User login
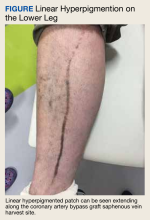
A 59-year-old man with a history of coronary artery bypass grafting (CABG), ischemic cardiomyopathy (ejection fraction, 15%-20%) with implantable cardioverter-defibrillator, recurrent paroxysmal ventricular tachycardia on amiodarone and mexiletine, and heart failure requiring left ventricular assist device (LVAD) placement presented for recurrent cellulitis and infection of the LVAD driveline exit site. He was initiated on minocycline 100 mg twice daily in combination with cefadroxil 500 mg twice daily. At his 8-week follow-up, the driveline site appeared improved with minimal erythema and no drainage. However, the patient developed a well-demarcated, linear, hyperpigmented patch along the length of the saphenous vein CABG harvest site and a few hyperpigmented macules medial to the harvest site (Figure).
Discussion
Hyperpigmentation presenting within scar tissue, as seen in this patient undergoing minocycline therapy, is a classic presentation of minocycline-induced hyperpigmentation (MIH) type I.
MIH is an uncommon, potentially cosmetically disfiguring adverse effect associated with systemic minocycline use. MIH can affect skin, teeth, nails, oral mucosa, sclera, and internal organs. The cumulative incidence of MIH in patients receiving minocycline over prolonged periods of time has been estimated from 2% to 15% in patients with acne and rosacea, to approximately 50% over 5 years in orthopedic patient populations.1-3 The risk for developing MIH increases with vitamin D deficiency, liver disease, concurrent use with other medications that can induce hyperpigmentation, and higher cumulative doses (> 70-100 g; more important for MIH types II and III).3,4
There are 3 distinct types of MIH. Type I MIH is characterized by blue-black macules and patches at sites of inflammation or prior scarring, most commonly described in facial acne scars.1,2,4 Type II is typified by blue-grey pigmentation on normal-appearing skin, most commonly on the shins, but also on sun-exposed sites.3 Biopsies of type I and II MIH demonstrate pigmented granules within macrophages or within the dermis.4,5 Both Perls iron stain and Fontana-Masson melanin stain are positive in type I and II MIH.5 Type III MIH presents as diffuse brownish hyperpigmentation on normal skin in chronically sun-exposed sites.3 Histopathology of type III MIH can be distinguished by increased melanin noted inside basal keratinocytes as well as dermal melanophages that stain positive for only Fontana-Masson.5 The current case exemplifies a unique presentation of type I MIH along the length of the saphenous vein CABG harvest site. The concomitant use of amiodarone with minocycline may have contributed to the presentation.
The differential diagnosis for MIH depends on the type of MIH. Blue-grey pigmentation within scars is fairly unique to minocycline but has been reported with other medications, including vandetanib.6 The differential diagnosis for diffuse blue-grey or brown hyperpigmentation in predominately sun-exposed sites is broader, including endocrine disorders (ie, Addison disease), heavy metal poisoning (ie, argyria), inherited conditions (ie, alkaptonuria, Wilson disease, and hemochromatosis), medication-induced hyperpigmentation (ie, antipsychotics, anticonvulsant, antimalarials, amiodarone, and cytotoxic drugs), as well as inflammatory dermatoses, such as erythema dyschromicum perstans.7
MIH typically fades over months to years following minocycline discontinuation, so prompt recognition and discontinuation is recommended. Unfortunately, some cases persist or only partially fade over time. While MIH is benign, it can be of aesthetic concern, cause anxiety, and impact patients’ quality of life.3,8 Persistent MIH is typically recalcitrant to topical hydroquinone.9 However, persistent MIH has been shown to improve with Q-switched, nanosecond lasers such as the 694 nm ruby, 755 nm alexandrite, and 1064 nm neodymium-doped yttrium aluminum garnet neodymium (Nd:YAG) lasers, as well as the 755 nm picosecond alexandrite laser.4,9,10
In our patient, minocycline therapy was discontinued and replaced with doxycycline 100 mg twice daily monotherapy. At a subsequent visit 12 weeks later, the hyperpigmentation remained unchanged.
Conclusions
Though uncommon, we hope to encourage clinician awareness of MIH through our case, as prompt diagnosis and the discontinuation of minocycline are preferred to improve patient outcomes.
1. Goulden V, Glass D, Cunliffe WJ. Safety of long-term high-dose minocycline in the treatment of acne. Br J Dermatol. 1996;134(4):693-695. doi:10.1111/j.1365-2133.1996.tb06972.x
2. Dwyer CM, Cuddihy AM, Kerr RE, Chapman RS, Allam BF. Skin pigmentation due to minocycline treatment of facial dermatoses. Br J Dermatol. 1993;129(2):158-162. doi:10.1111/j.1365-2133.1993.tb03519.x
3. Hanada Y, Berbari EF, Steckelberg JM. Minocycline-induced cutaneous hyperpigmentation in an orthopedic patient population. Open Forum Infect Dis. 2016;3(1):ofv107. doi:10.1093/ofid/ofv107
4. Eisen D, Hakim MD. Minocycline-induced pigmentation. Incidence, prevention and management. Drug Saf. 1998;18(6):431-440. doi:10.2165/00002018-199818060-00004
5. Bowen AR, McCalmont TH. The histopathology of subcutaneous minocycline pigmentation. J Am Acad Dermatol. 2007;57(5):836-839. doi:10.1016/j.jaad.2007.04.028
6. Perlmutter JW, Cogan RC, Wiseman MC. Blue-grey hyperpigmentation in acne after vandetanib therapy and doxycycline use: a case report. SAGE Open Med Case Rep. 2022;10:2050313X221086316. doi:10.1177/2050313X221086316
7. Judson T, Mihara K. Minocycline-induced hyperpigmentation. J Gen Intern Med. 2017;32(1):133. doi:10.1007/s11606-016-3735-x
8. Li Y, Zhen X, Yao X, Lu J. Successful treatment of minocycline-induced facial hyperpigmentation with a combination of chemical peels and intense pulsed light. Clin Cosmet Investig Dermatol. 2023;16:253-256. doi:10.2147/CCID.S394754
9. Sasaki K, Ohshiro T, Ohshiro T, et al. Type 2 Minocycline-induced hyperpigmentation successfully treated with the novel 755 nm picosecond alexandrite laser – a case report. Laser Ther. 2017;26(2):137-144. doi:10.5978/islsm.17-CR-03
10. Nisar MS, Iyer K, Brodell RT, Lloyd JR, Shin TM, Ahmad A. Minocycline-induced hyperpigmentation: comparison of 3 Q-switched lasers to reverse its effects. Clin Cosmet Investig Dermatol. 2013;6:159-162. doi:10.2147/CCID.S42166
A 59-year-old man with a history of coronary artery bypass grafting (CABG), ischemic cardiomyopathy (ejection fraction, 15%-20%) with implantable cardioverter-defibrillator, recurrent paroxysmal ventricular tachycardia on amiodarone and mexiletine, and heart failure requiring left ventricular assist device (LVAD) placement presented for recurrent cellulitis and infection of the LVAD driveline exit site. He was initiated on minocycline 100 mg twice daily in combination with cefadroxil 500 mg twice daily. At his 8-week follow-up, the driveline site appeared improved with minimal erythema and no drainage. However, the patient developed a well-demarcated, linear, hyperpigmented patch along the length of the saphenous vein CABG harvest site and a few hyperpigmented macules medial to the harvest site (Figure).
Discussion
Hyperpigmentation presenting within scar tissue, as seen in this patient undergoing minocycline therapy, is a classic presentation of minocycline-induced hyperpigmentation (MIH) type I.
MIH is an uncommon, potentially cosmetically disfiguring adverse effect associated with systemic minocycline use. MIH can affect skin, teeth, nails, oral mucosa, sclera, and internal organs. The cumulative incidence of MIH in patients receiving minocycline over prolonged periods of time has been estimated from 2% to 15% in patients with acne and rosacea, to approximately 50% over 5 years in orthopedic patient populations.1-3 The risk for developing MIH increases with vitamin D deficiency, liver disease, concurrent use with other medications that can induce hyperpigmentation, and higher cumulative doses (> 70-100 g; more important for MIH types II and III).3,4
There are 3 distinct types of MIH. Type I MIH is characterized by blue-black macules and patches at sites of inflammation or prior scarring, most commonly described in facial acne scars.1,2,4 Type II is typified by blue-grey pigmentation on normal-appearing skin, most commonly on the shins, but also on sun-exposed sites.3 Biopsies of type I and II MIH demonstrate pigmented granules within macrophages or within the dermis.4,5 Both Perls iron stain and Fontana-Masson melanin stain are positive in type I and II MIH.5 Type III MIH presents as diffuse brownish hyperpigmentation on normal skin in chronically sun-exposed sites.3 Histopathology of type III MIH can be distinguished by increased melanin noted inside basal keratinocytes as well as dermal melanophages that stain positive for only Fontana-Masson.5 The current case exemplifies a unique presentation of type I MIH along the length of the saphenous vein CABG harvest site. The concomitant use of amiodarone with minocycline may have contributed to the presentation.
The differential diagnosis for MIH depends on the type of MIH. Blue-grey pigmentation within scars is fairly unique to minocycline but has been reported with other medications, including vandetanib.6 The differential diagnosis for diffuse blue-grey or brown hyperpigmentation in predominately sun-exposed sites is broader, including endocrine disorders (ie, Addison disease), heavy metal poisoning (ie, argyria), inherited conditions (ie, alkaptonuria, Wilson disease, and hemochromatosis), medication-induced hyperpigmentation (ie, antipsychotics, anticonvulsant, antimalarials, amiodarone, and cytotoxic drugs), as well as inflammatory dermatoses, such as erythema dyschromicum perstans.7
MIH typically fades over months to years following minocycline discontinuation, so prompt recognition and discontinuation is recommended. Unfortunately, some cases persist or only partially fade over time. While MIH is benign, it can be of aesthetic concern, cause anxiety, and impact patients’ quality of life.3,8 Persistent MIH is typically recalcitrant to topical hydroquinone.9 However, persistent MIH has been shown to improve with Q-switched, nanosecond lasers such as the 694 nm ruby, 755 nm alexandrite, and 1064 nm neodymium-doped yttrium aluminum garnet neodymium (Nd:YAG) lasers, as well as the 755 nm picosecond alexandrite laser.4,9,10
In our patient, minocycline therapy was discontinued and replaced with doxycycline 100 mg twice daily monotherapy. At a subsequent visit 12 weeks later, the hyperpigmentation remained unchanged.
Conclusions
Though uncommon, we hope to encourage clinician awareness of MIH through our case, as prompt diagnosis and the discontinuation of minocycline are preferred to improve patient outcomes.
A 59-year-old man with a history of coronary artery bypass grafting (CABG), ischemic cardiomyopathy (ejection fraction, 15%-20%) with implantable cardioverter-defibrillator, recurrent paroxysmal ventricular tachycardia on amiodarone and mexiletine, and heart failure requiring left ventricular assist device (LVAD) placement presented for recurrent cellulitis and infection of the LVAD driveline exit site. He was initiated on minocycline 100 mg twice daily in combination with cefadroxil 500 mg twice daily. At his 8-week follow-up, the driveline site appeared improved with minimal erythema and no drainage. However, the patient developed a well-demarcated, linear, hyperpigmented patch along the length of the saphenous vein CABG harvest site and a few hyperpigmented macules medial to the harvest site (Figure).
Discussion
Hyperpigmentation presenting within scar tissue, as seen in this patient undergoing minocycline therapy, is a classic presentation of minocycline-induced hyperpigmentation (MIH) type I.
MIH is an uncommon, potentially cosmetically disfiguring adverse effect associated with systemic minocycline use. MIH can affect skin, teeth, nails, oral mucosa, sclera, and internal organs. The cumulative incidence of MIH in patients receiving minocycline over prolonged periods of time has been estimated from 2% to 15% in patients with acne and rosacea, to approximately 50% over 5 years in orthopedic patient populations.1-3 The risk for developing MIH increases with vitamin D deficiency, liver disease, concurrent use with other medications that can induce hyperpigmentation, and higher cumulative doses (> 70-100 g; more important for MIH types II and III).3,4
There are 3 distinct types of MIH. Type I MIH is characterized by blue-black macules and patches at sites of inflammation or prior scarring, most commonly described in facial acne scars.1,2,4 Type II is typified by blue-grey pigmentation on normal-appearing skin, most commonly on the shins, but also on sun-exposed sites.3 Biopsies of type I and II MIH demonstrate pigmented granules within macrophages or within the dermis.4,5 Both Perls iron stain and Fontana-Masson melanin stain are positive in type I and II MIH.5 Type III MIH presents as diffuse brownish hyperpigmentation on normal skin in chronically sun-exposed sites.3 Histopathology of type III MIH can be distinguished by increased melanin noted inside basal keratinocytes as well as dermal melanophages that stain positive for only Fontana-Masson.5 The current case exemplifies a unique presentation of type I MIH along the length of the saphenous vein CABG harvest site. The concomitant use of amiodarone with minocycline may have contributed to the presentation.
The differential diagnosis for MIH depends on the type of MIH. Blue-grey pigmentation within scars is fairly unique to minocycline but has been reported with other medications, including vandetanib.6 The differential diagnosis for diffuse blue-grey or brown hyperpigmentation in predominately sun-exposed sites is broader, including endocrine disorders (ie, Addison disease), heavy metal poisoning (ie, argyria), inherited conditions (ie, alkaptonuria, Wilson disease, and hemochromatosis), medication-induced hyperpigmentation (ie, antipsychotics, anticonvulsant, antimalarials, amiodarone, and cytotoxic drugs), as well as inflammatory dermatoses, such as erythema dyschromicum perstans.7
MIH typically fades over months to years following minocycline discontinuation, so prompt recognition and discontinuation is recommended. Unfortunately, some cases persist or only partially fade over time. While MIH is benign, it can be of aesthetic concern, cause anxiety, and impact patients’ quality of life.3,8 Persistent MIH is typically recalcitrant to topical hydroquinone.9 However, persistent MIH has been shown to improve with Q-switched, nanosecond lasers such as the 694 nm ruby, 755 nm alexandrite, and 1064 nm neodymium-doped yttrium aluminum garnet neodymium (Nd:YAG) lasers, as well as the 755 nm picosecond alexandrite laser.4,9,10
In our patient, minocycline therapy was discontinued and replaced with doxycycline 100 mg twice daily monotherapy. At a subsequent visit 12 weeks later, the hyperpigmentation remained unchanged.
Conclusions
Though uncommon, we hope to encourage clinician awareness of MIH through our case, as prompt diagnosis and the discontinuation of minocycline are preferred to improve patient outcomes.
1. Goulden V, Glass D, Cunliffe WJ. Safety of long-term high-dose minocycline in the treatment of acne. Br J Dermatol. 1996;134(4):693-695. doi:10.1111/j.1365-2133.1996.tb06972.x
2. Dwyer CM, Cuddihy AM, Kerr RE, Chapman RS, Allam BF. Skin pigmentation due to minocycline treatment of facial dermatoses. Br J Dermatol. 1993;129(2):158-162. doi:10.1111/j.1365-2133.1993.tb03519.x
3. Hanada Y, Berbari EF, Steckelberg JM. Minocycline-induced cutaneous hyperpigmentation in an orthopedic patient population. Open Forum Infect Dis. 2016;3(1):ofv107. doi:10.1093/ofid/ofv107
4. Eisen D, Hakim MD. Minocycline-induced pigmentation. Incidence, prevention and management. Drug Saf. 1998;18(6):431-440. doi:10.2165/00002018-199818060-00004
5. Bowen AR, McCalmont TH. The histopathology of subcutaneous minocycline pigmentation. J Am Acad Dermatol. 2007;57(5):836-839. doi:10.1016/j.jaad.2007.04.028
6. Perlmutter JW, Cogan RC, Wiseman MC. Blue-grey hyperpigmentation in acne after vandetanib therapy and doxycycline use: a case report. SAGE Open Med Case Rep. 2022;10:2050313X221086316. doi:10.1177/2050313X221086316
7. Judson T, Mihara K. Minocycline-induced hyperpigmentation. J Gen Intern Med. 2017;32(1):133. doi:10.1007/s11606-016-3735-x
8. Li Y, Zhen X, Yao X, Lu J. Successful treatment of minocycline-induced facial hyperpigmentation with a combination of chemical peels and intense pulsed light. Clin Cosmet Investig Dermatol. 2023;16:253-256. doi:10.2147/CCID.S394754
9. Sasaki K, Ohshiro T, Ohshiro T, et al. Type 2 Minocycline-induced hyperpigmentation successfully treated with the novel 755 nm picosecond alexandrite laser – a case report. Laser Ther. 2017;26(2):137-144. doi:10.5978/islsm.17-CR-03
10. Nisar MS, Iyer K, Brodell RT, Lloyd JR, Shin TM, Ahmad A. Minocycline-induced hyperpigmentation: comparison of 3 Q-switched lasers to reverse its effects. Clin Cosmet Investig Dermatol. 2013;6:159-162. doi:10.2147/CCID.S42166
1. Goulden V, Glass D, Cunliffe WJ. Safety of long-term high-dose minocycline in the treatment of acne. Br J Dermatol. 1996;134(4):693-695. doi:10.1111/j.1365-2133.1996.tb06972.x
2. Dwyer CM, Cuddihy AM, Kerr RE, Chapman RS, Allam BF. Skin pigmentation due to minocycline treatment of facial dermatoses. Br J Dermatol. 1993;129(2):158-162. doi:10.1111/j.1365-2133.1993.tb03519.x
3. Hanada Y, Berbari EF, Steckelberg JM. Minocycline-induced cutaneous hyperpigmentation in an orthopedic patient population. Open Forum Infect Dis. 2016;3(1):ofv107. doi:10.1093/ofid/ofv107
4. Eisen D, Hakim MD. Minocycline-induced pigmentation. Incidence, prevention and management. Drug Saf. 1998;18(6):431-440. doi:10.2165/00002018-199818060-00004
5. Bowen AR, McCalmont TH. The histopathology of subcutaneous minocycline pigmentation. J Am Acad Dermatol. 2007;57(5):836-839. doi:10.1016/j.jaad.2007.04.028
6. Perlmutter JW, Cogan RC, Wiseman MC. Blue-grey hyperpigmentation in acne after vandetanib therapy and doxycycline use: a case report. SAGE Open Med Case Rep. 2022;10:2050313X221086316. doi:10.1177/2050313X221086316
7. Judson T, Mihara K. Minocycline-induced hyperpigmentation. J Gen Intern Med. 2017;32(1):133. doi:10.1007/s11606-016-3735-x
8. Li Y, Zhen X, Yao X, Lu J. Successful treatment of minocycline-induced facial hyperpigmentation with a combination of chemical peels and intense pulsed light. Clin Cosmet Investig Dermatol. 2023;16:253-256. doi:10.2147/CCID.S394754
9. Sasaki K, Ohshiro T, Ohshiro T, et al. Type 2 Minocycline-induced hyperpigmentation successfully treated with the novel 755 nm picosecond alexandrite laser – a case report. Laser Ther. 2017;26(2):137-144. doi:10.5978/islsm.17-CR-03
10. Nisar MS, Iyer K, Brodell RT, Lloyd JR, Shin TM, Ahmad A. Minocycline-induced hyperpigmentation: comparison of 3 Q-switched lasers to reverse its effects. Clin Cosmet Investig Dermatol. 2013;6:159-162. doi:10.2147/CCID.S42166