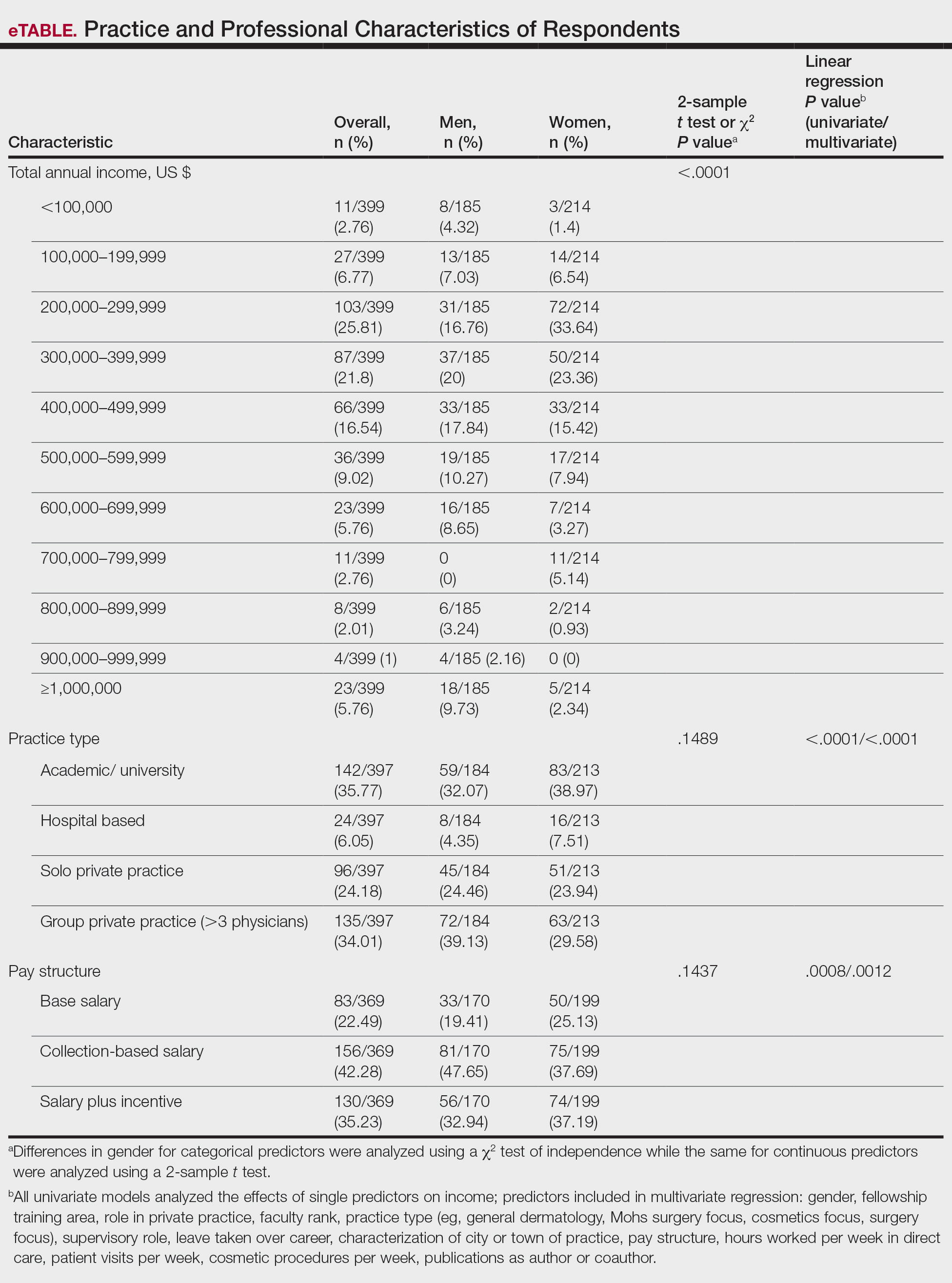
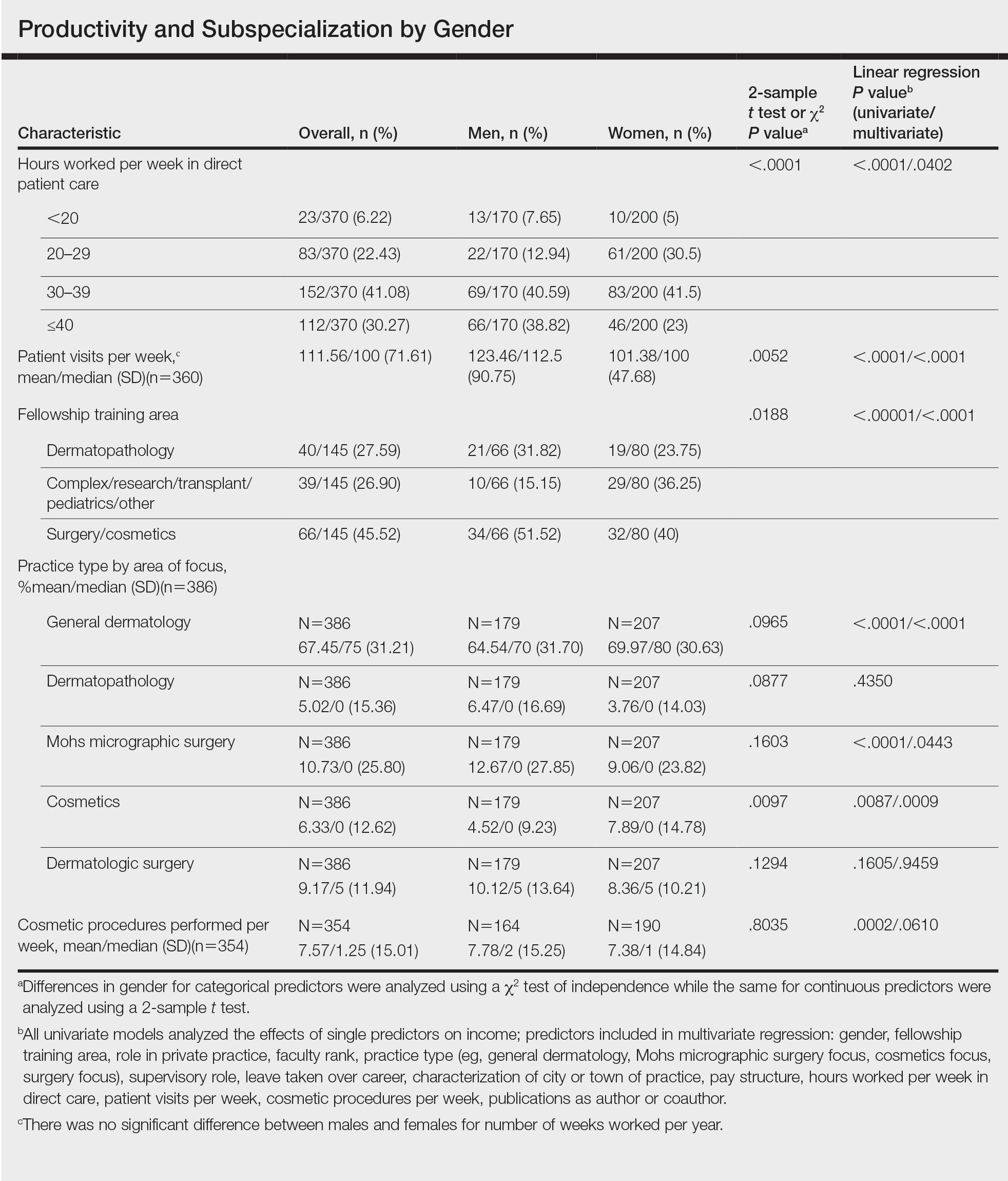
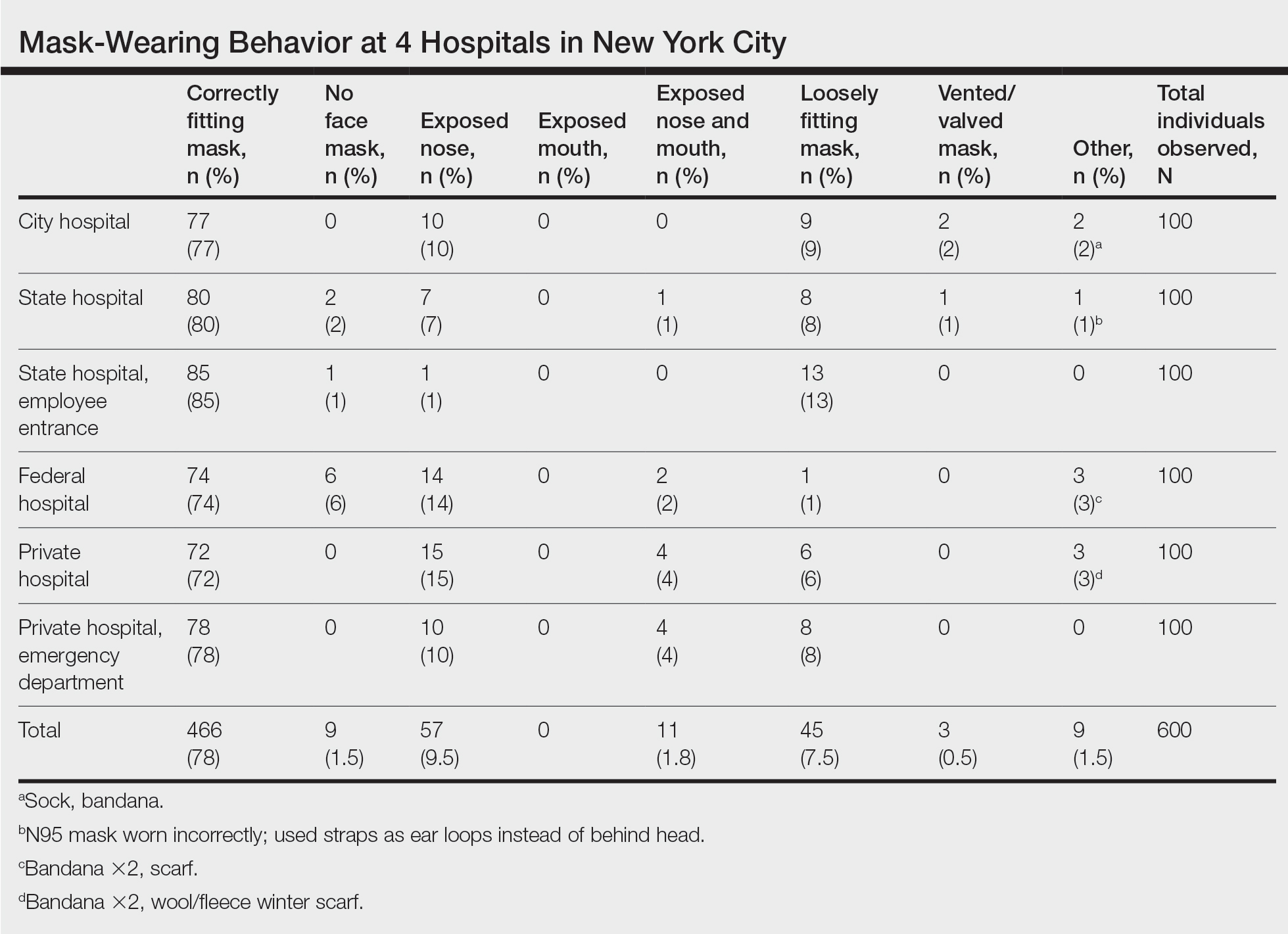
User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
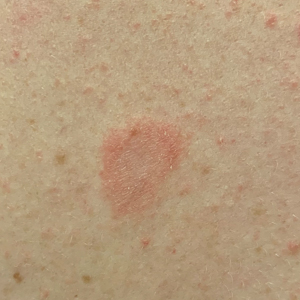
Tender Subcutaneous Nodule in a Prepubescent Boy
The Diagnosis: Dermatomyofibroma
Dermatomyofibroma is an uncommon, benign, cutaneous mesenchymal neoplasm composed of fibroblasts and myofibroblasts.1-3 This skin tumor was first described in 1991 by Hugel4 in the German literature as plaquelike fibromatosis. Pediatric dermatomyofibromas are exceedingly rare, with pediatric patients ranging in age from infants to teenagers.1
Clinically, dermatomyofibromas appear as long-standing, isolated, ill-demarcated, flesh-colored, slightly hyperpigmented or erythematous nodules or plaques that may be raised or indurated.1 Dermatomyofibromas may present with constant mild pain or pruritus, though in most cases the lesions are asymptomatic.1,3 The clinical presentation of dermatomyofibroma has a few distinct differences in children compared to adults. In adulthood, dermatomyofibroma has a strong female predominance and most commonly is located on the shoulder and adjacent upper body regions, including the axilla, neck, upper arm, and upper trunk.1-3 In childhood, the majority of dermatomyofibromas occur in young boys and usually are located on the neck with other upper body regions occurring less frequently.1,2 A shared characteristic includes the tendency for dermatomyofibromas to have an initial period of enlargement followed by stabilization or slow growth.1 Reported pediatric lesions have ranged in size from 4 to 60 mm with an average size of 14.9 mm (median, 12 mm).2
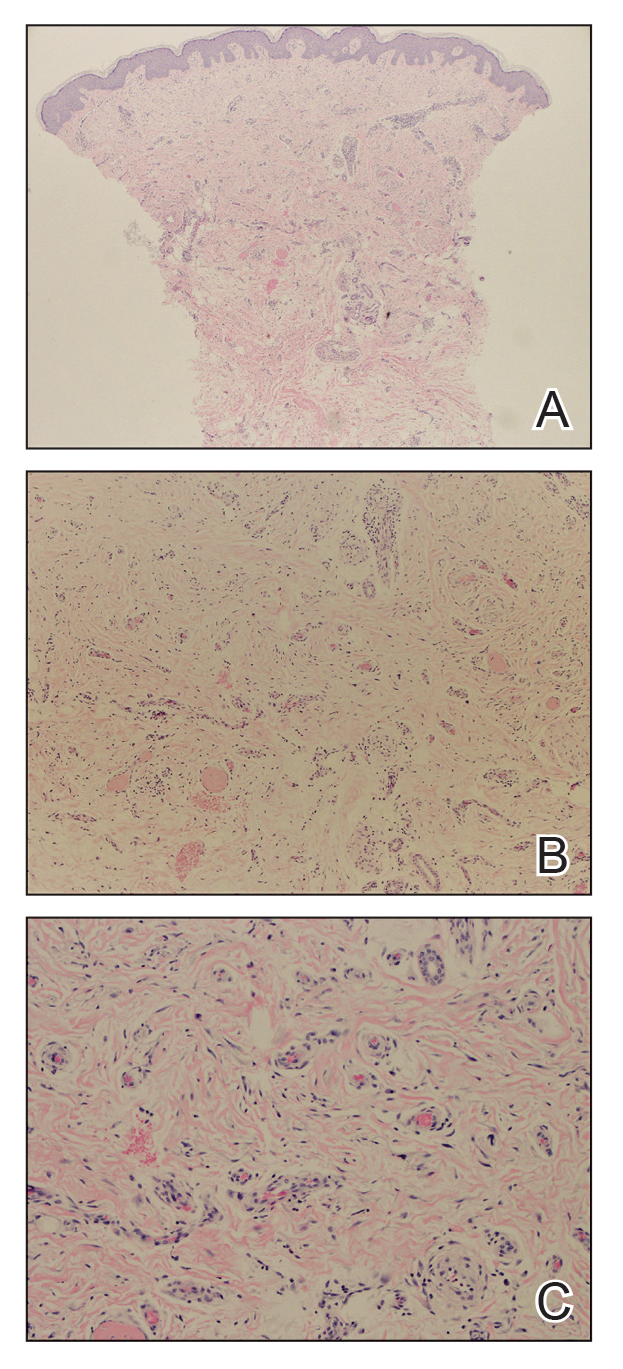
The diagnosis of dermatomyofibroma is based on histopathologic features in addition to clinical presentation. Histology from punch biopsy usually reveals a noninvasive dermal proliferation of bland, uniform, slender spindle cells oriented parallel to the overlying epidermis with increased and fragmented elastic fibers.1,3 Infiltration into the mid or deep dermis is common. The adnexal structures usually are spared; the stroma contains collagen and increased small blood vessels; and there typically is no inflammatory infiltrate, except for occasional scattered mast cells.2 Cytologically, the monomorphic spindleshaped tumor cells have an ill-defined, pale, eosinophilic cytoplasm and nuclei that are elongated with tapered edges.3 Dermatomyofibroma has a variable immunohistochemical profile, as it may stain focally positive for CD34 or smooth muscle actin, with occasional staining of factor XIIIa, desmin, calponin, or vimentin.1-3 Normal to increased levels of often fragmented elastic fibers is a helpful clue in distinguishing dermatomyofibroma from dermatofibroma, hypertrophic scar, dermatofibrosarcoma protuberans, and pilar leiomyoma, in which elastic fibers typically are reduced.3 Differential diagnoses of mesenchymal tumors in children include desmoid fibromatosis, connective tissue nevus, myofibromatosis, and smooth muscle hamartoma.1
A punch biopsy with clinical observation and followup is recommended for the management of lesions in cosmetically sensitive areas or in very young children who may not tolerate surgery. In symptomatic or cosmetically unappealing cases of dermatomyofibroma, simple surgical excision remains a viable treatment option. Recurrence is uncommon, even if only partially excised, and no instances of metastasis have been reported.1-5
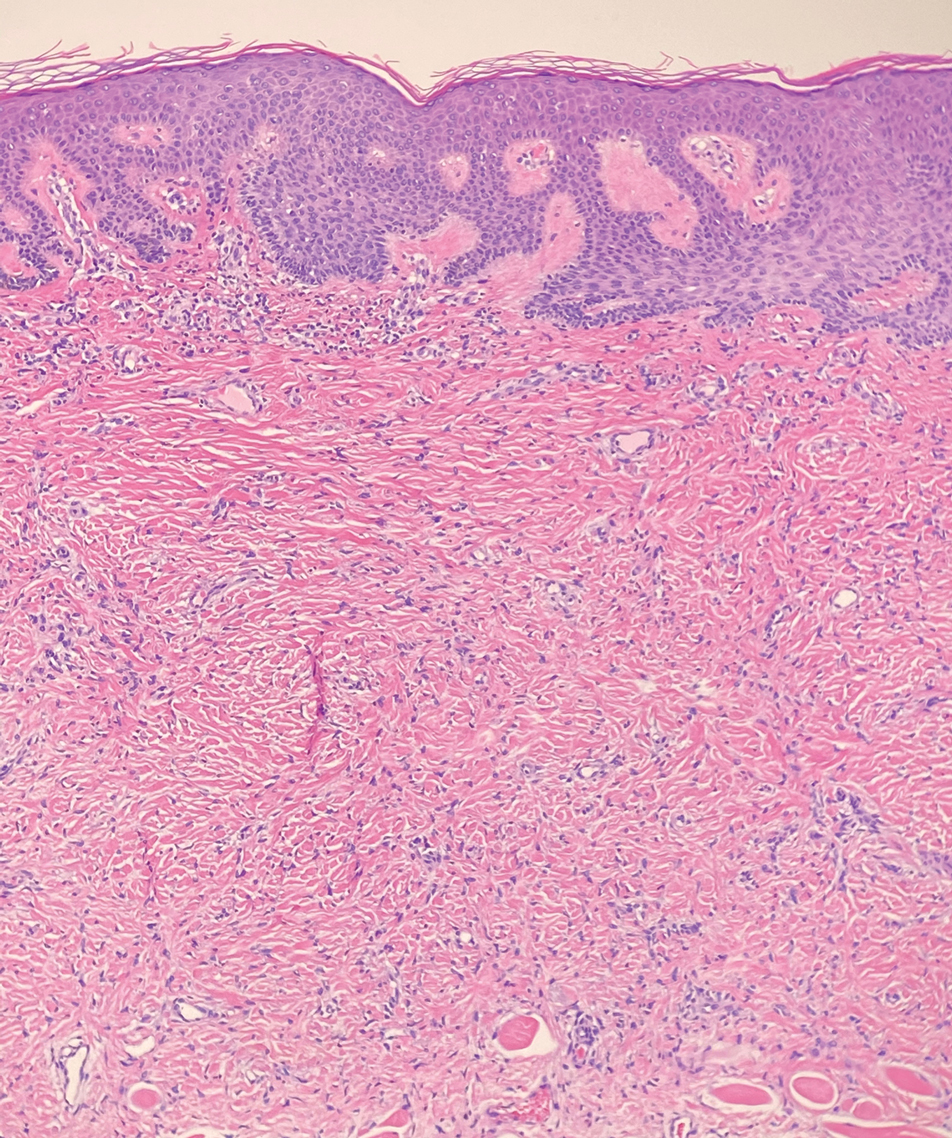
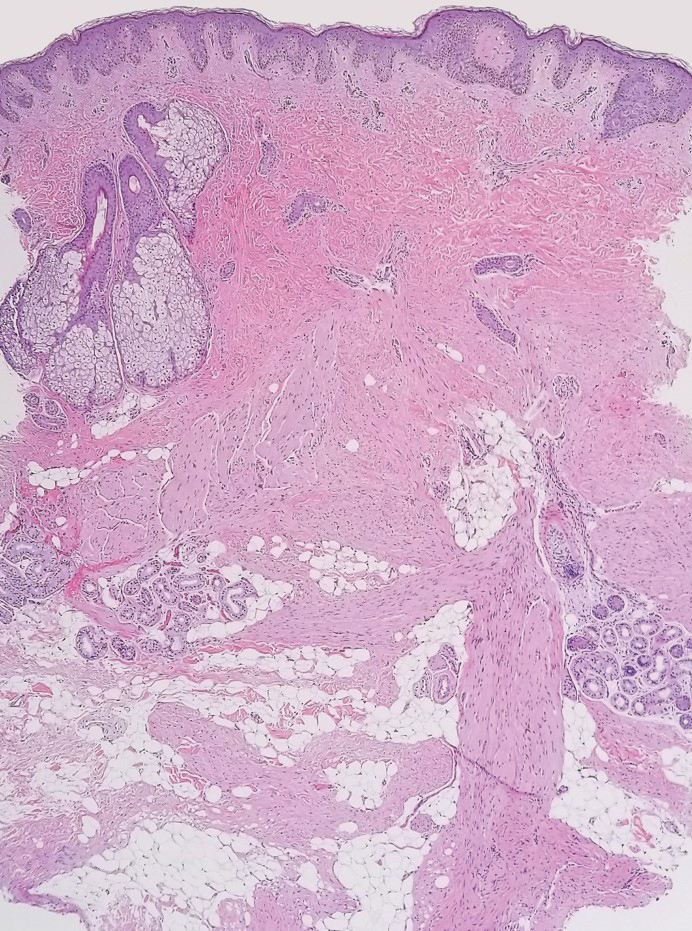
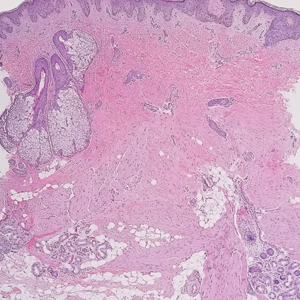
Dermatomyofibromas may be mistaken for several other entities both benign and malignant. For example, the benign dermatofibroma is the second most common fibrohistiocytic tumor of the skin and presents as a firm, nontender, minimally elevated to dome-shaped papule that usually measures less than or equal to 1 cm in diameter with or without overlying skin changes.5,6 It primarily is seen in adults with a slight female predominance and favors the lower extremities.5 Patients usually are asymptomatic but often report a history of local trauma at the lesion site.6 Histologically, dermatofibroma is characterized by a nodular dermal proliferation of spindleshaped fibrous cells and histiocytes in a storiform pattern (Figure 1).6 Epidermal induction with acanthosis overlying the tumor often is found with occasional basilar hyperpigmentation.5 Dermatofibroma also characteristically has trapped collagen (“collagen balls”) seen at the periphery.5,6
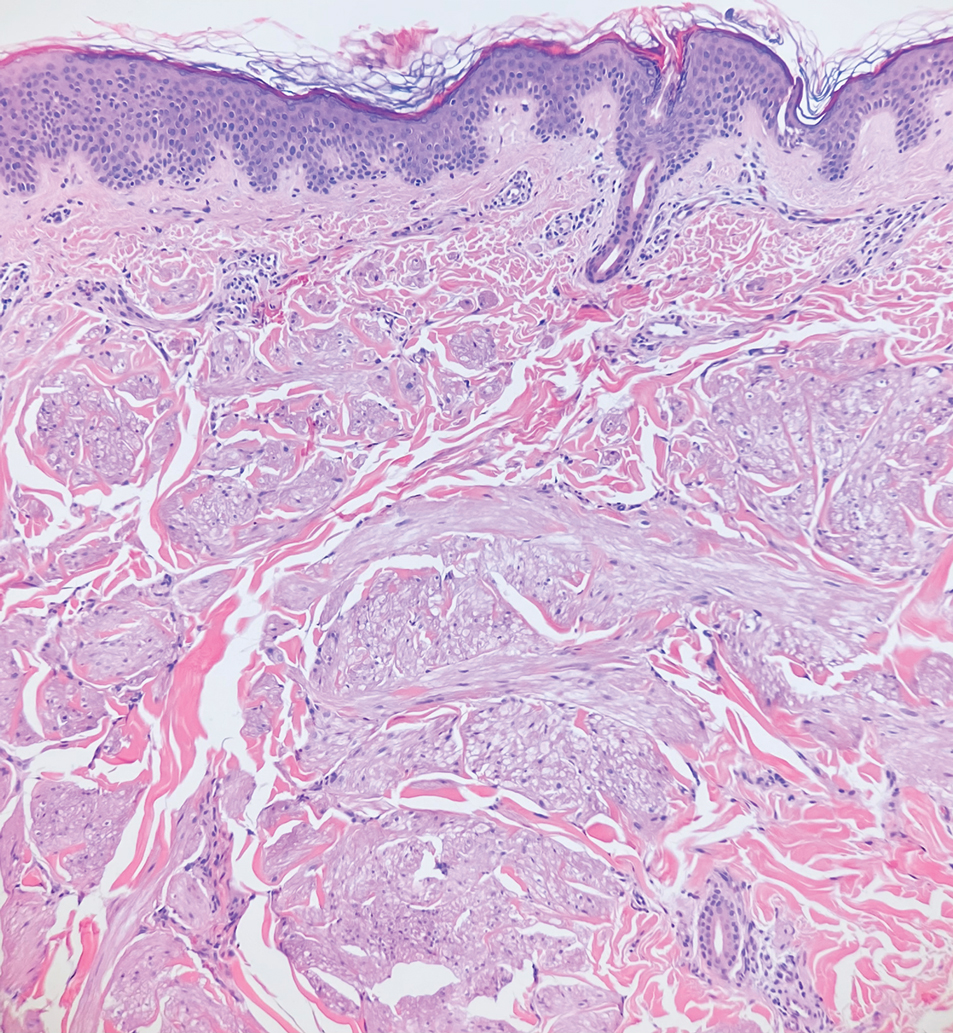
Piloleiomyomas are benign smooth muscle tumors arising from arrector pili muscles that may be solitary or multiple.5 Clinically, they typically present as firm, reddish-brown to flesh-colored papules or nodules that develop more commonly in adulthood.5,7 Piloleiomyomas favor the extremities and trunk, particularly the shoulder, and can be associated with spontaneous or induced pain. Histologically, piloleiomyomas are well circumscribed and centered within the reticular dermis situated closely to hair follicles (Figure 2).5 They are composed of numerous interlacing fascicles or whorls of smooth muscle cells with abundant eosinophilic cytoplasm and blunt-ended, cigar-shaped nuclei.5,7
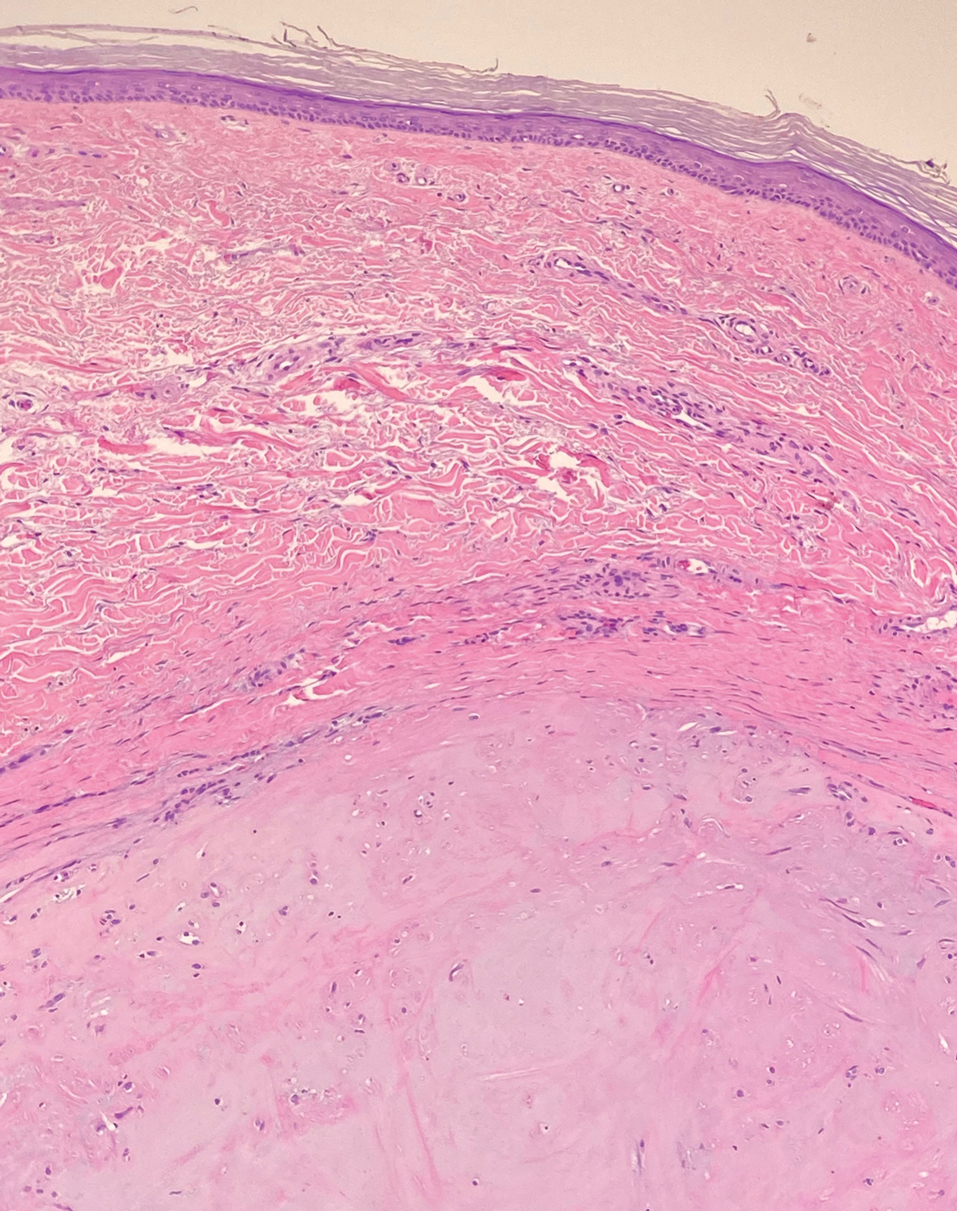
Solitary cutaneous myofibroma is a benign fibrous tumor found in adolescents and adults and is the counterpart to infantile myofibromatosis.8 Clinically, myofibromas typically present as painless, slow-growing, firm nodules with an occasional bluish hue. Histologically, solitary cutaneous myofibromas appear in a biphasic pattern, with hemangiopericytomatous components as well as spindle cells arranged in short bundles and fascicles resembling leiomyoma (Figure 3). The spindle cells also have abundant eosinophilic cytoplasm with short plump nuclei; the random, irregularly intersecting angles can be used to help differentiate myofibromas from smooth muscle lesions.8 Solitary cutaneous myofibroma is in the differential diagnosis for dermatomyofibroma because of their shared myofibroblastic nature.9
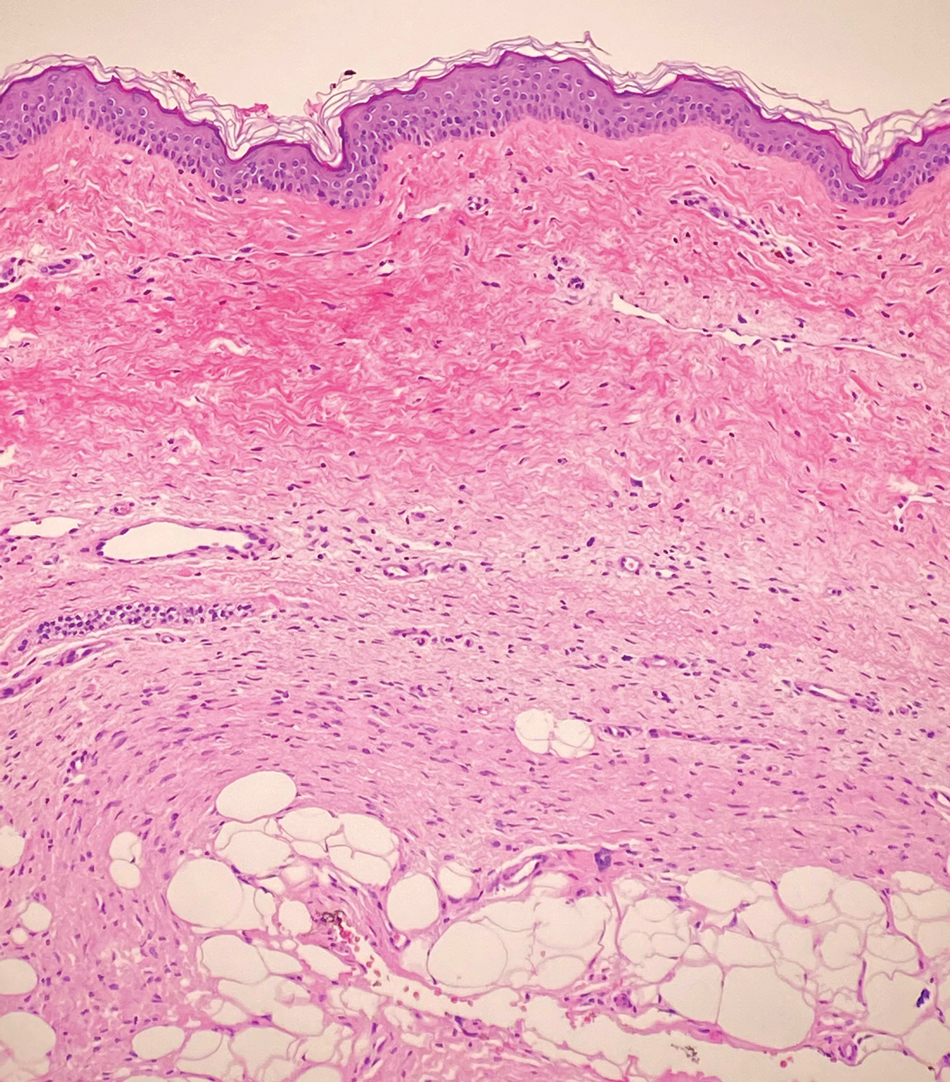
Dermatofibrosarcoma protuberans (DFSP) is an uncommon, locally invasive sarcoma with a high recurrence rate that favors young to middle-aged adults, with rare childhood onset reported.5,10,11 Clinically, DFSP typically presents as an asymptomatic, slow-growing, firm, flesh-colored, indurated plaque that develops into a violaceous to reddish-brown nodule.5 The atrophic variant of DFSP is characterized by a nonprotuberant lesion and can be especially difficult to distinguish from other entities such as dermatomyofibroma.11 The majority of DFSP lesions occur on the trunk, particularly in the shoulder or pelvic region.5 Histologically, early plaque lesions are comprised of monomorphic spindle cells arranged in long fascicles (parallel to the skin surface), infiltrating adnexal structures, and subcutaneous adipocytes in a multilayered honeycomb pattern; the spindle cells of late nodular lesions are arranged in short fascicles in a matted or storiform pattern (Figure 4).5,10 Early stages of DFSP as well as variations in childhood-onset DFSP can easily be misdiagnosed and incompletely excised.5
- Ma JE, Wieland CN, Tollefson MM. Dermatomyofibromas arising in children: report of two new cases and review of the literature. Pediatr Dermatol. 2017;34:347-351.
- Tardio JC, Azorin D, Hernandez-Nunez A, et al. Dermatomyofibromas presenting in pediatric patients: clinicopathologic characteristics and differential diagnosis. J Cutan Pathol. 2011;38:967-972.
- Mentzel T, Kutzner H. Dermatomyofibroma: clinicopathologic and immunohistochemical analysis of 56 cases and reappraisal of a rare and distinct cutaneous neoplasm. Am J Dermatopathol. 2009;31:44-49.
- Hugel H. Plaque-like dermal fibromatosis. Hautarzt. 1991;42:223-226.
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. WB Saunders Co; 2012.
- Myers DJ, Fillman EP. Dermatofibroma. StatPearls [Internet]. StatPearls Publishing; 2020.
- Dilek N, Yuksel D, Sehitoglu I, et al. Cutaneous leiomyoma in a child: a case report. Oncol Lett. 2013;5:1163-1164.
- Roh HS, Paek JO, Yu HJ, et al. Solitary cutaneous myofibroma on the sole: an unusual localization. Ann Dermatol. 2012;24:220-222.
- Weedon D, Strutton G, Rubin AI, et al. Weedon’s Skin Pathology. Churchill Livingstone/Elsevier; 2010.
- Mendenhall WM, Zlotecki RA, Scarborough MT. Dermatofibrosarcoma protuberans. Cancer. 2004;101:2503-2508.
- Akay BN, Unlu E, Erdem C, et al. Dermatoscopic findings of atrophic dermatofibrosarcoma protuberans. Dermatol Pract Concept. 2015;5:71-73.
The Diagnosis: Dermatomyofibroma
Dermatomyofibroma is an uncommon, benign, cutaneous mesenchymal neoplasm composed of fibroblasts and myofibroblasts.1-3 This skin tumor was first described in 1991 by Hugel4 in the German literature as plaquelike fibromatosis. Pediatric dermatomyofibromas are exceedingly rare, with pediatric patients ranging in age from infants to teenagers.1
Clinically, dermatomyofibromas appear as long-standing, isolated, ill-demarcated, flesh-colored, slightly hyperpigmented or erythematous nodules or plaques that may be raised or indurated.1 Dermatomyofibromas may present with constant mild pain or pruritus, though in most cases the lesions are asymptomatic.1,3 The clinical presentation of dermatomyofibroma has a few distinct differences in children compared to adults. In adulthood, dermatomyofibroma has a strong female predominance and most commonly is located on the shoulder and adjacent upper body regions, including the axilla, neck, upper arm, and upper trunk.1-3 In childhood, the majority of dermatomyofibromas occur in young boys and usually are located on the neck with other upper body regions occurring less frequently.1,2 A shared characteristic includes the tendency for dermatomyofibromas to have an initial period of enlargement followed by stabilization or slow growth.1 Reported pediatric lesions have ranged in size from 4 to 60 mm with an average size of 14.9 mm (median, 12 mm).2
The diagnosis of dermatomyofibroma is based on histopathologic features in addition to clinical presentation. Histology from punch biopsy usually reveals a noninvasive dermal proliferation of bland, uniform, slender spindle cells oriented parallel to the overlying epidermis with increased and fragmented elastic fibers.1,3 Infiltration into the mid or deep dermis is common. The adnexal structures usually are spared; the stroma contains collagen and increased small blood vessels; and there typically is no inflammatory infiltrate, except for occasional scattered mast cells.2 Cytologically, the monomorphic spindleshaped tumor cells have an ill-defined, pale, eosinophilic cytoplasm and nuclei that are elongated with tapered edges.3 Dermatomyofibroma has a variable immunohistochemical profile, as it may stain focally positive for CD34 or smooth muscle actin, with occasional staining of factor XIIIa, desmin, calponin, or vimentin.1-3 Normal to increased levels of often fragmented elastic fibers is a helpful clue in distinguishing dermatomyofibroma from dermatofibroma, hypertrophic scar, dermatofibrosarcoma protuberans, and pilar leiomyoma, in which elastic fibers typically are reduced.3 Differential diagnoses of mesenchymal tumors in children include desmoid fibromatosis, connective tissue nevus, myofibromatosis, and smooth muscle hamartoma.1
A punch biopsy with clinical observation and followup is recommended for the management of lesions in cosmetically sensitive areas or in very young children who may not tolerate surgery. In symptomatic or cosmetically unappealing cases of dermatomyofibroma, simple surgical excision remains a viable treatment option. Recurrence is uncommon, even if only partially excised, and no instances of metastasis have been reported.1-5
Dermatomyofibromas may be mistaken for several other entities both benign and malignant. For example, the benign dermatofibroma is the second most common fibrohistiocytic tumor of the skin and presents as a firm, nontender, minimally elevated to dome-shaped papule that usually measures less than or equal to 1 cm in diameter with or without overlying skin changes.5,6 It primarily is seen in adults with a slight female predominance and favors the lower extremities.5 Patients usually are asymptomatic but often report a history of local trauma at the lesion site.6 Histologically, dermatofibroma is characterized by a nodular dermal proliferation of spindleshaped fibrous cells and histiocytes in a storiform pattern (Figure 1).6 Epidermal induction with acanthosis overlying the tumor often is found with occasional basilar hyperpigmentation.5 Dermatofibroma also characteristically has trapped collagen (“collagen balls”) seen at the periphery.5,6
Piloleiomyomas are benign smooth muscle tumors arising from arrector pili muscles that may be solitary or multiple.5 Clinically, they typically present as firm, reddish-brown to flesh-colored papules or nodules that develop more commonly in adulthood.5,7 Piloleiomyomas favor the extremities and trunk, particularly the shoulder, and can be associated with spontaneous or induced pain. Histologically, piloleiomyomas are well circumscribed and centered within the reticular dermis situated closely to hair follicles (Figure 2).5 They are composed of numerous interlacing fascicles or whorls of smooth muscle cells with abundant eosinophilic cytoplasm and blunt-ended, cigar-shaped nuclei.5,7
Solitary cutaneous myofibroma is a benign fibrous tumor found in adolescents and adults and is the counterpart to infantile myofibromatosis.8 Clinically, myofibromas typically present as painless, slow-growing, firm nodules with an occasional bluish hue. Histologically, solitary cutaneous myofibromas appear in a biphasic pattern, with hemangiopericytomatous components as well as spindle cells arranged in short bundles and fascicles resembling leiomyoma (Figure 3). The spindle cells also have abundant eosinophilic cytoplasm with short plump nuclei; the random, irregularly intersecting angles can be used to help differentiate myofibromas from smooth muscle lesions.8 Solitary cutaneous myofibroma is in the differential diagnosis for dermatomyofibroma because of their shared myofibroblastic nature.9
Dermatofibrosarcoma protuberans (DFSP) is an uncommon, locally invasive sarcoma with a high recurrence rate that favors young to middle-aged adults, with rare childhood onset reported.5,10,11 Clinically, DFSP typically presents as an asymptomatic, slow-growing, firm, flesh-colored, indurated plaque that develops into a violaceous to reddish-brown nodule.5 The atrophic variant of DFSP is characterized by a nonprotuberant lesion and can be especially difficult to distinguish from other entities such as dermatomyofibroma.11 The majority of DFSP lesions occur on the trunk, particularly in the shoulder or pelvic region.5 Histologically, early plaque lesions are comprised of monomorphic spindle cells arranged in long fascicles (parallel to the skin surface), infiltrating adnexal structures, and subcutaneous adipocytes in a multilayered honeycomb pattern; the spindle cells of late nodular lesions are arranged in short fascicles in a matted or storiform pattern (Figure 4).5,10 Early stages of DFSP as well as variations in childhood-onset DFSP can easily be misdiagnosed and incompletely excised.5
The Diagnosis: Dermatomyofibroma
Dermatomyofibroma is an uncommon, benign, cutaneous mesenchymal neoplasm composed of fibroblasts and myofibroblasts.1-3 This skin tumor was first described in 1991 by Hugel4 in the German literature as plaquelike fibromatosis. Pediatric dermatomyofibromas are exceedingly rare, with pediatric patients ranging in age from infants to teenagers.1
Clinically, dermatomyofibromas appear as long-standing, isolated, ill-demarcated, flesh-colored, slightly hyperpigmented or erythematous nodules or plaques that may be raised or indurated.1 Dermatomyofibromas may present with constant mild pain or pruritus, though in most cases the lesions are asymptomatic.1,3 The clinical presentation of dermatomyofibroma has a few distinct differences in children compared to adults. In adulthood, dermatomyofibroma has a strong female predominance and most commonly is located on the shoulder and adjacent upper body regions, including the axilla, neck, upper arm, and upper trunk.1-3 In childhood, the majority of dermatomyofibromas occur in young boys and usually are located on the neck with other upper body regions occurring less frequently.1,2 A shared characteristic includes the tendency for dermatomyofibromas to have an initial period of enlargement followed by stabilization or slow growth.1 Reported pediatric lesions have ranged in size from 4 to 60 mm with an average size of 14.9 mm (median, 12 mm).2
The diagnosis of dermatomyofibroma is based on histopathologic features in addition to clinical presentation. Histology from punch biopsy usually reveals a noninvasive dermal proliferation of bland, uniform, slender spindle cells oriented parallel to the overlying epidermis with increased and fragmented elastic fibers.1,3 Infiltration into the mid or deep dermis is common. The adnexal structures usually are spared; the stroma contains collagen and increased small blood vessels; and there typically is no inflammatory infiltrate, except for occasional scattered mast cells.2 Cytologically, the monomorphic spindleshaped tumor cells have an ill-defined, pale, eosinophilic cytoplasm and nuclei that are elongated with tapered edges.3 Dermatomyofibroma has a variable immunohistochemical profile, as it may stain focally positive for CD34 or smooth muscle actin, with occasional staining of factor XIIIa, desmin, calponin, or vimentin.1-3 Normal to increased levels of often fragmented elastic fibers is a helpful clue in distinguishing dermatomyofibroma from dermatofibroma, hypertrophic scar, dermatofibrosarcoma protuberans, and pilar leiomyoma, in which elastic fibers typically are reduced.3 Differential diagnoses of mesenchymal tumors in children include desmoid fibromatosis, connective tissue nevus, myofibromatosis, and smooth muscle hamartoma.1
A punch biopsy with clinical observation and followup is recommended for the management of lesions in cosmetically sensitive areas or in very young children who may not tolerate surgery. In symptomatic or cosmetically unappealing cases of dermatomyofibroma, simple surgical excision remains a viable treatment option. Recurrence is uncommon, even if only partially excised, and no instances of metastasis have been reported.1-5
Dermatomyofibromas may be mistaken for several other entities both benign and malignant. For example, the benign dermatofibroma is the second most common fibrohistiocytic tumor of the skin and presents as a firm, nontender, minimally elevated to dome-shaped papule that usually measures less than or equal to 1 cm in diameter with or without overlying skin changes.5,6 It primarily is seen in adults with a slight female predominance and favors the lower extremities.5 Patients usually are asymptomatic but often report a history of local trauma at the lesion site.6 Histologically, dermatofibroma is characterized by a nodular dermal proliferation of spindleshaped fibrous cells and histiocytes in a storiform pattern (Figure 1).6 Epidermal induction with acanthosis overlying the tumor often is found with occasional basilar hyperpigmentation.5 Dermatofibroma also characteristically has trapped collagen (“collagen balls”) seen at the periphery.5,6
Piloleiomyomas are benign smooth muscle tumors arising from arrector pili muscles that may be solitary or multiple.5 Clinically, they typically present as firm, reddish-brown to flesh-colored papules or nodules that develop more commonly in adulthood.5,7 Piloleiomyomas favor the extremities and trunk, particularly the shoulder, and can be associated with spontaneous or induced pain. Histologically, piloleiomyomas are well circumscribed and centered within the reticular dermis situated closely to hair follicles (Figure 2).5 They are composed of numerous interlacing fascicles or whorls of smooth muscle cells with abundant eosinophilic cytoplasm and blunt-ended, cigar-shaped nuclei.5,7
Solitary cutaneous myofibroma is a benign fibrous tumor found in adolescents and adults and is the counterpart to infantile myofibromatosis.8 Clinically, myofibromas typically present as painless, slow-growing, firm nodules with an occasional bluish hue. Histologically, solitary cutaneous myofibromas appear in a biphasic pattern, with hemangiopericytomatous components as well as spindle cells arranged in short bundles and fascicles resembling leiomyoma (Figure 3). The spindle cells also have abundant eosinophilic cytoplasm with short plump nuclei; the random, irregularly intersecting angles can be used to help differentiate myofibromas from smooth muscle lesions.8 Solitary cutaneous myofibroma is in the differential diagnosis for dermatomyofibroma because of their shared myofibroblastic nature.9
Dermatofibrosarcoma protuberans (DFSP) is an uncommon, locally invasive sarcoma with a high recurrence rate that favors young to middle-aged adults, with rare childhood onset reported.5,10,11 Clinically, DFSP typically presents as an asymptomatic, slow-growing, firm, flesh-colored, indurated plaque that develops into a violaceous to reddish-brown nodule.5 The atrophic variant of DFSP is characterized by a nonprotuberant lesion and can be especially difficult to distinguish from other entities such as dermatomyofibroma.11 The majority of DFSP lesions occur on the trunk, particularly in the shoulder or pelvic region.5 Histologically, early plaque lesions are comprised of monomorphic spindle cells arranged in long fascicles (parallel to the skin surface), infiltrating adnexal structures, and subcutaneous adipocytes in a multilayered honeycomb pattern; the spindle cells of late nodular lesions are arranged in short fascicles in a matted or storiform pattern (Figure 4).5,10 Early stages of DFSP as well as variations in childhood-onset DFSP can easily be misdiagnosed and incompletely excised.5
- Ma JE, Wieland CN, Tollefson MM. Dermatomyofibromas arising in children: report of two new cases and review of the literature. Pediatr Dermatol. 2017;34:347-351.
- Tardio JC, Azorin D, Hernandez-Nunez A, et al. Dermatomyofibromas presenting in pediatric patients: clinicopathologic characteristics and differential diagnosis. J Cutan Pathol. 2011;38:967-972.
- Mentzel T, Kutzner H. Dermatomyofibroma: clinicopathologic and immunohistochemical analysis of 56 cases and reappraisal of a rare and distinct cutaneous neoplasm. Am J Dermatopathol. 2009;31:44-49.
- Hugel H. Plaque-like dermal fibromatosis. Hautarzt. 1991;42:223-226.
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. WB Saunders Co; 2012.
- Myers DJ, Fillman EP. Dermatofibroma. StatPearls [Internet]. StatPearls Publishing; 2020.
- Dilek N, Yuksel D, Sehitoglu I, et al. Cutaneous leiomyoma in a child: a case report. Oncol Lett. 2013;5:1163-1164.
- Roh HS, Paek JO, Yu HJ, et al. Solitary cutaneous myofibroma on the sole: an unusual localization. Ann Dermatol. 2012;24:220-222.
- Weedon D, Strutton G, Rubin AI, et al. Weedon’s Skin Pathology. Churchill Livingstone/Elsevier; 2010.
- Mendenhall WM, Zlotecki RA, Scarborough MT. Dermatofibrosarcoma protuberans. Cancer. 2004;101:2503-2508.
- Akay BN, Unlu E, Erdem C, et al. Dermatoscopic findings of atrophic dermatofibrosarcoma protuberans. Dermatol Pract Concept. 2015;5:71-73.
- Ma JE, Wieland CN, Tollefson MM. Dermatomyofibromas arising in children: report of two new cases and review of the literature. Pediatr Dermatol. 2017;34:347-351.
- Tardio JC, Azorin D, Hernandez-Nunez A, et al. Dermatomyofibromas presenting in pediatric patients: clinicopathologic characteristics and differential diagnosis. J Cutan Pathol. 2011;38:967-972.
- Mentzel T, Kutzner H. Dermatomyofibroma: clinicopathologic and immunohistochemical analysis of 56 cases and reappraisal of a rare and distinct cutaneous neoplasm. Am J Dermatopathol. 2009;31:44-49.
- Hugel H. Plaque-like dermal fibromatosis. Hautarzt. 1991;42:223-226.
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. WB Saunders Co; 2012.
- Myers DJ, Fillman EP. Dermatofibroma. StatPearls [Internet]. StatPearls Publishing; 2020.
- Dilek N, Yuksel D, Sehitoglu I, et al. Cutaneous leiomyoma in a child: a case report. Oncol Lett. 2013;5:1163-1164.
- Roh HS, Paek JO, Yu HJ, et al. Solitary cutaneous myofibroma on the sole: an unusual localization. Ann Dermatol. 2012;24:220-222.
- Weedon D, Strutton G, Rubin AI, et al. Weedon’s Skin Pathology. Churchill Livingstone/Elsevier; 2010.
- Mendenhall WM, Zlotecki RA, Scarborough MT. Dermatofibrosarcoma protuberans. Cancer. 2004;101:2503-2508.
- Akay BN, Unlu E, Erdem C, et al. Dermatoscopic findings of atrophic dermatofibrosarcoma protuberans. Dermatol Pract Concept. 2015;5:71-73.
A 12-year-old boy with olive skin presented with a tender subcutaneous nodule on the back of 6 months’ duration. He reported the lesion initially grew rapidly with increasing pain for approximately 3 months with subsequent stabilization in size and modest resolution of his symptoms. Physical examination revealed a solitary, 15-mm, ill-defined, indurated, tender, subcutaneous nodule with subtle overlying hyperpigmentation on the left side of the upper back. Hematoxylin and eosin staining of a 4-mm punch biopsy revealed a nonencapsulated mass of monomorphic eosinophilic spindle cells organized into fascicles arranged predominantly parallel to the skin surface. The mass extended from the mid reticular dermis to the upper subcutis, sparing adnexal structures.
Characterizing Counterfeit Dermatologic Devices Sold on Popular E-commerce Websites
To the Editor:
Approved medical devices on the market are substantial capital investments for practitioners. E-commerce websites, such as Alibaba.com (https://www.alibaba.com/) and DHgate.com (https://www.dhgate.com/), sell sham medical devices at a fraction of the cost of authentic products, with sellers often echoing the same treatment claims as legitimate devices that have been cleared by the US Food and Drug Administration (FDA).
In dermatology, devices claiming to perform cryolipolysis, laser skin resurfacing, radiofrequency skin tightening, and more exist on e-commerce websites. These counterfeit medical devices might differ from legitimate devices in ways that affect patient safety and treatment efficacy.1,2 The degree of difference between counterfeit and legitimate devices remains unknown, and potential harm from so-called knockoff devices needs to be critically examined by providers.
In this exploratory study, we characterize counterfeit listings of devices commonly used in dermatology. Using the trademark name of devices as the key terms, we searched Alibaba.com and DHgate.com for listings of counterfeit products. We recorded the total number of listings; the listing name, catalog number, and unit price; and claims of FDA certification. Characteristics of counterfeit listings were summarized using standard descriptive statistics in Microsoft Excel. Continuous variables were summarized with means and ranges.
Six medical devices that had been cleared by the FDA between 2002 and 2012 for use in dermatology were explored, including systems for picosecond and fractionated lasers, monopolar and bipolar radiofrequency skin tightening, cryolipolysis, and nonablative radiofrequency skin resurfacing. Our search of these 6 representative dermatologic devices revealed 47,055 counterfeit product listings on Alibaba.com and DHgate.com. Upon searching these popular e-commerce websites using the device name as the search term, the number of listings varied considerably between the 2 e-commerce websites for the same device and from device to device on the same e-commerce website. On Alibaba.com, the greatest number of listings resulted for picosecond laser (23,622 listings), fractionated laser (15,269), and radiofrequency skin tightening devices (3555); cryolipolysis and nonablative radiofrequency resurfacing devices had notably fewer listings (35 and 38, respectively). On DHGate.com, a similar trend was noted with the most numerous listings for picosecond and fractionated laser systems (2429 and 1345, respectively).
Among the first 10 listings of products on Alibaba.com and DHgate.com for these 6 devices, 10.7% (11 of 103) had advertised claims of FDA clearance on the listing page. Of 103 counterfeit products, China was the country of origin for 100; South Korea for 2; and Thailand for 1. Unit pricing was heterogeneous between the 2 e-commerce websites for the counterfeit listings; pricing for duplicate fractionated laser systems was particularly dissimilar, with an average price on Alibab.com of US $8105.80 and an average price on DHgate.com of US $3409.14. Even on the same e-commerce website, the range of unit pricing differed greatly for dermatologic devices. For example, among the first 10 listings on Alibaba.com for a fractionated laser system, the price ranged from US $2300 to US $32,000.
Counterfeit medical devices are on the rise in dermatology.1,3 Although devices such as radiofrequency and laser systems had thousands of knockoff listings on 2 e-commerce websites, other devices, such as cryolipolysis and body contouring systems, had fewer listings, suggesting heterogeneity in the prevalence of different counterfeit dermatologic devices on the market.
The varied pricing of the top 10 listings for each product and spurious claims of FDA clearance for some listings highlight the lack of regulatory authority over consistent product information on e-commerce websites. Furthermore, differences between characteristics of counterfeit device listings can impede efforts to trace suppliers and increase the opacity of counterfeit purchasing.
Three criteria have been proposed for a device to be considered counterfeit3:
• The device has no proven safety or efficacy among consumers. For example, the substantial threat of copycat devices in dermatology has been demonstrated by reports of burns caused by fake cryolipolysis devices.2
• The device violates patent rights or copy trademarks. Due to the regional nature of intellectual property rights, country-specific filings of patents and trademarks are required if protections are sought internationally. In this study, counterfeit devices originated in China, South Korea, and Thailand, where patent and trademark protections for the original devices do not extend.
• The device is falsely claimed to have been cleared by the FDA or other clinical regulatory authorities. Legitimate medical devices are subject to rounds of safety and compatibility testing using standards set by regulatory bodies, such as the FDA’s Center for Devices and Radiological Health, the International Organization of Standardization, and the International Electrotechnical Commission. Compliance with these safety standards is lost, however, among unregulated internet sales of medical devices. Our search revealed that 10.7% of the top 10 counterfeit device listings for each product explicitly mentioned FDA clearance in the product description. Among the thousands of listings on e-commerce sites, even a fraction that make spurious FDA-clearance claims can mislead consumers.
The issue of counterfeit medical devices has not gone unrecognized globally. In 2013, the World Health Organization created the Global Surveillance and Monitoring System to unify international efforts for reporting substandard, unlicensed, or falsified medical products.4 Although universal monitoring systems can improve detection of counterfeit products, we highlight the alarming continuing ease of purchasing counterfeit dermatologic devices through e-commerce websites. Due to the widespread nature of counterfeiting across all domains of medicine, the onus of curbing counterfeit dermatologic devices might be on dermatology providers to recognize and report such occurrences.
This exploration of counterfeit dermatologic devices revealed a lack of consistency throughout product listings on 2 popular e-commerce websites, Alibaba.com and DHgate.com. Given the alarming availability of these devices on the internet, practitioners should approach the purchase of any device with concern about counterfeiting. Future avenues of study might explore the prevalence of counterfeit devices used in dermatology practices and offer insight on regulation and consumer safety efforts.
- Wang JV, Zachary CB, Saedi N. Counterfeit esthetic devices and patient safety in dermatology. J Cosmet Dermatol. 2018;17:396-397. doi:10.1111/jocd.12526
- Biesman BS, Patel N. Physician alert: beware of counterfeit medical devices. Lasers Surg Med. 2014;46:528‐530. doi:10.1002/lsm.22275
- Stevens WG, Spring MA, Macias LH. Counterfeit medical devices: the money you save up front will cost you big in the end. Aesthet Surg J. 2014;34:786‐788. doi:10.1177/1090820X14529960
- Pisani E. WHO Global Surveillance and Monitoring System for Substandard and Falsified Medical Products. World Health Organization; 2017. Accessed November 21, 2021. https://www.who.int/medicines/regulation/ssffc/publications/GSMSreport_EN.pdf?ua=1
To the Editor:
Approved medical devices on the market are substantial capital investments for practitioners. E-commerce websites, such as Alibaba.com (https://www.alibaba.com/) and DHgate.com (https://www.dhgate.com/), sell sham medical devices at a fraction of the cost of authentic products, with sellers often echoing the same treatment claims as legitimate devices that have been cleared by the US Food and Drug Administration (FDA).
In dermatology, devices claiming to perform cryolipolysis, laser skin resurfacing, radiofrequency skin tightening, and more exist on e-commerce websites. These counterfeit medical devices might differ from legitimate devices in ways that affect patient safety and treatment efficacy.1,2 The degree of difference between counterfeit and legitimate devices remains unknown, and potential harm from so-called knockoff devices needs to be critically examined by providers.
In this exploratory study, we characterize counterfeit listings of devices commonly used in dermatology. Using the trademark name of devices as the key terms, we searched Alibaba.com and DHgate.com for listings of counterfeit products. We recorded the total number of listings; the listing name, catalog number, and unit price; and claims of FDA certification. Characteristics of counterfeit listings were summarized using standard descriptive statistics in Microsoft Excel. Continuous variables were summarized with means and ranges.
Six medical devices that had been cleared by the FDA between 2002 and 2012 for use in dermatology were explored, including systems for picosecond and fractionated lasers, monopolar and bipolar radiofrequency skin tightening, cryolipolysis, and nonablative radiofrequency skin resurfacing. Our search of these 6 representative dermatologic devices revealed 47,055 counterfeit product listings on Alibaba.com and DHgate.com. Upon searching these popular e-commerce websites using the device name as the search term, the number of listings varied considerably between the 2 e-commerce websites for the same device and from device to device on the same e-commerce website. On Alibaba.com, the greatest number of listings resulted for picosecond laser (23,622 listings), fractionated laser (15,269), and radiofrequency skin tightening devices (3555); cryolipolysis and nonablative radiofrequency resurfacing devices had notably fewer listings (35 and 38, respectively). On DHGate.com, a similar trend was noted with the most numerous listings for picosecond and fractionated laser systems (2429 and 1345, respectively).
Among the first 10 listings of products on Alibaba.com and DHgate.com for these 6 devices, 10.7% (11 of 103) had advertised claims of FDA clearance on the listing page. Of 103 counterfeit products, China was the country of origin for 100; South Korea for 2; and Thailand for 1. Unit pricing was heterogeneous between the 2 e-commerce websites for the counterfeit listings; pricing for duplicate fractionated laser systems was particularly dissimilar, with an average price on Alibab.com of US $8105.80 and an average price on DHgate.com of US $3409.14. Even on the same e-commerce website, the range of unit pricing differed greatly for dermatologic devices. For example, among the first 10 listings on Alibaba.com for a fractionated laser system, the price ranged from US $2300 to US $32,000.
Counterfeit medical devices are on the rise in dermatology.1,3 Although devices such as radiofrequency and laser systems had thousands of knockoff listings on 2 e-commerce websites, other devices, such as cryolipolysis and body contouring systems, had fewer listings, suggesting heterogeneity in the prevalence of different counterfeit dermatologic devices on the market.
The varied pricing of the top 10 listings for each product and spurious claims of FDA clearance for some listings highlight the lack of regulatory authority over consistent product information on e-commerce websites. Furthermore, differences between characteristics of counterfeit device listings can impede efforts to trace suppliers and increase the opacity of counterfeit purchasing.
Three criteria have been proposed for a device to be considered counterfeit3:
• The device has no proven safety or efficacy among consumers. For example, the substantial threat of copycat devices in dermatology has been demonstrated by reports of burns caused by fake cryolipolysis devices.2
• The device violates patent rights or copy trademarks. Due to the regional nature of intellectual property rights, country-specific filings of patents and trademarks are required if protections are sought internationally. In this study, counterfeit devices originated in China, South Korea, and Thailand, where patent and trademark protections for the original devices do not extend.
• The device is falsely claimed to have been cleared by the FDA or other clinical regulatory authorities. Legitimate medical devices are subject to rounds of safety and compatibility testing using standards set by regulatory bodies, such as the FDA’s Center for Devices and Radiological Health, the International Organization of Standardization, and the International Electrotechnical Commission. Compliance with these safety standards is lost, however, among unregulated internet sales of medical devices. Our search revealed that 10.7% of the top 10 counterfeit device listings for each product explicitly mentioned FDA clearance in the product description. Among the thousands of listings on e-commerce sites, even a fraction that make spurious FDA-clearance claims can mislead consumers.
The issue of counterfeit medical devices has not gone unrecognized globally. In 2013, the World Health Organization created the Global Surveillance and Monitoring System to unify international efforts for reporting substandard, unlicensed, or falsified medical products.4 Although universal monitoring systems can improve detection of counterfeit products, we highlight the alarming continuing ease of purchasing counterfeit dermatologic devices through e-commerce websites. Due to the widespread nature of counterfeiting across all domains of medicine, the onus of curbing counterfeit dermatologic devices might be on dermatology providers to recognize and report such occurrences.
This exploration of counterfeit dermatologic devices revealed a lack of consistency throughout product listings on 2 popular e-commerce websites, Alibaba.com and DHgate.com. Given the alarming availability of these devices on the internet, practitioners should approach the purchase of any device with concern about counterfeiting. Future avenues of study might explore the prevalence of counterfeit devices used in dermatology practices and offer insight on regulation and consumer safety efforts.
To the Editor:
Approved medical devices on the market are substantial capital investments for practitioners. E-commerce websites, such as Alibaba.com (https://www.alibaba.com/) and DHgate.com (https://www.dhgate.com/), sell sham medical devices at a fraction of the cost of authentic products, with sellers often echoing the same treatment claims as legitimate devices that have been cleared by the US Food and Drug Administration (FDA).
In dermatology, devices claiming to perform cryolipolysis, laser skin resurfacing, radiofrequency skin tightening, and more exist on e-commerce websites. These counterfeit medical devices might differ from legitimate devices in ways that affect patient safety and treatment efficacy.1,2 The degree of difference between counterfeit and legitimate devices remains unknown, and potential harm from so-called knockoff devices needs to be critically examined by providers.
In this exploratory study, we characterize counterfeit listings of devices commonly used in dermatology. Using the trademark name of devices as the key terms, we searched Alibaba.com and DHgate.com for listings of counterfeit products. We recorded the total number of listings; the listing name, catalog number, and unit price; and claims of FDA certification. Characteristics of counterfeit listings were summarized using standard descriptive statistics in Microsoft Excel. Continuous variables were summarized with means and ranges.
Six medical devices that had been cleared by the FDA between 2002 and 2012 for use in dermatology were explored, including systems for picosecond and fractionated lasers, monopolar and bipolar radiofrequency skin tightening, cryolipolysis, and nonablative radiofrequency skin resurfacing. Our search of these 6 representative dermatologic devices revealed 47,055 counterfeit product listings on Alibaba.com and DHgate.com. Upon searching these popular e-commerce websites using the device name as the search term, the number of listings varied considerably between the 2 e-commerce websites for the same device and from device to device on the same e-commerce website. On Alibaba.com, the greatest number of listings resulted for picosecond laser (23,622 listings), fractionated laser (15,269), and radiofrequency skin tightening devices (3555); cryolipolysis and nonablative radiofrequency resurfacing devices had notably fewer listings (35 and 38, respectively). On DHGate.com, a similar trend was noted with the most numerous listings for picosecond and fractionated laser systems (2429 and 1345, respectively).
Among the first 10 listings of products on Alibaba.com and DHgate.com for these 6 devices, 10.7% (11 of 103) had advertised claims of FDA clearance on the listing page. Of 103 counterfeit products, China was the country of origin for 100; South Korea for 2; and Thailand for 1. Unit pricing was heterogeneous between the 2 e-commerce websites for the counterfeit listings; pricing for duplicate fractionated laser systems was particularly dissimilar, with an average price on Alibab.com of US $8105.80 and an average price on DHgate.com of US $3409.14. Even on the same e-commerce website, the range of unit pricing differed greatly for dermatologic devices. For example, among the first 10 listings on Alibaba.com for a fractionated laser system, the price ranged from US $2300 to US $32,000.
Counterfeit medical devices are on the rise in dermatology.1,3 Although devices such as radiofrequency and laser systems had thousands of knockoff listings on 2 e-commerce websites, other devices, such as cryolipolysis and body contouring systems, had fewer listings, suggesting heterogeneity in the prevalence of different counterfeit dermatologic devices on the market.
The varied pricing of the top 10 listings for each product and spurious claims of FDA clearance for some listings highlight the lack of regulatory authority over consistent product information on e-commerce websites. Furthermore, differences between characteristics of counterfeit device listings can impede efforts to trace suppliers and increase the opacity of counterfeit purchasing.
Three criteria have been proposed for a device to be considered counterfeit3:
• The device has no proven safety or efficacy among consumers. For example, the substantial threat of copycat devices in dermatology has been demonstrated by reports of burns caused by fake cryolipolysis devices.2
• The device violates patent rights or copy trademarks. Due to the regional nature of intellectual property rights, country-specific filings of patents and trademarks are required if protections are sought internationally. In this study, counterfeit devices originated in China, South Korea, and Thailand, where patent and trademark protections for the original devices do not extend.
• The device is falsely claimed to have been cleared by the FDA or other clinical regulatory authorities. Legitimate medical devices are subject to rounds of safety and compatibility testing using standards set by regulatory bodies, such as the FDA’s Center for Devices and Radiological Health, the International Organization of Standardization, and the International Electrotechnical Commission. Compliance with these safety standards is lost, however, among unregulated internet sales of medical devices. Our search revealed that 10.7% of the top 10 counterfeit device listings for each product explicitly mentioned FDA clearance in the product description. Among the thousands of listings on e-commerce sites, even a fraction that make spurious FDA-clearance claims can mislead consumers.
The issue of counterfeit medical devices has not gone unrecognized globally. In 2013, the World Health Organization created the Global Surveillance and Monitoring System to unify international efforts for reporting substandard, unlicensed, or falsified medical products.4 Although universal monitoring systems can improve detection of counterfeit products, we highlight the alarming continuing ease of purchasing counterfeit dermatologic devices through e-commerce websites. Due to the widespread nature of counterfeiting across all domains of medicine, the onus of curbing counterfeit dermatologic devices might be on dermatology providers to recognize and report such occurrences.
This exploration of counterfeit dermatologic devices revealed a lack of consistency throughout product listings on 2 popular e-commerce websites, Alibaba.com and DHgate.com. Given the alarming availability of these devices on the internet, practitioners should approach the purchase of any device with concern about counterfeiting. Future avenues of study might explore the prevalence of counterfeit devices used in dermatology practices and offer insight on regulation and consumer safety efforts.
- Wang JV, Zachary CB, Saedi N. Counterfeit esthetic devices and patient safety in dermatology. J Cosmet Dermatol. 2018;17:396-397. doi:10.1111/jocd.12526
- Biesman BS, Patel N. Physician alert: beware of counterfeit medical devices. Lasers Surg Med. 2014;46:528‐530. doi:10.1002/lsm.22275
- Stevens WG, Spring MA, Macias LH. Counterfeit medical devices: the money you save up front will cost you big in the end. Aesthet Surg J. 2014;34:786‐788. doi:10.1177/1090820X14529960
- Pisani E. WHO Global Surveillance and Monitoring System for Substandard and Falsified Medical Products. World Health Organization; 2017. Accessed November 21, 2021. https://www.who.int/medicines/regulation/ssffc/publications/GSMSreport_EN.pdf?ua=1
- Wang JV, Zachary CB, Saedi N. Counterfeit esthetic devices and patient safety in dermatology. J Cosmet Dermatol. 2018;17:396-397. doi:10.1111/jocd.12526
- Biesman BS, Patel N. Physician alert: beware of counterfeit medical devices. Lasers Surg Med. 2014;46:528‐530. doi:10.1002/lsm.22275
- Stevens WG, Spring MA, Macias LH. Counterfeit medical devices: the money you save up front will cost you big in the end. Aesthet Surg J. 2014;34:786‐788. doi:10.1177/1090820X14529960
- Pisani E. WHO Global Surveillance and Monitoring System for Substandard and Falsified Medical Products. World Health Organization; 2017. Accessed November 21, 2021. https://www.who.int/medicines/regulation/ssffc/publications/GSMSreport_EN.pdf?ua=1
Practice Points
- Among thousands of counterfeit dermatologic listings, there is great heterogeneity in the number of listings per different subtypes of dermatologic devices, device descriptions, and unit pricing, along with false claims of US Food and Drug Administration clearance.
- Given the prevalence of counterfeit medical devices readily available for purchase online, dermatology practitioners should be wary of the authenticity of any medical device purchased for clinical use.
What’s Eating You? Caterpillars
Causes of Lepidopterism
Caterpillars are wormlike organisms that serve as the larval stage of moths and butterflies, which belong to the order Lepidoptera. There are almost 165,000 discovered species, with 13,000 found in the United States.1,2 Roughly 150 species are known to have the potential to cause an adverse reaction in humans, with 50 of these in the United States.1Lepidopterism describes systemic and cutaneous reactions to moths, butterflies, and caterpillars; erucism describes strictly cutaneous reactions.1
Although the rate of lepidopterism is thought to be underreported because it often is self-limited and of a mild nature, a review found caterpillars to be the cause of roughly 2.2% of reported bites and stings annually.2 Cases increase in number with seasonal increases in caterpillars, which vary by region and species. For example, the Megalopyge opercularis (southern flannel moth) caterpillar was noted to have 2 peaks in a Texas-based study: 12% of reported stings occurred in July; 59% from October through November.3 In general, the likelihood of exposure increases during warmer months, and exposure is more common in people who work outdoors in a rural area or in a suburban area where there are many caterpillar-infested trees.4
Most cases of lepidopterism are caused by caterpillars, not by adult butterflies and moths, because the former have many tubular, or porous, hairlike structures called setae that are embedded in the integument. Setae were once thought to be connected to poison-secreting glandular cells, but current belief is that venomous caterpillars lack specialized gland cells and instead produce venom through secretory epithelial cells located above the integument.1 Venom accumulates in the hemolymph and is stored in the setae or other types of bristles, such as scoli (wartlike bumps that bear setae) or spines.5 With a large amount of chitin, bristles have a tendency to fracture and release venom upon contact.1 It is thought that some species of caterpillars formulate venom by ingesting toxins or toxin precursors from plants; for example, the tiger moth (family Arctiidae) is known to produce venom containing biogenic amines, pyrrolizidine, alkaloids, and cardiac glycosides obtained through food sources.5
Even if a caterpillar does not produce venom, its setae might embed into skin or mucous membranes and cause an adverse irritant reaction.1 Setae also might dislodge and be transported in the air to embed in objects—some remaining stable in the environment for longer than a year.2 In contrast to setae, spines are permanently fixed into the integument; for that reason, only direct contact with the caterpillar can result in an adverse reaction. Although it is mostly caterpillars that contain setae and spines, certain species of moths also might contain these structures or might acquire them as they emerge from the cocoon, which often contains incorporated setae.2
Reactions in Humans
Lepidopterism encompasses 3 principal reactions in humans: sting reaction, hypersensitivity reaction, and lonomism (a hemorrhagic diathesis produced by Lonomia caterpillars). The type and severity of the reaction depends on (1) the species of caterpillar or moth and (2) the individual patient.2 There are approximately 12 families of caterpillars, mainly of the moth variety, that can cause an adverse reaction in humans.1 Tables 1 and 2 list examples of species that cause each type of reaction.6
Chemicals and toxins contained in the poison of setae and spines vary by species of caterpillar. Numerous kinds have been isolated from different venoms,1,2 including several peptides, histamine, histamine-releasing substances, acetylcholine, phospholipase A, hyaluronidase, formic acid, proteins with trypsinlike activity, serine proteases such as kallikrein, and other enzymes with vasodegenerative and fibrinolytic properties
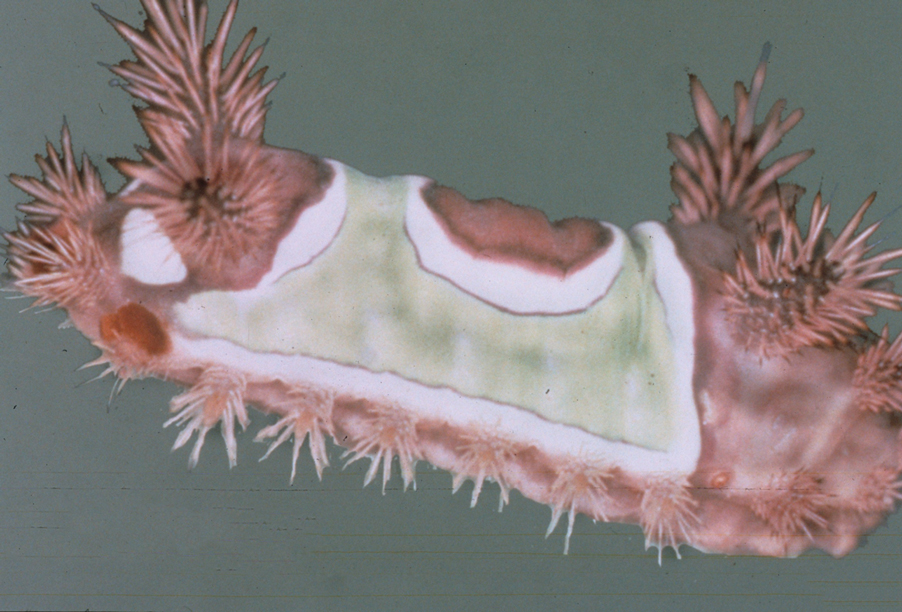
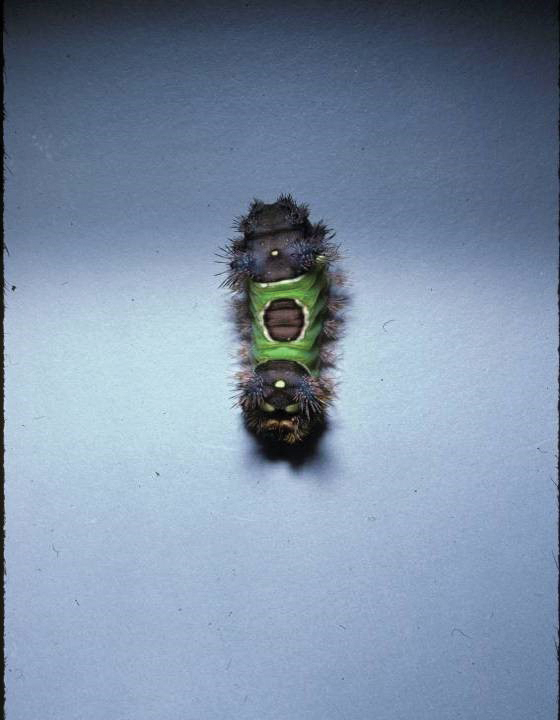
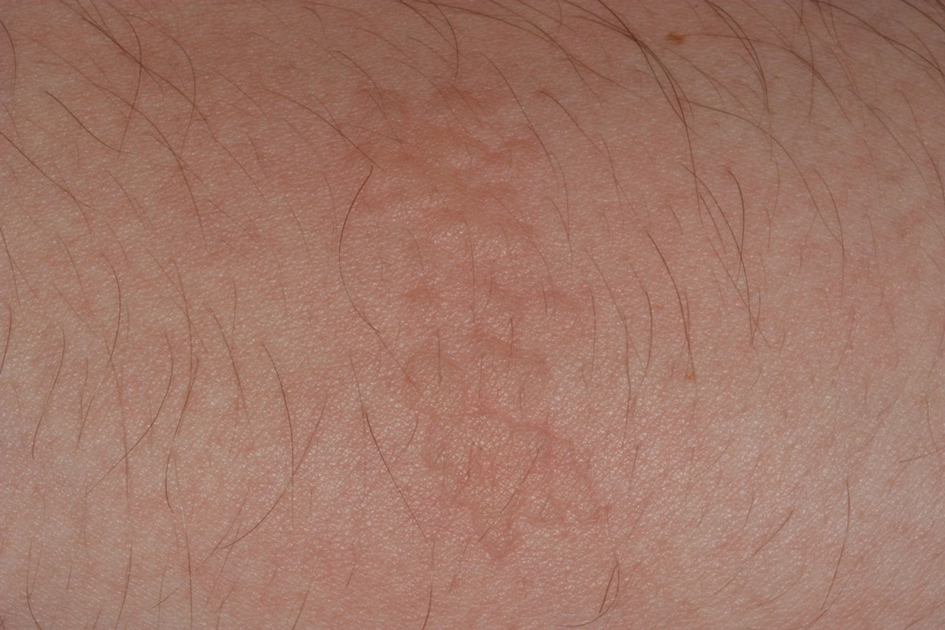
Stings: An Immediate Adverse Reaction—Depending on the venom, a sting might result in mild to severe burning pain, accompanied by welts, vesicles, and red papules or plaques.2 Figure 1 demonstrates a particularly mild sting from a caterpillar of the family Automeris, examples of which are seen in Figures 2 and 3 and eFigure 1. Components of the venom determine the mechanism of the sting and the pain that accompanies it. For example, a recent study demonstrated that the venom of the Latoia consocia caterpillar induces pain through the ion-channel receptor known as transient receptor potential vanilloid 1, which integrates and sends painful stimuli from the peripheral nervous system to the central nervous system.7 It is thought that a variety of ion channels are targets of the venom of caterpillars.
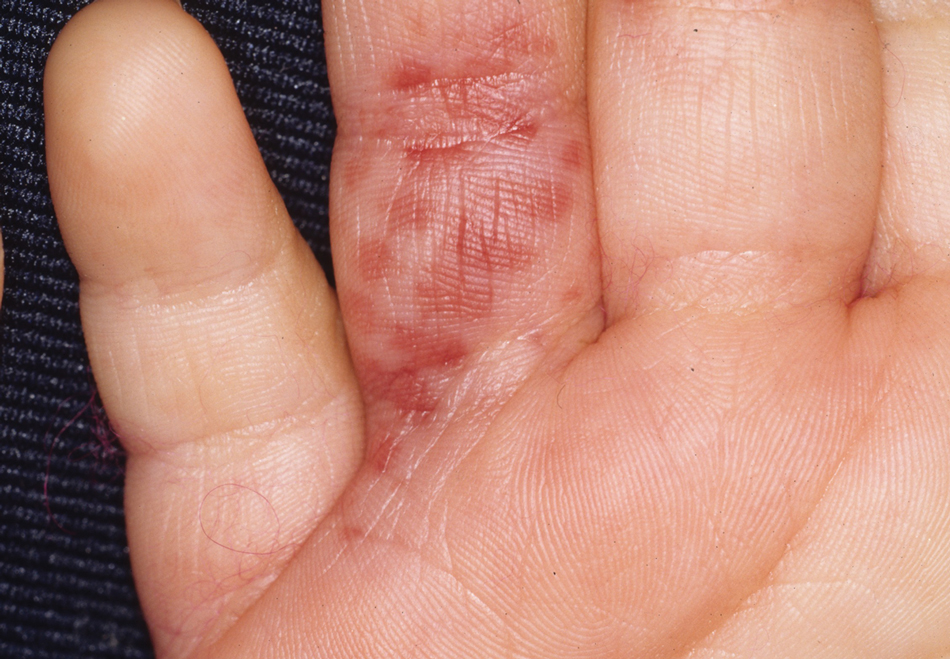
One of the most characteristic sting patterns is that of the caterpillar of family Megalopygidae (flannel moth)(eFigures 2 and 3). The stings of these caterpillars create a unique tram-track pattern of hemorrhagic macules or papules (Figure 4).4 A study found that 90% of reported M opercularis envenomations consist primarily of cutaneous symptoms, with 84% of those symptoms being irritation or pain; 45% a puncture or wound; 29% erythema; and 15% edema.3 Systemic findings can include headache, fever, adenopathy, nausea, vomiting, abdominal pain, and chest pain.4 Symptoms normally are self-limited, though they can last minutes or hours.
Hypersensitivity Reaction—Studies demonstrate that the symptoms of this reaction are a mixture of type I hypersensitivity, type IV hypersensitivity, and a foreign-body response.2 The specific hypersensitivity reaction depends on the venom and the exposed individual—most commonly including a combination of pruritic papules, urticarial wheals, flares, and dermatitis.2 A reaction that is a result of direct contact with the caterpillar or moth will appear on exposed areas; however, because setae embed in linens and clothing, they might cause a reaction anywhere on the body. Although usually self-limited, a hypersensitivity reaction might develop within minutes and can last for days or weeks.
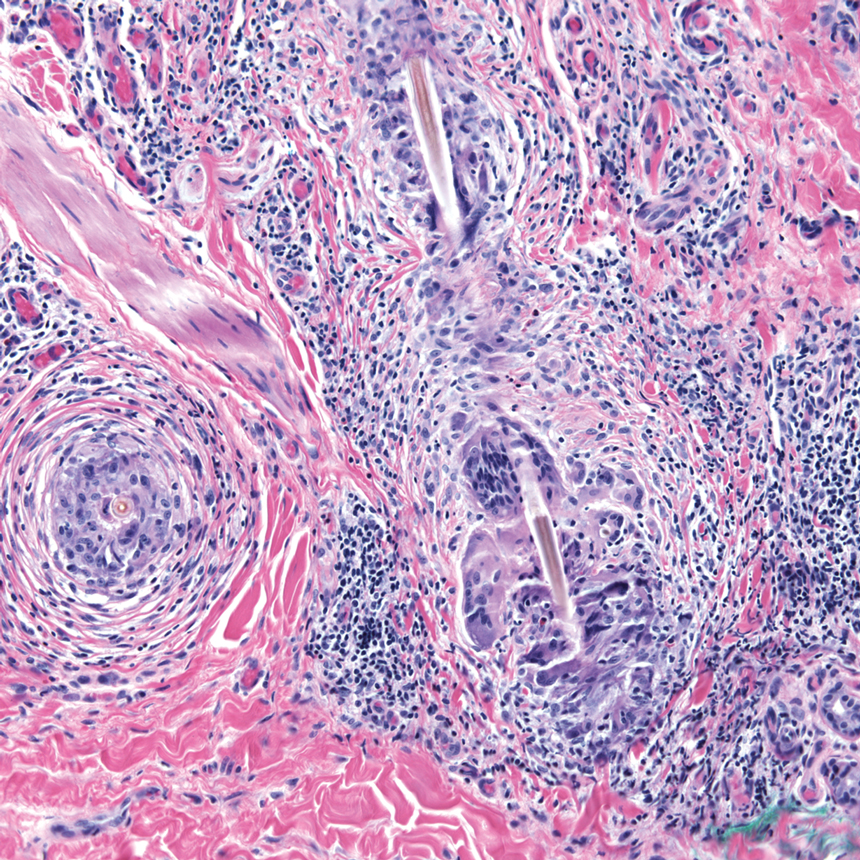
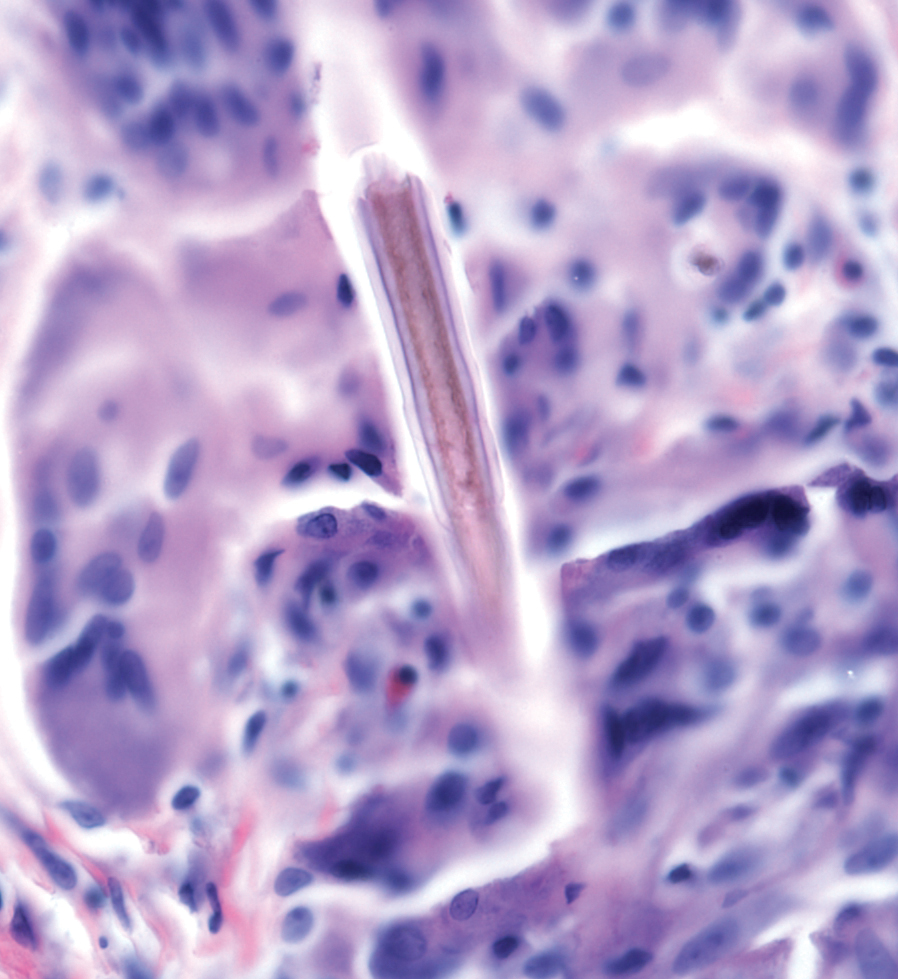
Stings and hypersensitivity reactions to caterpillars and moths tend to lead to a nonspecific histologic presentation characterized by epidermal edema and a superficial perivascular lymphocytic infiltrate, often with eosinophils.6 After approximately 1 week, a foreign-body response to setae can lead to tuberculoid granulomas accompanied by neutrophils in the dermis and occasionally in subcutaneous tissues (Figures 5 and 6).8 If setae have not yet been removed, they also might be visible in skin scrapings.
Additional complications can accompany the hypersensitivity reaction to setae or spines. Type I hypersensitivity reactions can lead to severe reactions on second contact due to previously sensitized IgE antibodies. Although the first reaction appears mild, second contact might result in angioedema, wheezing, dyspnea, or anaphylaxis, or a combination of these findings.9 In addition, some patients who come in contact with Dendrolimus caterpillars might develop a condition known as dendrolimiasis, characterized by dermatitis in addition to arthritis or chondritis.6 The arthritis is normally monoarticular and can result in complete destruction of the joint. Pararamose, a condition with a similar presentation, is caused by the Brazilian moth Premolis semirufa.6
Contact of setae or spines with mucous membranes or inhalation of setae also might result in edema, dysphagia, dyspnea, drooling, rhinitis, or conjunctivitis, or a combination of these findings.6 In addition, setae can embed in the eye and cause an inflammatory reaction—ophthalmia nodosa—most commonly caused by caterpillars of the pine processionary moth (Thaumetopoea pityocampa) and characterized by immediate chemosis, which can progress to liquefactive necrosis and hypopyon, later developing into a granulomatous foreign-body response.2,10 The process is thought to be the result of a combination of the thaumetopoein toxin in the setae and an IgE-mediated response to other proteins.10
Due to their harpoon shape and forward-only motion, setae might migrate deeper, potentially even to the optic nerve.11 Because migration might take years and the barbed shape of setae does not always allow removal, some patients require lifetime monitoring with slit-lamp examination.Chronic problems, such as cataracts and persistent posterior uveitis, have been reported.10,11
Lonomism—One of the most serious (though rarest) reactions to caterpillars is lonomism, a condition caused by the caterpillars of Lonomia achelous and Lonomia obliqua moths. These caterpillars have a unique combination of toxins filling their branched spines, which ultimately leads to the same outcome: a hemorrhagic diathesis.
The toxin of L achelous comprises several proteases that degrade fibrin, fibrinogen, and factor XIII while activating prothrombin. In contrast, L obliqua poison causes a hemorrhagic diathesis by promoting a consumptive coagulopathy through enzymes that activate factor X and prothrombin.
With initial contact with either of these Lonomia caterpillars, the patient experiences severe pain accompanied by systemic symptoms, including headache, nausea, and vomiting. Shortly afterward, symptoms of a hemorrhagic diathesis manifest, including bleeding gums, hematuria, bleeding from prior wounds, and epistaxis.5 Serious complications of the hemorrhagic diathesis, such as hemorrhage of major organs, leads to death in 4% of patients.5 A reported case of a patient whose Lonomia caterpillar sting went unrecognized until a week after the accident ended with progression to stage V chronic renal disease.12
Recent research has focused on the specific mechanism of injury caused by Lonomia species. A study found that the venom of L obliqua causes cytoskeleton rearrangement and migration in vascular smooth muscle cells (VSMCs) by inducing formation of reactive oxygen species through activation of nicotinamide adenine dinucleotide phosphate oxidase.13 Thus, the venom directly contributes to the proinflammatory phenotype of endothelial cells seen following envenomation. The same study also demonstrated that elevated reactive oxygen species trigger extracellular signal-regulated kinase pathway activation in VSMCs, leading to cell proliferation, re-stenosis, and ischemia.13 This finding was confirmed by another study,14 which demonstrated an increase in Rac1, a signaling protein involved in the extracellular signal-regulated kinase pathway, in VSMCs upon exposure to L obliqua venom. These studies propose potential new targets for treatment to prevent vascular damage.
Reactions to Adult Organisms—Although it is more common for the caterpillar form of these organisms to cause an adverse reaction, the adult moth also might be capable of causing a similar reaction by retaining setae from the cocoon or by their own spines. The most notable example of this is female moths of the genus Hylesia, which possess spines attached to glands on the abdomen. The poison in these spines—a mixture of proteases and chitinase—causes a dermatitis known as Caripito itch—the name derived from a river port in Venezuela where this moth caused a memorable epidemic of moth-induced dermatitis.7,15 Caripito itch is known for intense pruritus that most commonly lasts days or weeks, possibly longer than 1 year.
Diagnostic Difficulties
The challenge of diagnosing a caterpillar- or moth-induced reaction in humans arises from (1) the lack of clinical history (the caterpillar might not be seen at all by the patient or the examiner) and (2) the similarity of these reactions to those with more common triggers.
When setae remain embedded in the skin or mucous membranes, skin scrapings allow accelerated diagnosis. On a skin scraping prepared with 20% potassium hydroxide, setae appear as tapered and barbed hairlike structures, which allows them to be distinguished from other similar-appearing but differently shaped structures, such as glass fibers.
When setae do not remain embedded in the skin or when the cause of the reaction is due to spines, the physician is left with a nonspecific histologic picture and a large differential diagnosis to be narrowed down based on the history and occasionally the pattern of the skin lesion.
A challenge in sting diagnosis is differentiating a caterpillar or moth sting from that of another organism. In certain cases, such as those of the family Megalopygidae, specific patterns of stings might assist in making the diagnosis. Hypersensitivity reactions are associated with a wider differential diagnosis, including irritant or allergic dermatitis from other causes, scabies, eczema, lichen planus, lichen simplex chronicus, seborrheic dermatitis, and tinea corporis, to name a few.6 Skin scrapings can be examined for other features, such as burrows in the case of scabies, to further narrow the differential.
Stings and hypersensitivity reactions lacking a proper history and associated with more severe systemic symptoms have caused misdiagnosis or led to a workup for the wrong condition; for example, the picture of abdominal pain, nausea, vomiting, tachycardia, leukocytosis, hypokalemia, and metabolic acidosis can simulate appendicitis.16 Upon discovery of a puss caterpillar sting in a patient, her symptoms resolved after treatment with ondansetron, morphine, and intravenous fluids.16
In lonomism, the diagnosis must be established by laboratory measurement of the fibrinogen level, clotting factors, prothrombin time, and activated partial thromboplastin time.4 The differential diagnosis associated with lonomism includes disseminated intravascular coagulation (DIC), snakebite, and a hereditary bleeding disorder.4 The combination of laboratory tests and an extensive medical history allows a diagnosis. Absence of a personal or family history of bleeding excludes a diagnosis of hereditary bleeding disorder, whereas the absence of known causes of DIC or thrombocytopenia allows DIC to be excluded from the differential.
Treatment Options and Prevention
Treatment—The first step is to remove any embedded setae from the skin or mucous membranes. The stepwise recommendation is to remove any constricted clothing, detach setae with adhesive tape, wash with soap and water, and dry without touching the skin.1 Any remaining setae can be removed with additional tape or forceps; setae tend to be fragile and are difficult to remove in their entirety.
Other than removal of the setae, skin reactions are treated symptomatically. Ice packs and isopropyl alcohol have been utilized to cool burning or stinging areas. Pain, pruritus, and inflammation have been alleviated with antihistamines and topical corticosteroids.1 When pain is severe, oral codeine or local injection of anesthetic can be used. For severe and persistent skin lesions, a course of an oral glucocorticoid can be administered. Intramuscular triamcinolone acetonide has been shown to treat pain, dermatitis, and subcutaneous nodules otherwise refractory to treatment.8
Antivenin specific for L obliqua exists to treat lonomism and is therefore effective only when lonomism is caused by that species. Lonomism caused by L achelous is treated with cryoprecipitate, purified fibrinogen, and antifibrinolytic drugs, such as aprotinin.6 Whole blood and fresh-frozen plasma have been noted to make hemorrhage worse when utilized to treat lonomism. Because the mechanism of action of the venom of Lonomia species is based, in part, on inducing a proinflammatory profile in endothelial cells, studies have demonstrated that inhibition of kallikrein might prevent vascular injury and thus prevent serious adverse effects, such as renal failure.17
Prevention—People should wear proper protective clothing when outdoors in potentially infested areas. Measures should be taken to ensure that linens and clothing are not left outside in areas where setae might be carried on the wind. Infestation control is necessary if the population of caterpillars reaches a high enough level.
Conclusion
Several species of caterpillars and moths cause adverse reactions in humans: stings, hypersensitivity reactions, and lonomism. Although most reactions are self-limited, some might have more serious effects, including organ failure and death. Mechanisms of injury vary by species of caterpillar, moth, and butterfly; current research is focused on further defining venom components and signaling pathways to isolate potential targets to aid in the diagnosis and treatment of lepidopterism.
- Goldman BS, Bragg BN. Caterpillar and moth bites. Stat Pearls [Internet]. StatPearls Publishing. Updated August 3, 2021. Accessed November 4, 2021. https://www.ncbi.nlm.nih.gov/books/NBK539851/
- Hossler EW. Caterpillars and moths: part I. Dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:1-10. doi:10.1016/j.jaad.2009.08.060
- Forrester MB. Megalopyge opercularis caterpillar stings reported to Texas poison centers. Wilderness Environ Med. 2018;29:215-220. doi:10.1016/j.wem.2018.02.002
- Hossler EW. Lepidopterism: skin disorders secondary to caterpillars and moths. UpToDate website. Published October 20, 2021. Accessed November 18, 2021. https://www.uptodate.com/contents/lepidopterism-skin-disorders-secondary-to-caterpillars-and-moths
- Villas-Boas IM, Bonfá G, Tambourgi DV. Venomous caterpillars: from inoculation apparatus to venom composition and envenomation. Toxicon. 2018;153:39-52. doi:10.1016/j.toxicon.2018.08.007
- Hossler EW. Caterpillars and moths: part II. dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:13-28. doi:10.1016/j.jaad.2009.08.061
- Yao Z, Kamau PM, Han Y, et al. The Latoia consocia caterpillar induces pain by targeting nociceptive ion channel TRPV1. Toxins (Basel). 2019;11:695. doi:10.3390/toxins11120695
- Paniz-Mondolfi AE, Pérez-Alvarez AM, Lundberg U, et al. Cutaneous lepidopterism: dermatitis from contact with moths of Hylesia metabus (Cramer 1775) (Lepidoptera: Saturniidae), the causative agent of caripito itch. Int J Dermatol. 2011;50:535-541. doi:10.1111/j.1365-4632.2010.04683.x
- Santos-Magadán S, González de Olano D, Bartolomé-Zavala B, et al. Adverse reactions to the processionary caterpillar: irritant or allergic mechanism? Contact Dermatitis. 2009;60:109-110. doi:10.1111/j.1600-0536.2008.01464.x
- González-Martín-Moro J, Contreras-Martín I, Castro-Rebollo M, et al. Focal cortical cataract due to caterpillar hair migration. Clin Exp Optom. 2019;102:89-90. doi:10.1111/cxo.12809
- Singh A, Behera UC, Agrawal H. Intra-lenticular caterpillar seta in ophthalmia nodosa. Eur J Ophthalmol. 2021;31:NP109-NP111. doi:10.1177/1120672119858899
- Schmitberger PA, Fernandes TC, Santos RC, et al. Probable chronic renal failure caused by Lonomia caterpillar envenomation. J Venom Anim Toxins Incl Trop Dis. 2013;19:14. doi:10.1186/1678-9199-19-14
- Moraes JA, Rodrigues G, Nascimento-Silva V, et al. Effects of Lonomia obliqua venom on vascular smooth muscle cells: contribution of NADPH oxidase-derived reactive oxygen species. Toxins (Basel). 2017;9:360. doi:10.3390/toxins9110360
- Bernardi L, Pinto AFM, Mendes E, et al. Lonomia obliqua bristle extract modulates Rac1 activation, membrane dynamics and cell adhesion properties. Toxicon. 2019;162:32-39. doi:10.1016/j.toxicon.2019.02.019
- Cabrera G, Lundberg U, Rodríguez-Ulloa A, et al. Protein content of the Hylesia metabus egg nest setae (Cramer [1775]) (Lepidoptera: Saturniidae) and its association with the parental investment for the reproductive success and lepidopterism. J Proteomics. 2017;150:183-200. doi:10.1016/j.jprot.2016.08.010
- Greene SC, Carey JM. Puss caterpillar envenomation: erucism mimicking appendicitis in a young child. Pediatr Emerg Care. 2020;36:E732-E734. doi:10.1097/PEC.0000000000001514
- Berger M, de Moraes JA, Beys-da-Silva WO, et al. Renal and vascular effects of kallikrein inhibition in a model of Lonomia obliqua venom-induced acute kidney injury. PLoS Negl Trop Dis. 2019;13:e0007197. doi:10.1371/journal.pntd.0007197
Causes of Lepidopterism
Caterpillars are wormlike organisms that serve as the larval stage of moths and butterflies, which belong to the order Lepidoptera. There are almost 165,000 discovered species, with 13,000 found in the United States.1,2 Roughly 150 species are known to have the potential to cause an adverse reaction in humans, with 50 of these in the United States.1Lepidopterism describes systemic and cutaneous reactions to moths, butterflies, and caterpillars; erucism describes strictly cutaneous reactions.1
Although the rate of lepidopterism is thought to be underreported because it often is self-limited and of a mild nature, a review found caterpillars to be the cause of roughly 2.2% of reported bites and stings annually.2 Cases increase in number with seasonal increases in caterpillars, which vary by region and species. For example, the Megalopyge opercularis (southern flannel moth) caterpillar was noted to have 2 peaks in a Texas-based study: 12% of reported stings occurred in July; 59% from October through November.3 In general, the likelihood of exposure increases during warmer months, and exposure is more common in people who work outdoors in a rural area or in a suburban area where there are many caterpillar-infested trees.4
Most cases of lepidopterism are caused by caterpillars, not by adult butterflies and moths, because the former have many tubular, or porous, hairlike structures called setae that are embedded in the integument. Setae were once thought to be connected to poison-secreting glandular cells, but current belief is that venomous caterpillars lack specialized gland cells and instead produce venom through secretory epithelial cells located above the integument.1 Venom accumulates in the hemolymph and is stored in the setae or other types of bristles, such as scoli (wartlike bumps that bear setae) or spines.5 With a large amount of chitin, bristles have a tendency to fracture and release venom upon contact.1 It is thought that some species of caterpillars formulate venom by ingesting toxins or toxin precursors from plants; for example, the tiger moth (family Arctiidae) is known to produce venom containing biogenic amines, pyrrolizidine, alkaloids, and cardiac glycosides obtained through food sources.5
Even if a caterpillar does not produce venom, its setae might embed into skin or mucous membranes and cause an adverse irritant reaction.1 Setae also might dislodge and be transported in the air to embed in objects—some remaining stable in the environment for longer than a year.2 In contrast to setae, spines are permanently fixed into the integument; for that reason, only direct contact with the caterpillar can result in an adverse reaction. Although it is mostly caterpillars that contain setae and spines, certain species of moths also might contain these structures or might acquire them as they emerge from the cocoon, which often contains incorporated setae.2
Reactions in Humans
Lepidopterism encompasses 3 principal reactions in humans: sting reaction, hypersensitivity reaction, and lonomism (a hemorrhagic diathesis produced by Lonomia caterpillars). The type and severity of the reaction depends on (1) the species of caterpillar or moth and (2) the individual patient.2 There are approximately 12 families of caterpillars, mainly of the moth variety, that can cause an adverse reaction in humans.1 Tables 1 and 2 list examples of species that cause each type of reaction.6
Chemicals and toxins contained in the poison of setae and spines vary by species of caterpillar. Numerous kinds have been isolated from different venoms,1,2 including several peptides, histamine, histamine-releasing substances, acetylcholine, phospholipase A, hyaluronidase, formic acid, proteins with trypsinlike activity, serine proteases such as kallikrein, and other enzymes with vasodegenerative and fibrinolytic properties
Stings: An Immediate Adverse Reaction—Depending on the venom, a sting might result in mild to severe burning pain, accompanied by welts, vesicles, and red papules or plaques.2 Figure 1 demonstrates a particularly mild sting from a caterpillar of the family Automeris, examples of which are seen in Figures 2 and 3 and eFigure 1. Components of the venom determine the mechanism of the sting and the pain that accompanies it. For example, a recent study demonstrated that the venom of the Latoia consocia caterpillar induces pain through the ion-channel receptor known as transient receptor potential vanilloid 1, which integrates and sends painful stimuli from the peripheral nervous system to the central nervous system.7 It is thought that a variety of ion channels are targets of the venom of caterpillars.
One of the most characteristic sting patterns is that of the caterpillar of family Megalopygidae (flannel moth)(eFigures 2 and 3). The stings of these caterpillars create a unique tram-track pattern of hemorrhagic macules or papules (Figure 4).4 A study found that 90% of reported M opercularis envenomations consist primarily of cutaneous symptoms, with 84% of those symptoms being irritation or pain; 45% a puncture or wound; 29% erythema; and 15% edema.3 Systemic findings can include headache, fever, adenopathy, nausea, vomiting, abdominal pain, and chest pain.4 Symptoms normally are self-limited, though they can last minutes or hours.
Hypersensitivity Reaction—Studies demonstrate that the symptoms of this reaction are a mixture of type I hypersensitivity, type IV hypersensitivity, and a foreign-body response.2 The specific hypersensitivity reaction depends on the venom and the exposed individual—most commonly including a combination of pruritic papules, urticarial wheals, flares, and dermatitis.2 A reaction that is a result of direct contact with the caterpillar or moth will appear on exposed areas; however, because setae embed in linens and clothing, they might cause a reaction anywhere on the body. Although usually self-limited, a hypersensitivity reaction might develop within minutes and can last for days or weeks.
Stings and hypersensitivity reactions to caterpillars and moths tend to lead to a nonspecific histologic presentation characterized by epidermal edema and a superficial perivascular lymphocytic infiltrate, often with eosinophils.6 After approximately 1 week, a foreign-body response to setae can lead to tuberculoid granulomas accompanied by neutrophils in the dermis and occasionally in subcutaneous tissues (Figures 5 and 6).8 If setae have not yet been removed, they also might be visible in skin scrapings.
Additional complications can accompany the hypersensitivity reaction to setae or spines. Type I hypersensitivity reactions can lead to severe reactions on second contact due to previously sensitized IgE antibodies. Although the first reaction appears mild, second contact might result in angioedema, wheezing, dyspnea, or anaphylaxis, or a combination of these findings.9 In addition, some patients who come in contact with Dendrolimus caterpillars might develop a condition known as dendrolimiasis, characterized by dermatitis in addition to arthritis or chondritis.6 The arthritis is normally monoarticular and can result in complete destruction of the joint. Pararamose, a condition with a similar presentation, is caused by the Brazilian moth Premolis semirufa.6
Contact of setae or spines with mucous membranes or inhalation of setae also might result in edema, dysphagia, dyspnea, drooling, rhinitis, or conjunctivitis, or a combination of these findings.6 In addition, setae can embed in the eye and cause an inflammatory reaction—ophthalmia nodosa—most commonly caused by caterpillars of the pine processionary moth (Thaumetopoea pityocampa) and characterized by immediate chemosis, which can progress to liquefactive necrosis and hypopyon, later developing into a granulomatous foreign-body response.2,10 The process is thought to be the result of a combination of the thaumetopoein toxin in the setae and an IgE-mediated response to other proteins.10
Due to their harpoon shape and forward-only motion, setae might migrate deeper, potentially even to the optic nerve.11 Because migration might take years and the barbed shape of setae does not always allow removal, some patients require lifetime monitoring with slit-lamp examination.Chronic problems, such as cataracts and persistent posterior uveitis, have been reported.10,11
Lonomism—One of the most serious (though rarest) reactions to caterpillars is lonomism, a condition caused by the caterpillars of Lonomia achelous and Lonomia obliqua moths. These caterpillars have a unique combination of toxins filling their branched spines, which ultimately leads to the same outcome: a hemorrhagic diathesis.
The toxin of L achelous comprises several proteases that degrade fibrin, fibrinogen, and factor XIII while activating prothrombin. In contrast, L obliqua poison causes a hemorrhagic diathesis by promoting a consumptive coagulopathy through enzymes that activate factor X and prothrombin.
With initial contact with either of these Lonomia caterpillars, the patient experiences severe pain accompanied by systemic symptoms, including headache, nausea, and vomiting. Shortly afterward, symptoms of a hemorrhagic diathesis manifest, including bleeding gums, hematuria, bleeding from prior wounds, and epistaxis.5 Serious complications of the hemorrhagic diathesis, such as hemorrhage of major organs, leads to death in 4% of patients.5 A reported case of a patient whose Lonomia caterpillar sting went unrecognized until a week after the accident ended with progression to stage V chronic renal disease.12
Recent research has focused on the specific mechanism of injury caused by Lonomia species. A study found that the venom of L obliqua causes cytoskeleton rearrangement and migration in vascular smooth muscle cells (VSMCs) by inducing formation of reactive oxygen species through activation of nicotinamide adenine dinucleotide phosphate oxidase.13 Thus, the venom directly contributes to the proinflammatory phenotype of endothelial cells seen following envenomation. The same study also demonstrated that elevated reactive oxygen species trigger extracellular signal-regulated kinase pathway activation in VSMCs, leading to cell proliferation, re-stenosis, and ischemia.13 This finding was confirmed by another study,14 which demonstrated an increase in Rac1, a signaling protein involved in the extracellular signal-regulated kinase pathway, in VSMCs upon exposure to L obliqua venom. These studies propose potential new targets for treatment to prevent vascular damage.
Reactions to Adult Organisms—Although it is more common for the caterpillar form of these organisms to cause an adverse reaction, the adult moth also might be capable of causing a similar reaction by retaining setae from the cocoon or by their own spines. The most notable example of this is female moths of the genus Hylesia, which possess spines attached to glands on the abdomen. The poison in these spines—a mixture of proteases and chitinase—causes a dermatitis known as Caripito itch—the name derived from a river port in Venezuela where this moth caused a memorable epidemic of moth-induced dermatitis.7,15 Caripito itch is known for intense pruritus that most commonly lasts days or weeks, possibly longer than 1 year.
Diagnostic Difficulties
The challenge of diagnosing a caterpillar- or moth-induced reaction in humans arises from (1) the lack of clinical history (the caterpillar might not be seen at all by the patient or the examiner) and (2) the similarity of these reactions to those with more common triggers.
When setae remain embedded in the skin or mucous membranes, skin scrapings allow accelerated diagnosis. On a skin scraping prepared with 20% potassium hydroxide, setae appear as tapered and barbed hairlike structures, which allows them to be distinguished from other similar-appearing but differently shaped structures, such as glass fibers.
When setae do not remain embedded in the skin or when the cause of the reaction is due to spines, the physician is left with a nonspecific histologic picture and a large differential diagnosis to be narrowed down based on the history and occasionally the pattern of the skin lesion.
A challenge in sting diagnosis is differentiating a caterpillar or moth sting from that of another organism. In certain cases, such as those of the family Megalopygidae, specific patterns of stings might assist in making the diagnosis. Hypersensitivity reactions are associated with a wider differential diagnosis, including irritant or allergic dermatitis from other causes, scabies, eczema, lichen planus, lichen simplex chronicus, seborrheic dermatitis, and tinea corporis, to name a few.6 Skin scrapings can be examined for other features, such as burrows in the case of scabies, to further narrow the differential.
Stings and hypersensitivity reactions lacking a proper history and associated with more severe systemic symptoms have caused misdiagnosis or led to a workup for the wrong condition; for example, the picture of abdominal pain, nausea, vomiting, tachycardia, leukocytosis, hypokalemia, and metabolic acidosis can simulate appendicitis.16 Upon discovery of a puss caterpillar sting in a patient, her symptoms resolved after treatment with ondansetron, morphine, and intravenous fluids.16
In lonomism, the diagnosis must be established by laboratory measurement of the fibrinogen level, clotting factors, prothrombin time, and activated partial thromboplastin time.4 The differential diagnosis associated with lonomism includes disseminated intravascular coagulation (DIC), snakebite, and a hereditary bleeding disorder.4 The combination of laboratory tests and an extensive medical history allows a diagnosis. Absence of a personal or family history of bleeding excludes a diagnosis of hereditary bleeding disorder, whereas the absence of known causes of DIC or thrombocytopenia allows DIC to be excluded from the differential.
Treatment Options and Prevention
Treatment—The first step is to remove any embedded setae from the skin or mucous membranes. The stepwise recommendation is to remove any constricted clothing, detach setae with adhesive tape, wash with soap and water, and dry without touching the skin.1 Any remaining setae can be removed with additional tape or forceps; setae tend to be fragile and are difficult to remove in their entirety.
Other than removal of the setae, skin reactions are treated symptomatically. Ice packs and isopropyl alcohol have been utilized to cool burning or stinging areas. Pain, pruritus, and inflammation have been alleviated with antihistamines and topical corticosteroids.1 When pain is severe, oral codeine or local injection of anesthetic can be used. For severe and persistent skin lesions, a course of an oral glucocorticoid can be administered. Intramuscular triamcinolone acetonide has been shown to treat pain, dermatitis, and subcutaneous nodules otherwise refractory to treatment.8
Antivenin specific for L obliqua exists to treat lonomism and is therefore effective only when lonomism is caused by that species. Lonomism caused by L achelous is treated with cryoprecipitate, purified fibrinogen, and antifibrinolytic drugs, such as aprotinin.6 Whole blood and fresh-frozen plasma have been noted to make hemorrhage worse when utilized to treat lonomism. Because the mechanism of action of the venom of Lonomia species is based, in part, on inducing a proinflammatory profile in endothelial cells, studies have demonstrated that inhibition of kallikrein might prevent vascular injury and thus prevent serious adverse effects, such as renal failure.17
Prevention—People should wear proper protective clothing when outdoors in potentially infested areas. Measures should be taken to ensure that linens and clothing are not left outside in areas where setae might be carried on the wind. Infestation control is necessary if the population of caterpillars reaches a high enough level.
Conclusion
Several species of caterpillars and moths cause adverse reactions in humans: stings, hypersensitivity reactions, and lonomism. Although most reactions are self-limited, some might have more serious effects, including organ failure and death. Mechanisms of injury vary by species of caterpillar, moth, and butterfly; current research is focused on further defining venom components and signaling pathways to isolate potential targets to aid in the diagnosis and treatment of lepidopterism.
Causes of Lepidopterism
Caterpillars are wormlike organisms that serve as the larval stage of moths and butterflies, which belong to the order Lepidoptera. There are almost 165,000 discovered species, with 13,000 found in the United States.1,2 Roughly 150 species are known to have the potential to cause an adverse reaction in humans, with 50 of these in the United States.1Lepidopterism describes systemic and cutaneous reactions to moths, butterflies, and caterpillars; erucism describes strictly cutaneous reactions.1
Although the rate of lepidopterism is thought to be underreported because it often is self-limited and of a mild nature, a review found caterpillars to be the cause of roughly 2.2% of reported bites and stings annually.2 Cases increase in number with seasonal increases in caterpillars, which vary by region and species. For example, the Megalopyge opercularis (southern flannel moth) caterpillar was noted to have 2 peaks in a Texas-based study: 12% of reported stings occurred in July; 59% from October through November.3 In general, the likelihood of exposure increases during warmer months, and exposure is more common in people who work outdoors in a rural area or in a suburban area where there are many caterpillar-infested trees.4
Most cases of lepidopterism are caused by caterpillars, not by adult butterflies and moths, because the former have many tubular, or porous, hairlike structures called setae that are embedded in the integument. Setae were once thought to be connected to poison-secreting glandular cells, but current belief is that venomous caterpillars lack specialized gland cells and instead produce venom through secretory epithelial cells located above the integument.1 Venom accumulates in the hemolymph and is stored in the setae or other types of bristles, such as scoli (wartlike bumps that bear setae) or spines.5 With a large amount of chitin, bristles have a tendency to fracture and release venom upon contact.1 It is thought that some species of caterpillars formulate venom by ingesting toxins or toxin precursors from plants; for example, the tiger moth (family Arctiidae) is known to produce venom containing biogenic amines, pyrrolizidine, alkaloids, and cardiac glycosides obtained through food sources.5
Even if a caterpillar does not produce venom, its setae might embed into skin or mucous membranes and cause an adverse irritant reaction.1 Setae also might dislodge and be transported in the air to embed in objects—some remaining stable in the environment for longer than a year.2 In contrast to setae, spines are permanently fixed into the integument; for that reason, only direct contact with the caterpillar can result in an adverse reaction. Although it is mostly caterpillars that contain setae and spines, certain species of moths also might contain these structures or might acquire them as they emerge from the cocoon, which often contains incorporated setae.2
Reactions in Humans
Lepidopterism encompasses 3 principal reactions in humans: sting reaction, hypersensitivity reaction, and lonomism (a hemorrhagic diathesis produced by Lonomia caterpillars). The type and severity of the reaction depends on (1) the species of caterpillar or moth and (2) the individual patient.2 There are approximately 12 families of caterpillars, mainly of the moth variety, that can cause an adverse reaction in humans.1 Tables 1 and 2 list examples of species that cause each type of reaction.6
Chemicals and toxins contained in the poison of setae and spines vary by species of caterpillar. Numerous kinds have been isolated from different venoms,1,2 including several peptides, histamine, histamine-releasing substances, acetylcholine, phospholipase A, hyaluronidase, formic acid, proteins with trypsinlike activity, serine proteases such as kallikrein, and other enzymes with vasodegenerative and fibrinolytic properties
Stings: An Immediate Adverse Reaction—Depending on the venom, a sting might result in mild to severe burning pain, accompanied by welts, vesicles, and red papules or plaques.2 Figure 1 demonstrates a particularly mild sting from a caterpillar of the family Automeris, examples of which are seen in Figures 2 and 3 and eFigure 1. Components of the venom determine the mechanism of the sting and the pain that accompanies it. For example, a recent study demonstrated that the venom of the Latoia consocia caterpillar induces pain through the ion-channel receptor known as transient receptor potential vanilloid 1, which integrates and sends painful stimuli from the peripheral nervous system to the central nervous system.7 It is thought that a variety of ion channels are targets of the venom of caterpillars.
One of the most characteristic sting patterns is that of the caterpillar of family Megalopygidae (flannel moth)(eFigures 2 and 3). The stings of these caterpillars create a unique tram-track pattern of hemorrhagic macules or papules (Figure 4).4 A study found that 90% of reported M opercularis envenomations consist primarily of cutaneous symptoms, with 84% of those symptoms being irritation or pain; 45% a puncture or wound; 29% erythema; and 15% edema.3 Systemic findings can include headache, fever, adenopathy, nausea, vomiting, abdominal pain, and chest pain.4 Symptoms normally are self-limited, though they can last minutes or hours.
Hypersensitivity Reaction—Studies demonstrate that the symptoms of this reaction are a mixture of type I hypersensitivity, type IV hypersensitivity, and a foreign-body response.2 The specific hypersensitivity reaction depends on the venom and the exposed individual—most commonly including a combination of pruritic papules, urticarial wheals, flares, and dermatitis.2 A reaction that is a result of direct contact with the caterpillar or moth will appear on exposed areas; however, because setae embed in linens and clothing, they might cause a reaction anywhere on the body. Although usually self-limited, a hypersensitivity reaction might develop within minutes and can last for days or weeks.
Stings and hypersensitivity reactions to caterpillars and moths tend to lead to a nonspecific histologic presentation characterized by epidermal edema and a superficial perivascular lymphocytic infiltrate, often with eosinophils.6 After approximately 1 week, a foreign-body response to setae can lead to tuberculoid granulomas accompanied by neutrophils in the dermis and occasionally in subcutaneous tissues (Figures 5 and 6).8 If setae have not yet been removed, they also might be visible in skin scrapings.
Additional complications can accompany the hypersensitivity reaction to setae or spines. Type I hypersensitivity reactions can lead to severe reactions on second contact due to previously sensitized IgE antibodies. Although the first reaction appears mild, second contact might result in angioedema, wheezing, dyspnea, or anaphylaxis, or a combination of these findings.9 In addition, some patients who come in contact with Dendrolimus caterpillars might develop a condition known as dendrolimiasis, characterized by dermatitis in addition to arthritis or chondritis.6 The arthritis is normally monoarticular and can result in complete destruction of the joint. Pararamose, a condition with a similar presentation, is caused by the Brazilian moth Premolis semirufa.6
Contact of setae or spines with mucous membranes or inhalation of setae also might result in edema, dysphagia, dyspnea, drooling, rhinitis, or conjunctivitis, or a combination of these findings.6 In addition, setae can embed in the eye and cause an inflammatory reaction—ophthalmia nodosa—most commonly caused by caterpillars of the pine processionary moth (Thaumetopoea pityocampa) and characterized by immediate chemosis, which can progress to liquefactive necrosis and hypopyon, later developing into a granulomatous foreign-body response.2,10 The process is thought to be the result of a combination of the thaumetopoein toxin in the setae and an IgE-mediated response to other proteins.10
Due to their harpoon shape and forward-only motion, setae might migrate deeper, potentially even to the optic nerve.11 Because migration might take years and the barbed shape of setae does not always allow removal, some patients require lifetime monitoring with slit-lamp examination.Chronic problems, such as cataracts and persistent posterior uveitis, have been reported.10,11
Lonomism—One of the most serious (though rarest) reactions to caterpillars is lonomism, a condition caused by the caterpillars of Lonomia achelous and Lonomia obliqua moths. These caterpillars have a unique combination of toxins filling their branched spines, which ultimately leads to the same outcome: a hemorrhagic diathesis.
The toxin of L achelous comprises several proteases that degrade fibrin, fibrinogen, and factor XIII while activating prothrombin. In contrast, L obliqua poison causes a hemorrhagic diathesis by promoting a consumptive coagulopathy through enzymes that activate factor X and prothrombin.
With initial contact with either of these Lonomia caterpillars, the patient experiences severe pain accompanied by systemic symptoms, including headache, nausea, and vomiting. Shortly afterward, symptoms of a hemorrhagic diathesis manifest, including bleeding gums, hematuria, bleeding from prior wounds, and epistaxis.5 Serious complications of the hemorrhagic diathesis, such as hemorrhage of major organs, leads to death in 4% of patients.5 A reported case of a patient whose Lonomia caterpillar sting went unrecognized until a week after the accident ended with progression to stage V chronic renal disease.12
Recent research has focused on the specific mechanism of injury caused by Lonomia species. A study found that the venom of L obliqua causes cytoskeleton rearrangement and migration in vascular smooth muscle cells (VSMCs) by inducing formation of reactive oxygen species through activation of nicotinamide adenine dinucleotide phosphate oxidase.13 Thus, the venom directly contributes to the proinflammatory phenotype of endothelial cells seen following envenomation. The same study also demonstrated that elevated reactive oxygen species trigger extracellular signal-regulated kinase pathway activation in VSMCs, leading to cell proliferation, re-stenosis, and ischemia.13 This finding was confirmed by another study,14 which demonstrated an increase in Rac1, a signaling protein involved in the extracellular signal-regulated kinase pathway, in VSMCs upon exposure to L obliqua venom. These studies propose potential new targets for treatment to prevent vascular damage.
Reactions to Adult Organisms—Although it is more common for the caterpillar form of these organisms to cause an adverse reaction, the adult moth also might be capable of causing a similar reaction by retaining setae from the cocoon or by their own spines. The most notable example of this is female moths of the genus Hylesia, which possess spines attached to glands on the abdomen. The poison in these spines—a mixture of proteases and chitinase—causes a dermatitis known as Caripito itch—the name derived from a river port in Venezuela where this moth caused a memorable epidemic of moth-induced dermatitis.7,15 Caripito itch is known for intense pruritus that most commonly lasts days or weeks, possibly longer than 1 year.
Diagnostic Difficulties
The challenge of diagnosing a caterpillar- or moth-induced reaction in humans arises from (1) the lack of clinical history (the caterpillar might not be seen at all by the patient or the examiner) and (2) the similarity of these reactions to those with more common triggers.
When setae remain embedded in the skin or mucous membranes, skin scrapings allow accelerated diagnosis. On a skin scraping prepared with 20% potassium hydroxide, setae appear as tapered and barbed hairlike structures, which allows them to be distinguished from other similar-appearing but differently shaped structures, such as glass fibers.
When setae do not remain embedded in the skin or when the cause of the reaction is due to spines, the physician is left with a nonspecific histologic picture and a large differential diagnosis to be narrowed down based on the history and occasionally the pattern of the skin lesion.
A challenge in sting diagnosis is differentiating a caterpillar or moth sting from that of another organism. In certain cases, such as those of the family Megalopygidae, specific patterns of stings might assist in making the diagnosis. Hypersensitivity reactions are associated with a wider differential diagnosis, including irritant or allergic dermatitis from other causes, scabies, eczema, lichen planus, lichen simplex chronicus, seborrheic dermatitis, and tinea corporis, to name a few.6 Skin scrapings can be examined for other features, such as burrows in the case of scabies, to further narrow the differential.
Stings and hypersensitivity reactions lacking a proper history and associated with more severe systemic symptoms have caused misdiagnosis or led to a workup for the wrong condition; for example, the picture of abdominal pain, nausea, vomiting, tachycardia, leukocytosis, hypokalemia, and metabolic acidosis can simulate appendicitis.16 Upon discovery of a puss caterpillar sting in a patient, her symptoms resolved after treatment with ondansetron, morphine, and intravenous fluids.16
In lonomism, the diagnosis must be established by laboratory measurement of the fibrinogen level, clotting factors, prothrombin time, and activated partial thromboplastin time.4 The differential diagnosis associated with lonomism includes disseminated intravascular coagulation (DIC), snakebite, and a hereditary bleeding disorder.4 The combination of laboratory tests and an extensive medical history allows a diagnosis. Absence of a personal or family history of bleeding excludes a diagnosis of hereditary bleeding disorder, whereas the absence of known causes of DIC or thrombocytopenia allows DIC to be excluded from the differential.
Treatment Options and Prevention
Treatment—The first step is to remove any embedded setae from the skin or mucous membranes. The stepwise recommendation is to remove any constricted clothing, detach setae with adhesive tape, wash with soap and water, and dry without touching the skin.1 Any remaining setae can be removed with additional tape or forceps; setae tend to be fragile and are difficult to remove in their entirety.
Other than removal of the setae, skin reactions are treated symptomatically. Ice packs and isopropyl alcohol have been utilized to cool burning or stinging areas. Pain, pruritus, and inflammation have been alleviated with antihistamines and topical corticosteroids.1 When pain is severe, oral codeine or local injection of anesthetic can be used. For severe and persistent skin lesions, a course of an oral glucocorticoid can be administered. Intramuscular triamcinolone acetonide has been shown to treat pain, dermatitis, and subcutaneous nodules otherwise refractory to treatment.8
Antivenin specific for L obliqua exists to treat lonomism and is therefore effective only when lonomism is caused by that species. Lonomism caused by L achelous is treated with cryoprecipitate, purified fibrinogen, and antifibrinolytic drugs, such as aprotinin.6 Whole blood and fresh-frozen plasma have been noted to make hemorrhage worse when utilized to treat lonomism. Because the mechanism of action of the venom of Lonomia species is based, in part, on inducing a proinflammatory profile in endothelial cells, studies have demonstrated that inhibition of kallikrein might prevent vascular injury and thus prevent serious adverse effects, such as renal failure.17
Prevention—People should wear proper protective clothing when outdoors in potentially infested areas. Measures should be taken to ensure that linens and clothing are not left outside in areas where setae might be carried on the wind. Infestation control is necessary if the population of caterpillars reaches a high enough level.
Conclusion
Several species of caterpillars and moths cause adverse reactions in humans: stings, hypersensitivity reactions, and lonomism. Although most reactions are self-limited, some might have more serious effects, including organ failure and death. Mechanisms of injury vary by species of caterpillar, moth, and butterfly; current research is focused on further defining venom components and signaling pathways to isolate potential targets to aid in the diagnosis and treatment of lepidopterism.
- Goldman BS, Bragg BN. Caterpillar and moth bites. Stat Pearls [Internet]. StatPearls Publishing. Updated August 3, 2021. Accessed November 4, 2021. https://www.ncbi.nlm.nih.gov/books/NBK539851/
- Hossler EW. Caterpillars and moths: part I. Dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:1-10. doi:10.1016/j.jaad.2009.08.060
- Forrester MB. Megalopyge opercularis caterpillar stings reported to Texas poison centers. Wilderness Environ Med. 2018;29:215-220. doi:10.1016/j.wem.2018.02.002
- Hossler EW. Lepidopterism: skin disorders secondary to caterpillars and moths. UpToDate website. Published October 20, 2021. Accessed November 18, 2021. https://www.uptodate.com/contents/lepidopterism-skin-disorders-secondary-to-caterpillars-and-moths
- Villas-Boas IM, Bonfá G, Tambourgi DV. Venomous caterpillars: from inoculation apparatus to venom composition and envenomation. Toxicon. 2018;153:39-52. doi:10.1016/j.toxicon.2018.08.007
- Hossler EW. Caterpillars and moths: part II. dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:13-28. doi:10.1016/j.jaad.2009.08.061
- Yao Z, Kamau PM, Han Y, et al. The Latoia consocia caterpillar induces pain by targeting nociceptive ion channel TRPV1. Toxins (Basel). 2019;11:695. doi:10.3390/toxins11120695
- Paniz-Mondolfi AE, Pérez-Alvarez AM, Lundberg U, et al. Cutaneous lepidopterism: dermatitis from contact with moths of Hylesia metabus (Cramer 1775) (Lepidoptera: Saturniidae), the causative agent of caripito itch. Int J Dermatol. 2011;50:535-541. doi:10.1111/j.1365-4632.2010.04683.x
- Santos-Magadán S, González de Olano D, Bartolomé-Zavala B, et al. Adverse reactions to the processionary caterpillar: irritant or allergic mechanism? Contact Dermatitis. 2009;60:109-110. doi:10.1111/j.1600-0536.2008.01464.x
- González-Martín-Moro J, Contreras-Martín I, Castro-Rebollo M, et al. Focal cortical cataract due to caterpillar hair migration. Clin Exp Optom. 2019;102:89-90. doi:10.1111/cxo.12809
- Singh A, Behera UC, Agrawal H. Intra-lenticular caterpillar seta in ophthalmia nodosa. Eur J Ophthalmol. 2021;31:NP109-NP111. doi:10.1177/1120672119858899
- Schmitberger PA, Fernandes TC, Santos RC, et al. Probable chronic renal failure caused by Lonomia caterpillar envenomation. J Venom Anim Toxins Incl Trop Dis. 2013;19:14. doi:10.1186/1678-9199-19-14
- Moraes JA, Rodrigues G, Nascimento-Silva V, et al. Effects of Lonomia obliqua venom on vascular smooth muscle cells: contribution of NADPH oxidase-derived reactive oxygen species. Toxins (Basel). 2017;9:360. doi:10.3390/toxins9110360
- Bernardi L, Pinto AFM, Mendes E, et al. Lonomia obliqua bristle extract modulates Rac1 activation, membrane dynamics and cell adhesion properties. Toxicon. 2019;162:32-39. doi:10.1016/j.toxicon.2019.02.019
- Cabrera G, Lundberg U, Rodríguez-Ulloa A, et al. Protein content of the Hylesia metabus egg nest setae (Cramer [1775]) (Lepidoptera: Saturniidae) and its association with the parental investment for the reproductive success and lepidopterism. J Proteomics. 2017;150:183-200. doi:10.1016/j.jprot.2016.08.010
- Greene SC, Carey JM. Puss caterpillar envenomation: erucism mimicking appendicitis in a young child. Pediatr Emerg Care. 2020;36:E732-E734. doi:10.1097/PEC.0000000000001514
- Berger M, de Moraes JA, Beys-da-Silva WO, et al. Renal and vascular effects of kallikrein inhibition in a model of Lonomia obliqua venom-induced acute kidney injury. PLoS Negl Trop Dis. 2019;13:e0007197. doi:10.1371/journal.pntd.0007197
- Goldman BS, Bragg BN. Caterpillar and moth bites. Stat Pearls [Internet]. StatPearls Publishing. Updated August 3, 2021. Accessed November 4, 2021. https://www.ncbi.nlm.nih.gov/books/NBK539851/
- Hossler EW. Caterpillars and moths: part I. Dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:1-10. doi:10.1016/j.jaad.2009.08.060
- Forrester MB. Megalopyge opercularis caterpillar stings reported to Texas poison centers. Wilderness Environ Med. 2018;29:215-220. doi:10.1016/j.wem.2018.02.002
- Hossler EW. Lepidopterism: skin disorders secondary to caterpillars and moths. UpToDate website. Published October 20, 2021. Accessed November 18, 2021. https://www.uptodate.com/contents/lepidopterism-skin-disorders-secondary-to-caterpillars-and-moths
- Villas-Boas IM, Bonfá G, Tambourgi DV. Venomous caterpillars: from inoculation apparatus to venom composition and envenomation. Toxicon. 2018;153:39-52. doi:10.1016/j.toxicon.2018.08.007
- Hossler EW. Caterpillars and moths: part II. dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:13-28. doi:10.1016/j.jaad.2009.08.061
- Yao Z, Kamau PM, Han Y, et al. The Latoia consocia caterpillar induces pain by targeting nociceptive ion channel TRPV1. Toxins (Basel). 2019;11:695. doi:10.3390/toxins11120695
- Paniz-Mondolfi AE, Pérez-Alvarez AM, Lundberg U, et al. Cutaneous lepidopterism: dermatitis from contact with moths of Hylesia metabus (Cramer 1775) (Lepidoptera: Saturniidae), the causative agent of caripito itch. Int J Dermatol. 2011;50:535-541. doi:10.1111/j.1365-4632.2010.04683.x
- Santos-Magadán S, González de Olano D, Bartolomé-Zavala B, et al. Adverse reactions to the processionary caterpillar: irritant or allergic mechanism? Contact Dermatitis. 2009;60:109-110. doi:10.1111/j.1600-0536.2008.01464.x
- González-Martín-Moro J, Contreras-Martín I, Castro-Rebollo M, et al. Focal cortical cataract due to caterpillar hair migration. Clin Exp Optom. 2019;102:89-90. doi:10.1111/cxo.12809
- Singh A, Behera UC, Agrawal H. Intra-lenticular caterpillar seta in ophthalmia nodosa. Eur J Ophthalmol. 2021;31:NP109-NP111. doi:10.1177/1120672119858899
- Schmitberger PA, Fernandes TC, Santos RC, et al. Probable chronic renal failure caused by Lonomia caterpillar envenomation. J Venom Anim Toxins Incl Trop Dis. 2013;19:14. doi:10.1186/1678-9199-19-14
- Moraes JA, Rodrigues G, Nascimento-Silva V, et al. Effects of Lonomia obliqua venom on vascular smooth muscle cells: contribution of NADPH oxidase-derived reactive oxygen species. Toxins (Basel). 2017;9:360. doi:10.3390/toxins9110360
- Bernardi L, Pinto AFM, Mendes E, et al. Lonomia obliqua bristle extract modulates Rac1 activation, membrane dynamics and cell adhesion properties. Toxicon. 2019;162:32-39. doi:10.1016/j.toxicon.2019.02.019
- Cabrera G, Lundberg U, Rodríguez-Ulloa A, et al. Protein content of the Hylesia metabus egg nest setae (Cramer [1775]) (Lepidoptera: Saturniidae) and its association with the parental investment for the reproductive success and lepidopterism. J Proteomics. 2017;150:183-200. doi:10.1016/j.jprot.2016.08.010
- Greene SC, Carey JM. Puss caterpillar envenomation: erucism mimicking appendicitis in a young child. Pediatr Emerg Care. 2020;36:E732-E734. doi:10.1097/PEC.0000000000001514
- Berger M, de Moraes JA, Beys-da-Silva WO, et al. Renal and vascular effects of kallikrein inhibition in a model of Lonomia obliqua venom-induced acute kidney injury. PLoS Negl Trop Dis. 2019;13:e0007197. doi:10.1371/journal.pntd.0007197
Practice Points
- Lepidopterism describes adverse reactions caused by the stings, hypersensitivity reactions, and lonomism (a hemorrhagic diathesis) of caterpillars, moths, and butterflies.
- Caterpillars can induce an adverse reaction by injecting venom stored in their bristles, inducing a foreign-body reaction to embedded bristles, or a combination of these mechanisms.
- A thorough history, skin scrapings, relevant examination of affected body parts (such as slit-lamp examination, in the case of eyes), and laboratory testing should be conducted to narrow the wide differential diagnosis associated with lepidopterism.
Diet in Wound Care: Can Nutrition Impact Healing?
Dermatologists commonly manage a variety of wounds in the outpatient setting. Wound healing requires a multifaceted approach that often includes topical and oral therapies, adjustment of mechanical factors, and behavioral and lifestyle modifications. Physiologically, wound healing requires an inflammatory phase, a proliferative phase, and a remodeling phase. Chronic wounds undergo a prolonged inflammatory response hindered by decreased growth factors and increased wound bioburden.1 Malnutrition has been routinely associated with wound chronicity and serves as a modifiable risk factor that may improve wound healing outcomes.2
Although the causes of wounds encountered in dermatology vary extensively, the importance of nutrition underlies all wound healing. Caloric needs in wound healing have been estimated at 30 to 40 kcal/kg dependent on baseline body weight, age, medical comorbidities, activity level, stage of wound healing, wound size, and number of wounds.1,3,4 Nutritional supplementation is patient dependent, but this article serves to review the existing literature on macronutrient and micronutrient supplementation to clarify the potentially complementary role for nutritional support in chronic wounds. All patients should be screened with a thorough history, review of systems, and physical examination for existing nutrient deficiencies. Patients with age-related or chronic diseases are at increased risk for nutritional deficiency, and focused laboratory testing may be warranted. Supplementation for specific deficiencies with help from a registered dietician is recommended.
Macronutrients for Wound Healing
Protein—Protein is the most widely known macronutrient required for wound healing. The primary function of dietary protein is to provide amino acids to perform physiologic functions.5 Not only does cutaneous injury increase the metabolic needs of the wounded area, but large amounts of protein can be continually lost through wound exudates. Protein is necessary for the immune response required to transition from inflammatory to proliferative phases of wound healing.6 Protein energy deficiency has been reported to reduce fibroblast activity, delay angiogenesis, and decrease collagen formation.7 Additionally, protein is required for the formation of inflammatory cells and maintenance of oncotic pressure, specifically in venous insufficiency wounds.1
The current recommended dietary allowance for protein in healthy adults is 0.8 g/kg daily of body weight. In patients with pressure ulcerations, a goal recommended dietary allowance of 1.25 to 2.0 g/kg daily of body weight, dependent on ulceration size, has been recommended by the National Pressure Ulcer Advisory Panel and European Pressure Ulcer Advisory Panel.8 This recommendation was based on a series of studies that reported enhanced healing rates in patients with pressure ulcers receiving higher-protein diets.9 The largest study to date was double-blinded and included 89 residents of long-term care facilities with stage II to stage IV pressure ulcers.10 Participants were randomized to receive commercial protein supplementation vs placebo. At the end of 8 weeks, a statistically significant difference was seen in mean (SD) pressure ulcer scale for healing scores (3.55 [4.66] vs 3.22 [4.11]; P<.05).10 A 2014 Cochrane review failed to identify benefit associated with nutritional interventions for either the prevention and/or treatment of pressure ulcers.11 Specific recommendations on protein intake for other types of chronic wounds have not been proposed. Protein supplementation generally is provided orally, if tolerated. Liquid supplements such as Boost (Nestlé), Carnation Breakfast Essentials (Nestlé), NuBasics (SupremeMed), Resource (Nestlé Health Science), and Ensure (Abbott Laboratories) are frequently used to supplement both protein and caloric intake. Protein oversupplementation has not been associated with improved outcomes and may cause or exacerbate other medical comorbidities.
Fatty Acids for Wound Healing
Wound healing is an anabolic process that requires adequate intake of substrates such as glucose and fat. Carbohydrates serve as the major energy source required for wound healing, while fats are thought to play roles in cell membrane development and modulation of cellular signaling.1 Fats utilize a unique pathway for energy production through beta-oxidation and the production of adenosine triphosphate, allowing available protein to be harnessed for wound healing.1 Omega-3 and omega-6 fatty acids serve as precursors to prostaglandins, leukotrienes, and thromboxane—all key mediators of the inflammatory phase of wound healing.3 Omega-3 fatty acids are thought to downregulate genes involved in proinflammatory pathways,12 as well as to diminish lymphocyte proliferation and levels of IL-1β, tumor necrosis factor α, and IL-6 in vitro.13 In vivo studies assessing the impact of omega-3 fatty acid supplementation on wound healing are minimal, and the role of dietary supplementation for this indication remains unknown. Fish oil contains the omega-3 fatty acid–rich eicosapentaenoic acid and docosahexaenoic acid, which has been compared to mineral oil supplementation for wound healing in healthy adults. When fish oil was supplemented for 4 weeks, no significant differences were identified in time to complete wound healing between groups. Interestingly, significantly higher levels of the proinflammatory cytokine IL-1β were identified in blister fluid at 24 hours after blistering vs the placebo group (t=2.52, df=25, P<.05).14 Prior studies evaluating wound healing in animal models similarly identified longer times to re-epithelialization after omega-3 polyunsaturated fatty acid supplementation orally and topically.15,16 The fatty acid quality and composition consumed also may impact wound healing, as high-fat diets that are not rich in omega-3 fatty acids have been shown to promote inflammation and impair wound healing in rats, but this has not been thoroughly explored in human trials.17 Although adequate intake of these macronutrients is important, excessive intake may be harmful. Larger prospective trials are needed to shed light on the dose and composition of fatty acid supplementation that may optimize wound healing.
Vitamins and Micronutrients Required for Wound Healing
Vitamin A—Many vitamins serve as cofactors for the enzymatic processes required in wound healing. Vitamin A is an essential fat-soluble vitamin that serves a variety of dermatologic functions and promotes wound healing through stimulation of fibroblasts and ground substance, and it facilitates epithelial cell differentiation when applied topically.3,18 Vitamin A works through the activation of retinoid receptors on endothelial cells, fibroblasts, keratinocytes, melanocytes, and sebocytes, and has purported anti-inflammatory effects that aid the healing of open wounds.3 Additionally, vitamin A is thought to enhance cytokine release in the inflammatory phase of wound healing.19 Supplemental vitamin A has been associated with positive effects on acute wound healing, burns, and radiation injuries.3 The utility of vitamin A supplementation in chronic wounds remains unknown; however, it has been shown to be beneficial in patients with inflammatory disease, such as rheumatoid arthritis, on corticosteroid therapy. Vitamin A supplementation in this population has been shown to counteract the negative effects of corticosteroids on wound healing via downregulation of transforming growth factor β and insulinlike growth factor 1.20 Vitamin A deficiency has been associated with impaired progression through inflammatory and remodeling phases of healing due to altered B-cell and T-cell function and antibody production.1 Some experts recommend short courses of oral vitamin A supplementation to enhance wound healing at doses between 10,000 and 25,000 IU daily.2,3 Large, population-based studies are needed, and the safety supporting this recommendation in all patients remains unknown.
Vitamin C—Vitamin C is widely known for its role in collagen formation, immunomodulation, and antioxidant capacity.1 Although vitamin C deficiency is associated with decreased collagen synthesis and impaired wound healing,21 the utility of long-term supplementation in patients who are not deficient remains unexplored. A systematic review evaluating interventional studies utilizing vitamin C supplementation on pressure ulcerations and surgical wound healing concluded that convincing evidence exists only for supplementation with at least 500 mg of vitamin C. The authors noted, “There is little evidence for improved healing of surgical wounds by high-dose single vitamin C supplementation (1–3 g/day).”22 In a prospective, randomized, controlled trial, 20 patients with pressure ulcerations were supplemented with vitamin C vs placebo with a mean reduction in pressure-sore area of 84% after 1 month in the vitamin C–supplemented group compared to 42.7% in the placebo group (P<.005). A limitation of this study is the small population.23 One current recommendation for vitamin C supplementation in chronic wounds is for 500 mg daily in uncomplicated wounds to 2 g daily in severe wounds.3 Additional studies have suggested that the benefits of vitamin C supplementation are maximized when given in combination with zinc and arginine.22 At this time, evaluation for vitamin C deficiency and appropriate supplementation in patients with chronic wounds is needed.
Zinc—Minerals similarly play important roles in enzymatic regulation. Hundreds of zinc-containing enzymes are involved in wound healing and are required in tissue repair, growth, antioxidant capacity, and immune function.1,24 Zinc is specifically critical to collagen, DNA, RNA, and protein synthesis, as well as cellular proliferation.4 Zinc deficiency has been encountered in the setting of chronic wounds with extensive drainage, decreased dietary intake, or excessive gastrointestinal losses.25 Although many studies exist evaluating the utility of zinc supplementation on wound healing, many are confounded by multinutrient supplementation. No studies to date support zinc supplementation when zinc deficiency is absent. Patient assessment for medications or conditions that may impact zinc metabolism should be completed. Importantly, zinc supplementation can interfere with the absorption of other cations, so excessive supplementation should be avoided.1
Amino Acids for Wound Healing
Arginine—Arginine is an essential amino acid that serves as a substrate for cellular proliferation, collagen deposition, and lymphocyte function.8,26,27 Arginine serves as the biologic precursor for nitric oxide (NO), a substrate that has important wound healing properties. Nitric oxide metabolites have been shown to positively regulate wound repair while NO metabolites are reduced in wound environments in diabetic ulcerations.28,29 Arginine also is a proline precursor, an essential building block for collagen synthesis,6,30 and a stimulator of growth hormone and T cells.30,31 Animal studies have suggested L-arginine supplementation may reverse impaired NO synthesis in diabetic wounds.28 A single randomized trial assessing differing doses of arginine supplementation on stage II or stage IV pressure ulcers noted an almost two-fold improvement in healing time.32 However, human studies have not shown increased rates of re-epithelialization of skin graft donor sites when provided oral or parenteral arginine supplementation.33 Inadequate data currently exist to support regular arginine supplementation for all types of wounds, and no safe dose of daily arginine intake has been established.
Glutamine—Similarly, glutamine supplementation has been proposed to accelerate wound healing due to its role as a primary metabolic fuel source for rapidly proliferating cells such as epithelial cells and fibroblasts.8 Glutamine is thought to induce expression of heat-shock proteins and protect against inflammatory and infectious wound complications.34 Additionally, glutamine is thought to increase tissue insulin sensitivity, which may prove beneficial in wounds, as topical insulin previously has been shown in animal and human models to promote healing.35 Glutamine is thought to play a role in the inflammatory phase of wound healing via superoxide production, leukocyte apoptosis, and phagocytosis.6,34,36 Unfortunately, numerous randomized trials on glutamine supplementation have resulted in conflicting evidence confounded by multisupplementation within the same trial.37,38 A double-blind, randomized, controlled trial of 270 participants assessed the effect of oral supplementation with arginine, glutamine, or β-hydroxy-β-methylbutyrate vs control in the healing time of diabetic foot ulcerations. Significant differences in wound closure time at week 16 were only identified in participants with low albumin levels (≤40 g/L) who were supplemented (50.8%) vs the control group (34.9%; P=.0325) and in those with poor limb perfusion (ankle-brachial index of <1.0) who were supplemented (60.3%) vs the control group (39.3%; P=.0079).39 Ongoing clinical trials evaluating the effects of glutamine supplementation on differing wound types will hopefully shed light on the efficacy of supplementation.
Final Thoughts
Wound healing is multifactorial and should consider the health status and medical comorbidities of each patient treated. We propose an individualized approach to wound healing that includes exploration of specific macronutrient and micronutrient deficiencies, as malnutrition has been associated with wound chronicity and serves as a modifiable risk factor to improve healing.2 The evidence backing specific nutrient supplementation in patients with deficiencies is strong and should be considered in patients with chronic wounds. Adequate caloric intake and protein content should be recommended for most wound patients; however, excessive protein intake has not been beneficial in wound healing. The data behind specific amino acid and vitamin supplementation are limited at this time. As with other therapeutics, there is likely an appropriate dose for supplementation that has not yet been elucidated. Consideration of wound type, size, depth, exudate, and underlying cause are important to optimize healing and tailor nutritional supplementation to each patient. We hope future studies will illuminate the complementary role of dietary intake and nutrient supplementation for the treatment of chronic nonhealing wounds.
- Quain AM, Khardori NM. Nutrition in wound care management: a comprehensive overview. Wounds. 2015;27:327-335.
- Stechmiller JK. Understanding the role of nutrition and wound healing. Nutr Clin Pract. 2010;25:61-68. doi:10.1177/0884533609358997
- Molnar JA, Underdown MJ, Clark WA. Nutrition and chronic wounds. Adv Wound Care (New Rochelle). 2014;3:663-681. doi:10.1089/wound.2014.0530
- Dorner B, Posthauer ME, Thomas D; Panel NPUA. The role of nutrition in pressure ulcer prevention and treatment: National Pressure Ulcer Advisory Panel white paper. Adv Skin Wound Care. 2009;22:212-221. doi:10.1097/01.ASW.0000350838.11854.0a
- Collins N. Protein and wound healing. Adv Skin Wound Care. 2001;14:288-289. doi:10.1097/00129334-200111000-00008
- Barchitta M, Maugeri A, Favara G, et al. Nutrition and wound healing: an overview focusing on the beneficial effects of curcumin [published online March 5, 2019]. Int J Mol Sci. doi:10.3390/ijms20051119
- Harris CL, Fraser C. Malnutrition in the institutionalized elderly: the effects on wound healing. Ostomy Wound Manage. 2004;50:54-63.
- Saghaleini SH, Dehghan K, Shadvar K, et al. Pressure ulcer and nutrition. Indian J Crit Care Med. 2018;22:283-289. doi:10.4103/ijccm.IJCCM_277_17
- Breslow RA, Hallfrisch J, Guy DG, et al. The importance of dietary protein in healing pressure ulcers. J Am Geriatr Soc. 1993;41:357-362. doi:10.1111/j.1532-5415.1993.tb06940.x
- Lee SK, Posthauer ME, Dorner B, et al. Pressure ulcer healing with a concentrated, fortified, collagen protein hydrolysate supplement: a randomized controlled trial. Adv Skin Wound Care. 2006;19:92-96. doi:10.1097/00129334-200603000-00011
- Langer G, Fink A. Nutritional interventions for preventing and treating pressure ulcers. Cochrane Database Syst Rev. 2014;6:CD003216. doi:10.1002/14651858.CD003216.pub2
- Bouwens M, van de Rest O, Dellschaft N, et al. Fish-oil supplementation induces antiinflammatory gene expression profiles in human blood mononuclear cells. Am J Clin Nutr. 2009;90:415-424. doi:10.3945/ajcn.2009.27680
- Meydani SN, Endres S, Woods MM, et al. Oral (n-3) fatty acid supplementation suppresses cytokine production and lymphocyte proliferation: comparison between young and older women. J Nutr. 1991;121:547-555. doi:10.1093/jn/121.4.547
- McDaniel JC, Belury M, Ahijevych K, et al. Omega-3 fatty acids effect on wound healing. Wound Repair Regen. 2008;16:337-345. doi:10.1111/j.1524-475X.2008.00388.x
- Mooney MA, Vaughn DM, Reinhart GA, et al. Evaluation of the effects of omega-3 fatty acid-containing diets on the inflammatory stage of wound healing in dogs. Am J Vet Res. 1998;59:859-863.
- Cardoso CR, Souza MA, Ferro EA, et al. Influence of topical administration of n-3 and n-6 essential and n-9 nonessential fatty acids on the healing of cutaneous wounds. Wound Repair Regen. 2004;12:235-243. doi:10.1111/j.1067-1927.2004.012216.x
- Rosa DF, Sarandy MM, Novaes RD, et al. High-fat diet and alcohol intake promotes inflammation and impairs skin wound healing in Wistar rats. Mediators Inflamm. 2018;2018:4658583. doi:10.1155/2018/4658583
- Levenson SM, Gruber CA, Rettura G, et al. Supplemental vitamin A prevents the acute radiation-induced defect in wound healing. Ann Surg. 1984;200:494-512. doi:10.1097/00000658-198410000-00011
- Palmieri B, Vadalà M, Laurino C. Nutrition in wound healing: investigation of the molecular mechanisms, a narrative review. J Wound Care. 2019;28:683-693. doi:10.12968/jowc.2019.28.10.683
- Ehrlich HP, Hunt TK. Effects of cortisone and vitamin A on wound healing. Ann Surg. 1968;167:324-328. doi:10.1097/00000658-196803000-00004
- Pullar JM, Carr AC, Vissers MCM. The roles of vitamin C in skin health [published online August 12, 2017]. Nutrients. doi:10.3390/nu9080866
- Ellinger S, Stehle P. Efficacy of vitamin supplementation in situations with wound healing disorders: results from clinical intervention studies. Curr Opin Clin Nutr Metab Care. 2009;12:588-595. doi:10.1097/MCO.0b013e328331a5b5
- Taylor TV, Rimmer S, Day B, et al. Ascorbic acid supplementation in the treatment of pressure-sores. Lancet. 1974;2:544-546. doi:10.1016/s0140-6736(74)91874-1
- Ibs KH, Rink L. Zinc-altered immune function. J Nutr. 2003;133(5 suppl 1):1452S-1456S. doi:10.1093/jn/133.5.1452S
- Hoffman M, Micheletti RG, Shields BE. Nutritional dermatoses in the hospitalized patient. Cutis. 2020;105:296-302, 308, E1-E5.
- Chow O, Barbul A. Immunonutrition: role in wound healing and tissue regeneration. Adv Wound Care (New Rochelle). 2014;3:46-53. doi:10.1089/wound.2012.0415
- Singh K, Coburn LA, Barry DP, et al. L-arginine uptake by cationic amino acid transporter 2 is essential for colonic epithelial cell restitution. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1061-G1073. doi:10.1152/ajpgi.00544.2011
- Witte MB, Thornton FJ, Tantry U, et al. L-Arginine supplementation enhances diabetic wound healing: involvement of the nitric oxide synthase and arginase pathways. Metabolism. 2002;51:1269-1273. doi:10.1053/meta.2002.35185
- Witte MB, Barbul A. Role of nitric oxide in wound repair. Am J Surg. 2002;183:406-412. doi:10.1016/s0002-9610(02)00815-2
- Barbul A. Proline precursors to sustain Mammalian collagen synthesis. J Nutr. 2008;138:2021S-2024S. doi:10.1093/jn/138.10.2021S
- Wu G, Bazer FW, Davis TA, et al. Arginine metabolism and nutrition in growth, health and disease. Amino Acids. 2009;37:153-168. doi:10.1007/s00726-008-0210-y
- Leigh B, Desneves K, Rafferty J, et al. The effect of different doses of an arginine-containing supplement on the healing of pressure ulcers. J Wound Care. 2012;21:150-156. doi:10.12968/jowc.2012.21.3.150
- Debats IB, Koeneman MM, Booi DI, et al. Intravenous arginine and human skin graft donor site healing: a randomized controlled trial. Burns. 2011;37:420-426. doi:10.1016/j.burns.2010.06.003
- Wischmeyer PE. Glutamine and heat shock protein expression. Nutrition. 2002;18:225-228. doi:10.1016/s0899-9007(01)00796-1
- Wang J, Xu J. Effects of topical insulin on wound healing: a review of animal and human evidences. Diabetes Metab Syndr Obes. 2020;13:719-727. doi:10.2147/DMSO.S237294
- Newsholme P. Why is L-glutamine metabolism important to cells of the immune system in health, postinjury, surgery or infection?J Nutr. 2001;131(9 suppl):2515S-2522S; discussion 2523S-2524S. doi:10.1093/jn/131.9.2515S
- Aquino VM, Harvey AR, Garvin JH, et al. A double-blind randomized placebo-controlled study of oral glutamine in the prevention of mucositis in children undergoing hematopoietic stem cell transplantation: a pediatric blood and marrow transplant consortium study. Bone Marrow Transplant. 2005;36:611-616. doi:10.1038/sj.bmt.1705084
- Ward E, Smith M, Henderson M, et al. The effect of high-dose enteral glutamine on the incidence and severity of mucositis in paediatric oncology patients. Eur J Clin Nutr. 2009;63:134-140. doi:10.1038/sj.ejcn.1602894
- Armstrong DG, Hanft JR, Driver VR, et al. Effect of oral nutritional supplementation on wound healing in diabetic foot ulcers: a prospective randomized controlled trial. Diabet Med. 2014;31:1069-1077. doi:10.1111/dme.12509
Dermatologists commonly manage a variety of wounds in the outpatient setting. Wound healing requires a multifaceted approach that often includes topical and oral therapies, adjustment of mechanical factors, and behavioral and lifestyle modifications. Physiologically, wound healing requires an inflammatory phase, a proliferative phase, and a remodeling phase. Chronic wounds undergo a prolonged inflammatory response hindered by decreased growth factors and increased wound bioburden.1 Malnutrition has been routinely associated with wound chronicity and serves as a modifiable risk factor that may improve wound healing outcomes.2
Although the causes of wounds encountered in dermatology vary extensively, the importance of nutrition underlies all wound healing. Caloric needs in wound healing have been estimated at 30 to 40 kcal/kg dependent on baseline body weight, age, medical comorbidities, activity level, stage of wound healing, wound size, and number of wounds.1,3,4 Nutritional supplementation is patient dependent, but this article serves to review the existing literature on macronutrient and micronutrient supplementation to clarify the potentially complementary role for nutritional support in chronic wounds. All patients should be screened with a thorough history, review of systems, and physical examination for existing nutrient deficiencies. Patients with age-related or chronic diseases are at increased risk for nutritional deficiency, and focused laboratory testing may be warranted. Supplementation for specific deficiencies with help from a registered dietician is recommended.
Macronutrients for Wound Healing
Protein—Protein is the most widely known macronutrient required for wound healing. The primary function of dietary protein is to provide amino acids to perform physiologic functions.5 Not only does cutaneous injury increase the metabolic needs of the wounded area, but large amounts of protein can be continually lost through wound exudates. Protein is necessary for the immune response required to transition from inflammatory to proliferative phases of wound healing.6 Protein energy deficiency has been reported to reduce fibroblast activity, delay angiogenesis, and decrease collagen formation.7 Additionally, protein is required for the formation of inflammatory cells and maintenance of oncotic pressure, specifically in venous insufficiency wounds.1
The current recommended dietary allowance for protein in healthy adults is 0.8 g/kg daily of body weight. In patients with pressure ulcerations, a goal recommended dietary allowance of 1.25 to 2.0 g/kg daily of body weight, dependent on ulceration size, has been recommended by the National Pressure Ulcer Advisory Panel and European Pressure Ulcer Advisory Panel.8 This recommendation was based on a series of studies that reported enhanced healing rates in patients with pressure ulcers receiving higher-protein diets.9 The largest study to date was double-blinded and included 89 residents of long-term care facilities with stage II to stage IV pressure ulcers.10 Participants were randomized to receive commercial protein supplementation vs placebo. At the end of 8 weeks, a statistically significant difference was seen in mean (SD) pressure ulcer scale for healing scores (3.55 [4.66] vs 3.22 [4.11]; P<.05).10 A 2014 Cochrane review failed to identify benefit associated with nutritional interventions for either the prevention and/or treatment of pressure ulcers.11 Specific recommendations on protein intake for other types of chronic wounds have not been proposed. Protein supplementation generally is provided orally, if tolerated. Liquid supplements such as Boost (Nestlé), Carnation Breakfast Essentials (Nestlé), NuBasics (SupremeMed), Resource (Nestlé Health Science), and Ensure (Abbott Laboratories) are frequently used to supplement both protein and caloric intake. Protein oversupplementation has not been associated with improved outcomes and may cause or exacerbate other medical comorbidities.
Fatty Acids for Wound Healing
Wound healing is an anabolic process that requires adequate intake of substrates such as glucose and fat. Carbohydrates serve as the major energy source required for wound healing, while fats are thought to play roles in cell membrane development and modulation of cellular signaling.1 Fats utilize a unique pathway for energy production through beta-oxidation and the production of adenosine triphosphate, allowing available protein to be harnessed for wound healing.1 Omega-3 and omega-6 fatty acids serve as precursors to prostaglandins, leukotrienes, and thromboxane—all key mediators of the inflammatory phase of wound healing.3 Omega-3 fatty acids are thought to downregulate genes involved in proinflammatory pathways,12 as well as to diminish lymphocyte proliferation and levels of IL-1β, tumor necrosis factor α, and IL-6 in vitro.13 In vivo studies assessing the impact of omega-3 fatty acid supplementation on wound healing are minimal, and the role of dietary supplementation for this indication remains unknown. Fish oil contains the omega-3 fatty acid–rich eicosapentaenoic acid and docosahexaenoic acid, which has been compared to mineral oil supplementation for wound healing in healthy adults. When fish oil was supplemented for 4 weeks, no significant differences were identified in time to complete wound healing between groups. Interestingly, significantly higher levels of the proinflammatory cytokine IL-1β were identified in blister fluid at 24 hours after blistering vs the placebo group (t=2.52, df=25, P<.05).14 Prior studies evaluating wound healing in animal models similarly identified longer times to re-epithelialization after omega-3 polyunsaturated fatty acid supplementation orally and topically.15,16 The fatty acid quality and composition consumed also may impact wound healing, as high-fat diets that are not rich in omega-3 fatty acids have been shown to promote inflammation and impair wound healing in rats, but this has not been thoroughly explored in human trials.17 Although adequate intake of these macronutrients is important, excessive intake may be harmful. Larger prospective trials are needed to shed light on the dose and composition of fatty acid supplementation that may optimize wound healing.
Vitamins and Micronutrients Required for Wound Healing
Vitamin A—Many vitamins serve as cofactors for the enzymatic processes required in wound healing. Vitamin A is an essential fat-soluble vitamin that serves a variety of dermatologic functions and promotes wound healing through stimulation of fibroblasts and ground substance, and it facilitates epithelial cell differentiation when applied topically.3,18 Vitamin A works through the activation of retinoid receptors on endothelial cells, fibroblasts, keratinocytes, melanocytes, and sebocytes, and has purported anti-inflammatory effects that aid the healing of open wounds.3 Additionally, vitamin A is thought to enhance cytokine release in the inflammatory phase of wound healing.19 Supplemental vitamin A has been associated with positive effects on acute wound healing, burns, and radiation injuries.3 The utility of vitamin A supplementation in chronic wounds remains unknown; however, it has been shown to be beneficial in patients with inflammatory disease, such as rheumatoid arthritis, on corticosteroid therapy. Vitamin A supplementation in this population has been shown to counteract the negative effects of corticosteroids on wound healing via downregulation of transforming growth factor β and insulinlike growth factor 1.20 Vitamin A deficiency has been associated with impaired progression through inflammatory and remodeling phases of healing due to altered B-cell and T-cell function and antibody production.1 Some experts recommend short courses of oral vitamin A supplementation to enhance wound healing at doses between 10,000 and 25,000 IU daily.2,3 Large, population-based studies are needed, and the safety supporting this recommendation in all patients remains unknown.
Vitamin C—Vitamin C is widely known for its role in collagen formation, immunomodulation, and antioxidant capacity.1 Although vitamin C deficiency is associated with decreased collagen synthesis and impaired wound healing,21 the utility of long-term supplementation in patients who are not deficient remains unexplored. A systematic review evaluating interventional studies utilizing vitamin C supplementation on pressure ulcerations and surgical wound healing concluded that convincing evidence exists only for supplementation with at least 500 mg of vitamin C. The authors noted, “There is little evidence for improved healing of surgical wounds by high-dose single vitamin C supplementation (1–3 g/day).”22 In a prospective, randomized, controlled trial, 20 patients with pressure ulcerations were supplemented with vitamin C vs placebo with a mean reduction in pressure-sore area of 84% after 1 month in the vitamin C–supplemented group compared to 42.7% in the placebo group (P<.005). A limitation of this study is the small population.23 One current recommendation for vitamin C supplementation in chronic wounds is for 500 mg daily in uncomplicated wounds to 2 g daily in severe wounds.3 Additional studies have suggested that the benefits of vitamin C supplementation are maximized when given in combination with zinc and arginine.22 At this time, evaluation for vitamin C deficiency and appropriate supplementation in patients with chronic wounds is needed.
Zinc—Minerals similarly play important roles in enzymatic regulation. Hundreds of zinc-containing enzymes are involved in wound healing and are required in tissue repair, growth, antioxidant capacity, and immune function.1,24 Zinc is specifically critical to collagen, DNA, RNA, and protein synthesis, as well as cellular proliferation.4 Zinc deficiency has been encountered in the setting of chronic wounds with extensive drainage, decreased dietary intake, or excessive gastrointestinal losses.25 Although many studies exist evaluating the utility of zinc supplementation on wound healing, many are confounded by multinutrient supplementation. No studies to date support zinc supplementation when zinc deficiency is absent. Patient assessment for medications or conditions that may impact zinc metabolism should be completed. Importantly, zinc supplementation can interfere with the absorption of other cations, so excessive supplementation should be avoided.1
Amino Acids for Wound Healing
Arginine—Arginine is an essential amino acid that serves as a substrate for cellular proliferation, collagen deposition, and lymphocyte function.8,26,27 Arginine serves as the biologic precursor for nitric oxide (NO), a substrate that has important wound healing properties. Nitric oxide metabolites have been shown to positively regulate wound repair while NO metabolites are reduced in wound environments in diabetic ulcerations.28,29 Arginine also is a proline precursor, an essential building block for collagen synthesis,6,30 and a stimulator of growth hormone and T cells.30,31 Animal studies have suggested L-arginine supplementation may reverse impaired NO synthesis in diabetic wounds.28 A single randomized trial assessing differing doses of arginine supplementation on stage II or stage IV pressure ulcers noted an almost two-fold improvement in healing time.32 However, human studies have not shown increased rates of re-epithelialization of skin graft donor sites when provided oral or parenteral arginine supplementation.33 Inadequate data currently exist to support regular arginine supplementation for all types of wounds, and no safe dose of daily arginine intake has been established.
Glutamine—Similarly, glutamine supplementation has been proposed to accelerate wound healing due to its role as a primary metabolic fuel source for rapidly proliferating cells such as epithelial cells and fibroblasts.8 Glutamine is thought to induce expression of heat-shock proteins and protect against inflammatory and infectious wound complications.34 Additionally, glutamine is thought to increase tissue insulin sensitivity, which may prove beneficial in wounds, as topical insulin previously has been shown in animal and human models to promote healing.35 Glutamine is thought to play a role in the inflammatory phase of wound healing via superoxide production, leukocyte apoptosis, and phagocytosis.6,34,36 Unfortunately, numerous randomized trials on glutamine supplementation have resulted in conflicting evidence confounded by multisupplementation within the same trial.37,38 A double-blind, randomized, controlled trial of 270 participants assessed the effect of oral supplementation with arginine, glutamine, or β-hydroxy-β-methylbutyrate vs control in the healing time of diabetic foot ulcerations. Significant differences in wound closure time at week 16 were only identified in participants with low albumin levels (≤40 g/L) who were supplemented (50.8%) vs the control group (34.9%; P=.0325) and in those with poor limb perfusion (ankle-brachial index of <1.0) who were supplemented (60.3%) vs the control group (39.3%; P=.0079).39 Ongoing clinical trials evaluating the effects of glutamine supplementation on differing wound types will hopefully shed light on the efficacy of supplementation.
Final Thoughts
Wound healing is multifactorial and should consider the health status and medical comorbidities of each patient treated. We propose an individualized approach to wound healing that includes exploration of specific macronutrient and micronutrient deficiencies, as malnutrition has been associated with wound chronicity and serves as a modifiable risk factor to improve healing.2 The evidence backing specific nutrient supplementation in patients with deficiencies is strong and should be considered in patients with chronic wounds. Adequate caloric intake and protein content should be recommended for most wound patients; however, excessive protein intake has not been beneficial in wound healing. The data behind specific amino acid and vitamin supplementation are limited at this time. As with other therapeutics, there is likely an appropriate dose for supplementation that has not yet been elucidated. Consideration of wound type, size, depth, exudate, and underlying cause are important to optimize healing and tailor nutritional supplementation to each patient. We hope future studies will illuminate the complementary role of dietary intake and nutrient supplementation for the treatment of chronic nonhealing wounds.
Dermatologists commonly manage a variety of wounds in the outpatient setting. Wound healing requires a multifaceted approach that often includes topical and oral therapies, adjustment of mechanical factors, and behavioral and lifestyle modifications. Physiologically, wound healing requires an inflammatory phase, a proliferative phase, and a remodeling phase. Chronic wounds undergo a prolonged inflammatory response hindered by decreased growth factors and increased wound bioburden.1 Malnutrition has been routinely associated with wound chronicity and serves as a modifiable risk factor that may improve wound healing outcomes.2
Although the causes of wounds encountered in dermatology vary extensively, the importance of nutrition underlies all wound healing. Caloric needs in wound healing have been estimated at 30 to 40 kcal/kg dependent on baseline body weight, age, medical comorbidities, activity level, stage of wound healing, wound size, and number of wounds.1,3,4 Nutritional supplementation is patient dependent, but this article serves to review the existing literature on macronutrient and micronutrient supplementation to clarify the potentially complementary role for nutritional support in chronic wounds. All patients should be screened with a thorough history, review of systems, and physical examination for existing nutrient deficiencies. Patients with age-related or chronic diseases are at increased risk for nutritional deficiency, and focused laboratory testing may be warranted. Supplementation for specific deficiencies with help from a registered dietician is recommended.
Macronutrients for Wound Healing
Protein—Protein is the most widely known macronutrient required for wound healing. The primary function of dietary protein is to provide amino acids to perform physiologic functions.5 Not only does cutaneous injury increase the metabolic needs of the wounded area, but large amounts of protein can be continually lost through wound exudates. Protein is necessary for the immune response required to transition from inflammatory to proliferative phases of wound healing.6 Protein energy deficiency has been reported to reduce fibroblast activity, delay angiogenesis, and decrease collagen formation.7 Additionally, protein is required for the formation of inflammatory cells and maintenance of oncotic pressure, specifically in venous insufficiency wounds.1
The current recommended dietary allowance for protein in healthy adults is 0.8 g/kg daily of body weight. In patients with pressure ulcerations, a goal recommended dietary allowance of 1.25 to 2.0 g/kg daily of body weight, dependent on ulceration size, has been recommended by the National Pressure Ulcer Advisory Panel and European Pressure Ulcer Advisory Panel.8 This recommendation was based on a series of studies that reported enhanced healing rates in patients with pressure ulcers receiving higher-protein diets.9 The largest study to date was double-blinded and included 89 residents of long-term care facilities with stage II to stage IV pressure ulcers.10 Participants were randomized to receive commercial protein supplementation vs placebo. At the end of 8 weeks, a statistically significant difference was seen in mean (SD) pressure ulcer scale for healing scores (3.55 [4.66] vs 3.22 [4.11]; P<.05).10 A 2014 Cochrane review failed to identify benefit associated with nutritional interventions for either the prevention and/or treatment of pressure ulcers.11 Specific recommendations on protein intake for other types of chronic wounds have not been proposed. Protein supplementation generally is provided orally, if tolerated. Liquid supplements such as Boost (Nestlé), Carnation Breakfast Essentials (Nestlé), NuBasics (SupremeMed), Resource (Nestlé Health Science), and Ensure (Abbott Laboratories) are frequently used to supplement both protein and caloric intake. Protein oversupplementation has not been associated with improved outcomes and may cause or exacerbate other medical comorbidities.
Fatty Acids for Wound Healing
Wound healing is an anabolic process that requires adequate intake of substrates such as glucose and fat. Carbohydrates serve as the major energy source required for wound healing, while fats are thought to play roles in cell membrane development and modulation of cellular signaling.1 Fats utilize a unique pathway for energy production through beta-oxidation and the production of adenosine triphosphate, allowing available protein to be harnessed for wound healing.1 Omega-3 and omega-6 fatty acids serve as precursors to prostaglandins, leukotrienes, and thromboxane—all key mediators of the inflammatory phase of wound healing.3 Omega-3 fatty acids are thought to downregulate genes involved in proinflammatory pathways,12 as well as to diminish lymphocyte proliferation and levels of IL-1β, tumor necrosis factor α, and IL-6 in vitro.13 In vivo studies assessing the impact of omega-3 fatty acid supplementation on wound healing are minimal, and the role of dietary supplementation for this indication remains unknown. Fish oil contains the omega-3 fatty acid–rich eicosapentaenoic acid and docosahexaenoic acid, which has been compared to mineral oil supplementation for wound healing in healthy adults. When fish oil was supplemented for 4 weeks, no significant differences were identified in time to complete wound healing between groups. Interestingly, significantly higher levels of the proinflammatory cytokine IL-1β were identified in blister fluid at 24 hours after blistering vs the placebo group (t=2.52, df=25, P<.05).14 Prior studies evaluating wound healing in animal models similarly identified longer times to re-epithelialization after omega-3 polyunsaturated fatty acid supplementation orally and topically.15,16 The fatty acid quality and composition consumed also may impact wound healing, as high-fat diets that are not rich in omega-3 fatty acids have been shown to promote inflammation and impair wound healing in rats, but this has not been thoroughly explored in human trials.17 Although adequate intake of these macronutrients is important, excessive intake may be harmful. Larger prospective trials are needed to shed light on the dose and composition of fatty acid supplementation that may optimize wound healing.
Vitamins and Micronutrients Required for Wound Healing
Vitamin A—Many vitamins serve as cofactors for the enzymatic processes required in wound healing. Vitamin A is an essential fat-soluble vitamin that serves a variety of dermatologic functions and promotes wound healing through stimulation of fibroblasts and ground substance, and it facilitates epithelial cell differentiation when applied topically.3,18 Vitamin A works through the activation of retinoid receptors on endothelial cells, fibroblasts, keratinocytes, melanocytes, and sebocytes, and has purported anti-inflammatory effects that aid the healing of open wounds.3 Additionally, vitamin A is thought to enhance cytokine release in the inflammatory phase of wound healing.19 Supplemental vitamin A has been associated with positive effects on acute wound healing, burns, and radiation injuries.3 The utility of vitamin A supplementation in chronic wounds remains unknown; however, it has been shown to be beneficial in patients with inflammatory disease, such as rheumatoid arthritis, on corticosteroid therapy. Vitamin A supplementation in this population has been shown to counteract the negative effects of corticosteroids on wound healing via downregulation of transforming growth factor β and insulinlike growth factor 1.20 Vitamin A deficiency has been associated with impaired progression through inflammatory and remodeling phases of healing due to altered B-cell and T-cell function and antibody production.1 Some experts recommend short courses of oral vitamin A supplementation to enhance wound healing at doses between 10,000 and 25,000 IU daily.2,3 Large, population-based studies are needed, and the safety supporting this recommendation in all patients remains unknown.
Vitamin C—Vitamin C is widely known for its role in collagen formation, immunomodulation, and antioxidant capacity.1 Although vitamin C deficiency is associated with decreased collagen synthesis and impaired wound healing,21 the utility of long-term supplementation in patients who are not deficient remains unexplored. A systematic review evaluating interventional studies utilizing vitamin C supplementation on pressure ulcerations and surgical wound healing concluded that convincing evidence exists only for supplementation with at least 500 mg of vitamin C. The authors noted, “There is little evidence for improved healing of surgical wounds by high-dose single vitamin C supplementation (1–3 g/day).”22 In a prospective, randomized, controlled trial, 20 patients with pressure ulcerations were supplemented with vitamin C vs placebo with a mean reduction in pressure-sore area of 84% after 1 month in the vitamin C–supplemented group compared to 42.7% in the placebo group (P<.005). A limitation of this study is the small population.23 One current recommendation for vitamin C supplementation in chronic wounds is for 500 mg daily in uncomplicated wounds to 2 g daily in severe wounds.3 Additional studies have suggested that the benefits of vitamin C supplementation are maximized when given in combination with zinc and arginine.22 At this time, evaluation for vitamin C deficiency and appropriate supplementation in patients with chronic wounds is needed.
Zinc—Minerals similarly play important roles in enzymatic regulation. Hundreds of zinc-containing enzymes are involved in wound healing and are required in tissue repair, growth, antioxidant capacity, and immune function.1,24 Zinc is specifically critical to collagen, DNA, RNA, and protein synthesis, as well as cellular proliferation.4 Zinc deficiency has been encountered in the setting of chronic wounds with extensive drainage, decreased dietary intake, or excessive gastrointestinal losses.25 Although many studies exist evaluating the utility of zinc supplementation on wound healing, many are confounded by multinutrient supplementation. No studies to date support zinc supplementation when zinc deficiency is absent. Patient assessment for medications or conditions that may impact zinc metabolism should be completed. Importantly, zinc supplementation can interfere with the absorption of other cations, so excessive supplementation should be avoided.1
Amino Acids for Wound Healing
Arginine—Arginine is an essential amino acid that serves as a substrate for cellular proliferation, collagen deposition, and lymphocyte function.8,26,27 Arginine serves as the biologic precursor for nitric oxide (NO), a substrate that has important wound healing properties. Nitric oxide metabolites have been shown to positively regulate wound repair while NO metabolites are reduced in wound environments in diabetic ulcerations.28,29 Arginine also is a proline precursor, an essential building block for collagen synthesis,6,30 and a stimulator of growth hormone and T cells.30,31 Animal studies have suggested L-arginine supplementation may reverse impaired NO synthesis in diabetic wounds.28 A single randomized trial assessing differing doses of arginine supplementation on stage II or stage IV pressure ulcers noted an almost two-fold improvement in healing time.32 However, human studies have not shown increased rates of re-epithelialization of skin graft donor sites when provided oral or parenteral arginine supplementation.33 Inadequate data currently exist to support regular arginine supplementation for all types of wounds, and no safe dose of daily arginine intake has been established.
Glutamine—Similarly, glutamine supplementation has been proposed to accelerate wound healing due to its role as a primary metabolic fuel source for rapidly proliferating cells such as epithelial cells and fibroblasts.8 Glutamine is thought to induce expression of heat-shock proteins and protect against inflammatory and infectious wound complications.34 Additionally, glutamine is thought to increase tissue insulin sensitivity, which may prove beneficial in wounds, as topical insulin previously has been shown in animal and human models to promote healing.35 Glutamine is thought to play a role in the inflammatory phase of wound healing via superoxide production, leukocyte apoptosis, and phagocytosis.6,34,36 Unfortunately, numerous randomized trials on glutamine supplementation have resulted in conflicting evidence confounded by multisupplementation within the same trial.37,38 A double-blind, randomized, controlled trial of 270 participants assessed the effect of oral supplementation with arginine, glutamine, or β-hydroxy-β-methylbutyrate vs control in the healing time of diabetic foot ulcerations. Significant differences in wound closure time at week 16 were only identified in participants with low albumin levels (≤40 g/L) who were supplemented (50.8%) vs the control group (34.9%; P=.0325) and in those with poor limb perfusion (ankle-brachial index of <1.0) who were supplemented (60.3%) vs the control group (39.3%; P=.0079).39 Ongoing clinical trials evaluating the effects of glutamine supplementation on differing wound types will hopefully shed light on the efficacy of supplementation.
Final Thoughts
Wound healing is multifactorial and should consider the health status and medical comorbidities of each patient treated. We propose an individualized approach to wound healing that includes exploration of specific macronutrient and micronutrient deficiencies, as malnutrition has been associated with wound chronicity and serves as a modifiable risk factor to improve healing.2 The evidence backing specific nutrient supplementation in patients with deficiencies is strong and should be considered in patients with chronic wounds. Adequate caloric intake and protein content should be recommended for most wound patients; however, excessive protein intake has not been beneficial in wound healing. The data behind specific amino acid and vitamin supplementation are limited at this time. As with other therapeutics, there is likely an appropriate dose for supplementation that has not yet been elucidated. Consideration of wound type, size, depth, exudate, and underlying cause are important to optimize healing and tailor nutritional supplementation to each patient. We hope future studies will illuminate the complementary role of dietary intake and nutrient supplementation for the treatment of chronic nonhealing wounds.
- Quain AM, Khardori NM. Nutrition in wound care management: a comprehensive overview. Wounds. 2015;27:327-335.
- Stechmiller JK. Understanding the role of nutrition and wound healing. Nutr Clin Pract. 2010;25:61-68. doi:10.1177/0884533609358997
- Molnar JA, Underdown MJ, Clark WA. Nutrition and chronic wounds. Adv Wound Care (New Rochelle). 2014;3:663-681. doi:10.1089/wound.2014.0530
- Dorner B, Posthauer ME, Thomas D; Panel NPUA. The role of nutrition in pressure ulcer prevention and treatment: National Pressure Ulcer Advisory Panel white paper. Adv Skin Wound Care. 2009;22:212-221. doi:10.1097/01.ASW.0000350838.11854.0a
- Collins N. Protein and wound healing. Adv Skin Wound Care. 2001;14:288-289. doi:10.1097/00129334-200111000-00008
- Barchitta M, Maugeri A, Favara G, et al. Nutrition and wound healing: an overview focusing on the beneficial effects of curcumin [published online March 5, 2019]. Int J Mol Sci. doi:10.3390/ijms20051119
- Harris CL, Fraser C. Malnutrition in the institutionalized elderly: the effects on wound healing. Ostomy Wound Manage. 2004;50:54-63.
- Saghaleini SH, Dehghan K, Shadvar K, et al. Pressure ulcer and nutrition. Indian J Crit Care Med. 2018;22:283-289. doi:10.4103/ijccm.IJCCM_277_17
- Breslow RA, Hallfrisch J, Guy DG, et al. The importance of dietary protein in healing pressure ulcers. J Am Geriatr Soc. 1993;41:357-362. doi:10.1111/j.1532-5415.1993.tb06940.x
- Lee SK, Posthauer ME, Dorner B, et al. Pressure ulcer healing with a concentrated, fortified, collagen protein hydrolysate supplement: a randomized controlled trial. Adv Skin Wound Care. 2006;19:92-96. doi:10.1097/00129334-200603000-00011
- Langer G, Fink A. Nutritional interventions for preventing and treating pressure ulcers. Cochrane Database Syst Rev. 2014;6:CD003216. doi:10.1002/14651858.CD003216.pub2
- Bouwens M, van de Rest O, Dellschaft N, et al. Fish-oil supplementation induces antiinflammatory gene expression profiles in human blood mononuclear cells. Am J Clin Nutr. 2009;90:415-424. doi:10.3945/ajcn.2009.27680
- Meydani SN, Endres S, Woods MM, et al. Oral (n-3) fatty acid supplementation suppresses cytokine production and lymphocyte proliferation: comparison between young and older women. J Nutr. 1991;121:547-555. doi:10.1093/jn/121.4.547
- McDaniel JC, Belury M, Ahijevych K, et al. Omega-3 fatty acids effect on wound healing. Wound Repair Regen. 2008;16:337-345. doi:10.1111/j.1524-475X.2008.00388.x
- Mooney MA, Vaughn DM, Reinhart GA, et al. Evaluation of the effects of omega-3 fatty acid-containing diets on the inflammatory stage of wound healing in dogs. Am J Vet Res. 1998;59:859-863.
- Cardoso CR, Souza MA, Ferro EA, et al. Influence of topical administration of n-3 and n-6 essential and n-9 nonessential fatty acids on the healing of cutaneous wounds. Wound Repair Regen. 2004;12:235-243. doi:10.1111/j.1067-1927.2004.012216.x
- Rosa DF, Sarandy MM, Novaes RD, et al. High-fat diet and alcohol intake promotes inflammation and impairs skin wound healing in Wistar rats. Mediators Inflamm. 2018;2018:4658583. doi:10.1155/2018/4658583
- Levenson SM, Gruber CA, Rettura G, et al. Supplemental vitamin A prevents the acute radiation-induced defect in wound healing. Ann Surg. 1984;200:494-512. doi:10.1097/00000658-198410000-00011
- Palmieri B, Vadalà M, Laurino C. Nutrition in wound healing: investigation of the molecular mechanisms, a narrative review. J Wound Care. 2019;28:683-693. doi:10.12968/jowc.2019.28.10.683
- Ehrlich HP, Hunt TK. Effects of cortisone and vitamin A on wound healing. Ann Surg. 1968;167:324-328. doi:10.1097/00000658-196803000-00004
- Pullar JM, Carr AC, Vissers MCM. The roles of vitamin C in skin health [published online August 12, 2017]. Nutrients. doi:10.3390/nu9080866
- Ellinger S, Stehle P. Efficacy of vitamin supplementation in situations with wound healing disorders: results from clinical intervention studies. Curr Opin Clin Nutr Metab Care. 2009;12:588-595. doi:10.1097/MCO.0b013e328331a5b5
- Taylor TV, Rimmer S, Day B, et al. Ascorbic acid supplementation in the treatment of pressure-sores. Lancet. 1974;2:544-546. doi:10.1016/s0140-6736(74)91874-1
- Ibs KH, Rink L. Zinc-altered immune function. J Nutr. 2003;133(5 suppl 1):1452S-1456S. doi:10.1093/jn/133.5.1452S
- Hoffman M, Micheletti RG, Shields BE. Nutritional dermatoses in the hospitalized patient. Cutis. 2020;105:296-302, 308, E1-E5.
- Chow O, Barbul A. Immunonutrition: role in wound healing and tissue regeneration. Adv Wound Care (New Rochelle). 2014;3:46-53. doi:10.1089/wound.2012.0415
- Singh K, Coburn LA, Barry DP, et al. L-arginine uptake by cationic amino acid transporter 2 is essential for colonic epithelial cell restitution. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1061-G1073. doi:10.1152/ajpgi.00544.2011
- Witte MB, Thornton FJ, Tantry U, et al. L-Arginine supplementation enhances diabetic wound healing: involvement of the nitric oxide synthase and arginase pathways. Metabolism. 2002;51:1269-1273. doi:10.1053/meta.2002.35185
- Witte MB, Barbul A. Role of nitric oxide in wound repair. Am J Surg. 2002;183:406-412. doi:10.1016/s0002-9610(02)00815-2
- Barbul A. Proline precursors to sustain Mammalian collagen synthesis. J Nutr. 2008;138:2021S-2024S. doi:10.1093/jn/138.10.2021S
- Wu G, Bazer FW, Davis TA, et al. Arginine metabolism and nutrition in growth, health and disease. Amino Acids. 2009;37:153-168. doi:10.1007/s00726-008-0210-y
- Leigh B, Desneves K, Rafferty J, et al. The effect of different doses of an arginine-containing supplement on the healing of pressure ulcers. J Wound Care. 2012;21:150-156. doi:10.12968/jowc.2012.21.3.150
- Debats IB, Koeneman MM, Booi DI, et al. Intravenous arginine and human skin graft donor site healing: a randomized controlled trial. Burns. 2011;37:420-426. doi:10.1016/j.burns.2010.06.003
- Wischmeyer PE. Glutamine and heat shock protein expression. Nutrition. 2002;18:225-228. doi:10.1016/s0899-9007(01)00796-1
- Wang J, Xu J. Effects of topical insulin on wound healing: a review of animal and human evidences. Diabetes Metab Syndr Obes. 2020;13:719-727. doi:10.2147/DMSO.S237294
- Newsholme P. Why is L-glutamine metabolism important to cells of the immune system in health, postinjury, surgery or infection?J Nutr. 2001;131(9 suppl):2515S-2522S; discussion 2523S-2524S. doi:10.1093/jn/131.9.2515S
- Aquino VM, Harvey AR, Garvin JH, et al. A double-blind randomized placebo-controlled study of oral glutamine in the prevention of mucositis in children undergoing hematopoietic stem cell transplantation: a pediatric blood and marrow transplant consortium study. Bone Marrow Transplant. 2005;36:611-616. doi:10.1038/sj.bmt.1705084
- Ward E, Smith M, Henderson M, et al. The effect of high-dose enteral glutamine on the incidence and severity of mucositis in paediatric oncology patients. Eur J Clin Nutr. 2009;63:134-140. doi:10.1038/sj.ejcn.1602894
- Armstrong DG, Hanft JR, Driver VR, et al. Effect of oral nutritional supplementation on wound healing in diabetic foot ulcers: a prospective randomized controlled trial. Diabet Med. 2014;31:1069-1077. doi:10.1111/dme.12509
- Quain AM, Khardori NM. Nutrition in wound care management: a comprehensive overview. Wounds. 2015;27:327-335.
- Stechmiller JK. Understanding the role of nutrition and wound healing. Nutr Clin Pract. 2010;25:61-68. doi:10.1177/0884533609358997
- Molnar JA, Underdown MJ, Clark WA. Nutrition and chronic wounds. Adv Wound Care (New Rochelle). 2014;3:663-681. doi:10.1089/wound.2014.0530
- Dorner B, Posthauer ME, Thomas D; Panel NPUA. The role of nutrition in pressure ulcer prevention and treatment: National Pressure Ulcer Advisory Panel white paper. Adv Skin Wound Care. 2009;22:212-221. doi:10.1097/01.ASW.0000350838.11854.0a
- Collins N. Protein and wound healing. Adv Skin Wound Care. 2001;14:288-289. doi:10.1097/00129334-200111000-00008
- Barchitta M, Maugeri A, Favara G, et al. Nutrition and wound healing: an overview focusing on the beneficial effects of curcumin [published online March 5, 2019]. Int J Mol Sci. doi:10.3390/ijms20051119
- Harris CL, Fraser C. Malnutrition in the institutionalized elderly: the effects on wound healing. Ostomy Wound Manage. 2004;50:54-63.
- Saghaleini SH, Dehghan K, Shadvar K, et al. Pressure ulcer and nutrition. Indian J Crit Care Med. 2018;22:283-289. doi:10.4103/ijccm.IJCCM_277_17
- Breslow RA, Hallfrisch J, Guy DG, et al. The importance of dietary protein in healing pressure ulcers. J Am Geriatr Soc. 1993;41:357-362. doi:10.1111/j.1532-5415.1993.tb06940.x
- Lee SK, Posthauer ME, Dorner B, et al. Pressure ulcer healing with a concentrated, fortified, collagen protein hydrolysate supplement: a randomized controlled trial. Adv Skin Wound Care. 2006;19:92-96. doi:10.1097/00129334-200603000-00011
- Langer G, Fink A. Nutritional interventions for preventing and treating pressure ulcers. Cochrane Database Syst Rev. 2014;6:CD003216. doi:10.1002/14651858.CD003216.pub2
- Bouwens M, van de Rest O, Dellschaft N, et al. Fish-oil supplementation induces antiinflammatory gene expression profiles in human blood mononuclear cells. Am J Clin Nutr. 2009;90:415-424. doi:10.3945/ajcn.2009.27680
- Meydani SN, Endres S, Woods MM, et al. Oral (n-3) fatty acid supplementation suppresses cytokine production and lymphocyte proliferation: comparison between young and older women. J Nutr. 1991;121:547-555. doi:10.1093/jn/121.4.547
- McDaniel JC, Belury M, Ahijevych K, et al. Omega-3 fatty acids effect on wound healing. Wound Repair Regen. 2008;16:337-345. doi:10.1111/j.1524-475X.2008.00388.x
- Mooney MA, Vaughn DM, Reinhart GA, et al. Evaluation of the effects of omega-3 fatty acid-containing diets on the inflammatory stage of wound healing in dogs. Am J Vet Res. 1998;59:859-863.
- Cardoso CR, Souza MA, Ferro EA, et al. Influence of topical administration of n-3 and n-6 essential and n-9 nonessential fatty acids on the healing of cutaneous wounds. Wound Repair Regen. 2004;12:235-243. doi:10.1111/j.1067-1927.2004.012216.x
- Rosa DF, Sarandy MM, Novaes RD, et al. High-fat diet and alcohol intake promotes inflammation and impairs skin wound healing in Wistar rats. Mediators Inflamm. 2018;2018:4658583. doi:10.1155/2018/4658583
- Levenson SM, Gruber CA, Rettura G, et al. Supplemental vitamin A prevents the acute radiation-induced defect in wound healing. Ann Surg. 1984;200:494-512. doi:10.1097/00000658-198410000-00011
- Palmieri B, Vadalà M, Laurino C. Nutrition in wound healing: investigation of the molecular mechanisms, a narrative review. J Wound Care. 2019;28:683-693. doi:10.12968/jowc.2019.28.10.683
- Ehrlich HP, Hunt TK. Effects of cortisone and vitamin A on wound healing. Ann Surg. 1968;167:324-328. doi:10.1097/00000658-196803000-00004
- Pullar JM, Carr AC, Vissers MCM. The roles of vitamin C in skin health [published online August 12, 2017]. Nutrients. doi:10.3390/nu9080866
- Ellinger S, Stehle P. Efficacy of vitamin supplementation in situations with wound healing disorders: results from clinical intervention studies. Curr Opin Clin Nutr Metab Care. 2009;12:588-595. doi:10.1097/MCO.0b013e328331a5b5
- Taylor TV, Rimmer S, Day B, et al. Ascorbic acid supplementation in the treatment of pressure-sores. Lancet. 1974;2:544-546. doi:10.1016/s0140-6736(74)91874-1
- Ibs KH, Rink L. Zinc-altered immune function. J Nutr. 2003;133(5 suppl 1):1452S-1456S. doi:10.1093/jn/133.5.1452S
- Hoffman M, Micheletti RG, Shields BE. Nutritional dermatoses in the hospitalized patient. Cutis. 2020;105:296-302, 308, E1-E5.
- Chow O, Barbul A. Immunonutrition: role in wound healing and tissue regeneration. Adv Wound Care (New Rochelle). 2014;3:46-53. doi:10.1089/wound.2012.0415
- Singh K, Coburn LA, Barry DP, et al. L-arginine uptake by cationic amino acid transporter 2 is essential for colonic epithelial cell restitution. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1061-G1073. doi:10.1152/ajpgi.00544.2011
- Witte MB, Thornton FJ, Tantry U, et al. L-Arginine supplementation enhances diabetic wound healing: involvement of the nitric oxide synthase and arginase pathways. Metabolism. 2002;51:1269-1273. doi:10.1053/meta.2002.35185
- Witte MB, Barbul A. Role of nitric oxide in wound repair. Am J Surg. 2002;183:406-412. doi:10.1016/s0002-9610(02)00815-2
- Barbul A. Proline precursors to sustain Mammalian collagen synthesis. J Nutr. 2008;138:2021S-2024S. doi:10.1093/jn/138.10.2021S
- Wu G, Bazer FW, Davis TA, et al. Arginine metabolism and nutrition in growth, health and disease. Amino Acids. 2009;37:153-168. doi:10.1007/s00726-008-0210-y
- Leigh B, Desneves K, Rafferty J, et al. The effect of different doses of an arginine-containing supplement on the healing of pressure ulcers. J Wound Care. 2012;21:150-156. doi:10.12968/jowc.2012.21.3.150
- Debats IB, Koeneman MM, Booi DI, et al. Intravenous arginine and human skin graft donor site healing: a randomized controlled trial. Burns. 2011;37:420-426. doi:10.1016/j.burns.2010.06.003
- Wischmeyer PE. Glutamine and heat shock protein expression. Nutrition. 2002;18:225-228. doi:10.1016/s0899-9007(01)00796-1
- Wang J, Xu J. Effects of topical insulin on wound healing: a review of animal and human evidences. Diabetes Metab Syndr Obes. 2020;13:719-727. doi:10.2147/DMSO.S237294
- Newsholme P. Why is L-glutamine metabolism important to cells of the immune system in health, postinjury, surgery or infection?J Nutr. 2001;131(9 suppl):2515S-2522S; discussion 2523S-2524S. doi:10.1093/jn/131.9.2515S
- Aquino VM, Harvey AR, Garvin JH, et al. A double-blind randomized placebo-controlled study of oral glutamine in the prevention of mucositis in children undergoing hematopoietic stem cell transplantation: a pediatric blood and marrow transplant consortium study. Bone Marrow Transplant. 2005;36:611-616. doi:10.1038/sj.bmt.1705084
- Ward E, Smith M, Henderson M, et al. The effect of high-dose enteral glutamine on the incidence and severity of mucositis in paediatric oncology patients. Eur J Clin Nutr. 2009;63:134-140. doi:10.1038/sj.ejcn.1602894
- Armstrong DG, Hanft JR, Driver VR, et al. Effect of oral nutritional supplementation on wound healing in diabetic foot ulcers: a prospective randomized controlled trial. Diabet Med. 2014;31:1069-1077. doi:10.1111/dme.12509
Practice Points
- Optimizing wound healing requires local and systemic therapies as well as adequate nutritional support.
- Malnutrition is a potentially modifiable risk factor that may contribute to impaired wound healing.
- Patients with chronic wounds and specific nutrient deficiencies should supplement to optimize healing.
Can Artificial Intelligence Technology Replace Human Scribes?
The personal connection between patients and physicians has evolved over the last decade with advances in medicine, technology, and the overwhelming impact of electronic medical records (EMRs). The average primary care physician spends 5.9 hours of their 11.4-hour workday doing various tasks in the EMR.1 With approximately half of a physician’s workday dedicated to writing patient notes, billing, and managing their inbox, the other half of the day needs to be sparingly allotted across their total patient load.
This progression of increased EMR time demands and reduced time interacting with patients has led to the development of various advantageous strategies to minimize the physician’s workload and shift the focus back to the patient. Two paramount examples that can maximize the physician’s time and the patient’s individualized care are the use of medical scribes as well as technology to write notes and accomplish various office tasks. Both reduce the physician’s workload and allow for more patient-focused interactions but via different methods. When considering which practice to employ, a physician must weigh the positive and negative aspects of both modalities, particularly dermatologists who utilize these options to streamline high patient loads.
Medical Scribes in Dermatology
A scribe is defined as a staff member who records patient-physician interactions in real time and functions as the “physician’s partner in the clinical encounter.”2 A variety of staff members can serve as scribes, such as medical assistants and registered nurses (RNs), but the majority of scribes are prehealth students (eg, premedical, prenursing, pre–physician assistant).3 In this modality of patient information recording, the physician brings the scribe into the examination room and introduces them to the patient, and the scribe proceeds to record the encounter directly into the EMR. After the encounter, the physician then is able to review the completed notes and make the necessary changes before finalized submission. This process drastically reduces the physician’s workload and also may have a lasting impact on the scribe. Aside from financial compensation, scribes also are offered a very in-depth clinical experience. Especially for prehealth students, scribing can be an eye-opening phase of their progression toward a future career in medicine. These students are able to immerse themselves in the clinical setting and truly experience the medical field through active participation in patient care. Robert et al2 commented on the professional development of prehealth students through scribing and self-reflection on their clinical experiences involving human suffering, empathy, power dynamics, and social inequality. Scribing allows prehealth students to begin to develop the critical skills necessary to succeed in the medical field at an earlier stage of their career development through real-time clinical engagement. This can be a motivational learning experience and can help these students to become more empathetic, understanding, and well-rounded providers in their future careers.
It is important to consider that human scribes currently are the status quo. They have been used reliably in the clinical setting for more than a decade, and it has been proven that their use is advantageous for physicians. Overall, the increased productivity and long-term effects of the immersive experiences that scribes encounter on a daily basis are important considerations when physicians decide to seek assistance in reducing their workload.
Virtual Technology and Artificial Intelligence in Dermatology
Another way to reduce the physician’s daily workload is through virtual technology and artificial intelligence (AI)–based programs. There have been many varieties of technology developed over the last decade to coincide with the rising EMR work requirements. Virtual technology allows for a wide variety of utilization in the medical clinic that can vary from virtual assistants who record patient encounters, such as Hello Rache (Temark International, Inc), to audio programs such as DeepScribe (DeepScribe Inc) that listen to the patient-physician interaction and utilize an AI-based machine to concurrently convert the audio to written documentation in the EMR.
Among the available options, the most similar to the scribe method seems to be programs such as Hello Rache that provide a virtual assistant—often an RN—who can assist in completing a multitude of tasks, such as referrals, telephone calls, transcription of dictation, and other office needs. Similar to scribing, the virtual assistant can be brought into the room to chart the notes from the visit in real time into the EMR. Although this seems similar to conventional scribing, there are 3 glaring differences in the virtual approach. The first is that the use of a tablet, computer, or other technology source is required to bring the virtual assistant in the room to listen and observe the patient interaction. This increases ease of use and allows the physician to move seamlessly between patient encounters. However, the utilization of technology also adds a layer of potential problems to the physician’s workflow, such as unreliable Internet connection, the need for battery power, and data storage requirements. The second major difference is the fact that the virtual assistant recording the notes into the EMR is not physically present and therefore is unable to move around the room to observe the physical examination. Lastly, the population of virtual assistants employed by Hello Rache seems to be restricted to specifically trained RNs in the Philippines. These virtual assistants are specially vetted for working in the medical field, and their position as a virtual assistant is their career, which provides a specialized workforce to help physicians be more effective in their work. It also shows stark contrast to the prehealth professionals that make up the majority of conventional scribes for whom scribing is a stepping stone into the medical field rather than a career path. This offers a more comprehensive approach to reducing the physician’s workload but also contributes to a more detached clinical experience for the virtual assistant.
Final Thoughts
Both conventional and virtual scribing modalities provide assistance to maximize efficiency and reduce the physician’s workload.3 Both methods achieve the same goal, but they have unique long-term impact on the physician, scribe, and most importantly the patient. Artificial intelligence provides an intriguing approach to minimizing work in the medical setting, but it does not have the successful history of utilization and longitudinal clinical impact on the scribe that is achieved through traditional scribing. It is important to consider the personal and professional growth that early clinical experiences provide for scribes, especially because the majority pursue a career in the medical field. Human scribes will continue to be the status quo when opposing the increased requirements of the EMR, but the implementation of AI sparks the need for more in-depth research and comparisons. Lastly, it is essential to uncover what the patient may prefer. Conventional scribing has been successfully utilized and accepted by patients in the clinical setting for years, but investigations of the efficacy and satisfaction of virtual scribing are still needed. Although both provide an advantageous approach to maximizing the patient-physician time in the dermatology clinic, one cannot say for certain that AI will be welcomed the same way as modern-day human scribes.
- Arndt BG, Beasley JW, Watkinson MD, et al. Tethered to the EHR: primary care physician workload assessment using EHR event log data and time-motion observations [published online September 2017]. Ann Fam Med. doi:10.1370/afm.2121
- Robert J, Piemonte N, Truten J. The reflective scribe: encouraging critical self-reflection and professional development in pre-health education. J Med Humanit. 2018;39:447-454. doi:10.1007/s10912-018-9541-1
- Berger E. Medical scribe industry booms: rapid rise leads to questioning. Ann Emerg Med. 2015;65:A13. doi:10.1016/j.annemergmed.2015.02.016
The personal connection between patients and physicians has evolved over the last decade with advances in medicine, technology, and the overwhelming impact of electronic medical records (EMRs). The average primary care physician spends 5.9 hours of their 11.4-hour workday doing various tasks in the EMR.1 With approximately half of a physician’s workday dedicated to writing patient notes, billing, and managing their inbox, the other half of the day needs to be sparingly allotted across their total patient load.
This progression of increased EMR time demands and reduced time interacting with patients has led to the development of various advantageous strategies to minimize the physician’s workload and shift the focus back to the patient. Two paramount examples that can maximize the physician’s time and the patient’s individualized care are the use of medical scribes as well as technology to write notes and accomplish various office tasks. Both reduce the physician’s workload and allow for more patient-focused interactions but via different methods. When considering which practice to employ, a physician must weigh the positive and negative aspects of both modalities, particularly dermatologists who utilize these options to streamline high patient loads.
Medical Scribes in Dermatology
A scribe is defined as a staff member who records patient-physician interactions in real time and functions as the “physician’s partner in the clinical encounter.”2 A variety of staff members can serve as scribes, such as medical assistants and registered nurses (RNs), but the majority of scribes are prehealth students (eg, premedical, prenursing, pre–physician assistant).3 In this modality of patient information recording, the physician brings the scribe into the examination room and introduces them to the patient, and the scribe proceeds to record the encounter directly into the EMR. After the encounter, the physician then is able to review the completed notes and make the necessary changes before finalized submission. This process drastically reduces the physician’s workload and also may have a lasting impact on the scribe. Aside from financial compensation, scribes also are offered a very in-depth clinical experience. Especially for prehealth students, scribing can be an eye-opening phase of their progression toward a future career in medicine. These students are able to immerse themselves in the clinical setting and truly experience the medical field through active participation in patient care. Robert et al2 commented on the professional development of prehealth students through scribing and self-reflection on their clinical experiences involving human suffering, empathy, power dynamics, and social inequality. Scribing allows prehealth students to begin to develop the critical skills necessary to succeed in the medical field at an earlier stage of their career development through real-time clinical engagement. This can be a motivational learning experience and can help these students to become more empathetic, understanding, and well-rounded providers in their future careers.
It is important to consider that human scribes currently are the status quo. They have been used reliably in the clinical setting for more than a decade, and it has been proven that their use is advantageous for physicians. Overall, the increased productivity and long-term effects of the immersive experiences that scribes encounter on a daily basis are important considerations when physicians decide to seek assistance in reducing their workload.
Virtual Technology and Artificial Intelligence in Dermatology
Another way to reduce the physician’s daily workload is through virtual technology and artificial intelligence (AI)–based programs. There have been many varieties of technology developed over the last decade to coincide with the rising EMR work requirements. Virtual technology allows for a wide variety of utilization in the medical clinic that can vary from virtual assistants who record patient encounters, such as Hello Rache (Temark International, Inc), to audio programs such as DeepScribe (DeepScribe Inc) that listen to the patient-physician interaction and utilize an AI-based machine to concurrently convert the audio to written documentation in the EMR.
Among the available options, the most similar to the scribe method seems to be programs such as Hello Rache that provide a virtual assistant—often an RN—who can assist in completing a multitude of tasks, such as referrals, telephone calls, transcription of dictation, and other office needs. Similar to scribing, the virtual assistant can be brought into the room to chart the notes from the visit in real time into the EMR. Although this seems similar to conventional scribing, there are 3 glaring differences in the virtual approach. The first is that the use of a tablet, computer, or other technology source is required to bring the virtual assistant in the room to listen and observe the patient interaction. This increases ease of use and allows the physician to move seamlessly between patient encounters. However, the utilization of technology also adds a layer of potential problems to the physician’s workflow, such as unreliable Internet connection, the need for battery power, and data storage requirements. The second major difference is the fact that the virtual assistant recording the notes into the EMR is not physically present and therefore is unable to move around the room to observe the physical examination. Lastly, the population of virtual assistants employed by Hello Rache seems to be restricted to specifically trained RNs in the Philippines. These virtual assistants are specially vetted for working in the medical field, and their position as a virtual assistant is their career, which provides a specialized workforce to help physicians be more effective in their work. It also shows stark contrast to the prehealth professionals that make up the majority of conventional scribes for whom scribing is a stepping stone into the medical field rather than a career path. This offers a more comprehensive approach to reducing the physician’s workload but also contributes to a more detached clinical experience for the virtual assistant.
Final Thoughts
Both conventional and virtual scribing modalities provide assistance to maximize efficiency and reduce the physician’s workload.3 Both methods achieve the same goal, but they have unique long-term impact on the physician, scribe, and most importantly the patient. Artificial intelligence provides an intriguing approach to minimizing work in the medical setting, but it does not have the successful history of utilization and longitudinal clinical impact on the scribe that is achieved through traditional scribing. It is important to consider the personal and professional growth that early clinical experiences provide for scribes, especially because the majority pursue a career in the medical field. Human scribes will continue to be the status quo when opposing the increased requirements of the EMR, but the implementation of AI sparks the need for more in-depth research and comparisons. Lastly, it is essential to uncover what the patient may prefer. Conventional scribing has been successfully utilized and accepted by patients in the clinical setting for years, but investigations of the efficacy and satisfaction of virtual scribing are still needed. Although both provide an advantageous approach to maximizing the patient-physician time in the dermatology clinic, one cannot say for certain that AI will be welcomed the same way as modern-day human scribes.
The personal connection between patients and physicians has evolved over the last decade with advances in medicine, technology, and the overwhelming impact of electronic medical records (EMRs). The average primary care physician spends 5.9 hours of their 11.4-hour workday doing various tasks in the EMR.1 With approximately half of a physician’s workday dedicated to writing patient notes, billing, and managing their inbox, the other half of the day needs to be sparingly allotted across their total patient load.
This progression of increased EMR time demands and reduced time interacting with patients has led to the development of various advantageous strategies to minimize the physician’s workload and shift the focus back to the patient. Two paramount examples that can maximize the physician’s time and the patient’s individualized care are the use of medical scribes as well as technology to write notes and accomplish various office tasks. Both reduce the physician’s workload and allow for more patient-focused interactions but via different methods. When considering which practice to employ, a physician must weigh the positive and negative aspects of both modalities, particularly dermatologists who utilize these options to streamline high patient loads.
Medical Scribes in Dermatology
A scribe is defined as a staff member who records patient-physician interactions in real time and functions as the “physician’s partner in the clinical encounter.”2 A variety of staff members can serve as scribes, such as medical assistants and registered nurses (RNs), but the majority of scribes are prehealth students (eg, premedical, prenursing, pre–physician assistant).3 In this modality of patient information recording, the physician brings the scribe into the examination room and introduces them to the patient, and the scribe proceeds to record the encounter directly into the EMR. After the encounter, the physician then is able to review the completed notes and make the necessary changes before finalized submission. This process drastically reduces the physician’s workload and also may have a lasting impact on the scribe. Aside from financial compensation, scribes also are offered a very in-depth clinical experience. Especially for prehealth students, scribing can be an eye-opening phase of their progression toward a future career in medicine. These students are able to immerse themselves in the clinical setting and truly experience the medical field through active participation in patient care. Robert et al2 commented on the professional development of prehealth students through scribing and self-reflection on their clinical experiences involving human suffering, empathy, power dynamics, and social inequality. Scribing allows prehealth students to begin to develop the critical skills necessary to succeed in the medical field at an earlier stage of their career development through real-time clinical engagement. This can be a motivational learning experience and can help these students to become more empathetic, understanding, and well-rounded providers in their future careers.
It is important to consider that human scribes currently are the status quo. They have been used reliably in the clinical setting for more than a decade, and it has been proven that their use is advantageous for physicians. Overall, the increased productivity and long-term effects of the immersive experiences that scribes encounter on a daily basis are important considerations when physicians decide to seek assistance in reducing their workload.
Virtual Technology and Artificial Intelligence in Dermatology
Another way to reduce the physician’s daily workload is through virtual technology and artificial intelligence (AI)–based programs. There have been many varieties of technology developed over the last decade to coincide with the rising EMR work requirements. Virtual technology allows for a wide variety of utilization in the medical clinic that can vary from virtual assistants who record patient encounters, such as Hello Rache (Temark International, Inc), to audio programs such as DeepScribe (DeepScribe Inc) that listen to the patient-physician interaction and utilize an AI-based machine to concurrently convert the audio to written documentation in the EMR.
Among the available options, the most similar to the scribe method seems to be programs such as Hello Rache that provide a virtual assistant—often an RN—who can assist in completing a multitude of tasks, such as referrals, telephone calls, transcription of dictation, and other office needs. Similar to scribing, the virtual assistant can be brought into the room to chart the notes from the visit in real time into the EMR. Although this seems similar to conventional scribing, there are 3 glaring differences in the virtual approach. The first is that the use of a tablet, computer, or other technology source is required to bring the virtual assistant in the room to listen and observe the patient interaction. This increases ease of use and allows the physician to move seamlessly between patient encounters. However, the utilization of technology also adds a layer of potential problems to the physician’s workflow, such as unreliable Internet connection, the need for battery power, and data storage requirements. The second major difference is the fact that the virtual assistant recording the notes into the EMR is not physically present and therefore is unable to move around the room to observe the physical examination. Lastly, the population of virtual assistants employed by Hello Rache seems to be restricted to specifically trained RNs in the Philippines. These virtual assistants are specially vetted for working in the medical field, and their position as a virtual assistant is their career, which provides a specialized workforce to help physicians be more effective in their work. It also shows stark contrast to the prehealth professionals that make up the majority of conventional scribes for whom scribing is a stepping stone into the medical field rather than a career path. This offers a more comprehensive approach to reducing the physician’s workload but also contributes to a more detached clinical experience for the virtual assistant.
Final Thoughts
Both conventional and virtual scribing modalities provide assistance to maximize efficiency and reduce the physician’s workload.3 Both methods achieve the same goal, but they have unique long-term impact on the physician, scribe, and most importantly the patient. Artificial intelligence provides an intriguing approach to minimizing work in the medical setting, but it does not have the successful history of utilization and longitudinal clinical impact on the scribe that is achieved through traditional scribing. It is important to consider the personal and professional growth that early clinical experiences provide for scribes, especially because the majority pursue a career in the medical field. Human scribes will continue to be the status quo when opposing the increased requirements of the EMR, but the implementation of AI sparks the need for more in-depth research and comparisons. Lastly, it is essential to uncover what the patient may prefer. Conventional scribing has been successfully utilized and accepted by patients in the clinical setting for years, but investigations of the efficacy and satisfaction of virtual scribing are still needed. Although both provide an advantageous approach to maximizing the patient-physician time in the dermatology clinic, one cannot say for certain that AI will be welcomed the same way as modern-day human scribes.
- Arndt BG, Beasley JW, Watkinson MD, et al. Tethered to the EHR: primary care physician workload assessment using EHR event log data and time-motion observations [published online September 2017]. Ann Fam Med. doi:10.1370/afm.2121
- Robert J, Piemonte N, Truten J. The reflective scribe: encouraging critical self-reflection and professional development in pre-health education. J Med Humanit. 2018;39:447-454. doi:10.1007/s10912-018-9541-1
- Berger E. Medical scribe industry booms: rapid rise leads to questioning. Ann Emerg Med. 2015;65:A13. doi:10.1016/j.annemergmed.2015.02.016
- Arndt BG, Beasley JW, Watkinson MD, et al. Tethered to the EHR: primary care physician workload assessment using EHR event log data and time-motion observations [published online September 2017]. Ann Fam Med. doi:10.1370/afm.2121
- Robert J, Piemonte N, Truten J. The reflective scribe: encouraging critical self-reflection and professional development in pre-health education. J Med Humanit. 2018;39:447-454. doi:10.1007/s10912-018-9541-1
- Berger E. Medical scribe industry booms: rapid rise leads to questioning. Ann Emerg Med. 2015;65:A13. doi:10.1016/j.annemergmed.2015.02.016
A Contrasting Dark Background for Nail Sampling
Practice Gap
Mycologic testing is necessary and cost-effective1 for appropriate diagnosis and treatment of onychomycosis. Empiric treatment of onychodystrophy for presumed onychomycosis can result in misdiagnosis, treatment failure, or potential adverse effects caused by medications.2 Collection of ample subungual debris facilitates the sensitivity and specificity of fungal culture and fungal polymerase chain reaction. However, the naturally pale hue of subungual debris makes specimen estimation challenging, particularly when using a similarly light-colored gauze or piece of paper for collection (Figure, A).
The Technique
A sheet from a black sticky notepad (widely available and cost-effective) can be adapted for making a diagnosis of onychomycosis (Figure, B).
Practical Implication
Use of a dark background that contrasts with light-hued nail debris is valuable to ensure an adequate specimen for fungal culture and polymerase chain reaction.
- Gupta AK, Versteeg SG, Shear NH. Confirmatory testing prior to initiating onychomycosis therapy is cost effective. J Cutan Med Surg. 2018;22:129-141. doi:10.1177/1203475417733461
- Lipner SR, Scher RK. Onychomycosis—a small step for quality of care. Curr Med Res Opin. 2016;32:865-867. doi:10.1185/03007995.2016.1147026
Practice Gap
Mycologic testing is necessary and cost-effective1 for appropriate diagnosis and treatment of onychomycosis. Empiric treatment of onychodystrophy for presumed onychomycosis can result in misdiagnosis, treatment failure, or potential adverse effects caused by medications.2 Collection of ample subungual debris facilitates the sensitivity and specificity of fungal culture and fungal polymerase chain reaction. However, the naturally pale hue of subungual debris makes specimen estimation challenging, particularly when using a similarly light-colored gauze or piece of paper for collection (Figure, A).
The Technique
A sheet from a black sticky notepad (widely available and cost-effective) can be adapted for making a diagnosis of onychomycosis (Figure, B).
Practical Implication
Use of a dark background that contrasts with light-hued nail debris is valuable to ensure an adequate specimen for fungal culture and polymerase chain reaction.
Practice Gap
Mycologic testing is necessary and cost-effective1 for appropriate diagnosis and treatment of onychomycosis. Empiric treatment of onychodystrophy for presumed onychomycosis can result in misdiagnosis, treatment failure, or potential adverse effects caused by medications.2 Collection of ample subungual debris facilitates the sensitivity and specificity of fungal culture and fungal polymerase chain reaction. However, the naturally pale hue of subungual debris makes specimen estimation challenging, particularly when using a similarly light-colored gauze or piece of paper for collection (Figure, A).
The Technique
A sheet from a black sticky notepad (widely available and cost-effective) can be adapted for making a diagnosis of onychomycosis (Figure, B).
Practical Implication
Use of a dark background that contrasts with light-hued nail debris is valuable to ensure an adequate specimen for fungal culture and polymerase chain reaction.
- Gupta AK, Versteeg SG, Shear NH. Confirmatory testing prior to initiating onychomycosis therapy is cost effective. J Cutan Med Surg. 2018;22:129-141. doi:10.1177/1203475417733461
- Lipner SR, Scher RK. Onychomycosis—a small step for quality of care. Curr Med Res Opin. 2016;32:865-867. doi:10.1185/03007995.2016.1147026
- Gupta AK, Versteeg SG, Shear NH. Confirmatory testing prior to initiating onychomycosis therapy is cost effective. J Cutan Med Surg. 2018;22:129-141. doi:10.1177/1203475417733461
- Lipner SR, Scher RK. Onychomycosis—a small step for quality of care. Curr Med Res Opin. 2016;32:865-867. doi:10.1185/03007995.2016.1147026
Gender Disparities in Income Among Board-Certified Dermatologists
Although the number of female graduates from US medical schools has steadily increased,1 several studies since the 1970s indicate that a disparity exists in salary, academic rank, and promotion among female and male physicians across multiple specialties.2-8 Proposed explanations include women working fewer hours, having lower productivity rates, undernegotiating compensation, and underbilling for the same services. However, when controlling for variables such as time, experience, specialty, rank, and research activities, this gap unequivocally persists. There are limited data on this topic in dermatology, a field in which women comprise more than half of the working population.6,7 Most analyses of gender disparities in dermatology are based on data primarily from academic dermatologists, which may not be representative of the larger population of dermatologists.8,9 The purpose of this study is to determine if an income disparity exists between male and female physicians in dermatology, including those in private practice and those who are specialty trained.
Methods
Population—We performed a cross-sectional self-reported survey to examine compensation of male and female board-certified dermatologists (MDs/DOs). Several populations of dermatologists were surveyed in August and September 2018. Approximately 20% of the members of the American Academy of Dermatology were randomly selected and sent a link to the survey. Additionally, a survey link was emailed to members of the Association of Professors of Dermatology, American College of Mohs Surgery, and American Society for Dermatologic Surgery. A link to the survey also was published on “The Board Certified Dermatologists” Facebook group.
Statistical Analysis—Descriptive statistics were used to summarize the distribution of variables overall and within gender (male or female). Not all respondents completed every section, and duplicates and incomplete responses were removed. Variables were compared between genders using t tests (continuous), the Pearson χ2 test (nominal), or the Cochran-Mantel-Haenszel test (ordinal). For categorical variables with small cell counts, an exact χ2 test for small samples was used. For continuous variables, t test P values were calculated using either pooled or Satterthwaithe approximation.
To analyze the effect of different variables on total income using multivariate and univariate linear regression, the income variable was transformed into a continuous variable by using midpoints of the categories. Univariate linear regression was used to assess the effect and significance of each variable on total annual income. Variables that were found to have a P value of less than .05 (α=.05) were deemed as significant predictors of total annual income. These variables were added to a multivariate linear regression model to determine their effect on income when adjusting for other significant (and approaching significance) factors. In addition, variables that were found to have a P value of less than .2 (α=.05) were added to the multivariate linear regression model to assess significance of these specific variables when adjusting for other factors. In this way, we tested and accounted for a multitude of variables as potential sources of confounding.
Results
Demographics—Our survey was emailed to 3079 members of the American Academy of Dermatology, and 277 responses were received. Approximately 144 additional responses were obtained collectively from links sent to the directories of the Association of Professors of Dermatology, American College of Mohs Surgery, and American Society for Dermatologic Surgery and from social media. Of these respondents, 53.65% (213/397) were female and 46.35% (184/397) were male. When stratifying by race/ethnicity, 77.33% identified as White; 13.85% identified as Asian; 6.3% identified as Black or African American, Hispanic/Latino, and Native American; and 2.52% chose not to respond. Although most male and female respondents were White, a significantly higher proportion of female respondents identified as Asian or Black/African American/Hispanic/Latino/Native American (P=.0006). We found that race/ethnicity did not significantly impact income (P=.2736). All US Census regions were represented in this study, and geographic distribution as well as population density of practice location (ie, rural, suburban, urban setting) did not differ significantly between males and females (P=.5982 and P=.1007, respectively) and did not significantly impact income (P=.3225 and P=.10663, respectively).
Income—Total annual income was defined as the aggregate sum of all types of financial compensation received in 1 calendar year (eg, salary, bonuses, benefits) and was elicited as an ordinal variable in income brackets of US $100,000. Overall, χ2 analysis showed a statistically significant difference in annual total income between male and female dermatologists (P<.0001), with a higher proportion of males in the highest pay bracket (Figure). Gender remained a statistically significant predictor of income on both univariate and multivariate linear regression analyses (P=.0002 and P<.0001, respectively), indicating that gender has a significant impact on compensation, even after controlling for other variables (eTable). Of note, males in this sample were on average older and in practice longer than females (approximately 6 years, P<.0001). However, when univariate linear regression was performed, both age (P=.8281) and number of years since residency or fellowship completion (P=.8743) were not significant predictors of income.
Practice Type—There were no statistically significant differences between men and women in practice type (P=.1489), including academic/university, hospital based, and solo and group private practice; pay structure (P=.1437), including base salary, collection-based salary, or salary plus incentive; holding a supervisory role (P=.0846); or having ownership of a practice (P=.3565)(eTable). Most respondents were in solo or group private practice (58.2%) and had a component of productivity-based compensation (77.5%). In addition, 62% of private practice dermatologists (133/212) had an ownership interest in their practice. As expected, univariate and multivariate regression analyses showed that practice type, pay structure, supervisory roles, and employee vs ownership roles were significant predictors of income (P<.05)(eTable).
Work Productivity—Statistically significant differences were found between men and women in hours worked per week in direct patient care (P<.0001) and in patient visits per week (P=.0052), with a higher percentage of men working more than 40 hours per week and men seeing an average of approximately 22 more patients per week than women. In the subgroup of all dermatologists working more than 40 hours per week, a statistically significant difference in income persisted between males and females (P=.0001). Hours worked per week and patient visits per week were statistically significant predictors of income on both univariate and multivariate regression analyses (P<.05)(Table).
Education and Fellowship Training—No significant difference existed between males and females in type of undergraduate school attended, namely public or private institutions (P=.1090), but a significant difference existed within type of medical school education, with a higher percentage of females attending private medical schools (53.03%) compared to males (38.24%)(P=.0045). However, type of undergraduate or medical school attended had no impact on income (P=.9103). A higher percentage of males (27.32%) completed additional advanced degrees, such as a master of business administration or a master of public health, compared to females (16.9%)(P=.0122). However, the completion of additional advanced degrees had no significant impact on income (P=.2379). No statistical significance existed between males and females in number of residencies completed (P=.3236), and residencies completed had no significant impact on income (P=.4584).
Of 397 respondents, approximately one-third of respondents completed fellowship training (36.5%). Fellowships included dermatopathology, surgery/cosmetics, and other (encompassing complex medical, research, transplant, and pediatric dermatology). Although similar percentages of men and women completed fellowship training, men and women differed significantly by type of fellowship completed (P=.0188). There were similar rates of dermatopathology and surgical fellowship completion between genders but almost 3 times the number of females who completed other fellowships. Type of fellowship training was a statistically significant predictor of income on both univariate and multivariate regression analyses (P<.00001 and P<.0001, respectively).
Work Activity—Respondents were asked to estimate the amount of time devoted to general dermatology, dermatopathology, Mohs micrographic surgery, cosmetics, and dermatologic surgery in their practices (Table). Women devoted a significantly higher average percentage of time to cosmetics (7.89%) compared to men (4.52%)(P=.0097). The number of cosmetic procedures performed per week was not statistically significantly different between men and women (P=.8035) but was a significant factor for income on univariate regression analysis (P=.0002). Time spent performing dermatologic surgery, general dermatology, or Mohs micrographic surgery did not significantly differ between men and women but was found to significantly influence income.
Academic Dermatology—Among the respondents working in academic settings, χ2 analysis identified a significant difference in the faculty rank between males and females, with a tendency for lower academic rank in females (P=.0508). Assistant professorship was comprised of 35% of men vs 51% of women, whereas full professorship consisted of 26% of men but only 13% of women. Academic rank was found to be a significant predictor of income, with higher rank associated with higher income (P<.0001 on univariate regression analysis). However, when adjusting for other factors, academic rank was no longer a significant predictor of income (P=.0840 on multivariate regression analysis). No significant difference existed between men and women in funding received from the National Institutes of Health, conduction of clinical trials, or authorship of scientific publications, and these factors were not found to have a significant impact on income.
Work Leave—Male and female dermatologists showed a statistically significant difference in maternity or Family and Medical Leave Act (FMLA) leave taken over their careers, with 56.03% of females reporting leave taken compared to 6.78% of males (P<.0001). Women reported a significantly higher average number of weeks of maternity or FMLA leave taken over their careers (12.92 weeks) compared to men (2.42 weeks) (P<.0001). However, upon univariate regression analysis, whether or not maternity or FMLA leave was taken over their careers (P=.2005), the number of times that maternity or FMLA leave was taken (P=.4350), and weeks of maternity or FMLA leave taken (P=.4057) were all not significant predictors of income.
Comment
This study sought to investigate the relationship between income and gender in dermatology, and our results demonstrated that statistically significant differences in total annual income exist between male and female dermatologists, with male dermatologists earning a significantly higher income, approximately an additional $80,000. Our results are consistent with other studies of US physician income, which have found a gender gap ranging from $13,399 to $82,000 that persists even when controlling for factors such as specialty choice, practice setting, rank and role in practice, work hours, vacation/leave taken, and others.2-7,10-15
There was a significant difference in rank of male and female academic dermatologists, with fewer females at higher academic ranks. These results are consistent with numerous studies in academic dermatology that show underrepresentation of women at higher academic ranks and leadership positions.8,9,16-18 Poor negotiation may contribute to differences in both rank and income.19,20 There are conflicting data on research productivity of academic dermatologists and length of career, first and senior authorship, and quality and academic impact, all of which add complexity to this topic.8,9,12,16-18,20-23Male and female dermatologists reported significant differences in productivity, with male dermatologists working more hours and seeing more patients per week than female dermatologists. These results are consistent with other studies of dermatologists4,24 and other physicians.12 Regardless, gender was still found to have a significant impact on income even when controlling for differences in productivity and FMLA leave taken. These results are consistent with numerous studies of US physicians that found a gender gap in income even when controlling for hours worked.12,23 Although fellowship training as a whole was found to significantly impact income, our results do not characterize whether the impact on income was positive or negative for each type of fellowship. Fellowship training in specialties such as internal medicine or general surgery likewise has variable effects on income.24,25
A comprehensive survey design and significant data elicited from dermatologists working in private practice for the first time served as the main strengths of this study. Limitations included self-reported design, categorical ranges, and limited sample size in subgroups. Future directions include deeper analysis of subgroups, including fellowship-trained dermatologists, dermatologists working more than 40 hours per week, and female dermatologists by race/ethnicity.
Conclusion
We have demonstrated that self-reported discrepancies in salary between male and female dermatologists exist, with male dermatologists earning a significantly higher annual salary than their female counterparts. This study identified and stratified several career factors that comprise the broad field and practice of dermatology. Even when controlling for these variations, we have demonstrated that gender alone remains a significant predictor of income, indicating that an unexplained income gap between the 2 genders exists in dermatology.
- Association of American Medical Colleges. Table B-2.2: Total Graduates by U.S. Medical School and Sex, 2015-2016 through 2019-2020. December 3, 2020. Accessed October 12, 2021. https://www.aamc.org/download/321532/data/factstableb2-2.pdf
- Willett LL, Halvorsen AJ, McDonald FS, et al. Gender differences in salary of internal medicine residency directors: a national survey. Am J Med. 2015;128:659-665.
- Weeks WB, Wallace AE, Mackenzie TA. Gender differences in anesthesiologists’ annual incomes. Anesthesiology. 2007;106:806-811.
- Weeks WB, Wallace AE. Gender differences in ophthalmologists’ annual incomes. Ophthalmology. 2007;114:1696-1701.
- Singh A, Burke CA, Larive B, et al. Do gender disparities persist in gastroenterology after 10 years of practice? Am J Gastroenterol. 2008;103:1589-1595.
- Desai T, Ali S, Fang X, et al. Equal work for unequal pay: the gender reimbursement gap for healthcare providers in the United States. Postgrad Med J. 2016;92:571-575.
- Jena AB, Olenski AR, Blumenthal DM. Sex differences in physician salary in US public medical schools. JAMA Intern Med. 2016;176:1294-1304.
- John AM, Gupta AB, John ES, et al. A gender-based comparison of promotion and research productivity in academic dermatology. Dermatol Online J. 2016;22:13030/qt1hx610pf.
- Sadeghpour M, Bernstein I, Ko C, et al. Role of sex in academic dermatology: results from a national survey. Arch Dermatol. 2012;148:809-814.
- Gilbert SB, Allshouse A, Skaznik-Wikiel ME. Gender inequality in salaries among reproductive endocrinology and infertility subspecialists in the United States. Fertil Steril. 2019;111:1194-1200.
- Jagsi R, Griffith KA, Stewart A, et al. Gender differences in the salaries of physician researchers. JAMA. 2012;307:2410-2417. doi:10.1001/jama.2012.6183
- Apaydin EA, Chen PGC, Friedberg MW, et al. Differences in physician income by gender in a multiregion survey. J Gen Intern Med. 2018;33:1574-1581.
- Read S, Butkus R, Weissman A, et al. Compensation disparities by gender in internal medicine. Ann Intern Med. 2018;169:658-661.
- Guss ZD, Chen Q, Hu C, et al. Differences in physician compensation between men and women at United States public academic radiation oncology departments. Int J Radiat Oncol Biol Phys. 2019;103:314-319.
- Lo Sasso AT, Richards MR, Chou CF, et al. The $16,819 pay gap for newly trained physicians: the unexplained trend of men earning more than women. Health Aff (Millwood). 2011;30:193-201.
- Shah A, Jalal S, Khosa F. Influences for gender disparity in dermatology in North America. Int J Dermatol. 2018;57:171-176.
- Shi CR, Olbricht S, Vleugels RA, et al. Sex and leadership in academic dermatology: a nationwide survey. J Am Acad Dermatol. 2017;77:782-784.
- Shih AF, Sun W, Yick C, et al. Trends in scholarly productivity of dermatology faculty by academic status and gender. J Am Acad Dermatol. 2019;80:1774-1776.
- Sarfaty S, Kolb D, Barnett R, et al. Negotiation in academic medicine: a necessary career skill. J Womens Health (Larchmt). 2007;16:235-244.
- Jacobson CC, Nguyen JC, Kimball AB. Gender and parenting significantly affect work hours of recent dermatology program graduates. Arch Dermatol. 2004;140:191-196.
- Feramisco JD, Leitenberger JJ, Redfern SI, et al. A gender gap in the dermatology literature? Cross-sectional analysis of manuscript authorship trends in dermatology journals during 3 decades. J Am Acad Dermatol. 2009;60:63-69.
- Bendels MHK, Dietz MC, Brüggmann D, et al. Gender disparities in high-quality dermatology research: a descriptive bibliometric study on scientific authorships. BMJ Open. 2018;8:e020089.
- Seabury SA, Chandra A, Jena AB. Trends in the earnings of male and female health care professionals in the United States, 1987 to 2010. JAMA Intern Med. 2013;173:1748-1750.
- Baimas-George M, Fleischer B, Slakey D, et al. Is it all about the money? Not all surgical subspecialization leads to higher lifetime revenue when compared to general surgery. J Surg Educ. 2017;74:E62-E66.
- Leigh JP, Tancredi D, Jerant A, et al. Lifetime earnings for physicians across specialties. Med Care. 2012;50:1093-1101.
Although the number of female graduates from US medical schools has steadily increased,1 several studies since the 1970s indicate that a disparity exists in salary, academic rank, and promotion among female and male physicians across multiple specialties.2-8 Proposed explanations include women working fewer hours, having lower productivity rates, undernegotiating compensation, and underbilling for the same services. However, when controlling for variables such as time, experience, specialty, rank, and research activities, this gap unequivocally persists. There are limited data on this topic in dermatology, a field in which women comprise more than half of the working population.6,7 Most analyses of gender disparities in dermatology are based on data primarily from academic dermatologists, which may not be representative of the larger population of dermatologists.8,9 The purpose of this study is to determine if an income disparity exists between male and female physicians in dermatology, including those in private practice and those who are specialty trained.
Methods
Population—We performed a cross-sectional self-reported survey to examine compensation of male and female board-certified dermatologists (MDs/DOs). Several populations of dermatologists were surveyed in August and September 2018. Approximately 20% of the members of the American Academy of Dermatology were randomly selected and sent a link to the survey. Additionally, a survey link was emailed to members of the Association of Professors of Dermatology, American College of Mohs Surgery, and American Society for Dermatologic Surgery. A link to the survey also was published on “The Board Certified Dermatologists” Facebook group.
Statistical Analysis—Descriptive statistics were used to summarize the distribution of variables overall and within gender (male or female). Not all respondents completed every section, and duplicates and incomplete responses were removed. Variables were compared between genders using t tests (continuous), the Pearson χ2 test (nominal), or the Cochran-Mantel-Haenszel test (ordinal). For categorical variables with small cell counts, an exact χ2 test for small samples was used. For continuous variables, t test P values were calculated using either pooled or Satterthwaithe approximation.
To analyze the effect of different variables on total income using multivariate and univariate linear regression, the income variable was transformed into a continuous variable by using midpoints of the categories. Univariate linear regression was used to assess the effect and significance of each variable on total annual income. Variables that were found to have a P value of less than .05 (α=.05) were deemed as significant predictors of total annual income. These variables were added to a multivariate linear regression model to determine their effect on income when adjusting for other significant (and approaching significance) factors. In addition, variables that were found to have a P value of less than .2 (α=.05) were added to the multivariate linear regression model to assess significance of these specific variables when adjusting for other factors. In this way, we tested and accounted for a multitude of variables as potential sources of confounding.
Results
Demographics—Our survey was emailed to 3079 members of the American Academy of Dermatology, and 277 responses were received. Approximately 144 additional responses were obtained collectively from links sent to the directories of the Association of Professors of Dermatology, American College of Mohs Surgery, and American Society for Dermatologic Surgery and from social media. Of these respondents, 53.65% (213/397) were female and 46.35% (184/397) were male. When stratifying by race/ethnicity, 77.33% identified as White; 13.85% identified as Asian; 6.3% identified as Black or African American, Hispanic/Latino, and Native American; and 2.52% chose not to respond. Although most male and female respondents were White, a significantly higher proportion of female respondents identified as Asian or Black/African American/Hispanic/Latino/Native American (P=.0006). We found that race/ethnicity did not significantly impact income (P=.2736). All US Census regions were represented in this study, and geographic distribution as well as population density of practice location (ie, rural, suburban, urban setting) did not differ significantly between males and females (P=.5982 and P=.1007, respectively) and did not significantly impact income (P=.3225 and P=.10663, respectively).
Income—Total annual income was defined as the aggregate sum of all types of financial compensation received in 1 calendar year (eg, salary, bonuses, benefits) and was elicited as an ordinal variable in income brackets of US $100,000. Overall, χ2 analysis showed a statistically significant difference in annual total income between male and female dermatologists (P<.0001), with a higher proportion of males in the highest pay bracket (Figure). Gender remained a statistically significant predictor of income on both univariate and multivariate linear regression analyses (P=.0002 and P<.0001, respectively), indicating that gender has a significant impact on compensation, even after controlling for other variables (eTable). Of note, males in this sample were on average older and in practice longer than females (approximately 6 years, P<.0001). However, when univariate linear regression was performed, both age (P=.8281) and number of years since residency or fellowship completion (P=.8743) were not significant predictors of income.
Practice Type—There were no statistically significant differences between men and women in practice type (P=.1489), including academic/university, hospital based, and solo and group private practice; pay structure (P=.1437), including base salary, collection-based salary, or salary plus incentive; holding a supervisory role (P=.0846); or having ownership of a practice (P=.3565)(eTable). Most respondents were in solo or group private practice (58.2%) and had a component of productivity-based compensation (77.5%). In addition, 62% of private practice dermatologists (133/212) had an ownership interest in their practice. As expected, univariate and multivariate regression analyses showed that practice type, pay structure, supervisory roles, and employee vs ownership roles were significant predictors of income (P<.05)(eTable).
Work Productivity—Statistically significant differences were found between men and women in hours worked per week in direct patient care (P<.0001) and in patient visits per week (P=.0052), with a higher percentage of men working more than 40 hours per week and men seeing an average of approximately 22 more patients per week than women. In the subgroup of all dermatologists working more than 40 hours per week, a statistically significant difference in income persisted between males and females (P=.0001). Hours worked per week and patient visits per week were statistically significant predictors of income on both univariate and multivariate regression analyses (P<.05)(Table).
Education and Fellowship Training—No significant difference existed between males and females in type of undergraduate school attended, namely public or private institutions (P=.1090), but a significant difference existed within type of medical school education, with a higher percentage of females attending private medical schools (53.03%) compared to males (38.24%)(P=.0045). However, type of undergraduate or medical school attended had no impact on income (P=.9103). A higher percentage of males (27.32%) completed additional advanced degrees, such as a master of business administration or a master of public health, compared to females (16.9%)(P=.0122). However, the completion of additional advanced degrees had no significant impact on income (P=.2379). No statistical significance existed between males and females in number of residencies completed (P=.3236), and residencies completed had no significant impact on income (P=.4584).
Of 397 respondents, approximately one-third of respondents completed fellowship training (36.5%). Fellowships included dermatopathology, surgery/cosmetics, and other (encompassing complex medical, research, transplant, and pediatric dermatology). Although similar percentages of men and women completed fellowship training, men and women differed significantly by type of fellowship completed (P=.0188). There were similar rates of dermatopathology and surgical fellowship completion between genders but almost 3 times the number of females who completed other fellowships. Type of fellowship training was a statistically significant predictor of income on both univariate and multivariate regression analyses (P<.00001 and P<.0001, respectively).
Work Activity—Respondents were asked to estimate the amount of time devoted to general dermatology, dermatopathology, Mohs micrographic surgery, cosmetics, and dermatologic surgery in their practices (Table). Women devoted a significantly higher average percentage of time to cosmetics (7.89%) compared to men (4.52%)(P=.0097). The number of cosmetic procedures performed per week was not statistically significantly different between men and women (P=.8035) but was a significant factor for income on univariate regression analysis (P=.0002). Time spent performing dermatologic surgery, general dermatology, or Mohs micrographic surgery did not significantly differ between men and women but was found to significantly influence income.
Academic Dermatology—Among the respondents working in academic settings, χ2 analysis identified a significant difference in the faculty rank between males and females, with a tendency for lower academic rank in females (P=.0508). Assistant professorship was comprised of 35% of men vs 51% of women, whereas full professorship consisted of 26% of men but only 13% of women. Academic rank was found to be a significant predictor of income, with higher rank associated with higher income (P<.0001 on univariate regression analysis). However, when adjusting for other factors, academic rank was no longer a significant predictor of income (P=.0840 on multivariate regression analysis). No significant difference existed between men and women in funding received from the National Institutes of Health, conduction of clinical trials, or authorship of scientific publications, and these factors were not found to have a significant impact on income.
Work Leave—Male and female dermatologists showed a statistically significant difference in maternity or Family and Medical Leave Act (FMLA) leave taken over their careers, with 56.03% of females reporting leave taken compared to 6.78% of males (P<.0001). Women reported a significantly higher average number of weeks of maternity or FMLA leave taken over their careers (12.92 weeks) compared to men (2.42 weeks) (P<.0001). However, upon univariate regression analysis, whether or not maternity or FMLA leave was taken over their careers (P=.2005), the number of times that maternity or FMLA leave was taken (P=.4350), and weeks of maternity or FMLA leave taken (P=.4057) were all not significant predictors of income.
Comment
This study sought to investigate the relationship between income and gender in dermatology, and our results demonstrated that statistically significant differences in total annual income exist between male and female dermatologists, with male dermatologists earning a significantly higher income, approximately an additional $80,000. Our results are consistent with other studies of US physician income, which have found a gender gap ranging from $13,399 to $82,000 that persists even when controlling for factors such as specialty choice, practice setting, rank and role in practice, work hours, vacation/leave taken, and others.2-7,10-15
There was a significant difference in rank of male and female academic dermatologists, with fewer females at higher academic ranks. These results are consistent with numerous studies in academic dermatology that show underrepresentation of women at higher academic ranks and leadership positions.8,9,16-18 Poor negotiation may contribute to differences in both rank and income.19,20 There are conflicting data on research productivity of academic dermatologists and length of career, first and senior authorship, and quality and academic impact, all of which add complexity to this topic.8,9,12,16-18,20-23Male and female dermatologists reported significant differences in productivity, with male dermatologists working more hours and seeing more patients per week than female dermatologists. These results are consistent with other studies of dermatologists4,24 and other physicians.12 Regardless, gender was still found to have a significant impact on income even when controlling for differences in productivity and FMLA leave taken. These results are consistent with numerous studies of US physicians that found a gender gap in income even when controlling for hours worked.12,23 Although fellowship training as a whole was found to significantly impact income, our results do not characterize whether the impact on income was positive or negative for each type of fellowship. Fellowship training in specialties such as internal medicine or general surgery likewise has variable effects on income.24,25
A comprehensive survey design and significant data elicited from dermatologists working in private practice for the first time served as the main strengths of this study. Limitations included self-reported design, categorical ranges, and limited sample size in subgroups. Future directions include deeper analysis of subgroups, including fellowship-trained dermatologists, dermatologists working more than 40 hours per week, and female dermatologists by race/ethnicity.
Conclusion
We have demonstrated that self-reported discrepancies in salary between male and female dermatologists exist, with male dermatologists earning a significantly higher annual salary than their female counterparts. This study identified and stratified several career factors that comprise the broad field and practice of dermatology. Even when controlling for these variations, we have demonstrated that gender alone remains a significant predictor of income, indicating that an unexplained income gap between the 2 genders exists in dermatology.
Although the number of female graduates from US medical schools has steadily increased,1 several studies since the 1970s indicate that a disparity exists in salary, academic rank, and promotion among female and male physicians across multiple specialties.2-8 Proposed explanations include women working fewer hours, having lower productivity rates, undernegotiating compensation, and underbilling for the same services. However, when controlling for variables such as time, experience, specialty, rank, and research activities, this gap unequivocally persists. There are limited data on this topic in dermatology, a field in which women comprise more than half of the working population.6,7 Most analyses of gender disparities in dermatology are based on data primarily from academic dermatologists, which may not be representative of the larger population of dermatologists.8,9 The purpose of this study is to determine if an income disparity exists between male and female physicians in dermatology, including those in private practice and those who are specialty trained.
Methods
Population—We performed a cross-sectional self-reported survey to examine compensation of male and female board-certified dermatologists (MDs/DOs). Several populations of dermatologists were surveyed in August and September 2018. Approximately 20% of the members of the American Academy of Dermatology were randomly selected and sent a link to the survey. Additionally, a survey link was emailed to members of the Association of Professors of Dermatology, American College of Mohs Surgery, and American Society for Dermatologic Surgery. A link to the survey also was published on “The Board Certified Dermatologists” Facebook group.
Statistical Analysis—Descriptive statistics were used to summarize the distribution of variables overall and within gender (male or female). Not all respondents completed every section, and duplicates and incomplete responses were removed. Variables were compared between genders using t tests (continuous), the Pearson χ2 test (nominal), or the Cochran-Mantel-Haenszel test (ordinal). For categorical variables with small cell counts, an exact χ2 test for small samples was used. For continuous variables, t test P values were calculated using either pooled or Satterthwaithe approximation.
To analyze the effect of different variables on total income using multivariate and univariate linear regression, the income variable was transformed into a continuous variable by using midpoints of the categories. Univariate linear regression was used to assess the effect and significance of each variable on total annual income. Variables that were found to have a P value of less than .05 (α=.05) were deemed as significant predictors of total annual income. These variables were added to a multivariate linear regression model to determine their effect on income when adjusting for other significant (and approaching significance) factors. In addition, variables that were found to have a P value of less than .2 (α=.05) were added to the multivariate linear regression model to assess significance of these specific variables when adjusting for other factors. In this way, we tested and accounted for a multitude of variables as potential sources of confounding.
Results
Demographics—Our survey was emailed to 3079 members of the American Academy of Dermatology, and 277 responses were received. Approximately 144 additional responses were obtained collectively from links sent to the directories of the Association of Professors of Dermatology, American College of Mohs Surgery, and American Society for Dermatologic Surgery and from social media. Of these respondents, 53.65% (213/397) were female and 46.35% (184/397) were male. When stratifying by race/ethnicity, 77.33% identified as White; 13.85% identified as Asian; 6.3% identified as Black or African American, Hispanic/Latino, and Native American; and 2.52% chose not to respond. Although most male and female respondents were White, a significantly higher proportion of female respondents identified as Asian or Black/African American/Hispanic/Latino/Native American (P=.0006). We found that race/ethnicity did not significantly impact income (P=.2736). All US Census regions were represented in this study, and geographic distribution as well as population density of practice location (ie, rural, suburban, urban setting) did not differ significantly between males and females (P=.5982 and P=.1007, respectively) and did not significantly impact income (P=.3225 and P=.10663, respectively).
Income—Total annual income was defined as the aggregate sum of all types of financial compensation received in 1 calendar year (eg, salary, bonuses, benefits) and was elicited as an ordinal variable in income brackets of US $100,000. Overall, χ2 analysis showed a statistically significant difference in annual total income between male and female dermatologists (P<.0001), with a higher proportion of males in the highest pay bracket (Figure). Gender remained a statistically significant predictor of income on both univariate and multivariate linear regression analyses (P=.0002 and P<.0001, respectively), indicating that gender has a significant impact on compensation, even after controlling for other variables (eTable). Of note, males in this sample were on average older and in practice longer than females (approximately 6 years, P<.0001). However, when univariate linear regression was performed, both age (P=.8281) and number of years since residency or fellowship completion (P=.8743) were not significant predictors of income.
Practice Type—There were no statistically significant differences between men and women in practice type (P=.1489), including academic/university, hospital based, and solo and group private practice; pay structure (P=.1437), including base salary, collection-based salary, or salary plus incentive; holding a supervisory role (P=.0846); or having ownership of a practice (P=.3565)(eTable). Most respondents were in solo or group private practice (58.2%) and had a component of productivity-based compensation (77.5%). In addition, 62% of private practice dermatologists (133/212) had an ownership interest in their practice. As expected, univariate and multivariate regression analyses showed that practice type, pay structure, supervisory roles, and employee vs ownership roles were significant predictors of income (P<.05)(eTable).
Work Productivity—Statistically significant differences were found between men and women in hours worked per week in direct patient care (P<.0001) and in patient visits per week (P=.0052), with a higher percentage of men working more than 40 hours per week and men seeing an average of approximately 22 more patients per week than women. In the subgroup of all dermatologists working more than 40 hours per week, a statistically significant difference in income persisted between males and females (P=.0001). Hours worked per week and patient visits per week were statistically significant predictors of income on both univariate and multivariate regression analyses (P<.05)(Table).
Education and Fellowship Training—No significant difference existed between males and females in type of undergraduate school attended, namely public or private institutions (P=.1090), but a significant difference existed within type of medical school education, with a higher percentage of females attending private medical schools (53.03%) compared to males (38.24%)(P=.0045). However, type of undergraduate or medical school attended had no impact on income (P=.9103). A higher percentage of males (27.32%) completed additional advanced degrees, such as a master of business administration or a master of public health, compared to females (16.9%)(P=.0122). However, the completion of additional advanced degrees had no significant impact on income (P=.2379). No statistical significance existed between males and females in number of residencies completed (P=.3236), and residencies completed had no significant impact on income (P=.4584).
Of 397 respondents, approximately one-third of respondents completed fellowship training (36.5%). Fellowships included dermatopathology, surgery/cosmetics, and other (encompassing complex medical, research, transplant, and pediatric dermatology). Although similar percentages of men and women completed fellowship training, men and women differed significantly by type of fellowship completed (P=.0188). There were similar rates of dermatopathology and surgical fellowship completion between genders but almost 3 times the number of females who completed other fellowships. Type of fellowship training was a statistically significant predictor of income on both univariate and multivariate regression analyses (P<.00001 and P<.0001, respectively).
Work Activity—Respondents were asked to estimate the amount of time devoted to general dermatology, dermatopathology, Mohs micrographic surgery, cosmetics, and dermatologic surgery in their practices (Table). Women devoted a significantly higher average percentage of time to cosmetics (7.89%) compared to men (4.52%)(P=.0097). The number of cosmetic procedures performed per week was not statistically significantly different between men and women (P=.8035) but was a significant factor for income on univariate regression analysis (P=.0002). Time spent performing dermatologic surgery, general dermatology, or Mohs micrographic surgery did not significantly differ between men and women but was found to significantly influence income.
Academic Dermatology—Among the respondents working in academic settings, χ2 analysis identified a significant difference in the faculty rank between males and females, with a tendency for lower academic rank in females (P=.0508). Assistant professorship was comprised of 35% of men vs 51% of women, whereas full professorship consisted of 26% of men but only 13% of women. Academic rank was found to be a significant predictor of income, with higher rank associated with higher income (P<.0001 on univariate regression analysis). However, when adjusting for other factors, academic rank was no longer a significant predictor of income (P=.0840 on multivariate regression analysis). No significant difference existed between men and women in funding received from the National Institutes of Health, conduction of clinical trials, or authorship of scientific publications, and these factors were not found to have a significant impact on income.
Work Leave—Male and female dermatologists showed a statistically significant difference in maternity or Family and Medical Leave Act (FMLA) leave taken over their careers, with 56.03% of females reporting leave taken compared to 6.78% of males (P<.0001). Women reported a significantly higher average number of weeks of maternity or FMLA leave taken over their careers (12.92 weeks) compared to men (2.42 weeks) (P<.0001). However, upon univariate regression analysis, whether or not maternity or FMLA leave was taken over their careers (P=.2005), the number of times that maternity or FMLA leave was taken (P=.4350), and weeks of maternity or FMLA leave taken (P=.4057) were all not significant predictors of income.
Comment
This study sought to investigate the relationship between income and gender in dermatology, and our results demonstrated that statistically significant differences in total annual income exist between male and female dermatologists, with male dermatologists earning a significantly higher income, approximately an additional $80,000. Our results are consistent with other studies of US physician income, which have found a gender gap ranging from $13,399 to $82,000 that persists even when controlling for factors such as specialty choice, practice setting, rank and role in practice, work hours, vacation/leave taken, and others.2-7,10-15
There was a significant difference in rank of male and female academic dermatologists, with fewer females at higher academic ranks. These results are consistent with numerous studies in academic dermatology that show underrepresentation of women at higher academic ranks and leadership positions.8,9,16-18 Poor negotiation may contribute to differences in both rank and income.19,20 There are conflicting data on research productivity of academic dermatologists and length of career, first and senior authorship, and quality and academic impact, all of which add complexity to this topic.8,9,12,16-18,20-23Male and female dermatologists reported significant differences in productivity, with male dermatologists working more hours and seeing more patients per week than female dermatologists. These results are consistent with other studies of dermatologists4,24 and other physicians.12 Regardless, gender was still found to have a significant impact on income even when controlling for differences in productivity and FMLA leave taken. These results are consistent with numerous studies of US physicians that found a gender gap in income even when controlling for hours worked.12,23 Although fellowship training as a whole was found to significantly impact income, our results do not characterize whether the impact on income was positive or negative for each type of fellowship. Fellowship training in specialties such as internal medicine or general surgery likewise has variable effects on income.24,25
A comprehensive survey design and significant data elicited from dermatologists working in private practice for the first time served as the main strengths of this study. Limitations included self-reported design, categorical ranges, and limited sample size in subgroups. Future directions include deeper analysis of subgroups, including fellowship-trained dermatologists, dermatologists working more than 40 hours per week, and female dermatologists by race/ethnicity.
Conclusion
We have demonstrated that self-reported discrepancies in salary between male and female dermatologists exist, with male dermatologists earning a significantly higher annual salary than their female counterparts. This study identified and stratified several career factors that comprise the broad field and practice of dermatology. Even when controlling for these variations, we have demonstrated that gender alone remains a significant predictor of income, indicating that an unexplained income gap between the 2 genders exists in dermatology.
- Association of American Medical Colleges. Table B-2.2: Total Graduates by U.S. Medical School and Sex, 2015-2016 through 2019-2020. December 3, 2020. Accessed October 12, 2021. https://www.aamc.org/download/321532/data/factstableb2-2.pdf
- Willett LL, Halvorsen AJ, McDonald FS, et al. Gender differences in salary of internal medicine residency directors: a national survey. Am J Med. 2015;128:659-665.
- Weeks WB, Wallace AE, Mackenzie TA. Gender differences in anesthesiologists’ annual incomes. Anesthesiology. 2007;106:806-811.
- Weeks WB, Wallace AE. Gender differences in ophthalmologists’ annual incomes. Ophthalmology. 2007;114:1696-1701.
- Singh A, Burke CA, Larive B, et al. Do gender disparities persist in gastroenterology after 10 years of practice? Am J Gastroenterol. 2008;103:1589-1595.
- Desai T, Ali S, Fang X, et al. Equal work for unequal pay: the gender reimbursement gap for healthcare providers in the United States. Postgrad Med J. 2016;92:571-575.
- Jena AB, Olenski AR, Blumenthal DM. Sex differences in physician salary in US public medical schools. JAMA Intern Med. 2016;176:1294-1304.
- John AM, Gupta AB, John ES, et al. A gender-based comparison of promotion and research productivity in academic dermatology. Dermatol Online J. 2016;22:13030/qt1hx610pf.
- Sadeghpour M, Bernstein I, Ko C, et al. Role of sex in academic dermatology: results from a national survey. Arch Dermatol. 2012;148:809-814.
- Gilbert SB, Allshouse A, Skaznik-Wikiel ME. Gender inequality in salaries among reproductive endocrinology and infertility subspecialists in the United States. Fertil Steril. 2019;111:1194-1200.
- Jagsi R, Griffith KA, Stewart A, et al. Gender differences in the salaries of physician researchers. JAMA. 2012;307:2410-2417. doi:10.1001/jama.2012.6183
- Apaydin EA, Chen PGC, Friedberg MW, et al. Differences in physician income by gender in a multiregion survey. J Gen Intern Med. 2018;33:1574-1581.
- Read S, Butkus R, Weissman A, et al. Compensation disparities by gender in internal medicine. Ann Intern Med. 2018;169:658-661.
- Guss ZD, Chen Q, Hu C, et al. Differences in physician compensation between men and women at United States public academic radiation oncology departments. Int J Radiat Oncol Biol Phys. 2019;103:314-319.
- Lo Sasso AT, Richards MR, Chou CF, et al. The $16,819 pay gap for newly trained physicians: the unexplained trend of men earning more than women. Health Aff (Millwood). 2011;30:193-201.
- Shah A, Jalal S, Khosa F. Influences for gender disparity in dermatology in North America. Int J Dermatol. 2018;57:171-176.
- Shi CR, Olbricht S, Vleugels RA, et al. Sex and leadership in academic dermatology: a nationwide survey. J Am Acad Dermatol. 2017;77:782-784.
- Shih AF, Sun W, Yick C, et al. Trends in scholarly productivity of dermatology faculty by academic status and gender. J Am Acad Dermatol. 2019;80:1774-1776.
- Sarfaty S, Kolb D, Barnett R, et al. Negotiation in academic medicine: a necessary career skill. J Womens Health (Larchmt). 2007;16:235-244.
- Jacobson CC, Nguyen JC, Kimball AB. Gender and parenting significantly affect work hours of recent dermatology program graduates. Arch Dermatol. 2004;140:191-196.
- Feramisco JD, Leitenberger JJ, Redfern SI, et al. A gender gap in the dermatology literature? Cross-sectional analysis of manuscript authorship trends in dermatology journals during 3 decades. J Am Acad Dermatol. 2009;60:63-69.
- Bendels MHK, Dietz MC, Brüggmann D, et al. Gender disparities in high-quality dermatology research: a descriptive bibliometric study on scientific authorships. BMJ Open. 2018;8:e020089.
- Seabury SA, Chandra A, Jena AB. Trends in the earnings of male and female health care professionals in the United States, 1987 to 2010. JAMA Intern Med. 2013;173:1748-1750.
- Baimas-George M, Fleischer B, Slakey D, et al. Is it all about the money? Not all surgical subspecialization leads to higher lifetime revenue when compared to general surgery. J Surg Educ. 2017;74:E62-E66.
- Leigh JP, Tancredi D, Jerant A, et al. Lifetime earnings for physicians across specialties. Med Care. 2012;50:1093-1101.
- Association of American Medical Colleges. Table B-2.2: Total Graduates by U.S. Medical School and Sex, 2015-2016 through 2019-2020. December 3, 2020. Accessed October 12, 2021. https://www.aamc.org/download/321532/data/factstableb2-2.pdf
- Willett LL, Halvorsen AJ, McDonald FS, et al. Gender differences in salary of internal medicine residency directors: a national survey. Am J Med. 2015;128:659-665.
- Weeks WB, Wallace AE, Mackenzie TA. Gender differences in anesthesiologists’ annual incomes. Anesthesiology. 2007;106:806-811.
- Weeks WB, Wallace AE. Gender differences in ophthalmologists’ annual incomes. Ophthalmology. 2007;114:1696-1701.
- Singh A, Burke CA, Larive B, et al. Do gender disparities persist in gastroenterology after 10 years of practice? Am J Gastroenterol. 2008;103:1589-1595.
- Desai T, Ali S, Fang X, et al. Equal work for unequal pay: the gender reimbursement gap for healthcare providers in the United States. Postgrad Med J. 2016;92:571-575.
- Jena AB, Olenski AR, Blumenthal DM. Sex differences in physician salary in US public medical schools. JAMA Intern Med. 2016;176:1294-1304.
- John AM, Gupta AB, John ES, et al. A gender-based comparison of promotion and research productivity in academic dermatology. Dermatol Online J. 2016;22:13030/qt1hx610pf.
- Sadeghpour M, Bernstein I, Ko C, et al. Role of sex in academic dermatology: results from a national survey. Arch Dermatol. 2012;148:809-814.
- Gilbert SB, Allshouse A, Skaznik-Wikiel ME. Gender inequality in salaries among reproductive endocrinology and infertility subspecialists in the United States. Fertil Steril. 2019;111:1194-1200.
- Jagsi R, Griffith KA, Stewart A, et al. Gender differences in the salaries of physician researchers. JAMA. 2012;307:2410-2417. doi:10.1001/jama.2012.6183
- Apaydin EA, Chen PGC, Friedberg MW, et al. Differences in physician income by gender in a multiregion survey. J Gen Intern Med. 2018;33:1574-1581.
- Read S, Butkus R, Weissman A, et al. Compensation disparities by gender in internal medicine. Ann Intern Med. 2018;169:658-661.
- Guss ZD, Chen Q, Hu C, et al. Differences in physician compensation between men and women at United States public academic radiation oncology departments. Int J Radiat Oncol Biol Phys. 2019;103:314-319.
- Lo Sasso AT, Richards MR, Chou CF, et al. The $16,819 pay gap for newly trained physicians: the unexplained trend of men earning more than women. Health Aff (Millwood). 2011;30:193-201.
- Shah A, Jalal S, Khosa F. Influences for gender disparity in dermatology in North America. Int J Dermatol. 2018;57:171-176.
- Shi CR, Olbricht S, Vleugels RA, et al. Sex and leadership in academic dermatology: a nationwide survey. J Am Acad Dermatol. 2017;77:782-784.
- Shih AF, Sun W, Yick C, et al. Trends in scholarly productivity of dermatology faculty by academic status and gender. J Am Acad Dermatol. 2019;80:1774-1776.
- Sarfaty S, Kolb D, Barnett R, et al. Negotiation in academic medicine: a necessary career skill. J Womens Health (Larchmt). 2007;16:235-244.
- Jacobson CC, Nguyen JC, Kimball AB. Gender and parenting significantly affect work hours of recent dermatology program graduates. Arch Dermatol. 2004;140:191-196.
- Feramisco JD, Leitenberger JJ, Redfern SI, et al. A gender gap in the dermatology literature? Cross-sectional analysis of manuscript authorship trends in dermatology journals during 3 decades. J Am Acad Dermatol. 2009;60:63-69.
- Bendels MHK, Dietz MC, Brüggmann D, et al. Gender disparities in high-quality dermatology research: a descriptive bibliometric study on scientific authorships. BMJ Open. 2018;8:e020089.
- Seabury SA, Chandra A, Jena AB. Trends in the earnings of male and female health care professionals in the United States, 1987 to 2010. JAMA Intern Med. 2013;173:1748-1750.
- Baimas-George M, Fleischer B, Slakey D, et al. Is it all about the money? Not all surgical subspecialization leads to higher lifetime revenue when compared to general surgery. J Surg Educ. 2017;74:E62-E66.
- Leigh JP, Tancredi D, Jerant A, et al. Lifetime earnings for physicians across specialties. Med Care. 2012;50:1093-1101.
Practice Points
- In this survey-based cross-sectional study, a statistically significant income disparity between male and female dermatologists was found.
- Although several differences were identified between male and female dermatologists that contribute to income, gender remained a statistically significant predictor of income, and this disparity could not be explained by other factors.
Proper Use and Compliance of Facial Masks During the COVID-19 Pandemic: An Observational Study of Hospitals in New York City
Although the universal use of masks by both health care professionals and the general public now appears routine, widely differing recommendations were distributed by different health organizations early in the pandemic. In April 2020, the World Health Organization (WHO) stated that there was no evidence that healthy individuals wearing a medical mask in the community prevented COVID-19 infection.1 However, these recommendations must be placed in the context of a national shortage of personal protective equipment early in the pandemic. The WHO guidance released on June 5, 2020, recommended continuous use of masks for health care workers in the clinical setting.2 Additional recommendations included mask replacement when wet, soiled, or damaged, and when the wearer touched the mask. The WHO also recommended mask usage by those with underlying medical comorbidities and those living in high population–density areas and in settings where physical distancing was not possible.2
The Centers for Disease Control and Prevention (CDC) officially recommended the use of face coverings for the general public to prevent COVID-19 transmission on April 3, 2020.3 The CDC highlighted that masks should not be worn by children younger than 2 years; individuals with respiratory compromise; and patients who are unconscious, incapacitated, or unable to remove a mask without assistance.4 Medical masks and respirators were only recommended for health care workers. Importantly, masks with valves/vents were not recommended, as respiratory droplets can be emitted, defeating the purpose of source control.4 New York State mandated mask usage in public places starting on April 15, 2020.
These recommendations were based on the hypothesis that COVID-19 transmission occurs primarily via droplets and contact. In reality, SARS-CoV-2 transmission more likely occurs in a continuum from larger droplets to miniscule aerosols expelled from an infected person when talking, coughing, or sneezing.5,6 It should be noted that there was a formal suggestion of the potential for airborne transmission of SARS-CoV-2 by the CDC in a statement on September 18, 2020, that was subsequently retracted 3 days later.7,8 The CDC, reversing their prior recommendations, updated their guidance on October 5, 2020, endorsing prior reports that SARS-CoV-2 can be spread through aerosol transmission.8
Mask usage helps prevent viral spread by all individuals, especially those who are presymptomatic and asymptomatic. Presymptomatic individuals account for approximately 40% to 60% of transmissions, and asymptomatic individuals account for approximately 4% to 30% of infections by some models, which suggest these individuals are the drivers of the pandemic, more so than symptomatic individuals.9-15 Additionally, masking also may in effect reduce the amount of SARS-CoV-2 to which individuals are being exposed in the community.14 Universal masking is a relatively low-cost, low-risk intervention that may provide moderate benefit to the individual but substantial benefit to communities at large.10-13 Universal masking in other countries also has clearly demonstrated major benefits during the pandemic. Implementation of universal masking in Taiwan resulted in only approximately 440 COVID-19 cases and less than 10 deaths, despite a population of 23 million.16 South Korea, having experience with Middle East respiratory syndrome, also was able to quickly institute a mask policy for its citizens, resulting in approximately 94% compliance.17 Moreover, several mathematical models have shown that even imperfect use of masks on a population level can prevent disease transmission and should be instituted.18
Given the importance and potential benefits of mask usage, we investigated compliance and proper utilization of facial masks in New York City (NYC), once the epicenter of the pandemic in the United States. New York City and the rest of New York State experienced more than 1.13 million and 1.46 million cases of COVID-19, respectively, as of early November 2021.19 Nationwide, NYC had the greatest absolute death count of more than 34,634 and the greatest rate of death per 100,000 individuals of 412. In contrast, New York State, excluding NYC, had an absolute death count of more than 21,646 and a death rate per 100,000 individuals of 195 as of early November 2021.19 Now entering 20 months since the first case of COVID-19 in NYC, it continues to be vital for facial mask protocols to be emphasized as part of a comprehensive infection prevention protocol, especially in light of continued vaccine resistance, to help stall continued spread of SARS-CoV-2.20
We seek to show that despite months of policies for universal masking in NYC, there is still considerable mask noncompliance by the general public in health care settings where the use of masks is particularly imperative. We conducted an observational study investigating proper use of face masks of adults entering the main entrance of 4 hospitals located in NYC.
Methods
We observed mask usage in adults entering 4 hospitals in September 2020 (postsurge in NYC and prior to the availability of COVID-19 vaccinations). Hospitals were chosen to represent several types of health care delivery systems available in the United States and included a city, state, federal, and private hospital. Data collection was completed during peak traffic hours (8:00
Mask usage was observed and classified into several categories: correctly fitting mask over the nose and mouth, no face mask, mask usage with nose exposed, mask usage with mouth exposed, mask usage with both nose and mouth exposed (ie, mask on the chin/neck area), loosely fitting mask, vented/valved mask, or other form of face covering (eg, bandana, scarf).
Results
We observed a consistent rate of mask compliance between 72% and 85%, with an average of 78% of the 600 individuals observed wearing correctly fitting masks across the 4 hospitals included in this study (Table). The employee entrance included in this study had the highest compliance rate of 85%. An overall low rate of complete mask noncompliance was observed, with only 9 individuals (1.5%) in the entire study not wearing any mask. The federal hospital had the highest rate of mask noncompliance. We also observed a low rate of nose and mouth exposure, with 1.8% of individuals wearing a mask with the nose and mouth exposed (ie, mask tucked under the chin). No individuals were observed with the mouth exposed but with the nose covered by a mask. Additionally, only 3 individuals (0.5%) wore a mask with a vent/valve. The most common way that masks were worn incorrectly was with the nose exposed, accounting for 9.5% of individuals observed. Overall, only 9 individuals (1.5%) wore a nontraditional face covering, with a bandana being the most commonly observed makeshift mask.
Signage regarding the requirement to wear masks and to social distance was universally instituted at all hospital entry points (both inside and outside the hospital) in this study. However, there were no illustrations demonstrating correct and incorrect forms of mask usage. All signage merely displayed a graphic of a facial mask noting the requirement to wear a mask prior to entering the building. Hospital staff also had face masks available for patients who failed to bring a mask or who wore an inappropriate mask (ie, vented/valved masks).
Comment
Mask Effectiveness—Masks reduce the spread of SARS-CoV-2 by preventing both droplets and potentially virus-bearing aerosols.6,21,22 It has been demonstrated that well-fitted cotton homemade masks and medical masks provide the most effective method of reducing droplet dispersion. Loosely fitted masks as well as bandana-style facial coverings minimally reduce small aerosolized droplets, and an uncovered mouth and nose can disperse particles at a distance much greater than 6 feet.22
Mask Compliance—We report an overall high compliance rate with mask wearing among individuals visiting a hospital; however, compliance was still imperfect. Overall, 78% of observed individuals wore a correctly fitting mask when entering a hospital, even with hospital staff positioned at entry points to ensure proper mask usage. With all the resources available at health care centers, we anticipated a much higher compliance rate for correctly fitting masks at hospital entrances. We hypothesize that given only 78% of individuals showed proper mask compliance in a setting with enforcement by health care personnel, the mask compliance rate in the larger community is likely much lower. It is imperative to enforce continued mask compliance in medical centers and other public areas given notable vaccine noncompliance in certain parts of the country.
Tools to Prevent Disease Transmission—Mask usage by the general public in NYC helped in its response to the COVID-19 pandemic. Yang et al23 demonstrated through mathematical modeling that mask usage in NYC was associated with a 6.6% reduction in transmission overall and a 20% decrease in transmission for individuals 65 years and older during the first month of the universal mask policy going into effect. The authors extrapolated these data during the NYC reopening and found that universal masking reduced transmission by approximately 9% to 11%, accounting for the increase in hours spent outside home quarantine. The authors also hypothesized that if universal masking was as effective in its reduction of transmission for everyone in NYC as it was for older adults, the potential reduction in transmission of SARS-CoV-2 could be as high as 28% to 32%.23
Temperature checks at entrance barricades were standard protocol during the observation period. Although the main purpose of this study was to investigate compliance with and proper use of facial masks in a health care setting, it should be mentioned that, although temperature checks were being done on almost every person entering a hospital, the uniformity and practicality of this intervention has not been backed by substantial evidence. Although many nontouch thermometers are intended to capture a forehead temperature for the most accurate reading, the authors will share that in their observation, medical personnel screening individuals at hospital entrances were observed checking temperatures at any easily accessible body part, such as the forearm, hand, or neck. Furthermore, it has been reported that only approximately 40% of individuals with COVID-19 present with a fever.24 Many hospitals, including the 4 that were included in this investigation, have formal protocols for patients presenting with a fever, especially those presenting to an ambulatory center. Patients are usually instructed to call ahead if they have a fever, and a decision regarding next steps will be discussed with a health care provider. In addition, 1 meta-analysis on the symptoms of COVID-19 suggested that approximately 12% of infected patients are asymptomatic, likely a conservative estimate.25 Although we do not suggest that hospitals stop temperature checks, consistent temperature checks in anatomic locations intended for the specific thermometer used must be employed. Alternatively, a thermographic camera system that could detect heat signatures may be a way to screen faster, only necessitating that those above a threshold be assessed further.
The results of this study suggest that much greater effort is being placed on these temperature checks than on other equally important components of the entrance health assessment. This initial encounter at hospital entrances should serve as an opportunity for education on proper choice and use of masks with clear instructions that masks should not be removed unless directed by a health care provider and in a designated area, such as an examination room. The COVID-19 pandemic in the United States is likely the first time an individual is wearing these types of masks. Reiterating when and how often a mask should be changed (eg, when wet or soiled), how a soiled mask is not an effective mask, how a used mask should be discarded, ways to prevent self-contamination (ie, proper donning and doffing), and the importance of other infection-prevention behaviors—hand hygiene; social distancing; avoidance of touching the eyes, nose, and mouth with unwashed hands; and regular disinfecting of surfaces—should be practiced.11,26-29 Extended use and reuse of masks also can result in transmission of infection.30
Throughout the pandemic, our personal experience is that some patients often overtly refuse to wear a mask, citing underlying respiratory issues. The implications of patients not wearing a mask in a medical office and endangering other patients and staff are beyond the scope of this analysis. We will, however, comment briefly on the evidence behind this common concern. Matuschek et al31 found substantial adverse changes in respiratory rate, oxygen saturation, and CO2 levels in patients with severe chronic obstructive pulmonary disease who were wearing N95 respirators during a 6-minute walk test. Another study by Chan et al32 showed that nonmedical masks in healthy older adults in the community setting had no impact on oxygen saturation. Ultimately, the most effective mask a patient can wear is a mask that will be worn consistently.32
Populations With Limited Access to Masks—The COVID-19 pandemic disproportionately impacted disadvantaged populations, both in socioeconomic status and minority status. A disproportionate number of COVID-19 hospitalizations and deaths occurred in lower-income and minority populations.10 In fact, Lamb et al33 reported that NYC neighborhoods with a larger proportion of uninsured individuals with limited access to health care and overall lower socioeconomic status had a higher rate of SARS-CoV-2 positivity. A retrospective study in Louisiana showed that Black individuals accounted for 77% of hospitalizations and 71% of deaths due to COVID-19 in a population where only 31% of individuals identified as Black.10 Chu et al6 even asserted that policies should be put into place to address equity issues for populations with limited access to masks. We agree that policies should be put into action to ensure that individuals lacking the means to obtain appropriate masks or unable to obtain an adequate supply of masks be provided this new necessity. It has been calculated that the impact of masks in reducing virus transmission would be greatest if mask availability to disadvantaged populations is ensured.18 We support a plan for masks to be covered by government-sponsored health plans.
Study Limitations—Several limitations exist in our study that should be discussed. Although the data collectors observed a large number of individuals, each hospital entrance was only observed for 1 half-day morning session. There may be variations in the number of people wearing a mask at different times of day and different days of the week with fluctuations in hospital traffic. Although data were collected at a variety of hospitals representing the diverse health care delivery models available in the United States, the NYC hospitals included in this study may have different resources available for infection-prevention strategies than hospitals across the country, given NYC’s unique population density and demographics.
Study Strengths—The generalizability of the study should be recognized. Data were collected by all major health care delivery models available in the United States—private, state, city, and federal hospital systems. This study can be easily replicated in other health care delivery systems to further investigate potential gaps in mask usage and infection prevention. Repeating this study in areas where a large portion of the population does not believe in the virus also will likely show lower levels of mask use.
Conclusion
As the country grapples with vaccine hesitancy and with the new variants of SARS-CoV-2, continued universal masking is still imperative. The effectiveness of universal masking has been demonstrated, and with the combination of vaccinations, we can be assured that the world will continue to emerge from the pandemic.
- World Health Organization. Advice on the use of masks in the context of COVID-19. Interim guidance (6 April 2020). Accessed November 8, 2021. https://apps.who.int/iris/bitstream/handle/10665/331693/WHO-2019-nCov-IPC_Masks-2020.3-eng.pdf?sequence=1ceisAllowed=y
- World Health Organization. Advice on the use of masks in the context of COVID-19. Interim guidance (5 June 2020). Accessed November 8, 2021. https://apps.who.int/iris/bitstream/handle/10665/332293/WHO- 2019-nCov-IPC_Masks-2020.4-eng.pdf?sequence=1&isAllowed=y
- Fisher KA, Barile JP, Guerin RJ, et al. Factors associated with cloth face covering use among adults during the COVID-19 pandemic—United States, April and May 2020. MMWR Morb Mortal Wkly Rep. 2020;69:933-937.
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19). Considerations for wearing masks (19 April 2021). Accessed November 10, 2021. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/cloth-face-cover-guidance.html
- Conly J, Seto WH, Pittet D, et al. Use of medical face masks versus particulate respirators as a component of personal protective equipment for health care workers in the context of the COVID-19 pandemic. Antimicrob Resist Infect Control. 2020;9:126.
- Chu DK, Akl EA, Duda S, et al; COVID-19 Systematic Urgent Review Group Effort (SURGE) study authors. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395:1973-1987.
- Huang, P. Coronavirus FAQs: Why can’t the CDC make up its mind about airborne transmission? NPR. September 25, 2020. Accessed November 8, 2021. https://www.npr.org/sections/goatsandsoda/2020/09/25/916624967/coronavirus-faqs-why-cant-the-cdc-make-up-its-mind-about-airborne-transmission
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19). How COVID-19 spreads (14 July 2021). Accessed November 10, 2021. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/how-covid-spreads.html
- Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782-793.
- Klompas M, Morris CA, Shenoy ES. Universal masking in the covid-19 era. N Engl J Med. 2020;383:E9.
- Middleton JD, Lopes H. Face masks in the covid-19 crisis: caveats, limits, and priorities. BMJ. 2020;369:m2030.
- Cheng KK, Lam TH, Leung CC. Wearing face masks in the community during the COVID-19 pandemic: altruism and solidarity [published online April 16, 2020]. Lancet. doi:10.1016/S0140-6736(20)30918-1
- Javid B, Weekes MP, Matheson NJ. Covid-19: should the public wear face masks? BMJ. 2020;369:m1442.
- Gandhi M, Beyrer C, Goosby E. Masks do more than protect others during COVID-19: reducing the inoculum of SARS-CoV-2 to protect the wearer. J Gen Intern Med. 2020;35:3063-3066.
- Ngonghala CN, Iboi EA, Gumel AB. Could masks curtail the post-lockdown resurgence of COVID-19 in the US? Math Biosci. 2020;329:108452. doi:10.1016/j.mbs.2020.108452
- Yi-Fong Su V, Yen YF, Yang KY, et al. Masks and medical care: two keys to Taiwan’s success in preventing COVID-19 spread. Travel Med Infect Dis. 2020;38:101780.
- Lim S, Yoon HI, Song KH, et al. Face masks and containment of COVID-19: experience from South Korea. J Hosp Infect. 2020;106:206-207.
- Fisman DN, Greer AL, Tuite AR. Bidirectional impact of imperfect mask use on reproduction number of COVID-19: a next generation matrix approach. Infect Dis Model. 2020;5:405-408.
- Centers for Disease Control and Prevention. COVID data tracker. United States COVID-19 cases, deaths, and laboratory testing (NAATs) by state, territory, and jurisdiction. Accessed July 6, 2021. https://covid.cdc.gov/covid-data-tracker/#cases_totalcases
- Francescani C. Timeline: the first 100 days of New York Gov. Andrew Cuomo’s COVID-19 response. ABC News. June 17, 2020. Accessed November 8, 2021. https://abcnews.go.com/US/News/timeline-100-days-york-gov-andrew-cuomos-covid/story?id=71292880
- Zhang R, Li Y, Zhang AL, et al. Identifying airborne transmission as the dominant route for the spread of COVID-19. Proc Natl Acad Sci U S A. 2020;117:14857-14863.
- Verma S, Dhanak M, Frankenfield J. Visualizing the effectiveness of face masks in obstructing respiratory jets. Phys Fluids (1994). 2020;32:061708.
- Yang W, Shaff J, Shaman J. COVID-19 transmission dynamics and effectiveness of public health interventions in New York City during the 2020 spring pandemic wave. medRxiv. Preprint posted online September 9, 2020. doi:10.1101/2020.09.08.20190710
- Zavascki AP, Falci DR. Clinical characteristics of covid-19 in China. N Engl J Med. 2020;382:1859.
- Zhu J, Ji P, Pang J, et al. Clinical characteristics of 3062 COVID-19 patients: a meta-analysis. J Med Virol. 2020;92:1902-1914. doi:10.1002/jmv.25884
- Sommerstein R, Fux CA, Vuichard-Gysin D, et al. Risk of SARS-CoV-2 transmission by aerosols, the rational use of masks, and protection of healthcare workers from COVID-19. Antimicrob Resist Infect Control. 2020;9:100.
- Stone TE, Kunaviktikul W, Omura M, et al. Facemasks and the covid 19 pandemic: what advice should health professionals be giving the general public about the wearing of facemasks? Nurs Health Sci. 2020;22:339-342.
- Tam VC, Tam SY, Poon WK, et al. A reality check on the use of face masks during the COVID-19 outbreak in Hong Kong. EClinicalMedicine. 2020;22:100356.
- Chen YJ, Qin G, Chen J, et al. Comparison of face-touching behaviors before and during the coronavirus disease 2019 pandemic. JAMA Netw Open. 2020;3:e2016924.
- O’Dowd K, Nair KM, Forouzandeh P, et al. Face masks and respirators in the fight against the COVID-19 pandemic: a review of current materials, advances and future perspectives. Materials (Basel). 2020;13:3363.
- Matuschek C, Moll F, Fangerau H, et al. Face masks: benefits and risks during the COVID-19 crisis. Eur J Med Res. 2020;25:32.
- Chan NC, Li K, Hirsh J. Peripheral oxygen saturation in older persons wearing nonmedical face masks in community settings. JAMA. 2020;324:2323-2324. doi:10.1001/jama.2020.21905
- , , . Differential COVID‐19 case positivity in New York City neighborhoods: socioeconomic factors and mobility. Influenza Other Respir Viruses. 2021;15:209-217. doi:10.1111/irv.12816
Although the universal use of masks by both health care professionals and the general public now appears routine, widely differing recommendations were distributed by different health organizations early in the pandemic. In April 2020, the World Health Organization (WHO) stated that there was no evidence that healthy individuals wearing a medical mask in the community prevented COVID-19 infection.1 However, these recommendations must be placed in the context of a national shortage of personal protective equipment early in the pandemic. The WHO guidance released on June 5, 2020, recommended continuous use of masks for health care workers in the clinical setting.2 Additional recommendations included mask replacement when wet, soiled, or damaged, and when the wearer touched the mask. The WHO also recommended mask usage by those with underlying medical comorbidities and those living in high population–density areas and in settings where physical distancing was not possible.2
The Centers for Disease Control and Prevention (CDC) officially recommended the use of face coverings for the general public to prevent COVID-19 transmission on April 3, 2020.3 The CDC highlighted that masks should not be worn by children younger than 2 years; individuals with respiratory compromise; and patients who are unconscious, incapacitated, or unable to remove a mask without assistance.4 Medical masks and respirators were only recommended for health care workers. Importantly, masks with valves/vents were not recommended, as respiratory droplets can be emitted, defeating the purpose of source control.4 New York State mandated mask usage in public places starting on April 15, 2020.
These recommendations were based on the hypothesis that COVID-19 transmission occurs primarily via droplets and contact. In reality, SARS-CoV-2 transmission more likely occurs in a continuum from larger droplets to miniscule aerosols expelled from an infected person when talking, coughing, or sneezing.5,6 It should be noted that there was a formal suggestion of the potential for airborne transmission of SARS-CoV-2 by the CDC in a statement on September 18, 2020, that was subsequently retracted 3 days later.7,8 The CDC, reversing their prior recommendations, updated their guidance on October 5, 2020, endorsing prior reports that SARS-CoV-2 can be spread through aerosol transmission.8
Mask usage helps prevent viral spread by all individuals, especially those who are presymptomatic and asymptomatic. Presymptomatic individuals account for approximately 40% to 60% of transmissions, and asymptomatic individuals account for approximately 4% to 30% of infections by some models, which suggest these individuals are the drivers of the pandemic, more so than symptomatic individuals.9-15 Additionally, masking also may in effect reduce the amount of SARS-CoV-2 to which individuals are being exposed in the community.14 Universal masking is a relatively low-cost, low-risk intervention that may provide moderate benefit to the individual but substantial benefit to communities at large.10-13 Universal masking in other countries also has clearly demonstrated major benefits during the pandemic. Implementation of universal masking in Taiwan resulted in only approximately 440 COVID-19 cases and less than 10 deaths, despite a population of 23 million.16 South Korea, having experience with Middle East respiratory syndrome, also was able to quickly institute a mask policy for its citizens, resulting in approximately 94% compliance.17 Moreover, several mathematical models have shown that even imperfect use of masks on a population level can prevent disease transmission and should be instituted.18
Given the importance and potential benefits of mask usage, we investigated compliance and proper utilization of facial masks in New York City (NYC), once the epicenter of the pandemic in the United States. New York City and the rest of New York State experienced more than 1.13 million and 1.46 million cases of COVID-19, respectively, as of early November 2021.19 Nationwide, NYC had the greatest absolute death count of more than 34,634 and the greatest rate of death per 100,000 individuals of 412. In contrast, New York State, excluding NYC, had an absolute death count of more than 21,646 and a death rate per 100,000 individuals of 195 as of early November 2021.19 Now entering 20 months since the first case of COVID-19 in NYC, it continues to be vital for facial mask protocols to be emphasized as part of a comprehensive infection prevention protocol, especially in light of continued vaccine resistance, to help stall continued spread of SARS-CoV-2.20
We seek to show that despite months of policies for universal masking in NYC, there is still considerable mask noncompliance by the general public in health care settings where the use of masks is particularly imperative. We conducted an observational study investigating proper use of face masks of adults entering the main entrance of 4 hospitals located in NYC.
Methods
We observed mask usage in adults entering 4 hospitals in September 2020 (postsurge in NYC and prior to the availability of COVID-19 vaccinations). Hospitals were chosen to represent several types of health care delivery systems available in the United States and included a city, state, federal, and private hospital. Data collection was completed during peak traffic hours (8:00
Mask usage was observed and classified into several categories: correctly fitting mask over the nose and mouth, no face mask, mask usage with nose exposed, mask usage with mouth exposed, mask usage with both nose and mouth exposed (ie, mask on the chin/neck area), loosely fitting mask, vented/valved mask, or other form of face covering (eg, bandana, scarf).
Results
We observed a consistent rate of mask compliance between 72% and 85%, with an average of 78% of the 600 individuals observed wearing correctly fitting masks across the 4 hospitals included in this study (Table). The employee entrance included in this study had the highest compliance rate of 85%. An overall low rate of complete mask noncompliance was observed, with only 9 individuals (1.5%) in the entire study not wearing any mask. The federal hospital had the highest rate of mask noncompliance. We also observed a low rate of nose and mouth exposure, with 1.8% of individuals wearing a mask with the nose and mouth exposed (ie, mask tucked under the chin). No individuals were observed with the mouth exposed but with the nose covered by a mask. Additionally, only 3 individuals (0.5%) wore a mask with a vent/valve. The most common way that masks were worn incorrectly was with the nose exposed, accounting for 9.5% of individuals observed. Overall, only 9 individuals (1.5%) wore a nontraditional face covering, with a bandana being the most commonly observed makeshift mask.
Signage regarding the requirement to wear masks and to social distance was universally instituted at all hospital entry points (both inside and outside the hospital) in this study. However, there were no illustrations demonstrating correct and incorrect forms of mask usage. All signage merely displayed a graphic of a facial mask noting the requirement to wear a mask prior to entering the building. Hospital staff also had face masks available for patients who failed to bring a mask or who wore an inappropriate mask (ie, vented/valved masks).
Comment
Mask Effectiveness—Masks reduce the spread of SARS-CoV-2 by preventing both droplets and potentially virus-bearing aerosols.6,21,22 It has been demonstrated that well-fitted cotton homemade masks and medical masks provide the most effective method of reducing droplet dispersion. Loosely fitted masks as well as bandana-style facial coverings minimally reduce small aerosolized droplets, and an uncovered mouth and nose can disperse particles at a distance much greater than 6 feet.22
Mask Compliance—We report an overall high compliance rate with mask wearing among individuals visiting a hospital; however, compliance was still imperfect. Overall, 78% of observed individuals wore a correctly fitting mask when entering a hospital, even with hospital staff positioned at entry points to ensure proper mask usage. With all the resources available at health care centers, we anticipated a much higher compliance rate for correctly fitting masks at hospital entrances. We hypothesize that given only 78% of individuals showed proper mask compliance in a setting with enforcement by health care personnel, the mask compliance rate in the larger community is likely much lower. It is imperative to enforce continued mask compliance in medical centers and other public areas given notable vaccine noncompliance in certain parts of the country.
Tools to Prevent Disease Transmission—Mask usage by the general public in NYC helped in its response to the COVID-19 pandemic. Yang et al23 demonstrated through mathematical modeling that mask usage in NYC was associated with a 6.6% reduction in transmission overall and a 20% decrease in transmission for individuals 65 years and older during the first month of the universal mask policy going into effect. The authors extrapolated these data during the NYC reopening and found that universal masking reduced transmission by approximately 9% to 11%, accounting for the increase in hours spent outside home quarantine. The authors also hypothesized that if universal masking was as effective in its reduction of transmission for everyone in NYC as it was for older adults, the potential reduction in transmission of SARS-CoV-2 could be as high as 28% to 32%.23
Temperature checks at entrance barricades were standard protocol during the observation period. Although the main purpose of this study was to investigate compliance with and proper use of facial masks in a health care setting, it should be mentioned that, although temperature checks were being done on almost every person entering a hospital, the uniformity and practicality of this intervention has not been backed by substantial evidence. Although many nontouch thermometers are intended to capture a forehead temperature for the most accurate reading, the authors will share that in their observation, medical personnel screening individuals at hospital entrances were observed checking temperatures at any easily accessible body part, such as the forearm, hand, or neck. Furthermore, it has been reported that only approximately 40% of individuals with COVID-19 present with a fever.24 Many hospitals, including the 4 that were included in this investigation, have formal protocols for patients presenting with a fever, especially those presenting to an ambulatory center. Patients are usually instructed to call ahead if they have a fever, and a decision regarding next steps will be discussed with a health care provider. In addition, 1 meta-analysis on the symptoms of COVID-19 suggested that approximately 12% of infected patients are asymptomatic, likely a conservative estimate.25 Although we do not suggest that hospitals stop temperature checks, consistent temperature checks in anatomic locations intended for the specific thermometer used must be employed. Alternatively, a thermographic camera system that could detect heat signatures may be a way to screen faster, only necessitating that those above a threshold be assessed further.
The results of this study suggest that much greater effort is being placed on these temperature checks than on other equally important components of the entrance health assessment. This initial encounter at hospital entrances should serve as an opportunity for education on proper choice and use of masks with clear instructions that masks should not be removed unless directed by a health care provider and in a designated area, such as an examination room. The COVID-19 pandemic in the United States is likely the first time an individual is wearing these types of masks. Reiterating when and how often a mask should be changed (eg, when wet or soiled), how a soiled mask is not an effective mask, how a used mask should be discarded, ways to prevent self-contamination (ie, proper donning and doffing), and the importance of other infection-prevention behaviors—hand hygiene; social distancing; avoidance of touching the eyes, nose, and mouth with unwashed hands; and regular disinfecting of surfaces—should be practiced.11,26-29 Extended use and reuse of masks also can result in transmission of infection.30
Throughout the pandemic, our personal experience is that some patients often overtly refuse to wear a mask, citing underlying respiratory issues. The implications of patients not wearing a mask in a medical office and endangering other patients and staff are beyond the scope of this analysis. We will, however, comment briefly on the evidence behind this common concern. Matuschek et al31 found substantial adverse changes in respiratory rate, oxygen saturation, and CO2 levels in patients with severe chronic obstructive pulmonary disease who were wearing N95 respirators during a 6-minute walk test. Another study by Chan et al32 showed that nonmedical masks in healthy older adults in the community setting had no impact on oxygen saturation. Ultimately, the most effective mask a patient can wear is a mask that will be worn consistently.32
Populations With Limited Access to Masks—The COVID-19 pandemic disproportionately impacted disadvantaged populations, both in socioeconomic status and minority status. A disproportionate number of COVID-19 hospitalizations and deaths occurred in lower-income and minority populations.10 In fact, Lamb et al33 reported that NYC neighborhoods with a larger proportion of uninsured individuals with limited access to health care and overall lower socioeconomic status had a higher rate of SARS-CoV-2 positivity. A retrospective study in Louisiana showed that Black individuals accounted for 77% of hospitalizations and 71% of deaths due to COVID-19 in a population where only 31% of individuals identified as Black.10 Chu et al6 even asserted that policies should be put into place to address equity issues for populations with limited access to masks. We agree that policies should be put into action to ensure that individuals lacking the means to obtain appropriate masks or unable to obtain an adequate supply of masks be provided this new necessity. It has been calculated that the impact of masks in reducing virus transmission would be greatest if mask availability to disadvantaged populations is ensured.18 We support a plan for masks to be covered by government-sponsored health plans.
Study Limitations—Several limitations exist in our study that should be discussed. Although the data collectors observed a large number of individuals, each hospital entrance was only observed for 1 half-day morning session. There may be variations in the number of people wearing a mask at different times of day and different days of the week with fluctuations in hospital traffic. Although data were collected at a variety of hospitals representing the diverse health care delivery models available in the United States, the NYC hospitals included in this study may have different resources available for infection-prevention strategies than hospitals across the country, given NYC’s unique population density and demographics.
Study Strengths—The generalizability of the study should be recognized. Data were collected by all major health care delivery models available in the United States—private, state, city, and federal hospital systems. This study can be easily replicated in other health care delivery systems to further investigate potential gaps in mask usage and infection prevention. Repeating this study in areas where a large portion of the population does not believe in the virus also will likely show lower levels of mask use.
Conclusion
As the country grapples with vaccine hesitancy and with the new variants of SARS-CoV-2, continued universal masking is still imperative. The effectiveness of universal masking has been demonstrated, and with the combination of vaccinations, we can be assured that the world will continue to emerge from the pandemic.
Although the universal use of masks by both health care professionals and the general public now appears routine, widely differing recommendations were distributed by different health organizations early in the pandemic. In April 2020, the World Health Organization (WHO) stated that there was no evidence that healthy individuals wearing a medical mask in the community prevented COVID-19 infection.1 However, these recommendations must be placed in the context of a national shortage of personal protective equipment early in the pandemic. The WHO guidance released on June 5, 2020, recommended continuous use of masks for health care workers in the clinical setting.2 Additional recommendations included mask replacement when wet, soiled, or damaged, and when the wearer touched the mask. The WHO also recommended mask usage by those with underlying medical comorbidities and those living in high population–density areas and in settings where physical distancing was not possible.2
The Centers for Disease Control and Prevention (CDC) officially recommended the use of face coverings for the general public to prevent COVID-19 transmission on April 3, 2020.3 The CDC highlighted that masks should not be worn by children younger than 2 years; individuals with respiratory compromise; and patients who are unconscious, incapacitated, or unable to remove a mask without assistance.4 Medical masks and respirators were only recommended for health care workers. Importantly, masks with valves/vents were not recommended, as respiratory droplets can be emitted, defeating the purpose of source control.4 New York State mandated mask usage in public places starting on April 15, 2020.
These recommendations were based on the hypothesis that COVID-19 transmission occurs primarily via droplets and contact. In reality, SARS-CoV-2 transmission more likely occurs in a continuum from larger droplets to miniscule aerosols expelled from an infected person when talking, coughing, or sneezing.5,6 It should be noted that there was a formal suggestion of the potential for airborne transmission of SARS-CoV-2 by the CDC in a statement on September 18, 2020, that was subsequently retracted 3 days later.7,8 The CDC, reversing their prior recommendations, updated their guidance on October 5, 2020, endorsing prior reports that SARS-CoV-2 can be spread through aerosol transmission.8
Mask usage helps prevent viral spread by all individuals, especially those who are presymptomatic and asymptomatic. Presymptomatic individuals account for approximately 40% to 60% of transmissions, and asymptomatic individuals account for approximately 4% to 30% of infections by some models, which suggest these individuals are the drivers of the pandemic, more so than symptomatic individuals.9-15 Additionally, masking also may in effect reduce the amount of SARS-CoV-2 to which individuals are being exposed in the community.14 Universal masking is a relatively low-cost, low-risk intervention that may provide moderate benefit to the individual but substantial benefit to communities at large.10-13 Universal masking in other countries also has clearly demonstrated major benefits during the pandemic. Implementation of universal masking in Taiwan resulted in only approximately 440 COVID-19 cases and less than 10 deaths, despite a population of 23 million.16 South Korea, having experience with Middle East respiratory syndrome, also was able to quickly institute a mask policy for its citizens, resulting in approximately 94% compliance.17 Moreover, several mathematical models have shown that even imperfect use of masks on a population level can prevent disease transmission and should be instituted.18
Given the importance and potential benefits of mask usage, we investigated compliance and proper utilization of facial masks in New York City (NYC), once the epicenter of the pandemic in the United States. New York City and the rest of New York State experienced more than 1.13 million and 1.46 million cases of COVID-19, respectively, as of early November 2021.19 Nationwide, NYC had the greatest absolute death count of more than 34,634 and the greatest rate of death per 100,000 individuals of 412. In contrast, New York State, excluding NYC, had an absolute death count of more than 21,646 and a death rate per 100,000 individuals of 195 as of early November 2021.19 Now entering 20 months since the first case of COVID-19 in NYC, it continues to be vital for facial mask protocols to be emphasized as part of a comprehensive infection prevention protocol, especially in light of continued vaccine resistance, to help stall continued spread of SARS-CoV-2.20
We seek to show that despite months of policies for universal masking in NYC, there is still considerable mask noncompliance by the general public in health care settings where the use of masks is particularly imperative. We conducted an observational study investigating proper use of face masks of adults entering the main entrance of 4 hospitals located in NYC.
Methods
We observed mask usage in adults entering 4 hospitals in September 2020 (postsurge in NYC and prior to the availability of COVID-19 vaccinations). Hospitals were chosen to represent several types of health care delivery systems available in the United States and included a city, state, federal, and private hospital. Data collection was completed during peak traffic hours (8:00
Mask usage was observed and classified into several categories: correctly fitting mask over the nose and mouth, no face mask, mask usage with nose exposed, mask usage with mouth exposed, mask usage with both nose and mouth exposed (ie, mask on the chin/neck area), loosely fitting mask, vented/valved mask, or other form of face covering (eg, bandana, scarf).
Results
We observed a consistent rate of mask compliance between 72% and 85%, with an average of 78% of the 600 individuals observed wearing correctly fitting masks across the 4 hospitals included in this study (Table). The employee entrance included in this study had the highest compliance rate of 85%. An overall low rate of complete mask noncompliance was observed, with only 9 individuals (1.5%) in the entire study not wearing any mask. The federal hospital had the highest rate of mask noncompliance. We also observed a low rate of nose and mouth exposure, with 1.8% of individuals wearing a mask with the nose and mouth exposed (ie, mask tucked under the chin). No individuals were observed with the mouth exposed but with the nose covered by a mask. Additionally, only 3 individuals (0.5%) wore a mask with a vent/valve. The most common way that masks were worn incorrectly was with the nose exposed, accounting for 9.5% of individuals observed. Overall, only 9 individuals (1.5%) wore a nontraditional face covering, with a bandana being the most commonly observed makeshift mask.
Signage regarding the requirement to wear masks and to social distance was universally instituted at all hospital entry points (both inside and outside the hospital) in this study. However, there were no illustrations demonstrating correct and incorrect forms of mask usage. All signage merely displayed a graphic of a facial mask noting the requirement to wear a mask prior to entering the building. Hospital staff also had face masks available for patients who failed to bring a mask or who wore an inappropriate mask (ie, vented/valved masks).
Comment
Mask Effectiveness—Masks reduce the spread of SARS-CoV-2 by preventing both droplets and potentially virus-bearing aerosols.6,21,22 It has been demonstrated that well-fitted cotton homemade masks and medical masks provide the most effective method of reducing droplet dispersion. Loosely fitted masks as well as bandana-style facial coverings minimally reduce small aerosolized droplets, and an uncovered mouth and nose can disperse particles at a distance much greater than 6 feet.22
Mask Compliance—We report an overall high compliance rate with mask wearing among individuals visiting a hospital; however, compliance was still imperfect. Overall, 78% of observed individuals wore a correctly fitting mask when entering a hospital, even with hospital staff positioned at entry points to ensure proper mask usage. With all the resources available at health care centers, we anticipated a much higher compliance rate for correctly fitting masks at hospital entrances. We hypothesize that given only 78% of individuals showed proper mask compliance in a setting with enforcement by health care personnel, the mask compliance rate in the larger community is likely much lower. It is imperative to enforce continued mask compliance in medical centers and other public areas given notable vaccine noncompliance in certain parts of the country.
Tools to Prevent Disease Transmission—Mask usage by the general public in NYC helped in its response to the COVID-19 pandemic. Yang et al23 demonstrated through mathematical modeling that mask usage in NYC was associated with a 6.6% reduction in transmission overall and a 20% decrease in transmission for individuals 65 years and older during the first month of the universal mask policy going into effect. The authors extrapolated these data during the NYC reopening and found that universal masking reduced transmission by approximately 9% to 11%, accounting for the increase in hours spent outside home quarantine. The authors also hypothesized that if universal masking was as effective in its reduction of transmission for everyone in NYC as it was for older adults, the potential reduction in transmission of SARS-CoV-2 could be as high as 28% to 32%.23
Temperature checks at entrance barricades were standard protocol during the observation period. Although the main purpose of this study was to investigate compliance with and proper use of facial masks in a health care setting, it should be mentioned that, although temperature checks were being done on almost every person entering a hospital, the uniformity and practicality of this intervention has not been backed by substantial evidence. Although many nontouch thermometers are intended to capture a forehead temperature for the most accurate reading, the authors will share that in their observation, medical personnel screening individuals at hospital entrances were observed checking temperatures at any easily accessible body part, such as the forearm, hand, or neck. Furthermore, it has been reported that only approximately 40% of individuals with COVID-19 present with a fever.24 Many hospitals, including the 4 that were included in this investigation, have formal protocols for patients presenting with a fever, especially those presenting to an ambulatory center. Patients are usually instructed to call ahead if they have a fever, and a decision regarding next steps will be discussed with a health care provider. In addition, 1 meta-analysis on the symptoms of COVID-19 suggested that approximately 12% of infected patients are asymptomatic, likely a conservative estimate.25 Although we do not suggest that hospitals stop temperature checks, consistent temperature checks in anatomic locations intended for the specific thermometer used must be employed. Alternatively, a thermographic camera system that could detect heat signatures may be a way to screen faster, only necessitating that those above a threshold be assessed further.
The results of this study suggest that much greater effort is being placed on these temperature checks than on other equally important components of the entrance health assessment. This initial encounter at hospital entrances should serve as an opportunity for education on proper choice and use of masks with clear instructions that masks should not be removed unless directed by a health care provider and in a designated area, such as an examination room. The COVID-19 pandemic in the United States is likely the first time an individual is wearing these types of masks. Reiterating when and how often a mask should be changed (eg, when wet or soiled), how a soiled mask is not an effective mask, how a used mask should be discarded, ways to prevent self-contamination (ie, proper donning and doffing), and the importance of other infection-prevention behaviors—hand hygiene; social distancing; avoidance of touching the eyes, nose, and mouth with unwashed hands; and regular disinfecting of surfaces—should be practiced.11,26-29 Extended use and reuse of masks also can result in transmission of infection.30
Throughout the pandemic, our personal experience is that some patients often overtly refuse to wear a mask, citing underlying respiratory issues. The implications of patients not wearing a mask in a medical office and endangering other patients and staff are beyond the scope of this analysis. We will, however, comment briefly on the evidence behind this common concern. Matuschek et al31 found substantial adverse changes in respiratory rate, oxygen saturation, and CO2 levels in patients with severe chronic obstructive pulmonary disease who were wearing N95 respirators during a 6-minute walk test. Another study by Chan et al32 showed that nonmedical masks in healthy older adults in the community setting had no impact on oxygen saturation. Ultimately, the most effective mask a patient can wear is a mask that will be worn consistently.32
Populations With Limited Access to Masks—The COVID-19 pandemic disproportionately impacted disadvantaged populations, both in socioeconomic status and minority status. A disproportionate number of COVID-19 hospitalizations and deaths occurred in lower-income and minority populations.10 In fact, Lamb et al33 reported that NYC neighborhoods with a larger proportion of uninsured individuals with limited access to health care and overall lower socioeconomic status had a higher rate of SARS-CoV-2 positivity. A retrospective study in Louisiana showed that Black individuals accounted for 77% of hospitalizations and 71% of deaths due to COVID-19 in a population where only 31% of individuals identified as Black.10 Chu et al6 even asserted that policies should be put into place to address equity issues for populations with limited access to masks. We agree that policies should be put into action to ensure that individuals lacking the means to obtain appropriate masks or unable to obtain an adequate supply of masks be provided this new necessity. It has been calculated that the impact of masks in reducing virus transmission would be greatest if mask availability to disadvantaged populations is ensured.18 We support a plan for masks to be covered by government-sponsored health plans.
Study Limitations—Several limitations exist in our study that should be discussed. Although the data collectors observed a large number of individuals, each hospital entrance was only observed for 1 half-day morning session. There may be variations in the number of people wearing a mask at different times of day and different days of the week with fluctuations in hospital traffic. Although data were collected at a variety of hospitals representing the diverse health care delivery models available in the United States, the NYC hospitals included in this study may have different resources available for infection-prevention strategies than hospitals across the country, given NYC’s unique population density and demographics.
Study Strengths—The generalizability of the study should be recognized. Data were collected by all major health care delivery models available in the United States—private, state, city, and federal hospital systems. This study can be easily replicated in other health care delivery systems to further investigate potential gaps in mask usage and infection prevention. Repeating this study in areas where a large portion of the population does not believe in the virus also will likely show lower levels of mask use.
Conclusion
As the country grapples with vaccine hesitancy and with the new variants of SARS-CoV-2, continued universal masking is still imperative. The effectiveness of universal masking has been demonstrated, and with the combination of vaccinations, we can be assured that the world will continue to emerge from the pandemic.
- World Health Organization. Advice on the use of masks in the context of COVID-19. Interim guidance (6 April 2020). Accessed November 8, 2021. https://apps.who.int/iris/bitstream/handle/10665/331693/WHO-2019-nCov-IPC_Masks-2020.3-eng.pdf?sequence=1ceisAllowed=y
- World Health Organization. Advice on the use of masks in the context of COVID-19. Interim guidance (5 June 2020). Accessed November 8, 2021. https://apps.who.int/iris/bitstream/handle/10665/332293/WHO- 2019-nCov-IPC_Masks-2020.4-eng.pdf?sequence=1&isAllowed=y
- Fisher KA, Barile JP, Guerin RJ, et al. Factors associated with cloth face covering use among adults during the COVID-19 pandemic—United States, April and May 2020. MMWR Morb Mortal Wkly Rep. 2020;69:933-937.
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19). Considerations for wearing masks (19 April 2021). Accessed November 10, 2021. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/cloth-face-cover-guidance.html
- Conly J, Seto WH, Pittet D, et al. Use of medical face masks versus particulate respirators as a component of personal protective equipment for health care workers in the context of the COVID-19 pandemic. Antimicrob Resist Infect Control. 2020;9:126.
- Chu DK, Akl EA, Duda S, et al; COVID-19 Systematic Urgent Review Group Effort (SURGE) study authors. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395:1973-1987.
- Huang, P. Coronavirus FAQs: Why can’t the CDC make up its mind about airborne transmission? NPR. September 25, 2020. Accessed November 8, 2021. https://www.npr.org/sections/goatsandsoda/2020/09/25/916624967/coronavirus-faqs-why-cant-the-cdc-make-up-its-mind-about-airborne-transmission
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19). How COVID-19 spreads (14 July 2021). Accessed November 10, 2021. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/how-covid-spreads.html
- Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782-793.
- Klompas M, Morris CA, Shenoy ES. Universal masking in the covid-19 era. N Engl J Med. 2020;383:E9.
- Middleton JD, Lopes H. Face masks in the covid-19 crisis: caveats, limits, and priorities. BMJ. 2020;369:m2030.
- Cheng KK, Lam TH, Leung CC. Wearing face masks in the community during the COVID-19 pandemic: altruism and solidarity [published online April 16, 2020]. Lancet. doi:10.1016/S0140-6736(20)30918-1
- Javid B, Weekes MP, Matheson NJ. Covid-19: should the public wear face masks? BMJ. 2020;369:m1442.
- Gandhi M, Beyrer C, Goosby E. Masks do more than protect others during COVID-19: reducing the inoculum of SARS-CoV-2 to protect the wearer. J Gen Intern Med. 2020;35:3063-3066.
- Ngonghala CN, Iboi EA, Gumel AB. Could masks curtail the post-lockdown resurgence of COVID-19 in the US? Math Biosci. 2020;329:108452. doi:10.1016/j.mbs.2020.108452
- Yi-Fong Su V, Yen YF, Yang KY, et al. Masks and medical care: two keys to Taiwan’s success in preventing COVID-19 spread. Travel Med Infect Dis. 2020;38:101780.
- Lim S, Yoon HI, Song KH, et al. Face masks and containment of COVID-19: experience from South Korea. J Hosp Infect. 2020;106:206-207.
- Fisman DN, Greer AL, Tuite AR. Bidirectional impact of imperfect mask use on reproduction number of COVID-19: a next generation matrix approach. Infect Dis Model. 2020;5:405-408.
- Centers for Disease Control and Prevention. COVID data tracker. United States COVID-19 cases, deaths, and laboratory testing (NAATs) by state, territory, and jurisdiction. Accessed July 6, 2021. https://covid.cdc.gov/covid-data-tracker/#cases_totalcases
- Francescani C. Timeline: the first 100 days of New York Gov. Andrew Cuomo’s COVID-19 response. ABC News. June 17, 2020. Accessed November 8, 2021. https://abcnews.go.com/US/News/timeline-100-days-york-gov-andrew-cuomos-covid/story?id=71292880
- Zhang R, Li Y, Zhang AL, et al. Identifying airborne transmission as the dominant route for the spread of COVID-19. Proc Natl Acad Sci U S A. 2020;117:14857-14863.
- Verma S, Dhanak M, Frankenfield J. Visualizing the effectiveness of face masks in obstructing respiratory jets. Phys Fluids (1994). 2020;32:061708.
- Yang W, Shaff J, Shaman J. COVID-19 transmission dynamics and effectiveness of public health interventions in New York City during the 2020 spring pandemic wave. medRxiv. Preprint posted online September 9, 2020. doi:10.1101/2020.09.08.20190710
- Zavascki AP, Falci DR. Clinical characteristics of covid-19 in China. N Engl J Med. 2020;382:1859.
- Zhu J, Ji P, Pang J, et al. Clinical characteristics of 3062 COVID-19 patients: a meta-analysis. J Med Virol. 2020;92:1902-1914. doi:10.1002/jmv.25884
- Sommerstein R, Fux CA, Vuichard-Gysin D, et al. Risk of SARS-CoV-2 transmission by aerosols, the rational use of masks, and protection of healthcare workers from COVID-19. Antimicrob Resist Infect Control. 2020;9:100.
- Stone TE, Kunaviktikul W, Omura M, et al. Facemasks and the covid 19 pandemic: what advice should health professionals be giving the general public about the wearing of facemasks? Nurs Health Sci. 2020;22:339-342.
- Tam VC, Tam SY, Poon WK, et al. A reality check on the use of face masks during the COVID-19 outbreak in Hong Kong. EClinicalMedicine. 2020;22:100356.
- Chen YJ, Qin G, Chen J, et al. Comparison of face-touching behaviors before and during the coronavirus disease 2019 pandemic. JAMA Netw Open. 2020;3:e2016924.
- O’Dowd K, Nair KM, Forouzandeh P, et al. Face masks and respirators in the fight against the COVID-19 pandemic: a review of current materials, advances and future perspectives. Materials (Basel). 2020;13:3363.
- Matuschek C, Moll F, Fangerau H, et al. Face masks: benefits and risks during the COVID-19 crisis. Eur J Med Res. 2020;25:32.
- Chan NC, Li K, Hirsh J. Peripheral oxygen saturation in older persons wearing nonmedical face masks in community settings. JAMA. 2020;324:2323-2324. doi:10.1001/jama.2020.21905
- , , . Differential COVID‐19 case positivity in New York City neighborhoods: socioeconomic factors and mobility. Influenza Other Respir Viruses. 2021;15:209-217. doi:10.1111/irv.12816
- World Health Organization. Advice on the use of masks in the context of COVID-19. Interim guidance (6 April 2020). Accessed November 8, 2021. https://apps.who.int/iris/bitstream/handle/10665/331693/WHO-2019-nCov-IPC_Masks-2020.3-eng.pdf?sequence=1ceisAllowed=y
- World Health Organization. Advice on the use of masks in the context of COVID-19. Interim guidance (5 June 2020). Accessed November 8, 2021. https://apps.who.int/iris/bitstream/handle/10665/332293/WHO- 2019-nCov-IPC_Masks-2020.4-eng.pdf?sequence=1&isAllowed=y
- Fisher KA, Barile JP, Guerin RJ, et al. Factors associated with cloth face covering use among adults during the COVID-19 pandemic—United States, April and May 2020. MMWR Morb Mortal Wkly Rep. 2020;69:933-937.
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19). Considerations for wearing masks (19 April 2021). Accessed November 10, 2021. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/cloth-face-cover-guidance.html
- Conly J, Seto WH, Pittet D, et al. Use of medical face masks versus particulate respirators as a component of personal protective equipment for health care workers in the context of the COVID-19 pandemic. Antimicrob Resist Infect Control. 2020;9:126.
- Chu DK, Akl EA, Duda S, et al; COVID-19 Systematic Urgent Review Group Effort (SURGE) study authors. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395:1973-1987.
- Huang, P. Coronavirus FAQs: Why can’t the CDC make up its mind about airborne transmission? NPR. September 25, 2020. Accessed November 8, 2021. https://www.npr.org/sections/goatsandsoda/2020/09/25/916624967/coronavirus-faqs-why-cant-the-cdc-make-up-its-mind-about-airborne-transmission
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19). How COVID-19 spreads (14 July 2021). Accessed November 10, 2021. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/how-covid-spreads.html
- Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782-793.
- Klompas M, Morris CA, Shenoy ES. Universal masking in the covid-19 era. N Engl J Med. 2020;383:E9.
- Middleton JD, Lopes H. Face masks in the covid-19 crisis: caveats, limits, and priorities. BMJ. 2020;369:m2030.
- Cheng KK, Lam TH, Leung CC. Wearing face masks in the community during the COVID-19 pandemic: altruism and solidarity [published online April 16, 2020]. Lancet. doi:10.1016/S0140-6736(20)30918-1
- Javid B, Weekes MP, Matheson NJ. Covid-19: should the public wear face masks? BMJ. 2020;369:m1442.
- Gandhi M, Beyrer C, Goosby E. Masks do more than protect others during COVID-19: reducing the inoculum of SARS-CoV-2 to protect the wearer. J Gen Intern Med. 2020;35:3063-3066.
- Ngonghala CN, Iboi EA, Gumel AB. Could masks curtail the post-lockdown resurgence of COVID-19 in the US? Math Biosci. 2020;329:108452. doi:10.1016/j.mbs.2020.108452
- Yi-Fong Su V, Yen YF, Yang KY, et al. Masks and medical care: two keys to Taiwan’s success in preventing COVID-19 spread. Travel Med Infect Dis. 2020;38:101780.
- Lim S, Yoon HI, Song KH, et al. Face masks and containment of COVID-19: experience from South Korea. J Hosp Infect. 2020;106:206-207.
- Fisman DN, Greer AL, Tuite AR. Bidirectional impact of imperfect mask use on reproduction number of COVID-19: a next generation matrix approach. Infect Dis Model. 2020;5:405-408.
- Centers for Disease Control and Prevention. COVID data tracker. United States COVID-19 cases, deaths, and laboratory testing (NAATs) by state, territory, and jurisdiction. Accessed July 6, 2021. https://covid.cdc.gov/covid-data-tracker/#cases_totalcases
- Francescani C. Timeline: the first 100 days of New York Gov. Andrew Cuomo’s COVID-19 response. ABC News. June 17, 2020. Accessed November 8, 2021. https://abcnews.go.com/US/News/timeline-100-days-york-gov-andrew-cuomos-covid/story?id=71292880
- Zhang R, Li Y, Zhang AL, et al. Identifying airborne transmission as the dominant route for the spread of COVID-19. Proc Natl Acad Sci U S A. 2020;117:14857-14863.
- Verma S, Dhanak M, Frankenfield J. Visualizing the effectiveness of face masks in obstructing respiratory jets. Phys Fluids (1994). 2020;32:061708.
- Yang W, Shaff J, Shaman J. COVID-19 transmission dynamics and effectiveness of public health interventions in New York City during the 2020 spring pandemic wave. medRxiv. Preprint posted online September 9, 2020. doi:10.1101/2020.09.08.20190710
- Zavascki AP, Falci DR. Clinical characteristics of covid-19 in China. N Engl J Med. 2020;382:1859.
- Zhu J, Ji P, Pang J, et al. Clinical characteristics of 3062 COVID-19 patients: a meta-analysis. J Med Virol. 2020;92:1902-1914. doi:10.1002/jmv.25884
- Sommerstein R, Fux CA, Vuichard-Gysin D, et al. Risk of SARS-CoV-2 transmission by aerosols, the rational use of masks, and protection of healthcare workers from COVID-19. Antimicrob Resist Infect Control. 2020;9:100.
- Stone TE, Kunaviktikul W, Omura M, et al. Facemasks and the covid 19 pandemic: what advice should health professionals be giving the general public about the wearing of facemasks? Nurs Health Sci. 2020;22:339-342.
- Tam VC, Tam SY, Poon WK, et al. A reality check on the use of face masks during the COVID-19 outbreak in Hong Kong. EClinicalMedicine. 2020;22:100356.
- Chen YJ, Qin G, Chen J, et al. Comparison of face-touching behaviors before and during the coronavirus disease 2019 pandemic. JAMA Netw Open. 2020;3:e2016924.
- O’Dowd K, Nair KM, Forouzandeh P, et al. Face masks and respirators in the fight against the COVID-19 pandemic: a review of current materials, advances and future perspectives. Materials (Basel). 2020;13:3363.
- Matuschek C, Moll F, Fangerau H, et al. Face masks: benefits and risks during the COVID-19 crisis. Eur J Med Res. 2020;25:32.
- Chan NC, Li K, Hirsh J. Peripheral oxygen saturation in older persons wearing nonmedical face masks in community settings. JAMA. 2020;324:2323-2324. doi:10.1001/jama.2020.21905
- , , . Differential COVID‐19 case positivity in New York City neighborhoods: socioeconomic factors and mobility. Influenza Other Respir Viruses. 2021;15:209-217. doi:10.1111/irv.12816
Practice Points
- Enormous financial and human resources have been utilized by health care systems to prevent the spread of COVID-19 in health care settings, including universal temperature checks, clinical symptom triage, and masking policies. Despite these mitigation practices, mask noncompliance continues to be a major problem in hospitals.
- Mask compliance among 600 individuals entering 4 New York City hospitals was observed to be 78%, despite months of policies for universal masking and the city’s high mortality rates during the first COVID-19 wave.
- Masks have been shown to reduce the spread of COVID-19, and proper mask compliance is an important issue that must be addressed by health care administrations and governmental agencies.
Nephrogenic Systemic Fibrosis in the Setting of Transient Renal Insufficiency
Nephrogenic systemic fibrosis (NSF) is a rare debilitating disorder characterized by dermal plaques, joint contractures, and fibrosis of the skin with possible involvement of muscles and internal organs.1-3 Originally identified in 1997 as nephrogenic fibrosing dermopathy to describe its characteristic cutaneous thickening and hardening, the name was changed to NSF to more accurately reflect the noncutaneous manifestations present in other organ tissues.2,4,5 Nephrogenic systemic fibrosis occurs in patients with a history of renal insufficiency and exposure to gadolinium-based contrast agents (GBCAs) used in magnetic resonance angiography and magnetic resonance imaging. There is no predilection for age, sex, or ethnicity.
Nephrogenic systemic fibrosis may develop over a period of days to several weeks. However, there have been cases of NSF developing 10 years after gadolinium exposure.2 In most cases, patients have a history of severe chronic renal disease requiring hemodialysis. There have been a few reported cases of NSF occurring in patients with resolved acute kidney injury or resolved acute on chronic renal disease.1,6-10 We present a case of NSF occurring in a patient with resolved transient renal insufficiency and no history of chronic renal disease.
Case Report
A 68-year-old woman presented with new dark, painless, pink plaques on the right thigh and calf. The patient stated the condition started and got worse after she was hospitalized 12 years prior for lower extremity cellulitis, sepsis, and acute renal failure. The patient developed complications during that hospital stay and underwent a renal biopsy and renal artery embolization requiring use of a GBCA. After the procedure, she noticed skin hardening in the extremities and decreased mobility in both legs while she was still in the hospital. It was thought that the lower leg changes were due to cellulitis. Therefore, when the renal issues resolved, she was discharged. Her skin and joint changes remained stable until 6 years later when she noticed new pink plaques appearing. Her medical history was positive for breast cancer, which was surgically and medically treated 16 years prior to presentation.
On presentation, physical examination revealed dark pink, hyperpigmented plaques on the right leg and a firm hypopigmented broad linear plaque on the right forearm. Palpation of the legs revealed thickened sclerotic plaques from the thighs down to the ankles (Figure 1). The plaques were not tender to palpation. She did have a decreased range of motion with eversion and inversion of the feet and ankles.
Biopsies from the right medial leg and right volar forearm showed increased bland dermal spindle cellularity associated with numerous round to ovoid osteoid aggregates encircling elastic fibers and surrounded by osteoblasts (Figure 2). CD34 immunohistochemistry showed general retention of staining within the dermal fibroblast population, and elastin stain showed general retention of elastic fiber bundles and thickening.
Laboratory workup included a complete blood cell count, comprehensive metabolic panel, thyroid-stimulating hormone level, and serum protein electrophoresis; results were all within reference range. The patient also had a urine element profile from an outside provider 1 month after presenting to our office that showed an elevated urine gadolinium level of 4.146 μg/g (reference range, 0–0.019 μg/g). The patient’s skin lesions have remained stable, and she is now working with physical therapy to help with her range of motion.
Comment
Gadolinium Causing Fibrosis—The incidence of NSF varies according to the severity of renal impairment, dosage level of GBCA used, and the history of GBCA use. In patients with normal renal function, gadolinium is excreted within 90 minutes. In patients with severe renal disease, the half-life can increase to up to 34.3 hours.11 Reduced renal clearance and increased half-life of gadolinium lead to prolonged excretion, causing the GBCA to become unstable and dissociate into its constituents, leading to tissue deposition of Gd3+ cations. This dissociation is thought to be due to differences in the stability of the various chelation complexes among the different formulations of GBCAs.12 The mechanism by which the dissociated gadolinium causes the fibrosis in the skin or other organs of the body is still unknown. Furthermore, even patients with normal renal function who undergo repeated administration of GBCA have been found to have higher levels of Gd3+ in their tissues, even in the absence of symptoms.13
Diagnosing NSF—In 2011, Girardi et al14 created a clinical and histopathological scoring system to help diagnose NSF. Clinical findings can be broken down into major criteria and minor criteria. Major criteria consist of patterned plaques, joint contractures, cobblestoning, marked induration, or peau d’orange change. Minor criteria consist of puckering, linear banding, superficial plaques or patches, dermal papules, and scleral plaques. Histopathologic findings include increased dermal cellularity (score +1), CD34+ cells with tram tracking (score +1), thickened or thin collagen bundles (score +1), preserved elastic fibers (score −1), septal involvement (score +1), and osseous metaplasia (score +3)(eTable).14
Differential Diagnosis—The differential diagnosis of NSF includes scleromyxedema, scleroderma, eosinophilic fasciitis, eosinophilia-myalgia syndrome, lipodermatosclerosis, morphea, and chronic graft-vs-host disease. Histopathologic examination of scleromyxedema can look identical to NSF. Therefore, a review of the patient’s medical history, prior hospitalizations, and prior gadolinium exposure is important. Appropriate laboratory workups should be ordered to rule out the other differential diagnoses.
NSF and Kidney Injury—A PubMed search of articles indexed for MEDLINE using the terms NSF with kidney injury revealed 7 cases of NSF occurring in patients who either had resolved acute kidney injury or resolved acute on chronic kidney disease.1,6-10 Of those cases, 3 reported NSF occurring in patients with completely resolved acute kidney injury.6,7,10 One of those cases involved a 65-year-old man who developed acute renal failure due to acute tubular necrosis.7 He had no history of renal disease prior to hospitalization. His skin lesions continued to improve as his renal function normalized back to baseline after discharge.7 The second case involved a 42-year-old man who had repeated exposure to GBCAs during a brief period of acute kidney injury.6 Nephrogenic systemic fibrosis developed after his renal function normalized. The authors did not mention if there was clinical improvement.6 The third case involved a 22-year-old man who developed acute renal failure after ingestion of hair dye. He did not have a history of chronic renal disease, and as he recovered from the acute kidney injury, almost all of the skin lesions cleared after 1 year.10
Our patient did not have a history of chronic renal disease when she presented to the hospital for sepsis and acute tubular necrosis. Unlike 2 of the prior cases, she did not notice improvement of the skin lesions as the renal function returned to baseline. She continued to experience changes in the skin, even up to 5 years after, and then stabilized. Throughout that time, her renal function was normal. Interestingly, despite having a normal creatinine level, the patient had an elevated gadolinium level on the urine gadolinium test, which typically is not a standard test for NSF. However, the elevated value does shed light on the persistence of gadolinium in the patient despite her exposure having been more than 10 years earlier.
Treatment of NSF—There is no gold standard treatment of NSF, and reversing the fibrosis has proven to be difficult. Avoidance of GBCAs in acute kidney injury or chronic severe renal disease, as recommended by the US Food and Drug Administration, is key to preventing this debilitating disease.15 Restoration of renal function is essential for excreting the gadolinium and improvement in NSF.12 Physical and occupational therapy can improve joint mobility. Therapies such as extracorporeal photopheresis, sodium thiosulfate, pentoxifylline, glucocorticoids, plasmapheresis, intravenous immunoglobulin, cyclophosphamide, imatinib mesylate, intralesional interferon alfa, topical calcipotriene, corticosteroids, and UVA1 light therapy have been used with varying results.12 It has been suggested that renal transplantation can stop the progression of NSF. However, in the cases we reviewed, renal transplantation would not have benefited those patients because their renal function normalized.6,7,10 Additionally, even though our patient’s renal function normalized after discharge from the hospital, she continued to see more skin lesions developing, likely due to the accumulated gadolinium that was already in her tissue. The possibility of chelation therapy to remove the gadolinium has been proposed. In 1 case study involving deferoxamine injected intramuscularly in a patient with NSF, the urine excretion of gadolinium increased almost 2-fold, but there was no change in the serum concentration level of gadolinium or improvement in the patient’s clinical symptoms.16 We anticipate that our patient’s symptoms will slowly improve, as her body is still excreting the gadolinium. Our patient also was added to the International NSF Registry that was created by Dr. Shawn E. Cowper at the Yale School of Medicine (New Haven, Connecticut).
Conclusion
We report a rare case of NSF occurring in a patient with resolved acute kidney injury and no history of chronic renal disease. Our patient initially did not improve after her renal function normalized, as she continued to develop lesions 10 years after the exposure. Her elevated urine gadolinium excretion level also sheds light on the persistence of gadolinium in her body despite her normal renal function 10 years after her exposure. Although her clinical symptoms have stabilized, our case reiterates the complex pathology of this entity and challenge regarding treatment options. Physicians should be aware that NSF can still occur in healthy patients with no chronic renal disease who have had an episode of acute renal insufficiency along with exposure to a GBCA.
- Cowper SE, Su LD, Bhawan J, et al. Nephrogenic fibrosing dermopathy. Am J Dermatopathol. 2001;23:383-393.
- Grobner T. Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21:1104-1108.
- Larson KN, Gagnon AL, Darling MD, et al. Nephrogenic systemic fibrosis manifesting a decade after exposure to gadolinium. JAMA Dermatol. 2015;151:1117-1120.
- Mendoza FA, Artlett CM, Sandorfi N, et al. Description of 12 cases of nephrogenic fibrosing dermopathy and review of the literature. Semin Arthritis Rheum. 2006;35:238-249.
- Ting WW, Stone MS, Madison KC, et al. Nephrogenic fibrosing dermopathy with systemic involvement. Arch Dermatol. 2003;139:903-906.
- Lu CF, Hsiao CH, Tjiu JW. Nephrogenic systemic fibrosis developed after recovery from acute renal failure: gadolinium as a possible aetiological factor. J Eur Acad Dermatol Venereol. 2009;23:339-340.
- Cassis TB, Jackson JM, Sonnier GB, et al. Nephrogenic fibrosing dermopathy in a patient with acute renal failure never requiring dialysis. Int J Dermatol. 2006;45:56-59.
- Swartz RD, Crofford LJ, Phan SH, et al. Nephrogenic fibrosing dermopathy: a novel cutaneous fibrosing disorder in patients with renal failure. Am J Med. 2003;114:563-572.
- Mackay-Wiggan JM, Cohen DJ, Hardy MA, et al. Nephrogenic fibrosing dermopathy (scleromyxedema-like illness of renal disease). J Am Acad Dermatol. 2003;48:55-60.
- Reddy IS, Somani VK, Swarnalata G, et al. Nephrogenic systemic fibrosis following hair-dye ingestion induced acute renal failure. Indian J Dermatol Venereol Leprol. 2006;76:400-403.
- Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol. 2006;17:2359-2362.
- Cheong BYC, Muthupillai R. Nephrogenic systemic fibrosis: a concise review for cardiologists. Texas Heart Inst J. 2010;37:508-515.
- Rogosnitzky M, Branch S. Gadolinium-based contrast agent toxicity: a review of known and proposed mechanisms. BioMetals. 2016;29:365-376.
- Girardi M, Kay J, Elston DM, et al. Nephrogenic systemic fibrosis: clinicopathological definition and workup recommendations. J Am Acad Dermatol. 2011;65:1095-1106.
- US Food and Drug Administration. FDA Drug Safety Communication: new warnings for using gadolinium-based contrast agents in patients with kidney dysfunction. Updated February 6, 2018. Accessed November 22, 2021. http://www.fda.gov/Drugs/DrugSafety/ucm223966.htm
- Leung N, Pittelkow MR, Lee CU, et al. Chelation of gadolinium with deferoxamine in a patient with nephrogenic systemic fibrosis. NDT Plus. 2009;2:309-311.
Nephrogenic systemic fibrosis (NSF) is a rare debilitating disorder characterized by dermal plaques, joint contractures, and fibrosis of the skin with possible involvement of muscles and internal organs.1-3 Originally identified in 1997 as nephrogenic fibrosing dermopathy to describe its characteristic cutaneous thickening and hardening, the name was changed to NSF to more accurately reflect the noncutaneous manifestations present in other organ tissues.2,4,5 Nephrogenic systemic fibrosis occurs in patients with a history of renal insufficiency and exposure to gadolinium-based contrast agents (GBCAs) used in magnetic resonance angiography and magnetic resonance imaging. There is no predilection for age, sex, or ethnicity.
Nephrogenic systemic fibrosis may develop over a period of days to several weeks. However, there have been cases of NSF developing 10 years after gadolinium exposure.2 In most cases, patients have a history of severe chronic renal disease requiring hemodialysis. There have been a few reported cases of NSF occurring in patients with resolved acute kidney injury or resolved acute on chronic renal disease.1,6-10 We present a case of NSF occurring in a patient with resolved transient renal insufficiency and no history of chronic renal disease.
Case Report
A 68-year-old woman presented with new dark, painless, pink plaques on the right thigh and calf. The patient stated the condition started and got worse after she was hospitalized 12 years prior for lower extremity cellulitis, sepsis, and acute renal failure. The patient developed complications during that hospital stay and underwent a renal biopsy and renal artery embolization requiring use of a GBCA. After the procedure, she noticed skin hardening in the extremities and decreased mobility in both legs while she was still in the hospital. It was thought that the lower leg changes were due to cellulitis. Therefore, when the renal issues resolved, she was discharged. Her skin and joint changes remained stable until 6 years later when she noticed new pink plaques appearing. Her medical history was positive for breast cancer, which was surgically and medically treated 16 years prior to presentation.
On presentation, physical examination revealed dark pink, hyperpigmented plaques on the right leg and a firm hypopigmented broad linear plaque on the right forearm. Palpation of the legs revealed thickened sclerotic plaques from the thighs down to the ankles (Figure 1). The plaques were not tender to palpation. She did have a decreased range of motion with eversion and inversion of the feet and ankles.
Biopsies from the right medial leg and right volar forearm showed increased bland dermal spindle cellularity associated with numerous round to ovoid osteoid aggregates encircling elastic fibers and surrounded by osteoblasts (Figure 2). CD34 immunohistochemistry showed general retention of staining within the dermal fibroblast population, and elastin stain showed general retention of elastic fiber bundles and thickening.
Laboratory workup included a complete blood cell count, comprehensive metabolic panel, thyroid-stimulating hormone level, and serum protein electrophoresis; results were all within reference range. The patient also had a urine element profile from an outside provider 1 month after presenting to our office that showed an elevated urine gadolinium level of 4.146 μg/g (reference range, 0–0.019 μg/g). The patient’s skin lesions have remained stable, and she is now working with physical therapy to help with her range of motion.
Comment
Gadolinium Causing Fibrosis—The incidence of NSF varies according to the severity of renal impairment, dosage level of GBCA used, and the history of GBCA use. In patients with normal renal function, gadolinium is excreted within 90 minutes. In patients with severe renal disease, the half-life can increase to up to 34.3 hours.11 Reduced renal clearance and increased half-life of gadolinium lead to prolonged excretion, causing the GBCA to become unstable and dissociate into its constituents, leading to tissue deposition of Gd3+ cations. This dissociation is thought to be due to differences in the stability of the various chelation complexes among the different formulations of GBCAs.12 The mechanism by which the dissociated gadolinium causes the fibrosis in the skin or other organs of the body is still unknown. Furthermore, even patients with normal renal function who undergo repeated administration of GBCA have been found to have higher levels of Gd3+ in their tissues, even in the absence of symptoms.13
Diagnosing NSF—In 2011, Girardi et al14 created a clinical and histopathological scoring system to help diagnose NSF. Clinical findings can be broken down into major criteria and minor criteria. Major criteria consist of patterned plaques, joint contractures, cobblestoning, marked induration, or peau d’orange change. Minor criteria consist of puckering, linear banding, superficial plaques or patches, dermal papules, and scleral plaques. Histopathologic findings include increased dermal cellularity (score +1), CD34+ cells with tram tracking (score +1), thickened or thin collagen bundles (score +1), preserved elastic fibers (score −1), septal involvement (score +1), and osseous metaplasia (score +3)(eTable).14
Differential Diagnosis—The differential diagnosis of NSF includes scleromyxedema, scleroderma, eosinophilic fasciitis, eosinophilia-myalgia syndrome, lipodermatosclerosis, morphea, and chronic graft-vs-host disease. Histopathologic examination of scleromyxedema can look identical to NSF. Therefore, a review of the patient’s medical history, prior hospitalizations, and prior gadolinium exposure is important. Appropriate laboratory workups should be ordered to rule out the other differential diagnoses.
NSF and Kidney Injury—A PubMed search of articles indexed for MEDLINE using the terms NSF with kidney injury revealed 7 cases of NSF occurring in patients who either had resolved acute kidney injury or resolved acute on chronic kidney disease.1,6-10 Of those cases, 3 reported NSF occurring in patients with completely resolved acute kidney injury.6,7,10 One of those cases involved a 65-year-old man who developed acute renal failure due to acute tubular necrosis.7 He had no history of renal disease prior to hospitalization. His skin lesions continued to improve as his renal function normalized back to baseline after discharge.7 The second case involved a 42-year-old man who had repeated exposure to GBCAs during a brief period of acute kidney injury.6 Nephrogenic systemic fibrosis developed after his renal function normalized. The authors did not mention if there was clinical improvement.6 The third case involved a 22-year-old man who developed acute renal failure after ingestion of hair dye. He did not have a history of chronic renal disease, and as he recovered from the acute kidney injury, almost all of the skin lesions cleared after 1 year.10
Our patient did not have a history of chronic renal disease when she presented to the hospital for sepsis and acute tubular necrosis. Unlike 2 of the prior cases, she did not notice improvement of the skin lesions as the renal function returned to baseline. She continued to experience changes in the skin, even up to 5 years after, and then stabilized. Throughout that time, her renal function was normal. Interestingly, despite having a normal creatinine level, the patient had an elevated gadolinium level on the urine gadolinium test, which typically is not a standard test for NSF. However, the elevated value does shed light on the persistence of gadolinium in the patient despite her exposure having been more than 10 years earlier.
Treatment of NSF—There is no gold standard treatment of NSF, and reversing the fibrosis has proven to be difficult. Avoidance of GBCAs in acute kidney injury or chronic severe renal disease, as recommended by the US Food and Drug Administration, is key to preventing this debilitating disease.15 Restoration of renal function is essential for excreting the gadolinium and improvement in NSF.12 Physical and occupational therapy can improve joint mobility. Therapies such as extracorporeal photopheresis, sodium thiosulfate, pentoxifylline, glucocorticoids, plasmapheresis, intravenous immunoglobulin, cyclophosphamide, imatinib mesylate, intralesional interferon alfa, topical calcipotriene, corticosteroids, and UVA1 light therapy have been used with varying results.12 It has been suggested that renal transplantation can stop the progression of NSF. However, in the cases we reviewed, renal transplantation would not have benefited those patients because their renal function normalized.6,7,10 Additionally, even though our patient’s renal function normalized after discharge from the hospital, she continued to see more skin lesions developing, likely due to the accumulated gadolinium that was already in her tissue. The possibility of chelation therapy to remove the gadolinium has been proposed. In 1 case study involving deferoxamine injected intramuscularly in a patient with NSF, the urine excretion of gadolinium increased almost 2-fold, but there was no change in the serum concentration level of gadolinium or improvement in the patient’s clinical symptoms.16 We anticipate that our patient’s symptoms will slowly improve, as her body is still excreting the gadolinium. Our patient also was added to the International NSF Registry that was created by Dr. Shawn E. Cowper at the Yale School of Medicine (New Haven, Connecticut).
Conclusion
We report a rare case of NSF occurring in a patient with resolved acute kidney injury and no history of chronic renal disease. Our patient initially did not improve after her renal function normalized, as she continued to develop lesions 10 years after the exposure. Her elevated urine gadolinium excretion level also sheds light on the persistence of gadolinium in her body despite her normal renal function 10 years after her exposure. Although her clinical symptoms have stabilized, our case reiterates the complex pathology of this entity and challenge regarding treatment options. Physicians should be aware that NSF can still occur in healthy patients with no chronic renal disease who have had an episode of acute renal insufficiency along with exposure to a GBCA.
Nephrogenic systemic fibrosis (NSF) is a rare debilitating disorder characterized by dermal plaques, joint contractures, and fibrosis of the skin with possible involvement of muscles and internal organs.1-3 Originally identified in 1997 as nephrogenic fibrosing dermopathy to describe its characteristic cutaneous thickening and hardening, the name was changed to NSF to more accurately reflect the noncutaneous manifestations present in other organ tissues.2,4,5 Nephrogenic systemic fibrosis occurs in patients with a history of renal insufficiency and exposure to gadolinium-based contrast agents (GBCAs) used in magnetic resonance angiography and magnetic resonance imaging. There is no predilection for age, sex, or ethnicity.
Nephrogenic systemic fibrosis may develop over a period of days to several weeks. However, there have been cases of NSF developing 10 years after gadolinium exposure.2 In most cases, patients have a history of severe chronic renal disease requiring hemodialysis. There have been a few reported cases of NSF occurring in patients with resolved acute kidney injury or resolved acute on chronic renal disease.1,6-10 We present a case of NSF occurring in a patient with resolved transient renal insufficiency and no history of chronic renal disease.
Case Report
A 68-year-old woman presented with new dark, painless, pink plaques on the right thigh and calf. The patient stated the condition started and got worse after she was hospitalized 12 years prior for lower extremity cellulitis, sepsis, and acute renal failure. The patient developed complications during that hospital stay and underwent a renal biopsy and renal artery embolization requiring use of a GBCA. After the procedure, she noticed skin hardening in the extremities and decreased mobility in both legs while she was still in the hospital. It was thought that the lower leg changes were due to cellulitis. Therefore, when the renal issues resolved, she was discharged. Her skin and joint changes remained stable until 6 years later when she noticed new pink plaques appearing. Her medical history was positive for breast cancer, which was surgically and medically treated 16 years prior to presentation.
On presentation, physical examination revealed dark pink, hyperpigmented plaques on the right leg and a firm hypopigmented broad linear plaque on the right forearm. Palpation of the legs revealed thickened sclerotic plaques from the thighs down to the ankles (Figure 1). The plaques were not tender to palpation. She did have a decreased range of motion with eversion and inversion of the feet and ankles.
Biopsies from the right medial leg and right volar forearm showed increased bland dermal spindle cellularity associated with numerous round to ovoid osteoid aggregates encircling elastic fibers and surrounded by osteoblasts (Figure 2). CD34 immunohistochemistry showed general retention of staining within the dermal fibroblast population, and elastin stain showed general retention of elastic fiber bundles and thickening.
Laboratory workup included a complete blood cell count, comprehensive metabolic panel, thyroid-stimulating hormone level, and serum protein electrophoresis; results were all within reference range. The patient also had a urine element profile from an outside provider 1 month after presenting to our office that showed an elevated urine gadolinium level of 4.146 μg/g (reference range, 0–0.019 μg/g). The patient’s skin lesions have remained stable, and she is now working with physical therapy to help with her range of motion.
Comment
Gadolinium Causing Fibrosis—The incidence of NSF varies according to the severity of renal impairment, dosage level of GBCA used, and the history of GBCA use. In patients with normal renal function, gadolinium is excreted within 90 minutes. In patients with severe renal disease, the half-life can increase to up to 34.3 hours.11 Reduced renal clearance and increased half-life of gadolinium lead to prolonged excretion, causing the GBCA to become unstable and dissociate into its constituents, leading to tissue deposition of Gd3+ cations. This dissociation is thought to be due to differences in the stability of the various chelation complexes among the different formulations of GBCAs.12 The mechanism by which the dissociated gadolinium causes the fibrosis in the skin or other organs of the body is still unknown. Furthermore, even patients with normal renal function who undergo repeated administration of GBCA have been found to have higher levels of Gd3+ in their tissues, even in the absence of symptoms.13
Diagnosing NSF—In 2011, Girardi et al14 created a clinical and histopathological scoring system to help diagnose NSF. Clinical findings can be broken down into major criteria and minor criteria. Major criteria consist of patterned plaques, joint contractures, cobblestoning, marked induration, or peau d’orange change. Minor criteria consist of puckering, linear banding, superficial plaques or patches, dermal papules, and scleral plaques. Histopathologic findings include increased dermal cellularity (score +1), CD34+ cells with tram tracking (score +1), thickened or thin collagen bundles (score +1), preserved elastic fibers (score −1), septal involvement (score +1), and osseous metaplasia (score +3)(eTable).14
Differential Diagnosis—The differential diagnosis of NSF includes scleromyxedema, scleroderma, eosinophilic fasciitis, eosinophilia-myalgia syndrome, lipodermatosclerosis, morphea, and chronic graft-vs-host disease. Histopathologic examination of scleromyxedema can look identical to NSF. Therefore, a review of the patient’s medical history, prior hospitalizations, and prior gadolinium exposure is important. Appropriate laboratory workups should be ordered to rule out the other differential diagnoses.
NSF and Kidney Injury—A PubMed search of articles indexed for MEDLINE using the terms NSF with kidney injury revealed 7 cases of NSF occurring in patients who either had resolved acute kidney injury or resolved acute on chronic kidney disease.1,6-10 Of those cases, 3 reported NSF occurring in patients with completely resolved acute kidney injury.6,7,10 One of those cases involved a 65-year-old man who developed acute renal failure due to acute tubular necrosis.7 He had no history of renal disease prior to hospitalization. His skin lesions continued to improve as his renal function normalized back to baseline after discharge.7 The second case involved a 42-year-old man who had repeated exposure to GBCAs during a brief period of acute kidney injury.6 Nephrogenic systemic fibrosis developed after his renal function normalized. The authors did not mention if there was clinical improvement.6 The third case involved a 22-year-old man who developed acute renal failure after ingestion of hair dye. He did not have a history of chronic renal disease, and as he recovered from the acute kidney injury, almost all of the skin lesions cleared after 1 year.10
Our patient did not have a history of chronic renal disease when she presented to the hospital for sepsis and acute tubular necrosis. Unlike 2 of the prior cases, she did not notice improvement of the skin lesions as the renal function returned to baseline. She continued to experience changes in the skin, even up to 5 years after, and then stabilized. Throughout that time, her renal function was normal. Interestingly, despite having a normal creatinine level, the patient had an elevated gadolinium level on the urine gadolinium test, which typically is not a standard test for NSF. However, the elevated value does shed light on the persistence of gadolinium in the patient despite her exposure having been more than 10 years earlier.
Treatment of NSF—There is no gold standard treatment of NSF, and reversing the fibrosis has proven to be difficult. Avoidance of GBCAs in acute kidney injury or chronic severe renal disease, as recommended by the US Food and Drug Administration, is key to preventing this debilitating disease.15 Restoration of renal function is essential for excreting the gadolinium and improvement in NSF.12 Physical and occupational therapy can improve joint mobility. Therapies such as extracorporeal photopheresis, sodium thiosulfate, pentoxifylline, glucocorticoids, plasmapheresis, intravenous immunoglobulin, cyclophosphamide, imatinib mesylate, intralesional interferon alfa, topical calcipotriene, corticosteroids, and UVA1 light therapy have been used with varying results.12 It has been suggested that renal transplantation can stop the progression of NSF. However, in the cases we reviewed, renal transplantation would not have benefited those patients because their renal function normalized.6,7,10 Additionally, even though our patient’s renal function normalized after discharge from the hospital, she continued to see more skin lesions developing, likely due to the accumulated gadolinium that was already in her tissue. The possibility of chelation therapy to remove the gadolinium has been proposed. In 1 case study involving deferoxamine injected intramuscularly in a patient with NSF, the urine excretion of gadolinium increased almost 2-fold, but there was no change in the serum concentration level of gadolinium or improvement in the patient’s clinical symptoms.16 We anticipate that our patient’s symptoms will slowly improve, as her body is still excreting the gadolinium. Our patient also was added to the International NSF Registry that was created by Dr. Shawn E. Cowper at the Yale School of Medicine (New Haven, Connecticut).
Conclusion
We report a rare case of NSF occurring in a patient with resolved acute kidney injury and no history of chronic renal disease. Our patient initially did not improve after her renal function normalized, as she continued to develop lesions 10 years after the exposure. Her elevated urine gadolinium excretion level also sheds light on the persistence of gadolinium in her body despite her normal renal function 10 years after her exposure. Although her clinical symptoms have stabilized, our case reiterates the complex pathology of this entity and challenge regarding treatment options. Physicians should be aware that NSF can still occur in healthy patients with no chronic renal disease who have had an episode of acute renal insufficiency along with exposure to a GBCA.
- Cowper SE, Su LD, Bhawan J, et al. Nephrogenic fibrosing dermopathy. Am J Dermatopathol. 2001;23:383-393.
- Grobner T. Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21:1104-1108.
- Larson KN, Gagnon AL, Darling MD, et al. Nephrogenic systemic fibrosis manifesting a decade after exposure to gadolinium. JAMA Dermatol. 2015;151:1117-1120.
- Mendoza FA, Artlett CM, Sandorfi N, et al. Description of 12 cases of nephrogenic fibrosing dermopathy and review of the literature. Semin Arthritis Rheum. 2006;35:238-249.
- Ting WW, Stone MS, Madison KC, et al. Nephrogenic fibrosing dermopathy with systemic involvement. Arch Dermatol. 2003;139:903-906.
- Lu CF, Hsiao CH, Tjiu JW. Nephrogenic systemic fibrosis developed after recovery from acute renal failure: gadolinium as a possible aetiological factor. J Eur Acad Dermatol Venereol. 2009;23:339-340.
- Cassis TB, Jackson JM, Sonnier GB, et al. Nephrogenic fibrosing dermopathy in a patient with acute renal failure never requiring dialysis. Int J Dermatol. 2006;45:56-59.
- Swartz RD, Crofford LJ, Phan SH, et al. Nephrogenic fibrosing dermopathy: a novel cutaneous fibrosing disorder in patients with renal failure. Am J Med. 2003;114:563-572.
- Mackay-Wiggan JM, Cohen DJ, Hardy MA, et al. Nephrogenic fibrosing dermopathy (scleromyxedema-like illness of renal disease). J Am Acad Dermatol. 2003;48:55-60.
- Reddy IS, Somani VK, Swarnalata G, et al. Nephrogenic systemic fibrosis following hair-dye ingestion induced acute renal failure. Indian J Dermatol Venereol Leprol. 2006;76:400-403.
- Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol. 2006;17:2359-2362.
- Cheong BYC, Muthupillai R. Nephrogenic systemic fibrosis: a concise review for cardiologists. Texas Heart Inst J. 2010;37:508-515.
- Rogosnitzky M, Branch S. Gadolinium-based contrast agent toxicity: a review of known and proposed mechanisms. BioMetals. 2016;29:365-376.
- Girardi M, Kay J, Elston DM, et al. Nephrogenic systemic fibrosis: clinicopathological definition and workup recommendations. J Am Acad Dermatol. 2011;65:1095-1106.
- US Food and Drug Administration. FDA Drug Safety Communication: new warnings for using gadolinium-based contrast agents in patients with kidney dysfunction. Updated February 6, 2018. Accessed November 22, 2021. http://www.fda.gov/Drugs/DrugSafety/ucm223966.htm
- Leung N, Pittelkow MR, Lee CU, et al. Chelation of gadolinium with deferoxamine in a patient with nephrogenic systemic fibrosis. NDT Plus. 2009;2:309-311.
- Cowper SE, Su LD, Bhawan J, et al. Nephrogenic fibrosing dermopathy. Am J Dermatopathol. 2001;23:383-393.
- Grobner T. Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21:1104-1108.
- Larson KN, Gagnon AL, Darling MD, et al. Nephrogenic systemic fibrosis manifesting a decade after exposure to gadolinium. JAMA Dermatol. 2015;151:1117-1120.
- Mendoza FA, Artlett CM, Sandorfi N, et al. Description of 12 cases of nephrogenic fibrosing dermopathy and review of the literature. Semin Arthritis Rheum. 2006;35:238-249.
- Ting WW, Stone MS, Madison KC, et al. Nephrogenic fibrosing dermopathy with systemic involvement. Arch Dermatol. 2003;139:903-906.
- Lu CF, Hsiao CH, Tjiu JW. Nephrogenic systemic fibrosis developed after recovery from acute renal failure: gadolinium as a possible aetiological factor. J Eur Acad Dermatol Venereol. 2009;23:339-340.
- Cassis TB, Jackson JM, Sonnier GB, et al. Nephrogenic fibrosing dermopathy in a patient with acute renal failure never requiring dialysis. Int J Dermatol. 2006;45:56-59.
- Swartz RD, Crofford LJ, Phan SH, et al. Nephrogenic fibrosing dermopathy: a novel cutaneous fibrosing disorder in patients with renal failure. Am J Med. 2003;114:563-572.
- Mackay-Wiggan JM, Cohen DJ, Hardy MA, et al. Nephrogenic fibrosing dermopathy (scleromyxedema-like illness of renal disease). J Am Acad Dermatol. 2003;48:55-60.
- Reddy IS, Somani VK, Swarnalata G, et al. Nephrogenic systemic fibrosis following hair-dye ingestion induced acute renal failure. Indian J Dermatol Venereol Leprol. 2006;76:400-403.
- Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol. 2006;17:2359-2362.
- Cheong BYC, Muthupillai R. Nephrogenic systemic fibrosis: a concise review for cardiologists. Texas Heart Inst J. 2010;37:508-515.
- Rogosnitzky M, Branch S. Gadolinium-based contrast agent toxicity: a review of known and proposed mechanisms. BioMetals. 2016;29:365-376.
- Girardi M, Kay J, Elston DM, et al. Nephrogenic systemic fibrosis: clinicopathological definition and workup recommendations. J Am Acad Dermatol. 2011;65:1095-1106.
- US Food and Drug Administration. FDA Drug Safety Communication: new warnings for using gadolinium-based contrast agents in patients with kidney dysfunction. Updated February 6, 2018. Accessed November 22, 2021. http://www.fda.gov/Drugs/DrugSafety/ucm223966.htm
- Leung N, Pittelkow MR, Lee CU, et al. Chelation of gadolinium with deferoxamine in a patient with nephrogenic systemic fibrosis. NDT Plus. 2009;2:309-311.
Practice Points
- Nephrogenic systemic fibrosis may occur in patients with a history of renal insufficiency and exposure to gadolinium-based contrast agents.
- Nephrogenic systemic fibrosis may develop over a period of days to several years after exposure.
- Symptoms of this rare disease can progress and get worse even after renal function normalizes.
Pityriasis Rosea Associated With COVID-19 Vaccination: A Common Rash Following Administration of a Novel Vaccine
Pityriasis rosea is a papulosquamous eruption that favors the trunk and proximal extremities. It occurs most commonly in adolescents and young adults.1 The rash typically presents with a solitary lesion, known as a “herald patch,” which is followed by a scaly erythematous eruption along the cleavage lines of the skin. The condition is self-limited and often resolves in 6 to 8 weeks. Recent evidence suggests that viral reactivation of human herpesvirus 6 and human herpesvirus 7 may play a role in the development of skin lesions.2 Pityriasis rosea also has been reported following the administration of new medications and vaccinations.1-3 We report a case of a 30-year-old woman who developed pityriasis rosea 3 days after receiving the second dose of the COVID-19 vaccine.
Case Report
A 30-year-old woman presented to the dermatology office for evaluation of a rash on the trunk and upper extremities that had been present for 5 days. She reported an initial solitary lesion on the left upper back, subsequently followed by the appearance of a mildly pruritic rash on the trunk and upper extremities. The rash first appeared 3 days after she received the second dose of the Pfizer-BioNTech COVID-19 vaccine. She was otherwise asymptomatic after vaccination and denied fever, chills, headache, and myalgia. She denied any rash following her first dose of the COVID-19 vaccine, history of known COVID-19 infection or exposures, or new medications. Notably, the patient worked in health care.
Physical examination revealed a 2-cm, erythematous, thin, scaly plaque over the left side of the upper back (Figure, A). Erythematous, scaly, thin papules of varying sizes were distributed along the cleavage lines of the trunk and upper extremities (Figure, B). No biopsy was performed because of the classic clinical presentation of this self-limited condition and the patient’s history of hypertrophic scarring. No additional laboratory workup was performed. She was prescribed triamcinolone cream 0.1% as needed for pruritus and was reassured about the benign nature of this cutaneous eruption.
Comment
A broad spectrum of cutaneous manifestations has been reported in association with acute COVID-19 infection, including a papulovesicular rash, perniolike eruptions, urticaria, livedo reticularis, and petechiae.4 Several cases of pityriasis rosea in association with acute COVID-19 infection also have been reported.5 COVID-19 infection has been linked to reactivation of the herpesvirus, which may explain the connection between acute COVID-19 infection and the development of pityriasis rosea.6 Pityriasis rosea associated with administration of the COVID-19 vaccine is a rare complication with few reports in the literature.7 Similar to our patient, there are reports of pityriasis rosea developing after the second dose of the vaccine, with some patients reporting a reactivation of skin lesions.8 There is a paucity of reports describing pityriasis rosea associated with the influenza vaccine, hepatitis B vaccine, and human papillomavirus vaccine.3 In such cases, the onset of skin lesions was thought to be related to vaccine-induced stimulation of the immune system or a component of the vaccine.
Conclusion
We presented a unique case of pityriasis rosea following COVID-19 vaccination. Because additional laboratory workup and a skin biopsy were not performed, we are unable to infer causation. However, the classic clinical presentation, rash development within 3 days of vaccination, and prior reports of vaccine-associated pityriasis rosea strengthen the aforementioned association. We hope this case adds to the growing understanding of the novel COVID-19 vaccine. As more individuals become vaccinated, both clinicians and patients should be aware of this benign cutaneous eruption that can develop following COVID-19 vaccination.
- Papakostas D, Stavropoulos PG, Papafragkaki D, et al. An atypical case of pityriasis rosea gigantea after influenza vaccination. Case Rep Dermatol. 2014;6:119-123.
- Chen FJ, Chian CP, Chen YF, et al. Pityriasis rosea following influenza (H1N1) vaccination. J Chin Med Assoc. 2011;74:280-282.
- Li A, Li P, Li Y, et al. Recurrent pityriasis rosea: a case report. Hum Vaccin Immunother. 2018;4:1024-1026.
- Ng SM. Prolonged dermatological manifestation 4 weeks following recovery of COVID-19 in a child. BMJ Case Rep. 2020;13:e237056. doi:10.1136/bcr-2020-237056
- Johansen M, Chisolm SS, Aspey LD, et al. Pityriasis rosea in otherwise asymptomatic confirmed COVID-19-positive patients: a report of 2 cases. JAAD Case Rep. 2021;7:93-94.
- Dursun R, Temiz SA. The clinics of HHV-6 infection in COVID-19 pandemic: pityriasis rosea and Kawasaki disease. Dermatol Ther. 2020;33:e13730. doi:10.1111/dth.13730
- Leerunyakul K, Pakornphadungsit K, Suchonwanit P. Case report: pityriasis rosea-like eruption following COVID-19 vaccination [published online September 7, 2021]. Front Med. doi:10.3389/fmed.2021.752443
- Marcantonio-Santa Cruz OY, Vidal-Navarro A, Pesqué D, et al. Pityriasis rosea developing after COVID-19 vaccination. J Eur Acad Dermatol Venereol. 2021;35:E721-E722. doi:10.1111/jdv.17498
Pityriasis rosea is a papulosquamous eruption that favors the trunk and proximal extremities. It occurs most commonly in adolescents and young adults.1 The rash typically presents with a solitary lesion, known as a “herald patch,” which is followed by a scaly erythematous eruption along the cleavage lines of the skin. The condition is self-limited and often resolves in 6 to 8 weeks. Recent evidence suggests that viral reactivation of human herpesvirus 6 and human herpesvirus 7 may play a role in the development of skin lesions.2 Pityriasis rosea also has been reported following the administration of new medications and vaccinations.1-3 We report a case of a 30-year-old woman who developed pityriasis rosea 3 days after receiving the second dose of the COVID-19 vaccine.
Case Report
A 30-year-old woman presented to the dermatology office for evaluation of a rash on the trunk and upper extremities that had been present for 5 days. She reported an initial solitary lesion on the left upper back, subsequently followed by the appearance of a mildly pruritic rash on the trunk and upper extremities. The rash first appeared 3 days after she received the second dose of the Pfizer-BioNTech COVID-19 vaccine. She was otherwise asymptomatic after vaccination and denied fever, chills, headache, and myalgia. She denied any rash following her first dose of the COVID-19 vaccine, history of known COVID-19 infection or exposures, or new medications. Notably, the patient worked in health care.
Physical examination revealed a 2-cm, erythematous, thin, scaly plaque over the left side of the upper back (Figure, A). Erythematous, scaly, thin papules of varying sizes were distributed along the cleavage lines of the trunk and upper extremities (Figure, B). No biopsy was performed because of the classic clinical presentation of this self-limited condition and the patient’s history of hypertrophic scarring. No additional laboratory workup was performed. She was prescribed triamcinolone cream 0.1% as needed for pruritus and was reassured about the benign nature of this cutaneous eruption.
Comment
A broad spectrum of cutaneous manifestations has been reported in association with acute COVID-19 infection, including a papulovesicular rash, perniolike eruptions, urticaria, livedo reticularis, and petechiae.4 Several cases of pityriasis rosea in association with acute COVID-19 infection also have been reported.5 COVID-19 infection has been linked to reactivation of the herpesvirus, which may explain the connection between acute COVID-19 infection and the development of pityriasis rosea.6 Pityriasis rosea associated with administration of the COVID-19 vaccine is a rare complication with few reports in the literature.7 Similar to our patient, there are reports of pityriasis rosea developing after the second dose of the vaccine, with some patients reporting a reactivation of skin lesions.8 There is a paucity of reports describing pityriasis rosea associated with the influenza vaccine, hepatitis B vaccine, and human papillomavirus vaccine.3 In such cases, the onset of skin lesions was thought to be related to vaccine-induced stimulation of the immune system or a component of the vaccine.
Conclusion
We presented a unique case of pityriasis rosea following COVID-19 vaccination. Because additional laboratory workup and a skin biopsy were not performed, we are unable to infer causation. However, the classic clinical presentation, rash development within 3 days of vaccination, and prior reports of vaccine-associated pityriasis rosea strengthen the aforementioned association. We hope this case adds to the growing understanding of the novel COVID-19 vaccine. As more individuals become vaccinated, both clinicians and patients should be aware of this benign cutaneous eruption that can develop following COVID-19 vaccination.
Pityriasis rosea is a papulosquamous eruption that favors the trunk and proximal extremities. It occurs most commonly in adolescents and young adults.1 The rash typically presents with a solitary lesion, known as a “herald patch,” which is followed by a scaly erythematous eruption along the cleavage lines of the skin. The condition is self-limited and often resolves in 6 to 8 weeks. Recent evidence suggests that viral reactivation of human herpesvirus 6 and human herpesvirus 7 may play a role in the development of skin lesions.2 Pityriasis rosea also has been reported following the administration of new medications and vaccinations.1-3 We report a case of a 30-year-old woman who developed pityriasis rosea 3 days after receiving the second dose of the COVID-19 vaccine.
Case Report
A 30-year-old woman presented to the dermatology office for evaluation of a rash on the trunk and upper extremities that had been present for 5 days. She reported an initial solitary lesion on the left upper back, subsequently followed by the appearance of a mildly pruritic rash on the trunk and upper extremities. The rash first appeared 3 days after she received the second dose of the Pfizer-BioNTech COVID-19 vaccine. She was otherwise asymptomatic after vaccination and denied fever, chills, headache, and myalgia. She denied any rash following her first dose of the COVID-19 vaccine, history of known COVID-19 infection or exposures, or new medications. Notably, the patient worked in health care.
Physical examination revealed a 2-cm, erythematous, thin, scaly plaque over the left side of the upper back (Figure, A). Erythematous, scaly, thin papules of varying sizes were distributed along the cleavage lines of the trunk and upper extremities (Figure, B). No biopsy was performed because of the classic clinical presentation of this self-limited condition and the patient’s history of hypertrophic scarring. No additional laboratory workup was performed. She was prescribed triamcinolone cream 0.1% as needed for pruritus and was reassured about the benign nature of this cutaneous eruption.
Comment
A broad spectrum of cutaneous manifestations has been reported in association with acute COVID-19 infection, including a papulovesicular rash, perniolike eruptions, urticaria, livedo reticularis, and petechiae.4 Several cases of pityriasis rosea in association with acute COVID-19 infection also have been reported.5 COVID-19 infection has been linked to reactivation of the herpesvirus, which may explain the connection between acute COVID-19 infection and the development of pityriasis rosea.6 Pityriasis rosea associated with administration of the COVID-19 vaccine is a rare complication with few reports in the literature.7 Similar to our patient, there are reports of pityriasis rosea developing after the second dose of the vaccine, with some patients reporting a reactivation of skin lesions.8 There is a paucity of reports describing pityriasis rosea associated with the influenza vaccine, hepatitis B vaccine, and human papillomavirus vaccine.3 In such cases, the onset of skin lesions was thought to be related to vaccine-induced stimulation of the immune system or a component of the vaccine.
Conclusion
We presented a unique case of pityriasis rosea following COVID-19 vaccination. Because additional laboratory workup and a skin biopsy were not performed, we are unable to infer causation. However, the classic clinical presentation, rash development within 3 days of vaccination, and prior reports of vaccine-associated pityriasis rosea strengthen the aforementioned association. We hope this case adds to the growing understanding of the novel COVID-19 vaccine. As more individuals become vaccinated, both clinicians and patients should be aware of this benign cutaneous eruption that can develop following COVID-19 vaccination.
- Papakostas D, Stavropoulos PG, Papafragkaki D, et al. An atypical case of pityriasis rosea gigantea after influenza vaccination. Case Rep Dermatol. 2014;6:119-123.
- Chen FJ, Chian CP, Chen YF, et al. Pityriasis rosea following influenza (H1N1) vaccination. J Chin Med Assoc. 2011;74:280-282.
- Li A, Li P, Li Y, et al. Recurrent pityriasis rosea: a case report. Hum Vaccin Immunother. 2018;4:1024-1026.
- Ng SM. Prolonged dermatological manifestation 4 weeks following recovery of COVID-19 in a child. BMJ Case Rep. 2020;13:e237056. doi:10.1136/bcr-2020-237056
- Johansen M, Chisolm SS, Aspey LD, et al. Pityriasis rosea in otherwise asymptomatic confirmed COVID-19-positive patients: a report of 2 cases. JAAD Case Rep. 2021;7:93-94.
- Dursun R, Temiz SA. The clinics of HHV-6 infection in COVID-19 pandemic: pityriasis rosea and Kawasaki disease. Dermatol Ther. 2020;33:e13730. doi:10.1111/dth.13730
- Leerunyakul K, Pakornphadungsit K, Suchonwanit P. Case report: pityriasis rosea-like eruption following COVID-19 vaccination [published online September 7, 2021]. Front Med. doi:10.3389/fmed.2021.752443
- Marcantonio-Santa Cruz OY, Vidal-Navarro A, Pesqué D, et al. Pityriasis rosea developing after COVID-19 vaccination. J Eur Acad Dermatol Venereol. 2021;35:E721-E722. doi:10.1111/jdv.17498
- Papakostas D, Stavropoulos PG, Papafragkaki D, et al. An atypical case of pityriasis rosea gigantea after influenza vaccination. Case Rep Dermatol. 2014;6:119-123.
- Chen FJ, Chian CP, Chen YF, et al. Pityriasis rosea following influenza (H1N1) vaccination. J Chin Med Assoc. 2011;74:280-282.
- Li A, Li P, Li Y, et al. Recurrent pityriasis rosea: a case report. Hum Vaccin Immunother. 2018;4:1024-1026.
- Ng SM. Prolonged dermatological manifestation 4 weeks following recovery of COVID-19 in a child. BMJ Case Rep. 2020;13:e237056. doi:10.1136/bcr-2020-237056
- Johansen M, Chisolm SS, Aspey LD, et al. Pityriasis rosea in otherwise asymptomatic confirmed COVID-19-positive patients: a report of 2 cases. JAAD Case Rep. 2021;7:93-94.
- Dursun R, Temiz SA. The clinics of HHV-6 infection in COVID-19 pandemic: pityriasis rosea and Kawasaki disease. Dermatol Ther. 2020;33:e13730. doi:10.1111/dth.13730
- Leerunyakul K, Pakornphadungsit K, Suchonwanit P. Case report: pityriasis rosea-like eruption following COVID-19 vaccination [published online September 7, 2021]. Front Med. doi:10.3389/fmed.2021.752443
- Marcantonio-Santa Cruz OY, Vidal-Navarro A, Pesqué D, et al. Pityriasis rosea developing after COVID-19 vaccination. J Eur Acad Dermatol Venereol. 2021;35:E721-E722. doi:10.1111/jdv.17498
Practice Points
- Clinicians should be aware of the association between COVID-19 vaccination and the development of pityriasis rosea.
- Pityriasis rosea has been linked to reactivation of human herpesvirus 6 and human herpesvirus 7 and has been reported following administration of the influenza and human papillomavirus vaccines.
- Pityriasis rosea is a self-limited, cutaneous eruption that resolves within 6 to 8 weeks, and patients should be educated on the benign nature of this condition.