User login
Introduction
Chronic obstructive pulmonary disease (COPD) is common, often seen in primary care daily practice, and places a substantial burden on patients, their families, and society.1-4 Although dyspnea, cough, wheezing, chest tightness, and/or sputum production are typical symptoms of COPD, some patients present with less obvious issues, such as a highly sedentary lifestyle, adjusted to match their limitations and fatigue.5-7
Both pharmacologic and nonpharmacologic treatment options can reduce symptoms, treat comorbidities, prevent exacerbation, and improve quality of life, exercise tolerance, and health status in patients with COPD.3 Patients require initial therapy based on symptoms, history, and their own treatment goals, with regular monitoring to determine when to enhance or discontinue unnecessary therapy, and when to refer to a pulmonologist.
Primary care physicians manage the care of approximately 80% of patients with COPD.8 This provides the opportunity to engage patients in management goal-setting that facilitates more tailored treatments, and can improve adherence to therapy, which is historically poor in patients with COPD, thereby improving outcomes.9-11
Current COPD management guidelines
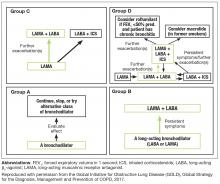
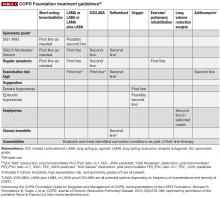
Both the Global Initiative for Obstructive Lung Disease (GOLD) and COPD Foundation guidelines recommend individualized care for patients with COPD.3,12 This individualized care is based on comprehensive assessment of symptoms (including assessment of whether symptoms are persistent or worsening) and/or continuation of exacerbations to escalate therapy. COPD phenotypes, such as individuals with frequent exacerbations, chronic bronchitis, and asthma–COPD overlap syndrome (ACO) can also guide treatment.13-15
GOLD 2017 strategy: key updates
- GOLD A – low symptoms, low exacerbation frequency
- GOLD B – high symptoms, low exacerbation frequency
- GOLD C – low symptoms, high exacerbation frequency
- GOLD D – high symptoms, high exacerbation frequency.
Postbronchodilator spirometry confirms the diagnosis of COPD by a forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC) ratio of less than 0.7, and denotes levels of airflow limitation severity based on the postbronchodilator FEV1 percentage predicted (Figure 1). Repeated spirometry assessment can identify individuals with rapidly declining lung function who are appropriate for referral to a pulmonologist.
Nonpharmacologic treatment approaches
Smoking cessation and pulmonary rehabilitation are central to effective COPD disease management.3 Smoking cessation has the greatest capacity to influence the natural history of COPD.3 Nicotine replacement products, as well as varenicline and bupropion, have been shown to increase long-term smoking cessation rates.16
Pulmonary rehabilitation (which includes exercise training, education, and self-management interventions aimed at behavior change) should be considered a fundamental part of COPD care.3 Pulmonary rehabilitation is recommended for any COPD patient of GOLD grades B–D (postbronchodilator FEV1/FVC ratio <0.70 and FEV1 <80% of predicted).3 The 2015 Cochrane Review of pulmonary rehabilitation for COPD assessed 65 randomized controlled trials involving 3822 participants, and concluded that pulmonary rehabilitation relieved dyspnea and fatigue, resulting in statistically improved functional exercise, maximal exercise capacity, and quality of life.17 Inclusion of pulmonary rehabilitation in treatment regimens may provide greater benefit than other more commonly used therapies alone.17
Long-term oxygen therapy has been shown to improve survival in COPD patients with severe resting hypoxemia (defined as a partial pressure of arterial oxygen [PaO2] of ≤55 mm Hg, or an oxyhemoglobin saturation level [SpO2] of ≤88%18), and is recommended in the current GOLD guidelines for selected patients.3 However, there is no clinical evidence demonstrating a mortality benefit with oxygen therapy in patients with stable COPD who have only moderate arterial oxygen desaturation (PaO2 of 56–59 mm Hg or SpO2 between 88%–90%18) at rest or with exercise.3 The Long-Term Oxygen Treatment Trial (LOTT) investigated the impact of the prescription of long-term supplemental oxygen in 738 patients with COPD and moderate resting (SpO2 between 89%–93%) or exercise-induced (SpO2 ≥80% for ≥5 min and <90% for ≥10 seconds during exercise) desaturation. Long-term oxygen supplementation did not result in either a longer time to death or first hospitalization.19 In a Cochrane Review published in 2016, Ekström et al conclude with moderate confidence that oxygen can relieve breathlessness when given during exercise to mildly hypoxemic and nonhypoxemic individuals with COPD, but does not improve health-related quality of life.20 Consultation with a pulmonologist is appropriate if when and how to prescribe oxygen therapy is not clear.
Pharmacologic treatment recommendations
Recent updates of the GOLD recommendations acknowledge the discordance between lung function and symptoms in patients with COPD. The 2017 recommendations use symptoms and exacerbation risk to define the ABCD categories that guide therapy selection. However, the GOLD authors still acknowledge the importance of spirometry in diagnosis, prognostic evaluation, and treatment with nonpharmacologic interventions in patients with COPD.3
- GOLD A patients: initial treatment with a short- or long-acting bronchodilator
- GOLD B patients: initial treatment with a single long-acting muscarinic receptor antagonist (LAMA) or long-acting β2-agonist (LABA). If symptoms (such as dyspnea) are severe at initiation of therapy, or persistent with use of 1 long-acting bronchodilator, LAMA/LABA combination is recommended
- GOLD C patients: initial treatment with a LAMA (LAMA is the preferred treatment due to superior exacerbation prevention versus LABA), with preferred escalation to LAMA/LABA if further exacerbations occur. Escalation to ICS/LABA combination may be considered (although is not preferred due to possible risk of pneumonia21)
- GOLD D patients: initial treatment with LAMA/LABA; initial treatment with ICS/LABA may be preferred in patients with a history and/or findings suggestive of asthma–COPD overlap or high blood eosinophil counts (but consider the risk of pneumonia). Escalation to ICS/LAMA/LABA triple therapy may be considered if symptoms persist or further exacerbations occur.
GOLD grades provide a valuable guide for initiating therapy and continuing assessment and care. Initial therapy may provide sufficient disease control in some patients, but disease progression and persistent symptoms despite therapy often require treatment escalation. Assessing and escalating therapy should be based on changes in functional status and symptom burden, which can be identified by asking appropriate questions, or performing tests to evaluate functional capacity, such as the 6-minute walk test.3 The modified Medical Research Council (mMRC) dyspnea scale is also a good example of a quick tool for baseline assessment of the patient’s functional status. This assessment must be coupled with appropriate follow-up. During follow-up visits, it is important to ask patients about their typical daily activities, and assess how these compare to what has been reported previously. Follow-up visits can also be an opportunity to check that a patient is using their inhaler device correctly.
Regular assessment of patients’ health status is important for optimal disease management.22 The COPD Assessment Test (CAT) is a short, simple, COPD patient-completed questionnaire, designed to inform the clinician about the severity and impact of a patient’s disease. Changes in patients’ functional abilities and symptoms over time can be monitored with regular use of the CAT at COPD visits.23 Although the CAT test facilitates prediction of COPD exacerbations,24 it is not intended to identify comorbidities; for example, the mental health comorbidities of COPD (including anxiety, sleep disturbances, and depression) are often unreported by patients and so can be difficult for clinicians to detect.25 Awareness of possible comorbid conditions, and appropriate screening for conditions such as depression (PHQ-2), anxiety (GAD-7), or osteoporosis (BMD) is recommended.26 Further details of PHQ-2 and GAD-7 are provided in the second article (Anxiety and Depression in Chronic Obstructive Pulmonary Disease: Recognition and Management) of this supplement.
Physicians need to make decisions about whether (and how) treatment should be escalated using parameters in addition to frequency of exacerbations, such as a lack of improvement or worsening of symptoms or functional status.3 For example, the addition of a second bronchodilator is recommended for a GOLD B patient with continued breathlessness on a single bronchodilator, and escalating from 1 to 2 long-acting bronchodilators is recommended for GOLD C patients with persistent exacerbations despite monotherapy with a LABA or LAMA. LAMA/LABA combinations that are currently approved for the treatment of COPD by the US Food and Drug Administration are umeclidinium/vilanterol, tiotropium/olodaterol, glycopyrrolate/formoterol, and glycopyrrolate/indacaterol.27-30
For patients with high symptom burden (mMRC ≥2, CAT ≥10) experiencing frequent exacerbations, defined as 2 or more exacerbations per year, or 1 or more exacerbations per year that lead to a hospitalization (ie, GOLD D patients), LAMA/LABA is recommended as first-choice treatment. A recent study showed LAMA/LABA to be superior to ICS/LABA for preventing exacerbations; while it should be noted that the majority of exacerbations in this study were mild, LAMA/LABA was also found to be significantly more effective at reducing exacerbations classed as moderate or severe than ICS/LABA.31 However, these findings may not be broadly generalizable, owing to limitations associated with the study’s exclusion criteria and the high discontinuation rate reported during the study’s run-in phase, which may have introduced a selection bias.31
ICS/LABA may be considered for treating persistent exacerbations in some GOLD C patients, and may be first choice in GOLD D patients with asthma-like features, or possibly high blood eosinophil counts.3 Patients who remain symptomatic on LAMA/LABA may also be considered for triple therapy (ICS/LAMA/LABA), as per the GOLD recommendations.3 Care must be taken to use ICS appropriately, as ICS treatment may increase a patient’s risk of developing pneumonia, although risk profiles for pneumonia vary depending on the ICS treatment selected.32 Increased risk of other adverse effects associated with ICS treatment should also be considered, including oral candidiasis (odds ratio [OR], 2.65; 95% confidence interval [CI], 2.03–3.46 [note, oral candidiasis can be avoided by mouth-rinsing33]), hoarse voice (OR, 1.95; 95% CI, 1.41–2.70), and skin bruising (OR, 1.63; 95% CI, 1.31–2.03) compared with placebo in patients with COPD.21 Nonetheless, use of ICS is not associated with a mortality risk,34 and a 2017 study by Crim et al reported that the risk of pneumonia was not increased with ICS compared with placebo in patients with moderate airflow limitation who had/were at high risk of cardiovascular disease.35 Physicians should therefore consider both the potential risks and benefits of ICS before prescribing them to patients with COPD.
While careful consideration of ICS is warranted, ICS/LABA combinations are often prescribed inappropriately in many patients with COPD in clinical practice, including those at low exacerbation risk.15 Treatment de-escalation by stopping ICS may be appropriate in patients receiving ICS/LAMA/LABA who suffer from fewer than 2 exacerbations per year (ie, receiving ICS inappropriately),36 or in those who continue to experience persistent exacerbations despite ICS.3 The use of systemic steroids in stable COPD is not recommended.37
At any stage of disease, patients may benefit from a referral by primary care to a pulmonologist for further evaluation.38 Reasons include uncertain diagnosis, severe COPD, assessment for oxygen therapy, trouble finding or referring to pulmonary rehabilitation, and COPD in patients younger than 40 years of age (who may be suffering from α1-antitrypsin deficiency).38 Referring patients with significant emphysema or other co-existing lung diseases also allows evaluation for surgical interventions such as lung transplantation, lung volume reduction surgery (LVRS), or other therapies.
Patients with COPD may gain particular benefit from comanagement by primary care physicians and pulmonologists.39 For example, primary care physicians may require guidance from pulmonologists regarding the management of patients with severe disease whose therapy requirements are becoming more complex. Similarly, pulmonologists may not be comfortable managing the comorbidities often encountered in COPD (eg, anxiety and depression), so would require support from the primary care physician to provide the patients with effective, holistic management.
Surgical and bronchoscopic interventions
Surgical and bronchoscopic interventions have the potential to significantly benefit carefully selected patient groups with emphysema.3 LVRS resects parts of the lungs to reduce hyperinflation, and improves lung function and reduces exacerbations in patients with advanced emphysema.3 It can prolong mortality in selected patients,40 but can increase the risk of death in those with low FEV1 and either homogenous emphysema or very low carbon monoxide diffusing capacity.41
Nonsurgical bronchoscopic interventions continue to improve; they have been designed to achieve similar results to LVRS (but with less morbidity), and provide a possible intervention for patients with heterogenous or homogenous emphysema, and significant hyperinflation refractory to optimized medical care.3 Use of endobronchial one-way valves and lung volume reduction coils has resulted in significant improvements in patients’ quality of life, exercise capacity, and pulmonary function for select patients with severe emphysema.42,43 Other therapies, such as adhesives (where a biologic sealant collapses targeted areas of the lung to induce the formation of scar tissue, thus reducing lung tissue volume), and vapor therapy (where heated water vapor is used to deliver thermal energy to the lungs, inducing an inflammatory response that causes contraction fibrosis and atelectasis, and subsequently lung volume reduction) are also in development.44 Consideration of surgical or nonsurgical interventions require referral to a pulmonologist.
Lung transplantation may be an option for patients with very severe COPD without significant comorbidities. Lung transplantation improves quality of life, but does not prolong survival.3,45,46 The procedure is limited by donor availability, high cost, and potential complications.3
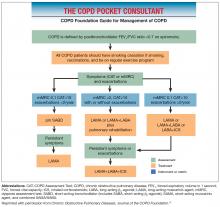
COPD Foundation guidelines
The COPD Foundation guidelines note that some spirometry results are normal, but do not rule out the presence of chronic bronchitis, emphysema, or other lung disease; or are neither normal nor consistent with COPD or other lung disease. The guidelines therefore define 2 additional spirometric grades, referred to as SG 0 (representing patients with normal spirometry) and SG U (representing patients who have a FEV1/FVC ratio >0.7 but FEV1 <80% predicted). At present, neither SG 0 nor SG U are associated with therapeutic options distinct from other spirometric grades, but this may change as we learn more from clinical studies.47,48
Importance of managing COPD comorbidities
Comorbidities are common among patients with COPD, and COPD itself may increase the risk of developing other diseases.3,49-52 It can be difficult to recognize the many comorbidities in patients with COPD, due to the diverse nature of these comorbidities, a lack of understanding of their underlying causes, patients’ failure to recognize or share symptoms, or misdiagnosing them as adverse effects associated with COPD medication.53 Failure to recognize and treat comorbidities can increase risk of hospitalizations or exacerbations, worsen prognosis, increase morbidity, lower the chances of treatment adherence, and place a greater burden on the patient, family, and health care resources.51,52,54-56 Common comorbidities include cardiovascular disease, musculoskeletal dysfunction, metabolic syndrome, anxiety/depression, osteoporosis, lung cancer, and heart failure.3,51,52
The value of effectively managing comorbidities in improving outcomes and adherence to therapy is well documented. For example, personalized management of patients with COPD and comorbid anxiety and/or depression has been shown to reduce both the mental health symptoms and COPD-related outcomes (eg, exercise tolerance, disability).57-59
Comorbidity burden may impact adherence to COPD medication. Depression, for instance, is a known risk factor for nonadherence to treatment. Patients with multiple untreated or uncontrolled comorbid conditions may also be less likely to benefit from pulmonary rehabilitation.60 It is therefore important that comorbidities are managed effectively to improve adherence to therapy, and enhance the benefits of pulmonary rehabilitation.
Patient monitoring
Routine follow-up of patients with COPD is essential as lung function may worsen over time, even with the best available care.3 Worsening of symptoms, activity limitation, and disease progression should be monitored closely to determine when to modify management/pharmacotherapy, and to identify any complications and/or comorbidities that may develop.3 When patients with COPD do not receive the appropriate level of treatment or monitoring, it can be due to: under-reporting of disease severity, symptoms, and exacerbations during consultation; lack of information on the impact of the disease on the patient’s quality of life; and failure to recognize comorbidities.23,25,53 Continued use of the patient questionnaires described previously is recommended, and the GOLD strategy advises that symptoms are assessed at each visit. These follow-up visits also provide an opportunity to monitor patients with COPD for key comorbidities, including heart failure, ischemic heart disease, arrhythmias, osteoporosis, depression/anxiety, and lung cancer, as well as to determine a patient’s current smoking status, taking appropriate action as needed.3
Unmet needs
COPD remains underdiagnosed in the United States, with only 50% of individuals with impaired lung function reported to receive a formal diagnosis of COPD.61,62 Opportunities for diagnosing COPD earlier in its course are being missed; 85% of patients consult primary care for lower respiratory symptoms in the 5 years before diagnosis of COPD, and might have been candidates for further evaluation of those symptoms, including spirometry testing.63 Initiating treatment at early stages of COPD has the potential to improve patients’ health-related quality of life, and may provide opportunities to slow disease progression through interventions such as smoking cessation.64 Practical approaches to improving early diagnosis in primary care involve the use of questionnaires and clinical suspicion to identify those appropriate for spirometry, the most reliable method for identifying patients with COPD.3,9,65 Such methodology is currently under investigation, with early studies demonstrating the potential benefit of the COPD Assessment in Primary Care To Identify Undiagnosed Respiratory Disease and Exacerbation Risk (CAPTURE) questionnaire in conjunction with peak expiratory flow to gauge whether a patient requires further diagnostic evaluation.66
In addition, the GOLD strategy and COPD Foundation guidelines emphasize that correct assessment of symptoms is of paramount importance in determining the most appropriate therapy (both pharmacologic and nonpharmacologic) for patients with COPD, but traditionally has not been used to inform management choices. Both guidelines therefore highlight the importance of symptom assessment ahead of therapeutic decision-making.
Poor adherence to prescribed therapies and inadequate patient monitoring also need addressing. Two studies analyzing refill adherence data in patients with COPD and asthma in Sweden reported that only 28%–29% of prescribed treatments were dispensed with refill adherence that covered more than 80% of prescribed treatment time67,68; a study in 5504 patients in the United States with a prescription of fluticasone propionate/salmeterol combination therapy found that more than half of patients only refilled their prescription once over the course of the 1-year study.69 With studies showing incorrect use of inhalers in more than 50% of patients with COPD, incorrect inhaler technique is a significant contributor to poor treatment adherence.70,71 Inhaler technique should be reviewed regularly with direct observation of patients’ technique. Assessment of the patients’ ability to use their current prescribed inhaler(s) is recommended before considering a change in treatment.70 Errors in inhaler use are also associated with an increased rate of severe COPD exacerbations, increased risk of hospitalization, and poor disease control.71,72 Important factors affecting inhaler use include age, education, product design, costs (copays and deductibles) for medications, and instruction and inhaler technique education from the health care providers.70,72,73 Recent data support improvements in product design, training by the health care provider, and “self-training” by the patient (assisted by instructional video or other digital media) to increase adherence and reduce the frequency of handling errors.10,70,74 Electronic monitoring devices, messaging systems, and cell phone applications are also being considered as ways to increase adherence.75
Maintenance medication is an essential component of COPD management. However, patients with COPD often report that their preference is for medication that they can “feel” working, which may be implicated in their motivation to adhere to therapy.76 Conversely, while maintenance medication may reduce exacerbations, and lessen a patient’s decline in lung function,77 it may not have a significant impact on how they “feel.” As a result, patients may not take it as prescribed, contributing to poor adherence. It is therefore important for primary care physicians to acknowledge that the impact of taking the maintenance medication may not be felt immediately, and articulate the importance of maintenance therapy to their patients, as failure to adhere to treatment can have significant implications for longer-term outcomes such as symptom burden, quality of life, and exacerbation risk.11
Regular patient follow-up is necessary to reinforce such information: patients with milder or stable COPD may be followed at 6-month intervals, while patients with severe or frequent exacerbations, or patients who have recently been hospitalized, require follow-up at 2- to 4-week intervals.78
Conclusions
Defining personal treatment goals for patients with COPD can enhance patient and physician communication and encourage continued collaboration to improve adherence and outcomes. Regularly monitoring symptoms, exacerbations, and comorbidities via patient-focused questionnaires, and closely examining patient adherence and technique, form a fundamental part of care for patients with COPD. Recent updates to the GOLD and the COPD Foundation guidelines have emphasized the importance of symptom assessment in initiating COPD therapy, and continued assessment to appropriately escalate treatment. Nonpharmacologic therapies such as smoking cessation and pulmonary rehabilitation are recommended at all stages of COPD alongside pharmacologic treatment.
- Janson C, Marks G, Buist S, et al. The impact of COPD on health status: findings from the BOLD study. Eur Respir J. 2013;42(6):1472-1483.
- Buist AS, Vollmer WM, McBurnie MA. Worldwide burden of COPD in high- and low-income countries. Part I. The burden of obstructive lung disease (BOLD) initiative. Int J Tuberc Lung Dis. 2008;12(7):703-708.
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management and Prevention of COPD. 2017. Available from: http://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd. Accessed July 2017.
- López-Campos JL, Tan W, Soriano JB. Global burden of COPD. Respirology. 2016;21(1):14-23.
- Wheaton AG, Cunningham TJ, Ford ES, Croft JB; Centers for Disease Control and Prevention (CDC). Employment and activity limitations among adults with chronic obstructive pulmonary disease--United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64(11):289-295.
- Rennard S, Decramer M, Calverley PM, et al. Impact of COPD in North America and Europe in 2000: subjects’ perspective of Confronting COPD International Survey. Eur Respir J. 2002;20(4):799-805.
- Troosters T, van der Molen T, Polkey M, et al. Improving physical activity in COPD: towards a new paradigm. Respir Res. 2013;14:115.
- Perez X, Wisnivesky JP, Lurslurchachai L, Kleinman LC, Kronish IM. Barriers to adherence to COPD guidelines among primary care providers. Respir Med. 2012;106(3):374-381.
- Price D, Crockett A, Arne M, et al. Spirometry in primary care case-identification, diagnosis and management of COPD. Prim Care Respir J. 2009;18(3):216-223.
- van Boven JF, Ryan D, Eakin MN, Canonica GW, Barot A, Foster JM; Respiratory Effectiveness Group. Enhancing respiratory medication adherence: the role of health care professionals and cost-effectiveness considerations. J Allergy Clin Immunol Pract. 2016;4(5):835-846.
- van Boven JF, Chavannes NH, van der Molen T, Rutten-van Mölken MP, Postma MJ, Vegter S. Clinical and economic impact of non-adherence in COPD: a systematic review. Respir Med. 2014;108(1):103-113.
- COPD Foundation. Pocket Consultant Guide for the Diagnosis and Management of COPD. 2016.
- Lange P, Halpin DM, O’Donnell DE, MacNee W. Diagnosis, assessment, and phenotyping of COPD: beyond FEV1. Int J Chron Obstruct Pulmon Dis. 2016;11 Spec Iss3-12.
- Miravitlles M, Soler-Cataluña JJ, Calle M, et al. A new approach to grading and treating COPD based on clinical phenotypes: summary of the Spanish COPD guidelines (GesEPOC). Prim Care Respir J. 2013;22(1):117-121.
- Patalano F, Banerji D, D’Andrea P, Fogel R, Altman P, Colthorpe P. Addressing unmet needs in the treatment of COPD. Eur Respir Rev. 2014;23(133):333-344.
- van Eerd EAM, van der Meer RM, van Schayck OC, Kotz D. Smoking cessation for people with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2016(8):CD010744.
- McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;(2):CD003793.
- Ekström M. Clinical usefulness of long-term oxygen therapy in adults. N Engl J Med. 2016;375(17):1683-1684.
- Albert RK, Au DH, Blackford AL, et al; Long-Term Oxygen Treatment Trial Research Group. A randomized trial of long-term oxygen for COPD with moderate desaturation. N Engl J Med. 2016;375(17):1617-1627.
- Ekström M, Ahmadi Z, Bornefalk-Hermansson A, Abernethy A, Currow D. Oxygen for breathlessness in patients with chronic obstructive pulmonary disease who do not qualify for home oxygen therapy. Cochrane Database Syst Rev. 2016;(11):CD006429.
- Yang IA, Clarke MS, Sim EH, Fong KM. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;(7):CD002991.
- Jones PW, Price D, van der Molen T. Role of clinical questionnaires in optimizing everyday care of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2011;6:289-296.
- Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648-654.
- Lee SD, Huang MS, Kang J, et al; Investigators of the Predictive Ability of CAT in Acute Exacerbations of COPD (PACE) Study. The COPD assessment test (CAT) assists prediction of COPD exacerbations in high-risk patients. Respir Med. 2014;108(4):600-608.
- Sonetti DA, Hospenthal AC, Adams SG. Integrated management strategies for chronic obstructive pulmonary disease. J Multidiscip Healthc. 2010;3:181-188.
- Miyazaki M, Nakamura H, Chubachi S, et al; Keio COPD Comorbidity Research (K-CCR) Group. Analysis of comorbid factors that increase the COPD assessment test scores. Respir Res. 2014;15:13.
- Anoro Ellipta [highlights of prescribing info]. Research Triangle Park, NC: GlaxoSmithKline group of companies; 2013.
- Stiolto Respimat [highlights of prescribing info]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc.; 2015.
- Bevespi Aerosphere [highlights of prescribing info]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2015.
- Utibron Neohaler [highlights of prescribing info]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2015.
- Wedzicha JA, Banerji D, Chapman KR, et al; FLAME Investigators. Indacaterol-glycopyrronium versus salmeterol-fluticasone for COPD. N Engl J Med. 2016;374(23):2222-2234.
- Suissa S, Patenaude V, Lapi F, Ernst P. Inhaled corticosteroids in COPD and the risk of serious pneumonia. Thorax. 2013;68(11):1029-1036.
- Dempsey OJ, Coutie WJ, Wilson AM, Williams P, Lipworth BJ. Evaluation of the buccal component of systemic absorption with inhaled fluticasone propionate. Thorax. 1999;54(7):614-617.
- Drummond MB, Dasenbrook EC, Pitz MW, Murphy DJ, Fan E. Inhaled corticosteroids in patients with stable chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA. 2008;300(20):2407-2416.
- Crim C, Calverley PMA, Anderson JA, et al; SUMMIT Investigators. Pneumonia risk with inhaled fluticasone furoate and vilanterol in COPD patients with moderate airflow limitation: The SUMMIT trial. Respir Med. 2017;131:27-34.
- Rossi A, Guerriero M, Corrado A, OPTIMO/AIPO Study Group. Withdrawal of inhaled corticosteroids can be safe in COPD patients at low risk of exacerbation: a real-life study on the appropriateness of treatment in moderate COPD patients (OPTIMO). Respir Res. 2014;15:77.
- Falk JA, Minai OA, Mosenifar Z. Inhaled and systemic corticosteroids in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5(4):506-512.
- British Thoracic Society Standards of Care Committee. BTS statement on criteria for specialist referral, admission, discharge and follow-up for adults with respiratory disease. Thorax. 2008;63(Suppl 1):i1-i16.
- Benfante A, Messina R, Milazzo V, Scichilone N. How to unveil chronic respiratory diseases in clinical practice? A model of alliance between general practitioners and pulmonologists. Pulm Pharmacol Ther. 2017;44:106-110.
- Fishman A, Martinez F, Naunheim K, et al; National Emphysema Treatment Trial Research Group. A randomized trial comparing lung-volume–reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348(21):2059-2073.
- Fishman A, Fessler H, Martinez F, et al. Patients at high risk of death after lung-volume-reduction surgery. N Engl J Med. 2001;345(15):1075-1083.
- Deslee G, Klooster K, Hetzel M, et al. Lung volume reduction coil treatment for patients with severe emphysema: a European multicentre trial. Thorax. 2014;69(11):980-986.
- Slebos DJ, Shah PL, Herth FJ, Valipour A. Endobronchial valves for endoscopic lung volume reduction: best practice recommendations from expert panel on endoscopic lung volume reduction. Respiration. 2017;93(2):138-150.
- Browning RF, Parrish S, Sarkar S, et al. Bronchoscopic interventions for severe COPD. J Thorac Dis. 2014;6(suppl 4):S407-S415.
- Stavem K, Bjørtuft Ø, Borgan Ø, Geiran O, Boe J. Lung transplantation in patients with chronic obstructive pulmonary disease in a national cohort is without obvious survival benefit. J Heart Lung Transplant. 2006;25(1):75-84.
- Hosenpud JD, Bennett LE, Keck BM, Edwards EB, Novick RJ. Effect of diagnosis on survival benefit of lung transplantation for end-stage lung disease. Lancet. 1998;351(9095):24-27.
- Yawn B, Thomashow DM, Mannino D, et al. A statement of the COPD Foundation: The 2017 update to the COPD Foundation COPD Pocket Consultant Guide. Chronic Obstr Pulm Dis. 2017;4(3):177-185.
- Rennard S, Thomashow B, Crapo J, et al. Introducing the COPD Foundation Guide for Diagnosis and Management of COPD, recommendations of the COPD Foundation. COPD. 2013;10(3):378-389.
- Dal Negro RW, Bonadiman L, Turco P. Prevalence of different comorbidities in COPD patients by gender and GOLD stage. Multidiscip Respir Med. 2015;10(1):24.
- Chetty U, McLean G, Morrison D, Agur K, Guthrie B, Mercer SW. Chronic obstructive pulmonary disease and comorbidities: a large cross-sectional study in primary care. Br J Gen Pract. 2017;67(658):e321-e328.
- Westerik JA, Metting EI, van Boven JF, Tiersma W, Kocks JW, Schermer TR. Associations between chronic comorbidity and exacerbation risk in primary care patients with COPD. Respir Res. 2017;18(1):31.
- Putcha N, Han MK, Martinez CH, et al; the COPDGene Investigators. Comorbidities of COPD have a major impact on clinical outcomes, particularly in African Americans. Chronic Obstr Pulm Dis. 2014;1(1):105-114.
- Koskela J, Kilpeläinen M, Kupiainen H, et al. Co-morbidities are the key nominators of the health related quality of life in mild and moderate COPD. BMC Pulm Med. 2014;14:102.
- Clini EM, Boschetto P, Lainscak M, Janssens W. Comorbidities in chronic obstructive pulmonary disease from assessment to treatment. Biomed Res Int. 2014;2014:414928.
- Mannino DM, Thorn D, Swensen A, Holguin F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J. 2008;32(4):962-969.
- Schwab P, Dhamane AD, Hopson SD, et al. Impact of comorbid conditions in COPD patients on health care resource utilization and costs in a predominantly Medicare population. Int J Chron Obstruct Pulmon Dis. 2017;12:735-744.
- Yohannes AM, Alexopoulos GS. Depression and anxiety in patients with COPD. Eur Respir Rev. 2014;23(133):345-349.
- Alexopoulos GS, Kiosses DN, Sirey JA, et al. Untangling therapeutic ingredients of a personalized intervention for patients with depression and severe COPD. Am J Geriatr Psychiatry. 2014;22(11):1316-1324.
- Eiser N, Harte R, Spiros K, Phillips C, Isaac MT. Effect of treating depression on quality-of-life and exercise tolerance in severe COPD. COPD. 2005;2(2):233-241.
- Crisafulli E, Costi S, Luppi F, et al. Role of comorbidities in a cohort of patients with COPD undergoing pulmonary rehabilitation. Thorax. 2008;63(6):487-492.
- Mannino DM, Homa DM, Akinbami LJ, Ford ES, Redd SC. Chronic obstructive pulmonary disease surveillance—United States, 1971-2000. Respir Care. 2002;47(10):1184-1199.
- Ford ES, Croft JB, Mannino DM, Wheaton AG, Zhang X, Giles WH. COPD surveillance—United States, 1999-2011. Chest. 2013;144(1):284-305.
- Jones RC, Price D, Ryan D, et al; Respiratory Effectiveness Group. Opportunities to diagnose chronic obstructive pulmonary disease in routine care in the UK: a retrospective study of a clinical cohort. Lancet Respir Med. 2014;2(4):267-276.
- Welte T, Vogelmeier C, Papi A. COPD: early diagnosis and treatment to slow disease progression. Int J Clin Pract. 2015;69(3):336-349.
- Price D, Freeman D, Cleland J, Kaplan A, Cerasoli F. Earlier diagnosis and earlier treatment of COPD in primary care. Prim Care Respir J. 2011;20(1):15-22.
- Martinez FJ, Mannino D, Leidy NK, et al; High-Risk-COPD Screening Study Group. A new approach for identifying patients with undiagnosed chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;195(6):748-756.
- Krigsman K, Nilsson JL, Ring L. Refill adherence for patients with asthma and COPD: comparison of a pharmacy record database with manually collected repeat prescriptions. Pharmacoepidemiol Drug Saf. 2007;16(4):441-448.
- Krigsman K, Moen J, Nilsson JL, Ring L. Refill adherence by the elderly for asthma/chronic obstructive pulmonary disease drugs dispensed over a 10-year period. J Clin Pharm Ther. 2007;32(6):603-611.
- Bender BG, Pedan A, Varasteh LT. Adherence and persistence with fluticasone propionate/salmeterol combination therapy. J Allergy Clin Immunol. 2006;118(4):899-904.
- Chrystyn H, Price DB, Molimard M, et al. Comparison of serious inhaler technique errors made by device-naïve patients using three different dry powder inhalers: a randomised, crossover, open-label study. BMC Pulm Med. 2016;16:12.
- Molimard M, Raherison C, Lignot S, et al. Chronic obstructive pulmonary disease exacerbation and inhaler device handling: real-life assessment of 2935 patients. Eur Respir J. 2017;49(2):pii: 1601794.
- Melani AS, Bonavia M, Cilenti V, et al; Gruppo Educazionale Associazione Italiana Pneumologi Ospedalieri. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med. 2011;105(6):930-938.
- Han MK, Martinez CH, Au DH, et al. Meeting the challenge of COPD care delivery in the USA: a multiprovider perspective. Lancet Respir Med. 2016;4(6):473-526.
- Plaza V, Peiró M, Torrejón M, et al; PROMETHEUS Study Group. A repeated short educational intervention improves asthma control and quality of life. Eur Respir J. 2015;46(5):1298-1307.
- Craven VE, Morton RW, Spencer S, Devadason SG, Everard ML. Electronic monitoring and reminding devices for improving adherence to inhaled therapy in patients with asthma. Cochrane Database Syst Rev. 2015;(3):CD011554.
- Kawata AK, Kleinman L, Harding G, Ramachandran S. Evaluation of patient preference and willingness to pay for attributes of maintenance medication for chronic obstructive pulmonary disease (COPD). Patient. 2014;7(4):413-426.
- Ferguson GT. Maintenance pharmacotherapy of mild and moderate COPD: what is the evidence? Respir Med. 2011;105(9):1268-1274.
- BMJ Best Practice. COPD. http://bestpractice.bmj.com/best-practice/monograph/7.html. Updated November 2017. Accessed May 30, 2017.
Introduction
Chronic obstructive pulmonary disease (COPD) is common, often seen in primary care daily practice, and places a substantial burden on patients, their families, and society.1-4 Although dyspnea, cough, wheezing, chest tightness, and/or sputum production are typical symptoms of COPD, some patients present with less obvious issues, such as a highly sedentary lifestyle, adjusted to match their limitations and fatigue.5-7
Both pharmacologic and nonpharmacologic treatment options can reduce symptoms, treat comorbidities, prevent exacerbation, and improve quality of life, exercise tolerance, and health status in patients with COPD.3 Patients require initial therapy based on symptoms, history, and their own treatment goals, with regular monitoring to determine when to enhance or discontinue unnecessary therapy, and when to refer to a pulmonologist.
Primary care physicians manage the care of approximately 80% of patients with COPD.8 This provides the opportunity to engage patients in management goal-setting that facilitates more tailored treatments, and can improve adherence to therapy, which is historically poor in patients with COPD, thereby improving outcomes.9-11
Current COPD management guidelines
Both the Global Initiative for Obstructive Lung Disease (GOLD) and COPD Foundation guidelines recommend individualized care for patients with COPD.3,12 This individualized care is based on comprehensive assessment of symptoms (including assessment of whether symptoms are persistent or worsening) and/or continuation of exacerbations to escalate therapy. COPD phenotypes, such as individuals with frequent exacerbations, chronic bronchitis, and asthma–COPD overlap syndrome (ACO) can also guide treatment.13-15
GOLD 2017 strategy: key updates
- GOLD A – low symptoms, low exacerbation frequency
- GOLD B – high symptoms, low exacerbation frequency
- GOLD C – low symptoms, high exacerbation frequency
- GOLD D – high symptoms, high exacerbation frequency.
Postbronchodilator spirometry confirms the diagnosis of COPD by a forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC) ratio of less than 0.7, and denotes levels of airflow limitation severity based on the postbronchodilator FEV1 percentage predicted (Figure 1). Repeated spirometry assessment can identify individuals with rapidly declining lung function who are appropriate for referral to a pulmonologist.
Nonpharmacologic treatment approaches
Smoking cessation and pulmonary rehabilitation are central to effective COPD disease management.3 Smoking cessation has the greatest capacity to influence the natural history of COPD.3 Nicotine replacement products, as well as varenicline and bupropion, have been shown to increase long-term smoking cessation rates.16
Pulmonary rehabilitation (which includes exercise training, education, and self-management interventions aimed at behavior change) should be considered a fundamental part of COPD care.3 Pulmonary rehabilitation is recommended for any COPD patient of GOLD grades B–D (postbronchodilator FEV1/FVC ratio <0.70 and FEV1 <80% of predicted).3 The 2015 Cochrane Review of pulmonary rehabilitation for COPD assessed 65 randomized controlled trials involving 3822 participants, and concluded that pulmonary rehabilitation relieved dyspnea and fatigue, resulting in statistically improved functional exercise, maximal exercise capacity, and quality of life.17 Inclusion of pulmonary rehabilitation in treatment regimens may provide greater benefit than other more commonly used therapies alone.17
Long-term oxygen therapy has been shown to improve survival in COPD patients with severe resting hypoxemia (defined as a partial pressure of arterial oxygen [PaO2] of ≤55 mm Hg, or an oxyhemoglobin saturation level [SpO2] of ≤88%18), and is recommended in the current GOLD guidelines for selected patients.3 However, there is no clinical evidence demonstrating a mortality benefit with oxygen therapy in patients with stable COPD who have only moderate arterial oxygen desaturation (PaO2 of 56–59 mm Hg or SpO2 between 88%–90%18) at rest or with exercise.3 The Long-Term Oxygen Treatment Trial (LOTT) investigated the impact of the prescription of long-term supplemental oxygen in 738 patients with COPD and moderate resting (SpO2 between 89%–93%) or exercise-induced (SpO2 ≥80% for ≥5 min and <90% for ≥10 seconds during exercise) desaturation. Long-term oxygen supplementation did not result in either a longer time to death or first hospitalization.19 In a Cochrane Review published in 2016, Ekström et al conclude with moderate confidence that oxygen can relieve breathlessness when given during exercise to mildly hypoxemic and nonhypoxemic individuals with COPD, but does not improve health-related quality of life.20 Consultation with a pulmonologist is appropriate if when and how to prescribe oxygen therapy is not clear.
Pharmacologic treatment recommendations
Recent updates of the GOLD recommendations acknowledge the discordance between lung function and symptoms in patients with COPD. The 2017 recommendations use symptoms and exacerbation risk to define the ABCD categories that guide therapy selection. However, the GOLD authors still acknowledge the importance of spirometry in diagnosis, prognostic evaluation, and treatment with nonpharmacologic interventions in patients with COPD.3
- GOLD A patients: initial treatment with a short- or long-acting bronchodilator
- GOLD B patients: initial treatment with a single long-acting muscarinic receptor antagonist (LAMA) or long-acting β2-agonist (LABA). If symptoms (such as dyspnea) are severe at initiation of therapy, or persistent with use of 1 long-acting bronchodilator, LAMA/LABA combination is recommended
- GOLD C patients: initial treatment with a LAMA (LAMA is the preferred treatment due to superior exacerbation prevention versus LABA), with preferred escalation to LAMA/LABA if further exacerbations occur. Escalation to ICS/LABA combination may be considered (although is not preferred due to possible risk of pneumonia21)
- GOLD D patients: initial treatment with LAMA/LABA; initial treatment with ICS/LABA may be preferred in patients with a history and/or findings suggestive of asthma–COPD overlap or high blood eosinophil counts (but consider the risk of pneumonia). Escalation to ICS/LAMA/LABA triple therapy may be considered if symptoms persist or further exacerbations occur.
GOLD grades provide a valuable guide for initiating therapy and continuing assessment and care. Initial therapy may provide sufficient disease control in some patients, but disease progression and persistent symptoms despite therapy often require treatment escalation. Assessing and escalating therapy should be based on changes in functional status and symptom burden, which can be identified by asking appropriate questions, or performing tests to evaluate functional capacity, such as the 6-minute walk test.3 The modified Medical Research Council (mMRC) dyspnea scale is also a good example of a quick tool for baseline assessment of the patient’s functional status. This assessment must be coupled with appropriate follow-up. During follow-up visits, it is important to ask patients about their typical daily activities, and assess how these compare to what has been reported previously. Follow-up visits can also be an opportunity to check that a patient is using their inhaler device correctly.
Regular assessment of patients’ health status is important for optimal disease management.22 The COPD Assessment Test (CAT) is a short, simple, COPD patient-completed questionnaire, designed to inform the clinician about the severity and impact of a patient’s disease. Changes in patients’ functional abilities and symptoms over time can be monitored with regular use of the CAT at COPD visits.23 Although the CAT test facilitates prediction of COPD exacerbations,24 it is not intended to identify comorbidities; for example, the mental health comorbidities of COPD (including anxiety, sleep disturbances, and depression) are often unreported by patients and so can be difficult for clinicians to detect.25 Awareness of possible comorbid conditions, and appropriate screening for conditions such as depression (PHQ-2), anxiety (GAD-7), or osteoporosis (BMD) is recommended.26 Further details of PHQ-2 and GAD-7 are provided in the second article (Anxiety and Depression in Chronic Obstructive Pulmonary Disease: Recognition and Management) of this supplement.
Physicians need to make decisions about whether (and how) treatment should be escalated using parameters in addition to frequency of exacerbations, such as a lack of improvement or worsening of symptoms or functional status.3 For example, the addition of a second bronchodilator is recommended for a GOLD B patient with continued breathlessness on a single bronchodilator, and escalating from 1 to 2 long-acting bronchodilators is recommended for GOLD C patients with persistent exacerbations despite monotherapy with a LABA or LAMA. LAMA/LABA combinations that are currently approved for the treatment of COPD by the US Food and Drug Administration are umeclidinium/vilanterol, tiotropium/olodaterol, glycopyrrolate/formoterol, and glycopyrrolate/indacaterol.27-30
For patients with high symptom burden (mMRC ≥2, CAT ≥10) experiencing frequent exacerbations, defined as 2 or more exacerbations per year, or 1 or more exacerbations per year that lead to a hospitalization (ie, GOLD D patients), LAMA/LABA is recommended as first-choice treatment. A recent study showed LAMA/LABA to be superior to ICS/LABA for preventing exacerbations; while it should be noted that the majority of exacerbations in this study were mild, LAMA/LABA was also found to be significantly more effective at reducing exacerbations classed as moderate or severe than ICS/LABA.31 However, these findings may not be broadly generalizable, owing to limitations associated with the study’s exclusion criteria and the high discontinuation rate reported during the study’s run-in phase, which may have introduced a selection bias.31
ICS/LABA may be considered for treating persistent exacerbations in some GOLD C patients, and may be first choice in GOLD D patients with asthma-like features, or possibly high blood eosinophil counts.3 Patients who remain symptomatic on LAMA/LABA may also be considered for triple therapy (ICS/LAMA/LABA), as per the GOLD recommendations.3 Care must be taken to use ICS appropriately, as ICS treatment may increase a patient’s risk of developing pneumonia, although risk profiles for pneumonia vary depending on the ICS treatment selected.32 Increased risk of other adverse effects associated with ICS treatment should also be considered, including oral candidiasis (odds ratio [OR], 2.65; 95% confidence interval [CI], 2.03–3.46 [note, oral candidiasis can be avoided by mouth-rinsing33]), hoarse voice (OR, 1.95; 95% CI, 1.41–2.70), and skin bruising (OR, 1.63; 95% CI, 1.31–2.03) compared with placebo in patients with COPD.21 Nonetheless, use of ICS is not associated with a mortality risk,34 and a 2017 study by Crim et al reported that the risk of pneumonia was not increased with ICS compared with placebo in patients with moderate airflow limitation who had/were at high risk of cardiovascular disease.35 Physicians should therefore consider both the potential risks and benefits of ICS before prescribing them to patients with COPD.
While careful consideration of ICS is warranted, ICS/LABA combinations are often prescribed inappropriately in many patients with COPD in clinical practice, including those at low exacerbation risk.15 Treatment de-escalation by stopping ICS may be appropriate in patients receiving ICS/LAMA/LABA who suffer from fewer than 2 exacerbations per year (ie, receiving ICS inappropriately),36 or in those who continue to experience persistent exacerbations despite ICS.3 The use of systemic steroids in stable COPD is not recommended.37
At any stage of disease, patients may benefit from a referral by primary care to a pulmonologist for further evaluation.38 Reasons include uncertain diagnosis, severe COPD, assessment for oxygen therapy, trouble finding or referring to pulmonary rehabilitation, and COPD in patients younger than 40 years of age (who may be suffering from α1-antitrypsin deficiency).38 Referring patients with significant emphysema or other co-existing lung diseases also allows evaluation for surgical interventions such as lung transplantation, lung volume reduction surgery (LVRS), or other therapies.
Patients with COPD may gain particular benefit from comanagement by primary care physicians and pulmonologists.39 For example, primary care physicians may require guidance from pulmonologists regarding the management of patients with severe disease whose therapy requirements are becoming more complex. Similarly, pulmonologists may not be comfortable managing the comorbidities often encountered in COPD (eg, anxiety and depression), so would require support from the primary care physician to provide the patients with effective, holistic management.
Surgical and bronchoscopic interventions
Surgical and bronchoscopic interventions have the potential to significantly benefit carefully selected patient groups with emphysema.3 LVRS resects parts of the lungs to reduce hyperinflation, and improves lung function and reduces exacerbations in patients with advanced emphysema.3 It can prolong mortality in selected patients,40 but can increase the risk of death in those with low FEV1 and either homogenous emphysema or very low carbon monoxide diffusing capacity.41
Nonsurgical bronchoscopic interventions continue to improve; they have been designed to achieve similar results to LVRS (but with less morbidity), and provide a possible intervention for patients with heterogenous or homogenous emphysema, and significant hyperinflation refractory to optimized medical care.3 Use of endobronchial one-way valves and lung volume reduction coils has resulted in significant improvements in patients’ quality of life, exercise capacity, and pulmonary function for select patients with severe emphysema.42,43 Other therapies, such as adhesives (where a biologic sealant collapses targeted areas of the lung to induce the formation of scar tissue, thus reducing lung tissue volume), and vapor therapy (where heated water vapor is used to deliver thermal energy to the lungs, inducing an inflammatory response that causes contraction fibrosis and atelectasis, and subsequently lung volume reduction) are also in development.44 Consideration of surgical or nonsurgical interventions require referral to a pulmonologist.
Lung transplantation may be an option for patients with very severe COPD without significant comorbidities. Lung transplantation improves quality of life, but does not prolong survival.3,45,46 The procedure is limited by donor availability, high cost, and potential complications.3
COPD Foundation guidelines
The COPD Foundation guidelines note that some spirometry results are normal, but do not rule out the presence of chronic bronchitis, emphysema, or other lung disease; or are neither normal nor consistent with COPD or other lung disease. The guidelines therefore define 2 additional spirometric grades, referred to as SG 0 (representing patients with normal spirometry) and SG U (representing patients who have a FEV1/FVC ratio >0.7 but FEV1 <80% predicted). At present, neither SG 0 nor SG U are associated with therapeutic options distinct from other spirometric grades, but this may change as we learn more from clinical studies.47,48
Importance of managing COPD comorbidities
Comorbidities are common among patients with COPD, and COPD itself may increase the risk of developing other diseases.3,49-52 It can be difficult to recognize the many comorbidities in patients with COPD, due to the diverse nature of these comorbidities, a lack of understanding of their underlying causes, patients’ failure to recognize or share symptoms, or misdiagnosing them as adverse effects associated with COPD medication.53 Failure to recognize and treat comorbidities can increase risk of hospitalizations or exacerbations, worsen prognosis, increase morbidity, lower the chances of treatment adherence, and place a greater burden on the patient, family, and health care resources.51,52,54-56 Common comorbidities include cardiovascular disease, musculoskeletal dysfunction, metabolic syndrome, anxiety/depression, osteoporosis, lung cancer, and heart failure.3,51,52
The value of effectively managing comorbidities in improving outcomes and adherence to therapy is well documented. For example, personalized management of patients with COPD and comorbid anxiety and/or depression has been shown to reduce both the mental health symptoms and COPD-related outcomes (eg, exercise tolerance, disability).57-59
Comorbidity burden may impact adherence to COPD medication. Depression, for instance, is a known risk factor for nonadherence to treatment. Patients with multiple untreated or uncontrolled comorbid conditions may also be less likely to benefit from pulmonary rehabilitation.60 It is therefore important that comorbidities are managed effectively to improve adherence to therapy, and enhance the benefits of pulmonary rehabilitation.
Patient monitoring
Routine follow-up of patients with COPD is essential as lung function may worsen over time, even with the best available care.3 Worsening of symptoms, activity limitation, and disease progression should be monitored closely to determine when to modify management/pharmacotherapy, and to identify any complications and/or comorbidities that may develop.3 When patients with COPD do not receive the appropriate level of treatment or monitoring, it can be due to: under-reporting of disease severity, symptoms, and exacerbations during consultation; lack of information on the impact of the disease on the patient’s quality of life; and failure to recognize comorbidities.23,25,53 Continued use of the patient questionnaires described previously is recommended, and the GOLD strategy advises that symptoms are assessed at each visit. These follow-up visits also provide an opportunity to monitor patients with COPD for key comorbidities, including heart failure, ischemic heart disease, arrhythmias, osteoporosis, depression/anxiety, and lung cancer, as well as to determine a patient’s current smoking status, taking appropriate action as needed.3
Unmet needs
COPD remains underdiagnosed in the United States, with only 50% of individuals with impaired lung function reported to receive a formal diagnosis of COPD.61,62 Opportunities for diagnosing COPD earlier in its course are being missed; 85% of patients consult primary care for lower respiratory symptoms in the 5 years before diagnosis of COPD, and might have been candidates for further evaluation of those symptoms, including spirometry testing.63 Initiating treatment at early stages of COPD has the potential to improve patients’ health-related quality of life, and may provide opportunities to slow disease progression through interventions such as smoking cessation.64 Practical approaches to improving early diagnosis in primary care involve the use of questionnaires and clinical suspicion to identify those appropriate for spirometry, the most reliable method for identifying patients with COPD.3,9,65 Such methodology is currently under investigation, with early studies demonstrating the potential benefit of the COPD Assessment in Primary Care To Identify Undiagnosed Respiratory Disease and Exacerbation Risk (CAPTURE) questionnaire in conjunction with peak expiratory flow to gauge whether a patient requires further diagnostic evaluation.66
In addition, the GOLD strategy and COPD Foundation guidelines emphasize that correct assessment of symptoms is of paramount importance in determining the most appropriate therapy (both pharmacologic and nonpharmacologic) for patients with COPD, but traditionally has not been used to inform management choices. Both guidelines therefore highlight the importance of symptom assessment ahead of therapeutic decision-making.
Poor adherence to prescribed therapies and inadequate patient monitoring also need addressing. Two studies analyzing refill adherence data in patients with COPD and asthma in Sweden reported that only 28%–29% of prescribed treatments were dispensed with refill adherence that covered more than 80% of prescribed treatment time67,68; a study in 5504 patients in the United States with a prescription of fluticasone propionate/salmeterol combination therapy found that more than half of patients only refilled their prescription once over the course of the 1-year study.69 With studies showing incorrect use of inhalers in more than 50% of patients with COPD, incorrect inhaler technique is a significant contributor to poor treatment adherence.70,71 Inhaler technique should be reviewed regularly with direct observation of patients’ technique. Assessment of the patients’ ability to use their current prescribed inhaler(s) is recommended before considering a change in treatment.70 Errors in inhaler use are also associated with an increased rate of severe COPD exacerbations, increased risk of hospitalization, and poor disease control.71,72 Important factors affecting inhaler use include age, education, product design, costs (copays and deductibles) for medications, and instruction and inhaler technique education from the health care providers.70,72,73 Recent data support improvements in product design, training by the health care provider, and “self-training” by the patient (assisted by instructional video or other digital media) to increase adherence and reduce the frequency of handling errors.10,70,74 Electronic monitoring devices, messaging systems, and cell phone applications are also being considered as ways to increase adherence.75
Maintenance medication is an essential component of COPD management. However, patients with COPD often report that their preference is for medication that they can “feel” working, which may be implicated in their motivation to adhere to therapy.76 Conversely, while maintenance medication may reduce exacerbations, and lessen a patient’s decline in lung function,77 it may not have a significant impact on how they “feel.” As a result, patients may not take it as prescribed, contributing to poor adherence. It is therefore important for primary care physicians to acknowledge that the impact of taking the maintenance medication may not be felt immediately, and articulate the importance of maintenance therapy to their patients, as failure to adhere to treatment can have significant implications for longer-term outcomes such as symptom burden, quality of life, and exacerbation risk.11
Regular patient follow-up is necessary to reinforce such information: patients with milder or stable COPD may be followed at 6-month intervals, while patients with severe or frequent exacerbations, or patients who have recently been hospitalized, require follow-up at 2- to 4-week intervals.78
Conclusions
Defining personal treatment goals for patients with COPD can enhance patient and physician communication and encourage continued collaboration to improve adherence and outcomes. Regularly monitoring symptoms, exacerbations, and comorbidities via patient-focused questionnaires, and closely examining patient adherence and technique, form a fundamental part of care for patients with COPD. Recent updates to the GOLD and the COPD Foundation guidelines have emphasized the importance of symptom assessment in initiating COPD therapy, and continued assessment to appropriately escalate treatment. Nonpharmacologic therapies such as smoking cessation and pulmonary rehabilitation are recommended at all stages of COPD alongside pharmacologic treatment.
Introduction
Chronic obstructive pulmonary disease (COPD) is common, often seen in primary care daily practice, and places a substantial burden on patients, their families, and society.1-4 Although dyspnea, cough, wheezing, chest tightness, and/or sputum production are typical symptoms of COPD, some patients present with less obvious issues, such as a highly sedentary lifestyle, adjusted to match their limitations and fatigue.5-7
Both pharmacologic and nonpharmacologic treatment options can reduce symptoms, treat comorbidities, prevent exacerbation, and improve quality of life, exercise tolerance, and health status in patients with COPD.3 Patients require initial therapy based on symptoms, history, and their own treatment goals, with regular monitoring to determine when to enhance or discontinue unnecessary therapy, and when to refer to a pulmonologist.
Primary care physicians manage the care of approximately 80% of patients with COPD.8 This provides the opportunity to engage patients in management goal-setting that facilitates more tailored treatments, and can improve adherence to therapy, which is historically poor in patients with COPD, thereby improving outcomes.9-11
Current COPD management guidelines
Both the Global Initiative for Obstructive Lung Disease (GOLD) and COPD Foundation guidelines recommend individualized care for patients with COPD.3,12 This individualized care is based on comprehensive assessment of symptoms (including assessment of whether symptoms are persistent or worsening) and/or continuation of exacerbations to escalate therapy. COPD phenotypes, such as individuals with frequent exacerbations, chronic bronchitis, and asthma–COPD overlap syndrome (ACO) can also guide treatment.13-15
GOLD 2017 strategy: key updates
- GOLD A – low symptoms, low exacerbation frequency
- GOLD B – high symptoms, low exacerbation frequency
- GOLD C – low symptoms, high exacerbation frequency
- GOLD D – high symptoms, high exacerbation frequency.
Postbronchodilator spirometry confirms the diagnosis of COPD by a forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC) ratio of less than 0.7, and denotes levels of airflow limitation severity based on the postbronchodilator FEV1 percentage predicted (Figure 1). Repeated spirometry assessment can identify individuals with rapidly declining lung function who are appropriate for referral to a pulmonologist.
Nonpharmacologic treatment approaches
Smoking cessation and pulmonary rehabilitation are central to effective COPD disease management.3 Smoking cessation has the greatest capacity to influence the natural history of COPD.3 Nicotine replacement products, as well as varenicline and bupropion, have been shown to increase long-term smoking cessation rates.16
Pulmonary rehabilitation (which includes exercise training, education, and self-management interventions aimed at behavior change) should be considered a fundamental part of COPD care.3 Pulmonary rehabilitation is recommended for any COPD patient of GOLD grades B–D (postbronchodilator FEV1/FVC ratio <0.70 and FEV1 <80% of predicted).3 The 2015 Cochrane Review of pulmonary rehabilitation for COPD assessed 65 randomized controlled trials involving 3822 participants, and concluded that pulmonary rehabilitation relieved dyspnea and fatigue, resulting in statistically improved functional exercise, maximal exercise capacity, and quality of life.17 Inclusion of pulmonary rehabilitation in treatment regimens may provide greater benefit than other more commonly used therapies alone.17
Long-term oxygen therapy has been shown to improve survival in COPD patients with severe resting hypoxemia (defined as a partial pressure of arterial oxygen [PaO2] of ≤55 mm Hg, or an oxyhemoglobin saturation level [SpO2] of ≤88%18), and is recommended in the current GOLD guidelines for selected patients.3 However, there is no clinical evidence demonstrating a mortality benefit with oxygen therapy in patients with stable COPD who have only moderate arterial oxygen desaturation (PaO2 of 56–59 mm Hg or SpO2 between 88%–90%18) at rest or with exercise.3 The Long-Term Oxygen Treatment Trial (LOTT) investigated the impact of the prescription of long-term supplemental oxygen in 738 patients with COPD and moderate resting (SpO2 between 89%–93%) or exercise-induced (SpO2 ≥80% for ≥5 min and <90% for ≥10 seconds during exercise) desaturation. Long-term oxygen supplementation did not result in either a longer time to death or first hospitalization.19 In a Cochrane Review published in 2016, Ekström et al conclude with moderate confidence that oxygen can relieve breathlessness when given during exercise to mildly hypoxemic and nonhypoxemic individuals with COPD, but does not improve health-related quality of life.20 Consultation with a pulmonologist is appropriate if when and how to prescribe oxygen therapy is not clear.
Pharmacologic treatment recommendations
Recent updates of the GOLD recommendations acknowledge the discordance between lung function and symptoms in patients with COPD. The 2017 recommendations use symptoms and exacerbation risk to define the ABCD categories that guide therapy selection. However, the GOLD authors still acknowledge the importance of spirometry in diagnosis, prognostic evaluation, and treatment with nonpharmacologic interventions in patients with COPD.3
- GOLD A patients: initial treatment with a short- or long-acting bronchodilator
- GOLD B patients: initial treatment with a single long-acting muscarinic receptor antagonist (LAMA) or long-acting β2-agonist (LABA). If symptoms (such as dyspnea) are severe at initiation of therapy, or persistent with use of 1 long-acting bronchodilator, LAMA/LABA combination is recommended
- GOLD C patients: initial treatment with a LAMA (LAMA is the preferred treatment due to superior exacerbation prevention versus LABA), with preferred escalation to LAMA/LABA if further exacerbations occur. Escalation to ICS/LABA combination may be considered (although is not preferred due to possible risk of pneumonia21)
- GOLD D patients: initial treatment with LAMA/LABA; initial treatment with ICS/LABA may be preferred in patients with a history and/or findings suggestive of asthma–COPD overlap or high blood eosinophil counts (but consider the risk of pneumonia). Escalation to ICS/LAMA/LABA triple therapy may be considered if symptoms persist or further exacerbations occur.
GOLD grades provide a valuable guide for initiating therapy and continuing assessment and care. Initial therapy may provide sufficient disease control in some patients, but disease progression and persistent symptoms despite therapy often require treatment escalation. Assessing and escalating therapy should be based on changes in functional status and symptom burden, which can be identified by asking appropriate questions, or performing tests to evaluate functional capacity, such as the 6-minute walk test.3 The modified Medical Research Council (mMRC) dyspnea scale is also a good example of a quick tool for baseline assessment of the patient’s functional status. This assessment must be coupled with appropriate follow-up. During follow-up visits, it is important to ask patients about their typical daily activities, and assess how these compare to what has been reported previously. Follow-up visits can also be an opportunity to check that a patient is using their inhaler device correctly.
Regular assessment of patients’ health status is important for optimal disease management.22 The COPD Assessment Test (CAT) is a short, simple, COPD patient-completed questionnaire, designed to inform the clinician about the severity and impact of a patient’s disease. Changes in patients’ functional abilities and symptoms over time can be monitored with regular use of the CAT at COPD visits.23 Although the CAT test facilitates prediction of COPD exacerbations,24 it is not intended to identify comorbidities; for example, the mental health comorbidities of COPD (including anxiety, sleep disturbances, and depression) are often unreported by patients and so can be difficult for clinicians to detect.25 Awareness of possible comorbid conditions, and appropriate screening for conditions such as depression (PHQ-2), anxiety (GAD-7), or osteoporosis (BMD) is recommended.26 Further details of PHQ-2 and GAD-7 are provided in the second article (Anxiety and Depression in Chronic Obstructive Pulmonary Disease: Recognition and Management) of this supplement.
Physicians need to make decisions about whether (and how) treatment should be escalated using parameters in addition to frequency of exacerbations, such as a lack of improvement or worsening of symptoms or functional status.3 For example, the addition of a second bronchodilator is recommended for a GOLD B patient with continued breathlessness on a single bronchodilator, and escalating from 1 to 2 long-acting bronchodilators is recommended for GOLD C patients with persistent exacerbations despite monotherapy with a LABA or LAMA. LAMA/LABA combinations that are currently approved for the treatment of COPD by the US Food and Drug Administration are umeclidinium/vilanterol, tiotropium/olodaterol, glycopyrrolate/formoterol, and glycopyrrolate/indacaterol.27-30
For patients with high symptom burden (mMRC ≥2, CAT ≥10) experiencing frequent exacerbations, defined as 2 or more exacerbations per year, or 1 or more exacerbations per year that lead to a hospitalization (ie, GOLD D patients), LAMA/LABA is recommended as first-choice treatment. A recent study showed LAMA/LABA to be superior to ICS/LABA for preventing exacerbations; while it should be noted that the majority of exacerbations in this study were mild, LAMA/LABA was also found to be significantly more effective at reducing exacerbations classed as moderate or severe than ICS/LABA.31 However, these findings may not be broadly generalizable, owing to limitations associated with the study’s exclusion criteria and the high discontinuation rate reported during the study’s run-in phase, which may have introduced a selection bias.31
ICS/LABA may be considered for treating persistent exacerbations in some GOLD C patients, and may be first choice in GOLD D patients with asthma-like features, or possibly high blood eosinophil counts.3 Patients who remain symptomatic on LAMA/LABA may also be considered for triple therapy (ICS/LAMA/LABA), as per the GOLD recommendations.3 Care must be taken to use ICS appropriately, as ICS treatment may increase a patient’s risk of developing pneumonia, although risk profiles for pneumonia vary depending on the ICS treatment selected.32 Increased risk of other adverse effects associated with ICS treatment should also be considered, including oral candidiasis (odds ratio [OR], 2.65; 95% confidence interval [CI], 2.03–3.46 [note, oral candidiasis can be avoided by mouth-rinsing33]), hoarse voice (OR, 1.95; 95% CI, 1.41–2.70), and skin bruising (OR, 1.63; 95% CI, 1.31–2.03) compared with placebo in patients with COPD.21 Nonetheless, use of ICS is not associated with a mortality risk,34 and a 2017 study by Crim et al reported that the risk of pneumonia was not increased with ICS compared with placebo in patients with moderate airflow limitation who had/were at high risk of cardiovascular disease.35 Physicians should therefore consider both the potential risks and benefits of ICS before prescribing them to patients with COPD.
While careful consideration of ICS is warranted, ICS/LABA combinations are often prescribed inappropriately in many patients with COPD in clinical practice, including those at low exacerbation risk.15 Treatment de-escalation by stopping ICS may be appropriate in patients receiving ICS/LAMA/LABA who suffer from fewer than 2 exacerbations per year (ie, receiving ICS inappropriately),36 or in those who continue to experience persistent exacerbations despite ICS.3 The use of systemic steroids in stable COPD is not recommended.37
At any stage of disease, patients may benefit from a referral by primary care to a pulmonologist for further evaluation.38 Reasons include uncertain diagnosis, severe COPD, assessment for oxygen therapy, trouble finding or referring to pulmonary rehabilitation, and COPD in patients younger than 40 years of age (who may be suffering from α1-antitrypsin deficiency).38 Referring patients with significant emphysema or other co-existing lung diseases also allows evaluation for surgical interventions such as lung transplantation, lung volume reduction surgery (LVRS), or other therapies.
Patients with COPD may gain particular benefit from comanagement by primary care physicians and pulmonologists.39 For example, primary care physicians may require guidance from pulmonologists regarding the management of patients with severe disease whose therapy requirements are becoming more complex. Similarly, pulmonologists may not be comfortable managing the comorbidities often encountered in COPD (eg, anxiety and depression), so would require support from the primary care physician to provide the patients with effective, holistic management.
Surgical and bronchoscopic interventions
Surgical and bronchoscopic interventions have the potential to significantly benefit carefully selected patient groups with emphysema.3 LVRS resects parts of the lungs to reduce hyperinflation, and improves lung function and reduces exacerbations in patients with advanced emphysema.3 It can prolong mortality in selected patients,40 but can increase the risk of death in those with low FEV1 and either homogenous emphysema or very low carbon monoxide diffusing capacity.41
Nonsurgical bronchoscopic interventions continue to improve; they have been designed to achieve similar results to LVRS (but with less morbidity), and provide a possible intervention for patients with heterogenous or homogenous emphysema, and significant hyperinflation refractory to optimized medical care.3 Use of endobronchial one-way valves and lung volume reduction coils has resulted in significant improvements in patients’ quality of life, exercise capacity, and pulmonary function for select patients with severe emphysema.42,43 Other therapies, such as adhesives (where a biologic sealant collapses targeted areas of the lung to induce the formation of scar tissue, thus reducing lung tissue volume), and vapor therapy (where heated water vapor is used to deliver thermal energy to the lungs, inducing an inflammatory response that causes contraction fibrosis and atelectasis, and subsequently lung volume reduction) are also in development.44 Consideration of surgical or nonsurgical interventions require referral to a pulmonologist.
Lung transplantation may be an option for patients with very severe COPD without significant comorbidities. Lung transplantation improves quality of life, but does not prolong survival.3,45,46 The procedure is limited by donor availability, high cost, and potential complications.3
COPD Foundation guidelines
The COPD Foundation guidelines note that some spirometry results are normal, but do not rule out the presence of chronic bronchitis, emphysema, or other lung disease; or are neither normal nor consistent with COPD or other lung disease. The guidelines therefore define 2 additional spirometric grades, referred to as SG 0 (representing patients with normal spirometry) and SG U (representing patients who have a FEV1/FVC ratio >0.7 but FEV1 <80% predicted). At present, neither SG 0 nor SG U are associated with therapeutic options distinct from other spirometric grades, but this may change as we learn more from clinical studies.47,48
Importance of managing COPD comorbidities
Comorbidities are common among patients with COPD, and COPD itself may increase the risk of developing other diseases.3,49-52 It can be difficult to recognize the many comorbidities in patients with COPD, due to the diverse nature of these comorbidities, a lack of understanding of their underlying causes, patients’ failure to recognize or share symptoms, or misdiagnosing them as adverse effects associated with COPD medication.53 Failure to recognize and treat comorbidities can increase risk of hospitalizations or exacerbations, worsen prognosis, increase morbidity, lower the chances of treatment adherence, and place a greater burden on the patient, family, and health care resources.51,52,54-56 Common comorbidities include cardiovascular disease, musculoskeletal dysfunction, metabolic syndrome, anxiety/depression, osteoporosis, lung cancer, and heart failure.3,51,52
The value of effectively managing comorbidities in improving outcomes and adherence to therapy is well documented. For example, personalized management of patients with COPD and comorbid anxiety and/or depression has been shown to reduce both the mental health symptoms and COPD-related outcomes (eg, exercise tolerance, disability).57-59
Comorbidity burden may impact adherence to COPD medication. Depression, for instance, is a known risk factor for nonadherence to treatment. Patients with multiple untreated or uncontrolled comorbid conditions may also be less likely to benefit from pulmonary rehabilitation.60 It is therefore important that comorbidities are managed effectively to improve adherence to therapy, and enhance the benefits of pulmonary rehabilitation.
Patient monitoring
Routine follow-up of patients with COPD is essential as lung function may worsen over time, even with the best available care.3 Worsening of symptoms, activity limitation, and disease progression should be monitored closely to determine when to modify management/pharmacotherapy, and to identify any complications and/or comorbidities that may develop.3 When patients with COPD do not receive the appropriate level of treatment or monitoring, it can be due to: under-reporting of disease severity, symptoms, and exacerbations during consultation; lack of information on the impact of the disease on the patient’s quality of life; and failure to recognize comorbidities.23,25,53 Continued use of the patient questionnaires described previously is recommended, and the GOLD strategy advises that symptoms are assessed at each visit. These follow-up visits also provide an opportunity to monitor patients with COPD for key comorbidities, including heart failure, ischemic heart disease, arrhythmias, osteoporosis, depression/anxiety, and lung cancer, as well as to determine a patient’s current smoking status, taking appropriate action as needed.3
Unmet needs
COPD remains underdiagnosed in the United States, with only 50% of individuals with impaired lung function reported to receive a formal diagnosis of COPD.61,62 Opportunities for diagnosing COPD earlier in its course are being missed; 85% of patients consult primary care for lower respiratory symptoms in the 5 years before diagnosis of COPD, and might have been candidates for further evaluation of those symptoms, including spirometry testing.63 Initiating treatment at early stages of COPD has the potential to improve patients’ health-related quality of life, and may provide opportunities to slow disease progression through interventions such as smoking cessation.64 Practical approaches to improving early diagnosis in primary care involve the use of questionnaires and clinical suspicion to identify those appropriate for spirometry, the most reliable method for identifying patients with COPD.3,9,65 Such methodology is currently under investigation, with early studies demonstrating the potential benefit of the COPD Assessment in Primary Care To Identify Undiagnosed Respiratory Disease and Exacerbation Risk (CAPTURE) questionnaire in conjunction with peak expiratory flow to gauge whether a patient requires further diagnostic evaluation.66
In addition, the GOLD strategy and COPD Foundation guidelines emphasize that correct assessment of symptoms is of paramount importance in determining the most appropriate therapy (both pharmacologic and nonpharmacologic) for patients with COPD, but traditionally has not been used to inform management choices. Both guidelines therefore highlight the importance of symptom assessment ahead of therapeutic decision-making.
Poor adherence to prescribed therapies and inadequate patient monitoring also need addressing. Two studies analyzing refill adherence data in patients with COPD and asthma in Sweden reported that only 28%–29% of prescribed treatments were dispensed with refill adherence that covered more than 80% of prescribed treatment time67,68; a study in 5504 patients in the United States with a prescription of fluticasone propionate/salmeterol combination therapy found that more than half of patients only refilled their prescription once over the course of the 1-year study.69 With studies showing incorrect use of inhalers in more than 50% of patients with COPD, incorrect inhaler technique is a significant contributor to poor treatment adherence.70,71 Inhaler technique should be reviewed regularly with direct observation of patients’ technique. Assessment of the patients’ ability to use their current prescribed inhaler(s) is recommended before considering a change in treatment.70 Errors in inhaler use are also associated with an increased rate of severe COPD exacerbations, increased risk of hospitalization, and poor disease control.71,72 Important factors affecting inhaler use include age, education, product design, costs (copays and deductibles) for medications, and instruction and inhaler technique education from the health care providers.70,72,73 Recent data support improvements in product design, training by the health care provider, and “self-training” by the patient (assisted by instructional video or other digital media) to increase adherence and reduce the frequency of handling errors.10,70,74 Electronic monitoring devices, messaging systems, and cell phone applications are also being considered as ways to increase adherence.75
Maintenance medication is an essential component of COPD management. However, patients with COPD often report that their preference is for medication that they can “feel” working, which may be implicated in their motivation to adhere to therapy.76 Conversely, while maintenance medication may reduce exacerbations, and lessen a patient’s decline in lung function,77 it may not have a significant impact on how they “feel.” As a result, patients may not take it as prescribed, contributing to poor adherence. It is therefore important for primary care physicians to acknowledge that the impact of taking the maintenance medication may not be felt immediately, and articulate the importance of maintenance therapy to their patients, as failure to adhere to treatment can have significant implications for longer-term outcomes such as symptom burden, quality of life, and exacerbation risk.11
Regular patient follow-up is necessary to reinforce such information: patients with milder or stable COPD may be followed at 6-month intervals, while patients with severe or frequent exacerbations, or patients who have recently been hospitalized, require follow-up at 2- to 4-week intervals.78
Conclusions
Defining personal treatment goals for patients with COPD can enhance patient and physician communication and encourage continued collaboration to improve adherence and outcomes. Regularly monitoring symptoms, exacerbations, and comorbidities via patient-focused questionnaires, and closely examining patient adherence and technique, form a fundamental part of care for patients with COPD. Recent updates to the GOLD and the COPD Foundation guidelines have emphasized the importance of symptom assessment in initiating COPD therapy, and continued assessment to appropriately escalate treatment. Nonpharmacologic therapies such as smoking cessation and pulmonary rehabilitation are recommended at all stages of COPD alongside pharmacologic treatment.
- Janson C, Marks G, Buist S, et al. The impact of COPD on health status: findings from the BOLD study. Eur Respir J. 2013;42(6):1472-1483.
- Buist AS, Vollmer WM, McBurnie MA. Worldwide burden of COPD in high- and low-income countries. Part I. The burden of obstructive lung disease (BOLD) initiative. Int J Tuberc Lung Dis. 2008;12(7):703-708.
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management and Prevention of COPD. 2017. Available from: http://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd. Accessed July 2017.
- López-Campos JL, Tan W, Soriano JB. Global burden of COPD. Respirology. 2016;21(1):14-23.
- Wheaton AG, Cunningham TJ, Ford ES, Croft JB; Centers for Disease Control and Prevention (CDC). Employment and activity limitations among adults with chronic obstructive pulmonary disease--United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64(11):289-295.
- Rennard S, Decramer M, Calverley PM, et al. Impact of COPD in North America and Europe in 2000: subjects’ perspective of Confronting COPD International Survey. Eur Respir J. 2002;20(4):799-805.
- Troosters T, van der Molen T, Polkey M, et al. Improving physical activity in COPD: towards a new paradigm. Respir Res. 2013;14:115.
- Perez X, Wisnivesky JP, Lurslurchachai L, Kleinman LC, Kronish IM. Barriers to adherence to COPD guidelines among primary care providers. Respir Med. 2012;106(3):374-381.
- Price D, Crockett A, Arne M, et al. Spirometry in primary care case-identification, diagnosis and management of COPD. Prim Care Respir J. 2009;18(3):216-223.
- van Boven JF, Ryan D, Eakin MN, Canonica GW, Barot A, Foster JM; Respiratory Effectiveness Group. Enhancing respiratory medication adherence: the role of health care professionals and cost-effectiveness considerations. J Allergy Clin Immunol Pract. 2016;4(5):835-846.
- van Boven JF, Chavannes NH, van der Molen T, Rutten-van Mölken MP, Postma MJ, Vegter S. Clinical and economic impact of non-adherence in COPD: a systematic review. Respir Med. 2014;108(1):103-113.
- COPD Foundation. Pocket Consultant Guide for the Diagnosis and Management of COPD. 2016.
- Lange P, Halpin DM, O’Donnell DE, MacNee W. Diagnosis, assessment, and phenotyping of COPD: beyond FEV1. Int J Chron Obstruct Pulmon Dis. 2016;11 Spec Iss3-12.
- Miravitlles M, Soler-Cataluña JJ, Calle M, et al. A new approach to grading and treating COPD based on clinical phenotypes: summary of the Spanish COPD guidelines (GesEPOC). Prim Care Respir J. 2013;22(1):117-121.
- Patalano F, Banerji D, D’Andrea P, Fogel R, Altman P, Colthorpe P. Addressing unmet needs in the treatment of COPD. Eur Respir Rev. 2014;23(133):333-344.
- van Eerd EAM, van der Meer RM, van Schayck OC, Kotz D. Smoking cessation for people with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2016(8):CD010744.
- McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;(2):CD003793.
- Ekström M. Clinical usefulness of long-term oxygen therapy in adults. N Engl J Med. 2016;375(17):1683-1684.
- Albert RK, Au DH, Blackford AL, et al; Long-Term Oxygen Treatment Trial Research Group. A randomized trial of long-term oxygen for COPD with moderate desaturation. N Engl J Med. 2016;375(17):1617-1627.
- Ekström M, Ahmadi Z, Bornefalk-Hermansson A, Abernethy A, Currow D. Oxygen for breathlessness in patients with chronic obstructive pulmonary disease who do not qualify for home oxygen therapy. Cochrane Database Syst Rev. 2016;(11):CD006429.
- Yang IA, Clarke MS, Sim EH, Fong KM. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;(7):CD002991.
- Jones PW, Price D, van der Molen T. Role of clinical questionnaires in optimizing everyday care of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2011;6:289-296.
- Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648-654.
- Lee SD, Huang MS, Kang J, et al; Investigators of the Predictive Ability of CAT in Acute Exacerbations of COPD (PACE) Study. The COPD assessment test (CAT) assists prediction of COPD exacerbations in high-risk patients. Respir Med. 2014;108(4):600-608.
- Sonetti DA, Hospenthal AC, Adams SG. Integrated management strategies for chronic obstructive pulmonary disease. J Multidiscip Healthc. 2010;3:181-188.
- Miyazaki M, Nakamura H, Chubachi S, et al; Keio COPD Comorbidity Research (K-CCR) Group. Analysis of comorbid factors that increase the COPD assessment test scores. Respir Res. 2014;15:13.
- Anoro Ellipta [highlights of prescribing info]. Research Triangle Park, NC: GlaxoSmithKline group of companies; 2013.
- Stiolto Respimat [highlights of prescribing info]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc.; 2015.
- Bevespi Aerosphere [highlights of prescribing info]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2015.
- Utibron Neohaler [highlights of prescribing info]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2015.
- Wedzicha JA, Banerji D, Chapman KR, et al; FLAME Investigators. Indacaterol-glycopyrronium versus salmeterol-fluticasone for COPD. N Engl J Med. 2016;374(23):2222-2234.
- Suissa S, Patenaude V, Lapi F, Ernst P. Inhaled corticosteroids in COPD and the risk of serious pneumonia. Thorax. 2013;68(11):1029-1036.
- Dempsey OJ, Coutie WJ, Wilson AM, Williams P, Lipworth BJ. Evaluation of the buccal component of systemic absorption with inhaled fluticasone propionate. Thorax. 1999;54(7):614-617.
- Drummond MB, Dasenbrook EC, Pitz MW, Murphy DJ, Fan E. Inhaled corticosteroids in patients with stable chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA. 2008;300(20):2407-2416.
- Crim C, Calverley PMA, Anderson JA, et al; SUMMIT Investigators. Pneumonia risk with inhaled fluticasone furoate and vilanterol in COPD patients with moderate airflow limitation: The SUMMIT trial. Respir Med. 2017;131:27-34.
- Rossi A, Guerriero M, Corrado A, OPTIMO/AIPO Study Group. Withdrawal of inhaled corticosteroids can be safe in COPD patients at low risk of exacerbation: a real-life study on the appropriateness of treatment in moderate COPD patients (OPTIMO). Respir Res. 2014;15:77.
- Falk JA, Minai OA, Mosenifar Z. Inhaled and systemic corticosteroids in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5(4):506-512.
- British Thoracic Society Standards of Care Committee. BTS statement on criteria for specialist referral, admission, discharge and follow-up for adults with respiratory disease. Thorax. 2008;63(Suppl 1):i1-i16.
- Benfante A, Messina R, Milazzo V, Scichilone N. How to unveil chronic respiratory diseases in clinical practice? A model of alliance between general practitioners and pulmonologists. Pulm Pharmacol Ther. 2017;44:106-110.
- Fishman A, Martinez F, Naunheim K, et al; National Emphysema Treatment Trial Research Group. A randomized trial comparing lung-volume–reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348(21):2059-2073.
- Fishman A, Fessler H, Martinez F, et al. Patients at high risk of death after lung-volume-reduction surgery. N Engl J Med. 2001;345(15):1075-1083.
- Deslee G, Klooster K, Hetzel M, et al. Lung volume reduction coil treatment for patients with severe emphysema: a European multicentre trial. Thorax. 2014;69(11):980-986.
- Slebos DJ, Shah PL, Herth FJ, Valipour A. Endobronchial valves for endoscopic lung volume reduction: best practice recommendations from expert panel on endoscopic lung volume reduction. Respiration. 2017;93(2):138-150.
- Browning RF, Parrish S, Sarkar S, et al. Bronchoscopic interventions for severe COPD. J Thorac Dis. 2014;6(suppl 4):S407-S415.
- Stavem K, Bjørtuft Ø, Borgan Ø, Geiran O, Boe J. Lung transplantation in patients with chronic obstructive pulmonary disease in a national cohort is without obvious survival benefit. J Heart Lung Transplant. 2006;25(1):75-84.
- Hosenpud JD, Bennett LE, Keck BM, Edwards EB, Novick RJ. Effect of diagnosis on survival benefit of lung transplantation for end-stage lung disease. Lancet. 1998;351(9095):24-27.
- Yawn B, Thomashow DM, Mannino D, et al. A statement of the COPD Foundation: The 2017 update to the COPD Foundation COPD Pocket Consultant Guide. Chronic Obstr Pulm Dis. 2017;4(3):177-185.
- Rennard S, Thomashow B, Crapo J, et al. Introducing the COPD Foundation Guide for Diagnosis and Management of COPD, recommendations of the COPD Foundation. COPD. 2013;10(3):378-389.
- Dal Negro RW, Bonadiman L, Turco P. Prevalence of different comorbidities in COPD patients by gender and GOLD stage. Multidiscip Respir Med. 2015;10(1):24.
- Chetty U, McLean G, Morrison D, Agur K, Guthrie B, Mercer SW. Chronic obstructive pulmonary disease and comorbidities: a large cross-sectional study in primary care. Br J Gen Pract. 2017;67(658):e321-e328.
- Westerik JA, Metting EI, van Boven JF, Tiersma W, Kocks JW, Schermer TR. Associations between chronic comorbidity and exacerbation risk in primary care patients with COPD. Respir Res. 2017;18(1):31.
- Putcha N, Han MK, Martinez CH, et al; the COPDGene Investigators. Comorbidities of COPD have a major impact on clinical outcomes, particularly in African Americans. Chronic Obstr Pulm Dis. 2014;1(1):105-114.
- Koskela J, Kilpeläinen M, Kupiainen H, et al. Co-morbidities are the key nominators of the health related quality of life in mild and moderate COPD. BMC Pulm Med. 2014;14:102.
- Clini EM, Boschetto P, Lainscak M, Janssens W. Comorbidities in chronic obstructive pulmonary disease from assessment to treatment. Biomed Res Int. 2014;2014:414928.
- Mannino DM, Thorn D, Swensen A, Holguin F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J. 2008;32(4):962-969.
- Schwab P, Dhamane AD, Hopson SD, et al. Impact of comorbid conditions in COPD patients on health care resource utilization and costs in a predominantly Medicare population. Int J Chron Obstruct Pulmon Dis. 2017;12:735-744.
- Yohannes AM, Alexopoulos GS. Depression and anxiety in patients with COPD. Eur Respir Rev. 2014;23(133):345-349.
- Alexopoulos GS, Kiosses DN, Sirey JA, et al. Untangling therapeutic ingredients of a personalized intervention for patients with depression and severe COPD. Am J Geriatr Psychiatry. 2014;22(11):1316-1324.
- Eiser N, Harte R, Spiros K, Phillips C, Isaac MT. Effect of treating depression on quality-of-life and exercise tolerance in severe COPD. COPD. 2005;2(2):233-241.
- Crisafulli E, Costi S, Luppi F, et al. Role of comorbidities in a cohort of patients with COPD undergoing pulmonary rehabilitation. Thorax. 2008;63(6):487-492.
- Mannino DM, Homa DM, Akinbami LJ, Ford ES, Redd SC. Chronic obstructive pulmonary disease surveillance—United States, 1971-2000. Respir Care. 2002;47(10):1184-1199.
- Ford ES, Croft JB, Mannino DM, Wheaton AG, Zhang X, Giles WH. COPD surveillance—United States, 1999-2011. Chest. 2013;144(1):284-305.
- Jones RC, Price D, Ryan D, et al; Respiratory Effectiveness Group. Opportunities to diagnose chronic obstructive pulmonary disease in routine care in the UK: a retrospective study of a clinical cohort. Lancet Respir Med. 2014;2(4):267-276.
- Welte T, Vogelmeier C, Papi A. COPD: early diagnosis and treatment to slow disease progression. Int J Clin Pract. 2015;69(3):336-349.
- Price D, Freeman D, Cleland J, Kaplan A, Cerasoli F. Earlier diagnosis and earlier treatment of COPD in primary care. Prim Care Respir J. 2011;20(1):15-22.
- Martinez FJ, Mannino D, Leidy NK, et al; High-Risk-COPD Screening Study Group. A new approach for identifying patients with undiagnosed chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;195(6):748-756.
- Krigsman K, Nilsson JL, Ring L. Refill adherence for patients with asthma and COPD: comparison of a pharmacy record database with manually collected repeat prescriptions. Pharmacoepidemiol Drug Saf. 2007;16(4):441-448.
- Krigsman K, Moen J, Nilsson JL, Ring L. Refill adherence by the elderly for asthma/chronic obstructive pulmonary disease drugs dispensed over a 10-year period. J Clin Pharm Ther. 2007;32(6):603-611.
- Bender BG, Pedan A, Varasteh LT. Adherence and persistence with fluticasone propionate/salmeterol combination therapy. J Allergy Clin Immunol. 2006;118(4):899-904.
- Chrystyn H, Price DB, Molimard M, et al. Comparison of serious inhaler technique errors made by device-naïve patients using three different dry powder inhalers: a randomised, crossover, open-label study. BMC Pulm Med. 2016;16:12.
- Molimard M, Raherison C, Lignot S, et al. Chronic obstructive pulmonary disease exacerbation and inhaler device handling: real-life assessment of 2935 patients. Eur Respir J. 2017;49(2):pii: 1601794.
- Melani AS, Bonavia M, Cilenti V, et al; Gruppo Educazionale Associazione Italiana Pneumologi Ospedalieri. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med. 2011;105(6):930-938.
- Han MK, Martinez CH, Au DH, et al. Meeting the challenge of COPD care delivery in the USA: a multiprovider perspective. Lancet Respir Med. 2016;4(6):473-526.
- Plaza V, Peiró M, Torrejón M, et al; PROMETHEUS Study Group. A repeated short educational intervention improves asthma control and quality of life. Eur Respir J. 2015;46(5):1298-1307.
- Craven VE, Morton RW, Spencer S, Devadason SG, Everard ML. Electronic monitoring and reminding devices for improving adherence to inhaled therapy in patients with asthma. Cochrane Database Syst Rev. 2015;(3):CD011554.
- Kawata AK, Kleinman L, Harding G, Ramachandran S. Evaluation of patient preference and willingness to pay for attributes of maintenance medication for chronic obstructive pulmonary disease (COPD). Patient. 2014;7(4):413-426.
- Ferguson GT. Maintenance pharmacotherapy of mild and moderate COPD: what is the evidence? Respir Med. 2011;105(9):1268-1274.
- BMJ Best Practice. COPD. http://bestpractice.bmj.com/best-practice/monograph/7.html. Updated November 2017. Accessed May 30, 2017.
- Janson C, Marks G, Buist S, et al. The impact of COPD on health status: findings from the BOLD study. Eur Respir J. 2013;42(6):1472-1483.
- Buist AS, Vollmer WM, McBurnie MA. Worldwide burden of COPD in high- and low-income countries. Part I. The burden of obstructive lung disease (BOLD) initiative. Int J Tuberc Lung Dis. 2008;12(7):703-708.
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management and Prevention of COPD. 2017. Available from: http://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd. Accessed July 2017.
- López-Campos JL, Tan W, Soriano JB. Global burden of COPD. Respirology. 2016;21(1):14-23.
- Wheaton AG, Cunningham TJ, Ford ES, Croft JB; Centers for Disease Control and Prevention (CDC). Employment and activity limitations among adults with chronic obstructive pulmonary disease--United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64(11):289-295.
- Rennard S, Decramer M, Calverley PM, et al. Impact of COPD in North America and Europe in 2000: subjects’ perspective of Confronting COPD International Survey. Eur Respir J. 2002;20(4):799-805.
- Troosters T, van der Molen T, Polkey M, et al. Improving physical activity in COPD: towards a new paradigm. Respir Res. 2013;14:115.
- Perez X, Wisnivesky JP, Lurslurchachai L, Kleinman LC, Kronish IM. Barriers to adherence to COPD guidelines among primary care providers. Respir Med. 2012;106(3):374-381.
- Price D, Crockett A, Arne M, et al. Spirometry in primary care case-identification, diagnosis and management of COPD. Prim Care Respir J. 2009;18(3):216-223.
- van Boven JF, Ryan D, Eakin MN, Canonica GW, Barot A, Foster JM; Respiratory Effectiveness Group. Enhancing respiratory medication adherence: the role of health care professionals and cost-effectiveness considerations. J Allergy Clin Immunol Pract. 2016;4(5):835-846.
- van Boven JF, Chavannes NH, van der Molen T, Rutten-van Mölken MP, Postma MJ, Vegter S. Clinical and economic impact of non-adherence in COPD: a systematic review. Respir Med. 2014;108(1):103-113.
- COPD Foundation. Pocket Consultant Guide for the Diagnosis and Management of COPD. 2016.
- Lange P, Halpin DM, O’Donnell DE, MacNee W. Diagnosis, assessment, and phenotyping of COPD: beyond FEV1. Int J Chron Obstruct Pulmon Dis. 2016;11 Spec Iss3-12.
- Miravitlles M, Soler-Cataluña JJ, Calle M, et al. A new approach to grading and treating COPD based on clinical phenotypes: summary of the Spanish COPD guidelines (GesEPOC). Prim Care Respir J. 2013;22(1):117-121.
- Patalano F, Banerji D, D’Andrea P, Fogel R, Altman P, Colthorpe P. Addressing unmet needs in the treatment of COPD. Eur Respir Rev. 2014;23(133):333-344.
- van Eerd EAM, van der Meer RM, van Schayck OC, Kotz D. Smoking cessation for people with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2016(8):CD010744.
- McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;(2):CD003793.
- Ekström M. Clinical usefulness of long-term oxygen therapy in adults. N Engl J Med. 2016;375(17):1683-1684.
- Albert RK, Au DH, Blackford AL, et al; Long-Term Oxygen Treatment Trial Research Group. A randomized trial of long-term oxygen for COPD with moderate desaturation. N Engl J Med. 2016;375(17):1617-1627.
- Ekström M, Ahmadi Z, Bornefalk-Hermansson A, Abernethy A, Currow D. Oxygen for breathlessness in patients with chronic obstructive pulmonary disease who do not qualify for home oxygen therapy. Cochrane Database Syst Rev. 2016;(11):CD006429.
- Yang IA, Clarke MS, Sim EH, Fong KM. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;(7):CD002991.
- Jones PW, Price D, van der Molen T. Role of clinical questionnaires in optimizing everyday care of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2011;6:289-296.
- Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648-654.
- Lee SD, Huang MS, Kang J, et al; Investigators of the Predictive Ability of CAT in Acute Exacerbations of COPD (PACE) Study. The COPD assessment test (CAT) assists prediction of COPD exacerbations in high-risk patients. Respir Med. 2014;108(4):600-608.
- Sonetti DA, Hospenthal AC, Adams SG. Integrated management strategies for chronic obstructive pulmonary disease. J Multidiscip Healthc. 2010;3:181-188.
- Miyazaki M, Nakamura H, Chubachi S, et al; Keio COPD Comorbidity Research (K-CCR) Group. Analysis of comorbid factors that increase the COPD assessment test scores. Respir Res. 2014;15:13.
- Anoro Ellipta [highlights of prescribing info]. Research Triangle Park, NC: GlaxoSmithKline group of companies; 2013.
- Stiolto Respimat [highlights of prescribing info]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc.; 2015.
- Bevespi Aerosphere [highlights of prescribing info]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2015.
- Utibron Neohaler [highlights of prescribing info]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2015.
- Wedzicha JA, Banerji D, Chapman KR, et al; FLAME Investigators. Indacaterol-glycopyrronium versus salmeterol-fluticasone for COPD. N Engl J Med. 2016;374(23):2222-2234.
- Suissa S, Patenaude V, Lapi F, Ernst P. Inhaled corticosteroids in COPD and the risk of serious pneumonia. Thorax. 2013;68(11):1029-1036.
- Dempsey OJ, Coutie WJ, Wilson AM, Williams P, Lipworth BJ. Evaluation of the buccal component of systemic absorption with inhaled fluticasone propionate. Thorax. 1999;54(7):614-617.
- Drummond MB, Dasenbrook EC, Pitz MW, Murphy DJ, Fan E. Inhaled corticosteroids in patients with stable chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA. 2008;300(20):2407-2416.
- Crim C, Calverley PMA, Anderson JA, et al; SUMMIT Investigators. Pneumonia risk with inhaled fluticasone furoate and vilanterol in COPD patients with moderate airflow limitation: The SUMMIT trial. Respir Med. 2017;131:27-34.
- Rossi A, Guerriero M, Corrado A, OPTIMO/AIPO Study Group. Withdrawal of inhaled corticosteroids can be safe in COPD patients at low risk of exacerbation: a real-life study on the appropriateness of treatment in moderate COPD patients (OPTIMO). Respir Res. 2014;15:77.
- Falk JA, Minai OA, Mosenifar Z. Inhaled and systemic corticosteroids in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5(4):506-512.
- British Thoracic Society Standards of Care Committee. BTS statement on criteria for specialist referral, admission, discharge and follow-up for adults with respiratory disease. Thorax. 2008;63(Suppl 1):i1-i16.
- Benfante A, Messina R, Milazzo V, Scichilone N. How to unveil chronic respiratory diseases in clinical practice? A model of alliance between general practitioners and pulmonologists. Pulm Pharmacol Ther. 2017;44:106-110.
- Fishman A, Martinez F, Naunheim K, et al; National Emphysema Treatment Trial Research Group. A randomized trial comparing lung-volume–reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348(21):2059-2073.
- Fishman A, Fessler H, Martinez F, et al. Patients at high risk of death after lung-volume-reduction surgery. N Engl J Med. 2001;345(15):1075-1083.
- Deslee G, Klooster K, Hetzel M, et al. Lung volume reduction coil treatment for patients with severe emphysema: a European multicentre trial. Thorax. 2014;69(11):980-986.
- Slebos DJ, Shah PL, Herth FJ, Valipour A. Endobronchial valves for endoscopic lung volume reduction: best practice recommendations from expert panel on endoscopic lung volume reduction. Respiration. 2017;93(2):138-150.
- Browning RF, Parrish S, Sarkar S, et al. Bronchoscopic interventions for severe COPD. J Thorac Dis. 2014;6(suppl 4):S407-S415.
- Stavem K, Bjørtuft Ø, Borgan Ø, Geiran O, Boe J. Lung transplantation in patients with chronic obstructive pulmonary disease in a national cohort is without obvious survival benefit. J Heart Lung Transplant. 2006;25(1):75-84.
- Hosenpud JD, Bennett LE, Keck BM, Edwards EB, Novick RJ. Effect of diagnosis on survival benefit of lung transplantation for end-stage lung disease. Lancet. 1998;351(9095):24-27.
- Yawn B, Thomashow DM, Mannino D, et al. A statement of the COPD Foundation: The 2017 update to the COPD Foundation COPD Pocket Consultant Guide. Chronic Obstr Pulm Dis. 2017;4(3):177-185.
- Rennard S, Thomashow B, Crapo J, et al. Introducing the COPD Foundation Guide for Diagnosis and Management of COPD, recommendations of the COPD Foundation. COPD. 2013;10(3):378-389.
- Dal Negro RW, Bonadiman L, Turco P. Prevalence of different comorbidities in COPD patients by gender and GOLD stage. Multidiscip Respir Med. 2015;10(1):24.
- Chetty U, McLean G, Morrison D, Agur K, Guthrie B, Mercer SW. Chronic obstructive pulmonary disease and comorbidities: a large cross-sectional study in primary care. Br J Gen Pract. 2017;67(658):e321-e328.
- Westerik JA, Metting EI, van Boven JF, Tiersma W, Kocks JW, Schermer TR. Associations between chronic comorbidity and exacerbation risk in primary care patients with COPD. Respir Res. 2017;18(1):31.
- Putcha N, Han MK, Martinez CH, et al; the COPDGene Investigators. Comorbidities of COPD have a major impact on clinical outcomes, particularly in African Americans. Chronic Obstr Pulm Dis. 2014;1(1):105-114.
- Koskela J, Kilpeläinen M, Kupiainen H, et al. Co-morbidities are the key nominators of the health related quality of life in mild and moderate COPD. BMC Pulm Med. 2014;14:102.
- Clini EM, Boschetto P, Lainscak M, Janssens W. Comorbidities in chronic obstructive pulmonary disease from assessment to treatment. Biomed Res Int. 2014;2014:414928.
- Mannino DM, Thorn D, Swensen A, Holguin F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J. 2008;32(4):962-969.
- Schwab P, Dhamane AD, Hopson SD, et al. Impact of comorbid conditions in COPD patients on health care resource utilization and costs in a predominantly Medicare population. Int J Chron Obstruct Pulmon Dis. 2017;12:735-744.
- Yohannes AM, Alexopoulos GS. Depression and anxiety in patients with COPD. Eur Respir Rev. 2014;23(133):345-349.
- Alexopoulos GS, Kiosses DN, Sirey JA, et al. Untangling therapeutic ingredients of a personalized intervention for patients with depression and severe COPD. Am J Geriatr Psychiatry. 2014;22(11):1316-1324.
- Eiser N, Harte R, Spiros K, Phillips C, Isaac MT. Effect of treating depression on quality-of-life and exercise tolerance in severe COPD. COPD. 2005;2(2):233-241.
- Crisafulli E, Costi S, Luppi F, et al. Role of comorbidities in a cohort of patients with COPD undergoing pulmonary rehabilitation. Thorax. 2008;63(6):487-492.
- Mannino DM, Homa DM, Akinbami LJ, Ford ES, Redd SC. Chronic obstructive pulmonary disease surveillance—United States, 1971-2000. Respir Care. 2002;47(10):1184-1199.
- Ford ES, Croft JB, Mannino DM, Wheaton AG, Zhang X, Giles WH. COPD surveillance—United States, 1999-2011. Chest. 2013;144(1):284-305.
- Jones RC, Price D, Ryan D, et al; Respiratory Effectiveness Group. Opportunities to diagnose chronic obstructive pulmonary disease in routine care in the UK: a retrospective study of a clinical cohort. Lancet Respir Med. 2014;2(4):267-276.
- Welte T, Vogelmeier C, Papi A. COPD: early diagnosis and treatment to slow disease progression. Int J Clin Pract. 2015;69(3):336-349.
- Price D, Freeman D, Cleland J, Kaplan A, Cerasoli F. Earlier diagnosis and earlier treatment of COPD in primary care. Prim Care Respir J. 2011;20(1):15-22.
- Martinez FJ, Mannino D, Leidy NK, et al; High-Risk-COPD Screening Study Group. A new approach for identifying patients with undiagnosed chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;195(6):748-756.
- Krigsman K, Nilsson JL, Ring L. Refill adherence for patients with asthma and COPD: comparison of a pharmacy record database with manually collected repeat prescriptions. Pharmacoepidemiol Drug Saf. 2007;16(4):441-448.
- Krigsman K, Moen J, Nilsson JL, Ring L. Refill adherence by the elderly for asthma/chronic obstructive pulmonary disease drugs dispensed over a 10-year period. J Clin Pharm Ther. 2007;32(6):603-611.
- Bender BG, Pedan A, Varasteh LT. Adherence and persistence with fluticasone propionate/salmeterol combination therapy. J Allergy Clin Immunol. 2006;118(4):899-904.
- Chrystyn H, Price DB, Molimard M, et al. Comparison of serious inhaler technique errors made by device-naïve patients using three different dry powder inhalers: a randomised, crossover, open-label study. BMC Pulm Med. 2016;16:12.
- Molimard M, Raherison C, Lignot S, et al. Chronic obstructive pulmonary disease exacerbation and inhaler device handling: real-life assessment of 2935 patients. Eur Respir J. 2017;49(2):pii: 1601794.
- Melani AS, Bonavia M, Cilenti V, et al; Gruppo Educazionale Associazione Italiana Pneumologi Ospedalieri. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med. 2011;105(6):930-938.
- Han MK, Martinez CH, Au DH, et al. Meeting the challenge of COPD care delivery in the USA: a multiprovider perspective. Lancet Respir Med. 2016;4(6):473-526.
- Plaza V, Peiró M, Torrejón M, et al; PROMETHEUS Study Group. A repeated short educational intervention improves asthma control and quality of life. Eur Respir J. 2015;46(5):1298-1307.
- Craven VE, Morton RW, Spencer S, Devadason SG, Everard ML. Electronic monitoring and reminding devices for improving adherence to inhaled therapy in patients with asthma. Cochrane Database Syst Rev. 2015;(3):CD011554.
- Kawata AK, Kleinman L, Harding G, Ramachandran S. Evaluation of patient preference and willingness to pay for attributes of maintenance medication for chronic obstructive pulmonary disease (COPD). Patient. 2014;7(4):413-426.
- Ferguson GT. Maintenance pharmacotherapy of mild and moderate COPD: what is the evidence? Respir Med. 2011;105(9):1268-1274.
- BMJ Best Practice. COPD. http://bestpractice.bmj.com/best-practice/monograph/7.html. Updated November 2017. Accessed May 30, 2017.