User login
Preventive health: Getting rid of the middleman (uh-oh, that’s us!)
As physicians, we find that preventive health is, frankly, really difficult. It requires thinking about a changing list of recommendations unprompted by the symptoms for which patients present. Compounding that challenge is that, in doing preventive health well, we need to have personalized discussions with our patients and this requires they come into the office, which doesn’t always happen on a regular basis. Furthermore, when patients do come in, they usually are presenting for an acute care visit, so there is little time set aside to discuss preventive health.
For all these reasons and many others, the data suggest that we are not particularly good at performing preventive health maintenance. We are much better at figuring out diagnostic dilemmas and choosing among competing medications or procedures to most effectively address acute and chronic medical problems. Let’s examine the data to see if there is a shred of truth in what we are saying; then let’s look at a potential solution to the dilemma of preventive health that we all believe in and that we carry out less frequently than any of us would like.
First, let’s look at recent data on cancer screening reported by the CDC1:
- Mammography: 72% of women aged 50–74 years reported having had a mammogram within the past 2 years.
- Pap test: 83% of women reported being up to date with cervical cancer screening.
- Colorectal cancer screening: 62% of men and women reported colorectal cancer screening test use consistent with USPSTF recommendations.
Of note, colorectal cancer screening has improved dramatically over he last 15 years, while screening for breast and cervical cancer has largely plateaued.1
Our success with cancer screening – or lack thereof depending upon one’s perspective – looks quite good next to national vaccination rates for adults. The immunization rate for commonly recommended vaccines are as follows2:
- The Tdap vaccination rate is 20%.
- The tetanus-diphtheria vaccination rate is 62%.
- The herpes zoster vaccination rate is 28%.
- The influenza vaccination rate is 43%.
- The pneumococcal vaccination rate among high-risk persons aged 19-64 years is 20% and among adults aged greater than or equal to 65 years is 61%.
Of adults who had health insurance and at least 10 physician contacts within the past year, 23.8%-88.8% reported not having received vaccinations that were recommended.
In the business literature there is a great deal of disagreement about the value of the “middleman.” The term middleman describes someone who brings the product from the producer, or factory, to the consumer. On the one hand, if the factory can sell the product directly to the consumer, the consumer can save money and the factory can make more money. On the other hand, if the middleman can help the consumer make a better choice among the variety of products available, then the middleman provides value and the consumer benefits.3
Traditionally, clinicians have served the role of the middleman for preventive health activities, knowing what to recommend to patients and informing them of the correct preventive health choices that fit their needs. The problem with this concept is that preventive health recommendations are largely demographically based, are tied to population-based risk assessment, and usually require very little individual judgment.
We as physicians are good at – and I believe truly enjoy – exercising judgment. We love thinking things through and helping the person in front of us. We are not as good at remembering unprompted information in the middle of busy visits that are often made for unrelated reasons. Most of the people who have not had a colonoscopy or pneumococcal vaccine have not decided against the procedure after a detailed discussion with their physician. On the contrary, the service was never recommended, or it was recommended, but the patient did not follow up to have the procedure performed.
Let’s now imagine another approach. You’re a patient and once a year you click on an email that shows up in your inbox from your doctor with the words “Preventive Health” in the subject line. The EHR – based on your gender, age, and a query of what has been documented in your chart – has determined the preventive health activities that are recommended for you. You can choose to pursue, opt out, or get more information for each of the recommended preventive services as you read through them.
If you choose to have more information, it is provided in a structured format that allows you to drill down to the level of detail that you desire. In all probability, you will find a greater level of detail and accuracy of information about each preventive service than could possibly be provided during a routine office visit. Specifics about the risks and benefits of the procedure will also be more extensive, as it is unlikely your care providers are able to keep all of the details and risk ratios in their heads. If desired, you as a patient can take your time to read and digest the information, sleep on it, and come back to it to make an informed decision. This is not something you can do during a routine office visit.
If you choose to opt out of the procedure, just click the “declined” box. Otherwise, when you’ve made all of your decisions and indicate that you’re done, the necessary prescriptions for blood work and x-rays, as well as referrals to the appropriate specialists, will print out. An entry will also be made in the electronic record showing you’ve been provided preventive health recommendations that are appropriate for your age and sex and made your preferred choices. At any point, if you feel you’d like further discussion with your physician, you can make an appointment electronically through the interface.
The hurdles for implementing such a system are real, but they are solvable, and the development of such an approach is inevitable, enviable, and will ultimately be good for both patients and their providers. Patients will get more predictable and complete recommendations for preventive care and providers will have more time to do what we enjoy and are most skilled at – talking with patients to clarify diagnoses, decide upon treatment, and clarify questions that come up about preventive health recommendations.
Dr. Skolnik is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records.
References
1. White A et al. Cancer screening test use – United States, 2015. MMWR Morb Mortal Wkly Rep. 2017 Mar 3;66(8):201-6.
2. Williams WW et al. Surveillance of vaccination coverage among adult populations – United States, 2014. MMWR Surveill Summ. 2016 Feb 5;65(1):1-36.
3. Conerly B. Don’t eliminate the middleman – He’s much too valuable. Forbes. Oct 28, 2015.
As physicians, we find that preventive health is, frankly, really difficult. It requires thinking about a changing list of recommendations unprompted by the symptoms for which patients present. Compounding that challenge is that, in doing preventive health well, we need to have personalized discussions with our patients and this requires they come into the office, which doesn’t always happen on a regular basis. Furthermore, when patients do come in, they usually are presenting for an acute care visit, so there is little time set aside to discuss preventive health.
For all these reasons and many others, the data suggest that we are not particularly good at performing preventive health maintenance. We are much better at figuring out diagnostic dilemmas and choosing among competing medications or procedures to most effectively address acute and chronic medical problems. Let’s examine the data to see if there is a shred of truth in what we are saying; then let’s look at a potential solution to the dilemma of preventive health that we all believe in and that we carry out less frequently than any of us would like.
First, let’s look at recent data on cancer screening reported by the CDC1:
- Mammography: 72% of women aged 50–74 years reported having had a mammogram within the past 2 years.
- Pap test: 83% of women reported being up to date with cervical cancer screening.
- Colorectal cancer screening: 62% of men and women reported colorectal cancer screening test use consistent with USPSTF recommendations.
Of note, colorectal cancer screening has improved dramatically over he last 15 years, while screening for breast and cervical cancer has largely plateaued.1
Our success with cancer screening – or lack thereof depending upon one’s perspective – looks quite good next to national vaccination rates for adults. The immunization rate for commonly recommended vaccines are as follows2:
- The Tdap vaccination rate is 20%.
- The tetanus-diphtheria vaccination rate is 62%.
- The herpes zoster vaccination rate is 28%.
- The influenza vaccination rate is 43%.
- The pneumococcal vaccination rate among high-risk persons aged 19-64 years is 20% and among adults aged greater than or equal to 65 years is 61%.
Of adults who had health insurance and at least 10 physician contacts within the past year, 23.8%-88.8% reported not having received vaccinations that were recommended.
In the business literature there is a great deal of disagreement about the value of the “middleman.” The term middleman describes someone who brings the product from the producer, or factory, to the consumer. On the one hand, if the factory can sell the product directly to the consumer, the consumer can save money and the factory can make more money. On the other hand, if the middleman can help the consumer make a better choice among the variety of products available, then the middleman provides value and the consumer benefits.3
Traditionally, clinicians have served the role of the middleman for preventive health activities, knowing what to recommend to patients and informing them of the correct preventive health choices that fit their needs. The problem with this concept is that preventive health recommendations are largely demographically based, are tied to population-based risk assessment, and usually require very little individual judgment.
We as physicians are good at – and I believe truly enjoy – exercising judgment. We love thinking things through and helping the person in front of us. We are not as good at remembering unprompted information in the middle of busy visits that are often made for unrelated reasons. Most of the people who have not had a colonoscopy or pneumococcal vaccine have not decided against the procedure after a detailed discussion with their physician. On the contrary, the service was never recommended, or it was recommended, but the patient did not follow up to have the procedure performed.
Let’s now imagine another approach. You’re a patient and once a year you click on an email that shows up in your inbox from your doctor with the words “Preventive Health” in the subject line. The EHR – based on your gender, age, and a query of what has been documented in your chart – has determined the preventive health activities that are recommended for you. You can choose to pursue, opt out, or get more information for each of the recommended preventive services as you read through them.
If you choose to have more information, it is provided in a structured format that allows you to drill down to the level of detail that you desire. In all probability, you will find a greater level of detail and accuracy of information about each preventive service than could possibly be provided during a routine office visit. Specifics about the risks and benefits of the procedure will also be more extensive, as it is unlikely your care providers are able to keep all of the details and risk ratios in their heads. If desired, you as a patient can take your time to read and digest the information, sleep on it, and come back to it to make an informed decision. This is not something you can do during a routine office visit.
If you choose to opt out of the procedure, just click the “declined” box. Otherwise, when you’ve made all of your decisions and indicate that you’re done, the necessary prescriptions for blood work and x-rays, as well as referrals to the appropriate specialists, will print out. An entry will also be made in the electronic record showing you’ve been provided preventive health recommendations that are appropriate for your age and sex and made your preferred choices. At any point, if you feel you’d like further discussion with your physician, you can make an appointment electronically through the interface.
The hurdles for implementing such a system are real, but they are solvable, and the development of such an approach is inevitable, enviable, and will ultimately be good for both patients and their providers. Patients will get more predictable and complete recommendations for preventive care and providers will have more time to do what we enjoy and are most skilled at – talking with patients to clarify diagnoses, decide upon treatment, and clarify questions that come up about preventive health recommendations.
Dr. Skolnik is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records.
References
1. White A et al. Cancer screening test use – United States, 2015. MMWR Morb Mortal Wkly Rep. 2017 Mar 3;66(8):201-6.
2. Williams WW et al. Surveillance of vaccination coverage among adult populations – United States, 2014. MMWR Surveill Summ. 2016 Feb 5;65(1):1-36.
3. Conerly B. Don’t eliminate the middleman – He’s much too valuable. Forbes. Oct 28, 2015.
As physicians, we find that preventive health is, frankly, really difficult. It requires thinking about a changing list of recommendations unprompted by the symptoms for which patients present. Compounding that challenge is that, in doing preventive health well, we need to have personalized discussions with our patients and this requires they come into the office, which doesn’t always happen on a regular basis. Furthermore, when patients do come in, they usually are presenting for an acute care visit, so there is little time set aside to discuss preventive health.
For all these reasons and many others, the data suggest that we are not particularly good at performing preventive health maintenance. We are much better at figuring out diagnostic dilemmas and choosing among competing medications or procedures to most effectively address acute and chronic medical problems. Let’s examine the data to see if there is a shred of truth in what we are saying; then let’s look at a potential solution to the dilemma of preventive health that we all believe in and that we carry out less frequently than any of us would like.
First, let’s look at recent data on cancer screening reported by the CDC1:
- Mammography: 72% of women aged 50–74 years reported having had a mammogram within the past 2 years.
- Pap test: 83% of women reported being up to date with cervical cancer screening.
- Colorectal cancer screening: 62% of men and women reported colorectal cancer screening test use consistent with USPSTF recommendations.
Of note, colorectal cancer screening has improved dramatically over he last 15 years, while screening for breast and cervical cancer has largely plateaued.1
Our success with cancer screening – or lack thereof depending upon one’s perspective – looks quite good next to national vaccination rates for adults. The immunization rate for commonly recommended vaccines are as follows2:
- The Tdap vaccination rate is 20%.
- The tetanus-diphtheria vaccination rate is 62%.
- The herpes zoster vaccination rate is 28%.
- The influenza vaccination rate is 43%.
- The pneumococcal vaccination rate among high-risk persons aged 19-64 years is 20% and among adults aged greater than or equal to 65 years is 61%.
Of adults who had health insurance and at least 10 physician contacts within the past year, 23.8%-88.8% reported not having received vaccinations that were recommended.
In the business literature there is a great deal of disagreement about the value of the “middleman.” The term middleman describes someone who brings the product from the producer, or factory, to the consumer. On the one hand, if the factory can sell the product directly to the consumer, the consumer can save money and the factory can make more money. On the other hand, if the middleman can help the consumer make a better choice among the variety of products available, then the middleman provides value and the consumer benefits.3
Traditionally, clinicians have served the role of the middleman for preventive health activities, knowing what to recommend to patients and informing them of the correct preventive health choices that fit their needs. The problem with this concept is that preventive health recommendations are largely demographically based, are tied to population-based risk assessment, and usually require very little individual judgment.
We as physicians are good at – and I believe truly enjoy – exercising judgment. We love thinking things through and helping the person in front of us. We are not as good at remembering unprompted information in the middle of busy visits that are often made for unrelated reasons. Most of the people who have not had a colonoscopy or pneumococcal vaccine have not decided against the procedure after a detailed discussion with their physician. On the contrary, the service was never recommended, or it was recommended, but the patient did not follow up to have the procedure performed.
Let’s now imagine another approach. You’re a patient and once a year you click on an email that shows up in your inbox from your doctor with the words “Preventive Health” in the subject line. The EHR – based on your gender, age, and a query of what has been documented in your chart – has determined the preventive health activities that are recommended for you. You can choose to pursue, opt out, or get more information for each of the recommended preventive services as you read through them.
If you choose to have more information, it is provided in a structured format that allows you to drill down to the level of detail that you desire. In all probability, you will find a greater level of detail and accuracy of information about each preventive service than could possibly be provided during a routine office visit. Specifics about the risks and benefits of the procedure will also be more extensive, as it is unlikely your care providers are able to keep all of the details and risk ratios in their heads. If desired, you as a patient can take your time to read and digest the information, sleep on it, and come back to it to make an informed decision. This is not something you can do during a routine office visit.
If you choose to opt out of the procedure, just click the “declined” box. Otherwise, when you’ve made all of your decisions and indicate that you’re done, the necessary prescriptions for blood work and x-rays, as well as referrals to the appropriate specialists, will print out. An entry will also be made in the electronic record showing you’ve been provided preventive health recommendations that are appropriate for your age and sex and made your preferred choices. At any point, if you feel you’d like further discussion with your physician, you can make an appointment electronically through the interface.
The hurdles for implementing such a system are real, but they are solvable, and the development of such an approach is inevitable, enviable, and will ultimately be good for both patients and their providers. Patients will get more predictable and complete recommendations for preventive care and providers will have more time to do what we enjoy and are most skilled at – talking with patients to clarify diagnoses, decide upon treatment, and clarify questions that come up about preventive health recommendations.
Dr. Skolnik is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records.
References
1. White A et al. Cancer screening test use – United States, 2015. MMWR Morb Mortal Wkly Rep. 2017 Mar 3;66(8):201-6.
2. Williams WW et al. Surveillance of vaccination coverage among adult populations – United States, 2014. MMWR Surveill Summ. 2016 Feb 5;65(1):1-36.
3. Conerly B. Don’t eliminate the middleman – He’s much too valuable. Forbes. Oct 28, 2015.
Considerations for Optimal Inhaler Device Selection in Chronic Obstructive Pulmonary Disease
Device considerations
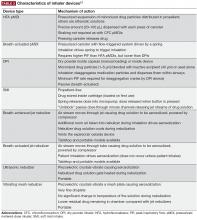
The SMI delivers the aerosol as a fine mist with slow velocity lasting >1 second, which is considerably slower than spray delivery with pMDIs.14 The aim of this design is to make it easier for patients to coordinate actuation with inhalation, but it is important to note that some coordination is still required for SMI devices to function correctly.14 In addition, the SMI is not dependent on a patient’s ability to generate sufficient PIF for effective drug delivery. A limitation of the SMI is the need to assemble the device, as patients with poor manual dexterity may encounter difficulty when attempting to load the drug cartridge.15
Nebulizers deliver aerosolized drug in a fine mist. Newer-generation portable vibrating mesh nebulizers can deliver a dose over a period of ~2 minutes, compared with 10 minutes for conventional pneumatic devices.16 Patients find them effective and easy to use, and the newer generation devices overcome problems with portability and length of treatment, which may be an issue during the daytime for ambulatory patients, along with the requirement for cleaning after each dose.4,8 However, drug delivery may be somewhat compromised with nebulizers compared with other inhalation devices, as medication can be dispersed into the atmosphere and lost, rather than inhaled.7 An additional point to consider is medication availability; some medications, particularly fixed-dose combination maintenance therapies, are currently unavailable in a nebulized format.16
The most important device-related factors influencing the site of deposition within the lungs are aerosol velocity and particle size of the inhaled drug.3,7,17 To maximize clinical effectiveness, adequate distribution throughout the lung is required to reach target sites of action for β2-agonists, anticholinergics, and corticosteroids.17 Particle size differs between inhaler device types, but all available devices generate drug particles sufficient for deposition throughout the lower airways and lung periphery, ie, within the range of 1–5 microns.3,18-21 Extra fine particles of <1 micron (or “submicron particles”) can be deposited deeper in the pulmonary acinus, but a higher fraction of such particles may be exhaled compared with particles 1–5 microns in size.3,20,22 In contrast, particles >5 microns deposit in the oropharynx and may be swallowed, potentially leading to systemic adverse effects.3,20,22
When more than one drug is required, it may be preferable to deliver them via a single device where possible to facilitate patient compliance with correct technique, and decrease confusion about how to use different inhalers.23 The inhaler device ideally serves as a platform on which many treatments are available; the greater the number of devices employed by the patient, the greater the likelihood of making an error with the usage of each device.24
Importance of proper inhaler technique
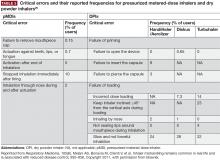
Errors relating to device handling are common in patients with COPD. The results of a meta-analysis by Chrystyn et al reported that overall error rates were high across all devices in patients with COPD and asthma, ranging from 50%–100%25; the reported frequencies of patients with at least one error were 86.8% and 60.9% for pMDIs and DPIs, respectively. However, the authors note that heterogeneity between the studies used in the analysis was high, and suggest that future investigations should look to use a more standardized approach in assessment of inhaler device errors.25 Moreover, further studies to investigate the frequency of errors in SMI devices, and to establish the relationship between critical errors in device handling and device efficacy, are warranted.
Handling errors are directly linked to compromised drug delivery and reduced treatment efficacy.3 This may lead to more frequent or inappropriate medication use that, in turn, could result in unnecessary dose increases by the physician due to perceived lack of efficacy, and subsequently more adverse effects.3,26-28 However, these errors can be addressed through proper training and demonstration.29-32
Common device-handling errors include4,26,27,32,33:
- pMDIs: not shaking the inhaler (for suspensions), not exhaling fully before actuation, inhaling too forcefully, and not holding their breath for long enough after inhalation.
- DPIs: exhaling into the device mouthpiece, not exhaling fully before inhalation, not inhaling deeply or forcefully enough, and not holding their breath after inhalation.
- SMIs: not rotating the inhaler with mouth cap facing upwards, rotating the inhaler while looking into the spray nozzle with the cap open (before inhalation), and not maintaining inhalation with drug spray.
Incorrect inhaler use is a common cause of secondary nonadherence (ie, relating to incorrect medication use) among patients with COPD.4,34 Compromised inhaler technique and medication nonadherence jeopardize health outcomes and add to the economic burden of COPD.8,12,26
A 2005 study estimated that over 20% of the $25 billion spent on inhalers annually in the United States is wasted as a direct consequence of incorrect device handling.35
Failing to inhale correctly to achieve the optimal inspiratory flow for the specific device being used—deep and slow for pMDIs, or forceful, quick and deep for DPIs—is a critical handling error for inhaler devices.26 Significant associations between critical errors and clinical outcomes (hospitalization, emergency department visits, antibiotic courses, and corticosteroid courses) have been reported in COPD patients.26 In a retrospective analysis of COPD inpatients, suboptimal PIF rates with DPIs were associated with worse scores on the COPD Assessment Test, higher COPD and all-cause readmission rates, and shorter time to next COPD exacerbation.12
Patient considerations
Poor inhaler technique is frequently reported in patients with COPD or asthma, irrespective of the device used and with considerable variability in handling error rates for each individual device.25,26,35,45 Although clinical evidence is limited,25 research to date indicates that some DPIs may require less training than pMDIs.23,29,45,46 Therefore, DPI devices may be viewed as a more appropriate option for patients who encounter difficulty in coordinating the inhalation and actuation required for effective operation of a pMDI device. Alternatively, use of a spacer with pMDIs appears to reduce handling errors compared with pMDIs alone, but whether a pMDI plus spacer improves technique versus DPIs remains unclear.25,46,47 Lack of device training appears to be a key reason for inhaler handling errors across device types.26
Elderly patients need special consideration when selecting an inhaler and ensuring it is used correctly.48 Reduced physical ability and cognitive function due to age-related conditions (eg, dementia, depression, neuromuscular and cerebrovascular diseases) are the main reasons for suboptimal inhaler use in older patients, but other factors may also contribute (eg, multiple comorbid conditions, consequent complicated medication regimens).15 Older age is strongly associated with inhaler misuse,26 and has also been shown to have a negative correlation with PIF, independent of COPD severity.41 When compared with younger patients, older patients make more attempts before mastering the inhalation technique for a specific device, and need longer instruction time from trained health care professionals to correct inhaler mishandling.49,50 In elderly patients with adequate cognitive and manual ability, the most important factors in selecting a device are availability, convenience, ease of use, patient preference, and cost.8,23
Device continuity is a key consideration when multiple inhaled medications are needed.23 Lack of continuity of device type for different clinical needs means that patients may need to master the different techniques for each device.3 For instance, a patient may have a pMDI rescue medication, one or more DPIs for their maintenance therapy, and a nebulizer for additional bronchodilation, which may lead to confusion and incorrect device usage. Device continuity has been shown to improve disease control compared with using multiple inhalers in patients with asthma.51
A full summary of patient- and physician-related considerations for device selection, along with suggestions for how these can be addressed, is provided in Table 5.
Inhaler device training for patients and physicians
Comprehensive instruction, including practical demonstration, is important for ensuring patients with COPD use the correct inhaler technique, with regular review and repeated instruction generally needed for continued correct use.1,23,32,42 Lack of instruction is significantly associated with inhaler misuse in patients with COPD or asthma.26 Verbal training on inhalation technique increased the number of patients achieving the minimum inhalation flow rate required for a range of different DPIs.39 Similarly, training helped patients using a pMDI to slow their inhalation rate to <90 L/min, as recommended for this type of device.39 The ‘teach-back’ method, where patients are asked to demonstrate correct usage of their inhaler after instruction from a health care professional,52 has shown to be particularly effective in pharmacist-led patient device training.53 Educational interventions that incorporated a physical demonstration significantly improved inhaler technique in patients with COPD and asthma compared with patients receiving written and verbal information alone.53 Proper device training in primary care settings should also include education about why the inhaler is needed.3
Face-to-face instruction from trained caregivers for approximately 5 to 10 minutes improves the use of MDIs and DPIs by patients.49 However, clinical research indicates that learning correct handling and use may be easier and quicker for some devices than for others.31,49 For example, patients naïve to the PulmoJet (a DPI device not currently available in the United States) were found to have fewer serious errors after training than those using Diskus or Turbuhaler devices.24 In another study, it took less time to correct errors in inhaler use with the Diskus compared with the HandiHaler.44 Health care professionals themselves may lack training or knowledge on correct use of inhaler devices,35,36,54 with 1 study finding that up to 67% of nurses, doctors, and respiratory therapists were unable to describe or perform critical steps for using inhalers.35
A range of resources is available to aid in training patients and health care professionals in inhaler techniques:
- Tools such as the In-Check DIAL inspiratory flow meter (Clement Clarke International Ltd, Harlow, UK), TurbuHaler Trainer (AstraZeneca, Lund, Sweden), Diskus/Accuhaler Training Device (Vitalograph, Ennis, Ireland), and 2Tone Trainer (Canday Medical Ltd, Newmarket, UK) can be used to evaluate a patient’s physical ability to use a specific inhaler.55
- The emergence of electronic monitoring devices, such as SmartTrack, SmartTurbo, and SmartMat (all developed by Adherium Ltd, Auckland New Zealand), can provide objective and detailed adherence data to support clinical decision-making.56
- It is essential that patients and physicians alike utilize the instructions and video demonstrations available online to understand how to use a device correctly, and avoid errors. These resources can be found on a number of organizations’ websites (eg, COPD Foundation, Allergy and Asthma Network, Centers for Disease Control and Prevention, National Jewish Health, Asthma UK, Centre for Pharmacy Postgraduate Education) and on manufacturers’ websites for individual inhalers or treatments (eg, https://www.advair.com/how-to-use-advair.html, https://www.incruse.com/how-to-use-incruse.html, https://www.mysymbicort.com/copd/taking-symbicort/how-to-use-the-inhaler.html, https://www.tudorzahcp.com/tudorza-instructions-dosing.html, www.us.respimat.com (“How to Use the RESPIMAT Inhaler”), https://www.utibron.com/how-to-use.html).
Conclusions
A number of inhalation devices are available for the treatment of COPD. However, incorrect usage or a poor match between the patient and the device may lead to confusion, suboptimal treatment, and increased cost to the patient and health care system. Considering both patient- and health care system-related factors can ensure that appropriate inhaler section and usage can be optimized.
- Global Initiative for Chronic Obstructive Lung Disease. GOLD 2017 Global Strategy for the Diagnosis, Management and Prevention of COPD. http://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd. Accessed July 2017.
- Dolovich MB, Dhand R. Aerosol drug delivery: developments in device design and clinical use. Lancet. 2011;377(9770):1032-1045.
- Bonini M, Usmani OS. The importance of inhaler devices in the treatment of COPD. COPD Res Pract. 2015;1(1):9.
- Restrepo RD, Alvarez MT, Wittnebel LD, et al. Medication adherence issues in patients treated for COPD. Int J Chron Obstruct Pulmon Dis. 2008;3(3):371-384.
- Rogliani P, Calzetta L, Coppola A, et al. Optimizing drug delivery in COPD: the role of inhaler devices. Respir Med. 2017;124:6-14.
- Lavorini F, Fontana GA, Usmani OS. New inhaler devices - the good, the bad and the ugly. Respiration. 2014;88(1):3-15.
- Ibrahim M, Verma R, Garcia-Contreras L. Inhalation drug delivery devices: technology update. Med Devices (Auckl). 2015;8:131-139.
- Barrons R, Pegram A, Borries A. Inhaler device selection: special considerations in elderly patients with chronic obstructive pulmonary disease. Am J Health Syst Pharm. 2011;68(13):1221-1232.
- Dal Negro RW. Dry powder inhalers and the right things to remember: a concept review. Multidiscip Respir Med. 2015;10(1):13.
- Mahler DA, Waterman LA, Gifford AH. Prevalence and COPD phenotype for a suboptimal peak inspiratory flow rate against the simulated resistance of the Diskus® dry powder inhaler. J Aerosol Med Pulm Drug Deliv. 2013;26(3):174-179.
- Sharma G, Mahler DA, Mayorga VM, Deering KL, Harshaw O, Ganapathy V. Prevalence of low peak inspiratory flow rate at discharge in patients hospitalized for COPD exacerbation. Chronic Obstr Pulm Dis. 2017;4(3):217-224.
- Loh CH, Peters SP, Lovings TM, Ohar JA. Suboptimal inspiratory flow rates are associated with chronic obstructive pulmonary disease and all cause readmissions. Ann Am Thorac Soc. 2017;14(8):1305-1311.
- Le V, Hoang Thi TH, Robins E, Flament M. Dry powder inhalers: study of the parameters influencing adhesion and dispersion of fluticasone propionate. AAPS PharmSciTech. 2012;13(2):477-484.
- Dalby RN, Eicher J, Zierenberg B. Development of Respimat® Soft Mist™ Inhaler and its clinical utility in respiratory disorders. Med Devices (Auckl). 2011;4:145-155.
- Lavorini F, Mannini C, Chellini E, Fontana GA. Optimising inhaled pharmacotherapy for elderly patients with chronic obstructive pulmonary disease: the importance of delivery devices. Drugs Aging. 2016;33(7):461-473.
- Tashkin DP. A review of nebulized drug delivery in COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:2585-2596.
- Labiris NR, Dolovich MB. Pulmonary drug delivery. Part I: physiological factors affecting therapeutic effectiveness of aerosolized medications. Br J Clin Pharmacol. 2003;56(6):588-599.
- Chrystyn H. Anatomy and physiology in delivery: can we define our targets? Allergy. 1999;54(suppl 49):82-87.
- Biddiscombe M, Meah S, Barnes P, Usmani O. Drug particle size and lung deposition in COPD. Eur Respir J. 2016;48(suppl 60):Abstract. doi:10.1183/13993003.congress-13992016.PA13993313.
- Demoly P, Hagedoorn P, de Boer AH, Frijlink HW. The clinical relevance of dry powder inhaler performance for drug delivery. Respir Med. 2014;108(8):1195-1203.
- Dhand R. Inhaled drug therapy 2016: the year in review. Respir Care. 2017;62(7):978-996.
- de Boer AH, Gjaltema D, Hagedoorn P, Frijlink HW. Can ‘extrafine’ dry powder aerosols improve lung deposition? Eur J Pharm Biopharm. 2015;96:143-151.
- Vincken W, Dekhuijzen PR, Barnes P; ADMIT Group. The ADMIT series - Issues in inhalation therapy. 4) How to choose inhaler devices for the treatment of COPD. Prim Care Respir J. 2010;19(1):10-20.
- Roggeri A, Micheletto C, Roggeri DP. Inhalation errors due to device switch in patients with chronic obstructive pulmonary disease and asthma: critical health and economic issues. Int J Chron Obstruct Pulmon Dis. 2016;11:597-602.
- Chrystyn H, van der Palen J, Sharma R, et al. Device errors in asthma and COPD: systematic literature review and meta-analysis. NPJ Prim Care Respir Med. 2017;27(1):22.
- Melani AS, Bonavia M, Cilenti V, et al; Gruppo Educazionale Associazione Italiana Pneumologi Ospedalieri. Inhaler mishandling remains common in real life and is associated with reduced disease control [published correction appears in Respir Med. 2012;106(5):757]. Respir Med. 2011;105(6):930-938.
- Sanchis J, Gich I, Pedersen S; Aerosol Drug Management Improvement Team (ADMIT). Systematic review of errors in inhaler use: has patient technique improved over time? Chest. 2016;150(2):394-406.
- Sulaiman I, Seheult J, Sadasivuni N, et al. The impact of common inhaler errors on drug delivery: investigating critical errors with a dry powder inhaler. J Aerosol Med Pulm Drug Deliv. 2017;30(4):247-255.
- Chapman KR, Love L, Brubaker H. A comparison of breath-actuated and conventional metered-dose inhaler inhalation techniques in elderly subjects. Chest. 1993;104(5):1332-1337.
- van der Palen J, Thomas M, Chrystyn H, et al. A randomised open-label cross-over study of inhaler errors, preference and time to achieve correct inhaler use in patients with COPD or asthma: comparison of ELLIPTA with other inhaler devices. NPJ Prim Care Respir Med. 2016;26:16079.
- Chrystyn H, Price DB, Molimard M, et al. Comparison of serious inhaler technique errors made by device-naïve patients using three different dry powder inhalers: a randomised, crossover, open-label study. BMC Pulm Med. 2016;16:12.
- Crane MA, Jenkins CR, Goeman DP, Douglass JA. Inhaler device technique can be improved in older adults through tailored education: findings from a randomised controlled trial. NPJ Prim Care Respir Med. 2014;24:14034.
- Ohbayashi H, Kudo S, Ishikawa M. Inhaler operability and patient satisfaction regarding Genuair® and Respimat® inhalers for chronic obstructive pulmonary disease: a randomized crossover sudy. Pulmon Ther. 2017;3(1):173-185.
- Bourbeau J, Bartlett SJ. Patient adherence in COPD. Thorax. 2008;63(9):831-838.
- Fink JB, Rubin BK. Problems with inhaler use: a call for improved clinician and patient education. Respir Care. 2005;50(10):1360-1374; discussion 1374-1375.
- Yawn BP, Colice GL, Hodder R. Practical aspects of inhaler use in the management of chronic obstructive pulmonary disease in the primary care setting. Int J Chron Obstruct Pulmon Dis. 2012;7:495-502.
- Dhand R, Dolovich M, Chipps B, Myers TR, Restrepo R, Farrar JR. The role of nebulized therapy in the management of COPD: evidence and recommendations. COPD. 2012;9(1):58-72.
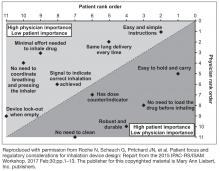
- Roche N, Gerhard S, Pritchard JN, et al. Patient focus and regulatory considerations for inhalation device design: report from the 2015 IPAC-RS/ISAM Workshop. J Aerosol Med Pulm Drug Deliv. 2017;30(1):1-13.
- Al-Showair RA, Tarsin WY, Assi KH, Pearson SB, Chrystyn H. Can all patients with COPD use the correct inhalation flow with all inhalers and does training help? Respir Med. 2007;101(11):2395-2401.
- Janssens W, VandenBrande P, Hardeman E, et al. Inspiratory flow rates at different levels of resistance in elderly COPD patients. Eur Respir J. 2008;31(1):78-83.
- Jarvis S, Ind PW, Shiner RJ. Inhaled therapy in elderly COPD patients; time for re-evaluation? Age Ageing. 2007;36(2):213-218.
- Lavorini F, Levy ML, Corrigan C, Crompton G; ADMIT Working Group. The ADMIT series - issues in inhalation therapy. 6) Training tools for inhalation devices. Prim Care Respir J. 2010;19(4):335-341.
- Pauwels R, Newman S, Borgström L. Airway deposition and airway effects of antiasthma drugs delivered from metered-dose inhalers. Eur Respir J. 1997;10(9):2127-2138.
- Everard ML, Devadason SG, Le Souëf PN. Flow early in the inspiratory manoeuvre affects the aerosol particle size distribution from a Turbuhaler. Respir Med. 1997;91(10):624-628.
- Molimard M, Raherison C, Lignot S, et al. Chronic obstructive pulmonary disease exacerbation and inhaler device handling: real-life assessment of 2935 patients. Eur Respir J. 2017;49(2):doi: 10.1183/13993003.13901794-2016.
- Jones V, Fernandez C, Diggory P. A comparison of large volume spacer, breath-activated and dry powder inhalers in older people. Age Ageing. 1999;28(5):481-484.
- Ho SF, O’Mahony MS, Steward JA, Breay P, Burr ML. Inhaler technique in older people in the community. Age Ageing. 2004;33(2):185-188.
- Taffet GE, Donohue JF, Altman PR. Considerations for managing chronic obstructive pulmonary disease in the elderly. Clin Interv Aging. 2014;9:23-30.
- Melani AS, Bonavia M, Mastropasqua E, et al; Gruppo Educazionale Associazione Italiana Pneumologi Ospedalieri (AIPO). Time required to rectify inhaler errors among experienced subjects with faulty technique. Respir Care. 2017;62(4):409-414.
- Dal Negro RW, Povero M. Dry-powder inhalers in patients with persistent airflow limitation: usability and preference. Multidiscip Respir Med. 2016;11(1):31.
- Price D, Chrystyn H, Kaplan A, et al. Effectiveness of same versus mixed asthma inhaler devices: a retrospective observational study in primary care. Allergy Asthma Immunol Res. 2012;4(4):184-191.
- Dantic DE. A critical review of the effectiveness of ‘teach-back’ technique in teaching COPD patients self-management using respiratory inhalers. Health Ed J. 2014;73(1):41-50.
- Bosnic-Anticevich SZ, Sinha H, So S, Reddel HK. Metered-dose inhaler technique: the effect of two educational interventions delivered in community pharmacy over time. J Asthma. 2010;47(3):251-256.
- Adnan M, Karim S, Khan S, Al Wabel N. Critical errors found during metered dose inhaler technique demonstration by pharmacists. Saudi Pharm J. 2016;24(5):625.
- Capstick TG, Clifton IJ. Inhaler technique and training in people with chronic obstructive pulmonary disease and asthma. Expert Rev Respir Med. 2012;6(1):91-101; quiz 102-103.
- Chan AH, Harrison J, Black PN, Mitchell EA, Foster JM. Using electronic monitoring devices to measure inhaler adherence: a practical guide for clinicians. J Allergy Clin Immunol Pract. 2015;3(3):335-349.e1-e5.
Device considerations
The SMI delivers the aerosol as a fine mist with slow velocity lasting >1 second, which is considerably slower than spray delivery with pMDIs.14 The aim of this design is to make it easier for patients to coordinate actuation with inhalation, but it is important to note that some coordination is still required for SMI devices to function correctly.14 In addition, the SMI is not dependent on a patient’s ability to generate sufficient PIF for effective drug delivery. A limitation of the SMI is the need to assemble the device, as patients with poor manual dexterity may encounter difficulty when attempting to load the drug cartridge.15
Nebulizers deliver aerosolized drug in a fine mist. Newer-generation portable vibrating mesh nebulizers can deliver a dose over a period of ~2 minutes, compared with 10 minutes for conventional pneumatic devices.16 Patients find them effective and easy to use, and the newer generation devices overcome problems with portability and length of treatment, which may be an issue during the daytime for ambulatory patients, along with the requirement for cleaning after each dose.4,8 However, drug delivery may be somewhat compromised with nebulizers compared with other inhalation devices, as medication can be dispersed into the atmosphere and lost, rather than inhaled.7 An additional point to consider is medication availability; some medications, particularly fixed-dose combination maintenance therapies, are currently unavailable in a nebulized format.16
The most important device-related factors influencing the site of deposition within the lungs are aerosol velocity and particle size of the inhaled drug.3,7,17 To maximize clinical effectiveness, adequate distribution throughout the lung is required to reach target sites of action for β2-agonists, anticholinergics, and corticosteroids.17 Particle size differs between inhaler device types, but all available devices generate drug particles sufficient for deposition throughout the lower airways and lung periphery, ie, within the range of 1–5 microns.3,18-21 Extra fine particles of <1 micron (or “submicron particles”) can be deposited deeper in the pulmonary acinus, but a higher fraction of such particles may be exhaled compared with particles 1–5 microns in size.3,20,22 In contrast, particles >5 microns deposit in the oropharynx and may be swallowed, potentially leading to systemic adverse effects.3,20,22
When more than one drug is required, it may be preferable to deliver them via a single device where possible to facilitate patient compliance with correct technique, and decrease confusion about how to use different inhalers.23 The inhaler device ideally serves as a platform on which many treatments are available; the greater the number of devices employed by the patient, the greater the likelihood of making an error with the usage of each device.24
Importance of proper inhaler technique
Errors relating to device handling are common in patients with COPD. The results of a meta-analysis by Chrystyn et al reported that overall error rates were high across all devices in patients with COPD and asthma, ranging from 50%–100%25; the reported frequencies of patients with at least one error were 86.8% and 60.9% for pMDIs and DPIs, respectively. However, the authors note that heterogeneity between the studies used in the analysis was high, and suggest that future investigations should look to use a more standardized approach in assessment of inhaler device errors.25 Moreover, further studies to investigate the frequency of errors in SMI devices, and to establish the relationship between critical errors in device handling and device efficacy, are warranted.
Handling errors are directly linked to compromised drug delivery and reduced treatment efficacy.3 This may lead to more frequent or inappropriate medication use that, in turn, could result in unnecessary dose increases by the physician due to perceived lack of efficacy, and subsequently more adverse effects.3,26-28 However, these errors can be addressed through proper training and demonstration.29-32
Common device-handling errors include4,26,27,32,33:
- pMDIs: not shaking the inhaler (for suspensions), not exhaling fully before actuation, inhaling too forcefully, and not holding their breath for long enough after inhalation.
- DPIs: exhaling into the device mouthpiece, not exhaling fully before inhalation, not inhaling deeply or forcefully enough, and not holding their breath after inhalation.
- SMIs: not rotating the inhaler with mouth cap facing upwards, rotating the inhaler while looking into the spray nozzle with the cap open (before inhalation), and not maintaining inhalation with drug spray.
Incorrect inhaler use is a common cause of secondary nonadherence (ie, relating to incorrect medication use) among patients with COPD.4,34 Compromised inhaler technique and medication nonadherence jeopardize health outcomes and add to the economic burden of COPD.8,12,26
A 2005 study estimated that over 20% of the $25 billion spent on inhalers annually in the United States is wasted as a direct consequence of incorrect device handling.35
Failing to inhale correctly to achieve the optimal inspiratory flow for the specific device being used—deep and slow for pMDIs, or forceful, quick and deep for DPIs—is a critical handling error for inhaler devices.26 Significant associations between critical errors and clinical outcomes (hospitalization, emergency department visits, antibiotic courses, and corticosteroid courses) have been reported in COPD patients.26 In a retrospective analysis of COPD inpatients, suboptimal PIF rates with DPIs were associated with worse scores on the COPD Assessment Test, higher COPD and all-cause readmission rates, and shorter time to next COPD exacerbation.12
Patient considerations
Poor inhaler technique is frequently reported in patients with COPD or asthma, irrespective of the device used and with considerable variability in handling error rates for each individual device.25,26,35,45 Although clinical evidence is limited,25 research to date indicates that some DPIs may require less training than pMDIs.23,29,45,46 Therefore, DPI devices may be viewed as a more appropriate option for patients who encounter difficulty in coordinating the inhalation and actuation required for effective operation of a pMDI device. Alternatively, use of a spacer with pMDIs appears to reduce handling errors compared with pMDIs alone, but whether a pMDI plus spacer improves technique versus DPIs remains unclear.25,46,47 Lack of device training appears to be a key reason for inhaler handling errors across device types.26
Elderly patients need special consideration when selecting an inhaler and ensuring it is used correctly.48 Reduced physical ability and cognitive function due to age-related conditions (eg, dementia, depression, neuromuscular and cerebrovascular diseases) are the main reasons for suboptimal inhaler use in older patients, but other factors may also contribute (eg, multiple comorbid conditions, consequent complicated medication regimens).15 Older age is strongly associated with inhaler misuse,26 and has also been shown to have a negative correlation with PIF, independent of COPD severity.41 When compared with younger patients, older patients make more attempts before mastering the inhalation technique for a specific device, and need longer instruction time from trained health care professionals to correct inhaler mishandling.49,50 In elderly patients with adequate cognitive and manual ability, the most important factors in selecting a device are availability, convenience, ease of use, patient preference, and cost.8,23
Device continuity is a key consideration when multiple inhaled medications are needed.23 Lack of continuity of device type for different clinical needs means that patients may need to master the different techniques for each device.3 For instance, a patient may have a pMDI rescue medication, one or more DPIs for their maintenance therapy, and a nebulizer for additional bronchodilation, which may lead to confusion and incorrect device usage. Device continuity has been shown to improve disease control compared with using multiple inhalers in patients with asthma.51
A full summary of patient- and physician-related considerations for device selection, along with suggestions for how these can be addressed, is provided in Table 5.
Inhaler device training for patients and physicians
Comprehensive instruction, including practical demonstration, is important for ensuring patients with COPD use the correct inhaler technique, with regular review and repeated instruction generally needed for continued correct use.1,23,32,42 Lack of instruction is significantly associated with inhaler misuse in patients with COPD or asthma.26 Verbal training on inhalation technique increased the number of patients achieving the minimum inhalation flow rate required for a range of different DPIs.39 Similarly, training helped patients using a pMDI to slow their inhalation rate to <90 L/min, as recommended for this type of device.39 The ‘teach-back’ method, where patients are asked to demonstrate correct usage of their inhaler after instruction from a health care professional,52 has shown to be particularly effective in pharmacist-led patient device training.53 Educational interventions that incorporated a physical demonstration significantly improved inhaler technique in patients with COPD and asthma compared with patients receiving written and verbal information alone.53 Proper device training in primary care settings should also include education about why the inhaler is needed.3
Face-to-face instruction from trained caregivers for approximately 5 to 10 minutes improves the use of MDIs and DPIs by patients.49 However, clinical research indicates that learning correct handling and use may be easier and quicker for some devices than for others.31,49 For example, patients naïve to the PulmoJet (a DPI device not currently available in the United States) were found to have fewer serious errors after training than those using Diskus or Turbuhaler devices.24 In another study, it took less time to correct errors in inhaler use with the Diskus compared with the HandiHaler.44 Health care professionals themselves may lack training or knowledge on correct use of inhaler devices,35,36,54 with 1 study finding that up to 67% of nurses, doctors, and respiratory therapists were unable to describe or perform critical steps for using inhalers.35
A range of resources is available to aid in training patients and health care professionals in inhaler techniques:
- Tools such as the In-Check DIAL inspiratory flow meter (Clement Clarke International Ltd, Harlow, UK), TurbuHaler Trainer (AstraZeneca, Lund, Sweden), Diskus/Accuhaler Training Device (Vitalograph, Ennis, Ireland), and 2Tone Trainer (Canday Medical Ltd, Newmarket, UK) can be used to evaluate a patient’s physical ability to use a specific inhaler.55
- The emergence of electronic monitoring devices, such as SmartTrack, SmartTurbo, and SmartMat (all developed by Adherium Ltd, Auckland New Zealand), can provide objective and detailed adherence data to support clinical decision-making.56
- It is essential that patients and physicians alike utilize the instructions and video demonstrations available online to understand how to use a device correctly, and avoid errors. These resources can be found on a number of organizations’ websites (eg, COPD Foundation, Allergy and Asthma Network, Centers for Disease Control and Prevention, National Jewish Health, Asthma UK, Centre for Pharmacy Postgraduate Education) and on manufacturers’ websites for individual inhalers or treatments (eg, https://www.advair.com/how-to-use-advair.html, https://www.incruse.com/how-to-use-incruse.html, https://www.mysymbicort.com/copd/taking-symbicort/how-to-use-the-inhaler.html, https://www.tudorzahcp.com/tudorza-instructions-dosing.html, www.us.respimat.com (“How to Use the RESPIMAT Inhaler”), https://www.utibron.com/how-to-use.html).
Conclusions
A number of inhalation devices are available for the treatment of COPD. However, incorrect usage or a poor match between the patient and the device may lead to confusion, suboptimal treatment, and increased cost to the patient and health care system. Considering both patient- and health care system-related factors can ensure that appropriate inhaler section and usage can be optimized.
Device considerations
The SMI delivers the aerosol as a fine mist with slow velocity lasting >1 second, which is considerably slower than spray delivery with pMDIs.14 The aim of this design is to make it easier for patients to coordinate actuation with inhalation, but it is important to note that some coordination is still required for SMI devices to function correctly.14 In addition, the SMI is not dependent on a patient’s ability to generate sufficient PIF for effective drug delivery. A limitation of the SMI is the need to assemble the device, as patients with poor manual dexterity may encounter difficulty when attempting to load the drug cartridge.15
Nebulizers deliver aerosolized drug in a fine mist. Newer-generation portable vibrating mesh nebulizers can deliver a dose over a period of ~2 minutes, compared with 10 minutes for conventional pneumatic devices.16 Patients find them effective and easy to use, and the newer generation devices overcome problems with portability and length of treatment, which may be an issue during the daytime for ambulatory patients, along with the requirement for cleaning after each dose.4,8 However, drug delivery may be somewhat compromised with nebulizers compared with other inhalation devices, as medication can be dispersed into the atmosphere and lost, rather than inhaled.7 An additional point to consider is medication availability; some medications, particularly fixed-dose combination maintenance therapies, are currently unavailable in a nebulized format.16
The most important device-related factors influencing the site of deposition within the lungs are aerosol velocity and particle size of the inhaled drug.3,7,17 To maximize clinical effectiveness, adequate distribution throughout the lung is required to reach target sites of action for β2-agonists, anticholinergics, and corticosteroids.17 Particle size differs between inhaler device types, but all available devices generate drug particles sufficient for deposition throughout the lower airways and lung periphery, ie, within the range of 1–5 microns.3,18-21 Extra fine particles of <1 micron (or “submicron particles”) can be deposited deeper in the pulmonary acinus, but a higher fraction of such particles may be exhaled compared with particles 1–5 microns in size.3,20,22 In contrast, particles >5 microns deposit in the oropharynx and may be swallowed, potentially leading to systemic adverse effects.3,20,22
When more than one drug is required, it may be preferable to deliver them via a single device where possible to facilitate patient compliance with correct technique, and decrease confusion about how to use different inhalers.23 The inhaler device ideally serves as a platform on which many treatments are available; the greater the number of devices employed by the patient, the greater the likelihood of making an error with the usage of each device.24
Importance of proper inhaler technique
Errors relating to device handling are common in patients with COPD. The results of a meta-analysis by Chrystyn et al reported that overall error rates were high across all devices in patients with COPD and asthma, ranging from 50%–100%25; the reported frequencies of patients with at least one error were 86.8% and 60.9% for pMDIs and DPIs, respectively. However, the authors note that heterogeneity between the studies used in the analysis was high, and suggest that future investigations should look to use a more standardized approach in assessment of inhaler device errors.25 Moreover, further studies to investigate the frequency of errors in SMI devices, and to establish the relationship between critical errors in device handling and device efficacy, are warranted.
Handling errors are directly linked to compromised drug delivery and reduced treatment efficacy.3 This may lead to more frequent or inappropriate medication use that, in turn, could result in unnecessary dose increases by the physician due to perceived lack of efficacy, and subsequently more adverse effects.3,26-28 However, these errors can be addressed through proper training and demonstration.29-32
Common device-handling errors include4,26,27,32,33:
- pMDIs: not shaking the inhaler (for suspensions), not exhaling fully before actuation, inhaling too forcefully, and not holding their breath for long enough after inhalation.
- DPIs: exhaling into the device mouthpiece, not exhaling fully before inhalation, not inhaling deeply or forcefully enough, and not holding their breath after inhalation.
- SMIs: not rotating the inhaler with mouth cap facing upwards, rotating the inhaler while looking into the spray nozzle with the cap open (before inhalation), and not maintaining inhalation with drug spray.
Incorrect inhaler use is a common cause of secondary nonadherence (ie, relating to incorrect medication use) among patients with COPD.4,34 Compromised inhaler technique and medication nonadherence jeopardize health outcomes and add to the economic burden of COPD.8,12,26
A 2005 study estimated that over 20% of the $25 billion spent on inhalers annually in the United States is wasted as a direct consequence of incorrect device handling.35
Failing to inhale correctly to achieve the optimal inspiratory flow for the specific device being used—deep and slow for pMDIs, or forceful, quick and deep for DPIs—is a critical handling error for inhaler devices.26 Significant associations between critical errors and clinical outcomes (hospitalization, emergency department visits, antibiotic courses, and corticosteroid courses) have been reported in COPD patients.26 In a retrospective analysis of COPD inpatients, suboptimal PIF rates with DPIs were associated with worse scores on the COPD Assessment Test, higher COPD and all-cause readmission rates, and shorter time to next COPD exacerbation.12
Patient considerations
Poor inhaler technique is frequently reported in patients with COPD or asthma, irrespective of the device used and with considerable variability in handling error rates for each individual device.25,26,35,45 Although clinical evidence is limited,25 research to date indicates that some DPIs may require less training than pMDIs.23,29,45,46 Therefore, DPI devices may be viewed as a more appropriate option for patients who encounter difficulty in coordinating the inhalation and actuation required for effective operation of a pMDI device. Alternatively, use of a spacer with pMDIs appears to reduce handling errors compared with pMDIs alone, but whether a pMDI plus spacer improves technique versus DPIs remains unclear.25,46,47 Lack of device training appears to be a key reason for inhaler handling errors across device types.26
Elderly patients need special consideration when selecting an inhaler and ensuring it is used correctly.48 Reduced physical ability and cognitive function due to age-related conditions (eg, dementia, depression, neuromuscular and cerebrovascular diseases) are the main reasons for suboptimal inhaler use in older patients, but other factors may also contribute (eg, multiple comorbid conditions, consequent complicated medication regimens).15 Older age is strongly associated with inhaler misuse,26 and has also been shown to have a negative correlation with PIF, independent of COPD severity.41 When compared with younger patients, older patients make more attempts before mastering the inhalation technique for a specific device, and need longer instruction time from trained health care professionals to correct inhaler mishandling.49,50 In elderly patients with adequate cognitive and manual ability, the most important factors in selecting a device are availability, convenience, ease of use, patient preference, and cost.8,23
Device continuity is a key consideration when multiple inhaled medications are needed.23 Lack of continuity of device type for different clinical needs means that patients may need to master the different techniques for each device.3 For instance, a patient may have a pMDI rescue medication, one or more DPIs for their maintenance therapy, and a nebulizer for additional bronchodilation, which may lead to confusion and incorrect device usage. Device continuity has been shown to improve disease control compared with using multiple inhalers in patients with asthma.51
A full summary of patient- and physician-related considerations for device selection, along with suggestions for how these can be addressed, is provided in Table 5.
Inhaler device training for patients and physicians
Comprehensive instruction, including practical demonstration, is important for ensuring patients with COPD use the correct inhaler technique, with regular review and repeated instruction generally needed for continued correct use.1,23,32,42 Lack of instruction is significantly associated with inhaler misuse in patients with COPD or asthma.26 Verbal training on inhalation technique increased the number of patients achieving the minimum inhalation flow rate required for a range of different DPIs.39 Similarly, training helped patients using a pMDI to slow their inhalation rate to <90 L/min, as recommended for this type of device.39 The ‘teach-back’ method, where patients are asked to demonstrate correct usage of their inhaler after instruction from a health care professional,52 has shown to be particularly effective in pharmacist-led patient device training.53 Educational interventions that incorporated a physical demonstration significantly improved inhaler technique in patients with COPD and asthma compared with patients receiving written and verbal information alone.53 Proper device training in primary care settings should also include education about why the inhaler is needed.3
Face-to-face instruction from trained caregivers for approximately 5 to 10 minutes improves the use of MDIs and DPIs by patients.49 However, clinical research indicates that learning correct handling and use may be easier and quicker for some devices than for others.31,49 For example, patients naïve to the PulmoJet (a DPI device not currently available in the United States) were found to have fewer serious errors after training than those using Diskus or Turbuhaler devices.24 In another study, it took less time to correct errors in inhaler use with the Diskus compared with the HandiHaler.44 Health care professionals themselves may lack training or knowledge on correct use of inhaler devices,35,36,54 with 1 study finding that up to 67% of nurses, doctors, and respiratory therapists were unable to describe or perform critical steps for using inhalers.35
A range of resources is available to aid in training patients and health care professionals in inhaler techniques:
- Tools such as the In-Check DIAL inspiratory flow meter (Clement Clarke International Ltd, Harlow, UK), TurbuHaler Trainer (AstraZeneca, Lund, Sweden), Diskus/Accuhaler Training Device (Vitalograph, Ennis, Ireland), and 2Tone Trainer (Canday Medical Ltd, Newmarket, UK) can be used to evaluate a patient’s physical ability to use a specific inhaler.55
- The emergence of electronic monitoring devices, such as SmartTrack, SmartTurbo, and SmartMat (all developed by Adherium Ltd, Auckland New Zealand), can provide objective and detailed adherence data to support clinical decision-making.56
- It is essential that patients and physicians alike utilize the instructions and video demonstrations available online to understand how to use a device correctly, and avoid errors. These resources can be found on a number of organizations’ websites (eg, COPD Foundation, Allergy and Asthma Network, Centers for Disease Control and Prevention, National Jewish Health, Asthma UK, Centre for Pharmacy Postgraduate Education) and on manufacturers’ websites for individual inhalers or treatments (eg, https://www.advair.com/how-to-use-advair.html, https://www.incruse.com/how-to-use-incruse.html, https://www.mysymbicort.com/copd/taking-symbicort/how-to-use-the-inhaler.html, https://www.tudorzahcp.com/tudorza-instructions-dosing.html, www.us.respimat.com (“How to Use the RESPIMAT Inhaler”), https://www.utibron.com/how-to-use.html).
Conclusions
A number of inhalation devices are available for the treatment of COPD. However, incorrect usage or a poor match between the patient and the device may lead to confusion, suboptimal treatment, and increased cost to the patient and health care system. Considering both patient- and health care system-related factors can ensure that appropriate inhaler section and usage can be optimized.
- Global Initiative for Chronic Obstructive Lung Disease. GOLD 2017 Global Strategy for the Diagnosis, Management and Prevention of COPD. http://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd. Accessed July 2017.
- Dolovich MB, Dhand R. Aerosol drug delivery: developments in device design and clinical use. Lancet. 2011;377(9770):1032-1045.
- Bonini M, Usmani OS. The importance of inhaler devices in the treatment of COPD. COPD Res Pract. 2015;1(1):9.
- Restrepo RD, Alvarez MT, Wittnebel LD, et al. Medication adherence issues in patients treated for COPD. Int J Chron Obstruct Pulmon Dis. 2008;3(3):371-384.
- Rogliani P, Calzetta L, Coppola A, et al. Optimizing drug delivery in COPD: the role of inhaler devices. Respir Med. 2017;124:6-14.
- Lavorini F, Fontana GA, Usmani OS. New inhaler devices - the good, the bad and the ugly. Respiration. 2014;88(1):3-15.
- Ibrahim M, Verma R, Garcia-Contreras L. Inhalation drug delivery devices: technology update. Med Devices (Auckl). 2015;8:131-139.
- Barrons R, Pegram A, Borries A. Inhaler device selection: special considerations in elderly patients with chronic obstructive pulmonary disease. Am J Health Syst Pharm. 2011;68(13):1221-1232.
- Dal Negro RW. Dry powder inhalers and the right things to remember: a concept review. Multidiscip Respir Med. 2015;10(1):13.
- Mahler DA, Waterman LA, Gifford AH. Prevalence and COPD phenotype for a suboptimal peak inspiratory flow rate against the simulated resistance of the Diskus® dry powder inhaler. J Aerosol Med Pulm Drug Deliv. 2013;26(3):174-179.
- Sharma G, Mahler DA, Mayorga VM, Deering KL, Harshaw O, Ganapathy V. Prevalence of low peak inspiratory flow rate at discharge in patients hospitalized for COPD exacerbation. Chronic Obstr Pulm Dis. 2017;4(3):217-224.
- Loh CH, Peters SP, Lovings TM, Ohar JA. Suboptimal inspiratory flow rates are associated with chronic obstructive pulmonary disease and all cause readmissions. Ann Am Thorac Soc. 2017;14(8):1305-1311.
- Le V, Hoang Thi TH, Robins E, Flament M. Dry powder inhalers: study of the parameters influencing adhesion and dispersion of fluticasone propionate. AAPS PharmSciTech. 2012;13(2):477-484.
- Dalby RN, Eicher J, Zierenberg B. Development of Respimat® Soft Mist™ Inhaler and its clinical utility in respiratory disorders. Med Devices (Auckl). 2011;4:145-155.
- Lavorini F, Mannini C, Chellini E, Fontana GA. Optimising inhaled pharmacotherapy for elderly patients with chronic obstructive pulmonary disease: the importance of delivery devices. Drugs Aging. 2016;33(7):461-473.
- Tashkin DP. A review of nebulized drug delivery in COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:2585-2596.
- Labiris NR, Dolovich MB. Pulmonary drug delivery. Part I: physiological factors affecting therapeutic effectiveness of aerosolized medications. Br J Clin Pharmacol. 2003;56(6):588-599.
- Chrystyn H. Anatomy and physiology in delivery: can we define our targets? Allergy. 1999;54(suppl 49):82-87.
- Biddiscombe M, Meah S, Barnes P, Usmani O. Drug particle size and lung deposition in COPD. Eur Respir J. 2016;48(suppl 60):Abstract. doi:10.1183/13993003.congress-13992016.PA13993313.
- Demoly P, Hagedoorn P, de Boer AH, Frijlink HW. The clinical relevance of dry powder inhaler performance for drug delivery. Respir Med. 2014;108(8):1195-1203.
- Dhand R. Inhaled drug therapy 2016: the year in review. Respir Care. 2017;62(7):978-996.
- de Boer AH, Gjaltema D, Hagedoorn P, Frijlink HW. Can ‘extrafine’ dry powder aerosols improve lung deposition? Eur J Pharm Biopharm. 2015;96:143-151.
- Vincken W, Dekhuijzen PR, Barnes P; ADMIT Group. The ADMIT series - Issues in inhalation therapy. 4) How to choose inhaler devices for the treatment of COPD. Prim Care Respir J. 2010;19(1):10-20.
- Roggeri A, Micheletto C, Roggeri DP. Inhalation errors due to device switch in patients with chronic obstructive pulmonary disease and asthma: critical health and economic issues. Int J Chron Obstruct Pulmon Dis. 2016;11:597-602.
- Chrystyn H, van der Palen J, Sharma R, et al. Device errors in asthma and COPD: systematic literature review and meta-analysis. NPJ Prim Care Respir Med. 2017;27(1):22.
- Melani AS, Bonavia M, Cilenti V, et al; Gruppo Educazionale Associazione Italiana Pneumologi Ospedalieri. Inhaler mishandling remains common in real life and is associated with reduced disease control [published correction appears in Respir Med. 2012;106(5):757]. Respir Med. 2011;105(6):930-938.
- Sanchis J, Gich I, Pedersen S; Aerosol Drug Management Improvement Team (ADMIT). Systematic review of errors in inhaler use: has patient technique improved over time? Chest. 2016;150(2):394-406.
- Sulaiman I, Seheult J, Sadasivuni N, et al. The impact of common inhaler errors on drug delivery: investigating critical errors with a dry powder inhaler. J Aerosol Med Pulm Drug Deliv. 2017;30(4):247-255.
- Chapman KR, Love L, Brubaker H. A comparison of breath-actuated and conventional metered-dose inhaler inhalation techniques in elderly subjects. Chest. 1993;104(5):1332-1337.
- van der Palen J, Thomas M, Chrystyn H, et al. A randomised open-label cross-over study of inhaler errors, preference and time to achieve correct inhaler use in patients with COPD or asthma: comparison of ELLIPTA with other inhaler devices. NPJ Prim Care Respir Med. 2016;26:16079.
- Chrystyn H, Price DB, Molimard M, et al. Comparison of serious inhaler technique errors made by device-naïve patients using three different dry powder inhalers: a randomised, crossover, open-label study. BMC Pulm Med. 2016;16:12.
- Crane MA, Jenkins CR, Goeman DP, Douglass JA. Inhaler device technique can be improved in older adults through tailored education: findings from a randomised controlled trial. NPJ Prim Care Respir Med. 2014;24:14034.
- Ohbayashi H, Kudo S, Ishikawa M. Inhaler operability and patient satisfaction regarding Genuair® and Respimat® inhalers for chronic obstructive pulmonary disease: a randomized crossover sudy. Pulmon Ther. 2017;3(1):173-185.
- Bourbeau J, Bartlett SJ. Patient adherence in COPD. Thorax. 2008;63(9):831-838.
- Fink JB, Rubin BK. Problems with inhaler use: a call for improved clinician and patient education. Respir Care. 2005;50(10):1360-1374; discussion 1374-1375.
- Yawn BP, Colice GL, Hodder R. Practical aspects of inhaler use in the management of chronic obstructive pulmonary disease in the primary care setting. Int J Chron Obstruct Pulmon Dis. 2012;7:495-502.
- Dhand R, Dolovich M, Chipps B, Myers TR, Restrepo R, Farrar JR. The role of nebulized therapy in the management of COPD: evidence and recommendations. COPD. 2012;9(1):58-72.
- Roche N, Gerhard S, Pritchard JN, et al. Patient focus and regulatory considerations for inhalation device design: report from the 2015 IPAC-RS/ISAM Workshop. J Aerosol Med Pulm Drug Deliv. 2017;30(1):1-13.
- Al-Showair RA, Tarsin WY, Assi KH, Pearson SB, Chrystyn H. Can all patients with COPD use the correct inhalation flow with all inhalers and does training help? Respir Med. 2007;101(11):2395-2401.
- Janssens W, VandenBrande P, Hardeman E, et al. Inspiratory flow rates at different levels of resistance in elderly COPD patients. Eur Respir J. 2008;31(1):78-83.
- Jarvis S, Ind PW, Shiner RJ. Inhaled therapy in elderly COPD patients; time for re-evaluation? Age Ageing. 2007;36(2):213-218.
- Lavorini F, Levy ML, Corrigan C, Crompton G; ADMIT Working Group. The ADMIT series - issues in inhalation therapy. 6) Training tools for inhalation devices. Prim Care Respir J. 2010;19(4):335-341.
- Pauwels R, Newman S, Borgström L. Airway deposition and airway effects of antiasthma drugs delivered from metered-dose inhalers. Eur Respir J. 1997;10(9):2127-2138.
- Everard ML, Devadason SG, Le Souëf PN. Flow early in the inspiratory manoeuvre affects the aerosol particle size distribution from a Turbuhaler. Respir Med. 1997;91(10):624-628.
- Molimard M, Raherison C, Lignot S, et al. Chronic obstructive pulmonary disease exacerbation and inhaler device handling: real-life assessment of 2935 patients. Eur Respir J. 2017;49(2):doi: 10.1183/13993003.13901794-2016.
- Jones V, Fernandez C, Diggory P. A comparison of large volume spacer, breath-activated and dry powder inhalers in older people. Age Ageing. 1999;28(5):481-484.
- Ho SF, O’Mahony MS, Steward JA, Breay P, Burr ML. Inhaler technique in older people in the community. Age Ageing. 2004;33(2):185-188.
- Taffet GE, Donohue JF, Altman PR. Considerations for managing chronic obstructive pulmonary disease in the elderly. Clin Interv Aging. 2014;9:23-30.
- Melani AS, Bonavia M, Mastropasqua E, et al; Gruppo Educazionale Associazione Italiana Pneumologi Ospedalieri (AIPO). Time required to rectify inhaler errors among experienced subjects with faulty technique. Respir Care. 2017;62(4):409-414.
- Dal Negro RW, Povero M. Dry-powder inhalers in patients with persistent airflow limitation: usability and preference. Multidiscip Respir Med. 2016;11(1):31.
- Price D, Chrystyn H, Kaplan A, et al. Effectiveness of same versus mixed asthma inhaler devices: a retrospective observational study in primary care. Allergy Asthma Immunol Res. 2012;4(4):184-191.
- Dantic DE. A critical review of the effectiveness of ‘teach-back’ technique in teaching COPD patients self-management using respiratory inhalers. Health Ed J. 2014;73(1):41-50.
- Bosnic-Anticevich SZ, Sinha H, So S, Reddel HK. Metered-dose inhaler technique: the effect of two educational interventions delivered in community pharmacy over time. J Asthma. 2010;47(3):251-256.
- Adnan M, Karim S, Khan S, Al Wabel N. Critical errors found during metered dose inhaler technique demonstration by pharmacists. Saudi Pharm J. 2016;24(5):625.
- Capstick TG, Clifton IJ. Inhaler technique and training in people with chronic obstructive pulmonary disease and asthma. Expert Rev Respir Med. 2012;6(1):91-101; quiz 102-103.
- Chan AH, Harrison J, Black PN, Mitchell EA, Foster JM. Using electronic monitoring devices to measure inhaler adherence: a practical guide for clinicians. J Allergy Clin Immunol Pract. 2015;3(3):335-349.e1-e5.
- Global Initiative for Chronic Obstructive Lung Disease. GOLD 2017 Global Strategy for the Diagnosis, Management and Prevention of COPD. http://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd. Accessed July 2017.
- Dolovich MB, Dhand R. Aerosol drug delivery: developments in device design and clinical use. Lancet. 2011;377(9770):1032-1045.
- Bonini M, Usmani OS. The importance of inhaler devices in the treatment of COPD. COPD Res Pract. 2015;1(1):9.
- Restrepo RD, Alvarez MT, Wittnebel LD, et al. Medication adherence issues in patients treated for COPD. Int J Chron Obstruct Pulmon Dis. 2008;3(3):371-384.
- Rogliani P, Calzetta L, Coppola A, et al. Optimizing drug delivery in COPD: the role of inhaler devices. Respir Med. 2017;124:6-14.
- Lavorini F, Fontana GA, Usmani OS. New inhaler devices - the good, the bad and the ugly. Respiration. 2014;88(1):3-15.
- Ibrahim M, Verma R, Garcia-Contreras L. Inhalation drug delivery devices: technology update. Med Devices (Auckl). 2015;8:131-139.
- Barrons R, Pegram A, Borries A. Inhaler device selection: special considerations in elderly patients with chronic obstructive pulmonary disease. Am J Health Syst Pharm. 2011;68(13):1221-1232.
- Dal Negro RW. Dry powder inhalers and the right things to remember: a concept review. Multidiscip Respir Med. 2015;10(1):13.
- Mahler DA, Waterman LA, Gifford AH. Prevalence and COPD phenotype for a suboptimal peak inspiratory flow rate against the simulated resistance of the Diskus® dry powder inhaler. J Aerosol Med Pulm Drug Deliv. 2013;26(3):174-179.
- Sharma G, Mahler DA, Mayorga VM, Deering KL, Harshaw O, Ganapathy V. Prevalence of low peak inspiratory flow rate at discharge in patients hospitalized for COPD exacerbation. Chronic Obstr Pulm Dis. 2017;4(3):217-224.
- Loh CH, Peters SP, Lovings TM, Ohar JA. Suboptimal inspiratory flow rates are associated with chronic obstructive pulmonary disease and all cause readmissions. Ann Am Thorac Soc. 2017;14(8):1305-1311.
- Le V, Hoang Thi TH, Robins E, Flament M. Dry powder inhalers: study of the parameters influencing adhesion and dispersion of fluticasone propionate. AAPS PharmSciTech. 2012;13(2):477-484.
- Dalby RN, Eicher J, Zierenberg B. Development of Respimat® Soft Mist™ Inhaler and its clinical utility in respiratory disorders. Med Devices (Auckl). 2011;4:145-155.
- Lavorini F, Mannini C, Chellini E, Fontana GA. Optimising inhaled pharmacotherapy for elderly patients with chronic obstructive pulmonary disease: the importance of delivery devices. Drugs Aging. 2016;33(7):461-473.
- Tashkin DP. A review of nebulized drug delivery in COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:2585-2596.
- Labiris NR, Dolovich MB. Pulmonary drug delivery. Part I: physiological factors affecting therapeutic effectiveness of aerosolized medications. Br J Clin Pharmacol. 2003;56(6):588-599.
- Chrystyn H. Anatomy and physiology in delivery: can we define our targets? Allergy. 1999;54(suppl 49):82-87.
- Biddiscombe M, Meah S, Barnes P, Usmani O. Drug particle size and lung deposition in COPD. Eur Respir J. 2016;48(suppl 60):Abstract. doi:10.1183/13993003.congress-13992016.PA13993313.
- Demoly P, Hagedoorn P, de Boer AH, Frijlink HW. The clinical relevance of dry powder inhaler performance for drug delivery. Respir Med. 2014;108(8):1195-1203.
- Dhand R. Inhaled drug therapy 2016: the year in review. Respir Care. 2017;62(7):978-996.
- de Boer AH, Gjaltema D, Hagedoorn P, Frijlink HW. Can ‘extrafine’ dry powder aerosols improve lung deposition? Eur J Pharm Biopharm. 2015;96:143-151.
- Vincken W, Dekhuijzen PR, Barnes P; ADMIT Group. The ADMIT series - Issues in inhalation therapy. 4) How to choose inhaler devices for the treatment of COPD. Prim Care Respir J. 2010;19(1):10-20.
- Roggeri A, Micheletto C, Roggeri DP. Inhalation errors due to device switch in patients with chronic obstructive pulmonary disease and asthma: critical health and economic issues. Int J Chron Obstruct Pulmon Dis. 2016;11:597-602.
- Chrystyn H, van der Palen J, Sharma R, et al. Device errors in asthma and COPD: systematic literature review and meta-analysis. NPJ Prim Care Respir Med. 2017;27(1):22.
- Melani AS, Bonavia M, Cilenti V, et al; Gruppo Educazionale Associazione Italiana Pneumologi Ospedalieri. Inhaler mishandling remains common in real life and is associated with reduced disease control [published correction appears in Respir Med. 2012;106(5):757]. Respir Med. 2011;105(6):930-938.
- Sanchis J, Gich I, Pedersen S; Aerosol Drug Management Improvement Team (ADMIT). Systematic review of errors in inhaler use: has patient technique improved over time? Chest. 2016;150(2):394-406.
- Sulaiman I, Seheult J, Sadasivuni N, et al. The impact of common inhaler errors on drug delivery: investigating critical errors with a dry powder inhaler. J Aerosol Med Pulm Drug Deliv. 2017;30(4):247-255.
- Chapman KR, Love L, Brubaker H. A comparison of breath-actuated and conventional metered-dose inhaler inhalation techniques in elderly subjects. Chest. 1993;104(5):1332-1337.
- van der Palen J, Thomas M, Chrystyn H, et al. A randomised open-label cross-over study of inhaler errors, preference and time to achieve correct inhaler use in patients with COPD or asthma: comparison of ELLIPTA with other inhaler devices. NPJ Prim Care Respir Med. 2016;26:16079.
- Chrystyn H, Price DB, Molimard M, et al. Comparison of serious inhaler technique errors made by device-naïve patients using three different dry powder inhalers: a randomised, crossover, open-label study. BMC Pulm Med. 2016;16:12.
- Crane MA, Jenkins CR, Goeman DP, Douglass JA. Inhaler device technique can be improved in older adults through tailored education: findings from a randomised controlled trial. NPJ Prim Care Respir Med. 2014;24:14034.
- Ohbayashi H, Kudo S, Ishikawa M. Inhaler operability and patient satisfaction regarding Genuair® and Respimat® inhalers for chronic obstructive pulmonary disease: a randomized crossover sudy. Pulmon Ther. 2017;3(1):173-185.
- Bourbeau J, Bartlett SJ. Patient adherence in COPD. Thorax. 2008;63(9):831-838.
- Fink JB, Rubin BK. Problems with inhaler use: a call for improved clinician and patient education. Respir Care. 2005;50(10):1360-1374; discussion 1374-1375.
- Yawn BP, Colice GL, Hodder R. Practical aspects of inhaler use in the management of chronic obstructive pulmonary disease in the primary care setting. Int J Chron Obstruct Pulmon Dis. 2012;7:495-502.
- Dhand R, Dolovich M, Chipps B, Myers TR, Restrepo R, Farrar JR. The role of nebulized therapy in the management of COPD: evidence and recommendations. COPD. 2012;9(1):58-72.
- Roche N, Gerhard S, Pritchard JN, et al. Patient focus and regulatory considerations for inhalation device design: report from the 2015 IPAC-RS/ISAM Workshop. J Aerosol Med Pulm Drug Deliv. 2017;30(1):1-13.
- Al-Showair RA, Tarsin WY, Assi KH, Pearson SB, Chrystyn H. Can all patients with COPD use the correct inhalation flow with all inhalers and does training help? Respir Med. 2007;101(11):2395-2401.
- Janssens W, VandenBrande P, Hardeman E, et al. Inspiratory flow rates at different levels of resistance in elderly COPD patients. Eur Respir J. 2008;31(1):78-83.
- Jarvis S, Ind PW, Shiner RJ. Inhaled therapy in elderly COPD patients; time for re-evaluation? Age Ageing. 2007;36(2):213-218.
- Lavorini F, Levy ML, Corrigan C, Crompton G; ADMIT Working Group. The ADMIT series - issues in inhalation therapy. 6) Training tools for inhalation devices. Prim Care Respir J. 2010;19(4):335-341.
- Pauwels R, Newman S, Borgström L. Airway deposition and airway effects of antiasthma drugs delivered from metered-dose inhalers. Eur Respir J. 1997;10(9):2127-2138.
- Everard ML, Devadason SG, Le Souëf PN. Flow early in the inspiratory manoeuvre affects the aerosol particle size distribution from a Turbuhaler. Respir Med. 1997;91(10):624-628.
- Molimard M, Raherison C, Lignot S, et al. Chronic obstructive pulmonary disease exacerbation and inhaler device handling: real-life assessment of 2935 patients. Eur Respir J. 2017;49(2):doi: 10.1183/13993003.13901794-2016.
- Jones V, Fernandez C, Diggory P. A comparison of large volume spacer, breath-activated and dry powder inhalers in older people. Age Ageing. 1999;28(5):481-484.
- Ho SF, O’Mahony MS, Steward JA, Breay P, Burr ML. Inhaler technique in older people in the community. Age Ageing. 2004;33(2):185-188.
- Taffet GE, Donohue JF, Altman PR. Considerations for managing chronic obstructive pulmonary disease in the elderly. Clin Interv Aging. 2014;9:23-30.
- Melani AS, Bonavia M, Mastropasqua E, et al; Gruppo Educazionale Associazione Italiana Pneumologi Ospedalieri (AIPO). Time required to rectify inhaler errors among experienced subjects with faulty technique. Respir Care. 2017;62(4):409-414.
- Dal Negro RW, Povero M. Dry-powder inhalers in patients with persistent airflow limitation: usability and preference. Multidiscip Respir Med. 2016;11(1):31.
- Price D, Chrystyn H, Kaplan A, et al. Effectiveness of same versus mixed asthma inhaler devices: a retrospective observational study in primary care. Allergy Asthma Immunol Res. 2012;4(4):184-191.
- Dantic DE. A critical review of the effectiveness of ‘teach-back’ technique in teaching COPD patients self-management using respiratory inhalers. Health Ed J. 2014;73(1):41-50.
- Bosnic-Anticevich SZ, Sinha H, So S, Reddel HK. Metered-dose inhaler technique: the effect of two educational interventions delivered in community pharmacy over time. J Asthma. 2010;47(3):251-256.
- Adnan M, Karim S, Khan S, Al Wabel N. Critical errors found during metered dose inhaler technique demonstration by pharmacists. Saudi Pharm J. 2016;24(5):625.
- Capstick TG, Clifton IJ. Inhaler technique and training in people with chronic obstructive pulmonary disease and asthma. Expert Rev Respir Med. 2012;6(1):91-101; quiz 102-103.
- Chan AH, Harrison J, Black PN, Mitchell EA, Foster JM. Using electronic monitoring devices to measure inhaler adherence: a practical guide for clinicians. J Allergy Clin Immunol Pract. 2015;3(3):335-349.e1-e5.
Use of non–vitamin K antagonist oral anticoagulants in the acute care, periprocedural settings
Non–vitamin K antagonist anticoagulants (NOACs, also called novel or direct oral anticoagulants) are commonly used to treat and prevent venous thromboembolism (VTE) and to prevent ischemic stroke in patients with nonvalvular atrial fibrillation. These agents, which include the factor Xa inhibitors rivaroxaban (Xarelto), apixaban (Eliquis), and edoxaban (Savaysa), and the competitive thrombin inhibitor dabigatran (Pradaxa), often are preferred over warfarin because of their more predictable pharmacokinetics, comparable efficacy, comparable or lower risk of major bleeding complications, fewer drug interactions, and lack of need for frequent monitoring. However, the acute care of patients taking NOACS can be challenging, because only dabigatran has an approved reversal agent, and none have readily available, reliable measurement assays. The American Heart Association (AHA) published a statement on the periprocedural and acute care management of patients taking NOACs. Here are the findings and recommendations of the AHA that are most relevant to primary care physicians.
Measurement
While all NOACs affect coagulation tests, their effect on prothrombin time and activated partial thromboplastin time is neither predictable nor an accurate reflection of the degree of anticoagulation. Instead, use the time of last drug ingestion and the patient’s creatinine clearance to estimate the anticoagulation effect. Dabigatran takes 1 hour to reach peak effect, or 2 hours if taken with food. Its half-life is 12-17 hours, on the higher end in the elderly and in those with moderate renal impairment. In those with severe renal impairment, half-life can be 28 hours. Rivaroxaban’s time to peak is 2-4 hours, and its half-life is 5-9 hours or up to 13 in the elderly. Apixaban’s time to peak is 3-4 hours and its half-life is about 12 hours. An antifactor Xa activity assay does provide a quantitative assessment of the factor Xa inhibitors.
Kidney injury
Acute kidney injury increases risk of bleeding while taking a NOAC. Monitor these patients closely and consider temporarily switching to a different anticoagulant in the setting of kidney injury.
Bleeding
Lack of reversibility is a common concern. Use 5 g of IV idarucizumab (Praxbind) to reverse dabigatran within minutes in a patient experiencing major bleeding. Hemodialysis, which removes about half of dabigatran in 4 hours, is a suitable option in acute kidney injury or in patients with a creatinine clearance under 30mL/min.
Options are more limited for the Xa inhibitors, because there are no available reversal agents and hemodialysis does not clear these highly protein-bound drugs. While data are limited, prothrombin complex concentrate may be given for patients on rivaroxaban, apixaban, or edoxaban who are experiencing an intracranial hemorrhage or other form of severe bleeding. Simply holding the NOAC is acceptable for minor bleeding.
Overdose
Activated charcoal to induce vomiting will work within 1-2 hours of drug ingestion.
Intracranial hemorrhage
Assume that a patient taking a NOAC who displays any acute neurologic change is experiencing an intracranial hemorrhage until proven otherwise. After CT confirmation, reverse dabigatran with idarucizumab, or give prothrombin complex concentrate to patients on other NOACs.
Ischemic stroke
Patients who suffer an ischemic stroke despite NOAC therapy are not candidates for tissue plasminogen activators.
The primary care physician is likely to be involved in the decision of whether, when, and for how long to resume anticoagulation therapy after a stroke. The statement says, “guidelines support withholding oral anticoagulation until 1-2 weeks after stroke among individuals with NVAF [nonvalvular atrial fibrillation], with shorter times for those with transient ischemic attack or small, nondisabling strokes and longer times for moderate to severe strokes.” In addition, it is worthwhile to consider medication nonadherence if no other etiology for the stroke is found; patients who miss doses may benefit more from warfarin because of its longer half-life.
Procedures and surgeries
Each year approximately 10% of patients on anticoagulation require surgery or other invasive procedures, and 20% require a minor procedure. To determine whether to interrupt NOAC therapy prior to a procedure, first determine the procedure’s bleeding risk. Patients undergoing procedures with low risk of bleeding, including minor dental, dermatologic, and ophthalmologic procedures, and endoscopies without biopsies, do not require interruption. For procedures with a moderate bleeding risk (including cardiac ablation, endoscopy with biopsies, radial artery catheterization) or high bleeding risk (including major surgery and cardiac catheterization via femoral artery), the patient’s thromboembolic risk should be evaluated using the medical history and the CHA2DS2 VASc score. NOACs should be stopped for 24-48 hours prior to the moderate to high-risk procedures. Dabigatran should be held for 72 hours for patients with creatinine clearance less than 50mL/min. Bridging therapy with heparin is not recommended for patients taking NOACS who are to have surgery. The decision about when to restart NOAC is based on the risk of thromboembolism and the bleeding risk of surgery.
Spinal or epidural anesthesia
Anesthesia guidelines recommend holding NOACs 3-5 days prior to the intervention, however, this increases risk of TE and studies have shown a very low incidence of hematoma in patients anticoagulated with a NOAC. For patients with a high risk of VTE, the NOAC can be resumed 12 hours post-procedure.
The bottom line
NOACS are commonly used for treatment and prophylaxis of VTE and atrial fibrillation and are often preferred over warfarin due to their more predictable pharmacokinetics, comparable efficacy, comparable or lower risk of major bleeding complications, fewer drug interactions, and lack of need for frequent monitoring. The AHA scientific statement gives guidance on managing NOACS in the face of acute bleeding as well as during and after procedures. NOACS should be stopped 24-48 hours prior to major surgeries and may be restarted based on weighing the risk of bleeding and risk of thromboembolism.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Oh is a third-year resident in the family medicine residency program at Abington Jefferson Health.
Reference
Raval AN et al. Management of patients on non–vitamin K antagonist oral anticoagulants in the acute care and periprocedural setting: A scientific statement from the American Heart Association. Circulation. 2017 Feb 6;135[10]:e604-e33. doi: 10.1161/CIR.0000000000000477
Non–vitamin K antagonist anticoagulants (NOACs, also called novel or direct oral anticoagulants) are commonly used to treat and prevent venous thromboembolism (VTE) and to prevent ischemic stroke in patients with nonvalvular atrial fibrillation. These agents, which include the factor Xa inhibitors rivaroxaban (Xarelto), apixaban (Eliquis), and edoxaban (Savaysa), and the competitive thrombin inhibitor dabigatran (Pradaxa), often are preferred over warfarin because of their more predictable pharmacokinetics, comparable efficacy, comparable or lower risk of major bleeding complications, fewer drug interactions, and lack of need for frequent monitoring. However, the acute care of patients taking NOACS can be challenging, because only dabigatran has an approved reversal agent, and none have readily available, reliable measurement assays. The American Heart Association (AHA) published a statement on the periprocedural and acute care management of patients taking NOACs. Here are the findings and recommendations of the AHA that are most relevant to primary care physicians.
Measurement
While all NOACs affect coagulation tests, their effect on prothrombin time and activated partial thromboplastin time is neither predictable nor an accurate reflection of the degree of anticoagulation. Instead, use the time of last drug ingestion and the patient’s creatinine clearance to estimate the anticoagulation effect. Dabigatran takes 1 hour to reach peak effect, or 2 hours if taken with food. Its half-life is 12-17 hours, on the higher end in the elderly and in those with moderate renal impairment. In those with severe renal impairment, half-life can be 28 hours. Rivaroxaban’s time to peak is 2-4 hours, and its half-life is 5-9 hours or up to 13 in the elderly. Apixaban’s time to peak is 3-4 hours and its half-life is about 12 hours. An antifactor Xa activity assay does provide a quantitative assessment of the factor Xa inhibitors.
Kidney injury
Acute kidney injury increases risk of bleeding while taking a NOAC. Monitor these patients closely and consider temporarily switching to a different anticoagulant in the setting of kidney injury.
Bleeding
Lack of reversibility is a common concern. Use 5 g of IV idarucizumab (Praxbind) to reverse dabigatran within minutes in a patient experiencing major bleeding. Hemodialysis, which removes about half of dabigatran in 4 hours, is a suitable option in acute kidney injury or in patients with a creatinine clearance under 30mL/min.
Options are more limited for the Xa inhibitors, because there are no available reversal agents and hemodialysis does not clear these highly protein-bound drugs. While data are limited, prothrombin complex concentrate may be given for patients on rivaroxaban, apixaban, or edoxaban who are experiencing an intracranial hemorrhage or other form of severe bleeding. Simply holding the NOAC is acceptable for minor bleeding.
Overdose
Activated charcoal to induce vomiting will work within 1-2 hours of drug ingestion.
Intracranial hemorrhage
Assume that a patient taking a NOAC who displays any acute neurologic change is experiencing an intracranial hemorrhage until proven otherwise. After CT confirmation, reverse dabigatran with idarucizumab, or give prothrombin complex concentrate to patients on other NOACs.
Ischemic stroke
Patients who suffer an ischemic stroke despite NOAC therapy are not candidates for tissue plasminogen activators.
The primary care physician is likely to be involved in the decision of whether, when, and for how long to resume anticoagulation therapy after a stroke. The statement says, “guidelines support withholding oral anticoagulation until 1-2 weeks after stroke among individuals with NVAF [nonvalvular atrial fibrillation], with shorter times for those with transient ischemic attack or small, nondisabling strokes and longer times for moderate to severe strokes.” In addition, it is worthwhile to consider medication nonadherence if no other etiology for the stroke is found; patients who miss doses may benefit more from warfarin because of its longer half-life.
Procedures and surgeries
Each year approximately 10% of patients on anticoagulation require surgery or other invasive procedures, and 20% require a minor procedure. To determine whether to interrupt NOAC therapy prior to a procedure, first determine the procedure’s bleeding risk. Patients undergoing procedures with low risk of bleeding, including minor dental, dermatologic, and ophthalmologic procedures, and endoscopies without biopsies, do not require interruption. For procedures with a moderate bleeding risk (including cardiac ablation, endoscopy with biopsies, radial artery catheterization) or high bleeding risk (including major surgery and cardiac catheterization via femoral artery), the patient’s thromboembolic risk should be evaluated using the medical history and the CHA2DS2 VASc score. NOACs should be stopped for 24-48 hours prior to the moderate to high-risk procedures. Dabigatran should be held for 72 hours for patients with creatinine clearance less than 50mL/min. Bridging therapy with heparin is not recommended for patients taking NOACS who are to have surgery. The decision about when to restart NOAC is based on the risk of thromboembolism and the bleeding risk of surgery.
Spinal or epidural anesthesia
Anesthesia guidelines recommend holding NOACs 3-5 days prior to the intervention, however, this increases risk of TE and studies have shown a very low incidence of hematoma in patients anticoagulated with a NOAC. For patients with a high risk of VTE, the NOAC can be resumed 12 hours post-procedure.
The bottom line
NOACS are commonly used for treatment and prophylaxis of VTE and atrial fibrillation and are often preferred over warfarin due to their more predictable pharmacokinetics, comparable efficacy, comparable or lower risk of major bleeding complications, fewer drug interactions, and lack of need for frequent monitoring. The AHA scientific statement gives guidance on managing NOACS in the face of acute bleeding as well as during and after procedures. NOACS should be stopped 24-48 hours prior to major surgeries and may be restarted based on weighing the risk of bleeding and risk of thromboembolism.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Oh is a third-year resident in the family medicine residency program at Abington Jefferson Health.
Reference
Raval AN et al. Management of patients on non–vitamin K antagonist oral anticoagulants in the acute care and periprocedural setting: A scientific statement from the American Heart Association. Circulation. 2017 Feb 6;135[10]:e604-e33. doi: 10.1161/CIR.0000000000000477
Non–vitamin K antagonist anticoagulants (NOACs, also called novel or direct oral anticoagulants) are commonly used to treat and prevent venous thromboembolism (VTE) and to prevent ischemic stroke in patients with nonvalvular atrial fibrillation. These agents, which include the factor Xa inhibitors rivaroxaban (Xarelto), apixaban (Eliquis), and edoxaban (Savaysa), and the competitive thrombin inhibitor dabigatran (Pradaxa), often are preferred over warfarin because of their more predictable pharmacokinetics, comparable efficacy, comparable or lower risk of major bleeding complications, fewer drug interactions, and lack of need for frequent monitoring. However, the acute care of patients taking NOACS can be challenging, because only dabigatran has an approved reversal agent, and none have readily available, reliable measurement assays. The American Heart Association (AHA) published a statement on the periprocedural and acute care management of patients taking NOACs. Here are the findings and recommendations of the AHA that are most relevant to primary care physicians.
Measurement
While all NOACs affect coagulation tests, their effect on prothrombin time and activated partial thromboplastin time is neither predictable nor an accurate reflection of the degree of anticoagulation. Instead, use the time of last drug ingestion and the patient’s creatinine clearance to estimate the anticoagulation effect. Dabigatran takes 1 hour to reach peak effect, or 2 hours if taken with food. Its half-life is 12-17 hours, on the higher end in the elderly and in those with moderate renal impairment. In those with severe renal impairment, half-life can be 28 hours. Rivaroxaban’s time to peak is 2-4 hours, and its half-life is 5-9 hours or up to 13 in the elderly. Apixaban’s time to peak is 3-4 hours and its half-life is about 12 hours. An antifactor Xa activity assay does provide a quantitative assessment of the factor Xa inhibitors.
Kidney injury
Acute kidney injury increases risk of bleeding while taking a NOAC. Monitor these patients closely and consider temporarily switching to a different anticoagulant in the setting of kidney injury.
Bleeding
Lack of reversibility is a common concern. Use 5 g of IV idarucizumab (Praxbind) to reverse dabigatran within minutes in a patient experiencing major bleeding. Hemodialysis, which removes about half of dabigatran in 4 hours, is a suitable option in acute kidney injury or in patients with a creatinine clearance under 30mL/min.
Options are more limited for the Xa inhibitors, because there are no available reversal agents and hemodialysis does not clear these highly protein-bound drugs. While data are limited, prothrombin complex concentrate may be given for patients on rivaroxaban, apixaban, or edoxaban who are experiencing an intracranial hemorrhage or other form of severe bleeding. Simply holding the NOAC is acceptable for minor bleeding.
Overdose
Activated charcoal to induce vomiting will work within 1-2 hours of drug ingestion.
Intracranial hemorrhage
Assume that a patient taking a NOAC who displays any acute neurologic change is experiencing an intracranial hemorrhage until proven otherwise. After CT confirmation, reverse dabigatran with idarucizumab, or give prothrombin complex concentrate to patients on other NOACs.
Ischemic stroke
Patients who suffer an ischemic stroke despite NOAC therapy are not candidates for tissue plasminogen activators.
The primary care physician is likely to be involved in the decision of whether, when, and for how long to resume anticoagulation therapy after a stroke. The statement says, “guidelines support withholding oral anticoagulation until 1-2 weeks after stroke among individuals with NVAF [nonvalvular atrial fibrillation], with shorter times for those with transient ischemic attack or small, nondisabling strokes and longer times for moderate to severe strokes.” In addition, it is worthwhile to consider medication nonadherence if no other etiology for the stroke is found; patients who miss doses may benefit more from warfarin because of its longer half-life.
Procedures and surgeries
Each year approximately 10% of patients on anticoagulation require surgery or other invasive procedures, and 20% require a minor procedure. To determine whether to interrupt NOAC therapy prior to a procedure, first determine the procedure’s bleeding risk. Patients undergoing procedures with low risk of bleeding, including minor dental, dermatologic, and ophthalmologic procedures, and endoscopies without biopsies, do not require interruption. For procedures with a moderate bleeding risk (including cardiac ablation, endoscopy with biopsies, radial artery catheterization) or high bleeding risk (including major surgery and cardiac catheterization via femoral artery), the patient’s thromboembolic risk should be evaluated using the medical history and the CHA2DS2 VASc score. NOACs should be stopped for 24-48 hours prior to the moderate to high-risk procedures. Dabigatran should be held for 72 hours for patients with creatinine clearance less than 50mL/min. Bridging therapy with heparin is not recommended for patients taking NOACS who are to have surgery. The decision about when to restart NOAC is based on the risk of thromboembolism and the bleeding risk of surgery.
Spinal or epidural anesthesia
Anesthesia guidelines recommend holding NOACs 3-5 days prior to the intervention, however, this increases risk of TE and studies have shown a very low incidence of hematoma in patients anticoagulated with a NOAC. For patients with a high risk of VTE, the NOAC can be resumed 12 hours post-procedure.
The bottom line
NOACS are commonly used for treatment and prophylaxis of VTE and atrial fibrillation and are often preferred over warfarin due to their more predictable pharmacokinetics, comparable efficacy, comparable or lower risk of major bleeding complications, fewer drug interactions, and lack of need for frequent monitoring. The AHA scientific statement gives guidance on managing NOACS in the face of acute bleeding as well as during and after procedures. NOACS should be stopped 24-48 hours prior to major surgeries and may be restarted based on weighing the risk of bleeding and risk of thromboembolism.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Oh is a third-year resident in the family medicine residency program at Abington Jefferson Health.
Reference
Raval AN et al. Management of patients on non–vitamin K antagonist oral anticoagulants in the acute care and periprocedural setting: A scientific statement from the American Heart Association. Circulation. 2017 Feb 6;135[10]:e604-e33. doi: 10.1161/CIR.0000000000000477
Testing for latent tuberculosis infection
While cases of active tuberculosis are relatively rare in the United States, TB is a major cause of morbidity and mortality worldwide. In the United States, there are an estimated 11 million individuals who have latent TB infection (LTBI). Without prophylactic treatment, somewhere between 4%-6% of individuals with LTBI will develop active disease during their lifetimes; roughly half of these cases will occur within a few years of the initial infection. Treatment of LTBI reduces – but does not eliminate – the risk for active disease, decreasing the consequences of active disease for the patient and the risk of transmitting infection to others.
Diagnostic tests for LTBI
The tuberculin skin test (TST) has been the standard method of diagnosing LTBI. It involves measuring induration caused by a delayed-type hypersensitivity reaction to Mycobacterium tuberculosis (Mtb) 2 or 3 days after injecting the reagent into the skin. The TST can result in false positives when detecting antibodies to BCG and nontuberculous mycobacteria, and false negatives when the patient does not demonstrate a robust immune response. A newer testing method is the Interferon Gamma Release Assay (IGRA), which involves phlebotomy, followed by a series of laboratory procedures that measure IFN-gamma release by T cells that have been sensitized to Mtb. The sensitivity of IGRA is similar to the TST, but it has better specificity; it is much less likely to react to antigens from BCG or nontuberculous mycobacteria. As detailed below, this guideline suggests a significantly more prominent role for IGRA, compared with previous recommendations.
Recommendation 1. Perform an IGRA, rather than a TST, in individuals 5 years or older who meet the following criteria: 1) are likely to be infected with Mtb; 2) have a low or intermediate risk of disease progression; 3) in whom it has been decided that testing for LTBI is warranted. A TST is an acceptable alternative, particularly if an IGRA is not available, is too costly, or is too burdensome. If an individual either has a history of BCG vaccination or is unlikely to return to have their TST read, then it is strongly recommended to use the IGRA as the test of choice.
Recommendation 2. There are insufficient data to recommend a preference for either a TST or an IGRA as the first-line diagnostic test in individuals 5 years or older who are likely to be infected with Mtb, who have a high risk of progression to active disease, and in whom it has been determined that diagnostic testing for LTBI infection is warranted; either test would be acceptable. In very high-risk patients, consider dual testing, with a positive result from either test (TST or IGRA) being considered positive.
Recommendation 3. Guidelines do not recommend testing for persons at low risk for Mtb infection. However, the authors recognize that testing in such persons may nevertheless be mandated in certain situations (for example in some school or child care settings). In these cases, the authors recommend performing an IGRA instead of a TST, to minimize the chance of a false-positive result, although a TST is an acceptable alternative. Furthermore, if the initial test is positive, they suggest performing a confirmatory test (either an IGRA or TST) and considering the person infected only if both tests are positive.
Recommendation 4. The authors suggest performing a TST rather than an IGRA in healthy children less than 5 years of age for whom it has been decided that diagnostic testing for LTBI is warranted. This recommendation reflects the limited body of evidence regarding IGRA testing in young children and the apparent decreased sensitivity (i.e. more false negatives) in this population, compared with TST use.
In the area of serial testing for TB infection, often done in health care and institutional settings, the guideline points out areas of uncertainty with IGRA testing. Specifically, the IGRA test is subject to variability in readings and boosting with antigen exposure that can complicate interpretation of apparent conversion on repeat testing. One longitudinal study showed conversion rates with IGRA to be six to nine times higher than that seen for the TST, and those conversions were thought to represent false positive tests. The guideline concludes that, “There is insufficient information available to guide the establishment of definitive criteria for the conversion.” The committee thought that a positive test in a low-risk individual was likely to be a false-positive result and recommended repeat testing. Because of the possibility of boosting with antigen exposure in situations where dual testing is anticipated, it may be preferable to obtain a specimen for IGRA prior to, or concurrently with TST placement.
Bottom line
Current guidelines suggest a more prominent role for IGRA in testing for LTBI, particularly when the likelihood of exposure is low and in situations where a person may have received BCG vaccination, or would be unlikely to return for TST reading.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Clark is associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
Reference
Lewisohn DM et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of Tuberculosis in Adults and Children. Clin Inf Dis. 2017;64(2):111-5.
While cases of active tuberculosis are relatively rare in the United States, TB is a major cause of morbidity and mortality worldwide. In the United States, there are an estimated 11 million individuals who have latent TB infection (LTBI). Without prophylactic treatment, somewhere between 4%-6% of individuals with LTBI will develop active disease during their lifetimes; roughly half of these cases will occur within a few years of the initial infection. Treatment of LTBI reduces – but does not eliminate – the risk for active disease, decreasing the consequences of active disease for the patient and the risk of transmitting infection to others.
Diagnostic tests for LTBI
The tuberculin skin test (TST) has been the standard method of diagnosing LTBI. It involves measuring induration caused by a delayed-type hypersensitivity reaction to Mycobacterium tuberculosis (Mtb) 2 or 3 days after injecting the reagent into the skin. The TST can result in false positives when detecting antibodies to BCG and nontuberculous mycobacteria, and false negatives when the patient does not demonstrate a robust immune response. A newer testing method is the Interferon Gamma Release Assay (IGRA), which involves phlebotomy, followed by a series of laboratory procedures that measure IFN-gamma release by T cells that have been sensitized to Mtb. The sensitivity of IGRA is similar to the TST, but it has better specificity; it is much less likely to react to antigens from BCG or nontuberculous mycobacteria. As detailed below, this guideline suggests a significantly more prominent role for IGRA, compared with previous recommendations.
Recommendation 1. Perform an IGRA, rather than a TST, in individuals 5 years or older who meet the following criteria: 1) are likely to be infected with Mtb; 2) have a low or intermediate risk of disease progression; 3) in whom it has been decided that testing for LTBI is warranted. A TST is an acceptable alternative, particularly if an IGRA is not available, is too costly, or is too burdensome. If an individual either has a history of BCG vaccination or is unlikely to return to have their TST read, then it is strongly recommended to use the IGRA as the test of choice.
Recommendation 2. There are insufficient data to recommend a preference for either a TST or an IGRA as the first-line diagnostic test in individuals 5 years or older who are likely to be infected with Mtb, who have a high risk of progression to active disease, and in whom it has been determined that diagnostic testing for LTBI infection is warranted; either test would be acceptable. In very high-risk patients, consider dual testing, with a positive result from either test (TST or IGRA) being considered positive.
Recommendation 3. Guidelines do not recommend testing for persons at low risk for Mtb infection. However, the authors recognize that testing in such persons may nevertheless be mandated in certain situations (for example in some school or child care settings). In these cases, the authors recommend performing an IGRA instead of a TST, to minimize the chance of a false-positive result, although a TST is an acceptable alternative. Furthermore, if the initial test is positive, they suggest performing a confirmatory test (either an IGRA or TST) and considering the person infected only if both tests are positive.
Recommendation 4. The authors suggest performing a TST rather than an IGRA in healthy children less than 5 years of age for whom it has been decided that diagnostic testing for LTBI is warranted. This recommendation reflects the limited body of evidence regarding IGRA testing in young children and the apparent decreased sensitivity (i.e. more false negatives) in this population, compared with TST use.
In the area of serial testing for TB infection, often done in health care and institutional settings, the guideline points out areas of uncertainty with IGRA testing. Specifically, the IGRA test is subject to variability in readings and boosting with antigen exposure that can complicate interpretation of apparent conversion on repeat testing. One longitudinal study showed conversion rates with IGRA to be six to nine times higher than that seen for the TST, and those conversions were thought to represent false positive tests. The guideline concludes that, “There is insufficient information available to guide the establishment of definitive criteria for the conversion.” The committee thought that a positive test in a low-risk individual was likely to be a false-positive result and recommended repeat testing. Because of the possibility of boosting with antigen exposure in situations where dual testing is anticipated, it may be preferable to obtain a specimen for IGRA prior to, or concurrently with TST placement.
Bottom line
Current guidelines suggest a more prominent role for IGRA in testing for LTBI, particularly when the likelihood of exposure is low and in situations where a person may have received BCG vaccination, or would be unlikely to return for TST reading.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Clark is associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
Reference
Lewisohn DM et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of Tuberculosis in Adults and Children. Clin Inf Dis. 2017;64(2):111-5.
While cases of active tuberculosis are relatively rare in the United States, TB is a major cause of morbidity and mortality worldwide. In the United States, there are an estimated 11 million individuals who have latent TB infection (LTBI). Without prophylactic treatment, somewhere between 4%-6% of individuals with LTBI will develop active disease during their lifetimes; roughly half of these cases will occur within a few years of the initial infection. Treatment of LTBI reduces – but does not eliminate – the risk for active disease, decreasing the consequences of active disease for the patient and the risk of transmitting infection to others.
Diagnostic tests for LTBI
The tuberculin skin test (TST) has been the standard method of diagnosing LTBI. It involves measuring induration caused by a delayed-type hypersensitivity reaction to Mycobacterium tuberculosis (Mtb) 2 or 3 days after injecting the reagent into the skin. The TST can result in false positives when detecting antibodies to BCG and nontuberculous mycobacteria, and false negatives when the patient does not demonstrate a robust immune response. A newer testing method is the Interferon Gamma Release Assay (IGRA), which involves phlebotomy, followed by a series of laboratory procedures that measure IFN-gamma release by T cells that have been sensitized to Mtb. The sensitivity of IGRA is similar to the TST, but it has better specificity; it is much less likely to react to antigens from BCG or nontuberculous mycobacteria. As detailed below, this guideline suggests a significantly more prominent role for IGRA, compared with previous recommendations.
Recommendation 1. Perform an IGRA, rather than a TST, in individuals 5 years or older who meet the following criteria: 1) are likely to be infected with Mtb; 2) have a low or intermediate risk of disease progression; 3) in whom it has been decided that testing for LTBI is warranted. A TST is an acceptable alternative, particularly if an IGRA is not available, is too costly, or is too burdensome. If an individual either has a history of BCG vaccination or is unlikely to return to have their TST read, then it is strongly recommended to use the IGRA as the test of choice.
Recommendation 2. There are insufficient data to recommend a preference for either a TST or an IGRA as the first-line diagnostic test in individuals 5 years or older who are likely to be infected with Mtb, who have a high risk of progression to active disease, and in whom it has been determined that diagnostic testing for LTBI infection is warranted; either test would be acceptable. In very high-risk patients, consider dual testing, with a positive result from either test (TST or IGRA) being considered positive.
Recommendation 3. Guidelines do not recommend testing for persons at low risk for Mtb infection. However, the authors recognize that testing in such persons may nevertheless be mandated in certain situations (for example in some school or child care settings). In these cases, the authors recommend performing an IGRA instead of a TST, to minimize the chance of a false-positive result, although a TST is an acceptable alternative. Furthermore, if the initial test is positive, they suggest performing a confirmatory test (either an IGRA or TST) and considering the person infected only if both tests are positive.
Recommendation 4. The authors suggest performing a TST rather than an IGRA in healthy children less than 5 years of age for whom it has been decided that diagnostic testing for LTBI is warranted. This recommendation reflects the limited body of evidence regarding IGRA testing in young children and the apparent decreased sensitivity (i.e. more false negatives) in this population, compared with TST use.
In the area of serial testing for TB infection, often done in health care and institutional settings, the guideline points out areas of uncertainty with IGRA testing. Specifically, the IGRA test is subject to variability in readings and boosting with antigen exposure that can complicate interpretation of apparent conversion on repeat testing. One longitudinal study showed conversion rates with IGRA to be six to nine times higher than that seen for the TST, and those conversions were thought to represent false positive tests. The guideline concludes that, “There is insufficient information available to guide the establishment of definitive criteria for the conversion.” The committee thought that a positive test in a low-risk individual was likely to be a false-positive result and recommended repeat testing. Because of the possibility of boosting with antigen exposure in situations where dual testing is anticipated, it may be preferable to obtain a specimen for IGRA prior to, or concurrently with TST placement.
Bottom line
Current guidelines suggest a more prominent role for IGRA in testing for LTBI, particularly when the likelihood of exposure is low and in situations where a person may have received BCG vaccination, or would be unlikely to return for TST reading.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Clark is associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
Reference
Lewisohn DM et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of Tuberculosis in Adults and Children. Clin Inf Dis. 2017;64(2):111-5.
“I’m sorry, doctor, I’m afraid I can’t do that”
In “2001: A Space Odyssey,” the epic 1968 film by Stanley Kubrick and Arthur C. Clarke, humanity makes first contact with an alien intelligence, and the course of history is irreversibly altered. Hailed as a watershed moment in science fiction, “2001” was considered way ahead of its time and raised a number of philosophical questions about what would happen if we ever encountered another form of life. Interestingly, the most noteworthy character in the film isn’t human or alien, but instead a new form of life altogether: an artificial intelligence (AI) known as the Heuristically programmed ALgorithmic computer 9000. HAL (as he is known colloquially) operates the Discovery One spacecraft, ferrying several scientists bound for Jupiter on a mission of exploration. Stating that he is “foolproof and incapable of error,” HAL’s superiority complex leads him to become the film’s antagonist, as he believes that human error is the cause of the difficulties they encounter. He eventually concludes that the best way to complete the mission is to eliminate human interference. When asked by scientist Dr. David Bowman to perform a simple function essential to the survival of the crew, HAL simply states “I’m sorry, Dave, I’m afraid I can’t do that.” Bowman is forced to disconnect HAL’s higher intellectual capabilities, reverting the computer to its most basic functions to ensure human survival.
Kubrick and Clarke may have been overly ambitious in predicting the progress of human space flight, but their call for concern over the risks of artificial intelligence seems quite prescient. Recently, billionaire entrepreneur Elon Musk (CEO of Tesla Motors and SpaceX) raised his concerns about AI, warning that, left unchecked, AI could be mankind’s final invention – one that could eventually destroy us. Other giants of the tech industry, including Bill Gates and Mark Zuckerberg, disagree. They believe AI represents tremendous promise for humanity and could usher in innovations unlike any we have ever seen.
A few weeks ago, we attended a national electronic health records conference where a well-known EHR vendor unveiled the new features in the upcoming release of their software. One of the most noteworthy additions was an intelligent virtual assistant, designed to help providers care for patients. While this is not the first time AI has ventured into health care (see IBM’s “Watson”), it is the first time the idea has become mainstream and fully integrated into physician workflow. Much like the virtual assistants mentioned above, this one can use voice or mouse/keyboard interaction to find clinical information, simplify common tasks, and help with medical decision-making.
While exciting at first, the idea of artificially intelligent EHRs may sound terrifying to some who aren’t yet ready to trust any patient care to machines. Reassuringly, while the integrated virtual assistant mentioned above can make suggestions to guide physicians to the right data or offer decision support when available, it is primarily focused on interface enhancement to improve work flow. It is not yet capable of making true clinical decisions that remove the physician from care delivery, but computers that do the diagnostic work of physicians may be closer than you think.
Research done at Jefferson University in Philadelphia and published in the August 2017 edition of Radiology1 investigated the ability of deep-learning algorithms to interpret chest radiographs for the diagnosis of tuberculosis. The computers achieved an impressive reliability of 99%. While at first radiograph interpretation seems quite different than the diagnostic decision-making done in primary care, the fundamental skill required for both is similar: pattern recognition. To build those patterns, artificial intelligence requires an enormous number of data points, but that’s hardly a problem thanks to the continual collection of patient data through electronic health records. The amount of raw information available to these algorithms is growing exponentially by the day, and with time their predictive ability will be unmatched. So where will that leave us, the physicians, entrusted for generations with the responsibility of diagnosis? Possibly more satisfied than we are today.
There was a time – not long ago – when the body of available medical knowledge was incredibly limited. Diagnostic testing was primitive and often inaccurate, and the treatment provided by physicians was focused on supporting, communicating, and genuinely caring for patients and their families. In the past 50 years, medical knowledge has exploded, and diagnostic testing has become incredibly advanced. Sadly, at the same time physicians have begun to feel more like clerical workers: entering data, writing prescriptions, and filling out forms. As artificial intelligence assumes some of this busywork and takes much of the guesswork out of diagnosis, physicians may find greater job satisfaction as they provide the skills a computer never can: a human touch, a personal and reflective interpretation of a patient’s diagnosis, and a true emotional connection. Ask this of a computer, and the response will always be the same: “I’m sorry, doctor, I’m afraid I can’t do that.”
Reference
1. Lakhani, Paras & Sundaram, Baskaran, “Deep Learning at Chest Radiography: Automated Classification of Pulmonary Tuberculosis by Using Convolutional Neural Networks,” Radiology. 2017 Aug;284:574-82.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is also a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington Jefferson Health.
In “2001: A Space Odyssey,” the epic 1968 film by Stanley Kubrick and Arthur C. Clarke, humanity makes first contact with an alien intelligence, and the course of history is irreversibly altered. Hailed as a watershed moment in science fiction, “2001” was considered way ahead of its time and raised a number of philosophical questions about what would happen if we ever encountered another form of life. Interestingly, the most noteworthy character in the film isn’t human or alien, but instead a new form of life altogether: an artificial intelligence (AI) known as the Heuristically programmed ALgorithmic computer 9000. HAL (as he is known colloquially) operates the Discovery One spacecraft, ferrying several scientists bound for Jupiter on a mission of exploration. Stating that he is “foolproof and incapable of error,” HAL’s superiority complex leads him to become the film’s antagonist, as he believes that human error is the cause of the difficulties they encounter. He eventually concludes that the best way to complete the mission is to eliminate human interference. When asked by scientist Dr. David Bowman to perform a simple function essential to the survival of the crew, HAL simply states “I’m sorry, Dave, I’m afraid I can’t do that.” Bowman is forced to disconnect HAL’s higher intellectual capabilities, reverting the computer to its most basic functions to ensure human survival.
Kubrick and Clarke may have been overly ambitious in predicting the progress of human space flight, but their call for concern over the risks of artificial intelligence seems quite prescient. Recently, billionaire entrepreneur Elon Musk (CEO of Tesla Motors and SpaceX) raised his concerns about AI, warning that, left unchecked, AI could be mankind’s final invention – one that could eventually destroy us. Other giants of the tech industry, including Bill Gates and Mark Zuckerberg, disagree. They believe AI represents tremendous promise for humanity and could usher in innovations unlike any we have ever seen.
A few weeks ago, we attended a national electronic health records conference where a well-known EHR vendor unveiled the new features in the upcoming release of their software. One of the most noteworthy additions was an intelligent virtual assistant, designed to help providers care for patients. While this is not the first time AI has ventured into health care (see IBM’s “Watson”), it is the first time the idea has become mainstream and fully integrated into physician workflow. Much like the virtual assistants mentioned above, this one can use voice or mouse/keyboard interaction to find clinical information, simplify common tasks, and help with medical decision-making.
While exciting at first, the idea of artificially intelligent EHRs may sound terrifying to some who aren’t yet ready to trust any patient care to machines. Reassuringly, while the integrated virtual assistant mentioned above can make suggestions to guide physicians to the right data or offer decision support when available, it is primarily focused on interface enhancement to improve work flow. It is not yet capable of making true clinical decisions that remove the physician from care delivery, but computers that do the diagnostic work of physicians may be closer than you think.
Research done at Jefferson University in Philadelphia and published in the August 2017 edition of Radiology1 investigated the ability of deep-learning algorithms to interpret chest radiographs for the diagnosis of tuberculosis. The computers achieved an impressive reliability of 99%. While at first radiograph interpretation seems quite different than the diagnostic decision-making done in primary care, the fundamental skill required for both is similar: pattern recognition. To build those patterns, artificial intelligence requires an enormous number of data points, but that’s hardly a problem thanks to the continual collection of patient data through electronic health records. The amount of raw information available to these algorithms is growing exponentially by the day, and with time their predictive ability will be unmatched. So where will that leave us, the physicians, entrusted for generations with the responsibility of diagnosis? Possibly more satisfied than we are today.
There was a time – not long ago – when the body of available medical knowledge was incredibly limited. Diagnostic testing was primitive and often inaccurate, and the treatment provided by physicians was focused on supporting, communicating, and genuinely caring for patients and their families. In the past 50 years, medical knowledge has exploded, and diagnostic testing has become incredibly advanced. Sadly, at the same time physicians have begun to feel more like clerical workers: entering data, writing prescriptions, and filling out forms. As artificial intelligence assumes some of this busywork and takes much of the guesswork out of diagnosis, physicians may find greater job satisfaction as they provide the skills a computer never can: a human touch, a personal and reflective interpretation of a patient’s diagnosis, and a true emotional connection. Ask this of a computer, and the response will always be the same: “I’m sorry, doctor, I’m afraid I can’t do that.”
Reference
1. Lakhani, Paras & Sundaram, Baskaran, “Deep Learning at Chest Radiography: Automated Classification of Pulmonary Tuberculosis by Using Convolutional Neural Networks,” Radiology. 2017 Aug;284:574-82.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is also a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington Jefferson Health.
In “2001: A Space Odyssey,” the epic 1968 film by Stanley Kubrick and Arthur C. Clarke, humanity makes first contact with an alien intelligence, and the course of history is irreversibly altered. Hailed as a watershed moment in science fiction, “2001” was considered way ahead of its time and raised a number of philosophical questions about what would happen if we ever encountered another form of life. Interestingly, the most noteworthy character in the film isn’t human or alien, but instead a new form of life altogether: an artificial intelligence (AI) known as the Heuristically programmed ALgorithmic computer 9000. HAL (as he is known colloquially) operates the Discovery One spacecraft, ferrying several scientists bound for Jupiter on a mission of exploration. Stating that he is “foolproof and incapable of error,” HAL’s superiority complex leads him to become the film’s antagonist, as he believes that human error is the cause of the difficulties they encounter. He eventually concludes that the best way to complete the mission is to eliminate human interference. When asked by scientist Dr. David Bowman to perform a simple function essential to the survival of the crew, HAL simply states “I’m sorry, Dave, I’m afraid I can’t do that.” Bowman is forced to disconnect HAL’s higher intellectual capabilities, reverting the computer to its most basic functions to ensure human survival.
Kubrick and Clarke may have been overly ambitious in predicting the progress of human space flight, but their call for concern over the risks of artificial intelligence seems quite prescient. Recently, billionaire entrepreneur Elon Musk (CEO of Tesla Motors and SpaceX) raised his concerns about AI, warning that, left unchecked, AI could be mankind’s final invention – one that could eventually destroy us. Other giants of the tech industry, including Bill Gates and Mark Zuckerberg, disagree. They believe AI represents tremendous promise for humanity and could usher in innovations unlike any we have ever seen.
A few weeks ago, we attended a national electronic health records conference where a well-known EHR vendor unveiled the new features in the upcoming release of their software. One of the most noteworthy additions was an intelligent virtual assistant, designed to help providers care for patients. While this is not the first time AI has ventured into health care (see IBM’s “Watson”), it is the first time the idea has become mainstream and fully integrated into physician workflow. Much like the virtual assistants mentioned above, this one can use voice or mouse/keyboard interaction to find clinical information, simplify common tasks, and help with medical decision-making.
While exciting at first, the idea of artificially intelligent EHRs may sound terrifying to some who aren’t yet ready to trust any patient care to machines. Reassuringly, while the integrated virtual assistant mentioned above can make suggestions to guide physicians to the right data or offer decision support when available, it is primarily focused on interface enhancement to improve work flow. It is not yet capable of making true clinical decisions that remove the physician from care delivery, but computers that do the diagnostic work of physicians may be closer than you think.
Research done at Jefferson University in Philadelphia and published in the August 2017 edition of Radiology1 investigated the ability of deep-learning algorithms to interpret chest radiographs for the diagnosis of tuberculosis. The computers achieved an impressive reliability of 99%. While at first radiograph interpretation seems quite different than the diagnostic decision-making done in primary care, the fundamental skill required for both is similar: pattern recognition. To build those patterns, artificial intelligence requires an enormous number of data points, but that’s hardly a problem thanks to the continual collection of patient data through electronic health records. The amount of raw information available to these algorithms is growing exponentially by the day, and with time their predictive ability will be unmatched. So where will that leave us, the physicians, entrusted for generations with the responsibility of diagnosis? Possibly more satisfied than we are today.
There was a time – not long ago – when the body of available medical knowledge was incredibly limited. Diagnostic testing was primitive and often inaccurate, and the treatment provided by physicians was focused on supporting, communicating, and genuinely caring for patients and their families. In the past 50 years, medical knowledge has exploded, and diagnostic testing has become incredibly advanced. Sadly, at the same time physicians have begun to feel more like clerical workers: entering data, writing prescriptions, and filling out forms. As artificial intelligence assumes some of this busywork and takes much of the guesswork out of diagnosis, physicians may find greater job satisfaction as they provide the skills a computer never can: a human touch, a personal and reflective interpretation of a patient’s diagnosis, and a true emotional connection. Ask this of a computer, and the response will always be the same: “I’m sorry, doctor, I’m afraid I can’t do that.”
Reference
1. Lakhani, Paras & Sundaram, Baskaran, “Deep Learning at Chest Radiography: Automated Classification of Pulmonary Tuberculosis by Using Convolutional Neural Networks,” Radiology. 2017 Aug;284:574-82.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is also a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington Jefferson Health.
The Inflection Point
In the early 1600s, the French playwright Molière wrote one of the great satires of all time, “The Doctor in Spite of Himself.” In that play the main character, Sganarelle, is a woodcutter who wastes all his money on alcohol, so his wife Martine decides she will teach him a lesson. As she is plotting her revenge, Martine overhears two peasants discussing how they have been trying to find a doctor for their rich employer’s daughter, who has become suddenly mute. Martine seizes the opportunity to tell the peasants that her husband is a brilliant – though eccentric – doctor who usually hides his identity. Learning this, the peasants find Sganarelle and beg him to see their master’s daughter. Though he initially refuses, they berate him until he can take it no more, and he finally says that he is a doctor and agrees to assess the ill young woman.
Sganarelle does his best to impersonate a doctor while examining the young woman, and as he is doing so it becomes apparent even to him that she is not truly ill. She is pretending to be mute because she’s being forced to marry a wealthy man she does not love. Sganarelle discusses the diagnosis with her father, stating, “this impediment to the action of the tongue is caused by certain humors.” He goes on to say that her muteness was triggered by, “the vapors that pass from the left side, where the liver resides, to the right side, where the heart dwells.” The rich aristocrat listens intently and accepts the diagnosis, though he seems puzzled about one thing. “Isn’t the heart on the left side of the chest?” he asks. To this insightful and obvious question Sganarelle replies, “Yes, that used to be true; but we’ve changed all that, and we practice medicine now according to a whole new method.”
It is astonishing that Molière, in a farcical comedy written in the 1600s, could have anticipated the dizzying rate of change in modern medicine. While the heart and liver have not changed sides, the ways we are practicing medicine have undergone landmark shifts over the past 10 years. Just look at the new ways in which we record documentation, learn new information, send in prescriptions, manage populations in addition to individual patients, and so many other aspects of care. At times this evolution has its own satirical feel to it. For example, the notion that refusing to refill an opioid prescription for a patient that broke their opioid contract could lead to a bad review on Yelp or points off on a Press Ganey satisfaction survey does not seem reasonable, but it is real.
When we started this column about 10 years ago, we regularly received emails (and even letters written in fine penmanship and mailed in envelopes) from physicians who felt that the EHR was ruining their practice and their lives. Many of the letters talked about early retirement. Some physicians ended up retiring early. Many of these physicians were smart, able people who we believe took great care of patients. But as Leon C. Megginson, interpreting the work of Darwin, observed, “It is not the strongest of the species that survives, nor the most intelligent, but the one most responsive to change.” Adaptability favors the young; the young have fewer habits to break, few preconceived ideas of how things should be, and perhaps more energy to give to new tasks.
We believe we have now reached the inflection point – a time in the history of an industry where an event (in this case the advent of the EHR) so fundamentally impacts the industry that the industry is changed from that point forward. The industry, and more importantly those who work in the industry, must adopt new approaches and attitudes in order to survive in the changed environment. Andrew Grove, the former CEO of Intel, talked about Strategic Inflection Points in a keynote address to the Academy of Management: “…what is common to [inflection points] and what is key is that they require a fundamental change in business strategy.” Grove also said, “That change can mean an opportunity to rise to new heights. But it may just as likely signal the beginning of the end.”
Up until recently, the introduction of the EHR lead to discussions about what was good and what was bad about the advent of EHRs. That time is past. We no longer receive letters from physicians expressing their concerns about the EHR, as many of those physicians have taken the change as a signal of the end of their careers, and chosen to retire. The rest have adapted to a new world. And in this new world we are certainly rising to new heights. We are forward-focused and looking at the multi-fold ways that our new technologies can accomplish their many missions – to improve the health of the population, to serve as a source of data to assess the real-world effectiveness of novel therapies, to evaluate and affect the quality of care given by practices and individual physicians, and to take excellent personalized care of individual patients. While we are physicians, not wood cutters as in Molière’s play, it remains incumbent upon us never to stop listening to our patients’ hearts, and to interpret their symptoms and signs with common sense, empathy and even humor when appropriate, all the while embracing approaches that move the health care of our patients forward to new heights.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington Jefferson Health. Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is also a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records.
In the early 1600s, the French playwright Molière wrote one of the great satires of all time, “The Doctor in Spite of Himself.” In that play the main character, Sganarelle, is a woodcutter who wastes all his money on alcohol, so his wife Martine decides she will teach him a lesson. As she is plotting her revenge, Martine overhears two peasants discussing how they have been trying to find a doctor for their rich employer’s daughter, who has become suddenly mute. Martine seizes the opportunity to tell the peasants that her husband is a brilliant – though eccentric – doctor who usually hides his identity. Learning this, the peasants find Sganarelle and beg him to see their master’s daughter. Though he initially refuses, they berate him until he can take it no more, and he finally says that he is a doctor and agrees to assess the ill young woman.
Sganarelle does his best to impersonate a doctor while examining the young woman, and as he is doing so it becomes apparent even to him that she is not truly ill. She is pretending to be mute because she’s being forced to marry a wealthy man she does not love. Sganarelle discusses the diagnosis with her father, stating, “this impediment to the action of the tongue is caused by certain humors.” He goes on to say that her muteness was triggered by, “the vapors that pass from the left side, where the liver resides, to the right side, where the heart dwells.” The rich aristocrat listens intently and accepts the diagnosis, though he seems puzzled about one thing. “Isn’t the heart on the left side of the chest?” he asks. To this insightful and obvious question Sganarelle replies, “Yes, that used to be true; but we’ve changed all that, and we practice medicine now according to a whole new method.”
It is astonishing that Molière, in a farcical comedy written in the 1600s, could have anticipated the dizzying rate of change in modern medicine. While the heart and liver have not changed sides, the ways we are practicing medicine have undergone landmark shifts over the past 10 years. Just look at the new ways in which we record documentation, learn new information, send in prescriptions, manage populations in addition to individual patients, and so many other aspects of care. At times this evolution has its own satirical feel to it. For example, the notion that refusing to refill an opioid prescription for a patient that broke their opioid contract could lead to a bad review on Yelp or points off on a Press Ganey satisfaction survey does not seem reasonable, but it is real.
When we started this column about 10 years ago, we regularly received emails (and even letters written in fine penmanship and mailed in envelopes) from physicians who felt that the EHR was ruining their practice and their lives. Many of the letters talked about early retirement. Some physicians ended up retiring early. Many of these physicians were smart, able people who we believe took great care of patients. But as Leon C. Megginson, interpreting the work of Darwin, observed, “It is not the strongest of the species that survives, nor the most intelligent, but the one most responsive to change.” Adaptability favors the young; the young have fewer habits to break, few preconceived ideas of how things should be, and perhaps more energy to give to new tasks.
We believe we have now reached the inflection point – a time in the history of an industry where an event (in this case the advent of the EHR) so fundamentally impacts the industry that the industry is changed from that point forward. The industry, and more importantly those who work in the industry, must adopt new approaches and attitudes in order to survive in the changed environment. Andrew Grove, the former CEO of Intel, talked about Strategic Inflection Points in a keynote address to the Academy of Management: “…what is common to [inflection points] and what is key is that they require a fundamental change in business strategy.” Grove also said, “That change can mean an opportunity to rise to new heights. But it may just as likely signal the beginning of the end.”
Up until recently, the introduction of the EHR lead to discussions about what was good and what was bad about the advent of EHRs. That time is past. We no longer receive letters from physicians expressing their concerns about the EHR, as many of those physicians have taken the change as a signal of the end of their careers, and chosen to retire. The rest have adapted to a new world. And in this new world we are certainly rising to new heights. We are forward-focused and looking at the multi-fold ways that our new technologies can accomplish their many missions – to improve the health of the population, to serve as a source of data to assess the real-world effectiveness of novel therapies, to evaluate and affect the quality of care given by practices and individual physicians, and to take excellent personalized care of individual patients. While we are physicians, not wood cutters as in Molière’s play, it remains incumbent upon us never to stop listening to our patients’ hearts, and to interpret their symptoms and signs with common sense, empathy and even humor when appropriate, all the while embracing approaches that move the health care of our patients forward to new heights.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington Jefferson Health. Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is also a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records.
In the early 1600s, the French playwright Molière wrote one of the great satires of all time, “The Doctor in Spite of Himself.” In that play the main character, Sganarelle, is a woodcutter who wastes all his money on alcohol, so his wife Martine decides she will teach him a lesson. As she is plotting her revenge, Martine overhears two peasants discussing how they have been trying to find a doctor for their rich employer’s daughter, who has become suddenly mute. Martine seizes the opportunity to tell the peasants that her husband is a brilliant – though eccentric – doctor who usually hides his identity. Learning this, the peasants find Sganarelle and beg him to see their master’s daughter. Though he initially refuses, they berate him until he can take it no more, and he finally says that he is a doctor and agrees to assess the ill young woman.
Sganarelle does his best to impersonate a doctor while examining the young woman, and as he is doing so it becomes apparent even to him that she is not truly ill. She is pretending to be mute because she’s being forced to marry a wealthy man she does not love. Sganarelle discusses the diagnosis with her father, stating, “this impediment to the action of the tongue is caused by certain humors.” He goes on to say that her muteness was triggered by, “the vapors that pass from the left side, where the liver resides, to the right side, where the heart dwells.” The rich aristocrat listens intently and accepts the diagnosis, though he seems puzzled about one thing. “Isn’t the heart on the left side of the chest?” he asks. To this insightful and obvious question Sganarelle replies, “Yes, that used to be true; but we’ve changed all that, and we practice medicine now according to a whole new method.”
It is astonishing that Molière, in a farcical comedy written in the 1600s, could have anticipated the dizzying rate of change in modern medicine. While the heart and liver have not changed sides, the ways we are practicing medicine have undergone landmark shifts over the past 10 years. Just look at the new ways in which we record documentation, learn new information, send in prescriptions, manage populations in addition to individual patients, and so many other aspects of care. At times this evolution has its own satirical feel to it. For example, the notion that refusing to refill an opioid prescription for a patient that broke their opioid contract could lead to a bad review on Yelp or points off on a Press Ganey satisfaction survey does not seem reasonable, but it is real.
When we started this column about 10 years ago, we regularly received emails (and even letters written in fine penmanship and mailed in envelopes) from physicians who felt that the EHR was ruining their practice and their lives. Many of the letters talked about early retirement. Some physicians ended up retiring early. Many of these physicians were smart, able people who we believe took great care of patients. But as Leon C. Megginson, interpreting the work of Darwin, observed, “It is not the strongest of the species that survives, nor the most intelligent, but the one most responsive to change.” Adaptability favors the young; the young have fewer habits to break, few preconceived ideas of how things should be, and perhaps more energy to give to new tasks.
We believe we have now reached the inflection point – a time in the history of an industry where an event (in this case the advent of the EHR) so fundamentally impacts the industry that the industry is changed from that point forward. The industry, and more importantly those who work in the industry, must adopt new approaches and attitudes in order to survive in the changed environment. Andrew Grove, the former CEO of Intel, talked about Strategic Inflection Points in a keynote address to the Academy of Management: “…what is common to [inflection points] and what is key is that they require a fundamental change in business strategy.” Grove also said, “That change can mean an opportunity to rise to new heights. But it may just as likely signal the beginning of the end.”
Up until recently, the introduction of the EHR lead to discussions about what was good and what was bad about the advent of EHRs. That time is past. We no longer receive letters from physicians expressing their concerns about the EHR, as many of those physicians have taken the change as a signal of the end of their careers, and chosen to retire. The rest have adapted to a new world. And in this new world we are certainly rising to new heights. We are forward-focused and looking at the multi-fold ways that our new technologies can accomplish their many missions – to improve the health of the population, to serve as a source of data to assess the real-world effectiveness of novel therapies, to evaluate and affect the quality of care given by practices and individual physicians, and to take excellent personalized care of individual patients. While we are physicians, not wood cutters as in Molière’s play, it remains incumbent upon us never to stop listening to our patients’ hearts, and to interpret their symptoms and signs with common sense, empathy and even humor when appropriate, all the while embracing approaches that move the health care of our patients forward to new heights.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington Jefferson Health. Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is also a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records.
Management of adults with syncope
Syncope is characterized by sudden transient loss of consciousness due to cerebral hypoperfusion and is typically associated with an inability to maintain postural tone. There are many different causes and clinical presentations of syncope and the incidence varies depending on the population. Estimated lifetime prevalence rates are as high as 41% for a single episode of syncope, with recurrent syncope occurring in 13.5% of the general population. Incidence follows a trimodal distribution with peaks at age 20, 60, and 80 years for both men and women. The National Hospital Ambulatory Medical Care Survey reported 6.7 million episodes of syncope in the emergency department, which is where most patients with syncope initially present. However, patients may also present to the primary care outpatient setting, and providers should be equipped for initial evaluation and management.
Previous and current treatment guidelines
Although there have been general reviews published by general and specialty societies, there were no comprehensive guidelines on the evaluation and management of syncope until recently. The 2017 guideline from the American College of Cardiology, American Heart Association, and Heart Rhythm Society is intended to provide guidance on evaluation and management of syncope, specifically in the context of different clinical settings, specific causes, or selected circumstances.1
What primary care providers should know
A detailed history and physical exam should be performed in all patients with syncope. Useful details include the setting in which syncope occurs, prodromal symptoms, witness reports, postevent symptoms, comorbidities, medication use, past medical history, and family history. The physical exam should include orthostatic vital signs, cardiac exam, neurologic exam, and any other relevant systems. A resting 12-lead ECG in the initial evaluation is recommended to detect underlying arrhythmia or structural heart disease (Class I recommendation – strong).
There are many different causes of syncope (see Table 1). Vasovagal syncope, a form of reflex syncope mediated by the vasovagal reflex, is the most common cause of syncope and a frequent reason for emergency department visits. There is often a prodrome of diaphoresis, warmth, nausea, and/or pallor, often followed by fatigue. The diagnosis can be made by the history, physical exam, and eyewitness observation.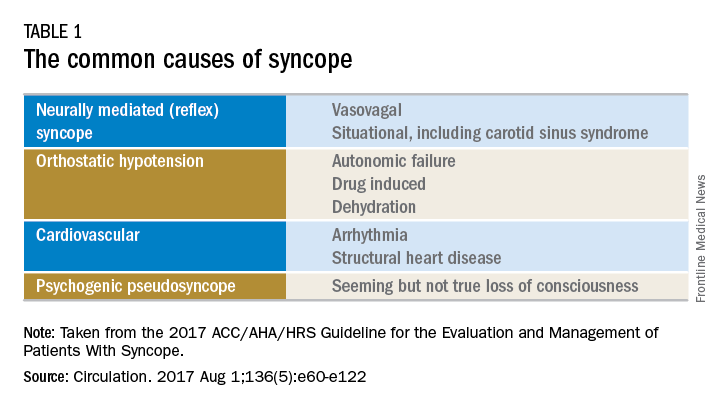
Once the initial evaluation is complete, further evaluation and management depends on the presence of risk factors presented in Table 2. Outpatient management is reasonable for patients with presumptive reflex-mediated syncope when there is an absence of serious medical conditions such as cardiac disease or comorbid neurologic disease. While hospital-based evaluation has not been shown to improve outcomes in patients with a low risk profile, hospital-based evaluation and treatment are recommended for patients presenting with syncope who have a serious medical condition potentially relevant to the cause of syncope.2 Serious medical conditions that require hospital management include arrhythmia, cardiac ischemia, severe aortic stenosis, hypertrophic cardiomyopathy, aortic dissection, acute heart failure, severe anemia, or major traumatic injury. Finally, patients with intermediate risk may benefit from an observational protocol in the emergency department.
Routine and comprehensive laboratory testing is not useful in syncope work-up (Class III recommendation – no benefit). Routine cardiac imaging is not recommended unless a cardiac etiology is suspected and routine neurological imaging and EEG are not recommended in the absence of focal neurologic findings. Additional work-up may be indicated if initial evaluation suggests a more specific etiology. If the initial evaluation suggests neurogenic orthostatic hypotension but the diagnosis is not clear, then referral for an autonomic evaluation is reasonable. If reflex syncope is suspected, tilt-table testing may be helpful to confirm the diagnosis. Lastly, if a cardiovascular etiology is suspected, it is recommended that the patient have cardiac monitoring in the acute care setting. In this later group, stress testing, transthoracic echocardiogram, electrophysiology study, and/or MRI or CT may be useful. Electrophysiologic testing is reasonable in patients with suspected arrhythmia as the etiology for syncope (Class IIa recommendation – moderate strength). The guideline provides a convenient summary algorithm to approach the initial and subsequent evaluations for syncope based on the initial evaluation and presenting symptoms.
Special populations
There are specific considerations for certain populations. In the pediatric population, the vast majority of syncopal episodes are reflex syncope but breath-holding spells should also be considered. In the geriatric population, particularly individuals older than 75 years, the incidence of syncope is high, the differential diagnosis is broad, and the diagnosis may be imprecise given amnesia, falls, lack of witnesses, and polypharmacy. In this group, morbidity is high because of multimorbidity and frailty. A careful history and physical exam with orthostatic vital signs is important, as is a multidisciplinary approach with geriatric consultation when needed.
Summary
Syncope is a common clinical syndrome often presenting to the emergency department or primary care setting. There are many causes, the most common being vasovagal syncope. In the initial evaluation, providers should perform a detailed history and physical exam, check orthostatic signs and perform a 12-lead ECG. Patients can be evaluated and managed safely in the outpatient setting in the absence of risk factors. Routine comprehensive laboratory testing and cardiac imaging are often not needed. For patients with defined risk factors, a more detailed evaluation in the hospital is recommended.
Dr. Li is a second-year resident in the family medicine residency program in the department of family and community medicine at the Sidney Kimmel Medical College at Thomas Jefferson University, Philadelphia. Dr. Mills is assistant residency program director and assistant professor in the departments of family and community medicine and physiology at the Sidney Kimmel Medical College. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
References
1. Shen W, Sheldon RS, Benditt DG, et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope. Circulation. 2017 Aug 1;136(5):e60-e122. doi: 10.1161/CIR.0000000000000499. Epub 2017 Mar 9.
2. Soteriades ES, Evans JC, Larson MG, et al. Incidence and prognosis of syncope. N Engl J Med. 2002;347(12):878-85.
Syncope is characterized by sudden transient loss of consciousness due to cerebral hypoperfusion and is typically associated with an inability to maintain postural tone. There are many different causes and clinical presentations of syncope and the incidence varies depending on the population. Estimated lifetime prevalence rates are as high as 41% for a single episode of syncope, with recurrent syncope occurring in 13.5% of the general population. Incidence follows a trimodal distribution with peaks at age 20, 60, and 80 years for both men and women. The National Hospital Ambulatory Medical Care Survey reported 6.7 million episodes of syncope in the emergency department, which is where most patients with syncope initially present. However, patients may also present to the primary care outpatient setting, and providers should be equipped for initial evaluation and management.
Previous and current treatment guidelines
Although there have been general reviews published by general and specialty societies, there were no comprehensive guidelines on the evaluation and management of syncope until recently. The 2017 guideline from the American College of Cardiology, American Heart Association, and Heart Rhythm Society is intended to provide guidance on evaluation and management of syncope, specifically in the context of different clinical settings, specific causes, or selected circumstances.1
What primary care providers should know
A detailed history and physical exam should be performed in all patients with syncope. Useful details include the setting in which syncope occurs, prodromal symptoms, witness reports, postevent symptoms, comorbidities, medication use, past medical history, and family history. The physical exam should include orthostatic vital signs, cardiac exam, neurologic exam, and any other relevant systems. A resting 12-lead ECG in the initial evaluation is recommended to detect underlying arrhythmia or structural heart disease (Class I recommendation – strong).
There are many different causes of syncope (see Table 1). Vasovagal syncope, a form of reflex syncope mediated by the vasovagal reflex, is the most common cause of syncope and a frequent reason for emergency department visits. There is often a prodrome of diaphoresis, warmth, nausea, and/or pallor, often followed by fatigue. The diagnosis can be made by the history, physical exam, and eyewitness observation.
Once the initial evaluation is complete, further evaluation and management depends on the presence of risk factors presented in Table 2. Outpatient management is reasonable for patients with presumptive reflex-mediated syncope when there is an absence of serious medical conditions such as cardiac disease or comorbid neurologic disease. While hospital-based evaluation has not been shown to improve outcomes in patients with a low risk profile, hospital-based evaluation and treatment are recommended for patients presenting with syncope who have a serious medical condition potentially relevant to the cause of syncope.2 Serious medical conditions that require hospital management include arrhythmia, cardiac ischemia, severe aortic stenosis, hypertrophic cardiomyopathy, aortic dissection, acute heart failure, severe anemia, or major traumatic injury. Finally, patients with intermediate risk may benefit from an observational protocol in the emergency department.
Routine and comprehensive laboratory testing is not useful in syncope work-up (Class III recommendation – no benefit). Routine cardiac imaging is not recommended unless a cardiac etiology is suspected and routine neurological imaging and EEG are not recommended in the absence of focal neurologic findings. Additional work-up may be indicated if initial evaluation suggests a more specific etiology. If the initial evaluation suggests neurogenic orthostatic hypotension but the diagnosis is not clear, then referral for an autonomic evaluation is reasonable. If reflex syncope is suspected, tilt-table testing may be helpful to confirm the diagnosis. Lastly, if a cardiovascular etiology is suspected, it is recommended that the patient have cardiac monitoring in the acute care setting. In this later group, stress testing, transthoracic echocardiogram, electrophysiology study, and/or MRI or CT may be useful. Electrophysiologic testing is reasonable in patients with suspected arrhythmia as the etiology for syncope (Class IIa recommendation – moderate strength). The guideline provides a convenient summary algorithm to approach the initial and subsequent evaluations for syncope based on the initial evaluation and presenting symptoms.
Special populations
There are specific considerations for certain populations. In the pediatric population, the vast majority of syncopal episodes are reflex syncope but breath-holding spells should also be considered. In the geriatric population, particularly individuals older than 75 years, the incidence of syncope is high, the differential diagnosis is broad, and the diagnosis may be imprecise given amnesia, falls, lack of witnesses, and polypharmacy. In this group, morbidity is high because of multimorbidity and frailty. A careful history and physical exam with orthostatic vital signs is important, as is a multidisciplinary approach with geriatric consultation when needed.
Summary
Syncope is a common clinical syndrome often presenting to the emergency department or primary care setting. There are many causes, the most common being vasovagal syncope. In the initial evaluation, providers should perform a detailed history and physical exam, check orthostatic signs and perform a 12-lead ECG. Patients can be evaluated and managed safely in the outpatient setting in the absence of risk factors. Routine comprehensive laboratory testing and cardiac imaging are often not needed. For patients with defined risk factors, a more detailed evaluation in the hospital is recommended.
Dr. Li is a second-year resident in the family medicine residency program in the department of family and community medicine at the Sidney Kimmel Medical College at Thomas Jefferson University, Philadelphia. Dr. Mills is assistant residency program director and assistant professor in the departments of family and community medicine and physiology at the Sidney Kimmel Medical College. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
References
1. Shen W, Sheldon RS, Benditt DG, et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope. Circulation. 2017 Aug 1;136(5):e60-e122. doi: 10.1161/CIR.0000000000000499. Epub 2017 Mar 9.
2. Soteriades ES, Evans JC, Larson MG, et al. Incidence and prognosis of syncope. N Engl J Med. 2002;347(12):878-85.
Syncope is characterized by sudden transient loss of consciousness due to cerebral hypoperfusion and is typically associated with an inability to maintain postural tone. There are many different causes and clinical presentations of syncope and the incidence varies depending on the population. Estimated lifetime prevalence rates are as high as 41% for a single episode of syncope, with recurrent syncope occurring in 13.5% of the general population. Incidence follows a trimodal distribution with peaks at age 20, 60, and 80 years for both men and women. The National Hospital Ambulatory Medical Care Survey reported 6.7 million episodes of syncope in the emergency department, which is where most patients with syncope initially present. However, patients may also present to the primary care outpatient setting, and providers should be equipped for initial evaluation and management.
Previous and current treatment guidelines
Although there have been general reviews published by general and specialty societies, there were no comprehensive guidelines on the evaluation and management of syncope until recently. The 2017 guideline from the American College of Cardiology, American Heart Association, and Heart Rhythm Society is intended to provide guidance on evaluation and management of syncope, specifically in the context of different clinical settings, specific causes, or selected circumstances.1
What primary care providers should know
A detailed history and physical exam should be performed in all patients with syncope. Useful details include the setting in which syncope occurs, prodromal symptoms, witness reports, postevent symptoms, comorbidities, medication use, past medical history, and family history. The physical exam should include orthostatic vital signs, cardiac exam, neurologic exam, and any other relevant systems. A resting 12-lead ECG in the initial evaluation is recommended to detect underlying arrhythmia or structural heart disease (Class I recommendation – strong).
There are many different causes of syncope (see Table 1). Vasovagal syncope, a form of reflex syncope mediated by the vasovagal reflex, is the most common cause of syncope and a frequent reason for emergency department visits. There is often a prodrome of diaphoresis, warmth, nausea, and/or pallor, often followed by fatigue. The diagnosis can be made by the history, physical exam, and eyewitness observation.
Once the initial evaluation is complete, further evaluation and management depends on the presence of risk factors presented in Table 2. Outpatient management is reasonable for patients with presumptive reflex-mediated syncope when there is an absence of serious medical conditions such as cardiac disease or comorbid neurologic disease. While hospital-based evaluation has not been shown to improve outcomes in patients with a low risk profile, hospital-based evaluation and treatment are recommended for patients presenting with syncope who have a serious medical condition potentially relevant to the cause of syncope.2 Serious medical conditions that require hospital management include arrhythmia, cardiac ischemia, severe aortic stenosis, hypertrophic cardiomyopathy, aortic dissection, acute heart failure, severe anemia, or major traumatic injury. Finally, patients with intermediate risk may benefit from an observational protocol in the emergency department.
Routine and comprehensive laboratory testing is not useful in syncope work-up (Class III recommendation – no benefit). Routine cardiac imaging is not recommended unless a cardiac etiology is suspected and routine neurological imaging and EEG are not recommended in the absence of focal neurologic findings. Additional work-up may be indicated if initial evaluation suggests a more specific etiology. If the initial evaluation suggests neurogenic orthostatic hypotension but the diagnosis is not clear, then referral for an autonomic evaluation is reasonable. If reflex syncope is suspected, tilt-table testing may be helpful to confirm the diagnosis. Lastly, if a cardiovascular etiology is suspected, it is recommended that the patient have cardiac monitoring in the acute care setting. In this later group, stress testing, transthoracic echocardiogram, electrophysiology study, and/or MRI or CT may be useful. Electrophysiologic testing is reasonable in patients with suspected arrhythmia as the etiology for syncope (Class IIa recommendation – moderate strength). The guideline provides a convenient summary algorithm to approach the initial and subsequent evaluations for syncope based on the initial evaluation and presenting symptoms.
Special populations
There are specific considerations for certain populations. In the pediatric population, the vast majority of syncopal episodes are reflex syncope but breath-holding spells should also be considered. In the geriatric population, particularly individuals older than 75 years, the incidence of syncope is high, the differential diagnosis is broad, and the diagnosis may be imprecise given amnesia, falls, lack of witnesses, and polypharmacy. In this group, morbidity is high because of multimorbidity and frailty. A careful history and physical exam with orthostatic vital signs is important, as is a multidisciplinary approach with geriatric consultation when needed.
Summary
Syncope is a common clinical syndrome often presenting to the emergency department or primary care setting. There are many causes, the most common being vasovagal syncope. In the initial evaluation, providers should perform a detailed history and physical exam, check orthostatic signs and perform a 12-lead ECG. Patients can be evaluated and managed safely in the outpatient setting in the absence of risk factors. Routine comprehensive laboratory testing and cardiac imaging are often not needed. For patients with defined risk factors, a more detailed evaluation in the hospital is recommended.
Dr. Li is a second-year resident in the family medicine residency program in the department of family and community medicine at the Sidney Kimmel Medical College at Thomas Jefferson University, Philadelphia. Dr. Mills is assistant residency program director and assistant professor in the departments of family and community medicine and physiology at the Sidney Kimmel Medical College. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
References
1. Shen W, Sheldon RS, Benditt DG, et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope. Circulation. 2017 Aug 1;136(5):e60-e122. doi: 10.1161/CIR.0000000000000499. Epub 2017 Mar 9.
2. Soteriades ES, Evans JC, Larson MG, et al. Incidence and prognosis of syncope. N Engl J Med. 2002;347(12):878-85.
Now boarding: How we can skip coach and bump our patients up to first class
Cruising above the earth at 37,000 feet on the way back from vacation, my mind starts wandering. The impending reality of returning to work is setting in, and I can’t help but reflect on how the experience of a weary traveler trying to get home is like that of a weary patient trying to navigate modern health care. As it turns out, there are more than a few similarities, and that is not necessarily a good thing.
The modern airline industry is often cited by experts as a model for safety, efficiency, and innovation, though just a few decades ago this wasn’t the case. Several factors (for example, catastrophic crashes; the events of September 11th, 2001; the economic downturn) forced airlines to make radical improvements in how they operated – many of which I am quite thankful for as I gaze down upon America’s heartland from my window seat. Still, there are many who would say that in spite of (and sometimes because of) these improvements, air travel is the worst it’s ever been; airport lines are longer than ever, costs have steadily increased, and customer service has become little more than a quaint idea from a bygone era.
Most people deride the frustrations of air travel yet accept them as normal. The same expectations have unfortunately been set in health care. Patients wait, though waiting only contributes to anxiety and leads them to question the quality of their care. They also expect their journey to have many layover stops, though these involve even more waiting and often unnecessary redundancy. We need to streamline the care delivery process, and this is where technology can help.
First of all, we need to address the waiting. In health care, we tend to call this “access,” an ever-present problem for patients and providers. Thankfully, some recent innovations have helped significantly. The first of these innovations is online scheduling, which allows patients to find openings and schedule visits without the need to pick up the phone. Much like the ability to book a dinner reservation online, this is becoming an expectation for health care consumers. Participating practices and health systems can also use it as a marketing advantage; it is a fantastic way to recruit new patients as they search for a new provider online (that is, seeing that a physician has immediate openings may make the decision easier).
There are several companies providing third-party online scheduling services, and many of these can interface directly with electronic health records. EHR vendors themselves also provide this functionality to existing patients through an online web portal or mobile app. Either way, if you haven’t considered it yet, you should. It’s a great way to fill last-minute schedule openings and increase your patient base, all while improving access and patient satisfaction.
Another way to improve access is through telemedicine. We’ve written about this in prior columns, but it has certainly become more prevalent and available since then. Now more insurers are reimbursing for telemedicine services, and consumers are starting to embrace it as well. Consider some advantages: it’s more convenient for patients and often less expensive for those without insurance – cash prices tend to be in the $50-$75 range. It can also be more convenient for providers, as the typical telemedicine visit lasts only about 10 minutes and can be easily fit in last-minute. Better still, telemedicine can be a way for providers to now be paid for services they might have previously provided for free by telephone. It is critical to choose patients and conditions appropriate for these “virtual visits.” Medication checks, lab follow-ups, or rash evaluations are just a few examples, but with a little bit of thought it is easy to find dozens of other opportunities to use telemedicine to improve access.
In addition to access, we need to look for ways to improve efficiency and decrease redundancy when sending patients for testing and consultations. Recently, I had the experience of visiting a specialist for a minor medical issue. In spite of the fact that the specialist was a member of the same health system as my PCP, I still spent the first 15 minutes of my visit filling out paperwork that requested information easily available from my health record. There must be a better way.
Patients are beginning to question why, in the world of ubiquitous social media and connectivity, our computerized medical records can’t communicate. This is especially true when they are seeing physicians who are part of the same health system (as in my case). Thankfully, vendors have gotten the message and have begun allowing providers to collaborate, not only with physicians using the same software, but also with those using other EHRs through Health Information Exchanges (HIEs). Unfortunately, this alone won’t be enough. We must continue to promote the notion of patient-owned medical records, as that will be the only way to ensure true patient-centered care. In a future column, we’ll explore this concept in greater detail, but for now we’ll confirm our belief that universal interoperability is reasonable and possible.
As we are getting ready to land, I reflect on the wonderful vacation I just had and the tasks ahead at home, most of which I enjoy. Patients aren’t always as lucky; they are accessing medical care because they have to, not because they want to. Their “destination” is all too often an unfortunate diagnosis, unexpected surgical procedure, or lifetime of chronic discomfort. It is therefore incumbent on us, their care providers, to use the tools at our disposal to offer them the most efficient, most comfortable, and most connected journey possible.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is also a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington Jefferson Health.
Cruising above the earth at 37,000 feet on the way back from vacation, my mind starts wandering. The impending reality of returning to work is setting in, and I can’t help but reflect on how the experience of a weary traveler trying to get home is like that of a weary patient trying to navigate modern health care. As it turns out, there are more than a few similarities, and that is not necessarily a good thing.
The modern airline industry is often cited by experts as a model for safety, efficiency, and innovation, though just a few decades ago this wasn’t the case. Several factors (for example, catastrophic crashes; the events of September 11th, 2001; the economic downturn) forced airlines to make radical improvements in how they operated – many of which I am quite thankful for as I gaze down upon America’s heartland from my window seat. Still, there are many who would say that in spite of (and sometimes because of) these improvements, air travel is the worst it’s ever been; airport lines are longer than ever, costs have steadily increased, and customer service has become little more than a quaint idea from a bygone era.
Most people deride the frustrations of air travel yet accept them as normal. The same expectations have unfortunately been set in health care. Patients wait, though waiting only contributes to anxiety and leads them to question the quality of their care. They also expect their journey to have many layover stops, though these involve even more waiting and often unnecessary redundancy. We need to streamline the care delivery process, and this is where technology can help.
First of all, we need to address the waiting. In health care, we tend to call this “access,” an ever-present problem for patients and providers. Thankfully, some recent innovations have helped significantly. The first of these innovations is online scheduling, which allows patients to find openings and schedule visits without the need to pick up the phone. Much like the ability to book a dinner reservation online, this is becoming an expectation for health care consumers. Participating practices and health systems can also use it as a marketing advantage; it is a fantastic way to recruit new patients as they search for a new provider online (that is, seeing that a physician has immediate openings may make the decision easier).
There are several companies providing third-party online scheduling services, and many of these can interface directly with electronic health records. EHR vendors themselves also provide this functionality to existing patients through an online web portal or mobile app. Either way, if you haven’t considered it yet, you should. It’s a great way to fill last-minute schedule openings and increase your patient base, all while improving access and patient satisfaction.
Another way to improve access is through telemedicine. We’ve written about this in prior columns, but it has certainly become more prevalent and available since then. Now more insurers are reimbursing for telemedicine services, and consumers are starting to embrace it as well. Consider some advantages: it’s more convenient for patients and often less expensive for those without insurance – cash prices tend to be in the $50-$75 range. It can also be more convenient for providers, as the typical telemedicine visit lasts only about 10 minutes and can be easily fit in last-minute. Better still, telemedicine can be a way for providers to now be paid for services they might have previously provided for free by telephone. It is critical to choose patients and conditions appropriate for these “virtual visits.” Medication checks, lab follow-ups, or rash evaluations are just a few examples, but with a little bit of thought it is easy to find dozens of other opportunities to use telemedicine to improve access.
In addition to access, we need to look for ways to improve efficiency and decrease redundancy when sending patients for testing and consultations. Recently, I had the experience of visiting a specialist for a minor medical issue. In spite of the fact that the specialist was a member of the same health system as my PCP, I still spent the first 15 minutes of my visit filling out paperwork that requested information easily available from my health record. There must be a better way.
Patients are beginning to question why, in the world of ubiquitous social media and connectivity, our computerized medical records can’t communicate. This is especially true when they are seeing physicians who are part of the same health system (as in my case). Thankfully, vendors have gotten the message and have begun allowing providers to collaborate, not only with physicians using the same software, but also with those using other EHRs through Health Information Exchanges (HIEs). Unfortunately, this alone won’t be enough. We must continue to promote the notion of patient-owned medical records, as that will be the only way to ensure true patient-centered care. In a future column, we’ll explore this concept in greater detail, but for now we’ll confirm our belief that universal interoperability is reasonable and possible.
As we are getting ready to land, I reflect on the wonderful vacation I just had and the tasks ahead at home, most of which I enjoy. Patients aren’t always as lucky; they are accessing medical care because they have to, not because they want to. Their “destination” is all too often an unfortunate diagnosis, unexpected surgical procedure, or lifetime of chronic discomfort. It is therefore incumbent on us, their care providers, to use the tools at our disposal to offer them the most efficient, most comfortable, and most connected journey possible.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is also a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington Jefferson Health.
Cruising above the earth at 37,000 feet on the way back from vacation, my mind starts wandering. The impending reality of returning to work is setting in, and I can’t help but reflect on how the experience of a weary traveler trying to get home is like that of a weary patient trying to navigate modern health care. As it turns out, there are more than a few similarities, and that is not necessarily a good thing.
The modern airline industry is often cited by experts as a model for safety, efficiency, and innovation, though just a few decades ago this wasn’t the case. Several factors (for example, catastrophic crashes; the events of September 11th, 2001; the economic downturn) forced airlines to make radical improvements in how they operated – many of which I am quite thankful for as I gaze down upon America’s heartland from my window seat. Still, there are many who would say that in spite of (and sometimes because of) these improvements, air travel is the worst it’s ever been; airport lines are longer than ever, costs have steadily increased, and customer service has become little more than a quaint idea from a bygone era.
Most people deride the frustrations of air travel yet accept them as normal. The same expectations have unfortunately been set in health care. Patients wait, though waiting only contributes to anxiety and leads them to question the quality of their care. They also expect their journey to have many layover stops, though these involve even more waiting and often unnecessary redundancy. We need to streamline the care delivery process, and this is where technology can help.
First of all, we need to address the waiting. In health care, we tend to call this “access,” an ever-present problem for patients and providers. Thankfully, some recent innovations have helped significantly. The first of these innovations is online scheduling, which allows patients to find openings and schedule visits without the need to pick up the phone. Much like the ability to book a dinner reservation online, this is becoming an expectation for health care consumers. Participating practices and health systems can also use it as a marketing advantage; it is a fantastic way to recruit new patients as they search for a new provider online (that is, seeing that a physician has immediate openings may make the decision easier).
There are several companies providing third-party online scheduling services, and many of these can interface directly with electronic health records. EHR vendors themselves also provide this functionality to existing patients through an online web portal or mobile app. Either way, if you haven’t considered it yet, you should. It’s a great way to fill last-minute schedule openings and increase your patient base, all while improving access and patient satisfaction.
Another way to improve access is through telemedicine. We’ve written about this in prior columns, but it has certainly become more prevalent and available since then. Now more insurers are reimbursing for telemedicine services, and consumers are starting to embrace it as well. Consider some advantages: it’s more convenient for patients and often less expensive for those without insurance – cash prices tend to be in the $50-$75 range. It can also be more convenient for providers, as the typical telemedicine visit lasts only about 10 minutes and can be easily fit in last-minute. Better still, telemedicine can be a way for providers to now be paid for services they might have previously provided for free by telephone. It is critical to choose patients and conditions appropriate for these “virtual visits.” Medication checks, lab follow-ups, or rash evaluations are just a few examples, but with a little bit of thought it is easy to find dozens of other opportunities to use telemedicine to improve access.
In addition to access, we need to look for ways to improve efficiency and decrease redundancy when sending patients for testing and consultations. Recently, I had the experience of visiting a specialist for a minor medical issue. In spite of the fact that the specialist was a member of the same health system as my PCP, I still spent the first 15 minutes of my visit filling out paperwork that requested information easily available from my health record. There must be a better way.
Patients are beginning to question why, in the world of ubiquitous social media and connectivity, our computerized medical records can’t communicate. This is especially true when they are seeing physicians who are part of the same health system (as in my case). Thankfully, vendors have gotten the message and have begun allowing providers to collaborate, not only with physicians using the same software, but also with those using other EHRs through Health Information Exchanges (HIEs). Unfortunately, this alone won’t be enough. We must continue to promote the notion of patient-owned medical records, as that will be the only way to ensure true patient-centered care. In a future column, we’ll explore this concept in greater detail, but for now we’ll confirm our belief that universal interoperability is reasonable and possible.
As we are getting ready to land, I reflect on the wonderful vacation I just had and the tasks ahead at home, most of which I enjoy. Patients aren’t always as lucky; they are accessing medical care because they have to, not because they want to. Their “destination” is all too often an unfortunate diagnosis, unexpected surgical procedure, or lifetime of chronic discomfort. It is therefore incumbent on us, their care providers, to use the tools at our disposal to offer them the most efficient, most comfortable, and most connected journey possible.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is also a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington Jefferson Health.
Treatment of low bone density or osteoporosis to prevent fractures in men and women
Osteoporosis is defined by a clinically diagnosed fragility fracture or a bone mineral density (BMD) of at least 2.5 SD below the mean for young female adults, usually measured by dual-energy x-ray absorptiometry. Risk factors include age, female sex, post-menopause, hypogonadism or premature ovarian failure, history of cigarette smoking or alcohol consumption (3 or more drinks daily), rheumatoid arthritis, or medications including glucocorticoids, anticoagulants, anticonvulsants, and aromatase inhibitors.
This guideline update focuses on treatment with bisphosphonates (alendronate, risedronate, ibandronate, zoledronic acid) and denosumab. Denosumab, a human monoclonal antibody against RANK-ligand, approved by the Food and Drug Administration for treatment of osteoporosis, has been added to the list of allowed medications since publication of the 2008 guideline. Several therapies have been excluded from the update, including calcitonin, which is no longer widely used for osteoporosis treatment, and etidronate and pamidronate, neither of which are FDA-approved for the prevention of fractures or treatment of osteoporosis. It should be noted that the evidence continues to be insufficient regarding the effectiveness of therapies to prevent fractures or to treat osteoporosis in men.
Bisphosphonates are associated with mild upper GI symptoms, atypical subtrochanteric fracture, and rare osteonecrosis of the jaw. There is no significant association between bisphosphonate use and total cardiovascular adverse events. Evidence is insufficient to associate bisphosphonates with increased cancer risk. Zoledronic acid is associated with atrial fibrillation, arthritis/arthralgias, headaches, hypocalcemia, influenza-like symptoms, and an increased incidence of uveitis/episcleritis. Denosumab is associated with mild upper GI symptoms, rash/eczema, and cellulitis.
While in the past additional medications were recommended for osteoporosis, the current guidelines recommend against using raloxifene, ibandronate, teriparatide, menopausal estrogen therapy, or menopausal estrogen plus progesterone therapy for first-line pharmacologic treatment.
The overall effect of calcium, vitamin D, or exercise alone on fracture risk is uncertain. Calcium and vitamin D may be added to treatment regimens, as a majority of trials with bisphosphonate therapy added this supplementation. Dosages should be considered because excessive dosing has been associated with hypercalcemia. Although previous data suggested an association between calcium supplementation and increased risk for myocardial infarction, moderate-quality evidence shows no association, though there is a risk of kidney stones.
Recommendation: Women who have osteoporosis and receive pharmacologic treatment should be treated for 5 years (weak recommendation; low-quality evidence). The evidence to determine the length of treatment is not strong, so recommendation is an extrapolation from existing evidence. High-risk patients may benefit from more than 5 years of treatment. Data suggests that patients treated with alendronate who had preexisting fractures or those with a BMD of –2.5 or less after 5 years of initial therapy may benefit from continued treatment, because these patients experienced a decreased incidence of new clinical vertebral fractures.
Recommendation: Pharmacologic treatment with bisphosphonates to reduce the risk for vertebral fracture can be offered to men who have clinically recognized osteoporosis (weak recommendation, low-quality evidence). No evidence suggests that outcomes associated with pharmacologic treatment would differ between men and women if based on similar BMDs.
Recommendation: Bone density monitoring is not recommended during the 5-year pharmacologic treatment period for osteoporosis in women (weak recommendation, low-quality evidence). Data showed that most women with normal dual-energy x-ray absorptiometry scores did not progress to osteoporosis within 15 years. Data also does not support monitoring BMD during the initial 5 years of treatment in patients taking pharmacologic agents to treat osteoporosis. Several studies showed that women treated with antiresorptive treatment benefited from reduced fractures with treatment even if BMD did not increase.
Only 10% of women with normal or mild osteopenia develop osteoporosis within 15 years; 10% of women with moderate osteopenia develop osteoporosis within 5 years, and 10% of women with advanced osteopenia develop osteoporosis within 1 year.
Recommendation: The decision about whether to treat osteopenic women older then 65 years of age who are at a high risk for fracture should be based on a discussion of with the patient about their risk of fracture and the risk and benefits of treatment. Clinicians can use their judgment regarding the qualitative risk for fracture, or a validated tool such as the FRAX tool that gives 10-year risk of any major osteoporotic fracture and of hip fracture. The FRAX site recommends consideration of treatment for individuals with low bone mass (T-score between –1.0 and –2.5 at the femoral neck or spine) and a 10-year probability of a hip fracture of at least 3% or a 10-year probability of a major osteoporosis-related fracture greater than 20%.
Bottom line:
Clinicians should offer pharmacologic treatment with alendronate, risedronate, zoledronic acid, or denosumab to reduce the risk of hip and vertebral fractures in women who have known osteoporosis diagnosed as a T score less than –2.5 or those with a fragility fracture. Pharmacologic therapy should be used for 5 years; however, high risk patients may benefit from longer treatment. There is no benefit to bone density monitoring during the 5-year pharmacologic treatment period. In addition, bisphosphonates should be considered in men who have clinically recognized osteoporosis.
Reference:
Qaseem, A, Forciea, MA, McLean RM, Denberg TD. Treatment of Low Bone Density or Osteoporosis to Prevent Fractures in Men and Women: A Clinical Practice Guideline Update From the American College of Physicians. Ann Int Med. 2017;166(11):818-39.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Meizinger is a second year resident in the Family Medicine Residency Program at Abington Jefferson Health.
Osteoporosis is defined by a clinically diagnosed fragility fracture or a bone mineral density (BMD) of at least 2.5 SD below the mean for young female adults, usually measured by dual-energy x-ray absorptiometry. Risk factors include age, female sex, post-menopause, hypogonadism or premature ovarian failure, history of cigarette smoking or alcohol consumption (3 or more drinks daily), rheumatoid arthritis, or medications including glucocorticoids, anticoagulants, anticonvulsants, and aromatase inhibitors.
This guideline update focuses on treatment with bisphosphonates (alendronate, risedronate, ibandronate, zoledronic acid) and denosumab. Denosumab, a human monoclonal antibody against RANK-ligand, approved by the Food and Drug Administration for treatment of osteoporosis, has been added to the list of allowed medications since publication of the 2008 guideline. Several therapies have been excluded from the update, including calcitonin, which is no longer widely used for osteoporosis treatment, and etidronate and pamidronate, neither of which are FDA-approved for the prevention of fractures or treatment of osteoporosis. It should be noted that the evidence continues to be insufficient regarding the effectiveness of therapies to prevent fractures or to treat osteoporosis in men.
Bisphosphonates are associated with mild upper GI symptoms, atypical subtrochanteric fracture, and rare osteonecrosis of the jaw. There is no significant association between bisphosphonate use and total cardiovascular adverse events. Evidence is insufficient to associate bisphosphonates with increased cancer risk. Zoledronic acid is associated with atrial fibrillation, arthritis/arthralgias, headaches, hypocalcemia, influenza-like symptoms, and an increased incidence of uveitis/episcleritis. Denosumab is associated with mild upper GI symptoms, rash/eczema, and cellulitis.
While in the past additional medications were recommended for osteoporosis, the current guidelines recommend against using raloxifene, ibandronate, teriparatide, menopausal estrogen therapy, or menopausal estrogen plus progesterone therapy for first-line pharmacologic treatment.
The overall effect of calcium, vitamin D, or exercise alone on fracture risk is uncertain. Calcium and vitamin D may be added to treatment regimens, as a majority of trials with bisphosphonate therapy added this supplementation. Dosages should be considered because excessive dosing has been associated with hypercalcemia. Although previous data suggested an association between calcium supplementation and increased risk for myocardial infarction, moderate-quality evidence shows no association, though there is a risk of kidney stones.
Recommendation: Women who have osteoporosis and receive pharmacologic treatment should be treated for 5 years (weak recommendation; low-quality evidence). The evidence to determine the length of treatment is not strong, so recommendation is an extrapolation from existing evidence. High-risk patients may benefit from more than 5 years of treatment. Data suggests that patients treated with alendronate who had preexisting fractures or those with a BMD of –2.5 or less after 5 years of initial therapy may benefit from continued treatment, because these patients experienced a decreased incidence of new clinical vertebral fractures.
Recommendation: Pharmacologic treatment with bisphosphonates to reduce the risk for vertebral fracture can be offered to men who have clinically recognized osteoporosis (weak recommendation, low-quality evidence). No evidence suggests that outcomes associated with pharmacologic treatment would differ between men and women if based on similar BMDs.
Recommendation: Bone density monitoring is not recommended during the 5-year pharmacologic treatment period for osteoporosis in women (weak recommendation, low-quality evidence). Data showed that most women with normal dual-energy x-ray absorptiometry scores did not progress to osteoporosis within 15 years. Data also does not support monitoring BMD during the initial 5 years of treatment in patients taking pharmacologic agents to treat osteoporosis. Several studies showed that women treated with antiresorptive treatment benefited from reduced fractures with treatment even if BMD did not increase.
Only 10% of women with normal or mild osteopenia develop osteoporosis within 15 years; 10% of women with moderate osteopenia develop osteoporosis within 5 years, and 10% of women with advanced osteopenia develop osteoporosis within 1 year.
Recommendation: The decision about whether to treat osteopenic women older then 65 years of age who are at a high risk for fracture should be based on a discussion of with the patient about their risk of fracture and the risk and benefits of treatment. Clinicians can use their judgment regarding the qualitative risk for fracture, or a validated tool such as the FRAX tool that gives 10-year risk of any major osteoporotic fracture and of hip fracture. The FRAX site recommends consideration of treatment for individuals with low bone mass (T-score between –1.0 and –2.5 at the femoral neck or spine) and a 10-year probability of a hip fracture of at least 3% or a 10-year probability of a major osteoporosis-related fracture greater than 20%.
Bottom line:
Clinicians should offer pharmacologic treatment with alendronate, risedronate, zoledronic acid, or denosumab to reduce the risk of hip and vertebral fractures in women who have known osteoporosis diagnosed as a T score less than –2.5 or those with a fragility fracture. Pharmacologic therapy should be used for 5 years; however, high risk patients may benefit from longer treatment. There is no benefit to bone density monitoring during the 5-year pharmacologic treatment period. In addition, bisphosphonates should be considered in men who have clinically recognized osteoporosis.
Reference:
Qaseem, A, Forciea, MA, McLean RM, Denberg TD. Treatment of Low Bone Density or Osteoporosis to Prevent Fractures in Men and Women: A Clinical Practice Guideline Update From the American College of Physicians. Ann Int Med. 2017;166(11):818-39.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Meizinger is a second year resident in the Family Medicine Residency Program at Abington Jefferson Health.
Osteoporosis is defined by a clinically diagnosed fragility fracture or a bone mineral density (BMD) of at least 2.5 SD below the mean for young female adults, usually measured by dual-energy x-ray absorptiometry. Risk factors include age, female sex, post-menopause, hypogonadism or premature ovarian failure, history of cigarette smoking or alcohol consumption (3 or more drinks daily), rheumatoid arthritis, or medications including glucocorticoids, anticoagulants, anticonvulsants, and aromatase inhibitors.
This guideline update focuses on treatment with bisphosphonates (alendronate, risedronate, ibandronate, zoledronic acid) and denosumab. Denosumab, a human monoclonal antibody against RANK-ligand, approved by the Food and Drug Administration for treatment of osteoporosis, has been added to the list of allowed medications since publication of the 2008 guideline. Several therapies have been excluded from the update, including calcitonin, which is no longer widely used for osteoporosis treatment, and etidronate and pamidronate, neither of which are FDA-approved for the prevention of fractures or treatment of osteoporosis. It should be noted that the evidence continues to be insufficient regarding the effectiveness of therapies to prevent fractures or to treat osteoporosis in men.
Bisphosphonates are associated with mild upper GI symptoms, atypical subtrochanteric fracture, and rare osteonecrosis of the jaw. There is no significant association between bisphosphonate use and total cardiovascular adverse events. Evidence is insufficient to associate bisphosphonates with increased cancer risk. Zoledronic acid is associated with atrial fibrillation, arthritis/arthralgias, headaches, hypocalcemia, influenza-like symptoms, and an increased incidence of uveitis/episcleritis. Denosumab is associated with mild upper GI symptoms, rash/eczema, and cellulitis.
While in the past additional medications were recommended for osteoporosis, the current guidelines recommend against using raloxifene, ibandronate, teriparatide, menopausal estrogen therapy, or menopausal estrogen plus progesterone therapy for first-line pharmacologic treatment.
The overall effect of calcium, vitamin D, or exercise alone on fracture risk is uncertain. Calcium and vitamin D may be added to treatment regimens, as a majority of trials with bisphosphonate therapy added this supplementation. Dosages should be considered because excessive dosing has been associated with hypercalcemia. Although previous data suggested an association between calcium supplementation and increased risk for myocardial infarction, moderate-quality evidence shows no association, though there is a risk of kidney stones.
Recommendation: Women who have osteoporosis and receive pharmacologic treatment should be treated for 5 years (weak recommendation; low-quality evidence). The evidence to determine the length of treatment is not strong, so recommendation is an extrapolation from existing evidence. High-risk patients may benefit from more than 5 years of treatment. Data suggests that patients treated with alendronate who had preexisting fractures or those with a BMD of –2.5 or less after 5 years of initial therapy may benefit from continued treatment, because these patients experienced a decreased incidence of new clinical vertebral fractures.
Recommendation: Pharmacologic treatment with bisphosphonates to reduce the risk for vertebral fracture can be offered to men who have clinically recognized osteoporosis (weak recommendation, low-quality evidence). No evidence suggests that outcomes associated with pharmacologic treatment would differ between men and women if based on similar BMDs.
Recommendation: Bone density monitoring is not recommended during the 5-year pharmacologic treatment period for osteoporosis in women (weak recommendation, low-quality evidence). Data showed that most women with normal dual-energy x-ray absorptiometry scores did not progress to osteoporosis within 15 years. Data also does not support monitoring BMD during the initial 5 years of treatment in patients taking pharmacologic agents to treat osteoporosis. Several studies showed that women treated with antiresorptive treatment benefited from reduced fractures with treatment even if BMD did not increase.
Only 10% of women with normal or mild osteopenia develop osteoporosis within 15 years; 10% of women with moderate osteopenia develop osteoporosis within 5 years, and 10% of women with advanced osteopenia develop osteoporosis within 1 year.
Recommendation: The decision about whether to treat osteopenic women older then 65 years of age who are at a high risk for fracture should be based on a discussion of with the patient about their risk of fracture and the risk and benefits of treatment. Clinicians can use their judgment regarding the qualitative risk for fracture, or a validated tool such as the FRAX tool that gives 10-year risk of any major osteoporotic fracture and of hip fracture. The FRAX site recommends consideration of treatment for individuals with low bone mass (T-score between –1.0 and –2.5 at the femoral neck or spine) and a 10-year probability of a hip fracture of at least 3% or a 10-year probability of a major osteoporosis-related fracture greater than 20%.
Bottom line:
Clinicians should offer pharmacologic treatment with alendronate, risedronate, zoledronic acid, or denosumab to reduce the risk of hip and vertebral fractures in women who have known osteoporosis diagnosed as a T score less than –2.5 or those with a fragility fracture. Pharmacologic therapy should be used for 5 years; however, high risk patients may benefit from longer treatment. There is no benefit to bone density monitoring during the 5-year pharmacologic treatment period. In addition, bisphosphonates should be considered in men who have clinically recognized osteoporosis.
Reference:
Qaseem, A, Forciea, MA, McLean RM, Denberg TD. Treatment of Low Bone Density or Osteoporosis to Prevent Fractures in Men and Women: A Clinical Practice Guideline Update From the American College of Physicians. Ann Int Med. 2017;166(11):818-39.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Meizinger is a second year resident in the Family Medicine Residency Program at Abington Jefferson Health.
EHR Report: Don’t let the electronic health record do the driving
The secret to the care of the patient ... is in caring for the patient.
-Francis W. Peabody, MD1
Last month I received a call from a man who was upset about the way he was treated in our office. He had presented with depression and felt insulted by one of our resident physicians in the way he had interacted with him during his visit. I offered to see him the next day.
When I walked into the exam room, I noticed that his eyes were bloodshot and he was fidgeting in his chair. He explained that it was difficult for him to address this issue, but he had been taken aback at his previous visit to our office when the doctor who saw him, after introducing himself, proceeded to sit down, open his computer, and start typing. The patient went on to describe that the physician – while staring at his computer screen – first acknowledged that he was being seen for depression and then immediately asked him if he had any plans to commit suicide. He did not have any suicidal plans, but he felt strongly that being asked about suicide as the first question in the doctor’s interview missed the point of his visit. He was having trouble concentrating, he felt down, and he was having difficulty sleeping at night, all contributing to trouble both at work and in his personal life. Suicide was not a concern of his. He shook his head. He said he understood that we, as doctors, had to put information into the computer, but he also felt that the doctor’s main goal during that visit appeared to be to get through the forms on the computer rather than taking care of him. He admonished that physicians also need to remember that there is a patient in the room and that we should pay attention to the patient first. The computer should be second. I couldn’t have said it better myself. I told him that I would look into what happened, and then we continued with his visit.
You can already see where this discussion is going. The odd thing about the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA), Medicare’s quality payment program, is that, unless we are careful, the result of the program may be the opposite of what it’s intended to accomplish. By leading to an over-focus on documentation of the quality of care, we are at risk of diminishing the quality of care itself. In essence, many of the requirements appear to simply be more advanced versions of the meaningful (meaningless?) use provisions with which we have previously grappled. It is clear that we should assess the quality of care that is given and that physician payment should be influenced by that care. It is also clear that the only reasonable way to measure the care provided is by collecting data from the EHR. The problem is that the sophistication of the EHR has not caught up to the sophistication of our goals.
Our challenge as physicians who care for patients therefore occurs at an individual level for each of us. How do we provide the necessary documentation scattered throughout our digital charts to satisfy reporting requirements, yet still meet the very real needs of patients to have their voices heard and their emotions acknowledged? The Physician Charter by the American Board of Internal Medicine discusses “the primacy of patient welfare” as a core tenant of medical practice. It goes on to state that “administrative exigencies must not compromise this principle.”2 Given competing demands, how do we continue to accomplish these goals which are often in conflict with one another?
We cannot provide an answer to this question because unfortunately – or perhaps fortunately – the answer does not come in the form of a clear algorithm of behaviors or a form that we can click on. However that does not mean that it cannot be done. Simply being mindful of how important personal interaction is to our patients will help us stay focused on patient needs. In fact, one of the most exciting aspects of our digital age (and our use of EHRs) is that the need to actually connect with people is more important than ever, and prioritizing this stands to reward those individuals who continue to pay attention to patients. In a future column, we will discuss suggestions and strategies for integrating the EHR into truly patient-centered care. In the early 1920s, Dr. Francis W. Peabody said, “The treatment of a disease may be entirely impersonal: the care of the patient must be completely personal.”1 Medical competency is essential and documentation is required, but neither alone is sufficient for the care of patients.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Notte is a family physician and clinical informaticist for Abington Memorial Hospital. He is also a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records.
References
1. Peabody FW. The care of the patient. JAMA. 1927;88:877-82.
2. The Physician Charter. American Board of Internal Medicine Foundation at http://abimfoundation.org/what-we-do/physician-charter.
The secret to the care of the patient ... is in caring for the patient.
-Francis W. Peabody, MD1
Last month I received a call from a man who was upset about the way he was treated in our office. He had presented with depression and felt insulted by one of our resident physicians in the way he had interacted with him during his visit. I offered to see him the next day.
When I walked into the exam room, I noticed that his eyes were bloodshot and he was fidgeting in his chair. He explained that it was difficult for him to address this issue, but he had been taken aback at his previous visit to our office when the doctor who saw him, after introducing himself, proceeded to sit down, open his computer, and start typing. The patient went on to describe that the physician – while staring at his computer screen – first acknowledged that he was being seen for depression and then immediately asked him if he had any plans to commit suicide. He did not have any suicidal plans, but he felt strongly that being asked about suicide as the first question in the doctor’s interview missed the point of his visit. He was having trouble concentrating, he felt down, and he was having difficulty sleeping at night, all contributing to trouble both at work and in his personal life. Suicide was not a concern of his. He shook his head. He said he understood that we, as doctors, had to put information into the computer, but he also felt that the doctor’s main goal during that visit appeared to be to get through the forms on the computer rather than taking care of him. He admonished that physicians also need to remember that there is a patient in the room and that we should pay attention to the patient first. The computer should be second. I couldn’t have said it better myself. I told him that I would look into what happened, and then we continued with his visit.
You can already see where this discussion is going. The odd thing about the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA), Medicare’s quality payment program, is that, unless we are careful, the result of the program may be the opposite of what it’s intended to accomplish. By leading to an over-focus on documentation of the quality of care, we are at risk of diminishing the quality of care itself. In essence, many of the requirements appear to simply be more advanced versions of the meaningful (meaningless?) use provisions with which we have previously grappled. It is clear that we should assess the quality of care that is given and that physician payment should be influenced by that care. It is also clear that the only reasonable way to measure the care provided is by collecting data from the EHR. The problem is that the sophistication of the EHR has not caught up to the sophistication of our goals.
Our challenge as physicians who care for patients therefore occurs at an individual level for each of us. How do we provide the necessary documentation scattered throughout our digital charts to satisfy reporting requirements, yet still meet the very real needs of patients to have their voices heard and their emotions acknowledged? The Physician Charter by the American Board of Internal Medicine discusses “the primacy of patient welfare” as a core tenant of medical practice. It goes on to state that “administrative exigencies must not compromise this principle.”2 Given competing demands, how do we continue to accomplish these goals which are often in conflict with one another?
We cannot provide an answer to this question because unfortunately – or perhaps fortunately – the answer does not come in the form of a clear algorithm of behaviors or a form that we can click on. However that does not mean that it cannot be done. Simply being mindful of how important personal interaction is to our patients will help us stay focused on patient needs. In fact, one of the most exciting aspects of our digital age (and our use of EHRs) is that the need to actually connect with people is more important than ever, and prioritizing this stands to reward those individuals who continue to pay attention to patients. In a future column, we will discuss suggestions and strategies for integrating the EHR into truly patient-centered care. In the early 1920s, Dr. Francis W. Peabody said, “The treatment of a disease may be entirely impersonal: the care of the patient must be completely personal.”1 Medical competency is essential and documentation is required, but neither alone is sufficient for the care of patients.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Notte is a family physician and clinical informaticist for Abington Memorial Hospital. He is also a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records.
References
1. Peabody FW. The care of the patient. JAMA. 1927;88:877-82.
2. The Physician Charter. American Board of Internal Medicine Foundation at http://abimfoundation.org/what-we-do/physician-charter.
The secret to the care of the patient ... is in caring for the patient.
-Francis W. Peabody, MD1
Last month I received a call from a man who was upset about the way he was treated in our office. He had presented with depression and felt insulted by one of our resident physicians in the way he had interacted with him during his visit. I offered to see him the next day.
When I walked into the exam room, I noticed that his eyes were bloodshot and he was fidgeting in his chair. He explained that it was difficult for him to address this issue, but he had been taken aback at his previous visit to our office when the doctor who saw him, after introducing himself, proceeded to sit down, open his computer, and start typing. The patient went on to describe that the physician – while staring at his computer screen – first acknowledged that he was being seen for depression and then immediately asked him if he had any plans to commit suicide. He did not have any suicidal plans, but he felt strongly that being asked about suicide as the first question in the doctor’s interview missed the point of his visit. He was having trouble concentrating, he felt down, and he was having difficulty sleeping at night, all contributing to trouble both at work and in his personal life. Suicide was not a concern of his. He shook his head. He said he understood that we, as doctors, had to put information into the computer, but he also felt that the doctor’s main goal during that visit appeared to be to get through the forms on the computer rather than taking care of him. He admonished that physicians also need to remember that there is a patient in the room and that we should pay attention to the patient first. The computer should be second. I couldn’t have said it better myself. I told him that I would look into what happened, and then we continued with his visit.
You can already see where this discussion is going. The odd thing about the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA), Medicare’s quality payment program, is that, unless we are careful, the result of the program may be the opposite of what it’s intended to accomplish. By leading to an over-focus on documentation of the quality of care, we are at risk of diminishing the quality of care itself. In essence, many of the requirements appear to simply be more advanced versions of the meaningful (meaningless?) use provisions with which we have previously grappled. It is clear that we should assess the quality of care that is given and that physician payment should be influenced by that care. It is also clear that the only reasonable way to measure the care provided is by collecting data from the EHR. The problem is that the sophistication of the EHR has not caught up to the sophistication of our goals.
Our challenge as physicians who care for patients therefore occurs at an individual level for each of us. How do we provide the necessary documentation scattered throughout our digital charts to satisfy reporting requirements, yet still meet the very real needs of patients to have their voices heard and their emotions acknowledged? The Physician Charter by the American Board of Internal Medicine discusses “the primacy of patient welfare” as a core tenant of medical practice. It goes on to state that “administrative exigencies must not compromise this principle.”2 Given competing demands, how do we continue to accomplish these goals which are often in conflict with one another?
We cannot provide an answer to this question because unfortunately – or perhaps fortunately – the answer does not come in the form of a clear algorithm of behaviors or a form that we can click on. However that does not mean that it cannot be done. Simply being mindful of how important personal interaction is to our patients will help us stay focused on patient needs. In fact, one of the most exciting aspects of our digital age (and our use of EHRs) is that the need to actually connect with people is more important than ever, and prioritizing this stands to reward those individuals who continue to pay attention to patients. In a future column, we will discuss suggestions and strategies for integrating the EHR into truly patient-centered care. In the early 1920s, Dr. Francis W. Peabody said, “The treatment of a disease may be entirely impersonal: the care of the patient must be completely personal.”1 Medical competency is essential and documentation is required, but neither alone is sufficient for the care of patients.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Notte is a family physician and clinical informaticist for Abington Memorial Hospital. He is also a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records.
References
1. Peabody FW. The care of the patient. JAMA. 1927;88:877-82.
2. The Physician Charter. American Board of Internal Medicine Foundation at http://abimfoundation.org/what-we-do/physician-charter.