User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Treat-to-Target Outcomes With Tapinarof Cream 1% in Phase 3 Trials for Plaque Psoriasis
Psoriasis is a chronic inflammatory disease affecting approximately 8 million adults in the United States and 2% of the global population.1,2 Psoriasis causes pain, itching, and disfigurement and is associated with a physical, psychological, and economic burden that substantially affects health-related quality of life.3-5
Setting treatment goals and treating to target are evidence-based approaches that have been successfully applied to several chronic diseases to improve patient outcomes, including diabetes, hypertension, and rheumatoid arthritis.6-9 Treat-to-target strategies generally set low disease activity (or remission) as an overall goal and seek to achieve this using available therapeutic options as necessary. Introduced following the availability of biologics and targeted systemic therapies, treat-to-target strategies generally provide guidance on expectations of treatment but not specific treatments, as personalized treatment decisions depend on an assessment of individual patients and consider clinical and demographic features as well as preferences for available therapeutic options. If targets are not achieved in the assigned time span, adjustments can be made to the treatment approach in close consultation with the patient. If the target is reached, follow-up visits can be scheduled to ensure improvement is maintained or to establish if more aggressive goals could be selected.
Treat-to-target strategies for the management of psoriasis developed by the National Psoriasis Foundation (NPF) Medical Board include reducing the extent of psoriasis to 1% or lower total body surface area (BSA) after 3 months of treatment.10 Treatment targets endorsed by the European Academy of Dermatology and Venereology (EADV) in guidelines on the use of systemic therapies in psoriasis include achieving a 75% or greater reduction in Psoriasis Area and Severity Index (PASI) score within 3 to 4 months of treatment.11
In clinical practice, many patients do not achieve these treatment targets, and topical treatments alone generally are insufficient in achieving treatment goals for psoriasis.12,13 Moreover, conventional topical treatments (eg, topical corticosteroids) used by most patients with psoriasis regardless of disease severity are associated with adverse events that can limit their use. Most topical corticosteroids have US Food and Drug Administration label restrictions relating to sites of application, duration and extent of use, and frequency of administration.14,15
Tapinarof cream 1% (VTAMA [Dermavant Sciences, Inc]) is a first-in-class topical nonsteroidal aryl hydrocarbon receptor agonist that was approved by the US Food and Drug Administration for the treatment of plaque psoriasis in adults16 and is being studied for the treatment of plaque psoriasis in children 2 years and older as well as for atopic dermatitis in adults and children 2 years and older. In PSOARING 1 (ClinicalTrials .gov identifier NCT03956355) and PSOARING 2 (NCT03983980)—identical 12-week pivotal phase 3 trials—monotherapy with tapinarof cream 1% once daily (QD) demonstrated statistically significant efficacy vs vehicle cream and was well tolerated in adults with mild to severe plaque psoriasis (Supplementary Figure S1).17 Lebwohl et al17 reported that significantly higher PASI75 responses were observed at week 12 with tapinarof cream vs vehicle in PSOARING 1 and PSOARING 2 (36% and 48% vs 10% and 7%, respectively; both P<.0001). A significantly higher PASI90 response of 19% and 21% at week 12 also was observed with tapinarof cream vs 2% and 3% with vehicle in PSOARING 1 and PSOARING 2, respectively (P=.0005 and P<.0001).17
In PSOARING 3 (NCT04053387)—the long-term extension trial (Supplementary Figure S1)—efficacy continued to improve or was maintained beyond the two 12-week trials, with improvements in total BSA affected and PASI scores for up to 52 weeks.18 Tapinarof cream 1% QD demonstrated positive, rapid, and durable outcomes in PSOARING 3, including high rates of complete disease clearance (Physician Global Assessment [PGA] score=0 [clear])(40.9% [312/763]), durability of response on treatment with no evidence of tachyphylaxis, and a remittive effect of approximately 4 months when off therapy (defined as maintenance of a PGA score of 0 [clear] or 1 [almost clear] after first achieving a PGA score of 0).18
Herein, we report absolute treatment targets for patients with plaque psoriasis who received tapinarof cream 1% QD in the PSOARING trials that are at least as stringent as the corresponding NPF and EADV targets of achieving a total BSA affected of 1% or lower or a PASI75 response within 3 to 4 months, respectively.
METHODS
Study Design
The pooled efficacy analyses included all patients with a baseline PGA score of 2 or higher (mild or worse) before treatment with tapinarof cream 1% QD in the PSOARING trials. This included patients who received tapinarof cream 1% in PSOARING 1 and PSOARING 2 who may or may not have continued into PSOARING 3, as well as those who received the vehicle in PSOARING 1 and PSOARING 2 who enrolled in PSOARING 3 and had a PGA score of 2 or higher before receiving tapinarof cream 1%.
Trial Participants
Full methods, including inclusion and exclusion criteria, for the PSOARING trials have been previously reported.17,18 Patients were aged 18 to 75 years and had chronic plaque psoriasis that was stable for at least 6 months before randomization; 3% to 20% total BSA affected (excluding the scalp, palms, fingernails, toenails, and soles); and a PGA score of 2 (mild), 3 (moderate), or 4 (severe) at baseline.
The clinical trials were conducted in compliance with the guidelines for Good Clinical Practice and the Declaration of Helsinki. Approval was obtained from local ethics committees or institutional review boards at each center. All patients provided written informed consent.
Trial Treatment
In PSOARING 1 and PSOARING 2, patients were randomized (2:1) to receive tapinarof cream 1% or vehicle QD for 12 weeks. In PSOARING 3 (the long-term extension trial), patients received up to 40 weeks of open-label tapinarof, followed by 4 weeks of follow-up off treatment. Patients received intermittent or continuous treatment with tapinarof cream 1% in PSOARING 3 based on PGA score: those entering the trial with a PGA score of 1 or higher received tapinarof cream 1% until complete disease clearance was achieved (defined as a PGA score of 0 [clear]). Those entering PSOARING 3 with or achieving a PGA score of 0 (clear) discontinued treatment and were observed for the duration of maintenance of a PGA score of 0 (clear) or 1 (almost clear) while off therapy (the protocol-defined “duration of remittive effect”). If disease worsening (defined as a PGA score 2 or higher) occurred, tapinarof cream 1% was restarted and continued until a PGA score of 0 (clear) was achieved. This pattern of treatment, discontinuation on achieving a PGA score of 0 (clear), and retreatment on disease worsening continued until the end of the trial. As a result, patients in PSOARING 3 could receive tapinarof cream 1% continuously or intermittently for 40 weeks.
Outcome Measures and Statistical Analyses
The assessment of total BSA affected by plaque psoriasis is an estimate of the total extent of disease as a percentage of total skin area. In the PSOARING trials, the skin surface of one hand (palm and digits) was assumed to be approximately equivalent to 1% BSA. The total BSA affected by psoriasis was evaluated from 0% to 100%, with greater total BSA affected being an indication of more extensive disease. The BSA efficacy outcomes used in these analyses were based post hoc on the proportion of patients who achieved a 1% or lower or 0.5% or lower total BSA affected.
Psoriasis Area and Severity Index scores assess both the severity and extent of psoriasis. A PASI score lower than 5 often is considered indicative of mild psoriasis, a score of 5 to 10 indicates moderate disease, and a score higher than 10 indicates severe disease.19 The maximum PASI score is 72. The PASI efficacy outcomes used in these analyses were based post hoc on the proportion of patients who achieved an absolute total PASI score of 3 or lower, 2 or lower, and 1 or lower.
Efficacy analyses were based on pooled data for all patients in the PSOARING trials who had a PGA score of 2 to 4 (mild to severe) before treatment with tapinarof cream 1% in the intention-to-treat population using observed cases. Time-to-target analyses were based on Kaplan-Meier (KM) estimates using observed cases.
Safety analyses included the incidence and frequency of adverse events and were based on all patients who received tapinarof cream 1% in the PSOARING trials.
RESULTS
Baseline Patient Demographics and Disease Characteristics
The pooled efficacy analyses included 915 eligible patients (Table). At baseline, the mean (SD) age was 50.2 (13.25) years, 58.7% were male, the mean (SD) weight was 92.2 (23.67) kg, and the mean (SD) body mass index was 31.6 (7.53) kg/m2. The percentage of patients with a PGA score of 2 (mild), 3 (moderate), or 4 (severe) was 13.9%, 78.1%, and 8.0%, respectively. The mean (SD) PASI score was 8.7 (4.23) and mean (SD) total BSA affected was 7.8% (4.98).
Efficacy
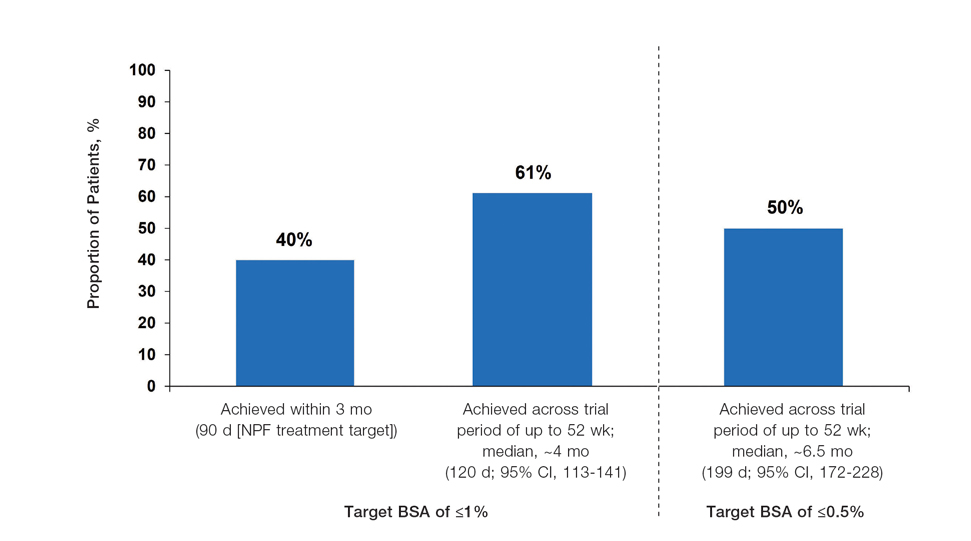
Achievement of BSA-Affected Targets—
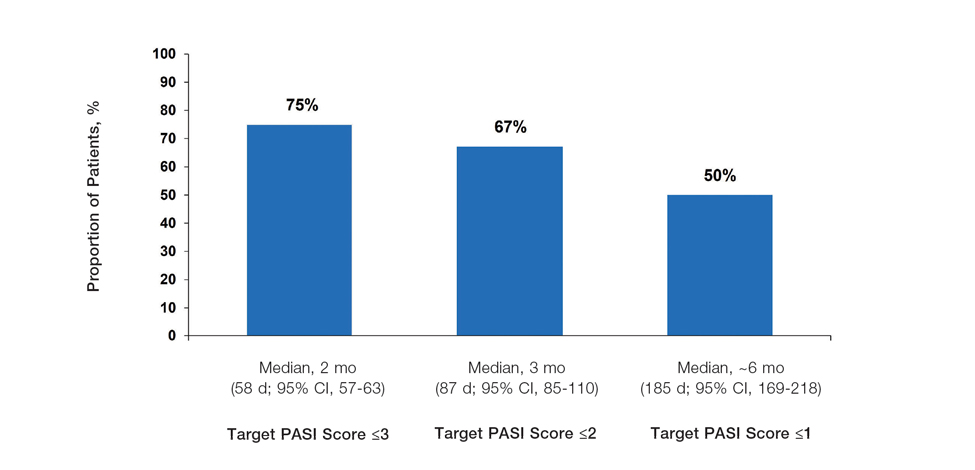
Achievement of Absolute PASI Targets—Across the total trial period (up to 52 weeks), an absolute total PASI score of 3 or lower was achieved by 75% of patients (686/915), with a median time to achieve this of 2 months (KM estimate: 58 days [95% CI, 57-63]); approximately 67% of patients (612/915) achieved a total PASI score of 2 or lower, with a median time to achieve of 3 months (KM estimate: 87 days [95% CI, 85-110])(Figure 2; Supplementary Figures S3a and S3b). A PASI score of 1 or lower was achieved by approximately 50% of patients (460/915), with a median time to achieve of approximately 6 months (KM estimate: 185 days [95% CI, 169-218])(Figure 2, Supplementary Figure S3c).
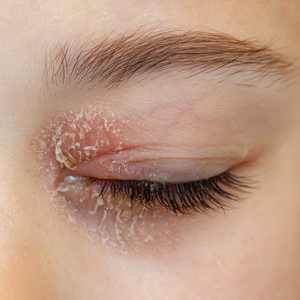
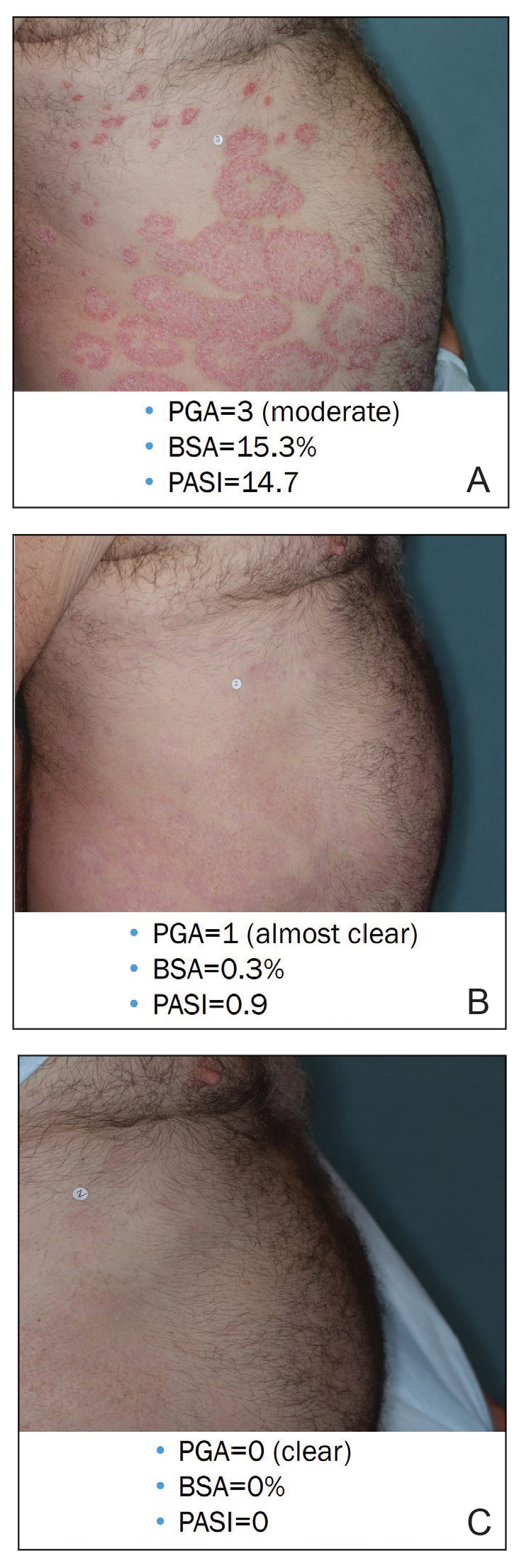
Illustrative Case—Case photography showing the clinical response in a 63-year-old man with moderate plaque psoriasis in PSOARING 2 is shown in Figure 3. After 12 weeks of treatment with tapinarof cream 1% QD, the patient achieved all primary and secondary efficacy end points. In addition to achieving the regulatory end point of a PGA score of 0 (clear) or 1 (almost clear) and a decrease from baseline of at least 2 points, achievement of 0% total BSA affected and a total PASI score of 0 at week 12 exceeded the NPF and EADV consensus treatment targets.10,11 Targets were achieved as early as week 4, with a total BSA affected of 0.5% or lower and a total PASI score of 1 or lower, illustrated by marked skin clearing and only faint residual erythema that completely resolved at week 12, with the absence of postinflammatory hyperpigmentation.
Safety
Safety data for the PSOARING trials have been previously reported.17,18 The most common treatment-emergent adverse events were folliculitis, contact dermatitis, upper respiratory tract infection, and nasopharyngitis. Treatment-emergent adverse events generally were mild or moderate in severity and did not lead to trial discontinuation.17,18
COMMENT
Treat-to-target management approaches aim to improve patient outcomes by striving to achieve optimal goals. The treat-to-target approach supports shared decision-making between clinicians and patients based on common expectations of what constitutes treatment success.
The findings of this analysis based on pooled data from a large cohort of patients demonstrate that a high proportion of patients can achieve or exceed recommended treatment targets with tapinarof cream 1% QD and maintain improvements long-term. The NPF-recommended treatment target of 1% or lower BSA affected within approximately 3 months (90 days) of treatment was achieved by 40% of tapinarof-treated patients. In addition, 1% or lower BSA affected at any time during the trials was achieved by 61% of patients (median, approximately 4 months). The analyses also indicated that PASI total scores of 3 or lower and 2 or lower were achieved by 75% and 67% of tapinarof-treated patients, respectively, within 2 to 3 months.
These findings support the previously reported efficacy of tapinarof cream, including high rates of complete disease clearance (40.9% [312/763]), durable response following treatment interruption, an off-therapy remittive effect of approximately 4 months, and good disease control on therapy with no evidence of tachyphylaxis.17,18
CONCLUSION
Taken together with previously reported tapinarof efficacy and safety results, our findings demonstrate that a high proportion of patients treated with tapinarof cream as monotherapy can achieve aggressive treatment targets set by both US and European guidelines developed for systemic and biologic therapies. Tapinarof cream 1% QD is an effective topical treatment option for patients with plaque psoriasis that has been approved without restrictions relating to severity or extent of disease treated, duration of use, or application sites, including application to sensitive and intertriginous skin.
Acknowledgments—Editorial and medical writing support under the guidance of the authors was provided by Melanie Govender, MSc (Med), ApotheCom (United Kingdom), and was funded by Dermavant Sciences, Inc, in accordance with Good Publication Practice (GPP) guidelines.
- Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157:940-946.
- Parisi R, Iskandar IYK, Kontopantelis E, et al. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ. 2020;369:m1590.
- Pilon D, Teeple A, Zhdanava M, et al. The economic burden of psoriasis with high comorbidity among privately insured patients in the United States. J Med Econ. 2019;22:196-203.
- Singh S, Taylor C, Kornmehl H, et al. Psoriasis and suicidality: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:425-440.e2.
- Feldman SR, Goffe B, Rice G, et al. The challenge of managing psoriasis: unmet medical needs and stakeholder perspectives. Am Health Drug Benefits. 2016;9:504-513.
- Ford JA, Solomon DH. Challenges in implementing treat-to-target strategies in rheumatology. Rheum Dis Clin North Am. 2019;45:101-112.
- Sitbon O, Galiè N. Treat-to-target strategies in pulmonary arterial hypertension: the importance of using multiple goals. Eur Respir Rev. 2010;19:272-278.
- Smolen JS, Aletaha D, Bijlsma JW, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2010;69:631-637.
- Wangnoo SK, Sethi B, Sahay RK, et al. Treat-to-target trials in diabetes. Indian J Endocrinol Metab. 2014;18:166-174.
- Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76:290-298.
- Pathirana D, Ormerod AD, Saiag P, et al. European S3-guidelines on the systemic treatment of psoriasis vulgaris. J Eur Acad Dermatol Venereol. 2009;23(Suppl 2):1-70.
- Strober BE, van der Walt JM, Armstrong AW, et al. Clinical goals and barriers to effective psoriasis care. Dermatol Ther (Heidelb). 2019; 9:5-18.
- Bagel J, Gold LS. Combining topical psoriasis treatment to enhance systemic and phototherapy: a review of the literature. J Drugs Dermatol. 2017;16:1209-1222.
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470.
- Stein Gold LF. Topical therapies for psoriasis: improving management strategies and patient adherence. Semin Cutan Med Surg. 2016;35 (2 Suppl 2):S36-S44; quiz S45.
- VTAMA® (tapinarof) cream. Prescribing information. Dermavant Sciences; 2022. Accessed September 13, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215272s000lbl.pdf
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229 and supplementary appendix.
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806.
- Clinical Review Report: Guselkumab (Tremfya) [Internet]. Canadian Agency for Drugs and Technologies in Health; 2018. Accessed September 13, 2024. https://www.ncbi.nlm.nih.gov/books/NBK534047/pdf/Bookshelf_NBK534047.pdf
Psoriasis is a chronic inflammatory disease affecting approximately 8 million adults in the United States and 2% of the global population.1,2 Psoriasis causes pain, itching, and disfigurement and is associated with a physical, psychological, and economic burden that substantially affects health-related quality of life.3-5
Setting treatment goals and treating to target are evidence-based approaches that have been successfully applied to several chronic diseases to improve patient outcomes, including diabetes, hypertension, and rheumatoid arthritis.6-9 Treat-to-target strategies generally set low disease activity (or remission) as an overall goal and seek to achieve this using available therapeutic options as necessary. Introduced following the availability of biologics and targeted systemic therapies, treat-to-target strategies generally provide guidance on expectations of treatment but not specific treatments, as personalized treatment decisions depend on an assessment of individual patients and consider clinical and demographic features as well as preferences for available therapeutic options. If targets are not achieved in the assigned time span, adjustments can be made to the treatment approach in close consultation with the patient. If the target is reached, follow-up visits can be scheduled to ensure improvement is maintained or to establish if more aggressive goals could be selected.
Treat-to-target strategies for the management of psoriasis developed by the National Psoriasis Foundation (NPF) Medical Board include reducing the extent of psoriasis to 1% or lower total body surface area (BSA) after 3 months of treatment.10 Treatment targets endorsed by the European Academy of Dermatology and Venereology (EADV) in guidelines on the use of systemic therapies in psoriasis include achieving a 75% or greater reduction in Psoriasis Area and Severity Index (PASI) score within 3 to 4 months of treatment.11
In clinical practice, many patients do not achieve these treatment targets, and topical treatments alone generally are insufficient in achieving treatment goals for psoriasis.12,13 Moreover, conventional topical treatments (eg, topical corticosteroids) used by most patients with psoriasis regardless of disease severity are associated with adverse events that can limit their use. Most topical corticosteroids have US Food and Drug Administration label restrictions relating to sites of application, duration and extent of use, and frequency of administration.14,15
Tapinarof cream 1% (VTAMA [Dermavant Sciences, Inc]) is a first-in-class topical nonsteroidal aryl hydrocarbon receptor agonist that was approved by the US Food and Drug Administration for the treatment of plaque psoriasis in adults16 and is being studied for the treatment of plaque psoriasis in children 2 years and older as well as for atopic dermatitis in adults and children 2 years and older. In PSOARING 1 (ClinicalTrials .gov identifier NCT03956355) and PSOARING 2 (NCT03983980)—identical 12-week pivotal phase 3 trials—monotherapy with tapinarof cream 1% once daily (QD) demonstrated statistically significant efficacy vs vehicle cream and was well tolerated in adults with mild to severe plaque psoriasis (Supplementary Figure S1).17 Lebwohl et al17 reported that significantly higher PASI75 responses were observed at week 12 with tapinarof cream vs vehicle in PSOARING 1 and PSOARING 2 (36% and 48% vs 10% and 7%, respectively; both P<.0001). A significantly higher PASI90 response of 19% and 21% at week 12 also was observed with tapinarof cream vs 2% and 3% with vehicle in PSOARING 1 and PSOARING 2, respectively (P=.0005 and P<.0001).17
In PSOARING 3 (NCT04053387)—the long-term extension trial (Supplementary Figure S1)—efficacy continued to improve or was maintained beyond the two 12-week trials, with improvements in total BSA affected and PASI scores for up to 52 weeks.18 Tapinarof cream 1% QD demonstrated positive, rapid, and durable outcomes in PSOARING 3, including high rates of complete disease clearance (Physician Global Assessment [PGA] score=0 [clear])(40.9% [312/763]), durability of response on treatment with no evidence of tachyphylaxis, and a remittive effect of approximately 4 months when off therapy (defined as maintenance of a PGA score of 0 [clear] or 1 [almost clear] after first achieving a PGA score of 0).18
Herein, we report absolute treatment targets for patients with plaque psoriasis who received tapinarof cream 1% QD in the PSOARING trials that are at least as stringent as the corresponding NPF and EADV targets of achieving a total BSA affected of 1% or lower or a PASI75 response within 3 to 4 months, respectively.
METHODS
Study Design
The pooled efficacy analyses included all patients with a baseline PGA score of 2 or higher (mild or worse) before treatment with tapinarof cream 1% QD in the PSOARING trials. This included patients who received tapinarof cream 1% in PSOARING 1 and PSOARING 2 who may or may not have continued into PSOARING 3, as well as those who received the vehicle in PSOARING 1 and PSOARING 2 who enrolled in PSOARING 3 and had a PGA score of 2 or higher before receiving tapinarof cream 1%.
Trial Participants
Full methods, including inclusion and exclusion criteria, for the PSOARING trials have been previously reported.17,18 Patients were aged 18 to 75 years and had chronic plaque psoriasis that was stable for at least 6 months before randomization; 3% to 20% total BSA affected (excluding the scalp, palms, fingernails, toenails, and soles); and a PGA score of 2 (mild), 3 (moderate), or 4 (severe) at baseline.
The clinical trials were conducted in compliance with the guidelines for Good Clinical Practice and the Declaration of Helsinki. Approval was obtained from local ethics committees or institutional review boards at each center. All patients provided written informed consent.
Trial Treatment
In PSOARING 1 and PSOARING 2, patients were randomized (2:1) to receive tapinarof cream 1% or vehicle QD for 12 weeks. In PSOARING 3 (the long-term extension trial), patients received up to 40 weeks of open-label tapinarof, followed by 4 weeks of follow-up off treatment. Patients received intermittent or continuous treatment with tapinarof cream 1% in PSOARING 3 based on PGA score: those entering the trial with a PGA score of 1 or higher received tapinarof cream 1% until complete disease clearance was achieved (defined as a PGA score of 0 [clear]). Those entering PSOARING 3 with or achieving a PGA score of 0 (clear) discontinued treatment and were observed for the duration of maintenance of a PGA score of 0 (clear) or 1 (almost clear) while off therapy (the protocol-defined “duration of remittive effect”). If disease worsening (defined as a PGA score 2 or higher) occurred, tapinarof cream 1% was restarted and continued until a PGA score of 0 (clear) was achieved. This pattern of treatment, discontinuation on achieving a PGA score of 0 (clear), and retreatment on disease worsening continued until the end of the trial. As a result, patients in PSOARING 3 could receive tapinarof cream 1% continuously or intermittently for 40 weeks.
Outcome Measures and Statistical Analyses
The assessment of total BSA affected by plaque psoriasis is an estimate of the total extent of disease as a percentage of total skin area. In the PSOARING trials, the skin surface of one hand (palm and digits) was assumed to be approximately equivalent to 1% BSA. The total BSA affected by psoriasis was evaluated from 0% to 100%, with greater total BSA affected being an indication of more extensive disease. The BSA efficacy outcomes used in these analyses were based post hoc on the proportion of patients who achieved a 1% or lower or 0.5% or lower total BSA affected.
Psoriasis Area and Severity Index scores assess both the severity and extent of psoriasis. A PASI score lower than 5 often is considered indicative of mild psoriasis, a score of 5 to 10 indicates moderate disease, and a score higher than 10 indicates severe disease.19 The maximum PASI score is 72. The PASI efficacy outcomes used in these analyses were based post hoc on the proportion of patients who achieved an absolute total PASI score of 3 or lower, 2 or lower, and 1 or lower.
Efficacy analyses were based on pooled data for all patients in the PSOARING trials who had a PGA score of 2 to 4 (mild to severe) before treatment with tapinarof cream 1% in the intention-to-treat population using observed cases. Time-to-target analyses were based on Kaplan-Meier (KM) estimates using observed cases.
Safety analyses included the incidence and frequency of adverse events and were based on all patients who received tapinarof cream 1% in the PSOARING trials.
RESULTS
Baseline Patient Demographics and Disease Characteristics
The pooled efficacy analyses included 915 eligible patients (Table). At baseline, the mean (SD) age was 50.2 (13.25) years, 58.7% were male, the mean (SD) weight was 92.2 (23.67) kg, and the mean (SD) body mass index was 31.6 (7.53) kg/m2. The percentage of patients with a PGA score of 2 (mild), 3 (moderate), or 4 (severe) was 13.9%, 78.1%, and 8.0%, respectively. The mean (SD) PASI score was 8.7 (4.23) and mean (SD) total BSA affected was 7.8% (4.98).
Efficacy
Achievement of BSA-Affected Targets—



Achievement of Absolute PASI Targets—Across the total trial period (up to 52 weeks), an absolute total PASI score of 3 or lower was achieved by 75% of patients (686/915), with a median time to achieve this of 2 months (KM estimate: 58 days [95% CI, 57-63]); approximately 67% of patients (612/915) achieved a total PASI score of 2 or lower, with a median time to achieve of 3 months (KM estimate: 87 days [95% CI, 85-110])(Figure 2; Supplementary Figures S3a and S3b). A PASI score of 1 or lower was achieved by approximately 50% of patients (460/915), with a median time to achieve of approximately 6 months (KM estimate: 185 days [95% CI, 169-218])(Figure 2, Supplementary Figure S3c).
Illustrative Case—Case photography showing the clinical response in a 63-year-old man with moderate plaque psoriasis in PSOARING 2 is shown in Figure 3. After 12 weeks of treatment with tapinarof cream 1% QD, the patient achieved all primary and secondary efficacy end points. In addition to achieving the regulatory end point of a PGA score of 0 (clear) or 1 (almost clear) and a decrease from baseline of at least 2 points, achievement of 0% total BSA affected and a total PASI score of 0 at week 12 exceeded the NPF and EADV consensus treatment targets.10,11 Targets were achieved as early as week 4, with a total BSA affected of 0.5% or lower and a total PASI score of 1 or lower, illustrated by marked skin clearing and only faint residual erythema that completely resolved at week 12, with the absence of postinflammatory hyperpigmentation.
Safety
Safety data for the PSOARING trials have been previously reported.17,18 The most common treatment-emergent adverse events were folliculitis, contact dermatitis, upper respiratory tract infection, and nasopharyngitis. Treatment-emergent adverse events generally were mild or moderate in severity and did not lead to trial discontinuation.17,18

COMMENT
Treat-to-target management approaches aim to improve patient outcomes by striving to achieve optimal goals. The treat-to-target approach supports shared decision-making between clinicians and patients based on common expectations of what constitutes treatment success.
The findings of this analysis based on pooled data from a large cohort of patients demonstrate that a high proportion of patients can achieve or exceed recommended treatment targets with tapinarof cream 1% QD and maintain improvements long-term. The NPF-recommended treatment target of 1% or lower BSA affected within approximately 3 months (90 days) of treatment was achieved by 40% of tapinarof-treated patients. In addition, 1% or lower BSA affected at any time during the trials was achieved by 61% of patients (median, approximately 4 months). The analyses also indicated that PASI total scores of 3 or lower and 2 or lower were achieved by 75% and 67% of tapinarof-treated patients, respectively, within 2 to 3 months.
These findings support the previously reported efficacy of tapinarof cream, including high rates of complete disease clearance (40.9% [312/763]), durable response following treatment interruption, an off-therapy remittive effect of approximately 4 months, and good disease control on therapy with no evidence of tachyphylaxis.17,18
CONCLUSION
Taken together with previously reported tapinarof efficacy and safety results, our findings demonstrate that a high proportion of patients treated with tapinarof cream as monotherapy can achieve aggressive treatment targets set by both US and European guidelines developed for systemic and biologic therapies. Tapinarof cream 1% QD is an effective topical treatment option for patients with plaque psoriasis that has been approved without restrictions relating to severity or extent of disease treated, duration of use, or application sites, including application to sensitive and intertriginous skin.
Acknowledgments—Editorial and medical writing support under the guidance of the authors was provided by Melanie Govender, MSc (Med), ApotheCom (United Kingdom), and was funded by Dermavant Sciences, Inc, in accordance with Good Publication Practice (GPP) guidelines.
Psoriasis is a chronic inflammatory disease affecting approximately 8 million adults in the United States and 2% of the global population.1,2 Psoriasis causes pain, itching, and disfigurement and is associated with a physical, psychological, and economic burden that substantially affects health-related quality of life.3-5
Setting treatment goals and treating to target are evidence-based approaches that have been successfully applied to several chronic diseases to improve patient outcomes, including diabetes, hypertension, and rheumatoid arthritis.6-9 Treat-to-target strategies generally set low disease activity (or remission) as an overall goal and seek to achieve this using available therapeutic options as necessary. Introduced following the availability of biologics and targeted systemic therapies, treat-to-target strategies generally provide guidance on expectations of treatment but not specific treatments, as personalized treatment decisions depend on an assessment of individual patients and consider clinical and demographic features as well as preferences for available therapeutic options. If targets are not achieved in the assigned time span, adjustments can be made to the treatment approach in close consultation with the patient. If the target is reached, follow-up visits can be scheduled to ensure improvement is maintained or to establish if more aggressive goals could be selected.
Treat-to-target strategies for the management of psoriasis developed by the National Psoriasis Foundation (NPF) Medical Board include reducing the extent of psoriasis to 1% or lower total body surface area (BSA) after 3 months of treatment.10 Treatment targets endorsed by the European Academy of Dermatology and Venereology (EADV) in guidelines on the use of systemic therapies in psoriasis include achieving a 75% or greater reduction in Psoriasis Area and Severity Index (PASI) score within 3 to 4 months of treatment.11
In clinical practice, many patients do not achieve these treatment targets, and topical treatments alone generally are insufficient in achieving treatment goals for psoriasis.12,13 Moreover, conventional topical treatments (eg, topical corticosteroids) used by most patients with psoriasis regardless of disease severity are associated with adverse events that can limit their use. Most topical corticosteroids have US Food and Drug Administration label restrictions relating to sites of application, duration and extent of use, and frequency of administration.14,15
Tapinarof cream 1% (VTAMA [Dermavant Sciences, Inc]) is a first-in-class topical nonsteroidal aryl hydrocarbon receptor agonist that was approved by the US Food and Drug Administration for the treatment of plaque psoriasis in adults16 and is being studied for the treatment of plaque psoriasis in children 2 years and older as well as for atopic dermatitis in adults and children 2 years and older. In PSOARING 1 (ClinicalTrials .gov identifier NCT03956355) and PSOARING 2 (NCT03983980)—identical 12-week pivotal phase 3 trials—monotherapy with tapinarof cream 1% once daily (QD) demonstrated statistically significant efficacy vs vehicle cream and was well tolerated in adults with mild to severe plaque psoriasis (Supplementary Figure S1).17 Lebwohl et al17 reported that significantly higher PASI75 responses were observed at week 12 with tapinarof cream vs vehicle in PSOARING 1 and PSOARING 2 (36% and 48% vs 10% and 7%, respectively; both P<.0001). A significantly higher PASI90 response of 19% and 21% at week 12 also was observed with tapinarof cream vs 2% and 3% with vehicle in PSOARING 1 and PSOARING 2, respectively (P=.0005 and P<.0001).17
In PSOARING 3 (NCT04053387)—the long-term extension trial (Supplementary Figure S1)—efficacy continued to improve or was maintained beyond the two 12-week trials, with improvements in total BSA affected and PASI scores for up to 52 weeks.18 Tapinarof cream 1% QD demonstrated positive, rapid, and durable outcomes in PSOARING 3, including high rates of complete disease clearance (Physician Global Assessment [PGA] score=0 [clear])(40.9% [312/763]), durability of response on treatment with no evidence of tachyphylaxis, and a remittive effect of approximately 4 months when off therapy (defined as maintenance of a PGA score of 0 [clear] or 1 [almost clear] after first achieving a PGA score of 0).18
Herein, we report absolute treatment targets for patients with plaque psoriasis who received tapinarof cream 1% QD in the PSOARING trials that are at least as stringent as the corresponding NPF and EADV targets of achieving a total BSA affected of 1% or lower or a PASI75 response within 3 to 4 months, respectively.
METHODS
Study Design
The pooled efficacy analyses included all patients with a baseline PGA score of 2 or higher (mild or worse) before treatment with tapinarof cream 1% QD in the PSOARING trials. This included patients who received tapinarof cream 1% in PSOARING 1 and PSOARING 2 who may or may not have continued into PSOARING 3, as well as those who received the vehicle in PSOARING 1 and PSOARING 2 who enrolled in PSOARING 3 and had a PGA score of 2 or higher before receiving tapinarof cream 1%.
Trial Participants
Full methods, including inclusion and exclusion criteria, for the PSOARING trials have been previously reported.17,18 Patients were aged 18 to 75 years and had chronic plaque psoriasis that was stable for at least 6 months before randomization; 3% to 20% total BSA affected (excluding the scalp, palms, fingernails, toenails, and soles); and a PGA score of 2 (mild), 3 (moderate), or 4 (severe) at baseline.
The clinical trials were conducted in compliance with the guidelines for Good Clinical Practice and the Declaration of Helsinki. Approval was obtained from local ethics committees or institutional review boards at each center. All patients provided written informed consent.
Trial Treatment
In PSOARING 1 and PSOARING 2, patients were randomized (2:1) to receive tapinarof cream 1% or vehicle QD for 12 weeks. In PSOARING 3 (the long-term extension trial), patients received up to 40 weeks of open-label tapinarof, followed by 4 weeks of follow-up off treatment. Patients received intermittent or continuous treatment with tapinarof cream 1% in PSOARING 3 based on PGA score: those entering the trial with a PGA score of 1 or higher received tapinarof cream 1% until complete disease clearance was achieved (defined as a PGA score of 0 [clear]). Those entering PSOARING 3 with or achieving a PGA score of 0 (clear) discontinued treatment and were observed for the duration of maintenance of a PGA score of 0 (clear) or 1 (almost clear) while off therapy (the protocol-defined “duration of remittive effect”). If disease worsening (defined as a PGA score 2 or higher) occurred, tapinarof cream 1% was restarted and continued until a PGA score of 0 (clear) was achieved. This pattern of treatment, discontinuation on achieving a PGA score of 0 (clear), and retreatment on disease worsening continued until the end of the trial. As a result, patients in PSOARING 3 could receive tapinarof cream 1% continuously or intermittently for 40 weeks.
Outcome Measures and Statistical Analyses
The assessment of total BSA affected by plaque psoriasis is an estimate of the total extent of disease as a percentage of total skin area. In the PSOARING trials, the skin surface of one hand (palm and digits) was assumed to be approximately equivalent to 1% BSA. The total BSA affected by psoriasis was evaluated from 0% to 100%, with greater total BSA affected being an indication of more extensive disease. The BSA efficacy outcomes used in these analyses were based post hoc on the proportion of patients who achieved a 1% or lower or 0.5% or lower total BSA affected.
Psoriasis Area and Severity Index scores assess both the severity and extent of psoriasis. A PASI score lower than 5 often is considered indicative of mild psoriasis, a score of 5 to 10 indicates moderate disease, and a score higher than 10 indicates severe disease.19 The maximum PASI score is 72. The PASI efficacy outcomes used in these analyses were based post hoc on the proportion of patients who achieved an absolute total PASI score of 3 or lower, 2 or lower, and 1 or lower.
Efficacy analyses were based on pooled data for all patients in the PSOARING trials who had a PGA score of 2 to 4 (mild to severe) before treatment with tapinarof cream 1% in the intention-to-treat population using observed cases. Time-to-target analyses were based on Kaplan-Meier (KM) estimates using observed cases.
Safety analyses included the incidence and frequency of adverse events and were based on all patients who received tapinarof cream 1% in the PSOARING trials.
RESULTS
Baseline Patient Demographics and Disease Characteristics
The pooled efficacy analyses included 915 eligible patients (Table). At baseline, the mean (SD) age was 50.2 (13.25) years, 58.7% were male, the mean (SD) weight was 92.2 (23.67) kg, and the mean (SD) body mass index was 31.6 (7.53) kg/m2. The percentage of patients with a PGA score of 2 (mild), 3 (moderate), or 4 (severe) was 13.9%, 78.1%, and 8.0%, respectively. The mean (SD) PASI score was 8.7 (4.23) and mean (SD) total BSA affected was 7.8% (4.98).
Efficacy
Achievement of BSA-Affected Targets—



Achievement of Absolute PASI Targets—Across the total trial period (up to 52 weeks), an absolute total PASI score of 3 or lower was achieved by 75% of patients (686/915), with a median time to achieve this of 2 months (KM estimate: 58 days [95% CI, 57-63]); approximately 67% of patients (612/915) achieved a total PASI score of 2 or lower, with a median time to achieve of 3 months (KM estimate: 87 days [95% CI, 85-110])(Figure 2; Supplementary Figures S3a and S3b). A PASI score of 1 or lower was achieved by approximately 50% of patients (460/915), with a median time to achieve of approximately 6 months (KM estimate: 185 days [95% CI, 169-218])(Figure 2, Supplementary Figure S3c).
Illustrative Case—Case photography showing the clinical response in a 63-year-old man with moderate plaque psoriasis in PSOARING 2 is shown in Figure 3. After 12 weeks of treatment with tapinarof cream 1% QD, the patient achieved all primary and secondary efficacy end points. In addition to achieving the regulatory end point of a PGA score of 0 (clear) or 1 (almost clear) and a decrease from baseline of at least 2 points, achievement of 0% total BSA affected and a total PASI score of 0 at week 12 exceeded the NPF and EADV consensus treatment targets.10,11 Targets were achieved as early as week 4, with a total BSA affected of 0.5% or lower and a total PASI score of 1 or lower, illustrated by marked skin clearing and only faint residual erythema that completely resolved at week 12, with the absence of postinflammatory hyperpigmentation.
Safety
Safety data for the PSOARING trials have been previously reported.17,18 The most common treatment-emergent adverse events were folliculitis, contact dermatitis, upper respiratory tract infection, and nasopharyngitis. Treatment-emergent adverse events generally were mild or moderate in severity and did not lead to trial discontinuation.17,18

COMMENT
Treat-to-target management approaches aim to improve patient outcomes by striving to achieve optimal goals. The treat-to-target approach supports shared decision-making between clinicians and patients based on common expectations of what constitutes treatment success.
The findings of this analysis based on pooled data from a large cohort of patients demonstrate that a high proportion of patients can achieve or exceed recommended treatment targets with tapinarof cream 1% QD and maintain improvements long-term. The NPF-recommended treatment target of 1% or lower BSA affected within approximately 3 months (90 days) of treatment was achieved by 40% of tapinarof-treated patients. In addition, 1% or lower BSA affected at any time during the trials was achieved by 61% of patients (median, approximately 4 months). The analyses also indicated that PASI total scores of 3 or lower and 2 or lower were achieved by 75% and 67% of tapinarof-treated patients, respectively, within 2 to 3 months.
These findings support the previously reported efficacy of tapinarof cream, including high rates of complete disease clearance (40.9% [312/763]), durable response following treatment interruption, an off-therapy remittive effect of approximately 4 months, and good disease control on therapy with no evidence of tachyphylaxis.17,18
CONCLUSION
Taken together with previously reported tapinarof efficacy and safety results, our findings demonstrate that a high proportion of patients treated with tapinarof cream as monotherapy can achieve aggressive treatment targets set by both US and European guidelines developed for systemic and biologic therapies. Tapinarof cream 1% QD is an effective topical treatment option for patients with plaque psoriasis that has been approved without restrictions relating to severity or extent of disease treated, duration of use, or application sites, including application to sensitive and intertriginous skin.
Acknowledgments—Editorial and medical writing support under the guidance of the authors was provided by Melanie Govender, MSc (Med), ApotheCom (United Kingdom), and was funded by Dermavant Sciences, Inc, in accordance with Good Publication Practice (GPP) guidelines.
- Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157:940-946.
- Parisi R, Iskandar IYK, Kontopantelis E, et al. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ. 2020;369:m1590.
- Pilon D, Teeple A, Zhdanava M, et al. The economic burden of psoriasis with high comorbidity among privately insured patients in the United States. J Med Econ. 2019;22:196-203.
- Singh S, Taylor C, Kornmehl H, et al. Psoriasis and suicidality: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:425-440.e2.
- Feldman SR, Goffe B, Rice G, et al. The challenge of managing psoriasis: unmet medical needs and stakeholder perspectives. Am Health Drug Benefits. 2016;9:504-513.
- Ford JA, Solomon DH. Challenges in implementing treat-to-target strategies in rheumatology. Rheum Dis Clin North Am. 2019;45:101-112.
- Sitbon O, Galiè N. Treat-to-target strategies in pulmonary arterial hypertension: the importance of using multiple goals. Eur Respir Rev. 2010;19:272-278.
- Smolen JS, Aletaha D, Bijlsma JW, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2010;69:631-637.
- Wangnoo SK, Sethi B, Sahay RK, et al. Treat-to-target trials in diabetes. Indian J Endocrinol Metab. 2014;18:166-174.
- Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76:290-298.
- Pathirana D, Ormerod AD, Saiag P, et al. European S3-guidelines on the systemic treatment of psoriasis vulgaris. J Eur Acad Dermatol Venereol. 2009;23(Suppl 2):1-70.
- Strober BE, van der Walt JM, Armstrong AW, et al. Clinical goals and barriers to effective psoriasis care. Dermatol Ther (Heidelb). 2019; 9:5-18.
- Bagel J, Gold LS. Combining topical psoriasis treatment to enhance systemic and phototherapy: a review of the literature. J Drugs Dermatol. 2017;16:1209-1222.
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470.
- Stein Gold LF. Topical therapies for psoriasis: improving management strategies and patient adherence. Semin Cutan Med Surg. 2016;35 (2 Suppl 2):S36-S44; quiz S45.
- VTAMA® (tapinarof) cream. Prescribing information. Dermavant Sciences; 2022. Accessed September 13, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215272s000lbl.pdf
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229 and supplementary appendix.
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806.
- Clinical Review Report: Guselkumab (Tremfya) [Internet]. Canadian Agency for Drugs and Technologies in Health; 2018. Accessed September 13, 2024. https://www.ncbi.nlm.nih.gov/books/NBK534047/pdf/Bookshelf_NBK534047.pdf
- Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157:940-946.
- Parisi R, Iskandar IYK, Kontopantelis E, et al. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ. 2020;369:m1590.
- Pilon D, Teeple A, Zhdanava M, et al. The economic burden of psoriasis with high comorbidity among privately insured patients in the United States. J Med Econ. 2019;22:196-203.
- Singh S, Taylor C, Kornmehl H, et al. Psoriasis and suicidality: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:425-440.e2.
- Feldman SR, Goffe B, Rice G, et al. The challenge of managing psoriasis: unmet medical needs and stakeholder perspectives. Am Health Drug Benefits. 2016;9:504-513.
- Ford JA, Solomon DH. Challenges in implementing treat-to-target strategies in rheumatology. Rheum Dis Clin North Am. 2019;45:101-112.
- Sitbon O, Galiè N. Treat-to-target strategies in pulmonary arterial hypertension: the importance of using multiple goals. Eur Respir Rev. 2010;19:272-278.
- Smolen JS, Aletaha D, Bijlsma JW, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2010;69:631-637.
- Wangnoo SK, Sethi B, Sahay RK, et al. Treat-to-target trials in diabetes. Indian J Endocrinol Metab. 2014;18:166-174.
- Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76:290-298.
- Pathirana D, Ormerod AD, Saiag P, et al. European S3-guidelines on the systemic treatment of psoriasis vulgaris. J Eur Acad Dermatol Venereol. 2009;23(Suppl 2):1-70.
- Strober BE, van der Walt JM, Armstrong AW, et al. Clinical goals and barriers to effective psoriasis care. Dermatol Ther (Heidelb). 2019; 9:5-18.
- Bagel J, Gold LS. Combining topical psoriasis treatment to enhance systemic and phototherapy: a review of the literature. J Drugs Dermatol. 2017;16:1209-1222.
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470.
- Stein Gold LF. Topical therapies for psoriasis: improving management strategies and patient adherence. Semin Cutan Med Surg. 2016;35 (2 Suppl 2):S36-S44; quiz S45.
- VTAMA® (tapinarof) cream. Prescribing information. Dermavant Sciences; 2022. Accessed September 13, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215272s000lbl.pdf
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229 and supplementary appendix.
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806.
- Clinical Review Report: Guselkumab (Tremfya) [Internet]. Canadian Agency for Drugs and Technologies in Health; 2018. Accessed September 13, 2024. https://www.ncbi.nlm.nih.gov/books/NBK534047/pdf/Bookshelf_NBK534047.pdf
Practice Points
- In clinical practice, many patients with psoriasis do not achieve treatment targets set forth by the National Psoriasis Foundation and the European Academy of Dermatology and Venereology, and topical treatments alone generally are insufficient in achieving treatment goals for psoriasis.
- Tapinarof cream 1% is a nonsteroidal aryl hydrocarbon receptor agonist approved by the US Food and Drug Administration for the treatment of plaque psoriasis in adults; it also is being studied for the treatment of plaque psoriasis in children 2 years and older.
- Tapinarof cream 1% is an effective topical treatment option for patients with plaque psoriasis of any severity, with no limitations on treatment duration, total extent of use, or application sites, including intertriginous skin and sensitive areas.
Hairless Scalp Lesion
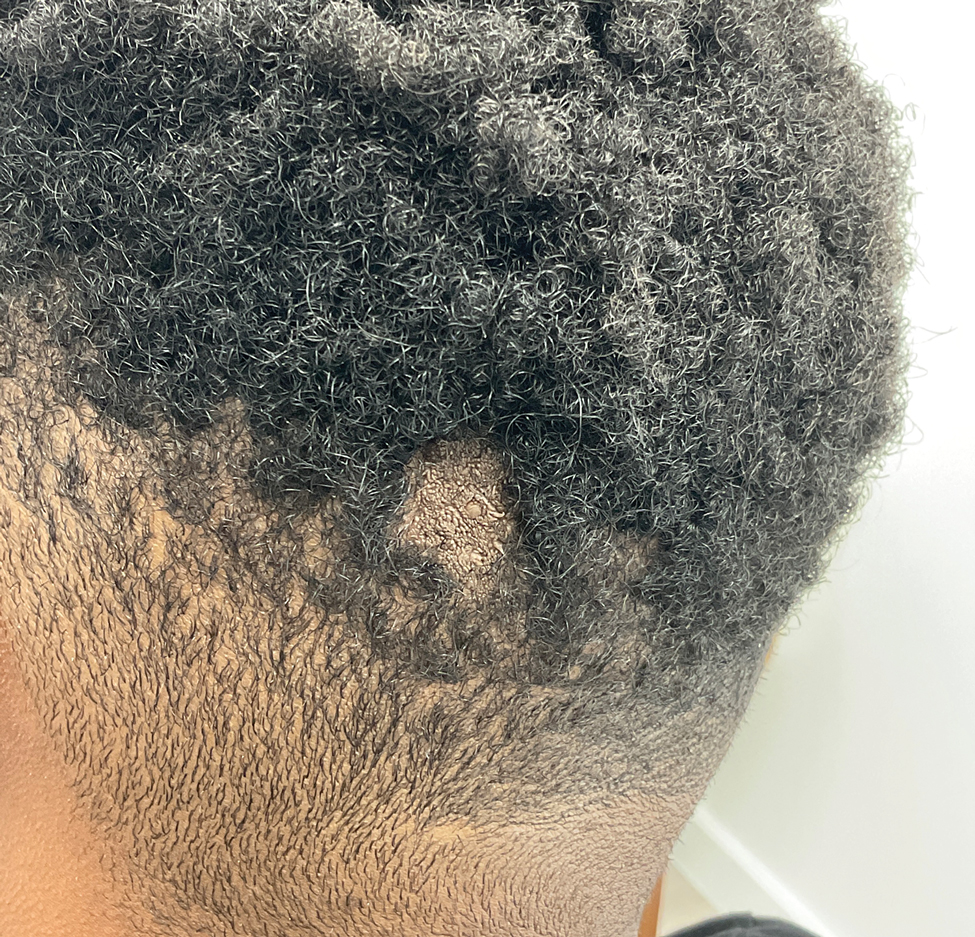
The Diagnosis: Nevus Sebaceus of Jadassohn
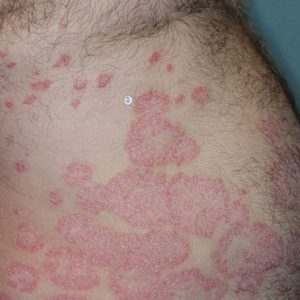
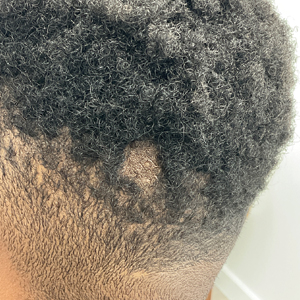
The diagnosis of nevus sebaceus of Jadassohn was made clinically based on the lesion’s appearance and presence since birth as well as the absence of systemic symptoms. Clinically, nevus sebaceus of Jadassohn typically manifests as a well-demarcated, yellow- brown plaque often located on the scalp, as was seen in our patient. The lack of pruritus and pain further supported the diagnosis in our patient. No biopsy was performed, as the presentation was considered classic for this condition. Our patient opted to forgo surgery and will be routinely monitored for any changes, as nevus sebaceus has a potential risk, albeit low, for malignant transformation later in life. No changes have been observed since the initial presentation, and regular follow-ups are planned to monitor for future developments.
Nevus sebaceus of Jadassohn is a hamartomatous lesion involving the pilosebaceous follicle and adjacent adnexal structures.1-3 It most commonly forms on the scalp (59.3%) and is accompanied by partial or total alopecia. 3,4 It is seen less often on the face, periauricular area, or neck1,4; thorax or limbs5; and oral or genital mucosae.6 Nevus sebaceus of Jadassohn affects approximately 0.3% of newborns,1 usually as a solitary lesion that can form an extensive plaque. The male-to-female occurrence ratio has been reported as equal to slightly more predominant in females; all races and ethnicities are affected.1,5
Nevus sebaceus of Jadassohn follows 3 stages of clinical development: infantile, adolescent, and adulthood. It manifests at birth or shortly afterward as a smooth hairless patch or plaque that is yellowish and can be hyperpigmented in Black patients.5 It may have an oval or linear configuration, typically is asymptomatic, and often arises along the Blaschko lines when it occurs as multiple lesions (a rare manifestation).1 During puberty, hormonal changes cause accelerated growth, sebaceous gland maturation, and epidermal hyperplasia. 7 Nevus sebaceus of Jadassohn often is not identified until this stage, when its classic wartlike appearance has fully developed.1
Patients with nevus sebaceus of Jadassohn have a 10% to 20% risk for tumor development in adulthood.2,7 Trichoblastoma and syringocystadenoma papilliferum are the most frequently described neoplasms.8 Basal cell carcinoma is the most common malignant secondary neoplasm with an occurrence rate of 0.8%.6,9 However, basal cell carcinoma and trichoblastoma may share histopathologic features, which may lead to misdiagnosis and a higher reported incidence of basal cell carcinoma in adults than is accurate.2
Early prophylactic surgical removal of nevus sebaceus of Jadassohn has been recommended; however, surgical management is controversial because the risk for a benign secondary neoplasm remains relatively high while the risk for malignancy is much lower.2,7 Surgical excision remains an acceptable option once the patient is mature enough to tolerate the procedure.1 However, patient education regarding watchful waiting vs a surgical approach— and the risks of each—is critical to ensure shared decision-making and a management plan tailored to the individual.
The differential diagnosis includes hypertrophic lichen planus, Langerhans cell histiocytosis (Letterer-Siwe disease type), epidermal nevus, and seborrheic keratosis. Hypertrophic lichen planus often occurs symmetrically on the dorsal feet and shins with thick, scaly, and extremely pruritic plaques. The lesions often persist for an average of 6 years and may lead to multiple keratoacanthomas or follicular base squamous cell carcinomas. Langerhans cell histiocytosis (Letterer-Siwe disease type) manifests with acute, disseminated, visceral, and cutaneous lesions before 2 years of age. These lesions appear as 1- to 2-mm, pink, seborrheic papules, pustules, or vesicles on the scalp, flexural neck, axilla, perineum, and trunk; they often are associated with petechiae, purpura, scale, crust, erosion, impetiginization, and tender fissures. Epidermal nevus occurs within the first year of life and is a hamartoma of the epidermis and papillary dermis. It manifests as papillomatous pigmented linear lines along the Blaschko lines. Seborrheic keratosis manifests as well-demarcated, waxy/verrucous, brown papules with a “stuck on” appearance on hair-bearing skin sparing the mucosae. They are common benign lesions associated with sun exposure and often manifest in the fourth decade of life.10
- Baigrie D, Troxell T, Cook C. Nevus sebaceus. StatPearls [Internet]. Updated August 16, 2023. Accessed September 12, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482493/
- Terenzi V, Indrizzi E, Buonaccorsi S, et al. Nevus sebaceus of Jadassohn. J Craniofac Surg. 2006;17:1234-1239. doi:10.1097/01 .scs.0000221531.56529.cc
- Kelati A, Baybay H, Gallouj S, et al. Dermoscopic analysis of nevus sebaceus of Jadassohn: a study of 13 cases. Skin Appendage Disord. 2017;3:83-91. doi:10.1159/000460258
- Ugras N, Ozgun G, Adim SB, et al. Nevus sebaceous at unusual location: a rare presentation. Indian J Pathol Microbiol. 2012;55:419-420. doi:10.4103/0377-4929.101768
- Serpas de Lopez RM, Hernandez-Perez E. Jadassohn’s sebaceous nevus. J Dermatol Surg Oncol. 1985;11:68-72. doi:10.1111/j.1524-4725 .1985.tb02893.x
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42(2 pt 1):263-268. doi:10.1016/S0190-9622(00)90136-1
- Santibanez-Gallerani A, Marshall D, Duarte AM, et al. Should nevus sebaceus of Jadassohn in children be excised? a study of 757 cases, and literature review. J Craniofac Surg. 2003;14:658-660. doi:10.1097/00001665-200309000-00010
- Chahboun F, Eljazouly M, Elomari M, et al. Trichoblastoma arising from the nevus sebaceus of Jadassohn. Cureus. 2021;13:E15325. doi:10.7759/cureus.15325
- Cazzato G, Cimmino A, Colagrande A, et al. The multiple faces of nodular trichoblastoma: review of the literature with case presentation. Dermatopathology (Basel). 2021;8:265-270. doi:10.3390 /dermatopathology8030032
- Dandekar MN, Gandhi RK. Neoplastic dermatology. In: Alikhan A, Hocker TLH (eds). Review of Dermatology. Elsevier; 2016: 321-366.
The Diagnosis: Nevus Sebaceus of Jadassohn
The diagnosis of nevus sebaceus of Jadassohn was made clinically based on the lesion’s appearance and presence since birth as well as the absence of systemic symptoms. Clinically, nevus sebaceus of Jadassohn typically manifests as a well-demarcated, yellow- brown plaque often located on the scalp, as was seen in our patient. The lack of pruritus and pain further supported the diagnosis in our patient. No biopsy was performed, as the presentation was considered classic for this condition. Our patient opted to forgo surgery and will be routinely monitored for any changes, as nevus sebaceus has a potential risk, albeit low, for malignant transformation later in life. No changes have been observed since the initial presentation, and regular follow-ups are planned to monitor for future developments.
Nevus sebaceus of Jadassohn is a hamartomatous lesion involving the pilosebaceous follicle and adjacent adnexal structures.1-3 It most commonly forms on the scalp (59.3%) and is accompanied by partial or total alopecia. 3,4 It is seen less often on the face, periauricular area, or neck1,4; thorax or limbs5; and oral or genital mucosae.6 Nevus sebaceus of Jadassohn affects approximately 0.3% of newborns,1 usually as a solitary lesion that can form an extensive plaque. The male-to-female occurrence ratio has been reported as equal to slightly more predominant in females; all races and ethnicities are affected.1,5
Nevus sebaceus of Jadassohn follows 3 stages of clinical development: infantile, adolescent, and adulthood. It manifests at birth or shortly afterward as a smooth hairless patch or plaque that is yellowish and can be hyperpigmented in Black patients.5 It may have an oval or linear configuration, typically is asymptomatic, and often arises along the Blaschko lines when it occurs as multiple lesions (a rare manifestation).1 During puberty, hormonal changes cause accelerated growth, sebaceous gland maturation, and epidermal hyperplasia. 7 Nevus sebaceus of Jadassohn often is not identified until this stage, when its classic wartlike appearance has fully developed.1
Patients with nevus sebaceus of Jadassohn have a 10% to 20% risk for tumor development in adulthood.2,7 Trichoblastoma and syringocystadenoma papilliferum are the most frequently described neoplasms.8 Basal cell carcinoma is the most common malignant secondary neoplasm with an occurrence rate of 0.8%.6,9 However, basal cell carcinoma and trichoblastoma may share histopathologic features, which may lead to misdiagnosis and a higher reported incidence of basal cell carcinoma in adults than is accurate.2
Early prophylactic surgical removal of nevus sebaceus of Jadassohn has been recommended; however, surgical management is controversial because the risk for a benign secondary neoplasm remains relatively high while the risk for malignancy is much lower.2,7 Surgical excision remains an acceptable option once the patient is mature enough to tolerate the procedure.1 However, patient education regarding watchful waiting vs a surgical approach— and the risks of each—is critical to ensure shared decision-making and a management plan tailored to the individual.
The differential diagnosis includes hypertrophic lichen planus, Langerhans cell histiocytosis (Letterer-Siwe disease type), epidermal nevus, and seborrheic keratosis. Hypertrophic lichen planus often occurs symmetrically on the dorsal feet and shins with thick, scaly, and extremely pruritic plaques. The lesions often persist for an average of 6 years and may lead to multiple keratoacanthomas or follicular base squamous cell carcinomas. Langerhans cell histiocytosis (Letterer-Siwe disease type) manifests with acute, disseminated, visceral, and cutaneous lesions before 2 years of age. These lesions appear as 1- to 2-mm, pink, seborrheic papules, pustules, or vesicles on the scalp, flexural neck, axilla, perineum, and trunk; they often are associated with petechiae, purpura, scale, crust, erosion, impetiginization, and tender fissures. Epidermal nevus occurs within the first year of life and is a hamartoma of the epidermis and papillary dermis. It manifests as papillomatous pigmented linear lines along the Blaschko lines. Seborrheic keratosis manifests as well-demarcated, waxy/verrucous, brown papules with a “stuck on” appearance on hair-bearing skin sparing the mucosae. They are common benign lesions associated with sun exposure and often manifest in the fourth decade of life.10
The Diagnosis: Nevus Sebaceus of Jadassohn
The diagnosis of nevus sebaceus of Jadassohn was made clinically based on the lesion’s appearance and presence since birth as well as the absence of systemic symptoms. Clinically, nevus sebaceus of Jadassohn typically manifests as a well-demarcated, yellow- brown plaque often located on the scalp, as was seen in our patient. The lack of pruritus and pain further supported the diagnosis in our patient. No biopsy was performed, as the presentation was considered classic for this condition. Our patient opted to forgo surgery and will be routinely monitored for any changes, as nevus sebaceus has a potential risk, albeit low, for malignant transformation later in life. No changes have been observed since the initial presentation, and regular follow-ups are planned to monitor for future developments.
Nevus sebaceus of Jadassohn is a hamartomatous lesion involving the pilosebaceous follicle and adjacent adnexal structures.1-3 It most commonly forms on the scalp (59.3%) and is accompanied by partial or total alopecia. 3,4 It is seen less often on the face, periauricular area, or neck1,4; thorax or limbs5; and oral or genital mucosae.6 Nevus sebaceus of Jadassohn affects approximately 0.3% of newborns,1 usually as a solitary lesion that can form an extensive plaque. The male-to-female occurrence ratio has been reported as equal to slightly more predominant in females; all races and ethnicities are affected.1,5
Nevus sebaceus of Jadassohn follows 3 stages of clinical development: infantile, adolescent, and adulthood. It manifests at birth or shortly afterward as a smooth hairless patch or plaque that is yellowish and can be hyperpigmented in Black patients.5 It may have an oval or linear configuration, typically is asymptomatic, and often arises along the Blaschko lines when it occurs as multiple lesions (a rare manifestation).1 During puberty, hormonal changes cause accelerated growth, sebaceous gland maturation, and epidermal hyperplasia. 7 Nevus sebaceus of Jadassohn often is not identified until this stage, when its classic wartlike appearance has fully developed.1
Patients with nevus sebaceus of Jadassohn have a 10% to 20% risk for tumor development in adulthood.2,7 Trichoblastoma and syringocystadenoma papilliferum are the most frequently described neoplasms.8 Basal cell carcinoma is the most common malignant secondary neoplasm with an occurrence rate of 0.8%.6,9 However, basal cell carcinoma and trichoblastoma may share histopathologic features, which may lead to misdiagnosis and a higher reported incidence of basal cell carcinoma in adults than is accurate.2
Early prophylactic surgical removal of nevus sebaceus of Jadassohn has been recommended; however, surgical management is controversial because the risk for a benign secondary neoplasm remains relatively high while the risk for malignancy is much lower.2,7 Surgical excision remains an acceptable option once the patient is mature enough to tolerate the procedure.1 However, patient education regarding watchful waiting vs a surgical approach— and the risks of each—is critical to ensure shared decision-making and a management plan tailored to the individual.
The differential diagnosis includes hypertrophic lichen planus, Langerhans cell histiocytosis (Letterer-Siwe disease type), epidermal nevus, and seborrheic keratosis. Hypertrophic lichen planus often occurs symmetrically on the dorsal feet and shins with thick, scaly, and extremely pruritic plaques. The lesions often persist for an average of 6 years and may lead to multiple keratoacanthomas or follicular base squamous cell carcinomas. Langerhans cell histiocytosis (Letterer-Siwe disease type) manifests with acute, disseminated, visceral, and cutaneous lesions before 2 years of age. These lesions appear as 1- to 2-mm, pink, seborrheic papules, pustules, or vesicles on the scalp, flexural neck, axilla, perineum, and trunk; they often are associated with petechiae, purpura, scale, crust, erosion, impetiginization, and tender fissures. Epidermal nevus occurs within the first year of life and is a hamartoma of the epidermis and papillary dermis. It manifests as papillomatous pigmented linear lines along the Blaschko lines. Seborrheic keratosis manifests as well-demarcated, waxy/verrucous, brown papules with a “stuck on” appearance on hair-bearing skin sparing the mucosae. They are common benign lesions associated with sun exposure and often manifest in the fourth decade of life.10
- Baigrie D, Troxell T, Cook C. Nevus sebaceus. StatPearls [Internet]. Updated August 16, 2023. Accessed September 12, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482493/
- Terenzi V, Indrizzi E, Buonaccorsi S, et al. Nevus sebaceus of Jadassohn. J Craniofac Surg. 2006;17:1234-1239. doi:10.1097/01 .scs.0000221531.56529.cc
- Kelati A, Baybay H, Gallouj S, et al. Dermoscopic analysis of nevus sebaceus of Jadassohn: a study of 13 cases. Skin Appendage Disord. 2017;3:83-91. doi:10.1159/000460258
- Ugras N, Ozgun G, Adim SB, et al. Nevus sebaceous at unusual location: a rare presentation. Indian J Pathol Microbiol. 2012;55:419-420. doi:10.4103/0377-4929.101768
- Serpas de Lopez RM, Hernandez-Perez E. Jadassohn’s sebaceous nevus. J Dermatol Surg Oncol. 1985;11:68-72. doi:10.1111/j.1524-4725 .1985.tb02893.x
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42(2 pt 1):263-268. doi:10.1016/S0190-9622(00)90136-1
- Santibanez-Gallerani A, Marshall D, Duarte AM, et al. Should nevus sebaceus of Jadassohn in children be excised? a study of 757 cases, and literature review. J Craniofac Surg. 2003;14:658-660. doi:10.1097/00001665-200309000-00010
- Chahboun F, Eljazouly M, Elomari M, et al. Trichoblastoma arising from the nevus sebaceus of Jadassohn. Cureus. 2021;13:E15325. doi:10.7759/cureus.15325
- Cazzato G, Cimmino A, Colagrande A, et al. The multiple faces of nodular trichoblastoma: review of the literature with case presentation. Dermatopathology (Basel). 2021;8:265-270. doi:10.3390 /dermatopathology8030032
- Dandekar MN, Gandhi RK. Neoplastic dermatology. In: Alikhan A, Hocker TLH (eds). Review of Dermatology. Elsevier; 2016: 321-366.
- Baigrie D, Troxell T, Cook C. Nevus sebaceus. StatPearls [Internet]. Updated August 16, 2023. Accessed September 12, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482493/
- Terenzi V, Indrizzi E, Buonaccorsi S, et al. Nevus sebaceus of Jadassohn. J Craniofac Surg. 2006;17:1234-1239. doi:10.1097/01 .scs.0000221531.56529.cc
- Kelati A, Baybay H, Gallouj S, et al. Dermoscopic analysis of nevus sebaceus of Jadassohn: a study of 13 cases. Skin Appendage Disord. 2017;3:83-91. doi:10.1159/000460258
- Ugras N, Ozgun G, Adim SB, et al. Nevus sebaceous at unusual location: a rare presentation. Indian J Pathol Microbiol. 2012;55:419-420. doi:10.4103/0377-4929.101768
- Serpas de Lopez RM, Hernandez-Perez E. Jadassohn’s sebaceous nevus. J Dermatol Surg Oncol. 1985;11:68-72. doi:10.1111/j.1524-4725 .1985.tb02893.x
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42(2 pt 1):263-268. doi:10.1016/S0190-9622(00)90136-1
- Santibanez-Gallerani A, Marshall D, Duarte AM, et al. Should nevus sebaceus of Jadassohn in children be excised? a study of 757 cases, and literature review. J Craniofac Surg. 2003;14:658-660. doi:10.1097/00001665-200309000-00010
- Chahboun F, Eljazouly M, Elomari M, et al. Trichoblastoma arising from the nevus sebaceus of Jadassohn. Cureus. 2021;13:E15325. doi:10.7759/cureus.15325
- Cazzato G, Cimmino A, Colagrande A, et al. The multiple faces of nodular trichoblastoma: review of the literature with case presentation. Dermatopathology (Basel). 2021;8:265-270. doi:10.3390 /dermatopathology8030032
- Dandekar MN, Gandhi RK. Neoplastic dermatology. In: Alikhan A, Hocker TLH (eds). Review of Dermatology. Elsevier; 2016: 321-366.
A 23-year-old man presented to the dermatology clinic with hair loss on the scalp of several years’ duration. The patient reported persistent pigmented bumps on the back of the scalp. He denied any pruritus or pain and had no systemic symptoms or comorbidities. Physical examination revealed a 1×1.5-cm, yellow-brown, hairless plaque on the left parietal scalp.
Dermatomyositis Cancer Screening Guidelines Get Real-World Validation
Newly issued guidelines for cancer screening in patients with dermatomyositis had 100% sensitivity in a single institution’s cohort, though most of the cancers found would have been detected with standard cancer screenings recommended for the general population, according to a research letter published in JAMA Dermatology.
“These early results emphasize the continued need to refine risk assessment and cancer screening for patients with dermatomyositis while balancing resource use and outcomes,” concluded Caroline J. Stone and her colleagues at the Department of Dermatology, Perelman School of Medicine, University of Pennsylvania, Philadelphia.
Patients with dermatomyositis have approximately a 4.7 times greater risk for cancer than those without it, according to a 2016 meta-analysis. Despite the well-established link between cancer and dermatomyositis, cancer in people with idiopathic inflammatory myopathies is commonly diagnosed at a later stage and is the leading cause of death in people with these conditions.
Guidelines First Presented in 2022 and Published in 2023
A wide variability in screening practices eventually led the International Myositis Assessment & Clinical Studies Group (IMACS) to present the first evidence-based and consensus-based guidelines for cancer screening of patients with idiopathic inflammatory myopathies, including those with dermatomyositis, at the 2022 annual meeting of the American College of Rheumatology and publish them in 2023 in Nature Reviews Rheumatology. The guidelines advise low-risk patients to undergo basic cancer screening with routine blood and urine studies, liver function tests, plain chest radiography, and age- and sex-appropriate cancer screening.
Intermediate- and high-risk patients are recommended to undergo enhanced screening that can include mammography, Pap tests, endoscopy/colonoscopy, pelvic and transvaginal ultrasonography, prostate-specific antigen or cancer antigen 125 blood tests, fecal occult blood tests, and CT of the neck, thorax, abdomen, and pelvis.
But because the guidelines are new, little evidence exists regarding their validation in real-world cohorts. Researchers, therefore, assessed the IMACS guidelines in 370 patients, aged 18-80 years, who visited the University of Pennsylvania rheumatology-dermatology specialty clinic between July 2008 and January 2024. All participants had dermatomyositis and at least 3 years of follow-up and were an average 48 years old. The vast majority were women (87%) and White participants (89%).
Most (68.6%) had myositis-specific autoantibody test results, one of the factors included in the guidelines for determining whether the patient should be classified as low, intermediate, or high risk. Other factors for risk stratification included myositis subtype, age at disease onset, and clinical features. About half (49.2%) had classic dermatomyositis, 42.4% had amyopathic dermatomyositis, 3.8% had juvenile dermatomyositis, 3.2% had hypomyopathic dermatomyositis, 0.8% had antisynthetase syndrome, and 0.5% had immune-mediated necrotizing myopathy.
Just over half the patients (54%) were classified as high risk, while 37.3% were classified as intermediate risk and 8.9% as low risk using the guidelines. Among the 18 patients (4.9%) with paraneoplastic dermatomyositis, 15 were classified as high risk and 3 as intermediate risk.
Of the patients diagnosed with cancer, 55% of cases were diagnosed about a year before their dermatomyositis diagnosis. In three patients, symptoms “suggestive of cancer at the time of dermatomyositis diagnosis, including lymphadenopathy and unexplained weight loss,” led to diagnostic testing that found an underlying cancer.
In the eight patients diagnosed with cancer after their dermatomyositis diagnosis, 75% of the cancers were identified during the first year of follow-up and 25% in the second year. Five were identified based on basic cancer screening and three on enhanced screening.
A total of 11 patients (3%) developed intravenous contrast allergies, and no other adverse events were reported to be associated with cancer screening, but the study was not designed to capture other types of adverse screening effects, such as cost, quality of life, or risk from radiation exposure.
The most common neoplasm identified was breast cancer, found in nine (50%) of the patients using mammography. Two patients had lung cancer identified with chest radiography and two had ovarian cancer identified with abdominal radiography and CT. The remaining five patients included one each with bladder cancer, papillary thyroid cancer, renal cell carcinoma, non-Hodgkin lymphoma, and adenocarcinoma with unknown primary.
The sensitivity of the guidelines in detecting cancer related to dermatomyositis was 100%, though the authors noted that the “IMACS risk-stratification scheme may overestimate cancer risk and encourage enhanced screening protocols of unclear benefit.” Most of the cancers found after dermatomyositis diagnosis were detected with routine age- and sex-related screening that already falls under basic cancer screening recommendations for the general population. Nonetheless, 90% of the participants fell into the intermediate- and high-risk groups, warranting a more comprehensive and costly enhanced screening protocol.
Will the Guidelines Lead to Overscreening?
The 4.9% cancer prevalence is considerably lower than the typical 15%-25% prevalence among patients with dermatomyositis, but the findings, regardless, suggest the guidelines will lead to overscreening, wrote Andrea D. Maderal, MD, University of Miami Miller School of Medicine in Florida, and Alisa Femia, MD, New York University Grossman School of Medicine, New York City, in an accompanying editorial. Given that the median age in patients with cancer in the study was 58 years — 18 years older than the age cutoff for high-risk criteria — one way to refine the guidelines may be to increase the age for the high-risk category, they suggested.
“While these guidelines led to many ultimately unnecessary screening tests based on currently recommended designations of intermediate-risk and high-risk patients, these guidelines reflect a more conservative approach to screening than was previously performed,” Dr. Maderal and Dr. Femia wrote.
Jeff Gehlhausen, MD, PhD, an assistant professor of dermatology at Yale School of Medicine, New Haven, Connecticut, said he is not concerned about overscreening in patients, however, and is “very enthusiastic” about the findings.
“Patients are very anxious for good reason,” given the typical cancer prevalence of 25% in this population, he said in an interview. “I think therein lies the challenge — with that risk, what is ‘enough’ screening?” Yet this “incredibly impressive” study “provides real insights into the applicability of the IMACS screenings to our dermatomyositis management,” including relevance to his own patients. “Their findings are instructive for how to better evaluate these patients in a more mindful fashion,” he said, and they are particularly welcome, given how widely variable practice has historically been before the guidelines were issued.
“This question has been an outstanding one for decades, and nearly every doctor has a different answer,” Dr. Gehlhausen said. “The introduction of the guidelines alone are now much more actionable with this study, and that’s why it’s such an important one for our community.”
Benedict Wu, DO, PhD, director of Inpatient Dermatology and an assistant professor at Montefiore Einstein and a member of the Montefiore Einstein Comprehensive Cancer Center in New York City, similarly regarded the findings as reassuring, though he was surprised at the low prevalence of cancer in the patients.
“The most reassuring finding was that the detection of most malignancies was possible by using routine age- and sex-related screening combined with basic cancer screening,” Wu said in an interview. “Basic cancer screening can reduce costs while keeping patients safe.”
He also found it reassuring that all the paraneoplastic dermatomyositis was in intermediate- or high-risk patients, and while he does not see the IMACS guidelines as overestimating cancer risk, he does think “the risk stratification and recommended screening tests could be revised to be less ‘aggressive.’ ”
The overall low rate of cancer in the group “calls into question the need for stringent and annual cancer screening,” he said. “In this large cohort of patients, the fact that malignancy was detected within 2 years of dermatomyositis diagnosis will help guide us with long-term screening recommendations.”
Despite the study’s small size and single-center design, the demographics of the patients nearly represents exactly what is found in the United States more broadly, Wu noted. He also drew attention to how many patients lacked the myositis antibody profile performed, and he agreed with the authors that more extensive and prospective studies need to be conducted. He also emphasized the need to keep in mind that “the primary goal of dermatomyositis management should focus on controlling/reducing the disease burden.”
The research was funded by the National Institutes of Health and the US Department of Veterans Affairs. The authors had no disclosures. Dr. Maderal reported personal fees from argenx. No disclosures were noted for Dr. Gehlhausen and Dr. Wu.
A version of this article appeared on Medscape.com.
Newly issued guidelines for cancer screening in patients with dermatomyositis had 100% sensitivity in a single institution’s cohort, though most of the cancers found would have been detected with standard cancer screenings recommended for the general population, according to a research letter published in JAMA Dermatology.
“These early results emphasize the continued need to refine risk assessment and cancer screening for patients with dermatomyositis while balancing resource use and outcomes,” concluded Caroline J. Stone and her colleagues at the Department of Dermatology, Perelman School of Medicine, University of Pennsylvania, Philadelphia.
Patients with dermatomyositis have approximately a 4.7 times greater risk for cancer than those without it, according to a 2016 meta-analysis. Despite the well-established link between cancer and dermatomyositis, cancer in people with idiopathic inflammatory myopathies is commonly diagnosed at a later stage and is the leading cause of death in people with these conditions.
Guidelines First Presented in 2022 and Published in 2023
A wide variability in screening practices eventually led the International Myositis Assessment & Clinical Studies Group (IMACS) to present the first evidence-based and consensus-based guidelines for cancer screening of patients with idiopathic inflammatory myopathies, including those with dermatomyositis, at the 2022 annual meeting of the American College of Rheumatology and publish them in 2023 in Nature Reviews Rheumatology. The guidelines advise low-risk patients to undergo basic cancer screening with routine blood and urine studies, liver function tests, plain chest radiography, and age- and sex-appropriate cancer screening.
Intermediate- and high-risk patients are recommended to undergo enhanced screening that can include mammography, Pap tests, endoscopy/colonoscopy, pelvic and transvaginal ultrasonography, prostate-specific antigen or cancer antigen 125 blood tests, fecal occult blood tests, and CT of the neck, thorax, abdomen, and pelvis.
But because the guidelines are new, little evidence exists regarding their validation in real-world cohorts. Researchers, therefore, assessed the IMACS guidelines in 370 patients, aged 18-80 years, who visited the University of Pennsylvania rheumatology-dermatology specialty clinic between July 2008 and January 2024. All participants had dermatomyositis and at least 3 years of follow-up and were an average 48 years old. The vast majority were women (87%) and White participants (89%).
Most (68.6%) had myositis-specific autoantibody test results, one of the factors included in the guidelines for determining whether the patient should be classified as low, intermediate, or high risk. Other factors for risk stratification included myositis subtype, age at disease onset, and clinical features. About half (49.2%) had classic dermatomyositis, 42.4% had amyopathic dermatomyositis, 3.8% had juvenile dermatomyositis, 3.2% had hypomyopathic dermatomyositis, 0.8% had antisynthetase syndrome, and 0.5% had immune-mediated necrotizing myopathy.
Just over half the patients (54%) were classified as high risk, while 37.3% were classified as intermediate risk and 8.9% as low risk using the guidelines. Among the 18 patients (4.9%) with paraneoplastic dermatomyositis, 15 were classified as high risk and 3 as intermediate risk.
Of the patients diagnosed with cancer, 55% of cases were diagnosed about a year before their dermatomyositis diagnosis. In three patients, symptoms “suggestive of cancer at the time of dermatomyositis diagnosis, including lymphadenopathy and unexplained weight loss,” led to diagnostic testing that found an underlying cancer.
In the eight patients diagnosed with cancer after their dermatomyositis diagnosis, 75% of the cancers were identified during the first year of follow-up and 25% in the second year. Five were identified based on basic cancer screening and three on enhanced screening.
A total of 11 patients (3%) developed intravenous contrast allergies, and no other adverse events were reported to be associated with cancer screening, but the study was not designed to capture other types of adverse screening effects, such as cost, quality of life, or risk from radiation exposure.
The most common neoplasm identified was breast cancer, found in nine (50%) of the patients using mammography. Two patients had lung cancer identified with chest radiography and two had ovarian cancer identified with abdominal radiography and CT. The remaining five patients included one each with bladder cancer, papillary thyroid cancer, renal cell carcinoma, non-Hodgkin lymphoma, and adenocarcinoma with unknown primary.
The sensitivity of the guidelines in detecting cancer related to dermatomyositis was 100%, though the authors noted that the “IMACS risk-stratification scheme may overestimate cancer risk and encourage enhanced screening protocols of unclear benefit.” Most of the cancers found after dermatomyositis diagnosis were detected with routine age- and sex-related screening that already falls under basic cancer screening recommendations for the general population. Nonetheless, 90% of the participants fell into the intermediate- and high-risk groups, warranting a more comprehensive and costly enhanced screening protocol.
Will the Guidelines Lead to Overscreening?
The 4.9% cancer prevalence is considerably lower than the typical 15%-25% prevalence among patients with dermatomyositis, but the findings, regardless, suggest the guidelines will lead to overscreening, wrote Andrea D. Maderal, MD, University of Miami Miller School of Medicine in Florida, and Alisa Femia, MD, New York University Grossman School of Medicine, New York City, in an accompanying editorial. Given that the median age in patients with cancer in the study was 58 years — 18 years older than the age cutoff for high-risk criteria — one way to refine the guidelines may be to increase the age for the high-risk category, they suggested.
“While these guidelines led to many ultimately unnecessary screening tests based on currently recommended designations of intermediate-risk and high-risk patients, these guidelines reflect a more conservative approach to screening than was previously performed,” Dr. Maderal and Dr. Femia wrote.
Jeff Gehlhausen, MD, PhD, an assistant professor of dermatology at Yale School of Medicine, New Haven, Connecticut, said he is not concerned about overscreening in patients, however, and is “very enthusiastic” about the findings.
“Patients are very anxious for good reason,” given the typical cancer prevalence of 25% in this population, he said in an interview. “I think therein lies the challenge — with that risk, what is ‘enough’ screening?” Yet this “incredibly impressive” study “provides real insights into the applicability of the IMACS screenings to our dermatomyositis management,” including relevance to his own patients. “Their findings are instructive for how to better evaluate these patients in a more mindful fashion,” he said, and they are particularly welcome, given how widely variable practice has historically been before the guidelines were issued.
“This question has been an outstanding one for decades, and nearly every doctor has a different answer,” Dr. Gehlhausen said. “The introduction of the guidelines alone are now much more actionable with this study, and that’s why it’s such an important one for our community.”
Benedict Wu, DO, PhD, director of Inpatient Dermatology and an assistant professor at Montefiore Einstein and a member of the Montefiore Einstein Comprehensive Cancer Center in New York City, similarly regarded the findings as reassuring, though he was surprised at the low prevalence of cancer in the patients.
“The most reassuring finding was that the detection of most malignancies was possible by using routine age- and sex-related screening combined with basic cancer screening,” Wu said in an interview. “Basic cancer screening can reduce costs while keeping patients safe.”
He also found it reassuring that all the paraneoplastic dermatomyositis was in intermediate- or high-risk patients, and while he does not see the IMACS guidelines as overestimating cancer risk, he does think “the risk stratification and recommended screening tests could be revised to be less ‘aggressive.’ ”
The overall low rate of cancer in the group “calls into question the need for stringent and annual cancer screening,” he said. “In this large cohort of patients, the fact that malignancy was detected within 2 years of dermatomyositis diagnosis will help guide us with long-term screening recommendations.”
Despite the study’s small size and single-center design, the demographics of the patients nearly represents exactly what is found in the United States more broadly, Wu noted. He also drew attention to how many patients lacked the myositis antibody profile performed, and he agreed with the authors that more extensive and prospective studies need to be conducted. He also emphasized the need to keep in mind that “the primary goal of dermatomyositis management should focus on controlling/reducing the disease burden.”
The research was funded by the National Institutes of Health and the US Department of Veterans Affairs. The authors had no disclosures. Dr. Maderal reported personal fees from argenx. No disclosures were noted for Dr. Gehlhausen and Dr. Wu.
A version of this article appeared on Medscape.com.
Newly issued guidelines for cancer screening in patients with dermatomyositis had 100% sensitivity in a single institution’s cohort, though most of the cancers found would have been detected with standard cancer screenings recommended for the general population, according to a research letter published in JAMA Dermatology.
“These early results emphasize the continued need to refine risk assessment and cancer screening for patients with dermatomyositis while balancing resource use and outcomes,” concluded Caroline J. Stone and her colleagues at the Department of Dermatology, Perelman School of Medicine, University of Pennsylvania, Philadelphia.
Patients with dermatomyositis have approximately a 4.7 times greater risk for cancer than those without it, according to a 2016 meta-analysis. Despite the well-established link between cancer and dermatomyositis, cancer in people with idiopathic inflammatory myopathies is commonly diagnosed at a later stage and is the leading cause of death in people with these conditions.
Guidelines First Presented in 2022 and Published in 2023
A wide variability in screening practices eventually led the International Myositis Assessment & Clinical Studies Group (IMACS) to present the first evidence-based and consensus-based guidelines for cancer screening of patients with idiopathic inflammatory myopathies, including those with dermatomyositis, at the 2022 annual meeting of the American College of Rheumatology and publish them in 2023 in Nature Reviews Rheumatology. The guidelines advise low-risk patients to undergo basic cancer screening with routine blood and urine studies, liver function tests, plain chest radiography, and age- and sex-appropriate cancer screening.
Intermediate- and high-risk patients are recommended to undergo enhanced screening that can include mammography, Pap tests, endoscopy/colonoscopy, pelvic and transvaginal ultrasonography, prostate-specific antigen or cancer antigen 125 blood tests, fecal occult blood tests, and CT of the neck, thorax, abdomen, and pelvis.
But because the guidelines are new, little evidence exists regarding their validation in real-world cohorts. Researchers, therefore, assessed the IMACS guidelines in 370 patients, aged 18-80 years, who visited the University of Pennsylvania rheumatology-dermatology specialty clinic between July 2008 and January 2024. All participants had dermatomyositis and at least 3 years of follow-up and were an average 48 years old. The vast majority were women (87%) and White participants (89%).
Most (68.6%) had myositis-specific autoantibody test results, one of the factors included in the guidelines for determining whether the patient should be classified as low, intermediate, or high risk. Other factors for risk stratification included myositis subtype, age at disease onset, and clinical features. About half (49.2%) had classic dermatomyositis, 42.4% had amyopathic dermatomyositis, 3.8% had juvenile dermatomyositis, 3.2% had hypomyopathic dermatomyositis, 0.8% had antisynthetase syndrome, and 0.5% had immune-mediated necrotizing myopathy.
Just over half the patients (54%) were classified as high risk, while 37.3% were classified as intermediate risk and 8.9% as low risk using the guidelines. Among the 18 patients (4.9%) with paraneoplastic dermatomyositis, 15 were classified as high risk and 3 as intermediate risk.
Of the patients diagnosed with cancer, 55% of cases were diagnosed about a year before their dermatomyositis diagnosis. In three patients, symptoms “suggestive of cancer at the time of dermatomyositis diagnosis, including lymphadenopathy and unexplained weight loss,” led to diagnostic testing that found an underlying cancer.
In the eight patients diagnosed with cancer after their dermatomyositis diagnosis, 75% of the cancers were identified during the first year of follow-up and 25% in the second year. Five were identified based on basic cancer screening and three on enhanced screening.
A total of 11 patients (3%) developed intravenous contrast allergies, and no other adverse events were reported to be associated with cancer screening, but the study was not designed to capture other types of adverse screening effects, such as cost, quality of life, or risk from radiation exposure.
The most common neoplasm identified was breast cancer, found in nine (50%) of the patients using mammography. Two patients had lung cancer identified with chest radiography and two had ovarian cancer identified with abdominal radiography and CT. The remaining five patients included one each with bladder cancer, papillary thyroid cancer, renal cell carcinoma, non-Hodgkin lymphoma, and adenocarcinoma with unknown primary.
The sensitivity of the guidelines in detecting cancer related to dermatomyositis was 100%, though the authors noted that the “IMACS risk-stratification scheme may overestimate cancer risk and encourage enhanced screening protocols of unclear benefit.” Most of the cancers found after dermatomyositis diagnosis were detected with routine age- and sex-related screening that already falls under basic cancer screening recommendations for the general population. Nonetheless, 90% of the participants fell into the intermediate- and high-risk groups, warranting a more comprehensive and costly enhanced screening protocol.
Will the Guidelines Lead to Overscreening?
The 4.9% cancer prevalence is considerably lower than the typical 15%-25% prevalence among patients with dermatomyositis, but the findings, regardless, suggest the guidelines will lead to overscreening, wrote Andrea D. Maderal, MD, University of Miami Miller School of Medicine in Florida, and Alisa Femia, MD, New York University Grossman School of Medicine, New York City, in an accompanying editorial. Given that the median age in patients with cancer in the study was 58 years — 18 years older than the age cutoff for high-risk criteria — one way to refine the guidelines may be to increase the age for the high-risk category, they suggested.
“While these guidelines led to many ultimately unnecessary screening tests based on currently recommended designations of intermediate-risk and high-risk patients, these guidelines reflect a more conservative approach to screening than was previously performed,” Dr. Maderal and Dr. Femia wrote.
Jeff Gehlhausen, MD, PhD, an assistant professor of dermatology at Yale School of Medicine, New Haven, Connecticut, said he is not concerned about overscreening in patients, however, and is “very enthusiastic” about the findings.
“Patients are very anxious for good reason,” given the typical cancer prevalence of 25% in this population, he said in an interview. “I think therein lies the challenge — with that risk, what is ‘enough’ screening?” Yet this “incredibly impressive” study “provides real insights into the applicability of the IMACS screenings to our dermatomyositis management,” including relevance to his own patients. “Their findings are instructive for how to better evaluate these patients in a more mindful fashion,” he said, and they are particularly welcome, given how widely variable practice has historically been before the guidelines were issued.
“This question has been an outstanding one for decades, and nearly every doctor has a different answer,” Dr. Gehlhausen said. “The introduction of the guidelines alone are now much more actionable with this study, and that’s why it’s such an important one for our community.”
Benedict Wu, DO, PhD, director of Inpatient Dermatology and an assistant professor at Montefiore Einstein and a member of the Montefiore Einstein Comprehensive Cancer Center in New York City, similarly regarded the findings as reassuring, though he was surprised at the low prevalence of cancer in the patients.
“The most reassuring finding was that the detection of most malignancies was possible by using routine age- and sex-related screening combined with basic cancer screening,” Wu said in an interview. “Basic cancer screening can reduce costs while keeping patients safe.”
He also found it reassuring that all the paraneoplastic dermatomyositis was in intermediate- or high-risk patients, and while he does not see the IMACS guidelines as overestimating cancer risk, he does think “the risk stratification and recommended screening tests could be revised to be less ‘aggressive.’ ”
The overall low rate of cancer in the group “calls into question the need for stringent and annual cancer screening,” he said. “In this large cohort of patients, the fact that malignancy was detected within 2 years of dermatomyositis diagnosis will help guide us with long-term screening recommendations.”
Despite the study’s small size and single-center design, the demographics of the patients nearly represents exactly what is found in the United States more broadly, Wu noted. He also drew attention to how many patients lacked the myositis antibody profile performed, and he agreed with the authors that more extensive and prospective studies need to be conducted. He also emphasized the need to keep in mind that “the primary goal of dermatomyositis management should focus on controlling/reducing the disease burden.”
The research was funded by the National Institutes of Health and the US Department of Veterans Affairs. The authors had no disclosures. Dr. Maderal reported personal fees from argenx. No disclosures were noted for Dr. Gehlhausen and Dr. Wu.
A version of this article appeared on Medscape.com.
FROM JAMA DERMATOLOGY
Study Supports Efficacy of Home-Based Phototherapy for Psoriasis
TOPLINE:
study.
METHODOLOGY:
- The pragmatic, investigator-initiated, open-label, noninferiority, randomized trial compared the effectiveness of 12 weeks of treatment with narrow-band ultraviolet B phototherapy administered at home (n = 393) vs at the doctor’s office (n = 390).
- Overall, 783 patients with plaque or guttate psoriasis (mean age, 48 years; 48% women) were enrolled at 42 academic and private clinical dermatology practices in the United States from March 1, 2019, to December 4, 2023, and were followed up through June 2024. At baseline, the mean Physician Global Assessment (PGA) and the mean Dermatology Life Quality Index (DLQI) scores were 2.7 and 12.2, respectively.
- The two co-primary endpoints were a PGA score ≤ 1 indicating clear or almost clear skin and a DLQI score ≤ 5.
TAKEAWAY:
- At 12 weeks, a PGA score ≤ 1 was achieved in 32.8% of patients using home-based phototherapy and in 25.6% of those who received office-based phototherapy (P < .001).
- At 12 weeks, a DLQI score ≤ 5 was achieved in 52.4% and 33.6% of home- and office-treated patients, respectively (P < .001).
- Similar benefits were seen across all Fitzpatrick skin types.
- A higher percentage of patients were adherent to home-based (51.4%) vs office-based (15.9%) phototherapy (P < .001).
IN PRACTICE:
“These data support the use of home phototherapy as a first-line treatment option for psoriasis,” and “efforts are needed to make home and office phototherapy more available to patients,” said the study’s lead author.
SOURCE:
Joel M. Gelfand, MD, director of the Psoriasis and Phototherapy Treatment Center at the University of Pennsylvania, Philadelphia, presented the findings at the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis meeting during the annual meeting of the European Academy of Dermatology and Venereology, with simultaneous publication in JAMA Dermatology.
LIMITATIONS:
This was an open-label trial and because of its pragmatic design, outcome data were missing. The cost of the home-based phototherapy equipment used in the study was $6040.88, which was mostly covered by Medicare, but direct costs to patients may have varied depending on their insurance plan.
DISCLOSURES:
The Patient-Centered Outcomes Research Institute funded the study. Daavlin provided and shipped machines for home-based phototherapy to patients at no cost. Dr. Gelfand disclosed serving as a consultant for AbbVie, Artax, Bristol-Myers Squibb, Boehringer Ingelheim, Celldex, and other companies. The full list of author disclosures can be found in the published study.
A version of this article first appeared on Medscape.com.
TOPLINE:
study.
METHODOLOGY:
- The pragmatic, investigator-initiated, open-label, noninferiority, randomized trial compared the effectiveness of 12 weeks of treatment with narrow-band ultraviolet B phototherapy administered at home (n = 393) vs at the doctor’s office (n = 390).
- Overall, 783 patients with plaque or guttate psoriasis (mean age, 48 years; 48% women) were enrolled at 42 academic and private clinical dermatology practices in the United States from March 1, 2019, to December 4, 2023, and were followed up through June 2024. At baseline, the mean Physician Global Assessment (PGA) and the mean Dermatology Life Quality Index (DLQI) scores were 2.7 and 12.2, respectively.
- The two co-primary endpoints were a PGA score ≤ 1 indicating clear or almost clear skin and a DLQI score ≤ 5.
TAKEAWAY:
- At 12 weeks, a PGA score ≤ 1 was achieved in 32.8% of patients using home-based phototherapy and in 25.6% of those who received office-based phototherapy (P < .001).
- At 12 weeks, a DLQI score ≤ 5 was achieved in 52.4% and 33.6% of home- and office-treated patients, respectively (P < .001).
- Similar benefits were seen across all Fitzpatrick skin types.
- A higher percentage of patients were adherent to home-based (51.4%) vs office-based (15.9%) phototherapy (P < .001).
IN PRACTICE:
“These data support the use of home phototherapy as a first-line treatment option for psoriasis,” and “efforts are needed to make home and office phototherapy more available to patients,” said the study’s lead author.
SOURCE:
Joel M. Gelfand, MD, director of the Psoriasis and Phototherapy Treatment Center at the University of Pennsylvania, Philadelphia, presented the findings at the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis meeting during the annual meeting of the European Academy of Dermatology and Venereology, with simultaneous publication in JAMA Dermatology.
LIMITATIONS:
This was an open-label trial and because of its pragmatic design, outcome data were missing. The cost of the home-based phototherapy equipment used in the study was $6040.88, which was mostly covered by Medicare, but direct costs to patients may have varied depending on their insurance plan.
DISCLOSURES:
The Patient-Centered Outcomes Research Institute funded the study. Daavlin provided and shipped machines for home-based phototherapy to patients at no cost. Dr. Gelfand disclosed serving as a consultant for AbbVie, Artax, Bristol-Myers Squibb, Boehringer Ingelheim, Celldex, and other companies. The full list of author disclosures can be found in the published study.
A version of this article first appeared on Medscape.com.
TOPLINE:
study.
METHODOLOGY:
- The pragmatic, investigator-initiated, open-label, noninferiority, randomized trial compared the effectiveness of 12 weeks of treatment with narrow-band ultraviolet B phototherapy administered at home (n = 393) vs at the doctor’s office (n = 390).
- Overall, 783 patients with plaque or guttate psoriasis (mean age, 48 years; 48% women) were enrolled at 42 academic and private clinical dermatology practices in the United States from March 1, 2019, to December 4, 2023, and were followed up through June 2024. At baseline, the mean Physician Global Assessment (PGA) and the mean Dermatology Life Quality Index (DLQI) scores were 2.7 and 12.2, respectively.
- The two co-primary endpoints were a PGA score ≤ 1 indicating clear or almost clear skin and a DLQI score ≤ 5.
TAKEAWAY:
- At 12 weeks, a PGA score ≤ 1 was achieved in 32.8% of patients using home-based phototherapy and in 25.6% of those who received office-based phototherapy (P < .001).
- At 12 weeks, a DLQI score ≤ 5 was achieved in 52.4% and 33.6% of home- and office-treated patients, respectively (P < .001).
- Similar benefits were seen across all Fitzpatrick skin types.
- A higher percentage of patients were adherent to home-based (51.4%) vs office-based (15.9%) phototherapy (P < .001).
IN PRACTICE:
“These data support the use of home phototherapy as a first-line treatment option for psoriasis,” and “efforts are needed to make home and office phototherapy more available to patients,” said the study’s lead author.
SOURCE:
Joel M. Gelfand, MD, director of the Psoriasis and Phototherapy Treatment Center at the University of Pennsylvania, Philadelphia, presented the findings at the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis meeting during the annual meeting of the European Academy of Dermatology and Venereology, with simultaneous publication in JAMA Dermatology.
LIMITATIONS:
This was an open-label trial and because of its pragmatic design, outcome data were missing. The cost of the home-based phototherapy equipment used in the study was $6040.88, which was mostly covered by Medicare, but direct costs to patients may have varied depending on their insurance plan.
DISCLOSURES:
The Patient-Centered Outcomes Research Institute funded the study. Daavlin provided and shipped machines for home-based phototherapy to patients at no cost. Dr. Gelfand disclosed serving as a consultant for AbbVie, Artax, Bristol-Myers Squibb, Boehringer Ingelheim, Celldex, and other companies. The full list of author disclosures can be found in the published study.
A version of this article first appeared on Medscape.com.
‘Cancer Doesn’t Wait’: How Prior Authorization Harms Care
Fantine Giap, MD, sat across from a 21-year-old with a rare sarcoma at the base of her skull.
Despite the large tumor, nestled in a sensitive area, the Boston-based radiation oncologist could envision a bright future for her patient.
She and the other members of the patient’s care team had an impressive cancer-fighting arsenal at her fingertips. The team had recommended surgery, followed by proton therapy — a sophisticated tool able to deliver concentrated, razor-focused radiation to the once apple-sized growth, while sparing the fragile brain stem, optic nerve, and spinal cord.
Surgery went as planned. But as the days and weeks wore on and insurance prior authorization for the proton therapy never came, the tumor roared back, leading to more surgeries and more complications. Ultimately, the young woman needed a tracheostomy and a feeding tube.
By the time insurance said yes, more than 1 year from her initial visit, the future the team had envisioned seemed out of reach.
“Unfortunately for this patient, it went from a potentially curable situation to a likely not curable situation,” recalled Dr. Giap, a clinician at Massachusetts General Hospital and instructor at Harvard Medical School, Boston. “I wanted to cry every day that she waited.’’
While a stark example, such insurance delays are not uncommon, according to new research published in JAMA Network Open.
Other studies have found that number to be even higher, with more than 86% of prior authorization requests ultimately approved with few changes.
‘’It gives you the idea that this entire process might be a little futile — that it’s just wasting people’s time,’’ said Fumiko Chino, MD, coauthor on the JAMA study and now an assistant professor in radiation oncology at MD Anderson Cancer Center in Houston. ‘’The problem is cancer doesn’t wait for bureaucracy.’’
Barriers at Every Step
As Dr. Chino and her study coauthors explained, advancements like intensity-modulated radiation therapy and stereotactic radiosurgery have allowed a new generation of specialists to treat previously untreatable cancers in ways that maximize tumor-killing power while minimizing collateral damage. But these tools require sophisticated planning, imaging, simulations and execution — all of which are subject to increased insurance scrutiny.
‘’We face barriers pretty much every step of the way for every patient,’’ said Dr. Chino.
To investigate how such barriers impact care, Dr. Chino and colleagues at Memorial Sloan Kettering Cancer Center — where she worked until July — looked at 206 cases in which payers denied prior authorization for radiation therapy from November 1, 2021 to December 8, 2022.
The team found that 62% were ultimately approved without any change to technique or dose, while 28% were authorized, but with lower doses or less sophisticated techniques. Four people, however, never got authorization at all — three abandoned treatment altogether, and one sought treatment at another institution.
Treatment delays ranged from 1 day to 49 days. Eighty-three patients died.
Would some of them have lived if it weren’t for prior authorization?
Dr. Chino cannot say for sure, but did note that certain cancers, like cervical cancer, can grow so quickly that every day of delayed treatment makes them harder to control.
Patients with metastatic or late-stage cancers are often denied more aggressive treatments by insurers who, in essence, “assume that they are going to die from their disease anyway,” Dr. Chino said.
She views this as tragically shortsighted.
‘’There’s actually a strong body of evidence to show that if you treat even metastatic stage IV diseases aggressively, you can prolong not just quality of life but also quantity,’’ she said.
In cases where the cancer is more localized and insurance mandates lower doses or cheaper techniques, the consequences can be equally heartbreaking.
‘’It’s like saying instead of taking an extra-strength Tylenol you can only have a baby aspirin,’’ she said. ‘’Their pain is less likely to be controlled, their disease is less likely to be controlled, and they are more likely to need retreatment.’’
Prior authorization delays can also significantly stress patients at the most vulnerable point of their lives.
In another recent study, Dr. Chino found that 69% of patients with cancer reported prior authorization-related delays in care, with one-third waiting a month or longer. One in five never got the care their doctors recommended, and 20% reported spending more than 11 hours on the phone haggling with their insurance companies.
Most patients rated the process as ‘’bad’’ or ‘’horrible,’’ and said it fueled anxiety.
Such delays can be hard on clinicians and the healthcare system too.
One 2022 study found that a typical academic radiation oncology practice spent about a half-million dollars per year seeking insurance preauthorization. Nationally, that number exceeds $40 million.
Then there is the burnout factor.
Dr. Giap, an early-career physician who specializes in rare, aggressive sarcomas, works at an institution that helped pioneer proton therapy. She says it pains her to tell a desperate patient, like the 21-year-old, who has traveled to her from out of state that they have to wait.
‘’Knowing that the majority of the cases are ultimately approved and that this wait is often unnecessary makes it even tougher,’’ she said.
Dr. Chino, a breast cancer specialist, has taken to warning patients before the alarming insurance letter arrives in the mail that their insurance may delay authorizing their care. But she tells patients that she will do everything she can to fight for them and develops a back-up plan to pivot to quickly, if needed.
‘’No one goes into medicine to spend their time talking to insurance companies,’’ said Dr. Chino.
The national trade group, America’s Health Insurance Plans (AHIP), did not return repeated requests for an interview for this story. But their official position, as stated on their website, is that “prior authorization is one of many tools health insurance providers use to promote safe, timely, evidence-based, affordable, and efficient care.”
Both Dr. Giap and Dr. Chino believe that prior authorization was developed with good intentions: to save healthcare costs and rein in treatments that don’t necessarily benefit patients.
But, in their specialty, the burden has proliferated to a point that Dr. Chino characterizes as ‘’unconscionable.’’
She believes that policy changes like the proposed Improving Seniors’ Timely Access to Care Act — which would require real-time decisions for procedures that are routinely approved — could go a long way in improving patient care.
Meanwhile, Dr. Giap said, more research and professional guidelines are necessary to bolster insurance company confidence in newer technologies, particularly for rare cancers.
Her patient ultimately got her proton therapy and is ‘’doing relatively well, all things considered.’’
But not all the stories end like this.
Dr. Chino will never forget a patient with a cancer growing so rapidly she could see it protruding through her chest wall. She called for an urgent PET scan to see where else in the body the cancer might be brewing and rushed the planning process for radiation therapy, both of which faced prior authorization barriers. That scan — which ultimately showed the cancer had spread — was delayed for months.*
If the team had had those imaging results upfront, she said, they would have recommended a completely different course of treatment.
And her patient might be alive today.
‘’Unfortunately,” Dr. Chino said, “the people with the very worst prior authorization stories aren’t here anymore to tell you about them.”
*Correction, 10/4/24: An earlier version of this article erroneously stated that Dr. Chino called for surgery for her patient. She actually called for a PET scan and an urgent radiation start.
A version of this article first appeared on Medscape.com.
Fantine Giap, MD, sat across from a 21-year-old with a rare sarcoma at the base of her skull.
Despite the large tumor, nestled in a sensitive area, the Boston-based radiation oncologist could envision a bright future for her patient.
She and the other members of the patient’s care team had an impressive cancer-fighting arsenal at her fingertips. The team had recommended surgery, followed by proton therapy — a sophisticated tool able to deliver concentrated, razor-focused radiation to the once apple-sized growth, while sparing the fragile brain stem, optic nerve, and spinal cord.
Surgery went as planned. But as the days and weeks wore on and insurance prior authorization for the proton therapy never came, the tumor roared back, leading to more surgeries and more complications. Ultimately, the young woman needed a tracheostomy and a feeding tube.
By the time insurance said yes, more than 1 year from her initial visit, the future the team had envisioned seemed out of reach.
“Unfortunately for this patient, it went from a potentially curable situation to a likely not curable situation,” recalled Dr. Giap, a clinician at Massachusetts General Hospital and instructor at Harvard Medical School, Boston. “I wanted to cry every day that she waited.’’
While a stark example, such insurance delays are not uncommon, according to new research published in JAMA Network Open.
Other studies have found that number to be even higher, with more than 86% of prior authorization requests ultimately approved with few changes.
‘’It gives you the idea that this entire process might be a little futile — that it’s just wasting people’s time,’’ said Fumiko Chino, MD, coauthor on the JAMA study and now an assistant professor in radiation oncology at MD Anderson Cancer Center in Houston. ‘’The problem is cancer doesn’t wait for bureaucracy.’’
Barriers at Every Step
As Dr. Chino and her study coauthors explained, advancements like intensity-modulated radiation therapy and stereotactic radiosurgery have allowed a new generation of specialists to treat previously untreatable cancers in ways that maximize tumor-killing power while minimizing collateral damage. But these tools require sophisticated planning, imaging, simulations and execution — all of which are subject to increased insurance scrutiny.
‘’We face barriers pretty much every step of the way for every patient,’’ said Dr. Chino.
To investigate how such barriers impact care, Dr. Chino and colleagues at Memorial Sloan Kettering Cancer Center — where she worked until July — looked at 206 cases in which payers denied prior authorization for radiation therapy from November 1, 2021 to December 8, 2022.
The team found that 62% were ultimately approved without any change to technique or dose, while 28% were authorized, but with lower doses or less sophisticated techniques. Four people, however, never got authorization at all — three abandoned treatment altogether, and one sought treatment at another institution.
Treatment delays ranged from 1 day to 49 days. Eighty-three patients died.
Would some of them have lived if it weren’t for prior authorization?
Dr. Chino cannot say for sure, but did note that certain cancers, like cervical cancer, can grow so quickly that every day of delayed treatment makes them harder to control.
Patients with metastatic or late-stage cancers are often denied more aggressive treatments by insurers who, in essence, “assume that they are going to die from their disease anyway,” Dr. Chino said.
She views this as tragically shortsighted.
‘’There’s actually a strong body of evidence to show that if you treat even metastatic stage IV diseases aggressively, you can prolong not just quality of life but also quantity,’’ she said.
In cases where the cancer is more localized and insurance mandates lower doses or cheaper techniques, the consequences can be equally heartbreaking.
‘’It’s like saying instead of taking an extra-strength Tylenol you can only have a baby aspirin,’’ she said. ‘’Their pain is less likely to be controlled, their disease is less likely to be controlled, and they are more likely to need retreatment.’’
Prior authorization delays can also significantly stress patients at the most vulnerable point of their lives.
In another recent study, Dr. Chino found that 69% of patients with cancer reported prior authorization-related delays in care, with one-third waiting a month or longer. One in five never got the care their doctors recommended, and 20% reported spending more than 11 hours on the phone haggling with their insurance companies.
Most patients rated the process as ‘’bad’’ or ‘’horrible,’’ and said it fueled anxiety.
Such delays can be hard on clinicians and the healthcare system too.
One 2022 study found that a typical academic radiation oncology practice spent about a half-million dollars per year seeking insurance preauthorization. Nationally, that number exceeds $40 million.
Then there is the burnout factor.
Dr. Giap, an early-career physician who specializes in rare, aggressive sarcomas, works at an institution that helped pioneer proton therapy. She says it pains her to tell a desperate patient, like the 21-year-old, who has traveled to her from out of state that they have to wait.
‘’Knowing that the majority of the cases are ultimately approved and that this wait is often unnecessary makes it even tougher,’’ she said.
Dr. Chino, a breast cancer specialist, has taken to warning patients before the alarming insurance letter arrives in the mail that their insurance may delay authorizing their care. But she tells patients that she will do everything she can to fight for them and develops a back-up plan to pivot to quickly, if needed.
‘’No one goes into medicine to spend their time talking to insurance companies,’’ said Dr. Chino.
The national trade group, America’s Health Insurance Plans (AHIP), did not return repeated requests for an interview for this story. But their official position, as stated on their website, is that “prior authorization is one of many tools health insurance providers use to promote safe, timely, evidence-based, affordable, and efficient care.”
Both Dr. Giap and Dr. Chino believe that prior authorization was developed with good intentions: to save healthcare costs and rein in treatments that don’t necessarily benefit patients.
But, in their specialty, the burden has proliferated to a point that Dr. Chino characterizes as ‘’unconscionable.’’
She believes that policy changes like the proposed Improving Seniors’ Timely Access to Care Act — which would require real-time decisions for procedures that are routinely approved — could go a long way in improving patient care.
Meanwhile, Dr. Giap said, more research and professional guidelines are necessary to bolster insurance company confidence in newer technologies, particularly for rare cancers.
Her patient ultimately got her proton therapy and is ‘’doing relatively well, all things considered.’’
But not all the stories end like this.
Dr. Chino will never forget a patient with a cancer growing so rapidly she could see it protruding through her chest wall. She called for an urgent PET scan to see where else in the body the cancer might be brewing and rushed the planning process for radiation therapy, both of which faced prior authorization barriers. That scan — which ultimately showed the cancer had spread — was delayed for months.*
If the team had had those imaging results upfront, she said, they would have recommended a completely different course of treatment.
And her patient might be alive today.
‘’Unfortunately,” Dr. Chino said, “the people with the very worst prior authorization stories aren’t here anymore to tell you about them.”
*Correction, 10/4/24: An earlier version of this article erroneously stated that Dr. Chino called for surgery for her patient. She actually called for a PET scan and an urgent radiation start.
A version of this article first appeared on Medscape.com.
Fantine Giap, MD, sat across from a 21-year-old with a rare sarcoma at the base of her skull.
Despite the large tumor, nestled in a sensitive area, the Boston-based radiation oncologist could envision a bright future for her patient.
She and the other members of the patient’s care team had an impressive cancer-fighting arsenal at her fingertips. The team had recommended surgery, followed by proton therapy — a sophisticated tool able to deliver concentrated, razor-focused radiation to the once apple-sized growth, while sparing the fragile brain stem, optic nerve, and spinal cord.
Surgery went as planned. But as the days and weeks wore on and insurance prior authorization for the proton therapy never came, the tumor roared back, leading to more surgeries and more complications. Ultimately, the young woman needed a tracheostomy and a feeding tube.
By the time insurance said yes, more than 1 year from her initial visit, the future the team had envisioned seemed out of reach.
“Unfortunately for this patient, it went from a potentially curable situation to a likely not curable situation,” recalled Dr. Giap, a clinician at Massachusetts General Hospital and instructor at Harvard Medical School, Boston. “I wanted to cry every day that she waited.’’
While a stark example, such insurance delays are not uncommon, according to new research published in JAMA Network Open.
Other studies have found that number to be even higher, with more than 86% of prior authorization requests ultimately approved with few changes.
‘’It gives you the idea that this entire process might be a little futile — that it’s just wasting people’s time,’’ said Fumiko Chino, MD, coauthor on the JAMA study and now an assistant professor in radiation oncology at MD Anderson Cancer Center in Houston. ‘’The problem is cancer doesn’t wait for bureaucracy.’’
Barriers at Every Step
As Dr. Chino and her study coauthors explained, advancements like intensity-modulated radiation therapy and stereotactic radiosurgery have allowed a new generation of specialists to treat previously untreatable cancers in ways that maximize tumor-killing power while minimizing collateral damage. But these tools require sophisticated planning, imaging, simulations and execution — all of which are subject to increased insurance scrutiny.
‘’We face barriers pretty much every step of the way for every patient,’’ said Dr. Chino.
To investigate how such barriers impact care, Dr. Chino and colleagues at Memorial Sloan Kettering Cancer Center — where she worked until July — looked at 206 cases in which payers denied prior authorization for radiation therapy from November 1, 2021 to December 8, 2022.
The team found that 62% were ultimately approved without any change to technique or dose, while 28% were authorized, but with lower doses or less sophisticated techniques. Four people, however, never got authorization at all — three abandoned treatment altogether, and one sought treatment at another institution.
Treatment delays ranged from 1 day to 49 days. Eighty-three patients died.
Would some of them have lived if it weren’t for prior authorization?
Dr. Chino cannot say for sure, but did note that certain cancers, like cervical cancer, can grow so quickly that every day of delayed treatment makes them harder to control.
Patients with metastatic or late-stage cancers are often denied more aggressive treatments by insurers who, in essence, “assume that they are going to die from their disease anyway,” Dr. Chino said.
She views this as tragically shortsighted.
‘’There’s actually a strong body of evidence to show that if you treat even metastatic stage IV diseases aggressively, you can prolong not just quality of life but also quantity,’’ she said.
In cases where the cancer is more localized and insurance mandates lower doses or cheaper techniques, the consequences can be equally heartbreaking.
‘’It’s like saying instead of taking an extra-strength Tylenol you can only have a baby aspirin,’’ she said. ‘’Their pain is less likely to be controlled, their disease is less likely to be controlled, and they are more likely to need retreatment.’’
Prior authorization delays can also significantly stress patients at the most vulnerable point of their lives.
In another recent study, Dr. Chino found that 69% of patients with cancer reported prior authorization-related delays in care, with one-third waiting a month or longer. One in five never got the care their doctors recommended, and 20% reported spending more than 11 hours on the phone haggling with their insurance companies.
Most patients rated the process as ‘’bad’’ or ‘’horrible,’’ and said it fueled anxiety.
Such delays can be hard on clinicians and the healthcare system too.
One 2022 study found that a typical academic radiation oncology practice spent about a half-million dollars per year seeking insurance preauthorization. Nationally, that number exceeds $40 million.
Then there is the burnout factor.
Dr. Giap, an early-career physician who specializes in rare, aggressive sarcomas, works at an institution that helped pioneer proton therapy. She says it pains her to tell a desperate patient, like the 21-year-old, who has traveled to her from out of state that they have to wait.
‘’Knowing that the majority of the cases are ultimately approved and that this wait is often unnecessary makes it even tougher,’’ she said.
Dr. Chino, a breast cancer specialist, has taken to warning patients before the alarming insurance letter arrives in the mail that their insurance may delay authorizing their care. But she tells patients that she will do everything she can to fight for them and develops a back-up plan to pivot to quickly, if needed.
‘’No one goes into medicine to spend their time talking to insurance companies,’’ said Dr. Chino.
The national trade group, America’s Health Insurance Plans (AHIP), did not return repeated requests for an interview for this story. But their official position, as stated on their website, is that “prior authorization is one of many tools health insurance providers use to promote safe, timely, evidence-based, affordable, and efficient care.”
Both Dr. Giap and Dr. Chino believe that prior authorization was developed with good intentions: to save healthcare costs and rein in treatments that don’t necessarily benefit patients.
But, in their specialty, the burden has proliferated to a point that Dr. Chino characterizes as ‘’unconscionable.’’
She believes that policy changes like the proposed Improving Seniors’ Timely Access to Care Act — which would require real-time decisions for procedures that are routinely approved — could go a long way in improving patient care.
Meanwhile, Dr. Giap said, more research and professional guidelines are necessary to bolster insurance company confidence in newer technologies, particularly for rare cancers.
Her patient ultimately got her proton therapy and is ‘’doing relatively well, all things considered.’’
But not all the stories end like this.
Dr. Chino will never forget a patient with a cancer growing so rapidly she could see it protruding through her chest wall. She called for an urgent PET scan to see where else in the body the cancer might be brewing and rushed the planning process for radiation therapy, both of which faced prior authorization barriers. That scan — which ultimately showed the cancer had spread — was delayed for months.*
If the team had had those imaging results upfront, she said, they would have recommended a completely different course of treatment.
And her patient might be alive today.
‘’Unfortunately,” Dr. Chino said, “the people with the very worst prior authorization stories aren’t here anymore to tell you about them.”
*Correction, 10/4/24: An earlier version of this article erroneously stated that Dr. Chino called for surgery for her patient. She actually called for a PET scan and an urgent radiation start.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Western Pygmy Rattlesnake Envenomation and Bite Management
There are 375 species of poisonous snakes, with approximately 20,000 deaths worldwide each year due to snakebites, mostly in Asia and Africa.1 The death rate in the United States is 14 to 20 cases per year. In the United States, a variety of rattlesnakes are poisonous. There are 2 genera of rattlesnakes: Sistrurus (3 species) and Crotalus (23 species). The pygmy rattlesnake belongs to the Sistrurus miliarius species that is divided into 3 subspecies: the Carolina pigmy rattlesnake (S miliarius miliarius), the western pygmy rattlesnake (S miliarius streckeri), and the dusky pygmy rattlesnake (S miliarius barbouri).2
The western pygmy rattlesnake belongs to the Crotalidae family. The rattlesnakes in this family also are known as pit vipers. All pit vipers have common characteristics for identification: triangular head, fangs, elliptical pupils, and a heat-sensing pit between the eyes. The western pygmy rattlesnake is found in Missouri, Arkansas, Oklahoma, Kentucky, and Tennessee.1 It is small bodied (15–20 inches)3 and grayish-brown, with a brown dorsal stripe with black blotches on its back. It is found in glades, second-growth forests near rock ledges, and areas where powerlines cut through dense forest.3 Its venom is hemorrhagic, causing tissue damage, but does not contain neurotoxins.4 Bites from the western pygmy rattlesnake often do not lead to death, but the venom, which contains numerous proteins and enzymes, does cause necrotic hemorrhagic ulceration at the site of envenomation and possible loss of digit.5,6
We present a case of a man who was bitten on the right third digit by a western pygmy rattlesnake. We describe the clinical course and treatment.
Case Report
A 56-year-old right-handed man presented to the emergency department with a rapidly swelling, painful hand following a snakebite to the dorsal aspect of the right third digit (Figure 1). He was able to capture a photograph of the snake at the time of injury, which helped identify it as a western pygmy rattlesnake (Figure 2). He also photographed the hand immediately after the bite occurred (Figure 3). Vitals on presentation included an elevated blood pressure of 161/100 mm Hg; no fever (temperature, 36.4 °C); and normal pulse oximetry of 98%, pulse of 86 beats per minute, and respiratory rate of 16 breaths per minute.

After the snakebite, the patient’s family called the Missouri Poison Center immediately. The family identified the snake species and shared this information with the poison center. Poison control recommended calling the nearest hospitals to determine if antivenom was available and make notification of arrival.
The patient’s tetanus toxoid immunization was updated immediately upon arrival. The hand was marked to monitor swelling. Initial laboratory test results revealed the following values: sodium, 133 mmol/L (reference range, 136–145 mmol/L); potassium, 3.4 mmol/L (3.6–5.2 mmol/L); lactic acid, 2.4 mmol/L (0.5–2.2 mmol/L); creatine kinase, 425 U/L (55–170 U/L); platelet count, 68/µL (150,000–450,000/µL); fibrinogen, 169 mg/dL (185–410 mg/dL); and glucose, 121 mg/dL (74–106 mg/dL). The remainder of the complete blood cell count and metabolic panel was unremarkable. Radiographs of the hand did not show any fractures, dislocations, or foreign bodies. Missouri Poison Center was consulted. Given the patient’s severe pain, edema beyond 40 cm, and developing ecchymosis on the inner arm, the bite was graded as a 3 on the traditional snakebite severity scale. Poison control recommended 4 to 6 vials of antivenom over 60 minutes. Six vials of Crotalidae polyvalent immune fab antivenom were given.
The patient’s complete blood cell count remained unremarkable throughout his admission. His metabolic panel returned to normal at 6 hours postadmission: sodium, 139 mmol/L; potassium, 4.0 mmol/L. His lactate and creatinine kinase were not rechecked. His fibrinogen was trending upward. Serial laboratory test results revealed fibrinogen levels of 153, 158, 161, 159, 173, and 216 mg/dL at 6, 12, 18, 24, 30, and 36 hours, respectively. Other laboratory test results including prothrombin time (11.0 s) and international normalized ratio (0.98) remained within reference range (11–13 s and 0.80–1.39, respectively) during serial monitoring.
The patient was hospitalized for 40 hours while waiting for his fibrinogen level to normalize. The local skin necrosis worsened acutely in this 40-hour window (Figure 4). Intravenous antibiotics were not administered during the hospital stay. Before discharge, the patient was evaluated by the surgery service, who did not recommend debridement.
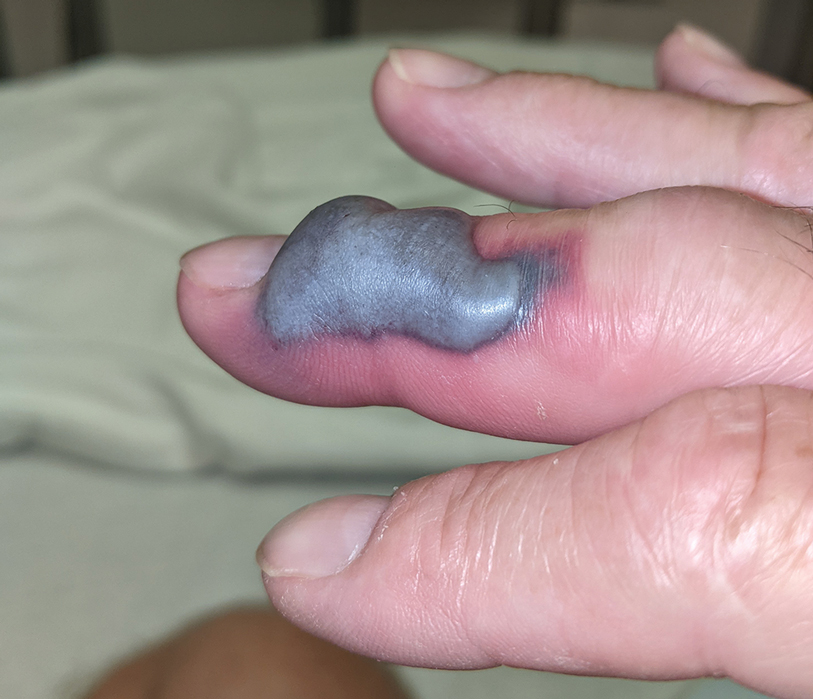
Following discharge, the patient consulted a wound care expert. The area of necrosis was unroofed and debrided in the outpatient setting (Figure 5). The patient was started on oral cefalexin 500 mg twice daily for 10 days and instructed to perform twice-daily dressing changes with silver sulfadiazine cream 1%. A hand surgeon was consulted for consideration of a reverse cross-finger flap, which was not recommended. Twice-daily dressing changes for the wound—consisting of application of silver sulfadiazine cream 1% directly to the wound followed by gauze, self-adhesive soft-rolled gauze, and elastic bandages—were performed for 2 weeks.
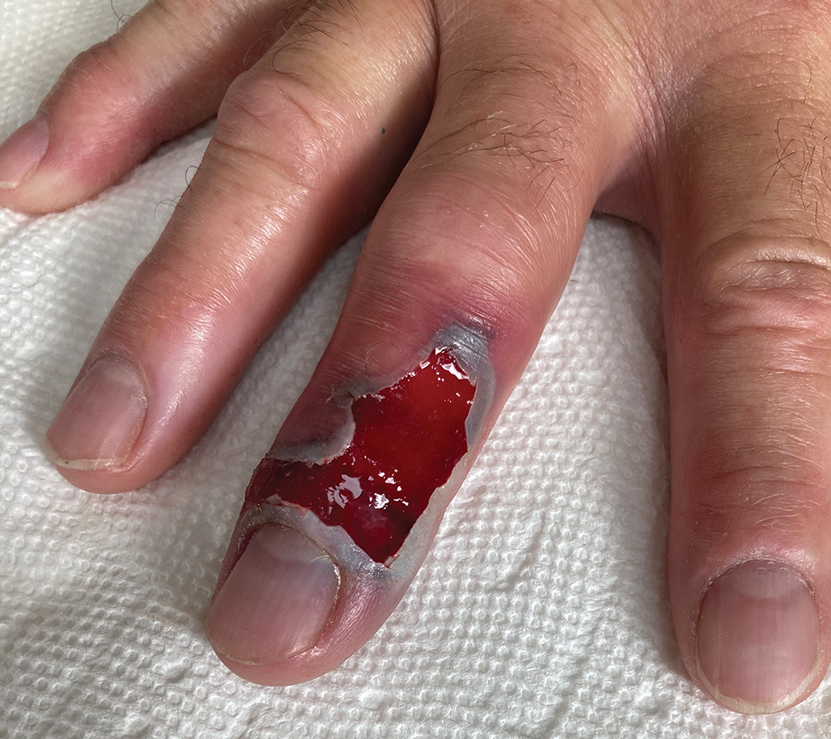
After 2 weeks, the wound was left open to the air and cleaned with soap and water as needed. At 6 weeks, the wound was completely healed via secondary intention, except for some minor remaining ulceration at the location of the fang entry point (Figure 6). The patient had no loss of finger function or sensation.
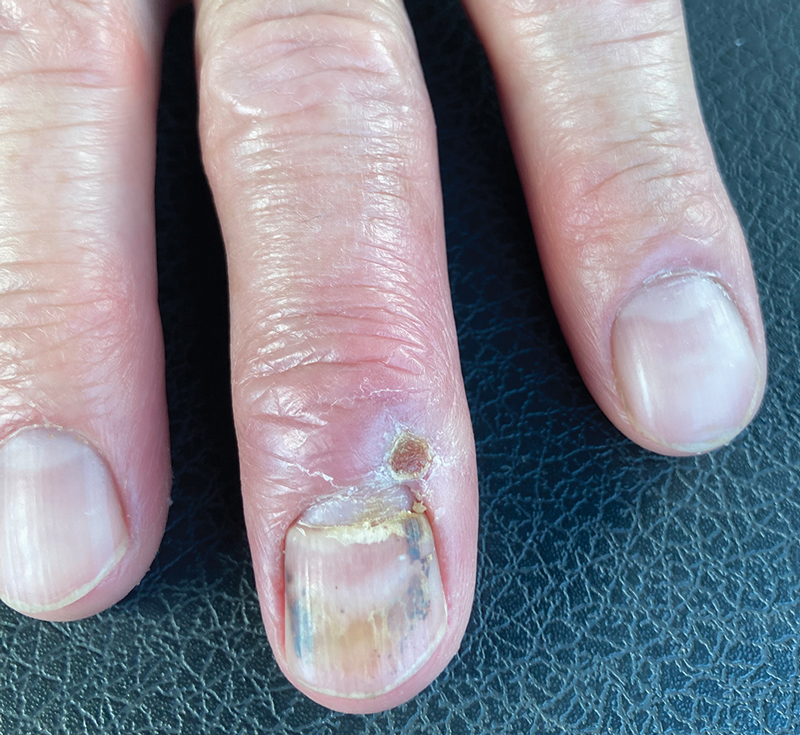
Surgical Management of Snakebites
The surgeon’s role in managing snakebites is controversial. Snakebites were once perceived as a surgical emergency due to symptoms mimicking compartment syndrome; however, snakebites rarely cause a true compartment syndrome.7 Prophylactic bite excision and fasciotomies are not recommended. Incision and suction of the fang marks may be beneficial if performed within 15 to 30 minutes from the time of the bite.8 With access to a surgeon in this short time period being nearly impossible, incision and suctioning of fang marks generally is not recommended.9 Retained snake fangs are a possibility, and the infection could spread to a nearby joint, causing septic arthritis,10 which would be an indication for surgical intervention. Bites to the finger often cause major swelling, and the benefits of dermotomy are documented.11 Generally, early administration of antivenom will decrease local tissue reaction and prevent additional tissue loss.12 In our patient, the decision to perform dermotomy was made when the area of necrosis had declared itself and the skin reached its elastic limit. Bozkurt et al13 described the neurovascular bundles within the digit as functioning as small compartments. When the skin of the digit reaches its elastic limit, pressure within the compartment may exceed the capillary closing pressure, and the integrity of small vessels and nerves may be compromised. Our case highlights the benefit of dermotomy as well as the functional and cosmetic results that can be achieved.
Wound Care for Snakebites
There is little published on the treatment of snakebites after patients are stabilized medically for hospital discharge. Venomous snakes inject toxins that predominantly consist of enzymes (eg, phospholipase A2, phosphodiesterase, hyaluronidase, peptidase, metalloproteinase) that cause tissue destruction through diverse mechanisms.14 The venom of western pygmy rattlesnakes is hemotoxic and can cause necrotic hemorrhagic ulceration,4 as was the case in our patient.
Silver sulfadiazine commonly is used to prevent infection in burn patients. Given the large surface area of exposed dermis after debridement and concern for infection, silver sulfadiazine was chosen in our patient for local wound care treatment. Silver sulfadiazine is a widely available and low-cost drug.15 Its antibacterial effects are due to the silver ions, which only act superficially and therefore limit systemic absorption.16 Application should be performed in a clean manner with minimal trauma to the tissue. This technique is best achieved by using sterile gloves and applying the medication manually. A 0.0625-inch layer should be applied to entirely cover the cleaned debrided area.17 When performing application with tongue blades or cotton swabs, it is important to never “double dip.” Patient education on proper administration is imperative to a successful outcome.
Final Thoughts
Our case demonstrates the safe use of Crotalidae polyvalent immune fab antivenom for the treatment of western pygmy rattlesnake (S miliarius streckeri) envenomation. Early administration of antivenom following pit viper rattlesnake envenomations is important to mitigate systemic effects and the extent of soft tissue damage. There are few studies on local wound care treatment after rattlesnake envenomation. This case highlights the role of dermotomy and wound care with silver sulfadiazine cream 1%.
- Biggers B. Management of Missouri snake bites. Mo Med. 2017;114:254-257.
- Stamm R. Sistrurus miliarius pigmy rattlesnake. University of Michigan Museum of Zoology. Accessed September 23, 2024. https://animaldiversity.org/accounts/Sistrurus_miliarius/
- Missouri Department of Conservation. Western pygmy rattlesnake. Accessed September 18, 2024. https://mdc.mo.gov/discover-nature/field-guide/western-pygmy-rattlesnake
- AnimalSake. Facts about the pigmy rattlesnake that are sure to surprise you. Accessed September 18, 2024. https://animalsake.com/pygmy-rattlesnake
- King AM, Crim WS, Menke NB, et al. Pygmy rattlesnake envenomation treated with crotalidae polyvalent immune fab antivenom. Toxicon. 2012;60:1287-1289.
- Juckett G, Hancox JG. Venomous snakebites in the United States: management review and update. Am Fam Physician. 2002;65:1367-1375.
- Toschlog EA, Bauer CR, Hall EL, et al. Surgical considerations in the management of pit viper snake envenomation. J Am Coll Surg. 2013;217:726-735.
- Cribari C. Management of poisonous snakebite. American College of Surgeons Committee on Trauma; 2004. https://www.hartcountyga.gov/documents/PoisonousSnakebiteTreatment.pdf
- Walker JP, Morrison RL. Current management of copperhead snakebite. J Am Coll Surg. 2011;212:470-474.
- Gelman D, Bates T, Nuelle JAV. Septic arthritis of the proximal interphalangeal joint after rattlesnake bite. J Hand Surg Am. 2022;47:484.e1-484.e4.
- Watt CH Jr. Treatment of poisonous snakebite with emphasis on digit dermotomy. South Med J. 1985;78:694-699.
- Corneille MG, Larson S, Stewart RM, et al. A large single-center experience with treatment of patients with crotalid envenomations: outcomes with and evolution of antivenin therapy. Am J Surg. 2006;192:848-852.
- Bozkurt M, Kulahci Y, Zor F, et al. The management of pit viper envenomation of the hand. Hand (NY). 2008;3:324-331.
- Aziz H, Rhee P, Pandit V, et al. The current concepts in management of animal (dog, cat, snake, scorpion) and human bite wounds. J Trauma Acute Care Surg. 2015;78:641-648.
- Hummel RP, MacMillan BG, Altemeier WA. Topical and systemic antibacterial agents in the treatment of burns. Ann Surg. 1970;172:370-384.
- Modak SM, Sampath L, Fox CL. Combined topical use of silver sulfadiazine and antibiotics as a possible solution to bacterial resistance in burn wounds. J Burn Care Rehabil. 1988;9:359-363.
- Oaks RJ, Cindass R. Silver sulfadiazine. StatPearls [Internet]. Updated January 22, 2023. Accessed September 23, 2024. https://www.ncbi.nlm.nih.gov/books/NBK556054/
There are 375 species of poisonous snakes, with approximately 20,000 deaths worldwide each year due to snakebites, mostly in Asia and Africa.1 The death rate in the United States is 14 to 20 cases per year. In the United States, a variety of rattlesnakes are poisonous. There are 2 genera of rattlesnakes: Sistrurus (3 species) and Crotalus (23 species). The pygmy rattlesnake belongs to the Sistrurus miliarius species that is divided into 3 subspecies: the Carolina pigmy rattlesnake (S miliarius miliarius), the western pygmy rattlesnake (S miliarius streckeri), and the dusky pygmy rattlesnake (S miliarius barbouri).2
The western pygmy rattlesnake belongs to the Crotalidae family. The rattlesnakes in this family also are known as pit vipers. All pit vipers have common characteristics for identification: triangular head, fangs, elliptical pupils, and a heat-sensing pit between the eyes. The western pygmy rattlesnake is found in Missouri, Arkansas, Oklahoma, Kentucky, and Tennessee.1 It is small bodied (15–20 inches)3 and grayish-brown, with a brown dorsal stripe with black blotches on its back. It is found in glades, second-growth forests near rock ledges, and areas where powerlines cut through dense forest.3 Its venom is hemorrhagic, causing tissue damage, but does not contain neurotoxins.4 Bites from the western pygmy rattlesnake often do not lead to death, but the venom, which contains numerous proteins and enzymes, does cause necrotic hemorrhagic ulceration at the site of envenomation and possible loss of digit.5,6
We present a case of a man who was bitten on the right third digit by a western pygmy rattlesnake. We describe the clinical course and treatment.
Case Report
A 56-year-old right-handed man presented to the emergency department with a rapidly swelling, painful hand following a snakebite to the dorsal aspect of the right third digit (Figure 1). He was able to capture a photograph of the snake at the time of injury, which helped identify it as a western pygmy rattlesnake (Figure 2). He also photographed the hand immediately after the bite occurred (Figure 3). Vitals on presentation included an elevated blood pressure of 161/100 mm Hg; no fever (temperature, 36.4 °C); and normal pulse oximetry of 98%, pulse of 86 beats per minute, and respiratory rate of 16 breaths per minute.



After the snakebite, the patient’s family called the Missouri Poison Center immediately. The family identified the snake species and shared this information with the poison center. Poison control recommended calling the nearest hospitals to determine if antivenom was available and make notification of arrival.
The patient’s tetanus toxoid immunization was updated immediately upon arrival. The hand was marked to monitor swelling. Initial laboratory test results revealed the following values: sodium, 133 mmol/L (reference range, 136–145 mmol/L); potassium, 3.4 mmol/L (3.6–5.2 mmol/L); lactic acid, 2.4 mmol/L (0.5–2.2 mmol/L); creatine kinase, 425 U/L (55–170 U/L); platelet count, 68/µL (150,000–450,000/µL); fibrinogen, 169 mg/dL (185–410 mg/dL); and glucose, 121 mg/dL (74–106 mg/dL). The remainder of the complete blood cell count and metabolic panel was unremarkable. Radiographs of the hand did not show any fractures, dislocations, or foreign bodies. Missouri Poison Center was consulted. Given the patient’s severe pain, edema beyond 40 cm, and developing ecchymosis on the inner arm, the bite was graded as a 3 on the traditional snakebite severity scale. Poison control recommended 4 to 6 vials of antivenom over 60 minutes. Six vials of Crotalidae polyvalent immune fab antivenom were given.
The patient’s complete blood cell count remained unremarkable throughout his admission. His metabolic panel returned to normal at 6 hours postadmission: sodium, 139 mmol/L; potassium, 4.0 mmol/L. His lactate and creatinine kinase were not rechecked. His fibrinogen was trending upward. Serial laboratory test results revealed fibrinogen levels of 153, 158, 161, 159, 173, and 216 mg/dL at 6, 12, 18, 24, 30, and 36 hours, respectively. Other laboratory test results including prothrombin time (11.0 s) and international normalized ratio (0.98) remained within reference range (11–13 s and 0.80–1.39, respectively) during serial monitoring.
The patient was hospitalized for 40 hours while waiting for his fibrinogen level to normalize. The local skin necrosis worsened acutely in this 40-hour window (Figure 4). Intravenous antibiotics were not administered during the hospital stay. Before discharge, the patient was evaluated by the surgery service, who did not recommend debridement.

Following discharge, the patient consulted a wound care expert. The area of necrosis was unroofed and debrided in the outpatient setting (Figure 5). The patient was started on oral cefalexin 500 mg twice daily for 10 days and instructed to perform twice-daily dressing changes with silver sulfadiazine cream 1%. A hand surgeon was consulted for consideration of a reverse cross-finger flap, which was not recommended. Twice-daily dressing changes for the wound—consisting of application of silver sulfadiazine cream 1% directly to the wound followed by gauze, self-adhesive soft-rolled gauze, and elastic bandages—were performed for 2 weeks.

After 2 weeks, the wound was left open to the air and cleaned with soap and water as needed. At 6 weeks, the wound was completely healed via secondary intention, except for some minor remaining ulceration at the location of the fang entry point (Figure 6). The patient had no loss of finger function or sensation.

Surgical Management of Snakebites
The surgeon’s role in managing snakebites is controversial. Snakebites were once perceived as a surgical emergency due to symptoms mimicking compartment syndrome; however, snakebites rarely cause a true compartment syndrome.7 Prophylactic bite excision and fasciotomies are not recommended. Incision and suction of the fang marks may be beneficial if performed within 15 to 30 minutes from the time of the bite.8 With access to a surgeon in this short time period being nearly impossible, incision and suctioning of fang marks generally is not recommended.9 Retained snake fangs are a possibility, and the infection could spread to a nearby joint, causing septic arthritis,10 which would be an indication for surgical intervention. Bites to the finger often cause major swelling, and the benefits of dermotomy are documented.11 Generally, early administration of antivenom will decrease local tissue reaction and prevent additional tissue loss.12 In our patient, the decision to perform dermotomy was made when the area of necrosis had declared itself and the skin reached its elastic limit. Bozkurt et al13 described the neurovascular bundles within the digit as functioning as small compartments. When the skin of the digit reaches its elastic limit, pressure within the compartment may exceed the capillary closing pressure, and the integrity of small vessels and nerves may be compromised. Our case highlights the benefit of dermotomy as well as the functional and cosmetic results that can be achieved.
Wound Care for Snakebites
There is little published on the treatment of snakebites after patients are stabilized medically for hospital discharge. Venomous snakes inject toxins that predominantly consist of enzymes (eg, phospholipase A2, phosphodiesterase, hyaluronidase, peptidase, metalloproteinase) that cause tissue destruction through diverse mechanisms.14 The venom of western pygmy rattlesnakes is hemotoxic and can cause necrotic hemorrhagic ulceration,4 as was the case in our patient.
Silver sulfadiazine commonly is used to prevent infection in burn patients. Given the large surface area of exposed dermis after debridement and concern for infection, silver sulfadiazine was chosen in our patient for local wound care treatment. Silver sulfadiazine is a widely available and low-cost drug.15 Its antibacterial effects are due to the silver ions, which only act superficially and therefore limit systemic absorption.16 Application should be performed in a clean manner with minimal trauma to the tissue. This technique is best achieved by using sterile gloves and applying the medication manually. A 0.0625-inch layer should be applied to entirely cover the cleaned debrided area.17 When performing application with tongue blades or cotton swabs, it is important to never “double dip.” Patient education on proper administration is imperative to a successful outcome.
Final Thoughts
Our case demonstrates the safe use of Crotalidae polyvalent immune fab antivenom for the treatment of western pygmy rattlesnake (S miliarius streckeri) envenomation. Early administration of antivenom following pit viper rattlesnake envenomations is important to mitigate systemic effects and the extent of soft tissue damage. There are few studies on local wound care treatment after rattlesnake envenomation. This case highlights the role of dermotomy and wound care with silver sulfadiazine cream 1%.
There are 375 species of poisonous snakes, with approximately 20,000 deaths worldwide each year due to snakebites, mostly in Asia and Africa.1 The death rate in the United States is 14 to 20 cases per year. In the United States, a variety of rattlesnakes are poisonous. There are 2 genera of rattlesnakes: Sistrurus (3 species) and Crotalus (23 species). The pygmy rattlesnake belongs to the Sistrurus miliarius species that is divided into 3 subspecies: the Carolina pigmy rattlesnake (S miliarius miliarius), the western pygmy rattlesnake (S miliarius streckeri), and the dusky pygmy rattlesnake (S miliarius barbouri).2
The western pygmy rattlesnake belongs to the Crotalidae family. The rattlesnakes in this family also are known as pit vipers. All pit vipers have common characteristics for identification: triangular head, fangs, elliptical pupils, and a heat-sensing pit between the eyes. The western pygmy rattlesnake is found in Missouri, Arkansas, Oklahoma, Kentucky, and Tennessee.1 It is small bodied (15–20 inches)3 and grayish-brown, with a brown dorsal stripe with black blotches on its back. It is found in glades, second-growth forests near rock ledges, and areas where powerlines cut through dense forest.3 Its venom is hemorrhagic, causing tissue damage, but does not contain neurotoxins.4 Bites from the western pygmy rattlesnake often do not lead to death, but the venom, which contains numerous proteins and enzymes, does cause necrotic hemorrhagic ulceration at the site of envenomation and possible loss of digit.5,6
We present a case of a man who was bitten on the right third digit by a western pygmy rattlesnake. We describe the clinical course and treatment.
Case Report
A 56-year-old right-handed man presented to the emergency department with a rapidly swelling, painful hand following a snakebite to the dorsal aspect of the right third digit (Figure 1). He was able to capture a photograph of the snake at the time of injury, which helped identify it as a western pygmy rattlesnake (Figure 2). He also photographed the hand immediately after the bite occurred (Figure 3). Vitals on presentation included an elevated blood pressure of 161/100 mm Hg; no fever (temperature, 36.4 °C); and normal pulse oximetry of 98%, pulse of 86 beats per minute, and respiratory rate of 16 breaths per minute.



After the snakebite, the patient’s family called the Missouri Poison Center immediately. The family identified the snake species and shared this information with the poison center. Poison control recommended calling the nearest hospitals to determine if antivenom was available and make notification of arrival.
The patient’s tetanus toxoid immunization was updated immediately upon arrival. The hand was marked to monitor swelling. Initial laboratory test results revealed the following values: sodium, 133 mmol/L (reference range, 136–145 mmol/L); potassium, 3.4 mmol/L (3.6–5.2 mmol/L); lactic acid, 2.4 mmol/L (0.5–2.2 mmol/L); creatine kinase, 425 U/L (55–170 U/L); platelet count, 68/µL (150,000–450,000/µL); fibrinogen, 169 mg/dL (185–410 mg/dL); and glucose, 121 mg/dL (74–106 mg/dL). The remainder of the complete blood cell count and metabolic panel was unremarkable. Radiographs of the hand did not show any fractures, dislocations, or foreign bodies. Missouri Poison Center was consulted. Given the patient’s severe pain, edema beyond 40 cm, and developing ecchymosis on the inner arm, the bite was graded as a 3 on the traditional snakebite severity scale. Poison control recommended 4 to 6 vials of antivenom over 60 minutes. Six vials of Crotalidae polyvalent immune fab antivenom were given.
The patient’s complete blood cell count remained unremarkable throughout his admission. His metabolic panel returned to normal at 6 hours postadmission: sodium, 139 mmol/L; potassium, 4.0 mmol/L. His lactate and creatinine kinase were not rechecked. His fibrinogen was trending upward. Serial laboratory test results revealed fibrinogen levels of 153, 158, 161, 159, 173, and 216 mg/dL at 6, 12, 18, 24, 30, and 36 hours, respectively. Other laboratory test results including prothrombin time (11.0 s) and international normalized ratio (0.98) remained within reference range (11–13 s and 0.80–1.39, respectively) during serial monitoring.
The patient was hospitalized for 40 hours while waiting for his fibrinogen level to normalize. The local skin necrosis worsened acutely in this 40-hour window (Figure 4). Intravenous antibiotics were not administered during the hospital stay. Before discharge, the patient was evaluated by the surgery service, who did not recommend debridement.

Following discharge, the patient consulted a wound care expert. The area of necrosis was unroofed and debrided in the outpatient setting (Figure 5). The patient was started on oral cefalexin 500 mg twice daily for 10 days and instructed to perform twice-daily dressing changes with silver sulfadiazine cream 1%. A hand surgeon was consulted for consideration of a reverse cross-finger flap, which was not recommended. Twice-daily dressing changes for the wound—consisting of application of silver sulfadiazine cream 1% directly to the wound followed by gauze, self-adhesive soft-rolled gauze, and elastic bandages—were performed for 2 weeks.

After 2 weeks, the wound was left open to the air and cleaned with soap and water as needed. At 6 weeks, the wound was completely healed via secondary intention, except for some minor remaining ulceration at the location of the fang entry point (Figure 6). The patient had no loss of finger function or sensation.

Surgical Management of Snakebites
The surgeon’s role in managing snakebites is controversial. Snakebites were once perceived as a surgical emergency due to symptoms mimicking compartment syndrome; however, snakebites rarely cause a true compartment syndrome.7 Prophylactic bite excision and fasciotomies are not recommended. Incision and suction of the fang marks may be beneficial if performed within 15 to 30 minutes from the time of the bite.8 With access to a surgeon in this short time period being nearly impossible, incision and suctioning of fang marks generally is not recommended.9 Retained snake fangs are a possibility, and the infection could spread to a nearby joint, causing septic arthritis,10 which would be an indication for surgical intervention. Bites to the finger often cause major swelling, and the benefits of dermotomy are documented.11 Generally, early administration of antivenom will decrease local tissue reaction and prevent additional tissue loss.12 In our patient, the decision to perform dermotomy was made when the area of necrosis had declared itself and the skin reached its elastic limit. Bozkurt et al13 described the neurovascular bundles within the digit as functioning as small compartments. When the skin of the digit reaches its elastic limit, pressure within the compartment may exceed the capillary closing pressure, and the integrity of small vessels and nerves may be compromised. Our case highlights the benefit of dermotomy as well as the functional and cosmetic results that can be achieved.
Wound Care for Snakebites
There is little published on the treatment of snakebites after patients are stabilized medically for hospital discharge. Venomous snakes inject toxins that predominantly consist of enzymes (eg, phospholipase A2, phosphodiesterase, hyaluronidase, peptidase, metalloproteinase) that cause tissue destruction through diverse mechanisms.14 The venom of western pygmy rattlesnakes is hemotoxic and can cause necrotic hemorrhagic ulceration,4 as was the case in our patient.
Silver sulfadiazine commonly is used to prevent infection in burn patients. Given the large surface area of exposed dermis after debridement and concern for infection, silver sulfadiazine was chosen in our patient for local wound care treatment. Silver sulfadiazine is a widely available and low-cost drug.15 Its antibacterial effects are due to the silver ions, which only act superficially and therefore limit systemic absorption.16 Application should be performed in a clean manner with minimal trauma to the tissue. This technique is best achieved by using sterile gloves and applying the medication manually. A 0.0625-inch layer should be applied to entirely cover the cleaned debrided area.17 When performing application with tongue blades or cotton swabs, it is important to never “double dip.” Patient education on proper administration is imperative to a successful outcome.
Final Thoughts
Our case demonstrates the safe use of Crotalidae polyvalent immune fab antivenom for the treatment of western pygmy rattlesnake (S miliarius streckeri) envenomation. Early administration of antivenom following pit viper rattlesnake envenomations is important to mitigate systemic effects and the extent of soft tissue damage. There are few studies on local wound care treatment after rattlesnake envenomation. This case highlights the role of dermotomy and wound care with silver sulfadiazine cream 1%.
- Biggers B. Management of Missouri snake bites. Mo Med. 2017;114:254-257.
- Stamm R. Sistrurus miliarius pigmy rattlesnake. University of Michigan Museum of Zoology. Accessed September 23, 2024. https://animaldiversity.org/accounts/Sistrurus_miliarius/
- Missouri Department of Conservation. Western pygmy rattlesnake. Accessed September 18, 2024. https://mdc.mo.gov/discover-nature/field-guide/western-pygmy-rattlesnake
- AnimalSake. Facts about the pigmy rattlesnake that are sure to surprise you. Accessed September 18, 2024. https://animalsake.com/pygmy-rattlesnake
- King AM, Crim WS, Menke NB, et al. Pygmy rattlesnake envenomation treated with crotalidae polyvalent immune fab antivenom. Toxicon. 2012;60:1287-1289.
- Juckett G, Hancox JG. Venomous snakebites in the United States: management review and update. Am Fam Physician. 2002;65:1367-1375.
- Toschlog EA, Bauer CR, Hall EL, et al. Surgical considerations in the management of pit viper snake envenomation. J Am Coll Surg. 2013;217:726-735.
- Cribari C. Management of poisonous snakebite. American College of Surgeons Committee on Trauma; 2004. https://www.hartcountyga.gov/documents/PoisonousSnakebiteTreatment.pdf
- Walker JP, Morrison RL. Current management of copperhead snakebite. J Am Coll Surg. 2011;212:470-474.
- Gelman D, Bates T, Nuelle JAV. Septic arthritis of the proximal interphalangeal joint after rattlesnake bite. J Hand Surg Am. 2022;47:484.e1-484.e4.
- Watt CH Jr. Treatment of poisonous snakebite with emphasis on digit dermotomy. South Med J. 1985;78:694-699.
- Corneille MG, Larson S, Stewart RM, et al. A large single-center experience with treatment of patients with crotalid envenomations: outcomes with and evolution of antivenin therapy. Am J Surg. 2006;192:848-852.
- Bozkurt M, Kulahci Y, Zor F, et al. The management of pit viper envenomation of the hand. Hand (NY). 2008;3:324-331.
- Aziz H, Rhee P, Pandit V, et al. The current concepts in management of animal (dog, cat, snake, scorpion) and human bite wounds. J Trauma Acute Care Surg. 2015;78:641-648.
- Hummel RP, MacMillan BG, Altemeier WA. Topical and systemic antibacterial agents in the treatment of burns. Ann Surg. 1970;172:370-384.
- Modak SM, Sampath L, Fox CL. Combined topical use of silver sulfadiazine and antibiotics as a possible solution to bacterial resistance in burn wounds. J Burn Care Rehabil. 1988;9:359-363.
- Oaks RJ, Cindass R. Silver sulfadiazine. StatPearls [Internet]. Updated January 22, 2023. Accessed September 23, 2024. https://www.ncbi.nlm.nih.gov/books/NBK556054/
- Biggers B. Management of Missouri snake bites. Mo Med. 2017;114:254-257.
- Stamm R. Sistrurus miliarius pigmy rattlesnake. University of Michigan Museum of Zoology. Accessed September 23, 2024. https://animaldiversity.org/accounts/Sistrurus_miliarius/
- Missouri Department of Conservation. Western pygmy rattlesnake. Accessed September 18, 2024. https://mdc.mo.gov/discover-nature/field-guide/western-pygmy-rattlesnake
- AnimalSake. Facts about the pigmy rattlesnake that are sure to surprise you. Accessed September 18, 2024. https://animalsake.com/pygmy-rattlesnake
- King AM, Crim WS, Menke NB, et al. Pygmy rattlesnake envenomation treated with crotalidae polyvalent immune fab antivenom. Toxicon. 2012;60:1287-1289.
- Juckett G, Hancox JG. Venomous snakebites in the United States: management review and update. Am Fam Physician. 2002;65:1367-1375.
- Toschlog EA, Bauer CR, Hall EL, et al. Surgical considerations in the management of pit viper snake envenomation. J Am Coll Surg. 2013;217:726-735.
- Cribari C. Management of poisonous snakebite. American College of Surgeons Committee on Trauma; 2004. https://www.hartcountyga.gov/documents/PoisonousSnakebiteTreatment.pdf
- Walker JP, Morrison RL. Current management of copperhead snakebite. J Am Coll Surg. 2011;212:470-474.
- Gelman D, Bates T, Nuelle JAV. Septic arthritis of the proximal interphalangeal joint after rattlesnake bite. J Hand Surg Am. 2022;47:484.e1-484.e4.
- Watt CH Jr. Treatment of poisonous snakebite with emphasis on digit dermotomy. South Med J. 1985;78:694-699.
- Corneille MG, Larson S, Stewart RM, et al. A large single-center experience with treatment of patients with crotalid envenomations: outcomes with and evolution of antivenin therapy. Am J Surg. 2006;192:848-852.
- Bozkurt M, Kulahci Y, Zor F, et al. The management of pit viper envenomation of the hand. Hand (NY). 2008;3:324-331.
- Aziz H, Rhee P, Pandit V, et al. The current concepts in management of animal (dog, cat, snake, scorpion) and human bite wounds. J Trauma Acute Care Surg. 2015;78:641-648.
- Hummel RP, MacMillan BG, Altemeier WA. Topical and systemic antibacterial agents in the treatment of burns. Ann Surg. 1970;172:370-384.
- Modak SM, Sampath L, Fox CL. Combined topical use of silver sulfadiazine and antibiotics as a possible solution to bacterial resistance in burn wounds. J Burn Care Rehabil. 1988;9:359-363.
- Oaks RJ, Cindass R. Silver sulfadiazine. StatPearls [Internet]. Updated January 22, 2023. Accessed September 23, 2024. https://www.ncbi.nlm.nih.gov/books/NBK556054/
Practice Points
- Patients should seek medical attention immediately for western pygmy rattlesnake bites for early initiation of antivenom treatment.
- Contact the closest emergency department to confirm they are equipped to treat rattlesnake bites and notify them of a pending arrival.
- Consider dermotomy or local debridement of bites involving the digits.
- Monitor the wound in the days and weeks following the bite to ensure adequate healing.
Multiple Painless Whitish Papules on the Vulva and Perianal Region
THE DIAGNOSIS: Papular Acantholytic Dyskeratosis
Histopathology of the lesion in our patient revealed hyperkeratosis, parakeratosis, dyskeratosis, and acantholysis of keratinocytes. The dermis showed variable chronic inflammatory cells. Corps ronds and grains in the acantholytic layer of the epidermis were identified. Hair follicles were not affected by acantholysis. Anti–desmoglein 1 and anti–desmoglein 3 serum antibodies were negative. Based on the combined clinical and histologic findings, the patient was diagnosed with papular acantholytic dyskeratosis (PAD) of the genitocrural area.
Although its typical histopathologic pattern mimics both Hailey-Hailey disease and Darier disease, PAD is a rare unique clinicopathologic entity recognized by dermatopathologists. It usually occurs in middle-aged women with no family history of similar conditions. The multiple localized, flesh-colored to whitish papules of PAD tend to coalesce into plaques in the anogenital and genitocrural regions. Plaques usually are asymptomatic but may be pruritic. Histopathologically, PAD will demonstrate hyperkeratosis, dyskeratosis, and acantholysis. Corps ronds and grains will be present in the acantholytic layer of the epidermis.1,2
The differential diagnosis for PAD includes pemphigus vegetans, Hailey-Hailey disease, Darier disease, and Grover disease. Patients usually develop pemphigus vegetans at an older age (typically 50–70 years).3 Histopathologically, it is characterized by pseudoepitheliomatous hyperplasia with an eosinophilic microabscess as well as acantholysis that involves the follicular epithelium (Figure 1),4 which were not seen in our patient. Direct immunofluorescence will show the intercellular pattern of the pemphigus group, and antidesmoglein antibodies can be detected by enzyme-linked immunosorbent assay.4,5
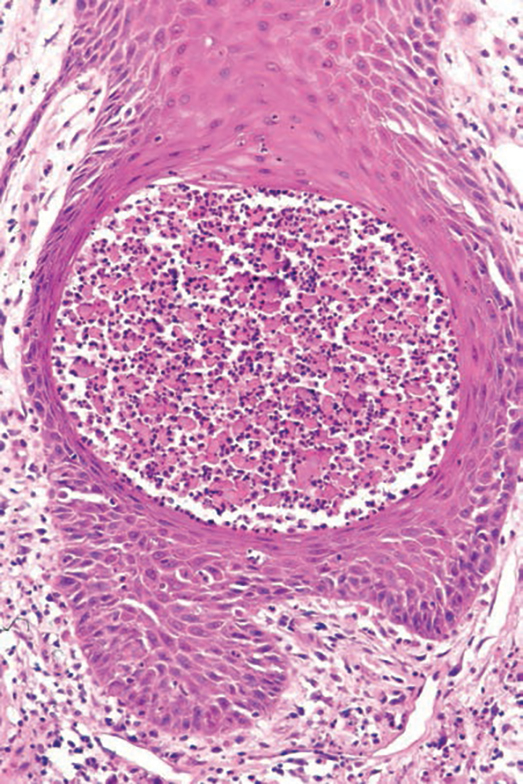
Hailey-Hailey disease (also known as benign familial pemphigus) typically manifests as itchy malodorous vesicles and erosions, especially in intertriginous areas. The most commonly affected sites are the groin, neck, under the breasts, and between the buttocks. In one study, two-thirds of affected patients reported a relevant family history.4 Histopathology will show minimal dyskeratosis and suprabasilar acantholysis with loss of intercellular bridges, classically described as resembling a dilapidated brick wall (Figure 2).4,5 There is no notable follicular involvement with acantholysis.4
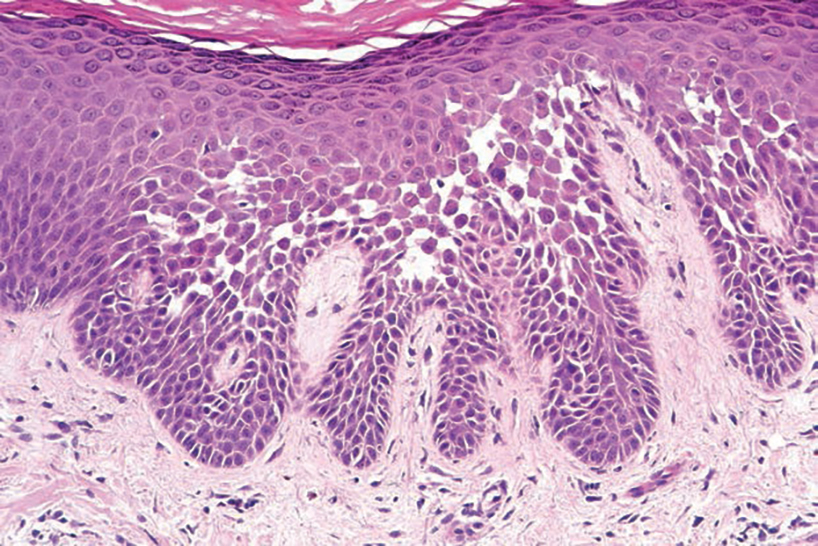
characteristic dilapidated brick wall appearance (H&E, original
magnification ×40).
Darier disease (also known as keratosis follicularis) typically is inherited in an autosomal-dominant pattern.4 It is found on the seborrheic areas such as the scalp, forehead, nasolabial folds, and upper chest. Characteristic features include distal notching of the nails, mucosal lesions, and palmoplantar papules. Histopathology will reveal acantholysis, dyskeratosis, suprabasilar acantholysis, and corps ronds and grains.4 Acantholysis in Darier disease can be in discrete foci and/or widespread (Figure 3).4 Darier disease demonstrates more dyskeratosis than Hailey-Hailey disease.4,5

Grover disease (also referred to as transient acantholytic dermatosis) is observed predominantly in individuals who are middle-aged or older, though occurrence in children has been rarely reported.4 It affects the trunk, neck, and proximal limbs but spares the genital area. Histopathology may reveal acantholysis (similar to Hailey-Hailey disease or pemphigus vulgaris), dyskeratosis (resembling Darier disease), spongiosis, parakeratosis, and a superficial perivascular lymphocytic infiltrate with eosinophils.4 A histologic clue to the diagnosis is small lesion size (1–3 mm). Usually, only 1 or 2 small discrete lesions that span a few rete ridges are noted (Figure 4).4 Grover disease can cause follicular or acrosyringeal involvement.4
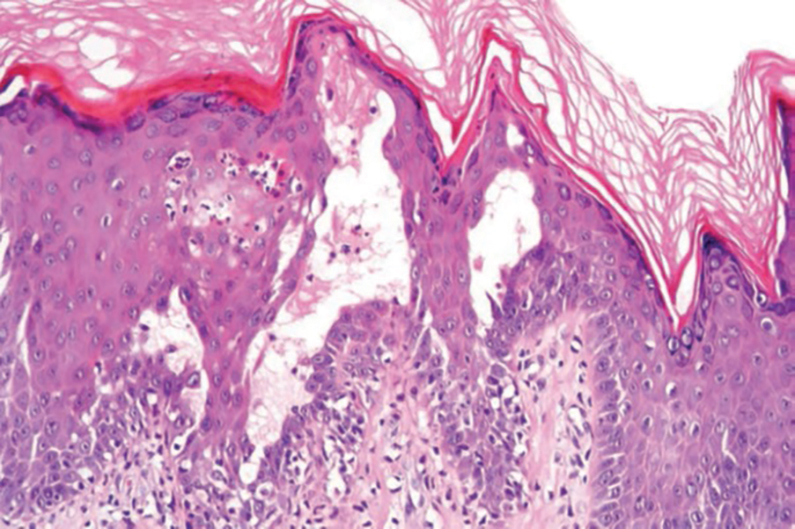
- Al-Muriesh M, Abdul-Fattah B, Wang X, et al. Papular acantholytic dyskeratosis of the anogenital and genitocrural area: case series and review of the literature. J Cutan Pathol. 2016;43:749-758. doi:10.1111/cup.12736
- Harrell J, Nielson C, Beers P, et al. Eruption on the vulva and groin. JAAD Case Reports. 2019;6:6-8. doi:10.1016/j.jdcr.2019.11.003
- Messersmith L, Krauland K. Pemphigus vegetans. StatPearls [Internet]. Updated June 26, 2023. Accessed September 18, 2024. https://www.ncbi.nlm.nih.gov/books/NBK545229
- Acantholytic disorders. In: Calonje E, Brenn T, Lazar A, et al, eds. McKee’s Pathology of the Skin: With Clinical Correlations. Elsevier/ Saunders; 2012:171-200.
- Mohr MR, Erdag G, Shada AL, et al. Two patients with Hailey- Hailey disease, multiple primary melanomas, and other cancers. Arch Dermatol. 2011;147:211215. doi:10.1001/archdermatol.2010.445
THE DIAGNOSIS: Papular Acantholytic Dyskeratosis
Histopathology of the lesion in our patient revealed hyperkeratosis, parakeratosis, dyskeratosis, and acantholysis of keratinocytes. The dermis showed variable chronic inflammatory cells. Corps ronds and grains in the acantholytic layer of the epidermis were identified. Hair follicles were not affected by acantholysis. Anti–desmoglein 1 and anti–desmoglein 3 serum antibodies were negative. Based on the combined clinical and histologic findings, the patient was diagnosed with papular acantholytic dyskeratosis (PAD) of the genitocrural area.
Although its typical histopathologic pattern mimics both Hailey-Hailey disease and Darier disease, PAD is a rare unique clinicopathologic entity recognized by dermatopathologists. It usually occurs in middle-aged women with no family history of similar conditions. The multiple localized, flesh-colored to whitish papules of PAD tend to coalesce into plaques in the anogenital and genitocrural regions. Plaques usually are asymptomatic but may be pruritic. Histopathologically, PAD will demonstrate hyperkeratosis, dyskeratosis, and acantholysis. Corps ronds and grains will be present in the acantholytic layer of the epidermis.1,2
The differential diagnosis for PAD includes pemphigus vegetans, Hailey-Hailey disease, Darier disease, and Grover disease. Patients usually develop pemphigus vegetans at an older age (typically 50–70 years).3 Histopathologically, it is characterized by pseudoepitheliomatous hyperplasia with an eosinophilic microabscess as well as acantholysis that involves the follicular epithelium (Figure 1),4 which were not seen in our patient. Direct immunofluorescence will show the intercellular pattern of the pemphigus group, and antidesmoglein antibodies can be detected by enzyme-linked immunosorbent assay.4,5

Hailey-Hailey disease (also known as benign familial pemphigus) typically manifests as itchy malodorous vesicles and erosions, especially in intertriginous areas. The most commonly affected sites are the groin, neck, under the breasts, and between the buttocks. In one study, two-thirds of affected patients reported a relevant family history.4 Histopathology will show minimal dyskeratosis and suprabasilar acantholysis with loss of intercellular bridges, classically described as resembling a dilapidated brick wall (Figure 2).4,5 There is no notable follicular involvement with acantholysis.4

characteristic dilapidated brick wall appearance (H&E, original
magnification ×40).
Darier disease (also known as keratosis follicularis) typically is inherited in an autosomal-dominant pattern.4 It is found on the seborrheic areas such as the scalp, forehead, nasolabial folds, and upper chest. Characteristic features include distal notching of the nails, mucosal lesions, and palmoplantar papules. Histopathology will reveal acantholysis, dyskeratosis, suprabasilar acantholysis, and corps ronds and grains.4 Acantholysis in Darier disease can be in discrete foci and/or widespread (Figure 3).4 Darier disease demonstrates more dyskeratosis than Hailey-Hailey disease.4,5

Grover disease (also referred to as transient acantholytic dermatosis) is observed predominantly in individuals who are middle-aged or older, though occurrence in children has been rarely reported.4 It affects the trunk, neck, and proximal limbs but spares the genital area. Histopathology may reveal acantholysis (similar to Hailey-Hailey disease or pemphigus vulgaris), dyskeratosis (resembling Darier disease), spongiosis, parakeratosis, and a superficial perivascular lymphocytic infiltrate with eosinophils.4 A histologic clue to the diagnosis is small lesion size (1–3 mm). Usually, only 1 or 2 small discrete lesions that span a few rete ridges are noted (Figure 4).4 Grover disease can cause follicular or acrosyringeal involvement.4

THE DIAGNOSIS: Papular Acantholytic Dyskeratosis
Histopathology of the lesion in our patient revealed hyperkeratosis, parakeratosis, dyskeratosis, and acantholysis of keratinocytes. The dermis showed variable chronic inflammatory cells. Corps ronds and grains in the acantholytic layer of the epidermis were identified. Hair follicles were not affected by acantholysis. Anti–desmoglein 1 and anti–desmoglein 3 serum antibodies were negative. Based on the combined clinical and histologic findings, the patient was diagnosed with papular acantholytic dyskeratosis (PAD) of the genitocrural area.
Although its typical histopathologic pattern mimics both Hailey-Hailey disease and Darier disease, PAD is a rare unique clinicopathologic entity recognized by dermatopathologists. It usually occurs in middle-aged women with no family history of similar conditions. The multiple localized, flesh-colored to whitish papules of PAD tend to coalesce into plaques in the anogenital and genitocrural regions. Plaques usually are asymptomatic but may be pruritic. Histopathologically, PAD will demonstrate hyperkeratosis, dyskeratosis, and acantholysis. Corps ronds and grains will be present in the acantholytic layer of the epidermis.1,2
The differential diagnosis for PAD includes pemphigus vegetans, Hailey-Hailey disease, Darier disease, and Grover disease. Patients usually develop pemphigus vegetans at an older age (typically 50–70 years).3 Histopathologically, it is characterized by pseudoepitheliomatous hyperplasia with an eosinophilic microabscess as well as acantholysis that involves the follicular epithelium (Figure 1),4 which were not seen in our patient. Direct immunofluorescence will show the intercellular pattern of the pemphigus group, and antidesmoglein antibodies can be detected by enzyme-linked immunosorbent assay.4,5

Hailey-Hailey disease (also known as benign familial pemphigus) typically manifests as itchy malodorous vesicles and erosions, especially in intertriginous areas. The most commonly affected sites are the groin, neck, under the breasts, and between the buttocks. In one study, two-thirds of affected patients reported a relevant family history.4 Histopathology will show minimal dyskeratosis and suprabasilar acantholysis with loss of intercellular bridges, classically described as resembling a dilapidated brick wall (Figure 2).4,5 There is no notable follicular involvement with acantholysis.4

characteristic dilapidated brick wall appearance (H&E, original
magnification ×40).
Darier disease (also known as keratosis follicularis) typically is inherited in an autosomal-dominant pattern.4 It is found on the seborrheic areas such as the scalp, forehead, nasolabial folds, and upper chest. Characteristic features include distal notching of the nails, mucosal lesions, and palmoplantar papules. Histopathology will reveal acantholysis, dyskeratosis, suprabasilar acantholysis, and corps ronds and grains.4 Acantholysis in Darier disease can be in discrete foci and/or widespread (Figure 3).4 Darier disease demonstrates more dyskeratosis than Hailey-Hailey disease.4,5

Grover disease (also referred to as transient acantholytic dermatosis) is observed predominantly in individuals who are middle-aged or older, though occurrence in children has been rarely reported.4 It affects the trunk, neck, and proximal limbs but spares the genital area. Histopathology may reveal acantholysis (similar to Hailey-Hailey disease or pemphigus vulgaris), dyskeratosis (resembling Darier disease), spongiosis, parakeratosis, and a superficial perivascular lymphocytic infiltrate with eosinophils.4 A histologic clue to the diagnosis is small lesion size (1–3 mm). Usually, only 1 or 2 small discrete lesions that span a few rete ridges are noted (Figure 4).4 Grover disease can cause follicular or acrosyringeal involvement.4

- Al-Muriesh M, Abdul-Fattah B, Wang X, et al. Papular acantholytic dyskeratosis of the anogenital and genitocrural area: case series and review of the literature. J Cutan Pathol. 2016;43:749-758. doi:10.1111/cup.12736
- Harrell J, Nielson C, Beers P, et al. Eruption on the vulva and groin. JAAD Case Reports. 2019;6:6-8. doi:10.1016/j.jdcr.2019.11.003
- Messersmith L, Krauland K. Pemphigus vegetans. StatPearls [Internet]. Updated June 26, 2023. Accessed September 18, 2024. https://www.ncbi.nlm.nih.gov/books/NBK545229
- Acantholytic disorders. In: Calonje E, Brenn T, Lazar A, et al, eds. McKee’s Pathology of the Skin: With Clinical Correlations. Elsevier/ Saunders; 2012:171-200.
- Mohr MR, Erdag G, Shada AL, et al. Two patients with Hailey- Hailey disease, multiple primary melanomas, and other cancers. Arch Dermatol. 2011;147:211215. doi:10.1001/archdermatol.2010.445
- Al-Muriesh M, Abdul-Fattah B, Wang X, et al. Papular acantholytic dyskeratosis of the anogenital and genitocrural area: case series and review of the literature. J Cutan Pathol. 2016;43:749-758. doi:10.1111/cup.12736
- Harrell J, Nielson C, Beers P, et al. Eruption on the vulva and groin. JAAD Case Reports. 2019;6:6-8. doi:10.1016/j.jdcr.2019.11.003
- Messersmith L, Krauland K. Pemphigus vegetans. StatPearls [Internet]. Updated June 26, 2023. Accessed September 18, 2024. https://www.ncbi.nlm.nih.gov/books/NBK545229
- Acantholytic disorders. In: Calonje E, Brenn T, Lazar A, et al, eds. McKee’s Pathology of the Skin: With Clinical Correlations. Elsevier/ Saunders; 2012:171-200.
- Mohr MR, Erdag G, Shada AL, et al. Two patients with Hailey- Hailey disease, multiple primary melanomas, and other cancers. Arch Dermatol. 2011;147:211215. doi:10.1001/archdermatol.2010.445
A 21-year-old woman presented with a chronic eruption in the anogenital region of 4 years’ duration. Clinical examination revealed numerous painless, mildly itchy, malodorous, whitish papules on an erythematous base that were distributed on the vulva and perianal region. There were no erosions, and no other areas were involved. Routine laboratory tests were within reference range. The patient had no sexual partner and no family history of similar lesions. A skin biopsy was performed.
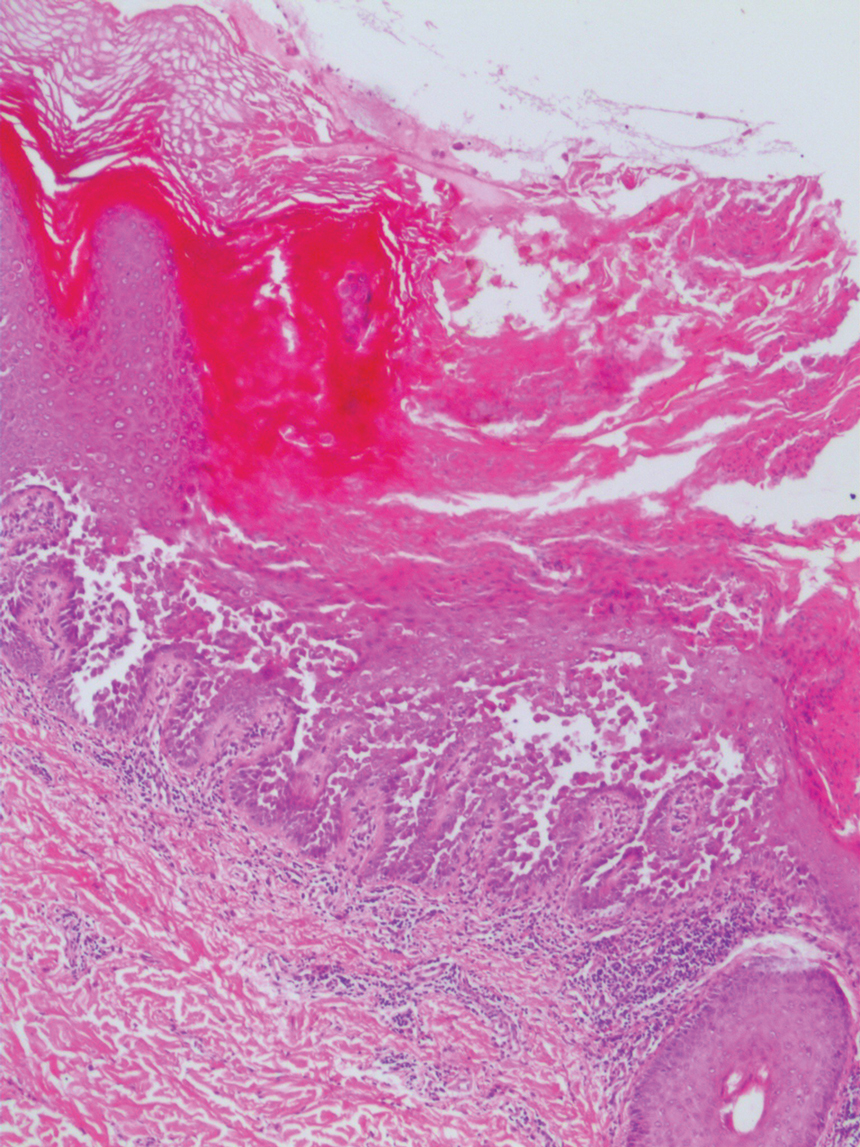
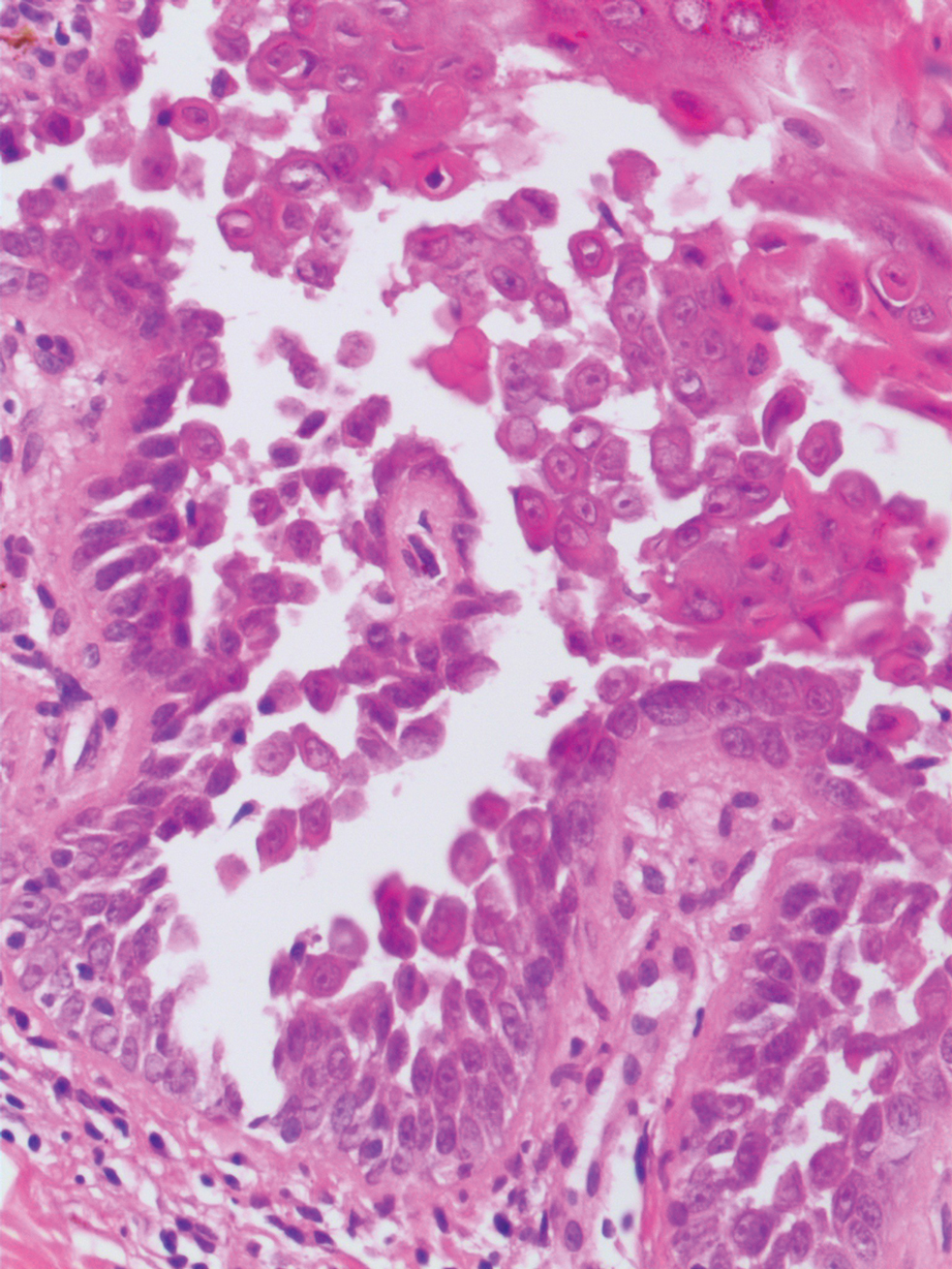
Pediatric Melanoma Outcomes by Race and Socioeconomic Factors
To the Editor:
Skin cancers are extremely common worldwide. Malignant melanomas comprise approximately 1 in 5 of these cancers. Exposure to UV radiation is postulated to be responsible for a global rise in melanoma cases over the past 50 years.1 Pediatric melanoma is a particularly rare condition that affects approximately 6 in every 1 million children.2 Melanoma incidence in children ranges by age, increasing by approximately 10-fold from age 1 to 4 years to age 15 to 19 years. Tumor ulceration is a feature more commonly seen among children younger than 10 years and is associated with worse outcomes. Tumor thickness and ulceration strongly predict sentinel lymph node metastases among children, which also is associated with a poor prognosis.3
A recent study evaluating stage IV melanoma survival rates in adolescents and young adults (AYAs) vs older adults found that survival is much worse among AYAs. Thicker tumors and public health insurance also were associated with worse survival rates for AYAs, while early detection was associated with better survival rates.4
Health disparities and their role in the prognosis of pediatric melanoma is another important factor. One study analyzed this relationship at the state level using Texas Cancer Registry data (1995-2009).5 Patients’ socioeconomic status (SES) and driving distance to the nearest pediatric cancer care center were included in the analysis. Hispanic children were found to be 3 times more likely to present with advanced disease than non-Hispanic White children. Although SES and distance to the nearest treatment center were not found to affect the melanoma stage at presentation, Hispanic ethnicity or being in the lowest SES quartile were correlated with a higher mortality risk.5
When considering specific subtypes of melanoma, acral lentiginous melanoma (ALM) is known to develop in patients with skin of color. A 2023 study by Holman et al6 reported that the percentage of melanomas that were ALMs ranged from 0.8% in non-Hispanic White individuals to 19.1% in Hispanic Black, American Indian/Alaska Native, and Asian/Pacific Islander individuals. However, ALM is rare in children. In a pooled cohort study with patient information retrieved from the nationwide Dutch Pathology Registry, only 1 child and 1 adolescent were found to have ALM across a total of 514 patients.7 We sought to analyze pediatric melanoma outcomes based on race and other barriers to appropriate care.
We conducted a search of the Surveillance, Epidemiology, and End Results (SEER) database from January 1995 to December 2016 for patients aged 21 years and younger with a primary melanoma diagnosis. The primary outcome was the 5-year survival rate. County-level SES variables were used to calculate a prosperity index. Kaplan-Meier analysis and Cox proportional hazards model were used to compare 5-year survival rates among the different racial/ethnic groups.
A sample of 2742 patients was identified during the study period and followed for 5 years. Eighty-two percent were White, 6% Hispanic, 2% Asian, 1% Black, and 5% classified as other/unknown race (data were missing for 4%). The cohort was predominantly female (61%). White patients were more likely to present with localized disease than any other race/ethnicity (83% vs 65% in Hispanic, 60% in Asian/Pacific Islander, and 45% in Black patients [P<.05]).
Black and Hispanic patients had the worst 5-year survival rates on bivariate analysis. On multivariate analysis, this finding remained significant for Hispanic patients when compared with White patients (hazard ratio, 2.37 [P<.05]). Increasing age, male sex, advanced stage at diagnosis, and failure to receive surgery were associated with increased odds of mortality.
Patients with regionalized and disseminated disease had increased odds of mortality (6.16 and 64.45, respectively; P<.05) compared with patients with localized disease. Socioeconomic status and urbanization were not found to influence 5-year survival rates.
Pediatric melanoma often presents a clinical challenge with special considerations. Pediatric-specific predisposing risk factors for melanoma and an atypical clinical presentation are some of the major concerns that necessitate a tailored approach to this malignancy, especially among different age groups, skin types, and racial and socioeconomic groups.5
Standard ABCDE criteria often are inadequate for accurate detection of pediatric melanomas. Initial lesions often manifest as raised, red, amelanotic lesions mimicking pyogenic granulomas. Lesions tend to be very small (<6 mm in diameter) and can be uniform in color, thereby making the melanoma more difficult to detect compared to the characteristic findings in adults.5 Bleeding or ulceration often can be a warning sign during physical examination.
With regard to incidence, pediatric melanoma is relatively rare. Since the 1970s, the incidence of pediatric melanoma has been increasing; however, a recent analysis of the SEER database showed a decreasing trend from 2000 to 2010.4
Our analysis of the SEER data showed an increased risk for pediatric melanoma in older adolescents. In addition, the incidence of pediatric melanoma was higher in females of all racial groups except Asian/Pacific Islander individuals. However, SES was not found to significantly influence the 5-year survival rate in pediatric melanoma.
White pediatric patients were more likely to present with localized disease compared with other races. Pediatric melanoma patients with regional disease had a 6-fold increase in mortality rate vs those with localized disease; those with disseminated disease had a 65-fold higher risk. Consistent with this, Black and Hispanic patients had the worst 5-year survival rates on bivariate analysis.
These findings suggest a relationship between race, melanoma spread, and disease severity. Patient education programs need to be directed specifically to minority groups to improve their knowledge on evolving skin lesions and sun protection practices. Physicians also need to have heightened suspicion and better knowledge of the unique traits of pediatric melanoma.5
Given the considerable influence these disparities can have on melanoma outcomes, further research is needed to characterize outcomes based on race and determine obstacles to appropriate care. Improved public outreach initiatives that accommodate specific cultural barriers (eg, language, traditional patterns of behavior) also are required to improve current circumstances.
- Arnold M, Singh D, Laversanne M, et al. Global burden of cutaneous melanoma in 2020 and projections to 2040. JAMA Dermatol. 2022;158:495-503.
- McCormack L, Hawryluk EB. Pediatric melanoma update. G Ital Dermatol Venereol. 2018;153:707-715.
- Saiyed FK, Hamilton EC, Austin MT. Pediatric melanoma: incidence, treatment, and prognosis. Pediatric Health Med Ther. 2017;8:39-45.
- Wojcik KY, Hawkins M, Anderson-Mellies A, et al. Melanoma survival by age group: population-based disparities for adolescent and young adult patients by stage, tumor thickness, and insurance type. J Am Acad Dermatol. 2023;88:831-840.
- Hamilton EC, Nguyen HT, Chang YC, et al. Health disparities influence childhood melanoma stage at diagnosis and outcome. J Pediatr. 2016;175:182-187.
- Holman DM, King JB, White A, et al. Acral lentiginous melanoma incidence by sex, race, ethnicity, and stage in the United States, 2010-2019. Prev Med. 2023;175:107692. doi:10.1016/j.ypmed.2023.107692
- El Sharouni MA, Rawson RV, Potter AJ, et al. Melanomas in children and adolescents: clinicopathologic features and survival outcomes. J Am Acad Dermatol. 2023;88:609-616. doi:10.1016/j.jaad.2022.08.067
To the Editor:
Skin cancers are extremely common worldwide. Malignant melanomas comprise approximately 1 in 5 of these cancers. Exposure to UV radiation is postulated to be responsible for a global rise in melanoma cases over the past 50 years.1 Pediatric melanoma is a particularly rare condition that affects approximately 6 in every 1 million children.2 Melanoma incidence in children ranges by age, increasing by approximately 10-fold from age 1 to 4 years to age 15 to 19 years. Tumor ulceration is a feature more commonly seen among children younger than 10 years and is associated with worse outcomes. Tumor thickness and ulceration strongly predict sentinel lymph node metastases among children, which also is associated with a poor prognosis.3
A recent study evaluating stage IV melanoma survival rates in adolescents and young adults (AYAs) vs older adults found that survival is much worse among AYAs. Thicker tumors and public health insurance also were associated with worse survival rates for AYAs, while early detection was associated with better survival rates.4
Health disparities and their role in the prognosis of pediatric melanoma is another important factor. One study analyzed this relationship at the state level using Texas Cancer Registry data (1995-2009).5 Patients’ socioeconomic status (SES) and driving distance to the nearest pediatric cancer care center were included in the analysis. Hispanic children were found to be 3 times more likely to present with advanced disease than non-Hispanic White children. Although SES and distance to the nearest treatment center were not found to affect the melanoma stage at presentation, Hispanic ethnicity or being in the lowest SES quartile were correlated with a higher mortality risk.5
When considering specific subtypes of melanoma, acral lentiginous melanoma (ALM) is known to develop in patients with skin of color. A 2023 study by Holman et al6 reported that the percentage of melanomas that were ALMs ranged from 0.8% in non-Hispanic White individuals to 19.1% in Hispanic Black, American Indian/Alaska Native, and Asian/Pacific Islander individuals. However, ALM is rare in children. In a pooled cohort study with patient information retrieved from the nationwide Dutch Pathology Registry, only 1 child and 1 adolescent were found to have ALM across a total of 514 patients.7 We sought to analyze pediatric melanoma outcomes based on race and other barriers to appropriate care.
We conducted a search of the Surveillance, Epidemiology, and End Results (SEER) database from January 1995 to December 2016 for patients aged 21 years and younger with a primary melanoma diagnosis. The primary outcome was the 5-year survival rate. County-level SES variables were used to calculate a prosperity index. Kaplan-Meier analysis and Cox proportional hazards model were used to compare 5-year survival rates among the different racial/ethnic groups.
A sample of 2742 patients was identified during the study period and followed for 5 years. Eighty-two percent were White, 6% Hispanic, 2% Asian, 1% Black, and 5% classified as other/unknown race (data were missing for 4%). The cohort was predominantly female (61%). White patients were more likely to present with localized disease than any other race/ethnicity (83% vs 65% in Hispanic, 60% in Asian/Pacific Islander, and 45% in Black patients [P<.05]).
Black and Hispanic patients had the worst 5-year survival rates on bivariate analysis. On multivariate analysis, this finding remained significant for Hispanic patients when compared with White patients (hazard ratio, 2.37 [P<.05]). Increasing age, male sex, advanced stage at diagnosis, and failure to receive surgery were associated with increased odds of mortality.
Patients with regionalized and disseminated disease had increased odds of mortality (6.16 and 64.45, respectively; P<.05) compared with patients with localized disease. Socioeconomic status and urbanization were not found to influence 5-year survival rates.
Pediatric melanoma often presents a clinical challenge with special considerations. Pediatric-specific predisposing risk factors for melanoma and an atypical clinical presentation are some of the major concerns that necessitate a tailored approach to this malignancy, especially among different age groups, skin types, and racial and socioeconomic groups.5
Standard ABCDE criteria often are inadequate for accurate detection of pediatric melanomas. Initial lesions often manifest as raised, red, amelanotic lesions mimicking pyogenic granulomas. Lesions tend to be very small (<6 mm in diameter) and can be uniform in color, thereby making the melanoma more difficult to detect compared to the characteristic findings in adults.5 Bleeding or ulceration often can be a warning sign during physical examination.
With regard to incidence, pediatric melanoma is relatively rare. Since the 1970s, the incidence of pediatric melanoma has been increasing; however, a recent analysis of the SEER database showed a decreasing trend from 2000 to 2010.4
Our analysis of the SEER data showed an increased risk for pediatric melanoma in older adolescents. In addition, the incidence of pediatric melanoma was higher in females of all racial groups except Asian/Pacific Islander individuals. However, SES was not found to significantly influence the 5-year survival rate in pediatric melanoma.
White pediatric patients were more likely to present with localized disease compared with other races. Pediatric melanoma patients with regional disease had a 6-fold increase in mortality rate vs those with localized disease; those with disseminated disease had a 65-fold higher risk. Consistent with this, Black and Hispanic patients had the worst 5-year survival rates on bivariate analysis.
These findings suggest a relationship between race, melanoma spread, and disease severity. Patient education programs need to be directed specifically to minority groups to improve their knowledge on evolving skin lesions and sun protection practices. Physicians also need to have heightened suspicion and better knowledge of the unique traits of pediatric melanoma.5
Given the considerable influence these disparities can have on melanoma outcomes, further research is needed to characterize outcomes based on race and determine obstacles to appropriate care. Improved public outreach initiatives that accommodate specific cultural barriers (eg, language, traditional patterns of behavior) also are required to improve current circumstances.
To the Editor:
Skin cancers are extremely common worldwide. Malignant melanomas comprise approximately 1 in 5 of these cancers. Exposure to UV radiation is postulated to be responsible for a global rise in melanoma cases over the past 50 years.1 Pediatric melanoma is a particularly rare condition that affects approximately 6 in every 1 million children.2 Melanoma incidence in children ranges by age, increasing by approximately 10-fold from age 1 to 4 years to age 15 to 19 years. Tumor ulceration is a feature more commonly seen among children younger than 10 years and is associated with worse outcomes. Tumor thickness and ulceration strongly predict sentinel lymph node metastases among children, which also is associated with a poor prognosis.3
A recent study evaluating stage IV melanoma survival rates in adolescents and young adults (AYAs) vs older adults found that survival is much worse among AYAs. Thicker tumors and public health insurance also were associated with worse survival rates for AYAs, while early detection was associated with better survival rates.4
Health disparities and their role in the prognosis of pediatric melanoma is another important factor. One study analyzed this relationship at the state level using Texas Cancer Registry data (1995-2009).5 Patients’ socioeconomic status (SES) and driving distance to the nearest pediatric cancer care center were included in the analysis. Hispanic children were found to be 3 times more likely to present with advanced disease than non-Hispanic White children. Although SES and distance to the nearest treatment center were not found to affect the melanoma stage at presentation, Hispanic ethnicity or being in the lowest SES quartile were correlated with a higher mortality risk.5
When considering specific subtypes of melanoma, acral lentiginous melanoma (ALM) is known to develop in patients with skin of color. A 2023 study by Holman et al6 reported that the percentage of melanomas that were ALMs ranged from 0.8% in non-Hispanic White individuals to 19.1% in Hispanic Black, American Indian/Alaska Native, and Asian/Pacific Islander individuals. However, ALM is rare in children. In a pooled cohort study with patient information retrieved from the nationwide Dutch Pathology Registry, only 1 child and 1 adolescent were found to have ALM across a total of 514 patients.7 We sought to analyze pediatric melanoma outcomes based on race and other barriers to appropriate care.
We conducted a search of the Surveillance, Epidemiology, and End Results (SEER) database from January 1995 to December 2016 for patients aged 21 years and younger with a primary melanoma diagnosis. The primary outcome was the 5-year survival rate. County-level SES variables were used to calculate a prosperity index. Kaplan-Meier analysis and Cox proportional hazards model were used to compare 5-year survival rates among the different racial/ethnic groups.
A sample of 2742 patients was identified during the study period and followed for 5 years. Eighty-two percent were White, 6% Hispanic, 2% Asian, 1% Black, and 5% classified as other/unknown race (data were missing for 4%). The cohort was predominantly female (61%). White patients were more likely to present with localized disease than any other race/ethnicity (83% vs 65% in Hispanic, 60% in Asian/Pacific Islander, and 45% in Black patients [P<.05]).
Black and Hispanic patients had the worst 5-year survival rates on bivariate analysis. On multivariate analysis, this finding remained significant for Hispanic patients when compared with White patients (hazard ratio, 2.37 [P<.05]). Increasing age, male sex, advanced stage at diagnosis, and failure to receive surgery were associated with increased odds of mortality.
Patients with regionalized and disseminated disease had increased odds of mortality (6.16 and 64.45, respectively; P<.05) compared with patients with localized disease. Socioeconomic status and urbanization were not found to influence 5-year survival rates.
Pediatric melanoma often presents a clinical challenge with special considerations. Pediatric-specific predisposing risk factors for melanoma and an atypical clinical presentation are some of the major concerns that necessitate a tailored approach to this malignancy, especially among different age groups, skin types, and racial and socioeconomic groups.5
Standard ABCDE criteria often are inadequate for accurate detection of pediatric melanomas. Initial lesions often manifest as raised, red, amelanotic lesions mimicking pyogenic granulomas. Lesions tend to be very small (<6 mm in diameter) and can be uniform in color, thereby making the melanoma more difficult to detect compared to the characteristic findings in adults.5 Bleeding or ulceration often can be a warning sign during physical examination.
With regard to incidence, pediatric melanoma is relatively rare. Since the 1970s, the incidence of pediatric melanoma has been increasing; however, a recent analysis of the SEER database showed a decreasing trend from 2000 to 2010.4
Our analysis of the SEER data showed an increased risk for pediatric melanoma in older adolescents. In addition, the incidence of pediatric melanoma was higher in females of all racial groups except Asian/Pacific Islander individuals. However, SES was not found to significantly influence the 5-year survival rate in pediatric melanoma.
White pediatric patients were more likely to present with localized disease compared with other races. Pediatric melanoma patients with regional disease had a 6-fold increase in mortality rate vs those with localized disease; those with disseminated disease had a 65-fold higher risk. Consistent with this, Black and Hispanic patients had the worst 5-year survival rates on bivariate analysis.
These findings suggest a relationship between race, melanoma spread, and disease severity. Patient education programs need to be directed specifically to minority groups to improve their knowledge on evolving skin lesions and sun protection practices. Physicians also need to have heightened suspicion and better knowledge of the unique traits of pediatric melanoma.5
Given the considerable influence these disparities can have on melanoma outcomes, further research is needed to characterize outcomes based on race and determine obstacles to appropriate care. Improved public outreach initiatives that accommodate specific cultural barriers (eg, language, traditional patterns of behavior) also are required to improve current circumstances.
- Arnold M, Singh D, Laversanne M, et al. Global burden of cutaneous melanoma in 2020 and projections to 2040. JAMA Dermatol. 2022;158:495-503.
- McCormack L, Hawryluk EB. Pediatric melanoma update. G Ital Dermatol Venereol. 2018;153:707-715.
- Saiyed FK, Hamilton EC, Austin MT. Pediatric melanoma: incidence, treatment, and prognosis. Pediatric Health Med Ther. 2017;8:39-45.
- Wojcik KY, Hawkins M, Anderson-Mellies A, et al. Melanoma survival by age group: population-based disparities for adolescent and young adult patients by stage, tumor thickness, and insurance type. J Am Acad Dermatol. 2023;88:831-840.
- Hamilton EC, Nguyen HT, Chang YC, et al. Health disparities influence childhood melanoma stage at diagnosis and outcome. J Pediatr. 2016;175:182-187.
- Holman DM, King JB, White A, et al. Acral lentiginous melanoma incidence by sex, race, ethnicity, and stage in the United States, 2010-2019. Prev Med. 2023;175:107692. doi:10.1016/j.ypmed.2023.107692
- El Sharouni MA, Rawson RV, Potter AJ, et al. Melanomas in children and adolescents: clinicopathologic features and survival outcomes. J Am Acad Dermatol. 2023;88:609-616. doi:10.1016/j.jaad.2022.08.067
- Arnold M, Singh D, Laversanne M, et al. Global burden of cutaneous melanoma in 2020 and projections to 2040. JAMA Dermatol. 2022;158:495-503.
- McCormack L, Hawryluk EB. Pediatric melanoma update. G Ital Dermatol Venereol. 2018;153:707-715.
- Saiyed FK, Hamilton EC, Austin MT. Pediatric melanoma: incidence, treatment, and prognosis. Pediatric Health Med Ther. 2017;8:39-45.
- Wojcik KY, Hawkins M, Anderson-Mellies A, et al. Melanoma survival by age group: population-based disparities for adolescent and young adult patients by stage, tumor thickness, and insurance type. J Am Acad Dermatol. 2023;88:831-840.
- Hamilton EC, Nguyen HT, Chang YC, et al. Health disparities influence childhood melanoma stage at diagnosis and outcome. J Pediatr. 2016;175:182-187.
- Holman DM, King JB, White A, et al. Acral lentiginous melanoma incidence by sex, race, ethnicity, and stage in the United States, 2010-2019. Prev Med. 2023;175:107692. doi:10.1016/j.ypmed.2023.107692
- El Sharouni MA, Rawson RV, Potter AJ, et al. Melanomas in children and adolescents: clinicopathologic features and survival outcomes. J Am Acad Dermatol. 2023;88:609-616. doi:10.1016/j.jaad.2022.08.067
Practice Points
- Pediatric melanoma is a unique clinical entity with a different clinical presentation than in adults.
- Thicker tumors and disseminated disease are associated with a worse prognosis, and these factors are more commonly seen in Black and Hispanic patients.
Eyelid Dermatitis: Common Patterns and Contact Allergens
Eyelid dermatitis is a common dermatologic concern representing a broad group of inflammatory dermatoses and typically presenting as eczematous lesions on the eyelids.1 One of the most common causes of eyelid dermatitis is thought to be allergic contact dermatitis (ACD), a type IV delayed hypersensitivity reaction caused by exposure to external allergens.2 Although ACD can occur anywhere on the body, dermatitis on the face and eyelids is quite common.1,2 This article aims to explore the clinical manifestation, evaluation, and management of eyelid ACD.
Pathophysiology of Eyelid ACD
Studies have shown that ACD is the most common cause of eyelid dermatitis, estimated to account for 46% to 72% of cases worldwide.3-6 Allergic contact dermatitis is a T cell–mediated type IV hypersensitivity reaction to external antigens that manifests as eczematous lesions at the site of contact with the allergen that may spread.7 Allergic contact dermatitis is a common condition, and it is estimated that at least 20% of the general worldwide population has a contact allergy.8,9 Histologically, ACD manifests as spongiotic dermatitis, though this is not unique and also may be seen in atopic dermatitis (AD) and irritant contact dermatitis.2 Allergic contact dermatitis is diagnosed via epicutaneous patch testing, and treatment involves allergen avoidance with or without adjuvant topical and/or systemic immunomodulatory treatments.7
The eyelids are uniquely prone to the development of ACD given their thinner epidermis and increased susceptibility to irritation. They frequently are exposed to allergens through the direct topical route as well as indirectly via airborne exposure, rinse-down products (eg, shampoos), and substances transferred from an individual’s own hands. The occluded skin folds of the eyelids facilitate increased exposure to trapped allergens.10,11 Additionally, the skin of the eyelids is thin, flexible, highly vascularized, and lacking in subcutaneous tissue, making this area more susceptible to antigen penetration than other locations on the body.1,2,10,12,13
Clinical Manifestations
Eyelid ACD is more common in females than males, which is thought to be related to increased use of cosmetics and fragrances.1,3,12,14-16 Clinical manifestations may resemble eczematous papules and plaques.1 Eyelid ACD commonly spreads beyond the eyelid margin, which helps to differentiate it from AD and irritant contact dermatitis. Symptoms of ACD on the eyelids typically include pruritus, redness, swelling, tearing, scaling, and pain.2 Persistent untreated eyelid dermatitis can lead to eyelash loss, damage to meibomian glands, and hyperpigmentation.2,17,18
Patterns of Eyelid ACD
Allergic contact dermatitis on the eyelids can occur due to direct application of allergens onto the skin of the eyelids, runoff of products from the hair/scalp (eg, shampoo), transfer of allergens from the hands, or contact with airborne allergens.1,2,11,12 Some reports have suggested that eyelid ACD more often is caused by products applied to the scalp or face rather than those applied directly to the eyelids.11 Because the scalp and face are less reactive to contact allergens, in some cases the eyelids may be the only affected site.10,12,13
The specific pattern of dermatitis on or around the eyelids can provide clues to the allergenic source. Dermatitis present around the eyelids and periorbital region with involvement of the bilateral upper and lower eyelids suggests direct exposure to a contact allergen, such as makeup or other cosmetic products.1 Unilateral involvement of only 1 eyelid can occur with ectopic transfer of allergens from the hands or nails.1,19 Involvement of the fingers or nails in addition to the eyelids may further suggest ectopic transfer, such as from allergens in nail polish.10 Unilateral eyelid dermatitis also could be caused by unique exposures such as a microscope or camera eyepiece.19 Distribution around the lower eyelids and upper cheeks is indicative of a drip or runoff pattern, which may result from an ophthalmic solution such as eye drops or contact lens solution.1,19 Finally, dermatitis affecting the upper eyelids along with the nasolabial folds and upper chest may suggest airborne contact dermatitis to fragrances or household cleaning products.1,11
Common Culprits of Eyelid ACD
Common causes of eyelid ACD include cosmetic products, ophthalmic medications, nail lacquers, and jewelry.10,13,20 Within the broader category of cosmetics, allergens may be found in makeup and makeup removers, cosmetic applicators and brushes, soaps and cleansers, creams and sunscreens, antiaging products, hair products, nail polish and files, and hair removal products, among many others.10,13,16,20 Additionally, ophthalmologic and topical medications are common sources of ACD, including eyedrops, contact lens solution, and topical antibiotics.10,13,21 Costume jewelry commonly contains allergenic metals, which also can be found in eyelash curlers, eyeglasses, toys, and other household items.22,23 Finally, contact allergens can be found in items such as goggles, gloves, textiles, and a variety of other occupational and household exposures.
Allergic contact dermatitis of the eyelids occurs predominantly—but not exclusively—in females.16,20,24 This finding has been attributed to the traditionally greater use of cosmetics and fragrances among women; however, the use of skin care products among men is increasing, and recent studies have shown the eyelids to be a common location of facial contact dermatitis among men.16,24 Although eyelid dermatitis has not been specifically analyzed by sex, a retrospective analysis of 1332 male patients with facial dermatitis found the most common sites to be the face (not otherwise specified)(48.9%), eyelids (23.5%), and lips (12.6%). In this cohort, the most common allergens were surfactants in shampoos and paraphenylenediamine in hair dyes.24
Common Allergens
Common contact allergens among patients with ACD of the eyelids include metals, fragrances, preservatives, acrylates, and topical medications.3,10,16,20,25-27 Sources of common contact allergens are reviewed in Table 1.
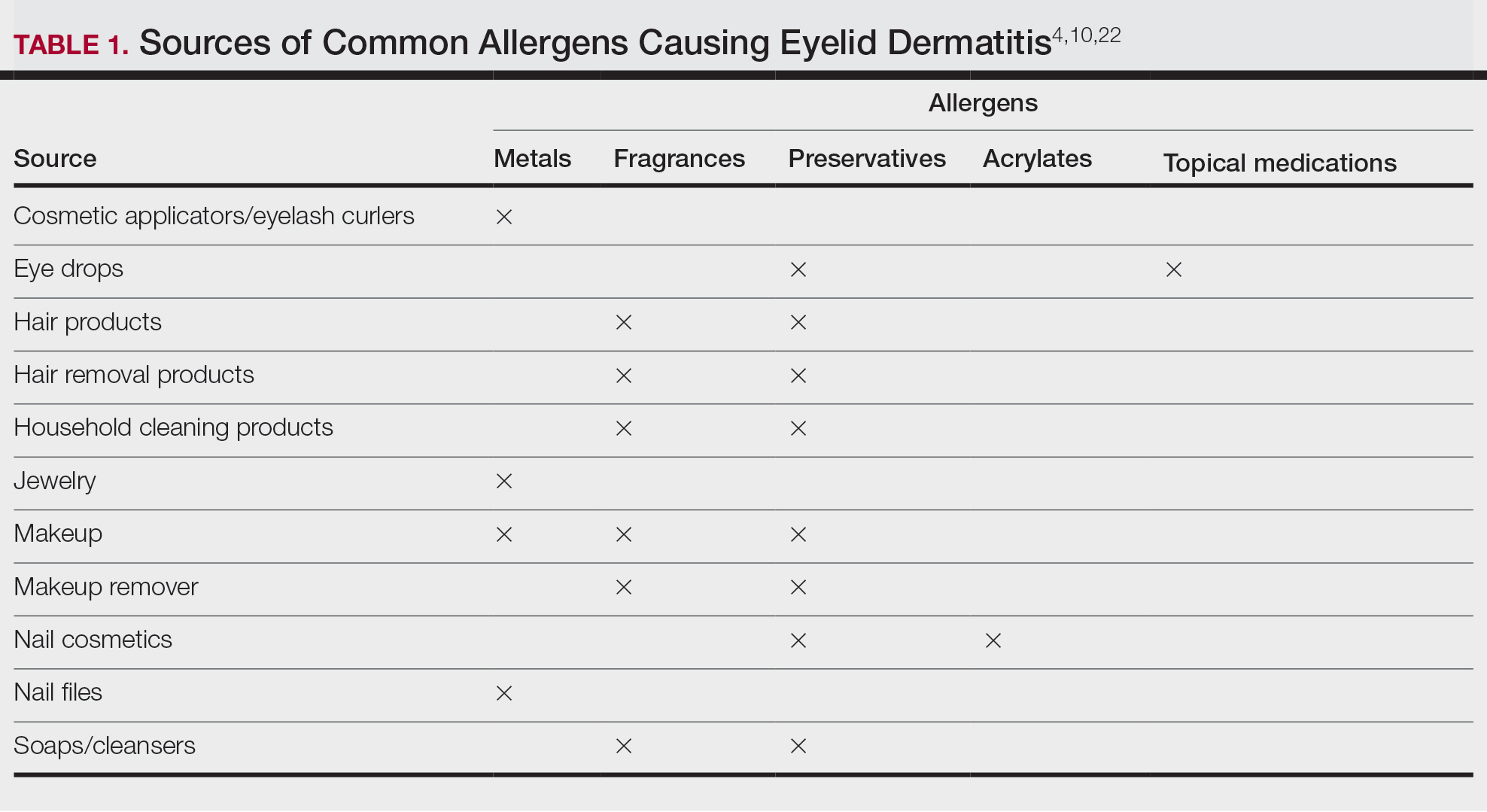
Metals—Metals are among the most common causes of ACD overall, and nickel frequently is reported as one of the top contact allergens in patients with eyelid dermatitis.16,27 A retrospective analysis of 2332 patients with eyelid dermatitis patch tested by the North American Contact Dermatitis Group from 1994 to 2016 found that 18.6% of patients with eyelid ACD had a clinically relevant nickel allergy. Sources of nickel exposure include jewelry, grooming devices, makeup and makeup applicators, and eyelash curlers, as well as direct transfer from the hands after contact with consumer products.16
Other metals that can cause ACD include cobalt (found in similar products to nickel) and gold. Gold often is associated with eyelid dermatitis, though its clinical relevance has been debated, as gold is a relatively inert metal that rarely is present in eye cosmetics and its ions are not displaced from objects and deposited on the skin via sweat in the same way as nickel.4,16,20,28-30 Despite this, studies have shown that gold is a common positive patch test reaction among patients with eyelid dermatitis, even in patients with no dermatitis at the site of contact with gold jewelry.20,29,31 Gold has been reported to be the most common allergen causing unilateral eyelid dermatitis via ectopic transfer.16,19,20,29 It has been proposed that titanium dioxide, present in many cosmetics and sunscreens, displaces gold allowing its release from jewelry, thereby liberating the fine gold ions and allowing them to desposit on the face and eyelids.30,31 Given the uncertain clinical relevance of positive patch test reactions to gold, Warshaw at al16 recommend a 2- to 3-month trial of gold jewelry avoidance to establish relevance, and Ehrlich and Gold29 noted that avoidance of gold leads to improvement.
Fragrances—Fragrances represent a broad category of naturally occurring and man-made components that often are combined to produce a desired scent in personal care products.32 Essential oils and botanicals are both examples of natural fragrances.33 Fragrances are found in numerous products including makeup, hair products, and household cleaning supplies and represent some of the most common contact allergens.32 Common fragrance allergens include fragrance mixes I and II, hydroperoxides of linalool, and balsam of Peru.12,32,34 Allergic contact dermatitis to fragrances typically manifests on the eyelids, face, or hands.33 Several studies have found fragrances to be among the top contact allergens in patients with eyelid dermatitis.3,12,20,25,34 Patch testing for fragrance allergy may include baseline series, supplemental fragrance series, and personal care products.32,35
Preservatives—Preservatives, including formaldehyde and formaldehyde releasers (eg, quaternium-15 and bronopol) and methylchloroisothiazolinone/methylisothiazolinone, may be found in personal care products such as makeup, makeup removers, emollients, shampoos, hair care products, and ophthalmologic solutions and are among the most common cosmetic sources of ACD.13,36-39 Preservatives are among the top allergens causing eyelid dermatitis.20 In particular, patch test positivity rates to methylchloroisothiazolinone/methylisothiazolinone have been increasing in North America.40 Sensitization to preservatives may occur through direct skin contact or transfer from the hands.41
Acrylates—Acrylates are compounds derived from acrylic acid that may be found in acrylic and gel nails, eyelash extensions, and other adhesives and are frequent causes of eyelid ACD.4,10,42 Acrylate exposure may be cosmetic among consumers or occupational (eg, aestheticians).42,43 Acrylates on the nails may cause eyelid dermatitis via ectopic transfer from the hands and also may cause periungual dermatitis manifesting as nail bed erythema.10 Hydroxyethyl methacrylate is one of the more common eyelid ACD allergens, and studies have shown increasing prevalence of positive reaction rates to hydroxyethylmethacrylate.10,44Topical Medications—Contact allergies to topical medications are quite common, estimated to occur in 10% to 17% of patients undergoing patch testing.45 Both active and inactive ingredients of topical medications may be culprits in eyelid ACD. The most common topical medication allergens include antibiotics, steroids, local anesthetics, and nonsteroidal anti-inflammatory drugs.45 Topical antibiotics such as neomycin and bacitracin represent some of the most common causes of eyelid dermatitis4,10 and may be found in a variety of products, including antibacterial ointments and eye drops.1 Many ophthalmologic medications also contain corticosteroids, with the most common allergenic steroids being tixocortol pivalate (a marker for hydrocortisone allergy) and budesonide.10,20 Topical steroids pose a particular dilemma, as they can be either the source of or a treatment for ACD.10 Eye drops also may contain anesthetics, β-blockers, and antihistamines, as well as the preservative benzalkonium chloride, all of which may be contact allergens.21,39
Differential Diagnosis of Eyelid Dermatitis
Although ACD is reported to be the most common cause of eyelid dermatitis, the differential diagnosis is broad, including endogenous inflammatory dermatoses and exogenous exposures (Table 2). Symptoms of eyelid ACD can be nonspecific (eg, erythema, pruritus), making diagnosis challenging.46
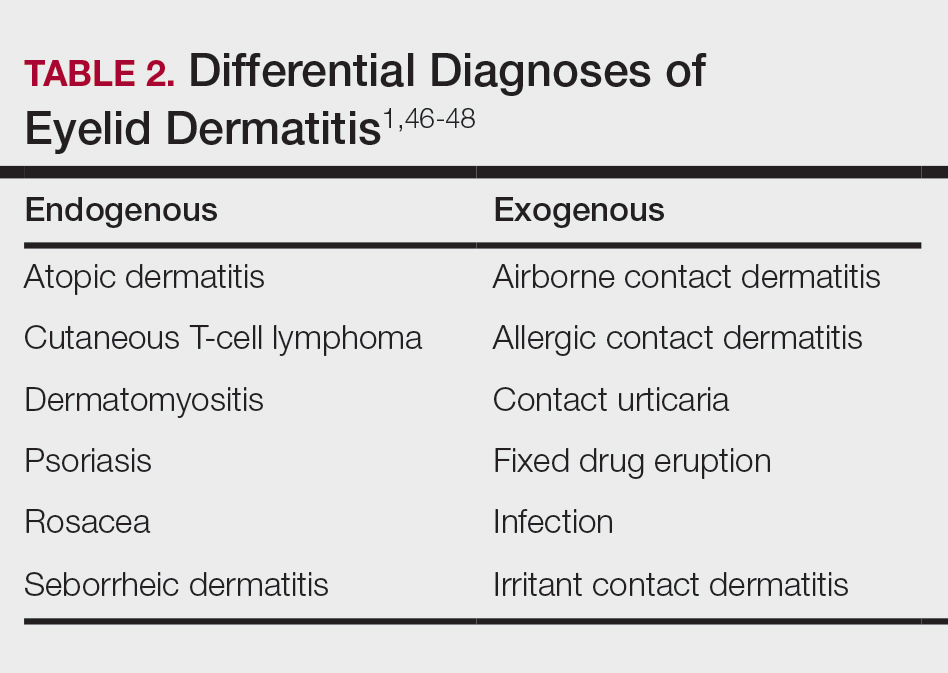
Atopic dermatitis represents another common cause of eyelid dermatitis, accounting for 14% to 39.5% of cases.3-5,49Atopic dermatitis of the eyelids classically manifests with lichenification of the medial aspects of the eyelids.50 Atopic dermatitis and ACD may be difficult to distinguish, as the 2 conditions appear clinically similar and can develop concomitantly.51 Additionally, atopic patients are likely to have comorbid allergic rhinitis and sensitivity to environmental allergens, which may lead to chronic eye scratching and lichenification.1,51 Clinical features of eyelid dermatitis suggesting allergic rhinitis and likely comorbid AD include creases in the lower eyelids (Dennie-Morgan lines) and periorbital hyperpigmentation (known as the allergic shiner) due to venous congestion.1,52
Seborrheic dermatitis is an inflammatory reaction to Malassezia yeast that occurs in sebaceous areas such as the groin, scalp, eyebrows, eyelids, and nasolabial folds.1,53,54
Irritant contact dermatitis, a nonspecific inflammatory reaction caused by direct cell damage from external irritants, also may affect the eyelids and appear similar to ACD.1 It typically manifests with a burning or stinging sensation, as opposed to pruritus, and generally develops and resolves more rapidly than ACD.1 Personal care products are common causes of eyelid irritant contact dermatitis.16
Patch Testing for Eyelid ACD
The gold standard for diagnosis of ACD is patch testing, outlined by the International Contact Dermatitis Research Group.55-57 Patch testing generally is performed with standardized panels of allergens and can be customized either with supplemental panels based on unique exposures or with the patient’s own personal care products to increase the sensitivity of testing. Therefore, a thorough history is crucial to identifying potential allergens in a patient’s environment.
False negatives are possible, as the skin on the back may be thicker and less sensitive than the skin at the location of dermatitis.2,58 This is particularly relevant when using patch testing to diagnose ACD of the eyelids, where the skin is particularly thin and sensitive.2 Additionally, ingredients of ophthalmic medications are known to have an especially high false-negative rate with standard patch testing and may require repeated testing with higher drug concentrations or modified patch testing procedures (eg, open testing, scratch-patch testing).1,59
Treatment
Management of ACD involves allergen avoidance, typically dictated by patch test results.10 Allergen avoidance may be facilitated using online resources such as the Contact Allergen Management Program (https://www.acdscamp.org/) created by the American Contact Dermatitis Society.10,18 Patient counseling following patch testing is crucial to educating patients about sources of potential allergen exposures and strategies for avoidance. In the case of eyelid dermatitis, it is particularly important to consider exposure to airborne allergens such as fragrances.16 Fragrance avoidance is uniquely difficult, as labelling standards in the United States currently do not require disclosure of specific fragrance components.33 Additionally, products labelled as unscented may still contain fragrances. As such, some patients with fragrance allergy may need to carefully avoid all products containing fragrances.33
In addition to allergen avoidance, eyelid ACD may be treated with topical medications (eg, steroids, calcineurin inhibitors, Janus kinase inhibitors); however, these same topical medications also can cause ACD due to some ingredients such as propylene glycol.10 Topical steroids should be used with caution on the eyelids given the risk for atrophy, cataracts, and glaucoma.1
Final Interpretation
Eyelid dermatitis is a common dermatologic condition most frequently caused by ACD due to exposure to allergens in cosmetic products, ophthalmic medications, nail lacquers, and jewelry, among many other potential sources. The most common allergens causing eyelid dermatitis include metals (particularly nickel), fragrances, preservatives, acrylates, and topical medications. Eyelid ACD is diagnosed via patch testing, and the mainstay of treatment is strict allergen avoidance. Patient counseling is vital for successful allergen avoidance and resolution of eyelid ACD.
- Hine AM, Waldman RA, Grzybowski A, et al. Allergic disorders of the eyelid. Clin Dermatol. 2023;41:476-480. doi:10.1016/j.clindermatol.2023.08.002
- Turkiewicz M, Shah A, Yang YW, et al. Allergic contact dermatitis of the eyelids: an interdisciplinary review. Ocul Surf. 2023;28:124-130. doi:10.1016/j.jtos.2023.03.001
- Valsecchi R, Imberti G, Martino D, et al. Eyelid dermatitis: an evaluation of 150 patients. Contact Dermatitis. 1992;27:143-147. doi:10.1111/j.1600-0536.1992.tb05242.x
- Guin JD. Eyelid dermatitis: experience in 203 cases. J Am Acad Dermatol. 2002;47:755-765. doi:10.1067/mjd.2002.122736
- Nethercott JR, Nield G, Holness DL. A review of 79 cases of eyelid dermatitis. J Am Acad Dermatol. 1989;21(2 pt 1):223-230. doi:10.1016/s0190-9622(89)70165-1
- Shah M, Lewis FM, Gawkrodger DJ. Facial dermatitis and eyelid dermatitis: a comparison of patch test results and final diagnoses. Contact Dermatitis. 1996;34:140-141. doi:10.1111/j.1600-0536.1996.tb02148.x
- Brites GS, Ferreira I, Sebastião AI, et al. Allergic contact dermatitis: from pathophysiology to development of new preventive strategies. Pharmacol Res. 2020;162:105282. doi:10.1016/j.phrs.2020.105282
- Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta-analysis. Contact Dermatitis. 2019;80:77-85. doi:10.1111/cod.13119
- Adler BL, DeLeo VA. Allergic contact dermatitis. JAMA Dermatol. 2021;157:364. doi:10.1001/jamadermatol.2020.5639
- Huang CX, Yiannias JA, Killian JM, et al. Seven common allergen groups causing eyelid dermatitis: education and avoidance strategies. Clin Ophthalmol Auckl NZ. 2021;15:1477-1490. doi:10.2147/OPTH.S297754
- Rozas-Muñoz E, Gamé D, Serra-Baldrich E. Allergic contact dermatitis by anatomical regions: diagnostic clues. Actas Dermo-Sifiliográficas Engl Ed. 2018;109:485-507. doi:10.1016/j.adengl.2018.05.016
- Amin KA, Belsito DV. The aetiology of eyelid dermatitis: a 10-year retrospective analysis. Contact Dermatitis. 2006;55:280-285. doi:10.1111/j.1600-0536.2006.00927.x
- Wolf R, Orion E, Tüzün Y. Periorbital (eyelid) dermatides. Clin Dermatol. 2014;32:131-140. doi:10.1016/j.clindermatol.2013.05.035
- Ockenfels HM, Seemann U, Goos M. Contact allergy in patients with periorbital eczema: an analysis of allergens. data recorded by the Information Network of the Departments of Dermatology. Dermatol Basel Switz. 1997;195:119-124. doi:10.1159/000245712
- Landeck L, John SM, Geier J. Periorbital dermatitis in 4779 patients—patch test results during a 10-year period. Contact Dermatitis. 2014;70:205-212. doi:10.1111/cod.12157
- Warshaw EM, Voller LM, Maibach HI, et al. Eyelid dermatitis in patients referred for patch testing: retrospective analysis of North American Contact Dermatitis Group data, 1994-2016. J Am Acad Dermatol. 2021;84:953-964. doi:10.1016/j.jaad.2020.07.020
- McMonnies CW. Management of chronic habits of abnormal eye rubbing. Contact Lens Anterior Eye. 2008;31:95-102. doi:10.1016/j.clae.2007.07.008
- Chisholm SAM, Couch SM, Custer PL. Etiology and management of allergic eyelid dermatitis. Ophthal Plast Reconstr Surg. 2017;33:248-250. doi:10.1097/IOP.0000000000000723
- Lewallen R, Feldman S, eds. Regional atlas of contact dermatitis. The Dermatologist. Accessed April 22, 2024. https://s3.amazonaws.com/HMP/hmp_ln/imported/Regional%20Atlas%20of%20Contact%20Dermatitis%20Book_lr.pdf
- Rietschel RL, Warshaw EM, Sasseville D, et al. Common contact allergens associated with eyelid dermatitis: data from the North American Contact Dermatitis Group 2003-2004 study period. Dermat Contact Atopic Occup Drug. 2007;18:78-81. doi:10.2310/6620.2007.06041
- Mughal AA, Kalavala M. Contact dermatitis to ophthalmic solutions. Clin Exp Dermatol. 2012;37:593-597; quiz 597-598. doi:10.1111/j.1365-2230.2012.04398.x
- Goossens A. Contact allergic reactions on the eyes and eyelids. Bull Soc Belge Ophtalmol. 2004;292:11-17.
- Silverberg NB, Pelletier JL, Jacob SE, et al. Nickel allergic contact dermatitis: identification, treatment, and prevention. Pediatrics. 2020;145:E20200628. doi:10.1542/peds.2020-0628
- Warshaw EM, Schlarbaum JP, Maibach HI, et al. Facial dermatitis in male patients referred for patch testing. JAMA Dermatol. 2020;156:79-84. doi:10.1001/jamadermatol.2019.3531
- Wenk KS, Ehrlich A. Fragrance series testing in eyelid dermatitis. Dermatitis. 2012;23:22-26. doi:10.1097/DER.0b013e31823d180f
- Crouse L, Ziemer C, Ziemer C, et al. Trends in eyelid dermatitis. Dermat Contact Atopic Occup Drug. 2018;29:96-97. doi:10.1097/DER.0000000000000338
- Yazdanparast T, Nassiri Kashani M, Shamsipour M, et al. Contact allergens responsible for eyelid dermatitis in adults. J Dermatol. 2024;51:691-695. doi:10.1111/1346-8138.17140
- Fowler J, Taylor J, Storrs F, et al. Gold allergy in North America. Am J Contact Dermat. 2001;12:3-5.
- Ehrlich A, Belsito DV. Allergic contact dermatitis to gold. Cutis. 2000;65:323-326.
- Danesh M, Murase JE. Titanium dioxide induces eyelid dermatitis in patients allergic to gold. J Am Acad Dermatol. 2015;73:E21. doi:10.1016/j.jaad.2015.03.046
- Katta R. Common misconceptions in contact dermatitis counseling. Dermatol Online J. 2008;14:2.
- De Groot AC. Fragrances: contact allergy and other adverse effects. Dermatitis. 2020;31:13-35. doi:10.1097/DER.0000000000000463
- Reeder MJ. Allergic contact dermatitis to fragrances. Dermatol Clin. 2020;38:371-377. doi:10.1016/j.det.2020.02.009
- Warshaw EM, Zhang AJ, DeKoven JG, et al. Epidemiology of nickel sensitivity: retrospective cross-sectional analysis of North American Contact Dermatitis Group data 1994-2014. J Am Acad Dermatol. 2019;80:701-713. doi:10.1016/j.jaad.2018.09.058
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society core allergen series: 2020 update. Dermatitis. 2020;31:279-282. doi:10.1097/DER.0000000000000621
- Yim E, Baquerizo Nole KL, Tosti A. Contact dermatitis caused by preservatives. Dermatitis. 2014;25:215-231. doi:10.1097/DER.0000000000000061
- Alani JI, Davis MDP, Yiannias JA. Allergy to cosmetics. Dermatitis. 2013;24:283-290. doi:10.1097/DER.0b013e3182a5d8bc
- Hamilton T, de Gannes GC. Allergic contact dermatitis to preservatives and fragrances in cosmetics. Skin Ther Lett. 2011;16:1-4.
- Ashton SJ, Mughal AA. Contact dermatitis to ophthalmic solutions: an update. Dermat Contact Atopic Occup Drug. 2023;34:480-483. doi:10.1089/derm.2023.0033
- Reeder MJ, Warshaw E, Aravamuthan S, et al. Trends in the prevalence of methylchloroisothiazolinone/methylisothiazolinone contact allergy in North America and Europe. JAMA Dermatol. 2023;159:267-274. doi:10.1001/jamadermatol.2022.5991
- Herro EM, Elsaie ML, Nijhawan RI, et al. Recommendations for a screening series for allergic contact eyelid dermatitis. Dermatitis. 2012;23:17-21. doi:10.1097/DER.0b013e31823d191f
- Kucharczyk M, Słowik-Rylska M, Cyran-Stemplewska S, et al. Acrylates as a significant cause of allergic contact dermatitis: new sources of exposure. Adv Dermatol Allergol Dermatol Alergol. 2021;38:555-560. doi:10.5114/ada.2020.95848
- Rodriguez I, George SE, Yu J, et al. Tackling acrylate allergy: the sticky truth. Cutis. 2023;112:282-286. doi:10.12788/cutis.0909
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group Patch Test Results: 2019–2020. Dermatitis. 2023;34:90-104. doi:10.1089/derm.2022.29017.jdk
- de Groot A. Allergic contact dermatitis from topical drugs: an overview. Dermatitis. 2021;32:197-213. doi:10.1097/DER.0000000000000737
- Zug KA, Palay DA, Rock B. Dermatologic diagnosis and treatment of itchy red eyelids. Surv Ophthalmol. 1996;40:293-306. doi:10.1016/s0039-6257(96)82004-2
- Beltrani VS. Eyelid dermatitis. Curr Allergy Asthma Rep. 2001;1:380-388. doi:10.1007/s11882-001-0052-0
- Hirji SH, Maeng MM, Tran AQ, et al. Cutaneous T-cell lymphoma of the eyelid masquerading as dermatitis. Orbit Amst Neth. 2021;40:75-78. doi:10.1080/01676830.2020.1739080
- Svensson A, Möller H. Eyelid dermatitis: the role of atopy and contact allergy. Contact Dermatitis. 1986;15:178-182. doi:10.1111/j.1600-0536.1986.tb01321.x
- Papier A, Tuttle DJ, Mahar TJ. Differential diagnosis of the swollen red eyelid. Am Fam Physician. 2007;76:1815-1824.
- Johnson H, Novack DE, Adler BL, et al. Can atopic dermatitis and allergic contact dermatitis coexist? Cutis. 2022;110:139-142. doi:10.12788cutis.0599
- Berger WE. Allergic rhinitis in children: diagnosis and management strategies. Paediatr Drugs. 2004;6:233-250. doi:10.2165/00148581-200406040-00003
- Singh A, Kansal NK, Kumawat D, et al. Ophthalmic manifestations of seborrheic dermatitis. Skinmed. 2023;21:397-401.
- Clark GW, Pope SM, Jaboori KA. Diagnosis and treatment of seborrheic dermatitis. Am Fam Physician. 2015;91:185-190.
- Lachapelle JM, Maibach HI. Patch Testing and Prick Testing. Springer; 2012.
- Fregert S. Manual of Contact Dermatitis: On Behalf of the International Contact Dermatitis Research Group. Munksgaard; 1974.
- Reeder M, Reck Atwater A. Patch testing 101, part 1: performing the test. Cutis. 2020;106:165-167. doi:10.12788/cutis.0093
- Wolf R, Perluk H. Failure of routine patch test results to detect eyelid dermatitis. Cutis. 1992;49:133-134.
- Grey KR, Warshaw EM. Allergic contact dermatitis to ophthalmic medications: relevant allergens and alternative testing methods. Dermat Contact Atopic Occup Drug. 2016;27:333-347. doi:10.1097/DER.0000000000000224
Eyelid dermatitis is a common dermatologic concern representing a broad group of inflammatory dermatoses and typically presenting as eczematous lesions on the eyelids.1 One of the most common causes of eyelid dermatitis is thought to be allergic contact dermatitis (ACD), a type IV delayed hypersensitivity reaction caused by exposure to external allergens.2 Although ACD can occur anywhere on the body, dermatitis on the face and eyelids is quite common.1,2 This article aims to explore the clinical manifestation, evaluation, and management of eyelid ACD.
Pathophysiology of Eyelid ACD
Studies have shown that ACD is the most common cause of eyelid dermatitis, estimated to account for 46% to 72% of cases worldwide.3-6 Allergic contact dermatitis is a T cell–mediated type IV hypersensitivity reaction to external antigens that manifests as eczematous lesions at the site of contact with the allergen that may spread.7 Allergic contact dermatitis is a common condition, and it is estimated that at least 20% of the general worldwide population has a contact allergy.8,9 Histologically, ACD manifests as spongiotic dermatitis, though this is not unique and also may be seen in atopic dermatitis (AD) and irritant contact dermatitis.2 Allergic contact dermatitis is diagnosed via epicutaneous patch testing, and treatment involves allergen avoidance with or without adjuvant topical and/or systemic immunomodulatory treatments.7
The eyelids are uniquely prone to the development of ACD given their thinner epidermis and increased susceptibility to irritation. They frequently are exposed to allergens through the direct topical route as well as indirectly via airborne exposure, rinse-down products (eg, shampoos), and substances transferred from an individual’s own hands. The occluded skin folds of the eyelids facilitate increased exposure to trapped allergens.10,11 Additionally, the skin of the eyelids is thin, flexible, highly vascularized, and lacking in subcutaneous tissue, making this area more susceptible to antigen penetration than other locations on the body.1,2,10,12,13
Clinical Manifestations
Eyelid ACD is more common in females than males, which is thought to be related to increased use of cosmetics and fragrances.1,3,12,14-16 Clinical manifestations may resemble eczematous papules and plaques.1 Eyelid ACD commonly spreads beyond the eyelid margin, which helps to differentiate it from AD and irritant contact dermatitis. Symptoms of ACD on the eyelids typically include pruritus, redness, swelling, tearing, scaling, and pain.2 Persistent untreated eyelid dermatitis can lead to eyelash loss, damage to meibomian glands, and hyperpigmentation.2,17,18
Patterns of Eyelid ACD
Allergic contact dermatitis on the eyelids can occur due to direct application of allergens onto the skin of the eyelids, runoff of products from the hair/scalp (eg, shampoo), transfer of allergens from the hands, or contact with airborne allergens.1,2,11,12 Some reports have suggested that eyelid ACD more often is caused by products applied to the scalp or face rather than those applied directly to the eyelids.11 Because the scalp and face are less reactive to contact allergens, in some cases the eyelids may be the only affected site.10,12,13
The specific pattern of dermatitis on or around the eyelids can provide clues to the allergenic source. Dermatitis present around the eyelids and periorbital region with involvement of the bilateral upper and lower eyelids suggests direct exposure to a contact allergen, such as makeup or other cosmetic products.1 Unilateral involvement of only 1 eyelid can occur with ectopic transfer of allergens from the hands or nails.1,19 Involvement of the fingers or nails in addition to the eyelids may further suggest ectopic transfer, such as from allergens in nail polish.10 Unilateral eyelid dermatitis also could be caused by unique exposures such as a microscope or camera eyepiece.19 Distribution around the lower eyelids and upper cheeks is indicative of a drip or runoff pattern, which may result from an ophthalmic solution such as eye drops or contact lens solution.1,19 Finally, dermatitis affecting the upper eyelids along with the nasolabial folds and upper chest may suggest airborne contact dermatitis to fragrances or household cleaning products.1,11
Common Culprits of Eyelid ACD
Common causes of eyelid ACD include cosmetic products, ophthalmic medications, nail lacquers, and jewelry.10,13,20 Within the broader category of cosmetics, allergens may be found in makeup and makeup removers, cosmetic applicators and brushes, soaps and cleansers, creams and sunscreens, antiaging products, hair products, nail polish and files, and hair removal products, among many others.10,13,16,20 Additionally, ophthalmologic and topical medications are common sources of ACD, including eyedrops, contact lens solution, and topical antibiotics.10,13,21 Costume jewelry commonly contains allergenic metals, which also can be found in eyelash curlers, eyeglasses, toys, and other household items.22,23 Finally, contact allergens can be found in items such as goggles, gloves, textiles, and a variety of other occupational and household exposures.
Allergic contact dermatitis of the eyelids occurs predominantly—but not exclusively—in females.16,20,24 This finding has been attributed to the traditionally greater use of cosmetics and fragrances among women; however, the use of skin care products among men is increasing, and recent studies have shown the eyelids to be a common location of facial contact dermatitis among men.16,24 Although eyelid dermatitis has not been specifically analyzed by sex, a retrospective analysis of 1332 male patients with facial dermatitis found the most common sites to be the face (not otherwise specified)(48.9%), eyelids (23.5%), and lips (12.6%). In this cohort, the most common allergens were surfactants in shampoos and paraphenylenediamine in hair dyes.24
Common Allergens
Common contact allergens among patients with ACD of the eyelids include metals, fragrances, preservatives, acrylates, and topical medications.3,10,16,20,25-27 Sources of common contact allergens are reviewed in Table 1.

Metals—Metals are among the most common causes of ACD overall, and nickel frequently is reported as one of the top contact allergens in patients with eyelid dermatitis.16,27 A retrospective analysis of 2332 patients with eyelid dermatitis patch tested by the North American Contact Dermatitis Group from 1994 to 2016 found that 18.6% of patients with eyelid ACD had a clinically relevant nickel allergy. Sources of nickel exposure include jewelry, grooming devices, makeup and makeup applicators, and eyelash curlers, as well as direct transfer from the hands after contact with consumer products.16
Other metals that can cause ACD include cobalt (found in similar products to nickel) and gold. Gold often is associated with eyelid dermatitis, though its clinical relevance has been debated, as gold is a relatively inert metal that rarely is present in eye cosmetics and its ions are not displaced from objects and deposited on the skin via sweat in the same way as nickel.4,16,20,28-30 Despite this, studies have shown that gold is a common positive patch test reaction among patients with eyelid dermatitis, even in patients with no dermatitis at the site of contact with gold jewelry.20,29,31 Gold has been reported to be the most common allergen causing unilateral eyelid dermatitis via ectopic transfer.16,19,20,29 It has been proposed that titanium dioxide, present in many cosmetics and sunscreens, displaces gold allowing its release from jewelry, thereby liberating the fine gold ions and allowing them to desposit on the face and eyelids.30,31 Given the uncertain clinical relevance of positive patch test reactions to gold, Warshaw at al16 recommend a 2- to 3-month trial of gold jewelry avoidance to establish relevance, and Ehrlich and Gold29 noted that avoidance of gold leads to improvement.
Fragrances—Fragrances represent a broad category of naturally occurring and man-made components that often are combined to produce a desired scent in personal care products.32 Essential oils and botanicals are both examples of natural fragrances.33 Fragrances are found in numerous products including makeup, hair products, and household cleaning supplies and represent some of the most common contact allergens.32 Common fragrance allergens include fragrance mixes I and II, hydroperoxides of linalool, and balsam of Peru.12,32,34 Allergic contact dermatitis to fragrances typically manifests on the eyelids, face, or hands.33 Several studies have found fragrances to be among the top contact allergens in patients with eyelid dermatitis.3,12,20,25,34 Patch testing for fragrance allergy may include baseline series, supplemental fragrance series, and personal care products.32,35
Preservatives—Preservatives, including formaldehyde and formaldehyde releasers (eg, quaternium-15 and bronopol) and methylchloroisothiazolinone/methylisothiazolinone, may be found in personal care products such as makeup, makeup removers, emollients, shampoos, hair care products, and ophthalmologic solutions and are among the most common cosmetic sources of ACD.13,36-39 Preservatives are among the top allergens causing eyelid dermatitis.20 In particular, patch test positivity rates to methylchloroisothiazolinone/methylisothiazolinone have been increasing in North America.40 Sensitization to preservatives may occur through direct skin contact or transfer from the hands.41
Acrylates—Acrylates are compounds derived from acrylic acid that may be found in acrylic and gel nails, eyelash extensions, and other adhesives and are frequent causes of eyelid ACD.4,10,42 Acrylate exposure may be cosmetic among consumers or occupational (eg, aestheticians).42,43 Acrylates on the nails may cause eyelid dermatitis via ectopic transfer from the hands and also may cause periungual dermatitis manifesting as nail bed erythema.10 Hydroxyethyl methacrylate is one of the more common eyelid ACD allergens, and studies have shown increasing prevalence of positive reaction rates to hydroxyethylmethacrylate.10,44Topical Medications—Contact allergies to topical medications are quite common, estimated to occur in 10% to 17% of patients undergoing patch testing.45 Both active and inactive ingredients of topical medications may be culprits in eyelid ACD. The most common topical medication allergens include antibiotics, steroids, local anesthetics, and nonsteroidal anti-inflammatory drugs.45 Topical antibiotics such as neomycin and bacitracin represent some of the most common causes of eyelid dermatitis4,10 and may be found in a variety of products, including antibacterial ointments and eye drops.1 Many ophthalmologic medications also contain corticosteroids, with the most common allergenic steroids being tixocortol pivalate (a marker for hydrocortisone allergy) and budesonide.10,20 Topical steroids pose a particular dilemma, as they can be either the source of or a treatment for ACD.10 Eye drops also may contain anesthetics, β-blockers, and antihistamines, as well as the preservative benzalkonium chloride, all of which may be contact allergens.21,39
Differential Diagnosis of Eyelid Dermatitis
Although ACD is reported to be the most common cause of eyelid dermatitis, the differential diagnosis is broad, including endogenous inflammatory dermatoses and exogenous exposures (Table 2). Symptoms of eyelid ACD can be nonspecific (eg, erythema, pruritus), making diagnosis challenging.46

Atopic dermatitis represents another common cause of eyelid dermatitis, accounting for 14% to 39.5% of cases.3-5,49Atopic dermatitis of the eyelids classically manifests with lichenification of the medial aspects of the eyelids.50 Atopic dermatitis and ACD may be difficult to distinguish, as the 2 conditions appear clinically similar and can develop concomitantly.51 Additionally, atopic patients are likely to have comorbid allergic rhinitis and sensitivity to environmental allergens, which may lead to chronic eye scratching and lichenification.1,51 Clinical features of eyelid dermatitis suggesting allergic rhinitis and likely comorbid AD include creases in the lower eyelids (Dennie-Morgan lines) and periorbital hyperpigmentation (known as the allergic shiner) due to venous congestion.1,52
Seborrheic dermatitis is an inflammatory reaction to Malassezia yeast that occurs in sebaceous areas such as the groin, scalp, eyebrows, eyelids, and nasolabial folds.1,53,54
Irritant contact dermatitis, a nonspecific inflammatory reaction caused by direct cell damage from external irritants, also may affect the eyelids and appear similar to ACD.1 It typically manifests with a burning or stinging sensation, as opposed to pruritus, and generally develops and resolves more rapidly than ACD.1 Personal care products are common causes of eyelid irritant contact dermatitis.16
Patch Testing for Eyelid ACD
The gold standard for diagnosis of ACD is patch testing, outlined by the International Contact Dermatitis Research Group.55-57 Patch testing generally is performed with standardized panels of allergens and can be customized either with supplemental panels based on unique exposures or with the patient’s own personal care products to increase the sensitivity of testing. Therefore, a thorough history is crucial to identifying potential allergens in a patient’s environment.
False negatives are possible, as the skin on the back may be thicker and less sensitive than the skin at the location of dermatitis.2,58 This is particularly relevant when using patch testing to diagnose ACD of the eyelids, where the skin is particularly thin and sensitive.2 Additionally, ingredients of ophthalmic medications are known to have an especially high false-negative rate with standard patch testing and may require repeated testing with higher drug concentrations or modified patch testing procedures (eg, open testing, scratch-patch testing).1,59
Treatment
Management of ACD involves allergen avoidance, typically dictated by patch test results.10 Allergen avoidance may be facilitated using online resources such as the Contact Allergen Management Program (https://www.acdscamp.org/) created by the American Contact Dermatitis Society.10,18 Patient counseling following patch testing is crucial to educating patients about sources of potential allergen exposures and strategies for avoidance. In the case of eyelid dermatitis, it is particularly important to consider exposure to airborne allergens such as fragrances.16 Fragrance avoidance is uniquely difficult, as labelling standards in the United States currently do not require disclosure of specific fragrance components.33 Additionally, products labelled as unscented may still contain fragrances. As such, some patients with fragrance allergy may need to carefully avoid all products containing fragrances.33
In addition to allergen avoidance, eyelid ACD may be treated with topical medications (eg, steroids, calcineurin inhibitors, Janus kinase inhibitors); however, these same topical medications also can cause ACD due to some ingredients such as propylene glycol.10 Topical steroids should be used with caution on the eyelids given the risk for atrophy, cataracts, and glaucoma.1
Final Interpretation
Eyelid dermatitis is a common dermatologic condition most frequently caused by ACD due to exposure to allergens in cosmetic products, ophthalmic medications, nail lacquers, and jewelry, among many other potential sources. The most common allergens causing eyelid dermatitis include metals (particularly nickel), fragrances, preservatives, acrylates, and topical medications. Eyelid ACD is diagnosed via patch testing, and the mainstay of treatment is strict allergen avoidance. Patient counseling is vital for successful allergen avoidance and resolution of eyelid ACD.
Eyelid dermatitis is a common dermatologic concern representing a broad group of inflammatory dermatoses and typically presenting as eczematous lesions on the eyelids.1 One of the most common causes of eyelid dermatitis is thought to be allergic contact dermatitis (ACD), a type IV delayed hypersensitivity reaction caused by exposure to external allergens.2 Although ACD can occur anywhere on the body, dermatitis on the face and eyelids is quite common.1,2 This article aims to explore the clinical manifestation, evaluation, and management of eyelid ACD.
Pathophysiology of Eyelid ACD
Studies have shown that ACD is the most common cause of eyelid dermatitis, estimated to account for 46% to 72% of cases worldwide.3-6 Allergic contact dermatitis is a T cell–mediated type IV hypersensitivity reaction to external antigens that manifests as eczematous lesions at the site of contact with the allergen that may spread.7 Allergic contact dermatitis is a common condition, and it is estimated that at least 20% of the general worldwide population has a contact allergy.8,9 Histologically, ACD manifests as spongiotic dermatitis, though this is not unique and also may be seen in atopic dermatitis (AD) and irritant contact dermatitis.2 Allergic contact dermatitis is diagnosed via epicutaneous patch testing, and treatment involves allergen avoidance with or without adjuvant topical and/or systemic immunomodulatory treatments.7
The eyelids are uniquely prone to the development of ACD given their thinner epidermis and increased susceptibility to irritation. They frequently are exposed to allergens through the direct topical route as well as indirectly via airborne exposure, rinse-down products (eg, shampoos), and substances transferred from an individual’s own hands. The occluded skin folds of the eyelids facilitate increased exposure to trapped allergens.10,11 Additionally, the skin of the eyelids is thin, flexible, highly vascularized, and lacking in subcutaneous tissue, making this area more susceptible to antigen penetration than other locations on the body.1,2,10,12,13
Clinical Manifestations
Eyelid ACD is more common in females than males, which is thought to be related to increased use of cosmetics and fragrances.1,3,12,14-16 Clinical manifestations may resemble eczematous papules and plaques.1 Eyelid ACD commonly spreads beyond the eyelid margin, which helps to differentiate it from AD and irritant contact dermatitis. Symptoms of ACD on the eyelids typically include pruritus, redness, swelling, tearing, scaling, and pain.2 Persistent untreated eyelid dermatitis can lead to eyelash loss, damage to meibomian glands, and hyperpigmentation.2,17,18
Patterns of Eyelid ACD
Allergic contact dermatitis on the eyelids can occur due to direct application of allergens onto the skin of the eyelids, runoff of products from the hair/scalp (eg, shampoo), transfer of allergens from the hands, or contact with airborne allergens.1,2,11,12 Some reports have suggested that eyelid ACD more often is caused by products applied to the scalp or face rather than those applied directly to the eyelids.11 Because the scalp and face are less reactive to contact allergens, in some cases the eyelids may be the only affected site.10,12,13
The specific pattern of dermatitis on or around the eyelids can provide clues to the allergenic source. Dermatitis present around the eyelids and periorbital region with involvement of the bilateral upper and lower eyelids suggests direct exposure to a contact allergen, such as makeup or other cosmetic products.1 Unilateral involvement of only 1 eyelid can occur with ectopic transfer of allergens from the hands or nails.1,19 Involvement of the fingers or nails in addition to the eyelids may further suggest ectopic transfer, such as from allergens in nail polish.10 Unilateral eyelid dermatitis also could be caused by unique exposures such as a microscope or camera eyepiece.19 Distribution around the lower eyelids and upper cheeks is indicative of a drip or runoff pattern, which may result from an ophthalmic solution such as eye drops or contact lens solution.1,19 Finally, dermatitis affecting the upper eyelids along with the nasolabial folds and upper chest may suggest airborne contact dermatitis to fragrances or household cleaning products.1,11
Common Culprits of Eyelid ACD
Common causes of eyelid ACD include cosmetic products, ophthalmic medications, nail lacquers, and jewelry.10,13,20 Within the broader category of cosmetics, allergens may be found in makeup and makeup removers, cosmetic applicators and brushes, soaps and cleansers, creams and sunscreens, antiaging products, hair products, nail polish and files, and hair removal products, among many others.10,13,16,20 Additionally, ophthalmologic and topical medications are common sources of ACD, including eyedrops, contact lens solution, and topical antibiotics.10,13,21 Costume jewelry commonly contains allergenic metals, which also can be found in eyelash curlers, eyeglasses, toys, and other household items.22,23 Finally, contact allergens can be found in items such as goggles, gloves, textiles, and a variety of other occupational and household exposures.
Allergic contact dermatitis of the eyelids occurs predominantly—but not exclusively—in females.16,20,24 This finding has been attributed to the traditionally greater use of cosmetics and fragrances among women; however, the use of skin care products among men is increasing, and recent studies have shown the eyelids to be a common location of facial contact dermatitis among men.16,24 Although eyelid dermatitis has not been specifically analyzed by sex, a retrospective analysis of 1332 male patients with facial dermatitis found the most common sites to be the face (not otherwise specified)(48.9%), eyelids (23.5%), and lips (12.6%). In this cohort, the most common allergens were surfactants in shampoos and paraphenylenediamine in hair dyes.24
Common Allergens
Common contact allergens among patients with ACD of the eyelids include metals, fragrances, preservatives, acrylates, and topical medications.3,10,16,20,25-27 Sources of common contact allergens are reviewed in Table 1.

Metals—Metals are among the most common causes of ACD overall, and nickel frequently is reported as one of the top contact allergens in patients with eyelid dermatitis.16,27 A retrospective analysis of 2332 patients with eyelid dermatitis patch tested by the North American Contact Dermatitis Group from 1994 to 2016 found that 18.6% of patients with eyelid ACD had a clinically relevant nickel allergy. Sources of nickel exposure include jewelry, grooming devices, makeup and makeup applicators, and eyelash curlers, as well as direct transfer from the hands after contact with consumer products.16
Other metals that can cause ACD include cobalt (found in similar products to nickel) and gold. Gold often is associated with eyelid dermatitis, though its clinical relevance has been debated, as gold is a relatively inert metal that rarely is present in eye cosmetics and its ions are not displaced from objects and deposited on the skin via sweat in the same way as nickel.4,16,20,28-30 Despite this, studies have shown that gold is a common positive patch test reaction among patients with eyelid dermatitis, even in patients with no dermatitis at the site of contact with gold jewelry.20,29,31 Gold has been reported to be the most common allergen causing unilateral eyelid dermatitis via ectopic transfer.16,19,20,29 It has been proposed that titanium dioxide, present in many cosmetics and sunscreens, displaces gold allowing its release from jewelry, thereby liberating the fine gold ions and allowing them to desposit on the face and eyelids.30,31 Given the uncertain clinical relevance of positive patch test reactions to gold, Warshaw at al16 recommend a 2- to 3-month trial of gold jewelry avoidance to establish relevance, and Ehrlich and Gold29 noted that avoidance of gold leads to improvement.
Fragrances—Fragrances represent a broad category of naturally occurring and man-made components that often are combined to produce a desired scent in personal care products.32 Essential oils and botanicals are both examples of natural fragrances.33 Fragrances are found in numerous products including makeup, hair products, and household cleaning supplies and represent some of the most common contact allergens.32 Common fragrance allergens include fragrance mixes I and II, hydroperoxides of linalool, and balsam of Peru.12,32,34 Allergic contact dermatitis to fragrances typically manifests on the eyelids, face, or hands.33 Several studies have found fragrances to be among the top contact allergens in patients with eyelid dermatitis.3,12,20,25,34 Patch testing for fragrance allergy may include baseline series, supplemental fragrance series, and personal care products.32,35
Preservatives—Preservatives, including formaldehyde and formaldehyde releasers (eg, quaternium-15 and bronopol) and methylchloroisothiazolinone/methylisothiazolinone, may be found in personal care products such as makeup, makeup removers, emollients, shampoos, hair care products, and ophthalmologic solutions and are among the most common cosmetic sources of ACD.13,36-39 Preservatives are among the top allergens causing eyelid dermatitis.20 In particular, patch test positivity rates to methylchloroisothiazolinone/methylisothiazolinone have been increasing in North America.40 Sensitization to preservatives may occur through direct skin contact or transfer from the hands.41
Acrylates—Acrylates are compounds derived from acrylic acid that may be found in acrylic and gel nails, eyelash extensions, and other adhesives and are frequent causes of eyelid ACD.4,10,42 Acrylate exposure may be cosmetic among consumers or occupational (eg, aestheticians).42,43 Acrylates on the nails may cause eyelid dermatitis via ectopic transfer from the hands and also may cause periungual dermatitis manifesting as nail bed erythema.10 Hydroxyethyl methacrylate is one of the more common eyelid ACD allergens, and studies have shown increasing prevalence of positive reaction rates to hydroxyethylmethacrylate.10,44Topical Medications—Contact allergies to topical medications are quite common, estimated to occur in 10% to 17% of patients undergoing patch testing.45 Both active and inactive ingredients of topical medications may be culprits in eyelid ACD. The most common topical medication allergens include antibiotics, steroids, local anesthetics, and nonsteroidal anti-inflammatory drugs.45 Topical antibiotics such as neomycin and bacitracin represent some of the most common causes of eyelid dermatitis4,10 and may be found in a variety of products, including antibacterial ointments and eye drops.1 Many ophthalmologic medications also contain corticosteroids, with the most common allergenic steroids being tixocortol pivalate (a marker for hydrocortisone allergy) and budesonide.10,20 Topical steroids pose a particular dilemma, as they can be either the source of or a treatment for ACD.10 Eye drops also may contain anesthetics, β-blockers, and antihistamines, as well as the preservative benzalkonium chloride, all of which may be contact allergens.21,39
Differential Diagnosis of Eyelid Dermatitis
Although ACD is reported to be the most common cause of eyelid dermatitis, the differential diagnosis is broad, including endogenous inflammatory dermatoses and exogenous exposures (Table 2). Symptoms of eyelid ACD can be nonspecific (eg, erythema, pruritus), making diagnosis challenging.46

Atopic dermatitis represents another common cause of eyelid dermatitis, accounting for 14% to 39.5% of cases.3-5,49Atopic dermatitis of the eyelids classically manifests with lichenification of the medial aspects of the eyelids.50 Atopic dermatitis and ACD may be difficult to distinguish, as the 2 conditions appear clinically similar and can develop concomitantly.51 Additionally, atopic patients are likely to have comorbid allergic rhinitis and sensitivity to environmental allergens, which may lead to chronic eye scratching and lichenification.1,51 Clinical features of eyelid dermatitis suggesting allergic rhinitis and likely comorbid AD include creases in the lower eyelids (Dennie-Morgan lines) and periorbital hyperpigmentation (known as the allergic shiner) due to venous congestion.1,52
Seborrheic dermatitis is an inflammatory reaction to Malassezia yeast that occurs in sebaceous areas such as the groin, scalp, eyebrows, eyelids, and nasolabial folds.1,53,54
Irritant contact dermatitis, a nonspecific inflammatory reaction caused by direct cell damage from external irritants, also may affect the eyelids and appear similar to ACD.1 It typically manifests with a burning or stinging sensation, as opposed to pruritus, and generally develops and resolves more rapidly than ACD.1 Personal care products are common causes of eyelid irritant contact dermatitis.16
Patch Testing for Eyelid ACD
The gold standard for diagnosis of ACD is patch testing, outlined by the International Contact Dermatitis Research Group.55-57 Patch testing generally is performed with standardized panels of allergens and can be customized either with supplemental panels based on unique exposures or with the patient’s own personal care products to increase the sensitivity of testing. Therefore, a thorough history is crucial to identifying potential allergens in a patient’s environment.
False negatives are possible, as the skin on the back may be thicker and less sensitive than the skin at the location of dermatitis.2,58 This is particularly relevant when using patch testing to diagnose ACD of the eyelids, where the skin is particularly thin and sensitive.2 Additionally, ingredients of ophthalmic medications are known to have an especially high false-negative rate with standard patch testing and may require repeated testing with higher drug concentrations or modified patch testing procedures (eg, open testing, scratch-patch testing).1,59
Treatment
Management of ACD involves allergen avoidance, typically dictated by patch test results.10 Allergen avoidance may be facilitated using online resources such as the Contact Allergen Management Program (https://www.acdscamp.org/) created by the American Contact Dermatitis Society.10,18 Patient counseling following patch testing is crucial to educating patients about sources of potential allergen exposures and strategies for avoidance. In the case of eyelid dermatitis, it is particularly important to consider exposure to airborne allergens such as fragrances.16 Fragrance avoidance is uniquely difficult, as labelling standards in the United States currently do not require disclosure of specific fragrance components.33 Additionally, products labelled as unscented may still contain fragrances. As such, some patients with fragrance allergy may need to carefully avoid all products containing fragrances.33
In addition to allergen avoidance, eyelid ACD may be treated with topical medications (eg, steroids, calcineurin inhibitors, Janus kinase inhibitors); however, these same topical medications also can cause ACD due to some ingredients such as propylene glycol.10 Topical steroids should be used with caution on the eyelids given the risk for atrophy, cataracts, and glaucoma.1
Final Interpretation
Eyelid dermatitis is a common dermatologic condition most frequently caused by ACD due to exposure to allergens in cosmetic products, ophthalmic medications, nail lacquers, and jewelry, among many other potential sources. The most common allergens causing eyelid dermatitis include metals (particularly nickel), fragrances, preservatives, acrylates, and topical medications. Eyelid ACD is diagnosed via patch testing, and the mainstay of treatment is strict allergen avoidance. Patient counseling is vital for successful allergen avoidance and resolution of eyelid ACD.
- Hine AM, Waldman RA, Grzybowski A, et al. Allergic disorders of the eyelid. Clin Dermatol. 2023;41:476-480. doi:10.1016/j.clindermatol.2023.08.002
- Turkiewicz M, Shah A, Yang YW, et al. Allergic contact dermatitis of the eyelids: an interdisciplinary review. Ocul Surf. 2023;28:124-130. doi:10.1016/j.jtos.2023.03.001
- Valsecchi R, Imberti G, Martino D, et al. Eyelid dermatitis: an evaluation of 150 patients. Contact Dermatitis. 1992;27:143-147. doi:10.1111/j.1600-0536.1992.tb05242.x
- Guin JD. Eyelid dermatitis: experience in 203 cases. J Am Acad Dermatol. 2002;47:755-765. doi:10.1067/mjd.2002.122736
- Nethercott JR, Nield G, Holness DL. A review of 79 cases of eyelid dermatitis. J Am Acad Dermatol. 1989;21(2 pt 1):223-230. doi:10.1016/s0190-9622(89)70165-1
- Shah M, Lewis FM, Gawkrodger DJ. Facial dermatitis and eyelid dermatitis: a comparison of patch test results and final diagnoses. Contact Dermatitis. 1996;34:140-141. doi:10.1111/j.1600-0536.1996.tb02148.x
- Brites GS, Ferreira I, Sebastião AI, et al. Allergic contact dermatitis: from pathophysiology to development of new preventive strategies. Pharmacol Res. 2020;162:105282. doi:10.1016/j.phrs.2020.105282
- Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta-analysis. Contact Dermatitis. 2019;80:77-85. doi:10.1111/cod.13119
- Adler BL, DeLeo VA. Allergic contact dermatitis. JAMA Dermatol. 2021;157:364. doi:10.1001/jamadermatol.2020.5639
- Huang CX, Yiannias JA, Killian JM, et al. Seven common allergen groups causing eyelid dermatitis: education and avoidance strategies. Clin Ophthalmol Auckl NZ. 2021;15:1477-1490. doi:10.2147/OPTH.S297754
- Rozas-Muñoz E, Gamé D, Serra-Baldrich E. Allergic contact dermatitis by anatomical regions: diagnostic clues. Actas Dermo-Sifiliográficas Engl Ed. 2018;109:485-507. doi:10.1016/j.adengl.2018.05.016
- Amin KA, Belsito DV. The aetiology of eyelid dermatitis: a 10-year retrospective analysis. Contact Dermatitis. 2006;55:280-285. doi:10.1111/j.1600-0536.2006.00927.x
- Wolf R, Orion E, Tüzün Y. Periorbital (eyelid) dermatides. Clin Dermatol. 2014;32:131-140. doi:10.1016/j.clindermatol.2013.05.035
- Ockenfels HM, Seemann U, Goos M. Contact allergy in patients with periorbital eczema: an analysis of allergens. data recorded by the Information Network of the Departments of Dermatology. Dermatol Basel Switz. 1997;195:119-124. doi:10.1159/000245712
- Landeck L, John SM, Geier J. Periorbital dermatitis in 4779 patients—patch test results during a 10-year period. Contact Dermatitis. 2014;70:205-212. doi:10.1111/cod.12157
- Warshaw EM, Voller LM, Maibach HI, et al. Eyelid dermatitis in patients referred for patch testing: retrospective analysis of North American Contact Dermatitis Group data, 1994-2016. J Am Acad Dermatol. 2021;84:953-964. doi:10.1016/j.jaad.2020.07.020
- McMonnies CW. Management of chronic habits of abnormal eye rubbing. Contact Lens Anterior Eye. 2008;31:95-102. doi:10.1016/j.clae.2007.07.008
- Chisholm SAM, Couch SM, Custer PL. Etiology and management of allergic eyelid dermatitis. Ophthal Plast Reconstr Surg. 2017;33:248-250. doi:10.1097/IOP.0000000000000723
- Lewallen R, Feldman S, eds. Regional atlas of contact dermatitis. The Dermatologist. Accessed April 22, 2024. https://s3.amazonaws.com/HMP/hmp_ln/imported/Regional%20Atlas%20of%20Contact%20Dermatitis%20Book_lr.pdf
- Rietschel RL, Warshaw EM, Sasseville D, et al. Common contact allergens associated with eyelid dermatitis: data from the North American Contact Dermatitis Group 2003-2004 study period. Dermat Contact Atopic Occup Drug. 2007;18:78-81. doi:10.2310/6620.2007.06041
- Mughal AA, Kalavala M. Contact dermatitis to ophthalmic solutions. Clin Exp Dermatol. 2012;37:593-597; quiz 597-598. doi:10.1111/j.1365-2230.2012.04398.x
- Goossens A. Contact allergic reactions on the eyes and eyelids. Bull Soc Belge Ophtalmol. 2004;292:11-17.
- Silverberg NB, Pelletier JL, Jacob SE, et al. Nickel allergic contact dermatitis: identification, treatment, and prevention. Pediatrics. 2020;145:E20200628. doi:10.1542/peds.2020-0628
- Warshaw EM, Schlarbaum JP, Maibach HI, et al. Facial dermatitis in male patients referred for patch testing. JAMA Dermatol. 2020;156:79-84. doi:10.1001/jamadermatol.2019.3531
- Wenk KS, Ehrlich A. Fragrance series testing in eyelid dermatitis. Dermatitis. 2012;23:22-26. doi:10.1097/DER.0b013e31823d180f
- Crouse L, Ziemer C, Ziemer C, et al. Trends in eyelid dermatitis. Dermat Contact Atopic Occup Drug. 2018;29:96-97. doi:10.1097/DER.0000000000000338
- Yazdanparast T, Nassiri Kashani M, Shamsipour M, et al. Contact allergens responsible for eyelid dermatitis in adults. J Dermatol. 2024;51:691-695. doi:10.1111/1346-8138.17140
- Fowler J, Taylor J, Storrs F, et al. Gold allergy in North America. Am J Contact Dermat. 2001;12:3-5.
- Ehrlich A, Belsito DV. Allergic contact dermatitis to gold. Cutis. 2000;65:323-326.
- Danesh M, Murase JE. Titanium dioxide induces eyelid dermatitis in patients allergic to gold. J Am Acad Dermatol. 2015;73:E21. doi:10.1016/j.jaad.2015.03.046
- Katta R. Common misconceptions in contact dermatitis counseling. Dermatol Online J. 2008;14:2.
- De Groot AC. Fragrances: contact allergy and other adverse effects. Dermatitis. 2020;31:13-35. doi:10.1097/DER.0000000000000463
- Reeder MJ. Allergic contact dermatitis to fragrances. Dermatol Clin. 2020;38:371-377. doi:10.1016/j.det.2020.02.009
- Warshaw EM, Zhang AJ, DeKoven JG, et al. Epidemiology of nickel sensitivity: retrospective cross-sectional analysis of North American Contact Dermatitis Group data 1994-2014. J Am Acad Dermatol. 2019;80:701-713. doi:10.1016/j.jaad.2018.09.058
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society core allergen series: 2020 update. Dermatitis. 2020;31:279-282. doi:10.1097/DER.0000000000000621
- Yim E, Baquerizo Nole KL, Tosti A. Contact dermatitis caused by preservatives. Dermatitis. 2014;25:215-231. doi:10.1097/DER.0000000000000061
- Alani JI, Davis MDP, Yiannias JA. Allergy to cosmetics. Dermatitis. 2013;24:283-290. doi:10.1097/DER.0b013e3182a5d8bc
- Hamilton T, de Gannes GC. Allergic contact dermatitis to preservatives and fragrances in cosmetics. Skin Ther Lett. 2011;16:1-4.
- Ashton SJ, Mughal AA. Contact dermatitis to ophthalmic solutions: an update. Dermat Contact Atopic Occup Drug. 2023;34:480-483. doi:10.1089/derm.2023.0033
- Reeder MJ, Warshaw E, Aravamuthan S, et al. Trends in the prevalence of methylchloroisothiazolinone/methylisothiazolinone contact allergy in North America and Europe. JAMA Dermatol. 2023;159:267-274. doi:10.1001/jamadermatol.2022.5991
- Herro EM, Elsaie ML, Nijhawan RI, et al. Recommendations for a screening series for allergic contact eyelid dermatitis. Dermatitis. 2012;23:17-21. doi:10.1097/DER.0b013e31823d191f
- Kucharczyk M, Słowik-Rylska M, Cyran-Stemplewska S, et al. Acrylates as a significant cause of allergic contact dermatitis: new sources of exposure. Adv Dermatol Allergol Dermatol Alergol. 2021;38:555-560. doi:10.5114/ada.2020.95848
- Rodriguez I, George SE, Yu J, et al. Tackling acrylate allergy: the sticky truth. Cutis. 2023;112:282-286. doi:10.12788/cutis.0909
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group Patch Test Results: 2019–2020. Dermatitis. 2023;34:90-104. doi:10.1089/derm.2022.29017.jdk
- de Groot A. Allergic contact dermatitis from topical drugs: an overview. Dermatitis. 2021;32:197-213. doi:10.1097/DER.0000000000000737
- Zug KA, Palay DA, Rock B. Dermatologic diagnosis and treatment of itchy red eyelids. Surv Ophthalmol. 1996;40:293-306. doi:10.1016/s0039-6257(96)82004-2
- Beltrani VS. Eyelid dermatitis. Curr Allergy Asthma Rep. 2001;1:380-388. doi:10.1007/s11882-001-0052-0
- Hirji SH, Maeng MM, Tran AQ, et al. Cutaneous T-cell lymphoma of the eyelid masquerading as dermatitis. Orbit Amst Neth. 2021;40:75-78. doi:10.1080/01676830.2020.1739080
- Svensson A, Möller H. Eyelid dermatitis: the role of atopy and contact allergy. Contact Dermatitis. 1986;15:178-182. doi:10.1111/j.1600-0536.1986.tb01321.x
- Papier A, Tuttle DJ, Mahar TJ. Differential diagnosis of the swollen red eyelid. Am Fam Physician. 2007;76:1815-1824.
- Johnson H, Novack DE, Adler BL, et al. Can atopic dermatitis and allergic contact dermatitis coexist? Cutis. 2022;110:139-142. doi:10.12788cutis.0599
- Berger WE. Allergic rhinitis in children: diagnosis and management strategies. Paediatr Drugs. 2004;6:233-250. doi:10.2165/00148581-200406040-00003
- Singh A, Kansal NK, Kumawat D, et al. Ophthalmic manifestations of seborrheic dermatitis. Skinmed. 2023;21:397-401.
- Clark GW, Pope SM, Jaboori KA. Diagnosis and treatment of seborrheic dermatitis. Am Fam Physician. 2015;91:185-190.
- Lachapelle JM, Maibach HI. Patch Testing and Prick Testing. Springer; 2012.
- Fregert S. Manual of Contact Dermatitis: On Behalf of the International Contact Dermatitis Research Group. Munksgaard; 1974.
- Reeder M, Reck Atwater A. Patch testing 101, part 1: performing the test. Cutis. 2020;106:165-167. doi:10.12788/cutis.0093
- Wolf R, Perluk H. Failure of routine patch test results to detect eyelid dermatitis. Cutis. 1992;49:133-134.
- Grey KR, Warshaw EM. Allergic contact dermatitis to ophthalmic medications: relevant allergens and alternative testing methods. Dermat Contact Atopic Occup Drug. 2016;27:333-347. doi:10.1097/DER.0000000000000224
- Hine AM, Waldman RA, Grzybowski A, et al. Allergic disorders of the eyelid. Clin Dermatol. 2023;41:476-480. doi:10.1016/j.clindermatol.2023.08.002
- Turkiewicz M, Shah A, Yang YW, et al. Allergic contact dermatitis of the eyelids: an interdisciplinary review. Ocul Surf. 2023;28:124-130. doi:10.1016/j.jtos.2023.03.001
- Valsecchi R, Imberti G, Martino D, et al. Eyelid dermatitis: an evaluation of 150 patients. Contact Dermatitis. 1992;27:143-147. doi:10.1111/j.1600-0536.1992.tb05242.x
- Guin JD. Eyelid dermatitis: experience in 203 cases. J Am Acad Dermatol. 2002;47:755-765. doi:10.1067/mjd.2002.122736
- Nethercott JR, Nield G, Holness DL. A review of 79 cases of eyelid dermatitis. J Am Acad Dermatol. 1989;21(2 pt 1):223-230. doi:10.1016/s0190-9622(89)70165-1
- Shah M, Lewis FM, Gawkrodger DJ. Facial dermatitis and eyelid dermatitis: a comparison of patch test results and final diagnoses. Contact Dermatitis. 1996;34:140-141. doi:10.1111/j.1600-0536.1996.tb02148.x
- Brites GS, Ferreira I, Sebastião AI, et al. Allergic contact dermatitis: from pathophysiology to development of new preventive strategies. Pharmacol Res. 2020;162:105282. doi:10.1016/j.phrs.2020.105282
- Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta-analysis. Contact Dermatitis. 2019;80:77-85. doi:10.1111/cod.13119
- Adler BL, DeLeo VA. Allergic contact dermatitis. JAMA Dermatol. 2021;157:364. doi:10.1001/jamadermatol.2020.5639
- Huang CX, Yiannias JA, Killian JM, et al. Seven common allergen groups causing eyelid dermatitis: education and avoidance strategies. Clin Ophthalmol Auckl NZ. 2021;15:1477-1490. doi:10.2147/OPTH.S297754
- Rozas-Muñoz E, Gamé D, Serra-Baldrich E. Allergic contact dermatitis by anatomical regions: diagnostic clues. Actas Dermo-Sifiliográficas Engl Ed. 2018;109:485-507. doi:10.1016/j.adengl.2018.05.016
- Amin KA, Belsito DV. The aetiology of eyelid dermatitis: a 10-year retrospective analysis. Contact Dermatitis. 2006;55:280-285. doi:10.1111/j.1600-0536.2006.00927.x
- Wolf R, Orion E, Tüzün Y. Periorbital (eyelid) dermatides. Clin Dermatol. 2014;32:131-140. doi:10.1016/j.clindermatol.2013.05.035
- Ockenfels HM, Seemann U, Goos M. Contact allergy in patients with periorbital eczema: an analysis of allergens. data recorded by the Information Network of the Departments of Dermatology. Dermatol Basel Switz. 1997;195:119-124. doi:10.1159/000245712
- Landeck L, John SM, Geier J. Periorbital dermatitis in 4779 patients—patch test results during a 10-year period. Contact Dermatitis. 2014;70:205-212. doi:10.1111/cod.12157
- Warshaw EM, Voller LM, Maibach HI, et al. Eyelid dermatitis in patients referred for patch testing: retrospective analysis of North American Contact Dermatitis Group data, 1994-2016. J Am Acad Dermatol. 2021;84:953-964. doi:10.1016/j.jaad.2020.07.020
- McMonnies CW. Management of chronic habits of abnormal eye rubbing. Contact Lens Anterior Eye. 2008;31:95-102. doi:10.1016/j.clae.2007.07.008
- Chisholm SAM, Couch SM, Custer PL. Etiology and management of allergic eyelid dermatitis. Ophthal Plast Reconstr Surg. 2017;33:248-250. doi:10.1097/IOP.0000000000000723
- Lewallen R, Feldman S, eds. Regional atlas of contact dermatitis. The Dermatologist. Accessed April 22, 2024. https://s3.amazonaws.com/HMP/hmp_ln/imported/Regional%20Atlas%20of%20Contact%20Dermatitis%20Book_lr.pdf
- Rietschel RL, Warshaw EM, Sasseville D, et al. Common contact allergens associated with eyelid dermatitis: data from the North American Contact Dermatitis Group 2003-2004 study period. Dermat Contact Atopic Occup Drug. 2007;18:78-81. doi:10.2310/6620.2007.06041
- Mughal AA, Kalavala M. Contact dermatitis to ophthalmic solutions. Clin Exp Dermatol. 2012;37:593-597; quiz 597-598. doi:10.1111/j.1365-2230.2012.04398.x
- Goossens A. Contact allergic reactions on the eyes and eyelids. Bull Soc Belge Ophtalmol. 2004;292:11-17.
- Silverberg NB, Pelletier JL, Jacob SE, et al. Nickel allergic contact dermatitis: identification, treatment, and prevention. Pediatrics. 2020;145:E20200628. doi:10.1542/peds.2020-0628
- Warshaw EM, Schlarbaum JP, Maibach HI, et al. Facial dermatitis in male patients referred for patch testing. JAMA Dermatol. 2020;156:79-84. doi:10.1001/jamadermatol.2019.3531
- Wenk KS, Ehrlich A. Fragrance series testing in eyelid dermatitis. Dermatitis. 2012;23:22-26. doi:10.1097/DER.0b013e31823d180f
- Crouse L, Ziemer C, Ziemer C, et al. Trends in eyelid dermatitis. Dermat Contact Atopic Occup Drug. 2018;29:96-97. doi:10.1097/DER.0000000000000338
- Yazdanparast T, Nassiri Kashani M, Shamsipour M, et al. Contact allergens responsible for eyelid dermatitis in adults. J Dermatol. 2024;51:691-695. doi:10.1111/1346-8138.17140
- Fowler J, Taylor J, Storrs F, et al. Gold allergy in North America. Am J Contact Dermat. 2001;12:3-5.
- Ehrlich A, Belsito DV. Allergic contact dermatitis to gold. Cutis. 2000;65:323-326.
- Danesh M, Murase JE. Titanium dioxide induces eyelid dermatitis in patients allergic to gold. J Am Acad Dermatol. 2015;73:E21. doi:10.1016/j.jaad.2015.03.046
- Katta R. Common misconceptions in contact dermatitis counseling. Dermatol Online J. 2008;14:2.
- De Groot AC. Fragrances: contact allergy and other adverse effects. Dermatitis. 2020;31:13-35. doi:10.1097/DER.0000000000000463
- Reeder MJ. Allergic contact dermatitis to fragrances. Dermatol Clin. 2020;38:371-377. doi:10.1016/j.det.2020.02.009
- Warshaw EM, Zhang AJ, DeKoven JG, et al. Epidemiology of nickel sensitivity: retrospective cross-sectional analysis of North American Contact Dermatitis Group data 1994-2014. J Am Acad Dermatol. 2019;80:701-713. doi:10.1016/j.jaad.2018.09.058
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society core allergen series: 2020 update. Dermatitis. 2020;31:279-282. doi:10.1097/DER.0000000000000621
- Yim E, Baquerizo Nole KL, Tosti A. Contact dermatitis caused by preservatives. Dermatitis. 2014;25:215-231. doi:10.1097/DER.0000000000000061
- Alani JI, Davis MDP, Yiannias JA. Allergy to cosmetics. Dermatitis. 2013;24:283-290. doi:10.1097/DER.0b013e3182a5d8bc
- Hamilton T, de Gannes GC. Allergic contact dermatitis to preservatives and fragrances in cosmetics. Skin Ther Lett. 2011;16:1-4.
- Ashton SJ, Mughal AA. Contact dermatitis to ophthalmic solutions: an update. Dermat Contact Atopic Occup Drug. 2023;34:480-483. doi:10.1089/derm.2023.0033
- Reeder MJ, Warshaw E, Aravamuthan S, et al. Trends in the prevalence of methylchloroisothiazolinone/methylisothiazolinone contact allergy in North America and Europe. JAMA Dermatol. 2023;159:267-274. doi:10.1001/jamadermatol.2022.5991
- Herro EM, Elsaie ML, Nijhawan RI, et al. Recommendations for a screening series for allergic contact eyelid dermatitis. Dermatitis. 2012;23:17-21. doi:10.1097/DER.0b013e31823d191f
- Kucharczyk M, Słowik-Rylska M, Cyran-Stemplewska S, et al. Acrylates as a significant cause of allergic contact dermatitis: new sources of exposure. Adv Dermatol Allergol Dermatol Alergol. 2021;38:555-560. doi:10.5114/ada.2020.95848
- Rodriguez I, George SE, Yu J, et al. Tackling acrylate allergy: the sticky truth. Cutis. 2023;112:282-286. doi:10.12788/cutis.0909
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group Patch Test Results: 2019–2020. Dermatitis. 2023;34:90-104. doi:10.1089/derm.2022.29017.jdk
- de Groot A. Allergic contact dermatitis from topical drugs: an overview. Dermatitis. 2021;32:197-213. doi:10.1097/DER.0000000000000737
- Zug KA, Palay DA, Rock B. Dermatologic diagnosis and treatment of itchy red eyelids. Surv Ophthalmol. 1996;40:293-306. doi:10.1016/s0039-6257(96)82004-2
- Beltrani VS. Eyelid dermatitis. Curr Allergy Asthma Rep. 2001;1:380-388. doi:10.1007/s11882-001-0052-0
- Hirji SH, Maeng MM, Tran AQ, et al. Cutaneous T-cell lymphoma of the eyelid masquerading as dermatitis. Orbit Amst Neth. 2021;40:75-78. doi:10.1080/01676830.2020.1739080
- Svensson A, Möller H. Eyelid dermatitis: the role of atopy and contact allergy. Contact Dermatitis. 1986;15:178-182. doi:10.1111/j.1600-0536.1986.tb01321.x
- Papier A, Tuttle DJ, Mahar TJ. Differential diagnosis of the swollen red eyelid. Am Fam Physician. 2007;76:1815-1824.
- Johnson H, Novack DE, Adler BL, et al. Can atopic dermatitis and allergic contact dermatitis coexist? Cutis. 2022;110:139-142. doi:10.12788cutis.0599
- Berger WE. Allergic rhinitis in children: diagnosis and management strategies. Paediatr Drugs. 2004;6:233-250. doi:10.2165/00148581-200406040-00003
- Singh A, Kansal NK, Kumawat D, et al. Ophthalmic manifestations of seborrheic dermatitis. Skinmed. 2023;21:397-401.
- Clark GW, Pope SM, Jaboori KA. Diagnosis and treatment of seborrheic dermatitis. Am Fam Physician. 2015;91:185-190.
- Lachapelle JM, Maibach HI. Patch Testing and Prick Testing. Springer; 2012.
- Fregert S. Manual of Contact Dermatitis: On Behalf of the International Contact Dermatitis Research Group. Munksgaard; 1974.
- Reeder M, Reck Atwater A. Patch testing 101, part 1: performing the test. Cutis. 2020;106:165-167. doi:10.12788/cutis.0093
- Wolf R, Perluk H. Failure of routine patch test results to detect eyelid dermatitis. Cutis. 1992;49:133-134.
- Grey KR, Warshaw EM. Allergic contact dermatitis to ophthalmic medications: relevant allergens and alternative testing methods. Dermat Contact Atopic Occup Drug. 2016;27:333-347. doi:10.1097/DER.0000000000000224
Practice Points
- Eyelid dermatitis is a common dermatologic concern representing a broad range of inflammatory dermatoses, most often caused by allergic contact dermatitis (ACD).
- The most common contact allergens associated with eyelid dermatitis are metals (particularly nickel), fragrances, preservatives, acrylates, and topical medications, which may be found in a variety of sources, including cosmetics, ophthalmic medications, nail lacquers, and jewelry.
- Eyelid ACD is diagnosed via patch testing, and management involves strict allergen avoidance.
A Few Rural Towns Are Bucking the Trend and Building New Hospitals
There’s a new morning ritual in Pinedale, Wyoming, a town of about 2000, nestled against the Wind River Mountains.
Friends and neighbors in the oil- and gas-rich community “take their morning coffee and pull up” to watch workers building the county’s first hospital, said Kari DeWitt, the project’s public relations director.
“I think it’s just gratitude,” Ms. DeWitt said.
Sublette County is the only one in Wyoming — where counties span thousands of square miles — without a hospital. The 10-bed, 40,000-square-foot hospital, with a similarly sized attached long-term care facility, is slated to open by the summer of 2025.
Ms. DeWitt, who also is executive director of the Sublette County Health Foundation, has an office at the town’s health clinic with a window view of the construction.
Pinedale’s residents have good reason to be excited. New full-service hospitals with inpatient beds are rare in rural America, where declining population has spurred decades of downsizing and closures. Yet, a few communities in Wyoming and others in Kansas and Georgia are defying the trend.
“To be honest with you, it even seems strange to me,” said Wyoming Hospital Association President Eric Boley. Small rural “hospitals are really struggling all across the country,” he said.
There is no official tally of new hospitals being built in rural America, but industry experts such as Mr. Boley said they’re rare. Typically, health-related construction projects in rural areas are for smaller urgent care centers or stand-alone emergency facilities or are replacements for old hospitals.
About half of rural hospitals lost money in the prior year, according to Chartis, a health analytics and consulting firm. And nearly 150 rural hospitals have closed or converted to smaller operations since 2010, according to data collected by the University of North Carolina’s Cecil G. Sheps Center for Health Services Research.
To stem the tide of closures, Congress created a new rural emergency hospital designation that allowed struggling hospitals to close their inpatient units and provide only outpatient and emergency services. Since January 2023, when the program took effect, 32 of the more than 1700 eligible rural hospitals — from Georgia to New Mexico — have joined the program, according to data from the Centers for Medicare & Medicaid Services.
Tony Breitlow is healthcare studio director for EUA, which has extensive experience working for rural health care systems. Mr. Breitlow said his national architecture and engineering firm’s work expands, replaces, or revamps older buildings, many of which were constructed during the middle of the last century.
The work, Mr. Breitlow said, is part of health care “systems figuring out how to remain robust and viable.”
Freeman Health System, based in Joplin, Missouri, announced plans last year to build a new 50-bed hospital across the state line in Kansas. Paula Baker, Freeman’s president and chief executive, said the system is building for patients in the southeastern corner of the state who travel 45 minutes or more to its bigger Joplin facilities for care.
Freeman’s new hospital, with construction on the building expected to begin in the spring, will be less than 10 miles away from an older, 64-bed hospital that has existed for decades. Kansas is one of more than a dozen states with no “certificate of need” law that would require health providers to obtain approval from the state before offering new services or building or expanding facilities.
Ms. Baker also said Freeman plans to operate emergency services and a small 10-bed outpost in Fort Scott, Kansas, opening early next year in a corner of a hospital that closed in late 2018. Residents there “cried, they cheered, they hugged me,” Ms. Baker said, adding that the “level of appreciation and gratitude that they felt and they displayed was overwhelming to me.”
Michael Topchik, executive director of the Chartis Center for Rural Health, said regional healthcare systems in the Upper Midwest have been particularly active in competing for patients by, among other things, building new hospitals.
And while private corporate money can drive construction, many rural hospital projects tap government programs, especially those supported by the US Department of Agriculture, Mr. Topchik said. That, he said, “surprises a lot of people.”
Since 2021, the USDA’s rural Community Facilities Programs have awarded $2.24 billion in loans and grants to 68 rural hospitals for work that was not related to an emergency or disaster, according to data analyzed by KFF Health News and confirmed by the agency. The federal program is funded through what is often known as the farm bill, which faces a September congressional renewal deadline.
Nearly all the projects are replacements or expansions and updates of older facilities.
The USDA confirmed that three new or planned Wyoming hospitals received federal funding. Hospital projects in Riverton and Saratoga received loans of $37.2 million and $18.3 million, respectively. Pinedale’s hospital received a $29.2 million loan from the agency.
Wyoming’s new construction is rare in a state where more than 80% of rural hospitals reported losses in the third quarter of 2023, according to Chartis. The state association’s Mr. Boley said he worries about several hospitals that have less than 10 days’ cash on hand “day and night.”
Pinedale’s project loan was approved after the community submitted a feasibility study to the USDA that included local clinics and a long-term care facility. “It’s pretty remote and right up in the mountains,” Mr. Boley said.
Pinedale’s Ms. DeWitt said the community was missing key services, such as blood transfusions, which are often necessary when there is a trauma like a car crash or if a pregnant woman faces severe complications. Local ambulances drove 94,000 miles last year, she said.
Ms. DeWitt began working to raise support for the new hospital after her own pregnancy-related trauma in 2014. She was bleeding heavily and arrived at the local health clinic believing it operated like a hospital.
“It was shocking to hear, ‘No, we’re not a hospital. We can’t do blood transfusions. We’re just going to have to pray you live for the next 45 minutes,’ ” Ms. DeWitt said.
Ms. DeWitt had to be airlifted to Idaho, where she delivered a few minutes after landing. When the hospital financing went on the ballot in 2020, Ms. DeWitt — fully recovered, with healthy grade-schoolers at home — began making five calls a night to rally support for a county tax increase to help fund the hospital.
“By improving health care, I think we improve everybody’s chances of survival. You know, it’s pretty basic,” Ms. DeWitt said.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.
There’s a new morning ritual in Pinedale, Wyoming, a town of about 2000, nestled against the Wind River Mountains.
Friends and neighbors in the oil- and gas-rich community “take their morning coffee and pull up” to watch workers building the county’s first hospital, said Kari DeWitt, the project’s public relations director.
“I think it’s just gratitude,” Ms. DeWitt said.
Sublette County is the only one in Wyoming — where counties span thousands of square miles — without a hospital. The 10-bed, 40,000-square-foot hospital, with a similarly sized attached long-term care facility, is slated to open by the summer of 2025.
Ms. DeWitt, who also is executive director of the Sublette County Health Foundation, has an office at the town’s health clinic with a window view of the construction.
Pinedale’s residents have good reason to be excited. New full-service hospitals with inpatient beds are rare in rural America, where declining population has spurred decades of downsizing and closures. Yet, a few communities in Wyoming and others in Kansas and Georgia are defying the trend.
“To be honest with you, it even seems strange to me,” said Wyoming Hospital Association President Eric Boley. Small rural “hospitals are really struggling all across the country,” he said.
There is no official tally of new hospitals being built in rural America, but industry experts such as Mr. Boley said they’re rare. Typically, health-related construction projects in rural areas are for smaller urgent care centers or stand-alone emergency facilities or are replacements for old hospitals.
About half of rural hospitals lost money in the prior year, according to Chartis, a health analytics and consulting firm. And nearly 150 rural hospitals have closed or converted to smaller operations since 2010, according to data collected by the University of North Carolina’s Cecil G. Sheps Center for Health Services Research.
To stem the tide of closures, Congress created a new rural emergency hospital designation that allowed struggling hospitals to close their inpatient units and provide only outpatient and emergency services. Since January 2023, when the program took effect, 32 of the more than 1700 eligible rural hospitals — from Georgia to New Mexico — have joined the program, according to data from the Centers for Medicare & Medicaid Services.
Tony Breitlow is healthcare studio director for EUA, which has extensive experience working for rural health care systems. Mr. Breitlow said his national architecture and engineering firm’s work expands, replaces, or revamps older buildings, many of which were constructed during the middle of the last century.
The work, Mr. Breitlow said, is part of health care “systems figuring out how to remain robust and viable.”
Freeman Health System, based in Joplin, Missouri, announced plans last year to build a new 50-bed hospital across the state line in Kansas. Paula Baker, Freeman’s president and chief executive, said the system is building for patients in the southeastern corner of the state who travel 45 minutes or more to its bigger Joplin facilities for care.
Freeman’s new hospital, with construction on the building expected to begin in the spring, will be less than 10 miles away from an older, 64-bed hospital that has existed for decades. Kansas is one of more than a dozen states with no “certificate of need” law that would require health providers to obtain approval from the state before offering new services or building or expanding facilities.
Ms. Baker also said Freeman plans to operate emergency services and a small 10-bed outpost in Fort Scott, Kansas, opening early next year in a corner of a hospital that closed in late 2018. Residents there “cried, they cheered, they hugged me,” Ms. Baker said, adding that the “level of appreciation and gratitude that they felt and they displayed was overwhelming to me.”
Michael Topchik, executive director of the Chartis Center for Rural Health, said regional healthcare systems in the Upper Midwest have been particularly active in competing for patients by, among other things, building new hospitals.
And while private corporate money can drive construction, many rural hospital projects tap government programs, especially those supported by the US Department of Agriculture, Mr. Topchik said. That, he said, “surprises a lot of people.”
Since 2021, the USDA’s rural Community Facilities Programs have awarded $2.24 billion in loans and grants to 68 rural hospitals for work that was not related to an emergency or disaster, according to data analyzed by KFF Health News and confirmed by the agency. The federal program is funded through what is often known as the farm bill, which faces a September congressional renewal deadline.
Nearly all the projects are replacements or expansions and updates of older facilities.
The USDA confirmed that three new or planned Wyoming hospitals received federal funding. Hospital projects in Riverton and Saratoga received loans of $37.2 million and $18.3 million, respectively. Pinedale’s hospital received a $29.2 million loan from the agency.
Wyoming’s new construction is rare in a state where more than 80% of rural hospitals reported losses in the third quarter of 2023, according to Chartis. The state association’s Mr. Boley said he worries about several hospitals that have less than 10 days’ cash on hand “day and night.”
Pinedale’s project loan was approved after the community submitted a feasibility study to the USDA that included local clinics and a long-term care facility. “It’s pretty remote and right up in the mountains,” Mr. Boley said.
Pinedale’s Ms. DeWitt said the community was missing key services, such as blood transfusions, which are often necessary when there is a trauma like a car crash or if a pregnant woman faces severe complications. Local ambulances drove 94,000 miles last year, she said.
Ms. DeWitt began working to raise support for the new hospital after her own pregnancy-related trauma in 2014. She was bleeding heavily and arrived at the local health clinic believing it operated like a hospital.
“It was shocking to hear, ‘No, we’re not a hospital. We can’t do blood transfusions. We’re just going to have to pray you live for the next 45 minutes,’ ” Ms. DeWitt said.
Ms. DeWitt had to be airlifted to Idaho, where she delivered a few minutes after landing. When the hospital financing went on the ballot in 2020, Ms. DeWitt — fully recovered, with healthy grade-schoolers at home — began making five calls a night to rally support for a county tax increase to help fund the hospital.
“By improving health care, I think we improve everybody’s chances of survival. You know, it’s pretty basic,” Ms. DeWitt said.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.
There’s a new morning ritual in Pinedale, Wyoming, a town of about 2000, nestled against the Wind River Mountains.
Friends and neighbors in the oil- and gas-rich community “take their morning coffee and pull up” to watch workers building the county’s first hospital, said Kari DeWitt, the project’s public relations director.
“I think it’s just gratitude,” Ms. DeWitt said.
Sublette County is the only one in Wyoming — where counties span thousands of square miles — without a hospital. The 10-bed, 40,000-square-foot hospital, with a similarly sized attached long-term care facility, is slated to open by the summer of 2025.
Ms. DeWitt, who also is executive director of the Sublette County Health Foundation, has an office at the town’s health clinic with a window view of the construction.
Pinedale’s residents have good reason to be excited. New full-service hospitals with inpatient beds are rare in rural America, where declining population has spurred decades of downsizing and closures. Yet, a few communities in Wyoming and others in Kansas and Georgia are defying the trend.
“To be honest with you, it even seems strange to me,” said Wyoming Hospital Association President Eric Boley. Small rural “hospitals are really struggling all across the country,” he said.
There is no official tally of new hospitals being built in rural America, but industry experts such as Mr. Boley said they’re rare. Typically, health-related construction projects in rural areas are for smaller urgent care centers or stand-alone emergency facilities or are replacements for old hospitals.
About half of rural hospitals lost money in the prior year, according to Chartis, a health analytics and consulting firm. And nearly 150 rural hospitals have closed or converted to smaller operations since 2010, according to data collected by the University of North Carolina’s Cecil G. Sheps Center for Health Services Research.
To stem the tide of closures, Congress created a new rural emergency hospital designation that allowed struggling hospitals to close their inpatient units and provide only outpatient and emergency services. Since January 2023, when the program took effect, 32 of the more than 1700 eligible rural hospitals — from Georgia to New Mexico — have joined the program, according to data from the Centers for Medicare & Medicaid Services.
Tony Breitlow is healthcare studio director for EUA, which has extensive experience working for rural health care systems. Mr. Breitlow said his national architecture and engineering firm’s work expands, replaces, or revamps older buildings, many of which were constructed during the middle of the last century.
The work, Mr. Breitlow said, is part of health care “systems figuring out how to remain robust and viable.”
Freeman Health System, based in Joplin, Missouri, announced plans last year to build a new 50-bed hospital across the state line in Kansas. Paula Baker, Freeman’s president and chief executive, said the system is building for patients in the southeastern corner of the state who travel 45 minutes or more to its bigger Joplin facilities for care.
Freeman’s new hospital, with construction on the building expected to begin in the spring, will be less than 10 miles away from an older, 64-bed hospital that has existed for decades. Kansas is one of more than a dozen states with no “certificate of need” law that would require health providers to obtain approval from the state before offering new services or building or expanding facilities.
Ms. Baker also said Freeman plans to operate emergency services and a small 10-bed outpost in Fort Scott, Kansas, opening early next year in a corner of a hospital that closed in late 2018. Residents there “cried, they cheered, they hugged me,” Ms. Baker said, adding that the “level of appreciation and gratitude that they felt and they displayed was overwhelming to me.”
Michael Topchik, executive director of the Chartis Center for Rural Health, said regional healthcare systems in the Upper Midwest have been particularly active in competing for patients by, among other things, building new hospitals.
And while private corporate money can drive construction, many rural hospital projects tap government programs, especially those supported by the US Department of Agriculture, Mr. Topchik said. That, he said, “surprises a lot of people.”
Since 2021, the USDA’s rural Community Facilities Programs have awarded $2.24 billion in loans and grants to 68 rural hospitals for work that was not related to an emergency or disaster, according to data analyzed by KFF Health News and confirmed by the agency. The federal program is funded through what is often known as the farm bill, which faces a September congressional renewal deadline.
Nearly all the projects are replacements or expansions and updates of older facilities.
The USDA confirmed that three new or planned Wyoming hospitals received federal funding. Hospital projects in Riverton and Saratoga received loans of $37.2 million and $18.3 million, respectively. Pinedale’s hospital received a $29.2 million loan from the agency.
Wyoming’s new construction is rare in a state where more than 80% of rural hospitals reported losses in the third quarter of 2023, according to Chartis. The state association’s Mr. Boley said he worries about several hospitals that have less than 10 days’ cash on hand “day and night.”
Pinedale’s project loan was approved after the community submitted a feasibility study to the USDA that included local clinics and a long-term care facility. “It’s pretty remote and right up in the mountains,” Mr. Boley said.
Pinedale’s Ms. DeWitt said the community was missing key services, such as blood transfusions, which are often necessary when there is a trauma like a car crash or if a pregnant woman faces severe complications. Local ambulances drove 94,000 miles last year, she said.
Ms. DeWitt began working to raise support for the new hospital after her own pregnancy-related trauma in 2014. She was bleeding heavily and arrived at the local health clinic believing it operated like a hospital.
“It was shocking to hear, ‘No, we’re not a hospital. We can’t do blood transfusions. We’re just going to have to pray you live for the next 45 minutes,’ ” Ms. DeWitt said.
Ms. DeWitt had to be airlifted to Idaho, where she delivered a few minutes after landing. When the hospital financing went on the ballot in 2020, Ms. DeWitt — fully recovered, with healthy grade-schoolers at home — began making five calls a night to rally support for a county tax increase to help fund the hospital.
“By improving health care, I think we improve everybody’s chances of survival. You know, it’s pretty basic,” Ms. DeWitt said.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.