User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Pityriasis Rosea Associated With COVID-19 Vaccination: A Common Rash Following Administration of a Novel Vaccine
Pityriasis rosea is a papulosquamous eruption that favors the trunk and proximal extremities. It occurs most commonly in adolescents and young adults.1 The rash typically presents with a solitary lesion, known as a “herald patch,” which is followed by a scaly erythematous eruption along the cleavage lines of the skin. The condition is self-limited and often resolves in 6 to 8 weeks. Recent evidence suggests that viral reactivation of human herpesvirus 6 and human herpesvirus 7 may play a role in the development of skin lesions.2 Pityriasis rosea also has been reported following the administration of new medications and vaccinations.1-3 We report a case of a 30-year-old woman who developed pityriasis rosea 3 days after receiving the second dose of the COVID-19 vaccine.
Case Report
A 30-year-old woman presented to the dermatology office for evaluation of a rash on the trunk and upper extremities that had been present for 5 days. She reported an initial solitary lesion on the left upper back, subsequently followed by the appearance of a mildly pruritic rash on the trunk and upper extremities. The rash first appeared 3 days after she received the second dose of the Pfizer-BioNTech COVID-19 vaccine. She was otherwise asymptomatic after vaccination and denied fever, chills, headache, and myalgia. She denied any rash following her first dose of the COVID-19 vaccine, history of known COVID-19 infection or exposures, or new medications. Notably, the patient worked in health care.
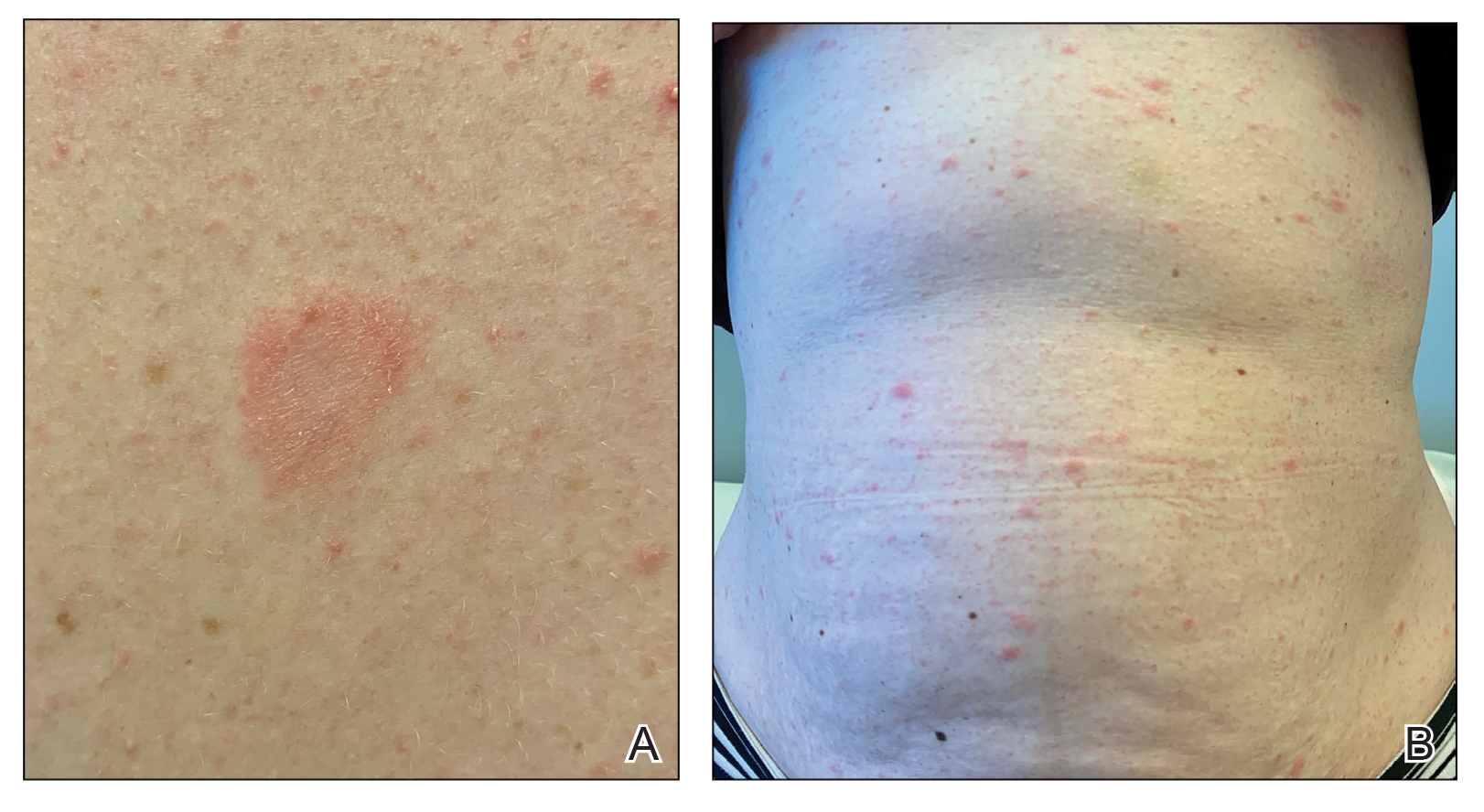
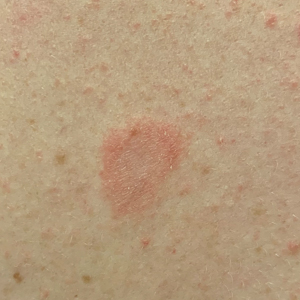
Physical examination revealed a 2-cm, erythematous, thin, scaly plaque over the left side of the upper back (Figure, A). Erythematous, scaly, thin papules of varying sizes were distributed along the cleavage lines of the trunk and upper extremities (Figure, B). No biopsy was performed because of the classic clinical presentation of this self-limited condition and the patient’s history of hypertrophic scarring. No additional laboratory workup was performed. She was prescribed triamcinolone cream 0.1% as needed for pruritus and was reassured about the benign nature of this cutaneous eruption.
Comment
A broad spectrum of cutaneous manifestations has been reported in association with acute COVID-19 infection, including a papulovesicular rash, perniolike eruptions, urticaria, livedo reticularis, and petechiae.4 Several cases of pityriasis rosea in association with acute COVID-19 infection also have been reported.5 COVID-19 infection has been linked to reactivation of the herpesvirus, which may explain the connection between acute COVID-19 infection and the development of pityriasis rosea.6 Pityriasis rosea associated with administration of the COVID-19 vaccine is a rare complication with few reports in the literature.7 Similar to our patient, there are reports of pityriasis rosea developing after the second dose of the vaccine, with some patients reporting a reactivation of skin lesions.8 There is a paucity of reports describing pityriasis rosea associated with the influenza vaccine, hepatitis B vaccine, and human papillomavirus vaccine.3 In such cases, the onset of skin lesions was thought to be related to vaccine-induced stimulation of the immune system or a component of the vaccine.
Conclusion
We presented a unique case of pityriasis rosea following COVID-19 vaccination. Because additional laboratory workup and a skin biopsy were not performed, we are unable to infer causation. However, the classic clinical presentation, rash development within 3 days of vaccination, and prior reports of vaccine-associated pityriasis rosea strengthen the aforementioned association. We hope this case adds to the growing understanding of the novel COVID-19 vaccine. As more individuals become vaccinated, both clinicians and patients should be aware of this benign cutaneous eruption that can develop following COVID-19 vaccination.
- Papakostas D, Stavropoulos PG, Papafragkaki D, et al. An atypical case of pityriasis rosea gigantea after influenza vaccination. Case Rep Dermatol. 2014;6:119-123.
- Chen FJ, Chian CP, Chen YF, et al. Pityriasis rosea following influenza (H1N1) vaccination. J Chin Med Assoc. 2011;74:280-282.
- Li A, Li P, Li Y, et al. Recurrent pityriasis rosea: a case report. Hum Vaccin Immunother. 2018;4:1024-1026.
- Ng SM. Prolonged dermatological manifestation 4 weeks following recovery of COVID-19 in a child. BMJ Case Rep. 2020;13:e237056. doi:10.1136/bcr-2020-237056
- Johansen M, Chisolm SS, Aspey LD, et al. Pityriasis rosea in otherwise asymptomatic confirmed COVID-19-positive patients: a report of 2 cases. JAAD Case Rep. 2021;7:93-94.
- Dursun R, Temiz SA. The clinics of HHV-6 infection in COVID-19 pandemic: pityriasis rosea and Kawasaki disease. Dermatol Ther. 2020;33:e13730. doi:10.1111/dth.13730
- Leerunyakul K, Pakornphadungsit K, Suchonwanit P. Case report: pityriasis rosea-like eruption following COVID-19 vaccination [published online September 7, 2021]. Front Med. doi:10.3389/fmed.2021.752443
- Marcantonio-Santa Cruz OY, Vidal-Navarro A, Pesqué D, et al. Pityriasis rosea developing after COVID-19 vaccination. J Eur Acad Dermatol Venereol. 2021;35:E721-E722. doi:10.1111/jdv.17498
Pityriasis rosea is a papulosquamous eruption that favors the trunk and proximal extremities. It occurs most commonly in adolescents and young adults.1 The rash typically presents with a solitary lesion, known as a “herald patch,” which is followed by a scaly erythematous eruption along the cleavage lines of the skin. The condition is self-limited and often resolves in 6 to 8 weeks. Recent evidence suggests that viral reactivation of human herpesvirus 6 and human herpesvirus 7 may play a role in the development of skin lesions.2 Pityriasis rosea also has been reported following the administration of new medications and vaccinations.1-3 We report a case of a 30-year-old woman who developed pityriasis rosea 3 days after receiving the second dose of the COVID-19 vaccine.
Case Report
A 30-year-old woman presented to the dermatology office for evaluation of a rash on the trunk and upper extremities that had been present for 5 days. She reported an initial solitary lesion on the left upper back, subsequently followed by the appearance of a mildly pruritic rash on the trunk and upper extremities. The rash first appeared 3 days after she received the second dose of the Pfizer-BioNTech COVID-19 vaccine. She was otherwise asymptomatic after vaccination and denied fever, chills, headache, and myalgia. She denied any rash following her first dose of the COVID-19 vaccine, history of known COVID-19 infection or exposures, or new medications. Notably, the patient worked in health care.
Physical examination revealed a 2-cm, erythematous, thin, scaly plaque over the left side of the upper back (Figure, A). Erythematous, scaly, thin papules of varying sizes were distributed along the cleavage lines of the trunk and upper extremities (Figure, B). No biopsy was performed because of the classic clinical presentation of this self-limited condition and the patient’s history of hypertrophic scarring. No additional laboratory workup was performed. She was prescribed triamcinolone cream 0.1% as needed for pruritus and was reassured about the benign nature of this cutaneous eruption.
Comment
A broad spectrum of cutaneous manifestations has been reported in association with acute COVID-19 infection, including a papulovesicular rash, perniolike eruptions, urticaria, livedo reticularis, and petechiae.4 Several cases of pityriasis rosea in association with acute COVID-19 infection also have been reported.5 COVID-19 infection has been linked to reactivation of the herpesvirus, which may explain the connection between acute COVID-19 infection and the development of pityriasis rosea.6 Pityriasis rosea associated with administration of the COVID-19 vaccine is a rare complication with few reports in the literature.7 Similar to our patient, there are reports of pityriasis rosea developing after the second dose of the vaccine, with some patients reporting a reactivation of skin lesions.8 There is a paucity of reports describing pityriasis rosea associated with the influenza vaccine, hepatitis B vaccine, and human papillomavirus vaccine.3 In such cases, the onset of skin lesions was thought to be related to vaccine-induced stimulation of the immune system or a component of the vaccine.
Conclusion
We presented a unique case of pityriasis rosea following COVID-19 vaccination. Because additional laboratory workup and a skin biopsy were not performed, we are unable to infer causation. However, the classic clinical presentation, rash development within 3 days of vaccination, and prior reports of vaccine-associated pityriasis rosea strengthen the aforementioned association. We hope this case adds to the growing understanding of the novel COVID-19 vaccine. As more individuals become vaccinated, both clinicians and patients should be aware of this benign cutaneous eruption that can develop following COVID-19 vaccination.
Pityriasis rosea is a papulosquamous eruption that favors the trunk and proximal extremities. It occurs most commonly in adolescents and young adults.1 The rash typically presents with a solitary lesion, known as a “herald patch,” which is followed by a scaly erythematous eruption along the cleavage lines of the skin. The condition is self-limited and often resolves in 6 to 8 weeks. Recent evidence suggests that viral reactivation of human herpesvirus 6 and human herpesvirus 7 may play a role in the development of skin lesions.2 Pityriasis rosea also has been reported following the administration of new medications and vaccinations.1-3 We report a case of a 30-year-old woman who developed pityriasis rosea 3 days after receiving the second dose of the COVID-19 vaccine.
Case Report
A 30-year-old woman presented to the dermatology office for evaluation of a rash on the trunk and upper extremities that had been present for 5 days. She reported an initial solitary lesion on the left upper back, subsequently followed by the appearance of a mildly pruritic rash on the trunk and upper extremities. The rash first appeared 3 days after she received the second dose of the Pfizer-BioNTech COVID-19 vaccine. She was otherwise asymptomatic after vaccination and denied fever, chills, headache, and myalgia. She denied any rash following her first dose of the COVID-19 vaccine, history of known COVID-19 infection or exposures, or new medications. Notably, the patient worked in health care.
Physical examination revealed a 2-cm, erythematous, thin, scaly plaque over the left side of the upper back (Figure, A). Erythematous, scaly, thin papules of varying sizes were distributed along the cleavage lines of the trunk and upper extremities (Figure, B). No biopsy was performed because of the classic clinical presentation of this self-limited condition and the patient’s history of hypertrophic scarring. No additional laboratory workup was performed. She was prescribed triamcinolone cream 0.1% as needed for pruritus and was reassured about the benign nature of this cutaneous eruption.
Comment
A broad spectrum of cutaneous manifestations has been reported in association with acute COVID-19 infection, including a papulovesicular rash, perniolike eruptions, urticaria, livedo reticularis, and petechiae.4 Several cases of pityriasis rosea in association with acute COVID-19 infection also have been reported.5 COVID-19 infection has been linked to reactivation of the herpesvirus, which may explain the connection between acute COVID-19 infection and the development of pityriasis rosea.6 Pityriasis rosea associated with administration of the COVID-19 vaccine is a rare complication with few reports in the literature.7 Similar to our patient, there are reports of pityriasis rosea developing after the second dose of the vaccine, with some patients reporting a reactivation of skin lesions.8 There is a paucity of reports describing pityriasis rosea associated with the influenza vaccine, hepatitis B vaccine, and human papillomavirus vaccine.3 In such cases, the onset of skin lesions was thought to be related to vaccine-induced stimulation of the immune system or a component of the vaccine.
Conclusion
We presented a unique case of pityriasis rosea following COVID-19 vaccination. Because additional laboratory workup and a skin biopsy were not performed, we are unable to infer causation. However, the classic clinical presentation, rash development within 3 days of vaccination, and prior reports of vaccine-associated pityriasis rosea strengthen the aforementioned association. We hope this case adds to the growing understanding of the novel COVID-19 vaccine. As more individuals become vaccinated, both clinicians and patients should be aware of this benign cutaneous eruption that can develop following COVID-19 vaccination.
- Papakostas D, Stavropoulos PG, Papafragkaki D, et al. An atypical case of pityriasis rosea gigantea after influenza vaccination. Case Rep Dermatol. 2014;6:119-123.
- Chen FJ, Chian CP, Chen YF, et al. Pityriasis rosea following influenza (H1N1) vaccination. J Chin Med Assoc. 2011;74:280-282.
- Li A, Li P, Li Y, et al. Recurrent pityriasis rosea: a case report. Hum Vaccin Immunother. 2018;4:1024-1026.
- Ng SM. Prolonged dermatological manifestation 4 weeks following recovery of COVID-19 in a child. BMJ Case Rep. 2020;13:e237056. doi:10.1136/bcr-2020-237056
- Johansen M, Chisolm SS, Aspey LD, et al. Pityriasis rosea in otherwise asymptomatic confirmed COVID-19-positive patients: a report of 2 cases. JAAD Case Rep. 2021;7:93-94.
- Dursun R, Temiz SA. The clinics of HHV-6 infection in COVID-19 pandemic: pityriasis rosea and Kawasaki disease. Dermatol Ther. 2020;33:e13730. doi:10.1111/dth.13730
- Leerunyakul K, Pakornphadungsit K, Suchonwanit P. Case report: pityriasis rosea-like eruption following COVID-19 vaccination [published online September 7, 2021]. Front Med. doi:10.3389/fmed.2021.752443
- Marcantonio-Santa Cruz OY, Vidal-Navarro A, Pesqué D, et al. Pityriasis rosea developing after COVID-19 vaccination. J Eur Acad Dermatol Venereol. 2021;35:E721-E722. doi:10.1111/jdv.17498
- Papakostas D, Stavropoulos PG, Papafragkaki D, et al. An atypical case of pityriasis rosea gigantea after influenza vaccination. Case Rep Dermatol. 2014;6:119-123.
- Chen FJ, Chian CP, Chen YF, et al. Pityriasis rosea following influenza (H1N1) vaccination. J Chin Med Assoc. 2011;74:280-282.
- Li A, Li P, Li Y, et al. Recurrent pityriasis rosea: a case report. Hum Vaccin Immunother. 2018;4:1024-1026.
- Ng SM. Prolonged dermatological manifestation 4 weeks following recovery of COVID-19 in a child. BMJ Case Rep. 2020;13:e237056. doi:10.1136/bcr-2020-237056
- Johansen M, Chisolm SS, Aspey LD, et al. Pityriasis rosea in otherwise asymptomatic confirmed COVID-19-positive patients: a report of 2 cases. JAAD Case Rep. 2021;7:93-94.
- Dursun R, Temiz SA. The clinics of HHV-6 infection in COVID-19 pandemic: pityriasis rosea and Kawasaki disease. Dermatol Ther. 2020;33:e13730. doi:10.1111/dth.13730
- Leerunyakul K, Pakornphadungsit K, Suchonwanit P. Case report: pityriasis rosea-like eruption following COVID-19 vaccination [published online September 7, 2021]. Front Med. doi:10.3389/fmed.2021.752443
- Marcantonio-Santa Cruz OY, Vidal-Navarro A, Pesqué D, et al. Pityriasis rosea developing after COVID-19 vaccination. J Eur Acad Dermatol Venereol. 2021;35:E721-E722. doi:10.1111/jdv.17498
Practice Points
- Clinicians should be aware of the association between COVID-19 vaccination and the development of pityriasis rosea.
- Pityriasis rosea has been linked to reactivation of human herpesvirus 6 and human herpesvirus 7 and has been reported following administration of the influenza and human papillomavirus vaccines.
- Pityriasis rosea is a self-limited, cutaneous eruption that resolves within 6 to 8 weeks, and patients should be educated on the benign nature of this condition.
Periungual Papules in an Elderly Woman
The Diagnosis: Multicentric Reticulohistiocytosis
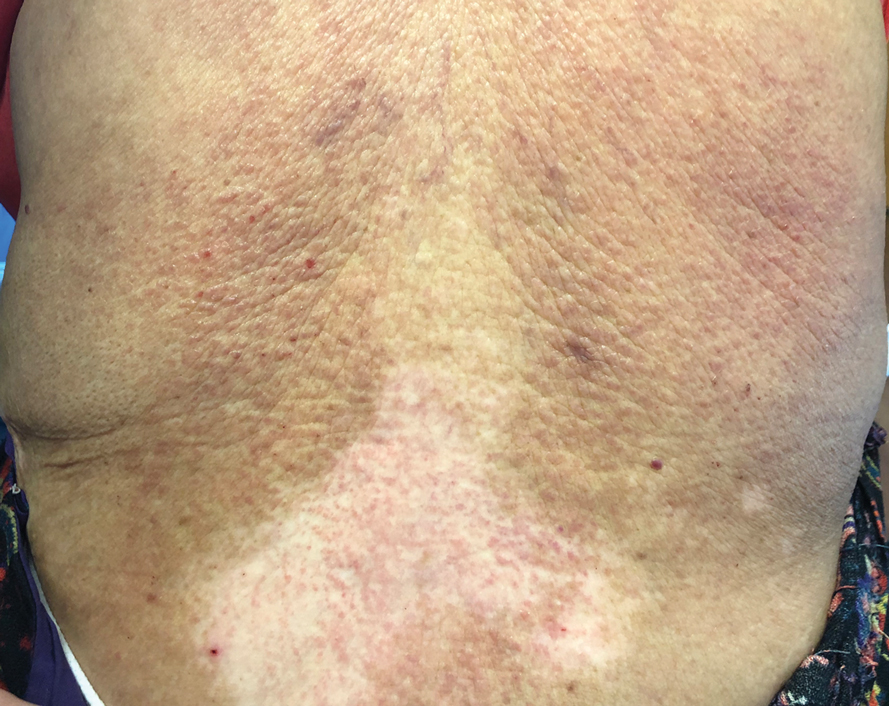
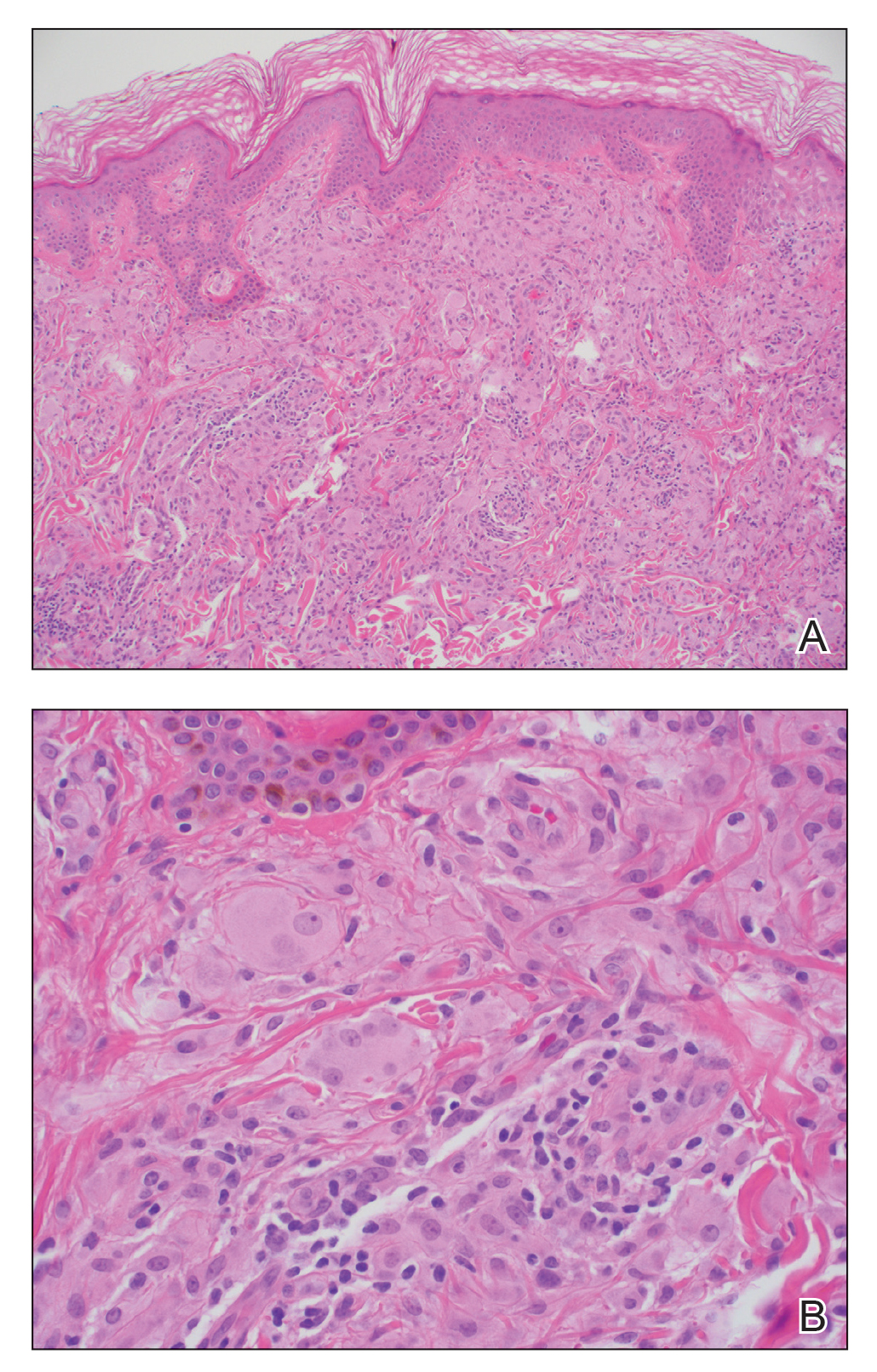
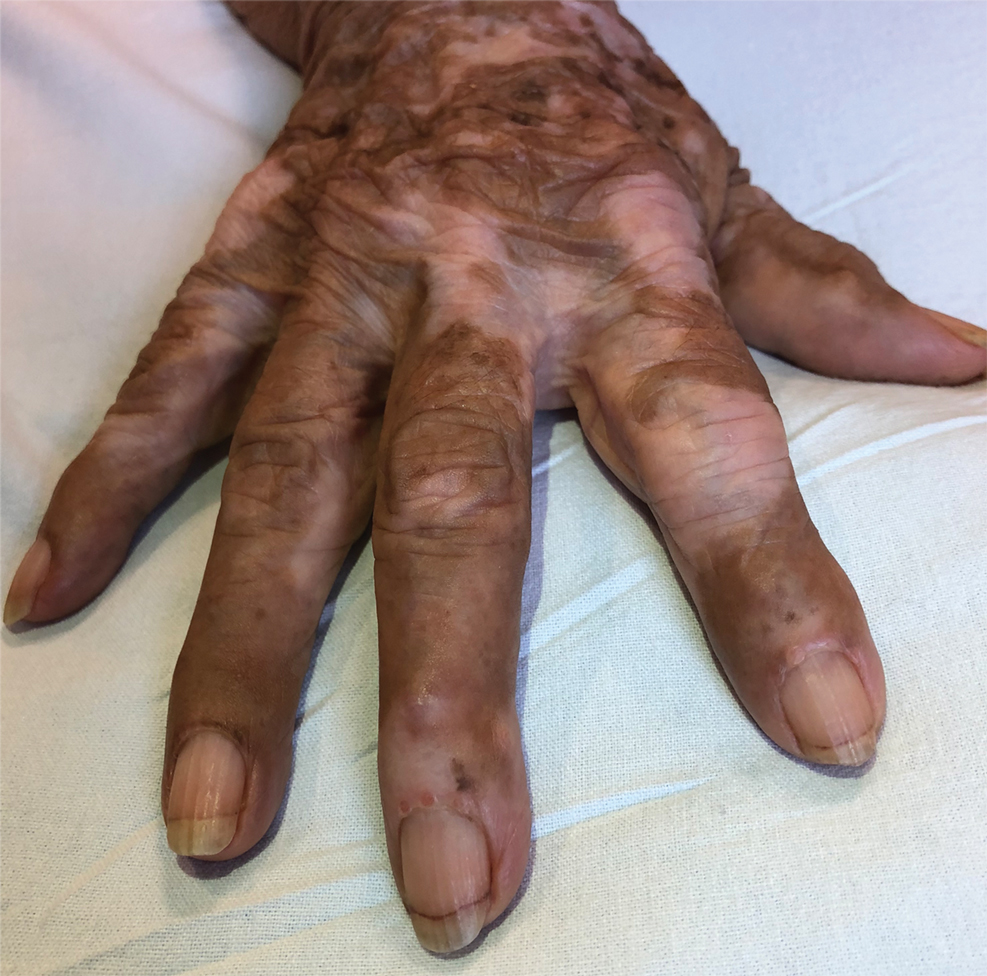
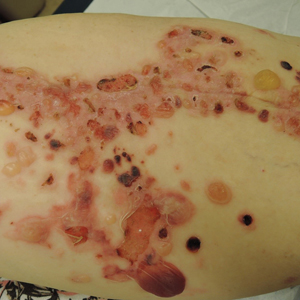
Te patient presented with pink papules coalescing into plaques on the upper chest and lower back (Figure 1) as well as a characteristic finding of periungual papules with a coral bead appearance. Histopathologic examination revealed a dense infiltrate of epithelioid histiocytes with amphophilic ground-glass cytoplasm in a nodular configuration (Figure 2). This pattern in conjunction with the clinical features seen in our patient was consistent with a diagnosis of multicentric reticulohistiocytosis (MRH).1-3 The cutaneous symptoms were managed with triamcinolone ointment 0.1% twice daily and oral hydroxyzine 10 mg 3 times daily as needed for itching with moderate improvement. She was referred to rheumatology for arthritis management, and the initial cancer screening was negative.
Multicentric reticulohistiocytosis is a rare granulomatous disease characterized by papulonodular cutaneous lesions and severe erosive arthritis. It has an insidious onset and most commonly affects middle-aged women.1 Multicentric reticulohistiocytosis typically presents as rounded pruritic papules or nodules that may be pink, red, or brown primarily affecting the face and distal upper extremities.1,3 Mucosal involvement occurs in more than half of patients and is characterized by multiple erythematous papules and nodules on the oral and nasopharyngeal mucosae that rarely can produce leonine facies.2 A hallmark feature of MRH is the presence of multiple shiny erythematous papules along the proximal and lateral nail folds that take on a coral bead appearance.1,3,4 Furthermore, nail changes such as atrophy, longitudinal ridging, brittleness, and hyperpigmentation can occur secondary to a synovial reaction that disturbs the nail matrix.4,5
Joint involvement precedes cutaneous involvement in most cases of MRH.1,5 Multicentric reticulohistiocytosis is associated with a symmetric destructive arthritis affecting the hands, knees, shoulders, and hips that often is associated with pain, stiffness, and swelling.1,3 The arthritis rapidly progresses in the early stages of the disease but then becomes less active over the subsequent 8 to 10 years.1 It has the potential to develop into arthritis mutilans, an end-stage form of arthritis also seen in psoriatic and rheumatoid arthritis that leads to severe joint deformity and debilitation.1,2
The etiology of MRH still is unknown, but it has an association with underlying malignancy in up to 25% of patients.6 Multicentric reticulohistiocytosis has been reported in the context of a wide variety of malignancies including melanoma; sarcoma; lymphoma; leukemia; and carcinomas of the breast, colon, and lung. In some cases, the diagnosis of MRH may even precede the diagnosis of cancer.3 Multicentric reticulohistiocytosis also may be associated with autoimmune conditions,3 as seen in our patient who had a history of both hypothyroidism and vitiligo.
Histopathologic examination is essential in distinguishing MRH from other autoimmune disorders associated with hand lesions, rash, and arthralgia. Erythema elevatum diutinum is associated with symmetric, violaceous, red or brown papules and plaques located on the extensor surfaces of the extremities and hands; however, histology reveals a leukocytoclastic vasculitis with a mixture of polymorphonuclear leukocytes and lymphocytes.7 Dermatomyositis may present with arthralgia, flattopped, erythematous (Gottron) papules localized over the proximal interphalangeal and distal interphalangeal joints, as well as proximal nail findings. The latter generally presents with periungual erythema associated with dilated capillary loops rather than the discrete orderly papules seen in MRH. Histologic examination of dermatomyositis shows mild epidermal atrophy, vacuolar changes in the basal keratinocyte layer, and a dermal perivascular lymphocytic infiltrate.8 Because MRH initially can present with joint symptoms and hand nodules, it may be confused with rheumatoid arthritis. However, rheumatoid arthritis typically is associated with severe osteopenia and tends to affect the metacarpophalangeal and proximal interphalangeal joints rather than the distal interphalangeal joints that most often are affected in MRH.1 Histologic examination of rheumatoid nodules reveals palisading granulomas surrounding a central area of fibrinoid necrosis.9 Sarcoidosis is a multisystem disease that can present with cutaneous involvement including erythema nodosum, skin plaques, subcutaneous nodules, and papular eruptions in addition to joint lesions.10 Sarcoidosis most frequently involves the lungs, manifesting as diffuse interstitial lung disease with bilateral hilar lymphadenopathy. Furthermore, histologic examination of lesions demonstrates classic noncaseating granulomas containing epithelioid cells, multinucleated giant cells with inclusion bodies, and lymphocytes.11
A skin biopsy is required to establish the diagnosis of MRH. In general, patients with MRH and no underlying malignancy have a good prognosis and respond to anti-inflammatory therapies such as nonsteroidal antiinflammatory drugs and corticosteroids. Other agents including methotrexate, cyclophosphamide, and tumor necrosis factor α inhibitors also have been effective in more severe cases.1,3,12 Finally, in addition to treating the cutaneous manifestations of MRH, it is important to screen patients for underlying malignancies and other autoimmune conditions.
- Tajirian AL, Malik MK, Robinson-Bostom L, et al. Multicentric reticulohistiocytosis. Clin Dermatol. 2006;24:486-492.
- Gold RH, Metzger AL, Mirra JM, et al. Multicentric reticulohistiocytosis (lipoid dermato-arthritis). an erosive polyarthritis with distinctive clinical, roentgenographic and pathologic features. Am J Roentgenol Radium Ther Nucl Med. 1975;124:610-624.
- Luz FB, Gaspar TAP, Kalil-Gaspar N, et al. Multicentric reticulohistiocytosis. J Eur Acad Dermatol Venereol. 2001;15:524-531.
- Barrow MV. The nails in multicentric reticulohistiocytosis. (lipoid dermato-arthritis). Arch Dermatol. 1967;95:200-201.
- Barrow MV, Holubar K. Multicentric reticulohistiocytosis. a review of 33 patients. Medicine (Baltimore). 1969;48:287-305.
- Snow JL, Muller SA. Malignancy-associated multicentric reticulohistiocytosis: a clinical, histological and immunophenotypic study. Br J Dermatol. 1995;133:71-76.
- Yiannias JA, el-Azhary RA, Gibson LE. Erythema elevatum diutinum: a clinical and histopathologic study of 13 patients. J Am Acad Dermatol. 1992;26:38-44.
- Smith ES, Hallman JR, DeLuca AM, et al. Dermatomyositis: a clinicopathological study of 40 patients. Am J Dermatopathol. 2009; 31:61-67.
- Athanasou NA, Quinn J, Woods CG, et al. Immunohistology of rheumatoid nodules and rheumatoid synovium. Ann Rheum Dis. 1988;47:398-403.
- Yanardag H, Pamuk ON, Karayel T. Cutaneous involvement in sarcoidosis: analysis of the features in 170 patients. Respir Med. 2003;97:978-982.
- Ma Y, Gal A, Koss MN. The pathology of pulmonary sarcoidosis: update. Semin Diagn Pathol. 2007;24:150-161.
- Kovach BT, Calamia KT, Walsh JS, et al. Treatment of multicentric reticulohistiocytosis with etanercept. Arch Dermatol. 2004;140:919-921.
The Diagnosis: Multicentric Reticulohistiocytosis
Te patient presented with pink papules coalescing into plaques on the upper chest and lower back (Figure 1) as well as a characteristic finding of periungual papules with a coral bead appearance. Histopathologic examination revealed a dense infiltrate of epithelioid histiocytes with amphophilic ground-glass cytoplasm in a nodular configuration (Figure 2). This pattern in conjunction with the clinical features seen in our patient was consistent with a diagnosis of multicentric reticulohistiocytosis (MRH).1-3 The cutaneous symptoms were managed with triamcinolone ointment 0.1% twice daily and oral hydroxyzine 10 mg 3 times daily as needed for itching with moderate improvement. She was referred to rheumatology for arthritis management, and the initial cancer screening was negative.
Multicentric reticulohistiocytosis is a rare granulomatous disease characterized by papulonodular cutaneous lesions and severe erosive arthritis. It has an insidious onset and most commonly affects middle-aged women.1 Multicentric reticulohistiocytosis typically presents as rounded pruritic papules or nodules that may be pink, red, or brown primarily affecting the face and distal upper extremities.1,3 Mucosal involvement occurs in more than half of patients and is characterized by multiple erythematous papules and nodules on the oral and nasopharyngeal mucosae that rarely can produce leonine facies.2 A hallmark feature of MRH is the presence of multiple shiny erythematous papules along the proximal and lateral nail folds that take on a coral bead appearance.1,3,4 Furthermore, nail changes such as atrophy, longitudinal ridging, brittleness, and hyperpigmentation can occur secondary to a synovial reaction that disturbs the nail matrix.4,5
Joint involvement precedes cutaneous involvement in most cases of MRH.1,5 Multicentric reticulohistiocytosis is associated with a symmetric destructive arthritis affecting the hands, knees, shoulders, and hips that often is associated with pain, stiffness, and swelling.1,3 The arthritis rapidly progresses in the early stages of the disease but then becomes less active over the subsequent 8 to 10 years.1 It has the potential to develop into arthritis mutilans, an end-stage form of arthritis also seen in psoriatic and rheumatoid arthritis that leads to severe joint deformity and debilitation.1,2
The etiology of MRH still is unknown, but it has an association with underlying malignancy in up to 25% of patients.6 Multicentric reticulohistiocytosis has been reported in the context of a wide variety of malignancies including melanoma; sarcoma; lymphoma; leukemia; and carcinomas of the breast, colon, and lung. In some cases, the diagnosis of MRH may even precede the diagnosis of cancer.3 Multicentric reticulohistiocytosis also may be associated with autoimmune conditions,3 as seen in our patient who had a history of both hypothyroidism and vitiligo.
Histopathologic examination is essential in distinguishing MRH from other autoimmune disorders associated with hand lesions, rash, and arthralgia. Erythema elevatum diutinum is associated with symmetric, violaceous, red or brown papules and plaques located on the extensor surfaces of the extremities and hands; however, histology reveals a leukocytoclastic vasculitis with a mixture of polymorphonuclear leukocytes and lymphocytes.7 Dermatomyositis may present with arthralgia, flattopped, erythematous (Gottron) papules localized over the proximal interphalangeal and distal interphalangeal joints, as well as proximal nail findings. The latter generally presents with periungual erythema associated with dilated capillary loops rather than the discrete orderly papules seen in MRH. Histologic examination of dermatomyositis shows mild epidermal atrophy, vacuolar changes in the basal keratinocyte layer, and a dermal perivascular lymphocytic infiltrate.8 Because MRH initially can present with joint symptoms and hand nodules, it may be confused with rheumatoid arthritis. However, rheumatoid arthritis typically is associated with severe osteopenia and tends to affect the metacarpophalangeal and proximal interphalangeal joints rather than the distal interphalangeal joints that most often are affected in MRH.1 Histologic examination of rheumatoid nodules reveals palisading granulomas surrounding a central area of fibrinoid necrosis.9 Sarcoidosis is a multisystem disease that can present with cutaneous involvement including erythema nodosum, skin plaques, subcutaneous nodules, and papular eruptions in addition to joint lesions.10 Sarcoidosis most frequently involves the lungs, manifesting as diffuse interstitial lung disease with bilateral hilar lymphadenopathy. Furthermore, histologic examination of lesions demonstrates classic noncaseating granulomas containing epithelioid cells, multinucleated giant cells with inclusion bodies, and lymphocytes.11
A skin biopsy is required to establish the diagnosis of MRH. In general, patients with MRH and no underlying malignancy have a good prognosis and respond to anti-inflammatory therapies such as nonsteroidal antiinflammatory drugs and corticosteroids. Other agents including methotrexate, cyclophosphamide, and tumor necrosis factor α inhibitors also have been effective in more severe cases.1,3,12 Finally, in addition to treating the cutaneous manifestations of MRH, it is important to screen patients for underlying malignancies and other autoimmune conditions.
The Diagnosis: Multicentric Reticulohistiocytosis
Te patient presented with pink papules coalescing into plaques on the upper chest and lower back (Figure 1) as well as a characteristic finding of periungual papules with a coral bead appearance. Histopathologic examination revealed a dense infiltrate of epithelioid histiocytes with amphophilic ground-glass cytoplasm in a nodular configuration (Figure 2). This pattern in conjunction with the clinical features seen in our patient was consistent with a diagnosis of multicentric reticulohistiocytosis (MRH).1-3 The cutaneous symptoms were managed with triamcinolone ointment 0.1% twice daily and oral hydroxyzine 10 mg 3 times daily as needed for itching with moderate improvement. She was referred to rheumatology for arthritis management, and the initial cancer screening was negative.
Multicentric reticulohistiocytosis is a rare granulomatous disease characterized by papulonodular cutaneous lesions and severe erosive arthritis. It has an insidious onset and most commonly affects middle-aged women.1 Multicentric reticulohistiocytosis typically presents as rounded pruritic papules or nodules that may be pink, red, or brown primarily affecting the face and distal upper extremities.1,3 Mucosal involvement occurs in more than half of patients and is characterized by multiple erythematous papules and nodules on the oral and nasopharyngeal mucosae that rarely can produce leonine facies.2 A hallmark feature of MRH is the presence of multiple shiny erythematous papules along the proximal and lateral nail folds that take on a coral bead appearance.1,3,4 Furthermore, nail changes such as atrophy, longitudinal ridging, brittleness, and hyperpigmentation can occur secondary to a synovial reaction that disturbs the nail matrix.4,5
Joint involvement precedes cutaneous involvement in most cases of MRH.1,5 Multicentric reticulohistiocytosis is associated with a symmetric destructive arthritis affecting the hands, knees, shoulders, and hips that often is associated with pain, stiffness, and swelling.1,3 The arthritis rapidly progresses in the early stages of the disease but then becomes less active over the subsequent 8 to 10 years.1 It has the potential to develop into arthritis mutilans, an end-stage form of arthritis also seen in psoriatic and rheumatoid arthritis that leads to severe joint deformity and debilitation.1,2
The etiology of MRH still is unknown, but it has an association with underlying malignancy in up to 25% of patients.6 Multicentric reticulohistiocytosis has been reported in the context of a wide variety of malignancies including melanoma; sarcoma; lymphoma; leukemia; and carcinomas of the breast, colon, and lung. In some cases, the diagnosis of MRH may even precede the diagnosis of cancer.3 Multicentric reticulohistiocytosis also may be associated with autoimmune conditions,3 as seen in our patient who had a history of both hypothyroidism and vitiligo.
Histopathologic examination is essential in distinguishing MRH from other autoimmune disorders associated with hand lesions, rash, and arthralgia. Erythema elevatum diutinum is associated with symmetric, violaceous, red or brown papules and plaques located on the extensor surfaces of the extremities and hands; however, histology reveals a leukocytoclastic vasculitis with a mixture of polymorphonuclear leukocytes and lymphocytes.7 Dermatomyositis may present with arthralgia, flattopped, erythematous (Gottron) papules localized over the proximal interphalangeal and distal interphalangeal joints, as well as proximal nail findings. The latter generally presents with periungual erythema associated with dilated capillary loops rather than the discrete orderly papules seen in MRH. Histologic examination of dermatomyositis shows mild epidermal atrophy, vacuolar changes in the basal keratinocyte layer, and a dermal perivascular lymphocytic infiltrate.8 Because MRH initially can present with joint symptoms and hand nodules, it may be confused with rheumatoid arthritis. However, rheumatoid arthritis typically is associated with severe osteopenia and tends to affect the metacarpophalangeal and proximal interphalangeal joints rather than the distal interphalangeal joints that most often are affected in MRH.1 Histologic examination of rheumatoid nodules reveals palisading granulomas surrounding a central area of fibrinoid necrosis.9 Sarcoidosis is a multisystem disease that can present with cutaneous involvement including erythema nodosum, skin plaques, subcutaneous nodules, and papular eruptions in addition to joint lesions.10 Sarcoidosis most frequently involves the lungs, manifesting as diffuse interstitial lung disease with bilateral hilar lymphadenopathy. Furthermore, histologic examination of lesions demonstrates classic noncaseating granulomas containing epithelioid cells, multinucleated giant cells with inclusion bodies, and lymphocytes.11
A skin biopsy is required to establish the diagnosis of MRH. In general, patients with MRH and no underlying malignancy have a good prognosis and respond to anti-inflammatory therapies such as nonsteroidal antiinflammatory drugs and corticosteroids. Other agents including methotrexate, cyclophosphamide, and tumor necrosis factor α inhibitors also have been effective in more severe cases.1,3,12 Finally, in addition to treating the cutaneous manifestations of MRH, it is important to screen patients for underlying malignancies and other autoimmune conditions.
- Tajirian AL, Malik MK, Robinson-Bostom L, et al. Multicentric reticulohistiocytosis. Clin Dermatol. 2006;24:486-492.
- Gold RH, Metzger AL, Mirra JM, et al. Multicentric reticulohistiocytosis (lipoid dermato-arthritis). an erosive polyarthritis with distinctive clinical, roentgenographic and pathologic features. Am J Roentgenol Radium Ther Nucl Med. 1975;124:610-624.
- Luz FB, Gaspar TAP, Kalil-Gaspar N, et al. Multicentric reticulohistiocytosis. J Eur Acad Dermatol Venereol. 2001;15:524-531.
- Barrow MV. The nails in multicentric reticulohistiocytosis. (lipoid dermato-arthritis). Arch Dermatol. 1967;95:200-201.
- Barrow MV, Holubar K. Multicentric reticulohistiocytosis. a review of 33 patients. Medicine (Baltimore). 1969;48:287-305.
- Snow JL, Muller SA. Malignancy-associated multicentric reticulohistiocytosis: a clinical, histological and immunophenotypic study. Br J Dermatol. 1995;133:71-76.
- Yiannias JA, el-Azhary RA, Gibson LE. Erythema elevatum diutinum: a clinical and histopathologic study of 13 patients. J Am Acad Dermatol. 1992;26:38-44.
- Smith ES, Hallman JR, DeLuca AM, et al. Dermatomyositis: a clinicopathological study of 40 patients. Am J Dermatopathol. 2009; 31:61-67.
- Athanasou NA, Quinn J, Woods CG, et al. Immunohistology of rheumatoid nodules and rheumatoid synovium. Ann Rheum Dis. 1988;47:398-403.
- Yanardag H, Pamuk ON, Karayel T. Cutaneous involvement in sarcoidosis: analysis of the features in 170 patients. Respir Med. 2003;97:978-982.
- Ma Y, Gal A, Koss MN. The pathology of pulmonary sarcoidosis: update. Semin Diagn Pathol. 2007;24:150-161.
- Kovach BT, Calamia KT, Walsh JS, et al. Treatment of multicentric reticulohistiocytosis with etanercept. Arch Dermatol. 2004;140:919-921.
- Tajirian AL, Malik MK, Robinson-Bostom L, et al. Multicentric reticulohistiocytosis. Clin Dermatol. 2006;24:486-492.
- Gold RH, Metzger AL, Mirra JM, et al. Multicentric reticulohistiocytosis (lipoid dermato-arthritis). an erosive polyarthritis with distinctive clinical, roentgenographic and pathologic features. Am J Roentgenol Radium Ther Nucl Med. 1975;124:610-624.
- Luz FB, Gaspar TAP, Kalil-Gaspar N, et al. Multicentric reticulohistiocytosis. J Eur Acad Dermatol Venereol. 2001;15:524-531.
- Barrow MV. The nails in multicentric reticulohistiocytosis. (lipoid dermato-arthritis). Arch Dermatol. 1967;95:200-201.
- Barrow MV, Holubar K. Multicentric reticulohistiocytosis. a review of 33 patients. Medicine (Baltimore). 1969;48:287-305.
- Snow JL, Muller SA. Malignancy-associated multicentric reticulohistiocytosis: a clinical, histological and immunophenotypic study. Br J Dermatol. 1995;133:71-76.
- Yiannias JA, el-Azhary RA, Gibson LE. Erythema elevatum diutinum: a clinical and histopathologic study of 13 patients. J Am Acad Dermatol. 1992;26:38-44.
- Smith ES, Hallman JR, DeLuca AM, et al. Dermatomyositis: a clinicopathological study of 40 patients. Am J Dermatopathol. 2009; 31:61-67.
- Athanasou NA, Quinn J, Woods CG, et al. Immunohistology of rheumatoid nodules and rheumatoid synovium. Ann Rheum Dis. 1988;47:398-403.
- Yanardag H, Pamuk ON, Karayel T. Cutaneous involvement in sarcoidosis: analysis of the features in 170 patients. Respir Med. 2003;97:978-982.
- Ma Y, Gal A, Koss MN. The pathology of pulmonary sarcoidosis: update. Semin Diagn Pathol. 2007;24:150-161.
- Kovach BT, Calamia KT, Walsh JS, et al. Treatment of multicentric reticulohistiocytosis with etanercept. Arch Dermatol. 2004;140:919-921.
A 79-year-old woman presented with pruritic papules and plaques on the chest, back, arms, hands, legs, and feet of 1 year’s duration. She reported a history of hypothyroidism, arthritis, and vitiligo but denied a history of cancer. Physical examination showed pink papules coalescing into plaques on the upper chest and lower back as well as lichenified plaques on the forearms and knees. Erythematous papules on the proximal nail folds of the right first and second digits also were noted. Multiple depigmented patches on the hands, wrists, arms, and lower back also were present, and deformities of the hands and bulbous-appearing knees were observed. Results from a complete blood cell count and blood chemistry analyses showed mild anemia but were otherwise normal. Radiography of the right knee showed degenerative changes and periarticular radiolucencies consistent with an inflammatory arthropathy. A 4-mm punch biopsy specimen from the back was obtained for histopathologic examination.
DRESS Syndrome Due to Cefdinir Mimicking Superinfected Eczema in a Pediatric Patient
To the Editor:
Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, or drug-induced hypersensitivity syndrome, is a serious and potentially fatal multiorgan drug hypersensitivity reaction. Drug reaction with eosinophilia and systemic symptoms syndrome shares many clinical features with viral exanthems and may be difficult to diagnose in the setting of atopic dermatitis (AD) in which children may have baseline eosinophilia from an atopic diathesis. The cutaneous exanthema also may be variable in presentation, further complicating diagnosis.1,2
A 3-year-old boy with AD since infancy and a history of anaphylaxis to peanuts presented to the emergency department with reported fever, rash, sore throat, and decreased oral intake. Ten days prior, the patient was treated for cellulitis of the left foot with a 7-day course of cefdinir with complete resolution of symptoms. Four days prior to admission, the patient started developing “bumps” on the face and fevers. He was seen at an outside facility, where a rapid test for Streptococcus was negative, and the patient was treated with ibuprofen and fluids for a presumed viral exanthem. The rash subsequently spread to involve the trunk and extremities. On the day of admission, the patient had a positive rapid test for Streptococcus and was referred to the emergency department with concern for superinfected eczema and eczema herpeticum. The patient recently traveled to Puerto Rico, where he had contact with an aunt with active herpes zoster but no other sick contacts. The patient’s immunizations were reported to be up-to-date.
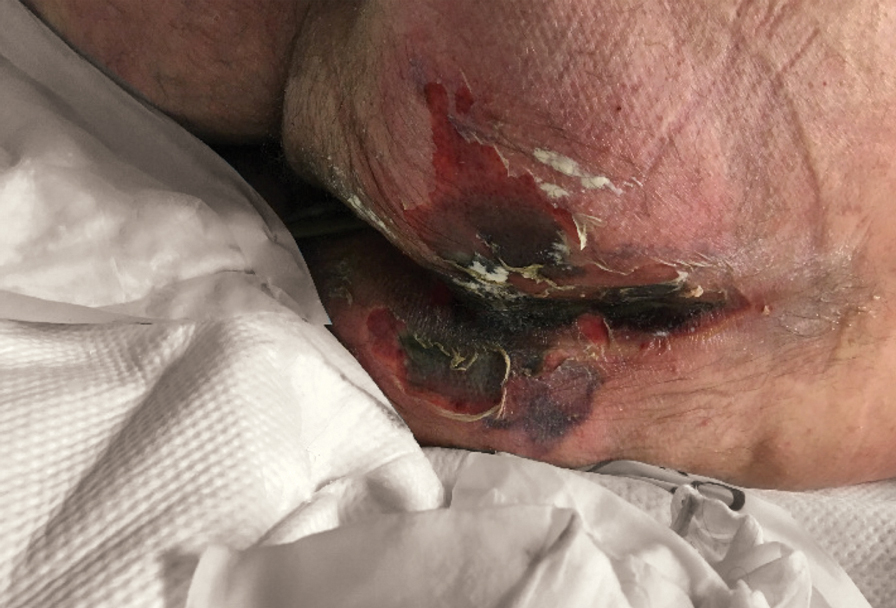
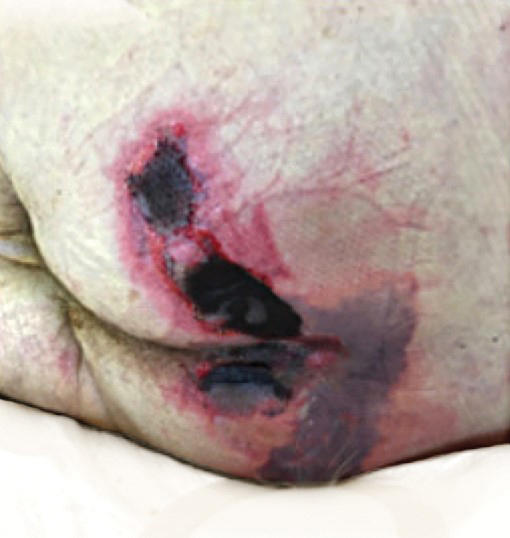
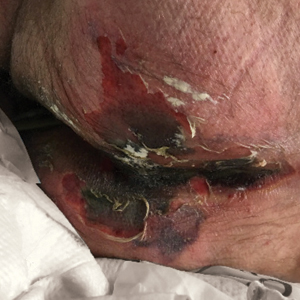
Physical examination revealed the patient was afebrile but irritable and had erythematous crusted papules and patches on the face, arms, and legs, as well as erythematous dry patches on the chest, abdomen, and back (Figure). There were no conjunctival erythematous or oral erosions. The patient was admitted to the hospital for presumed superinfected AD and possible eczema herpeticum. He was started on intravenous clindamycin and acyclovir.
The following day, the patient had new facial edema and fever (temperature, 102.8 °F [39.36 °C]) in addition to palpable mobile cervical, axillary, and inguinal lymphadenopathy. He also was noted to have notably worsening eosinophilia from 1288 (14%) to 2570 (29.2%) cells/µL (reference range, 0%–5%) and new-onset transaminitis. Herpes and varicella-zoster direct fluorescent antibody tests, culture, and serum polymerase chain reaction were all negative, and acyclovir was discontinued. Repeat laboratory tests 12 hours later showed a continued uptrend in transaminitis. Serologies for acute and chronic cytomegalovirus; Epstein-Barr virus; and hepatitis A, B, and C were all nonreactive. The patient was started on intravenous methylprednisolone 1 mg/kg daily for suspected DRESS syndrome likely due to cefdinir.
The patient’s eosinophilia completely resolved (from approximately 2600 to 100 cells/µL) after 1 dose of steroids, and his transaminitis trended down over the next few days. He remained afebrile for the remainder of his admission, and his facial swelling and rash continued to improve. Bacterial culture from the skin grew oxacillin-susceptible Staphylococcus aureus and group A Streptococcus pyogenes. A blood culture was negative. The patient was discharged home to complete a 10-day course of clindamycin and was given topical steroids for the eczema. He continued on oral prednisolone 1 mg/kg daily for 10 days, after which the dose was tapered down for a total 1-month course of systemic corticosteroids. At 1-month follow-up after completing the course of steroids, he was doing well with normal hepatic enzyme levels and no recurrence of fever, facial edema, or rash. He continues to be followed for management of the AD.
Drug reaction with eosinophilia and systemic symptoms syndrome is a serious systemic adverse drug reaction, with high morbidity and even mortality, estimated at 10% in the adult population, though more specific pediatric mortality data are not available.1,2 The exact pathogenesis of DRESS syndrome has not been elucidated. Certain human leukocyte antigen class I alleles are predisposed to the development of DRESS syndrome, but there has not been a human leukocyte antigen subtype identified with beta-lactam–associated DRESS syndrome. Some studies have demonstrated a reactivation of human herpesvirus 6, human herpesvirus 7, and Epstein-Barr virus.3 One study involving 40 patients with DRESS syndrome identified viremia in 76% (29/38) of patients and identified CD8+ T-cell populations directed toward viral epitopes.3 Finally, DRESS syndrome may be related to the slow detoxification and elimination of intermediary products of offending medications that serve as an immunogenic stimulus for the inflammatory cascade.2
In adults, DRESS syndrome was first identified in association with phenytoin, but more recently other drugs have been identified, including other aromatic anticonvulsants (ie, lamotrigine, phenobarbital, carbamazepine), allopurinol, sulfonamides, antiretrovirals (particularly abacavir), and minocycline.2 In a 3-year pediatric prospective study, 11 cases of DRESS syndrome were identified: 4 cases due to lamotrigine, and 3 caused by penicillins.4 The trigger in our patient’s case was the beta-lactam, third-generation cephalosporin cefdinir, and his symptoms developed within 6 days of starting the medication. Many articles report that beta-lactams are a rare cause of DRESS syndrome, with only a handful of cases reported.1,5,6
The diagnosis of DRESS syndrome often can be delayed, as children present acutely febrile and toxic appearing. Unlike many adverse drug reactions, DRESS syndrome does not show rapid resolution with withdrawal of the causative agent, further complicating the diagnosis. The typical onset of DRESS syndrome generally ranges from 2 to 6 weeks after the initiation of the offending drug; however, faster onset of symptoms, similar to our case, has been noted in antibiotic-triggered cases. In the prospective pediatric series by Sasidharanpillai et al,4 the average time to onset among 3 antibiotic-triggered DRESS cases was 5.8 days vs 23.9 days among the 4 cases of lamotrigine-associated DRESS syndrome.
Our patient demonstrated the classic features of DRESS syndrome, including fever, rash, lymphadenopathy, facial edema, peripheral eosinophilia, atypical lymphocytosis, and hepatitis. Based on the proposed RegiSCAR scoring system, our patient was classified as a “definite” case of DRESS syndrome.1,7 Other hematologic findings in DRESS syndrome may include thrombocytopenia and anemia. The liver is the most commonly affected internal organ in DRESS syndrome, with pneumonitis, carditis, and nephritis reported less frequently.1 The pattern of liver injury in our patient was mixed (hepatocellular and cholestatic), the second most common pattern in patients with DRESS syndrome (the cholestatic pattern is most common).8
The exanthem of DRESS syndrome can vary in morphology, with up to 7% of patients reported to have eczemalike lesions in the multinational prospective RegiSCAR study.1 Other entities in the differential diagnosis for our patient included Kawasaki disease, where conjunctivitis and strawberry tongue are classically present, as well as erythrodermic AD, where internal organ involvement is not common.2 Our patient’s exanthem initially was considered to be a flare of AD with superimposed bacterial infection and possible eczema herpeticum. Although bacterial cultures did grow Staphylococcus and Streptococcus, viral studies were all negative, and this alone would not have explained the facial edema, rapidly rising eosinophil count, and transaminitis. The dramatic drop in his eosinophil count and decrease in hepatic enzymes after 1 dose of intravenous methylprednisolone also supported the diagnosis of DRESS syndrome.
Treatment recommendations remain largely anecdotal. Early systemic steroids generally are accepted as the first line of therapy, with a slow taper. Although the average required duration of systemic steroids in 1 series of adults was reported at 50.1 days,9 the duration was shorter (21–35 days) in a series of pediatric patients.4 Our patient’s clinical symptoms and laboratory values normalized after completing a 1-month steroid taper. Other therapies have been tried for recalcitrant cases, including intravenous immunoglobulin, plasmapheresis, rituximab, and valganciclovir.2
Early clinical recognition of the signs and symptoms of DRESS syndrome in the setting of a new medication can decrease morbidity and mortality. Although DRESS syndrome in pediatric patients presents with many similar clinical features as in adults, it may be a greater diagnostic challenge. As in adult cases, timely administration of systemic corticosteroids and tapering based on clinical signs and symptoms can lead to resolution of the hypersensitivity syndrome.
- Kardaun SH, Sekula P, Valeyrie-Allanore L, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071-1080.
- Fernando SL. Drug-reaction eosinophilia and systemic symptoms and drug-induced hypersensitivity syndrome. Australas J Dermatol. 2014;55:15-23.
- Picard D, Janela B, Descamps V, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): a multiorgan antiviral T cell response. Sci Transl Med. 2010;2:46ra62.
- Sasidharanpillai S, Sabitha S, Riyaz N, et al. Drug reaction with eosinophilia and systemic symptoms in children: a prospective study. Pediatr Dermatol. 2016;33:E162-E165.
- Aouam K, Chaabane A, Toumi A, et al. Drug rash with eosinophilia and systemic symptoms (DRESS) probably induced by cefotaxime: a report of two cases. Clin Med Res. 2012;10:32-35.
- Guleria VS, Dhillon M, Gill S, et al. Ceftriaxone induced drug rash with eosinophilia and systemic symptoms. J Res Pharm Pract. 2014;3:72-74.
- Kardaun SH, Sidoroff A, Valeyrie-Allanore L, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol. 2007;156:609-611.
- Lin IC, Yang HC, Strong C, et al. Liver injury in patients with DRESS: a clinical study of 72 cases. J Am Acad Dermatol. 2015;72:984-991.
- Ang CC, Wang YS, Yoosuff EL, et al. Retrospective analysis of drug-induced hypersensitivity syndrome: a study of 27 patients. J Am Acad Dermatol. 2010;63:219-227.
To the Editor:
Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, or drug-induced hypersensitivity syndrome, is a serious and potentially fatal multiorgan drug hypersensitivity reaction. Drug reaction with eosinophilia and systemic symptoms syndrome shares many clinical features with viral exanthems and may be difficult to diagnose in the setting of atopic dermatitis (AD) in which children may have baseline eosinophilia from an atopic diathesis. The cutaneous exanthema also may be variable in presentation, further complicating diagnosis.1,2
A 3-year-old boy with AD since infancy and a history of anaphylaxis to peanuts presented to the emergency department with reported fever, rash, sore throat, and decreased oral intake. Ten days prior, the patient was treated for cellulitis of the left foot with a 7-day course of cefdinir with complete resolution of symptoms. Four days prior to admission, the patient started developing “bumps” on the face and fevers. He was seen at an outside facility, where a rapid test for Streptococcus was negative, and the patient was treated with ibuprofen and fluids for a presumed viral exanthem. The rash subsequently spread to involve the trunk and extremities. On the day of admission, the patient had a positive rapid test for Streptococcus and was referred to the emergency department with concern for superinfected eczema and eczema herpeticum. The patient recently traveled to Puerto Rico, where he had contact with an aunt with active herpes zoster but no other sick contacts. The patient’s immunizations were reported to be up-to-date.
Physical examination revealed the patient was afebrile but irritable and had erythematous crusted papules and patches on the face, arms, and legs, as well as erythematous dry patches on the chest, abdomen, and back (Figure). There were no conjunctival erythematous or oral erosions. The patient was admitted to the hospital for presumed superinfected AD and possible eczema herpeticum. He was started on intravenous clindamycin and acyclovir.
The following day, the patient had new facial edema and fever (temperature, 102.8 °F [39.36 °C]) in addition to palpable mobile cervical, axillary, and inguinal lymphadenopathy. He also was noted to have notably worsening eosinophilia from 1288 (14%) to 2570 (29.2%) cells/µL (reference range, 0%–5%) and new-onset transaminitis. Herpes and varicella-zoster direct fluorescent antibody tests, culture, and serum polymerase chain reaction were all negative, and acyclovir was discontinued. Repeat laboratory tests 12 hours later showed a continued uptrend in transaminitis. Serologies for acute and chronic cytomegalovirus; Epstein-Barr virus; and hepatitis A, B, and C were all nonreactive. The patient was started on intravenous methylprednisolone 1 mg/kg daily for suspected DRESS syndrome likely due to cefdinir.
The patient’s eosinophilia completely resolved (from approximately 2600 to 100 cells/µL) after 1 dose of steroids, and his transaminitis trended down over the next few days. He remained afebrile for the remainder of his admission, and his facial swelling and rash continued to improve. Bacterial culture from the skin grew oxacillin-susceptible Staphylococcus aureus and group A Streptococcus pyogenes. A blood culture was negative. The patient was discharged home to complete a 10-day course of clindamycin and was given topical steroids for the eczema. He continued on oral prednisolone 1 mg/kg daily for 10 days, after which the dose was tapered down for a total 1-month course of systemic corticosteroids. At 1-month follow-up after completing the course of steroids, he was doing well with normal hepatic enzyme levels and no recurrence of fever, facial edema, or rash. He continues to be followed for management of the AD.
Drug reaction with eosinophilia and systemic symptoms syndrome is a serious systemic adverse drug reaction, with high morbidity and even mortality, estimated at 10% in the adult population, though more specific pediatric mortality data are not available.1,2 The exact pathogenesis of DRESS syndrome has not been elucidated. Certain human leukocyte antigen class I alleles are predisposed to the development of DRESS syndrome, but there has not been a human leukocyte antigen subtype identified with beta-lactam–associated DRESS syndrome. Some studies have demonstrated a reactivation of human herpesvirus 6, human herpesvirus 7, and Epstein-Barr virus.3 One study involving 40 patients with DRESS syndrome identified viremia in 76% (29/38) of patients and identified CD8+ T-cell populations directed toward viral epitopes.3 Finally, DRESS syndrome may be related to the slow detoxification and elimination of intermediary products of offending medications that serve as an immunogenic stimulus for the inflammatory cascade.2
In adults, DRESS syndrome was first identified in association with phenytoin, but more recently other drugs have been identified, including other aromatic anticonvulsants (ie, lamotrigine, phenobarbital, carbamazepine), allopurinol, sulfonamides, antiretrovirals (particularly abacavir), and minocycline.2 In a 3-year pediatric prospective study, 11 cases of DRESS syndrome were identified: 4 cases due to lamotrigine, and 3 caused by penicillins.4 The trigger in our patient’s case was the beta-lactam, third-generation cephalosporin cefdinir, and his symptoms developed within 6 days of starting the medication. Many articles report that beta-lactams are a rare cause of DRESS syndrome, with only a handful of cases reported.1,5,6
The diagnosis of DRESS syndrome often can be delayed, as children present acutely febrile and toxic appearing. Unlike many adverse drug reactions, DRESS syndrome does not show rapid resolution with withdrawal of the causative agent, further complicating the diagnosis. The typical onset of DRESS syndrome generally ranges from 2 to 6 weeks after the initiation of the offending drug; however, faster onset of symptoms, similar to our case, has been noted in antibiotic-triggered cases. In the prospective pediatric series by Sasidharanpillai et al,4 the average time to onset among 3 antibiotic-triggered DRESS cases was 5.8 days vs 23.9 days among the 4 cases of lamotrigine-associated DRESS syndrome.
Our patient demonstrated the classic features of DRESS syndrome, including fever, rash, lymphadenopathy, facial edema, peripheral eosinophilia, atypical lymphocytosis, and hepatitis. Based on the proposed RegiSCAR scoring system, our patient was classified as a “definite” case of DRESS syndrome.1,7 Other hematologic findings in DRESS syndrome may include thrombocytopenia and anemia. The liver is the most commonly affected internal organ in DRESS syndrome, with pneumonitis, carditis, and nephritis reported less frequently.1 The pattern of liver injury in our patient was mixed (hepatocellular and cholestatic), the second most common pattern in patients with DRESS syndrome (the cholestatic pattern is most common).8
The exanthem of DRESS syndrome can vary in morphology, with up to 7% of patients reported to have eczemalike lesions in the multinational prospective RegiSCAR study.1 Other entities in the differential diagnosis for our patient included Kawasaki disease, where conjunctivitis and strawberry tongue are classically present, as well as erythrodermic AD, where internal organ involvement is not common.2 Our patient’s exanthem initially was considered to be a flare of AD with superimposed bacterial infection and possible eczema herpeticum. Although bacterial cultures did grow Staphylococcus and Streptococcus, viral studies were all negative, and this alone would not have explained the facial edema, rapidly rising eosinophil count, and transaminitis. The dramatic drop in his eosinophil count and decrease in hepatic enzymes after 1 dose of intravenous methylprednisolone also supported the diagnosis of DRESS syndrome.
Treatment recommendations remain largely anecdotal. Early systemic steroids generally are accepted as the first line of therapy, with a slow taper. Although the average required duration of systemic steroids in 1 series of adults was reported at 50.1 days,9 the duration was shorter (21–35 days) in a series of pediatric patients.4 Our patient’s clinical symptoms and laboratory values normalized after completing a 1-month steroid taper. Other therapies have been tried for recalcitrant cases, including intravenous immunoglobulin, plasmapheresis, rituximab, and valganciclovir.2
Early clinical recognition of the signs and symptoms of DRESS syndrome in the setting of a new medication can decrease morbidity and mortality. Although DRESS syndrome in pediatric patients presents with many similar clinical features as in adults, it may be a greater diagnostic challenge. As in adult cases, timely administration of systemic corticosteroids and tapering based on clinical signs and symptoms can lead to resolution of the hypersensitivity syndrome.
To the Editor:
Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, or drug-induced hypersensitivity syndrome, is a serious and potentially fatal multiorgan drug hypersensitivity reaction. Drug reaction with eosinophilia and systemic symptoms syndrome shares many clinical features with viral exanthems and may be difficult to diagnose in the setting of atopic dermatitis (AD) in which children may have baseline eosinophilia from an atopic diathesis. The cutaneous exanthema also may be variable in presentation, further complicating diagnosis.1,2
A 3-year-old boy with AD since infancy and a history of anaphylaxis to peanuts presented to the emergency department with reported fever, rash, sore throat, and decreased oral intake. Ten days prior, the patient was treated for cellulitis of the left foot with a 7-day course of cefdinir with complete resolution of symptoms. Four days prior to admission, the patient started developing “bumps” on the face and fevers. He was seen at an outside facility, where a rapid test for Streptococcus was negative, and the patient was treated with ibuprofen and fluids for a presumed viral exanthem. The rash subsequently spread to involve the trunk and extremities. On the day of admission, the patient had a positive rapid test for Streptococcus and was referred to the emergency department with concern for superinfected eczema and eczema herpeticum. The patient recently traveled to Puerto Rico, where he had contact with an aunt with active herpes zoster but no other sick contacts. The patient’s immunizations were reported to be up-to-date.
Physical examination revealed the patient was afebrile but irritable and had erythematous crusted papules and patches on the face, arms, and legs, as well as erythematous dry patches on the chest, abdomen, and back (Figure). There were no conjunctival erythematous or oral erosions. The patient was admitted to the hospital for presumed superinfected AD and possible eczema herpeticum. He was started on intravenous clindamycin and acyclovir.
The following day, the patient had new facial edema and fever (temperature, 102.8 °F [39.36 °C]) in addition to palpable mobile cervical, axillary, and inguinal lymphadenopathy. He also was noted to have notably worsening eosinophilia from 1288 (14%) to 2570 (29.2%) cells/µL (reference range, 0%–5%) and new-onset transaminitis. Herpes and varicella-zoster direct fluorescent antibody tests, culture, and serum polymerase chain reaction were all negative, and acyclovir was discontinued. Repeat laboratory tests 12 hours later showed a continued uptrend in transaminitis. Serologies for acute and chronic cytomegalovirus; Epstein-Barr virus; and hepatitis A, B, and C were all nonreactive. The patient was started on intravenous methylprednisolone 1 mg/kg daily for suspected DRESS syndrome likely due to cefdinir.
The patient’s eosinophilia completely resolved (from approximately 2600 to 100 cells/µL) after 1 dose of steroids, and his transaminitis trended down over the next few days. He remained afebrile for the remainder of his admission, and his facial swelling and rash continued to improve. Bacterial culture from the skin grew oxacillin-susceptible Staphylococcus aureus and group A Streptococcus pyogenes. A blood culture was negative. The patient was discharged home to complete a 10-day course of clindamycin and was given topical steroids for the eczema. He continued on oral prednisolone 1 mg/kg daily for 10 days, after which the dose was tapered down for a total 1-month course of systemic corticosteroids. At 1-month follow-up after completing the course of steroids, he was doing well with normal hepatic enzyme levels and no recurrence of fever, facial edema, or rash. He continues to be followed for management of the AD.
Drug reaction with eosinophilia and systemic symptoms syndrome is a serious systemic adverse drug reaction, with high morbidity and even mortality, estimated at 10% in the adult population, though more specific pediatric mortality data are not available.1,2 The exact pathogenesis of DRESS syndrome has not been elucidated. Certain human leukocyte antigen class I alleles are predisposed to the development of DRESS syndrome, but there has not been a human leukocyte antigen subtype identified with beta-lactam–associated DRESS syndrome. Some studies have demonstrated a reactivation of human herpesvirus 6, human herpesvirus 7, and Epstein-Barr virus.3 One study involving 40 patients with DRESS syndrome identified viremia in 76% (29/38) of patients and identified CD8+ T-cell populations directed toward viral epitopes.3 Finally, DRESS syndrome may be related to the slow detoxification and elimination of intermediary products of offending medications that serve as an immunogenic stimulus for the inflammatory cascade.2
In adults, DRESS syndrome was first identified in association with phenytoin, but more recently other drugs have been identified, including other aromatic anticonvulsants (ie, lamotrigine, phenobarbital, carbamazepine), allopurinol, sulfonamides, antiretrovirals (particularly abacavir), and minocycline.2 In a 3-year pediatric prospective study, 11 cases of DRESS syndrome were identified: 4 cases due to lamotrigine, and 3 caused by penicillins.4 The trigger in our patient’s case was the beta-lactam, third-generation cephalosporin cefdinir, and his symptoms developed within 6 days of starting the medication. Many articles report that beta-lactams are a rare cause of DRESS syndrome, with only a handful of cases reported.1,5,6
The diagnosis of DRESS syndrome often can be delayed, as children present acutely febrile and toxic appearing. Unlike many adverse drug reactions, DRESS syndrome does not show rapid resolution with withdrawal of the causative agent, further complicating the diagnosis. The typical onset of DRESS syndrome generally ranges from 2 to 6 weeks after the initiation of the offending drug; however, faster onset of symptoms, similar to our case, has been noted in antibiotic-triggered cases. In the prospective pediatric series by Sasidharanpillai et al,4 the average time to onset among 3 antibiotic-triggered DRESS cases was 5.8 days vs 23.9 days among the 4 cases of lamotrigine-associated DRESS syndrome.
Our patient demonstrated the classic features of DRESS syndrome, including fever, rash, lymphadenopathy, facial edema, peripheral eosinophilia, atypical lymphocytosis, and hepatitis. Based on the proposed RegiSCAR scoring system, our patient was classified as a “definite” case of DRESS syndrome.1,7 Other hematologic findings in DRESS syndrome may include thrombocytopenia and anemia. The liver is the most commonly affected internal organ in DRESS syndrome, with pneumonitis, carditis, and nephritis reported less frequently.1 The pattern of liver injury in our patient was mixed (hepatocellular and cholestatic), the second most common pattern in patients with DRESS syndrome (the cholestatic pattern is most common).8
The exanthem of DRESS syndrome can vary in morphology, with up to 7% of patients reported to have eczemalike lesions in the multinational prospective RegiSCAR study.1 Other entities in the differential diagnosis for our patient included Kawasaki disease, where conjunctivitis and strawberry tongue are classically present, as well as erythrodermic AD, where internal organ involvement is not common.2 Our patient’s exanthem initially was considered to be a flare of AD with superimposed bacterial infection and possible eczema herpeticum. Although bacterial cultures did grow Staphylococcus and Streptococcus, viral studies were all negative, and this alone would not have explained the facial edema, rapidly rising eosinophil count, and transaminitis. The dramatic drop in his eosinophil count and decrease in hepatic enzymes after 1 dose of intravenous methylprednisolone also supported the diagnosis of DRESS syndrome.
Treatment recommendations remain largely anecdotal. Early systemic steroids generally are accepted as the first line of therapy, with a slow taper. Although the average required duration of systemic steroids in 1 series of adults was reported at 50.1 days,9 the duration was shorter (21–35 days) in a series of pediatric patients.4 Our patient’s clinical symptoms and laboratory values normalized after completing a 1-month steroid taper. Other therapies have been tried for recalcitrant cases, including intravenous immunoglobulin, plasmapheresis, rituximab, and valganciclovir.2
Early clinical recognition of the signs and symptoms of DRESS syndrome in the setting of a new medication can decrease morbidity and mortality. Although DRESS syndrome in pediatric patients presents with many similar clinical features as in adults, it may be a greater diagnostic challenge. As in adult cases, timely administration of systemic corticosteroids and tapering based on clinical signs and symptoms can lead to resolution of the hypersensitivity syndrome.
- Kardaun SH, Sekula P, Valeyrie-Allanore L, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071-1080.
- Fernando SL. Drug-reaction eosinophilia and systemic symptoms and drug-induced hypersensitivity syndrome. Australas J Dermatol. 2014;55:15-23.
- Picard D, Janela B, Descamps V, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): a multiorgan antiviral T cell response. Sci Transl Med. 2010;2:46ra62.
- Sasidharanpillai S, Sabitha S, Riyaz N, et al. Drug reaction with eosinophilia and systemic symptoms in children: a prospective study. Pediatr Dermatol. 2016;33:E162-E165.
- Aouam K, Chaabane A, Toumi A, et al. Drug rash with eosinophilia and systemic symptoms (DRESS) probably induced by cefotaxime: a report of two cases. Clin Med Res. 2012;10:32-35.
- Guleria VS, Dhillon M, Gill S, et al. Ceftriaxone induced drug rash with eosinophilia and systemic symptoms. J Res Pharm Pract. 2014;3:72-74.
- Kardaun SH, Sidoroff A, Valeyrie-Allanore L, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol. 2007;156:609-611.
- Lin IC, Yang HC, Strong C, et al. Liver injury in patients with DRESS: a clinical study of 72 cases. J Am Acad Dermatol. 2015;72:984-991.
- Ang CC, Wang YS, Yoosuff EL, et al. Retrospective analysis of drug-induced hypersensitivity syndrome: a study of 27 patients. J Am Acad Dermatol. 2010;63:219-227.
- Kardaun SH, Sekula P, Valeyrie-Allanore L, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071-1080.
- Fernando SL. Drug-reaction eosinophilia and systemic symptoms and drug-induced hypersensitivity syndrome. Australas J Dermatol. 2014;55:15-23.
- Picard D, Janela B, Descamps V, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): a multiorgan antiviral T cell response. Sci Transl Med. 2010;2:46ra62.
- Sasidharanpillai S, Sabitha S, Riyaz N, et al. Drug reaction with eosinophilia and systemic symptoms in children: a prospective study. Pediatr Dermatol. 2016;33:E162-E165.
- Aouam K, Chaabane A, Toumi A, et al. Drug rash with eosinophilia and systemic symptoms (DRESS) probably induced by cefotaxime: a report of two cases. Clin Med Res. 2012;10:32-35.
- Guleria VS, Dhillon M, Gill S, et al. Ceftriaxone induced drug rash with eosinophilia and systemic symptoms. J Res Pharm Pract. 2014;3:72-74.
- Kardaun SH, Sidoroff A, Valeyrie-Allanore L, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol. 2007;156:609-611.
- Lin IC, Yang HC, Strong C, et al. Liver injury in patients with DRESS: a clinical study of 72 cases. J Am Acad Dermatol. 2015;72:984-991.
- Ang CC, Wang YS, Yoosuff EL, et al. Retrospective analysis of drug-induced hypersensitivity syndrome: a study of 27 patients. J Am Acad Dermatol. 2010;63:219-227.
Practice Points
- Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome shares many clinical features with viral exanthems and may be difficult to diagnose in the setting of atopic dermatitis in which children may have baseline eosinophilia from an atopic diathesis.
- Early clinical recognition of the signs and symptoms of DRESS syndrome in the setting of a new medication can decrease morbidity and mortality.
Acute Severe Urticaria From Minocycline
To the Editor:
Minocycline is a commonly prescribed semisynthetic tetracycline derivative used for long-term treatment of acne vulgaris.1 Given the continued popularity of minocycline and other tetracyclines in treating acne, more adverse side effects are being reported. We report a patient who experienced acute severe urticaria with angioedema from minocycline.
A 35-year-old woman with a history of acne vulgaris presented to the emergency department with urticaria and associated angioedema. Fifteen days after starting minocycline, she awoke with diffuse hives sparing only the abdomen that resolved with diphenhydramine. Later that day, she developed generalized pruritus, hives, and lip swelling. She received intravenous methylprednisolone, diphenhydramine, and famotidine in the emergency department. She returned to the emergency department the next day due to facial and lip swelling, diffuse urticaria that was most pronounced on the arms, and throat irritation. Intramuscular epinephrine was administered first followed by methylprednisolone, famotidine, and cetirizine. She was discharged and advised to start daily prednisone 50 mg and cetirizine 20 mg every evening.
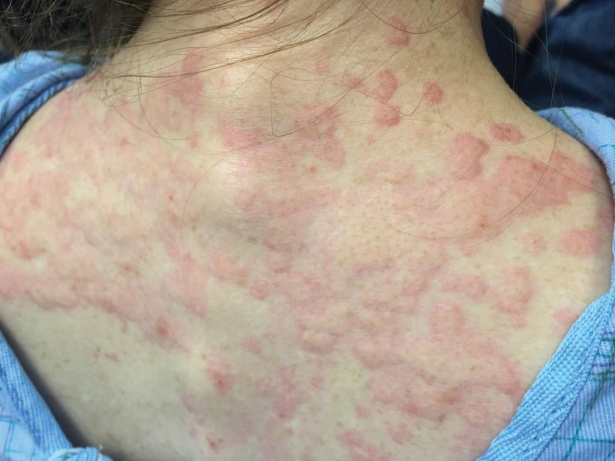
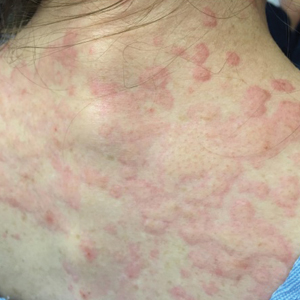
She returned to the emergency department the following morning due to worsening generalized urticaria and angioedema of the lips. She denied any associated respiratory, joint, or gastrointestinal tract symptoms. She had several urticarial plaques on the scalp, face, and body (Figure), only sparing the abdomen. Her hives were erythematous, raised, pruritic, and blanching. There was no residual purpura, ecchymosis, or hyperpigmentation associated with the urticaria, and each lesion was present for less than 24 hours. There was no swelling on examination. Additionally, she was afebrile. The C4 level was 18 mg/dL (reference range, 15–45 mg/dL). She did not develop eosinophilia (absolute eosinophil count, 0/µL [reference range, 50–500/µL]), lymphocytosis (absolute lymphocyte count, 1300/µL [reference range, 1000–4800/µL]), or abnormal liver or renal function. She was hospitalized for 3 days with severe urticaria and required 7 days of prednisone 40 to 50 mg, fexofenadine 360 mg, and cetirizine 20 mg. A viral infection was considered as a possible etiology; however, she had no supporting signs or symptoms of an upper respiratory illness or other viral illness.
The patient’s minocycline use was considered the most likely etiology, as an oral contraceptive was the only other medication. She was labelled allergic to minocycline and discharged with intramuscular epinephrine. She was evaluated in the outpatient allergy immunology clinic 9 days later, and all her symptoms had resolved. Due to the severity of our patient’s reaction and the possibility of further severe reactions, an oral challenge was not carried out. Our patient was not interested in pursuing any further minocycline or other tetracycline-based therapy for her acne. She also was not interested in pursuing any minocycline skin-prick testing or oral challenge. One limitation to this case is our patient declining a confirmatory drug challenge; however, given the severity of the symptoms, the physicians involved agreed the patient's safety outweighed the benefits of confirmatory testing.
A PubMed search of articles indexed for MEDLINE and a Google Scholar search using the terms minocycline, drug hypersensitivity, urticaria, anaphylaxis, minocycline allergy, and angioedema yielded only 16 articles and correspondences. Reported adverse effects of minocycline included drug-induced lupus erythematosus, vasculitis, nausea, photosensitivity, and DRESS-like (drug reaction with eosinophilia and systemic symptoms syndrome) conditions. Three case reports of anaphylaxis/anaphylactoid reactions have been published,2-4 but cases of urticaria attributable to minocycline appear to be exceedingly rare.2,3 Reports of serum sickness in patients aged 15 to 62 years were rare. Women were noted to experience a higher frequency of adverse effects compared to men.5 Symptoms typically presented 3 to 28 days after initiation of minocycline. Data currently suggest that the pathogenesis of hypersensitivity reactions to minocycline remains unknown6; however, one hypothesis is that minocycline or its metabolites act as a superantigen, resulting in lymphocyte overactivation and massive cytokine release.7
Minocycline generally is well tolerated by patients. Physicians should be aware that minocycline is a possible causative agent of allergic drug reactions. Our patient’s presentation of severe acute urticaria with angioedema of the face and lips is a rarity.
- Levenson T, Masood D, Patterson R. Minocycline-induced serum sickness. Allergy Asthma Proc. 1996;17:79-81.
- Okano M, Imai S. Anaphylactoid symptoms due to oral minocycline. Acta Derm Venereol. 1996;76:164.
- Jang JW, Bae Y-J, Kim YG, et al. A case of anaphylaxis to oral minocycline. J Korean Med Sci. 2010;25:1233.
- Nakamura R, Tanaka A, Kinoshita H, et al. Minocycline-induced anaphylaxis mediated by antigen-specific immunoglobulin E [published online November 9, 2021]. J Dermatol. doi:10.1111/1346-8138.16228
- MacNeil M, Haase DA, Tremaine R, et al. Fever, lymphadenopathy, eosinophilia, lymphocytosis, hepatitis, and dermatitis: a severe adverse reaction to minocycline. J Am Acad Dermatol. 1997;36:347-350.
- DePaz S, Perez A, Gomez M, et al. Severe hypersensitivity reaction to minocycline. J Invest Allergol Clin Immunol. 1999;9:403-404.
- Somech R, Arav-Boger R, Assia A, et al. Complications of minocycline therapy for acne vulgaris: case reports and review of the literature. Pediatr Dermatol. 1999;16:469-472.
To the Editor:
Minocycline is a commonly prescribed semisynthetic tetracycline derivative used for long-term treatment of acne vulgaris.1 Given the continued popularity of minocycline and other tetracyclines in treating acne, more adverse side effects are being reported. We report a patient who experienced acute severe urticaria with angioedema from minocycline.
A 35-year-old woman with a history of acne vulgaris presented to the emergency department with urticaria and associated angioedema. Fifteen days after starting minocycline, she awoke with diffuse hives sparing only the abdomen that resolved with diphenhydramine. Later that day, she developed generalized pruritus, hives, and lip swelling. She received intravenous methylprednisolone, diphenhydramine, and famotidine in the emergency department. She returned to the emergency department the next day due to facial and lip swelling, diffuse urticaria that was most pronounced on the arms, and throat irritation. Intramuscular epinephrine was administered first followed by methylprednisolone, famotidine, and cetirizine. She was discharged and advised to start daily prednisone 50 mg and cetirizine 20 mg every evening.
She returned to the emergency department the following morning due to worsening generalized urticaria and angioedema of the lips. She denied any associated respiratory, joint, or gastrointestinal tract symptoms. She had several urticarial plaques on the scalp, face, and body (Figure), only sparing the abdomen. Her hives were erythematous, raised, pruritic, and blanching. There was no residual purpura, ecchymosis, or hyperpigmentation associated with the urticaria, and each lesion was present for less than 24 hours. There was no swelling on examination. Additionally, she was afebrile. The C4 level was 18 mg/dL (reference range, 15–45 mg/dL). She did not develop eosinophilia (absolute eosinophil count, 0/µL [reference range, 50–500/µL]), lymphocytosis (absolute lymphocyte count, 1300/µL [reference range, 1000–4800/µL]), or abnormal liver or renal function. She was hospitalized for 3 days with severe urticaria and required 7 days of prednisone 40 to 50 mg, fexofenadine 360 mg, and cetirizine 20 mg. A viral infection was considered as a possible etiology; however, she had no supporting signs or symptoms of an upper respiratory illness or other viral illness.
The patient’s minocycline use was considered the most likely etiology, as an oral contraceptive was the only other medication. She was labelled allergic to minocycline and discharged with intramuscular epinephrine. She was evaluated in the outpatient allergy immunology clinic 9 days later, and all her symptoms had resolved. Due to the severity of our patient’s reaction and the possibility of further severe reactions, an oral challenge was not carried out. Our patient was not interested in pursuing any further minocycline or other tetracycline-based therapy for her acne. She also was not interested in pursuing any minocycline skin-prick testing or oral challenge. One limitation to this case is our patient declining a confirmatory drug challenge; however, given the severity of the symptoms, the physicians involved agreed the patient's safety outweighed the benefits of confirmatory testing.
A PubMed search of articles indexed for MEDLINE and a Google Scholar search using the terms minocycline, drug hypersensitivity, urticaria, anaphylaxis, minocycline allergy, and angioedema yielded only 16 articles and correspondences. Reported adverse effects of minocycline included drug-induced lupus erythematosus, vasculitis, nausea, photosensitivity, and DRESS-like (drug reaction with eosinophilia and systemic symptoms syndrome) conditions. Three case reports of anaphylaxis/anaphylactoid reactions have been published,2-4 but cases of urticaria attributable to minocycline appear to be exceedingly rare.2,3 Reports of serum sickness in patients aged 15 to 62 years were rare. Women were noted to experience a higher frequency of adverse effects compared to men.5 Symptoms typically presented 3 to 28 days after initiation of minocycline. Data currently suggest that the pathogenesis of hypersensitivity reactions to minocycline remains unknown6; however, one hypothesis is that minocycline or its metabolites act as a superantigen, resulting in lymphocyte overactivation and massive cytokine release.7
Minocycline generally is well tolerated by patients. Physicians should be aware that minocycline is a possible causative agent of allergic drug reactions. Our patient’s presentation of severe acute urticaria with angioedema of the face and lips is a rarity.
To the Editor:
Minocycline is a commonly prescribed semisynthetic tetracycline derivative used for long-term treatment of acne vulgaris.1 Given the continued popularity of minocycline and other tetracyclines in treating acne, more adverse side effects are being reported. We report a patient who experienced acute severe urticaria with angioedema from minocycline.
A 35-year-old woman with a history of acne vulgaris presented to the emergency department with urticaria and associated angioedema. Fifteen days after starting minocycline, she awoke with diffuse hives sparing only the abdomen that resolved with diphenhydramine. Later that day, she developed generalized pruritus, hives, and lip swelling. She received intravenous methylprednisolone, diphenhydramine, and famotidine in the emergency department. She returned to the emergency department the next day due to facial and lip swelling, diffuse urticaria that was most pronounced on the arms, and throat irritation. Intramuscular epinephrine was administered first followed by methylprednisolone, famotidine, and cetirizine. She was discharged and advised to start daily prednisone 50 mg and cetirizine 20 mg every evening.
She returned to the emergency department the following morning due to worsening generalized urticaria and angioedema of the lips. She denied any associated respiratory, joint, or gastrointestinal tract symptoms. She had several urticarial plaques on the scalp, face, and body (Figure), only sparing the abdomen. Her hives were erythematous, raised, pruritic, and blanching. There was no residual purpura, ecchymosis, or hyperpigmentation associated with the urticaria, and each lesion was present for less than 24 hours. There was no swelling on examination. Additionally, she was afebrile. The C4 level was 18 mg/dL (reference range, 15–45 mg/dL). She did not develop eosinophilia (absolute eosinophil count, 0/µL [reference range, 50–500/µL]), lymphocytosis (absolute lymphocyte count, 1300/µL [reference range, 1000–4800/µL]), or abnormal liver or renal function. She was hospitalized for 3 days with severe urticaria and required 7 days of prednisone 40 to 50 mg, fexofenadine 360 mg, and cetirizine 20 mg. A viral infection was considered as a possible etiology; however, she had no supporting signs or symptoms of an upper respiratory illness or other viral illness.
The patient’s minocycline use was considered the most likely etiology, as an oral contraceptive was the only other medication. She was labelled allergic to minocycline and discharged with intramuscular epinephrine. She was evaluated in the outpatient allergy immunology clinic 9 days later, and all her symptoms had resolved. Due to the severity of our patient’s reaction and the possibility of further severe reactions, an oral challenge was not carried out. Our patient was not interested in pursuing any further minocycline or other tetracycline-based therapy for her acne. She also was not interested in pursuing any minocycline skin-prick testing or oral challenge. One limitation to this case is our patient declining a confirmatory drug challenge; however, given the severity of the symptoms, the physicians involved agreed the patient's safety outweighed the benefits of confirmatory testing.
A PubMed search of articles indexed for MEDLINE and a Google Scholar search using the terms minocycline, drug hypersensitivity, urticaria, anaphylaxis, minocycline allergy, and angioedema yielded only 16 articles and correspondences. Reported adverse effects of minocycline included drug-induced lupus erythematosus, vasculitis, nausea, photosensitivity, and DRESS-like (drug reaction with eosinophilia and systemic symptoms syndrome) conditions. Three case reports of anaphylaxis/anaphylactoid reactions have been published,2-4 but cases of urticaria attributable to minocycline appear to be exceedingly rare.2,3 Reports of serum sickness in patients aged 15 to 62 years were rare. Women were noted to experience a higher frequency of adverse effects compared to men.5 Symptoms typically presented 3 to 28 days after initiation of minocycline. Data currently suggest that the pathogenesis of hypersensitivity reactions to minocycline remains unknown6; however, one hypothesis is that minocycline or its metabolites act as a superantigen, resulting in lymphocyte overactivation and massive cytokine release.7
Minocycline generally is well tolerated by patients. Physicians should be aware that minocycline is a possible causative agent of allergic drug reactions. Our patient’s presentation of severe acute urticaria with angioedema of the face and lips is a rarity.
- Levenson T, Masood D, Patterson R. Minocycline-induced serum sickness. Allergy Asthma Proc. 1996;17:79-81.
- Okano M, Imai S. Anaphylactoid symptoms due to oral minocycline. Acta Derm Venereol. 1996;76:164.
- Jang JW, Bae Y-J, Kim YG, et al. A case of anaphylaxis to oral minocycline. J Korean Med Sci. 2010;25:1233.
- Nakamura R, Tanaka A, Kinoshita H, et al. Minocycline-induced anaphylaxis mediated by antigen-specific immunoglobulin E [published online November 9, 2021]. J Dermatol. doi:10.1111/1346-8138.16228
- MacNeil M, Haase DA, Tremaine R, et al. Fever, lymphadenopathy, eosinophilia, lymphocytosis, hepatitis, and dermatitis: a severe adverse reaction to minocycline. J Am Acad Dermatol. 1997;36:347-350.
- DePaz S, Perez A, Gomez M, et al. Severe hypersensitivity reaction to minocycline. J Invest Allergol Clin Immunol. 1999;9:403-404.
- Somech R, Arav-Boger R, Assia A, et al. Complications of minocycline therapy for acne vulgaris: case reports and review of the literature. Pediatr Dermatol. 1999;16:469-472.
- Levenson T, Masood D, Patterson R. Minocycline-induced serum sickness. Allergy Asthma Proc. 1996;17:79-81.
- Okano M, Imai S. Anaphylactoid symptoms due to oral minocycline. Acta Derm Venereol. 1996;76:164.
- Jang JW, Bae Y-J, Kim YG, et al. A case of anaphylaxis to oral minocycline. J Korean Med Sci. 2010;25:1233.
- Nakamura R, Tanaka A, Kinoshita H, et al. Minocycline-induced anaphylaxis mediated by antigen-specific immunoglobulin E [published online November 9, 2021]. J Dermatol. doi:10.1111/1346-8138.16228
- MacNeil M, Haase DA, Tremaine R, et al. Fever, lymphadenopathy, eosinophilia, lymphocytosis, hepatitis, and dermatitis: a severe adverse reaction to minocycline. J Am Acad Dermatol. 1997;36:347-350.
- DePaz S, Perez A, Gomez M, et al. Severe hypersensitivity reaction to minocycline. J Invest Allergol Clin Immunol. 1999;9:403-404.
- Somech R, Arav-Boger R, Assia A, et al. Complications of minocycline therapy for acne vulgaris: case reports and review of the literature. Pediatr Dermatol. 1999;16:469-472.
Practice Points
- Minocycline is a commonly prescribed long-term treatment for acne vulgaris.
- Minocycline-induced acute urticaria and anaphylaxis are rare adverse events.
A Starter Guide to Immunofluorescence Testing in Dermatology
Direct immunofluorescence (DIF) is the go-to diagnostic test when evaluating vesiculobullous eruptions, connective tissue disease, and vasculitis. This specialized test allows visualization of autoantibodies and their reaction products in the epidermis and dermis (skin) and epithelium and subepithelium (mucosa). Indirect immunofluorescence (IIF) and enzyme-linked immunosorbent assay (ELISA) are additional tests that can help in the diagnosis of autoimmune blistering disease. In the blistering autoimmune diseases, the autoantibodies target components in skin and mucous membranes that are essential for cell-cell and cell-matrix adhesion causing separation within or beneath the epidermis, depending on where the target components are located. This article is intended to serve as a helpful primer for immunofluorescence testing in dermatology, with an overview of the tests available as well as pragmatic tips for optimal biopsy sites and specimen transport.
Direct Immunofluorescence
Immunofluorescence techniques date back to 1941 when Albert Coons, an American physician, pathologist, and immunologist, fluorescently labelled antibodies to visualize pneumococcal antigens in infected tissues.1-3 In dermatology, similar methodology was used to visualize the deposition of immunoglobulins and complement in the skin of patients with systemic lupus erythematosus in 1963.4 Basement membrane zone antibodies were first visualized via DIF in bullous pemphigoid in 1967.5 This elegant test utilizes specific antibodies labeled with fluorophores that are then incubated with the patient’s tissue, ultimately forming antibody-antigen conjugates that can be visualized with a fluorescent microscope. Antibodies usually include IgG, IgM, IgA, fibrinogen, and C3. Some institutions also evaluate for IgG4.
Transport medium is critical for proper evaluation of tissues using DIF. Inappropriate storage of tissue can degrade the antigen and confuse the interpretation of specimens. An acceptable medium for DIF includes Michel transport medium, which allows tissue to be stored for days while being transported at ambient temperature without loss of signal.6,7 Zeus medium also can be used and is more readily available. Alternatively, biopsy tissue can be snap frozen using liquid nitrogen. Specimens also may be stored on saline gauze but should be analyzed within 24 to 48 hours.8 Most importantly, do not place the specimen in formalin; even a brief soak in formalin can greatly alter results, especially when trying to diagnose pemphigus.9 Proper transport conditions are critical to prevent autolysis, mitigate putrefaction, and preserve morphology while maintaining antigenicity.10
Indirect Immunofluorescence
Indirect immunofluorescence can be helpful for detecting antibodies circulating in patient serum. Indirect immunofluorescence can be used to help diagnose pemphigoid, pemphigus, epidermolysis bullosa acquisita, bullous lupus erythematosus, and dermatitis herpetiformis. Serum testing also can be a helpful alternative when obtaining tissue is difficult, such as in children.
Indirect immunofluorescence is a 2-part technique that takes a bit longer to assay than DIF.11 The first step involves incubating prepared tissue substrates with patient serum. Unlabeled antibodies in the patient serum are allowed to bind to antigens in the substrate tissue for about 30 minutes. Doubling dilutions of patient serum can be performed to titer antibody levels. The second step uses fluorescein-labeled antihuman antibodies to recognize the antigen-antibody conjugates. Normal whole tissues (eg, monkey esophagus for pemphigus vulgaris, rat bladder for paraneoplastic pemphigus, salt-split normal human skin substrate for pemphigoid and epidermolysis bullosa) are the usual substrates for testing.11,12 Again, this test requires serum and should be collected in a red-top tube or serum-separator tube. Usually, a minimum of 0.5 mL is required for testing, but check with your preferred immunodermatology send-out laboratory before collecting.13
Indirect immunofluorescence usually involves an initial screening panel using 1 or 2 tissue substrates followed by individual antigen-specific assays that correspond to the clinical suspicion and IIF screening results.11 Salt-split skin is used to localize basement membrane zone autoantibodies to either the epidermal (roof) or dermal (floor) side. Although many dermatopathology laboratories offer DIF testing, IIF is more specialized and may be a send-out test at your institution.
Enzyme-linked Immunosorbent Assays
Another tool in the immunodermatology armamentarium is ELISA. Commercial ELISA systems are available for the detection of autoantibodies against bullous pemphigoid (BP) antigen 180, BP230, type VII collagen, desmoglein (Dsg) 1, Dsg3, and envoplakin.11 This test allows semiquantitative measurement of antibody levels and thus can be used to monitor response to treatment or identify relapse and treatment failure.11 For example, in BP, significantly increased baseline anti-BP180 IgG levels correlate with 1-year mortality rates (P=.001) and relapse rates (P=.041).14,15 Numerous additional studies support the observation that monitoring anti-BP180 as a potential marker of disease relapse can be helpful.16,17 In pemphigus, the presence or increase of autoantibodies at remission, either anti-Dsg3 or anti-Dsg1, may be a useful tool in predicting disease relapse.18 It is important for physicians to be aware of this to be able to offer guidance on prognosis.
Where Should I Biopsy?
Knowing where to biopsy can be confusing when beginning residency. But the short answer is, it depends. Let your clinical suspicion guide your specimen site. The Figure provides a quick reference for which location will give you the highest yield for a specific diagnosis.
A few cardinal rules should guide which site is biopsied. Avoid obtaining specimens from the lower extremities as much as possible, as this site has been linked with false-negative results, especially in bullous pemphigoid.19,20 As a dependent area prone to stasis, this site gets a lot of abuse and inflammatory changes secondary to everyday insults that can theoretically alter DIF findings, especially fibrinogen deposition.
Although tissue sent for hematoxylin and eosin staining should be lesional, biopsy for DIF ideally should not contain a new or active blister, ulcer, erosion, or bulla. Immunoreactants are more likely to be degraded in these areas, and DIF may be falsely negative.21
It is worthwhile to briefly discuss the definitions of the terms perilesional and nonlesional. Perilesional skin most frequently refers to skin adjacent to a bulla or vesicle. This skin can be erythematous/inflamed or appear normal. When obtaining tissue for a diagnosis of blistering disease, the general recommendation is to obtain the biopsy from lesional nonbullous skin or perilesional uninvolved skin within 1 cm of the bulla.22-24 The only exception to this is dermatitis herpetiformis, which is best diagnosed on tissue obtained from normal-appearing perilesional skin within 1 cm of an active lesion.25 Additionally, if your patient has oral disease, the recommendation is to obtain the biopsy from nonlesional buccal mucosa, especially if there is desquamative gingivitis.26,27
The ideal biopsy size is 4 or 5 mm. If considering both DIF and histopathology, it is best to procure 2 separate specimens. One larger biopsy can be carefully bisected in 2 but often is subject to more handling artifacts, which can affect findings. In the case of 1 biopsy bisected into 2 specimens, the punch should be at least 6 mm. Shave biopsies also can be performed as long as they extend into the reticular dermis.23
For vasculitis, biopsies for DIF should be taken from lesions that are less than 24 hours old for highest yield, as the level of tissue immunoreactants tends to decline over time.28 This guideline does differ from hematoxylin and eosin specimens sent for evaluation of vasculitis, which ideally should be lesional tissue over 72 hours old. When evaluating for lupus (including subacute cutaneous lupus, discoid lupus, and systemic lupus), DIF is more likely to be positive in well-established, active lesions.
Which Test Should I Order?
The answer to this question depends, but the use of all 3 tests has a specificity close to 100% when evaluating for autoantibody-associated diseases.23 For autoimmune blistering disease, DIF is considered the diagnostic standard. The sensitivity of DIF for diagnosing BP is in the range of 82% to 90.5%, while specificity is 98%.29-31 Other autoimmune blistering diseases, such as pemphigus or dermatitis herpetiformis, have even higher sensitivities and specificities. Direct immunofluorescence often is used as a screening test, but false negatives do occur.32,33 Although rare, false positives also can occur, especially in cases of infection, and should be suspected when there is a lack of clinicopathologic correlation.34 If DIF is negative but clinical suspicion remains high, IIF should be ordered to directly evaluate a patient’s serum for autoantibodies.
In acute cutaneous lupus, subacute cutaneous lupus, and discoid lupus, DIF of active lesions may be helpful if histopathologic examination of a cutaneous lupus erythematosus lesion is nondiagnostic. However, histopathologic examination of formalin-fixed tissue remains the standard for these diagnoses. In vasculitis, while DIF is not used for diagnosis, it is useful to evaluate for IgA deposition. This is important in adults, as IgA deposition has been associated with a greater risk for developing end-stage renal disease.35
Final Thoughts
This is an overview of the tests available for diagnosing autoimmune blistering diseases. Residents should keep in mind that these tests are just one part of the puzzle when it comes to diagnosing these diseases. Results of DIF, IIF, and ELISA testing should be considered in conjunction with patient history and physical examination as well as histopathologic examination of lesional tissue when evaluating for dermatologic diseases with autoantibodies.
- Arthur G. Albert Coons: harnessing the power of the antibody. Lancet Respir Med. 2016;4:181-182.
- Coons AH, Creech HJ, Jones RN. Immunological properties of an antibody containing a fluorescent group. Proc Soc Exp Biol Med. 1941;47:200-202.
- Coons AH, Creech HJ, Jones RN, et al. The demonstration of pneumococcal antigen in tissues by the use of fluorescent antibody. J Immunol. 1942;45:159-170.
- Burnham TK, Neblett TR, Fine G. The application of the fluorescent antibody technic to the investigation of lupus erythematosus and various dermatoses. J Invest Dermatol. 1963;41:451-456.
- Jordon RE, Beutner EH, Witebsky E, et al. Basement zone antibodies in bullous pemphigoid. JAMA. 1967;200:751-756.
- Vaughan Jones SA, Salas J, McGrath JA, et al. A retrospective analysis of tissue-fixed immunoreactants from skin biopsies maintained in Michel’s medium. Dermatology. 1994;189(suppl 1):131-132.
- Kim RH, Brinster NK. Practical direct immunofluorescence. Am J Dermatopathol. 2020;42:75-85.
- Vodegel RM, de Jong MC, Meijer HJ, et al. Enhanced diagnostic immunofluorescence using biopsies transported in saline. BMC Dermatol. 2004;4:10.
- Arbesman J, Grover R, Helm TN, et al. Can direct immunofluorescence testing still be accurate if performed on biopsy specimens after brief inadvertent immersion in formalin? J Am Acad Dermatol. 2011;65:106-111.
- Im K, Mareninov S, Diaz MFP, et al. An introduction to performing immunofluorescence staining. Methods Mol Biol. 2019;1897:299-311.
- Saschenbrecker S, Karl I, Komorowski L, et al. Serological diagnosis of autoimmune bullous skin diseases. Front Immunol. 2019;10:1974.
- Baum S, Sakka N, Artsi O, et al. Diagnosis and classification of autoimmune blistering diseases. Autoimmun Rev. 2014;13:482-489.
- Immunobullous disease panel, epithelial. ARUP Laboratories website. Accessed November 22, 2021. https://ltd.aruplab.com/Tests/Pub/3001409
- Monshi B, Gulz L, Piringer B, et al. Anti-BP180 autoantibody levels at diagnosis correlate with 1-year mortality rates in patients with bullous pemphigoid. J Eur Acad Dermatol Venereol. 2020;34:1583-1589.
- Koga H, Teye K, Ishii N, et al. High index values of enzyme-linked immunosorbent assay for BP180 at baseline predict relapse in patients with bullous pemphigoid. Front Med (Lausanne). 2018;5:139.
- Fichel F, Barbe C, Joly P, et al. Clinical and immunologic factors associated with bullous pemphigoid relapse during the first year of treatment: a multicenter, prospective study. JAMA Dermatol. 2014;150:25-33.
- Cai SC, Lim YL, Li W, et al. Anti-BP180 NC16A IgG titres as an indicator of disease activity and outcome in Asian patients with bullous pemphigoid. Ann Acad Med Singap. 2015;44:119-126.
- Genovese G, Maronese CA, Casazza G, et al. Clinical and serological predictors of relapse in pemphigus: a study of 143 patients [published online July 20, 2021]. Clin Exp Dermatol. doi:10.1111/ced.14854
- Weigand DA. Effect of anatomic region on immunofluorescence diagnosis of bullous pemphigoid. J Am Acad Dermatol. 1985;12(2, pt 1):274-278.
- Weigand DA, Clements MK. Direct immunofluorescence in bullous pemphigoid: effects of extent and location of lesions. J Am Acad Dermatol. 1989;20:437-440.
- Mutasim DF, Adams BB. Immunofluorescence in dermatology. J Am Acad Dermatol. 2001;45:803-822; quiz 822-824.
- Sladden C, Kirchhof MG, Crawford RI. Biopsy location for direct immunofluorescence in patients with suspected bullous pemphigoid impacts probability of a positive test result. J Cutan Med Surg. 2014;18:392-396.
- Elston DM, Stratman EJ, Miller SJ. Skin biopsy: biopsy issues in specific diseases. J Am Acad Dermatol. 2016;74:1-16; quiz 17-18.
- Seishima M, Izumi T, Kitajima Y. Antibody to bullous pemphigoid antigen 1 binds to the antigen at perilesional but not uninvolved skin, in localized bullous pemphigoid. Eur J Dermatol. 1999;9:39-42.
- Zone JJ, Meyer LJ, Petersen MJ. Deposition of granular IgA relative to clinical lesions in dermatitis herpetiformis. Arch Dermatol. 1996;132:912-918.
- Kamaguchi M, Iwata H, Ujiie I, et al. Direct immunofluorescence using non-lesional buccal mucosa in mucous membrane pemphigoid. Front Med (Lausanne). 2018;5:20.
- Carey B, Joshi S, Abdelghani A, et al. The optimal oral biopsy site for diagnosis of mucous membrane pemphigoid and pemphigus vulgaris. Br J Dermatol. 2020;182:747-753.
- Kulthanan K, Pinkaew S, Jiamton S, et al. Cutaneous leukocytoclastic vasculitis: the yield of direct immunofluorescence study. J Med Assoc Thai. 2004;87:531-535.
- Chaidemenos GC, Maltezos E, Chrysomallis F, et al. Value of routine diagnostic criteria of bullous pemphigoid. Int J Dermatol. 1998;37:206-210.
- Mysorekar VV, Sumathy TK, Shyam Prasad AL. Role of direct immunofluorescence in dermatological disorders. Indian Dermatol Online J. 2015;6:172-180.
- Fudge JG, Crawford RI. Bullous pemphigoid: a 10-year study of discordant results on direct immunofluorescence. J Cutan Med Surg. 2018;22:472-475.
- Sárdy M, Kostaki D, Varga R, et al. Comparative study of direct and indirect immunofluorescence and of bullous pemphigoid 180 and 230 enzyme-linked immunosorbent assays for diagnosis of bullous pemphigoid. J Am Acad Dermatol. 2013;69:748-753.
- Buch AC, Kumar H, Panicker N, et al. A cross-sectional study of direct immunofluorescence in the diagnosis of immunobullous dermatoses. Indian J Dermatol. 2014;59:364-368.
- Miller DD, Bhawan J. Bullous tinea pedis with direct immunofluorescence positivity: when is a positive result not autoimmune bullous disease? Am J Dermatopathol. 2013;35:587-594.
- Cao R, Lau S, Tan V, et al. Adult Henoch-Schönlein purpura: clinical and histopathological predictors of systemic disease and profound renal disease. Indian J Dermatol Venereol Leprol. 2017;83:577-582.
Direct immunofluorescence (DIF) is the go-to diagnostic test when evaluating vesiculobullous eruptions, connective tissue disease, and vasculitis. This specialized test allows visualization of autoantibodies and their reaction products in the epidermis and dermis (skin) and epithelium and subepithelium (mucosa). Indirect immunofluorescence (IIF) and enzyme-linked immunosorbent assay (ELISA) are additional tests that can help in the diagnosis of autoimmune blistering disease. In the blistering autoimmune diseases, the autoantibodies target components in skin and mucous membranes that are essential for cell-cell and cell-matrix adhesion causing separation within or beneath the epidermis, depending on where the target components are located. This article is intended to serve as a helpful primer for immunofluorescence testing in dermatology, with an overview of the tests available as well as pragmatic tips for optimal biopsy sites and specimen transport.
Direct Immunofluorescence
Immunofluorescence techniques date back to 1941 when Albert Coons, an American physician, pathologist, and immunologist, fluorescently labelled antibodies to visualize pneumococcal antigens in infected tissues.1-3 In dermatology, similar methodology was used to visualize the deposition of immunoglobulins and complement in the skin of patients with systemic lupus erythematosus in 1963.4 Basement membrane zone antibodies were first visualized via DIF in bullous pemphigoid in 1967.5 This elegant test utilizes specific antibodies labeled with fluorophores that are then incubated with the patient’s tissue, ultimately forming antibody-antigen conjugates that can be visualized with a fluorescent microscope. Antibodies usually include IgG, IgM, IgA, fibrinogen, and C3. Some institutions also evaluate for IgG4.
Transport medium is critical for proper evaluation of tissues using DIF. Inappropriate storage of tissue can degrade the antigen and confuse the interpretation of specimens. An acceptable medium for DIF includes Michel transport medium, which allows tissue to be stored for days while being transported at ambient temperature without loss of signal.6,7 Zeus medium also can be used and is more readily available. Alternatively, biopsy tissue can be snap frozen using liquid nitrogen. Specimens also may be stored on saline gauze but should be analyzed within 24 to 48 hours.8 Most importantly, do not place the specimen in formalin; even a brief soak in formalin can greatly alter results, especially when trying to diagnose pemphigus.9 Proper transport conditions are critical to prevent autolysis, mitigate putrefaction, and preserve morphology while maintaining antigenicity.10
Indirect Immunofluorescence
Indirect immunofluorescence can be helpful for detecting antibodies circulating in patient serum. Indirect immunofluorescence can be used to help diagnose pemphigoid, pemphigus, epidermolysis bullosa acquisita, bullous lupus erythematosus, and dermatitis herpetiformis. Serum testing also can be a helpful alternative when obtaining tissue is difficult, such as in children.
Indirect immunofluorescence is a 2-part technique that takes a bit longer to assay than DIF.11 The first step involves incubating prepared tissue substrates with patient serum. Unlabeled antibodies in the patient serum are allowed to bind to antigens in the substrate tissue for about 30 minutes. Doubling dilutions of patient serum can be performed to titer antibody levels. The second step uses fluorescein-labeled antihuman antibodies to recognize the antigen-antibody conjugates. Normal whole tissues (eg, monkey esophagus for pemphigus vulgaris, rat bladder for paraneoplastic pemphigus, salt-split normal human skin substrate for pemphigoid and epidermolysis bullosa) are the usual substrates for testing.11,12 Again, this test requires serum and should be collected in a red-top tube or serum-separator tube. Usually, a minimum of 0.5 mL is required for testing, but check with your preferred immunodermatology send-out laboratory before collecting.13
Indirect immunofluorescence usually involves an initial screening panel using 1 or 2 tissue substrates followed by individual antigen-specific assays that correspond to the clinical suspicion and IIF screening results.11 Salt-split skin is used to localize basement membrane zone autoantibodies to either the epidermal (roof) or dermal (floor) side. Although many dermatopathology laboratories offer DIF testing, IIF is more specialized and may be a send-out test at your institution.
Enzyme-linked Immunosorbent Assays
Another tool in the immunodermatology armamentarium is ELISA. Commercial ELISA systems are available for the detection of autoantibodies against bullous pemphigoid (BP) antigen 180, BP230, type VII collagen, desmoglein (Dsg) 1, Dsg3, and envoplakin.11 This test allows semiquantitative measurement of antibody levels and thus can be used to monitor response to treatment or identify relapse and treatment failure.11 For example, in BP, significantly increased baseline anti-BP180 IgG levels correlate with 1-year mortality rates (P=.001) and relapse rates (P=.041).14,15 Numerous additional studies support the observation that monitoring anti-BP180 as a potential marker of disease relapse can be helpful.16,17 In pemphigus, the presence or increase of autoantibodies at remission, either anti-Dsg3 or anti-Dsg1, may be a useful tool in predicting disease relapse.18 It is important for physicians to be aware of this to be able to offer guidance on prognosis.
Where Should I Biopsy?
Knowing where to biopsy can be confusing when beginning residency. But the short answer is, it depends. Let your clinical suspicion guide your specimen site. The Figure provides a quick reference for which location will give you the highest yield for a specific diagnosis.
A few cardinal rules should guide which site is biopsied. Avoid obtaining specimens from the lower extremities as much as possible, as this site has been linked with false-negative results, especially in bullous pemphigoid.19,20 As a dependent area prone to stasis, this site gets a lot of abuse and inflammatory changes secondary to everyday insults that can theoretically alter DIF findings, especially fibrinogen deposition.
Although tissue sent for hematoxylin and eosin staining should be lesional, biopsy for DIF ideally should not contain a new or active blister, ulcer, erosion, or bulla. Immunoreactants are more likely to be degraded in these areas, and DIF may be falsely negative.21
It is worthwhile to briefly discuss the definitions of the terms perilesional and nonlesional. Perilesional skin most frequently refers to skin adjacent to a bulla or vesicle. This skin can be erythematous/inflamed or appear normal. When obtaining tissue for a diagnosis of blistering disease, the general recommendation is to obtain the biopsy from lesional nonbullous skin or perilesional uninvolved skin within 1 cm of the bulla.22-24 The only exception to this is dermatitis herpetiformis, which is best diagnosed on tissue obtained from normal-appearing perilesional skin within 1 cm of an active lesion.25 Additionally, if your patient has oral disease, the recommendation is to obtain the biopsy from nonlesional buccal mucosa, especially if there is desquamative gingivitis.26,27
The ideal biopsy size is 4 or 5 mm. If considering both DIF and histopathology, it is best to procure 2 separate specimens. One larger biopsy can be carefully bisected in 2 but often is subject to more handling artifacts, which can affect findings. In the case of 1 biopsy bisected into 2 specimens, the punch should be at least 6 mm. Shave biopsies also can be performed as long as they extend into the reticular dermis.23
For vasculitis, biopsies for DIF should be taken from lesions that are less than 24 hours old for highest yield, as the level of tissue immunoreactants tends to decline over time.28 This guideline does differ from hematoxylin and eosin specimens sent for evaluation of vasculitis, which ideally should be lesional tissue over 72 hours old. When evaluating for lupus (including subacute cutaneous lupus, discoid lupus, and systemic lupus), DIF is more likely to be positive in well-established, active lesions.
Which Test Should I Order?
The answer to this question depends, but the use of all 3 tests has a specificity close to 100% when evaluating for autoantibody-associated diseases.23 For autoimmune blistering disease, DIF is considered the diagnostic standard. The sensitivity of DIF for diagnosing BP is in the range of 82% to 90.5%, while specificity is 98%.29-31 Other autoimmune blistering diseases, such as pemphigus or dermatitis herpetiformis, have even higher sensitivities and specificities. Direct immunofluorescence often is used as a screening test, but false negatives do occur.32,33 Although rare, false positives also can occur, especially in cases of infection, and should be suspected when there is a lack of clinicopathologic correlation.34 If DIF is negative but clinical suspicion remains high, IIF should be ordered to directly evaluate a patient’s serum for autoantibodies.
In acute cutaneous lupus, subacute cutaneous lupus, and discoid lupus, DIF of active lesions may be helpful if histopathologic examination of a cutaneous lupus erythematosus lesion is nondiagnostic. However, histopathologic examination of formalin-fixed tissue remains the standard for these diagnoses. In vasculitis, while DIF is not used for diagnosis, it is useful to evaluate for IgA deposition. This is important in adults, as IgA deposition has been associated with a greater risk for developing end-stage renal disease.35
Final Thoughts
This is an overview of the tests available for diagnosing autoimmune blistering diseases. Residents should keep in mind that these tests are just one part of the puzzle when it comes to diagnosing these diseases. Results of DIF, IIF, and ELISA testing should be considered in conjunction with patient history and physical examination as well as histopathologic examination of lesional tissue when evaluating for dermatologic diseases with autoantibodies.
Direct immunofluorescence (DIF) is the go-to diagnostic test when evaluating vesiculobullous eruptions, connective tissue disease, and vasculitis. This specialized test allows visualization of autoantibodies and their reaction products in the epidermis and dermis (skin) and epithelium and subepithelium (mucosa). Indirect immunofluorescence (IIF) and enzyme-linked immunosorbent assay (ELISA) are additional tests that can help in the diagnosis of autoimmune blistering disease. In the blistering autoimmune diseases, the autoantibodies target components in skin and mucous membranes that are essential for cell-cell and cell-matrix adhesion causing separation within or beneath the epidermis, depending on where the target components are located. This article is intended to serve as a helpful primer for immunofluorescence testing in dermatology, with an overview of the tests available as well as pragmatic tips for optimal biopsy sites and specimen transport.
Direct Immunofluorescence
Immunofluorescence techniques date back to 1941 when Albert Coons, an American physician, pathologist, and immunologist, fluorescently labelled antibodies to visualize pneumococcal antigens in infected tissues.1-3 In dermatology, similar methodology was used to visualize the deposition of immunoglobulins and complement in the skin of patients with systemic lupus erythematosus in 1963.4 Basement membrane zone antibodies were first visualized via DIF in bullous pemphigoid in 1967.5 This elegant test utilizes specific antibodies labeled with fluorophores that are then incubated with the patient’s tissue, ultimately forming antibody-antigen conjugates that can be visualized with a fluorescent microscope. Antibodies usually include IgG, IgM, IgA, fibrinogen, and C3. Some institutions also evaluate for IgG4.
Transport medium is critical for proper evaluation of tissues using DIF. Inappropriate storage of tissue can degrade the antigen and confuse the interpretation of specimens. An acceptable medium for DIF includes Michel transport medium, which allows tissue to be stored for days while being transported at ambient temperature without loss of signal.6,7 Zeus medium also can be used and is more readily available. Alternatively, biopsy tissue can be snap frozen using liquid nitrogen. Specimens also may be stored on saline gauze but should be analyzed within 24 to 48 hours.8 Most importantly, do not place the specimen in formalin; even a brief soak in formalin can greatly alter results, especially when trying to diagnose pemphigus.9 Proper transport conditions are critical to prevent autolysis, mitigate putrefaction, and preserve morphology while maintaining antigenicity.10
Indirect Immunofluorescence
Indirect immunofluorescence can be helpful for detecting antibodies circulating in patient serum. Indirect immunofluorescence can be used to help diagnose pemphigoid, pemphigus, epidermolysis bullosa acquisita, bullous lupus erythematosus, and dermatitis herpetiformis. Serum testing also can be a helpful alternative when obtaining tissue is difficult, such as in children.
Indirect immunofluorescence is a 2-part technique that takes a bit longer to assay than DIF.11 The first step involves incubating prepared tissue substrates with patient serum. Unlabeled antibodies in the patient serum are allowed to bind to antigens in the substrate tissue for about 30 minutes. Doubling dilutions of patient serum can be performed to titer antibody levels. The second step uses fluorescein-labeled antihuman antibodies to recognize the antigen-antibody conjugates. Normal whole tissues (eg, monkey esophagus for pemphigus vulgaris, rat bladder for paraneoplastic pemphigus, salt-split normal human skin substrate for pemphigoid and epidermolysis bullosa) are the usual substrates for testing.11,12 Again, this test requires serum and should be collected in a red-top tube or serum-separator tube. Usually, a minimum of 0.5 mL is required for testing, but check with your preferred immunodermatology send-out laboratory before collecting.13
Indirect immunofluorescence usually involves an initial screening panel using 1 or 2 tissue substrates followed by individual antigen-specific assays that correspond to the clinical suspicion and IIF screening results.11 Salt-split skin is used to localize basement membrane zone autoantibodies to either the epidermal (roof) or dermal (floor) side. Although many dermatopathology laboratories offer DIF testing, IIF is more specialized and may be a send-out test at your institution.
Enzyme-linked Immunosorbent Assays
Another tool in the immunodermatology armamentarium is ELISA. Commercial ELISA systems are available for the detection of autoantibodies against bullous pemphigoid (BP) antigen 180, BP230, type VII collagen, desmoglein (Dsg) 1, Dsg3, and envoplakin.11 This test allows semiquantitative measurement of antibody levels and thus can be used to monitor response to treatment or identify relapse and treatment failure.11 For example, in BP, significantly increased baseline anti-BP180 IgG levels correlate with 1-year mortality rates (P=.001) and relapse rates (P=.041).14,15 Numerous additional studies support the observation that monitoring anti-BP180 as a potential marker of disease relapse can be helpful.16,17 In pemphigus, the presence or increase of autoantibodies at remission, either anti-Dsg3 or anti-Dsg1, may be a useful tool in predicting disease relapse.18 It is important for physicians to be aware of this to be able to offer guidance on prognosis.
Where Should I Biopsy?
Knowing where to biopsy can be confusing when beginning residency. But the short answer is, it depends. Let your clinical suspicion guide your specimen site. The Figure provides a quick reference for which location will give you the highest yield for a specific diagnosis.
A few cardinal rules should guide which site is biopsied. Avoid obtaining specimens from the lower extremities as much as possible, as this site has been linked with false-negative results, especially in bullous pemphigoid.19,20 As a dependent area prone to stasis, this site gets a lot of abuse and inflammatory changes secondary to everyday insults that can theoretically alter DIF findings, especially fibrinogen deposition.
Although tissue sent for hematoxylin and eosin staining should be lesional, biopsy for DIF ideally should not contain a new or active blister, ulcer, erosion, or bulla. Immunoreactants are more likely to be degraded in these areas, and DIF may be falsely negative.21
It is worthwhile to briefly discuss the definitions of the terms perilesional and nonlesional. Perilesional skin most frequently refers to skin adjacent to a bulla or vesicle. This skin can be erythematous/inflamed or appear normal. When obtaining tissue for a diagnosis of blistering disease, the general recommendation is to obtain the biopsy from lesional nonbullous skin or perilesional uninvolved skin within 1 cm of the bulla.22-24 The only exception to this is dermatitis herpetiformis, which is best diagnosed on tissue obtained from normal-appearing perilesional skin within 1 cm of an active lesion.25 Additionally, if your patient has oral disease, the recommendation is to obtain the biopsy from nonlesional buccal mucosa, especially if there is desquamative gingivitis.26,27
The ideal biopsy size is 4 or 5 mm. If considering both DIF and histopathology, it is best to procure 2 separate specimens. One larger biopsy can be carefully bisected in 2 but often is subject to more handling artifacts, which can affect findings. In the case of 1 biopsy bisected into 2 specimens, the punch should be at least 6 mm. Shave biopsies also can be performed as long as they extend into the reticular dermis.23
For vasculitis, biopsies for DIF should be taken from lesions that are less than 24 hours old for highest yield, as the level of tissue immunoreactants tends to decline over time.28 This guideline does differ from hematoxylin and eosin specimens sent for evaluation of vasculitis, which ideally should be lesional tissue over 72 hours old. When evaluating for lupus (including subacute cutaneous lupus, discoid lupus, and systemic lupus), DIF is more likely to be positive in well-established, active lesions.
Which Test Should I Order?
The answer to this question depends, but the use of all 3 tests has a specificity close to 100% when evaluating for autoantibody-associated diseases.23 For autoimmune blistering disease, DIF is considered the diagnostic standard. The sensitivity of DIF for diagnosing BP is in the range of 82% to 90.5%, while specificity is 98%.29-31 Other autoimmune blistering diseases, such as pemphigus or dermatitis herpetiformis, have even higher sensitivities and specificities. Direct immunofluorescence often is used as a screening test, but false negatives do occur.32,33 Although rare, false positives also can occur, especially in cases of infection, and should be suspected when there is a lack of clinicopathologic correlation.34 If DIF is negative but clinical suspicion remains high, IIF should be ordered to directly evaluate a patient’s serum for autoantibodies.
In acute cutaneous lupus, subacute cutaneous lupus, and discoid lupus, DIF of active lesions may be helpful if histopathologic examination of a cutaneous lupus erythematosus lesion is nondiagnostic. However, histopathologic examination of formalin-fixed tissue remains the standard for these diagnoses. In vasculitis, while DIF is not used for diagnosis, it is useful to evaluate for IgA deposition. This is important in adults, as IgA deposition has been associated with a greater risk for developing end-stage renal disease.35
Final Thoughts
This is an overview of the tests available for diagnosing autoimmune blistering diseases. Residents should keep in mind that these tests are just one part of the puzzle when it comes to diagnosing these diseases. Results of DIF, IIF, and ELISA testing should be considered in conjunction with patient history and physical examination as well as histopathologic examination of lesional tissue when evaluating for dermatologic diseases with autoantibodies.
- Arthur G. Albert Coons: harnessing the power of the antibody. Lancet Respir Med. 2016;4:181-182.
- Coons AH, Creech HJ, Jones RN. Immunological properties of an antibody containing a fluorescent group. Proc Soc Exp Biol Med. 1941;47:200-202.
- Coons AH, Creech HJ, Jones RN, et al. The demonstration of pneumococcal antigen in tissues by the use of fluorescent antibody. J Immunol. 1942;45:159-170.
- Burnham TK, Neblett TR, Fine G. The application of the fluorescent antibody technic to the investigation of lupus erythematosus and various dermatoses. J Invest Dermatol. 1963;41:451-456.
- Jordon RE, Beutner EH, Witebsky E, et al. Basement zone antibodies in bullous pemphigoid. JAMA. 1967;200:751-756.
- Vaughan Jones SA, Salas J, McGrath JA, et al. A retrospective analysis of tissue-fixed immunoreactants from skin biopsies maintained in Michel’s medium. Dermatology. 1994;189(suppl 1):131-132.
- Kim RH, Brinster NK. Practical direct immunofluorescence. Am J Dermatopathol. 2020;42:75-85.
- Vodegel RM, de Jong MC, Meijer HJ, et al. Enhanced diagnostic immunofluorescence using biopsies transported in saline. BMC Dermatol. 2004;4:10.
- Arbesman J, Grover R, Helm TN, et al. Can direct immunofluorescence testing still be accurate if performed on biopsy specimens after brief inadvertent immersion in formalin? J Am Acad Dermatol. 2011;65:106-111.
- Im K, Mareninov S, Diaz MFP, et al. An introduction to performing immunofluorescence staining. Methods Mol Biol. 2019;1897:299-311.
- Saschenbrecker S, Karl I, Komorowski L, et al. Serological diagnosis of autoimmune bullous skin diseases. Front Immunol. 2019;10:1974.
- Baum S, Sakka N, Artsi O, et al. Diagnosis and classification of autoimmune blistering diseases. Autoimmun Rev. 2014;13:482-489.
- Immunobullous disease panel, epithelial. ARUP Laboratories website. Accessed November 22, 2021. https://ltd.aruplab.com/Tests/Pub/3001409
- Monshi B, Gulz L, Piringer B, et al. Anti-BP180 autoantibody levels at diagnosis correlate with 1-year mortality rates in patients with bullous pemphigoid. J Eur Acad Dermatol Venereol. 2020;34:1583-1589.
- Koga H, Teye K, Ishii N, et al. High index values of enzyme-linked immunosorbent assay for BP180 at baseline predict relapse in patients with bullous pemphigoid. Front Med (Lausanne). 2018;5:139.
- Fichel F, Barbe C, Joly P, et al. Clinical and immunologic factors associated with bullous pemphigoid relapse during the first year of treatment: a multicenter, prospective study. JAMA Dermatol. 2014;150:25-33.
- Cai SC, Lim YL, Li W, et al. Anti-BP180 NC16A IgG titres as an indicator of disease activity and outcome in Asian patients with bullous pemphigoid. Ann Acad Med Singap. 2015;44:119-126.
- Genovese G, Maronese CA, Casazza G, et al. Clinical and serological predictors of relapse in pemphigus: a study of 143 patients [published online July 20, 2021]. Clin Exp Dermatol. doi:10.1111/ced.14854
- Weigand DA. Effect of anatomic region on immunofluorescence diagnosis of bullous pemphigoid. J Am Acad Dermatol. 1985;12(2, pt 1):274-278.
- Weigand DA, Clements MK. Direct immunofluorescence in bullous pemphigoid: effects of extent and location of lesions. J Am Acad Dermatol. 1989;20:437-440.
- Mutasim DF, Adams BB. Immunofluorescence in dermatology. J Am Acad Dermatol. 2001;45:803-822; quiz 822-824.
- Sladden C, Kirchhof MG, Crawford RI. Biopsy location for direct immunofluorescence in patients with suspected bullous pemphigoid impacts probability of a positive test result. J Cutan Med Surg. 2014;18:392-396.
- Elston DM, Stratman EJ, Miller SJ. Skin biopsy: biopsy issues in specific diseases. J Am Acad Dermatol. 2016;74:1-16; quiz 17-18.
- Seishima M, Izumi T, Kitajima Y. Antibody to bullous pemphigoid antigen 1 binds to the antigen at perilesional but not uninvolved skin, in localized bullous pemphigoid. Eur J Dermatol. 1999;9:39-42.
- Zone JJ, Meyer LJ, Petersen MJ. Deposition of granular IgA relative to clinical lesions in dermatitis herpetiformis. Arch Dermatol. 1996;132:912-918.
- Kamaguchi M, Iwata H, Ujiie I, et al. Direct immunofluorescence using non-lesional buccal mucosa in mucous membrane pemphigoid. Front Med (Lausanne). 2018;5:20.
- Carey B, Joshi S, Abdelghani A, et al. The optimal oral biopsy site for diagnosis of mucous membrane pemphigoid and pemphigus vulgaris. Br J Dermatol. 2020;182:747-753.
- Kulthanan K, Pinkaew S, Jiamton S, et al. Cutaneous leukocytoclastic vasculitis: the yield of direct immunofluorescence study. J Med Assoc Thai. 2004;87:531-535.
- Chaidemenos GC, Maltezos E, Chrysomallis F, et al. Value of routine diagnostic criteria of bullous pemphigoid. Int J Dermatol. 1998;37:206-210.
- Mysorekar VV, Sumathy TK, Shyam Prasad AL. Role of direct immunofluorescence in dermatological disorders. Indian Dermatol Online J. 2015;6:172-180.
- Fudge JG, Crawford RI. Bullous pemphigoid: a 10-year study of discordant results on direct immunofluorescence. J Cutan Med Surg. 2018;22:472-475.
- Sárdy M, Kostaki D, Varga R, et al. Comparative study of direct and indirect immunofluorescence and of bullous pemphigoid 180 and 230 enzyme-linked immunosorbent assays for diagnosis of bullous pemphigoid. J Am Acad Dermatol. 2013;69:748-753.
- Buch AC, Kumar H, Panicker N, et al. A cross-sectional study of direct immunofluorescence in the diagnosis of immunobullous dermatoses. Indian J Dermatol. 2014;59:364-368.
- Miller DD, Bhawan J. Bullous tinea pedis with direct immunofluorescence positivity: when is a positive result not autoimmune bullous disease? Am J Dermatopathol. 2013;35:587-594.
- Cao R, Lau S, Tan V, et al. Adult Henoch-Schönlein purpura: clinical and histopathological predictors of systemic disease and profound renal disease. Indian J Dermatol Venereol Leprol. 2017;83:577-582.
- Arthur G. Albert Coons: harnessing the power of the antibody. Lancet Respir Med. 2016;4:181-182.
- Coons AH, Creech HJ, Jones RN. Immunological properties of an antibody containing a fluorescent group. Proc Soc Exp Biol Med. 1941;47:200-202.
- Coons AH, Creech HJ, Jones RN, et al. The demonstration of pneumococcal antigen in tissues by the use of fluorescent antibody. J Immunol. 1942;45:159-170.
- Burnham TK, Neblett TR, Fine G. The application of the fluorescent antibody technic to the investigation of lupus erythematosus and various dermatoses. J Invest Dermatol. 1963;41:451-456.
- Jordon RE, Beutner EH, Witebsky E, et al. Basement zone antibodies in bullous pemphigoid. JAMA. 1967;200:751-756.
- Vaughan Jones SA, Salas J, McGrath JA, et al. A retrospective analysis of tissue-fixed immunoreactants from skin biopsies maintained in Michel’s medium. Dermatology. 1994;189(suppl 1):131-132.
- Kim RH, Brinster NK. Practical direct immunofluorescence. Am J Dermatopathol. 2020;42:75-85.
- Vodegel RM, de Jong MC, Meijer HJ, et al. Enhanced diagnostic immunofluorescence using biopsies transported in saline. BMC Dermatol. 2004;4:10.
- Arbesman J, Grover R, Helm TN, et al. Can direct immunofluorescence testing still be accurate if performed on biopsy specimens after brief inadvertent immersion in formalin? J Am Acad Dermatol. 2011;65:106-111.
- Im K, Mareninov S, Diaz MFP, et al. An introduction to performing immunofluorescence staining. Methods Mol Biol. 2019;1897:299-311.
- Saschenbrecker S, Karl I, Komorowski L, et al. Serological diagnosis of autoimmune bullous skin diseases. Front Immunol. 2019;10:1974.
- Baum S, Sakka N, Artsi O, et al. Diagnosis and classification of autoimmune blistering diseases. Autoimmun Rev. 2014;13:482-489.
- Immunobullous disease panel, epithelial. ARUP Laboratories website. Accessed November 22, 2021. https://ltd.aruplab.com/Tests/Pub/3001409
- Monshi B, Gulz L, Piringer B, et al. Anti-BP180 autoantibody levels at diagnosis correlate with 1-year mortality rates in patients with bullous pemphigoid. J Eur Acad Dermatol Venereol. 2020;34:1583-1589.
- Koga H, Teye K, Ishii N, et al. High index values of enzyme-linked immunosorbent assay for BP180 at baseline predict relapse in patients with bullous pemphigoid. Front Med (Lausanne). 2018;5:139.
- Fichel F, Barbe C, Joly P, et al. Clinical and immunologic factors associated with bullous pemphigoid relapse during the first year of treatment: a multicenter, prospective study. JAMA Dermatol. 2014;150:25-33.
- Cai SC, Lim YL, Li W, et al. Anti-BP180 NC16A IgG titres as an indicator of disease activity and outcome in Asian patients with bullous pemphigoid. Ann Acad Med Singap. 2015;44:119-126.
- Genovese G, Maronese CA, Casazza G, et al. Clinical and serological predictors of relapse in pemphigus: a study of 143 patients [published online July 20, 2021]. Clin Exp Dermatol. doi:10.1111/ced.14854
- Weigand DA. Effect of anatomic region on immunofluorescence diagnosis of bullous pemphigoid. J Am Acad Dermatol. 1985;12(2, pt 1):274-278.
- Weigand DA, Clements MK. Direct immunofluorescence in bullous pemphigoid: effects of extent and location of lesions. J Am Acad Dermatol. 1989;20:437-440.
- Mutasim DF, Adams BB. Immunofluorescence in dermatology. J Am Acad Dermatol. 2001;45:803-822; quiz 822-824.
- Sladden C, Kirchhof MG, Crawford RI. Biopsy location for direct immunofluorescence in patients with suspected bullous pemphigoid impacts probability of a positive test result. J Cutan Med Surg. 2014;18:392-396.
- Elston DM, Stratman EJ, Miller SJ. Skin biopsy: biopsy issues in specific diseases. J Am Acad Dermatol. 2016;74:1-16; quiz 17-18.
- Seishima M, Izumi T, Kitajima Y. Antibody to bullous pemphigoid antigen 1 binds to the antigen at perilesional but not uninvolved skin, in localized bullous pemphigoid. Eur J Dermatol. 1999;9:39-42.
- Zone JJ, Meyer LJ, Petersen MJ. Deposition of granular IgA relative to clinical lesions in dermatitis herpetiformis. Arch Dermatol. 1996;132:912-918.
- Kamaguchi M, Iwata H, Ujiie I, et al. Direct immunofluorescence using non-lesional buccal mucosa in mucous membrane pemphigoid. Front Med (Lausanne). 2018;5:20.
- Carey B, Joshi S, Abdelghani A, et al. The optimal oral biopsy site for diagnosis of mucous membrane pemphigoid and pemphigus vulgaris. Br J Dermatol. 2020;182:747-753.
- Kulthanan K, Pinkaew S, Jiamton S, et al. Cutaneous leukocytoclastic vasculitis: the yield of direct immunofluorescence study. J Med Assoc Thai. 2004;87:531-535.
- Chaidemenos GC, Maltezos E, Chrysomallis F, et al. Value of routine diagnostic criteria of bullous pemphigoid. Int J Dermatol. 1998;37:206-210.
- Mysorekar VV, Sumathy TK, Shyam Prasad AL. Role of direct immunofluorescence in dermatological disorders. Indian Dermatol Online J. 2015;6:172-180.
- Fudge JG, Crawford RI. Bullous pemphigoid: a 10-year study of discordant results on direct immunofluorescence. J Cutan Med Surg. 2018;22:472-475.
- Sárdy M, Kostaki D, Varga R, et al. Comparative study of direct and indirect immunofluorescence and of bullous pemphigoid 180 and 230 enzyme-linked immunosorbent assays for diagnosis of bullous pemphigoid. J Am Acad Dermatol. 2013;69:748-753.
- Buch AC, Kumar H, Panicker N, et al. A cross-sectional study of direct immunofluorescence in the diagnosis of immunobullous dermatoses. Indian J Dermatol. 2014;59:364-368.
- Miller DD, Bhawan J. Bullous tinea pedis with direct immunofluorescence positivity: when is a positive result not autoimmune bullous disease? Am J Dermatopathol. 2013;35:587-594.
- Cao R, Lau S, Tan V, et al. Adult Henoch-Schönlein purpura: clinical and histopathological predictors of systemic disease and profound renal disease. Indian J Dermatol Venereol Leprol. 2017;83:577-582.
Resident Pearl
- Direct immunofluorescence, indirect immunofluorescence, and enzyme-linked immunosorbent assay are important tests for residents to have in their diagnostic tool box, especially when evaluating patients with blistering diseases.
Multiple Lesions With Recurrent Bleeding
The Diagnosis: Nevoid Basal Cell Carcinoma Syndrome
Nevoid basal cell carcinoma syndrome (NBCCS), also known as Gorlin syndrome, is a rare autosomal-dominant disorder that increases the risk for developing various carcinomas and affects multiple organ systems. Nevoid basal cell carcinoma syndrome is estimated at 1 per 40,000 to 60,000 individuals with no sexual predilection.1,2 Pathogenesis of NBCCS occurs through molecular alterations in the dormant hedgehog signaling pathway, causing constitutive signaling activity and a loss of function in the tumor suppressor patched 1 gene, PTCH1. As a result, the inhibition of smoothened oncogenes is released, Gli proteins are activated, and the hedgehog signaling pathway is no longer quiescent.2 Additional loss of function in the suppressor of fused homolog protein, a negative regulator of the hedgehog pathway, allows for further tumor proliferation. The crucial role these genes play in the hedgehog signaling pathway and their mutation association with NBCCS allows for molecular confirmation in the diagnosis of NBCCS. Allelic losses at the PTCH1 gene site are thought to occur in approximately 70% of NBCCS patients.2
Diagnosis of NBCCS is based on genetic testing to examine pathogenic gene variants, notably in the PTCH1 gene, and identification of characteristic clinical findings.2 Diagnosis of NBCCS requires either 2 minor suggestive criteria and 1 major suggestive criterion, 2 major suggestive criteria and 1 minor suggestive criterion, or 1 major suggestive criterion with molecular confirmation. The presence of basal cell carcinomas (BCCs) before 20 years of age or an excessive numbers of BCCs, keratocystic odontogenic tumors (KOTs), palmar or plantar pitting, and first-degree relatives with NBCCS are classified as major suggestive criteria.2 Nevoid basal cell carcinoma syndrome patients typically have BCCs that crust, ulcerate, or bleed. Minor suggestive criteria for NBCCS are rib abnormalities, skeletal malformations, macrocephaly, cleft lip or palate, and desmoplastic medulloblastoma.2-4 Suppressor of fused homolog protein mutations may increase the risk for desmoplastic medulloblastoma in NBCCS patients. Our patient had 4 of the major suggestive criteria, including a history of KOTs, multiple BCCs, first-degree relatives with NBCCS, and palmar or plantar pitting (bottom quiz image), while having 1 minor suggestive criterion of frontal bossing.
Patients with NBCCS have high phenotypic variability, as their skin carcinomas do not have the classic features of pearly surfaces or corkscrew telangiectasia that typically are associated with BCCs.1 Basal cell carcinomas in NBCCS-affected individuals usually are indistinguishable from sporadic lesions that arise in sun-exposed areas, making NBCCS difficult to diagnose. These sporadic lesions often are misdiagnosed as psoriatic or eczematous lesions, and additional subsequent examination is required. The findings of multiple papules and plaques spanning the body as well as lesions with rolled borders and ulcerated bases, indicative of BCCs, aid dermatologists in distinguishing benign lesions from those of NBCCS.1
Additional differential diagnoses are required to distinguish NBCCS from other similar inherited skin disorders that are characterized by BCCs. The presence of multiple incidental BCCs early in life remains a histopathologic clue for NBCCS diagnosis, as opposed to Rombo syndrome, in which BCCs often develop in adulthood.2,4 In addition, although both Bazex syndrome and Muir-Torre syndrome are characterized by the early onset of BCCs, the lack of skeletal abnormalities and palmar and plantar pitting distinguish these entities from NBCCS.2,4 Furthermore, though psoriasis also can present on the scalp, clinical presentation often includes well-demarcated and symmetric plaques that are erythematous and silvery, all of which were not present in our patient and typically are not seen in NBCCS.5
The recommended treatment of NBCCS is vismodegib, a specific oncogene inhibitor. This medication suppresses the hedgehog signaling pathway by inhibiting smoothened oncogenes and downstream target molecules, thereby decreasing tumor proliferation.6 In doing so, vismodegib inhibits the development of new BCCs while reducing the burden of present ones. Additionally, vismodegib appears to effectively treat KOTs. If successful, this medication may be able to suppress KOTs in patients with NBCCS and thus facilitate surgery.6 Additional hedgehog inhibitors include patidegib, sonidegib, and itraconazole. Patidegib gel 2% currently is in phase 3 clinical trials for evaluation of efficacy and safety in treatment of NBCCS. Sonidegib is approved for the treatment of locally advanced BCCs in the United States and the European Union and for both locally advanced BCCs and metastatic BCCs in Switzerland and Australia.7 Further research is needed before recommending antifungal itraconazole for NBCCS clinical use.8 Other medications for localized areas include topical application of 5-fluorouracil and imiquimod.2
- Sangehra R, Grewal P. Gorlin syndrome presentation and the importance of differential diagnosis of skin cancer: a case report. J Pharm Pharm Sci. 2018;21:222-224.
- Bresler S, Padwa B, Granter S. Nevoid basal cell carcinoma syndrome (Gorlin syndrome). Head Neck Pathol. 2016;10:119-124.
- Fujii K, Miyashita T. Gorlin syndrome (nevoid basal cell carcinoma syndrome): update and literature review. Pediatr Int. 2014;56:667-674.
- Evans G, Farndon P. Nevoid basal cell carcinoma syndrome. GeneReviews [Internet]. University of Washington; 1993-2020.
- Kim WB, Jerome D, Yeung J. Diagnosis and management of psoriasis. Can Fam Physician. 2017;63:278-285.
- Booms P, Harth M, Sader R, et al. Vismodegib hedgehog-signaling inhibition and treatment of basal cell carcinomas as well as keratocystic odontogenic tumors in Gorlin syndrome. Ann Maxillofac Surg. 2015;5:14-19.
- Gutzmer R, Soloon J. Hedgehog pathway inhibition for the treatment of basal cell carcinoma. Target Oncol. 2019;14:253-267.
- Leavitt E, Lask G, Martin S. Sonic hedgehog pathway inhibition in the treatment of advanced basal cell carcinoma. Curr Treat Options Oncol. 2019;20:84.
The Diagnosis: Nevoid Basal Cell Carcinoma Syndrome
Nevoid basal cell carcinoma syndrome (NBCCS), also known as Gorlin syndrome, is a rare autosomal-dominant disorder that increases the risk for developing various carcinomas and affects multiple organ systems. Nevoid basal cell carcinoma syndrome is estimated at 1 per 40,000 to 60,000 individuals with no sexual predilection.1,2 Pathogenesis of NBCCS occurs through molecular alterations in the dormant hedgehog signaling pathway, causing constitutive signaling activity and a loss of function in the tumor suppressor patched 1 gene, PTCH1. As a result, the inhibition of smoothened oncogenes is released, Gli proteins are activated, and the hedgehog signaling pathway is no longer quiescent.2 Additional loss of function in the suppressor of fused homolog protein, a negative regulator of the hedgehog pathway, allows for further tumor proliferation. The crucial role these genes play in the hedgehog signaling pathway and their mutation association with NBCCS allows for molecular confirmation in the diagnosis of NBCCS. Allelic losses at the PTCH1 gene site are thought to occur in approximately 70% of NBCCS patients.2
Diagnosis of NBCCS is based on genetic testing to examine pathogenic gene variants, notably in the PTCH1 gene, and identification of characteristic clinical findings.2 Diagnosis of NBCCS requires either 2 minor suggestive criteria and 1 major suggestive criterion, 2 major suggestive criteria and 1 minor suggestive criterion, or 1 major suggestive criterion with molecular confirmation. The presence of basal cell carcinomas (BCCs) before 20 years of age or an excessive numbers of BCCs, keratocystic odontogenic tumors (KOTs), palmar or plantar pitting, and first-degree relatives with NBCCS are classified as major suggestive criteria.2 Nevoid basal cell carcinoma syndrome patients typically have BCCs that crust, ulcerate, or bleed. Minor suggestive criteria for NBCCS are rib abnormalities, skeletal malformations, macrocephaly, cleft lip or palate, and desmoplastic medulloblastoma.2-4 Suppressor of fused homolog protein mutations may increase the risk for desmoplastic medulloblastoma in NBCCS patients. Our patient had 4 of the major suggestive criteria, including a history of KOTs, multiple BCCs, first-degree relatives with NBCCS, and palmar or plantar pitting (bottom quiz image), while having 1 minor suggestive criterion of frontal bossing.
Patients with NBCCS have high phenotypic variability, as their skin carcinomas do not have the classic features of pearly surfaces or corkscrew telangiectasia that typically are associated with BCCs.1 Basal cell carcinomas in NBCCS-affected individuals usually are indistinguishable from sporadic lesions that arise in sun-exposed areas, making NBCCS difficult to diagnose. These sporadic lesions often are misdiagnosed as psoriatic or eczematous lesions, and additional subsequent examination is required. The findings of multiple papules and plaques spanning the body as well as lesions with rolled borders and ulcerated bases, indicative of BCCs, aid dermatologists in distinguishing benign lesions from those of NBCCS.1
Additional differential diagnoses are required to distinguish NBCCS from other similar inherited skin disorders that are characterized by BCCs. The presence of multiple incidental BCCs early in life remains a histopathologic clue for NBCCS diagnosis, as opposed to Rombo syndrome, in which BCCs often develop in adulthood.2,4 In addition, although both Bazex syndrome and Muir-Torre syndrome are characterized by the early onset of BCCs, the lack of skeletal abnormalities and palmar and plantar pitting distinguish these entities from NBCCS.2,4 Furthermore, though psoriasis also can present on the scalp, clinical presentation often includes well-demarcated and symmetric plaques that are erythematous and silvery, all of which were not present in our patient and typically are not seen in NBCCS.5
The recommended treatment of NBCCS is vismodegib, a specific oncogene inhibitor. This medication suppresses the hedgehog signaling pathway by inhibiting smoothened oncogenes and downstream target molecules, thereby decreasing tumor proliferation.6 In doing so, vismodegib inhibits the development of new BCCs while reducing the burden of present ones. Additionally, vismodegib appears to effectively treat KOTs. If successful, this medication may be able to suppress KOTs in patients with NBCCS and thus facilitate surgery.6 Additional hedgehog inhibitors include patidegib, sonidegib, and itraconazole. Patidegib gel 2% currently is in phase 3 clinical trials for evaluation of efficacy and safety in treatment of NBCCS. Sonidegib is approved for the treatment of locally advanced BCCs in the United States and the European Union and for both locally advanced BCCs and metastatic BCCs in Switzerland and Australia.7 Further research is needed before recommending antifungal itraconazole for NBCCS clinical use.8 Other medications for localized areas include topical application of 5-fluorouracil and imiquimod.2
The Diagnosis: Nevoid Basal Cell Carcinoma Syndrome
Nevoid basal cell carcinoma syndrome (NBCCS), also known as Gorlin syndrome, is a rare autosomal-dominant disorder that increases the risk for developing various carcinomas and affects multiple organ systems. Nevoid basal cell carcinoma syndrome is estimated at 1 per 40,000 to 60,000 individuals with no sexual predilection.1,2 Pathogenesis of NBCCS occurs through molecular alterations in the dormant hedgehog signaling pathway, causing constitutive signaling activity and a loss of function in the tumor suppressor patched 1 gene, PTCH1. As a result, the inhibition of smoothened oncogenes is released, Gli proteins are activated, and the hedgehog signaling pathway is no longer quiescent.2 Additional loss of function in the suppressor of fused homolog protein, a negative regulator of the hedgehog pathway, allows for further tumor proliferation. The crucial role these genes play in the hedgehog signaling pathway and their mutation association with NBCCS allows for molecular confirmation in the diagnosis of NBCCS. Allelic losses at the PTCH1 gene site are thought to occur in approximately 70% of NBCCS patients.2
Diagnosis of NBCCS is based on genetic testing to examine pathogenic gene variants, notably in the PTCH1 gene, and identification of characteristic clinical findings.2 Diagnosis of NBCCS requires either 2 minor suggestive criteria and 1 major suggestive criterion, 2 major suggestive criteria and 1 minor suggestive criterion, or 1 major suggestive criterion with molecular confirmation. The presence of basal cell carcinomas (BCCs) before 20 years of age or an excessive numbers of BCCs, keratocystic odontogenic tumors (KOTs), palmar or plantar pitting, and first-degree relatives with NBCCS are classified as major suggestive criteria.2 Nevoid basal cell carcinoma syndrome patients typically have BCCs that crust, ulcerate, or bleed. Minor suggestive criteria for NBCCS are rib abnormalities, skeletal malformations, macrocephaly, cleft lip or palate, and desmoplastic medulloblastoma.2-4 Suppressor of fused homolog protein mutations may increase the risk for desmoplastic medulloblastoma in NBCCS patients. Our patient had 4 of the major suggestive criteria, including a history of KOTs, multiple BCCs, first-degree relatives with NBCCS, and palmar or plantar pitting (bottom quiz image), while having 1 minor suggestive criterion of frontal bossing.
Patients with NBCCS have high phenotypic variability, as their skin carcinomas do not have the classic features of pearly surfaces or corkscrew telangiectasia that typically are associated with BCCs.1 Basal cell carcinomas in NBCCS-affected individuals usually are indistinguishable from sporadic lesions that arise in sun-exposed areas, making NBCCS difficult to diagnose. These sporadic lesions often are misdiagnosed as psoriatic or eczematous lesions, and additional subsequent examination is required. The findings of multiple papules and plaques spanning the body as well as lesions with rolled borders and ulcerated bases, indicative of BCCs, aid dermatologists in distinguishing benign lesions from those of NBCCS.1
Additional differential diagnoses are required to distinguish NBCCS from other similar inherited skin disorders that are characterized by BCCs. The presence of multiple incidental BCCs early in life remains a histopathologic clue for NBCCS diagnosis, as opposed to Rombo syndrome, in which BCCs often develop in adulthood.2,4 In addition, although both Bazex syndrome and Muir-Torre syndrome are characterized by the early onset of BCCs, the lack of skeletal abnormalities and palmar and plantar pitting distinguish these entities from NBCCS.2,4 Furthermore, though psoriasis also can present on the scalp, clinical presentation often includes well-demarcated and symmetric plaques that are erythematous and silvery, all of which were not present in our patient and typically are not seen in NBCCS.5
The recommended treatment of NBCCS is vismodegib, a specific oncogene inhibitor. This medication suppresses the hedgehog signaling pathway by inhibiting smoothened oncogenes and downstream target molecules, thereby decreasing tumor proliferation.6 In doing so, vismodegib inhibits the development of new BCCs while reducing the burden of present ones. Additionally, vismodegib appears to effectively treat KOTs. If successful, this medication may be able to suppress KOTs in patients with NBCCS and thus facilitate surgery.6 Additional hedgehog inhibitors include patidegib, sonidegib, and itraconazole. Patidegib gel 2% currently is in phase 3 clinical trials for evaluation of efficacy and safety in treatment of NBCCS. Sonidegib is approved for the treatment of locally advanced BCCs in the United States and the European Union and for both locally advanced BCCs and metastatic BCCs in Switzerland and Australia.7 Further research is needed before recommending antifungal itraconazole for NBCCS clinical use.8 Other medications for localized areas include topical application of 5-fluorouracil and imiquimod.2
- Sangehra R, Grewal P. Gorlin syndrome presentation and the importance of differential diagnosis of skin cancer: a case report. J Pharm Pharm Sci. 2018;21:222-224.
- Bresler S, Padwa B, Granter S. Nevoid basal cell carcinoma syndrome (Gorlin syndrome). Head Neck Pathol. 2016;10:119-124.
- Fujii K, Miyashita T. Gorlin syndrome (nevoid basal cell carcinoma syndrome): update and literature review. Pediatr Int. 2014;56:667-674.
- Evans G, Farndon P. Nevoid basal cell carcinoma syndrome. GeneReviews [Internet]. University of Washington; 1993-2020.
- Kim WB, Jerome D, Yeung J. Diagnosis and management of psoriasis. Can Fam Physician. 2017;63:278-285.
- Booms P, Harth M, Sader R, et al. Vismodegib hedgehog-signaling inhibition and treatment of basal cell carcinomas as well as keratocystic odontogenic tumors in Gorlin syndrome. Ann Maxillofac Surg. 2015;5:14-19.
- Gutzmer R, Soloon J. Hedgehog pathway inhibition for the treatment of basal cell carcinoma. Target Oncol. 2019;14:253-267.
- Leavitt E, Lask G, Martin S. Sonic hedgehog pathway inhibition in the treatment of advanced basal cell carcinoma. Curr Treat Options Oncol. 2019;20:84.
- Sangehra R, Grewal P. Gorlin syndrome presentation and the importance of differential diagnosis of skin cancer: a case report. J Pharm Pharm Sci. 2018;21:222-224.
- Bresler S, Padwa B, Granter S. Nevoid basal cell carcinoma syndrome (Gorlin syndrome). Head Neck Pathol. 2016;10:119-124.
- Fujii K, Miyashita T. Gorlin syndrome (nevoid basal cell carcinoma syndrome): update and literature review. Pediatr Int. 2014;56:667-674.
- Evans G, Farndon P. Nevoid basal cell carcinoma syndrome. GeneReviews [Internet]. University of Washington; 1993-2020.
- Kim WB, Jerome D, Yeung J. Diagnosis and management of psoriasis. Can Fam Physician. 2017;63:278-285.
- Booms P, Harth M, Sader R, et al. Vismodegib hedgehog-signaling inhibition and treatment of basal cell carcinomas as well as keratocystic odontogenic tumors in Gorlin syndrome. Ann Maxillofac Surg. 2015;5:14-19.
- Gutzmer R, Soloon J. Hedgehog pathway inhibition for the treatment of basal cell carcinoma. Target Oncol. 2019;14:253-267.
- Leavitt E, Lask G, Martin S. Sonic hedgehog pathway inhibition in the treatment of advanced basal cell carcinoma. Curr Treat Options Oncol. 2019;20:84.
A 63-year-old man with frontal bossing and a history of jaw cysts presented with numerous lesions on the scalp, trunk, and legs with recurrent bleeding. Both of his siblings had similar findings. Many lesions had been present for at least 40 years. Physical examination revealed a large, irregular, atrophic, hyperpigmented plaque on the central scalp with multiple translucent hyperpigmented papules at the periphery (top). Similar papules and plaques were present at the temples, around the waist, and on the distal lower extremities, leading to surgical excision of the largest leg lesions. In addition, there were many pinpoint pits on both palms (bottom). A biopsy was submitted for review, which confirmed basal cell carcinomas on the scalp.
Large Leg Ulcers After Swimming in the Ocean
The Diagnosis: Vibrio vulnificus Infection
At the initial presentation, the differential diagnosis included infectious processes such as bacterial or angioinvasive fungal infections or an inflammatory process such as pyoderma gangrenosum. Blood cultures were found to be positive for pansensitive Vibrio vulnificus. He initially was treated with piperacillin-tazobactam and received surgical debridement of the affected tissues. Pathologic interpretation of the wound tissues revealed a diagnosis of necrotizing softtissue infection and positive Candida albicans growth. He received topical bacitracin on discharge as well as a 7-day course of amoxicillin-clavulanate and fluconazole. He continued to receive debridement procedures and skin grafts, followed by topical mupirocin treatment and silver sulfadiazine. He was seen 6 weeks after discharge with healing wounds and healthy-appearing granulation tissue at the base.
Our patient’s presentation of retiform purpura with stellate necrosis was consistent with a wide range of serious pathologies ranging from medium-vessel vasculitis to thromboembolic phenomena and angioinvasive fungal infections.1 Although Vibrio infection rarely is the first explanation that comes to mind when observing necrotic retiform purpura, the chronic nonhealing injury on the leg combined with the recent history of ocean swimming made V vulnificus stand out as a likely culprit. Although V vulnificus infection traditionally presents with cellulitis, edema, and hemorrhagic bulla,2 necrosis also has been observed.3Vibrio vulnificus produces multiple virulence factors, and it is believed that these severe cutaneous symptoms are attributable to the production of a specific metalloprotease that enhances vascular permeability, thereby inducing hemorrhage within the vascular basement membrane zone.2
Vibrio vulnificus is an opportunistic bacterial pathogen associated with consumption of contaminated seafood or swimming in ocean waters with open wounds. Infections are rare, with only approximately 100 cases reported annually in the United States.4 However, V vulnificus infections have demonstrated increasing incidence in recent years, especially infections of pre-existing wounds.4,5 Risk factors for their development include age over 40 years and underlying conditions including liver disease, diabetes mellitus, and immune dysfunction.4Vibrio vulnificus infections also demonstrate a strong male predilection, with almost 90% of infections occurring in males.4 Although the precise etiology of this sex discrepancy remains unknown, estrogen has been suggested to be a protective factor.6 Alternatively, behavioral differences also have been proposed as possible explanations for this discrepancy, with women less likely to consume seafood or go swimming. However, epidemiologic data reveal strong correlations between male sex and liver cirrhosis, a primary risk factor for V vulnificus infections, suggesting that male sex may simply be a confounding variable.7
Infections with V vulnificus are notable for their short incubation periods, with onset of symptoms occurring within 24 hours of exposure, making prompt diagnosis and treatment of high importance.8 Although rare, V vulnificus infections are associated with high mortality rates. From 1988 to 2010, nearly 600 deaths were reported secondary to V vulnificus infections.4 Wound infections carry a 17.6% fatality rate,4 while bloodborne V vulnificus infections exceed 50% fatality.8 Although sepsis secondary to V vulnificus usually is caused by ingestion of raw or undercooked shellfish, primarily oysters,4 our case highlights a rarer instance of both sepsis and localized infection stemming from ocean water exposure.
Vibrio vulnificus is an obligate halophile and therefore is found in marine environments rather than freshwater bodies. However, it rarely is isolated from bodies of water with salinities over 25 parts per thousand, such as the Mediterranean Sea; it usually is found in warmer waters, making it more common in the summer months from May to October.4 Given this proclivity for warmer environments, climate change has contributed to both a greater incidence and global distribution of V vulnificus. 9,10
Treatment of V vulnificus infections centers on antibiotic treatment, with Vibrio species generally demonstrating susceptibility to most antibiotics of human significance.11 However, some Vibrio isolates within the United States have demonstrated antibiotic resistance; 45% of a variety of clinical and environmental samples from South Carolina and Georgia demonstrated resistance to at least 3 antibiotic classes, and 17.3% resisted 8 or more classes of antibiotics.12 These included medications such as doxycycline, tetracycline, aminoglycosides, and cephalosporins—agents that normally are prescribed for V vulnificus infections. Although tetracyclines have long been touted as the preferred treatment of V vulnificus infections, the spread of antibiotic resistance may require greater reliance on alternative regimens such as combinations of cephalosporins and doxycycline or a single fluoroquinolone.13 Although rare, Vibrio infections can have rapidly fatal consequences and should be given serious consideration when evaluating patients with relevant risk factors.
The differential diagnosis included angioinvasive mucormycosis, calciphylaxis, pyoderma gangrenosum, and Stevens-Johnson syndrome/toxic epidermal necrolysis. Mucormycosis is a fungal infection caused by Mucorales fungi that most commonly is seen in patients with diabetes mellitus, hematologic malignancies, neutropenia, and immunocompromise.14 Calciphylaxis is a condition involving microvascular occlusion due to diffuse calcium deposition in cutaneous blood vessels. It typically presents as violaceous retiform patches and plaques commonly seen on areas such as the thighs, buttocks, or abdomen and usually is associated with chronic renal failure, hemodialysis, and/or secondary hyperparathyroidism.15 Pyoderma gangrenosum is an inflammatory condition involving neutrophilic ulceration of the skin that typically presents as ulceration with a classically undermined border. It frequently is considered a diagnosis of exclusion and therefore requires that providers rule out other causes of ulceration prior to diagnosis.16 Stevens-Johnson syndrome/toxic epidermal necrolysis is a rare drug reaction involving mucosal erosions and cutaneous detachment.17 This diagnosis is less likely given that our patient lacked mucosal involvement and did not have any notable medication exposures prior to symptom onset.
- Wysong A, Venkatesan P. An approach to the patient with retiform purpura. Dermatol Ther. 2011;24:151-172. doi:10.1111/j .1529-8019.2011.01392.x
- Miyoshi S-I. Vibrio vulnificus infection and metalloprotease. J Dermatol. 2006;33:589-595. doi:10.1111/j.1346-8138.2006.00139.x
- Patel VJ, Gardner E, Burton CS. Vibrio vulnificus septicemia and leg ulcer. J Am Acad Dermatol. 2002;46(5 suppl):S144-S145. doi:10.1067 /mjd.2002.107778
- Baker-Austin C, Oliver JD. Vibrio vulnificus: new insights into a deadly opportunistic pathogen. Environ Microbiol. 2018;20:423-430. doi:10.1111/1462-2920.13955
- Preliminary FoodNet data on the incidence of infection with pathogens transmitted commonly through food —10 states, 2009. CDC website. Published April 16, 2010. Accessed November 3, 2021. https://www.cdc .gov/mmwr/preview/mmwrhtml/mm5914a2.htm
- Merkel SM, Alexander S, Zufall E, et al. Essential role for estrogen in protection against Vibrio vulnificus-induced endotoxic shock. Infect Immun. 2001;69:6119-6122. doi:10.1128/IAI.69.10.6119 -6122.2001
- Scaglione S, Kliethermes S, Cao G, et al. The epidemiology of cirrhosis in the United States: a population-based study. J Clin Gastroenterol. 2015;49:690-696. doi:10.1097/MCG.0000000000000208
- Jones M, Oliver J. Vibrio vulnificus: disease and pathogenesis [published online December 20, 2020]. Infect Immun. https://doi.org/10.1128 /IAI.01046-08
- Paz S, Bisharat N, Paz E, et al. Climate change and the emergence of Vibrio vulnificus disease in Israel. Environ Res. 2007;103:390-396. doi:10.1016/j.envres.2006.07.002
- Martinez-Urtaza J, Bowers JC, Trinanes J, et al. Climate anomalies and the increasing risk of Vibrio parahaemolyticus and Vibrio vulnificus illnesses. Food Res Int. 2010;43:1780-1790. doi:10.1016/j. foodres.2010.04.001
- Oliver JD. Vibrio vulnificus. In: Thompson FL, Austin B, Swings J, eds. The Biology of Vibrios. ASM Press; 2006:349-366.
- Baker-Austin C, McArthur JV, Lindell AH, et al. Multi-site analysis reveals widespread antibiotic resistance in the marine pathogen Vibrio vulnificus. Microb Ecol. 2009;57:151-159. doi:10.1007 /s00248-008-9413-8
- Elmahdi S, DaSilva LV, Parveen S. Antibiotic resistance of Vibrio parahaemolyticus and Vibrio vulnificus in various countries: a review. Food Microbiol. 2016;57:128-134. doi:10.1016/j.fm.2016.02.008
- Prasad P, Wong V, Burgin S, et al. Mucormycosis. VisualDx website. Accessed November 13, 2021. https://www-visualdx-com.proxy.lib.ohio-state.edu/visualdx/diagnosis/mucormycosis?diagnosisId=51981 &moduleId=101
- Blum A, Song P, Tan B, et al. Calciphylaxis. VisualDx website. Accessed November 13, 2021. https://www-visualdx-com.proxy.lib.ohio-state.edu/visualdx/diagnosis/calciphylaxis?diagnosisId=51241&moduleId=101
- Cohen J, Wong V, Burgin S. Pyoderma gangrenosum. VisualDx website. Accessed November 13, 2021. https://www-visualdx-com.proxy.lib.ohio-state.edu/visualdx/diagnosis/pyoderma+gangrenosum?diagnosis Id=52242&moduleId=101
- Walls A, Burgin S. Stevens-Johnson syndrome. VisualDx website. Accessed November 13, 2021. https://www-visualdx-com.proxy.lib.ohio-state.edu/visualdx/diagnosis/stevens-johnson+syndrome?diagnosisId=52342&moduleId=101
The Diagnosis: Vibrio vulnificus Infection
At the initial presentation, the differential diagnosis included infectious processes such as bacterial or angioinvasive fungal infections or an inflammatory process such as pyoderma gangrenosum. Blood cultures were found to be positive for pansensitive Vibrio vulnificus. He initially was treated with piperacillin-tazobactam and received surgical debridement of the affected tissues. Pathologic interpretation of the wound tissues revealed a diagnosis of necrotizing softtissue infection and positive Candida albicans growth. He received topical bacitracin on discharge as well as a 7-day course of amoxicillin-clavulanate and fluconazole. He continued to receive debridement procedures and skin grafts, followed by topical mupirocin treatment and silver sulfadiazine. He was seen 6 weeks after discharge with healing wounds and healthy-appearing granulation tissue at the base.
Our patient’s presentation of retiform purpura with stellate necrosis was consistent with a wide range of serious pathologies ranging from medium-vessel vasculitis to thromboembolic phenomena and angioinvasive fungal infections.1 Although Vibrio infection rarely is the first explanation that comes to mind when observing necrotic retiform purpura, the chronic nonhealing injury on the leg combined with the recent history of ocean swimming made V vulnificus stand out as a likely culprit. Although V vulnificus infection traditionally presents with cellulitis, edema, and hemorrhagic bulla,2 necrosis also has been observed.3Vibrio vulnificus produces multiple virulence factors, and it is believed that these severe cutaneous symptoms are attributable to the production of a specific metalloprotease that enhances vascular permeability, thereby inducing hemorrhage within the vascular basement membrane zone.2
Vibrio vulnificus is an opportunistic bacterial pathogen associated with consumption of contaminated seafood or swimming in ocean waters with open wounds. Infections are rare, with only approximately 100 cases reported annually in the United States.4 However, V vulnificus infections have demonstrated increasing incidence in recent years, especially infections of pre-existing wounds.4,5 Risk factors for their development include age over 40 years and underlying conditions including liver disease, diabetes mellitus, and immune dysfunction.4Vibrio vulnificus infections also demonstrate a strong male predilection, with almost 90% of infections occurring in males.4 Although the precise etiology of this sex discrepancy remains unknown, estrogen has been suggested to be a protective factor.6 Alternatively, behavioral differences also have been proposed as possible explanations for this discrepancy, with women less likely to consume seafood or go swimming. However, epidemiologic data reveal strong correlations between male sex and liver cirrhosis, a primary risk factor for V vulnificus infections, suggesting that male sex may simply be a confounding variable.7
Infections with V vulnificus are notable for their short incubation periods, with onset of symptoms occurring within 24 hours of exposure, making prompt diagnosis and treatment of high importance.8 Although rare, V vulnificus infections are associated with high mortality rates. From 1988 to 2010, nearly 600 deaths were reported secondary to V vulnificus infections.4 Wound infections carry a 17.6% fatality rate,4 while bloodborne V vulnificus infections exceed 50% fatality.8 Although sepsis secondary to V vulnificus usually is caused by ingestion of raw or undercooked shellfish, primarily oysters,4 our case highlights a rarer instance of both sepsis and localized infection stemming from ocean water exposure.
Vibrio vulnificus is an obligate halophile and therefore is found in marine environments rather than freshwater bodies. However, it rarely is isolated from bodies of water with salinities over 25 parts per thousand, such as the Mediterranean Sea; it usually is found in warmer waters, making it more common in the summer months from May to October.4 Given this proclivity for warmer environments, climate change has contributed to both a greater incidence and global distribution of V vulnificus. 9,10
Treatment of V vulnificus infections centers on antibiotic treatment, with Vibrio species generally demonstrating susceptibility to most antibiotics of human significance.11 However, some Vibrio isolates within the United States have demonstrated antibiotic resistance; 45% of a variety of clinical and environmental samples from South Carolina and Georgia demonstrated resistance to at least 3 antibiotic classes, and 17.3% resisted 8 or more classes of antibiotics.12 These included medications such as doxycycline, tetracycline, aminoglycosides, and cephalosporins—agents that normally are prescribed for V vulnificus infections. Although tetracyclines have long been touted as the preferred treatment of V vulnificus infections, the spread of antibiotic resistance may require greater reliance on alternative regimens such as combinations of cephalosporins and doxycycline or a single fluoroquinolone.13 Although rare, Vibrio infections can have rapidly fatal consequences and should be given serious consideration when evaluating patients with relevant risk factors.
The differential diagnosis included angioinvasive mucormycosis, calciphylaxis, pyoderma gangrenosum, and Stevens-Johnson syndrome/toxic epidermal necrolysis. Mucormycosis is a fungal infection caused by Mucorales fungi that most commonly is seen in patients with diabetes mellitus, hematologic malignancies, neutropenia, and immunocompromise.14 Calciphylaxis is a condition involving microvascular occlusion due to diffuse calcium deposition in cutaneous blood vessels. It typically presents as violaceous retiform patches and plaques commonly seen on areas such as the thighs, buttocks, or abdomen and usually is associated with chronic renal failure, hemodialysis, and/or secondary hyperparathyroidism.15 Pyoderma gangrenosum is an inflammatory condition involving neutrophilic ulceration of the skin that typically presents as ulceration with a classically undermined border. It frequently is considered a diagnosis of exclusion and therefore requires that providers rule out other causes of ulceration prior to diagnosis.16 Stevens-Johnson syndrome/toxic epidermal necrolysis is a rare drug reaction involving mucosal erosions and cutaneous detachment.17 This diagnosis is less likely given that our patient lacked mucosal involvement and did not have any notable medication exposures prior to symptom onset.
The Diagnosis: Vibrio vulnificus Infection
At the initial presentation, the differential diagnosis included infectious processes such as bacterial or angioinvasive fungal infections or an inflammatory process such as pyoderma gangrenosum. Blood cultures were found to be positive for pansensitive Vibrio vulnificus. He initially was treated with piperacillin-tazobactam and received surgical debridement of the affected tissues. Pathologic interpretation of the wound tissues revealed a diagnosis of necrotizing softtissue infection and positive Candida albicans growth. He received topical bacitracin on discharge as well as a 7-day course of amoxicillin-clavulanate and fluconazole. He continued to receive debridement procedures and skin grafts, followed by topical mupirocin treatment and silver sulfadiazine. He was seen 6 weeks after discharge with healing wounds and healthy-appearing granulation tissue at the base.
Our patient’s presentation of retiform purpura with stellate necrosis was consistent with a wide range of serious pathologies ranging from medium-vessel vasculitis to thromboembolic phenomena and angioinvasive fungal infections.1 Although Vibrio infection rarely is the first explanation that comes to mind when observing necrotic retiform purpura, the chronic nonhealing injury on the leg combined with the recent history of ocean swimming made V vulnificus stand out as a likely culprit. Although V vulnificus infection traditionally presents with cellulitis, edema, and hemorrhagic bulla,2 necrosis also has been observed.3Vibrio vulnificus produces multiple virulence factors, and it is believed that these severe cutaneous symptoms are attributable to the production of a specific metalloprotease that enhances vascular permeability, thereby inducing hemorrhage within the vascular basement membrane zone.2
Vibrio vulnificus is an opportunistic bacterial pathogen associated with consumption of contaminated seafood or swimming in ocean waters with open wounds. Infections are rare, with only approximately 100 cases reported annually in the United States.4 However, V vulnificus infections have demonstrated increasing incidence in recent years, especially infections of pre-existing wounds.4,5 Risk factors for their development include age over 40 years and underlying conditions including liver disease, diabetes mellitus, and immune dysfunction.4Vibrio vulnificus infections also demonstrate a strong male predilection, with almost 90% of infections occurring in males.4 Although the precise etiology of this sex discrepancy remains unknown, estrogen has been suggested to be a protective factor.6 Alternatively, behavioral differences also have been proposed as possible explanations for this discrepancy, with women less likely to consume seafood or go swimming. However, epidemiologic data reveal strong correlations between male sex and liver cirrhosis, a primary risk factor for V vulnificus infections, suggesting that male sex may simply be a confounding variable.7
Infections with V vulnificus are notable for their short incubation periods, with onset of symptoms occurring within 24 hours of exposure, making prompt diagnosis and treatment of high importance.8 Although rare, V vulnificus infections are associated with high mortality rates. From 1988 to 2010, nearly 600 deaths were reported secondary to V vulnificus infections.4 Wound infections carry a 17.6% fatality rate,4 while bloodborne V vulnificus infections exceed 50% fatality.8 Although sepsis secondary to V vulnificus usually is caused by ingestion of raw or undercooked shellfish, primarily oysters,4 our case highlights a rarer instance of both sepsis and localized infection stemming from ocean water exposure.
Vibrio vulnificus is an obligate halophile and therefore is found in marine environments rather than freshwater bodies. However, it rarely is isolated from bodies of water with salinities over 25 parts per thousand, such as the Mediterranean Sea; it usually is found in warmer waters, making it more common in the summer months from May to October.4 Given this proclivity for warmer environments, climate change has contributed to both a greater incidence and global distribution of V vulnificus. 9,10
Treatment of V vulnificus infections centers on antibiotic treatment, with Vibrio species generally demonstrating susceptibility to most antibiotics of human significance.11 However, some Vibrio isolates within the United States have demonstrated antibiotic resistance; 45% of a variety of clinical and environmental samples from South Carolina and Georgia demonstrated resistance to at least 3 antibiotic classes, and 17.3% resisted 8 or more classes of antibiotics.12 These included medications such as doxycycline, tetracycline, aminoglycosides, and cephalosporins—agents that normally are prescribed for V vulnificus infections. Although tetracyclines have long been touted as the preferred treatment of V vulnificus infections, the spread of antibiotic resistance may require greater reliance on alternative regimens such as combinations of cephalosporins and doxycycline or a single fluoroquinolone.13 Although rare, Vibrio infections can have rapidly fatal consequences and should be given serious consideration when evaluating patients with relevant risk factors.
The differential diagnosis included angioinvasive mucormycosis, calciphylaxis, pyoderma gangrenosum, and Stevens-Johnson syndrome/toxic epidermal necrolysis. Mucormycosis is a fungal infection caused by Mucorales fungi that most commonly is seen in patients with diabetes mellitus, hematologic malignancies, neutropenia, and immunocompromise.14 Calciphylaxis is a condition involving microvascular occlusion due to diffuse calcium deposition in cutaneous blood vessels. It typically presents as violaceous retiform patches and plaques commonly seen on areas such as the thighs, buttocks, or abdomen and usually is associated with chronic renal failure, hemodialysis, and/or secondary hyperparathyroidism.15 Pyoderma gangrenosum is an inflammatory condition involving neutrophilic ulceration of the skin that typically presents as ulceration with a classically undermined border. It frequently is considered a diagnosis of exclusion and therefore requires that providers rule out other causes of ulceration prior to diagnosis.16 Stevens-Johnson syndrome/toxic epidermal necrolysis is a rare drug reaction involving mucosal erosions and cutaneous detachment.17 This diagnosis is less likely given that our patient lacked mucosal involvement and did not have any notable medication exposures prior to symptom onset.
- Wysong A, Venkatesan P. An approach to the patient with retiform purpura. Dermatol Ther. 2011;24:151-172. doi:10.1111/j .1529-8019.2011.01392.x
- Miyoshi S-I. Vibrio vulnificus infection and metalloprotease. J Dermatol. 2006;33:589-595. doi:10.1111/j.1346-8138.2006.00139.x
- Patel VJ, Gardner E, Burton CS. Vibrio vulnificus septicemia and leg ulcer. J Am Acad Dermatol. 2002;46(5 suppl):S144-S145. doi:10.1067 /mjd.2002.107778
- Baker-Austin C, Oliver JD. Vibrio vulnificus: new insights into a deadly opportunistic pathogen. Environ Microbiol. 2018;20:423-430. doi:10.1111/1462-2920.13955
- Preliminary FoodNet data on the incidence of infection with pathogens transmitted commonly through food —10 states, 2009. CDC website. Published April 16, 2010. Accessed November 3, 2021. https://www.cdc .gov/mmwr/preview/mmwrhtml/mm5914a2.htm
- Merkel SM, Alexander S, Zufall E, et al. Essential role for estrogen in protection against Vibrio vulnificus-induced endotoxic shock. Infect Immun. 2001;69:6119-6122. doi:10.1128/IAI.69.10.6119 -6122.2001
- Scaglione S, Kliethermes S, Cao G, et al. The epidemiology of cirrhosis in the United States: a population-based study. J Clin Gastroenterol. 2015;49:690-696. doi:10.1097/MCG.0000000000000208
- Jones M, Oliver J. Vibrio vulnificus: disease and pathogenesis [published online December 20, 2020]. Infect Immun. https://doi.org/10.1128 /IAI.01046-08
- Paz S, Bisharat N, Paz E, et al. Climate change and the emergence of Vibrio vulnificus disease in Israel. Environ Res. 2007;103:390-396. doi:10.1016/j.envres.2006.07.002
- Martinez-Urtaza J, Bowers JC, Trinanes J, et al. Climate anomalies and the increasing risk of Vibrio parahaemolyticus and Vibrio vulnificus illnesses. Food Res Int. 2010;43:1780-1790. doi:10.1016/j. foodres.2010.04.001
- Oliver JD. Vibrio vulnificus. In: Thompson FL, Austin B, Swings J, eds. The Biology of Vibrios. ASM Press; 2006:349-366.
- Baker-Austin C, McArthur JV, Lindell AH, et al. Multi-site analysis reveals widespread antibiotic resistance in the marine pathogen Vibrio vulnificus. Microb Ecol. 2009;57:151-159. doi:10.1007 /s00248-008-9413-8
- Elmahdi S, DaSilva LV, Parveen S. Antibiotic resistance of Vibrio parahaemolyticus and Vibrio vulnificus in various countries: a review. Food Microbiol. 2016;57:128-134. doi:10.1016/j.fm.2016.02.008
- Prasad P, Wong V, Burgin S, et al. Mucormycosis. VisualDx website. Accessed November 13, 2021. https://www-visualdx-com.proxy.lib.ohio-state.edu/visualdx/diagnosis/mucormycosis?diagnosisId=51981 &moduleId=101
- Blum A, Song P, Tan B, et al. Calciphylaxis. VisualDx website. Accessed November 13, 2021. https://www-visualdx-com.proxy.lib.ohio-state.edu/visualdx/diagnosis/calciphylaxis?diagnosisId=51241&moduleId=101
- Cohen J, Wong V, Burgin S. Pyoderma gangrenosum. VisualDx website. Accessed November 13, 2021. https://www-visualdx-com.proxy.lib.ohio-state.edu/visualdx/diagnosis/pyoderma+gangrenosum?diagnosis Id=52242&moduleId=101
- Walls A, Burgin S. Stevens-Johnson syndrome. VisualDx website. Accessed November 13, 2021. https://www-visualdx-com.proxy.lib.ohio-state.edu/visualdx/diagnosis/stevens-johnson+syndrome?diagnosisId=52342&moduleId=101
- Wysong A, Venkatesan P. An approach to the patient with retiform purpura. Dermatol Ther. 2011;24:151-172. doi:10.1111/j .1529-8019.2011.01392.x
- Miyoshi S-I. Vibrio vulnificus infection and metalloprotease. J Dermatol. 2006;33:589-595. doi:10.1111/j.1346-8138.2006.00139.x
- Patel VJ, Gardner E, Burton CS. Vibrio vulnificus septicemia and leg ulcer. J Am Acad Dermatol. 2002;46(5 suppl):S144-S145. doi:10.1067 /mjd.2002.107778
- Baker-Austin C, Oliver JD. Vibrio vulnificus: new insights into a deadly opportunistic pathogen. Environ Microbiol. 2018;20:423-430. doi:10.1111/1462-2920.13955
- Preliminary FoodNet data on the incidence of infection with pathogens transmitted commonly through food —10 states, 2009. CDC website. Published April 16, 2010. Accessed November 3, 2021. https://www.cdc .gov/mmwr/preview/mmwrhtml/mm5914a2.htm
- Merkel SM, Alexander S, Zufall E, et al. Essential role for estrogen in protection against Vibrio vulnificus-induced endotoxic shock. Infect Immun. 2001;69:6119-6122. doi:10.1128/IAI.69.10.6119 -6122.2001
- Scaglione S, Kliethermes S, Cao G, et al. The epidemiology of cirrhosis in the United States: a population-based study. J Clin Gastroenterol. 2015;49:690-696. doi:10.1097/MCG.0000000000000208
- Jones M, Oliver J. Vibrio vulnificus: disease and pathogenesis [published online December 20, 2020]. Infect Immun. https://doi.org/10.1128 /IAI.01046-08
- Paz S, Bisharat N, Paz E, et al. Climate change and the emergence of Vibrio vulnificus disease in Israel. Environ Res. 2007;103:390-396. doi:10.1016/j.envres.2006.07.002
- Martinez-Urtaza J, Bowers JC, Trinanes J, et al. Climate anomalies and the increasing risk of Vibrio parahaemolyticus and Vibrio vulnificus illnesses. Food Res Int. 2010;43:1780-1790. doi:10.1016/j. foodres.2010.04.001
- Oliver JD. Vibrio vulnificus. In: Thompson FL, Austin B, Swings J, eds. The Biology of Vibrios. ASM Press; 2006:349-366.
- Baker-Austin C, McArthur JV, Lindell AH, et al. Multi-site analysis reveals widespread antibiotic resistance in the marine pathogen Vibrio vulnificus. Microb Ecol. 2009;57:151-159. doi:10.1007 /s00248-008-9413-8
- Elmahdi S, DaSilva LV, Parveen S. Antibiotic resistance of Vibrio parahaemolyticus and Vibrio vulnificus in various countries: a review. Food Microbiol. 2016;57:128-134. doi:10.1016/j.fm.2016.02.008
- Prasad P, Wong V, Burgin S, et al. Mucormycosis. VisualDx website. Accessed November 13, 2021. https://www-visualdx-com.proxy.lib.ohio-state.edu/visualdx/diagnosis/mucormycosis?diagnosisId=51981 &moduleId=101
- Blum A, Song P, Tan B, et al. Calciphylaxis. VisualDx website. Accessed November 13, 2021. https://www-visualdx-com.proxy.lib.ohio-state.edu/visualdx/diagnosis/calciphylaxis?diagnosisId=51241&moduleId=101
- Cohen J, Wong V, Burgin S. Pyoderma gangrenosum. VisualDx website. Accessed November 13, 2021. https://www-visualdx-com.proxy.lib.ohio-state.edu/visualdx/diagnosis/pyoderma+gangrenosum?diagnosis Id=52242&moduleId=101
- Walls A, Burgin S. Stevens-Johnson syndrome. VisualDx website. Accessed November 13, 2021. https://www-visualdx-com.proxy.lib.ohio-state.edu/visualdx/diagnosis/stevens-johnson+syndrome?diagnosisId=52342&moduleId=101
A 48-year-old man presented to the emergency department with pain in both legs after swimming in the ocean surrounding Florida 1 month prior to presentation. His medical history included skin graft treatment of burns during childhood and a chronic lower extremity ulcer that developed after trauma. He received hemodialysis for acute renal failure approximately 1 month prior to the current presentation. At the current presentation he was found to be septic and quickly developed rapidly expanding regions of retiform purpura with stellate necrosis on the legs.
Bullous Pemphigoid Masquerading as a Prosthesis Allergy
To the Editor:
Bullous pemphigoid (BP) is an autoimmune bullous dermatosis characterized by tense subepidermal blisters. It primarily affects older individuals who typically report pruritus in the affected area. Subepidermal blisters are caused by a humoral and cellular autoimmune attack directed against 2 BP antigens—BP180 and BP230—which are 2 critical components of the hemidesmosome whose primary function is to anchor the epidermis to the underlying dermis. Although tense bullae typically prompt immediate consideration of BP in the differential diagnosis, early disease often is characterized by urticarial plaques that require a high degree of suspicion to make the appropriate diagnosis. Locus minoris resistentiae is a term used to describe the phenomenon of skin disease occurring at the point of least resistance.1
A 79-year-old woman with type 2 diabetes mellitus, peptic ulcer disease, and hypertension was referred to the dermatology clinic due to concern for allergic contact dermatitis limited to the area of and adjacent to a well-healed surgical wound. History and examination revealed that the patient had sustained a left femoral neck fracture 10 months prior to presentation that required closed reduction and surgical pinning. The surgical site healed well postoperatively; however, 7 months after surgery, she began to develop edema and erythema within and immediately adjacent to the surgical scar. She subsequently developed areas of superficial erosion within the erythema and was evaluated by her surgeon who was concerned for suture granuloma. Superficial wound debridement of the area was performed without improvement. Approximately 9 months after surgery, the patient developed bullae along the old surgical site, which raised concern for an allergic reaction to the implanted screws. Orthopedics elected to remove the hardware but also sent intraoperative tissue for pathologic examination, which revealed subepidermal bullae containing eosinophils and neutrophils, most consistent with a bullous drug eruption. During the ensuing weeks after hardware removal, the plaque spread along the old surgical wound, and several bullous lesions began to appear. The patient’s primary care physician became concerned for allergic contact dermatitis, possibly to the surgical scrub employed during hardware removal. He prescribed triamcinolone ointment 0.1% and referred the patient to dermatology.
Upon presentation to dermatology, the patient noted stinging pain and intense pruritus of the affected area. Examination revealed a pink edematous plaque distributed along a well-healed surgical wound (Figure). Numerous fluid-filled tense bullae were superimposed on this plaque as well as areas of superficial erosion with serum crust. An expanded examination revealed similar smaller lesions on the upper arms, inner thighs, and lateral breasts. A 4-mm punch biopsy of lesional and perilesional skin was sent for hematoxylin and eosin staining and direct immunofluorescence, which demonstrated a subepidermal bullous dermatosis with a predominance of neutrophilic inflammation as well as a band of linear IgG deposition at the dermal-epidermal junction. The patient was diagnosed with BP exhibiting a locus minoris resistentiae phenomenon within the surgical site. She was started on prednisone 1 mg/kg daily and doxycycline 100 mg twice daily and demonstrated rapid improvement.
Although the tense bullae seen in well-developed BP are fairly characteristic, the prodromal phase of this disease can present with urticarial plaques that are nonspecific. This progression is well described, but our case demonstrates the difficulty of considering BP when a patient presents with an urticarial plaque. As lesions progress to the bullous phase, they may be inappropriately diagnosed as allergic contact dermatitis, an error that may lead to unnecessary interventions (eg, removal of an implicated prosthesis). This case is a reminder that not all cutaneous eruptions in and around postsurgical scars are allergic in nature.
This case also depicts BP appearing in the locus minoris resistentiae, a well-healed surgical wound in our patient. Although many diseases have been shown to exhibit this type of isomorphic response, this phenomenon may pose diagnostic and management conundrums. Locus minoris resistentiae has been reported in many different diseases, both cutaneous and otherwise, but there likely are distinct disease- and case-specific mechanisms via which this occurs. Local phenomena reported to trigger BP include contact dermatitis, vaccination, radiation therapy, phototherapy, infection, and surgery.2 We suspect that the mechanism of locus minoris resistentiae in our patient was disruption of the architecture of the dermal-epidermal basement membrane zone due to surgical trauma. Disruption of this architecture may have resulted in exposure of previously occult antigens, recognition by T cells, T-cell stimulation of autoantibody production by B cells, binding of autoantibodies to BP180, complement deposition, recruitment of inflammatory cells, release of proteinases, and degradation of BP180 and extracellular matrix proteins.2
- Lo Schiavo A, Ruocco E, Russo T, et al. Locus minoris resistentiae: an old but still valid way of thinking in medicine. Clin Dermatol. 2014;32:553-556.
- Lo Schiavo A, Ruocco E, Brancaccio G, et al. Bullous pemphigoid: etiology, pathogenesis, and inducing factors: facts and controversies. Clin Dermatol. 2013;31:391-399.
To the Editor:
Bullous pemphigoid (BP) is an autoimmune bullous dermatosis characterized by tense subepidermal blisters. It primarily affects older individuals who typically report pruritus in the affected area. Subepidermal blisters are caused by a humoral and cellular autoimmune attack directed against 2 BP antigens—BP180 and BP230—which are 2 critical components of the hemidesmosome whose primary function is to anchor the epidermis to the underlying dermis. Although tense bullae typically prompt immediate consideration of BP in the differential diagnosis, early disease often is characterized by urticarial plaques that require a high degree of suspicion to make the appropriate diagnosis. Locus minoris resistentiae is a term used to describe the phenomenon of skin disease occurring at the point of least resistance.1
A 79-year-old woman with type 2 diabetes mellitus, peptic ulcer disease, and hypertension was referred to the dermatology clinic due to concern for allergic contact dermatitis limited to the area of and adjacent to a well-healed surgical wound. History and examination revealed that the patient had sustained a left femoral neck fracture 10 months prior to presentation that required closed reduction and surgical pinning. The surgical site healed well postoperatively; however, 7 months after surgery, she began to develop edema and erythema within and immediately adjacent to the surgical scar. She subsequently developed areas of superficial erosion within the erythema and was evaluated by her surgeon who was concerned for suture granuloma. Superficial wound debridement of the area was performed without improvement. Approximately 9 months after surgery, the patient developed bullae along the old surgical site, which raised concern for an allergic reaction to the implanted screws. Orthopedics elected to remove the hardware but also sent intraoperative tissue for pathologic examination, which revealed subepidermal bullae containing eosinophils and neutrophils, most consistent with a bullous drug eruption. During the ensuing weeks after hardware removal, the plaque spread along the old surgical wound, and several bullous lesions began to appear. The patient’s primary care physician became concerned for allergic contact dermatitis, possibly to the surgical scrub employed during hardware removal. He prescribed triamcinolone ointment 0.1% and referred the patient to dermatology.
Upon presentation to dermatology, the patient noted stinging pain and intense pruritus of the affected area. Examination revealed a pink edematous plaque distributed along a well-healed surgical wound (Figure). Numerous fluid-filled tense bullae were superimposed on this plaque as well as areas of superficial erosion with serum crust. An expanded examination revealed similar smaller lesions on the upper arms, inner thighs, and lateral breasts. A 4-mm punch biopsy of lesional and perilesional skin was sent for hematoxylin and eosin staining and direct immunofluorescence, which demonstrated a subepidermal bullous dermatosis with a predominance of neutrophilic inflammation as well as a band of linear IgG deposition at the dermal-epidermal junction. The patient was diagnosed with BP exhibiting a locus minoris resistentiae phenomenon within the surgical site. She was started on prednisone 1 mg/kg daily and doxycycline 100 mg twice daily and demonstrated rapid improvement.
Although the tense bullae seen in well-developed BP are fairly characteristic, the prodromal phase of this disease can present with urticarial plaques that are nonspecific. This progression is well described, but our case demonstrates the difficulty of considering BP when a patient presents with an urticarial plaque. As lesions progress to the bullous phase, they may be inappropriately diagnosed as allergic contact dermatitis, an error that may lead to unnecessary interventions (eg, removal of an implicated prosthesis). This case is a reminder that not all cutaneous eruptions in and around postsurgical scars are allergic in nature.
This case also depicts BP appearing in the locus minoris resistentiae, a well-healed surgical wound in our patient. Although many diseases have been shown to exhibit this type of isomorphic response, this phenomenon may pose diagnostic and management conundrums. Locus minoris resistentiae has been reported in many different diseases, both cutaneous and otherwise, but there likely are distinct disease- and case-specific mechanisms via which this occurs. Local phenomena reported to trigger BP include contact dermatitis, vaccination, radiation therapy, phototherapy, infection, and surgery.2 We suspect that the mechanism of locus minoris resistentiae in our patient was disruption of the architecture of the dermal-epidermal basement membrane zone due to surgical trauma. Disruption of this architecture may have resulted in exposure of previously occult antigens, recognition by T cells, T-cell stimulation of autoantibody production by B cells, binding of autoantibodies to BP180, complement deposition, recruitment of inflammatory cells, release of proteinases, and degradation of BP180 and extracellular matrix proteins.2
To the Editor:
Bullous pemphigoid (BP) is an autoimmune bullous dermatosis characterized by tense subepidermal blisters. It primarily affects older individuals who typically report pruritus in the affected area. Subepidermal blisters are caused by a humoral and cellular autoimmune attack directed against 2 BP antigens—BP180 and BP230—which are 2 critical components of the hemidesmosome whose primary function is to anchor the epidermis to the underlying dermis. Although tense bullae typically prompt immediate consideration of BP in the differential diagnosis, early disease often is characterized by urticarial plaques that require a high degree of suspicion to make the appropriate diagnosis. Locus minoris resistentiae is a term used to describe the phenomenon of skin disease occurring at the point of least resistance.1
A 79-year-old woman with type 2 diabetes mellitus, peptic ulcer disease, and hypertension was referred to the dermatology clinic due to concern for allergic contact dermatitis limited to the area of and adjacent to a well-healed surgical wound. History and examination revealed that the patient had sustained a left femoral neck fracture 10 months prior to presentation that required closed reduction and surgical pinning. The surgical site healed well postoperatively; however, 7 months after surgery, she began to develop edema and erythema within and immediately adjacent to the surgical scar. She subsequently developed areas of superficial erosion within the erythema and was evaluated by her surgeon who was concerned for suture granuloma. Superficial wound debridement of the area was performed without improvement. Approximately 9 months after surgery, the patient developed bullae along the old surgical site, which raised concern for an allergic reaction to the implanted screws. Orthopedics elected to remove the hardware but also sent intraoperative tissue for pathologic examination, which revealed subepidermal bullae containing eosinophils and neutrophils, most consistent with a bullous drug eruption. During the ensuing weeks after hardware removal, the plaque spread along the old surgical wound, and several bullous lesions began to appear. The patient’s primary care physician became concerned for allergic contact dermatitis, possibly to the surgical scrub employed during hardware removal. He prescribed triamcinolone ointment 0.1% and referred the patient to dermatology.
Upon presentation to dermatology, the patient noted stinging pain and intense pruritus of the affected area. Examination revealed a pink edematous plaque distributed along a well-healed surgical wound (Figure). Numerous fluid-filled tense bullae were superimposed on this plaque as well as areas of superficial erosion with serum crust. An expanded examination revealed similar smaller lesions on the upper arms, inner thighs, and lateral breasts. A 4-mm punch biopsy of lesional and perilesional skin was sent for hematoxylin and eosin staining and direct immunofluorescence, which demonstrated a subepidermal bullous dermatosis with a predominance of neutrophilic inflammation as well as a band of linear IgG deposition at the dermal-epidermal junction. The patient was diagnosed with BP exhibiting a locus minoris resistentiae phenomenon within the surgical site. She was started on prednisone 1 mg/kg daily and doxycycline 100 mg twice daily and demonstrated rapid improvement.
Although the tense bullae seen in well-developed BP are fairly characteristic, the prodromal phase of this disease can present with urticarial plaques that are nonspecific. This progression is well described, but our case demonstrates the difficulty of considering BP when a patient presents with an urticarial plaque. As lesions progress to the bullous phase, they may be inappropriately diagnosed as allergic contact dermatitis, an error that may lead to unnecessary interventions (eg, removal of an implicated prosthesis). This case is a reminder that not all cutaneous eruptions in and around postsurgical scars are allergic in nature.
This case also depicts BP appearing in the locus minoris resistentiae, a well-healed surgical wound in our patient. Although many diseases have been shown to exhibit this type of isomorphic response, this phenomenon may pose diagnostic and management conundrums. Locus minoris resistentiae has been reported in many different diseases, both cutaneous and otherwise, but there likely are distinct disease- and case-specific mechanisms via which this occurs. Local phenomena reported to trigger BP include contact dermatitis, vaccination, radiation therapy, phototherapy, infection, and surgery.2 We suspect that the mechanism of locus minoris resistentiae in our patient was disruption of the architecture of the dermal-epidermal basement membrane zone due to surgical trauma. Disruption of this architecture may have resulted in exposure of previously occult antigens, recognition by T cells, T-cell stimulation of autoantibody production by B cells, binding of autoantibodies to BP180, complement deposition, recruitment of inflammatory cells, release of proteinases, and degradation of BP180 and extracellular matrix proteins.2
- Lo Schiavo A, Ruocco E, Russo T, et al. Locus minoris resistentiae: an old but still valid way of thinking in medicine. Clin Dermatol. 2014;32:553-556.
- Lo Schiavo A, Ruocco E, Brancaccio G, et al. Bullous pemphigoid: etiology, pathogenesis, and inducing factors: facts and controversies. Clin Dermatol. 2013;31:391-399.
- Lo Schiavo A, Ruocco E, Russo T, et al. Locus minoris resistentiae: an old but still valid way of thinking in medicine. Clin Dermatol. 2014;32:553-556.
- Lo Schiavo A, Ruocco E, Brancaccio G, et al. Bullous pemphigoid: etiology, pathogenesis, and inducing factors: facts and controversies. Clin Dermatol. 2013;31:391-399.
Practice Points
- Bullous pemphigoid frequently presents with urticarial plaques without classic tense blisters in the early phase of disease.
- The phenomenon of locus minoris resistentiae can lead to the presentation of bullous pemphigoid in locations traumatized by surgery.
- Bullous pemphigoid can present as urticarial plaques at surgery sites mimicking allergic contact dermatitis or reaction to surgical sutures or hardware.
Retiform Purpura on the Buttocks in 6 Critically Ill COVID-19 Patients
To the Editor:
There is emerging evidence of skin findings in patients with COVID-19, including perniolike changes of the toes as well as urticarial and vesicular eruptions.1 Magro et al2 reported 3 cases of livedoid and purpuric skin eruptions in critically ill COVID-19 patients with evidence of thrombotic vasculopathy on skin biopsy, including a 32-year-old man with striking buttocks retiform purpura. Histopathologic analysis revealed thrombotic vasculopathy and pressure-induced ischemic necrosis. Since that patient was first evaluated (March 2020), we identified 6 more cases of critically ill COVID-19 patients from a single academic hospital in New York City with essentially identical clinical findings. Herein, we report those 6 cases of critically ill and intubated patients with COVID-19 who developed retiform purpura on the buttocks only, approximately 11 to 21 days after onset of COVID-19 symptoms.
We provided consultation for 5 men and 1 woman (age range, 42–78 years) who were critically ill with COVID-19 and developed retiform purpura on the buttocks (Figures 1 and 2). All had an elevated D-dimer concentration: 2 patients, >700 ng/mL; 2 patients, >2000 ng/mL; 2 patients, >6000 ng/mL (reference, 229 ng/mL). Three patients experienced a peak D-dimer concentration on the day retiform purpura was reported.
Further evidence of coagulopathy in these patients included 1 patient with a newly diagnosed left popliteal deep vein thrombosis and 1 patient with a known history of protein C deficiency and deep vein thromboses. Five patients were receiving anticoagulation on the day the skin changes were documented; anticoagulation was contraindicated in the sixth patient because of oropharyngeal bleeding. Anticoagulation was continued at the treatment dosage (enoxaparin 80 mg twice daily) in 3 patients, and in 2 patients receiving a prophylactic dose (enoxaparin 40 mg daily), anticoagulation was escalated to treatment dose due to rising D-dimer levels and newly diagnosed retiform purpura. Skin biopsy was deferred for all patients due to positional and ventilatory restrictions. At that point in their care, 3 patients remained admitted on medicine floors, 2 were in the intensive care unit, and 1 had died.
Although the differential diagnosis for retiform purpura is broad and should be fully considered in any patient with this finding, based on the elevated D-dimer concentration, critical illness secondary to COVID-19, and striking similarity to earlier reported case of buttocks retiform purpura with thrombotic vasculopathy and pressure injury noted histopathologically,2 we suspect the buttocks retiform purpura in our 6 cases also represent a combination of cutaneous thrombosis and pressure injury. In addition to acral livedoid eruptions (also reported by Magro and colleagues2), we suspect that this cutaneous manifestation might be associated with a hypercoagulable state in some patients, especially in the setting of a rising D-dimer concentration. One study found that 31% of 184 patients with severe COVID-19 had thrombotic complications,3 a clinical picture that portends a poor prognosis.4
COVID-19 patients presenting with retiform purpura should be fully evaluated based on the broad differential for this morphology. We present 6 cases of buttocks retiform purpura in critically ill COVID-19 patients—all with strikingly similar morphologic findings, an elevated D-dimer concentration, and critical illness due to COVID-19—to alert clinicians to this constellation of findings and propose that this cutaneous manifestation could indicate an associated hypercoaguable state and should prompt a hematology consultation. Additionally, biopsy of this skin finding should be considered, especially if biopsy results might serve to guide management; however, obtaining a biopsy specimen can be technically difficult because of ventilatory requirements.
Given the magnitude of the COVID-19 pandemic and the propensity of these patients to experience thrombotic events, recognition of this skin finding in COVID-19 is important and might allow timely intervention.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:e212-e213. doi:10.1111/jdv.16387
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13. doi:10.1016/j.trsl.2020.04.007
- Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. doi:10.1016/j.thromres.2020.04.013
- Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844-847. doi:10.1111/jth.14768
To the Editor:
There is emerging evidence of skin findings in patients with COVID-19, including perniolike changes of the toes as well as urticarial and vesicular eruptions.1 Magro et al2 reported 3 cases of livedoid and purpuric skin eruptions in critically ill COVID-19 patients with evidence of thrombotic vasculopathy on skin biopsy, including a 32-year-old man with striking buttocks retiform purpura. Histopathologic analysis revealed thrombotic vasculopathy and pressure-induced ischemic necrosis. Since that patient was first evaluated (March 2020), we identified 6 more cases of critically ill COVID-19 patients from a single academic hospital in New York City with essentially identical clinical findings. Herein, we report those 6 cases of critically ill and intubated patients with COVID-19 who developed retiform purpura on the buttocks only, approximately 11 to 21 days after onset of COVID-19 symptoms.
We provided consultation for 5 men and 1 woman (age range, 42–78 years) who were critically ill with COVID-19 and developed retiform purpura on the buttocks (Figures 1 and 2). All had an elevated D-dimer concentration: 2 patients, >700 ng/mL; 2 patients, >2000 ng/mL; 2 patients, >6000 ng/mL (reference, 229 ng/mL). Three patients experienced a peak D-dimer concentration on the day retiform purpura was reported.
Further evidence of coagulopathy in these patients included 1 patient with a newly diagnosed left popliteal deep vein thrombosis and 1 patient with a known history of protein C deficiency and deep vein thromboses. Five patients were receiving anticoagulation on the day the skin changes were documented; anticoagulation was contraindicated in the sixth patient because of oropharyngeal bleeding. Anticoagulation was continued at the treatment dosage (enoxaparin 80 mg twice daily) in 3 patients, and in 2 patients receiving a prophylactic dose (enoxaparin 40 mg daily), anticoagulation was escalated to treatment dose due to rising D-dimer levels and newly diagnosed retiform purpura. Skin biopsy was deferred for all patients due to positional and ventilatory restrictions. At that point in their care, 3 patients remained admitted on medicine floors, 2 were in the intensive care unit, and 1 had died.
Although the differential diagnosis for retiform purpura is broad and should be fully considered in any patient with this finding, based on the elevated D-dimer concentration, critical illness secondary to COVID-19, and striking similarity to earlier reported case of buttocks retiform purpura with thrombotic vasculopathy and pressure injury noted histopathologically,2 we suspect the buttocks retiform purpura in our 6 cases also represent a combination of cutaneous thrombosis and pressure injury. In addition to acral livedoid eruptions (also reported by Magro and colleagues2), we suspect that this cutaneous manifestation might be associated with a hypercoagulable state in some patients, especially in the setting of a rising D-dimer concentration. One study found that 31% of 184 patients with severe COVID-19 had thrombotic complications,3 a clinical picture that portends a poor prognosis.4
COVID-19 patients presenting with retiform purpura should be fully evaluated based on the broad differential for this morphology. We present 6 cases of buttocks retiform purpura in critically ill COVID-19 patients—all with strikingly similar morphologic findings, an elevated D-dimer concentration, and critical illness due to COVID-19—to alert clinicians to this constellation of findings and propose that this cutaneous manifestation could indicate an associated hypercoaguable state and should prompt a hematology consultation. Additionally, biopsy of this skin finding should be considered, especially if biopsy results might serve to guide management; however, obtaining a biopsy specimen can be technically difficult because of ventilatory requirements.
Given the magnitude of the COVID-19 pandemic and the propensity of these patients to experience thrombotic events, recognition of this skin finding in COVID-19 is important and might allow timely intervention.
To the Editor:
There is emerging evidence of skin findings in patients with COVID-19, including perniolike changes of the toes as well as urticarial and vesicular eruptions.1 Magro et al2 reported 3 cases of livedoid and purpuric skin eruptions in critically ill COVID-19 patients with evidence of thrombotic vasculopathy on skin biopsy, including a 32-year-old man with striking buttocks retiform purpura. Histopathologic analysis revealed thrombotic vasculopathy and pressure-induced ischemic necrosis. Since that patient was first evaluated (March 2020), we identified 6 more cases of critically ill COVID-19 patients from a single academic hospital in New York City with essentially identical clinical findings. Herein, we report those 6 cases of critically ill and intubated patients with COVID-19 who developed retiform purpura on the buttocks only, approximately 11 to 21 days after onset of COVID-19 symptoms.
We provided consultation for 5 men and 1 woman (age range, 42–78 years) who were critically ill with COVID-19 and developed retiform purpura on the buttocks (Figures 1 and 2). All had an elevated D-dimer concentration: 2 patients, >700 ng/mL; 2 patients, >2000 ng/mL; 2 patients, >6000 ng/mL (reference, 229 ng/mL). Three patients experienced a peak D-dimer concentration on the day retiform purpura was reported.
Further evidence of coagulopathy in these patients included 1 patient with a newly diagnosed left popliteal deep vein thrombosis and 1 patient with a known history of protein C deficiency and deep vein thromboses. Five patients were receiving anticoagulation on the day the skin changes were documented; anticoagulation was contraindicated in the sixth patient because of oropharyngeal bleeding. Anticoagulation was continued at the treatment dosage (enoxaparin 80 mg twice daily) in 3 patients, and in 2 patients receiving a prophylactic dose (enoxaparin 40 mg daily), anticoagulation was escalated to treatment dose due to rising D-dimer levels and newly diagnosed retiform purpura. Skin biopsy was deferred for all patients due to positional and ventilatory restrictions. At that point in their care, 3 patients remained admitted on medicine floors, 2 were in the intensive care unit, and 1 had died.
Although the differential diagnosis for retiform purpura is broad and should be fully considered in any patient with this finding, based on the elevated D-dimer concentration, critical illness secondary to COVID-19, and striking similarity to earlier reported case of buttocks retiform purpura with thrombotic vasculopathy and pressure injury noted histopathologically,2 we suspect the buttocks retiform purpura in our 6 cases also represent a combination of cutaneous thrombosis and pressure injury. In addition to acral livedoid eruptions (also reported by Magro and colleagues2), we suspect that this cutaneous manifestation might be associated with a hypercoagulable state in some patients, especially in the setting of a rising D-dimer concentration. One study found that 31% of 184 patients with severe COVID-19 had thrombotic complications,3 a clinical picture that portends a poor prognosis.4
COVID-19 patients presenting with retiform purpura should be fully evaluated based on the broad differential for this morphology. We present 6 cases of buttocks retiform purpura in critically ill COVID-19 patients—all with strikingly similar morphologic findings, an elevated D-dimer concentration, and critical illness due to COVID-19—to alert clinicians to this constellation of findings and propose that this cutaneous manifestation could indicate an associated hypercoaguable state and should prompt a hematology consultation. Additionally, biopsy of this skin finding should be considered, especially if biopsy results might serve to guide management; however, obtaining a biopsy specimen can be technically difficult because of ventilatory requirements.
Given the magnitude of the COVID-19 pandemic and the propensity of these patients to experience thrombotic events, recognition of this skin finding in COVID-19 is important and might allow timely intervention.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:e212-e213. doi:10.1111/jdv.16387
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13. doi:10.1016/j.trsl.2020.04.007
- Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. doi:10.1016/j.thromres.2020.04.013
- Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844-847. doi:10.1111/jth.14768
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:e212-e213. doi:10.1111/jdv.16387
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13. doi:10.1016/j.trsl.2020.04.007
- Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. doi:10.1016/j.thromres.2020.04.013
- Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844-847. doi:10.1111/jth.14768
Practice Points
- Retiform purpura in a severely ill patient with COVID-19 and a markedly elevated D-dimer concentration might be a cutaneous sign of systemic coagulopathy.
- This constellation of findings should prompt consideration of skin biopsy and hematology consultation.