User login
Special Report II: Tackling Burnout
Last month, we introduced the epidemic of burnout and the adverse consequences for both our vascular surgery patients and ourselves. Today we will outline a framework for addressing these issues. The foundation of this framework is informed by the social and neurosciences.
From the perspective of the social scientist: Christina Maslach, the originator of the widely used Maslach Burnout Inventory, theorized that burnout arises from a chronic mismatch between people and their work setting in some or all of the following domains: Workload (too much, wrong kind); control (lack of autonomy, or insufficient control over resources); reward (insufficient financial or social rewards commensurate with achievements); community (loss of positive connection with others); fairness (lack of perceived fairness, inequity of work, pay, or promotion); and values (conflict of personal and organizational values). The reality of practicing medicine in today’s business milieu – of achieving service efficiencies by meeting performance targets – brings many of these mismatches into sharp focus.
From the perspective of the neuroscientist: Recent advances, including functional MRI, have demonstrated that the human brain is hard wired for compassion. Compassion is the deep feeling that arises when confronted with another’s suffering, coupled with a strong desire to alleviate that suffering. There are at least two neural pathways: one activated during empathy, having us experience another’s pain; and the other activated during compassion, resulting in our sense of reward. Thus, burnout is thought to occur when you know what your patient needs but you are unable to deliver it. Compassionate medical care is purposeful work, which promotes a sense of reward and mitigates burnout.
Because burnout affects all caregivers (anyone who touches the patient), a successful program addressing workforce well-being must be comprehensive and organization wide, similar to successful patient safety, CPI [continuous process improvement] and LEAN [Six Sigma] initiatives.
There are no shortcuts. Creating a culture of compassionate, collaborative care requires an understanding of the interrelationships between the individual provider, the unit or team, and organizational leadership.
1) The individual provider: There is evidence to support the use of programs that build personal resilience. A recently published meta-analysis by West and colleagues concluded that while no specific physician burnout intervention has been shown to be better than other types of interventions, mindfulness, stress management, and small-group discussions can be effective approaches to reducing burnout scores. Strategies to build individual resilience, such as mindfulness and meditation, are easy to teach but place the burden for success on the individual. No amount of resilience can withstand an unsupportive or toxic workplace environment, so both individual and organizational strategies in combination are necessary.
2) The unit or team: Scheduling time for open and honest discussion of social and emotional issues that arise in caring for patients helps nourish caregiver to caregiver compassion. The Schwartz Center for Compassionate Healthcare is a national nonprofit leading the movement to bring compassion to every patient-caregiver interaction. More than 425 health care organization are Schwartz Center members and conduct Schwartz Rounds™ to bring doctors, nurses, and other caregivers together to discuss the human side of health care. (www.theschwartzcenter.org). Team member to team member support is essential for navigating the stressors of practice. With having lunch in front of your computer being the norm, and the disappearance of traditional spaces for colleagues to connect (for example, nurses’ lounge, physician dining rooms), the opportunity for caregivers to have a safe place to escape, a place to have their own humanity reaffirmed, a place to offer support to their peers, has been eliminated.
3) Organizational Leadership: Making compassion a core value, articulating it, and establishing metrics whereby it can be measured, is a good start. The barriers to a culture of compassion are related to our systems of care. There are burgeoning administrative and documentation tasks to be performed, and productivity expectations that turn our clinics and hospitals into assembly lines. No, we cannot expect the EMR [electronic medical records] to be eliminated, but workforce well-being cannot be sustainable in the context of inadequate resources. A culture of compassionate collaborative care requires programs and policies that are implemented by the organization itself. Examples of organization-wide initiatives that support workforce well-being and provider engagement include: screening for caregiver burnout, establishing policies for managing adverse events with an eye toward the second victim, and most importantly, supporting systems that preserve work control autonomy of physicians and nurses in clinical settings. The business sector has long recognized that workplace stress is a function of how demanding a person’s job is and how much control that person has over his or her responsibilities. The business community has also recognized that the experience of the worker (provider) drives the experience of the customer (patient). In a study of hospital compassionate practices and HCAHPS [the Hospital Consumer Assessment of Healthcare Providers and Systems], McClelland and Vogus reported that how well a hospital compassionately supports it employees and rewards compassionate acts is significantly and positively is associated with that hospital’s ratings and likelihood of patients recommending it.
How does the Society of Vascular Surgery, or any professional medical/nursing society for that matter, fit into this model?
We propose that the SVS find ways to empower their members to be agents for culture change within their own health care organizations. How might this be done:
- Teach organizational leadership skills, starting with the SVS Board of Directors, the presidential line, and the chairs of committees. Offer leadership courses at the Annual Meeting.
- Develop a community of caregivers committed to creating a compassionate collaborative culture. The SVS is a founding member of the Schwartz Center Healthcare Society Leadership Council, and you, as members of the SVS benefit from reduced registration at the Annual Compassion in Action Healthcare Conference, June 24-27, 2017 in Boston. (http://compassioninactionconference.org) This conference is designed to be highly experiential, using a hands-on “how to do it” model.
- The SVS should make improving the overall wellness of its members a specific goal and find specific metrics to monitor our progress towards this goal. Members can be provided with the tools to identify, monitor, and measure burnout and compassion. Each committee and council of the SVS can reexamine their objectives through the lens of reducing burnout and improving the wellness of vascular surgeons.
- Provide members with evidence-based programs that build personal resilience. This will not be a successful initiative unless our surgeons recognize and acknowledge the symptoms of burnout, and are willing to admit vulnerability. Without doing so, it is difficult to reach out for help.
- Redesign postgraduate resident and fellowship education. Standardizing clinical care may reduce variation and promote efficiency. However, when processes such as time-limited appointment scheduling, EMR templates, and protocols that drive physician-patient interactions are embedded in Resident and Fellowship education, the result may well be inflexibility in practice, reduced face time with patients, and interactions that lack compassion; all leading to burnout. Graduate Medical Education leaders must develop programs that support the learner’s ability to connect with patients and families, cultivate and role-model skills and behaviors that strengthen compassionate interactions, and strive to develop clinical practice models that increase Resident and Fellow work control autonomy.
The SVS should work proactively to optimize workload, fairness, and reward on a larger scale for its members as it relates to the EMR, reimbursement, and systems coverage. While we may be relatively small in size, as leaders, we are perfectly poised to address these larger, global issues. Perhaps working within the current system (i.e., PAC and APM task force) and considering innovative solutions at a national leadership scale, the SVS can direct real change!
Changing culture is not easy, nor quick, nor does it have an easy-to-follow blueprint. The first step is recognizing the need. The second is taking a leadership role. The third is thinking deeply about implementation.
*The authors extend their thanks and appreciation for the guidance, resources and support of Michael Goldberg, MD, scholar in residence, Schwartz Center for Compassionate Care, Boston and clinical professor of orthopedics at Seattle Children’s Hospital.
REFERENCES
1. J Managerial Psychol. (2007) 22:309-28
2. Annu Rev Neurosci. (2012) 35:1-23
3. Medicine. (2016) 44:583-5
4. J Health Organization Manag. (2015) 29:973-87
5. De Zulueta P Developing compassionate leadership in health care: an integrative review. J Healthcare Leadership. (2016) 8:1-10
6. Dolan ED, Morh D, Lempa M et al. Using a single item to measure burnout in primary care staff: A psychometry evaluation. J Gen Intern Med. (2015) 30:582-7
7. Karasek RA Job demands, job decision latitude, and mental strain: implications for job design. Administrative Sciences Quarterly (1979) 24: 285-308
8. Lee VS, Miller T, Daniels C, et al. Creating the exceptional patient experience in one academic health system. Acad Med. (2016) 91:338-44
9. Linzer M, Levine R, Meltzer D, et al. 10 bold steps to prevent burnout in general internal medicine. J Gen Intern Med. (2013) 29:18-20
10. Lown BA, Manning CF The Schwartz Center Rounds: Evaluation of an interdisciplinary approach to enhancing patient-centered communication, teamwork, and provider support. Acad Med. (2010) 85:1073-81
11. Lown BA, Muncer SJ, Chadwick R Can compassionate healthcare be measured? The Schwartz Center Compassionate Care Scale. Patient Education and Counseling (2015) 98:1005-10
12. Lown BA, McIntosh S, Gaines ME, et. al. Integrating compassionate collaborative care (“the Triple C”) into health professional education to advance the triple aim of health care. Acad Med (2016) 91:1-7
13. Lown BA A social neuroscience-informed model for teaching and practicing compassion in health care. Medical Education (2016) 50: 332-342
14. Maslach C, Schaufeli WG, Leiter MP Job burnout. Annu Rev Psychol (2001) 52:397-422
15. McClelland LE, Vogus TJ Compassion practices and HCAHPS: Does rewarding and supporting workplace compassion influence patient perceptions? HSR: Health Serv Res. (2014) 49:1670-83
16. Shanafelt TD, Noseworthy JH Executive leadership and physician well-being: Nine organizational strategies to promote engagement and reduce burnout. Mayo Clin Proc. (2016) 6:1-18
17. Shanafelt TD, Dyrbye LN, West CP Addressing physician burnout: the way forward. JAMA (2017) 317:901-2
18. Singer T, Klimecki OM Empathy and compassion Curr Biol. (2014) 24: R875-8
19. West CP, Dyrbye LN, Satele DV et. al. Concurrent validity of single-item measures of emotional exhaustion and depersonalization in burnout assessment. J Gen Intern Med. (2012) 27:1445-52
20. West CP, Dyrbye LN, Erwin PJ, et al. Interventions to address and reduce physician burnout: a systematic review and meta-analysis. Lancet. (2016) 388:2272-81
21. Wuest TK, Goldberg MJ, Kelly JD Clinical faceoff: Physician burnout-Fact, fantasy, or the fourth component of the triple aim? Clin Orthop Relat Res. (2016) doi: 10.1007/5-11999-016-5193-5
Last month, we introduced the epidemic of burnout and the adverse consequences for both our vascular surgery patients and ourselves. Today we will outline a framework for addressing these issues. The foundation of this framework is informed by the social and neurosciences.
From the perspective of the social scientist: Christina Maslach, the originator of the widely used Maslach Burnout Inventory, theorized that burnout arises from a chronic mismatch between people and their work setting in some or all of the following domains: Workload (too much, wrong kind); control (lack of autonomy, or insufficient control over resources); reward (insufficient financial or social rewards commensurate with achievements); community (loss of positive connection with others); fairness (lack of perceived fairness, inequity of work, pay, or promotion); and values (conflict of personal and organizational values). The reality of practicing medicine in today’s business milieu – of achieving service efficiencies by meeting performance targets – brings many of these mismatches into sharp focus.
From the perspective of the neuroscientist: Recent advances, including functional MRI, have demonstrated that the human brain is hard wired for compassion. Compassion is the deep feeling that arises when confronted with another’s suffering, coupled with a strong desire to alleviate that suffering. There are at least two neural pathways: one activated during empathy, having us experience another’s pain; and the other activated during compassion, resulting in our sense of reward. Thus, burnout is thought to occur when you know what your patient needs but you are unable to deliver it. Compassionate medical care is purposeful work, which promotes a sense of reward and mitigates burnout.
Because burnout affects all caregivers (anyone who touches the patient), a successful program addressing workforce well-being must be comprehensive and organization wide, similar to successful patient safety, CPI [continuous process improvement] and LEAN [Six Sigma] initiatives.
There are no shortcuts. Creating a culture of compassionate, collaborative care requires an understanding of the interrelationships between the individual provider, the unit or team, and organizational leadership.
1) The individual provider: There is evidence to support the use of programs that build personal resilience. A recently published meta-analysis by West and colleagues concluded that while no specific physician burnout intervention has been shown to be better than other types of interventions, mindfulness, stress management, and small-group discussions can be effective approaches to reducing burnout scores. Strategies to build individual resilience, such as mindfulness and meditation, are easy to teach but place the burden for success on the individual. No amount of resilience can withstand an unsupportive or toxic workplace environment, so both individual and organizational strategies in combination are necessary.
2) The unit or team: Scheduling time for open and honest discussion of social and emotional issues that arise in caring for patients helps nourish caregiver to caregiver compassion. The Schwartz Center for Compassionate Healthcare is a national nonprofit leading the movement to bring compassion to every patient-caregiver interaction. More than 425 health care organization are Schwartz Center members and conduct Schwartz Rounds™ to bring doctors, nurses, and other caregivers together to discuss the human side of health care. (www.theschwartzcenter.org). Team member to team member support is essential for navigating the stressors of practice. With having lunch in front of your computer being the norm, and the disappearance of traditional spaces for colleagues to connect (for example, nurses’ lounge, physician dining rooms), the opportunity for caregivers to have a safe place to escape, a place to have their own humanity reaffirmed, a place to offer support to their peers, has been eliminated.
3) Organizational Leadership: Making compassion a core value, articulating it, and establishing metrics whereby it can be measured, is a good start. The barriers to a culture of compassion are related to our systems of care. There are burgeoning administrative and documentation tasks to be performed, and productivity expectations that turn our clinics and hospitals into assembly lines. No, we cannot expect the EMR [electronic medical records] to be eliminated, but workforce well-being cannot be sustainable in the context of inadequate resources. A culture of compassionate collaborative care requires programs and policies that are implemented by the organization itself. Examples of organization-wide initiatives that support workforce well-being and provider engagement include: screening for caregiver burnout, establishing policies for managing adverse events with an eye toward the second victim, and most importantly, supporting systems that preserve work control autonomy of physicians and nurses in clinical settings. The business sector has long recognized that workplace stress is a function of how demanding a person’s job is and how much control that person has over his or her responsibilities. The business community has also recognized that the experience of the worker (provider) drives the experience of the customer (patient). In a study of hospital compassionate practices and HCAHPS [the Hospital Consumer Assessment of Healthcare Providers and Systems], McClelland and Vogus reported that how well a hospital compassionately supports it employees and rewards compassionate acts is significantly and positively is associated with that hospital’s ratings and likelihood of patients recommending it.
How does the Society of Vascular Surgery, or any professional medical/nursing society for that matter, fit into this model?
We propose that the SVS find ways to empower their members to be agents for culture change within their own health care organizations. How might this be done:
- Teach organizational leadership skills, starting with the SVS Board of Directors, the presidential line, and the chairs of committees. Offer leadership courses at the Annual Meeting.
- Develop a community of caregivers committed to creating a compassionate collaborative culture. The SVS is a founding member of the Schwartz Center Healthcare Society Leadership Council, and you, as members of the SVS benefit from reduced registration at the Annual Compassion in Action Healthcare Conference, June 24-27, 2017 in Boston. (http://compassioninactionconference.org) This conference is designed to be highly experiential, using a hands-on “how to do it” model.
- The SVS should make improving the overall wellness of its members a specific goal and find specific metrics to monitor our progress towards this goal. Members can be provided with the tools to identify, monitor, and measure burnout and compassion. Each committee and council of the SVS can reexamine their objectives through the lens of reducing burnout and improving the wellness of vascular surgeons.
- Provide members with evidence-based programs that build personal resilience. This will not be a successful initiative unless our surgeons recognize and acknowledge the symptoms of burnout, and are willing to admit vulnerability. Without doing so, it is difficult to reach out for help.
- Redesign postgraduate resident and fellowship education. Standardizing clinical care may reduce variation and promote efficiency. However, when processes such as time-limited appointment scheduling, EMR templates, and protocols that drive physician-patient interactions are embedded in Resident and Fellowship education, the result may well be inflexibility in practice, reduced face time with patients, and interactions that lack compassion; all leading to burnout. Graduate Medical Education leaders must develop programs that support the learner’s ability to connect with patients and families, cultivate and role-model skills and behaviors that strengthen compassionate interactions, and strive to develop clinical practice models that increase Resident and Fellow work control autonomy.
The SVS should work proactively to optimize workload, fairness, and reward on a larger scale for its members as it relates to the EMR, reimbursement, and systems coverage. While we may be relatively small in size, as leaders, we are perfectly poised to address these larger, global issues. Perhaps working within the current system (i.e., PAC and APM task force) and considering innovative solutions at a national leadership scale, the SVS can direct real change!
Changing culture is not easy, nor quick, nor does it have an easy-to-follow blueprint. The first step is recognizing the need. The second is taking a leadership role. The third is thinking deeply about implementation.
*The authors extend their thanks and appreciation for the guidance, resources and support of Michael Goldberg, MD, scholar in residence, Schwartz Center for Compassionate Care, Boston and clinical professor of orthopedics at Seattle Children’s Hospital.
REFERENCES
1. J Managerial Psychol. (2007) 22:309-28
2. Annu Rev Neurosci. (2012) 35:1-23
3. Medicine. (2016) 44:583-5
4. J Health Organization Manag. (2015) 29:973-87
5. De Zulueta P Developing compassionate leadership in health care: an integrative review. J Healthcare Leadership. (2016) 8:1-10
6. Dolan ED, Morh D, Lempa M et al. Using a single item to measure burnout in primary care staff: A psychometry evaluation. J Gen Intern Med. (2015) 30:582-7
7. Karasek RA Job demands, job decision latitude, and mental strain: implications for job design. Administrative Sciences Quarterly (1979) 24: 285-308
8. Lee VS, Miller T, Daniels C, et al. Creating the exceptional patient experience in one academic health system. Acad Med. (2016) 91:338-44
9. Linzer M, Levine R, Meltzer D, et al. 10 bold steps to prevent burnout in general internal medicine. J Gen Intern Med. (2013) 29:18-20
10. Lown BA, Manning CF The Schwartz Center Rounds: Evaluation of an interdisciplinary approach to enhancing patient-centered communication, teamwork, and provider support. Acad Med. (2010) 85:1073-81
11. Lown BA, Muncer SJ, Chadwick R Can compassionate healthcare be measured? The Schwartz Center Compassionate Care Scale. Patient Education and Counseling (2015) 98:1005-10
12. Lown BA, McIntosh S, Gaines ME, et. al. Integrating compassionate collaborative care (“the Triple C”) into health professional education to advance the triple aim of health care. Acad Med (2016) 91:1-7
13. Lown BA A social neuroscience-informed model for teaching and practicing compassion in health care. Medical Education (2016) 50: 332-342
14. Maslach C, Schaufeli WG, Leiter MP Job burnout. Annu Rev Psychol (2001) 52:397-422
15. McClelland LE, Vogus TJ Compassion practices and HCAHPS: Does rewarding and supporting workplace compassion influence patient perceptions? HSR: Health Serv Res. (2014) 49:1670-83
16. Shanafelt TD, Noseworthy JH Executive leadership and physician well-being: Nine organizational strategies to promote engagement and reduce burnout. Mayo Clin Proc. (2016) 6:1-18
17. Shanafelt TD, Dyrbye LN, West CP Addressing physician burnout: the way forward. JAMA (2017) 317:901-2
18. Singer T, Klimecki OM Empathy and compassion Curr Biol. (2014) 24: R875-8
19. West CP, Dyrbye LN, Satele DV et. al. Concurrent validity of single-item measures of emotional exhaustion and depersonalization in burnout assessment. J Gen Intern Med. (2012) 27:1445-52
20. West CP, Dyrbye LN, Erwin PJ, et al. Interventions to address and reduce physician burnout: a systematic review and meta-analysis. Lancet. (2016) 388:2272-81
21. Wuest TK, Goldberg MJ, Kelly JD Clinical faceoff: Physician burnout-Fact, fantasy, or the fourth component of the triple aim? Clin Orthop Relat Res. (2016) doi: 10.1007/5-11999-016-5193-5
Last month, we introduced the epidemic of burnout and the adverse consequences for both our vascular surgery patients and ourselves. Today we will outline a framework for addressing these issues. The foundation of this framework is informed by the social and neurosciences.
From the perspective of the social scientist: Christina Maslach, the originator of the widely used Maslach Burnout Inventory, theorized that burnout arises from a chronic mismatch between people and their work setting in some or all of the following domains: Workload (too much, wrong kind); control (lack of autonomy, or insufficient control over resources); reward (insufficient financial or social rewards commensurate with achievements); community (loss of positive connection with others); fairness (lack of perceived fairness, inequity of work, pay, or promotion); and values (conflict of personal and organizational values). The reality of practicing medicine in today’s business milieu – of achieving service efficiencies by meeting performance targets – brings many of these mismatches into sharp focus.
From the perspective of the neuroscientist: Recent advances, including functional MRI, have demonstrated that the human brain is hard wired for compassion. Compassion is the deep feeling that arises when confronted with another’s suffering, coupled with a strong desire to alleviate that suffering. There are at least two neural pathways: one activated during empathy, having us experience another’s pain; and the other activated during compassion, resulting in our sense of reward. Thus, burnout is thought to occur when you know what your patient needs but you are unable to deliver it. Compassionate medical care is purposeful work, which promotes a sense of reward and mitigates burnout.
Because burnout affects all caregivers (anyone who touches the patient), a successful program addressing workforce well-being must be comprehensive and organization wide, similar to successful patient safety, CPI [continuous process improvement] and LEAN [Six Sigma] initiatives.
There are no shortcuts. Creating a culture of compassionate, collaborative care requires an understanding of the interrelationships between the individual provider, the unit or team, and organizational leadership.
1) The individual provider: There is evidence to support the use of programs that build personal resilience. A recently published meta-analysis by West and colleagues concluded that while no specific physician burnout intervention has been shown to be better than other types of interventions, mindfulness, stress management, and small-group discussions can be effective approaches to reducing burnout scores. Strategies to build individual resilience, such as mindfulness and meditation, are easy to teach but place the burden for success on the individual. No amount of resilience can withstand an unsupportive or toxic workplace environment, so both individual and organizational strategies in combination are necessary.
2) The unit or team: Scheduling time for open and honest discussion of social and emotional issues that arise in caring for patients helps nourish caregiver to caregiver compassion. The Schwartz Center for Compassionate Healthcare is a national nonprofit leading the movement to bring compassion to every patient-caregiver interaction. More than 425 health care organization are Schwartz Center members and conduct Schwartz Rounds™ to bring doctors, nurses, and other caregivers together to discuss the human side of health care. (www.theschwartzcenter.org). Team member to team member support is essential for navigating the stressors of practice. With having lunch in front of your computer being the norm, and the disappearance of traditional spaces for colleagues to connect (for example, nurses’ lounge, physician dining rooms), the opportunity for caregivers to have a safe place to escape, a place to have their own humanity reaffirmed, a place to offer support to their peers, has been eliminated.
3) Organizational Leadership: Making compassion a core value, articulating it, and establishing metrics whereby it can be measured, is a good start. The barriers to a culture of compassion are related to our systems of care. There are burgeoning administrative and documentation tasks to be performed, and productivity expectations that turn our clinics and hospitals into assembly lines. No, we cannot expect the EMR [electronic medical records] to be eliminated, but workforce well-being cannot be sustainable in the context of inadequate resources. A culture of compassionate collaborative care requires programs and policies that are implemented by the organization itself. Examples of organization-wide initiatives that support workforce well-being and provider engagement include: screening for caregiver burnout, establishing policies for managing adverse events with an eye toward the second victim, and most importantly, supporting systems that preserve work control autonomy of physicians and nurses in clinical settings. The business sector has long recognized that workplace stress is a function of how demanding a person’s job is and how much control that person has over his or her responsibilities. The business community has also recognized that the experience of the worker (provider) drives the experience of the customer (patient). In a study of hospital compassionate practices and HCAHPS [the Hospital Consumer Assessment of Healthcare Providers and Systems], McClelland and Vogus reported that how well a hospital compassionately supports it employees and rewards compassionate acts is significantly and positively is associated with that hospital’s ratings and likelihood of patients recommending it.
How does the Society of Vascular Surgery, or any professional medical/nursing society for that matter, fit into this model?
We propose that the SVS find ways to empower their members to be agents for culture change within their own health care organizations. How might this be done:
- Teach organizational leadership skills, starting with the SVS Board of Directors, the presidential line, and the chairs of committees. Offer leadership courses at the Annual Meeting.
- Develop a community of caregivers committed to creating a compassionate collaborative culture. The SVS is a founding member of the Schwartz Center Healthcare Society Leadership Council, and you, as members of the SVS benefit from reduced registration at the Annual Compassion in Action Healthcare Conference, June 24-27, 2017 in Boston. (http://compassioninactionconference.org) This conference is designed to be highly experiential, using a hands-on “how to do it” model.
- The SVS should make improving the overall wellness of its members a specific goal and find specific metrics to monitor our progress towards this goal. Members can be provided with the tools to identify, monitor, and measure burnout and compassion. Each committee and council of the SVS can reexamine their objectives through the lens of reducing burnout and improving the wellness of vascular surgeons.
- Provide members with evidence-based programs that build personal resilience. This will not be a successful initiative unless our surgeons recognize and acknowledge the symptoms of burnout, and are willing to admit vulnerability. Without doing so, it is difficult to reach out for help.
- Redesign postgraduate resident and fellowship education. Standardizing clinical care may reduce variation and promote efficiency. However, when processes such as time-limited appointment scheduling, EMR templates, and protocols that drive physician-patient interactions are embedded in Resident and Fellowship education, the result may well be inflexibility in practice, reduced face time with patients, and interactions that lack compassion; all leading to burnout. Graduate Medical Education leaders must develop programs that support the learner’s ability to connect with patients and families, cultivate and role-model skills and behaviors that strengthen compassionate interactions, and strive to develop clinical practice models that increase Resident and Fellow work control autonomy.
The SVS should work proactively to optimize workload, fairness, and reward on a larger scale for its members as it relates to the EMR, reimbursement, and systems coverage. While we may be relatively small in size, as leaders, we are perfectly poised to address these larger, global issues. Perhaps working within the current system (i.e., PAC and APM task force) and considering innovative solutions at a national leadership scale, the SVS can direct real change!
Changing culture is not easy, nor quick, nor does it have an easy-to-follow blueprint. The first step is recognizing the need. The second is taking a leadership role. The third is thinking deeply about implementation.
*The authors extend their thanks and appreciation for the guidance, resources and support of Michael Goldberg, MD, scholar in residence, Schwartz Center for Compassionate Care, Boston and clinical professor of orthopedics at Seattle Children’s Hospital.
REFERENCES
1. J Managerial Psychol. (2007) 22:309-28
2. Annu Rev Neurosci. (2012) 35:1-23
3. Medicine. (2016) 44:583-5
4. J Health Organization Manag. (2015) 29:973-87
5. De Zulueta P Developing compassionate leadership in health care: an integrative review. J Healthcare Leadership. (2016) 8:1-10
6. Dolan ED, Morh D, Lempa M et al. Using a single item to measure burnout in primary care staff: A psychometry evaluation. J Gen Intern Med. (2015) 30:582-7
7. Karasek RA Job demands, job decision latitude, and mental strain: implications for job design. Administrative Sciences Quarterly (1979) 24: 285-308
8. Lee VS, Miller T, Daniels C, et al. Creating the exceptional patient experience in one academic health system. Acad Med. (2016) 91:338-44
9. Linzer M, Levine R, Meltzer D, et al. 10 bold steps to prevent burnout in general internal medicine. J Gen Intern Med. (2013) 29:18-20
10. Lown BA, Manning CF The Schwartz Center Rounds: Evaluation of an interdisciplinary approach to enhancing patient-centered communication, teamwork, and provider support. Acad Med. (2010) 85:1073-81
11. Lown BA, Muncer SJ, Chadwick R Can compassionate healthcare be measured? The Schwartz Center Compassionate Care Scale. Patient Education and Counseling (2015) 98:1005-10
12. Lown BA, McIntosh S, Gaines ME, et. al. Integrating compassionate collaborative care (“the Triple C”) into health professional education to advance the triple aim of health care. Acad Med (2016) 91:1-7
13. Lown BA A social neuroscience-informed model for teaching and practicing compassion in health care. Medical Education (2016) 50: 332-342
14. Maslach C, Schaufeli WG, Leiter MP Job burnout. Annu Rev Psychol (2001) 52:397-422
15. McClelland LE, Vogus TJ Compassion practices and HCAHPS: Does rewarding and supporting workplace compassion influence patient perceptions? HSR: Health Serv Res. (2014) 49:1670-83
16. Shanafelt TD, Noseworthy JH Executive leadership and physician well-being: Nine organizational strategies to promote engagement and reduce burnout. Mayo Clin Proc. (2016) 6:1-18
17. Shanafelt TD, Dyrbye LN, West CP Addressing physician burnout: the way forward. JAMA (2017) 317:901-2
18. Singer T, Klimecki OM Empathy and compassion Curr Biol. (2014) 24: R875-8
19. West CP, Dyrbye LN, Satele DV et. al. Concurrent validity of single-item measures of emotional exhaustion and depersonalization in burnout assessment. J Gen Intern Med. (2012) 27:1445-52
20. West CP, Dyrbye LN, Erwin PJ, et al. Interventions to address and reduce physician burnout: a systematic review and meta-analysis. Lancet. (2016) 388:2272-81
21. Wuest TK, Goldberg MJ, Kelly JD Clinical faceoff: Physician burnout-Fact, fantasy, or the fourth component of the triple aim? Clin Orthop Relat Res. (2016) doi: 10.1007/5-11999-016-5193-5
Endometrial Cancer: 5 Things to Know
Endometrial cancer is a common type of gynecologic cancer, and its incidence is rising steadily in the United States and globally. Most cases are endometrioid adenocarcinomas, arising from the inner lining of the uterus — the endometrium. While many patients are diagnosed early because of noticeable symptoms like abnormal bleeding, trends in both incidence and mortality are concerning, especially given the persistent racial and socioeconomic disparities in outcomes.
In addition to being the most common uterine malignancy, endometrial cancer is at the forefront of precision oncology in gynecology. The traditional classification systems based on histology and hormone dependence are now being augmented by molecular subtyping that better informs prognosis and treatment. As diagnostic tools, genetic testing, and therapeutic strategies advance, the management of endometrial cancer is becoming increasingly personalized.
Here are five things to know about endometrial cancer:
1. Endometrial cancer is one of the few cancers with increasing mortality.
Endometrial cancer accounts for the majority of uterine cancers in the United States with an overall lifetime risk for women of about 1 in 40. Since the mid-2000s, incidence rates have risen steadily, by > 1% per year, reflecting both lifestyle and environmental factors. Importantly, the disease tends to be diagnosed at an early stage due to the presence of warning signs like postmenopausal bleeding, which contributes to relatively favorable survival outcomes when caught early.
However, mortality trends continue to evolve. From 1999 to 2013, death rates from endometrial cancer in the US declined slightly, but since 2013, they have increased sharply — by > 8% annually — according to recent data. This upward trend in mortality disproportionately affects non-Hispanic Black women, who experience the highest mortality rate (4.7 per 100,000) among all racial and ethnic groups. This disparity is likely caused by a complex interplay of factors, including delays in diagnosis, more aggressive tumor biology, and inequities in access to care. Addressing these disparities remains a key priority in improving outcomes.
2. Risk factors go beyond hormones and age.
Risk factors for endometrial cancer include prolonged exposure to unopposed estrogen, which can result from estrogen-only hormone replacement therapy, higher BMI, and early menarche or late menopause. Nulliparity (having never been pregnant) and older age also increase risk, as does tamoxifen use — a medication commonly prescribed for breast cancer prevention. These factors cumulatively increase endometrial proliferation and the potential for atypical cellular changes. Endometrial hyperplasia, a known precursor to cancer, is often linked to these hormonal imbalances and may require surveillance or treatment.
Beyond estrogen’s influence, a growing body of research is uncovering additional risk contributors. Women with polycystic ovary syndrome (PCOS), metabolic syndrome, or diabetes face elevated risk of developing endometrial cancer. Genetic syndromes, particularly Lynch and Cowden syndromes, are associated with significantly increased lifetime risks of endometrial cancer. Environmental exposures, such as the use of hair relaxers, are being investigated as emerging risk factors. Additionally, race remains a risk marker, with Black women not only experiencing higher mortality but also more aggressive subtypes of the disease. These complex, overlapping risks highlight the importance of individualized risk assessment and early intervention strategies.
3. Postmenopausal bleeding is the hallmark symptom — but not the only one.
In endometrial cancer, the majority of cases are diagnosed at an early stage, largely because of the hallmark symptom of postmenopausal bleeding. In addition to bleeding, patients may present with vaginal discharge, pyometra, and even pain and abdominal distension in advanced disease. Any bleeding in a postmenopausal woman should prompt evaluation, as it may signal endometrial hyperplasia or carcinoma. In premenopausal women, irregular or heavy menstrual bleeding may raise suspicion, particularly when accompanied by risk factors such as PCOS.
The diagnostic workup for suspected endometrial cancer in women, particularly those presenting with postmenopausal bleeding, begins with a focused clinical assessment and frequently includes transvaginal ultrasound (TVUS) to evaluate the endometrium. While TVUS can aid in identifying structural abnormalities or suggest malignancy, endometrial sampling is warranted in all postmenopausal women with abnormal bleeding, regardless of endometrial thickness. Office-based biopsy is the preferred initial approach due to its convenience and diagnostic yield; however, if the sample is nondiagnostic or technically difficult to obtain, hysteroscopy with directed biopsy or dilation and curettage should be pursued.
4. Classification systems are evolving to include molecular subtypes.
Historically, endometrial cancers were classified using the World Health Organization system based on histology and by hormone dependence: Type 1 (estrogen-dependent, typically endometrioid and low grade) and Type 2 (non-estrogen dependent, often serous and high grade). Type 1 cancers tend to have a better prognosis and slower progression, while Type 2 cancers are more aggressive and require intensive treatment. While helpful, this binary classification does not fully capture the biological diversity or treatment responsiveness of the disease.
The field is now moving toward molecular classification, which offers a more nuanced understanding. The four main molecular subtypes include: polymerase epsilon (POLE)-mutant, mismatch repair (MMR)-deficient, p53-abnormal, and no specific molecular profile (NSMP). These groups differ in prognosis and therapeutic implications. POLE-mutant tumors with extremely high mutational burdens generally have excellent outcomes and may not require aggressive adjuvant therapy. In contrast, p53-abnormal tumors are associated with chromosomal instability, TP53 mutations, and poor outcomes, necessitating more aggressive multimodal treatment. MMR-deficient tumors are particularly responsive to immunotherapy. These molecular distinctions are changing how clinicians approach risk stratification and management in patients with endometrial cancer.
5. Treatment is increasingly personalized — and immunotherapy is expanding.
The cornerstone of treatment for early-stage endometrial cancer is surgical: total hysterectomy with bilateral salpingo-oophorectomy, often with sentinel node mapping or lymphadenectomy. Adjuvant therapy depends on factors such as stage, grade, histology, and molecular subtype. Fertility-sparing management with progestin therapy is an option for highly selected patients with early-stage, low-grade tumors. Clinical guidelines recommend that fertility desires be addressed prior to initiating treatment, as standard surgical management typically results in loss of reproductive capacity.
For advanced or recurrent disease, treatment becomes more complex and increasingly individualized. Chemotherapy, often with carboplatin and paclitaxel, is standard for stage III/IV and recurrent disease. Molecular findings now guide additional therapy: For instance, MMR-deficient tumors may respond to checkpoint inhibitors. As targeted agents and combination regimens continue to emerge, treatment of endometrial is increasingly focused on precision medicine.
Markman is professor of medical oncology and therapeutics research and President of Medicine & Science at City of Hope in Atlanta and Chicago. He has disclosed relevant financial relationships with AstraZeneca, GSK and Myriad.
A version of this article first appeared on Medscape.com.
Endometrial cancer is a common type of gynecologic cancer, and its incidence is rising steadily in the United States and globally. Most cases are endometrioid adenocarcinomas, arising from the inner lining of the uterus — the endometrium. While many patients are diagnosed early because of noticeable symptoms like abnormal bleeding, trends in both incidence and mortality are concerning, especially given the persistent racial and socioeconomic disparities in outcomes.
In addition to being the most common uterine malignancy, endometrial cancer is at the forefront of precision oncology in gynecology. The traditional classification systems based on histology and hormone dependence are now being augmented by molecular subtyping that better informs prognosis and treatment. As diagnostic tools, genetic testing, and therapeutic strategies advance, the management of endometrial cancer is becoming increasingly personalized.
Here are five things to know about endometrial cancer:
1. Endometrial cancer is one of the few cancers with increasing mortality.
Endometrial cancer accounts for the majority of uterine cancers in the United States with an overall lifetime risk for women of about 1 in 40. Since the mid-2000s, incidence rates have risen steadily, by > 1% per year, reflecting both lifestyle and environmental factors. Importantly, the disease tends to be diagnosed at an early stage due to the presence of warning signs like postmenopausal bleeding, which contributes to relatively favorable survival outcomes when caught early.
However, mortality trends continue to evolve. From 1999 to 2013, death rates from endometrial cancer in the US declined slightly, but since 2013, they have increased sharply — by > 8% annually — according to recent data. This upward trend in mortality disproportionately affects non-Hispanic Black women, who experience the highest mortality rate (4.7 per 100,000) among all racial and ethnic groups. This disparity is likely caused by a complex interplay of factors, including delays in diagnosis, more aggressive tumor biology, and inequities in access to care. Addressing these disparities remains a key priority in improving outcomes.
2. Risk factors go beyond hormones and age.
Risk factors for endometrial cancer include prolonged exposure to unopposed estrogen, which can result from estrogen-only hormone replacement therapy, higher BMI, and early menarche or late menopause. Nulliparity (having never been pregnant) and older age also increase risk, as does tamoxifen use — a medication commonly prescribed for breast cancer prevention. These factors cumulatively increase endometrial proliferation and the potential for atypical cellular changes. Endometrial hyperplasia, a known precursor to cancer, is often linked to these hormonal imbalances and may require surveillance or treatment.
Beyond estrogen’s influence, a growing body of research is uncovering additional risk contributors. Women with polycystic ovary syndrome (PCOS), metabolic syndrome, or diabetes face elevated risk of developing endometrial cancer. Genetic syndromes, particularly Lynch and Cowden syndromes, are associated with significantly increased lifetime risks of endometrial cancer. Environmental exposures, such as the use of hair relaxers, are being investigated as emerging risk factors. Additionally, race remains a risk marker, with Black women not only experiencing higher mortality but also more aggressive subtypes of the disease. These complex, overlapping risks highlight the importance of individualized risk assessment and early intervention strategies.
3. Postmenopausal bleeding is the hallmark symptom — but not the only one.
In endometrial cancer, the majority of cases are diagnosed at an early stage, largely because of the hallmark symptom of postmenopausal bleeding. In addition to bleeding, patients may present with vaginal discharge, pyometra, and even pain and abdominal distension in advanced disease. Any bleeding in a postmenopausal woman should prompt evaluation, as it may signal endometrial hyperplasia or carcinoma. In premenopausal women, irregular or heavy menstrual bleeding may raise suspicion, particularly when accompanied by risk factors such as PCOS.
The diagnostic workup for suspected endometrial cancer in women, particularly those presenting with postmenopausal bleeding, begins with a focused clinical assessment and frequently includes transvaginal ultrasound (TVUS) to evaluate the endometrium. While TVUS can aid in identifying structural abnormalities or suggest malignancy, endometrial sampling is warranted in all postmenopausal women with abnormal bleeding, regardless of endometrial thickness. Office-based biopsy is the preferred initial approach due to its convenience and diagnostic yield; however, if the sample is nondiagnostic or technically difficult to obtain, hysteroscopy with directed biopsy or dilation and curettage should be pursued.
4. Classification systems are evolving to include molecular subtypes.
Historically, endometrial cancers were classified using the World Health Organization system based on histology and by hormone dependence: Type 1 (estrogen-dependent, typically endometrioid and low grade) and Type 2 (non-estrogen dependent, often serous and high grade). Type 1 cancers tend to have a better prognosis and slower progression, while Type 2 cancers are more aggressive and require intensive treatment. While helpful, this binary classification does not fully capture the biological diversity or treatment responsiveness of the disease.
The field is now moving toward molecular classification, which offers a more nuanced understanding. The four main molecular subtypes include: polymerase epsilon (POLE)-mutant, mismatch repair (MMR)-deficient, p53-abnormal, and no specific molecular profile (NSMP). These groups differ in prognosis and therapeutic implications. POLE-mutant tumors with extremely high mutational burdens generally have excellent outcomes and may not require aggressive adjuvant therapy. In contrast, p53-abnormal tumors are associated with chromosomal instability, TP53 mutations, and poor outcomes, necessitating more aggressive multimodal treatment. MMR-deficient tumors are particularly responsive to immunotherapy. These molecular distinctions are changing how clinicians approach risk stratification and management in patients with endometrial cancer.
5. Treatment is increasingly personalized — and immunotherapy is expanding.
The cornerstone of treatment for early-stage endometrial cancer is surgical: total hysterectomy with bilateral salpingo-oophorectomy, often with sentinel node mapping or lymphadenectomy. Adjuvant therapy depends on factors such as stage, grade, histology, and molecular subtype. Fertility-sparing management with progestin therapy is an option for highly selected patients with early-stage, low-grade tumors. Clinical guidelines recommend that fertility desires be addressed prior to initiating treatment, as standard surgical management typically results in loss of reproductive capacity.
For advanced or recurrent disease, treatment becomes more complex and increasingly individualized. Chemotherapy, often with carboplatin and paclitaxel, is standard for stage III/IV and recurrent disease. Molecular findings now guide additional therapy: For instance, MMR-deficient tumors may respond to checkpoint inhibitors. As targeted agents and combination regimens continue to emerge, treatment of endometrial is increasingly focused on precision medicine.
Markman is professor of medical oncology and therapeutics research and President of Medicine & Science at City of Hope in Atlanta and Chicago. He has disclosed relevant financial relationships with AstraZeneca, GSK and Myriad.
A version of this article first appeared on Medscape.com.
Endometrial cancer is a common type of gynecologic cancer, and its incidence is rising steadily in the United States and globally. Most cases are endometrioid adenocarcinomas, arising from the inner lining of the uterus — the endometrium. While many patients are diagnosed early because of noticeable symptoms like abnormal bleeding, trends in both incidence and mortality are concerning, especially given the persistent racial and socioeconomic disparities in outcomes.
In addition to being the most common uterine malignancy, endometrial cancer is at the forefront of precision oncology in gynecology. The traditional classification systems based on histology and hormone dependence are now being augmented by molecular subtyping that better informs prognosis and treatment. As diagnostic tools, genetic testing, and therapeutic strategies advance, the management of endometrial cancer is becoming increasingly personalized.
Here are five things to know about endometrial cancer:
1. Endometrial cancer is one of the few cancers with increasing mortality.
Endometrial cancer accounts for the majority of uterine cancers in the United States with an overall lifetime risk for women of about 1 in 40. Since the mid-2000s, incidence rates have risen steadily, by > 1% per year, reflecting both lifestyle and environmental factors. Importantly, the disease tends to be diagnosed at an early stage due to the presence of warning signs like postmenopausal bleeding, which contributes to relatively favorable survival outcomes when caught early.
However, mortality trends continue to evolve. From 1999 to 2013, death rates from endometrial cancer in the US declined slightly, but since 2013, they have increased sharply — by > 8% annually — according to recent data. This upward trend in mortality disproportionately affects non-Hispanic Black women, who experience the highest mortality rate (4.7 per 100,000) among all racial and ethnic groups. This disparity is likely caused by a complex interplay of factors, including delays in diagnosis, more aggressive tumor biology, and inequities in access to care. Addressing these disparities remains a key priority in improving outcomes.
2. Risk factors go beyond hormones and age.
Risk factors for endometrial cancer include prolonged exposure to unopposed estrogen, which can result from estrogen-only hormone replacement therapy, higher BMI, and early menarche or late menopause. Nulliparity (having never been pregnant) and older age also increase risk, as does tamoxifen use — a medication commonly prescribed for breast cancer prevention. These factors cumulatively increase endometrial proliferation and the potential for atypical cellular changes. Endometrial hyperplasia, a known precursor to cancer, is often linked to these hormonal imbalances and may require surveillance or treatment.
Beyond estrogen’s influence, a growing body of research is uncovering additional risk contributors. Women with polycystic ovary syndrome (PCOS), metabolic syndrome, or diabetes face elevated risk of developing endometrial cancer. Genetic syndromes, particularly Lynch and Cowden syndromes, are associated with significantly increased lifetime risks of endometrial cancer. Environmental exposures, such as the use of hair relaxers, are being investigated as emerging risk factors. Additionally, race remains a risk marker, with Black women not only experiencing higher mortality but also more aggressive subtypes of the disease. These complex, overlapping risks highlight the importance of individualized risk assessment and early intervention strategies.
3. Postmenopausal bleeding is the hallmark symptom — but not the only one.
In endometrial cancer, the majority of cases are diagnosed at an early stage, largely because of the hallmark symptom of postmenopausal bleeding. In addition to bleeding, patients may present with vaginal discharge, pyometra, and even pain and abdominal distension in advanced disease. Any bleeding in a postmenopausal woman should prompt evaluation, as it may signal endometrial hyperplasia or carcinoma. In premenopausal women, irregular or heavy menstrual bleeding may raise suspicion, particularly when accompanied by risk factors such as PCOS.
The diagnostic workup for suspected endometrial cancer in women, particularly those presenting with postmenopausal bleeding, begins with a focused clinical assessment and frequently includes transvaginal ultrasound (TVUS) to evaluate the endometrium. While TVUS can aid in identifying structural abnormalities or suggest malignancy, endometrial sampling is warranted in all postmenopausal women with abnormal bleeding, regardless of endometrial thickness. Office-based biopsy is the preferred initial approach due to its convenience and diagnostic yield; however, if the sample is nondiagnostic or technically difficult to obtain, hysteroscopy with directed biopsy or dilation and curettage should be pursued.
4. Classification systems are evolving to include molecular subtypes.
Historically, endometrial cancers were classified using the World Health Organization system based on histology and by hormone dependence: Type 1 (estrogen-dependent, typically endometrioid and low grade) and Type 2 (non-estrogen dependent, often serous and high grade). Type 1 cancers tend to have a better prognosis and slower progression, while Type 2 cancers are more aggressive and require intensive treatment. While helpful, this binary classification does not fully capture the biological diversity or treatment responsiveness of the disease.
The field is now moving toward molecular classification, which offers a more nuanced understanding. The four main molecular subtypes include: polymerase epsilon (POLE)-mutant, mismatch repair (MMR)-deficient, p53-abnormal, and no specific molecular profile (NSMP). These groups differ in prognosis and therapeutic implications. POLE-mutant tumors with extremely high mutational burdens generally have excellent outcomes and may not require aggressive adjuvant therapy. In contrast, p53-abnormal tumors are associated with chromosomal instability, TP53 mutations, and poor outcomes, necessitating more aggressive multimodal treatment. MMR-deficient tumors are particularly responsive to immunotherapy. These molecular distinctions are changing how clinicians approach risk stratification and management in patients with endometrial cancer.
5. Treatment is increasingly personalized — and immunotherapy is expanding.
The cornerstone of treatment for early-stage endometrial cancer is surgical: total hysterectomy with bilateral salpingo-oophorectomy, often with sentinel node mapping or lymphadenectomy. Adjuvant therapy depends on factors such as stage, grade, histology, and molecular subtype. Fertility-sparing management with progestin therapy is an option for highly selected patients with early-stage, low-grade tumors. Clinical guidelines recommend that fertility desires be addressed prior to initiating treatment, as standard surgical management typically results in loss of reproductive capacity.
For advanced or recurrent disease, treatment becomes more complex and increasingly individualized. Chemotherapy, often with carboplatin and paclitaxel, is standard for stage III/IV and recurrent disease. Molecular findings now guide additional therapy: For instance, MMR-deficient tumors may respond to checkpoint inhibitors. As targeted agents and combination regimens continue to emerge, treatment of endometrial is increasingly focused on precision medicine.
Markman is professor of medical oncology and therapeutics research and President of Medicine & Science at City of Hope in Atlanta and Chicago. He has disclosed relevant financial relationships with AstraZeneca, GSK and Myriad.
A version of this article first appeared on Medscape.com.
A Cancer Patient’s Bittersweet Reminder
Recently, a 40-year-old woman took to Facebook to announce that she had died.
Rachel Davies, of Wales, wrote: “If you’re reading this, then it means I’m no longer here. What a life I’ve had, and surprisingly, since cancer entered my life. When I look through my photos, I’ve done and seen so much since cancer, and probably some of my best memories are from this period. In so many ways, I have to thank it for learning how to live fully. What I wish is that everyone can experience the same but without needing cancer. Get out there, experience life fully, and wear that dress!!! I’m so sad to leave my family and friends, I wish I never had to go. I’m so grateful to have had Charlie young so that I’ve watched him grow into the man he is today. I’m unbelievably proud of him. I am thankful I had the opportunity to have Kacey and Jacob in my life. Lastly, I was blessed to meet the love of my life, my husband, and my best friend. I have no regrets, I have had a wonderful life. So to all of you, don’t be sad I’ve gone. Live your life and live it well. Love, Rachel x.”
I didn’t know Ms. Davies, but am likely among many who wish I had. In a terrible situation she kept trying.
She had HER2 metastatic breast cancer, which can respond to the drug Enhertu (trastuzumab). Unfortunately, she never had the chance, because it wasn’t available to her in Wales. In the United Kingdom it’s available only in Scotland.
I’m not saying it was a cure. Statistically, it likely would have bought her another 6 months of family time. But that’s still another half year.
I’m not blaming the Welsh NHS, though they made the decision not to cover it because of cost. The jobs of such committees is a thankless one, trying to decide where the limited money goes — vaccines for many children that are proven to lessen morbidity and mortality over the course of a lifetime, or to add 6 months to the lives of comparatively fewer women with HER2 metastatic breast cancer.
I’m not blaming the company that makes Enhertu, though it was the cost that kept her from getting it. Bringing a drug to market, with all the labs and clinical research behind it, ain’t cheap. If the company can’t keep the lights on they’re not going to able to develop future pharmaceuticals to help others, though I do wonder if a better price could have been negotiated. (I’m not trying to justify the salaries of insurance CEOs — don’t even get me started on those.)
Money is always limited, and human suffering is infinite. Every health care organization, public or private, has to face that simple fact. There is no right place to draw the line, so we use the greatest good for the greatest many as our best guess.
In her last post, though, Ms. Davies didn’t dwell on any of this. She reflected on her joys and blessings, and encouraged others to live life fully. Things we should all focus on.
Thank you, Ms. Davies, for the reminder.
Allan M. Block, MD, has a solo neurology practice in Scottsdale, Arizona.
Recently, a 40-year-old woman took to Facebook to announce that she had died.
Rachel Davies, of Wales, wrote: “If you’re reading this, then it means I’m no longer here. What a life I’ve had, and surprisingly, since cancer entered my life. When I look through my photos, I’ve done and seen so much since cancer, and probably some of my best memories are from this period. In so many ways, I have to thank it for learning how to live fully. What I wish is that everyone can experience the same but without needing cancer. Get out there, experience life fully, and wear that dress!!! I’m so sad to leave my family and friends, I wish I never had to go. I’m so grateful to have had Charlie young so that I’ve watched him grow into the man he is today. I’m unbelievably proud of him. I am thankful I had the opportunity to have Kacey and Jacob in my life. Lastly, I was blessed to meet the love of my life, my husband, and my best friend. I have no regrets, I have had a wonderful life. So to all of you, don’t be sad I’ve gone. Live your life and live it well. Love, Rachel x.”
I didn’t know Ms. Davies, but am likely among many who wish I had. In a terrible situation she kept trying.
She had HER2 metastatic breast cancer, which can respond to the drug Enhertu (trastuzumab). Unfortunately, she never had the chance, because it wasn’t available to her in Wales. In the United Kingdom it’s available only in Scotland.
I’m not saying it was a cure. Statistically, it likely would have bought her another 6 months of family time. But that’s still another half year.
I’m not blaming the Welsh NHS, though they made the decision not to cover it because of cost. The jobs of such committees is a thankless one, trying to decide where the limited money goes — vaccines for many children that are proven to lessen morbidity and mortality over the course of a lifetime, or to add 6 months to the lives of comparatively fewer women with HER2 metastatic breast cancer.
I’m not blaming the company that makes Enhertu, though it was the cost that kept her from getting it. Bringing a drug to market, with all the labs and clinical research behind it, ain’t cheap. If the company can’t keep the lights on they’re not going to able to develop future pharmaceuticals to help others, though I do wonder if a better price could have been negotiated. (I’m not trying to justify the salaries of insurance CEOs — don’t even get me started on those.)
Money is always limited, and human suffering is infinite. Every health care organization, public or private, has to face that simple fact. There is no right place to draw the line, so we use the greatest good for the greatest many as our best guess.
In her last post, though, Ms. Davies didn’t dwell on any of this. She reflected on her joys and blessings, and encouraged others to live life fully. Things we should all focus on.
Thank you, Ms. Davies, for the reminder.
Allan M. Block, MD, has a solo neurology practice in Scottsdale, Arizona.
Recently, a 40-year-old woman took to Facebook to announce that she had died.
Rachel Davies, of Wales, wrote: “If you’re reading this, then it means I’m no longer here. What a life I’ve had, and surprisingly, since cancer entered my life. When I look through my photos, I’ve done and seen so much since cancer, and probably some of my best memories are from this period. In so many ways, I have to thank it for learning how to live fully. What I wish is that everyone can experience the same but without needing cancer. Get out there, experience life fully, and wear that dress!!! I’m so sad to leave my family and friends, I wish I never had to go. I’m so grateful to have had Charlie young so that I’ve watched him grow into the man he is today. I’m unbelievably proud of him. I am thankful I had the opportunity to have Kacey and Jacob in my life. Lastly, I was blessed to meet the love of my life, my husband, and my best friend. I have no regrets, I have had a wonderful life. So to all of you, don’t be sad I’ve gone. Live your life and live it well. Love, Rachel x.”
I didn’t know Ms. Davies, but am likely among many who wish I had. In a terrible situation she kept trying.
She had HER2 metastatic breast cancer, which can respond to the drug Enhertu (trastuzumab). Unfortunately, she never had the chance, because it wasn’t available to her in Wales. In the United Kingdom it’s available only in Scotland.
I’m not saying it was a cure. Statistically, it likely would have bought her another 6 months of family time. But that’s still another half year.
I’m not blaming the Welsh NHS, though they made the decision not to cover it because of cost. The jobs of such committees is a thankless one, trying to decide where the limited money goes — vaccines for many children that are proven to lessen morbidity and mortality over the course of a lifetime, or to add 6 months to the lives of comparatively fewer women with HER2 metastatic breast cancer.
I’m not blaming the company that makes Enhertu, though it was the cost that kept her from getting it. Bringing a drug to market, with all the labs and clinical research behind it, ain’t cheap. If the company can’t keep the lights on they’re not going to able to develop future pharmaceuticals to help others, though I do wonder if a better price could have been negotiated. (I’m not trying to justify the salaries of insurance CEOs — don’t even get me started on those.)
Money is always limited, and human suffering is infinite. Every health care organization, public or private, has to face that simple fact. There is no right place to draw the line, so we use the greatest good for the greatest many as our best guess.
In her last post, though, Ms. Davies didn’t dwell on any of this. She reflected on her joys and blessings, and encouraged others to live life fully. Things we should all focus on.
Thank you, Ms. Davies, for the reminder.
Allan M. Block, MD, has a solo neurology practice in Scottsdale, Arizona.
Have Your Cake and Eat It, Too: Findings Based on Ingredients in Christmas Desserts From The Great British Bake Off
This transcript has been edited for clarity.
Hello. I’m David Kerr, professor of cancer medicine at University of Oxford. As I become, sadly, older, I’ve become much more interested in the concept of cancer prevention than cancer treatment. Of course, I’m still a practicing cancer physician and researcher. That’s my daily bread and butter. But prevention is important.
There’s a really interesting article in the Christmas edition of The BMJ. This is an opportunity for us to take good science, but lighthearted science, to titillate and amuse our Christmas readers. This is a nice article from the States led by Joshua Wallach. As I say, this brings together good science in a sometimes absurd setting. I’ll read its title: “Association of Health Benefits and Harms of Christmas Dessert Ingredients in Recipes From The Great British Bake Off: Umbrella Review of Umbrella Reviews of Meta-analyses of Observational Studies.”
It’s obviously a very strong statistical underpinning from this group from Yale, predominantly — a half-decent university, as those of us from Oxford would have to admit. They used The Great British Bake Off website, Embase, Medline, and Scopus. They looked at the whole host of umbrella reviews and so on.
They were interested in looking at the relative balance of dangerous and protective ingredients that were recommended in Christmas desserts on this immensely popular television show called The Great British Bake Off. Some of you have watched it and have enjoyed watching the trials and tribulations of the various contestants.
They looked at 48 recipes for Christmas desserts, including cakes, biscuits, pastries, puddings, and conventional desserts. Of all these, there were 178 unique ingredients. Literature research then parsed whether these ingredients were good for you or bad for you.
It was very interesting that, when they put the summary together, the umbrella review of umbrella reviews of meta-analyses compressed together, it was good news for us all. Recipes for Christmas desserts, particularly from The Great British Bake Off — which should be enormously proud of this — tend to use ingredient groups that are associated with reductions rather than increases in the risk for disease. Hurrah!
This means that, clearly, Christmas is a time in which those of us who can, tend to overindulge in food. The granddad falling asleep with a full tummy, sitting with the family in front of a hot fire — all of us can remember and imagine all of that.
Perhaps the most important takeaway point from this observationally, critically important study is that, yes — at Christmas time, enjoy the dessert. You can have your cake and eat it, too. You heard it here. It’s philosophically true and statistically proven: You can have your cake and eat it.
Thanks for listening. I’d be very interested in your own recipes, and whether we think that the American Thanksgiving desserts correlate with British Christmas desserts in some way and are beneficial to your health.
Have a look at this article that is cleverly, wittily written. As always, Medscapers, for the time being, thanks for listening. Over and out.
Dr Kerr, Professor, Nuffield Department of Clinical Laboratory Science, University of Oxford; Professor of Cancer Medicine, Oxford Cancer Centre, Oxford, United Kingdom, has disclosed ties with Celleron Therapeutics, Oxford Cancer Biomarkers (Board of Directors); Afrox (charity; Trustee); GlaxoSmithKline and Bayer HealthCare Pharmaceuticals (Consultant); Genomic Health; Merck Serono, Roche.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Hello. I’m David Kerr, professor of cancer medicine at University of Oxford. As I become, sadly, older, I’ve become much more interested in the concept of cancer prevention than cancer treatment. Of course, I’m still a practicing cancer physician and researcher. That’s my daily bread and butter. But prevention is important.
There’s a really interesting article in the Christmas edition of The BMJ. This is an opportunity for us to take good science, but lighthearted science, to titillate and amuse our Christmas readers. This is a nice article from the States led by Joshua Wallach. As I say, this brings together good science in a sometimes absurd setting. I’ll read its title: “Association of Health Benefits and Harms of Christmas Dessert Ingredients in Recipes From The Great British Bake Off: Umbrella Review of Umbrella Reviews of Meta-analyses of Observational Studies.”
It’s obviously a very strong statistical underpinning from this group from Yale, predominantly — a half-decent university, as those of us from Oxford would have to admit. They used The Great British Bake Off website, Embase, Medline, and Scopus. They looked at the whole host of umbrella reviews and so on.
They were interested in looking at the relative balance of dangerous and protective ingredients that were recommended in Christmas desserts on this immensely popular television show called The Great British Bake Off. Some of you have watched it and have enjoyed watching the trials and tribulations of the various contestants.
They looked at 48 recipes for Christmas desserts, including cakes, biscuits, pastries, puddings, and conventional desserts. Of all these, there were 178 unique ingredients. Literature research then parsed whether these ingredients were good for you or bad for you.
It was very interesting that, when they put the summary together, the umbrella review of umbrella reviews of meta-analyses compressed together, it was good news for us all. Recipes for Christmas desserts, particularly from The Great British Bake Off — which should be enormously proud of this — tend to use ingredient groups that are associated with reductions rather than increases in the risk for disease. Hurrah!
This means that, clearly, Christmas is a time in which those of us who can, tend to overindulge in food. The granddad falling asleep with a full tummy, sitting with the family in front of a hot fire — all of us can remember and imagine all of that.
Perhaps the most important takeaway point from this observationally, critically important study is that, yes — at Christmas time, enjoy the dessert. You can have your cake and eat it, too. You heard it here. It’s philosophically true and statistically proven: You can have your cake and eat it.
Thanks for listening. I’d be very interested in your own recipes, and whether we think that the American Thanksgiving desserts correlate with British Christmas desserts in some way and are beneficial to your health.
Have a look at this article that is cleverly, wittily written. As always, Medscapers, for the time being, thanks for listening. Over and out.
Dr Kerr, Professor, Nuffield Department of Clinical Laboratory Science, University of Oxford; Professor of Cancer Medicine, Oxford Cancer Centre, Oxford, United Kingdom, has disclosed ties with Celleron Therapeutics, Oxford Cancer Biomarkers (Board of Directors); Afrox (charity; Trustee); GlaxoSmithKline and Bayer HealthCare Pharmaceuticals (Consultant); Genomic Health; Merck Serono, Roche.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Hello. I’m David Kerr, professor of cancer medicine at University of Oxford. As I become, sadly, older, I’ve become much more interested in the concept of cancer prevention than cancer treatment. Of course, I’m still a practicing cancer physician and researcher. That’s my daily bread and butter. But prevention is important.
There’s a really interesting article in the Christmas edition of The BMJ. This is an opportunity for us to take good science, but lighthearted science, to titillate and amuse our Christmas readers. This is a nice article from the States led by Joshua Wallach. As I say, this brings together good science in a sometimes absurd setting. I’ll read its title: “Association of Health Benefits and Harms of Christmas Dessert Ingredients in Recipes From The Great British Bake Off: Umbrella Review of Umbrella Reviews of Meta-analyses of Observational Studies.”
It’s obviously a very strong statistical underpinning from this group from Yale, predominantly — a half-decent university, as those of us from Oxford would have to admit. They used The Great British Bake Off website, Embase, Medline, and Scopus. They looked at the whole host of umbrella reviews and so on.
They were interested in looking at the relative balance of dangerous and protective ingredients that were recommended in Christmas desserts on this immensely popular television show called The Great British Bake Off. Some of you have watched it and have enjoyed watching the trials and tribulations of the various contestants.
They looked at 48 recipes for Christmas desserts, including cakes, biscuits, pastries, puddings, and conventional desserts. Of all these, there were 178 unique ingredients. Literature research then parsed whether these ingredients were good for you or bad for you.
It was very interesting that, when they put the summary together, the umbrella review of umbrella reviews of meta-analyses compressed together, it was good news for us all. Recipes for Christmas desserts, particularly from The Great British Bake Off — which should be enormously proud of this — tend to use ingredient groups that are associated with reductions rather than increases in the risk for disease. Hurrah!
This means that, clearly, Christmas is a time in which those of us who can, tend to overindulge in food. The granddad falling asleep with a full tummy, sitting with the family in front of a hot fire — all of us can remember and imagine all of that.
Perhaps the most important takeaway point from this observationally, critically important study is that, yes — at Christmas time, enjoy the dessert. You can have your cake and eat it, too. You heard it here. It’s philosophically true and statistically proven: You can have your cake and eat it.
Thanks for listening. I’d be very interested in your own recipes, and whether we think that the American Thanksgiving desserts correlate with British Christmas desserts in some way and are beneficial to your health.
Have a look at this article that is cleverly, wittily written. As always, Medscapers, for the time being, thanks for listening. Over and out.
Dr Kerr, Professor, Nuffield Department of Clinical Laboratory Science, University of Oxford; Professor of Cancer Medicine, Oxford Cancer Centre, Oxford, United Kingdom, has disclosed ties with Celleron Therapeutics, Oxford Cancer Biomarkers (Board of Directors); Afrox (charity; Trustee); GlaxoSmithKline and Bayer HealthCare Pharmaceuticals (Consultant); Genomic Health; Merck Serono, Roche.
A version of this article appeared on Medscape.com.
Primary Sclerosing Cholangitis (PSC) and Its Importance in Clinical Practice
Primary sclerosing cholangitis (PSC) is a rare, chronic, and progressive cholestatic liver disorder.1 Commonly associated with pruritus, an intense itch that significantly impacts patients’ lives, PSC is characterized by inflammation, fibrosis, and stricturing of the intrahepatic and/or extrahepatic bile ducts.1,2 The natural history of PSC is highly variable, but disease progression frequently leads to end-stage liver disease, with liver transplantation as the only currently available treatment option.1,2 PSC has a close association with inflammatory bowel disease (IBD), with approximately 60% to 80% of patients with PSC having a diagnosis of either ulcerative colitis or Crohn’s disease.1,3 Although the exact pathogenesis of PSC is still under investigation, evidence suggests a complex interplay of genetic susceptibility, immune dysregulation, and environmental factors may be responsible.4
PSC is considered a rare disease, with an estimated global median incidence of 0.7 to 0.8 per 100,000 and estimated prevalence of 10 cases per 100,000.5 PSC is more common in men (60% to 70%), with men having a 2-fold higher risk of developing PSC than women.2,6,7 The majority of patients are diagnosed between the ages of 30 to 40 years, with a median survival time after diagnosis without a liver transplant of 10 to 20 years.2,7-9
Signs and Symptoms of PSC
Approximately 50% of patients with PSC are asymptomatic when persistently abnormal liver function tests trigger further evaluation.1,2,10 Patients may complain of pruritus, which may be episodic; right upper quadrant pain; fatigue; and jaundice.2,7 Fevers, chills, and night sweats may also be present at the time of diagnosis.2
Pruritus and fatigue are common symptoms of PSC and can have a significant impact on the lives of patients.5 The pathogenesis of pruritus is complex and not completely understood but is believed to be caused by a toxic buildup of bile acids due to a decrease in bile flow related to inflammation, fibrosis, and stricturing resulting from PSC.11,12
Pruritus has been shown to have a substantial impact on patients’ health-related quality of life (QoL), with greater impairment seen with increased severity of pruritus.13 Specifically, patients with pruritus report physical limitations on QoL-specific questionnaires, as well as an impact on emotional, bodily pain, vitality, energy, and physical mobility measures.14
From a multinational survey on the impact of pruritus in PSC patients, 96% of respondents indicated that their itch was worst in the evening, with 58% indicating mood changes, including anxiety, irritability, and feelings of hopelessness due to their itch. Further, respondents reported that their pruritus disrupted their day-to-day responsibilities and that this disruption lasted 1 month or more.15
The psychological impact of living with PSC has not been well studied, although it has been found that individuals living with the disease demonstrated a greater number of depressive symptoms and poorer well-being, often coinciding with their stage of liver disease and comorbidity with IBD.16
In those living with PSC, mental health-related QoL has been shown to be influenced by liver disease, pruritus, social isolation, and depression. In one study, nearly 75% of patients expressed existential anxiety regarding disease progression and shortened life expectancy, with 25% disclosing social isolation.13
Diagnosing PSC
PSC should be considered in patients with a cholestatic pattern of liver test abnormalities, especially in those with underlying IBD. Abnormalities that may be detected on physical examination include jaundice, hepatomegaly, splenomegaly, and excoriations from scratching.3,5 PSC and autoimmune hepatitis (AIH) may coexist, particularly in younger patients, with serum biochemical tests and autoantibodies suggestive of AIH.2 Most patients demonstrate elevated serum alkaline phosphatase levels, as well as modest elevation of transaminases.2 Bilirubin and albumin levels may be normal at the time of diagnosis, although they may become increasingly abnormal as the disease progresses.2 A subset of patients (10%) may have elevated levels of immunoglobulin G4 (IgG4) and tend to progress more rapidly in the absence of treatment.2 Autoantibodies, which are characteristic of primary biliary cholangitis (PBC)—another rare, chronic, and progressive liver disease—are usually absent in PSC. When present, autoantibodies are of unknown clinical significance.2,17
Imaging, including cross-sectional imaging, particularly magnetic resonance cholangiopancreatography, is often used to the biliary tree in patients with persistently abnormal cholestatic tests.2 A diagnosis of PSC is typically established by the demonstration of characteristic multifocal stricturing and dilation of intrahepatic and/or extrahepatic bile ducts on cholangiography.5 The diagnosis of PSC is occasionally made on liver biopsy, which may reveal characteristic features of “onion skin fibrosis” and fibro-obliterative cholangitis when cholangiography is normal. In this circumstance, it is classified as “small-duct PSC.”5,18
Treatment and Management of PSC
Despite advances in our understanding of PSC, there are currently no approved drug therapies for PSC and no approved treatments for PSC-associated pruritus. The American Association for the Study of Liver Diseases (AASLD) published the most recent practice guidance for the treatment and management of PSC in 2022.7
Ursodeoxycholic acid (UDCA) has been widely studied as a potential PSC treatment. While UDCA demonstrates improvements in biochemical measures, there has been a lack of evidence demonstrating clinical improvement.19
The role of UDCA in the treatment of PSC is unclear and, at this time, is not supported by the American College of Gastroenterology or AASLD.2,7 Additional treatments, including immunosuppressive medications (methotrexate, tacrolimus), corticosteroids (prednisolone), and antibiotics (minocycline, vancomycin) have been explored but have not shown definitive clinical benefit.2
UDCA, if used, should not be prescribed at doses in excess of 20 mg/kg/day since high-dose UDCA (28-30 mg/kg) was associated with adverse liver outcomes.2
Although there are no therapies approved specifically to manage PSC-associated pruritus, cholestyramine and rifampin have been shown to be beneficial in relieving itch in some patients.22 In a survey of PSC patients, one in three reported suffering from pruritus during the previous week. It is possible that the prevalence and severity of pruritus in PSC may be under-recognized compared with PBC, given that patients and physicians may be focused on the many other medical issues that are often prioritized over symptoms, such as concern about cancer risk and need for frequent surveillance procedures.15,23 Discussions between patients and physicians are important to deepen our understanding of the prevalence of pruritus and its burden on the lives of patients.
Novel therapies for PSC and PSC-associated pruritus, including a selective inhibitor of the ileal bile acid transporter (IBAT), are currently being explored in clinical trials. Research suggests that the inhibition of IBAT blocks the recycling of bile acids, which reduces bile acids systemically and in the liver. Early clinical studies demonstrated on-target fecal bile acid excretion, a pharmacodynamic marker of IBAT inhibition, in addition to decreases in low-density lipoprotein cholesterol and increases in 7αC4, which are markers of bile acid synthesis.24
To learn more about ongoing clinical trials, please visit https://www.mirumclinicaltrials.com.
References
1. Karlsen TH, Folseraas T, Thorburn D, Vesterhus M. Primary sclerosing cholangitis – a comprehensive review. J Hepatol. 2017;67(6):1298-1323. doi:10.1016/j.jhep.2017.07.022
2. Lindor KD, Kowdley KV, Harrison EM. ACG clinical guideline: primary sclerosing cholangitis. Am. J. Gastroenterol. 110, 646–659 (2015).
3. Chapman R, Fevery J, Kalloo A, et al; American Association for the Study of Liver Diseases. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51(2):660-678. doi:10.1002/hep.23294
4. Jiang X, Karlsen TH. Genetics of primary sclerosing cholangitis and pathophysiological implications. Nat Rev Gastroenterol Hepatol. 2017;14(5):279-295. doi:10.1038/nrgastro.2016.154
5. Sohal A, Kayani S, Kowdley KV. Primary sclerosing cholangitis: epidemiology, diagnosis, and presentation. Clin Liver Dis. 2024;28(1):129-141. doi:10.1016/j.cld.2023.07.005
6. Molodecky NA, Kareemi H, Parab R, et al. Incidence of primary sclerosing cholangitis: a systematic review and meta-analysis. Hepatology. 2011;53(5):1590-1599. doi:10.1002/hep.24247
7. Bowlus CL, Arrivé L, Bergquist A, et al. AASLD practice guidance on primary sclerosing cholangitis and cholangiocarcinoma. Hepatology. 2023;77(2):659-702. doi:10.1002/hep.32771
8. Hirschfield GM, Karlsen TH, Lindor KD, Adams DH. Primary sclerosing cholangitis. Lancet. 2013;382(9904):1587-1599.
9. Trivedi PJ, Bowlus CL, Yimam KK, Razavi H, Estes C. Epidemiology, natural history, and outcomes of primary sclerosing cholangitis: a systematic review of population-based studies. Clin Gastroenterol Hepatol. 2022;20(8):1687-1700.e4. doi:10.1016/j.cgh.2021.08.039
10. Tischendorf JJ, Hecker H, Krüger M, Manns MP, Meier PN. Characterization, outcome, and prognosis in 273 patients with primary sclerosing cholangitis: a single center study. Am J Gastroenterol. 2007;102(1):107-114. doi:10.1111/j.1572-0241.2006.00872.x
11. Sanjel B, Shim WS. Recent advances in understanding the molecular mechanisms of cholestatic pruritus: a review. Biochim Biophys Acta Mol Basis Dis. 2020;1866(12):165958. doi:10.1016/j.bbadis.2020.16595
12. Patel SP, Vasavda C, Ho B, Meixiong J, Dong X, Kwatra SG. Cholestatic pruritus: emerging mechanisms and therapeutics. J Am Acad Dermatol. 2019;81(6):1371-1378. doi:10.1016/j.jaad.2019.04.035
13. Cheung AC, Patel H, Meza-Cardona J, Cino M, Sockalingam S, Hirschfield GM. Factors that influence health-related quality of life in patients with primary sclerosing cholangitis. Dig Dis Sci. 2016;61(6):1692-9. doi:10.1007/s10620-015-4013-1
14. Jin XY, Khan TM. Quality of life among patients suffering from cholestatic liver disease-induced pruritus: a systematic review. J Formos Med Assoc. 2016;115(9):689-702. doi:10.1016/j.jfma.2016.05.006
15. Kowdley K, et al. Impact of pruritus in primary sclerosing cholangitis (PSC): a multinational survey. J. Hepatol. 2022;(1)77:S312-S313.
16. Ranieri V, Kennedy E, Walmsley M, Thorburn D, McKay K. The Primary Sclerosing Cholangitis (PSC) Wellbeing Study: understanding psychological distress in those living with PSC and those who support them. PLoS One. 2020;15(7):e0234624.:10.1371/journal.pone.0234624
17. Hov JR, Boberg KM, Karlsen TH. Autoantibodies in primary sclerosing cholangitis. World J Gastroenterol. 2008;14(24):3781-91. doi:10.3748/wjg.14.3781
18. Cazzagon N, Sarcognato S, Catanzaro E, Bonaiuto E, Peviani M, Pezzato F, Motta R. Primary Sclerosing Cholangitis: Diagnostic Criteria. Tomography. 2024;10(1):47-65. doi:10.3390/tomography10010005
19. Lindor KD. Ursodiol for primary sclerosing cholangitis. Mayo Primary Sclerosing Cholangitis-Ursodeoxycholic Acid Study Group. N Engl J Med. 1997;336(10):691-695. doi:10.1056/NEJM199703063361003
20. Lee YM, Kaplan MM. Primary sclerosing cholangitis. N Engl J Med. 1995;332(14):924-33. doi:10.1056/NEJM199504063321406
21. Said K, Glaumann H, Bergquist A. Gallbladder disease in patients with primary sclerosing cholangitis. J Hepatol. 2008;48(4):598-605. doi:10.1016/j.jhep.2007.11.01
22. Basic PSC facts: basic facts. PSC Partners Seeking a Cure. Accessed October 14, 2024. https://pscpartners.org/about/the-disease/basic-facts.html
23. PSC support: patient insights report. Accessed October 14, 2024. https://pscsupport.org.uk/surveys/insights-living-with-psc/
24. Key C, McKibben A, Chien E. A phase 1 dose-ranging study assessing fecal bile acid excretion by volixibat, an apical sodium‑dependent bile acid transporter inhibitor, and coadministration with loperamide. Poster presented at The Liver Meeting Digital Experience™ (TLMdX), American Association for the Study of Liver Diseases (AASLD); November 13-16, 2020.
US-DS-2400079 December 2024
Neither of the editors of GI & Hepatology News® nor the Editorial Advisory Board nor the reporting staff contributed to this content.
Faculty Disclosure: Dr. Kowdley has been paid consulting fees by Mirum.
Primary sclerosing cholangitis (PSC) is a rare, chronic, and progressive cholestatic liver disorder.1 Commonly associated with pruritus, an intense itch that significantly impacts patients’ lives, PSC is characterized by inflammation, fibrosis, and stricturing of the intrahepatic and/or extrahepatic bile ducts.1,2 The natural history of PSC is highly variable, but disease progression frequently leads to end-stage liver disease, with liver transplantation as the only currently available treatment option.1,2 PSC has a close association with inflammatory bowel disease (IBD), with approximately 60% to 80% of patients with PSC having a diagnosis of either ulcerative colitis or Crohn’s disease.1,3 Although the exact pathogenesis of PSC is still under investigation, evidence suggests a complex interplay of genetic susceptibility, immune dysregulation, and environmental factors may be responsible.4
PSC is considered a rare disease, with an estimated global median incidence of 0.7 to 0.8 per 100,000 and estimated prevalence of 10 cases per 100,000.5 PSC is more common in men (60% to 70%), with men having a 2-fold higher risk of developing PSC than women.2,6,7 The majority of patients are diagnosed between the ages of 30 to 40 years, with a median survival time after diagnosis without a liver transplant of 10 to 20 years.2,7-9
Signs and Symptoms of PSC
Approximately 50% of patients with PSC are asymptomatic when persistently abnormal liver function tests trigger further evaluation.1,2,10 Patients may complain of pruritus, which may be episodic; right upper quadrant pain; fatigue; and jaundice.2,7 Fevers, chills, and night sweats may also be present at the time of diagnosis.2
Pruritus and fatigue are common symptoms of PSC and can have a significant impact on the lives of patients.5 The pathogenesis of pruritus is complex and not completely understood but is believed to be caused by a toxic buildup of bile acids due to a decrease in bile flow related to inflammation, fibrosis, and stricturing resulting from PSC.11,12
Pruritus has been shown to have a substantial impact on patients’ health-related quality of life (QoL), with greater impairment seen with increased severity of pruritus.13 Specifically, patients with pruritus report physical limitations on QoL-specific questionnaires, as well as an impact on emotional, bodily pain, vitality, energy, and physical mobility measures.14
From a multinational survey on the impact of pruritus in PSC patients, 96% of respondents indicated that their itch was worst in the evening, with 58% indicating mood changes, including anxiety, irritability, and feelings of hopelessness due to their itch. Further, respondents reported that their pruritus disrupted their day-to-day responsibilities and that this disruption lasted 1 month or more.15
The psychological impact of living with PSC has not been well studied, although it has been found that individuals living with the disease demonstrated a greater number of depressive symptoms and poorer well-being, often coinciding with their stage of liver disease and comorbidity with IBD.16
In those living with PSC, mental health-related QoL has been shown to be influenced by liver disease, pruritus, social isolation, and depression. In one study, nearly 75% of patients expressed existential anxiety regarding disease progression and shortened life expectancy, with 25% disclosing social isolation.13
Diagnosing PSC
PSC should be considered in patients with a cholestatic pattern of liver test abnormalities, especially in those with underlying IBD. Abnormalities that may be detected on physical examination include jaundice, hepatomegaly, splenomegaly, and excoriations from scratching.3,5 PSC and autoimmune hepatitis (AIH) may coexist, particularly in younger patients, with serum biochemical tests and autoantibodies suggestive of AIH.2 Most patients demonstrate elevated serum alkaline phosphatase levels, as well as modest elevation of transaminases.2 Bilirubin and albumin levels may be normal at the time of diagnosis, although they may become increasingly abnormal as the disease progresses.2 A subset of patients (10%) may have elevated levels of immunoglobulin G4 (IgG4) and tend to progress more rapidly in the absence of treatment.2 Autoantibodies, which are characteristic of primary biliary cholangitis (PBC)—another rare, chronic, and progressive liver disease—are usually absent in PSC. When present, autoantibodies are of unknown clinical significance.2,17
Imaging, including cross-sectional imaging, particularly magnetic resonance cholangiopancreatography, is often used to the biliary tree in patients with persistently abnormal cholestatic tests.2 A diagnosis of PSC is typically established by the demonstration of characteristic multifocal stricturing and dilation of intrahepatic and/or extrahepatic bile ducts on cholangiography.5 The diagnosis of PSC is occasionally made on liver biopsy, which may reveal characteristic features of “onion skin fibrosis” and fibro-obliterative cholangitis when cholangiography is normal. In this circumstance, it is classified as “small-duct PSC.”5,18
Treatment and Management of PSC
Despite advances in our understanding of PSC, there are currently no approved drug therapies for PSC and no approved treatments for PSC-associated pruritus. The American Association for the Study of Liver Diseases (AASLD) published the most recent practice guidance for the treatment and management of PSC in 2022.7
Ursodeoxycholic acid (UDCA) has been widely studied as a potential PSC treatment. While UDCA demonstrates improvements in biochemical measures, there has been a lack of evidence demonstrating clinical improvement.19
The role of UDCA in the treatment of PSC is unclear and, at this time, is not supported by the American College of Gastroenterology or AASLD.2,7 Additional treatments, including immunosuppressive medications (methotrexate, tacrolimus), corticosteroids (prednisolone), and antibiotics (minocycline, vancomycin) have been explored but have not shown definitive clinical benefit.2
UDCA, if used, should not be prescribed at doses in excess of 20 mg/kg/day since high-dose UDCA (28-30 mg/kg) was associated with adverse liver outcomes.2
Although there are no therapies approved specifically to manage PSC-associated pruritus, cholestyramine and rifampin have been shown to be beneficial in relieving itch in some patients.22 In a survey of PSC patients, one in three reported suffering from pruritus during the previous week. It is possible that the prevalence and severity of pruritus in PSC may be under-recognized compared with PBC, given that patients and physicians may be focused on the many other medical issues that are often prioritized over symptoms, such as concern about cancer risk and need for frequent surveillance procedures.15,23 Discussions between patients and physicians are important to deepen our understanding of the prevalence of pruritus and its burden on the lives of patients.
Novel therapies for PSC and PSC-associated pruritus, including a selective inhibitor of the ileal bile acid transporter (IBAT), are currently being explored in clinical trials. Research suggests that the inhibition of IBAT blocks the recycling of bile acids, which reduces bile acids systemically and in the liver. Early clinical studies demonstrated on-target fecal bile acid excretion, a pharmacodynamic marker of IBAT inhibition, in addition to decreases in low-density lipoprotein cholesterol and increases in 7αC4, which are markers of bile acid synthesis.24
To learn more about ongoing clinical trials, please visit https://www.mirumclinicaltrials.com.
References
1. Karlsen TH, Folseraas T, Thorburn D, Vesterhus M. Primary sclerosing cholangitis – a comprehensive review. J Hepatol. 2017;67(6):1298-1323. doi:10.1016/j.jhep.2017.07.022
2. Lindor KD, Kowdley KV, Harrison EM. ACG clinical guideline: primary sclerosing cholangitis. Am. J. Gastroenterol. 110, 646–659 (2015).
3. Chapman R, Fevery J, Kalloo A, et al; American Association for the Study of Liver Diseases. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51(2):660-678. doi:10.1002/hep.23294
4. Jiang X, Karlsen TH. Genetics of primary sclerosing cholangitis and pathophysiological implications. Nat Rev Gastroenterol Hepatol. 2017;14(5):279-295. doi:10.1038/nrgastro.2016.154
5. Sohal A, Kayani S, Kowdley KV. Primary sclerosing cholangitis: epidemiology, diagnosis, and presentation. Clin Liver Dis. 2024;28(1):129-141. doi:10.1016/j.cld.2023.07.005
6. Molodecky NA, Kareemi H, Parab R, et al. Incidence of primary sclerosing cholangitis: a systematic review and meta-analysis. Hepatology. 2011;53(5):1590-1599. doi:10.1002/hep.24247
7. Bowlus CL, Arrivé L, Bergquist A, et al. AASLD practice guidance on primary sclerosing cholangitis and cholangiocarcinoma. Hepatology. 2023;77(2):659-702. doi:10.1002/hep.32771
8. Hirschfield GM, Karlsen TH, Lindor KD, Adams DH. Primary sclerosing cholangitis. Lancet. 2013;382(9904):1587-1599.
9. Trivedi PJ, Bowlus CL, Yimam KK, Razavi H, Estes C. Epidemiology, natural history, and outcomes of primary sclerosing cholangitis: a systematic review of population-based studies. Clin Gastroenterol Hepatol. 2022;20(8):1687-1700.e4. doi:10.1016/j.cgh.2021.08.039
10. Tischendorf JJ, Hecker H, Krüger M, Manns MP, Meier PN. Characterization, outcome, and prognosis in 273 patients with primary sclerosing cholangitis: a single center study. Am J Gastroenterol. 2007;102(1):107-114. doi:10.1111/j.1572-0241.2006.00872.x
11. Sanjel B, Shim WS. Recent advances in understanding the molecular mechanisms of cholestatic pruritus: a review. Biochim Biophys Acta Mol Basis Dis. 2020;1866(12):165958. doi:10.1016/j.bbadis.2020.16595
12. Patel SP, Vasavda C, Ho B, Meixiong J, Dong X, Kwatra SG. Cholestatic pruritus: emerging mechanisms and therapeutics. J Am Acad Dermatol. 2019;81(6):1371-1378. doi:10.1016/j.jaad.2019.04.035
13. Cheung AC, Patel H, Meza-Cardona J, Cino M, Sockalingam S, Hirschfield GM. Factors that influence health-related quality of life in patients with primary sclerosing cholangitis. Dig Dis Sci. 2016;61(6):1692-9. doi:10.1007/s10620-015-4013-1
14. Jin XY, Khan TM. Quality of life among patients suffering from cholestatic liver disease-induced pruritus: a systematic review. J Formos Med Assoc. 2016;115(9):689-702. doi:10.1016/j.jfma.2016.05.006
15. Kowdley K, et al. Impact of pruritus in primary sclerosing cholangitis (PSC): a multinational survey. J. Hepatol. 2022;(1)77:S312-S313.
16. Ranieri V, Kennedy E, Walmsley M, Thorburn D, McKay K. The Primary Sclerosing Cholangitis (PSC) Wellbeing Study: understanding psychological distress in those living with PSC and those who support them. PLoS One. 2020;15(7):e0234624.:10.1371/journal.pone.0234624
17. Hov JR, Boberg KM, Karlsen TH. Autoantibodies in primary sclerosing cholangitis. World J Gastroenterol. 2008;14(24):3781-91. doi:10.3748/wjg.14.3781
18. Cazzagon N, Sarcognato S, Catanzaro E, Bonaiuto E, Peviani M, Pezzato F, Motta R. Primary Sclerosing Cholangitis: Diagnostic Criteria. Tomography. 2024;10(1):47-65. doi:10.3390/tomography10010005
19. Lindor KD. Ursodiol for primary sclerosing cholangitis. Mayo Primary Sclerosing Cholangitis-Ursodeoxycholic Acid Study Group. N Engl J Med. 1997;336(10):691-695. doi:10.1056/NEJM199703063361003
20. Lee YM, Kaplan MM. Primary sclerosing cholangitis. N Engl J Med. 1995;332(14):924-33. doi:10.1056/NEJM199504063321406
21. Said K, Glaumann H, Bergquist A. Gallbladder disease in patients with primary sclerosing cholangitis. J Hepatol. 2008;48(4):598-605. doi:10.1016/j.jhep.2007.11.01
22. Basic PSC facts: basic facts. PSC Partners Seeking a Cure. Accessed October 14, 2024. https://pscpartners.org/about/the-disease/basic-facts.html
23. PSC support: patient insights report. Accessed October 14, 2024. https://pscsupport.org.uk/surveys/insights-living-with-psc/
24. Key C, McKibben A, Chien E. A phase 1 dose-ranging study assessing fecal bile acid excretion by volixibat, an apical sodium‑dependent bile acid transporter inhibitor, and coadministration with loperamide. Poster presented at The Liver Meeting Digital Experience™ (TLMdX), American Association for the Study of Liver Diseases (AASLD); November 13-16, 2020.
US-DS-2400079 December 2024
Neither of the editors of GI & Hepatology News® nor the Editorial Advisory Board nor the reporting staff contributed to this content.
Faculty Disclosure: Dr. Kowdley has been paid consulting fees by Mirum.
Primary sclerosing cholangitis (PSC) is a rare, chronic, and progressive cholestatic liver disorder.1 Commonly associated with pruritus, an intense itch that significantly impacts patients’ lives, PSC is characterized by inflammation, fibrosis, and stricturing of the intrahepatic and/or extrahepatic bile ducts.1,2 The natural history of PSC is highly variable, but disease progression frequently leads to end-stage liver disease, with liver transplantation as the only currently available treatment option.1,2 PSC has a close association with inflammatory bowel disease (IBD), with approximately 60% to 80% of patients with PSC having a diagnosis of either ulcerative colitis or Crohn’s disease.1,3 Although the exact pathogenesis of PSC is still under investigation, evidence suggests a complex interplay of genetic susceptibility, immune dysregulation, and environmental factors may be responsible.4
PSC is considered a rare disease, with an estimated global median incidence of 0.7 to 0.8 per 100,000 and estimated prevalence of 10 cases per 100,000.5 PSC is more common in men (60% to 70%), with men having a 2-fold higher risk of developing PSC than women.2,6,7 The majority of patients are diagnosed between the ages of 30 to 40 years, with a median survival time after diagnosis without a liver transplant of 10 to 20 years.2,7-9
Signs and Symptoms of PSC
Approximately 50% of patients with PSC are asymptomatic when persistently abnormal liver function tests trigger further evaluation.1,2,10 Patients may complain of pruritus, which may be episodic; right upper quadrant pain; fatigue; and jaundice.2,7 Fevers, chills, and night sweats may also be present at the time of diagnosis.2
Pruritus and fatigue are common symptoms of PSC and can have a significant impact on the lives of patients.5 The pathogenesis of pruritus is complex and not completely understood but is believed to be caused by a toxic buildup of bile acids due to a decrease in bile flow related to inflammation, fibrosis, and stricturing resulting from PSC.11,12
Pruritus has been shown to have a substantial impact on patients’ health-related quality of life (QoL), with greater impairment seen with increased severity of pruritus.13 Specifically, patients with pruritus report physical limitations on QoL-specific questionnaires, as well as an impact on emotional, bodily pain, vitality, energy, and physical mobility measures.14
From a multinational survey on the impact of pruritus in PSC patients, 96% of respondents indicated that their itch was worst in the evening, with 58% indicating mood changes, including anxiety, irritability, and feelings of hopelessness due to their itch. Further, respondents reported that their pruritus disrupted their day-to-day responsibilities and that this disruption lasted 1 month or more.15
The psychological impact of living with PSC has not been well studied, although it has been found that individuals living with the disease demonstrated a greater number of depressive symptoms and poorer well-being, often coinciding with their stage of liver disease and comorbidity with IBD.16
In those living with PSC, mental health-related QoL has been shown to be influenced by liver disease, pruritus, social isolation, and depression. In one study, nearly 75% of patients expressed existential anxiety regarding disease progression and shortened life expectancy, with 25% disclosing social isolation.13
Diagnosing PSC
PSC should be considered in patients with a cholestatic pattern of liver test abnormalities, especially in those with underlying IBD. Abnormalities that may be detected on physical examination include jaundice, hepatomegaly, splenomegaly, and excoriations from scratching.3,5 PSC and autoimmune hepatitis (AIH) may coexist, particularly in younger patients, with serum biochemical tests and autoantibodies suggestive of AIH.2 Most patients demonstrate elevated serum alkaline phosphatase levels, as well as modest elevation of transaminases.2 Bilirubin and albumin levels may be normal at the time of diagnosis, although they may become increasingly abnormal as the disease progresses.2 A subset of patients (10%) may have elevated levels of immunoglobulin G4 (IgG4) and tend to progress more rapidly in the absence of treatment.2 Autoantibodies, which are characteristic of primary biliary cholangitis (PBC)—another rare, chronic, and progressive liver disease—are usually absent in PSC. When present, autoantibodies are of unknown clinical significance.2,17
Imaging, including cross-sectional imaging, particularly magnetic resonance cholangiopancreatography, is often used to the biliary tree in patients with persistently abnormal cholestatic tests.2 A diagnosis of PSC is typically established by the demonstration of characteristic multifocal stricturing and dilation of intrahepatic and/or extrahepatic bile ducts on cholangiography.5 The diagnosis of PSC is occasionally made on liver biopsy, which may reveal characteristic features of “onion skin fibrosis” and fibro-obliterative cholangitis when cholangiography is normal. In this circumstance, it is classified as “small-duct PSC.”5,18
Treatment and Management of PSC
Despite advances in our understanding of PSC, there are currently no approved drug therapies for PSC and no approved treatments for PSC-associated pruritus. The American Association for the Study of Liver Diseases (AASLD) published the most recent practice guidance for the treatment and management of PSC in 2022.7
Ursodeoxycholic acid (UDCA) has been widely studied as a potential PSC treatment. While UDCA demonstrates improvements in biochemical measures, there has been a lack of evidence demonstrating clinical improvement.19
The role of UDCA in the treatment of PSC is unclear and, at this time, is not supported by the American College of Gastroenterology or AASLD.2,7 Additional treatments, including immunosuppressive medications (methotrexate, tacrolimus), corticosteroids (prednisolone), and antibiotics (minocycline, vancomycin) have been explored but have not shown definitive clinical benefit.2
UDCA, if used, should not be prescribed at doses in excess of 20 mg/kg/day since high-dose UDCA (28-30 mg/kg) was associated with adverse liver outcomes.2
Although there are no therapies approved specifically to manage PSC-associated pruritus, cholestyramine and rifampin have been shown to be beneficial in relieving itch in some patients.22 In a survey of PSC patients, one in three reported suffering from pruritus during the previous week. It is possible that the prevalence and severity of pruritus in PSC may be under-recognized compared with PBC, given that patients and physicians may be focused on the many other medical issues that are often prioritized over symptoms, such as concern about cancer risk and need for frequent surveillance procedures.15,23 Discussions between patients and physicians are important to deepen our understanding of the prevalence of pruritus and its burden on the lives of patients.
Novel therapies for PSC and PSC-associated pruritus, including a selective inhibitor of the ileal bile acid transporter (IBAT), are currently being explored in clinical trials. Research suggests that the inhibition of IBAT blocks the recycling of bile acids, which reduces bile acids systemically and in the liver. Early clinical studies demonstrated on-target fecal bile acid excretion, a pharmacodynamic marker of IBAT inhibition, in addition to decreases in low-density lipoprotein cholesterol and increases in 7αC4, which are markers of bile acid synthesis.24
To learn more about ongoing clinical trials, please visit https://www.mirumclinicaltrials.com.
References
1. Karlsen TH, Folseraas T, Thorburn D, Vesterhus M. Primary sclerosing cholangitis – a comprehensive review. J Hepatol. 2017;67(6):1298-1323. doi:10.1016/j.jhep.2017.07.022
2. Lindor KD, Kowdley KV, Harrison EM. ACG clinical guideline: primary sclerosing cholangitis. Am. J. Gastroenterol. 110, 646–659 (2015).
3. Chapman R, Fevery J, Kalloo A, et al; American Association for the Study of Liver Diseases. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51(2):660-678. doi:10.1002/hep.23294
4. Jiang X, Karlsen TH. Genetics of primary sclerosing cholangitis and pathophysiological implications. Nat Rev Gastroenterol Hepatol. 2017;14(5):279-295. doi:10.1038/nrgastro.2016.154
5. Sohal A, Kayani S, Kowdley KV. Primary sclerosing cholangitis: epidemiology, diagnosis, and presentation. Clin Liver Dis. 2024;28(1):129-141. doi:10.1016/j.cld.2023.07.005
6. Molodecky NA, Kareemi H, Parab R, et al. Incidence of primary sclerosing cholangitis: a systematic review and meta-analysis. Hepatology. 2011;53(5):1590-1599. doi:10.1002/hep.24247
7. Bowlus CL, Arrivé L, Bergquist A, et al. AASLD practice guidance on primary sclerosing cholangitis and cholangiocarcinoma. Hepatology. 2023;77(2):659-702. doi:10.1002/hep.32771
8. Hirschfield GM, Karlsen TH, Lindor KD, Adams DH. Primary sclerosing cholangitis. Lancet. 2013;382(9904):1587-1599.
9. Trivedi PJ, Bowlus CL, Yimam KK, Razavi H, Estes C. Epidemiology, natural history, and outcomes of primary sclerosing cholangitis: a systematic review of population-based studies. Clin Gastroenterol Hepatol. 2022;20(8):1687-1700.e4. doi:10.1016/j.cgh.2021.08.039
10. Tischendorf JJ, Hecker H, Krüger M, Manns MP, Meier PN. Characterization, outcome, and prognosis in 273 patients with primary sclerosing cholangitis: a single center study. Am J Gastroenterol. 2007;102(1):107-114. doi:10.1111/j.1572-0241.2006.00872.x
11. Sanjel B, Shim WS. Recent advances in understanding the molecular mechanisms of cholestatic pruritus: a review. Biochim Biophys Acta Mol Basis Dis. 2020;1866(12):165958. doi:10.1016/j.bbadis.2020.16595
12. Patel SP, Vasavda C, Ho B, Meixiong J, Dong X, Kwatra SG. Cholestatic pruritus: emerging mechanisms and therapeutics. J Am Acad Dermatol. 2019;81(6):1371-1378. doi:10.1016/j.jaad.2019.04.035
13. Cheung AC, Patel H, Meza-Cardona J, Cino M, Sockalingam S, Hirschfield GM. Factors that influence health-related quality of life in patients with primary sclerosing cholangitis. Dig Dis Sci. 2016;61(6):1692-9. doi:10.1007/s10620-015-4013-1
14. Jin XY, Khan TM. Quality of life among patients suffering from cholestatic liver disease-induced pruritus: a systematic review. J Formos Med Assoc. 2016;115(9):689-702. doi:10.1016/j.jfma.2016.05.006
15. Kowdley K, et al. Impact of pruritus in primary sclerosing cholangitis (PSC): a multinational survey. J. Hepatol. 2022;(1)77:S312-S313.
16. Ranieri V, Kennedy E, Walmsley M, Thorburn D, McKay K. The Primary Sclerosing Cholangitis (PSC) Wellbeing Study: understanding psychological distress in those living with PSC and those who support them. PLoS One. 2020;15(7):e0234624.:10.1371/journal.pone.0234624
17. Hov JR, Boberg KM, Karlsen TH. Autoantibodies in primary sclerosing cholangitis. World J Gastroenterol. 2008;14(24):3781-91. doi:10.3748/wjg.14.3781
18. Cazzagon N, Sarcognato S, Catanzaro E, Bonaiuto E, Peviani M, Pezzato F, Motta R. Primary Sclerosing Cholangitis: Diagnostic Criteria. Tomography. 2024;10(1):47-65. doi:10.3390/tomography10010005
19. Lindor KD. Ursodiol for primary sclerosing cholangitis. Mayo Primary Sclerosing Cholangitis-Ursodeoxycholic Acid Study Group. N Engl J Med. 1997;336(10):691-695. doi:10.1056/NEJM199703063361003
20. Lee YM, Kaplan MM. Primary sclerosing cholangitis. N Engl J Med. 1995;332(14):924-33. doi:10.1056/NEJM199504063321406
21. Said K, Glaumann H, Bergquist A. Gallbladder disease in patients with primary sclerosing cholangitis. J Hepatol. 2008;48(4):598-605. doi:10.1016/j.jhep.2007.11.01
22. Basic PSC facts: basic facts. PSC Partners Seeking a Cure. Accessed October 14, 2024. https://pscpartners.org/about/the-disease/basic-facts.html
23. PSC support: patient insights report. Accessed October 14, 2024. https://pscsupport.org.uk/surveys/insights-living-with-psc/
24. Key C, McKibben A, Chien E. A phase 1 dose-ranging study assessing fecal bile acid excretion by volixibat, an apical sodium‑dependent bile acid transporter inhibitor, and coadministration with loperamide. Poster presented at The Liver Meeting Digital Experience™ (TLMdX), American Association for the Study of Liver Diseases (AASLD); November 13-16, 2020.
US-DS-2400079 December 2024
Neither of the editors of GI & Hepatology News® nor the Editorial Advisory Board nor the reporting staff contributed to this content.
Faculty Disclosure: Dr. Kowdley has been paid consulting fees by Mirum.
Revolutionizing Headache Medicine: The Role of Artificial Intelligence
As we move further into the 21st century, technology continues to revolutionize various facets of our lives. Healthcare is a prime example. Advances in technology have dramatically reshaped the way we develop medications, diagnose diseases, and enhance patient care. The rise of artificial intelligence (AI) and the widespread adoption of digital health technologies have marked a significant milestone in improving the quality of care. AI, with its ability to leverage algorithms, deep learning, and machine learning to process data, make decisions, and perform tasks autonomously, is becoming an integral part of modern society. It is embedded in various technologies that we rely on daily, from smartphones and smart home devices to content recommendations on streaming services and social media platforms.
In healthcare, AI has applications in numerous fields, such as radiology. AI streamlines processes such as organizing patient appointments, optimizing radiation protocols for safety and efficiency, and enhancing the documentation process through advanced image analysis. AI technology plays an integral role in imaging tasks like image enhancement, lesion detection, and precise measurement. In difficult-to-interpret radiologic studies, such as some mammography images, it can be a crucial aid to the radiologist. Additionally, the use of AI has significantly improved remote patient monitoring that enables healthcare professionals to monitor and assess patient conditions without needing in-person visits. Remote patient monitoring gained prominence during the COVID-19 pandemic and continues to be a valuable tool in post pandemic care. Study results have highlighted that AI-driven ambient dictation tools have increased provider engagement with patients during consultations while reducing the time spent documenting in electronic health records.
Like many other medical specialties, headache medicine also uses AI. Most prominently, AI has been used in models and engines in assisting with headache diagnoses. A noteworthy example of AI in headache medicine is the development of an online, computer-based diagnostic engine (CDE) by Rapoport et al, called BonTriage. This tool is designed to diagnose headaches by employing a rule set based on the International Classification of Headache Disorders-3 (ICHD-3) criteria for primary headache disorders while also evaluating secondary headaches and medication overuse headaches. By leveraging machine learning, the CDE has the potential to streamline the diagnostic process, reducing the number of questions needed to reach a diagnosis and making the experience more efficient. This information can then be printed as a PDF file and taken by the patient to a healthcare professional for further discussion, fostering a more accurate, fluid, and conversational consultation.
A study was conducted to evaluate the accuracy of the CDE. Participants were randomly assigned to 1 of 2 sequences: (1) using the CDE followed by a structured standard interview with a headache specialist using the same ICHD-3 criteria or (2) starting with the structured standard interview followed by the CDE. The results demonstrated nearly perfect agreement in diagnosing migraine and probable migraine between the CDE and structured standard interview (κ = 0.82, 95% CI: 0.74, 0.90). The CDE demonstrated a diagnostic accuracy of 91.6% (95% CI: 86.9%, 95.0%), a sensitivity rate of 89.0% (95% CI: 82.5%, 93.7%), and a specificity rate of 97.0% (95% CI: 89.5%, 99.6%).
A diagnostic engine such as this can save time that clinicians spend on documentation and allow more time for discussion with the patient. For instance, a patient can take the printout received from the CDE to an appointment; the printout gives a detailed history plus information about social and psychological issues, a list of medications taken, and results of previous testing. The CDE system was originally designed to help patients see a specialist in the environment of a nationwide lack of headache specialists. There are currently 45 million patients with headaches who are seeking treatment with only around 550 certified headache specialists in the United States. The CDE printed information can help a patient obtain a consultation from a clinician quickly and start evaluation and treatment earlier. This expert online consultation is currently free of charge.
Kwon et al developed a machine learning–based model designed to automatically classify headache disorders using data from a questionnaire. Their model was able to predict diagnoses for conditions such as migraine, tension-type headaches, trigeminal autonomic cephalalgia, epicranial headache, and thunderclap headaches. The model was trained on data from 2162 patients, all diagnosed by headache specialists, and achieved an overall accuracy of 81%, with a sensitivity of 88% and a specificity of 95% for diagnosing migraines. However, the model’s performance was less robust when applied to other headache disorders.
Katsuki et al developed an AI model to help non specialists accurately diagnose headaches. This model analyzed 17 variables and was trained on data from 2800 patients, with additional testing and refinement using data from another 200 patients. To evaluate its effectiveness, 2 groups of non-headache specialists each assessed 50 patients: 1 group relied solely on their expertise, while the other used the AI model. The group without AI assistance achieved an overall accuracy of 46% (κ = 0.21), while the group using the AI model significantly improved, reaching an overall accuracy of 83.2% (κ = 0.68).
Building on their work with AI for diagnosing headaches, Katsuki et al conducted a study using a smartphone application that tracked user-reported headache events alongside local weather data. The AI model revealed that lower barometric pressure, higher humidity, and increased rainfall were linked to the onset of headache attacks. The application also identified triggers for headaches in specific weather patterns, such as a drop in barometric pressure noted 6 hours before headache onset. The application of AI in monitoring weather changes could be crucial, especially given concerns that the rising frequency of severe weather events due to climate change may be exacerbating the severity and burden of migraine. Additionally, recent post hoc analyses of fremanezumab clinical trials have provided further evidence that weather changes can trigger headaches.
Rapoport and colleagues have also developed an application called Migraine Mentor, which accurately tracks headaches, triggers, health data, and response to medication on a smartphone. The patient spends 3 minutes a day answering a few questions about their day and whether they had a headache or took any medication. At 1 or 2 months, Migraine Mentor can generate a detailed report with data and current trends that is sent to the patient, which the patient can then share with the clinician. The application also reminds patients when to document data and take medication.
However, although the use of AI in headache medicine appears promising, caution must be exercised to ensure proper results and information are disseminated. One rapidly expanding application of AI is the widely popular ChatGPT. ChatGPT, which stands for generative pretraining transformer, is a type of large language model (LLM). An LLM is a deep learning algorithm designed to recognize, translate, predict, summarize, and generate text responses based on a given prompt. This model is trained on an extensive dataset that includes a diverse array of books, articles, and websites, exposing it to various language structures and styles. This training enables ChatGPT to generate responses that closely mimic human communication. LLMs are being used more and more in medicine to assist with generating patient documentation and educational materials.
However, Dr Fred Cohen published a perspective piece detailing how LLMs (such as ChatGPT) can produce misleading and inaccurate answers. In his example, he tasked ChatGPT to describe the epidemiology of migraines in penguins; the AI model generated a well-written and highly believable manuscript titled, “Migraine Under the Ice: Understanding Headaches in Antarctica's Feathered Friends.” The manuscript highlights that migraines are more prevalent in male penguins compared to females, with the peak age of onset occurring between 4 and 5 years. Additionally, emperor and king penguins are identified as being more susceptible to developing migraines compared to other penguin species. The paper was fictitious (as no studies on migraine in penguins have been written to date), exemplifying that these models can produce nonfactual materials.
For years, technological advancements have been reshaping many aspects of life, and medicine is no exception. AI has been successfully applied to streamline medical documentation, develop new drug targets, and deepen our understanding of various diseases. The field of headache medicine now also uses AI. Recent developments show significant promise, with AI aiding in the diagnosis of migraine and other headache disorders. AI models have even been used in the identification of potential drug targets for migraine treatment. Although there are still limitations to overcome, the future of AI in headache medicine appears bright.
If you would like to read more about Dr. Cohen’s work on AI and migraine, please visit fredcohenmd.com or TikTok @fredcohenmd.
As we move further into the 21st century, technology continues to revolutionize various facets of our lives. Healthcare is a prime example. Advances in technology have dramatically reshaped the way we develop medications, diagnose diseases, and enhance patient care. The rise of artificial intelligence (AI) and the widespread adoption of digital health technologies have marked a significant milestone in improving the quality of care. AI, with its ability to leverage algorithms, deep learning, and machine learning to process data, make decisions, and perform tasks autonomously, is becoming an integral part of modern society. It is embedded in various technologies that we rely on daily, from smartphones and smart home devices to content recommendations on streaming services and social media platforms.
In healthcare, AI has applications in numerous fields, such as radiology. AI streamlines processes such as organizing patient appointments, optimizing radiation protocols for safety and efficiency, and enhancing the documentation process through advanced image analysis. AI technology plays an integral role in imaging tasks like image enhancement, lesion detection, and precise measurement. In difficult-to-interpret radiologic studies, such as some mammography images, it can be a crucial aid to the radiologist. Additionally, the use of AI has significantly improved remote patient monitoring that enables healthcare professionals to monitor and assess patient conditions without needing in-person visits. Remote patient monitoring gained prominence during the COVID-19 pandemic and continues to be a valuable tool in post pandemic care. Study results have highlighted that AI-driven ambient dictation tools have increased provider engagement with patients during consultations while reducing the time spent documenting in electronic health records.
Like many other medical specialties, headache medicine also uses AI. Most prominently, AI has been used in models and engines in assisting with headache diagnoses. A noteworthy example of AI in headache medicine is the development of an online, computer-based diagnostic engine (CDE) by Rapoport et al, called BonTriage. This tool is designed to diagnose headaches by employing a rule set based on the International Classification of Headache Disorders-3 (ICHD-3) criteria for primary headache disorders while also evaluating secondary headaches and medication overuse headaches. By leveraging machine learning, the CDE has the potential to streamline the diagnostic process, reducing the number of questions needed to reach a diagnosis and making the experience more efficient. This information can then be printed as a PDF file and taken by the patient to a healthcare professional for further discussion, fostering a more accurate, fluid, and conversational consultation.
A study was conducted to evaluate the accuracy of the CDE. Participants were randomly assigned to 1 of 2 sequences: (1) using the CDE followed by a structured standard interview with a headache specialist using the same ICHD-3 criteria or (2) starting with the structured standard interview followed by the CDE. The results demonstrated nearly perfect agreement in diagnosing migraine and probable migraine between the CDE and structured standard interview (κ = 0.82, 95% CI: 0.74, 0.90). The CDE demonstrated a diagnostic accuracy of 91.6% (95% CI: 86.9%, 95.0%), a sensitivity rate of 89.0% (95% CI: 82.5%, 93.7%), and a specificity rate of 97.0% (95% CI: 89.5%, 99.6%).
A diagnostic engine such as this can save time that clinicians spend on documentation and allow more time for discussion with the patient. For instance, a patient can take the printout received from the CDE to an appointment; the printout gives a detailed history plus information about social and psychological issues, a list of medications taken, and results of previous testing. The CDE system was originally designed to help patients see a specialist in the environment of a nationwide lack of headache specialists. There are currently 45 million patients with headaches who are seeking treatment with only around 550 certified headache specialists in the United States. The CDE printed information can help a patient obtain a consultation from a clinician quickly and start evaluation and treatment earlier. This expert online consultation is currently free of charge.
Kwon et al developed a machine learning–based model designed to automatically classify headache disorders using data from a questionnaire. Their model was able to predict diagnoses for conditions such as migraine, tension-type headaches, trigeminal autonomic cephalalgia, epicranial headache, and thunderclap headaches. The model was trained on data from 2162 patients, all diagnosed by headache specialists, and achieved an overall accuracy of 81%, with a sensitivity of 88% and a specificity of 95% for diagnosing migraines. However, the model’s performance was less robust when applied to other headache disorders.
Katsuki et al developed an AI model to help non specialists accurately diagnose headaches. This model analyzed 17 variables and was trained on data from 2800 patients, with additional testing and refinement using data from another 200 patients. To evaluate its effectiveness, 2 groups of non-headache specialists each assessed 50 patients: 1 group relied solely on their expertise, while the other used the AI model. The group without AI assistance achieved an overall accuracy of 46% (κ = 0.21), while the group using the AI model significantly improved, reaching an overall accuracy of 83.2% (κ = 0.68).
Building on their work with AI for diagnosing headaches, Katsuki et al conducted a study using a smartphone application that tracked user-reported headache events alongside local weather data. The AI model revealed that lower barometric pressure, higher humidity, and increased rainfall were linked to the onset of headache attacks. The application also identified triggers for headaches in specific weather patterns, such as a drop in barometric pressure noted 6 hours before headache onset. The application of AI in monitoring weather changes could be crucial, especially given concerns that the rising frequency of severe weather events due to climate change may be exacerbating the severity and burden of migraine. Additionally, recent post hoc analyses of fremanezumab clinical trials have provided further evidence that weather changes can trigger headaches.
Rapoport and colleagues have also developed an application called Migraine Mentor, which accurately tracks headaches, triggers, health data, and response to medication on a smartphone. The patient spends 3 minutes a day answering a few questions about their day and whether they had a headache or took any medication. At 1 or 2 months, Migraine Mentor can generate a detailed report with data and current trends that is sent to the patient, which the patient can then share with the clinician. The application also reminds patients when to document data and take medication.
However, although the use of AI in headache medicine appears promising, caution must be exercised to ensure proper results and information are disseminated. One rapidly expanding application of AI is the widely popular ChatGPT. ChatGPT, which stands for generative pretraining transformer, is a type of large language model (LLM). An LLM is a deep learning algorithm designed to recognize, translate, predict, summarize, and generate text responses based on a given prompt. This model is trained on an extensive dataset that includes a diverse array of books, articles, and websites, exposing it to various language structures and styles. This training enables ChatGPT to generate responses that closely mimic human communication. LLMs are being used more and more in medicine to assist with generating patient documentation and educational materials.
However, Dr Fred Cohen published a perspective piece detailing how LLMs (such as ChatGPT) can produce misleading and inaccurate answers. In his example, he tasked ChatGPT to describe the epidemiology of migraines in penguins; the AI model generated a well-written and highly believable manuscript titled, “Migraine Under the Ice: Understanding Headaches in Antarctica's Feathered Friends.” The manuscript highlights that migraines are more prevalent in male penguins compared to females, with the peak age of onset occurring between 4 and 5 years. Additionally, emperor and king penguins are identified as being more susceptible to developing migraines compared to other penguin species. The paper was fictitious (as no studies on migraine in penguins have been written to date), exemplifying that these models can produce nonfactual materials.
For years, technological advancements have been reshaping many aspects of life, and medicine is no exception. AI has been successfully applied to streamline medical documentation, develop new drug targets, and deepen our understanding of various diseases. The field of headache medicine now also uses AI. Recent developments show significant promise, with AI aiding in the diagnosis of migraine and other headache disorders. AI models have even been used in the identification of potential drug targets for migraine treatment. Although there are still limitations to overcome, the future of AI in headache medicine appears bright.
If you would like to read more about Dr. Cohen’s work on AI and migraine, please visit fredcohenmd.com or TikTok @fredcohenmd.
As we move further into the 21st century, technology continues to revolutionize various facets of our lives. Healthcare is a prime example. Advances in technology have dramatically reshaped the way we develop medications, diagnose diseases, and enhance patient care. The rise of artificial intelligence (AI) and the widespread adoption of digital health technologies have marked a significant milestone in improving the quality of care. AI, with its ability to leverage algorithms, deep learning, and machine learning to process data, make decisions, and perform tasks autonomously, is becoming an integral part of modern society. It is embedded in various technologies that we rely on daily, from smartphones and smart home devices to content recommendations on streaming services and social media platforms.
In healthcare, AI has applications in numerous fields, such as radiology. AI streamlines processes such as organizing patient appointments, optimizing radiation protocols for safety and efficiency, and enhancing the documentation process through advanced image analysis. AI technology plays an integral role in imaging tasks like image enhancement, lesion detection, and precise measurement. In difficult-to-interpret radiologic studies, such as some mammography images, it can be a crucial aid to the radiologist. Additionally, the use of AI has significantly improved remote patient monitoring that enables healthcare professionals to monitor and assess patient conditions without needing in-person visits. Remote patient monitoring gained prominence during the COVID-19 pandemic and continues to be a valuable tool in post pandemic care. Study results have highlighted that AI-driven ambient dictation tools have increased provider engagement with patients during consultations while reducing the time spent documenting in electronic health records.
Like many other medical specialties, headache medicine also uses AI. Most prominently, AI has been used in models and engines in assisting with headache diagnoses. A noteworthy example of AI in headache medicine is the development of an online, computer-based diagnostic engine (CDE) by Rapoport et al, called BonTriage. This tool is designed to diagnose headaches by employing a rule set based on the International Classification of Headache Disorders-3 (ICHD-3) criteria for primary headache disorders while also evaluating secondary headaches and medication overuse headaches. By leveraging machine learning, the CDE has the potential to streamline the diagnostic process, reducing the number of questions needed to reach a diagnosis and making the experience more efficient. This information can then be printed as a PDF file and taken by the patient to a healthcare professional for further discussion, fostering a more accurate, fluid, and conversational consultation.
A study was conducted to evaluate the accuracy of the CDE. Participants were randomly assigned to 1 of 2 sequences: (1) using the CDE followed by a structured standard interview with a headache specialist using the same ICHD-3 criteria or (2) starting with the structured standard interview followed by the CDE. The results demonstrated nearly perfect agreement in diagnosing migraine and probable migraine between the CDE and structured standard interview (κ = 0.82, 95% CI: 0.74, 0.90). The CDE demonstrated a diagnostic accuracy of 91.6% (95% CI: 86.9%, 95.0%), a sensitivity rate of 89.0% (95% CI: 82.5%, 93.7%), and a specificity rate of 97.0% (95% CI: 89.5%, 99.6%).
A diagnostic engine such as this can save time that clinicians spend on documentation and allow more time for discussion with the patient. For instance, a patient can take the printout received from the CDE to an appointment; the printout gives a detailed history plus information about social and psychological issues, a list of medications taken, and results of previous testing. The CDE system was originally designed to help patients see a specialist in the environment of a nationwide lack of headache specialists. There are currently 45 million patients with headaches who are seeking treatment with only around 550 certified headache specialists in the United States. The CDE printed information can help a patient obtain a consultation from a clinician quickly and start evaluation and treatment earlier. This expert online consultation is currently free of charge.
Kwon et al developed a machine learning–based model designed to automatically classify headache disorders using data from a questionnaire. Their model was able to predict diagnoses for conditions such as migraine, tension-type headaches, trigeminal autonomic cephalalgia, epicranial headache, and thunderclap headaches. The model was trained on data from 2162 patients, all diagnosed by headache specialists, and achieved an overall accuracy of 81%, with a sensitivity of 88% and a specificity of 95% for diagnosing migraines. However, the model’s performance was less robust when applied to other headache disorders.
Katsuki et al developed an AI model to help non specialists accurately diagnose headaches. This model analyzed 17 variables and was trained on data from 2800 patients, with additional testing and refinement using data from another 200 patients. To evaluate its effectiveness, 2 groups of non-headache specialists each assessed 50 patients: 1 group relied solely on their expertise, while the other used the AI model. The group without AI assistance achieved an overall accuracy of 46% (κ = 0.21), while the group using the AI model significantly improved, reaching an overall accuracy of 83.2% (κ = 0.68).
Building on their work with AI for diagnosing headaches, Katsuki et al conducted a study using a smartphone application that tracked user-reported headache events alongside local weather data. The AI model revealed that lower barometric pressure, higher humidity, and increased rainfall were linked to the onset of headache attacks. The application also identified triggers for headaches in specific weather patterns, such as a drop in barometric pressure noted 6 hours before headache onset. The application of AI in monitoring weather changes could be crucial, especially given concerns that the rising frequency of severe weather events due to climate change may be exacerbating the severity and burden of migraine. Additionally, recent post hoc analyses of fremanezumab clinical trials have provided further evidence that weather changes can trigger headaches.
Rapoport and colleagues have also developed an application called Migraine Mentor, which accurately tracks headaches, triggers, health data, and response to medication on a smartphone. The patient spends 3 minutes a day answering a few questions about their day and whether they had a headache or took any medication. At 1 or 2 months, Migraine Mentor can generate a detailed report with data and current trends that is sent to the patient, which the patient can then share with the clinician. The application also reminds patients when to document data and take medication.
However, although the use of AI in headache medicine appears promising, caution must be exercised to ensure proper results and information are disseminated. One rapidly expanding application of AI is the widely popular ChatGPT. ChatGPT, which stands for generative pretraining transformer, is a type of large language model (LLM). An LLM is a deep learning algorithm designed to recognize, translate, predict, summarize, and generate text responses based on a given prompt. This model is trained on an extensive dataset that includes a diverse array of books, articles, and websites, exposing it to various language structures and styles. This training enables ChatGPT to generate responses that closely mimic human communication. LLMs are being used more and more in medicine to assist with generating patient documentation and educational materials.
However, Dr Fred Cohen published a perspective piece detailing how LLMs (such as ChatGPT) can produce misleading and inaccurate answers. In his example, he tasked ChatGPT to describe the epidemiology of migraines in penguins; the AI model generated a well-written and highly believable manuscript titled, “Migraine Under the Ice: Understanding Headaches in Antarctica's Feathered Friends.” The manuscript highlights that migraines are more prevalent in male penguins compared to females, with the peak age of onset occurring between 4 and 5 years. Additionally, emperor and king penguins are identified as being more susceptible to developing migraines compared to other penguin species. The paper was fictitious (as no studies on migraine in penguins have been written to date), exemplifying that these models can produce nonfactual materials.
For years, technological advancements have been reshaping many aspects of life, and medicine is no exception. AI has been successfully applied to streamline medical documentation, develop new drug targets, and deepen our understanding of various diseases. The field of headache medicine now also uses AI. Recent developments show significant promise, with AI aiding in the diagnosis of migraine and other headache disorders. AI models have even been used in the identification of potential drug targets for migraine treatment. Although there are still limitations to overcome, the future of AI in headache medicine appears bright.
If you would like to read more about Dr. Cohen’s work on AI and migraine, please visit fredcohenmd.com or TikTok @fredcohenmd.
A Clonal Complete Remission Induced by IDH1 Inhibitor Ivosidenib in a Myelodysplastic Syndrome (MDS) With Co-Mutations of IDH1 and the ZRSR2 RNA Splicing Gene
Background
IDH1 mutations are detected in 3-4% of MDS, nearly always with one or more co-mutations. Treatment with IDH1 inhibitor ivosidenib typically resulted in regression of the abnormal clone in 15 reported responders. However, in a few cases differentiation was restored from the abnormal clone. Here we report a durable MDS remission despite sustained proliferation of a clone with IDH1 and ZRSR2 mutations.
Case Presentation
A 49-year-old man developed severe neutropenia and macrocytic anemia in January 2019. Mild marrow dysplasia developed by March 2020 with IDH1 (31.1%) and splicing gene ZRSR2 (55.7%) mutations. In October 2022 biopsy showed MDS with 4% blasts, megakaryocytic/granulocytic hypoplasia, normal cytogenetics and 43% IDH1/89% ZRSR2. After azacytidine failure, ivosidenib was started in November 2023 following FDA approval. Within weeks ANCs increased from 170 to 1580 and hemoglobin from 7.9 to 11.6 with MCV 115, reticulocytes 1.72%. At 3 months a CBC was normal except for MCV 111. IDH1 and ZRSR2 were 36.4% and 71%. After 6 months, ANC was 2380, hemoglobin 14.7, MCV 108.6, reticulo-cytes 1.77%. IDH1 PCR showed a 33.1% allele frequency consistent with a clonal remission.
Discussion
IDH1 mutations in MDS/AML frequently co-occur with mutations in RNA splicing genes SRSF2 or ZRSR2. For ZRSR2, we previously reported that isolated mutations of this gene cause refractory macrocytic anemias without dysplasia, thus presenting as clonal cytopenias of undetermined significance (Fleischman et al., Leuk Res, 2017). In this MDS case, after ivosidenib treatment the ZRSR2 splicing defect sustained clonal dominance over polyclonal hematopoiesis while accounting for macrocytosis. Longitudinal data for two ivosidenib-treated IDH1/SRSF2 MDS cases are incomplete, but one case of IDH2/SRSF2 MDS treated with the inhibitor enasidenib similarly achieved complete remission without regression of the mutated clone for 12 months.
Conclusions
Following the FDA approval of ivosidenib, all cases of MDS should have DNA sequencing performed at diagnosis to identify IDH1 mutations. Treatment induces high rates of remission even when polyclonal hematopoiesis does not recover. Moreover, the restoration of hematopoietic differentiation by the abnormal clone provides unique insights into the clinical phenotype and fitness advantage conferred by the co-existing driver mutations.
Background
IDH1 mutations are detected in 3-4% of MDS, nearly always with one or more co-mutations. Treatment with IDH1 inhibitor ivosidenib typically resulted in regression of the abnormal clone in 15 reported responders. However, in a few cases differentiation was restored from the abnormal clone. Here we report a durable MDS remission despite sustained proliferation of a clone with IDH1 and ZRSR2 mutations.
Case Presentation
A 49-year-old man developed severe neutropenia and macrocytic anemia in January 2019. Mild marrow dysplasia developed by March 2020 with IDH1 (31.1%) and splicing gene ZRSR2 (55.7%) mutations. In October 2022 biopsy showed MDS with 4% blasts, megakaryocytic/granulocytic hypoplasia, normal cytogenetics and 43% IDH1/89% ZRSR2. After azacytidine failure, ivosidenib was started in November 2023 following FDA approval. Within weeks ANCs increased from 170 to 1580 and hemoglobin from 7.9 to 11.6 with MCV 115, reticulocytes 1.72%. At 3 months a CBC was normal except for MCV 111. IDH1 and ZRSR2 were 36.4% and 71%. After 6 months, ANC was 2380, hemoglobin 14.7, MCV 108.6, reticulo-cytes 1.77%. IDH1 PCR showed a 33.1% allele frequency consistent with a clonal remission.
Discussion
IDH1 mutations in MDS/AML frequently co-occur with mutations in RNA splicing genes SRSF2 or ZRSR2. For ZRSR2, we previously reported that isolated mutations of this gene cause refractory macrocytic anemias without dysplasia, thus presenting as clonal cytopenias of undetermined significance (Fleischman et al., Leuk Res, 2017). In this MDS case, after ivosidenib treatment the ZRSR2 splicing defect sustained clonal dominance over polyclonal hematopoiesis while accounting for macrocytosis. Longitudinal data for two ivosidenib-treated IDH1/SRSF2 MDS cases are incomplete, but one case of IDH2/SRSF2 MDS treated with the inhibitor enasidenib similarly achieved complete remission without regression of the mutated clone for 12 months.
Conclusions
Following the FDA approval of ivosidenib, all cases of MDS should have DNA sequencing performed at diagnosis to identify IDH1 mutations. Treatment induces high rates of remission even when polyclonal hematopoiesis does not recover. Moreover, the restoration of hematopoietic differentiation by the abnormal clone provides unique insights into the clinical phenotype and fitness advantage conferred by the co-existing driver mutations.
Background
IDH1 mutations are detected in 3-4% of MDS, nearly always with one or more co-mutations. Treatment with IDH1 inhibitor ivosidenib typically resulted in regression of the abnormal clone in 15 reported responders. However, in a few cases differentiation was restored from the abnormal clone. Here we report a durable MDS remission despite sustained proliferation of a clone with IDH1 and ZRSR2 mutations.
Case Presentation
A 49-year-old man developed severe neutropenia and macrocytic anemia in January 2019. Mild marrow dysplasia developed by March 2020 with IDH1 (31.1%) and splicing gene ZRSR2 (55.7%) mutations. In October 2022 biopsy showed MDS with 4% blasts, megakaryocytic/granulocytic hypoplasia, normal cytogenetics and 43% IDH1/89% ZRSR2. After azacytidine failure, ivosidenib was started in November 2023 following FDA approval. Within weeks ANCs increased from 170 to 1580 and hemoglobin from 7.9 to 11.6 with MCV 115, reticulocytes 1.72%. At 3 months a CBC was normal except for MCV 111. IDH1 and ZRSR2 were 36.4% and 71%. After 6 months, ANC was 2380, hemoglobin 14.7, MCV 108.6, reticulo-cytes 1.77%. IDH1 PCR showed a 33.1% allele frequency consistent with a clonal remission.
Discussion
IDH1 mutations in MDS/AML frequently co-occur with mutations in RNA splicing genes SRSF2 or ZRSR2. For ZRSR2, we previously reported that isolated mutations of this gene cause refractory macrocytic anemias without dysplasia, thus presenting as clonal cytopenias of undetermined significance (Fleischman et al., Leuk Res, 2017). In this MDS case, after ivosidenib treatment the ZRSR2 splicing defect sustained clonal dominance over polyclonal hematopoiesis while accounting for macrocytosis. Longitudinal data for two ivosidenib-treated IDH1/SRSF2 MDS cases are incomplete, but one case of IDH2/SRSF2 MDS treated with the inhibitor enasidenib similarly achieved complete remission without regression of the mutated clone for 12 months.
Conclusions
Following the FDA approval of ivosidenib, all cases of MDS should have DNA sequencing performed at diagnosis to identify IDH1 mutations. Treatment induces high rates of remission even when polyclonal hematopoiesis does not recover. Moreover, the restoration of hematopoietic differentiation by the abnormal clone provides unique insights into the clinical phenotype and fitness advantage conferred by the co-existing driver mutations.
Creating a Urology Prostate Cancer Note, a National Oncology and Surgery Office Collaboration for Prostate Cancer Clinical Pathway Utilization
Background
Prostate cancer is the most common non-cutaneous malignancy diagnosis within the Department of Veterans Affairs (VA). The Prostate Cancer Clinical Pathways (PCCP) were developed to enable providers to treat all Veterans with prostate cancer at subject matter expert level.
Discussion
The PCCP was launched in February 2021; however, provider documentation of PCCP is variable across the VA healthcare system and within the PCCP, specific flow maps have differential use. For example, the Very Low Risk flow map has seven unique Veterans entered, whereas the Molecular Testing flow map has over 3,900 unique Veterans entered. One clear reason for this disparity in pathway documentation use is that local prostate cancer is managed by urology and their documentation of the PCCP is not as widespread as the medical oncologists. The National Oncology Program developed clinical note templates to document PCCP that medical oncologist use which has increased utilization. To increase urology specific flow map use, a collaboration between the National Surgery Office and National Oncology Program was established to develop a Urology Prostate Cancer Note (UPCN). The UPCN was designed by urologists with assistance from a medical oncologist and a clinical applications coordinator. The UPCN will function as a working clinical note for urologists and has the PCCPs embedded into reminder dialog templates, which when completed generate health factors. The health factors that are generated from the UPCN are data mined to record PCCP use and to perform data analytics. The UPCN is in the testing phase at three pilot test sites and is scheduled to be deployed summer 2024. The collaborative effort is aligned with the VHA directives outlined in the Cleland Dole Act.
Background
Prostate cancer is the most common non-cutaneous malignancy diagnosis within the Department of Veterans Affairs (VA). The Prostate Cancer Clinical Pathways (PCCP) were developed to enable providers to treat all Veterans with prostate cancer at subject matter expert level.
Discussion
The PCCP was launched in February 2021; however, provider documentation of PCCP is variable across the VA healthcare system and within the PCCP, specific flow maps have differential use. For example, the Very Low Risk flow map has seven unique Veterans entered, whereas the Molecular Testing flow map has over 3,900 unique Veterans entered. One clear reason for this disparity in pathway documentation use is that local prostate cancer is managed by urology and their documentation of the PCCP is not as widespread as the medical oncologists. The National Oncology Program developed clinical note templates to document PCCP that medical oncologist use which has increased utilization. To increase urology specific flow map use, a collaboration between the National Surgery Office and National Oncology Program was established to develop a Urology Prostate Cancer Note (UPCN). The UPCN was designed by urologists with assistance from a medical oncologist and a clinical applications coordinator. The UPCN will function as a working clinical note for urologists and has the PCCPs embedded into reminder dialog templates, which when completed generate health factors. The health factors that are generated from the UPCN are data mined to record PCCP use and to perform data analytics. The UPCN is in the testing phase at three pilot test sites and is scheduled to be deployed summer 2024. The collaborative effort is aligned with the VHA directives outlined in the Cleland Dole Act.
Background
Prostate cancer is the most common non-cutaneous malignancy diagnosis within the Department of Veterans Affairs (VA). The Prostate Cancer Clinical Pathways (PCCP) were developed to enable providers to treat all Veterans with prostate cancer at subject matter expert level.
Discussion
The PCCP was launched in February 2021; however, provider documentation of PCCP is variable across the VA healthcare system and within the PCCP, specific flow maps have differential use. For example, the Very Low Risk flow map has seven unique Veterans entered, whereas the Molecular Testing flow map has over 3,900 unique Veterans entered. One clear reason for this disparity in pathway documentation use is that local prostate cancer is managed by urology and their documentation of the PCCP is not as widespread as the medical oncologists. The National Oncology Program developed clinical note templates to document PCCP that medical oncologist use which has increased utilization. To increase urology specific flow map use, a collaboration between the National Surgery Office and National Oncology Program was established to develop a Urology Prostate Cancer Note (UPCN). The UPCN was designed by urologists with assistance from a medical oncologist and a clinical applications coordinator. The UPCN will function as a working clinical note for urologists and has the PCCPs embedded into reminder dialog templates, which when completed generate health factors. The health factors that are generated from the UPCN are data mined to record PCCP use and to perform data analytics. The UPCN is in the testing phase at three pilot test sites and is scheduled to be deployed summer 2024. The collaborative effort is aligned with the VHA directives outlined in the Cleland Dole Act.
Migraine Treatment Outcomes
Outcomes of Acute and Preventive Migraine Therapy Based on Patient Sex
I previously have addressed myths about migraine as they pertain to men and women. When I found an interesting study recently published in Cephalalgia investigating the effectiveness of calcitonin gene-related peptide receptor (CGRP-R) antagonists (gepants) for acute care and prevention of episodic migraine and CGRP monoclonal antibodies for preventive treatment of episodic and chronic migraine in men and women, I thought I would discuss it here.
The study’s aim was to discern if patient sex contributed to outcomes in each specific treatment arm. Female sex hormones have been recognized as factors in promoting migraine, and women show increased severity, persistence, and comorbidity in migraine profiles, and increased prevalence of migraine relative to men.
Gepants used for acute therapy (ubrogepant, rimegepant, zavegepant) and preventive therapy (atogepant, rimegepant) were studied in this trial. Erenumab, fremanezumab, galcanezumab, and eptinezumab are monoclonal antibodies that either sit on the CGRP receptor (erenumab) or inactivate the CGRP ligand (fremanezumab, galcanezumab, and eptinezumab) and are used for migraine prevention. CGRP-based therapies are not effective in all patients and understanding which patient groups respond preferentially could reduce trial and error in treatment selection. The effectiveness of treatments targeting CGRP or the CGRP receptor may not be uniform in men and women, highlighting the need for further research and understanding of CGRP neurobiology in both sexes.
Key findings:
- In the trial by Porreca et al: In women, the 3 gepants approved by the FDA for the acute care of migraine (ubrogepant, rimegepant, zavegepant) produced a statistically significant drug effect for the 2-hour pain freedom (2h-PF) endpoint, with an average drug effect of 9.5% (CI: 7.4 to 11.6) and an average number needed to treat (NNT) of 11.
- Men did not show statistically significant effects with the acute use of gepants. The average drug effect was 2.8%, and the average NNT was 36.
- For both men and women, CGRP-targeting therapies for prevention of migraine (the 4 monoclonal antibodies) were equally effective; however, possible sex differences remain uncertain and need further study.
- In patients with chronic migraine, CGRP/CGRP-R antibodies were similarly effective in both men and women.
- For the 2-hour freedom from most bothersome symptom (2h-MBS) endpoint when gepants were given acutely, the effects were much better in women than men, with an average drug effect of 10.2% and an average NNT of 10.
- In men, these medications produced observed treatment effects on 2h-MBS with an average drug effect of 3.2% and an average NNT of 32.
- In men, 5 out of 12 estimates favored placebo over the active treatment, suggesting a treatment with little to no effect.
- The pooled treatment effects for women were 3 times as large, at 9.2% and 10.2%, respectively.
- The placebo response rates for 2 of the 3 ubrogepant studies and one of 2 zavegepant studies were higher in men than in women.
The study concludes that, while small molecule CGRP-R antagonists are dramatically effective for acute therapy of migraine in women, available data do not demonstrate effectiveness in men. The treatment effect was found to always favor active treatment numerically for both men and women for prevention of episodic and chronic migraine. The data highlight possible differential effects of CGRP-targeted therapies in different patient populations and the need for increased understanding of CGRP neurobiology in men and women. The study also emphasizes the need to understand which patient groups preferentially respond to CGRP-based therapies to reduce trial and error in treatment. Note that rimegepant data on prevention were not available for analysis at the time of the writing.
It would be interesting to perform a meta-analysis of multiple well-done, large, real-world studies to see if the same differences and similarities are found in men versus women for acute care of migraine and prevention of episodic and chronic migraine. I suspect that we would find that acute care results favor women but that some men do well.
The Effectiveness of Prednisolone for Treating Medication Overuse Headache
I often discuss medication overuse headache (MOH), as it is difficult to diagnose and treat, so I wanted to comment on another pertinent study. It is a post hoc analysis of the Registry for Load and Management of Medication Overuse Headache (RELEASE). The RELEASE trial is an ongoing, multicenter, observational, cohort study of MOH that has been conducted in Korea since April 2020. Findings were recently published in Headache by Lee et al.
MOH is a secondary headache disorder that develops in patients with a preexisting primary headache when they overuse acute care headache medications of any type except gepants. This includes prescription medications such as triptans, ergots, butalbital-containing medications; opioids; aspirin; acetaminophen; any type of combination medication often containing caffeine; or a combination of medications. This condition significantly impacts patients’ quality of life and productivity, usually increasing the frequency of headaches per month and leading to higher healthcare-related costs.
Treating MOH is challenging due to the lack of high-quality drug trials specifically designed for MOH and doctor inexperience. Current evidence is based largely on subgroup analyses of drug trials for the treatment of chronic migraine that contain these patient types.
Withdrawal of acute care headache medications that are being overused has traditionally been considered an important aspect of MOH treatment, although this may be changing. Withdrawal symptoms, such as increased intensity of headache pain, frequency of headaches, and other symptoms like agitation and sleep disturbance, can prevent patients from discontinuing overused medications. Systemic corticosteroids are widely used to reduce these withdrawal headaches, but clinical trials are sparse and have failed to meet proper endpoints. Despite this, corticosteroids have shown potential benefits, such as decreasing withdrawal headaches, reducing the use of rescue medications, and lowering headache intensity at certain time points after treatment.
Given these findings, this published study hypothesized that prednisolone may play a role in converting MOH to non-MOH at 3 months after treatment. The objective was to evaluate the outcome of prednisolone therapy in reversing medication overuse at 3 months posttreatment in patients with MOH using prospective multicenter registry data. Prednisolone was prescribed to 59 out of 309 patients (19.1%) enrolled during this observational study period, with doses ranging from 10 to 40 mg/day for 5-14 days. Of these patients, 228 (73.8%) completed the 3-month follow-up period.
Key findings:
- The MOH reversal rates at 3 months postbaseline were 76% (31/41) in the prednisolone group and 57.8% (108/187) in the no prednisolone group (p = 0.034).
- The steroid effect remained significant (adjusted odds ratio, 2.78; 95% confidence interval 1.27-6.1, p = 0.010) after adjusting for the number of monthly headache days at baseline, mode of discontinuation of overused medication, use of early preventive medications, and the number of combined preventive medications.
The study had several strengths, including the multicenter collection of data, prospective follow-ups, and comprehensiveness of data acquisition. However, it also had significant limitations, such as the noninterventional, observational nature of the study, potential bias in steroid prescription (every doctor prescribed what they wanted), and heterogeneity in the patient population. Also, there were a variety of treatments, and they were not standardized. Further external validation may be necessary before generalizing the study results.
Despite these limitations, the results do suggest that prednisolone may be one part of a valid treatment option for patients with MOH. I suspect, if the proper studies are done, we will see that using a good preventive medication, with few adverse events, and with careful education of the patient, formal detoxification will not be necessary when treating many patients with MOH. This has been my experience with MOH treatment utilizing the newer anti-CGRP preventive medications, including the older monoclonal antibodies and the newer gepants.
Outcomes of Acute and Preventive Migraine Therapy Based on Patient Sex
I previously have addressed myths about migraine as they pertain to men and women. When I found an interesting study recently published in Cephalalgia investigating the effectiveness of calcitonin gene-related peptide receptor (CGRP-R) antagonists (gepants) for acute care and prevention of episodic migraine and CGRP monoclonal antibodies for preventive treatment of episodic and chronic migraine in men and women, I thought I would discuss it here.
The study’s aim was to discern if patient sex contributed to outcomes in each specific treatment arm. Female sex hormones have been recognized as factors in promoting migraine, and women show increased severity, persistence, and comorbidity in migraine profiles, and increased prevalence of migraine relative to men.
Gepants used for acute therapy (ubrogepant, rimegepant, zavegepant) and preventive therapy (atogepant, rimegepant) were studied in this trial. Erenumab, fremanezumab, galcanezumab, and eptinezumab are monoclonal antibodies that either sit on the CGRP receptor (erenumab) or inactivate the CGRP ligand (fremanezumab, galcanezumab, and eptinezumab) and are used for migraine prevention. CGRP-based therapies are not effective in all patients and understanding which patient groups respond preferentially could reduce trial and error in treatment selection. The effectiveness of treatments targeting CGRP or the CGRP receptor may not be uniform in men and women, highlighting the need for further research and understanding of CGRP neurobiology in both sexes.
Key findings:
- In the trial by Porreca et al: In women, the 3 gepants approved by the FDA for the acute care of migraine (ubrogepant, rimegepant, zavegepant) produced a statistically significant drug effect for the 2-hour pain freedom (2h-PF) endpoint, with an average drug effect of 9.5% (CI: 7.4 to 11.6) and an average number needed to treat (NNT) of 11.
- Men did not show statistically significant effects with the acute use of gepants. The average drug effect was 2.8%, and the average NNT was 36.
- For both men and women, CGRP-targeting therapies for prevention of migraine (the 4 monoclonal antibodies) were equally effective; however, possible sex differences remain uncertain and need further study.
- In patients with chronic migraine, CGRP/CGRP-R antibodies were similarly effective in both men and women.
- For the 2-hour freedom from most bothersome symptom (2h-MBS) endpoint when gepants were given acutely, the effects were much better in women than men, with an average drug effect of 10.2% and an average NNT of 10.
- In men, these medications produced observed treatment effects on 2h-MBS with an average drug effect of 3.2% and an average NNT of 32.
- In men, 5 out of 12 estimates favored placebo over the active treatment, suggesting a treatment with little to no effect.
- The pooled treatment effects for women were 3 times as large, at 9.2% and 10.2%, respectively.
- The placebo response rates for 2 of the 3 ubrogepant studies and one of 2 zavegepant studies were higher in men than in women.
The study concludes that, while small molecule CGRP-R antagonists are dramatically effective for acute therapy of migraine in women, available data do not demonstrate effectiveness in men. The treatment effect was found to always favor active treatment numerically for both men and women for prevention of episodic and chronic migraine. The data highlight possible differential effects of CGRP-targeted therapies in different patient populations and the need for increased understanding of CGRP neurobiology in men and women. The study also emphasizes the need to understand which patient groups preferentially respond to CGRP-based therapies to reduce trial and error in treatment. Note that rimegepant data on prevention were not available for analysis at the time of the writing.
It would be interesting to perform a meta-analysis of multiple well-done, large, real-world studies to see if the same differences and similarities are found in men versus women for acute care of migraine and prevention of episodic and chronic migraine. I suspect that we would find that acute care results favor women but that some men do well.
The Effectiveness of Prednisolone for Treating Medication Overuse Headache
I often discuss medication overuse headache (MOH), as it is difficult to diagnose and treat, so I wanted to comment on another pertinent study. It is a post hoc analysis of the Registry for Load and Management of Medication Overuse Headache (RELEASE). The RELEASE trial is an ongoing, multicenter, observational, cohort study of MOH that has been conducted in Korea since April 2020. Findings were recently published in Headache by Lee et al.
MOH is a secondary headache disorder that develops in patients with a preexisting primary headache when they overuse acute care headache medications of any type except gepants. This includes prescription medications such as triptans, ergots, butalbital-containing medications; opioids; aspirin; acetaminophen; any type of combination medication often containing caffeine; or a combination of medications. This condition significantly impacts patients’ quality of life and productivity, usually increasing the frequency of headaches per month and leading to higher healthcare-related costs.
Treating MOH is challenging due to the lack of high-quality drug trials specifically designed for MOH and doctor inexperience. Current evidence is based largely on subgroup analyses of drug trials for the treatment of chronic migraine that contain these patient types.
Withdrawal of acute care headache medications that are being overused has traditionally been considered an important aspect of MOH treatment, although this may be changing. Withdrawal symptoms, such as increased intensity of headache pain, frequency of headaches, and other symptoms like agitation and sleep disturbance, can prevent patients from discontinuing overused medications. Systemic corticosteroids are widely used to reduce these withdrawal headaches, but clinical trials are sparse and have failed to meet proper endpoints. Despite this, corticosteroids have shown potential benefits, such as decreasing withdrawal headaches, reducing the use of rescue medications, and lowering headache intensity at certain time points after treatment.
Given these findings, this published study hypothesized that prednisolone may play a role in converting MOH to non-MOH at 3 months after treatment. The objective was to evaluate the outcome of prednisolone therapy in reversing medication overuse at 3 months posttreatment in patients with MOH using prospective multicenter registry data. Prednisolone was prescribed to 59 out of 309 patients (19.1%) enrolled during this observational study period, with doses ranging from 10 to 40 mg/day for 5-14 days. Of these patients, 228 (73.8%) completed the 3-month follow-up period.
Key findings:
- The MOH reversal rates at 3 months postbaseline were 76% (31/41) in the prednisolone group and 57.8% (108/187) in the no prednisolone group (p = 0.034).
- The steroid effect remained significant (adjusted odds ratio, 2.78; 95% confidence interval 1.27-6.1, p = 0.010) after adjusting for the number of monthly headache days at baseline, mode of discontinuation of overused medication, use of early preventive medications, and the number of combined preventive medications.
The study had several strengths, including the multicenter collection of data, prospective follow-ups, and comprehensiveness of data acquisition. However, it also had significant limitations, such as the noninterventional, observational nature of the study, potential bias in steroid prescription (every doctor prescribed what they wanted), and heterogeneity in the patient population. Also, there were a variety of treatments, and they were not standardized. Further external validation may be necessary before generalizing the study results.
Despite these limitations, the results do suggest that prednisolone may be one part of a valid treatment option for patients with MOH. I suspect, if the proper studies are done, we will see that using a good preventive medication, with few adverse events, and with careful education of the patient, formal detoxification will not be necessary when treating many patients with MOH. This has been my experience with MOH treatment utilizing the newer anti-CGRP preventive medications, including the older monoclonal antibodies and the newer gepants.
Outcomes of Acute and Preventive Migraine Therapy Based on Patient Sex
I previously have addressed myths about migraine as they pertain to men and women. When I found an interesting study recently published in Cephalalgia investigating the effectiveness of calcitonin gene-related peptide receptor (CGRP-R) antagonists (gepants) for acute care and prevention of episodic migraine and CGRP monoclonal antibodies for preventive treatment of episodic and chronic migraine in men and women, I thought I would discuss it here.
The study’s aim was to discern if patient sex contributed to outcomes in each specific treatment arm. Female sex hormones have been recognized as factors in promoting migraine, and women show increased severity, persistence, and comorbidity in migraine profiles, and increased prevalence of migraine relative to men.
Gepants used for acute therapy (ubrogepant, rimegepant, zavegepant) and preventive therapy (atogepant, rimegepant) were studied in this trial. Erenumab, fremanezumab, galcanezumab, and eptinezumab are monoclonal antibodies that either sit on the CGRP receptor (erenumab) or inactivate the CGRP ligand (fremanezumab, galcanezumab, and eptinezumab) and are used for migraine prevention. CGRP-based therapies are not effective in all patients and understanding which patient groups respond preferentially could reduce trial and error in treatment selection. The effectiveness of treatments targeting CGRP or the CGRP receptor may not be uniform in men and women, highlighting the need for further research and understanding of CGRP neurobiology in both sexes.
Key findings:
- In the trial by Porreca et al: In women, the 3 gepants approved by the FDA for the acute care of migraine (ubrogepant, rimegepant, zavegepant) produced a statistically significant drug effect for the 2-hour pain freedom (2h-PF) endpoint, with an average drug effect of 9.5% (CI: 7.4 to 11.6) and an average number needed to treat (NNT) of 11.
- Men did not show statistically significant effects with the acute use of gepants. The average drug effect was 2.8%, and the average NNT was 36.
- For both men and women, CGRP-targeting therapies for prevention of migraine (the 4 monoclonal antibodies) were equally effective; however, possible sex differences remain uncertain and need further study.
- In patients with chronic migraine, CGRP/CGRP-R antibodies were similarly effective in both men and women.
- For the 2-hour freedom from most bothersome symptom (2h-MBS) endpoint when gepants were given acutely, the effects were much better in women than men, with an average drug effect of 10.2% and an average NNT of 10.
- In men, these medications produced observed treatment effects on 2h-MBS with an average drug effect of 3.2% and an average NNT of 32.
- In men, 5 out of 12 estimates favored placebo over the active treatment, suggesting a treatment with little to no effect.
- The pooled treatment effects for women were 3 times as large, at 9.2% and 10.2%, respectively.
- The placebo response rates for 2 of the 3 ubrogepant studies and one of 2 zavegepant studies were higher in men than in women.
The study concludes that, while small molecule CGRP-R antagonists are dramatically effective for acute therapy of migraine in women, available data do not demonstrate effectiveness in men. The treatment effect was found to always favor active treatment numerically for both men and women for prevention of episodic and chronic migraine. The data highlight possible differential effects of CGRP-targeted therapies in different patient populations and the need for increased understanding of CGRP neurobiology in men and women. The study also emphasizes the need to understand which patient groups preferentially respond to CGRP-based therapies to reduce trial and error in treatment. Note that rimegepant data on prevention were not available for analysis at the time of the writing.
It would be interesting to perform a meta-analysis of multiple well-done, large, real-world studies to see if the same differences and similarities are found in men versus women for acute care of migraine and prevention of episodic and chronic migraine. I suspect that we would find that acute care results favor women but that some men do well.
The Effectiveness of Prednisolone for Treating Medication Overuse Headache
I often discuss medication overuse headache (MOH), as it is difficult to diagnose and treat, so I wanted to comment on another pertinent study. It is a post hoc analysis of the Registry for Load and Management of Medication Overuse Headache (RELEASE). The RELEASE trial is an ongoing, multicenter, observational, cohort study of MOH that has been conducted in Korea since April 2020. Findings were recently published in Headache by Lee et al.
MOH is a secondary headache disorder that develops in patients with a preexisting primary headache when they overuse acute care headache medications of any type except gepants. This includes prescription medications such as triptans, ergots, butalbital-containing medications; opioids; aspirin; acetaminophen; any type of combination medication often containing caffeine; or a combination of medications. This condition significantly impacts patients’ quality of life and productivity, usually increasing the frequency of headaches per month and leading to higher healthcare-related costs.
Treating MOH is challenging due to the lack of high-quality drug trials specifically designed for MOH and doctor inexperience. Current evidence is based largely on subgroup analyses of drug trials for the treatment of chronic migraine that contain these patient types.
Withdrawal of acute care headache medications that are being overused has traditionally been considered an important aspect of MOH treatment, although this may be changing. Withdrawal symptoms, such as increased intensity of headache pain, frequency of headaches, and other symptoms like agitation and sleep disturbance, can prevent patients from discontinuing overused medications. Systemic corticosteroids are widely used to reduce these withdrawal headaches, but clinical trials are sparse and have failed to meet proper endpoints. Despite this, corticosteroids have shown potential benefits, such as decreasing withdrawal headaches, reducing the use of rescue medications, and lowering headache intensity at certain time points after treatment.
Given these findings, this published study hypothesized that prednisolone may play a role in converting MOH to non-MOH at 3 months after treatment. The objective was to evaluate the outcome of prednisolone therapy in reversing medication overuse at 3 months posttreatment in patients with MOH using prospective multicenter registry data. Prednisolone was prescribed to 59 out of 309 patients (19.1%) enrolled during this observational study period, with doses ranging from 10 to 40 mg/day for 5-14 days. Of these patients, 228 (73.8%) completed the 3-month follow-up period.
Key findings:
- The MOH reversal rates at 3 months postbaseline were 76% (31/41) in the prednisolone group and 57.8% (108/187) in the no prednisolone group (p = 0.034).
- The steroid effect remained significant (adjusted odds ratio, 2.78; 95% confidence interval 1.27-6.1, p = 0.010) after adjusting for the number of monthly headache days at baseline, mode of discontinuation of overused medication, use of early preventive medications, and the number of combined preventive medications.
The study had several strengths, including the multicenter collection of data, prospective follow-ups, and comprehensiveness of data acquisition. However, it also had significant limitations, such as the noninterventional, observational nature of the study, potential bias in steroid prescription (every doctor prescribed what they wanted), and heterogeneity in the patient population. Also, there were a variety of treatments, and they were not standardized. Further external validation may be necessary before generalizing the study results.
Despite these limitations, the results do suggest that prednisolone may be one part of a valid treatment option for patients with MOH. I suspect, if the proper studies are done, we will see that using a good preventive medication, with few adverse events, and with careful education of the patient, formal detoxification will not be necessary when treating many patients with MOH. This has been my experience with MOH treatment utilizing the newer anti-CGRP preventive medications, including the older monoclonal antibodies and the newer gepants.
Tender Dermal Nodule on the Temple
The Diagnosis: Lymphoepithelioma-like Carcinoma
Lymphoepithelioma-like carcinoma (LELC) is a rare, poorly differentiated, primary cutaneous neoplasm that occurs on sun-exposed skin, particularly on the head and neck of elderly individuals. It often manifests as an asymptomatic, slow-growing, flesh-colored or erythematous dermal nodule, though ulceration and tenderness have been reported.1 Histopathologically, these neoplasms often are poorly circumscribed and can infiltrate surrounding subcutaneous and soft tissue. As a biphasic tumor, LELC is characterized by islands, nests, or trabeculae of epithelioid cells within the mid dermis surrounded by a dense lymphocytic infiltrate with plasma cells (Figure 1).1 The epithelial component rarely communicates with the overlying epidermis and is composed of atypical polygonal cells with eosinophilic cytoplasm, vesicular nuclei, prominent nucleoli, and frequent mitosis.2 These epithelial nests can be highlighted by pancytokeratin AE1/AE3 or other epithelial differentiation markers (eg, CAM 5.2, CK5/6, epithelial membrane antigen, high-molecular-weight cytokeratin), while the surrounding lymphocytic infiltrate consists of an admixture of T cells and B cells. Lymphoepithelioma-like carcinomas also can demonstrate sebaceous, eccrine, or follicular differentiations.3 The epithelial nests of LELC also are positive for p63 and epithelial membrane antigen.2
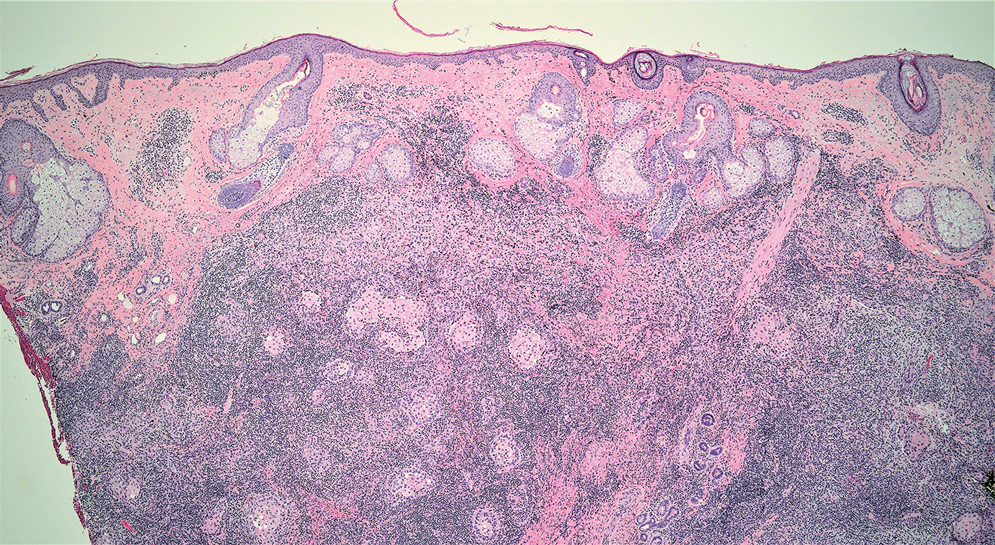
The usual treatment of LELC is wide local excision or Mohs micrographic surgery.1 Despite the poorly differentiated morphology of the tumor, LELC has a generally good prognosis with low metastatic potential and few reports of local recurrence after incomplete excision.3 Patients who are not candidates for surgery as well as recalcitrant cases are managed with radiotherapy.1
Cutaneous lymphadenoma (CL) is a benign adnexal neoplasm that manifests as a small, solitary, fleshcolored nodule usually in the head and neck region.4 Histologically, CL consists of well-circumscribed epithelial nests within the dermis that are peripherally outlined by palisading basaloid cells and filled with clear to eosinophilic epithelioid cells (Figure 2).5 The fibrotic tumor stroma often is infiltrated by numerous intralobular dendritic cells and lymphocytes that occasionally can be arranged in germinal center–like nodules.4 The lymphoepithelial nature of CL can be challenging to distinguish morphologically from LELC, and immunohistochemistry stains may be required. In CL, both the basaloid and epithelioid cells stain positive for pancytokeratin AE1/ AE3, but the peripheral palisaded basaloid cells also stain positive for BerEP4. Additionally, the fibrotic stroma can be highlighted by CD34 and the intralobular dendritic cells by S-100.4
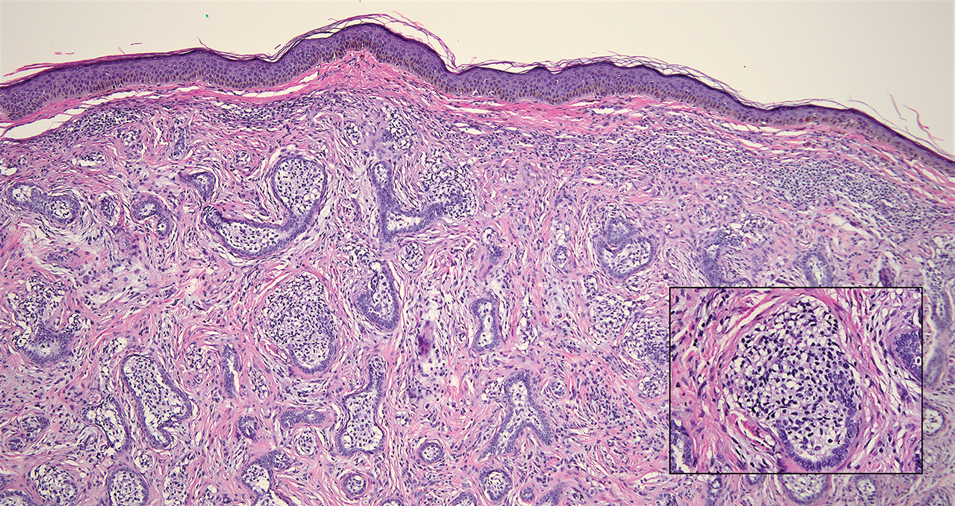
Nasopharyngeal carcinoma (NPC), formerly known as lymphoepithelioma, refers to carcinoma arising within the epithelium of the nasopharynx.6 Endemic to China, NPC manifests as an enlarging nasopharyngeal mass, causing clinical symptoms such as nasal obstruction and epistaxis.7 Histologically, nonkeratinizing NPC exhibits a biphasic morphology consisting of epithelioid neoplastic cells and background lymphocytic infiltrates (Figure 3). The epithelial component consists of round to oval neoplastic cells with amphophilic to eosinophilic cytoplasm, vesicular nuclei, and prominent nucleoli.6 Nasopharyngeal carcinoma is associated strongly with the Epstein-Barr virus while LELC is not; thus, Epstein- Barr encoding region in situ hybridization can reliably distinguish these entities. Metastatic NPC is rare but has been reported; therefore, it is highly recommended to perform an otolaryngologic examination in addition to testing for Epstein-Barr virus reactivity as part of a complete evaluation.8
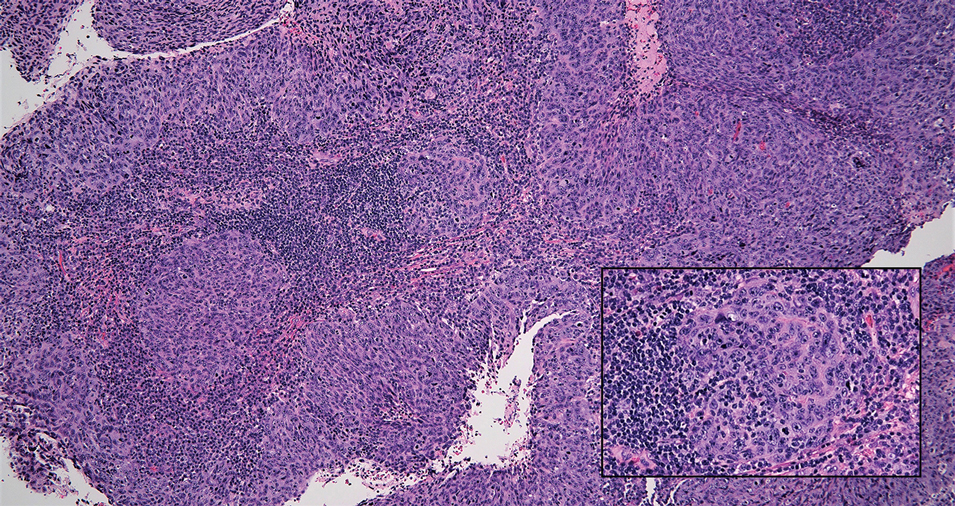
Cutaneous squamous cell carcinoma (SCC) is a common epidermal malignancy with multiple subtypes and variable morphology. The clinical presentation of SCC is similar to LELC—an enlarging hyperkeratotic papule or nodule on sun-exposed skin that often is ulcerated and tender.9 Histologically, poorly differentiated nonkeratinizing SCC can form nests and trabeculae of epithelioid cells that are stained by epithelial differentiation markers, resembling the epithelioid nests of LELC. Distinguishing between LELC and poorly differentiated SCC with robust inflammatory infiltrate can be challenging (Figure 4). In fact, some experts support LELC as an SCC variant rather than a separate entity.9 However, in contrast to LELC, the dermal nests of SCC usually maintain an epidermal connection and often are associated with an overlying area of SCC in situ or welldifferentiated SCC.3
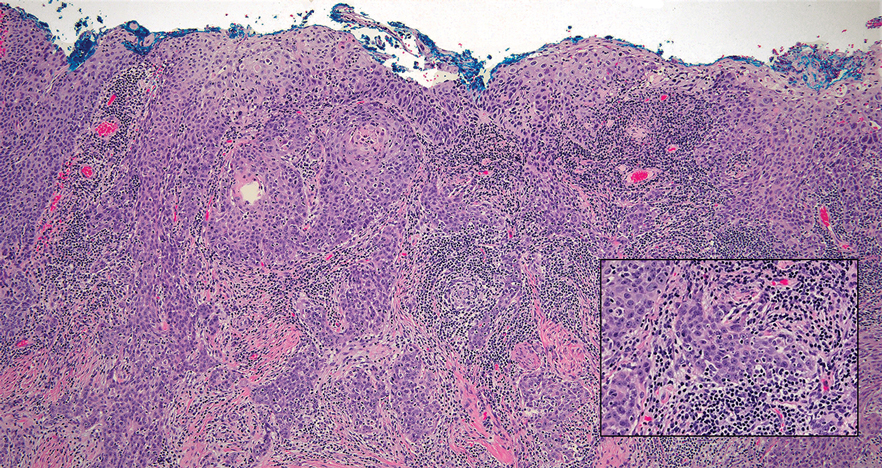
Mycosis fungoides (MF) is a primary cutaneous T-cell lymphoma. It is the most common type of cutaneous lymphoma, accounting for almost 50% of all reported cases.10 Classic MF has an indolent course and progresses through several clinical stages. Patches and plaques characterize early stages; lymphadenopathy indicates progression to later stages in which erythroderma may develop with coalescence of patches, plaques, and tumors; and MF present in blood or lymph nodes characterizes the late stage. Each stage of MF is different histologically—from a superficial lichenoid infiltrate with exocytosis of malignant T cells in the patch stage, to more robust epidermotropism and dermal infiltrate in the plaque stage, and finally a dense dermal infiltrate in the late stage.11 The rare syringotropic variant of MF clinically manifests as solitary or multiple erythematous lesions, often with overlying alopecia. Syringotropic MF uniquely exhibits folliculotropism and syringotropism along with syringometaplasia on histologic evaluation (Figure 5).12 The syringometaplasia can be difficult to distinguish from the epithelial nests of LELC, particularly with the lymphocytic background. Immunohistochemical panels for T-cell markers can highlight aberrant T cells in syringotropic MF through their usual loss of CD5 and CD7, in comparison to normal T cells in LELC.11 An elevated CD4:CD8 ratio of 4:1 and molecular analysis for T-cell receptor gene clonal rearrangements also can support the diagnosis of MF.12
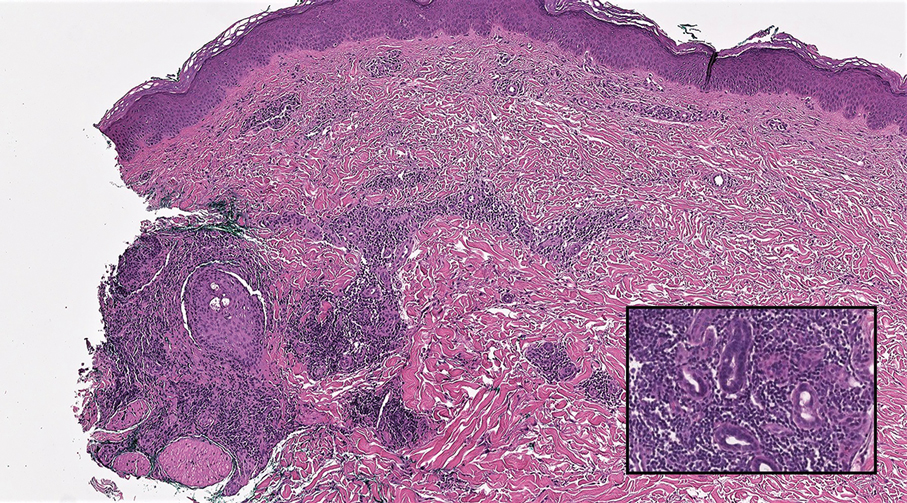
- Morteza Abedi S, Salama S, Alowami S. Lymphoepithelioma-like carcinoma of the skin: case report and approach to surgical pathology sign out. Rare Tumors. 2013;5:E47.
- Fisher JC, White RM, Hurd DS. Lymphoepithelioma-like carcinoma of the skin: a case of one patient presenting with two primary cutaneous neoplasms. J Am Osteopath Coll Dermatol. 2015;33:40-41.
- Welch PQ, Williams SB, Foss RD, et al. Lymphoepithelioma-like carcinoma of head and neck skin: a systematic analysis of 11 cases and review of literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:78-86.
- Yu R, Salama S, Alowami S. Cutaneous lymphadenoma: a rare case and brief review of a diagnostic pitfall. Rare Tumors. 2014;6:5358.
- Monteagudo C, Fúnez R, Sánchez-Sendra B, et al. Cutaneous lymphadenoma is a distinct trichoblastoma-like lymphoepithelial tumor with diffuse androgen receptor immunoreactivity, Notch1 ligand in Reed-Sternberg-like Cells, and common EGFR somatic mutations. Am J Surg Pathol. 2021;45:1382-1390.
- Stelow EB, Wenig BM. Update from the 4th edition of the World Health Organization classification of head and neck tumours: nasopharynx. Head Neck Pathol. 2017;11:16-22.
- Almomani MH, Zulfiqar H, Nagalli S. Nasopharyngeal carcinoma (NPC, lymphoepithelioma). StatPearls Publishing; 2022.
- Lassen CB, Lock-Andersen J. Lymphoepithelioma-like carcinoma of the skin: a case with perineural invasion. Plast Reconstr Surg Glob Open. 2014;2:E252.
- Motaparthi K, Kapil JP, Velazquez EF. Cutaneous squamous cell carcinoma: review of the eighth edition of the American Joint Committee on Cancer Staging Guidelines, Prognostic Factors, and Histopathologic Variants. Adv Anat Pathol. 2017;24:171-194.
- Pileri A, Facchetti F, Rütten A, et al. Syringotropic mycosis fungoides: a rare variant of the disease with peculiar clinicopathologic features. Am J Surg Pathol. 2011;35:100-109.
- Ryu HJ, Kim SI, Jang HO, et al. Evaluation of the International Society for Cutaneous Lymphoma Algorithm for the Diagnosis of Early Mycosis Fungoides [published October 15, 2021]. Cells. 2021;10:2758. doi:10.3390/cells10102758
- Lehmer LM, Amber KT, de Feraudy SM. Syringotropic mycosis fungoides: a rare form of cutaneous T-cell lymphoma enabling a histopathologic “sigh of relief.” Am J Dermatopathol. 2017;39:920-923.
The Diagnosis: Lymphoepithelioma-like Carcinoma
Lymphoepithelioma-like carcinoma (LELC) is a rare, poorly differentiated, primary cutaneous neoplasm that occurs on sun-exposed skin, particularly on the head and neck of elderly individuals. It often manifests as an asymptomatic, slow-growing, flesh-colored or erythematous dermal nodule, though ulceration and tenderness have been reported.1 Histopathologically, these neoplasms often are poorly circumscribed and can infiltrate surrounding subcutaneous and soft tissue. As a biphasic tumor, LELC is characterized by islands, nests, or trabeculae of epithelioid cells within the mid dermis surrounded by a dense lymphocytic infiltrate with plasma cells (Figure 1).1 The epithelial component rarely communicates with the overlying epidermis and is composed of atypical polygonal cells with eosinophilic cytoplasm, vesicular nuclei, prominent nucleoli, and frequent mitosis.2 These epithelial nests can be highlighted by pancytokeratin AE1/AE3 or other epithelial differentiation markers (eg, CAM 5.2, CK5/6, epithelial membrane antigen, high-molecular-weight cytokeratin), while the surrounding lymphocytic infiltrate consists of an admixture of T cells and B cells. Lymphoepithelioma-like carcinomas also can demonstrate sebaceous, eccrine, or follicular differentiations.3 The epithelial nests of LELC also are positive for p63 and epithelial membrane antigen.2

The usual treatment of LELC is wide local excision or Mohs micrographic surgery.1 Despite the poorly differentiated morphology of the tumor, LELC has a generally good prognosis with low metastatic potential and few reports of local recurrence after incomplete excision.3 Patients who are not candidates for surgery as well as recalcitrant cases are managed with radiotherapy.1
Cutaneous lymphadenoma (CL) is a benign adnexal neoplasm that manifests as a small, solitary, fleshcolored nodule usually in the head and neck region.4 Histologically, CL consists of well-circumscribed epithelial nests within the dermis that are peripherally outlined by palisading basaloid cells and filled with clear to eosinophilic epithelioid cells (Figure 2).5 The fibrotic tumor stroma often is infiltrated by numerous intralobular dendritic cells and lymphocytes that occasionally can be arranged in germinal center–like nodules.4 The lymphoepithelial nature of CL can be challenging to distinguish morphologically from LELC, and immunohistochemistry stains may be required. In CL, both the basaloid and epithelioid cells stain positive for pancytokeratin AE1/ AE3, but the peripheral palisaded basaloid cells also stain positive for BerEP4. Additionally, the fibrotic stroma can be highlighted by CD34 and the intralobular dendritic cells by S-100.4

Nasopharyngeal carcinoma (NPC), formerly known as lymphoepithelioma, refers to carcinoma arising within the epithelium of the nasopharynx.6 Endemic to China, NPC manifests as an enlarging nasopharyngeal mass, causing clinical symptoms such as nasal obstruction and epistaxis.7 Histologically, nonkeratinizing NPC exhibits a biphasic morphology consisting of epithelioid neoplastic cells and background lymphocytic infiltrates (Figure 3). The epithelial component consists of round to oval neoplastic cells with amphophilic to eosinophilic cytoplasm, vesicular nuclei, and prominent nucleoli.6 Nasopharyngeal carcinoma is associated strongly with the Epstein-Barr virus while LELC is not; thus, Epstein- Barr encoding region in situ hybridization can reliably distinguish these entities. Metastatic NPC is rare but has been reported; therefore, it is highly recommended to perform an otolaryngologic examination in addition to testing for Epstein-Barr virus reactivity as part of a complete evaluation.8

Cutaneous squamous cell carcinoma (SCC) is a common epidermal malignancy with multiple subtypes and variable morphology. The clinical presentation of SCC is similar to LELC—an enlarging hyperkeratotic papule or nodule on sun-exposed skin that often is ulcerated and tender.9 Histologically, poorly differentiated nonkeratinizing SCC can form nests and trabeculae of epithelioid cells that are stained by epithelial differentiation markers, resembling the epithelioid nests of LELC. Distinguishing between LELC and poorly differentiated SCC with robust inflammatory infiltrate can be challenging (Figure 4). In fact, some experts support LELC as an SCC variant rather than a separate entity.9 However, in contrast to LELC, the dermal nests of SCC usually maintain an epidermal connection and often are associated with an overlying area of SCC in situ or welldifferentiated SCC.3

Mycosis fungoides (MF) is a primary cutaneous T-cell lymphoma. It is the most common type of cutaneous lymphoma, accounting for almost 50% of all reported cases.10 Classic MF has an indolent course and progresses through several clinical stages. Patches and plaques characterize early stages; lymphadenopathy indicates progression to later stages in which erythroderma may develop with coalescence of patches, plaques, and tumors; and MF present in blood or lymph nodes characterizes the late stage. Each stage of MF is different histologically—from a superficial lichenoid infiltrate with exocytosis of malignant T cells in the patch stage, to more robust epidermotropism and dermal infiltrate in the plaque stage, and finally a dense dermal infiltrate in the late stage.11 The rare syringotropic variant of MF clinically manifests as solitary or multiple erythematous lesions, often with overlying alopecia. Syringotropic MF uniquely exhibits folliculotropism and syringotropism along with syringometaplasia on histologic evaluation (Figure 5).12 The syringometaplasia can be difficult to distinguish from the epithelial nests of LELC, particularly with the lymphocytic background. Immunohistochemical panels for T-cell markers can highlight aberrant T cells in syringotropic MF through their usual loss of CD5 and CD7, in comparison to normal T cells in LELC.11 An elevated CD4:CD8 ratio of 4:1 and molecular analysis for T-cell receptor gene clonal rearrangements also can support the diagnosis of MF.12

The Diagnosis: Lymphoepithelioma-like Carcinoma
Lymphoepithelioma-like carcinoma (LELC) is a rare, poorly differentiated, primary cutaneous neoplasm that occurs on sun-exposed skin, particularly on the head and neck of elderly individuals. It often manifests as an asymptomatic, slow-growing, flesh-colored or erythematous dermal nodule, though ulceration and tenderness have been reported.1 Histopathologically, these neoplasms often are poorly circumscribed and can infiltrate surrounding subcutaneous and soft tissue. As a biphasic tumor, LELC is characterized by islands, nests, or trabeculae of epithelioid cells within the mid dermis surrounded by a dense lymphocytic infiltrate with plasma cells (Figure 1).1 The epithelial component rarely communicates with the overlying epidermis and is composed of atypical polygonal cells with eosinophilic cytoplasm, vesicular nuclei, prominent nucleoli, and frequent mitosis.2 These epithelial nests can be highlighted by pancytokeratin AE1/AE3 or other epithelial differentiation markers (eg, CAM 5.2, CK5/6, epithelial membrane antigen, high-molecular-weight cytokeratin), while the surrounding lymphocytic infiltrate consists of an admixture of T cells and B cells. Lymphoepithelioma-like carcinomas also can demonstrate sebaceous, eccrine, or follicular differentiations.3 The epithelial nests of LELC also are positive for p63 and epithelial membrane antigen.2

The usual treatment of LELC is wide local excision or Mohs micrographic surgery.1 Despite the poorly differentiated morphology of the tumor, LELC has a generally good prognosis with low metastatic potential and few reports of local recurrence after incomplete excision.3 Patients who are not candidates for surgery as well as recalcitrant cases are managed with radiotherapy.1
Cutaneous lymphadenoma (CL) is a benign adnexal neoplasm that manifests as a small, solitary, fleshcolored nodule usually in the head and neck region.4 Histologically, CL consists of well-circumscribed epithelial nests within the dermis that are peripherally outlined by palisading basaloid cells and filled with clear to eosinophilic epithelioid cells (Figure 2).5 The fibrotic tumor stroma often is infiltrated by numerous intralobular dendritic cells and lymphocytes that occasionally can be arranged in germinal center–like nodules.4 The lymphoepithelial nature of CL can be challenging to distinguish morphologically from LELC, and immunohistochemistry stains may be required. In CL, both the basaloid and epithelioid cells stain positive for pancytokeratin AE1/ AE3, but the peripheral palisaded basaloid cells also stain positive for BerEP4. Additionally, the fibrotic stroma can be highlighted by CD34 and the intralobular dendritic cells by S-100.4

Nasopharyngeal carcinoma (NPC), formerly known as lymphoepithelioma, refers to carcinoma arising within the epithelium of the nasopharynx.6 Endemic to China, NPC manifests as an enlarging nasopharyngeal mass, causing clinical symptoms such as nasal obstruction and epistaxis.7 Histologically, nonkeratinizing NPC exhibits a biphasic morphology consisting of epithelioid neoplastic cells and background lymphocytic infiltrates (Figure 3). The epithelial component consists of round to oval neoplastic cells with amphophilic to eosinophilic cytoplasm, vesicular nuclei, and prominent nucleoli.6 Nasopharyngeal carcinoma is associated strongly with the Epstein-Barr virus while LELC is not; thus, Epstein- Barr encoding region in situ hybridization can reliably distinguish these entities. Metastatic NPC is rare but has been reported; therefore, it is highly recommended to perform an otolaryngologic examination in addition to testing for Epstein-Barr virus reactivity as part of a complete evaluation.8

Cutaneous squamous cell carcinoma (SCC) is a common epidermal malignancy with multiple subtypes and variable morphology. The clinical presentation of SCC is similar to LELC—an enlarging hyperkeratotic papule or nodule on sun-exposed skin that often is ulcerated and tender.9 Histologically, poorly differentiated nonkeratinizing SCC can form nests and trabeculae of epithelioid cells that are stained by epithelial differentiation markers, resembling the epithelioid nests of LELC. Distinguishing between LELC and poorly differentiated SCC with robust inflammatory infiltrate can be challenging (Figure 4). In fact, some experts support LELC as an SCC variant rather than a separate entity.9 However, in contrast to LELC, the dermal nests of SCC usually maintain an epidermal connection and often are associated with an overlying area of SCC in situ or welldifferentiated SCC.3

Mycosis fungoides (MF) is a primary cutaneous T-cell lymphoma. It is the most common type of cutaneous lymphoma, accounting for almost 50% of all reported cases.10 Classic MF has an indolent course and progresses through several clinical stages. Patches and plaques characterize early stages; lymphadenopathy indicates progression to later stages in which erythroderma may develop with coalescence of patches, plaques, and tumors; and MF present in blood or lymph nodes characterizes the late stage. Each stage of MF is different histologically—from a superficial lichenoid infiltrate with exocytosis of malignant T cells in the patch stage, to more robust epidermotropism and dermal infiltrate in the plaque stage, and finally a dense dermal infiltrate in the late stage.11 The rare syringotropic variant of MF clinically manifests as solitary or multiple erythematous lesions, often with overlying alopecia. Syringotropic MF uniquely exhibits folliculotropism and syringotropism along with syringometaplasia on histologic evaluation (Figure 5).12 The syringometaplasia can be difficult to distinguish from the epithelial nests of LELC, particularly with the lymphocytic background. Immunohistochemical panels for T-cell markers can highlight aberrant T cells in syringotropic MF through their usual loss of CD5 and CD7, in comparison to normal T cells in LELC.11 An elevated CD4:CD8 ratio of 4:1 and molecular analysis for T-cell receptor gene clonal rearrangements also can support the diagnosis of MF.12

- Morteza Abedi S, Salama S, Alowami S. Lymphoepithelioma-like carcinoma of the skin: case report and approach to surgical pathology sign out. Rare Tumors. 2013;5:E47.
- Fisher JC, White RM, Hurd DS. Lymphoepithelioma-like carcinoma of the skin: a case of one patient presenting with two primary cutaneous neoplasms. J Am Osteopath Coll Dermatol. 2015;33:40-41.
- Welch PQ, Williams SB, Foss RD, et al. Lymphoepithelioma-like carcinoma of head and neck skin: a systematic analysis of 11 cases and review of literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:78-86.
- Yu R, Salama S, Alowami S. Cutaneous lymphadenoma: a rare case and brief review of a diagnostic pitfall. Rare Tumors. 2014;6:5358.
- Monteagudo C, Fúnez R, Sánchez-Sendra B, et al. Cutaneous lymphadenoma is a distinct trichoblastoma-like lymphoepithelial tumor with diffuse androgen receptor immunoreactivity, Notch1 ligand in Reed-Sternberg-like Cells, and common EGFR somatic mutations. Am J Surg Pathol. 2021;45:1382-1390.
- Stelow EB, Wenig BM. Update from the 4th edition of the World Health Organization classification of head and neck tumours: nasopharynx. Head Neck Pathol. 2017;11:16-22.
- Almomani MH, Zulfiqar H, Nagalli S. Nasopharyngeal carcinoma (NPC, lymphoepithelioma). StatPearls Publishing; 2022.
- Lassen CB, Lock-Andersen J. Lymphoepithelioma-like carcinoma of the skin: a case with perineural invasion. Plast Reconstr Surg Glob Open. 2014;2:E252.
- Motaparthi K, Kapil JP, Velazquez EF. Cutaneous squamous cell carcinoma: review of the eighth edition of the American Joint Committee on Cancer Staging Guidelines, Prognostic Factors, and Histopathologic Variants. Adv Anat Pathol. 2017;24:171-194.
- Pileri A, Facchetti F, Rütten A, et al. Syringotropic mycosis fungoides: a rare variant of the disease with peculiar clinicopathologic features. Am J Surg Pathol. 2011;35:100-109.
- Ryu HJ, Kim SI, Jang HO, et al. Evaluation of the International Society for Cutaneous Lymphoma Algorithm for the Diagnosis of Early Mycosis Fungoides [published October 15, 2021]. Cells. 2021;10:2758. doi:10.3390/cells10102758
- Lehmer LM, Amber KT, de Feraudy SM. Syringotropic mycosis fungoides: a rare form of cutaneous T-cell lymphoma enabling a histopathologic “sigh of relief.” Am J Dermatopathol. 2017;39:920-923.
- Morteza Abedi S, Salama S, Alowami S. Lymphoepithelioma-like carcinoma of the skin: case report and approach to surgical pathology sign out. Rare Tumors. 2013;5:E47.
- Fisher JC, White RM, Hurd DS. Lymphoepithelioma-like carcinoma of the skin: a case of one patient presenting with two primary cutaneous neoplasms. J Am Osteopath Coll Dermatol. 2015;33:40-41.
- Welch PQ, Williams SB, Foss RD, et al. Lymphoepithelioma-like carcinoma of head and neck skin: a systematic analysis of 11 cases and review of literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:78-86.
- Yu R, Salama S, Alowami S. Cutaneous lymphadenoma: a rare case and brief review of a diagnostic pitfall. Rare Tumors. 2014;6:5358.
- Monteagudo C, Fúnez R, Sánchez-Sendra B, et al. Cutaneous lymphadenoma is a distinct trichoblastoma-like lymphoepithelial tumor with diffuse androgen receptor immunoreactivity, Notch1 ligand in Reed-Sternberg-like Cells, and common EGFR somatic mutations. Am J Surg Pathol. 2021;45:1382-1390.
- Stelow EB, Wenig BM. Update from the 4th edition of the World Health Organization classification of head and neck tumours: nasopharynx. Head Neck Pathol. 2017;11:16-22.
- Almomani MH, Zulfiqar H, Nagalli S. Nasopharyngeal carcinoma (NPC, lymphoepithelioma). StatPearls Publishing; 2022.
- Lassen CB, Lock-Andersen J. Lymphoepithelioma-like carcinoma of the skin: a case with perineural invasion. Plast Reconstr Surg Glob Open. 2014;2:E252.
- Motaparthi K, Kapil JP, Velazquez EF. Cutaneous squamous cell carcinoma: review of the eighth edition of the American Joint Committee on Cancer Staging Guidelines, Prognostic Factors, and Histopathologic Variants. Adv Anat Pathol. 2017;24:171-194.
- Pileri A, Facchetti F, Rütten A, et al. Syringotropic mycosis fungoides: a rare variant of the disease with peculiar clinicopathologic features. Am J Surg Pathol. 2011;35:100-109.
- Ryu HJ, Kim SI, Jang HO, et al. Evaluation of the International Society for Cutaneous Lymphoma Algorithm for the Diagnosis of Early Mycosis Fungoides [published October 15, 2021]. Cells. 2021;10:2758. doi:10.3390/cells10102758
- Lehmer LM, Amber KT, de Feraudy SM. Syringotropic mycosis fungoides: a rare form of cutaneous T-cell lymphoma enabling a histopathologic “sigh of relief.” Am J Dermatopathol. 2017;39:920-923.
A 77-year-old man presented with a 1.2-cm dermal nodule on the left temple of 1 year’s duration. The lesion had become tender and darker in color. An excision was performed and submitted for histologic examination. Additional immunohistochemistry staining for Epstein-Barr virus was negative.