User login
Punked By the Punctum: Domestically Acquired Cutaneous Myiasis
To the Editor:
Cutaneous myiasis is a skin infestation with dipterous larvae that feed on the host’s tissue and cause a wide range of manifestations depending on the location of infestation. Cutaneous myiasis, which includes furuncular, wound, and migratory types, is the most common clinical form of this condition.1 It is endemic to tropical and subtropical areas and is not common in the United States, thus it can pose a diagnostic challenge when presenting in nonendemic areas. We present the case of a woman from Michigan who acquired furuncular myiasis without travel history to a tropical or subtropical locale.
A 72-year-old woman presented to our clinic with a chief concern of a burning, pruritic, migratory skin lesion on the left arm of approximately 1 week’s duration. She had a medical history of squamous cell carcinoma, keratoacanthoma, and multiple tick bites. She reported that the lesion started on the distal aspect of the left arm as an eraser-sized, perfectly round, raised bruise with a dark pepperlike bump in the center. The lesion then spread proximally over the course of 1 week, creating 3 more identical lesions. As one lesion resolved, a new lesion appeared approximately 2 to 4 cm proximal to the preceding lesion. The patient had traveled to England, Scotland, and Ireland 2 months prior but otherwise denied leaving the state of Michigan. She reported frequent exposure to gardens, meadows, and wetlands in search of milkweed and monarch butterfly larvae that she raises in northeast Michigan. She denied any recent illness or associated systemic symptoms. Initial evaluation by a primary care physician resulted in a diagnosis of a furuncle or tick bite; she completed a 10-day course of amoxicillin and a methylprednisolone dose pack without improvement.
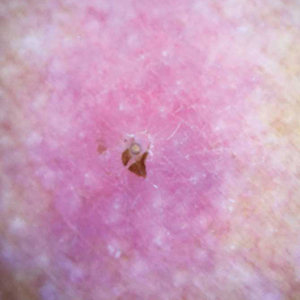
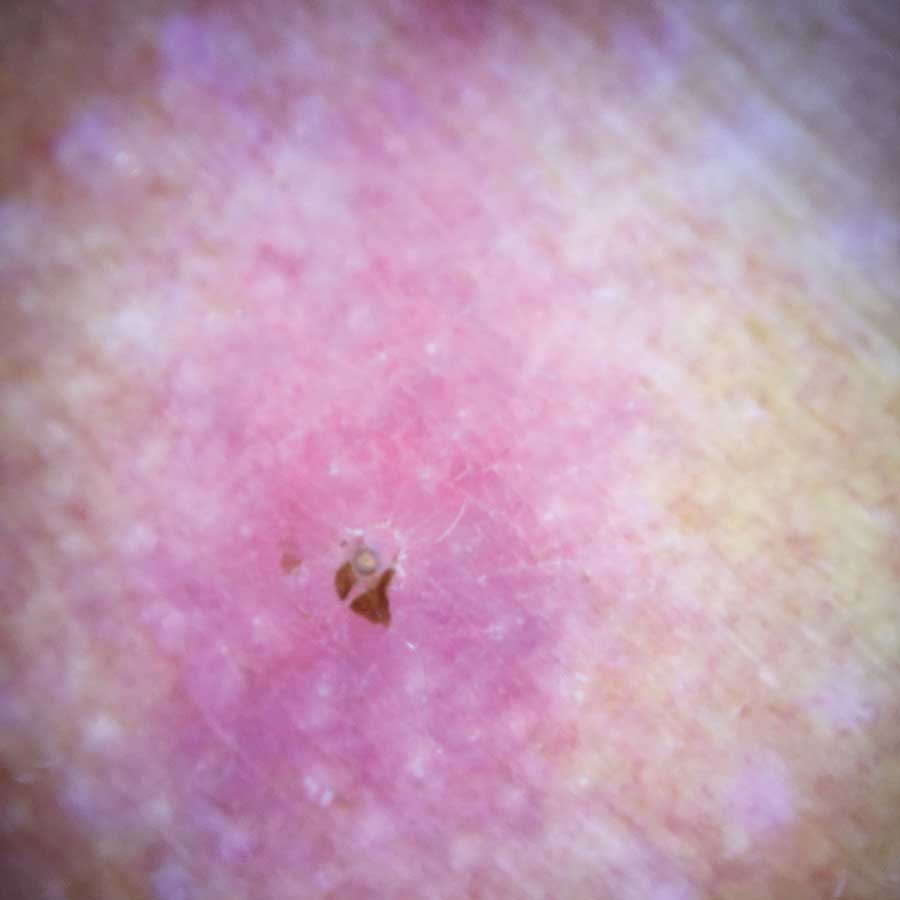
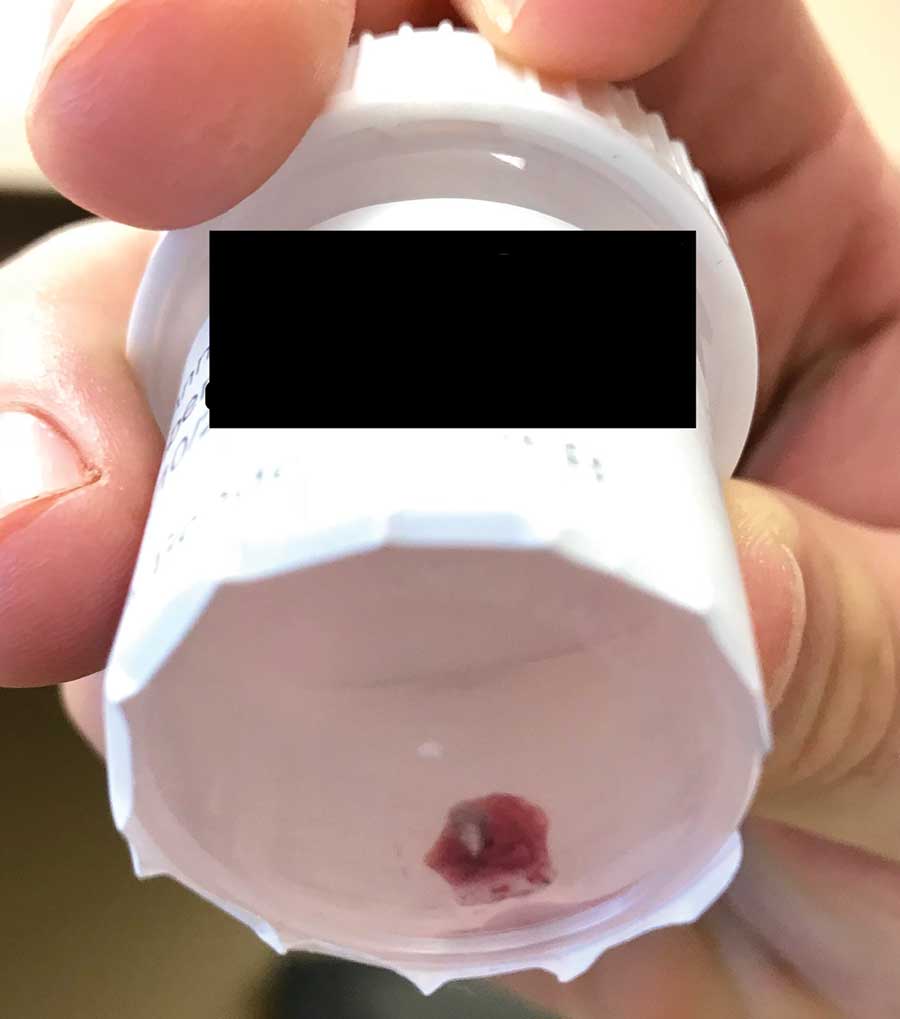
Physical examination revealed a 1-cm, firm, violaceous nodule with a small distinct central punctum and surrounding erythema on the proximal aspect of the left arm. Dermoscopy revealed a pulsating motion and expulsion of serosanguineous fluid from the central punctum (Figure 1). Further inspection of the patient’s left arm exposed several noninflammatory puncta distal to the primary lesion spaced at 2- to 4-cm intervals.
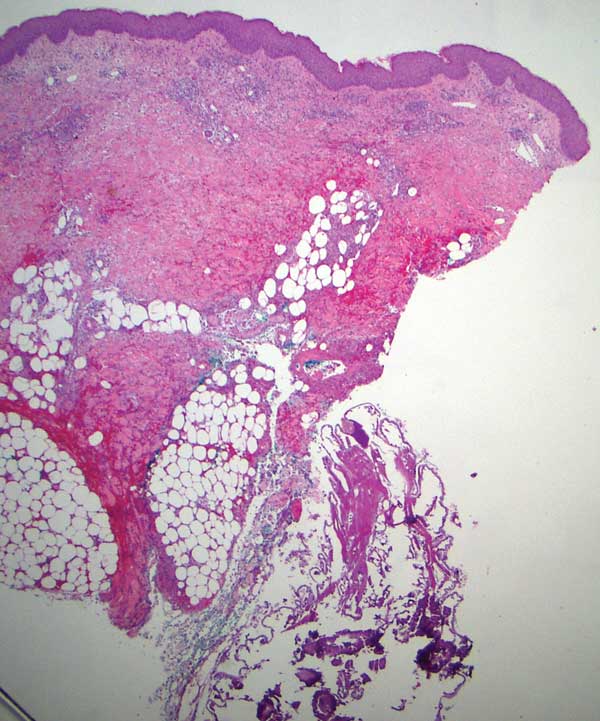
Gross examination of a 6-mm punch biopsy from the primary inflammatory nodule uncovered a small, motile, gray-white larval organism in the inferior portion of the specimen (Figure 2). Histopathology revealed superficial and deep eosinophil-rich inflammation, fibrosis, and hemorrhage. There was a complex wedge-shaped organism with extensive internal muscle bounded by a thin cuticle bearing rows of chitinous hooklets located at one side within the deep dermis (Figure 3). The findings were consistent with a diagnosis of cutaneous myiasis. No further treatment was required, as the organism was completely excised with the biopsy.
The most common causative agents of furuncular myiasis obtained from travelers returning from Mexico and Central and South America are Dermatobia hominis and Cordylobia anthropophaga. Cases of furuncular myiasis acquired in the United States without recent foreign travel are rare. Most of these cases are caused by larvae of the Cuterebra species (also known as the rabbit botfly or rodent botfly).2 In a 2003 literature review by Safdar et al3 on 56 cases of furuncular myiasis in the United States, the median age of patients was 14 years, 87% of cases occurred in August and September, and most involved exposure in rural or suburban settings; 53% of cases presented in the northeastern United States.
Furuncular myiasis occurs when the organism’s ova are deposited on the skin of a human host by the parent organism or a mosquito vector. The heat of the skin causes the eggs to hatch and the dipteran larvae must penetrate the skin within 20 days.1 Signs of infection typically are seen 6 to 10 days after infestation.3 The larvae then feed on human tissue and burrow deep in the dermis, forming an erythematous furunculoid nodule containing one or multiple maggots. After 5 to 10 weeks, the adult larvae drop to the ground, where they mature into adult organisms in the soil.1
The most reported symptoms of furuncular myiasis include pruritus, pain, and movement sensation, typically occurring suddenly at night.4 The most common presentation is a furunclelike lesion that exudes serosanguineous or purulent fluid,1 but there have been reports of vesicular, bullous, pustular, erosive, ecchymotic, and ulcerative lesions.5Dermatobia hominis usually presents on an exposed site, such as the scalp, face, and extremities. It may present with paroxysmal episodes of lancinating pain. Over time, the lesion usually heals without a scar, though hyperpigmentation and scarring can occur. The most reported complication is secondary bacterial infection.4 Local lymphadenopathy or systemic symptoms should raise concern for infection. Staphylococcus aureus and group B Streptococcus have been cultured from lesions.6,7
The differential diagnosis for myiasis should include furuncle, insect bite, insect prurigo, pyoderma, inflamed cyst, and tungiasis. Myiasis also can present similarly to severe soft tissue infections or cellulitis. If located on the breasts, it can be mistaken for periductal mastitis, a benign mass with microcalcification, or inflammatory carcinoma. Lastly, due to pain, erythema, pruritus, small vesicles, and crusting, it may be confused for herpes simplex virus.1
Furuncular myiasis typically is diagnosed based on clinical presentation, especially in endemic regions. In nonendemic areas, the patient’s history may reveal recent travel or predisposition to myiasis. In cases where there is uncertainty, dermoscopy may be used to identify the maggot in the lesion, or ultrasonography can be used to confirm myiasis through the detection of larval movement.8 Dermoscopy will reveal a furuncular lesion with a central opening surrounded by dilated blood vessels and a yellowish structure with black barblike spines.9 Within the dermis is a fibrous cystic sinus tract containing the dipteran larva. Laboratory studies typically are unremarkable. In chronic cases, a complete blood cell count and other laboratory tests may show systemic inflammation, peripheral eosinophilia, and elevated IgE.10 Biopsies of furuncular myiasis are not necessary for diagnosis. Histopathology reveals an ulcerated epidermis with or without hyperkeratosis and an inflammatory infiltrate composed of lymphocytes and neutrophils with eosinophils, fibroblasts, histiocytes, basophils, mast cells, plasma cells, and Langerhans cells within the dermis and subcutis.11
There are various approaches to treating furuncular myiasis, with the goal of complete removal of the larva and prevention of secondary infection. One treatment option is to apply a toxic substance to the larva, effectively killing it. Another approach is to force the larva to emerge via localized hypoxia, which can be done by occluding the punctum of the lesion for at least 24 hours. A complication of this method is suffocation of the larva without migration, leading to incomplete extraction and secondary infection.1 A third method is to surgically remove the larva, which allows for debridement of necrotic tissue surrounding the lesion if present.12 Ultrasonography also can be used therapeutically to aid in the removal of the larvae. The last method is to inject lidocaine into the base of the lesion, forcing the larva out of the punctum via fluid pressure.13 Oral treatments such as ivermectin are not recommended because they can result in the death of larvae within the lesion, leading to an inflammatory response.8
Furuncular myiasis is a form of cutaneous larvae infestation not commonly seen in individuals who do not live or travel in endemic, tropical, and subtropical regions. Diagnosis is based on clinical presentation, with imaging and laboratory studies available to supplement in unclear or atypical manifestations. Treatment involves complete removal of the larva, typically through forced evacuation via hypoxia or through surgical removal. Most cases resolve without notable scarring or other sequelae; however, in those who do have complications, the most common is secondary bacterial infection. Our patient’s absence of notable travel history and frequent environmental exposure in Michigan led us to believe the organism was from a domestic source. Our case underlines the importance of a thorough history and clinical examination of furuncular lesions including the use of dermoscopy to yield an appropriate diagnosis and treatment plan.
- Francesconi F, Lupi O. Myiasis. Clin Microbiol Rev. 2012;25:79-105. doi:10.1128/CMR.00010-11
- Schiff TA. Furuncular cutaneous myiasis caused by Cuterebra larva. J Am Acad Dermatol 1993;28:261-263.
- Safdar N, Young DK, Andes D. Autochthonous furuncular myiasis in the United States: case report and literature review. Clin Infect Dis. 2003;26:73-80.
- Mahal JJ, Sperling JD. Furuncular myiasis from Dermatobia hominus: a case of human botfly infestation. J Emerg Med. 2012;43:618-621.
- Francesconi F, Lupi O. Myiasis. In: Tyring SK, Lupi O, Hengge UR, eds. Tropical Dermatology. Elsevier; 2006:232-239.
- Gordon PM, Hepburn NC, Williams AE, et al. Cutaneous myiasis due to Dermatobia hominis: a report of six cases. Br J Dermatol. 1995;132:811-814.
- Hubler WR Jr, Rudolph AH, Dougherty EF. Dermal myiasis. Arch Dermatol. 1974;110:109-110.
- Quintanilla-Cedillo MR, León-Ureña H, Contreras-Ruiz J, et al. The value of Doppler ultrasound in diagnosis in 25 cases of furunculoid myiasis. Int J Dermatol. 2005;44:34-37.
- Bakos RM, Bakos L. Dermoscopic diagnosis of furuncular myiasis. Arch Dermatol. 2007;143:123-124.
- Varani S, Tassinari D, Elleri D, et al. A case of furuncular myiasis associated with systemic inflammation. Parasitol Int. 2007;56:330-333.
- Grogan TM, Payne CM, Spier C, et al. Cutaneous myiasis. immunohistologic and ultrastructural morphometric features of a human botfly lesion. Am J Dermatopathol. 1987;9:232-239.
- Krajewski A, Allen B, Hoss D, et al. Cutaneous myiasis. J Plast Reconstr Aesthet Surg. 2009;62:383-386.
- Lebwohl MG, Heymann WR, Berth-Jones J, et al. Myiasis: Treatment of Skin Diseases. Comprehensive Therapeutic Strategies. 2nd ed. Elsevier-Mosby; 2006.
To the Editor:
Cutaneous myiasis is a skin infestation with dipterous larvae that feed on the host’s tissue and cause a wide range of manifestations depending on the location of infestation. Cutaneous myiasis, which includes furuncular, wound, and migratory types, is the most common clinical form of this condition.1 It is endemic to tropical and subtropical areas and is not common in the United States, thus it can pose a diagnostic challenge when presenting in nonendemic areas. We present the case of a woman from Michigan who acquired furuncular myiasis without travel history to a tropical or subtropical locale.
A 72-year-old woman presented to our clinic with a chief concern of a burning, pruritic, migratory skin lesion on the left arm of approximately 1 week’s duration. She had a medical history of squamous cell carcinoma, keratoacanthoma, and multiple tick bites. She reported that the lesion started on the distal aspect of the left arm as an eraser-sized, perfectly round, raised bruise with a dark pepperlike bump in the center. The lesion then spread proximally over the course of 1 week, creating 3 more identical lesions. As one lesion resolved, a new lesion appeared approximately 2 to 4 cm proximal to the preceding lesion. The patient had traveled to England, Scotland, and Ireland 2 months prior but otherwise denied leaving the state of Michigan. She reported frequent exposure to gardens, meadows, and wetlands in search of milkweed and monarch butterfly larvae that she raises in northeast Michigan. She denied any recent illness or associated systemic symptoms. Initial evaluation by a primary care physician resulted in a diagnosis of a furuncle or tick bite; she completed a 10-day course of amoxicillin and a methylprednisolone dose pack without improvement.
Physical examination revealed a 1-cm, firm, violaceous nodule with a small distinct central punctum and surrounding erythema on the proximal aspect of the left arm. Dermoscopy revealed a pulsating motion and expulsion of serosanguineous fluid from the central punctum (Figure 1). Further inspection of the patient’s left arm exposed several noninflammatory puncta distal to the primary lesion spaced at 2- to 4-cm intervals.

Gross examination of a 6-mm punch biopsy from the primary inflammatory nodule uncovered a small, motile, gray-white larval organism in the inferior portion of the specimen (Figure 2). Histopathology revealed superficial and deep eosinophil-rich inflammation, fibrosis, and hemorrhage. There was a complex wedge-shaped organism with extensive internal muscle bounded by a thin cuticle bearing rows of chitinous hooklets located at one side within the deep dermis (Figure 3). The findings were consistent with a diagnosis of cutaneous myiasis. No further treatment was required, as the organism was completely excised with the biopsy.

The most common causative agents of furuncular myiasis obtained from travelers returning from Mexico and Central and South America are Dermatobia hominis and Cordylobia anthropophaga. Cases of furuncular myiasis acquired in the United States without recent foreign travel are rare. Most of these cases are caused by larvae of the Cuterebra species (also known as the rabbit botfly or rodent botfly).2 In a 2003 literature review by Safdar et al3 on 56 cases of furuncular myiasis in the United States, the median age of patients was 14 years, 87% of cases occurred in August and September, and most involved exposure in rural or suburban settings; 53% of cases presented in the northeastern United States.

Furuncular myiasis occurs when the organism’s ova are deposited on the skin of a human host by the parent organism or a mosquito vector. The heat of the skin causes the eggs to hatch and the dipteran larvae must penetrate the skin within 20 days.1 Signs of infection typically are seen 6 to 10 days after infestation.3 The larvae then feed on human tissue and burrow deep in the dermis, forming an erythematous furunculoid nodule containing one or multiple maggots. After 5 to 10 weeks, the adult larvae drop to the ground, where they mature into adult organisms in the soil.1
The most reported symptoms of furuncular myiasis include pruritus, pain, and movement sensation, typically occurring suddenly at night.4 The most common presentation is a furunclelike lesion that exudes serosanguineous or purulent fluid,1 but there have been reports of vesicular, bullous, pustular, erosive, ecchymotic, and ulcerative lesions.5Dermatobia hominis usually presents on an exposed site, such as the scalp, face, and extremities. It may present with paroxysmal episodes of lancinating pain. Over time, the lesion usually heals without a scar, though hyperpigmentation and scarring can occur. The most reported complication is secondary bacterial infection.4 Local lymphadenopathy or systemic symptoms should raise concern for infection. Staphylococcus aureus and group B Streptococcus have been cultured from lesions.6,7
The differential diagnosis for myiasis should include furuncle, insect bite, insect prurigo, pyoderma, inflamed cyst, and tungiasis. Myiasis also can present similarly to severe soft tissue infections or cellulitis. If located on the breasts, it can be mistaken for periductal mastitis, a benign mass with microcalcification, or inflammatory carcinoma. Lastly, due to pain, erythema, pruritus, small vesicles, and crusting, it may be confused for herpes simplex virus.1
Furuncular myiasis typically is diagnosed based on clinical presentation, especially in endemic regions. In nonendemic areas, the patient’s history may reveal recent travel or predisposition to myiasis. In cases where there is uncertainty, dermoscopy may be used to identify the maggot in the lesion, or ultrasonography can be used to confirm myiasis through the detection of larval movement.8 Dermoscopy will reveal a furuncular lesion with a central opening surrounded by dilated blood vessels and a yellowish structure with black barblike spines.9 Within the dermis is a fibrous cystic sinus tract containing the dipteran larva. Laboratory studies typically are unremarkable. In chronic cases, a complete blood cell count and other laboratory tests may show systemic inflammation, peripheral eosinophilia, and elevated IgE.10 Biopsies of furuncular myiasis are not necessary for diagnosis. Histopathology reveals an ulcerated epidermis with or without hyperkeratosis and an inflammatory infiltrate composed of lymphocytes and neutrophils with eosinophils, fibroblasts, histiocytes, basophils, mast cells, plasma cells, and Langerhans cells within the dermis and subcutis.11
There are various approaches to treating furuncular myiasis, with the goal of complete removal of the larva and prevention of secondary infection. One treatment option is to apply a toxic substance to the larva, effectively killing it. Another approach is to force the larva to emerge via localized hypoxia, which can be done by occluding the punctum of the lesion for at least 24 hours. A complication of this method is suffocation of the larva without migration, leading to incomplete extraction and secondary infection.1 A third method is to surgically remove the larva, which allows for debridement of necrotic tissue surrounding the lesion if present.12 Ultrasonography also can be used therapeutically to aid in the removal of the larvae. The last method is to inject lidocaine into the base of the lesion, forcing the larva out of the punctum via fluid pressure.13 Oral treatments such as ivermectin are not recommended because they can result in the death of larvae within the lesion, leading to an inflammatory response.8
Furuncular myiasis is a form of cutaneous larvae infestation not commonly seen in individuals who do not live or travel in endemic, tropical, and subtropical regions. Diagnosis is based on clinical presentation, with imaging and laboratory studies available to supplement in unclear or atypical manifestations. Treatment involves complete removal of the larva, typically through forced evacuation via hypoxia or through surgical removal. Most cases resolve without notable scarring or other sequelae; however, in those who do have complications, the most common is secondary bacterial infection. Our patient’s absence of notable travel history and frequent environmental exposure in Michigan led us to believe the organism was from a domestic source. Our case underlines the importance of a thorough history and clinical examination of furuncular lesions including the use of dermoscopy to yield an appropriate diagnosis and treatment plan.
To the Editor:
Cutaneous myiasis is a skin infestation with dipterous larvae that feed on the host’s tissue and cause a wide range of manifestations depending on the location of infestation. Cutaneous myiasis, which includes furuncular, wound, and migratory types, is the most common clinical form of this condition.1 It is endemic to tropical and subtropical areas and is not common in the United States, thus it can pose a diagnostic challenge when presenting in nonendemic areas. We present the case of a woman from Michigan who acquired furuncular myiasis without travel history to a tropical or subtropical locale.
A 72-year-old woman presented to our clinic with a chief concern of a burning, pruritic, migratory skin lesion on the left arm of approximately 1 week’s duration. She had a medical history of squamous cell carcinoma, keratoacanthoma, and multiple tick bites. She reported that the lesion started on the distal aspect of the left arm as an eraser-sized, perfectly round, raised bruise with a dark pepperlike bump in the center. The lesion then spread proximally over the course of 1 week, creating 3 more identical lesions. As one lesion resolved, a new lesion appeared approximately 2 to 4 cm proximal to the preceding lesion. The patient had traveled to England, Scotland, and Ireland 2 months prior but otherwise denied leaving the state of Michigan. She reported frequent exposure to gardens, meadows, and wetlands in search of milkweed and monarch butterfly larvae that she raises in northeast Michigan. She denied any recent illness or associated systemic symptoms. Initial evaluation by a primary care physician resulted in a diagnosis of a furuncle or tick bite; she completed a 10-day course of amoxicillin and a methylprednisolone dose pack without improvement.
Physical examination revealed a 1-cm, firm, violaceous nodule with a small distinct central punctum and surrounding erythema on the proximal aspect of the left arm. Dermoscopy revealed a pulsating motion and expulsion of serosanguineous fluid from the central punctum (Figure 1). Further inspection of the patient’s left arm exposed several noninflammatory puncta distal to the primary lesion spaced at 2- to 4-cm intervals.

Gross examination of a 6-mm punch biopsy from the primary inflammatory nodule uncovered a small, motile, gray-white larval organism in the inferior portion of the specimen (Figure 2). Histopathology revealed superficial and deep eosinophil-rich inflammation, fibrosis, and hemorrhage. There was a complex wedge-shaped organism with extensive internal muscle bounded by a thin cuticle bearing rows of chitinous hooklets located at one side within the deep dermis (Figure 3). The findings were consistent with a diagnosis of cutaneous myiasis. No further treatment was required, as the organism was completely excised with the biopsy.

The most common causative agents of furuncular myiasis obtained from travelers returning from Mexico and Central and South America are Dermatobia hominis and Cordylobia anthropophaga. Cases of furuncular myiasis acquired in the United States without recent foreign travel are rare. Most of these cases are caused by larvae of the Cuterebra species (also known as the rabbit botfly or rodent botfly).2 In a 2003 literature review by Safdar et al3 on 56 cases of furuncular myiasis in the United States, the median age of patients was 14 years, 87% of cases occurred in August and September, and most involved exposure in rural or suburban settings; 53% of cases presented in the northeastern United States.

Furuncular myiasis occurs when the organism’s ova are deposited on the skin of a human host by the parent organism or a mosquito vector. The heat of the skin causes the eggs to hatch and the dipteran larvae must penetrate the skin within 20 days.1 Signs of infection typically are seen 6 to 10 days after infestation.3 The larvae then feed on human tissue and burrow deep in the dermis, forming an erythematous furunculoid nodule containing one or multiple maggots. After 5 to 10 weeks, the adult larvae drop to the ground, where they mature into adult organisms in the soil.1
The most reported symptoms of furuncular myiasis include pruritus, pain, and movement sensation, typically occurring suddenly at night.4 The most common presentation is a furunclelike lesion that exudes serosanguineous or purulent fluid,1 but there have been reports of vesicular, bullous, pustular, erosive, ecchymotic, and ulcerative lesions.5Dermatobia hominis usually presents on an exposed site, such as the scalp, face, and extremities. It may present with paroxysmal episodes of lancinating pain. Over time, the lesion usually heals without a scar, though hyperpigmentation and scarring can occur. The most reported complication is secondary bacterial infection.4 Local lymphadenopathy or systemic symptoms should raise concern for infection. Staphylococcus aureus and group B Streptococcus have been cultured from lesions.6,7
The differential diagnosis for myiasis should include furuncle, insect bite, insect prurigo, pyoderma, inflamed cyst, and tungiasis. Myiasis also can present similarly to severe soft tissue infections or cellulitis. If located on the breasts, it can be mistaken for periductal mastitis, a benign mass with microcalcification, or inflammatory carcinoma. Lastly, due to pain, erythema, pruritus, small vesicles, and crusting, it may be confused for herpes simplex virus.1
Furuncular myiasis typically is diagnosed based on clinical presentation, especially in endemic regions. In nonendemic areas, the patient’s history may reveal recent travel or predisposition to myiasis. In cases where there is uncertainty, dermoscopy may be used to identify the maggot in the lesion, or ultrasonography can be used to confirm myiasis through the detection of larval movement.8 Dermoscopy will reveal a furuncular lesion with a central opening surrounded by dilated blood vessels and a yellowish structure with black barblike spines.9 Within the dermis is a fibrous cystic sinus tract containing the dipteran larva. Laboratory studies typically are unremarkable. In chronic cases, a complete blood cell count and other laboratory tests may show systemic inflammation, peripheral eosinophilia, and elevated IgE.10 Biopsies of furuncular myiasis are not necessary for diagnosis. Histopathology reveals an ulcerated epidermis with or without hyperkeratosis and an inflammatory infiltrate composed of lymphocytes and neutrophils with eosinophils, fibroblasts, histiocytes, basophils, mast cells, plasma cells, and Langerhans cells within the dermis and subcutis.11
There are various approaches to treating furuncular myiasis, with the goal of complete removal of the larva and prevention of secondary infection. One treatment option is to apply a toxic substance to the larva, effectively killing it. Another approach is to force the larva to emerge via localized hypoxia, which can be done by occluding the punctum of the lesion for at least 24 hours. A complication of this method is suffocation of the larva without migration, leading to incomplete extraction and secondary infection.1 A third method is to surgically remove the larva, which allows for debridement of necrotic tissue surrounding the lesion if present.12 Ultrasonography also can be used therapeutically to aid in the removal of the larvae. The last method is to inject lidocaine into the base of the lesion, forcing the larva out of the punctum via fluid pressure.13 Oral treatments such as ivermectin are not recommended because they can result in the death of larvae within the lesion, leading to an inflammatory response.8
Furuncular myiasis is a form of cutaneous larvae infestation not commonly seen in individuals who do not live or travel in endemic, tropical, and subtropical regions. Diagnosis is based on clinical presentation, with imaging and laboratory studies available to supplement in unclear or atypical manifestations. Treatment involves complete removal of the larva, typically through forced evacuation via hypoxia or through surgical removal. Most cases resolve without notable scarring or other sequelae; however, in those who do have complications, the most common is secondary bacterial infection. Our patient’s absence of notable travel history and frequent environmental exposure in Michigan led us to believe the organism was from a domestic source. Our case underlines the importance of a thorough history and clinical examination of furuncular lesions including the use of dermoscopy to yield an appropriate diagnosis and treatment plan.
- Francesconi F, Lupi O. Myiasis. Clin Microbiol Rev. 2012;25:79-105. doi:10.1128/CMR.00010-11
- Schiff TA. Furuncular cutaneous myiasis caused by Cuterebra larva. J Am Acad Dermatol 1993;28:261-263.
- Safdar N, Young DK, Andes D. Autochthonous furuncular myiasis in the United States: case report and literature review. Clin Infect Dis. 2003;26:73-80.
- Mahal JJ, Sperling JD. Furuncular myiasis from Dermatobia hominus: a case of human botfly infestation. J Emerg Med. 2012;43:618-621.
- Francesconi F, Lupi O. Myiasis. In: Tyring SK, Lupi O, Hengge UR, eds. Tropical Dermatology. Elsevier; 2006:232-239.
- Gordon PM, Hepburn NC, Williams AE, et al. Cutaneous myiasis due to Dermatobia hominis: a report of six cases. Br J Dermatol. 1995;132:811-814.
- Hubler WR Jr, Rudolph AH, Dougherty EF. Dermal myiasis. Arch Dermatol. 1974;110:109-110.
- Quintanilla-Cedillo MR, León-Ureña H, Contreras-Ruiz J, et al. The value of Doppler ultrasound in diagnosis in 25 cases of furunculoid myiasis. Int J Dermatol. 2005;44:34-37.
- Bakos RM, Bakos L. Dermoscopic diagnosis of furuncular myiasis. Arch Dermatol. 2007;143:123-124.
- Varani S, Tassinari D, Elleri D, et al. A case of furuncular myiasis associated with systemic inflammation. Parasitol Int. 2007;56:330-333.
- Grogan TM, Payne CM, Spier C, et al. Cutaneous myiasis. immunohistologic and ultrastructural morphometric features of a human botfly lesion. Am J Dermatopathol. 1987;9:232-239.
- Krajewski A, Allen B, Hoss D, et al. Cutaneous myiasis. J Plast Reconstr Aesthet Surg. 2009;62:383-386.
- Lebwohl MG, Heymann WR, Berth-Jones J, et al. Myiasis: Treatment of Skin Diseases. Comprehensive Therapeutic Strategies. 2nd ed. Elsevier-Mosby; 2006.
- Francesconi F, Lupi O. Myiasis. Clin Microbiol Rev. 2012;25:79-105. doi:10.1128/CMR.00010-11
- Schiff TA. Furuncular cutaneous myiasis caused by Cuterebra larva. J Am Acad Dermatol 1993;28:261-263.
- Safdar N, Young DK, Andes D. Autochthonous furuncular myiasis in the United States: case report and literature review. Clin Infect Dis. 2003;26:73-80.
- Mahal JJ, Sperling JD. Furuncular myiasis from Dermatobia hominus: a case of human botfly infestation. J Emerg Med. 2012;43:618-621.
- Francesconi F, Lupi O. Myiasis. In: Tyring SK, Lupi O, Hengge UR, eds. Tropical Dermatology. Elsevier; 2006:232-239.
- Gordon PM, Hepburn NC, Williams AE, et al. Cutaneous myiasis due to Dermatobia hominis: a report of six cases. Br J Dermatol. 1995;132:811-814.
- Hubler WR Jr, Rudolph AH, Dougherty EF. Dermal myiasis. Arch Dermatol. 1974;110:109-110.
- Quintanilla-Cedillo MR, León-Ureña H, Contreras-Ruiz J, et al. The value of Doppler ultrasound in diagnosis in 25 cases of furunculoid myiasis. Int J Dermatol. 2005;44:34-37.
- Bakos RM, Bakos L. Dermoscopic diagnosis of furuncular myiasis. Arch Dermatol. 2007;143:123-124.
- Varani S, Tassinari D, Elleri D, et al. A case of furuncular myiasis associated with systemic inflammation. Parasitol Int. 2007;56:330-333.
- Grogan TM, Payne CM, Spier C, et al. Cutaneous myiasis. immunohistologic and ultrastructural morphometric features of a human botfly lesion. Am J Dermatopathol. 1987;9:232-239.
- Krajewski A, Allen B, Hoss D, et al. Cutaneous myiasis. J Plast Reconstr Aesthet Surg. 2009;62:383-386.
- Lebwohl MG, Heymann WR, Berth-Jones J, et al. Myiasis: Treatment of Skin Diseases. Comprehensive Therapeutic Strategies. 2nd ed. Elsevier-Mosby; 2006.
Practice Points
- Cutaneous myiasis is a skin infestation with dipterous larvae that feed on the host’s tissue and cause a wide range of manifestations depending on the location of infestation. It consists of 3 types: furuncular, wound, and migratory forms.
- It is uncommon in the United States and not typically seen in patients who have no history of recent travel to tropical or subtropical areas.
- The most common cause of African furuncular myiasis acquired in the United States is larvae of the Cuterebra species (also known as the rabbit botfly or rodent botfly).
Pityriasis Rosea Associated With COVID-19 Vaccination: A Common Rash Following Administration of a Novel Vaccine
Pityriasis rosea is a papulosquamous eruption that favors the trunk and proximal extremities. It occurs most commonly in adolescents and young adults.1 The rash typically presents with a solitary lesion, known as a “herald patch,” which is followed by a scaly erythematous eruption along the cleavage lines of the skin. The condition is self-limited and often resolves in 6 to 8 weeks. Recent evidence suggests that viral reactivation of human herpesvirus 6 and human herpesvirus 7 may play a role in the development of skin lesions.2 Pityriasis rosea also has been reported following the administration of new medications and vaccinations.1-3 We report a case of a 30-year-old woman who developed pityriasis rosea 3 days after receiving the second dose of the COVID-19 vaccine.
Case Report
A 30-year-old woman presented to the dermatology office for evaluation of a rash on the trunk and upper extremities that had been present for 5 days. She reported an initial solitary lesion on the left upper back, subsequently followed by the appearance of a mildly pruritic rash on the trunk and upper extremities. The rash first appeared 3 days after she received the second dose of the Pfizer-BioNTech COVID-19 vaccine. She was otherwise asymptomatic after vaccination and denied fever, chills, headache, and myalgia. She denied any rash following her first dose of the COVID-19 vaccine, history of known COVID-19 infection or exposures, or new medications. Notably, the patient worked in health care.
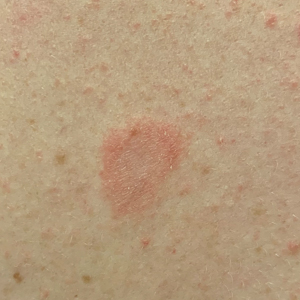
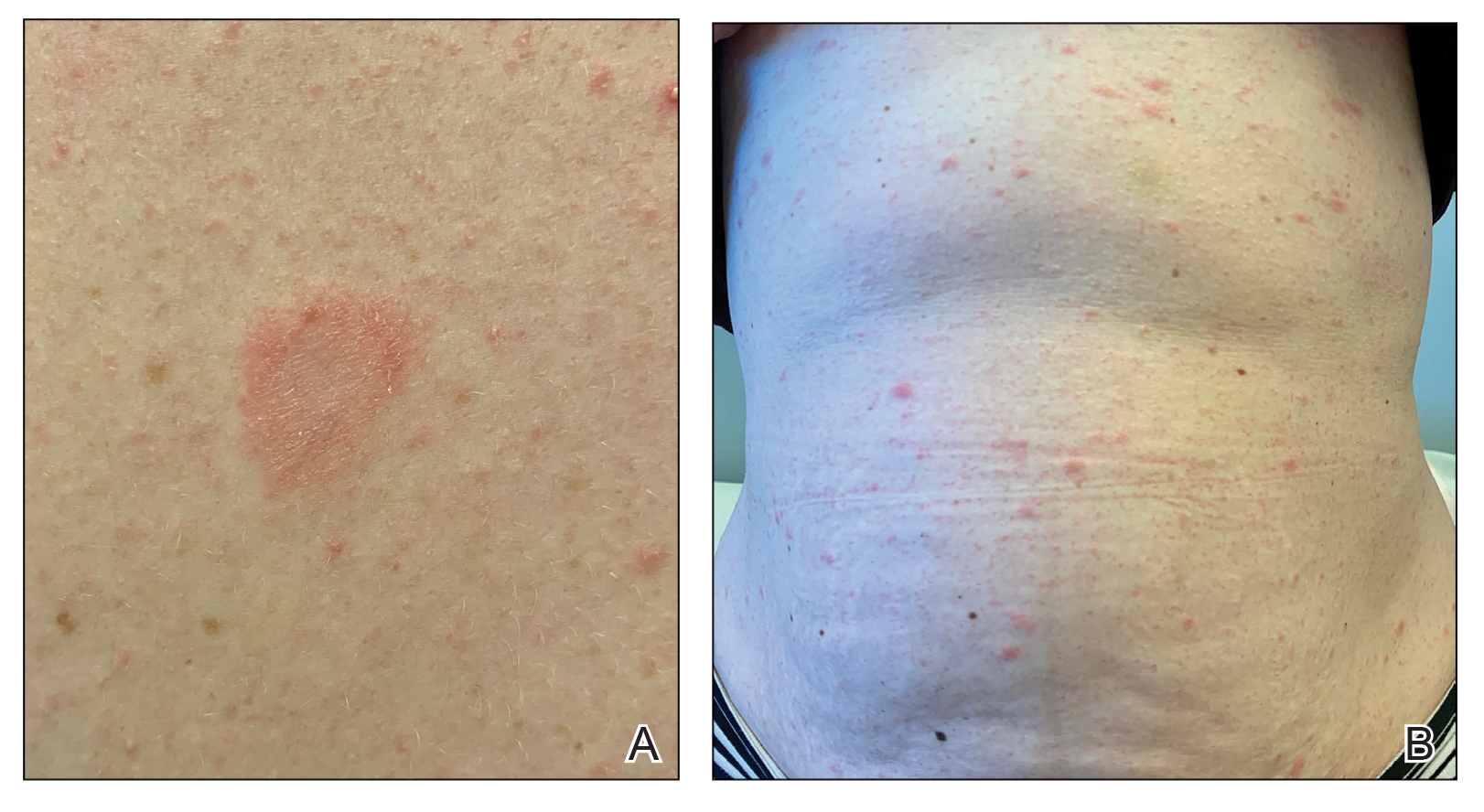
Physical examination revealed a 2-cm, erythematous, thin, scaly plaque over the left side of the upper back (Figure, A). Erythematous, scaly, thin papules of varying sizes were distributed along the cleavage lines of the trunk and upper extremities (Figure, B). No biopsy was performed because of the classic clinical presentation of this self-limited condition and the patient’s history of hypertrophic scarring. No additional laboratory workup was performed. She was prescribed triamcinolone cream 0.1% as needed for pruritus and was reassured about the benign nature of this cutaneous eruption.
Comment
A broad spectrum of cutaneous manifestations has been reported in association with acute COVID-19 infection, including a papulovesicular rash, perniolike eruptions, urticaria, livedo reticularis, and petechiae.4 Several cases of pityriasis rosea in association with acute COVID-19 infection also have been reported.5 COVID-19 infection has been linked to reactivation of the herpesvirus, which may explain the connection between acute COVID-19 infection and the development of pityriasis rosea.6 Pityriasis rosea associated with administration of the COVID-19 vaccine is a rare complication with few reports in the literature.7 Similar to our patient, there are reports of pityriasis rosea developing after the second dose of the vaccine, with some patients reporting a reactivation of skin lesions.8 There is a paucity of reports describing pityriasis rosea associated with the influenza vaccine, hepatitis B vaccine, and human papillomavirus vaccine.3 In such cases, the onset of skin lesions was thought to be related to vaccine-induced stimulation of the immune system or a component of the vaccine.
Conclusion
We presented a unique case of pityriasis rosea following COVID-19 vaccination. Because additional laboratory workup and a skin biopsy were not performed, we are unable to infer causation. However, the classic clinical presentation, rash development within 3 days of vaccination, and prior reports of vaccine-associated pityriasis rosea strengthen the aforementioned association. We hope this case adds to the growing understanding of the novel COVID-19 vaccine. As more individuals become vaccinated, both clinicians and patients should be aware of this benign cutaneous eruption that can develop following COVID-19 vaccination.
- Papakostas D, Stavropoulos PG, Papafragkaki D, et al. An atypical case of pityriasis rosea gigantea after influenza vaccination. Case Rep Dermatol. 2014;6:119-123.
- Chen FJ, Chian CP, Chen YF, et al. Pityriasis rosea following influenza (H1N1) vaccination. J Chin Med Assoc. 2011;74:280-282.
- Li A, Li P, Li Y, et al. Recurrent pityriasis rosea: a case report. Hum Vaccin Immunother. 2018;4:1024-1026.
- Ng SM. Prolonged dermatological manifestation 4 weeks following recovery of COVID-19 in a child. BMJ Case Rep. 2020;13:e237056. doi:10.1136/bcr-2020-237056
- Johansen M, Chisolm SS, Aspey LD, et al. Pityriasis rosea in otherwise asymptomatic confirmed COVID-19-positive patients: a report of 2 cases. JAAD Case Rep. 2021;7:93-94.
- Dursun R, Temiz SA. The clinics of HHV-6 infection in COVID-19 pandemic: pityriasis rosea and Kawasaki disease. Dermatol Ther. 2020;33:e13730. doi:10.1111/dth.13730
- Leerunyakul K, Pakornphadungsit K, Suchonwanit P. Case report: pityriasis rosea-like eruption following COVID-19 vaccination [published online September 7, 2021]. Front Med. doi:10.3389/fmed.2021.752443
- Marcantonio-Santa Cruz OY, Vidal-Navarro A, Pesqué D, et al. Pityriasis rosea developing after COVID-19 vaccination. J Eur Acad Dermatol Venereol. 2021;35:E721-E722. doi:10.1111/jdv.17498
Pityriasis rosea is a papulosquamous eruption that favors the trunk and proximal extremities. It occurs most commonly in adolescents and young adults.1 The rash typically presents with a solitary lesion, known as a “herald patch,” which is followed by a scaly erythematous eruption along the cleavage lines of the skin. The condition is self-limited and often resolves in 6 to 8 weeks. Recent evidence suggests that viral reactivation of human herpesvirus 6 and human herpesvirus 7 may play a role in the development of skin lesions.2 Pityriasis rosea also has been reported following the administration of new medications and vaccinations.1-3 We report a case of a 30-year-old woman who developed pityriasis rosea 3 days after receiving the second dose of the COVID-19 vaccine.
Case Report
A 30-year-old woman presented to the dermatology office for evaluation of a rash on the trunk and upper extremities that had been present for 5 days. She reported an initial solitary lesion on the left upper back, subsequently followed by the appearance of a mildly pruritic rash on the trunk and upper extremities. The rash first appeared 3 days after she received the second dose of the Pfizer-BioNTech COVID-19 vaccine. She was otherwise asymptomatic after vaccination and denied fever, chills, headache, and myalgia. She denied any rash following her first dose of the COVID-19 vaccine, history of known COVID-19 infection or exposures, or new medications. Notably, the patient worked in health care.
Physical examination revealed a 2-cm, erythematous, thin, scaly plaque over the left side of the upper back (Figure, A). Erythematous, scaly, thin papules of varying sizes were distributed along the cleavage lines of the trunk and upper extremities (Figure, B). No biopsy was performed because of the classic clinical presentation of this self-limited condition and the patient’s history of hypertrophic scarring. No additional laboratory workup was performed. She was prescribed triamcinolone cream 0.1% as needed for pruritus and was reassured about the benign nature of this cutaneous eruption.

Comment
A broad spectrum of cutaneous manifestations has been reported in association with acute COVID-19 infection, including a papulovesicular rash, perniolike eruptions, urticaria, livedo reticularis, and petechiae.4 Several cases of pityriasis rosea in association with acute COVID-19 infection also have been reported.5 COVID-19 infection has been linked to reactivation of the herpesvirus, which may explain the connection between acute COVID-19 infection and the development of pityriasis rosea.6 Pityriasis rosea associated with administration of the COVID-19 vaccine is a rare complication with few reports in the literature.7 Similar to our patient, there are reports of pityriasis rosea developing after the second dose of the vaccine, with some patients reporting a reactivation of skin lesions.8 There is a paucity of reports describing pityriasis rosea associated with the influenza vaccine, hepatitis B vaccine, and human papillomavirus vaccine.3 In such cases, the onset of skin lesions was thought to be related to vaccine-induced stimulation of the immune system or a component of the vaccine.
Conclusion
We presented a unique case of pityriasis rosea following COVID-19 vaccination. Because additional laboratory workup and a skin biopsy were not performed, we are unable to infer causation. However, the classic clinical presentation, rash development within 3 days of vaccination, and prior reports of vaccine-associated pityriasis rosea strengthen the aforementioned association. We hope this case adds to the growing understanding of the novel COVID-19 vaccine. As more individuals become vaccinated, both clinicians and patients should be aware of this benign cutaneous eruption that can develop following COVID-19 vaccination.
Pityriasis rosea is a papulosquamous eruption that favors the trunk and proximal extremities. It occurs most commonly in adolescents and young adults.1 The rash typically presents with a solitary lesion, known as a “herald patch,” which is followed by a scaly erythematous eruption along the cleavage lines of the skin. The condition is self-limited and often resolves in 6 to 8 weeks. Recent evidence suggests that viral reactivation of human herpesvirus 6 and human herpesvirus 7 may play a role in the development of skin lesions.2 Pityriasis rosea also has been reported following the administration of new medications and vaccinations.1-3 We report a case of a 30-year-old woman who developed pityriasis rosea 3 days after receiving the second dose of the COVID-19 vaccine.
Case Report
A 30-year-old woman presented to the dermatology office for evaluation of a rash on the trunk and upper extremities that had been present for 5 days. She reported an initial solitary lesion on the left upper back, subsequently followed by the appearance of a mildly pruritic rash on the trunk and upper extremities. The rash first appeared 3 days after she received the second dose of the Pfizer-BioNTech COVID-19 vaccine. She was otherwise asymptomatic after vaccination and denied fever, chills, headache, and myalgia. She denied any rash following her first dose of the COVID-19 vaccine, history of known COVID-19 infection or exposures, or new medications. Notably, the patient worked in health care.
Physical examination revealed a 2-cm, erythematous, thin, scaly plaque over the left side of the upper back (Figure, A). Erythematous, scaly, thin papules of varying sizes were distributed along the cleavage lines of the trunk and upper extremities (Figure, B). No biopsy was performed because of the classic clinical presentation of this self-limited condition and the patient’s history of hypertrophic scarring. No additional laboratory workup was performed. She was prescribed triamcinolone cream 0.1% as needed for pruritus and was reassured about the benign nature of this cutaneous eruption.

Comment
A broad spectrum of cutaneous manifestations has been reported in association with acute COVID-19 infection, including a papulovesicular rash, perniolike eruptions, urticaria, livedo reticularis, and petechiae.4 Several cases of pityriasis rosea in association with acute COVID-19 infection also have been reported.5 COVID-19 infection has been linked to reactivation of the herpesvirus, which may explain the connection between acute COVID-19 infection and the development of pityriasis rosea.6 Pityriasis rosea associated with administration of the COVID-19 vaccine is a rare complication with few reports in the literature.7 Similar to our patient, there are reports of pityriasis rosea developing after the second dose of the vaccine, with some patients reporting a reactivation of skin lesions.8 There is a paucity of reports describing pityriasis rosea associated with the influenza vaccine, hepatitis B vaccine, and human papillomavirus vaccine.3 In such cases, the onset of skin lesions was thought to be related to vaccine-induced stimulation of the immune system or a component of the vaccine.
Conclusion
We presented a unique case of pityriasis rosea following COVID-19 vaccination. Because additional laboratory workup and a skin biopsy were not performed, we are unable to infer causation. However, the classic clinical presentation, rash development within 3 days of vaccination, and prior reports of vaccine-associated pityriasis rosea strengthen the aforementioned association. We hope this case adds to the growing understanding of the novel COVID-19 vaccine. As more individuals become vaccinated, both clinicians and patients should be aware of this benign cutaneous eruption that can develop following COVID-19 vaccination.
- Papakostas D, Stavropoulos PG, Papafragkaki D, et al. An atypical case of pityriasis rosea gigantea after influenza vaccination. Case Rep Dermatol. 2014;6:119-123.
- Chen FJ, Chian CP, Chen YF, et al. Pityriasis rosea following influenza (H1N1) vaccination. J Chin Med Assoc. 2011;74:280-282.
- Li A, Li P, Li Y, et al. Recurrent pityriasis rosea: a case report. Hum Vaccin Immunother. 2018;4:1024-1026.
- Ng SM. Prolonged dermatological manifestation 4 weeks following recovery of COVID-19 in a child. BMJ Case Rep. 2020;13:e237056. doi:10.1136/bcr-2020-237056
- Johansen M, Chisolm SS, Aspey LD, et al. Pityriasis rosea in otherwise asymptomatic confirmed COVID-19-positive patients: a report of 2 cases. JAAD Case Rep. 2021;7:93-94.
- Dursun R, Temiz SA. The clinics of HHV-6 infection in COVID-19 pandemic: pityriasis rosea and Kawasaki disease. Dermatol Ther. 2020;33:e13730. doi:10.1111/dth.13730
- Leerunyakul K, Pakornphadungsit K, Suchonwanit P. Case report: pityriasis rosea-like eruption following COVID-19 vaccination [published online September 7, 2021]. Front Med. doi:10.3389/fmed.2021.752443
- Marcantonio-Santa Cruz OY, Vidal-Navarro A, Pesqué D, et al. Pityriasis rosea developing after COVID-19 vaccination. J Eur Acad Dermatol Venereol. 2021;35:E721-E722. doi:10.1111/jdv.17498
- Papakostas D, Stavropoulos PG, Papafragkaki D, et al. An atypical case of pityriasis rosea gigantea after influenza vaccination. Case Rep Dermatol. 2014;6:119-123.
- Chen FJ, Chian CP, Chen YF, et al. Pityriasis rosea following influenza (H1N1) vaccination. J Chin Med Assoc. 2011;74:280-282.
- Li A, Li P, Li Y, et al. Recurrent pityriasis rosea: a case report. Hum Vaccin Immunother. 2018;4:1024-1026.
- Ng SM. Prolonged dermatological manifestation 4 weeks following recovery of COVID-19 in a child. BMJ Case Rep. 2020;13:e237056. doi:10.1136/bcr-2020-237056
- Johansen M, Chisolm SS, Aspey LD, et al. Pityriasis rosea in otherwise asymptomatic confirmed COVID-19-positive patients: a report of 2 cases. JAAD Case Rep. 2021;7:93-94.
- Dursun R, Temiz SA. The clinics of HHV-6 infection in COVID-19 pandemic: pityriasis rosea and Kawasaki disease. Dermatol Ther. 2020;33:e13730. doi:10.1111/dth.13730
- Leerunyakul K, Pakornphadungsit K, Suchonwanit P. Case report: pityriasis rosea-like eruption following COVID-19 vaccination [published online September 7, 2021]. Front Med. doi:10.3389/fmed.2021.752443
- Marcantonio-Santa Cruz OY, Vidal-Navarro A, Pesqué D, et al. Pityriasis rosea developing after COVID-19 vaccination. J Eur Acad Dermatol Venereol. 2021;35:E721-E722. doi:10.1111/jdv.17498
Practice Points
- Clinicians should be aware of the association between COVID-19 vaccination and the development of pityriasis rosea.
- Pityriasis rosea has been linked to reactivation of human herpesvirus 6 and human herpesvirus 7 and has been reported following administration of the influenza and human papillomavirus vaccines.
- Pityriasis rosea is a self-limited, cutaneous eruption that resolves within 6 to 8 weeks, and patients should be educated on the benign nature of this condition.
Autoimmune Progesterone Dermatitis
To the Editor:
Autoimmune progesterone dermatitis (APD) is a rare dermatologic condition that can be challenging to diagnose. The associated skin lesions are not only variable in physical presentation but also in the timing of the outbreak. The skin disorder stems from an internal reaction to elevated levels of progesterone during the luteal phase of the menstrual cycle. Autoimmune progesterone dermatitis can be difficult to detect; although the typical menstrual cycle is 28 days, many women have longer or shorter hormonal phases, leading to cyclical irregularity that can cause the lesions to appear sporadic in nature when in fact they are not.1
A 34-year-old woman with a history of endometriosis, psoriasis, and malignant melanoma presented to our dermatology clinic 2 days after a brief hospitalization during which she was diagnosed with a hypersensitivity reaction. Two days prior to her hospital admission, the patient developed a rash on the lower back with associated myalgia. The rash progressively worsened, spreading laterally to the flanks, which prompted her to seek medical attention. Blood work included a complete blood cell count with differential, complete metabolic panel, antinuclear antibody test, and erythrocyte sedimentation rate, which all were within reference range. A 4-mm punch biopsy from the left lateral flank was performed and was consistent with a neutrophilic dermatosis. The patient’s symptoms diminished and she was discharged the next day with instructions to follow up with a dermatologist.
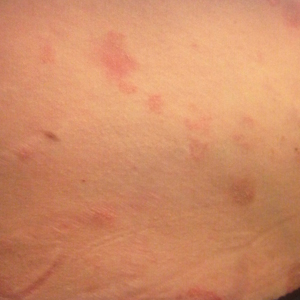
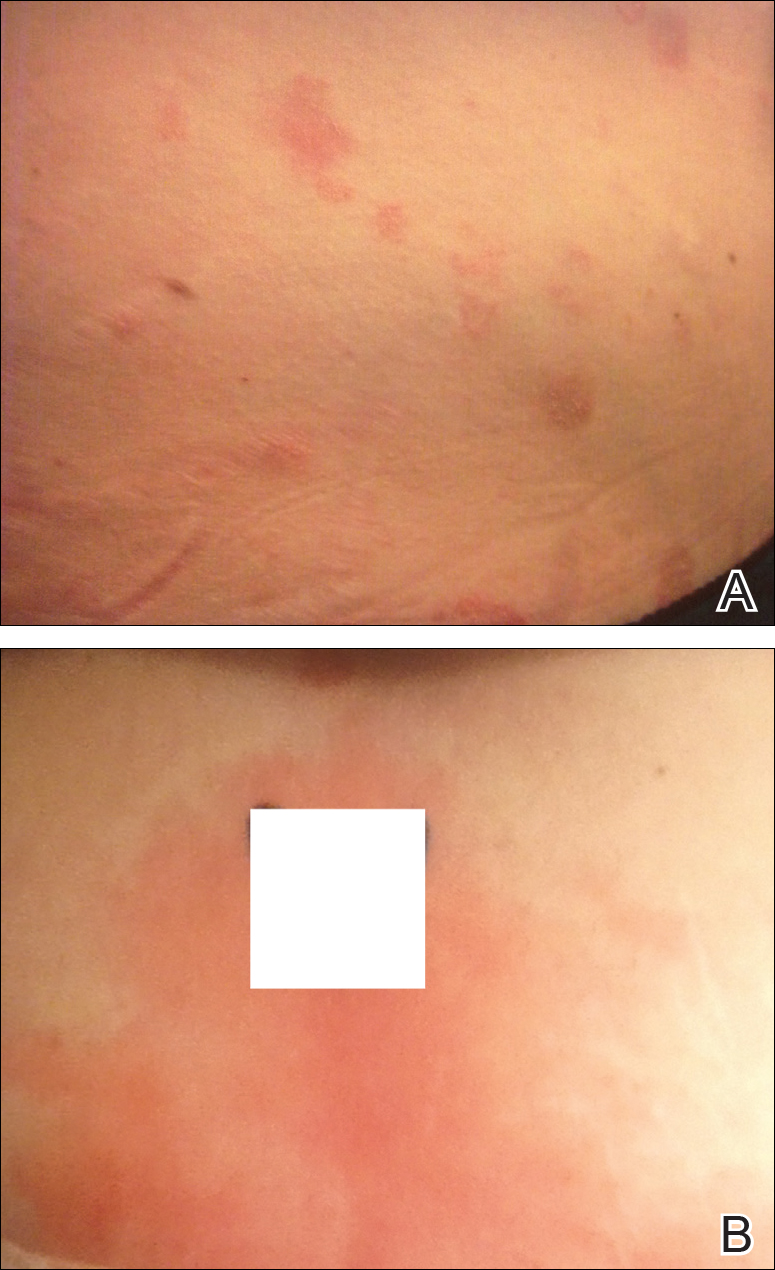
Physical examination at our clinic revealed multiple minimally indurated, erythematous plaques with superficial scaling along the left lower back and upper buttock (Figure 1). No other skin lesions were present, and palpation of the cervical, axillary, and inguinal lymph nodes was unremarkable. A repeat 6-mm punch biopsy was performed and she was sent for fasting blood work.
Histologic examination of the punch biopsy revealed a superficial and deep perivascular and interstitial dermatitis with scattered neutrophils and eosinophils. Findings were described as nonspecific, possibly representing a dermal hypersensitivity or urticarial reaction.
Glucose-6-phosphate dehydrogenase testing was within reference range, and therapy was initiated with oral dapsone 50 mg once daily as well as fexofenadine 180 mg once daily. The patient initially responded well to the oral therapy, but she experienced recurrence of the skin eruption at infrequent intervals over the next few months, requiring escalating doses of dapsone to control the symptoms. After further questioning at a subsequent visit a few months later, it was discovered that the eruption occurred near the onset of the patient’s irregular menstrual cycle.
Approximately 1 year after her initial presentation, the patient returned for intradermal hormone injections to test for hormonally induced hypersensitivities. An injection of0.1 mL of a 50-mg/mL progesterone solution was administered in the right forearm as well as 0.1 mL of a 5-mg/mL estradiol solution and 0.1 mL of saline in the left forearm as a control. One hour after the injections, a strong positive reaction consisting of a 15-mm indurated plaque with surrounding wheal was noted at the site of the progesterone injection. The estradiol and saline control sites were clear of any dermal reaction (Figure 2). A diagnosis of APD was established, and the patient was referred to her gynecologist for treatment.
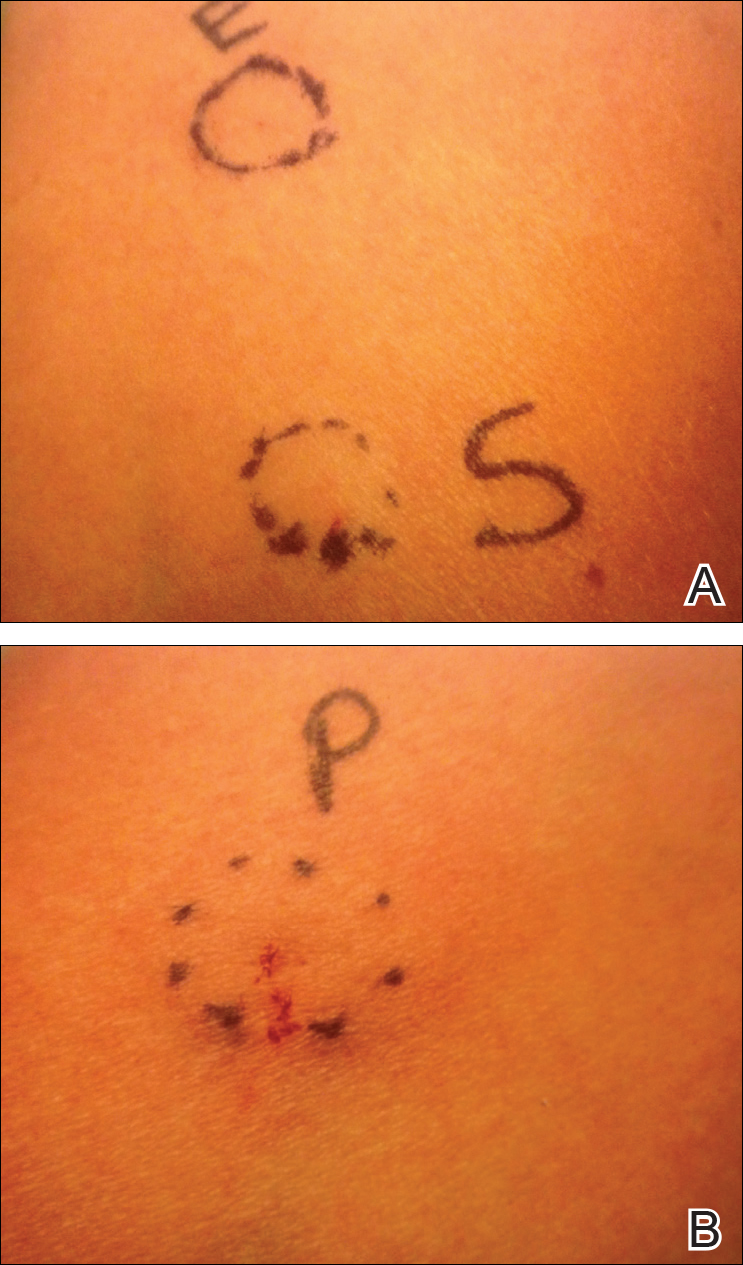
Due to the aggressive nature of her endometriosis, the gonadotropin-releasing hormone agonist leuprolide acetate was the first-line treatment prescribed by her gynecologist; however, after 8 months of therapy with leuprolide acetate, she was still experiencing breakthrough myalgia with her menstrual cycle and opted for a hysterectomy with a bilateral salpingo-oophorectomy. Within weeks of surgery, the myalgia ceased and the patient was completely asymptomatic.
Autoimmune progesterone dermatitis was first described in 1921.2 In affected women, the body reacts to the progesterone hormone surge during the luteal phase of the menstrual cycle. Symptoms begin approximately 3 to 4 days prior to menses and resolve 2 to 3 days after onset of flow. These progesterone hypersensitivity reactions can present within a spectrum of morphologies and severities. The lesions can appear eczematous, urticarial, as an angioedemalike reaction, as an erythema multiforme–like reaction with targetoid lesions, or in other nonspecific ways.1,3 Some patients experience a very mild, almost asymptomatic reaction, while others have a profound reaction progressing to anaphylaxis. Originally it was thought that exogenous exposure to progesterone led to a cross-reaction or hypersensitivity to the hormone; however, there have been cases reported in females as young as 12 years of age with no prior exposure.3,4 Reactions also can vary during pregnancy. There have been reports of spontaneous abortion in some affected females, but symptoms may dissipate in others, possibly due to a slow rise in progesterone causing a desensitization reaction.3,5
According to Bandino et al,6 there are 3 criteria for diagnosis of APD: (1) skin lesions related to the menstrual cycle, (2) positive response to intradermal testing with progesterone, and (3) symptomatic improvement after inhibiting progesterone secretions by suppressing ovulation.Areas checked with intradermal testing need to be evaluated 24 and 48 hours later for possible immediate or delayed-type hypersensitivity reactions. Biopsy typically is not helpful in this diagnosis because results usually are nonspecific.
Treatment of APD is targeted toward suppressing the internal hormonal surge. By suppressing the progesterone hormone, the symptoms are alleviated. The discomfort from the skin reaction typically is unresponsive to steroids or antihistamines. Oral contraceptives are first line in most cases because they suppress ovulation. Gonadotropin-releasing hormone analogues and tamoxifen also have been successful. For patients with severe disease that is recalcitrant to standard therapy or those who are postmenopausal, an oophorectemy is a curative option.2,4,5,7
Autoimmune progesterone dermatitis is a rare cyclical dermatologic condition in which the body responds to a surge of the patient’s own progesterone hormone. The disorder is difficult to diagnose because it can present with differing morphologies and biopsy is nonspecific. It also can be increasingly difficult to diagnose in women who do not have a typical 28-day menstrual cycle. In our patient, her irregular menstrual cycle may have caused a delay in diagnosis. Although the condition is rare, APD should be included in the differential diagnosis in females with a recurrent, cyclical, or recalcitrant cutaneous eruption.
- Wojnarowska F, Greaves MW, Peachey RD, et al. Progesterone-induced erythema multiforme. J R Soc Med. 1985;78:407-408.
- Lee MK, Lee WY, Yong SJ, et al. A case of autoimmune progesterone dermatitis misdiagnosed as allergic contact dermatitis [published online February 9, 2011]. Allergy Asthma Immunol Res. 2011;3:141-144.
- Baptist AP, Baldwin JL. Autoimmune progesterone dermatitis in a patient with endometriosis: a case report and review of the literature. Clin Mol Allergy. 2004;2:10.
- Baççıoğlu A, Kocak M, Bozdag O, et al. An unusual form of autoimmune progesterone dermatitis (ADP): the role of diagnostic challenge test. World Allergy Organ J. 2007;10:S52.
- George R, Badawy SZ. Autoimmune progesterone dermatitis: a case report [published online August 9, 2012]. Case Rep Obstet Gynecol. doi:10.1155/2012/757854.
- Bandino JP, Thoppil J, Kennedy JS, et al. Iatrogenic autoimmune progesterone dermatitis causes by 17α-hydroxyprogesterone caproate for preterm labor prevention. Cutis. 2011;88:241-243.
- Magen E, Feldman V. Autoimmune progesterone anaphylaxis in a 24-year-old woman. Isr Med Assoc J. 2012;14:518-519.
To the Editor:
Autoimmune progesterone dermatitis (APD) is a rare dermatologic condition that can be challenging to diagnose. The associated skin lesions are not only variable in physical presentation but also in the timing of the outbreak. The skin disorder stems from an internal reaction to elevated levels of progesterone during the luteal phase of the menstrual cycle. Autoimmune progesterone dermatitis can be difficult to detect; although the typical menstrual cycle is 28 days, many women have longer or shorter hormonal phases, leading to cyclical irregularity that can cause the lesions to appear sporadic in nature when in fact they are not.1
A 34-year-old woman with a history of endometriosis, psoriasis, and malignant melanoma presented to our dermatology clinic 2 days after a brief hospitalization during which she was diagnosed with a hypersensitivity reaction. Two days prior to her hospital admission, the patient developed a rash on the lower back with associated myalgia. The rash progressively worsened, spreading laterally to the flanks, which prompted her to seek medical attention. Blood work included a complete blood cell count with differential, complete metabolic panel, antinuclear antibody test, and erythrocyte sedimentation rate, which all were within reference range. A 4-mm punch biopsy from the left lateral flank was performed and was consistent with a neutrophilic dermatosis. The patient’s symptoms diminished and she was discharged the next day with instructions to follow up with a dermatologist.
Physical examination at our clinic revealed multiple minimally indurated, erythematous plaques with superficial scaling along the left lower back and upper buttock (Figure 1). No other skin lesions were present, and palpation of the cervical, axillary, and inguinal lymph nodes was unremarkable. A repeat 6-mm punch biopsy was performed and she was sent for fasting blood work.

Histologic examination of the punch biopsy revealed a superficial and deep perivascular and interstitial dermatitis with scattered neutrophils and eosinophils. Findings were described as nonspecific, possibly representing a dermal hypersensitivity or urticarial reaction.
Glucose-6-phosphate dehydrogenase testing was within reference range, and therapy was initiated with oral dapsone 50 mg once daily as well as fexofenadine 180 mg once daily. The patient initially responded well to the oral therapy, but she experienced recurrence of the skin eruption at infrequent intervals over the next few months, requiring escalating doses of dapsone to control the symptoms. After further questioning at a subsequent visit a few months later, it was discovered that the eruption occurred near the onset of the patient’s irregular menstrual cycle.
Approximately 1 year after her initial presentation, the patient returned for intradermal hormone injections to test for hormonally induced hypersensitivities. An injection of0.1 mL of a 50-mg/mL progesterone solution was administered in the right forearm as well as 0.1 mL of a 5-mg/mL estradiol solution and 0.1 mL of saline in the left forearm as a control. One hour after the injections, a strong positive reaction consisting of a 15-mm indurated plaque with surrounding wheal was noted at the site of the progesterone injection. The estradiol and saline control sites were clear of any dermal reaction (Figure 2). A diagnosis of APD was established, and the patient was referred to her gynecologist for treatment.

Due to the aggressive nature of her endometriosis, the gonadotropin-releasing hormone agonist leuprolide acetate was the first-line treatment prescribed by her gynecologist; however, after 8 months of therapy with leuprolide acetate, she was still experiencing breakthrough myalgia with her menstrual cycle and opted for a hysterectomy with a bilateral salpingo-oophorectomy. Within weeks of surgery, the myalgia ceased and the patient was completely asymptomatic.
Autoimmune progesterone dermatitis was first described in 1921.2 In affected women, the body reacts to the progesterone hormone surge during the luteal phase of the menstrual cycle. Symptoms begin approximately 3 to 4 days prior to menses and resolve 2 to 3 days after onset of flow. These progesterone hypersensitivity reactions can present within a spectrum of morphologies and severities. The lesions can appear eczematous, urticarial, as an angioedemalike reaction, as an erythema multiforme–like reaction with targetoid lesions, or in other nonspecific ways.1,3 Some patients experience a very mild, almost asymptomatic reaction, while others have a profound reaction progressing to anaphylaxis. Originally it was thought that exogenous exposure to progesterone led to a cross-reaction or hypersensitivity to the hormone; however, there have been cases reported in females as young as 12 years of age with no prior exposure.3,4 Reactions also can vary during pregnancy. There have been reports of spontaneous abortion in some affected females, but symptoms may dissipate in others, possibly due to a slow rise in progesterone causing a desensitization reaction.3,5
According to Bandino et al,6 there are 3 criteria for diagnosis of APD: (1) skin lesions related to the menstrual cycle, (2) positive response to intradermal testing with progesterone, and (3) symptomatic improvement after inhibiting progesterone secretions by suppressing ovulation.Areas checked with intradermal testing need to be evaluated 24 and 48 hours later for possible immediate or delayed-type hypersensitivity reactions. Biopsy typically is not helpful in this diagnosis because results usually are nonspecific.
Treatment of APD is targeted toward suppressing the internal hormonal surge. By suppressing the progesterone hormone, the symptoms are alleviated. The discomfort from the skin reaction typically is unresponsive to steroids or antihistamines. Oral contraceptives are first line in most cases because they suppress ovulation. Gonadotropin-releasing hormone analogues and tamoxifen also have been successful. For patients with severe disease that is recalcitrant to standard therapy or those who are postmenopausal, an oophorectemy is a curative option.2,4,5,7
Autoimmune progesterone dermatitis is a rare cyclical dermatologic condition in which the body responds to a surge of the patient’s own progesterone hormone. The disorder is difficult to diagnose because it can present with differing morphologies and biopsy is nonspecific. It also can be increasingly difficult to diagnose in women who do not have a typical 28-day menstrual cycle. In our patient, her irregular menstrual cycle may have caused a delay in diagnosis. Although the condition is rare, APD should be included in the differential diagnosis in females with a recurrent, cyclical, or recalcitrant cutaneous eruption.
To the Editor:
Autoimmune progesterone dermatitis (APD) is a rare dermatologic condition that can be challenging to diagnose. The associated skin lesions are not only variable in physical presentation but also in the timing of the outbreak. The skin disorder stems from an internal reaction to elevated levels of progesterone during the luteal phase of the menstrual cycle. Autoimmune progesterone dermatitis can be difficult to detect; although the typical menstrual cycle is 28 days, many women have longer or shorter hormonal phases, leading to cyclical irregularity that can cause the lesions to appear sporadic in nature when in fact they are not.1
A 34-year-old woman with a history of endometriosis, psoriasis, and malignant melanoma presented to our dermatology clinic 2 days after a brief hospitalization during which she was diagnosed with a hypersensitivity reaction. Two days prior to her hospital admission, the patient developed a rash on the lower back with associated myalgia. The rash progressively worsened, spreading laterally to the flanks, which prompted her to seek medical attention. Blood work included a complete blood cell count with differential, complete metabolic panel, antinuclear antibody test, and erythrocyte sedimentation rate, which all were within reference range. A 4-mm punch biopsy from the left lateral flank was performed and was consistent with a neutrophilic dermatosis. The patient’s symptoms diminished and she was discharged the next day with instructions to follow up with a dermatologist.
Physical examination at our clinic revealed multiple minimally indurated, erythematous plaques with superficial scaling along the left lower back and upper buttock (Figure 1). No other skin lesions were present, and palpation of the cervical, axillary, and inguinal lymph nodes was unremarkable. A repeat 6-mm punch biopsy was performed and she was sent for fasting blood work.

Histologic examination of the punch biopsy revealed a superficial and deep perivascular and interstitial dermatitis with scattered neutrophils and eosinophils. Findings were described as nonspecific, possibly representing a dermal hypersensitivity or urticarial reaction.
Glucose-6-phosphate dehydrogenase testing was within reference range, and therapy was initiated with oral dapsone 50 mg once daily as well as fexofenadine 180 mg once daily. The patient initially responded well to the oral therapy, but she experienced recurrence of the skin eruption at infrequent intervals over the next few months, requiring escalating doses of dapsone to control the symptoms. After further questioning at a subsequent visit a few months later, it was discovered that the eruption occurred near the onset of the patient’s irregular menstrual cycle.
Approximately 1 year after her initial presentation, the patient returned for intradermal hormone injections to test for hormonally induced hypersensitivities. An injection of0.1 mL of a 50-mg/mL progesterone solution was administered in the right forearm as well as 0.1 mL of a 5-mg/mL estradiol solution and 0.1 mL of saline in the left forearm as a control. One hour after the injections, a strong positive reaction consisting of a 15-mm indurated plaque with surrounding wheal was noted at the site of the progesterone injection. The estradiol and saline control sites were clear of any dermal reaction (Figure 2). A diagnosis of APD was established, and the patient was referred to her gynecologist for treatment.

Due to the aggressive nature of her endometriosis, the gonadotropin-releasing hormone agonist leuprolide acetate was the first-line treatment prescribed by her gynecologist; however, after 8 months of therapy with leuprolide acetate, she was still experiencing breakthrough myalgia with her menstrual cycle and opted for a hysterectomy with a bilateral salpingo-oophorectomy. Within weeks of surgery, the myalgia ceased and the patient was completely asymptomatic.
Autoimmune progesterone dermatitis was first described in 1921.2 In affected women, the body reacts to the progesterone hormone surge during the luteal phase of the menstrual cycle. Symptoms begin approximately 3 to 4 days prior to menses and resolve 2 to 3 days after onset of flow. These progesterone hypersensitivity reactions can present within a spectrum of morphologies and severities. The lesions can appear eczematous, urticarial, as an angioedemalike reaction, as an erythema multiforme–like reaction with targetoid lesions, or in other nonspecific ways.1,3 Some patients experience a very mild, almost asymptomatic reaction, while others have a profound reaction progressing to anaphylaxis. Originally it was thought that exogenous exposure to progesterone led to a cross-reaction or hypersensitivity to the hormone; however, there have been cases reported in females as young as 12 years of age with no prior exposure.3,4 Reactions also can vary during pregnancy. There have been reports of spontaneous abortion in some affected females, but symptoms may dissipate in others, possibly due to a slow rise in progesterone causing a desensitization reaction.3,5
According to Bandino et al,6 there are 3 criteria for diagnosis of APD: (1) skin lesions related to the menstrual cycle, (2) positive response to intradermal testing with progesterone, and (3) symptomatic improvement after inhibiting progesterone secretions by suppressing ovulation.Areas checked with intradermal testing need to be evaluated 24 and 48 hours later for possible immediate or delayed-type hypersensitivity reactions. Biopsy typically is not helpful in this diagnosis because results usually are nonspecific.
Treatment of APD is targeted toward suppressing the internal hormonal surge. By suppressing the progesterone hormone, the symptoms are alleviated. The discomfort from the skin reaction typically is unresponsive to steroids or antihistamines. Oral contraceptives are first line in most cases because they suppress ovulation. Gonadotropin-releasing hormone analogues and tamoxifen also have been successful. For patients with severe disease that is recalcitrant to standard therapy or those who are postmenopausal, an oophorectemy is a curative option.2,4,5,7
Autoimmune progesterone dermatitis is a rare cyclical dermatologic condition in which the body responds to a surge of the patient’s own progesterone hormone. The disorder is difficult to diagnose because it can present with differing morphologies and biopsy is nonspecific. It also can be increasingly difficult to diagnose in women who do not have a typical 28-day menstrual cycle. In our patient, her irregular menstrual cycle may have caused a delay in diagnosis. Although the condition is rare, APD should be included in the differential diagnosis in females with a recurrent, cyclical, or recalcitrant cutaneous eruption.
- Wojnarowska F, Greaves MW, Peachey RD, et al. Progesterone-induced erythema multiforme. J R Soc Med. 1985;78:407-408.
- Lee MK, Lee WY, Yong SJ, et al. A case of autoimmune progesterone dermatitis misdiagnosed as allergic contact dermatitis [published online February 9, 2011]. Allergy Asthma Immunol Res. 2011;3:141-144.
- Baptist AP, Baldwin JL. Autoimmune progesterone dermatitis in a patient with endometriosis: a case report and review of the literature. Clin Mol Allergy. 2004;2:10.
- Baççıoğlu A, Kocak M, Bozdag O, et al. An unusual form of autoimmune progesterone dermatitis (ADP): the role of diagnostic challenge test. World Allergy Organ J. 2007;10:S52.
- George R, Badawy SZ. Autoimmune progesterone dermatitis: a case report [published online August 9, 2012]. Case Rep Obstet Gynecol. doi:10.1155/2012/757854.
- Bandino JP, Thoppil J, Kennedy JS, et al. Iatrogenic autoimmune progesterone dermatitis causes by 17α-hydroxyprogesterone caproate for preterm labor prevention. Cutis. 2011;88:241-243.
- Magen E, Feldman V. Autoimmune progesterone anaphylaxis in a 24-year-old woman. Isr Med Assoc J. 2012;14:518-519.
- Wojnarowska F, Greaves MW, Peachey RD, et al. Progesterone-induced erythema multiforme. J R Soc Med. 1985;78:407-408.
- Lee MK, Lee WY, Yong SJ, et al. A case of autoimmune progesterone dermatitis misdiagnosed as allergic contact dermatitis [published online February 9, 2011]. Allergy Asthma Immunol Res. 2011;3:141-144.
- Baptist AP, Baldwin JL. Autoimmune progesterone dermatitis in a patient with endometriosis: a case report and review of the literature. Clin Mol Allergy. 2004;2:10.
- Baççıoğlu A, Kocak M, Bozdag O, et al. An unusual form of autoimmune progesterone dermatitis (ADP): the role of diagnostic challenge test. World Allergy Organ J. 2007;10:S52.
- George R, Badawy SZ. Autoimmune progesterone dermatitis: a case report [published online August 9, 2012]. Case Rep Obstet Gynecol. doi:10.1155/2012/757854.
- Bandino JP, Thoppil J, Kennedy JS, et al. Iatrogenic autoimmune progesterone dermatitis causes by 17α-hydroxyprogesterone caproate for preterm labor prevention. Cutis. 2011;88:241-243.
- Magen E, Feldman V. Autoimmune progesterone anaphylaxis in a 24-year-old woman. Isr Med Assoc J. 2012;14:518-519.
Practice Points
- Autoimmune progesterone dermatitis (APD) is a hypersensitivity reaction to the progesterone surge during a woman’s menstrual cycle.
- Patients with APD often are misdiagnosed for years due to the variability of each woman’s menstrual cycle, making the correlation difficult.
- It is important to keep APD in mind for any recalcitrant or recurrent rash in females. A thorough history is critical when formulating a diagnosis.