User login
-
Med students, doctor groups react to SCOTUS affirmative action ban
The U.S. Supreme Court ruled on June 29 that using race as a factor in college admissions is unconstitutional, rolling back more than 40 years of affirmative action standards and changing how medical schools evaluate applicants to attract students from diverse backgrounds.
Jesse M. Ehrenfeld, MD, MPH, president of the American Medical Association, said in a prepared statement that the Supreme Court ruling will result in a less diverse physician workforce, which is “bad for health care, bad for medicine, and undermines the health of our nation.” He cited the AMA’s recent adoption of a policy advising medical schools to increase enrollment of people from racial and ethnic groups traditionally underrepresented in medicine – even if that means considering race as a factor in admissions criteria.
“Supporting racial and ethnic diversity in the health professions – spanning classrooms, labs, and clinical settings – enriches the educational experiences of all medical and health professions students and the teaching experiences of faculty, and it is essential to improving the overall health of our nation,” the Association of American Medical Colleges (AAMC) said in a prepared statement.
The American Medical Student Association also denounced the Supreme Court decision. “As future physicians committed to justice and equality, we are profoundly outraged ... We strongly support increased representation of minority students in all levels of education, including colleges and medical schools. By fostering diversity and inclusion, institutions have the power to create more empathetic and inclusive learning environments,” the organization said in a press release.
“Diversity in the health care workforce not only benefits underserved patients but improves care for all patients” by increasing understanding and empathy for people of various cultures, Omar T. Atiq, MD, president of the American College of Physicians, said in a press release.
The Supreme Court ruling stems from a lawsuit by the Students for Fair Admissions against Harvard University and the University of North Carolina. The lawsuit alleges that considering race in the college admission process constitutes discrimination and violates the Equal Protection Clause.
Chief Justice John Roberts, who delivered the court’s decision, stated that an applicant’s personal experiences should carry the most weight in admission decisions and that historically, universities have “wrongly concluded that the touchstone of an individual’s identity is not challenges bested, skills built, or lessons learned, but the color of their skin. Our constitutional history does not tolerate that choice.”
Still, Justice Roberts said the opinion does not prohibit universities from considering how race has affected an applicant’s life, “be it through discrimination, inspiration, or otherwise.”
Diversity in medical schools increased last year, with more Black, Hispanic, and female students applying and enrolling. But continued diversity efforts were expected to prove challenging with affirmative action off the table, according to an amicus brief filed last year by the AMA, the AAMC, and dozens of other professional health care organizations.
The brief supported continued use of race in college admissions, stating that eliminating that factor could slow efforts to achieve greater health equity because fewer doctors would be training and working with colleagues from diverse backgrounds.
Several universities with medical programs, such as Yale and Johns Hopkins universities, filed a separate brief citing similar concerns. After the June 29 decision, Harvard and the University of North Carolina released statements stating they would comply with the ruling.
A version of this article first appeared on Medscape.com.
The U.S. Supreme Court ruled on June 29 that using race as a factor in college admissions is unconstitutional, rolling back more than 40 years of affirmative action standards and changing how medical schools evaluate applicants to attract students from diverse backgrounds.
Jesse M. Ehrenfeld, MD, MPH, president of the American Medical Association, said in a prepared statement that the Supreme Court ruling will result in a less diverse physician workforce, which is “bad for health care, bad for medicine, and undermines the health of our nation.” He cited the AMA’s recent adoption of a policy advising medical schools to increase enrollment of people from racial and ethnic groups traditionally underrepresented in medicine – even if that means considering race as a factor in admissions criteria.
“Supporting racial and ethnic diversity in the health professions – spanning classrooms, labs, and clinical settings – enriches the educational experiences of all medical and health professions students and the teaching experiences of faculty, and it is essential to improving the overall health of our nation,” the Association of American Medical Colleges (AAMC) said in a prepared statement.
The American Medical Student Association also denounced the Supreme Court decision. “As future physicians committed to justice and equality, we are profoundly outraged ... We strongly support increased representation of minority students in all levels of education, including colleges and medical schools. By fostering diversity and inclusion, institutions have the power to create more empathetic and inclusive learning environments,” the organization said in a press release.
“Diversity in the health care workforce not only benefits underserved patients but improves care for all patients” by increasing understanding and empathy for people of various cultures, Omar T. Atiq, MD, president of the American College of Physicians, said in a press release.
The Supreme Court ruling stems from a lawsuit by the Students for Fair Admissions against Harvard University and the University of North Carolina. The lawsuit alleges that considering race in the college admission process constitutes discrimination and violates the Equal Protection Clause.
Chief Justice John Roberts, who delivered the court’s decision, stated that an applicant’s personal experiences should carry the most weight in admission decisions and that historically, universities have “wrongly concluded that the touchstone of an individual’s identity is not challenges bested, skills built, or lessons learned, but the color of their skin. Our constitutional history does not tolerate that choice.”
Still, Justice Roberts said the opinion does not prohibit universities from considering how race has affected an applicant’s life, “be it through discrimination, inspiration, or otherwise.”
Diversity in medical schools increased last year, with more Black, Hispanic, and female students applying and enrolling. But continued diversity efforts were expected to prove challenging with affirmative action off the table, according to an amicus brief filed last year by the AMA, the AAMC, and dozens of other professional health care organizations.
The brief supported continued use of race in college admissions, stating that eliminating that factor could slow efforts to achieve greater health equity because fewer doctors would be training and working with colleagues from diverse backgrounds.
Several universities with medical programs, such as Yale and Johns Hopkins universities, filed a separate brief citing similar concerns. After the June 29 decision, Harvard and the University of North Carolina released statements stating they would comply with the ruling.
A version of this article first appeared on Medscape.com.
The U.S. Supreme Court ruled on June 29 that using race as a factor in college admissions is unconstitutional, rolling back more than 40 years of affirmative action standards and changing how medical schools evaluate applicants to attract students from diverse backgrounds.
Jesse M. Ehrenfeld, MD, MPH, president of the American Medical Association, said in a prepared statement that the Supreme Court ruling will result in a less diverse physician workforce, which is “bad for health care, bad for medicine, and undermines the health of our nation.” He cited the AMA’s recent adoption of a policy advising medical schools to increase enrollment of people from racial and ethnic groups traditionally underrepresented in medicine – even if that means considering race as a factor in admissions criteria.
“Supporting racial and ethnic diversity in the health professions – spanning classrooms, labs, and clinical settings – enriches the educational experiences of all medical and health professions students and the teaching experiences of faculty, and it is essential to improving the overall health of our nation,” the Association of American Medical Colleges (AAMC) said in a prepared statement.
The American Medical Student Association also denounced the Supreme Court decision. “As future physicians committed to justice and equality, we are profoundly outraged ... We strongly support increased representation of minority students in all levels of education, including colleges and medical schools. By fostering diversity and inclusion, institutions have the power to create more empathetic and inclusive learning environments,” the organization said in a press release.
“Diversity in the health care workforce not only benefits underserved patients but improves care for all patients” by increasing understanding and empathy for people of various cultures, Omar T. Atiq, MD, president of the American College of Physicians, said in a press release.
The Supreme Court ruling stems from a lawsuit by the Students for Fair Admissions against Harvard University and the University of North Carolina. The lawsuit alleges that considering race in the college admission process constitutes discrimination and violates the Equal Protection Clause.
Chief Justice John Roberts, who delivered the court’s decision, stated that an applicant’s personal experiences should carry the most weight in admission decisions and that historically, universities have “wrongly concluded that the touchstone of an individual’s identity is not challenges bested, skills built, or lessons learned, but the color of their skin. Our constitutional history does not tolerate that choice.”
Still, Justice Roberts said the opinion does not prohibit universities from considering how race has affected an applicant’s life, “be it through discrimination, inspiration, or otherwise.”
Diversity in medical schools increased last year, with more Black, Hispanic, and female students applying and enrolling. But continued diversity efforts were expected to prove challenging with affirmative action off the table, according to an amicus brief filed last year by the AMA, the AAMC, and dozens of other professional health care organizations.
The brief supported continued use of race in college admissions, stating that eliminating that factor could slow efforts to achieve greater health equity because fewer doctors would be training and working with colleagues from diverse backgrounds.
Several universities with medical programs, such as Yale and Johns Hopkins universities, filed a separate brief citing similar concerns. After the June 29 decision, Harvard and the University of North Carolina released statements stating they would comply with the ruling.
A version of this article first appeared on Medscape.com.
CLL combo treatment: Phase-3 study inconclusive
The difference in PFS between the IVO arm, 85%, versus 87% in the IO arm was statistically insignificant.
“Due to the early read-out and the futility boundaries being crossed, long-term follow-up will be critical to understand if there are any long-term benefits to IVO,” said study principal investigator Jennifer A. Woyach MD, professor in the division of hematology at The Ohio State University Comprehensive Care Center (OSUCCC – The James) in Columbus.
The 14-month follow-up data includes results from 465 CLL patients aged 65+ (median age 74 years, 67.5% male) who were treatment naive. The IO and IVO arms had 232 and 233 participants respectively, patients across both arms had Eastern Cooperative Oncology Group scores of 0-1 (97%), occurrence of Del (17p) was 13%, and a Rai stage status of III/IV was 55%, slightly more patients in the IO arm had unmutated IGHV 55% vs. 47% in the IVO arm. Researchers noted that, as expected, patients in the IVO group had a greater occurrence of hematologic adverse events graded at 3 or above, 61% VS 48% in the IO arm, P =.006.
The trial was spurred by the fact that many CLL patients on IO therapy must remain on treatment indefinitely, and an earlier phase II trial suggested that IVO therapy could lead to deep remission and therapy discontinuation.
Looking at the complete response (CR) rates and undetectable minimal residual disease (uMRD) rates across both arms suggested that there may be some hope that IVO could help CLL patients achieve deep remissions and discontinue therapy. Patients in the IVO arm had a CR of 68.5% and uMRD of 86.8% while only 31.3% of those in the IO arm had a CR and 33.3% achieved uMRD status.
“Despite the impressive CR and uMRD results, this study demonstrates that IVO is not superior to IO in terms of progression-free survival. However, because many patients in the IVO arm have discontinued treatment while those in the IO arm remain on ibrutinib, we think that it will be very important to continue to follow these patients long term, to see if there are advantages to this time limited therapy, especially in terms of toxicity, that we cannot appreciate with this follow-up,” said Dr. Woyach.
The Alliance for Clinical Trials in Oncology cooperative group, including OSUCCC James, is currently working to design the next frontline CLL study for older patients that builds on this work.
Dr. Woyach disclosed ties with Abbvie, AstraZeneca, Beigene, Genentech, Janssen, Loxo/Lilly, Merck, Newave, Pharmacyclics, and Schrodinger.
The difference in PFS between the IVO arm, 85%, versus 87% in the IO arm was statistically insignificant.
“Due to the early read-out and the futility boundaries being crossed, long-term follow-up will be critical to understand if there are any long-term benefits to IVO,” said study principal investigator Jennifer A. Woyach MD, professor in the division of hematology at The Ohio State University Comprehensive Care Center (OSUCCC – The James) in Columbus.
The 14-month follow-up data includes results from 465 CLL patients aged 65+ (median age 74 years, 67.5% male) who were treatment naive. The IO and IVO arms had 232 and 233 participants respectively, patients across both arms had Eastern Cooperative Oncology Group scores of 0-1 (97%), occurrence of Del (17p) was 13%, and a Rai stage status of III/IV was 55%, slightly more patients in the IO arm had unmutated IGHV 55% vs. 47% in the IVO arm. Researchers noted that, as expected, patients in the IVO group had a greater occurrence of hematologic adverse events graded at 3 or above, 61% VS 48% in the IO arm, P =.006.
The trial was spurred by the fact that many CLL patients on IO therapy must remain on treatment indefinitely, and an earlier phase II trial suggested that IVO therapy could lead to deep remission and therapy discontinuation.
Looking at the complete response (CR) rates and undetectable minimal residual disease (uMRD) rates across both arms suggested that there may be some hope that IVO could help CLL patients achieve deep remissions and discontinue therapy. Patients in the IVO arm had a CR of 68.5% and uMRD of 86.8% while only 31.3% of those in the IO arm had a CR and 33.3% achieved uMRD status.
“Despite the impressive CR and uMRD results, this study demonstrates that IVO is not superior to IO in terms of progression-free survival. However, because many patients in the IVO arm have discontinued treatment while those in the IO arm remain on ibrutinib, we think that it will be very important to continue to follow these patients long term, to see if there are advantages to this time limited therapy, especially in terms of toxicity, that we cannot appreciate with this follow-up,” said Dr. Woyach.
The Alliance for Clinical Trials in Oncology cooperative group, including OSUCCC James, is currently working to design the next frontline CLL study for older patients that builds on this work.
Dr. Woyach disclosed ties with Abbvie, AstraZeneca, Beigene, Genentech, Janssen, Loxo/Lilly, Merck, Newave, Pharmacyclics, and Schrodinger.
The difference in PFS between the IVO arm, 85%, versus 87% in the IO arm was statistically insignificant.
“Due to the early read-out and the futility boundaries being crossed, long-term follow-up will be critical to understand if there are any long-term benefits to IVO,” said study principal investigator Jennifer A. Woyach MD, professor in the division of hematology at The Ohio State University Comprehensive Care Center (OSUCCC – The James) in Columbus.
The 14-month follow-up data includes results from 465 CLL patients aged 65+ (median age 74 years, 67.5% male) who were treatment naive. The IO and IVO arms had 232 and 233 participants respectively, patients across both arms had Eastern Cooperative Oncology Group scores of 0-1 (97%), occurrence of Del (17p) was 13%, and a Rai stage status of III/IV was 55%, slightly more patients in the IO arm had unmutated IGHV 55% vs. 47% in the IVO arm. Researchers noted that, as expected, patients in the IVO group had a greater occurrence of hematologic adverse events graded at 3 or above, 61% VS 48% in the IO arm, P =.006.
The trial was spurred by the fact that many CLL patients on IO therapy must remain on treatment indefinitely, and an earlier phase II trial suggested that IVO therapy could lead to deep remission and therapy discontinuation.
Looking at the complete response (CR) rates and undetectable minimal residual disease (uMRD) rates across both arms suggested that there may be some hope that IVO could help CLL patients achieve deep remissions and discontinue therapy. Patients in the IVO arm had a CR of 68.5% and uMRD of 86.8% while only 31.3% of those in the IO arm had a CR and 33.3% achieved uMRD status.
“Despite the impressive CR and uMRD results, this study demonstrates that IVO is not superior to IO in terms of progression-free survival. However, because many patients in the IVO arm have discontinued treatment while those in the IO arm remain on ibrutinib, we think that it will be very important to continue to follow these patients long term, to see if there are advantages to this time limited therapy, especially in terms of toxicity, that we cannot appreciate with this follow-up,” said Dr. Woyach.
The Alliance for Clinical Trials in Oncology cooperative group, including OSUCCC James, is currently working to design the next frontline CLL study for older patients that builds on this work.
Dr. Woyach disclosed ties with Abbvie, AstraZeneca, Beigene, Genentech, Janssen, Loxo/Lilly, Merck, Newave, Pharmacyclics, and Schrodinger.
FDA approves first gene therapy for hemophilia A
Valoctocogene roxaparvovec, a one-time, single-dose IV infusion, is the first gene therapy approved in the United States for severe hemophilia A and will cost around $2.9 million. BioMarin has said the price tag reflects “the possibility of freedom from years” of infusions, which come to about $800,000 each year.
However, last December, the Institute for Clinical and Economic Review (ICER) set the upper bounds for the gene therapy at about $1.96 million. The extent to which the gene therapy will provide freedom from infusions, for how long, and in which patients are not completely understood.
Hemophilia A is caused by a mutation in the gene that produces a protein called coagulation factor VIII, which is essential for blood clotting. Valoctocogene roxaparvovec delivers a functional gene to liver cells via an adeno-associated virus serotype 5; the gene instructs the cells to make the missing clotting factor.
“Adults with severe hemophilia A face a lifelong burden, with frequent infusions and a high risk of health complications, including uncontrolled bleeding and irreversible joint damage,” Steven Pipe, MD, professor of pediatrics and pathology at the University of Michigan, Ann Arbor, and an investigator for the phase 3 study that led to the approval, said in a statement. The approval of valoctocogene roxaparvovec “has the potential to transform the way we treat adults based on years of bleed control following a single, one-time infusion.”
About 6,500 U.S. adults live with severe hemophilia A, and BioMarin said it anticipates approximately 2,500 will be eligible to receive the gene therapy following the approval. The U.S. indication is limited to patients without a history of factor VIII inhibitors and without detectable antibodies to the adeno-associated virus serotype 5.
Last August, the European Medicines Agency authorized the gene therapy for use in Europe, but according to Forbes and PharmaPhorum, uptake in Europe has been delayed because of reimbursement issues, given the cost of treatment and clinical uncertainties.
Data to date, however, are promising for most patients. Approval was based on BioMarin’s open-label, single-arm GENEr8-1 study in 134 men with severe congenital hemophilia A. Patients received a single infusion of 6 x 1013 vector genomes per kilogram.
Among the 132 patients available for 2-year evaluation, median factor VIII activity was in the range for mild hemophilia (6%-39% of normal) with an 84.5% reduction in bleeding events from baseline.
More than 80% of participants had no bleeding events requiring treatment, and there was a 98% reduction from baseline in mean use of exogenous factor VIII.
Overall, at 2 years, only 4.5% of patients had factor VIII activity consistent with severe hemophilia A; 9.1% had activity consistent with moderate disease; 59.8% had activity consistent with mild disease, and 26.5% had activity in the normal range above 40 IU/dL.
Trial investigators estimated that the typical half-life of the transgene-derived factor VIII production system is 123 weeks.
Among the six men who resumed prophylaxis, most had fewer bleeding events than when they were on prophylaxis before the infusion. All patients developed antibodies to the virus delivery vector, precluding retreatment.
Elevated alanine aminotransferase levels were the most common adverse event, occurring in 88.8% of patients, who were treated with immunosuppressants for a median of 33 weeks. Elevations persisted at 2 years in 29% of patients.
The other most common adverse events were headache (38.1%), nausea (37.3%), and increases in aspartate aminotransferase (35.1%).
A version of this article first appeared on Medscape.com.
Valoctocogene roxaparvovec, a one-time, single-dose IV infusion, is the first gene therapy approved in the United States for severe hemophilia A and will cost around $2.9 million. BioMarin has said the price tag reflects “the possibility of freedom from years” of infusions, which come to about $800,000 each year.
However, last December, the Institute for Clinical and Economic Review (ICER) set the upper bounds for the gene therapy at about $1.96 million. The extent to which the gene therapy will provide freedom from infusions, for how long, and in which patients are not completely understood.
Hemophilia A is caused by a mutation in the gene that produces a protein called coagulation factor VIII, which is essential for blood clotting. Valoctocogene roxaparvovec delivers a functional gene to liver cells via an adeno-associated virus serotype 5; the gene instructs the cells to make the missing clotting factor.
“Adults with severe hemophilia A face a lifelong burden, with frequent infusions and a high risk of health complications, including uncontrolled bleeding and irreversible joint damage,” Steven Pipe, MD, professor of pediatrics and pathology at the University of Michigan, Ann Arbor, and an investigator for the phase 3 study that led to the approval, said in a statement. The approval of valoctocogene roxaparvovec “has the potential to transform the way we treat adults based on years of bleed control following a single, one-time infusion.”
About 6,500 U.S. adults live with severe hemophilia A, and BioMarin said it anticipates approximately 2,500 will be eligible to receive the gene therapy following the approval. The U.S. indication is limited to patients without a history of factor VIII inhibitors and without detectable antibodies to the adeno-associated virus serotype 5.
Last August, the European Medicines Agency authorized the gene therapy for use in Europe, but according to Forbes and PharmaPhorum, uptake in Europe has been delayed because of reimbursement issues, given the cost of treatment and clinical uncertainties.
Data to date, however, are promising for most patients. Approval was based on BioMarin’s open-label, single-arm GENEr8-1 study in 134 men with severe congenital hemophilia A. Patients received a single infusion of 6 x 1013 vector genomes per kilogram.
Among the 132 patients available for 2-year evaluation, median factor VIII activity was in the range for mild hemophilia (6%-39% of normal) with an 84.5% reduction in bleeding events from baseline.
More than 80% of participants had no bleeding events requiring treatment, and there was a 98% reduction from baseline in mean use of exogenous factor VIII.
Overall, at 2 years, only 4.5% of patients had factor VIII activity consistent with severe hemophilia A; 9.1% had activity consistent with moderate disease; 59.8% had activity consistent with mild disease, and 26.5% had activity in the normal range above 40 IU/dL.
Trial investigators estimated that the typical half-life of the transgene-derived factor VIII production system is 123 weeks.
Among the six men who resumed prophylaxis, most had fewer bleeding events than when they were on prophylaxis before the infusion. All patients developed antibodies to the virus delivery vector, precluding retreatment.
Elevated alanine aminotransferase levels were the most common adverse event, occurring in 88.8% of patients, who were treated with immunosuppressants for a median of 33 weeks. Elevations persisted at 2 years in 29% of patients.
The other most common adverse events were headache (38.1%), nausea (37.3%), and increases in aspartate aminotransferase (35.1%).
A version of this article first appeared on Medscape.com.
Valoctocogene roxaparvovec, a one-time, single-dose IV infusion, is the first gene therapy approved in the United States for severe hemophilia A and will cost around $2.9 million. BioMarin has said the price tag reflects “the possibility of freedom from years” of infusions, which come to about $800,000 each year.
However, last December, the Institute for Clinical and Economic Review (ICER) set the upper bounds for the gene therapy at about $1.96 million. The extent to which the gene therapy will provide freedom from infusions, for how long, and in which patients are not completely understood.
Hemophilia A is caused by a mutation in the gene that produces a protein called coagulation factor VIII, which is essential for blood clotting. Valoctocogene roxaparvovec delivers a functional gene to liver cells via an adeno-associated virus serotype 5; the gene instructs the cells to make the missing clotting factor.
“Adults with severe hemophilia A face a lifelong burden, with frequent infusions and a high risk of health complications, including uncontrolled bleeding and irreversible joint damage,” Steven Pipe, MD, professor of pediatrics and pathology at the University of Michigan, Ann Arbor, and an investigator for the phase 3 study that led to the approval, said in a statement. The approval of valoctocogene roxaparvovec “has the potential to transform the way we treat adults based on years of bleed control following a single, one-time infusion.”
About 6,500 U.S. adults live with severe hemophilia A, and BioMarin said it anticipates approximately 2,500 will be eligible to receive the gene therapy following the approval. The U.S. indication is limited to patients without a history of factor VIII inhibitors and without detectable antibodies to the adeno-associated virus serotype 5.
Last August, the European Medicines Agency authorized the gene therapy for use in Europe, but according to Forbes and PharmaPhorum, uptake in Europe has been delayed because of reimbursement issues, given the cost of treatment and clinical uncertainties.
Data to date, however, are promising for most patients. Approval was based on BioMarin’s open-label, single-arm GENEr8-1 study in 134 men with severe congenital hemophilia A. Patients received a single infusion of 6 x 1013 vector genomes per kilogram.
Among the 132 patients available for 2-year evaluation, median factor VIII activity was in the range for mild hemophilia (6%-39% of normal) with an 84.5% reduction in bleeding events from baseline.
More than 80% of participants had no bleeding events requiring treatment, and there was a 98% reduction from baseline in mean use of exogenous factor VIII.
Overall, at 2 years, only 4.5% of patients had factor VIII activity consistent with severe hemophilia A; 9.1% had activity consistent with moderate disease; 59.8% had activity consistent with mild disease, and 26.5% had activity in the normal range above 40 IU/dL.
Trial investigators estimated that the typical half-life of the transgene-derived factor VIII production system is 123 weeks.
Among the six men who resumed prophylaxis, most had fewer bleeding events than when they were on prophylaxis before the infusion. All patients developed antibodies to the virus delivery vector, precluding retreatment.
Elevated alanine aminotransferase levels were the most common adverse event, occurring in 88.8% of patients, who were treated with immunosuppressants for a median of 33 weeks. Elevations persisted at 2 years in 29% of patients.
The other most common adverse events were headache (38.1%), nausea (37.3%), and increases in aspartate aminotransferase (35.1%).
A version of this article first appeared on Medscape.com.
Placebo effect can be found in a cup of coffee
The best part of waking up is placebo in your cup
Coffee makes the world go round. It’s impossible to picture any workplace without a cast of forlorn characters huddled around the office coffee maker on a Monday morning, imbibing their beverage du jour until they’ve been lifted out of their semi-zombified stupor.
Millions upon millions of people swear by their morning coffee. And if they don’t get that sweet, sweet caffeine boost, they’ll make Garfield and the Boomtown Rats’ opinions of Mondays look tame. And it only makes sense that they’d believe that. After all, caffeine is a stimulant. It helps your brain focus and kicks it into overdrive. Of course drinking a beverage full of caffeine wakes you up. Right?
Not so fast, a group of Portuguese researchers say. That morning cup of coffee? It may actually be a placebo. Cue the dramatic sound effect.
Here’s the scoop: After recruiting a group of coffee drinkers (at least one cup a day), the researchers kept their test subjects off of coffee for at least 3 hours, then performed a brief functional MRI scan on all test subjects. Half an hour later, study participants received either a standard cup of coffee or pure caffeine. Half an hour after consuming their respective study product, the subjects underwent a second MRI.
As expected, both people who consumed coffee and those who consumed pure caffeine showed decreased connectivity in the default mode network after consumption, indicating preparation in the brain to move from resting to working on tasks. However, those who had pure caffeine did not show increased connectivity in the visual and executive control networks, while those who had coffee did. Simply put, caffeine may wake you up, but it doesn’t make you any sharper. Only coffee gets you in shape for that oh-so-important Monday meeting.
This doesn’t make a lot of sense. How can the drug part of coffee not be responsible for every effect the drink gives you? That’s where the placebo comes in, according to the scientists. It’s possible the effect they saw was caused by withdrawal – after just 3 hours? Yikes, hope not – but it’s more likely it comes down to psychology. We expect coffee to wake us up and make us ready for the day, so that’s exactly what it does. Hey, if that’s all it takes, time to convince ourselves that eating an entire pizza is actually an incredibly effective weight loss tool. Don’t let us down now, placebo effect.
Bread, milk, toilet paper, AFib diagnosis
Now consider the shopping cart. It does its job of carrying stuff around the store well enough, but can it lift you out of a semi-zombified stupor in the morning? No. Can it identify undiagnosed atrial fibrillation? Again, no.
Not so fast, say the investigators conducting the SHOPS-AF (Supermarket/Hypermarket Opportunistic Screening for Atrial Fibrillation) study. They built a better shopping cart. Except they call it a trolley, not a cart, since the study was conducted in England, where they sometimes have funny names for things.
Their improved shopping trolley – we’re just going to call it a cart from here on – has an electrocardiogram sensor embedded into the handlebar, so it can effectively detect AFib in shoppers who held it for at least 60 seconds. The sensor lights up red if it detects an irregular heartbeat and green if it does not. Let’s see a cup of coffee do that.
They put 10 of these modified carts in four supermarkets in Liverpool to see what would happen. Would shoppers be able to tell that we secretly replaced the fine coffee they usually serve with Folger’s crystals? Oops. Sorry about that. Coffee on the brain, apparently. Back to the carts.
A total of 2,155 adult shoppers used one of the carts over 2 months, and electrocardiogram data were available for 220 participants who either had a red light on the sensor and/or an irregular pulse that suggested atrial fibrillation. After further review by the SHOPS-AF cardiologist, AFib was diagnosed in 59 shoppers, of whom 39 were previously undiagnosed.
They’re already working to cut the scan time to 30 seconds for SHOPS-AF II, but we’re wondering about a possible flaw in the whole health-care-delivery-through-shopping-cart scenario. When we go to the local super/hyper/megamart, it seems like half of the people trundling up and down the aisles are store employees filling orders for customers who won’t even set foot inside. Is the shopping cart on its way out? Maybe. Who wants to tell the SHOPS-AF II team? Not us.
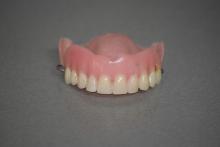
Put pneumonia where your mouth is
Getting dentures does not mean the end of dental care. If anything, new research reveals a huge reason for staying on top of one’s denture care: pneumonia.
It all started with swabs. Scientists in the United Kingdom took mouth, tongue, and denture specimens from frail elderly hospital patients who had pneumonia and wore dentures and from similar patients in care homes who wore dentures and did not have pneumonia. When they compared the microbial populations of the two groups, the investigators found about 20 times the number of respiratory pathogens on the dentures of those with pneumonia.
The research team suggested that dentures may play a role in causing pneumonia, but lead author Josh Twigg, BDS, PhD, also noted that “you certainly couldn’t say that people got pneumonia because they were wearing dentures. It’s just showing that there is an association there.” Improper cleaning, though, could lead to microbial colonization of the dentures, and patients could be inhaling those microbes into their lungs, thereby turning a dental issue into a respiratory issue.
More research needs to be done on the association between dentures and pneumonia, but Dr. Twigg hoped that the results of this study could be presented to the public. The message? “It is important to clean dentures thoroughly” and visit the dentist regularly, he said, but the best way to prevent denture-related infections is to avoid needing to wear dentures entirely.
The best part of waking up is placebo in your cup
Coffee makes the world go round. It’s impossible to picture any workplace without a cast of forlorn characters huddled around the office coffee maker on a Monday morning, imbibing their beverage du jour until they’ve been lifted out of their semi-zombified stupor.
Millions upon millions of people swear by their morning coffee. And if they don’t get that sweet, sweet caffeine boost, they’ll make Garfield and the Boomtown Rats’ opinions of Mondays look tame. And it only makes sense that they’d believe that. After all, caffeine is a stimulant. It helps your brain focus and kicks it into overdrive. Of course drinking a beverage full of caffeine wakes you up. Right?
Not so fast, a group of Portuguese researchers say. That morning cup of coffee? It may actually be a placebo. Cue the dramatic sound effect.
Here’s the scoop: After recruiting a group of coffee drinkers (at least one cup a day), the researchers kept their test subjects off of coffee for at least 3 hours, then performed a brief functional MRI scan on all test subjects. Half an hour later, study participants received either a standard cup of coffee or pure caffeine. Half an hour after consuming their respective study product, the subjects underwent a second MRI.
As expected, both people who consumed coffee and those who consumed pure caffeine showed decreased connectivity in the default mode network after consumption, indicating preparation in the brain to move from resting to working on tasks. However, those who had pure caffeine did not show increased connectivity in the visual and executive control networks, while those who had coffee did. Simply put, caffeine may wake you up, but it doesn’t make you any sharper. Only coffee gets you in shape for that oh-so-important Monday meeting.
This doesn’t make a lot of sense. How can the drug part of coffee not be responsible for every effect the drink gives you? That’s where the placebo comes in, according to the scientists. It’s possible the effect they saw was caused by withdrawal – after just 3 hours? Yikes, hope not – but it’s more likely it comes down to psychology. We expect coffee to wake us up and make us ready for the day, so that’s exactly what it does. Hey, if that’s all it takes, time to convince ourselves that eating an entire pizza is actually an incredibly effective weight loss tool. Don’t let us down now, placebo effect.
Bread, milk, toilet paper, AFib diagnosis
Now consider the shopping cart. It does its job of carrying stuff around the store well enough, but can it lift you out of a semi-zombified stupor in the morning? No. Can it identify undiagnosed atrial fibrillation? Again, no.
Not so fast, say the investigators conducting the SHOPS-AF (Supermarket/Hypermarket Opportunistic Screening for Atrial Fibrillation) study. They built a better shopping cart. Except they call it a trolley, not a cart, since the study was conducted in England, where they sometimes have funny names for things.
Their improved shopping trolley – we’re just going to call it a cart from here on – has an electrocardiogram sensor embedded into the handlebar, so it can effectively detect AFib in shoppers who held it for at least 60 seconds. The sensor lights up red if it detects an irregular heartbeat and green if it does not. Let’s see a cup of coffee do that.
They put 10 of these modified carts in four supermarkets in Liverpool to see what would happen. Would shoppers be able to tell that we secretly replaced the fine coffee they usually serve with Folger’s crystals? Oops. Sorry about that. Coffee on the brain, apparently. Back to the carts.
A total of 2,155 adult shoppers used one of the carts over 2 months, and electrocardiogram data were available for 220 participants who either had a red light on the sensor and/or an irregular pulse that suggested atrial fibrillation. After further review by the SHOPS-AF cardiologist, AFib was diagnosed in 59 shoppers, of whom 39 were previously undiagnosed.
They’re already working to cut the scan time to 30 seconds for SHOPS-AF II, but we’re wondering about a possible flaw in the whole health-care-delivery-through-shopping-cart scenario. When we go to the local super/hyper/megamart, it seems like half of the people trundling up and down the aisles are store employees filling orders for customers who won’t even set foot inside. Is the shopping cart on its way out? Maybe. Who wants to tell the SHOPS-AF II team? Not us.
Put pneumonia where your mouth is
Getting dentures does not mean the end of dental care. If anything, new research reveals a huge reason for staying on top of one’s denture care: pneumonia.
It all started with swabs. Scientists in the United Kingdom took mouth, tongue, and denture specimens from frail elderly hospital patients who had pneumonia and wore dentures and from similar patients in care homes who wore dentures and did not have pneumonia. When they compared the microbial populations of the two groups, the investigators found about 20 times the number of respiratory pathogens on the dentures of those with pneumonia.
The research team suggested that dentures may play a role in causing pneumonia, but lead author Josh Twigg, BDS, PhD, also noted that “you certainly couldn’t say that people got pneumonia because they were wearing dentures. It’s just showing that there is an association there.” Improper cleaning, though, could lead to microbial colonization of the dentures, and patients could be inhaling those microbes into their lungs, thereby turning a dental issue into a respiratory issue.
More research needs to be done on the association between dentures and pneumonia, but Dr. Twigg hoped that the results of this study could be presented to the public. The message? “It is important to clean dentures thoroughly” and visit the dentist regularly, he said, but the best way to prevent denture-related infections is to avoid needing to wear dentures entirely.
The best part of waking up is placebo in your cup
Coffee makes the world go round. It’s impossible to picture any workplace without a cast of forlorn characters huddled around the office coffee maker on a Monday morning, imbibing their beverage du jour until they’ve been lifted out of their semi-zombified stupor.
Millions upon millions of people swear by their morning coffee. And if they don’t get that sweet, sweet caffeine boost, they’ll make Garfield and the Boomtown Rats’ opinions of Mondays look tame. And it only makes sense that they’d believe that. After all, caffeine is a stimulant. It helps your brain focus and kicks it into overdrive. Of course drinking a beverage full of caffeine wakes you up. Right?
Not so fast, a group of Portuguese researchers say. That morning cup of coffee? It may actually be a placebo. Cue the dramatic sound effect.
Here’s the scoop: After recruiting a group of coffee drinkers (at least one cup a day), the researchers kept their test subjects off of coffee for at least 3 hours, then performed a brief functional MRI scan on all test subjects. Half an hour later, study participants received either a standard cup of coffee or pure caffeine. Half an hour after consuming their respective study product, the subjects underwent a second MRI.
As expected, both people who consumed coffee and those who consumed pure caffeine showed decreased connectivity in the default mode network after consumption, indicating preparation in the brain to move from resting to working on tasks. However, those who had pure caffeine did not show increased connectivity in the visual and executive control networks, while those who had coffee did. Simply put, caffeine may wake you up, but it doesn’t make you any sharper. Only coffee gets you in shape for that oh-so-important Monday meeting.
This doesn’t make a lot of sense. How can the drug part of coffee not be responsible for every effect the drink gives you? That’s where the placebo comes in, according to the scientists. It’s possible the effect they saw was caused by withdrawal – after just 3 hours? Yikes, hope not – but it’s more likely it comes down to psychology. We expect coffee to wake us up and make us ready for the day, so that’s exactly what it does. Hey, if that’s all it takes, time to convince ourselves that eating an entire pizza is actually an incredibly effective weight loss tool. Don’t let us down now, placebo effect.
Bread, milk, toilet paper, AFib diagnosis
Now consider the shopping cart. It does its job of carrying stuff around the store well enough, but can it lift you out of a semi-zombified stupor in the morning? No. Can it identify undiagnosed atrial fibrillation? Again, no.
Not so fast, say the investigators conducting the SHOPS-AF (Supermarket/Hypermarket Opportunistic Screening for Atrial Fibrillation) study. They built a better shopping cart. Except they call it a trolley, not a cart, since the study was conducted in England, where they sometimes have funny names for things.
Their improved shopping trolley – we’re just going to call it a cart from here on – has an electrocardiogram sensor embedded into the handlebar, so it can effectively detect AFib in shoppers who held it for at least 60 seconds. The sensor lights up red if it detects an irregular heartbeat and green if it does not. Let’s see a cup of coffee do that.
They put 10 of these modified carts in four supermarkets in Liverpool to see what would happen. Would shoppers be able to tell that we secretly replaced the fine coffee they usually serve with Folger’s crystals? Oops. Sorry about that. Coffee on the brain, apparently. Back to the carts.
A total of 2,155 adult shoppers used one of the carts over 2 months, and electrocardiogram data were available for 220 participants who either had a red light on the sensor and/or an irregular pulse that suggested atrial fibrillation. After further review by the SHOPS-AF cardiologist, AFib was diagnosed in 59 shoppers, of whom 39 were previously undiagnosed.
They’re already working to cut the scan time to 30 seconds for SHOPS-AF II, but we’re wondering about a possible flaw in the whole health-care-delivery-through-shopping-cart scenario. When we go to the local super/hyper/megamart, it seems like half of the people trundling up and down the aisles are store employees filling orders for customers who won’t even set foot inside. Is the shopping cart on its way out? Maybe. Who wants to tell the SHOPS-AF II team? Not us.
Put pneumonia where your mouth is
Getting dentures does not mean the end of dental care. If anything, new research reveals a huge reason for staying on top of one’s denture care: pneumonia.
It all started with swabs. Scientists in the United Kingdom took mouth, tongue, and denture specimens from frail elderly hospital patients who had pneumonia and wore dentures and from similar patients in care homes who wore dentures and did not have pneumonia. When they compared the microbial populations of the two groups, the investigators found about 20 times the number of respiratory pathogens on the dentures of those with pneumonia.
The research team suggested that dentures may play a role in causing pneumonia, but lead author Josh Twigg, BDS, PhD, also noted that “you certainly couldn’t say that people got pneumonia because they were wearing dentures. It’s just showing that there is an association there.” Improper cleaning, though, could lead to microbial colonization of the dentures, and patients could be inhaling those microbes into their lungs, thereby turning a dental issue into a respiratory issue.
More research needs to be done on the association between dentures and pneumonia, but Dr. Twigg hoped that the results of this study could be presented to the public. The message? “It is important to clean dentures thoroughly” and visit the dentist regularly, he said, but the best way to prevent denture-related infections is to avoid needing to wear dentures entirely.
ESMO helps hematologists assess new cancer drugs
It consists of 11 2- to 3-page forms with checklists to grade treatment trials on the extent to which they meet efficacy and safety thresholds. Each of the 11 forms covers a specific trial scenario, such as a randomized controlled trial with curative intent or a trial of a therapy that is not likely to be curative with a primary endpoint of overall survival.
Treatments with curative intent are graded A, B, or C, while treatments in the noncurative setting are graded on a descending scale from 5 to 1. Scores of A and B in the curative setting and 5 and 4 in the noncurative setting represent substantial benefit.
On the form for RCTs with curative intent, for instance, a survival improvement of 5% or more garners an A but an improvement of less than 3% gets a C. Scores are also annotated for serious acute and/or persistent toxicity if present.
The tool, dubbed the ESMO-MCBS:H (European Society for Medical Oncology Magnitude of Clinical Benefit Scale: Hematology), is explained in an article published in Annals of Oncology. The evaluation forms are available online.
The idea behind the work is to help health care professionals and others to more “accurately assess the value of and prioritise therapies for patients with blood cancers. For clinicians, ESMO-MCBS:H will aid in their clinical decision-making and in the development of evidence-based practice and guidelines,” ESMO said in a press release.
To develop ESMO-MCBS:H, the group tailored its tool for evaluating solid tumor therapies, the ESMO-MCBS, to account for the sometimes different endpoints used in hematologic malignancy trials and the very indolent nature of some blood cancers, such as follicular lymphoma, which hampers development of mature data.
Specific changes include adding a new evaluation form to grade single-arm trials with curative intent, such as those used for CAR-T-cell therapies; incorporating molecular surrogate endpoints used in CML trials; and adding a way to grade outcomes for indolent cancers, among others.
The development process included applying the solid tumor tool to 80 blood cancer studies to identify shortcomings and improve its applicability. The final tool was field tested with 51 international experts from EHA and ESMO who largely agreed on the reasonableness of the trial scores.
ESMO said it expects ESMO-MCBS:H will be useful. The solid tumor tool, first published in 2015, is used by the World Health Organization to screen medications for its essential medicines list as well as by ESMO to generate guidelines and oncology centers across Europe to help with resource allocation decisions.
It consists of 11 2- to 3-page forms with checklists to grade treatment trials on the extent to which they meet efficacy and safety thresholds. Each of the 11 forms covers a specific trial scenario, such as a randomized controlled trial with curative intent or a trial of a therapy that is not likely to be curative with a primary endpoint of overall survival.
Treatments with curative intent are graded A, B, or C, while treatments in the noncurative setting are graded on a descending scale from 5 to 1. Scores of A and B in the curative setting and 5 and 4 in the noncurative setting represent substantial benefit.
On the form for RCTs with curative intent, for instance, a survival improvement of 5% or more garners an A but an improvement of less than 3% gets a C. Scores are also annotated for serious acute and/or persistent toxicity if present.
The tool, dubbed the ESMO-MCBS:H (European Society for Medical Oncology Magnitude of Clinical Benefit Scale: Hematology), is explained in an article published in Annals of Oncology. The evaluation forms are available online.
The idea behind the work is to help health care professionals and others to more “accurately assess the value of and prioritise therapies for patients with blood cancers. For clinicians, ESMO-MCBS:H will aid in their clinical decision-making and in the development of evidence-based practice and guidelines,” ESMO said in a press release.
To develop ESMO-MCBS:H, the group tailored its tool for evaluating solid tumor therapies, the ESMO-MCBS, to account for the sometimes different endpoints used in hematologic malignancy trials and the very indolent nature of some blood cancers, such as follicular lymphoma, which hampers development of mature data.
Specific changes include adding a new evaluation form to grade single-arm trials with curative intent, such as those used for CAR-T-cell therapies; incorporating molecular surrogate endpoints used in CML trials; and adding a way to grade outcomes for indolent cancers, among others.
The development process included applying the solid tumor tool to 80 blood cancer studies to identify shortcomings and improve its applicability. The final tool was field tested with 51 international experts from EHA and ESMO who largely agreed on the reasonableness of the trial scores.
ESMO said it expects ESMO-MCBS:H will be useful. The solid tumor tool, first published in 2015, is used by the World Health Organization to screen medications for its essential medicines list as well as by ESMO to generate guidelines and oncology centers across Europe to help with resource allocation decisions.
It consists of 11 2- to 3-page forms with checklists to grade treatment trials on the extent to which they meet efficacy and safety thresholds. Each of the 11 forms covers a specific trial scenario, such as a randomized controlled trial with curative intent or a trial of a therapy that is not likely to be curative with a primary endpoint of overall survival.
Treatments with curative intent are graded A, B, or C, while treatments in the noncurative setting are graded on a descending scale from 5 to 1. Scores of A and B in the curative setting and 5 and 4 in the noncurative setting represent substantial benefit.
On the form for RCTs with curative intent, for instance, a survival improvement of 5% or more garners an A but an improvement of less than 3% gets a C. Scores are also annotated for serious acute and/or persistent toxicity if present.
The tool, dubbed the ESMO-MCBS:H (European Society for Medical Oncology Magnitude of Clinical Benefit Scale: Hematology), is explained in an article published in Annals of Oncology. The evaluation forms are available online.
The idea behind the work is to help health care professionals and others to more “accurately assess the value of and prioritise therapies for patients with blood cancers. For clinicians, ESMO-MCBS:H will aid in their clinical decision-making and in the development of evidence-based practice and guidelines,” ESMO said in a press release.
To develop ESMO-MCBS:H, the group tailored its tool for evaluating solid tumor therapies, the ESMO-MCBS, to account for the sometimes different endpoints used in hematologic malignancy trials and the very indolent nature of some blood cancers, such as follicular lymphoma, which hampers development of mature data.
Specific changes include adding a new evaluation form to grade single-arm trials with curative intent, such as those used for CAR-T-cell therapies; incorporating molecular surrogate endpoints used in CML trials; and adding a way to grade outcomes for indolent cancers, among others.
The development process included applying the solid tumor tool to 80 blood cancer studies to identify shortcomings and improve its applicability. The final tool was field tested with 51 international experts from EHA and ESMO who largely agreed on the reasonableness of the trial scores.
ESMO said it expects ESMO-MCBS:H will be useful. The solid tumor tool, first published in 2015, is used by the World Health Organization to screen medications for its essential medicines list as well as by ESMO to generate guidelines and oncology centers across Europe to help with resource allocation decisions.
FROM ANNALS OF ONCOLOGY
Multiprong strategy makes clinical trials less White
CHICAGO – Clinical trials are so White. Only a small percentage of eligible patients participate in clinical trials in the first place, and very few come from racial and ethnic minority groups.
For example, according to the Food and Drug Administration, in trials that resulted in drug approvals from 2017 to 2020, only 2%-5% of participants were Black patients.
When clinical trials lack diverse patient populations, those who are left out have fewer opportunities to get new therapies. Moreover, the scope of the research is limited by smaller phenotypic and genotypic samples, and the trial results are applicable only to more homogeneous patient groups.
There has been a push to include more underrepresented patients in clinical trials. One group reported its success in doing so here at the annual meeting of the American Society of Clinical Oncology.
a period that included a pandemic-induced hiatus in clinical trials in general.
Alliance member Electra D. Paskett, PhD, from the College of Public Health at the Ohio State University in Columbus, presented accrual data from 117 trials led by the Alliance from 2014 to 2022.
During this period, accrual of racial and ethnic minority patients increased from 13.6% to 25.3% for cancer treatment trials and from 13% to 21.5% for cancer control trials.
Overall, the recruitment program resulted in an absolute increase from 13.5 % to 23.6% of underrepresented populations, which translated into a relative 74.8% improvement.
“We’re focusing now on monitoring accrual of women, rural populations, younger AYAs [adolescents and young adults] and older patients, and we’ll see what strategies we need to implement,” Dr. Packett told this news organization.
The Alliance has implemented a real-time accrual dashboard on its website that allows individual sites to review accrual by trial and overall for all of the identified underrepresented populations, she noted.
Program to increase underrepresented patient accrual
The impetus for the program to increase enrollment of underrepresented patients came from the goal set by Monica M. Bertagnolli, MD, group chair of the Alliance from 2011 to 2022 and currently the director of the U.S. National Cancer Institute.
“Our leader, Dr. Bertagnolli, set out a group-wide goal for accrual of underrepresented minorities to our trials of 20%, and that gave us permission to implement a whole host of new strategies,” Dr. Paskett said in an interview.
“These strategies follow the Accrual of Clinical Trials framework, which essentially says that the interaction between the patient and the provider for going on a clinical trial is not just an interaction between the patient and provider but recognizes, for example, that the provider has coworkers and they have norms and beliefs and attitudes, and the patient comes from a family with their own values. And then there are system-level barriers, and there are community barriers that all relate to this interaction about going on a trial,” Dr. Packett said.
What works?
The study was presented as a poster at the meeting. During the poster discussion session, comoderator Victoria S. Blinder, MD, from Memorial Sloan Kettering Cancer Center in New York, asked Dr. Paskett, “If you had a certain amount of money and you really wanted to use that resource to focus on one area, where would you put that resource?”
“I’m going to violate the rules of your question,” Dr. Paskett replied.
“You cannot change this problem by focusing on one thing, and that’s what we showed in our Alliance poster, and what I’ve said is based on over 30 years of work in this area,” she said.
She cited what she considered as the two most important components for improving accrual of underrepresented populations: a commitment by leadership to a recruitment goal, and the development of protocols with specific accrual goals for minority populations.
Still, those are only two components of a comprehensive program that includes the aforementioned accrual goal set by Dr. Bertagnolli, as well as the following:
- Funding of minority junior investigators and research that focuses on issues of concern to underrepresented populations.
- Establishment of work groups that focus on specific populations with the Alliance health disparities committee.
- Translation of informational materials for patients.
- Opening studies at National Cancer Institute Community. Oncology Research Program–designated minority underserved sites.
- Real-time monitoring of accrual demographics by the Alliance and at the trial site.
- Closing protocol enrollment to majority populations.
- Increasing the study sample sizes to enroll additional minority participants and to allow for subgroup analyses.
The study was funded by the National Institutes of Health. Dr. Packett and Dr. Blinder reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
CHICAGO – Clinical trials are so White. Only a small percentage of eligible patients participate in clinical trials in the first place, and very few come from racial and ethnic minority groups.
For example, according to the Food and Drug Administration, in trials that resulted in drug approvals from 2017 to 2020, only 2%-5% of participants were Black patients.
When clinical trials lack diverse patient populations, those who are left out have fewer opportunities to get new therapies. Moreover, the scope of the research is limited by smaller phenotypic and genotypic samples, and the trial results are applicable only to more homogeneous patient groups.
There has been a push to include more underrepresented patients in clinical trials. One group reported its success in doing so here at the annual meeting of the American Society of Clinical Oncology.
a period that included a pandemic-induced hiatus in clinical trials in general.
Alliance member Electra D. Paskett, PhD, from the College of Public Health at the Ohio State University in Columbus, presented accrual data from 117 trials led by the Alliance from 2014 to 2022.
During this period, accrual of racial and ethnic minority patients increased from 13.6% to 25.3% for cancer treatment trials and from 13% to 21.5% for cancer control trials.
Overall, the recruitment program resulted in an absolute increase from 13.5 % to 23.6% of underrepresented populations, which translated into a relative 74.8% improvement.
“We’re focusing now on monitoring accrual of women, rural populations, younger AYAs [adolescents and young adults] and older patients, and we’ll see what strategies we need to implement,” Dr. Packett told this news organization.
The Alliance has implemented a real-time accrual dashboard on its website that allows individual sites to review accrual by trial and overall for all of the identified underrepresented populations, she noted.
Program to increase underrepresented patient accrual
The impetus for the program to increase enrollment of underrepresented patients came from the goal set by Monica M. Bertagnolli, MD, group chair of the Alliance from 2011 to 2022 and currently the director of the U.S. National Cancer Institute.
“Our leader, Dr. Bertagnolli, set out a group-wide goal for accrual of underrepresented minorities to our trials of 20%, and that gave us permission to implement a whole host of new strategies,” Dr. Paskett said in an interview.
“These strategies follow the Accrual of Clinical Trials framework, which essentially says that the interaction between the patient and the provider for going on a clinical trial is not just an interaction between the patient and provider but recognizes, for example, that the provider has coworkers and they have norms and beliefs and attitudes, and the patient comes from a family with their own values. And then there are system-level barriers, and there are community barriers that all relate to this interaction about going on a trial,” Dr. Packett said.
What works?
The study was presented as a poster at the meeting. During the poster discussion session, comoderator Victoria S. Blinder, MD, from Memorial Sloan Kettering Cancer Center in New York, asked Dr. Paskett, “If you had a certain amount of money and you really wanted to use that resource to focus on one area, where would you put that resource?”
“I’m going to violate the rules of your question,” Dr. Paskett replied.
“You cannot change this problem by focusing on one thing, and that’s what we showed in our Alliance poster, and what I’ve said is based on over 30 years of work in this area,” she said.
She cited what she considered as the two most important components for improving accrual of underrepresented populations: a commitment by leadership to a recruitment goal, and the development of protocols with specific accrual goals for minority populations.
Still, those are only two components of a comprehensive program that includes the aforementioned accrual goal set by Dr. Bertagnolli, as well as the following:
- Funding of minority junior investigators and research that focuses on issues of concern to underrepresented populations.
- Establishment of work groups that focus on specific populations with the Alliance health disparities committee.
- Translation of informational materials for patients.
- Opening studies at National Cancer Institute Community. Oncology Research Program–designated minority underserved sites.
- Real-time monitoring of accrual demographics by the Alliance and at the trial site.
- Closing protocol enrollment to majority populations.
- Increasing the study sample sizes to enroll additional minority participants and to allow for subgroup analyses.
The study was funded by the National Institutes of Health. Dr. Packett and Dr. Blinder reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
CHICAGO – Clinical trials are so White. Only a small percentage of eligible patients participate in clinical trials in the first place, and very few come from racial and ethnic minority groups.
For example, according to the Food and Drug Administration, in trials that resulted in drug approvals from 2017 to 2020, only 2%-5% of participants were Black patients.
When clinical trials lack diverse patient populations, those who are left out have fewer opportunities to get new therapies. Moreover, the scope of the research is limited by smaller phenotypic and genotypic samples, and the trial results are applicable only to more homogeneous patient groups.
There has been a push to include more underrepresented patients in clinical trials. One group reported its success in doing so here at the annual meeting of the American Society of Clinical Oncology.
a period that included a pandemic-induced hiatus in clinical trials in general.
Alliance member Electra D. Paskett, PhD, from the College of Public Health at the Ohio State University in Columbus, presented accrual data from 117 trials led by the Alliance from 2014 to 2022.
During this period, accrual of racial and ethnic minority patients increased from 13.6% to 25.3% for cancer treatment trials and from 13% to 21.5% for cancer control trials.
Overall, the recruitment program resulted in an absolute increase from 13.5 % to 23.6% of underrepresented populations, which translated into a relative 74.8% improvement.
“We’re focusing now on monitoring accrual of women, rural populations, younger AYAs [adolescents and young adults] and older patients, and we’ll see what strategies we need to implement,” Dr. Packett told this news organization.
The Alliance has implemented a real-time accrual dashboard on its website that allows individual sites to review accrual by trial and overall for all of the identified underrepresented populations, she noted.
Program to increase underrepresented patient accrual
The impetus for the program to increase enrollment of underrepresented patients came from the goal set by Monica M. Bertagnolli, MD, group chair of the Alliance from 2011 to 2022 and currently the director of the U.S. National Cancer Institute.
“Our leader, Dr. Bertagnolli, set out a group-wide goal for accrual of underrepresented minorities to our trials of 20%, and that gave us permission to implement a whole host of new strategies,” Dr. Paskett said in an interview.
“These strategies follow the Accrual of Clinical Trials framework, which essentially says that the interaction between the patient and the provider for going on a clinical trial is not just an interaction between the patient and provider but recognizes, for example, that the provider has coworkers and they have norms and beliefs and attitudes, and the patient comes from a family with their own values. And then there are system-level barriers, and there are community barriers that all relate to this interaction about going on a trial,” Dr. Packett said.
What works?
The study was presented as a poster at the meeting. During the poster discussion session, comoderator Victoria S. Blinder, MD, from Memorial Sloan Kettering Cancer Center in New York, asked Dr. Paskett, “If you had a certain amount of money and you really wanted to use that resource to focus on one area, where would you put that resource?”
“I’m going to violate the rules of your question,” Dr. Paskett replied.
“You cannot change this problem by focusing on one thing, and that’s what we showed in our Alliance poster, and what I’ve said is based on over 30 years of work in this area,” she said.
She cited what she considered as the two most important components for improving accrual of underrepresented populations: a commitment by leadership to a recruitment goal, and the development of protocols with specific accrual goals for minority populations.
Still, those are only two components of a comprehensive program that includes the aforementioned accrual goal set by Dr. Bertagnolli, as well as the following:
- Funding of minority junior investigators and research that focuses on issues of concern to underrepresented populations.
- Establishment of work groups that focus on specific populations with the Alliance health disparities committee.
- Translation of informational materials for patients.
- Opening studies at National Cancer Institute Community. Oncology Research Program–designated minority underserved sites.
- Real-time monitoring of accrual demographics by the Alliance and at the trial site.
- Closing protocol enrollment to majority populations.
- Increasing the study sample sizes to enroll additional minority participants and to allow for subgroup analyses.
The study was funded by the National Institutes of Health. Dr. Packett and Dr. Blinder reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
AT ASCO 2023
CBSM phone app eases anxiety, depression in cancer patients
CHICAGO – One-third of patients with cancer also experience anxiety or depression, and an estimated 70% of the 18 million patients with cancer and cancer survivors in the US experience emotional symptoms, including fear of recurrence.
Despite many having these symptoms, few patients with cancer have access to psycho-oncologic support.
A digital cognitive-behavioral stress management (CBSM) application may help to ease some of the burden, reported Allison Ramiller, MPH, of Blue Note Therapeutics in San Francisco, which developed the app version of the program.
In addition, patients assigned to the CBSM app were twice as likely as control persons to report that their symptoms were “much” or “very much” improved after using the app for 12 weeks, Ms. Ramiller reported at an oral abstract session at the annual meeting of the American Society of Clinical Oncology (ASCO).
However, the investigators did not report baseline characteristics of patients in each of the study arms, which might have helped to clarify the depth of the effects they saw.
The CBSM program was developed by Michael H. Antoni, PhD, and colleagues in the University of Miami Health System. It is based on cognitive-behavioral therapy but also includes stress management and relaxation techniques to help patients cope with cancer-specific stress.
“”It has been clinically validated and shown to benefit patients with cancer,” Ms. Ramiller said. “However, access is a problem,” she said.
“There aren’t enough qualified, trained providers for the need, and patients with cancer encounter barriers to in-person participation, including things like transportation or financial barriers. So to overcome this, we developed a digitized version of CBSM,” she explained.
Impressive and elegant
“Everything about [the study] I thought was very impressive, very elegant, very nicely done,” said invited discussant Raymond U. Osarogiagbon, MBBS, FACP, chief scientist at Baptist Memorial Health Care Corp in Memphis, Tenn.
“They showed efficacy, they showed safety – very nice – user friendliness – very good. Certainly they look like they’re trying to address a highly important, unmet need in a very elegant way. Certainly, they pointed out it needs longer follow-up to see sustainability. We need to see will this work in other settings. Will this be cost-effective? You’ve gotta believe it probably will be,” he said.
CBSM has previously been shown to help patients with cancer reduce stress, improve general and cancer-specific quality of life at various stages of treatment, reduce symptom burden, and improve coping skills, Ms. Ramiller said.
To see whether these benefits could be conveyed digitally rather than in face-to-face encounters, Ms. Ramiller and colleagues worked with Dr. Antoni to develop the CBSM app.
Patients using the app received therapeutic content over 10 sessions with audio, video, and interactive tools that mimicked the sessions they would have received during in-person interventions.
They then compared the app against the control educational app in the randomized, decentralized RESTORE study.
High-quality control
Ms. Ramiller said that the control app set “a high bar.”
“The control also offered 10 interactive self-guided sessions. Both treatment apps were professionally designed and visually similar in styling, and they were presented as digital therapeutic-specific for cancer patients. And they were also in a match condition, meaning they received the same attention from study staff and cadence of reminders, but importantly, only the intervention app was based on CBSM,” she explained.
A total of 449 patients with cancers of stage I–III who were undergoing active systemic treatment or were planning to undergo such treatment within 6 months were randomly assigned to the CBSM app or the control app.
The CBSM app was superior to the control app for the primary outcome of anxiety reduction over baseline, as measured at 4, 8 and 12 weeks by the Patient-Reported Outcomes Measurement Information System Anxiety Scale (PROMIS-A) (beta = -.03; P = .019).
CBSM was also significantly better than the control app for the secondary endpoints of reducing symptoms of depression, as measured by the PROMIS-D scale (beta = -.02, P = .042), and also at increasing the percentage of patients who reported improvement in anxiety and depression symptoms on the Patient Global Impression of Change instrument (P < .001)
An extension study of the durability of the effects at 3 and 6 months is underway.
The investigators noted that the incremental cost of management of anxiety or depression is greater than $17,000 per patient per year.
“One of the big promises of a digital therapeutic like this is that it could potentially reduce costs,” Ms. Ramiller told the audience, but she acknowledged, “More work is really needed, however, to directly test the potential savings.”
The RESTORE study is funded by Blue Note Therapeutics. Dr. Osarogiagbon owns stock in Gilead, Lilly, and Pfizer, has received honoraria from Biodesix and Medscape, and has a consulting or advisory role for the American Cancer Society AstraZeneca, Genentech/Roche, LUNGevity, National Cancer Institute, and Triptych Health Partners.
A version of this article originally appeared on Medscape.com.
CHICAGO – One-third of patients with cancer also experience anxiety or depression, and an estimated 70% of the 18 million patients with cancer and cancer survivors in the US experience emotional symptoms, including fear of recurrence.
Despite many having these symptoms, few patients with cancer have access to psycho-oncologic support.
A digital cognitive-behavioral stress management (CBSM) application may help to ease some of the burden, reported Allison Ramiller, MPH, of Blue Note Therapeutics in San Francisco, which developed the app version of the program.
In addition, patients assigned to the CBSM app were twice as likely as control persons to report that their symptoms were “much” or “very much” improved after using the app for 12 weeks, Ms. Ramiller reported at an oral abstract session at the annual meeting of the American Society of Clinical Oncology (ASCO).
However, the investigators did not report baseline characteristics of patients in each of the study arms, which might have helped to clarify the depth of the effects they saw.
The CBSM program was developed by Michael H. Antoni, PhD, and colleagues in the University of Miami Health System. It is based on cognitive-behavioral therapy but also includes stress management and relaxation techniques to help patients cope with cancer-specific stress.
“”It has been clinically validated and shown to benefit patients with cancer,” Ms. Ramiller said. “However, access is a problem,” she said.
“There aren’t enough qualified, trained providers for the need, and patients with cancer encounter barriers to in-person participation, including things like transportation or financial barriers. So to overcome this, we developed a digitized version of CBSM,” she explained.
Impressive and elegant
“Everything about [the study] I thought was very impressive, very elegant, very nicely done,” said invited discussant Raymond U. Osarogiagbon, MBBS, FACP, chief scientist at Baptist Memorial Health Care Corp in Memphis, Tenn.
“They showed efficacy, they showed safety – very nice – user friendliness – very good. Certainly they look like they’re trying to address a highly important, unmet need in a very elegant way. Certainly, they pointed out it needs longer follow-up to see sustainability. We need to see will this work in other settings. Will this be cost-effective? You’ve gotta believe it probably will be,” he said.
CBSM has previously been shown to help patients with cancer reduce stress, improve general and cancer-specific quality of life at various stages of treatment, reduce symptom burden, and improve coping skills, Ms. Ramiller said.
To see whether these benefits could be conveyed digitally rather than in face-to-face encounters, Ms. Ramiller and colleagues worked with Dr. Antoni to develop the CBSM app.
Patients using the app received therapeutic content over 10 sessions with audio, video, and interactive tools that mimicked the sessions they would have received during in-person interventions.
They then compared the app against the control educational app in the randomized, decentralized RESTORE study.
High-quality control
Ms. Ramiller said that the control app set “a high bar.”
“The control also offered 10 interactive self-guided sessions. Both treatment apps were professionally designed and visually similar in styling, and they were presented as digital therapeutic-specific for cancer patients. And they were also in a match condition, meaning they received the same attention from study staff and cadence of reminders, but importantly, only the intervention app was based on CBSM,” she explained.
A total of 449 patients with cancers of stage I–III who were undergoing active systemic treatment or were planning to undergo such treatment within 6 months were randomly assigned to the CBSM app or the control app.
The CBSM app was superior to the control app for the primary outcome of anxiety reduction over baseline, as measured at 4, 8 and 12 weeks by the Patient-Reported Outcomes Measurement Information System Anxiety Scale (PROMIS-A) (beta = -.03; P = .019).
CBSM was also significantly better than the control app for the secondary endpoints of reducing symptoms of depression, as measured by the PROMIS-D scale (beta = -.02, P = .042), and also at increasing the percentage of patients who reported improvement in anxiety and depression symptoms on the Patient Global Impression of Change instrument (P < .001)
An extension study of the durability of the effects at 3 and 6 months is underway.
The investigators noted that the incremental cost of management of anxiety or depression is greater than $17,000 per patient per year.
“One of the big promises of a digital therapeutic like this is that it could potentially reduce costs,” Ms. Ramiller told the audience, but she acknowledged, “More work is really needed, however, to directly test the potential savings.”
The RESTORE study is funded by Blue Note Therapeutics. Dr. Osarogiagbon owns stock in Gilead, Lilly, and Pfizer, has received honoraria from Biodesix and Medscape, and has a consulting or advisory role for the American Cancer Society AstraZeneca, Genentech/Roche, LUNGevity, National Cancer Institute, and Triptych Health Partners.
A version of this article originally appeared on Medscape.com.
CHICAGO – One-third of patients with cancer also experience anxiety or depression, and an estimated 70% of the 18 million patients with cancer and cancer survivors in the US experience emotional symptoms, including fear of recurrence.
Despite many having these symptoms, few patients with cancer have access to psycho-oncologic support.
A digital cognitive-behavioral stress management (CBSM) application may help to ease some of the burden, reported Allison Ramiller, MPH, of Blue Note Therapeutics in San Francisco, which developed the app version of the program.
In addition, patients assigned to the CBSM app were twice as likely as control persons to report that their symptoms were “much” or “very much” improved after using the app for 12 weeks, Ms. Ramiller reported at an oral abstract session at the annual meeting of the American Society of Clinical Oncology (ASCO).
However, the investigators did not report baseline characteristics of patients in each of the study arms, which might have helped to clarify the depth of the effects they saw.
The CBSM program was developed by Michael H. Antoni, PhD, and colleagues in the University of Miami Health System. It is based on cognitive-behavioral therapy but also includes stress management and relaxation techniques to help patients cope with cancer-specific stress.
“”It has been clinically validated and shown to benefit patients with cancer,” Ms. Ramiller said. “However, access is a problem,” she said.
“There aren’t enough qualified, trained providers for the need, and patients with cancer encounter barriers to in-person participation, including things like transportation or financial barriers. So to overcome this, we developed a digitized version of CBSM,” she explained.
Impressive and elegant
“Everything about [the study] I thought was very impressive, very elegant, very nicely done,” said invited discussant Raymond U. Osarogiagbon, MBBS, FACP, chief scientist at Baptist Memorial Health Care Corp in Memphis, Tenn.
“They showed efficacy, they showed safety – very nice – user friendliness – very good. Certainly they look like they’re trying to address a highly important, unmet need in a very elegant way. Certainly, they pointed out it needs longer follow-up to see sustainability. We need to see will this work in other settings. Will this be cost-effective? You’ve gotta believe it probably will be,” he said.
CBSM has previously been shown to help patients with cancer reduce stress, improve general and cancer-specific quality of life at various stages of treatment, reduce symptom burden, and improve coping skills, Ms. Ramiller said.
To see whether these benefits could be conveyed digitally rather than in face-to-face encounters, Ms. Ramiller and colleagues worked with Dr. Antoni to develop the CBSM app.
Patients using the app received therapeutic content over 10 sessions with audio, video, and interactive tools that mimicked the sessions they would have received during in-person interventions.
They then compared the app against the control educational app in the randomized, decentralized RESTORE study.
High-quality control
Ms. Ramiller said that the control app set “a high bar.”
“The control also offered 10 interactive self-guided sessions. Both treatment apps were professionally designed and visually similar in styling, and they were presented as digital therapeutic-specific for cancer patients. And they were also in a match condition, meaning they received the same attention from study staff and cadence of reminders, but importantly, only the intervention app was based on CBSM,” she explained.
A total of 449 patients with cancers of stage I–III who were undergoing active systemic treatment or were planning to undergo such treatment within 6 months were randomly assigned to the CBSM app or the control app.
The CBSM app was superior to the control app for the primary outcome of anxiety reduction over baseline, as measured at 4, 8 and 12 weeks by the Patient-Reported Outcomes Measurement Information System Anxiety Scale (PROMIS-A) (beta = -.03; P = .019).
CBSM was also significantly better than the control app for the secondary endpoints of reducing symptoms of depression, as measured by the PROMIS-D scale (beta = -.02, P = .042), and also at increasing the percentage of patients who reported improvement in anxiety and depression symptoms on the Patient Global Impression of Change instrument (P < .001)
An extension study of the durability of the effects at 3 and 6 months is underway.
The investigators noted that the incremental cost of management of anxiety or depression is greater than $17,000 per patient per year.
“One of the big promises of a digital therapeutic like this is that it could potentially reduce costs,” Ms. Ramiller told the audience, but she acknowledged, “More work is really needed, however, to directly test the potential savings.”
The RESTORE study is funded by Blue Note Therapeutics. Dr. Osarogiagbon owns stock in Gilead, Lilly, and Pfizer, has received honoraria from Biodesix and Medscape, and has a consulting or advisory role for the American Cancer Society AstraZeneca, Genentech/Roche, LUNGevity, National Cancer Institute, and Triptych Health Partners.
A version of this article originally appeared on Medscape.com.
AT ASCO 2023
CAR T-cell benefit in lenalidomide-refractory myeloma
New results show that such patients benefit from treatment with the chimeric antigen receptor T-cell (CAR T) construct ciltacabtagene autoleucel (cilta-cel) (Carvykti).
The finding comes from the phase 3 CARTITUDE-4 trial, which was reported at the annual meeting of the American Society of Clinical Oncology (ASCO) and was simultaneously published online in the New England Journal of Medicine.
Patients with lenalidomide-refractory multiple myeloma who received a single infusion of ciltacabtagene autoleucel demonstrated a 74% reduction in the risk for disease progression or death, compared with patients who received the standard of care.
The hazard ratio for death or progression with cilta-cel was 0.26 (P < .001), which “is the best hazard ratio ever reported in this patient population in a randomized clinical setting,” said principal investigator Binod Dhakal, MD, from the Medical College of Wisconsin, Milwaukee.
Dr. Dhakal reported data from the first analysis of the trial. At a median follow-up of 15.9 months, median progression-free survival (PFS), the primary endpoint, had not been reached among 208 patients who received cilta-cel; PFS was 11.8 months for the 211 patients assigned to receive standard of care, which consisted of the physician’s choice of either pomalidomide, bortezomib, and dexamethasone (PVd), or daratumumab, pomalidomide, and dexamethasone (DPd).
Twelve-month PFS rates were 75.9% and 48.6%, respectively, and both the overall response rate (ORR) and the complete response (CR) rate were higher with the CAR T construct than with the standard of care (ORR, 84.6% vs. 67.3%; CR rates, 73.1% and 21.8%, respectively).
“My perspective on Dr. Dakhal and colleague’s data is that myeloma treatment should be revisited in the light of this,” commented invited discussant Asher Chanan-Khan, MD, from the Mayo Clinic Cancer Center in Jacksonville, Fla.
“Early CAR Ts demonstrating efficacy and safety and prior lines of treatment impact survival from CAR T in myeloma. In lymphoma, CAR T is almost replacing, if not already, autotransplant. Can this also be true for multiple myeloma?” he asked.
Dr. Chanan-Khan noted that there are at least four ongoing trials with CAR T targeting either the B-cell maturation antigen (BCMA) alone or in combination with an anti-CD19 CAR T, immune checkpoint inhibitors, or with bortezomib, lenalidomide, and dexamethasone.
Also commenting on the new results, ASCO Expert Oreofe Odejide, MD, of the Dana-Farber Cancer Institute in Boston, said in a statement: “Lenalidomide has become a foundation of care for people with myeloma, but as its use has expanded, so has the number of patients whose disease will no longer respond to the treatment. Ciltacabtagene autoleucel has not only shown that it delivers remarkably effective outcomes, compared with patients’ current options, but also that it can be used safely earlier in the treatment phase.”
Already approved for refractory myeloma
Cilta-cel is a second-generation CAR T that contains two single-domain antibodies that target BCMA. This target was first described in myeloma in 2004 as a mechanism for the growth and survival of malignant plasma cells.
The product is already approved for use in myeloma; it was approved in March 2022 by the U.S. Food and Drug Administration for use in patients with refractory/relapsed multiple myeloma who have already tried four or more therapies. That approval was based on results from phase 1b/2 CARTITUDE-1 trial, which, as previously reported by this news organization, showed that early and deep responses with cilta-cel proved to be durable.
Final results of CARTITUDE-1, reported in a scientific poster at ASCO 2023, showed that almost half of patients (47.5%) who were treated with cilta-cel were free of disease progression at 3 years, and 59.8% had sustained, complete responses. In addition, the median PFS was longer than for any previously reported therapy for heavily pretreated patients with relapsed/refractory multiple myeloma, the authors said.
CARTITUDE-4 details
For the CARTITUDE-4 trial, the investigators enrolled patients aged 18 years or older with lenalidomide-refractory multiple myeloma who had experienced relapse after one to three prior lines of therapy that included a prosteasome inhibitor and immunomodulator. After stratification by the choice of PVd or DPd, Multiple Myeloma International Staging System, and number of prior lines of therapy, patients were randomly assigned to receive either cilta-cel or one of the two standard-of-care regimens previously described.
Patients assigned to cilta-cel received one or more cycles of either PVd or DPd as bridging therapy during the period from apheresis to infusion of the CAR T cells.
As already noted, cilta-cel showed superior PFS and response rates and was associated with a significantly higher rate of minimal residual disease (MRD) negativity, compared with standard of care, in the intention-to-treat population: 60.6% vs. 15.6%, which translates into an odds ratio for achieving MRD negativity with CAR T of 8.7 (P < .0001). Among the subset of patients evaluable for MRD, the respective rates were 87.5% and 32.7%.
Overall survival data were not mature at the time of presentation. In all, 39 patients in the cilta-cel arm and 47 in the standard-of-care arm died during the study.
Grade 3 or 4 adverse events occurred in 97% of patients who received cilta-cel and in 94% of those who received standard-of-care therapies. In the cilta-cel arm, 76.1% of patients had cytokine release syndrome (CRS), although only 1.1% of cases were of grade 3 or 4 in severity, and there were no CRS-associated deaths. Eight patients in this arm had immune effector cell–associated neurotoxicity syndrome, all of grade 1 or 2. One patient had grade 1 movement and neurocognitive symptoms, 16 had grade 2 or 3 cranial nerve palsy, and 5 patients had CAR T–related peripheral neuropathy of grade 1, 2, or 3.
The investigators plan to follow patients to determine the long-term effects of ciltacabtagene autoleucel and are currently performing analyses of health-related quality of life, subgroups, and biomarkers.
The study was funded by Janssen and Legend Biotech, which market ciltacabtagene autoleucel. Dr. Dhakal disclosed consulting, speaker’s bureau participation, and institutional research funding from Janssen and others. Several coauthors are employees of the study funders. Dr. Chanan-Khan’s relevant financial information was not available. Dr. Odejide reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New results show that such patients benefit from treatment with the chimeric antigen receptor T-cell (CAR T) construct ciltacabtagene autoleucel (cilta-cel) (Carvykti).
The finding comes from the phase 3 CARTITUDE-4 trial, which was reported at the annual meeting of the American Society of Clinical Oncology (ASCO) and was simultaneously published online in the New England Journal of Medicine.
Patients with lenalidomide-refractory multiple myeloma who received a single infusion of ciltacabtagene autoleucel demonstrated a 74% reduction in the risk for disease progression or death, compared with patients who received the standard of care.
The hazard ratio for death or progression with cilta-cel was 0.26 (P < .001), which “is the best hazard ratio ever reported in this patient population in a randomized clinical setting,” said principal investigator Binod Dhakal, MD, from the Medical College of Wisconsin, Milwaukee.
Dr. Dhakal reported data from the first analysis of the trial. At a median follow-up of 15.9 months, median progression-free survival (PFS), the primary endpoint, had not been reached among 208 patients who received cilta-cel; PFS was 11.8 months for the 211 patients assigned to receive standard of care, which consisted of the physician’s choice of either pomalidomide, bortezomib, and dexamethasone (PVd), or daratumumab, pomalidomide, and dexamethasone (DPd).
Twelve-month PFS rates were 75.9% and 48.6%, respectively, and both the overall response rate (ORR) and the complete response (CR) rate were higher with the CAR T construct than with the standard of care (ORR, 84.6% vs. 67.3%; CR rates, 73.1% and 21.8%, respectively).
“My perspective on Dr. Dakhal and colleague’s data is that myeloma treatment should be revisited in the light of this,” commented invited discussant Asher Chanan-Khan, MD, from the Mayo Clinic Cancer Center in Jacksonville, Fla.
“Early CAR Ts demonstrating efficacy and safety and prior lines of treatment impact survival from CAR T in myeloma. In lymphoma, CAR T is almost replacing, if not already, autotransplant. Can this also be true for multiple myeloma?” he asked.
Dr. Chanan-Khan noted that there are at least four ongoing trials with CAR T targeting either the B-cell maturation antigen (BCMA) alone or in combination with an anti-CD19 CAR T, immune checkpoint inhibitors, or with bortezomib, lenalidomide, and dexamethasone.
Also commenting on the new results, ASCO Expert Oreofe Odejide, MD, of the Dana-Farber Cancer Institute in Boston, said in a statement: “Lenalidomide has become a foundation of care for people with myeloma, but as its use has expanded, so has the number of patients whose disease will no longer respond to the treatment. Ciltacabtagene autoleucel has not only shown that it delivers remarkably effective outcomes, compared with patients’ current options, but also that it can be used safely earlier in the treatment phase.”
Already approved for refractory myeloma
Cilta-cel is a second-generation CAR T that contains two single-domain antibodies that target BCMA. This target was first described in myeloma in 2004 as a mechanism for the growth and survival of malignant plasma cells.
The product is already approved for use in myeloma; it was approved in March 2022 by the U.S. Food and Drug Administration for use in patients with refractory/relapsed multiple myeloma who have already tried four or more therapies. That approval was based on results from phase 1b/2 CARTITUDE-1 trial, which, as previously reported by this news organization, showed that early and deep responses with cilta-cel proved to be durable.
Final results of CARTITUDE-1, reported in a scientific poster at ASCO 2023, showed that almost half of patients (47.5%) who were treated with cilta-cel were free of disease progression at 3 years, and 59.8% had sustained, complete responses. In addition, the median PFS was longer than for any previously reported therapy for heavily pretreated patients with relapsed/refractory multiple myeloma, the authors said.
CARTITUDE-4 details
For the CARTITUDE-4 trial, the investigators enrolled patients aged 18 years or older with lenalidomide-refractory multiple myeloma who had experienced relapse after one to three prior lines of therapy that included a prosteasome inhibitor and immunomodulator. After stratification by the choice of PVd or DPd, Multiple Myeloma International Staging System, and number of prior lines of therapy, patients were randomly assigned to receive either cilta-cel or one of the two standard-of-care regimens previously described.
Patients assigned to cilta-cel received one or more cycles of either PVd or DPd as bridging therapy during the period from apheresis to infusion of the CAR T cells.
As already noted, cilta-cel showed superior PFS and response rates and was associated with a significantly higher rate of minimal residual disease (MRD) negativity, compared with standard of care, in the intention-to-treat population: 60.6% vs. 15.6%, which translates into an odds ratio for achieving MRD negativity with CAR T of 8.7 (P < .0001). Among the subset of patients evaluable for MRD, the respective rates were 87.5% and 32.7%.
Overall survival data were not mature at the time of presentation. In all, 39 patients in the cilta-cel arm and 47 in the standard-of-care arm died during the study.
Grade 3 or 4 adverse events occurred in 97% of patients who received cilta-cel and in 94% of those who received standard-of-care therapies. In the cilta-cel arm, 76.1% of patients had cytokine release syndrome (CRS), although only 1.1% of cases were of grade 3 or 4 in severity, and there were no CRS-associated deaths. Eight patients in this arm had immune effector cell–associated neurotoxicity syndrome, all of grade 1 or 2. One patient had grade 1 movement and neurocognitive symptoms, 16 had grade 2 or 3 cranial nerve palsy, and 5 patients had CAR T–related peripheral neuropathy of grade 1, 2, or 3.
The investigators plan to follow patients to determine the long-term effects of ciltacabtagene autoleucel and are currently performing analyses of health-related quality of life, subgroups, and biomarkers.
The study was funded by Janssen and Legend Biotech, which market ciltacabtagene autoleucel. Dr. Dhakal disclosed consulting, speaker’s bureau participation, and institutional research funding from Janssen and others. Several coauthors are employees of the study funders. Dr. Chanan-Khan’s relevant financial information was not available. Dr. Odejide reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New results show that such patients benefit from treatment with the chimeric antigen receptor T-cell (CAR T) construct ciltacabtagene autoleucel (cilta-cel) (Carvykti).
The finding comes from the phase 3 CARTITUDE-4 trial, which was reported at the annual meeting of the American Society of Clinical Oncology (ASCO) and was simultaneously published online in the New England Journal of Medicine.
Patients with lenalidomide-refractory multiple myeloma who received a single infusion of ciltacabtagene autoleucel demonstrated a 74% reduction in the risk for disease progression or death, compared with patients who received the standard of care.
The hazard ratio for death or progression with cilta-cel was 0.26 (P < .001), which “is the best hazard ratio ever reported in this patient population in a randomized clinical setting,” said principal investigator Binod Dhakal, MD, from the Medical College of Wisconsin, Milwaukee.
Dr. Dhakal reported data from the first analysis of the trial. At a median follow-up of 15.9 months, median progression-free survival (PFS), the primary endpoint, had not been reached among 208 patients who received cilta-cel; PFS was 11.8 months for the 211 patients assigned to receive standard of care, which consisted of the physician’s choice of either pomalidomide, bortezomib, and dexamethasone (PVd), or daratumumab, pomalidomide, and dexamethasone (DPd).
Twelve-month PFS rates were 75.9% and 48.6%, respectively, and both the overall response rate (ORR) and the complete response (CR) rate were higher with the CAR T construct than with the standard of care (ORR, 84.6% vs. 67.3%; CR rates, 73.1% and 21.8%, respectively).
“My perspective on Dr. Dakhal and colleague’s data is that myeloma treatment should be revisited in the light of this,” commented invited discussant Asher Chanan-Khan, MD, from the Mayo Clinic Cancer Center in Jacksonville, Fla.
“Early CAR Ts demonstrating efficacy and safety and prior lines of treatment impact survival from CAR T in myeloma. In lymphoma, CAR T is almost replacing, if not already, autotransplant. Can this also be true for multiple myeloma?” he asked.
Dr. Chanan-Khan noted that there are at least four ongoing trials with CAR T targeting either the B-cell maturation antigen (BCMA) alone or in combination with an anti-CD19 CAR T, immune checkpoint inhibitors, or with bortezomib, lenalidomide, and dexamethasone.
Also commenting on the new results, ASCO Expert Oreofe Odejide, MD, of the Dana-Farber Cancer Institute in Boston, said in a statement: “Lenalidomide has become a foundation of care for people with myeloma, but as its use has expanded, so has the number of patients whose disease will no longer respond to the treatment. Ciltacabtagene autoleucel has not only shown that it delivers remarkably effective outcomes, compared with patients’ current options, but also that it can be used safely earlier in the treatment phase.”
Already approved for refractory myeloma
Cilta-cel is a second-generation CAR T that contains two single-domain antibodies that target BCMA. This target was first described in myeloma in 2004 as a mechanism for the growth and survival of malignant plasma cells.
The product is already approved for use in myeloma; it was approved in March 2022 by the U.S. Food and Drug Administration for use in patients with refractory/relapsed multiple myeloma who have already tried four or more therapies. That approval was based on results from phase 1b/2 CARTITUDE-1 trial, which, as previously reported by this news organization, showed that early and deep responses with cilta-cel proved to be durable.
Final results of CARTITUDE-1, reported in a scientific poster at ASCO 2023, showed that almost half of patients (47.5%) who were treated with cilta-cel were free of disease progression at 3 years, and 59.8% had sustained, complete responses. In addition, the median PFS was longer than for any previously reported therapy for heavily pretreated patients with relapsed/refractory multiple myeloma, the authors said.
CARTITUDE-4 details
For the CARTITUDE-4 trial, the investigators enrolled patients aged 18 years or older with lenalidomide-refractory multiple myeloma who had experienced relapse after one to three prior lines of therapy that included a prosteasome inhibitor and immunomodulator. After stratification by the choice of PVd or DPd, Multiple Myeloma International Staging System, and number of prior lines of therapy, patients were randomly assigned to receive either cilta-cel or one of the two standard-of-care regimens previously described.
Patients assigned to cilta-cel received one or more cycles of either PVd or DPd as bridging therapy during the period from apheresis to infusion of the CAR T cells.
As already noted, cilta-cel showed superior PFS and response rates and was associated with a significantly higher rate of minimal residual disease (MRD) negativity, compared with standard of care, in the intention-to-treat population: 60.6% vs. 15.6%, which translates into an odds ratio for achieving MRD negativity with CAR T of 8.7 (P < .0001). Among the subset of patients evaluable for MRD, the respective rates were 87.5% and 32.7%.
Overall survival data were not mature at the time of presentation. In all, 39 patients in the cilta-cel arm and 47 in the standard-of-care arm died during the study.
Grade 3 or 4 adverse events occurred in 97% of patients who received cilta-cel and in 94% of those who received standard-of-care therapies. In the cilta-cel arm, 76.1% of patients had cytokine release syndrome (CRS), although only 1.1% of cases were of grade 3 or 4 in severity, and there were no CRS-associated deaths. Eight patients in this arm had immune effector cell–associated neurotoxicity syndrome, all of grade 1 or 2. One patient had grade 1 movement and neurocognitive symptoms, 16 had grade 2 or 3 cranial nerve palsy, and 5 patients had CAR T–related peripheral neuropathy of grade 1, 2, or 3.
The investigators plan to follow patients to determine the long-term effects of ciltacabtagene autoleucel and are currently performing analyses of health-related quality of life, subgroups, and biomarkers.
The study was funded by Janssen and Legend Biotech, which market ciltacabtagene autoleucel. Dr. Dhakal disclosed consulting, speaker’s bureau participation, and institutional research funding from Janssen and others. Several coauthors are employees of the study funders. Dr. Chanan-Khan’s relevant financial information was not available. Dr. Odejide reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ASCO 2023
Huge underuse of germline testing for cancer patients
Information from germline genetic testing could affect a patient’s cancer care. For example, such testing could indicate that targeted therapies would be beneficial, and it would have implications for close relatives who may carry the same genes.
The finding that so few patients with newly diagnosed cancer were tested comes from an analysis of data on more than 1.3 million individuals across two U.S. states. The data were taken from the Surveillance, Epidemiology, and End Results (SEER) registry.
The rate is “well below guideline recommendations,” said study presenter Allison W. Kurian, MD, department of medicine, Stanford (Calif.) University.
“Innovative care delivery” is needed to tackle the problem, including the streamlining of pretest counseling, making posttest counseling more widely available, and employing long-term follow-up to track patient outcomes, she suggested.
“I do think this is a time for creative solutions of a number of different kinds,” she said. She suggested that lessons could be learned from the use of telemedicine during the COVID-19 pandemic. She also noted that “there have been some interesting studies on embedding genetic counselors in oncology clinics.”
Dr. Kurian presented the study at the annual meeting of the American Society of Clinical Oncology (ASCO). The study was simultaneously published in the Journal of the American Medical Association.
The current results represent a “missed opportunity for decrease the population-level burden of cancer,” experts noted in an accompanying editorial.
“Clinicians should recommend testing to their patients and provide them with the information necessary to make informed decisions about whether to undergo testing,” Zsofia K. Stadler, MD, and Deborah Schrag, MD, MPH, of Memorial Sloan Kettering Cancer Center, New York, wrote in their editorial.
They suggested novel approaches to widen access, such as use of point-of-care testing, telecounseling, and, in the future, chatbots to respond to patient questions.
“With greater emphasis on overcoming both health system and patient-level barriers to genetic cancer susceptibility testing for patients with cancer, treatment outcomes will improve and cancer diagnoses and related deaths in family members will be prevented,” they concluded.
At the meeting, invited discussant Erin Frances Cobain, MD, assistant professor of medical oncology, University of Michigan Health, Ann Arbor, referring to breast cancer as an example, said that progress has “stagnated” in recent years.
The study found a higher rate of gene testing among patients with newly diagnosed breast cancer, at just over 20%.
Dr. Cobain argued that this was still too low. She pointed out that “a recent study suggested that over 60% of individuals with an incident cancer diagnosis would meet criteria for genetic testing by National Comprehensive Cancer Network guidelines.
“This may be because testing is not offered, there may be poor access to genetic counseling resources, or patients may be offered testing but decline it,” she suggested.
One compelling reason to conduct genetic testing for patients newly diagnosed with breast cancer is that it may show that they are candidates for treatment with PARP (poly[ADP]-ribose polymerase) inhibitors, which “may have a direct impact on cancer-related mortality,” she pointed out.
“We need increased awareness and access to genetic testing resources for patients with breast cancer, particularly for racial and ethnic minorities,” she said.
Dr. Cobain also noted that finding variants of uncertain significance (VUS) was more likely among patients from racial and ethnic minorities than among White patients. She said such a finding “increases patient and physician anxiety,” and there may be “unclear optimal management recommendations for these patients.”
Details of the study
Germline genetic testing is “increasingly essential for cancer care,” Dr. Kurian said.
It is central to risk-adapted screening and secondary prevention, the use of targeted therapies, including PARP and checkpoint inhibitors, and cascade testing to identify at-risk relatives.
She pointed out that in clinical practice, testing has “evolved rapidly.” Panels include more and more genes. In addition, the cost of these tests is falling, and guidelines have become “more expansive.”
However, “little is known about genetic testing use and results,” Dr. Kurian noted.
The team therefore undertook the SEER-GeneLINK initiative, which involved patients aged ≥ 20 years who were diagnosed with cancer between Jan. 1, 2013, and March 31, 2019, and who were reported to statewide SEER registries in California and Georgia.
The team looked for patients for whom germline genetic test results had been reported by the four laboratories that performed the majority of patient testing in the two states. Results were categorized as pathogenic, benign, or VUS.
The results were classified on the basis of current guidelines for testing and/or management as related to breast/ovarian cancer, gastrointestinal cancer, other hereditary cancers, or those with no guidelines for testing or management.
Dr. Kurian reported that from an overall population of 1,412,388 patients diagnosed with cancer, 1,369,660 were eligible for inclusion. Of those, about half (51.9%) were women, and the majority (86.3%) were aged 50 years or older.
Many of these patients (61.4%) were non-Hispanic White persons, and slightly fewer than half (49.8%) were deemed to be in medium or high poverty, as determined using U.S. Census tract levels.
Overall, germline genetic testing was performed in 93,052 (6.8%) of patients over the study period.
Women were more likely to have undergone germline mutation testing than men, at 13.9% vs. 2.2%, as were patients aged 20-49 years, at 22.1% vs. 8.2% for those aged 50-69 years, and 3.3% for those aged 70 years and older.
The number of genes for which testing was conducted increased from a median of 2 in 2013 to 34 in 2019. Rates of VUS increased more than that for pathologic variants and substantially more so in non-White patients.
By 2019, the ratio of VUS to pathologic variants stood at 1.7 among White patients, vs. 3.9 among Asian patients, 3.6 among Black patients, and 2.2 among Hispanic patients.
The majority of identified pathologic variants that were related to the diagnosed cancer and genes with testing and/or management guidelines accounted for 67.5% to 94.9% of such variants.
Regarding specific cancer diagnoses, Dr. Kurian said that over the course of the study period, testing rates consistently exceeded 50% only among male breast cancer patients.
There were rapid increases in testing for ovarian cancer, from 28.0% of cases in 2013 to 54.0% in 2019. For pancreatic cancer, rates increased from 1.0% to 19.0% over the same period, and for prostate cancer, rates increased from 0.1% to 4.0%. She suggested that these increases in rates may be related to the approval of PARP inhibitors for use in these indications.
However, there was little change in the rates of germline mutation testing for lung cancer patients, from 01% in 2013 to 0.8% in 2019, and for other cancers, from 0.3% to 2.0%.
The results also revealed racial and ethnic differences in testing after controlling for age, cancer type, and year. Over the course of the study period, 8.0% of White patients underwent genetic testing, compared with 6.0% each for Asian, Black, and Hispanic patients and 5.0% for other patients (P < .001).
With regard specifically to male and female breast cancer and ovarian cancer, testing rates were 31% among White patients, 22% for Asian patients, 25% for Black patients, and 23% for Hispanic patients (P < .001).
Dr. Kurian acknowledged that the study is limited by a lack of testing from other laboratories and direct-to-consumer test data, although a recent survey suggested that this represents fewer than 5% of all germline genetic tests.
She also noted that the SEER registries do not collect data on family history or tumor sequencing.
The study was funded by the National Institutes of Health, and the Centers for Disease Control and Prevention. Dr. Kurian has relationships with Adela, Ambry Genetics, Color Genomics, GeneDx/BioReference, Genentech, InVitae, and Myriad Genetics. Other authors report numerous relationships with industry. Dr. Cobain has ties with AstraZeneca, Daiichi Sankyo, Athenex, Ayala Pharmaceuticals, bioTheranostics, and Immunomedics. Dr. Schrag has relationships with Merck, JAMA, AACR, and Grail. Dr. Stadler has ties with Adverum Biotechnologies, Genentech, Neurogene, Novartis, Optos Plc, Outlook Therapeutics, and Regeneron Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Information from germline genetic testing could affect a patient’s cancer care. For example, such testing could indicate that targeted therapies would be beneficial, and it would have implications for close relatives who may carry the same genes.
The finding that so few patients with newly diagnosed cancer were tested comes from an analysis of data on more than 1.3 million individuals across two U.S. states. The data were taken from the Surveillance, Epidemiology, and End Results (SEER) registry.
The rate is “well below guideline recommendations,” said study presenter Allison W. Kurian, MD, department of medicine, Stanford (Calif.) University.
“Innovative care delivery” is needed to tackle the problem, including the streamlining of pretest counseling, making posttest counseling more widely available, and employing long-term follow-up to track patient outcomes, she suggested.
“I do think this is a time for creative solutions of a number of different kinds,” she said. She suggested that lessons could be learned from the use of telemedicine during the COVID-19 pandemic. She also noted that “there have been some interesting studies on embedding genetic counselors in oncology clinics.”
Dr. Kurian presented the study at the annual meeting of the American Society of Clinical Oncology (ASCO). The study was simultaneously published in the Journal of the American Medical Association.
The current results represent a “missed opportunity for decrease the population-level burden of cancer,” experts noted in an accompanying editorial.
“Clinicians should recommend testing to their patients and provide them with the information necessary to make informed decisions about whether to undergo testing,” Zsofia K. Stadler, MD, and Deborah Schrag, MD, MPH, of Memorial Sloan Kettering Cancer Center, New York, wrote in their editorial.
They suggested novel approaches to widen access, such as use of point-of-care testing, telecounseling, and, in the future, chatbots to respond to patient questions.
“With greater emphasis on overcoming both health system and patient-level barriers to genetic cancer susceptibility testing for patients with cancer, treatment outcomes will improve and cancer diagnoses and related deaths in family members will be prevented,” they concluded.
At the meeting, invited discussant Erin Frances Cobain, MD, assistant professor of medical oncology, University of Michigan Health, Ann Arbor, referring to breast cancer as an example, said that progress has “stagnated” in recent years.
The study found a higher rate of gene testing among patients with newly diagnosed breast cancer, at just over 20%.
Dr. Cobain argued that this was still too low. She pointed out that “a recent study suggested that over 60% of individuals with an incident cancer diagnosis would meet criteria for genetic testing by National Comprehensive Cancer Network guidelines.
“This may be because testing is not offered, there may be poor access to genetic counseling resources, or patients may be offered testing but decline it,” she suggested.
One compelling reason to conduct genetic testing for patients newly diagnosed with breast cancer is that it may show that they are candidates for treatment with PARP (poly[ADP]-ribose polymerase) inhibitors, which “may have a direct impact on cancer-related mortality,” she pointed out.
“We need increased awareness and access to genetic testing resources for patients with breast cancer, particularly for racial and ethnic minorities,” she said.
Dr. Cobain also noted that finding variants of uncertain significance (VUS) was more likely among patients from racial and ethnic minorities than among White patients. She said such a finding “increases patient and physician anxiety,” and there may be “unclear optimal management recommendations for these patients.”
Details of the study
Germline genetic testing is “increasingly essential for cancer care,” Dr. Kurian said.
It is central to risk-adapted screening and secondary prevention, the use of targeted therapies, including PARP and checkpoint inhibitors, and cascade testing to identify at-risk relatives.
She pointed out that in clinical practice, testing has “evolved rapidly.” Panels include more and more genes. In addition, the cost of these tests is falling, and guidelines have become “more expansive.”
However, “little is known about genetic testing use and results,” Dr. Kurian noted.
The team therefore undertook the SEER-GeneLINK initiative, which involved patients aged ≥ 20 years who were diagnosed with cancer between Jan. 1, 2013, and March 31, 2019, and who were reported to statewide SEER registries in California and Georgia.
The team looked for patients for whom germline genetic test results had been reported by the four laboratories that performed the majority of patient testing in the two states. Results were categorized as pathogenic, benign, or VUS.
The results were classified on the basis of current guidelines for testing and/or management as related to breast/ovarian cancer, gastrointestinal cancer, other hereditary cancers, or those with no guidelines for testing or management.
Dr. Kurian reported that from an overall population of 1,412,388 patients diagnosed with cancer, 1,369,660 were eligible for inclusion. Of those, about half (51.9%) were women, and the majority (86.3%) were aged 50 years or older.
Many of these patients (61.4%) were non-Hispanic White persons, and slightly fewer than half (49.8%) were deemed to be in medium or high poverty, as determined using U.S. Census tract levels.
Overall, germline genetic testing was performed in 93,052 (6.8%) of patients over the study period.
Women were more likely to have undergone germline mutation testing than men, at 13.9% vs. 2.2%, as were patients aged 20-49 years, at 22.1% vs. 8.2% for those aged 50-69 years, and 3.3% for those aged 70 years and older.
The number of genes for which testing was conducted increased from a median of 2 in 2013 to 34 in 2019. Rates of VUS increased more than that for pathologic variants and substantially more so in non-White patients.
By 2019, the ratio of VUS to pathologic variants stood at 1.7 among White patients, vs. 3.9 among Asian patients, 3.6 among Black patients, and 2.2 among Hispanic patients.
The majority of identified pathologic variants that were related to the diagnosed cancer and genes with testing and/or management guidelines accounted for 67.5% to 94.9% of such variants.
Regarding specific cancer diagnoses, Dr. Kurian said that over the course of the study period, testing rates consistently exceeded 50% only among male breast cancer patients.
There were rapid increases in testing for ovarian cancer, from 28.0% of cases in 2013 to 54.0% in 2019. For pancreatic cancer, rates increased from 1.0% to 19.0% over the same period, and for prostate cancer, rates increased from 0.1% to 4.0%. She suggested that these increases in rates may be related to the approval of PARP inhibitors for use in these indications.
However, there was little change in the rates of germline mutation testing for lung cancer patients, from 01% in 2013 to 0.8% in 2019, and for other cancers, from 0.3% to 2.0%.
The results also revealed racial and ethnic differences in testing after controlling for age, cancer type, and year. Over the course of the study period, 8.0% of White patients underwent genetic testing, compared with 6.0% each for Asian, Black, and Hispanic patients and 5.0% for other patients (P < .001).
With regard specifically to male and female breast cancer and ovarian cancer, testing rates were 31% among White patients, 22% for Asian patients, 25% for Black patients, and 23% for Hispanic patients (P < .001).
Dr. Kurian acknowledged that the study is limited by a lack of testing from other laboratories and direct-to-consumer test data, although a recent survey suggested that this represents fewer than 5% of all germline genetic tests.
She also noted that the SEER registries do not collect data on family history or tumor sequencing.
The study was funded by the National Institutes of Health, and the Centers for Disease Control and Prevention. Dr. Kurian has relationships with Adela, Ambry Genetics, Color Genomics, GeneDx/BioReference, Genentech, InVitae, and Myriad Genetics. Other authors report numerous relationships with industry. Dr. Cobain has ties with AstraZeneca, Daiichi Sankyo, Athenex, Ayala Pharmaceuticals, bioTheranostics, and Immunomedics. Dr. Schrag has relationships with Merck, JAMA, AACR, and Grail. Dr. Stadler has ties with Adverum Biotechnologies, Genentech, Neurogene, Novartis, Optos Plc, Outlook Therapeutics, and Regeneron Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Information from germline genetic testing could affect a patient’s cancer care. For example, such testing could indicate that targeted therapies would be beneficial, and it would have implications for close relatives who may carry the same genes.
The finding that so few patients with newly diagnosed cancer were tested comes from an analysis of data on more than 1.3 million individuals across two U.S. states. The data were taken from the Surveillance, Epidemiology, and End Results (SEER) registry.
The rate is “well below guideline recommendations,” said study presenter Allison W. Kurian, MD, department of medicine, Stanford (Calif.) University.
“Innovative care delivery” is needed to tackle the problem, including the streamlining of pretest counseling, making posttest counseling more widely available, and employing long-term follow-up to track patient outcomes, she suggested.
“I do think this is a time for creative solutions of a number of different kinds,” she said. She suggested that lessons could be learned from the use of telemedicine during the COVID-19 pandemic. She also noted that “there have been some interesting studies on embedding genetic counselors in oncology clinics.”
Dr. Kurian presented the study at the annual meeting of the American Society of Clinical Oncology (ASCO). The study was simultaneously published in the Journal of the American Medical Association.
The current results represent a “missed opportunity for decrease the population-level burden of cancer,” experts noted in an accompanying editorial.
“Clinicians should recommend testing to their patients and provide them with the information necessary to make informed decisions about whether to undergo testing,” Zsofia K. Stadler, MD, and Deborah Schrag, MD, MPH, of Memorial Sloan Kettering Cancer Center, New York, wrote in their editorial.
They suggested novel approaches to widen access, such as use of point-of-care testing, telecounseling, and, in the future, chatbots to respond to patient questions.
“With greater emphasis on overcoming both health system and patient-level barriers to genetic cancer susceptibility testing for patients with cancer, treatment outcomes will improve and cancer diagnoses and related deaths in family members will be prevented,” they concluded.
At the meeting, invited discussant Erin Frances Cobain, MD, assistant professor of medical oncology, University of Michigan Health, Ann Arbor, referring to breast cancer as an example, said that progress has “stagnated” in recent years.
The study found a higher rate of gene testing among patients with newly diagnosed breast cancer, at just over 20%.
Dr. Cobain argued that this was still too low. She pointed out that “a recent study suggested that over 60% of individuals with an incident cancer diagnosis would meet criteria for genetic testing by National Comprehensive Cancer Network guidelines.
“This may be because testing is not offered, there may be poor access to genetic counseling resources, or patients may be offered testing but decline it,” she suggested.
One compelling reason to conduct genetic testing for patients newly diagnosed with breast cancer is that it may show that they are candidates for treatment with PARP (poly[ADP]-ribose polymerase) inhibitors, which “may have a direct impact on cancer-related mortality,” she pointed out.
“We need increased awareness and access to genetic testing resources for patients with breast cancer, particularly for racial and ethnic minorities,” she said.
Dr. Cobain also noted that finding variants of uncertain significance (VUS) was more likely among patients from racial and ethnic minorities than among White patients. She said such a finding “increases patient and physician anxiety,” and there may be “unclear optimal management recommendations for these patients.”
Details of the study
Germline genetic testing is “increasingly essential for cancer care,” Dr. Kurian said.
It is central to risk-adapted screening and secondary prevention, the use of targeted therapies, including PARP and checkpoint inhibitors, and cascade testing to identify at-risk relatives.
She pointed out that in clinical practice, testing has “evolved rapidly.” Panels include more and more genes. In addition, the cost of these tests is falling, and guidelines have become “more expansive.”
However, “little is known about genetic testing use and results,” Dr. Kurian noted.
The team therefore undertook the SEER-GeneLINK initiative, which involved patients aged ≥ 20 years who were diagnosed with cancer between Jan. 1, 2013, and March 31, 2019, and who were reported to statewide SEER registries in California and Georgia.
The team looked for patients for whom germline genetic test results had been reported by the four laboratories that performed the majority of patient testing in the two states. Results were categorized as pathogenic, benign, or VUS.
The results were classified on the basis of current guidelines for testing and/or management as related to breast/ovarian cancer, gastrointestinal cancer, other hereditary cancers, or those with no guidelines for testing or management.
Dr. Kurian reported that from an overall population of 1,412,388 patients diagnosed with cancer, 1,369,660 were eligible for inclusion. Of those, about half (51.9%) were women, and the majority (86.3%) were aged 50 years or older.
Many of these patients (61.4%) were non-Hispanic White persons, and slightly fewer than half (49.8%) were deemed to be in medium or high poverty, as determined using U.S. Census tract levels.
Overall, germline genetic testing was performed in 93,052 (6.8%) of patients over the study period.
Women were more likely to have undergone germline mutation testing than men, at 13.9% vs. 2.2%, as were patients aged 20-49 years, at 22.1% vs. 8.2% for those aged 50-69 years, and 3.3% for those aged 70 years and older.
The number of genes for which testing was conducted increased from a median of 2 in 2013 to 34 in 2019. Rates of VUS increased more than that for pathologic variants and substantially more so in non-White patients.
By 2019, the ratio of VUS to pathologic variants stood at 1.7 among White patients, vs. 3.9 among Asian patients, 3.6 among Black patients, and 2.2 among Hispanic patients.
The majority of identified pathologic variants that were related to the diagnosed cancer and genes with testing and/or management guidelines accounted for 67.5% to 94.9% of such variants.
Regarding specific cancer diagnoses, Dr. Kurian said that over the course of the study period, testing rates consistently exceeded 50% only among male breast cancer patients.
There were rapid increases in testing for ovarian cancer, from 28.0% of cases in 2013 to 54.0% in 2019. For pancreatic cancer, rates increased from 1.0% to 19.0% over the same period, and for prostate cancer, rates increased from 0.1% to 4.0%. She suggested that these increases in rates may be related to the approval of PARP inhibitors for use in these indications.
However, there was little change in the rates of germline mutation testing for lung cancer patients, from 01% in 2013 to 0.8% in 2019, and for other cancers, from 0.3% to 2.0%.
The results also revealed racial and ethnic differences in testing after controlling for age, cancer type, and year. Over the course of the study period, 8.0% of White patients underwent genetic testing, compared with 6.0% each for Asian, Black, and Hispanic patients and 5.0% for other patients (P < .001).
With regard specifically to male and female breast cancer and ovarian cancer, testing rates were 31% among White patients, 22% for Asian patients, 25% for Black patients, and 23% for Hispanic patients (P < .001).
Dr. Kurian acknowledged that the study is limited by a lack of testing from other laboratories and direct-to-consumer test data, although a recent survey suggested that this represents fewer than 5% of all germline genetic tests.
She also noted that the SEER registries do not collect data on family history or tumor sequencing.
The study was funded by the National Institutes of Health, and the Centers for Disease Control and Prevention. Dr. Kurian has relationships with Adela, Ambry Genetics, Color Genomics, GeneDx/BioReference, Genentech, InVitae, and Myriad Genetics. Other authors report numerous relationships with industry. Dr. Cobain has ties with AstraZeneca, Daiichi Sankyo, Athenex, Ayala Pharmaceuticals, bioTheranostics, and Immunomedics. Dr. Schrag has relationships with Merck, JAMA, AACR, and Grail. Dr. Stadler has ties with Adverum Biotechnologies, Genentech, Neurogene, Novartis, Optos Plc, Outlook Therapeutics, and Regeneron Pharmaceuticals.
A version of this article first appeared on Medscape.com.
AT ASCO 2023
DEI training gives oncology fellows more confidence
The finding comes from a survey conducted after the introduction of DEI training within the Yale Medical Oncology-Hematology Fellowship Program. The study was reported by Norin Ansari, MD, MPH, of Yale Cancer Center, New Haven, Conn., at the annual meeting of the American Society of Clinical Oncology (ASCO).
Dr. Ansari emphasized the DEI curriculum in fellowship programs by highlighting the racial and gender disparities that exist among physicians.
“There is a significant representation problem – only 2%-3% of practicing oncologists are Black or Hispanic/Latino,” she said. “And that representation decreases with each stage in the pipeline of the workforce.”
Dr. Ansari also noted gender disparities in the oncologist workforce, reporting that about one-third of faculty positions are held by women.
The anonymous survey was sent to 29 fellows; 23 responded, including 8 first-year fellows and 13 senior fellows. Over 57% of respondents rated the importance of DEI education as 10 on a 10-point scale (mean, 8.6).
At the start of this year, the responses of senior fellows who had already received some DEI training during the previous year’s lecture series were compared with first-year fellows who had not had any fellowship DEI education.
First-year fellows reported a mean confidence score of 2.5/5 at navigating bias and microaggressions when experienced personally and a mean score of 2.9/5 when they were directed at others. Senior fellows reported mean confidence scores of 3 and 3.2, respectively.
Yale then compared longitudinal data on fellows’ comfort levels in navigating discrimination in 2021, 2022, and 2023 a month before the ASCO meeting.
Fellows were asked to rate their comfort level from 1 to 10 in navigating different types of discrimination, including racial inequality, sexual harassment, and gender discrimination. In these three categories, fellows rated comfortability as a 5 in 2021 and as 7 in 2023 after the DEI training.
“Our first goal is to normalize talking about DEI and to recognize that different people in our workforce have different experiences and how we can be allies for them and for our patients,” Dr. Ansari said. “And I think for long-term goals we want to take stock of who’s at the table, who’s making decisions, and how does that affect our field, our science, and our patients.”
Yale designed the 3-year longitudinal curriculum with two annual core topics: upstander training and journal club for discussion and reflection. An additional two to three training sessions per year will focus on either race, gender, LGBTQ+, disability, religion, or implicit bias training.
The most popular topics among fellows were upstander training, cancer treatment and outcomes disparities, recruitment and retention, and career promotion and pay disparities.
The preferred platforms of content delivery were lectures from experts in the field, affinity groups or mentorship links, small group discussions, and advocacy education.
Gerald Hsu, MD, PhD, with the San Francisco VA Medical Center, discussed the results of Yale’s DEI curriculum assessment, saying it represented “best practices” in the industry. However, he acknowledged that realistically, not everyone will be receptive to DEI training.
Dr. Hsu said that holding medical staff accountable is the only way to truly incorporate DEI into everyday practice.
“Collectively, we need to be holding ourselves to different standards or holding ourselves to some standard,” Dr. Hsu said. “Maybe we need to be setting goals to the degree to which we diversify our training programs and our faculty, and there needs to be consequences to not doing so.”
No funding for the study was reported.
A version of this article first appeared on Medscape.com.
The finding comes from a survey conducted after the introduction of DEI training within the Yale Medical Oncology-Hematology Fellowship Program. The study was reported by Norin Ansari, MD, MPH, of Yale Cancer Center, New Haven, Conn., at the annual meeting of the American Society of Clinical Oncology (ASCO).
Dr. Ansari emphasized the DEI curriculum in fellowship programs by highlighting the racial and gender disparities that exist among physicians.
“There is a significant representation problem – only 2%-3% of practicing oncologists are Black or Hispanic/Latino,” she said. “And that representation decreases with each stage in the pipeline of the workforce.”
Dr. Ansari also noted gender disparities in the oncologist workforce, reporting that about one-third of faculty positions are held by women.
The anonymous survey was sent to 29 fellows; 23 responded, including 8 first-year fellows and 13 senior fellows. Over 57% of respondents rated the importance of DEI education as 10 on a 10-point scale (mean, 8.6).
At the start of this year, the responses of senior fellows who had already received some DEI training during the previous year’s lecture series were compared with first-year fellows who had not had any fellowship DEI education.
First-year fellows reported a mean confidence score of 2.5/5 at navigating bias and microaggressions when experienced personally and a mean score of 2.9/5 when they were directed at others. Senior fellows reported mean confidence scores of 3 and 3.2, respectively.
Yale then compared longitudinal data on fellows’ comfort levels in navigating discrimination in 2021, 2022, and 2023 a month before the ASCO meeting.
Fellows were asked to rate their comfort level from 1 to 10 in navigating different types of discrimination, including racial inequality, sexual harassment, and gender discrimination. In these three categories, fellows rated comfortability as a 5 in 2021 and as 7 in 2023 after the DEI training.
“Our first goal is to normalize talking about DEI and to recognize that different people in our workforce have different experiences and how we can be allies for them and for our patients,” Dr. Ansari said. “And I think for long-term goals we want to take stock of who’s at the table, who’s making decisions, and how does that affect our field, our science, and our patients.”
Yale designed the 3-year longitudinal curriculum with two annual core topics: upstander training and journal club for discussion and reflection. An additional two to three training sessions per year will focus on either race, gender, LGBTQ+, disability, religion, or implicit bias training.
The most popular topics among fellows were upstander training, cancer treatment and outcomes disparities, recruitment and retention, and career promotion and pay disparities.
The preferred platforms of content delivery were lectures from experts in the field, affinity groups or mentorship links, small group discussions, and advocacy education.
Gerald Hsu, MD, PhD, with the San Francisco VA Medical Center, discussed the results of Yale’s DEI curriculum assessment, saying it represented “best practices” in the industry. However, he acknowledged that realistically, not everyone will be receptive to DEI training.
Dr. Hsu said that holding medical staff accountable is the only way to truly incorporate DEI into everyday practice.
“Collectively, we need to be holding ourselves to different standards or holding ourselves to some standard,” Dr. Hsu said. “Maybe we need to be setting goals to the degree to which we diversify our training programs and our faculty, and there needs to be consequences to not doing so.”
No funding for the study was reported.
A version of this article first appeared on Medscape.com.
The finding comes from a survey conducted after the introduction of DEI training within the Yale Medical Oncology-Hematology Fellowship Program. The study was reported by Norin Ansari, MD, MPH, of Yale Cancer Center, New Haven, Conn., at the annual meeting of the American Society of Clinical Oncology (ASCO).
Dr. Ansari emphasized the DEI curriculum in fellowship programs by highlighting the racial and gender disparities that exist among physicians.
“There is a significant representation problem – only 2%-3% of practicing oncologists are Black or Hispanic/Latino,” she said. “And that representation decreases with each stage in the pipeline of the workforce.”
Dr. Ansari also noted gender disparities in the oncologist workforce, reporting that about one-third of faculty positions are held by women.
The anonymous survey was sent to 29 fellows; 23 responded, including 8 first-year fellows and 13 senior fellows. Over 57% of respondents rated the importance of DEI education as 10 on a 10-point scale (mean, 8.6).
At the start of this year, the responses of senior fellows who had already received some DEI training during the previous year’s lecture series were compared with first-year fellows who had not had any fellowship DEI education.
First-year fellows reported a mean confidence score of 2.5/5 at navigating bias and microaggressions when experienced personally and a mean score of 2.9/5 when they were directed at others. Senior fellows reported mean confidence scores of 3 and 3.2, respectively.
Yale then compared longitudinal data on fellows’ comfort levels in navigating discrimination in 2021, 2022, and 2023 a month before the ASCO meeting.
Fellows were asked to rate their comfort level from 1 to 10 in navigating different types of discrimination, including racial inequality, sexual harassment, and gender discrimination. In these three categories, fellows rated comfortability as a 5 in 2021 and as 7 in 2023 after the DEI training.
“Our first goal is to normalize talking about DEI and to recognize that different people in our workforce have different experiences and how we can be allies for them and for our patients,” Dr. Ansari said. “And I think for long-term goals we want to take stock of who’s at the table, who’s making decisions, and how does that affect our field, our science, and our patients.”
Yale designed the 3-year longitudinal curriculum with two annual core topics: upstander training and journal club for discussion and reflection. An additional two to three training sessions per year will focus on either race, gender, LGBTQ+, disability, religion, or implicit bias training.
The most popular topics among fellows were upstander training, cancer treatment and outcomes disparities, recruitment and retention, and career promotion and pay disparities.
The preferred platforms of content delivery were lectures from experts in the field, affinity groups or mentorship links, small group discussions, and advocacy education.
Gerald Hsu, MD, PhD, with the San Francisco VA Medical Center, discussed the results of Yale’s DEI curriculum assessment, saying it represented “best practices” in the industry. However, he acknowledged that realistically, not everyone will be receptive to DEI training.
Dr. Hsu said that holding medical staff accountable is the only way to truly incorporate DEI into everyday practice.
“Collectively, we need to be holding ourselves to different standards or holding ourselves to some standard,” Dr. Hsu said. “Maybe we need to be setting goals to the degree to which we diversify our training programs and our faculty, and there needs to be consequences to not doing so.”
No funding for the study was reported.
A version of this article first appeared on Medscape.com.
FROM ASCO 2023