User login
Clinical Guidelines Hub only
VIP Boot Camp: Expanding the Impact of VA Primary Care Mental Health With a Transdiagnostic Modular Group Program
VIP Boot Camp: Expanding the Impact of VA Primary Care Mental Health With a Transdiagnostic Modular Group Program
Since 2007, Primary Care Mental Health Integration (PCMHI) at the Veterans Health Administration (VHA) has improved access to mental health care services for veterans by directly embedding mental health care professionals (HCPs) within primary care teams.1 Veterans referred to PCMHI often have co-occurring physical and mental health disorders.2 Untreated chronic physical and mental comorbidities can diminish the effectiveness of medical and mental health interventions. Growing evidence suggests that treatment of mental health conditions can improve physical health outcomes and management of physical conditions can improve mental health outcomes.2,3
Chronic pain and sleep disorders are common reasons patients present to primary care, and often coexist together with mental health comorbidities.4 Sleep disorders affect 50% to 88% of patients with chronic pain, and 40% of patients with sleep disorders report chronic pain.4 Research has found that chronic pain and sleep disorders increase the risk of suicide attempts and deaths by suicide. Addressing suicide prevention simultaneously with treating chronic pain and insomnia is encouraged.5
Background
PCMHI treats physical and mental health comorbidities with a collaborative framework and a biopsychosocial integrative model.6 PCMHI staff provide mental health services as members of primary care teams. An interdisciplinary PCMHI team can include, but is not limited to, psychologists, mental health social workers, psychiatrists, nurse practitioners, clinical pharmacists, and mental health nurses. Quality of care within this model is elevated, as mental and physical health are recognized as interconnected. Collaboration between primary care and mental health benefits veterans and the VHA by increasing access to mental health care, decreasing stigma associated with mental health treatment, improving health outcomes, and enhancing the likelihood of recovery, resulting in high patient satisfaction.6-8
In the existing PCMHI model, HCPs are encouraged to use short-term, evidence-based psychotherapies (EBPs).9 Veterans referred to PCMHI from primary care are typically able to attend 1 to 6 brief sessions of mental health treatment, often 20 to 30 minutes long. Most EBPs in PCMHI are disorder- specific, providing interventions focused on a single presenting problem (eg, insomnia, chronic pain, or posttraumatic stress disorder [PTSD]). For veterans with a single issue, this model can be very effective. 1,10 However, the high rate of co-occurrence of mental and physical health issues can make it difficult to fully treat interrelated problems if the focus is on 1 specific diagnosis. Veterans with a need for additional (more comprehensive or intensive) mental health treatment are frequently referred to a higher, more resource-intensive level of mental health care, either in the VHA or the community. Examples of higher levels of mental health care include the longer term behavioral health interdisciplinary program (BHIP), sometimes called a mental health clinic (MHC), or a specialty mental health program such as a PTSD clinic.
As PCMHI continues to grow, new challenges have emerged related to staffing shortages and gaps in the clinical delivery of mental health treatment within the VHA. At the same time, demand for VHA mental health treatment has increased. However, a mental health professional shortage severely limits the ability of the VHA to meet this demand. In many systems, this shortage may result in more referrals being made to a higher level of mental health care because of fewer resources to provide comprehensive treatment in a less intensive PCMHI setting.8,10,11 This referral pattern can overburden higher level care, often with long wait times for treatment and lengthy lag times between appointments. Furthermore, these gaps in the clinical delivery of care cannot be effectively addressed by hiring additional mental health professionals. This strain on resources can impede access to care and negatively affect outcomes.10
Recent congressional reports highlight these issues, noting that demand for mental health services continues to outpace the capacity of both PCMHI and higher levels of mental health care, leading to delays in treatment that may negatively affect outcomes.8,10,11 These delays can be particularly detrimental for individuals with conditions requiring timely intervention.8,11 Some veterans are willing to engage with PCMHI in a primary care setting but may be reluctant to engage in general mental health treatment. These veterans might not receive the mental health care they need without PCMHI.
Group Psychotherapy
A group psychotherapy format can address gaps in care delivery and provide advantages for patients, mental health professionals, and the VHA. Group psychotherapy aligns with the US Department of Veterans Affairs (VA) 2018 Blueprint for Excellence and 2018 to 2024 strategic plan, underscoring the need for more timely and efficient mental health services.12,13
Benefits of group psychotherapy include reductions in symptoms, decreased feelings of isolation, increased social support, decreased emotional suppression, and enhanced satisfaction with overall quality of life.14-17 Studies of veterans with PTSD have found less attrition among those who chose group therapy compared with individual therapy.14,18 Group psychotherapy improves access to care by enabling delivery to more patients.14 When compared with individual therapy, the group format allows for a large number of patients to be treated simultaneously, maximizing resources and reducing costs.3,19-21
VISN 9 CRH Innovation
The VA provides care to veterans through regionally distinct administrative systems known as Veterans Integrated Service Networks (VISNs). Clinical resource hubs (CRH) are VISN-based programs created to cover VA staffing shortages by virtually deploying HCPs into local VA systems until vacancies are filled. The national CRH vision of effectively using resources and innovative technologies to meet veterans’ health care needs, along with the above-referenced clinical gaps in the delivery of care, inspired the development of VIP Boot Camp within the VISN 9 CRH.22
Program Description
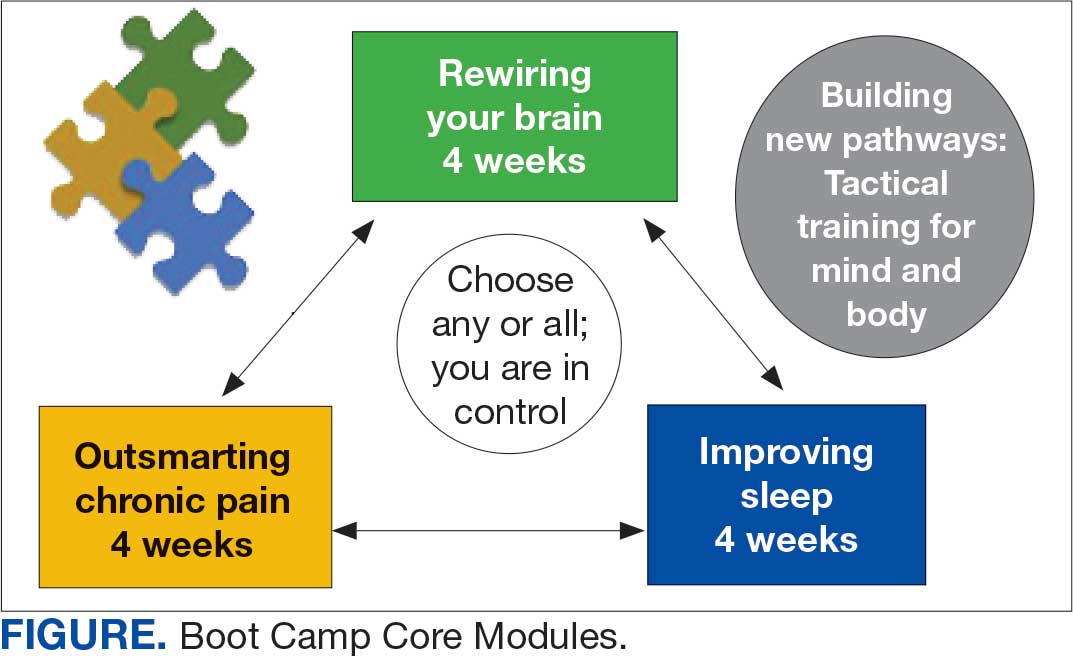
VIP Boot Camp is an evidence-informed group psychotherapy program designed to provide timely, brief, and comprehensive mental health treatment for veterans. VIP Boot Camp was developed to address the needs of veterans accessing PCMHI services who experience ≥ 1 of the often overlapping problems of anxiety/emotion regulation/stress, sleep difficulties, and chronic pain (Figure). VIP Boot Camp uses an integrative approach to highlight interconnections and similarities among these difficulties and their treatment. A primary vision of the program is to provide this comprehensive treatment within PCMHI (upstream) so additional referrals to higher levels of mental health care (downstream) may not be needed.
This design is intentional because it increases the number of individuals who can be treated upstream with comprehensive, preventive, and proactive care within PCMHI which, over time, frees up resources in the BHIP for individuals requiring higher levels of care. This approach also aligns with the importance of early treatment for chronic pain and sleep disturbances, which are linked to increased risk of suicide attempts and deaths by suicide for veterans.5 National interest for VIP Boot Camp grew during fiscal year 2024 after it received the Gold Medal Recognition for Most Adoptable and Greatest Potential for Impact during VHA National Access Sprint Wave 3—Mental Health Call of Champions.
History
VIP Boot Camp began in August 2021 at VISN 9 as a 6-week virtual group for veterans with chronic pain. It was established to assist a large VA medical center experiencing PCMHI staffing shortages and lacking available PCMHI groups. Many veterans in the chronic pain group discussed co-occurring issues such as sleep disturbances, anxiety, and stress. The CRH team considered launching 2 separate groups to address these additional PCMHI-level issues; however, in developing the group material which drew from multiple clinical approaches, the team recognized significant overlapping and interconnected themes.
The team discussed EBPs within the VHA and how certain interventions within these treatments could be helpful across many other co-occurring disorders. Integrated tactics (clinical interventions) were drawn from cognitive-behavioral therapy (for depression, insomnia, or chronic pain), acceptance and commitment therapy, prolonged exposure, cognitive processing therapy, dialectical behavior therapy, unified protocol, pain reprocessing therapy, emotional awareness and expression therapy, interpersonal neurobiology, and mindfulness. We collaborated with veterans during VIP Boot Camp groups to determine how to present and discuss complex interventions in ways that were clinically accurate, understandable, relatable, and relevant to their experiences.
To address accessibility issues, the chronic pain group was reduced to 4 weeks. A second 4-week module for anxiety, emotion regulation, and stress was developed, mirroring the tactics, language, and integrative approach of the revised chronic pain module. A similar integrative approach led to the development of the third and final 4-week module for sleep disturbances.
Current Program
The VIP Boot Camp consists of three 4-week integrated modules, each highlighting a critical area: sleep disturbances (Improving Sleep), chronic pain difficulties (Outsmarting Chronic Pain), and emotion regulation difficulties (Rewiring Your Brain). VIP Boot Camp is designed for veterans who are at the PCMHI level of care. Referrals are accepted for patients receiving treatment from primary care or PCMHI.
Guidelines for participation in VIP Boot Camp may differ across sites or VISNs. For example, a veteran who has been referred to the BHIP for medication management only or to a specialty MHC such as a pain clinic or PTSD clinic might also be appropriate and eligible for VIP Boot Camp.
Given the interconnectedness of foundational themes, elements, and practices across the VIP Boot Camp modules, the modules are offered in a rolling format with a veteran-centric “choose your own adventure” approach. Tactics are presented in the modules in a way that allows patients to begin with any 1 of the 3 modules and receive treatment that will help in the other areas. Participants choose their core module and initial treatment focus based on their values, needs, and goals. Individuals who complete a core module can end their VIP Boot Camp experience or continue to the next 4-week module for up to 3 modules.
The group is open to new individuals at the start of any 4-week module and closed for the remainder of its 4-week duration. This innovative rolling modular approach combines elements of open- and closed-group format, allowing for the flexibility and accessibility of an open group with the stability and peer support of a closed group.
Given the complicated and overlapping nature of chronic pain, emotion regulation/ stress, and sleep disturbances, VIP Boot Camp acknowledges that everything is interconnected and difficulties in 1 area may impact other areas. The 3 interconnected modules with repeating themes provide coherence and consistency. Veterans learn how interconnections across difficulties can be leveraged so that tactics learned and practiced in 1 area can assist in other areas, changing the cycle of suffering into a cycle of growth.
VIP Boot Camp sessions are 90 minutes long, once weekly for 4 weeks, with 2 mental health professionals trained to lead a dynamic group psychotherapy experience that aims to be fun for participants. VIP Boot Camp synthesizes evidence-based and evidence-informed interventions, as well as techniques from VHA complementary and integrative health programs, psychoeducation, and interpersonal interventions that model connection, playfulness, and healthy boundaries. These varied strategies combine to equip veterans with practical tactics for self-management outside of sessions, a process described as “finding puzzle pieces.” VIP Boot Camp is built on the idea that people are more likely to adopt and practice any tactic after being taught why that tactic is important, and how it fits into their larger interconnected puzzle. After each session, participants are provided with additional asynchronous educational material to help reinforce their learnings and practices.
Although individuals may hesitate to participate in a group setting, they often find the experience of community enhances and accelerates their treatment and gains. This involvement is highlighted in a core aspect of a VIP Boot Camp session called wins, during which participants learn how others on their Boot Camp team are implementing new skills and moving toward their personal values and objectives in a stepwise manner. Through these shared experiences, veterans discover how tactics working for others may serve as a model for their own personal objectives and plans for practice. The sense of relief described by many upon realizing they are not alone in their experiences, along with the satisfaction felt in discovering their ability to support others in Boot Camp, is described by many participants as deeply meaningful and in line with their personal values.
While developed as a fully virtual group program, VIP Boot Camp can also be conducted in person. The virtual program has been successful and continues to spread across VISN 9. There are 8 virtual VIP Boot Camps running in VISN 9, with plans for continued expansion. In the VISN 9 CRH, Boot Camps typically have 10 to 12 participants. Additionally, as VIP Boot Camp grows within a location there are frequently sufficient referrals to support a second rolling group, which enables staggering of the module offerings to allow for even more timely treatment.
Training Program
VISN 9 CRH also developed a VIP Boot Camp 3-day intensive training program for PCMHI HCPs that consists of learning and practicing VIP Boot Camp material for chronic pain, emotion regulation/ stress, sleep disturbances, mindfulness, and guided imagery, along with gaining experience as a VIP Boot Camp coleader. Feedback received from PCMHI HCPs who completed training has been positive. There is also a private Microsoft Teams channel for HCPs, which allows for resource sharing and community building among coleaders. More than 75 PCMHI HCPs have completed VIP Boot Camp training and > 25 VIP Boot Camps have been established at 4 additional VISNs.
The VISN 9 CRH VIP Boot Camp program initiated an implementation and effectiveness project with the Michael E. DeBakey VA Medical Center and the South Central Mental Illness Research, Education and Clinical Center. The focus of this collaboration is support for implementation and treatment effectiveness research with reports, articles, and a white paper on findings and best practices, alongside continued dissemination of the VIP Boot Camp program and training.
Conclusions
VIP Boot Camp is a PCMHI group program offering readily available, comprehensive, and integrative group psychotherapy services to veterans experiencing . 1 of the following: chronic pain, emotion regulation/ stress, and sleep disturbances. It was launched at the VISN 9 CRH with a goal of addressing clinical gaps in the delivery of mental health care, by increasing the number of patients treated within PCMHI. The VIP Boot Camp model provides veterans the opportunity to transform cycles of suffering into cycles of growth through a single approach that can address multiple presenting and interconnected issues.
A 3-day VIP Boot Camp training program provides a quick and effective path for a PCMHI program to train HCPs to launch a VIP Boot Camp. The VISN 9 CRH will continue to champion VIP Boot Camp as a model for the successful provision of comprehensive and integrative mental health treatment within PCMHI at the VA. Through readily available access to comprehensive mental health treatment in an environment that promotes participant empowerment and social engagement, VIP Boot Camp represents an integrative and innovative model of mental health treatment that offers benefits to veteran participants, HCPs, and the VHA.
- Leung LB, Yoon J, Escarce JJ, et al. Primary care-mental health integration in the VA: shifting mental health services for common mental illnesses to primary care. Psychiatr Serv. 2018;69:403-409. doi:10.1176/appi.ps.201700190
- Zhang A, Park S, Sullivan JE, et al. The effectiveness of problem-solving therapy for primary care patients’ depressive and/or anxiety disorders: a systematic review and meta-analysis. J Am Board Fam Med. 2018;31:139-150. doi:10.3122/jabfm.2018.01.170270
- Hundt NE, Barrera TL, Robinson A, et al. A systematic review of cognitive behavioral therapy for depression in veterans. Mil Med. 2014;179:942-949. doi:10.7205/milmed-d-14-00128
- Jank R, Gallee A, Boeckle M, et al. Chronic pain and sleep disorders in primary care. Pain Res Treat. 2017;2017:1-9. doi:10.1155/2017/9081802
- Ashrafioun L, Bishop TM, Pigeon WR. The relationship between pain severity, insomnia, and suicide attempts among a national veteran sample initiating pain care. Psychosom Med. 2021;83:733- 738. doi:10.1097/psy.0000000000000975
- Ramanuj P, Ferenchik E, Docherty M, et al. Evolving models of integrated behavioral health and primary care. Curr Psychiatry Rep. 2019;21:1. doi:10.1007/s11920-019-0985-4
- Post EP, Metzger M, Dumas P, et al. Integrating mental health into primary care within the Veterans Health Administration. Fam Syst Health. 2010;28:83-90. doi:10.1037/a0020130
- Smith TL, Kim B, Benzer JK, et al. FLOW: early results from a clinical demonstration project to improve the transition of patients with mental health disorders back to primary care. Psychol Serv. 2021;18:23-32. doi:10.1037/ser0000336
- Kearney LK, Post EP, Pomerantz AS, et al. Applying the interprofessional patient aligned care team in the department of veterans affairs transforming primary care. Am Psychol. 2014;69(4):399-408. doi:10.1037/a0035909
- US Government Accountability Office. Veterans health care: staffing challenges persist for fully integrating mental health and primary care services. December 15, 2022. Accessed July 9, 2025. https://www.gao.gov/products/gao-23-105372
- National Academies of Science and Engineering. Evaluation of the Department of Veterans Affairs Mental Health Services. National Academies Press; 2018. Accessed July 9, 2025. https://nap.nationalacademies.org/catalog/24915/evaluation-of-the-department-of-veterans-affairs-mental-health-services
- US Department of Veterans Affairs. Blueprint for excellence: achieving veterans’ excellence. October 6, 2014. Accessed July 9, 2025. https://www.volunteer.va.gov/docs/blueprintforexcellence_factsheet.PDF
- US Department of Veterans Affairs. Department of Veterans Affairs FY 2018-2024 strategic plan. Accessed July 9, 2025. https://www.calvet.ca.gov/Regulations/USDVA%20Strategic%20Plan%202018-2024.pdf
- Sripada RK, Bohnert KM, Ganoczy D, et al. Initial group versus individual therapy for posttraumatic stress disorder and subsequent follow-up treatment adequacy. Psychol Serv. 2016;13:349-355. doi:10.1037/ser0000077
- Burnett-Zeigler IE, Pfeiffer P, Zivin K, et al. Psychotherapy utilization for acute depression within the Veterans Affairs health care system. Psychol Serv. 2012;9:325-335. doi:10.1037/a0027957
- Kim JS, Prins A, Hirschhorn EW, et al. Preliminary investigation into the effectiveness of group webSTAIR for trauma-exposed veterans in primary care. Mil Med. 2024;189:e1403-e1408. doi:10.1093/milmed/usae052
- Jakupcak M, Blais RK, Grossbard J, et al. “Toughness” in association with mental health symptoms among Iraq and Afghanistan war veterans seeking Veterans Affairs health care. Psychol Men Masc. 2014;15:100-104. doi:10.1037/a0031508
- Stoycos SA, Berzenski SR, Beck JG, et al. Predictors of treatment completion in group psychotherapy for male veterans with posttraumatic stress disorder. J Trauma Stress. 2023;36:346-358. doi:10.1002/jts.22915
- Possemato K. The current state of intervention research for posttraumatic stress disorder within the primary care setting. J Clin Psychol Med Settings. 2011;18:268-280. doi:10.1007/s10880-011-9237-4
- Hunt MG, Rosenheck RA. Psychotherapy in mental health clinics of the Department of Veterans Affairs. J Clin Psychol. 2011;67:561-573. doi:10.1002/jclp.20788
- Khatri N, Marziali E, Tchernikov I, et al. Comparing telehealth-based and clinic-based group cognitive behavioral therapy for adults with depression and anxiety: a pilot study. Clin Interv Aging. 2014;9:765. doi:10.2147/cia.s57832
- Dangel J. Clinical resource hub increases veterans' access to care. VA News. January 12, 2025. Accessed September 3, 2025. https://news.va.gov/137439/clinical-resource-hub-increases-access-to-care/
Since 2007, Primary Care Mental Health Integration (PCMHI) at the Veterans Health Administration (VHA) has improved access to mental health care services for veterans by directly embedding mental health care professionals (HCPs) within primary care teams.1 Veterans referred to PCMHI often have co-occurring physical and mental health disorders.2 Untreated chronic physical and mental comorbidities can diminish the effectiveness of medical and mental health interventions. Growing evidence suggests that treatment of mental health conditions can improve physical health outcomes and management of physical conditions can improve mental health outcomes.2,3
Chronic pain and sleep disorders are common reasons patients present to primary care, and often coexist together with mental health comorbidities.4 Sleep disorders affect 50% to 88% of patients with chronic pain, and 40% of patients with sleep disorders report chronic pain.4 Research has found that chronic pain and sleep disorders increase the risk of suicide attempts and deaths by suicide. Addressing suicide prevention simultaneously with treating chronic pain and insomnia is encouraged.5
Background
PCMHI treats physical and mental health comorbidities with a collaborative framework and a biopsychosocial integrative model.6 PCMHI staff provide mental health services as members of primary care teams. An interdisciplinary PCMHI team can include, but is not limited to, psychologists, mental health social workers, psychiatrists, nurse practitioners, clinical pharmacists, and mental health nurses. Quality of care within this model is elevated, as mental and physical health are recognized as interconnected. Collaboration between primary care and mental health benefits veterans and the VHA by increasing access to mental health care, decreasing stigma associated with mental health treatment, improving health outcomes, and enhancing the likelihood of recovery, resulting in high patient satisfaction.6-8
In the existing PCMHI model, HCPs are encouraged to use short-term, evidence-based psychotherapies (EBPs).9 Veterans referred to PCMHI from primary care are typically able to attend 1 to 6 brief sessions of mental health treatment, often 20 to 30 minutes long. Most EBPs in PCMHI are disorder- specific, providing interventions focused on a single presenting problem (eg, insomnia, chronic pain, or posttraumatic stress disorder [PTSD]). For veterans with a single issue, this model can be very effective. 1,10 However, the high rate of co-occurrence of mental and physical health issues can make it difficult to fully treat interrelated problems if the focus is on 1 specific diagnosis. Veterans with a need for additional (more comprehensive or intensive) mental health treatment are frequently referred to a higher, more resource-intensive level of mental health care, either in the VHA or the community. Examples of higher levels of mental health care include the longer term behavioral health interdisciplinary program (BHIP), sometimes called a mental health clinic (MHC), or a specialty mental health program such as a PTSD clinic.
As PCMHI continues to grow, new challenges have emerged related to staffing shortages and gaps in the clinical delivery of mental health treatment within the VHA. At the same time, demand for VHA mental health treatment has increased. However, a mental health professional shortage severely limits the ability of the VHA to meet this demand. In many systems, this shortage may result in more referrals being made to a higher level of mental health care because of fewer resources to provide comprehensive treatment in a less intensive PCMHI setting.8,10,11 This referral pattern can overburden higher level care, often with long wait times for treatment and lengthy lag times between appointments. Furthermore, these gaps in the clinical delivery of care cannot be effectively addressed by hiring additional mental health professionals. This strain on resources can impede access to care and negatively affect outcomes.10
Recent congressional reports highlight these issues, noting that demand for mental health services continues to outpace the capacity of both PCMHI and higher levels of mental health care, leading to delays in treatment that may negatively affect outcomes.8,10,11 These delays can be particularly detrimental for individuals with conditions requiring timely intervention.8,11 Some veterans are willing to engage with PCMHI in a primary care setting but may be reluctant to engage in general mental health treatment. These veterans might not receive the mental health care they need without PCMHI.
Group Psychotherapy
A group psychotherapy format can address gaps in care delivery and provide advantages for patients, mental health professionals, and the VHA. Group psychotherapy aligns with the US Department of Veterans Affairs (VA) 2018 Blueprint for Excellence and 2018 to 2024 strategic plan, underscoring the need for more timely and efficient mental health services.12,13
Benefits of group psychotherapy include reductions in symptoms, decreased feelings of isolation, increased social support, decreased emotional suppression, and enhanced satisfaction with overall quality of life.14-17 Studies of veterans with PTSD have found less attrition among those who chose group therapy compared with individual therapy.14,18 Group psychotherapy improves access to care by enabling delivery to more patients.14 When compared with individual therapy, the group format allows for a large number of patients to be treated simultaneously, maximizing resources and reducing costs.3,19-21
VISN 9 CRH Innovation
The VA provides care to veterans through regionally distinct administrative systems known as Veterans Integrated Service Networks (VISNs). Clinical resource hubs (CRH) are VISN-based programs created to cover VA staffing shortages by virtually deploying HCPs into local VA systems until vacancies are filled. The national CRH vision of effectively using resources and innovative technologies to meet veterans’ health care needs, along with the above-referenced clinical gaps in the delivery of care, inspired the development of VIP Boot Camp within the VISN 9 CRH.22
Program Description
VIP Boot Camp is an evidence-informed group psychotherapy program designed to provide timely, brief, and comprehensive mental health treatment for veterans. VIP Boot Camp was developed to address the needs of veterans accessing PCMHI services who experience ≥ 1 of the often overlapping problems of anxiety/emotion regulation/stress, sleep difficulties, and chronic pain (Figure). VIP Boot Camp uses an integrative approach to highlight interconnections and similarities among these difficulties and their treatment. A primary vision of the program is to provide this comprehensive treatment within PCMHI (upstream) so additional referrals to higher levels of mental health care (downstream) may not be needed.

This design is intentional because it increases the number of individuals who can be treated upstream with comprehensive, preventive, and proactive care within PCMHI which, over time, frees up resources in the BHIP for individuals requiring higher levels of care. This approach also aligns with the importance of early treatment for chronic pain and sleep disturbances, which are linked to increased risk of suicide attempts and deaths by suicide for veterans.5 National interest for VIP Boot Camp grew during fiscal year 2024 after it received the Gold Medal Recognition for Most Adoptable and Greatest Potential for Impact during VHA National Access Sprint Wave 3—Mental Health Call of Champions.
History
VIP Boot Camp began in August 2021 at VISN 9 as a 6-week virtual group for veterans with chronic pain. It was established to assist a large VA medical center experiencing PCMHI staffing shortages and lacking available PCMHI groups. Many veterans in the chronic pain group discussed co-occurring issues such as sleep disturbances, anxiety, and stress. The CRH team considered launching 2 separate groups to address these additional PCMHI-level issues; however, in developing the group material which drew from multiple clinical approaches, the team recognized significant overlapping and interconnected themes.
The team discussed EBPs within the VHA and how certain interventions within these treatments could be helpful across many other co-occurring disorders. Integrated tactics (clinical interventions) were drawn from cognitive-behavioral therapy (for depression, insomnia, or chronic pain), acceptance and commitment therapy, prolonged exposure, cognitive processing therapy, dialectical behavior therapy, unified protocol, pain reprocessing therapy, emotional awareness and expression therapy, interpersonal neurobiology, and mindfulness. We collaborated with veterans during VIP Boot Camp groups to determine how to present and discuss complex interventions in ways that were clinically accurate, understandable, relatable, and relevant to their experiences.
To address accessibility issues, the chronic pain group was reduced to 4 weeks. A second 4-week module for anxiety, emotion regulation, and stress was developed, mirroring the tactics, language, and integrative approach of the revised chronic pain module. A similar integrative approach led to the development of the third and final 4-week module for sleep disturbances.
Current Program
The VIP Boot Camp consists of three 4-week integrated modules, each highlighting a critical area: sleep disturbances (Improving Sleep), chronic pain difficulties (Outsmarting Chronic Pain), and emotion regulation difficulties (Rewiring Your Brain). VIP Boot Camp is designed for veterans who are at the PCMHI level of care. Referrals are accepted for patients receiving treatment from primary care or PCMHI.
Guidelines for participation in VIP Boot Camp may differ across sites or VISNs. For example, a veteran who has been referred to the BHIP for medication management only or to a specialty MHC such as a pain clinic or PTSD clinic might also be appropriate and eligible for VIP Boot Camp.
Given the interconnectedness of foundational themes, elements, and practices across the VIP Boot Camp modules, the modules are offered in a rolling format with a veteran-centric “choose your own adventure” approach. Tactics are presented in the modules in a way that allows patients to begin with any 1 of the 3 modules and receive treatment that will help in the other areas. Participants choose their core module and initial treatment focus based on their values, needs, and goals. Individuals who complete a core module can end their VIP Boot Camp experience or continue to the next 4-week module for up to 3 modules.
The group is open to new individuals at the start of any 4-week module and closed for the remainder of its 4-week duration. This innovative rolling modular approach combines elements of open- and closed-group format, allowing for the flexibility and accessibility of an open group with the stability and peer support of a closed group.
Given the complicated and overlapping nature of chronic pain, emotion regulation/ stress, and sleep disturbances, VIP Boot Camp acknowledges that everything is interconnected and difficulties in 1 area may impact other areas. The 3 interconnected modules with repeating themes provide coherence and consistency. Veterans learn how interconnections across difficulties can be leveraged so that tactics learned and practiced in 1 area can assist in other areas, changing the cycle of suffering into a cycle of growth.
VIP Boot Camp sessions are 90 minutes long, once weekly for 4 weeks, with 2 mental health professionals trained to lead a dynamic group psychotherapy experience that aims to be fun for participants. VIP Boot Camp synthesizes evidence-based and evidence-informed interventions, as well as techniques from VHA complementary and integrative health programs, psychoeducation, and interpersonal interventions that model connection, playfulness, and healthy boundaries. These varied strategies combine to equip veterans with practical tactics for self-management outside of sessions, a process described as “finding puzzle pieces.” VIP Boot Camp is built on the idea that people are more likely to adopt and practice any tactic after being taught why that tactic is important, and how it fits into their larger interconnected puzzle. After each session, participants are provided with additional asynchronous educational material to help reinforce their learnings and practices.
Although individuals may hesitate to participate in a group setting, they often find the experience of community enhances and accelerates their treatment and gains. This involvement is highlighted in a core aspect of a VIP Boot Camp session called wins, during which participants learn how others on their Boot Camp team are implementing new skills and moving toward their personal values and objectives in a stepwise manner. Through these shared experiences, veterans discover how tactics working for others may serve as a model for their own personal objectives and plans for practice. The sense of relief described by many upon realizing they are not alone in their experiences, along with the satisfaction felt in discovering their ability to support others in Boot Camp, is described by many participants as deeply meaningful and in line with their personal values.
While developed as a fully virtual group program, VIP Boot Camp can also be conducted in person. The virtual program has been successful and continues to spread across VISN 9. There are 8 virtual VIP Boot Camps running in VISN 9, with plans for continued expansion. In the VISN 9 CRH, Boot Camps typically have 10 to 12 participants. Additionally, as VIP Boot Camp grows within a location there are frequently sufficient referrals to support a second rolling group, which enables staggering of the module offerings to allow for even more timely treatment.
Training Program
VISN 9 CRH also developed a VIP Boot Camp 3-day intensive training program for PCMHI HCPs that consists of learning and practicing VIP Boot Camp material for chronic pain, emotion regulation/ stress, sleep disturbances, mindfulness, and guided imagery, along with gaining experience as a VIP Boot Camp coleader. Feedback received from PCMHI HCPs who completed training has been positive. There is also a private Microsoft Teams channel for HCPs, which allows for resource sharing and community building among coleaders. More than 75 PCMHI HCPs have completed VIP Boot Camp training and > 25 VIP Boot Camps have been established at 4 additional VISNs.
The VISN 9 CRH VIP Boot Camp program initiated an implementation and effectiveness project with the Michael E. DeBakey VA Medical Center and the South Central Mental Illness Research, Education and Clinical Center. The focus of this collaboration is support for implementation and treatment effectiveness research with reports, articles, and a white paper on findings and best practices, alongside continued dissemination of the VIP Boot Camp program and training.
Conclusions
VIP Boot Camp is a PCMHI group program offering readily available, comprehensive, and integrative group psychotherapy services to veterans experiencing . 1 of the following: chronic pain, emotion regulation/ stress, and sleep disturbances. It was launched at the VISN 9 CRH with a goal of addressing clinical gaps in the delivery of mental health care, by increasing the number of patients treated within PCMHI. The VIP Boot Camp model provides veterans the opportunity to transform cycles of suffering into cycles of growth through a single approach that can address multiple presenting and interconnected issues.
A 3-day VIP Boot Camp training program provides a quick and effective path for a PCMHI program to train HCPs to launch a VIP Boot Camp. The VISN 9 CRH will continue to champion VIP Boot Camp as a model for the successful provision of comprehensive and integrative mental health treatment within PCMHI at the VA. Through readily available access to comprehensive mental health treatment in an environment that promotes participant empowerment and social engagement, VIP Boot Camp represents an integrative and innovative model of mental health treatment that offers benefits to veteran participants, HCPs, and the VHA.
Since 2007, Primary Care Mental Health Integration (PCMHI) at the Veterans Health Administration (VHA) has improved access to mental health care services for veterans by directly embedding mental health care professionals (HCPs) within primary care teams.1 Veterans referred to PCMHI often have co-occurring physical and mental health disorders.2 Untreated chronic physical and mental comorbidities can diminish the effectiveness of medical and mental health interventions. Growing evidence suggests that treatment of mental health conditions can improve physical health outcomes and management of physical conditions can improve mental health outcomes.2,3
Chronic pain and sleep disorders are common reasons patients present to primary care, and often coexist together with mental health comorbidities.4 Sleep disorders affect 50% to 88% of patients with chronic pain, and 40% of patients with sleep disorders report chronic pain.4 Research has found that chronic pain and sleep disorders increase the risk of suicide attempts and deaths by suicide. Addressing suicide prevention simultaneously with treating chronic pain and insomnia is encouraged.5
Background
PCMHI treats physical and mental health comorbidities with a collaborative framework and a biopsychosocial integrative model.6 PCMHI staff provide mental health services as members of primary care teams. An interdisciplinary PCMHI team can include, but is not limited to, psychologists, mental health social workers, psychiatrists, nurse practitioners, clinical pharmacists, and mental health nurses. Quality of care within this model is elevated, as mental and physical health are recognized as interconnected. Collaboration between primary care and mental health benefits veterans and the VHA by increasing access to mental health care, decreasing stigma associated with mental health treatment, improving health outcomes, and enhancing the likelihood of recovery, resulting in high patient satisfaction.6-8
In the existing PCMHI model, HCPs are encouraged to use short-term, evidence-based psychotherapies (EBPs).9 Veterans referred to PCMHI from primary care are typically able to attend 1 to 6 brief sessions of mental health treatment, often 20 to 30 minutes long. Most EBPs in PCMHI are disorder- specific, providing interventions focused on a single presenting problem (eg, insomnia, chronic pain, or posttraumatic stress disorder [PTSD]). For veterans with a single issue, this model can be very effective. 1,10 However, the high rate of co-occurrence of mental and physical health issues can make it difficult to fully treat interrelated problems if the focus is on 1 specific diagnosis. Veterans with a need for additional (more comprehensive or intensive) mental health treatment are frequently referred to a higher, more resource-intensive level of mental health care, either in the VHA or the community. Examples of higher levels of mental health care include the longer term behavioral health interdisciplinary program (BHIP), sometimes called a mental health clinic (MHC), or a specialty mental health program such as a PTSD clinic.
As PCMHI continues to grow, new challenges have emerged related to staffing shortages and gaps in the clinical delivery of mental health treatment within the VHA. At the same time, demand for VHA mental health treatment has increased. However, a mental health professional shortage severely limits the ability of the VHA to meet this demand. In many systems, this shortage may result in more referrals being made to a higher level of mental health care because of fewer resources to provide comprehensive treatment in a less intensive PCMHI setting.8,10,11 This referral pattern can overburden higher level care, often with long wait times for treatment and lengthy lag times between appointments. Furthermore, these gaps in the clinical delivery of care cannot be effectively addressed by hiring additional mental health professionals. This strain on resources can impede access to care and negatively affect outcomes.10
Recent congressional reports highlight these issues, noting that demand for mental health services continues to outpace the capacity of both PCMHI and higher levels of mental health care, leading to delays in treatment that may negatively affect outcomes.8,10,11 These delays can be particularly detrimental for individuals with conditions requiring timely intervention.8,11 Some veterans are willing to engage with PCMHI in a primary care setting but may be reluctant to engage in general mental health treatment. These veterans might not receive the mental health care they need without PCMHI.
Group Psychotherapy
A group psychotherapy format can address gaps in care delivery and provide advantages for patients, mental health professionals, and the VHA. Group psychotherapy aligns with the US Department of Veterans Affairs (VA) 2018 Blueprint for Excellence and 2018 to 2024 strategic plan, underscoring the need for more timely and efficient mental health services.12,13
Benefits of group psychotherapy include reductions in symptoms, decreased feelings of isolation, increased social support, decreased emotional suppression, and enhanced satisfaction with overall quality of life.14-17 Studies of veterans with PTSD have found less attrition among those who chose group therapy compared with individual therapy.14,18 Group psychotherapy improves access to care by enabling delivery to more patients.14 When compared with individual therapy, the group format allows for a large number of patients to be treated simultaneously, maximizing resources and reducing costs.3,19-21
VISN 9 CRH Innovation
The VA provides care to veterans through regionally distinct administrative systems known as Veterans Integrated Service Networks (VISNs). Clinical resource hubs (CRH) are VISN-based programs created to cover VA staffing shortages by virtually deploying HCPs into local VA systems until vacancies are filled. The national CRH vision of effectively using resources and innovative technologies to meet veterans’ health care needs, along with the above-referenced clinical gaps in the delivery of care, inspired the development of VIP Boot Camp within the VISN 9 CRH.22
Program Description
VIP Boot Camp is an evidence-informed group psychotherapy program designed to provide timely, brief, and comprehensive mental health treatment for veterans. VIP Boot Camp was developed to address the needs of veterans accessing PCMHI services who experience ≥ 1 of the often overlapping problems of anxiety/emotion regulation/stress, sleep difficulties, and chronic pain (Figure). VIP Boot Camp uses an integrative approach to highlight interconnections and similarities among these difficulties and their treatment. A primary vision of the program is to provide this comprehensive treatment within PCMHI (upstream) so additional referrals to higher levels of mental health care (downstream) may not be needed.

This design is intentional because it increases the number of individuals who can be treated upstream with comprehensive, preventive, and proactive care within PCMHI which, over time, frees up resources in the BHIP for individuals requiring higher levels of care. This approach also aligns with the importance of early treatment for chronic pain and sleep disturbances, which are linked to increased risk of suicide attempts and deaths by suicide for veterans.5 National interest for VIP Boot Camp grew during fiscal year 2024 after it received the Gold Medal Recognition for Most Adoptable and Greatest Potential for Impact during VHA National Access Sprint Wave 3—Mental Health Call of Champions.
History
VIP Boot Camp began in August 2021 at VISN 9 as a 6-week virtual group for veterans with chronic pain. It was established to assist a large VA medical center experiencing PCMHI staffing shortages and lacking available PCMHI groups. Many veterans in the chronic pain group discussed co-occurring issues such as sleep disturbances, anxiety, and stress. The CRH team considered launching 2 separate groups to address these additional PCMHI-level issues; however, in developing the group material which drew from multiple clinical approaches, the team recognized significant overlapping and interconnected themes.
The team discussed EBPs within the VHA and how certain interventions within these treatments could be helpful across many other co-occurring disorders. Integrated tactics (clinical interventions) were drawn from cognitive-behavioral therapy (for depression, insomnia, or chronic pain), acceptance and commitment therapy, prolonged exposure, cognitive processing therapy, dialectical behavior therapy, unified protocol, pain reprocessing therapy, emotional awareness and expression therapy, interpersonal neurobiology, and mindfulness. We collaborated with veterans during VIP Boot Camp groups to determine how to present and discuss complex interventions in ways that were clinically accurate, understandable, relatable, and relevant to their experiences.
To address accessibility issues, the chronic pain group was reduced to 4 weeks. A second 4-week module for anxiety, emotion regulation, and stress was developed, mirroring the tactics, language, and integrative approach of the revised chronic pain module. A similar integrative approach led to the development of the third and final 4-week module for sleep disturbances.
Current Program
The VIP Boot Camp consists of three 4-week integrated modules, each highlighting a critical area: sleep disturbances (Improving Sleep), chronic pain difficulties (Outsmarting Chronic Pain), and emotion regulation difficulties (Rewiring Your Brain). VIP Boot Camp is designed for veterans who are at the PCMHI level of care. Referrals are accepted for patients receiving treatment from primary care or PCMHI.
Guidelines for participation in VIP Boot Camp may differ across sites or VISNs. For example, a veteran who has been referred to the BHIP for medication management only or to a specialty MHC such as a pain clinic or PTSD clinic might also be appropriate and eligible for VIP Boot Camp.
Given the interconnectedness of foundational themes, elements, and practices across the VIP Boot Camp modules, the modules are offered in a rolling format with a veteran-centric “choose your own adventure” approach. Tactics are presented in the modules in a way that allows patients to begin with any 1 of the 3 modules and receive treatment that will help in the other areas. Participants choose their core module and initial treatment focus based on their values, needs, and goals. Individuals who complete a core module can end their VIP Boot Camp experience or continue to the next 4-week module for up to 3 modules.
The group is open to new individuals at the start of any 4-week module and closed for the remainder of its 4-week duration. This innovative rolling modular approach combines elements of open- and closed-group format, allowing for the flexibility and accessibility of an open group with the stability and peer support of a closed group.
Given the complicated and overlapping nature of chronic pain, emotion regulation/ stress, and sleep disturbances, VIP Boot Camp acknowledges that everything is interconnected and difficulties in 1 area may impact other areas. The 3 interconnected modules with repeating themes provide coherence and consistency. Veterans learn how interconnections across difficulties can be leveraged so that tactics learned and practiced in 1 area can assist in other areas, changing the cycle of suffering into a cycle of growth.
VIP Boot Camp sessions are 90 minutes long, once weekly for 4 weeks, with 2 mental health professionals trained to lead a dynamic group psychotherapy experience that aims to be fun for participants. VIP Boot Camp synthesizes evidence-based and evidence-informed interventions, as well as techniques from VHA complementary and integrative health programs, psychoeducation, and interpersonal interventions that model connection, playfulness, and healthy boundaries. These varied strategies combine to equip veterans with practical tactics for self-management outside of sessions, a process described as “finding puzzle pieces.” VIP Boot Camp is built on the idea that people are more likely to adopt and practice any tactic after being taught why that tactic is important, and how it fits into their larger interconnected puzzle. After each session, participants are provided with additional asynchronous educational material to help reinforce their learnings and practices.
Although individuals may hesitate to participate in a group setting, they often find the experience of community enhances and accelerates their treatment and gains. This involvement is highlighted in a core aspect of a VIP Boot Camp session called wins, during which participants learn how others on their Boot Camp team are implementing new skills and moving toward their personal values and objectives in a stepwise manner. Through these shared experiences, veterans discover how tactics working for others may serve as a model for their own personal objectives and plans for practice. The sense of relief described by many upon realizing they are not alone in their experiences, along with the satisfaction felt in discovering their ability to support others in Boot Camp, is described by many participants as deeply meaningful and in line with their personal values.
While developed as a fully virtual group program, VIP Boot Camp can also be conducted in person. The virtual program has been successful and continues to spread across VISN 9. There are 8 virtual VIP Boot Camps running in VISN 9, with plans for continued expansion. In the VISN 9 CRH, Boot Camps typically have 10 to 12 participants. Additionally, as VIP Boot Camp grows within a location there are frequently sufficient referrals to support a second rolling group, which enables staggering of the module offerings to allow for even more timely treatment.
Training Program
VISN 9 CRH also developed a VIP Boot Camp 3-day intensive training program for PCMHI HCPs that consists of learning and practicing VIP Boot Camp material for chronic pain, emotion regulation/ stress, sleep disturbances, mindfulness, and guided imagery, along with gaining experience as a VIP Boot Camp coleader. Feedback received from PCMHI HCPs who completed training has been positive. There is also a private Microsoft Teams channel for HCPs, which allows for resource sharing and community building among coleaders. More than 75 PCMHI HCPs have completed VIP Boot Camp training and > 25 VIP Boot Camps have been established at 4 additional VISNs.
The VISN 9 CRH VIP Boot Camp program initiated an implementation and effectiveness project with the Michael E. DeBakey VA Medical Center and the South Central Mental Illness Research, Education and Clinical Center. The focus of this collaboration is support for implementation and treatment effectiveness research with reports, articles, and a white paper on findings and best practices, alongside continued dissemination of the VIP Boot Camp program and training.
Conclusions
VIP Boot Camp is a PCMHI group program offering readily available, comprehensive, and integrative group psychotherapy services to veterans experiencing . 1 of the following: chronic pain, emotion regulation/ stress, and sleep disturbances. It was launched at the VISN 9 CRH with a goal of addressing clinical gaps in the delivery of mental health care, by increasing the number of patients treated within PCMHI. The VIP Boot Camp model provides veterans the opportunity to transform cycles of suffering into cycles of growth through a single approach that can address multiple presenting and interconnected issues.
A 3-day VIP Boot Camp training program provides a quick and effective path for a PCMHI program to train HCPs to launch a VIP Boot Camp. The VISN 9 CRH will continue to champion VIP Boot Camp as a model for the successful provision of comprehensive and integrative mental health treatment within PCMHI at the VA. Through readily available access to comprehensive mental health treatment in an environment that promotes participant empowerment and social engagement, VIP Boot Camp represents an integrative and innovative model of mental health treatment that offers benefits to veteran participants, HCPs, and the VHA.
- Leung LB, Yoon J, Escarce JJ, et al. Primary care-mental health integration in the VA: shifting mental health services for common mental illnesses to primary care. Psychiatr Serv. 2018;69:403-409. doi:10.1176/appi.ps.201700190
- Zhang A, Park S, Sullivan JE, et al. The effectiveness of problem-solving therapy for primary care patients’ depressive and/or anxiety disorders: a systematic review and meta-analysis. J Am Board Fam Med. 2018;31:139-150. doi:10.3122/jabfm.2018.01.170270
- Hundt NE, Barrera TL, Robinson A, et al. A systematic review of cognitive behavioral therapy for depression in veterans. Mil Med. 2014;179:942-949. doi:10.7205/milmed-d-14-00128
- Jank R, Gallee A, Boeckle M, et al. Chronic pain and sleep disorders in primary care. Pain Res Treat. 2017;2017:1-9. doi:10.1155/2017/9081802
- Ashrafioun L, Bishop TM, Pigeon WR. The relationship between pain severity, insomnia, and suicide attempts among a national veteran sample initiating pain care. Psychosom Med. 2021;83:733- 738. doi:10.1097/psy.0000000000000975
- Ramanuj P, Ferenchik E, Docherty M, et al. Evolving models of integrated behavioral health and primary care. Curr Psychiatry Rep. 2019;21:1. doi:10.1007/s11920-019-0985-4
- Post EP, Metzger M, Dumas P, et al. Integrating mental health into primary care within the Veterans Health Administration. Fam Syst Health. 2010;28:83-90. doi:10.1037/a0020130
- Smith TL, Kim B, Benzer JK, et al. FLOW: early results from a clinical demonstration project to improve the transition of patients with mental health disorders back to primary care. Psychol Serv. 2021;18:23-32. doi:10.1037/ser0000336
- Kearney LK, Post EP, Pomerantz AS, et al. Applying the interprofessional patient aligned care team in the department of veterans affairs transforming primary care. Am Psychol. 2014;69(4):399-408. doi:10.1037/a0035909
- US Government Accountability Office. Veterans health care: staffing challenges persist for fully integrating mental health and primary care services. December 15, 2022. Accessed July 9, 2025. https://www.gao.gov/products/gao-23-105372
- National Academies of Science and Engineering. Evaluation of the Department of Veterans Affairs Mental Health Services. National Academies Press; 2018. Accessed July 9, 2025. https://nap.nationalacademies.org/catalog/24915/evaluation-of-the-department-of-veterans-affairs-mental-health-services
- US Department of Veterans Affairs. Blueprint for excellence: achieving veterans’ excellence. October 6, 2014. Accessed July 9, 2025. https://www.volunteer.va.gov/docs/blueprintforexcellence_factsheet.PDF
- US Department of Veterans Affairs. Department of Veterans Affairs FY 2018-2024 strategic plan. Accessed July 9, 2025. https://www.calvet.ca.gov/Regulations/USDVA%20Strategic%20Plan%202018-2024.pdf
- Sripada RK, Bohnert KM, Ganoczy D, et al. Initial group versus individual therapy for posttraumatic stress disorder and subsequent follow-up treatment adequacy. Psychol Serv. 2016;13:349-355. doi:10.1037/ser0000077
- Burnett-Zeigler IE, Pfeiffer P, Zivin K, et al. Psychotherapy utilization for acute depression within the Veterans Affairs health care system. Psychol Serv. 2012;9:325-335. doi:10.1037/a0027957
- Kim JS, Prins A, Hirschhorn EW, et al. Preliminary investigation into the effectiveness of group webSTAIR for trauma-exposed veterans in primary care. Mil Med. 2024;189:e1403-e1408. doi:10.1093/milmed/usae052
- Jakupcak M, Blais RK, Grossbard J, et al. “Toughness” in association with mental health symptoms among Iraq and Afghanistan war veterans seeking Veterans Affairs health care. Psychol Men Masc. 2014;15:100-104. doi:10.1037/a0031508
- Stoycos SA, Berzenski SR, Beck JG, et al. Predictors of treatment completion in group psychotherapy for male veterans with posttraumatic stress disorder. J Trauma Stress. 2023;36:346-358. doi:10.1002/jts.22915
- Possemato K. The current state of intervention research for posttraumatic stress disorder within the primary care setting. J Clin Psychol Med Settings. 2011;18:268-280. doi:10.1007/s10880-011-9237-4
- Hunt MG, Rosenheck RA. Psychotherapy in mental health clinics of the Department of Veterans Affairs. J Clin Psychol. 2011;67:561-573. doi:10.1002/jclp.20788
- Khatri N, Marziali E, Tchernikov I, et al. Comparing telehealth-based and clinic-based group cognitive behavioral therapy for adults with depression and anxiety: a pilot study. Clin Interv Aging. 2014;9:765. doi:10.2147/cia.s57832
- Dangel J. Clinical resource hub increases veterans' access to care. VA News. January 12, 2025. Accessed September 3, 2025. https://news.va.gov/137439/clinical-resource-hub-increases-access-to-care/
- Leung LB, Yoon J, Escarce JJ, et al. Primary care-mental health integration in the VA: shifting mental health services for common mental illnesses to primary care. Psychiatr Serv. 2018;69:403-409. doi:10.1176/appi.ps.201700190
- Zhang A, Park S, Sullivan JE, et al. The effectiveness of problem-solving therapy for primary care patients’ depressive and/or anxiety disorders: a systematic review and meta-analysis. J Am Board Fam Med. 2018;31:139-150. doi:10.3122/jabfm.2018.01.170270
- Hundt NE, Barrera TL, Robinson A, et al. A systematic review of cognitive behavioral therapy for depression in veterans. Mil Med. 2014;179:942-949. doi:10.7205/milmed-d-14-00128
- Jank R, Gallee A, Boeckle M, et al. Chronic pain and sleep disorders in primary care. Pain Res Treat. 2017;2017:1-9. doi:10.1155/2017/9081802
- Ashrafioun L, Bishop TM, Pigeon WR. The relationship between pain severity, insomnia, and suicide attempts among a national veteran sample initiating pain care. Psychosom Med. 2021;83:733- 738. doi:10.1097/psy.0000000000000975
- Ramanuj P, Ferenchik E, Docherty M, et al. Evolving models of integrated behavioral health and primary care. Curr Psychiatry Rep. 2019;21:1. doi:10.1007/s11920-019-0985-4
- Post EP, Metzger M, Dumas P, et al. Integrating mental health into primary care within the Veterans Health Administration. Fam Syst Health. 2010;28:83-90. doi:10.1037/a0020130
- Smith TL, Kim B, Benzer JK, et al. FLOW: early results from a clinical demonstration project to improve the transition of patients with mental health disorders back to primary care. Psychol Serv. 2021;18:23-32. doi:10.1037/ser0000336
- Kearney LK, Post EP, Pomerantz AS, et al. Applying the interprofessional patient aligned care team in the department of veterans affairs transforming primary care. Am Psychol. 2014;69(4):399-408. doi:10.1037/a0035909
- US Government Accountability Office. Veterans health care: staffing challenges persist for fully integrating mental health and primary care services. December 15, 2022. Accessed July 9, 2025. https://www.gao.gov/products/gao-23-105372
- National Academies of Science and Engineering. Evaluation of the Department of Veterans Affairs Mental Health Services. National Academies Press; 2018. Accessed July 9, 2025. https://nap.nationalacademies.org/catalog/24915/evaluation-of-the-department-of-veterans-affairs-mental-health-services
- US Department of Veterans Affairs. Blueprint for excellence: achieving veterans’ excellence. October 6, 2014. Accessed July 9, 2025. https://www.volunteer.va.gov/docs/blueprintforexcellence_factsheet.PDF
- US Department of Veterans Affairs. Department of Veterans Affairs FY 2018-2024 strategic plan. Accessed July 9, 2025. https://www.calvet.ca.gov/Regulations/USDVA%20Strategic%20Plan%202018-2024.pdf
- Sripada RK, Bohnert KM, Ganoczy D, et al. Initial group versus individual therapy for posttraumatic stress disorder and subsequent follow-up treatment adequacy. Psychol Serv. 2016;13:349-355. doi:10.1037/ser0000077
- Burnett-Zeigler IE, Pfeiffer P, Zivin K, et al. Psychotherapy utilization for acute depression within the Veterans Affairs health care system. Psychol Serv. 2012;9:325-335. doi:10.1037/a0027957
- Kim JS, Prins A, Hirschhorn EW, et al. Preliminary investigation into the effectiveness of group webSTAIR for trauma-exposed veterans in primary care. Mil Med. 2024;189:e1403-e1408. doi:10.1093/milmed/usae052
- Jakupcak M, Blais RK, Grossbard J, et al. “Toughness” in association with mental health symptoms among Iraq and Afghanistan war veterans seeking Veterans Affairs health care. Psychol Men Masc. 2014;15:100-104. doi:10.1037/a0031508
- Stoycos SA, Berzenski SR, Beck JG, et al. Predictors of treatment completion in group psychotherapy for male veterans with posttraumatic stress disorder. J Trauma Stress. 2023;36:346-358. doi:10.1002/jts.22915
- Possemato K. The current state of intervention research for posttraumatic stress disorder within the primary care setting. J Clin Psychol Med Settings. 2011;18:268-280. doi:10.1007/s10880-011-9237-4
- Hunt MG, Rosenheck RA. Psychotherapy in mental health clinics of the Department of Veterans Affairs. J Clin Psychol. 2011;67:561-573. doi:10.1002/jclp.20788
- Khatri N, Marziali E, Tchernikov I, et al. Comparing telehealth-based and clinic-based group cognitive behavioral therapy for adults with depression and anxiety: a pilot study. Clin Interv Aging. 2014;9:765. doi:10.2147/cia.s57832
- Dangel J. Clinical resource hub increases veterans' access to care. VA News. January 12, 2025. Accessed September 3, 2025. https://news.va.gov/137439/clinical-resource-hub-increases-access-to-care/
VIP Boot Camp: Expanding the Impact of VA Primary Care Mental Health With a Transdiagnostic Modular Group Program
VIP Boot Camp: Expanding the Impact of VA Primary Care Mental Health With a Transdiagnostic Modular Group Program
E-Consults Bridge to Interdisciplinary Team Care for Rural Appalachian Veterans With Chronic Pain and Opioid Use Disorder
E-Consults Bridge to Interdisciplinary Team Care for Rural Appalachian Veterans With Chronic Pain and Opioid Use Disorder
Rural veterans are prescribed long-term opioid therapy for chronic pain at higher rates than urban veterans, increasing their risk of developing opioid use disorder (OUD).1,2 Veterans with co-occurring OUD and chronic pain have more severe health concerns, as well as higher rates of homelessness, psychoactive drug misuse, and mental health disorders, compared to veterans with either chronic pain or OUD alone.3 Interdisciplinary team (IDT) care is recommended for both chronic pain and OUD.4,5 Rural veterans with co-occurring chronic pain and OUD, however, are often unable to access IDTs due to long travel and wait times. As a result, these rural veterans often receive care from primary care practitioners (PCPs) who lack training in pain management and addiction and have low confidence in their ability to provide optimal treatment.6,7
In the Veterans Health Administration, electronic consultations (e-consults) provide support to PCPs by recommending evidence-based approaches such as buprenorphine for OUD and pain IDTs for chronic pain.5,8 However, research on the use of e-consults to connect to IDT care for co-occurring chronic pain and OUD are lacking, as well as studies on IDTs using innovative methods (eg, shared appointments) to overcome treatment barriers (eg, multiple appointments) for rural veterans at higher risk for co-occurring OUD and chronic pain.
This quality improvement study sought to determine the feasibility and impact of a pharmacy e-consult service that provided pain medication recommendations and subsequent referrals to RESTORE, a shared appointment program with an IDT, for assessment and treatment of chronic pain and OUD.
Methods
This retrospective chart review was approved as nonresearch by the Institutional Review Board Chair at the Salem Veterans Affairs Healthcare System (SVAHS), a low-complexity medical center in Virginia that primarily serves a rural and highly rural Central Appalachian veteran population.
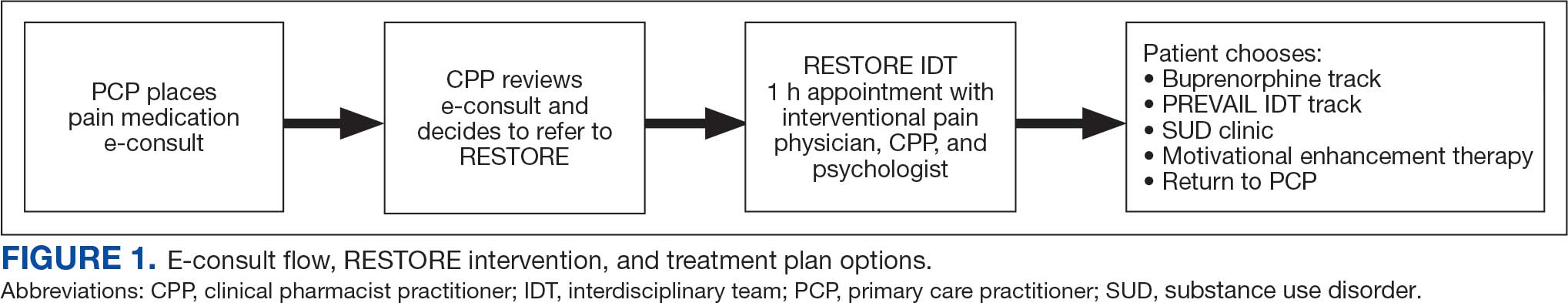
This study included veterans whose clinicians placed a pain medication e-consult requesting recommendations for medication adjustments and/or a referral to RESTORE from January 1, 2022, through January 6, 2023. Requests for services that could not be provided through an e-consult were excluded (Figure 1). Veterans who had a pain medication e-consult were identified in the SVAHS electronic medical record (EMR). Data extracted from the EMR included demographics, referral source, reason for referral, RESTORE appointment attendance, OUD diagnosis made during the RESTORE initial evaluation, implementation of medication recommendations by the referrer within 6 months, engagement in ≥ 3 pain education classes, and a shared appointment with a pain IDT within 6 months. Data were entered into a REDCap database, and descriptive statistics summarized the results. Feasibility was assessed by use of the e-consult by PCPs, attendance at the RESTORE appointment, and OUD diagnosis by the RESTORE team.
RESTORE Intervention
A pain medication e-consult was followed by referral to a shared appointment with the RESTORE IDT, with subsequent referrals to a pain IDT for chronic pain management if the veteran was amenable.
Pain medication e-consults in the EMR prompted a chart review by a clinical pharmacist practitioner (CPP). Recommendations for changes to medication regimens were documented in the EMR. At completion of the e-consult, the referring clinician received an automated view alert.
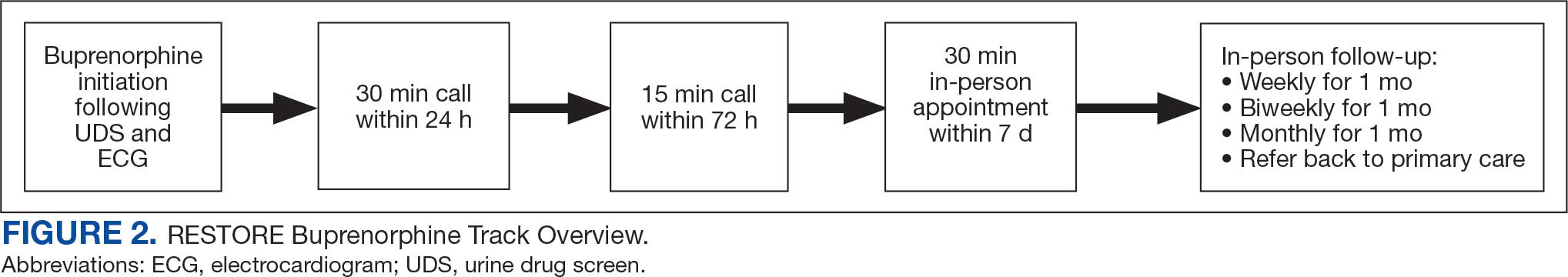
Veterans (and a support person, if preferred) were seen in a 60-minute, face-to-face shared appointment which included a psychologist, CPP, and pain physician. The psychologist conducted an OUD diagnostic interview, provided diagnostic feedback, and used motivational interviewing to provide psychoeducation on the biopsychosocial model of chronic pain, the IDT approach to chronic pain, and an overview of pain IDT care locally available. A CPP and physician then described medication options available to address OUD, if applicable. Together, the IDT and patient used shared decision making to determine a comprehensive treatment plan that may include a referral to the SVAHS PREVAIL Center for Chronic Pain IDT track (PREVAIL IDT track), a referral to substance use care in the case of polysubstance use, or medication initiation.9-11 If medication was prescribed, the patient was subsequently followed by the CPP through phone calls and face-to-face appointments at regularly scheduled intervals in coordination with the prescriber until they were stabilized. After stabilization, the prescription would be managed by their PCP (Figure 2). Veterans whose clinical condition changed significantly or worsened after returning to their PCP were invited to be reevaluated by the RESTORE team and restart care in that program. Individuals who were actively receiving RESTORE team care were discussed in a weekly care coordination meeting with all clinicians from both the PREVAIL and RESTORE teams.
Program Metrics
Pain medication e-consults were placed for 77 patients; 7 were excluded as inappropriate referral requests. Seventy (83%) e-consults were placed by PCPs (Table). Fifty-seven referring PCPs (81%) implemented ≥ 1 medication recommendation and 41 (59%) implemented all recommendations within 6 months. CPPs referred 19 individuals to RESTORE due to concerns related to high risk. All attended the initial evaluation appointment with the RESTORE team, 17 (89%) agreed to be referred to PREVAIL IDT track for nonpharmacologic pain care, and 9 (53%) engaged with that care within 6 months. Of those who attended RESTORE, 7 patients (37%) initiated buprenorphine for OUD with 6 (86%) being prescribed buprenorphine for ≥ 6 months.

Discussion
Most e-consults placed at SVAHS, which primarily serves a rural veteran population in Central Appalachia, resulted in veterans engaging in evidence-based treatment for co-occurring chronic pain and OUD. The use of e-consults and subsequent shared appointments with an IDT appears to be feasible, as the service was most often used by PCPs who often feel unequipped to manage chronic pain.7 The attendance rate for the RESTORE appointments was notable given the typically poor follow-up for patients with OUD. It supports the feasibility of a shared appointment approach which may overcome frequent barriers to care in this vulnerable population (ie, time, transportation). By attending 1 appointment with all clinicians present as opposed to multiple appointments, veterans experience fewer barriers than attending multiple appointments. RESTORE continues to be offered as an active clinical service whose implementation is now supported by changes to SVAHS policies. Since this study was conducted, the number of patients seen weekly has doubled and will soon be tripled based on high demand from PCPs.
While this study was limited to 1 site, had a small sample size, and was limited in scope, its results suggest that future research is warranted. Future studies using a larger sample size utilizing both a randomized control trial design and qualitative methods are needed to answer critical questions such as the role of patient characteristics on treatment effectiveness and the impact of the RESTORE model on long-term OUD medication adherence, patients’ perceptions and satisfaction, barriers to implementation, PCP confidence in providing pain care, and use of evidence-based nonpharmacologic pain management services.12-14
Conclusions
The results of this quality-improvement project suggest that e-consults may facilitate referrals to and patient follow-through with evidence-based treatment for co-occurring chronic pain and OUD among veterans living in rural communities in Central Appalachia who tend to experience significant barriers to traditional care and may require an innovative approach to facilitate effective treatment.
- Lund BC, Ohl ME, Hadlandsmyth K, et al. Regional and rural-urban variation in opioid prescribing in the Veterans Health Administration. Mil Med. 2019;184(11-12):894-900. doi:10.1093/milmed/usz104
- Edlund MJ, Martin BC, Russo JE, et al. The role of opioid prescription in incident opioid abuse and dependence among individuals with chronic noncancer pain: the role of opioid prescription. Clin J Pain. 2014;30(7):557-564. doi:10.1097/AJP.0000000000000021
- MacLean RR, Sofuoglu M, Stefanovics E, et al. Opioid use disorder with chronic pain increases disease burden and service use. Psychol Serv. 2023;20(1):157-165. doi:10.1037/ser0000607
- US Department of Veterans Affairs, US Department of Defense. VA/DoD clinical practice guidelines: use of opioids in the management of chronic pain. Version 4.0. Updated May 2022. Accessed August 4, 2025. https://www.healthquality.va.gov/guidelines/Pain/cot/VADoDOpioidsCPG.pdf
- US Department of Veterans Affairs, US Department of Defense. VA/DoD clinical practice guideline for the diagnosis and treatment of low back pain: the diagnosis and treatment of low back pain. Version 3.0. Updated February 2022. Accessed August 4, 2025. https://www.healthquality.va.gov/guidelines/Pain/lbp/VADoDLBPCPGFinal508.pdf
- Shipton EE, Bate F, Garrick R, et al. Systematic review of pain medicine content, teaching, and assessment in medical school curricula internationally. Pain Ther. 2018;7(2):139-161. doi:10.1007/s40122-018-0103-z
- Jamison RN, Scanlan E, Matthews ML, et al. Attitudes of primary care practitioners in managing chronic pain patients prescribed opioids for pain: a prospective longitudinal controlled trial. Pain Med. 2016;17(1):99-113. doi:10.1111/pme.12871
- Miller DM, Harvey TL. Pharmacist pain e-consults that result in a therapy change. Fed Pract. 2015;32(7):14-19.
- Courtney RE, Schadegg MJ. Chronic, noncancer pain care in the Veterans Administration: current trends and future directions. Anesthesiol Clin. 2023;41(2):519-529. doi:10.1016/j.anclin.2023.02.004
- Courtney RE, Schadegg MJ, Bolton R, et al. Using a whole health approach to build biopsychosocial-spiritual personal health plans for veterans with chronic pain. Pain Manag Nurs. 2024;25(1):69-74. doi:10.1016/j.pmn.2023.09.010
- Darnall BD, Edwards KA, Courtney RE, et al. Innovative treatment formats, technologies, and clinician trainings that improve access to behavioral pain treatment for youth and adults. Front Pain Res. 2023;4. doi:10.3389/fpain.2023.1223172
- Lister JJ, Weaver A, Ellis JD, et al. A systematic review of rural-specific barriers to medication treatment for opioid use disorder in the United States. Am J Drug Alcohol Abuse. 2020;46:273-288. doi:10.1080/00952990.2019.1694536
- Bhatraju EP, Radick AC, Leroux BG, et al. Buprenorphine adherence and illicit opioid use among patients in treatment for opioid use disorder. Am J Drug Alcohol Abuse. 2023;49. doi:10.1080/00952990.2023.2220876
- Courtney RE, Halsey E, Patil T, Mastronardi KV, Browne HS, Darnall BD. Prescription opioid tapering practices and outcomes at a rural VA health care system. Pain Med. 2024;25:480-482. doi:10.1093/pm/pnae013
Rural veterans are prescribed long-term opioid therapy for chronic pain at higher rates than urban veterans, increasing their risk of developing opioid use disorder (OUD).1,2 Veterans with co-occurring OUD and chronic pain have more severe health concerns, as well as higher rates of homelessness, psychoactive drug misuse, and mental health disorders, compared to veterans with either chronic pain or OUD alone.3 Interdisciplinary team (IDT) care is recommended for both chronic pain and OUD.4,5 Rural veterans with co-occurring chronic pain and OUD, however, are often unable to access IDTs due to long travel and wait times. As a result, these rural veterans often receive care from primary care practitioners (PCPs) who lack training in pain management and addiction and have low confidence in their ability to provide optimal treatment.6,7
In the Veterans Health Administration, electronic consultations (e-consults) provide support to PCPs by recommending evidence-based approaches such as buprenorphine for OUD and pain IDTs for chronic pain.5,8 However, research on the use of e-consults to connect to IDT care for co-occurring chronic pain and OUD are lacking, as well as studies on IDTs using innovative methods (eg, shared appointments) to overcome treatment barriers (eg, multiple appointments) for rural veterans at higher risk for co-occurring OUD and chronic pain.
This quality improvement study sought to determine the feasibility and impact of a pharmacy e-consult service that provided pain medication recommendations and subsequent referrals to RESTORE, a shared appointment program with an IDT, for assessment and treatment of chronic pain and OUD.
Methods
This retrospective chart review was approved as nonresearch by the Institutional Review Board Chair at the Salem Veterans Affairs Healthcare System (SVAHS), a low-complexity medical center in Virginia that primarily serves a rural and highly rural Central Appalachian veteran population.
This study included veterans whose clinicians placed a pain medication e-consult requesting recommendations for medication adjustments and/or a referral to RESTORE from January 1, 2022, through January 6, 2023. Requests for services that could not be provided through an e-consult were excluded (Figure 1). Veterans who had a pain medication e-consult were identified in the SVAHS electronic medical record (EMR). Data extracted from the EMR included demographics, referral source, reason for referral, RESTORE appointment attendance, OUD diagnosis made during the RESTORE initial evaluation, implementation of medication recommendations by the referrer within 6 months, engagement in ≥ 3 pain education classes, and a shared appointment with a pain IDT within 6 months. Data were entered into a REDCap database, and descriptive statistics summarized the results. Feasibility was assessed by use of the e-consult by PCPs, attendance at the RESTORE appointment, and OUD diagnosis by the RESTORE team.

RESTORE Intervention
A pain medication e-consult was followed by referral to a shared appointment with the RESTORE IDT, with subsequent referrals to a pain IDT for chronic pain management if the veteran was amenable.
Pain medication e-consults in the EMR prompted a chart review by a clinical pharmacist practitioner (CPP). Recommendations for changes to medication regimens were documented in the EMR. At completion of the e-consult, the referring clinician received an automated view alert.
Veterans (and a support person, if preferred) were seen in a 60-minute, face-to-face shared appointment which included a psychologist, CPP, and pain physician. The psychologist conducted an OUD diagnostic interview, provided diagnostic feedback, and used motivational interviewing to provide psychoeducation on the biopsychosocial model of chronic pain, the IDT approach to chronic pain, and an overview of pain IDT care locally available. A CPP and physician then described medication options available to address OUD, if applicable. Together, the IDT and patient used shared decision making to determine a comprehensive treatment plan that may include a referral to the SVAHS PREVAIL Center for Chronic Pain IDT track (PREVAIL IDT track), a referral to substance use care in the case of polysubstance use, or medication initiation.9-11 If medication was prescribed, the patient was subsequently followed by the CPP through phone calls and face-to-face appointments at regularly scheduled intervals in coordination with the prescriber until they were stabilized. After stabilization, the prescription would be managed by their PCP (Figure 2). Veterans whose clinical condition changed significantly or worsened after returning to their PCP were invited to be reevaluated by the RESTORE team and restart care in that program. Individuals who were actively receiving RESTORE team care were discussed in a weekly care coordination meeting with all clinicians from both the PREVAIL and RESTORE teams.

Program Metrics
Pain medication e-consults were placed for 77 patients; 7 were excluded as inappropriate referral requests. Seventy (83%) e-consults were placed by PCPs (Table). Fifty-seven referring PCPs (81%) implemented ≥ 1 medication recommendation and 41 (59%) implemented all recommendations within 6 months. CPPs referred 19 individuals to RESTORE due to concerns related to high risk. All attended the initial evaluation appointment with the RESTORE team, 17 (89%) agreed to be referred to PREVAIL IDT track for nonpharmacologic pain care, and 9 (53%) engaged with that care within 6 months. Of those who attended RESTORE, 7 patients (37%) initiated buprenorphine for OUD with 6 (86%) being prescribed buprenorphine for ≥ 6 months.

Discussion
Most e-consults placed at SVAHS, which primarily serves a rural veteran population in Central Appalachia, resulted in veterans engaging in evidence-based treatment for co-occurring chronic pain and OUD. The use of e-consults and subsequent shared appointments with an IDT appears to be feasible, as the service was most often used by PCPs who often feel unequipped to manage chronic pain.7 The attendance rate for the RESTORE appointments was notable given the typically poor follow-up for patients with OUD. It supports the feasibility of a shared appointment approach which may overcome frequent barriers to care in this vulnerable population (ie, time, transportation). By attending 1 appointment with all clinicians present as opposed to multiple appointments, veterans experience fewer barriers than attending multiple appointments. RESTORE continues to be offered as an active clinical service whose implementation is now supported by changes to SVAHS policies. Since this study was conducted, the number of patients seen weekly has doubled and will soon be tripled based on high demand from PCPs.
While this study was limited to 1 site, had a small sample size, and was limited in scope, its results suggest that future research is warranted. Future studies using a larger sample size utilizing both a randomized control trial design and qualitative methods are needed to answer critical questions such as the role of patient characteristics on treatment effectiveness and the impact of the RESTORE model on long-term OUD medication adherence, patients’ perceptions and satisfaction, barriers to implementation, PCP confidence in providing pain care, and use of evidence-based nonpharmacologic pain management services.12-14
Conclusions
The results of this quality-improvement project suggest that e-consults may facilitate referrals to and patient follow-through with evidence-based treatment for co-occurring chronic pain and OUD among veterans living in rural communities in Central Appalachia who tend to experience significant barriers to traditional care and may require an innovative approach to facilitate effective treatment.
Rural veterans are prescribed long-term opioid therapy for chronic pain at higher rates than urban veterans, increasing their risk of developing opioid use disorder (OUD).1,2 Veterans with co-occurring OUD and chronic pain have more severe health concerns, as well as higher rates of homelessness, psychoactive drug misuse, and mental health disorders, compared to veterans with either chronic pain or OUD alone.3 Interdisciplinary team (IDT) care is recommended for both chronic pain and OUD.4,5 Rural veterans with co-occurring chronic pain and OUD, however, are often unable to access IDTs due to long travel and wait times. As a result, these rural veterans often receive care from primary care practitioners (PCPs) who lack training in pain management and addiction and have low confidence in their ability to provide optimal treatment.6,7
In the Veterans Health Administration, electronic consultations (e-consults) provide support to PCPs by recommending evidence-based approaches such as buprenorphine for OUD and pain IDTs for chronic pain.5,8 However, research on the use of e-consults to connect to IDT care for co-occurring chronic pain and OUD are lacking, as well as studies on IDTs using innovative methods (eg, shared appointments) to overcome treatment barriers (eg, multiple appointments) for rural veterans at higher risk for co-occurring OUD and chronic pain.
This quality improvement study sought to determine the feasibility and impact of a pharmacy e-consult service that provided pain medication recommendations and subsequent referrals to RESTORE, a shared appointment program with an IDT, for assessment and treatment of chronic pain and OUD.
Methods
This retrospective chart review was approved as nonresearch by the Institutional Review Board Chair at the Salem Veterans Affairs Healthcare System (SVAHS), a low-complexity medical center in Virginia that primarily serves a rural and highly rural Central Appalachian veteran population.
This study included veterans whose clinicians placed a pain medication e-consult requesting recommendations for medication adjustments and/or a referral to RESTORE from January 1, 2022, through January 6, 2023. Requests for services that could not be provided through an e-consult were excluded (Figure 1). Veterans who had a pain medication e-consult were identified in the SVAHS electronic medical record (EMR). Data extracted from the EMR included demographics, referral source, reason for referral, RESTORE appointment attendance, OUD diagnosis made during the RESTORE initial evaluation, implementation of medication recommendations by the referrer within 6 months, engagement in ≥ 3 pain education classes, and a shared appointment with a pain IDT within 6 months. Data were entered into a REDCap database, and descriptive statistics summarized the results. Feasibility was assessed by use of the e-consult by PCPs, attendance at the RESTORE appointment, and OUD diagnosis by the RESTORE team.

RESTORE Intervention
A pain medication e-consult was followed by referral to a shared appointment with the RESTORE IDT, with subsequent referrals to a pain IDT for chronic pain management if the veteran was amenable.
Pain medication e-consults in the EMR prompted a chart review by a clinical pharmacist practitioner (CPP). Recommendations for changes to medication regimens were documented in the EMR. At completion of the e-consult, the referring clinician received an automated view alert.
Veterans (and a support person, if preferred) were seen in a 60-minute, face-to-face shared appointment which included a psychologist, CPP, and pain physician. The psychologist conducted an OUD diagnostic interview, provided diagnostic feedback, and used motivational interviewing to provide psychoeducation on the biopsychosocial model of chronic pain, the IDT approach to chronic pain, and an overview of pain IDT care locally available. A CPP and physician then described medication options available to address OUD, if applicable. Together, the IDT and patient used shared decision making to determine a comprehensive treatment plan that may include a referral to the SVAHS PREVAIL Center for Chronic Pain IDT track (PREVAIL IDT track), a referral to substance use care in the case of polysubstance use, or medication initiation.9-11 If medication was prescribed, the patient was subsequently followed by the CPP through phone calls and face-to-face appointments at regularly scheduled intervals in coordination with the prescriber until they were stabilized. After stabilization, the prescription would be managed by their PCP (Figure 2). Veterans whose clinical condition changed significantly or worsened after returning to their PCP were invited to be reevaluated by the RESTORE team and restart care in that program. Individuals who were actively receiving RESTORE team care were discussed in a weekly care coordination meeting with all clinicians from both the PREVAIL and RESTORE teams.

Program Metrics
Pain medication e-consults were placed for 77 patients; 7 were excluded as inappropriate referral requests. Seventy (83%) e-consults were placed by PCPs (Table). Fifty-seven referring PCPs (81%) implemented ≥ 1 medication recommendation and 41 (59%) implemented all recommendations within 6 months. CPPs referred 19 individuals to RESTORE due to concerns related to high risk. All attended the initial evaluation appointment with the RESTORE team, 17 (89%) agreed to be referred to PREVAIL IDT track for nonpharmacologic pain care, and 9 (53%) engaged with that care within 6 months. Of those who attended RESTORE, 7 patients (37%) initiated buprenorphine for OUD with 6 (86%) being prescribed buprenorphine for ≥ 6 months.

Discussion
Most e-consults placed at SVAHS, which primarily serves a rural veteran population in Central Appalachia, resulted in veterans engaging in evidence-based treatment for co-occurring chronic pain and OUD. The use of e-consults and subsequent shared appointments with an IDT appears to be feasible, as the service was most often used by PCPs who often feel unequipped to manage chronic pain.7 The attendance rate for the RESTORE appointments was notable given the typically poor follow-up for patients with OUD. It supports the feasibility of a shared appointment approach which may overcome frequent barriers to care in this vulnerable population (ie, time, transportation). By attending 1 appointment with all clinicians present as opposed to multiple appointments, veterans experience fewer barriers than attending multiple appointments. RESTORE continues to be offered as an active clinical service whose implementation is now supported by changes to SVAHS policies. Since this study was conducted, the number of patients seen weekly has doubled and will soon be tripled based on high demand from PCPs.
While this study was limited to 1 site, had a small sample size, and was limited in scope, its results suggest that future research is warranted. Future studies using a larger sample size utilizing both a randomized control trial design and qualitative methods are needed to answer critical questions such as the role of patient characteristics on treatment effectiveness and the impact of the RESTORE model on long-term OUD medication adherence, patients’ perceptions and satisfaction, barriers to implementation, PCP confidence in providing pain care, and use of evidence-based nonpharmacologic pain management services.12-14
Conclusions
The results of this quality-improvement project suggest that e-consults may facilitate referrals to and patient follow-through with evidence-based treatment for co-occurring chronic pain and OUD among veterans living in rural communities in Central Appalachia who tend to experience significant barriers to traditional care and may require an innovative approach to facilitate effective treatment.
- Lund BC, Ohl ME, Hadlandsmyth K, et al. Regional and rural-urban variation in opioid prescribing in the Veterans Health Administration. Mil Med. 2019;184(11-12):894-900. doi:10.1093/milmed/usz104
- Edlund MJ, Martin BC, Russo JE, et al. The role of opioid prescription in incident opioid abuse and dependence among individuals with chronic noncancer pain: the role of opioid prescription. Clin J Pain. 2014;30(7):557-564. doi:10.1097/AJP.0000000000000021
- MacLean RR, Sofuoglu M, Stefanovics E, et al. Opioid use disorder with chronic pain increases disease burden and service use. Psychol Serv. 2023;20(1):157-165. doi:10.1037/ser0000607
- US Department of Veterans Affairs, US Department of Defense. VA/DoD clinical practice guidelines: use of opioids in the management of chronic pain. Version 4.0. Updated May 2022. Accessed August 4, 2025. https://www.healthquality.va.gov/guidelines/Pain/cot/VADoDOpioidsCPG.pdf
- US Department of Veterans Affairs, US Department of Defense. VA/DoD clinical practice guideline for the diagnosis and treatment of low back pain: the diagnosis and treatment of low back pain. Version 3.0. Updated February 2022. Accessed August 4, 2025. https://www.healthquality.va.gov/guidelines/Pain/lbp/VADoDLBPCPGFinal508.pdf
- Shipton EE, Bate F, Garrick R, et al. Systematic review of pain medicine content, teaching, and assessment in medical school curricula internationally. Pain Ther. 2018;7(2):139-161. doi:10.1007/s40122-018-0103-z
- Jamison RN, Scanlan E, Matthews ML, et al. Attitudes of primary care practitioners in managing chronic pain patients prescribed opioids for pain: a prospective longitudinal controlled trial. Pain Med. 2016;17(1):99-113. doi:10.1111/pme.12871
- Miller DM, Harvey TL. Pharmacist pain e-consults that result in a therapy change. Fed Pract. 2015;32(7):14-19.
- Courtney RE, Schadegg MJ. Chronic, noncancer pain care in the Veterans Administration: current trends and future directions. Anesthesiol Clin. 2023;41(2):519-529. doi:10.1016/j.anclin.2023.02.004
- Courtney RE, Schadegg MJ, Bolton R, et al. Using a whole health approach to build biopsychosocial-spiritual personal health plans for veterans with chronic pain. Pain Manag Nurs. 2024;25(1):69-74. doi:10.1016/j.pmn.2023.09.010
- Darnall BD, Edwards KA, Courtney RE, et al. Innovative treatment formats, technologies, and clinician trainings that improve access to behavioral pain treatment for youth and adults. Front Pain Res. 2023;4. doi:10.3389/fpain.2023.1223172
- Lister JJ, Weaver A, Ellis JD, et al. A systematic review of rural-specific barriers to medication treatment for opioid use disorder in the United States. Am J Drug Alcohol Abuse. 2020;46:273-288. doi:10.1080/00952990.2019.1694536
- Bhatraju EP, Radick AC, Leroux BG, et al. Buprenorphine adherence and illicit opioid use among patients in treatment for opioid use disorder. Am J Drug Alcohol Abuse. 2023;49. doi:10.1080/00952990.2023.2220876
- Courtney RE, Halsey E, Patil T, Mastronardi KV, Browne HS, Darnall BD. Prescription opioid tapering practices and outcomes at a rural VA health care system. Pain Med. 2024;25:480-482. doi:10.1093/pm/pnae013
- Lund BC, Ohl ME, Hadlandsmyth K, et al. Regional and rural-urban variation in opioid prescribing in the Veterans Health Administration. Mil Med. 2019;184(11-12):894-900. doi:10.1093/milmed/usz104
- Edlund MJ, Martin BC, Russo JE, et al. The role of opioid prescription in incident opioid abuse and dependence among individuals with chronic noncancer pain: the role of opioid prescription. Clin J Pain. 2014;30(7):557-564. doi:10.1097/AJP.0000000000000021
- MacLean RR, Sofuoglu M, Stefanovics E, et al. Opioid use disorder with chronic pain increases disease burden and service use. Psychol Serv. 2023;20(1):157-165. doi:10.1037/ser0000607
- US Department of Veterans Affairs, US Department of Defense. VA/DoD clinical practice guidelines: use of opioids in the management of chronic pain. Version 4.0. Updated May 2022. Accessed August 4, 2025. https://www.healthquality.va.gov/guidelines/Pain/cot/VADoDOpioidsCPG.pdf
- US Department of Veterans Affairs, US Department of Defense. VA/DoD clinical practice guideline for the diagnosis and treatment of low back pain: the diagnosis and treatment of low back pain. Version 3.0. Updated February 2022. Accessed August 4, 2025. https://www.healthquality.va.gov/guidelines/Pain/lbp/VADoDLBPCPGFinal508.pdf
- Shipton EE, Bate F, Garrick R, et al. Systematic review of pain medicine content, teaching, and assessment in medical school curricula internationally. Pain Ther. 2018;7(2):139-161. doi:10.1007/s40122-018-0103-z
- Jamison RN, Scanlan E, Matthews ML, et al. Attitudes of primary care practitioners in managing chronic pain patients prescribed opioids for pain: a prospective longitudinal controlled trial. Pain Med. 2016;17(1):99-113. doi:10.1111/pme.12871
- Miller DM, Harvey TL. Pharmacist pain e-consults that result in a therapy change. Fed Pract. 2015;32(7):14-19.
- Courtney RE, Schadegg MJ. Chronic, noncancer pain care in the Veterans Administration: current trends and future directions. Anesthesiol Clin. 2023;41(2):519-529. doi:10.1016/j.anclin.2023.02.004
- Courtney RE, Schadegg MJ, Bolton R, et al. Using a whole health approach to build biopsychosocial-spiritual personal health plans for veterans with chronic pain. Pain Manag Nurs. 2024;25(1):69-74. doi:10.1016/j.pmn.2023.09.010
- Darnall BD, Edwards KA, Courtney RE, et al. Innovative treatment formats, technologies, and clinician trainings that improve access to behavioral pain treatment for youth and adults. Front Pain Res. 2023;4. doi:10.3389/fpain.2023.1223172
- Lister JJ, Weaver A, Ellis JD, et al. A systematic review of rural-specific barriers to medication treatment for opioid use disorder in the United States. Am J Drug Alcohol Abuse. 2020;46:273-288. doi:10.1080/00952990.2019.1694536
- Bhatraju EP, Radick AC, Leroux BG, et al. Buprenorphine adherence and illicit opioid use among patients in treatment for opioid use disorder. Am J Drug Alcohol Abuse. 2023;49. doi:10.1080/00952990.2023.2220876
- Courtney RE, Halsey E, Patil T, Mastronardi KV, Browne HS, Darnall BD. Prescription opioid tapering practices and outcomes at a rural VA health care system. Pain Med. 2024;25:480-482. doi:10.1093/pm/pnae013
E-Consults Bridge to Interdisciplinary Team Care for Rural Appalachian Veterans With Chronic Pain and Opioid Use Disorder
E-Consults Bridge to Interdisciplinary Team Care for Rural Appalachian Veterans With Chronic Pain and Opioid Use Disorder
Impact of Multisite Patient Education on Pharmacotherapy for Veterans With Alcohol Use Disorder
Impact of Multisite Patient Education on Pharmacotherapy for Veterans With Alcohol Use Disorder
Excessive alcohol use is one of the leading preventable causes of death in the United States, responsible for about 178,000 deaths annually and an average of 488 daily deaths in 2020 and 2021.1Alcohol-related deaths increased by 49% between 2006 and 2019.2 This trend continued during the COVID-19 pandemic, with death certificates that listed alcohol increasing by > 25% from 2019 to 2020, and another 10% in 2021.3 This increase of alcohol-related deaths includes those as a direct result of chronic alcohol use, such as alcoholic cardiomyopathy, alcoholic hepatitis and cirrhosis, and alcohol-induced pancreatitis, as well as a result of acute use such as alcohol poisoning, suicide by exposure to alcohol, and alcohol-impaired driving fatalities.4
Excessive alcohol consumption poses other serious risks, including cases when intake is abruptly reduced without proper management. Alcohol withdrawal syndrome (AWS) can vary in severity, with potentially life-threatening complications such as hallucinations, seizures, and delirium tremens.5
These risks highlight the importance of professional intervention and support, not only to mitigate risks associated with AWS, but provide a pathway towards recovery from alcohol use disorder (AUD).
According to the 2022 National Survey on Drug Use and Health, 28.8 million US adults had AUD in the prior year, yet only 7.6% of these individuals received treatment and an even smaller group (2.2%) received medication-assisted treatment for alcohol.6,7 This is despite American Psychiatric Association guidelines for the pharmacological treatment of patients with AUD, including the use of naltrexone, acamprosate, disulfiram, topiramate, or gabapentin, depending on therapy goals, past medication trials, medication contraindications, and patient preference.8 Several of these medications are approved by the US Food and Drug Administration (FDA) for the treatment of AUD and have support for effectiveness from randomized controlled trials and meta-analyses.9-11
Clinical practice guidelines for the management of substance use disorders (SUDs) from the US Department of Veterans Affairs (VA) and US Department of Defense have strong recommendations for naltrexone and topiramate as first-line pharmacotherapies for moderate to severe AUD. Acamprosate and disulfiram are weak recommendations as alternative options. Gabapentin is a weak recommendation for cases where first-line treatments are contraindicated or ineffective. The guidelines emphasize the importance of a comprehensive approach to AUD treatment, including psychosocial interventions in addition to pharmacotherapy.12
A 2023 national survey found veterans reported higher alcohol consumption than nonveterans.13 At the end of fiscal year 2023, > 4.4 million veterans—6% of Veterans Health Administration patients—had been diagnosed with AUD.14 However, > 87% of these patients nationally, and 88% of Veterans Integrated Service Network (VISN) 21 patients, were not receiving naltrexone, acamprosate, disulfiram, or topiramate as part of their treatment. The VA Academic Detailing Service (ADS) now includes AUD pharmacotherapy as a campaign focus, highlighting its importance. The ADS is a pharmacy educational outreach program that uses unbiased clinical guidelines to promote aligning prescribing behavior with best practices. Academic detailing methods include speaking with health care practitioners (HCPs), and direct-to-consumer (DTC) patient education.
ADS campaigns include DTC educational handouts. Past ADS projects and research using DTC have demonstrated a significant improvement in outcomes and positively influencing patients’ pharmacotherapy treatment. 15,16 A VA quality improvement project found a positive correlation between the initiation of AUD pharmacotherapy and engagement with mental health care following the distribution of AUD DTC patient education. 17 This project aimed to apply the same principles of prior research to explore the use of DTC across multiple facilities within VISN 21 to increase AUD pharmacotherapy. VISN 21 includes VA facilities and clinics across the Pacific Islands, Nevada, and California and serves about 350,000 veterans.
METHODS
A prospective cohort of VISN 21 veterans with or at high risk for AUD was identified using the VA ADS AUD Dashboard. The cohort included those not on acamprosate, disulfiram, naltrexone, topiramate, or gabapentin for treatment of AUD and had an elevated Alcohol Use Disorder Identification Test-Consumption (AUDIT-C) score of ≥ 6 (high risk) with an AUD diagnosis or ≥ 8 (severe risk) without a diagnosis. The AUDIT-C scores used in the dashboard are supported by the VA AUD clinician guide as the minimum scores when AUD pharmacotherapy should be offered to patients.18 Prescriptions filled outside the VA were not included in this dashboard.
Data and patient information were collected using the VA Corporate Data Warehouse. To be eligible, veterans needed a valid mailing address within the VISN 21 region and a primary care, mental health, or SUD clinician prescriber visit scheduled between October 1, 2023, and January 31, 2024. Veterans were excluded if they were in hospice, had a 1-year mortality risk score > 50% based on their Care Assessment Need (CAN) score, or facility leadership opted out of project involvement. Patients with both severe renal and hepatic impairments were excluded because they were ineligible for AUD pharmacotherapy. However, veterans with either renal or hepatic impairment (but not both) were included, as they could be potential candidates for ≥ 1 AUD pharmacotherapy option.
Initial correspondence with facilities was initiated through local academic detailers. A local champion was identified for the 1 facility without an academic detailer. Facilities could opt in or out of the project. Approval was provided by the local pharmacy and therapeutics committee, pharmacy, primary care, or psychiatry leadership. Approval process and clinician involvement varied by site.
Education
The selected AUD patient education was designed and approved by the national VA ADS (eappendix). The DTC patient education provided general knowledge about alcohol, including what constitutes a standard amount of alcohol, what is considered heavy drinking, risks of heavy drinking, creating a plan with a clinician to reduce and manage withdrawal symptoms, and additional resources. The DTC was accompanied by a cover letter that included a local facility contact number.
A centralized mailing facility was used for all materials. VA Northern California Health Care System provided the funding to cover the cost of postage. The list of veterans to be contacted was updated on a rolling basis and DTC education was mailed 2 weeks prior to their scheduled prescriber visit.
The eligible cohort of 1260 veterans received DTC education. A comparator group of 2048 veterans that did not receive DTC education was obtained retrospectively by using the same inclusion and exclusion criteria with a scheduled primary care, mental health, or SUD HCP visit from October 1, 2022, to January 31, 2023. The outcomes assessed were within 30 days of the scheduled visit, with the primary outcome as the initiation of AUD-related pharmacotherapy and the secondary outcome as the placement of a consultation for mental health or SUD services. Any consultations sent to Behavioral Health, Addiction, Mental Health, Psychiatric, and SUD services following the HCP visit, within the specified time frame, were used for the secondary outcome.
Matching and Analysis
A 1-to-1 nearest neighbor propensity score (PS) matching without replacement was used to pair the 1260 veterans from the intervention group with similarly scored comparator group veterans for a PS-matched final dataset of 2520 veterans. The PS model was a multivariate logistic regression with the outcome being exposure and comparator group status. Baseline characteristics used in the PS model were age, birth sex, race, facility of care, baseline AUDIT-C score, and days between project start and scheduled appointment. Covariate imbalance for the PS-matched sample was assessed to ensure the standardized mean difference for all covariates fell under a 0.1 threshold (Figure).19
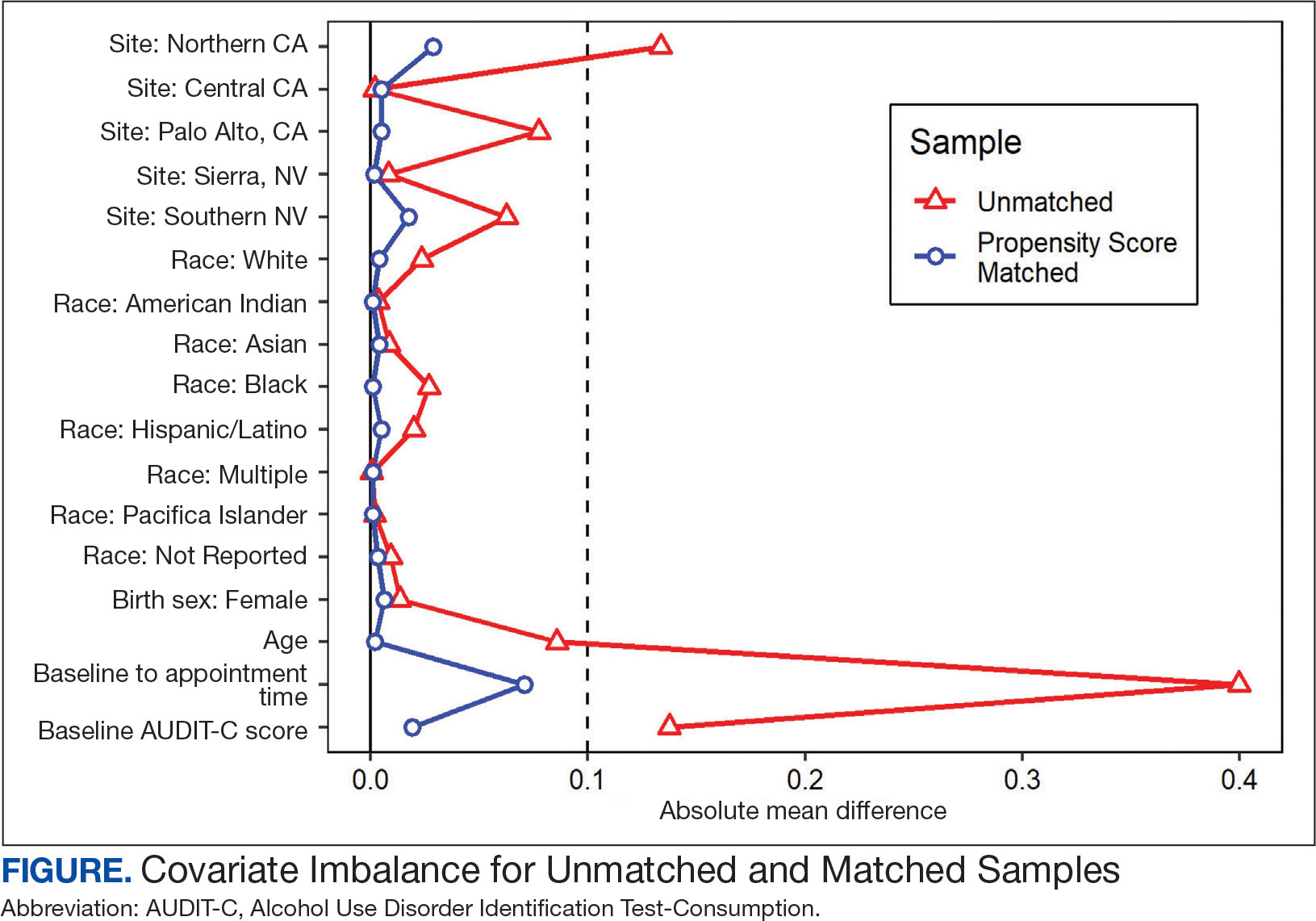
A frequency table was provided to compare the discrete distributions of the baseline characteristics in the intervention and comparator groups. Logistic regression analysis was performed to evaluate the association between DTC education exposure and pharmacotherapy initiation, while controlling for potential confounders. Univariate and multivariate P value results for each variable included in the model were reported along with the multivariate odds ratios (ORs) and their associated 95% CIs. Logistic regression analyses were run for both outcomes. Each model included the exposure and comparator group status as well as the baseline characteristics included in the PS model. Statistical significance was set at P < .05. All statistical analyses were performed with R version 4.2.1.
RESULTS
Two of 7 VISN 21 sites did not participate, and 3 had restrictions on participation. DTC education was mailed about 2 weeks prior to scheduled visit for 1260 veterans; 53.6% identified as White, 37.6% were aged 41 to 60 years, and 79.2% had an AUDIT-C ≥ 8 (Table 1). Of those mailed education, there were 173 no-show appointments (13.7%). Thirty-two veterans (2.5%) in the DTC group and 33 veterans (2.6%) in the comparator group received an AUD-related pharmacotherapy prescription (P = .88) (Table 2). One hundred seventy-one veterans (13.6%) in the DTC group and 160 veterans (12.7%) in the comparator group had a consult placed for mental health or SUD services within 30 days of their appointment (P = .59) (Table 3).
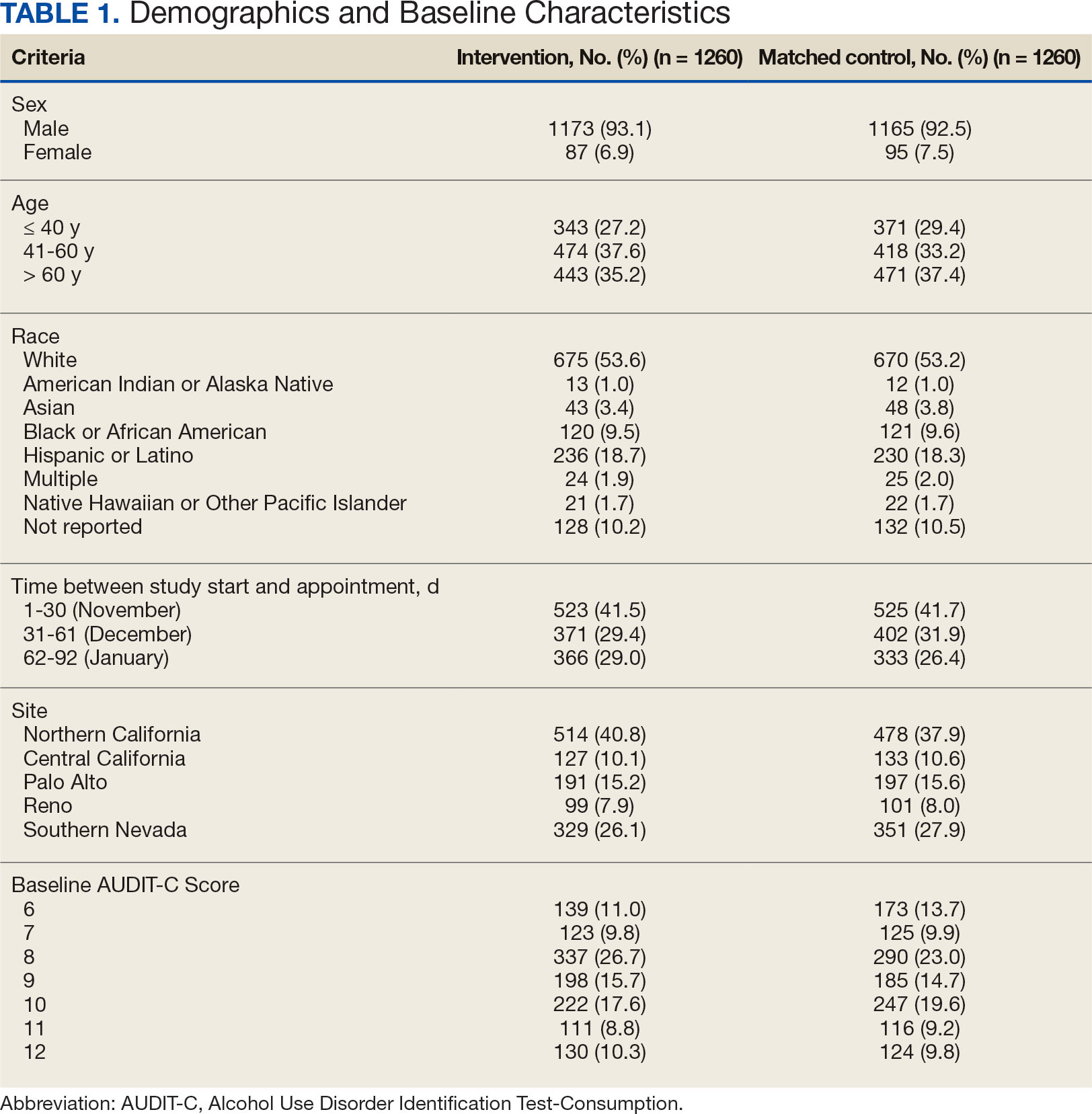
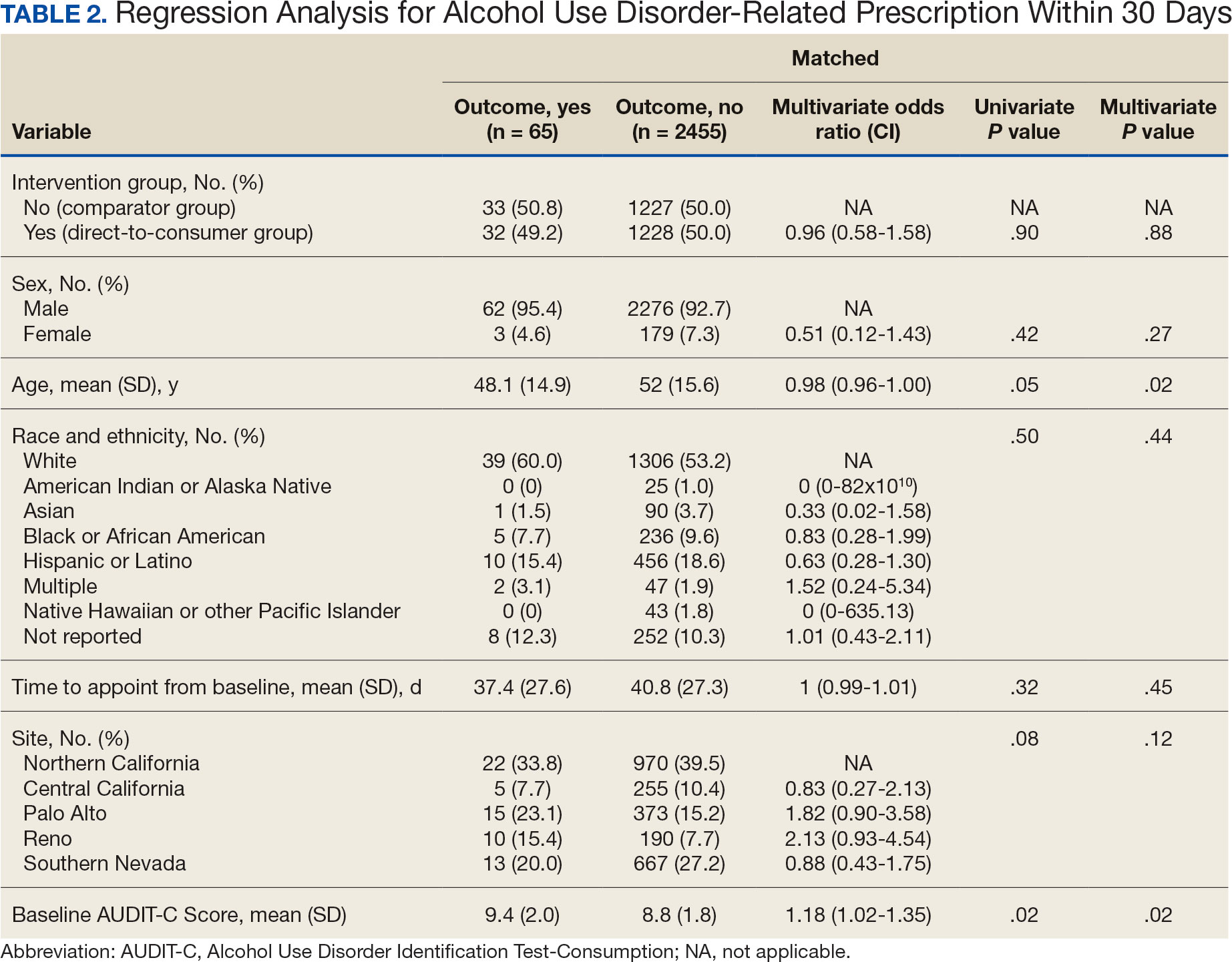
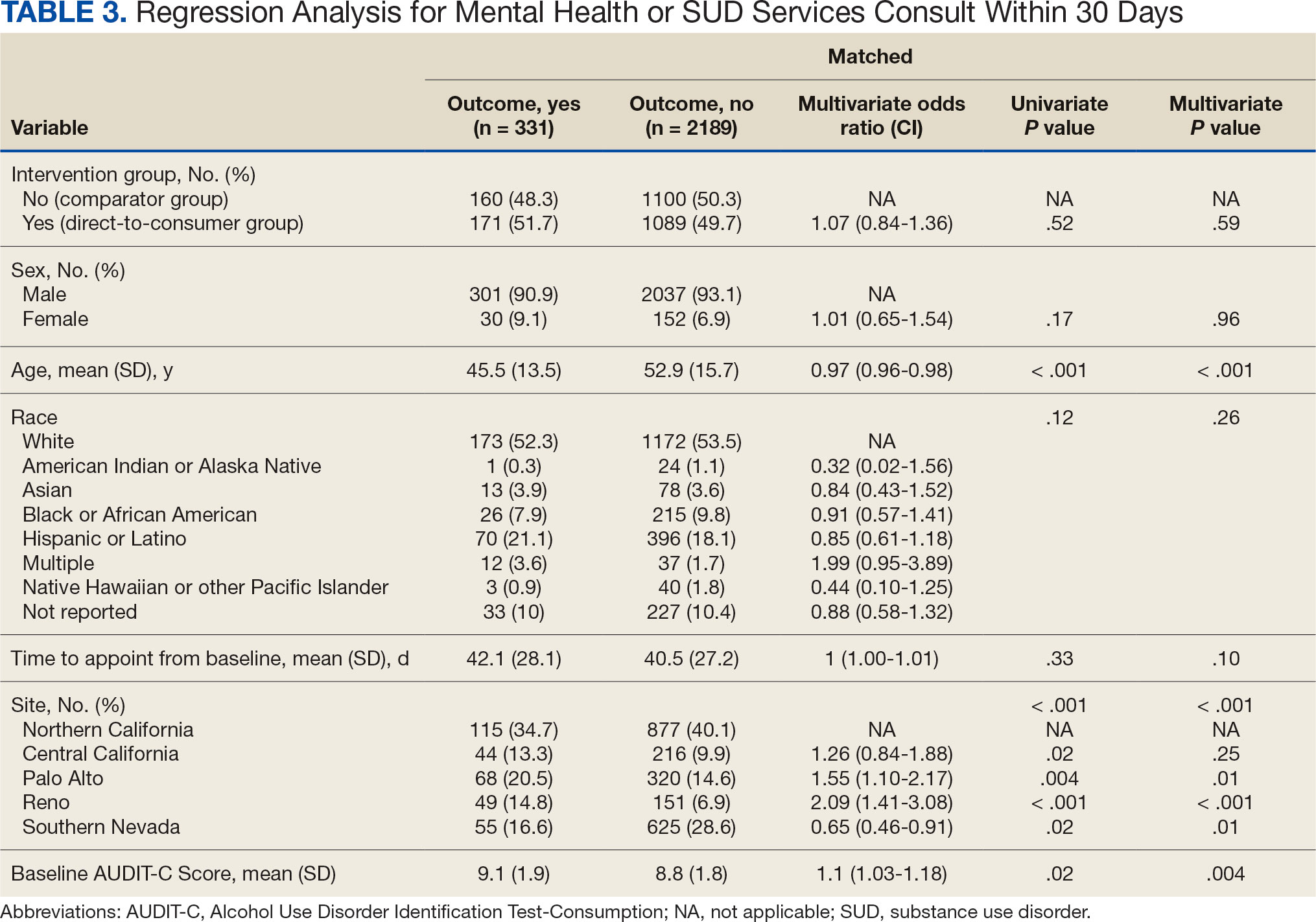
DISCUSSION
This project did not yield statistically significant differences in either the primary or secondary outcomes within the 30-day follow-up window and found limited impact from the DTC educational outreach to veterans. The percentage of veterans that received AUD-related pharmacotherapy or consultations for mental health or SUD services was similarly low in the DTC and comparator groups. These findings suggest that although DTC education may raise awareness, it may not be sufficient on its own to drive changes in prescribing behavior or referral patterns without system-level support.
Addiction is a complex disease faced with stigma and requiring readiness by both the HCP and patient to move forward in support and treatment. The consequences of stigma can be severe: the more stigma perceived by a person with AUD, the less likely they are to seek treatment.20 Stigma may exist even within HCPs and may lead to compromised care including shortened visits, less engagement, and less empathy.19 Cultural attitude towards alcohol use and intoxication can also be influenced through a wide range of sources including social media, movies, music, and television. Studies have shown targeted alcohol marketing may result in the development of positive beliefs about drinking and expand environments where alcohol use is socially acceptable and encouraged.21 These factors can impact drinking behavior, including the onset of drinking, binge drinking, and increased alcohol consumption.22
Three VISN 21 sites in this study had restrictions on or excluded primary care from participation. Leadership at some of these facilities were concerned that primary care teams did not have the bandwidth to take on additional items and/or there was variable primary care readiness for initiating AUD pharmacotherapy. Further attempts should be made to integrate primary care into the process of initiating AUD treatment as significant research suggests that integrated care models for AUD may be associated with improved process and outcome measures of care.23
There are several differences between this quality improvement project and prior research investigating the impact of DTC education for other conditions, such as the EMPOWER randomized controlled trial and VISN 22 project, which both demonstrated effectiveness of DTC education for reducing benzodiazepine use in geriatric veterans. 15,16 These studies focused on reducing or stopping pharmacotherapy use, whereas this project sought to promote the initiation of AUD pharmacotherapy. These studies evaluated outcomes at least 6 months postindex date, whereas this project evaluated outcomes within 30 days postappointment. Furthermore, the educational content varied significantly. Other projects provided patients with information focused on specific medications and interventions, such as benzodiazepine tapering, while this project mailed general information on heavy drinking, its risks, and strategies for cutting back, without mentioning pharmacotherapy. The DTC material used in this project was chosen because it was a preapproved national VA ADS resource, which expedited the project timeline by avoiding the need for additional approvals at each participating site. These differences may impact the observed effectiveness of DTC education in this project, especially regarding the primary outcome.
Strengths and Limitations
This quality improvement project sent a large sample of veterans DTC education in a clinical setting across multiple sites. Additionally, PS matching methods were used to balance covariates between the comparator and DTC education group, thereby simulating a randomized controlled trial and reducing selection bias. The project brought attention to the VISN 21 AUD treatment rates, stimulated conversation across sites about available treatments and resources for AUD, and sparked collaboration between academic detailing, mental health, and primary care services. The time frame for visits was selected during the winter; the National Institute on Alcohol Abuse and Alcoholism notes this is a time when people may be more likely to engage in excessive alcohol consumption than at other times of the year.24
The 30-day time frame for outcomes may have been too short to observe changes in prescribing or referral patterns. Additionally, the comparator group was comprised of veterans seen from October 1, 2022, to January 31, 2023, where seasonal timing may have influenced alcohol consumption behaviors and skewed the results. There were also no-show appointments in the DTC education group (13.7%), though it is likely some patients rescheduled and still received AUD pharmacotherapy within 30 days of the original appointment. Finally, it was not possible to confirm whether a patient opened and read the education that was mailed to them. This may be another reason to explore electronic distribution of DTC education. This all may have contributed to the lack of statistically significant differences in both the primary and secondary outcomes.
There was a high level of variability between facility participation in the project. Two of 7 sites did not participate, and 3 sites restricted primary care engagement. This represents a significant limitation, particularly for the secondary outcome of placing consultations for MH or SUD services. Facilities that only included mental health or SUD HCPs may have resulted in lower consultation rates due to their inherent specialization, reducing the likelihood of self-referrals.
The project may overestimate prescribed AUD pharmacotherapy in the primary outcome due to potential misclassification of medications. While the project adhered to the national VA ADS AUD dashboard’s definition of AUD pharmacotherapy, including acamprosate, disulfiram, naltrexone, topiramate, and gabapentin, some of these medications have multiple indications. For example, gabapentin is commonly prescribed for peripheral neuropathy, and topiramate is used to treat migraines and seizures. The multipurpose use adds uncertainty about whether they were prescribed specifically for AUD treatment, especially in cases where the HCP is responsible for treating a broad range of disease states, as in primary care.
CONCLUSIONS
Results of this quality improvement project did not show a statistically significant difference between patients sent DTC education and the comparator group for the initiation of AUD pharmacotherapy or placement of a consult to mental health or SUD services within 30 days of their scheduled visit. Future studies may seek to implement stricter criteria to confirm the intended use of topiramate and gabapentin, such as looking for keywords in the prescription instructions for use, performing chart reviews, and/or only including these medications if prescribed by a mental health or SUD HCP. Alternatively, future studies may consider limiting the analysis to only FDA-approved AUD medications: acamprosate, disulfiram, and naltrexone. It is vital to continue to enhance primary care HCP readiness to treat AUD, given the existing relationships and trust they often have with patients. Electronic methods for distributing DTC education could also be advantageous, as these methods may have the ability to track whether a message has been opened and read. Despite a lack of statistical significance, this project sparked crucial conversations and collaboration around AUD, available treatments, and addressing potential barriers to connecting patients to care within VISN 21.
- Centers for Disease Control and Prevention. Facts about U.S. deaths from excessive alcohol use. August 6, 2024. Accessed February 5, 2025. https://www.cdc.gov/alcohol/facts-stats/
- State Health Access Data Assistance Center. Escalating alcohol-involved death rates: trends and variation across the nation and in the states from 2006 to 2019. April 19, 2021. Accessed February 5, 2025. https://www.shadac.org/escalating-alcohol-involved-death-rates-trends-and-variation-across-nation-and-states-2006-2019
- National Institute on Alcohol Abuse and Alcoholism. Alcohol- related emergencies and deaths in the United States. Updated November 2024. Accessed February 5, 2025. https://www.niaaa.nih.gov/alcohols-effects-health/alcohol-topics/alcohol-facts-and-statistics/alcohol-related-emergencies-and-deaths-united-states
- Esser MB, Sherk A, Liu Y, Naimi TS. Deaths from excessive alcohol use - United States, 2016- 2021. MMWR Morb Mortal Wkly Rep. 2024;73(8):154-161. doi:10.15585/mmwr.mm7308a1
- Canver BR, Newman RK, Gomez AE. Alcohol Withdrawal Syndrome. In: StatPearls. StatPearls Publishing; 2024.
- National Institute on Alcohol Abuse and Alcoholism. Alcohol treatment in the United States. Updated January 2025. Accessed February 5, 2025. https://www.niaaa.nih.gov/alcohols-effects-health/alcohol-topics/alcohol-facts-and-statistics/alcohol-treatment-united-states
- National Institute on Alcohol Abuse and Alcoholism. Alcohol use disorder (AUD) in the United States: age groups and demographic characteristics. Updated September 2024. Accessed February 5, 2025. https://www.niaaa.nih.gov/alcohols-effects-health/alcohol-topics/alcohol-facts-and-statistics/alcohol-use-disorder-aud-united-states-age-groups-and-demographic-characteristics
- Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association practice guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018;175(1):86-90. doi:10.1176/appi.ajp.2017.1750101
- Blodgett JC, Del Re AC, Maisel NC, Finney JW. A meta-analysis of topiramate’s effects for individuals with alcohol use disorders. Alcohol Clin Exp Res. 2014;38(6):1481-1488. doi:10.1111/acer.12411
- Maisel NC, Blodgett JC, Wilbourne PL, Humphreys K, Finney JW. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108(2):275-293. doi:10.1111/j.1360-0443.2012.04054.x
- Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014;311(18):1889-1900. doi:10.1001/jama.2014.3628
- US Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for the management of substance use disorders. August 2021. Accessed February 5, 2025. https://www.healthquality.va.gov/guidelines/MH/sud/VADODSUDCPG.pdf
- Ranney RM, Bernhard PA, Vogt D, et al. Alcohol use and treatment utilization in a national sample of veterans and nonveterans. J Subst Use Addict Treat. 2023;146:208964. doi:10.1016/j.josat.2023.208964
- US Department of Veterans Affairs, Pharmacy Benefit Management Service, Academic Detailing Service. AUD Trend Report. https://vaww.pbi.cdw.va.gov/PBIRS/Pages/ReportViewer.aspx?/GPE/PBM_AD/SSRS/AUD/AUD_TrendReport
- Mendes MA, Smith JP, Marin JK, et al. Reducing benzodiazepine prescribing in older veterans: a direct-to-consumer educational brochure. Fed Pract. 2018;35(9):36-43.
- Tannenbaum C, Martin P, Tamblyn R, Benedetti A, Ahmed S. Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: the EMPOWER cluster randomized trial. JAMA Intern Med. 2014;174(6):890-898. doi:10.1001/jamainternmed.2014.949
- Maloney R, Funmilayo M. Acting on the AUDIT-C: implementation of direct-to-consumer education on unhealth alcohol use. Presented on March 31, 2023; Central Virginia Veterans Affairs Health Care System, Richmond, Virginia.
- US Department of Veterans Affairs, Pharmacy Benefit Management Service. Alcohol use disorder (AUD) – leading the charge in the treatment of AUD: a VA clinician’s guide. February 2022. Accessed February 5, 2025. https://www.pbm.va.gov/PBM/AcademicDetailingService/Documents/508/10-1530_AUD_ClinicianGuide_508Conformant.pdf
- Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399-424. doi:10.1080/00273171.2011.568786
- National Institute on Alcohol Abuse and Alcoholism. Stigma: overcoming a pervasive barrier to optimal care. Updated January 6, 2025. Accessed February 5, 2025. https://www.niaaa.nih.gov/health-professionals-communities/core-resource-on-alcohol/stigma-overcoming-pervasive-barrier-optimal-care
- Sudhinaraset M, Wigglesworth C, Takeuchi DT. Social and cultural contexts of alcohol use: influences in a socialecological framework. Alcohol Res. 2016;38(1):35-45.
- Tanski SE, McClure AC, Li Z, et al. Cued recall of alcohol advertising on television and underage drinking behavior. JAMA Pediatr. 2015;169(3):264-271. doi:10.1001/jamapediatrics.2014.3345
- Hyland CJ, McDowell MJ, Bain PA, Huskamp HA, Busch AB. Integration of pharmacotherapy for alcohol use disorder treatment in primary care settings: a scoping review. J Subst Abuse Treat. 2023;144:108919. doi:10.1016/j.jsat.2022.108919
- National Institute on Alcohol Abuse and Alcoholism. The truth about holiday spirits. Updated November 2023. Accessed February 5, 2025. ,a href="https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/truth-about-holiday-spirits">https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/truth-about-holiday-spirits
Excessive alcohol use is one of the leading preventable causes of death in the United States, responsible for about 178,000 deaths annually and an average of 488 daily deaths in 2020 and 2021.1Alcohol-related deaths increased by 49% between 2006 and 2019.2 This trend continued during the COVID-19 pandemic, with death certificates that listed alcohol increasing by > 25% from 2019 to 2020, and another 10% in 2021.3 This increase of alcohol-related deaths includes those as a direct result of chronic alcohol use, such as alcoholic cardiomyopathy, alcoholic hepatitis and cirrhosis, and alcohol-induced pancreatitis, as well as a result of acute use such as alcohol poisoning, suicide by exposure to alcohol, and alcohol-impaired driving fatalities.4
Excessive alcohol consumption poses other serious risks, including cases when intake is abruptly reduced without proper management. Alcohol withdrawal syndrome (AWS) can vary in severity, with potentially life-threatening complications such as hallucinations, seizures, and delirium tremens.5
These risks highlight the importance of professional intervention and support, not only to mitigate risks associated with AWS, but provide a pathway towards recovery from alcohol use disorder (AUD).
According to the 2022 National Survey on Drug Use and Health, 28.8 million US adults had AUD in the prior year, yet only 7.6% of these individuals received treatment and an even smaller group (2.2%) received medication-assisted treatment for alcohol.6,7 This is despite American Psychiatric Association guidelines for the pharmacological treatment of patients with AUD, including the use of naltrexone, acamprosate, disulfiram, topiramate, or gabapentin, depending on therapy goals, past medication trials, medication contraindications, and patient preference.8 Several of these medications are approved by the US Food and Drug Administration (FDA) for the treatment of AUD and have support for effectiveness from randomized controlled trials and meta-analyses.9-11
Clinical practice guidelines for the management of substance use disorders (SUDs) from the US Department of Veterans Affairs (VA) and US Department of Defense have strong recommendations for naltrexone and topiramate as first-line pharmacotherapies for moderate to severe AUD. Acamprosate and disulfiram are weak recommendations as alternative options. Gabapentin is a weak recommendation for cases where first-line treatments are contraindicated or ineffective. The guidelines emphasize the importance of a comprehensive approach to AUD treatment, including psychosocial interventions in addition to pharmacotherapy.12
A 2023 national survey found veterans reported higher alcohol consumption than nonveterans.13 At the end of fiscal year 2023, > 4.4 million veterans—6% of Veterans Health Administration patients—had been diagnosed with AUD.14 However, > 87% of these patients nationally, and 88% of Veterans Integrated Service Network (VISN) 21 patients, were not receiving naltrexone, acamprosate, disulfiram, or topiramate as part of their treatment. The VA Academic Detailing Service (ADS) now includes AUD pharmacotherapy as a campaign focus, highlighting its importance. The ADS is a pharmacy educational outreach program that uses unbiased clinical guidelines to promote aligning prescribing behavior with best practices. Academic detailing methods include speaking with health care practitioners (HCPs), and direct-to-consumer (DTC) patient education.
ADS campaigns include DTC educational handouts. Past ADS projects and research using DTC have demonstrated a significant improvement in outcomes and positively influencing patients’ pharmacotherapy treatment. 15,16 A VA quality improvement project found a positive correlation between the initiation of AUD pharmacotherapy and engagement with mental health care following the distribution of AUD DTC patient education. 17 This project aimed to apply the same principles of prior research to explore the use of DTC across multiple facilities within VISN 21 to increase AUD pharmacotherapy. VISN 21 includes VA facilities and clinics across the Pacific Islands, Nevada, and California and serves about 350,000 veterans.
METHODS
A prospective cohort of VISN 21 veterans with or at high risk for AUD was identified using the VA ADS AUD Dashboard. The cohort included those not on acamprosate, disulfiram, naltrexone, topiramate, or gabapentin for treatment of AUD and had an elevated Alcohol Use Disorder Identification Test-Consumption (AUDIT-C) score of ≥ 6 (high risk) with an AUD diagnosis or ≥ 8 (severe risk) without a diagnosis. The AUDIT-C scores used in the dashboard are supported by the VA AUD clinician guide as the minimum scores when AUD pharmacotherapy should be offered to patients.18 Prescriptions filled outside the VA were not included in this dashboard.
Data and patient information were collected using the VA Corporate Data Warehouse. To be eligible, veterans needed a valid mailing address within the VISN 21 region and a primary care, mental health, or SUD clinician prescriber visit scheduled between October 1, 2023, and January 31, 2024. Veterans were excluded if they were in hospice, had a 1-year mortality risk score > 50% based on their Care Assessment Need (CAN) score, or facility leadership opted out of project involvement. Patients with both severe renal and hepatic impairments were excluded because they were ineligible for AUD pharmacotherapy. However, veterans with either renal or hepatic impairment (but not both) were included, as they could be potential candidates for ≥ 1 AUD pharmacotherapy option.
Initial correspondence with facilities was initiated through local academic detailers. A local champion was identified for the 1 facility without an academic detailer. Facilities could opt in or out of the project. Approval was provided by the local pharmacy and therapeutics committee, pharmacy, primary care, or psychiatry leadership. Approval process and clinician involvement varied by site.
Education
The selected AUD patient education was designed and approved by the national VA ADS (eappendix). The DTC patient education provided general knowledge about alcohol, including what constitutes a standard amount of alcohol, what is considered heavy drinking, risks of heavy drinking, creating a plan with a clinician to reduce and manage withdrawal symptoms, and additional resources. The DTC was accompanied by a cover letter that included a local facility contact number.
A centralized mailing facility was used for all materials. VA Northern California Health Care System provided the funding to cover the cost of postage. The list of veterans to be contacted was updated on a rolling basis and DTC education was mailed 2 weeks prior to their scheduled prescriber visit.
The eligible cohort of 1260 veterans received DTC education. A comparator group of 2048 veterans that did not receive DTC education was obtained retrospectively by using the same inclusion and exclusion criteria with a scheduled primary care, mental health, or SUD HCP visit from October 1, 2022, to January 31, 2023. The outcomes assessed were within 30 days of the scheduled visit, with the primary outcome as the initiation of AUD-related pharmacotherapy and the secondary outcome as the placement of a consultation for mental health or SUD services. Any consultations sent to Behavioral Health, Addiction, Mental Health, Psychiatric, and SUD services following the HCP visit, within the specified time frame, were used for the secondary outcome.
Matching and Analysis
A 1-to-1 nearest neighbor propensity score (PS) matching without replacement was used to pair the 1260 veterans from the intervention group with similarly scored comparator group veterans for a PS-matched final dataset of 2520 veterans. The PS model was a multivariate logistic regression with the outcome being exposure and comparator group status. Baseline characteristics used in the PS model were age, birth sex, race, facility of care, baseline AUDIT-C score, and days between project start and scheduled appointment. Covariate imbalance for the PS-matched sample was assessed to ensure the standardized mean difference for all covariates fell under a 0.1 threshold (Figure).19

A frequency table was provided to compare the discrete distributions of the baseline characteristics in the intervention and comparator groups. Logistic regression analysis was performed to evaluate the association between DTC education exposure and pharmacotherapy initiation, while controlling for potential confounders. Univariate and multivariate P value results for each variable included in the model were reported along with the multivariate odds ratios (ORs) and their associated 95% CIs. Logistic regression analyses were run for both outcomes. Each model included the exposure and comparator group status as well as the baseline characteristics included in the PS model. Statistical significance was set at P < .05. All statistical analyses were performed with R version 4.2.1.
RESULTS
Two of 7 VISN 21 sites did not participate, and 3 had restrictions on participation. DTC education was mailed about 2 weeks prior to scheduled visit for 1260 veterans; 53.6% identified as White, 37.6% were aged 41 to 60 years, and 79.2% had an AUDIT-C ≥ 8 (Table 1). Of those mailed education, there were 173 no-show appointments (13.7%). Thirty-two veterans (2.5%) in the DTC group and 33 veterans (2.6%) in the comparator group received an AUD-related pharmacotherapy prescription (P = .88) (Table 2). One hundred seventy-one veterans (13.6%) in the DTC group and 160 veterans (12.7%) in the comparator group had a consult placed for mental health or SUD services within 30 days of their appointment (P = .59) (Table 3).



DISCUSSION
This project did not yield statistically significant differences in either the primary or secondary outcomes within the 30-day follow-up window and found limited impact from the DTC educational outreach to veterans. The percentage of veterans that received AUD-related pharmacotherapy or consultations for mental health or SUD services was similarly low in the DTC and comparator groups. These findings suggest that although DTC education may raise awareness, it may not be sufficient on its own to drive changes in prescribing behavior or referral patterns without system-level support.
Addiction is a complex disease faced with stigma and requiring readiness by both the HCP and patient to move forward in support and treatment. The consequences of stigma can be severe: the more stigma perceived by a person with AUD, the less likely they are to seek treatment.20 Stigma may exist even within HCPs and may lead to compromised care including shortened visits, less engagement, and less empathy.19 Cultural attitude towards alcohol use and intoxication can also be influenced through a wide range of sources including social media, movies, music, and television. Studies have shown targeted alcohol marketing may result in the development of positive beliefs about drinking and expand environments where alcohol use is socially acceptable and encouraged.21 These factors can impact drinking behavior, including the onset of drinking, binge drinking, and increased alcohol consumption.22
Three VISN 21 sites in this study had restrictions on or excluded primary care from participation. Leadership at some of these facilities were concerned that primary care teams did not have the bandwidth to take on additional items and/or there was variable primary care readiness for initiating AUD pharmacotherapy. Further attempts should be made to integrate primary care into the process of initiating AUD treatment as significant research suggests that integrated care models for AUD may be associated with improved process and outcome measures of care.23
There are several differences between this quality improvement project and prior research investigating the impact of DTC education for other conditions, such as the EMPOWER randomized controlled trial and VISN 22 project, which both demonstrated effectiveness of DTC education for reducing benzodiazepine use in geriatric veterans. 15,16 These studies focused on reducing or stopping pharmacotherapy use, whereas this project sought to promote the initiation of AUD pharmacotherapy. These studies evaluated outcomes at least 6 months postindex date, whereas this project evaluated outcomes within 30 days postappointment. Furthermore, the educational content varied significantly. Other projects provided patients with information focused on specific medications and interventions, such as benzodiazepine tapering, while this project mailed general information on heavy drinking, its risks, and strategies for cutting back, without mentioning pharmacotherapy. The DTC material used in this project was chosen because it was a preapproved national VA ADS resource, which expedited the project timeline by avoiding the need for additional approvals at each participating site. These differences may impact the observed effectiveness of DTC education in this project, especially regarding the primary outcome.
Strengths and Limitations
This quality improvement project sent a large sample of veterans DTC education in a clinical setting across multiple sites. Additionally, PS matching methods were used to balance covariates between the comparator and DTC education group, thereby simulating a randomized controlled trial and reducing selection bias. The project brought attention to the VISN 21 AUD treatment rates, stimulated conversation across sites about available treatments and resources for AUD, and sparked collaboration between academic detailing, mental health, and primary care services. The time frame for visits was selected during the winter; the National Institute on Alcohol Abuse and Alcoholism notes this is a time when people may be more likely to engage in excessive alcohol consumption than at other times of the year.24
The 30-day time frame for outcomes may have been too short to observe changes in prescribing or referral patterns. Additionally, the comparator group was comprised of veterans seen from October 1, 2022, to January 31, 2023, where seasonal timing may have influenced alcohol consumption behaviors and skewed the results. There were also no-show appointments in the DTC education group (13.7%), though it is likely some patients rescheduled and still received AUD pharmacotherapy within 30 days of the original appointment. Finally, it was not possible to confirm whether a patient opened and read the education that was mailed to them. This may be another reason to explore electronic distribution of DTC education. This all may have contributed to the lack of statistically significant differences in both the primary and secondary outcomes.
There was a high level of variability between facility participation in the project. Two of 7 sites did not participate, and 3 sites restricted primary care engagement. This represents a significant limitation, particularly for the secondary outcome of placing consultations for MH or SUD services. Facilities that only included mental health or SUD HCPs may have resulted in lower consultation rates due to their inherent specialization, reducing the likelihood of self-referrals.
The project may overestimate prescribed AUD pharmacotherapy in the primary outcome due to potential misclassification of medications. While the project adhered to the national VA ADS AUD dashboard’s definition of AUD pharmacotherapy, including acamprosate, disulfiram, naltrexone, topiramate, and gabapentin, some of these medications have multiple indications. For example, gabapentin is commonly prescribed for peripheral neuropathy, and topiramate is used to treat migraines and seizures. The multipurpose use adds uncertainty about whether they were prescribed specifically for AUD treatment, especially in cases where the HCP is responsible for treating a broad range of disease states, as in primary care.
CONCLUSIONS
Results of this quality improvement project did not show a statistically significant difference between patients sent DTC education and the comparator group for the initiation of AUD pharmacotherapy or placement of a consult to mental health or SUD services within 30 days of their scheduled visit. Future studies may seek to implement stricter criteria to confirm the intended use of topiramate and gabapentin, such as looking for keywords in the prescription instructions for use, performing chart reviews, and/or only including these medications if prescribed by a mental health or SUD HCP. Alternatively, future studies may consider limiting the analysis to only FDA-approved AUD medications: acamprosate, disulfiram, and naltrexone. It is vital to continue to enhance primary care HCP readiness to treat AUD, given the existing relationships and trust they often have with patients. Electronic methods for distributing DTC education could also be advantageous, as these methods may have the ability to track whether a message has been opened and read. Despite a lack of statistical significance, this project sparked crucial conversations and collaboration around AUD, available treatments, and addressing potential barriers to connecting patients to care within VISN 21.
Excessive alcohol use is one of the leading preventable causes of death in the United States, responsible for about 178,000 deaths annually and an average of 488 daily deaths in 2020 and 2021.1Alcohol-related deaths increased by 49% between 2006 and 2019.2 This trend continued during the COVID-19 pandemic, with death certificates that listed alcohol increasing by > 25% from 2019 to 2020, and another 10% in 2021.3 This increase of alcohol-related deaths includes those as a direct result of chronic alcohol use, such as alcoholic cardiomyopathy, alcoholic hepatitis and cirrhosis, and alcohol-induced pancreatitis, as well as a result of acute use such as alcohol poisoning, suicide by exposure to alcohol, and alcohol-impaired driving fatalities.4
Excessive alcohol consumption poses other serious risks, including cases when intake is abruptly reduced without proper management. Alcohol withdrawal syndrome (AWS) can vary in severity, with potentially life-threatening complications such as hallucinations, seizures, and delirium tremens.5
These risks highlight the importance of professional intervention and support, not only to mitigate risks associated with AWS, but provide a pathway towards recovery from alcohol use disorder (AUD).
According to the 2022 National Survey on Drug Use and Health, 28.8 million US adults had AUD in the prior year, yet only 7.6% of these individuals received treatment and an even smaller group (2.2%) received medication-assisted treatment for alcohol.6,7 This is despite American Psychiatric Association guidelines for the pharmacological treatment of patients with AUD, including the use of naltrexone, acamprosate, disulfiram, topiramate, or gabapentin, depending on therapy goals, past medication trials, medication contraindications, and patient preference.8 Several of these medications are approved by the US Food and Drug Administration (FDA) for the treatment of AUD and have support for effectiveness from randomized controlled trials and meta-analyses.9-11
Clinical practice guidelines for the management of substance use disorders (SUDs) from the US Department of Veterans Affairs (VA) and US Department of Defense have strong recommendations for naltrexone and topiramate as first-line pharmacotherapies for moderate to severe AUD. Acamprosate and disulfiram are weak recommendations as alternative options. Gabapentin is a weak recommendation for cases where first-line treatments are contraindicated or ineffective. The guidelines emphasize the importance of a comprehensive approach to AUD treatment, including psychosocial interventions in addition to pharmacotherapy.12
A 2023 national survey found veterans reported higher alcohol consumption than nonveterans.13 At the end of fiscal year 2023, > 4.4 million veterans—6% of Veterans Health Administration patients—had been diagnosed with AUD.14 However, > 87% of these patients nationally, and 88% of Veterans Integrated Service Network (VISN) 21 patients, were not receiving naltrexone, acamprosate, disulfiram, or topiramate as part of their treatment. The VA Academic Detailing Service (ADS) now includes AUD pharmacotherapy as a campaign focus, highlighting its importance. The ADS is a pharmacy educational outreach program that uses unbiased clinical guidelines to promote aligning prescribing behavior with best practices. Academic detailing methods include speaking with health care practitioners (HCPs), and direct-to-consumer (DTC) patient education.
ADS campaigns include DTC educational handouts. Past ADS projects and research using DTC have demonstrated a significant improvement in outcomes and positively influencing patients’ pharmacotherapy treatment. 15,16 A VA quality improvement project found a positive correlation between the initiation of AUD pharmacotherapy and engagement with mental health care following the distribution of AUD DTC patient education. 17 This project aimed to apply the same principles of prior research to explore the use of DTC across multiple facilities within VISN 21 to increase AUD pharmacotherapy. VISN 21 includes VA facilities and clinics across the Pacific Islands, Nevada, and California and serves about 350,000 veterans.
METHODS
A prospective cohort of VISN 21 veterans with or at high risk for AUD was identified using the VA ADS AUD Dashboard. The cohort included those not on acamprosate, disulfiram, naltrexone, topiramate, or gabapentin for treatment of AUD and had an elevated Alcohol Use Disorder Identification Test-Consumption (AUDIT-C) score of ≥ 6 (high risk) with an AUD diagnosis or ≥ 8 (severe risk) without a diagnosis. The AUDIT-C scores used in the dashboard are supported by the VA AUD clinician guide as the minimum scores when AUD pharmacotherapy should be offered to patients.18 Prescriptions filled outside the VA were not included in this dashboard.
Data and patient information were collected using the VA Corporate Data Warehouse. To be eligible, veterans needed a valid mailing address within the VISN 21 region and a primary care, mental health, or SUD clinician prescriber visit scheduled between October 1, 2023, and January 31, 2024. Veterans were excluded if they were in hospice, had a 1-year mortality risk score > 50% based on their Care Assessment Need (CAN) score, or facility leadership opted out of project involvement. Patients with both severe renal and hepatic impairments were excluded because they were ineligible for AUD pharmacotherapy. However, veterans with either renal or hepatic impairment (but not both) were included, as they could be potential candidates for ≥ 1 AUD pharmacotherapy option.
Initial correspondence with facilities was initiated through local academic detailers. A local champion was identified for the 1 facility without an academic detailer. Facilities could opt in or out of the project. Approval was provided by the local pharmacy and therapeutics committee, pharmacy, primary care, or psychiatry leadership. Approval process and clinician involvement varied by site.
Education
The selected AUD patient education was designed and approved by the national VA ADS (eappendix). The DTC patient education provided general knowledge about alcohol, including what constitutes a standard amount of alcohol, what is considered heavy drinking, risks of heavy drinking, creating a plan with a clinician to reduce and manage withdrawal symptoms, and additional resources. The DTC was accompanied by a cover letter that included a local facility contact number.
A centralized mailing facility was used for all materials. VA Northern California Health Care System provided the funding to cover the cost of postage. The list of veterans to be contacted was updated on a rolling basis and DTC education was mailed 2 weeks prior to their scheduled prescriber visit.
The eligible cohort of 1260 veterans received DTC education. A comparator group of 2048 veterans that did not receive DTC education was obtained retrospectively by using the same inclusion and exclusion criteria with a scheduled primary care, mental health, or SUD HCP visit from October 1, 2022, to January 31, 2023. The outcomes assessed were within 30 days of the scheduled visit, with the primary outcome as the initiation of AUD-related pharmacotherapy and the secondary outcome as the placement of a consultation for mental health or SUD services. Any consultations sent to Behavioral Health, Addiction, Mental Health, Psychiatric, and SUD services following the HCP visit, within the specified time frame, were used for the secondary outcome.
Matching and Analysis
A 1-to-1 nearest neighbor propensity score (PS) matching without replacement was used to pair the 1260 veterans from the intervention group with similarly scored comparator group veterans for a PS-matched final dataset of 2520 veterans. The PS model was a multivariate logistic regression with the outcome being exposure and comparator group status. Baseline characteristics used in the PS model were age, birth sex, race, facility of care, baseline AUDIT-C score, and days between project start and scheduled appointment. Covariate imbalance for the PS-matched sample was assessed to ensure the standardized mean difference for all covariates fell under a 0.1 threshold (Figure).19

A frequency table was provided to compare the discrete distributions of the baseline characteristics in the intervention and comparator groups. Logistic regression analysis was performed to evaluate the association between DTC education exposure and pharmacotherapy initiation, while controlling for potential confounders. Univariate and multivariate P value results for each variable included in the model were reported along with the multivariate odds ratios (ORs) and their associated 95% CIs. Logistic regression analyses were run for both outcomes. Each model included the exposure and comparator group status as well as the baseline characteristics included in the PS model. Statistical significance was set at P < .05. All statistical analyses were performed with R version 4.2.1.
RESULTS
Two of 7 VISN 21 sites did not participate, and 3 had restrictions on participation. DTC education was mailed about 2 weeks prior to scheduled visit for 1260 veterans; 53.6% identified as White, 37.6% were aged 41 to 60 years, and 79.2% had an AUDIT-C ≥ 8 (Table 1). Of those mailed education, there were 173 no-show appointments (13.7%). Thirty-two veterans (2.5%) in the DTC group and 33 veterans (2.6%) in the comparator group received an AUD-related pharmacotherapy prescription (P = .88) (Table 2). One hundred seventy-one veterans (13.6%) in the DTC group and 160 veterans (12.7%) in the comparator group had a consult placed for mental health or SUD services within 30 days of their appointment (P = .59) (Table 3).



DISCUSSION
This project did not yield statistically significant differences in either the primary or secondary outcomes within the 30-day follow-up window and found limited impact from the DTC educational outreach to veterans. The percentage of veterans that received AUD-related pharmacotherapy or consultations for mental health or SUD services was similarly low in the DTC and comparator groups. These findings suggest that although DTC education may raise awareness, it may not be sufficient on its own to drive changes in prescribing behavior or referral patterns without system-level support.
Addiction is a complex disease faced with stigma and requiring readiness by both the HCP and patient to move forward in support and treatment. The consequences of stigma can be severe: the more stigma perceived by a person with AUD, the less likely they are to seek treatment.20 Stigma may exist even within HCPs and may lead to compromised care including shortened visits, less engagement, and less empathy.19 Cultural attitude towards alcohol use and intoxication can also be influenced through a wide range of sources including social media, movies, music, and television. Studies have shown targeted alcohol marketing may result in the development of positive beliefs about drinking and expand environments where alcohol use is socially acceptable and encouraged.21 These factors can impact drinking behavior, including the onset of drinking, binge drinking, and increased alcohol consumption.22
Three VISN 21 sites in this study had restrictions on or excluded primary care from participation. Leadership at some of these facilities were concerned that primary care teams did not have the bandwidth to take on additional items and/or there was variable primary care readiness for initiating AUD pharmacotherapy. Further attempts should be made to integrate primary care into the process of initiating AUD treatment as significant research suggests that integrated care models for AUD may be associated with improved process and outcome measures of care.23
There are several differences between this quality improvement project and prior research investigating the impact of DTC education for other conditions, such as the EMPOWER randomized controlled trial and VISN 22 project, which both demonstrated effectiveness of DTC education for reducing benzodiazepine use in geriatric veterans. 15,16 These studies focused on reducing or stopping pharmacotherapy use, whereas this project sought to promote the initiation of AUD pharmacotherapy. These studies evaluated outcomes at least 6 months postindex date, whereas this project evaluated outcomes within 30 days postappointment. Furthermore, the educational content varied significantly. Other projects provided patients with information focused on specific medications and interventions, such as benzodiazepine tapering, while this project mailed general information on heavy drinking, its risks, and strategies for cutting back, without mentioning pharmacotherapy. The DTC material used in this project was chosen because it was a preapproved national VA ADS resource, which expedited the project timeline by avoiding the need for additional approvals at each participating site. These differences may impact the observed effectiveness of DTC education in this project, especially regarding the primary outcome.
Strengths and Limitations
This quality improvement project sent a large sample of veterans DTC education in a clinical setting across multiple sites. Additionally, PS matching methods were used to balance covariates between the comparator and DTC education group, thereby simulating a randomized controlled trial and reducing selection bias. The project brought attention to the VISN 21 AUD treatment rates, stimulated conversation across sites about available treatments and resources for AUD, and sparked collaboration between academic detailing, mental health, and primary care services. The time frame for visits was selected during the winter; the National Institute on Alcohol Abuse and Alcoholism notes this is a time when people may be more likely to engage in excessive alcohol consumption than at other times of the year.24
The 30-day time frame for outcomes may have been too short to observe changes in prescribing or referral patterns. Additionally, the comparator group was comprised of veterans seen from October 1, 2022, to January 31, 2023, where seasonal timing may have influenced alcohol consumption behaviors and skewed the results. There were also no-show appointments in the DTC education group (13.7%), though it is likely some patients rescheduled and still received AUD pharmacotherapy within 30 days of the original appointment. Finally, it was not possible to confirm whether a patient opened and read the education that was mailed to them. This may be another reason to explore electronic distribution of DTC education. This all may have contributed to the lack of statistically significant differences in both the primary and secondary outcomes.
There was a high level of variability between facility participation in the project. Two of 7 sites did not participate, and 3 sites restricted primary care engagement. This represents a significant limitation, particularly for the secondary outcome of placing consultations for MH or SUD services. Facilities that only included mental health or SUD HCPs may have resulted in lower consultation rates due to their inherent specialization, reducing the likelihood of self-referrals.
The project may overestimate prescribed AUD pharmacotherapy in the primary outcome due to potential misclassification of medications. While the project adhered to the national VA ADS AUD dashboard’s definition of AUD pharmacotherapy, including acamprosate, disulfiram, naltrexone, topiramate, and gabapentin, some of these medications have multiple indications. For example, gabapentin is commonly prescribed for peripheral neuropathy, and topiramate is used to treat migraines and seizures. The multipurpose use adds uncertainty about whether they were prescribed specifically for AUD treatment, especially in cases where the HCP is responsible for treating a broad range of disease states, as in primary care.
CONCLUSIONS
Results of this quality improvement project did not show a statistically significant difference between patients sent DTC education and the comparator group for the initiation of AUD pharmacotherapy or placement of a consult to mental health or SUD services within 30 days of their scheduled visit. Future studies may seek to implement stricter criteria to confirm the intended use of topiramate and gabapentin, such as looking for keywords in the prescription instructions for use, performing chart reviews, and/or only including these medications if prescribed by a mental health or SUD HCP. Alternatively, future studies may consider limiting the analysis to only FDA-approved AUD medications: acamprosate, disulfiram, and naltrexone. It is vital to continue to enhance primary care HCP readiness to treat AUD, given the existing relationships and trust they often have with patients. Electronic methods for distributing DTC education could also be advantageous, as these methods may have the ability to track whether a message has been opened and read. Despite a lack of statistical significance, this project sparked crucial conversations and collaboration around AUD, available treatments, and addressing potential barriers to connecting patients to care within VISN 21.
- Centers for Disease Control and Prevention. Facts about U.S. deaths from excessive alcohol use. August 6, 2024. Accessed February 5, 2025. https://www.cdc.gov/alcohol/facts-stats/
- State Health Access Data Assistance Center. Escalating alcohol-involved death rates: trends and variation across the nation and in the states from 2006 to 2019. April 19, 2021. Accessed February 5, 2025. https://www.shadac.org/escalating-alcohol-involved-death-rates-trends-and-variation-across-nation-and-states-2006-2019
- National Institute on Alcohol Abuse and Alcoholism. Alcohol- related emergencies and deaths in the United States. Updated November 2024. Accessed February 5, 2025. https://www.niaaa.nih.gov/alcohols-effects-health/alcohol-topics/alcohol-facts-and-statistics/alcohol-related-emergencies-and-deaths-united-states
- Esser MB, Sherk A, Liu Y, Naimi TS. Deaths from excessive alcohol use - United States, 2016- 2021. MMWR Morb Mortal Wkly Rep. 2024;73(8):154-161. doi:10.15585/mmwr.mm7308a1
- Canver BR, Newman RK, Gomez AE. Alcohol Withdrawal Syndrome. In: StatPearls. StatPearls Publishing; 2024.
- National Institute on Alcohol Abuse and Alcoholism. Alcohol treatment in the United States. Updated January 2025. Accessed February 5, 2025. https://www.niaaa.nih.gov/alcohols-effects-health/alcohol-topics/alcohol-facts-and-statistics/alcohol-treatment-united-states
- National Institute on Alcohol Abuse and Alcoholism. Alcohol use disorder (AUD) in the United States: age groups and demographic characteristics. Updated September 2024. Accessed February 5, 2025. https://www.niaaa.nih.gov/alcohols-effects-health/alcohol-topics/alcohol-facts-and-statistics/alcohol-use-disorder-aud-united-states-age-groups-and-demographic-characteristics
- Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association practice guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018;175(1):86-90. doi:10.1176/appi.ajp.2017.1750101
- Blodgett JC, Del Re AC, Maisel NC, Finney JW. A meta-analysis of topiramate’s effects for individuals with alcohol use disorders. Alcohol Clin Exp Res. 2014;38(6):1481-1488. doi:10.1111/acer.12411
- Maisel NC, Blodgett JC, Wilbourne PL, Humphreys K, Finney JW. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108(2):275-293. doi:10.1111/j.1360-0443.2012.04054.x
- Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014;311(18):1889-1900. doi:10.1001/jama.2014.3628
- US Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for the management of substance use disorders. August 2021. Accessed February 5, 2025. https://www.healthquality.va.gov/guidelines/MH/sud/VADODSUDCPG.pdf
- Ranney RM, Bernhard PA, Vogt D, et al. Alcohol use and treatment utilization in a national sample of veterans and nonveterans. J Subst Use Addict Treat. 2023;146:208964. doi:10.1016/j.josat.2023.208964
- US Department of Veterans Affairs, Pharmacy Benefit Management Service, Academic Detailing Service. AUD Trend Report. https://vaww.pbi.cdw.va.gov/PBIRS/Pages/ReportViewer.aspx?/GPE/PBM_AD/SSRS/AUD/AUD_TrendReport
- Mendes MA, Smith JP, Marin JK, et al. Reducing benzodiazepine prescribing in older veterans: a direct-to-consumer educational brochure. Fed Pract. 2018;35(9):36-43.
- Tannenbaum C, Martin P, Tamblyn R, Benedetti A, Ahmed S. Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: the EMPOWER cluster randomized trial. JAMA Intern Med. 2014;174(6):890-898. doi:10.1001/jamainternmed.2014.949
- Maloney R, Funmilayo M. Acting on the AUDIT-C: implementation of direct-to-consumer education on unhealth alcohol use. Presented on March 31, 2023; Central Virginia Veterans Affairs Health Care System, Richmond, Virginia.
- US Department of Veterans Affairs, Pharmacy Benefit Management Service. Alcohol use disorder (AUD) – leading the charge in the treatment of AUD: a VA clinician’s guide. February 2022. Accessed February 5, 2025. https://www.pbm.va.gov/PBM/AcademicDetailingService/Documents/508/10-1530_AUD_ClinicianGuide_508Conformant.pdf
- Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399-424. doi:10.1080/00273171.2011.568786
- National Institute on Alcohol Abuse and Alcoholism. Stigma: overcoming a pervasive barrier to optimal care. Updated January 6, 2025. Accessed February 5, 2025. https://www.niaaa.nih.gov/health-professionals-communities/core-resource-on-alcohol/stigma-overcoming-pervasive-barrier-optimal-care
- Sudhinaraset M, Wigglesworth C, Takeuchi DT. Social and cultural contexts of alcohol use: influences in a socialecological framework. Alcohol Res. 2016;38(1):35-45.
- Tanski SE, McClure AC, Li Z, et al. Cued recall of alcohol advertising on television and underage drinking behavior. JAMA Pediatr. 2015;169(3):264-271. doi:10.1001/jamapediatrics.2014.3345
- Hyland CJ, McDowell MJ, Bain PA, Huskamp HA, Busch AB. Integration of pharmacotherapy for alcohol use disorder treatment in primary care settings: a scoping review. J Subst Abuse Treat. 2023;144:108919. doi:10.1016/j.jsat.2022.108919
- National Institute on Alcohol Abuse and Alcoholism. The truth about holiday spirits. Updated November 2023. Accessed February 5, 2025. ,a href="https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/truth-about-holiday-spirits">https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/truth-about-holiday-spirits
- Centers for Disease Control and Prevention. Facts about U.S. deaths from excessive alcohol use. August 6, 2024. Accessed February 5, 2025. https://www.cdc.gov/alcohol/facts-stats/
- State Health Access Data Assistance Center. Escalating alcohol-involved death rates: trends and variation across the nation and in the states from 2006 to 2019. April 19, 2021. Accessed February 5, 2025. https://www.shadac.org/escalating-alcohol-involved-death-rates-trends-and-variation-across-nation-and-states-2006-2019
- National Institute on Alcohol Abuse and Alcoholism. Alcohol- related emergencies and deaths in the United States. Updated November 2024. Accessed February 5, 2025. https://www.niaaa.nih.gov/alcohols-effects-health/alcohol-topics/alcohol-facts-and-statistics/alcohol-related-emergencies-and-deaths-united-states
- Esser MB, Sherk A, Liu Y, Naimi TS. Deaths from excessive alcohol use - United States, 2016- 2021. MMWR Morb Mortal Wkly Rep. 2024;73(8):154-161. doi:10.15585/mmwr.mm7308a1
- Canver BR, Newman RK, Gomez AE. Alcohol Withdrawal Syndrome. In: StatPearls. StatPearls Publishing; 2024.
- National Institute on Alcohol Abuse and Alcoholism. Alcohol treatment in the United States. Updated January 2025. Accessed February 5, 2025. https://www.niaaa.nih.gov/alcohols-effects-health/alcohol-topics/alcohol-facts-and-statistics/alcohol-treatment-united-states
- National Institute on Alcohol Abuse and Alcoholism. Alcohol use disorder (AUD) in the United States: age groups and demographic characteristics. Updated September 2024. Accessed February 5, 2025. https://www.niaaa.nih.gov/alcohols-effects-health/alcohol-topics/alcohol-facts-and-statistics/alcohol-use-disorder-aud-united-states-age-groups-and-demographic-characteristics
- Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association practice guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018;175(1):86-90. doi:10.1176/appi.ajp.2017.1750101
- Blodgett JC, Del Re AC, Maisel NC, Finney JW. A meta-analysis of topiramate’s effects for individuals with alcohol use disorders. Alcohol Clin Exp Res. 2014;38(6):1481-1488. doi:10.1111/acer.12411
- Maisel NC, Blodgett JC, Wilbourne PL, Humphreys K, Finney JW. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108(2):275-293. doi:10.1111/j.1360-0443.2012.04054.x
- Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014;311(18):1889-1900. doi:10.1001/jama.2014.3628
- US Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for the management of substance use disorders. August 2021. Accessed February 5, 2025. https://www.healthquality.va.gov/guidelines/MH/sud/VADODSUDCPG.pdf
- Ranney RM, Bernhard PA, Vogt D, et al. Alcohol use and treatment utilization in a national sample of veterans and nonveterans. J Subst Use Addict Treat. 2023;146:208964. doi:10.1016/j.josat.2023.208964
- US Department of Veterans Affairs, Pharmacy Benefit Management Service, Academic Detailing Service. AUD Trend Report. https://vaww.pbi.cdw.va.gov/PBIRS/Pages/ReportViewer.aspx?/GPE/PBM_AD/SSRS/AUD/AUD_TrendReport
- Mendes MA, Smith JP, Marin JK, et al. Reducing benzodiazepine prescribing in older veterans: a direct-to-consumer educational brochure. Fed Pract. 2018;35(9):36-43.
- Tannenbaum C, Martin P, Tamblyn R, Benedetti A, Ahmed S. Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: the EMPOWER cluster randomized trial. JAMA Intern Med. 2014;174(6):890-898. doi:10.1001/jamainternmed.2014.949
- Maloney R, Funmilayo M. Acting on the AUDIT-C: implementation of direct-to-consumer education on unhealth alcohol use. Presented on March 31, 2023; Central Virginia Veterans Affairs Health Care System, Richmond, Virginia.
- US Department of Veterans Affairs, Pharmacy Benefit Management Service. Alcohol use disorder (AUD) – leading the charge in the treatment of AUD: a VA clinician’s guide. February 2022. Accessed February 5, 2025. https://www.pbm.va.gov/PBM/AcademicDetailingService/Documents/508/10-1530_AUD_ClinicianGuide_508Conformant.pdf
- Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399-424. doi:10.1080/00273171.2011.568786
- National Institute on Alcohol Abuse and Alcoholism. Stigma: overcoming a pervasive barrier to optimal care. Updated January 6, 2025. Accessed February 5, 2025. https://www.niaaa.nih.gov/health-professionals-communities/core-resource-on-alcohol/stigma-overcoming-pervasive-barrier-optimal-care
- Sudhinaraset M, Wigglesworth C, Takeuchi DT. Social and cultural contexts of alcohol use: influences in a socialecological framework. Alcohol Res. 2016;38(1):35-45.
- Tanski SE, McClure AC, Li Z, et al. Cued recall of alcohol advertising on television and underage drinking behavior. JAMA Pediatr. 2015;169(3):264-271. doi:10.1001/jamapediatrics.2014.3345
- Hyland CJ, McDowell MJ, Bain PA, Huskamp HA, Busch AB. Integration of pharmacotherapy for alcohol use disorder treatment in primary care settings: a scoping review. J Subst Abuse Treat. 2023;144:108919. doi:10.1016/j.jsat.2022.108919
- National Institute on Alcohol Abuse and Alcoholism. The truth about holiday spirits. Updated November 2023. Accessed February 5, 2025. ,a href="https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/truth-about-holiday-spirits">https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/truth-about-holiday-spirits
Impact of Multisite Patient Education on Pharmacotherapy for Veterans With Alcohol Use Disorder
Impact of Multisite Patient Education on Pharmacotherapy for Veterans With Alcohol Use Disorder
Treatment of low bone density or osteoporosis to prevent fractures in men and women
Osteoporosis is defined by a clinically diagnosed fragility fracture or a bone mineral density (BMD) of at least 2.5 SD below the mean for young female adults, usually measured by dual-energy x-ray absorptiometry. Risk factors include age, female sex, post-menopause, hypogonadism or premature ovarian failure, history of cigarette smoking or alcohol consumption (3 or more drinks daily), rheumatoid arthritis, or medications including glucocorticoids, anticoagulants, anticonvulsants, and aromatase inhibitors.
This guideline update focuses on treatment with bisphosphonates (alendronate, risedronate, ibandronate, zoledronic acid) and denosumab. Denosumab, a human monoclonal antibody against RANK-ligand, approved by the Food and Drug Administration for treatment of osteoporosis, has been added to the list of allowed medications since publication of the 2008 guideline. Several therapies have been excluded from the update, including calcitonin, which is no longer widely used for osteoporosis treatment, and etidronate and pamidronate, neither of which are FDA-approved for the prevention of fractures or treatment of osteoporosis. It should be noted that the evidence continues to be insufficient regarding the effectiveness of therapies to prevent fractures or to treat osteoporosis in men.
Bisphosphonates are associated with mild upper GI symptoms, atypical subtrochanteric fracture, and rare osteonecrosis of the jaw. There is no significant association between bisphosphonate use and total cardiovascular adverse events. Evidence is insufficient to associate bisphosphonates with increased cancer risk. Zoledronic acid is associated with atrial fibrillation, arthritis/arthralgias, headaches, hypocalcemia, influenza-like symptoms, and an increased incidence of uveitis/episcleritis. Denosumab is associated with mild upper GI symptoms, rash/eczema, and cellulitis.
While in the past additional medications were recommended for osteoporosis, the current guidelines recommend against using raloxifene, ibandronate, teriparatide, menopausal estrogen therapy, or menopausal estrogen plus progesterone therapy for first-line pharmacologic treatment.
The overall effect of calcium, vitamin D, or exercise alone on fracture risk is uncertain. Calcium and vitamin D may be added to treatment regimens, as a majority of trials with bisphosphonate therapy added this supplementation. Dosages should be considered because excessive dosing has been associated with hypercalcemia. Although previous data suggested an association between calcium supplementation and increased risk for myocardial infarction, moderate-quality evidence shows no association, though there is a risk of kidney stones.
Recommendation: Women who have osteoporosis and receive pharmacologic treatment should be treated for 5 years (weak recommendation; low-quality evidence). The evidence to determine the length of treatment is not strong, so recommendation is an extrapolation from existing evidence. High-risk patients may benefit from more than 5 years of treatment. Data suggests that patients treated with alendronate who had preexisting fractures or those with a BMD of –2.5 or less after 5 years of initial therapy may benefit from continued treatment, because these patients experienced a decreased incidence of new clinical vertebral fractures.
Recommendation: Pharmacologic treatment with bisphosphonates to reduce the risk for vertebral fracture can be offered to men who have clinically recognized osteoporosis (weak recommendation, low-quality evidence). No evidence suggests that outcomes associated with pharmacologic treatment would differ between men and women if based on similar BMDs.
Recommendation: Bone density monitoring is not recommended during the 5-year pharmacologic treatment period for osteoporosis in women (weak recommendation, low-quality evidence). Data showed that most women with normal dual-energy x-ray absorptiometry scores did not progress to osteoporosis within 15 years. Data also does not support monitoring BMD during the initial 5 years of treatment in patients taking pharmacologic agents to treat osteoporosis. Several studies showed that women treated with antiresorptive treatment benefited from reduced fractures with treatment even if BMD did not increase.
Only 10% of women with normal or mild osteopenia develop osteoporosis within 15 years; 10% of women with moderate osteopenia develop osteoporosis within 5 years, and 10% of women with advanced osteopenia develop osteoporosis within 1 year.
Recommendation: The decision about whether to treat osteopenic women older then 65 years of age who are at a high risk for fracture should be based on a discussion of with the patient about their risk of fracture and the risk and benefits of treatment. Clinicians can use their judgment regarding the qualitative risk for fracture, or a validated tool such as the FRAX tool that gives 10-year risk of any major osteoporotic fracture and of hip fracture. The FRAX site recommends consideration of treatment for individuals with low bone mass (T-score between –1.0 and –2.5 at the femoral neck or spine) and a 10-year probability of a hip fracture of at least 3% or a 10-year probability of a major osteoporosis-related fracture greater than 20%.
Bottom line:
Clinicians should offer pharmacologic treatment with alendronate, risedronate, zoledronic acid, or denosumab to reduce the risk of hip and vertebral fractures in women who have known osteoporosis diagnosed as a T score less than –2.5 or those with a fragility fracture. Pharmacologic therapy should be used for 5 years; however, high risk patients may benefit from longer treatment. There is no benefit to bone density monitoring during the 5-year pharmacologic treatment period. In addition, bisphosphonates should be considered in men who have clinically recognized osteoporosis.
Reference:
Qaseem, A, Forciea, MA, McLean RM, Denberg TD. Treatment of Low Bone Density or Osteoporosis to Prevent Fractures in Men and Women: A Clinical Practice Guideline Update From the American College of Physicians. Ann Int Med. 2017;166(11):818-39.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Meizinger is a second year resident in the Family Medicine Residency Program at Abington Jefferson Health.
Osteoporosis is defined by a clinically diagnosed fragility fracture or a bone mineral density (BMD) of at least 2.5 SD below the mean for young female adults, usually measured by dual-energy x-ray absorptiometry. Risk factors include age, female sex, post-menopause, hypogonadism or premature ovarian failure, history of cigarette smoking or alcohol consumption (3 or more drinks daily), rheumatoid arthritis, or medications including glucocorticoids, anticoagulants, anticonvulsants, and aromatase inhibitors.
This guideline update focuses on treatment with bisphosphonates (alendronate, risedronate, ibandronate, zoledronic acid) and denosumab. Denosumab, a human monoclonal antibody against RANK-ligand, approved by the Food and Drug Administration for treatment of osteoporosis, has been added to the list of allowed medications since publication of the 2008 guideline. Several therapies have been excluded from the update, including calcitonin, which is no longer widely used for osteoporosis treatment, and etidronate and pamidronate, neither of which are FDA-approved for the prevention of fractures or treatment of osteoporosis. It should be noted that the evidence continues to be insufficient regarding the effectiveness of therapies to prevent fractures or to treat osteoporosis in men.
Bisphosphonates are associated with mild upper GI symptoms, atypical subtrochanteric fracture, and rare osteonecrosis of the jaw. There is no significant association between bisphosphonate use and total cardiovascular adverse events. Evidence is insufficient to associate bisphosphonates with increased cancer risk. Zoledronic acid is associated with atrial fibrillation, arthritis/arthralgias, headaches, hypocalcemia, influenza-like symptoms, and an increased incidence of uveitis/episcleritis. Denosumab is associated with mild upper GI symptoms, rash/eczema, and cellulitis.
While in the past additional medications were recommended for osteoporosis, the current guidelines recommend against using raloxifene, ibandronate, teriparatide, menopausal estrogen therapy, or menopausal estrogen plus progesterone therapy for first-line pharmacologic treatment.
The overall effect of calcium, vitamin D, or exercise alone on fracture risk is uncertain. Calcium and vitamin D may be added to treatment regimens, as a majority of trials with bisphosphonate therapy added this supplementation. Dosages should be considered because excessive dosing has been associated with hypercalcemia. Although previous data suggested an association between calcium supplementation and increased risk for myocardial infarction, moderate-quality evidence shows no association, though there is a risk of kidney stones.
Recommendation: Women who have osteoporosis and receive pharmacologic treatment should be treated for 5 years (weak recommendation; low-quality evidence). The evidence to determine the length of treatment is not strong, so recommendation is an extrapolation from existing evidence. High-risk patients may benefit from more than 5 years of treatment. Data suggests that patients treated with alendronate who had preexisting fractures or those with a BMD of –2.5 or less after 5 years of initial therapy may benefit from continued treatment, because these patients experienced a decreased incidence of new clinical vertebral fractures.
Recommendation: Pharmacologic treatment with bisphosphonates to reduce the risk for vertebral fracture can be offered to men who have clinically recognized osteoporosis (weak recommendation, low-quality evidence). No evidence suggests that outcomes associated with pharmacologic treatment would differ between men and women if based on similar BMDs.
Recommendation: Bone density monitoring is not recommended during the 5-year pharmacologic treatment period for osteoporosis in women (weak recommendation, low-quality evidence). Data showed that most women with normal dual-energy x-ray absorptiometry scores did not progress to osteoporosis within 15 years. Data also does not support monitoring BMD during the initial 5 years of treatment in patients taking pharmacologic agents to treat osteoporosis. Several studies showed that women treated with antiresorptive treatment benefited from reduced fractures with treatment even if BMD did not increase.
Only 10% of women with normal or mild osteopenia develop osteoporosis within 15 years; 10% of women with moderate osteopenia develop osteoporosis within 5 years, and 10% of women with advanced osteopenia develop osteoporosis within 1 year.
Recommendation: The decision about whether to treat osteopenic women older then 65 years of age who are at a high risk for fracture should be based on a discussion of with the patient about their risk of fracture and the risk and benefits of treatment. Clinicians can use their judgment regarding the qualitative risk for fracture, or a validated tool such as the FRAX tool that gives 10-year risk of any major osteoporotic fracture and of hip fracture. The FRAX site recommends consideration of treatment for individuals with low bone mass (T-score between –1.0 and –2.5 at the femoral neck or spine) and a 10-year probability of a hip fracture of at least 3% or a 10-year probability of a major osteoporosis-related fracture greater than 20%.
Bottom line:
Clinicians should offer pharmacologic treatment with alendronate, risedronate, zoledronic acid, or denosumab to reduce the risk of hip and vertebral fractures in women who have known osteoporosis diagnosed as a T score less than –2.5 or those with a fragility fracture. Pharmacologic therapy should be used for 5 years; however, high risk patients may benefit from longer treatment. There is no benefit to bone density monitoring during the 5-year pharmacologic treatment period. In addition, bisphosphonates should be considered in men who have clinically recognized osteoporosis.
Reference:
Qaseem, A, Forciea, MA, McLean RM, Denberg TD. Treatment of Low Bone Density or Osteoporosis to Prevent Fractures in Men and Women: A Clinical Practice Guideline Update From the American College of Physicians. Ann Int Med. 2017;166(11):818-39.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Meizinger is a second year resident in the Family Medicine Residency Program at Abington Jefferson Health.
Osteoporosis is defined by a clinically diagnosed fragility fracture or a bone mineral density (BMD) of at least 2.5 SD below the mean for young female adults, usually measured by dual-energy x-ray absorptiometry. Risk factors include age, female sex, post-menopause, hypogonadism or premature ovarian failure, history of cigarette smoking or alcohol consumption (3 or more drinks daily), rheumatoid arthritis, or medications including glucocorticoids, anticoagulants, anticonvulsants, and aromatase inhibitors.
This guideline update focuses on treatment with bisphosphonates (alendronate, risedronate, ibandronate, zoledronic acid) and denosumab. Denosumab, a human monoclonal antibody against RANK-ligand, approved by the Food and Drug Administration for treatment of osteoporosis, has been added to the list of allowed medications since publication of the 2008 guideline. Several therapies have been excluded from the update, including calcitonin, which is no longer widely used for osteoporosis treatment, and etidronate and pamidronate, neither of which are FDA-approved for the prevention of fractures or treatment of osteoporosis. It should be noted that the evidence continues to be insufficient regarding the effectiveness of therapies to prevent fractures or to treat osteoporosis in men.
Bisphosphonates are associated with mild upper GI symptoms, atypical subtrochanteric fracture, and rare osteonecrosis of the jaw. There is no significant association between bisphosphonate use and total cardiovascular adverse events. Evidence is insufficient to associate bisphosphonates with increased cancer risk. Zoledronic acid is associated with atrial fibrillation, arthritis/arthralgias, headaches, hypocalcemia, influenza-like symptoms, and an increased incidence of uveitis/episcleritis. Denosumab is associated with mild upper GI symptoms, rash/eczema, and cellulitis.
While in the past additional medications were recommended for osteoporosis, the current guidelines recommend against using raloxifene, ibandronate, teriparatide, menopausal estrogen therapy, or menopausal estrogen plus progesterone therapy for first-line pharmacologic treatment.
The overall effect of calcium, vitamin D, or exercise alone on fracture risk is uncertain. Calcium and vitamin D may be added to treatment regimens, as a majority of trials with bisphosphonate therapy added this supplementation. Dosages should be considered because excessive dosing has been associated with hypercalcemia. Although previous data suggested an association between calcium supplementation and increased risk for myocardial infarction, moderate-quality evidence shows no association, though there is a risk of kidney stones.
Recommendation: Women who have osteoporosis and receive pharmacologic treatment should be treated for 5 years (weak recommendation; low-quality evidence). The evidence to determine the length of treatment is not strong, so recommendation is an extrapolation from existing evidence. High-risk patients may benefit from more than 5 years of treatment. Data suggests that patients treated with alendronate who had preexisting fractures or those with a BMD of –2.5 or less after 5 years of initial therapy may benefit from continued treatment, because these patients experienced a decreased incidence of new clinical vertebral fractures.
Recommendation: Pharmacologic treatment with bisphosphonates to reduce the risk for vertebral fracture can be offered to men who have clinically recognized osteoporosis (weak recommendation, low-quality evidence). No evidence suggests that outcomes associated with pharmacologic treatment would differ between men and women if based on similar BMDs.
Recommendation: Bone density monitoring is not recommended during the 5-year pharmacologic treatment period for osteoporosis in women (weak recommendation, low-quality evidence). Data showed that most women with normal dual-energy x-ray absorptiometry scores did not progress to osteoporosis within 15 years. Data also does not support monitoring BMD during the initial 5 years of treatment in patients taking pharmacologic agents to treat osteoporosis. Several studies showed that women treated with antiresorptive treatment benefited from reduced fractures with treatment even if BMD did not increase.
Only 10% of women with normal or mild osteopenia develop osteoporosis within 15 years; 10% of women with moderate osteopenia develop osteoporosis within 5 years, and 10% of women with advanced osteopenia develop osteoporosis within 1 year.
Recommendation: The decision about whether to treat osteopenic women older then 65 years of age who are at a high risk for fracture should be based on a discussion of with the patient about their risk of fracture and the risk and benefits of treatment. Clinicians can use their judgment regarding the qualitative risk for fracture, or a validated tool such as the FRAX tool that gives 10-year risk of any major osteoporotic fracture and of hip fracture. The FRAX site recommends consideration of treatment for individuals with low bone mass (T-score between –1.0 and –2.5 at the femoral neck or spine) and a 10-year probability of a hip fracture of at least 3% or a 10-year probability of a major osteoporosis-related fracture greater than 20%.
Bottom line:
Clinicians should offer pharmacologic treatment with alendronate, risedronate, zoledronic acid, or denosumab to reduce the risk of hip and vertebral fractures in women who have known osteoporosis diagnosed as a T score less than –2.5 or those with a fragility fracture. Pharmacologic therapy should be used for 5 years; however, high risk patients may benefit from longer treatment. There is no benefit to bone density monitoring during the 5-year pharmacologic treatment period. In addition, bisphosphonates should be considered in men who have clinically recognized osteoporosis.
Reference:
Qaseem, A, Forciea, MA, McLean RM, Denberg TD. Treatment of Low Bone Density or Osteoporosis to Prevent Fractures in Men and Women: A Clinical Practice Guideline Update From the American College of Physicians. Ann Int Med. 2017;166(11):818-39.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Meizinger is a second year resident in the Family Medicine Residency Program at Abington Jefferson Health.
GOLD guidelines for the management of COPD – 2017 update
Chronic obstructive lung disease (COPD) is the third leading cause of death in the United States1 and a major cause of mortality and morbidity around the world. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) released a new “2017 Report”2 with modified recommendations for the diagnosis, management, and prevention of COPD. The report contains several changes that are relevant to the primary care provider that will be outlined below.
Redefining COPD
GOLD’s definition of COPD was changed in its 2017 Report: “COPD is a common, preventable, and treatable disease that is characterized by persistent respiratory symptoms and airflow limitations that are due to airway and/or alveolar abnormalities usually caused by significant exposure to noxious particles or gases.” The report emphasizes that “COPD may be punctuated by periods of acute worsening of respiratory symptoms, called exacerbations.” Note that the terms “emphysema” and “chronic bronchitis” have been removed in favor of a more comprehensive description of the pathophysiology of COPD. Importantly, the report states that cough and sputum production for at least 3 months in each of 2 consecutive years, previously accepted as diagnostic criteria, are present in only a minority of patients. It is noted that chronic respiratory symptoms may exist without spirometric changes and many patients (usually smokers) have structural evidence of COPD without airflow limitation.
Changes to COPD initial assessment
The primary criterion for diagnosis is unchanged: post-bronchodilator forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) less than 0.70. Spirometry remains important to confirm the diagnosis in those with classic symptoms of dyspnea, chronic cough, and/or sputum production with a history of exposure to noxious particles or gases.
The GOLD assessment system previously incorporated spirometry and included an “ABCD” system such that patients in group A are least severe. Spirometry has been progressively deemphasized in favor of symptom-based classification and the 2017 Report, for the first time, dissociates spirometric findings from severity classification.
The new system uses symptom severity and exacerbation risk to classify COPD. Two specific standardized COPD symptom measurement tools, The Modified British Medical Research Council (mMRC) questionnaire and COPD Assessment Test (CAT), are reported by GOLD as the most widely used. Low symptom severity is considered an mMRC less than or equal to 1 or CAT less than or equal to 9, high symptom severity is considered an mMRC greater than or equal to 2 or CAT greater than or equal to 10. Low risk of exacerbation is defined as no more than one exacerbation not resulting in hospital admission in the last 12 months; high risk of exacerbation is defined as at least two exacerbations or any exacerbations resulting in hospital admission in the last 12 months. Symptom severity and exacerbation risk is divided into four quadrants:
• GOLD group A: Low symptom severity, low exacerbation risk.
• GOLD group B: High symptom severity, low exacerbation risk.
• GOLD group C: Low symptom severity, high exacerbation risk.
• GOLD group D: High symptom severity, high exacerbation risk.
Changes to prevention and management of stable COPD
Smoking cessation remains important in the prevention of COPD. The 2017 Report reflects the U.S. Preventive Services Task Force’s guidelines for smoking cessation: Offer nicotine replacement, cessation counseling, and pharmacotherapy (varenicline, bupropion or nortriptyline). There is insufficient evidence to support the use of e-cigarettes. Influenza and pneumococcal vaccinations are recommended. Pulmonary rehabilitation remains important.
The 2017 Report includes an expanded discussion of COPD medications. The role of short-acting bronchodilators (SABD) in COPD remains prominent. Changes include a stronger recommendation to use combination short-acting beta-agonists and short-acting muscarinic antagonists (SABA/SAMA) as these seem to be superior to SABD monotherapy in improving symptoms and FEV1.
There were several changes to the pharmacologic treatment algorithm. For the first time, GOLD proposes escalation strategies. Preference is given to LABA/LAMA (long-acting beta-agonist/long-acting muscarinic antagonists) combinations over LABA/ICS (long-acting beta-agonist/inhaled corticosteroid) combinations as a mainstay of treatment. The rationale for this change is that LABA/LAMAs give greater bronchodilation compared with LABA/ICS, and one study showed a decreased rate of exacerbations compared to LABA/ICS in patients with a history of exacerbations. In addition, patients with COPD who receive ICS appear to have a higher risk of developing pneumonia. GOLD recommendations are:
• Group A: Start with single bronchodilator (short- or long-acting), escalate to alternative class of bronchodilator if necessary.
• Group B: Start with LABA or LAMA, escalate to LABA/LAMA if symptoms persist.
• Group C: Start with LAMA, escalate to LABA/LAMA (preferred) or LABA/ICS if exacerbations continue.
• Group D: Start with LABA/LAMA (preferred) or LAMA monotherapy, escalate to LABA/LAMA/ICS (preferred) or try LABA/ICS before escalating to LAMA/LABA/ICS if symptoms persist or exacerbations continue; roflumilast and/or a macrolide may be considered if further exacerbations occur with LABA/LAMA/ICS.
Bottom line
1. GOLD classification of COPD severity is now based on clinical criteria alone: symptom assessment and risk for exacerbation.
2. SABA/SAMA combination therapy seems to be superior to either SABA or SAMA alone.
3. Patients in group A (milder symptoms, low exacerbation risk) may be initiated on either short- or long-acting bronchodilator therapy.
4. Patients in group B (milder symptoms, increased exacerbation risk) should be initiated on LAMA monotherapy.
5. LABA/LAMA combination therapy seems to be superior to LABA/ICS combination therapy and should be used when long-acting bronchodilator monotherapy fails to control symptoms or reduce exacerbations.
References
1. CDC MMWR 11/23/12
2. Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017 at http://goldcopd.org (accessed 3/10/2017)
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital. Dr. Lent is chief resident in the program.
Chronic obstructive lung disease (COPD) is the third leading cause of death in the United States1 and a major cause of mortality and morbidity around the world. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) released a new “2017 Report”2 with modified recommendations for the diagnosis, management, and prevention of COPD. The report contains several changes that are relevant to the primary care provider that will be outlined below.
Redefining COPD
GOLD’s definition of COPD was changed in its 2017 Report: “COPD is a common, preventable, and treatable disease that is characterized by persistent respiratory symptoms and airflow limitations that are due to airway and/or alveolar abnormalities usually caused by significant exposure to noxious particles or gases.” The report emphasizes that “COPD may be punctuated by periods of acute worsening of respiratory symptoms, called exacerbations.” Note that the terms “emphysema” and “chronic bronchitis” have been removed in favor of a more comprehensive description of the pathophysiology of COPD. Importantly, the report states that cough and sputum production for at least 3 months in each of 2 consecutive years, previously accepted as diagnostic criteria, are present in only a minority of patients. It is noted that chronic respiratory symptoms may exist without spirometric changes and many patients (usually smokers) have structural evidence of COPD without airflow limitation.
Changes to COPD initial assessment
The primary criterion for diagnosis is unchanged: post-bronchodilator forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) less than 0.70. Spirometry remains important to confirm the diagnosis in those with classic symptoms of dyspnea, chronic cough, and/or sputum production with a history of exposure to noxious particles or gases.
The GOLD assessment system previously incorporated spirometry and included an “ABCD” system such that patients in group A are least severe. Spirometry has been progressively deemphasized in favor of symptom-based classification and the 2017 Report, for the first time, dissociates spirometric findings from severity classification.
The new system uses symptom severity and exacerbation risk to classify COPD. Two specific standardized COPD symptom measurement tools, The Modified British Medical Research Council (mMRC) questionnaire and COPD Assessment Test (CAT), are reported by GOLD as the most widely used. Low symptom severity is considered an mMRC less than or equal to 1 or CAT less than or equal to 9, high symptom severity is considered an mMRC greater than or equal to 2 or CAT greater than or equal to 10. Low risk of exacerbation is defined as no more than one exacerbation not resulting in hospital admission in the last 12 months; high risk of exacerbation is defined as at least two exacerbations or any exacerbations resulting in hospital admission in the last 12 months. Symptom severity and exacerbation risk is divided into four quadrants:
• GOLD group A: Low symptom severity, low exacerbation risk.
• GOLD group B: High symptom severity, low exacerbation risk.
• GOLD group C: Low symptom severity, high exacerbation risk.
• GOLD group D: High symptom severity, high exacerbation risk.
Changes to prevention and management of stable COPD
Smoking cessation remains important in the prevention of COPD. The 2017 Report reflects the U.S. Preventive Services Task Force’s guidelines for smoking cessation: Offer nicotine replacement, cessation counseling, and pharmacotherapy (varenicline, bupropion or nortriptyline). There is insufficient evidence to support the use of e-cigarettes. Influenza and pneumococcal vaccinations are recommended. Pulmonary rehabilitation remains important.
The 2017 Report includes an expanded discussion of COPD medications. The role of short-acting bronchodilators (SABD) in COPD remains prominent. Changes include a stronger recommendation to use combination short-acting beta-agonists and short-acting muscarinic antagonists (SABA/SAMA) as these seem to be superior to SABD monotherapy in improving symptoms and FEV1.
There were several changes to the pharmacologic treatment algorithm. For the first time, GOLD proposes escalation strategies. Preference is given to LABA/LAMA (long-acting beta-agonist/long-acting muscarinic antagonists) combinations over LABA/ICS (long-acting beta-agonist/inhaled corticosteroid) combinations as a mainstay of treatment. The rationale for this change is that LABA/LAMAs give greater bronchodilation compared with LABA/ICS, and one study showed a decreased rate of exacerbations compared to LABA/ICS in patients with a history of exacerbations. In addition, patients with COPD who receive ICS appear to have a higher risk of developing pneumonia. GOLD recommendations are:
• Group A: Start with single bronchodilator (short- or long-acting), escalate to alternative class of bronchodilator if necessary.
• Group B: Start with LABA or LAMA, escalate to LABA/LAMA if symptoms persist.
• Group C: Start with LAMA, escalate to LABA/LAMA (preferred) or LABA/ICS if exacerbations continue.
• Group D: Start with LABA/LAMA (preferred) or LAMA monotherapy, escalate to LABA/LAMA/ICS (preferred) or try LABA/ICS before escalating to LAMA/LABA/ICS if symptoms persist or exacerbations continue; roflumilast and/or a macrolide may be considered if further exacerbations occur with LABA/LAMA/ICS.
Bottom line
1. GOLD classification of COPD severity is now based on clinical criteria alone: symptom assessment and risk for exacerbation.
2. SABA/SAMA combination therapy seems to be superior to either SABA or SAMA alone.
3. Patients in group A (milder symptoms, low exacerbation risk) may be initiated on either short- or long-acting bronchodilator therapy.
4. Patients in group B (milder symptoms, increased exacerbation risk) should be initiated on LAMA monotherapy.
5. LABA/LAMA combination therapy seems to be superior to LABA/ICS combination therapy and should be used when long-acting bronchodilator monotherapy fails to control symptoms or reduce exacerbations.
References
1. CDC MMWR 11/23/12
2. Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017 at http://goldcopd.org (accessed 3/10/2017)
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital. Dr. Lent is chief resident in the program.
Chronic obstructive lung disease (COPD) is the third leading cause of death in the United States1 and a major cause of mortality and morbidity around the world. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) released a new “2017 Report”2 with modified recommendations for the diagnosis, management, and prevention of COPD. The report contains several changes that are relevant to the primary care provider that will be outlined below.
Redefining COPD
GOLD’s definition of COPD was changed in its 2017 Report: “COPD is a common, preventable, and treatable disease that is characterized by persistent respiratory symptoms and airflow limitations that are due to airway and/or alveolar abnormalities usually caused by significant exposure to noxious particles or gases.” The report emphasizes that “COPD may be punctuated by periods of acute worsening of respiratory symptoms, called exacerbations.” Note that the terms “emphysema” and “chronic bronchitis” have been removed in favor of a more comprehensive description of the pathophysiology of COPD. Importantly, the report states that cough and sputum production for at least 3 months in each of 2 consecutive years, previously accepted as diagnostic criteria, are present in only a minority of patients. It is noted that chronic respiratory symptoms may exist without spirometric changes and many patients (usually smokers) have structural evidence of COPD without airflow limitation.
Changes to COPD initial assessment
The primary criterion for diagnosis is unchanged: post-bronchodilator forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) less than 0.70. Spirometry remains important to confirm the diagnosis in those with classic symptoms of dyspnea, chronic cough, and/or sputum production with a history of exposure to noxious particles or gases.
The GOLD assessment system previously incorporated spirometry and included an “ABCD” system such that patients in group A are least severe. Spirometry has been progressively deemphasized in favor of symptom-based classification and the 2017 Report, for the first time, dissociates spirometric findings from severity classification.
The new system uses symptom severity and exacerbation risk to classify COPD. Two specific standardized COPD symptom measurement tools, The Modified British Medical Research Council (mMRC) questionnaire and COPD Assessment Test (CAT), are reported by GOLD as the most widely used. Low symptom severity is considered an mMRC less than or equal to 1 or CAT less than or equal to 9, high symptom severity is considered an mMRC greater than or equal to 2 or CAT greater than or equal to 10. Low risk of exacerbation is defined as no more than one exacerbation not resulting in hospital admission in the last 12 months; high risk of exacerbation is defined as at least two exacerbations or any exacerbations resulting in hospital admission in the last 12 months. Symptom severity and exacerbation risk is divided into four quadrants:
• GOLD group A: Low symptom severity, low exacerbation risk.
• GOLD group B: High symptom severity, low exacerbation risk.
• GOLD group C: Low symptom severity, high exacerbation risk.
• GOLD group D: High symptom severity, high exacerbation risk.
Changes to prevention and management of stable COPD
Smoking cessation remains important in the prevention of COPD. The 2017 Report reflects the U.S. Preventive Services Task Force’s guidelines for smoking cessation: Offer nicotine replacement, cessation counseling, and pharmacotherapy (varenicline, bupropion or nortriptyline). There is insufficient evidence to support the use of e-cigarettes. Influenza and pneumococcal vaccinations are recommended. Pulmonary rehabilitation remains important.
The 2017 Report includes an expanded discussion of COPD medications. The role of short-acting bronchodilators (SABD) in COPD remains prominent. Changes include a stronger recommendation to use combination short-acting beta-agonists and short-acting muscarinic antagonists (SABA/SAMA) as these seem to be superior to SABD monotherapy in improving symptoms and FEV1.
There were several changes to the pharmacologic treatment algorithm. For the first time, GOLD proposes escalation strategies. Preference is given to LABA/LAMA (long-acting beta-agonist/long-acting muscarinic antagonists) combinations over LABA/ICS (long-acting beta-agonist/inhaled corticosteroid) combinations as a mainstay of treatment. The rationale for this change is that LABA/LAMAs give greater bronchodilation compared with LABA/ICS, and one study showed a decreased rate of exacerbations compared to LABA/ICS in patients with a history of exacerbations. In addition, patients with COPD who receive ICS appear to have a higher risk of developing pneumonia. GOLD recommendations are:
• Group A: Start with single bronchodilator (short- or long-acting), escalate to alternative class of bronchodilator if necessary.
• Group B: Start with LABA or LAMA, escalate to LABA/LAMA if symptoms persist.
• Group C: Start with LAMA, escalate to LABA/LAMA (preferred) or LABA/ICS if exacerbations continue.
• Group D: Start with LABA/LAMA (preferred) or LAMA monotherapy, escalate to LABA/LAMA/ICS (preferred) or try LABA/ICS before escalating to LAMA/LABA/ICS if symptoms persist or exacerbations continue; roflumilast and/or a macrolide may be considered if further exacerbations occur with LABA/LAMA/ICS.
Bottom line
1. GOLD classification of COPD severity is now based on clinical criteria alone: symptom assessment and risk for exacerbation.
2. SABA/SAMA combination therapy seems to be superior to either SABA or SAMA alone.
3. Patients in group A (milder symptoms, low exacerbation risk) may be initiated on either short- or long-acting bronchodilator therapy.
4. Patients in group B (milder symptoms, increased exacerbation risk) should be initiated on LAMA monotherapy.
5. LABA/LAMA combination therapy seems to be superior to LABA/ICS combination therapy and should be used when long-acting bronchodilator monotherapy fails to control symptoms or reduce exacerbations.
References
1. CDC MMWR 11/23/12
2. Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017 at http://goldcopd.org (accessed 3/10/2017)
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital. Dr. Lent is chief resident in the program.
AGA Guideline: Transient elastography in liver fibrosis, most used and most accurate
Vibration-controlled transient elastography (VCTE) can accurately diagnose cirrhosis in most patients with chronic liver disease, particularly those with chronic hepatitis B or C, states a new guideline from the AGA Institute, published in the May issue of Gastroenterology (doi: 10.1053/j.gastro.2017.03.017).
However, magnetic resonance elastography (MRE) is somewhat more accurate for detecting cirrhosis in nonalcoholic fatty liver disease, wrote Joseph K. Lim, MD, AGAF, of Yale University in New Haven, Conn., with his associates from the Clinical Guidelines Committee of the AGA. VCTE is convenient but performs unevenly in some liver conditions and is especially unreliable in patients with acute hepatitis, alcohol abuse, food intake within 2-3 hours, congestive heart failure, or extrahepatic cholestasis, the guideline notes. Yet, VCTE remains the most common imaging tool for diagnosing fibrosis in the United States, and the guideline addresses “focused, clinically relevant questions” to guide its use.
When possible, clinicians should use VCTE instead of noninvasive serum tests for cirrhosis in patients with chronic hepatitis C, the guideline asserts. In pooled analyses of 62 studies, VCTE detected about 89% of cirrhosis cases (95% confidence interval, 84%-92%), Fibrosis-4 test (FIB-4) detected 87% (95% CI, 74%-94%), and aspartate aminotransferase to platelet ratio index (APRI) detected 77% (95% CI, 73%-81%). The specificity of VCTE (91%) also equaled or exceeded that of FIB-4 (91%) or APRI (78%), the guideline noted.
For chronic hepatitis C, MRE had “poorer specificity with higher false-positive rates, suggesting poorer diagnostic performance,” compared with VCTE. Lower cost and lower point-of-care availability make VCTE “an attractive solution compared to MRE,” the guideline adds. It conditionally recommends VCTE cutoffs of 12.5 kPa for cirrhosis and 9.5 kPa for advanced (F3-F4) liver fibrosis after patients have a sustained virologic response to therapy. The 9.5-kPa cutoff would misclassify only 1% of low-risk patients and 7% of high-risk patients, but noncirrhotic patients (less than 9.5 kPa) may reasonably choose to continue specialty care if they prioritize avoiding “the small risk” of hepatocellular carcinoma over the “inconvenience and risks of continued laboratory and fibrosis testing.”
For chronic hepatitis B, the guideline conditionally recommends VCTE with an 11.0-kPa cutoff over APRI or FIB-4. In a pooled analysis of 28 studies, VCTE detected cirrhosis with a sensitivity of 86% and a specificity of 85%, compared with 66% and 74%, respectively, for APRI, and 87% and 65%, respectively, for FIB-4. However, the overall diagnostic performance of VCTE resembled that of the serum tests, and clinicians should interpret VCTE in the context of other clinical cirrhosis data, the guideline states.
Among 17 studies of VCTE cutoffs in hepatitis B, an 11.0-kPa threshold diagnosed cirrhosis with a sensitivity of 81% and a specificity of 83%. This cutoff would miss cirrhosis in less than 1% of low-risk patients and about 5% of high-risk patients and would yield false positives in 10%-15% of patients. Thus, its cutoff minimizes false negatives, reflecting “a judgment that the harm of missing cirrhosis is greater than the harm of over diagnosis,” the authors write.
For chronic alcoholic liver disease, the AGA conditionally recommends VCTE with a cirrhosis cutoff of 12.5 kPa. In pooled analyses, this value had a sensitivity of 95% and a specificity of 71%. For suspected compensated cirrhosis, the guideline conditionally suggests a 19.5-kPa cutoff when assessing the need for esophagogastroduodenoscopy (EGD) to identify high-risk esophageal varices. Patients who fall below this cutoff can reasonably pursue screening endoscopy if they are concerned about the small risk of acute variceal hemorrhage, the guideline adds.
The guideline also conditionally recommends a 17-kPa cutoff to detect clinically significant portal hypertension in patients with suspected chronic liver disease who are undergoing elective nonhepatic surgeries. This cutoff will miss about 0.1% of very low-risk patients, 0.8% of low-risk patients, and 7% of high-risk patients. Because the failure to detect portal hypertension contributes to operative morbidity and mortality, higher-risk patients might “reasonably” pursue screening endoscopy even if their kPa is below the cutoff, the guideline states.
The guideline made no recommendation about VCTE versus APRI or FIB-4 in adults with nonalcoholic fatty liver disease (NAFLD), citing “unacceptable bias” in 12 studies that excluded obese patients, used per-protocol rather than intention-to-diagnose analyses, and ignored “unsuccessful or inadequate” liver stiffness measurements, which are relatively common in NAFLD, the guideline notes. It conditionally recommends MRE over VCTE in high-risk adults with NAFLD, including those who are older, diabetic, or obese (especially with central adiposity) or who have alanine levels more than twice the upper limit of normal. However, it cites insufficient evidence to extend this recommendation to low-risk patients who only have imaging evidence of fatty liver.
Overall, the guideline focuses on “routine clinical management issues, and [does] not address comparisons with proprietary serum fibrosis assays, other emerging imaging-based fibrosis assessment techniques, or combinations of more than one noninvasive fibrosis test,” the authors note. They also limited VCTE cutoffs to single thresholds that prioritized sensitivity over specificity. “Additional studies are needed to further define the role of VCTE, MRE, and emerging diagnostic studies in the assessment of liver fibrosis, for which a significant unmet medical need remains, particularly in conditions such as NAFLD/[nonalcoholic steatohepatitis],” they add. “In particular, defining the implications for serial liver stiffness measurements over time on management decisions is of great interest.”
Dr. Muir has served as a consultant for AbbVie, Bristol-Myers Squibb, Gilead, and Merck. Dr. Lim has served as a consultant for Bristol Myers-Squibb, Gilead, Merck, and Boehringer Ingelheim. Dr. Flamm has served as a consultant or received research support from Gilead, Bristol-Myers Squibb, AbbVie, Salix Pharmaceuticals, and Intercept Pharmaceuticals. Dr. Dieterich has presented lectures for Gilead and Merck products. The rest of the authors disclosed no conflicts related to the content of this guideline.
Vibration-controlled transient elastography (VCTE) can accurately diagnose cirrhosis in most patients with chronic liver disease, particularly those with chronic hepatitis B or C, states a new guideline from the AGA Institute, published in the May issue of Gastroenterology (doi: 10.1053/j.gastro.2017.03.017).
However, magnetic resonance elastography (MRE) is somewhat more accurate for detecting cirrhosis in nonalcoholic fatty liver disease, wrote Joseph K. Lim, MD, AGAF, of Yale University in New Haven, Conn., with his associates from the Clinical Guidelines Committee of the AGA. VCTE is convenient but performs unevenly in some liver conditions and is especially unreliable in patients with acute hepatitis, alcohol abuse, food intake within 2-3 hours, congestive heart failure, or extrahepatic cholestasis, the guideline notes. Yet, VCTE remains the most common imaging tool for diagnosing fibrosis in the United States, and the guideline addresses “focused, clinically relevant questions” to guide its use.
When possible, clinicians should use VCTE instead of noninvasive serum tests for cirrhosis in patients with chronic hepatitis C, the guideline asserts. In pooled analyses of 62 studies, VCTE detected about 89% of cirrhosis cases (95% confidence interval, 84%-92%), Fibrosis-4 test (FIB-4) detected 87% (95% CI, 74%-94%), and aspartate aminotransferase to platelet ratio index (APRI) detected 77% (95% CI, 73%-81%). The specificity of VCTE (91%) also equaled or exceeded that of FIB-4 (91%) or APRI (78%), the guideline noted.
For chronic hepatitis C, MRE had “poorer specificity with higher false-positive rates, suggesting poorer diagnostic performance,” compared with VCTE. Lower cost and lower point-of-care availability make VCTE “an attractive solution compared to MRE,” the guideline adds. It conditionally recommends VCTE cutoffs of 12.5 kPa for cirrhosis and 9.5 kPa for advanced (F3-F4) liver fibrosis after patients have a sustained virologic response to therapy. The 9.5-kPa cutoff would misclassify only 1% of low-risk patients and 7% of high-risk patients, but noncirrhotic patients (less than 9.5 kPa) may reasonably choose to continue specialty care if they prioritize avoiding “the small risk” of hepatocellular carcinoma over the “inconvenience and risks of continued laboratory and fibrosis testing.”
For chronic hepatitis B, the guideline conditionally recommends VCTE with an 11.0-kPa cutoff over APRI or FIB-4. In a pooled analysis of 28 studies, VCTE detected cirrhosis with a sensitivity of 86% and a specificity of 85%, compared with 66% and 74%, respectively, for APRI, and 87% and 65%, respectively, for FIB-4. However, the overall diagnostic performance of VCTE resembled that of the serum tests, and clinicians should interpret VCTE in the context of other clinical cirrhosis data, the guideline states.
Among 17 studies of VCTE cutoffs in hepatitis B, an 11.0-kPa threshold diagnosed cirrhosis with a sensitivity of 81% and a specificity of 83%. This cutoff would miss cirrhosis in less than 1% of low-risk patients and about 5% of high-risk patients and would yield false positives in 10%-15% of patients. Thus, its cutoff minimizes false negatives, reflecting “a judgment that the harm of missing cirrhosis is greater than the harm of over diagnosis,” the authors write.
For chronic alcoholic liver disease, the AGA conditionally recommends VCTE with a cirrhosis cutoff of 12.5 kPa. In pooled analyses, this value had a sensitivity of 95% and a specificity of 71%. For suspected compensated cirrhosis, the guideline conditionally suggests a 19.5-kPa cutoff when assessing the need for esophagogastroduodenoscopy (EGD) to identify high-risk esophageal varices. Patients who fall below this cutoff can reasonably pursue screening endoscopy if they are concerned about the small risk of acute variceal hemorrhage, the guideline adds.
The guideline also conditionally recommends a 17-kPa cutoff to detect clinically significant portal hypertension in patients with suspected chronic liver disease who are undergoing elective nonhepatic surgeries. This cutoff will miss about 0.1% of very low-risk patients, 0.8% of low-risk patients, and 7% of high-risk patients. Because the failure to detect portal hypertension contributes to operative morbidity and mortality, higher-risk patients might “reasonably” pursue screening endoscopy even if their kPa is below the cutoff, the guideline states.
The guideline made no recommendation about VCTE versus APRI or FIB-4 in adults with nonalcoholic fatty liver disease (NAFLD), citing “unacceptable bias” in 12 studies that excluded obese patients, used per-protocol rather than intention-to-diagnose analyses, and ignored “unsuccessful or inadequate” liver stiffness measurements, which are relatively common in NAFLD, the guideline notes. It conditionally recommends MRE over VCTE in high-risk adults with NAFLD, including those who are older, diabetic, or obese (especially with central adiposity) or who have alanine levels more than twice the upper limit of normal. However, it cites insufficient evidence to extend this recommendation to low-risk patients who only have imaging evidence of fatty liver.
Overall, the guideline focuses on “routine clinical management issues, and [does] not address comparisons with proprietary serum fibrosis assays, other emerging imaging-based fibrosis assessment techniques, or combinations of more than one noninvasive fibrosis test,” the authors note. They also limited VCTE cutoffs to single thresholds that prioritized sensitivity over specificity. “Additional studies are needed to further define the role of VCTE, MRE, and emerging diagnostic studies in the assessment of liver fibrosis, for which a significant unmet medical need remains, particularly in conditions such as NAFLD/[nonalcoholic steatohepatitis],” they add. “In particular, defining the implications for serial liver stiffness measurements over time on management decisions is of great interest.”
Dr. Muir has served as a consultant for AbbVie, Bristol-Myers Squibb, Gilead, and Merck. Dr. Lim has served as a consultant for Bristol Myers-Squibb, Gilead, Merck, and Boehringer Ingelheim. Dr. Flamm has served as a consultant or received research support from Gilead, Bristol-Myers Squibb, AbbVie, Salix Pharmaceuticals, and Intercept Pharmaceuticals. Dr. Dieterich has presented lectures for Gilead and Merck products. The rest of the authors disclosed no conflicts related to the content of this guideline.
Vibration-controlled transient elastography (VCTE) can accurately diagnose cirrhosis in most patients with chronic liver disease, particularly those with chronic hepatitis B or C, states a new guideline from the AGA Institute, published in the May issue of Gastroenterology (doi: 10.1053/j.gastro.2017.03.017).
However, magnetic resonance elastography (MRE) is somewhat more accurate for detecting cirrhosis in nonalcoholic fatty liver disease, wrote Joseph K. Lim, MD, AGAF, of Yale University in New Haven, Conn., with his associates from the Clinical Guidelines Committee of the AGA. VCTE is convenient but performs unevenly in some liver conditions and is especially unreliable in patients with acute hepatitis, alcohol abuse, food intake within 2-3 hours, congestive heart failure, or extrahepatic cholestasis, the guideline notes. Yet, VCTE remains the most common imaging tool for diagnosing fibrosis in the United States, and the guideline addresses “focused, clinically relevant questions” to guide its use.
When possible, clinicians should use VCTE instead of noninvasive serum tests for cirrhosis in patients with chronic hepatitis C, the guideline asserts. In pooled analyses of 62 studies, VCTE detected about 89% of cirrhosis cases (95% confidence interval, 84%-92%), Fibrosis-4 test (FIB-4) detected 87% (95% CI, 74%-94%), and aspartate aminotransferase to platelet ratio index (APRI) detected 77% (95% CI, 73%-81%). The specificity of VCTE (91%) also equaled or exceeded that of FIB-4 (91%) or APRI (78%), the guideline noted.
For chronic hepatitis C, MRE had “poorer specificity with higher false-positive rates, suggesting poorer diagnostic performance,” compared with VCTE. Lower cost and lower point-of-care availability make VCTE “an attractive solution compared to MRE,” the guideline adds. It conditionally recommends VCTE cutoffs of 12.5 kPa for cirrhosis and 9.5 kPa for advanced (F3-F4) liver fibrosis after patients have a sustained virologic response to therapy. The 9.5-kPa cutoff would misclassify only 1% of low-risk patients and 7% of high-risk patients, but noncirrhotic patients (less than 9.5 kPa) may reasonably choose to continue specialty care if they prioritize avoiding “the small risk” of hepatocellular carcinoma over the “inconvenience and risks of continued laboratory and fibrosis testing.”
For chronic hepatitis B, the guideline conditionally recommends VCTE with an 11.0-kPa cutoff over APRI or FIB-4. In a pooled analysis of 28 studies, VCTE detected cirrhosis with a sensitivity of 86% and a specificity of 85%, compared with 66% and 74%, respectively, for APRI, and 87% and 65%, respectively, for FIB-4. However, the overall diagnostic performance of VCTE resembled that of the serum tests, and clinicians should interpret VCTE in the context of other clinical cirrhosis data, the guideline states.
Among 17 studies of VCTE cutoffs in hepatitis B, an 11.0-kPa threshold diagnosed cirrhosis with a sensitivity of 81% and a specificity of 83%. This cutoff would miss cirrhosis in less than 1% of low-risk patients and about 5% of high-risk patients and would yield false positives in 10%-15% of patients. Thus, its cutoff minimizes false negatives, reflecting “a judgment that the harm of missing cirrhosis is greater than the harm of over diagnosis,” the authors write.
For chronic alcoholic liver disease, the AGA conditionally recommends VCTE with a cirrhosis cutoff of 12.5 kPa. In pooled analyses, this value had a sensitivity of 95% and a specificity of 71%. For suspected compensated cirrhosis, the guideline conditionally suggests a 19.5-kPa cutoff when assessing the need for esophagogastroduodenoscopy (EGD) to identify high-risk esophageal varices. Patients who fall below this cutoff can reasonably pursue screening endoscopy if they are concerned about the small risk of acute variceal hemorrhage, the guideline adds.
The guideline also conditionally recommends a 17-kPa cutoff to detect clinically significant portal hypertension in patients with suspected chronic liver disease who are undergoing elective nonhepatic surgeries. This cutoff will miss about 0.1% of very low-risk patients, 0.8% of low-risk patients, and 7% of high-risk patients. Because the failure to detect portal hypertension contributes to operative morbidity and mortality, higher-risk patients might “reasonably” pursue screening endoscopy even if their kPa is below the cutoff, the guideline states.
The guideline made no recommendation about VCTE versus APRI or FIB-4 in adults with nonalcoholic fatty liver disease (NAFLD), citing “unacceptable bias” in 12 studies that excluded obese patients, used per-protocol rather than intention-to-diagnose analyses, and ignored “unsuccessful or inadequate” liver stiffness measurements, which are relatively common in NAFLD, the guideline notes. It conditionally recommends MRE over VCTE in high-risk adults with NAFLD, including those who are older, diabetic, or obese (especially with central adiposity) or who have alanine levels more than twice the upper limit of normal. However, it cites insufficient evidence to extend this recommendation to low-risk patients who only have imaging evidence of fatty liver.
Overall, the guideline focuses on “routine clinical management issues, and [does] not address comparisons with proprietary serum fibrosis assays, other emerging imaging-based fibrosis assessment techniques, or combinations of more than one noninvasive fibrosis test,” the authors note. They also limited VCTE cutoffs to single thresholds that prioritized sensitivity over specificity. “Additional studies are needed to further define the role of VCTE, MRE, and emerging diagnostic studies in the assessment of liver fibrosis, for which a significant unmet medical need remains, particularly in conditions such as NAFLD/[nonalcoholic steatohepatitis],” they add. “In particular, defining the implications for serial liver stiffness measurements over time on management decisions is of great interest.”
Dr. Muir has served as a consultant for AbbVie, Bristol-Myers Squibb, Gilead, and Merck. Dr. Lim has served as a consultant for Bristol Myers-Squibb, Gilead, Merck, and Boehringer Ingelheim. Dr. Flamm has served as a consultant or received research support from Gilead, Bristol-Myers Squibb, AbbVie, Salix Pharmaceuticals, and Intercept Pharmaceuticals. Dr. Dieterich has presented lectures for Gilead and Merck products. The rest of the authors disclosed no conflicts related to the content of this guideline.
FROM GASTROENTEROLOGY
AGA Clinical Practice Update: Expert review recommendations on post-SVR hepatitis C care
The AGA Institute issued a clinical practice update for managing hepatitis C virus–infected patients who achieve a sustained virologic response after antiviral therapy, who still require ongoing care for their liver disease. The expert review appears in the May issue of Gastroenterology (doi: 10.1053/j.gastro.2017.03.018).
Even though direct-acting antiviral regimens have produced remarkably high sustained virologic response (SVR) rates and it appears that fewer than 1% of patients relapse, and even though liver fibrosis and cirrhosis may regress with this therapy, continued surveillance and even intervention may be needed “to reduce complications arising from liver damage that has already accrued by the time SVR was attained,” said Ira M. Jacobson, MD, AGAF, chair of the department of medicine, Mount Sinai Beth Israel Medical Center, New York, and his associates.
Dr. Jacobson and his associates at the AGA Institute reviewed the current literature and expert opinion to formulate 11 best-practice recommendations for managing this patient population. Among their recommendations:
SVR should be confirmed by hepatitis C virus RNA testing at 12 weeks after completion of an all-oral direct-acting antiviral regimen, and routine confirmation after 48 weeks is also “prudent.” Further testing for later virologic relapse is not supported by the available evidence. However, further periodic testing is advised for patients at risk for reinfection, such as those who continue to use IV drugs.
All patients with stage 3 or higher liver fibrosis or cirrhosis before achieving SVR should continue to be monitored by liver imaging (with or without serum alpha fetoprotein testing) twice a year “for an indefinite duration.” At present, there is no evidence of a finite point beyond which the risk of hepatocellular carcinoma is reduced to the level of people who don’t have a history of liver disease. And there have been documented cases of hepatocellular carcinoma developing more than 5 years after attaining SVR.
Regardless of SVR status, all patients with liver cirrhosis should undergo endoscopic screening for esophagogastric varices. If no varices or only small varices are detected, repeat endoscopy should be done 2-3 years after achieving SVR. If no varices are identified then, “cessation of further endoscopic screening may be considered on an individual patient basis if there are no risk factors for progressive cirrhosis.”
Noninvasive assessment of fibrosis, such as liver elastography, may be considered on an individual basis after SVR is attained, to assess whether fibrosis has progressed or regressed or to guide clinical management.
All patients who achieve SVR must be counseled regarding factors that could further injure the liver and contribute to the progression of fibrosis, hepatic decompensation, or the development of hepatocellular carcinoma. These include alcohol consumption, fatty liver, diabetes, and potential toxins. If serum liver enzyme levels rise, all patients should be evaluated for possible liver injury.
The AGA Institute issued a clinical practice update for managing hepatitis C virus–infected patients who achieve a sustained virologic response after antiviral therapy, who still require ongoing care for their liver disease. The expert review appears in the May issue of Gastroenterology (doi: 10.1053/j.gastro.2017.03.018).
Even though direct-acting antiviral regimens have produced remarkably high sustained virologic response (SVR) rates and it appears that fewer than 1% of patients relapse, and even though liver fibrosis and cirrhosis may regress with this therapy, continued surveillance and even intervention may be needed “to reduce complications arising from liver damage that has already accrued by the time SVR was attained,” said Ira M. Jacobson, MD, AGAF, chair of the department of medicine, Mount Sinai Beth Israel Medical Center, New York, and his associates.
Dr. Jacobson and his associates at the AGA Institute reviewed the current literature and expert opinion to formulate 11 best-practice recommendations for managing this patient population. Among their recommendations:
SVR should be confirmed by hepatitis C virus RNA testing at 12 weeks after completion of an all-oral direct-acting antiviral regimen, and routine confirmation after 48 weeks is also “prudent.” Further testing for later virologic relapse is not supported by the available evidence. However, further periodic testing is advised for patients at risk for reinfection, such as those who continue to use IV drugs.
All patients with stage 3 or higher liver fibrosis or cirrhosis before achieving SVR should continue to be monitored by liver imaging (with or without serum alpha fetoprotein testing) twice a year “for an indefinite duration.” At present, there is no evidence of a finite point beyond which the risk of hepatocellular carcinoma is reduced to the level of people who don’t have a history of liver disease. And there have been documented cases of hepatocellular carcinoma developing more than 5 years after attaining SVR.
Regardless of SVR status, all patients with liver cirrhosis should undergo endoscopic screening for esophagogastric varices. If no varices or only small varices are detected, repeat endoscopy should be done 2-3 years after achieving SVR. If no varices are identified then, “cessation of further endoscopic screening may be considered on an individual patient basis if there are no risk factors for progressive cirrhosis.”
Noninvasive assessment of fibrosis, such as liver elastography, may be considered on an individual basis after SVR is attained, to assess whether fibrosis has progressed or regressed or to guide clinical management.
All patients who achieve SVR must be counseled regarding factors that could further injure the liver and contribute to the progression of fibrosis, hepatic decompensation, or the development of hepatocellular carcinoma. These include alcohol consumption, fatty liver, diabetes, and potential toxins. If serum liver enzyme levels rise, all patients should be evaluated for possible liver injury.
The AGA Institute issued a clinical practice update for managing hepatitis C virus–infected patients who achieve a sustained virologic response after antiviral therapy, who still require ongoing care for their liver disease. The expert review appears in the May issue of Gastroenterology (doi: 10.1053/j.gastro.2017.03.018).
Even though direct-acting antiviral regimens have produced remarkably high sustained virologic response (SVR) rates and it appears that fewer than 1% of patients relapse, and even though liver fibrosis and cirrhosis may regress with this therapy, continued surveillance and even intervention may be needed “to reduce complications arising from liver damage that has already accrued by the time SVR was attained,” said Ira M. Jacobson, MD, AGAF, chair of the department of medicine, Mount Sinai Beth Israel Medical Center, New York, and his associates.
Dr. Jacobson and his associates at the AGA Institute reviewed the current literature and expert opinion to formulate 11 best-practice recommendations for managing this patient population. Among their recommendations:
SVR should be confirmed by hepatitis C virus RNA testing at 12 weeks after completion of an all-oral direct-acting antiviral regimen, and routine confirmation after 48 weeks is also “prudent.” Further testing for later virologic relapse is not supported by the available evidence. However, further periodic testing is advised for patients at risk for reinfection, such as those who continue to use IV drugs.
All patients with stage 3 or higher liver fibrosis or cirrhosis before achieving SVR should continue to be monitored by liver imaging (with or without serum alpha fetoprotein testing) twice a year “for an indefinite duration.” At present, there is no evidence of a finite point beyond which the risk of hepatocellular carcinoma is reduced to the level of people who don’t have a history of liver disease. And there have been documented cases of hepatocellular carcinoma developing more than 5 years after attaining SVR.
Regardless of SVR status, all patients with liver cirrhosis should undergo endoscopic screening for esophagogastric varices. If no varices or only small varices are detected, repeat endoscopy should be done 2-3 years after achieving SVR. If no varices are identified then, “cessation of further endoscopic screening may be considered on an individual patient basis if there are no risk factors for progressive cirrhosis.”
Noninvasive assessment of fibrosis, such as liver elastography, may be considered on an individual basis after SVR is attained, to assess whether fibrosis has progressed or regressed or to guide clinical management.
All patients who achieve SVR must be counseled regarding factors that could further injure the liver and contribute to the progression of fibrosis, hepatic decompensation, or the development of hepatocellular carcinoma. These include alcohol consumption, fatty liver, diabetes, and potential toxins. If serum liver enzyme levels rise, all patients should be evaluated for possible liver injury.
Key clinical point: The AGA Institute issued a clinical practice update for managing HCV patients who achieve a sustained virologic response after antiviral therapy, who still require ongoing care for their liver disease.
Major finding: SVR should be confirmed by HCV RNA testing at 12 weeks after completion of an all-oral direct-acting antiviral regimen, and routine confirmation after 48 weeks is also “prudent.”
Data source: A review of the literature and of expert opinion to compile 11 best-practice recommendations for managing post-SVR HCV care.
Disclosures: This work was supported by the AGA Institute. Dr. Jacobson reported ties to AbbVie, Bristol-Myers Squibb, Gilead, Intercept, Janssen, Merck, and Trek; one of his associates reported ties to those groups and to Target PharmaSolutions.
New guideline: Address GTCS frequency to reduce SUDEP risk
BOSTON – Generalized tonic-clonic seizures (GTCS) are a major risk factor for sudden unexpected death in epilepsy (SUDEP), which underscores the importance of advising people with epilepsy about controlling such seizures, according to a new practice guideline from the American Academy of Neurology and the American Epilepsy Society.
Though SUDEP is rare, with an incidence rate of 0.22/1,000 patient-years in children with epilepsy and 1.2/1,000 patient-years in adults with epilepsy, the guideline committee found that people with three or more GTCS per year are 15 times more likely to die suddenly than are those without this seizure type. The risk increases with increasing GTCS frequency. This translates to an absolute risk of up to 18 deaths per 1,000 patient-years for people with epilepsy who have frequent GTCS.
Specifically, the guideline recommends that , she said, adding that the guideline shows that “being free of seizures, particularly tonic-clonic seizures, is strongly associated with a decreased risk.”
Guideline coauthor, Elizabeth Donner, MD, added that, for this reason, the guideline recommends “that health professionals work with people who continue to have, specifically, these kind of seizures to try and reduce them with medications or with epilepsy surgery, actively weighing the risks and benefits of any new approach to seizure management.”
The recommendations are based on moderate (Level B) evidence.
The team also looked at numerous other risk factors for SUDEP and found that the strength of the evidence was too weak to support additional recommendations, said Dr. Donner, director of the comprehensive epilepsy program at The Hospital for Sick Children in Toronto and chair of the American Epilepsy Society SUDEP Task Force.
“More research is now needed to identify other preventable risk factors for SUDEP so that we can focus future studies on finding ways to reduce how often SUDEP occurs,” she added.
While the message regarding the importance of reducing seizure frequency is not new, it is important that this message be reiterated in the context of SUDEP, Dr. Donner said.
“It’s very important for it to be clear that the risk of frequent generalized tonic-clonic seizures – and we’re not talking about really frequent here; we’re talking about significant increased risk of death with only three per year – is not related only to maintaining a driver’s license, maintaining work, or other outcomes like that. It’s actually related to risk of death,” she said, noting that she hopes this is a motivator for pursuing treatments beyond medication when medication isn’t successful for treating seizures.
The guideline, which is endorsed by the International Child Neurology Association, is available online and in print (Neurology. 2017;88:1674–80).
Dr. Harden receives royalties from Wiley and Up-to-Date. Dr. Donner has received research support from the Canadian Institutes of Health Research, Dravet Canada, and SUDEP Aware. Other guideline authors reported numerous disclosures, including many industry sources.
BOSTON – Generalized tonic-clonic seizures (GTCS) are a major risk factor for sudden unexpected death in epilepsy (SUDEP), which underscores the importance of advising people with epilepsy about controlling such seizures, according to a new practice guideline from the American Academy of Neurology and the American Epilepsy Society.
Though SUDEP is rare, with an incidence rate of 0.22/1,000 patient-years in children with epilepsy and 1.2/1,000 patient-years in adults with epilepsy, the guideline committee found that people with three or more GTCS per year are 15 times more likely to die suddenly than are those without this seizure type. The risk increases with increasing GTCS frequency. This translates to an absolute risk of up to 18 deaths per 1,000 patient-years for people with epilepsy who have frequent GTCS.
Specifically, the guideline recommends that , she said, adding that the guideline shows that “being free of seizures, particularly tonic-clonic seizures, is strongly associated with a decreased risk.”
Guideline coauthor, Elizabeth Donner, MD, added that, for this reason, the guideline recommends “that health professionals work with people who continue to have, specifically, these kind of seizures to try and reduce them with medications or with epilepsy surgery, actively weighing the risks and benefits of any new approach to seizure management.”
The recommendations are based on moderate (Level B) evidence.
The team also looked at numerous other risk factors for SUDEP and found that the strength of the evidence was too weak to support additional recommendations, said Dr. Donner, director of the comprehensive epilepsy program at The Hospital for Sick Children in Toronto and chair of the American Epilepsy Society SUDEP Task Force.
“More research is now needed to identify other preventable risk factors for SUDEP so that we can focus future studies on finding ways to reduce how often SUDEP occurs,” she added.
While the message regarding the importance of reducing seizure frequency is not new, it is important that this message be reiterated in the context of SUDEP, Dr. Donner said.
“It’s very important for it to be clear that the risk of frequent generalized tonic-clonic seizures – and we’re not talking about really frequent here; we’re talking about significant increased risk of death with only three per year – is not related only to maintaining a driver’s license, maintaining work, or other outcomes like that. It’s actually related to risk of death,” she said, noting that she hopes this is a motivator for pursuing treatments beyond medication when medication isn’t successful for treating seizures.
The guideline, which is endorsed by the International Child Neurology Association, is available online and in print (Neurology. 2017;88:1674–80).
Dr. Harden receives royalties from Wiley and Up-to-Date. Dr. Donner has received research support from the Canadian Institutes of Health Research, Dravet Canada, and SUDEP Aware. Other guideline authors reported numerous disclosures, including many industry sources.
BOSTON – Generalized tonic-clonic seizures (GTCS) are a major risk factor for sudden unexpected death in epilepsy (SUDEP), which underscores the importance of advising people with epilepsy about controlling such seizures, according to a new practice guideline from the American Academy of Neurology and the American Epilepsy Society.
Though SUDEP is rare, with an incidence rate of 0.22/1,000 patient-years in children with epilepsy and 1.2/1,000 patient-years in adults with epilepsy, the guideline committee found that people with three or more GTCS per year are 15 times more likely to die suddenly than are those without this seizure type. The risk increases with increasing GTCS frequency. This translates to an absolute risk of up to 18 deaths per 1,000 patient-years for people with epilepsy who have frequent GTCS.
Specifically, the guideline recommends that , she said, adding that the guideline shows that “being free of seizures, particularly tonic-clonic seizures, is strongly associated with a decreased risk.”
Guideline coauthor, Elizabeth Donner, MD, added that, for this reason, the guideline recommends “that health professionals work with people who continue to have, specifically, these kind of seizures to try and reduce them with medications or with epilepsy surgery, actively weighing the risks and benefits of any new approach to seizure management.”
The recommendations are based on moderate (Level B) evidence.
The team also looked at numerous other risk factors for SUDEP and found that the strength of the evidence was too weak to support additional recommendations, said Dr. Donner, director of the comprehensive epilepsy program at The Hospital for Sick Children in Toronto and chair of the American Epilepsy Society SUDEP Task Force.
“More research is now needed to identify other preventable risk factors for SUDEP so that we can focus future studies on finding ways to reduce how often SUDEP occurs,” she added.
While the message regarding the importance of reducing seizure frequency is not new, it is important that this message be reiterated in the context of SUDEP, Dr. Donner said.
“It’s very important for it to be clear that the risk of frequent generalized tonic-clonic seizures – and we’re not talking about really frequent here; we’re talking about significant increased risk of death with only three per year – is not related only to maintaining a driver’s license, maintaining work, or other outcomes like that. It’s actually related to risk of death,” she said, noting that she hopes this is a motivator for pursuing treatments beyond medication when medication isn’t successful for treating seizures.
The guideline, which is endorsed by the International Child Neurology Association, is available online and in print (Neurology. 2017;88:1674–80).
Dr. Harden receives royalties from Wiley and Up-to-Date. Dr. Donner has received research support from the Canadian Institutes of Health Research, Dravet Canada, and SUDEP Aware. Other guideline authors reported numerous disclosures, including many industry sources.
AT AAN 2017
NCCN myelofibrosis guideline: Patient voice is key
ORLANDO – Referral to a specialized center with expertise in the management of myeloproliferative neoplasms is strongly recommended for all patients diagnosed with myelofibrosis, according to a new treatment guideline from the National Comprehensive Cancer Network.
The guideline is the first in a series addressing myeloproliferative neoplasms (MPNs), and it focuses on the diagnostic work-up of MPNs, as well as the treatment of myelofibrosis. The guideline panel, led by panel chair Ruben A. Mesa, MD, is working next on guidelines for the other two “core classic” Philadelphia chromosome–negative MPNs: polycythemia vera, and essential thrombocythemia.
Nearly two-thirds of myelofibrosis patients have intermediate-risk 2 or high-risk disease, and treatment decisions in these patients are complex and require patient input – particularly in candidates for allogeneic hematopoietic stem cell transplantation, he said.
“These diseases can be a little different than other malignant diseases,” Dr. Mesa said, explaining that while there is a clear risk of progression to acute myeloid leukemia, and from polycythemia vera and essential thrombocythemia to myelofibrosis, and while the diseases can be fatal, the burden patients face is not solely related to mortality.
There are implications in terms of health that are independent of that, such as the risk of thrombosis and bleeding, the potential for cytopenia, and severe splenomegaly that results in significant symptoms, he said.
Further, while molecular mutations and their implications for prognosis are a “rapidly moving part of the discussion,” the care of patients with MPNs involves far more than a molecular understanding of the disease.
In fact, the role of molecular changes in these patients is speculative, he said.
While such changes can be assessed and used for patient stratification, their role in myelofibrosis – unlike in other diseases such as chronic myeloid leukemia where the level of change in a target gene is highly relevant and prognostic, is not yet clear.
Thus, a core aspect of the guideline is inclusion of the voice of the patient in individualizing care, he said, noting that many factors should be considered, including how well the patient metabolizes drugs, and the symptom profile, psychosocial circumstances, support structure, and personal beliefs.
“It’s not solely about the tumor,” he stressed.
In fact, the answer to the question of whether a patient can be symptomatic enough to require a specific treatment is “no,” because of the potential for side effects, risk, expense, and other considerations.
“So the voice of the patient is always a key part [of the decision],” he said, noting also that as with all NCCN guidelines, this guideline is a partnership with the treating physician; deciding who is a transplant candidate is a nuanced issue for which the panel provides “discussion and guidance.”
“But clearly, these guidelines are the most useful and helpful in the setting of experienced providers bringing all of their experiences to bear,” he said.
In general, however, the guidelines call for allogeneic hematopoietic stem cell transplantation (HCT) in those with intermediate-risk 2 or high-risk disease who are transplant candidates, and treatment based on assessment of symptom burden (using the MPN–Symptom Assessment Form Total Symptom Score–10 Items) in those who are not HCT candidates. Those with platelets at 50,000 or below should be considered for clinical trial enrollment, and those with platelets above 50,000 should be considered for a clinical trial or treatment with the oral JAK1 and JAK2 inhibitor ruxolitinib, which has been shown to have beneficial effects on both symptoms and survival and which is approved for patients with platelets above 50,000. .
Treated patients should be monitored for response and for signs and symptoms of disease progression every 3-6 months. Treatment should continue in those who respond, as well as in those who do not – as long as there is no disease progression.
Those with progressive disease include patients who are moving toward acute leukemia, and those with overt acute leukemia.
“Here is where the key decision occurs. Are they or are they not a transplant candidate? If they are a candidate, we have a potentially curative track which would include cytoreduction followed by transplant,” Dr. Mesa said.
Cytoreduction can involve hypomethylating agents if the patient doesn’t have excess blast cells or too high a burden of disease.
Acute myeloid leukemia–like induction chemotherapy followed by allogeneic HCT is also an option in these patients.
As for treatment of low-risk myelofibrosis, the guideline states that asymptomatic patients can be observed or enrolled in a clinical trial and monitored for progression every 3-6 months, and that symptomatic patients should receive ruxolitinib or interferons (which are used off label), or be enrolled in a clinical trial. Treatment is important for patients with particularly difficult symptoms, he said, noting that some patients have had pruritus so severe that they have committed suicide. Treatment should continue unless monitoring shows signs of progression to intermediate risk 1, intermediate risk 2/high-risk, or advanced stage disease.
For those with intermediate risk 1 disease, the guideline calls for observation or ruxolitinib in those who are symptomatic, or clinical trial enrollment or allogeneic HCT. Treatment should continue unless monitoring shows disease progression, in which case the appropriate algorithm should be considered.
The guideline also addresses several special circumstances, including the management of anemia in myelofibrosis patients, which can be a difficult issue, he said.
Since the guideline was first published in December, two updates have been incorporated, and Dr. Mesa said that he anticipates regular updates given the rapidly evolving understanding of MPNs and new findings with respect to potential treatment strategies.
He noted that a number of drugs are currently in clinical trials involving patients with myelofibrosis, including the JAK2/FLT3 inhibitor pacritinib, the JAK1/JAK2 inhibitor momelotinib, the active antifibrosing agent PRM-151, and the telomerase inhibitor imetelstat, as well as numerous drug combinations.
Going forward, the guideline panel will be focusing on four different areas of assessment, including new therapies and new genetic therapies, improving transplant outcomes, MPN symptom and quality of life assessment, and nonpharmacologic interventions such as yoga.
“We certainly hope to complement things over time, to look not only at pharmacologic interventions, but others that patients may be able to utilize from a toolkit of resources,” he said.
COMFORT-1 update: ruxolitinib responses durable in myelofibrosis
To date, ruxolitinib is the only Food and Drug Administration–approved drug for the treatment of myelofibrosis.
The randomized controlled phase III COMFORT I and II trials conducted in the United States and Europe, respectively, demonstrated that the oral JAK1/JAK2 inhibitor has a rapid, beneficial impact on both survival and disease-associated enlargement of the spleen and improvement in related symptoms, Dr. Mesa said.
A 5-year update on data from 309 patients in the COMFORT-1 trial, as reported at the annual meeting of the American Society of Clinical Oncology in 2016, confirmed the durability of treatment responses to ruxolitinib in patients initially randomized to receive the drug, he said.
“We were able to demonstrate a continued survival advantage for those individuals receiving ruxolitinib,” he added.
At weeks 24 and 264, the mean spleen volume reduction was 31.6% and 37.6%, respectively, in those originally randomized to ruxolitinib. The median duration of at least 35% spleen volume reduction was 168.3 weeks.
Overall survival favored ruxolitinib (hazard ratio, 0.69). Median overall survival in the ruxolitinib group had not yet been reached.
“But we realize our work is not done. The survival curve does not plateau; we are not curing these patients. We’re having meaningful impact, but we have room to continue to improve,” he said.
Also, there is an initial drop in platelet counts that tends to stabilize, but not improve, and there is worsening of anemia (new onset grade 3 or 4 anemia was 25.2% with ruxolitinib, and 26.1% in 111 of 154 patients who crossed over from the placebo group), and although this tends to improve, these are among areas of unmet need, he added.
Further, long-term risks of treatment include cutaneous malignancies (basal cell carcinoma occurred in 7.7% and 9.0% of treatment and crossover patients, respectively), which are difficult to separate from baseline hydroxyurea use, and increased risk of herpes zoster (which occurred in 10.3% and 13.5% of treated and crossover patients).
However, there appears to be no increased risk – and there may be a slight decreased risk – of progression to acute leukemia, Dr. Mesa said.
Dr. Mesa disclosed that he has received consulting fees, honoraria, and/or grant/research support from ARIAD Pharmaceuticals, Celgene, CTI BioPharma, Galena Biopharma, Gilead Sciences, Incyte, Novartis Pharmaceuticals, and Promedior.
A step toward harmonizing treatment
Myelofibrosis is a rare chronic leukemia with a complex biology. Disease heterogeneity poses several challenges in the appropriate selection and timing of treatments in this disorder. The NCCN Practice Guidelines in Myelofibrosis is an important step towards harmonizing clinical practice for treating this disease and improving the care of patients.
Vikas Gupta, MD, FRCP, FRCPath, is Director of The Elizabeth and Tony Comper MPN Program at Princess Margaret Cancer Centre in Toronto and a member of the editorial advisory board of Hematology News.
A step toward harmonizing treatment
Myelofibrosis is a rare chronic leukemia with a complex biology. Disease heterogeneity poses several challenges in the appropriate selection and timing of treatments in this disorder. The NCCN Practice Guidelines in Myelofibrosis is an important step towards harmonizing clinical practice for treating this disease and improving the care of patients.
Vikas Gupta, MD, FRCP, FRCPath, is Director of The Elizabeth and Tony Comper MPN Program at Princess Margaret Cancer Centre in Toronto and a member of the editorial advisory board of Hematology News.
A step toward harmonizing treatment
Myelofibrosis is a rare chronic leukemia with a complex biology. Disease heterogeneity poses several challenges in the appropriate selection and timing of treatments in this disorder. The NCCN Practice Guidelines in Myelofibrosis is an important step towards harmonizing clinical practice for treating this disease and improving the care of patients.
Vikas Gupta, MD, FRCP, FRCPath, is Director of The Elizabeth and Tony Comper MPN Program at Princess Margaret Cancer Centre in Toronto and a member of the editorial advisory board of Hematology News.
ORLANDO – Referral to a specialized center with expertise in the management of myeloproliferative neoplasms is strongly recommended for all patients diagnosed with myelofibrosis, according to a new treatment guideline from the National Comprehensive Cancer Network.
The guideline is the first in a series addressing myeloproliferative neoplasms (MPNs), and it focuses on the diagnostic work-up of MPNs, as well as the treatment of myelofibrosis. The guideline panel, led by panel chair Ruben A. Mesa, MD, is working next on guidelines for the other two “core classic” Philadelphia chromosome–negative MPNs: polycythemia vera, and essential thrombocythemia.
Nearly two-thirds of myelofibrosis patients have intermediate-risk 2 or high-risk disease, and treatment decisions in these patients are complex and require patient input – particularly in candidates for allogeneic hematopoietic stem cell transplantation, he said.
“These diseases can be a little different than other malignant diseases,” Dr. Mesa said, explaining that while there is a clear risk of progression to acute myeloid leukemia, and from polycythemia vera and essential thrombocythemia to myelofibrosis, and while the diseases can be fatal, the burden patients face is not solely related to mortality.
There are implications in terms of health that are independent of that, such as the risk of thrombosis and bleeding, the potential for cytopenia, and severe splenomegaly that results in significant symptoms, he said.
Further, while molecular mutations and their implications for prognosis are a “rapidly moving part of the discussion,” the care of patients with MPNs involves far more than a molecular understanding of the disease.
In fact, the role of molecular changes in these patients is speculative, he said.
While such changes can be assessed and used for patient stratification, their role in myelofibrosis – unlike in other diseases such as chronic myeloid leukemia where the level of change in a target gene is highly relevant and prognostic, is not yet clear.
Thus, a core aspect of the guideline is inclusion of the voice of the patient in individualizing care, he said, noting that many factors should be considered, including how well the patient metabolizes drugs, and the symptom profile, psychosocial circumstances, support structure, and personal beliefs.
“It’s not solely about the tumor,” he stressed.
In fact, the answer to the question of whether a patient can be symptomatic enough to require a specific treatment is “no,” because of the potential for side effects, risk, expense, and other considerations.
“So the voice of the patient is always a key part [of the decision],” he said, noting also that as with all NCCN guidelines, this guideline is a partnership with the treating physician; deciding who is a transplant candidate is a nuanced issue for which the panel provides “discussion and guidance.”
“But clearly, these guidelines are the most useful and helpful in the setting of experienced providers bringing all of their experiences to bear,” he said.
In general, however, the guidelines call for allogeneic hematopoietic stem cell transplantation (HCT) in those with intermediate-risk 2 or high-risk disease who are transplant candidates, and treatment based on assessment of symptom burden (using the MPN–Symptom Assessment Form Total Symptom Score–10 Items) in those who are not HCT candidates. Those with platelets at 50,000 or below should be considered for clinical trial enrollment, and those with platelets above 50,000 should be considered for a clinical trial or treatment with the oral JAK1 and JAK2 inhibitor ruxolitinib, which has been shown to have beneficial effects on both symptoms and survival and which is approved for patients with platelets above 50,000. .
Treated patients should be monitored for response and for signs and symptoms of disease progression every 3-6 months. Treatment should continue in those who respond, as well as in those who do not – as long as there is no disease progression.
Those with progressive disease include patients who are moving toward acute leukemia, and those with overt acute leukemia.
“Here is where the key decision occurs. Are they or are they not a transplant candidate? If they are a candidate, we have a potentially curative track which would include cytoreduction followed by transplant,” Dr. Mesa said.
Cytoreduction can involve hypomethylating agents if the patient doesn’t have excess blast cells or too high a burden of disease.
Acute myeloid leukemia–like induction chemotherapy followed by allogeneic HCT is also an option in these patients.
As for treatment of low-risk myelofibrosis, the guideline states that asymptomatic patients can be observed or enrolled in a clinical trial and monitored for progression every 3-6 months, and that symptomatic patients should receive ruxolitinib or interferons (which are used off label), or be enrolled in a clinical trial. Treatment is important for patients with particularly difficult symptoms, he said, noting that some patients have had pruritus so severe that they have committed suicide. Treatment should continue unless monitoring shows signs of progression to intermediate risk 1, intermediate risk 2/high-risk, or advanced stage disease.
For those with intermediate risk 1 disease, the guideline calls for observation or ruxolitinib in those who are symptomatic, or clinical trial enrollment or allogeneic HCT. Treatment should continue unless monitoring shows disease progression, in which case the appropriate algorithm should be considered.
The guideline also addresses several special circumstances, including the management of anemia in myelofibrosis patients, which can be a difficult issue, he said.
Since the guideline was first published in December, two updates have been incorporated, and Dr. Mesa said that he anticipates regular updates given the rapidly evolving understanding of MPNs and new findings with respect to potential treatment strategies.
He noted that a number of drugs are currently in clinical trials involving patients with myelofibrosis, including the JAK2/FLT3 inhibitor pacritinib, the JAK1/JAK2 inhibitor momelotinib, the active antifibrosing agent PRM-151, and the telomerase inhibitor imetelstat, as well as numerous drug combinations.
Going forward, the guideline panel will be focusing on four different areas of assessment, including new therapies and new genetic therapies, improving transplant outcomes, MPN symptom and quality of life assessment, and nonpharmacologic interventions such as yoga.
“We certainly hope to complement things over time, to look not only at pharmacologic interventions, but others that patients may be able to utilize from a toolkit of resources,” he said.
COMFORT-1 update: ruxolitinib responses durable in myelofibrosis
To date, ruxolitinib is the only Food and Drug Administration–approved drug for the treatment of myelofibrosis.
The randomized controlled phase III COMFORT I and II trials conducted in the United States and Europe, respectively, demonstrated that the oral JAK1/JAK2 inhibitor has a rapid, beneficial impact on both survival and disease-associated enlargement of the spleen and improvement in related symptoms, Dr. Mesa said.
A 5-year update on data from 309 patients in the COMFORT-1 trial, as reported at the annual meeting of the American Society of Clinical Oncology in 2016, confirmed the durability of treatment responses to ruxolitinib in patients initially randomized to receive the drug, he said.
“We were able to demonstrate a continued survival advantage for those individuals receiving ruxolitinib,” he added.
At weeks 24 and 264, the mean spleen volume reduction was 31.6% and 37.6%, respectively, in those originally randomized to ruxolitinib. The median duration of at least 35% spleen volume reduction was 168.3 weeks.
Overall survival favored ruxolitinib (hazard ratio, 0.69). Median overall survival in the ruxolitinib group had not yet been reached.
“But we realize our work is not done. The survival curve does not plateau; we are not curing these patients. We’re having meaningful impact, but we have room to continue to improve,” he said.
Also, there is an initial drop in platelet counts that tends to stabilize, but not improve, and there is worsening of anemia (new onset grade 3 or 4 anemia was 25.2% with ruxolitinib, and 26.1% in 111 of 154 patients who crossed over from the placebo group), and although this tends to improve, these are among areas of unmet need, he added.
Further, long-term risks of treatment include cutaneous malignancies (basal cell carcinoma occurred in 7.7% and 9.0% of treatment and crossover patients, respectively), which are difficult to separate from baseline hydroxyurea use, and increased risk of herpes zoster (which occurred in 10.3% and 13.5% of treated and crossover patients).
However, there appears to be no increased risk – and there may be a slight decreased risk – of progression to acute leukemia, Dr. Mesa said.
Dr. Mesa disclosed that he has received consulting fees, honoraria, and/or grant/research support from ARIAD Pharmaceuticals, Celgene, CTI BioPharma, Galena Biopharma, Gilead Sciences, Incyte, Novartis Pharmaceuticals, and Promedior.
ORLANDO – Referral to a specialized center with expertise in the management of myeloproliferative neoplasms is strongly recommended for all patients diagnosed with myelofibrosis, according to a new treatment guideline from the National Comprehensive Cancer Network.
The guideline is the first in a series addressing myeloproliferative neoplasms (MPNs), and it focuses on the diagnostic work-up of MPNs, as well as the treatment of myelofibrosis. The guideline panel, led by panel chair Ruben A. Mesa, MD, is working next on guidelines for the other two “core classic” Philadelphia chromosome–negative MPNs: polycythemia vera, and essential thrombocythemia.
Nearly two-thirds of myelofibrosis patients have intermediate-risk 2 or high-risk disease, and treatment decisions in these patients are complex and require patient input – particularly in candidates for allogeneic hematopoietic stem cell transplantation, he said.
“These diseases can be a little different than other malignant diseases,” Dr. Mesa said, explaining that while there is a clear risk of progression to acute myeloid leukemia, and from polycythemia vera and essential thrombocythemia to myelofibrosis, and while the diseases can be fatal, the burden patients face is not solely related to mortality.
There are implications in terms of health that are independent of that, such as the risk of thrombosis and bleeding, the potential for cytopenia, and severe splenomegaly that results in significant symptoms, he said.
Further, while molecular mutations and their implications for prognosis are a “rapidly moving part of the discussion,” the care of patients with MPNs involves far more than a molecular understanding of the disease.
In fact, the role of molecular changes in these patients is speculative, he said.
While such changes can be assessed and used for patient stratification, their role in myelofibrosis – unlike in other diseases such as chronic myeloid leukemia where the level of change in a target gene is highly relevant and prognostic, is not yet clear.
Thus, a core aspect of the guideline is inclusion of the voice of the patient in individualizing care, he said, noting that many factors should be considered, including how well the patient metabolizes drugs, and the symptom profile, psychosocial circumstances, support structure, and personal beliefs.
“It’s not solely about the tumor,” he stressed.
In fact, the answer to the question of whether a patient can be symptomatic enough to require a specific treatment is “no,” because of the potential for side effects, risk, expense, and other considerations.
“So the voice of the patient is always a key part [of the decision],” he said, noting also that as with all NCCN guidelines, this guideline is a partnership with the treating physician; deciding who is a transplant candidate is a nuanced issue for which the panel provides “discussion and guidance.”
“But clearly, these guidelines are the most useful and helpful in the setting of experienced providers bringing all of their experiences to bear,” he said.
In general, however, the guidelines call for allogeneic hematopoietic stem cell transplantation (HCT) in those with intermediate-risk 2 or high-risk disease who are transplant candidates, and treatment based on assessment of symptom burden (using the MPN–Symptom Assessment Form Total Symptom Score–10 Items) in those who are not HCT candidates. Those with platelets at 50,000 or below should be considered for clinical trial enrollment, and those with platelets above 50,000 should be considered for a clinical trial or treatment with the oral JAK1 and JAK2 inhibitor ruxolitinib, which has been shown to have beneficial effects on both symptoms and survival and which is approved for patients with platelets above 50,000. .
Treated patients should be monitored for response and for signs and symptoms of disease progression every 3-6 months. Treatment should continue in those who respond, as well as in those who do not – as long as there is no disease progression.
Those with progressive disease include patients who are moving toward acute leukemia, and those with overt acute leukemia.
“Here is where the key decision occurs. Are they or are they not a transplant candidate? If they are a candidate, we have a potentially curative track which would include cytoreduction followed by transplant,” Dr. Mesa said.
Cytoreduction can involve hypomethylating agents if the patient doesn’t have excess blast cells or too high a burden of disease.
Acute myeloid leukemia–like induction chemotherapy followed by allogeneic HCT is also an option in these patients.
As for treatment of low-risk myelofibrosis, the guideline states that asymptomatic patients can be observed or enrolled in a clinical trial and monitored for progression every 3-6 months, and that symptomatic patients should receive ruxolitinib or interferons (which are used off label), or be enrolled in a clinical trial. Treatment is important for patients with particularly difficult symptoms, he said, noting that some patients have had pruritus so severe that they have committed suicide. Treatment should continue unless monitoring shows signs of progression to intermediate risk 1, intermediate risk 2/high-risk, or advanced stage disease.
For those with intermediate risk 1 disease, the guideline calls for observation or ruxolitinib in those who are symptomatic, or clinical trial enrollment or allogeneic HCT. Treatment should continue unless monitoring shows disease progression, in which case the appropriate algorithm should be considered.
The guideline also addresses several special circumstances, including the management of anemia in myelofibrosis patients, which can be a difficult issue, he said.
Since the guideline was first published in December, two updates have been incorporated, and Dr. Mesa said that he anticipates regular updates given the rapidly evolving understanding of MPNs and new findings with respect to potential treatment strategies.
He noted that a number of drugs are currently in clinical trials involving patients with myelofibrosis, including the JAK2/FLT3 inhibitor pacritinib, the JAK1/JAK2 inhibitor momelotinib, the active antifibrosing agent PRM-151, and the telomerase inhibitor imetelstat, as well as numerous drug combinations.
Going forward, the guideline panel will be focusing on four different areas of assessment, including new therapies and new genetic therapies, improving transplant outcomes, MPN symptom and quality of life assessment, and nonpharmacologic interventions such as yoga.
“We certainly hope to complement things over time, to look not only at pharmacologic interventions, but others that patients may be able to utilize from a toolkit of resources,” he said.
COMFORT-1 update: ruxolitinib responses durable in myelofibrosis
To date, ruxolitinib is the only Food and Drug Administration–approved drug for the treatment of myelofibrosis.
The randomized controlled phase III COMFORT I and II trials conducted in the United States and Europe, respectively, demonstrated that the oral JAK1/JAK2 inhibitor has a rapid, beneficial impact on both survival and disease-associated enlargement of the spleen and improvement in related symptoms, Dr. Mesa said.
A 5-year update on data from 309 patients in the COMFORT-1 trial, as reported at the annual meeting of the American Society of Clinical Oncology in 2016, confirmed the durability of treatment responses to ruxolitinib in patients initially randomized to receive the drug, he said.
“We were able to demonstrate a continued survival advantage for those individuals receiving ruxolitinib,” he added.
At weeks 24 and 264, the mean spleen volume reduction was 31.6% and 37.6%, respectively, in those originally randomized to ruxolitinib. The median duration of at least 35% spleen volume reduction was 168.3 weeks.
Overall survival favored ruxolitinib (hazard ratio, 0.69). Median overall survival in the ruxolitinib group had not yet been reached.
“But we realize our work is not done. The survival curve does not plateau; we are not curing these patients. We’re having meaningful impact, but we have room to continue to improve,” he said.
Also, there is an initial drop in platelet counts that tends to stabilize, but not improve, and there is worsening of anemia (new onset grade 3 or 4 anemia was 25.2% with ruxolitinib, and 26.1% in 111 of 154 patients who crossed over from the placebo group), and although this tends to improve, these are among areas of unmet need, he added.
Further, long-term risks of treatment include cutaneous malignancies (basal cell carcinoma occurred in 7.7% and 9.0% of treatment and crossover patients, respectively), which are difficult to separate from baseline hydroxyurea use, and increased risk of herpes zoster (which occurred in 10.3% and 13.5% of treated and crossover patients).
However, there appears to be no increased risk – and there may be a slight decreased risk – of progression to acute leukemia, Dr. Mesa said.
Dr. Mesa disclosed that he has received consulting fees, honoraria, and/or grant/research support from ARIAD Pharmaceuticals, Celgene, CTI BioPharma, Galena Biopharma, Gilead Sciences, Incyte, Novartis Pharmaceuticals, and Promedior.
EXPERT ANALYSIS AT THE NCCN ANNUAL CONFERENCE
Eliminating hepatitis in the United States: A road map
An ambitious new report by the National Academies of Sciences, Engineering, and Medicine lays out a detailed path by which some 90,000 deaths from hepatitis B and C infection could be prevented by 2030.
The National Academies, a group of nongovernmental advisory bodies that includes the former Institute of Medicine, said that “the tools to prevent these deaths” exist – namely vaccination to prevent new hepatitis B infections and antiviral drugs, including new oral medications that can cure chronic hepatitis C infections within months.
The authors of the 200-plus-page report, led by Brian Strom, MD, MPH, of Rutgers University in Newark, NJ, calculate that deaths from hepatitis B infection could be halved by 2030 if 90% of patients are diagnosed, if 90% of those diagnosed are connected to care, and if 80% of those for whom treatment is indicated receive it. Treating everyone with chronic hepatitis C would reduce new infections by 90% by 2030, while reducing related deaths by 65%, Dr. Strom and his colleagues estimate.
But the authors also concede that drastic changes to current health policy would be required to reach these goals. These include the adoption of “aggressive testing, diagnosis, treatment, and prevention methods, such as needle exchange.”
They propose that the federal government seek a unique licensing arrangement with one or more manufacturers to bring down the notoriously high cost of direct-acting drugs used in hepatitis C, as a way of raising treatment rates. Currently, fewer than half the patients on Medicaid who are eligible for hepatitis C treatment receive it, and fewer than 1% of prisoners, who have high rates of infection.
Dr. Joseph Lim, director of the viral hepatitis program at Yale University in New Haven. Conn., who was not involved in the National Academies report, called it helpful in the sense that “it casts a spotlight on something that those of us involved in the care of people with viral hepatitis have long known – which is that this is a national and global public health burden that has been under the radar and in the shadow of other important health priorities.”
Both hepatitis B and C increase the risk of liver cancer and are associated with significant morbidity and mortality. Though approximately 4 million people in the United States are estimated to be infected with chronic hepatitis B (1.3 million) or C (2.7 million), these diseases account for less than 1% of the research budget at the National Institutes of Health, the report said. This compares unfavorably to funding for HIV, which affects about 1 million Americans.
As the report states, the tools to radically reduce hepatitis B and C deaths already exist. However, Dr. Lim cautioned in an interview, “the public health infrastructure to address viral hepatitis has been woefully inadequate.” In the United States, he noted, most states receive federal funding for at most a single person in charge of viral hepatitis epidemiology. “The resources currently available are in no way adequate to achieve the very aggressive goals described in the report,” he said.
Even among people with a known diagnosis of hepatitis B or C, only some receive confirmatory testing, Dr. Lim said. And of those with confirmed infections, “only a fraction are linked to care from the diagnosing clinician to a provider with the capacity to assess the state of liver disease and determine whether antiviral therapy is warranted.” Finally, he said, “many patients continue to face barriers to curative therapy due to cost and restrictions by public and private payers.”
Among the recommendations contained in the report is that unrestricted, mass treatment of hepatitis C infections be undertaken – regardless of disease stage. Currently, direct-acting antiviral agents remain costly and are poorly covered, notably by Medicaid. The National Academies advise that the government rectify this by purchasing “a license or assignment to the patent on a direct-acting antiviral drug, and use it only in those market segments where the government pays for treatment and access is now limited, such as Medicaid and prisons.”
Dr. Lim called the licensing proposal “very novel and bold,” but noted that there is no precedent in the United States for diseases such as hepatitis C. “If it could be done it would be an incredible model of government-pharma partnership for the public health good, and have a very significant impact.”
Steven Flamm, MD, chief of the liver transplantation program at Northwestern University in Chicago, who like Dr. Lim was not involved in the creation of the report, said in an interview that it contained innovative ideas and helped underscore the fact that “hepatitis has been given short shrift. The NIH and other agencies do not devote time and energy to this particular medical issue for reasons that are not completely clear.”
But “the problem with these kinds of analyses,” he said, “is that carrying them out is harder than making the recommendations.”
Dr. Flamm echoed Dr. Lim’s concerns about the practicability of implementing some of the recommendations in what he considers a resource-deprived health care environment for viral hepatitis.
“Is elimination possible or can you take a big bite out of it? The answer to that question is yes. We now have agents that can treat chronic viral hepatitis well, which we didn’t have a few years ago.”
Still, he emphasized, having the tools is only one part of the picture. Hepatitis C diagnostic tests have been available since the early 1990s. Yet, Dr. Flamm pointed out, fewer than half of patients have been diagnosed. “If the new CDC screening guidelines gain traction, we will do better than that.”
Dr. Flamm said that he considered the report’s call for a unique government licensing agreement for hepatitis C drugs a tall order. The drugs are already heavily discounted by manufacturers in many cases, he said, yet remain unavailable to those in need of them. In Illinois, Dr. Flamm said, few Medicaid patients with confirmed hepatitis C are given the short-acting antivirals that have revolutionized treatment. “The vast majority have no access to the therapy at all,” he said.
One of the report’s strengths, he said, is in detailing innovative prevention strategies such as delivering and promoting hepatitis B vaccinations to adults through local pharmacies, after the model of influenza vaccinations, and also conducting needle exchanges through pharmacies for intravenous drug users, who are at high risk of contracting both hepatitis B and C.
“Many of these strategies are not very costly,” he said. “The problem is you run into moral platitudes – to eliminate hepatitis, we will have to overcome that,” Dr. Flamm said, something that cannot be taken for granted in the current political environment.
But even if the goals outlined in the report seem ambitious, its authors have done an important service in underscoring the burden of viral hepatitis and laying out how some barriers to prevention, diagnosis, and treatment might be broken, he said.
Viral hepatitis “is a big deal, and it does cost a tremendous amount of money,” he added. “Everybody focuses on the therapeutic cost, but nobody focuses on the costs, direct and indirect, of all the sick people that are out there.”
An ambitious new report by the National Academies of Sciences, Engineering, and Medicine lays out a detailed path by which some 90,000 deaths from hepatitis B and C infection could be prevented by 2030.
The National Academies, a group of nongovernmental advisory bodies that includes the former Institute of Medicine, said that “the tools to prevent these deaths” exist – namely vaccination to prevent new hepatitis B infections and antiviral drugs, including new oral medications that can cure chronic hepatitis C infections within months.
The authors of the 200-plus-page report, led by Brian Strom, MD, MPH, of Rutgers University in Newark, NJ, calculate that deaths from hepatitis B infection could be halved by 2030 if 90% of patients are diagnosed, if 90% of those diagnosed are connected to care, and if 80% of those for whom treatment is indicated receive it. Treating everyone with chronic hepatitis C would reduce new infections by 90% by 2030, while reducing related deaths by 65%, Dr. Strom and his colleagues estimate.
But the authors also concede that drastic changes to current health policy would be required to reach these goals. These include the adoption of “aggressive testing, diagnosis, treatment, and prevention methods, such as needle exchange.”
They propose that the federal government seek a unique licensing arrangement with one or more manufacturers to bring down the notoriously high cost of direct-acting drugs used in hepatitis C, as a way of raising treatment rates. Currently, fewer than half the patients on Medicaid who are eligible for hepatitis C treatment receive it, and fewer than 1% of prisoners, who have high rates of infection.
Dr. Joseph Lim, director of the viral hepatitis program at Yale University in New Haven. Conn., who was not involved in the National Academies report, called it helpful in the sense that “it casts a spotlight on something that those of us involved in the care of people with viral hepatitis have long known – which is that this is a national and global public health burden that has been under the radar and in the shadow of other important health priorities.”
Both hepatitis B and C increase the risk of liver cancer and are associated with significant morbidity and mortality. Though approximately 4 million people in the United States are estimated to be infected with chronic hepatitis B (1.3 million) or C (2.7 million), these diseases account for less than 1% of the research budget at the National Institutes of Health, the report said. This compares unfavorably to funding for HIV, which affects about 1 million Americans.
As the report states, the tools to radically reduce hepatitis B and C deaths already exist. However, Dr. Lim cautioned in an interview, “the public health infrastructure to address viral hepatitis has been woefully inadequate.” In the United States, he noted, most states receive federal funding for at most a single person in charge of viral hepatitis epidemiology. “The resources currently available are in no way adequate to achieve the very aggressive goals described in the report,” he said.
Even among people with a known diagnosis of hepatitis B or C, only some receive confirmatory testing, Dr. Lim said. And of those with confirmed infections, “only a fraction are linked to care from the diagnosing clinician to a provider with the capacity to assess the state of liver disease and determine whether antiviral therapy is warranted.” Finally, he said, “many patients continue to face barriers to curative therapy due to cost and restrictions by public and private payers.”
Among the recommendations contained in the report is that unrestricted, mass treatment of hepatitis C infections be undertaken – regardless of disease stage. Currently, direct-acting antiviral agents remain costly and are poorly covered, notably by Medicaid. The National Academies advise that the government rectify this by purchasing “a license or assignment to the patent on a direct-acting antiviral drug, and use it only in those market segments where the government pays for treatment and access is now limited, such as Medicaid and prisons.”
Dr. Lim called the licensing proposal “very novel and bold,” but noted that there is no precedent in the United States for diseases such as hepatitis C. “If it could be done it would be an incredible model of government-pharma partnership for the public health good, and have a very significant impact.”
Steven Flamm, MD, chief of the liver transplantation program at Northwestern University in Chicago, who like Dr. Lim was not involved in the creation of the report, said in an interview that it contained innovative ideas and helped underscore the fact that “hepatitis has been given short shrift. The NIH and other agencies do not devote time and energy to this particular medical issue for reasons that are not completely clear.”
But “the problem with these kinds of analyses,” he said, “is that carrying them out is harder than making the recommendations.”
Dr. Flamm echoed Dr. Lim’s concerns about the practicability of implementing some of the recommendations in what he considers a resource-deprived health care environment for viral hepatitis.
“Is elimination possible or can you take a big bite out of it? The answer to that question is yes. We now have agents that can treat chronic viral hepatitis well, which we didn’t have a few years ago.”
Still, he emphasized, having the tools is only one part of the picture. Hepatitis C diagnostic tests have been available since the early 1990s. Yet, Dr. Flamm pointed out, fewer than half of patients have been diagnosed. “If the new CDC screening guidelines gain traction, we will do better than that.”
Dr. Flamm said that he considered the report’s call for a unique government licensing agreement for hepatitis C drugs a tall order. The drugs are already heavily discounted by manufacturers in many cases, he said, yet remain unavailable to those in need of them. In Illinois, Dr. Flamm said, few Medicaid patients with confirmed hepatitis C are given the short-acting antivirals that have revolutionized treatment. “The vast majority have no access to the therapy at all,” he said.
One of the report’s strengths, he said, is in detailing innovative prevention strategies such as delivering and promoting hepatitis B vaccinations to adults through local pharmacies, after the model of influenza vaccinations, and also conducting needle exchanges through pharmacies for intravenous drug users, who are at high risk of contracting both hepatitis B and C.
“Many of these strategies are not very costly,” he said. “The problem is you run into moral platitudes – to eliminate hepatitis, we will have to overcome that,” Dr. Flamm said, something that cannot be taken for granted in the current political environment.
But even if the goals outlined in the report seem ambitious, its authors have done an important service in underscoring the burden of viral hepatitis and laying out how some barriers to prevention, diagnosis, and treatment might be broken, he said.
Viral hepatitis “is a big deal, and it does cost a tremendous amount of money,” he added. “Everybody focuses on the therapeutic cost, but nobody focuses on the costs, direct and indirect, of all the sick people that are out there.”
An ambitious new report by the National Academies of Sciences, Engineering, and Medicine lays out a detailed path by which some 90,000 deaths from hepatitis B and C infection could be prevented by 2030.
The National Academies, a group of nongovernmental advisory bodies that includes the former Institute of Medicine, said that “the tools to prevent these deaths” exist – namely vaccination to prevent new hepatitis B infections and antiviral drugs, including new oral medications that can cure chronic hepatitis C infections within months.
The authors of the 200-plus-page report, led by Brian Strom, MD, MPH, of Rutgers University in Newark, NJ, calculate that deaths from hepatitis B infection could be halved by 2030 if 90% of patients are diagnosed, if 90% of those diagnosed are connected to care, and if 80% of those for whom treatment is indicated receive it. Treating everyone with chronic hepatitis C would reduce new infections by 90% by 2030, while reducing related deaths by 65%, Dr. Strom and his colleagues estimate.
But the authors also concede that drastic changes to current health policy would be required to reach these goals. These include the adoption of “aggressive testing, diagnosis, treatment, and prevention methods, such as needle exchange.”
They propose that the federal government seek a unique licensing arrangement with one or more manufacturers to bring down the notoriously high cost of direct-acting drugs used in hepatitis C, as a way of raising treatment rates. Currently, fewer than half the patients on Medicaid who are eligible for hepatitis C treatment receive it, and fewer than 1% of prisoners, who have high rates of infection.
Dr. Joseph Lim, director of the viral hepatitis program at Yale University in New Haven. Conn., who was not involved in the National Academies report, called it helpful in the sense that “it casts a spotlight on something that those of us involved in the care of people with viral hepatitis have long known – which is that this is a national and global public health burden that has been under the radar and in the shadow of other important health priorities.”
Both hepatitis B and C increase the risk of liver cancer and are associated with significant morbidity and mortality. Though approximately 4 million people in the United States are estimated to be infected with chronic hepatitis B (1.3 million) or C (2.7 million), these diseases account for less than 1% of the research budget at the National Institutes of Health, the report said. This compares unfavorably to funding for HIV, which affects about 1 million Americans.
As the report states, the tools to radically reduce hepatitis B and C deaths already exist. However, Dr. Lim cautioned in an interview, “the public health infrastructure to address viral hepatitis has been woefully inadequate.” In the United States, he noted, most states receive federal funding for at most a single person in charge of viral hepatitis epidemiology. “The resources currently available are in no way adequate to achieve the very aggressive goals described in the report,” he said.
Even among people with a known diagnosis of hepatitis B or C, only some receive confirmatory testing, Dr. Lim said. And of those with confirmed infections, “only a fraction are linked to care from the diagnosing clinician to a provider with the capacity to assess the state of liver disease and determine whether antiviral therapy is warranted.” Finally, he said, “many patients continue to face barriers to curative therapy due to cost and restrictions by public and private payers.”
Among the recommendations contained in the report is that unrestricted, mass treatment of hepatitis C infections be undertaken – regardless of disease stage. Currently, direct-acting antiviral agents remain costly and are poorly covered, notably by Medicaid. The National Academies advise that the government rectify this by purchasing “a license or assignment to the patent on a direct-acting antiviral drug, and use it only in those market segments where the government pays for treatment and access is now limited, such as Medicaid and prisons.”
Dr. Lim called the licensing proposal “very novel and bold,” but noted that there is no precedent in the United States for diseases such as hepatitis C. “If it could be done it would be an incredible model of government-pharma partnership for the public health good, and have a very significant impact.”
Steven Flamm, MD, chief of the liver transplantation program at Northwestern University in Chicago, who like Dr. Lim was not involved in the creation of the report, said in an interview that it contained innovative ideas and helped underscore the fact that “hepatitis has been given short shrift. The NIH and other agencies do not devote time and energy to this particular medical issue for reasons that are not completely clear.”
But “the problem with these kinds of analyses,” he said, “is that carrying them out is harder than making the recommendations.”
Dr. Flamm echoed Dr. Lim’s concerns about the practicability of implementing some of the recommendations in what he considers a resource-deprived health care environment for viral hepatitis.
“Is elimination possible or can you take a big bite out of it? The answer to that question is yes. We now have agents that can treat chronic viral hepatitis well, which we didn’t have a few years ago.”
Still, he emphasized, having the tools is only one part of the picture. Hepatitis C diagnostic tests have been available since the early 1990s. Yet, Dr. Flamm pointed out, fewer than half of patients have been diagnosed. “If the new CDC screening guidelines gain traction, we will do better than that.”
Dr. Flamm said that he considered the report’s call for a unique government licensing agreement for hepatitis C drugs a tall order. The drugs are already heavily discounted by manufacturers in many cases, he said, yet remain unavailable to those in need of them. In Illinois, Dr. Flamm said, few Medicaid patients with confirmed hepatitis C are given the short-acting antivirals that have revolutionized treatment. “The vast majority have no access to the therapy at all,” he said.
One of the report’s strengths, he said, is in detailing innovative prevention strategies such as delivering and promoting hepatitis B vaccinations to adults through local pharmacies, after the model of influenza vaccinations, and also conducting needle exchanges through pharmacies for intravenous drug users, who are at high risk of contracting both hepatitis B and C.
“Many of these strategies are not very costly,” he said. “The problem is you run into moral platitudes – to eliminate hepatitis, we will have to overcome that,” Dr. Flamm said, something that cannot be taken for granted in the current political environment.
But even if the goals outlined in the report seem ambitious, its authors have done an important service in underscoring the burden of viral hepatitis and laying out how some barriers to prevention, diagnosis, and treatment might be broken, he said.
Viral hepatitis “is a big deal, and it does cost a tremendous amount of money,” he added. “Everybody focuses on the therapeutic cost, but nobody focuses on the costs, direct and indirect, of all the sick people that are out there.”
FROM THE NATIONAL ACADEMIES OF SCIENCES, ENGINEERING, AND MEDICINE