User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Statins tied to lower ICH risk regardless of bleed location
A new study has provided further reassurance on questions about the risk of intracerebral hemorrhage (ICH) with statins.
The Danish case-control study, which compared statin use in 2,164 case patients with ICH and in 86,255 matched control persons, found that current statin use was associated with a lower risk of having a first ICH and that the risk was further reduced with longer duration of statin use.
The study also showed that statin use was linked to a lower risk of ICH in the more superficial lobar areas of the brain and in the deeper, nonlobar locations. There was no difference in the magnitude of risk reduction between the two locations.
“Although this study is observational, I feel these data are strong, and the results are reassuring. It certainly does not suggest any increased risk of ICH with statins,” senior author David Gaist, PhD, Odense University Hospital, Denmark, said in an interview.
“On the contrary, it indicates a lower risk, which seems to be independent of the location of the bleed.”
The study was published online in Neurology.
The authors note that statins effectively reduce the occurrence of cardiovascular events and ischemic stroke in high-risk populations, but early randomized trials raised concerns of an increased risk of ICH among statin users who have a history of stroke.
Subsequent observational studies, including four meta-analyses, included patients with and those without prior stroke. The results were inconsistent, although most found no increase in bleeding. More recent studies have found a lower risk of ICH among statin users; the risk was inversely associated with the duration and intensity of statin treatment.
However, the researchers point out that few studies have assessed the association between statin use and the location of ICH. Hemorrhages that occur in the lobar region of the brain and those that occur in the nonlobar areas can have different pathophysiologies. Arteriolosclerosis, which is strongly associated with hypertension, is a common histologic finding in patients with ICH, regardless of hemorrhage location, while cerebral amyloid angiopathy (CAA) is associated with lobar but not nonlobar ICH.
The current study was conducted to look more closely at the relationship between statin use and hematoma location as a reflection of differences in the underlying pathophysiologies of lobar versus nonlobar ICH.
The researchers used Danish registries to identify all first-ever cases of spontaneous ICH that occurred between 2009 and 2018 in persons older than 55 years in the Southern Denmark region. Patients with traumatic ICH or ICH related to vascular malformations and tumors were excluded.
These cases were verified through medical records. ICH diagnoses were classified as having a lobar or nonlobar location, and patients were matched for age, sex, and calendar year to general population control persons. The nationwide prescription registry was also analyzed to ascertain use of statins and other medications.
The study included 989 patients with lobar ICH who were matched to 39,500 control persons and 1,175 patients with nonlobar ICH who were matched to 46,755 control persons.
Results showed that current statin use was associated with a 16%-17% relative reduction in ICH risk. There was no difference with respect to ICH location.
For lobar ICH, statin use showed an adjusted odds ratio of 0.83 (95% confidence interval, 0.70-0.98); for nonlobar ICH, the adjusted odds ratio was 0.84 (95% CI, 0.72-0.98).
Longer duration of statin use was associated with a greater reduction in risk of ICH; use for more than 5 years was associated with a relative reduction of ICH of 33%-38%, again with no difference with regard to ICH location.
For lobar ICH, statin use for more than 5 years showed an adjusted odds ratio of 0.67 (95% CI, 0.51-0.87); and for nonlobar ICH, the adjusted odds ratio was 0.62 (95% CI, 0.48-0.80).
“We suspected that statins may have more of an effect in reducing nonlobar ICH, as this type is considered to be more associated with arteriosclerosis, compared with lobar ICH,” Dr. Gaist explained. “But we didn’t find that. We found that taking statins was associated with a similar reduction in risk of both lobar and nonlobar ICH.”
Although amyloid angiopathy can contribute to lobar ICH, arteriosclerosis is still involved in the majority of cases, he noted. He cited a recent population-based U.K. study that showed that while histologically verified CAA was present in 58% of patients with a lobar ICH, most also had evidence of arteriosclerosis, with only 13% having isolated CAA pathology.
“If statins exert their effect on reducing ICH by reducing arteriosclerosis, which is likely, then this observation of arteriosclerosis pathology being prevalent in both lobar and nonlobar ICH locations would explain our results,” Dr. Gaist commented.
“Strengths of our study include the large numbers involved and the fact that the patients are unselected. We tried to find everyone who had had a first ICH in a well-defined region of Denmark, so issues of selection are less of a concern than in some other studies,” he noted.
He also pointed out that all the ICH diagnoses were verified from medical records and that in a substudy, brain scans were evaluated, with investigators masked to clinical data to evaluate the location and characteristics of the hematoma. In addition, data on statin use were collected prospectively from a nationwide prescription registry.
Interaction with antihypertensives, anticoagulants?
Other results from the study suggest a possible interaction between statin use and antihypertensive and anticoagulant drugs.
Data showed that the lower ICH risk was restricted to patients who received statins and antihypertensive drugs concurrently. Conversely, only patients who were not concurrently taking anticoagulants had a lower risk of ICH in association with statin use.
Dr. Gaist suggested that the lack of a reduction in ICH with statins among patients taking anticoagulants could be because the increased risk of ICH with anticoagulants was stronger than the reduced risk with statins.
Regarding the fact that the reduced risk of ICH with statins was only observed among individuals who were also taking antihypertensive medication, Dr. Gaist noted that because hypertension is such an important risk factor for ICH, “it may be that to get the true benefit of statins, patients have to have their hypertension controlled.”
However, an alternative explanation could that the finding is a result of “healthy adherer” bias, in which people who take antihypertensive medication and follow a healthy lifestyle as advised would be more likely to take statins.
“The observational nature of our study does not allow us to determine the extent to which associations are causal,” the authors say.
Dr. Gaist also noted that an important caveat in this study is that they focused on individuals who had had a first ICH.
“This data does not inform us about those who have already had an ICH and are taking statins. But we are planning to look at this in our next study,” he said.
The study was funded by the Novo Nordisk Foundation. Dr. Gaist has received speaker honorarium from Bristol-Myers Squibb and Pfizer unrelated to this work.
A version of this article first appeared on Medscape.com.
A new study has provided further reassurance on questions about the risk of intracerebral hemorrhage (ICH) with statins.
The Danish case-control study, which compared statin use in 2,164 case patients with ICH and in 86,255 matched control persons, found that current statin use was associated with a lower risk of having a first ICH and that the risk was further reduced with longer duration of statin use.
The study also showed that statin use was linked to a lower risk of ICH in the more superficial lobar areas of the brain and in the deeper, nonlobar locations. There was no difference in the magnitude of risk reduction between the two locations.
“Although this study is observational, I feel these data are strong, and the results are reassuring. It certainly does not suggest any increased risk of ICH with statins,” senior author David Gaist, PhD, Odense University Hospital, Denmark, said in an interview.
“On the contrary, it indicates a lower risk, which seems to be independent of the location of the bleed.”
The study was published online in Neurology.
The authors note that statins effectively reduce the occurrence of cardiovascular events and ischemic stroke in high-risk populations, but early randomized trials raised concerns of an increased risk of ICH among statin users who have a history of stroke.
Subsequent observational studies, including four meta-analyses, included patients with and those without prior stroke. The results were inconsistent, although most found no increase in bleeding. More recent studies have found a lower risk of ICH among statin users; the risk was inversely associated with the duration and intensity of statin treatment.
However, the researchers point out that few studies have assessed the association between statin use and the location of ICH. Hemorrhages that occur in the lobar region of the brain and those that occur in the nonlobar areas can have different pathophysiologies. Arteriolosclerosis, which is strongly associated with hypertension, is a common histologic finding in patients with ICH, regardless of hemorrhage location, while cerebral amyloid angiopathy (CAA) is associated with lobar but not nonlobar ICH.
The current study was conducted to look more closely at the relationship between statin use and hematoma location as a reflection of differences in the underlying pathophysiologies of lobar versus nonlobar ICH.
The researchers used Danish registries to identify all first-ever cases of spontaneous ICH that occurred between 2009 and 2018 in persons older than 55 years in the Southern Denmark region. Patients with traumatic ICH or ICH related to vascular malformations and tumors were excluded.
These cases were verified through medical records. ICH diagnoses were classified as having a lobar or nonlobar location, and patients were matched for age, sex, and calendar year to general population control persons. The nationwide prescription registry was also analyzed to ascertain use of statins and other medications.
The study included 989 patients with lobar ICH who were matched to 39,500 control persons and 1,175 patients with nonlobar ICH who were matched to 46,755 control persons.
Results showed that current statin use was associated with a 16%-17% relative reduction in ICH risk. There was no difference with respect to ICH location.
For lobar ICH, statin use showed an adjusted odds ratio of 0.83 (95% confidence interval, 0.70-0.98); for nonlobar ICH, the adjusted odds ratio was 0.84 (95% CI, 0.72-0.98).
Longer duration of statin use was associated with a greater reduction in risk of ICH; use for more than 5 years was associated with a relative reduction of ICH of 33%-38%, again with no difference with regard to ICH location.
For lobar ICH, statin use for more than 5 years showed an adjusted odds ratio of 0.67 (95% CI, 0.51-0.87); and for nonlobar ICH, the adjusted odds ratio was 0.62 (95% CI, 0.48-0.80).
“We suspected that statins may have more of an effect in reducing nonlobar ICH, as this type is considered to be more associated with arteriosclerosis, compared with lobar ICH,” Dr. Gaist explained. “But we didn’t find that. We found that taking statins was associated with a similar reduction in risk of both lobar and nonlobar ICH.”
Although amyloid angiopathy can contribute to lobar ICH, arteriosclerosis is still involved in the majority of cases, he noted. He cited a recent population-based U.K. study that showed that while histologically verified CAA was present in 58% of patients with a lobar ICH, most also had evidence of arteriosclerosis, with only 13% having isolated CAA pathology.
“If statins exert their effect on reducing ICH by reducing arteriosclerosis, which is likely, then this observation of arteriosclerosis pathology being prevalent in both lobar and nonlobar ICH locations would explain our results,” Dr. Gaist commented.
“Strengths of our study include the large numbers involved and the fact that the patients are unselected. We tried to find everyone who had had a first ICH in a well-defined region of Denmark, so issues of selection are less of a concern than in some other studies,” he noted.
He also pointed out that all the ICH diagnoses were verified from medical records and that in a substudy, brain scans were evaluated, with investigators masked to clinical data to evaluate the location and characteristics of the hematoma. In addition, data on statin use were collected prospectively from a nationwide prescription registry.
Interaction with antihypertensives, anticoagulants?
Other results from the study suggest a possible interaction between statin use and antihypertensive and anticoagulant drugs.
Data showed that the lower ICH risk was restricted to patients who received statins and antihypertensive drugs concurrently. Conversely, only patients who were not concurrently taking anticoagulants had a lower risk of ICH in association with statin use.
Dr. Gaist suggested that the lack of a reduction in ICH with statins among patients taking anticoagulants could be because the increased risk of ICH with anticoagulants was stronger than the reduced risk with statins.
Regarding the fact that the reduced risk of ICH with statins was only observed among individuals who were also taking antihypertensive medication, Dr. Gaist noted that because hypertension is such an important risk factor for ICH, “it may be that to get the true benefit of statins, patients have to have their hypertension controlled.”
However, an alternative explanation could that the finding is a result of “healthy adherer” bias, in which people who take antihypertensive medication and follow a healthy lifestyle as advised would be more likely to take statins.
“The observational nature of our study does not allow us to determine the extent to which associations are causal,” the authors say.
Dr. Gaist also noted that an important caveat in this study is that they focused on individuals who had had a first ICH.
“This data does not inform us about those who have already had an ICH and are taking statins. But we are planning to look at this in our next study,” he said.
The study was funded by the Novo Nordisk Foundation. Dr. Gaist has received speaker honorarium from Bristol-Myers Squibb and Pfizer unrelated to this work.
A version of this article first appeared on Medscape.com.
A new study has provided further reassurance on questions about the risk of intracerebral hemorrhage (ICH) with statins.
The Danish case-control study, which compared statin use in 2,164 case patients with ICH and in 86,255 matched control persons, found that current statin use was associated with a lower risk of having a first ICH and that the risk was further reduced with longer duration of statin use.
The study also showed that statin use was linked to a lower risk of ICH in the more superficial lobar areas of the brain and in the deeper, nonlobar locations. There was no difference in the magnitude of risk reduction between the two locations.
“Although this study is observational, I feel these data are strong, and the results are reassuring. It certainly does not suggest any increased risk of ICH with statins,” senior author David Gaist, PhD, Odense University Hospital, Denmark, said in an interview.
“On the contrary, it indicates a lower risk, which seems to be independent of the location of the bleed.”
The study was published online in Neurology.
The authors note that statins effectively reduce the occurrence of cardiovascular events and ischemic stroke in high-risk populations, but early randomized trials raised concerns of an increased risk of ICH among statin users who have a history of stroke.
Subsequent observational studies, including four meta-analyses, included patients with and those without prior stroke. The results were inconsistent, although most found no increase in bleeding. More recent studies have found a lower risk of ICH among statin users; the risk was inversely associated with the duration and intensity of statin treatment.
However, the researchers point out that few studies have assessed the association between statin use and the location of ICH. Hemorrhages that occur in the lobar region of the brain and those that occur in the nonlobar areas can have different pathophysiologies. Arteriolosclerosis, which is strongly associated with hypertension, is a common histologic finding in patients with ICH, regardless of hemorrhage location, while cerebral amyloid angiopathy (CAA) is associated with lobar but not nonlobar ICH.
The current study was conducted to look more closely at the relationship between statin use and hematoma location as a reflection of differences in the underlying pathophysiologies of lobar versus nonlobar ICH.
The researchers used Danish registries to identify all first-ever cases of spontaneous ICH that occurred between 2009 and 2018 in persons older than 55 years in the Southern Denmark region. Patients with traumatic ICH or ICH related to vascular malformations and tumors were excluded.
These cases were verified through medical records. ICH diagnoses were classified as having a lobar or nonlobar location, and patients were matched for age, sex, and calendar year to general population control persons. The nationwide prescription registry was also analyzed to ascertain use of statins and other medications.
The study included 989 patients with lobar ICH who were matched to 39,500 control persons and 1,175 patients with nonlobar ICH who were matched to 46,755 control persons.
Results showed that current statin use was associated with a 16%-17% relative reduction in ICH risk. There was no difference with respect to ICH location.
For lobar ICH, statin use showed an adjusted odds ratio of 0.83 (95% confidence interval, 0.70-0.98); for nonlobar ICH, the adjusted odds ratio was 0.84 (95% CI, 0.72-0.98).
Longer duration of statin use was associated with a greater reduction in risk of ICH; use for more than 5 years was associated with a relative reduction of ICH of 33%-38%, again with no difference with regard to ICH location.
For lobar ICH, statin use for more than 5 years showed an adjusted odds ratio of 0.67 (95% CI, 0.51-0.87); and for nonlobar ICH, the adjusted odds ratio was 0.62 (95% CI, 0.48-0.80).
“We suspected that statins may have more of an effect in reducing nonlobar ICH, as this type is considered to be more associated with arteriosclerosis, compared with lobar ICH,” Dr. Gaist explained. “But we didn’t find that. We found that taking statins was associated with a similar reduction in risk of both lobar and nonlobar ICH.”
Although amyloid angiopathy can contribute to lobar ICH, arteriosclerosis is still involved in the majority of cases, he noted. He cited a recent population-based U.K. study that showed that while histologically verified CAA was present in 58% of patients with a lobar ICH, most also had evidence of arteriosclerosis, with only 13% having isolated CAA pathology.
“If statins exert their effect on reducing ICH by reducing arteriosclerosis, which is likely, then this observation of arteriosclerosis pathology being prevalent in both lobar and nonlobar ICH locations would explain our results,” Dr. Gaist commented.
“Strengths of our study include the large numbers involved and the fact that the patients are unselected. We tried to find everyone who had had a first ICH in a well-defined region of Denmark, so issues of selection are less of a concern than in some other studies,” he noted.
He also pointed out that all the ICH diagnoses were verified from medical records and that in a substudy, brain scans were evaluated, with investigators masked to clinical data to evaluate the location and characteristics of the hematoma. In addition, data on statin use were collected prospectively from a nationwide prescription registry.
Interaction with antihypertensives, anticoagulants?
Other results from the study suggest a possible interaction between statin use and antihypertensive and anticoagulant drugs.
Data showed that the lower ICH risk was restricted to patients who received statins and antihypertensive drugs concurrently. Conversely, only patients who were not concurrently taking anticoagulants had a lower risk of ICH in association with statin use.
Dr. Gaist suggested that the lack of a reduction in ICH with statins among patients taking anticoagulants could be because the increased risk of ICH with anticoagulants was stronger than the reduced risk with statins.
Regarding the fact that the reduced risk of ICH with statins was only observed among individuals who were also taking antihypertensive medication, Dr. Gaist noted that because hypertension is such an important risk factor for ICH, “it may be that to get the true benefit of statins, patients have to have their hypertension controlled.”
However, an alternative explanation could that the finding is a result of “healthy adherer” bias, in which people who take antihypertensive medication and follow a healthy lifestyle as advised would be more likely to take statins.
“The observational nature of our study does not allow us to determine the extent to which associations are causal,” the authors say.
Dr. Gaist also noted that an important caveat in this study is that they focused on individuals who had had a first ICH.
“This data does not inform us about those who have already had an ICH and are taking statins. But we are planning to look at this in our next study,” he said.
The study was funded by the Novo Nordisk Foundation. Dr. Gaist has received speaker honorarium from Bristol-Myers Squibb and Pfizer unrelated to this work.
A version of this article first appeared on Medscape.com.
Cold water immersion can have benefits
according to researchers from the Arctic University of Norway and the University Hospital of North Norway.
What to know
- Immersion in cold water has a major impact on the body. It elevates the heart rate and has positive effects on brown adipose tissue, a type of “good” body fat that is activated by cold and may protect against and cardiovascular disease.
- Exposure to cold water or cold air also appears to increase the production of the protein adiponectin by adipose tissue. Adiponectin plays a key role in protecting against , diabetes, and other diseases.
- Repeated cold-water immersions by inexperienced as well as experienced swimmers during the winter months significantly increased sensitivity and decreased insulin concentrations.
- Numerous health and well-being claims from regular exposure to the cold, such as weight loss, better mental health, and increased libido, may be explained by other factors, including an active lifestyle, trained stress handling, and social interactions, as well as a positive mindset.
- Those seeking to voluntarily practice cold-water immersion need to be educated about possible health risks associated with taking a dip in icy water, which include the consequences of hypothermia, and of heart and lung problems, which are often related to the shock from the cold.
This is a summary of the article, “Health effects of voluntary exposure to cold water – a continuing subject of debate,” published by the International Journal of Circumpolar Health.
A version of this article first appeared on Medscape.com.
according to researchers from the Arctic University of Norway and the University Hospital of North Norway.
What to know
- Immersion in cold water has a major impact on the body. It elevates the heart rate and has positive effects on brown adipose tissue, a type of “good” body fat that is activated by cold and may protect against and cardiovascular disease.
- Exposure to cold water or cold air also appears to increase the production of the protein adiponectin by adipose tissue. Adiponectin plays a key role in protecting against , diabetes, and other diseases.
- Repeated cold-water immersions by inexperienced as well as experienced swimmers during the winter months significantly increased sensitivity and decreased insulin concentrations.
- Numerous health and well-being claims from regular exposure to the cold, such as weight loss, better mental health, and increased libido, may be explained by other factors, including an active lifestyle, trained stress handling, and social interactions, as well as a positive mindset.
- Those seeking to voluntarily practice cold-water immersion need to be educated about possible health risks associated with taking a dip in icy water, which include the consequences of hypothermia, and of heart and lung problems, which are often related to the shock from the cold.
This is a summary of the article, “Health effects of voluntary exposure to cold water – a continuing subject of debate,” published by the International Journal of Circumpolar Health.
A version of this article first appeared on Medscape.com.
according to researchers from the Arctic University of Norway and the University Hospital of North Norway.
What to know
- Immersion in cold water has a major impact on the body. It elevates the heart rate and has positive effects on brown adipose tissue, a type of “good” body fat that is activated by cold and may protect against and cardiovascular disease.
- Exposure to cold water or cold air also appears to increase the production of the protein adiponectin by adipose tissue. Adiponectin plays a key role in protecting against , diabetes, and other diseases.
- Repeated cold-water immersions by inexperienced as well as experienced swimmers during the winter months significantly increased sensitivity and decreased insulin concentrations.
- Numerous health and well-being claims from regular exposure to the cold, such as weight loss, better mental health, and increased libido, may be explained by other factors, including an active lifestyle, trained stress handling, and social interactions, as well as a positive mindset.
- Those seeking to voluntarily practice cold-water immersion need to be educated about possible health risks associated with taking a dip in icy water, which include the consequences of hypothermia, and of heart and lung problems, which are often related to the shock from the cold.
This is a summary of the article, “Health effects of voluntary exposure to cold water – a continuing subject of debate,” published by the International Journal of Circumpolar Health.
A version of this article first appeared on Medscape.com.
FROM THE INTERNATIONAL JOURNAL OF CIRCUMPOLAR HEALTH
Guideline stresses new strategies for hypoglycemia management
The Endocrine Society has issued an updated clinical practice guideline on the prevention and management of hypoglycemia in patients with diabetes who are at high risk, addressing the wide variety of treatment advances, such as insulin pumps and continuous glucose monitoring (CGM) systems, that have appeared since the publication of the society’s last guideline on hypoglycemia, in 2009.
“CGM and insulin pumps have been much more commonly used in the last decade among people with diabetes, including children, and there are new forms of glucagon available,” said Anthony L. McCall, MD, PhD, chair of the panel that wrote the guideline.
“We had to update our guideline to match these developments in the diabetes field,” noted Dr. McCall, University of Virginia, Charlottesville, in a press statement.
The new guideline, developed by a multidisciplinary panel of clinical experts and published in the Journal of Clinical Endocrinology and Metabolism, addresses 10 key clinical questions regarding current issues relevant to hypoglycemia prevention and treatment in adult or pediatric patients with either type 1 or type 2 diabetes in the outpatient or inpatient setting.
Key guideline recommendations
The recommendations are based on factors including critical outcomes, implementation feasibility, and patient preferences.
Key guideline recommendations that are considered “strong,” based on evidence, include:
- The use of CGM rather than self-monitoring of blood glucose by fingerstick for patients with type 1 diabetes receiving multiple daily injections. The panel underscored that “comprehensive patient education on how to use and troubleshoot CGM devices and interpret these data is critically important for maximum benefit and successful outcomes.”
The use of a structured program for patient education versus unstructured advice for adult and pediatric outpatients with type 1 diabetes or type 2 diabetes receiving insulin therapy.
- Structured education on how to avoid repeated hypoglycemia is critical, and this education should be performed by experienced diabetes clinicians,” the panel asserts. “Moreover, insurance coverage for education should be available for all insulin-using patients.”
- The use of glucagon preparations that do not have to be reconstituted, as opposed to those that do (that is, available as a powder and diluent) in the treatment of outpatients with severe hypoglycemia.
Guideline recommendations that received conditional recommendations include:
- Use of real-time CGM and algorithm-driven insulin pumps in people with type 1 diabetes.
- Use of CGM for outpatients with type 2 diabetes at high risk for hypoglycemia.
- Use of long-acting and rapid-acting insulin analogs for patients at high risk for hypoglycemia.
Noting that there is “moderate-certainty” evidence for severe hypoglycemia reduction as an outcome in those using long-acting analog insulins versus human neutral protamine Hagedorn (NPH) insulin, the panel cautions that “most studies of long-acting analog insulins do not assess for significant adverse effects, including cardiovascular outcomes, and that many studies were designed to demonstrate noninferiority of analog insulin, compared with human NPH insulin.”
- Initiation of and continuation of CGM for select inpatient populations at high risk for hypoglycemia.
Hypoglycemia: One of top three preventable adverse drug reactions
The updated guidelines are especially important considering the common incidence of hypoglycemia, which the U.S. Department of Health and Human Services has determined to be one of the top 3 preventable adverse drug reactions, the panel says.
They note that between January 2007 and December 2011, emergency department visits for therapy-associated hypoglycemia among Medicare beneficiaries resulted in more than $600 million in spending.
Meanwhile, many people with type 1 or 2 diabetes may not experience or recognize the symptoms of hypoglycemia, which, in severe cases, can lead to unconsciousness or seizures, in addition to affecting quality of life, social life, work productivity, and ability to drive safely.
The key to accurate diagnosis of those patients is assessment of the three levels of hypoglycemia, described in a 2018 consensus statement:
- Level 1: Glucose less than 70 mg/dL (3.9 mmol/L) and greater than or equal to 54 mg/dL (3.0 mmol/L). This level of hypoglycemia should alert patients that they may need to ingest carbohydrate to prevent progressive hypoglycemia.
- Level 2: Glucose less than 54 mg/dL (3.0 mmol/L). This level of hypoglycemia is associated with increased risk for cognitive dysfunction and mortality.
- Level 3: A severe event characterized by altered mental and/or physical status requiring assistance. This level of hypoglycemia is life-threatening and requires emergent treatment, typically with glucagon.
Ultimately, “new technology and medications will help reduce hypoglycemia, and [clinicians] can better treat patients now with new, easier glucagons,” Dr. McCall told this news organization.
“People with diabetes, their caregivers, and diabetes specialists will all benefit from our guideline with a better understanding of best practices and interventions,” the panel notes.
Disparities still exist in access to insulin pumps
Separately, new research shows that while use of insulin pumps to manage type 1 diabetes has grown over 20 years, there has been no improvement in racial, ethnic, and socioeconomic disparities in their use in the United States. The findings are reported in Diabetes Technology & Therapeutics.
Using data from the SEARCH for Diabetes Youth Study across four time periods between 2001 and 2019, the researchers show that by the end of the period studied, insulin pump use was 67% among non-Hispanic White people, 41% among Hispanic people, 29% among Black people, and 46% among other racial and ethnic groups.
In addition, 70% of people with bachelor’s degrees or higher used the pumps, compared with 56% among those with some college, 40% among holders of high school degrees, and 18% among those with no high school education. By income level, 74% of those with household incomes of $75,000 or more, 66% with $50,000-$74,999, 51% with $25,000-$49,999, and 41% with less than $25,000 used the pumps.
“Diabetes technology has numerous benefits for patients with type 1 diabetes, but the problem is that there is a huge divide in who actually has access to these technologies,” said study lead Estelle Everett, MD, assistant professor of medicine in the division of endocrinology, diabetes & metabolism at the University of California, Los Angeles.
A version of this article first appeared on Medscape.com.
The Endocrine Society has issued an updated clinical practice guideline on the prevention and management of hypoglycemia in patients with diabetes who are at high risk, addressing the wide variety of treatment advances, such as insulin pumps and continuous glucose monitoring (CGM) systems, that have appeared since the publication of the society’s last guideline on hypoglycemia, in 2009.
“CGM and insulin pumps have been much more commonly used in the last decade among people with diabetes, including children, and there are new forms of glucagon available,” said Anthony L. McCall, MD, PhD, chair of the panel that wrote the guideline.
“We had to update our guideline to match these developments in the diabetes field,” noted Dr. McCall, University of Virginia, Charlottesville, in a press statement.
The new guideline, developed by a multidisciplinary panel of clinical experts and published in the Journal of Clinical Endocrinology and Metabolism, addresses 10 key clinical questions regarding current issues relevant to hypoglycemia prevention and treatment in adult or pediatric patients with either type 1 or type 2 diabetes in the outpatient or inpatient setting.
Key guideline recommendations
The recommendations are based on factors including critical outcomes, implementation feasibility, and patient preferences.
Key guideline recommendations that are considered “strong,” based on evidence, include:
- The use of CGM rather than self-monitoring of blood glucose by fingerstick for patients with type 1 diabetes receiving multiple daily injections. The panel underscored that “comprehensive patient education on how to use and troubleshoot CGM devices and interpret these data is critically important for maximum benefit and successful outcomes.”
The use of a structured program for patient education versus unstructured advice for adult and pediatric outpatients with type 1 diabetes or type 2 diabetes receiving insulin therapy.
- Structured education on how to avoid repeated hypoglycemia is critical, and this education should be performed by experienced diabetes clinicians,” the panel asserts. “Moreover, insurance coverage for education should be available for all insulin-using patients.”
- The use of glucagon preparations that do not have to be reconstituted, as opposed to those that do (that is, available as a powder and diluent) in the treatment of outpatients with severe hypoglycemia.
Guideline recommendations that received conditional recommendations include:
- Use of real-time CGM and algorithm-driven insulin pumps in people with type 1 diabetes.
- Use of CGM for outpatients with type 2 diabetes at high risk for hypoglycemia.
- Use of long-acting and rapid-acting insulin analogs for patients at high risk for hypoglycemia.
Noting that there is “moderate-certainty” evidence for severe hypoglycemia reduction as an outcome in those using long-acting analog insulins versus human neutral protamine Hagedorn (NPH) insulin, the panel cautions that “most studies of long-acting analog insulins do not assess for significant adverse effects, including cardiovascular outcomes, and that many studies were designed to demonstrate noninferiority of analog insulin, compared with human NPH insulin.”
- Initiation of and continuation of CGM for select inpatient populations at high risk for hypoglycemia.
Hypoglycemia: One of top three preventable adverse drug reactions
The updated guidelines are especially important considering the common incidence of hypoglycemia, which the U.S. Department of Health and Human Services has determined to be one of the top 3 preventable adverse drug reactions, the panel says.
They note that between January 2007 and December 2011, emergency department visits for therapy-associated hypoglycemia among Medicare beneficiaries resulted in more than $600 million in spending.
Meanwhile, many people with type 1 or 2 diabetes may not experience or recognize the symptoms of hypoglycemia, which, in severe cases, can lead to unconsciousness or seizures, in addition to affecting quality of life, social life, work productivity, and ability to drive safely.
The key to accurate diagnosis of those patients is assessment of the three levels of hypoglycemia, described in a 2018 consensus statement:
- Level 1: Glucose less than 70 mg/dL (3.9 mmol/L) and greater than or equal to 54 mg/dL (3.0 mmol/L). This level of hypoglycemia should alert patients that they may need to ingest carbohydrate to prevent progressive hypoglycemia.
- Level 2: Glucose less than 54 mg/dL (3.0 mmol/L). This level of hypoglycemia is associated with increased risk for cognitive dysfunction and mortality.
- Level 3: A severe event characterized by altered mental and/or physical status requiring assistance. This level of hypoglycemia is life-threatening and requires emergent treatment, typically with glucagon.
Ultimately, “new technology and medications will help reduce hypoglycemia, and [clinicians] can better treat patients now with new, easier glucagons,” Dr. McCall told this news organization.
“People with diabetes, their caregivers, and diabetes specialists will all benefit from our guideline with a better understanding of best practices and interventions,” the panel notes.
Disparities still exist in access to insulin pumps
Separately, new research shows that while use of insulin pumps to manage type 1 diabetes has grown over 20 years, there has been no improvement in racial, ethnic, and socioeconomic disparities in their use in the United States. The findings are reported in Diabetes Technology & Therapeutics.
Using data from the SEARCH for Diabetes Youth Study across four time periods between 2001 and 2019, the researchers show that by the end of the period studied, insulin pump use was 67% among non-Hispanic White people, 41% among Hispanic people, 29% among Black people, and 46% among other racial and ethnic groups.
In addition, 70% of people with bachelor’s degrees or higher used the pumps, compared with 56% among those with some college, 40% among holders of high school degrees, and 18% among those with no high school education. By income level, 74% of those with household incomes of $75,000 or more, 66% with $50,000-$74,999, 51% with $25,000-$49,999, and 41% with less than $25,000 used the pumps.
“Diabetes technology has numerous benefits for patients with type 1 diabetes, but the problem is that there is a huge divide in who actually has access to these technologies,” said study lead Estelle Everett, MD, assistant professor of medicine in the division of endocrinology, diabetes & metabolism at the University of California, Los Angeles.
A version of this article first appeared on Medscape.com.
The Endocrine Society has issued an updated clinical practice guideline on the prevention and management of hypoglycemia in patients with diabetes who are at high risk, addressing the wide variety of treatment advances, such as insulin pumps and continuous glucose monitoring (CGM) systems, that have appeared since the publication of the society’s last guideline on hypoglycemia, in 2009.
“CGM and insulin pumps have been much more commonly used in the last decade among people with diabetes, including children, and there are new forms of glucagon available,” said Anthony L. McCall, MD, PhD, chair of the panel that wrote the guideline.
“We had to update our guideline to match these developments in the diabetes field,” noted Dr. McCall, University of Virginia, Charlottesville, in a press statement.
The new guideline, developed by a multidisciplinary panel of clinical experts and published in the Journal of Clinical Endocrinology and Metabolism, addresses 10 key clinical questions regarding current issues relevant to hypoglycemia prevention and treatment in adult or pediatric patients with either type 1 or type 2 diabetes in the outpatient or inpatient setting.
Key guideline recommendations
The recommendations are based on factors including critical outcomes, implementation feasibility, and patient preferences.
Key guideline recommendations that are considered “strong,” based on evidence, include:
- The use of CGM rather than self-monitoring of blood glucose by fingerstick for patients with type 1 diabetes receiving multiple daily injections. The panel underscored that “comprehensive patient education on how to use and troubleshoot CGM devices and interpret these data is critically important for maximum benefit and successful outcomes.”
The use of a structured program for patient education versus unstructured advice for adult and pediatric outpatients with type 1 diabetes or type 2 diabetes receiving insulin therapy.
- Structured education on how to avoid repeated hypoglycemia is critical, and this education should be performed by experienced diabetes clinicians,” the panel asserts. “Moreover, insurance coverage for education should be available for all insulin-using patients.”
- The use of glucagon preparations that do not have to be reconstituted, as opposed to those that do (that is, available as a powder and diluent) in the treatment of outpatients with severe hypoglycemia.
Guideline recommendations that received conditional recommendations include:
- Use of real-time CGM and algorithm-driven insulin pumps in people with type 1 diabetes.
- Use of CGM for outpatients with type 2 diabetes at high risk for hypoglycemia.
- Use of long-acting and rapid-acting insulin analogs for patients at high risk for hypoglycemia.
Noting that there is “moderate-certainty” evidence for severe hypoglycemia reduction as an outcome in those using long-acting analog insulins versus human neutral protamine Hagedorn (NPH) insulin, the panel cautions that “most studies of long-acting analog insulins do not assess for significant adverse effects, including cardiovascular outcomes, and that many studies were designed to demonstrate noninferiority of analog insulin, compared with human NPH insulin.”
- Initiation of and continuation of CGM for select inpatient populations at high risk for hypoglycemia.
Hypoglycemia: One of top three preventable adverse drug reactions
The updated guidelines are especially important considering the common incidence of hypoglycemia, which the U.S. Department of Health and Human Services has determined to be one of the top 3 preventable adverse drug reactions, the panel says.
They note that between January 2007 and December 2011, emergency department visits for therapy-associated hypoglycemia among Medicare beneficiaries resulted in more than $600 million in spending.
Meanwhile, many people with type 1 or 2 diabetes may not experience or recognize the symptoms of hypoglycemia, which, in severe cases, can lead to unconsciousness or seizures, in addition to affecting quality of life, social life, work productivity, and ability to drive safely.
The key to accurate diagnosis of those patients is assessment of the three levels of hypoglycemia, described in a 2018 consensus statement:
- Level 1: Glucose less than 70 mg/dL (3.9 mmol/L) and greater than or equal to 54 mg/dL (3.0 mmol/L). This level of hypoglycemia should alert patients that they may need to ingest carbohydrate to prevent progressive hypoglycemia.
- Level 2: Glucose less than 54 mg/dL (3.0 mmol/L). This level of hypoglycemia is associated with increased risk for cognitive dysfunction and mortality.
- Level 3: A severe event characterized by altered mental and/or physical status requiring assistance. This level of hypoglycemia is life-threatening and requires emergent treatment, typically with glucagon.
Ultimately, “new technology and medications will help reduce hypoglycemia, and [clinicians] can better treat patients now with new, easier glucagons,” Dr. McCall told this news organization.
“People with diabetes, their caregivers, and diabetes specialists will all benefit from our guideline with a better understanding of best practices and interventions,” the panel notes.
Disparities still exist in access to insulin pumps
Separately, new research shows that while use of insulin pumps to manage type 1 diabetes has grown over 20 years, there has been no improvement in racial, ethnic, and socioeconomic disparities in their use in the United States. The findings are reported in Diabetes Technology & Therapeutics.
Using data from the SEARCH for Diabetes Youth Study across four time periods between 2001 and 2019, the researchers show that by the end of the period studied, insulin pump use was 67% among non-Hispanic White people, 41% among Hispanic people, 29% among Black people, and 46% among other racial and ethnic groups.
In addition, 70% of people with bachelor’s degrees or higher used the pumps, compared with 56% among those with some college, 40% among holders of high school degrees, and 18% among those with no high school education. By income level, 74% of those with household incomes of $75,000 or more, 66% with $50,000-$74,999, 51% with $25,000-$49,999, and 41% with less than $25,000 used the pumps.
“Diabetes technology has numerous benefits for patients with type 1 diabetes, but the problem is that there is a huge divide in who actually has access to these technologies,” said study lead Estelle Everett, MD, assistant professor of medicine in the division of endocrinology, diabetes & metabolism at the University of California, Los Angeles.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY AND METABOLISM
Subset of patients with melanoma have very low mortality risk
Although melanoma is the most serious skin cancer, most patients do have high chances of survival. New research has now identified a subset of patients with early disease who have a very low risk of dying from the disease.
In a cohort of almost 11,600 patients, the overall 7-year rate of death from melanoma was 2.5%, but the risk in a subset of 25% of patients was below 1%. Conversely, the study authors were also able to identify a small subset of high‐risk patients with a greater than 20% risk for death.
and may help to begin to address the problem of overdiagnosis, they note.
“While the topic of very low-risk melanomas has been presented at national and international meetings, there have been no formal discussions to define the classification of ‘melanocytic neoplasms of low malignant potential’ at this time,” first author Megan M. Eguchi, MPH, of the department of medicine, University of California, Los Angeles, said in an interview. “Criteria would need to be established using study designs beyond those available using SEER data.”
She emphasized that currently, they do not propose any change to treatment of these lesions, just a change to the terminology. “A diagnosis of ‘MNLMP’ rather than ‘melanoma’ may potentially alleviate people’s concerns related to prognosis and begin to address the problem of overdiagnosis,” said Ms. Eguchi. The study was recently published online in Cancer.
Even though melanoma is considered to be the most common potentially lethal tumor of the skin, prognosis is often very good for those with T1 tumors, the lowest risk category. Prognostic modeling has been used to predict survival in patients with melanoma and identify prognostic variables, the authors note, with the most prominent attributes being Breslow thickness and ulceration of the primary tumor, which form the basis of the current American Joint Committee on Cancer (AJCC) staging system.
There is evidence that the increasing incidence of melanoma is partly due to overdiagnosis, meaning the diagnosis of lesions that will not lead to symptoms or death. The authors write that they were interested in identifying lesions that are currently diagnosed as melanoma but might lack the capacity for metastasis, cases that could potentially be part of the phenomenon of overdiagnosis.
Subsets with low and high risk for death
In the study, Ms. Eguchi and colleagues analyzed information from the United States Surveillance, Epidemiology, and End Results (SEER) database and identified 11,594 patients who were diagnosed in 2010 and 2011 with stage 1 melanoma that was less than or equal to 1.0 mm in thickness and had not spread to the lymph nodes. Prognostic models for risk for death from melanoma in patients with low-risk melanomas were developed, then the ability of the models to identify very‐low risk subsets of patients with melanoma‐specific survival surpassing that of T1 overall was evaluated.
The median age of the patients was 58 years, the median Breslow thickness was 0.45 mm (interquartile range, 0.30-0.65 mm), and 71% were assigned stage IA. Ulceration was present in 4% of cases, 27% were mitogenic, and 45% were Clark level II, and within this cohort, 292 (2.5%) patients died of melanoma within 7 years. In the training data set, 177 of 7,652 (2.3%) patients died of melanoma within 7 years, and numbers were similar in the testing set (115 of 3,942; 2.9%).
Overall, the investigators identified three large subsets of patients who were in the AJCC seventh edition classification for stage I (“thin”) melanoma, who had a risk for death of approximately less than 1%. This was a marked improvement from the rate of the overall sample. In the simplest model (Model 1A), patients who were younger than 70 years at diagnosis with Clark level II invasion were deemed as very low risk.
In Model 1B, the same initial classification was used, but it was further refined and limited to patients who were either age 43 years or younger or 44-69 years with Breslow thickness less than 0.40 mm. At 10 years postdiagnosis, this subset also showed a less than 1% risk for death from melanoma. The logistic regression model (Model 2) was similar, as it identified about 25% of patients with a predicted risk for death of less than 0.5%, incorporating patient age, sex, mitogenicity, Clark level, and ulceration. Model 2 was also able to further identify a small subset of patients with no deaths.
The logistic regression model was also able to identify a very small subset (0.7% and 0.8%) of patients who had a risk for death that exceeded 20%, which was markedly higher, compared with most patients with T1b tumors.
This study was supported by the National Cancer Institute. Ms. Eguchi had no disclosures to report.
A version of this article first appeared on Medscape.com.
Although melanoma is the most serious skin cancer, most patients do have high chances of survival. New research has now identified a subset of patients with early disease who have a very low risk of dying from the disease.
In a cohort of almost 11,600 patients, the overall 7-year rate of death from melanoma was 2.5%, but the risk in a subset of 25% of patients was below 1%. Conversely, the study authors were also able to identify a small subset of high‐risk patients with a greater than 20% risk for death.
and may help to begin to address the problem of overdiagnosis, they note.
“While the topic of very low-risk melanomas has been presented at national and international meetings, there have been no formal discussions to define the classification of ‘melanocytic neoplasms of low malignant potential’ at this time,” first author Megan M. Eguchi, MPH, of the department of medicine, University of California, Los Angeles, said in an interview. “Criteria would need to be established using study designs beyond those available using SEER data.”
She emphasized that currently, they do not propose any change to treatment of these lesions, just a change to the terminology. “A diagnosis of ‘MNLMP’ rather than ‘melanoma’ may potentially alleviate people’s concerns related to prognosis and begin to address the problem of overdiagnosis,” said Ms. Eguchi. The study was recently published online in Cancer.
Even though melanoma is considered to be the most common potentially lethal tumor of the skin, prognosis is often very good for those with T1 tumors, the lowest risk category. Prognostic modeling has been used to predict survival in patients with melanoma and identify prognostic variables, the authors note, with the most prominent attributes being Breslow thickness and ulceration of the primary tumor, which form the basis of the current American Joint Committee on Cancer (AJCC) staging system.
There is evidence that the increasing incidence of melanoma is partly due to overdiagnosis, meaning the diagnosis of lesions that will not lead to symptoms or death. The authors write that they were interested in identifying lesions that are currently diagnosed as melanoma but might lack the capacity for metastasis, cases that could potentially be part of the phenomenon of overdiagnosis.
Subsets with low and high risk for death
In the study, Ms. Eguchi and colleagues analyzed information from the United States Surveillance, Epidemiology, and End Results (SEER) database and identified 11,594 patients who were diagnosed in 2010 and 2011 with stage 1 melanoma that was less than or equal to 1.0 mm in thickness and had not spread to the lymph nodes. Prognostic models for risk for death from melanoma in patients with low-risk melanomas were developed, then the ability of the models to identify very‐low risk subsets of patients with melanoma‐specific survival surpassing that of T1 overall was evaluated.
The median age of the patients was 58 years, the median Breslow thickness was 0.45 mm (interquartile range, 0.30-0.65 mm), and 71% were assigned stage IA. Ulceration was present in 4% of cases, 27% were mitogenic, and 45% were Clark level II, and within this cohort, 292 (2.5%) patients died of melanoma within 7 years. In the training data set, 177 of 7,652 (2.3%) patients died of melanoma within 7 years, and numbers were similar in the testing set (115 of 3,942; 2.9%).
Overall, the investigators identified three large subsets of patients who were in the AJCC seventh edition classification for stage I (“thin”) melanoma, who had a risk for death of approximately less than 1%. This was a marked improvement from the rate of the overall sample. In the simplest model (Model 1A), patients who were younger than 70 years at diagnosis with Clark level II invasion were deemed as very low risk.
In Model 1B, the same initial classification was used, but it was further refined and limited to patients who were either age 43 years or younger or 44-69 years with Breslow thickness less than 0.40 mm. At 10 years postdiagnosis, this subset also showed a less than 1% risk for death from melanoma. The logistic regression model (Model 2) was similar, as it identified about 25% of patients with a predicted risk for death of less than 0.5%, incorporating patient age, sex, mitogenicity, Clark level, and ulceration. Model 2 was also able to further identify a small subset of patients with no deaths.
The logistic regression model was also able to identify a very small subset (0.7% and 0.8%) of patients who had a risk for death that exceeded 20%, which was markedly higher, compared with most patients with T1b tumors.
This study was supported by the National Cancer Institute. Ms. Eguchi had no disclosures to report.
A version of this article first appeared on Medscape.com.
Although melanoma is the most serious skin cancer, most patients do have high chances of survival. New research has now identified a subset of patients with early disease who have a very low risk of dying from the disease.
In a cohort of almost 11,600 patients, the overall 7-year rate of death from melanoma was 2.5%, but the risk in a subset of 25% of patients was below 1%. Conversely, the study authors were also able to identify a small subset of high‐risk patients with a greater than 20% risk for death.
and may help to begin to address the problem of overdiagnosis, they note.
“While the topic of very low-risk melanomas has been presented at national and international meetings, there have been no formal discussions to define the classification of ‘melanocytic neoplasms of low malignant potential’ at this time,” first author Megan M. Eguchi, MPH, of the department of medicine, University of California, Los Angeles, said in an interview. “Criteria would need to be established using study designs beyond those available using SEER data.”
She emphasized that currently, they do not propose any change to treatment of these lesions, just a change to the terminology. “A diagnosis of ‘MNLMP’ rather than ‘melanoma’ may potentially alleviate people’s concerns related to prognosis and begin to address the problem of overdiagnosis,” said Ms. Eguchi. The study was recently published online in Cancer.
Even though melanoma is considered to be the most common potentially lethal tumor of the skin, prognosis is often very good for those with T1 tumors, the lowest risk category. Prognostic modeling has been used to predict survival in patients with melanoma and identify prognostic variables, the authors note, with the most prominent attributes being Breslow thickness and ulceration of the primary tumor, which form the basis of the current American Joint Committee on Cancer (AJCC) staging system.
There is evidence that the increasing incidence of melanoma is partly due to overdiagnosis, meaning the diagnosis of lesions that will not lead to symptoms or death. The authors write that they were interested in identifying lesions that are currently diagnosed as melanoma but might lack the capacity for metastasis, cases that could potentially be part of the phenomenon of overdiagnosis.
Subsets with low and high risk for death
In the study, Ms. Eguchi and colleagues analyzed information from the United States Surveillance, Epidemiology, and End Results (SEER) database and identified 11,594 patients who were diagnosed in 2010 and 2011 with stage 1 melanoma that was less than or equal to 1.0 mm in thickness and had not spread to the lymph nodes. Prognostic models for risk for death from melanoma in patients with low-risk melanomas were developed, then the ability of the models to identify very‐low risk subsets of patients with melanoma‐specific survival surpassing that of T1 overall was evaluated.
The median age of the patients was 58 years, the median Breslow thickness was 0.45 mm (interquartile range, 0.30-0.65 mm), and 71% were assigned stage IA. Ulceration was present in 4% of cases, 27% were mitogenic, and 45% were Clark level II, and within this cohort, 292 (2.5%) patients died of melanoma within 7 years. In the training data set, 177 of 7,652 (2.3%) patients died of melanoma within 7 years, and numbers were similar in the testing set (115 of 3,942; 2.9%).
Overall, the investigators identified three large subsets of patients who were in the AJCC seventh edition classification for stage I (“thin”) melanoma, who had a risk for death of approximately less than 1%. This was a marked improvement from the rate of the overall sample. In the simplest model (Model 1A), patients who were younger than 70 years at diagnosis with Clark level II invasion were deemed as very low risk.
In Model 1B, the same initial classification was used, but it was further refined and limited to patients who were either age 43 years or younger or 44-69 years with Breslow thickness less than 0.40 mm. At 10 years postdiagnosis, this subset also showed a less than 1% risk for death from melanoma. The logistic regression model (Model 2) was similar, as it identified about 25% of patients with a predicted risk for death of less than 0.5%, incorporating patient age, sex, mitogenicity, Clark level, and ulceration. Model 2 was also able to further identify a small subset of patients with no deaths.
The logistic regression model was also able to identify a very small subset (0.7% and 0.8%) of patients who had a risk for death that exceeded 20%, which was markedly higher, compared with most patients with T1b tumors.
This study was supported by the National Cancer Institute. Ms. Eguchi had no disclosures to report.
A version of this article first appeared on Medscape.com.
FROM CANCER
Blame IBS on gravity intolerance?
The precise cause of irritable bowel syndrome (IBS) remains a mystery. A novel new hypothesis suggests that IBS could result from the body’s inability to manage gravity.
Gravity may be the “unifying factor in multiple seemingly disparate and mutually incompatible theories of IBS,” Brennan Spiegel, MD, director of Health Services Research at Cedars-Sinai Medical Center, Los Angeles, told this news organization. Dr. Spiegel’s gravity hypothesis of IBS is described in an article published in the December issue of American Journal of Gastroenterology.
A human being’s relationship to gravity is not unlike the relationship of a fish to water, he explained.
“We live our entire life in it, are shaped by it, yet hardly notice its ever-present influence on our body. Every fiber of our body is affected by gravity every day, including our gastrointestinal tract,” said Dr. Spiegel.
The abdominal contents are like a sack of heavy potatoes that we’re destined to carry around for our entire lives. To meet this demand, our body evolved to support the abdominal load with a set of mechanisms that hoist the viscera against gravity in an upright posture, Dr. Spiegel explained.
A failure of these mechanisms could lead to a host of problems, including motility problems or bacterial overgrowth in the gut and symptoms of IBS.
Dr. Spiegel’s gravity hypothesis, however, goes beyond the gastrointestinal tract.
“Our nervous system has evolved its own ways of managing gravity and how gut feelings arise when our nervous system detects gravity challenges, like getting ‘butterflies’ when falling on a roller coaster or in a turbulent airplane,” Dr. Spiegel said.
“Even our neuropsychological orientation to gravity is found in our language, like when people talk about feeling down in the dumps, feeling low, [or say they] can’t get out of bed. These are directional metaphors that we use that refer to the fact that there is something about getting pulled down that’s obviously negative,” he noted.
‘A big ask’
Dr. Spiegel said his gravity theory of IBS draws from “extensive literature to build a hypothesis that IBS may result from ineffective anatomical, physiological, and neuropsychological gravity-management systems designed to optimize GI form and function, protect body integrity, and maximize survival in a gravity-bound world.”
He acknowledged that it’s “a big ask” to get people to consider a unifying theory of anything. “But when we dig down deep, it’s not terribly controversial to me to suggest that our health has something to do with gravity. How could it not?” he said.
Dr. Spiegel also thinks this line of thinking has clinical implications.
“While we can’t change gravity, we can change our relationship to gravity in different ways,” he said.
“For starters, we can bolster our body to manage gravity better, through losing weight, exercise, and strengthening the anti-gravity extensor muscles along the back, which supports the spine, which is the chassis that holds up the whole body and includes maintaining the shape of the abdominal cavity,” Dr. Spiegel said.
The reason physical therapy and exercise are effective for IBS could be because these interventions strengthen the GI support systems, he said.
Testable theory
Before Dr. Spiegel “got up the courage” to submit his paper, he sent it to leading IBS researchers in the United States to get their honest opinion, he said.
“To my surprise, they wrote back and said this makes sense. And a few said this could have implications for other diseases,” he said in an interview.
Some of his patients with IBS have told him how the paper resonates and the specific ways they have noticed the impact of gravity and related air pressure on their IBS symptoms.
Some have reported that their symptoms get better when they scuba dive but worsen when they get out of the ocean; others said they feel much better up in the mountains versus at sea level; another said doing a headstand during yoga eases their GI symptoms.
“These may just be anecdotes, but they’re really striking,” Dr. Spiegel said.
His theory is not meant to replace any of the many existing theories of IBS, Dr. Spiegel emphasized. Rather, it’s an attempt to pull together the different theories under a single, potentially unifying explanation.
His paper includes a list of research projects that might help explore the gravity theory of IBS.
“It may be that none of this ends up being true, or bits and pieces of it are kind of true,” Dr. Spiegel said.
A research challenge
Dr. Spiegel has given researchers an “intriguing and interesting thought experiment and kind of a challenge to go out there and determine whether or not this hypothesis may actually be true,” Millie Long, MD, co–editor-in-chief of the American Journal of Gastroenterology, said in a podcast.
Dr. Long, a gastroenterologist and professor at the University of North Carolina at Chapel Hill School of Medicine, encouraged listeners to “dig deep into this hypothesis.”
The gravity hypothesis is provocative, “but the best thing about it is that it is testable,” Shelly Lu, MD, director of the Division of Digestive and Liver Diseases at Cedars-Sinai, Los Angeles, said in a news release issued by the medical center.
“If proved correct, it is a major paradigm shift in the way we think about IBS and possibly treatment as well,” said Dr. Lu.
Also weighing in, Brian Lacy, MD, PhD, a gastroenterologist with the Mayo Clinic, Jacksonville, Fla., noted that “our understanding of the etiopathophysiology of IBS has evolved over the past 50 years.”
“Once thought to be a psychiatric disorder (‘nervous colitis’) or a disorder simply of gut spasms (‘spastic colitis’), we now understand that symptoms of IBS develop for a multitude of reasons, including alterations in the gut microbiome, changes in gut sensation and motility, and modulation of the brain-gut axis, to name just a few,” Dr. Lacy said in an interview.
“Dr. Spiegel’s intriguing manuscript opens the door for us to think about IBS in a completely different way,” said Dr. Lacy.
“His novel hypothesis is a superb challenge to researchers and clinicians who can directly test his theory with a number of intriguing experiments. The results of these experiments may completely change the treatment paradigm for IBS patients,” Dr. Lacy added.
This research had no financial support. Dr. Spiegel and Dr. Lacy report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The precise cause of irritable bowel syndrome (IBS) remains a mystery. A novel new hypothesis suggests that IBS could result from the body’s inability to manage gravity.
Gravity may be the “unifying factor in multiple seemingly disparate and mutually incompatible theories of IBS,” Brennan Spiegel, MD, director of Health Services Research at Cedars-Sinai Medical Center, Los Angeles, told this news organization. Dr. Spiegel’s gravity hypothesis of IBS is described in an article published in the December issue of American Journal of Gastroenterology.
A human being’s relationship to gravity is not unlike the relationship of a fish to water, he explained.
“We live our entire life in it, are shaped by it, yet hardly notice its ever-present influence on our body. Every fiber of our body is affected by gravity every day, including our gastrointestinal tract,” said Dr. Spiegel.
The abdominal contents are like a sack of heavy potatoes that we’re destined to carry around for our entire lives. To meet this demand, our body evolved to support the abdominal load with a set of mechanisms that hoist the viscera against gravity in an upright posture, Dr. Spiegel explained.
A failure of these mechanisms could lead to a host of problems, including motility problems or bacterial overgrowth in the gut and symptoms of IBS.
Dr. Spiegel’s gravity hypothesis, however, goes beyond the gastrointestinal tract.
“Our nervous system has evolved its own ways of managing gravity and how gut feelings arise when our nervous system detects gravity challenges, like getting ‘butterflies’ when falling on a roller coaster or in a turbulent airplane,” Dr. Spiegel said.
“Even our neuropsychological orientation to gravity is found in our language, like when people talk about feeling down in the dumps, feeling low, [or say they] can’t get out of bed. These are directional metaphors that we use that refer to the fact that there is something about getting pulled down that’s obviously negative,” he noted.
‘A big ask’
Dr. Spiegel said his gravity theory of IBS draws from “extensive literature to build a hypothesis that IBS may result from ineffective anatomical, physiological, and neuropsychological gravity-management systems designed to optimize GI form and function, protect body integrity, and maximize survival in a gravity-bound world.”
He acknowledged that it’s “a big ask” to get people to consider a unifying theory of anything. “But when we dig down deep, it’s not terribly controversial to me to suggest that our health has something to do with gravity. How could it not?” he said.
Dr. Spiegel also thinks this line of thinking has clinical implications.
“While we can’t change gravity, we can change our relationship to gravity in different ways,” he said.
“For starters, we can bolster our body to manage gravity better, through losing weight, exercise, and strengthening the anti-gravity extensor muscles along the back, which supports the spine, which is the chassis that holds up the whole body and includes maintaining the shape of the abdominal cavity,” Dr. Spiegel said.
The reason physical therapy and exercise are effective for IBS could be because these interventions strengthen the GI support systems, he said.
Testable theory
Before Dr. Spiegel “got up the courage” to submit his paper, he sent it to leading IBS researchers in the United States to get their honest opinion, he said.
“To my surprise, they wrote back and said this makes sense. And a few said this could have implications for other diseases,” he said in an interview.
Some of his patients with IBS have told him how the paper resonates and the specific ways they have noticed the impact of gravity and related air pressure on their IBS symptoms.
Some have reported that their symptoms get better when they scuba dive but worsen when they get out of the ocean; others said they feel much better up in the mountains versus at sea level; another said doing a headstand during yoga eases their GI symptoms.
“These may just be anecdotes, but they’re really striking,” Dr. Spiegel said.
His theory is not meant to replace any of the many existing theories of IBS, Dr. Spiegel emphasized. Rather, it’s an attempt to pull together the different theories under a single, potentially unifying explanation.
His paper includes a list of research projects that might help explore the gravity theory of IBS.
“It may be that none of this ends up being true, or bits and pieces of it are kind of true,” Dr. Spiegel said.
A research challenge
Dr. Spiegel has given researchers an “intriguing and interesting thought experiment and kind of a challenge to go out there and determine whether or not this hypothesis may actually be true,” Millie Long, MD, co–editor-in-chief of the American Journal of Gastroenterology, said in a podcast.
Dr. Long, a gastroenterologist and professor at the University of North Carolina at Chapel Hill School of Medicine, encouraged listeners to “dig deep into this hypothesis.”
The gravity hypothesis is provocative, “but the best thing about it is that it is testable,” Shelly Lu, MD, director of the Division of Digestive and Liver Diseases at Cedars-Sinai, Los Angeles, said in a news release issued by the medical center.
“If proved correct, it is a major paradigm shift in the way we think about IBS and possibly treatment as well,” said Dr. Lu.
Also weighing in, Brian Lacy, MD, PhD, a gastroenterologist with the Mayo Clinic, Jacksonville, Fla., noted that “our understanding of the etiopathophysiology of IBS has evolved over the past 50 years.”
“Once thought to be a psychiatric disorder (‘nervous colitis’) or a disorder simply of gut spasms (‘spastic colitis’), we now understand that symptoms of IBS develop for a multitude of reasons, including alterations in the gut microbiome, changes in gut sensation and motility, and modulation of the brain-gut axis, to name just a few,” Dr. Lacy said in an interview.
“Dr. Spiegel’s intriguing manuscript opens the door for us to think about IBS in a completely different way,” said Dr. Lacy.
“His novel hypothesis is a superb challenge to researchers and clinicians who can directly test his theory with a number of intriguing experiments. The results of these experiments may completely change the treatment paradigm for IBS patients,” Dr. Lacy added.
This research had no financial support. Dr. Spiegel and Dr. Lacy report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The precise cause of irritable bowel syndrome (IBS) remains a mystery. A novel new hypothesis suggests that IBS could result from the body’s inability to manage gravity.
Gravity may be the “unifying factor in multiple seemingly disparate and mutually incompatible theories of IBS,” Brennan Spiegel, MD, director of Health Services Research at Cedars-Sinai Medical Center, Los Angeles, told this news organization. Dr. Spiegel’s gravity hypothesis of IBS is described in an article published in the December issue of American Journal of Gastroenterology.
A human being’s relationship to gravity is not unlike the relationship of a fish to water, he explained.
“We live our entire life in it, are shaped by it, yet hardly notice its ever-present influence on our body. Every fiber of our body is affected by gravity every day, including our gastrointestinal tract,” said Dr. Spiegel.
The abdominal contents are like a sack of heavy potatoes that we’re destined to carry around for our entire lives. To meet this demand, our body evolved to support the abdominal load with a set of mechanisms that hoist the viscera against gravity in an upright posture, Dr. Spiegel explained.
A failure of these mechanisms could lead to a host of problems, including motility problems or bacterial overgrowth in the gut and symptoms of IBS.
Dr. Spiegel’s gravity hypothesis, however, goes beyond the gastrointestinal tract.
“Our nervous system has evolved its own ways of managing gravity and how gut feelings arise when our nervous system detects gravity challenges, like getting ‘butterflies’ when falling on a roller coaster or in a turbulent airplane,” Dr. Spiegel said.
“Even our neuropsychological orientation to gravity is found in our language, like when people talk about feeling down in the dumps, feeling low, [or say they] can’t get out of bed. These are directional metaphors that we use that refer to the fact that there is something about getting pulled down that’s obviously negative,” he noted.
‘A big ask’
Dr. Spiegel said his gravity theory of IBS draws from “extensive literature to build a hypothesis that IBS may result from ineffective anatomical, physiological, and neuropsychological gravity-management systems designed to optimize GI form and function, protect body integrity, and maximize survival in a gravity-bound world.”
He acknowledged that it’s “a big ask” to get people to consider a unifying theory of anything. “But when we dig down deep, it’s not terribly controversial to me to suggest that our health has something to do with gravity. How could it not?” he said.
Dr. Spiegel also thinks this line of thinking has clinical implications.
“While we can’t change gravity, we can change our relationship to gravity in different ways,” he said.
“For starters, we can bolster our body to manage gravity better, through losing weight, exercise, and strengthening the anti-gravity extensor muscles along the back, which supports the spine, which is the chassis that holds up the whole body and includes maintaining the shape of the abdominal cavity,” Dr. Spiegel said.
The reason physical therapy and exercise are effective for IBS could be because these interventions strengthen the GI support systems, he said.
Testable theory
Before Dr. Spiegel “got up the courage” to submit his paper, he sent it to leading IBS researchers in the United States to get their honest opinion, he said.
“To my surprise, they wrote back and said this makes sense. And a few said this could have implications for other diseases,” he said in an interview.
Some of his patients with IBS have told him how the paper resonates and the specific ways they have noticed the impact of gravity and related air pressure on their IBS symptoms.
Some have reported that their symptoms get better when they scuba dive but worsen when they get out of the ocean; others said they feel much better up in the mountains versus at sea level; another said doing a headstand during yoga eases their GI symptoms.
“These may just be anecdotes, but they’re really striking,” Dr. Spiegel said.
His theory is not meant to replace any of the many existing theories of IBS, Dr. Spiegel emphasized. Rather, it’s an attempt to pull together the different theories under a single, potentially unifying explanation.
His paper includes a list of research projects that might help explore the gravity theory of IBS.
“It may be that none of this ends up being true, or bits and pieces of it are kind of true,” Dr. Spiegel said.
A research challenge
Dr. Spiegel has given researchers an “intriguing and interesting thought experiment and kind of a challenge to go out there and determine whether or not this hypothesis may actually be true,” Millie Long, MD, co–editor-in-chief of the American Journal of Gastroenterology, said in a podcast.
Dr. Long, a gastroenterologist and professor at the University of North Carolina at Chapel Hill School of Medicine, encouraged listeners to “dig deep into this hypothesis.”
The gravity hypothesis is provocative, “but the best thing about it is that it is testable,” Shelly Lu, MD, director of the Division of Digestive and Liver Diseases at Cedars-Sinai, Los Angeles, said in a news release issued by the medical center.
“If proved correct, it is a major paradigm shift in the way we think about IBS and possibly treatment as well,” said Dr. Lu.
Also weighing in, Brian Lacy, MD, PhD, a gastroenterologist with the Mayo Clinic, Jacksonville, Fla., noted that “our understanding of the etiopathophysiology of IBS has evolved over the past 50 years.”
“Once thought to be a psychiatric disorder (‘nervous colitis’) or a disorder simply of gut spasms (‘spastic colitis’), we now understand that symptoms of IBS develop for a multitude of reasons, including alterations in the gut microbiome, changes in gut sensation and motility, and modulation of the brain-gut axis, to name just a few,” Dr. Lacy said in an interview.
“Dr. Spiegel’s intriguing manuscript opens the door for us to think about IBS in a completely different way,” said Dr. Lacy.
“His novel hypothesis is a superb challenge to researchers and clinicians who can directly test his theory with a number of intriguing experiments. The results of these experiments may completely change the treatment paradigm for IBS patients,” Dr. Lacy added.
This research had no financial support. Dr. Spiegel and Dr. Lacy report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF GASTROENTEROLOGY
Study eyes sunscreens marketed to individuals with skin of color
, and more than 40% contain a UV blocker that may create a white cast.
Those are among the findings from a study by Michelle Xiong, a medical student at Brown University, Providence, R.I., and Erin M. Warshaw, MD, of the department of dermatology at Park Nicollet/Health Partners Health Services, Minneapolis, which was published online in the Journal of the American Academy of Dermatology.
“There is increasing awareness of the negative effects of ultraviolet (UV) light in individuals with skin of color (SOC), especially in regards to pigmentation disorders induced and/or exacerbated by UV exposure,” the authors wrote. “As a result, there has been a surge in sunscreens marketed to this population. We aimed to characterize cost, marketing claims, and potential allergenic ingredients in sunscreens marketed to individuals with SOC.”
Between December 2021 and October 2022, the researchers used the following search terms on Google: “sunscreen” plus “skin of 36 color,” “dark skin,” “brown skin,” “LatinX skin,” and/or “Black skin.” They extracted price, marketing claims, and ingredients from manufacturers’ websites and used 90 allergens contained in the American Contact Dermatitis Society 2020 Core series to identify potential allergens. Next, they combined cross-reactors/synonyms into allergen categories based on ACDS Contact Allergen Management Plan (CAMP) cross-reactor classification. If multiple ingredients in a sunscreen were represented by a single allergen category, it was counted only once. A similar approach was utilized for marketing categories.
A total of 12 sunscreens were included in the analysis: Absolute Joi, Black Girl Sunscreen, Black Girl Sunscreen Make It Matte, Bolden SPF Brightening Moisturizer, Eleven on the Defense Unrivaled Sun Serum, Kinlo Golden Rays Sunscreen, Live Tinted Hueguard 3-in-1 Mineral Sunscreen, Mele Dew The Most Sheer Moisturizer SPF30 Broad Spectrum Sunscreen, Mele No Shade Sunscreen Oil, Specific Beauty Active Radiance Day Moi, Unsun Mineral Sunscreen, and Urban Skin Rx Complexion Protection. Their average cost was $19.30 per ounce (range, $6.33-$50.00) and common marketing claims for these products were “no white cast” (91.7%), being free of an ingredient (83.3%), and “moisturizing” (75%).
Of the 12 sunscreens, 7 (58.3%) contained a chemical sunscreen agent, 5 (41.7%) contained a physical UV blocker, and all contained at least one allergen. The average number of allergens per product was 4.7, most commonly fragrance/botanicals (83.3%), tocopherol (83.3%), sodium benzoates/derivatives (58.3%), and sorbitan sesquiolate/derivatives (58.3%).
“Average cost of sunscreens marketed to individuals with SOC was $19.30/oz, much higher than the median price of $3.32/oz reported in a separate study of 65 popular sunscreens,” the study authors wrote. “As many of the sunscreens in our study were sold by smaller businesses, higher prices may be due to higher production costs or a perceived smaller market.”
The authors expressed surprise that five sunscreens marketed to individuals with SOC contained a physical UV blocker which may create a white cast. They contacted the manufacturers of these five sunscreens and confirmed that three used micronized formulations. “While ingested/inhaled nanoparticles of titanium dioxide may cause tissue effects, most studies of topical products show excellent safety,” they wrote.
They also noted that the average of 4.7 allergens per product observed in the analysis was similar to the average of 4.9 seen in a separate study of 52 popular sunscreens. “However, that study only included 34 allergens while this study evaluated 90 allergens,” the authors wrote. “Consumers and providers should be aware sunscreens marketed to individuals with SOC may cause allergic contact dermatitis,” they commented.
“It is interesting to see how costly these products are now compared to store bought and general commercially available sunscreens several years ago,” said Lawrence J. Green, clinical professor of dermatology at George Washington University, Washington, who was asked to comment on the study. “However, to me that is not surprising as products marketed and targeted to specific populations are often priced at a premium. It wasn’t clear to me how many of these specialized online SOC sunscreens are tinted. I wish the authors had compared the cost of tinted sunscreens in general to nontinted sunscreens because tinted ones are more useful for SOC, because when rubbed in, they can readily match SOC and can also offer protection in the visible light spectrum.”
The authors reported having no financial disclosures; the study had no funding source. Dr. Green disclosed that he is a speaker, consultant, or investigator for many pharmaceutical companies.
, and more than 40% contain a UV blocker that may create a white cast.
Those are among the findings from a study by Michelle Xiong, a medical student at Brown University, Providence, R.I., and Erin M. Warshaw, MD, of the department of dermatology at Park Nicollet/Health Partners Health Services, Minneapolis, which was published online in the Journal of the American Academy of Dermatology.
“There is increasing awareness of the negative effects of ultraviolet (UV) light in individuals with skin of color (SOC), especially in regards to pigmentation disorders induced and/or exacerbated by UV exposure,” the authors wrote. “As a result, there has been a surge in sunscreens marketed to this population. We aimed to characterize cost, marketing claims, and potential allergenic ingredients in sunscreens marketed to individuals with SOC.”
Between December 2021 and October 2022, the researchers used the following search terms on Google: “sunscreen” plus “skin of 36 color,” “dark skin,” “brown skin,” “LatinX skin,” and/or “Black skin.” They extracted price, marketing claims, and ingredients from manufacturers’ websites and used 90 allergens contained in the American Contact Dermatitis Society 2020 Core series to identify potential allergens. Next, they combined cross-reactors/synonyms into allergen categories based on ACDS Contact Allergen Management Plan (CAMP) cross-reactor classification. If multiple ingredients in a sunscreen were represented by a single allergen category, it was counted only once. A similar approach was utilized for marketing categories.
A total of 12 sunscreens were included in the analysis: Absolute Joi, Black Girl Sunscreen, Black Girl Sunscreen Make It Matte, Bolden SPF Brightening Moisturizer, Eleven on the Defense Unrivaled Sun Serum, Kinlo Golden Rays Sunscreen, Live Tinted Hueguard 3-in-1 Mineral Sunscreen, Mele Dew The Most Sheer Moisturizer SPF30 Broad Spectrum Sunscreen, Mele No Shade Sunscreen Oil, Specific Beauty Active Radiance Day Moi, Unsun Mineral Sunscreen, and Urban Skin Rx Complexion Protection. Their average cost was $19.30 per ounce (range, $6.33-$50.00) and common marketing claims for these products were “no white cast” (91.7%), being free of an ingredient (83.3%), and “moisturizing” (75%).
Of the 12 sunscreens, 7 (58.3%) contained a chemical sunscreen agent, 5 (41.7%) contained a physical UV blocker, and all contained at least one allergen. The average number of allergens per product was 4.7, most commonly fragrance/botanicals (83.3%), tocopherol (83.3%), sodium benzoates/derivatives (58.3%), and sorbitan sesquiolate/derivatives (58.3%).
“Average cost of sunscreens marketed to individuals with SOC was $19.30/oz, much higher than the median price of $3.32/oz reported in a separate study of 65 popular sunscreens,” the study authors wrote. “As many of the sunscreens in our study were sold by smaller businesses, higher prices may be due to higher production costs or a perceived smaller market.”
The authors expressed surprise that five sunscreens marketed to individuals with SOC contained a physical UV blocker which may create a white cast. They contacted the manufacturers of these five sunscreens and confirmed that three used micronized formulations. “While ingested/inhaled nanoparticles of titanium dioxide may cause tissue effects, most studies of topical products show excellent safety,” they wrote.
They also noted that the average of 4.7 allergens per product observed in the analysis was similar to the average of 4.9 seen in a separate study of 52 popular sunscreens. “However, that study only included 34 allergens while this study evaluated 90 allergens,” the authors wrote. “Consumers and providers should be aware sunscreens marketed to individuals with SOC may cause allergic contact dermatitis,” they commented.
“It is interesting to see how costly these products are now compared to store bought and general commercially available sunscreens several years ago,” said Lawrence J. Green, clinical professor of dermatology at George Washington University, Washington, who was asked to comment on the study. “However, to me that is not surprising as products marketed and targeted to specific populations are often priced at a premium. It wasn’t clear to me how many of these specialized online SOC sunscreens are tinted. I wish the authors had compared the cost of tinted sunscreens in general to nontinted sunscreens because tinted ones are more useful for SOC, because when rubbed in, they can readily match SOC and can also offer protection in the visible light spectrum.”
The authors reported having no financial disclosures; the study had no funding source. Dr. Green disclosed that he is a speaker, consultant, or investigator for many pharmaceutical companies.
, and more than 40% contain a UV blocker that may create a white cast.
Those are among the findings from a study by Michelle Xiong, a medical student at Brown University, Providence, R.I., and Erin M. Warshaw, MD, of the department of dermatology at Park Nicollet/Health Partners Health Services, Minneapolis, which was published online in the Journal of the American Academy of Dermatology.
“There is increasing awareness of the negative effects of ultraviolet (UV) light in individuals with skin of color (SOC), especially in regards to pigmentation disorders induced and/or exacerbated by UV exposure,” the authors wrote. “As a result, there has been a surge in sunscreens marketed to this population. We aimed to characterize cost, marketing claims, and potential allergenic ingredients in sunscreens marketed to individuals with SOC.”
Between December 2021 and October 2022, the researchers used the following search terms on Google: “sunscreen” plus “skin of 36 color,” “dark skin,” “brown skin,” “LatinX skin,” and/or “Black skin.” They extracted price, marketing claims, and ingredients from manufacturers’ websites and used 90 allergens contained in the American Contact Dermatitis Society 2020 Core series to identify potential allergens. Next, they combined cross-reactors/synonyms into allergen categories based on ACDS Contact Allergen Management Plan (CAMP) cross-reactor classification. If multiple ingredients in a sunscreen were represented by a single allergen category, it was counted only once. A similar approach was utilized for marketing categories.
A total of 12 sunscreens were included in the analysis: Absolute Joi, Black Girl Sunscreen, Black Girl Sunscreen Make It Matte, Bolden SPF Brightening Moisturizer, Eleven on the Defense Unrivaled Sun Serum, Kinlo Golden Rays Sunscreen, Live Tinted Hueguard 3-in-1 Mineral Sunscreen, Mele Dew The Most Sheer Moisturizer SPF30 Broad Spectrum Sunscreen, Mele No Shade Sunscreen Oil, Specific Beauty Active Radiance Day Moi, Unsun Mineral Sunscreen, and Urban Skin Rx Complexion Protection. Their average cost was $19.30 per ounce (range, $6.33-$50.00) and common marketing claims for these products were “no white cast” (91.7%), being free of an ingredient (83.3%), and “moisturizing” (75%).
Of the 12 sunscreens, 7 (58.3%) contained a chemical sunscreen agent, 5 (41.7%) contained a physical UV blocker, and all contained at least one allergen. The average number of allergens per product was 4.7, most commonly fragrance/botanicals (83.3%), tocopherol (83.3%), sodium benzoates/derivatives (58.3%), and sorbitan sesquiolate/derivatives (58.3%).
“Average cost of sunscreens marketed to individuals with SOC was $19.30/oz, much higher than the median price of $3.32/oz reported in a separate study of 65 popular sunscreens,” the study authors wrote. “As many of the sunscreens in our study were sold by smaller businesses, higher prices may be due to higher production costs or a perceived smaller market.”
The authors expressed surprise that five sunscreens marketed to individuals with SOC contained a physical UV blocker which may create a white cast. They contacted the manufacturers of these five sunscreens and confirmed that three used micronized formulations. “While ingested/inhaled nanoparticles of titanium dioxide may cause tissue effects, most studies of topical products show excellent safety,” they wrote.
They also noted that the average of 4.7 allergens per product observed in the analysis was similar to the average of 4.9 seen in a separate study of 52 popular sunscreens. “However, that study only included 34 allergens while this study evaluated 90 allergens,” the authors wrote. “Consumers and providers should be aware sunscreens marketed to individuals with SOC may cause allergic contact dermatitis,” they commented.
“It is interesting to see how costly these products are now compared to store bought and general commercially available sunscreens several years ago,” said Lawrence J. Green, clinical professor of dermatology at George Washington University, Washington, who was asked to comment on the study. “However, to me that is not surprising as products marketed and targeted to specific populations are often priced at a premium. It wasn’t clear to me how many of these specialized online SOC sunscreens are tinted. I wish the authors had compared the cost of tinted sunscreens in general to nontinted sunscreens because tinted ones are more useful for SOC, because when rubbed in, they can readily match SOC and can also offer protection in the visible light spectrum.”
The authors reported having no financial disclosures; the study had no funding source. Dr. Green disclosed that he is a speaker, consultant, or investigator for many pharmaceutical companies.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
How a cheap liver drug may be the key to preventing COVID
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
As soon as the pandemic started, the search was on for a medication that could stave off infection, or at least the worst consequences of infection.
One that would be cheap to make, safe, easy to distribute, and, ideally, was already available. The search had a quest-like quality, like something from a fairy tale. Society, poisoned by COVID, would find the antidote out there, somewhere, if we looked hard enough.
You know the story. There were some pretty dramatic failures: hydroxychloroquine, ivermectin. There were some successes, like dexamethasone.
I’m not here today to tell you that the antidote has been found – no, it takes large randomized trials to figure that out. But
How do you make a case that an existing drug – UDCA, in this case – might be useful to prevent or treat COVID? In contrast to prior basic-science studies, like the original ivermectin study, which essentially took a bunch of cells and virus in a tube filled with varying concentrations of the antiparasitic agent, the authors of this paper appearing in Nature give us multiple, complementary lines of evidence. Let me walk you through it.
All good science starts with a biologically plausible hypothesis. In this case, the authors recognized that SARS-CoV-2, in all its variants, requires the presence of the ACE2 receptor on the surface of cells to bind.
That is the doorway to infection. Vaccines and antibodies block the key to this door, the spike protein and its receptor binding domain. But what if you could get rid of the doors altogether?
The authors first showed that ACE2 expression is controlled by a certain transcription factor known as the farnesoid X receptor, or FXR. Reducing the binding of FXR should therefore reduce ACE2 expression.
As luck would have it, UDCA – Actigall – reduces the levels of FXR and thus the expression of ACE2 in cells.
Okay. So we have a drug that can reduce ACE2, and we know that ACE2 is necessary for the virus to infect cells. Would UDCA prevent viral infection?
They started with test tubes, showing that cells were less likely to be infected by SARS-CoV-2 in the presence of UDCA at concentrations similar to what humans achieve in their blood after standard dosing. The red staining here is spike protein; you can see that it is markedly lower in the cells exposed to UDCA.
So far, so good. But test tubes aren’t people. So they moved up to mice and Syrian golden hamsters. These cute fellows are quite susceptible to human COVID and have been a model organism in countless studies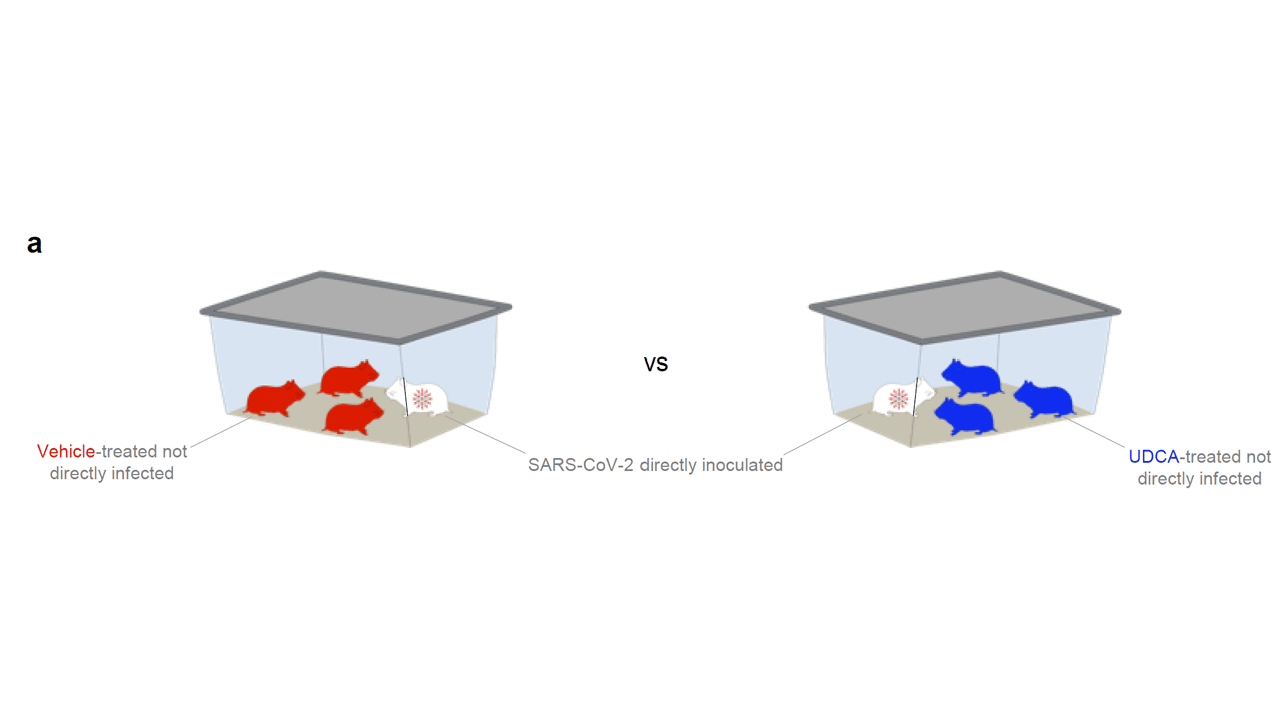
Mice and hamsters treated with UDCA in the presence of littermates with COVID infections were less likely to become infected themselves compared with mice not so treated. They also showed that mice and hamsters treated with UDCA had lower levels of ACE2 in their nasal passages.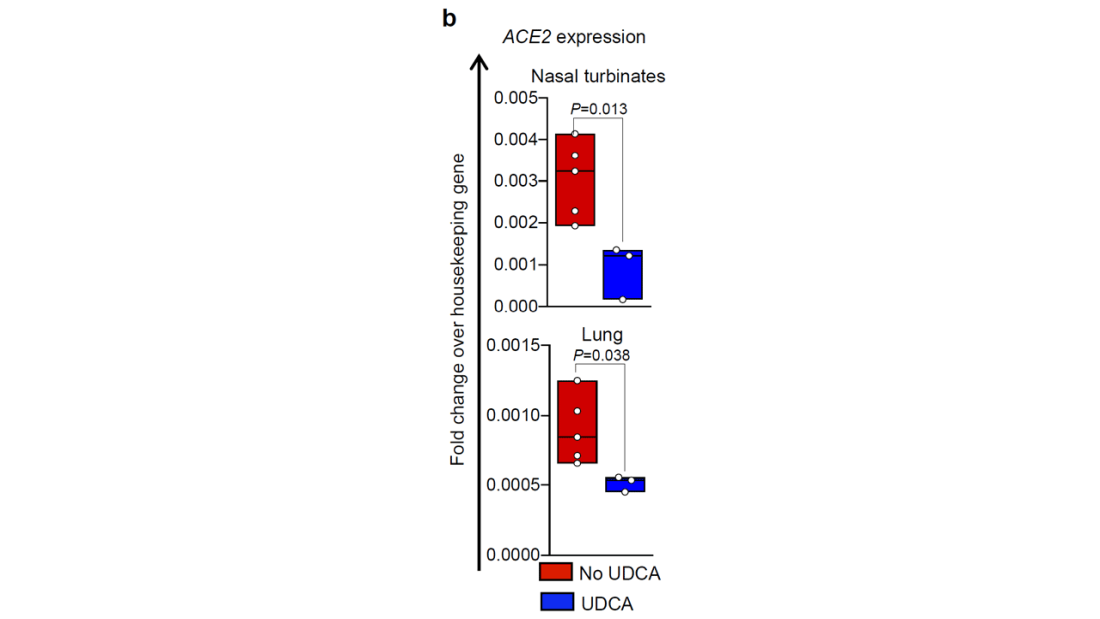
Of course, mice aren’t humans either. So the researchers didn’t stop there.
To determine the effects of UDCA on human tissue, they utilized perfused human lungs that had been declined for transplantation. The lungs were perfused with a special fluid to keep them viable, and were mechanically ventilated. One lung was exposed to UDCA and the other served as a control. The authors were able to show that ACE2 levels went down in the exposed lung. And, importantly, when samples of tissue from both lungs were exposed to SARS-CoV-2, the lung tissue exposed to UDCA had lower levels of viral infection.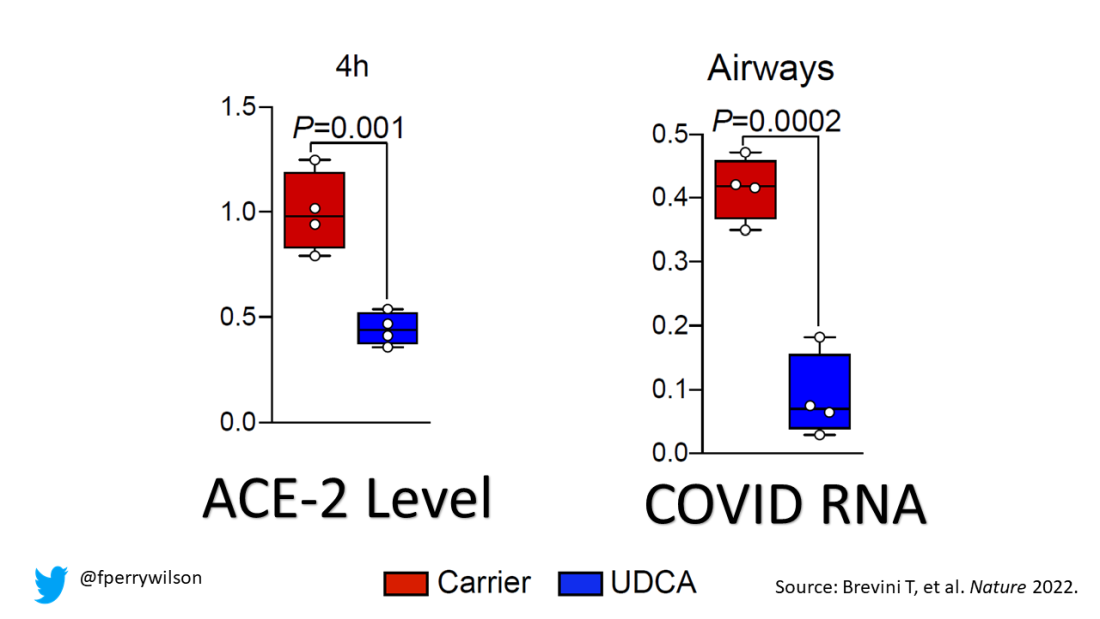
They didn’t stop there.
Eight human volunteers were recruited to take UDCA for 5 days. ACE2 levels in the nasal passages went down over the course of treatment. They confirmed those results from a proteomics dataset with several hundred people who had received UDCA for clinical reasons. Treated individuals had lower ACE2 levels.
Finally, they looked at the epidemiologic effect. They examined a dataset that contained information on over 1,000 patients with liver disease who had contracted COVID-19, 31 of whom had been receiving UDCA. Even after adjustment for baseline differences, those receiving UDCA were less likely to be hospitalized, require an ICU, or die.
Okay, we’ll stop there. Reading this study, all I could think was, Yes! This is how you generate evidence that you have a drug that might work – step by careful step.
But let’s be careful as well. Does this study show that taking Actigall will prevent COVID? Of course not. It doesn’t show that it will treat COVID either. But I bring it up because the rigor of this study stands in contrast to those that generated huge enthusiasm earlier in the pandemic only to let us down in randomized trials. If there has been a drug out there this whole time which will prevent or treat COVID, this is how we’ll find it. The next step? Test it in a randomized trial.
For Medscape, I’m Perry Wilson.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. He disclosed no relevant financial relationships.
A version of this video transcript first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
As soon as the pandemic started, the search was on for a medication that could stave off infection, or at least the worst consequences of infection.
One that would be cheap to make, safe, easy to distribute, and, ideally, was already available. The search had a quest-like quality, like something from a fairy tale. Society, poisoned by COVID, would find the antidote out there, somewhere, if we looked hard enough.
You know the story. There were some pretty dramatic failures: hydroxychloroquine, ivermectin. There were some successes, like dexamethasone.
I’m not here today to tell you that the antidote has been found – no, it takes large randomized trials to figure that out. But
How do you make a case that an existing drug – UDCA, in this case – might be useful to prevent or treat COVID? In contrast to prior basic-science studies, like the original ivermectin study, which essentially took a bunch of cells and virus in a tube filled with varying concentrations of the antiparasitic agent, the authors of this paper appearing in Nature give us multiple, complementary lines of evidence. Let me walk you through it.
All good science starts with a biologically plausible hypothesis. In this case, the authors recognized that SARS-CoV-2, in all its variants, requires the presence of the ACE2 receptor on the surface of cells to bind.
That is the doorway to infection. Vaccines and antibodies block the key to this door, the spike protein and its receptor binding domain. But what if you could get rid of the doors altogether?
The authors first showed that ACE2 expression is controlled by a certain transcription factor known as the farnesoid X receptor, or FXR. Reducing the binding of FXR should therefore reduce ACE2 expression.
As luck would have it, UDCA – Actigall – reduces the levels of FXR and thus the expression of ACE2 in cells.
Okay. So we have a drug that can reduce ACE2, and we know that ACE2 is necessary for the virus to infect cells. Would UDCA prevent viral infection?
They started with test tubes, showing that cells were less likely to be infected by SARS-CoV-2 in the presence of UDCA at concentrations similar to what humans achieve in their blood after standard dosing. The red staining here is spike protein; you can see that it is markedly lower in the cells exposed to UDCA.
So far, so good. But test tubes aren’t people. So they moved up to mice and Syrian golden hamsters. These cute fellows are quite susceptible to human COVID and have been a model organism in countless studies
Mice and hamsters treated with UDCA in the presence of littermates with COVID infections were less likely to become infected themselves compared with mice not so treated. They also showed that mice and hamsters treated with UDCA had lower levels of ACE2 in their nasal passages.
Of course, mice aren’t humans either. So the researchers didn’t stop there.
To determine the effects of UDCA on human tissue, they utilized perfused human lungs that had been declined for transplantation. The lungs were perfused with a special fluid to keep them viable, and were mechanically ventilated. One lung was exposed to UDCA and the other served as a control. The authors were able to show that ACE2 levels went down in the exposed lung. And, importantly, when samples of tissue from both lungs were exposed to SARS-CoV-2, the lung tissue exposed to UDCA had lower levels of viral infection.
They didn’t stop there.
Eight human volunteers were recruited to take UDCA for 5 days. ACE2 levels in the nasal passages went down over the course of treatment. They confirmed those results from a proteomics dataset with several hundred people who had received UDCA for clinical reasons. Treated individuals had lower ACE2 levels.
Finally, they looked at the epidemiologic effect. They examined a dataset that contained information on over 1,000 patients with liver disease who had contracted COVID-19, 31 of whom had been receiving UDCA. Even after adjustment for baseline differences, those receiving UDCA were less likely to be hospitalized, require an ICU, or die.
Okay, we’ll stop there. Reading this study, all I could think was, Yes! This is how you generate evidence that you have a drug that might work – step by careful step.
But let’s be careful as well. Does this study show that taking Actigall will prevent COVID? Of course not. It doesn’t show that it will treat COVID either. But I bring it up because the rigor of this study stands in contrast to those that generated huge enthusiasm earlier in the pandemic only to let us down in randomized trials. If there has been a drug out there this whole time which will prevent or treat COVID, this is how we’ll find it. The next step? Test it in a randomized trial.
For Medscape, I’m Perry Wilson.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. He disclosed no relevant financial relationships.
A version of this video transcript first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
As soon as the pandemic started, the search was on for a medication that could stave off infection, or at least the worst consequences of infection.
One that would be cheap to make, safe, easy to distribute, and, ideally, was already available. The search had a quest-like quality, like something from a fairy tale. Society, poisoned by COVID, would find the antidote out there, somewhere, if we looked hard enough.
You know the story. There were some pretty dramatic failures: hydroxychloroquine, ivermectin. There were some successes, like dexamethasone.
I’m not here today to tell you that the antidote has been found – no, it takes large randomized trials to figure that out. But
How do you make a case that an existing drug – UDCA, in this case – might be useful to prevent or treat COVID? In contrast to prior basic-science studies, like the original ivermectin study, which essentially took a bunch of cells and virus in a tube filled with varying concentrations of the antiparasitic agent, the authors of this paper appearing in Nature give us multiple, complementary lines of evidence. Let me walk you through it.
All good science starts with a biologically plausible hypothesis. In this case, the authors recognized that SARS-CoV-2, in all its variants, requires the presence of the ACE2 receptor on the surface of cells to bind.
That is the doorway to infection. Vaccines and antibodies block the key to this door, the spike protein and its receptor binding domain. But what if you could get rid of the doors altogether?
The authors first showed that ACE2 expression is controlled by a certain transcription factor known as the farnesoid X receptor, or FXR. Reducing the binding of FXR should therefore reduce ACE2 expression.
As luck would have it, UDCA – Actigall – reduces the levels of FXR and thus the expression of ACE2 in cells.
Okay. So we have a drug that can reduce ACE2, and we know that ACE2 is necessary for the virus to infect cells. Would UDCA prevent viral infection?
They started with test tubes, showing that cells were less likely to be infected by SARS-CoV-2 in the presence of UDCA at concentrations similar to what humans achieve in their blood after standard dosing. The red staining here is spike protein; you can see that it is markedly lower in the cells exposed to UDCA.
So far, so good. But test tubes aren’t people. So they moved up to mice and Syrian golden hamsters. These cute fellows are quite susceptible to human COVID and have been a model organism in countless studies
Mice and hamsters treated with UDCA in the presence of littermates with COVID infections were less likely to become infected themselves compared with mice not so treated. They also showed that mice and hamsters treated with UDCA had lower levels of ACE2 in their nasal passages.
Of course, mice aren’t humans either. So the researchers didn’t stop there.
To determine the effects of UDCA on human tissue, they utilized perfused human lungs that had been declined for transplantation. The lungs were perfused with a special fluid to keep them viable, and were mechanically ventilated. One lung was exposed to UDCA and the other served as a control. The authors were able to show that ACE2 levels went down in the exposed lung. And, importantly, when samples of tissue from both lungs were exposed to SARS-CoV-2, the lung tissue exposed to UDCA had lower levels of viral infection.
They didn’t stop there.
Eight human volunteers were recruited to take UDCA for 5 days. ACE2 levels in the nasal passages went down over the course of treatment. They confirmed those results from a proteomics dataset with several hundred people who had received UDCA for clinical reasons. Treated individuals had lower ACE2 levels.
Finally, they looked at the epidemiologic effect. They examined a dataset that contained information on over 1,000 patients with liver disease who had contracted COVID-19, 31 of whom had been receiving UDCA. Even after adjustment for baseline differences, those receiving UDCA were less likely to be hospitalized, require an ICU, or die.
Okay, we’ll stop there. Reading this study, all I could think was, Yes! This is how you generate evidence that you have a drug that might work – step by careful step.
But let’s be careful as well. Does this study show that taking Actigall will prevent COVID? Of course not. It doesn’t show that it will treat COVID either. But I bring it up because the rigor of this study stands in contrast to those that generated huge enthusiasm earlier in the pandemic only to let us down in randomized trials. If there has been a drug out there this whole time which will prevent or treat COVID, this is how we’ll find it. The next step? Test it in a randomized trial.
For Medscape, I’m Perry Wilson.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. He disclosed no relevant financial relationships.
A version of this video transcript first appeared on Medscape.com.
Know the right resuscitation for right-sided heart failure
Amado Alejandro Baez, MD, said in a presentation at the 2022 scientific assembly of the American College of Emergency Physicians.
The patient arrived on day 20 after a radical cystoprostatectomy. He had driven 4 hours from another city for a urology follow-up visit. On arrival, he developed respiratory distress symptoms and presented to the emergency department, said Dr. Baez, professor of emergency medicine and epidemiology at the Medical College of Georgia/Augusta University and triple-board certified in EMS, emergency medicine, and critical care.
The patient developed a massive pulmonary embolism with acute cor pulmonale (right-sided heart failure). An electrocardiogram showed an S1Q3T3, demonstrating the distinctive nature of right ventricular failure, said Dr. Baez.
Research has demonstrated the differences in physiology between the right and left ventricles, he said.
Dr. Baez highlighted some of the features of right ventricle (RV) failure and how to manage it. Notably, the RV is thinner and less resilient. “RV failure patients may fall off the Starling curve,” in contrast to patients with isolated left ventricle (LV) failure.
RV pressure overload is associated with a range of conditions, such as pericardial disease, pulmonary embolism, acute respiratory distress syndrome, and pulmonary arterial hypertension. When combined with RV overload, patients may develop intracardiac shunting or coronary heart disease, Dr. Baez said. Decreased contractility associated with RV failure can result from sepsis, right ventricular myocardial infarction, myocarditis, and arrhythmia.
Dr. Baez cited the 2018 scientific statement from the American Heart Association on the evaluation and management of right-sided heart failure. The authors of the statement noted that the complicated geometry of the right heart makes functional assessment a challenge. They wrote that various hemodynamic and biochemical markers can help guide clinical assessment and therapeutic decision-making.
Increased RV afterload drives multiple factors that can ultimately lead to cardiogenic shock and death, said Dr. Baez. These factors include decreased RV oxygen delivery, decreased RV coronary perfusion, decreased systemic blood pressure, and low carbon monoxide levels. RV afterload also leads to decreased RV contractility, an increase in RV oxygen demand, and tension in the RV wall, and it may contribute to tricuspid valve insufficiency, neurohormonal activation, and RV ischemia.
Treatment strategies involve improving symptoms and stopping disease progression, said Baez. In its scientific statement, the AHA recommends steps for assessing RV and LV function so as to identify RV failure as soon as possible, he said. After excluding pericardial disease, the AHA advises diagnosis and treatment of etiology-specific causes, such as right ventricular MI, pulmonary embolism, and sepsis. For arrhythmias, it recommends maintaining sinus rhythm when possible and considering a pacemaker to maintain atrioventricular synchrony and to avoid excessive bradycardia.
In its statement, the AHA also recommends optimizing preload with right arterial pressure/central venous pressure of 8-12 mm Hg, said Dr. Baez. Preload optimization combined with afterload reduction and improved contractility are hallmarks of care for patients with RV failure.
Avoiding systemic hypotension can prevent sequelae, such as myocardial ischemia and further hypotension, he said.
Optimization of fluid status is another key to managing RV failure, said Dr. Baez. Right heart coronary perfusion pressure can be protected by maintaining mean arterial pressure, and consideration should be given to reducing the RV afterload. Other strategies include inotropic medications and rhythm stabilization.
In general, for RV failure patients, “correct hypoxia, hypercarbia, and acidosis and avoid intubation when possible,” he said. Extracorporeal membrane oxygenation (ECMO) may be an option, depending on how many mechanical ventilator settings need to be adjusted.
In a study by Dr. Baez and colleagues published in Critical Care Medicine, the authors presented a Bayesian probability model for plasma lactate and severity of illness in cases of acute pulmonary embolism. “This Bayesian model demonstrated that the combination of shock index and lactate yield superior diagnostic gains than those compare to the sPESI and lactate,” Dr. Baez said.
The care model needs to be specific to the etiology, he added. Volume management in congested pulmonary hypertension involves a “squeeze and diurese” strategy.
According to the Internet Book of Critical Care, for patients with mean arterial pressure (MAP) of 60 mm Hg, central venous pressure (CVP) of 25 mm Hg, renal perfusion pressure of 25 mm Hg, and no urine output, a vasopressor should be added to treatment, Dr. Baez said. In cases in which the MAP 75 mm Hg, the CVP is 25 mm Hg, the renal perfusion pressure is 50 mm Hg, and the patient has good urine output, vasopressors should be continued and fluid should be removed through use of a diuretic. For patients with a MAP of 75 mm Hg, a CVP of 12 mm Hg, and renal perfusion pressure of 63 mm Hg who have good urine output, the diuretic and the vasopressor should be discontinued.
Dr. Baez also reviewed several clinical studies of the utility of acute mechanical circulatory support systems for RV failure.
In two small studies involving a heart pump and a right ventricular assistive device, the 30-day survival rate was approximately 72%-73%. A study of 179 patients involving ECMO showed an in-hospital mortality rate of 38.6%, he said.
Overall, “prompt diagnosis, hemodynamic support, and initiation of specific treatment” are the foundations of managing RV failure, he concluded.
Dr. Baez disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Amado Alejandro Baez, MD, said in a presentation at the 2022 scientific assembly of the American College of Emergency Physicians.
The patient arrived on day 20 after a radical cystoprostatectomy. He had driven 4 hours from another city for a urology follow-up visit. On arrival, he developed respiratory distress symptoms and presented to the emergency department, said Dr. Baez, professor of emergency medicine and epidemiology at the Medical College of Georgia/Augusta University and triple-board certified in EMS, emergency medicine, and critical care.
The patient developed a massive pulmonary embolism with acute cor pulmonale (right-sided heart failure). An electrocardiogram showed an S1Q3T3, demonstrating the distinctive nature of right ventricular failure, said Dr. Baez.
Research has demonstrated the differences in physiology between the right and left ventricles, he said.
Dr. Baez highlighted some of the features of right ventricle (RV) failure and how to manage it. Notably, the RV is thinner and less resilient. “RV failure patients may fall off the Starling curve,” in contrast to patients with isolated left ventricle (LV) failure.
RV pressure overload is associated with a range of conditions, such as pericardial disease, pulmonary embolism, acute respiratory distress syndrome, and pulmonary arterial hypertension. When combined with RV overload, patients may develop intracardiac shunting or coronary heart disease, Dr. Baez said. Decreased contractility associated with RV failure can result from sepsis, right ventricular myocardial infarction, myocarditis, and arrhythmia.
Dr. Baez cited the 2018 scientific statement from the American Heart Association on the evaluation and management of right-sided heart failure. The authors of the statement noted that the complicated geometry of the right heart makes functional assessment a challenge. They wrote that various hemodynamic and biochemical markers can help guide clinical assessment and therapeutic decision-making.
Increased RV afterload drives multiple factors that can ultimately lead to cardiogenic shock and death, said Dr. Baez. These factors include decreased RV oxygen delivery, decreased RV coronary perfusion, decreased systemic blood pressure, and low carbon monoxide levels. RV afterload also leads to decreased RV contractility, an increase in RV oxygen demand, and tension in the RV wall, and it may contribute to tricuspid valve insufficiency, neurohormonal activation, and RV ischemia.
Treatment strategies involve improving symptoms and stopping disease progression, said Baez. In its scientific statement, the AHA recommends steps for assessing RV and LV function so as to identify RV failure as soon as possible, he said. After excluding pericardial disease, the AHA advises diagnosis and treatment of etiology-specific causes, such as right ventricular MI, pulmonary embolism, and sepsis. For arrhythmias, it recommends maintaining sinus rhythm when possible and considering a pacemaker to maintain atrioventricular synchrony and to avoid excessive bradycardia.
In its statement, the AHA also recommends optimizing preload with right arterial pressure/central venous pressure of 8-12 mm Hg, said Dr. Baez. Preload optimization combined with afterload reduction and improved contractility are hallmarks of care for patients with RV failure.
Avoiding systemic hypotension can prevent sequelae, such as myocardial ischemia and further hypotension, he said.
Optimization of fluid status is another key to managing RV failure, said Dr. Baez. Right heart coronary perfusion pressure can be protected by maintaining mean arterial pressure, and consideration should be given to reducing the RV afterload. Other strategies include inotropic medications and rhythm stabilization.
In general, for RV failure patients, “correct hypoxia, hypercarbia, and acidosis and avoid intubation when possible,” he said. Extracorporeal membrane oxygenation (ECMO) may be an option, depending on how many mechanical ventilator settings need to be adjusted.
In a study by Dr. Baez and colleagues published in Critical Care Medicine, the authors presented a Bayesian probability model for plasma lactate and severity of illness in cases of acute pulmonary embolism. “This Bayesian model demonstrated that the combination of shock index and lactate yield superior diagnostic gains than those compare to the sPESI and lactate,” Dr. Baez said.
The care model needs to be specific to the etiology, he added. Volume management in congested pulmonary hypertension involves a “squeeze and diurese” strategy.
According to the Internet Book of Critical Care, for patients with mean arterial pressure (MAP) of 60 mm Hg, central venous pressure (CVP) of 25 mm Hg, renal perfusion pressure of 25 mm Hg, and no urine output, a vasopressor should be added to treatment, Dr. Baez said. In cases in which the MAP 75 mm Hg, the CVP is 25 mm Hg, the renal perfusion pressure is 50 mm Hg, and the patient has good urine output, vasopressors should be continued and fluid should be removed through use of a diuretic. For patients with a MAP of 75 mm Hg, a CVP of 12 mm Hg, and renal perfusion pressure of 63 mm Hg who have good urine output, the diuretic and the vasopressor should be discontinued.
Dr. Baez also reviewed several clinical studies of the utility of acute mechanical circulatory support systems for RV failure.
In two small studies involving a heart pump and a right ventricular assistive device, the 30-day survival rate was approximately 72%-73%. A study of 179 patients involving ECMO showed an in-hospital mortality rate of 38.6%, he said.
Overall, “prompt diagnosis, hemodynamic support, and initiation of specific treatment” are the foundations of managing RV failure, he concluded.
Dr. Baez disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Amado Alejandro Baez, MD, said in a presentation at the 2022 scientific assembly of the American College of Emergency Physicians.
The patient arrived on day 20 after a radical cystoprostatectomy. He had driven 4 hours from another city for a urology follow-up visit. On arrival, he developed respiratory distress symptoms and presented to the emergency department, said Dr. Baez, professor of emergency medicine and epidemiology at the Medical College of Georgia/Augusta University and triple-board certified in EMS, emergency medicine, and critical care.
The patient developed a massive pulmonary embolism with acute cor pulmonale (right-sided heart failure). An electrocardiogram showed an S1Q3T3, demonstrating the distinctive nature of right ventricular failure, said Dr. Baez.
Research has demonstrated the differences in physiology between the right and left ventricles, he said.
Dr. Baez highlighted some of the features of right ventricle (RV) failure and how to manage it. Notably, the RV is thinner and less resilient. “RV failure patients may fall off the Starling curve,” in contrast to patients with isolated left ventricle (LV) failure.
RV pressure overload is associated with a range of conditions, such as pericardial disease, pulmonary embolism, acute respiratory distress syndrome, and pulmonary arterial hypertension. When combined with RV overload, patients may develop intracardiac shunting or coronary heart disease, Dr. Baez said. Decreased contractility associated with RV failure can result from sepsis, right ventricular myocardial infarction, myocarditis, and arrhythmia.
Dr. Baez cited the 2018 scientific statement from the American Heart Association on the evaluation and management of right-sided heart failure. The authors of the statement noted that the complicated geometry of the right heart makes functional assessment a challenge. They wrote that various hemodynamic and biochemical markers can help guide clinical assessment and therapeutic decision-making.
Increased RV afterload drives multiple factors that can ultimately lead to cardiogenic shock and death, said Dr. Baez. These factors include decreased RV oxygen delivery, decreased RV coronary perfusion, decreased systemic blood pressure, and low carbon monoxide levels. RV afterload also leads to decreased RV contractility, an increase in RV oxygen demand, and tension in the RV wall, and it may contribute to tricuspid valve insufficiency, neurohormonal activation, and RV ischemia.
Treatment strategies involve improving symptoms and stopping disease progression, said Baez. In its scientific statement, the AHA recommends steps for assessing RV and LV function so as to identify RV failure as soon as possible, he said. After excluding pericardial disease, the AHA advises diagnosis and treatment of etiology-specific causes, such as right ventricular MI, pulmonary embolism, and sepsis. For arrhythmias, it recommends maintaining sinus rhythm when possible and considering a pacemaker to maintain atrioventricular synchrony and to avoid excessive bradycardia.
In its statement, the AHA also recommends optimizing preload with right arterial pressure/central venous pressure of 8-12 mm Hg, said Dr. Baez. Preload optimization combined with afterload reduction and improved contractility are hallmarks of care for patients with RV failure.
Avoiding systemic hypotension can prevent sequelae, such as myocardial ischemia and further hypotension, he said.
Optimization of fluid status is another key to managing RV failure, said Dr. Baez. Right heart coronary perfusion pressure can be protected by maintaining mean arterial pressure, and consideration should be given to reducing the RV afterload. Other strategies include inotropic medications and rhythm stabilization.
In general, for RV failure patients, “correct hypoxia, hypercarbia, and acidosis and avoid intubation when possible,” he said. Extracorporeal membrane oxygenation (ECMO) may be an option, depending on how many mechanical ventilator settings need to be adjusted.
In a study by Dr. Baez and colleagues published in Critical Care Medicine, the authors presented a Bayesian probability model for plasma lactate and severity of illness in cases of acute pulmonary embolism. “This Bayesian model demonstrated that the combination of shock index and lactate yield superior diagnostic gains than those compare to the sPESI and lactate,” Dr. Baez said.
The care model needs to be specific to the etiology, he added. Volume management in congested pulmonary hypertension involves a “squeeze and diurese” strategy.
According to the Internet Book of Critical Care, for patients with mean arterial pressure (MAP) of 60 mm Hg, central venous pressure (CVP) of 25 mm Hg, renal perfusion pressure of 25 mm Hg, and no urine output, a vasopressor should be added to treatment, Dr. Baez said. In cases in which the MAP 75 mm Hg, the CVP is 25 mm Hg, the renal perfusion pressure is 50 mm Hg, and the patient has good urine output, vasopressors should be continued and fluid should be removed through use of a diuretic. For patients with a MAP of 75 mm Hg, a CVP of 12 mm Hg, and renal perfusion pressure of 63 mm Hg who have good urine output, the diuretic and the vasopressor should be discontinued.
Dr. Baez also reviewed several clinical studies of the utility of acute mechanical circulatory support systems for RV failure.
In two small studies involving a heart pump and a right ventricular assistive device, the 30-day survival rate was approximately 72%-73%. A study of 179 patients involving ECMO showed an in-hospital mortality rate of 38.6%, he said.
Overall, “prompt diagnosis, hemodynamic support, and initiation of specific treatment” are the foundations of managing RV failure, he concluded.
Dr. Baez disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ACEP 2022
Current alopecia areata options include old and new therapies
LAS VEGAS – in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
“Some patients don’t have alopecia, but they have been managed for it,” he said. “Whenever there is an ounce of doubt, take a biopsy,” he advised.
Assessing disease severity in patients with alopecia areata (AA) is especially important as new therapies become available, said Dr. King, associate professor of dermatology at Yale University, New Haven, Conn. The Severity of Alopecia Tool (SALT) Score has been available since 2004, and remains a useful tool to estimate percent hair loss. The SALT Score divides the scalp into four sections: 18% each for the right and left sides, 40% for the top of the head, and 24% for the back of the head, said Dr. King. However, the SALT Score can be enhanced or modified based on a holistic approach to disease severity that categorizes alopecia as mild (scalp hair loss of 20% or less), moderate (scalp hair loss of 21 to 49%), or severe (scalp hair loss of 50% or more).
For example, if a patient’s hair loss based on SALT Score is mild or moderate, increase the severity by 1 level (from mild to moderate, or moderate to severe) if any of the following conditions apply: Noticeable eyebrow or eyelash involvement, inadequate treatment response after 6 months, diffuse positive hair pull test consistent with rapid progression of AA, or a negative impact on psychosocial functioning because of AA, he said.
Treatment advances
Understanding of the pathogenesis of AA has been slow to evolve, Dr. King noted. “We haven’t been able to shake this concept that people are causing the disease by being depressed,” as noted in the literature from the 1950s.
In 2014, breakthrough research changed the game by identifying the roles of interferon gamma and interleukin 15, Dr. King said. Since then, more research has been conducted on Janus kinase (JAK) inhibitors for AA. Dr. King was a coinvestigator on a 2014 case report in which a patient with psoriasis and alopecia universalis experienced regrowth of most of his body hair after 8 months of daily oral tofacitinib, a JAK inhibitor.
However, despite the dramatic results in some patients, “tofacitinib doesn’t always work,” said Dr. King. In his experience, patients for whom tofacitinib didn’t work were those with complete or nearly complete scalp hair loss for more than 10 years.
Approval of baricitinib
Dr. King’s recent work supported the approval in June 2022 of oral baricitinib, a JAK inhibitor, for AA. He reviewed data from his late-breaker abstract presented at the annual meeting of the American Academy of Dermatology in March 2022, where he reported that almost 40% of adults with AA treated with 4 mg of baricitinib daily had significant hair regrowth over 52 weeks.
Two other oral JAK inhibitors in the pipeline for AA are deuruxolitinib and ritlecitinib, which significantly increased the proportion of patients achieving SALT scores of 20 or less, compared with patients on placebo in early clinical trials. Data on both were presented at the annual meeting of the European Academy of Dermatology and Venereology.
So far, topical JAK inhibitors have not shown success in hair regrowth for AA patients, said Dr. King. Phase 2 studies of both ruxolitinib 1.5% cream and delgocitinib ointment were ineffective for AA.
Emerging role for oral minoxidil
Oral minoxidil has had a recent resurgence as an adjunct therapy to the new JAK inhibitors. A study published in 1987 found that, with oral minoxidil monotherapy, a cosmetic response was seen in 18% of patients with AA, Dr. King said.
In a study published in the Journal of the American Academy of Dermatology, Dr. King and colleagues noted that dose escalation is sometimes needed for effective treatment of AA with tofacitinib. They examined the effect of adding oral minoxidil to tofacitinib in patients with severe AA as a way to increase efficacy without increasing tofacitinib dosage. They reviewed data from 12 patients ages 18-51 years who were prescribed 5 mg of tofacitinib twice daily, plus 2.5 mg oral minoxidil daily for women and 2.5 mg of minoxidil twice daily for men; women received a lower dose to minimize the side effect of hypertrichosis.
After 6 months, 67% (eight patients) achieved at least 75% hair regrowth; of those eight patients, seven (58% of the total) had hair regrowth on a twice-daily dose of 5 mg tofacitinib with no need for dose escalation, Dr. King said.
More research is needed, but oral minoxidil may be a useful adjunct treatment for some patients with AA, he added.
During a question and answer session, Dr. King was asked to elaborate on the mechanism of minoxidil in combination with JAK inhibitors. “The truth is that I just don’t know” why the combination works for some patients. However, the majority of patients who succeed with this combination regrow hair by 4 months. “There is something special about that combination.”
Dr. King disclosed serving as a consultant or adviser for AbbVie, AltruBio, Almirall, AnaptysBio, Arena Pharmaceuticals, Bioniz, Bristol Myers Squibb, Concert Pharmaceuticals, Horizon, Incyte, Leo Pharma, Eli Lilly, Otsuka, Pfizer, Regeneron, Sanofi Genzyme, Twi Biotechnology, Viela Bio, and Visterra; serving as a speaker or as a member of the speakers bureau for Incyte, Pfizer, Regeneron, Sanofi Genzyme; and receiving research funding from Concert Pharmaceuticals, Eli Lilly, and Pfizer.
MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
“Some patients don’t have alopecia, but they have been managed for it,” he said. “Whenever there is an ounce of doubt, take a biopsy,” he advised.
Assessing disease severity in patients with alopecia areata (AA) is especially important as new therapies become available, said Dr. King, associate professor of dermatology at Yale University, New Haven, Conn. The Severity of Alopecia Tool (SALT) Score has been available since 2004, and remains a useful tool to estimate percent hair loss. The SALT Score divides the scalp into four sections: 18% each for the right and left sides, 40% for the top of the head, and 24% for the back of the head, said Dr. King. However, the SALT Score can be enhanced or modified based on a holistic approach to disease severity that categorizes alopecia as mild (scalp hair loss of 20% or less), moderate (scalp hair loss of 21 to 49%), or severe (scalp hair loss of 50% or more).
For example, if a patient’s hair loss based on SALT Score is mild or moderate, increase the severity by 1 level (from mild to moderate, or moderate to severe) if any of the following conditions apply: Noticeable eyebrow or eyelash involvement, inadequate treatment response after 6 months, diffuse positive hair pull test consistent with rapid progression of AA, or a negative impact on psychosocial functioning because of AA, he said.
Treatment advances
Understanding of the pathogenesis of AA has been slow to evolve, Dr. King noted. “We haven’t been able to shake this concept that people are causing the disease by being depressed,” as noted in the literature from the 1950s.
In 2014, breakthrough research changed the game by identifying the roles of interferon gamma and interleukin 15, Dr. King said. Since then, more research has been conducted on Janus kinase (JAK) inhibitors for AA. Dr. King was a coinvestigator on a 2014 case report in which a patient with psoriasis and alopecia universalis experienced regrowth of most of his body hair after 8 months of daily oral tofacitinib, a JAK inhibitor.
However, despite the dramatic results in some patients, “tofacitinib doesn’t always work,” said Dr. King. In his experience, patients for whom tofacitinib didn’t work were those with complete or nearly complete scalp hair loss for more than 10 years.
Approval of baricitinib
Dr. King’s recent work supported the approval in June 2022 of oral baricitinib, a JAK inhibitor, for AA. He reviewed data from his late-breaker abstract presented at the annual meeting of the American Academy of Dermatology in March 2022, where he reported that almost 40% of adults with AA treated with 4 mg of baricitinib daily had significant hair regrowth over 52 weeks.
Two other oral JAK inhibitors in the pipeline for AA are deuruxolitinib and ritlecitinib, which significantly increased the proportion of patients achieving SALT scores of 20 or less, compared with patients on placebo in early clinical trials. Data on both were presented at the annual meeting of the European Academy of Dermatology and Venereology.
So far, topical JAK inhibitors have not shown success in hair regrowth for AA patients, said Dr. King. Phase 2 studies of both ruxolitinib 1.5% cream and delgocitinib ointment were ineffective for AA.
Emerging role for oral minoxidil
Oral minoxidil has had a recent resurgence as an adjunct therapy to the new JAK inhibitors. A study published in 1987 found that, with oral minoxidil monotherapy, a cosmetic response was seen in 18% of patients with AA, Dr. King said.
In a study published in the Journal of the American Academy of Dermatology, Dr. King and colleagues noted that dose escalation is sometimes needed for effective treatment of AA with tofacitinib. They examined the effect of adding oral minoxidil to tofacitinib in patients with severe AA as a way to increase efficacy without increasing tofacitinib dosage. They reviewed data from 12 patients ages 18-51 years who were prescribed 5 mg of tofacitinib twice daily, plus 2.5 mg oral minoxidil daily for women and 2.5 mg of minoxidil twice daily for men; women received a lower dose to minimize the side effect of hypertrichosis.
After 6 months, 67% (eight patients) achieved at least 75% hair regrowth; of those eight patients, seven (58% of the total) had hair regrowth on a twice-daily dose of 5 mg tofacitinib with no need for dose escalation, Dr. King said.
More research is needed, but oral minoxidil may be a useful adjunct treatment for some patients with AA, he added.
During a question and answer session, Dr. King was asked to elaborate on the mechanism of minoxidil in combination with JAK inhibitors. “The truth is that I just don’t know” why the combination works for some patients. However, the majority of patients who succeed with this combination regrow hair by 4 months. “There is something special about that combination.”
Dr. King disclosed serving as a consultant or adviser for AbbVie, AltruBio, Almirall, AnaptysBio, Arena Pharmaceuticals, Bioniz, Bristol Myers Squibb, Concert Pharmaceuticals, Horizon, Incyte, Leo Pharma, Eli Lilly, Otsuka, Pfizer, Regeneron, Sanofi Genzyme, Twi Biotechnology, Viela Bio, and Visterra; serving as a speaker or as a member of the speakers bureau for Incyte, Pfizer, Regeneron, Sanofi Genzyme; and receiving research funding from Concert Pharmaceuticals, Eli Lilly, and Pfizer.
MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
“Some patients don’t have alopecia, but they have been managed for it,” he said. “Whenever there is an ounce of doubt, take a biopsy,” he advised.
Assessing disease severity in patients with alopecia areata (AA) is especially important as new therapies become available, said Dr. King, associate professor of dermatology at Yale University, New Haven, Conn. The Severity of Alopecia Tool (SALT) Score has been available since 2004, and remains a useful tool to estimate percent hair loss. The SALT Score divides the scalp into four sections: 18% each for the right and left sides, 40% for the top of the head, and 24% for the back of the head, said Dr. King. However, the SALT Score can be enhanced or modified based on a holistic approach to disease severity that categorizes alopecia as mild (scalp hair loss of 20% or less), moderate (scalp hair loss of 21 to 49%), or severe (scalp hair loss of 50% or more).
For example, if a patient’s hair loss based on SALT Score is mild or moderate, increase the severity by 1 level (from mild to moderate, or moderate to severe) if any of the following conditions apply: Noticeable eyebrow or eyelash involvement, inadequate treatment response after 6 months, diffuse positive hair pull test consistent with rapid progression of AA, or a negative impact on psychosocial functioning because of AA, he said.
Treatment advances
Understanding of the pathogenesis of AA has been slow to evolve, Dr. King noted. “We haven’t been able to shake this concept that people are causing the disease by being depressed,” as noted in the literature from the 1950s.
In 2014, breakthrough research changed the game by identifying the roles of interferon gamma and interleukin 15, Dr. King said. Since then, more research has been conducted on Janus kinase (JAK) inhibitors for AA. Dr. King was a coinvestigator on a 2014 case report in which a patient with psoriasis and alopecia universalis experienced regrowth of most of his body hair after 8 months of daily oral tofacitinib, a JAK inhibitor.
However, despite the dramatic results in some patients, “tofacitinib doesn’t always work,” said Dr. King. In his experience, patients for whom tofacitinib didn’t work were those with complete or nearly complete scalp hair loss for more than 10 years.
Approval of baricitinib
Dr. King’s recent work supported the approval in June 2022 of oral baricitinib, a JAK inhibitor, for AA. He reviewed data from his late-breaker abstract presented at the annual meeting of the American Academy of Dermatology in March 2022, where he reported that almost 40% of adults with AA treated with 4 mg of baricitinib daily had significant hair regrowth over 52 weeks.
Two other oral JAK inhibitors in the pipeline for AA are deuruxolitinib and ritlecitinib, which significantly increased the proportion of patients achieving SALT scores of 20 or less, compared with patients on placebo in early clinical trials. Data on both were presented at the annual meeting of the European Academy of Dermatology and Venereology.
So far, topical JAK inhibitors have not shown success in hair regrowth for AA patients, said Dr. King. Phase 2 studies of both ruxolitinib 1.5% cream and delgocitinib ointment were ineffective for AA.
Emerging role for oral minoxidil
Oral minoxidil has had a recent resurgence as an adjunct therapy to the new JAK inhibitors. A study published in 1987 found that, with oral minoxidil monotherapy, a cosmetic response was seen in 18% of patients with AA, Dr. King said.
In a study published in the Journal of the American Academy of Dermatology, Dr. King and colleagues noted that dose escalation is sometimes needed for effective treatment of AA with tofacitinib. They examined the effect of adding oral minoxidil to tofacitinib in patients with severe AA as a way to increase efficacy without increasing tofacitinib dosage. They reviewed data from 12 patients ages 18-51 years who were prescribed 5 mg of tofacitinib twice daily, plus 2.5 mg oral minoxidil daily for women and 2.5 mg of minoxidil twice daily for men; women received a lower dose to minimize the side effect of hypertrichosis.
After 6 months, 67% (eight patients) achieved at least 75% hair regrowth; of those eight patients, seven (58% of the total) had hair regrowth on a twice-daily dose of 5 mg tofacitinib with no need for dose escalation, Dr. King said.
More research is needed, but oral minoxidil may be a useful adjunct treatment for some patients with AA, he added.
During a question and answer session, Dr. King was asked to elaborate on the mechanism of minoxidil in combination with JAK inhibitors. “The truth is that I just don’t know” why the combination works for some patients. However, the majority of patients who succeed with this combination regrow hair by 4 months. “There is something special about that combination.”
Dr. King disclosed serving as a consultant or adviser for AbbVie, AltruBio, Almirall, AnaptysBio, Arena Pharmaceuticals, Bioniz, Bristol Myers Squibb, Concert Pharmaceuticals, Horizon, Incyte, Leo Pharma, Eli Lilly, Otsuka, Pfizer, Regeneron, Sanofi Genzyme, Twi Biotechnology, Viela Bio, and Visterra; serving as a speaker or as a member of the speakers bureau for Incyte, Pfizer, Regeneron, Sanofi Genzyme; and receiving research funding from Concert Pharmaceuticals, Eli Lilly, and Pfizer.
MedscapeLive and this news organization are owned by the same parent company.
AT INNOVATIONS IN DERMATOLOGY
Paxlovid has been free so far. Next year, sticker shock awaits
Nearly 6 million Americans have taken Paxlovid for free, courtesy of the federal government. The Pfizer pill has helped prevent many people infected with COVID-19 from being hospitalized or dying, and it may even reduce the risk of developing long COVID.
And that means fewer people will get the potentially lifesaving treatments, experts said.
“I think the numbers will go way down,” said Jill Rosenthal, director of public health policy at the Center for American Progress, a left-leaning think tank. A bill for several hundred dollars or more would lead many people to decide the medication isn’t worth the price, she said.
In response to the unprecedented public health crisis caused by COVID, the federal government spent billions of dollars on developing new vaccines and treatments, to swift success: Less than a year after the pandemic was declared, medical workers got their first vaccines. But as many people have refused the shots and stopped wearing masks, the virus still rages and mutates. In 2022 alone, 250,000 Americans have died from COVID, more than from strokes or diabetes.
But soon the Department of Health & Human Services will stop supplying COVID treatments, and pharmacies will purchase and bill for them the same way they do for antibiotic pills or asthma inhalers. Paxlovid is expected to hit the private market in mid-2023, according to HHS plans shared in an October meeting with state health officials and clinicians. Merck’s Lagevrio, a less-effective COVID treatment pill, and AstraZeneca’s Evusheld, a preventive therapy for the immunocompromised, are on track to be commercialized sooner, sometime in the winter.
The U.S. government has so far purchased 20 million courses of Paxlovid, priced at about $530 each, a discount for buying in bulk that Pfizer CEO Albert Bourla called “really very attractive” to the federal government in a July earnings call. The drug will cost far more on the private market, although in a statement to Kaiser Health News, Pfizer declined to share the planned price. The government will also stop paying for the company’s COVID vaccine next year – those shots will quadruple in price, from the discount rate the government pays of $30 to about $120.
Mr. Bourla told investors in November that he expects the move will make Paxlovid and its COVID vaccine “a multibillion-dollars franchise.”
Nearly 9 in 10 people dying from the virus now are 65 or older. Yet federal law restricts Medicare Part D – the prescription drug program that covers nearly 50 million seniors – from covering the COVID treatment pills. The medications are meant for those most at risk of serious illness, including seniors.
Paxlovid and the other treatments are currently available under an emergency use authorization from the FDA, a fast-track review used in extraordinary situations. Although Pfizer applied for full approval in June, the process can take anywhere from several months to years. And Medicare Part D can’t cover any medications without that full stamp of approval.
Paying out-of-pocket would be “a substantial barrier” for seniors on Medicare – the very people who would benefit most from the drug, wrote federal health experts.
“From a public health perspective, and even from a health care capacity and cost perspective, it would just defy reason to not continue to make these drugs readily available,” said Dr. Larry Madoff, medical director of Massachusetts’s Bureau of Infectious Disease and Laboratory Sciences. He’s hopeful that the federal health agency will find a way to set aside unused doses for seniors and people without insurance.
In mid-November, the White House requested that Congress approve an additional $2.5 billion for COVID therapeutics and vaccines to make sure people can afford the medications when they’re no longer free. But there’s little hope it will be approved – the Senate voted that same day to end the public health emergency and denied similar requests in recent months.
Many Americans have already faced hurdles just getting a prescription for COVID treatment. Although the federal government doesn’t track who’s gotten the drug, a Centers for Disease Control and Prevention study using data from 30 medical centers found that Black and Hispanic patients with COVID were much less likely to receive Paxlovid than White patients. (Hispanic people can be of any race or combination of races.) And when the government is no longer picking up the tab, experts predict that these gaps by race, income, and geography will widen.
People in Northeastern states used the drug far more often than those in the rest of the country, according to a KHN analysis of Paxlovid use in September and October. But it wasn’t because people in the region were getting sick from COVID at much higher rates – instead, many of those states offered better access to health care to begin with and created special programs to get Paxlovid to their residents.
About 10 mostly Democratic states and several large counties in the Northeast and elsewhere created free “test-to-treat” programs that allow their residents to get an immediate doctor visit and prescription for treatment after testing positive for COVID. In Massachusetts, more than 20,000 residents have used the state’s video and phone hotline, which is available 7 days a week in 13 languages. Massachusetts, which has the highest insurance rate in the country and relatively low travel times to pharmacies, had the second-highest Paxlovid usage rate among states this fall.
States with higher COVID death rates, like Florida and Kentucky, where residents must travel farther for health care and are more likely to be uninsured, used the drug less often. Without no-cost test-to-treat options, residents have struggled to get prescriptions even though the drug itself is still free.
“If you look at access to medications for people who are uninsured, I think that there’s no question that will widen those disparities,” Ms. Rosenthal said.
People who get insurance through their jobs could face high copays at the register, too, just as they do for insulin and other expensive or brand-name drugs.
Most private insurance companies will end up covering COVID therapeutics to some extent, said Sabrina Corlette, a research professor at Georgetown University’s Center on Health Insurance Reforms. After all, the pills are cheaper than a hospital stay. But for most people who get insurance through their jobs, there are “really no rules at all,” she said. Some insurers could take months to add the drugs to their plans or decide not to pay for them.
And the additional cost means many people will go without the medication. “We know from lots of research that when people face cost sharing for these drugs that they need to take, they will often forgo or cut back,” Ms. Corlette said.
One group doesn’t need to worry about sticker shock. Medicaid, the public insurance program for low-income adults and children, will cover the treatments in full until at least early 2024.
HHS officials could set aside any leftover taxpayer-funded medication for people who can’t afford to pay the full cost, but they haven’t shared any concrete plans to do so. The government purchased 20 million courses of Paxlovid and 3 million of Lagevrio. Fewer than a third have been used, and usage has fallen in recent months, according to KHN’s analysis of the data from HHS.
Sixty percent of the government’s supply of Evusheld is also still available, although the COVID prevention therapy is less effective against new strains of the virus. The health department in one state, New Mexico, has recommended against using it.
HHS did not make officials available for an interview or answer written questions about the commercialization plans.
The government created a potential workaround when they moved bebtelovimab, another COVID treatment, to the private market this summer. It now retails for $2,100 per patient. The agency set aside the remaining 60,000 government-purchased doses that hospitals could use to treat uninsured patients in a convoluted dose-replacement process. But it’s hard to tell how well that setup would work for Paxlovid: Bebtelovimab was already much less popular, and the FDA halted its use on Nov. 30 because it’s less effective against current strains of the virus.
Federal officials and insurance companies would have good reason to make sure patients can continue to afford COVID drugs: They’re far cheaper than if patients land in the emergency room.
“The medications are so worthwhile,” said Dr. Madoff, the Massachusetts health official. “They’re not expensive in the grand scheme of health care costs.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Nearly 6 million Americans have taken Paxlovid for free, courtesy of the federal government. The Pfizer pill has helped prevent many people infected with COVID-19 from being hospitalized or dying, and it may even reduce the risk of developing long COVID.
And that means fewer people will get the potentially lifesaving treatments, experts said.
“I think the numbers will go way down,” said Jill Rosenthal, director of public health policy at the Center for American Progress, a left-leaning think tank. A bill for several hundred dollars or more would lead many people to decide the medication isn’t worth the price, she said.
In response to the unprecedented public health crisis caused by COVID, the federal government spent billions of dollars on developing new vaccines and treatments, to swift success: Less than a year after the pandemic was declared, medical workers got their first vaccines. But as many people have refused the shots and stopped wearing masks, the virus still rages and mutates. In 2022 alone, 250,000 Americans have died from COVID, more than from strokes or diabetes.
But soon the Department of Health & Human Services will stop supplying COVID treatments, and pharmacies will purchase and bill for them the same way they do for antibiotic pills or asthma inhalers. Paxlovid is expected to hit the private market in mid-2023, according to HHS plans shared in an October meeting with state health officials and clinicians. Merck’s Lagevrio, a less-effective COVID treatment pill, and AstraZeneca’s Evusheld, a preventive therapy for the immunocompromised, are on track to be commercialized sooner, sometime in the winter.
The U.S. government has so far purchased 20 million courses of Paxlovid, priced at about $530 each, a discount for buying in bulk that Pfizer CEO Albert Bourla called “really very attractive” to the federal government in a July earnings call. The drug will cost far more on the private market, although in a statement to Kaiser Health News, Pfizer declined to share the planned price. The government will also stop paying for the company’s COVID vaccine next year – those shots will quadruple in price, from the discount rate the government pays of $30 to about $120.
Mr. Bourla told investors in November that he expects the move will make Paxlovid and its COVID vaccine “a multibillion-dollars franchise.”
Nearly 9 in 10 people dying from the virus now are 65 or older. Yet federal law restricts Medicare Part D – the prescription drug program that covers nearly 50 million seniors – from covering the COVID treatment pills. The medications are meant for those most at risk of serious illness, including seniors.
Paxlovid and the other treatments are currently available under an emergency use authorization from the FDA, a fast-track review used in extraordinary situations. Although Pfizer applied for full approval in June, the process can take anywhere from several months to years. And Medicare Part D can’t cover any medications without that full stamp of approval.
Paying out-of-pocket would be “a substantial barrier” for seniors on Medicare – the very people who would benefit most from the drug, wrote federal health experts.
“From a public health perspective, and even from a health care capacity and cost perspective, it would just defy reason to not continue to make these drugs readily available,” said Dr. Larry Madoff, medical director of Massachusetts’s Bureau of Infectious Disease and Laboratory Sciences. He’s hopeful that the federal health agency will find a way to set aside unused doses for seniors and people without insurance.
In mid-November, the White House requested that Congress approve an additional $2.5 billion for COVID therapeutics and vaccines to make sure people can afford the medications when they’re no longer free. But there’s little hope it will be approved – the Senate voted that same day to end the public health emergency and denied similar requests in recent months.
Many Americans have already faced hurdles just getting a prescription for COVID treatment. Although the federal government doesn’t track who’s gotten the drug, a Centers for Disease Control and Prevention study using data from 30 medical centers found that Black and Hispanic patients with COVID were much less likely to receive Paxlovid than White patients. (Hispanic people can be of any race or combination of races.) And when the government is no longer picking up the tab, experts predict that these gaps by race, income, and geography will widen.
People in Northeastern states used the drug far more often than those in the rest of the country, according to a KHN analysis of Paxlovid use in September and October. But it wasn’t because people in the region were getting sick from COVID at much higher rates – instead, many of those states offered better access to health care to begin with and created special programs to get Paxlovid to their residents.
About 10 mostly Democratic states and several large counties in the Northeast and elsewhere created free “test-to-treat” programs that allow their residents to get an immediate doctor visit and prescription for treatment after testing positive for COVID. In Massachusetts, more than 20,000 residents have used the state’s video and phone hotline, which is available 7 days a week in 13 languages. Massachusetts, which has the highest insurance rate in the country and relatively low travel times to pharmacies, had the second-highest Paxlovid usage rate among states this fall.
States with higher COVID death rates, like Florida and Kentucky, where residents must travel farther for health care and are more likely to be uninsured, used the drug less often. Without no-cost test-to-treat options, residents have struggled to get prescriptions even though the drug itself is still free.
“If you look at access to medications for people who are uninsured, I think that there’s no question that will widen those disparities,” Ms. Rosenthal said.
People who get insurance through their jobs could face high copays at the register, too, just as they do for insulin and other expensive or brand-name drugs.
Most private insurance companies will end up covering COVID therapeutics to some extent, said Sabrina Corlette, a research professor at Georgetown University’s Center on Health Insurance Reforms. After all, the pills are cheaper than a hospital stay. But for most people who get insurance through their jobs, there are “really no rules at all,” she said. Some insurers could take months to add the drugs to their plans or decide not to pay for them.
And the additional cost means many people will go without the medication. “We know from lots of research that when people face cost sharing for these drugs that they need to take, they will often forgo or cut back,” Ms. Corlette said.
One group doesn’t need to worry about sticker shock. Medicaid, the public insurance program for low-income adults and children, will cover the treatments in full until at least early 2024.
HHS officials could set aside any leftover taxpayer-funded medication for people who can’t afford to pay the full cost, but they haven’t shared any concrete plans to do so. The government purchased 20 million courses of Paxlovid and 3 million of Lagevrio. Fewer than a third have been used, and usage has fallen in recent months, according to KHN’s analysis of the data from HHS.
Sixty percent of the government’s supply of Evusheld is also still available, although the COVID prevention therapy is less effective against new strains of the virus. The health department in one state, New Mexico, has recommended against using it.
HHS did not make officials available for an interview or answer written questions about the commercialization plans.
The government created a potential workaround when they moved bebtelovimab, another COVID treatment, to the private market this summer. It now retails for $2,100 per patient. The agency set aside the remaining 60,000 government-purchased doses that hospitals could use to treat uninsured patients in a convoluted dose-replacement process. But it’s hard to tell how well that setup would work for Paxlovid: Bebtelovimab was already much less popular, and the FDA halted its use on Nov. 30 because it’s less effective against current strains of the virus.
Federal officials and insurance companies would have good reason to make sure patients can continue to afford COVID drugs: They’re far cheaper than if patients land in the emergency room.
“The medications are so worthwhile,” said Dr. Madoff, the Massachusetts health official. “They’re not expensive in the grand scheme of health care costs.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Nearly 6 million Americans have taken Paxlovid for free, courtesy of the federal government. The Pfizer pill has helped prevent many people infected with COVID-19 from being hospitalized or dying, and it may even reduce the risk of developing long COVID.
And that means fewer people will get the potentially lifesaving treatments, experts said.
“I think the numbers will go way down,” said Jill Rosenthal, director of public health policy at the Center for American Progress, a left-leaning think tank. A bill for several hundred dollars or more would lead many people to decide the medication isn’t worth the price, she said.
In response to the unprecedented public health crisis caused by COVID, the federal government spent billions of dollars on developing new vaccines and treatments, to swift success: Less than a year after the pandemic was declared, medical workers got their first vaccines. But as many people have refused the shots and stopped wearing masks, the virus still rages and mutates. In 2022 alone, 250,000 Americans have died from COVID, more than from strokes or diabetes.
But soon the Department of Health & Human Services will stop supplying COVID treatments, and pharmacies will purchase and bill for them the same way they do for antibiotic pills or asthma inhalers. Paxlovid is expected to hit the private market in mid-2023, according to HHS plans shared in an October meeting with state health officials and clinicians. Merck’s Lagevrio, a less-effective COVID treatment pill, and AstraZeneca’s Evusheld, a preventive therapy for the immunocompromised, are on track to be commercialized sooner, sometime in the winter.
The U.S. government has so far purchased 20 million courses of Paxlovid, priced at about $530 each, a discount for buying in bulk that Pfizer CEO Albert Bourla called “really very attractive” to the federal government in a July earnings call. The drug will cost far more on the private market, although in a statement to Kaiser Health News, Pfizer declined to share the planned price. The government will also stop paying for the company’s COVID vaccine next year – those shots will quadruple in price, from the discount rate the government pays of $30 to about $120.
Mr. Bourla told investors in November that he expects the move will make Paxlovid and its COVID vaccine “a multibillion-dollars franchise.”
Nearly 9 in 10 people dying from the virus now are 65 or older. Yet federal law restricts Medicare Part D – the prescription drug program that covers nearly 50 million seniors – from covering the COVID treatment pills. The medications are meant for those most at risk of serious illness, including seniors.
Paxlovid and the other treatments are currently available under an emergency use authorization from the FDA, a fast-track review used in extraordinary situations. Although Pfizer applied for full approval in June, the process can take anywhere from several months to years. And Medicare Part D can’t cover any medications without that full stamp of approval.
Paying out-of-pocket would be “a substantial barrier” for seniors on Medicare – the very people who would benefit most from the drug, wrote federal health experts.
“From a public health perspective, and even from a health care capacity and cost perspective, it would just defy reason to not continue to make these drugs readily available,” said Dr. Larry Madoff, medical director of Massachusetts’s Bureau of Infectious Disease and Laboratory Sciences. He’s hopeful that the federal health agency will find a way to set aside unused doses for seniors and people without insurance.
In mid-November, the White House requested that Congress approve an additional $2.5 billion for COVID therapeutics and vaccines to make sure people can afford the medications when they’re no longer free. But there’s little hope it will be approved – the Senate voted that same day to end the public health emergency and denied similar requests in recent months.
Many Americans have already faced hurdles just getting a prescription for COVID treatment. Although the federal government doesn’t track who’s gotten the drug, a Centers for Disease Control and Prevention study using data from 30 medical centers found that Black and Hispanic patients with COVID were much less likely to receive Paxlovid than White patients. (Hispanic people can be of any race or combination of races.) And when the government is no longer picking up the tab, experts predict that these gaps by race, income, and geography will widen.
People in Northeastern states used the drug far more often than those in the rest of the country, according to a KHN analysis of Paxlovid use in September and October. But it wasn’t because people in the region were getting sick from COVID at much higher rates – instead, many of those states offered better access to health care to begin with and created special programs to get Paxlovid to their residents.
About 10 mostly Democratic states and several large counties in the Northeast and elsewhere created free “test-to-treat” programs that allow their residents to get an immediate doctor visit and prescription for treatment after testing positive for COVID. In Massachusetts, more than 20,000 residents have used the state’s video and phone hotline, which is available 7 days a week in 13 languages. Massachusetts, which has the highest insurance rate in the country and relatively low travel times to pharmacies, had the second-highest Paxlovid usage rate among states this fall.
States with higher COVID death rates, like Florida and Kentucky, where residents must travel farther for health care and are more likely to be uninsured, used the drug less often. Without no-cost test-to-treat options, residents have struggled to get prescriptions even though the drug itself is still free.
“If you look at access to medications for people who are uninsured, I think that there’s no question that will widen those disparities,” Ms. Rosenthal said.
People who get insurance through their jobs could face high copays at the register, too, just as they do for insulin and other expensive or brand-name drugs.
Most private insurance companies will end up covering COVID therapeutics to some extent, said Sabrina Corlette, a research professor at Georgetown University’s Center on Health Insurance Reforms. After all, the pills are cheaper than a hospital stay. But for most people who get insurance through their jobs, there are “really no rules at all,” she said. Some insurers could take months to add the drugs to their plans or decide not to pay for them.
And the additional cost means many people will go without the medication. “We know from lots of research that when people face cost sharing for these drugs that they need to take, they will often forgo or cut back,” Ms. Corlette said.
One group doesn’t need to worry about sticker shock. Medicaid, the public insurance program for low-income adults and children, will cover the treatments in full until at least early 2024.
HHS officials could set aside any leftover taxpayer-funded medication for people who can’t afford to pay the full cost, but they haven’t shared any concrete plans to do so. The government purchased 20 million courses of Paxlovid and 3 million of Lagevrio. Fewer than a third have been used, and usage has fallen in recent months, according to KHN’s analysis of the data from HHS.
Sixty percent of the government’s supply of Evusheld is also still available, although the COVID prevention therapy is less effective against new strains of the virus. The health department in one state, New Mexico, has recommended against using it.
HHS did not make officials available for an interview or answer written questions about the commercialization plans.
The government created a potential workaround when they moved bebtelovimab, another COVID treatment, to the private market this summer. It now retails for $2,100 per patient. The agency set aside the remaining 60,000 government-purchased doses that hospitals could use to treat uninsured patients in a convoluted dose-replacement process. But it’s hard to tell how well that setup would work for Paxlovid: Bebtelovimab was already much less popular, and the FDA halted its use on Nov. 30 because it’s less effective against current strains of the virus.
Federal officials and insurance companies would have good reason to make sure patients can continue to afford COVID drugs: They’re far cheaper than if patients land in the emergency room.
“The medications are so worthwhile,” said Dr. Madoff, the Massachusetts health official. “They’re not expensive in the grand scheme of health care costs.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.