User login
E-Consults in Dermatology: A Retrospective Analysis
Dermatologic conditions affect approximately one-third of individuals in the United States.1,2 Nearly 1 in 4 physician office visits in the United States are for skin conditions, and less than one-third of these visits are with dermatologists. Although many of these patients may prefer to see a dermatologist for their concerns, they may not be able to access specialist care.3 The limited supply and urban-focused distribution of dermatologists along with reduced acceptance of state-funded insurance plans and long appointment wait times all pose considerable challenges to individuals seeking dermatologic care.2 Electronic consultations (e-consults) have emerged as a promising solution to overcoming these barriers while providing high-quality dermatologic care to a large diverse patient population.2,4 Although e-consults can be of service to all dermatology patients, this modality may be especially beneficial to underserved populations, such as the uninsured and Medicaid patients—groups that historically have experienced limited access to dermatology care due to the low reimbursement rates and high administrative burdens accompanying care delivery.4 This limited access leads to inequity in care, as timely access to dermatology is associated with improved diagnostic accuracy and disease outcomes.3 E-consult implementation can facilitate timely access for these underserved populations and bypass additional barriers to care such as lack of transportation or time off work. Prior e-consult studies have demonstrated relatively high numbers of Medicaid patients utilizing e-consult services.3,5
Although in-person visits remain the gold standard for diagnosis and treatment of dermatologic conditions, e-consults placed by primary care providers (PCPs) can improve access and help triage patients who require in-person dermatology visits.6 In this study, we conducted a retrospective chart review to characterize the e-consults requested of the dermatology department at a large tertiary care medical center in Winston-Salem, North Carolina.
Methods
The electronic health record (EHR) of Atrium Health Wake Forest Baptist (Winston-Salem, North Carolina) was screened for eligible patients from January 1, 2020, to May 31, 2021. Patients—both adult (aged ≥18 years) and pediatric (aged <18 years)—were included if they underwent a dermatology e-consult within this time frame. Provider notes in the medical records were reviewed to determine the nature of the lesion, how long the dermatologist took to complete the e-consult, whether an in-person appointment was recommended, and whether the patient was seen by dermatology within 90 days of the e-consult. Institutional review board approval was obtained.
For each e-consult, the PCP obtained clinical photographs of the lesion in question either through the EHR mobile application or by having patients upload their own photographs directly to their medical records. The referring PCP then completed a brief template regarding the patient’s clinical question and medical history and then sent the completed information to the consulting dermatologist’s EHR inbox. From there, the dermatologist could view the clinical question, documented photographs, and patient medical record to create a brief consult note with recommendations. The note was then sent back via EHR to the PCP to follow up with the patient. Patients were not charged for the e-consult.
Results
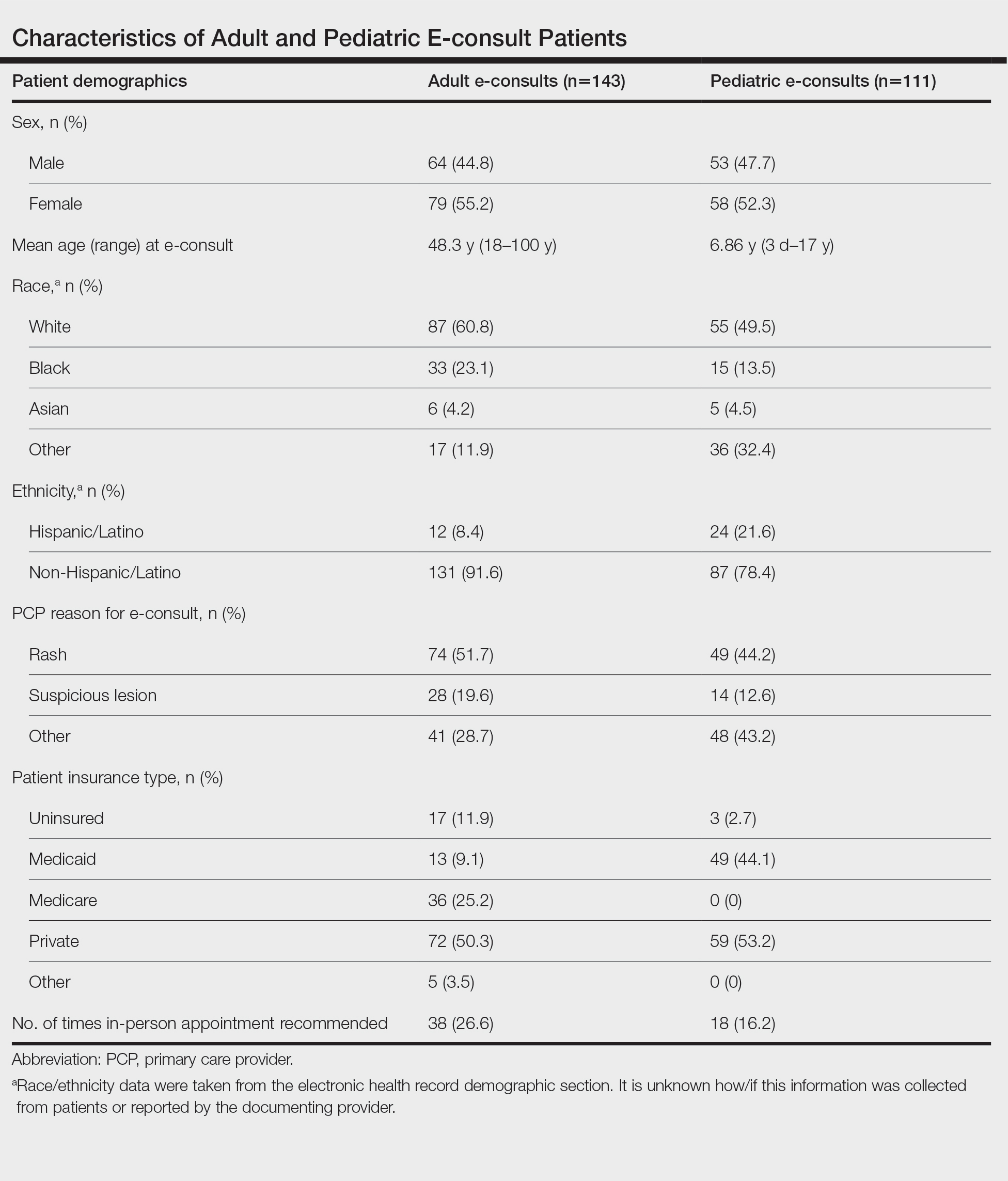
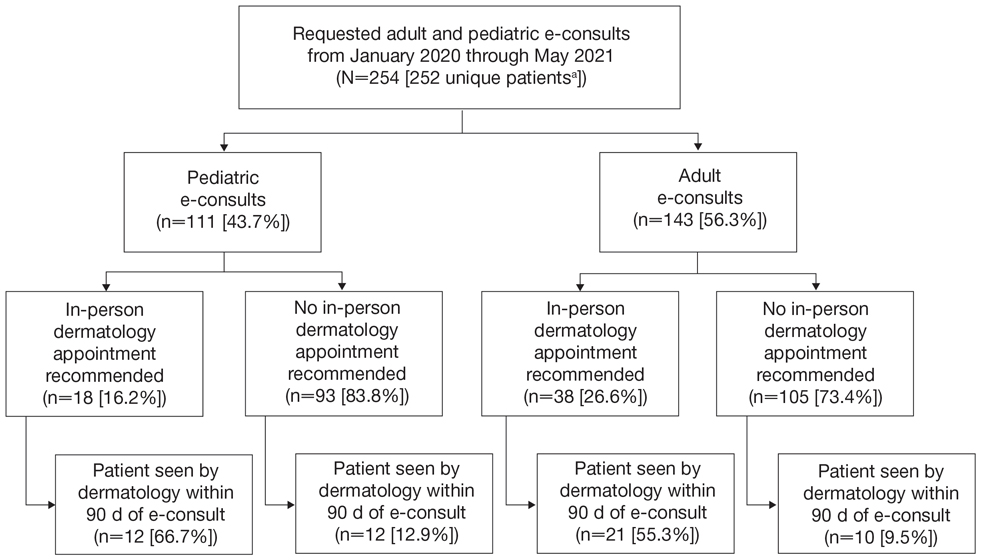
Two hundred fifty-four dermatology e-consults were requested by providers at the study center (eTable), which included 252 unique patients (2 patients had 2 separate e-consults regarding different clinical questions). The median time for completion of the e-consult—from submission of the PCP’s e-consult request to dermatologist completion—was 0.37 days. Fifty-six patients (22.0%) were recommended for an in-person appointment (Figure), 33 (58.9%) of whom ultimately scheduled the in-person appointment, and the median length of time between the completion of the e-consult and the in-person appointment was 16.5 days. The remaining 198 patients (78.0%) were not triaged to receive an in-person appointment following the e-consult,but 2 patients (8.7%) were ultimately seen in-person anyway via other referral pathways, with a median length of 33 days between e-consult completion and the in-person appointment. One hundred seventy-six patients (69.8%) avoided an in-person dermatology visit, although 38 (21.6%) of those patients were fewer than 90 days out from their e-consults at the time of data collection. The 254 e-consults included patients from 50 different zip codes, 49 (98.0%) of which were in North Carolina.
Comment
An e-consult is an asynchronous telehealth modality through which PCPs can request specialty evaluation to provide diagnostic and therapeutic guidance, facilitate PCP-specialist coordination of care, and increase access to specialty care with reduced wait times.7,8 Increased care access is especially important, as specialty referral can decrease overall health care expenditure; however, the demand for specialists often exceeds the availability.8 Our e-consult program drastically reduced the time from patients’ initial presentation at their PCP’s office to dermatologist recommendations for treatment or need for in-person dermatology follow-up.
In our analysis, patients were of different racial, ethnic, and socioeconomic backgrounds and lived across a variety of zip codes, predominantly in central and western North Carolina. Almost three-quarters of the patients resided in zip codes where the average income was less than the North Carolina median household income ($66,196).9 Additionally, 82 patients (32.3%) were uninsured or on Medicaid (eTable). These economically disadvantaged patient populations historically have had limited access to dermatologic care.4 One study showed that privately insured individuals were accepted as new patients by dermatologists 91% of the time compared to a 29.8% acceptance rate for publicly insured individuals.10 Uninsured and Medicaid patients also have to wait 34% longer for an appointment compared to individuals with Medicare or private insurance.2 Considering these patients may already be at an economic disadvantage when it comes to seeing and paying for dermatologic services, e-consults may reduce patient travel and appointment expenses while increasing access to specialty care. Based on a 2020 study, each e-consult generates an estimated savings of $80 out-of-pocket per patient per avoided in-person visit.11
In our study, the most common condition for an e-consult in both adult and pediatric patients was rash, which is consistent with prior e-consult studies.5,11 We found that most e-consult patients were not recommended for an in-person dermatology visit, and for those who were recommended to have an in-person visit, the wait time was reduced (Figure). These results corroborate that e-consults may be used as an important triage tool for determining whether a specialist appointment is indicated as well as a public health tool, as timely evaluation is associated with better dermatologic health care outcomes.3 However, the number of patients who did not present for an in-person appointment in our study may be overestimated, as 38 patients’ (21.6%) e-consults were conducted fewer than 90 days before our data collection. Although none of these patients had been seen in person, it is possible they requested an in-person visit after their medical records were reviewed for this study. Additionally, it is possible patients sought care from outside providers not documented in the EHR.
With regard to the payment model for the e-consult program, Atrium Health Wake Forest Baptist initially piloted the e-consult system through a partnership with the American Academy of Medical Colleges’ Project CORE: Coordinating Optimal Referral Experiences (https://www.aamc.org/what-we-do/mission-areas/health-care/project-core). Grant funding through Project CORE allowed both the referring PCP and the specialist completing the e-consult to each receive approximately 0.5 relative value units in payment for each consult completed. Based on early adoption successes, the institution has created additional internal funding to support the continued expansion of the e-consult system and is incentivized to continue funding, as proper utilization of e-consults improves patient access to timely specialist care, avoids no-shows or last-minute cancellations for specialist appointments, and decreases back-door access to specialist care through the emergency department and urgent care facilities.5 Although 0.5 relative value units is not equivalent compensation to an in-person office visit, our study showed that e-consults can be completed much more quickly and efficiently and do not utilize nursing staff or other office resources.
Conclusion
E-consults are an effective telehealth modality that can increase patients’ access to dermatologic specialty care.
Acknowledgments—The authors thank the Wake Forest University School of Medicine Department of Medical Education and Department of Dermatology (Winston-Salem, North Carolina) for their contributions to this research study as well as the Wake Forest Clinical and Translational Science Institute (Winston-Salem, North Carolina) for their help extracting EHR data.
- Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134:1527-1534.
- Naka F, Lu J, Porto A, et al. Impact of dermatology econsults on access to care and skin cancer screening in underserved populations: a model for teledermatology services in community health centers. J Am Acad Dermatol. 2018;78:293-302.
- Mulcahy A, Mehrotra A, Edison K, et al. Variation in dermatologist visits by sociodemographic characteristics. J Am Acad Dermatol. 2017;76:918-924.
- Yang X, Barbieri JS, Kovarik CL. Cost analysis of a store-and-forward teledermatology consult system in Philadelphia. J Am Acad Dermatol. 2019;81:758-764.
- Wang RF, Trinidad J, Lawrence J, et al. Improved patient access and outcomes with the integration of an econsult program (teledermatology) within a large academic medical center. J Am Acad Dermatol. 2020;83:1633-1638.
- Lee KJ, Finnane A, Soyer HP. Recent trends in teledermatology and teledermoscopy. Dermatol Pract Concept. 2018;8:214-223.
- Parikh PJ, Mowrey C, Gallimore J, et al. Evaluating e-consultation implementations based on use and time-line across various specialties. Int J Med Inform. 2017;108:42-48.
- Wasfy JH, Rao SK, Kalwani N, et al. Longer-term impact of cardiology e-consults. Am Heart J. 2016;173:86-93.
- United States Census Bureau. QuickFacts: North Carolina; United States. Accessed February 26, 2024. https://www.census.gov/quickfacts/fact/table/NC,US/PST045222
- Alghothani L, Jacks SK, Vander Horst A, et al. Disparities in access to dermatologic care according to insurance type. Arch Dermatol. 2012;148:956-957.
- Seiger K, Hawryluk EB, Kroshinsky D, et al. Pediatric dermatology econsults: reduced wait times and dermatology office visits. Pediatr Dermatol. 2020;37:804-810.
Dermatologic conditions affect approximately one-third of individuals in the United States.1,2 Nearly 1 in 4 physician office visits in the United States are for skin conditions, and less than one-third of these visits are with dermatologists. Although many of these patients may prefer to see a dermatologist for their concerns, they may not be able to access specialist care.3 The limited supply and urban-focused distribution of dermatologists along with reduced acceptance of state-funded insurance plans and long appointment wait times all pose considerable challenges to individuals seeking dermatologic care.2 Electronic consultations (e-consults) have emerged as a promising solution to overcoming these barriers while providing high-quality dermatologic care to a large diverse patient population.2,4 Although e-consults can be of service to all dermatology patients, this modality may be especially beneficial to underserved populations, such as the uninsured and Medicaid patients—groups that historically have experienced limited access to dermatology care due to the low reimbursement rates and high administrative burdens accompanying care delivery.4 This limited access leads to inequity in care, as timely access to dermatology is associated with improved diagnostic accuracy and disease outcomes.3 E-consult implementation can facilitate timely access for these underserved populations and bypass additional barriers to care such as lack of transportation or time off work. Prior e-consult studies have demonstrated relatively high numbers of Medicaid patients utilizing e-consult services.3,5
Although in-person visits remain the gold standard for diagnosis and treatment of dermatologic conditions, e-consults placed by primary care providers (PCPs) can improve access and help triage patients who require in-person dermatology visits.6 In this study, we conducted a retrospective chart review to characterize the e-consults requested of the dermatology department at a large tertiary care medical center in Winston-Salem, North Carolina.
Methods
The electronic health record (EHR) of Atrium Health Wake Forest Baptist (Winston-Salem, North Carolina) was screened for eligible patients from January 1, 2020, to May 31, 2021. Patients—both adult (aged ≥18 years) and pediatric (aged <18 years)—were included if they underwent a dermatology e-consult within this time frame. Provider notes in the medical records were reviewed to determine the nature of the lesion, how long the dermatologist took to complete the e-consult, whether an in-person appointment was recommended, and whether the patient was seen by dermatology within 90 days of the e-consult. Institutional review board approval was obtained.
For each e-consult, the PCP obtained clinical photographs of the lesion in question either through the EHR mobile application or by having patients upload their own photographs directly to their medical records. The referring PCP then completed a brief template regarding the patient’s clinical question and medical history and then sent the completed information to the consulting dermatologist’s EHR inbox. From there, the dermatologist could view the clinical question, documented photographs, and patient medical record to create a brief consult note with recommendations. The note was then sent back via EHR to the PCP to follow up with the patient. Patients were not charged for the e-consult.

Results
Two hundred fifty-four dermatology e-consults were requested by providers at the study center (eTable), which included 252 unique patients (2 patients had 2 separate e-consults regarding different clinical questions). The median time for completion of the e-consult—from submission of the PCP’s e-consult request to dermatologist completion—was 0.37 days. Fifty-six patients (22.0%) were recommended for an in-person appointment (Figure), 33 (58.9%) of whom ultimately scheduled the in-person appointment, and the median length of time between the completion of the e-consult and the in-person appointment was 16.5 days. The remaining 198 patients (78.0%) were not triaged to receive an in-person appointment following the e-consult,but 2 patients (8.7%) were ultimately seen in-person anyway via other referral pathways, with a median length of 33 days between e-consult completion and the in-person appointment. One hundred seventy-six patients (69.8%) avoided an in-person dermatology visit, although 38 (21.6%) of those patients were fewer than 90 days out from their e-consults at the time of data collection. The 254 e-consults included patients from 50 different zip codes, 49 (98.0%) of which were in North Carolina.

Comment
An e-consult is an asynchronous telehealth modality through which PCPs can request specialty evaluation to provide diagnostic and therapeutic guidance, facilitate PCP-specialist coordination of care, and increase access to specialty care with reduced wait times.7,8 Increased care access is especially important, as specialty referral can decrease overall health care expenditure; however, the demand for specialists often exceeds the availability.8 Our e-consult program drastically reduced the time from patients’ initial presentation at their PCP’s office to dermatologist recommendations for treatment or need for in-person dermatology follow-up.
In our analysis, patients were of different racial, ethnic, and socioeconomic backgrounds and lived across a variety of zip codes, predominantly in central and western North Carolina. Almost three-quarters of the patients resided in zip codes where the average income was less than the North Carolina median household income ($66,196).9 Additionally, 82 patients (32.3%) were uninsured or on Medicaid (eTable). These economically disadvantaged patient populations historically have had limited access to dermatologic care.4 One study showed that privately insured individuals were accepted as new patients by dermatologists 91% of the time compared to a 29.8% acceptance rate for publicly insured individuals.10 Uninsured and Medicaid patients also have to wait 34% longer for an appointment compared to individuals with Medicare or private insurance.2 Considering these patients may already be at an economic disadvantage when it comes to seeing and paying for dermatologic services, e-consults may reduce patient travel and appointment expenses while increasing access to specialty care. Based on a 2020 study, each e-consult generates an estimated savings of $80 out-of-pocket per patient per avoided in-person visit.11
In our study, the most common condition for an e-consult in both adult and pediatric patients was rash, which is consistent with prior e-consult studies.5,11 We found that most e-consult patients were not recommended for an in-person dermatology visit, and for those who were recommended to have an in-person visit, the wait time was reduced (Figure). These results corroborate that e-consults may be used as an important triage tool for determining whether a specialist appointment is indicated as well as a public health tool, as timely evaluation is associated with better dermatologic health care outcomes.3 However, the number of patients who did not present for an in-person appointment in our study may be overestimated, as 38 patients’ (21.6%) e-consults were conducted fewer than 90 days before our data collection. Although none of these patients had been seen in person, it is possible they requested an in-person visit after their medical records were reviewed for this study. Additionally, it is possible patients sought care from outside providers not documented in the EHR.
With regard to the payment model for the e-consult program, Atrium Health Wake Forest Baptist initially piloted the e-consult system through a partnership with the American Academy of Medical Colleges’ Project CORE: Coordinating Optimal Referral Experiences (https://www.aamc.org/what-we-do/mission-areas/health-care/project-core). Grant funding through Project CORE allowed both the referring PCP and the specialist completing the e-consult to each receive approximately 0.5 relative value units in payment for each consult completed. Based on early adoption successes, the institution has created additional internal funding to support the continued expansion of the e-consult system and is incentivized to continue funding, as proper utilization of e-consults improves patient access to timely specialist care, avoids no-shows or last-minute cancellations for specialist appointments, and decreases back-door access to specialist care through the emergency department and urgent care facilities.5 Although 0.5 relative value units is not equivalent compensation to an in-person office visit, our study showed that e-consults can be completed much more quickly and efficiently and do not utilize nursing staff or other office resources.
Conclusion
E-consults are an effective telehealth modality that can increase patients’ access to dermatologic specialty care.
Acknowledgments—The authors thank the Wake Forest University School of Medicine Department of Medical Education and Department of Dermatology (Winston-Salem, North Carolina) for their contributions to this research study as well as the Wake Forest Clinical and Translational Science Institute (Winston-Salem, North Carolina) for their help extracting EHR data.
Dermatologic conditions affect approximately one-third of individuals in the United States.1,2 Nearly 1 in 4 physician office visits in the United States are for skin conditions, and less than one-third of these visits are with dermatologists. Although many of these patients may prefer to see a dermatologist for their concerns, they may not be able to access specialist care.3 The limited supply and urban-focused distribution of dermatologists along with reduced acceptance of state-funded insurance plans and long appointment wait times all pose considerable challenges to individuals seeking dermatologic care.2 Electronic consultations (e-consults) have emerged as a promising solution to overcoming these barriers while providing high-quality dermatologic care to a large diverse patient population.2,4 Although e-consults can be of service to all dermatology patients, this modality may be especially beneficial to underserved populations, such as the uninsured and Medicaid patients—groups that historically have experienced limited access to dermatology care due to the low reimbursement rates and high administrative burdens accompanying care delivery.4 This limited access leads to inequity in care, as timely access to dermatology is associated with improved diagnostic accuracy and disease outcomes.3 E-consult implementation can facilitate timely access for these underserved populations and bypass additional barriers to care such as lack of transportation or time off work. Prior e-consult studies have demonstrated relatively high numbers of Medicaid patients utilizing e-consult services.3,5
Although in-person visits remain the gold standard for diagnosis and treatment of dermatologic conditions, e-consults placed by primary care providers (PCPs) can improve access and help triage patients who require in-person dermatology visits.6 In this study, we conducted a retrospective chart review to characterize the e-consults requested of the dermatology department at a large tertiary care medical center in Winston-Salem, North Carolina.
Methods
The electronic health record (EHR) of Atrium Health Wake Forest Baptist (Winston-Salem, North Carolina) was screened for eligible patients from January 1, 2020, to May 31, 2021. Patients—both adult (aged ≥18 years) and pediatric (aged <18 years)—were included if they underwent a dermatology e-consult within this time frame. Provider notes in the medical records were reviewed to determine the nature of the lesion, how long the dermatologist took to complete the e-consult, whether an in-person appointment was recommended, and whether the patient was seen by dermatology within 90 days of the e-consult. Institutional review board approval was obtained.
For each e-consult, the PCP obtained clinical photographs of the lesion in question either through the EHR mobile application or by having patients upload their own photographs directly to their medical records. The referring PCP then completed a brief template regarding the patient’s clinical question and medical history and then sent the completed information to the consulting dermatologist’s EHR inbox. From there, the dermatologist could view the clinical question, documented photographs, and patient medical record to create a brief consult note with recommendations. The note was then sent back via EHR to the PCP to follow up with the patient. Patients were not charged for the e-consult.

Results
Two hundred fifty-four dermatology e-consults were requested by providers at the study center (eTable), which included 252 unique patients (2 patients had 2 separate e-consults regarding different clinical questions). The median time for completion of the e-consult—from submission of the PCP’s e-consult request to dermatologist completion—was 0.37 days. Fifty-six patients (22.0%) were recommended for an in-person appointment (Figure), 33 (58.9%) of whom ultimately scheduled the in-person appointment, and the median length of time between the completion of the e-consult and the in-person appointment was 16.5 days. The remaining 198 patients (78.0%) were not triaged to receive an in-person appointment following the e-consult,but 2 patients (8.7%) were ultimately seen in-person anyway via other referral pathways, with a median length of 33 days between e-consult completion and the in-person appointment. One hundred seventy-six patients (69.8%) avoided an in-person dermatology visit, although 38 (21.6%) of those patients were fewer than 90 days out from their e-consults at the time of data collection. The 254 e-consults included patients from 50 different zip codes, 49 (98.0%) of which were in North Carolina.

Comment
An e-consult is an asynchronous telehealth modality through which PCPs can request specialty evaluation to provide diagnostic and therapeutic guidance, facilitate PCP-specialist coordination of care, and increase access to specialty care with reduced wait times.7,8 Increased care access is especially important, as specialty referral can decrease overall health care expenditure; however, the demand for specialists often exceeds the availability.8 Our e-consult program drastically reduced the time from patients’ initial presentation at their PCP’s office to dermatologist recommendations for treatment or need for in-person dermatology follow-up.
In our analysis, patients were of different racial, ethnic, and socioeconomic backgrounds and lived across a variety of zip codes, predominantly in central and western North Carolina. Almost three-quarters of the patients resided in zip codes where the average income was less than the North Carolina median household income ($66,196).9 Additionally, 82 patients (32.3%) were uninsured or on Medicaid (eTable). These economically disadvantaged patient populations historically have had limited access to dermatologic care.4 One study showed that privately insured individuals were accepted as new patients by dermatologists 91% of the time compared to a 29.8% acceptance rate for publicly insured individuals.10 Uninsured and Medicaid patients also have to wait 34% longer for an appointment compared to individuals with Medicare or private insurance.2 Considering these patients may already be at an economic disadvantage when it comes to seeing and paying for dermatologic services, e-consults may reduce patient travel and appointment expenses while increasing access to specialty care. Based on a 2020 study, each e-consult generates an estimated savings of $80 out-of-pocket per patient per avoided in-person visit.11
In our study, the most common condition for an e-consult in both adult and pediatric patients was rash, which is consistent with prior e-consult studies.5,11 We found that most e-consult patients were not recommended for an in-person dermatology visit, and for those who were recommended to have an in-person visit, the wait time was reduced (Figure). These results corroborate that e-consults may be used as an important triage tool for determining whether a specialist appointment is indicated as well as a public health tool, as timely evaluation is associated with better dermatologic health care outcomes.3 However, the number of patients who did not present for an in-person appointment in our study may be overestimated, as 38 patients’ (21.6%) e-consults were conducted fewer than 90 days before our data collection. Although none of these patients had been seen in person, it is possible they requested an in-person visit after their medical records were reviewed for this study. Additionally, it is possible patients sought care from outside providers not documented in the EHR.
With regard to the payment model for the e-consult program, Atrium Health Wake Forest Baptist initially piloted the e-consult system through a partnership with the American Academy of Medical Colleges’ Project CORE: Coordinating Optimal Referral Experiences (https://www.aamc.org/what-we-do/mission-areas/health-care/project-core). Grant funding through Project CORE allowed both the referring PCP and the specialist completing the e-consult to each receive approximately 0.5 relative value units in payment for each consult completed. Based on early adoption successes, the institution has created additional internal funding to support the continued expansion of the e-consult system and is incentivized to continue funding, as proper utilization of e-consults improves patient access to timely specialist care, avoids no-shows or last-minute cancellations for specialist appointments, and decreases back-door access to specialist care through the emergency department and urgent care facilities.5 Although 0.5 relative value units is not equivalent compensation to an in-person office visit, our study showed that e-consults can be completed much more quickly and efficiently and do not utilize nursing staff or other office resources.
Conclusion
E-consults are an effective telehealth modality that can increase patients’ access to dermatologic specialty care.
Acknowledgments—The authors thank the Wake Forest University School of Medicine Department of Medical Education and Department of Dermatology (Winston-Salem, North Carolina) for their contributions to this research study as well as the Wake Forest Clinical and Translational Science Institute (Winston-Salem, North Carolina) for their help extracting EHR data.
- Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134:1527-1534.
- Naka F, Lu J, Porto A, et al. Impact of dermatology econsults on access to care and skin cancer screening in underserved populations: a model for teledermatology services in community health centers. J Am Acad Dermatol. 2018;78:293-302.
- Mulcahy A, Mehrotra A, Edison K, et al. Variation in dermatologist visits by sociodemographic characteristics. J Am Acad Dermatol. 2017;76:918-924.
- Yang X, Barbieri JS, Kovarik CL. Cost analysis of a store-and-forward teledermatology consult system in Philadelphia. J Am Acad Dermatol. 2019;81:758-764.
- Wang RF, Trinidad J, Lawrence J, et al. Improved patient access and outcomes with the integration of an econsult program (teledermatology) within a large academic medical center. J Am Acad Dermatol. 2020;83:1633-1638.
- Lee KJ, Finnane A, Soyer HP. Recent trends in teledermatology and teledermoscopy. Dermatol Pract Concept. 2018;8:214-223.
- Parikh PJ, Mowrey C, Gallimore J, et al. Evaluating e-consultation implementations based on use and time-line across various specialties. Int J Med Inform. 2017;108:42-48.
- Wasfy JH, Rao SK, Kalwani N, et al. Longer-term impact of cardiology e-consults. Am Heart J. 2016;173:86-93.
- United States Census Bureau. QuickFacts: North Carolina; United States. Accessed February 26, 2024. https://www.census.gov/quickfacts/fact/table/NC,US/PST045222
- Alghothani L, Jacks SK, Vander Horst A, et al. Disparities in access to dermatologic care according to insurance type. Arch Dermatol. 2012;148:956-957.
- Seiger K, Hawryluk EB, Kroshinsky D, et al. Pediatric dermatology econsults: reduced wait times and dermatology office visits. Pediatr Dermatol. 2020;37:804-810.
- Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134:1527-1534.
- Naka F, Lu J, Porto A, et al. Impact of dermatology econsults on access to care and skin cancer screening in underserved populations: a model for teledermatology services in community health centers. J Am Acad Dermatol. 2018;78:293-302.
- Mulcahy A, Mehrotra A, Edison K, et al. Variation in dermatologist visits by sociodemographic characteristics. J Am Acad Dermatol. 2017;76:918-924.
- Yang X, Barbieri JS, Kovarik CL. Cost analysis of a store-and-forward teledermatology consult system in Philadelphia. J Am Acad Dermatol. 2019;81:758-764.
- Wang RF, Trinidad J, Lawrence J, et al. Improved patient access and outcomes with the integration of an econsult program (teledermatology) within a large academic medical center. J Am Acad Dermatol. 2020;83:1633-1638.
- Lee KJ, Finnane A, Soyer HP. Recent trends in teledermatology and teledermoscopy. Dermatol Pract Concept. 2018;8:214-223.
- Parikh PJ, Mowrey C, Gallimore J, et al. Evaluating e-consultation implementations based on use and time-line across various specialties. Int J Med Inform. 2017;108:42-48.
- Wasfy JH, Rao SK, Kalwani N, et al. Longer-term impact of cardiology e-consults. Am Heart J. 2016;173:86-93.
- United States Census Bureau. QuickFacts: North Carolina; United States. Accessed February 26, 2024. https://www.census.gov/quickfacts/fact/table/NC,US/PST045222
- Alghothani L, Jacks SK, Vander Horst A, et al. Disparities in access to dermatologic care according to insurance type. Arch Dermatol. 2012;148:956-957.
- Seiger K, Hawryluk EB, Kroshinsky D, et al. Pediatric dermatology econsults: reduced wait times and dermatology office visits. Pediatr Dermatol. 2020;37:804-810.
Practice Points
- Most electronic consult patients may be able to avoid in-person dermatology appointments.
- E-consults can increase patient access to dermatologic specialty care.
Commentary: PPI Dosing, Biomarkers, and Eating Behaviors in Patients With EoE, March 2024
This study provides compelling evidence that a twice-daily dosing regimen of moderate-dose proton pump inhibitors (PPIs) is superior to a once-daily regimen for inducing histologic remission in eosinophilic esophagitis (EoE). This finding suggests a significant paradigm shift in EoE management, challenging the current standard treatment guideline that recommends a PPI trial of 20-40 mg twice daily. The limited data on various dosing regimens for EoE treatment underscores the importance of this research. Dr Muftah and colleagues from Brigham and Women's Hospital have conducted a novel retrospective cohort study to address the question: Does a twice-daily PPI dose induce a higher remission rate in EoE than a once-daily regimen does regardless of the total daily dose?
The study enrolled adult patients with newly-diagnosed treatment-naive EoE at a tertiary care center, dividing participants into four groups on the basis of their treatment regimen: once-daily standard dose (20 mg omeprazole), once-daily moderate dose (40 mg), twice-daily moderate dose (20 mg), and twice-daily high dose (40 mg). Patients underwent endoscopy 8-12 weeks after initiating PPI treatment, with the primary outcome being the histologic response to PPI, defined as fewer than 15 eosinophils/high power field in repeat esophageal biopsies.
Out of 305 patients (54.6% men, mean age 44.7 ± 16.7 years), 42.3% achieved a histologic response to PPI treatment. Patients receiving the standard PPI dose (20 mg omeprazole once daily) vs those on twice-daily moderate and high doses showed significantly higher histologic response rates (52.8% vs 11.8%, P < .0001; and 54.3% vs 11.8%, P < .0001; respectively). Multivariable analysis revealed that twice-daily moderate and high doses were significantly more effective (adjusted odds ration [aOR] 6.75; CI 2.53-18.0, P = .0008; and aOR 12.8, CI 4.69-34.8, P < .001; respectively).
However, the study's retrospective design limits its ability to establish causality and may introduce selection bias. In addition, the lack of specified adjustments for PPI dosing based on diet and lifestyle factors across the cohort could influence treatment response and outcomes. Last, as a single-center study, the results may not generalize across diverse patient populations, particularly those with different demographics or disease severities.
This research heralds a shift toward a more effective treatment strategy in EoE management, suggesting that a twice-daily PPI regimen may be more beneficial than once-daily dosing is for inducing histologic remission, especially in patients inadequately responding to once-daily PPI treatment. It advocates for a personalized treatment approach, considering factors such as symptom severity, previous PPI response, and potential for adherence to a twice-daily regimen.
Distinguishing between inflammatory bowel disease (IBD)–induced eosinophilia and EoE poses a significant challenge for clinicians. Given that the incidence of EoE is 3-5 times higher in patients with IBD compared with the general population, there is a pressing need for new biomarkers to differentiate between these two conditions. In response to this need, Dr Butzke and colleagues at Nemours Children's Health in Wilmington, Delaware, conducted a retrospective study to evaluate the roles of Major Basic Protein (MBP) and interleukin (IL)-13 in distinguishing these diseases. The study included participants who underwent esophagogastroduodenoscopy with esophageal biopsies for IBD workup or suspicion of EoE. It comprised 27 patients with EoE-IBD, 39 with EoE, 29 with IBD eosinophilia, 30 with IBD only, and 30 control patients. The biopsies were stained with MBP and IL-13 antibodies, and the results (percent staining/total tissue area), demographic, and clinical findings were compared among the groups.
The study revealed that MBP staining levels among patients with EoE-IBD were 3.8 units, which is significantly lower than those in the EoE group at 52.8 units and higher than those with IBD eosinophilia at 0.2 units (P < .001). IL-13 expression was significantly higher only compared with the IBD and control groups and not with EoE-IBD or IBD eosinophilia. MBP predicted EoE with 100% sensitivity and 99% specificity, whereas IL-13 demonstrated 83% sensitivity and 90% specificity using a cutoff point from the cohort of patients without EoE-IBD. Based on the MBP cutoff point of 3.49 units that distinguished between EoE and non-EoE cases, 100% of patients with EoE were MBP-positive compared with 3% of patients with IBD-associated eosinophilia (P < .05).
To implement this new biomarker into clinical practice, guidelines for interpreting MBP staining results should be developed and established, including defining cutoff points for positive and negative results. However, this study faces several limitations, such as not evaluating the differences in MBP results based on EoE-IBD type and disease activity. The retrospective nature of the study and its small sample size limit its power. In addition, the study did not assess how different treatments and disease activity affect MBP levels nor did it address the lack of longitudinal evaluation in assessing MBP levels.
Despite these limitations, the study presents a compelling case for the use of MBP as a biomarker to distinguish true EoE from EoE-IBD. This differentiation is crucial because it can guide therapeutic approaches, influencing medication choices and dietary interventions. MBP shows promise as an excellent biomarker for distinguishing true EoE from eosinophilia caused by IBD. When combined with endoscopic and histologic changes, MBP can assist with the diagnosis of EoE in IBD patients, thereby reducing the possibility of misdiagnosis.
Being diagnosed with EoE poses a challenging and life-altering experience for patients and their families. They face numerous challenges, from undergoing diagnostic procedures and treatments to adapting daily diets. Limited information is available on the eating habits of patients diagnosed with EoE. In this study, Dr Kennedy and colleagues explored how a diagnosis of EoE affects eating behaviors among pediatric patients.
The researchers conducted a prospective study involving 27 patients diagnosed with EoE and compared their eating behaviors to those of 25 healthy control participants. The participants were evaluated on the basis of their responses to four food textures (puree, soft solid, chewable, and hard solid), focusing on the number of chews per bite, sips of fluid per food, and consumption time.
The study found that, on average, patients with EoE (63.5% boys, mean age 11 years) required more chews per bite across several food textures (soft solid P = .031; chewable P = .047; and hard solid P = .037) and demonstrated increased consumption time for soft solid (P = .002), chewable (P = .005), and hard solid foods (P = .034) compared to healthy controls. In addition, endoscopic reference scores positively correlated with consumption time (r = 0.53; P = .008) and the number of chews (r = 0.45; P = .027) for chewable foods as well as with the number of chews (r = 0.44; P = .043) for hard solid foods. Increased consumption time also correlated with increased eosinophil counts (r = 0.42; P = .050) and decreased esophageal distensibility (r = -0.82; P < .0001).
Though these findings open promising avenues for the noninvasive assessment and personalized management of EoE, further research with larger, longitudinal studies is essential to validate these behaviors as reliable clinical biomarkers. Increasing the sample size would enhance the study's power and broaden the generalizability of its findings to a wider pediatric EoE population. The study's cross-sectional nature limits the ability to assess how eating behaviors change over time with treatment or disease progression.
This study underscores the potential of eating behaviors as clinical markers for pediatric patients with EoE, enabling early identification through increased chewing and consumption times, especially with harder textures. Such markers could prompt diagnostic evaluations in settings where endoscopy and biopsy are gold standards for diagnosing EoE. Moreover, eating patterns could assist in monitoring disease activity and progression, offering a noninvasive means of assessing disease status and response to therapy, thus allowing for more frequent assessments of disease status without the need for invasive procedures. Understanding these behaviors allows healthcare providers to tailor dietary advice and interventions, potentially enhancing treatment compliance and improving the quality of life for pediatric patients with EoE.
This study provides compelling evidence that a twice-daily dosing regimen of moderate-dose proton pump inhibitors (PPIs) is superior to a once-daily regimen for inducing histologic remission in eosinophilic esophagitis (EoE). This finding suggests a significant paradigm shift in EoE management, challenging the current standard treatment guideline that recommends a PPI trial of 20-40 mg twice daily. The limited data on various dosing regimens for EoE treatment underscores the importance of this research. Dr Muftah and colleagues from Brigham and Women's Hospital have conducted a novel retrospective cohort study to address the question: Does a twice-daily PPI dose induce a higher remission rate in EoE than a once-daily regimen does regardless of the total daily dose?
The study enrolled adult patients with newly-diagnosed treatment-naive EoE at a tertiary care center, dividing participants into four groups on the basis of their treatment regimen: once-daily standard dose (20 mg omeprazole), once-daily moderate dose (40 mg), twice-daily moderate dose (20 mg), and twice-daily high dose (40 mg). Patients underwent endoscopy 8-12 weeks after initiating PPI treatment, with the primary outcome being the histologic response to PPI, defined as fewer than 15 eosinophils/high power field in repeat esophageal biopsies.
Out of 305 patients (54.6% men, mean age 44.7 ± 16.7 years), 42.3% achieved a histologic response to PPI treatment. Patients receiving the standard PPI dose (20 mg omeprazole once daily) vs those on twice-daily moderate and high doses showed significantly higher histologic response rates (52.8% vs 11.8%, P < .0001; and 54.3% vs 11.8%, P < .0001; respectively). Multivariable analysis revealed that twice-daily moderate and high doses were significantly more effective (adjusted odds ration [aOR] 6.75; CI 2.53-18.0, P = .0008; and aOR 12.8, CI 4.69-34.8, P < .001; respectively).
However, the study's retrospective design limits its ability to establish causality and may introduce selection bias. In addition, the lack of specified adjustments for PPI dosing based on diet and lifestyle factors across the cohort could influence treatment response and outcomes. Last, as a single-center study, the results may not generalize across diverse patient populations, particularly those with different demographics or disease severities.
This research heralds a shift toward a more effective treatment strategy in EoE management, suggesting that a twice-daily PPI regimen may be more beneficial than once-daily dosing is for inducing histologic remission, especially in patients inadequately responding to once-daily PPI treatment. It advocates for a personalized treatment approach, considering factors such as symptom severity, previous PPI response, and potential for adherence to a twice-daily regimen.
Distinguishing between inflammatory bowel disease (IBD)–induced eosinophilia and EoE poses a significant challenge for clinicians. Given that the incidence of EoE is 3-5 times higher in patients with IBD compared with the general population, there is a pressing need for new biomarkers to differentiate between these two conditions. In response to this need, Dr Butzke and colleagues at Nemours Children's Health in Wilmington, Delaware, conducted a retrospective study to evaluate the roles of Major Basic Protein (MBP) and interleukin (IL)-13 in distinguishing these diseases. The study included participants who underwent esophagogastroduodenoscopy with esophageal biopsies for IBD workup or suspicion of EoE. It comprised 27 patients with EoE-IBD, 39 with EoE, 29 with IBD eosinophilia, 30 with IBD only, and 30 control patients. The biopsies were stained with MBP and IL-13 antibodies, and the results (percent staining/total tissue area), demographic, and clinical findings were compared among the groups.
The study revealed that MBP staining levels among patients with EoE-IBD were 3.8 units, which is significantly lower than those in the EoE group at 52.8 units and higher than those with IBD eosinophilia at 0.2 units (P < .001). IL-13 expression was significantly higher only compared with the IBD and control groups and not with EoE-IBD or IBD eosinophilia. MBP predicted EoE with 100% sensitivity and 99% specificity, whereas IL-13 demonstrated 83% sensitivity and 90% specificity using a cutoff point from the cohort of patients without EoE-IBD. Based on the MBP cutoff point of 3.49 units that distinguished between EoE and non-EoE cases, 100% of patients with EoE were MBP-positive compared with 3% of patients with IBD-associated eosinophilia (P < .05).
To implement this new biomarker into clinical practice, guidelines for interpreting MBP staining results should be developed and established, including defining cutoff points for positive and negative results. However, this study faces several limitations, such as not evaluating the differences in MBP results based on EoE-IBD type and disease activity. The retrospective nature of the study and its small sample size limit its power. In addition, the study did not assess how different treatments and disease activity affect MBP levels nor did it address the lack of longitudinal evaluation in assessing MBP levels.
Despite these limitations, the study presents a compelling case for the use of MBP as a biomarker to distinguish true EoE from EoE-IBD. This differentiation is crucial because it can guide therapeutic approaches, influencing medication choices and dietary interventions. MBP shows promise as an excellent biomarker for distinguishing true EoE from eosinophilia caused by IBD. When combined with endoscopic and histologic changes, MBP can assist with the diagnosis of EoE in IBD patients, thereby reducing the possibility of misdiagnosis.
Being diagnosed with EoE poses a challenging and life-altering experience for patients and their families. They face numerous challenges, from undergoing diagnostic procedures and treatments to adapting daily diets. Limited information is available on the eating habits of patients diagnosed with EoE. In this study, Dr Kennedy and colleagues explored how a diagnosis of EoE affects eating behaviors among pediatric patients.
The researchers conducted a prospective study involving 27 patients diagnosed with EoE and compared their eating behaviors to those of 25 healthy control participants. The participants were evaluated on the basis of their responses to four food textures (puree, soft solid, chewable, and hard solid), focusing on the number of chews per bite, sips of fluid per food, and consumption time.
The study found that, on average, patients with EoE (63.5% boys, mean age 11 years) required more chews per bite across several food textures (soft solid P = .031; chewable P = .047; and hard solid P = .037) and demonstrated increased consumption time for soft solid (P = .002), chewable (P = .005), and hard solid foods (P = .034) compared to healthy controls. In addition, endoscopic reference scores positively correlated with consumption time (r = 0.53; P = .008) and the number of chews (r = 0.45; P = .027) for chewable foods as well as with the number of chews (r = 0.44; P = .043) for hard solid foods. Increased consumption time also correlated with increased eosinophil counts (r = 0.42; P = .050) and decreased esophageal distensibility (r = -0.82; P < .0001).
Though these findings open promising avenues for the noninvasive assessment and personalized management of EoE, further research with larger, longitudinal studies is essential to validate these behaviors as reliable clinical biomarkers. Increasing the sample size would enhance the study's power and broaden the generalizability of its findings to a wider pediatric EoE population. The study's cross-sectional nature limits the ability to assess how eating behaviors change over time with treatment or disease progression.
This study underscores the potential of eating behaviors as clinical markers for pediatric patients with EoE, enabling early identification through increased chewing and consumption times, especially with harder textures. Such markers could prompt diagnostic evaluations in settings where endoscopy and biopsy are gold standards for diagnosing EoE. Moreover, eating patterns could assist in monitoring disease activity and progression, offering a noninvasive means of assessing disease status and response to therapy, thus allowing for more frequent assessments of disease status without the need for invasive procedures. Understanding these behaviors allows healthcare providers to tailor dietary advice and interventions, potentially enhancing treatment compliance and improving the quality of life for pediatric patients with EoE.
This study provides compelling evidence that a twice-daily dosing regimen of moderate-dose proton pump inhibitors (PPIs) is superior to a once-daily regimen for inducing histologic remission in eosinophilic esophagitis (EoE). This finding suggests a significant paradigm shift in EoE management, challenging the current standard treatment guideline that recommends a PPI trial of 20-40 mg twice daily. The limited data on various dosing regimens for EoE treatment underscores the importance of this research. Dr Muftah and colleagues from Brigham and Women's Hospital have conducted a novel retrospective cohort study to address the question: Does a twice-daily PPI dose induce a higher remission rate in EoE than a once-daily regimen does regardless of the total daily dose?
The study enrolled adult patients with newly-diagnosed treatment-naive EoE at a tertiary care center, dividing participants into four groups on the basis of their treatment regimen: once-daily standard dose (20 mg omeprazole), once-daily moderate dose (40 mg), twice-daily moderate dose (20 mg), and twice-daily high dose (40 mg). Patients underwent endoscopy 8-12 weeks after initiating PPI treatment, with the primary outcome being the histologic response to PPI, defined as fewer than 15 eosinophils/high power field in repeat esophageal biopsies.
Out of 305 patients (54.6% men, mean age 44.7 ± 16.7 years), 42.3% achieved a histologic response to PPI treatment. Patients receiving the standard PPI dose (20 mg omeprazole once daily) vs those on twice-daily moderate and high doses showed significantly higher histologic response rates (52.8% vs 11.8%, P < .0001; and 54.3% vs 11.8%, P < .0001; respectively). Multivariable analysis revealed that twice-daily moderate and high doses were significantly more effective (adjusted odds ration [aOR] 6.75; CI 2.53-18.0, P = .0008; and aOR 12.8, CI 4.69-34.8, P < .001; respectively).
However, the study's retrospective design limits its ability to establish causality and may introduce selection bias. In addition, the lack of specified adjustments for PPI dosing based on diet and lifestyle factors across the cohort could influence treatment response and outcomes. Last, as a single-center study, the results may not generalize across diverse patient populations, particularly those with different demographics or disease severities.
This research heralds a shift toward a more effective treatment strategy in EoE management, suggesting that a twice-daily PPI regimen may be more beneficial than once-daily dosing is for inducing histologic remission, especially in patients inadequately responding to once-daily PPI treatment. It advocates for a personalized treatment approach, considering factors such as symptom severity, previous PPI response, and potential for adherence to a twice-daily regimen.
Distinguishing between inflammatory bowel disease (IBD)–induced eosinophilia and EoE poses a significant challenge for clinicians. Given that the incidence of EoE is 3-5 times higher in patients with IBD compared with the general population, there is a pressing need for new biomarkers to differentiate between these two conditions. In response to this need, Dr Butzke and colleagues at Nemours Children's Health in Wilmington, Delaware, conducted a retrospective study to evaluate the roles of Major Basic Protein (MBP) and interleukin (IL)-13 in distinguishing these diseases. The study included participants who underwent esophagogastroduodenoscopy with esophageal biopsies for IBD workup or suspicion of EoE. It comprised 27 patients with EoE-IBD, 39 with EoE, 29 with IBD eosinophilia, 30 with IBD only, and 30 control patients. The biopsies were stained with MBP and IL-13 antibodies, and the results (percent staining/total tissue area), demographic, and clinical findings were compared among the groups.
The study revealed that MBP staining levels among patients with EoE-IBD were 3.8 units, which is significantly lower than those in the EoE group at 52.8 units and higher than those with IBD eosinophilia at 0.2 units (P < .001). IL-13 expression was significantly higher only compared with the IBD and control groups and not with EoE-IBD or IBD eosinophilia. MBP predicted EoE with 100% sensitivity and 99% specificity, whereas IL-13 demonstrated 83% sensitivity and 90% specificity using a cutoff point from the cohort of patients without EoE-IBD. Based on the MBP cutoff point of 3.49 units that distinguished between EoE and non-EoE cases, 100% of patients with EoE were MBP-positive compared with 3% of patients with IBD-associated eosinophilia (P < .05).
To implement this new biomarker into clinical practice, guidelines for interpreting MBP staining results should be developed and established, including defining cutoff points for positive and negative results. However, this study faces several limitations, such as not evaluating the differences in MBP results based on EoE-IBD type and disease activity. The retrospective nature of the study and its small sample size limit its power. In addition, the study did not assess how different treatments and disease activity affect MBP levels nor did it address the lack of longitudinal evaluation in assessing MBP levels.
Despite these limitations, the study presents a compelling case for the use of MBP as a biomarker to distinguish true EoE from EoE-IBD. This differentiation is crucial because it can guide therapeutic approaches, influencing medication choices and dietary interventions. MBP shows promise as an excellent biomarker for distinguishing true EoE from eosinophilia caused by IBD. When combined with endoscopic and histologic changes, MBP can assist with the diagnosis of EoE in IBD patients, thereby reducing the possibility of misdiagnosis.
Being diagnosed with EoE poses a challenging and life-altering experience for patients and their families. They face numerous challenges, from undergoing diagnostic procedures and treatments to adapting daily diets. Limited information is available on the eating habits of patients diagnosed with EoE. In this study, Dr Kennedy and colleagues explored how a diagnosis of EoE affects eating behaviors among pediatric patients.
The researchers conducted a prospective study involving 27 patients diagnosed with EoE and compared their eating behaviors to those of 25 healthy control participants. The participants were evaluated on the basis of their responses to four food textures (puree, soft solid, chewable, and hard solid), focusing on the number of chews per bite, sips of fluid per food, and consumption time.
The study found that, on average, patients with EoE (63.5% boys, mean age 11 years) required more chews per bite across several food textures (soft solid P = .031; chewable P = .047; and hard solid P = .037) and demonstrated increased consumption time for soft solid (P = .002), chewable (P = .005), and hard solid foods (P = .034) compared to healthy controls. In addition, endoscopic reference scores positively correlated with consumption time (r = 0.53; P = .008) and the number of chews (r = 0.45; P = .027) for chewable foods as well as with the number of chews (r = 0.44; P = .043) for hard solid foods. Increased consumption time also correlated with increased eosinophil counts (r = 0.42; P = .050) and decreased esophageal distensibility (r = -0.82; P < .0001).
Though these findings open promising avenues for the noninvasive assessment and personalized management of EoE, further research with larger, longitudinal studies is essential to validate these behaviors as reliable clinical biomarkers. Increasing the sample size would enhance the study's power and broaden the generalizability of its findings to a wider pediatric EoE population. The study's cross-sectional nature limits the ability to assess how eating behaviors change over time with treatment or disease progression.
This study underscores the potential of eating behaviors as clinical markers for pediatric patients with EoE, enabling early identification through increased chewing and consumption times, especially with harder textures. Such markers could prompt diagnostic evaluations in settings where endoscopy and biopsy are gold standards for diagnosing EoE. Moreover, eating patterns could assist in monitoring disease activity and progression, offering a noninvasive means of assessing disease status and response to therapy, thus allowing for more frequent assessments of disease status without the need for invasive procedures. Understanding these behaviors allows healthcare providers to tailor dietary advice and interventions, potentially enhancing treatment compliance and improving the quality of life for pediatric patients with EoE.
Commentary: New Research on BC Chemotherapies, March 2024
The phase 3 KEYNOTE-355 trial established the role of chemotherapy in combination with pembrolizumab in the first-line setting for programmed death-ligand 1 (PD-L1)–positive advanced triple-negative breast cancer (TNBC). Patients unselected for PD-L1 status in this trial who received platinum- or taxane-based chemotherapy with placebo had a median progression-free survival of 5.6 months.[3] Strategies to improve upon efficacy and tolerability are desired in this space, and various trials have evaluated "switch maintenance" that involves receipt of an intensive induction regimen followed by a switch to an alternative/more tolerable regimen after response is achieved.[4] The phase II DORA trial randomized 45 patients with advanced TNBC and ongoing stable disease or complete or partial response from first- or second-line platinum-based chemotherapy to a maintenance regimen of olaparib (300 mg orally twice daily) with or without durvalumab (1500 mg on day 1 and every 4 weeks) (Tan et al). At a median follow-up of 9.8 months, median progression-free survival was 4.0 months (95% CI 2.6-6.1) with olaparib and 6.1 months (95% CI 3.7-10.1) with the combination; both were significantly longer than the historical control of continued platinum-based therapy (P = .0023 and P < .0001, respectively). Durable disease control appeared more pronounced in patients with complete or partial response to prior platinum therapy, and no new safety signals were observed. Future efforts to study this approach include the phase 2/3 KEYLYNK-009 trial, which is evaluating olaparib plus pembrolizumab maintenance therapy after first-line chemotherapy plus pembrolizumab for TNBC.[5]
TNBC is a heterogenous subtype, characterized by aggressive biology, and it benefits from chemotherapy and immunotherapy treatment approaches. Presently, the management of early-stage TNBC often involves neoadjuvant systemic therapy; however, a proportion of patients receive treatment in the postoperative setting, highlighting the relevance of time to initiation of adjuvant therapy as well.[6] Various prior studies have showed that delayed administration of adjuvant chemotherapy for EBC can lead to adverse survival outcomes. Furthermore, this effect is subtype-dependent, with more aggressive tumors (luminal B, triple-negative, human epidermal growth factor receptor 2 [HER2]-positive) exhibiting inferior outcomes with delayed chemotherapy.[7] A retrospective cohort study that included 245 patients with early TNBC who received adjuvant chemotherapy after surgery evaluated the impact of time to initiation of adjuvant therapy in this population (Hatzipanagiotou et al). Superior survival outcomes were observed for the group receiving systemic therapy 22-28 days after surgery (median overall survival 10.2 years) compared with those receiving adjuvant chemotherapy at later time points (29-35 days, 36-42 days, and >6 weeks after surgery; median overall survival 8.3 years, 7.8 years, and 6.9 years, respectively). Patients receiving chemotherapy 22-28 days after surgery had significantly better survival than those receiving chemotherapy 29-35 days (P = .043) and >6 weeks (P = 0.033) postoperatively. This study emphasizes the importance of timely administration of adjuvant chemotherapy for early TNBC, and efforts aimed to identify potential challenges and propose solutions to optimize outcomes in this space are valuable.
Additional References
- Gnant M, Frantal S, Pfeiler G, et al, for the Austrian Breast & Colorectal Cancer Study Group. Long-term outcomes of adjuvant denosumab in breast cancer. NEJM Evid. 2022;1:EVIDoa2200162. doi: 10.1056/EVIDoa2200162 Source
- Fassio A, Idolazzi L, Rossini M, et al. The obesity paradox and osteoporosis. Eating and Weight Disorders - Studies on Anorexia, Bulimia and Obesity. 2018;23:293-30 doi: 10.1007/s40519-018-0505-2 Source
- Cortes J, Cescon DW, Rugo HS, et al, for the KEYNOTE-355 Investigators. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): A randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396:1817-1828. doi: 10.1016/S0140-6736(20)32531-9 Source
- Bachelot T, Filleron T, Bieche I, et al. Durvalumab compared to maintenance chemotherapy in metastatic breast cancer: The randomized phase II SAFIR02-BREAST IMMUNO trial. Nat Med. 2021;27:250-255. doi: 10.1038/s41591-020-01189-2 Source
- Saji S, Cussac AL, Andre F, et al. 68TiP KEYLYNK-009: a phase II/III, open-label, randomized study of pembrolizumab (pembro) + olaparib (ola) vs pembro + chemotherapy after induction with first-line (1L) pembro + chemo in patients (pts) with locally recurrent inoperable or metastatic TNBC (abstract). Ann Oncol. 2020;31(Suppl 6):S1268. doi: 10.1016/j.annonc.2020.10.088 Source
- Ortmann O, Blohmer JU, Sibert NT, et al for 55 breast cancer centers certified by the German Cancer Society. Current clinical practice and outcome of neoadjuvant chemotherapy for early breast cancer: Analysis of individual data from 94,638 patients treated in 55 breast cancer centers. J Cancer Res Clin Oncol. 2023;149:1195-1209. doi: 10.1007/s00432-022-03938-x Source
- Yu KD, Fan L, Qiu LX, et al. Influence of delayed initiation of adjuvant chemotherapy on breast cancer survival is subtype-dependent. Oncotarget. 2017;8:46549-46556. doi: 10.18632/oncotarget.10551 Source
The phase 3 KEYNOTE-355 trial established the role of chemotherapy in combination with pembrolizumab in the first-line setting for programmed death-ligand 1 (PD-L1)–positive advanced triple-negative breast cancer (TNBC). Patients unselected for PD-L1 status in this trial who received platinum- or taxane-based chemotherapy with placebo had a median progression-free survival of 5.6 months.[3] Strategies to improve upon efficacy and tolerability are desired in this space, and various trials have evaluated "switch maintenance" that involves receipt of an intensive induction regimen followed by a switch to an alternative/more tolerable regimen after response is achieved.[4] The phase II DORA trial randomized 45 patients with advanced TNBC and ongoing stable disease or complete or partial response from first- or second-line platinum-based chemotherapy to a maintenance regimen of olaparib (300 mg orally twice daily) with or without durvalumab (1500 mg on day 1 and every 4 weeks) (Tan et al). At a median follow-up of 9.8 months, median progression-free survival was 4.0 months (95% CI 2.6-6.1) with olaparib and 6.1 months (95% CI 3.7-10.1) with the combination; both were significantly longer than the historical control of continued platinum-based therapy (P = .0023 and P < .0001, respectively). Durable disease control appeared more pronounced in patients with complete or partial response to prior platinum therapy, and no new safety signals were observed. Future efforts to study this approach include the phase 2/3 KEYLYNK-009 trial, which is evaluating olaparib plus pembrolizumab maintenance therapy after first-line chemotherapy plus pembrolizumab for TNBC.[5]
TNBC is a heterogenous subtype, characterized by aggressive biology, and it benefits from chemotherapy and immunotherapy treatment approaches. Presently, the management of early-stage TNBC often involves neoadjuvant systemic therapy; however, a proportion of patients receive treatment in the postoperative setting, highlighting the relevance of time to initiation of adjuvant therapy as well.[6] Various prior studies have showed that delayed administration of adjuvant chemotherapy for EBC can lead to adverse survival outcomes. Furthermore, this effect is subtype-dependent, with more aggressive tumors (luminal B, triple-negative, human epidermal growth factor receptor 2 [HER2]-positive) exhibiting inferior outcomes with delayed chemotherapy.[7] A retrospective cohort study that included 245 patients with early TNBC who received adjuvant chemotherapy after surgery evaluated the impact of time to initiation of adjuvant therapy in this population (Hatzipanagiotou et al). Superior survival outcomes were observed for the group receiving systemic therapy 22-28 days after surgery (median overall survival 10.2 years) compared with those receiving adjuvant chemotherapy at later time points (29-35 days, 36-42 days, and >6 weeks after surgery; median overall survival 8.3 years, 7.8 years, and 6.9 years, respectively). Patients receiving chemotherapy 22-28 days after surgery had significantly better survival than those receiving chemotherapy 29-35 days (P = .043) and >6 weeks (P = 0.033) postoperatively. This study emphasizes the importance of timely administration of adjuvant chemotherapy for early TNBC, and efforts aimed to identify potential challenges and propose solutions to optimize outcomes in this space are valuable.
Additional References
- Gnant M, Frantal S, Pfeiler G, et al, for the Austrian Breast & Colorectal Cancer Study Group. Long-term outcomes of adjuvant denosumab in breast cancer. NEJM Evid. 2022;1:EVIDoa2200162. doi: 10.1056/EVIDoa2200162 Source
- Fassio A, Idolazzi L, Rossini M, et al. The obesity paradox and osteoporosis. Eating and Weight Disorders - Studies on Anorexia, Bulimia and Obesity. 2018;23:293-30 doi: 10.1007/s40519-018-0505-2 Source
- Cortes J, Cescon DW, Rugo HS, et al, for the KEYNOTE-355 Investigators. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): A randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396:1817-1828. doi: 10.1016/S0140-6736(20)32531-9 Source
- Bachelot T, Filleron T, Bieche I, et al. Durvalumab compared to maintenance chemotherapy in metastatic breast cancer: The randomized phase II SAFIR02-BREAST IMMUNO trial. Nat Med. 2021;27:250-255. doi: 10.1038/s41591-020-01189-2 Source
- Saji S, Cussac AL, Andre F, et al. 68TiP KEYLYNK-009: a phase II/III, open-label, randomized study of pembrolizumab (pembro) + olaparib (ola) vs pembro + chemotherapy after induction with first-line (1L) pembro + chemo in patients (pts) with locally recurrent inoperable or metastatic TNBC (abstract). Ann Oncol. 2020;31(Suppl 6):S1268. doi: 10.1016/j.annonc.2020.10.088 Source
- Ortmann O, Blohmer JU, Sibert NT, et al for 55 breast cancer centers certified by the German Cancer Society. Current clinical practice and outcome of neoadjuvant chemotherapy for early breast cancer: Analysis of individual data from 94,638 patients treated in 55 breast cancer centers. J Cancer Res Clin Oncol. 2023;149:1195-1209. doi: 10.1007/s00432-022-03938-x Source
- Yu KD, Fan L, Qiu LX, et al. Influence of delayed initiation of adjuvant chemotherapy on breast cancer survival is subtype-dependent. Oncotarget. 2017;8:46549-46556. doi: 10.18632/oncotarget.10551 Source
The phase 3 KEYNOTE-355 trial established the role of chemotherapy in combination with pembrolizumab in the first-line setting for programmed death-ligand 1 (PD-L1)–positive advanced triple-negative breast cancer (TNBC). Patients unselected for PD-L1 status in this trial who received platinum- or taxane-based chemotherapy with placebo had a median progression-free survival of 5.6 months.[3] Strategies to improve upon efficacy and tolerability are desired in this space, and various trials have evaluated "switch maintenance" that involves receipt of an intensive induction regimen followed by a switch to an alternative/more tolerable regimen after response is achieved.[4] The phase II DORA trial randomized 45 patients with advanced TNBC and ongoing stable disease or complete or partial response from first- or second-line platinum-based chemotherapy to a maintenance regimen of olaparib (300 mg orally twice daily) with or without durvalumab (1500 mg on day 1 and every 4 weeks) (Tan et al). At a median follow-up of 9.8 months, median progression-free survival was 4.0 months (95% CI 2.6-6.1) with olaparib and 6.1 months (95% CI 3.7-10.1) with the combination; both were significantly longer than the historical control of continued platinum-based therapy (P = .0023 and P < .0001, respectively). Durable disease control appeared more pronounced in patients with complete or partial response to prior platinum therapy, and no new safety signals were observed. Future efforts to study this approach include the phase 2/3 KEYLYNK-009 trial, which is evaluating olaparib plus pembrolizumab maintenance therapy after first-line chemotherapy plus pembrolizumab for TNBC.[5]
TNBC is a heterogenous subtype, characterized by aggressive biology, and it benefits from chemotherapy and immunotherapy treatment approaches. Presently, the management of early-stage TNBC often involves neoadjuvant systemic therapy; however, a proportion of patients receive treatment in the postoperative setting, highlighting the relevance of time to initiation of adjuvant therapy as well.[6] Various prior studies have showed that delayed administration of adjuvant chemotherapy for EBC can lead to adverse survival outcomes. Furthermore, this effect is subtype-dependent, with more aggressive tumors (luminal B, triple-negative, human epidermal growth factor receptor 2 [HER2]-positive) exhibiting inferior outcomes with delayed chemotherapy.[7] A retrospective cohort study that included 245 patients with early TNBC who received adjuvant chemotherapy after surgery evaluated the impact of time to initiation of adjuvant therapy in this population (Hatzipanagiotou et al). Superior survival outcomes were observed for the group receiving systemic therapy 22-28 days after surgery (median overall survival 10.2 years) compared with those receiving adjuvant chemotherapy at later time points (29-35 days, 36-42 days, and >6 weeks after surgery; median overall survival 8.3 years, 7.8 years, and 6.9 years, respectively). Patients receiving chemotherapy 22-28 days after surgery had significantly better survival than those receiving chemotherapy 29-35 days (P = .043) and >6 weeks (P = 0.033) postoperatively. This study emphasizes the importance of timely administration of adjuvant chemotherapy for early TNBC, and efforts aimed to identify potential challenges and propose solutions to optimize outcomes in this space are valuable.
Additional References
- Gnant M, Frantal S, Pfeiler G, et al, for the Austrian Breast & Colorectal Cancer Study Group. Long-term outcomes of adjuvant denosumab in breast cancer. NEJM Evid. 2022;1:EVIDoa2200162. doi: 10.1056/EVIDoa2200162 Source
- Fassio A, Idolazzi L, Rossini M, et al. The obesity paradox and osteoporosis. Eating and Weight Disorders - Studies on Anorexia, Bulimia and Obesity. 2018;23:293-30 doi: 10.1007/s40519-018-0505-2 Source
- Cortes J, Cescon DW, Rugo HS, et al, for the KEYNOTE-355 Investigators. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): A randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396:1817-1828. doi: 10.1016/S0140-6736(20)32531-9 Source
- Bachelot T, Filleron T, Bieche I, et al. Durvalumab compared to maintenance chemotherapy in metastatic breast cancer: The randomized phase II SAFIR02-BREAST IMMUNO trial. Nat Med. 2021;27:250-255. doi: 10.1038/s41591-020-01189-2 Source
- Saji S, Cussac AL, Andre F, et al. 68TiP KEYLYNK-009: a phase II/III, open-label, randomized study of pembrolizumab (pembro) + olaparib (ola) vs pembro + chemotherapy after induction with first-line (1L) pembro + chemo in patients (pts) with locally recurrent inoperable or metastatic TNBC (abstract). Ann Oncol. 2020;31(Suppl 6):S1268. doi: 10.1016/j.annonc.2020.10.088 Source
- Ortmann O, Blohmer JU, Sibert NT, et al for 55 breast cancer centers certified by the German Cancer Society. Current clinical practice and outcome of neoadjuvant chemotherapy for early breast cancer: Analysis of individual data from 94,638 patients treated in 55 breast cancer centers. J Cancer Res Clin Oncol. 2023;149:1195-1209. doi: 10.1007/s00432-022-03938-x Source
- Yu KD, Fan L, Qiu LX, et al. Influence of delayed initiation of adjuvant chemotherapy on breast cancer survival is subtype-dependent. Oncotarget. 2017;8:46549-46556. doi: 10.18632/oncotarget.10551 Source
Commentary: Medication Timing and Other Dupilumab Concerns, March 2024
When skin diseases affect the palm or sole, they can have a disproportionately large negative effect on patients' lives. Hand and foot dermatitis can be disabling. Simpson and colleagues find that dupilumab is an effective treatment for AD of the hands and feet. Having safe and effective treatment for hand and foot dermatitis will be life-changing for many of our patients.
Patients often do very well with biologic treatment. When they do, they often wonder, Do I need to continue taking the medication? Lasheras-Pérez and colleagues found that the great majority of patients doing well taking dupilumab for AD could stretch out their dosing interval. I suspect a lot of our patients are doing this already. I used to worry that stretching out the dosing interval might lead to antidrug antibodies and loss of activity. Such loss of activity doesn't appear common. Because we also have multiple alternative treatments for severe AD, I think it may be quite reasonable for patients to try spreading out their doses after their disease has been well controlled for a good long time.
Superficial skin infections aren't rare in children, particularly children with AD. Paller and colleagues' study is informative about the safety of dupilumab in children. The drug, which blocks a pathway of the immune system, was associated with fewer infections. This is good news. The reduction in infections could be through restoring "immune balance" (whatever that means) or by improving skin barrier function. Perhaps the low rate of infection explains why dupilumab is not considered immunosuppressive.
I love studies of drug survival because I think that knowing the percentage of patients who stay with drug treatment is a good measure of overall safety and efficacy. Pezzolo and colleagues found — perhaps not surprisingly given the extraordinary efficacy of upadacitinib for AD — that almost no one discontinued the drug over 1.5 years due to lack of efficacy. There were patients who discontinued due to adverse events (and additional patients lost to follow-up who perhaps also discontinued the drug), but 80% of patients were still in the study at the end of 1.5 years. Three patients who weren't vaccinated for shingles developed shingles; encouraging patients to get the shingles vaccine may be a prudent measure when starting patients taking upadacitinib.
When skin diseases affect the palm or sole, they can have a disproportionately large negative effect on patients' lives. Hand and foot dermatitis can be disabling. Simpson and colleagues find that dupilumab is an effective treatment for AD of the hands and feet. Having safe and effective treatment for hand and foot dermatitis will be life-changing for many of our patients.
Patients often do very well with biologic treatment. When they do, they often wonder, Do I need to continue taking the medication? Lasheras-Pérez and colleagues found that the great majority of patients doing well taking dupilumab for AD could stretch out their dosing interval. I suspect a lot of our patients are doing this already. I used to worry that stretching out the dosing interval might lead to antidrug antibodies and loss of activity. Such loss of activity doesn't appear common. Because we also have multiple alternative treatments for severe AD, I think it may be quite reasonable for patients to try spreading out their doses after their disease has been well controlled for a good long time.
Superficial skin infections aren't rare in children, particularly children with AD. Paller and colleagues' study is informative about the safety of dupilumab in children. The drug, which blocks a pathway of the immune system, was associated with fewer infections. This is good news. The reduction in infections could be through restoring "immune balance" (whatever that means) or by improving skin barrier function. Perhaps the low rate of infection explains why dupilumab is not considered immunosuppressive.
I love studies of drug survival because I think that knowing the percentage of patients who stay with drug treatment is a good measure of overall safety and efficacy. Pezzolo and colleagues found — perhaps not surprisingly given the extraordinary efficacy of upadacitinib for AD — that almost no one discontinued the drug over 1.5 years due to lack of efficacy. There were patients who discontinued due to adverse events (and additional patients lost to follow-up who perhaps also discontinued the drug), but 80% of patients were still in the study at the end of 1.5 years. Three patients who weren't vaccinated for shingles developed shingles; encouraging patients to get the shingles vaccine may be a prudent measure when starting patients taking upadacitinib.
When skin diseases affect the palm or sole, they can have a disproportionately large negative effect on patients' lives. Hand and foot dermatitis can be disabling. Simpson and colleagues find that dupilumab is an effective treatment for AD of the hands and feet. Having safe and effective treatment for hand and foot dermatitis will be life-changing for many of our patients.
Patients often do very well with biologic treatment. When they do, they often wonder, Do I need to continue taking the medication? Lasheras-Pérez and colleagues found that the great majority of patients doing well taking dupilumab for AD could stretch out their dosing interval. I suspect a lot of our patients are doing this already. I used to worry that stretching out the dosing interval might lead to antidrug antibodies and loss of activity. Such loss of activity doesn't appear common. Because we also have multiple alternative treatments for severe AD, I think it may be quite reasonable for patients to try spreading out their doses after their disease has been well controlled for a good long time.
Superficial skin infections aren't rare in children, particularly children with AD. Paller and colleagues' study is informative about the safety of dupilumab in children. The drug, which blocks a pathway of the immune system, was associated with fewer infections. This is good news. The reduction in infections could be through restoring "immune balance" (whatever that means) or by improving skin barrier function. Perhaps the low rate of infection explains why dupilumab is not considered immunosuppressive.
I love studies of drug survival because I think that knowing the percentage of patients who stay with drug treatment is a good measure of overall safety and efficacy. Pezzolo and colleagues found — perhaps not surprisingly given the extraordinary efficacy of upadacitinib for AD — that almost no one discontinued the drug over 1.5 years due to lack of efficacy. There were patients who discontinued due to adverse events (and additional patients lost to follow-up who perhaps also discontinued the drug), but 80% of patients were still in the study at the end of 1.5 years. Three patients who weren't vaccinated for shingles developed shingles; encouraging patients to get the shingles vaccine may be a prudent measure when starting patients taking upadacitinib.
Commentary: Allergies, EDN, and the Psychosocial Burden of EoE, February 2024
A significant gap in our understanding of eosinophilic esophagitis (EoE) lies in how environmental factors, such as allergens or food, influence the response to proton pump inhibitor (PPI) therapy. While PPI achieve histologic remission in approximately 50% of patients, the response in the remaining 50% remains unclear. Addressing this, Muftah and colleagues conducted a study to evaluate the relationship between environmental and food allergies and PPI response in newly diagnosed EoE patients.
Between 2012 and 2016, adult patients newly diagnosed with EoE were tested for environmental and food allergies. Following diagnosis, patients participated in an 8-week trial of twice-daily PPI therapy. The treatment's effectiveness was assessed through repeated upper endoscopies with esophageal biopsies.
The study's primary outcome was the histologic remission of EoE, defined as a decrease in eosinophils to < 15 eosinophils/high-powered field (eos/hpf) in all esophageal biopsy samples during repeat endoscopy. Out of 61 patients, 21 achieved histologic remission, while 40 were classified as having PPI-nonresponding EoE. Among PPI-nonresponding EoE patients, positive food allergen testing was significantly more prevalent compared with PPI-responding EoE patients (82.5% vs 42.9%; P = .0003). Additionally, patients with >10 positive environmental allergen tests were significantly less likely to be PPI-responding EoE patients than those with <10 positive results (21% vs 53.9%; P = .03). A similar trend was observed in patients with >5 positive environmental allergens.
This study is not without limitations. It may exhibit a selection bias toward more severe cases and has a relatively small sample size, affecting its statistical power and generalizability.
This research supports the idea of more tailored management for EoE patients, focusing on their allergen profile, potentially leading to more effective treatment strategies and reducing unnecessary PPI trials. The statistically significant results pave the way for further research, providing an additional tool to predict PPI responsiveness and prevent delays in achieving remission.
Clinicians should consider patient characteristics, particularly positive food allergen tests, that might affect treatment response. More studies are needed, however, to understand the effect of environmental allergies on PPI response fully. A notable finding is that specific aeroallergens, such as oak, birch, Hormodendrum mold, dust mite (Dermatophagoides pteronyssinus), tree mix, and grass mix allergens, are associated with a lack of PPI response. This raises questions about whether exposure to these allergens during peak seasons could worsen PPI response in allergic EoE patients.
Key takeaways from this study include: (1) the importance of integrating allergen testing in EoE patients, especially those unresponsive to standard PPI therapy or suspected as having allergic phenotypes; (2) the need to monitor and adjust therapy based on clinical and histologic responses; and (3) the necessity of staying abreast of emerging research in this area.
EoE diagnosis presents unique challenges, particularly when patients exhibit exclusive distal esophageal eosinophilia or when discrepancies arise between endoscopic and histologic findings. Eosinophil-derived neurotoxin (EDN), a molecule previously studied for its role in monitoring allergy-mediated inflammatory diseases such as asthma and eczema, can shed light on these diagnostic difficulties.
Thomas and coworkers conducted a retrospective study in which they reviewed 231 pediatric patients, obtaining a minimum of four biopsies from at least two different levels of the esophagus. The study aimed to evaluate whether EDN concentrations, determined through esophageal epithelial brushing at the time of biopsy, could serve as an adjunctive diagnostic tool for EoE.
EDN levels proved sensitive (84.4%) and specific (94.6%) in evaluating active EoE when several measures of EoE were used in patients with active EoE compared with those with inactive EoE and the control group. Previous studies at the same institution had found EDN useful for differentiating EoE patients from non-EoE patients. Moreover, an EDN concentration > 10 μg/mL, when collected through esophageal epithelial brushing, was highly sensitive (97%) and specific (89%) for active EoE. This finding suggests the potential for using EDN as a biochemical marker, enhancing diagnostic accuracy and reducing the need for additional interventions in complex cases.
EDN as a biomarker could be invaluable for distinguishing difficult cases, such as those involving distal eosinophilia, active vs nonactive EoE, or non-EoE conditions, such as gastroesophageal reflux disease. Of note, lower EDN levels were observed in pediatric EoE patients who responded to PPI, suggesting EDN's potential utility in predicting PPI responsiveness. Incorporating the measurement of eosinophilic activity could add a new dimension to existing criteria, equipping clinicians with more precise diagnostic tools and reducing the reliance on multiple procedures. This approach would strengthen the correlation between symptomatic, endoscopic, and histologic data.
The study by Jensen and colleagues sheds light on a crucial aspect of EoE management: the psychosocial burden. A recent EoE diagnosis can be associated with increased symptom burden, somatization, and anxiety in patients and families, underscoring the need for a multidisciplinary approach to patient care that considers both physical and mental health. To date, numerous studies have focused on understanding the disease, its follow-up, and treatment. However, there has been limited exploration of the psychosocial burden and patient-associated factors in EoE.
In this context, this team aimed to enhance our understanding of the burden of EoE by evaluating psychosocial comorbidities, such as disordered sleep, anxiety, and somatization, in a pediatric population with EoE. The study included 87 patients of age 8-18 years who completed validated assessments during routine clinic visits, encompassing EoE symptoms (Pediatric Eosinophilic Esophagitis Symptom Scores, PEESSv2.0), quality of life (PedsQL-EoE), anxiety state and trait (State-Trait Anxiety Inventory for Children, STAI-C), somatization (Children's Somatic Symptoms Inventory-24, CSSI-24), and sleep-disordered breathing (University of Michigan Pediatric Sleep Questionnaire, PSQ).
The mean age of the participants was 12.8 years, highlighting the importance of addressing psychosocial distress in this age group, which undergoes crucial developmental stages. Most patients (82%, 71) had been diagnosed with EoE at least 12 months prior, and 60% (52) were treated with multiple approaches. Additionally, 34% (29) had undergone seven or more esophagogastroduodenoscopies, and nearly one third (33%, 27) had experienced a gastrointestinal-related emergency department visit. These factors potentially increase patient stress due to the continuous need for repeat procedures and hospital visits. An intriguing finding was that patients with shorter disease durations (6-12 months since diagnosis) experienced higher symptom burdens (P = .03). Patients with public insurance had less favorable scores for sleep-disordered breathing (P = .01).
Significantly, patients with neurodevelopmental comorbidities had higher scores for somatic symptoms, trait anxiety, and sleep-disordered breathing, and lower quality-of-life scores, compared with those without such comorbidities (P < .01 for all), suggesting that patients with neurodevelopmental issues might particularly benefit from tailored treatments addressing these aspects of the disease. Furthermore, patients with shorter disease durations since diagnosis exhibited higher somatic symptoms and trait anxiety (both P < .01). The study also revealed that patients with fewer esophagogastroduodenoscopies (1-3) had higher somatic symptom scores (P < .01), state anxiety (P = .02), and trait anxiety (P = .03). EoE-associated symptom burden was significantly correlated with increased somatic symptoms (0.34; 95% CI 0.23-0.45) and decreased quality of life (-0.42; 95% CI -0.59 to -0.25). Concerns about eating food and EoE-associated symptoms were both linked to the EoE-associated symptom burden.
This study has several limitations, including a relatively small sample size, which decreases the power and limits inferences for smaller groups within the sample. There was also an imbalance in gender distribution, with only 26% of patients being female, potentially limiting the generalizability of the findings. Moreover, the study included only EoE patients, lacking a control group for comparison to the general pediatric population.
Highlighting a significant aspect of pediatric EoE treatment, this study illuminates an area that might affect patients' long-term quality of life. It underscores the need for multidisciplinary care for EoE patients, where mental health professionals, such as psychologists or psychiatrists, can play a vital role in improving mental health through early identification and intervention for anxiety and somatization disorders. They can also provide education for patients and families on coping strategies. Peer support groups for children and adolescents could be another beneficial tool, allowing them to share experiences and reduce feelings of isolation.
Physicians who treat chronic diseases such as EoE should consider psychosocial factors, as they can affect both physical and mental quality of life. Using screening tools (such as PEESSv2.0, PedsQL-EoE, STAI-C, CSSI-24, or PSQ) during clinic visits can facilitate a more comprehensive evaluation.
A significant gap in our understanding of eosinophilic esophagitis (EoE) lies in how environmental factors, such as allergens or food, influence the response to proton pump inhibitor (PPI) therapy. While PPI achieve histologic remission in approximately 50% of patients, the response in the remaining 50% remains unclear. Addressing this, Muftah and colleagues conducted a study to evaluate the relationship between environmental and food allergies and PPI response in newly diagnosed EoE patients.
Between 2012 and 2016, adult patients newly diagnosed with EoE were tested for environmental and food allergies. Following diagnosis, patients participated in an 8-week trial of twice-daily PPI therapy. The treatment's effectiveness was assessed through repeated upper endoscopies with esophageal biopsies.
The study's primary outcome was the histologic remission of EoE, defined as a decrease in eosinophils to < 15 eosinophils/high-powered field (eos/hpf) in all esophageal biopsy samples during repeat endoscopy. Out of 61 patients, 21 achieved histologic remission, while 40 were classified as having PPI-nonresponding EoE. Among PPI-nonresponding EoE patients, positive food allergen testing was significantly more prevalent compared with PPI-responding EoE patients (82.5% vs 42.9%; P = .0003). Additionally, patients with >10 positive environmental allergen tests were significantly less likely to be PPI-responding EoE patients than those with <10 positive results (21% vs 53.9%; P = .03). A similar trend was observed in patients with >5 positive environmental allergens.
This study is not without limitations. It may exhibit a selection bias toward more severe cases and has a relatively small sample size, affecting its statistical power and generalizability.
This research supports the idea of more tailored management for EoE patients, focusing on their allergen profile, potentially leading to more effective treatment strategies and reducing unnecessary PPI trials. The statistically significant results pave the way for further research, providing an additional tool to predict PPI responsiveness and prevent delays in achieving remission.
Clinicians should consider patient characteristics, particularly positive food allergen tests, that might affect treatment response. More studies are needed, however, to understand the effect of environmental allergies on PPI response fully. A notable finding is that specific aeroallergens, such as oak, birch, Hormodendrum mold, dust mite (Dermatophagoides pteronyssinus), tree mix, and grass mix allergens, are associated with a lack of PPI response. This raises questions about whether exposure to these allergens during peak seasons could worsen PPI response in allergic EoE patients.
Key takeaways from this study include: (1) the importance of integrating allergen testing in EoE patients, especially those unresponsive to standard PPI therapy or suspected as having allergic phenotypes; (2) the need to monitor and adjust therapy based on clinical and histologic responses; and (3) the necessity of staying abreast of emerging research in this area.
EoE diagnosis presents unique challenges, particularly when patients exhibit exclusive distal esophageal eosinophilia or when discrepancies arise between endoscopic and histologic findings. Eosinophil-derived neurotoxin (EDN), a molecule previously studied for its role in monitoring allergy-mediated inflammatory diseases such as asthma and eczema, can shed light on these diagnostic difficulties.
Thomas and coworkers conducted a retrospective study in which they reviewed 231 pediatric patients, obtaining a minimum of four biopsies from at least two different levels of the esophagus. The study aimed to evaluate whether EDN concentrations, determined through esophageal epithelial brushing at the time of biopsy, could serve as an adjunctive diagnostic tool for EoE.
EDN levels proved sensitive (84.4%) and specific (94.6%) in evaluating active EoE when several measures of EoE were used in patients with active EoE compared with those with inactive EoE and the control group. Previous studies at the same institution had found EDN useful for differentiating EoE patients from non-EoE patients. Moreover, an EDN concentration > 10 μg/mL, when collected through esophageal epithelial brushing, was highly sensitive (97%) and specific (89%) for active EoE. This finding suggests the potential for using EDN as a biochemical marker, enhancing diagnostic accuracy and reducing the need for additional interventions in complex cases.
EDN as a biomarker could be invaluable for distinguishing difficult cases, such as those involving distal eosinophilia, active vs nonactive EoE, or non-EoE conditions, such as gastroesophageal reflux disease. Of note, lower EDN levels were observed in pediatric EoE patients who responded to PPI, suggesting EDN's potential utility in predicting PPI responsiveness. Incorporating the measurement of eosinophilic activity could add a new dimension to existing criteria, equipping clinicians with more precise diagnostic tools and reducing the reliance on multiple procedures. This approach would strengthen the correlation between symptomatic, endoscopic, and histologic data.
The study by Jensen and colleagues sheds light on a crucial aspect of EoE management: the psychosocial burden. A recent EoE diagnosis can be associated with increased symptom burden, somatization, and anxiety in patients and families, underscoring the need for a multidisciplinary approach to patient care that considers both physical and mental health. To date, numerous studies have focused on understanding the disease, its follow-up, and treatment. However, there has been limited exploration of the psychosocial burden and patient-associated factors in EoE.
In this context, this team aimed to enhance our understanding of the burden of EoE by evaluating psychosocial comorbidities, such as disordered sleep, anxiety, and somatization, in a pediatric population with EoE. The study included 87 patients of age 8-18 years who completed validated assessments during routine clinic visits, encompassing EoE symptoms (Pediatric Eosinophilic Esophagitis Symptom Scores, PEESSv2.0), quality of life (PedsQL-EoE), anxiety state and trait (State-Trait Anxiety Inventory for Children, STAI-C), somatization (Children's Somatic Symptoms Inventory-24, CSSI-24), and sleep-disordered breathing (University of Michigan Pediatric Sleep Questionnaire, PSQ).
The mean age of the participants was 12.8 years, highlighting the importance of addressing psychosocial distress in this age group, which undergoes crucial developmental stages. Most patients (82%, 71) had been diagnosed with EoE at least 12 months prior, and 60% (52) were treated with multiple approaches. Additionally, 34% (29) had undergone seven or more esophagogastroduodenoscopies, and nearly one third (33%, 27) had experienced a gastrointestinal-related emergency department visit. These factors potentially increase patient stress due to the continuous need for repeat procedures and hospital visits. An intriguing finding was that patients with shorter disease durations (6-12 months since diagnosis) experienced higher symptom burdens (P = .03). Patients with public insurance had less favorable scores for sleep-disordered breathing (P = .01).
Significantly, patients with neurodevelopmental comorbidities had higher scores for somatic symptoms, trait anxiety, and sleep-disordered breathing, and lower quality-of-life scores, compared with those without such comorbidities (P < .01 for all), suggesting that patients with neurodevelopmental issues might particularly benefit from tailored treatments addressing these aspects of the disease. Furthermore, patients with shorter disease durations since diagnosis exhibited higher somatic symptoms and trait anxiety (both P < .01). The study also revealed that patients with fewer esophagogastroduodenoscopies (1-3) had higher somatic symptom scores (P < .01), state anxiety (P = .02), and trait anxiety (P = .03). EoE-associated symptom burden was significantly correlated with increased somatic symptoms (0.34; 95% CI 0.23-0.45) and decreased quality of life (-0.42; 95% CI -0.59 to -0.25). Concerns about eating food and EoE-associated symptoms were both linked to the EoE-associated symptom burden.
This study has several limitations, including a relatively small sample size, which decreases the power and limits inferences for smaller groups within the sample. There was also an imbalance in gender distribution, with only 26% of patients being female, potentially limiting the generalizability of the findings. Moreover, the study included only EoE patients, lacking a control group for comparison to the general pediatric population.
Highlighting a significant aspect of pediatric EoE treatment, this study illuminates an area that might affect patients' long-term quality of life. It underscores the need for multidisciplinary care for EoE patients, where mental health professionals, such as psychologists or psychiatrists, can play a vital role in improving mental health through early identification and intervention for anxiety and somatization disorders. They can also provide education for patients and families on coping strategies. Peer support groups for children and adolescents could be another beneficial tool, allowing them to share experiences and reduce feelings of isolation.
Physicians who treat chronic diseases such as EoE should consider psychosocial factors, as they can affect both physical and mental quality of life. Using screening tools (such as PEESSv2.0, PedsQL-EoE, STAI-C, CSSI-24, or PSQ) during clinic visits can facilitate a more comprehensive evaluation.
A significant gap in our understanding of eosinophilic esophagitis (EoE) lies in how environmental factors, such as allergens or food, influence the response to proton pump inhibitor (PPI) therapy. While PPI achieve histologic remission in approximately 50% of patients, the response in the remaining 50% remains unclear. Addressing this, Muftah and colleagues conducted a study to evaluate the relationship between environmental and food allergies and PPI response in newly diagnosed EoE patients.
Between 2012 and 2016, adult patients newly diagnosed with EoE were tested for environmental and food allergies. Following diagnosis, patients participated in an 8-week trial of twice-daily PPI therapy. The treatment's effectiveness was assessed through repeated upper endoscopies with esophageal biopsies.
The study's primary outcome was the histologic remission of EoE, defined as a decrease in eosinophils to < 15 eosinophils/high-powered field (eos/hpf) in all esophageal biopsy samples during repeat endoscopy. Out of 61 patients, 21 achieved histologic remission, while 40 were classified as having PPI-nonresponding EoE. Among PPI-nonresponding EoE patients, positive food allergen testing was significantly more prevalent compared with PPI-responding EoE patients (82.5% vs 42.9%; P = .0003). Additionally, patients with >10 positive environmental allergen tests were significantly less likely to be PPI-responding EoE patients than those with <10 positive results (21% vs 53.9%; P = .03). A similar trend was observed in patients with >5 positive environmental allergens.
This study is not without limitations. It may exhibit a selection bias toward more severe cases and has a relatively small sample size, affecting its statistical power and generalizability.
This research supports the idea of more tailored management for EoE patients, focusing on their allergen profile, potentially leading to more effective treatment strategies and reducing unnecessary PPI trials. The statistically significant results pave the way for further research, providing an additional tool to predict PPI responsiveness and prevent delays in achieving remission.
Clinicians should consider patient characteristics, particularly positive food allergen tests, that might affect treatment response. More studies are needed, however, to understand the effect of environmental allergies on PPI response fully. A notable finding is that specific aeroallergens, such as oak, birch, Hormodendrum mold, dust mite (Dermatophagoides pteronyssinus), tree mix, and grass mix allergens, are associated with a lack of PPI response. This raises questions about whether exposure to these allergens during peak seasons could worsen PPI response in allergic EoE patients.
Key takeaways from this study include: (1) the importance of integrating allergen testing in EoE patients, especially those unresponsive to standard PPI therapy or suspected as having allergic phenotypes; (2) the need to monitor and adjust therapy based on clinical and histologic responses; and (3) the necessity of staying abreast of emerging research in this area.
EoE diagnosis presents unique challenges, particularly when patients exhibit exclusive distal esophageal eosinophilia or when discrepancies arise between endoscopic and histologic findings. Eosinophil-derived neurotoxin (EDN), a molecule previously studied for its role in monitoring allergy-mediated inflammatory diseases such as asthma and eczema, can shed light on these diagnostic difficulties.
Thomas and coworkers conducted a retrospective study in which they reviewed 231 pediatric patients, obtaining a minimum of four biopsies from at least two different levels of the esophagus. The study aimed to evaluate whether EDN concentrations, determined through esophageal epithelial brushing at the time of biopsy, could serve as an adjunctive diagnostic tool for EoE.
EDN levels proved sensitive (84.4%) and specific (94.6%) in evaluating active EoE when several measures of EoE were used in patients with active EoE compared with those with inactive EoE and the control group. Previous studies at the same institution had found EDN useful for differentiating EoE patients from non-EoE patients. Moreover, an EDN concentration > 10 μg/mL, when collected through esophageal epithelial brushing, was highly sensitive (97%) and specific (89%) for active EoE. This finding suggests the potential for using EDN as a biochemical marker, enhancing diagnostic accuracy and reducing the need for additional interventions in complex cases.
EDN as a biomarker could be invaluable for distinguishing difficult cases, such as those involving distal eosinophilia, active vs nonactive EoE, or non-EoE conditions, such as gastroesophageal reflux disease. Of note, lower EDN levels were observed in pediatric EoE patients who responded to PPI, suggesting EDN's potential utility in predicting PPI responsiveness. Incorporating the measurement of eosinophilic activity could add a new dimension to existing criteria, equipping clinicians with more precise diagnostic tools and reducing the reliance on multiple procedures. This approach would strengthen the correlation between symptomatic, endoscopic, and histologic data.
The study by Jensen and colleagues sheds light on a crucial aspect of EoE management: the psychosocial burden. A recent EoE diagnosis can be associated with increased symptom burden, somatization, and anxiety in patients and families, underscoring the need for a multidisciplinary approach to patient care that considers both physical and mental health. To date, numerous studies have focused on understanding the disease, its follow-up, and treatment. However, there has been limited exploration of the psychosocial burden and patient-associated factors in EoE.
In this context, this team aimed to enhance our understanding of the burden of EoE by evaluating psychosocial comorbidities, such as disordered sleep, anxiety, and somatization, in a pediatric population with EoE. The study included 87 patients of age 8-18 years who completed validated assessments during routine clinic visits, encompassing EoE symptoms (Pediatric Eosinophilic Esophagitis Symptom Scores, PEESSv2.0), quality of life (PedsQL-EoE), anxiety state and trait (State-Trait Anxiety Inventory for Children, STAI-C), somatization (Children's Somatic Symptoms Inventory-24, CSSI-24), and sleep-disordered breathing (University of Michigan Pediatric Sleep Questionnaire, PSQ).
The mean age of the participants was 12.8 years, highlighting the importance of addressing psychosocial distress in this age group, which undergoes crucial developmental stages. Most patients (82%, 71) had been diagnosed with EoE at least 12 months prior, and 60% (52) were treated with multiple approaches. Additionally, 34% (29) had undergone seven or more esophagogastroduodenoscopies, and nearly one third (33%, 27) had experienced a gastrointestinal-related emergency department visit. These factors potentially increase patient stress due to the continuous need for repeat procedures and hospital visits. An intriguing finding was that patients with shorter disease durations (6-12 months since diagnosis) experienced higher symptom burdens (P = .03). Patients with public insurance had less favorable scores for sleep-disordered breathing (P = .01).
Significantly, patients with neurodevelopmental comorbidities had higher scores for somatic symptoms, trait anxiety, and sleep-disordered breathing, and lower quality-of-life scores, compared with those without such comorbidities (P < .01 for all), suggesting that patients with neurodevelopmental issues might particularly benefit from tailored treatments addressing these aspects of the disease. Furthermore, patients with shorter disease durations since diagnosis exhibited higher somatic symptoms and trait anxiety (both P < .01). The study also revealed that patients with fewer esophagogastroduodenoscopies (1-3) had higher somatic symptom scores (P < .01), state anxiety (P = .02), and trait anxiety (P = .03). EoE-associated symptom burden was significantly correlated with increased somatic symptoms (0.34; 95% CI 0.23-0.45) and decreased quality of life (-0.42; 95% CI -0.59 to -0.25). Concerns about eating food and EoE-associated symptoms were both linked to the EoE-associated symptom burden.
This study has several limitations, including a relatively small sample size, which decreases the power and limits inferences for smaller groups within the sample. There was also an imbalance in gender distribution, with only 26% of patients being female, potentially limiting the generalizability of the findings. Moreover, the study included only EoE patients, lacking a control group for comparison to the general pediatric population.
Highlighting a significant aspect of pediatric EoE treatment, this study illuminates an area that might affect patients' long-term quality of life. It underscores the need for multidisciplinary care for EoE patients, where mental health professionals, such as psychologists or psychiatrists, can play a vital role in improving mental health through early identification and intervention for anxiety and somatization disorders. They can also provide education for patients and families on coping strategies. Peer support groups for children and adolescents could be another beneficial tool, allowing them to share experiences and reduce feelings of isolation.
Physicians who treat chronic diseases such as EoE should consider psychosocial factors, as they can affect both physical and mental quality of life. Using screening tools (such as PEESSv2.0, PedsQL-EoE, STAI-C, CSSI-24, or PSQ) during clinic visits can facilitate a more comprehensive evaluation.
Commentary: Risks for Eosinophilic Esophagitis: IBD, Eczema, Diet, and Acid Suppressants, January 2024
Although there exists a recognized association between IBD and secondary EoE diagnoses, studies focusing on the primary diagnosis of EoE alongside IBD have yielded conflicting results. Dr Amiko Uchida, from the University of Utah Department of Medicine, working with colleagues from the Department of Medical Epidemiology and Biostatistics at Karolinska Institutet in Stockholm, Sweden, led by Dr Jonas F. Ludvigsson, conducted a comprehensive study spanning 1990-2019 to explore the relationship between these two diseases.
Dr Uchida and colleagues assessed the association among Swedish patients diagnosed with biopsy-verified EoE (n = 1587) between 1990 and 2017. These patients were age- and sex-matched with up to five reference individuals from the general population (n = 7808). The primary focus was to discern the relationship between the primary diagnosis of EoE and the subsequent diagnosis of IBD.
The study's findings underscore the importance of heightened awareness among healthcare professionals regarding the potential association between EoE and the development of subsequent IBD. Collaborative efforts between physicians, gastroenterologists, and specialists in allergic diseases are crucial to ensure comprehensive care and timely identification of gastrointestinal complications in patients with EoE.
These results indicate a potential interplay between EoE and the pathogenesis of IBD, particularly Crohn's disease. In patients with EoE, careful consideration of gastrointestinal symptoms suggestive of IBD, such as abdominal pain, diarrhea, and rectal bleeding, is pivotal, necessitating further evaluation and appropriate monitoring for early detection and management of IBD.
Upon diagnosis, clinicians must develop a management plan for this chronic and critical disease. This study aids in planning future screenings for these patients, because one third of those diagnosed with EoE are at risk of developing IBD within a year. Therefore, primary physicians must remain vigilant for the development of gastrointestinal symptoms leading to a diagnosis of Crohn's disease or ulcerative colitis. Additionally, family awareness is crucial owing to observed associations between siblings, suggesting a potential role of genetics or early environmental factors in EoE development and future IBD diagnoses.
Further research is necessary to elucidate the shared pathophysiologic mechanisms connecting EoE and IBD. It is important to consider certain details, however; for instance, 31% of all subsequent IBD cases were diagnosed within the first year after an EoE diagnosis, potentially indicating a role of detection bias in these findings. Using a validated nationwide cohort and comparing study individuals with their siblings helped control for potential intrafamilial confounders as well as some environmental confounders, minimizing such biases as selection bias due to socioeconomic status. This strengthens the observed association in this study.
These insights into the increased risk for IBD, notably Crohn's disease, among patients with EoE underscore the need for thorough clinical evaluation and vigilant monitoring for gastrointestinal complications in this population.
A retrospective study conducted in pediatric patients presenting with an aerodigestive manifestation aimed to assess the factors associated with EoE and the diagnostic role of triple endoscopy. The results suggested a potential association between a family history of eczema and a diet lacking allergenic foods with a future diagnosis of EoE.
This study by Sheila Moran and colleagues, led by Dr Christina J. Yang from the Department of Otorhinolaryngology, Head and Neck Surgery at the Albert Einstein College of Medicine, Bronx, New York, aimed to identify preoperative risk factors linked to an EoE diagnosis in children undergoing triple endoscopy. They evaluated 119 pediatric patients aged 0-21 years who underwent triple endoscopy (including flexible bronchoscopy, rigid direct laryngoscopy and bronchoscopy, and esophagoscopy with biopsy) at the Children's Hospital at Montefiore between January 1, 2015, and December 31, 2019.
The study underscored the significance of both genetic predisposition and dietary influences in EoE development. Understanding the interplay between familial atopic conditions and dietary choices among pediatric patients with aerodigestive dysfunction is crucial for early identification and implementing preventive strategies against EoE.
As clinicians, it's essential to consider rare diseases like EoE when patients present with mixed symptoms, including aerodigestive symptoms, a family history of eczema, and a history of environmental allergies, given the association between these conditions. The potential link between a family history of eczema and increased EoE risk suggests a shared genetic susceptibility among allergic conditions. Therefore, clinicians evaluating children with aerodigestive dysfunction, particularly those with a familial history of eczema, should maintain a high index of suspicion for EoE, prompting vigilant monitoring and appropriate diagnostic assessments.
When contemplating advanced procedures, such as triple endoscopy or biopsy sampling, considering the patient's previous medical history and the effect of dietary modifications, such as incorporating or excluding dairy from the diet, warrants further investigation in the context of EoE prevention. Clinicians should consider providing dietary counseling and personalized nutritional plans based on evidence-based approaches to potentially mitigate EoE risk in susceptible pediatric populations.
Additionally, it's crucial to consider EoE in minority racial groups and underserved communities and encourage the use of diagnostic tests, such as triple endoscopy, to facilitate early diagnosis. Healthcare providers should contemplate integrating family history assessments, particularly regarding eczema, into the evaluation of children with aerodigestive dysfunction. This information can assist in risk assessment and early identification of individuals at a higher risk of developing EoE, enabling prompt intervention and management.
The increasing incidence of EoE across different nations, including the United States, has underscored the need for a deeper understanding of its causes, early diagnosis, and treatment. A novel population-based study conducted in Denmark aimed to explore the relationship between maternal and infant use of antibiotics and acid suppressants in the development of EoE. The study yielded significant results based on a population of 392 cases. Dr Elizabeth T. Jensen, MPH, PhD, from the Department of Epidemiology & Prevention at Wake Forest University School of Medicine, Winston-Salem, North Carolina, conducted a comprehensive study spanning the last 20 years to decipher potential causes contributing to the rising incidence of EoE.
Dr Jensen and her colleagues evaluated the association between maternal and infant use of antibiotics and acid suppressants in Denmark. They used pathology, prescription, birth, inpatient, and outpatient health registry data, ensuring complete ascertainment of all EoE cases among Danish residents born between 1997 and 2018. The research obtained a census of cases from a registered sample of approximately 1.4 million children, matching EoE cases to controls using a 1:10 ratio through incidence density sampling. A total of 392 patients with EoE and 3637 control patients were enrolled. The primary outcome of the study focused on the development of EoE, revealing a dose-response association between maternal and infant antibiotic and acid suppressant use and increased EoE risk.
This study demonstrated a robust correlation between the dosage of antibiotics and acid suppressants and the development of EoE in offspring during childhood. These findings hold significance because these medications represent some of the most common prescriptions in clinical practice. Pregnancy triggers significant physiologic changes in women, including increased hormonal effects and abdominal pressure on the lower esophageal sphincter, making pregnant individuals more prone to esophageal reflux and necessitating the use of gastric acid suppressants. As clinicians, it's crucial to consider lifestyle modifications and dietary adjustments before resorting to acid suppressants, reserving their use for only when absolutely necessary.
Postpregnancy, emphasizing exclusive breastfeeding for the first 6 months and proper feeding techniques can aid in reducing the likelihood of reflux disease in newborns. Acid suppressants have been linked to alterations in infant microbiome colonization, potentially increasing the susceptibility to immunoreactive diseases, such as asthma, atopic dermatitis, and allergic rhinitis. Given that exclusive breastfeeding in the initial 6 months has demonstrated preventive benefits against such diseases, primary physicians play a crucial role in advocating its importance. Although gastric acid suppressants and antibiotics are essential for managing various health conditions, including infections and gastroesophageal reflux disease (GERD), their potential impact on EoE development should not be overlooked.
Though this study had a relatively small sample size, the strong population registry of Denmark significantly reduced recall bias. However, cultural differences and over-the-counter access to drugs, such as acid suppressants, in other countries, including the United States, warrant further research to ascertain their effect on EoE development.
In light of these findings, clinicians should carefully weigh the risks and benefits of prescribing antibiotics and acid suppressants during pregnancy and infancy. Adherence to evidence-based guidelines and considering alternative treatment options, such as lifestyle modifications, should be prioritized. Prescribing antibiotics only when medically necessary and using nonpharmacologic strategies for managing GERD in infants should be considered to mitigate potential risks associated with these medications.
Although there exists a recognized association between IBD and secondary EoE diagnoses, studies focusing on the primary diagnosis of EoE alongside IBD have yielded conflicting results. Dr Amiko Uchida, from the University of Utah Department of Medicine, working with colleagues from the Department of Medical Epidemiology and Biostatistics at Karolinska Institutet in Stockholm, Sweden, led by Dr Jonas F. Ludvigsson, conducted a comprehensive study spanning 1990-2019 to explore the relationship between these two diseases.
Dr Uchida and colleagues assessed the association among Swedish patients diagnosed with biopsy-verified EoE (n = 1587) between 1990 and 2017. These patients were age- and sex-matched with up to five reference individuals from the general population (n = 7808). The primary focus was to discern the relationship between the primary diagnosis of EoE and the subsequent diagnosis of IBD.
The study's findings underscore the importance of heightened awareness among healthcare professionals regarding the potential association between EoE and the development of subsequent IBD. Collaborative efforts between physicians, gastroenterologists, and specialists in allergic diseases are crucial to ensure comprehensive care and timely identification of gastrointestinal complications in patients with EoE.
These results indicate a potential interplay between EoE and the pathogenesis of IBD, particularly Crohn's disease. In patients with EoE, careful consideration of gastrointestinal symptoms suggestive of IBD, such as abdominal pain, diarrhea, and rectal bleeding, is pivotal, necessitating further evaluation and appropriate monitoring for early detection and management of IBD.
Upon diagnosis, clinicians must develop a management plan for this chronic and critical disease. This study aids in planning future screenings for these patients, because one third of those diagnosed with EoE are at risk of developing IBD within a year. Therefore, primary physicians must remain vigilant for the development of gastrointestinal symptoms leading to a diagnosis of Crohn's disease or ulcerative colitis. Additionally, family awareness is crucial owing to observed associations between siblings, suggesting a potential role of genetics or early environmental factors in EoE development and future IBD diagnoses.
Further research is necessary to elucidate the shared pathophysiologic mechanisms connecting EoE and IBD. It is important to consider certain details, however; for instance, 31% of all subsequent IBD cases were diagnosed within the first year after an EoE diagnosis, potentially indicating a role of detection bias in these findings. Using a validated nationwide cohort and comparing study individuals with their siblings helped control for potential intrafamilial confounders as well as some environmental confounders, minimizing such biases as selection bias due to socioeconomic status. This strengthens the observed association in this study.
These insights into the increased risk for IBD, notably Crohn's disease, among patients with EoE underscore the need for thorough clinical evaluation and vigilant monitoring for gastrointestinal complications in this population.
A retrospective study conducted in pediatric patients presenting with an aerodigestive manifestation aimed to assess the factors associated with EoE and the diagnostic role of triple endoscopy. The results suggested a potential association between a family history of eczema and a diet lacking allergenic foods with a future diagnosis of EoE.
This study by Sheila Moran and colleagues, led by Dr Christina J. Yang from the Department of Otorhinolaryngology, Head and Neck Surgery at the Albert Einstein College of Medicine, Bronx, New York, aimed to identify preoperative risk factors linked to an EoE diagnosis in children undergoing triple endoscopy. They evaluated 119 pediatric patients aged 0-21 years who underwent triple endoscopy (including flexible bronchoscopy, rigid direct laryngoscopy and bronchoscopy, and esophagoscopy with biopsy) at the Children's Hospital at Montefiore between January 1, 2015, and December 31, 2019.
The study underscored the significance of both genetic predisposition and dietary influences in EoE development. Understanding the interplay between familial atopic conditions and dietary choices among pediatric patients with aerodigestive dysfunction is crucial for early identification and implementing preventive strategies against EoE.
As clinicians, it's essential to consider rare diseases like EoE when patients present with mixed symptoms, including aerodigestive symptoms, a family history of eczema, and a history of environmental allergies, given the association between these conditions. The potential link between a family history of eczema and increased EoE risk suggests a shared genetic susceptibility among allergic conditions. Therefore, clinicians evaluating children with aerodigestive dysfunction, particularly those with a familial history of eczema, should maintain a high index of suspicion for EoE, prompting vigilant monitoring and appropriate diagnostic assessments.
When contemplating advanced procedures, such as triple endoscopy or biopsy sampling, considering the patient's previous medical history and the effect of dietary modifications, such as incorporating or excluding dairy from the diet, warrants further investigation in the context of EoE prevention. Clinicians should consider providing dietary counseling and personalized nutritional plans based on evidence-based approaches to potentially mitigate EoE risk in susceptible pediatric populations.
Additionally, it's crucial to consider EoE in minority racial groups and underserved communities and encourage the use of diagnostic tests, such as triple endoscopy, to facilitate early diagnosis. Healthcare providers should contemplate integrating family history assessments, particularly regarding eczema, into the evaluation of children with aerodigestive dysfunction. This information can assist in risk assessment and early identification of individuals at a higher risk of developing EoE, enabling prompt intervention and management.
The increasing incidence of EoE across different nations, including the United States, has underscored the need for a deeper understanding of its causes, early diagnosis, and treatment. A novel population-based study conducted in Denmark aimed to explore the relationship between maternal and infant use of antibiotics and acid suppressants in the development of EoE. The study yielded significant results based on a population of 392 cases. Dr Elizabeth T. Jensen, MPH, PhD, from the Department of Epidemiology & Prevention at Wake Forest University School of Medicine, Winston-Salem, North Carolina, conducted a comprehensive study spanning the last 20 years to decipher potential causes contributing to the rising incidence of EoE.
Dr Jensen and her colleagues evaluated the association between maternal and infant use of antibiotics and acid suppressants in Denmark. They used pathology, prescription, birth, inpatient, and outpatient health registry data, ensuring complete ascertainment of all EoE cases among Danish residents born between 1997 and 2018. The research obtained a census of cases from a registered sample of approximately 1.4 million children, matching EoE cases to controls using a 1:10 ratio through incidence density sampling. A total of 392 patients with EoE and 3637 control patients were enrolled. The primary outcome of the study focused on the development of EoE, revealing a dose-response association between maternal and infant antibiotic and acid suppressant use and increased EoE risk.
This study demonstrated a robust correlation between the dosage of antibiotics and acid suppressants and the development of EoE in offspring during childhood. These findings hold significance because these medications represent some of the most common prescriptions in clinical practice. Pregnancy triggers significant physiologic changes in women, including increased hormonal effects and abdominal pressure on the lower esophageal sphincter, making pregnant individuals more prone to esophageal reflux and necessitating the use of gastric acid suppressants. As clinicians, it's crucial to consider lifestyle modifications and dietary adjustments before resorting to acid suppressants, reserving their use for only when absolutely necessary.
Postpregnancy, emphasizing exclusive breastfeeding for the first 6 months and proper feeding techniques can aid in reducing the likelihood of reflux disease in newborns. Acid suppressants have been linked to alterations in infant microbiome colonization, potentially increasing the susceptibility to immunoreactive diseases, such as asthma, atopic dermatitis, and allergic rhinitis. Given that exclusive breastfeeding in the initial 6 months has demonstrated preventive benefits against such diseases, primary physicians play a crucial role in advocating its importance. Although gastric acid suppressants and antibiotics are essential for managing various health conditions, including infections and gastroesophageal reflux disease (GERD), their potential impact on EoE development should not be overlooked.
Though this study had a relatively small sample size, the strong population registry of Denmark significantly reduced recall bias. However, cultural differences and over-the-counter access to drugs, such as acid suppressants, in other countries, including the United States, warrant further research to ascertain their effect on EoE development.
In light of these findings, clinicians should carefully weigh the risks and benefits of prescribing antibiotics and acid suppressants during pregnancy and infancy. Adherence to evidence-based guidelines and considering alternative treatment options, such as lifestyle modifications, should be prioritized. Prescribing antibiotics only when medically necessary and using nonpharmacologic strategies for managing GERD in infants should be considered to mitigate potential risks associated with these medications.
Although there exists a recognized association between IBD and secondary EoE diagnoses, studies focusing on the primary diagnosis of EoE alongside IBD have yielded conflicting results. Dr Amiko Uchida, from the University of Utah Department of Medicine, working with colleagues from the Department of Medical Epidemiology and Biostatistics at Karolinska Institutet in Stockholm, Sweden, led by Dr Jonas F. Ludvigsson, conducted a comprehensive study spanning 1990-2019 to explore the relationship between these two diseases.
Dr Uchida and colleagues assessed the association among Swedish patients diagnosed with biopsy-verified EoE (n = 1587) between 1990 and 2017. These patients were age- and sex-matched with up to five reference individuals from the general population (n = 7808). The primary focus was to discern the relationship between the primary diagnosis of EoE and the subsequent diagnosis of IBD.
The study's findings underscore the importance of heightened awareness among healthcare professionals regarding the potential association between EoE and the development of subsequent IBD. Collaborative efforts between physicians, gastroenterologists, and specialists in allergic diseases are crucial to ensure comprehensive care and timely identification of gastrointestinal complications in patients with EoE.
These results indicate a potential interplay between EoE and the pathogenesis of IBD, particularly Crohn's disease. In patients with EoE, careful consideration of gastrointestinal symptoms suggestive of IBD, such as abdominal pain, diarrhea, and rectal bleeding, is pivotal, necessitating further evaluation and appropriate monitoring for early detection and management of IBD.
Upon diagnosis, clinicians must develop a management plan for this chronic and critical disease. This study aids in planning future screenings for these patients, because one third of those diagnosed with EoE are at risk of developing IBD within a year. Therefore, primary physicians must remain vigilant for the development of gastrointestinal symptoms leading to a diagnosis of Crohn's disease or ulcerative colitis. Additionally, family awareness is crucial owing to observed associations between siblings, suggesting a potential role of genetics or early environmental factors in EoE development and future IBD diagnoses.
Further research is necessary to elucidate the shared pathophysiologic mechanisms connecting EoE and IBD. It is important to consider certain details, however; for instance, 31% of all subsequent IBD cases were diagnosed within the first year after an EoE diagnosis, potentially indicating a role of detection bias in these findings. Using a validated nationwide cohort and comparing study individuals with their siblings helped control for potential intrafamilial confounders as well as some environmental confounders, minimizing such biases as selection bias due to socioeconomic status. This strengthens the observed association in this study.
These insights into the increased risk for IBD, notably Crohn's disease, among patients with EoE underscore the need for thorough clinical evaluation and vigilant monitoring for gastrointestinal complications in this population.
A retrospective study conducted in pediatric patients presenting with an aerodigestive manifestation aimed to assess the factors associated with EoE and the diagnostic role of triple endoscopy. The results suggested a potential association between a family history of eczema and a diet lacking allergenic foods with a future diagnosis of EoE.
This study by Sheila Moran and colleagues, led by Dr Christina J. Yang from the Department of Otorhinolaryngology, Head and Neck Surgery at the Albert Einstein College of Medicine, Bronx, New York, aimed to identify preoperative risk factors linked to an EoE diagnosis in children undergoing triple endoscopy. They evaluated 119 pediatric patients aged 0-21 years who underwent triple endoscopy (including flexible bronchoscopy, rigid direct laryngoscopy and bronchoscopy, and esophagoscopy with biopsy) at the Children's Hospital at Montefiore between January 1, 2015, and December 31, 2019.
The study underscored the significance of both genetic predisposition and dietary influences in EoE development. Understanding the interplay between familial atopic conditions and dietary choices among pediatric patients with aerodigestive dysfunction is crucial for early identification and implementing preventive strategies against EoE.
As clinicians, it's essential to consider rare diseases like EoE when patients present with mixed symptoms, including aerodigestive symptoms, a family history of eczema, and a history of environmental allergies, given the association between these conditions. The potential link between a family history of eczema and increased EoE risk suggests a shared genetic susceptibility among allergic conditions. Therefore, clinicians evaluating children with aerodigestive dysfunction, particularly those with a familial history of eczema, should maintain a high index of suspicion for EoE, prompting vigilant monitoring and appropriate diagnostic assessments.
When contemplating advanced procedures, such as triple endoscopy or biopsy sampling, considering the patient's previous medical history and the effect of dietary modifications, such as incorporating or excluding dairy from the diet, warrants further investigation in the context of EoE prevention. Clinicians should consider providing dietary counseling and personalized nutritional plans based on evidence-based approaches to potentially mitigate EoE risk in susceptible pediatric populations.
Additionally, it's crucial to consider EoE in minority racial groups and underserved communities and encourage the use of diagnostic tests, such as triple endoscopy, to facilitate early diagnosis. Healthcare providers should contemplate integrating family history assessments, particularly regarding eczema, into the evaluation of children with aerodigestive dysfunction. This information can assist in risk assessment and early identification of individuals at a higher risk of developing EoE, enabling prompt intervention and management.
The increasing incidence of EoE across different nations, including the United States, has underscored the need for a deeper understanding of its causes, early diagnosis, and treatment. A novel population-based study conducted in Denmark aimed to explore the relationship between maternal and infant use of antibiotics and acid suppressants in the development of EoE. The study yielded significant results based on a population of 392 cases. Dr Elizabeth T. Jensen, MPH, PhD, from the Department of Epidemiology & Prevention at Wake Forest University School of Medicine, Winston-Salem, North Carolina, conducted a comprehensive study spanning the last 20 years to decipher potential causes contributing to the rising incidence of EoE.
Dr Jensen and her colleagues evaluated the association between maternal and infant use of antibiotics and acid suppressants in Denmark. They used pathology, prescription, birth, inpatient, and outpatient health registry data, ensuring complete ascertainment of all EoE cases among Danish residents born between 1997 and 2018. The research obtained a census of cases from a registered sample of approximately 1.4 million children, matching EoE cases to controls using a 1:10 ratio through incidence density sampling. A total of 392 patients with EoE and 3637 control patients were enrolled. The primary outcome of the study focused on the development of EoE, revealing a dose-response association between maternal and infant antibiotic and acid suppressant use and increased EoE risk.
This study demonstrated a robust correlation between the dosage of antibiotics and acid suppressants and the development of EoE in offspring during childhood. These findings hold significance because these medications represent some of the most common prescriptions in clinical practice. Pregnancy triggers significant physiologic changes in women, including increased hormonal effects and abdominal pressure on the lower esophageal sphincter, making pregnant individuals more prone to esophageal reflux and necessitating the use of gastric acid suppressants. As clinicians, it's crucial to consider lifestyle modifications and dietary adjustments before resorting to acid suppressants, reserving their use for only when absolutely necessary.
Postpregnancy, emphasizing exclusive breastfeeding for the first 6 months and proper feeding techniques can aid in reducing the likelihood of reflux disease in newborns. Acid suppressants have been linked to alterations in infant microbiome colonization, potentially increasing the susceptibility to immunoreactive diseases, such as asthma, atopic dermatitis, and allergic rhinitis. Given that exclusive breastfeeding in the initial 6 months has demonstrated preventive benefits against such diseases, primary physicians play a crucial role in advocating its importance. Although gastric acid suppressants and antibiotics are essential for managing various health conditions, including infections and gastroesophageal reflux disease (GERD), their potential impact on EoE development should not be overlooked.
Though this study had a relatively small sample size, the strong population registry of Denmark significantly reduced recall bias. However, cultural differences and over-the-counter access to drugs, such as acid suppressants, in other countries, including the United States, warrant further research to ascertain their effect on EoE development.
In light of these findings, clinicians should carefully weigh the risks and benefits of prescribing antibiotics and acid suppressants during pregnancy and infancy. Adherence to evidence-based guidelines and considering alternative treatment options, such as lifestyle modifications, should be prioritized. Prescribing antibiotics only when medically necessary and using nonpharmacologic strategies for managing GERD in infants should be considered to mitigate potential risks associated with these medications.
Coronary Artery Bypass Graft Saphenous Vein Harvest Site Hyperpigmentation
A 59-year-old man with a history of coronary artery bypass grafting (CABG), ischemic cardiomyopathy (ejection fraction, 15%-20%) with implantable cardioverter-defibrillator, recurrent paroxysmal ventricular tachycardia on amiodarone and mexiletine, and heart failure requiring left ventricular assist device (LVAD) placement presented for recurrent cellulitis and infection of the LVAD driveline exit site. He was initiated on minocycline 100 mg twice daily in combination with cefadroxil 500 mg twice daily. At his 8-week follow-up, the driveline site appeared improved with minimal erythema and no drainage. However, the patient developed a well-demarcated, linear, hyperpigmented patch along the length of the saphenous vein CABG harvest site and a few hyperpigmented macules medial to the harvest site (Figure).
Discussion
Hyperpigmentation presenting within scar tissue, as seen in this patient undergoing minocycline therapy, is a classic presentation of minocycline-induced hyperpigmentation (MIH) type I.
MIH is an uncommon, potentially cosmetically disfiguring adverse effect associated with systemic minocycline use. MIH can affect skin, teeth, nails, oral mucosa, sclera, and internal organs. The cumulative incidence of MIH in patients receiving minocycline over prolonged periods of time has been estimated from 2% to 15% in patients with acne and rosacea, to approximately 50% over 5 years in orthopedic patient populations.1-3 The risk for developing MIH increases with vitamin D deficiency, liver disease, concurrent use with other medications that can induce hyperpigmentation, and higher cumulative doses (> 70-100 g; more important for MIH types II and III).3,4
There are 3 distinct types of MIH. Type I MIH is characterized by blue-black macules and patches at sites of inflammation or prior scarring, most commonly described in facial acne scars.1,2,4 Type II is typified by blue-grey pigmentation on normal-appearing skin, most commonly on the shins, but also on sun-exposed sites.3 Biopsies of type I and II MIH demonstrate pigmented granules within macrophages or within the dermis.4,5 Both Perls iron stain and Fontana-Masson melanin stain are positive in type I and II MIH.5 Type III MIH presents as diffuse brownish hyperpigmentation on normal skin in chronically sun-exposed sites.3 Histopathology of type III MIH can be distinguished by increased melanin noted inside basal keratinocytes as well as dermal melanophages that stain positive for only Fontana-Masson.5 The current case exemplifies a unique presentation of type I MIH along the length of the saphenous vein CABG harvest site. The concomitant use of amiodarone with minocycline may have contributed to the presentation.
The differential diagnosis for MIH depends on the type of MIH. Blue-grey pigmentation within scars is fairly unique to minocycline but has been reported with other medications, including vandetanib.6 The differential diagnosis for diffuse blue-grey or brown hyperpigmentation in predominately sun-exposed sites is broader, including endocrine disorders (ie, Addison disease), heavy metal poisoning (ie, argyria), inherited conditions (ie, alkaptonuria, Wilson disease, and hemochromatosis), medication-induced hyperpigmentation (ie, antipsychotics, anticonvulsant, antimalarials, amiodarone, and cytotoxic drugs), as well as inflammatory dermatoses, such as erythema dyschromicum perstans.7
MIH typically fades over months to years following minocycline discontinuation, so prompt recognition and discontinuation is recommended. Unfortunately, some cases persist or only partially fade over time. While MIH is benign, it can be of aesthetic concern, cause anxiety, and impact patients’ quality of life.3,8 Persistent MIH is typically recalcitrant to topical hydroquinone.9 However, persistent MIH has been shown to improve with Q-switched, nanosecond lasers such as the 694 nm ruby, 755 nm alexandrite, and 1064 nm neodymium-doped yttrium aluminum garnet neodymium (Nd:YAG) lasers, as well as the 755 nm picosecond alexandrite laser.4,9,10
In our patient, minocycline therapy was discontinued and replaced with doxycycline 100 mg twice daily monotherapy. At a subsequent visit 12 weeks later, the hyperpigmentation remained unchanged.
Conclusions
Though uncommon, we hope to encourage clinician awareness of MIH through our case, as prompt diagnosis and the discontinuation of minocycline are preferred to improve patient outcomes.
1. Goulden V, Glass D, Cunliffe WJ. Safety of long-term high-dose minocycline in the treatment of acne. Br J Dermatol. 1996;134(4):693-695. doi:10.1111/j.1365-2133.1996.tb06972.x
2. Dwyer CM, Cuddihy AM, Kerr RE, Chapman RS, Allam BF. Skin pigmentation due to minocycline treatment of facial dermatoses. Br J Dermatol. 1993;129(2):158-162. doi:10.1111/j.1365-2133.1993.tb03519.x
3. Hanada Y, Berbari EF, Steckelberg JM. Minocycline-induced cutaneous hyperpigmentation in an orthopedic patient population. Open Forum Infect Dis. 2016;3(1):ofv107. doi:10.1093/ofid/ofv107
4. Eisen D, Hakim MD. Minocycline-induced pigmentation. Incidence, prevention and management. Drug Saf. 1998;18(6):431-440. doi:10.2165/00002018-199818060-00004
5. Bowen AR, McCalmont TH. The histopathology of subcutaneous minocycline pigmentation. J Am Acad Dermatol. 2007;57(5):836-839. doi:10.1016/j.jaad.2007.04.028
6. Perlmutter JW, Cogan RC, Wiseman MC. Blue-grey hyperpigmentation in acne after vandetanib therapy and doxycycline use: a case report. SAGE Open Med Case Rep. 2022;10:2050313X221086316. doi:10.1177/2050313X221086316
7. Judson T, Mihara K. Minocycline-induced hyperpigmentation. J Gen Intern Med. 2017;32(1):133. doi:10.1007/s11606-016-3735-x
8. Li Y, Zhen X, Yao X, Lu J. Successful treatment of minocycline-induced facial hyperpigmentation with a combination of chemical peels and intense pulsed light. Clin Cosmet Investig Dermatol. 2023;16:253-256. doi:10.2147/CCID.S394754
9. Sasaki K, Ohshiro T, Ohshiro T, et al. Type 2 Minocycline-induced hyperpigmentation successfully treated with the novel 755 nm picosecond alexandrite laser – a case report. Laser Ther. 2017;26(2):137-144. doi:10.5978/islsm.17-CR-03
10. Nisar MS, Iyer K, Brodell RT, Lloyd JR, Shin TM, Ahmad A. Minocycline-induced hyperpigmentation: comparison of 3 Q-switched lasers to reverse its effects. Clin Cosmet Investig Dermatol. 2013;6:159-162. doi:10.2147/CCID.S42166
A 59-year-old man with a history of coronary artery bypass grafting (CABG), ischemic cardiomyopathy (ejection fraction, 15%-20%) with implantable cardioverter-defibrillator, recurrent paroxysmal ventricular tachycardia on amiodarone and mexiletine, and heart failure requiring left ventricular assist device (LVAD) placement presented for recurrent cellulitis and infection of the LVAD driveline exit site. He was initiated on minocycline 100 mg twice daily in combination with cefadroxil 500 mg twice daily. At his 8-week follow-up, the driveline site appeared improved with minimal erythema and no drainage. However, the patient developed a well-demarcated, linear, hyperpigmented patch along the length of the saphenous vein CABG harvest site and a few hyperpigmented macules medial to the harvest site (Figure).
Discussion
Hyperpigmentation presenting within scar tissue, as seen in this patient undergoing minocycline therapy, is a classic presentation of minocycline-induced hyperpigmentation (MIH) type I.
MIH is an uncommon, potentially cosmetically disfiguring adverse effect associated with systemic minocycline use. MIH can affect skin, teeth, nails, oral mucosa, sclera, and internal organs. The cumulative incidence of MIH in patients receiving minocycline over prolonged periods of time has been estimated from 2% to 15% in patients with acne and rosacea, to approximately 50% over 5 years in orthopedic patient populations.1-3 The risk for developing MIH increases with vitamin D deficiency, liver disease, concurrent use with other medications that can induce hyperpigmentation, and higher cumulative doses (> 70-100 g; more important for MIH types II and III).3,4
There are 3 distinct types of MIH. Type I MIH is characterized by blue-black macules and patches at sites of inflammation or prior scarring, most commonly described in facial acne scars.1,2,4 Type II is typified by blue-grey pigmentation on normal-appearing skin, most commonly on the shins, but also on sun-exposed sites.3 Biopsies of type I and II MIH demonstrate pigmented granules within macrophages or within the dermis.4,5 Both Perls iron stain and Fontana-Masson melanin stain are positive in type I and II MIH.5 Type III MIH presents as diffuse brownish hyperpigmentation on normal skin in chronically sun-exposed sites.3 Histopathology of type III MIH can be distinguished by increased melanin noted inside basal keratinocytes as well as dermal melanophages that stain positive for only Fontana-Masson.5 The current case exemplifies a unique presentation of type I MIH along the length of the saphenous vein CABG harvest site. The concomitant use of amiodarone with minocycline may have contributed to the presentation.
The differential diagnosis for MIH depends on the type of MIH. Blue-grey pigmentation within scars is fairly unique to minocycline but has been reported with other medications, including vandetanib.6 The differential diagnosis for diffuse blue-grey or brown hyperpigmentation in predominately sun-exposed sites is broader, including endocrine disorders (ie, Addison disease), heavy metal poisoning (ie, argyria), inherited conditions (ie, alkaptonuria, Wilson disease, and hemochromatosis), medication-induced hyperpigmentation (ie, antipsychotics, anticonvulsant, antimalarials, amiodarone, and cytotoxic drugs), as well as inflammatory dermatoses, such as erythema dyschromicum perstans.7
MIH typically fades over months to years following minocycline discontinuation, so prompt recognition and discontinuation is recommended. Unfortunately, some cases persist or only partially fade over time. While MIH is benign, it can be of aesthetic concern, cause anxiety, and impact patients’ quality of life.3,8 Persistent MIH is typically recalcitrant to topical hydroquinone.9 However, persistent MIH has been shown to improve with Q-switched, nanosecond lasers such as the 694 nm ruby, 755 nm alexandrite, and 1064 nm neodymium-doped yttrium aluminum garnet neodymium (Nd:YAG) lasers, as well as the 755 nm picosecond alexandrite laser.4,9,10
In our patient, minocycline therapy was discontinued and replaced with doxycycline 100 mg twice daily monotherapy. At a subsequent visit 12 weeks later, the hyperpigmentation remained unchanged.
Conclusions
Though uncommon, we hope to encourage clinician awareness of MIH through our case, as prompt diagnosis and the discontinuation of minocycline are preferred to improve patient outcomes.
A 59-year-old man with a history of coronary artery bypass grafting (CABG), ischemic cardiomyopathy (ejection fraction, 15%-20%) with implantable cardioverter-defibrillator, recurrent paroxysmal ventricular tachycardia on amiodarone and mexiletine, and heart failure requiring left ventricular assist device (LVAD) placement presented for recurrent cellulitis and infection of the LVAD driveline exit site. He was initiated on minocycline 100 mg twice daily in combination with cefadroxil 500 mg twice daily. At his 8-week follow-up, the driveline site appeared improved with minimal erythema and no drainage. However, the patient developed a well-demarcated, linear, hyperpigmented patch along the length of the saphenous vein CABG harvest site and a few hyperpigmented macules medial to the harvest site (Figure).
Discussion
Hyperpigmentation presenting within scar tissue, as seen in this patient undergoing minocycline therapy, is a classic presentation of minocycline-induced hyperpigmentation (MIH) type I.
MIH is an uncommon, potentially cosmetically disfiguring adverse effect associated with systemic minocycline use. MIH can affect skin, teeth, nails, oral mucosa, sclera, and internal organs. The cumulative incidence of MIH in patients receiving minocycline over prolonged periods of time has been estimated from 2% to 15% in patients with acne and rosacea, to approximately 50% over 5 years in orthopedic patient populations.1-3 The risk for developing MIH increases with vitamin D deficiency, liver disease, concurrent use with other medications that can induce hyperpigmentation, and higher cumulative doses (> 70-100 g; more important for MIH types II and III).3,4
There are 3 distinct types of MIH. Type I MIH is characterized by blue-black macules and patches at sites of inflammation or prior scarring, most commonly described in facial acne scars.1,2,4 Type II is typified by blue-grey pigmentation on normal-appearing skin, most commonly on the shins, but also on sun-exposed sites.3 Biopsies of type I and II MIH demonstrate pigmented granules within macrophages or within the dermis.4,5 Both Perls iron stain and Fontana-Masson melanin stain are positive in type I and II MIH.5 Type III MIH presents as diffuse brownish hyperpigmentation on normal skin in chronically sun-exposed sites.3 Histopathology of type III MIH can be distinguished by increased melanin noted inside basal keratinocytes as well as dermal melanophages that stain positive for only Fontana-Masson.5 The current case exemplifies a unique presentation of type I MIH along the length of the saphenous vein CABG harvest site. The concomitant use of amiodarone with minocycline may have contributed to the presentation.
The differential diagnosis for MIH depends on the type of MIH. Blue-grey pigmentation within scars is fairly unique to minocycline but has been reported with other medications, including vandetanib.6 The differential diagnosis for diffuse blue-grey or brown hyperpigmentation in predominately sun-exposed sites is broader, including endocrine disorders (ie, Addison disease), heavy metal poisoning (ie, argyria), inherited conditions (ie, alkaptonuria, Wilson disease, and hemochromatosis), medication-induced hyperpigmentation (ie, antipsychotics, anticonvulsant, antimalarials, amiodarone, and cytotoxic drugs), as well as inflammatory dermatoses, such as erythema dyschromicum perstans.7
MIH typically fades over months to years following minocycline discontinuation, so prompt recognition and discontinuation is recommended. Unfortunately, some cases persist or only partially fade over time. While MIH is benign, it can be of aesthetic concern, cause anxiety, and impact patients’ quality of life.3,8 Persistent MIH is typically recalcitrant to topical hydroquinone.9 However, persistent MIH has been shown to improve with Q-switched, nanosecond lasers such as the 694 nm ruby, 755 nm alexandrite, and 1064 nm neodymium-doped yttrium aluminum garnet neodymium (Nd:YAG) lasers, as well as the 755 nm picosecond alexandrite laser.4,9,10
In our patient, minocycline therapy was discontinued and replaced with doxycycline 100 mg twice daily monotherapy. At a subsequent visit 12 weeks later, the hyperpigmentation remained unchanged.
Conclusions
Though uncommon, we hope to encourage clinician awareness of MIH through our case, as prompt diagnosis and the discontinuation of minocycline are preferred to improve patient outcomes.
1. Goulden V, Glass D, Cunliffe WJ. Safety of long-term high-dose minocycline in the treatment of acne. Br J Dermatol. 1996;134(4):693-695. doi:10.1111/j.1365-2133.1996.tb06972.x
2. Dwyer CM, Cuddihy AM, Kerr RE, Chapman RS, Allam BF. Skin pigmentation due to minocycline treatment of facial dermatoses. Br J Dermatol. 1993;129(2):158-162. doi:10.1111/j.1365-2133.1993.tb03519.x
3. Hanada Y, Berbari EF, Steckelberg JM. Minocycline-induced cutaneous hyperpigmentation in an orthopedic patient population. Open Forum Infect Dis. 2016;3(1):ofv107. doi:10.1093/ofid/ofv107
4. Eisen D, Hakim MD. Minocycline-induced pigmentation. Incidence, prevention and management. Drug Saf. 1998;18(6):431-440. doi:10.2165/00002018-199818060-00004
5. Bowen AR, McCalmont TH. The histopathology of subcutaneous minocycline pigmentation. J Am Acad Dermatol. 2007;57(5):836-839. doi:10.1016/j.jaad.2007.04.028
6. Perlmutter JW, Cogan RC, Wiseman MC. Blue-grey hyperpigmentation in acne after vandetanib therapy and doxycycline use: a case report. SAGE Open Med Case Rep. 2022;10:2050313X221086316. doi:10.1177/2050313X221086316
7. Judson T, Mihara K. Minocycline-induced hyperpigmentation. J Gen Intern Med. 2017;32(1):133. doi:10.1007/s11606-016-3735-x
8. Li Y, Zhen X, Yao X, Lu J. Successful treatment of minocycline-induced facial hyperpigmentation with a combination of chemical peels and intense pulsed light. Clin Cosmet Investig Dermatol. 2023;16:253-256. doi:10.2147/CCID.S394754
9. Sasaki K, Ohshiro T, Ohshiro T, et al. Type 2 Minocycline-induced hyperpigmentation successfully treated with the novel 755 nm picosecond alexandrite laser – a case report. Laser Ther. 2017;26(2):137-144. doi:10.5978/islsm.17-CR-03
10. Nisar MS, Iyer K, Brodell RT, Lloyd JR, Shin TM, Ahmad A. Minocycline-induced hyperpigmentation: comparison of 3 Q-switched lasers to reverse its effects. Clin Cosmet Investig Dermatol. 2013;6:159-162. doi:10.2147/CCID.S42166
1. Goulden V, Glass D, Cunliffe WJ. Safety of long-term high-dose minocycline in the treatment of acne. Br J Dermatol. 1996;134(4):693-695. doi:10.1111/j.1365-2133.1996.tb06972.x
2. Dwyer CM, Cuddihy AM, Kerr RE, Chapman RS, Allam BF. Skin pigmentation due to minocycline treatment of facial dermatoses. Br J Dermatol. 1993;129(2):158-162. doi:10.1111/j.1365-2133.1993.tb03519.x
3. Hanada Y, Berbari EF, Steckelberg JM. Minocycline-induced cutaneous hyperpigmentation in an orthopedic patient population. Open Forum Infect Dis. 2016;3(1):ofv107. doi:10.1093/ofid/ofv107
4. Eisen D, Hakim MD. Minocycline-induced pigmentation. Incidence, prevention and management. Drug Saf. 1998;18(6):431-440. doi:10.2165/00002018-199818060-00004
5. Bowen AR, McCalmont TH. The histopathology of subcutaneous minocycline pigmentation. J Am Acad Dermatol. 2007;57(5):836-839. doi:10.1016/j.jaad.2007.04.028
6. Perlmutter JW, Cogan RC, Wiseman MC. Blue-grey hyperpigmentation in acne after vandetanib therapy and doxycycline use: a case report. SAGE Open Med Case Rep. 2022;10:2050313X221086316. doi:10.1177/2050313X221086316
7. Judson T, Mihara K. Minocycline-induced hyperpigmentation. J Gen Intern Med. 2017;32(1):133. doi:10.1007/s11606-016-3735-x
8. Li Y, Zhen X, Yao X, Lu J. Successful treatment of minocycline-induced facial hyperpigmentation with a combination of chemical peels and intense pulsed light. Clin Cosmet Investig Dermatol. 2023;16:253-256. doi:10.2147/CCID.S394754
9. Sasaki K, Ohshiro T, Ohshiro T, et al. Type 2 Minocycline-induced hyperpigmentation successfully treated with the novel 755 nm picosecond alexandrite laser – a case report. Laser Ther. 2017;26(2):137-144. doi:10.5978/islsm.17-CR-03
10. Nisar MS, Iyer K, Brodell RT, Lloyd JR, Shin TM, Ahmad A. Minocycline-induced hyperpigmentation: comparison of 3 Q-switched lasers to reverse its effects. Clin Cosmet Investig Dermatol. 2013;6:159-162. doi:10.2147/CCID.S42166
Dupilumab for Dyshidrotic Eczema With Secondary Improvement in Eosinophilic Interstitial Lung Disease
To the Editor:
Biologic medications are increasingly utilized in adults with moderate to severe atopic dermatitis (AD) that is inadequately controlled with topical medication. By targeting the IL-4 receptor alpha subunit, dupilumab inhibits the biologic effects of IL-4 and IL-13, resulting in remarkable improvement in disease and quality of life for many patients with refractory AD.1
In 2017, the US Food and Drug Administration approved dupilumab for use in AD, asthma, and chronic rhinosinusitis. However, there is evidence of the drug’s off-label efficacy in conditions such as eosinophilic annular erythema.2 We present a patient with dyshidrotic eczema treated with dupilumab who experienced contemporaneous secondary improvement in chronic eosinophilic pneumonia (CEP) and interstitial lung disease (ILD).
A 45-year-old man was referred to our dermatology clinic for chronic hand dermatitis refractory to increasing strengths of topical corticosteroids. He had a history of progressive shortness of breath of unknown cause, which began 2 years prior, and he was being followed at our institution’s ILD clinic. Earlier pulmonary function testing revealed a restrictive pattern with interstitial infiltrates seen on chest computed tomography. A lung biopsy demonstrated features of fibrotic nonspecific interstitial pneumonitis with superimposed eosinophilic pneumonia. His pulmonary symptoms had progressively worsened; over a period of several months, the supplemental oxygen requirement had increased to 6 L at rest and 12 L upon exertion. Prednisone therapy was initiated, which alleviated respiratory symptoms; however, the patient was unable to tolerate a gradual wean of the medication, which rendered him steroid dependent at 30 mg/d.
Along with respiratory symptoms, the patient reported symptoms consistent with an autoimmune process, including dry eyes. Muscle weakness and tenderness also were noted. Ultimately, a diagnosis of anti–PL-7 (anti-threonyl-transfer RNA synthetase) antisynthetase syndrome was rendered by identification of anti–PL-7 antibodies and an elevated level of creatinine kinase.
Physical examination at our clinic revealed subtle palmar scaling on the hands and multiple small clear vesicles on the lateral aspects of the digits (Figure, A), consistent with dyshidrotic eczema. He initially was treated with clobetasol propionate ointment 0.05%. Despite adherence to this high-potency topical corticosteroid, he experienced only minimal improvement over a period of 3 months. Dupilumab was started at standard dosing—600 mg at initiation, followed by 300 mg every 2 weeks. The patient reported rapid improvement in dyshidrotic eczema over several months with near-complete resolution (Figure, B).
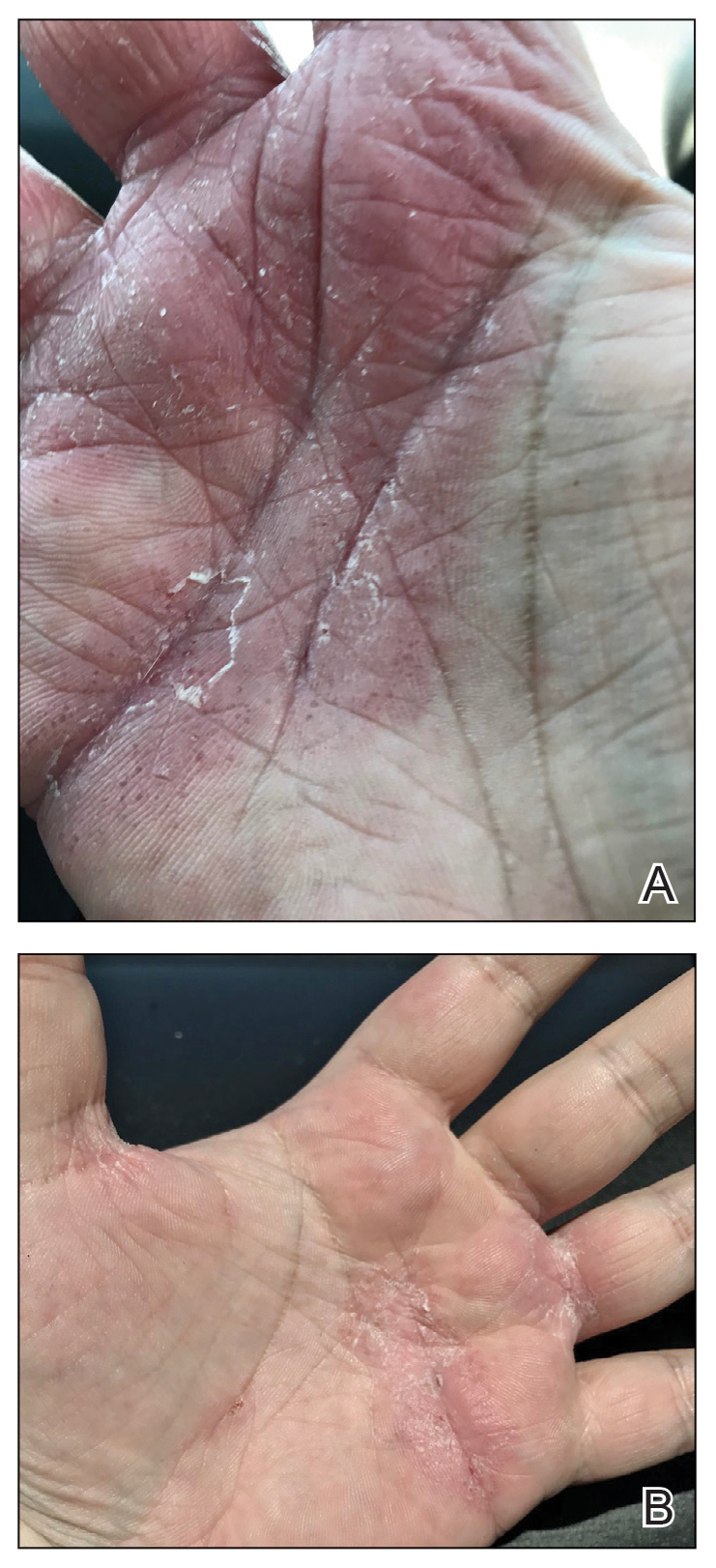
Concurrent with initiation and continued use of dupilumab, without other changes in his medication regimen, the patient noted gradual improvement in respiratory symptoms. At 6-month follow-up he reported notable improvement in respiratory function and quality of life. He then tolerated a gradual wean of prednisone to 10 mg/d, with a similar reduction in supplemental oxygen.
Off-label use of dupilumab for various eosinophilic conditions has shown promising efficacy. Our patient experienced improvement in CEP shortly after initiation of dupilumab, enabling weaning of prednisone, which has a well established adverse effect profile associated with long term use.3,4 In comparison, dupilumab generally is well tolerated, with rare ophthalmologic complications and injection-site reactions.5
One case report suggested that CEP may represent a potential rare adverse effect of dupilumab initiation.6 However, prior to initiation of dupilumab, that patient had poorly controlled asthma requiring frequent oral corticosteroid therapy. It is possible that CEP was subclinical prior to initiation of dupilumab and became more noticeable once the patient was weaned from corticosteroids, which had served as an indirect treatment.6 Nonetheless, more research is needed to definitively establish the efficacy of dupilumab in CEP prior to more widespread use.
Irrespective of the potential efficacy of dupilumab for the treatment of CEP, our case highlights the growing body of evidence that dupilumab should be considered in the treatment of dyshidrotic eczema, particularly in cases refractory to topical treatment.7 When a systemic medication is preferred, dupilumab likely represents an option with a relatively well-tolerated adverse effect profile compared to traditional systemic treatments for dyshidrotic eczema.
1. Barbarot S, Wollenberg A, Silverberg JI, et al. Dupilumab provides rapid and sustained improvement in SCORAD outcomes in adults with moderate-to-severe atopic dermatitis: combined results ofour randomized phase 3 trials. J Dermatolog Treat. 2022;33:266-277. doi:10.1080/09546634.2020.1750550
2. Gordon SC, Robinson SN, Abudu M, et al. Eosinophilic annular erythema treated with dupilumab. Pediatr Dermatol. 2018;35:E255-E256. doi:10.1111/pde.13533
3. Callaghan DJ 3rd. Use of Google Trends to examine interest in Mohs micrographic surgery: 2004 to 2016. Dermatol Surg. 2018;44:186-192. doi:10.1097/DSS.0000000000001270
4. Fowler C, Hoover W. Dupilumab for chronic eosinophilic pneumonia. Pediatr Pulmonol. 2020;55:3229-3230. doi:10.1002/ppul.25096
5. Simpson EL, Akinlade B, Ardeleanu M. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2017;376:1090-1091. doi:10.1056/NEJMc1700366
6. Menzella F, Montanari G, Patricelli G, et al. A case of chronic eosinophilic pneumonia in a patient treated with dupilumab. Ther Clin Risk Manag. 2019;15:869-875. doi:10.2147/TCRM.S207402
7. Waldman RA, DeWane ME, Sloan B, et al. Dupilumab for the treatment of dyshidrotic eczema in 15 consecutive patients. J Am Acad Dermatol. 2020;82:1251-1252. doi:10.1016/j.jaad.2019.12.053
To the Editor:
Biologic medications are increasingly utilized in adults with moderate to severe atopic dermatitis (AD) that is inadequately controlled with topical medication. By targeting the IL-4 receptor alpha subunit, dupilumab inhibits the biologic effects of IL-4 and IL-13, resulting in remarkable improvement in disease and quality of life for many patients with refractory AD.1
In 2017, the US Food and Drug Administration approved dupilumab for use in AD, asthma, and chronic rhinosinusitis. However, there is evidence of the drug’s off-label efficacy in conditions such as eosinophilic annular erythema.2 We present a patient with dyshidrotic eczema treated with dupilumab who experienced contemporaneous secondary improvement in chronic eosinophilic pneumonia (CEP) and interstitial lung disease (ILD).
A 45-year-old man was referred to our dermatology clinic for chronic hand dermatitis refractory to increasing strengths of topical corticosteroids. He had a history of progressive shortness of breath of unknown cause, which began 2 years prior, and he was being followed at our institution’s ILD clinic. Earlier pulmonary function testing revealed a restrictive pattern with interstitial infiltrates seen on chest computed tomography. A lung biopsy demonstrated features of fibrotic nonspecific interstitial pneumonitis with superimposed eosinophilic pneumonia. His pulmonary symptoms had progressively worsened; over a period of several months, the supplemental oxygen requirement had increased to 6 L at rest and 12 L upon exertion. Prednisone therapy was initiated, which alleviated respiratory symptoms; however, the patient was unable to tolerate a gradual wean of the medication, which rendered him steroid dependent at 30 mg/d.
Along with respiratory symptoms, the patient reported symptoms consistent with an autoimmune process, including dry eyes. Muscle weakness and tenderness also were noted. Ultimately, a diagnosis of anti–PL-7 (anti-threonyl-transfer RNA synthetase) antisynthetase syndrome was rendered by identification of anti–PL-7 antibodies and an elevated level of creatinine kinase.
Physical examination at our clinic revealed subtle palmar scaling on the hands and multiple small clear vesicles on the lateral aspects of the digits (Figure, A), consistent with dyshidrotic eczema. He initially was treated with clobetasol propionate ointment 0.05%. Despite adherence to this high-potency topical corticosteroid, he experienced only minimal improvement over a period of 3 months. Dupilumab was started at standard dosing—600 mg at initiation, followed by 300 mg every 2 weeks. The patient reported rapid improvement in dyshidrotic eczema over several months with near-complete resolution (Figure, B).

Concurrent with initiation and continued use of dupilumab, without other changes in his medication regimen, the patient noted gradual improvement in respiratory symptoms. At 6-month follow-up he reported notable improvement in respiratory function and quality of life. He then tolerated a gradual wean of prednisone to 10 mg/d, with a similar reduction in supplemental oxygen.
Off-label use of dupilumab for various eosinophilic conditions has shown promising efficacy. Our patient experienced improvement in CEP shortly after initiation of dupilumab, enabling weaning of prednisone, which has a well established adverse effect profile associated with long term use.3,4 In comparison, dupilumab generally is well tolerated, with rare ophthalmologic complications and injection-site reactions.5
One case report suggested that CEP may represent a potential rare adverse effect of dupilumab initiation.6 However, prior to initiation of dupilumab, that patient had poorly controlled asthma requiring frequent oral corticosteroid therapy. It is possible that CEP was subclinical prior to initiation of dupilumab and became more noticeable once the patient was weaned from corticosteroids, which had served as an indirect treatment.6 Nonetheless, more research is needed to definitively establish the efficacy of dupilumab in CEP prior to more widespread use.
Irrespective of the potential efficacy of dupilumab for the treatment of CEP, our case highlights the growing body of evidence that dupilumab should be considered in the treatment of dyshidrotic eczema, particularly in cases refractory to topical treatment.7 When a systemic medication is preferred, dupilumab likely represents an option with a relatively well-tolerated adverse effect profile compared to traditional systemic treatments for dyshidrotic eczema.
To the Editor:
Biologic medications are increasingly utilized in adults with moderate to severe atopic dermatitis (AD) that is inadequately controlled with topical medication. By targeting the IL-4 receptor alpha subunit, dupilumab inhibits the biologic effects of IL-4 and IL-13, resulting in remarkable improvement in disease and quality of life for many patients with refractory AD.1
In 2017, the US Food and Drug Administration approved dupilumab for use in AD, asthma, and chronic rhinosinusitis. However, there is evidence of the drug’s off-label efficacy in conditions such as eosinophilic annular erythema.2 We present a patient with dyshidrotic eczema treated with dupilumab who experienced contemporaneous secondary improvement in chronic eosinophilic pneumonia (CEP) and interstitial lung disease (ILD).
A 45-year-old man was referred to our dermatology clinic for chronic hand dermatitis refractory to increasing strengths of topical corticosteroids. He had a history of progressive shortness of breath of unknown cause, which began 2 years prior, and he was being followed at our institution’s ILD clinic. Earlier pulmonary function testing revealed a restrictive pattern with interstitial infiltrates seen on chest computed tomography. A lung biopsy demonstrated features of fibrotic nonspecific interstitial pneumonitis with superimposed eosinophilic pneumonia. His pulmonary symptoms had progressively worsened; over a period of several months, the supplemental oxygen requirement had increased to 6 L at rest and 12 L upon exertion. Prednisone therapy was initiated, which alleviated respiratory symptoms; however, the patient was unable to tolerate a gradual wean of the medication, which rendered him steroid dependent at 30 mg/d.
Along with respiratory symptoms, the patient reported symptoms consistent with an autoimmune process, including dry eyes. Muscle weakness and tenderness also were noted. Ultimately, a diagnosis of anti–PL-7 (anti-threonyl-transfer RNA synthetase) antisynthetase syndrome was rendered by identification of anti–PL-7 antibodies and an elevated level of creatinine kinase.
Physical examination at our clinic revealed subtle palmar scaling on the hands and multiple small clear vesicles on the lateral aspects of the digits (Figure, A), consistent with dyshidrotic eczema. He initially was treated with clobetasol propionate ointment 0.05%. Despite adherence to this high-potency topical corticosteroid, he experienced only minimal improvement over a period of 3 months. Dupilumab was started at standard dosing—600 mg at initiation, followed by 300 mg every 2 weeks. The patient reported rapid improvement in dyshidrotic eczema over several months with near-complete resolution (Figure, B).

Concurrent with initiation and continued use of dupilumab, without other changes in his medication regimen, the patient noted gradual improvement in respiratory symptoms. At 6-month follow-up he reported notable improvement in respiratory function and quality of life. He then tolerated a gradual wean of prednisone to 10 mg/d, with a similar reduction in supplemental oxygen.
Off-label use of dupilumab for various eosinophilic conditions has shown promising efficacy. Our patient experienced improvement in CEP shortly after initiation of dupilumab, enabling weaning of prednisone, which has a well established adverse effect profile associated with long term use.3,4 In comparison, dupilumab generally is well tolerated, with rare ophthalmologic complications and injection-site reactions.5
One case report suggested that CEP may represent a potential rare adverse effect of dupilumab initiation.6 However, prior to initiation of dupilumab, that patient had poorly controlled asthma requiring frequent oral corticosteroid therapy. It is possible that CEP was subclinical prior to initiation of dupilumab and became more noticeable once the patient was weaned from corticosteroids, which had served as an indirect treatment.6 Nonetheless, more research is needed to definitively establish the efficacy of dupilumab in CEP prior to more widespread use.
Irrespective of the potential efficacy of dupilumab for the treatment of CEP, our case highlights the growing body of evidence that dupilumab should be considered in the treatment of dyshidrotic eczema, particularly in cases refractory to topical treatment.7 When a systemic medication is preferred, dupilumab likely represents an option with a relatively well-tolerated adverse effect profile compared to traditional systemic treatments for dyshidrotic eczema.
1. Barbarot S, Wollenberg A, Silverberg JI, et al. Dupilumab provides rapid and sustained improvement in SCORAD outcomes in adults with moderate-to-severe atopic dermatitis: combined results ofour randomized phase 3 trials. J Dermatolog Treat. 2022;33:266-277. doi:10.1080/09546634.2020.1750550
2. Gordon SC, Robinson SN, Abudu M, et al. Eosinophilic annular erythema treated with dupilumab. Pediatr Dermatol. 2018;35:E255-E256. doi:10.1111/pde.13533
3. Callaghan DJ 3rd. Use of Google Trends to examine interest in Mohs micrographic surgery: 2004 to 2016. Dermatol Surg. 2018;44:186-192. doi:10.1097/DSS.0000000000001270
4. Fowler C, Hoover W. Dupilumab for chronic eosinophilic pneumonia. Pediatr Pulmonol. 2020;55:3229-3230. doi:10.1002/ppul.25096
5. Simpson EL, Akinlade B, Ardeleanu M. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2017;376:1090-1091. doi:10.1056/NEJMc1700366
6. Menzella F, Montanari G, Patricelli G, et al. A case of chronic eosinophilic pneumonia in a patient treated with dupilumab. Ther Clin Risk Manag. 2019;15:869-875. doi:10.2147/TCRM.S207402
7. Waldman RA, DeWane ME, Sloan B, et al. Dupilumab for the treatment of dyshidrotic eczema in 15 consecutive patients. J Am Acad Dermatol. 2020;82:1251-1252. doi:10.1016/j.jaad.2019.12.053
1. Barbarot S, Wollenberg A, Silverberg JI, et al. Dupilumab provides rapid and sustained improvement in SCORAD outcomes in adults with moderate-to-severe atopic dermatitis: combined results ofour randomized phase 3 trials. J Dermatolog Treat. 2022;33:266-277. doi:10.1080/09546634.2020.1750550
2. Gordon SC, Robinson SN, Abudu M, et al. Eosinophilic annular erythema treated with dupilumab. Pediatr Dermatol. 2018;35:E255-E256. doi:10.1111/pde.13533
3. Callaghan DJ 3rd. Use of Google Trends to examine interest in Mohs micrographic surgery: 2004 to 2016. Dermatol Surg. 2018;44:186-192. doi:10.1097/DSS.0000000000001270
4. Fowler C, Hoover W. Dupilumab for chronic eosinophilic pneumonia. Pediatr Pulmonol. 2020;55:3229-3230. doi:10.1002/ppul.25096
5. Simpson EL, Akinlade B, Ardeleanu M. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2017;376:1090-1091. doi:10.1056/NEJMc1700366
6. Menzella F, Montanari G, Patricelli G, et al. A case of chronic eosinophilic pneumonia in a patient treated with dupilumab. Ther Clin Risk Manag. 2019;15:869-875. doi:10.2147/TCRM.S207402
7. Waldman RA, DeWane ME, Sloan B, et al. Dupilumab for the treatment of dyshidrotic eczema in 15 consecutive patients. J Am Acad Dermatol. 2020;82:1251-1252. doi:10.1016/j.jaad.2019.12.053
Practice Points
- Dupilumab can be considered for treatment of refractory dyshidrotic eczema.
- Dupilumab may provide secondary efficacy in patients with dyshidrotic eczema who also have an eosinophilic condition such as eosinophilic pneumonia.
Quality Improvement Project of All Resected Lung Specimens for Pathologic Findings and Synoptic Surgical Reports for Accuracy in Staging: A Critical Review of 91 Specimens
BACKGROUND
In 2017, the Thoracic Tumor Board realized that there were patients whose lung resections had critical review of the slides and reports prior to presentation. Errors were found which resulted in a change of the pathology Tumor Nodal Metastases (pTNM) staging for the patient. The impacts were important for determining appropriate therapy. It was decided to systematically review all lung cancer resections for accuracy before determining definitive therapy recommendations.
METHODS
All lung resections for malignancy were examined prior and up to 2 days of completion for accuracy of tumor type, tumor size, tumor grade, lymph node metastases and pathologic stage (pTNM). Any errors found were given to the original pathologist for a change in the report before release or for a modified report to be issued.
RESULTS
From June 2017 to December 2020, there were 91 lung resections with 28 (30.77%) errors. Errors included: 16 incorrect pathologic staging, 5 missed tumors in lung and lymph nodes, 2 unexamined stapled surgical margins, 1 wrong site, 1 incorrect lymph node number and 2 missed tumor vascular invasion.
IMPLICATIONS
Quality improvement (QI) review of lung resections by a second pathologist is important and may clearly improve pathologic staging for lung cancer patients. It can be added to QI programs currently used in Surgical Pathology. It is important in directing appropriate postsurgical therapies.
BACKGROUND
In 2017, the Thoracic Tumor Board realized that there were patients whose lung resections had critical review of the slides and reports prior to presentation. Errors were found which resulted in a change of the pathology Tumor Nodal Metastases (pTNM) staging for the patient. The impacts were important for determining appropriate therapy. It was decided to systematically review all lung cancer resections for accuracy before determining definitive therapy recommendations.
METHODS
All lung resections for malignancy were examined prior and up to 2 days of completion for accuracy of tumor type, tumor size, tumor grade, lymph node metastases and pathologic stage (pTNM). Any errors found were given to the original pathologist for a change in the report before release or for a modified report to be issued.
RESULTS
From June 2017 to December 2020, there were 91 lung resections with 28 (30.77%) errors. Errors included: 16 incorrect pathologic staging, 5 missed tumors in lung and lymph nodes, 2 unexamined stapled surgical margins, 1 wrong site, 1 incorrect lymph node number and 2 missed tumor vascular invasion.
IMPLICATIONS
Quality improvement (QI) review of lung resections by a second pathologist is important and may clearly improve pathologic staging for lung cancer patients. It can be added to QI programs currently used in Surgical Pathology. It is important in directing appropriate postsurgical therapies.
BACKGROUND
In 2017, the Thoracic Tumor Board realized that there were patients whose lung resections had critical review of the slides and reports prior to presentation. Errors were found which resulted in a change of the pathology Tumor Nodal Metastases (pTNM) staging for the patient. The impacts were important for determining appropriate therapy. It was decided to systematically review all lung cancer resections for accuracy before determining definitive therapy recommendations.
METHODS
All lung resections for malignancy were examined prior and up to 2 days of completion for accuracy of tumor type, tumor size, tumor grade, lymph node metastases and pathologic stage (pTNM). Any errors found were given to the original pathologist for a change in the report before release or for a modified report to be issued.
RESULTS
From June 2017 to December 2020, there were 91 lung resections with 28 (30.77%) errors. Errors included: 16 incorrect pathologic staging, 5 missed tumors in lung and lymph nodes, 2 unexamined stapled surgical margins, 1 wrong site, 1 incorrect lymph node number and 2 missed tumor vascular invasion.
IMPLICATIONS
Quality improvement (QI) review of lung resections by a second pathologist is important and may clearly improve pathologic staging for lung cancer patients. It can be added to QI programs currently used in Surgical Pathology. It is important in directing appropriate postsurgical therapies.
A Rare Case of Leptomeningeal Carcinomatosis From Gastroesophageal Adenocarcinoma Masquerading as Polyneuropathy
INTRODUCTION
Leptomeningeal metastasis (LM) is an extremely rare complication of gastroesophageal (GE) cancer. Diagnosis is challenging due to frequently nonspecific clinical presentations, limited sensitivity of diagnostic testing, and potential overlap with neurologic immune-related adverse events (irAE). We describe a case of metastatic gastroesophageal cancer on immunotherapy presenting with LM masquerading as polyneuropathy.
CASE REPORT
A 74-year-old male with HER2+ GE junction cancer with peritoneal metastases diagnosed 6 months ago, on maintenance trastuzumab/pembrolizumab and with no previous history of cranial or spinal disease, presented with worsening ataxia, headache, and diplopia for one month with multiple negative outpatient MRIs. Examination showed left abducens nerve palsy, dysmetria and absent deep tendon reflexes in upper and lower extremities. CT head was unremarkable, and MRI showed non-specific mild enhancement of the right optic nerve, symmetrical lumbosacral nerve roots and cauda equina concerning for paraneoplastic versus immunotherapy-related polyneuropathy. He was started on empiric high-dose corticosteroids. PET-CT was negative for FDG-avid lesions. Cerebrospinal fluid (CSF) analysis revealed moderate pleocytosis with many large atypical cells, elevated protein (118 mg/dL) and LDH (28 IU/L). Immunohistochemistry was positive for CDX2, CA 19-9, CK7, and pankeratin, consistent with metastatic adenocarcinoma, negative for HER2 in contrast to the original tumor. He subsequently developed hydrocephalus requiring a ventriculoperitoneal shunt. He received ten fractions of whole brain irradiation before electing to pursue hospice care.
DISCUSSION
LM is an extremely rare complication of GE cancer with an incidence of <0.2% and carries a poor prognosis. Differentiation between LM and irAE in patients on immunotherapy can be challenging. Diagnosis relies mostly on CSF cytology, and lumbar puncture should not be delayed in patients with new neurologic symptoms. Treatment options are intrathecal chemotherapy, radiation and steroids. A recent phase II trial has shown promise for intrathecal trastuzumab in patients with HER2+ cancers, but options for HER2 negative disease remain mostly palliative.
CONCLUSIONS
Our case highlights the need for suspecting this rare metastatic site, as early diagnosis and genetic characterization allow for exploring more treatment options including targeted therapies which may improve overall survival and quality of life.
INTRODUCTION
Leptomeningeal metastasis (LM) is an extremely rare complication of gastroesophageal (GE) cancer. Diagnosis is challenging due to frequently nonspecific clinical presentations, limited sensitivity of diagnostic testing, and potential overlap with neurologic immune-related adverse events (irAE). We describe a case of metastatic gastroesophageal cancer on immunotherapy presenting with LM masquerading as polyneuropathy.
CASE REPORT
A 74-year-old male with HER2+ GE junction cancer with peritoneal metastases diagnosed 6 months ago, on maintenance trastuzumab/pembrolizumab and with no previous history of cranial or spinal disease, presented with worsening ataxia, headache, and diplopia for one month with multiple negative outpatient MRIs. Examination showed left abducens nerve palsy, dysmetria and absent deep tendon reflexes in upper and lower extremities. CT head was unremarkable, and MRI showed non-specific mild enhancement of the right optic nerve, symmetrical lumbosacral nerve roots and cauda equina concerning for paraneoplastic versus immunotherapy-related polyneuropathy. He was started on empiric high-dose corticosteroids. PET-CT was negative for FDG-avid lesions. Cerebrospinal fluid (CSF) analysis revealed moderate pleocytosis with many large atypical cells, elevated protein (118 mg/dL) and LDH (28 IU/L). Immunohistochemistry was positive for CDX2, CA 19-9, CK7, and pankeratin, consistent with metastatic adenocarcinoma, negative for HER2 in contrast to the original tumor. He subsequently developed hydrocephalus requiring a ventriculoperitoneal shunt. He received ten fractions of whole brain irradiation before electing to pursue hospice care.
DISCUSSION
LM is an extremely rare complication of GE cancer with an incidence of <0.2% and carries a poor prognosis. Differentiation between LM and irAE in patients on immunotherapy can be challenging. Diagnosis relies mostly on CSF cytology, and lumbar puncture should not be delayed in patients with new neurologic symptoms. Treatment options are intrathecal chemotherapy, radiation and steroids. A recent phase II trial has shown promise for intrathecal trastuzumab in patients with HER2+ cancers, but options for HER2 negative disease remain mostly palliative.
CONCLUSIONS
Our case highlights the need for suspecting this rare metastatic site, as early diagnosis and genetic characterization allow for exploring more treatment options including targeted therapies which may improve overall survival and quality of life.
INTRODUCTION
Leptomeningeal metastasis (LM) is an extremely rare complication of gastroesophageal (GE) cancer. Diagnosis is challenging due to frequently nonspecific clinical presentations, limited sensitivity of diagnostic testing, and potential overlap with neurologic immune-related adverse events (irAE). We describe a case of metastatic gastroesophageal cancer on immunotherapy presenting with LM masquerading as polyneuropathy.
CASE REPORT
A 74-year-old male with HER2+ GE junction cancer with peritoneal metastases diagnosed 6 months ago, on maintenance trastuzumab/pembrolizumab and with no previous history of cranial or spinal disease, presented with worsening ataxia, headache, and diplopia for one month with multiple negative outpatient MRIs. Examination showed left abducens nerve palsy, dysmetria and absent deep tendon reflexes in upper and lower extremities. CT head was unremarkable, and MRI showed non-specific mild enhancement of the right optic nerve, symmetrical lumbosacral nerve roots and cauda equina concerning for paraneoplastic versus immunotherapy-related polyneuropathy. He was started on empiric high-dose corticosteroids. PET-CT was negative for FDG-avid lesions. Cerebrospinal fluid (CSF) analysis revealed moderate pleocytosis with many large atypical cells, elevated protein (118 mg/dL) and LDH (28 IU/L). Immunohistochemistry was positive for CDX2, CA 19-9, CK7, and pankeratin, consistent with metastatic adenocarcinoma, negative for HER2 in contrast to the original tumor. He subsequently developed hydrocephalus requiring a ventriculoperitoneal shunt. He received ten fractions of whole brain irradiation before electing to pursue hospice care.
DISCUSSION
LM is an extremely rare complication of GE cancer with an incidence of <0.2% and carries a poor prognosis. Differentiation between LM and irAE in patients on immunotherapy can be challenging. Diagnosis relies mostly on CSF cytology, and lumbar puncture should not be delayed in patients with new neurologic symptoms. Treatment options are intrathecal chemotherapy, radiation and steroids. A recent phase II trial has shown promise for intrathecal trastuzumab in patients with HER2+ cancers, but options for HER2 negative disease remain mostly palliative.
CONCLUSIONS
Our case highlights the need for suspecting this rare metastatic site, as early diagnosis and genetic characterization allow for exploring more treatment options including targeted therapies which may improve overall survival and quality of life.