from 11 hospitals in Massachusetts during the 2018 calendar year. The hospitals ranged in size from fewer than 100 beds to more than 700 beds; all patients were aged 18 years and older. A panel of nine nurses reviewed the admissions records to identify potential adverse events, and eight physicians reviewed the adverse event summaries and either agreed or disagreed with the adverse event type. The severity of each event was ranked using a general severity scale into categories of significant, serious, life-threatening, or fatal.
Overall, at least one adverse event was identified in 23.6% of the hospital admissions. A total of 978 adverse events were deemed to have occurred during the index admission, and 222 of these (22.7%) were deemed preventable. Among the preventable adverse events, 19.7% were classified as serious, 3.3% as life-threatening, and 0.5% as fatal.
A total of 523 admissions (18.6%) involved at least one significant adverse event, defined as an event that caused unnecessary harm but from which recovery was rapid. A total of 211 admissions involved a serious adverse event, defined as harm resulting in substantial intervention or prolonged recovery; 34 included at least one life-threatening event; and seven admissions involved a fatal adverse event.
A total of 191 admissions involved at least one adverse event deemed preventable. Of those, 29 involved at least one preventable adverse event that was serious, life-threatening, or fatal, the researchers write. Of the seven deaths in the study population, one was deemed preventable.
The most common adverse events were adverse drug events, which accounted for 39.0% of the adverse events; surgical or other procedural events accounted for 30.4%; patient care events (including falls and pressure ulcers) accounted for 15.0%; and health care–associated infections accounted for 11.9%.
Overcoming barriers to better safety
“The overall level of harm, with nearly 1 in 4 patients suffering an adverse event, was higher than I expected it might be,” Dr. Bates told this news organization. However, techniques for identifying adverse events have improved, and “it is easier to find them in electronic records than in paper records,” he noted.
“Hospitals have many issues they are currently dealing with since COVID, and one issue is simply prioritization,” Dr. Bates said. “But it is now possible to measure harm for all patients using electronic tools, and if hospitals know how much harm they are having in specific areas, they can make choices about which ones to focus on.”
“We now have effective prevention strategies for most of the main kinds of harm,” he said. Generally, rates of harm are high because these strategies are not being used effectively, he said. “In addition, there are new tools that can be used – for example, to identify patients who are decompensating earlier,” he noted.
As for additional research, some specific types of harm that have been resistant to interventions, such as pressure ulcers, deserve more attention, said Dr. Bates. “In addition, diagnostic errors appear to cause a great deal of harm, but we don’t yet have good strategies for preventing these,” he said.
The study findings were limited by several factors, including the use of data from hospitals that might not represent hospitals at large and by the inclusion mainly of patients with private insurance, the researchers write. Other limitations include the likelihood that some adverse events were missed and the level of agreement on adverse events between adjudicators was only fair.
However, the findings serve as a reminder to health care professionals of the need for continued attention to improving patient safety, and measuring adverse events remains a critical part of guiding these improvements, the researchers conclude.
Timely reassessment and opportunities to improve
In the decades since the publication of the report, “To Err Is Human,” by the National Academies in 2000, significant attention has been paid to improving patient safety during hospitalizations, and health care systems have increased in both system and disease complexity, Said Suman Pal, MBBS, a specialist in hospital medicine at the University of New Mexico, Albuquerque, said in an interview. “Therefore, this study is important in reassessing the safety of inpatient care at the current time,” he said.
“The findings of this study showing preventable adverse events in approximately 7% of all admissions; while concerning, is not surprising, as it is consistent with other studies over time, as the authors have also noted in their discussion,” said Dr. Pal. The current findings “underscore the importance of continuous quality improvement efforts to increase the safety of patient care for hospitalized patients,” he noted.
“The increasing complexity of medical care, fragmentation of health care, structural inequities of health systems, and more recent widespread public health challenges such as the COVID-19 pandemic have been, in my opinion, barriers to improving patient safety,” Dr. Pal said. “The use of innovation and an interdisciplinary approach to patient safety and quality improvement in hospital-based care, such as the use of machine learning to monitor trends and predict the individualized risk of harm, could be a potential way out” to help reduce barriers and improve safety, he said.
“Additional research is needed to understand the key drivers of preventable harm for hospitalized patients in the United States,” said Dr. Pal. “When planning for change, keen attention must be paid to understanding how these [drivers] may differ for patients who have been historically marginalized or are otherwise underserved so as to not exacerbate health care inequities,” he added.
The study was funded by the Controlled Risk Insurance Company and the Risk Management Foundation of the Harvard Medical Institutions. Dr. Bates owns stock options with AESOP, Clew, FeelBetter, Guided Clinical Solutions, MDClone, and ValeraHealth and has grants/contracts from IBM Watson and EarlySense. He has also served as a consultant for CDI Negev. Dr. Pal has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
as indicated from data from 2,809 admissions at 11 hospitals.
The 1991 Harvard Medical Practice Study, which focused on medical injury and litigation, documented an adverse event rate of 3.7 events per 100 admissions; 28% of those events were attributed to negligence, write David W. Bates, MD, of Brigham and Women’s Hospital, Boston, and colleagues.
Although patient safety has changed significantly since 1991, documenting improvements has been challenging, the researchers say. Several reports have shown a decrease in health care–associated infections. However, other aspects of safety – notably, adverse drug events, defined as injuries resulting from drugs taken – are not easily measured and tracked, the researchers say.
“We have not had good estimates of how much harm is being caused by care in hospitals in an ongoing way that looked across all types of adverse events,” and the current review is therefore important, Dr. Bates said in an interview.
In a study recently published in the New England Journal of Medicine, the researchers analyzed a random sample of 2,809 hospital admissions from 11 hospitals in Massachusetts during the 2018 calendar year. The hospitals ranged in size from fewer than 100 beds to more than 700 beds; all patients were aged 18 years and older. A panel of nine nurses reviewed the admissions records to identify potential adverse events, and eight physicians reviewed the adverse event summaries and either agreed or disagreed with the adverse event type. The severity of each event was ranked using a general severity scale into categories of significant, serious, life-threatening, or fatal.
Overall, at least one adverse event was identified in 23.6% of the hospital admissions. A total of 978 adverse events were deemed to have occurred during the index admission, and 222 of these (22.7%) were deemed preventable. Among the preventable adverse events, 19.7% were classified as serious, 3.3% as life-threatening, and 0.5% as fatal.
A total of 523 admissions (18.6%) involved at least one significant adverse event, defined as an event that caused unnecessary harm but from which recovery was rapid. A total of 211 admissions involved a serious adverse event, defined as harm resulting in substantial intervention or prolonged recovery; 34 included at least one life-threatening event; and seven admissions involved a fatal adverse event.
A total of 191 admissions involved at least one adverse event deemed preventable. Of those, 29 involved at least one preventable adverse event that was serious, life-threatening, or fatal, the researchers write. Of the seven deaths in the study population, one was deemed preventable.
The most common adverse events were adverse drug events, which accounted for 39.0% of the adverse events; surgical or other procedural events accounted for 30.4%; patient care events (including falls and pressure ulcers) accounted for 15.0%; and health care–associated infections accounted for 11.9%.
Overcoming barriers to better safety
“The overall level of harm, with nearly 1 in 4 patients suffering an adverse event, was higher than I expected it might be,” Dr. Bates told this news organization. However, techniques for identifying adverse events have improved, and “it is easier to find them in electronic records than in paper records,” he noted.
“Hospitals have many issues they are currently dealing with since COVID, and one issue is simply prioritization,” Dr. Bates said. “But it is now possible to measure harm for all patients using electronic tools, and if hospitals know how much harm they are having in specific areas, they can make choices about which ones to focus on.”
“We now have effective prevention strategies for most of the main kinds of harm,” he said. Generally, rates of harm are high because these strategies are not being used effectively, he said. “In addition, there are new tools that can be used – for example, to identify patients who are decompensating earlier,” he noted.
As for additional research, some specific types of harm that have been resistant to interventions, such as pressure ulcers, deserve more attention, said Dr. Bates. “In addition, diagnostic errors appear to cause a great deal of harm, but we don’t yet have good strategies for preventing these,” he said.
The study findings were limited by several factors, including the use of data from hospitals that might not represent hospitals at large and by the inclusion mainly of patients with private insurance, the researchers write. Other limitations include the likelihood that some adverse events were missed and the level of agreement on adverse events between adjudicators was only fair.
However, the findings serve as a reminder to health care professionals of the need for continued attention to improving patient safety, and measuring adverse events remains a critical part of guiding these improvements, the researchers conclude.
Timely reassessment and opportunities to improve
In the decades since the publication of the report, “To Err Is Human,” by the National Academies in 2000, significant attention has been paid to improving patient safety during hospitalizations, and health care systems have increased in both system and disease complexity, Said Suman Pal, MBBS, a specialist in hospital medicine at the University of New Mexico, Albuquerque, said in an interview. “Therefore, this study is important in reassessing the safety of inpatient care at the current time,” he said.
“The findings of this study showing preventable adverse events in approximately 7% of all admissions; while concerning, is not surprising, as it is consistent with other studies over time, as the authors have also noted in their discussion,” said Dr. Pal. The current findings “underscore the importance of continuous quality improvement efforts to increase the safety of patient care for hospitalized patients,” he noted.
“The increasing complexity of medical care, fragmentation of health care, structural inequities of health systems, and more recent widespread public health challenges such as the COVID-19 pandemic have been, in my opinion, barriers to improving patient safety,” Dr. Pal said. “The use of innovation and an interdisciplinary approach to patient safety and quality improvement in hospital-based care, such as the use of machine learning to monitor trends and predict the individualized risk of harm, could be a potential way out” to help reduce barriers and improve safety, he said.
“Additional research is needed to understand the key drivers of preventable harm for hospitalized patients in the United States,” said Dr. Pal. “When planning for change, keen attention must be paid to understanding how these [drivers] may differ for patients who have been historically marginalized or are otherwise underserved so as to not exacerbate health care inequities,” he added.
The study was funded by the Controlled Risk Insurance Company and the Risk Management Foundation of the Harvard Medical Institutions. Dr. Bates owns stock options with AESOP, Clew, FeelBetter, Guided Clinical Solutions, MDClone, and ValeraHealth and has grants/contracts from IBM Watson and EarlySense. He has also served as a consultant for CDI Negev. Dr. Pal has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
as indicated from data from 2,809 admissions at 11 hospitals.
The 1991 Harvard Medical Practice Study, which focused on medical injury and litigation, documented an adverse event rate of 3.7 events per 100 admissions; 28% of those events were attributed to negligence, write David W. Bates, MD, of Brigham and Women’s Hospital, Boston, and colleagues.
Although patient safety has changed significantly since 1991, documenting improvements has been challenging, the researchers say. Several reports have shown a decrease in health care–associated infections. However, other aspects of safety – notably, adverse drug events, defined as injuries resulting from drugs taken – are not easily measured and tracked, the researchers say.
“We have not had good estimates of how much harm is being caused by care in hospitals in an ongoing way that looked across all types of adverse events,” and the current review is therefore important, Dr. Bates said in an interview.
In a study recently published in the New England Journal of Medicine, the researchers analyzed a random sample of 2,809 hospital admissions from 11 hospitals in Massachusetts during the 2018 calendar year. The hospitals ranged in size from fewer than 100 beds to more than 700 beds; all patients were aged 18 years and older. A panel of nine nurses reviewed the admissions records to identify potential adverse events, and eight physicians reviewed the adverse event summaries and either agreed or disagreed with the adverse event type. The severity of each event was ranked using a general severity scale into categories of significant, serious, life-threatening, or fatal.
Overall, at least one adverse event was identified in 23.6% of the hospital admissions. A total of 978 adverse events were deemed to have occurred during the index admission, and 222 of these (22.7%) were deemed preventable. Among the preventable adverse events, 19.7% were classified as serious, 3.3% as life-threatening, and 0.5% as fatal.
A total of 523 admissions (18.6%) involved at least one significant adverse event, defined as an event that caused unnecessary harm but from which recovery was rapid. A total of 211 admissions involved a serious adverse event, defined as harm resulting in substantial intervention or prolonged recovery; 34 included at least one life-threatening event; and seven admissions involved a fatal adverse event.
A total of 191 admissions involved at least one adverse event deemed preventable. Of those, 29 involved at least one preventable adverse event that was serious, life-threatening, or fatal, the researchers write. Of the seven deaths in the study population, one was deemed preventable.
The most common adverse events were adverse drug events, which accounted for 39.0% of the adverse events; surgical or other procedural events accounted for 30.4%; patient care events (including falls and pressure ulcers) accounted for 15.0%; and health care–associated infections accounted for 11.9%.
Overcoming barriers to better safety
“The overall level of harm, with nearly 1 in 4 patients suffering an adverse event, was higher than I expected it might be,” Dr. Bates told this news organization. However, techniques for identifying adverse events have improved, and “it is easier to find them in electronic records than in paper records,” he noted.
“Hospitals have many issues they are currently dealing with since COVID, and one issue is simply prioritization,” Dr. Bates said. “But it is now possible to measure harm for all patients using electronic tools, and if hospitals know how much harm they are having in specific areas, they can make choices about which ones to focus on.”
“We now have effective prevention strategies for most of the main kinds of harm,” he said. Generally, rates of harm are high because these strategies are not being used effectively, he said. “In addition, there are new tools that can be used – for example, to identify patients who are decompensating earlier,” he noted.
As for additional research, some specific types of harm that have been resistant to interventions, such as pressure ulcers, deserve more attention, said Dr. Bates. “In addition, diagnostic errors appear to cause a great deal of harm, but we don’t yet have good strategies for preventing these,” he said.
The study findings were limited by several factors, including the use of data from hospitals that might not represent hospitals at large and by the inclusion mainly of patients with private insurance, the researchers write. Other limitations include the likelihood that some adverse events were missed and the level of agreement on adverse events between adjudicators was only fair.
However, the findings serve as a reminder to health care professionals of the need for continued attention to improving patient safety, and measuring adverse events remains a critical part of guiding these improvements, the researchers conclude.
Timely reassessment and opportunities to improve
In the decades since the publication of the report, “To Err Is Human,” by the National Academies in 2000, significant attention has been paid to improving patient safety during hospitalizations, and health care systems have increased in both system and disease complexity, Said Suman Pal, MBBS, a specialist in hospital medicine at the University of New Mexico, Albuquerque, said in an interview. “Therefore, this study is important in reassessing the safety of inpatient care at the current time,” he said.
“The findings of this study showing preventable adverse events in approximately 7% of all admissions; while concerning, is not surprising, as it is consistent with other studies over time, as the authors have also noted in their discussion,” said Dr. Pal. The current findings “underscore the importance of continuous quality improvement efforts to increase the safety of patient care for hospitalized patients,” he noted.
“The increasing complexity of medical care, fragmentation of health care, structural inequities of health systems, and more recent widespread public health challenges such as the COVID-19 pandemic have been, in my opinion, barriers to improving patient safety,” Dr. Pal said. “The use of innovation and an interdisciplinary approach to patient safety and quality improvement in hospital-based care, such as the use of machine learning to monitor trends and predict the individualized risk of harm, could be a potential way out” to help reduce barriers and improve safety, he said.
“Additional research is needed to understand the key drivers of preventable harm for hospitalized patients in the United States,” said Dr. Pal. “When planning for change, keen attention must be paid to understanding how these [drivers] may differ for patients who have been historically marginalized or are otherwise underserved so as to not exacerbate health care inequities,” he added.
The study was funded by the Controlled Risk Insurance Company and the Risk Management Foundation of the Harvard Medical Institutions. Dr. Bates owns stock options with AESOP, Clew, FeelBetter, Guided Clinical Solutions, MDClone, and ValeraHealth and has grants/contracts from IBM Watson and EarlySense. He has also served as a consultant for CDI Negev. Dr. Pal has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Possible bivalent vaccine link to strokes in people over 65
who got the shot, the Centers for Disease Control and Prevention and the Food and Drug Administration said in a joint news release.
The release did not recommend people change their vaccine practices, saying the database finding probably did not represent a “true clinical risk.” The CDC said everybody, including people over 65, should stay up to date on their COVID vaccines, including the bivalent booster.
The news release said the Vaccine Safety Datalink (VSD), “a near real-time surveillance system,” raised a safety concern about the Pfizer/BioNTech booster.
“Rapid-response investigation of the signal in the VSD raised a question of whether people 65 and older who have received the Pfizer-BioNTech COVID-19 Vaccine, Bivalent were more likely to have an ischemic stroke in the 21 days following vaccination compared with days 22-44 following vaccination,” the news release said.
Ischemic strokes are blockages of blood to the brain, often caused by blood clots.
“Although the totality of the data currently suggests that it is very unlikely that the signal in VSD (Vaccine Safety Datalink) represents a true clinical risk, we believe it is important to share this information with the public, as we have in the past, when one of our safety monitoring systems detects a signal,” the release said.
No higher likelihood of strokes linked to the Pfizer bivalent vaccine had been found by Pfizer/BioNTech, the Department of Veterans Affairs, the Vaccine Adverse Event Reporting System maintained by the CDC and the FDA, or other agencies that monitor reactions of vaccines, the news release said. No safety issues about strokes have been identified with the Moderna bivalent vaccine.
CNN, citing a CDC official, reported that about 550,000 seniors who got Pfizer bivalent boosters were tracked by the VSD, and 130 of them had strokes within 3 weeks of getting the shot. None of those 130 people died, CNN said. The official spoke on the condition of anonymity because they weren’t authorized to share the data.
The issue will be discussed at the January meeting of the FDA’s Vaccines and Related Biological Products Advisory Committee.
In a joint statement, Pfizer and BioNTech said: “Neither Pfizer and BioNTech nor the CDC or FDA have observed similar findings across numerous other monitoring systems in the U.S. and globally and there is no evidence to conclude that ischemic stroke is associated with the use of the companies’ COVID-19 vaccines.”
Bivalent boosters contain two strains of vaccine – one to protect against the original COVID-19 virus and another targeting Omicron subvariants.
A version of this article first appeared on WebMD.com.
who got the shot, the Centers for Disease Control and Prevention and the Food and Drug Administration said in a joint news release.
The release did not recommend people change their vaccine practices, saying the database finding probably did not represent a “true clinical risk.” The CDC said everybody, including people over 65, should stay up to date on their COVID vaccines, including the bivalent booster.
The news release said the Vaccine Safety Datalink (VSD), “a near real-time surveillance system,” raised a safety concern about the Pfizer/BioNTech booster.
“Rapid-response investigation of the signal in the VSD raised a question of whether people 65 and older who have received the Pfizer-BioNTech COVID-19 Vaccine, Bivalent were more likely to have an ischemic stroke in the 21 days following vaccination compared with days 22-44 following vaccination,” the news release said.
Ischemic strokes are blockages of blood to the brain, often caused by blood clots.
“Although the totality of the data currently suggests that it is very unlikely that the signal in VSD (Vaccine Safety Datalink) represents a true clinical risk, we believe it is important to share this information with the public, as we have in the past, when one of our safety monitoring systems detects a signal,” the release said.
No higher likelihood of strokes linked to the Pfizer bivalent vaccine had been found by Pfizer/BioNTech, the Department of Veterans Affairs, the Vaccine Adverse Event Reporting System maintained by the CDC and the FDA, or other agencies that monitor reactions of vaccines, the news release said. No safety issues about strokes have been identified with the Moderna bivalent vaccine.
CNN, citing a CDC official, reported that about 550,000 seniors who got Pfizer bivalent boosters were tracked by the VSD, and 130 of them had strokes within 3 weeks of getting the shot. None of those 130 people died, CNN said. The official spoke on the condition of anonymity because they weren’t authorized to share the data.
The issue will be discussed at the January meeting of the FDA’s Vaccines and Related Biological Products Advisory Committee.
In a joint statement, Pfizer and BioNTech said: “Neither Pfizer and BioNTech nor the CDC or FDA have observed similar findings across numerous other monitoring systems in the U.S. and globally and there is no evidence to conclude that ischemic stroke is associated with the use of the companies’ COVID-19 vaccines.”
Bivalent boosters contain two strains of vaccine – one to protect against the original COVID-19 virus and another targeting Omicron subvariants.
A version of this article first appeared on WebMD.com.
who got the shot, the Centers for Disease Control and Prevention and the Food and Drug Administration said in a joint news release.
The release did not recommend people change their vaccine practices, saying the database finding probably did not represent a “true clinical risk.” The CDC said everybody, including people over 65, should stay up to date on their COVID vaccines, including the bivalent booster.
The news release said the Vaccine Safety Datalink (VSD), “a near real-time surveillance system,” raised a safety concern about the Pfizer/BioNTech booster.
“Rapid-response investigation of the signal in the VSD raised a question of whether people 65 and older who have received the Pfizer-BioNTech COVID-19 Vaccine, Bivalent were more likely to have an ischemic stroke in the 21 days following vaccination compared with days 22-44 following vaccination,” the news release said.
Ischemic strokes are blockages of blood to the brain, often caused by blood clots.
“Although the totality of the data currently suggests that it is very unlikely that the signal in VSD (Vaccine Safety Datalink) represents a true clinical risk, we believe it is important to share this information with the public, as we have in the past, when one of our safety monitoring systems detects a signal,” the release said.
No higher likelihood of strokes linked to the Pfizer bivalent vaccine had been found by Pfizer/BioNTech, the Department of Veterans Affairs, the Vaccine Adverse Event Reporting System maintained by the CDC and the FDA, or other agencies that monitor reactions of vaccines, the news release said. No safety issues about strokes have been identified with the Moderna bivalent vaccine.
CNN, citing a CDC official, reported that about 550,000 seniors who got Pfizer bivalent boosters were tracked by the VSD, and 130 of them had strokes within 3 weeks of getting the shot. None of those 130 people died, CNN said. The official spoke on the condition of anonymity because they weren’t authorized to share the data.
The issue will be discussed at the January meeting of the FDA’s Vaccines and Related Biological Products Advisory Committee.
In a joint statement, Pfizer and BioNTech said: “Neither Pfizer and BioNTech nor the CDC or FDA have observed similar findings across numerous other monitoring systems in the U.S. and globally and there is no evidence to conclude that ischemic stroke is associated with the use of the companies’ COVID-19 vaccines.”
Bivalent boosters contain two strains of vaccine – one to protect against the original COVID-19 virus and another targeting Omicron subvariants.
A version of this article first appeared on WebMD.com.
Nearly 50% of patients with dementia experience falls
, suggests new research that also identifies multiple risk factors for these falls.
In a study of more than 5,500 participants, 45.5% of those with dementia experienced one or more falls, compared with 30.9% of their peers without dementia.
Vision impairment and living with a spouse were among the strongest predictors of future fall risk among participants living with dementia. Interestingly, high neighborhood social deprivation, which is reflected by such things as income and education, was associated with lower odds of falling.
Overall, the results highlight the need for a multidisciplinary approach to preventing falls among elderly individuals with dementia, said lead author Safiyyah M. Okoye, PhD, assistant professor, College of Nursing and Health Professions, Drexel University, Philadelphia.
“We need to consider different dimensions and figure out how we can try to go beyond the clinic in our interactions,” she said.
Dr. Okoye noted that in addition to reviewing medications that may contribute to falls and screening for vision problems, clinicians might also consider resources to improve the home environment and ensure that families have appropriate caregiving.
The findings were published online in Alzheimer’s and Dementia: The Journal of the Alzheimer’s Association.
No ‘silver bullet’
Every year, falls cause millions of injuries in older adults, and those with dementia are especially vulnerable. This population has twice the risk of falling and up to three times the risk of incurring serious fall-related injuries, such as fractures, the researchers noted.
Falls are a leading cause of hospitalization among those with dementia. Previous evidence has shown that persons with dementia are more likely to experience negative health consequences, such as delirium, while in hospital, compared with those without dementia. Even minor fall-related injuries are associated with the patient’s being discharged to a nursing home rather than returning home.
Dr. Okoye stressed that many factors contribute to falls, including health status; function, such as the ability to walk and balance; medications; home environment; and activity level.
“There are multidimensional aspects, and we can’t just find one silver bullet to address falls. It should be addressed comprehensively,” she said.
Existing studies “overwhelmingly” focus on factors related to health and function that could be addressed in the doctor’s office or with a referral, rather than on environmental and social factors, Dr. Okoye noted.
And even though the risk of falling is high among community-dwelling seniors with dementia, very few studies have addressed the risk of falls among these adults, she added.
The new analysis included a nationally representative sample of 5,581 community-dwelling adults who participated in both the 2015 and 2016 National Health and Aging Trends Study (NHATS). The NHATS is a population-based survey of health and disability trends and trajectories among Americans aged 65 years and older.
During interviews, participants were asked, personally or by proxy, about falls during the previous 12 months. Having fallen at baseline was evaluated as a possible predictor of falls in the subsequent 12 months.
To determine probable dementia, researchers asked whether a doctor had ever told the participants that they had dementia or Alzheimer’s disease. They also used a dementia screening questionnaire and neuropsychological tests of memory, orientation, and executive function.
Of the total sample, most (n = 5,093) did not have dementia.
Physical environmental factors that were assessed included conditions at home, such as clutter, tripping hazards, and structural issues, as well as neighborhood social and economic deprivation – such as income, education levels, and employment status.
Fall rates and counterintuitive findings
Results showed that significantly more of those with dementia than without experienced one or more falls (45.5% vs. 30.9%; P < .001).
In addition, a history of falling was significantly associated with subsequent falls among those with dementia (odds ratio, 6.20; 95% confidence interval, 3.81-10.09), as was vision impairment (OR, 2.22; 95% CI, 1.12-4.40) and living with a spouse versus alone (OR, 2.43; 95% CI, 1.09-5.43).
A possible explanation for higher fall risk among those living with a partner is that those living alone usually have better functioning, the investigators noted. Also, live-in partners tend to be of a similar age as the person with dementia and may have challenges of their own.
Interestingly, high neighborhood social deprivation was associated with lower odds of falling (OR, 0.55 for the highest deprivation scores; 95% CI, 0.31-0.98), a finding Dr. Okoye said was “counterintuitive.”
This result could be related to the social environment, she noted. “Maybe there are more people around in the house, more people with eyes on the person, or more people in the community who know the person. Despite the low economic resources, there could be social resources there,” she said.
The new findings underscore the idea that falling is a multidimensional phenomenon among older adults with dementia as well as those without dementia, Dr. Okoye noted.
Doctors can play a role in reducing falls among patients with dementia by asking about falls, possibly eliminating medications that are associated with risk of falling, and screening for and correcting vision and hearing impairments, she suggested.
They may also help determine household hazards for a patient, such as clutter and poor lighting, and ensure that these are addressed, Dr. Okoye added.
No surprise
Commenting on the study, David S. Knopman, MD, a clinical neurologist at Mayo Clinic, Rochester, Minn., said the finding that visual impairment and a prior history of falling are predictive of subsequent falls “comes as no surprise.”
Dr. Knopman, whose research focuses on late-life cognitive disorders, was not involved with the current study.
Risk reduction is “of course” a key management goal, he said. “Vigilance and optimizing the patient’s living space to reduce fall risks are the major strategies,” he added.
Dr. Knopman reiterated that falls among those with dementia are associated with higher mortality and often lead to loss of the capacity to live outside of an institution.
The study was supported by the National Institute on Aging. The investigators and Dr. Knopman report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, suggests new research that also identifies multiple risk factors for these falls.
In a study of more than 5,500 participants, 45.5% of those with dementia experienced one or more falls, compared with 30.9% of their peers without dementia.
Vision impairment and living with a spouse were among the strongest predictors of future fall risk among participants living with dementia. Interestingly, high neighborhood social deprivation, which is reflected by such things as income and education, was associated with lower odds of falling.
Overall, the results highlight the need for a multidisciplinary approach to preventing falls among elderly individuals with dementia, said lead author Safiyyah M. Okoye, PhD, assistant professor, College of Nursing and Health Professions, Drexel University, Philadelphia.
“We need to consider different dimensions and figure out how we can try to go beyond the clinic in our interactions,” she said.
Dr. Okoye noted that in addition to reviewing medications that may contribute to falls and screening for vision problems, clinicians might also consider resources to improve the home environment and ensure that families have appropriate caregiving.
The findings were published online in Alzheimer’s and Dementia: The Journal of the Alzheimer’s Association.
No ‘silver bullet’
Every year, falls cause millions of injuries in older adults, and those with dementia are especially vulnerable. This population has twice the risk of falling and up to three times the risk of incurring serious fall-related injuries, such as fractures, the researchers noted.
Falls are a leading cause of hospitalization among those with dementia. Previous evidence has shown that persons with dementia are more likely to experience negative health consequences, such as delirium, while in hospital, compared with those without dementia. Even minor fall-related injuries are associated with the patient’s being discharged to a nursing home rather than returning home.
Dr. Okoye stressed that many factors contribute to falls, including health status; function, such as the ability to walk and balance; medications; home environment; and activity level.
“There are multidimensional aspects, and we can’t just find one silver bullet to address falls. It should be addressed comprehensively,” she said.
Existing studies “overwhelmingly” focus on factors related to health and function that could be addressed in the doctor’s office or with a referral, rather than on environmental and social factors, Dr. Okoye noted.
And even though the risk of falling is high among community-dwelling seniors with dementia, very few studies have addressed the risk of falls among these adults, she added.
The new analysis included a nationally representative sample of 5,581 community-dwelling adults who participated in both the 2015 and 2016 National Health and Aging Trends Study (NHATS). The NHATS is a population-based survey of health and disability trends and trajectories among Americans aged 65 years and older.
During interviews, participants were asked, personally or by proxy, about falls during the previous 12 months. Having fallen at baseline was evaluated as a possible predictor of falls in the subsequent 12 months.
To determine probable dementia, researchers asked whether a doctor had ever told the participants that they had dementia or Alzheimer’s disease. They also used a dementia screening questionnaire and neuropsychological tests of memory, orientation, and executive function.
Of the total sample, most (n = 5,093) did not have dementia.
Physical environmental factors that were assessed included conditions at home, such as clutter, tripping hazards, and structural issues, as well as neighborhood social and economic deprivation – such as income, education levels, and employment status.
Fall rates and counterintuitive findings
Results showed that significantly more of those with dementia than without experienced one or more falls (45.5% vs. 30.9%; P < .001).
In addition, a history of falling was significantly associated with subsequent falls among those with dementia (odds ratio, 6.20; 95% confidence interval, 3.81-10.09), as was vision impairment (OR, 2.22; 95% CI, 1.12-4.40) and living with a spouse versus alone (OR, 2.43; 95% CI, 1.09-5.43).
A possible explanation for higher fall risk among those living with a partner is that those living alone usually have better functioning, the investigators noted. Also, live-in partners tend to be of a similar age as the person with dementia and may have challenges of their own.
Interestingly, high neighborhood social deprivation was associated with lower odds of falling (OR, 0.55 for the highest deprivation scores; 95% CI, 0.31-0.98), a finding Dr. Okoye said was “counterintuitive.”
This result could be related to the social environment, she noted. “Maybe there are more people around in the house, more people with eyes on the person, or more people in the community who know the person. Despite the low economic resources, there could be social resources there,” she said.
The new findings underscore the idea that falling is a multidimensional phenomenon among older adults with dementia as well as those without dementia, Dr. Okoye noted.
Doctors can play a role in reducing falls among patients with dementia by asking about falls, possibly eliminating medications that are associated with risk of falling, and screening for and correcting vision and hearing impairments, she suggested.
They may also help determine household hazards for a patient, such as clutter and poor lighting, and ensure that these are addressed, Dr. Okoye added.
No surprise
Commenting on the study, David S. Knopman, MD, a clinical neurologist at Mayo Clinic, Rochester, Minn., said the finding that visual impairment and a prior history of falling are predictive of subsequent falls “comes as no surprise.”
Dr. Knopman, whose research focuses on late-life cognitive disorders, was not involved with the current study.
Risk reduction is “of course” a key management goal, he said. “Vigilance and optimizing the patient’s living space to reduce fall risks are the major strategies,” he added.
Dr. Knopman reiterated that falls among those with dementia are associated with higher mortality and often lead to loss of the capacity to live outside of an institution.
The study was supported by the National Institute on Aging. The investigators and Dr. Knopman report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, suggests new research that also identifies multiple risk factors for these falls.
In a study of more than 5,500 participants, 45.5% of those with dementia experienced one or more falls, compared with 30.9% of their peers without dementia.
Vision impairment and living with a spouse were among the strongest predictors of future fall risk among participants living with dementia. Interestingly, high neighborhood social deprivation, which is reflected by such things as income and education, was associated with lower odds of falling.
Overall, the results highlight the need for a multidisciplinary approach to preventing falls among elderly individuals with dementia, said lead author Safiyyah M. Okoye, PhD, assistant professor, College of Nursing and Health Professions, Drexel University, Philadelphia.
“We need to consider different dimensions and figure out how we can try to go beyond the clinic in our interactions,” she said.
Dr. Okoye noted that in addition to reviewing medications that may contribute to falls and screening for vision problems, clinicians might also consider resources to improve the home environment and ensure that families have appropriate caregiving.
The findings were published online in Alzheimer’s and Dementia: The Journal of the Alzheimer’s Association.
No ‘silver bullet’
Every year, falls cause millions of injuries in older adults, and those with dementia are especially vulnerable. This population has twice the risk of falling and up to three times the risk of incurring serious fall-related injuries, such as fractures, the researchers noted.
Falls are a leading cause of hospitalization among those with dementia. Previous evidence has shown that persons with dementia are more likely to experience negative health consequences, such as delirium, while in hospital, compared with those without dementia. Even minor fall-related injuries are associated with the patient’s being discharged to a nursing home rather than returning home.
Dr. Okoye stressed that many factors contribute to falls, including health status; function, such as the ability to walk and balance; medications; home environment; and activity level.
“There are multidimensional aspects, and we can’t just find one silver bullet to address falls. It should be addressed comprehensively,” she said.
Existing studies “overwhelmingly” focus on factors related to health and function that could be addressed in the doctor’s office or with a referral, rather than on environmental and social factors, Dr. Okoye noted.
And even though the risk of falling is high among community-dwelling seniors with dementia, very few studies have addressed the risk of falls among these adults, she added.
The new analysis included a nationally representative sample of 5,581 community-dwelling adults who participated in both the 2015 and 2016 National Health and Aging Trends Study (NHATS). The NHATS is a population-based survey of health and disability trends and trajectories among Americans aged 65 years and older.
During interviews, participants were asked, personally or by proxy, about falls during the previous 12 months. Having fallen at baseline was evaluated as a possible predictor of falls in the subsequent 12 months.
To determine probable dementia, researchers asked whether a doctor had ever told the participants that they had dementia or Alzheimer’s disease. They also used a dementia screening questionnaire and neuropsychological tests of memory, orientation, and executive function.
Of the total sample, most (n = 5,093) did not have dementia.
Physical environmental factors that were assessed included conditions at home, such as clutter, tripping hazards, and structural issues, as well as neighborhood social and economic deprivation – such as income, education levels, and employment status.
Fall rates and counterintuitive findings
Results showed that significantly more of those with dementia than without experienced one or more falls (45.5% vs. 30.9%; P < .001).
In addition, a history of falling was significantly associated with subsequent falls among those with dementia (odds ratio, 6.20; 95% confidence interval, 3.81-10.09), as was vision impairment (OR, 2.22; 95% CI, 1.12-4.40) and living with a spouse versus alone (OR, 2.43; 95% CI, 1.09-5.43).
A possible explanation for higher fall risk among those living with a partner is that those living alone usually have better functioning, the investigators noted. Also, live-in partners tend to be of a similar age as the person with dementia and may have challenges of their own.
Interestingly, high neighborhood social deprivation was associated with lower odds of falling (OR, 0.55 for the highest deprivation scores; 95% CI, 0.31-0.98), a finding Dr. Okoye said was “counterintuitive.”
This result could be related to the social environment, she noted. “Maybe there are more people around in the house, more people with eyes on the person, or more people in the community who know the person. Despite the low economic resources, there could be social resources there,” she said.
The new findings underscore the idea that falling is a multidimensional phenomenon among older adults with dementia as well as those without dementia, Dr. Okoye noted.
Doctors can play a role in reducing falls among patients with dementia by asking about falls, possibly eliminating medications that are associated with risk of falling, and screening for and correcting vision and hearing impairments, she suggested.
They may also help determine household hazards for a patient, such as clutter and poor lighting, and ensure that these are addressed, Dr. Okoye added.
No surprise
Commenting on the study, David S. Knopman, MD, a clinical neurologist at Mayo Clinic, Rochester, Minn., said the finding that visual impairment and a prior history of falling are predictive of subsequent falls “comes as no surprise.”
Dr. Knopman, whose research focuses on late-life cognitive disorders, was not involved with the current study.
Risk reduction is “of course” a key management goal, he said. “Vigilance and optimizing the patient’s living space to reduce fall risks are the major strategies,” he added.
Dr. Knopman reiterated that falls among those with dementia are associated with higher mortality and often lead to loss of the capacity to live outside of an institution.
The study was supported by the National Institute on Aging. The investigators and Dr. Knopman report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ALZHEIMER’S AND DEMENTIA
Children and COVID: ED visits and hospitalizations start to fall again
Emergency department visits and hospitalizations for COVID-19 in children appear to be following the declining trend set by weekly cases since early December, based on data from the Centers for Disease Control and Prevention.
. New cases took a different path that had the weekly total falling through November before taking a big jump during the week of Nov. 27 to Dec. 3 – the count doubled from 30,000 the previous week to 63,000 – and then decreased again, the CDC reported.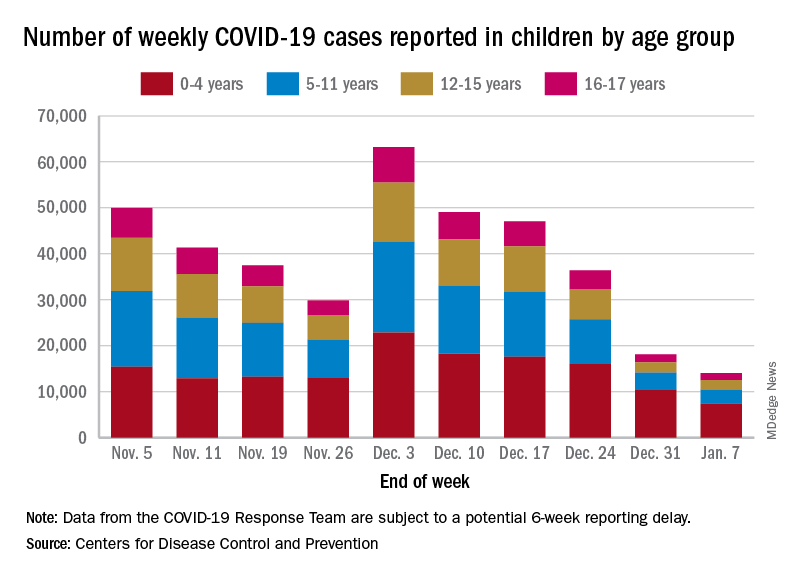
The proportion of ED visits with COVID, which was down to 1.0% of all ED visits (7-day average) for children aged 0-4 years on Nov. 4, was up to 3.2% on Jan. 3 but slipped to 2.5% as of Jan. 10. The patterns for older children are similar, with some differences in timing and lower peaks (1.7% for 12- to 15-year-olds and 1.9% for those aged 16-17), according to the CDC’s COVID Data Tracker.
The trend for new hospital admissions of children with confirmed COVID showed a similar rise through December, and the latest data for the very beginning of January suggest an even faster drop, although there is more of a reporting lag with hospitalization data, compared with ED visits, the CDC noted.
The most current data (Dec. 30 to Jan. 5) available from the American Academy of Pediatrics and the Children’s Hospital Association show less volatility in the number of weekly cases through November and December, with the peak being about 48,000 in mid-December. The AAP/CHA totals for the last 2 weeks, however, were both higher than the CDC’s corresponding counts, which are more preliminary and subject to revision.
The CDC puts the total number of COVID cases in children at 16.7 million – about 17.2% of all cases – as of Jan. 11, with 1,981 deaths reported so far. The AAP and CHA are not tracking deaths, but their case total as of Jan. 5 was 15.2 million, which represents 18.1% of cases in all ages. The AAP/CHA report is based on data reported publicly by an ever-decreasing number of states and territories.
Emergency department visits and hospitalizations for COVID-19 in children appear to be following the declining trend set by weekly cases since early December, based on data from the Centers for Disease Control and Prevention.
. New cases took a different path that had the weekly total falling through November before taking a big jump during the week of Nov. 27 to Dec. 3 – the count doubled from 30,000 the previous week to 63,000 – and then decreased again, the CDC reported.
The proportion of ED visits with COVID, which was down to 1.0% of all ED visits (7-day average) for children aged 0-4 years on Nov. 4, was up to 3.2% on Jan. 3 but slipped to 2.5% as of Jan. 10. The patterns for older children are similar, with some differences in timing and lower peaks (1.7% for 12- to 15-year-olds and 1.9% for those aged 16-17), according to the CDC’s COVID Data Tracker.
The trend for new hospital admissions of children with confirmed COVID showed a similar rise through December, and the latest data for the very beginning of January suggest an even faster drop, although there is more of a reporting lag with hospitalization data, compared with ED visits, the CDC noted.
The most current data (Dec. 30 to Jan. 5) available from the American Academy of Pediatrics and the Children’s Hospital Association show less volatility in the number of weekly cases through November and December, with the peak being about 48,000 in mid-December. The AAP/CHA totals for the last 2 weeks, however, were both higher than the CDC’s corresponding counts, which are more preliminary and subject to revision.
The CDC puts the total number of COVID cases in children at 16.7 million – about 17.2% of all cases – as of Jan. 11, with 1,981 deaths reported so far. The AAP and CHA are not tracking deaths, but their case total as of Jan. 5 was 15.2 million, which represents 18.1% of cases in all ages. The AAP/CHA report is based on data reported publicly by an ever-decreasing number of states and territories.
Emergency department visits and hospitalizations for COVID-19 in children appear to be following the declining trend set by weekly cases since early December, based on data from the Centers for Disease Control and Prevention.
. New cases took a different path that had the weekly total falling through November before taking a big jump during the week of Nov. 27 to Dec. 3 – the count doubled from 30,000 the previous week to 63,000 – and then decreased again, the CDC reported.
The proportion of ED visits with COVID, which was down to 1.0% of all ED visits (7-day average) for children aged 0-4 years on Nov. 4, was up to 3.2% on Jan. 3 but slipped to 2.5% as of Jan. 10. The patterns for older children are similar, with some differences in timing and lower peaks (1.7% for 12- to 15-year-olds and 1.9% for those aged 16-17), according to the CDC’s COVID Data Tracker.
The trend for new hospital admissions of children with confirmed COVID showed a similar rise through December, and the latest data for the very beginning of January suggest an even faster drop, although there is more of a reporting lag with hospitalization data, compared with ED visits, the CDC noted.
The most current data (Dec. 30 to Jan. 5) available from the American Academy of Pediatrics and the Children’s Hospital Association show less volatility in the number of weekly cases through November and December, with the peak being about 48,000 in mid-December. The AAP/CHA totals for the last 2 weeks, however, were both higher than the CDC’s corresponding counts, which are more preliminary and subject to revision.
The CDC puts the total number of COVID cases in children at 16.7 million – about 17.2% of all cases – as of Jan. 11, with 1,981 deaths reported so far. The AAP and CHA are not tracking deaths, but their case total as of Jan. 5 was 15.2 million, which represents 18.1% of cases in all ages. The AAP/CHA report is based on data reported publicly by an ever-decreasing number of states and territories.
Manicure gone wrong leads to cancer diagnosis
. Now, she and her doctor are spreading the word about her ordeal as a lesson that speed and persistence in seeking treatment are the keys that make her type of cancer – squamous cell carcinoma – completely curable.
“She cut me, and the cut wasn’t just a regular cuticle cut. She cut me deep, and that was one of the first times that happened to me,” Grace Garcia, 50, told TODAY.com, recalling the November 2021 incident.
Ms. Garcia had been getting her nails done regularly for 20 years, she said, but happened to go to a different salon than her usual spot because she couldn’t get an appointment during the busy pre-Thanksgiving season. She doesn’t recall whether the technician opened packaging that signals unused tools.
She put antibiotic ointment on the cut, but it didn’t heal after a few days. Eventually, the skin closed and a darkened bump formed. It was painful. She went to her doctor, who said it was a “callus from writing,” she told TODAY.com. But it was on her ring finger, which didn’t seem connected to writing. Her doctor said to keep an eye on it.
Five months after the cut occurred, she mentioned it during a gynecology appointment and was referred to a dermatologist, who also advised keeping an eye on it. A wart developed. She went back to her primary care physician and then to another dermatologist. The spot was biopsied.
Squamous cell carcinoma is a common type of skin cancer, according to the American Academy of Dermatology. It can have many causes, but the cause in Ms. Garcia’s case was both very common and very rare: human papillomavirus, or HPV. HPV is a virus that infects millions of people every year, but it’s not a typical cause of skin cancer.
“It’s pretty rare for several reasons. Generally speaking, the strains that cause cancer from an HPV standpoint tend to be more sexually transmitted,” dermatologist Teo Soleymani told TODAY.com. “In Grace’s case, she had an injury, which became the portal of entry. So that thick skin that we have on our hands and feet that acts as a natural barrier against infections and things like that was no longer the case, and the virus was able to infect her skin.”
Dr. Soleymani said Ms. Garcia’s persistence to get answers likely saved her from losing a finger.
“Your outcomes are entirely dictated by how early you catch them, and very often they’re completely curable,” he said. “Her persistence – not only was she able to have a great outcome, she probably saved herself from having her finger amputated.”
. Now, she and her doctor are spreading the word about her ordeal as a lesson that speed and persistence in seeking treatment are the keys that make her type of cancer – squamous cell carcinoma – completely curable.
“She cut me, and the cut wasn’t just a regular cuticle cut. She cut me deep, and that was one of the first times that happened to me,” Grace Garcia, 50, told TODAY.com, recalling the November 2021 incident.
Ms. Garcia had been getting her nails done regularly for 20 years, she said, but happened to go to a different salon than her usual spot because she couldn’t get an appointment during the busy pre-Thanksgiving season. She doesn’t recall whether the technician opened packaging that signals unused tools.
She put antibiotic ointment on the cut, but it didn’t heal after a few days. Eventually, the skin closed and a darkened bump formed. It was painful. She went to her doctor, who said it was a “callus from writing,” she told TODAY.com. But it was on her ring finger, which didn’t seem connected to writing. Her doctor said to keep an eye on it.
Five months after the cut occurred, she mentioned it during a gynecology appointment and was referred to a dermatologist, who also advised keeping an eye on it. A wart developed. She went back to her primary care physician and then to another dermatologist. The spot was biopsied.
Squamous cell carcinoma is a common type of skin cancer, according to the American Academy of Dermatology. It can have many causes, but the cause in Ms. Garcia’s case was both very common and very rare: human papillomavirus, or HPV. HPV is a virus that infects millions of people every year, but it’s not a typical cause of skin cancer.
“It’s pretty rare for several reasons. Generally speaking, the strains that cause cancer from an HPV standpoint tend to be more sexually transmitted,” dermatologist Teo Soleymani told TODAY.com. “In Grace’s case, she had an injury, which became the portal of entry. So that thick skin that we have on our hands and feet that acts as a natural barrier against infections and things like that was no longer the case, and the virus was able to infect her skin.”
Dr. Soleymani said Ms. Garcia’s persistence to get answers likely saved her from losing a finger.
“Your outcomes are entirely dictated by how early you catch them, and very often they’re completely curable,” he said. “Her persistence – not only was she able to have a great outcome, she probably saved herself from having her finger amputated.”
. Now, she and her doctor are spreading the word about her ordeal as a lesson that speed and persistence in seeking treatment are the keys that make her type of cancer – squamous cell carcinoma – completely curable.
“She cut me, and the cut wasn’t just a regular cuticle cut. She cut me deep, and that was one of the first times that happened to me,” Grace Garcia, 50, told TODAY.com, recalling the November 2021 incident.
Ms. Garcia had been getting her nails done regularly for 20 years, she said, but happened to go to a different salon than her usual spot because she couldn’t get an appointment during the busy pre-Thanksgiving season. She doesn’t recall whether the technician opened packaging that signals unused tools.
She put antibiotic ointment on the cut, but it didn’t heal after a few days. Eventually, the skin closed and a darkened bump formed. It was painful. She went to her doctor, who said it was a “callus from writing,” she told TODAY.com. But it was on her ring finger, which didn’t seem connected to writing. Her doctor said to keep an eye on it.
Five months after the cut occurred, she mentioned it during a gynecology appointment and was referred to a dermatologist, who also advised keeping an eye on it. A wart developed. She went back to her primary care physician and then to another dermatologist. The spot was biopsied.
Squamous cell carcinoma is a common type of skin cancer, according to the American Academy of Dermatology. It can have many causes, but the cause in Ms. Garcia’s case was both very common and very rare: human papillomavirus, or HPV. HPV is a virus that infects millions of people every year, but it’s not a typical cause of skin cancer.
“It’s pretty rare for several reasons. Generally speaking, the strains that cause cancer from an HPV standpoint tend to be more sexually transmitted,” dermatologist Teo Soleymani told TODAY.com. “In Grace’s case, she had an injury, which became the portal of entry. So that thick skin that we have on our hands and feet that acts as a natural barrier against infections and things like that was no longer the case, and the virus was able to infect her skin.”
Dr. Soleymani said Ms. Garcia’s persistence to get answers likely saved her from losing a finger.
“Your outcomes are entirely dictated by how early you catch them, and very often they’re completely curable,” he said. “Her persistence – not only was she able to have a great outcome, she probably saved herself from having her finger amputated.”
Hearing loss strongly tied to increased dementia risk
, new national data show. Investigators also found that even mild hearing loss was associated with increased dementia risk, although it was not statistically significant, and that hearing aid use was tied to a 32% decrease in dementia prevalence.
“Every 10-decibel increase in hearing loss was associated with 16% greater prevalence of dementia, such that prevalence of dementia in older adults with moderate or greater hearing loss was 61% higher than prevalence in those with normal hearing,” said lead investigator Alison Huang, PhD, senior research associate in epidemiology at Johns Hopkins Bloomberg School of Public Health and core faculty in the Cochlear Center for Hearing and Public Health, Baltimore.
The findings were published online in JAMA.
Dose dependent effect
For their study, researchers analyzed data on 2,413 community-dwelling participants in the National Health and Aging Trends Study, a nationally representative, continuous panel study of U.S. Medicare beneficiaries aged 65 and older.
Data from the study was collected during in-home interviews, setting it apart from previous work that relied on data collected in a clinical setting, Dr. Huang said.
“This study was able to capture more vulnerable populations, such as the oldest old and older adults with disabilities, typically excluded from prior epidemiologic studies of the hearing loss–dementia association that use clinic-based data collection, which only captures people who have the ability and means to get to clinics,” Dr. Huang said.
Weighted hearing loss prevalence was 36.7% for mild and 29.8% for moderate to severe hearing loss, and weighted prevalence of dementia was 10.3%.
Those with moderate to severe hearing loss were 61% more likely to have dementia than were those with normal hearing (prevalence ratio, 1.61; 95% confidence interval [CI], 1.09-2.38).
Dementia prevalence increased with increasing severity of hearing loss: Normal hearing: 6.19% (95% CI, 4.31-8.80); mild hearing loss: 8.93% (95% CI, 6.99-11.34); moderate to severe hearing loss: 16.52% (95% CI, 13.81-19.64). But only moderate to severe hearing loss showed a statistically significant association with dementia (P = .02).
Dementia prevalence increased 16% per 10-decibel increase in hearing loss (prevalence ratio 1.16; P < .001).
Among the 853 individuals in the study with moderate to severe hearing loss, those who used hearing aids (n = 414) had a 32% lower risk of dementia compared with those who didn’t use assisted devices (prevalence ratio, 0.68; 95% CI, 0.47-1.00). Similar data were published in JAMA Neurology, suggesting that hearing aids reduce dementia risk.
“With this study, we were able to refine our understanding of the strength of the hearing loss–dementia association in a study more representative of older adults in the United States,” said Dr. Huang.
Robust association
Commenting on the findings, Justin S. Golub, MD, associate professor in the department of otolaryngology–head and neck surgery at Columbia University, New York, said the study supports earlier research and suggests a “robust” association between hearing loss and dementia.
“The particular advantage of this study was that it was high quality and nationally representative,” Dr. Golub said. “It is also among a smaller set of studies that have shown hearing aid use to be associated with lower risk of dementia.”
Although not statistically significant, researchers did find increasing prevalence of dementia among people with only mild hearing loss, and clinicians should take note, said Dr. Golub, who was not involved with this study.
“We would expect the relationship between mild hearing loss and dementia to be weaker than severe hearing loss and dementia and, as a result, it might take more participants to show an association among the mild group,” Dr. Golub said.
“Even though this particular study did not specifically find a relationship between mild hearing loss and dementia, I would still recommend people to start treating their hearing loss when it is early,” Dr. Golub added.
The study was funded by the National Institute on Aging. Dr. Golub reports no relevant financial relationships. Full disclosures for study authors are included in the original article.
A version of this article first appeared on Medscape.com.
, new national data show. Investigators also found that even mild hearing loss was associated with increased dementia risk, although it was not statistically significant, and that hearing aid use was tied to a 32% decrease in dementia prevalence.
“Every 10-decibel increase in hearing loss was associated with 16% greater prevalence of dementia, such that prevalence of dementia in older adults with moderate or greater hearing loss was 61% higher than prevalence in those with normal hearing,” said lead investigator Alison Huang, PhD, senior research associate in epidemiology at Johns Hopkins Bloomberg School of Public Health and core faculty in the Cochlear Center for Hearing and Public Health, Baltimore.
The findings were published online in JAMA.
Dose dependent effect
For their study, researchers analyzed data on 2,413 community-dwelling participants in the National Health and Aging Trends Study, a nationally representative, continuous panel study of U.S. Medicare beneficiaries aged 65 and older.
Data from the study was collected during in-home interviews, setting it apart from previous work that relied on data collected in a clinical setting, Dr. Huang said.
“This study was able to capture more vulnerable populations, such as the oldest old and older adults with disabilities, typically excluded from prior epidemiologic studies of the hearing loss–dementia association that use clinic-based data collection, which only captures people who have the ability and means to get to clinics,” Dr. Huang said.
Weighted hearing loss prevalence was 36.7% for mild and 29.8% for moderate to severe hearing loss, and weighted prevalence of dementia was 10.3%.
Those with moderate to severe hearing loss were 61% more likely to have dementia than were those with normal hearing (prevalence ratio, 1.61; 95% confidence interval [CI], 1.09-2.38).
Dementia prevalence increased with increasing severity of hearing loss: Normal hearing: 6.19% (95% CI, 4.31-8.80); mild hearing loss: 8.93% (95% CI, 6.99-11.34); moderate to severe hearing loss: 16.52% (95% CI, 13.81-19.64). But only moderate to severe hearing loss showed a statistically significant association with dementia (P = .02).
Dementia prevalence increased 16% per 10-decibel increase in hearing loss (prevalence ratio 1.16; P < .001).
Among the 853 individuals in the study with moderate to severe hearing loss, those who used hearing aids (n = 414) had a 32% lower risk of dementia compared with those who didn’t use assisted devices (prevalence ratio, 0.68; 95% CI, 0.47-1.00). Similar data were published in JAMA Neurology, suggesting that hearing aids reduce dementia risk.
“With this study, we were able to refine our understanding of the strength of the hearing loss–dementia association in a study more representative of older adults in the United States,” said Dr. Huang.
Robust association
Commenting on the findings, Justin S. Golub, MD, associate professor in the department of otolaryngology–head and neck surgery at Columbia University, New York, said the study supports earlier research and suggests a “robust” association between hearing loss and dementia.
“The particular advantage of this study was that it was high quality and nationally representative,” Dr. Golub said. “It is also among a smaller set of studies that have shown hearing aid use to be associated with lower risk of dementia.”
Although not statistically significant, researchers did find increasing prevalence of dementia among people with only mild hearing loss, and clinicians should take note, said Dr. Golub, who was not involved with this study.
“We would expect the relationship between mild hearing loss and dementia to be weaker than severe hearing loss and dementia and, as a result, it might take more participants to show an association among the mild group,” Dr. Golub said.
“Even though this particular study did not specifically find a relationship between mild hearing loss and dementia, I would still recommend people to start treating their hearing loss when it is early,” Dr. Golub added.
The study was funded by the National Institute on Aging. Dr. Golub reports no relevant financial relationships. Full disclosures for study authors are included in the original article.
A version of this article first appeared on Medscape.com.
, new national data show. Investigators also found that even mild hearing loss was associated with increased dementia risk, although it was not statistically significant, and that hearing aid use was tied to a 32% decrease in dementia prevalence.
“Every 10-decibel increase in hearing loss was associated with 16% greater prevalence of dementia, such that prevalence of dementia in older adults with moderate or greater hearing loss was 61% higher than prevalence in those with normal hearing,” said lead investigator Alison Huang, PhD, senior research associate in epidemiology at Johns Hopkins Bloomberg School of Public Health and core faculty in the Cochlear Center for Hearing and Public Health, Baltimore.
The findings were published online in JAMA.
Dose dependent effect
For their study, researchers analyzed data on 2,413 community-dwelling participants in the National Health and Aging Trends Study, a nationally representative, continuous panel study of U.S. Medicare beneficiaries aged 65 and older.
Data from the study was collected during in-home interviews, setting it apart from previous work that relied on data collected in a clinical setting, Dr. Huang said.
“This study was able to capture more vulnerable populations, such as the oldest old and older adults with disabilities, typically excluded from prior epidemiologic studies of the hearing loss–dementia association that use clinic-based data collection, which only captures people who have the ability and means to get to clinics,” Dr. Huang said.
Weighted hearing loss prevalence was 36.7% for mild and 29.8% for moderate to severe hearing loss, and weighted prevalence of dementia was 10.3%.
Those with moderate to severe hearing loss were 61% more likely to have dementia than were those with normal hearing (prevalence ratio, 1.61; 95% confidence interval [CI], 1.09-2.38).
Dementia prevalence increased with increasing severity of hearing loss: Normal hearing: 6.19% (95% CI, 4.31-8.80); mild hearing loss: 8.93% (95% CI, 6.99-11.34); moderate to severe hearing loss: 16.52% (95% CI, 13.81-19.64). But only moderate to severe hearing loss showed a statistically significant association with dementia (P = .02).
Dementia prevalence increased 16% per 10-decibel increase in hearing loss (prevalence ratio 1.16; P < .001).
Among the 853 individuals in the study with moderate to severe hearing loss, those who used hearing aids (n = 414) had a 32% lower risk of dementia compared with those who didn’t use assisted devices (prevalence ratio, 0.68; 95% CI, 0.47-1.00). Similar data were published in JAMA Neurology, suggesting that hearing aids reduce dementia risk.
“With this study, we were able to refine our understanding of the strength of the hearing loss–dementia association in a study more representative of older adults in the United States,” said Dr. Huang.
Robust association
Commenting on the findings, Justin S. Golub, MD, associate professor in the department of otolaryngology–head and neck surgery at Columbia University, New York, said the study supports earlier research and suggests a “robust” association between hearing loss and dementia.
“The particular advantage of this study was that it was high quality and nationally representative,” Dr. Golub said. “It is also among a smaller set of studies that have shown hearing aid use to be associated with lower risk of dementia.”
Although not statistically significant, researchers did find increasing prevalence of dementia among people with only mild hearing loss, and clinicians should take note, said Dr. Golub, who was not involved with this study.
“We would expect the relationship between mild hearing loss and dementia to be weaker than severe hearing loss and dementia and, as a result, it might take more participants to show an association among the mild group,” Dr. Golub said.
“Even though this particular study did not specifically find a relationship between mild hearing loss and dementia, I would still recommend people to start treating their hearing loss when it is early,” Dr. Golub added.
The study was funded by the National Institute on Aging. Dr. Golub reports no relevant financial relationships. Full disclosures for study authors are included in the original article.
A version of this article first appeared on Medscape.com.
Add this to the list of long COVID symptoms: Stigma
Most people with long COVID find they’re facing stigma due to their condition, according to a new report from researchers in the United Kingdom. In short: Relatives and friends may not believe they’re truly sick.
The U.K. team found that more than three-quarters of people studied had experienced stigma often or always.
In fact, 95% of people with long COVID faced at least one type of stigma at least sometimes, according to the study, published in November in the journal PLOS One.
Those conclusions had surprised the study’s lead researcher, Marija Pantelic, PhD, a public health lecturer at Brighton and Sussex Medical School, England.
“After years of working on HIV-related stigma, I was shocked to see how many people were turning a blind eye to and dismissing the difficulties experienced by people with long COVID,” Dr. Pantelic says. “It has also been clear to me from the start that this stigma is detrimental not just for people’s dignity, but also public health.”
Even some doctors argue that the growing attention paid to long COVID is excessive.
“It’s often normal to experience mild fatigue or weaknesses for weeks after being sick and inactive and not eating well. Calling these cases long COVID is the medicalization of modern life,” Marty Makary, MD, a surgeon and public policy researcher at Johns Hopkins University, Baltimore, wrote in a commentary in the Wall Street Journal.
Other doctors strongly disagree, including Alba Azola, MD, codirector of the Johns Hopkins Post-Acute COVID-19 Team and an expert in the stigma surrounding long COVID.
“Putting that spin on things, it’s just hurting people,” she says.
One example is people who cannot return to work.
“A lot of their family members tell me that they’re being lazy,” Dr. Azola says. “That’s part of the public stigma, that these are people just trying to get out of work.”
Some experts say the U.K. study represents a landmark.
“When you have data like this on long COVID stigma, it becomes more difficult to deny its existence or address it,” says Naomi Torres-Mackie, PhD, a clinical psychologist at Lenox Hill Hospital in New York. She also is head of research at the New York–based Mental Health Coalition, a group of experts working to end the stigma surrounding mental health.
She recalls her first patient with long COVID.
“She experienced the discomfort and pain itself, and then she had this crushing feeling that it wasn’t valid, or real. She felt very alone in it,” Dr. Torres-Mackie says.
Another one of her patients is working at her job from home but facing doubt about her condition from her employers.
“Every month, her medical doctor has to produce a letter confirming her medical condition,” Dr. Torres-Mackie says.
Taking part in the British stigma survey were 1,166 people, including 966 residents of the United Kingdom, with the average age of 48. Nearly 85% were female, and more than three-quarters were educated at the university level or higher.
Half of them said they had a clinical diagnosis of long COVID.
More than 60% of them said that at least some of the time, they were cautious about who they talked to about their condition. And fully 34% of those who did disclose their diagnosis said that they regretted having done so.
That’s a difficult experience for those with long COVID, says Leonard Jason, PhD, a professor of psychology at DePaul University in Chicago.
“It’s like they’re traumatized by the initial experience of being sick, and retraumatized by the response of others to them,” he says.
Unexplained illnesses are not well-regarded by the general public, Dr. Jason says.
He gave the example of multiple sclerosis. Before the 1980s, those with MS were considered to have a psychological illness, he says. “Then, in the 1980s, there were biomarkers that said, ‘Here’s the evidence.’ ”
The British study described three types of stigma stemming from the long COVID diagnosis of those questioned:
- Enacted stigma: People were directly treated unfairly because of their condition.
- Internalized stigma: People felt embarrassed by that condition.
- Anticipated stigma: People expected they would be treated poorly because of their diagnosis.
Dr. Azola calls the medical community a major problem when it comes to dealing with long COVID.
“What I see with my patients is medical trauma,” she says. They may have symptoms that send them to the emergency room, and then the tests come back negative. “Instead of tracking the patients’ symptoms, patients get told, ‘Everything looks good, you can go home, this is a panic attack,’ ” she says.
Some people go online to search for treatments, sometimes launching GoFundMe campaigns to raise money for unreliable treatments.
Long COVID patients may have gone through 5 to 10 doctors before they arrive for treatment with the Johns Hopkins Post-Acute COVID-19 Team. The clinic began in April 2020 remotely and in August of that year in person.
Today, the clinic staff spends an hour with a first-time long COVID patient, hearing their stories and helping relieve anxiety, Dr. Azola says.
The phenomenon of long COVID is similar to what patients have had with chronic fatigue syndrome, lupus, or fibromyalgia, where people have symptoms that are hard to explain, says Jennifer Chevinsky, MD, deputy public health officer for Riverside County, Calif.
“Stigma within medicine or health care is nothing new,” she says.
In Chicago, Dr. Jason notes that the federal government’s decision to invest hundreds of millions of dollars in long COVID research “shows the government is helping destigmatize it.”
Dr. Pantelic says she and her colleagues are continuing their research.
“We are interested in understanding the impacts of this stigma, and how to mitigate any adverse outcomes for patients and services,” she says.
A version of this article first appeared on WebMD.com.
Most people with long COVID find they’re facing stigma due to their condition, according to a new report from researchers in the United Kingdom. In short: Relatives and friends may not believe they’re truly sick.
The U.K. team found that more than three-quarters of people studied had experienced stigma often or always.
In fact, 95% of people with long COVID faced at least one type of stigma at least sometimes, according to the study, published in November in the journal PLOS One.
Those conclusions had surprised the study’s lead researcher, Marija Pantelic, PhD, a public health lecturer at Brighton and Sussex Medical School, England.
“After years of working on HIV-related stigma, I was shocked to see how many people were turning a blind eye to and dismissing the difficulties experienced by people with long COVID,” Dr. Pantelic says. “It has also been clear to me from the start that this stigma is detrimental not just for people’s dignity, but also public health.”
Even some doctors argue that the growing attention paid to long COVID is excessive.
“It’s often normal to experience mild fatigue or weaknesses for weeks after being sick and inactive and not eating well. Calling these cases long COVID is the medicalization of modern life,” Marty Makary, MD, a surgeon and public policy researcher at Johns Hopkins University, Baltimore, wrote in a commentary in the Wall Street Journal.
Other doctors strongly disagree, including Alba Azola, MD, codirector of the Johns Hopkins Post-Acute COVID-19 Team and an expert in the stigma surrounding long COVID.
“Putting that spin on things, it’s just hurting people,” she says.
One example is people who cannot return to work.
“A lot of their family members tell me that they’re being lazy,” Dr. Azola says. “That’s part of the public stigma, that these are people just trying to get out of work.”
Some experts say the U.K. study represents a landmark.
“When you have data like this on long COVID stigma, it becomes more difficult to deny its existence or address it,” says Naomi Torres-Mackie, PhD, a clinical psychologist at Lenox Hill Hospital in New York. She also is head of research at the New York–based Mental Health Coalition, a group of experts working to end the stigma surrounding mental health.
She recalls her first patient with long COVID.
“She experienced the discomfort and pain itself, and then she had this crushing feeling that it wasn’t valid, or real. She felt very alone in it,” Dr. Torres-Mackie says.
Another one of her patients is working at her job from home but facing doubt about her condition from her employers.
“Every month, her medical doctor has to produce a letter confirming her medical condition,” Dr. Torres-Mackie says.
Taking part in the British stigma survey were 1,166 people, including 966 residents of the United Kingdom, with the average age of 48. Nearly 85% were female, and more than three-quarters were educated at the university level or higher.
Half of them said they had a clinical diagnosis of long COVID.
More than 60% of them said that at least some of the time, they were cautious about who they talked to about their condition. And fully 34% of those who did disclose their diagnosis said that they regretted having done so.
That’s a difficult experience for those with long COVID, says Leonard Jason, PhD, a professor of psychology at DePaul University in Chicago.
“It’s like they’re traumatized by the initial experience of being sick, and retraumatized by the response of others to them,” he says.
Unexplained illnesses are not well-regarded by the general public, Dr. Jason says.
He gave the example of multiple sclerosis. Before the 1980s, those with MS were considered to have a psychological illness, he says. “Then, in the 1980s, there were biomarkers that said, ‘Here’s the evidence.’ ”
The British study described three types of stigma stemming from the long COVID diagnosis of those questioned:
- Enacted stigma: People were directly treated unfairly because of their condition.
- Internalized stigma: People felt embarrassed by that condition.
- Anticipated stigma: People expected they would be treated poorly because of their diagnosis.
Dr. Azola calls the medical community a major problem when it comes to dealing with long COVID.
“What I see with my patients is medical trauma,” she says. They may have symptoms that send them to the emergency room, and then the tests come back negative. “Instead of tracking the patients’ symptoms, patients get told, ‘Everything looks good, you can go home, this is a panic attack,’ ” she says.
Some people go online to search for treatments, sometimes launching GoFundMe campaigns to raise money for unreliable treatments.
Long COVID patients may have gone through 5 to 10 doctors before they arrive for treatment with the Johns Hopkins Post-Acute COVID-19 Team. The clinic began in April 2020 remotely and in August of that year in person.
Today, the clinic staff spends an hour with a first-time long COVID patient, hearing their stories and helping relieve anxiety, Dr. Azola says.
The phenomenon of long COVID is similar to what patients have had with chronic fatigue syndrome, lupus, or fibromyalgia, where people have symptoms that are hard to explain, says Jennifer Chevinsky, MD, deputy public health officer for Riverside County, Calif.
“Stigma within medicine or health care is nothing new,” she says.
In Chicago, Dr. Jason notes that the federal government’s decision to invest hundreds of millions of dollars in long COVID research “shows the government is helping destigmatize it.”
Dr. Pantelic says she and her colleagues are continuing their research.
“We are interested in understanding the impacts of this stigma, and how to mitigate any adverse outcomes for patients and services,” she says.
A version of this article first appeared on WebMD.com.
Most people with long COVID find they’re facing stigma due to their condition, according to a new report from researchers in the United Kingdom. In short: Relatives and friends may not believe they’re truly sick.
The U.K. team found that more than three-quarters of people studied had experienced stigma often or always.
In fact, 95% of people with long COVID faced at least one type of stigma at least sometimes, according to the study, published in November in the journal PLOS One.
Those conclusions had surprised the study’s lead researcher, Marija Pantelic, PhD, a public health lecturer at Brighton and Sussex Medical School, England.
“After years of working on HIV-related stigma, I was shocked to see how many people were turning a blind eye to and dismissing the difficulties experienced by people with long COVID,” Dr. Pantelic says. “It has also been clear to me from the start that this stigma is detrimental not just for people’s dignity, but also public health.”
Even some doctors argue that the growing attention paid to long COVID is excessive.
“It’s often normal to experience mild fatigue or weaknesses for weeks after being sick and inactive and not eating well. Calling these cases long COVID is the medicalization of modern life,” Marty Makary, MD, a surgeon and public policy researcher at Johns Hopkins University, Baltimore, wrote in a commentary in the Wall Street Journal.
Other doctors strongly disagree, including Alba Azola, MD, codirector of the Johns Hopkins Post-Acute COVID-19 Team and an expert in the stigma surrounding long COVID.
“Putting that spin on things, it’s just hurting people,” she says.
One example is people who cannot return to work.
“A lot of their family members tell me that they’re being lazy,” Dr. Azola says. “That’s part of the public stigma, that these are people just trying to get out of work.”
Some experts say the U.K. study represents a landmark.
“When you have data like this on long COVID stigma, it becomes more difficult to deny its existence or address it,” says Naomi Torres-Mackie, PhD, a clinical psychologist at Lenox Hill Hospital in New York. She also is head of research at the New York–based Mental Health Coalition, a group of experts working to end the stigma surrounding mental health.
She recalls her first patient with long COVID.
“She experienced the discomfort and pain itself, and then she had this crushing feeling that it wasn’t valid, or real. She felt very alone in it,” Dr. Torres-Mackie says.
Another one of her patients is working at her job from home but facing doubt about her condition from her employers.
“Every month, her medical doctor has to produce a letter confirming her medical condition,” Dr. Torres-Mackie says.
Taking part in the British stigma survey were 1,166 people, including 966 residents of the United Kingdom, with the average age of 48. Nearly 85% were female, and more than three-quarters were educated at the university level or higher.
Half of them said they had a clinical diagnosis of long COVID.
More than 60% of them said that at least some of the time, they were cautious about who they talked to about their condition. And fully 34% of those who did disclose their diagnosis said that they regretted having done so.
That’s a difficult experience for those with long COVID, says Leonard Jason, PhD, a professor of psychology at DePaul University in Chicago.
“It’s like they’re traumatized by the initial experience of being sick, and retraumatized by the response of others to them,” he says.
Unexplained illnesses are not well-regarded by the general public, Dr. Jason says.
He gave the example of multiple sclerosis. Before the 1980s, those with MS were considered to have a psychological illness, he says. “Then, in the 1980s, there were biomarkers that said, ‘Here’s the evidence.’ ”
The British study described three types of stigma stemming from the long COVID diagnosis of those questioned:
- Enacted stigma: People were directly treated unfairly because of their condition.
- Internalized stigma: People felt embarrassed by that condition.
- Anticipated stigma: People expected they would be treated poorly because of their diagnosis.
Dr. Azola calls the medical community a major problem when it comes to dealing with long COVID.
“What I see with my patients is medical trauma,” she says. They may have symptoms that send them to the emergency room, and then the tests come back negative. “Instead of tracking the patients’ symptoms, patients get told, ‘Everything looks good, you can go home, this is a panic attack,’ ” she says.
Some people go online to search for treatments, sometimes launching GoFundMe campaigns to raise money for unreliable treatments.
Long COVID patients may have gone through 5 to 10 doctors before they arrive for treatment with the Johns Hopkins Post-Acute COVID-19 Team. The clinic began in April 2020 remotely and in August of that year in person.
Today, the clinic staff spends an hour with a first-time long COVID patient, hearing their stories and helping relieve anxiety, Dr. Azola says.
The phenomenon of long COVID is similar to what patients have had with chronic fatigue syndrome, lupus, or fibromyalgia, where people have symptoms that are hard to explain, says Jennifer Chevinsky, MD, deputy public health officer for Riverside County, Calif.
“Stigma within medicine or health care is nothing new,” she says.
In Chicago, Dr. Jason notes that the federal government’s decision to invest hundreds of millions of dollars in long COVID research “shows the government is helping destigmatize it.”
Dr. Pantelic says she and her colleagues are continuing their research.
“We are interested in understanding the impacts of this stigma, and how to mitigate any adverse outcomes for patients and services,” she says.
A version of this article first appeared on WebMD.com.
PLOS ONE


