User login
There are 375 species of poisonous snakes, with approximately 20,000 deaths worldwide each year due to snakebites, mostly in Asia and Africa.1 The death rate in the United States is 14 to 20 cases per year. In the United States, a variety of rattlesnakes are poisonous. There are 2 genera of rattlesnakes: Sistrurus (3 species) and Crotalus (23 species). The pygmy rattlesnake belongs to the Sistrurus miliarius species that is divided into 3 subspecies: the Carolina pigmy rattlesnake (S miliarius miliarius), the western pygmy rattlesnake (S miliarius streckeri), and the dusky pygmy rattlesnake (S miliarius barbouri).2
The western pygmy rattlesnake belongs to the Crotalidae family. The rattlesnakes in this family also are known as pit vipers. All pit vipers have common characteristics for identification: triangular head, fangs, elliptical pupils, and a heat-sensing pit between the eyes. The western pygmy rattlesnake is found in Missouri, Arkansas, Oklahoma, Kentucky, and Tennessee.1 It is small bodied (15–20 inches)3 and grayish-brown, with a brown dorsal stripe with black blotches on its back. It is found in glades, second-growth forests near rock ledges, and areas where powerlines cut through dense forest.3 Its venom is hemorrhagic, causing tissue damage, but does not contain neurotoxins.4 Bites from the western pygmy rattlesnake often do not lead to death, but the venom, which contains numerous proteins and enzymes, does cause necrotic hemorrhagic ulceration at the site of envenomation and possible loss of digit.5,6
We present a case of a man who was bitten on the right third digit by a western pygmy rattlesnake. We describe the clinical course and treatment.
Case Report
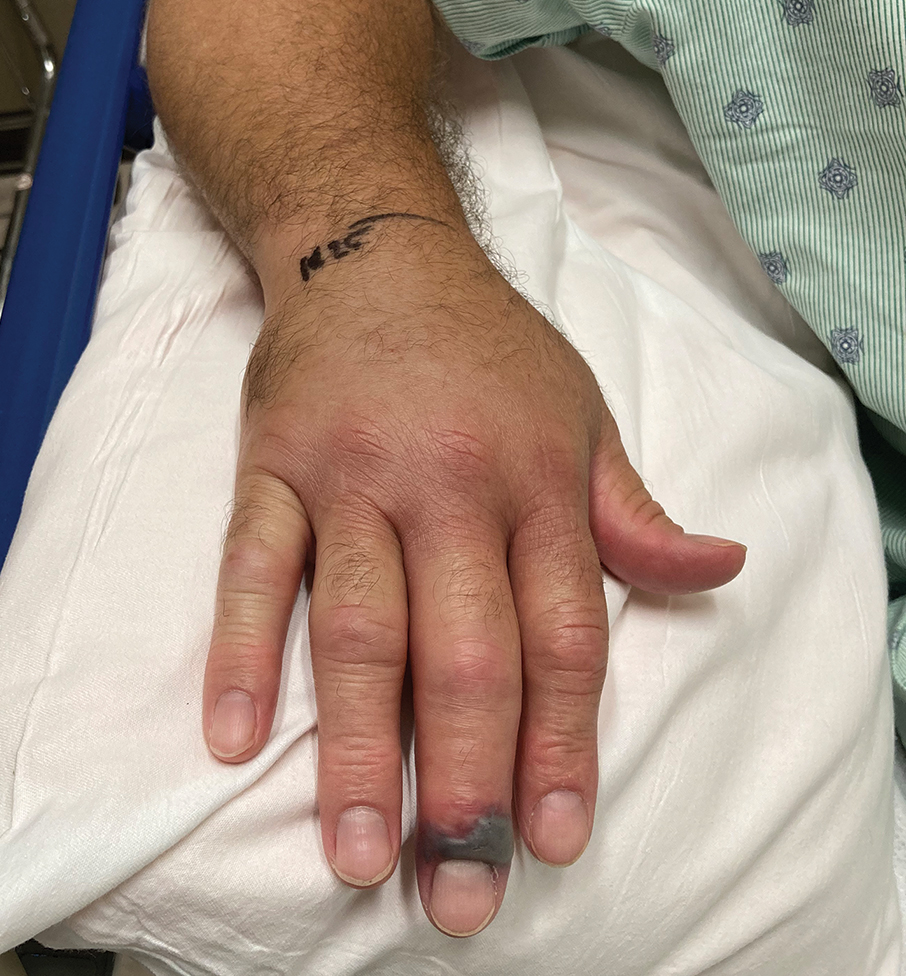
A 56-year-old right-handed man presented to the emergency department with a rapidly swelling, painful hand following a snakebite to the dorsal aspect of the right third digit (Figure 1). He was able to capture a photograph of the snake at the time of injury, which helped identify it as a western pygmy rattlesnake (Figure 2). He also photographed the hand immediately after the bite occurred (Figure 3). Vitals on presentation included an elevated blood pressure of 161/100 mm Hg; no fever (temperature, 36.4 °C); and normal pulse oximetry of 98%, pulse of 86 beats per minute, and respiratory rate of 16 breaths per minute.

After the snakebite, the patient’s family called the Missouri Poison Center immediately. The family identified the snake species and shared this information with the poison center. Poison control recommended calling the nearest hospitals to determine if antivenom was available and make notification of arrival.
The patient’s tetanus toxoid immunization was updated immediately upon arrival. The hand was marked to monitor swelling. Initial laboratory test results revealed the following values: sodium, 133 mmol/L (reference range, 136–145 mmol/L); potassium, 3.4 mmol/L (3.6–5.2 mmol/L); lactic acid, 2.4 mmol/L (0.5–2.2 mmol/L); creatine kinase, 425 U/L (55–170 U/L); platelet count, 68/µL (150,000–450,000/µL); fibrinogen, 169 mg/dL (185–410 mg/dL); and glucose, 121 mg/dL (74–106 mg/dL). The remainder of the complete blood cell count and metabolic panel was unremarkable. Radiographs of the hand did not show any fractures, dislocations, or foreign bodies. Missouri Poison Center was consulted. Given the patient’s severe pain, edema beyond 40 cm, and developing ecchymosis on the inner arm, the bite was graded as a 3 on the traditional snakebite severity scale. Poison control recommended 4 to 6 vials of antivenom over 60 minutes. Six vials of Crotalidae polyvalent immune fab antivenom were given.
The patient’s complete blood cell count remained unremarkable throughout his admission. His metabolic panel returned to normal at 6 hours postadmission: sodium, 139 mmol/L; potassium, 4.0 mmol/L. His lactate and creatinine kinase were not rechecked. His fibrinogen was trending upward. Serial laboratory test results revealed fibrinogen levels of 153, 158, 161, 159, 173, and 216 mg/dL at 6, 12, 18, 24, 30, and 36 hours, respectively. Other laboratory test results including prothrombin time (11.0 s) and international normalized ratio (0.98) remained within reference range (11–13 s and 0.80–1.39, respectively) during serial monitoring.
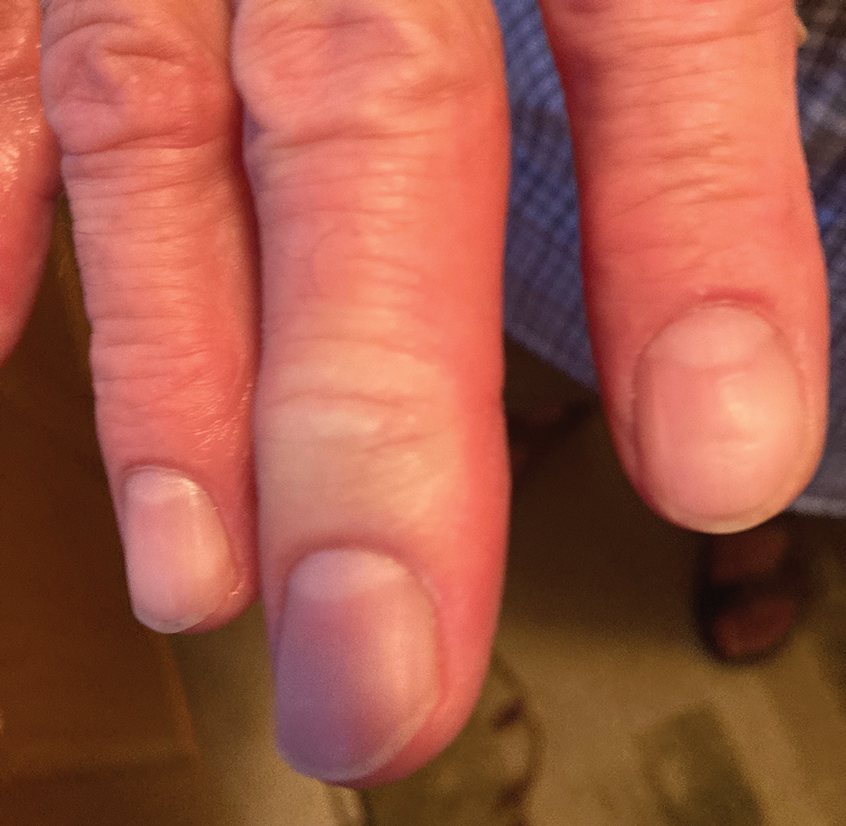
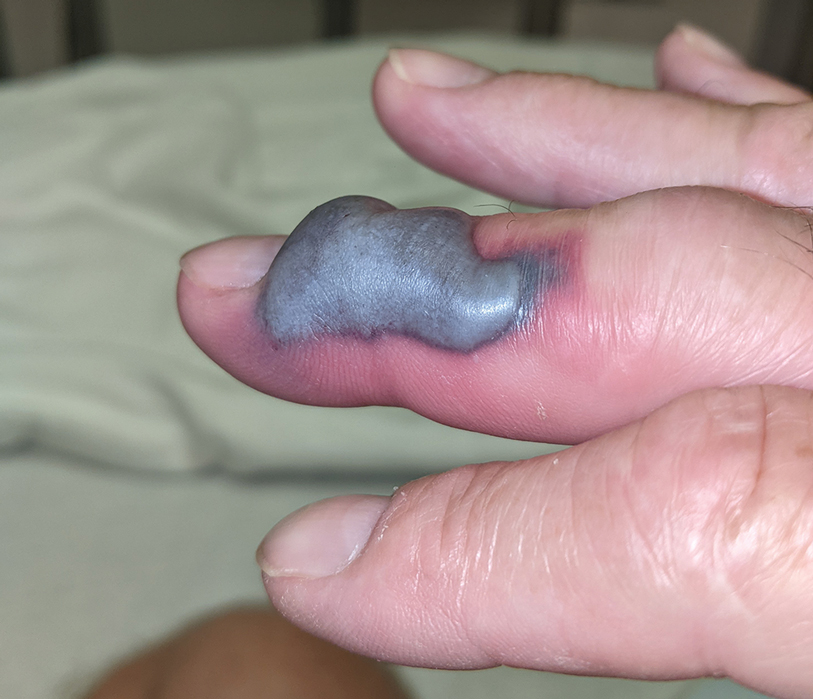
The patient was hospitalized for 40 hours while waiting for his fibrinogen level to normalize. The local skin necrosis worsened acutely in this 40-hour window (Figure 4). Intravenous antibiotics were not administered during the hospital stay. Before discharge, the patient was evaluated by the surgery service, who did not recommend debridement.
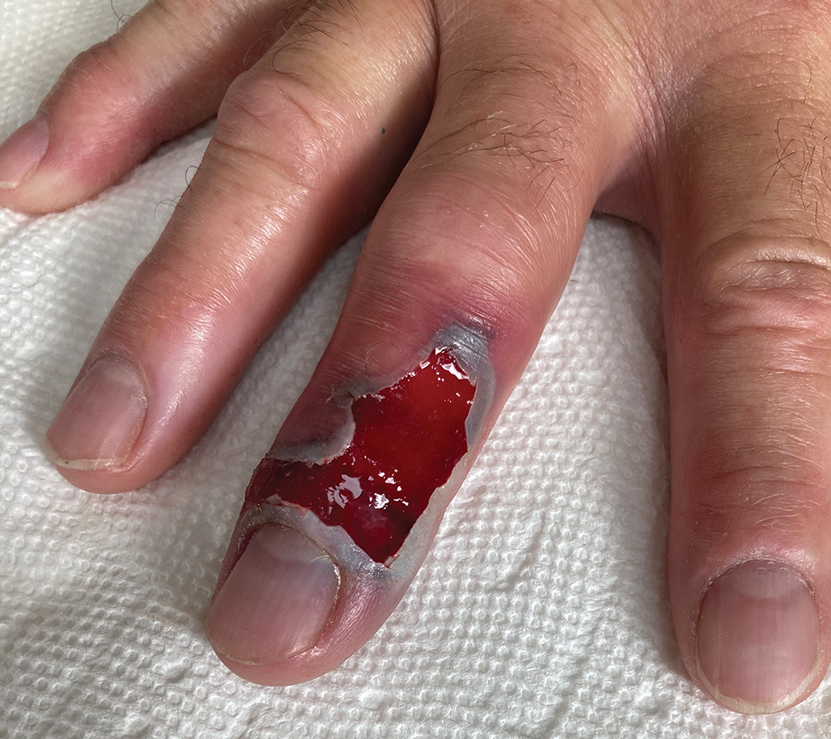
Following discharge, the patient consulted a wound care expert. The area of necrosis was unroofed and debrided in the outpatient setting (Figure 5). The patient was started on oral cefalexin 500 mg twice daily for 10 days and instructed to perform twice-daily dressing changes with silver sulfadiazine cream 1%. A hand surgeon was consulted for consideration of a reverse cross-finger flap, which was not recommended. Twice-daily dressing changes for the wound—consisting of application of silver sulfadiazine cream 1% directly to the wound followed by gauze, self-adhesive soft-rolled gauze, and elastic bandages—were performed for 2 weeks.
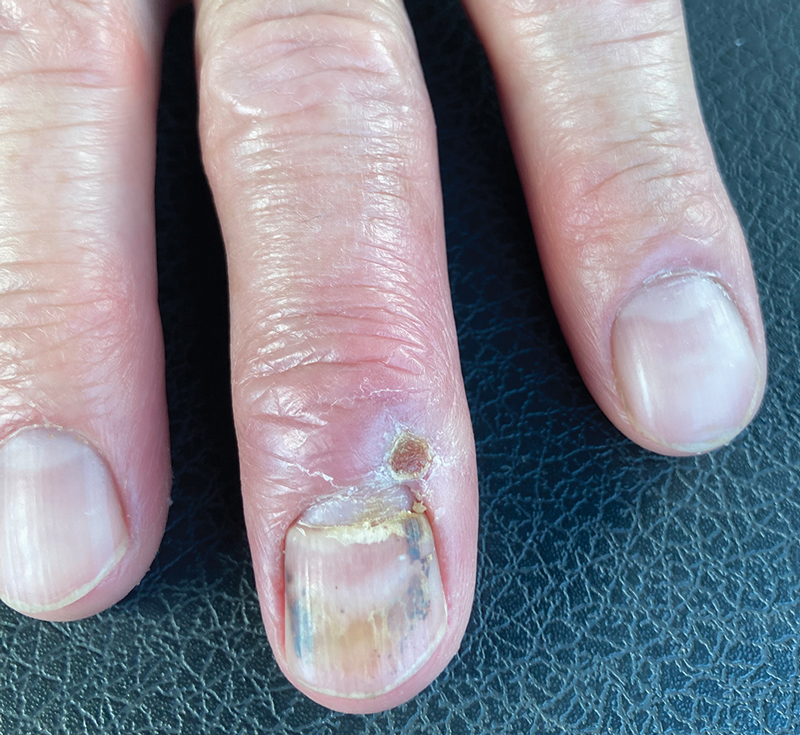
After 2 weeks, the wound was left open to the air and cleaned with soap and water as needed. At 6 weeks, the wound was completely healed via secondary intention, except for some minor remaining ulceration at the location of the fang entry point (Figure 6). The patient had no loss of finger function or sensation.
Surgical Management of Snakebites
The surgeon’s role in managing snakebites is controversial. Snakebites were once perceived as a surgical emergency due to symptoms mimicking compartment syndrome; however, snakebites rarely cause a true compartment syndrome.7 Prophylactic bite excision and fasciotomies are not recommended. Incision and suction of the fang marks may be beneficial if performed within 15 to 30 minutes from the time of the bite.8 With access to a surgeon in this short time period being nearly impossible, incision and suctioning of fang marks generally is not recommended.9 Retained snake fangs are a possibility, and the infection could spread to a nearby joint, causing septic arthritis,10 which would be an indication for surgical intervention. Bites to the finger often cause major swelling, and the benefits of dermotomy are documented.11 Generally, early administration of antivenom will decrease local tissue reaction and prevent additional tissue loss.12 In our patient, the decision to perform dermotomy was made when the area of necrosis had declared itself and the skin reached its elastic limit. Bozkurt et al13 described the neurovascular bundles within the digit as functioning as small compartments. When the skin of the digit reaches its elastic limit, pressure within the compartment may exceed the capillary closing pressure, and the integrity of small vessels and nerves may be compromised. Our case highlights the benefit of dermotomy as well as the functional and cosmetic results that can be achieved.
Wound Care for Snakebites
There is little published on the treatment of snakebites after patients are stabilized medically for hospital discharge. Venomous snakes inject toxins that predominantly consist of enzymes (eg, phospholipase A2, phosphodiesterase, hyaluronidase, peptidase, metalloproteinase) that cause tissue destruction through diverse mechanisms.14 The venom of western pygmy rattlesnakes is hemotoxic and can cause necrotic hemorrhagic ulceration,4 as was the case in our patient.
Silver sulfadiazine commonly is used to prevent infection in burn patients. Given the large surface area of exposed dermis after debridement and concern for infection, silver sulfadiazine was chosen in our patient for local wound care treatment. Silver sulfadiazine is a widely available and low-cost drug.15 Its antibacterial effects are due to the silver ions, which only act superficially and therefore limit systemic absorption.16 Application should be performed in a clean manner with minimal trauma to the tissue. This technique is best achieved by using sterile gloves and applying the medication manually. A 0.0625-inch layer should be applied to entirely cover the cleaned debrided area.17 When performing application with tongue blades or cotton swabs, it is important to never “double dip.” Patient education on proper administration is imperative to a successful outcome.
Final Thoughts
Our case demonstrates the safe use of Crotalidae polyvalent immune fab antivenom for the treatment of western pygmy rattlesnake (S miliarius streckeri) envenomation. Early administration of antivenom following pit viper rattlesnake envenomations is important to mitigate systemic effects and the extent of soft tissue damage. There are few studies on local wound care treatment after rattlesnake envenomation. This case highlights the role of dermotomy and wound care with silver sulfadiazine cream 1%.
- Biggers B. Management of Missouri snake bites. Mo Med. 2017;114:254-257.
- Stamm R. Sistrurus miliarius pigmy rattlesnake. University of Michigan Museum of Zoology. Accessed September 23, 2024. https://animaldiversity.org/accounts/Sistrurus_miliarius/
- Missouri Department of Conservation. Western pygmy rattlesnake. Accessed September 18, 2024. https://mdc.mo.gov/discover-nature/field-guide/western-pygmy-rattlesnake
- AnimalSake. Facts about the pigmy rattlesnake that are sure to surprise you. Accessed September 18, 2024. https://animalsake.com/pygmy-rattlesnake
- King AM, Crim WS, Menke NB, et al. Pygmy rattlesnake envenomation treated with crotalidae polyvalent immune fab antivenom. Toxicon. 2012;60:1287-1289.
- Juckett G, Hancox JG. Venomous snakebites in the United States: management review and update. Am Fam Physician. 2002;65:1367-1375.
- Toschlog EA, Bauer CR, Hall EL, et al. Surgical considerations in the management of pit viper snake envenomation. J Am Coll Surg. 2013;217:726-735.
- Cribari C. Management of poisonous snakebite. American College of Surgeons Committee on Trauma; 2004. https://www.hartcountyga.gov/documents/PoisonousSnakebiteTreatment.pdf
- Walker JP, Morrison RL. Current management of copperhead snakebite. J Am Coll Surg. 2011;212:470-474.
- Gelman D, Bates T, Nuelle JAV. Septic arthritis of the proximal interphalangeal joint after rattlesnake bite. J Hand Surg Am. 2022;47:484.e1-484.e4.
- Watt CH Jr. Treatment of poisonous snakebite with emphasis on digit dermotomy. South Med J. 1985;78:694-699.
- Corneille MG, Larson S, Stewart RM, et al. A large single-center experience with treatment of patients with crotalid envenomations: outcomes with and evolution of antivenin therapy. Am J Surg. 2006;192:848-852.
- Bozkurt M, Kulahci Y, Zor F, et al. The management of pit viper envenomation of the hand. Hand (NY). 2008;3:324-331.
- Aziz H, Rhee P, Pandit V, et al. The current concepts in management of animal (dog, cat, snake, scorpion) and human bite wounds. J Trauma Acute Care Surg. 2015;78:641-648.
- Hummel RP, MacMillan BG, Altemeier WA. Topical and systemic antibacterial agents in the treatment of burns. Ann Surg. 1970;172:370-384.
- Modak SM, Sampath L, Fox CL. Combined topical use of silver sulfadiazine and antibiotics as a possible solution to bacterial resistance in burn wounds. J Burn Care Rehabil. 1988;9:359-363.
- Oaks RJ, Cindass R. Silver sulfadiazine. StatPearls [Internet]. Updated January 22, 2023. Accessed September 23, 2024. https://www.ncbi.nlm.nih.gov/books/NBK556054/
There are 375 species of poisonous snakes, with approximately 20,000 deaths worldwide each year due to snakebites, mostly in Asia and Africa.1 The death rate in the United States is 14 to 20 cases per year. In the United States, a variety of rattlesnakes are poisonous. There are 2 genera of rattlesnakes: Sistrurus (3 species) and Crotalus (23 species). The pygmy rattlesnake belongs to the Sistrurus miliarius species that is divided into 3 subspecies: the Carolina pigmy rattlesnake (S miliarius miliarius), the western pygmy rattlesnake (S miliarius streckeri), and the dusky pygmy rattlesnake (S miliarius barbouri).2
The western pygmy rattlesnake belongs to the Crotalidae family. The rattlesnakes in this family also are known as pit vipers. All pit vipers have common characteristics for identification: triangular head, fangs, elliptical pupils, and a heat-sensing pit between the eyes. The western pygmy rattlesnake is found in Missouri, Arkansas, Oklahoma, Kentucky, and Tennessee.1 It is small bodied (15–20 inches)3 and grayish-brown, with a brown dorsal stripe with black blotches on its back. It is found in glades, second-growth forests near rock ledges, and areas where powerlines cut through dense forest.3 Its venom is hemorrhagic, causing tissue damage, but does not contain neurotoxins.4 Bites from the western pygmy rattlesnake often do not lead to death, but the venom, which contains numerous proteins and enzymes, does cause necrotic hemorrhagic ulceration at the site of envenomation and possible loss of digit.5,6
We present a case of a man who was bitten on the right third digit by a western pygmy rattlesnake. We describe the clinical course and treatment.
Case Report
A 56-year-old right-handed man presented to the emergency department with a rapidly swelling, painful hand following a snakebite to the dorsal aspect of the right third digit (Figure 1). He was able to capture a photograph of the snake at the time of injury, which helped identify it as a western pygmy rattlesnake (Figure 2). He also photographed the hand immediately after the bite occurred (Figure 3). Vitals on presentation included an elevated blood pressure of 161/100 mm Hg; no fever (temperature, 36.4 °C); and normal pulse oximetry of 98%, pulse of 86 beats per minute, and respiratory rate of 16 breaths per minute.



After the snakebite, the patient’s family called the Missouri Poison Center immediately. The family identified the snake species and shared this information with the poison center. Poison control recommended calling the nearest hospitals to determine if antivenom was available and make notification of arrival.
The patient’s tetanus toxoid immunization was updated immediately upon arrival. The hand was marked to monitor swelling. Initial laboratory test results revealed the following values: sodium, 133 mmol/L (reference range, 136–145 mmol/L); potassium, 3.4 mmol/L (3.6–5.2 mmol/L); lactic acid, 2.4 mmol/L (0.5–2.2 mmol/L); creatine kinase, 425 U/L (55–170 U/L); platelet count, 68/µL (150,000–450,000/µL); fibrinogen, 169 mg/dL (185–410 mg/dL); and glucose, 121 mg/dL (74–106 mg/dL). The remainder of the complete blood cell count and metabolic panel was unremarkable. Radiographs of the hand did not show any fractures, dislocations, or foreign bodies. Missouri Poison Center was consulted. Given the patient’s severe pain, edema beyond 40 cm, and developing ecchymosis on the inner arm, the bite was graded as a 3 on the traditional snakebite severity scale. Poison control recommended 4 to 6 vials of antivenom over 60 minutes. Six vials of Crotalidae polyvalent immune fab antivenom were given.
The patient’s complete blood cell count remained unremarkable throughout his admission. His metabolic panel returned to normal at 6 hours postadmission: sodium, 139 mmol/L; potassium, 4.0 mmol/L. His lactate and creatinine kinase were not rechecked. His fibrinogen was trending upward. Serial laboratory test results revealed fibrinogen levels of 153, 158, 161, 159, 173, and 216 mg/dL at 6, 12, 18, 24, 30, and 36 hours, respectively. Other laboratory test results including prothrombin time (11.0 s) and international normalized ratio (0.98) remained within reference range (11–13 s and 0.80–1.39, respectively) during serial monitoring.
The patient was hospitalized for 40 hours while waiting for his fibrinogen level to normalize. The local skin necrosis worsened acutely in this 40-hour window (Figure 4). Intravenous antibiotics were not administered during the hospital stay. Before discharge, the patient was evaluated by the surgery service, who did not recommend debridement.

Following discharge, the patient consulted a wound care expert. The area of necrosis was unroofed and debrided in the outpatient setting (Figure 5). The patient was started on oral cefalexin 500 mg twice daily for 10 days and instructed to perform twice-daily dressing changes with silver sulfadiazine cream 1%. A hand surgeon was consulted for consideration of a reverse cross-finger flap, which was not recommended. Twice-daily dressing changes for the wound—consisting of application of silver sulfadiazine cream 1% directly to the wound followed by gauze, self-adhesive soft-rolled gauze, and elastic bandages—were performed for 2 weeks.

After 2 weeks, the wound was left open to the air and cleaned with soap and water as needed. At 6 weeks, the wound was completely healed via secondary intention, except for some minor remaining ulceration at the location of the fang entry point (Figure 6). The patient had no loss of finger function or sensation.

Surgical Management of Snakebites
The surgeon’s role in managing snakebites is controversial. Snakebites were once perceived as a surgical emergency due to symptoms mimicking compartment syndrome; however, snakebites rarely cause a true compartment syndrome.7 Prophylactic bite excision and fasciotomies are not recommended. Incision and suction of the fang marks may be beneficial if performed within 15 to 30 minutes from the time of the bite.8 With access to a surgeon in this short time period being nearly impossible, incision and suctioning of fang marks generally is not recommended.9 Retained snake fangs are a possibility, and the infection could spread to a nearby joint, causing septic arthritis,10 which would be an indication for surgical intervention. Bites to the finger often cause major swelling, and the benefits of dermotomy are documented.11 Generally, early administration of antivenom will decrease local tissue reaction and prevent additional tissue loss.12 In our patient, the decision to perform dermotomy was made when the area of necrosis had declared itself and the skin reached its elastic limit. Bozkurt et al13 described the neurovascular bundles within the digit as functioning as small compartments. When the skin of the digit reaches its elastic limit, pressure within the compartment may exceed the capillary closing pressure, and the integrity of small vessels and nerves may be compromised. Our case highlights the benefit of dermotomy as well as the functional and cosmetic results that can be achieved.
Wound Care for Snakebites
There is little published on the treatment of snakebites after patients are stabilized medically for hospital discharge. Venomous snakes inject toxins that predominantly consist of enzymes (eg, phospholipase A2, phosphodiesterase, hyaluronidase, peptidase, metalloproteinase) that cause tissue destruction through diverse mechanisms.14 The venom of western pygmy rattlesnakes is hemotoxic and can cause necrotic hemorrhagic ulceration,4 as was the case in our patient.
Silver sulfadiazine commonly is used to prevent infection in burn patients. Given the large surface area of exposed dermis after debridement and concern for infection, silver sulfadiazine was chosen in our patient for local wound care treatment. Silver sulfadiazine is a widely available and low-cost drug.15 Its antibacterial effects are due to the silver ions, which only act superficially and therefore limit systemic absorption.16 Application should be performed in a clean manner with minimal trauma to the tissue. This technique is best achieved by using sterile gloves and applying the medication manually. A 0.0625-inch layer should be applied to entirely cover the cleaned debrided area.17 When performing application with tongue blades or cotton swabs, it is important to never “double dip.” Patient education on proper administration is imperative to a successful outcome.
Final Thoughts
Our case demonstrates the safe use of Crotalidae polyvalent immune fab antivenom for the treatment of western pygmy rattlesnake (S miliarius streckeri) envenomation. Early administration of antivenom following pit viper rattlesnake envenomations is important to mitigate systemic effects and the extent of soft tissue damage. There are few studies on local wound care treatment after rattlesnake envenomation. This case highlights the role of dermotomy and wound care with silver sulfadiazine cream 1%.
There are 375 species of poisonous snakes, with approximately 20,000 deaths worldwide each year due to snakebites, mostly in Asia and Africa.1 The death rate in the United States is 14 to 20 cases per year. In the United States, a variety of rattlesnakes are poisonous. There are 2 genera of rattlesnakes: Sistrurus (3 species) and Crotalus (23 species). The pygmy rattlesnake belongs to the Sistrurus miliarius species that is divided into 3 subspecies: the Carolina pigmy rattlesnake (S miliarius miliarius), the western pygmy rattlesnake (S miliarius streckeri), and the dusky pygmy rattlesnake (S miliarius barbouri).2
The western pygmy rattlesnake belongs to the Crotalidae family. The rattlesnakes in this family also are known as pit vipers. All pit vipers have common characteristics for identification: triangular head, fangs, elliptical pupils, and a heat-sensing pit between the eyes. The western pygmy rattlesnake is found in Missouri, Arkansas, Oklahoma, Kentucky, and Tennessee.1 It is small bodied (15–20 inches)3 and grayish-brown, with a brown dorsal stripe with black blotches on its back. It is found in glades, second-growth forests near rock ledges, and areas where powerlines cut through dense forest.3 Its venom is hemorrhagic, causing tissue damage, but does not contain neurotoxins.4 Bites from the western pygmy rattlesnake often do not lead to death, but the venom, which contains numerous proteins and enzymes, does cause necrotic hemorrhagic ulceration at the site of envenomation and possible loss of digit.5,6
We present a case of a man who was bitten on the right third digit by a western pygmy rattlesnake. We describe the clinical course and treatment.
Case Report
A 56-year-old right-handed man presented to the emergency department with a rapidly swelling, painful hand following a snakebite to the dorsal aspect of the right third digit (Figure 1). He was able to capture a photograph of the snake at the time of injury, which helped identify it as a western pygmy rattlesnake (Figure 2). He also photographed the hand immediately after the bite occurred (Figure 3). Vitals on presentation included an elevated blood pressure of 161/100 mm Hg; no fever (temperature, 36.4 °C); and normal pulse oximetry of 98%, pulse of 86 beats per minute, and respiratory rate of 16 breaths per minute.



After the snakebite, the patient’s family called the Missouri Poison Center immediately. The family identified the snake species and shared this information with the poison center. Poison control recommended calling the nearest hospitals to determine if antivenom was available and make notification of arrival.
The patient’s tetanus toxoid immunization was updated immediately upon arrival. The hand was marked to monitor swelling. Initial laboratory test results revealed the following values: sodium, 133 mmol/L (reference range, 136–145 mmol/L); potassium, 3.4 mmol/L (3.6–5.2 mmol/L); lactic acid, 2.4 mmol/L (0.5–2.2 mmol/L); creatine kinase, 425 U/L (55–170 U/L); platelet count, 68/µL (150,000–450,000/µL); fibrinogen, 169 mg/dL (185–410 mg/dL); and glucose, 121 mg/dL (74–106 mg/dL). The remainder of the complete blood cell count and metabolic panel was unremarkable. Radiographs of the hand did not show any fractures, dislocations, or foreign bodies. Missouri Poison Center was consulted. Given the patient’s severe pain, edema beyond 40 cm, and developing ecchymosis on the inner arm, the bite was graded as a 3 on the traditional snakebite severity scale. Poison control recommended 4 to 6 vials of antivenom over 60 minutes. Six vials of Crotalidae polyvalent immune fab antivenom were given.
The patient’s complete blood cell count remained unremarkable throughout his admission. His metabolic panel returned to normal at 6 hours postadmission: sodium, 139 mmol/L; potassium, 4.0 mmol/L. His lactate and creatinine kinase were not rechecked. His fibrinogen was trending upward. Serial laboratory test results revealed fibrinogen levels of 153, 158, 161, 159, 173, and 216 mg/dL at 6, 12, 18, 24, 30, and 36 hours, respectively. Other laboratory test results including prothrombin time (11.0 s) and international normalized ratio (0.98) remained within reference range (11–13 s and 0.80–1.39, respectively) during serial monitoring.
The patient was hospitalized for 40 hours while waiting for his fibrinogen level to normalize. The local skin necrosis worsened acutely in this 40-hour window (Figure 4). Intravenous antibiotics were not administered during the hospital stay. Before discharge, the patient was evaluated by the surgery service, who did not recommend debridement.

Following discharge, the patient consulted a wound care expert. The area of necrosis was unroofed and debrided in the outpatient setting (Figure 5). The patient was started on oral cefalexin 500 mg twice daily for 10 days and instructed to perform twice-daily dressing changes with silver sulfadiazine cream 1%. A hand surgeon was consulted for consideration of a reverse cross-finger flap, which was not recommended. Twice-daily dressing changes for the wound—consisting of application of silver sulfadiazine cream 1% directly to the wound followed by gauze, self-adhesive soft-rolled gauze, and elastic bandages—were performed for 2 weeks.

After 2 weeks, the wound was left open to the air and cleaned with soap and water as needed. At 6 weeks, the wound was completely healed via secondary intention, except for some minor remaining ulceration at the location of the fang entry point (Figure 6). The patient had no loss of finger function or sensation.

Surgical Management of Snakebites
The surgeon’s role in managing snakebites is controversial. Snakebites were once perceived as a surgical emergency due to symptoms mimicking compartment syndrome; however, snakebites rarely cause a true compartment syndrome.7 Prophylactic bite excision and fasciotomies are not recommended. Incision and suction of the fang marks may be beneficial if performed within 15 to 30 minutes from the time of the bite.8 With access to a surgeon in this short time period being nearly impossible, incision and suctioning of fang marks generally is not recommended.9 Retained snake fangs are a possibility, and the infection could spread to a nearby joint, causing septic arthritis,10 which would be an indication for surgical intervention. Bites to the finger often cause major swelling, and the benefits of dermotomy are documented.11 Generally, early administration of antivenom will decrease local tissue reaction and prevent additional tissue loss.12 In our patient, the decision to perform dermotomy was made when the area of necrosis had declared itself and the skin reached its elastic limit. Bozkurt et al13 described the neurovascular bundles within the digit as functioning as small compartments. When the skin of the digit reaches its elastic limit, pressure within the compartment may exceed the capillary closing pressure, and the integrity of small vessels and nerves may be compromised. Our case highlights the benefit of dermotomy as well as the functional and cosmetic results that can be achieved.
Wound Care for Snakebites
There is little published on the treatment of snakebites after patients are stabilized medically for hospital discharge. Venomous snakes inject toxins that predominantly consist of enzymes (eg, phospholipase A2, phosphodiesterase, hyaluronidase, peptidase, metalloproteinase) that cause tissue destruction through diverse mechanisms.14 The venom of western pygmy rattlesnakes is hemotoxic and can cause necrotic hemorrhagic ulceration,4 as was the case in our patient.
Silver sulfadiazine commonly is used to prevent infection in burn patients. Given the large surface area of exposed dermis after debridement and concern for infection, silver sulfadiazine was chosen in our patient for local wound care treatment. Silver sulfadiazine is a widely available and low-cost drug.15 Its antibacterial effects are due to the silver ions, which only act superficially and therefore limit systemic absorption.16 Application should be performed in a clean manner with minimal trauma to the tissue. This technique is best achieved by using sterile gloves and applying the medication manually. A 0.0625-inch layer should be applied to entirely cover the cleaned debrided area.17 When performing application with tongue blades or cotton swabs, it is important to never “double dip.” Patient education on proper administration is imperative to a successful outcome.
Final Thoughts
Our case demonstrates the safe use of Crotalidae polyvalent immune fab antivenom for the treatment of western pygmy rattlesnake (S miliarius streckeri) envenomation. Early administration of antivenom following pit viper rattlesnake envenomations is important to mitigate systemic effects and the extent of soft tissue damage. There are few studies on local wound care treatment after rattlesnake envenomation. This case highlights the role of dermotomy and wound care with silver sulfadiazine cream 1%.
- Biggers B. Management of Missouri snake bites. Mo Med. 2017;114:254-257.
- Stamm R. Sistrurus miliarius pigmy rattlesnake. University of Michigan Museum of Zoology. Accessed September 23, 2024. https://animaldiversity.org/accounts/Sistrurus_miliarius/
- Missouri Department of Conservation. Western pygmy rattlesnake. Accessed September 18, 2024. https://mdc.mo.gov/discover-nature/field-guide/western-pygmy-rattlesnake
- AnimalSake. Facts about the pigmy rattlesnake that are sure to surprise you. Accessed September 18, 2024. https://animalsake.com/pygmy-rattlesnake
- King AM, Crim WS, Menke NB, et al. Pygmy rattlesnake envenomation treated with crotalidae polyvalent immune fab antivenom. Toxicon. 2012;60:1287-1289.
- Juckett G, Hancox JG. Venomous snakebites in the United States: management review and update. Am Fam Physician. 2002;65:1367-1375.
- Toschlog EA, Bauer CR, Hall EL, et al. Surgical considerations in the management of pit viper snake envenomation. J Am Coll Surg. 2013;217:726-735.
- Cribari C. Management of poisonous snakebite. American College of Surgeons Committee on Trauma; 2004. https://www.hartcountyga.gov/documents/PoisonousSnakebiteTreatment.pdf
- Walker JP, Morrison RL. Current management of copperhead snakebite. J Am Coll Surg. 2011;212:470-474.
- Gelman D, Bates T, Nuelle JAV. Septic arthritis of the proximal interphalangeal joint after rattlesnake bite. J Hand Surg Am. 2022;47:484.e1-484.e4.
- Watt CH Jr. Treatment of poisonous snakebite with emphasis on digit dermotomy. South Med J. 1985;78:694-699.
- Corneille MG, Larson S, Stewart RM, et al. A large single-center experience with treatment of patients with crotalid envenomations: outcomes with and evolution of antivenin therapy. Am J Surg. 2006;192:848-852.
- Bozkurt M, Kulahci Y, Zor F, et al. The management of pit viper envenomation of the hand. Hand (NY). 2008;3:324-331.
- Aziz H, Rhee P, Pandit V, et al. The current concepts in management of animal (dog, cat, snake, scorpion) and human bite wounds. J Trauma Acute Care Surg. 2015;78:641-648.
- Hummel RP, MacMillan BG, Altemeier WA. Topical and systemic antibacterial agents in the treatment of burns. Ann Surg. 1970;172:370-384.
- Modak SM, Sampath L, Fox CL. Combined topical use of silver sulfadiazine and antibiotics as a possible solution to bacterial resistance in burn wounds. J Burn Care Rehabil. 1988;9:359-363.
- Oaks RJ, Cindass R. Silver sulfadiazine. StatPearls [Internet]. Updated January 22, 2023. Accessed September 23, 2024. https://www.ncbi.nlm.nih.gov/books/NBK556054/
- Biggers B. Management of Missouri snake bites. Mo Med. 2017;114:254-257.
- Stamm R. Sistrurus miliarius pigmy rattlesnake. University of Michigan Museum of Zoology. Accessed September 23, 2024. https://animaldiversity.org/accounts/Sistrurus_miliarius/
- Missouri Department of Conservation. Western pygmy rattlesnake. Accessed September 18, 2024. https://mdc.mo.gov/discover-nature/field-guide/western-pygmy-rattlesnake
- AnimalSake. Facts about the pigmy rattlesnake that are sure to surprise you. Accessed September 18, 2024. https://animalsake.com/pygmy-rattlesnake
- King AM, Crim WS, Menke NB, et al. Pygmy rattlesnake envenomation treated with crotalidae polyvalent immune fab antivenom. Toxicon. 2012;60:1287-1289.
- Juckett G, Hancox JG. Venomous snakebites in the United States: management review and update. Am Fam Physician. 2002;65:1367-1375.
- Toschlog EA, Bauer CR, Hall EL, et al. Surgical considerations in the management of pit viper snake envenomation. J Am Coll Surg. 2013;217:726-735.
- Cribari C. Management of poisonous snakebite. American College of Surgeons Committee on Trauma; 2004. https://www.hartcountyga.gov/documents/PoisonousSnakebiteTreatment.pdf
- Walker JP, Morrison RL. Current management of copperhead snakebite. J Am Coll Surg. 2011;212:470-474.
- Gelman D, Bates T, Nuelle JAV. Septic arthritis of the proximal interphalangeal joint after rattlesnake bite. J Hand Surg Am. 2022;47:484.e1-484.e4.
- Watt CH Jr. Treatment of poisonous snakebite with emphasis on digit dermotomy. South Med J. 1985;78:694-699.
- Corneille MG, Larson S, Stewart RM, et al. A large single-center experience with treatment of patients with crotalid envenomations: outcomes with and evolution of antivenin therapy. Am J Surg. 2006;192:848-852.
- Bozkurt M, Kulahci Y, Zor F, et al. The management of pit viper envenomation of the hand. Hand (NY). 2008;3:324-331.
- Aziz H, Rhee P, Pandit V, et al. The current concepts in management of animal (dog, cat, snake, scorpion) and human bite wounds. J Trauma Acute Care Surg. 2015;78:641-648.
- Hummel RP, MacMillan BG, Altemeier WA. Topical and systemic antibacterial agents in the treatment of burns. Ann Surg. 1970;172:370-384.
- Modak SM, Sampath L, Fox CL. Combined topical use of silver sulfadiazine and antibiotics as a possible solution to bacterial resistance in burn wounds. J Burn Care Rehabil. 1988;9:359-363.
- Oaks RJ, Cindass R. Silver sulfadiazine. StatPearls [Internet]. Updated January 22, 2023. Accessed September 23, 2024. https://www.ncbi.nlm.nih.gov/books/NBK556054/
Practice Points
- Patients should seek medical attention immediately for western pygmy rattlesnake bites for early initiation of antivenom treatment.
- Contact the closest emergency department to confirm they are equipped to treat rattlesnake bites and notify them of a pending arrival.
- Consider dermotomy or local debridement of bites involving the digits.
- Monitor the wound in the days and weeks following the bite to ensure adequate healing.