User login
Considerations for Optimal Inhaler Device Selection in Chronic Obstructive Pulmonary Disease
Device considerations
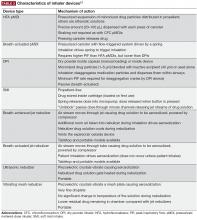
The SMI delivers the aerosol as a fine mist with slow velocity lasting >1 second, which is considerably slower than spray delivery with pMDIs.14 The aim of this design is to make it easier for patients to coordinate actuation with inhalation, but it is important to note that some coordination is still required for SMI devices to function correctly.14 In addition, the SMI is not dependent on a patient’s ability to generate sufficient PIF for effective drug delivery. A limitation of the SMI is the need to assemble the device, as patients with poor manual dexterity may encounter difficulty when attempting to load the drug cartridge.15
Nebulizers deliver aerosolized drug in a fine mist. Newer-generation portable vibrating mesh nebulizers can deliver a dose over a period of ~2 minutes, compared with 10 minutes for conventional pneumatic devices.16 Patients find them effective and easy to use, and the newer generation devices overcome problems with portability and length of treatment, which may be an issue during the daytime for ambulatory patients, along with the requirement for cleaning after each dose.4,8 However, drug delivery may be somewhat compromised with nebulizers compared with other inhalation devices, as medication can be dispersed into the atmosphere and lost, rather than inhaled.7 An additional point to consider is medication availability; some medications, particularly fixed-dose combination maintenance therapies, are currently unavailable in a nebulized format.16
The most important device-related factors influencing the site of deposition within the lungs are aerosol velocity and particle size of the inhaled drug.3,7,17 To maximize clinical effectiveness, adequate distribution throughout the lung is required to reach target sites of action for β2-agonists, anticholinergics, and corticosteroids.17 Particle size differs between inhaler device types, but all available devices generate drug particles sufficient for deposition throughout the lower airways and lung periphery, ie, within the range of 1–5 microns.3,18-21 Extra fine particles of <1 micron (or “submicron particles”) can be deposited deeper in the pulmonary acinus, but a higher fraction of such particles may be exhaled compared with particles 1–5 microns in size.3,20,22 In contrast, particles >5 microns deposit in the oropharynx and may be swallowed, potentially leading to systemic adverse effects.3,20,22
When more than one drug is required, it may be preferable to deliver them via a single device where possible to facilitate patient compliance with correct technique, and decrease confusion about how to use different inhalers.23 The inhaler device ideally serves as a platform on which many treatments are available; the greater the number of devices employed by the patient, the greater the likelihood of making an error with the usage of each device.24
Importance of proper inhaler technique
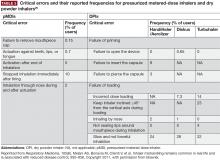
Errors relating to device handling are common in patients with COPD. The results of a meta-analysis by Chrystyn et al reported that overall error rates were high across all devices in patients with COPD and asthma, ranging from 50%–100%25; the reported frequencies of patients with at least one error were 86.8% and 60.9% for pMDIs and DPIs, respectively. However, the authors note that heterogeneity between the studies used in the analysis was high, and suggest that future investigations should look to use a more standardized approach in assessment of inhaler device errors.25 Moreover, further studies to investigate the frequency of errors in SMI devices, and to establish the relationship between critical errors in device handling and device efficacy, are warranted.
Handling errors are directly linked to compromised drug delivery and reduced treatment efficacy.3 This may lead to more frequent or inappropriate medication use that, in turn, could result in unnecessary dose increases by the physician due to perceived lack of efficacy, and subsequently more adverse effects.3,26-28 However, these errors can be addressed through proper training and demonstration.29-32
Common device-handling errors include4,26,27,32,33:
- pMDIs: not shaking the inhaler (for suspensions), not exhaling fully before actuation, inhaling too forcefully, and not holding their breath for long enough after inhalation.
- DPIs: exhaling into the device mouthpiece, not exhaling fully before inhalation, not inhaling deeply or forcefully enough, and not holding their breath after inhalation.
- SMIs: not rotating the inhaler with mouth cap facing upwards, rotating the inhaler while looking into the spray nozzle with the cap open (before inhalation), and not maintaining inhalation with drug spray.
Incorrect inhaler use is a common cause of secondary nonadherence (ie, relating to incorrect medication use) among patients with COPD.4,34 Compromised inhaler technique and medication nonadherence jeopardize health outcomes and add to the economic burden of COPD.8,12,26
A 2005 study estimated that over 20% of the $25 billion spent on inhalers annually in the United States is wasted as a direct consequence of incorrect device handling.35
Failing to inhale correctly to achieve the optimal inspiratory flow for the specific device being used—deep and slow for pMDIs, or forceful, quick and deep for DPIs—is a critical handling error for inhaler devices.26 Significant associations between critical errors and clinical outcomes (hospitalization, emergency department visits, antibiotic courses, and corticosteroid courses) have been reported in COPD patients.26 In a retrospective analysis of COPD inpatients, suboptimal PIF rates with DPIs were associated with worse scores on the COPD Assessment Test, higher COPD and all-cause readmission rates, and shorter time to next COPD exacerbation.12
Patient considerations
Poor inhaler technique is frequently reported in patients with COPD or asthma, irrespective of the device used and with considerable variability in handling error rates for each individual device.25,26,35,45 Although clinical evidence is limited,25 research to date indicates that some DPIs may require less training than pMDIs.23,29,45,46 Therefore, DPI devices may be viewed as a more appropriate option for patients who encounter difficulty in coordinating the inhalation and actuation required for effective operation of a pMDI device. Alternatively, use of a spacer with pMDIs appears to reduce handling errors compared with pMDIs alone, but whether a pMDI plus spacer improves technique versus DPIs remains unclear.25,46,47 Lack of device training appears to be a key reason for inhaler handling errors across device types.26
Elderly patients need special consideration when selecting an inhaler and ensuring it is used correctly.48 Reduced physical ability and cognitive function due to age-related conditions (eg, dementia, depression, neuromuscular and cerebrovascular diseases) are the main reasons for suboptimal inhaler use in older patients, but other factors may also contribute (eg, multiple comorbid conditions, consequent complicated medication regimens).15 Older age is strongly associated with inhaler misuse,26 and has also been shown to have a negative correlation with PIF, independent of COPD severity.41 When compared with younger patients, older patients make more attempts before mastering the inhalation technique for a specific device, and need longer instruction time from trained health care professionals to correct inhaler mishandling.49,50 In elderly patients with adequate cognitive and manual ability, the most important factors in selecting a device are availability, convenience, ease of use, patient preference, and cost.8,23
Device continuity is a key consideration when multiple inhaled medications are needed.23 Lack of continuity of device type for different clinical needs means that patients may need to master the different techniques for each device.3 For instance, a patient may have a pMDI rescue medication, one or more DPIs for their maintenance therapy, and a nebulizer for additional bronchodilation, which may lead to confusion and incorrect device usage. Device continuity has been shown to improve disease control compared with using multiple inhalers in patients with asthma.51
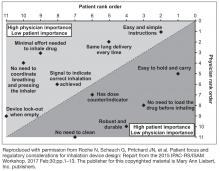
A full summary of patient- and physician-related considerations for device selection, along with suggestions for how these can be addressed, is provided in Table 5.
Inhaler device training for patients and physicians
Comprehensive instruction, including practical demonstration, is important for ensuring patients with COPD use the correct inhaler technique, with regular review and repeated instruction generally needed for continued correct use.1,23,32,42 Lack of instruction is significantly associated with inhaler misuse in patients with COPD or asthma.26 Verbal training on inhalation technique increased the number of patients achieving the minimum inhalation flow rate required for a range of different DPIs.39 Similarly, training helped patients using a pMDI to slow their inhalation rate to <90 L/min, as recommended for this type of device.39 The ‘teach-back’ method, where patients are asked to demonstrate correct usage of their inhaler after instruction from a health care professional,52 has shown to be particularly effective in pharmacist-led patient device training.53 Educational interventions that incorporated a physical demonstration significantly improved inhaler technique in patients with COPD and asthma compared with patients receiving written and verbal information alone.53 Proper device training in primary care settings should also include education about why the inhaler is needed.3
Face-to-face instruction from trained caregivers for approximately 5 to 10 minutes improves the use of MDIs and DPIs by patients.49 However, clinical research indicates that learning correct handling and use may be easier and quicker for some devices than for others.31,49 For example, patients naïve to the PulmoJet (a DPI device not currently available in the United States) were found to have fewer serious errors after training than those using Diskus or Turbuhaler devices.24 In another study, it took less time to correct errors in inhaler use with the Diskus compared with the HandiHaler.44 Health care professionals themselves may lack training or knowledge on correct use of inhaler devices,35,36,54 with 1 study finding that up to 67% of nurses, doctors, and respiratory therapists were unable to describe or perform critical steps for using inhalers.35
A range of resources is available to aid in training patients and health care professionals in inhaler techniques:
- Tools such as the In-Check DIAL inspiratory flow meter (Clement Clarke International Ltd, Harlow, UK), TurbuHaler Trainer (AstraZeneca, Lund, Sweden), Diskus/Accuhaler Training Device (Vitalograph, Ennis, Ireland), and 2Tone Trainer (Canday Medical Ltd, Newmarket, UK) can be used to evaluate a patient’s physical ability to use a specific inhaler.55
- The emergence of electronic monitoring devices, such as SmartTrack, SmartTurbo, and SmartMat (all developed by Adherium Ltd, Auckland New Zealand), can provide objective and detailed adherence data to support clinical decision-making.56
- It is essential that patients and physicians alike utilize the instructions and video demonstrations available online to understand how to use a device correctly, and avoid errors. These resources can be found on a number of organizations’ websites (eg, COPD Foundation, Allergy and Asthma Network, Centers for Disease Control and Prevention, National Jewish Health, Asthma UK, Centre for Pharmacy Postgraduate Education) and on manufacturers’ websites for individual inhalers or treatments (eg, https://www.advair.com/how-to-use-advair.html, https://www.incruse.com/how-to-use-incruse.html, https://www.mysymbicort.com/copd/taking-symbicort/how-to-use-the-inhaler.html, https://www.tudorzahcp.com/tudorza-instructions-dosing.html, www.us.respimat.com (“How to Use the RESPIMAT Inhaler”), https://www.utibron.com/how-to-use.html).
Conclusions
A number of inhalation devices are available for the treatment of COPD. However, incorrect usage or a poor match between the patient and the device may lead to confusion, suboptimal treatment, and increased cost to the patient and health care system. Considering both patient- and health care system-related factors can ensure that appropriate inhaler section and usage can be optimized.
- Global Initiative for Chronic Obstructive Lung Disease. GOLD 2017 Global Strategy for the Diagnosis, Management and Prevention of COPD. http://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd. Accessed July 2017.
- Dolovich MB, Dhand R. Aerosol drug delivery: developments in device design and clinical use. Lancet. 2011;377(9770):1032-1045.
- Bonini M, Usmani OS. The importance of inhaler devices in the treatment of COPD. COPD Res Pract. 2015;1(1):9.
- Restrepo RD, Alvarez MT, Wittnebel LD, et al. Medication adherence issues in patients treated for COPD. Int J Chron Obstruct Pulmon Dis. 2008;3(3):371-384.
- Rogliani P, Calzetta L, Coppola A, et al. Optimizing drug delivery in COPD: the role of inhaler devices. Respir Med. 2017;124:6-14.
- Lavorini F, Fontana GA, Usmani OS. New inhaler devices - the good, the bad and the ugly. Respiration. 2014;88(1):3-15.
- Ibrahim M, Verma R, Garcia-Contreras L. Inhalation drug delivery devices: technology update. Med Devices (Auckl). 2015;8:131-139.
- Barrons R, Pegram A, Borries A. Inhaler device selection: special considerations in elderly patients with chronic obstructive pulmonary disease. Am J Health Syst Pharm. 2011;68(13):1221-1232.
- Dal Negro RW. Dry powder inhalers and the right things to remember: a concept review. Multidiscip Respir Med. 2015;10(1):13.
- Mahler DA, Waterman LA, Gifford AH. Prevalence and COPD phenotype for a suboptimal peak inspiratory flow rate against the simulated resistance of the Diskus® dry powder inhaler. J Aerosol Med Pulm Drug Deliv. 2013;26(3):174-179.
- Sharma G, Mahler DA, Mayorga VM, Deering KL, Harshaw O, Ganapathy V. Prevalence of low peak inspiratory flow rate at discharge in patients hospitalized for COPD exacerbation. Chronic Obstr Pulm Dis. 2017;4(3):217-224.
- Loh CH, Peters SP, Lovings TM, Ohar JA. Suboptimal inspiratory flow rates are associated with chronic obstructive pulmonary disease and all cause readmissions. Ann Am Thorac Soc. 2017;14(8):1305-1311.
- Le V, Hoang Thi TH, Robins E, Flament M. Dry powder inhalers: study of the parameters influencing adhesion and dispersion of fluticasone propionate. AAPS PharmSciTech. 2012;13(2):477-484.
- Dalby RN, Eicher J, Zierenberg B. Development of Respimat® Soft Mist™ Inhaler and its clinical utility in respiratory disorders. Med Devices (Auckl). 2011;4:145-155.
- Lavorini F, Mannini C, Chellini E, Fontana GA. Optimising inhaled pharmacotherapy for elderly patients with chronic obstructive pulmonary disease: the importance of delivery devices. Drugs Aging. 2016;33(7):461-473.
- Tashkin DP. A review of nebulized drug delivery in COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:2585-2596.
- Labiris NR, Dolovich MB. Pulmonary drug delivery. Part I: physiological factors affecting therapeutic effectiveness of aerosolized medications. Br J Clin Pharmacol. 2003;56(6):588-599.
- Chrystyn H. Anatomy and physiology in delivery: can we define our targets? Allergy. 1999;54(suppl 49):82-87.
- Biddiscombe M, Meah S, Barnes P, Usmani O. Drug particle size and lung deposition in COPD. Eur Respir J. 2016;48(suppl 60):Abstract. doi:10.1183/13993003.congress-13992016.PA13993313.
- Demoly P, Hagedoorn P, de Boer AH, Frijlink HW. The clinical relevance of dry powder inhaler performance for drug delivery. Respir Med. 2014;108(8):1195-1203.
- Dhand R. Inhaled drug therapy 2016: the year in review. Respir Care. 2017;62(7):978-996.
- de Boer AH, Gjaltema D, Hagedoorn P, Frijlink HW. Can ‘extrafine’ dry powder aerosols improve lung deposition? Eur J Pharm Biopharm. 2015;96:143-151.
- Vincken W, Dekhuijzen PR, Barnes P; ADMIT Group. The ADMIT series - Issues in inhalation therapy. 4) How to choose inhaler devices for the treatment of COPD. Prim Care Respir J. 2010;19(1):10-20.
- Roggeri A, Micheletto C, Roggeri DP. Inhalation errors due to device switch in patients with chronic obstructive pulmonary disease and asthma: critical health and economic issues. Int J Chron Obstruct Pulmon Dis. 2016;11:597-602.
- Chrystyn H, van der Palen J, Sharma R, et al. Device errors in asthma and COPD: systematic literature review and meta-analysis. NPJ Prim Care Respir Med. 2017;27(1):22.
- Melani AS, Bonavia M, Cilenti V, et al; Gruppo Educazionale Associazione Italiana Pneumologi Ospedalieri. Inhaler mishandling remains common in real life and is associated with reduced disease control [published correction appears in Respir Med. 2012;106(5):757]. Respir Med. 2011;105(6):930-938.
- Sanchis J, Gich I, Pedersen S; Aerosol Drug Management Improvement Team (ADMIT). Systematic review of errors in inhaler use: has patient technique improved over time? Chest. 2016;150(2):394-406.
- Sulaiman I, Seheult J, Sadasivuni N, et al. The impact of common inhaler errors on drug delivery: investigating critical errors with a dry powder inhaler. J Aerosol Med Pulm Drug Deliv. 2017;30(4):247-255.
- Chapman KR, Love L, Brubaker H. A comparison of breath-actuated and conventional metered-dose inhaler inhalation techniques in elderly subjects. Chest. 1993;104(5):1332-1337.
- van der Palen J, Thomas M, Chrystyn H, et al. A randomised open-label cross-over study of inhaler errors, preference and time to achieve correct inhaler use in patients with COPD or asthma: comparison of ELLIPTA with other inhaler devices. NPJ Prim Care Respir Med. 2016;26:16079.
- Chrystyn H, Price DB, Molimard M, et al. Comparison of serious inhaler technique errors made by device-naïve patients using three different dry powder inhalers: a randomised, crossover, open-label study. BMC Pulm Med. 2016;16:12.
- Crane MA, Jenkins CR, Goeman DP, Douglass JA. Inhaler device technique can be improved in older adults through tailored education: findings from a randomised controlled trial. NPJ Prim Care Respir Med. 2014;24:14034.
- Ohbayashi H, Kudo S, Ishikawa M. Inhaler operability and patient satisfaction regarding Genuair® and Respimat® inhalers for chronic obstructive pulmonary disease: a randomized crossover sudy. Pulmon Ther. 2017;3(1):173-185.
- Bourbeau J, Bartlett SJ. Patient adherence in COPD. Thorax. 2008;63(9):831-838.
- Fink JB, Rubin BK. Problems with inhaler use: a call for improved clinician and patient education. Respir Care. 2005;50(10):1360-1374; discussion 1374-1375.
- Yawn BP, Colice GL, Hodder R. Practical aspects of inhaler use in the management of chronic obstructive pulmonary disease in the primary care setting. Int J Chron Obstruct Pulmon Dis. 2012;7:495-502.
- Dhand R, Dolovich M, Chipps B, Myers TR, Restrepo R, Farrar JR. The role of nebulized therapy in the management of COPD: evidence and recommendations. COPD. 2012;9(1):58-72.
- Roche N, Gerhard S, Pritchard JN, et al. Patient focus and regulatory considerations for inhalation device design: report from the 2015 IPAC-RS/ISAM Workshop. J Aerosol Med Pulm Drug Deliv. 2017;30(1):1-13.
- Al-Showair RA, Tarsin WY, Assi KH, Pearson SB, Chrystyn H. Can all patients with COPD use the correct inhalation flow with all inhalers and does training help? Respir Med. 2007;101(11):2395-2401.
- Janssens W, VandenBrande P, Hardeman E, et al. Inspiratory flow rates at different levels of resistance in elderly COPD patients. Eur Respir J. 2008;31(1):78-83.
- Jarvis S, Ind PW, Shiner RJ. Inhaled therapy in elderly COPD patients; time for re-evaluation? Age Ageing. 2007;36(2):213-218.
- Lavorini F, Levy ML, Corrigan C, Crompton G; ADMIT Working Group. The ADMIT series - issues in inhalation therapy. 6) Training tools for inhalation devices. Prim Care Respir J. 2010;19(4):335-341.
- Pauwels R, Newman S, Borgström L. Airway deposition and airway effects of antiasthma drugs delivered from metered-dose inhalers. Eur Respir J. 1997;10(9):2127-2138.
- Everard ML, Devadason SG, Le Souëf PN. Flow early in the inspiratory manoeuvre affects the aerosol particle size distribution from a Turbuhaler. Respir Med. 1997;91(10):624-628.
- Molimard M, Raherison C, Lignot S, et al. Chronic obstructive pulmonary disease exacerbation and inhaler device handling: real-life assessment of 2935 patients. Eur Respir J. 2017;49(2):doi: 10.1183/13993003.13901794-2016.
- Jones V, Fernandez C, Diggory P. A comparison of large volume spacer, breath-activated and dry powder inhalers in older people. Age Ageing. 1999;28(5):481-484.
- Ho SF, O’Mahony MS, Steward JA, Breay P, Burr ML. Inhaler technique in older people in the community. Age Ageing. 2004;33(2):185-188.
- Taffet GE, Donohue JF, Altman PR. Considerations for managing chronic obstructive pulmonary disease in the elderly. Clin Interv Aging. 2014;9:23-30.
- Melani AS, Bonavia M, Mastropasqua E, et al; Gruppo Educazionale Associazione Italiana Pneumologi Ospedalieri (AIPO). Time required to rectify inhaler errors among experienced subjects with faulty technique. Respir Care. 2017;62(4):409-414.
- Dal Negro RW, Povero M. Dry-powder inhalers in patients with persistent airflow limitation: usability and preference. Multidiscip Respir Med. 2016;11(1):31.
- Price D, Chrystyn H, Kaplan A, et al. Effectiveness of same versus mixed asthma inhaler devices: a retrospective observational study in primary care. Allergy Asthma Immunol Res. 2012;4(4):184-191.
- Dantic DE. A critical review of the effectiveness of ‘teach-back’ technique in teaching COPD patients self-management using respiratory inhalers. Health Ed J. 2014;73(1):41-50.
- Bosnic-Anticevich SZ, Sinha H, So S, Reddel HK. Metered-dose inhaler technique: the effect of two educational interventions delivered in community pharmacy over time. J Asthma. 2010;47(3):251-256.
- Adnan M, Karim S, Khan S, Al Wabel N. Critical errors found during metered dose inhaler technique demonstration by pharmacists. Saudi Pharm J. 2016;24(5):625.
- Capstick TG, Clifton IJ. Inhaler technique and training in people with chronic obstructive pulmonary disease and asthma. Expert Rev Respir Med. 2012;6(1):91-101; quiz 102-103.
- Chan AH, Harrison J, Black PN, Mitchell EA, Foster JM. Using electronic monitoring devices to measure inhaler adherence: a practical guide for clinicians. J Allergy Clin Immunol Pract. 2015;3(3):335-349.e1-e5.
Device considerations
The SMI delivers the aerosol as a fine mist with slow velocity lasting >1 second, which is considerably slower than spray delivery with pMDIs.14 The aim of this design is to make it easier for patients to coordinate actuation with inhalation, but it is important to note that some coordination is still required for SMI devices to function correctly.14 In addition, the SMI is not dependent on a patient’s ability to generate sufficient PIF for effective drug delivery. A limitation of the SMI is the need to assemble the device, as patients with poor manual dexterity may encounter difficulty when attempting to load the drug cartridge.15
Nebulizers deliver aerosolized drug in a fine mist. Newer-generation portable vibrating mesh nebulizers can deliver a dose over a period of ~2 minutes, compared with 10 minutes for conventional pneumatic devices.16 Patients find them effective and easy to use, and the newer generation devices overcome problems with portability and length of treatment, which may be an issue during the daytime for ambulatory patients, along with the requirement for cleaning after each dose.4,8 However, drug delivery may be somewhat compromised with nebulizers compared with other inhalation devices, as medication can be dispersed into the atmosphere and lost, rather than inhaled.7 An additional point to consider is medication availability; some medications, particularly fixed-dose combination maintenance therapies, are currently unavailable in a nebulized format.16
The most important device-related factors influencing the site of deposition within the lungs are aerosol velocity and particle size of the inhaled drug.3,7,17 To maximize clinical effectiveness, adequate distribution throughout the lung is required to reach target sites of action for β2-agonists, anticholinergics, and corticosteroids.17 Particle size differs between inhaler device types, but all available devices generate drug particles sufficient for deposition throughout the lower airways and lung periphery, ie, within the range of 1–5 microns.3,18-21 Extra fine particles of <1 micron (or “submicron particles”) can be deposited deeper in the pulmonary acinus, but a higher fraction of such particles may be exhaled compared with particles 1–5 microns in size.3,20,22 In contrast, particles >5 microns deposit in the oropharynx and may be swallowed, potentially leading to systemic adverse effects.3,20,22
When more than one drug is required, it may be preferable to deliver them via a single device where possible to facilitate patient compliance with correct technique, and decrease confusion about how to use different inhalers.23 The inhaler device ideally serves as a platform on which many treatments are available; the greater the number of devices employed by the patient, the greater the likelihood of making an error with the usage of each device.24
Importance of proper inhaler technique
Errors relating to device handling are common in patients with COPD. The results of a meta-analysis by Chrystyn et al reported that overall error rates were high across all devices in patients with COPD and asthma, ranging from 50%–100%25; the reported frequencies of patients with at least one error were 86.8% and 60.9% for pMDIs and DPIs, respectively. However, the authors note that heterogeneity between the studies used in the analysis was high, and suggest that future investigations should look to use a more standardized approach in assessment of inhaler device errors.25 Moreover, further studies to investigate the frequency of errors in SMI devices, and to establish the relationship between critical errors in device handling and device efficacy, are warranted.
Handling errors are directly linked to compromised drug delivery and reduced treatment efficacy.3 This may lead to more frequent or inappropriate medication use that, in turn, could result in unnecessary dose increases by the physician due to perceived lack of efficacy, and subsequently more adverse effects.3,26-28 However, these errors can be addressed through proper training and demonstration.29-32
Common device-handling errors include4,26,27,32,33:
- pMDIs: not shaking the inhaler (for suspensions), not exhaling fully before actuation, inhaling too forcefully, and not holding their breath for long enough after inhalation.
- DPIs: exhaling into the device mouthpiece, not exhaling fully before inhalation, not inhaling deeply or forcefully enough, and not holding their breath after inhalation.
- SMIs: not rotating the inhaler with mouth cap facing upwards, rotating the inhaler while looking into the spray nozzle with the cap open (before inhalation), and not maintaining inhalation with drug spray.
Incorrect inhaler use is a common cause of secondary nonadherence (ie, relating to incorrect medication use) among patients with COPD.4,34 Compromised inhaler technique and medication nonadherence jeopardize health outcomes and add to the economic burden of COPD.8,12,26
A 2005 study estimated that over 20% of the $25 billion spent on inhalers annually in the United States is wasted as a direct consequence of incorrect device handling.35
Failing to inhale correctly to achieve the optimal inspiratory flow for the specific device being used—deep and slow for pMDIs, or forceful, quick and deep for DPIs—is a critical handling error for inhaler devices.26 Significant associations between critical errors and clinical outcomes (hospitalization, emergency department visits, antibiotic courses, and corticosteroid courses) have been reported in COPD patients.26 In a retrospective analysis of COPD inpatients, suboptimal PIF rates with DPIs were associated with worse scores on the COPD Assessment Test, higher COPD and all-cause readmission rates, and shorter time to next COPD exacerbation.12
Patient considerations
Poor inhaler technique is frequently reported in patients with COPD or asthma, irrespective of the device used and with considerable variability in handling error rates for each individual device.25,26,35,45 Although clinical evidence is limited,25 research to date indicates that some DPIs may require less training than pMDIs.23,29,45,46 Therefore, DPI devices may be viewed as a more appropriate option for patients who encounter difficulty in coordinating the inhalation and actuation required for effective operation of a pMDI device. Alternatively, use of a spacer with pMDIs appears to reduce handling errors compared with pMDIs alone, but whether a pMDI plus spacer improves technique versus DPIs remains unclear.25,46,47 Lack of device training appears to be a key reason for inhaler handling errors across device types.26
Elderly patients need special consideration when selecting an inhaler and ensuring it is used correctly.48 Reduced physical ability and cognitive function due to age-related conditions (eg, dementia, depression, neuromuscular and cerebrovascular diseases) are the main reasons for suboptimal inhaler use in older patients, but other factors may also contribute (eg, multiple comorbid conditions, consequent complicated medication regimens).15 Older age is strongly associated with inhaler misuse,26 and has also been shown to have a negative correlation with PIF, independent of COPD severity.41 When compared with younger patients, older patients make more attempts before mastering the inhalation technique for a specific device, and need longer instruction time from trained health care professionals to correct inhaler mishandling.49,50 In elderly patients with adequate cognitive and manual ability, the most important factors in selecting a device are availability, convenience, ease of use, patient preference, and cost.8,23
Device continuity is a key consideration when multiple inhaled medications are needed.23 Lack of continuity of device type for different clinical needs means that patients may need to master the different techniques for each device.3 For instance, a patient may have a pMDI rescue medication, one or more DPIs for their maintenance therapy, and a nebulizer for additional bronchodilation, which may lead to confusion and incorrect device usage. Device continuity has been shown to improve disease control compared with using multiple inhalers in patients with asthma.51
A full summary of patient- and physician-related considerations for device selection, along with suggestions for how these can be addressed, is provided in Table 5.
Inhaler device training for patients and physicians
Comprehensive instruction, including practical demonstration, is important for ensuring patients with COPD use the correct inhaler technique, with regular review and repeated instruction generally needed for continued correct use.1,23,32,42 Lack of instruction is significantly associated with inhaler misuse in patients with COPD or asthma.26 Verbal training on inhalation technique increased the number of patients achieving the minimum inhalation flow rate required for a range of different DPIs.39 Similarly, training helped patients using a pMDI to slow their inhalation rate to <90 L/min, as recommended for this type of device.39 The ‘teach-back’ method, where patients are asked to demonstrate correct usage of their inhaler after instruction from a health care professional,52 has shown to be particularly effective in pharmacist-led patient device training.53 Educational interventions that incorporated a physical demonstration significantly improved inhaler technique in patients with COPD and asthma compared with patients receiving written and verbal information alone.53 Proper device training in primary care settings should also include education about why the inhaler is needed.3
Face-to-face instruction from trained caregivers for approximately 5 to 10 minutes improves the use of MDIs and DPIs by patients.49 However, clinical research indicates that learning correct handling and use may be easier and quicker for some devices than for others.31,49 For example, patients naïve to the PulmoJet (a DPI device not currently available in the United States) were found to have fewer serious errors after training than those using Diskus or Turbuhaler devices.24 In another study, it took less time to correct errors in inhaler use with the Diskus compared with the HandiHaler.44 Health care professionals themselves may lack training or knowledge on correct use of inhaler devices,35,36,54 with 1 study finding that up to 67% of nurses, doctors, and respiratory therapists were unable to describe or perform critical steps for using inhalers.35
A range of resources is available to aid in training patients and health care professionals in inhaler techniques:
- Tools such as the In-Check DIAL inspiratory flow meter (Clement Clarke International Ltd, Harlow, UK), TurbuHaler Trainer (AstraZeneca, Lund, Sweden), Diskus/Accuhaler Training Device (Vitalograph, Ennis, Ireland), and 2Tone Trainer (Canday Medical Ltd, Newmarket, UK) can be used to evaluate a patient’s physical ability to use a specific inhaler.55
- The emergence of electronic monitoring devices, such as SmartTrack, SmartTurbo, and SmartMat (all developed by Adherium Ltd, Auckland New Zealand), can provide objective and detailed adherence data to support clinical decision-making.56
- It is essential that patients and physicians alike utilize the instructions and video demonstrations available online to understand how to use a device correctly, and avoid errors. These resources can be found on a number of organizations’ websites (eg, COPD Foundation, Allergy and Asthma Network, Centers for Disease Control and Prevention, National Jewish Health, Asthma UK, Centre for Pharmacy Postgraduate Education) and on manufacturers’ websites for individual inhalers or treatments (eg, https://www.advair.com/how-to-use-advair.html, https://www.incruse.com/how-to-use-incruse.html, https://www.mysymbicort.com/copd/taking-symbicort/how-to-use-the-inhaler.html, https://www.tudorzahcp.com/tudorza-instructions-dosing.html, www.us.respimat.com (“How to Use the RESPIMAT Inhaler”), https://www.utibron.com/how-to-use.html).
Conclusions
A number of inhalation devices are available for the treatment of COPD. However, incorrect usage or a poor match between the patient and the device may lead to confusion, suboptimal treatment, and increased cost to the patient and health care system. Considering both patient- and health care system-related factors can ensure that appropriate inhaler section and usage can be optimized.
Device considerations
The SMI delivers the aerosol as a fine mist with slow velocity lasting >1 second, which is considerably slower than spray delivery with pMDIs.14 The aim of this design is to make it easier for patients to coordinate actuation with inhalation, but it is important to note that some coordination is still required for SMI devices to function correctly.14 In addition, the SMI is not dependent on a patient’s ability to generate sufficient PIF for effective drug delivery. A limitation of the SMI is the need to assemble the device, as patients with poor manual dexterity may encounter difficulty when attempting to load the drug cartridge.15
Nebulizers deliver aerosolized drug in a fine mist. Newer-generation portable vibrating mesh nebulizers can deliver a dose over a period of ~2 minutes, compared with 10 minutes for conventional pneumatic devices.16 Patients find them effective and easy to use, and the newer generation devices overcome problems with portability and length of treatment, which may be an issue during the daytime for ambulatory patients, along with the requirement for cleaning after each dose.4,8 However, drug delivery may be somewhat compromised with nebulizers compared with other inhalation devices, as medication can be dispersed into the atmosphere and lost, rather than inhaled.7 An additional point to consider is medication availability; some medications, particularly fixed-dose combination maintenance therapies, are currently unavailable in a nebulized format.16
The most important device-related factors influencing the site of deposition within the lungs are aerosol velocity and particle size of the inhaled drug.3,7,17 To maximize clinical effectiveness, adequate distribution throughout the lung is required to reach target sites of action for β2-agonists, anticholinergics, and corticosteroids.17 Particle size differs between inhaler device types, but all available devices generate drug particles sufficient for deposition throughout the lower airways and lung periphery, ie, within the range of 1–5 microns.3,18-21 Extra fine particles of <1 micron (or “submicron particles”) can be deposited deeper in the pulmonary acinus, but a higher fraction of such particles may be exhaled compared with particles 1–5 microns in size.3,20,22 In contrast, particles >5 microns deposit in the oropharynx and may be swallowed, potentially leading to systemic adverse effects.3,20,22
When more than one drug is required, it may be preferable to deliver them via a single device where possible to facilitate patient compliance with correct technique, and decrease confusion about how to use different inhalers.23 The inhaler device ideally serves as a platform on which many treatments are available; the greater the number of devices employed by the patient, the greater the likelihood of making an error with the usage of each device.24
Importance of proper inhaler technique
Errors relating to device handling are common in patients with COPD. The results of a meta-analysis by Chrystyn et al reported that overall error rates were high across all devices in patients with COPD and asthma, ranging from 50%–100%25; the reported frequencies of patients with at least one error were 86.8% and 60.9% for pMDIs and DPIs, respectively. However, the authors note that heterogeneity between the studies used in the analysis was high, and suggest that future investigations should look to use a more standardized approach in assessment of inhaler device errors.25 Moreover, further studies to investigate the frequency of errors in SMI devices, and to establish the relationship between critical errors in device handling and device efficacy, are warranted.
Handling errors are directly linked to compromised drug delivery and reduced treatment efficacy.3 This may lead to more frequent or inappropriate medication use that, in turn, could result in unnecessary dose increases by the physician due to perceived lack of efficacy, and subsequently more adverse effects.3,26-28 However, these errors can be addressed through proper training and demonstration.29-32
Common device-handling errors include4,26,27,32,33:
- pMDIs: not shaking the inhaler (for suspensions), not exhaling fully before actuation, inhaling too forcefully, and not holding their breath for long enough after inhalation.
- DPIs: exhaling into the device mouthpiece, not exhaling fully before inhalation, not inhaling deeply or forcefully enough, and not holding their breath after inhalation.
- SMIs: not rotating the inhaler with mouth cap facing upwards, rotating the inhaler while looking into the spray nozzle with the cap open (before inhalation), and not maintaining inhalation with drug spray.
Incorrect inhaler use is a common cause of secondary nonadherence (ie, relating to incorrect medication use) among patients with COPD.4,34 Compromised inhaler technique and medication nonadherence jeopardize health outcomes and add to the economic burden of COPD.8,12,26
A 2005 study estimated that over 20% of the $25 billion spent on inhalers annually in the United States is wasted as a direct consequence of incorrect device handling.35
Failing to inhale correctly to achieve the optimal inspiratory flow for the specific device being used—deep and slow for pMDIs, or forceful, quick and deep for DPIs—is a critical handling error for inhaler devices.26 Significant associations between critical errors and clinical outcomes (hospitalization, emergency department visits, antibiotic courses, and corticosteroid courses) have been reported in COPD patients.26 In a retrospective analysis of COPD inpatients, suboptimal PIF rates with DPIs were associated with worse scores on the COPD Assessment Test, higher COPD and all-cause readmission rates, and shorter time to next COPD exacerbation.12
Patient considerations
Poor inhaler technique is frequently reported in patients with COPD or asthma, irrespective of the device used and with considerable variability in handling error rates for each individual device.25,26,35,45 Although clinical evidence is limited,25 research to date indicates that some DPIs may require less training than pMDIs.23,29,45,46 Therefore, DPI devices may be viewed as a more appropriate option for patients who encounter difficulty in coordinating the inhalation and actuation required for effective operation of a pMDI device. Alternatively, use of a spacer with pMDIs appears to reduce handling errors compared with pMDIs alone, but whether a pMDI plus spacer improves technique versus DPIs remains unclear.25,46,47 Lack of device training appears to be a key reason for inhaler handling errors across device types.26
Elderly patients need special consideration when selecting an inhaler and ensuring it is used correctly.48 Reduced physical ability and cognitive function due to age-related conditions (eg, dementia, depression, neuromuscular and cerebrovascular diseases) are the main reasons for suboptimal inhaler use in older patients, but other factors may also contribute (eg, multiple comorbid conditions, consequent complicated medication regimens).15 Older age is strongly associated with inhaler misuse,26 and has also been shown to have a negative correlation with PIF, independent of COPD severity.41 When compared with younger patients, older patients make more attempts before mastering the inhalation technique for a specific device, and need longer instruction time from trained health care professionals to correct inhaler mishandling.49,50 In elderly patients with adequate cognitive and manual ability, the most important factors in selecting a device are availability, convenience, ease of use, patient preference, and cost.8,23
Device continuity is a key consideration when multiple inhaled medications are needed.23 Lack of continuity of device type for different clinical needs means that patients may need to master the different techniques for each device.3 For instance, a patient may have a pMDI rescue medication, one or more DPIs for their maintenance therapy, and a nebulizer for additional bronchodilation, which may lead to confusion and incorrect device usage. Device continuity has been shown to improve disease control compared with using multiple inhalers in patients with asthma.51
A full summary of patient- and physician-related considerations for device selection, along with suggestions for how these can be addressed, is provided in Table 5.
Inhaler device training for patients and physicians
Comprehensive instruction, including practical demonstration, is important for ensuring patients with COPD use the correct inhaler technique, with regular review and repeated instruction generally needed for continued correct use.1,23,32,42 Lack of instruction is significantly associated with inhaler misuse in patients with COPD or asthma.26 Verbal training on inhalation technique increased the number of patients achieving the minimum inhalation flow rate required for a range of different DPIs.39 Similarly, training helped patients using a pMDI to slow their inhalation rate to <90 L/min, as recommended for this type of device.39 The ‘teach-back’ method, where patients are asked to demonstrate correct usage of their inhaler after instruction from a health care professional,52 has shown to be particularly effective in pharmacist-led patient device training.53 Educational interventions that incorporated a physical demonstration significantly improved inhaler technique in patients with COPD and asthma compared with patients receiving written and verbal information alone.53 Proper device training in primary care settings should also include education about why the inhaler is needed.3
Face-to-face instruction from trained caregivers for approximately 5 to 10 minutes improves the use of MDIs and DPIs by patients.49 However, clinical research indicates that learning correct handling and use may be easier and quicker for some devices than for others.31,49 For example, patients naïve to the PulmoJet (a DPI device not currently available in the United States) were found to have fewer serious errors after training than those using Diskus or Turbuhaler devices.24 In another study, it took less time to correct errors in inhaler use with the Diskus compared with the HandiHaler.44 Health care professionals themselves may lack training or knowledge on correct use of inhaler devices,35,36,54 with 1 study finding that up to 67% of nurses, doctors, and respiratory therapists were unable to describe or perform critical steps for using inhalers.35
A range of resources is available to aid in training patients and health care professionals in inhaler techniques:
- Tools such as the In-Check DIAL inspiratory flow meter (Clement Clarke International Ltd, Harlow, UK), TurbuHaler Trainer (AstraZeneca, Lund, Sweden), Diskus/Accuhaler Training Device (Vitalograph, Ennis, Ireland), and 2Tone Trainer (Canday Medical Ltd, Newmarket, UK) can be used to evaluate a patient’s physical ability to use a specific inhaler.55
- The emergence of electronic monitoring devices, such as SmartTrack, SmartTurbo, and SmartMat (all developed by Adherium Ltd, Auckland New Zealand), can provide objective and detailed adherence data to support clinical decision-making.56
- It is essential that patients and physicians alike utilize the instructions and video demonstrations available online to understand how to use a device correctly, and avoid errors. These resources can be found on a number of organizations’ websites (eg, COPD Foundation, Allergy and Asthma Network, Centers for Disease Control and Prevention, National Jewish Health, Asthma UK, Centre for Pharmacy Postgraduate Education) and on manufacturers’ websites for individual inhalers or treatments (eg, https://www.advair.com/how-to-use-advair.html, https://www.incruse.com/how-to-use-incruse.html, https://www.mysymbicort.com/copd/taking-symbicort/how-to-use-the-inhaler.html, https://www.tudorzahcp.com/tudorza-instructions-dosing.html, www.us.respimat.com (“How to Use the RESPIMAT Inhaler”), https://www.utibron.com/how-to-use.html).
Conclusions
A number of inhalation devices are available for the treatment of COPD. However, incorrect usage or a poor match between the patient and the device may lead to confusion, suboptimal treatment, and increased cost to the patient and health care system. Considering both patient- and health care system-related factors can ensure that appropriate inhaler section and usage can be optimized.
- Global Initiative for Chronic Obstructive Lung Disease. GOLD 2017 Global Strategy for the Diagnosis, Management and Prevention of COPD. http://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd. Accessed July 2017.
- Dolovich MB, Dhand R. Aerosol drug delivery: developments in device design and clinical use. Lancet. 2011;377(9770):1032-1045.
- Bonini M, Usmani OS. The importance of inhaler devices in the treatment of COPD. COPD Res Pract. 2015;1(1):9.
- Restrepo RD, Alvarez MT, Wittnebel LD, et al. Medication adherence issues in patients treated for COPD. Int J Chron Obstruct Pulmon Dis. 2008;3(3):371-384.
- Rogliani P, Calzetta L, Coppola A, et al. Optimizing drug delivery in COPD: the role of inhaler devices. Respir Med. 2017;124:6-14.
- Lavorini F, Fontana GA, Usmani OS. New inhaler devices - the good, the bad and the ugly. Respiration. 2014;88(1):3-15.
- Ibrahim M, Verma R, Garcia-Contreras L. Inhalation drug delivery devices: technology update. Med Devices (Auckl). 2015;8:131-139.
- Barrons R, Pegram A, Borries A. Inhaler device selection: special considerations in elderly patients with chronic obstructive pulmonary disease. Am J Health Syst Pharm. 2011;68(13):1221-1232.
- Dal Negro RW. Dry powder inhalers and the right things to remember: a concept review. Multidiscip Respir Med. 2015;10(1):13.
- Mahler DA, Waterman LA, Gifford AH. Prevalence and COPD phenotype for a suboptimal peak inspiratory flow rate against the simulated resistance of the Diskus® dry powder inhaler. J Aerosol Med Pulm Drug Deliv. 2013;26(3):174-179.
- Sharma G, Mahler DA, Mayorga VM, Deering KL, Harshaw O, Ganapathy V. Prevalence of low peak inspiratory flow rate at discharge in patients hospitalized for COPD exacerbation. Chronic Obstr Pulm Dis. 2017;4(3):217-224.
- Loh CH, Peters SP, Lovings TM, Ohar JA. Suboptimal inspiratory flow rates are associated with chronic obstructive pulmonary disease and all cause readmissions. Ann Am Thorac Soc. 2017;14(8):1305-1311.
- Le V, Hoang Thi TH, Robins E, Flament M. Dry powder inhalers: study of the parameters influencing adhesion and dispersion of fluticasone propionate. AAPS PharmSciTech. 2012;13(2):477-484.
- Dalby RN, Eicher J, Zierenberg B. Development of Respimat® Soft Mist™ Inhaler and its clinical utility in respiratory disorders. Med Devices (Auckl). 2011;4:145-155.
- Lavorini F, Mannini C, Chellini E, Fontana GA. Optimising inhaled pharmacotherapy for elderly patients with chronic obstructive pulmonary disease: the importance of delivery devices. Drugs Aging. 2016;33(7):461-473.
- Tashkin DP. A review of nebulized drug delivery in COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:2585-2596.
- Labiris NR, Dolovich MB. Pulmonary drug delivery. Part I: physiological factors affecting therapeutic effectiveness of aerosolized medications. Br J Clin Pharmacol. 2003;56(6):588-599.
- Chrystyn H. Anatomy and physiology in delivery: can we define our targets? Allergy. 1999;54(suppl 49):82-87.
- Biddiscombe M, Meah S, Barnes P, Usmani O. Drug particle size and lung deposition in COPD. Eur Respir J. 2016;48(suppl 60):Abstract. doi:10.1183/13993003.congress-13992016.PA13993313.
- Demoly P, Hagedoorn P, de Boer AH, Frijlink HW. The clinical relevance of dry powder inhaler performance for drug delivery. Respir Med. 2014;108(8):1195-1203.
- Dhand R. Inhaled drug therapy 2016: the year in review. Respir Care. 2017;62(7):978-996.
- de Boer AH, Gjaltema D, Hagedoorn P, Frijlink HW. Can ‘extrafine’ dry powder aerosols improve lung deposition? Eur J Pharm Biopharm. 2015;96:143-151.
- Vincken W, Dekhuijzen PR, Barnes P; ADMIT Group. The ADMIT series - Issues in inhalation therapy. 4) How to choose inhaler devices for the treatment of COPD. Prim Care Respir J. 2010;19(1):10-20.
- Roggeri A, Micheletto C, Roggeri DP. Inhalation errors due to device switch in patients with chronic obstructive pulmonary disease and asthma: critical health and economic issues. Int J Chron Obstruct Pulmon Dis. 2016;11:597-602.
- Chrystyn H, van der Palen J, Sharma R, et al. Device errors in asthma and COPD: systematic literature review and meta-analysis. NPJ Prim Care Respir Med. 2017;27(1):22.
- Melani AS, Bonavia M, Cilenti V, et al; Gruppo Educazionale Associazione Italiana Pneumologi Ospedalieri. Inhaler mishandling remains common in real life and is associated with reduced disease control [published correction appears in Respir Med. 2012;106(5):757]. Respir Med. 2011;105(6):930-938.
- Sanchis J, Gich I, Pedersen S; Aerosol Drug Management Improvement Team (ADMIT). Systematic review of errors in inhaler use: has patient technique improved over time? Chest. 2016;150(2):394-406.
- Sulaiman I, Seheult J, Sadasivuni N, et al. The impact of common inhaler errors on drug delivery: investigating critical errors with a dry powder inhaler. J Aerosol Med Pulm Drug Deliv. 2017;30(4):247-255.
- Chapman KR, Love L, Brubaker H. A comparison of breath-actuated and conventional metered-dose inhaler inhalation techniques in elderly subjects. Chest. 1993;104(5):1332-1337.
- van der Palen J, Thomas M, Chrystyn H, et al. A randomised open-label cross-over study of inhaler errors, preference and time to achieve correct inhaler use in patients with COPD or asthma: comparison of ELLIPTA with other inhaler devices. NPJ Prim Care Respir Med. 2016;26:16079.
- Chrystyn H, Price DB, Molimard M, et al. Comparison of serious inhaler technique errors made by device-naïve patients using three different dry powder inhalers: a randomised, crossover, open-label study. BMC Pulm Med. 2016;16:12.
- Crane MA, Jenkins CR, Goeman DP, Douglass JA. Inhaler device technique can be improved in older adults through tailored education: findings from a randomised controlled trial. NPJ Prim Care Respir Med. 2014;24:14034.
- Ohbayashi H, Kudo S, Ishikawa M. Inhaler operability and patient satisfaction regarding Genuair® and Respimat® inhalers for chronic obstructive pulmonary disease: a randomized crossover sudy. Pulmon Ther. 2017;3(1):173-185.
- Bourbeau J, Bartlett SJ. Patient adherence in COPD. Thorax. 2008;63(9):831-838.
- Fink JB, Rubin BK. Problems with inhaler use: a call for improved clinician and patient education. Respir Care. 2005;50(10):1360-1374; discussion 1374-1375.
- Yawn BP, Colice GL, Hodder R. Practical aspects of inhaler use in the management of chronic obstructive pulmonary disease in the primary care setting. Int J Chron Obstruct Pulmon Dis. 2012;7:495-502.
- Dhand R, Dolovich M, Chipps B, Myers TR, Restrepo R, Farrar JR. The role of nebulized therapy in the management of COPD: evidence and recommendations. COPD. 2012;9(1):58-72.
- Roche N, Gerhard S, Pritchard JN, et al. Patient focus and regulatory considerations for inhalation device design: report from the 2015 IPAC-RS/ISAM Workshop. J Aerosol Med Pulm Drug Deliv. 2017;30(1):1-13.
- Al-Showair RA, Tarsin WY, Assi KH, Pearson SB, Chrystyn H. Can all patients with COPD use the correct inhalation flow with all inhalers and does training help? Respir Med. 2007;101(11):2395-2401.
- Janssens W, VandenBrande P, Hardeman E, et al. Inspiratory flow rates at different levels of resistance in elderly COPD patients. Eur Respir J. 2008;31(1):78-83.
- Jarvis S, Ind PW, Shiner RJ. Inhaled therapy in elderly COPD patients; time for re-evaluation? Age Ageing. 2007;36(2):213-218.
- Lavorini F, Levy ML, Corrigan C, Crompton G; ADMIT Working Group. The ADMIT series - issues in inhalation therapy. 6) Training tools for inhalation devices. Prim Care Respir J. 2010;19(4):335-341.
- Pauwels R, Newman S, Borgström L. Airway deposition and airway effects of antiasthma drugs delivered from metered-dose inhalers. Eur Respir J. 1997;10(9):2127-2138.
- Everard ML, Devadason SG, Le Souëf PN. Flow early in the inspiratory manoeuvre affects the aerosol particle size distribution from a Turbuhaler. Respir Med. 1997;91(10):624-628.
- Molimard M, Raherison C, Lignot S, et al. Chronic obstructive pulmonary disease exacerbation and inhaler device handling: real-life assessment of 2935 patients. Eur Respir J. 2017;49(2):doi: 10.1183/13993003.13901794-2016.
- Jones V, Fernandez C, Diggory P. A comparison of large volume spacer, breath-activated and dry powder inhalers in older people. Age Ageing. 1999;28(5):481-484.
- Ho SF, O’Mahony MS, Steward JA, Breay P, Burr ML. Inhaler technique in older people in the community. Age Ageing. 2004;33(2):185-188.
- Taffet GE, Donohue JF, Altman PR. Considerations for managing chronic obstructive pulmonary disease in the elderly. Clin Interv Aging. 2014;9:23-30.
- Melani AS, Bonavia M, Mastropasqua E, et al; Gruppo Educazionale Associazione Italiana Pneumologi Ospedalieri (AIPO). Time required to rectify inhaler errors among experienced subjects with faulty technique. Respir Care. 2017;62(4):409-414.
- Dal Negro RW, Povero M. Dry-powder inhalers in patients with persistent airflow limitation: usability and preference. Multidiscip Respir Med. 2016;11(1):31.
- Price D, Chrystyn H, Kaplan A, et al. Effectiveness of same versus mixed asthma inhaler devices: a retrospective observational study in primary care. Allergy Asthma Immunol Res. 2012;4(4):184-191.
- Dantic DE. A critical review of the effectiveness of ‘teach-back’ technique in teaching COPD patients self-management using respiratory inhalers. Health Ed J. 2014;73(1):41-50.
- Bosnic-Anticevich SZ, Sinha H, So S, Reddel HK. Metered-dose inhaler technique: the effect of two educational interventions delivered in community pharmacy over time. J Asthma. 2010;47(3):251-256.
- Adnan M, Karim S, Khan S, Al Wabel N. Critical errors found during metered dose inhaler technique demonstration by pharmacists. Saudi Pharm J. 2016;24(5):625.
- Capstick TG, Clifton IJ. Inhaler technique and training in people with chronic obstructive pulmonary disease and asthma. Expert Rev Respir Med. 2012;6(1):91-101; quiz 102-103.
- Chan AH, Harrison J, Black PN, Mitchell EA, Foster JM. Using electronic monitoring devices to measure inhaler adherence: a practical guide for clinicians. J Allergy Clin Immunol Pract. 2015;3(3):335-349.e1-e5.
- Global Initiative for Chronic Obstructive Lung Disease. GOLD 2017 Global Strategy for the Diagnosis, Management and Prevention of COPD. http://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd. Accessed July 2017.
- Dolovich MB, Dhand R. Aerosol drug delivery: developments in device design and clinical use. Lancet. 2011;377(9770):1032-1045.
- Bonini M, Usmani OS. The importance of inhaler devices in the treatment of COPD. COPD Res Pract. 2015;1(1):9.
- Restrepo RD, Alvarez MT, Wittnebel LD, et al. Medication adherence issues in patients treated for COPD. Int J Chron Obstruct Pulmon Dis. 2008;3(3):371-384.
- Rogliani P, Calzetta L, Coppola A, et al. Optimizing drug delivery in COPD: the role of inhaler devices. Respir Med. 2017;124:6-14.
- Lavorini F, Fontana GA, Usmani OS. New inhaler devices - the good, the bad and the ugly. Respiration. 2014;88(1):3-15.
- Ibrahim M, Verma R, Garcia-Contreras L. Inhalation drug delivery devices: technology update. Med Devices (Auckl). 2015;8:131-139.
- Barrons R, Pegram A, Borries A. Inhaler device selection: special considerations in elderly patients with chronic obstructive pulmonary disease. Am J Health Syst Pharm. 2011;68(13):1221-1232.
- Dal Negro RW. Dry powder inhalers and the right things to remember: a concept review. Multidiscip Respir Med. 2015;10(1):13.
- Mahler DA, Waterman LA, Gifford AH. Prevalence and COPD phenotype for a suboptimal peak inspiratory flow rate against the simulated resistance of the Diskus® dry powder inhaler. J Aerosol Med Pulm Drug Deliv. 2013;26(3):174-179.
- Sharma G, Mahler DA, Mayorga VM, Deering KL, Harshaw O, Ganapathy V. Prevalence of low peak inspiratory flow rate at discharge in patients hospitalized for COPD exacerbation. Chronic Obstr Pulm Dis. 2017;4(3):217-224.
- Loh CH, Peters SP, Lovings TM, Ohar JA. Suboptimal inspiratory flow rates are associated with chronic obstructive pulmonary disease and all cause readmissions. Ann Am Thorac Soc. 2017;14(8):1305-1311.
- Le V, Hoang Thi TH, Robins E, Flament M. Dry powder inhalers: study of the parameters influencing adhesion and dispersion of fluticasone propionate. AAPS PharmSciTech. 2012;13(2):477-484.
- Dalby RN, Eicher J, Zierenberg B. Development of Respimat® Soft Mist™ Inhaler and its clinical utility in respiratory disorders. Med Devices (Auckl). 2011;4:145-155.
- Lavorini F, Mannini C, Chellini E, Fontana GA. Optimising inhaled pharmacotherapy for elderly patients with chronic obstructive pulmonary disease: the importance of delivery devices. Drugs Aging. 2016;33(7):461-473.
- Tashkin DP. A review of nebulized drug delivery in COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:2585-2596.
- Labiris NR, Dolovich MB. Pulmonary drug delivery. Part I: physiological factors affecting therapeutic effectiveness of aerosolized medications. Br J Clin Pharmacol. 2003;56(6):588-599.
- Chrystyn H. Anatomy and physiology in delivery: can we define our targets? Allergy. 1999;54(suppl 49):82-87.
- Biddiscombe M, Meah S, Barnes P, Usmani O. Drug particle size and lung deposition in COPD. Eur Respir J. 2016;48(suppl 60):Abstract. doi:10.1183/13993003.congress-13992016.PA13993313.
- Demoly P, Hagedoorn P, de Boer AH, Frijlink HW. The clinical relevance of dry powder inhaler performance for drug delivery. Respir Med. 2014;108(8):1195-1203.
- Dhand R. Inhaled drug therapy 2016: the year in review. Respir Care. 2017;62(7):978-996.
- de Boer AH, Gjaltema D, Hagedoorn P, Frijlink HW. Can ‘extrafine’ dry powder aerosols improve lung deposition? Eur J Pharm Biopharm. 2015;96:143-151.
- Vincken W, Dekhuijzen PR, Barnes P; ADMIT Group. The ADMIT series - Issues in inhalation therapy. 4) How to choose inhaler devices for the treatment of COPD. Prim Care Respir J. 2010;19(1):10-20.
- Roggeri A, Micheletto C, Roggeri DP. Inhalation errors due to device switch in patients with chronic obstructive pulmonary disease and asthma: critical health and economic issues. Int J Chron Obstruct Pulmon Dis. 2016;11:597-602.
- Chrystyn H, van der Palen J, Sharma R, et al. Device errors in asthma and COPD: systematic literature review and meta-analysis. NPJ Prim Care Respir Med. 2017;27(1):22.
- Melani AS, Bonavia M, Cilenti V, et al; Gruppo Educazionale Associazione Italiana Pneumologi Ospedalieri. Inhaler mishandling remains common in real life and is associated with reduced disease control [published correction appears in Respir Med. 2012;106(5):757]. Respir Med. 2011;105(6):930-938.
- Sanchis J, Gich I, Pedersen S; Aerosol Drug Management Improvement Team (ADMIT). Systematic review of errors in inhaler use: has patient technique improved over time? Chest. 2016;150(2):394-406.
- Sulaiman I, Seheult J, Sadasivuni N, et al. The impact of common inhaler errors on drug delivery: investigating critical errors with a dry powder inhaler. J Aerosol Med Pulm Drug Deliv. 2017;30(4):247-255.
- Chapman KR, Love L, Brubaker H. A comparison of breath-actuated and conventional metered-dose inhaler inhalation techniques in elderly subjects. Chest. 1993;104(5):1332-1337.
- van der Palen J, Thomas M, Chrystyn H, et al. A randomised open-label cross-over study of inhaler errors, preference and time to achieve correct inhaler use in patients with COPD or asthma: comparison of ELLIPTA with other inhaler devices. NPJ Prim Care Respir Med. 2016;26:16079.
- Chrystyn H, Price DB, Molimard M, et al. Comparison of serious inhaler technique errors made by device-naïve patients using three different dry powder inhalers: a randomised, crossover, open-label study. BMC Pulm Med. 2016;16:12.
- Crane MA, Jenkins CR, Goeman DP, Douglass JA. Inhaler device technique can be improved in older adults through tailored education: findings from a randomised controlled trial. NPJ Prim Care Respir Med. 2014;24:14034.
- Ohbayashi H, Kudo S, Ishikawa M. Inhaler operability and patient satisfaction regarding Genuair® and Respimat® inhalers for chronic obstructive pulmonary disease: a randomized crossover sudy. Pulmon Ther. 2017;3(1):173-185.
- Bourbeau J, Bartlett SJ. Patient adherence in COPD. Thorax. 2008;63(9):831-838.
- Fink JB, Rubin BK. Problems with inhaler use: a call for improved clinician and patient education. Respir Care. 2005;50(10):1360-1374; discussion 1374-1375.
- Yawn BP, Colice GL, Hodder R. Practical aspects of inhaler use in the management of chronic obstructive pulmonary disease in the primary care setting. Int J Chron Obstruct Pulmon Dis. 2012;7:495-502.
- Dhand R, Dolovich M, Chipps B, Myers TR, Restrepo R, Farrar JR. The role of nebulized therapy in the management of COPD: evidence and recommendations. COPD. 2012;9(1):58-72.
- Roche N, Gerhard S, Pritchard JN, et al. Patient focus and regulatory considerations for inhalation device design: report from the 2015 IPAC-RS/ISAM Workshop. J Aerosol Med Pulm Drug Deliv. 2017;30(1):1-13.
- Al-Showair RA, Tarsin WY, Assi KH, Pearson SB, Chrystyn H. Can all patients with COPD use the correct inhalation flow with all inhalers and does training help? Respir Med. 2007;101(11):2395-2401.
- Janssens W, VandenBrande P, Hardeman E, et al. Inspiratory flow rates at different levels of resistance in elderly COPD patients. Eur Respir J. 2008;31(1):78-83.
- Jarvis S, Ind PW, Shiner RJ. Inhaled therapy in elderly COPD patients; time for re-evaluation? Age Ageing. 2007;36(2):213-218.
- Lavorini F, Levy ML, Corrigan C, Crompton G; ADMIT Working Group. The ADMIT series - issues in inhalation therapy. 6) Training tools for inhalation devices. Prim Care Respir J. 2010;19(4):335-341.
- Pauwels R, Newman S, Borgström L. Airway deposition and airway effects of antiasthma drugs delivered from metered-dose inhalers. Eur Respir J. 1997;10(9):2127-2138.
- Everard ML, Devadason SG, Le Souëf PN. Flow early in the inspiratory manoeuvre affects the aerosol particle size distribution from a Turbuhaler. Respir Med. 1997;91(10):624-628.
- Molimard M, Raherison C, Lignot S, et al. Chronic obstructive pulmonary disease exacerbation and inhaler device handling: real-life assessment of 2935 patients. Eur Respir J. 2017;49(2):doi: 10.1183/13993003.13901794-2016.
- Jones V, Fernandez C, Diggory P. A comparison of large volume spacer, breath-activated and dry powder inhalers in older people. Age Ageing. 1999;28(5):481-484.
- Ho SF, O’Mahony MS, Steward JA, Breay P, Burr ML. Inhaler technique in older people in the community. Age Ageing. 2004;33(2):185-188.
- Taffet GE, Donohue JF, Altman PR. Considerations for managing chronic obstructive pulmonary disease in the elderly. Clin Interv Aging. 2014;9:23-30.
- Melani AS, Bonavia M, Mastropasqua E, et al; Gruppo Educazionale Associazione Italiana Pneumologi Ospedalieri (AIPO). Time required to rectify inhaler errors among experienced subjects with faulty technique. Respir Care. 2017;62(4):409-414.
- Dal Negro RW, Povero M. Dry-powder inhalers in patients with persistent airflow limitation: usability and preference. Multidiscip Respir Med. 2016;11(1):31.
- Price D, Chrystyn H, Kaplan A, et al. Effectiveness of same versus mixed asthma inhaler devices: a retrospective observational study in primary care. Allergy Asthma Immunol Res. 2012;4(4):184-191.
- Dantic DE. A critical review of the effectiveness of ‘teach-back’ technique in teaching COPD patients self-management using respiratory inhalers. Health Ed J. 2014;73(1):41-50.
- Bosnic-Anticevich SZ, Sinha H, So S, Reddel HK. Metered-dose inhaler technique: the effect of two educational interventions delivered in community pharmacy over time. J Asthma. 2010;47(3):251-256.
- Adnan M, Karim S, Khan S, Al Wabel N. Critical errors found during metered dose inhaler technique demonstration by pharmacists. Saudi Pharm J. 2016;24(5):625.
- Capstick TG, Clifton IJ. Inhaler technique and training in people with chronic obstructive pulmonary disease and asthma. Expert Rev Respir Med. 2012;6(1):91-101; quiz 102-103.
- Chan AH, Harrison J, Black PN, Mitchell EA, Foster JM. Using electronic monitoring devices to measure inhaler adherence: a practical guide for clinicians. J Allergy Clin Immunol Pract. 2015;3(3):335-349.e1-e5.