User login
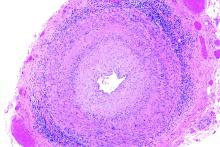
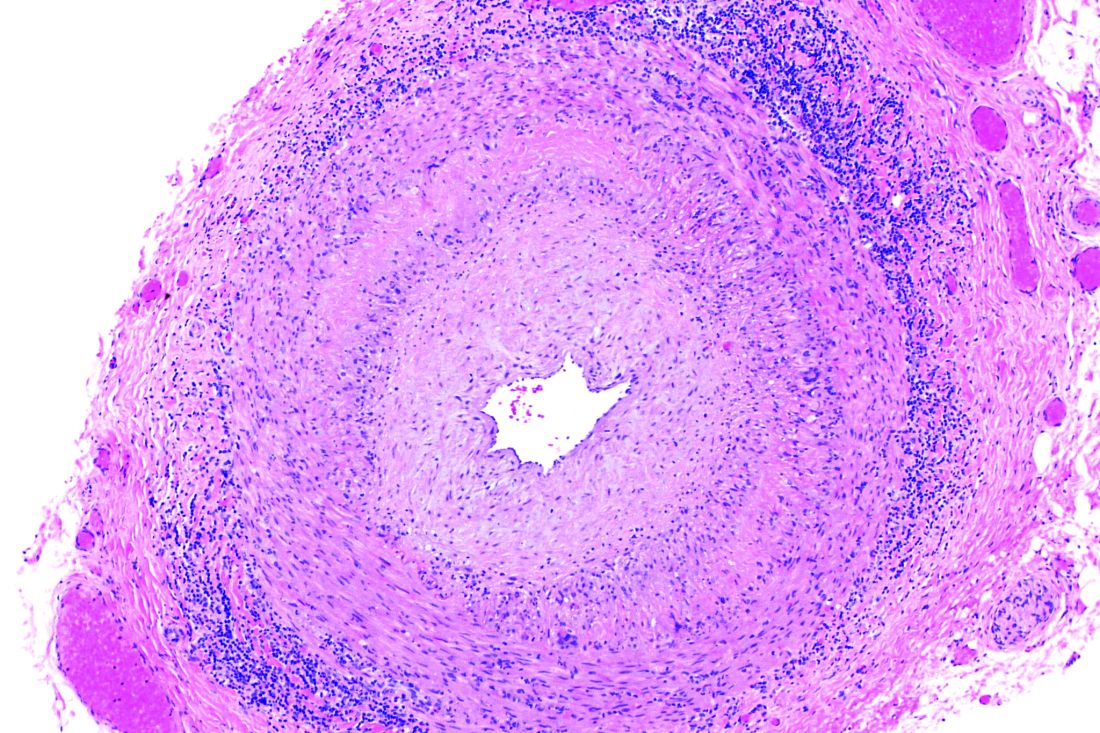
White and black patients have similar rates of giant cell arteritis
To determine the incidence of biopsy-proven GCA (BP-GCA) in a racially diverse cohort, Anna M. Gruener of Nottingham (England) University Hospitals NHS Trust and coauthors analyzed the medical records of more than 10 years of patients who underwent temporal artery biopsy at Johns Hopkins Wilmer Eye Institute in Baltimore. Of the 586 patients in the study, 167 (28.5%) were black, 382 (65.2%) were white, and 37 (6.3%) were other or unknown. The mean age was 70.5 years.
Of the 573 patients who were aged 50 years and older, 92 (16.1%) had a positive biopsy for BP-GCA; 14 were black (8.4% of all black patients), 75 were white (19.6% of all white patients), and 3 were other or unknown. The population-adjusted, age- and sex-standardized incidence rates per 100,000 were 3.1 (95% confidence interval, 1.0-5.2) for black patients and 3.6 (95% CI, 2.5-4.7) for white patients.
Overall, BP-GCA occurred more frequently in women than in men (incidence rate ratio, 1.9; 95% CI, 1.1-3.4; P = .03) but at similar levels in white and black patients (IRR, 1.2; 95% CI, 0.6-2.4; P = .66).
In an accompanying editorial, Michael K. Yoon, MD, and Joseph F. Rizzo III, MD, of Harvard Medical School, Boston, praised the researchers for conducting their study in a population with a large percentage of black patients, a noted weakness of earlier studies in this area (JAMA Ophthalmol. 2019 Aug 8. doi: 10.1001/jamaophthalmol.2019.2933). That said, the two doctors also recognized the limitations of the work done by Gruener et al., including relying on U.S. Census data to calculate adjusted incidence rates instead of local racial distribution and also the potentially problematic choice to count patients with healed arteritis as having BP-GCA.
Still, Dr. Yoon and Dr. Rizzo commended Gruener et al. for questioning previous findings on GCA rates. “Although the authors’ methods are imperfect,” they wrote, “the studies that had previously established a low incidence of GCA in black patients were also flawed in design.”
The study had no outside funding source, and no conflicts of interest were reported.
SOURCE: Gruener AM et al. JAMA Ophthalmol. 2019 Aug 8. doi: 10.1001/jamaophthalmol.2019.2919.
To determine the incidence of biopsy-proven GCA (BP-GCA) in a racially diverse cohort, Anna M. Gruener of Nottingham (England) University Hospitals NHS Trust and coauthors analyzed the medical records of more than 10 years of patients who underwent temporal artery biopsy at Johns Hopkins Wilmer Eye Institute in Baltimore. Of the 586 patients in the study, 167 (28.5%) were black, 382 (65.2%) were white, and 37 (6.3%) were other or unknown. The mean age was 70.5 years.
Of the 573 patients who were aged 50 years and older, 92 (16.1%) had a positive biopsy for BP-GCA; 14 were black (8.4% of all black patients), 75 were white (19.6% of all white patients), and 3 were other or unknown. The population-adjusted, age- and sex-standardized incidence rates per 100,000 were 3.1 (95% confidence interval, 1.0-5.2) for black patients and 3.6 (95% CI, 2.5-4.7) for white patients.
Overall, BP-GCA occurred more frequently in women than in men (incidence rate ratio, 1.9; 95% CI, 1.1-3.4; P = .03) but at similar levels in white and black patients (IRR, 1.2; 95% CI, 0.6-2.4; P = .66).
In an accompanying editorial, Michael K. Yoon, MD, and Joseph F. Rizzo III, MD, of Harvard Medical School, Boston, praised the researchers for conducting their study in a population with a large percentage of black patients, a noted weakness of earlier studies in this area (JAMA Ophthalmol. 2019 Aug 8. doi: 10.1001/jamaophthalmol.2019.2933). That said, the two doctors also recognized the limitations of the work done by Gruener et al., including relying on U.S. Census data to calculate adjusted incidence rates instead of local racial distribution and also the potentially problematic choice to count patients with healed arteritis as having BP-GCA.
Still, Dr. Yoon and Dr. Rizzo commended Gruener et al. for questioning previous findings on GCA rates. “Although the authors’ methods are imperfect,” they wrote, “the studies that had previously established a low incidence of GCA in black patients were also flawed in design.”
The study had no outside funding source, and no conflicts of interest were reported.
SOURCE: Gruener AM et al. JAMA Ophthalmol. 2019 Aug 8. doi: 10.1001/jamaophthalmol.2019.2919.
To determine the incidence of biopsy-proven GCA (BP-GCA) in a racially diverse cohort, Anna M. Gruener of Nottingham (England) University Hospitals NHS Trust and coauthors analyzed the medical records of more than 10 years of patients who underwent temporal artery biopsy at Johns Hopkins Wilmer Eye Institute in Baltimore. Of the 586 patients in the study, 167 (28.5%) were black, 382 (65.2%) were white, and 37 (6.3%) were other or unknown. The mean age was 70.5 years.
Of the 573 patients who were aged 50 years and older, 92 (16.1%) had a positive biopsy for BP-GCA; 14 were black (8.4% of all black patients), 75 were white (19.6% of all white patients), and 3 were other or unknown. The population-adjusted, age- and sex-standardized incidence rates per 100,000 were 3.1 (95% confidence interval, 1.0-5.2) for black patients and 3.6 (95% CI, 2.5-4.7) for white patients.
Overall, BP-GCA occurred more frequently in women than in men (incidence rate ratio, 1.9; 95% CI, 1.1-3.4; P = .03) but at similar levels in white and black patients (IRR, 1.2; 95% CI, 0.6-2.4; P = .66).
In an accompanying editorial, Michael K. Yoon, MD, and Joseph F. Rizzo III, MD, of Harvard Medical School, Boston, praised the researchers for conducting their study in a population with a large percentage of black patients, a noted weakness of earlier studies in this area (JAMA Ophthalmol. 2019 Aug 8. doi: 10.1001/jamaophthalmol.2019.2933). That said, the two doctors also recognized the limitations of the work done by Gruener et al., including relying on U.S. Census data to calculate adjusted incidence rates instead of local racial distribution and also the potentially problematic choice to count patients with healed arteritis as having BP-GCA.
Still, Dr. Yoon and Dr. Rizzo commended Gruener et al. for questioning previous findings on GCA rates. “Although the authors’ methods are imperfect,” they wrote, “the studies that had previously established a low incidence of GCA in black patients were also flawed in design.”
The study had no outside funding source, and no conflicts of interest were reported.
SOURCE: Gruener AM et al. JAMA Ophthalmol. 2019 Aug 8. doi: 10.1001/jamaophthalmol.2019.2919.
FROM JAMA OPHTHALMOLOGY
Professional coaching keeps doctors in the game
Physicians who receive professional coaching are less emotionally exhausted and less vulnerable to burnout, according to the results of a pilot study.
“This intervention adds to the growing literature of evidence-based approaches to promote physician well-being and should be considered a complementary strategy to be deployed in combination with other organizational approaches to improve system-level drivers of work-related stressors,” wrote Liselotte N. Dyrbye, MD, of the Mayo Clinic in Rochester, Minn., and coauthors in JAMA Internal Medicine.
Dr. Dyrbye and colleagues conducted a randomized pilot study of 88 Mayo Clinic physicians in the departments of medicine, family medicine, and pediatrics. Half (n = 44) received 3.5 hours of sessions facilitated by a professional coach. The other half (n = 44) served as controls. Participants’ well-being – in regard to burnout, quality of life, resilience, job satisfaction, engagement, and meaning at work – was surveyed at baseline and the study’s completion.
Physicians in the coaching group participated in a 1-hour initial telephone session, designed to establish a relationship between the physician and coach, as well as to assess needs, set goals, identify values, and create an action plan. During follow-up sessions, coaches would check in, help plan and set goals, and suggest strategies/changes to incorporate into daily life. Physicians were permitted to ask for support on any issue, but also were expected to see as many patients as their colleagues outside of the study.
After 6 months, physicians in the coaching group saw a significant decrease in emotional exhaustion by a mean of 5.2 points, compared with an increase of 1.5 points in the control group. At 5 months, absolute rates of high emotional exhaustion decreased by 19.5% in the coaching group and increased by 9.8% in the control group and absolute rates of overall burnout decreased by 17.1% in the coaching group and increased by 4.9% in the control group. Quality of life and resilience scores also improved, though there were no notable differences between groups in measures of job satisfaction, engagement, and meaning at work.
The authors noted their study’s limitations, which included a modest sample size and a volunteer group of participants.
In addition, the lower percentage of men in the study – 48 of 88 participants were women – may be a result of factors that deserve further investigation. Finally, burnout rates among volunteers were higher than those among other physicians, suggesting that “the study appealed to those in greatest need of the intervention.”
The study was funded by the Mayo Clinic department of medicine’s Program on Physician Well-Being and the Physician Foundation. Two of the authors – Dr. Dyrbye and Tait D. Shanafelt, MD, of Stanford (Calif.) University – reported being the coinventors of, and receiving royalties for, the Physician Well-Being Index, Medical Student Well-Being Index, Nurse Well-Being Index, and the Well-Being Index.
SOURCE: Dyrbye LN et al. JAMA Intern Med. 2019 Aug 5. doi: 10.1001/jamainternmed.2019.2425.
Physicians who receive professional coaching are less emotionally exhausted and less vulnerable to burnout, according to the results of a pilot study.
“This intervention adds to the growing literature of evidence-based approaches to promote physician well-being and should be considered a complementary strategy to be deployed in combination with other organizational approaches to improve system-level drivers of work-related stressors,” wrote Liselotte N. Dyrbye, MD, of the Mayo Clinic in Rochester, Minn., and coauthors in JAMA Internal Medicine.
Dr. Dyrbye and colleagues conducted a randomized pilot study of 88 Mayo Clinic physicians in the departments of medicine, family medicine, and pediatrics. Half (n = 44) received 3.5 hours of sessions facilitated by a professional coach. The other half (n = 44) served as controls. Participants’ well-being – in regard to burnout, quality of life, resilience, job satisfaction, engagement, and meaning at work – was surveyed at baseline and the study’s completion.
Physicians in the coaching group participated in a 1-hour initial telephone session, designed to establish a relationship between the physician and coach, as well as to assess needs, set goals, identify values, and create an action plan. During follow-up sessions, coaches would check in, help plan and set goals, and suggest strategies/changes to incorporate into daily life. Physicians were permitted to ask for support on any issue, but also were expected to see as many patients as their colleagues outside of the study.
After 6 months, physicians in the coaching group saw a significant decrease in emotional exhaustion by a mean of 5.2 points, compared with an increase of 1.5 points in the control group. At 5 months, absolute rates of high emotional exhaustion decreased by 19.5% in the coaching group and increased by 9.8% in the control group and absolute rates of overall burnout decreased by 17.1% in the coaching group and increased by 4.9% in the control group. Quality of life and resilience scores also improved, though there were no notable differences between groups in measures of job satisfaction, engagement, and meaning at work.
The authors noted their study’s limitations, which included a modest sample size and a volunteer group of participants.
In addition, the lower percentage of men in the study – 48 of 88 participants were women – may be a result of factors that deserve further investigation. Finally, burnout rates among volunteers were higher than those among other physicians, suggesting that “the study appealed to those in greatest need of the intervention.”
The study was funded by the Mayo Clinic department of medicine’s Program on Physician Well-Being and the Physician Foundation. Two of the authors – Dr. Dyrbye and Tait D. Shanafelt, MD, of Stanford (Calif.) University – reported being the coinventors of, and receiving royalties for, the Physician Well-Being Index, Medical Student Well-Being Index, Nurse Well-Being Index, and the Well-Being Index.
SOURCE: Dyrbye LN et al. JAMA Intern Med. 2019 Aug 5. doi: 10.1001/jamainternmed.2019.2425.
Physicians who receive professional coaching are less emotionally exhausted and less vulnerable to burnout, according to the results of a pilot study.
“This intervention adds to the growing literature of evidence-based approaches to promote physician well-being and should be considered a complementary strategy to be deployed in combination with other organizational approaches to improve system-level drivers of work-related stressors,” wrote Liselotte N. Dyrbye, MD, of the Mayo Clinic in Rochester, Minn., and coauthors in JAMA Internal Medicine.
Dr. Dyrbye and colleagues conducted a randomized pilot study of 88 Mayo Clinic physicians in the departments of medicine, family medicine, and pediatrics. Half (n = 44) received 3.5 hours of sessions facilitated by a professional coach. The other half (n = 44) served as controls. Participants’ well-being – in regard to burnout, quality of life, resilience, job satisfaction, engagement, and meaning at work – was surveyed at baseline and the study’s completion.
Physicians in the coaching group participated in a 1-hour initial telephone session, designed to establish a relationship between the physician and coach, as well as to assess needs, set goals, identify values, and create an action plan. During follow-up sessions, coaches would check in, help plan and set goals, and suggest strategies/changes to incorporate into daily life. Physicians were permitted to ask for support on any issue, but also were expected to see as many patients as their colleagues outside of the study.
After 6 months, physicians in the coaching group saw a significant decrease in emotional exhaustion by a mean of 5.2 points, compared with an increase of 1.5 points in the control group. At 5 months, absolute rates of high emotional exhaustion decreased by 19.5% in the coaching group and increased by 9.8% in the control group and absolute rates of overall burnout decreased by 17.1% in the coaching group and increased by 4.9% in the control group. Quality of life and resilience scores also improved, though there were no notable differences between groups in measures of job satisfaction, engagement, and meaning at work.
The authors noted their study’s limitations, which included a modest sample size and a volunteer group of participants.
In addition, the lower percentage of men in the study – 48 of 88 participants were women – may be a result of factors that deserve further investigation. Finally, burnout rates among volunteers were higher than those among other physicians, suggesting that “the study appealed to those in greatest need of the intervention.”
The study was funded by the Mayo Clinic department of medicine’s Program on Physician Well-Being and the Physician Foundation. Two of the authors – Dr. Dyrbye and Tait D. Shanafelt, MD, of Stanford (Calif.) University – reported being the coinventors of, and receiving royalties for, the Physician Well-Being Index, Medical Student Well-Being Index, Nurse Well-Being Index, and the Well-Being Index.
SOURCE: Dyrbye LN et al. JAMA Intern Med. 2019 Aug 5. doi: 10.1001/jamainternmed.2019.2425.
FROM JAMA INTERNAL MEDICINE
Older patients who stop statins may be increasing their cardiovascular risk
Discontinuing statins was associated with an increased risk of hospital admission for a cardiovascular event, according to a study of elderly French patients with no history of heart disease.
“The results of this study suggest potential cardiovascular risk reduction associated with continuing statin therapy after the age of 75 years in persons already taking these drugs for primary prevention,” wrote Philippe Giral, MD, of Hôpital La Pitié Salpêtrière (France) and coauthors. The study was published in the European Heart Journal.
To determine if statins are a cardiovascular benefit or detriment to older people, the researchers reviewed data from 120,173 patients in French health care databases who turned 75 during 2012-2014. Patients with a diagnosis of cardiovascular disease in the previous 2 years were excluded, and all eligible patients were required to have a statin medication possession ratio of at least 80% in each of the previous 2 years.
Over a follow-up period that averaged 2.4 years, 17,204 patients (14.3%) discontinued statins and 5,396 (4.5%) were admitted for a cardiovascular event. The adjusted hazard ratios for admissions after statin discontinuation were 1.33 (95% confidence interval, 1.18-1.50) for a cardiovascular event, 1.46 (95% CI, 1.21-1.75) for a coronary event, 1.26 (95% CI, 1.05-1.51) for a cerebrovascular event, and 1.02 (95% CI, 0.74-1.40) for other vascular events, respectively.
The coauthors acknowledged their study’s limitations, including being unable to account for certain cardiovascular risk factors such as baseline LDL cholesterol level, tobacco use, obesity, and frailty markers. In addition, no information was available as to why patients discontinued statins. However, the presence of other major cardiovascular risk factors was investigated and accounted for, as was discontinuation of other cardiovascular drug therapies.
The study was not funded, and the authors declared no conflicts of interest.
SOURCE: Giral P at al. Eur Heart J. 2019 July 31. doi: 10.1093/eurheartj/ehz458.
Discontinuing statins was associated with an increased risk of hospital admission for a cardiovascular event, according to a study of elderly French patients with no history of heart disease.
“The results of this study suggest potential cardiovascular risk reduction associated with continuing statin therapy after the age of 75 years in persons already taking these drugs for primary prevention,” wrote Philippe Giral, MD, of Hôpital La Pitié Salpêtrière (France) and coauthors. The study was published in the European Heart Journal.
To determine if statins are a cardiovascular benefit or detriment to older people, the researchers reviewed data from 120,173 patients in French health care databases who turned 75 during 2012-2014. Patients with a diagnosis of cardiovascular disease in the previous 2 years were excluded, and all eligible patients were required to have a statin medication possession ratio of at least 80% in each of the previous 2 years.
Over a follow-up period that averaged 2.4 years, 17,204 patients (14.3%) discontinued statins and 5,396 (4.5%) were admitted for a cardiovascular event. The adjusted hazard ratios for admissions after statin discontinuation were 1.33 (95% confidence interval, 1.18-1.50) for a cardiovascular event, 1.46 (95% CI, 1.21-1.75) for a coronary event, 1.26 (95% CI, 1.05-1.51) for a cerebrovascular event, and 1.02 (95% CI, 0.74-1.40) for other vascular events, respectively.
The coauthors acknowledged their study’s limitations, including being unable to account for certain cardiovascular risk factors such as baseline LDL cholesterol level, tobacco use, obesity, and frailty markers. In addition, no information was available as to why patients discontinued statins. However, the presence of other major cardiovascular risk factors was investigated and accounted for, as was discontinuation of other cardiovascular drug therapies.
The study was not funded, and the authors declared no conflicts of interest.
SOURCE: Giral P at al. Eur Heart J. 2019 July 31. doi: 10.1093/eurheartj/ehz458.
Discontinuing statins was associated with an increased risk of hospital admission for a cardiovascular event, according to a study of elderly French patients with no history of heart disease.
“The results of this study suggest potential cardiovascular risk reduction associated with continuing statin therapy after the age of 75 years in persons already taking these drugs for primary prevention,” wrote Philippe Giral, MD, of Hôpital La Pitié Salpêtrière (France) and coauthors. The study was published in the European Heart Journal.
To determine if statins are a cardiovascular benefit or detriment to older people, the researchers reviewed data from 120,173 patients in French health care databases who turned 75 during 2012-2014. Patients with a diagnosis of cardiovascular disease in the previous 2 years were excluded, and all eligible patients were required to have a statin medication possession ratio of at least 80% in each of the previous 2 years.
Over a follow-up period that averaged 2.4 years, 17,204 patients (14.3%) discontinued statins and 5,396 (4.5%) were admitted for a cardiovascular event. The adjusted hazard ratios for admissions after statin discontinuation were 1.33 (95% confidence interval, 1.18-1.50) for a cardiovascular event, 1.46 (95% CI, 1.21-1.75) for a coronary event, 1.26 (95% CI, 1.05-1.51) for a cerebrovascular event, and 1.02 (95% CI, 0.74-1.40) for other vascular events, respectively.
The coauthors acknowledged their study’s limitations, including being unable to account for certain cardiovascular risk factors such as baseline LDL cholesterol level, tobacco use, obesity, and frailty markers. In addition, no information was available as to why patients discontinued statins. However, the presence of other major cardiovascular risk factors was investigated and accounted for, as was discontinuation of other cardiovascular drug therapies.
The study was not funded, and the authors declared no conflicts of interest.
SOURCE: Giral P at al. Eur Heart J. 2019 July 31. doi: 10.1093/eurheartj/ehz458.
FROM THE EUROPEAN HEART JOURNAL
Inadequate glycemic control in type 1 diabetes leads to increased fracture risk
A single percentage increase in the level of hemoglobin A1c (HbA1c) in patients with newly diagnosed type 1 diabetes is significantly associated with an increase in fracture risk, according to findings in a study published in Diabetic Medicine.
To determine the effect of glycemic control on fracture risk, Rasiah Thayakaran, PhD, of the University of Birmingham (England) and colleagues analyzed data from 5,368 patients with newly diagnosed type 1 diabetes in the United Kingdom. HbA1c measurements were collected until either fracture or the end of the study, and were then converted from percentages to mmol/mol. Patient age ranged between 1 and 60 years, and the mean age was 22 years.
During 37,830 person‐years of follow‐up, 525 fractures were observed, with an incidence rate of 14 per 1,000 person‐years. The rate among men was 15 per 1,000 person‐years, compared with 12 per 1,000 person‐years among women. There was a significant association between hemoglobin level and risk of fractures (adjusted hazard ratio, 1.007 mmol/mol; 95% confidence interval, 1.002-1.011 mmol/mol), representing an increase of 7% in risk for fracture for each percentage increase in hemoglobin level.
“When assessing an individual with newly diagnosed type 1 diabetes and high HbA1c, increased clinical awareness about the fracture risk may be incorporated in decision‐making regarding the clinical management and even in prompting early antiosteoporotic intervention,” Dr. Thayakaran and coauthors wrote.
The researchers acknowledged the study’s limitations, including a possibility of residual confounding because of their use of observational data. In addition, they could not confirm whether the increase in fracture risk should be attributed to bone fragility or to increased risk of falls. Finally, though they noted using a comprehensive list of codes to identify fractures, they could not verify “completeness of recording ... and therefore reported overall fracture incidence should be interpreted with caution.”
The study was not funded. The authors reported no conflicts of interest.
SOURCE: Thayakaran R et al. Diab Med. 2019 Mar 8. doi: 10.1111/dme.13945.
A single percentage increase in the level of hemoglobin A1c (HbA1c) in patients with newly diagnosed type 1 diabetes is significantly associated with an increase in fracture risk, according to findings in a study published in Diabetic Medicine.
To determine the effect of glycemic control on fracture risk, Rasiah Thayakaran, PhD, of the University of Birmingham (England) and colleagues analyzed data from 5,368 patients with newly diagnosed type 1 diabetes in the United Kingdom. HbA1c measurements were collected until either fracture or the end of the study, and were then converted from percentages to mmol/mol. Patient age ranged between 1 and 60 years, and the mean age was 22 years.
During 37,830 person‐years of follow‐up, 525 fractures were observed, with an incidence rate of 14 per 1,000 person‐years. The rate among men was 15 per 1,000 person‐years, compared with 12 per 1,000 person‐years among women. There was a significant association between hemoglobin level and risk of fractures (adjusted hazard ratio, 1.007 mmol/mol; 95% confidence interval, 1.002-1.011 mmol/mol), representing an increase of 7% in risk for fracture for each percentage increase in hemoglobin level.
“When assessing an individual with newly diagnosed type 1 diabetes and high HbA1c, increased clinical awareness about the fracture risk may be incorporated in decision‐making regarding the clinical management and even in prompting early antiosteoporotic intervention,” Dr. Thayakaran and coauthors wrote.
The researchers acknowledged the study’s limitations, including a possibility of residual confounding because of their use of observational data. In addition, they could not confirm whether the increase in fracture risk should be attributed to bone fragility or to increased risk of falls. Finally, though they noted using a comprehensive list of codes to identify fractures, they could not verify “completeness of recording ... and therefore reported overall fracture incidence should be interpreted with caution.”
The study was not funded. The authors reported no conflicts of interest.
SOURCE: Thayakaran R et al. Diab Med. 2019 Mar 8. doi: 10.1111/dme.13945.
A single percentage increase in the level of hemoglobin A1c (HbA1c) in patients with newly diagnosed type 1 diabetes is significantly associated with an increase in fracture risk, according to findings in a study published in Diabetic Medicine.
To determine the effect of glycemic control on fracture risk, Rasiah Thayakaran, PhD, of the University of Birmingham (England) and colleagues analyzed data from 5,368 patients with newly diagnosed type 1 diabetes in the United Kingdom. HbA1c measurements were collected until either fracture or the end of the study, and were then converted from percentages to mmol/mol. Patient age ranged between 1 and 60 years, and the mean age was 22 years.
During 37,830 person‐years of follow‐up, 525 fractures were observed, with an incidence rate of 14 per 1,000 person‐years. The rate among men was 15 per 1,000 person‐years, compared with 12 per 1,000 person‐years among women. There was a significant association between hemoglobin level and risk of fractures (adjusted hazard ratio, 1.007 mmol/mol; 95% confidence interval, 1.002-1.011 mmol/mol), representing an increase of 7% in risk for fracture for each percentage increase in hemoglobin level.
“When assessing an individual with newly diagnosed type 1 diabetes and high HbA1c, increased clinical awareness about the fracture risk may be incorporated in decision‐making regarding the clinical management and even in prompting early antiosteoporotic intervention,” Dr. Thayakaran and coauthors wrote.
The researchers acknowledged the study’s limitations, including a possibility of residual confounding because of their use of observational data. In addition, they could not confirm whether the increase in fracture risk should be attributed to bone fragility or to increased risk of falls. Finally, though they noted using a comprehensive list of codes to identify fractures, they could not verify “completeness of recording ... and therefore reported overall fracture incidence should be interpreted with caution.”
The study was not funded. The authors reported no conflicts of interest.
SOURCE: Thayakaran R et al. Diab Med. 2019 Mar 8. doi: 10.1111/dme.13945.
FROM DIABETIC MEDICINE
First-time fathers at risk of postnatal depressive symptoms
First-time fathers may be at risk of experiencing depressive symptoms as they transition to parenthood – especially if risk factors such as poor sleep are present, results of a prospective study of more than 600 new fathers show.
“Strategies to promote better sleep, mobilize social support, and strengthen the couple relationship may be important to address in innovative interventions tailored to new fathers at risk for depression during the perinatal period,” wrote Deborah Da Costa, PhD, of McGill University, Montreal, and colleagues. The study was published in the Journal of Affective Disorders.
To determine the prevalence of depressive symptoms in first-time fathers and identify notable risk factors, the researchers surveyed 622 Canadian men during their partner’s third trimester. The same group was surveyed again at 2 and 6 months postpartum. Depression was assessed via the Edinburgh Postnatal Depression Scale (EPDS), and additional variables such as sleep quality, social support, and stress were gathered as well.
Of the initial 622 men surveyed, 487 (78.3%) and 375 (60.3%) completed the questionnaires at 2 and 6 months postpartum, respectively. The prevalence of paternal depressive symptoms was 13.76% (95% confidence interval, 10.70-16.82) at 2 months and 13.6% (95% CI, 10.13-17.07) at 6 months. Of the men who reported depressive symptoms at 2 months postpartum, 40.3% also experienced symptoms during the third trimester. Of the men who reported depressive symptoms at 6 months postpartum, 24% experienced symptoms during the third trimester and after 2 months.
At 2 months, the risk of depressive symptoms increased for men with worse sleep quality (odds ratio, 1.25; 95% CI, 1.10-1.42), poorer couple relationship adjustment (OR, 0.97; 95% CI, 0.94-0.99), and higher parenting stress (OR, 1.07; 95% CI, 1.02-1.11). At 6 months, there was a significant association between paternal depressive symptoms and unemployment (OR, 3.75; 95% CI, 1.00-13.72), poorer sleep quality (OR, 1.37; 95% CI, 1.16-1.65), lower social support (OR, 0.92; 95% CI, 0.84-1.00), poorer couple relationship adjustment (OR, 0.95; 95% CI, 0.92-0.98), and higher financial stress (OR, 1.21; 95% CI, 1.04-1.42).
The authors acknowledged their study’s limitations, including a middling response rate that could affect the accuracy of prevalence estimates and a well-educated, largely middle-class sample that could limit generalizability. In addition, they assessed depressive symptoms by self-report and not diagnostic clinical interviews. However, they also noted that “the EPDS is the most widely used tool to assess depressive symptoms in parents during the perinatal period and was validated in expectant and new fathers.”
The study was funded by the Canadian Institutes of Health Research. No conflicts of interest were reported.
SOURCE: Da Costa D et al. J Affect Disord. 2019 Apr 15;249:371-7.
First-time fathers may be at risk of experiencing depressive symptoms as they transition to parenthood – especially if risk factors such as poor sleep are present, results of a prospective study of more than 600 new fathers show.
“Strategies to promote better sleep, mobilize social support, and strengthen the couple relationship may be important to address in innovative interventions tailored to new fathers at risk for depression during the perinatal period,” wrote Deborah Da Costa, PhD, of McGill University, Montreal, and colleagues. The study was published in the Journal of Affective Disorders.
To determine the prevalence of depressive symptoms in first-time fathers and identify notable risk factors, the researchers surveyed 622 Canadian men during their partner’s third trimester. The same group was surveyed again at 2 and 6 months postpartum. Depression was assessed via the Edinburgh Postnatal Depression Scale (EPDS), and additional variables such as sleep quality, social support, and stress were gathered as well.
Of the initial 622 men surveyed, 487 (78.3%) and 375 (60.3%) completed the questionnaires at 2 and 6 months postpartum, respectively. The prevalence of paternal depressive symptoms was 13.76% (95% confidence interval, 10.70-16.82) at 2 months and 13.6% (95% CI, 10.13-17.07) at 6 months. Of the men who reported depressive symptoms at 2 months postpartum, 40.3% also experienced symptoms during the third trimester. Of the men who reported depressive symptoms at 6 months postpartum, 24% experienced symptoms during the third trimester and after 2 months.
At 2 months, the risk of depressive symptoms increased for men with worse sleep quality (odds ratio, 1.25; 95% CI, 1.10-1.42), poorer couple relationship adjustment (OR, 0.97; 95% CI, 0.94-0.99), and higher parenting stress (OR, 1.07; 95% CI, 1.02-1.11). At 6 months, there was a significant association between paternal depressive symptoms and unemployment (OR, 3.75; 95% CI, 1.00-13.72), poorer sleep quality (OR, 1.37; 95% CI, 1.16-1.65), lower social support (OR, 0.92; 95% CI, 0.84-1.00), poorer couple relationship adjustment (OR, 0.95; 95% CI, 0.92-0.98), and higher financial stress (OR, 1.21; 95% CI, 1.04-1.42).
The authors acknowledged their study’s limitations, including a middling response rate that could affect the accuracy of prevalence estimates and a well-educated, largely middle-class sample that could limit generalizability. In addition, they assessed depressive symptoms by self-report and not diagnostic clinical interviews. However, they also noted that “the EPDS is the most widely used tool to assess depressive symptoms in parents during the perinatal period and was validated in expectant and new fathers.”
The study was funded by the Canadian Institutes of Health Research. No conflicts of interest were reported.
SOURCE: Da Costa D et al. J Affect Disord. 2019 Apr 15;249:371-7.
First-time fathers may be at risk of experiencing depressive symptoms as they transition to parenthood – especially if risk factors such as poor sleep are present, results of a prospective study of more than 600 new fathers show.
“Strategies to promote better sleep, mobilize social support, and strengthen the couple relationship may be important to address in innovative interventions tailored to new fathers at risk for depression during the perinatal period,” wrote Deborah Da Costa, PhD, of McGill University, Montreal, and colleagues. The study was published in the Journal of Affective Disorders.
To determine the prevalence of depressive symptoms in first-time fathers and identify notable risk factors, the researchers surveyed 622 Canadian men during their partner’s third trimester. The same group was surveyed again at 2 and 6 months postpartum. Depression was assessed via the Edinburgh Postnatal Depression Scale (EPDS), and additional variables such as sleep quality, social support, and stress were gathered as well.
Of the initial 622 men surveyed, 487 (78.3%) and 375 (60.3%) completed the questionnaires at 2 and 6 months postpartum, respectively. The prevalence of paternal depressive symptoms was 13.76% (95% confidence interval, 10.70-16.82) at 2 months and 13.6% (95% CI, 10.13-17.07) at 6 months. Of the men who reported depressive symptoms at 2 months postpartum, 40.3% also experienced symptoms during the third trimester. Of the men who reported depressive symptoms at 6 months postpartum, 24% experienced symptoms during the third trimester and after 2 months.
At 2 months, the risk of depressive symptoms increased for men with worse sleep quality (odds ratio, 1.25; 95% CI, 1.10-1.42), poorer couple relationship adjustment (OR, 0.97; 95% CI, 0.94-0.99), and higher parenting stress (OR, 1.07; 95% CI, 1.02-1.11). At 6 months, there was a significant association between paternal depressive symptoms and unemployment (OR, 3.75; 95% CI, 1.00-13.72), poorer sleep quality (OR, 1.37; 95% CI, 1.16-1.65), lower social support (OR, 0.92; 95% CI, 0.84-1.00), poorer couple relationship adjustment (OR, 0.95; 95% CI, 0.92-0.98), and higher financial stress (OR, 1.21; 95% CI, 1.04-1.42).
The authors acknowledged their study’s limitations, including a middling response rate that could affect the accuracy of prevalence estimates and a well-educated, largely middle-class sample that could limit generalizability. In addition, they assessed depressive symptoms by self-report and not diagnostic clinical interviews. However, they also noted that “the EPDS is the most widely used tool to assess depressive symptoms in parents during the perinatal period and was validated in expectant and new fathers.”
The study was funded by the Canadian Institutes of Health Research. No conflicts of interest were reported.
SOURCE: Da Costa D et al. J Affect Disord. 2019 Apr 15;249:371-7.
FROM THE JOURNAL OF AFFECTIVE DISORDERS
For African Americans with MDD, more education means more benefits of friendships
A new analysis has found that, for African Americans with major depressive disorder (MDD), friendship’s mitigating benefits can vary based on education levels.
“These findings underscore the complexity of social support as a possible intervening process in depression,” wrote Ann W. Nguyen, PhD, of Case Western Reserve University in Cleveland, and her coauthors. The study was published in the Journal of Affective Disorders.
To determine how certain elements of friendship affect MDD, the researchers analyzed 3,434 responses from African Americans to the National Survey of American Life. They assessed variables such as “subjective closeness to friends,” “frequency of contact with friends,” “receipt of support from friends,” and “provision of support to friends” via responses to related questions, along with factoring in the impact of education level on 12-month MDD. Analysis was performed via logistic regression.
Among all respondents, the 12-month prevalence of MDD was 6.7%. Overall, subjective closeness and frequency of contact with friends were negatively associated with 12-month MDD. those with lower levels of education saw no association between frequency of contact and 12-month MDD. There was a similar association between receipt of support or provision of support and education: The high education group saw their probability of MDD decrease as receipt or provision of support increased.
The authors acknowledged their study’s limitations, including the impossibility of causal inferences because of its cross-sectional design. In addition, the survey only included community-dwelling adults, meaning its findings cannot be extended to institutionalized and homeless individuals. Also, each friendship variable was assessed through a single question. “Future research,” they noted, “should assess these relationship dimensions using multi-item scales, as they tend to be more stable, reliable, and precise.”
No conflicts of interest were reported.
SOURCE: Nguyen AW et al. J Affect Disord. 2019 Jun 15. doi: 10.1016/j.jad.2019.04.013
A new analysis has found that, for African Americans with major depressive disorder (MDD), friendship’s mitigating benefits can vary based on education levels.
“These findings underscore the complexity of social support as a possible intervening process in depression,” wrote Ann W. Nguyen, PhD, of Case Western Reserve University in Cleveland, and her coauthors. The study was published in the Journal of Affective Disorders.
To determine how certain elements of friendship affect MDD, the researchers analyzed 3,434 responses from African Americans to the National Survey of American Life. They assessed variables such as “subjective closeness to friends,” “frequency of contact with friends,” “receipt of support from friends,” and “provision of support to friends” via responses to related questions, along with factoring in the impact of education level on 12-month MDD. Analysis was performed via logistic regression.
Among all respondents, the 12-month prevalence of MDD was 6.7%. Overall, subjective closeness and frequency of contact with friends were negatively associated with 12-month MDD. those with lower levels of education saw no association between frequency of contact and 12-month MDD. There was a similar association between receipt of support or provision of support and education: The high education group saw their probability of MDD decrease as receipt or provision of support increased.
The authors acknowledged their study’s limitations, including the impossibility of causal inferences because of its cross-sectional design. In addition, the survey only included community-dwelling adults, meaning its findings cannot be extended to institutionalized and homeless individuals. Also, each friendship variable was assessed through a single question. “Future research,” they noted, “should assess these relationship dimensions using multi-item scales, as they tend to be more stable, reliable, and precise.”
No conflicts of interest were reported.
SOURCE: Nguyen AW et al. J Affect Disord. 2019 Jun 15. doi: 10.1016/j.jad.2019.04.013
A new analysis has found that, for African Americans with major depressive disorder (MDD), friendship’s mitigating benefits can vary based on education levels.
“These findings underscore the complexity of social support as a possible intervening process in depression,” wrote Ann W. Nguyen, PhD, of Case Western Reserve University in Cleveland, and her coauthors. The study was published in the Journal of Affective Disorders.
To determine how certain elements of friendship affect MDD, the researchers analyzed 3,434 responses from African Americans to the National Survey of American Life. They assessed variables such as “subjective closeness to friends,” “frequency of contact with friends,” “receipt of support from friends,” and “provision of support to friends” via responses to related questions, along with factoring in the impact of education level on 12-month MDD. Analysis was performed via logistic regression.
Among all respondents, the 12-month prevalence of MDD was 6.7%. Overall, subjective closeness and frequency of contact with friends were negatively associated with 12-month MDD. those with lower levels of education saw no association between frequency of contact and 12-month MDD. There was a similar association between receipt of support or provision of support and education: The high education group saw their probability of MDD decrease as receipt or provision of support increased.
The authors acknowledged their study’s limitations, including the impossibility of causal inferences because of its cross-sectional design. In addition, the survey only included community-dwelling adults, meaning its findings cannot be extended to institutionalized and homeless individuals. Also, each friendship variable was assessed through a single question. “Future research,” they noted, “should assess these relationship dimensions using multi-item scales, as they tend to be more stable, reliable, and precise.”
No conflicts of interest were reported.
SOURCE: Nguyen AW et al. J Affect Disord. 2019 Jun 15. doi: 10.1016/j.jad.2019.04.013
FROM THE JOURNAL OF AFFECTIVE DISORDERS
Almost one-third of ED patients with gout are prescribed opioids
Patients with gout who visit the emergency department are regularly prescribed opioids, based on a review of electronic medical records.
“In addition to regulatory changes, the burden of opioid prescription could be potentially reduced by creating prompts for providers in electronic record systems to avoid prescribing opioids in opioid-naive patients or using lower intensity and shorter duration of prescription,” wrote Deepan S. Dalal, MD, of Brown University, Providence, R.I., and coauthors. The study was published in Arthritis Care & Research.
To determine frequency, dose, and duration of opioid prescription at ED discharge, the researchers reviewed the records of 456 patients with acute gout who were discharged in Rhode Island between March 30, 2015, and Sept. 30, 2017. All data were gathered via electronic medical system records.
Of the 456 discharged patients, 129 (28.3%) were prescribed opioids; 102 (79%) were not on opioids at the time. A full prescription description was available for 119 of the 129 patients; 96 (81%) were prescribed oxycodone or oxycodone combinations. Hydrocodone was prescribed for 9 patients (8%) and tramadol was prescribed for 11 patients (9%).
The median duration of each prescription was 8 days (interquartile range, 5-14 days) and the average daily dose was 37.9 mg of morphine equivalent. Patients who were prescribed opioids tended to be younger and male. After multivariable analysis, diabetes, polyarticular gout attack, and prior opioid use were all associated with a more than 100% higher odds of receiving an opioid prescription.
The authors acknowledged their study’s limitations, including their inability to determine the physicians’ reasoning behind each prescription or the prescribing habits of each provider. In addition, they were only able to assess the prescriptions as being written and not the number of pills actually taken or not taken.
No conflicts of interest were reported.
SOURCE: Dalal DS et al. Arthritis Care Res. 2019 Jul 3. doi: 10.1002/acr.23928.
Patients with gout who visit the emergency department are regularly prescribed opioids, based on a review of electronic medical records.
“In addition to regulatory changes, the burden of opioid prescription could be potentially reduced by creating prompts for providers in electronic record systems to avoid prescribing opioids in opioid-naive patients or using lower intensity and shorter duration of prescription,” wrote Deepan S. Dalal, MD, of Brown University, Providence, R.I., and coauthors. The study was published in Arthritis Care & Research.
To determine frequency, dose, and duration of opioid prescription at ED discharge, the researchers reviewed the records of 456 patients with acute gout who were discharged in Rhode Island between March 30, 2015, and Sept. 30, 2017. All data were gathered via electronic medical system records.
Of the 456 discharged patients, 129 (28.3%) were prescribed opioids; 102 (79%) were not on opioids at the time. A full prescription description was available for 119 of the 129 patients; 96 (81%) were prescribed oxycodone or oxycodone combinations. Hydrocodone was prescribed for 9 patients (8%) and tramadol was prescribed for 11 patients (9%).
The median duration of each prescription was 8 days (interquartile range, 5-14 days) and the average daily dose was 37.9 mg of morphine equivalent. Patients who were prescribed opioids tended to be younger and male. After multivariable analysis, diabetes, polyarticular gout attack, and prior opioid use were all associated with a more than 100% higher odds of receiving an opioid prescription.
The authors acknowledged their study’s limitations, including their inability to determine the physicians’ reasoning behind each prescription or the prescribing habits of each provider. In addition, they were only able to assess the prescriptions as being written and not the number of pills actually taken or not taken.
No conflicts of interest were reported.
SOURCE: Dalal DS et al. Arthritis Care Res. 2019 Jul 3. doi: 10.1002/acr.23928.
Patients with gout who visit the emergency department are regularly prescribed opioids, based on a review of electronic medical records.
“In addition to regulatory changes, the burden of opioid prescription could be potentially reduced by creating prompts for providers in electronic record systems to avoid prescribing opioids in opioid-naive patients or using lower intensity and shorter duration of prescription,” wrote Deepan S. Dalal, MD, of Brown University, Providence, R.I., and coauthors. The study was published in Arthritis Care & Research.
To determine frequency, dose, and duration of opioid prescription at ED discharge, the researchers reviewed the records of 456 patients with acute gout who were discharged in Rhode Island between March 30, 2015, and Sept. 30, 2017. All data were gathered via electronic medical system records.
Of the 456 discharged patients, 129 (28.3%) were prescribed opioids; 102 (79%) were not on opioids at the time. A full prescription description was available for 119 of the 129 patients; 96 (81%) were prescribed oxycodone or oxycodone combinations. Hydrocodone was prescribed for 9 patients (8%) and tramadol was prescribed for 11 patients (9%).
The median duration of each prescription was 8 days (interquartile range, 5-14 days) and the average daily dose was 37.9 mg of morphine equivalent. Patients who were prescribed opioids tended to be younger and male. After multivariable analysis, diabetes, polyarticular gout attack, and prior opioid use were all associated with a more than 100% higher odds of receiving an opioid prescription.
The authors acknowledged their study’s limitations, including their inability to determine the physicians’ reasoning behind each prescription or the prescribing habits of each provider. In addition, they were only able to assess the prescriptions as being written and not the number of pills actually taken or not taken.
No conflicts of interest were reported.
SOURCE: Dalal DS et al. Arthritis Care Res. 2019 Jul 3. doi: 10.1002/acr.23928.
FROM ARTHRITIS CARE & RESEARCH
Key clinical point: Though there are other effective conventional treatments, opioids are often prescribed for patients who present to the ED with gout.
Major finding: After multivariable analysis, diabetes, polyarticular gout attack, and prior opioid use were all associated with a more than 100% higher odds of opioid prescription.
Study details: A retrospective cohort study of 456 patients with acute gout discharged from EDs in Rhode Island.
Disclosures: The authors reported no conflicts of interest.
Source: Dalal DS et al. Arthritis Care Res. 2019 Jul 3. doi: 10.1002/acr.23928.
Breast density alone should not prompt supplemental imaging discussions
Breast density should be a factor in assessing breast cancer risk and recommending supplemental imaging, but not the primary factor, according to a study of women who were screened for the disease.
“Counseling strategies that identified women for supplemental imaging based on breast density and BCSC 5-year risk were more efficient compared with strategies based on age and density or density alone,” wrote Karla Kerlikowske, MD, of the Department of Veterans Affairs, and her coauthors. The study was published in JAMA Internal Medicine.
To assess breast cancer risk and strategies for recommending supplemental screening, the researchers assembled a cohort of 638,856 women aged 40 to 74 years who received mammograms at Breast Cancer Surveillance Consortium (BCSC) facilities from Jan. 3, 2005, to Dec. 31, 2014. Participants were identified as high risk via combinations of Breast Imaging Reporting and Data System (BI-RADS) breast density, BCSC 5-year breast cancer risk, and age.
Women with dense breasts made up 47% of those screened, and 60% of those with advanced cancers. Low advanced cancer rates (less than .61 per 1,000 mammograms) occurred in 34.5% of women with dense breasts, while high advanced cancer rates (greater than or equal to .61 cases per 1,000 mammograms) occurred in women with heterogeneously dense breasts and a 5-year risk of 2.5% or higher (6.0% of screened women) and those with extremely dense breasts and a 5-year risk of 1.0% or higher (6.5% of screened women).
In a hypothetical cohort of 100,000 women, supplemental imaging for all 47,012 women with dense breasts would mean a ratio of 1,866 supplemental imaging discussions per potential advanced breast cancer prevented. If imaging was considered based on a combination of density plus BCSC 5-year risk, the number of women screened would be reduced to 12,506 and the ratio would become 1,097 supplemental imaging discussions per potential advanced cancer prevented.
The coauthors acknowledged their study’s limitations, including their lack of ability to determine if women at high risk of advanced cancer would benefit from supplemental screening. In addition, they were unable to evaluate digital breast tomosynthesis outcomes, though they noted that, to their knowledge, “no published evidence indicates that advanced cancer rates differ for digital mammography vs. tomosynthesis according to breast density.”
The study was funded by the Patient-Centered Outcomes Research Institute, the Breast Cancer Surveillance Consortium, the National Cancer Institute, the Agency for Health Research and Quality, and the Lake Champlain Cancer Research Organization. The authors reported several potential conflicts of interest, including being members of various working groups, advisory boards, committees, task forces, and panels.
SOURCE: Kerlikowske K et al. JAMA Intern Med. 2019 Jul 1. doi:10.1001/jamainternmed.2019.1758 .
Identifying women at risk of breast cancer is key, but physicians and policymakers should pause and reassess how exactly to go about it, according to Ilana B. Richman, MD, and Susan H. Busch, PhD of the Yale School of Medicine.
The latest proposal from the U.S. Food and Drug Administration focuses on recommending additional screening for women with dense breasts, but that can be too broad of a stroke. “Breast density is only one aspect of breast cancer risk,” the coauthors noted, and limiting supplemental screening recommendations to women with dense breasts may leave out many others at legitimate risk.
So how should supplemental screening be handled moving forward? In their accompanying study, Kerlikowske et al. rejected 2 strategies while embracing elements of 3 others, but none of them were recognized as the proper path to take.
At the same time, the coauthors asked, “Why legislate this particular area of medicine?” And what is the exact opportunity cost of supplemental screening? There is no simple answer, which highlights “both the overall inefficiency of supplemental screening and the insensitivity of a targeted approach.” In short, more work is needed.
These comments are adapted from an accompanying editorial (JAMA Intern Med. 2019 Jul 1. doi:10.1001/jamainternmed.2019.1737 ). Dr. Richman reported receiving funding from the Centers for Medicare and Medicaid Services to develop quality measures, along with funding from the National Center for Advancing Translational Sciences.
Identifying women at risk of breast cancer is key, but physicians and policymakers should pause and reassess how exactly to go about it, according to Ilana B. Richman, MD, and Susan H. Busch, PhD of the Yale School of Medicine.
The latest proposal from the U.S. Food and Drug Administration focuses on recommending additional screening for women with dense breasts, but that can be too broad of a stroke. “Breast density is only one aspect of breast cancer risk,” the coauthors noted, and limiting supplemental screening recommendations to women with dense breasts may leave out many others at legitimate risk.
So how should supplemental screening be handled moving forward? In their accompanying study, Kerlikowske et al. rejected 2 strategies while embracing elements of 3 others, but none of them were recognized as the proper path to take.
At the same time, the coauthors asked, “Why legislate this particular area of medicine?” And what is the exact opportunity cost of supplemental screening? There is no simple answer, which highlights “both the overall inefficiency of supplemental screening and the insensitivity of a targeted approach.” In short, more work is needed.
These comments are adapted from an accompanying editorial (JAMA Intern Med. 2019 Jul 1. doi:10.1001/jamainternmed.2019.1737 ). Dr. Richman reported receiving funding from the Centers for Medicare and Medicaid Services to develop quality measures, along with funding from the National Center for Advancing Translational Sciences.
Identifying women at risk of breast cancer is key, but physicians and policymakers should pause and reassess how exactly to go about it, according to Ilana B. Richman, MD, and Susan H. Busch, PhD of the Yale School of Medicine.
The latest proposal from the U.S. Food and Drug Administration focuses on recommending additional screening for women with dense breasts, but that can be too broad of a stroke. “Breast density is only one aspect of breast cancer risk,” the coauthors noted, and limiting supplemental screening recommendations to women with dense breasts may leave out many others at legitimate risk.
So how should supplemental screening be handled moving forward? In their accompanying study, Kerlikowske et al. rejected 2 strategies while embracing elements of 3 others, but none of them were recognized as the proper path to take.
At the same time, the coauthors asked, “Why legislate this particular area of medicine?” And what is the exact opportunity cost of supplemental screening? There is no simple answer, which highlights “both the overall inefficiency of supplemental screening and the insensitivity of a targeted approach.” In short, more work is needed.
These comments are adapted from an accompanying editorial (JAMA Intern Med. 2019 Jul 1. doi:10.1001/jamainternmed.2019.1737 ). Dr. Richman reported receiving funding from the Centers for Medicare and Medicaid Services to develop quality measures, along with funding from the National Center for Advancing Translational Sciences.
Breast density should be a factor in assessing breast cancer risk and recommending supplemental imaging, but not the primary factor, according to a study of women who were screened for the disease.
“Counseling strategies that identified women for supplemental imaging based on breast density and BCSC 5-year risk were more efficient compared with strategies based on age and density or density alone,” wrote Karla Kerlikowske, MD, of the Department of Veterans Affairs, and her coauthors. The study was published in JAMA Internal Medicine.
To assess breast cancer risk and strategies for recommending supplemental screening, the researchers assembled a cohort of 638,856 women aged 40 to 74 years who received mammograms at Breast Cancer Surveillance Consortium (BCSC) facilities from Jan. 3, 2005, to Dec. 31, 2014. Participants were identified as high risk via combinations of Breast Imaging Reporting and Data System (BI-RADS) breast density, BCSC 5-year breast cancer risk, and age.
Women with dense breasts made up 47% of those screened, and 60% of those with advanced cancers. Low advanced cancer rates (less than .61 per 1,000 mammograms) occurred in 34.5% of women with dense breasts, while high advanced cancer rates (greater than or equal to .61 cases per 1,000 mammograms) occurred in women with heterogeneously dense breasts and a 5-year risk of 2.5% or higher (6.0% of screened women) and those with extremely dense breasts and a 5-year risk of 1.0% or higher (6.5% of screened women).
In a hypothetical cohort of 100,000 women, supplemental imaging for all 47,012 women with dense breasts would mean a ratio of 1,866 supplemental imaging discussions per potential advanced breast cancer prevented. If imaging was considered based on a combination of density plus BCSC 5-year risk, the number of women screened would be reduced to 12,506 and the ratio would become 1,097 supplemental imaging discussions per potential advanced cancer prevented.
The coauthors acknowledged their study’s limitations, including their lack of ability to determine if women at high risk of advanced cancer would benefit from supplemental screening. In addition, they were unable to evaluate digital breast tomosynthesis outcomes, though they noted that, to their knowledge, “no published evidence indicates that advanced cancer rates differ for digital mammography vs. tomosynthesis according to breast density.”
The study was funded by the Patient-Centered Outcomes Research Institute, the Breast Cancer Surveillance Consortium, the National Cancer Institute, the Agency for Health Research and Quality, and the Lake Champlain Cancer Research Organization. The authors reported several potential conflicts of interest, including being members of various working groups, advisory boards, committees, task forces, and panels.
SOURCE: Kerlikowske K et al. JAMA Intern Med. 2019 Jul 1. doi:10.1001/jamainternmed.2019.1758 .
Breast density should be a factor in assessing breast cancer risk and recommending supplemental imaging, but not the primary factor, according to a study of women who were screened for the disease.
“Counseling strategies that identified women for supplemental imaging based on breast density and BCSC 5-year risk were more efficient compared with strategies based on age and density or density alone,” wrote Karla Kerlikowske, MD, of the Department of Veterans Affairs, and her coauthors. The study was published in JAMA Internal Medicine.
To assess breast cancer risk and strategies for recommending supplemental screening, the researchers assembled a cohort of 638,856 women aged 40 to 74 years who received mammograms at Breast Cancer Surveillance Consortium (BCSC) facilities from Jan. 3, 2005, to Dec. 31, 2014. Participants were identified as high risk via combinations of Breast Imaging Reporting and Data System (BI-RADS) breast density, BCSC 5-year breast cancer risk, and age.
Women with dense breasts made up 47% of those screened, and 60% of those with advanced cancers. Low advanced cancer rates (less than .61 per 1,000 mammograms) occurred in 34.5% of women with dense breasts, while high advanced cancer rates (greater than or equal to .61 cases per 1,000 mammograms) occurred in women with heterogeneously dense breasts and a 5-year risk of 2.5% or higher (6.0% of screened women) and those with extremely dense breasts and a 5-year risk of 1.0% or higher (6.5% of screened women).
In a hypothetical cohort of 100,000 women, supplemental imaging for all 47,012 women with dense breasts would mean a ratio of 1,866 supplemental imaging discussions per potential advanced breast cancer prevented. If imaging was considered based on a combination of density plus BCSC 5-year risk, the number of women screened would be reduced to 12,506 and the ratio would become 1,097 supplemental imaging discussions per potential advanced cancer prevented.
The coauthors acknowledged their study’s limitations, including their lack of ability to determine if women at high risk of advanced cancer would benefit from supplemental screening. In addition, they were unable to evaluate digital breast tomosynthesis outcomes, though they noted that, to their knowledge, “no published evidence indicates that advanced cancer rates differ for digital mammography vs. tomosynthesis according to breast density.”
The study was funded by the Patient-Centered Outcomes Research Institute, the Breast Cancer Surveillance Consortium, the National Cancer Institute, the Agency for Health Research and Quality, and the Lake Champlain Cancer Research Organization. The authors reported several potential conflicts of interest, including being members of various working groups, advisory boards, committees, task forces, and panels.
SOURCE: Kerlikowske K et al. JAMA Intern Med. 2019 Jul 1. doi:10.1001/jamainternmed.2019.1758 .
FROM JAMA INTERNAL MEDICINE
Budesonide tablets considerably outperformed placebo for active EoE
Budesonide orodispersible tablets (BOTs) are highly effective in inducing disease remission in adults with active eosinophilic esophagitis (EOE), according to a study of the tablets versus placebo in European patients.
“A 6-week treatment with 1 mg budesonide twice daily was highly superior over placebo with regard to all predefined primary and secondary outcomes,” wrote Alfredo J. Lucendo, MD, of Hospital General de Tomelloso in Real, Spain, and his coauthors. The study was published in Gastroenterology.
To assess the effectiveness and tolerability of BOT in adults with EOE, Dr. Lucendo and his fellow researchers launched a randomized, placebo-controlled trial made up of 88 European adults with active EoE. Patients were assigned to either a group that received BOT twice daily (n = 59) or a group that received placebo (n = 29). The primary endpoint was complete remission.
After 6 weeks, 34 of 59 patients (58%) receiving BOT had achieved complete remission, compared with 0 patients receiving placebo (P less than .0001). After 12 weeks, 50 of 59 patients (85%) in the BOT group had achieved complete remission. BOT was also well tolerated; no serious adverse event was reported, and no differences were observed between groups with regard to commonly reported adverse events.
The coauthors acknowledged their study’s limitations, including the fact that it was designed to demonstrate budesonide’s superiority to placebo at 6 weeks, not to identify the time of its maximal effect. In addition, the researchers did not identify a minimally effective dose; they did, however, note their belief that a lower dose could still achieve similar rates of remission and “a higher dose would not achieve a higher clinico-remission rate.”
The study was funded by Dr. Falk Pharma. The authors reported numerous conflicts of interest, including receiving research funding and speaker fees from various pharmaceutical companies and foundations.
SOURCE: Lucendo AJ et al. Gastroenterology. 2019 Mar 25. doi: 10.1053/j.gastro.2019.03.025.
Budesonide orodispersible tablets (BOTs) are highly effective in inducing disease remission in adults with active eosinophilic esophagitis (EOE), according to a study of the tablets versus placebo in European patients.
“A 6-week treatment with 1 mg budesonide twice daily was highly superior over placebo with regard to all predefined primary and secondary outcomes,” wrote Alfredo J. Lucendo, MD, of Hospital General de Tomelloso in Real, Spain, and his coauthors. The study was published in Gastroenterology.
To assess the effectiveness and tolerability of BOT in adults with EOE, Dr. Lucendo and his fellow researchers launched a randomized, placebo-controlled trial made up of 88 European adults with active EoE. Patients were assigned to either a group that received BOT twice daily (n = 59) or a group that received placebo (n = 29). The primary endpoint was complete remission.
After 6 weeks, 34 of 59 patients (58%) receiving BOT had achieved complete remission, compared with 0 patients receiving placebo (P less than .0001). After 12 weeks, 50 of 59 patients (85%) in the BOT group had achieved complete remission. BOT was also well tolerated; no serious adverse event was reported, and no differences were observed between groups with regard to commonly reported adverse events.
The coauthors acknowledged their study’s limitations, including the fact that it was designed to demonstrate budesonide’s superiority to placebo at 6 weeks, not to identify the time of its maximal effect. In addition, the researchers did not identify a minimally effective dose; they did, however, note their belief that a lower dose could still achieve similar rates of remission and “a higher dose would not achieve a higher clinico-remission rate.”
The study was funded by Dr. Falk Pharma. The authors reported numerous conflicts of interest, including receiving research funding and speaker fees from various pharmaceutical companies and foundations.
SOURCE: Lucendo AJ et al. Gastroenterology. 2019 Mar 25. doi: 10.1053/j.gastro.2019.03.025.
Budesonide orodispersible tablets (BOTs) are highly effective in inducing disease remission in adults with active eosinophilic esophagitis (EOE), according to a study of the tablets versus placebo in European patients.
“A 6-week treatment with 1 mg budesonide twice daily was highly superior over placebo with regard to all predefined primary and secondary outcomes,” wrote Alfredo J. Lucendo, MD, of Hospital General de Tomelloso in Real, Spain, and his coauthors. The study was published in Gastroenterology.
To assess the effectiveness and tolerability of BOT in adults with EOE, Dr. Lucendo and his fellow researchers launched a randomized, placebo-controlled trial made up of 88 European adults with active EoE. Patients were assigned to either a group that received BOT twice daily (n = 59) or a group that received placebo (n = 29). The primary endpoint was complete remission.
After 6 weeks, 34 of 59 patients (58%) receiving BOT had achieved complete remission, compared with 0 patients receiving placebo (P less than .0001). After 12 weeks, 50 of 59 patients (85%) in the BOT group had achieved complete remission. BOT was also well tolerated; no serious adverse event was reported, and no differences were observed between groups with regard to commonly reported adverse events.
The coauthors acknowledged their study’s limitations, including the fact that it was designed to demonstrate budesonide’s superiority to placebo at 6 weeks, not to identify the time of its maximal effect. In addition, the researchers did not identify a minimally effective dose; they did, however, note their belief that a lower dose could still achieve similar rates of remission and “a higher dose would not achieve a higher clinico-remission rate.”
The study was funded by Dr. Falk Pharma. The authors reported numerous conflicts of interest, including receiving research funding and speaker fees from various pharmaceutical companies and foundations.
SOURCE: Lucendo AJ et al. Gastroenterology. 2019 Mar 25. doi: 10.1053/j.gastro.2019.03.025.
FROM GASTROENTEROLOGY
COPD rates reflect current smoking prevalence
, according to a Centers for Disease Control and Prevention analysis of respondents to a behavioral risk factor survey.
“Population-based strategies for smoking prevention and control have the potential to decrease the prevalence of COPD in the United States,” wrote Anne G. Wheaton, PhD, of the CDC’s National Center for Chronic Disease Prevention and Health Promotion and coauthors. The study was published in the Morbidity and Mortality Weekly Report.
Dr. Wheaton and her fellow researchers analyzed data from 418,378 adult respondents to the 2017 Behavioral Risk Factor Surveillance System survey. Responses came from all 50 states and Washington, D.C.; respondents who had smoked less than 100 lifetime cigarettes were categorized as “never smoked,” while those who had smoked at least 100 cigarettes but no longer smoked were categorized as “former smokers.” Anyone who had smoked at least 100 cigarettes and currently smoked was categorized as a “current smoker.”
The age-adjusted prevalence of COPD among U.S. adults was 6.2% (95% confidence interval, 6.0%-6.3%) in 2017. Current cigarette smokers had a prevalence of 15.2% (95% CI, 14.7%-15.7%); this dipped to 7.6% (95% CI, 7.3%-8.0%) among former smokers and 2.8% (95% CI, 2.7%-2.9%) among adults who had never smoked. Patterns were visible within states: Current smokers had a state-level prevalence of COPD that was strongly correlated with state-level current smoking prevalence (Pearson correlation coefficient, 0.69; P less than .001). State-level COPD prevalence among former smokers (Pearson correlation coefficient, 0.71; P less than .001) and those who never smoked (Pearson correlation coefficient, 0.64; P less than .001) were also strongly correlated with the current smoking prevalence, indicating secondhand smoke as a risk factor for COPD.
The coauthors acknowledged the study’s limitations, including relying on self-reporting for both COPD and smoking status. They also noted that there was no way to measure exposure to secondhand smoke, other indoor or outdoor air pollutants, or respiratory infection history, “all of which might contribute to COPD risk.”
No conflicts of interest were reported.
SOURCE: Wheaton AG et al. MMWR Morb Mortal Wkly Rep. 2019 Jun 21;68(24):533-8.
, according to a Centers for Disease Control and Prevention analysis of respondents to a behavioral risk factor survey.
“Population-based strategies for smoking prevention and control have the potential to decrease the prevalence of COPD in the United States,” wrote Anne G. Wheaton, PhD, of the CDC’s National Center for Chronic Disease Prevention and Health Promotion and coauthors. The study was published in the Morbidity and Mortality Weekly Report.
Dr. Wheaton and her fellow researchers analyzed data from 418,378 adult respondents to the 2017 Behavioral Risk Factor Surveillance System survey. Responses came from all 50 states and Washington, D.C.; respondents who had smoked less than 100 lifetime cigarettes were categorized as “never smoked,” while those who had smoked at least 100 cigarettes but no longer smoked were categorized as “former smokers.” Anyone who had smoked at least 100 cigarettes and currently smoked was categorized as a “current smoker.”
The age-adjusted prevalence of COPD among U.S. adults was 6.2% (95% confidence interval, 6.0%-6.3%) in 2017. Current cigarette smokers had a prevalence of 15.2% (95% CI, 14.7%-15.7%); this dipped to 7.6% (95% CI, 7.3%-8.0%) among former smokers and 2.8% (95% CI, 2.7%-2.9%) among adults who had never smoked. Patterns were visible within states: Current smokers had a state-level prevalence of COPD that was strongly correlated with state-level current smoking prevalence (Pearson correlation coefficient, 0.69; P less than .001). State-level COPD prevalence among former smokers (Pearson correlation coefficient, 0.71; P less than .001) and those who never smoked (Pearson correlation coefficient, 0.64; P less than .001) were also strongly correlated with the current smoking prevalence, indicating secondhand smoke as a risk factor for COPD.
The coauthors acknowledged the study’s limitations, including relying on self-reporting for both COPD and smoking status. They also noted that there was no way to measure exposure to secondhand smoke, other indoor or outdoor air pollutants, or respiratory infection history, “all of which might contribute to COPD risk.”
No conflicts of interest were reported.
SOURCE: Wheaton AG et al. MMWR Morb Mortal Wkly Rep. 2019 Jun 21;68(24):533-8.
, according to a Centers for Disease Control and Prevention analysis of respondents to a behavioral risk factor survey.
“Population-based strategies for smoking prevention and control have the potential to decrease the prevalence of COPD in the United States,” wrote Anne G. Wheaton, PhD, of the CDC’s National Center for Chronic Disease Prevention and Health Promotion and coauthors. The study was published in the Morbidity and Mortality Weekly Report.
Dr. Wheaton and her fellow researchers analyzed data from 418,378 adult respondents to the 2017 Behavioral Risk Factor Surveillance System survey. Responses came from all 50 states and Washington, D.C.; respondents who had smoked less than 100 lifetime cigarettes were categorized as “never smoked,” while those who had smoked at least 100 cigarettes but no longer smoked were categorized as “former smokers.” Anyone who had smoked at least 100 cigarettes and currently smoked was categorized as a “current smoker.”
The age-adjusted prevalence of COPD among U.S. adults was 6.2% (95% confidence interval, 6.0%-6.3%) in 2017. Current cigarette smokers had a prevalence of 15.2% (95% CI, 14.7%-15.7%); this dipped to 7.6% (95% CI, 7.3%-8.0%) among former smokers and 2.8% (95% CI, 2.7%-2.9%) among adults who had never smoked. Patterns were visible within states: Current smokers had a state-level prevalence of COPD that was strongly correlated with state-level current smoking prevalence (Pearson correlation coefficient, 0.69; P less than .001). State-level COPD prevalence among former smokers (Pearson correlation coefficient, 0.71; P less than .001) and those who never smoked (Pearson correlation coefficient, 0.64; P less than .001) were also strongly correlated with the current smoking prevalence, indicating secondhand smoke as a risk factor for COPD.
The coauthors acknowledged the study’s limitations, including relying on self-reporting for both COPD and smoking status. They also noted that there was no way to measure exposure to secondhand smoke, other indoor or outdoor air pollutants, or respiratory infection history, “all of which might contribute to COPD risk.”
No conflicts of interest were reported.
SOURCE: Wheaton AG et al. MMWR Morb Mortal Wkly Rep. 2019 Jun 21;68(24):533-8.
FROM MMWR