User login
New guideline conditionally recommends long-term home NIV for COPD patients
from a European Respiratory Society task force.
“Our recommendations, based on the best available evidence, can guide the management of chronic hypercapnic respiratory failure in COPD patients aimed at improving patient outcomes,” wrote Begum Ergan, MD, of Dokuz Eylul University, Izmir, Turkey, and coauthors. The guideline was published in the European Respiratory Journal.
To provide insight into the clinical application of LTH-NIV, the European Respiratory Society convened a task force of 20 clinicians, methodologists, and experts. Their four recommendations were developed based on the GRADE (Grading, Recommendation, Assessment, Development and Evaluation) methodology.
The first recommendation was to use LTH-NIV for patients with chronic stable hypercapnic COPD. Though an analysis of randomized, controlled trials showed little effect on mortality or hospitalizations, pooled analyses showed that NIV may decrease dyspnea scores (standardized mean difference, –0.51; 95% confidence interval, –0.06 to –0.95) and increase health-related quality of life (SMD, 0.49; 95% CI, –0.01 to 0.98).
The second was to use LTH-NIV in patients with COPD following a life-threatening episode of acute hypercapnic respiratory failure requiring acute NIV, if hypercapnia persists. Though it was not associated with a reduction in mortality (risk ratio, 0.92; 95% CI, 0.67-1.25), it was found to potentially reduce exacerbations (SMD, 0.19; 95% CI, –0.40 to 0.01) and hospitalizations (RR, 0.61; 95% CI, 0.30-1.24).
The third was to titrate LTH-NIV to normalize or reduce PaCO2 levels in patients with COPD. While this recommendation was issued with a very low certainty of evidence, it was driven by the “minimal potential harms of targeted PaCO2 reduction.”
The fourth was to use fixed pressure support mode as first-choice ventilator mode in patients with COPD using LTH-NIV. The six trials on this subject did not provide insight into long-term outcomes, nor were there significant improvements seen in health-related quality of life, sleep quality, or exercise tolerance. As such, it was also issued with a very low certainty of evidence.
The authors acknowledged all four recommendations as weak and conditional, “due to limitations in the certainty of the available evidence.” As such, they noted that their recommendations “require consideration of individual preferences, resource considerations, technical expertise, and clinical circumstances prior to implementation in clinical practice.”
The authors reported numerous disclosures, including receiving grants and personal fees from various medical supply companies.
SOURCE: Ergan B et al. Eur Respir J. 2019 Aug 29. doi: 10.1183/13993003.01003-2019.
from a European Respiratory Society task force.
“Our recommendations, based on the best available evidence, can guide the management of chronic hypercapnic respiratory failure in COPD patients aimed at improving patient outcomes,” wrote Begum Ergan, MD, of Dokuz Eylul University, Izmir, Turkey, and coauthors. The guideline was published in the European Respiratory Journal.
To provide insight into the clinical application of LTH-NIV, the European Respiratory Society convened a task force of 20 clinicians, methodologists, and experts. Their four recommendations were developed based on the GRADE (Grading, Recommendation, Assessment, Development and Evaluation) methodology.
The first recommendation was to use LTH-NIV for patients with chronic stable hypercapnic COPD. Though an analysis of randomized, controlled trials showed little effect on mortality or hospitalizations, pooled analyses showed that NIV may decrease dyspnea scores (standardized mean difference, –0.51; 95% confidence interval, –0.06 to –0.95) and increase health-related quality of life (SMD, 0.49; 95% CI, –0.01 to 0.98).
The second was to use LTH-NIV in patients with COPD following a life-threatening episode of acute hypercapnic respiratory failure requiring acute NIV, if hypercapnia persists. Though it was not associated with a reduction in mortality (risk ratio, 0.92; 95% CI, 0.67-1.25), it was found to potentially reduce exacerbations (SMD, 0.19; 95% CI, –0.40 to 0.01) and hospitalizations (RR, 0.61; 95% CI, 0.30-1.24).
The third was to titrate LTH-NIV to normalize or reduce PaCO2 levels in patients with COPD. While this recommendation was issued with a very low certainty of evidence, it was driven by the “minimal potential harms of targeted PaCO2 reduction.”
The fourth was to use fixed pressure support mode as first-choice ventilator mode in patients with COPD using LTH-NIV. The six trials on this subject did not provide insight into long-term outcomes, nor were there significant improvements seen in health-related quality of life, sleep quality, or exercise tolerance. As such, it was also issued with a very low certainty of evidence.
The authors acknowledged all four recommendations as weak and conditional, “due to limitations in the certainty of the available evidence.” As such, they noted that their recommendations “require consideration of individual preferences, resource considerations, technical expertise, and clinical circumstances prior to implementation in clinical practice.”
The authors reported numerous disclosures, including receiving grants and personal fees from various medical supply companies.
SOURCE: Ergan B et al. Eur Respir J. 2019 Aug 29. doi: 10.1183/13993003.01003-2019.
from a European Respiratory Society task force.
“Our recommendations, based on the best available evidence, can guide the management of chronic hypercapnic respiratory failure in COPD patients aimed at improving patient outcomes,” wrote Begum Ergan, MD, of Dokuz Eylul University, Izmir, Turkey, and coauthors. The guideline was published in the European Respiratory Journal.
To provide insight into the clinical application of LTH-NIV, the European Respiratory Society convened a task force of 20 clinicians, methodologists, and experts. Their four recommendations were developed based on the GRADE (Grading, Recommendation, Assessment, Development and Evaluation) methodology.
The first recommendation was to use LTH-NIV for patients with chronic stable hypercapnic COPD. Though an analysis of randomized, controlled trials showed little effect on mortality or hospitalizations, pooled analyses showed that NIV may decrease dyspnea scores (standardized mean difference, –0.51; 95% confidence interval, –0.06 to –0.95) and increase health-related quality of life (SMD, 0.49; 95% CI, –0.01 to 0.98).
The second was to use LTH-NIV in patients with COPD following a life-threatening episode of acute hypercapnic respiratory failure requiring acute NIV, if hypercapnia persists. Though it was not associated with a reduction in mortality (risk ratio, 0.92; 95% CI, 0.67-1.25), it was found to potentially reduce exacerbations (SMD, 0.19; 95% CI, –0.40 to 0.01) and hospitalizations (RR, 0.61; 95% CI, 0.30-1.24).
The third was to titrate LTH-NIV to normalize or reduce PaCO2 levels in patients with COPD. While this recommendation was issued with a very low certainty of evidence, it was driven by the “minimal potential harms of targeted PaCO2 reduction.”
The fourth was to use fixed pressure support mode as first-choice ventilator mode in patients with COPD using LTH-NIV. The six trials on this subject did not provide insight into long-term outcomes, nor were there significant improvements seen in health-related quality of life, sleep quality, or exercise tolerance. As such, it was also issued with a very low certainty of evidence.
The authors acknowledged all four recommendations as weak and conditional, “due to limitations in the certainty of the available evidence.” As such, they noted that their recommendations “require consideration of individual preferences, resource considerations, technical expertise, and clinical circumstances prior to implementation in clinical practice.”
The authors reported numerous disclosures, including receiving grants and personal fees from various medical supply companies.
SOURCE: Ergan B et al. Eur Respir J. 2019 Aug 29. doi: 10.1183/13993003.01003-2019.
FROM THE EUROPEAN RESPIRATORY JOURNAL
Increased levels of a bacterial strain may cause nonalcoholic fatty liver disease
A new study involving both human patients and mice has confirmed a long-believed association between nonalcoholic fatty liver disease (NAFLD) and an alteration in the gut microbiome that produces high levels of alcohol.
The study was initiated after the treatment of a rare case: a patient who presented with severe nonalcoholic steatohepatitis (NASH) plus auto-brewery syndrome. The patient had a very high blood alcohol concentration but an alcohol-free, high-carbohydrate diet. It was determined that strains of high alcohol-producing Klebsiella pneumoniae (HiAlc Kpn) rather than a fungal infection were the catalyst for the high blood alcohol level. As such, Jing Yuan of the Capital Institute of Pediatrics in Beijing and coauthors attempted to “connect these commensal HiAlc Kpn to the pathogenesis of hepatic damage” through this study, which was published in Cell Metabolism.
The researchers began by examining 43 patients with NAFLD and 48 healthy controls. Among the patients with NAFLD, 11 had nonalcoholic fatty liver and 32 had NASH. Specifically, they analyzed the presence and effects of HiAlc Kpn, determining that the abundance of Klebsiella pneumoniae was slightly higher in the feces of NAFLD patients, compared with healthy patients, but that their alcohol-producing ability in NAFLD patients was significantly stronger. Of the patients with NAFLD, 61% carried HiAlc and medium-alcohol-producing Kpn, compared with 6.25% of the controls.
Another phase of their study involved feeding specific pathogen-free mice either HiAlc Kpn, ethanol, or yeast extract peptone dextrose medium (pair-fed) for 4, 6, and 8 weeks. The mice that were fed HiAlc Kpn or ethanol showed clear microsteatosis and macrosteatosis in their livers at 4 and 8 weeks, compared with the pair-fed mice. In addition, the HiAlc-Kpn-fed and ethanol-fed mice had increased levels of aspartate transaminase and alanine transaminase in their serum and increased levels of triglycerides and thiobarbituric acid reactive substances in their liver. The results overall indicated that the HiAlc-Kpn-fed mice had developed hepatic steatosis.
An additional phase included the intestinal flora from a NASH patient with a specific Kpn strain being fed to germ-free mice. At the same time, two types of intestinal flora from mice with NAFLD were transplanted into healthy mice: one induced by two other specific Kpn strains and one in which those strains had been selectively eliminated. The results saw obvious steatosis in the mice who received the flora from either mice with NAFLD induced by Kpn or the NASH patient at 4 weeks and 8 weeks, respectively. The mice who received the flora win which Kpn had been eliminated saw no fat-related changes in the liver. “These results further suggest that HiAlc Kpn might be one of the major causes of NAFLD development,” the researchers wrote.
The authors acknowledged the study’s limitations, chiefly including the lack of a clinical cohort of individuals with auto brewery syndrome but without NAFLD that could be used as a control. However, they also noted a belief that “causality was shown by the transfer experiments of HiAlc Kpn” while adding that “the further analysis of the impact of ethanol in ABS [auto brewery syndrome] patients should be investigated.”
The study was funded by grants from the National Natural Science Foundation for Key Programs of China, the National Natural Science Foundation of China, Megaprojects of Science and Technology Research of China, and CAMS Innovation Fund for Medical Sciences. The authors reported no conflicts of interest.
SOURCE: Yuan J et al. Cell Metab. 2019 Sep 19. doi: 10.1016/j.cmet.2019.08.018.
A new study involving both human patients and mice has confirmed a long-believed association between nonalcoholic fatty liver disease (NAFLD) and an alteration in the gut microbiome that produces high levels of alcohol.
The study was initiated after the treatment of a rare case: a patient who presented with severe nonalcoholic steatohepatitis (NASH) plus auto-brewery syndrome. The patient had a very high blood alcohol concentration but an alcohol-free, high-carbohydrate diet. It was determined that strains of high alcohol-producing Klebsiella pneumoniae (HiAlc Kpn) rather than a fungal infection were the catalyst for the high blood alcohol level. As such, Jing Yuan of the Capital Institute of Pediatrics in Beijing and coauthors attempted to “connect these commensal HiAlc Kpn to the pathogenesis of hepatic damage” through this study, which was published in Cell Metabolism.
The researchers began by examining 43 patients with NAFLD and 48 healthy controls. Among the patients with NAFLD, 11 had nonalcoholic fatty liver and 32 had NASH. Specifically, they analyzed the presence and effects of HiAlc Kpn, determining that the abundance of Klebsiella pneumoniae was slightly higher in the feces of NAFLD patients, compared with healthy patients, but that their alcohol-producing ability in NAFLD patients was significantly stronger. Of the patients with NAFLD, 61% carried HiAlc and medium-alcohol-producing Kpn, compared with 6.25% of the controls.
Another phase of their study involved feeding specific pathogen-free mice either HiAlc Kpn, ethanol, or yeast extract peptone dextrose medium (pair-fed) for 4, 6, and 8 weeks. The mice that were fed HiAlc Kpn or ethanol showed clear microsteatosis and macrosteatosis in their livers at 4 and 8 weeks, compared with the pair-fed mice. In addition, the HiAlc-Kpn-fed and ethanol-fed mice had increased levels of aspartate transaminase and alanine transaminase in their serum and increased levels of triglycerides and thiobarbituric acid reactive substances in their liver. The results overall indicated that the HiAlc-Kpn-fed mice had developed hepatic steatosis.
An additional phase included the intestinal flora from a NASH patient with a specific Kpn strain being fed to germ-free mice. At the same time, two types of intestinal flora from mice with NAFLD were transplanted into healthy mice: one induced by two other specific Kpn strains and one in which those strains had been selectively eliminated. The results saw obvious steatosis in the mice who received the flora from either mice with NAFLD induced by Kpn or the NASH patient at 4 weeks and 8 weeks, respectively. The mice who received the flora win which Kpn had been eliminated saw no fat-related changes in the liver. “These results further suggest that HiAlc Kpn might be one of the major causes of NAFLD development,” the researchers wrote.
The authors acknowledged the study’s limitations, chiefly including the lack of a clinical cohort of individuals with auto brewery syndrome but without NAFLD that could be used as a control. However, they also noted a belief that “causality was shown by the transfer experiments of HiAlc Kpn” while adding that “the further analysis of the impact of ethanol in ABS [auto brewery syndrome] patients should be investigated.”
The study was funded by grants from the National Natural Science Foundation for Key Programs of China, the National Natural Science Foundation of China, Megaprojects of Science and Technology Research of China, and CAMS Innovation Fund for Medical Sciences. The authors reported no conflicts of interest.
SOURCE: Yuan J et al. Cell Metab. 2019 Sep 19. doi: 10.1016/j.cmet.2019.08.018.
A new study involving both human patients and mice has confirmed a long-believed association between nonalcoholic fatty liver disease (NAFLD) and an alteration in the gut microbiome that produces high levels of alcohol.
The study was initiated after the treatment of a rare case: a patient who presented with severe nonalcoholic steatohepatitis (NASH) plus auto-brewery syndrome. The patient had a very high blood alcohol concentration but an alcohol-free, high-carbohydrate diet. It was determined that strains of high alcohol-producing Klebsiella pneumoniae (HiAlc Kpn) rather than a fungal infection were the catalyst for the high blood alcohol level. As such, Jing Yuan of the Capital Institute of Pediatrics in Beijing and coauthors attempted to “connect these commensal HiAlc Kpn to the pathogenesis of hepatic damage” through this study, which was published in Cell Metabolism.
The researchers began by examining 43 patients with NAFLD and 48 healthy controls. Among the patients with NAFLD, 11 had nonalcoholic fatty liver and 32 had NASH. Specifically, they analyzed the presence and effects of HiAlc Kpn, determining that the abundance of Klebsiella pneumoniae was slightly higher in the feces of NAFLD patients, compared with healthy patients, but that their alcohol-producing ability in NAFLD patients was significantly stronger. Of the patients with NAFLD, 61% carried HiAlc and medium-alcohol-producing Kpn, compared with 6.25% of the controls.
Another phase of their study involved feeding specific pathogen-free mice either HiAlc Kpn, ethanol, or yeast extract peptone dextrose medium (pair-fed) for 4, 6, and 8 weeks. The mice that were fed HiAlc Kpn or ethanol showed clear microsteatosis and macrosteatosis in their livers at 4 and 8 weeks, compared with the pair-fed mice. In addition, the HiAlc-Kpn-fed and ethanol-fed mice had increased levels of aspartate transaminase and alanine transaminase in their serum and increased levels of triglycerides and thiobarbituric acid reactive substances in their liver. The results overall indicated that the HiAlc-Kpn-fed mice had developed hepatic steatosis.
An additional phase included the intestinal flora from a NASH patient with a specific Kpn strain being fed to germ-free mice. At the same time, two types of intestinal flora from mice with NAFLD were transplanted into healthy mice: one induced by two other specific Kpn strains and one in which those strains had been selectively eliminated. The results saw obvious steatosis in the mice who received the flora from either mice with NAFLD induced by Kpn or the NASH patient at 4 weeks and 8 weeks, respectively. The mice who received the flora win which Kpn had been eliminated saw no fat-related changes in the liver. “These results further suggest that HiAlc Kpn might be one of the major causes of NAFLD development,” the researchers wrote.
The authors acknowledged the study’s limitations, chiefly including the lack of a clinical cohort of individuals with auto brewery syndrome but without NAFLD that could be used as a control. However, they also noted a belief that “causality was shown by the transfer experiments of HiAlc Kpn” while adding that “the further analysis of the impact of ethanol in ABS [auto brewery syndrome] patients should be investigated.”
The study was funded by grants from the National Natural Science Foundation for Key Programs of China, the National Natural Science Foundation of China, Megaprojects of Science and Technology Research of China, and CAMS Innovation Fund for Medical Sciences. The authors reported no conflicts of interest.
SOURCE: Yuan J et al. Cell Metab. 2019 Sep 19. doi: 10.1016/j.cmet.2019.08.018.
FROM CELL METABOLISM
Key clinical point: Nonalcoholic fatty liver disease can be caused or exacerbated by excess levels of a high-alcohol-producing bacterial strain.
Major finding: 61% of patients with NAFLD carried high alcohol- and medium alcohol-producing Klebsiella pneumoniae (HiAlc Kpn), compared to 6.25% of healthy controls.
Study details: A multiphase study that included analysis of a 43-patient cohort with nonalcoholic fatty liver disease as well as experiments with mice and HiAlc Kpn.
Disclosures: The study was funded by grants from the National Natural Science Foundation for Key Programs of China, the National Natural Science Foundation of China, Megaprojects of Science and Technology Research of China, and CAMS Innovation Fund for Medical Sciences. The authors reported no conflicts of interest.
Source: Yuan J et al. Cell Metab. 2019 Sep 19. doi: 10.1016/j.cmet.2019.08.018.
Rates of off-label prescribing for children continue to increase
Physicians continue to prescribe off-label drugs for children, with rates increasing over a 10-year period from 2006 to 2015, according to findings from a new study.
The increase occurred despite recent legislation aimed at encouraging pediatric clinical trials, with the intention of improving the “quality of evidence and the number of drugs approved for children,” Divya Hoon of Rutgers University in New Brunswick, N.J., and colleagues wrote in Pediatrics.
“[Our] results can help inform ongoing education, research, and policies around efficacious, effective, and safe use of medications in children,” the researchers said.
To determine trends in, and categories of, drugs prescribed off label, the researchers used data from the National Ambulatory Medical Care Surveys for all pediatric visits and subsequent drug orders from 2006 to 2015. They focused on 141 drugs that are predominantly or exclusively used in systemic formulations and that had been ordered at least 30 times.
At least one off-label systemic drug order occurred at 18.5% of the 1.74 billion estimated ambulatory pediatric visits (95% confidence interval, 17.7%-19.3%), totaling 41.2 million off-label orders per year. The primary reason for a drug being considered off label was that it was for an unapproved condition (74.6%), followed by patient age (17.6%) and weight (0.6%). Absolute and relative rates of off-label ordering increased throughout the study, especially in regard to antihistamines and psychotropic drugs, the investigators said.
In an accompanying editorial, Katelyn Yackey, MD, of the University of Kentucky Children’s Hospital, Lexington, and Rachel Stanley, MD, of Nationwide Children’s Hospital, Columbus, Ohio, stated that “off label is not synonymous with off evidence” and emphasized the need for more clinical trials of medications for children (Pediatrics. 2019 Sep 16. doi: 10.1542/peds.2019-1571).
“Although drugs are often used off label, there may be sufficient preliminary research about a medical condition and particular drugs to support their use,” they wrote. While recognizing that evaluating medications in pediatric patients has been challenging, they added that “children continue to receive medications off label and for unapproved conditions,” so studies that evaluate “safety, efficacy, pharmacokinetics, and optimal dosing in pediatric patients” remain a necessity.
Though the research featured a long study period and large study population, the authors recognized its possible limitations, including the exclusion of less commonly ordered drugs, the inability to determine drug formulation or dosage, and the fact that the survey data captured only ordered medicines and not whether they were actually dispensed or consumed.
The study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the Rutgers Robert Wood Johnson Medical School Summer Research Fellowship. The authors reported no conflicts of interest.
SOURCE: Hoon D et al. Pediatrics. 2019 Sep 16. doi: 10.1542/peds.2019-0896.
Physicians continue to prescribe off-label drugs for children, with rates increasing over a 10-year period from 2006 to 2015, according to findings from a new study.
The increase occurred despite recent legislation aimed at encouraging pediatric clinical trials, with the intention of improving the “quality of evidence and the number of drugs approved for children,” Divya Hoon of Rutgers University in New Brunswick, N.J., and colleagues wrote in Pediatrics.
“[Our] results can help inform ongoing education, research, and policies around efficacious, effective, and safe use of medications in children,” the researchers said.
To determine trends in, and categories of, drugs prescribed off label, the researchers used data from the National Ambulatory Medical Care Surveys for all pediatric visits and subsequent drug orders from 2006 to 2015. They focused on 141 drugs that are predominantly or exclusively used in systemic formulations and that had been ordered at least 30 times.
At least one off-label systemic drug order occurred at 18.5% of the 1.74 billion estimated ambulatory pediatric visits (95% confidence interval, 17.7%-19.3%), totaling 41.2 million off-label orders per year. The primary reason for a drug being considered off label was that it was for an unapproved condition (74.6%), followed by patient age (17.6%) and weight (0.6%). Absolute and relative rates of off-label ordering increased throughout the study, especially in regard to antihistamines and psychotropic drugs, the investigators said.
In an accompanying editorial, Katelyn Yackey, MD, of the University of Kentucky Children’s Hospital, Lexington, and Rachel Stanley, MD, of Nationwide Children’s Hospital, Columbus, Ohio, stated that “off label is not synonymous with off evidence” and emphasized the need for more clinical trials of medications for children (Pediatrics. 2019 Sep 16. doi: 10.1542/peds.2019-1571).
“Although drugs are often used off label, there may be sufficient preliminary research about a medical condition and particular drugs to support their use,” they wrote. While recognizing that evaluating medications in pediatric patients has been challenging, they added that “children continue to receive medications off label and for unapproved conditions,” so studies that evaluate “safety, efficacy, pharmacokinetics, and optimal dosing in pediatric patients” remain a necessity.
Though the research featured a long study period and large study population, the authors recognized its possible limitations, including the exclusion of less commonly ordered drugs, the inability to determine drug formulation or dosage, and the fact that the survey data captured only ordered medicines and not whether they were actually dispensed or consumed.
The study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the Rutgers Robert Wood Johnson Medical School Summer Research Fellowship. The authors reported no conflicts of interest.
SOURCE: Hoon D et al. Pediatrics. 2019 Sep 16. doi: 10.1542/peds.2019-0896.
Physicians continue to prescribe off-label drugs for children, with rates increasing over a 10-year period from 2006 to 2015, according to findings from a new study.
The increase occurred despite recent legislation aimed at encouraging pediatric clinical trials, with the intention of improving the “quality of evidence and the number of drugs approved for children,” Divya Hoon of Rutgers University in New Brunswick, N.J., and colleagues wrote in Pediatrics.
“[Our] results can help inform ongoing education, research, and policies around efficacious, effective, and safe use of medications in children,” the researchers said.
To determine trends in, and categories of, drugs prescribed off label, the researchers used data from the National Ambulatory Medical Care Surveys for all pediatric visits and subsequent drug orders from 2006 to 2015. They focused on 141 drugs that are predominantly or exclusively used in systemic formulations and that had been ordered at least 30 times.
At least one off-label systemic drug order occurred at 18.5% of the 1.74 billion estimated ambulatory pediatric visits (95% confidence interval, 17.7%-19.3%), totaling 41.2 million off-label orders per year. The primary reason for a drug being considered off label was that it was for an unapproved condition (74.6%), followed by patient age (17.6%) and weight (0.6%). Absolute and relative rates of off-label ordering increased throughout the study, especially in regard to antihistamines and psychotropic drugs, the investigators said.
In an accompanying editorial, Katelyn Yackey, MD, of the University of Kentucky Children’s Hospital, Lexington, and Rachel Stanley, MD, of Nationwide Children’s Hospital, Columbus, Ohio, stated that “off label is not synonymous with off evidence” and emphasized the need for more clinical trials of medications for children (Pediatrics. 2019 Sep 16. doi: 10.1542/peds.2019-1571).
“Although drugs are often used off label, there may be sufficient preliminary research about a medical condition and particular drugs to support their use,” they wrote. While recognizing that evaluating medications in pediatric patients has been challenging, they added that “children continue to receive medications off label and for unapproved conditions,” so studies that evaluate “safety, efficacy, pharmacokinetics, and optimal dosing in pediatric patients” remain a necessity.
Though the research featured a long study period and large study population, the authors recognized its possible limitations, including the exclusion of less commonly ordered drugs, the inability to determine drug formulation or dosage, and the fact that the survey data captured only ordered medicines and not whether they were actually dispensed or consumed.
The study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the Rutgers Robert Wood Johnson Medical School Summer Research Fellowship. The authors reported no conflicts of interest.
SOURCE: Hoon D et al. Pediatrics. 2019 Sep 16. doi: 10.1542/peds.2019-0896.
FROM PEDIATRICS
Key clinical point:
Major finding: At least one off-label systemic drug order occurred at 18.5% of the 1.74 billion estimated ambulatory pediatric visits (95% CI, 17.7%-19.3%).
Study details: A retrospective study of serial, cross-sectional data from the National Ambulatory Medical Care Surveys (2006-2015).
Disclosures: The study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the Rutgers Robert Wood Johnson Medical School Summer Research Fellowship. The authors reported no conflicts of interest.
Source: Hoon D et al. Pediatrics. 2019 Sep 16. doi: 10.1542/peds.2019-0896.
Cancer patients increasingly being discharged to subacute rehabilitation facilities
As immunotherapy has become more widely available, cancer patients have been referred to subacute rehabilitation (SAR) facilities at an increasing rate, to become well enough to tolerate treatment, investigators report.
However, “many patients never receive additional therapy and are readmitted or deceased within a short time, marking this population as one with strong needs for concurrent palliative and usual oncology care,” wrote Jonathan C. Yeh, MD, of Johns Hopkins University, Baltimore, and coauthors. Their report is in Journal of Oncology Practice.
To determine if the advent of immunotherapy contributed to an increase in referrals to SAR facilities – and which patients survived long enough to benefit – the researchers reviewed the electronic charts of 358 patients who were referred to such facilities. The number of referrals increased gradually over the 8-year period of the study.
Of these 358 patients, 174 (49%) were seen again in the oncology clinic before readmission or death and 117 (33%) ever received additional cancer-directed treatment. The patients most likely to receive additional treatment were those with leukemia, lymphoma, or localized solid disease.
Of the 413 total discharges, 116 (28%) resulted in hospital readmission within 30 days of discharge. Seventy-four (21%) of the patients were deceased within 30 days; 212 (59%) were deceased within 180 days. Only 123 (30%) of the initial admissions involved a palliative care specialist; this involvement was associated with increases in documented goals of care, completion of advance directives, and election of do-not-resuscitate status.
The authors noted their study’s limitations, including all of the data coming from a single tertiary cancer center. In addition, the data are observational, which made the researchers “unable to control for key patient characteristics such as performance status, patient goals, insurance coverage of trials, and the like.”
Dr. Smith reported being employed by UpToDate and receiving royalties as coeditor of the Oxford Textbook of Cancer Communication. No other conflicts of interest were reported.
SOURCE: Yeh JC et al. J Oncol Pract. 2019 Aug 29. doi: 10.1200/JOP.19.00044.
As immunotherapy has become more widely available, cancer patients have been referred to subacute rehabilitation (SAR) facilities at an increasing rate, to become well enough to tolerate treatment, investigators report.
However, “many patients never receive additional therapy and are readmitted or deceased within a short time, marking this population as one with strong needs for concurrent palliative and usual oncology care,” wrote Jonathan C. Yeh, MD, of Johns Hopkins University, Baltimore, and coauthors. Their report is in Journal of Oncology Practice.
To determine if the advent of immunotherapy contributed to an increase in referrals to SAR facilities – and which patients survived long enough to benefit – the researchers reviewed the electronic charts of 358 patients who were referred to such facilities. The number of referrals increased gradually over the 8-year period of the study.
Of these 358 patients, 174 (49%) were seen again in the oncology clinic before readmission or death and 117 (33%) ever received additional cancer-directed treatment. The patients most likely to receive additional treatment were those with leukemia, lymphoma, or localized solid disease.
Of the 413 total discharges, 116 (28%) resulted in hospital readmission within 30 days of discharge. Seventy-four (21%) of the patients were deceased within 30 days; 212 (59%) were deceased within 180 days. Only 123 (30%) of the initial admissions involved a palliative care specialist; this involvement was associated with increases in documented goals of care, completion of advance directives, and election of do-not-resuscitate status.
The authors noted their study’s limitations, including all of the data coming from a single tertiary cancer center. In addition, the data are observational, which made the researchers “unable to control for key patient characteristics such as performance status, patient goals, insurance coverage of trials, and the like.”
Dr. Smith reported being employed by UpToDate and receiving royalties as coeditor of the Oxford Textbook of Cancer Communication. No other conflicts of interest were reported.
SOURCE: Yeh JC et al. J Oncol Pract. 2019 Aug 29. doi: 10.1200/JOP.19.00044.
As immunotherapy has become more widely available, cancer patients have been referred to subacute rehabilitation (SAR) facilities at an increasing rate, to become well enough to tolerate treatment, investigators report.
However, “many patients never receive additional therapy and are readmitted or deceased within a short time, marking this population as one with strong needs for concurrent palliative and usual oncology care,” wrote Jonathan C. Yeh, MD, of Johns Hopkins University, Baltimore, and coauthors. Their report is in Journal of Oncology Practice.
To determine if the advent of immunotherapy contributed to an increase in referrals to SAR facilities – and which patients survived long enough to benefit – the researchers reviewed the electronic charts of 358 patients who were referred to such facilities. The number of referrals increased gradually over the 8-year period of the study.
Of these 358 patients, 174 (49%) were seen again in the oncology clinic before readmission or death and 117 (33%) ever received additional cancer-directed treatment. The patients most likely to receive additional treatment were those with leukemia, lymphoma, or localized solid disease.
Of the 413 total discharges, 116 (28%) resulted in hospital readmission within 30 days of discharge. Seventy-four (21%) of the patients were deceased within 30 days; 212 (59%) were deceased within 180 days. Only 123 (30%) of the initial admissions involved a palliative care specialist; this involvement was associated with increases in documented goals of care, completion of advance directives, and election of do-not-resuscitate status.
The authors noted their study’s limitations, including all of the data coming from a single tertiary cancer center. In addition, the data are observational, which made the researchers “unable to control for key patient characteristics such as performance status, patient goals, insurance coverage of trials, and the like.”
Dr. Smith reported being employed by UpToDate and receiving royalties as coeditor of the Oxford Textbook of Cancer Communication. No other conflicts of interest were reported.
SOURCE: Yeh JC et al. J Oncol Pract. 2019 Aug 29. doi: 10.1200/JOP.19.00044.
FROM THE JOURNAL OF ONCOLOGY PRACTICE
High-dose teriparatide tops standard dose in boosting BMD in postmenopausal women
A high dose of teriparatide in combination with denosumab increases bone mineral density (BMD) in postmenopausal women with osteoporosis to a greater extent than a lower-dose regimen.
The latest findings suggest that high-dose teriparatide “stimulates even greater separation between bone resorption and formation than that of standard-dose teriparatide,” wrote Joy N. Tsai, MD, of Harvard Medical School, Boston, and her coauthors in Lancet Diabetes & Endocrinology.
Previously, findings from the Denosumab and Teriparatide Administration (DATA) study showed that a combination of teriparatide and denosumab increased both BMD and estimated bone strength more than either drug alone. To determine if a higher dose of teriparatide plus denosumab would result in larger increases in BMD, investigators in the DATA-HD study randomly assigned 76 postmenopausal women with osteoporosis to receive either 40 μg of teriparatide daily (higher dose; n = 37) or 20 μg of teriparatide daily (standard dose; n = 39). At 3 months, patients in both groups were also started on 60 mg of denosumab every 6 months. Of the initial participants, 69 completed at least one postbaseline visit and were included in the analysis.
At the 15-month follow-up, areal BMD (aBMD) had increased in both groups in all measured sites – lumbar spine, femoral neck, total hip, and distal radius. Patients in the 40-μg group had a significantly higher increase in mean lumbar spine aBMD (17.5%), compared with those in the 20-μg or standard-dose group (9.5%; 95% confidence interval, 5.5-10.6; P less than .0001).
There was also a greater increase in mean femoral neck aBMD in patients in the 40-μg group (6.8%), compared with those in the 20-μg group (4.3; 95% CI, 0.5-4.5; P = .04) and in mean total hip aBMD (6.1% vs. 3.9%, 95% CI; 0.6-3.8, P less than .0001).
In all, 29 participants in the 40-μg group (78%) and 30 participants in the 20-μg group (77%) had adverse events, but with the exceptions of headache and rash, all serious adverse events were considered unrelated to treatment.
The authors noted the limitations of their study, including that it was conducted at a single site with a predominantly white population. In addition, the authors acknowledged that the small sample size did not allow for “direct assessment of fracture benefit [or] for rigorous evaluation of the tolerability and safety of this treatment.”
In an accompanying editorial, Sundeep Khosla, MD, of the Mayo Clinic, Rochester, Minn., wrote that the benefits of personalizing treatment for osteoporosis patients at high risk of fracture seemed to be coming into focus (Lancet Diabetes Endocrinol. 2019 Aug 22. doi: 10.1016/S2213-8587(19)30266-9).
Although the DATA and DATA-HD studies of teriparatide and denosumab reported by Dr. Tsai and colleagues have their limitations – including a small sample size for DATA-HD – they indicate the “possibility of refining treatment for patients with osteoporosis at high risk of fracture and personalizing treatment for these patients beyond the one-size-fits-all approach currently used,” Dr. Khosla wrote. Rather than offer bisphosphonates at standardized doses, patients at high risk could now be considered for the newly recommended high-dose teriparatide and denosumab combination, he said.
Dr. Khosla also noted that price remains an issue, given the estimated cost of $76,000 for 15 months of this proposed combination. However, the benefits in regard to at least bone mineral density are clear, he added, and that might prove sufficient enough for high-risk patients in need of an alternative therapy.
The study was supported by the Dart Family Foundation, the National Institutes of Health, and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Eli Lilly and Amgen supplied the drugs. The authors reported numerous conflicts of interest, including receiving grants, reimbursements, and personal fees from various pharmaceutical companies, committees, and research institutes. Dr. Khosla reported no conflicts of interest.
SOURCE: Tsai JN et al. Lancet Diabetes Endocrinol. 2019 Aug 22. doi: 10.1016/S2213-8587(19)30255-4.
A high dose of teriparatide in combination with denosumab increases bone mineral density (BMD) in postmenopausal women with osteoporosis to a greater extent than a lower-dose regimen.
The latest findings suggest that high-dose teriparatide “stimulates even greater separation between bone resorption and formation than that of standard-dose teriparatide,” wrote Joy N. Tsai, MD, of Harvard Medical School, Boston, and her coauthors in Lancet Diabetes & Endocrinology.
Previously, findings from the Denosumab and Teriparatide Administration (DATA) study showed that a combination of teriparatide and denosumab increased both BMD and estimated bone strength more than either drug alone. To determine if a higher dose of teriparatide plus denosumab would result in larger increases in BMD, investigators in the DATA-HD study randomly assigned 76 postmenopausal women with osteoporosis to receive either 40 μg of teriparatide daily (higher dose; n = 37) or 20 μg of teriparatide daily (standard dose; n = 39). At 3 months, patients in both groups were also started on 60 mg of denosumab every 6 months. Of the initial participants, 69 completed at least one postbaseline visit and were included in the analysis.
At the 15-month follow-up, areal BMD (aBMD) had increased in both groups in all measured sites – lumbar spine, femoral neck, total hip, and distal radius. Patients in the 40-μg group had a significantly higher increase in mean lumbar spine aBMD (17.5%), compared with those in the 20-μg or standard-dose group (9.5%; 95% confidence interval, 5.5-10.6; P less than .0001).
There was also a greater increase in mean femoral neck aBMD in patients in the 40-μg group (6.8%), compared with those in the 20-μg group (4.3; 95% CI, 0.5-4.5; P = .04) and in mean total hip aBMD (6.1% vs. 3.9%, 95% CI; 0.6-3.8, P less than .0001).
In all, 29 participants in the 40-μg group (78%) and 30 participants in the 20-μg group (77%) had adverse events, but with the exceptions of headache and rash, all serious adverse events were considered unrelated to treatment.
The authors noted the limitations of their study, including that it was conducted at a single site with a predominantly white population. In addition, the authors acknowledged that the small sample size did not allow for “direct assessment of fracture benefit [or] for rigorous evaluation of the tolerability and safety of this treatment.”
In an accompanying editorial, Sundeep Khosla, MD, of the Mayo Clinic, Rochester, Minn., wrote that the benefits of personalizing treatment for osteoporosis patients at high risk of fracture seemed to be coming into focus (Lancet Diabetes Endocrinol. 2019 Aug 22. doi: 10.1016/S2213-8587(19)30266-9).
Although the DATA and DATA-HD studies of teriparatide and denosumab reported by Dr. Tsai and colleagues have their limitations – including a small sample size for DATA-HD – they indicate the “possibility of refining treatment for patients with osteoporosis at high risk of fracture and personalizing treatment for these patients beyond the one-size-fits-all approach currently used,” Dr. Khosla wrote. Rather than offer bisphosphonates at standardized doses, patients at high risk could now be considered for the newly recommended high-dose teriparatide and denosumab combination, he said.
Dr. Khosla also noted that price remains an issue, given the estimated cost of $76,000 for 15 months of this proposed combination. However, the benefits in regard to at least bone mineral density are clear, he added, and that might prove sufficient enough for high-risk patients in need of an alternative therapy.
The study was supported by the Dart Family Foundation, the National Institutes of Health, and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Eli Lilly and Amgen supplied the drugs. The authors reported numerous conflicts of interest, including receiving grants, reimbursements, and personal fees from various pharmaceutical companies, committees, and research institutes. Dr. Khosla reported no conflicts of interest.
SOURCE: Tsai JN et al. Lancet Diabetes Endocrinol. 2019 Aug 22. doi: 10.1016/S2213-8587(19)30255-4.
A high dose of teriparatide in combination with denosumab increases bone mineral density (BMD) in postmenopausal women with osteoporosis to a greater extent than a lower-dose regimen.
The latest findings suggest that high-dose teriparatide “stimulates even greater separation between bone resorption and formation than that of standard-dose teriparatide,” wrote Joy N. Tsai, MD, of Harvard Medical School, Boston, and her coauthors in Lancet Diabetes & Endocrinology.
Previously, findings from the Denosumab and Teriparatide Administration (DATA) study showed that a combination of teriparatide and denosumab increased both BMD and estimated bone strength more than either drug alone. To determine if a higher dose of teriparatide plus denosumab would result in larger increases in BMD, investigators in the DATA-HD study randomly assigned 76 postmenopausal women with osteoporosis to receive either 40 μg of teriparatide daily (higher dose; n = 37) or 20 μg of teriparatide daily (standard dose; n = 39). At 3 months, patients in both groups were also started on 60 mg of denosumab every 6 months. Of the initial participants, 69 completed at least one postbaseline visit and were included in the analysis.
At the 15-month follow-up, areal BMD (aBMD) had increased in both groups in all measured sites – lumbar spine, femoral neck, total hip, and distal radius. Patients in the 40-μg group had a significantly higher increase in mean lumbar spine aBMD (17.5%), compared with those in the 20-μg or standard-dose group (9.5%; 95% confidence interval, 5.5-10.6; P less than .0001).
There was also a greater increase in mean femoral neck aBMD in patients in the 40-μg group (6.8%), compared with those in the 20-μg group (4.3; 95% CI, 0.5-4.5; P = .04) and in mean total hip aBMD (6.1% vs. 3.9%, 95% CI; 0.6-3.8, P less than .0001).
In all, 29 participants in the 40-μg group (78%) and 30 participants in the 20-μg group (77%) had adverse events, but with the exceptions of headache and rash, all serious adverse events were considered unrelated to treatment.
The authors noted the limitations of their study, including that it was conducted at a single site with a predominantly white population. In addition, the authors acknowledged that the small sample size did not allow for “direct assessment of fracture benefit [or] for rigorous evaluation of the tolerability and safety of this treatment.”
In an accompanying editorial, Sundeep Khosla, MD, of the Mayo Clinic, Rochester, Minn., wrote that the benefits of personalizing treatment for osteoporosis patients at high risk of fracture seemed to be coming into focus (Lancet Diabetes Endocrinol. 2019 Aug 22. doi: 10.1016/S2213-8587(19)30266-9).
Although the DATA and DATA-HD studies of teriparatide and denosumab reported by Dr. Tsai and colleagues have their limitations – including a small sample size for DATA-HD – they indicate the “possibility of refining treatment for patients with osteoporosis at high risk of fracture and personalizing treatment for these patients beyond the one-size-fits-all approach currently used,” Dr. Khosla wrote. Rather than offer bisphosphonates at standardized doses, patients at high risk could now be considered for the newly recommended high-dose teriparatide and denosumab combination, he said.
Dr. Khosla also noted that price remains an issue, given the estimated cost of $76,000 for 15 months of this proposed combination. However, the benefits in regard to at least bone mineral density are clear, he added, and that might prove sufficient enough for high-risk patients in need of an alternative therapy.
The study was supported by the Dart Family Foundation, the National Institutes of Health, and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Eli Lilly and Amgen supplied the drugs. The authors reported numerous conflicts of interest, including receiving grants, reimbursements, and personal fees from various pharmaceutical companies, committees, and research institutes. Dr. Khosla reported no conflicts of interest.
SOURCE: Tsai JN et al. Lancet Diabetes Endocrinol. 2019 Aug 22. doi: 10.1016/S2213-8587(19)30255-4.
FROM LANCET DIABETES & ENDOCRINOLOGY
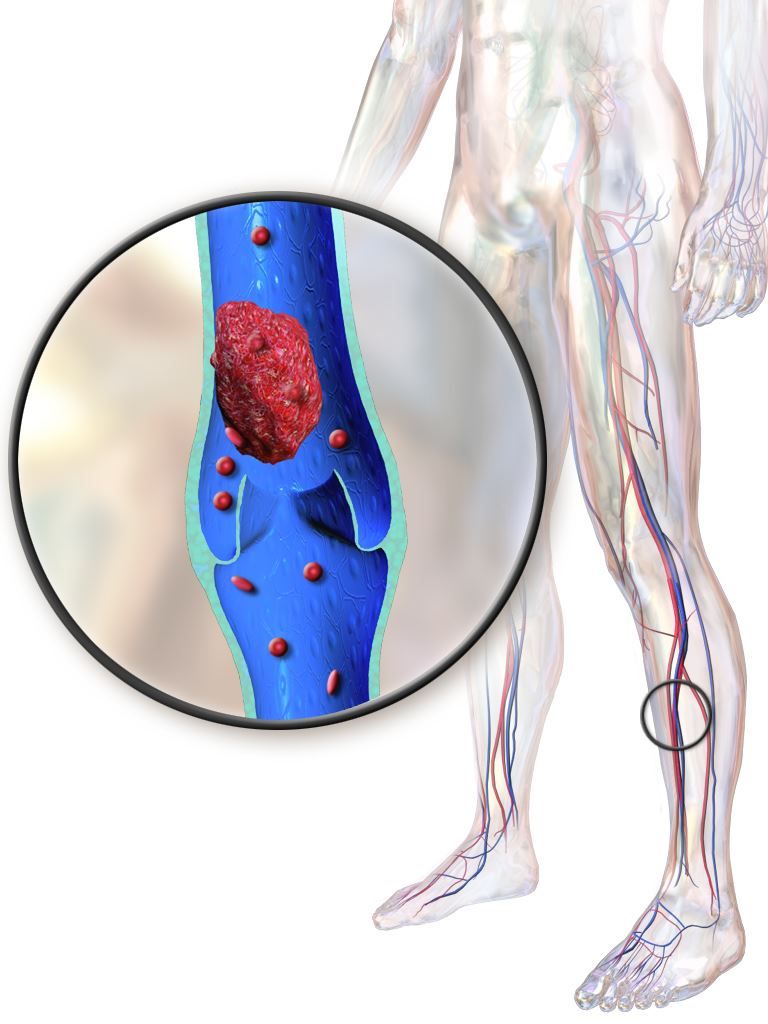
Older IBD patients are most at risk of postdischarge VTE
Hospitalized patients with inflammatory bowel diseases (IBD) are most likely to be readmitted for venous thromboembolism (VTE) within 60 days of discharge, according to a new study that analyzed 5 years of U.S. readmissions data.
“Given increased thrombotic risk postdischarge, as well as overall safety of VTE prophylaxis, extending prophylaxis for those at highest risk may have significant benefits,” wrote Adam S. Faye, MD, of Columbia University, and coauthors. The study was published in Clinical Gastroenterology and Hepatology.
To determine which IBD patients would be most in need of postdischarge VTE prophylaxis, as well as when to administer it, the researchers analyzed 2010-2014 data from the Nationwide Readmissions Database (NRD). They found a total of 872,122 index admissions for IBD patients; 4% of those patients had a prior VTE. Of the index admissions, 1,160 led to a VTE readmission within 90 days. Readmitted patients had a relatively equal proportion of ulcerative colitis (n = 522) and Crohn’s disease (n = 638).
More than 90% of VTE readmissions occurred within 60 days of discharge; the risk was highest over the first 10 days and then decreased in each ensuing 10-day period until a slight increase at the 81- to 90-day period. All patients over age 30 had higher rates of readmission than those of patients under age 18, with the highest risk in patients between the ages of 66 and 80 years (risk ratio 4.04; 95% confidence interval, 2.54-6.44, P less than .01). Women were at lower risk (RR 0.82; 95% CI, 0.73-0.92, P less than .01). Higher risks of readmission were also associated with being on Medicare (RR 1.39; 95% CI, 1.23-1.58, P less than .01) compared with being on private insurance and being cared for at a large hospital (RR 1.26; 95% CI, 1.04-1.52, P = .02) compared with a small hospital.
The highest risk of VTE readmission was associated with a prior history of VTE (RR 2.89; 95% CI, 2.40-3.48, P less than .01), having two or more comorbidities (RR 2.57; 95% CI, 2.11-3.12, P less than .01) and having a Clostridioides difficile infection as of index admission (RR 1.90; 95% CI, 1.51-2.38, P less than .01). In addition, increased risk was associated with being discharged to a nursing or care facility (RR 1.85; 95% CI, 1.56-2.20, P less than .01) or home with health services (RR 2.05; 95% CI, 1.78-2.38, P less than .01) compared with a routine discharge.
In their multivariable analysis, similar factors such as a history of VTE (adjusted RR 2.41; 95% CI, 1.99-2.90, P less than .01), two or more comorbidities (aRR 1.78; 95% CI, 1.44-2.20, P less than .01) and C. difficile infection (aRR 1.47; 95% CI, 1.17-1.85, P less than.01) continued to be associated with higher risk of VTE readmission.
Though they emphasized that the use of NRD data offered the impressive ability to “review over 15 million discharges across the U.S. annually,” Dr. Faye and coauthors acknowledged that their study did have limitations. These included the inability to verify via chart review the study’s outcomes and covariates. In addition, they were unable to assess potential contributing risk factors such as medication use, use of VTE prophylaxis during hospitalization, disease severity, and family history. Finally, though unlikely, they admitted the possibility that patients could be counted more than once if they were readmitted with a VTE each year of the study.
The authors reported being supported by grants from the National Institutes of Health and various pharmaceutical companies, as well as receiving honoraria and serving as consultants.
SOURCE: Faye AS et al. Clin Gastroenterol Hepatol. 2019 July 20. doi: 10.1016/j.cgh.2019.07.028.
Hospitalized patients with inflammatory bowel diseases (IBD) are most likely to be readmitted for venous thromboembolism (VTE) within 60 days of discharge, according to a new study that analyzed 5 years of U.S. readmissions data.
“Given increased thrombotic risk postdischarge, as well as overall safety of VTE prophylaxis, extending prophylaxis for those at highest risk may have significant benefits,” wrote Adam S. Faye, MD, of Columbia University, and coauthors. The study was published in Clinical Gastroenterology and Hepatology.
To determine which IBD patients would be most in need of postdischarge VTE prophylaxis, as well as when to administer it, the researchers analyzed 2010-2014 data from the Nationwide Readmissions Database (NRD). They found a total of 872,122 index admissions for IBD patients; 4% of those patients had a prior VTE. Of the index admissions, 1,160 led to a VTE readmission within 90 days. Readmitted patients had a relatively equal proportion of ulcerative colitis (n = 522) and Crohn’s disease (n = 638).
More than 90% of VTE readmissions occurred within 60 days of discharge; the risk was highest over the first 10 days and then decreased in each ensuing 10-day period until a slight increase at the 81- to 90-day period. All patients over age 30 had higher rates of readmission than those of patients under age 18, with the highest risk in patients between the ages of 66 and 80 years (risk ratio 4.04; 95% confidence interval, 2.54-6.44, P less than .01). Women were at lower risk (RR 0.82; 95% CI, 0.73-0.92, P less than .01). Higher risks of readmission were also associated with being on Medicare (RR 1.39; 95% CI, 1.23-1.58, P less than .01) compared with being on private insurance and being cared for at a large hospital (RR 1.26; 95% CI, 1.04-1.52, P = .02) compared with a small hospital.
The highest risk of VTE readmission was associated with a prior history of VTE (RR 2.89; 95% CI, 2.40-3.48, P less than .01), having two or more comorbidities (RR 2.57; 95% CI, 2.11-3.12, P less than .01) and having a Clostridioides difficile infection as of index admission (RR 1.90; 95% CI, 1.51-2.38, P less than .01). In addition, increased risk was associated with being discharged to a nursing or care facility (RR 1.85; 95% CI, 1.56-2.20, P less than .01) or home with health services (RR 2.05; 95% CI, 1.78-2.38, P less than .01) compared with a routine discharge.
In their multivariable analysis, similar factors such as a history of VTE (adjusted RR 2.41; 95% CI, 1.99-2.90, P less than .01), two or more comorbidities (aRR 1.78; 95% CI, 1.44-2.20, P less than .01) and C. difficile infection (aRR 1.47; 95% CI, 1.17-1.85, P less than.01) continued to be associated with higher risk of VTE readmission.
Though they emphasized that the use of NRD data offered the impressive ability to “review over 15 million discharges across the U.S. annually,” Dr. Faye and coauthors acknowledged that their study did have limitations. These included the inability to verify via chart review the study’s outcomes and covariates. In addition, they were unable to assess potential contributing risk factors such as medication use, use of VTE prophylaxis during hospitalization, disease severity, and family history. Finally, though unlikely, they admitted the possibility that patients could be counted more than once if they were readmitted with a VTE each year of the study.
The authors reported being supported by grants from the National Institutes of Health and various pharmaceutical companies, as well as receiving honoraria and serving as consultants.
SOURCE: Faye AS et al. Clin Gastroenterol Hepatol. 2019 July 20. doi: 10.1016/j.cgh.2019.07.028.
Hospitalized patients with inflammatory bowel diseases (IBD) are most likely to be readmitted for venous thromboembolism (VTE) within 60 days of discharge, according to a new study that analyzed 5 years of U.S. readmissions data.
“Given increased thrombotic risk postdischarge, as well as overall safety of VTE prophylaxis, extending prophylaxis for those at highest risk may have significant benefits,” wrote Adam S. Faye, MD, of Columbia University, and coauthors. The study was published in Clinical Gastroenterology and Hepatology.
To determine which IBD patients would be most in need of postdischarge VTE prophylaxis, as well as when to administer it, the researchers analyzed 2010-2014 data from the Nationwide Readmissions Database (NRD). They found a total of 872,122 index admissions for IBD patients; 4% of those patients had a prior VTE. Of the index admissions, 1,160 led to a VTE readmission within 90 days. Readmitted patients had a relatively equal proportion of ulcerative colitis (n = 522) and Crohn’s disease (n = 638).
More than 90% of VTE readmissions occurred within 60 days of discharge; the risk was highest over the first 10 days and then decreased in each ensuing 10-day period until a slight increase at the 81- to 90-day period. All patients over age 30 had higher rates of readmission than those of patients under age 18, with the highest risk in patients between the ages of 66 and 80 years (risk ratio 4.04; 95% confidence interval, 2.54-6.44, P less than .01). Women were at lower risk (RR 0.82; 95% CI, 0.73-0.92, P less than .01). Higher risks of readmission were also associated with being on Medicare (RR 1.39; 95% CI, 1.23-1.58, P less than .01) compared with being on private insurance and being cared for at a large hospital (RR 1.26; 95% CI, 1.04-1.52, P = .02) compared with a small hospital.
The highest risk of VTE readmission was associated with a prior history of VTE (RR 2.89; 95% CI, 2.40-3.48, P less than .01), having two or more comorbidities (RR 2.57; 95% CI, 2.11-3.12, P less than .01) and having a Clostridioides difficile infection as of index admission (RR 1.90; 95% CI, 1.51-2.38, P less than .01). In addition, increased risk was associated with being discharged to a nursing or care facility (RR 1.85; 95% CI, 1.56-2.20, P less than .01) or home with health services (RR 2.05; 95% CI, 1.78-2.38, P less than .01) compared with a routine discharge.
In their multivariable analysis, similar factors such as a history of VTE (adjusted RR 2.41; 95% CI, 1.99-2.90, P less than .01), two or more comorbidities (aRR 1.78; 95% CI, 1.44-2.20, P less than .01) and C. difficile infection (aRR 1.47; 95% CI, 1.17-1.85, P less than.01) continued to be associated with higher risk of VTE readmission.
Though they emphasized that the use of NRD data offered the impressive ability to “review over 15 million discharges across the U.S. annually,” Dr. Faye and coauthors acknowledged that their study did have limitations. These included the inability to verify via chart review the study’s outcomes and covariates. In addition, they were unable to assess potential contributing risk factors such as medication use, use of VTE prophylaxis during hospitalization, disease severity, and family history. Finally, though unlikely, they admitted the possibility that patients could be counted more than once if they were readmitted with a VTE each year of the study.
The authors reported being supported by grants from the National Institutes of Health and various pharmaceutical companies, as well as receiving honoraria and serving as consultants.
SOURCE: Faye AS et al. Clin Gastroenterol Hepatol. 2019 July 20. doi: 10.1016/j.cgh.2019.07.028.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Readmission for VTE in patients with inflammatory bowel diseases most often occurs within 60 days of discharge.
Major finding: The highest readmission risk was in patients between the ages of 66 and 80 (risk ratio 4.04; 95% confidence interval, 2.54-6.44, P less than .01).
Study details: A retrospective cohort study of 1,160 IBD patients who had VTE readmissions via 2010-2014 data from the Nationwide Readmissions Database.
Disclosures: The authors reported being supported by grants from the National Institutes of Health and various pharmaceutical companies, as well as receiving honoraria and serving as consultants.
Source: Faye AS et al. Clin Gastroenterol Hepatol. 2019 July 20. doi: 10.1016/j.cgh.2019.07.028.
BPA substitutes bisphenol S and bisphenol F linked to obesity
Though exposure to the obesogen bisphenol A (BPA) is decreasing, a new study has linked substitute chemicals bisphenol S (BPS) and bisphenol F (BPF) to obesity as well.
“The potential health effects of BPS and other BPA replacement compounds should be monitored going forward, given that human exposure to these compounds is likely to continue to increase in the future,” wrote Melanie H. Jacobson, PhD, of New York University, and coauthors. Their report is in the Journal of the Endocrine Society.
BPA is one of the best known synthetic chemical obesogens, the authors noted. “It enlarges adipocytes and enhances differentiation from mesenchymal cells to adipocytes, inhibits adiponectin function, and is a synthetic estrogen and thereby can have sex-specific effects on body mass,” they explained.
To determine if the BPA analogues, BPS and BPF, could also induce obesity, the researchers analyzed data from 1,831 children and adolescents gathered through the U.S. National Health and Nutrition Examination Surveys from 2013 to 2016. Concentrations of BPA, BPS, and BPF were measured in spot urine samples and they were detected in 97.5%, 87.8%, and 55.2% of samples, respectively.
Log-transformed BPS concentrations were associated with an increased prevalence of general obesity (odds ratio, 1.16; 95% confidence interval, 1.02-1.32), which was defined as being greater than or equal to the 95th percentile of standardized body mass index z scores. BPS concentrations were also associated with an increased prevalence of abdominal obesity (OR, 1.13; 95% CI, 1.02-1.27), as was BPF detection (OR, 1.29; 95% CI, 1.01-1.64).
BPA and total bisphenols were not significantly associated with general or abdominal obesity.
“Though tissue and animal studies of the replacements are lacking, [BPS and BPF] have shown estrogenic activity. Further, BPS has been shown to promote preadipocyte differentiation, raising the possibility that these BPA replacements may induce the same obesogenic effects in humans,” the authors wrote.
They acknowledged that their results should be interpreted cautiously, because they were not able to determine if exposure to bisphenols influences weight gain or if obese children are merely more exposed to those compounds. In addition, because BPS and BPF are metabolized rapidly, spot urine samples cannot accurately reflect long-term exposure levels. Finally, because a good deal of food and beverage packaging contains bisphenols, “those who consume more of these products are more likely to have higher exposure levels,” they wrote.
The study was funded by grants from the National Institutes of Environmental Health Sciences. The authors reported no conflicts of interest.
SOURCE: Jacobson MH et al. J Endocr Soc. 2019 Jul 25. doi: 10.1210/js.2019-00201.
Though exposure to the obesogen bisphenol A (BPA) is decreasing, a new study has linked substitute chemicals bisphenol S (BPS) and bisphenol F (BPF) to obesity as well.
“The potential health effects of BPS and other BPA replacement compounds should be monitored going forward, given that human exposure to these compounds is likely to continue to increase in the future,” wrote Melanie H. Jacobson, PhD, of New York University, and coauthors. Their report is in the Journal of the Endocrine Society.
BPA is one of the best known synthetic chemical obesogens, the authors noted. “It enlarges adipocytes and enhances differentiation from mesenchymal cells to adipocytes, inhibits adiponectin function, and is a synthetic estrogen and thereby can have sex-specific effects on body mass,” they explained.
To determine if the BPA analogues, BPS and BPF, could also induce obesity, the researchers analyzed data from 1,831 children and adolescents gathered through the U.S. National Health and Nutrition Examination Surveys from 2013 to 2016. Concentrations of BPA, BPS, and BPF were measured in spot urine samples and they were detected in 97.5%, 87.8%, and 55.2% of samples, respectively.
Log-transformed BPS concentrations were associated with an increased prevalence of general obesity (odds ratio, 1.16; 95% confidence interval, 1.02-1.32), which was defined as being greater than or equal to the 95th percentile of standardized body mass index z scores. BPS concentrations were also associated with an increased prevalence of abdominal obesity (OR, 1.13; 95% CI, 1.02-1.27), as was BPF detection (OR, 1.29; 95% CI, 1.01-1.64).
BPA and total bisphenols were not significantly associated with general or abdominal obesity.
“Though tissue and animal studies of the replacements are lacking, [BPS and BPF] have shown estrogenic activity. Further, BPS has been shown to promote preadipocyte differentiation, raising the possibility that these BPA replacements may induce the same obesogenic effects in humans,” the authors wrote.
They acknowledged that their results should be interpreted cautiously, because they were not able to determine if exposure to bisphenols influences weight gain or if obese children are merely more exposed to those compounds. In addition, because BPS and BPF are metabolized rapidly, spot urine samples cannot accurately reflect long-term exposure levels. Finally, because a good deal of food and beverage packaging contains bisphenols, “those who consume more of these products are more likely to have higher exposure levels,” they wrote.
The study was funded by grants from the National Institutes of Environmental Health Sciences. The authors reported no conflicts of interest.
SOURCE: Jacobson MH et al. J Endocr Soc. 2019 Jul 25. doi: 10.1210/js.2019-00201.
Though exposure to the obesogen bisphenol A (BPA) is decreasing, a new study has linked substitute chemicals bisphenol S (BPS) and bisphenol F (BPF) to obesity as well.
“The potential health effects of BPS and other BPA replacement compounds should be monitored going forward, given that human exposure to these compounds is likely to continue to increase in the future,” wrote Melanie H. Jacobson, PhD, of New York University, and coauthors. Their report is in the Journal of the Endocrine Society.
BPA is one of the best known synthetic chemical obesogens, the authors noted. “It enlarges adipocytes and enhances differentiation from mesenchymal cells to adipocytes, inhibits adiponectin function, and is a synthetic estrogen and thereby can have sex-specific effects on body mass,” they explained.
To determine if the BPA analogues, BPS and BPF, could also induce obesity, the researchers analyzed data from 1,831 children and adolescents gathered through the U.S. National Health and Nutrition Examination Surveys from 2013 to 2016. Concentrations of BPA, BPS, and BPF were measured in spot urine samples and they were detected in 97.5%, 87.8%, and 55.2% of samples, respectively.
Log-transformed BPS concentrations were associated with an increased prevalence of general obesity (odds ratio, 1.16; 95% confidence interval, 1.02-1.32), which was defined as being greater than or equal to the 95th percentile of standardized body mass index z scores. BPS concentrations were also associated with an increased prevalence of abdominal obesity (OR, 1.13; 95% CI, 1.02-1.27), as was BPF detection (OR, 1.29; 95% CI, 1.01-1.64).
BPA and total bisphenols were not significantly associated with general or abdominal obesity.
“Though tissue and animal studies of the replacements are lacking, [BPS and BPF] have shown estrogenic activity. Further, BPS has been shown to promote preadipocyte differentiation, raising the possibility that these BPA replacements may induce the same obesogenic effects in humans,” the authors wrote.
They acknowledged that their results should be interpreted cautiously, because they were not able to determine if exposure to bisphenols influences weight gain or if obese children are merely more exposed to those compounds. In addition, because BPS and BPF are metabolized rapidly, spot urine samples cannot accurately reflect long-term exposure levels. Finally, because a good deal of food and beverage packaging contains bisphenols, “those who consume more of these products are more likely to have higher exposure levels,” they wrote.
The study was funded by grants from the National Institutes of Environmental Health Sciences. The authors reported no conflicts of interest.
SOURCE: Jacobson MH et al. J Endocr Soc. 2019 Jul 25. doi: 10.1210/js.2019-00201.
FROM JOURNAL OF THE ENDOCRINE SOCIETY
Cephalosporins remain empiric therapy for skin infections in pediatric AD
“Clindamycin, tetracyclines, or TMP‐SMX can be considered in patients suspected to have, or with a history of, MRSA [methicillin‐resistant S. aureus] infection,” wrote Cristopher C. Briscoe, MD, of the Washington University School of Medicine in St. Louis, Missouri, and his coauthors. The study was published in Pediatric Dermatology.
To determine the optimal empiric antibiotic for pediatric AD patients with skin infections, the researchers analyzed skin cultures from 106 patients seen at Saint Louis Children’s Hospital (SLCH). The results were also compared to cultures from pediatric patients who presented at the SLCH emergency department (ED) with S. aureus skin abscesses.
Of the 170 cultures that grew S. aureus, 130 (77.8%) grew MSSA, and 37 (22.2%) grew MRSA. Three of the cultures grew both. The prevalence of MRSA in the cohort differed from the prevalence in the ED patients (44%). The prevalence of either infection did not differ significantly by age, sex or race, though the average number of cultures in African American patients topped the average for Caucasian patients (1.8 vs. 1.2, P less than .003).
All patients with MSSA – in both the cohort and the ED – proved 100% susceptible to cefazolin. Cohort patients with MSSA saw lower susceptibility to doxycycline compared to the ED patients (89.4% vs. 97%), as did MRSA cohort patients to trimethoprim‐sulfamethoxazole (92% vs. 98%).
“When a patient with AD walks into your office and looks like they have an infection of their eczema, your go-to antibiotic is going to be one that targets MSSA,” said coauthor Carrie Coughlin, MD, of the Washington University School of Medicine in an interview. “You’ll still do a culture to prove or disprove that assumption, but it gives you a guide to help make that patient better in the short term while you work things up.”
“Also, remember that MSSA is not ‘better’ to have than MRSA,” she added. “You can now see some of the virulence factors from MRSA strains in MSSA strains, so treating both of them is important.”
The authors acknowledged their study’s limitations, including the limited generalizability of a single-center design and a lack of information as to the body sites from which the cultures were obtained. They were also unable to reliably determine prior antibiotic exposure, noting that “future work could examine whether prior exposure differed significantly in the MRSA and MSSA groups.”
The study was funded by grants from the Agency for Healthcare Research and Quality. The authors reported no conflicts of interest.
SOURCE: Briscoe CC et al. Pediatr Dermatol. 2019 May 24. doi: 10.1111/pde.13867.
“Clindamycin, tetracyclines, or TMP‐SMX can be considered in patients suspected to have, or with a history of, MRSA [methicillin‐resistant S. aureus] infection,” wrote Cristopher C. Briscoe, MD, of the Washington University School of Medicine in St. Louis, Missouri, and his coauthors. The study was published in Pediatric Dermatology.
To determine the optimal empiric antibiotic for pediatric AD patients with skin infections, the researchers analyzed skin cultures from 106 patients seen at Saint Louis Children’s Hospital (SLCH). The results were also compared to cultures from pediatric patients who presented at the SLCH emergency department (ED) with S. aureus skin abscesses.
Of the 170 cultures that grew S. aureus, 130 (77.8%) grew MSSA, and 37 (22.2%) grew MRSA. Three of the cultures grew both. The prevalence of MRSA in the cohort differed from the prevalence in the ED patients (44%). The prevalence of either infection did not differ significantly by age, sex or race, though the average number of cultures in African American patients topped the average for Caucasian patients (1.8 vs. 1.2, P less than .003).
All patients with MSSA – in both the cohort and the ED – proved 100% susceptible to cefazolin. Cohort patients with MSSA saw lower susceptibility to doxycycline compared to the ED patients (89.4% vs. 97%), as did MRSA cohort patients to trimethoprim‐sulfamethoxazole (92% vs. 98%).
“When a patient with AD walks into your office and looks like they have an infection of their eczema, your go-to antibiotic is going to be one that targets MSSA,” said coauthor Carrie Coughlin, MD, of the Washington University School of Medicine in an interview. “You’ll still do a culture to prove or disprove that assumption, but it gives you a guide to help make that patient better in the short term while you work things up.”
“Also, remember that MSSA is not ‘better’ to have than MRSA,” she added. “You can now see some of the virulence factors from MRSA strains in MSSA strains, so treating both of them is important.”
The authors acknowledged their study’s limitations, including the limited generalizability of a single-center design and a lack of information as to the body sites from which the cultures were obtained. They were also unable to reliably determine prior antibiotic exposure, noting that “future work could examine whether prior exposure differed significantly in the MRSA and MSSA groups.”
The study was funded by grants from the Agency for Healthcare Research and Quality. The authors reported no conflicts of interest.
SOURCE: Briscoe CC et al. Pediatr Dermatol. 2019 May 24. doi: 10.1111/pde.13867.
“Clindamycin, tetracyclines, or TMP‐SMX can be considered in patients suspected to have, or with a history of, MRSA [methicillin‐resistant S. aureus] infection,” wrote Cristopher C. Briscoe, MD, of the Washington University School of Medicine in St. Louis, Missouri, and his coauthors. The study was published in Pediatric Dermatology.
To determine the optimal empiric antibiotic for pediatric AD patients with skin infections, the researchers analyzed skin cultures from 106 patients seen at Saint Louis Children’s Hospital (SLCH). The results were also compared to cultures from pediatric patients who presented at the SLCH emergency department (ED) with S. aureus skin abscesses.
Of the 170 cultures that grew S. aureus, 130 (77.8%) grew MSSA, and 37 (22.2%) grew MRSA. Three of the cultures grew both. The prevalence of MRSA in the cohort differed from the prevalence in the ED patients (44%). The prevalence of either infection did not differ significantly by age, sex or race, though the average number of cultures in African American patients topped the average for Caucasian patients (1.8 vs. 1.2, P less than .003).
All patients with MSSA – in both the cohort and the ED – proved 100% susceptible to cefazolin. Cohort patients with MSSA saw lower susceptibility to doxycycline compared to the ED patients (89.4% vs. 97%), as did MRSA cohort patients to trimethoprim‐sulfamethoxazole (92% vs. 98%).
“When a patient with AD walks into your office and looks like they have an infection of their eczema, your go-to antibiotic is going to be one that targets MSSA,” said coauthor Carrie Coughlin, MD, of the Washington University School of Medicine in an interview. “You’ll still do a culture to prove or disprove that assumption, but it gives you a guide to help make that patient better in the short term while you work things up.”
“Also, remember that MSSA is not ‘better’ to have than MRSA,” she added. “You can now see some of the virulence factors from MRSA strains in MSSA strains, so treating both of them is important.”
The authors acknowledged their study’s limitations, including the limited generalizability of a single-center design and a lack of information as to the body sites from which the cultures were obtained. They were also unable to reliably determine prior antibiotic exposure, noting that “future work could examine whether prior exposure differed significantly in the MRSA and MSSA groups.”
The study was funded by grants from the Agency for Healthcare Research and Quality. The authors reported no conflicts of interest.
SOURCE: Briscoe CC et al. Pediatr Dermatol. 2019 May 24. doi: 10.1111/pde.13867.
FROM PEDIATRIC DERMATOLOGY
Endoscopic therapy decreases recurrence of intestinal metaplasia, dysplasia in patients with Barrett’s esophagus
A study of patients with Barrett’s esophagus found that, although intestinal metaplasia and dysplasia in the cardia were common before treatment, they were more frequently present at higher levels and successful endoscopic eradication therapy lessened the risk.
“The results of this study provide evidence to suggest that, in Barrett’s esophagus patients who have achieved CEIM [complete eradication of intestinal metaplasia], it is sufficient to perform a close examination of the cardia and, in the absence of visible abnormalities, to randomly biopsy only at the level of TGF [top of gastric folds], rather than deeper into the cardia, during surveillance exams,” wrote Swathi Eluri, MD, of the University of North Carolina in Chapel Hill and coauthors. The study was published in Clinical Gastroenterology and Hepatology.
To determine the prevalence of intestinal metaplasia or dysplasia in the cardia of patients with Barrett’s esophagus who successfully underwent endoscopic eradication therapy (EET), along with the incidence of cardia intestinal metaplasia or dysplasia in patients undergoing EET, this single-center study examined two groups: a cross-sectional group of 116 patients who had achieved CEIM, and a longitudinal group of 42 treatment-naive patients who were receiving EET and subsequently achieved CEIM.
Along with clinical biopsies, the cross-sectional group underwent standardized biopsies from four quadrants in four locations: the distal esophagus, 1 cm proximal to top of gastric folds (TGF–1); at TGF; 1 cm into the gastric cardia (TGF+1); and 2 cm into the cardia (TGF+2). The longitudinal group also underwent 16 biopsies in the same areas; after CEIM was achieved, they underwent standard research biopsies of the distal esophagus and cardia at 6- and 18-month follow-ups.
Within the cross-sectional group, 15% of patients (n = 17) had intestinal metaplasia or dysplasia in the cardia after CEIM. Of those 17 patients, 12 had intestinal metaplasia, 2 were indefinite for dysplasia, and 3 had low-grade dysplasia. Of the 12 patients with cardia intestinal metaplasia, 83% had it at the level of TGF; 50% at TGF+1; and 25% at TGF+2.
Within the longitudinal group, 28% of patients (n = 12) had intestinal metaplasia or dysplasia in the cardia before ablation. Of those 12 patients, 9 had dysplastic intestinal metaplasia. Cases of pretreatment dysplasia were all found at the level of TGF, with one case extended to TGF+1. All patients achieved CEIM; at 18 months post CEIM, two patients had intestinal metaplasia and none had dysplasia.
The authors shared their study’s limitations, which included the lack of generalizability of a single-center study and a notable number of dropouts in the longitudinal group. They also acknowledged using multiple ablation modalities, although they added that most patients in both groups underwent radiofrequency ablation, the most commonly used treatment method and one that made “the results of the study more applicable to real-world practice.”
In turn, the authors noted their study’s strengths, which included the collection of data in a standardized manner and the availability of complete ablation history for all patients. Theirs was also the first study to systematically sample the cardia at multiple levels, which allows for “a more granular understanding of the location of initial and incident cardia lesions, which can guide depth of ablation during EET.”
The study was funded by an American Gastroenterological Association Research Scholar Award and CSA Medical. The authors reported no conflicts of interest.
SOURCE: Eluri S et al. Clin Gastroenterol Hepatol. 2019 May 8. doi: 10.1016/j.cgh.2019.04.065.
A study of patients with Barrett’s esophagus found that, although intestinal metaplasia and dysplasia in the cardia were common before treatment, they were more frequently present at higher levels and successful endoscopic eradication therapy lessened the risk.
“The results of this study provide evidence to suggest that, in Barrett’s esophagus patients who have achieved CEIM [complete eradication of intestinal metaplasia], it is sufficient to perform a close examination of the cardia and, in the absence of visible abnormalities, to randomly biopsy only at the level of TGF [top of gastric folds], rather than deeper into the cardia, during surveillance exams,” wrote Swathi Eluri, MD, of the University of North Carolina in Chapel Hill and coauthors. The study was published in Clinical Gastroenterology and Hepatology.
To determine the prevalence of intestinal metaplasia or dysplasia in the cardia of patients with Barrett’s esophagus who successfully underwent endoscopic eradication therapy (EET), along with the incidence of cardia intestinal metaplasia or dysplasia in patients undergoing EET, this single-center study examined two groups: a cross-sectional group of 116 patients who had achieved CEIM, and a longitudinal group of 42 treatment-naive patients who were receiving EET and subsequently achieved CEIM.
Along with clinical biopsies, the cross-sectional group underwent standardized biopsies from four quadrants in four locations: the distal esophagus, 1 cm proximal to top of gastric folds (TGF–1); at TGF; 1 cm into the gastric cardia (TGF+1); and 2 cm into the cardia (TGF+2). The longitudinal group also underwent 16 biopsies in the same areas; after CEIM was achieved, they underwent standard research biopsies of the distal esophagus and cardia at 6- and 18-month follow-ups.
Within the cross-sectional group, 15% of patients (n = 17) had intestinal metaplasia or dysplasia in the cardia after CEIM. Of those 17 patients, 12 had intestinal metaplasia, 2 were indefinite for dysplasia, and 3 had low-grade dysplasia. Of the 12 patients with cardia intestinal metaplasia, 83% had it at the level of TGF; 50% at TGF+1; and 25% at TGF+2.
Within the longitudinal group, 28% of patients (n = 12) had intestinal metaplasia or dysplasia in the cardia before ablation. Of those 12 patients, 9 had dysplastic intestinal metaplasia. Cases of pretreatment dysplasia were all found at the level of TGF, with one case extended to TGF+1. All patients achieved CEIM; at 18 months post CEIM, two patients had intestinal metaplasia and none had dysplasia.
The authors shared their study’s limitations, which included the lack of generalizability of a single-center study and a notable number of dropouts in the longitudinal group. They also acknowledged using multiple ablation modalities, although they added that most patients in both groups underwent radiofrequency ablation, the most commonly used treatment method and one that made “the results of the study more applicable to real-world practice.”
In turn, the authors noted their study’s strengths, which included the collection of data in a standardized manner and the availability of complete ablation history for all patients. Theirs was also the first study to systematically sample the cardia at multiple levels, which allows for “a more granular understanding of the location of initial and incident cardia lesions, which can guide depth of ablation during EET.”
The study was funded by an American Gastroenterological Association Research Scholar Award and CSA Medical. The authors reported no conflicts of interest.
SOURCE: Eluri S et al. Clin Gastroenterol Hepatol. 2019 May 8. doi: 10.1016/j.cgh.2019.04.065.
A study of patients with Barrett’s esophagus found that, although intestinal metaplasia and dysplasia in the cardia were common before treatment, they were more frequently present at higher levels and successful endoscopic eradication therapy lessened the risk.
“The results of this study provide evidence to suggest that, in Barrett’s esophagus patients who have achieved CEIM [complete eradication of intestinal metaplasia], it is sufficient to perform a close examination of the cardia and, in the absence of visible abnormalities, to randomly biopsy only at the level of TGF [top of gastric folds], rather than deeper into the cardia, during surveillance exams,” wrote Swathi Eluri, MD, of the University of North Carolina in Chapel Hill and coauthors. The study was published in Clinical Gastroenterology and Hepatology.
To determine the prevalence of intestinal metaplasia or dysplasia in the cardia of patients with Barrett’s esophagus who successfully underwent endoscopic eradication therapy (EET), along with the incidence of cardia intestinal metaplasia or dysplasia in patients undergoing EET, this single-center study examined two groups: a cross-sectional group of 116 patients who had achieved CEIM, and a longitudinal group of 42 treatment-naive patients who were receiving EET and subsequently achieved CEIM.
Along with clinical biopsies, the cross-sectional group underwent standardized biopsies from four quadrants in four locations: the distal esophagus, 1 cm proximal to top of gastric folds (TGF–1); at TGF; 1 cm into the gastric cardia (TGF+1); and 2 cm into the cardia (TGF+2). The longitudinal group also underwent 16 biopsies in the same areas; after CEIM was achieved, they underwent standard research biopsies of the distal esophagus and cardia at 6- and 18-month follow-ups.
Within the cross-sectional group, 15% of patients (n = 17) had intestinal metaplasia or dysplasia in the cardia after CEIM. Of those 17 patients, 12 had intestinal metaplasia, 2 were indefinite for dysplasia, and 3 had low-grade dysplasia. Of the 12 patients with cardia intestinal metaplasia, 83% had it at the level of TGF; 50% at TGF+1; and 25% at TGF+2.
Within the longitudinal group, 28% of patients (n = 12) had intestinal metaplasia or dysplasia in the cardia before ablation. Of those 12 patients, 9 had dysplastic intestinal metaplasia. Cases of pretreatment dysplasia were all found at the level of TGF, with one case extended to TGF+1. All patients achieved CEIM; at 18 months post CEIM, two patients had intestinal metaplasia and none had dysplasia.
The authors shared their study’s limitations, which included the lack of generalizability of a single-center study and a notable number of dropouts in the longitudinal group. They also acknowledged using multiple ablation modalities, although they added that most patients in both groups underwent radiofrequency ablation, the most commonly used treatment method and one that made “the results of the study more applicable to real-world practice.”
In turn, the authors noted their study’s strengths, which included the collection of data in a standardized manner and the availability of complete ablation history for all patients. Theirs was also the first study to systematically sample the cardia at multiple levels, which allows for “a more granular understanding of the location of initial and incident cardia lesions, which can guide depth of ablation during EET.”
The study was funded by an American Gastroenterological Association Research Scholar Award and CSA Medical. The authors reported no conflicts of interest.
SOURCE: Eluri S et al. Clin Gastroenterol Hepatol. 2019 May 8. doi: 10.1016/j.cgh.2019.04.065.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Exposure to outdoor air pollutants linked to increased emphysema
Long-term exposure to ambient air pollutants was significantly associated with increases in emphysema and decreases in lung function, according to a diverse cohort study of six U.S. metropolitan areas.
“These associations in a community-based population demonstrate novel evidence that air pollution contributes to worsening lung health,” wrote Meng Wang, PhD, of the University of Washington, Seattle, and coauthors. The study was published in JAMA.
To determine whether exposure to outdoor air pollutants was associated with emphysema progression and change in lung function, the Multiethnic Study of Atherosclerosis (MESA) assessed 6,860 participants from six areas: Winston-Salem, N.C.; New York City; Baltimore; St. Paul, Minn.; Chicago; and Los Angeles. Percent emphysema was calculated based on all available CT scans; lung function was assessed via spirometry.
Spatiotemporal exposure models were developed for ozone, fine particulate matter less than 2.5 mcm in aerodynamic diameter, and oxides of nitrogen in each area based on Environmental Protection Agency measurements and the study’s cohort-specific monitoring. Annual mean concentrations of fine particulate matter and nitrogen decreased during follow-up, while ozone concentrations did not.
All participants underwent a cardiac CT scan at baseline, and 5,780 had at least one follow-up CT scan over a median period of 10 years. Ambient concentrations of ozone, fine particulate matter, nitrogen, and black carbon at baseline were significantly associated with greater increases in percent emphysema per 10 years, as were concentrations of zone and nitrogen during follow-up, reported Dr. Wang, formerly with the State University of New York at Buffalo.
Of the 3,636 participants who had at least one spirometric assessment, there was a mean decline in forced expiratory volume in 1 second (FEV1) of 309 mL (95% CI, 299-319 mL) and in forced vital capacity of 331 mL (95% CI, 317-345 mL) over 10 years. Ambient concentrations of ozone were significantly associated with a decline in FEV1, both at baseline and during follow-up.
The coauthors acknowledged their study’s limitations, including that general outdoor air pollution concentrations might not reflect individual exposure or concentrations indoors, where people spend most of their time. In addition, percent emphysema was only measured in the lower two-thirds of the lung, though they noted that “percent emphysema measured in the lower two-thirds of the lung correlates well with full-lung percent emphysema in this cohort and a cohort of smokers.”
This article was developed by the EPA and the University of Washington Center for Clean Air Research. MESA was funded by the National Institutes of Health and the National Heart, Lung, and Blood Institute, and supported by the National Institute of Environmental Health Sciences. The authors reported numerous conflicts of interest, including receiving grants and fees from the University of Washington, the EPA, the NIH, and various other pharmaceutical companies, foundations, and governmental entities.
SOURCE: Wang M et al. JAMA. 2019 Aug 13. doi: 10.1001/jama.2019.10255
Long-term exposure to ambient air pollutants was significantly associated with increases in emphysema and decreases in lung function, according to a diverse cohort study of six U.S. metropolitan areas.
“These associations in a community-based population demonstrate novel evidence that air pollution contributes to worsening lung health,” wrote Meng Wang, PhD, of the University of Washington, Seattle, and coauthors. The study was published in JAMA.
To determine whether exposure to outdoor air pollutants was associated with emphysema progression and change in lung function, the Multiethnic Study of Atherosclerosis (MESA) assessed 6,860 participants from six areas: Winston-Salem, N.C.; New York City; Baltimore; St. Paul, Minn.; Chicago; and Los Angeles. Percent emphysema was calculated based on all available CT scans; lung function was assessed via spirometry.
Spatiotemporal exposure models were developed for ozone, fine particulate matter less than 2.5 mcm in aerodynamic diameter, and oxides of nitrogen in each area based on Environmental Protection Agency measurements and the study’s cohort-specific monitoring. Annual mean concentrations of fine particulate matter and nitrogen decreased during follow-up, while ozone concentrations did not.
All participants underwent a cardiac CT scan at baseline, and 5,780 had at least one follow-up CT scan over a median period of 10 years. Ambient concentrations of ozone, fine particulate matter, nitrogen, and black carbon at baseline were significantly associated with greater increases in percent emphysema per 10 years, as were concentrations of zone and nitrogen during follow-up, reported Dr. Wang, formerly with the State University of New York at Buffalo.
Of the 3,636 participants who had at least one spirometric assessment, there was a mean decline in forced expiratory volume in 1 second (FEV1) of 309 mL (95% CI, 299-319 mL) and in forced vital capacity of 331 mL (95% CI, 317-345 mL) over 10 years. Ambient concentrations of ozone were significantly associated with a decline in FEV1, both at baseline and during follow-up.
The coauthors acknowledged their study’s limitations, including that general outdoor air pollution concentrations might not reflect individual exposure or concentrations indoors, where people spend most of their time. In addition, percent emphysema was only measured in the lower two-thirds of the lung, though they noted that “percent emphysema measured in the lower two-thirds of the lung correlates well with full-lung percent emphysema in this cohort and a cohort of smokers.”
This article was developed by the EPA and the University of Washington Center for Clean Air Research. MESA was funded by the National Institutes of Health and the National Heart, Lung, and Blood Institute, and supported by the National Institute of Environmental Health Sciences. The authors reported numerous conflicts of interest, including receiving grants and fees from the University of Washington, the EPA, the NIH, and various other pharmaceutical companies, foundations, and governmental entities.
SOURCE: Wang M et al. JAMA. 2019 Aug 13. doi: 10.1001/jama.2019.10255
Long-term exposure to ambient air pollutants was significantly associated with increases in emphysema and decreases in lung function, according to a diverse cohort study of six U.S. metropolitan areas.
“These associations in a community-based population demonstrate novel evidence that air pollution contributes to worsening lung health,” wrote Meng Wang, PhD, of the University of Washington, Seattle, and coauthors. The study was published in JAMA.
To determine whether exposure to outdoor air pollutants was associated with emphysema progression and change in lung function, the Multiethnic Study of Atherosclerosis (MESA) assessed 6,860 participants from six areas: Winston-Salem, N.C.; New York City; Baltimore; St. Paul, Minn.; Chicago; and Los Angeles. Percent emphysema was calculated based on all available CT scans; lung function was assessed via spirometry.
Spatiotemporal exposure models were developed for ozone, fine particulate matter less than 2.5 mcm in aerodynamic diameter, and oxides of nitrogen in each area based on Environmental Protection Agency measurements and the study’s cohort-specific monitoring. Annual mean concentrations of fine particulate matter and nitrogen decreased during follow-up, while ozone concentrations did not.
All participants underwent a cardiac CT scan at baseline, and 5,780 had at least one follow-up CT scan over a median period of 10 years. Ambient concentrations of ozone, fine particulate matter, nitrogen, and black carbon at baseline were significantly associated with greater increases in percent emphysema per 10 years, as were concentrations of zone and nitrogen during follow-up, reported Dr. Wang, formerly with the State University of New York at Buffalo.
Of the 3,636 participants who had at least one spirometric assessment, there was a mean decline in forced expiratory volume in 1 second (FEV1) of 309 mL (95% CI, 299-319 mL) and in forced vital capacity of 331 mL (95% CI, 317-345 mL) over 10 years. Ambient concentrations of ozone were significantly associated with a decline in FEV1, both at baseline and during follow-up.
The coauthors acknowledged their study’s limitations, including that general outdoor air pollution concentrations might not reflect individual exposure or concentrations indoors, where people spend most of their time. In addition, percent emphysema was only measured in the lower two-thirds of the lung, though they noted that “percent emphysema measured in the lower two-thirds of the lung correlates well with full-lung percent emphysema in this cohort and a cohort of smokers.”
This article was developed by the EPA and the University of Washington Center for Clean Air Research. MESA was funded by the National Institutes of Health and the National Heart, Lung, and Blood Institute, and supported by the National Institute of Environmental Health Sciences. The authors reported numerous conflicts of interest, including receiving grants and fees from the University of Washington, the EPA, the NIH, and various other pharmaceutical companies, foundations, and governmental entities.
SOURCE: Wang M et al. JAMA. 2019 Aug 13. doi: 10.1001/jama.2019.10255
FROM JAMA